User login
DMARDs may hamper pneumococcal vaccine response in systemic sclerosis patients
Patients taking disease-modifying antirheumatic medications for systemic sclerosis appear to have a decreased response to pneumococcal vaccines, a Swedish study has determined.
Those not taking disease-modifying antirheumatic medications (DMARDs), however, had a normal immune response, suggesting that it’s the immunomodulating medications, not the disease itself, that is affecting antibody levels, Roger Hesselstrand, MD, of Lund (Sweden) University and his colleagues reported online in Rheumatology.
“The currently recommended prime-boost vaccination strategy using a dose of PCV13 [13-valent pneumococcal conjugate vaccine] followed by a dose of PPV23 [23-valent pneumococcal polysaccharide vaccine] might be a possible way of enhancing the vaccine immunogenicity in immunosuppressed patients,” Dr. Hesselstrand and his coauthors wrote.
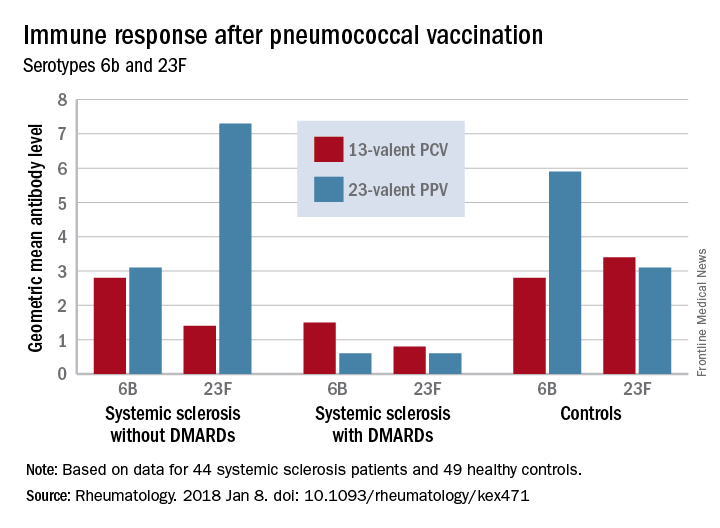
The study comprised 44 subjects with systemic sclerosis, 12 of whom were taking a DMARD (mycophenolate mofetil, azathioprine, or hydroxychloroquine), and 49 healthy controls; all underwent pneumococcal vaccination. The first 13 got a single dose of PPV23 intramuscularly. PCV13 was then licensed for adults in Sweden, and the remaining 31 patients received this vaccine. The primary outcome was 6-week change from baseline in the level of pneumococcal IgG to Streptococcus pneumoniae serotypes 23F and 6B.
Both vaccines were safe and well-tolerated by all patients, including those taking a DMARD.
Before vaccination, antibody levels to both serotypes were similar between the groups. After vaccination, antibody levels for both serotypes increased significantly in systemic sclerosis patients not taking a DMARD and in controls. However, patients taking a DMARD mounted only an adequate response to serotype 6B.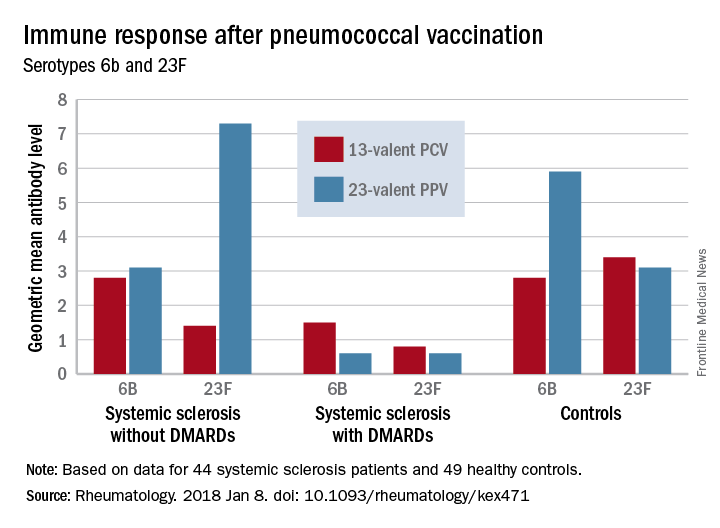
There were fewer responders among those taking DMARDs, whether they received the PCV13 or the PPV23 vaccine. An increase from prevaccination antibody levels of at least twofold occurred in fewer patients taking DMARDs than did in patients not taking DMARDs and in controls, regardless of vaccine type (PPV23, 50% vs. about 55% and 50%, respectively; PCV13, about 17% vs. 57% and 100%, respectively).
“We demonstrated that the antibody response ... as well as functionality of antibodies in [systemic sclerosis] patients not receiving DMARDs was as good as in controls regardless of vaccine type,” the investigators concluded. “Systemic sclerosis patients treated with DMARDs, however, had lower proportion of patients with positive antibody response, although the functionality of the antibodies was preserved. These results suggest that immunomodulating drugs but not systemic sclerosis itself and/or immunological disturbance as a part of this disease affect the ability to produce a sufficient amount of vaccine-specific antibodies, but not their function.”
None of the authors had conflicts of interest to disclose.
SOURCE: Hesselstrand R et al. Rheumatology [Oxford]. 2018 Jan 8. doi: 10.1093/rheumatology/kex471.
Patients taking disease-modifying antirheumatic medications for systemic sclerosis appear to have a decreased response to pneumococcal vaccines, a Swedish study has determined.
Those not taking disease-modifying antirheumatic medications (DMARDs), however, had a normal immune response, suggesting that it’s the immunomodulating medications, not the disease itself, that is affecting antibody levels, Roger Hesselstrand, MD, of Lund (Sweden) University and his colleagues reported online in Rheumatology.
“The currently recommended prime-boost vaccination strategy using a dose of PCV13 [13-valent pneumococcal conjugate vaccine] followed by a dose of PPV23 [23-valent pneumococcal polysaccharide vaccine] might be a possible way of enhancing the vaccine immunogenicity in immunosuppressed patients,” Dr. Hesselstrand and his coauthors wrote.
The study comprised 44 subjects with systemic sclerosis, 12 of whom were taking a DMARD (mycophenolate mofetil, azathioprine, or hydroxychloroquine), and 49 healthy controls; all underwent pneumococcal vaccination. The first 13 got a single dose of PPV23 intramuscularly. PCV13 was then licensed for adults in Sweden, and the remaining 31 patients received this vaccine. The primary outcome was 6-week change from baseline in the level of pneumococcal IgG to Streptococcus pneumoniae serotypes 23F and 6B.
Both vaccines were safe and well-tolerated by all patients, including those taking a DMARD.
Before vaccination, antibody levels to both serotypes were similar between the groups. After vaccination, antibody levels for both serotypes increased significantly in systemic sclerosis patients not taking a DMARD and in controls. However, patients taking a DMARD mounted only an adequate response to serotype 6B.
There were fewer responders among those taking DMARDs, whether they received the PCV13 or the PPV23 vaccine. An increase from prevaccination antibody levels of at least twofold occurred in fewer patients taking DMARDs than did in patients not taking DMARDs and in controls, regardless of vaccine type (PPV23, 50% vs. about 55% and 50%, respectively; PCV13, about 17% vs. 57% and 100%, respectively).
“We demonstrated that the antibody response ... as well as functionality of antibodies in [systemic sclerosis] patients not receiving DMARDs was as good as in controls regardless of vaccine type,” the investigators concluded. “Systemic sclerosis patients treated with DMARDs, however, had lower proportion of patients with positive antibody response, although the functionality of the antibodies was preserved. These results suggest that immunomodulating drugs but not systemic sclerosis itself and/or immunological disturbance as a part of this disease affect the ability to produce a sufficient amount of vaccine-specific antibodies, but not their function.”
None of the authors had conflicts of interest to disclose.
SOURCE: Hesselstrand R et al. Rheumatology [Oxford]. 2018 Jan 8. doi: 10.1093/rheumatology/kex471.
Patients taking disease-modifying antirheumatic medications for systemic sclerosis appear to have a decreased response to pneumococcal vaccines, a Swedish study has determined.
Those not taking disease-modifying antirheumatic medications (DMARDs), however, had a normal immune response, suggesting that it’s the immunomodulating medications, not the disease itself, that is affecting antibody levels, Roger Hesselstrand, MD, of Lund (Sweden) University and his colleagues reported online in Rheumatology.
“The currently recommended prime-boost vaccination strategy using a dose of PCV13 [13-valent pneumococcal conjugate vaccine] followed by a dose of PPV23 [23-valent pneumococcal polysaccharide vaccine] might be a possible way of enhancing the vaccine immunogenicity in immunosuppressed patients,” Dr. Hesselstrand and his coauthors wrote.
The study comprised 44 subjects with systemic sclerosis, 12 of whom were taking a DMARD (mycophenolate mofetil, azathioprine, or hydroxychloroquine), and 49 healthy controls; all underwent pneumococcal vaccination. The first 13 got a single dose of PPV23 intramuscularly. PCV13 was then licensed for adults in Sweden, and the remaining 31 patients received this vaccine. The primary outcome was 6-week change from baseline in the level of pneumococcal IgG to Streptococcus pneumoniae serotypes 23F and 6B.
Both vaccines were safe and well-tolerated by all patients, including those taking a DMARD.
Before vaccination, antibody levels to both serotypes were similar between the groups. After vaccination, antibody levels for both serotypes increased significantly in systemic sclerosis patients not taking a DMARD and in controls. However, patients taking a DMARD mounted only an adequate response to serotype 6B.
There were fewer responders among those taking DMARDs, whether they received the PCV13 or the PPV23 vaccine. An increase from prevaccination antibody levels of at least twofold occurred in fewer patients taking DMARDs than did in patients not taking DMARDs and in controls, regardless of vaccine type (PPV23, 50% vs. about 55% and 50%, respectively; PCV13, about 17% vs. 57% and 100%, respectively).
“We demonstrated that the antibody response ... as well as functionality of antibodies in [systemic sclerosis] patients not receiving DMARDs was as good as in controls regardless of vaccine type,” the investigators concluded. “Systemic sclerosis patients treated with DMARDs, however, had lower proportion of patients with positive antibody response, although the functionality of the antibodies was preserved. These results suggest that immunomodulating drugs but not systemic sclerosis itself and/or immunological disturbance as a part of this disease affect the ability to produce a sufficient amount of vaccine-specific antibodies, but not their function.”
None of the authors had conflicts of interest to disclose.
SOURCE: Hesselstrand R et al. Rheumatology [Oxford]. 2018 Jan 8. doi: 10.1093/rheumatology/kex471.
FROM RHEUMATOLOGY
Key clinical point:
Major finding: An increase in prevaccination antibody levels of at least twofold occurred in significantly fewer patients taking DMARDs than in patients not taking DMARDs and controls, regardless of vaccine type (PPV23, 50% vs. about 55% and 50%, respectively; PCV13, about 17% vs. 57% and 100%, respectively).
Study details: The prospective study comprised 44 systemic sclerosis patients and 49 healthy controls.
Disclosures: None of the authors had conflicts of interest to disclose.
Source: Hesselstrand R et al. Rheumatology [Oxford]. 2018 Jan 8. doi: 10.1093/rheumatology/kex471
Clinical Trial Begins for Long-Acting Anti-HIV Injectable
A clinical trial to test a new, potentially more convenient HIV prophylaxis for women is starting in Africa. It is a long-acting form of the investigational drug cabotegravir and could give sexually active women a choice of biomedical HIV prevention tools for the first time, similar to the choices available for contraception, says Sinead Delany-Moretiwe, PhD, chair of the protocol.
The trial, HPTN 084, will enroll about 3,200 women aged 18 to 45 years at 20 sites in 7 countries. The women will be randomly assigned to either cabotegravir and a placebo pill or Truvada, which is a combination of emtricitabine and tenofovir disoproxil fumarate. Truvada, currently the only drug licensed for HIV pre-exposure prophylaxis, must be taken every day to achieve and maintain protective drug concentrations. The women will start with 2 cabotegravir injections 4 weeks apart, then receive injections once every 8 weeks for an average of 2.6 years. After completing the injections, participants will be offered 48 weeks of PrEP with daily oral Truvada.
The NIAID is sponsoring the phase 3 clinical trial and cofunding it in a unique partnership with ViiV Healthcare (which is providing the study medications with Gilead Sciences) and the Bill & Melinda Gates Foundation.
Participants will receive HIV prevention counseling, condoms and lubricant, and counseling to support adherence to the daily pill. Anyone who becomes HIV infected during the trial will stop receiving the study products and be referred to local medical providers for care and treatment.
The study also will evaluate how women experience long-acting injectable cabotegravir, the researchers say. They are hoping to get a better understanding of the types of safe and effective HIV prevention that also fit best in women’s lives.
A clinical trial to test a new, potentially more convenient HIV prophylaxis for women is starting in Africa. It is a long-acting form of the investigational drug cabotegravir and could give sexually active women a choice of biomedical HIV prevention tools for the first time, similar to the choices available for contraception, says Sinead Delany-Moretiwe, PhD, chair of the protocol.
The trial, HPTN 084, will enroll about 3,200 women aged 18 to 45 years at 20 sites in 7 countries. The women will be randomly assigned to either cabotegravir and a placebo pill or Truvada, which is a combination of emtricitabine and tenofovir disoproxil fumarate. Truvada, currently the only drug licensed for HIV pre-exposure prophylaxis, must be taken every day to achieve and maintain protective drug concentrations. The women will start with 2 cabotegravir injections 4 weeks apart, then receive injections once every 8 weeks for an average of 2.6 years. After completing the injections, participants will be offered 48 weeks of PrEP with daily oral Truvada.
The NIAID is sponsoring the phase 3 clinical trial and cofunding it in a unique partnership with ViiV Healthcare (which is providing the study medications with Gilead Sciences) and the Bill & Melinda Gates Foundation.
Participants will receive HIV prevention counseling, condoms and lubricant, and counseling to support adherence to the daily pill. Anyone who becomes HIV infected during the trial will stop receiving the study products and be referred to local medical providers for care and treatment.
The study also will evaluate how women experience long-acting injectable cabotegravir, the researchers say. They are hoping to get a better understanding of the types of safe and effective HIV prevention that also fit best in women’s lives.
A clinical trial to test a new, potentially more convenient HIV prophylaxis for women is starting in Africa. It is a long-acting form of the investigational drug cabotegravir and could give sexually active women a choice of biomedical HIV prevention tools for the first time, similar to the choices available for contraception, says Sinead Delany-Moretiwe, PhD, chair of the protocol.
The trial, HPTN 084, will enroll about 3,200 women aged 18 to 45 years at 20 sites in 7 countries. The women will be randomly assigned to either cabotegravir and a placebo pill or Truvada, which is a combination of emtricitabine and tenofovir disoproxil fumarate. Truvada, currently the only drug licensed for HIV pre-exposure prophylaxis, must be taken every day to achieve and maintain protective drug concentrations. The women will start with 2 cabotegravir injections 4 weeks apart, then receive injections once every 8 weeks for an average of 2.6 years. After completing the injections, participants will be offered 48 weeks of PrEP with daily oral Truvada.
The NIAID is sponsoring the phase 3 clinical trial and cofunding it in a unique partnership with ViiV Healthcare (which is providing the study medications with Gilead Sciences) and the Bill & Melinda Gates Foundation.
Participants will receive HIV prevention counseling, condoms and lubricant, and counseling to support adherence to the daily pill. Anyone who becomes HIV infected during the trial will stop receiving the study products and be referred to local medical providers for care and treatment.
The study also will evaluate how women experience long-acting injectable cabotegravir, the researchers say. They are hoping to get a better understanding of the types of safe and effective HIV prevention that also fit best in women’s lives.
Dental Health: What It Means in Kidney Disease
Q) I teach nephrology at a local PA program, and they want us to integrate dental care into each module. What’s the connection between the two?
Dental health is frequently overlooked in the medical realm, as many clinicians feel that dental issues are out of our purview. Hematuria worries us, but bleeding gums and other signs of periodontal disease are often ignored. Surprisingly, many patients don’t seem to mind when their gums bleed every time they brush; they believe that this is normal, when really, it’s not.
Growing evidence supports associations between dental health and multiple medical issues—chronic kidney disease (CKD) among them. Periodontal disease is one of several inflammatory diseases caused by an interaction between gram-negative periodontal bacterial species and the immune system. It manifests with sore, red, bleeding gums and can lead to tooth loss if left untreated.
Chronic inflammation in the gums is a good indicator of inflammation elsewhere in the body. In and of itself, periodontitis can set off an inflammatory cascade in the body. Poor dentition can also lead to poor nutrition, which then causes a feedback loop, leading to even more inflammation.
Patients with periodontal disease have higher levels of C-reactive protein and a higher erythrocyte sedimentation rate than those without the disease.1 And a recent study by Zhang et al showed that periodontal disease increased risk for all-cause mortality in patients with CKD.2
The high cost of CKD from both a financial and personal view makes any intervention worth exploring, as the risk factors are difficult to modify and the CKD population is growing worldwide. We, as medical providers, should reiterate what our dental colleagues have been saying for years: Encourage patients with CKD to practice good dental hygiene by brushing twice a day and flossing daily, in an attempt to improve their overall outcomes.
LCDR Julie Taylor, PA-C
United States Public Health Service, Boston
1. Zhang J, Jiang H, Sun M, Chen J. Association between periodontal disease and mortality in people with CKD: a meta-analysis of cohort studies. BMC Nephrol. 2017;18(1):269.
2. Chen YT, Shin CJ, Ou SM, et al; Taiwan Geriatric Kidney Disease (TGKD) Research Group. Periodontal disease and risks of kidney function decline and mortality in older people: a community-based cohort study. Am J Kidney Dis. 2015; 66(2):223-230.
Q) I teach nephrology at a local PA program, and they want us to integrate dental care into each module. What’s the connection between the two?
Dental health is frequently overlooked in the medical realm, as many clinicians feel that dental issues are out of our purview. Hematuria worries us, but bleeding gums and other signs of periodontal disease are often ignored. Surprisingly, many patients don’t seem to mind when their gums bleed every time they brush; they believe that this is normal, when really, it’s not.
Growing evidence supports associations between dental health and multiple medical issues—chronic kidney disease (CKD) among them. Periodontal disease is one of several inflammatory diseases caused by an interaction between gram-negative periodontal bacterial species and the immune system. It manifests with sore, red, bleeding gums and can lead to tooth loss if left untreated.
Chronic inflammation in the gums is a good indicator of inflammation elsewhere in the body. In and of itself, periodontitis can set off an inflammatory cascade in the body. Poor dentition can also lead to poor nutrition, which then causes a feedback loop, leading to even more inflammation.
Patients with periodontal disease have higher levels of C-reactive protein and a higher erythrocyte sedimentation rate than those without the disease.1 And a recent study by Zhang et al showed that periodontal disease increased risk for all-cause mortality in patients with CKD.2
The high cost of CKD from both a financial and personal view makes any intervention worth exploring, as the risk factors are difficult to modify and the CKD population is growing worldwide. We, as medical providers, should reiterate what our dental colleagues have been saying for years: Encourage patients with CKD to practice good dental hygiene by brushing twice a day and flossing daily, in an attempt to improve their overall outcomes.
LCDR Julie Taylor, PA-C
United States Public Health Service, Boston
Q) I teach nephrology at a local PA program, and they want us to integrate dental care into each module. What’s the connection between the two?
Dental health is frequently overlooked in the medical realm, as many clinicians feel that dental issues are out of our purview. Hematuria worries us, but bleeding gums and other signs of periodontal disease are often ignored. Surprisingly, many patients don’t seem to mind when their gums bleed every time they brush; they believe that this is normal, when really, it’s not.
Growing evidence supports associations between dental health and multiple medical issues—chronic kidney disease (CKD) among them. Periodontal disease is one of several inflammatory diseases caused by an interaction between gram-negative periodontal bacterial species and the immune system. It manifests with sore, red, bleeding gums and can lead to tooth loss if left untreated.
Chronic inflammation in the gums is a good indicator of inflammation elsewhere in the body. In and of itself, periodontitis can set off an inflammatory cascade in the body. Poor dentition can also lead to poor nutrition, which then causes a feedback loop, leading to even more inflammation.
Patients with periodontal disease have higher levels of C-reactive protein and a higher erythrocyte sedimentation rate than those without the disease.1 And a recent study by Zhang et al showed that periodontal disease increased risk for all-cause mortality in patients with CKD.2
The high cost of CKD from both a financial and personal view makes any intervention worth exploring, as the risk factors are difficult to modify and the CKD population is growing worldwide. We, as medical providers, should reiterate what our dental colleagues have been saying for years: Encourage patients with CKD to practice good dental hygiene by brushing twice a day and flossing daily, in an attempt to improve their overall outcomes.
LCDR Julie Taylor, PA-C
United States Public Health Service, Boston
1. Zhang J, Jiang H, Sun M, Chen J. Association between periodontal disease and mortality in people with CKD: a meta-analysis of cohort studies. BMC Nephrol. 2017;18(1):269.
2. Chen YT, Shin CJ, Ou SM, et al; Taiwan Geriatric Kidney Disease (TGKD) Research Group. Periodontal disease and risks of kidney function decline and mortality in older people: a community-based cohort study. Am J Kidney Dis. 2015; 66(2):223-230.
1. Zhang J, Jiang H, Sun M, Chen J. Association between periodontal disease and mortality in people with CKD: a meta-analysis of cohort studies. BMC Nephrol. 2017;18(1):269.
2. Chen YT, Shin CJ, Ou SM, et al; Taiwan Geriatric Kidney Disease (TGKD) Research Group. Periodontal disease and risks of kidney function decline and mortality in older people: a community-based cohort study. Am J Kidney Dis. 2015; 66(2):223-230.
Novel herpes zoster vaccine is more cost effective than old vaccine
The novel herpes zoster subunit vaccine (HZ/su) is more effective and less expensive than the currently used live attenuated virus (ZVL), according to a study from the Center for Value-Based Research.
Phuc Le, PhD, of the Cleveland Clinic and her colleague Michael Rothberg, MD, conducted an economic analysis of vaccine strategies from the societal perspective. This included the direct medical costs and productivity losses associated with HZ disease and complications.
The one-, two-, and three-way sensitivity analyses examined how different variables affected the cost-effectiveness of different vaccine strategies. The one-way analysis examined the association of input variables and cost-effectiveness. This included HZ/su prices, waning rate and initial efficacy of a dose of HZ/su, and the adherence rate. This analysis revealed that, compared with no vaccination, HZ/su would provide cost savings up to a price of $160, or $80 per dose.
Regardless of circumstance, HZ/su was always more effective than ZVL according to the two-way sensitivity analysis. This analysis took into account the joint effect of price, adherence to two doses of HZ/su, efficacy, and the waning rate of one dose and two doses of HZ/su and ZVL. Compared with ZVL, HZ/su would be less costly up to a price of $350 per series.
Adherence rates to vaccination schedules were important in determining the efficacy and waning rate which ultimately effected cost-effectiveness. The three-way sensitivity analysis found that, if HZ/su adherence to the second dose was greater than 56.8%, results were insensitive to the variation of single-dose efficacy and waning rate of HZ/su. But if adherence rates fell below 40%, combinations of waning rate and lower efficacy made HZ/su cost ineffective. Most importantly, ZVL was never cost effective for 60-year-old patients.
Despite a projected price of $280 per series, HZ/su is still more effective and less expensive than ZVL for adults 60 years or older. According to Dr. Le and Dr. Rothberg, the assumptions about the vaccine’s efficacy duration and price were reasonable. But, if the vaccine price were to rise in the future, or a single dose becomes much less effective than reported by GlaxoSmithKline, or if adherence to the second dose was remarkably low, the results of the study would be changed.
“An ACIP recommendation stating a preference for HZ/su over ZVL could lead to future price increases, which would render the vaccine no longer cost effective” wrote Dr. Le and Dr. Rothberg. “Therefore, a recommendation linked to periodic reassessment of cost-effectiveness based on the vaccine price might help to mitigate the effect of the recommendation on vaccine affordability.”
Dr. Le and Dr. Rothberg reported having no conflicts of interest.
The HZ/su vaccine is not yet approved by the U.S. Food and Drug Administration, but in 2017, the FDA Advisory Committee unanimously voted in favor of its use in adults age 50 years and older.
SOURCE: Phuc L et al. JAMA Intern Med. 2018 Jan 2. doi: 10.1001/jamainternmed.2017.7431. Najafzadeh M. JAMA Intern Med. 2018. doi: 10.1001/jamainternmed.2017.7442.
The herpes zoster (HZ) virus disproportionately affects elderly populations. As the U.S. population ages, tools and mechanisms to reduce the clinical and economic burden of HZ will be needed in the coming years.
The work of Dr. Le and Dr. Rothberg presents the results of an economic evaluation on randomized clinical trials of a yet-to-be-approved novel HZ subunit vaccine (HZ/su) to determine the economic and clinical benefit of the new vaccine, compared with the currently used vaccine. Although HZ/su is intended to be a two-dose vaccine, the study focused on a one-time vaccine strategy because booster vaccines are unpopular and not recommended by the Advisory Committee on Immunization Practices.
“If priced at $280 per two required doses, HZ/su appears to be a cost-saving option, compared with ZVL and a cost-effective option, compared with no-vaccine strategies,” wrote Dr. Najafzadeh. “However, the value of HZ/su vaccine would be even higher if it could be marketed at a price comparable to that of ZVL.” An added benefit of the HZ/su vaccine is that it can be used in immunocompromised patients.
Mehdi Najafzadeh, PhD , is an instructor in medicine at Harvard Medical School, Boston. He also serves as an associate statistician/epidemiologist in the division of pharmacoepidemiology and pharmacoeconomics at Brigham and Women’s Hospital, Boston.
The herpes zoster (HZ) virus disproportionately affects elderly populations. As the U.S. population ages, tools and mechanisms to reduce the clinical and economic burden of HZ will be needed in the coming years.
The work of Dr. Le and Dr. Rothberg presents the results of an economic evaluation on randomized clinical trials of a yet-to-be-approved novel HZ subunit vaccine (HZ/su) to determine the economic and clinical benefit of the new vaccine, compared with the currently used vaccine. Although HZ/su is intended to be a two-dose vaccine, the study focused on a one-time vaccine strategy because booster vaccines are unpopular and not recommended by the Advisory Committee on Immunization Practices.
“If priced at $280 per two required doses, HZ/su appears to be a cost-saving option, compared with ZVL and a cost-effective option, compared with no-vaccine strategies,” wrote Dr. Najafzadeh. “However, the value of HZ/su vaccine would be even higher if it could be marketed at a price comparable to that of ZVL.” An added benefit of the HZ/su vaccine is that it can be used in immunocompromised patients.
Mehdi Najafzadeh, PhD , is an instructor in medicine at Harvard Medical School, Boston. He also serves as an associate statistician/epidemiologist in the division of pharmacoepidemiology and pharmacoeconomics at Brigham and Women’s Hospital, Boston.
The herpes zoster (HZ) virus disproportionately affects elderly populations. As the U.S. population ages, tools and mechanisms to reduce the clinical and economic burden of HZ will be needed in the coming years.
The work of Dr. Le and Dr. Rothberg presents the results of an economic evaluation on randomized clinical trials of a yet-to-be-approved novel HZ subunit vaccine (HZ/su) to determine the economic and clinical benefit of the new vaccine, compared with the currently used vaccine. Although HZ/su is intended to be a two-dose vaccine, the study focused on a one-time vaccine strategy because booster vaccines are unpopular and not recommended by the Advisory Committee on Immunization Practices.
“If priced at $280 per two required doses, HZ/su appears to be a cost-saving option, compared with ZVL and a cost-effective option, compared with no-vaccine strategies,” wrote Dr. Najafzadeh. “However, the value of HZ/su vaccine would be even higher if it could be marketed at a price comparable to that of ZVL.” An added benefit of the HZ/su vaccine is that it can be used in immunocompromised patients.
Mehdi Najafzadeh, PhD , is an instructor in medicine at Harvard Medical School, Boston. He also serves as an associate statistician/epidemiologist in the division of pharmacoepidemiology and pharmacoeconomics at Brigham and Women’s Hospital, Boston.
The novel herpes zoster subunit vaccine (HZ/su) is more effective and less expensive than the currently used live attenuated virus (ZVL), according to a study from the Center for Value-Based Research.
Phuc Le, PhD, of the Cleveland Clinic and her colleague Michael Rothberg, MD, conducted an economic analysis of vaccine strategies from the societal perspective. This included the direct medical costs and productivity losses associated with HZ disease and complications.
The one-, two-, and three-way sensitivity analyses examined how different variables affected the cost-effectiveness of different vaccine strategies. The one-way analysis examined the association of input variables and cost-effectiveness. This included HZ/su prices, waning rate and initial efficacy of a dose of HZ/su, and the adherence rate. This analysis revealed that, compared with no vaccination, HZ/su would provide cost savings up to a price of $160, or $80 per dose.
Regardless of circumstance, HZ/su was always more effective than ZVL according to the two-way sensitivity analysis. This analysis took into account the joint effect of price, adherence to two doses of HZ/su, efficacy, and the waning rate of one dose and two doses of HZ/su and ZVL. Compared with ZVL, HZ/su would be less costly up to a price of $350 per series.
Adherence rates to vaccination schedules were important in determining the efficacy and waning rate which ultimately effected cost-effectiveness. The three-way sensitivity analysis found that, if HZ/su adherence to the second dose was greater than 56.8%, results were insensitive to the variation of single-dose efficacy and waning rate of HZ/su. But if adherence rates fell below 40%, combinations of waning rate and lower efficacy made HZ/su cost ineffective. Most importantly, ZVL was never cost effective for 60-year-old patients.
Despite a projected price of $280 per series, HZ/su is still more effective and less expensive than ZVL for adults 60 years or older. According to Dr. Le and Dr. Rothberg, the assumptions about the vaccine’s efficacy duration and price were reasonable. But, if the vaccine price were to rise in the future, or a single dose becomes much less effective than reported by GlaxoSmithKline, or if adherence to the second dose was remarkably low, the results of the study would be changed.
“An ACIP recommendation stating a preference for HZ/su over ZVL could lead to future price increases, which would render the vaccine no longer cost effective” wrote Dr. Le and Dr. Rothberg. “Therefore, a recommendation linked to periodic reassessment of cost-effectiveness based on the vaccine price might help to mitigate the effect of the recommendation on vaccine affordability.”
Dr. Le and Dr. Rothberg reported having no conflicts of interest.
The HZ/su vaccine is not yet approved by the U.S. Food and Drug Administration, but in 2017, the FDA Advisory Committee unanimously voted in favor of its use in adults age 50 years and older.
SOURCE: Phuc L et al. JAMA Intern Med. 2018 Jan 2. doi: 10.1001/jamainternmed.2017.7431. Najafzadeh M. JAMA Intern Med. 2018. doi: 10.1001/jamainternmed.2017.7442.
The novel herpes zoster subunit vaccine (HZ/su) is more effective and less expensive than the currently used live attenuated virus (ZVL), according to a study from the Center for Value-Based Research.
Phuc Le, PhD, of the Cleveland Clinic and her colleague Michael Rothberg, MD, conducted an economic analysis of vaccine strategies from the societal perspective. This included the direct medical costs and productivity losses associated with HZ disease and complications.
The one-, two-, and three-way sensitivity analyses examined how different variables affected the cost-effectiveness of different vaccine strategies. The one-way analysis examined the association of input variables and cost-effectiveness. This included HZ/su prices, waning rate and initial efficacy of a dose of HZ/su, and the adherence rate. This analysis revealed that, compared with no vaccination, HZ/su would provide cost savings up to a price of $160, or $80 per dose.
Regardless of circumstance, HZ/su was always more effective than ZVL according to the two-way sensitivity analysis. This analysis took into account the joint effect of price, adherence to two doses of HZ/su, efficacy, and the waning rate of one dose and two doses of HZ/su and ZVL. Compared with ZVL, HZ/su would be less costly up to a price of $350 per series.
Adherence rates to vaccination schedules were important in determining the efficacy and waning rate which ultimately effected cost-effectiveness. The three-way sensitivity analysis found that, if HZ/su adherence to the second dose was greater than 56.8%, results were insensitive to the variation of single-dose efficacy and waning rate of HZ/su. But if adherence rates fell below 40%, combinations of waning rate and lower efficacy made HZ/su cost ineffective. Most importantly, ZVL was never cost effective for 60-year-old patients.
Despite a projected price of $280 per series, HZ/su is still more effective and less expensive than ZVL for adults 60 years or older. According to Dr. Le and Dr. Rothberg, the assumptions about the vaccine’s efficacy duration and price were reasonable. But, if the vaccine price were to rise in the future, or a single dose becomes much less effective than reported by GlaxoSmithKline, or if adherence to the second dose was remarkably low, the results of the study would be changed.
“An ACIP recommendation stating a preference for HZ/su over ZVL could lead to future price increases, which would render the vaccine no longer cost effective” wrote Dr. Le and Dr. Rothberg. “Therefore, a recommendation linked to periodic reassessment of cost-effectiveness based on the vaccine price might help to mitigate the effect of the recommendation on vaccine affordability.”
Dr. Le and Dr. Rothberg reported having no conflicts of interest.
The HZ/su vaccine is not yet approved by the U.S. Food and Drug Administration, but in 2017, the FDA Advisory Committee unanimously voted in favor of its use in adults age 50 years and older.
SOURCE: Phuc L et al. JAMA Intern Med. 2018 Jan 2. doi: 10.1001/jamainternmed.2017.7431. Najafzadeh M. JAMA Intern Med. 2018. doi: 10.1001/jamainternmed.2017.7442.
FROM JAMA INTERNAL MEDICINE
Key clinical point: Cost-effectiveness can be an important factor in considering patient treatment for herpes zoster.
Major finding:
Study details: The study was based on U.S. medical literature. Data were derived from adults 60 years or older in patient groups of 100-30,000 from July 1 to July 31, 2017.
Disclosures: None of the researchers had conflicts of interest to report.
Source: L. Phuc et al. JAMA Intern Med. 2018 Jan 2. doi: 10.1001/jamainternmed.2017.7431; Najafzadeh M. JAMA Intern Med. 2018. doi: 10.1001/jamainternmed.2017.7442.
For Patients With CKD, Don’t Wait—Vaccinate!
Q) What can I tell my kidney patients to increase acceptance of the influenza and pneumonia vaccines during cold and flu season?
The CDC recommends that everyone ages 6 months and older receive an annual flu vaccination, unless contraindicated.1 Additionally, administration of either the 13-valent pneumococcal conjugate vaccine (PCV13) or the 23-valent pneumococcal polysaccharide vaccine (PPSV23) is recommended for all adults ages 65 and older and for younger adults (ages 19 to 64) with diabetes, chronic kidney disease (CKD), chronic heart disease, and/or solid organ transplant.1 Despite these recommendations, patients often decline vaccination. What they may not realize is that CKD increases their risk for infection.
In a cohort of more than 1 million Swedish patients, researchers found that any stage of CKD increased risk for community-acquired infection and that the risk for lower respiratory tract infection increased as glomerular filtration rate declined.2 Patients on hemodialysis have an increased risk for pneumonia and an incidence of pneumonia-related mortality that is up to 16 times higher than that of the general population.3 Pneumonia also increases the risk for cardiovascular events among all patients with CKD, regardless of stage.4
So, can vaccines reduce these risks in our kidney patients? McGrath and colleagues found that patients with end-stage renal disease (ESRD) who were vaccinated against the flu had lower mortality rates than those who were not vaccinated—even when the vaccine was poorly matched to the circulating virus strain.5 Additional research has demonstrated that for patients with any stage of CKD, including those on dialysis, the flu vaccine is safe and effective, and its protection may be durable over time.6
For pneumonia vaccines, antibody response in patients with CKD may be suboptimal; however, Medicare data have demonstrated that patients with ESRD who are vaccinated against pneumonia have lower rates of all-cause and cardiovascular mortality than unvaccinated patients do.5 Given their increased vulnerability to vaccine-preventable respiratory illnesses, it is imperative that our kidney patients receive both the flu and pneumonia vaccines.
Nicole DeFeo McCormick, DNP, MBA, NP-C, CCTC
Assistant Professor
School of Medicine at the University of Colorado
1. CDC. Recommended immunization schedule for adults aged 19 years or older, United States, 2017. www.cdc.gov/vaccines/schedules/hcp/index.html. Accessed November 22, 2017.
2. Xu H, Gasparini A, Ishigami J, et al. eGFR and the risk of community-acquired infections. Clin J Am Soc Nephrol. 2017; 12(9):1399-1408.
3. Sarnak MJ, Jaber BL. Pulmonary infectious mortality among patients with end-stage renal disease. Chest. 2001;120(6): 1883-1887.
4. Mathew R, Mason D, Kennedy JS. Vaccination issues in patients with chronic kidney disease. Expert Rev Vaccines. 2014;13(2):285-298.
5. McGrath LJ, Kshirsagar AV, Cole SR, et al. Evaluating influenza vaccine effectiveness among hemodialysis patients using a natural experiment. Arch Intern Med. 2012;172(7): 548-554.
6. Janus N, Vacher L, Karie S, et al. Vaccination and chronic kidney disease. Nephrol Dial Transplant. 2008;23(3):800-807.
Q) What can I tell my kidney patients to increase acceptance of the influenza and pneumonia vaccines during cold and flu season?
The CDC recommends that everyone ages 6 months and older receive an annual flu vaccination, unless contraindicated.1 Additionally, administration of either the 13-valent pneumococcal conjugate vaccine (PCV13) or the 23-valent pneumococcal polysaccharide vaccine (PPSV23) is recommended for all adults ages 65 and older and for younger adults (ages 19 to 64) with diabetes, chronic kidney disease (CKD), chronic heart disease, and/or solid organ transplant.1 Despite these recommendations, patients often decline vaccination. What they may not realize is that CKD increases their risk for infection.
In a cohort of more than 1 million Swedish patients, researchers found that any stage of CKD increased risk for community-acquired infection and that the risk for lower respiratory tract infection increased as glomerular filtration rate declined.2 Patients on hemodialysis have an increased risk for pneumonia and an incidence of pneumonia-related mortality that is up to 16 times higher than that of the general population.3 Pneumonia also increases the risk for cardiovascular events among all patients with CKD, regardless of stage.4
So, can vaccines reduce these risks in our kidney patients? McGrath and colleagues found that patients with end-stage renal disease (ESRD) who were vaccinated against the flu had lower mortality rates than those who were not vaccinated—even when the vaccine was poorly matched to the circulating virus strain.5 Additional research has demonstrated that for patients with any stage of CKD, including those on dialysis, the flu vaccine is safe and effective, and its protection may be durable over time.6
For pneumonia vaccines, antibody response in patients with CKD may be suboptimal; however, Medicare data have demonstrated that patients with ESRD who are vaccinated against pneumonia have lower rates of all-cause and cardiovascular mortality than unvaccinated patients do.5 Given their increased vulnerability to vaccine-preventable respiratory illnesses, it is imperative that our kidney patients receive both the flu and pneumonia vaccines.
Nicole DeFeo McCormick, DNP, MBA, NP-C, CCTC
Assistant Professor
School of Medicine at the University of Colorado
Q) What can I tell my kidney patients to increase acceptance of the influenza and pneumonia vaccines during cold and flu season?
The CDC recommends that everyone ages 6 months and older receive an annual flu vaccination, unless contraindicated.1 Additionally, administration of either the 13-valent pneumococcal conjugate vaccine (PCV13) or the 23-valent pneumococcal polysaccharide vaccine (PPSV23) is recommended for all adults ages 65 and older and for younger adults (ages 19 to 64) with diabetes, chronic kidney disease (CKD), chronic heart disease, and/or solid organ transplant.1 Despite these recommendations, patients often decline vaccination. What they may not realize is that CKD increases their risk for infection.
In a cohort of more than 1 million Swedish patients, researchers found that any stage of CKD increased risk for community-acquired infection and that the risk for lower respiratory tract infection increased as glomerular filtration rate declined.2 Patients on hemodialysis have an increased risk for pneumonia and an incidence of pneumonia-related mortality that is up to 16 times higher than that of the general population.3 Pneumonia also increases the risk for cardiovascular events among all patients with CKD, regardless of stage.4
So, can vaccines reduce these risks in our kidney patients? McGrath and colleagues found that patients with end-stage renal disease (ESRD) who were vaccinated against the flu had lower mortality rates than those who were not vaccinated—even when the vaccine was poorly matched to the circulating virus strain.5 Additional research has demonstrated that for patients with any stage of CKD, including those on dialysis, the flu vaccine is safe and effective, and its protection may be durable over time.6
For pneumonia vaccines, antibody response in patients with CKD may be suboptimal; however, Medicare data have demonstrated that patients with ESRD who are vaccinated against pneumonia have lower rates of all-cause and cardiovascular mortality than unvaccinated patients do.5 Given their increased vulnerability to vaccine-preventable respiratory illnesses, it is imperative that our kidney patients receive both the flu and pneumonia vaccines.
Nicole DeFeo McCormick, DNP, MBA, NP-C, CCTC
Assistant Professor
School of Medicine at the University of Colorado
1. CDC. Recommended immunization schedule for adults aged 19 years or older, United States, 2017. www.cdc.gov/vaccines/schedules/hcp/index.html. Accessed November 22, 2017.
2. Xu H, Gasparini A, Ishigami J, et al. eGFR and the risk of community-acquired infections. Clin J Am Soc Nephrol. 2017; 12(9):1399-1408.
3. Sarnak MJ, Jaber BL. Pulmonary infectious mortality among patients with end-stage renal disease. Chest. 2001;120(6): 1883-1887.
4. Mathew R, Mason D, Kennedy JS. Vaccination issues in patients with chronic kidney disease. Expert Rev Vaccines. 2014;13(2):285-298.
5. McGrath LJ, Kshirsagar AV, Cole SR, et al. Evaluating influenza vaccine effectiveness among hemodialysis patients using a natural experiment. Arch Intern Med. 2012;172(7): 548-554.
6. Janus N, Vacher L, Karie S, et al. Vaccination and chronic kidney disease. Nephrol Dial Transplant. 2008;23(3):800-807.
1. CDC. Recommended immunization schedule for adults aged 19 years or older, United States, 2017. www.cdc.gov/vaccines/schedules/hcp/index.html. Accessed November 22, 2017.
2. Xu H, Gasparini A, Ishigami J, et al. eGFR and the risk of community-acquired infections. Clin J Am Soc Nephrol. 2017; 12(9):1399-1408.
3. Sarnak MJ, Jaber BL. Pulmonary infectious mortality among patients with end-stage renal disease. Chest. 2001;120(6): 1883-1887.
4. Mathew R, Mason D, Kennedy JS. Vaccination issues in patients with chronic kidney disease. Expert Rev Vaccines. 2014;13(2):285-298.
5. McGrath LJ, Kshirsagar AV, Cole SR, et al. Evaluating influenza vaccine effectiveness among hemodialysis patients using a natural experiment. Arch Intern Med. 2012;172(7): 548-554.
6. Janus N, Vacher L, Karie S, et al. Vaccination and chronic kidney disease. Nephrol Dial Transplant. 2008;23(3):800-807.
Don’t give up on influenza vaccine
I suspect most health care providers have heard the complaint, “The vaccine doesn’t work. One year I got the vaccine, and I still came down with the flu.”
Over the years, I’ve polished my responses to vaccine naysayers.
Influenza vaccine doesn’t protect you against every virus that can cause cold and flu symptoms. It only prevents influenza. It’s possible you had a different virus, such as adenovirus, coronavirus, parainfluenza virus, or respiratory syncytial virus.
Some years, the vaccine works better than others because there is a mismatch between the viruses chosen for the vaccine, and the viruses that end up circulating. Even when it doesn’t prevent flu, the vaccine can potentially reduce the severity of illness.
The discussion became a little more complicated in 2016 when the Centers for Disease Control and Prevention Advisory Committee on Immunization Practices withdrew its support for the live attenuated influenza virus vaccine (LAIV4) because of concerns about effectiveness. During the 2015-2016 influenza season, LAIV4 demonstrated no statistically significant effectiveness in children 2-17 years of age against H1N1pdm09, the predominant influenza strain. Fortunately, inactivated injectable vaccine did offer protection. An estimated 41.8 million children aged 6 months to 17 years ultimately received this vaccine during the 2016-2017 influenza season.
Now with the 2017-2018 influenza season in full swing, some media reports are proclaiming the influenza vaccine is only 10% effective this year. This claim is based on an interim analysis of data from the most recent flu season in Australia and the effectiveness of the vaccine against the circulating H3N2 virus strain. News from the U.S. CDC is more encouraging. The H3N2 virus contained in this year’s vaccine is the same as that used last year, and so far, circulating H3N2 viruses in the United States are similar to the vaccine virus. Public health officials suggest that we can hope that the vaccine works as well as it did last year, when overall vaccine effectiveness against all circulating flu viruses was 39%, and effectiveness against the H3N2 virus specifically was 32%.
I’m upping my game when talking to parents about flu vaccine. I mention one study conducted between 2010 and 2012 in which influenza immunization reduced a child’s risk of being admitted to an intensive care unit with flu by 74% (J Infect Dis. 2014 Sep 1;210[5]:674-83). I emphasize that flu vaccine reduces the chance that a child will die from flu. According to a study published in 2017, influenza vaccine reduced the risk of death from flu by 65% in healthy children and 51% in children with high-risk medical conditions (Pediatrics. 2017 May. doi: 10.1542/peds.2016-4244).
When I’m talking to trainees, I no longer just focus on the match between circulating strains of flu and vaccine strains. I mention that viruses used to produce most seasonal flu vaccines are grown in eggs, a process that can result in minor antigenic changes in the hemagglutinin protein, especially in H3N2 viruses. These “egg-adapted changes” may result in a vaccine that stimulates a less effective immune response, even with a good match between circulating strains and vaccine strains. For example, Zost et al. found that the H3N2 virus that emerged during the 2014-2015 season possessed a new hemagglutinin-associated glycosylation site (Proc Natl Acad Sci U S A. 2017 Nov 21;114[47]:12578-83). Although this virus was represented in the 2016-2017 influenza vaccine, the egg-adapted version lost the glycosylation site, resulting in decreased vaccine immunogenicity and less protection against H3N2 viruses circulating in the community.
The real take-home message here is that we need better flu vaccines. In the short term, cell-based flu vaccines that use virus grown in animal cells are a potential alternative to egg-based vaccines. In the long term, we need a universal flu vaccine. The National Institute of Allergy and Infectious Diseases is prioritizing work on a vaccine that could provide long-lasting protection against multiple subtypes of the virus. According to a report on the National Institutes of Health website, such a vaccine could “eliminate the need to update and administer the seasonal flu vaccine each year and could provide protection against newly emerging flu strains,” including those with the potential to cause a pandemic. The NIH researchers acknowledge, however, that achieving this goal will require “a broad range of expertise and substantial resources.”
Until new vaccines are available, we need to do a better job of using available, albeit imperfect, flu vaccines. During the 2016-2017 season, only 59% of children 6 months to 17 years were immunized, and there were 110 influenza-associated deaths in children, according to the CDC. It’s likely that some of these were preventable.
The total magnitude of suffering associated with flu is more difficult to quantify, but anecdotes can be illuminating. A friend recently diagnosed with influenza shared her experience via Facebook. “Rough night. I’m seconds away from a meltdown. My body aches so bad that I can’t get comfortable on the couch or my bed. Can’t breathe, and I cough until I vomit. My head is about to burst along with my ears. Just took a hot bath hoping that would help. I don’t know what else to do. The flu really sucks.”
Indeed. Even a 1 in 10 chance of preventing the flu is better than no chance at all.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital in Louisville. She said she had no relevant financial disclosures. Email her at pdnews@frontlinemedcom.com.
I suspect most health care providers have heard the complaint, “The vaccine doesn’t work. One year I got the vaccine, and I still came down with the flu.”
Over the years, I’ve polished my responses to vaccine naysayers.
Influenza vaccine doesn’t protect you against every virus that can cause cold and flu symptoms. It only prevents influenza. It’s possible you had a different virus, such as adenovirus, coronavirus, parainfluenza virus, or respiratory syncytial virus.
Some years, the vaccine works better than others because there is a mismatch between the viruses chosen for the vaccine, and the viruses that end up circulating. Even when it doesn’t prevent flu, the vaccine can potentially reduce the severity of illness.
The discussion became a little more complicated in 2016 when the Centers for Disease Control and Prevention Advisory Committee on Immunization Practices withdrew its support for the live attenuated influenza virus vaccine (LAIV4) because of concerns about effectiveness. During the 2015-2016 influenza season, LAIV4 demonstrated no statistically significant effectiveness in children 2-17 years of age against H1N1pdm09, the predominant influenza strain. Fortunately, inactivated injectable vaccine did offer protection. An estimated 41.8 million children aged 6 months to 17 years ultimately received this vaccine during the 2016-2017 influenza season.
Now with the 2017-2018 influenza season in full swing, some media reports are proclaiming the influenza vaccine is only 10% effective this year. This claim is based on an interim analysis of data from the most recent flu season in Australia and the effectiveness of the vaccine against the circulating H3N2 virus strain. News from the U.S. CDC is more encouraging. The H3N2 virus contained in this year’s vaccine is the same as that used last year, and so far, circulating H3N2 viruses in the United States are similar to the vaccine virus. Public health officials suggest that we can hope that the vaccine works as well as it did last year, when overall vaccine effectiveness against all circulating flu viruses was 39%, and effectiveness against the H3N2 virus specifically was 32%.
I’m upping my game when talking to parents about flu vaccine. I mention one study conducted between 2010 and 2012 in which influenza immunization reduced a child’s risk of being admitted to an intensive care unit with flu by 74% (J Infect Dis. 2014 Sep 1;210[5]:674-83). I emphasize that flu vaccine reduces the chance that a child will die from flu. According to a study published in 2017, influenza vaccine reduced the risk of death from flu by 65% in healthy children and 51% in children with high-risk medical conditions (Pediatrics. 2017 May. doi: 10.1542/peds.2016-4244).
When I’m talking to trainees, I no longer just focus on the match between circulating strains of flu and vaccine strains. I mention that viruses used to produce most seasonal flu vaccines are grown in eggs, a process that can result in minor antigenic changes in the hemagglutinin protein, especially in H3N2 viruses. These “egg-adapted changes” may result in a vaccine that stimulates a less effective immune response, even with a good match between circulating strains and vaccine strains. For example, Zost et al. found that the H3N2 virus that emerged during the 2014-2015 season possessed a new hemagglutinin-associated glycosylation site (Proc Natl Acad Sci U S A. 2017 Nov 21;114[47]:12578-83). Although this virus was represented in the 2016-2017 influenza vaccine, the egg-adapted version lost the glycosylation site, resulting in decreased vaccine immunogenicity and less protection against H3N2 viruses circulating in the community.
The real take-home message here is that we need better flu vaccines. In the short term, cell-based flu vaccines that use virus grown in animal cells are a potential alternative to egg-based vaccines. In the long term, we need a universal flu vaccine. The National Institute of Allergy and Infectious Diseases is prioritizing work on a vaccine that could provide long-lasting protection against multiple subtypes of the virus. According to a report on the National Institutes of Health website, such a vaccine could “eliminate the need to update and administer the seasonal flu vaccine each year and could provide protection against newly emerging flu strains,” including those with the potential to cause a pandemic. The NIH researchers acknowledge, however, that achieving this goal will require “a broad range of expertise and substantial resources.”
Until new vaccines are available, we need to do a better job of using available, albeit imperfect, flu vaccines. During the 2016-2017 season, only 59% of children 6 months to 17 years were immunized, and there were 110 influenza-associated deaths in children, according to the CDC. It’s likely that some of these were preventable.
The total magnitude of suffering associated with flu is more difficult to quantify, but anecdotes can be illuminating. A friend recently diagnosed with influenza shared her experience via Facebook. “Rough night. I’m seconds away from a meltdown. My body aches so bad that I can’t get comfortable on the couch or my bed. Can’t breathe, and I cough until I vomit. My head is about to burst along with my ears. Just took a hot bath hoping that would help. I don’t know what else to do. The flu really sucks.”
Indeed. Even a 1 in 10 chance of preventing the flu is better than no chance at all.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital in Louisville. She said she had no relevant financial disclosures. Email her at pdnews@frontlinemedcom.com.
I suspect most health care providers have heard the complaint, “The vaccine doesn’t work. One year I got the vaccine, and I still came down with the flu.”
Over the years, I’ve polished my responses to vaccine naysayers.
Influenza vaccine doesn’t protect you against every virus that can cause cold and flu symptoms. It only prevents influenza. It’s possible you had a different virus, such as adenovirus, coronavirus, parainfluenza virus, or respiratory syncytial virus.
Some years, the vaccine works better than others because there is a mismatch between the viruses chosen for the vaccine, and the viruses that end up circulating. Even when it doesn’t prevent flu, the vaccine can potentially reduce the severity of illness.
The discussion became a little more complicated in 2016 when the Centers for Disease Control and Prevention Advisory Committee on Immunization Practices withdrew its support for the live attenuated influenza virus vaccine (LAIV4) because of concerns about effectiveness. During the 2015-2016 influenza season, LAIV4 demonstrated no statistically significant effectiveness in children 2-17 years of age against H1N1pdm09, the predominant influenza strain. Fortunately, inactivated injectable vaccine did offer protection. An estimated 41.8 million children aged 6 months to 17 years ultimately received this vaccine during the 2016-2017 influenza season.
Now with the 2017-2018 influenza season in full swing, some media reports are proclaiming the influenza vaccine is only 10% effective this year. This claim is based on an interim analysis of data from the most recent flu season in Australia and the effectiveness of the vaccine against the circulating H3N2 virus strain. News from the U.S. CDC is more encouraging. The H3N2 virus contained in this year’s vaccine is the same as that used last year, and so far, circulating H3N2 viruses in the United States are similar to the vaccine virus. Public health officials suggest that we can hope that the vaccine works as well as it did last year, when overall vaccine effectiveness against all circulating flu viruses was 39%, and effectiveness against the H3N2 virus specifically was 32%.
I’m upping my game when talking to parents about flu vaccine. I mention one study conducted between 2010 and 2012 in which influenza immunization reduced a child’s risk of being admitted to an intensive care unit with flu by 74% (J Infect Dis. 2014 Sep 1;210[5]:674-83). I emphasize that flu vaccine reduces the chance that a child will die from flu. According to a study published in 2017, influenza vaccine reduced the risk of death from flu by 65% in healthy children and 51% in children with high-risk medical conditions (Pediatrics. 2017 May. doi: 10.1542/peds.2016-4244).
When I’m talking to trainees, I no longer just focus on the match between circulating strains of flu and vaccine strains. I mention that viruses used to produce most seasonal flu vaccines are grown in eggs, a process that can result in minor antigenic changes in the hemagglutinin protein, especially in H3N2 viruses. These “egg-adapted changes” may result in a vaccine that stimulates a less effective immune response, even with a good match between circulating strains and vaccine strains. For example, Zost et al. found that the H3N2 virus that emerged during the 2014-2015 season possessed a new hemagglutinin-associated glycosylation site (Proc Natl Acad Sci U S A. 2017 Nov 21;114[47]:12578-83). Although this virus was represented in the 2016-2017 influenza vaccine, the egg-adapted version lost the glycosylation site, resulting in decreased vaccine immunogenicity and less protection against H3N2 viruses circulating in the community.
The real take-home message here is that we need better flu vaccines. In the short term, cell-based flu vaccines that use virus grown in animal cells are a potential alternative to egg-based vaccines. In the long term, we need a universal flu vaccine. The National Institute of Allergy and Infectious Diseases is prioritizing work on a vaccine that could provide long-lasting protection against multiple subtypes of the virus. According to a report on the National Institutes of Health website, such a vaccine could “eliminate the need to update and administer the seasonal flu vaccine each year and could provide protection against newly emerging flu strains,” including those with the potential to cause a pandemic. The NIH researchers acknowledge, however, that achieving this goal will require “a broad range of expertise and substantial resources.”
Until new vaccines are available, we need to do a better job of using available, albeit imperfect, flu vaccines. During the 2016-2017 season, only 59% of children 6 months to 17 years were immunized, and there were 110 influenza-associated deaths in children, according to the CDC. It’s likely that some of these were preventable.
The total magnitude of suffering associated with flu is more difficult to quantify, but anecdotes can be illuminating. A friend recently diagnosed with influenza shared her experience via Facebook. “Rough night. I’m seconds away from a meltdown. My body aches so bad that I can’t get comfortable on the couch or my bed. Can’t breathe, and I cough until I vomit. My head is about to burst along with my ears. Just took a hot bath hoping that would help. I don’t know what else to do. The flu really sucks.”
Indeed. Even a 1 in 10 chance of preventing the flu is better than no chance at all.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital in Louisville. She said she had no relevant financial disclosures. Email her at pdnews@frontlinemedcom.com.
What is the hepatitis B vaccination regimen in chronic kidney disease?
For patients age 16 and older with advanced chronic kidney disease (CKD), including those undergoing hemodialysis, we recommend a higher dose of hepatitis B virus (HBV) vaccine, more doses, or both. Vaccination with a higher dose may improve the immune response. The hepatitis B surface antibody (anti-HBs) titer should be monitored 1 to 2 months after completion of the vaccination schedule and annually thereafter, with a target titer of 10 IU/mL or greater. For patients who do not develop a protective antibody titer after completing the initial vaccination schedule, the vaccination schedule should be repeated.
RECOMMENDED DOSES AND SCHEDULES
Recommendation 1
Give higher vaccine doses, increase the number of doses, or both.
Recommendation 2
A 4-dose regimen may provide a better antibody response than a 3-dose regimen. (Note: This recommendation applies only to Engerix-B; 4 doses of Recombivax-HB would be an off-label use.)
Rationale. The US Centers for Disease Control and Prevention reported that after completion of a 3-dose vaccination schedule, the median proportion of patients developing a protective antibody response was 64% (range 34%–88%) vs a median of 86% (range 40%–98%) after a 4-dose schedule.3
Lacson et al5 compared antibody response rates after 3 doses of Recombivax-HB and after 4 doses of Engerix-B and found a better response rate with the 4-dose schedule. The rate of persistent protective anti-HBs titer after 1 year was 77% for Engerix-B vs 53% for Recombivax-HB.
Agarwal et al6 evaluated response rates in patients who had mild CKD (serum creatinine levels 1.5–3.0 mg/dL), moderate CKD (creatinine 3.0–6.0 mg/dL), and severe CKD (creatinine > 6.0 mg/dL). The seroconversion rates after 3 doses of 40-μg HBV vaccine were 87.5% in those with mild CKD, 66.6% in those with moderate CKD, and 35.7% in those with severe disease. After a fourth dose, rates improved significantly to 100%, 77%, and 36.4%, respectively.
Recommendation 3
In patients with CKD, vaccination should be done early, before they become dependent on hemodialysis.
Rationale. Patients with advanced CKD may have a lower seroconversion rate. Fraser et al7 found that after a 4-dose series, the seroprotection rate in adult prehemodialysis patients with serum creatinine levels of 4 mg/dL or less was 86%, compared with 37% in patients with serum creatinine levels above 4 mg/dL, of whom 88% were on hemodialysis.7
In a 2003 prospective cohort study by DaRoza et al,8 patients with higher levels of kidney function were more likely to respond to HBV vaccination, and the level of kidney function was found to be an independent predictor of seroconversion.8
A 2012 prospective study by Ghadiani et al9 compared seroconversion rates in patients with stage 3 or 4 CKD vs patients on hemodialysis, with medical staff as controls. The authors reported seroprotection rates of 26.1% in patients on hemodialysis, 55.2% in patients with stage 3 or 4 CKD, and 96.2% in controls. They concluded that vaccination is more likely to induce seroconversion in earlier stages of kidney disease.9
MONITORING THE RESPONSE TO VACCINATION AND REVACCINATION
Testing after vaccination is recommended to determine response. Testing should be done 1 to 2 months after the last dose of the vaccination schedule.1–3 Anti-HBs levels 10 IU/mL and greater are considered protective.10
Revaccination with a full vaccination series is recommended for patients who do not develop adequate levels of protective antibodies after completion of the vaccination schedule.2 Reported response rates to revaccination have varied from 40% to 50% after 2 or 3 additional intramuscular doses of 40 µg, to 64% after 4 additional intramuscular doses of 10 µg.3 Serologic testing should be repeated after the last dose of the vaccination series, as serologic testing after only 1 or 2 additional doses appears to be no more cost-effective.2,3
To the best of our knowledge, no data exist to indicate that in nonresponders, further doses given after completion of 2 full vaccination schedules would induce an antibody response.
ANTIBODY PERSISTENCE AND BOOSTER DOSES
Antibody levels fall with time in patients on hemodialysis. Limited data suggest that in patients who respond to the primary vaccination series, antibodies remain detectable for 6 months in 80% to 100% (median 100%) of patients and for 12 months in 58% to 100% (median 70%) of patients.3 The need for booster doses should be assessed by annual monitoring.2,11 Booster doses should be given when the anti-HBs titer declines to below 10 IU/mL. Limited data indicate that nearly all such patients would respond to a booster dose.3
OTHER WAYS TO IMPROVE VACCINE RESPONSE
Other strategies to improve vaccine response, such as the addition of adjuvants or immunostimulants, have shown variable success.12 Intradermal HBV vaccination in patients on chronic hemodialysis has also been proposed. The efficacy of intradermal vaccination may be related to the dense network of immunologic dendritic cells within the dermis. After intradermal administration, the antigen is taken up by dendritic cells residing in the dermis, which mature and travel to the regional lymph node where further immunostimulation takes place.13
In a systematic review of four prospective trials with a total of 204 hemodialysis patients,13 a significantly higher proportion of patients achieved seroconversion with intradermal HBV vaccine administration than with intramuscular administration. The authors concluded that the intradermal route in primary nonresponders undergoing hemodialysis provides an effective alternative to the intramuscular route to protect against HBV infection in this highly susceptible population.
Additional well-designed, double-blinded, randomized trials are needed to establish clear guidelines on intradermal HBV vaccine dosing and vaccination schedules.
- Grzegorzewska AE. Hepatitis B vaccination in chronic kidney disease: review of evidence in non-dialyzed patients. Hepat Mon 2012; 12:e7359.
- Chi C, Patel P, Pilishvili T, Moore M, Murphy T, Strikas R. Guidelines for vaccinating kidney dialysis patients and patients with chronic kidney disease. www.cdc.gov/dialysis/PDFs/Vaccinating_Dialysis_Patients_and_Patients_dec2012.pdf. Accessed September 6, 2017.
- Recommendations for preventing transmission of infections among chronic hemodialysis patients. MMWR Recomm Rep 2001; 50:1–43.
- Kim DK, Riley LE, Harriman KH, Hunter P, Bridges CB; Advisory Committee on Immunization Practices. Recommended immunization schedule for adults aged 19 years or older, United States, 2017. Ann Intern Med 2017; 166:209–219.
- Lacson E, Teng M, Ong J, Vienneau L, Ofsthun N, Lazarus JM. Antibody response to Engerix-B and Recombivax-HB hepatitis B vaccination in end-stage renal disease. Hemodialysis international. Hemodial Int 2005; 9:367–375.
- Agarwal SK, Irshad M, Dash SC. Comparison of two schedules of hepatitis B vaccination in patients with mild, moderate and severe renal failure. J Assoc Physicians India 1999; 47:183–185.
- Fraser GM, Ochana N, Fenyves D, et al. Increasing serum creatinine and age reduce the response to hepatitis B vaccine in renal failure patients. J Hepatol 1994; 21:450–454.
- DaRoza G, Loewen A, Djurdjev O, et al. Stage of chronic kidney disease predicts seroconversion after hepatitis B immunization: earlier is better. Am J Kidney Dis 2003; 42:1184–1192.
- Ghadiani MH, Besharati S, Mousavinasab N, Jalalzadeh M. Response rates to HB vaccine in CKD stages 3-4 and hemodialysis patients. J Res Med Sci 2012; 17:527–533.
- Jack AD, Hall AJ, Maine N, Mendy M, Whittle HC. What level of hepatitis B antibody is protective? J Infect Dis 1999; 179:489–492.
- Guidelines for vaccination in patients with chronic kidney disease. Indian J Nephrol 2016; 26(suppl 1):S15–S18.
- Somi MH, Hajipour B. Improving hepatitis B vaccine efficacy in end-stage renal diseases patients and role of adjuvants. ISRN Gastroenterol 2012; 2012:960413.
- Yousaf F, Gandham S, Galler M, Spinowitz B, Charytan C. Systematic review of the efficacy and safety of intradermal versus intramuscular hepatitis B vaccination in end-stage renal disease population unresponsive to primary vaccination series. Ren Fail 2015; 37:1080–1088.
For patients age 16 and older with advanced chronic kidney disease (CKD), including those undergoing hemodialysis, we recommend a higher dose of hepatitis B virus (HBV) vaccine, more doses, or both. Vaccination with a higher dose may improve the immune response. The hepatitis B surface antibody (anti-HBs) titer should be monitored 1 to 2 months after completion of the vaccination schedule and annually thereafter, with a target titer of 10 IU/mL or greater. For patients who do not develop a protective antibody titer after completing the initial vaccination schedule, the vaccination schedule should be repeated.
RECOMMENDED DOSES AND SCHEDULES
Recommendation 1
Give higher vaccine doses, increase the number of doses, or both.
Recommendation 2
A 4-dose regimen may provide a better antibody response than a 3-dose regimen. (Note: This recommendation applies only to Engerix-B; 4 doses of Recombivax-HB would be an off-label use.)
Rationale. The US Centers for Disease Control and Prevention reported that after completion of a 3-dose vaccination schedule, the median proportion of patients developing a protective antibody response was 64% (range 34%–88%) vs a median of 86% (range 40%–98%) after a 4-dose schedule.3
Lacson et al5 compared antibody response rates after 3 doses of Recombivax-HB and after 4 doses of Engerix-B and found a better response rate with the 4-dose schedule. The rate of persistent protective anti-HBs titer after 1 year was 77% for Engerix-B vs 53% for Recombivax-HB.
Agarwal et al6 evaluated response rates in patients who had mild CKD (serum creatinine levels 1.5–3.0 mg/dL), moderate CKD (creatinine 3.0–6.0 mg/dL), and severe CKD (creatinine > 6.0 mg/dL). The seroconversion rates after 3 doses of 40-μg HBV vaccine were 87.5% in those with mild CKD, 66.6% in those with moderate CKD, and 35.7% in those with severe disease. After a fourth dose, rates improved significantly to 100%, 77%, and 36.4%, respectively.
Recommendation 3
In patients with CKD, vaccination should be done early, before they become dependent on hemodialysis.
Rationale. Patients with advanced CKD may have a lower seroconversion rate. Fraser et al7 found that after a 4-dose series, the seroprotection rate in adult prehemodialysis patients with serum creatinine levels of 4 mg/dL or less was 86%, compared with 37% in patients with serum creatinine levels above 4 mg/dL, of whom 88% were on hemodialysis.7
In a 2003 prospective cohort study by DaRoza et al,8 patients with higher levels of kidney function were more likely to respond to HBV vaccination, and the level of kidney function was found to be an independent predictor of seroconversion.8
A 2012 prospective study by Ghadiani et al9 compared seroconversion rates in patients with stage 3 or 4 CKD vs patients on hemodialysis, with medical staff as controls. The authors reported seroprotection rates of 26.1% in patients on hemodialysis, 55.2% in patients with stage 3 or 4 CKD, and 96.2% in controls. They concluded that vaccination is more likely to induce seroconversion in earlier stages of kidney disease.9
MONITORING THE RESPONSE TO VACCINATION AND REVACCINATION
Testing after vaccination is recommended to determine response. Testing should be done 1 to 2 months after the last dose of the vaccination schedule.1–3 Anti-HBs levels 10 IU/mL and greater are considered protective.10
Revaccination with a full vaccination series is recommended for patients who do not develop adequate levels of protective antibodies after completion of the vaccination schedule.2 Reported response rates to revaccination have varied from 40% to 50% after 2 or 3 additional intramuscular doses of 40 µg, to 64% after 4 additional intramuscular doses of 10 µg.3 Serologic testing should be repeated after the last dose of the vaccination series, as serologic testing after only 1 or 2 additional doses appears to be no more cost-effective.2,3
To the best of our knowledge, no data exist to indicate that in nonresponders, further doses given after completion of 2 full vaccination schedules would induce an antibody response.
ANTIBODY PERSISTENCE AND BOOSTER DOSES
Antibody levels fall with time in patients on hemodialysis. Limited data suggest that in patients who respond to the primary vaccination series, antibodies remain detectable for 6 months in 80% to 100% (median 100%) of patients and for 12 months in 58% to 100% (median 70%) of patients.3 The need for booster doses should be assessed by annual monitoring.2,11 Booster doses should be given when the anti-HBs titer declines to below 10 IU/mL. Limited data indicate that nearly all such patients would respond to a booster dose.3
OTHER WAYS TO IMPROVE VACCINE RESPONSE
Other strategies to improve vaccine response, such as the addition of adjuvants or immunostimulants, have shown variable success.12 Intradermal HBV vaccination in patients on chronic hemodialysis has also been proposed. The efficacy of intradermal vaccination may be related to the dense network of immunologic dendritic cells within the dermis. After intradermal administration, the antigen is taken up by dendritic cells residing in the dermis, which mature and travel to the regional lymph node where further immunostimulation takes place.13
In a systematic review of four prospective trials with a total of 204 hemodialysis patients,13 a significantly higher proportion of patients achieved seroconversion with intradermal HBV vaccine administration than with intramuscular administration. The authors concluded that the intradermal route in primary nonresponders undergoing hemodialysis provides an effective alternative to the intramuscular route to protect against HBV infection in this highly susceptible population.
Additional well-designed, double-blinded, randomized trials are needed to establish clear guidelines on intradermal HBV vaccine dosing and vaccination schedules.
For patients age 16 and older with advanced chronic kidney disease (CKD), including those undergoing hemodialysis, we recommend a higher dose of hepatitis B virus (HBV) vaccine, more doses, or both. Vaccination with a higher dose may improve the immune response. The hepatitis B surface antibody (anti-HBs) titer should be monitored 1 to 2 months after completion of the vaccination schedule and annually thereafter, with a target titer of 10 IU/mL or greater. For patients who do not develop a protective antibody titer after completing the initial vaccination schedule, the vaccination schedule should be repeated.
RECOMMENDED DOSES AND SCHEDULES
Recommendation 1
Give higher vaccine doses, increase the number of doses, or both.
Recommendation 2
A 4-dose regimen may provide a better antibody response than a 3-dose regimen. (Note: This recommendation applies only to Engerix-B; 4 doses of Recombivax-HB would be an off-label use.)
Rationale. The US Centers for Disease Control and Prevention reported that after completion of a 3-dose vaccination schedule, the median proportion of patients developing a protective antibody response was 64% (range 34%–88%) vs a median of 86% (range 40%–98%) after a 4-dose schedule.3
Lacson et al5 compared antibody response rates after 3 doses of Recombivax-HB and after 4 doses of Engerix-B and found a better response rate with the 4-dose schedule. The rate of persistent protective anti-HBs titer after 1 year was 77% for Engerix-B vs 53% for Recombivax-HB.
Agarwal et al6 evaluated response rates in patients who had mild CKD (serum creatinine levels 1.5–3.0 mg/dL), moderate CKD (creatinine 3.0–6.0 mg/dL), and severe CKD (creatinine > 6.0 mg/dL). The seroconversion rates after 3 doses of 40-μg HBV vaccine were 87.5% in those with mild CKD, 66.6% in those with moderate CKD, and 35.7% in those with severe disease. After a fourth dose, rates improved significantly to 100%, 77%, and 36.4%, respectively.
Recommendation 3
In patients with CKD, vaccination should be done early, before they become dependent on hemodialysis.
Rationale. Patients with advanced CKD may have a lower seroconversion rate. Fraser et al7 found that after a 4-dose series, the seroprotection rate in adult prehemodialysis patients with serum creatinine levels of 4 mg/dL or less was 86%, compared with 37% in patients with serum creatinine levels above 4 mg/dL, of whom 88% were on hemodialysis.7
In a 2003 prospective cohort study by DaRoza et al,8 patients with higher levels of kidney function were more likely to respond to HBV vaccination, and the level of kidney function was found to be an independent predictor of seroconversion.8
A 2012 prospective study by Ghadiani et al9 compared seroconversion rates in patients with stage 3 or 4 CKD vs patients on hemodialysis, with medical staff as controls. The authors reported seroprotection rates of 26.1% in patients on hemodialysis, 55.2% in patients with stage 3 or 4 CKD, and 96.2% in controls. They concluded that vaccination is more likely to induce seroconversion in earlier stages of kidney disease.9
MONITORING THE RESPONSE TO VACCINATION AND REVACCINATION
Testing after vaccination is recommended to determine response. Testing should be done 1 to 2 months after the last dose of the vaccination schedule.1–3 Anti-HBs levels 10 IU/mL and greater are considered protective.10
Revaccination with a full vaccination series is recommended for patients who do not develop adequate levels of protective antibodies after completion of the vaccination schedule.2 Reported response rates to revaccination have varied from 40% to 50% after 2 or 3 additional intramuscular doses of 40 µg, to 64% after 4 additional intramuscular doses of 10 µg.3 Serologic testing should be repeated after the last dose of the vaccination series, as serologic testing after only 1 or 2 additional doses appears to be no more cost-effective.2,3
To the best of our knowledge, no data exist to indicate that in nonresponders, further doses given after completion of 2 full vaccination schedules would induce an antibody response.
ANTIBODY PERSISTENCE AND BOOSTER DOSES
Antibody levels fall with time in patients on hemodialysis. Limited data suggest that in patients who respond to the primary vaccination series, antibodies remain detectable for 6 months in 80% to 100% (median 100%) of patients and for 12 months in 58% to 100% (median 70%) of patients.3 The need for booster doses should be assessed by annual monitoring.2,11 Booster doses should be given when the anti-HBs titer declines to below 10 IU/mL. Limited data indicate that nearly all such patients would respond to a booster dose.3
OTHER WAYS TO IMPROVE VACCINE RESPONSE
Other strategies to improve vaccine response, such as the addition of adjuvants or immunostimulants, have shown variable success.12 Intradermal HBV vaccination in patients on chronic hemodialysis has also been proposed. The efficacy of intradermal vaccination may be related to the dense network of immunologic dendritic cells within the dermis. After intradermal administration, the antigen is taken up by dendritic cells residing in the dermis, which mature and travel to the regional lymph node where further immunostimulation takes place.13
In a systematic review of four prospective trials with a total of 204 hemodialysis patients,13 a significantly higher proportion of patients achieved seroconversion with intradermal HBV vaccine administration than with intramuscular administration. The authors concluded that the intradermal route in primary nonresponders undergoing hemodialysis provides an effective alternative to the intramuscular route to protect against HBV infection in this highly susceptible population.
Additional well-designed, double-blinded, randomized trials are needed to establish clear guidelines on intradermal HBV vaccine dosing and vaccination schedules.
- Grzegorzewska AE. Hepatitis B vaccination in chronic kidney disease: review of evidence in non-dialyzed patients. Hepat Mon 2012; 12:e7359.
- Chi C, Patel P, Pilishvili T, Moore M, Murphy T, Strikas R. Guidelines for vaccinating kidney dialysis patients and patients with chronic kidney disease. www.cdc.gov/dialysis/PDFs/Vaccinating_Dialysis_Patients_and_Patients_dec2012.pdf. Accessed September 6, 2017.
- Recommendations for preventing transmission of infections among chronic hemodialysis patients. MMWR Recomm Rep 2001; 50:1–43.
- Kim DK, Riley LE, Harriman KH, Hunter P, Bridges CB; Advisory Committee on Immunization Practices. Recommended immunization schedule for adults aged 19 years or older, United States, 2017. Ann Intern Med 2017; 166:209–219.
- Lacson E, Teng M, Ong J, Vienneau L, Ofsthun N, Lazarus JM. Antibody response to Engerix-B and Recombivax-HB hepatitis B vaccination in end-stage renal disease. Hemodialysis international. Hemodial Int 2005; 9:367–375.
- Agarwal SK, Irshad M, Dash SC. Comparison of two schedules of hepatitis B vaccination in patients with mild, moderate and severe renal failure. J Assoc Physicians India 1999; 47:183–185.
- Fraser GM, Ochana N, Fenyves D, et al. Increasing serum creatinine and age reduce the response to hepatitis B vaccine in renal failure patients. J Hepatol 1994; 21:450–454.
- DaRoza G, Loewen A, Djurdjev O, et al. Stage of chronic kidney disease predicts seroconversion after hepatitis B immunization: earlier is better. Am J Kidney Dis 2003; 42:1184–1192.
- Ghadiani MH, Besharati S, Mousavinasab N, Jalalzadeh M. Response rates to HB vaccine in CKD stages 3-4 and hemodialysis patients. J Res Med Sci 2012; 17:527–533.
- Jack AD, Hall AJ, Maine N, Mendy M, Whittle HC. What level of hepatitis B antibody is protective? J Infect Dis 1999; 179:489–492.
- Guidelines for vaccination in patients with chronic kidney disease. Indian J Nephrol 2016; 26(suppl 1):S15–S18.
- Somi MH, Hajipour B. Improving hepatitis B vaccine efficacy in end-stage renal diseases patients and role of adjuvants. ISRN Gastroenterol 2012; 2012:960413.
- Yousaf F, Gandham S, Galler M, Spinowitz B, Charytan C. Systematic review of the efficacy and safety of intradermal versus intramuscular hepatitis B vaccination in end-stage renal disease population unresponsive to primary vaccination series. Ren Fail 2015; 37:1080–1088.
- Grzegorzewska AE. Hepatitis B vaccination in chronic kidney disease: review of evidence in non-dialyzed patients. Hepat Mon 2012; 12:e7359.
- Chi C, Patel P, Pilishvili T, Moore M, Murphy T, Strikas R. Guidelines for vaccinating kidney dialysis patients and patients with chronic kidney disease. www.cdc.gov/dialysis/PDFs/Vaccinating_Dialysis_Patients_and_Patients_dec2012.pdf. Accessed September 6, 2017.
- Recommendations for preventing transmission of infections among chronic hemodialysis patients. MMWR Recomm Rep 2001; 50:1–43.
- Kim DK, Riley LE, Harriman KH, Hunter P, Bridges CB; Advisory Committee on Immunization Practices. Recommended immunization schedule for adults aged 19 years or older, United States, 2017. Ann Intern Med 2017; 166:209–219.
- Lacson E, Teng M, Ong J, Vienneau L, Ofsthun N, Lazarus JM. Antibody response to Engerix-B and Recombivax-HB hepatitis B vaccination in end-stage renal disease. Hemodialysis international. Hemodial Int 2005; 9:367–375.
- Agarwal SK, Irshad M, Dash SC. Comparison of two schedules of hepatitis B vaccination in patients with mild, moderate and severe renal failure. J Assoc Physicians India 1999; 47:183–185.
- Fraser GM, Ochana N, Fenyves D, et al. Increasing serum creatinine and age reduce the response to hepatitis B vaccine in renal failure patients. J Hepatol 1994; 21:450–454.
- DaRoza G, Loewen A, Djurdjev O, et al. Stage of chronic kidney disease predicts seroconversion after hepatitis B immunization: earlier is better. Am J Kidney Dis 2003; 42:1184–1192.
- Ghadiani MH, Besharati S, Mousavinasab N, Jalalzadeh M. Response rates to HB vaccine in CKD stages 3-4 and hemodialysis patients. J Res Med Sci 2012; 17:527–533.
- Jack AD, Hall AJ, Maine N, Mendy M, Whittle HC. What level of hepatitis B antibody is protective? J Infect Dis 1999; 179:489–492.
- Guidelines for vaccination in patients with chronic kidney disease. Indian J Nephrol 2016; 26(suppl 1):S15–S18.
- Somi MH, Hajipour B. Improving hepatitis B vaccine efficacy in end-stage renal diseases patients and role of adjuvants. ISRN Gastroenterol 2012; 2012:960413.
- Yousaf F, Gandham S, Galler M, Spinowitz B, Charytan C. Systematic review of the efficacy and safety of intradermal versus intramuscular hepatitis B vaccination in end-stage renal disease population unresponsive to primary vaccination series. Ren Fail 2015; 37:1080–1088.
CDC provides advice on recent hepatitis A outbreaks
The epidemiology of hepatitis A virus (HAV) disease has changed. Since July 2016, there have been 5 large outbreaks of infection involving more than 1600 cases,1 with affected states requiring assistance from the Centers for Disease Control and Prevention (CDC). Two of these outbreaks were foodborne, and 3 involved person-to-person transmission.1
Before 2016, the number of outbreaks had been very low, and were predominantly associated with contaminated food, infected food handlers, and other food service-related exposures. Total annual cases of HAV infection had been declining steadily in all age groups since 1995 when HAV vaccine became available, from an estimated 271,000 cases resulting in 100 deaths2 to an estimated 2800 cases (with 1390 reported) resulting in 67 deaths in 2015 (FIGURE).3
Extent of the outbreaks
The largest hepatitis A outbreak involving person-to-person transmission in the United States in the past 20 years is occurring now in California. Predominantly affected are the homeless and users of illicit drugs, whose risk of infection is compounded by exposure to fecally-contaminated environments. As of December 1, the largest number of cases were recorded in San Diego (567), Santa Cruz (76), and Los Angeles (11).4 Adding 18 cases from other locations, the total has reached 672, resulting in 430 hospitalizations (64%) and 21 deaths (3%).4 In San Diego, 20% of those infected also had chronic hepatitis C and 5% had chronic hepatitis B.1
In southeastern Michigan, 555 cases have been reported, with 457 hospitalizations (82%) and 20 deaths (4%).5 In Utah, 91 cases and 53 hospitalizations (58%) have been documented.6 In these regions, the predominant risk factors have been homelessness and illicit drug use. And many of those infected have had chronic hepatitis C (27.5%), hepatitis B (13.2%), or both (9.9%).6 In 2 of the 3 states just described, the outbreaks have involved HAV genotype 1B.1
In New York City, an outbreak starting in January 2017 resulted in 51 cases. The epidemiology of this outbreak has been different from the others, involving men who have sex with men (MSM) and the HAV genotype 1A that matches a strain circulating among MSM in Europe.7
Low adult immunity is behind the outbreaks
These outbreaks have occurred in an adult US population that has low levels of immunity to HAV. In 2012 only 12.2% of adults ages 19 to 49 years had received 2 doses of HAV vaccine8 and only 24.2% of adults had antibodies to HAV,9 showing that most adults had never been infected with the virus or vaccinated. The reduction in HAV incidence previously described is due to the introduction of targeted, and then universal, child HAV vaccination recommendations by the Advisory Committee on Immunization Practices.
As the incidence of HAV disease declined, fewer individuals became infected as children, leading later to a susceptible pool of adults who had not been infected as children and who did not receive the vaccine in adulthood. Most of these adults will not be exposed to HAV due to decreased rates of infection in children, which, historically, has been the predominant means of adult exposure. The high hospitalization and death rates encountered in the recent and ongoing large outbreaks are explained by the multiple comorbidities of those infected.
Who should be vaccinated against HAV
The CDC recommends giving HAV vaccine to all children at age one year, and to the following groups:2,10,11
- residents of a community that has a high rate of hepatitis A infection
- household members or other close personal contacts (eg, regular babysitters) of adopted children newly arrived from countries with high or intermediate hepatitis A endemicity
- men who have sex with other men
- users of illicit injection and noninjection drugs
- workers in, or travelers to, countries with high rates of hepatitis A infection
- individuals with chronic liver disease
- individuals who work with HAV-infected animals or with HAV in a research setting.
Outbreak-specific vaccine recommendations
The CDC has additionally recommended that, during outbreaks, health care providers should consider taking the following 4 steps:12,13
- Increase the availability of HAV vaccine to the homeless and to those who use illicit drugs; to anyone who has ongoing, close contact with people who are homeless or who use injection and non-injection drugs; and as post-exposure prophylaxis for unvaccinated people who have been exposed to HAV in the previous 2 weeks.
- Defer the second dose of HAV vaccine if it is in short supply.
- Perform pre-vaccination serologic testing to identify those who are immune, thereby preserving vaccine and reducing costs.
- Use TWINRIX if other HAV vaccines are unavailable, keeping in mind that a single dose of TWINRIX achieves 94% protection against HAV but only 31% against hepatitis B virus (HBV). Three doses of TWINRIX are needed for full protection against HBV.
Available vaccines
Three vaccines are available for protection against HAV (TABLE2,14). Post-exposure prevention of HAV can be achieved with HAV vaccine or immune globulin.15 Vaccine is preferred for individuals up to age 40 years and can be used for older individuals if immune globulin is unavailable.
The CDC reports that the supply of adult HAV vaccine is being strained by these large outbreaks.16 Physicians will need to stay in touch with their local public health departments regarding vaccine availability in the community and any local recommendations being made regarding vaccine administration, as well as to the status of any local HAV outbreaks.
1. Nelson N. Hepatitis A outbreaks. Presented at: Advisory Committee on Immunization Practices; October 25, 2017; Atlanta, GA. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2017-10/hepatitis-04-nelson.pdf. Accessed December 5, 2017.
2. CDC. Prevention of hepatitis A through passive or active immunization. Recommendations of the Advisory Committee on Immunization Practices. Available at: https://www.cdc.gov/mmwr/preview/mmwrhtml/rr5507a1.htm. Accessed November 28, 2017.
3. CDC. Viral hepatitis surveillance—United States, 2015. Available at: https://www.cdc.gov/hepatitis/statistics/2015surveillance/pdfs/2015HepSurveillanceRpt.pdf. Accessed November 28, 2017.
4. California Department of Public Health. Hepatitis A outbreak in California. Available at: https://www.cdph.ca.gov/Programs/CID/DCDC/Pages/Immunization/Hepatitis-A-Outbreak.aspx. Accessed November 28, 2017.
5. Michigan Department of Health & Human Services. Hepatitis A southeast Michigan outbreak. Available at: http://www.michigan.gov/mdhhs/0,5885,7-339-71550_2955_2976_82305_82310-447907--,00.html. Accessed November 28, 2017.
6. Utah Department of Health. Hepatitis A outbreak. Available at: http://health.utah.gov/epi/diseases/hepatitisA/HAVoutbreak_2017. Accessed November 28, 2017.
7. Latash J, Dorsinville M, Del Rosso P, et al. Notes from the field: increase in reported hepatitis A infections among men who have sex with men–New York City, January-August 2017. MMWR Morb Mortal Wkly Rep. 2017;66:999-1000.
8. CDC. Murphy TV, Denniston MM, Hill HA, et al. Progress toward eliminating hepatitis A disease in the United States. MMWR Morb Mortal Wkly Rep. 2016;65:29-41.
9. Klevens RM, Denniston MM, Jiles-Chapman RB, et al. Decreasing immunity to hepatitis A virus infection among US adults: findings from the National Health and Nutrition Examination Survey (NHANES), 1999-2012. Vaccine. 2015;33:6192-6198.
10. CDC. Vaccines and preventable diseases. Hepatitis A in-short. Available at: https://www.cdc.gov/vaccines/vpd/hepa/public/in-short-adult.html#who. Accessed November 20, 2017.
11. CDC. Updated recommendations from the Advisory Committee on Immunization Practices (ACIP) for use of hepatitis A vaccine in close contacts of newly arriving international adoptees. MMWR Morb Mortal Wkly Rep. 2009;58:1006-1007.
12. CDC. Interim outbreak-specific guidance on hepatitis A vaccine administration. Available at: https://www.cdc.gov/hepatitis/outbreaks/InterimOutbreakGuidance-HAV-VaccineAdmin.htm. Accessed November 20, 2017.
13. CDC. 2017–Outbreaks of hepatitis A in multiple states among people who are homeless and people who use drugs. Available at: https://www.cdc.gov/hepatitis/outbreaks/2017March-HepatitisA.htm. Accessed December 11, 2017.
14. CDC. Notice to readers: FDA approval of an alternate dosing schedule for a combined hepatitis A and B vaccine (Twinrix). Available at: https://www.cdc.gov/mmwr/preview/mmwrhtml/mm5640a5.htm. Accessed December 8, 2017.
15. CDC. Update: prevention of hepatitis A after exposure to hepatitis A virus and in International Travelers. Updated recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2007;56:1080-1084.
16. CDC. Current vaccine shortages and delays. Available at: https://www.cdc.gov/vaccines/hcp/clinical-resources/shortages.html. Accessed November 28, 2017.
The epidemiology of hepatitis A virus (HAV) disease has changed. Since July 2016, there have been 5 large outbreaks of infection involving more than 1600 cases,1 with affected states requiring assistance from the Centers for Disease Control and Prevention (CDC). Two of these outbreaks were foodborne, and 3 involved person-to-person transmission.1
Before 2016, the number of outbreaks had been very low, and were predominantly associated with contaminated food, infected food handlers, and other food service-related exposures. Total annual cases of HAV infection had been declining steadily in all age groups since 1995 when HAV vaccine became available, from an estimated 271,000 cases resulting in 100 deaths2 to an estimated 2800 cases (with 1390 reported) resulting in 67 deaths in 2015 (FIGURE).3
Extent of the outbreaks
The largest hepatitis A outbreak involving person-to-person transmission in the United States in the past 20 years is occurring now in California. Predominantly affected are the homeless and users of illicit drugs, whose risk of infection is compounded by exposure to fecally-contaminated environments. As of December 1, the largest number of cases were recorded in San Diego (567), Santa Cruz (76), and Los Angeles (11).4 Adding 18 cases from other locations, the total has reached 672, resulting in 430 hospitalizations (64%) and 21 deaths (3%).4 In San Diego, 20% of those infected also had chronic hepatitis C and 5% had chronic hepatitis B.1
In southeastern Michigan, 555 cases have been reported, with 457 hospitalizations (82%) and 20 deaths (4%).5 In Utah, 91 cases and 53 hospitalizations (58%) have been documented.6 In these regions, the predominant risk factors have been homelessness and illicit drug use. And many of those infected have had chronic hepatitis C (27.5%), hepatitis B (13.2%), or both (9.9%).6 In 2 of the 3 states just described, the outbreaks have involved HAV genotype 1B.1
In New York City, an outbreak starting in January 2017 resulted in 51 cases. The epidemiology of this outbreak has been different from the others, involving men who have sex with men (MSM) and the HAV genotype 1A that matches a strain circulating among MSM in Europe.7
Low adult immunity is behind the outbreaks
These outbreaks have occurred in an adult US population that has low levels of immunity to HAV. In 2012 only 12.2% of adults ages 19 to 49 years had received 2 doses of HAV vaccine8 and only 24.2% of adults had antibodies to HAV,9 showing that most adults had never been infected with the virus or vaccinated. The reduction in HAV incidence previously described is due to the introduction of targeted, and then universal, child HAV vaccination recommendations by the Advisory Committee on Immunization Practices.
As the incidence of HAV disease declined, fewer individuals became infected as children, leading later to a susceptible pool of adults who had not been infected as children and who did not receive the vaccine in adulthood. Most of these adults will not be exposed to HAV due to decreased rates of infection in children, which, historically, has been the predominant means of adult exposure. The high hospitalization and death rates encountered in the recent and ongoing large outbreaks are explained by the multiple comorbidities of those infected.
Who should be vaccinated against HAV
The CDC recommends giving HAV vaccine to all children at age one year, and to the following groups:2,10,11
- residents of a community that has a high rate of hepatitis A infection
- household members or other close personal contacts (eg, regular babysitters) of adopted children newly arrived from countries with high or intermediate hepatitis A endemicity
- men who have sex with other men
- users of illicit injection and noninjection drugs
- workers in, or travelers to, countries with high rates of hepatitis A infection
- individuals with chronic liver disease
- individuals who work with HAV-infected animals or with HAV in a research setting.
Outbreak-specific vaccine recommendations
The CDC has additionally recommended that, during outbreaks, health care providers should consider taking the following 4 steps:12,13
- Increase the availability of HAV vaccine to the homeless and to those who use illicit drugs; to anyone who has ongoing, close contact with people who are homeless or who use injection and non-injection drugs; and as post-exposure prophylaxis for unvaccinated people who have been exposed to HAV in the previous 2 weeks.
- Defer the second dose of HAV vaccine if it is in short supply.
- Perform pre-vaccination serologic testing to identify those who are immune, thereby preserving vaccine and reducing costs.
- Use TWINRIX if other HAV vaccines are unavailable, keeping in mind that a single dose of TWINRIX achieves 94% protection against HAV but only 31% against hepatitis B virus (HBV). Three doses of TWINRIX are needed for full protection against HBV.
Available vaccines
Three vaccines are available for protection against HAV (TABLE2,14). Post-exposure prevention of HAV can be achieved with HAV vaccine or immune globulin.15 Vaccine is preferred for individuals up to age 40 years and can be used for older individuals if immune globulin is unavailable.
The CDC reports that the supply of adult HAV vaccine is being strained by these large outbreaks.16 Physicians will need to stay in touch with their local public health departments regarding vaccine availability in the community and any local recommendations being made regarding vaccine administration, as well as to the status of any local HAV outbreaks.
The epidemiology of hepatitis A virus (HAV) disease has changed. Since July 2016, there have been 5 large outbreaks of infection involving more than 1600 cases,1 with affected states requiring assistance from the Centers for Disease Control and Prevention (CDC). Two of these outbreaks were foodborne, and 3 involved person-to-person transmission.1
Before 2016, the number of outbreaks had been very low, and were predominantly associated with contaminated food, infected food handlers, and other food service-related exposures. Total annual cases of HAV infection had been declining steadily in all age groups since 1995 when HAV vaccine became available, from an estimated 271,000 cases resulting in 100 deaths2 to an estimated 2800 cases (with 1390 reported) resulting in 67 deaths in 2015 (FIGURE).3
Extent of the outbreaks
The largest hepatitis A outbreak involving person-to-person transmission in the United States in the past 20 years is occurring now in California. Predominantly affected are the homeless and users of illicit drugs, whose risk of infection is compounded by exposure to fecally-contaminated environments. As of December 1, the largest number of cases were recorded in San Diego (567), Santa Cruz (76), and Los Angeles (11).4 Adding 18 cases from other locations, the total has reached 672, resulting in 430 hospitalizations (64%) and 21 deaths (3%).4 In San Diego, 20% of those infected also had chronic hepatitis C and 5% had chronic hepatitis B.1
In southeastern Michigan, 555 cases have been reported, with 457 hospitalizations (82%) and 20 deaths (4%).5 In Utah, 91 cases and 53 hospitalizations (58%) have been documented.6 In these regions, the predominant risk factors have been homelessness and illicit drug use. And many of those infected have had chronic hepatitis C (27.5%), hepatitis B (13.2%), or both (9.9%).6 In 2 of the 3 states just described, the outbreaks have involved HAV genotype 1B.1
In New York City, an outbreak starting in January 2017 resulted in 51 cases. The epidemiology of this outbreak has been different from the others, involving men who have sex with men (MSM) and the HAV genotype 1A that matches a strain circulating among MSM in Europe.7
Low adult immunity is behind the outbreaks
These outbreaks have occurred in an adult US population that has low levels of immunity to HAV. In 2012 only 12.2% of adults ages 19 to 49 years had received 2 doses of HAV vaccine8 and only 24.2% of adults had antibodies to HAV,9 showing that most adults had never been infected with the virus or vaccinated. The reduction in HAV incidence previously described is due to the introduction of targeted, and then universal, child HAV vaccination recommendations by the Advisory Committee on Immunization Practices.
As the incidence of HAV disease declined, fewer individuals became infected as children, leading later to a susceptible pool of adults who had not been infected as children and who did not receive the vaccine in adulthood. Most of these adults will not be exposed to HAV due to decreased rates of infection in children, which, historically, has been the predominant means of adult exposure. The high hospitalization and death rates encountered in the recent and ongoing large outbreaks are explained by the multiple comorbidities of those infected.
Who should be vaccinated against HAV
The CDC recommends giving HAV vaccine to all children at age one year, and to the following groups:2,10,11
- residents of a community that has a high rate of hepatitis A infection
- household members or other close personal contacts (eg, regular babysitters) of adopted children newly arrived from countries with high or intermediate hepatitis A endemicity
- men who have sex with other men
- users of illicit injection and noninjection drugs
- workers in, or travelers to, countries with high rates of hepatitis A infection
- individuals with chronic liver disease
- individuals who work with HAV-infected animals or with HAV in a research setting.
Outbreak-specific vaccine recommendations
The CDC has additionally recommended that, during outbreaks, health care providers should consider taking the following 4 steps:12,13
- Increase the availability of HAV vaccine to the homeless and to those who use illicit drugs; to anyone who has ongoing, close contact with people who are homeless or who use injection and non-injection drugs; and as post-exposure prophylaxis for unvaccinated people who have been exposed to HAV in the previous 2 weeks.
- Defer the second dose of HAV vaccine if it is in short supply.
- Perform pre-vaccination serologic testing to identify those who are immune, thereby preserving vaccine and reducing costs.
- Use TWINRIX if other HAV vaccines are unavailable, keeping in mind that a single dose of TWINRIX achieves 94% protection against HAV but only 31% against hepatitis B virus (HBV). Three doses of TWINRIX are needed for full protection against HBV.
Available vaccines
Three vaccines are available for protection against HAV (TABLE2,14). Post-exposure prevention of HAV can be achieved with HAV vaccine or immune globulin.15 Vaccine is preferred for individuals up to age 40 years and can be used for older individuals if immune globulin is unavailable.
The CDC reports that the supply of adult HAV vaccine is being strained by these large outbreaks.16 Physicians will need to stay in touch with their local public health departments regarding vaccine availability in the community and any local recommendations being made regarding vaccine administration, as well as to the status of any local HAV outbreaks.
1. Nelson N. Hepatitis A outbreaks. Presented at: Advisory Committee on Immunization Practices; October 25, 2017; Atlanta, GA. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2017-10/hepatitis-04-nelson.pdf. Accessed December 5, 2017.
2. CDC. Prevention of hepatitis A through passive or active immunization. Recommendations of the Advisory Committee on Immunization Practices. Available at: https://www.cdc.gov/mmwr/preview/mmwrhtml/rr5507a1.htm. Accessed November 28, 2017.
3. CDC. Viral hepatitis surveillance—United States, 2015. Available at: https://www.cdc.gov/hepatitis/statistics/2015surveillance/pdfs/2015HepSurveillanceRpt.pdf. Accessed November 28, 2017.
4. California Department of Public Health. Hepatitis A outbreak in California. Available at: https://www.cdph.ca.gov/Programs/CID/DCDC/Pages/Immunization/Hepatitis-A-Outbreak.aspx. Accessed November 28, 2017.
5. Michigan Department of Health & Human Services. Hepatitis A southeast Michigan outbreak. Available at: http://www.michigan.gov/mdhhs/0,5885,7-339-71550_2955_2976_82305_82310-447907--,00.html. Accessed November 28, 2017.
6. Utah Department of Health. Hepatitis A outbreak. Available at: http://health.utah.gov/epi/diseases/hepatitisA/HAVoutbreak_2017. Accessed November 28, 2017.
7. Latash J, Dorsinville M, Del Rosso P, et al. Notes from the field: increase in reported hepatitis A infections among men who have sex with men–New York City, January-August 2017. MMWR Morb Mortal Wkly Rep. 2017;66:999-1000.
8. CDC. Murphy TV, Denniston MM, Hill HA, et al. Progress toward eliminating hepatitis A disease in the United States. MMWR Morb Mortal Wkly Rep. 2016;65:29-41.
9. Klevens RM, Denniston MM, Jiles-Chapman RB, et al. Decreasing immunity to hepatitis A virus infection among US adults: findings from the National Health and Nutrition Examination Survey (NHANES), 1999-2012. Vaccine. 2015;33:6192-6198.
10. CDC. Vaccines and preventable diseases. Hepatitis A in-short. Available at: https://www.cdc.gov/vaccines/vpd/hepa/public/in-short-adult.html#who. Accessed November 20, 2017.
11. CDC. Updated recommendations from the Advisory Committee on Immunization Practices (ACIP) for use of hepatitis A vaccine in close contacts of newly arriving international adoptees. MMWR Morb Mortal Wkly Rep. 2009;58:1006-1007.
12. CDC. Interim outbreak-specific guidance on hepatitis A vaccine administration. Available at: https://www.cdc.gov/hepatitis/outbreaks/InterimOutbreakGuidance-HAV-VaccineAdmin.htm. Accessed November 20, 2017.
13. CDC. 2017–Outbreaks of hepatitis A in multiple states among people who are homeless and people who use drugs. Available at: https://www.cdc.gov/hepatitis/outbreaks/2017March-HepatitisA.htm. Accessed December 11, 2017.
14. CDC. Notice to readers: FDA approval of an alternate dosing schedule for a combined hepatitis A and B vaccine (Twinrix). Available at: https://www.cdc.gov/mmwr/preview/mmwrhtml/mm5640a5.htm. Accessed December 8, 2017.
15. CDC. Update: prevention of hepatitis A after exposure to hepatitis A virus and in International Travelers. Updated recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2007;56:1080-1084.
16. CDC. Current vaccine shortages and delays. Available at: https://www.cdc.gov/vaccines/hcp/clinical-resources/shortages.html. Accessed November 28, 2017.
1. Nelson N. Hepatitis A outbreaks. Presented at: Advisory Committee on Immunization Practices; October 25, 2017; Atlanta, GA. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2017-10/hepatitis-04-nelson.pdf. Accessed December 5, 2017.
2. CDC. Prevention of hepatitis A through passive or active immunization. Recommendations of the Advisory Committee on Immunization Practices. Available at: https://www.cdc.gov/mmwr/preview/mmwrhtml/rr5507a1.htm. Accessed November 28, 2017.
3. CDC. Viral hepatitis surveillance—United States, 2015. Available at: https://www.cdc.gov/hepatitis/statistics/2015surveillance/pdfs/2015HepSurveillanceRpt.pdf. Accessed November 28, 2017.
4. California Department of Public Health. Hepatitis A outbreak in California. Available at: https://www.cdph.ca.gov/Programs/CID/DCDC/Pages/Immunization/Hepatitis-A-Outbreak.aspx. Accessed November 28, 2017.
5. Michigan Department of Health & Human Services. Hepatitis A southeast Michigan outbreak. Available at: http://www.michigan.gov/mdhhs/0,5885,7-339-71550_2955_2976_82305_82310-447907--,00.html. Accessed November 28, 2017.
6. Utah Department of Health. Hepatitis A outbreak. Available at: http://health.utah.gov/epi/diseases/hepatitisA/HAVoutbreak_2017. Accessed November 28, 2017.
7. Latash J, Dorsinville M, Del Rosso P, et al. Notes from the field: increase in reported hepatitis A infections among men who have sex with men–New York City, January-August 2017. MMWR Morb Mortal Wkly Rep. 2017;66:999-1000.
8. CDC. Murphy TV, Denniston MM, Hill HA, et al. Progress toward eliminating hepatitis A disease in the United States. MMWR Morb Mortal Wkly Rep. 2016;65:29-41.
9. Klevens RM, Denniston MM, Jiles-Chapman RB, et al. Decreasing immunity to hepatitis A virus infection among US adults: findings from the National Health and Nutrition Examination Survey (NHANES), 1999-2012. Vaccine. 2015;33:6192-6198.
10. CDC. Vaccines and preventable diseases. Hepatitis A in-short. Available at: https://www.cdc.gov/vaccines/vpd/hepa/public/in-short-adult.html#who. Accessed November 20, 2017.
11. CDC. Updated recommendations from the Advisory Committee on Immunization Practices (ACIP) for use of hepatitis A vaccine in close contacts of newly arriving international adoptees. MMWR Morb Mortal Wkly Rep. 2009;58:1006-1007.
12. CDC. Interim outbreak-specific guidance on hepatitis A vaccine administration. Available at: https://www.cdc.gov/hepatitis/outbreaks/InterimOutbreakGuidance-HAV-VaccineAdmin.htm. Accessed November 20, 2017.
13. CDC. 2017–Outbreaks of hepatitis A in multiple states among people who are homeless and people who use drugs. Available at: https://www.cdc.gov/hepatitis/outbreaks/2017March-HepatitisA.htm. Accessed December 11, 2017.
14. CDC. Notice to readers: FDA approval of an alternate dosing schedule for a combined hepatitis A and B vaccine (Twinrix). Available at: https://www.cdc.gov/mmwr/preview/mmwrhtml/mm5640a5.htm. Accessed December 8, 2017.
15. CDC. Update: prevention of hepatitis A after exposure to hepatitis A virus and in International Travelers. Updated recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2007;56:1080-1084.
16. CDC. Current vaccine shortages and delays. Available at: https://www.cdc.gov/vaccines/hcp/clinical-resources/shortages.html. Accessed November 28, 2017.
Practice changing events of 2017
Members of the Pediatric News Editorial Advisory Board share some of the events and findings of 2017 that they believe have or will have the most impact on pediatric practice.
Francis Rushton Jr., MD, practiced pediatrics in Beaufort, S.C. for 32 years, and currently is the medical director of S.C. Quality through Technology and Innovation in Pediatrics (QTIP), funded by the South Carolina Department of Health and Human Services.
Dropping the human papillomavirus (HPV) regimen to two shots from three shots, as recommended by the Centers for Disease Control and Prevention, appears to have really improved uptake of HPV immunization.
Preventive oral health in the pediatrician’s office is not really a new recommendation from 2017; we have been talking about fluoride varnish for over a decade. What is new is that we gradually are seeing fluoride varnish move into practice, up from 1,000 applications in pediatric offices in 2011 to close to 20,000 applications in South Carolina alone.
Pediatricians are being asked to screen more and more. We’re asked to do developmental screening, postpartum depression screening, autism screening, behavioral health screening, social determinants of health screening, parental concerns screening, etc. As a result, we now have multiple different screens with different schedules. The Survey of Well-Being of Young Children screening tool does it all – one screen at each preschool well visit from birth to age 5 years.
A different approach is to use CHADIS (Child Health and Development Interactive System), a for-profit venture where all the screens are loaded electronically.
Howard Smart, MD, is chairman of pediatrics at Sharp Rees-Stealy Medical Group, San Diego.
The switch to a two-dose schedule for HPV vaccination has improved both acceptance of the vaccine and the likelihood of timely completion of the HPV series.
Kelly Curran, MD, MA, is an assistant professor of pediatrics at the University of Oklahoma Health Sciences Center, Oklahoma City, practicing adolescent medicine.
The news from Australia is that the older meningitis B vaccine (MeNZB) provides some protection against Neisseria gonorrhoeae, as reported in the Lancet (2017 July 10. doi: 10.1016/S0140-6736[17]31449-6)! The newer version of the meningitis B vaccine Bexsero also contains the same outer membrane vesicle antigen. Given increasing bacterial resistance – and pan-resistant gonorrhea organisms already in some parts of the world – this is exciting news for the future!
The increased use of the reverse screening algorithm for syphilis is exciting. Although this has been “available” for several years, increasingly more physicians/laboratories are using this in practice. Our academic center – in a relatively high prevalence area for syphilis – recently switched to this screening method.
M. Susan Jay, MD, is a professor of pediatrics and section chief of adolescent medicine at the Medical College of Wisconsin and program director of adolescent health and medicine at the Children’s Hospital of Wisconsin, both in Milwaukee.
In adolescent medicine, the addition of long-acting reversible contraceptives has been wonderful as an aid to both menstrual management and contraception. Specifically, Liletta, a new IUD that is smaller in size and remains in place for 5 years as well as being considerably more cost effective, has changed care for adolescent females.
Suzanne C. Boulter, MD, is adjunct professor of pediatrics and community and family medicine at the Geisel School of Medicine at Dartmouth in Hanover, N.H.
I was very impressed with the recent American Academy of Pediatrics policy on human trafficking published in Pediatrics (2017 November. doi: 10.1542/peds.2017-3138), and think this is a new area of knowledge of which pediatricians need to be aware.
With all the news and social media about sexual misconduct by persons in power, I’m a bit concerned that there could be a fallout on pediatricians performing appropriate examinations on their patients that could be interpreted as something else.
Timothy J. Joos, MD, MPH, is a practicing clinician in combined internal medicine/pediatrics in Seattle. For the last decade, he has worked at a federally qualified community health center in Seattle serving a largely low-income and immigrant population.
With regard to practice-changing events for 2017, I don’t wish to downplay the numerous research advances over the year, but the advances cannot be made without funding, and they are not going to be practice-changing if they can’t reach the patients. We can’t ignore the uncertainty that the current political situation in 2017 has caused for our patients and their families, as well as for research and for the health care industry in general.
It is impossible to deny the important role government health care programs play in the health of our own patients and the health of the whole country. According to numbers from the Kaiser Family Foundation website, currently 38% of the estimated 74 million kids in this country are covered by Medicaid and CHIP programs. The numbers of uninsured children are at all-time lows at 5% (adults 10%). The current uncertainty of government funding is felt strongly by safety net providers such as community health centers that have traditionally seen the uninsured patients. The community health center where I work went from 35% of its patients being uninsured before the Affordable Care Act to about 15% now.
Efforts to dismantle the Affordable Care Act and reverse Medicaid expansions, as well as delays on funding to the CHIP program, have created uncertainty and anxiety across health care from the administrators and insurance companies to us – the providers – and the families we take care of. In addition, National Institutes of Health funding is threatened to be cut by 20%. 2017 will go down in history as the year of health care toxic stress (that is, unless 2018 is worse). As we celebrate the end of the year, we all deserve a Xanax and a Zantac.
Members of the Pediatric News Editorial Advisory Board share some of the events and findings of 2017 that they believe have or will have the most impact on pediatric practice.
Francis Rushton Jr., MD, practiced pediatrics in Beaufort, S.C. for 32 years, and currently is the medical director of S.C. Quality through Technology and Innovation in Pediatrics (QTIP), funded by the South Carolina Department of Health and Human Services.
Dropping the human papillomavirus (HPV) regimen to two shots from three shots, as recommended by the Centers for Disease Control and Prevention, appears to have really improved uptake of HPV immunization.
Preventive oral health in the pediatrician’s office is not really a new recommendation from 2017; we have been talking about fluoride varnish for over a decade. What is new is that we gradually are seeing fluoride varnish move into practice, up from 1,000 applications in pediatric offices in 2011 to close to 20,000 applications in South Carolina alone.
Pediatricians are being asked to screen more and more. We’re asked to do developmental screening, postpartum depression screening, autism screening, behavioral health screening, social determinants of health screening, parental concerns screening, etc. As a result, we now have multiple different screens with different schedules. The Survey of Well-Being of Young Children screening tool does it all – one screen at each preschool well visit from birth to age 5 years.
A different approach is to use CHADIS (Child Health and Development Interactive System), a for-profit venture where all the screens are loaded electronically.
Howard Smart, MD, is chairman of pediatrics at Sharp Rees-Stealy Medical Group, San Diego.
The switch to a two-dose schedule for HPV vaccination has improved both acceptance of the vaccine and the likelihood of timely completion of the HPV series.
Kelly Curran, MD, MA, is an assistant professor of pediatrics at the University of Oklahoma Health Sciences Center, Oklahoma City, practicing adolescent medicine.
The news from Australia is that the older meningitis B vaccine (MeNZB) provides some protection against Neisseria gonorrhoeae, as reported in the Lancet (2017 July 10. doi: 10.1016/S0140-6736[17]31449-6)! The newer version of the meningitis B vaccine Bexsero also contains the same outer membrane vesicle antigen. Given increasing bacterial resistance – and pan-resistant gonorrhea organisms already in some parts of the world – this is exciting news for the future!
The increased use of the reverse screening algorithm for syphilis is exciting. Although this has been “available” for several years, increasingly more physicians/laboratories are using this in practice. Our academic center – in a relatively high prevalence area for syphilis – recently switched to this screening method.
M. Susan Jay, MD, is a professor of pediatrics and section chief of adolescent medicine at the Medical College of Wisconsin and program director of adolescent health and medicine at the Children’s Hospital of Wisconsin, both in Milwaukee.
In adolescent medicine, the addition of long-acting reversible contraceptives has been wonderful as an aid to both menstrual management and contraception. Specifically, Liletta, a new IUD that is smaller in size and remains in place for 5 years as well as being considerably more cost effective, has changed care for adolescent females.
Suzanne C. Boulter, MD, is adjunct professor of pediatrics and community and family medicine at the Geisel School of Medicine at Dartmouth in Hanover, N.H.
I was very impressed with the recent American Academy of Pediatrics policy on human trafficking published in Pediatrics (2017 November. doi: 10.1542/peds.2017-3138), and think this is a new area of knowledge of which pediatricians need to be aware.
With all the news and social media about sexual misconduct by persons in power, I’m a bit concerned that there could be a fallout on pediatricians performing appropriate examinations on their patients that could be interpreted as something else.
Timothy J. Joos, MD, MPH, is a practicing clinician in combined internal medicine/pediatrics in Seattle. For the last decade, he has worked at a federally qualified community health center in Seattle serving a largely low-income and immigrant population.
With regard to practice-changing events for 2017, I don’t wish to downplay the numerous research advances over the year, but the advances cannot be made without funding, and they are not going to be practice-changing if they can’t reach the patients. We can’t ignore the uncertainty that the current political situation in 2017 has caused for our patients and their families, as well as for research and for the health care industry in general.
It is impossible to deny the important role government health care programs play in the health of our own patients and the health of the whole country. According to numbers from the Kaiser Family Foundation website, currently 38% of the estimated 74 million kids in this country are covered by Medicaid and CHIP programs. The numbers of uninsured children are at all-time lows at 5% (adults 10%). The current uncertainty of government funding is felt strongly by safety net providers such as community health centers that have traditionally seen the uninsured patients. The community health center where I work went from 35% of its patients being uninsured before the Affordable Care Act to about 15% now.
Efforts to dismantle the Affordable Care Act and reverse Medicaid expansions, as well as delays on funding to the CHIP program, have created uncertainty and anxiety across health care from the administrators and insurance companies to us – the providers – and the families we take care of. In addition, National Institutes of Health funding is threatened to be cut by 20%. 2017 will go down in history as the year of health care toxic stress (that is, unless 2018 is worse). As we celebrate the end of the year, we all deserve a Xanax and a Zantac.
Members of the Pediatric News Editorial Advisory Board share some of the events and findings of 2017 that they believe have or will have the most impact on pediatric practice.
Francis Rushton Jr., MD, practiced pediatrics in Beaufort, S.C. for 32 years, and currently is the medical director of S.C. Quality through Technology and Innovation in Pediatrics (QTIP), funded by the South Carolina Department of Health and Human Services.
Dropping the human papillomavirus (HPV) regimen to two shots from three shots, as recommended by the Centers for Disease Control and Prevention, appears to have really improved uptake of HPV immunization.
Preventive oral health in the pediatrician’s office is not really a new recommendation from 2017; we have been talking about fluoride varnish for over a decade. What is new is that we gradually are seeing fluoride varnish move into practice, up from 1,000 applications in pediatric offices in 2011 to close to 20,000 applications in South Carolina alone.
Pediatricians are being asked to screen more and more. We’re asked to do developmental screening, postpartum depression screening, autism screening, behavioral health screening, social determinants of health screening, parental concerns screening, etc. As a result, we now have multiple different screens with different schedules. The Survey of Well-Being of Young Children screening tool does it all – one screen at each preschool well visit from birth to age 5 years.
A different approach is to use CHADIS (Child Health and Development Interactive System), a for-profit venture where all the screens are loaded electronically.
Howard Smart, MD, is chairman of pediatrics at Sharp Rees-Stealy Medical Group, San Diego.
The switch to a two-dose schedule for HPV vaccination has improved both acceptance of the vaccine and the likelihood of timely completion of the HPV series.
Kelly Curran, MD, MA, is an assistant professor of pediatrics at the University of Oklahoma Health Sciences Center, Oklahoma City, practicing adolescent medicine.
The news from Australia is that the older meningitis B vaccine (MeNZB) provides some protection against Neisseria gonorrhoeae, as reported in the Lancet (2017 July 10. doi: 10.1016/S0140-6736[17]31449-6)! The newer version of the meningitis B vaccine Bexsero also contains the same outer membrane vesicle antigen. Given increasing bacterial resistance – and pan-resistant gonorrhea organisms already in some parts of the world – this is exciting news for the future!
The increased use of the reverse screening algorithm for syphilis is exciting. Although this has been “available” for several years, increasingly more physicians/laboratories are using this in practice. Our academic center – in a relatively high prevalence area for syphilis – recently switched to this screening method.
M. Susan Jay, MD, is a professor of pediatrics and section chief of adolescent medicine at the Medical College of Wisconsin and program director of adolescent health and medicine at the Children’s Hospital of Wisconsin, both in Milwaukee.
In adolescent medicine, the addition of long-acting reversible contraceptives has been wonderful as an aid to both menstrual management and contraception. Specifically, Liletta, a new IUD that is smaller in size and remains in place for 5 years as well as being considerably more cost effective, has changed care for adolescent females.
Suzanne C. Boulter, MD, is adjunct professor of pediatrics and community and family medicine at the Geisel School of Medicine at Dartmouth in Hanover, N.H.
I was very impressed with the recent American Academy of Pediatrics policy on human trafficking published in Pediatrics (2017 November. doi: 10.1542/peds.2017-3138), and think this is a new area of knowledge of which pediatricians need to be aware.
With all the news and social media about sexual misconduct by persons in power, I’m a bit concerned that there could be a fallout on pediatricians performing appropriate examinations on their patients that could be interpreted as something else.
Timothy J. Joos, MD, MPH, is a practicing clinician in combined internal medicine/pediatrics in Seattle. For the last decade, he has worked at a federally qualified community health center in Seattle serving a largely low-income and immigrant population.
With regard to practice-changing events for 2017, I don’t wish to downplay the numerous research advances over the year, but the advances cannot be made without funding, and they are not going to be practice-changing if they can’t reach the patients. We can’t ignore the uncertainty that the current political situation in 2017 has caused for our patients and their families, as well as for research and for the health care industry in general.
It is impossible to deny the important role government health care programs play in the health of our own patients and the health of the whole country. According to numbers from the Kaiser Family Foundation website, currently 38% of the estimated 74 million kids in this country are covered by Medicaid and CHIP programs. The numbers of uninsured children are at all-time lows at 5% (adults 10%). The current uncertainty of government funding is felt strongly by safety net providers such as community health centers that have traditionally seen the uninsured patients. The community health center where I work went from 35% of its patients being uninsured before the Affordable Care Act to about 15% now.
Efforts to dismantle the Affordable Care Act and reverse Medicaid expansions, as well as delays on funding to the CHIP program, have created uncertainty and anxiety across health care from the administrators and insurance companies to us – the providers – and the families we take care of. In addition, National Institutes of Health funding is threatened to be cut by 20%. 2017 will go down in history as the year of health care toxic stress (that is, unless 2018 is worse). As we celebrate the end of the year, we all deserve a Xanax and a Zantac.
Counseling parents may curb nonmedical vaccine exemptions
Nonmedical vaccine exemptions for children in Washington State decreased by 40% after the implementation of a law requiring parent counseling and a signed form from a medical provider, based on data from a regression analysis of kindergarten students during time periods before and after the law took effect.
The Washington State Senate Bill 5005 (SB5005), implemented in 2011, requires parents seeking exemptions to file a certificate of exemption (COE) signed by a Washington-licensed health care provider. It documents that the parents have discussed “the benefits and risks of immunizations” with the provider, the researchers wrote.
The researchers examined the effect of the parent counseling and signature requirement on exemption rates by reviewing data on kindergarten students.
Overall, the significant relative decrease of 40% translated to a significant absolute reduction of 2.9% in immunization exemption rates, and vaccine coverage increased or remained the same across all vaccines required for school. The greatest decline in exemption rates occurred in geographic areas with historically high rates before the bill was passed, the researchers said.
Based on the Washington findings, “states in the United States and jurisdictions in other countries should consider adding parental counseling as a requirement for obtaining exemptions to vaccination requirements,” they concluded.
Dr. Omer had no financial conflicts to disclose. One of the coauthors disclosed ties to vaccine manufacturers, and another’s organization had such ties. The study was supported by the Robert Wood Johnson Foundation.
Restoring community immunity requires diligence on the part of medical professionals, policymakers, and parents, wrote California state senator Richard J. Pan, MD, MPH, in an accompanying editorial.
Laws requiring that children be vaccinated before starting school are designed to protect the vaccinated children and the community at large, wrote Dr. Pan, but the passage of certain laws resulted in nonmedical exemptions that can reduce the effectiveness of childhood vaccinations on community health. In the past, parents rarely chose nonmedical exemptions because they recognized the dangers of diseases such as polio and measles and acknowledged the safety and effectiveness of the vaccines to protect against them, said Dr. Pan.
“However, some saw the opportunity to exploit these circumstances for personal gain by spreading vaccine misinformation over the Internet and social media to fuel parental anxiety and promote sales of their supplements and books, leading to increased use of nonmedical exemptions,” he said.
In Dr. Pan’s view, community immunity can be restored by creating stricter policies for vaccination and eliminating nonmedical exemptions. He authored a bill in California to abolish these exemptions, and the vaccination rate was 96% among kindergartners in California during the first year the policy was in place.
But antivaccine groups are well organized. One study found that “half of all Twitter posts about vaccines contain antivaccine beliefs. Just this year [2017] in Minnesota, antivaccine groups targeted a community, causing a significant drop in vaccination rates. The resulting measles outbreak exposed more than 8,000 people, sickened 79 (of which 73 were less than 10 years old), and hospitalized 22,” Dr. Pan said.
Pediatricians and other child health advocates must continue to work to address medical exemptions as well and to define the standards for what constitutes a medical exemption, Dr. Pan said. By educating and working with parents, legislators, and other health care professionals, “pediatricians need to build the political will to pass effective vaccine policy,” he said.
Dr. Pan is a California state senator in Sacramento. He had no financial conflicts to disclose. He commented in an editorial accompanying the study by Omer et al. (Pediatrics. 2017 Dec 18;141[1]:e20173449).
Restoring community immunity requires diligence on the part of medical professionals, policymakers, and parents, wrote California state senator Richard J. Pan, MD, MPH, in an accompanying editorial.
Laws requiring that children be vaccinated before starting school are designed to protect the vaccinated children and the community at large, wrote Dr. Pan, but the passage of certain laws resulted in nonmedical exemptions that can reduce the effectiveness of childhood vaccinations on community health. In the past, parents rarely chose nonmedical exemptions because they recognized the dangers of diseases such as polio and measles and acknowledged the safety and effectiveness of the vaccines to protect against them, said Dr. Pan.
“However, some saw the opportunity to exploit these circumstances for personal gain by spreading vaccine misinformation over the Internet and social media to fuel parental anxiety and promote sales of their supplements and books, leading to increased use of nonmedical exemptions,” he said.
In Dr. Pan’s view, community immunity can be restored by creating stricter policies for vaccination and eliminating nonmedical exemptions. He authored a bill in California to abolish these exemptions, and the vaccination rate was 96% among kindergartners in California during the first year the policy was in place.
But antivaccine groups are well organized. One study found that “half of all Twitter posts about vaccines contain antivaccine beliefs. Just this year [2017] in Minnesota, antivaccine groups targeted a community, causing a significant drop in vaccination rates. The resulting measles outbreak exposed more than 8,000 people, sickened 79 (of which 73 were less than 10 years old), and hospitalized 22,” Dr. Pan said.
Pediatricians and other child health advocates must continue to work to address medical exemptions as well and to define the standards for what constitutes a medical exemption, Dr. Pan said. By educating and working with parents, legislators, and other health care professionals, “pediatricians need to build the political will to pass effective vaccine policy,” he said.
Dr. Pan is a California state senator in Sacramento. He had no financial conflicts to disclose. He commented in an editorial accompanying the study by Omer et al. (Pediatrics. 2017 Dec 18;141[1]:e20173449).
Restoring community immunity requires diligence on the part of medical professionals, policymakers, and parents, wrote California state senator Richard J. Pan, MD, MPH, in an accompanying editorial.
Laws requiring that children be vaccinated before starting school are designed to protect the vaccinated children and the community at large, wrote Dr. Pan, but the passage of certain laws resulted in nonmedical exemptions that can reduce the effectiveness of childhood vaccinations on community health. In the past, parents rarely chose nonmedical exemptions because they recognized the dangers of diseases such as polio and measles and acknowledged the safety and effectiveness of the vaccines to protect against them, said Dr. Pan.
“However, some saw the opportunity to exploit these circumstances for personal gain by spreading vaccine misinformation over the Internet and social media to fuel parental anxiety and promote sales of their supplements and books, leading to increased use of nonmedical exemptions,” he said.
In Dr. Pan’s view, community immunity can be restored by creating stricter policies for vaccination and eliminating nonmedical exemptions. He authored a bill in California to abolish these exemptions, and the vaccination rate was 96% among kindergartners in California during the first year the policy was in place.
But antivaccine groups are well organized. One study found that “half of all Twitter posts about vaccines contain antivaccine beliefs. Just this year [2017] in Minnesota, antivaccine groups targeted a community, causing a significant drop in vaccination rates. The resulting measles outbreak exposed more than 8,000 people, sickened 79 (of which 73 were less than 10 years old), and hospitalized 22,” Dr. Pan said.
Pediatricians and other child health advocates must continue to work to address medical exemptions as well and to define the standards for what constitutes a medical exemption, Dr. Pan said. By educating and working with parents, legislators, and other health care professionals, “pediatricians need to build the political will to pass effective vaccine policy,” he said.
Dr. Pan is a California state senator in Sacramento. He had no financial conflicts to disclose. He commented in an editorial accompanying the study by Omer et al. (Pediatrics. 2017 Dec 18;141[1]:e20173449).
Nonmedical vaccine exemptions for children in Washington State decreased by 40% after the implementation of a law requiring parent counseling and a signed form from a medical provider, based on data from a regression analysis of kindergarten students during time periods before and after the law took effect.
The Washington State Senate Bill 5005 (SB5005), implemented in 2011, requires parents seeking exemptions to file a certificate of exemption (COE) signed by a Washington-licensed health care provider. It documents that the parents have discussed “the benefits and risks of immunizations” with the provider, the researchers wrote.
The researchers examined the effect of the parent counseling and signature requirement on exemption rates by reviewing data on kindergarten students.
Overall, the significant relative decrease of 40% translated to a significant absolute reduction of 2.9% in immunization exemption rates, and vaccine coverage increased or remained the same across all vaccines required for school. The greatest decline in exemption rates occurred in geographic areas with historically high rates before the bill was passed, the researchers said.
Based on the Washington findings, “states in the United States and jurisdictions in other countries should consider adding parental counseling as a requirement for obtaining exemptions to vaccination requirements,” they concluded.
Dr. Omer had no financial conflicts to disclose. One of the coauthors disclosed ties to vaccine manufacturers, and another’s organization had such ties. The study was supported by the Robert Wood Johnson Foundation.
Nonmedical vaccine exemptions for children in Washington State decreased by 40% after the implementation of a law requiring parent counseling and a signed form from a medical provider, based on data from a regression analysis of kindergarten students during time periods before and after the law took effect.
The Washington State Senate Bill 5005 (SB5005), implemented in 2011, requires parents seeking exemptions to file a certificate of exemption (COE) signed by a Washington-licensed health care provider. It documents that the parents have discussed “the benefits and risks of immunizations” with the provider, the researchers wrote.
The researchers examined the effect of the parent counseling and signature requirement on exemption rates by reviewing data on kindergarten students.
Overall, the significant relative decrease of 40% translated to a significant absolute reduction of 2.9% in immunization exemption rates, and vaccine coverage increased or remained the same across all vaccines required for school. The greatest decline in exemption rates occurred in geographic areas with historically high rates before the bill was passed, the researchers said.
Based on the Washington findings, “states in the United States and jurisdictions in other countries should consider adding parental counseling as a requirement for obtaining exemptions to vaccination requirements,” they concluded.
Dr. Omer had no financial conflicts to disclose. One of the coauthors disclosed ties to vaccine manufacturers, and another’s organization had such ties. The study was supported by the Robert Wood Johnson Foundation.
FROM PEDIATRICS
Key clinical point:
Major finding: After the implementation of SB5005 in Washington State, the rate of exemptions decreased by 40%.
Data source: The data come from a regression analysis of immunization coverage and exemption rates in Washington State for the school years 1997-1998 through 2013-2014.
Disclosures: Dr. Omer had no financial conflicts to disclose. One of the coauthors disclosed ties to vaccine manufacturers, and another’s organization had such ties. The study was supported by the Robert Wood Johnson Foundation.
Source: Omer SB et al. Pediatrics. 2018;141(1):e20172364.