User login
One-Dose HPV Vaccine Program Would Be Efficient in Canada
In Canada, switching to a one-dose, gender-neutral vaccination program for human papillomavirus (HPV) could use vaccine doses more efficiently and prevent a similar number of cervical cancer cases, compared with a two-dose program, according to a new modeling analysis.
If vaccine protection remains high during the ages of peak sexual activity, all one-dose vaccination options are projected to be “substantially more efficient” than two-dose programs, even in the most pessimistic scenarios, the study authors wrote.
In addition, the scenarios projected the elimination of cervical cancer in Canada between 2032 and 2040. HPV can also lead to oral, throat, and penile cancers, and most are preventable through vaccination.
“The COVID-19 pandemic has impacted HPV vaccination in Canada, particularly among vulnerable population subgroups,” said study author Chantal Sauvageau, MD, a consultant in infectious diseases at the National Institute of Public Health of Quebec and associate professor of social and preventive medicine at the University of Laval, Quebec City, Canada.
Switching to one-dose vaccination would offer potential economic savings and programmatic flexibility, she added. The change also could enable investments aimed at increasing vaccination rates in regions where coverage is suboptimal, as well as in subgroups with a high HPV burden. Such initiatives could mitigate the pandemic’s impact on health programs and reduce inequalities.
The study was published online in CMAJ.
Vaccination Program Changes
Globally, countries have been investigating whether to shift from a two-dose to a one-dose HPV vaccine strategy since the World Health Organization’s Strategic Advisory Group of Experts on Immunization issued a single-dose recommendation in 2022.
In July, Canada’s National Advisory Committee on Immunization (NACI) updated its guidelines to recommend the single-dose approach for ages 9-20 years. The change aligns Canada with 35 other countries, including Australia and the United Kingdom. Canada›s vaccine advisory group still recommends two doses for ages 21-26 years and three doses for patients who are immunocompromised or have HIV.
To help inform new NACI policies, Sauvageau and colleagues modeled several one-dose and two-dose strategies using HPV-ADVISE, an individual-based transmission-dynamic model of HPV infections and diseases. They looked at vaccination programs in Quebec, which has a high HPV vaccine coverage rate of around 85%, and Ontario, which has lower coverage of around 65%.
For one-dose programs, the researchers analyzed noninferior (98% efficacy) and pessimistic (90% efficacy) scenarios and different average vaccine duration periods, including lifelong, 30-year, and 25-year coverage. They compared the scenarios with a two-dose program with 98% efficacy and lifelong duration, estimating the relative reduction in HPV-16 infection and cervical cancer incidence and the number of doses needed to prevent one cervical cancer case.
Overall, the model projected that gender-neutral HPV vaccine programs with either two doses or a noninferior one dose would nearly eliminate HPV-16 infection by 2040-2045 in Quebec and reduce infection by more than 90% in Ontario. Under a one-dose strategy with 90% vaccine efficacy, rebounds in HPV-16 infection would start more than 25-30 years after a switch to a lower-dose strategy, thus providing time for officials to detect any signs of waning efficacy and change policies, if needed, the authors wrote.
In addition, the model projected that a noninferior one-dose, gender-neutral HPV vaccination program would avert a similar number of cervical cancer cases, compared with a two-dose program. The reduction would be about 60% in Quebec and 55% in Ontario, compared with no vaccination. Under the most pessimistic scenario with 25-year vaccine duration, a one-dose program would be slightly less effective in averting cancer: about 3% lower than a two-dose program over 100 years.
All one-dose scenarios were projected to lead to the elimination of cervical cancer in 8-16 years — at fewer than four cervical cancer cases per 100,000 female-years.
One-dose programs would also lead to more efficient use of vaccine doses, with about 800-1000 doses needed to prevent one cervical cancer case in a one-dose program and more than 10,000 incremental doses needed to prevent one additional cervical cancer case in a two-dose program.
What Next?
In Canada, the HPV vaccine is authorized for patients aged 9-45 years. Current immunization coverage among adolescents and young adults varies across provinces and falls below the national target of 90%. In its July 2024 update, NACI estimated that 76% of 14-year-olds of both genders received at least one vaccine dose and that 67% received two doses in 2023. Vaccine uptake was slightly higher among girls than boys.
To boost the coverage rate, shifting to a one-dose schedule could appeal to young people, as well as maintain vaccination efficacy.
“When you look at the studies that have been published worldwide, the effectiveness of one dose of the HPV vaccine is actually quite high,” said Caroline Quach-Thanh, MD, professor of microbiology, infectious diseases, immunology, and pediatrics at the University of Montreal, Quebec, Canada.
Quach-Thanh, who wasn’t involved with this study, previously served as NACI chair and now serves as chair of the Quebec Immunization Committee.
“In terms of prevention of HPV infections that may lead to cancer, whether you give one dose or two doses basically gives you the same amount of protection,” she said.
However, not all physicians agree about the switch in vaccination approaches. In early October, the Federation of Medical Women of Canada released a report with 12 recommendations to increase HPV vaccination rates, including a call for healthcare providers to continue with multidose immunization schedules for now.
“Vaccination is the most powerful action we can take in preventing HPV-related cancers. Canada is falling behind, but we can get back on track if we act quickly,” said Vivien Brown, MD, chair of the group’s HPV Immunization Task Force, chair and cofounder of HPV Prevention Week in Canada, and a past president of the federation.
After the NACI update in July, the task force evaluated the risks and benefits of a single-dose vaccine regimen, she said. They concluded that a multidose schedule should continue at this time because of its proven effectiveness.
“Until more research on the efficacy of a single-dose schedule becomes available, healthcare providers and public health agencies should continue to offer patients a multidose schedule,” said Brown. “This is the only way to ensure individuals are protected against HPV infection and cancer over the long term.”
The study was supported by the Public Health Agency of Canada, the Canadian Institutes of Health Research, the Bill & Melinda Gates Foundation, and Canadian Immunization Research Network. Sauvageau, Quach-Thanh, and Brown declared no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
In Canada, switching to a one-dose, gender-neutral vaccination program for human papillomavirus (HPV) could use vaccine doses more efficiently and prevent a similar number of cervical cancer cases, compared with a two-dose program, according to a new modeling analysis.
If vaccine protection remains high during the ages of peak sexual activity, all one-dose vaccination options are projected to be “substantially more efficient” than two-dose programs, even in the most pessimistic scenarios, the study authors wrote.
In addition, the scenarios projected the elimination of cervical cancer in Canada between 2032 and 2040. HPV can also lead to oral, throat, and penile cancers, and most are preventable through vaccination.
“The COVID-19 pandemic has impacted HPV vaccination in Canada, particularly among vulnerable population subgroups,” said study author Chantal Sauvageau, MD, a consultant in infectious diseases at the National Institute of Public Health of Quebec and associate professor of social and preventive medicine at the University of Laval, Quebec City, Canada.
Switching to one-dose vaccination would offer potential economic savings and programmatic flexibility, she added. The change also could enable investments aimed at increasing vaccination rates in regions where coverage is suboptimal, as well as in subgroups with a high HPV burden. Such initiatives could mitigate the pandemic’s impact on health programs and reduce inequalities.
The study was published online in CMAJ.
Vaccination Program Changes
Globally, countries have been investigating whether to shift from a two-dose to a one-dose HPV vaccine strategy since the World Health Organization’s Strategic Advisory Group of Experts on Immunization issued a single-dose recommendation in 2022.
In July, Canada’s National Advisory Committee on Immunization (NACI) updated its guidelines to recommend the single-dose approach for ages 9-20 years. The change aligns Canada with 35 other countries, including Australia and the United Kingdom. Canada›s vaccine advisory group still recommends two doses for ages 21-26 years and three doses for patients who are immunocompromised or have HIV.
To help inform new NACI policies, Sauvageau and colleagues modeled several one-dose and two-dose strategies using HPV-ADVISE, an individual-based transmission-dynamic model of HPV infections and diseases. They looked at vaccination programs in Quebec, which has a high HPV vaccine coverage rate of around 85%, and Ontario, which has lower coverage of around 65%.
For one-dose programs, the researchers analyzed noninferior (98% efficacy) and pessimistic (90% efficacy) scenarios and different average vaccine duration periods, including lifelong, 30-year, and 25-year coverage. They compared the scenarios with a two-dose program with 98% efficacy and lifelong duration, estimating the relative reduction in HPV-16 infection and cervical cancer incidence and the number of doses needed to prevent one cervical cancer case.
Overall, the model projected that gender-neutral HPV vaccine programs with either two doses or a noninferior one dose would nearly eliminate HPV-16 infection by 2040-2045 in Quebec and reduce infection by more than 90% in Ontario. Under a one-dose strategy with 90% vaccine efficacy, rebounds in HPV-16 infection would start more than 25-30 years after a switch to a lower-dose strategy, thus providing time for officials to detect any signs of waning efficacy and change policies, if needed, the authors wrote.
In addition, the model projected that a noninferior one-dose, gender-neutral HPV vaccination program would avert a similar number of cervical cancer cases, compared with a two-dose program. The reduction would be about 60% in Quebec and 55% in Ontario, compared with no vaccination. Under the most pessimistic scenario with 25-year vaccine duration, a one-dose program would be slightly less effective in averting cancer: about 3% lower than a two-dose program over 100 years.
All one-dose scenarios were projected to lead to the elimination of cervical cancer in 8-16 years — at fewer than four cervical cancer cases per 100,000 female-years.
One-dose programs would also lead to more efficient use of vaccine doses, with about 800-1000 doses needed to prevent one cervical cancer case in a one-dose program and more than 10,000 incremental doses needed to prevent one additional cervical cancer case in a two-dose program.
What Next?
In Canada, the HPV vaccine is authorized for patients aged 9-45 years. Current immunization coverage among adolescents and young adults varies across provinces and falls below the national target of 90%. In its July 2024 update, NACI estimated that 76% of 14-year-olds of both genders received at least one vaccine dose and that 67% received two doses in 2023. Vaccine uptake was slightly higher among girls than boys.
To boost the coverage rate, shifting to a one-dose schedule could appeal to young people, as well as maintain vaccination efficacy.
“When you look at the studies that have been published worldwide, the effectiveness of one dose of the HPV vaccine is actually quite high,” said Caroline Quach-Thanh, MD, professor of microbiology, infectious diseases, immunology, and pediatrics at the University of Montreal, Quebec, Canada.
Quach-Thanh, who wasn’t involved with this study, previously served as NACI chair and now serves as chair of the Quebec Immunization Committee.
“In terms of prevention of HPV infections that may lead to cancer, whether you give one dose or two doses basically gives you the same amount of protection,” she said.
However, not all physicians agree about the switch in vaccination approaches. In early October, the Federation of Medical Women of Canada released a report with 12 recommendations to increase HPV vaccination rates, including a call for healthcare providers to continue with multidose immunization schedules for now.
“Vaccination is the most powerful action we can take in preventing HPV-related cancers. Canada is falling behind, but we can get back on track if we act quickly,” said Vivien Brown, MD, chair of the group’s HPV Immunization Task Force, chair and cofounder of HPV Prevention Week in Canada, and a past president of the federation.
After the NACI update in July, the task force evaluated the risks and benefits of a single-dose vaccine regimen, she said. They concluded that a multidose schedule should continue at this time because of its proven effectiveness.
“Until more research on the efficacy of a single-dose schedule becomes available, healthcare providers and public health agencies should continue to offer patients a multidose schedule,” said Brown. “This is the only way to ensure individuals are protected against HPV infection and cancer over the long term.”
The study was supported by the Public Health Agency of Canada, the Canadian Institutes of Health Research, the Bill & Melinda Gates Foundation, and Canadian Immunization Research Network. Sauvageau, Quach-Thanh, and Brown declared no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
In Canada, switching to a one-dose, gender-neutral vaccination program for human papillomavirus (HPV) could use vaccine doses more efficiently and prevent a similar number of cervical cancer cases, compared with a two-dose program, according to a new modeling analysis.
If vaccine protection remains high during the ages of peak sexual activity, all one-dose vaccination options are projected to be “substantially more efficient” than two-dose programs, even in the most pessimistic scenarios, the study authors wrote.
In addition, the scenarios projected the elimination of cervical cancer in Canada between 2032 and 2040. HPV can also lead to oral, throat, and penile cancers, and most are preventable through vaccination.
“The COVID-19 pandemic has impacted HPV vaccination in Canada, particularly among vulnerable population subgroups,” said study author Chantal Sauvageau, MD, a consultant in infectious diseases at the National Institute of Public Health of Quebec and associate professor of social and preventive medicine at the University of Laval, Quebec City, Canada.
Switching to one-dose vaccination would offer potential economic savings and programmatic flexibility, she added. The change also could enable investments aimed at increasing vaccination rates in regions where coverage is suboptimal, as well as in subgroups with a high HPV burden. Such initiatives could mitigate the pandemic’s impact on health programs and reduce inequalities.
The study was published online in CMAJ.
Vaccination Program Changes
Globally, countries have been investigating whether to shift from a two-dose to a one-dose HPV vaccine strategy since the World Health Organization’s Strategic Advisory Group of Experts on Immunization issued a single-dose recommendation in 2022.
In July, Canada’s National Advisory Committee on Immunization (NACI) updated its guidelines to recommend the single-dose approach for ages 9-20 years. The change aligns Canada with 35 other countries, including Australia and the United Kingdom. Canada›s vaccine advisory group still recommends two doses for ages 21-26 years and three doses for patients who are immunocompromised or have HIV.
To help inform new NACI policies, Sauvageau and colleagues modeled several one-dose and two-dose strategies using HPV-ADVISE, an individual-based transmission-dynamic model of HPV infections and diseases. They looked at vaccination programs in Quebec, which has a high HPV vaccine coverage rate of around 85%, and Ontario, which has lower coverage of around 65%.
For one-dose programs, the researchers analyzed noninferior (98% efficacy) and pessimistic (90% efficacy) scenarios and different average vaccine duration periods, including lifelong, 30-year, and 25-year coverage. They compared the scenarios with a two-dose program with 98% efficacy and lifelong duration, estimating the relative reduction in HPV-16 infection and cervical cancer incidence and the number of doses needed to prevent one cervical cancer case.
Overall, the model projected that gender-neutral HPV vaccine programs with either two doses or a noninferior one dose would nearly eliminate HPV-16 infection by 2040-2045 in Quebec and reduce infection by more than 90% in Ontario. Under a one-dose strategy with 90% vaccine efficacy, rebounds in HPV-16 infection would start more than 25-30 years after a switch to a lower-dose strategy, thus providing time for officials to detect any signs of waning efficacy and change policies, if needed, the authors wrote.
In addition, the model projected that a noninferior one-dose, gender-neutral HPV vaccination program would avert a similar number of cervical cancer cases, compared with a two-dose program. The reduction would be about 60% in Quebec and 55% in Ontario, compared with no vaccination. Under the most pessimistic scenario with 25-year vaccine duration, a one-dose program would be slightly less effective in averting cancer: about 3% lower than a two-dose program over 100 years.
All one-dose scenarios were projected to lead to the elimination of cervical cancer in 8-16 years — at fewer than four cervical cancer cases per 100,000 female-years.
One-dose programs would also lead to more efficient use of vaccine doses, with about 800-1000 doses needed to prevent one cervical cancer case in a one-dose program and more than 10,000 incremental doses needed to prevent one additional cervical cancer case in a two-dose program.
What Next?
In Canada, the HPV vaccine is authorized for patients aged 9-45 years. Current immunization coverage among adolescents and young adults varies across provinces and falls below the national target of 90%. In its July 2024 update, NACI estimated that 76% of 14-year-olds of both genders received at least one vaccine dose and that 67% received two doses in 2023. Vaccine uptake was slightly higher among girls than boys.
To boost the coverage rate, shifting to a one-dose schedule could appeal to young people, as well as maintain vaccination efficacy.
“When you look at the studies that have been published worldwide, the effectiveness of one dose of the HPV vaccine is actually quite high,” said Caroline Quach-Thanh, MD, professor of microbiology, infectious diseases, immunology, and pediatrics at the University of Montreal, Quebec, Canada.
Quach-Thanh, who wasn’t involved with this study, previously served as NACI chair and now serves as chair of the Quebec Immunization Committee.
“In terms of prevention of HPV infections that may lead to cancer, whether you give one dose or two doses basically gives you the same amount of protection,” she said.
However, not all physicians agree about the switch in vaccination approaches. In early October, the Federation of Medical Women of Canada released a report with 12 recommendations to increase HPV vaccination rates, including a call for healthcare providers to continue with multidose immunization schedules for now.
“Vaccination is the most powerful action we can take in preventing HPV-related cancers. Canada is falling behind, but we can get back on track if we act quickly,” said Vivien Brown, MD, chair of the group’s HPV Immunization Task Force, chair and cofounder of HPV Prevention Week in Canada, and a past president of the federation.
After the NACI update in July, the task force evaluated the risks and benefits of a single-dose vaccine regimen, she said. They concluded that a multidose schedule should continue at this time because of its proven effectiveness.
“Until more research on the efficacy of a single-dose schedule becomes available, healthcare providers and public health agencies should continue to offer patients a multidose schedule,” said Brown. “This is the only way to ensure individuals are protected against HPV infection and cancer over the long term.”
The study was supported by the Public Health Agency of Canada, the Canadian Institutes of Health Research, the Bill & Melinda Gates Foundation, and Canadian Immunization Research Network. Sauvageau, Quach-Thanh, and Brown declared no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
FROM CMAJ
Heart Attack, Stroke Survivors at High Risk for Long COVID
Primary care doctors and specialists should advise patients who have already experienced a heart attack or stroke that they are at a higher risk for long COVID and need to take steps to avoid contracting the virus, according to new research.
The study, led by researchers at Columbia University, New York City, suggests that anyone with cardiovascular disease (CVD) — defined as having experienced a heart attack or stroke — should consider getting the updated COVID vaccine boosters. They also suggest patients with CVD take other steps to avoid an acute infection, such as avoiding crowded indoor spaces.
There is no specific test or treatment for long COVID, which can become disabling and chronic. Long COVID is defined by the failure to recover from acute COVID-19 in 90 days.
The scientists used data from nearly 5000 people enrolled in 14 established, ongoing research programs, including the 76-year-old Framingham Heart Study. The results of the analysis of the “mega-cohort” were published in JAMA Network Open.
Most of the 14 studies already had 10-20 years of data on the cardiac health of thousands of enrollees, said Norrina B. Allen, one of the authors and a cardiac epidemiologist at Northwestern University Feinberg School of Medicine in Chicago, Illinois.
“This is a particularly strong study that looked at risk factors — or individual health — prior to developing COVID and their impact on the likely of recovering from COVID,” she said.
In addition to those with CVD, women and adults with preexisting chronic illnesses took longer to recover.
More than 20% of those in the large, racially and ethnically diverse US population–based study did not recover from COVID in 90 days. The researchers found that the median self-reported time to recovery from acute infection was 20 days.
While women and those with chronic illness had a higher risk for long COVID, vaccination and infection with the Omicron variant wave were associated with shorter recovery times.
These findings make sense, said Ziyad Al-Aly, MD, chief of research at Veterans Affairs St. Louis Health Care System and clinical epidemiologist at Washington University in St. Louis, Missouri.
“We also see that COVID-19 can lead to new-onset cardiovascular disease,” said Al-Aly, who was not involved in the study. “There is clearly a (link) between COVID and cardiovascular disease. These two seem to be intimately intertwined. In my view, this emphasizes the importance of targeting these individuals for vaccination and potentially antivirals (when they get infected) to help reduce their risk of adverse events and ameliorate their chance of full and fast recovery.”
The study used data from the Collaborative Cohort of Cohorts for COVID-19 Research. The long list of researchers contributing to this study includes epidemiologists, biostatisticians, neurologists, pulmonologists, and cardiologists. The data come from a list of cohorts like the Framingham Heart Study, which identified key risk factors for CVD, including cholesterol levels. Other studies include the Atherosclerosis Risk in Communities study, which began in the mid-1980s. Researchers there recruited a cohort of 15,792 men and women in rural North Carolina and Mississippi and suburban Minneapolis. They enrolled a high number of African American participants, who have been underrepresented in past studies. Other cohorts focused on young adults with CVD and Hispanics, while another focused on people with chronic obstructive pulmonary disease.
Lead author Elizabeth C. Oelsner, MD, of Columbia University Irving Medical Center in New York City, said she was not surprised by the CVD-long COVID link.
“We were aware that individuals with CVD were at higher risk of a more severe acute infection,” she said. “We were also seeing evidence that long and severe infection led to persistent symptoms.”
Oelsner noted that many patients still take more than 3 months to recover, even during the Omicron wave.
“While that has improved over the course of the pandemic, many individuals are taking a very long time to recover, and that can have a huge burden on the patient,” she said.
She encourages healthcare providers to tell patients at higher risk to take steps to avoid the virus, including vaccination and boosters.
A version of this article first appeared on Medscape.com.
Primary care doctors and specialists should advise patients who have already experienced a heart attack or stroke that they are at a higher risk for long COVID and need to take steps to avoid contracting the virus, according to new research.
The study, led by researchers at Columbia University, New York City, suggests that anyone with cardiovascular disease (CVD) — defined as having experienced a heart attack or stroke — should consider getting the updated COVID vaccine boosters. They also suggest patients with CVD take other steps to avoid an acute infection, such as avoiding crowded indoor spaces.
There is no specific test or treatment for long COVID, which can become disabling and chronic. Long COVID is defined by the failure to recover from acute COVID-19 in 90 days.
The scientists used data from nearly 5000 people enrolled in 14 established, ongoing research programs, including the 76-year-old Framingham Heart Study. The results of the analysis of the “mega-cohort” were published in JAMA Network Open.
Most of the 14 studies already had 10-20 years of data on the cardiac health of thousands of enrollees, said Norrina B. Allen, one of the authors and a cardiac epidemiologist at Northwestern University Feinberg School of Medicine in Chicago, Illinois.
“This is a particularly strong study that looked at risk factors — or individual health — prior to developing COVID and their impact on the likely of recovering from COVID,” she said.
In addition to those with CVD, women and adults with preexisting chronic illnesses took longer to recover.
More than 20% of those in the large, racially and ethnically diverse US population–based study did not recover from COVID in 90 days. The researchers found that the median self-reported time to recovery from acute infection was 20 days.
While women and those with chronic illness had a higher risk for long COVID, vaccination and infection with the Omicron variant wave were associated with shorter recovery times.
These findings make sense, said Ziyad Al-Aly, MD, chief of research at Veterans Affairs St. Louis Health Care System and clinical epidemiologist at Washington University in St. Louis, Missouri.
“We also see that COVID-19 can lead to new-onset cardiovascular disease,” said Al-Aly, who was not involved in the study. “There is clearly a (link) between COVID and cardiovascular disease. These two seem to be intimately intertwined. In my view, this emphasizes the importance of targeting these individuals for vaccination and potentially antivirals (when they get infected) to help reduce their risk of adverse events and ameliorate their chance of full and fast recovery.”
The study used data from the Collaborative Cohort of Cohorts for COVID-19 Research. The long list of researchers contributing to this study includes epidemiologists, biostatisticians, neurologists, pulmonologists, and cardiologists. The data come from a list of cohorts like the Framingham Heart Study, which identified key risk factors for CVD, including cholesterol levels. Other studies include the Atherosclerosis Risk in Communities study, which began in the mid-1980s. Researchers there recruited a cohort of 15,792 men and women in rural North Carolina and Mississippi and suburban Minneapolis. They enrolled a high number of African American participants, who have been underrepresented in past studies. Other cohorts focused on young adults with CVD and Hispanics, while another focused on people with chronic obstructive pulmonary disease.
Lead author Elizabeth C. Oelsner, MD, of Columbia University Irving Medical Center in New York City, said she was not surprised by the CVD-long COVID link.
“We were aware that individuals with CVD were at higher risk of a more severe acute infection,” she said. “We were also seeing evidence that long and severe infection led to persistent symptoms.”
Oelsner noted that many patients still take more than 3 months to recover, even during the Omicron wave.
“While that has improved over the course of the pandemic, many individuals are taking a very long time to recover, and that can have a huge burden on the patient,” she said.
She encourages healthcare providers to tell patients at higher risk to take steps to avoid the virus, including vaccination and boosters.
A version of this article first appeared on Medscape.com.
Primary care doctors and specialists should advise patients who have already experienced a heart attack or stroke that they are at a higher risk for long COVID and need to take steps to avoid contracting the virus, according to new research.
The study, led by researchers at Columbia University, New York City, suggests that anyone with cardiovascular disease (CVD) — defined as having experienced a heart attack or stroke — should consider getting the updated COVID vaccine boosters. They also suggest patients with CVD take other steps to avoid an acute infection, such as avoiding crowded indoor spaces.
There is no specific test or treatment for long COVID, which can become disabling and chronic. Long COVID is defined by the failure to recover from acute COVID-19 in 90 days.
The scientists used data from nearly 5000 people enrolled in 14 established, ongoing research programs, including the 76-year-old Framingham Heart Study. The results of the analysis of the “mega-cohort” were published in JAMA Network Open.
Most of the 14 studies already had 10-20 years of data on the cardiac health of thousands of enrollees, said Norrina B. Allen, one of the authors and a cardiac epidemiologist at Northwestern University Feinberg School of Medicine in Chicago, Illinois.
“This is a particularly strong study that looked at risk factors — or individual health — prior to developing COVID and their impact on the likely of recovering from COVID,” she said.
In addition to those with CVD, women and adults with preexisting chronic illnesses took longer to recover.
More than 20% of those in the large, racially and ethnically diverse US population–based study did not recover from COVID in 90 days. The researchers found that the median self-reported time to recovery from acute infection was 20 days.
While women and those with chronic illness had a higher risk for long COVID, vaccination and infection with the Omicron variant wave were associated with shorter recovery times.
These findings make sense, said Ziyad Al-Aly, MD, chief of research at Veterans Affairs St. Louis Health Care System and clinical epidemiologist at Washington University in St. Louis, Missouri.
“We also see that COVID-19 can lead to new-onset cardiovascular disease,” said Al-Aly, who was not involved in the study. “There is clearly a (link) between COVID and cardiovascular disease. These two seem to be intimately intertwined. In my view, this emphasizes the importance of targeting these individuals for vaccination and potentially antivirals (when they get infected) to help reduce their risk of adverse events and ameliorate their chance of full and fast recovery.”
The study used data from the Collaborative Cohort of Cohorts for COVID-19 Research. The long list of researchers contributing to this study includes epidemiologists, biostatisticians, neurologists, pulmonologists, and cardiologists. The data come from a list of cohorts like the Framingham Heart Study, which identified key risk factors for CVD, including cholesterol levels. Other studies include the Atherosclerosis Risk in Communities study, which began in the mid-1980s. Researchers there recruited a cohort of 15,792 men and women in rural North Carolina and Mississippi and suburban Minneapolis. They enrolled a high number of African American participants, who have been underrepresented in past studies. Other cohorts focused on young adults with CVD and Hispanics, while another focused on people with chronic obstructive pulmonary disease.
Lead author Elizabeth C. Oelsner, MD, of Columbia University Irving Medical Center in New York City, said she was not surprised by the CVD-long COVID link.
“We were aware that individuals with CVD were at higher risk of a more severe acute infection,” she said. “We were also seeing evidence that long and severe infection led to persistent symptoms.”
Oelsner noted that many patients still take more than 3 months to recover, even during the Omicron wave.
“While that has improved over the course of the pandemic, many individuals are taking a very long time to recover, and that can have a huge burden on the patient,” she said.
She encourages healthcare providers to tell patients at higher risk to take steps to avoid the virus, including vaccination and boosters.
A version of this article first appeared on Medscape.com.
Live Rotavirus Vaccine Safe for Newborns of Biologic-Treated Moms With IBD
No adverse events or impairment of the immune system emerged in babies at 7 days, 1 month, and 9 months post vaccination, in findings from a small Canadian study published in Clinical Gastroenterology and Hepatology.
The study found normal extended immune function testing in infants despite third-trimester maternal biologic therapy and regardless of circulating drug levels. The data provide reassurance about live rotavirus vaccination in this population and may also offer insights into the safety of other live vaccines in biologic-exposed individuals, wrote investigators led by gastroenterologist Cynthia H. Seow, MD, a professor in the Cumming School of Medicine at the University of Calgary in Alberta, Canada.
“Despite the well-established safety and effectiveness of non–live vaccination in individuals with IBD, including those on immunomodulators and biologic therapy, vaccine uptake in pregnant women with IBD and their infants remains suboptimal,” Seow said in an interview. This largely arises from maternal and physician concerns regarding transplacental transfer of IBD therapies and their impact on the safety of vaccination.
“These concerns were heightened after reports emerged of five fatal outcomes following the administration of the live Bacille Calmette-Guérin [BCG] vaccine in biologic-exposed infants. However, it had already been reported that inadvertent administration of the live oral rotavirus vaccine, a very different vaccine in terms of target and mechanism of action, in biologic-exposed individuals had not been associated with significant adverse effects,” she said.
They undertook their analysis with the hypothesis that vaccination would carry low risk, although the live oral vaccine is not currently recommended in biologic-exposed infants. “Yet rotavirus is a leading cause of severe, dehydrating diarrhea in children under the age of 5 years globally, and vaccination has led to significant reductions in hospitalizations and mortality,” Seow added.
Provision of the vaccine to anti–tumor necrosis factor (TNF)–exposed infants has been incorporated into the Canadian Public Health and Immunization guidelines, as the majority of the biologic-exposed infants were exposed to anti-TNF agents. “And with collection of further data, we expect that this will be extended to other biologic agent exposure. These data are important to pregnant women with IBD as they help to normalize their care. Pregnancy is difficult enough without having to remember exceptions to care,” Seow said.
“Before some of the studies came out, broad guidelines recommended that live vaccines should not be used in biologic-exposed infants, but this had been thought to be overly zealous and too conservative, and the risk was thought to be low,” said Elizabeth Spencer, MD, an assistant professor of pediatrics in the Division of Pediatric Gastroenterology at the Icahn School of Medicine at Mount Sinai in New York City, in an interview. Spencer was not involved in the Canadian study.
“At our center, we had some moms on biologics during pregnancy who forgot and had their babies vaccinated for rotavirus, and the babies were all fine,” she said.
The safety of this vaccine has been confirmed by several small studies and recently the PIANO Helmsley Global Consensus on Pregnancy and Inflammatory Bowel Disease, which was presented at Digestive Disease Week 2024. The consensus encompasses preconception counseling and the safety of IBD medications during pregnancy and lactation.
“Another concern, however, was that giving a live GI bug like rotavirus to babies might overstimulate their immune systems and provoke IBD,” Spencer added. “While a number of population-based studies in the US and Europe showed that was not the case, at least in the general population, there was a suggestion that, down the road, vaccination might be mildly protective against IBD in some cases.”
She added the caveat that these studies were not done in mothers and their babies with IBD, who might be inherently at greater risk for IBD. “So, a question for future research would be, ‘Is immune stimulation of the gut in IBD moms and their babies a good or a bad thing for their gut?’ ”
Spencer conceded that “the data present a bit of a blurry picture, but I think it’s always better just to vaccinate according to the regular schedule. The current data say there is no added risk, but it would be nice to look specifically at risk in moms with IBD and their children.”
The Study
The prospective cohort study is a substudy of a larger 2023 one that included biologic use in a range of maternal illnesses, not just IBD.
For the current study, Seow and colleagues identified 57 infants born to 52 mothers with IBD attending a pregnancy clinic at the University of Calgary in the period 2019-2023. Almost 81% of the mothers had Crohn’s disease, and the median duration of IBD was 10 years. The median gestational age at delivery was 39 weeks, and almost 60% of deliveries were vaginal. The infants had been exposed in utero to infliximab (n = 21), adalimumab (n = 19), vedolizumab (n = 10), and ustekinumab (n = 7) in the third trimester.
The 57 biologic-exposed infants underwent standardized clinical assessments, drug concentration, and immune function testing. The live oral rotavirus vaccine series was provided to 50 infants, with the first dose at a median of 13 weeks of age. Immunologic assessments validated for age were normal in all infants despite median infliximab concentrations of 6.1 μg/mL (range, 0.4-28.8 μg/mL), adalimumab concentrations of 1.7 μg/mL (range, 0.7-7.9 μg/mL), ustekinumab concentrations of 0.6 μg/mL (range, 0-1.1), and undetectable for vedolizumab at 10.7 weeks of age.
As anticipated, infant immune function was normal regardless of circulating drug levels.
The overall message, said Seow, is “healthy mum equals healthy baby. Be more concerned regarding active inflammation than active medications. In almost all circumstances, treat to target in pregnancy as you would in the nonpregnant state.” She added, however, that further studies are needed to determine the safety and optimal timing of other live vaccines, such as the BCG, in the presence of biologic therapy.
This study was funded by the Alberta Children’s Hospital Research Institute. Seow reported advisory/speaker’s fees for Janssen, AbbVie, Takeda, Pfizer, Fresenius Kabi, Bristol-Myers Squibb, Pharmascience, and Lilly, as well as funding from Alberta Children’s Hospital Research Institute, Crohn’s and Colitis Canada, the Canadian Institutes of Health Research, and Calgary Health Trust, and data safety monitoring from New South Wales Government Health, Australia. Multiple coauthors disclosed similar consulting or speaker relationships with private industry. Spencer had no competing interests with regard to her comments.
A version of this article first appeared on Medscape.com.
No adverse events or impairment of the immune system emerged in babies at 7 days, 1 month, and 9 months post vaccination, in findings from a small Canadian study published in Clinical Gastroenterology and Hepatology.
The study found normal extended immune function testing in infants despite third-trimester maternal biologic therapy and regardless of circulating drug levels. The data provide reassurance about live rotavirus vaccination in this population and may also offer insights into the safety of other live vaccines in biologic-exposed individuals, wrote investigators led by gastroenterologist Cynthia H. Seow, MD, a professor in the Cumming School of Medicine at the University of Calgary in Alberta, Canada.
“Despite the well-established safety and effectiveness of non–live vaccination in individuals with IBD, including those on immunomodulators and biologic therapy, vaccine uptake in pregnant women with IBD and their infants remains suboptimal,” Seow said in an interview. This largely arises from maternal and physician concerns regarding transplacental transfer of IBD therapies and their impact on the safety of vaccination.
“These concerns were heightened after reports emerged of five fatal outcomes following the administration of the live Bacille Calmette-Guérin [BCG] vaccine in biologic-exposed infants. However, it had already been reported that inadvertent administration of the live oral rotavirus vaccine, a very different vaccine in terms of target and mechanism of action, in biologic-exposed individuals had not been associated with significant adverse effects,” she said.
They undertook their analysis with the hypothesis that vaccination would carry low risk, although the live oral vaccine is not currently recommended in biologic-exposed infants. “Yet rotavirus is a leading cause of severe, dehydrating diarrhea in children under the age of 5 years globally, and vaccination has led to significant reductions in hospitalizations and mortality,” Seow added.
Provision of the vaccine to anti–tumor necrosis factor (TNF)–exposed infants has been incorporated into the Canadian Public Health and Immunization guidelines, as the majority of the biologic-exposed infants were exposed to anti-TNF agents. “And with collection of further data, we expect that this will be extended to other biologic agent exposure. These data are important to pregnant women with IBD as they help to normalize their care. Pregnancy is difficult enough without having to remember exceptions to care,” Seow said.
“Before some of the studies came out, broad guidelines recommended that live vaccines should not be used in biologic-exposed infants, but this had been thought to be overly zealous and too conservative, and the risk was thought to be low,” said Elizabeth Spencer, MD, an assistant professor of pediatrics in the Division of Pediatric Gastroenterology at the Icahn School of Medicine at Mount Sinai in New York City, in an interview. Spencer was not involved in the Canadian study.
“At our center, we had some moms on biologics during pregnancy who forgot and had their babies vaccinated for rotavirus, and the babies were all fine,” she said.
The safety of this vaccine has been confirmed by several small studies and recently the PIANO Helmsley Global Consensus on Pregnancy and Inflammatory Bowel Disease, which was presented at Digestive Disease Week 2024. The consensus encompasses preconception counseling and the safety of IBD medications during pregnancy and lactation.
“Another concern, however, was that giving a live GI bug like rotavirus to babies might overstimulate their immune systems and provoke IBD,” Spencer added. “While a number of population-based studies in the US and Europe showed that was not the case, at least in the general population, there was a suggestion that, down the road, vaccination might be mildly protective against IBD in some cases.”
She added the caveat that these studies were not done in mothers and their babies with IBD, who might be inherently at greater risk for IBD. “So, a question for future research would be, ‘Is immune stimulation of the gut in IBD moms and their babies a good or a bad thing for their gut?’ ”
Spencer conceded that “the data present a bit of a blurry picture, but I think it’s always better just to vaccinate according to the regular schedule. The current data say there is no added risk, but it would be nice to look specifically at risk in moms with IBD and their children.”
The Study
The prospective cohort study is a substudy of a larger 2023 one that included biologic use in a range of maternal illnesses, not just IBD.
For the current study, Seow and colleagues identified 57 infants born to 52 mothers with IBD attending a pregnancy clinic at the University of Calgary in the period 2019-2023. Almost 81% of the mothers had Crohn’s disease, and the median duration of IBD was 10 years. The median gestational age at delivery was 39 weeks, and almost 60% of deliveries were vaginal. The infants had been exposed in utero to infliximab (n = 21), adalimumab (n = 19), vedolizumab (n = 10), and ustekinumab (n = 7) in the third trimester.
The 57 biologic-exposed infants underwent standardized clinical assessments, drug concentration, and immune function testing. The live oral rotavirus vaccine series was provided to 50 infants, with the first dose at a median of 13 weeks of age. Immunologic assessments validated for age were normal in all infants despite median infliximab concentrations of 6.1 μg/mL (range, 0.4-28.8 μg/mL), adalimumab concentrations of 1.7 μg/mL (range, 0.7-7.9 μg/mL), ustekinumab concentrations of 0.6 μg/mL (range, 0-1.1), and undetectable for vedolizumab at 10.7 weeks of age.
As anticipated, infant immune function was normal regardless of circulating drug levels.
The overall message, said Seow, is “healthy mum equals healthy baby. Be more concerned regarding active inflammation than active medications. In almost all circumstances, treat to target in pregnancy as you would in the nonpregnant state.” She added, however, that further studies are needed to determine the safety and optimal timing of other live vaccines, such as the BCG, in the presence of biologic therapy.
This study was funded by the Alberta Children’s Hospital Research Institute. Seow reported advisory/speaker’s fees for Janssen, AbbVie, Takeda, Pfizer, Fresenius Kabi, Bristol-Myers Squibb, Pharmascience, and Lilly, as well as funding from Alberta Children’s Hospital Research Institute, Crohn’s and Colitis Canada, the Canadian Institutes of Health Research, and Calgary Health Trust, and data safety monitoring from New South Wales Government Health, Australia. Multiple coauthors disclosed similar consulting or speaker relationships with private industry. Spencer had no competing interests with regard to her comments.
A version of this article first appeared on Medscape.com.
No adverse events or impairment of the immune system emerged in babies at 7 days, 1 month, and 9 months post vaccination, in findings from a small Canadian study published in Clinical Gastroenterology and Hepatology.
The study found normal extended immune function testing in infants despite third-trimester maternal biologic therapy and regardless of circulating drug levels. The data provide reassurance about live rotavirus vaccination in this population and may also offer insights into the safety of other live vaccines in biologic-exposed individuals, wrote investigators led by gastroenterologist Cynthia H. Seow, MD, a professor in the Cumming School of Medicine at the University of Calgary in Alberta, Canada.
“Despite the well-established safety and effectiveness of non–live vaccination in individuals with IBD, including those on immunomodulators and biologic therapy, vaccine uptake in pregnant women with IBD and their infants remains suboptimal,” Seow said in an interview. This largely arises from maternal and physician concerns regarding transplacental transfer of IBD therapies and their impact on the safety of vaccination.
“These concerns were heightened after reports emerged of five fatal outcomes following the administration of the live Bacille Calmette-Guérin [BCG] vaccine in biologic-exposed infants. However, it had already been reported that inadvertent administration of the live oral rotavirus vaccine, a very different vaccine in terms of target and mechanism of action, in biologic-exposed individuals had not been associated with significant adverse effects,” she said.
They undertook their analysis with the hypothesis that vaccination would carry low risk, although the live oral vaccine is not currently recommended in biologic-exposed infants. “Yet rotavirus is a leading cause of severe, dehydrating diarrhea in children under the age of 5 years globally, and vaccination has led to significant reductions in hospitalizations and mortality,” Seow added.
Provision of the vaccine to anti–tumor necrosis factor (TNF)–exposed infants has been incorporated into the Canadian Public Health and Immunization guidelines, as the majority of the biologic-exposed infants were exposed to anti-TNF agents. “And with collection of further data, we expect that this will be extended to other biologic agent exposure. These data are important to pregnant women with IBD as they help to normalize their care. Pregnancy is difficult enough without having to remember exceptions to care,” Seow said.
“Before some of the studies came out, broad guidelines recommended that live vaccines should not be used in biologic-exposed infants, but this had been thought to be overly zealous and too conservative, and the risk was thought to be low,” said Elizabeth Spencer, MD, an assistant professor of pediatrics in the Division of Pediatric Gastroenterology at the Icahn School of Medicine at Mount Sinai in New York City, in an interview. Spencer was not involved in the Canadian study.
“At our center, we had some moms on biologics during pregnancy who forgot and had their babies vaccinated for rotavirus, and the babies were all fine,” she said.
The safety of this vaccine has been confirmed by several small studies and recently the PIANO Helmsley Global Consensus on Pregnancy and Inflammatory Bowel Disease, which was presented at Digestive Disease Week 2024. The consensus encompasses preconception counseling and the safety of IBD medications during pregnancy and lactation.
“Another concern, however, was that giving a live GI bug like rotavirus to babies might overstimulate their immune systems and provoke IBD,” Spencer added. “While a number of population-based studies in the US and Europe showed that was not the case, at least in the general population, there was a suggestion that, down the road, vaccination might be mildly protective against IBD in some cases.”
She added the caveat that these studies were not done in mothers and their babies with IBD, who might be inherently at greater risk for IBD. “So, a question for future research would be, ‘Is immune stimulation of the gut in IBD moms and their babies a good or a bad thing for their gut?’ ”
Spencer conceded that “the data present a bit of a blurry picture, but I think it’s always better just to vaccinate according to the regular schedule. The current data say there is no added risk, but it would be nice to look specifically at risk in moms with IBD and their children.”
The Study
The prospective cohort study is a substudy of a larger 2023 one that included biologic use in a range of maternal illnesses, not just IBD.
For the current study, Seow and colleagues identified 57 infants born to 52 mothers with IBD attending a pregnancy clinic at the University of Calgary in the period 2019-2023. Almost 81% of the mothers had Crohn’s disease, and the median duration of IBD was 10 years. The median gestational age at delivery was 39 weeks, and almost 60% of deliveries were vaginal. The infants had been exposed in utero to infliximab (n = 21), adalimumab (n = 19), vedolizumab (n = 10), and ustekinumab (n = 7) in the third trimester.
The 57 biologic-exposed infants underwent standardized clinical assessments, drug concentration, and immune function testing. The live oral rotavirus vaccine series was provided to 50 infants, with the first dose at a median of 13 weeks of age. Immunologic assessments validated for age were normal in all infants despite median infliximab concentrations of 6.1 μg/mL (range, 0.4-28.8 μg/mL), adalimumab concentrations of 1.7 μg/mL (range, 0.7-7.9 μg/mL), ustekinumab concentrations of 0.6 μg/mL (range, 0-1.1), and undetectable for vedolizumab at 10.7 weeks of age.
As anticipated, infant immune function was normal regardless of circulating drug levels.
The overall message, said Seow, is “healthy mum equals healthy baby. Be more concerned regarding active inflammation than active medications. In almost all circumstances, treat to target in pregnancy as you would in the nonpregnant state.” She added, however, that further studies are needed to determine the safety and optimal timing of other live vaccines, such as the BCG, in the presence of biologic therapy.
This study was funded by the Alberta Children’s Hospital Research Institute. Seow reported advisory/speaker’s fees for Janssen, AbbVie, Takeda, Pfizer, Fresenius Kabi, Bristol-Myers Squibb, Pharmascience, and Lilly, as well as funding from Alberta Children’s Hospital Research Institute, Crohn’s and Colitis Canada, the Canadian Institutes of Health Research, and Calgary Health Trust, and data safety monitoring from New South Wales Government Health, Australia. Multiple coauthors disclosed similar consulting or speaker relationships with private industry. Spencer had no competing interests with regard to her comments.
A version of this article first appeared on Medscape.com.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Too Few Immunocompromised Veterans Are Getting Zoster Vaccinations
TOPLINE:
the low rate of herpes zoster vaccination in this immunocompromised group, especially among younger individuals, is concerning.
METHODOLOGY:
- In 2021, the Food and Drug Administration authorized the use of RZV for adults aged 18 years or older on chronic immunosuppressive medications because of their high risk for herpes zoster and its related complications, followed by updated guidance from the Centers for Disease Control and Prevention and American College of Rheumatology in 2021 and 2022, respectively.
- This study aimed to assess the receipt of RZV among veterans receiving immunosuppressive medications within the Veterans Health Administration (VHA) healthcare system before and after the expanded indications in February 2022.
- It included 190,162 veterans who were prescribed one or more immunosuppressive medications for at least 90 days at 130 medical facilities between January 1, 2018, and June 30, 2023.
- A total of 23,295 veterans (12.3%) were younger than 50 years by the end of the study period.
- The outcome measured was the percentage of veterans with one or more doses of RZV documented during the study period.
TAKEAWAY:
- Among veterans aged 50 years or older, 36.2% and 49.8% received an RZV before the expanded indication and by mid-2023, respectively. Even though the rate of vaccination is higher than that observed in the 2021 National Health Interview Survey, significant room for improvement remains.
- Among veterans younger than 50 years, very few (2.8%) received an RZV before the expanded indication, and only 13.4% received it by mid-2023.
- Demographic factors associated with lower odds of vaccination included male sex, African American or unknown race, and nonurban residence (P ≤ .004 for all).
- Those who received targeted synthetic disease-modifying antirheumatic drugs (DMARDs) alone or in combination with other drugs or those who received other vaccines were more likely to receive RZV than those who received conventional synthetic DMARD monotherapy (P < .001 for both).
IN PRACTICE:
“Future work to improve RZV vaccination in patients at high risk should focus on creating informatics tools to identify individuals at high risk and standardizing vaccination guidelines across subspecialties,” the authors wrote.
SOURCE:
This study was led by Sharon Abada, MD, University of California, San Francisco. It was published online on October 11, 2024, in JAMA Network Open.
LIMITATIONS:
This study may not be generalizable to nonveteran populations or countries outside the United States. Limitations also included difficulty with capturing vaccinations not administered within the VHA system, which may have resulted in an underestimation of the percentage of patients vaccinated.
DISCLOSURES:
This work was funded by grants from the VA Quality Enhancement Research Initiative and the Agency for Healthcare Research and Quality. Some authors reported receiving grants from institutions and pharmaceutical companies.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
the low rate of herpes zoster vaccination in this immunocompromised group, especially among younger individuals, is concerning.
METHODOLOGY:
- In 2021, the Food and Drug Administration authorized the use of RZV for adults aged 18 years or older on chronic immunosuppressive medications because of their high risk for herpes zoster and its related complications, followed by updated guidance from the Centers for Disease Control and Prevention and American College of Rheumatology in 2021 and 2022, respectively.
- This study aimed to assess the receipt of RZV among veterans receiving immunosuppressive medications within the Veterans Health Administration (VHA) healthcare system before and after the expanded indications in February 2022.
- It included 190,162 veterans who were prescribed one or more immunosuppressive medications for at least 90 days at 130 medical facilities between January 1, 2018, and June 30, 2023.
- A total of 23,295 veterans (12.3%) were younger than 50 years by the end of the study period.
- The outcome measured was the percentage of veterans with one or more doses of RZV documented during the study period.
TAKEAWAY:
- Among veterans aged 50 years or older, 36.2% and 49.8% received an RZV before the expanded indication and by mid-2023, respectively. Even though the rate of vaccination is higher than that observed in the 2021 National Health Interview Survey, significant room for improvement remains.
- Among veterans younger than 50 years, very few (2.8%) received an RZV before the expanded indication, and only 13.4% received it by mid-2023.
- Demographic factors associated with lower odds of vaccination included male sex, African American or unknown race, and nonurban residence (P ≤ .004 for all).
- Those who received targeted synthetic disease-modifying antirheumatic drugs (DMARDs) alone or in combination with other drugs or those who received other vaccines were more likely to receive RZV than those who received conventional synthetic DMARD monotherapy (P < .001 for both).
IN PRACTICE:
“Future work to improve RZV vaccination in patients at high risk should focus on creating informatics tools to identify individuals at high risk and standardizing vaccination guidelines across subspecialties,” the authors wrote.
SOURCE:
This study was led by Sharon Abada, MD, University of California, San Francisco. It was published online on October 11, 2024, in JAMA Network Open.
LIMITATIONS:
This study may not be generalizable to nonveteran populations or countries outside the United States. Limitations also included difficulty with capturing vaccinations not administered within the VHA system, which may have resulted in an underestimation of the percentage of patients vaccinated.
DISCLOSURES:
This work was funded by grants from the VA Quality Enhancement Research Initiative and the Agency for Healthcare Research and Quality. Some authors reported receiving grants from institutions and pharmaceutical companies.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
the low rate of herpes zoster vaccination in this immunocompromised group, especially among younger individuals, is concerning.
METHODOLOGY:
- In 2021, the Food and Drug Administration authorized the use of RZV for adults aged 18 years or older on chronic immunosuppressive medications because of their high risk for herpes zoster and its related complications, followed by updated guidance from the Centers for Disease Control and Prevention and American College of Rheumatology in 2021 and 2022, respectively.
- This study aimed to assess the receipt of RZV among veterans receiving immunosuppressive medications within the Veterans Health Administration (VHA) healthcare system before and after the expanded indications in February 2022.
- It included 190,162 veterans who were prescribed one or more immunosuppressive medications for at least 90 days at 130 medical facilities between January 1, 2018, and June 30, 2023.
- A total of 23,295 veterans (12.3%) were younger than 50 years by the end of the study period.
- The outcome measured was the percentage of veterans with one or more doses of RZV documented during the study period.
TAKEAWAY:
- Among veterans aged 50 years or older, 36.2% and 49.8% received an RZV before the expanded indication and by mid-2023, respectively. Even though the rate of vaccination is higher than that observed in the 2021 National Health Interview Survey, significant room for improvement remains.
- Among veterans younger than 50 years, very few (2.8%) received an RZV before the expanded indication, and only 13.4% received it by mid-2023.
- Demographic factors associated with lower odds of vaccination included male sex, African American or unknown race, and nonurban residence (P ≤ .004 for all).
- Those who received targeted synthetic disease-modifying antirheumatic drugs (DMARDs) alone or in combination with other drugs or those who received other vaccines were more likely to receive RZV than those who received conventional synthetic DMARD monotherapy (P < .001 for both).
IN PRACTICE:
“Future work to improve RZV vaccination in patients at high risk should focus on creating informatics tools to identify individuals at high risk and standardizing vaccination guidelines across subspecialties,” the authors wrote.
SOURCE:
This study was led by Sharon Abada, MD, University of California, San Francisco. It was published online on October 11, 2024, in JAMA Network Open.
LIMITATIONS:
This study may not be generalizable to nonveteran populations or countries outside the United States. Limitations also included difficulty with capturing vaccinations not administered within the VHA system, which may have resulted in an underestimation of the percentage of patients vaccinated.
DISCLOSURES:
This work was funded by grants from the VA Quality Enhancement Research Initiative and the Agency for Healthcare Research and Quality. Some authors reported receiving grants from institutions and pharmaceutical companies.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Maternal Immunization to Prevent Serious Respiratory Illness
Editor’s Note: Sadly, this is the last column in the Master Class Obstetrics series. This award-winning column has been part of Ob.Gyn. News for 20 years. The deep discussion of cutting-edge topics in obstetrics by specialists and researchers will be missed as will the leadership and curation of topics by Dr. E. Albert Reece.
Introduction: The Need for Increased Vigilance About Maternal Immunization
Viruses are becoming increasingly prevalent in our world and the consequences of viral infections are implicated in a growing number of disease states. It is well established that certain cancers are caused by viruses and it is increasingly evident that viral infections can trigger the development of chronic illness. In pregnant women, viruses such as cytomegalovirus can cause infection in utero and lead to long-term impairments for the baby.
Likewise, it appears that the virulence of viruses is increasing, whether it be the respiratory syncytial virus (RSV) in children or the severe acute respiratory syndrome (SARS) coronaviruses in adults. Clearly, our environment is changing, with increases in population growth and urbanization, for instance, and an intensification of climate change and its effects. Viruses are part of this changing background.
Vaccines are our most powerful tool to protect people of all ages against viral threats, and fortunately, we benefit from increasing expertise in vaccinology. Since 1974, the University of Maryland School of Medicine has a Center for Vaccine Development and Global Health that has conducted research on vaccines to defend against the Zika virus, H1N1, Ebola, and SARS-CoV-2.
We’re not alone. Other vaccinology centers across the country — as well as the National Institutes of Health at the national level, through its National Institute of Allergy and Infectious Diseases — are doing research and developing vaccines to combat viral diseases.
In this column, we are focused on viral diseases in pregnancy and the role that vaccines can play in preventing serious respiratory illness in mothers and their newborns. I have invited Laura E. Riley, MD, the Given Foundation Professor and Chair of Obstetrics and Gynecology at Weill Cornell Medicine, to address the importance of maternal immunization and how we can best counsel our patients and improve immunization rates.
As Dr. Riley explains, we are in a new era, and it behooves us all to be more vigilant about recommending vaccines, combating misperceptions, addressing patients’ knowledge gaps, and administering vaccines whenever possible.
Dr. Reece is the former Dean of Medicine & University Executive VP, and The Distinguished University and Endowed Professor & Director of the Center for Advanced Research Training and Innovation (CARTI) at the University of Maryland School of Medicine, as well as senior scientist at the Center for Birth Defects Research.
The alarming decline in maternal immunization rates that occurred in the wake of the COVID-19 pandemic means that, now more than ever, we must fully embrace our responsibility to recommend immunizations in pregnancy and to communicate what is known about their efficacy and safety. Data show that vaccination rates drop when we do not offer vaccines in our offices, so whenever possible, we should administer them as well.
The ob.gyn. is the patient’s most trusted person in pregnancy. When patients decline or express hesitancy about vaccines, it is incumbent upon us to ask why. Oftentimes, we can identify areas in which patients lack knowledge or have misperceptions and we can successfully educate the patient or change their perspective or misunderstanding concerning the importance of vaccination for themselves and their babies. (See Table 1.) We can also successfully address concerns about safety.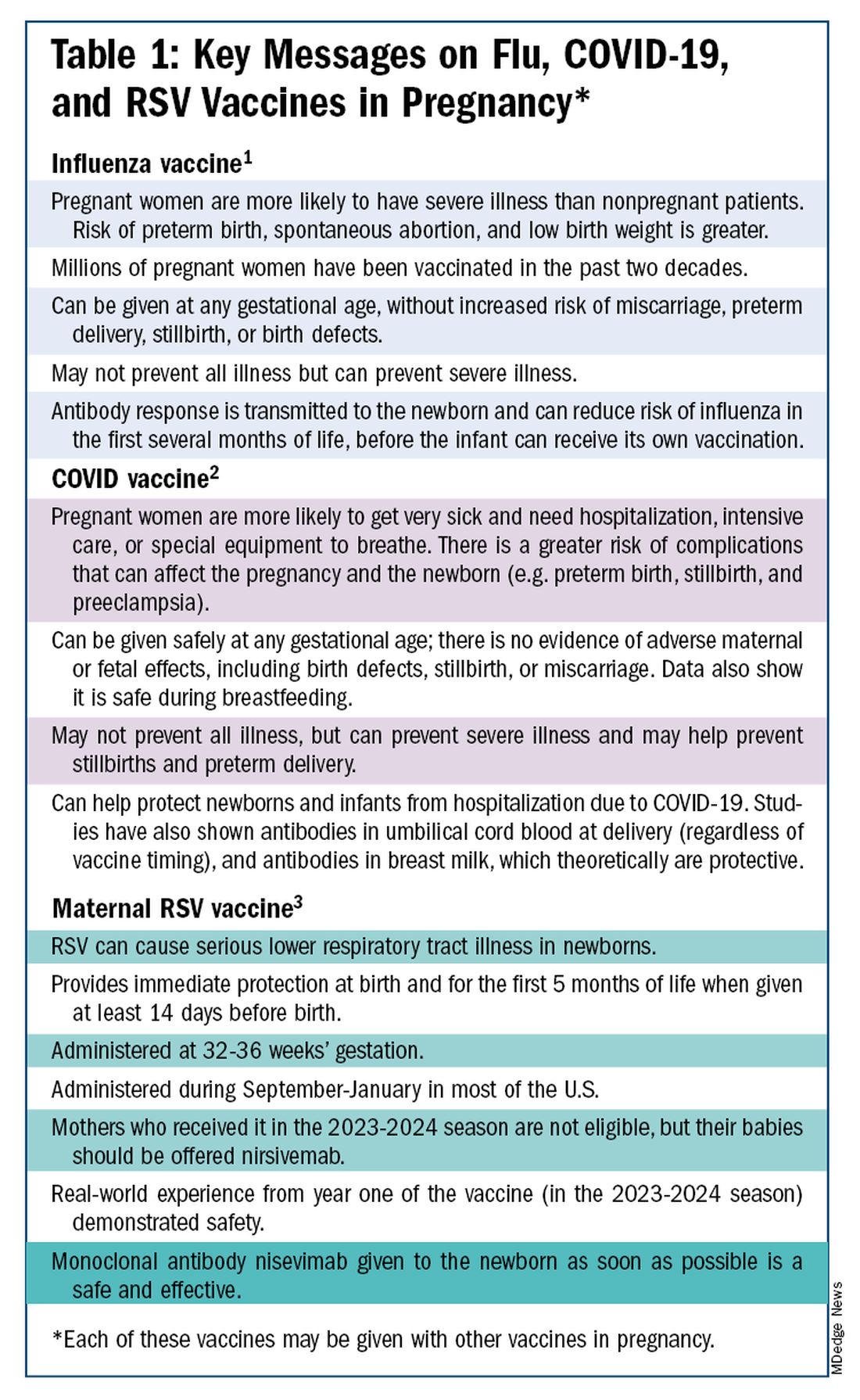
The safety of COVID-19 vaccinations in pregnancy is now backed by several years of data from multiple studies showing no increase in birth defects, preterm delivery, miscarriage, or stillbirth.
Data also show that pregnant patients are more likely than patients who are not pregnant to need hospitalization and intensive care when infected with SARS-CoV-2 and are at risk of having complications that can affect pregnancy and the newborn, including preterm birth and stillbirth. Vaccination has been shown to reduce the risk of severe illness and the risk of such adverse obstetrical outcomes, in addition to providing protection for the infant early on.
Similarly, influenza has long been more likely to be severe in pregnant patients, with an increased risk of poor obstetrical outcomes. Vaccines similarly provide “two for one protection,” protecting both mother and baby, and are, of course, backed by many years of safety and efficacy data.
With the new maternal respiratory syncytial virus (RSV) vaccine, now in its second year of availability, the goal is to protect the baby from RSV-caused serious lower respiratory tract illness. The illness has contributed to tens of thousands of annual hospitalizations and up to several hundred deaths every year in children younger than 5 years — particularly in those under age 6 months.
The RSV monoclonal antibody nirsevimab is available for the newborn as an alternative to maternal immunization but the maternal vaccine is optimal in that it will provide immediate rather than delayed protection for the newborn. The maternal vaccine is recommended during weeks 32-36 of pregnancy in mothers who were not vaccinated during last year’s RSV season. With real-world experience from year one, the available safety data are reassuring.
Counseling About Influenza and COVID-19 Vaccination
The COVID-19 pandemic took a toll on vaccination interest/receptivity broadly in pregnant and nonpregnant people. Among pregnant individuals, influenza vaccination coverage declined from 71% in the 2019-2020 influenza season to 56% in the 2021-2022 season, according to data from the Centers for Disease Control and Prevention’s Vaccine Safety Datalink.4 Coverage for the 2022-2023 and 2023-2024 influenza seasons was even worse: well under 50%.5
Fewer pregnant women have received updated COVID-19 vaccines. Only 13% of pregnant persons overall received the updated 2023-2024 COVID-19 booster vaccine (through March 30, 2024), according to the CDC.6
Maternal immunization for influenza has been recommended in the United States since 2004 (part of the recommendation that everyone over the age of 6 months receive an annual flu vaccine), and flu vaccines have been given to millions of pregnant women, but the H1N1 pandemic of 2009 reinforced its value as a priority for prenatal care. Most of the women who became severely ill from the H1N1 virus were young and healthy, without co-existing conditions known to increase risk.7
It became clearer during the H1N1 pandemic that pregnancy itself — which is associated with physiologic changes such as decreased lung capacity, increased nasal congestion and changes in the immune system – is its own significant risk factor for severe illness from the influenza virus. This increased risk applies to COVID-19 as well.
As COVID-19 has become endemic, with hospitalizations and deaths not reaching the levels of previous surges — and with mask-wearing and other preventive measures having declined — patients understandably have become more complacent. Some patients are vaccine deniers, but in my practice, these patients are a much smaller group than those who believe COVID-19 “is no big deal,” especially if they have had infections recently.
This is why it’s important to actively listen to concerns and to ask patients who decline a vaccination why they are hesitant. Blanket messages about vaccine efficacy and safety are the first step, but individualized, more pointed conversations based on the patient’s personal experiences and beliefs have become increasingly important.
I routinely tell pregnant patients about the risks of COVID-19 and I explain that it has been difficult to predict who will develop severe illness. Sometimes more conversation is needed. For those who are still hesitant or who tell me they feel protected by a recent infection, for instance, I provide more detail on the unique risks of pregnancy — the fact that “pregnancy is different” — and that natural immunity wanes while the protection afforded by immunization is believed to last longer. Many women are also concerned about the safety of the COVID-19 vaccine, so having safety data at your fingertips is helpful. (See Table 2.)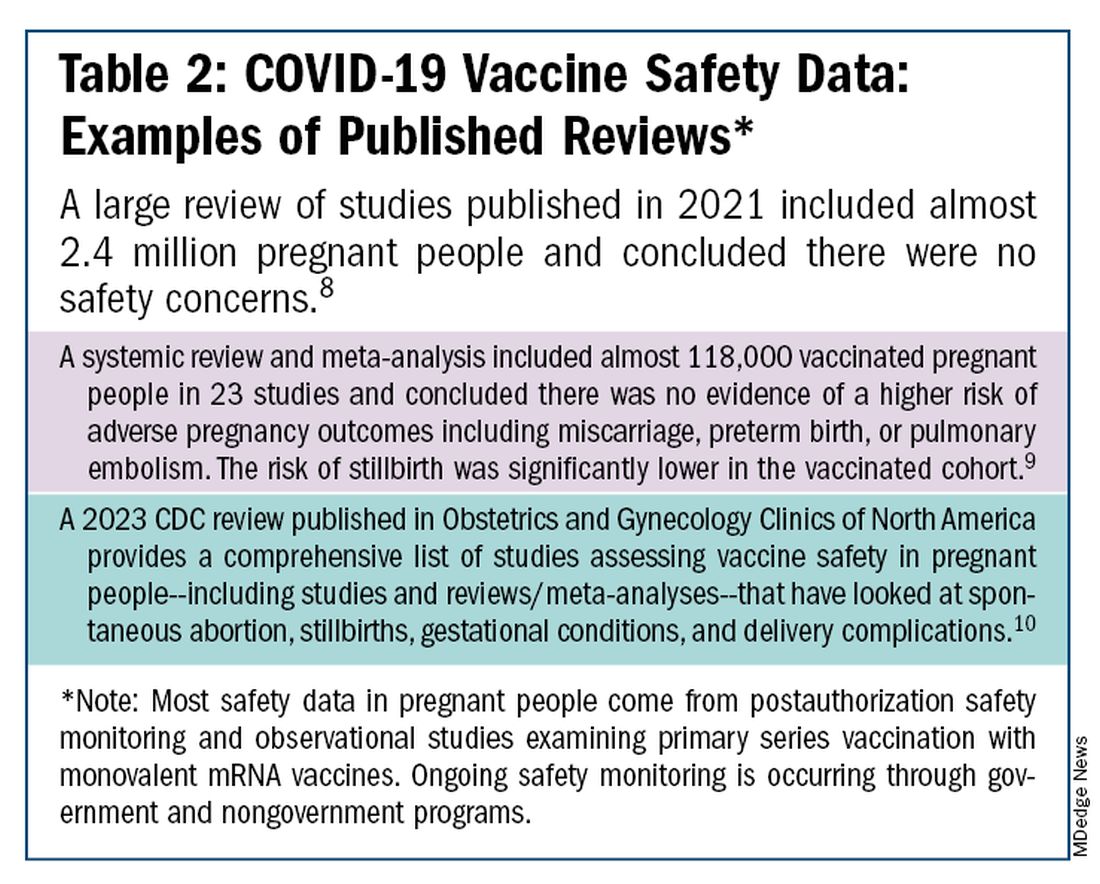
The fact that influenza and COVID-19 vaccination protect the newborn as well as the mother is something that I find is underappreciated by many patients. Explaining that infants likely benefit from the passage of antibodies across the placenta should be part of patient counseling.
Counseling About RSV Vaccination
Importantly, for the 2024-2025 RSV season, the maternal RSV vaccine (Abrysvo, Pfizer) is recommended only for pregnant women who did not receive the vaccine during the 2023-2024 season. When more research is done and more data are obtained showing how long the immune response persists post vaccination, it may be that the US Food and Drug Administration (FDA) will approve the maternal RSV vaccine for use in every pregnancy.
The later timing of the vaccination recommendation — 32-36 weeks’ gestation — reflects a conservative approach taken by the FDA in response to data from one of the pivotal trials showing a numerical trend toward more preterm deliveries among vaccinated compared with unvaccinated patients. This imbalance in the original trial, which administered the vaccine during 24-36 weeks of gestation, was seen only in low-income countries with no temporal association, however.
In our experience at two Weill Cornell Medical College–associated hospitals we did not see this trend. Our cohort study of almost 3000 pregnant patients who delivered at 32 weeks’ gestation or later found no increased risk of preterm birth among the 35% of patients who received the RSV vaccine during the 2023-2024 RSV season. We also did not see any difference in preeclampsia, in contrast with original trial data that showed a signal for increased risk.11
When fewer than 2 weeks have elapsed between maternal vaccination and delivery, the monoclonal antibody nirsevimab is recommended for the newborn — ideally before the newborn leaves the hospital. Nirsevimab is also recommended for newborns of mothers who decline vaccination or were not candidates (e.g. vaccinated in a previous pregnancy), or when there is concern about the adequacy of the maternal immune response to the vaccine (e.g. in cases of immunosuppression).
While there was a limited supply of the monoclonal antibody last year, limitations are not expected this year, especially after October.
The ultimate goal is that patients choose the vaccine or the immunoglobulin, given the severity of RSV disease. Patient preferences should be considered. However, given that it takes 2 weeks after vaccination for protection to build up, I stress to patients that if they’ve vaccinated themselves, their newborn will leave the hospital with protection. If nirsevimab is relied upon, I explain, their newborn may not be protected for some period of time.
Take-home Messages
- When patients decline or are hesitant about vaccines, ask why. Listen actively, and work to correct misperceptions and knowledge gaps.
- Whenever possible, offer vaccines in your practice. Vaccination rates drop when this does not occur.
- COVID-vaccine safety is backed by many studies showing no increase in birth defects, preterm delivery, miscarriage, or stillbirth.
- Pregnant women are more likely to have severe illness from the influenza and SARS-CoV-2 viruses. Vaccines can prevent severe illness and can protect the newborn as well as the mother.
- Recommend/administer the maternal RSV vaccine at 32-36 weeks’ gestation in women who did not receive the vaccine in the 2023-2024 season. If mothers aren’t eligible their babies should be offered nirsevimab.
Dr. Riley is the Given Foundation Professor and Chair of Obstetrics and Gynecology at Weill Cornell Medicine and the obstetrician and gynecologist-in-chief at New York Presbyterian Hospital. She disclosed that she has provided one-time consultations to Pfizer (Abrysvo RSV vaccine) and GSK (cytomegalovirus vaccine), and is providing consultant education on CMV for Moderna. She is chair of ACOG’s task force on immunization and emerging infectious diseases, serves on the medical advisory board for MAVEN, and serves as an editor or editorial board member for several medical publications.
References
1. ACOG Committee Opinion No. 741: Maternal Immunization. Obstet Gynecol. 2018;131(6):e214-e217.
2. Centers for Disease Control and Prevention. COVID-19 Vaccination for People Who are Pregnant or Breastfeeding. https://www.cdc.gov/covid/vaccines/pregnant-or-breastfeeding.html.
3. ACOG Practice Advisory on Maternal Respiratory Syncytial Virus Vaccination, September 2023. (Updated August 2024).4. Irving S et al. Open Forum Infect Dis. 2023;10(Suppl 2):ofad500.1002.
5. Flu Vaccination Dashboard, CDC, National Center for Immunization and Respiratory Diseases.
6. Weekly COVID-19 Vaccination Dashboard, CDC. https://www.cdc.gov/covidvaxview/weekly-dashboard/index.html
7. Louie JK et al. N Engl J Med. 2010;362:27-35. 8. Ciapponi A et al. Vaccine. 2021;39(40):5891-908.
9. Prasad S et al. Nature Communications. 2022;13:2414. 10. Fleming-Dutra KE et al. Obstet Gynecol Clin North Am 2023;50(2):279-97. 11. Mouen S et al. JAMA Network Open 2024;7(7):e2419268.
Editor’s Note: Sadly, this is the last column in the Master Class Obstetrics series. This award-winning column has been part of Ob.Gyn. News for 20 years. The deep discussion of cutting-edge topics in obstetrics by specialists and researchers will be missed as will the leadership and curation of topics by Dr. E. Albert Reece.
Introduction: The Need for Increased Vigilance About Maternal Immunization
Viruses are becoming increasingly prevalent in our world and the consequences of viral infections are implicated in a growing number of disease states. It is well established that certain cancers are caused by viruses and it is increasingly evident that viral infections can trigger the development of chronic illness. In pregnant women, viruses such as cytomegalovirus can cause infection in utero and lead to long-term impairments for the baby.
Likewise, it appears that the virulence of viruses is increasing, whether it be the respiratory syncytial virus (RSV) in children or the severe acute respiratory syndrome (SARS) coronaviruses in adults. Clearly, our environment is changing, with increases in population growth and urbanization, for instance, and an intensification of climate change and its effects. Viruses are part of this changing background.
Vaccines are our most powerful tool to protect people of all ages against viral threats, and fortunately, we benefit from increasing expertise in vaccinology. Since 1974, the University of Maryland School of Medicine has a Center for Vaccine Development and Global Health that has conducted research on vaccines to defend against the Zika virus, H1N1, Ebola, and SARS-CoV-2.
We’re not alone. Other vaccinology centers across the country — as well as the National Institutes of Health at the national level, through its National Institute of Allergy and Infectious Diseases — are doing research and developing vaccines to combat viral diseases.
In this column, we are focused on viral diseases in pregnancy and the role that vaccines can play in preventing serious respiratory illness in mothers and their newborns. I have invited Laura E. Riley, MD, the Given Foundation Professor and Chair of Obstetrics and Gynecology at Weill Cornell Medicine, to address the importance of maternal immunization and how we can best counsel our patients and improve immunization rates.
As Dr. Riley explains, we are in a new era, and it behooves us all to be more vigilant about recommending vaccines, combating misperceptions, addressing patients’ knowledge gaps, and administering vaccines whenever possible.
Dr. Reece is the former Dean of Medicine & University Executive VP, and The Distinguished University and Endowed Professor & Director of the Center for Advanced Research Training and Innovation (CARTI) at the University of Maryland School of Medicine, as well as senior scientist at the Center for Birth Defects Research.
The alarming decline in maternal immunization rates that occurred in the wake of the COVID-19 pandemic means that, now more than ever, we must fully embrace our responsibility to recommend immunizations in pregnancy and to communicate what is known about their efficacy and safety. Data show that vaccination rates drop when we do not offer vaccines in our offices, so whenever possible, we should administer them as well.
The ob.gyn. is the patient’s most trusted person in pregnancy. When patients decline or express hesitancy about vaccines, it is incumbent upon us to ask why. Oftentimes, we can identify areas in which patients lack knowledge or have misperceptions and we can successfully educate the patient or change their perspective or misunderstanding concerning the importance of vaccination for themselves and their babies. (See Table 1.) We can also successfully address concerns about safety.
The safety of COVID-19 vaccinations in pregnancy is now backed by several years of data from multiple studies showing no increase in birth defects, preterm delivery, miscarriage, or stillbirth.
Data also show that pregnant patients are more likely than patients who are not pregnant to need hospitalization and intensive care when infected with SARS-CoV-2 and are at risk of having complications that can affect pregnancy and the newborn, including preterm birth and stillbirth. Vaccination has been shown to reduce the risk of severe illness and the risk of such adverse obstetrical outcomes, in addition to providing protection for the infant early on.
Similarly, influenza has long been more likely to be severe in pregnant patients, with an increased risk of poor obstetrical outcomes. Vaccines similarly provide “two for one protection,” protecting both mother and baby, and are, of course, backed by many years of safety and efficacy data.
With the new maternal respiratory syncytial virus (RSV) vaccine, now in its second year of availability, the goal is to protect the baby from RSV-caused serious lower respiratory tract illness. The illness has contributed to tens of thousands of annual hospitalizations and up to several hundred deaths every year in children younger than 5 years — particularly in those under age 6 months.
The RSV monoclonal antibody nirsevimab is available for the newborn as an alternative to maternal immunization but the maternal vaccine is optimal in that it will provide immediate rather than delayed protection for the newborn. The maternal vaccine is recommended during weeks 32-36 of pregnancy in mothers who were not vaccinated during last year’s RSV season. With real-world experience from year one, the available safety data are reassuring.
Counseling About Influenza and COVID-19 Vaccination
The COVID-19 pandemic took a toll on vaccination interest/receptivity broadly in pregnant and nonpregnant people. Among pregnant individuals, influenza vaccination coverage declined from 71% in the 2019-2020 influenza season to 56% in the 2021-2022 season, according to data from the Centers for Disease Control and Prevention’s Vaccine Safety Datalink.4 Coverage for the 2022-2023 and 2023-2024 influenza seasons was even worse: well under 50%.5
Fewer pregnant women have received updated COVID-19 vaccines. Only 13% of pregnant persons overall received the updated 2023-2024 COVID-19 booster vaccine (through March 30, 2024), according to the CDC.6
Maternal immunization for influenza has been recommended in the United States since 2004 (part of the recommendation that everyone over the age of 6 months receive an annual flu vaccine), and flu vaccines have been given to millions of pregnant women, but the H1N1 pandemic of 2009 reinforced its value as a priority for prenatal care. Most of the women who became severely ill from the H1N1 virus were young and healthy, without co-existing conditions known to increase risk.7
It became clearer during the H1N1 pandemic that pregnancy itself — which is associated with physiologic changes such as decreased lung capacity, increased nasal congestion and changes in the immune system – is its own significant risk factor for severe illness from the influenza virus. This increased risk applies to COVID-19 as well.
As COVID-19 has become endemic, with hospitalizations and deaths not reaching the levels of previous surges — and with mask-wearing and other preventive measures having declined — patients understandably have become more complacent. Some patients are vaccine deniers, but in my practice, these patients are a much smaller group than those who believe COVID-19 “is no big deal,” especially if they have had infections recently.
This is why it’s important to actively listen to concerns and to ask patients who decline a vaccination why they are hesitant. Blanket messages about vaccine efficacy and safety are the first step, but individualized, more pointed conversations based on the patient’s personal experiences and beliefs have become increasingly important.
I routinely tell pregnant patients about the risks of COVID-19 and I explain that it has been difficult to predict who will develop severe illness. Sometimes more conversation is needed. For those who are still hesitant or who tell me they feel protected by a recent infection, for instance, I provide more detail on the unique risks of pregnancy — the fact that “pregnancy is different” — and that natural immunity wanes while the protection afforded by immunization is believed to last longer. Many women are also concerned about the safety of the COVID-19 vaccine, so having safety data at your fingertips is helpful. (See Table 2.)
The fact that influenza and COVID-19 vaccination protect the newborn as well as the mother is something that I find is underappreciated by many patients. Explaining that infants likely benefit from the passage of antibodies across the placenta should be part of patient counseling.
Counseling About RSV Vaccination
Importantly, for the 2024-2025 RSV season, the maternal RSV vaccine (Abrysvo, Pfizer) is recommended only for pregnant women who did not receive the vaccine during the 2023-2024 season. When more research is done and more data are obtained showing how long the immune response persists post vaccination, it may be that the US Food and Drug Administration (FDA) will approve the maternal RSV vaccine for use in every pregnancy.
The later timing of the vaccination recommendation — 32-36 weeks’ gestation — reflects a conservative approach taken by the FDA in response to data from one of the pivotal trials showing a numerical trend toward more preterm deliveries among vaccinated compared with unvaccinated patients. This imbalance in the original trial, which administered the vaccine during 24-36 weeks of gestation, was seen only in low-income countries with no temporal association, however.
In our experience at two Weill Cornell Medical College–associated hospitals we did not see this trend. Our cohort study of almost 3000 pregnant patients who delivered at 32 weeks’ gestation or later found no increased risk of preterm birth among the 35% of patients who received the RSV vaccine during the 2023-2024 RSV season. We also did not see any difference in preeclampsia, in contrast with original trial data that showed a signal for increased risk.11
When fewer than 2 weeks have elapsed between maternal vaccination and delivery, the monoclonal antibody nirsevimab is recommended for the newborn — ideally before the newborn leaves the hospital. Nirsevimab is also recommended for newborns of mothers who decline vaccination or were not candidates (e.g. vaccinated in a previous pregnancy), or when there is concern about the adequacy of the maternal immune response to the vaccine (e.g. in cases of immunosuppression).
While there was a limited supply of the monoclonal antibody last year, limitations are not expected this year, especially after October.
The ultimate goal is that patients choose the vaccine or the immunoglobulin, given the severity of RSV disease. Patient preferences should be considered. However, given that it takes 2 weeks after vaccination for protection to build up, I stress to patients that if they’ve vaccinated themselves, their newborn will leave the hospital with protection. If nirsevimab is relied upon, I explain, their newborn may not be protected for some period of time.
Take-home Messages
- When patients decline or are hesitant about vaccines, ask why. Listen actively, and work to correct misperceptions and knowledge gaps.
- Whenever possible, offer vaccines in your practice. Vaccination rates drop when this does not occur.
- COVID-vaccine safety is backed by many studies showing no increase in birth defects, preterm delivery, miscarriage, or stillbirth.
- Pregnant women are more likely to have severe illness from the influenza and SARS-CoV-2 viruses. Vaccines can prevent severe illness and can protect the newborn as well as the mother.
- Recommend/administer the maternal RSV vaccine at 32-36 weeks’ gestation in women who did not receive the vaccine in the 2023-2024 season. If mothers aren’t eligible their babies should be offered nirsevimab.
Dr. Riley is the Given Foundation Professor and Chair of Obstetrics and Gynecology at Weill Cornell Medicine and the obstetrician and gynecologist-in-chief at New York Presbyterian Hospital. She disclosed that she has provided one-time consultations to Pfizer (Abrysvo RSV vaccine) and GSK (cytomegalovirus vaccine), and is providing consultant education on CMV for Moderna. She is chair of ACOG’s task force on immunization and emerging infectious diseases, serves on the medical advisory board for MAVEN, and serves as an editor or editorial board member for several medical publications.
References
1. ACOG Committee Opinion No. 741: Maternal Immunization. Obstet Gynecol. 2018;131(6):e214-e217.
2. Centers for Disease Control and Prevention. COVID-19 Vaccination for People Who are Pregnant or Breastfeeding. https://www.cdc.gov/covid/vaccines/pregnant-or-breastfeeding.html.
3. ACOG Practice Advisory on Maternal Respiratory Syncytial Virus Vaccination, September 2023. (Updated August 2024).4. Irving S et al. Open Forum Infect Dis. 2023;10(Suppl 2):ofad500.1002.
5. Flu Vaccination Dashboard, CDC, National Center for Immunization and Respiratory Diseases.
6. Weekly COVID-19 Vaccination Dashboard, CDC. https://www.cdc.gov/covidvaxview/weekly-dashboard/index.html
7. Louie JK et al. N Engl J Med. 2010;362:27-35. 8. Ciapponi A et al. Vaccine. 2021;39(40):5891-908.
9. Prasad S et al. Nature Communications. 2022;13:2414. 10. Fleming-Dutra KE et al. Obstet Gynecol Clin North Am 2023;50(2):279-97. 11. Mouen S et al. JAMA Network Open 2024;7(7):e2419268.
Editor’s Note: Sadly, this is the last column in the Master Class Obstetrics series. This award-winning column has been part of Ob.Gyn. News for 20 years. The deep discussion of cutting-edge topics in obstetrics by specialists and researchers will be missed as will the leadership and curation of topics by Dr. E. Albert Reece.
Introduction: The Need for Increased Vigilance About Maternal Immunization
Viruses are becoming increasingly prevalent in our world and the consequences of viral infections are implicated in a growing number of disease states. It is well established that certain cancers are caused by viruses and it is increasingly evident that viral infections can trigger the development of chronic illness. In pregnant women, viruses such as cytomegalovirus can cause infection in utero and lead to long-term impairments for the baby.
Likewise, it appears that the virulence of viruses is increasing, whether it be the respiratory syncytial virus (RSV) in children or the severe acute respiratory syndrome (SARS) coronaviruses in adults. Clearly, our environment is changing, with increases in population growth and urbanization, for instance, and an intensification of climate change and its effects. Viruses are part of this changing background.
Vaccines are our most powerful tool to protect people of all ages against viral threats, and fortunately, we benefit from increasing expertise in vaccinology. Since 1974, the University of Maryland School of Medicine has a Center for Vaccine Development and Global Health that has conducted research on vaccines to defend against the Zika virus, H1N1, Ebola, and SARS-CoV-2.
We’re not alone. Other vaccinology centers across the country — as well as the National Institutes of Health at the national level, through its National Institute of Allergy and Infectious Diseases — are doing research and developing vaccines to combat viral diseases.
In this column, we are focused on viral diseases in pregnancy and the role that vaccines can play in preventing serious respiratory illness in mothers and their newborns. I have invited Laura E. Riley, MD, the Given Foundation Professor and Chair of Obstetrics and Gynecology at Weill Cornell Medicine, to address the importance of maternal immunization and how we can best counsel our patients and improve immunization rates.
As Dr. Riley explains, we are in a new era, and it behooves us all to be more vigilant about recommending vaccines, combating misperceptions, addressing patients’ knowledge gaps, and administering vaccines whenever possible.
Dr. Reece is the former Dean of Medicine & University Executive VP, and The Distinguished University and Endowed Professor & Director of the Center for Advanced Research Training and Innovation (CARTI) at the University of Maryland School of Medicine, as well as senior scientist at the Center for Birth Defects Research.
The alarming decline in maternal immunization rates that occurred in the wake of the COVID-19 pandemic means that, now more than ever, we must fully embrace our responsibility to recommend immunizations in pregnancy and to communicate what is known about their efficacy and safety. Data show that vaccination rates drop when we do not offer vaccines in our offices, so whenever possible, we should administer them as well.
The ob.gyn. is the patient’s most trusted person in pregnancy. When patients decline or express hesitancy about vaccines, it is incumbent upon us to ask why. Oftentimes, we can identify areas in which patients lack knowledge or have misperceptions and we can successfully educate the patient or change their perspective or misunderstanding concerning the importance of vaccination for themselves and their babies. (See Table 1.) We can also successfully address concerns about safety.
The safety of COVID-19 vaccinations in pregnancy is now backed by several years of data from multiple studies showing no increase in birth defects, preterm delivery, miscarriage, or stillbirth.
Data also show that pregnant patients are more likely than patients who are not pregnant to need hospitalization and intensive care when infected with SARS-CoV-2 and are at risk of having complications that can affect pregnancy and the newborn, including preterm birth and stillbirth. Vaccination has been shown to reduce the risk of severe illness and the risk of such adverse obstetrical outcomes, in addition to providing protection for the infant early on.
Similarly, influenza has long been more likely to be severe in pregnant patients, with an increased risk of poor obstetrical outcomes. Vaccines similarly provide “two for one protection,” protecting both mother and baby, and are, of course, backed by many years of safety and efficacy data.
With the new maternal respiratory syncytial virus (RSV) vaccine, now in its second year of availability, the goal is to protect the baby from RSV-caused serious lower respiratory tract illness. The illness has contributed to tens of thousands of annual hospitalizations and up to several hundred deaths every year in children younger than 5 years — particularly in those under age 6 months.
The RSV monoclonal antibody nirsevimab is available for the newborn as an alternative to maternal immunization but the maternal vaccine is optimal in that it will provide immediate rather than delayed protection for the newborn. The maternal vaccine is recommended during weeks 32-36 of pregnancy in mothers who were not vaccinated during last year’s RSV season. With real-world experience from year one, the available safety data are reassuring.
Counseling About Influenza and COVID-19 Vaccination
The COVID-19 pandemic took a toll on vaccination interest/receptivity broadly in pregnant and nonpregnant people. Among pregnant individuals, influenza vaccination coverage declined from 71% in the 2019-2020 influenza season to 56% in the 2021-2022 season, according to data from the Centers for Disease Control and Prevention’s Vaccine Safety Datalink.4 Coverage for the 2022-2023 and 2023-2024 influenza seasons was even worse: well under 50%.5
Fewer pregnant women have received updated COVID-19 vaccines. Only 13% of pregnant persons overall received the updated 2023-2024 COVID-19 booster vaccine (through March 30, 2024), according to the CDC.6
Maternal immunization for influenza has been recommended in the United States since 2004 (part of the recommendation that everyone over the age of 6 months receive an annual flu vaccine), and flu vaccines have been given to millions of pregnant women, but the H1N1 pandemic of 2009 reinforced its value as a priority for prenatal care. Most of the women who became severely ill from the H1N1 virus were young and healthy, without co-existing conditions known to increase risk.7
It became clearer during the H1N1 pandemic that pregnancy itself — which is associated with physiologic changes such as decreased lung capacity, increased nasal congestion and changes in the immune system – is its own significant risk factor for severe illness from the influenza virus. This increased risk applies to COVID-19 as well.
As COVID-19 has become endemic, with hospitalizations and deaths not reaching the levels of previous surges — and with mask-wearing and other preventive measures having declined — patients understandably have become more complacent. Some patients are vaccine deniers, but in my practice, these patients are a much smaller group than those who believe COVID-19 “is no big deal,” especially if they have had infections recently.
This is why it’s important to actively listen to concerns and to ask patients who decline a vaccination why they are hesitant. Blanket messages about vaccine efficacy and safety are the first step, but individualized, more pointed conversations based on the patient’s personal experiences and beliefs have become increasingly important.
I routinely tell pregnant patients about the risks of COVID-19 and I explain that it has been difficult to predict who will develop severe illness. Sometimes more conversation is needed. For those who are still hesitant or who tell me they feel protected by a recent infection, for instance, I provide more detail on the unique risks of pregnancy — the fact that “pregnancy is different” — and that natural immunity wanes while the protection afforded by immunization is believed to last longer. Many women are also concerned about the safety of the COVID-19 vaccine, so having safety data at your fingertips is helpful. (See Table 2.)
The fact that influenza and COVID-19 vaccination protect the newborn as well as the mother is something that I find is underappreciated by many patients. Explaining that infants likely benefit from the passage of antibodies across the placenta should be part of patient counseling.
Counseling About RSV Vaccination
Importantly, for the 2024-2025 RSV season, the maternal RSV vaccine (Abrysvo, Pfizer) is recommended only for pregnant women who did not receive the vaccine during the 2023-2024 season. When more research is done and more data are obtained showing how long the immune response persists post vaccination, it may be that the US Food and Drug Administration (FDA) will approve the maternal RSV vaccine for use in every pregnancy.
The later timing of the vaccination recommendation — 32-36 weeks’ gestation — reflects a conservative approach taken by the FDA in response to data from one of the pivotal trials showing a numerical trend toward more preterm deliveries among vaccinated compared with unvaccinated patients. This imbalance in the original trial, which administered the vaccine during 24-36 weeks of gestation, was seen only in low-income countries with no temporal association, however.
In our experience at two Weill Cornell Medical College–associated hospitals we did not see this trend. Our cohort study of almost 3000 pregnant patients who delivered at 32 weeks’ gestation or later found no increased risk of preterm birth among the 35% of patients who received the RSV vaccine during the 2023-2024 RSV season. We also did not see any difference in preeclampsia, in contrast with original trial data that showed a signal for increased risk.11
When fewer than 2 weeks have elapsed between maternal vaccination and delivery, the monoclonal antibody nirsevimab is recommended for the newborn — ideally before the newborn leaves the hospital. Nirsevimab is also recommended for newborns of mothers who decline vaccination or were not candidates (e.g. vaccinated in a previous pregnancy), or when there is concern about the adequacy of the maternal immune response to the vaccine (e.g. in cases of immunosuppression).
While there was a limited supply of the monoclonal antibody last year, limitations are not expected this year, especially after October.
The ultimate goal is that patients choose the vaccine or the immunoglobulin, given the severity of RSV disease. Patient preferences should be considered. However, given that it takes 2 weeks after vaccination for protection to build up, I stress to patients that if they’ve vaccinated themselves, their newborn will leave the hospital with protection. If nirsevimab is relied upon, I explain, their newborn may not be protected for some period of time.
Take-home Messages
- When patients decline or are hesitant about vaccines, ask why. Listen actively, and work to correct misperceptions and knowledge gaps.
- Whenever possible, offer vaccines in your practice. Vaccination rates drop when this does not occur.
- COVID-vaccine safety is backed by many studies showing no increase in birth defects, preterm delivery, miscarriage, or stillbirth.
- Pregnant women are more likely to have severe illness from the influenza and SARS-CoV-2 viruses. Vaccines can prevent severe illness and can protect the newborn as well as the mother.
- Recommend/administer the maternal RSV vaccine at 32-36 weeks’ gestation in women who did not receive the vaccine in the 2023-2024 season. If mothers aren’t eligible their babies should be offered nirsevimab.
Dr. Riley is the Given Foundation Professor and Chair of Obstetrics and Gynecology at Weill Cornell Medicine and the obstetrician and gynecologist-in-chief at New York Presbyterian Hospital. She disclosed that she has provided one-time consultations to Pfizer (Abrysvo RSV vaccine) and GSK (cytomegalovirus vaccine), and is providing consultant education on CMV for Moderna. She is chair of ACOG’s task force on immunization and emerging infectious diseases, serves on the medical advisory board for MAVEN, and serves as an editor or editorial board member for several medical publications.
References
1. ACOG Committee Opinion No. 741: Maternal Immunization. Obstet Gynecol. 2018;131(6):e214-e217.
2. Centers for Disease Control and Prevention. COVID-19 Vaccination for People Who are Pregnant or Breastfeeding. https://www.cdc.gov/covid/vaccines/pregnant-or-breastfeeding.html.
3. ACOG Practice Advisory on Maternal Respiratory Syncytial Virus Vaccination, September 2023. (Updated August 2024).4. Irving S et al. Open Forum Infect Dis. 2023;10(Suppl 2):ofad500.1002.
5. Flu Vaccination Dashboard, CDC, National Center for Immunization and Respiratory Diseases.
6. Weekly COVID-19 Vaccination Dashboard, CDC. https://www.cdc.gov/covidvaxview/weekly-dashboard/index.html
7. Louie JK et al. N Engl J Med. 2010;362:27-35. 8. Ciapponi A et al. Vaccine. 2021;39(40):5891-908.
9. Prasad S et al. Nature Communications. 2022;13:2414. 10. Fleming-Dutra KE et al. Obstet Gynecol Clin North Am 2023;50(2):279-97. 11. Mouen S et al. JAMA Network Open 2024;7(7):e2419268.
Anticipated Effects of Pneumococcal Vaccines on Otitis
Acute otitis media (AOM) is caused by Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis. Since the introduction of pneumococcal conjugate vaccines (PCVs) shifts in the proportion of these three bacteria as causes of AOM and their antibiotic susceptibility profiles and strain diversity have occurred due to multiple factors including the PCVs and antibiotic selection pressure.
The 7-valent PCV (PCV7) was introduced in 2000 and was proven to be efficacious in preventing AOM, but no subsequent PCV has received an indication for prevention of AOM because the FDA required a tympanocentesis study to prove efficacy and that approval was not achieved for PCV13, PCV15, or PCV20. This is a little known fact. After introduction of PCV7, replacement pneumococcal strains expressing serotypes not in PCV7 emerged and antibiotic non-susceptible strains became predominant causes of AOM, especially antibiotic-resistant serotype 19A. To address the phenomena of pneumococcal serotype replacement, PCV13 was introduced in 2010. But serotype replacement continued to occur under PCV13 pressure, replacement serotypes increasingly caused AOM, and antibiotic-resistant serotype 35B emerged. Now we have two new higher valency PCVs: PCV15 (Merck) where serotypes 22F and 33F were added to the PCV13 serotypes and PCV20 (Pfizer) where 22F, 33F, 8, 10A, 11A, 12F, 15B were added to PCV13. Note that neither PCV15 nor PCV20 includes the most common serotype causing AOM – serotype 35B.1
While PCV15 and PCV20 should provide protection against more pneumococcal serotypes, increasing serotypes in both vaccines decreased immunogenicity of certain shared serotypes, more so with the addition of seven more in PCV20 than two more in PCV15, compared with PCV13. Whether lower antibody concentrations will make a difference clinically in terms of vaccine failure to prevent nasopharyngeal colonization, AOM, and/or invasive pneumococcal infections is currently unknown.
Our group from greater Rochester, New York, is the only one in the United States performing tympanocentesis to determine the etiology of AOM infections. Children between ages 6 and 36 months are studied. We recently reported our results for the time span September 2021 to September 2023, the immediate 2 years prior to recommendations for use of PCV15 and PCV20 in young children.2 Tympanocentesis was performed in 139 (78%) of 179 episodes of AOM, yielding 216 middle ear fluid samples (the higher number of middle ear fluids was due to bilateral tympanocentesis in some children). H. influenzae (40%) was the most common bacterial isolate, followed by S. pneumonia (19%) and M. catarrhalis (17%), with the remainder no growth. Polymerase chain reactions (PCR) was positive in many of those culture negative samples, suggesting prior use of antibiotics before tympanocentesis was performed. Among the pneumococcal isolates, 46% were oxacillin non-susceptible. Among the H. influenzae isolates, 27% were beta-lactamase producing and all M. catarrhalis were beta-lactamase-producing.
As we previously reported,1 we once again found that serotype 35B was the most frequent non-PCV15, non-PCV20, serotype. Other frequently detected non-PCV20 pneumococcal serotypes were 23A, 23B, 35D, 35F and 15C.2
Projected Pneumococcal Serotype Coverage by PCV15 and PCV20
PCV13 serotypes were identified in 9% of middle ear fluids, consistent with vaccine failure. Assuming 100% vaccine-type effectiveness, PCV15 will provide about 11% coverage of pneumococci causing AOM, the same PCV13 and PCV20 will provide 30% coverage, leaving 70% of pneumococci causing AOM in young children uncovered (Figure).
Thus, the high proportion of pneumococcal serotype 35B and other non-PCV15 or non-PCV20 serotypes will result in a relatively small incremental benefit over PCV13 in young children for AOM.
AOM is the most common cause of pediatric outpatient visits and antibiotic prescriptions in the United States that contributes to selection of antibiotic-resistant microbes.3 The economic burden of AOM is high, estimated at about $3 billion annually in the United States, when direct and indirect costs are calculated,4 thereby making AOM a major factor in calculations of cost effectiveness analyses of PCV immunizations in children.
While PCV15 and PCV20 include common serotypes associated with invasive pneumococcal diseases, their effectiveness in preventing AOM, acute sinusitis, and non-bacteremic community-acquired pneumonia is currently unknown because these vaccines were licensed based on safety and immunogenicity data, not proven efficacy.
The data on antibiotic susceptibility of pneumococci and H. influenza and M. catarrhalis isolated in the late post PCV13 era from young children in a pediatric primary-care setting raise a question about empiric antibiotic choice for AOM today. For penicillin non-susceptible pneumococcal strains, higher dosages of amoxicillin can improve eradication. However, higher dosages of amoxicillin cannot overcome beta-lactamase production by H. influenza and M. catarrhalis. Based on the mix of pathogens causing AOM and the antibiotic susceptibility of those bacteria, high-dose amoxicillin/clavulanate or alternative cephalosporin drugs active against pneumococci and beta-lactamase producing H. influenza and M. catarrhalis would be a better empiric choice over high-dose amoxicillin.
Limitations of our study include that it occurred in one center in New York, although we have previously shown results of tympanocentesis at our center are similar to those in Virginia and Pennsylvania5 and our study population was composed of children living in urban, suburban, and rural households of all economic levels. Because this study was conducted during a relatively short time frame (2021-2023), the numbers of subjects and samples were sometimes insufficient to identify statistically significant differences in some comparisons. Some children were lost to follow-up, and not every participant was consented for tympanocentesis. Some participants received antibiotics prior to middle ear fluid specimen collection.
Dr. Pichichero is a specialist in pediatric infectious diseases, Center for Infectious Diseases and Immunology, and director of the Research Institute, at Rochester (N.Y.) General Hospital. He has no conflicts of interest to declare.
References
1. Kaur R et al. Dynamic Changes in Otopathogens Colonizing the Nasopharynx and Causing Acute Otitis Media in Children After 13-Valent (PCV13) Pneumococcal Conjugate Vaccination During 2015-2019. Eur J Clin Microbiol Infect Dis. 2022 Jan;41(1):37-44. doi: 10.1007/s10096-021-04324-0.
2. Kaur R et al. Anticipated Effects of Higher-valency Pneumococcal Conjugate Vaccines on Colonization and Acute Otitis Media. Pediatr Infect Dis J. 2024 Oct 1;43(10):1004-1010. doi: 10.1097/INF.0000000000004413.
3. King LM et al. Pediatric Outpatient Visits and Antibiotic Use Attributable to Higher Valency Pneumococcal Conjugate Vaccine Serotypes. medRxiv [Preprint]. 2023 Aug 25:2023.08.24.23294570. doi: 10.1101/2023.08.24.23294570.
4. Ahmed S et al. Incremental Health Care Utilization and Costs for Acute Otitis Media in Children. Laryngoscope. 2014 Jan;124(1):301-5. doi: 10.1002/lary.24190.
5. Pichichero ME et al. Pathogens Causing Recurrent and Difficult-to-Treat Acute Otitis Media, 2003-2006. Clin Pediatr (Phila). 2008 Nov;47(9):901-6. doi: 10.1177/0009922808319966.
Acute otitis media (AOM) is caused by Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis. Since the introduction of pneumococcal conjugate vaccines (PCVs) shifts in the proportion of these three bacteria as causes of AOM and their antibiotic susceptibility profiles and strain diversity have occurred due to multiple factors including the PCVs and antibiotic selection pressure.
The 7-valent PCV (PCV7) was introduced in 2000 and was proven to be efficacious in preventing AOM, but no subsequent PCV has received an indication for prevention of AOM because the FDA required a tympanocentesis study to prove efficacy and that approval was not achieved for PCV13, PCV15, or PCV20. This is a little known fact. After introduction of PCV7, replacement pneumococcal strains expressing serotypes not in PCV7 emerged and antibiotic non-susceptible strains became predominant causes of AOM, especially antibiotic-resistant serotype 19A. To address the phenomena of pneumococcal serotype replacement, PCV13 was introduced in 2010. But serotype replacement continued to occur under PCV13 pressure, replacement serotypes increasingly caused AOM, and antibiotic-resistant serotype 35B emerged. Now we have two new higher valency PCVs: PCV15 (Merck) where serotypes 22F and 33F were added to the PCV13 serotypes and PCV20 (Pfizer) where 22F, 33F, 8, 10A, 11A, 12F, 15B were added to PCV13. Note that neither PCV15 nor PCV20 includes the most common serotype causing AOM – serotype 35B.1
While PCV15 and PCV20 should provide protection against more pneumococcal serotypes, increasing serotypes in both vaccines decreased immunogenicity of certain shared serotypes, more so with the addition of seven more in PCV20 than two more in PCV15, compared with PCV13. Whether lower antibody concentrations will make a difference clinically in terms of vaccine failure to prevent nasopharyngeal colonization, AOM, and/or invasive pneumococcal infections is currently unknown.
Our group from greater Rochester, New York, is the only one in the United States performing tympanocentesis to determine the etiology of AOM infections. Children between ages 6 and 36 months are studied. We recently reported our results for the time span September 2021 to September 2023, the immediate 2 years prior to recommendations for use of PCV15 and PCV20 in young children.2 Tympanocentesis was performed in 139 (78%) of 179 episodes of AOM, yielding 216 middle ear fluid samples (the higher number of middle ear fluids was due to bilateral tympanocentesis in some children). H. influenzae (40%) was the most common bacterial isolate, followed by S. pneumonia (19%) and M. catarrhalis (17%), with the remainder no growth. Polymerase chain reactions (PCR) was positive in many of those culture negative samples, suggesting prior use of antibiotics before tympanocentesis was performed. Among the pneumococcal isolates, 46% were oxacillin non-susceptible. Among the H. influenzae isolates, 27% were beta-lactamase producing and all M. catarrhalis were beta-lactamase-producing.
As we previously reported,1 we once again found that serotype 35B was the most frequent non-PCV15, non-PCV20, serotype. Other frequently detected non-PCV20 pneumococcal serotypes were 23A, 23B, 35D, 35F and 15C.2
Projected Pneumococcal Serotype Coverage by PCV15 and PCV20
PCV13 serotypes were identified in 9% of middle ear fluids, consistent with vaccine failure. Assuming 100% vaccine-type effectiveness, PCV15 will provide about 11% coverage of pneumococci causing AOM, the same PCV13 and PCV20 will provide 30% coverage, leaving 70% of pneumococci causing AOM in young children uncovered (Figure).

Thus, the high proportion of pneumococcal serotype 35B and other non-PCV15 or non-PCV20 serotypes will result in a relatively small incremental benefit over PCV13 in young children for AOM.
AOM is the most common cause of pediatric outpatient visits and antibiotic prescriptions in the United States that contributes to selection of antibiotic-resistant microbes.3 The economic burden of AOM is high, estimated at about $3 billion annually in the United States, when direct and indirect costs are calculated,4 thereby making AOM a major factor in calculations of cost effectiveness analyses of PCV immunizations in children.
While PCV15 and PCV20 include common serotypes associated with invasive pneumococcal diseases, their effectiveness in preventing AOM, acute sinusitis, and non-bacteremic community-acquired pneumonia is currently unknown because these vaccines were licensed based on safety and immunogenicity data, not proven efficacy.
The data on antibiotic susceptibility of pneumococci and H. influenza and M. catarrhalis isolated in the late post PCV13 era from young children in a pediatric primary-care setting raise a question about empiric antibiotic choice for AOM today. For penicillin non-susceptible pneumococcal strains, higher dosages of amoxicillin can improve eradication. However, higher dosages of amoxicillin cannot overcome beta-lactamase production by H. influenza and M. catarrhalis. Based on the mix of pathogens causing AOM and the antibiotic susceptibility of those bacteria, high-dose amoxicillin/clavulanate or alternative cephalosporin drugs active against pneumococci and beta-lactamase producing H. influenza and M. catarrhalis would be a better empiric choice over high-dose amoxicillin.
Limitations of our study include that it occurred in one center in New York, although we have previously shown results of tympanocentesis at our center are similar to those in Virginia and Pennsylvania5 and our study population was composed of children living in urban, suburban, and rural households of all economic levels. Because this study was conducted during a relatively short time frame (2021-2023), the numbers of subjects and samples were sometimes insufficient to identify statistically significant differences in some comparisons. Some children were lost to follow-up, and not every participant was consented for tympanocentesis. Some participants received antibiotics prior to middle ear fluid specimen collection.
Dr. Pichichero is a specialist in pediatric infectious diseases, Center for Infectious Diseases and Immunology, and director of the Research Institute, at Rochester (N.Y.) General Hospital. He has no conflicts of interest to declare.
References
1. Kaur R et al. Dynamic Changes in Otopathogens Colonizing the Nasopharynx and Causing Acute Otitis Media in Children After 13-Valent (PCV13) Pneumococcal Conjugate Vaccination During 2015-2019. Eur J Clin Microbiol Infect Dis. 2022 Jan;41(1):37-44. doi: 10.1007/s10096-021-04324-0.
2. Kaur R et al. Anticipated Effects of Higher-valency Pneumococcal Conjugate Vaccines on Colonization and Acute Otitis Media. Pediatr Infect Dis J. 2024 Oct 1;43(10):1004-1010. doi: 10.1097/INF.0000000000004413.
3. King LM et al. Pediatric Outpatient Visits and Antibiotic Use Attributable to Higher Valency Pneumococcal Conjugate Vaccine Serotypes. medRxiv [Preprint]. 2023 Aug 25:2023.08.24.23294570. doi: 10.1101/2023.08.24.23294570.
4. Ahmed S et al. Incremental Health Care Utilization and Costs for Acute Otitis Media in Children. Laryngoscope. 2014 Jan;124(1):301-5. doi: 10.1002/lary.24190.
5. Pichichero ME et al. Pathogens Causing Recurrent and Difficult-to-Treat Acute Otitis Media, 2003-2006. Clin Pediatr (Phila). 2008 Nov;47(9):901-6. doi: 10.1177/0009922808319966.
Acute otitis media (AOM) is caused by Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis. Since the introduction of pneumococcal conjugate vaccines (PCVs) shifts in the proportion of these three bacteria as causes of AOM and their antibiotic susceptibility profiles and strain diversity have occurred due to multiple factors including the PCVs and antibiotic selection pressure.
The 7-valent PCV (PCV7) was introduced in 2000 and was proven to be efficacious in preventing AOM, but no subsequent PCV has received an indication for prevention of AOM because the FDA required a tympanocentesis study to prove efficacy and that approval was not achieved for PCV13, PCV15, or PCV20. This is a little known fact. After introduction of PCV7, replacement pneumococcal strains expressing serotypes not in PCV7 emerged and antibiotic non-susceptible strains became predominant causes of AOM, especially antibiotic-resistant serotype 19A. To address the phenomena of pneumococcal serotype replacement, PCV13 was introduced in 2010. But serotype replacement continued to occur under PCV13 pressure, replacement serotypes increasingly caused AOM, and antibiotic-resistant serotype 35B emerged. Now we have two new higher valency PCVs: PCV15 (Merck) where serotypes 22F and 33F were added to the PCV13 serotypes and PCV20 (Pfizer) where 22F, 33F, 8, 10A, 11A, 12F, 15B were added to PCV13. Note that neither PCV15 nor PCV20 includes the most common serotype causing AOM – serotype 35B.1
While PCV15 and PCV20 should provide protection against more pneumococcal serotypes, increasing serotypes in both vaccines decreased immunogenicity of certain shared serotypes, more so with the addition of seven more in PCV20 than two more in PCV15, compared with PCV13. Whether lower antibody concentrations will make a difference clinically in terms of vaccine failure to prevent nasopharyngeal colonization, AOM, and/or invasive pneumococcal infections is currently unknown.
Our group from greater Rochester, New York, is the only one in the United States performing tympanocentesis to determine the etiology of AOM infections. Children between ages 6 and 36 months are studied. We recently reported our results for the time span September 2021 to September 2023, the immediate 2 years prior to recommendations for use of PCV15 and PCV20 in young children.2 Tympanocentesis was performed in 139 (78%) of 179 episodes of AOM, yielding 216 middle ear fluid samples (the higher number of middle ear fluids was due to bilateral tympanocentesis in some children). H. influenzae (40%) was the most common bacterial isolate, followed by S. pneumonia (19%) and M. catarrhalis (17%), with the remainder no growth. Polymerase chain reactions (PCR) was positive in many of those culture negative samples, suggesting prior use of antibiotics before tympanocentesis was performed. Among the pneumococcal isolates, 46% were oxacillin non-susceptible. Among the H. influenzae isolates, 27% were beta-lactamase producing and all M. catarrhalis were beta-lactamase-producing.
As we previously reported,1 we once again found that serotype 35B was the most frequent non-PCV15, non-PCV20, serotype. Other frequently detected non-PCV20 pneumococcal serotypes were 23A, 23B, 35D, 35F and 15C.2
Projected Pneumococcal Serotype Coverage by PCV15 and PCV20
PCV13 serotypes were identified in 9% of middle ear fluids, consistent with vaccine failure. Assuming 100% vaccine-type effectiveness, PCV15 will provide about 11% coverage of pneumococci causing AOM, the same PCV13 and PCV20 will provide 30% coverage, leaving 70% of pneumococci causing AOM in young children uncovered (Figure).

Thus, the high proportion of pneumococcal serotype 35B and other non-PCV15 or non-PCV20 serotypes will result in a relatively small incremental benefit over PCV13 in young children for AOM.
AOM is the most common cause of pediatric outpatient visits and antibiotic prescriptions in the United States that contributes to selection of antibiotic-resistant microbes.3 The economic burden of AOM is high, estimated at about $3 billion annually in the United States, when direct and indirect costs are calculated,4 thereby making AOM a major factor in calculations of cost effectiveness analyses of PCV immunizations in children.
While PCV15 and PCV20 include common serotypes associated with invasive pneumococcal diseases, their effectiveness in preventing AOM, acute sinusitis, and non-bacteremic community-acquired pneumonia is currently unknown because these vaccines were licensed based on safety and immunogenicity data, not proven efficacy.
The data on antibiotic susceptibility of pneumococci and H. influenza and M. catarrhalis isolated in the late post PCV13 era from young children in a pediatric primary-care setting raise a question about empiric antibiotic choice for AOM today. For penicillin non-susceptible pneumococcal strains, higher dosages of amoxicillin can improve eradication. However, higher dosages of amoxicillin cannot overcome beta-lactamase production by H. influenza and M. catarrhalis. Based on the mix of pathogens causing AOM and the antibiotic susceptibility of those bacteria, high-dose amoxicillin/clavulanate or alternative cephalosporin drugs active against pneumococci and beta-lactamase producing H. influenza and M. catarrhalis would be a better empiric choice over high-dose amoxicillin.
Limitations of our study include that it occurred in one center in New York, although we have previously shown results of tympanocentesis at our center are similar to those in Virginia and Pennsylvania5 and our study population was composed of children living in urban, suburban, and rural households of all economic levels. Because this study was conducted during a relatively short time frame (2021-2023), the numbers of subjects and samples were sometimes insufficient to identify statistically significant differences in some comparisons. Some children were lost to follow-up, and not every participant was consented for tympanocentesis. Some participants received antibiotics prior to middle ear fluid specimen collection.
Dr. Pichichero is a specialist in pediatric infectious diseases, Center for Infectious Diseases and Immunology, and director of the Research Institute, at Rochester (N.Y.) General Hospital. He has no conflicts of interest to declare.
References
1. Kaur R et al. Dynamic Changes in Otopathogens Colonizing the Nasopharynx and Causing Acute Otitis Media in Children After 13-Valent (PCV13) Pneumococcal Conjugate Vaccination During 2015-2019. Eur J Clin Microbiol Infect Dis. 2022 Jan;41(1):37-44. doi: 10.1007/s10096-021-04324-0.
2. Kaur R et al. Anticipated Effects of Higher-valency Pneumococcal Conjugate Vaccines on Colonization and Acute Otitis Media. Pediatr Infect Dis J. 2024 Oct 1;43(10):1004-1010. doi: 10.1097/INF.0000000000004413.
3. King LM et al. Pediatric Outpatient Visits and Antibiotic Use Attributable to Higher Valency Pneumococcal Conjugate Vaccine Serotypes. medRxiv [Preprint]. 2023 Aug 25:2023.08.24.23294570. doi: 10.1101/2023.08.24.23294570.
4. Ahmed S et al. Incremental Health Care Utilization and Costs for Acute Otitis Media in Children. Laryngoscope. 2014 Jan;124(1):301-5. doi: 10.1002/lary.24190.
5. Pichichero ME et al. Pathogens Causing Recurrent and Difficult-to-Treat Acute Otitis Media, 2003-2006. Clin Pediatr (Phila). 2008 Nov;47(9):901-6. doi: 10.1177/0009922808319966.
How Doctors Can Overcome Vaccine Hesitancy Through Empathy, Storytelling, and Patient-Centered Communication
When Kimberly Fisher, MD, was a junior doctor, she got fired up when patients showed hesitancy about vaccines. She responded by providing numbers, data, and facts that proved vaccines were safe and effective in preventing life-threatening diseases. But she soon realized that regurgitating scientific evidence wasn’t a winning strategy. “I’ve made the mistake of launching into a let me tell you all the things that I know that you don’t know kind of lecture,” Dr. Fisher, now an associate professor of medicine at UMass Chan Medical School, Worcester, Massachusetts, a pulmonary physician, and a researcher interested in patient-provider communication, told this news organization. “Through experience and research, I have learned that when you do that, they stop listening.”
She said when patients give reasons for not getting vaccinated that are factually wrong and rooted in misinformation, the most common reaction is to correct that information and not let it stand. “That is important; it just can’t be the first thing you do,” she said.
Diane Arnaout, MD, a pediatrician at Cook Children’s Pediatrics in Fort Worth, Texas, said listening to some patients explaining why vaccine injections are poisonous or a conspiracy can be exhausting and frustrating, but she agrees that presenting scientific facts alone won’t change people’s minds. “Even in my worst days, I take the time to stop talking for a moment and let the parents talk about what concerns them because if you just get mad and put a wall up, then that trust is gone, possibly forever, not just about vaccines.”
The Default Option
Since the start of the COVID-19 pandemic, Dr. Fisher has dedicated much of her time researching vaccine hesitancy. One of the most “fascinating and unexpected” findings of her work was that people are more likely to get vaccinated if a healthcare provider recommends that they get vaccinated in a “presumptive style,” which means that the provider uses language that presupposes that the person’s going to get vaccinated. “Rather than asking whether they wanted to get the vaccine conveying that the option of not getting it is just as valid, you make vaccination the default option,” she suggested.
The strategy wins many undecided, but it might not work on the most reluctant. “The presumptive recommendation is very directive, and if that works, great, but if it doesn’t, you need to shift to almost the opposite strategy, showing empathy and understanding about the person’s reasons for not wanting to be vaccinated,” Dr. Fisher said.
Find One Thing to Agree On
During a focus group on COVID-19 vaccine hesitancy that Dr. Fisher conducted in December 2021, most physicians expressed frustration that some patients remained resistant despite their best efforts. However, one participant shared an approach she found effective with even the most hesitant patients. The physician would listen carefully and express understanding, and even if what the patient said wasn’t accurate, she would find a kernel of truth to agree with and align herself with the patient. By doing this, she made patients feel like they were a team.
The example she gave was if a patient said, “I don’t know. I’ve heard different things and don’t feel comfortable taking the vaccine,” she might respond with something like, “I think it’s great that you’re thinking critically about this before making a decision. I was the same way — I wanted to fully understand the data before getting vaccinated. I also wouldn’t want to take something if I thought it wasn’t safe. It’s good that you’re being thorough.” Acknowledging their careful thought process, the physician helped patients feel seen and understood only after she introduced additional information to guide them toward understanding why the vaccine might be beneficial.
Focus on the Disease
Dr. Arnaout’s frustration grows when at the end of an appointment some parents object to vaccines with irrational and misguided concerns. “You’ve trusted me with everything else we’ve discussed today — whether it’s a diaper rash or an ear infection — so why wouldn’t you trust me on this? Sometimes it feels almost offensive — why trust my medical expertise on everything else but not vaccines?” she said.
The answer, she believes, is that vaccines are preventive, and when the threat of disease feels distant, it’s hard to see the necessity of a painful shot for your healthy child. “But if your baby were dying from meningitis, the needles we use to deliver life-saving medications in the hospital would feel absolutely necessary. It’s hard as a parent to inflict pain for something you’ve never personally seen.”
Dr. Arnaout thinks it is important to bring the focus on the disease the vaccine prevents. “Let’s talk about measles — how if a baby in my waiting room has measles and coughs, the virus can stay suspended in the air for 2 hours, and 100% of unvaccinated people in that room will get measles.”
She said sharing personal stories can also help physicians connect with their patients. “I talk to parents every day about their vaccine concerns, and I’ve found that if I take the time to explain why we vaccinate, they start to understand. I also tell them, ‘I vaccinated my children for everything on time and give them the flu shot every year. Why would I offer your child something I wouldn’t give my own?’ That personal decision, made without hesitation, resonates with parents.”
Wired for Stories
Medical professionals have a professional necessity to think and speak with precision. Their training is based on analyzing studies and data, and they develop a specialized vocabulary to describe their findings accurately.
But the human brain is naturally inclined to process and make sense of information through the structure and narrative of stories. We instinctively organize reality into a “shape of a story” rather than just isolated facts, explained Ben Riggs, senior communications specialist at Kettering Health, Dayton, Ohio, a nonfiction writing coach and author. Storytelling also taps into the emotional, rather than just the rational, parts of the brain. This emotional connection helps make the information more memorable and impactful for the listener.
Mr. Riggs said that moving from this world of precision and accuracy to one that also requires effective communication with those who haven’t had that same training is much like learning a new language. “If they can’t speak in a way that non-scientists understand, it’s like the old saying: If a tree falls in the woods and no one hears it, does it make a sound?”
Metaphors can help doctors translate scientific facts into language that meets people where they are, allowing patients to make informed decisions about their health. They can help physicians transform abstract concepts into vivid, tangible mental images that are easier for people to understand and relate to, Mr. Riggs explained. “We are predominantly concrete thinkers. Metaphors can create concrete scenes and do much of the heavy lifting when communicating complex ideas.”
“It’s important to align yourself with the other person by showing that you care, that you’re truly listening, and understand their perspective,” concluded Dr. Fisher. “Acknowledge their point of view and emphasize that they have autonomy in the decision-making process. This can open people up to hearing your perspective. You also need to know when to let go don’t cause a rift in the relationship.”
Dr. Fisher, Dr. Arnaout, and Mr. Riggs reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
When Kimberly Fisher, MD, was a junior doctor, she got fired up when patients showed hesitancy about vaccines. She responded by providing numbers, data, and facts that proved vaccines were safe and effective in preventing life-threatening diseases. But she soon realized that regurgitating scientific evidence wasn’t a winning strategy. “I’ve made the mistake of launching into a let me tell you all the things that I know that you don’t know kind of lecture,” Dr. Fisher, now an associate professor of medicine at UMass Chan Medical School, Worcester, Massachusetts, a pulmonary physician, and a researcher interested in patient-provider communication, told this news organization. “Through experience and research, I have learned that when you do that, they stop listening.”
She said when patients give reasons for not getting vaccinated that are factually wrong and rooted in misinformation, the most common reaction is to correct that information and not let it stand. “That is important; it just can’t be the first thing you do,” she said.
Diane Arnaout, MD, a pediatrician at Cook Children’s Pediatrics in Fort Worth, Texas, said listening to some patients explaining why vaccine injections are poisonous or a conspiracy can be exhausting and frustrating, but she agrees that presenting scientific facts alone won’t change people’s minds. “Even in my worst days, I take the time to stop talking for a moment and let the parents talk about what concerns them because if you just get mad and put a wall up, then that trust is gone, possibly forever, not just about vaccines.”
The Default Option
Since the start of the COVID-19 pandemic, Dr. Fisher has dedicated much of her time researching vaccine hesitancy. One of the most “fascinating and unexpected” findings of her work was that people are more likely to get vaccinated if a healthcare provider recommends that they get vaccinated in a “presumptive style,” which means that the provider uses language that presupposes that the person’s going to get vaccinated. “Rather than asking whether they wanted to get the vaccine conveying that the option of not getting it is just as valid, you make vaccination the default option,” she suggested.
The strategy wins many undecided, but it might not work on the most reluctant. “The presumptive recommendation is very directive, and if that works, great, but if it doesn’t, you need to shift to almost the opposite strategy, showing empathy and understanding about the person’s reasons for not wanting to be vaccinated,” Dr. Fisher said.
Find One Thing to Agree On
During a focus group on COVID-19 vaccine hesitancy that Dr. Fisher conducted in December 2021, most physicians expressed frustration that some patients remained resistant despite their best efforts. However, one participant shared an approach she found effective with even the most hesitant patients. The physician would listen carefully and express understanding, and even if what the patient said wasn’t accurate, she would find a kernel of truth to agree with and align herself with the patient. By doing this, she made patients feel like they were a team.
The example she gave was if a patient said, “I don’t know. I’ve heard different things and don’t feel comfortable taking the vaccine,” she might respond with something like, “I think it’s great that you’re thinking critically about this before making a decision. I was the same way — I wanted to fully understand the data before getting vaccinated. I also wouldn’t want to take something if I thought it wasn’t safe. It’s good that you’re being thorough.” Acknowledging their careful thought process, the physician helped patients feel seen and understood only after she introduced additional information to guide them toward understanding why the vaccine might be beneficial.
Focus on the Disease
Dr. Arnaout’s frustration grows when at the end of an appointment some parents object to vaccines with irrational and misguided concerns. “You’ve trusted me with everything else we’ve discussed today — whether it’s a diaper rash or an ear infection — so why wouldn’t you trust me on this? Sometimes it feels almost offensive — why trust my medical expertise on everything else but not vaccines?” she said.
The answer, she believes, is that vaccines are preventive, and when the threat of disease feels distant, it’s hard to see the necessity of a painful shot for your healthy child. “But if your baby were dying from meningitis, the needles we use to deliver life-saving medications in the hospital would feel absolutely necessary. It’s hard as a parent to inflict pain for something you’ve never personally seen.”
Dr. Arnaout thinks it is important to bring the focus on the disease the vaccine prevents. “Let’s talk about measles — how if a baby in my waiting room has measles and coughs, the virus can stay suspended in the air for 2 hours, and 100% of unvaccinated people in that room will get measles.”
She said sharing personal stories can also help physicians connect with their patients. “I talk to parents every day about their vaccine concerns, and I’ve found that if I take the time to explain why we vaccinate, they start to understand. I also tell them, ‘I vaccinated my children for everything on time and give them the flu shot every year. Why would I offer your child something I wouldn’t give my own?’ That personal decision, made without hesitation, resonates with parents.”
Wired for Stories
Medical professionals have a professional necessity to think and speak with precision. Their training is based on analyzing studies and data, and they develop a specialized vocabulary to describe their findings accurately.
But the human brain is naturally inclined to process and make sense of information through the structure and narrative of stories. We instinctively organize reality into a “shape of a story” rather than just isolated facts, explained Ben Riggs, senior communications specialist at Kettering Health, Dayton, Ohio, a nonfiction writing coach and author. Storytelling also taps into the emotional, rather than just the rational, parts of the brain. This emotional connection helps make the information more memorable and impactful for the listener.
Mr. Riggs said that moving from this world of precision and accuracy to one that also requires effective communication with those who haven’t had that same training is much like learning a new language. “If they can’t speak in a way that non-scientists understand, it’s like the old saying: If a tree falls in the woods and no one hears it, does it make a sound?”
Metaphors can help doctors translate scientific facts into language that meets people where they are, allowing patients to make informed decisions about their health. They can help physicians transform abstract concepts into vivid, tangible mental images that are easier for people to understand and relate to, Mr. Riggs explained. “We are predominantly concrete thinkers. Metaphors can create concrete scenes and do much of the heavy lifting when communicating complex ideas.”
“It’s important to align yourself with the other person by showing that you care, that you’re truly listening, and understand their perspective,” concluded Dr. Fisher. “Acknowledge their point of view and emphasize that they have autonomy in the decision-making process. This can open people up to hearing your perspective. You also need to know when to let go don’t cause a rift in the relationship.”
Dr. Fisher, Dr. Arnaout, and Mr. Riggs reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
When Kimberly Fisher, MD, was a junior doctor, she got fired up when patients showed hesitancy about vaccines. She responded by providing numbers, data, and facts that proved vaccines were safe and effective in preventing life-threatening diseases. But she soon realized that regurgitating scientific evidence wasn’t a winning strategy. “I’ve made the mistake of launching into a let me tell you all the things that I know that you don’t know kind of lecture,” Dr. Fisher, now an associate professor of medicine at UMass Chan Medical School, Worcester, Massachusetts, a pulmonary physician, and a researcher interested in patient-provider communication, told this news organization. “Through experience and research, I have learned that when you do that, they stop listening.”
She said when patients give reasons for not getting vaccinated that are factually wrong and rooted in misinformation, the most common reaction is to correct that information and not let it stand. “That is important; it just can’t be the first thing you do,” she said.
Diane Arnaout, MD, a pediatrician at Cook Children’s Pediatrics in Fort Worth, Texas, said listening to some patients explaining why vaccine injections are poisonous or a conspiracy can be exhausting and frustrating, but she agrees that presenting scientific facts alone won’t change people’s minds. “Even in my worst days, I take the time to stop talking for a moment and let the parents talk about what concerns them because if you just get mad and put a wall up, then that trust is gone, possibly forever, not just about vaccines.”
The Default Option
Since the start of the COVID-19 pandemic, Dr. Fisher has dedicated much of her time researching vaccine hesitancy. One of the most “fascinating and unexpected” findings of her work was that people are more likely to get vaccinated if a healthcare provider recommends that they get vaccinated in a “presumptive style,” which means that the provider uses language that presupposes that the person’s going to get vaccinated. “Rather than asking whether they wanted to get the vaccine conveying that the option of not getting it is just as valid, you make vaccination the default option,” she suggested.
The strategy wins many undecided, but it might not work on the most reluctant. “The presumptive recommendation is very directive, and if that works, great, but if it doesn’t, you need to shift to almost the opposite strategy, showing empathy and understanding about the person’s reasons for not wanting to be vaccinated,” Dr. Fisher said.
Find One Thing to Agree On
During a focus group on COVID-19 vaccine hesitancy that Dr. Fisher conducted in December 2021, most physicians expressed frustration that some patients remained resistant despite their best efforts. However, one participant shared an approach she found effective with even the most hesitant patients. The physician would listen carefully and express understanding, and even if what the patient said wasn’t accurate, she would find a kernel of truth to agree with and align herself with the patient. By doing this, she made patients feel like they were a team.
The example she gave was if a patient said, “I don’t know. I’ve heard different things and don’t feel comfortable taking the vaccine,” she might respond with something like, “I think it’s great that you’re thinking critically about this before making a decision. I was the same way — I wanted to fully understand the data before getting vaccinated. I also wouldn’t want to take something if I thought it wasn’t safe. It’s good that you’re being thorough.” Acknowledging their careful thought process, the physician helped patients feel seen and understood only after she introduced additional information to guide them toward understanding why the vaccine might be beneficial.
Focus on the Disease
Dr. Arnaout’s frustration grows when at the end of an appointment some parents object to vaccines with irrational and misguided concerns. “You’ve trusted me with everything else we’ve discussed today — whether it’s a diaper rash or an ear infection — so why wouldn’t you trust me on this? Sometimes it feels almost offensive — why trust my medical expertise on everything else but not vaccines?” she said.
The answer, she believes, is that vaccines are preventive, and when the threat of disease feels distant, it’s hard to see the necessity of a painful shot for your healthy child. “But if your baby were dying from meningitis, the needles we use to deliver life-saving medications in the hospital would feel absolutely necessary. It’s hard as a parent to inflict pain for something you’ve never personally seen.”
Dr. Arnaout thinks it is important to bring the focus on the disease the vaccine prevents. “Let’s talk about measles — how if a baby in my waiting room has measles and coughs, the virus can stay suspended in the air for 2 hours, and 100% of unvaccinated people in that room will get measles.”
She said sharing personal stories can also help physicians connect with their patients. “I talk to parents every day about their vaccine concerns, and I’ve found that if I take the time to explain why we vaccinate, they start to understand. I also tell them, ‘I vaccinated my children for everything on time and give them the flu shot every year. Why would I offer your child something I wouldn’t give my own?’ That personal decision, made without hesitation, resonates with parents.”
Wired for Stories
Medical professionals have a professional necessity to think and speak with precision. Their training is based on analyzing studies and data, and they develop a specialized vocabulary to describe their findings accurately.
But the human brain is naturally inclined to process and make sense of information through the structure and narrative of stories. We instinctively organize reality into a “shape of a story” rather than just isolated facts, explained Ben Riggs, senior communications specialist at Kettering Health, Dayton, Ohio, a nonfiction writing coach and author. Storytelling also taps into the emotional, rather than just the rational, parts of the brain. This emotional connection helps make the information more memorable and impactful for the listener.
Mr. Riggs said that moving from this world of precision and accuracy to one that also requires effective communication with those who haven’t had that same training is much like learning a new language. “If they can’t speak in a way that non-scientists understand, it’s like the old saying: If a tree falls in the woods and no one hears it, does it make a sound?”
Metaphors can help doctors translate scientific facts into language that meets people where they are, allowing patients to make informed decisions about their health. They can help physicians transform abstract concepts into vivid, tangible mental images that are easier for people to understand and relate to, Mr. Riggs explained. “We are predominantly concrete thinkers. Metaphors can create concrete scenes and do much of the heavy lifting when communicating complex ideas.”
“It’s important to align yourself with the other person by showing that you care, that you’re truly listening, and understand their perspective,” concluded Dr. Fisher. “Acknowledge their point of view and emphasize that they have autonomy in the decision-making process. This can open people up to hearing your perspective. You also need to know when to let go don’t cause a rift in the relationship.”
Dr. Fisher, Dr. Arnaout, and Mr. Riggs reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Reduced Vaccination Rates Contribute to Rising Pertussis Numbers
New data from the Centers for Disease Control and Prevention (CDC) show significant spikes in pertussis cases compared with last year, especially in several urban areas including New York, Illinois, Florida, and Colorado.
Notably, the current pertussis case count in Illinois as of September 21, 2024, was five times higher than the total cases in 2023 (1058 vs 50). New York City alone had reported 624 cases as of September 21, compared with 38 cases in 2023.
Additional data from the CDC on vaccination coverage and exemptions of school-aged children showed an increase from 3.0% last year to 3.3% in 2024 of children who were exempted from recommended vaccination requirements. Although nearly 93% of kindergarteners in the United States received recommended vaccines (including Tdap), similar to last year, this number shows a steady decline from 94% in the 2021-2021 school year and 93% in the 2021-2022 school year, according to previous CDC reports.
What’s Happening in the Clinic
Clinical experience and the most recent CDC data point to under vaccination as a driver of the increased pertussis cases this year, David J. Cennimo, MD, associate professor of medicine and pediatrics in the division of infectious disease at Rutgers New Jersey Medical School, Newark, New Jersey, said in an interview.
Although the pertussis vaccination rates in infancy are still very good, clinicians are seeing a drop-off in school-aged children and adults, and the lingering anti-vaccine efforts from the COVID-19 pandemic period are undoubtedly playing a part, said Dr. Cennimo. “Unfortunately, pertussis is contagious, and the vaccine effectiveness wears off. Having decreased numbers of people protected results in more rapid spread,” he said.
Dr. Cennimo agreed that the number of cases in the United States is underreported, and even higher than the data suggest. “I’m sure of it; the initial clinical presentation may be mistaken for a viral upper respiratory tract infection (common cold),” he told this news organization.
Many older children and adults with pertussis do not manifest the classic “whooping cough” seen in infants and young children, so making a clinical diagnosis can be difficult, he said. “One classical component of the illness is a prolonged cough. I have wondered if some people now reporting a lingering cough had pertussis that was missed,” Dr. Cennimo noted.
“Clinicians should stress the value of boosters in a vaccine-preventable illness where we know immunity wanes overtime,” Dr. Cennimo said. “We have a great remedy in the Tdap vaccine, which we should all be getting very 10 years,” he said.
He also emphasized that clinicians remind pregnant women of the current recommendations to receive the Tdap vaccine for every pregnancy. “Vaccination during pregnancy is the best way to protect both the pregnant person and the newborn.
Even for the vaccine hesitant, this vaccine has a long track record of safety so should not be a significant concern,” he said.
The ultimate take-home message is not a new one, and applies to all illnesses, Dr. Cennimo told this news organization. Simply put, “Stay home if you are sick. Social distancing is not just for COVID-19,” he said.
Dr. Cennimo had no financial conflicts to disclose.
A version of this article first appeared on Medscape.com.
New data from the Centers for Disease Control and Prevention (CDC) show significant spikes in pertussis cases compared with last year, especially in several urban areas including New York, Illinois, Florida, and Colorado.
Notably, the current pertussis case count in Illinois as of September 21, 2024, was five times higher than the total cases in 2023 (1058 vs 50). New York City alone had reported 624 cases as of September 21, compared with 38 cases in 2023.
Additional data from the CDC on vaccination coverage and exemptions of school-aged children showed an increase from 3.0% last year to 3.3% in 2024 of children who were exempted from recommended vaccination requirements. Although nearly 93% of kindergarteners in the United States received recommended vaccines (including Tdap), similar to last year, this number shows a steady decline from 94% in the 2021-2021 school year and 93% in the 2021-2022 school year, according to previous CDC reports.
What’s Happening in the Clinic
Clinical experience and the most recent CDC data point to under vaccination as a driver of the increased pertussis cases this year, David J. Cennimo, MD, associate professor of medicine and pediatrics in the division of infectious disease at Rutgers New Jersey Medical School, Newark, New Jersey, said in an interview.
Although the pertussis vaccination rates in infancy are still very good, clinicians are seeing a drop-off in school-aged children and adults, and the lingering anti-vaccine efforts from the COVID-19 pandemic period are undoubtedly playing a part, said Dr. Cennimo. “Unfortunately, pertussis is contagious, and the vaccine effectiveness wears off. Having decreased numbers of people protected results in more rapid spread,” he said.
Dr. Cennimo agreed that the number of cases in the United States is underreported, and even higher than the data suggest. “I’m sure of it; the initial clinical presentation may be mistaken for a viral upper respiratory tract infection (common cold),” he told this news organization.
Many older children and adults with pertussis do not manifest the classic “whooping cough” seen in infants and young children, so making a clinical diagnosis can be difficult, he said. “One classical component of the illness is a prolonged cough. I have wondered if some people now reporting a lingering cough had pertussis that was missed,” Dr. Cennimo noted.
“Clinicians should stress the value of boosters in a vaccine-preventable illness where we know immunity wanes overtime,” Dr. Cennimo said. “We have a great remedy in the Tdap vaccine, which we should all be getting very 10 years,” he said.
He also emphasized that clinicians remind pregnant women of the current recommendations to receive the Tdap vaccine for every pregnancy. “Vaccination during pregnancy is the best way to protect both the pregnant person and the newborn.
Even for the vaccine hesitant, this vaccine has a long track record of safety so should not be a significant concern,” he said.
The ultimate take-home message is not a new one, and applies to all illnesses, Dr. Cennimo told this news organization. Simply put, “Stay home if you are sick. Social distancing is not just for COVID-19,” he said.
Dr. Cennimo had no financial conflicts to disclose.
A version of this article first appeared on Medscape.com.
New data from the Centers for Disease Control and Prevention (CDC) show significant spikes in pertussis cases compared with last year, especially in several urban areas including New York, Illinois, Florida, and Colorado.
Notably, the current pertussis case count in Illinois as of September 21, 2024, was five times higher than the total cases in 2023 (1058 vs 50). New York City alone had reported 624 cases as of September 21, compared with 38 cases in 2023.
Additional data from the CDC on vaccination coverage and exemptions of school-aged children showed an increase from 3.0% last year to 3.3% in 2024 of children who were exempted from recommended vaccination requirements. Although nearly 93% of kindergarteners in the United States received recommended vaccines (including Tdap), similar to last year, this number shows a steady decline from 94% in the 2021-2021 school year and 93% in the 2021-2022 school year, according to previous CDC reports.
What’s Happening in the Clinic
Clinical experience and the most recent CDC data point to under vaccination as a driver of the increased pertussis cases this year, David J. Cennimo, MD, associate professor of medicine and pediatrics in the division of infectious disease at Rutgers New Jersey Medical School, Newark, New Jersey, said in an interview.
Although the pertussis vaccination rates in infancy are still very good, clinicians are seeing a drop-off in school-aged children and adults, and the lingering anti-vaccine efforts from the COVID-19 pandemic period are undoubtedly playing a part, said Dr. Cennimo. “Unfortunately, pertussis is contagious, and the vaccine effectiveness wears off. Having decreased numbers of people protected results in more rapid spread,” he said.
Dr. Cennimo agreed that the number of cases in the United States is underreported, and even higher than the data suggest. “I’m sure of it; the initial clinical presentation may be mistaken for a viral upper respiratory tract infection (common cold),” he told this news organization.
Many older children and adults with pertussis do not manifest the classic “whooping cough” seen in infants and young children, so making a clinical diagnosis can be difficult, he said. “One classical component of the illness is a prolonged cough. I have wondered if some people now reporting a lingering cough had pertussis that was missed,” Dr. Cennimo noted.
“Clinicians should stress the value of boosters in a vaccine-preventable illness where we know immunity wanes overtime,” Dr. Cennimo said. “We have a great remedy in the Tdap vaccine, which we should all be getting very 10 years,” he said.
He also emphasized that clinicians remind pregnant women of the current recommendations to receive the Tdap vaccine for every pregnancy. “Vaccination during pregnancy is the best way to protect both the pregnant person and the newborn.
Even for the vaccine hesitant, this vaccine has a long track record of safety so should not be a significant concern,” he said.
The ultimate take-home message is not a new one, and applies to all illnesses, Dr. Cennimo told this news organization. Simply put, “Stay home if you are sick. Social distancing is not just for COVID-19,” he said.
Dr. Cennimo had no financial conflicts to disclose.
A version of this article first appeared on Medscape.com.
Guidance for Practicing Primary Care: World Health Organization’s Updated Influenza Guidelines for 2024
As primary care physicians, we are often the first ones patients see when they become infected with influenza. According to Centers for Disease Control and Prevention statistics, approximately 5%-20% of the US population will be infected with influenza every year. Additionally, more than 200,000 of these patients will be hospitalized because of complications related to influenza.
Earlier in September, the World Health Organization (WHO) issued its latest clinical practice guidelines for influenza for the 2024-2025 season. This is a 213-page document aimed at healthcare providers who treat patients infected with influenza. It includes treatment for those with severe and nonsevere influenza infections, those in both the outpatient and hospitalized setting, as well as medication prophylaxis for those exposed to the virus. Additionally, it defines risk estimates for those who are at risk of being hospitalized or dying. In contrast, previous updates focused on management of severe influenza or those at risk of severe influenza.
These guidelines cover recommendations regarding all the antiviral medications for treating influenza used around the world. For the purpose of this article, we will focus on those most commonly used in the United States.
A newer medication discussed was baloxavir. It is recommended to be used for patients with nonsevere influenza who are at high risk for progression to severe disease. The advice is to not use it for those with little risk of progression to severe disease. Oseltamivir is recommended for those with severe infection.
The guidelines recommend against using antibiotics for those who have a low likelihood of having a bacterial coinfection. As primary care doctors, we often prescribe medications to help with symptoms. These guidelines recommend against the use of corticosteroids and antibiotics but did advise that NSAIDs could be used for symptom relief.
One of the important parts of these guidelines is prevention in patients who have been exposed but are asymptomatic. They recommend baloxavir or oseltamivir but only for those patients who are at high risk of being hospitalized if they were to become infected. Any of the antivirals can be used for patients who are exposed to the novel influenza A, which is associated with a higher mortality rate. Caution when prescribing antivirals is recommended in immunocompromised patients because there is more drug resistance seen in these patients.
These updates also discuss the use of different influenza tests. In the outpatient setting, primary doctors don’t have time for test results that may take 2 days to come back. Only rapid tests make the sense in the primary care setting. Additionally, in the age of COVID, it is important to make an accurate diagnosis so we should be testing patients. There is resistance seen with the antivirals we prescribe for influenza so prescribing them empirically without a confirmed diagnosis of influenza may be doing more harm than good.
One gap in these recommendations is vaccination. This topic was not covered at all. It would be helpful to have a strategy in place to prevent infection in populations rather than focusing just on exposed individuals. A discussion of when and who and to vaccinate would be helpful. Research into the effectiveness of vaccines is key and more accurate development of a season’s influenza vaccine would be beneficial. Currently, there is much vaccine misinformation being spread around. Education and information regarding influenza vaccines, especially coming from WHO, is crucial.
Another failure of these recommendations is that the guidelines apply only to those who present within a few days of becoming symptomatic. As family doctors, we know many of our patients self-treat or consult Google. They often don’t come for medical care until they’ve been sick for a week or longer. There are no guidelines for these patients.
In general, these guidelines are comprehensive and do a great job discussing the current medications available. However, more is needed to increase vaccination rates. Patients need to know that if they may be sick with influenza, they need to seek medical care as soon as possible. We, as family doctors, need to do a better job of risk-stratifying our patients and prescribing prophylactic medication when suitable. Every infection we prevent aids in the health of our community and the global population at large.
Dr. Girgis practices family medicine in South River, New Jersey, and is a clinical assistant professor of family medicine at Robert Wood Johnson Medical School, New Brunswick, New Jersey. She has no relevant conflicts of interest.
As primary care physicians, we are often the first ones patients see when they become infected with influenza. According to Centers for Disease Control and Prevention statistics, approximately 5%-20% of the US population will be infected with influenza every year. Additionally, more than 200,000 of these patients will be hospitalized because of complications related to influenza.
Earlier in September, the World Health Organization (WHO) issued its latest clinical practice guidelines for influenza for the 2024-2025 season. This is a 213-page document aimed at healthcare providers who treat patients infected with influenza. It includes treatment for those with severe and nonsevere influenza infections, those in both the outpatient and hospitalized setting, as well as medication prophylaxis for those exposed to the virus. Additionally, it defines risk estimates for those who are at risk of being hospitalized or dying. In contrast, previous updates focused on management of severe influenza or those at risk of severe influenza.
These guidelines cover recommendations regarding all the antiviral medications for treating influenza used around the world. For the purpose of this article, we will focus on those most commonly used in the United States.
A newer medication discussed was baloxavir. It is recommended to be used for patients with nonsevere influenza who are at high risk for progression to severe disease. The advice is to not use it for those with little risk of progression to severe disease. Oseltamivir is recommended for those with severe infection.
The guidelines recommend against using antibiotics for those who have a low likelihood of having a bacterial coinfection. As primary care doctors, we often prescribe medications to help with symptoms. These guidelines recommend against the use of corticosteroids and antibiotics but did advise that NSAIDs could be used for symptom relief.
One of the important parts of these guidelines is prevention in patients who have been exposed but are asymptomatic. They recommend baloxavir or oseltamivir but only for those patients who are at high risk of being hospitalized if they were to become infected. Any of the antivirals can be used for patients who are exposed to the novel influenza A, which is associated with a higher mortality rate. Caution when prescribing antivirals is recommended in immunocompromised patients because there is more drug resistance seen in these patients.
These updates also discuss the use of different influenza tests. In the outpatient setting, primary doctors don’t have time for test results that may take 2 days to come back. Only rapid tests make the sense in the primary care setting. Additionally, in the age of COVID, it is important to make an accurate diagnosis so we should be testing patients. There is resistance seen with the antivirals we prescribe for influenza so prescribing them empirically without a confirmed diagnosis of influenza may be doing more harm than good.
One gap in these recommendations is vaccination. This topic was not covered at all. It would be helpful to have a strategy in place to prevent infection in populations rather than focusing just on exposed individuals. A discussion of when and who and to vaccinate would be helpful. Research into the effectiveness of vaccines is key and more accurate development of a season’s influenza vaccine would be beneficial. Currently, there is much vaccine misinformation being spread around. Education and information regarding influenza vaccines, especially coming from WHO, is crucial.
Another failure of these recommendations is that the guidelines apply only to those who present within a few days of becoming symptomatic. As family doctors, we know many of our patients self-treat or consult Google. They often don’t come for medical care until they’ve been sick for a week or longer. There are no guidelines for these patients.
In general, these guidelines are comprehensive and do a great job discussing the current medications available. However, more is needed to increase vaccination rates. Patients need to know that if they may be sick with influenza, they need to seek medical care as soon as possible. We, as family doctors, need to do a better job of risk-stratifying our patients and prescribing prophylactic medication when suitable. Every infection we prevent aids in the health of our community and the global population at large.
Dr. Girgis practices family medicine in South River, New Jersey, and is a clinical assistant professor of family medicine at Robert Wood Johnson Medical School, New Brunswick, New Jersey. She has no relevant conflicts of interest.
As primary care physicians, we are often the first ones patients see when they become infected with influenza. According to Centers for Disease Control and Prevention statistics, approximately 5%-20% of the US population will be infected with influenza every year. Additionally, more than 200,000 of these patients will be hospitalized because of complications related to influenza.
Earlier in September, the World Health Organization (WHO) issued its latest clinical practice guidelines for influenza for the 2024-2025 season. This is a 213-page document aimed at healthcare providers who treat patients infected with influenza. It includes treatment for those with severe and nonsevere influenza infections, those in both the outpatient and hospitalized setting, as well as medication prophylaxis for those exposed to the virus. Additionally, it defines risk estimates for those who are at risk of being hospitalized or dying. In contrast, previous updates focused on management of severe influenza or those at risk of severe influenza.
These guidelines cover recommendations regarding all the antiviral medications for treating influenza used around the world. For the purpose of this article, we will focus on those most commonly used in the United States.
A newer medication discussed was baloxavir. It is recommended to be used for patients with nonsevere influenza who are at high risk for progression to severe disease. The advice is to not use it for those with little risk of progression to severe disease. Oseltamivir is recommended for those with severe infection.
The guidelines recommend against using antibiotics for those who have a low likelihood of having a bacterial coinfection. As primary care doctors, we often prescribe medications to help with symptoms. These guidelines recommend against the use of corticosteroids and antibiotics but did advise that NSAIDs could be used for symptom relief.
One of the important parts of these guidelines is prevention in patients who have been exposed but are asymptomatic. They recommend baloxavir or oseltamivir but only for those patients who are at high risk of being hospitalized if they were to become infected. Any of the antivirals can be used for patients who are exposed to the novel influenza A, which is associated with a higher mortality rate. Caution when prescribing antivirals is recommended in immunocompromised patients because there is more drug resistance seen in these patients.
These updates also discuss the use of different influenza tests. In the outpatient setting, primary doctors don’t have time for test results that may take 2 days to come back. Only rapid tests make the sense in the primary care setting. Additionally, in the age of COVID, it is important to make an accurate diagnosis so we should be testing patients. There is resistance seen with the antivirals we prescribe for influenza so prescribing them empirically without a confirmed diagnosis of influenza may be doing more harm than good.
One gap in these recommendations is vaccination. This topic was not covered at all. It would be helpful to have a strategy in place to prevent infection in populations rather than focusing just on exposed individuals. A discussion of when and who and to vaccinate would be helpful. Research into the effectiveness of vaccines is key and more accurate development of a season’s influenza vaccine would be beneficial. Currently, there is much vaccine misinformation being spread around. Education and information regarding influenza vaccines, especially coming from WHO, is crucial.
Another failure of these recommendations is that the guidelines apply only to those who present within a few days of becoming symptomatic. As family doctors, we know many of our patients self-treat or consult Google. They often don’t come for medical care until they’ve been sick for a week or longer. There are no guidelines for these patients.
In general, these guidelines are comprehensive and do a great job discussing the current medications available. However, more is needed to increase vaccination rates. Patients need to know that if they may be sick with influenza, they need to seek medical care as soon as possible. We, as family doctors, need to do a better job of risk-stratifying our patients and prescribing prophylactic medication when suitable. Every infection we prevent aids in the health of our community and the global population at large.
Dr. Girgis practices family medicine in South River, New Jersey, and is a clinical assistant professor of family medicine at Robert Wood Johnson Medical School, New Brunswick, New Jersey. She has no relevant conflicts of interest.
Pertussis Rates Up Compared With Recent Years
, according to data from the Centers for Disease Control and Prevention (CDC). Reports from several states illustrate this trend, thought to be due to reduced immunity across the country.
The Alaska Department of Health issued a statement on its website about the significant increase in pertussis cases in the state during the summer, with 90 cases in July and 61 in August, compared with 24 in June and a total of 26 cases in 2023.
Similarly, the Florida Department of Health reported a pertussis increase in July 2024 that was higher than the June 2024 case count and also above the previous 5-year average.
Experts in these and other states suggest that several factors are driving the nationwide increase, including the fact that fewer people are consistently wearing masks. The mass masking during the COVID-19 pandemic caused a significant drop in pertussis, but the latest data suggest a return to prepandemic levels, and waning immunity likely plays a role as well.
Pertussis, also known as whooping cough, typically begins with symptoms similar to those of the common cold, including runny nose, sneezing, mild fever, and cough, according to the CDC. However, babies with whooping cough may experience trouble breathing rather than a cough. The coughing fits often associated with pertussis may not start until 2 weeks after the onset of other symptoms, according to the CDC.
Those who have been vaccinated against pertussis can still become infected, but the risk is lower, and the illness, if it occurs, is likely to be milder. Complications such as apnea, pneumonia, and convulsions can occur in babies younger than 1 year, especially if they have not been vaccinated, according to the CDC.
Beyond Easing Pandemic Precautions
Many respiratory-based infections dipped during the COVID-19 pandemic, almost certainly from the multifactorial interventions of masking, distancing, and the general lack of comingling, said David J. Cennimo, MD, associate professor of medicine & pediatrics in the Division of Infectious Diseases at Rutgers New Jersey Medical School, Newark, New Jersey, in an interview.
The number of cases of many of these diseases returned to previous levels after COVID-19 restrictions were lifted, he said.
“However, we know pertussis immunity wanes over time. Children get DTaP at 2, 4, 6, and 15 months, and a Tdap booster at 11-12 years old gets them to adulthood,” Dr. Cennimo said. Adults should be getting a Tdap every 10 years, he added.
The latest available CDC data indicate that Tdap vaccine coverage in adults is approximately 40%, which means that there may be a large number of susceptible people who can become infected and propagate to others, said Dr. Cennimo.
Not Just the Young Ones
A recent pertussis outbreak among college students in Virginia highlighted the fact that the infection can affect all ages, and that the effectiveness of childhood vaccines may decrease over time. The majority of the recently diagnosed cases occurred in individuals who had been previously vaccinated, according to a press release from the Virginia Department of Health.
Clinical Clues
The initial stage of pertussis infection looks like a common cold with symptoms of upper respiratory infection, Dr. Cennimo told this news organization. “Unless there is reason to suspect pertussis exposure, it would almost certainly be missed,” he noted.
The characteristic barking/seal-like cough is mostly seen in children, said Dr. Cennimo. Adults and children can experience coughing fits that can lead to shortness of breath and/or vomiting, which would raise suspicion for pertussis, but is not universally present, he said. The convalescent stage of pertussis can be prolonged and is characterized by chronic coughing. “In the past, pertussis had been called the 100-day cough,” and at that point, treatment is ineffective, Dr. Cennimo said.
In clinical practice, “I advise everyone to get the Tdap vaccine every 10 years,” and remember that the “Td” is the every 10-year tetanus shot as well, Dr. Cennimo told this news organization. Reassure patients that the Tdap can be given with other vaccines, he said, and remind patients that, as with any of the respiratory illnesses, they should stay home if sick, cover a cough, consider wearing a mask in public, and wash hands frequently, he said.
Dr. Cennimo had no financial conflicts to disclose.
A version of this article first appeared on Medscape.com.
, according to data from the Centers for Disease Control and Prevention (CDC). Reports from several states illustrate this trend, thought to be due to reduced immunity across the country.
The Alaska Department of Health issued a statement on its website about the significant increase in pertussis cases in the state during the summer, with 90 cases in July and 61 in August, compared with 24 in June and a total of 26 cases in 2023.
Similarly, the Florida Department of Health reported a pertussis increase in July 2024 that was higher than the June 2024 case count and also above the previous 5-year average.
Experts in these and other states suggest that several factors are driving the nationwide increase, including the fact that fewer people are consistently wearing masks. The mass masking during the COVID-19 pandemic caused a significant drop in pertussis, but the latest data suggest a return to prepandemic levels, and waning immunity likely plays a role as well.
Pertussis, also known as whooping cough, typically begins with symptoms similar to those of the common cold, including runny nose, sneezing, mild fever, and cough, according to the CDC. However, babies with whooping cough may experience trouble breathing rather than a cough. The coughing fits often associated with pertussis may not start until 2 weeks after the onset of other symptoms, according to the CDC.
Those who have been vaccinated against pertussis can still become infected, but the risk is lower, and the illness, if it occurs, is likely to be milder. Complications such as apnea, pneumonia, and convulsions can occur in babies younger than 1 year, especially if they have not been vaccinated, according to the CDC.
Beyond Easing Pandemic Precautions
Many respiratory-based infections dipped during the COVID-19 pandemic, almost certainly from the multifactorial interventions of masking, distancing, and the general lack of comingling, said David J. Cennimo, MD, associate professor of medicine & pediatrics in the Division of Infectious Diseases at Rutgers New Jersey Medical School, Newark, New Jersey, in an interview.
The number of cases of many of these diseases returned to previous levels after COVID-19 restrictions were lifted, he said.
“However, we know pertussis immunity wanes over time. Children get DTaP at 2, 4, 6, and 15 months, and a Tdap booster at 11-12 years old gets them to adulthood,” Dr. Cennimo said. Adults should be getting a Tdap every 10 years, he added.
The latest available CDC data indicate that Tdap vaccine coverage in adults is approximately 40%, which means that there may be a large number of susceptible people who can become infected and propagate to others, said Dr. Cennimo.
Not Just the Young Ones
A recent pertussis outbreak among college students in Virginia highlighted the fact that the infection can affect all ages, and that the effectiveness of childhood vaccines may decrease over time. The majority of the recently diagnosed cases occurred in individuals who had been previously vaccinated, according to a press release from the Virginia Department of Health.
Clinical Clues
The initial stage of pertussis infection looks like a common cold with symptoms of upper respiratory infection, Dr. Cennimo told this news organization. “Unless there is reason to suspect pertussis exposure, it would almost certainly be missed,” he noted.
The characteristic barking/seal-like cough is mostly seen in children, said Dr. Cennimo. Adults and children can experience coughing fits that can lead to shortness of breath and/or vomiting, which would raise suspicion for pertussis, but is not universally present, he said. The convalescent stage of pertussis can be prolonged and is characterized by chronic coughing. “In the past, pertussis had been called the 100-day cough,” and at that point, treatment is ineffective, Dr. Cennimo said.
In clinical practice, “I advise everyone to get the Tdap vaccine every 10 years,” and remember that the “Td” is the every 10-year tetanus shot as well, Dr. Cennimo told this news organization. Reassure patients that the Tdap can be given with other vaccines, he said, and remind patients that, as with any of the respiratory illnesses, they should stay home if sick, cover a cough, consider wearing a mask in public, and wash hands frequently, he said.
Dr. Cennimo had no financial conflicts to disclose.
A version of this article first appeared on Medscape.com.
, according to data from the Centers for Disease Control and Prevention (CDC). Reports from several states illustrate this trend, thought to be due to reduced immunity across the country.
The Alaska Department of Health issued a statement on its website about the significant increase in pertussis cases in the state during the summer, with 90 cases in July and 61 in August, compared with 24 in June and a total of 26 cases in 2023.
Similarly, the Florida Department of Health reported a pertussis increase in July 2024 that was higher than the June 2024 case count and also above the previous 5-year average.
Experts in these and other states suggest that several factors are driving the nationwide increase, including the fact that fewer people are consistently wearing masks. The mass masking during the COVID-19 pandemic caused a significant drop in pertussis, but the latest data suggest a return to prepandemic levels, and waning immunity likely plays a role as well.
Pertussis, also known as whooping cough, typically begins with symptoms similar to those of the common cold, including runny nose, sneezing, mild fever, and cough, according to the CDC. However, babies with whooping cough may experience trouble breathing rather than a cough. The coughing fits often associated with pertussis may not start until 2 weeks after the onset of other symptoms, according to the CDC.
Those who have been vaccinated against pertussis can still become infected, but the risk is lower, and the illness, if it occurs, is likely to be milder. Complications such as apnea, pneumonia, and convulsions can occur in babies younger than 1 year, especially if they have not been vaccinated, according to the CDC.
Beyond Easing Pandemic Precautions
Many respiratory-based infections dipped during the COVID-19 pandemic, almost certainly from the multifactorial interventions of masking, distancing, and the general lack of comingling, said David J. Cennimo, MD, associate professor of medicine & pediatrics in the Division of Infectious Diseases at Rutgers New Jersey Medical School, Newark, New Jersey, in an interview.
The number of cases of many of these diseases returned to previous levels after COVID-19 restrictions were lifted, he said.
“However, we know pertussis immunity wanes over time. Children get DTaP at 2, 4, 6, and 15 months, and a Tdap booster at 11-12 years old gets them to adulthood,” Dr. Cennimo said. Adults should be getting a Tdap every 10 years, he added.
The latest available CDC data indicate that Tdap vaccine coverage in adults is approximately 40%, which means that there may be a large number of susceptible people who can become infected and propagate to others, said Dr. Cennimo.
Not Just the Young Ones
A recent pertussis outbreak among college students in Virginia highlighted the fact that the infection can affect all ages, and that the effectiveness of childhood vaccines may decrease over time. The majority of the recently diagnosed cases occurred in individuals who had been previously vaccinated, according to a press release from the Virginia Department of Health.
Clinical Clues
The initial stage of pertussis infection looks like a common cold with symptoms of upper respiratory infection, Dr. Cennimo told this news organization. “Unless there is reason to suspect pertussis exposure, it would almost certainly be missed,” he noted.
The characteristic barking/seal-like cough is mostly seen in children, said Dr. Cennimo. Adults and children can experience coughing fits that can lead to shortness of breath and/or vomiting, which would raise suspicion for pertussis, but is not universally present, he said. The convalescent stage of pertussis can be prolonged and is characterized by chronic coughing. “In the past, pertussis had been called the 100-day cough,” and at that point, treatment is ineffective, Dr. Cennimo said.
In clinical practice, “I advise everyone to get the Tdap vaccine every 10 years,” and remember that the “Td” is the every 10-year tetanus shot as well, Dr. Cennimo told this news organization. Reassure patients that the Tdap can be given with other vaccines, he said, and remind patients that, as with any of the respiratory illnesses, they should stay home if sick, cover a cough, consider wearing a mask in public, and wash hands frequently, he said.
Dr. Cennimo had no financial conflicts to disclose.
A version of this article first appeared on Medscape.com.








