User login
Teenagers get in the queue for COVID-19 vaccines
The vaccinations can’t come soon enough for parents like Stacy Hillenburg, a developmental therapist in Aurora, Ill., whose 9-year-old son takes immunosuppressants because he had a heart transplant when he was 7 weeks old. Although school-age children aren’t yet included in clinical trials, if her 12- and 13-year-old daughters could get vaccinated, along with both parents, then the family could relax some of the protocols they currently follow to prevent infection.
Whenever they are around other people, even masked and socially distanced, they come home and immediately shower and change their clothes. So far, no one in the family has been infected with COVID, but the anxiety is ever-present. “I can’t wait for it to come out,” Ms. Hillenburg said of a pediatric COVID vaccine. “It will ease my mind so much.”
She isn’t alone in that anticipation. In the fall, the American Academy of Pediatrics and other pediatric vaccine experts urged faster action on pediatric vaccine trials and worried that children would be left behind as adults gained protection from COVID. But recent developments have eased those concerns.
“Over the next couple of months, we will be doing trials in an age-deescalation manner,” with studies moving gradually to younger children, Anthony S. Fauci, MD, chief medical adviser on COVID-19 to the president, said in a coronavirus response team briefing on Jan. 29. “So that hopefully, as we get to the late spring and summer, we will have children being able to be vaccinated.”
Pfizer completed enrollment of 2,259 teens aged 12-15 years in late January and expects to move forward with a separate pediatric trial of children aged 5-11 years by this spring, Keanna Ghazvini, senior associate for global media relations at Pfizer, said in an interview.
Enrollment in Moderna’s TeenCove study of adolescents ages 12-17 years began slowly in late December, but the pace has since picked up, said company spokesperson Colleen Hussey. “We continue to bring clinical trial sites online, and we are on track to provide updated data around mid-year 2021.” A trial extension in children 11 years and younger is expected to begin later in 2021.
Johnson & Johnson and AstraZeneca said they expect to begin adolescent trials in early 2021, according to data shared by the Advisory Committee on Immunization Practices. An interim analysis of J&J’s Janssen COVID-19 vaccine trial data, released on Jan. 29, showed it was 72% effective in US participants aged 18 years or older. AstraZeneca’s U.S. trial in adults is ongoing.
Easing the burden
Vaccination could lessen children’s risk of severe disease as well as the social and emotional burdens of the pandemic, says James Campbell, MD, a pediatric infectious disease specialist at the University of Maryland’s Center for Vaccine Development in Baltimore, which was involved in the Moderna and early-phase Pfizer trials. He coauthored a September 2020 article in Clinical Infectious Diseases titled: “Warp Speed for COVID-19 vaccines: Why are children stuck in neutral?”
The adolescent trials are a vital step to ensure timely vaccine access for teens and younger children. “It is reasonable, when you have limited vaccine, that your rollout goes to the highest priority and then moves to lower and lower priorities. In adults, we’re just saying: ‘Wait your turn,’ ” he said of the current vaccination effort. “If we didn’t have the [vaccine trial] data in children, we’d be saying: ‘You don’t have a turn.’ ”
As the pandemic has worn on, the burden on children has grown. As of Tuesday, 269 children had died of COVID-19. That is well above the highest annual death toll recorded during a regular flu season – 188 flu deaths among children and adolescents under 18 in the 2019-2020 and 2017-2018 flu seasons.
Children are less likely to transmit COVID-19 in their household than adults, according to a meta-analysis of 54 studies published in JAMA Network Open. But that does not necessarily mean children are less infectious, the authors said, noting that unmeasured factors could have affected the spread of infection among adults.
Moreover, children and adolescents need protection from COVID infection – and from the potential for severe disease or lingering effects – and, given that there are 74 million children and teens in the United States, their vaccination is an important part of stopping the pandemic, said Grace Lee, MD, professor of pediatrics at Stanford (Calif.) University, and cochair of ACIP’s COVID-19 Vaccine Safety Technical Subgroup.
“In order to interrupt transmission, I don’t see how we’re going to do that without vaccinating children and adolescents,” she said.
Dr. Lee said her 16-year-old daughter misses the normal teenage social life and is excited about getting the vaccine when she is eligible. (Adolescents without high-risk conditions are in the lowest vaccination tier, according to ACIP recommendations.) “There is truly individual protection to be gained,” Dr. Lee said.
She noted that researchers continue to assess the immune responses to the adult vaccines – even looking at immune characteristics of the small percentage of people who aren’t protected from infection – and that information helps in the evaluation of the pediatric immune responses. As the trials expand to younger children and infants, dosing will be a major focus. “How many doses do they need they need to receive the same immunity? Safety considerations will be critically important,” she said.
Teen trials underway
Pfizer/BioNTech extended its adult trial to 16- and 17-year-olds in October, which enabled older teens to be included in its emergency-use authorization. They and younger teens, ages 12-15, receive the same dose as adults.
The ongoing trials with Pfizer and Moderna vaccines are immunobridging trials, designed to study safety and immunogenicity. Investigators will compare the teens’ immune response with the findings from the larger adult trials. When the trials expand to school-age children (6-12 years), protocols call for testing the safety and immunogenicity of a half-dose vaccine as well as the full dose.
Children ages 2-5 years and infants and toddlers will be enrolled in future trials, studying safety and immunogenicity of full, half, or even quarter dosages. The Pediatric Research Equity Act of 2003 requires licensed vaccines to be tested for safety and efficacy in children, unless they are not appropriate for a pediatric population.
Demand for the teen trials has been strong. At Cincinnati Children’s Hospital Medical Center, 259 teenagers joined the Pfizer/BioNTech trial, but some teenagers were turned away when the trial’s national enrollment closed in late January.
“Many of the children are having no side effects, and if they are, they’re having the same [effects] as the young adults – local redness or pain, fatigue, and headaches,” said Robert Frenck, MD, director of the Cincinnati Children’s Gamble Program for Clinical Studies.
Parents may share some of the vaccine hesitancy that has affected adult vaccination. But that is balanced by the hope that vaccines will end the pandemic and usher in a new normal. “If it looks like [vaccines] will increase the likelihood of children returning to school safely, that may be a motivating factor,” Dr. Frenck said.
Cody Meissner, MD, chief of the pediatric infectious disease service at Tufts Medical Center, Boston, was initially cautious about the extension of vaccination to adolescents. A member of the Vaccine and Related Biological Products Advisory Committee, which evaluates data and makes recommendations to the Food and Drug Administration, Dr. Meissner initially abstained in the vote on the Pfizer/BioNTech emergency-use authorization for people 16 and older.
He noted that, at the time the committee reviewed the Pfizer vaccine, the company had data available for just 134 teenagers, half of whom received a placebo. But the vaccination of 34 million adults has provided robust data about the vaccine’s safety, and the trial expansion into adolescents is important.
“I’m comfortable with the way these trials are going now,” he said. “This is the way I was hoping they would go.”
Ms. Hillenburg is on the parent advisory board of Voices for Vaccines, an organization of parents supporting vaccination that is affiliated with the Task Force for Global Health, an Atlanta-based independent public health organization. Dr. Campbell’s institution has received funds to conduct clinical trials from the National Institutes of Health and several companies, including Merck, GlaxoSmithKline, Sanofi, Pfizer, and Moderna. He has served pro bono on many safety and data monitoring committees. Dr. Frenck’s institution is receiving funds to conduct the Pfizer trial. In the past 5 years, he has also participated in clinical trials for GlaxoSmithKline, Merck, and Meissa vaccines. Dr. Lee and Dr. Meissner disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The vaccinations can’t come soon enough for parents like Stacy Hillenburg, a developmental therapist in Aurora, Ill., whose 9-year-old son takes immunosuppressants because he had a heart transplant when he was 7 weeks old. Although school-age children aren’t yet included in clinical trials, if her 12- and 13-year-old daughters could get vaccinated, along with both parents, then the family could relax some of the protocols they currently follow to prevent infection.
Whenever they are around other people, even masked and socially distanced, they come home and immediately shower and change their clothes. So far, no one in the family has been infected with COVID, but the anxiety is ever-present. “I can’t wait for it to come out,” Ms. Hillenburg said of a pediatric COVID vaccine. “It will ease my mind so much.”
She isn’t alone in that anticipation. In the fall, the American Academy of Pediatrics and other pediatric vaccine experts urged faster action on pediatric vaccine trials and worried that children would be left behind as adults gained protection from COVID. But recent developments have eased those concerns.
“Over the next couple of months, we will be doing trials in an age-deescalation manner,” with studies moving gradually to younger children, Anthony S. Fauci, MD, chief medical adviser on COVID-19 to the president, said in a coronavirus response team briefing on Jan. 29. “So that hopefully, as we get to the late spring and summer, we will have children being able to be vaccinated.”
Pfizer completed enrollment of 2,259 teens aged 12-15 years in late January and expects to move forward with a separate pediatric trial of children aged 5-11 years by this spring, Keanna Ghazvini, senior associate for global media relations at Pfizer, said in an interview.
Enrollment in Moderna’s TeenCove study of adolescents ages 12-17 years began slowly in late December, but the pace has since picked up, said company spokesperson Colleen Hussey. “We continue to bring clinical trial sites online, and we are on track to provide updated data around mid-year 2021.” A trial extension in children 11 years and younger is expected to begin later in 2021.
Johnson & Johnson and AstraZeneca said they expect to begin adolescent trials in early 2021, according to data shared by the Advisory Committee on Immunization Practices. An interim analysis of J&J’s Janssen COVID-19 vaccine trial data, released on Jan. 29, showed it was 72% effective in US participants aged 18 years or older. AstraZeneca’s U.S. trial in adults is ongoing.
Easing the burden
Vaccination could lessen children’s risk of severe disease as well as the social and emotional burdens of the pandemic, says James Campbell, MD, a pediatric infectious disease specialist at the University of Maryland’s Center for Vaccine Development in Baltimore, which was involved in the Moderna and early-phase Pfizer trials. He coauthored a September 2020 article in Clinical Infectious Diseases titled: “Warp Speed for COVID-19 vaccines: Why are children stuck in neutral?”
The adolescent trials are a vital step to ensure timely vaccine access for teens and younger children. “It is reasonable, when you have limited vaccine, that your rollout goes to the highest priority and then moves to lower and lower priorities. In adults, we’re just saying: ‘Wait your turn,’ ” he said of the current vaccination effort. “If we didn’t have the [vaccine trial] data in children, we’d be saying: ‘You don’t have a turn.’ ”
As the pandemic has worn on, the burden on children has grown. As of Tuesday, 269 children had died of COVID-19. That is well above the highest annual death toll recorded during a regular flu season – 188 flu deaths among children and adolescents under 18 in the 2019-2020 and 2017-2018 flu seasons.
Children are less likely to transmit COVID-19 in their household than adults, according to a meta-analysis of 54 studies published in JAMA Network Open. But that does not necessarily mean children are less infectious, the authors said, noting that unmeasured factors could have affected the spread of infection among adults.
Moreover, children and adolescents need protection from COVID infection – and from the potential for severe disease or lingering effects – and, given that there are 74 million children and teens in the United States, their vaccination is an important part of stopping the pandemic, said Grace Lee, MD, professor of pediatrics at Stanford (Calif.) University, and cochair of ACIP’s COVID-19 Vaccine Safety Technical Subgroup.
“In order to interrupt transmission, I don’t see how we’re going to do that without vaccinating children and adolescents,” she said.
Dr. Lee said her 16-year-old daughter misses the normal teenage social life and is excited about getting the vaccine when she is eligible. (Adolescents without high-risk conditions are in the lowest vaccination tier, according to ACIP recommendations.) “There is truly individual protection to be gained,” Dr. Lee said.
She noted that researchers continue to assess the immune responses to the adult vaccines – even looking at immune characteristics of the small percentage of people who aren’t protected from infection – and that information helps in the evaluation of the pediatric immune responses. As the trials expand to younger children and infants, dosing will be a major focus. “How many doses do they need they need to receive the same immunity? Safety considerations will be critically important,” she said.
Teen trials underway
Pfizer/BioNTech extended its adult trial to 16- and 17-year-olds in October, which enabled older teens to be included in its emergency-use authorization. They and younger teens, ages 12-15, receive the same dose as adults.
The ongoing trials with Pfizer and Moderna vaccines are immunobridging trials, designed to study safety and immunogenicity. Investigators will compare the teens’ immune response with the findings from the larger adult trials. When the trials expand to school-age children (6-12 years), protocols call for testing the safety and immunogenicity of a half-dose vaccine as well as the full dose.
Children ages 2-5 years and infants and toddlers will be enrolled in future trials, studying safety and immunogenicity of full, half, or even quarter dosages. The Pediatric Research Equity Act of 2003 requires licensed vaccines to be tested for safety and efficacy in children, unless they are not appropriate for a pediatric population.
Demand for the teen trials has been strong. At Cincinnati Children’s Hospital Medical Center, 259 teenagers joined the Pfizer/BioNTech trial, but some teenagers were turned away when the trial’s national enrollment closed in late January.
“Many of the children are having no side effects, and if they are, they’re having the same [effects] as the young adults – local redness or pain, fatigue, and headaches,” said Robert Frenck, MD, director of the Cincinnati Children’s Gamble Program for Clinical Studies.
Parents may share some of the vaccine hesitancy that has affected adult vaccination. But that is balanced by the hope that vaccines will end the pandemic and usher in a new normal. “If it looks like [vaccines] will increase the likelihood of children returning to school safely, that may be a motivating factor,” Dr. Frenck said.
Cody Meissner, MD, chief of the pediatric infectious disease service at Tufts Medical Center, Boston, was initially cautious about the extension of vaccination to adolescents. A member of the Vaccine and Related Biological Products Advisory Committee, which evaluates data and makes recommendations to the Food and Drug Administration, Dr. Meissner initially abstained in the vote on the Pfizer/BioNTech emergency-use authorization for people 16 and older.
He noted that, at the time the committee reviewed the Pfizer vaccine, the company had data available for just 134 teenagers, half of whom received a placebo. But the vaccination of 34 million adults has provided robust data about the vaccine’s safety, and the trial expansion into adolescents is important.
“I’m comfortable with the way these trials are going now,” he said. “This is the way I was hoping they would go.”
Ms. Hillenburg is on the parent advisory board of Voices for Vaccines, an organization of parents supporting vaccination that is affiliated with the Task Force for Global Health, an Atlanta-based independent public health organization. Dr. Campbell’s institution has received funds to conduct clinical trials from the National Institutes of Health and several companies, including Merck, GlaxoSmithKline, Sanofi, Pfizer, and Moderna. He has served pro bono on many safety and data monitoring committees. Dr. Frenck’s institution is receiving funds to conduct the Pfizer trial. In the past 5 years, he has also participated in clinical trials for GlaxoSmithKline, Merck, and Meissa vaccines. Dr. Lee and Dr. Meissner disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The vaccinations can’t come soon enough for parents like Stacy Hillenburg, a developmental therapist in Aurora, Ill., whose 9-year-old son takes immunosuppressants because he had a heart transplant when he was 7 weeks old. Although school-age children aren’t yet included in clinical trials, if her 12- and 13-year-old daughters could get vaccinated, along with both parents, then the family could relax some of the protocols they currently follow to prevent infection.
Whenever they are around other people, even masked and socially distanced, they come home and immediately shower and change their clothes. So far, no one in the family has been infected with COVID, but the anxiety is ever-present. “I can’t wait for it to come out,” Ms. Hillenburg said of a pediatric COVID vaccine. “It will ease my mind so much.”
She isn’t alone in that anticipation. In the fall, the American Academy of Pediatrics and other pediatric vaccine experts urged faster action on pediatric vaccine trials and worried that children would be left behind as adults gained protection from COVID. But recent developments have eased those concerns.
“Over the next couple of months, we will be doing trials in an age-deescalation manner,” with studies moving gradually to younger children, Anthony S. Fauci, MD, chief medical adviser on COVID-19 to the president, said in a coronavirus response team briefing on Jan. 29. “So that hopefully, as we get to the late spring and summer, we will have children being able to be vaccinated.”
Pfizer completed enrollment of 2,259 teens aged 12-15 years in late January and expects to move forward with a separate pediatric trial of children aged 5-11 years by this spring, Keanna Ghazvini, senior associate for global media relations at Pfizer, said in an interview.
Enrollment in Moderna’s TeenCove study of adolescents ages 12-17 years began slowly in late December, but the pace has since picked up, said company spokesperson Colleen Hussey. “We continue to bring clinical trial sites online, and we are on track to provide updated data around mid-year 2021.” A trial extension in children 11 years and younger is expected to begin later in 2021.
Johnson & Johnson and AstraZeneca said they expect to begin adolescent trials in early 2021, according to data shared by the Advisory Committee on Immunization Practices. An interim analysis of J&J’s Janssen COVID-19 vaccine trial data, released on Jan. 29, showed it was 72% effective in US participants aged 18 years or older. AstraZeneca’s U.S. trial in adults is ongoing.
Easing the burden
Vaccination could lessen children’s risk of severe disease as well as the social and emotional burdens of the pandemic, says James Campbell, MD, a pediatric infectious disease specialist at the University of Maryland’s Center for Vaccine Development in Baltimore, which was involved in the Moderna and early-phase Pfizer trials. He coauthored a September 2020 article in Clinical Infectious Diseases titled: “Warp Speed for COVID-19 vaccines: Why are children stuck in neutral?”
The adolescent trials are a vital step to ensure timely vaccine access for teens and younger children. “It is reasonable, when you have limited vaccine, that your rollout goes to the highest priority and then moves to lower and lower priorities. In adults, we’re just saying: ‘Wait your turn,’ ” he said of the current vaccination effort. “If we didn’t have the [vaccine trial] data in children, we’d be saying: ‘You don’t have a turn.’ ”
As the pandemic has worn on, the burden on children has grown. As of Tuesday, 269 children had died of COVID-19. That is well above the highest annual death toll recorded during a regular flu season – 188 flu deaths among children and adolescents under 18 in the 2019-2020 and 2017-2018 flu seasons.
Children are less likely to transmit COVID-19 in their household than adults, according to a meta-analysis of 54 studies published in JAMA Network Open. But that does not necessarily mean children are less infectious, the authors said, noting that unmeasured factors could have affected the spread of infection among adults.
Moreover, children and adolescents need protection from COVID infection – and from the potential for severe disease or lingering effects – and, given that there are 74 million children and teens in the United States, their vaccination is an important part of stopping the pandemic, said Grace Lee, MD, professor of pediatrics at Stanford (Calif.) University, and cochair of ACIP’s COVID-19 Vaccine Safety Technical Subgroup.
“In order to interrupt transmission, I don’t see how we’re going to do that without vaccinating children and adolescents,” she said.
Dr. Lee said her 16-year-old daughter misses the normal teenage social life and is excited about getting the vaccine when she is eligible. (Adolescents without high-risk conditions are in the lowest vaccination tier, according to ACIP recommendations.) “There is truly individual protection to be gained,” Dr. Lee said.
She noted that researchers continue to assess the immune responses to the adult vaccines – even looking at immune characteristics of the small percentage of people who aren’t protected from infection – and that information helps in the evaluation of the pediatric immune responses. As the trials expand to younger children and infants, dosing will be a major focus. “How many doses do they need they need to receive the same immunity? Safety considerations will be critically important,” she said.
Teen trials underway
Pfizer/BioNTech extended its adult trial to 16- and 17-year-olds in October, which enabled older teens to be included in its emergency-use authorization. They and younger teens, ages 12-15, receive the same dose as adults.
The ongoing trials with Pfizer and Moderna vaccines are immunobridging trials, designed to study safety and immunogenicity. Investigators will compare the teens’ immune response with the findings from the larger adult trials. When the trials expand to school-age children (6-12 years), protocols call for testing the safety and immunogenicity of a half-dose vaccine as well as the full dose.
Children ages 2-5 years and infants and toddlers will be enrolled in future trials, studying safety and immunogenicity of full, half, or even quarter dosages. The Pediatric Research Equity Act of 2003 requires licensed vaccines to be tested for safety and efficacy in children, unless they are not appropriate for a pediatric population.
Demand for the teen trials has been strong. At Cincinnati Children’s Hospital Medical Center, 259 teenagers joined the Pfizer/BioNTech trial, but some teenagers were turned away when the trial’s national enrollment closed in late January.
“Many of the children are having no side effects, and if they are, they’re having the same [effects] as the young adults – local redness or pain, fatigue, and headaches,” said Robert Frenck, MD, director of the Cincinnati Children’s Gamble Program for Clinical Studies.
Parents may share some of the vaccine hesitancy that has affected adult vaccination. But that is balanced by the hope that vaccines will end the pandemic and usher in a new normal. “If it looks like [vaccines] will increase the likelihood of children returning to school safely, that may be a motivating factor,” Dr. Frenck said.
Cody Meissner, MD, chief of the pediatric infectious disease service at Tufts Medical Center, Boston, was initially cautious about the extension of vaccination to adolescents. A member of the Vaccine and Related Biological Products Advisory Committee, which evaluates data and makes recommendations to the Food and Drug Administration, Dr. Meissner initially abstained in the vote on the Pfizer/BioNTech emergency-use authorization for people 16 and older.
He noted that, at the time the committee reviewed the Pfizer vaccine, the company had data available for just 134 teenagers, half of whom received a placebo. But the vaccination of 34 million adults has provided robust data about the vaccine’s safety, and the trial expansion into adolescents is important.
“I’m comfortable with the way these trials are going now,” he said. “This is the way I was hoping they would go.”
Ms. Hillenburg is on the parent advisory board of Voices for Vaccines, an organization of parents supporting vaccination that is affiliated with the Task Force for Global Health, an Atlanta-based independent public health organization. Dr. Campbell’s institution has received funds to conduct clinical trials from the National Institutes of Health and several companies, including Merck, GlaxoSmithKline, Sanofi, Pfizer, and Moderna. He has served pro bono on many safety and data monitoring committees. Dr. Frenck’s institution is receiving funds to conduct the Pfizer trial. In the past 5 years, he has also participated in clinical trials for GlaxoSmithKline, Merck, and Meissa vaccines. Dr. Lee and Dr. Meissner disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
CLL, MBL had lower response rates to flu vaccination, compared with healthy adults
Immunogenicity of the high-dose influenza vaccine (HD IIV3) in patients with chronic lymphocytic leukemia (CLL) and monoclonal B-cell lymphocytosis (MBL, the precursor state to CLL) was found lower than reported in healthy adults according to a report in Vaccine.
In addition, immunogenicity to influenza B was found to be greater in those patients with MBL, compared with those with CLL.
“Acute and chronic leukemia patients hospitalized with influenza infection document a case fatality rate of 25%-37%,” according to Jennifer A. Whitaker, MD, of the Mayo Clinic, Rochester, Minn., and colleagues in pointing out the importance of their study.
The prospective pilot study assessed the humoral immune responses of patients to the 2013-2014 and 2014-2015 HD IIV3 (Fluzone High-Dose; Sanofi Pasteur), which was administered as part of routine clinical care in 30 patients (17 with previously untreated CLL and 13 with MBL). The median patient age was 69.5 years.
The primary outcomes were seroconversion and seroprotection, as measured by hemagglutination inhibition assay (HAI).
Lower response rate
At day 28 post vaccination, the seroprotection rates for the overall cohort were 19/30 (63.3%) for A/H1N1, 21/23 (91.3%) for A/H3N2, and 13/30 (43.3%) for influenza B. Patients with MBL achieved significantly higher day 28 HAI geometric mean titers (GMT), compared with CLL patients (54.1 vs. 12.1]; P = .01), In addition, MBL patients achieved higher day 28 seroprotection rates against the influenza B vaccine strain virus than did those with CLL (76.9% vs. 17.6%; P = .002). Seroconversion rates for the overall cohort were 3/30 (10%) for A/H1N1; 5/23 (21.7%) for A/H3N2; and 3/30 (10%) for influenza B. No individual with CLL demonstrated seroconversion for influenza B, according to the researchers.
“Our studies reinforce rigorous adherence to vaccination strategies in patients with hematologic malignancy, including those with CLL, given the increased risk of serious complications among those experiencing influenza infection,” the authors stated.
“Even suboptimal responses to influenza vaccination can provide partial protection, reduce hospitalization rates, and/or prevent serious disease complications. Given the recent major issue with novel and aggressive viruses such COVID-19, we absolutely must continue with larger prospective studies to confirm these findings and evaluate vaccine effectiveness in preventing influenza or other novel viruses in these populations,” the researchers concluded.
This study was funded by the National Institutes of Health. Dr. Whitaker reported having no disclosures. Several of the coauthors reported financial relationships with a variety of pharmaceutical and biotechnology companies.
Immunogenicity of the high-dose influenza vaccine (HD IIV3) in patients with chronic lymphocytic leukemia (CLL) and monoclonal B-cell lymphocytosis (MBL, the precursor state to CLL) was found lower than reported in healthy adults according to a report in Vaccine.
In addition, immunogenicity to influenza B was found to be greater in those patients with MBL, compared with those with CLL.
“Acute and chronic leukemia patients hospitalized with influenza infection document a case fatality rate of 25%-37%,” according to Jennifer A. Whitaker, MD, of the Mayo Clinic, Rochester, Minn., and colleagues in pointing out the importance of their study.
The prospective pilot study assessed the humoral immune responses of patients to the 2013-2014 and 2014-2015 HD IIV3 (Fluzone High-Dose; Sanofi Pasteur), which was administered as part of routine clinical care in 30 patients (17 with previously untreated CLL and 13 with MBL). The median patient age was 69.5 years.
The primary outcomes were seroconversion and seroprotection, as measured by hemagglutination inhibition assay (HAI).
Lower response rate
At day 28 post vaccination, the seroprotection rates for the overall cohort were 19/30 (63.3%) for A/H1N1, 21/23 (91.3%) for A/H3N2, and 13/30 (43.3%) for influenza B. Patients with MBL achieved significantly higher day 28 HAI geometric mean titers (GMT), compared with CLL patients (54.1 vs. 12.1]; P = .01), In addition, MBL patients achieved higher day 28 seroprotection rates against the influenza B vaccine strain virus than did those with CLL (76.9% vs. 17.6%; P = .002). Seroconversion rates for the overall cohort were 3/30 (10%) for A/H1N1; 5/23 (21.7%) for A/H3N2; and 3/30 (10%) for influenza B. No individual with CLL demonstrated seroconversion for influenza B, according to the researchers.
“Our studies reinforce rigorous adherence to vaccination strategies in patients with hematologic malignancy, including those with CLL, given the increased risk of serious complications among those experiencing influenza infection,” the authors stated.
“Even suboptimal responses to influenza vaccination can provide partial protection, reduce hospitalization rates, and/or prevent serious disease complications. Given the recent major issue with novel and aggressive viruses such COVID-19, we absolutely must continue with larger prospective studies to confirm these findings and evaluate vaccine effectiveness in preventing influenza or other novel viruses in these populations,” the researchers concluded.
This study was funded by the National Institutes of Health. Dr. Whitaker reported having no disclosures. Several of the coauthors reported financial relationships with a variety of pharmaceutical and biotechnology companies.
Immunogenicity of the high-dose influenza vaccine (HD IIV3) in patients with chronic lymphocytic leukemia (CLL) and monoclonal B-cell lymphocytosis (MBL, the precursor state to CLL) was found lower than reported in healthy adults according to a report in Vaccine.
In addition, immunogenicity to influenza B was found to be greater in those patients with MBL, compared with those with CLL.
“Acute and chronic leukemia patients hospitalized with influenza infection document a case fatality rate of 25%-37%,” according to Jennifer A. Whitaker, MD, of the Mayo Clinic, Rochester, Minn., and colleagues in pointing out the importance of their study.
The prospective pilot study assessed the humoral immune responses of patients to the 2013-2014 and 2014-2015 HD IIV3 (Fluzone High-Dose; Sanofi Pasteur), which was administered as part of routine clinical care in 30 patients (17 with previously untreated CLL and 13 with MBL). The median patient age was 69.5 years.
The primary outcomes were seroconversion and seroprotection, as measured by hemagglutination inhibition assay (HAI).
Lower response rate
At day 28 post vaccination, the seroprotection rates for the overall cohort were 19/30 (63.3%) for A/H1N1, 21/23 (91.3%) for A/H3N2, and 13/30 (43.3%) for influenza B. Patients with MBL achieved significantly higher day 28 HAI geometric mean titers (GMT), compared with CLL patients (54.1 vs. 12.1]; P = .01), In addition, MBL patients achieved higher day 28 seroprotection rates against the influenza B vaccine strain virus than did those with CLL (76.9% vs. 17.6%; P = .002). Seroconversion rates for the overall cohort were 3/30 (10%) for A/H1N1; 5/23 (21.7%) for A/H3N2; and 3/30 (10%) for influenza B. No individual with CLL demonstrated seroconversion for influenza B, according to the researchers.
“Our studies reinforce rigorous adherence to vaccination strategies in patients with hematologic malignancy, including those with CLL, given the increased risk of serious complications among those experiencing influenza infection,” the authors stated.
“Even suboptimal responses to influenza vaccination can provide partial protection, reduce hospitalization rates, and/or prevent serious disease complications. Given the recent major issue with novel and aggressive viruses such COVID-19, we absolutely must continue with larger prospective studies to confirm these findings and evaluate vaccine effectiveness in preventing influenza or other novel viruses in these populations,” the researchers concluded.
This study was funded by the National Institutes of Health. Dr. Whitaker reported having no disclosures. Several of the coauthors reported financial relationships with a variety of pharmaceutical and biotechnology companies.
FROM VACCINE
The Veterans Health Administration Approach to COVID-19 Vaccine Allocation—Balancing Utility and Equity
The Veterans Health Administration (VHA) COVID-19 vaccine allocation plan showcases several lessons for government and health care leaders in planning for future pandemics.1 Many state governments—underresourced and overwhelmed with other COVID-19 demands—have struggled to get COVID-19 vaccines into the arms of their residents.2 In contrast, the VHA was able to mobilize early to identify vaccine allocation guidelines and proactively prepare facilities to vaccinate VHA staff and veterans as soon as vaccines were approved under Emergency Use Authorization by the US Food and Drug Administration.3,4
In August 2020, VHA formed a COVID-19 Vaccine Integrated Project Team, composed of 6 subgroups: communications, distribution, education, measurement, policy, prioritization, and vaccine safety. The National Center for Ethics in Health Care weighed in on the ethical justification for the developed vaccination risk stratification framework, which was informed by, but not identical to, that recommended by the Centers for Disease Control and Prevention Advisory Committee on Immunization Practices.5
Prioritizing who gets early access to a potentially life-saving vaccine weighs heavily on those leaders charged with making such decisions. The ethics of scarce resource allocation and triage protocols that may be necessary in a pandemic are often in tension with the patient-centered clinical ethics that health care practitioners (HCPs) encounter. HCPs require assistance in appreciating the ethical rationale for this shift in focus from the preference of the individual to the common good. The same is true for the risk stratification criteria required when there is not sufficient vaccine for all those who could benefit from immunization. Decisions must be transparent to ensure widespread acceptance and trust in the vaccination process. The ethical reasoning and values that are the basis for allocation criteria must be clearly, compassionately, and consistently communicated to the public, as outlined below. Ethical questions or concerns involve a conflict between core values: one of the central tasks of ethical analysis is to identify the available ethical options to resolve value conflicts. Several ethical frameworks for vaccine allocation are available—each balances and weighs the primary values of equity, dignity, beneficence, and utility slightly differently.6
For example, utilitarian ethics looks to produce the most good and avoid the most harm for the greatest number of people. Within this framework, there can be different notions of “good,” for example, saving the most lives, the most life years, the most quality life years, or the lives of those who have more life “innings” ahead. The approach of the US Department of Veterans Affairs (VA) focuses on saving the most lives in combination with avoiding suffering from serious illness, minimizing contagion, and preserving the essential workforce. Frameworks that give primacy to 1 notion of the good (ie, saving the most lives) may deprioritize other beneficial outcomes, such as allowing earlier return to work, school, and leisure activities that many find integral to human flourishing. Other ethical theories and principles may be used to support various allocation frameworks. For example, a pragmatic ethics approach might emphasize the importance of adapting the approach based on the evolving science and innovation surrounding COVID-19. Having more than 1 ethically defensible approach is common; the goal in ethics work is to be open to diversity of thought and reflect on the strength of one’s reasoning in resolving a core values conflict. We identify 2 central tenets of pandemic ethics that inform vaccine allocation.
1. Pandemic Ethics Requires Proactive Planning and Reevaluation of Continually Evolving Facts
There is an oft quoted saying among bioethicists: “Good ethics begins with good facts.” One obvious challenge during the COVID-19 pandemic has been the difficulty accessing up-to-date facts to inform decision making. If a main goal of a vaccination plan is to minimize the incidence of serious or fatal COVID-19 disease and contagion, myriad data points are needed to identify the best way to do this. For example, if 2 doses of the same vaccine are needed, this impacts the logistics of identifying, inviting, and scheduling eligible individuals and staffing vaccine clinics as well as ensuring that sufficient personal protective equipment and rescue equipment/medication are available to treat allergic reactions. If the adverse effects of vaccines lead to staff absenteeism or vaccine hesitancy, this needs to be factored into logistics.7 Tailored messaging is important to reduce appointment no-shows and vaccine nonadopters.8 Transportation to vaccination sites is a relevant factor: how a vaccine is stored, thawed, and reconstituted and its shelf life impacts whether it can be transported after thawing and what must be provided on site.
Consideration of the multifaceted factors influencing a successful vaccination campaign requires proactive planning and the readiness to pivot when new information is revealed. For example, vaccine appointment no-shows should be anticipated along with a fair process for allocating unused vaccine that would otherwise be wasted. This is an example of responsible stewardship of a scarce and life-saving resource. A higher than anticipated no-show rate would require revisiting a facility’s approach to ensuring that waste is avoided while the process is perceived to be fair and transparent. Ethical theories and principles cannot do all the work here; mindful attention to detail and proactive, informed planning are critical. Fortunately, the VA is well resourced in this domain, whereas many state health departments floundered in their response, causing unnecessary vaccination delays.9
2. Utility: Necessary But Insufficient
Most ethical approaches recognize to some extent that seeking good and minimizing harm is of value. However, a strictly utilitarian approach is insufficient to address the core values in conflict surrounding how best to allocate limited doses of COVID-19 vaccine. For example, some may argue that prioritizing the elderly or those in long-term care facilities like VA’s community living centers because they have the highest COVID-19 mortality rate produces less net benefit than prioritizing younger veterans with comorbidities or certain higher risk essential workers. There are 2 important points to make here.
First, the VHA vaccination plan balances utility with other ethical principles, namely, treating people with equal concern, and addressing health inequities, including a focus on justice and valuing the worth and dignity of each person. Rather than giving everyone an equal chance via lottery, the prioritization plan recognizes that some people have greater need or would stand to better mitigate viral contagion and preserve the essential workforce if they were vaccinated earlier. However, the principle of justice requires that efforts are made to treat like cases the same to avoid perceptions of bias, and to demonstrate respect for the dignity of each individual by way of promoting a fair vaccination process.
This requires transparency, consistency, and delivery of respectful and accurate communication. For example, the VA recognizes that lifetime exposure to social injustice produces health inequities that make Black, Hispanic, and Native American persons more susceptible to contracting COVID-19 and suffering serious or fatal illness. The approach to addressing this inequity is by giving priority to those with higher risk factors. Again, this is an example of blending and balancing ethical principles of utility and justice—that is, recognizing and remedying social injustice is of value both because it will help achieve better outcomes for persons of color and because it is inherently worthwhile to oppose injustice.
However, contrary to some news reports, the VHA approach does not allocate by race/ethnicity alone, as it does by age.10,11 Doing so would present logistical challenges—for example, race/ethnicity is not an objective classification as is age, and reconciling individuals’ self-reports could create confusion or chaos that is antithetical to a fair, streamlined vaccination program. Putting veterans of color at the front of the vaccination line could backfire by amplifying worries that they are being exposed to vaccine that is not fully tested (a common contributor to vaccine hesitancy, particularly among communities of color familiar with prior exploitation and abuse in the name of science).
Discriminating based on race/ethnicity alone in the spirit of achieving equity would be precedent setting for the VA and would require a strong ethical justification. The decision to prioritize for vaccine based on risk factors strives to achieve this balance of equity and utility, as it encompasses VA staff and veterans of color by way of their status as essential workers or those with comorbidities. However, it is important to address race-based access barriers and vaccine hesitancy to satisfy the equity demands. This effort is underway (eg, engaging community champions and developing tailored educational resources to reach diverse communities).
In addition, pragmatic ethics recognizes that an overly granular, complicated allocation plan would be inefficient to implement. While it might be true that some veterans who are aged < 65 years may be at higher risk from COVID-19 than some elderly veterans, achieving the goals of fairness and transparency requires establishing a vaccine prioritization plan that is both ethically defensible and feasibly implementable (ie, achieves its goal of getting “needles into arms”). For example, veterans aged ≥ 65 years may be invited to schedule their vaccination before younger veterans, but any veteran may be accepted “on-call” for vaccine appointment no-shows via first-come, first-served or by lottery. Flexibility of response is crucial. This played out in adding flexibility around the decision to vaccinate veterans aged ≥ 75 years before those aged 65 to 74 years, after revisiting how this prioritization might affect feasibility and throughput and opting to allow the opportunity to include those aged ≥ 65 years.
There will no doubt be additional modifications to the vaccine allocation plan as more data become available. Since the danger of fueling suspicion and distrust is high (ie, that certain privileged people are jumping the line, as we heard reports of in some non-VA facilities).12 There is an obvious ethical duty to explain why the chosen approach is ethically defensible. VA facility leaders should be able to answer how their approach achieves the goals of avoiding serious or fatal illness, reducing contagion, and preserving the essential workforce while ensuring a fair, respectful, evidence-based, and transparent process.
1. US Department of Veterans Affairs. COVID-19 vaccination plan for the Veterans Health Administration. Version 2.0, Published December 14, 2020. Accessed February 3, 2021. https://www.publichealth.va.gov/docs/n-coronavirus/VHA-COVID-Vaccine-Plan-14Dec2020.pdf
2. Hennigann WJ, Park A, Ducharme J. The U.S. fumbled its early vaccine rollout. Will the Biden Administration put America back on track? TIME. January 21, 2021. Accessed February 3, 2021. https://time.com/5932028/vaccine-rollout-joe-biden/
3. US Food and Drug Administration. FDA take key action in fight against COVID-19 by issuing emergency use authorization for first COVID-19 vaccine [press release]. Published December 11, 2020. Accessed February 3, 2021. https://www.fda.gov/news-events/press-announcements/fda-takes-key-action-fight-against-covid-19-issuing-emergency-use-authorization-first-covid-19
4. US Food and Drug Administration. FDA takes additional action in fight against COVID-19 by Issuing emergency use authorization for second COVID-19 vaccine [press release]. Published December 18, 2020. Accessed February 3, 2021. https://www.fda.gov/news-events/press-announcements/fda-takes-additional-action-fight-against-covid-19-issuing-emergency-use-authorization-second-covid
5. McClung N, Chamberland M, Kinlaw K, et al. The Advisory Committee on Immunization Practices’ Ethical Principles for Allocating Initial Supplies of COVID-19 Vaccine-United States, 2020. Am J Transplant. 2021;21(1):420-425. doi:10.1111/ajt.16437
6. National Academies of Sciences, Engineering, and Medicine. 2020. Framework for equitable allocation of COVID-19 vaccine. The National Academies Press; 2020. doi:10.17226/25917
7 . Wood S, Schulman K. Beyond Politics - Promoting Covid-19 vaccination in the United States [published online ahead of print, 2021 Jan 6]. N Engl J Med. 2021;10.1056/NEJMms2033790. doi:10.1056/NEJMms2033790
8 . Matrajt L, Eaton J, Leung T, Brown ER. Vaccine optimization for COVID-19, who to vaccinate first? medRxiv . 2020 Aug 16. doi:10.1101/2020.08.14.20175257
9 . Makary M. Hospitals: stop playing vaccine games and show leadership. Published January 12, 2021. Accessed February 3, 2021. https://www.medpagetoday.com/blogs/marty-makary/90649
10 . Wentling N. Minority veterans to receive priority for coronavirus vaccines. Stars and Stripes. December 10, 2020. Accessed February 3, 2021. https://www.stripes.com/news/us/minority-veterans-to-receive-priority-for-coronavirus-vaccines-1.654624
11 . Kime, P. Minority veterans on VA’s priority list for COVID-19 vaccine distribution. Published December 8, 2020. Accessed February 3, 2021. https://www.military.com/daily-news/2020/12/08/minority-veterans-vas-priority-list-covid-19-vaccine-distribution.html
12 . Rosenthal, E. Yes, it matters that people are jumping the vaccine line. The New York Times . Published January 28, 2021. Accessed February 3, 2021. https://www.nytimes.com/2021/01/28/opinion/covid-vaccine-line.html
The Veterans Health Administration (VHA) COVID-19 vaccine allocation plan showcases several lessons for government and health care leaders in planning for future pandemics.1 Many state governments—underresourced and overwhelmed with other COVID-19 demands—have struggled to get COVID-19 vaccines into the arms of their residents.2 In contrast, the VHA was able to mobilize early to identify vaccine allocation guidelines and proactively prepare facilities to vaccinate VHA staff and veterans as soon as vaccines were approved under Emergency Use Authorization by the US Food and Drug Administration.3,4
In August 2020, VHA formed a COVID-19 Vaccine Integrated Project Team, composed of 6 subgroups: communications, distribution, education, measurement, policy, prioritization, and vaccine safety. The National Center for Ethics in Health Care weighed in on the ethical justification for the developed vaccination risk stratification framework, which was informed by, but not identical to, that recommended by the Centers for Disease Control and Prevention Advisory Committee on Immunization Practices.5
Prioritizing who gets early access to a potentially life-saving vaccine weighs heavily on those leaders charged with making such decisions. The ethics of scarce resource allocation and triage protocols that may be necessary in a pandemic are often in tension with the patient-centered clinical ethics that health care practitioners (HCPs) encounter. HCPs require assistance in appreciating the ethical rationale for this shift in focus from the preference of the individual to the common good. The same is true for the risk stratification criteria required when there is not sufficient vaccine for all those who could benefit from immunization. Decisions must be transparent to ensure widespread acceptance and trust in the vaccination process. The ethical reasoning and values that are the basis for allocation criteria must be clearly, compassionately, and consistently communicated to the public, as outlined below. Ethical questions or concerns involve a conflict between core values: one of the central tasks of ethical analysis is to identify the available ethical options to resolve value conflicts. Several ethical frameworks for vaccine allocation are available—each balances and weighs the primary values of equity, dignity, beneficence, and utility slightly differently.6
For example, utilitarian ethics looks to produce the most good and avoid the most harm for the greatest number of people. Within this framework, there can be different notions of “good,” for example, saving the most lives, the most life years, the most quality life years, or the lives of those who have more life “innings” ahead. The approach of the US Department of Veterans Affairs (VA) focuses on saving the most lives in combination with avoiding suffering from serious illness, minimizing contagion, and preserving the essential workforce. Frameworks that give primacy to 1 notion of the good (ie, saving the most lives) may deprioritize other beneficial outcomes, such as allowing earlier return to work, school, and leisure activities that many find integral to human flourishing. Other ethical theories and principles may be used to support various allocation frameworks. For example, a pragmatic ethics approach might emphasize the importance of adapting the approach based on the evolving science and innovation surrounding COVID-19. Having more than 1 ethically defensible approach is common; the goal in ethics work is to be open to diversity of thought and reflect on the strength of one’s reasoning in resolving a core values conflict. We identify 2 central tenets of pandemic ethics that inform vaccine allocation.
1. Pandemic Ethics Requires Proactive Planning and Reevaluation of Continually Evolving Facts
There is an oft quoted saying among bioethicists: “Good ethics begins with good facts.” One obvious challenge during the COVID-19 pandemic has been the difficulty accessing up-to-date facts to inform decision making. If a main goal of a vaccination plan is to minimize the incidence of serious or fatal COVID-19 disease and contagion, myriad data points are needed to identify the best way to do this. For example, if 2 doses of the same vaccine are needed, this impacts the logistics of identifying, inviting, and scheduling eligible individuals and staffing vaccine clinics as well as ensuring that sufficient personal protective equipment and rescue equipment/medication are available to treat allergic reactions. If the adverse effects of vaccines lead to staff absenteeism or vaccine hesitancy, this needs to be factored into logistics.7 Tailored messaging is important to reduce appointment no-shows and vaccine nonadopters.8 Transportation to vaccination sites is a relevant factor: how a vaccine is stored, thawed, and reconstituted and its shelf life impacts whether it can be transported after thawing and what must be provided on site.
Consideration of the multifaceted factors influencing a successful vaccination campaign requires proactive planning and the readiness to pivot when new information is revealed. For example, vaccine appointment no-shows should be anticipated along with a fair process for allocating unused vaccine that would otherwise be wasted. This is an example of responsible stewardship of a scarce and life-saving resource. A higher than anticipated no-show rate would require revisiting a facility’s approach to ensuring that waste is avoided while the process is perceived to be fair and transparent. Ethical theories and principles cannot do all the work here; mindful attention to detail and proactive, informed planning are critical. Fortunately, the VA is well resourced in this domain, whereas many state health departments floundered in their response, causing unnecessary vaccination delays.9
2. Utility: Necessary But Insufficient
Most ethical approaches recognize to some extent that seeking good and minimizing harm is of value. However, a strictly utilitarian approach is insufficient to address the core values in conflict surrounding how best to allocate limited doses of COVID-19 vaccine. For example, some may argue that prioritizing the elderly or those in long-term care facilities like VA’s community living centers because they have the highest COVID-19 mortality rate produces less net benefit than prioritizing younger veterans with comorbidities or certain higher risk essential workers. There are 2 important points to make here.
First, the VHA vaccination plan balances utility with other ethical principles, namely, treating people with equal concern, and addressing health inequities, including a focus on justice and valuing the worth and dignity of each person. Rather than giving everyone an equal chance via lottery, the prioritization plan recognizes that some people have greater need or would stand to better mitigate viral contagion and preserve the essential workforce if they were vaccinated earlier. However, the principle of justice requires that efforts are made to treat like cases the same to avoid perceptions of bias, and to demonstrate respect for the dignity of each individual by way of promoting a fair vaccination process.
This requires transparency, consistency, and delivery of respectful and accurate communication. For example, the VA recognizes that lifetime exposure to social injustice produces health inequities that make Black, Hispanic, and Native American persons more susceptible to contracting COVID-19 and suffering serious or fatal illness. The approach to addressing this inequity is by giving priority to those with higher risk factors. Again, this is an example of blending and balancing ethical principles of utility and justice—that is, recognizing and remedying social injustice is of value both because it will help achieve better outcomes for persons of color and because it is inherently worthwhile to oppose injustice.
However, contrary to some news reports, the VHA approach does not allocate by race/ethnicity alone, as it does by age.10,11 Doing so would present logistical challenges—for example, race/ethnicity is not an objective classification as is age, and reconciling individuals’ self-reports could create confusion or chaos that is antithetical to a fair, streamlined vaccination program. Putting veterans of color at the front of the vaccination line could backfire by amplifying worries that they are being exposed to vaccine that is not fully tested (a common contributor to vaccine hesitancy, particularly among communities of color familiar with prior exploitation and abuse in the name of science).
Discriminating based on race/ethnicity alone in the spirit of achieving equity would be precedent setting for the VA and would require a strong ethical justification. The decision to prioritize for vaccine based on risk factors strives to achieve this balance of equity and utility, as it encompasses VA staff and veterans of color by way of their status as essential workers or those with comorbidities. However, it is important to address race-based access barriers and vaccine hesitancy to satisfy the equity demands. This effort is underway (eg, engaging community champions and developing tailored educational resources to reach diverse communities).
In addition, pragmatic ethics recognizes that an overly granular, complicated allocation plan would be inefficient to implement. While it might be true that some veterans who are aged < 65 years may be at higher risk from COVID-19 than some elderly veterans, achieving the goals of fairness and transparency requires establishing a vaccine prioritization plan that is both ethically defensible and feasibly implementable (ie, achieves its goal of getting “needles into arms”). For example, veterans aged ≥ 65 years may be invited to schedule their vaccination before younger veterans, but any veteran may be accepted “on-call” for vaccine appointment no-shows via first-come, first-served or by lottery. Flexibility of response is crucial. This played out in adding flexibility around the decision to vaccinate veterans aged ≥ 75 years before those aged 65 to 74 years, after revisiting how this prioritization might affect feasibility and throughput and opting to allow the opportunity to include those aged ≥ 65 years.
There will no doubt be additional modifications to the vaccine allocation plan as more data become available. Since the danger of fueling suspicion and distrust is high (ie, that certain privileged people are jumping the line, as we heard reports of in some non-VA facilities).12 There is an obvious ethical duty to explain why the chosen approach is ethically defensible. VA facility leaders should be able to answer how their approach achieves the goals of avoiding serious or fatal illness, reducing contagion, and preserving the essential workforce while ensuring a fair, respectful, evidence-based, and transparent process.
The Veterans Health Administration (VHA) COVID-19 vaccine allocation plan showcases several lessons for government and health care leaders in planning for future pandemics.1 Many state governments—underresourced and overwhelmed with other COVID-19 demands—have struggled to get COVID-19 vaccines into the arms of their residents.2 In contrast, the VHA was able to mobilize early to identify vaccine allocation guidelines and proactively prepare facilities to vaccinate VHA staff and veterans as soon as vaccines were approved under Emergency Use Authorization by the US Food and Drug Administration.3,4
In August 2020, VHA formed a COVID-19 Vaccine Integrated Project Team, composed of 6 subgroups: communications, distribution, education, measurement, policy, prioritization, and vaccine safety. The National Center for Ethics in Health Care weighed in on the ethical justification for the developed vaccination risk stratification framework, which was informed by, but not identical to, that recommended by the Centers for Disease Control and Prevention Advisory Committee on Immunization Practices.5
Prioritizing who gets early access to a potentially life-saving vaccine weighs heavily on those leaders charged with making such decisions. The ethics of scarce resource allocation and triage protocols that may be necessary in a pandemic are often in tension with the patient-centered clinical ethics that health care practitioners (HCPs) encounter. HCPs require assistance in appreciating the ethical rationale for this shift in focus from the preference of the individual to the common good. The same is true for the risk stratification criteria required when there is not sufficient vaccine for all those who could benefit from immunization. Decisions must be transparent to ensure widespread acceptance and trust in the vaccination process. The ethical reasoning and values that are the basis for allocation criteria must be clearly, compassionately, and consistently communicated to the public, as outlined below. Ethical questions or concerns involve a conflict between core values: one of the central tasks of ethical analysis is to identify the available ethical options to resolve value conflicts. Several ethical frameworks for vaccine allocation are available—each balances and weighs the primary values of equity, dignity, beneficence, and utility slightly differently.6
For example, utilitarian ethics looks to produce the most good and avoid the most harm for the greatest number of people. Within this framework, there can be different notions of “good,” for example, saving the most lives, the most life years, the most quality life years, or the lives of those who have more life “innings” ahead. The approach of the US Department of Veterans Affairs (VA) focuses on saving the most lives in combination with avoiding suffering from serious illness, minimizing contagion, and preserving the essential workforce. Frameworks that give primacy to 1 notion of the good (ie, saving the most lives) may deprioritize other beneficial outcomes, such as allowing earlier return to work, school, and leisure activities that many find integral to human flourishing. Other ethical theories and principles may be used to support various allocation frameworks. For example, a pragmatic ethics approach might emphasize the importance of adapting the approach based on the evolving science and innovation surrounding COVID-19. Having more than 1 ethically defensible approach is common; the goal in ethics work is to be open to diversity of thought and reflect on the strength of one’s reasoning in resolving a core values conflict. We identify 2 central tenets of pandemic ethics that inform vaccine allocation.
1. Pandemic Ethics Requires Proactive Planning and Reevaluation of Continually Evolving Facts
There is an oft quoted saying among bioethicists: “Good ethics begins with good facts.” One obvious challenge during the COVID-19 pandemic has been the difficulty accessing up-to-date facts to inform decision making. If a main goal of a vaccination plan is to minimize the incidence of serious or fatal COVID-19 disease and contagion, myriad data points are needed to identify the best way to do this. For example, if 2 doses of the same vaccine are needed, this impacts the logistics of identifying, inviting, and scheduling eligible individuals and staffing vaccine clinics as well as ensuring that sufficient personal protective equipment and rescue equipment/medication are available to treat allergic reactions. If the adverse effects of vaccines lead to staff absenteeism or vaccine hesitancy, this needs to be factored into logistics.7 Tailored messaging is important to reduce appointment no-shows and vaccine nonadopters.8 Transportation to vaccination sites is a relevant factor: how a vaccine is stored, thawed, and reconstituted and its shelf life impacts whether it can be transported after thawing and what must be provided on site.
Consideration of the multifaceted factors influencing a successful vaccination campaign requires proactive planning and the readiness to pivot when new information is revealed. For example, vaccine appointment no-shows should be anticipated along with a fair process for allocating unused vaccine that would otherwise be wasted. This is an example of responsible stewardship of a scarce and life-saving resource. A higher than anticipated no-show rate would require revisiting a facility’s approach to ensuring that waste is avoided while the process is perceived to be fair and transparent. Ethical theories and principles cannot do all the work here; mindful attention to detail and proactive, informed planning are critical. Fortunately, the VA is well resourced in this domain, whereas many state health departments floundered in their response, causing unnecessary vaccination delays.9
2. Utility: Necessary But Insufficient
Most ethical approaches recognize to some extent that seeking good and minimizing harm is of value. However, a strictly utilitarian approach is insufficient to address the core values in conflict surrounding how best to allocate limited doses of COVID-19 vaccine. For example, some may argue that prioritizing the elderly or those in long-term care facilities like VA’s community living centers because they have the highest COVID-19 mortality rate produces less net benefit than prioritizing younger veterans with comorbidities or certain higher risk essential workers. There are 2 important points to make here.
First, the VHA vaccination plan balances utility with other ethical principles, namely, treating people with equal concern, and addressing health inequities, including a focus on justice and valuing the worth and dignity of each person. Rather than giving everyone an equal chance via lottery, the prioritization plan recognizes that some people have greater need or would stand to better mitigate viral contagion and preserve the essential workforce if they were vaccinated earlier. However, the principle of justice requires that efforts are made to treat like cases the same to avoid perceptions of bias, and to demonstrate respect for the dignity of each individual by way of promoting a fair vaccination process.
This requires transparency, consistency, and delivery of respectful and accurate communication. For example, the VA recognizes that lifetime exposure to social injustice produces health inequities that make Black, Hispanic, and Native American persons more susceptible to contracting COVID-19 and suffering serious or fatal illness. The approach to addressing this inequity is by giving priority to those with higher risk factors. Again, this is an example of blending and balancing ethical principles of utility and justice—that is, recognizing and remedying social injustice is of value both because it will help achieve better outcomes for persons of color and because it is inherently worthwhile to oppose injustice.
However, contrary to some news reports, the VHA approach does not allocate by race/ethnicity alone, as it does by age.10,11 Doing so would present logistical challenges—for example, race/ethnicity is not an objective classification as is age, and reconciling individuals’ self-reports could create confusion or chaos that is antithetical to a fair, streamlined vaccination program. Putting veterans of color at the front of the vaccination line could backfire by amplifying worries that they are being exposed to vaccine that is not fully tested (a common contributor to vaccine hesitancy, particularly among communities of color familiar with prior exploitation and abuse in the name of science).
Discriminating based on race/ethnicity alone in the spirit of achieving equity would be precedent setting for the VA and would require a strong ethical justification. The decision to prioritize for vaccine based on risk factors strives to achieve this balance of equity and utility, as it encompasses VA staff and veterans of color by way of their status as essential workers or those with comorbidities. However, it is important to address race-based access barriers and vaccine hesitancy to satisfy the equity demands. This effort is underway (eg, engaging community champions and developing tailored educational resources to reach diverse communities).
In addition, pragmatic ethics recognizes that an overly granular, complicated allocation plan would be inefficient to implement. While it might be true that some veterans who are aged < 65 years may be at higher risk from COVID-19 than some elderly veterans, achieving the goals of fairness and transparency requires establishing a vaccine prioritization plan that is both ethically defensible and feasibly implementable (ie, achieves its goal of getting “needles into arms”). For example, veterans aged ≥ 65 years may be invited to schedule their vaccination before younger veterans, but any veteran may be accepted “on-call” for vaccine appointment no-shows via first-come, first-served or by lottery. Flexibility of response is crucial. This played out in adding flexibility around the decision to vaccinate veterans aged ≥ 75 years before those aged 65 to 74 years, after revisiting how this prioritization might affect feasibility and throughput and opting to allow the opportunity to include those aged ≥ 65 years.
There will no doubt be additional modifications to the vaccine allocation plan as more data become available. Since the danger of fueling suspicion and distrust is high (ie, that certain privileged people are jumping the line, as we heard reports of in some non-VA facilities).12 There is an obvious ethical duty to explain why the chosen approach is ethically defensible. VA facility leaders should be able to answer how their approach achieves the goals of avoiding serious or fatal illness, reducing contagion, and preserving the essential workforce while ensuring a fair, respectful, evidence-based, and transparent process.
1. US Department of Veterans Affairs. COVID-19 vaccination plan for the Veterans Health Administration. Version 2.0, Published December 14, 2020. Accessed February 3, 2021. https://www.publichealth.va.gov/docs/n-coronavirus/VHA-COVID-Vaccine-Plan-14Dec2020.pdf
2. Hennigann WJ, Park A, Ducharme J. The U.S. fumbled its early vaccine rollout. Will the Biden Administration put America back on track? TIME. January 21, 2021. Accessed February 3, 2021. https://time.com/5932028/vaccine-rollout-joe-biden/
3. US Food and Drug Administration. FDA take key action in fight against COVID-19 by issuing emergency use authorization for first COVID-19 vaccine [press release]. Published December 11, 2020. Accessed February 3, 2021. https://www.fda.gov/news-events/press-announcements/fda-takes-key-action-fight-against-covid-19-issuing-emergency-use-authorization-first-covid-19
4. US Food and Drug Administration. FDA takes additional action in fight against COVID-19 by Issuing emergency use authorization for second COVID-19 vaccine [press release]. Published December 18, 2020. Accessed February 3, 2021. https://www.fda.gov/news-events/press-announcements/fda-takes-additional-action-fight-against-covid-19-issuing-emergency-use-authorization-second-covid
5. McClung N, Chamberland M, Kinlaw K, et al. The Advisory Committee on Immunization Practices’ Ethical Principles for Allocating Initial Supplies of COVID-19 Vaccine-United States, 2020. Am J Transplant. 2021;21(1):420-425. doi:10.1111/ajt.16437
6. National Academies of Sciences, Engineering, and Medicine. 2020. Framework for equitable allocation of COVID-19 vaccine. The National Academies Press; 2020. doi:10.17226/25917
7 . Wood S, Schulman K. Beyond Politics - Promoting Covid-19 vaccination in the United States [published online ahead of print, 2021 Jan 6]. N Engl J Med. 2021;10.1056/NEJMms2033790. doi:10.1056/NEJMms2033790
8 . Matrajt L, Eaton J, Leung T, Brown ER. Vaccine optimization for COVID-19, who to vaccinate first? medRxiv . 2020 Aug 16. doi:10.1101/2020.08.14.20175257
9 . Makary M. Hospitals: stop playing vaccine games and show leadership. Published January 12, 2021. Accessed February 3, 2021. https://www.medpagetoday.com/blogs/marty-makary/90649
10 . Wentling N. Minority veterans to receive priority for coronavirus vaccines. Stars and Stripes. December 10, 2020. Accessed February 3, 2021. https://www.stripes.com/news/us/minority-veterans-to-receive-priority-for-coronavirus-vaccines-1.654624
11 . Kime, P. Minority veterans on VA’s priority list for COVID-19 vaccine distribution. Published December 8, 2020. Accessed February 3, 2021. https://www.military.com/daily-news/2020/12/08/minority-veterans-vas-priority-list-covid-19-vaccine-distribution.html
12 . Rosenthal, E. Yes, it matters that people are jumping the vaccine line. The New York Times . Published January 28, 2021. Accessed February 3, 2021. https://www.nytimes.com/2021/01/28/opinion/covid-vaccine-line.html
1. US Department of Veterans Affairs. COVID-19 vaccination plan for the Veterans Health Administration. Version 2.0, Published December 14, 2020. Accessed February 3, 2021. https://www.publichealth.va.gov/docs/n-coronavirus/VHA-COVID-Vaccine-Plan-14Dec2020.pdf
2. Hennigann WJ, Park A, Ducharme J. The U.S. fumbled its early vaccine rollout. Will the Biden Administration put America back on track? TIME. January 21, 2021. Accessed February 3, 2021. https://time.com/5932028/vaccine-rollout-joe-biden/
3. US Food and Drug Administration. FDA take key action in fight against COVID-19 by issuing emergency use authorization for first COVID-19 vaccine [press release]. Published December 11, 2020. Accessed February 3, 2021. https://www.fda.gov/news-events/press-announcements/fda-takes-key-action-fight-against-covid-19-issuing-emergency-use-authorization-first-covid-19
4. US Food and Drug Administration. FDA takes additional action in fight against COVID-19 by Issuing emergency use authorization for second COVID-19 vaccine [press release]. Published December 18, 2020. Accessed February 3, 2021. https://www.fda.gov/news-events/press-announcements/fda-takes-additional-action-fight-against-covid-19-issuing-emergency-use-authorization-second-covid
5. McClung N, Chamberland M, Kinlaw K, et al. The Advisory Committee on Immunization Practices’ Ethical Principles for Allocating Initial Supplies of COVID-19 Vaccine-United States, 2020. Am J Transplant. 2021;21(1):420-425. doi:10.1111/ajt.16437
6. National Academies of Sciences, Engineering, and Medicine. 2020. Framework for equitable allocation of COVID-19 vaccine. The National Academies Press; 2020. doi:10.17226/25917
7 . Wood S, Schulman K. Beyond Politics - Promoting Covid-19 vaccination in the United States [published online ahead of print, 2021 Jan 6]. N Engl J Med. 2021;10.1056/NEJMms2033790. doi:10.1056/NEJMms2033790
8 . Matrajt L, Eaton J, Leung T, Brown ER. Vaccine optimization for COVID-19, who to vaccinate first? medRxiv . 2020 Aug 16. doi:10.1101/2020.08.14.20175257
9 . Makary M. Hospitals: stop playing vaccine games and show leadership. Published January 12, 2021. Accessed February 3, 2021. https://www.medpagetoday.com/blogs/marty-makary/90649
10 . Wentling N. Minority veterans to receive priority for coronavirus vaccines. Stars and Stripes. December 10, 2020. Accessed February 3, 2021. https://www.stripes.com/news/us/minority-veterans-to-receive-priority-for-coronavirus-vaccines-1.654624
11 . Kime, P. Minority veterans on VA’s priority list for COVID-19 vaccine distribution. Published December 8, 2020. Accessed February 3, 2021. https://www.military.com/daily-news/2020/12/08/minority-veterans-vas-priority-list-covid-19-vaccine-distribution.html
12 . Rosenthal, E. Yes, it matters that people are jumping the vaccine line. The New York Times . Published January 28, 2021. Accessed February 3, 2021. https://www.nytimes.com/2021/01/28/opinion/covid-vaccine-line.html
Is it an Allergic Reaction to the COVID-19 Vaccine—or COVID-19?
As of January 10, 2021, a reported 4,041,396 first doses of Moderna’s COVID-19 vaccine had been administered in the US. Reports of 1,266 (0.03%) adverse effects (AEs) after receipt of the vaccine were submitted to the Vaccine Adverse Event Reporting System (VAERS), according to researchers from the Centers for Disease Control and Prevention (CDC) COVID-19 Response Team and Food and Drug Administration in a Morbidity and Mortality Weekly Report early release.
The researchers screened VAERS reports that described suspected severe allergic reactions and anaphylaxis and collected information from medical records and outreach to healthcare facilities, providers, and recipients. They identified 108 reports for further review as possible severe allergic reaction, including anaphylaxis, a rare vaccination reaction. Ten cases were determined to be anaphylaxis—or a rate of 2.5 cases per million vaccine doses administered. Nine of the cases were people with a documented history of allergies or allergic reactions; 5 had a history of anaphylaxis.
The median interval from vaccine receipt to symptom onset was 7.5 minutes. Eight people had follow-up information available; all had recovered or were discharged. Of the case reports that were determined not to be anaphylaxis, 47 were assessed as nonanaphylactic allergic reactions and 47 were considered nonallergic adverse events. Four cases lacked enough information to be determined.
Based on those preliminary findings, it appears anaphylaxis is rare after the Moderna vaccination, but the researchers note that comparisons with other non–COVID-19 vaccines are constrained due to the limited information available this early in the vaccination program. They did cite an analysis of the Pfizer-BioNTech COVID-19 vaccine, also an mRNA vaccine, which estimated an initial rate of 11.1 cases per million doses after the first shot.
The researchers found a “strong female predominance” of anaphylaxis for both vaccines. All 10 anaphylaxis cases reported with the Moderna vaccine were in women. However, during the analytic period, 61% of first doses were given to women, vs 36% to men. Similarly, two thirds of first doses of the Pfizer-BioNTech vaccine were administered to women, and women were more affected.
Postvaccine COVID-19 Infections
But patients shouldn’t be too hasty to assume that symptoms after the vaccination are vaccine related, researchers at Israel’s Sheba Medical Center warn. The mere availability of a vaccine may lead to a certain laxity of precautions and a consequent rise in COVID-19 cases. “Thus, almost every physical complaint after vaccination poses a true diagnostic dilemma,” they point out, “as to whether an adverse reaction or a new COVID-19 infection is the cause.”
They studied 4,081 healthcare workers given the Pfizer-BioNTech vaccine. Of the vaccinated healthcare workers, 22 (0.54%) later had laboratory-confirmed COVID-19. Thirteen were tested because they had symptoms, usually an influenza-like illness that included fever, chills, cough, headache, myalgia, and sore throat. The median time between the first dose of vaccine and first symptoms was 3.5 days (one HCW had symptoms before immunization).
The vaccine, BNT162b2, is not likely to protect against clinical disease during the first days after receipt of the first dose, the researchers say. Efficacy was 52% a week after the first dose and positive COVID-19 cases were described among vaccinees even early after the second dose.
Clinicians should have a high level of suspicion of reported symptoms, the researchers advise, and avoid dismissing complaints as vaccine related until true infection is ruled out and the vaccine recipient is tested.
As of January 10, 2021, a reported 4,041,396 first doses of Moderna’s COVID-19 vaccine had been administered in the US. Reports of 1,266 (0.03%) adverse effects (AEs) after receipt of the vaccine were submitted to the Vaccine Adverse Event Reporting System (VAERS), according to researchers from the Centers for Disease Control and Prevention (CDC) COVID-19 Response Team and Food and Drug Administration in a Morbidity and Mortality Weekly Report early release.
The researchers screened VAERS reports that described suspected severe allergic reactions and anaphylaxis and collected information from medical records and outreach to healthcare facilities, providers, and recipients. They identified 108 reports for further review as possible severe allergic reaction, including anaphylaxis, a rare vaccination reaction. Ten cases were determined to be anaphylaxis—or a rate of 2.5 cases per million vaccine doses administered. Nine of the cases were people with a documented history of allergies or allergic reactions; 5 had a history of anaphylaxis.
The median interval from vaccine receipt to symptom onset was 7.5 minutes. Eight people had follow-up information available; all had recovered or were discharged. Of the case reports that were determined not to be anaphylaxis, 47 were assessed as nonanaphylactic allergic reactions and 47 were considered nonallergic adverse events. Four cases lacked enough information to be determined.
Based on those preliminary findings, it appears anaphylaxis is rare after the Moderna vaccination, but the researchers note that comparisons with other non–COVID-19 vaccines are constrained due to the limited information available this early in the vaccination program. They did cite an analysis of the Pfizer-BioNTech COVID-19 vaccine, also an mRNA vaccine, which estimated an initial rate of 11.1 cases per million doses after the first shot.
The researchers found a “strong female predominance” of anaphylaxis for both vaccines. All 10 anaphylaxis cases reported with the Moderna vaccine were in women. However, during the analytic period, 61% of first doses were given to women, vs 36% to men. Similarly, two thirds of first doses of the Pfizer-BioNTech vaccine were administered to women, and women were more affected.
Postvaccine COVID-19 Infections
But patients shouldn’t be too hasty to assume that symptoms after the vaccination are vaccine related, researchers at Israel’s Sheba Medical Center warn. The mere availability of a vaccine may lead to a certain laxity of precautions and a consequent rise in COVID-19 cases. “Thus, almost every physical complaint after vaccination poses a true diagnostic dilemma,” they point out, “as to whether an adverse reaction or a new COVID-19 infection is the cause.”
They studied 4,081 healthcare workers given the Pfizer-BioNTech vaccine. Of the vaccinated healthcare workers, 22 (0.54%) later had laboratory-confirmed COVID-19. Thirteen were tested because they had symptoms, usually an influenza-like illness that included fever, chills, cough, headache, myalgia, and sore throat. The median time between the first dose of vaccine and first symptoms was 3.5 days (one HCW had symptoms before immunization).
The vaccine, BNT162b2, is not likely to protect against clinical disease during the first days after receipt of the first dose, the researchers say. Efficacy was 52% a week after the first dose and positive COVID-19 cases were described among vaccinees even early after the second dose.
Clinicians should have a high level of suspicion of reported symptoms, the researchers advise, and avoid dismissing complaints as vaccine related until true infection is ruled out and the vaccine recipient is tested.
As of January 10, 2021, a reported 4,041,396 first doses of Moderna’s COVID-19 vaccine had been administered in the US. Reports of 1,266 (0.03%) adverse effects (AEs) after receipt of the vaccine were submitted to the Vaccine Adverse Event Reporting System (VAERS), according to researchers from the Centers for Disease Control and Prevention (CDC) COVID-19 Response Team and Food and Drug Administration in a Morbidity and Mortality Weekly Report early release.
The researchers screened VAERS reports that described suspected severe allergic reactions and anaphylaxis and collected information from medical records and outreach to healthcare facilities, providers, and recipients. They identified 108 reports for further review as possible severe allergic reaction, including anaphylaxis, a rare vaccination reaction. Ten cases were determined to be anaphylaxis—or a rate of 2.5 cases per million vaccine doses administered. Nine of the cases were people with a documented history of allergies or allergic reactions; 5 had a history of anaphylaxis.
The median interval from vaccine receipt to symptom onset was 7.5 minutes. Eight people had follow-up information available; all had recovered or were discharged. Of the case reports that were determined not to be anaphylaxis, 47 were assessed as nonanaphylactic allergic reactions and 47 were considered nonallergic adverse events. Four cases lacked enough information to be determined.
Based on those preliminary findings, it appears anaphylaxis is rare after the Moderna vaccination, but the researchers note that comparisons with other non–COVID-19 vaccines are constrained due to the limited information available this early in the vaccination program. They did cite an analysis of the Pfizer-BioNTech COVID-19 vaccine, also an mRNA vaccine, which estimated an initial rate of 11.1 cases per million doses after the first shot.
The researchers found a “strong female predominance” of anaphylaxis for both vaccines. All 10 anaphylaxis cases reported with the Moderna vaccine were in women. However, during the analytic period, 61% of first doses were given to women, vs 36% to men. Similarly, two thirds of first doses of the Pfizer-BioNTech vaccine were administered to women, and women were more affected.
Postvaccine COVID-19 Infections
But patients shouldn’t be too hasty to assume that symptoms after the vaccination are vaccine related, researchers at Israel’s Sheba Medical Center warn. The mere availability of a vaccine may lead to a certain laxity of precautions and a consequent rise in COVID-19 cases. “Thus, almost every physical complaint after vaccination poses a true diagnostic dilemma,” they point out, “as to whether an adverse reaction or a new COVID-19 infection is the cause.”
They studied 4,081 healthcare workers given the Pfizer-BioNTech vaccine. Of the vaccinated healthcare workers, 22 (0.54%) later had laboratory-confirmed COVID-19. Thirteen were tested because they had symptoms, usually an influenza-like illness that included fever, chills, cough, headache, myalgia, and sore throat. The median time between the first dose of vaccine and first symptoms was 3.5 days (one HCW had symptoms before immunization).
The vaccine, BNT162b2, is not likely to protect against clinical disease during the first days after receipt of the first dose, the researchers say. Efficacy was 52% a week after the first dose and positive COVID-19 cases were described among vaccinees even early after the second dose.
Clinicians should have a high level of suspicion of reported symptoms, the researchers advise, and avoid dismissing complaints as vaccine related until true infection is ruled out and the vaccine recipient is tested.
New campaign fights COVID-19 vaccine disinformation
As health care providers work against the clock to administer as many COVID-19 vaccine doses as soon as possible, logistics aren’t the only thing standing in their way.
Misinformation – which has hampered the nation’s coronavirus response – is now hurting vaccination efforts, too.
About one in five Americans say they won’t take a COVID-19 vaccine, according to the Kaiser Family Foundation’s COVID-19 Vaccine Monitor. Even a third of health care workers have voiced their hesitance.
The spread of COVID-19 vaccine misinformation creates “a really powerful parallel pandemic to the real pandemic,” Imran Ahmed, CEO of the Center for Countering Digital Hate, told NPR. The center has tracked the links between vaccine misinformation and vaccine hesitancy during the past year.
The “infodemic” is essentially “working in concert to really undermine our capacity to contain COVID,” Mr. Ahmed said.
To help combat vaccine misinformation and address lingering concerns that people have, corporate, nonprofit, and media leaders, including this news organization, are joining a public service campaign called VaxFacts. Led by HealthGuard, the goal of the campaign is to provide facts and tools to help consumers make informed decisions about vaccines.
Steven Brill, co-CEO of HealthGuard, said credible information that comes from trusted messengers is critical to counter vaccine hesitancy.
“There’s traditionally a lot of skepticism about vaccines. That has really ramped up in the last few years based on campaigns about the measles vaccine. ... And now you have the COVID vaccine, which by everybody’s understanding has been ‘rushed,’ ” Mr. Brill said during an interview on Coronavirus in Context, a video series hosted by John Whyte, MD, chief medical officer for WebMD.
“There may be less understanding of the nature of what rushed really means. It’s still gone through the clinical trials it needs to go through.”
HealthGuard is a browser extension that flags health hoaxes, provides credibility ratings for hundreds of websites, and guides users to sources that offer trusted information. The tool is a new service from NewsGuard, which veteran journalists Mr. Brill and co-CEO Gordon Crovitz created in 2018 to combat misinformation in the news. HealthGuard, which is free for users globally through June, is specifically aimed at informing readers about health myths related to vaccines and COVID-19. It will cost $35 per year after that.
The HealthGuard Coronavirus Tracking Center has flagged nearly 400 websites for publishing misinformation about the coronavirus, including several top myths about COVID-19 vaccines:
- The mRNA vaccines can alter human DNA.
- Vaccines will use microchip surveillance technology.
- COVID-19 vaccines cause infertility.
- The vaccine developed by Oxford University will turn people into monkeys.
- COVID-19 vaccines contain aborted human fetal tissue.
As a partner, this news organization will feature continuing coverage of COVID-19 vaccine misinformation, including articles and videos.
There will be other efforts this year. Google has launched a $3 million fund to back fact-checking organizations to counter vaccine misinformation, and social media platforms are monitoring posts that actively promote disinformation around vaccines.
The United States has distributed nearly 50 million vaccine doses, and states have administered more than 32 million of them, including 5.9 million second doses in the two-shot vaccines, according to the latest CDC update.
To reach herd immunity, about 75%-85% of Americans will need to receive a vaccine, Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, said in December 2020.
Vaccine skepticism has increased in recent years, which has led to a decline in vaccination rates and the highest annual number of measles cases in the United States in more than 25 years, according to the Pew Research Center. In 2019, the World Health Organization named vaccine hesitancy as 1 of 10 threats to global health.
With the COVID-19 vaccines in particular, people have voiced concerns about their safety and how well they work, given their accelerated development, according to Kaiser’s poll. They’re also worried about potential side effects, the perceived role of politics in the development process, and a lack of trust in government. Others don’t trust vaccines in general or believe they may contract COVID-19 from a vaccine, the Kaiser poll found, “suggesting that messages combating particular types of misinformation may be especially important for increasing vaccine confidence.”
A version of this article first appeared on WebMD.com.
As health care providers work against the clock to administer as many COVID-19 vaccine doses as soon as possible, logistics aren’t the only thing standing in their way.
Misinformation – which has hampered the nation’s coronavirus response – is now hurting vaccination efforts, too.
About one in five Americans say they won’t take a COVID-19 vaccine, according to the Kaiser Family Foundation’s COVID-19 Vaccine Monitor. Even a third of health care workers have voiced their hesitance.
The spread of COVID-19 vaccine misinformation creates “a really powerful parallel pandemic to the real pandemic,” Imran Ahmed, CEO of the Center for Countering Digital Hate, told NPR. The center has tracked the links between vaccine misinformation and vaccine hesitancy during the past year.
The “infodemic” is essentially “working in concert to really undermine our capacity to contain COVID,” Mr. Ahmed said.
To help combat vaccine misinformation and address lingering concerns that people have, corporate, nonprofit, and media leaders, including this news organization, are joining a public service campaign called VaxFacts. Led by HealthGuard, the goal of the campaign is to provide facts and tools to help consumers make informed decisions about vaccines.
Steven Brill, co-CEO of HealthGuard, said credible information that comes from trusted messengers is critical to counter vaccine hesitancy.
“There’s traditionally a lot of skepticism about vaccines. That has really ramped up in the last few years based on campaigns about the measles vaccine. ... And now you have the COVID vaccine, which by everybody’s understanding has been ‘rushed,’ ” Mr. Brill said during an interview on Coronavirus in Context, a video series hosted by John Whyte, MD, chief medical officer for WebMD.
“There may be less understanding of the nature of what rushed really means. It’s still gone through the clinical trials it needs to go through.”
HealthGuard is a browser extension that flags health hoaxes, provides credibility ratings for hundreds of websites, and guides users to sources that offer trusted information. The tool is a new service from NewsGuard, which veteran journalists Mr. Brill and co-CEO Gordon Crovitz created in 2018 to combat misinformation in the news. HealthGuard, which is free for users globally through June, is specifically aimed at informing readers about health myths related to vaccines and COVID-19. It will cost $35 per year after that.
The HealthGuard Coronavirus Tracking Center has flagged nearly 400 websites for publishing misinformation about the coronavirus, including several top myths about COVID-19 vaccines:
- The mRNA vaccines can alter human DNA.
- Vaccines will use microchip surveillance technology.
- COVID-19 vaccines cause infertility.
- The vaccine developed by Oxford University will turn people into monkeys.
- COVID-19 vaccines contain aborted human fetal tissue.
As a partner, this news organization will feature continuing coverage of COVID-19 vaccine misinformation, including articles and videos.
There will be other efforts this year. Google has launched a $3 million fund to back fact-checking organizations to counter vaccine misinformation, and social media platforms are monitoring posts that actively promote disinformation around vaccines.
The United States has distributed nearly 50 million vaccine doses, and states have administered more than 32 million of them, including 5.9 million second doses in the two-shot vaccines, according to the latest CDC update.
To reach herd immunity, about 75%-85% of Americans will need to receive a vaccine, Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, said in December 2020.
Vaccine skepticism has increased in recent years, which has led to a decline in vaccination rates and the highest annual number of measles cases in the United States in more than 25 years, according to the Pew Research Center. In 2019, the World Health Organization named vaccine hesitancy as 1 of 10 threats to global health.
With the COVID-19 vaccines in particular, people have voiced concerns about their safety and how well they work, given their accelerated development, according to Kaiser’s poll. They’re also worried about potential side effects, the perceived role of politics in the development process, and a lack of trust in government. Others don’t trust vaccines in general or believe they may contract COVID-19 from a vaccine, the Kaiser poll found, “suggesting that messages combating particular types of misinformation may be especially important for increasing vaccine confidence.”
A version of this article first appeared on WebMD.com.
As health care providers work against the clock to administer as many COVID-19 vaccine doses as soon as possible, logistics aren’t the only thing standing in their way.
Misinformation – which has hampered the nation’s coronavirus response – is now hurting vaccination efforts, too.
About one in five Americans say they won’t take a COVID-19 vaccine, according to the Kaiser Family Foundation’s COVID-19 Vaccine Monitor. Even a third of health care workers have voiced their hesitance.
The spread of COVID-19 vaccine misinformation creates “a really powerful parallel pandemic to the real pandemic,” Imran Ahmed, CEO of the Center for Countering Digital Hate, told NPR. The center has tracked the links between vaccine misinformation and vaccine hesitancy during the past year.
The “infodemic” is essentially “working in concert to really undermine our capacity to contain COVID,” Mr. Ahmed said.
To help combat vaccine misinformation and address lingering concerns that people have, corporate, nonprofit, and media leaders, including this news organization, are joining a public service campaign called VaxFacts. Led by HealthGuard, the goal of the campaign is to provide facts and tools to help consumers make informed decisions about vaccines.
Steven Brill, co-CEO of HealthGuard, said credible information that comes from trusted messengers is critical to counter vaccine hesitancy.
“There’s traditionally a lot of skepticism about vaccines. That has really ramped up in the last few years based on campaigns about the measles vaccine. ... And now you have the COVID vaccine, which by everybody’s understanding has been ‘rushed,’ ” Mr. Brill said during an interview on Coronavirus in Context, a video series hosted by John Whyte, MD, chief medical officer for WebMD.
“There may be less understanding of the nature of what rushed really means. It’s still gone through the clinical trials it needs to go through.”
HealthGuard is a browser extension that flags health hoaxes, provides credibility ratings for hundreds of websites, and guides users to sources that offer trusted information. The tool is a new service from NewsGuard, which veteran journalists Mr. Brill and co-CEO Gordon Crovitz created in 2018 to combat misinformation in the news. HealthGuard, which is free for users globally through June, is specifically aimed at informing readers about health myths related to vaccines and COVID-19. It will cost $35 per year after that.
The HealthGuard Coronavirus Tracking Center has flagged nearly 400 websites for publishing misinformation about the coronavirus, including several top myths about COVID-19 vaccines:
- The mRNA vaccines can alter human DNA.
- Vaccines will use microchip surveillance technology.
- COVID-19 vaccines cause infertility.
- The vaccine developed by Oxford University will turn people into monkeys.
- COVID-19 vaccines contain aborted human fetal tissue.
As a partner, this news organization will feature continuing coverage of COVID-19 vaccine misinformation, including articles and videos.
There will be other efforts this year. Google has launched a $3 million fund to back fact-checking organizations to counter vaccine misinformation, and social media platforms are monitoring posts that actively promote disinformation around vaccines.
The United States has distributed nearly 50 million vaccine doses, and states have administered more than 32 million of them, including 5.9 million second doses in the two-shot vaccines, according to the latest CDC update.
To reach herd immunity, about 75%-85% of Americans will need to receive a vaccine, Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, said in December 2020.
Vaccine skepticism has increased in recent years, which has led to a decline in vaccination rates and the highest annual number of measles cases in the United States in more than 25 years, according to the Pew Research Center. In 2019, the World Health Organization named vaccine hesitancy as 1 of 10 threats to global health.
With the COVID-19 vaccines in particular, people have voiced concerns about their safety and how well they work, given their accelerated development, according to Kaiser’s poll. They’re also worried about potential side effects, the perceived role of politics in the development process, and a lack of trust in government. Others don’t trust vaccines in general or believe they may contract COVID-19 from a vaccine, the Kaiser poll found, “suggesting that messages combating particular types of misinformation may be especially important for increasing vaccine confidence.”
A version of this article first appeared on WebMD.com.
Global Ebola vaccine stockpile established
The International Coordinating Group (ICG) on Vaccine Provision announced the establishment of a global Ebola vaccine stockpile initiative.
The ICG, which was established in 1997, is made up of the World Health Organization, the United Nations Children’s Fund, the International Federation of Red Cross and Red Crescent Societies, and Médecins Sans Frontières.
The stockpile was created in order to make the single-dose Ebola vaccine (rVSV∆G-ZEBOV-GP, live; trade name Everbo) rapidly available at the start of the next Ebola outbreak anywhere in the world. The vaccine was developed and is marketed by Merck Sharp & Dohme, with financial support from the United States.
The stockpile, which is maintained in Switzerland and managed by UNICEF, is designed to be readily deployed to other countries whenever there is an outbreak. The ICG will be the decision-making body for the vaccine’s allocation and release, as is also the case with previously created stockpiles of cholera, meningitis, and yellow fever vaccines.
“The decision to allocate the vaccine will be made within 48 hours of receiving a request from a country; vaccines will be made available together with ultra-cold chain packaging by the manufacturer for shipment to countries within 48 hours of the decision. The targeted overall delivery time from the stockpile to countries is 7 days,” according to the WHO press release.
Currently 6,890 doses are available for outbreak response, with further quantities to be delivered into the stockpile throughout 2021 and beyond. Initial use of the vaccine will be directed to health care and frontline workers. It is expected that it will take 2-3 years to reach the Strategic Advisory Group of Experts on Immunization–recommended level of 500,000 doses for the stockpile of Ebola vaccines.
The International Coordinating Group (ICG) on Vaccine Provision announced the establishment of a global Ebola vaccine stockpile initiative.
The ICG, which was established in 1997, is made up of the World Health Organization, the United Nations Children’s Fund, the International Federation of Red Cross and Red Crescent Societies, and Médecins Sans Frontières.
The stockpile was created in order to make the single-dose Ebola vaccine (rVSV∆G-ZEBOV-GP, live; trade name Everbo) rapidly available at the start of the next Ebola outbreak anywhere in the world. The vaccine was developed and is marketed by Merck Sharp & Dohme, with financial support from the United States.
The stockpile, which is maintained in Switzerland and managed by UNICEF, is designed to be readily deployed to other countries whenever there is an outbreak. The ICG will be the decision-making body for the vaccine’s allocation and release, as is also the case with previously created stockpiles of cholera, meningitis, and yellow fever vaccines.
“The decision to allocate the vaccine will be made within 48 hours of receiving a request from a country; vaccines will be made available together with ultra-cold chain packaging by the manufacturer for shipment to countries within 48 hours of the decision. The targeted overall delivery time from the stockpile to countries is 7 days,” according to the WHO press release.
Currently 6,890 doses are available for outbreak response, with further quantities to be delivered into the stockpile throughout 2021 and beyond. Initial use of the vaccine will be directed to health care and frontline workers. It is expected that it will take 2-3 years to reach the Strategic Advisory Group of Experts on Immunization–recommended level of 500,000 doses for the stockpile of Ebola vaccines.
The International Coordinating Group (ICG) on Vaccine Provision announced the establishment of a global Ebola vaccine stockpile initiative.
The ICG, which was established in 1997, is made up of the World Health Organization, the United Nations Children’s Fund, the International Federation of Red Cross and Red Crescent Societies, and Médecins Sans Frontières.
The stockpile was created in order to make the single-dose Ebola vaccine (rVSV∆G-ZEBOV-GP, live; trade name Everbo) rapidly available at the start of the next Ebola outbreak anywhere in the world. The vaccine was developed and is marketed by Merck Sharp & Dohme, with financial support from the United States.
The stockpile, which is maintained in Switzerland and managed by UNICEF, is designed to be readily deployed to other countries whenever there is an outbreak. The ICG will be the decision-making body for the vaccine’s allocation and release, as is also the case with previously created stockpiles of cholera, meningitis, and yellow fever vaccines.
“The decision to allocate the vaccine will be made within 48 hours of receiving a request from a country; vaccines will be made available together with ultra-cold chain packaging by the manufacturer for shipment to countries within 48 hours of the decision. The targeted overall delivery time from the stockpile to countries is 7 days,” according to the WHO press release.
Currently 6,890 doses are available for outbreak response, with further quantities to be delivered into the stockpile throughout 2021 and beyond. Initial use of the vaccine will be directed to health care and frontline workers. It is expected that it will take 2-3 years to reach the Strategic Advisory Group of Experts on Immunization–recommended level of 500,000 doses for the stockpile of Ebola vaccines.
Moderna needs more kids for COVID vaccine trials
according to the company CEO and a federal official.
The Moderna vaccine was authorized for use in December and is now being given to people 18 and over. But children would receive lower doses, so new clinical trials must be done, Moderna CEO Stephane Bancel said at the JPMorgan virtual Health Care Conference on Monday.
Clinical trials on children 11 and younger “will take much longer, because we have to age deescalate and start at a lower dose. So we should not anticipate clinical data in 2021, but more in 2022,” Ms. Bancel said, according to Business Insider.
Moderna’s clinical trials for 12- to 17-year-olds started 4 weeks ago, but the company is having trouble getting enough participants, said Moncef Slaoui, PhD, the scientific head of Operation Warp Speed, the U.S. government’s vaccine effort. That could delay Food and Drug Administration approval, he said.
“It’s really very important for all of us, for all the population in America, to realize that we can’t have that indication unless adolescents aged 12-18 decide to participate,” Dr. Slaoui said, according to USA Today.
He said the adolescent trials are getting only about 800 volunteers a month, but need at least 3,000 volunteers to complete the study, USA Today reported. Parents interested in having their child participate can check eligibility and sign at this website.
The Pfizer/BioNTech vaccine won authorization for use in 16- to 17-year-olds as well as adults.
The coronavirus doesn’t appear to have as serious complications for children as for adults.
“At this time, it appears that severe illness due to COVID-19 is rare among children,” the American Association of Pediatrics says. “However, there is an urgent need to collect more data on longer-term impacts of the pandemic on children, including ways the virus may harm the long-term physical health of infected children, as well as its emotional and mental health effects.”
The association says 179 children had died of COVID-related reasons in 43 states and New York City as of Dec. 31, 2020. That’s about 0.06% of total COVID deaths, it says.
But children do get sick. As of Jan. 7, 2021, nearly 2.3 million children had tested positive for COVID-19 since the start of the pandemic, the association says.
A version of this article first appeared on WebMD.com.
according to the company CEO and a federal official.
The Moderna vaccine was authorized for use in December and is now being given to people 18 and over. But children would receive lower doses, so new clinical trials must be done, Moderna CEO Stephane Bancel said at the JPMorgan virtual Health Care Conference on Monday.
Clinical trials on children 11 and younger “will take much longer, because we have to age deescalate and start at a lower dose. So we should not anticipate clinical data in 2021, but more in 2022,” Ms. Bancel said, according to Business Insider.
Moderna’s clinical trials for 12- to 17-year-olds started 4 weeks ago, but the company is having trouble getting enough participants, said Moncef Slaoui, PhD, the scientific head of Operation Warp Speed, the U.S. government’s vaccine effort. That could delay Food and Drug Administration approval, he said.
“It’s really very important for all of us, for all the population in America, to realize that we can’t have that indication unless adolescents aged 12-18 decide to participate,” Dr. Slaoui said, according to USA Today.
He said the adolescent trials are getting only about 800 volunteers a month, but need at least 3,000 volunteers to complete the study, USA Today reported. Parents interested in having their child participate can check eligibility and sign at this website.
The Pfizer/BioNTech vaccine won authorization for use in 16- to 17-year-olds as well as adults.
The coronavirus doesn’t appear to have as serious complications for children as for adults.
“At this time, it appears that severe illness due to COVID-19 is rare among children,” the American Association of Pediatrics says. “However, there is an urgent need to collect more data on longer-term impacts of the pandemic on children, including ways the virus may harm the long-term physical health of infected children, as well as its emotional and mental health effects.”
The association says 179 children had died of COVID-related reasons in 43 states and New York City as of Dec. 31, 2020. That’s about 0.06% of total COVID deaths, it says.
But children do get sick. As of Jan. 7, 2021, nearly 2.3 million children had tested positive for COVID-19 since the start of the pandemic, the association says.
A version of this article first appeared on WebMD.com.
according to the company CEO and a federal official.
The Moderna vaccine was authorized for use in December and is now being given to people 18 and over. But children would receive lower doses, so new clinical trials must be done, Moderna CEO Stephane Bancel said at the JPMorgan virtual Health Care Conference on Monday.
Clinical trials on children 11 and younger “will take much longer, because we have to age deescalate and start at a lower dose. So we should not anticipate clinical data in 2021, but more in 2022,” Ms. Bancel said, according to Business Insider.
Moderna’s clinical trials for 12- to 17-year-olds started 4 weeks ago, but the company is having trouble getting enough participants, said Moncef Slaoui, PhD, the scientific head of Operation Warp Speed, the U.S. government’s vaccine effort. That could delay Food and Drug Administration approval, he said.
“It’s really very important for all of us, for all the population in America, to realize that we can’t have that indication unless adolescents aged 12-18 decide to participate,” Dr. Slaoui said, according to USA Today.
He said the adolescent trials are getting only about 800 volunteers a month, but need at least 3,000 volunteers to complete the study, USA Today reported. Parents interested in having their child participate can check eligibility and sign at this website.
The Pfizer/BioNTech vaccine won authorization for use in 16- to 17-year-olds as well as adults.
The coronavirus doesn’t appear to have as serious complications for children as for adults.
“At this time, it appears that severe illness due to COVID-19 is rare among children,” the American Association of Pediatrics says. “However, there is an urgent need to collect more data on longer-term impacts of the pandemic on children, including ways the virus may harm the long-term physical health of infected children, as well as its emotional and mental health effects.”
The association says 179 children had died of COVID-related reasons in 43 states and New York City as of Dec. 31, 2020. That’s about 0.06% of total COVID deaths, it says.
But children do get sick. As of Jan. 7, 2021, nearly 2.3 million children had tested positive for COVID-19 since the start of the pandemic, the association says.
A version of this article first appeared on WebMD.com.
Family physicians can help achieve national goals on STIs
Among these are the U.S. Department of Health and Human Services’ first “Sexually Transmitted Infections (STIs) National Strategic Plan for the United States,” which has a strong encompassing vision.
“The United States will be a place where sexually transmitted infections are prevented and where every person has high-quality STI prevention care, and treatment while living free from stigma and discrimination. The vision includes all people, regardless of age, sex, gender identity, sexual orientation, race, ethnicity, religion, disability, geographic location, or socioeconomic circumstance,” the new HHS plan states.1
Family physicians can and should play important roles in helping our country meet this plan’s goals particularly by following two important updated clinical guidelines, one from the U.S. Preventive Services Task Force (USPSTF) and another from the Centers for Disease Control and Prevention (CDC).
This strategic plan includes the following five overarching goals with associated objectives:
- Prevent New STIs.
- Improve the health of people by reducing adverse outcomes of STIs.
- Accelerate progress in STI research, technology, and innovation.
- Reduce STI-related health disparities and health inequities.
- Achieve integrated, coordinated efforts that address the STI epidemic.1
In my opinion, family physicians have important roles to play in order for each of these goals to be achieved.Unfortunately, there are approximately 20 million new cases of STIs each year, and the U.S. has seen increases in the rates of STIs in the past decade.
“Sexually transmitted infections are frequently asymptomatic, which may delay diagnosis and treatment and lead persons to unknowingly transmit STIs to others,” according to a new recommendation statement from the USPSTF.2 STIs may lead to serious health consequences for patients, cause harms to a mother and infant during pregnancy, and lead to cases of cancer among other concerning outcomes. As such, following the HHS new national strategic plan is critical for us to address the needs of our communities.
Preventing new STIs
Family physicians can be vital in achieving the first goal of the plan by helping to prevent new STIs. In August 2020, the USPSTF updated its guideline on behavioral counseling interventions to prevent STIs. In my opinion, the USPSTF offers some practical improvements from the earlier version of this guideline.
The task force provides a grade B recommendation that all sexually active adolescents and adults at increased risk for STIs be provided with behavioral counseling to prevent STIs. The guideline indicates that behavioral counseling interventions reduce the likelihood of those at increased risk for acquiring STIs.2
The 2014 guideline had recommended intensive interventions with a minimum of 30 minutes of counseling. Many family physicians may have found this previous recommendation impractical to implement. These updated recommendations now include a variety of interventions, such as those that take less than 30 minutes.
Although interventions with more than 120 minutes of contact time had the most effect, those with less than 30 minutes still demonstrated statistically significant fewer acquisitions of STIs during follow-up. These options include in-person counseling, and providing written materials, websites, videos, and telephone and text support to patients. These interventions can be delivered directly by the family physician, or patients may be referred to other settings or the media interventions.
The task force’s updated recommendation statement refers to a variety of resources that can be used to identify these interventions. Many of the studies reviewed for this guideline were conducted in STI clinics, and the guideline authors recommended further studies in primary care as opportunities for more generalizability.
In addition to behavioral counseling for STI prevention, family physicians can help prevent STIs in their patients through HPV vaccination and HIV pre-exposure prophylaxis (PrEP provision) within their practices. As the first contact for health care for many patients, we have an opportunity to significantly impact this first goal of prevention.
Treating STIs
Within the second goal of the national strategic plan is treatment of STIs, which family physicians should include in their practices as well as the diagnosis of STIs.
In December 2020, an update to the CDC’s treatment guideline for gonococcal infection was released. Prior to the publishing of this updated recommendation, the CDC recommended combination therapy of 250 mg intramuscular (IM) dose of ceftriaxone and either doxycycline or azithromycin. This recommendation has been changed to a single 500-mg IM dose of ceftriaxone for uncomplicated urogenital, anorectal, and pharyngeal gonorrhea. If chlamydia cannot be excluded, then the addition of oral doxycycline 100 mg twice daily for 7 days is recommended for nonpregnant persons, and 1 g oral azithromycin for pregnant persons. The previous treatment was recommended based on a concern for gonococcal resistance.
This updated guideline reflects increasing concerns for antimicrobial stewardship and emerging azithromycin resistance. It does not recommend a test-of-cure for urogenital or rectal gonorrhea, though did recommend a test-of-cure 7-14 days after treatment of pharyngeal gonorrhea. The guideline also recommends testing for reinfection 3-12 months after treatment as the rate of reinfection ranges from 7% to 12% among those previously treated.3
For some offices, the provision of the IM injection may be challenging, though having this medication in stock with the possibility of provision can greatly improve access and ease of treatment for patients. Family physicians can incorporate these updated recommendations along with those for other STIs such as chlamydia and syphilis with standing orders for treatment and testing within their offices.
Accelerating progress in STI research
Family physicians can also support the national strategic plan by participating in studies looking at the impact of behavioral counseling in the primary care office as opposed to in STI clinics. In addition, by following the STI treatment and screening guidelines, family physicians will contribute to the body of knowledge of prevalence, treatment failure, and reinfection rates of STIs. We can also help advance the research by providing feedback on interventions that have success within our practices.
Reducing STI-related health disparities and inequities
Family physicians are also in important places to support the strategic plan’s fourth goal of reducing health disparities and health inequities.
If we continue to ask the questions to identify those at high risk and ensure that we are offering appropriate STI prevention, care, and treatment services within our clinics, we can expand access to all who need services and improve equity. By offering these services within the primary care office, we may be able to decrease the stigma some may feel going to an STI clinic for services.
By incorporating additional screening and counseling in our practices we may identify some patients who were not aware that they were at risk for an STI and offer them preventive services.
Achieving integrated and coordinated efforts
Finally, as many family physicians have integrated practices, we are uniquely poised to support the fifth goal of the strategic plan of achieving integrated and coordinated efforts addressing the STI epidemic. In our practices we can participate in, lead, and refer to programs for substance use disorders, viral hepatitis, STIs, and HIV as part of full scope primary care.
Family physicians and other primary care providers should work to support the entire strategic plan to ensure that we are fully caring for our patients and communities and stopping the past decade’s increase in STIs. We have an opportunity to use this strategy and make a large impact in our communities.
Dr. Wheat is a family physician at Erie Family Health Center in Chicago. She is program director of Northwestern’s McGaw Family Medicine residency program at Humboldt Park, Chicago. Dr. Wheat serves on the editorial advisory board of Family Practice News. You can contact her at fpnews@mdedge.com.
References
1. U.S. Department of Health and Human Services. 2020. Sexually Transmitted Infections National Strategic Plan for the United States: 2021-2025. Washington.
2. U.S. Preventive Services Task Force. Behavioral counseling interventions to prevent sexually transmitted infections: U.S. Preventive Services Task Force Recommendation Statement. JAMA. 2020;324(7):674-81. doi: 10.1001/jama.2020.13095.
3. St. Cyr S et al. Update to CDC’s Treatment Guideline for Gonococcal Infection, 2020. MMWR Morb Mortal Wkly Rep 2020;69:1911-6. doi: 10.15585/mmwr.mm6950a6external_icon.
Among these are the U.S. Department of Health and Human Services’ first “Sexually Transmitted Infections (STIs) National Strategic Plan for the United States,” which has a strong encompassing vision.
“The United States will be a place where sexually transmitted infections are prevented and where every person has high-quality STI prevention care, and treatment while living free from stigma and discrimination. The vision includes all people, regardless of age, sex, gender identity, sexual orientation, race, ethnicity, religion, disability, geographic location, or socioeconomic circumstance,” the new HHS plan states.1
Family physicians can and should play important roles in helping our country meet this plan’s goals particularly by following two important updated clinical guidelines, one from the U.S. Preventive Services Task Force (USPSTF) and another from the Centers for Disease Control and Prevention (CDC).
This strategic plan includes the following five overarching goals with associated objectives:
- Prevent New STIs.
- Improve the health of people by reducing adverse outcomes of STIs.
- Accelerate progress in STI research, technology, and innovation.
- Reduce STI-related health disparities and health inequities.
- Achieve integrated, coordinated efforts that address the STI epidemic.1
In my opinion, family physicians have important roles to play in order for each of these goals to be achieved.Unfortunately, there are approximately 20 million new cases of STIs each year, and the U.S. has seen increases in the rates of STIs in the past decade.
“Sexually transmitted infections are frequently asymptomatic, which may delay diagnosis and treatment and lead persons to unknowingly transmit STIs to others,” according to a new recommendation statement from the USPSTF.2 STIs may lead to serious health consequences for patients, cause harms to a mother and infant during pregnancy, and lead to cases of cancer among other concerning outcomes. As such, following the HHS new national strategic plan is critical for us to address the needs of our communities.
Preventing new STIs
Family physicians can be vital in achieving the first goal of the plan by helping to prevent new STIs. In August 2020, the USPSTF updated its guideline on behavioral counseling interventions to prevent STIs. In my opinion, the USPSTF offers some practical improvements from the earlier version of this guideline.
The task force provides a grade B recommendation that all sexually active adolescents and adults at increased risk for STIs be provided with behavioral counseling to prevent STIs. The guideline indicates that behavioral counseling interventions reduce the likelihood of those at increased risk for acquiring STIs.2
The 2014 guideline had recommended intensive interventions with a minimum of 30 minutes of counseling. Many family physicians may have found this previous recommendation impractical to implement. These updated recommendations now include a variety of interventions, such as those that take less than 30 minutes.
Although interventions with more than 120 minutes of contact time had the most effect, those with less than 30 minutes still demonstrated statistically significant fewer acquisitions of STIs during follow-up. These options include in-person counseling, and providing written materials, websites, videos, and telephone and text support to patients. These interventions can be delivered directly by the family physician, or patients may be referred to other settings or the media interventions.
The task force’s updated recommendation statement refers to a variety of resources that can be used to identify these interventions. Many of the studies reviewed for this guideline were conducted in STI clinics, and the guideline authors recommended further studies in primary care as opportunities for more generalizability.
In addition to behavioral counseling for STI prevention, family physicians can help prevent STIs in their patients through HPV vaccination and HIV pre-exposure prophylaxis (PrEP provision) within their practices. As the first contact for health care for many patients, we have an opportunity to significantly impact this first goal of prevention.
Treating STIs
Within the second goal of the national strategic plan is treatment of STIs, which family physicians should include in their practices as well as the diagnosis of STIs.
In December 2020, an update to the CDC’s treatment guideline for gonococcal infection was released. Prior to the publishing of this updated recommendation, the CDC recommended combination therapy of 250 mg intramuscular (IM) dose of ceftriaxone and either doxycycline or azithromycin. This recommendation has been changed to a single 500-mg IM dose of ceftriaxone for uncomplicated urogenital, anorectal, and pharyngeal gonorrhea. If chlamydia cannot be excluded, then the addition of oral doxycycline 100 mg twice daily for 7 days is recommended for nonpregnant persons, and 1 g oral azithromycin for pregnant persons. The previous treatment was recommended based on a concern for gonococcal resistance.
This updated guideline reflects increasing concerns for antimicrobial stewardship and emerging azithromycin resistance. It does not recommend a test-of-cure for urogenital or rectal gonorrhea, though did recommend a test-of-cure 7-14 days after treatment of pharyngeal gonorrhea. The guideline also recommends testing for reinfection 3-12 months after treatment as the rate of reinfection ranges from 7% to 12% among those previously treated.3
For some offices, the provision of the IM injection may be challenging, though having this medication in stock with the possibility of provision can greatly improve access and ease of treatment for patients. Family physicians can incorporate these updated recommendations along with those for other STIs such as chlamydia and syphilis with standing orders for treatment and testing within their offices.
Accelerating progress in STI research
Family physicians can also support the national strategic plan by participating in studies looking at the impact of behavioral counseling in the primary care office as opposed to in STI clinics. In addition, by following the STI treatment and screening guidelines, family physicians will contribute to the body of knowledge of prevalence, treatment failure, and reinfection rates of STIs. We can also help advance the research by providing feedback on interventions that have success within our practices.
Reducing STI-related health disparities and inequities
Family physicians are also in important places to support the strategic plan’s fourth goal of reducing health disparities and health inequities.
If we continue to ask the questions to identify those at high risk and ensure that we are offering appropriate STI prevention, care, and treatment services within our clinics, we can expand access to all who need services and improve equity. By offering these services within the primary care office, we may be able to decrease the stigma some may feel going to an STI clinic for services.
By incorporating additional screening and counseling in our practices we may identify some patients who were not aware that they were at risk for an STI and offer them preventive services.
Achieving integrated and coordinated efforts
Finally, as many family physicians have integrated practices, we are uniquely poised to support the fifth goal of the strategic plan of achieving integrated and coordinated efforts addressing the STI epidemic. In our practices we can participate in, lead, and refer to programs for substance use disorders, viral hepatitis, STIs, and HIV as part of full scope primary care.
Family physicians and other primary care providers should work to support the entire strategic plan to ensure that we are fully caring for our patients and communities and stopping the past decade’s increase in STIs. We have an opportunity to use this strategy and make a large impact in our communities.
Dr. Wheat is a family physician at Erie Family Health Center in Chicago. She is program director of Northwestern’s McGaw Family Medicine residency program at Humboldt Park, Chicago. Dr. Wheat serves on the editorial advisory board of Family Practice News. You can contact her at fpnews@mdedge.com.
References
1. U.S. Department of Health and Human Services. 2020. Sexually Transmitted Infections National Strategic Plan for the United States: 2021-2025. Washington.
2. U.S. Preventive Services Task Force. Behavioral counseling interventions to prevent sexually transmitted infections: U.S. Preventive Services Task Force Recommendation Statement. JAMA. 2020;324(7):674-81. doi: 10.1001/jama.2020.13095.
3. St. Cyr S et al. Update to CDC’s Treatment Guideline for Gonococcal Infection, 2020. MMWR Morb Mortal Wkly Rep 2020;69:1911-6. doi: 10.15585/mmwr.mm6950a6external_icon.
Among these are the U.S. Department of Health and Human Services’ first “Sexually Transmitted Infections (STIs) National Strategic Plan for the United States,” which has a strong encompassing vision.
“The United States will be a place where sexually transmitted infections are prevented and where every person has high-quality STI prevention care, and treatment while living free from stigma and discrimination. The vision includes all people, regardless of age, sex, gender identity, sexual orientation, race, ethnicity, religion, disability, geographic location, or socioeconomic circumstance,” the new HHS plan states.1
Family physicians can and should play important roles in helping our country meet this plan’s goals particularly by following two important updated clinical guidelines, one from the U.S. Preventive Services Task Force (USPSTF) and another from the Centers for Disease Control and Prevention (CDC).
This strategic plan includes the following five overarching goals with associated objectives:
- Prevent New STIs.
- Improve the health of people by reducing adverse outcomes of STIs.
- Accelerate progress in STI research, technology, and innovation.
- Reduce STI-related health disparities and health inequities.
- Achieve integrated, coordinated efforts that address the STI epidemic.1
In my opinion, family physicians have important roles to play in order for each of these goals to be achieved.Unfortunately, there are approximately 20 million new cases of STIs each year, and the U.S. has seen increases in the rates of STIs in the past decade.
“Sexually transmitted infections are frequently asymptomatic, which may delay diagnosis and treatment and lead persons to unknowingly transmit STIs to others,” according to a new recommendation statement from the USPSTF.2 STIs may lead to serious health consequences for patients, cause harms to a mother and infant during pregnancy, and lead to cases of cancer among other concerning outcomes. As such, following the HHS new national strategic plan is critical for us to address the needs of our communities.
Preventing new STIs
Family physicians can be vital in achieving the first goal of the plan by helping to prevent new STIs. In August 2020, the USPSTF updated its guideline on behavioral counseling interventions to prevent STIs. In my opinion, the USPSTF offers some practical improvements from the earlier version of this guideline.
The task force provides a grade B recommendation that all sexually active adolescents and adults at increased risk for STIs be provided with behavioral counseling to prevent STIs. The guideline indicates that behavioral counseling interventions reduce the likelihood of those at increased risk for acquiring STIs.2
The 2014 guideline had recommended intensive interventions with a minimum of 30 minutes of counseling. Many family physicians may have found this previous recommendation impractical to implement. These updated recommendations now include a variety of interventions, such as those that take less than 30 minutes.
Although interventions with more than 120 minutes of contact time had the most effect, those with less than 30 minutes still demonstrated statistically significant fewer acquisitions of STIs during follow-up. These options include in-person counseling, and providing written materials, websites, videos, and telephone and text support to patients. These interventions can be delivered directly by the family physician, or patients may be referred to other settings or the media interventions.
The task force’s updated recommendation statement refers to a variety of resources that can be used to identify these interventions. Many of the studies reviewed for this guideline were conducted in STI clinics, and the guideline authors recommended further studies in primary care as opportunities for more generalizability.
In addition to behavioral counseling for STI prevention, family physicians can help prevent STIs in their patients through HPV vaccination and HIV pre-exposure prophylaxis (PrEP provision) within their practices. As the first contact for health care for many patients, we have an opportunity to significantly impact this first goal of prevention.
Treating STIs
Within the second goal of the national strategic plan is treatment of STIs, which family physicians should include in their practices as well as the diagnosis of STIs.
In December 2020, an update to the CDC’s treatment guideline for gonococcal infection was released. Prior to the publishing of this updated recommendation, the CDC recommended combination therapy of 250 mg intramuscular (IM) dose of ceftriaxone and either doxycycline or azithromycin. This recommendation has been changed to a single 500-mg IM dose of ceftriaxone for uncomplicated urogenital, anorectal, and pharyngeal gonorrhea. If chlamydia cannot be excluded, then the addition of oral doxycycline 100 mg twice daily for 7 days is recommended for nonpregnant persons, and 1 g oral azithromycin for pregnant persons. The previous treatment was recommended based on a concern for gonococcal resistance.
This updated guideline reflects increasing concerns for antimicrobial stewardship and emerging azithromycin resistance. It does not recommend a test-of-cure for urogenital or rectal gonorrhea, though did recommend a test-of-cure 7-14 days after treatment of pharyngeal gonorrhea. The guideline also recommends testing for reinfection 3-12 months after treatment as the rate of reinfection ranges from 7% to 12% among those previously treated.3
For some offices, the provision of the IM injection may be challenging, though having this medication in stock with the possibility of provision can greatly improve access and ease of treatment for patients. Family physicians can incorporate these updated recommendations along with those for other STIs such as chlamydia and syphilis with standing orders for treatment and testing within their offices.
Accelerating progress in STI research
Family physicians can also support the national strategic plan by participating in studies looking at the impact of behavioral counseling in the primary care office as opposed to in STI clinics. In addition, by following the STI treatment and screening guidelines, family physicians will contribute to the body of knowledge of prevalence, treatment failure, and reinfection rates of STIs. We can also help advance the research by providing feedback on interventions that have success within our practices.
Reducing STI-related health disparities and inequities
Family physicians are also in important places to support the strategic plan’s fourth goal of reducing health disparities and health inequities.
If we continue to ask the questions to identify those at high risk and ensure that we are offering appropriate STI prevention, care, and treatment services within our clinics, we can expand access to all who need services and improve equity. By offering these services within the primary care office, we may be able to decrease the stigma some may feel going to an STI clinic for services.
By incorporating additional screening and counseling in our practices we may identify some patients who were not aware that they were at risk for an STI and offer them preventive services.
Achieving integrated and coordinated efforts
Finally, as many family physicians have integrated practices, we are uniquely poised to support the fifth goal of the strategic plan of achieving integrated and coordinated efforts addressing the STI epidemic. In our practices we can participate in, lead, and refer to programs for substance use disorders, viral hepatitis, STIs, and HIV as part of full scope primary care.
Family physicians and other primary care providers should work to support the entire strategic plan to ensure that we are fully caring for our patients and communities and stopping the past decade’s increase in STIs. We have an opportunity to use this strategy and make a large impact in our communities.
Dr. Wheat is a family physician at Erie Family Health Center in Chicago. She is program director of Northwestern’s McGaw Family Medicine residency program at Humboldt Park, Chicago. Dr. Wheat serves on the editorial advisory board of Family Practice News. You can contact her at fpnews@mdedge.com.
References
1. U.S. Department of Health and Human Services. 2020. Sexually Transmitted Infections National Strategic Plan for the United States: 2021-2025. Washington.
2. U.S. Preventive Services Task Force. Behavioral counseling interventions to prevent sexually transmitted infections: U.S. Preventive Services Task Force Recommendation Statement. JAMA. 2020;324(7):674-81. doi: 10.1001/jama.2020.13095.
3. St. Cyr S et al. Update to CDC’s Treatment Guideline for Gonococcal Infection, 2020. MMWR Morb Mortal Wkly Rep 2020;69:1911-6. doi: 10.15585/mmwr.mm6950a6external_icon.
Coping with vaccine refusal
Do you accept new families into your practice who have already chosen to not have their children immunized? What about families who have been in your practice for several months or years? In 2016 the American Academy of Pediatrics published a clinical report in which it stated that, under some circumstances, dismissing families who refuse to vaccinate is permissible. Have you felt sufficiently supported by that statement and dismissed any families after multiple attempts at education on your part?
In a Pediatrics Perspective article in the December issue of Pediatrics, two philosophers and a physician make the argument that, while in some situations dismissing a family who refuses vaccines may be “an ethically acceptable option” refusing to accept a family with the same philosophy is not. It is an interesting paper and worth reading regardless of whether or not you already accept and continue to tolerate vaccine deniers in your practice.
The Pediatrics Perspective is certainly not the last word on the ethics of caring for families who deny their children care that we believe is critical to their health and the welfare of the community at large. There has been a lot of discussion about the issue but little has been written about how we as the physicians on the front line are coping emotionally with what the authors of the paper call the “burdens associated with treating” families who refuse to follow our guidance.
It is hard not to feel angry when a family you have invested valuable office time in discussing the benefits and safety of vaccines continues to disregard what you see as the facts. The time you have spent with them is not just income-generating time for your practice, it is time stolen from other families who are more willing to follow your recommendations. In how many visits will you continue to raise the issue? Unless I saw a glimmer of hope I would usually stop after two wasted encounters. But, the issue would still linger as the elephant in the examination room for as long as I continued to see the patient.
How have you expressed your anger? Have you been argumentative or rude? You may have been able maintain your composure and remain civil and appear caring, but I suspect the anger is still gnawing at you. And, there is still the frustration and feeling of impotence. You may have questioned your ability as an educator. You should get over that notion quickly. There is ample evidence that most vaccine deniers are not going to be convinced by even the most carefully presented information. I suggest you leave it to others to try their hands at education. Let them invest their time while you tend to the needs of your other patients. You can try being a fear monger and, while fear can be effective, you have better ways to spend your office day than telling horror stories.
If vaccine denial makes you feel powerless, you should get over that pretty quickly as well and accept the fact that you are simply an advisor. If you believe that most of the families in your practice are following your recommendations as though you had presented them on stone tablets, it is time for a wakeup call.
Finally, there is the most troubling emotion associated with vaccine refusal and that is fear, the fear of being sued. Establishing a relationship with a family is one that requires mutual trust and certainly vaccine refusal will put that trust in question, particularly if you have done a less than adequate job of hiding your anger and frustration with their unfortunate decision.
For now, vaccine refusal is just another one of those crosses that those of us in primary care must bear together wearing the best face we can put forward. That doesn’t mean we can’t share those emotions with our peers. Misery does love company.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at pdnews@mdedge.com.
Do you accept new families into your practice who have already chosen to not have their children immunized? What about families who have been in your practice for several months or years? In 2016 the American Academy of Pediatrics published a clinical report in which it stated that, under some circumstances, dismissing families who refuse to vaccinate is permissible. Have you felt sufficiently supported by that statement and dismissed any families after multiple attempts at education on your part?
In a Pediatrics Perspective article in the December issue of Pediatrics, two philosophers and a physician make the argument that, while in some situations dismissing a family who refuses vaccines may be “an ethically acceptable option” refusing to accept a family with the same philosophy is not. It is an interesting paper and worth reading regardless of whether or not you already accept and continue to tolerate vaccine deniers in your practice.
The Pediatrics Perspective is certainly not the last word on the ethics of caring for families who deny their children care that we believe is critical to their health and the welfare of the community at large. There has been a lot of discussion about the issue but little has been written about how we as the physicians on the front line are coping emotionally with what the authors of the paper call the “burdens associated with treating” families who refuse to follow our guidance.
It is hard not to feel angry when a family you have invested valuable office time in discussing the benefits and safety of vaccines continues to disregard what you see as the facts. The time you have spent with them is not just income-generating time for your practice, it is time stolen from other families who are more willing to follow your recommendations. In how many visits will you continue to raise the issue? Unless I saw a glimmer of hope I would usually stop after two wasted encounters. But, the issue would still linger as the elephant in the examination room for as long as I continued to see the patient.
How have you expressed your anger? Have you been argumentative or rude? You may have been able maintain your composure and remain civil and appear caring, but I suspect the anger is still gnawing at you. And, there is still the frustration and feeling of impotence. You may have questioned your ability as an educator. You should get over that notion quickly. There is ample evidence that most vaccine deniers are not going to be convinced by even the most carefully presented information. I suggest you leave it to others to try their hands at education. Let them invest their time while you tend to the needs of your other patients. You can try being a fear monger and, while fear can be effective, you have better ways to spend your office day than telling horror stories.
If vaccine denial makes you feel powerless, you should get over that pretty quickly as well and accept the fact that you are simply an advisor. If you believe that most of the families in your practice are following your recommendations as though you had presented them on stone tablets, it is time for a wakeup call.
Finally, there is the most troubling emotion associated with vaccine refusal and that is fear, the fear of being sued. Establishing a relationship with a family is one that requires mutual trust and certainly vaccine refusal will put that trust in question, particularly if you have done a less than adequate job of hiding your anger and frustration with their unfortunate decision.
For now, vaccine refusal is just another one of those crosses that those of us in primary care must bear together wearing the best face we can put forward. That doesn’t mean we can’t share those emotions with our peers. Misery does love company.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at pdnews@mdedge.com.
Do you accept new families into your practice who have already chosen to not have their children immunized? What about families who have been in your practice for several months or years? In 2016 the American Academy of Pediatrics published a clinical report in which it stated that, under some circumstances, dismissing families who refuse to vaccinate is permissible. Have you felt sufficiently supported by that statement and dismissed any families after multiple attempts at education on your part?
In a Pediatrics Perspective article in the December issue of Pediatrics, two philosophers and a physician make the argument that, while in some situations dismissing a family who refuses vaccines may be “an ethically acceptable option” refusing to accept a family with the same philosophy is not. It is an interesting paper and worth reading regardless of whether or not you already accept and continue to tolerate vaccine deniers in your practice.
The Pediatrics Perspective is certainly not the last word on the ethics of caring for families who deny their children care that we believe is critical to their health and the welfare of the community at large. There has been a lot of discussion about the issue but little has been written about how we as the physicians on the front line are coping emotionally with what the authors of the paper call the “burdens associated with treating” families who refuse to follow our guidance.
It is hard not to feel angry when a family you have invested valuable office time in discussing the benefits and safety of vaccines continues to disregard what you see as the facts. The time you have spent with them is not just income-generating time for your practice, it is time stolen from other families who are more willing to follow your recommendations. In how many visits will you continue to raise the issue? Unless I saw a glimmer of hope I would usually stop after two wasted encounters. But, the issue would still linger as the elephant in the examination room for as long as I continued to see the patient.
How have you expressed your anger? Have you been argumentative or rude? You may have been able maintain your composure and remain civil and appear caring, but I suspect the anger is still gnawing at you. And, there is still the frustration and feeling of impotence. You may have questioned your ability as an educator. You should get over that notion quickly. There is ample evidence that most vaccine deniers are not going to be convinced by even the most carefully presented information. I suggest you leave it to others to try their hands at education. Let them invest their time while you tend to the needs of your other patients. You can try being a fear monger and, while fear can be effective, you have better ways to spend your office day than telling horror stories.
If vaccine denial makes you feel powerless, you should get over that pretty quickly as well and accept the fact that you are simply an advisor. If you believe that most of the families in your practice are following your recommendations as though you had presented them on stone tablets, it is time for a wakeup call.
Finally, there is the most troubling emotion associated with vaccine refusal and that is fear, the fear of being sued. Establishing a relationship with a family is one that requires mutual trust and certainly vaccine refusal will put that trust in question, particularly if you have done a less than adequate job of hiding your anger and frustration with their unfortunate decision.
For now, vaccine refusal is just another one of those crosses that those of us in primary care must bear together wearing the best face we can put forward. That doesn’t mean we can’t share those emotions with our peers. Misery does love company.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at pdnews@mdedge.com.
Waiting for the COVID 19 vaccine, or not?
A shot of relief. A shot of hope. Those are the words used to describe COVID-19 vaccines on a television commercial running in prime time in Kentucky.
“We all can’t get the vaccine at once,” the announcer says solemnly, “but we’ll all get a turn.”
For some of us, that turn came quickly. In December, the Advisory Committee on Immunization Practices recommended that health care personnel (HCP) and long-term care facility residents be the first to be immunized with COVID-19 vaccines (see table). 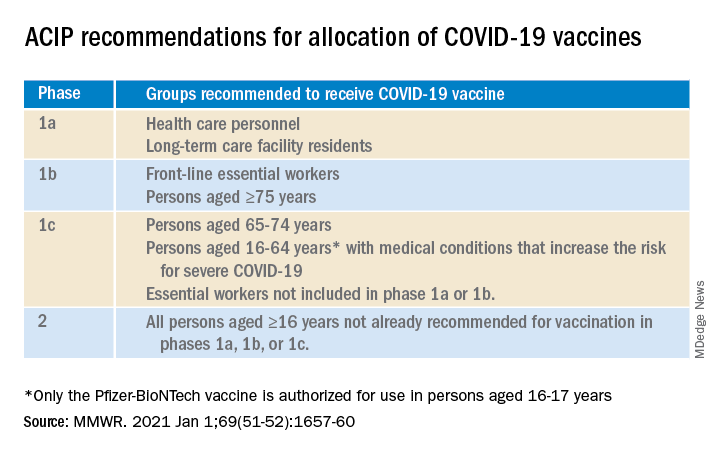
On Dec. 14, 2020, Sandra Lindsay, a nurse and director of patient care services in the intensive care unit at Long Island Jewish Medical Center, was the first person in the United States to receive a COVID-19 vaccine outside a clinical trial.
In subsequent days, social media sites were quickly flooded with photos of HCP rolling up their sleeves or flashing their immunization cards. There was jubilation ... and perhaps a little bit of jealousy. There were tears of joy and some tears of frustration.
There are more than 21 million HCP in the United States and to date, there have not been enough vaccines nor adequate infrastructure to immunize all of them. According to the Centers for Disease Control and Prevention Data Tracker, as of Jan. 7, 2021, 21,419,800 doses of vaccine had been distributed to states to immunize everyone identified in phase 1a, but only 5,919,418 people had received a first dose. Limited supply has necessitated prioritization of subgroups of HCP; those in the front of the line have varied by state, and even by hospital or health care systems within states. Both the American Academy of Pediatrics and the American Academy of Family Physicians have noted that primary care providers not employed by a hospital may have more difficulty accessing vaccine.
The mismatch between supply and demand has created an intense focus on improving supply and distribution. Soon though, we’re going to shift our attention to how we increase demand. We don’t have good data on those who being are offered COVID-19 vaccine and declining, but several studies that predate the Emergency Use Authorization for the Pfizer-BioNTech and Moderna vaccines suggest significant COVID-19 vaccine hesitancy among adults in the United States.
One large, longitudinal Internet-based study of U.S. adults found that the proportion who reported they were “somewhat or very likely” to receive COVID-19 vaccine declined from 74% in early April to 56% in early December.
In the Understanding America Study, self-reported likelihood of being vaccinated with COVID-19 vaccine was lower among Black compared to White respondents (38% vs. 59%; aRR, 0.7 [95% confidence interval, 0.6-0.8]), and lower among women compared to men (51% vs. 62%; aRR, 0.9 [95% CI, 0.8-0.9]). Those 65 years of age and older were more likely to report a willingness to be vaccinated than were those 18-49 years of age, as were those with at least a bachelor’s degree compared to those with a high school education or less.
A study conducted by the Pew Research Center in November – before any COVID-19 vaccines were available – found that only 60% of American adults said they would “definitely or probably get a vaccine for coronavirus” if one were available. That was an increase from 51% in September, but and overall decrease of 72% in May. Of the remaining 40%, just over half said they did not intend to get vaccinated and were “pretty certain” that more information would not change their minds.
Concern about acquiring a serious case of COVID-19 and trust in the vaccine development process were associated with an intent to receive vaccine, as was a personal history of receiving a flu shot annually. Willingness to be vaccinated varied by age, race, and family income, with Black respondents, women, and those with a lower family incomes less likely to accept a vaccine.
To date, few data are available about HCP and willingness to receive COVID-19 vaccine. A preprint posted at medrxiv.org reports on a cross-sectional study of more than 3,400 HCP surveyed between Oct. 7 and Nov. 9, 2020. In that study, only 36% of respondents voiced a willingness to be immunized as soon as vaccine is available. Vaccine acceptance increased with increasing age, income level, and education. As in other studies, self-reported willingness to accept vaccine was lower in women and Black individuals. While vaccine acceptance was higher in direct medical care providers than others, it was still only 49%.
So here’s the paradox: Even as limited supplies of vaccine are available and many are frustrated about lack of access, we need to promote the value of immunization to those who are hesitant. Pediatricians are trusted sources of vaccine information and we are in a good position to educate our colleagues, our staff, the parents of our patients and the community at-large.
A useful resource for those ready to take that step it is the CDC’s COVID-19 Vaccination Communication Toolkit. While this collection is designed to build vaccine confidence and promote immunization among health care providers, many of the strategies will be easily adapted for use with patients.
It’s not clear when we might have a COVID 19 vaccine for most children. The Pfizer-BioNTech vaccine emergency use authorization includes those as young as 16 years of age, and 16- and 17-year-olds with high risk medical conditions are included in phase 1c of vaccine allocation. Pfizer is currently enrolling children as young as 12 years of age in clinical trials, and Moderna and Janssen are poised to do the same. It is conceivable but far from certain that we could have a vaccine for children late this year. Are parents going to be ready to vaccinate their children?
Limited data about parental acceptance of vaccine for their children mirrors what was seen in the Understanding America Study and the Pew Research Study. In December 2020, the National Parents Union surveyed 1,008 parents of public school students enrolled in kindergarten through 12th grade. Sixty percent of parents said they would allow their children to receive a COVID-19 vaccine, while 25% would not and 15% were unsure. This suggests that now is the time to begin building vaccine confidence with parents. One conversation starter might be, “I am going to be vaccinated as soon as the vaccine is available.” Ideally, many of you will soon be able to say what I do: “I am excited to tell you that I have been immunized with the COVID-19 vaccine. I did this to protect myself, my family, and our community. I’m hopeful that vaccine will soon be available for all of us.”
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She said she had no relevant financial disclosures. Email her at pdnews@mdedge.com.
A shot of relief. A shot of hope. Those are the words used to describe COVID-19 vaccines on a television commercial running in prime time in Kentucky.
“We all can’t get the vaccine at once,” the announcer says solemnly, “but we’ll all get a turn.”
For some of us, that turn came quickly. In December, the Advisory Committee on Immunization Practices recommended that health care personnel (HCP) and long-term care facility residents be the first to be immunized with COVID-19 vaccines (see table). 
On Dec. 14, 2020, Sandra Lindsay, a nurse and director of patient care services in the intensive care unit at Long Island Jewish Medical Center, was the first person in the United States to receive a COVID-19 vaccine outside a clinical trial.
In subsequent days, social media sites were quickly flooded with photos of HCP rolling up their sleeves or flashing their immunization cards. There was jubilation ... and perhaps a little bit of jealousy. There were tears of joy and some tears of frustration.
There are more than 21 million HCP in the United States and to date, there have not been enough vaccines nor adequate infrastructure to immunize all of them. According to the Centers for Disease Control and Prevention Data Tracker, as of Jan. 7, 2021, 21,419,800 doses of vaccine had been distributed to states to immunize everyone identified in phase 1a, but only 5,919,418 people had received a first dose. Limited supply has necessitated prioritization of subgroups of HCP; those in the front of the line have varied by state, and even by hospital or health care systems within states. Both the American Academy of Pediatrics and the American Academy of Family Physicians have noted that primary care providers not employed by a hospital may have more difficulty accessing vaccine.
The mismatch between supply and demand has created an intense focus on improving supply and distribution. Soon though, we’re going to shift our attention to how we increase demand. We don’t have good data on those who being are offered COVID-19 vaccine and declining, but several studies that predate the Emergency Use Authorization for the Pfizer-BioNTech and Moderna vaccines suggest significant COVID-19 vaccine hesitancy among adults in the United States.
One large, longitudinal Internet-based study of U.S. adults found that the proportion who reported they were “somewhat or very likely” to receive COVID-19 vaccine declined from 74% in early April to 56% in early December.
In the Understanding America Study, self-reported likelihood of being vaccinated with COVID-19 vaccine was lower among Black compared to White respondents (38% vs. 59%; aRR, 0.7 [95% confidence interval, 0.6-0.8]), and lower among women compared to men (51% vs. 62%; aRR, 0.9 [95% CI, 0.8-0.9]). Those 65 years of age and older were more likely to report a willingness to be vaccinated than were those 18-49 years of age, as were those with at least a bachelor’s degree compared to those with a high school education or less.
A study conducted by the Pew Research Center in November – before any COVID-19 vaccines were available – found that only 60% of American adults said they would “definitely or probably get a vaccine for coronavirus” if one were available. That was an increase from 51% in September, but and overall decrease of 72% in May. Of the remaining 40%, just over half said they did not intend to get vaccinated and were “pretty certain” that more information would not change their minds.
Concern about acquiring a serious case of COVID-19 and trust in the vaccine development process were associated with an intent to receive vaccine, as was a personal history of receiving a flu shot annually. Willingness to be vaccinated varied by age, race, and family income, with Black respondents, women, and those with a lower family incomes less likely to accept a vaccine.
To date, few data are available about HCP and willingness to receive COVID-19 vaccine. A preprint posted at medrxiv.org reports on a cross-sectional study of more than 3,400 HCP surveyed between Oct. 7 and Nov. 9, 2020. In that study, only 36% of respondents voiced a willingness to be immunized as soon as vaccine is available. Vaccine acceptance increased with increasing age, income level, and education. As in other studies, self-reported willingness to accept vaccine was lower in women and Black individuals. While vaccine acceptance was higher in direct medical care providers than others, it was still only 49%.
So here’s the paradox: Even as limited supplies of vaccine are available and many are frustrated about lack of access, we need to promote the value of immunization to those who are hesitant. Pediatricians are trusted sources of vaccine information and we are in a good position to educate our colleagues, our staff, the parents of our patients and the community at-large.
A useful resource for those ready to take that step it is the CDC’s COVID-19 Vaccination Communication Toolkit. While this collection is designed to build vaccine confidence and promote immunization among health care providers, many of the strategies will be easily adapted for use with patients.
It’s not clear when we might have a COVID 19 vaccine for most children. The Pfizer-BioNTech vaccine emergency use authorization includes those as young as 16 years of age, and 16- and 17-year-olds with high risk medical conditions are included in phase 1c of vaccine allocation. Pfizer is currently enrolling children as young as 12 years of age in clinical trials, and Moderna and Janssen are poised to do the same. It is conceivable but far from certain that we could have a vaccine for children late this year. Are parents going to be ready to vaccinate their children?
Limited data about parental acceptance of vaccine for their children mirrors what was seen in the Understanding America Study and the Pew Research Study. In December 2020, the National Parents Union surveyed 1,008 parents of public school students enrolled in kindergarten through 12th grade. Sixty percent of parents said they would allow their children to receive a COVID-19 vaccine, while 25% would not and 15% were unsure. This suggests that now is the time to begin building vaccine confidence with parents. One conversation starter might be, “I am going to be vaccinated as soon as the vaccine is available.” Ideally, many of you will soon be able to say what I do: “I am excited to tell you that I have been immunized with the COVID-19 vaccine. I did this to protect myself, my family, and our community. I’m hopeful that vaccine will soon be available for all of us.”
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She said she had no relevant financial disclosures. Email her at pdnews@mdedge.com.
A shot of relief. A shot of hope. Those are the words used to describe COVID-19 vaccines on a television commercial running in prime time in Kentucky.
“We all can’t get the vaccine at once,” the announcer says solemnly, “but we’ll all get a turn.”
For some of us, that turn came quickly. In December, the Advisory Committee on Immunization Practices recommended that health care personnel (HCP) and long-term care facility residents be the first to be immunized with COVID-19 vaccines (see table). 
On Dec. 14, 2020, Sandra Lindsay, a nurse and director of patient care services in the intensive care unit at Long Island Jewish Medical Center, was the first person in the United States to receive a COVID-19 vaccine outside a clinical trial.
In subsequent days, social media sites were quickly flooded with photos of HCP rolling up their sleeves or flashing their immunization cards. There was jubilation ... and perhaps a little bit of jealousy. There were tears of joy and some tears of frustration.
There are more than 21 million HCP in the United States and to date, there have not been enough vaccines nor adequate infrastructure to immunize all of them. According to the Centers for Disease Control and Prevention Data Tracker, as of Jan. 7, 2021, 21,419,800 doses of vaccine had been distributed to states to immunize everyone identified in phase 1a, but only 5,919,418 people had received a first dose. Limited supply has necessitated prioritization of subgroups of HCP; those in the front of the line have varied by state, and even by hospital or health care systems within states. Both the American Academy of Pediatrics and the American Academy of Family Physicians have noted that primary care providers not employed by a hospital may have more difficulty accessing vaccine.
The mismatch between supply and demand has created an intense focus on improving supply and distribution. Soon though, we’re going to shift our attention to how we increase demand. We don’t have good data on those who being are offered COVID-19 vaccine and declining, but several studies that predate the Emergency Use Authorization for the Pfizer-BioNTech and Moderna vaccines suggest significant COVID-19 vaccine hesitancy among adults in the United States.
One large, longitudinal Internet-based study of U.S. adults found that the proportion who reported they were “somewhat or very likely” to receive COVID-19 vaccine declined from 74% in early April to 56% in early December.
In the Understanding America Study, self-reported likelihood of being vaccinated with COVID-19 vaccine was lower among Black compared to White respondents (38% vs. 59%; aRR, 0.7 [95% confidence interval, 0.6-0.8]), and lower among women compared to men (51% vs. 62%; aRR, 0.9 [95% CI, 0.8-0.9]). Those 65 years of age and older were more likely to report a willingness to be vaccinated than were those 18-49 years of age, as were those with at least a bachelor’s degree compared to those with a high school education or less.
A study conducted by the Pew Research Center in November – before any COVID-19 vaccines were available – found that only 60% of American adults said they would “definitely or probably get a vaccine for coronavirus” if one were available. That was an increase from 51% in September, but and overall decrease of 72% in May. Of the remaining 40%, just over half said they did not intend to get vaccinated and were “pretty certain” that more information would not change their minds.
Concern about acquiring a serious case of COVID-19 and trust in the vaccine development process were associated with an intent to receive vaccine, as was a personal history of receiving a flu shot annually. Willingness to be vaccinated varied by age, race, and family income, with Black respondents, women, and those with a lower family incomes less likely to accept a vaccine.
To date, few data are available about HCP and willingness to receive COVID-19 vaccine. A preprint posted at medrxiv.org reports on a cross-sectional study of more than 3,400 HCP surveyed between Oct. 7 and Nov. 9, 2020. In that study, only 36% of respondents voiced a willingness to be immunized as soon as vaccine is available. Vaccine acceptance increased with increasing age, income level, and education. As in other studies, self-reported willingness to accept vaccine was lower in women and Black individuals. While vaccine acceptance was higher in direct medical care providers than others, it was still only 49%.
So here’s the paradox: Even as limited supplies of vaccine are available and many are frustrated about lack of access, we need to promote the value of immunization to those who are hesitant. Pediatricians are trusted sources of vaccine information and we are in a good position to educate our colleagues, our staff, the parents of our patients and the community at-large.
A useful resource for those ready to take that step it is the CDC’s COVID-19 Vaccination Communication Toolkit. While this collection is designed to build vaccine confidence and promote immunization among health care providers, many of the strategies will be easily adapted for use with patients.
It’s not clear when we might have a COVID 19 vaccine for most children. The Pfizer-BioNTech vaccine emergency use authorization includes those as young as 16 years of age, and 16- and 17-year-olds with high risk medical conditions are included in phase 1c of vaccine allocation. Pfizer is currently enrolling children as young as 12 years of age in clinical trials, and Moderna and Janssen are poised to do the same. It is conceivable but far from certain that we could have a vaccine for children late this year. Are parents going to be ready to vaccinate their children?
Limited data about parental acceptance of vaccine for their children mirrors what was seen in the Understanding America Study and the Pew Research Study. In December 2020, the National Parents Union surveyed 1,008 parents of public school students enrolled in kindergarten through 12th grade. Sixty percent of parents said they would allow their children to receive a COVID-19 vaccine, while 25% would not and 15% were unsure. This suggests that now is the time to begin building vaccine confidence with parents. One conversation starter might be, “I am going to be vaccinated as soon as the vaccine is available.” Ideally, many of you will soon be able to say what I do: “I am excited to tell you that I have been immunized with the COVID-19 vaccine. I did this to protect myself, my family, and our community. I’m hopeful that vaccine will soon be available for all of us.”
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She said she had no relevant financial disclosures. Email her at pdnews@mdedge.com.




