User login
Heavy cannabis use in pregnancy correlates with risks to infant
Cannabis use that interferes with a woman’s ability to function during pregnancy is a risk factor for severe health problems in the child, new research indicates.
Pregnant women with cannabis use disorder are more likely to have children with low birth weights and children who die within 1 year of birth, compared with matched controls, according to a study published online in Addiction.
The death rate among infants exposed to prenatal cannabis use disorder was 0.98%, compared with 0.75% among infants whose mothers did not have this diagnosis.
Cannabis use disorder during pregnancy “has increased dramatically in the past two decades,” but few studies have examined the health impacts on offspring, study author Yuyan Shi, PhD, said in an interview. “It is particularly concerning in states with cannabis legalization where cannabis is increasingly available.”
Dr. Shi, a researcher at the Herbert Wertheim School of Public Health and Human Longevity Science at the University of California, San Diego, and colleagues analyzed data from more than 4.8 million mothers who delivered a live singleton birth in California between 2001 and 2012 and their infants. They focused on 20,237 mothers who had a diagnosis of cannabis use disorder at delivery. The disorder is defined by continued use of the drug despite impairments in physical, psychological, and social functioning.
The researchers matched mothers with cannabis use disorder 1:2 to mothers who did not have this diagnosis. They aimed to balance factors such as maternal age, educational attainment, health insurance, physical and mental health conditions, prenatal care, and alcohol and opioid use disorder.
An increasingly common diagnosis
Over the study period, the rate of cannabis use disorder increased from 2.8 cases per 1,000 deliveries in 2001 to 6.9 cases per 1,000 deliveries in 2012.
Cannabis use disorder was associated with increased odds of preterm birth (odds ratio, 1.06), small for gestational age (OR, 1.13), low birth weight (OR, 1.13), and death within 1 year of birth (OR, 1.35), according to the researchers’ estimates. Cannabis use disorder was associated with lower odds of hospitalization within 1 year of birth, however (OR, 0.91).
“The most notable observation is that exposed infants were 35% more likely to die within 1 year of birth than unexposed infants,” Dr. Shi and colleagues wrote. More research is needed to understand the causes of death at different stages of infancy, they said.
The results “imply that cannabis use disorder screening as well as appropriate education, counseling, or referral to substance abuse treatment services should be encouraged among pregnant women,” Dr. Shi said.
The study does not establish that cannabis use disorder causes adverse effects, and it is not clear how the results might apply to mothers who use cannabis but do not meet diagnostic criteria for the disorder, the authors noted.
“Presumably the health consequences of mothers who use cannabis but do not meet the criteria ... are less severe than mothers with cannabis use disorder,” Dr. Shi said. “Unfortunately, no research has been conducted to test this hypothesis.”
Enough data to recommend abstaining
Many clinicians may not feel equipped to make a diagnosis of cannabis use disorder, said Jamie Lo, MD, assistant professor of obstetrics and gynecology at Oregon Health and Science University in Portland.
Although many clinicians ask patients about substance use in general, specifically screening for cannabis use is not necessarily routine practice. “I think people are starting to adopt that, but it probably will take a little bit of time,” Dr. Lo said.
Dr. Lo, who was not involved in the study, researches the effects of marijuana during pregnancy.
Confounding factors such as frequent co-use of tobacco have so far made it “difficult to suss out” whether observed effects are directly from cannabis use, other substances or exposures, or a combination, said Dr. Lo. The possibility that stigma may lead to inaccurate self-reporting poses another challenge. And the range of cannabis delivery devices further complicates matters.
“It is hard to compare smoking a bowl versus a joint versus using the oils or CBD or edibles,” Dr. Lo said. The data regarding cigarettes and alcohol are cleaner and more precise, in comparison.
Still, federal agencies and professional societies agree that “what we do know is enough to recommend that pregnant women abstain from using cannabis during pregnancy,” Dr. Lo said.
The National Institute on Drug Abuse, which funded the study, said the results add to the evidence that prenatal exposure to cannabis may be associated with poor birth outcomes and infant health.
“While we cannot establish that cannabis use caused negative outcomes in this study, these data reinforce the case for caution around using cannabis during pregnancy,” Nora D. Volkow, MD, the director of the agency, said in a news release.
“Careful analysis of data like these is one way we can responsibly study how cannabis use affects the developing child, all while a natural experiment is playing out across our country in places where cannabis is becoming widely available to pregnant consumers.”
The study authors and Dr. Lo had no disclosures.
Cannabis use that interferes with a woman’s ability to function during pregnancy is a risk factor for severe health problems in the child, new research indicates.
Pregnant women with cannabis use disorder are more likely to have children with low birth weights and children who die within 1 year of birth, compared with matched controls, according to a study published online in Addiction.
The death rate among infants exposed to prenatal cannabis use disorder was 0.98%, compared with 0.75% among infants whose mothers did not have this diagnosis.
Cannabis use disorder during pregnancy “has increased dramatically in the past two decades,” but few studies have examined the health impacts on offspring, study author Yuyan Shi, PhD, said in an interview. “It is particularly concerning in states with cannabis legalization where cannabis is increasingly available.”
Dr. Shi, a researcher at the Herbert Wertheim School of Public Health and Human Longevity Science at the University of California, San Diego, and colleagues analyzed data from more than 4.8 million mothers who delivered a live singleton birth in California between 2001 and 2012 and their infants. They focused on 20,237 mothers who had a diagnosis of cannabis use disorder at delivery. The disorder is defined by continued use of the drug despite impairments in physical, psychological, and social functioning.
The researchers matched mothers with cannabis use disorder 1:2 to mothers who did not have this diagnosis. They aimed to balance factors such as maternal age, educational attainment, health insurance, physical and mental health conditions, prenatal care, and alcohol and opioid use disorder.
An increasingly common diagnosis
Over the study period, the rate of cannabis use disorder increased from 2.8 cases per 1,000 deliveries in 2001 to 6.9 cases per 1,000 deliveries in 2012.
Cannabis use disorder was associated with increased odds of preterm birth (odds ratio, 1.06), small for gestational age (OR, 1.13), low birth weight (OR, 1.13), and death within 1 year of birth (OR, 1.35), according to the researchers’ estimates. Cannabis use disorder was associated with lower odds of hospitalization within 1 year of birth, however (OR, 0.91).
“The most notable observation is that exposed infants were 35% more likely to die within 1 year of birth than unexposed infants,” Dr. Shi and colleagues wrote. More research is needed to understand the causes of death at different stages of infancy, they said.
The results “imply that cannabis use disorder screening as well as appropriate education, counseling, or referral to substance abuse treatment services should be encouraged among pregnant women,” Dr. Shi said.
The study does not establish that cannabis use disorder causes adverse effects, and it is not clear how the results might apply to mothers who use cannabis but do not meet diagnostic criteria for the disorder, the authors noted.
“Presumably the health consequences of mothers who use cannabis but do not meet the criteria ... are less severe than mothers with cannabis use disorder,” Dr. Shi said. “Unfortunately, no research has been conducted to test this hypothesis.”
Enough data to recommend abstaining
Many clinicians may not feel equipped to make a diagnosis of cannabis use disorder, said Jamie Lo, MD, assistant professor of obstetrics and gynecology at Oregon Health and Science University in Portland.
Although many clinicians ask patients about substance use in general, specifically screening for cannabis use is not necessarily routine practice. “I think people are starting to adopt that, but it probably will take a little bit of time,” Dr. Lo said.
Dr. Lo, who was not involved in the study, researches the effects of marijuana during pregnancy.
Confounding factors such as frequent co-use of tobacco have so far made it “difficult to suss out” whether observed effects are directly from cannabis use, other substances or exposures, or a combination, said Dr. Lo. The possibility that stigma may lead to inaccurate self-reporting poses another challenge. And the range of cannabis delivery devices further complicates matters.
“It is hard to compare smoking a bowl versus a joint versus using the oils or CBD or edibles,” Dr. Lo said. The data regarding cigarettes and alcohol are cleaner and more precise, in comparison.
Still, federal agencies and professional societies agree that “what we do know is enough to recommend that pregnant women abstain from using cannabis during pregnancy,” Dr. Lo said.
The National Institute on Drug Abuse, which funded the study, said the results add to the evidence that prenatal exposure to cannabis may be associated with poor birth outcomes and infant health.
“While we cannot establish that cannabis use caused negative outcomes in this study, these data reinforce the case for caution around using cannabis during pregnancy,” Nora D. Volkow, MD, the director of the agency, said in a news release.
“Careful analysis of data like these is one way we can responsibly study how cannabis use affects the developing child, all while a natural experiment is playing out across our country in places where cannabis is becoming widely available to pregnant consumers.”
The study authors and Dr. Lo had no disclosures.
Cannabis use that interferes with a woman’s ability to function during pregnancy is a risk factor for severe health problems in the child, new research indicates.
Pregnant women with cannabis use disorder are more likely to have children with low birth weights and children who die within 1 year of birth, compared with matched controls, according to a study published online in Addiction.
The death rate among infants exposed to prenatal cannabis use disorder was 0.98%, compared with 0.75% among infants whose mothers did not have this diagnosis.
Cannabis use disorder during pregnancy “has increased dramatically in the past two decades,” but few studies have examined the health impacts on offspring, study author Yuyan Shi, PhD, said in an interview. “It is particularly concerning in states with cannabis legalization where cannabis is increasingly available.”
Dr. Shi, a researcher at the Herbert Wertheim School of Public Health and Human Longevity Science at the University of California, San Diego, and colleagues analyzed data from more than 4.8 million mothers who delivered a live singleton birth in California between 2001 and 2012 and their infants. They focused on 20,237 mothers who had a diagnosis of cannabis use disorder at delivery. The disorder is defined by continued use of the drug despite impairments in physical, psychological, and social functioning.
The researchers matched mothers with cannabis use disorder 1:2 to mothers who did not have this diagnosis. They aimed to balance factors such as maternal age, educational attainment, health insurance, physical and mental health conditions, prenatal care, and alcohol and opioid use disorder.
An increasingly common diagnosis
Over the study period, the rate of cannabis use disorder increased from 2.8 cases per 1,000 deliveries in 2001 to 6.9 cases per 1,000 deliveries in 2012.
Cannabis use disorder was associated with increased odds of preterm birth (odds ratio, 1.06), small for gestational age (OR, 1.13), low birth weight (OR, 1.13), and death within 1 year of birth (OR, 1.35), according to the researchers’ estimates. Cannabis use disorder was associated with lower odds of hospitalization within 1 year of birth, however (OR, 0.91).
“The most notable observation is that exposed infants were 35% more likely to die within 1 year of birth than unexposed infants,” Dr. Shi and colleagues wrote. More research is needed to understand the causes of death at different stages of infancy, they said.
The results “imply that cannabis use disorder screening as well as appropriate education, counseling, or referral to substance abuse treatment services should be encouraged among pregnant women,” Dr. Shi said.
The study does not establish that cannabis use disorder causes adverse effects, and it is not clear how the results might apply to mothers who use cannabis but do not meet diagnostic criteria for the disorder, the authors noted.
“Presumably the health consequences of mothers who use cannabis but do not meet the criteria ... are less severe than mothers with cannabis use disorder,” Dr. Shi said. “Unfortunately, no research has been conducted to test this hypothesis.”
Enough data to recommend abstaining
Many clinicians may not feel equipped to make a diagnosis of cannabis use disorder, said Jamie Lo, MD, assistant professor of obstetrics and gynecology at Oregon Health and Science University in Portland.
Although many clinicians ask patients about substance use in general, specifically screening for cannabis use is not necessarily routine practice. “I think people are starting to adopt that, but it probably will take a little bit of time,” Dr. Lo said.
Dr. Lo, who was not involved in the study, researches the effects of marijuana during pregnancy.
Confounding factors such as frequent co-use of tobacco have so far made it “difficult to suss out” whether observed effects are directly from cannabis use, other substances or exposures, or a combination, said Dr. Lo. The possibility that stigma may lead to inaccurate self-reporting poses another challenge. And the range of cannabis delivery devices further complicates matters.
“It is hard to compare smoking a bowl versus a joint versus using the oils or CBD or edibles,” Dr. Lo said. The data regarding cigarettes and alcohol are cleaner and more precise, in comparison.
Still, federal agencies and professional societies agree that “what we do know is enough to recommend that pregnant women abstain from using cannabis during pregnancy,” Dr. Lo said.
The National Institute on Drug Abuse, which funded the study, said the results add to the evidence that prenatal exposure to cannabis may be associated with poor birth outcomes and infant health.
“While we cannot establish that cannabis use caused negative outcomes in this study, these data reinforce the case for caution around using cannabis during pregnancy,” Nora D. Volkow, MD, the director of the agency, said in a news release.
“Careful analysis of data like these is one way we can responsibly study how cannabis use affects the developing child, all while a natural experiment is playing out across our country in places where cannabis is becoming widely available to pregnant consumers.”
The study authors and Dr. Lo had no disclosures.
55 new chemicals found in pregnant women, their newborns
Fifty-five chemicals never before reported in humans were found in pregnant women, according to a study from the University of California, San Francisco. The chemicals likely come from consumer products or industrial sources, researchers say.
Findings were published online in Environmental Science and Technology.
Co-first authors Aolin Wang, PhD, and Dimitri Panagopoulos Abrahamsson, PhD, postdoctoral fellows in UCSF’s obstetrics and gynecology department, and colleagues found 109 chemicals in the blood of pregnant women, including 42 “mystery chemicals” whose sources and uses are not known.
The chemicals were also found in their newborns, tests from umbilical cord blood show, suggesting the chemicals cross through the placenta.
Among the chemicals, 40 are used as plasticizers, 28 are used in cosmetics, another 25 are used in consumer products, 29 as pharmaceuticals, 23 as pesticides, three as flame retardants, and seven are PFAS [per- and polyfluoroalkyl substances] compounds used in multiple applications including carpeting and upholstery, the authors report.
Senior author Tracey Woodruff, PhD, MPH, characterized their discoveries as “disturbing.”
She told this news organization that it’s not only frustrating to know the chemicals are present but to know so little about them.
“We know it’s a chemical registered to be manufactured, and it’s used in commerce, but we don’t know where,” she explained. “That’s very disturbing, that we can’t trace them, and that shows a failure in public policy and government.”
“Exposures are occurring without our consent,” said Ms. Woodruff, a former U.S. Environmental Protection Agency scientist, who directs the Program on Reproductive Health and the Environment (PRHE) and the Environmental Research and Translation for Health (EaRTH) Center, both at UCSF.
She said researchers know from previous studies that when the U.S. government acts to remove harmful chemicals from the marketplace, the levels of those chemicals measured in people drop.
“Examples include lead, certain PFAS, flame retardant chemicals, and certain phthalates,” she said. “So public policies can be effective in preventing exposures that can be harmful.”
Technological advances led to the discoveries
The team used high-resolution mass spectrometry (HRMS) to identify human-made chemicals in people.
Dr. Abrahamsson said in an interview that the technology is relatively new in research and had not previously been used to scan for chemicals in pregnant women and their infants.
Because scientists often study what other scientists have studied, he said, the same chemicals tend to get attention. The wider scope made possible by the new technology helps illumine where to focus future research, he said.
A benefit of the technology is that now researchers don’t have to know which chemicals they are looking for when they scan blood samples, but they can observe whatever appears, he said.
Ms. Woodruff said, “We hope this is further data and evidence that support government policies that require industries to tell us where they are using their chemicals and how we might be exposed to them.”
She said this research will also help identify which chemicals to prioritize for monitoring in the environment.
Average age of the women in the study was 32 years. Nearly half were Hispanic; 37% were non-Hispanic Whites; and 17% were non-Hispanic Asians, Pacific Islanders, and African Americans. Half of the participants were born outside the United States and had lived in the U.S. for an average 22 years.
Sean Palfrey, MD, a professor of clinical pediatrics and public health at Boston University, said more chemical discoveries like these will come as technology continues to evolve.
Dr. Palfrey, who was not involved in the study, agrees with the authors that there is a lack of oversight as to what substances are used in products.
“Our industrial regulations are very poor and therefore our industries get away with using new and untested substances in their products,” he told this news organization.
“This lack of regulation is really important when it results in us not recognizing that known and serious toxins are being put into foods or other products, or when a new class of toxin has been invented which is a serious poison. Most of the toxins, though, are discovered in products in very low levels,” he said.
Dr. Palfrey said, however, that focus should stay on the known and serious toxins that seep into the environment from common products.
“It has taken us decades to ban certain flame retardants from home products,” he said. “TOSCA [the Toxic Substances Control Act passed by Congress in 1976] was too limited when it was passed decades ago and is now fearfully out of date. Unless we discover a COVID among the toxins discovered in studies like this, we should focus on the big stuff.”
The authors and Dr. Palfrey have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Fifty-five chemicals never before reported in humans were found in pregnant women, according to a study from the University of California, San Francisco. The chemicals likely come from consumer products or industrial sources, researchers say.
Findings were published online in Environmental Science and Technology.
Co-first authors Aolin Wang, PhD, and Dimitri Panagopoulos Abrahamsson, PhD, postdoctoral fellows in UCSF’s obstetrics and gynecology department, and colleagues found 109 chemicals in the blood of pregnant women, including 42 “mystery chemicals” whose sources and uses are not known.
The chemicals were also found in their newborns, tests from umbilical cord blood show, suggesting the chemicals cross through the placenta.
Among the chemicals, 40 are used as plasticizers, 28 are used in cosmetics, another 25 are used in consumer products, 29 as pharmaceuticals, 23 as pesticides, three as flame retardants, and seven are PFAS [per- and polyfluoroalkyl substances] compounds used in multiple applications including carpeting and upholstery, the authors report.
Senior author Tracey Woodruff, PhD, MPH, characterized their discoveries as “disturbing.”
She told this news organization that it’s not only frustrating to know the chemicals are present but to know so little about them.
“We know it’s a chemical registered to be manufactured, and it’s used in commerce, but we don’t know where,” she explained. “That’s very disturbing, that we can’t trace them, and that shows a failure in public policy and government.”
“Exposures are occurring without our consent,” said Ms. Woodruff, a former U.S. Environmental Protection Agency scientist, who directs the Program on Reproductive Health and the Environment (PRHE) and the Environmental Research and Translation for Health (EaRTH) Center, both at UCSF.
She said researchers know from previous studies that when the U.S. government acts to remove harmful chemicals from the marketplace, the levels of those chemicals measured in people drop.
“Examples include lead, certain PFAS, flame retardant chemicals, and certain phthalates,” she said. “So public policies can be effective in preventing exposures that can be harmful.”
Technological advances led to the discoveries
The team used high-resolution mass spectrometry (HRMS) to identify human-made chemicals in people.
Dr. Abrahamsson said in an interview that the technology is relatively new in research and had not previously been used to scan for chemicals in pregnant women and their infants.
Because scientists often study what other scientists have studied, he said, the same chemicals tend to get attention. The wider scope made possible by the new technology helps illumine where to focus future research, he said.
A benefit of the technology is that now researchers don’t have to know which chemicals they are looking for when they scan blood samples, but they can observe whatever appears, he said.
Ms. Woodruff said, “We hope this is further data and evidence that support government policies that require industries to tell us where they are using their chemicals and how we might be exposed to them.”
She said this research will also help identify which chemicals to prioritize for monitoring in the environment.
Average age of the women in the study was 32 years. Nearly half were Hispanic; 37% were non-Hispanic Whites; and 17% were non-Hispanic Asians, Pacific Islanders, and African Americans. Half of the participants were born outside the United States and had lived in the U.S. for an average 22 years.
Sean Palfrey, MD, a professor of clinical pediatrics and public health at Boston University, said more chemical discoveries like these will come as technology continues to evolve.
Dr. Palfrey, who was not involved in the study, agrees with the authors that there is a lack of oversight as to what substances are used in products.
“Our industrial regulations are very poor and therefore our industries get away with using new and untested substances in their products,” he told this news organization.
“This lack of regulation is really important when it results in us not recognizing that known and serious toxins are being put into foods or other products, or when a new class of toxin has been invented which is a serious poison. Most of the toxins, though, are discovered in products in very low levels,” he said.
Dr. Palfrey said, however, that focus should stay on the known and serious toxins that seep into the environment from common products.
“It has taken us decades to ban certain flame retardants from home products,” he said. “TOSCA [the Toxic Substances Control Act passed by Congress in 1976] was too limited when it was passed decades ago and is now fearfully out of date. Unless we discover a COVID among the toxins discovered in studies like this, we should focus on the big stuff.”
The authors and Dr. Palfrey have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Fifty-five chemicals never before reported in humans were found in pregnant women, according to a study from the University of California, San Francisco. The chemicals likely come from consumer products or industrial sources, researchers say.
Findings were published online in Environmental Science and Technology.
Co-first authors Aolin Wang, PhD, and Dimitri Panagopoulos Abrahamsson, PhD, postdoctoral fellows in UCSF’s obstetrics and gynecology department, and colleagues found 109 chemicals in the blood of pregnant women, including 42 “mystery chemicals” whose sources and uses are not known.
The chemicals were also found in their newborns, tests from umbilical cord blood show, suggesting the chemicals cross through the placenta.
Among the chemicals, 40 are used as plasticizers, 28 are used in cosmetics, another 25 are used in consumer products, 29 as pharmaceuticals, 23 as pesticides, three as flame retardants, and seven are PFAS [per- and polyfluoroalkyl substances] compounds used in multiple applications including carpeting and upholstery, the authors report.
Senior author Tracey Woodruff, PhD, MPH, characterized their discoveries as “disturbing.”
She told this news organization that it’s not only frustrating to know the chemicals are present but to know so little about them.
“We know it’s a chemical registered to be manufactured, and it’s used in commerce, but we don’t know where,” she explained. “That’s very disturbing, that we can’t trace them, and that shows a failure in public policy and government.”
“Exposures are occurring without our consent,” said Ms. Woodruff, a former U.S. Environmental Protection Agency scientist, who directs the Program on Reproductive Health and the Environment (PRHE) and the Environmental Research and Translation for Health (EaRTH) Center, both at UCSF.
She said researchers know from previous studies that when the U.S. government acts to remove harmful chemicals from the marketplace, the levels of those chemicals measured in people drop.
“Examples include lead, certain PFAS, flame retardant chemicals, and certain phthalates,” she said. “So public policies can be effective in preventing exposures that can be harmful.”
Technological advances led to the discoveries
The team used high-resolution mass spectrometry (HRMS) to identify human-made chemicals in people.
Dr. Abrahamsson said in an interview that the technology is relatively new in research and had not previously been used to scan for chemicals in pregnant women and their infants.
Because scientists often study what other scientists have studied, he said, the same chemicals tend to get attention. The wider scope made possible by the new technology helps illumine where to focus future research, he said.
A benefit of the technology is that now researchers don’t have to know which chemicals they are looking for when they scan blood samples, but they can observe whatever appears, he said.
Ms. Woodruff said, “We hope this is further data and evidence that support government policies that require industries to tell us where they are using their chemicals and how we might be exposed to them.”
She said this research will also help identify which chemicals to prioritize for monitoring in the environment.
Average age of the women in the study was 32 years. Nearly half were Hispanic; 37% were non-Hispanic Whites; and 17% were non-Hispanic Asians, Pacific Islanders, and African Americans. Half of the participants were born outside the United States and had lived in the U.S. for an average 22 years.
Sean Palfrey, MD, a professor of clinical pediatrics and public health at Boston University, said more chemical discoveries like these will come as technology continues to evolve.
Dr. Palfrey, who was not involved in the study, agrees with the authors that there is a lack of oversight as to what substances are used in products.
“Our industrial regulations are very poor and therefore our industries get away with using new and untested substances in their products,” he told this news organization.
“This lack of regulation is really important when it results in us not recognizing that known and serious toxins are being put into foods or other products, or when a new class of toxin has been invented which is a serious poison. Most of the toxins, though, are discovered in products in very low levels,” he said.
Dr. Palfrey said, however, that focus should stay on the known and serious toxins that seep into the environment from common products.
“It has taken us decades to ban certain flame retardants from home products,” he said. “TOSCA [the Toxic Substances Control Act passed by Congress in 1976] was too limited when it was passed decades ago and is now fearfully out of date. Unless we discover a COVID among the toxins discovered in studies like this, we should focus on the big stuff.”
The authors and Dr. Palfrey have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Self-harm is a leading cause of death for new moms
Death by self-harm through suicide or overdose is a leading cause of death for women in the first year post partum, data indicate. Many of these deaths may be preventable, said Adrienne Griffen, MPP, executive director of the Maternal Mental Health Leadership Alliance.
Ms. Griffen discussed these findings and ways clinicians may be able to help at the 2021 virtual meeting of the American College of Obstetricians and Gynecologists.
Women “visit a health care provider an average of 25 times during a healthy pregnancy and first year of baby’s life,” she said. “Obstetric and primary care providers who serve pregnant and postpartum women are uniquely positioned to intervene effectively to screen and assess women for mental health disorders.”
To that end, clinicians should discuss mental health “early and often,” Ms. Griffen said.
“Asking about mental health issues and suicide will not cause women to think these thoughts,” she said. “We cannot wait for women to raise their hand and ask for help because by the time they do that, they needed help many weeks ago.”
For example, a doctor might tell a patient: “Your mental health is just as important as your physical health, and anxiety and depression are the most common complications of pregnancy and childbirth,” Ms. Griffen suggested. “Every time I see you, I’m going to ask you how you are doing, and we’ll do a formal screening assessment periodically over the course of the pregnancy. … Your job is to answer us honestly so that we can connect you with resources as soon as possible to minimize the impact on you and your baby.”
Although the obstetric provider should introduce this topic, a nurse, lactation consultant, or social worker may conduct screenings and help patients who are experiencing distress, she said.
During the past decade, several medical associations have issued new guidance around screening new mothers for anxiety and depression. One recent ACOG committee opinion recommends screening for depression at least once during pregnancy and once post partum, and encourages doctors to initiate medical therapy if possible and provide resources and referrals.
Another committee opinion suggests that doctors should have contact with a patient between 2 and 3 weeks post partum, primarily to assess for mental health.
Limited data
In discussing maternal suicide statistics, Ms. Griffen focused on data from Maternal Mortality Review Committees (MMRCs).
Two other sources of data about maternal mortality – the National Vital Statistics System and the Pregnancy Mortality Surveillance System – do not include information about suicide, which may be a reason this cause of death is not discussed more often, Ms. Griffen noted.
MMRCs, on the other hand, include information about suicide and self-harm. About half of the states in the United States have these multidisciplinary committees. Committee members review deaths of all women during pregnancy or within 1 year of pregnancy. Members consider a range of clinical and nonclinical data, including reports from social services and police, to try to understand the circumstances of each death.
A report that examined pregnancy-related deaths using data from 14 U.S. MMRCs between 2008 and 2017 showed that mental health conditions were the leading cause of death for non-Hispanic White women. In all, 34% of pregnancy-related suicide deaths had a documented prior suicide attempt, and the majority of suicides happened in the late postpartum time frame (43-365 days post partum).
Some physicians cite a lack of education, time, reimbursement, or referral resources as barriers to maternal mental health screening and treatment, but there may be useful options available, Ms. Griffen said. Postpartum Support International provides resources for physicians, as well as mothers. The National Curriculum in Reproductive Psychiatry and the Seleni Institute also have educational resources.
Some states have psychiatry access programs, where psychiatrists educate obstetricians, family physicians, and pediatricians about how to assess for and treat maternal mental health issues, Ms. Griffen noted.
Self care, social support, and talk therapy may help patients. “Sometimes medication is needed, but a combination of all of these things … can help women recover from maternal mental health conditions,” Ms. Griffen said.
Need to intervene
Although medical societies have emphasized the importance of maternal mental health screening and treatment in recent years, the risk of self-harm has been a concern for obstetricians and gynecologists long before then, said Marc Alan Landsberg, MD, a member of the meeting’s scientific committee who moderated the session.
“We have been talking about this at ACOG for a long time,” Dr. Landsberg said in an interview.
The presentation highlighted why obstetricians, gynecologists, and other doctors who deliver babies and care for women post partum “have got to screen these people,” he said. The finding that 34% of pregnancy-related suicide deaths had a prior suicide attempt indicates that clinicians may be able to identify these patients, Dr. Landsberg said. Suicide and overdose are leading causes of death in the first year post partum and “probably 100% of these are preventable,” he said.
As a first step, screening may be relatively simple. The Edinburgh Postnatal Depression Scale, highlighted during the talk, is an easy and quick tool to use, Dr. Landsberg said. It contains 10 items and assesses for anxiety and depression. It also specifically asks about suicide.
Ms. Griffen and Dr. Landsberg had no conflicts of interest.
Death by self-harm through suicide or overdose is a leading cause of death for women in the first year post partum, data indicate. Many of these deaths may be preventable, said Adrienne Griffen, MPP, executive director of the Maternal Mental Health Leadership Alliance.
Ms. Griffen discussed these findings and ways clinicians may be able to help at the 2021 virtual meeting of the American College of Obstetricians and Gynecologists.
Women “visit a health care provider an average of 25 times during a healthy pregnancy and first year of baby’s life,” she said. “Obstetric and primary care providers who serve pregnant and postpartum women are uniquely positioned to intervene effectively to screen and assess women for mental health disorders.”
To that end, clinicians should discuss mental health “early and often,” Ms. Griffen said.
“Asking about mental health issues and suicide will not cause women to think these thoughts,” she said. “We cannot wait for women to raise their hand and ask for help because by the time they do that, they needed help many weeks ago.”
For example, a doctor might tell a patient: “Your mental health is just as important as your physical health, and anxiety and depression are the most common complications of pregnancy and childbirth,” Ms. Griffen suggested. “Every time I see you, I’m going to ask you how you are doing, and we’ll do a formal screening assessment periodically over the course of the pregnancy. … Your job is to answer us honestly so that we can connect you with resources as soon as possible to minimize the impact on you and your baby.”
Although the obstetric provider should introduce this topic, a nurse, lactation consultant, or social worker may conduct screenings and help patients who are experiencing distress, she said.
During the past decade, several medical associations have issued new guidance around screening new mothers for anxiety and depression. One recent ACOG committee opinion recommends screening for depression at least once during pregnancy and once post partum, and encourages doctors to initiate medical therapy if possible and provide resources and referrals.
Another committee opinion suggests that doctors should have contact with a patient between 2 and 3 weeks post partum, primarily to assess for mental health.
Limited data
In discussing maternal suicide statistics, Ms. Griffen focused on data from Maternal Mortality Review Committees (MMRCs).
Two other sources of data about maternal mortality – the National Vital Statistics System and the Pregnancy Mortality Surveillance System – do not include information about suicide, which may be a reason this cause of death is not discussed more often, Ms. Griffen noted.
MMRCs, on the other hand, include information about suicide and self-harm. About half of the states in the United States have these multidisciplinary committees. Committee members review deaths of all women during pregnancy or within 1 year of pregnancy. Members consider a range of clinical and nonclinical data, including reports from social services and police, to try to understand the circumstances of each death.
A report that examined pregnancy-related deaths using data from 14 U.S. MMRCs between 2008 and 2017 showed that mental health conditions were the leading cause of death for non-Hispanic White women. In all, 34% of pregnancy-related suicide deaths had a documented prior suicide attempt, and the majority of suicides happened in the late postpartum time frame (43-365 days post partum).
Some physicians cite a lack of education, time, reimbursement, or referral resources as barriers to maternal mental health screening and treatment, but there may be useful options available, Ms. Griffen said. Postpartum Support International provides resources for physicians, as well as mothers. The National Curriculum in Reproductive Psychiatry and the Seleni Institute also have educational resources.
Some states have psychiatry access programs, where psychiatrists educate obstetricians, family physicians, and pediatricians about how to assess for and treat maternal mental health issues, Ms. Griffen noted.
Self care, social support, and talk therapy may help patients. “Sometimes medication is needed, but a combination of all of these things … can help women recover from maternal mental health conditions,” Ms. Griffen said.
Need to intervene
Although medical societies have emphasized the importance of maternal mental health screening and treatment in recent years, the risk of self-harm has been a concern for obstetricians and gynecologists long before then, said Marc Alan Landsberg, MD, a member of the meeting’s scientific committee who moderated the session.
“We have been talking about this at ACOG for a long time,” Dr. Landsberg said in an interview.
The presentation highlighted why obstetricians, gynecologists, and other doctors who deliver babies and care for women post partum “have got to screen these people,” he said. The finding that 34% of pregnancy-related suicide deaths had a prior suicide attempt indicates that clinicians may be able to identify these patients, Dr. Landsberg said. Suicide and overdose are leading causes of death in the first year post partum and “probably 100% of these are preventable,” he said.
As a first step, screening may be relatively simple. The Edinburgh Postnatal Depression Scale, highlighted during the talk, is an easy and quick tool to use, Dr. Landsberg said. It contains 10 items and assesses for anxiety and depression. It also specifically asks about suicide.
Ms. Griffen and Dr. Landsberg had no conflicts of interest.
Death by self-harm through suicide or overdose is a leading cause of death for women in the first year post partum, data indicate. Many of these deaths may be preventable, said Adrienne Griffen, MPP, executive director of the Maternal Mental Health Leadership Alliance.
Ms. Griffen discussed these findings and ways clinicians may be able to help at the 2021 virtual meeting of the American College of Obstetricians and Gynecologists.
Women “visit a health care provider an average of 25 times during a healthy pregnancy and first year of baby’s life,” she said. “Obstetric and primary care providers who serve pregnant and postpartum women are uniquely positioned to intervene effectively to screen and assess women for mental health disorders.”
To that end, clinicians should discuss mental health “early and often,” Ms. Griffen said.
“Asking about mental health issues and suicide will not cause women to think these thoughts,” she said. “We cannot wait for women to raise their hand and ask for help because by the time they do that, they needed help many weeks ago.”
For example, a doctor might tell a patient: “Your mental health is just as important as your physical health, and anxiety and depression are the most common complications of pregnancy and childbirth,” Ms. Griffen suggested. “Every time I see you, I’m going to ask you how you are doing, and we’ll do a formal screening assessment periodically over the course of the pregnancy. … Your job is to answer us honestly so that we can connect you with resources as soon as possible to minimize the impact on you and your baby.”
Although the obstetric provider should introduce this topic, a nurse, lactation consultant, or social worker may conduct screenings and help patients who are experiencing distress, she said.
During the past decade, several medical associations have issued new guidance around screening new mothers for anxiety and depression. One recent ACOG committee opinion recommends screening for depression at least once during pregnancy and once post partum, and encourages doctors to initiate medical therapy if possible and provide resources and referrals.
Another committee opinion suggests that doctors should have contact with a patient between 2 and 3 weeks post partum, primarily to assess for mental health.
Limited data
In discussing maternal suicide statistics, Ms. Griffen focused on data from Maternal Mortality Review Committees (MMRCs).
Two other sources of data about maternal mortality – the National Vital Statistics System and the Pregnancy Mortality Surveillance System – do not include information about suicide, which may be a reason this cause of death is not discussed more often, Ms. Griffen noted.
MMRCs, on the other hand, include information about suicide and self-harm. About half of the states in the United States have these multidisciplinary committees. Committee members review deaths of all women during pregnancy or within 1 year of pregnancy. Members consider a range of clinical and nonclinical data, including reports from social services and police, to try to understand the circumstances of each death.
A report that examined pregnancy-related deaths using data from 14 U.S. MMRCs between 2008 and 2017 showed that mental health conditions were the leading cause of death for non-Hispanic White women. In all, 34% of pregnancy-related suicide deaths had a documented prior suicide attempt, and the majority of suicides happened in the late postpartum time frame (43-365 days post partum).
Some physicians cite a lack of education, time, reimbursement, or referral resources as barriers to maternal mental health screening and treatment, but there may be useful options available, Ms. Griffen said. Postpartum Support International provides resources for physicians, as well as mothers. The National Curriculum in Reproductive Psychiatry and the Seleni Institute also have educational resources.
Some states have psychiatry access programs, where psychiatrists educate obstetricians, family physicians, and pediatricians about how to assess for and treat maternal mental health issues, Ms. Griffen noted.
Self care, social support, and talk therapy may help patients. “Sometimes medication is needed, but a combination of all of these things … can help women recover from maternal mental health conditions,” Ms. Griffen said.
Need to intervene
Although medical societies have emphasized the importance of maternal mental health screening and treatment in recent years, the risk of self-harm has been a concern for obstetricians and gynecologists long before then, said Marc Alan Landsberg, MD, a member of the meeting’s scientific committee who moderated the session.
“We have been talking about this at ACOG for a long time,” Dr. Landsberg said in an interview.
The presentation highlighted why obstetricians, gynecologists, and other doctors who deliver babies and care for women post partum “have got to screen these people,” he said. The finding that 34% of pregnancy-related suicide deaths had a prior suicide attempt indicates that clinicians may be able to identify these patients, Dr. Landsberg said. Suicide and overdose are leading causes of death in the first year post partum and “probably 100% of these are preventable,” he said.
As a first step, screening may be relatively simple. The Edinburgh Postnatal Depression Scale, highlighted during the talk, is an easy and quick tool to use, Dr. Landsberg said. It contains 10 items and assesses for anxiety and depression. It also specifically asks about suicide.
Ms. Griffen and Dr. Landsberg had no conflicts of interest.
FROM ACOG 2021
BERENICE: Further evidence of heart safety of dual HER2 blockade
Dual HER2 blockade with pertuzumab (Perjeta) and trastuzumab (Herceptin) on top of anthracycline-based neoadjuvant chemotherapy for early-stage breast cancer was associated with a low rate of clinically relevant cardiac events in the final follow-up of the BERENICE study.
After more than 5 years, 1.0%-1.5% of patients who had locally advanced, inflammatory, or early-stage breast cancer developed heart failure, and around 12%-13% showed any significant changes in left ventricular ejection fraction (LVEF).
Importantly, “there were no new safety concerns that arose during long-term follow-up,” study investigator Chau Dang, MD, said in presenting the findings at the European Society for Medical Oncology: Breast Cancer virtual meeting.
Dr. Dang, a medical oncologist at Memorial Sloan Kettering Cancer Centre in New York, reported that the most common cause of death was disease progression.
BERENICE was designed as a cardiac safety study and so not powered to look at long-term efficacy, which Dr. Dang was clear in reporting. Nevertheless event-free survival (EFS), invasive disease-free survival (IDFS), and overall survival (OS) rates at 5 years were all high, at least a respective 89.2%, 91%, and 93.8%, she said. “The medians have not been reached,” she observed.
“These data support the use of dual HER2 blockade with pertuzumab-trastuzumab–based regimens, including in combination with dose-dense, anthracycline-based chemotherapy, across the neoadjuvant and adjuvant treatment settings for the complete treatment of patients with HER2-positive early-stage breast cancer,” Dr. Dang said.
Evandro de Azambuja, MD, PhD, the invited discussant for the trial agreed that the regimens tested appeared “safe from a cardiac standpoint.” However, “you cannot forget that today we are using much less anthracyclines in our patient population.”
Patients in trials are also very different from those treated in clinical practice, often being younger and much fitter, he said. Therefore, it may be important to look at the baseline cardiac medications and comorbidities, Dr. de Azambuja, a medical oncologist at the Institut Jules Bordet in Brussels, Belgium, suggested.
That said, the BERENICE findings sit well with other trials that have been conducted, Dr. de Azambuja pointed out.
“If we look at other trials that have also tested dual HER2 blockade with anthracycline or nonanthracycline regimens, all of them reassure that dual blockade is not more cardiotoxic than single blockade,” he said. This includes trials such as TRYPHAENA, APHINITY, KRISTINE, NeoSphere and PEONY.
The 3-year IDFS rate of 91% in BERENICE also compares well to that seen in APHINITY (94%), Dr. de Azambuja said.
BERENICE study design
BERENICE was a multicenter, open-label, nonrandomized and noncomparative phase 2 trial that recruited 400 patients across 75 centers in 12 countries.
Eligibility criteria were that participants had to have been centrally confirmed HER2-positive locally advanced, inflammatory or early breast cancer, with the latter defined as tumors bigger than 2 cm or greater than 5 mm in size, and be node-positive. Patients also had to have a starting LVEF of 55% or higher.
Patients were allocated to one of two neoadjuvant chemotherapy regimens depending on the choice of their physician. One group received a regimen of dose-dense doxorubicin and cyclophosphamide (ddAC) given every 2 weeks for four cycles and then paclitaxel every week for 12 cycles. The other group received 5-fluorouracil, epirubicin, and cyclophosphamide (FEC) every 3 weeks for four cycles and then docetaxel every 3 weeks for four cycles.
Pertuzumab and trastuzumab were started at the same time as the taxanes in both groups and given every 3 weeks for four cycles. Patients then underwent surgery and continued pertuzumab/trastuzumab treatment alone for a further 13 cycles.
The co-primary endpoints were the incidence of New York Heart Association class III or IV heart failure and incidence of symptomatic and asymptomatic LVEF decline of 10% or more.
The primary analysis of the trial was published in 2018 and, at that time, it was reported that three patients in the ddAC cohort and none in the FEC cohort experienced heart failure. LVEF decline was observed in a respective 6.5% and 2% of patients.
Discussion points
Dr. de Azambuja noted that the contribution of the chemotherapy to the efficacy cannot be assessed because of the nonrandomized trial design. That should not matter, pointed out Sybille Loibl, MD, PhD, during discussion.
“I think it compares nicely to other trials that looked at dose-dense chemotherapy,” said Dr. Loibl, who is an associate professor at the University of Frankfurt in Germany. “It seems that, in the light of what we consider today probably one of the best anti-HER2 treatments, the chemotherapy is less relevant, and that’s why a dose-dense regimen doesn’t add so much on a standard anthracycline taxane-containing regimen.”
Dr. de Azambuja also commented on the assessment of cardiotoxicity and the use of reduced LVEF as a measure: LVEF decline is a late effect of cardiotoxicity, he observed, and he suggested a different approach in future trials.
“If you use Global Longitudinal Strain, this could be an optimal parameter to detect early subclinical LVEF dysfunction and you should consider it for the next trials looking for cardiac safety. Also, cardiac biomarkers. This was not implemented in this trial, and I strongly recommend this should be for the next trial.”
The BERENICE trial was funded by F. Hoffmann-La Roche. Dr. Dang disclosed receiving consultancy fees from F. Hoffmann-La Roche, Genentech, Daiichi Sankyo, Lilly, and Puma Biotechnology. Dr. de Azambuja was not involved in the study but disclosed receiving honoraria, travel grants, research grants from Roche and Genentech as well as from other companies. Dr. Loibl was one of the cochairs of the session and, among disclosures regarding many other companies, has been an invited speaker for Roche and received reimbursement via her institution for a writing engagement.
Dual HER2 blockade with pertuzumab (Perjeta) and trastuzumab (Herceptin) on top of anthracycline-based neoadjuvant chemotherapy for early-stage breast cancer was associated with a low rate of clinically relevant cardiac events in the final follow-up of the BERENICE study.
After more than 5 years, 1.0%-1.5% of patients who had locally advanced, inflammatory, or early-stage breast cancer developed heart failure, and around 12%-13% showed any significant changes in left ventricular ejection fraction (LVEF).
Importantly, “there were no new safety concerns that arose during long-term follow-up,” study investigator Chau Dang, MD, said in presenting the findings at the European Society for Medical Oncology: Breast Cancer virtual meeting.
Dr. Dang, a medical oncologist at Memorial Sloan Kettering Cancer Centre in New York, reported that the most common cause of death was disease progression.
BERENICE was designed as a cardiac safety study and so not powered to look at long-term efficacy, which Dr. Dang was clear in reporting. Nevertheless event-free survival (EFS), invasive disease-free survival (IDFS), and overall survival (OS) rates at 5 years were all high, at least a respective 89.2%, 91%, and 93.8%, she said. “The medians have not been reached,” she observed.
“These data support the use of dual HER2 blockade with pertuzumab-trastuzumab–based regimens, including in combination with dose-dense, anthracycline-based chemotherapy, across the neoadjuvant and adjuvant treatment settings for the complete treatment of patients with HER2-positive early-stage breast cancer,” Dr. Dang said.
Evandro de Azambuja, MD, PhD, the invited discussant for the trial agreed that the regimens tested appeared “safe from a cardiac standpoint.” However, “you cannot forget that today we are using much less anthracyclines in our patient population.”
Patients in trials are also very different from those treated in clinical practice, often being younger and much fitter, he said. Therefore, it may be important to look at the baseline cardiac medications and comorbidities, Dr. de Azambuja, a medical oncologist at the Institut Jules Bordet in Brussels, Belgium, suggested.
That said, the BERENICE findings sit well with other trials that have been conducted, Dr. de Azambuja pointed out.
“If we look at other trials that have also tested dual HER2 blockade with anthracycline or nonanthracycline regimens, all of them reassure that dual blockade is not more cardiotoxic than single blockade,” he said. This includes trials such as TRYPHAENA, APHINITY, KRISTINE, NeoSphere and PEONY.
The 3-year IDFS rate of 91% in BERENICE also compares well to that seen in APHINITY (94%), Dr. de Azambuja said.
BERENICE study design
BERENICE was a multicenter, open-label, nonrandomized and noncomparative phase 2 trial that recruited 400 patients across 75 centers in 12 countries.
Eligibility criteria were that participants had to have been centrally confirmed HER2-positive locally advanced, inflammatory or early breast cancer, with the latter defined as tumors bigger than 2 cm or greater than 5 mm in size, and be node-positive. Patients also had to have a starting LVEF of 55% or higher.
Patients were allocated to one of two neoadjuvant chemotherapy regimens depending on the choice of their physician. One group received a regimen of dose-dense doxorubicin and cyclophosphamide (ddAC) given every 2 weeks for four cycles and then paclitaxel every week for 12 cycles. The other group received 5-fluorouracil, epirubicin, and cyclophosphamide (FEC) every 3 weeks for four cycles and then docetaxel every 3 weeks for four cycles.
Pertuzumab and trastuzumab were started at the same time as the taxanes in both groups and given every 3 weeks for four cycles. Patients then underwent surgery and continued pertuzumab/trastuzumab treatment alone for a further 13 cycles.
The co-primary endpoints were the incidence of New York Heart Association class III or IV heart failure and incidence of symptomatic and asymptomatic LVEF decline of 10% or more.
The primary analysis of the trial was published in 2018 and, at that time, it was reported that three patients in the ddAC cohort and none in the FEC cohort experienced heart failure. LVEF decline was observed in a respective 6.5% and 2% of patients.
Discussion points
Dr. de Azambuja noted that the contribution of the chemotherapy to the efficacy cannot be assessed because of the nonrandomized trial design. That should not matter, pointed out Sybille Loibl, MD, PhD, during discussion.
“I think it compares nicely to other trials that looked at dose-dense chemotherapy,” said Dr. Loibl, who is an associate professor at the University of Frankfurt in Germany. “It seems that, in the light of what we consider today probably one of the best anti-HER2 treatments, the chemotherapy is less relevant, and that’s why a dose-dense regimen doesn’t add so much on a standard anthracycline taxane-containing regimen.”
Dr. de Azambuja also commented on the assessment of cardiotoxicity and the use of reduced LVEF as a measure: LVEF decline is a late effect of cardiotoxicity, he observed, and he suggested a different approach in future trials.
“If you use Global Longitudinal Strain, this could be an optimal parameter to detect early subclinical LVEF dysfunction and you should consider it for the next trials looking for cardiac safety. Also, cardiac biomarkers. This was not implemented in this trial, and I strongly recommend this should be for the next trial.”
The BERENICE trial was funded by F. Hoffmann-La Roche. Dr. Dang disclosed receiving consultancy fees from F. Hoffmann-La Roche, Genentech, Daiichi Sankyo, Lilly, and Puma Biotechnology. Dr. de Azambuja was not involved in the study but disclosed receiving honoraria, travel grants, research grants from Roche and Genentech as well as from other companies. Dr. Loibl was one of the cochairs of the session and, among disclosures regarding many other companies, has been an invited speaker for Roche and received reimbursement via her institution for a writing engagement.
Dual HER2 blockade with pertuzumab (Perjeta) and trastuzumab (Herceptin) on top of anthracycline-based neoadjuvant chemotherapy for early-stage breast cancer was associated with a low rate of clinically relevant cardiac events in the final follow-up of the BERENICE study.
After more than 5 years, 1.0%-1.5% of patients who had locally advanced, inflammatory, or early-stage breast cancer developed heart failure, and around 12%-13% showed any significant changes in left ventricular ejection fraction (LVEF).
Importantly, “there were no new safety concerns that arose during long-term follow-up,” study investigator Chau Dang, MD, said in presenting the findings at the European Society for Medical Oncology: Breast Cancer virtual meeting.
Dr. Dang, a medical oncologist at Memorial Sloan Kettering Cancer Centre in New York, reported that the most common cause of death was disease progression.
BERENICE was designed as a cardiac safety study and so not powered to look at long-term efficacy, which Dr. Dang was clear in reporting. Nevertheless event-free survival (EFS), invasive disease-free survival (IDFS), and overall survival (OS) rates at 5 years were all high, at least a respective 89.2%, 91%, and 93.8%, she said. “The medians have not been reached,” she observed.
“These data support the use of dual HER2 blockade with pertuzumab-trastuzumab–based regimens, including in combination with dose-dense, anthracycline-based chemotherapy, across the neoadjuvant and adjuvant treatment settings for the complete treatment of patients with HER2-positive early-stage breast cancer,” Dr. Dang said.
Evandro de Azambuja, MD, PhD, the invited discussant for the trial agreed that the regimens tested appeared “safe from a cardiac standpoint.” However, “you cannot forget that today we are using much less anthracyclines in our patient population.”
Patients in trials are also very different from those treated in clinical practice, often being younger and much fitter, he said. Therefore, it may be important to look at the baseline cardiac medications and comorbidities, Dr. de Azambuja, a medical oncologist at the Institut Jules Bordet in Brussels, Belgium, suggested.
That said, the BERENICE findings sit well with other trials that have been conducted, Dr. de Azambuja pointed out.
“If we look at other trials that have also tested dual HER2 blockade with anthracycline or nonanthracycline regimens, all of them reassure that dual blockade is not more cardiotoxic than single blockade,” he said. This includes trials such as TRYPHAENA, APHINITY, KRISTINE, NeoSphere and PEONY.
The 3-year IDFS rate of 91% in BERENICE also compares well to that seen in APHINITY (94%), Dr. de Azambuja said.
BERENICE study design
BERENICE was a multicenter, open-label, nonrandomized and noncomparative phase 2 trial that recruited 400 patients across 75 centers in 12 countries.
Eligibility criteria were that participants had to have been centrally confirmed HER2-positive locally advanced, inflammatory or early breast cancer, with the latter defined as tumors bigger than 2 cm or greater than 5 mm in size, and be node-positive. Patients also had to have a starting LVEF of 55% or higher.
Patients were allocated to one of two neoadjuvant chemotherapy regimens depending on the choice of their physician. One group received a regimen of dose-dense doxorubicin and cyclophosphamide (ddAC) given every 2 weeks for four cycles and then paclitaxel every week for 12 cycles. The other group received 5-fluorouracil, epirubicin, and cyclophosphamide (FEC) every 3 weeks for four cycles and then docetaxel every 3 weeks for four cycles.
Pertuzumab and trastuzumab were started at the same time as the taxanes in both groups and given every 3 weeks for four cycles. Patients then underwent surgery and continued pertuzumab/trastuzumab treatment alone for a further 13 cycles.
The co-primary endpoints were the incidence of New York Heart Association class III or IV heart failure and incidence of symptomatic and asymptomatic LVEF decline of 10% or more.
The primary analysis of the trial was published in 2018 and, at that time, it was reported that three patients in the ddAC cohort and none in the FEC cohort experienced heart failure. LVEF decline was observed in a respective 6.5% and 2% of patients.
Discussion points
Dr. de Azambuja noted that the contribution of the chemotherapy to the efficacy cannot be assessed because of the nonrandomized trial design. That should not matter, pointed out Sybille Loibl, MD, PhD, during discussion.
“I think it compares nicely to other trials that looked at dose-dense chemotherapy,” said Dr. Loibl, who is an associate professor at the University of Frankfurt in Germany. “It seems that, in the light of what we consider today probably one of the best anti-HER2 treatments, the chemotherapy is less relevant, and that’s why a dose-dense regimen doesn’t add so much on a standard anthracycline taxane-containing regimen.”
Dr. de Azambuja also commented on the assessment of cardiotoxicity and the use of reduced LVEF as a measure: LVEF decline is a late effect of cardiotoxicity, he observed, and he suggested a different approach in future trials.
“If you use Global Longitudinal Strain, this could be an optimal parameter to detect early subclinical LVEF dysfunction and you should consider it for the next trials looking for cardiac safety. Also, cardiac biomarkers. This was not implemented in this trial, and I strongly recommend this should be for the next trial.”
The BERENICE trial was funded by F. Hoffmann-La Roche. Dr. Dang disclosed receiving consultancy fees from F. Hoffmann-La Roche, Genentech, Daiichi Sankyo, Lilly, and Puma Biotechnology. Dr. de Azambuja was not involved in the study but disclosed receiving honoraria, travel grants, research grants from Roche and Genentech as well as from other companies. Dr. Loibl was one of the cochairs of the session and, among disclosures regarding many other companies, has been an invited speaker for Roche and received reimbursement via her institution for a writing engagement.
FROM ESMO BREAST CANCER 2021
A review of the latest USPSTF recommendations
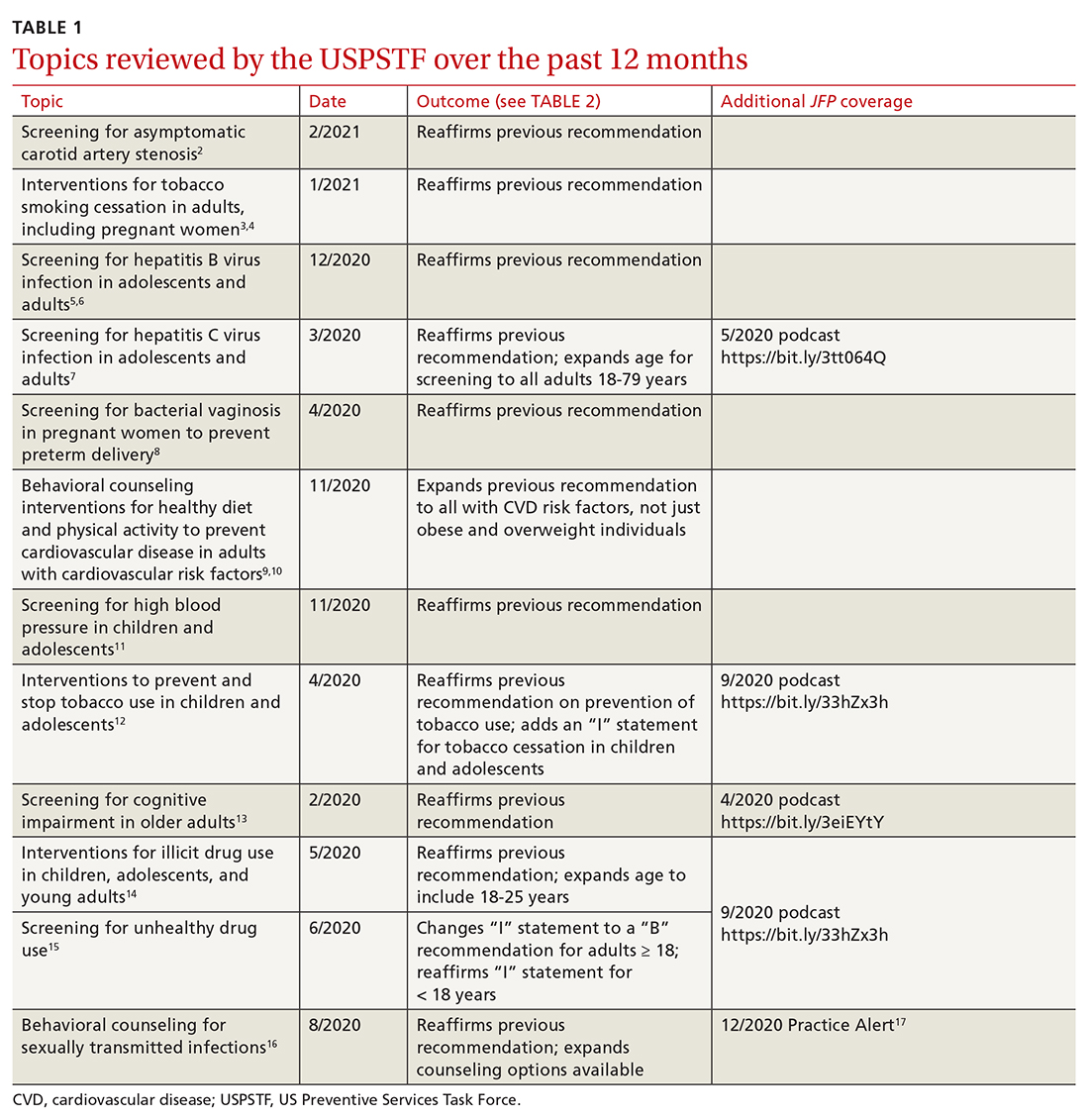
Since the last Practice Alert update on recommendations made by the US Preventive Services Task Force,1 the Task Force has completed work on 12 topics (TABLE 1).2-17 Five of these topics have been discussed in JFP audio recordings, and the links are provided in TABLE 1.
This latest Task Force endeavor resulted in 18 recommendations (TABLE 2), all of which reaffirm previous recommendations on these topics and expand the scope of 2. There were 2 “A” recommendations, 6 “B” recommendations, 2 “D” recommendations, and 8 “I” statements, indicating that there was insufficient evidence to assess effectiveness or harms. The willingness to make “I” statements when there is little or no evidence on the intervention being assessed distinguishes the USPSTF from other clinical guideline committees.
Screening for carotid artery stenosis
One of the “D” recommendations this past year reaffirms the prior recommendation against screening for carotid artery stenosis in asymptomatic adults—ie, those without a history of transient ischemic attack, stroke, or neurologic signs or symptoms that might be caused by carotid artery stenosis.2 The screening tests the Task Force researched included carotid duplex ultrasonography (DUS), magnetic resonance angiography, and computed tomography angiography. The Task Force did not look at the value of auscultation for carotid bruits because it has been proven to be inaccurate and they do not consider it to be a useful screening tool.
The Task Force based its “D” recommendation on a lack of evidence for any benefit in detecting asymptomatic carotid artery stenosis, and on evidence that screening can lead to harms through false-positive tests and potential complications from carotid endarterectomy and carotid artery angioplasty and stenting. In its clinical considerations, the Task Force emphasized the primary prevention of atherosclerotic disease by focusing on the following actions:
- screening for high blood pressure in adults
- encouraging tobacco smoking cessation in adults
- promoting a healthy diet and physical activity in adults with cardiovascular risk factors
- recommending aspirin use to prevent cardiovascular disease and colorectal cancer
- advising statin use for the primary prevention of cardiovascular disease in adults ages 45 to 75 years who have 1 or more risk factors (hyperlipidemia, diabetes, hypertension, smoking) and those with a 10-year risk of a cardiovascular event of 10% or greater.
This “D” recommendation differs from recommendations made by other professional organizations, some of which recommend testing with DUS for asymptomatic patients with a carotid bruit, and others that recommend DUS screening in patients with multiple risk factors for stroke and in those with known peripheral artery disease or other cardiovascular disease.18,19
Smoking cessation in adults
Smoking tobacco is the leading preventable cause of death in the United States, causing about 480,000 deaths annually.3 Smoking during pregnancy increases the risk of complications including miscarriage, congenital anomalies, stillbirth, fetal growth restriction, preterm birth, and placental abruption.
The Task Force published recommendations earlier this year advising all clinicians to ask all adult patients about tobacco use; and, for those who smoke, to provide (or refer them to) smoking cessation behavioral therapy. The Task Force also recommends prescribing pharmacotherapy approved by the Food and Drug Administration (FDA) for smoking cessation for nonpregnant adults. (There is a lack of information to assess the harms and benefits of smoking cessation pharmacotherapy during pregnancy.)
Continue to: FDA-approved medications...
FDA-approved medications for treating tobacco smoking dependence are nicotine replacement therapy (NRT), bupropion hydrochloride, and varenicline.3 NRT is available in transdermal patches, lozenges, gum, inhalers, and nasal sprays.
In addition, the Task Force indicates that there is insufficient evidence to assess the benefits and harms of e-cigarettes when used as a method of achieving smoking cessation: “Few randomized trials have evaluated the effectiveness of e-cigarettes to increase tobacco smoking cessation in nonpregnant adults, and no trials have evaluated e-cigarettes for tobacco smoking cessation in pregnant persons.”4
Hepatitis B infection screening
The Task Force reaffirmed a previous recommendation to screen for hepatitis B virus (HBV) infection only in adults who are at high risk,5 rather than universal screening that it recommends for hepatitis C virus infection (HCV).7 (See: https://bit.ly/3tt064Q). The Task Force has a separate recommendation to screen all pregnant women for hepatitis B at the first prenatal visit.6
Those at high risk for hepatitis B who should be screened include individuals born in countries or regions of the world with a hepatitis B surface antigen (HBsAg) prevalence ≥ 2% and individuals born in the United States who have not received HBV vaccine and whose parents were born in regions with an HBsAg prevalence ≥ 8%.5 (A table listing countries with HBsAg ≥ 8%—as well as those in lower prevalence categories—is included with the recommendation.5)
HBV screening should also be offered to other high-risk groups that have a prevalence of positive HBsAg ≥ 2%: those who have injected drugs in the past or are currently injecting drugs; men who have sex with men; individuals with HIV; and sex partners, needle-sharing contacts, and household contacts of people known to be HBsAg positive.5
Continue to: It is estimated that...
It is estimated that > 860,000 people in the United States have chronic HBV infection and that close to two-thirds of them are unaware of their infection.5 The screening test for HBV is highly accurate; sensitivity and specificity are both > 98%.5 While there is no direct evidence that screening, detecting, and treating asymptomatic HBV infection reduces morbidity and mortality, the Task Force felt that the evidence for improvement in multiple outcomes in those with HBV when treated with antiviral regimens was sufficient to support the recommendation.
Screening for bacterial vaginosis in pregnancy
While bacterial vaginosis (BV) is associated with a two-fold risk of preterm delivery, treating BV during pregnancy does not seem to reduce this risk, indicating that some other variable is involved.8 In addition, studies that looked at screening for, and treatment of, asymptomatic BV in pregnant women at high risk for preterm delivery (defined primarily as those with a previous preterm delivery) have shown inconsistent results. There is the potential for harm in treating BV in pregnancy, chiefly involving gastrointestinal upset caused by metronidazole or clindamycin.
Given that there are no benefits—and some harms—resulting from treatment, the Task Force recommends against screening for BV in non-high-risk pregnant women. A lack of sufficient information to assess any potential benefits to screening in high-risk pregnancies led the Task Force to an “I” statement on this question.8
Behavioral counseling on healthy diet, exercise for adults with CV risks
Cardiovascular disease (CVD) remains the number one cause of death in the United States. The major risk factors for CVD, which can be modified, are high blood pressure, hyperlipidemia, diabetes, smoking, obesity or overweight, and lack of physical activity.
The Task Force has previously recommended intensive behavioral interventions to improve nutrition and physical activity in those who are overweight/obese and in those with abnormal blood glucose levels,9 and has addressed smoking prevention and cessation.4 This new recommendation applies to those with other CVD risks such as high blood pressure and/or hyperlipidemia and those with an estimated 10-year CVD risk of ≥ 7.5%.10
Continue to: Behavioral interventions...
Behavioral interventions included in the Task Force analysis employed a median of 12 contacts and an estimated 6 hours of contact time over 6 to 18 months.10 Most interventions involved motivational interviewing and instruction on behavioral change methods. These interventions can be provided by primary care clinicians, as well as a wide range of other trained professionals. The Affordable Care Act dictates that all “A” and “B” recommendations must be provided by commercial health plans at no out-of-pocket expense for the patient.
Nutritional advice should include reductions in saturated fats, salt, and sugars and increases in fruits, vegetables, and whole grains. The Mediterranean diet and the Dietary Approaches to Stop Hypertension (DASH) diet are often recommended.10 Physical activity counseling should advocate for 90 to 180 minutes per week of moderate to vigorous activity.
This new recommendation, along with the previous ones pertaining to behavioral interventions for lifestyle changes, make it clear that intensive interventions are needed to achieve meaningful change. Simple advice from a clinician will have little to no effect.
Task Force reviews evidence on HTN, smoking cessation in young people
In 2020 the Task Force completed reviews of evidence relevant to screening for high blood pressure11 and
The 2 “I” statements are in disagreement with recommendations of other professional organizations. The American Academy of Pediatrics (AAP) and the American Heart Association recommend routine screening for high blood pressure starting at age 3 years. And the AAP recommends screening teenagers for tobacco use and offering tobacco dependence treatment, referral, or both (including pharmacotherapy) when indicated. E-cigarettes are not recommended as a treatment for tobacco dependence.20
Continue to: The difference between...
The difference between the methods used by the Task Force and other guideline-producing organizations becomes apparent when it comes to recommendations pertaining to children and adolescents, for whom long-term outcome-oriented studies on prevention issues are rare. The Task Force is unwilling to make recommendations when evidence does not exist. The AAP often makes recommendations based on expert opinion consensus in such situations. One notable part of each Task Force recommendation statement is a discussion of what other organizations recommend on the same topic so that these differences can be openly described.
Better Task Force funding could expand topic coverage
It is worth revisiting 2 issues that were pointed out in last year’s USPSTF summary in this column.1 First, the Task Force methods are robust and evidence based, and recommendations therefore are rarely changed once they are made at an “A”, “B”, or “D” level. Second, Task Force resources are finite, and thus, the group is currently unable to update previous recommendations with greater frequency or to consider many new topics. In the past 2 years, the Task Force has developed recommendations on only 2 completely new topics. Hopefully, its budget can be expanded so that new topics can be added in the future.
1. Campos-Outcalt D. USPSTF roundup. J Fam Pract. 2020;69:201-204.
2. USPSTF. Screening for asymptomatic carotid artery stenosis. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/carotid-artery-stenosis-screening
3. USPSTF. Interventions for tobacco smoking cessation in adults, including pregnant persons. Accessed April 30, 2021. www.uspreventiveservicestaskforce.org/uspstf/recommendation/tobacco-use-in-adults-and-pregnant-women-counseling-and-interventions
4. USPSTF. Interventions for tobacco smoking cessation in adults, including pregnant persons. JAMA. 2021;325:265-279.
5. USPSTF. Screening for Hepatitis B virus infection in adolescents and adults. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/hepatitis-b-virus-infection-screening
6. USPSTF. Hepatitis B virus infection in pregnant women: screening. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/hepatitis-b-virus-infection-in-pregnant-women-screening
7. USPSTF. Hepatitis C virus infection in adolescents and adults: screening. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/hepatitis-c-screening
8. USPSTF; Owens DK, Davidson KW, Krisk AH, et al. Screening for bacterial vaginosis in pregnant persons to prevent preterm delivery: US Preventive Services Task Force recommendation statement. JAMA. 2020;323:1286-1292.
9. Behavioral counseling to promote a healthful diet and physical activity for cardiovascular disease prevention in adults with cardiovascular risk factors: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;161:587-593.
10. USPSTF. Behavioral counseling interventions to promote a healthy and physical activity for cardiovascular disease prevention in adults with cardiovascular risk factors: US Preventive Services Task Force recommendation statement. JAMA. 2020;324:2069-2075.
11. USPSTF. High blood pressure in children and adolescents: screening. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/blood-pressure-in-children-and-adolescents-hypertension-screening
12. USPSTF. Prevention and cessation of tobacco use in children and adolescents: primary care interventions. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/tobacco-and-nicotine-use-prevention-in-children-and-adolescents-primary-care-interventions
13. USPSTF. Cognitive impairment in older adults: screening. Accessed March 26, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/cognitive-impairment-in-older-adults-screening
14. USPSTF. Illicit drug use in children, adolescents, and young adults: primary care-based interventions. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/drug-use-illicit-primary-care-interventions-for-children-and-adolescents
15. USPSTF. Unhealthy drug use: screening. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/drug-use-illicit-screening
16. USPSTF. Sexually transmitted infections: behavioral counseling. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/sexually-transmitted-infections-behavioral-counseling.
17. Campos-Outcalt D. USPSTF update on sexually transmitted infections. J Fam Pract. 2020;69:514-517.
18. Brott TG, Halperin JL, Abbara S, et al; ASA/ACCF/AHA/AANN/AANS/ACR/ASNR/CNS/SAIP/SCAI/SIR/SNIS/SVM/SVS guideline on the management of patients with extracranial carotid and vertebral artery disease. Catheter Cardiovasc Interv. 2013;81:E76-E123.
19. Ricotta JJ, Aburahma A, Ascher E, et al; Society for Vascular Surgery. Updated Society for Vascular Surgery guidelines for management of extracranial carotid disease. J Vasc Surg. 2011;54:e1-e31.
20. Farber HJ, Walley SC, Groner JA, et al; Section on Tobacco Control. Clinical practice policy to protect children from tobacco, nicotine, and tobacco smoke. Pediatrics. 2015;136:1008-1017.
Since the last Practice Alert update on recommendations made by the US Preventive Services Task Force,1 the Task Force has completed work on 12 topics (TABLE 1).2-17 Five of these topics have been discussed in JFP audio recordings, and the links are provided in TABLE 1.
This latest Task Force endeavor resulted in 18 recommendations (TABLE 2), all of which reaffirm previous recommendations on these topics and expand the scope of 2. There were 2 “A” recommendations, 6 “B” recommendations, 2 “D” recommendations, and 8 “I” statements, indicating that there was insufficient evidence to assess effectiveness or harms. The willingness to make “I” statements when there is little or no evidence on the intervention being assessed distinguishes the USPSTF from other clinical guideline committees.
Screening for carotid artery stenosis
One of the “D” recommendations this past year reaffirms the prior recommendation against screening for carotid artery stenosis in asymptomatic adults—ie, those without a history of transient ischemic attack, stroke, or neurologic signs or symptoms that might be caused by carotid artery stenosis.2 The screening tests the Task Force researched included carotid duplex ultrasonography (DUS), magnetic resonance angiography, and computed tomography angiography. The Task Force did not look at the value of auscultation for carotid bruits because it has been proven to be inaccurate and they do not consider it to be a useful screening tool.
The Task Force based its “D” recommendation on a lack of evidence for any benefit in detecting asymptomatic carotid artery stenosis, and on evidence that screening can lead to harms through false-positive tests and potential complications from carotid endarterectomy and carotid artery angioplasty and stenting. In its clinical considerations, the Task Force emphasized the primary prevention of atherosclerotic disease by focusing on the following actions:
- screening for high blood pressure in adults
- encouraging tobacco smoking cessation in adults
- promoting a healthy diet and physical activity in adults with cardiovascular risk factors
- recommending aspirin use to prevent cardiovascular disease and colorectal cancer
- advising statin use for the primary prevention of cardiovascular disease in adults ages 45 to 75 years who have 1 or more risk factors (hyperlipidemia, diabetes, hypertension, smoking) and those with a 10-year risk of a cardiovascular event of 10% or greater.
This “D” recommendation differs from recommendations made by other professional organizations, some of which recommend testing with DUS for asymptomatic patients with a carotid bruit, and others that recommend DUS screening in patients with multiple risk factors for stroke and in those with known peripheral artery disease or other cardiovascular disease.18,19
Smoking cessation in adults
Smoking tobacco is the leading preventable cause of death in the United States, causing about 480,000 deaths annually.3 Smoking during pregnancy increases the risk of complications including miscarriage, congenital anomalies, stillbirth, fetal growth restriction, preterm birth, and placental abruption.
The Task Force published recommendations earlier this year advising all clinicians to ask all adult patients about tobacco use; and, for those who smoke, to provide (or refer them to) smoking cessation behavioral therapy. The Task Force also recommends prescribing pharmacotherapy approved by the Food and Drug Administration (FDA) for smoking cessation for nonpregnant adults. (There is a lack of information to assess the harms and benefits of smoking cessation pharmacotherapy during pregnancy.)
Continue to: FDA-approved medications...
FDA-approved medications for treating tobacco smoking dependence are nicotine replacement therapy (NRT), bupropion hydrochloride, and varenicline.3 NRT is available in transdermal patches, lozenges, gum, inhalers, and nasal sprays.
In addition, the Task Force indicates that there is insufficient evidence to assess the benefits and harms of e-cigarettes when used as a method of achieving smoking cessation: “Few randomized trials have evaluated the effectiveness of e-cigarettes to increase tobacco smoking cessation in nonpregnant adults, and no trials have evaluated e-cigarettes for tobacco smoking cessation in pregnant persons.”4
Hepatitis B infection screening
The Task Force reaffirmed a previous recommendation to screen for hepatitis B virus (HBV) infection only in adults who are at high risk,5 rather than universal screening that it recommends for hepatitis C virus infection (HCV).7 (See: https://bit.ly/3tt064Q). The Task Force has a separate recommendation to screen all pregnant women for hepatitis B at the first prenatal visit.6
Those at high risk for hepatitis B who should be screened include individuals born in countries or regions of the world with a hepatitis B surface antigen (HBsAg) prevalence ≥ 2% and individuals born in the United States who have not received HBV vaccine and whose parents were born in regions with an HBsAg prevalence ≥ 8%.5 (A table listing countries with HBsAg ≥ 8%—as well as those in lower prevalence categories—is included with the recommendation.5)
HBV screening should also be offered to other high-risk groups that have a prevalence of positive HBsAg ≥ 2%: those who have injected drugs in the past or are currently injecting drugs; men who have sex with men; individuals with HIV; and sex partners, needle-sharing contacts, and household contacts of people known to be HBsAg positive.5
Continue to: It is estimated that...
It is estimated that > 860,000 people in the United States have chronic HBV infection and that close to two-thirds of them are unaware of their infection.5 The screening test for HBV is highly accurate; sensitivity and specificity are both > 98%.5 While there is no direct evidence that screening, detecting, and treating asymptomatic HBV infection reduces morbidity and mortality, the Task Force felt that the evidence for improvement in multiple outcomes in those with HBV when treated with antiviral regimens was sufficient to support the recommendation.
Screening for bacterial vaginosis in pregnancy
While bacterial vaginosis (BV) is associated with a two-fold risk of preterm delivery, treating BV during pregnancy does not seem to reduce this risk, indicating that some other variable is involved.8 In addition, studies that looked at screening for, and treatment of, asymptomatic BV in pregnant women at high risk for preterm delivery (defined primarily as those with a previous preterm delivery) have shown inconsistent results. There is the potential for harm in treating BV in pregnancy, chiefly involving gastrointestinal upset caused by metronidazole or clindamycin.
Given that there are no benefits—and some harms—resulting from treatment, the Task Force recommends against screening for BV in non-high-risk pregnant women. A lack of sufficient information to assess any potential benefits to screening in high-risk pregnancies led the Task Force to an “I” statement on this question.8
Behavioral counseling on healthy diet, exercise for adults with CV risks
Cardiovascular disease (CVD) remains the number one cause of death in the United States. The major risk factors for CVD, which can be modified, are high blood pressure, hyperlipidemia, diabetes, smoking, obesity or overweight, and lack of physical activity.
The Task Force has previously recommended intensive behavioral interventions to improve nutrition and physical activity in those who are overweight/obese and in those with abnormal blood glucose levels,9 and has addressed smoking prevention and cessation.4 This new recommendation applies to those with other CVD risks such as high blood pressure and/or hyperlipidemia and those with an estimated 10-year CVD risk of ≥ 7.5%.10
Continue to: Behavioral interventions...
Behavioral interventions included in the Task Force analysis employed a median of 12 contacts and an estimated 6 hours of contact time over 6 to 18 months.10 Most interventions involved motivational interviewing and instruction on behavioral change methods. These interventions can be provided by primary care clinicians, as well as a wide range of other trained professionals. The Affordable Care Act dictates that all “A” and “B” recommendations must be provided by commercial health plans at no out-of-pocket expense for the patient.
Nutritional advice should include reductions in saturated fats, salt, and sugars and increases in fruits, vegetables, and whole grains. The Mediterranean diet and the Dietary Approaches to Stop Hypertension (DASH) diet are often recommended.10 Physical activity counseling should advocate for 90 to 180 minutes per week of moderate to vigorous activity.
This new recommendation, along with the previous ones pertaining to behavioral interventions for lifestyle changes, make it clear that intensive interventions are needed to achieve meaningful change. Simple advice from a clinician will have little to no effect.
Task Force reviews evidence on HTN, smoking cessation in young people
In 2020 the Task Force completed reviews of evidence relevant to screening for high blood pressure11 and
The 2 “I” statements are in disagreement with recommendations of other professional organizations. The American Academy of Pediatrics (AAP) and the American Heart Association recommend routine screening for high blood pressure starting at age 3 years. And the AAP recommends screening teenagers for tobacco use and offering tobacco dependence treatment, referral, or both (including pharmacotherapy) when indicated. E-cigarettes are not recommended as a treatment for tobacco dependence.20
Continue to: The difference between...
The difference between the methods used by the Task Force and other guideline-producing organizations becomes apparent when it comes to recommendations pertaining to children and adolescents, for whom long-term outcome-oriented studies on prevention issues are rare. The Task Force is unwilling to make recommendations when evidence does not exist. The AAP often makes recommendations based on expert opinion consensus in such situations. One notable part of each Task Force recommendation statement is a discussion of what other organizations recommend on the same topic so that these differences can be openly described.
Better Task Force funding could expand topic coverage
It is worth revisiting 2 issues that were pointed out in last year’s USPSTF summary in this column.1 First, the Task Force methods are robust and evidence based, and recommendations therefore are rarely changed once they are made at an “A”, “B”, or “D” level. Second, Task Force resources are finite, and thus, the group is currently unable to update previous recommendations with greater frequency or to consider many new topics. In the past 2 years, the Task Force has developed recommendations on only 2 completely new topics. Hopefully, its budget can be expanded so that new topics can be added in the future.
Since the last Practice Alert update on recommendations made by the US Preventive Services Task Force,1 the Task Force has completed work on 12 topics (TABLE 1).2-17 Five of these topics have been discussed in JFP audio recordings, and the links are provided in TABLE 1.
This latest Task Force endeavor resulted in 18 recommendations (TABLE 2), all of which reaffirm previous recommendations on these topics and expand the scope of 2. There were 2 “A” recommendations, 6 “B” recommendations, 2 “D” recommendations, and 8 “I” statements, indicating that there was insufficient evidence to assess effectiveness or harms. The willingness to make “I” statements when there is little or no evidence on the intervention being assessed distinguishes the USPSTF from other clinical guideline committees.
Screening for carotid artery stenosis
One of the “D” recommendations this past year reaffirms the prior recommendation against screening for carotid artery stenosis in asymptomatic adults—ie, those without a history of transient ischemic attack, stroke, or neurologic signs or symptoms that might be caused by carotid artery stenosis.2 The screening tests the Task Force researched included carotid duplex ultrasonography (DUS), magnetic resonance angiography, and computed tomography angiography. The Task Force did not look at the value of auscultation for carotid bruits because it has been proven to be inaccurate and they do not consider it to be a useful screening tool.
The Task Force based its “D” recommendation on a lack of evidence for any benefit in detecting asymptomatic carotid artery stenosis, and on evidence that screening can lead to harms through false-positive tests and potential complications from carotid endarterectomy and carotid artery angioplasty and stenting. In its clinical considerations, the Task Force emphasized the primary prevention of atherosclerotic disease by focusing on the following actions:
- screening for high blood pressure in adults
- encouraging tobacco smoking cessation in adults
- promoting a healthy diet and physical activity in adults with cardiovascular risk factors
- recommending aspirin use to prevent cardiovascular disease and colorectal cancer
- advising statin use for the primary prevention of cardiovascular disease in adults ages 45 to 75 years who have 1 or more risk factors (hyperlipidemia, diabetes, hypertension, smoking) and those with a 10-year risk of a cardiovascular event of 10% or greater.
This “D” recommendation differs from recommendations made by other professional organizations, some of which recommend testing with DUS for asymptomatic patients with a carotid bruit, and others that recommend DUS screening in patients with multiple risk factors for stroke and in those with known peripheral artery disease or other cardiovascular disease.18,19
Smoking cessation in adults
Smoking tobacco is the leading preventable cause of death in the United States, causing about 480,000 deaths annually.3 Smoking during pregnancy increases the risk of complications including miscarriage, congenital anomalies, stillbirth, fetal growth restriction, preterm birth, and placental abruption.
The Task Force published recommendations earlier this year advising all clinicians to ask all adult patients about tobacco use; and, for those who smoke, to provide (or refer them to) smoking cessation behavioral therapy. The Task Force also recommends prescribing pharmacotherapy approved by the Food and Drug Administration (FDA) for smoking cessation for nonpregnant adults. (There is a lack of information to assess the harms and benefits of smoking cessation pharmacotherapy during pregnancy.)
Continue to: FDA-approved medications...
FDA-approved medications for treating tobacco smoking dependence are nicotine replacement therapy (NRT), bupropion hydrochloride, and varenicline.3 NRT is available in transdermal patches, lozenges, gum, inhalers, and nasal sprays.
In addition, the Task Force indicates that there is insufficient evidence to assess the benefits and harms of e-cigarettes when used as a method of achieving smoking cessation: “Few randomized trials have evaluated the effectiveness of e-cigarettes to increase tobacco smoking cessation in nonpregnant adults, and no trials have evaluated e-cigarettes for tobacco smoking cessation in pregnant persons.”4
Hepatitis B infection screening
The Task Force reaffirmed a previous recommendation to screen for hepatitis B virus (HBV) infection only in adults who are at high risk,5 rather than universal screening that it recommends for hepatitis C virus infection (HCV).7 (See: https://bit.ly/3tt064Q). The Task Force has a separate recommendation to screen all pregnant women for hepatitis B at the first prenatal visit.6
Those at high risk for hepatitis B who should be screened include individuals born in countries or regions of the world with a hepatitis B surface antigen (HBsAg) prevalence ≥ 2% and individuals born in the United States who have not received HBV vaccine and whose parents were born in regions with an HBsAg prevalence ≥ 8%.5 (A table listing countries with HBsAg ≥ 8%—as well as those in lower prevalence categories—is included with the recommendation.5)
HBV screening should also be offered to other high-risk groups that have a prevalence of positive HBsAg ≥ 2%: those who have injected drugs in the past or are currently injecting drugs; men who have sex with men; individuals with HIV; and sex partners, needle-sharing contacts, and household contacts of people known to be HBsAg positive.5
Continue to: It is estimated that...
It is estimated that > 860,000 people in the United States have chronic HBV infection and that close to two-thirds of them are unaware of their infection.5 The screening test for HBV is highly accurate; sensitivity and specificity are both > 98%.5 While there is no direct evidence that screening, detecting, and treating asymptomatic HBV infection reduces morbidity and mortality, the Task Force felt that the evidence for improvement in multiple outcomes in those with HBV when treated with antiviral regimens was sufficient to support the recommendation.
Screening for bacterial vaginosis in pregnancy
While bacterial vaginosis (BV) is associated with a two-fold risk of preterm delivery, treating BV during pregnancy does not seem to reduce this risk, indicating that some other variable is involved.8 In addition, studies that looked at screening for, and treatment of, asymptomatic BV in pregnant women at high risk for preterm delivery (defined primarily as those with a previous preterm delivery) have shown inconsistent results. There is the potential for harm in treating BV in pregnancy, chiefly involving gastrointestinal upset caused by metronidazole or clindamycin.
Given that there are no benefits—and some harms—resulting from treatment, the Task Force recommends against screening for BV in non-high-risk pregnant women. A lack of sufficient information to assess any potential benefits to screening in high-risk pregnancies led the Task Force to an “I” statement on this question.8
Behavioral counseling on healthy diet, exercise for adults with CV risks
Cardiovascular disease (CVD) remains the number one cause of death in the United States. The major risk factors for CVD, which can be modified, are high blood pressure, hyperlipidemia, diabetes, smoking, obesity or overweight, and lack of physical activity.
The Task Force has previously recommended intensive behavioral interventions to improve nutrition and physical activity in those who are overweight/obese and in those with abnormal blood glucose levels,9 and has addressed smoking prevention and cessation.4 This new recommendation applies to those with other CVD risks such as high blood pressure and/or hyperlipidemia and those with an estimated 10-year CVD risk of ≥ 7.5%.10
Continue to: Behavioral interventions...
Behavioral interventions included in the Task Force analysis employed a median of 12 contacts and an estimated 6 hours of contact time over 6 to 18 months.10 Most interventions involved motivational interviewing and instruction on behavioral change methods. These interventions can be provided by primary care clinicians, as well as a wide range of other trained professionals. The Affordable Care Act dictates that all “A” and “B” recommendations must be provided by commercial health plans at no out-of-pocket expense for the patient.
Nutritional advice should include reductions in saturated fats, salt, and sugars and increases in fruits, vegetables, and whole grains. The Mediterranean diet and the Dietary Approaches to Stop Hypertension (DASH) diet are often recommended.10 Physical activity counseling should advocate for 90 to 180 minutes per week of moderate to vigorous activity.
This new recommendation, along with the previous ones pertaining to behavioral interventions for lifestyle changes, make it clear that intensive interventions are needed to achieve meaningful change. Simple advice from a clinician will have little to no effect.
Task Force reviews evidence on HTN, smoking cessation in young people
In 2020 the Task Force completed reviews of evidence relevant to screening for high blood pressure11 and
The 2 “I” statements are in disagreement with recommendations of other professional organizations. The American Academy of Pediatrics (AAP) and the American Heart Association recommend routine screening for high blood pressure starting at age 3 years. And the AAP recommends screening teenagers for tobacco use and offering tobacco dependence treatment, referral, or both (including pharmacotherapy) when indicated. E-cigarettes are not recommended as a treatment for tobacco dependence.20
Continue to: The difference between...
The difference between the methods used by the Task Force and other guideline-producing organizations becomes apparent when it comes to recommendations pertaining to children and adolescents, for whom long-term outcome-oriented studies on prevention issues are rare. The Task Force is unwilling to make recommendations when evidence does not exist. The AAP often makes recommendations based on expert opinion consensus in such situations. One notable part of each Task Force recommendation statement is a discussion of what other organizations recommend on the same topic so that these differences can be openly described.
Better Task Force funding could expand topic coverage
It is worth revisiting 2 issues that were pointed out in last year’s USPSTF summary in this column.1 First, the Task Force methods are robust and evidence based, and recommendations therefore are rarely changed once they are made at an “A”, “B”, or “D” level. Second, Task Force resources are finite, and thus, the group is currently unable to update previous recommendations with greater frequency or to consider many new topics. In the past 2 years, the Task Force has developed recommendations on only 2 completely new topics. Hopefully, its budget can be expanded so that new topics can be added in the future.
1. Campos-Outcalt D. USPSTF roundup. J Fam Pract. 2020;69:201-204.
2. USPSTF. Screening for asymptomatic carotid artery stenosis. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/carotid-artery-stenosis-screening
3. USPSTF. Interventions for tobacco smoking cessation in adults, including pregnant persons. Accessed April 30, 2021. www.uspreventiveservicestaskforce.org/uspstf/recommendation/tobacco-use-in-adults-and-pregnant-women-counseling-and-interventions
4. USPSTF. Interventions for tobacco smoking cessation in adults, including pregnant persons. JAMA. 2021;325:265-279.
5. USPSTF. Screening for Hepatitis B virus infection in adolescents and adults. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/hepatitis-b-virus-infection-screening
6. USPSTF. Hepatitis B virus infection in pregnant women: screening. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/hepatitis-b-virus-infection-in-pregnant-women-screening
7. USPSTF. Hepatitis C virus infection in adolescents and adults: screening. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/hepatitis-c-screening
8. USPSTF; Owens DK, Davidson KW, Krisk AH, et al. Screening for bacterial vaginosis in pregnant persons to prevent preterm delivery: US Preventive Services Task Force recommendation statement. JAMA. 2020;323:1286-1292.
9. Behavioral counseling to promote a healthful diet and physical activity for cardiovascular disease prevention in adults with cardiovascular risk factors: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;161:587-593.
10. USPSTF. Behavioral counseling interventions to promote a healthy and physical activity for cardiovascular disease prevention in adults with cardiovascular risk factors: US Preventive Services Task Force recommendation statement. JAMA. 2020;324:2069-2075.
11. USPSTF. High blood pressure in children and adolescents: screening. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/blood-pressure-in-children-and-adolescents-hypertension-screening
12. USPSTF. Prevention and cessation of tobacco use in children and adolescents: primary care interventions. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/tobacco-and-nicotine-use-prevention-in-children-and-adolescents-primary-care-interventions
13. USPSTF. Cognitive impairment in older adults: screening. Accessed March 26, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/cognitive-impairment-in-older-adults-screening
14. USPSTF. Illicit drug use in children, adolescents, and young adults: primary care-based interventions. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/drug-use-illicit-primary-care-interventions-for-children-and-adolescents
15. USPSTF. Unhealthy drug use: screening. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/drug-use-illicit-screening
16. USPSTF. Sexually transmitted infections: behavioral counseling. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/sexually-transmitted-infections-behavioral-counseling.
17. Campos-Outcalt D. USPSTF update on sexually transmitted infections. J Fam Pract. 2020;69:514-517.
18. Brott TG, Halperin JL, Abbara S, et al; ASA/ACCF/AHA/AANN/AANS/ACR/ASNR/CNS/SAIP/SCAI/SIR/SNIS/SVM/SVS guideline on the management of patients with extracranial carotid and vertebral artery disease. Catheter Cardiovasc Interv. 2013;81:E76-E123.
19. Ricotta JJ, Aburahma A, Ascher E, et al; Society for Vascular Surgery. Updated Society for Vascular Surgery guidelines for management of extracranial carotid disease. J Vasc Surg. 2011;54:e1-e31.
20. Farber HJ, Walley SC, Groner JA, et al; Section on Tobacco Control. Clinical practice policy to protect children from tobacco, nicotine, and tobacco smoke. Pediatrics. 2015;136:1008-1017.
1. Campos-Outcalt D. USPSTF roundup. J Fam Pract. 2020;69:201-204.
2. USPSTF. Screening for asymptomatic carotid artery stenosis. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/carotid-artery-stenosis-screening
3. USPSTF. Interventions for tobacco smoking cessation in adults, including pregnant persons. Accessed April 30, 2021. www.uspreventiveservicestaskforce.org/uspstf/recommendation/tobacco-use-in-adults-and-pregnant-women-counseling-and-interventions
4. USPSTF. Interventions for tobacco smoking cessation in adults, including pregnant persons. JAMA. 2021;325:265-279.
5. USPSTF. Screening for Hepatitis B virus infection in adolescents and adults. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/hepatitis-b-virus-infection-screening
6. USPSTF. Hepatitis B virus infection in pregnant women: screening. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/hepatitis-b-virus-infection-in-pregnant-women-screening
7. USPSTF. Hepatitis C virus infection in adolescents and adults: screening. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/hepatitis-c-screening
8. USPSTF; Owens DK, Davidson KW, Krisk AH, et al. Screening for bacterial vaginosis in pregnant persons to prevent preterm delivery: US Preventive Services Task Force recommendation statement. JAMA. 2020;323:1286-1292.
9. Behavioral counseling to promote a healthful diet and physical activity for cardiovascular disease prevention in adults with cardiovascular risk factors: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;161:587-593.
10. USPSTF. Behavioral counseling interventions to promote a healthy and physical activity for cardiovascular disease prevention in adults with cardiovascular risk factors: US Preventive Services Task Force recommendation statement. JAMA. 2020;324:2069-2075.
11. USPSTF. High blood pressure in children and adolescents: screening. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/blood-pressure-in-children-and-adolescents-hypertension-screening
12. USPSTF. Prevention and cessation of tobacco use in children and adolescents: primary care interventions. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/tobacco-and-nicotine-use-prevention-in-children-and-adolescents-primary-care-interventions
13. USPSTF. Cognitive impairment in older adults: screening. Accessed March 26, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/cognitive-impairment-in-older-adults-screening
14. USPSTF. Illicit drug use in children, adolescents, and young adults: primary care-based interventions. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/drug-use-illicit-primary-care-interventions-for-children-and-adolescents
15. USPSTF. Unhealthy drug use: screening. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/drug-use-illicit-screening
16. USPSTF. Sexually transmitted infections: behavioral counseling. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/sexually-transmitted-infections-behavioral-counseling.
17. Campos-Outcalt D. USPSTF update on sexually transmitted infections. J Fam Pract. 2020;69:514-517.
18. Brott TG, Halperin JL, Abbara S, et al; ASA/ACCF/AHA/AANN/AANS/ACR/ASNR/CNS/SAIP/SCAI/SIR/SNIS/SVM/SVS guideline on the management of patients with extracranial carotid and vertebral artery disease. Catheter Cardiovasc Interv. 2013;81:E76-E123.
19. Ricotta JJ, Aburahma A, Ascher E, et al; Society for Vascular Surgery. Updated Society for Vascular Surgery guidelines for management of extracranial carotid disease. J Vasc Surg. 2011;54:e1-e31.
20. Farber HJ, Walley SC, Groner JA, et al; Section on Tobacco Control. Clinical practice policy to protect children from tobacco, nicotine, and tobacco smoke. Pediatrics. 2015;136:1008-1017.
Screening High-Risk Women Veterans for Breast Cancer
The number of women seeking care from the Veterans Health Administration (VHA) is increasing.1 In 2015, there were 2 million women veterans in the United States, which is 9.4% of the total veteran population. This group is expected to increase at an average of about 18,000 women per year for the next 10 years.2 The percentage of women veterans who are US Department of Veterans Affairs (VA) users aged 45 to 64 years rose 46% from 2000 to 2015.1,3-4 It is estimated that 15% of veterans who used VA services in 2020 were women.1 Nineteen percent of women veterans are Black.1 The median age of women veterans in 2015 was 50 years.5 Breast cancer is the leading cancer affecting female veterans, and data suggest they have an increased risk of breast cancer based on unique service-related exposures.1,6-9
In the US, about 10 million women are eligible for breast cancer preventive therapy, including, but not limited to, medications, surgery, or lifestyle changes.10 Secondary prevention options include change in surveillance that can reduce their risk or identify cancer at an earlier stage when treatment is more effective. The United States Preventive Services Task Force, the National Comprehensive Cancer Network, the American Society for Clinical Oncology, the National Institute for Health and Care Excellence, and the Oncology Nursing Society recommend screening women aged ≥ 35 years to assess breast cancer risk.11-18 If a woman is at increased risk, she may be a candidate for chemoprevention, prozphylactic surgery, and possibly an enhanced screening regimen.
Urban and minority women are an understudied population. Most veterans (75%) live in urban or suburban settings.19,20 Urban veteran women constitute an important potential study population.
Chemoprevention measures have been underused because of factors involving both women and their health care providers. A large proportion of women are unaware of their higher risk status due to lack of adequate screening and risk assessment.21,22 In addition to patient lack of awareness of their high-risk status, primary care physicians are also reluctant to prescribe chemopreventive agents due to a lack of comfort or familiarity with the risks and benefits.23-26 The STAR2015, BCPT2005, IBIS2014, MAP3 2011, IBIS-I 2014, and IBIS II 2014 studies clearly demonstrate a 49 to 62% reduction in risk for women using chemoprevention such as selective estrogen receptor modulators or aromatase inhibitors, respectively.27-32 Yet only 4 to 9% of high-risk women not enrolled in a clinical trial are using chemoprevention.33-39
The possibility of developing breast cancer also may be increased because of a positive family history or being a member of a family in which there is a known susceptibility gene mutation.40 Based on these risk factors, women may be eligible for tailored follow-up and genetic counseling.41-44
Nationally, 7 to 10% of the civilian US population will experience posttraumatic stress disorder (PTSD).45 The rates are remarkably higher for women veterans, with roughly 20% diagnosed with PTSD.46,47 Anxiety and PTSD have been implicated in poor adherence to medical advice.48,49
In 2014, a national VA multidisciplinary group focused on breast cancer prevention, detection, treatment, and research to address breast health in the growing population of women veterans. High-risk breast cancer screenings are not routinely carried out by the VA in primary care, women’s health, or oncology services. Furthermore, the recording of screening questionnaire results was not synchronized until a standard questionnaire was created and approved as a template by this group in the VA electronic medical record (EMR) in 2015.
Several prediction models can identify which women are at an increased risk of developing breast cancer. The most commonly used risk assessment model, the Gail breast cancer risk assessment tool (BCRAT), has been refined to include women of additional ethnicities (https://www.cancer.gov/bcrisktool).
This pilot project was launched to identify an effective manner to screen women veterans regarding their risk of developing breast cancer and refer them for chemoprevention education or genetic counseling as appropriate.
Methods
A high-risk breast cancer screening questionnaire based on the Gail BCRAT and including lifestyle questions was developed and included as a note template in the VA EMR. The James J. Peters VA Medical Center, Bronx, NY (JJPVAMC) and the Washington DC VA Medical Center (DCVAMC) ran a pilot study between 2015 and 2018 using this breast cancer screening questionnaire to collect data from women veterans. Quality Executive Committee and institutional review board approvals were granted respectively.
Eligibility criteria included women aged ≥ 35 years with no personal history of breast cancer. Most patients were self-referred, but participants also were recruited during VA Breast Cancer Awareness month events, health fairs, or at informational tables in the hospital lobbies. After completing the 20 multiple choice questionnaire with a study team member, either in person or over the phone, a 5-year and lifetime risk of invasive breast cancer was calculated using the Gail BCRAT. A woman is considered high risk and eligible for chemoprevention if her 5-year risk is > 1.66% or her lifetime risk is ≥ 20%. Eligibility for genetic counseling is based on the Breast Cancer Referral Screening Tool, which includes a personal or family history of breast or ovarian cancer and Jewish ancestry.
All patients were notified of their average or high risk status by a clinician. Those who were deemed to be average risk received a follow-up letter in the mail with instructions (eg, to follow-up with a yearly mammogram). Those who were deemed to be high risk for developing breast cancer were asked to come in for an appointment with the study principal investigator (a VA oncologist/breast cancer specialist) to discuss prevention options, further screening, or referrals to genetic counseling. Depending on a patient’s other health factors, a woman at high risk for developing breast cancer also may be a candidate for chemoprevention with tamoxifen, raloxifene, exemestane, anastrozole, or letrozole.
Data on the participant’s lifestyle, including exercise, diet, and smoking, were evaluated to determine whether these factors had an impact on risk status.
Results
The JJP and DC VAMCs screened 103 women veterans between 2015 and 2018. Four patients were excluded for nonveteran (spousal) status, leaving 99 women veterans with a mean age of 54 years. The most common self-reported races were Black (60%), non-Hispanic White (14%), and Hispanic or Latino (13%) (Table 1).
Women veterans in our study were nearly 3-times more likely than the general population were to receive a high-risk Gail Score/BCRAT (35% vs 13%, respectively).50,51 Of this subset, 46% had breast biopsies, and 86% had a positive family history. Thirty-one percent of Black women in our study were high risk, while nationally, 8.2 to 13.3% of Black women aged 50 to 59 years are considered high risk.50,51 Of the Black high-risk group with a high Gail/BCRAT score, 94% had a positive family history, and 33% had a history of breast biopsy (Table 2).
Of the 35 high-risk patients 26 (74%) patients accepted consultations for chemoprevention and 5 (19%) started chemoprevention. Of this high-risk group, 13 (37%) patients were referred for genetic counseling (Table 3).44 The prevalence of PTSD was present in 31% of high-risk women and 29% of the cohort (Figure).The lifestyle questions indicated that, among all participants, 79% had an overweight or obese body mass index; 58% exercised weekly; 51% consumed alcohol; 14% were smokers; and 21% consumed 3 to 4 servings of fruits/vegetables daily.
Discussion
Breast cancer is the most common cancer in women.52 The number of women with breast cancer in the VHA has more than tripled from 1995 to 2012.1 The lifetime risk of developing breast cancer in the general population is about 13%.50 This rate can be affected by risk factors including age, hormone exposure, family history, radiation exposure, and lifestyle factors, such as weight and alcohol use.6,52-56 In the United States, invasive breast cancer affects 1 in 8 women.50,52,57
Our screened population showed nearly 3 times as many women veterans were at an increased risk for breast cancer when compared with historical averages in US women. This difference may be based on a high rate of prior breast biopsies or positive family history, although a provocative study using the Surveillance, Epidemiology, and End Results database showed military women to have higher rates of breast cancer as well.9 Historically, Blacks are vastly understudied in clinical research with only 5% representation on a national level.5,58 The urban locations of both pilot sites (Washington, DC and Bronx, NY) allowed for the inclusion of minority patients in our study. We found that the rates of breast cancer in Black women veterans to be higher than seen nationally, possibly prompting further screening initiatives for this understudied population.
Our pilot study’s chemoprevention utilization (19%) was double the < 10% seen in the national population.33-35 The presence of a knowledgeable breast health practitioner to recruit study participants and offer personalized counseling to women veterans is a likely factor in overcoming barriers to chemopreventive acceptance. These participants may have been motivated to seek care for their high-risk status given a strong family history and prior breast biopsies.
Interestingly, a 3-fold higher PTSD rate was seen in this pilot population (29%) when compared with PTSD rates in the general female population (7-10%) and still one-third higher than the general population of women veterans (20%).45-47 Mental health, anxiety, and PTSD have been barriers to patients who sought treatment and have been implicated in poor adherence to medical advice.48,49 Cancer screening can induce anxiety in patients, and it may be amplified in patients with PTSD. It was remarkable that although adherence with screening recommendations is decreased when PTSD is present, our patient population demonstrated a higher rate of screening adherence.
Women who are seen at the VA often use multiple clinical specialties, and their EMR can be accessed across VA medical centers nationwide. Therefore, identifying women veterans who meet screening criteria is easily attainable within the VA.
When comparing high-risk with average risk women, the lifestyle results (BMI, smoking history, exercise and consumption of fruits, vegetables and alcohol) were essentially the same. Lifestyle factors were similar to national population rates and were unlikely to impact risk levels.
Limitations
Study limitations included a high number of self-referrals and the large percentage of patients with a family history of breast cancer, making them more likely to seek screening. The higher-than-average risk of breast cancer may be driven by a high rate of breast biopsies and a strong family history. Lifestyle metrics could not be accurately compared to other national assessments of lifestyle factors due to the difference in data points that we used or the format of our questions.
Conclusions
As the number of women veterans increases and the incidence of breast cancer in women veterans rise, chemoprevention options should follow national guidelines. To our knowledge, this is the only oncology study with 60% Black women veterans. This study had a higher participation rate for Black women veterans than is typically seen in national research studies and shows the VA to be a germane source for further understanding of an understudied population that may benefit from increased screening for breast cancer.
A team-based, multidisciplinary model that meets the unique healthcare needs of women veterans results in a patient-centric delivery of care for assessing breast cancer risk status and prevention options. This model can be replicated nationally by directing primary care physicians and women’s health practitioners to a risk-assessment questionnaire and referring high-risk women for appropriate preventative care. Given that these results show chemoprevention adherence rates doubled those seen nationally, perhaps techniques used within this VA pilot study may be adapted to decrease breast cancer incidence nationally.
Since the rate of PTSD among women veterans is triple the national average, we would expect adherence rates to be lower in our patient cohort. However, the multidisciplinary approach we used in this study (eg, 1:1 consultation with oncologist; genetic counseling referrals; mental health support available), may have improved adherence rates. Perhaps the high rates of PTSD seen in the VA patient population can be a useful way to explore patient adherence rates in those with mental illness and medical conditions.
Future research with a larger cohort may lead to greater insight into the correlation between PTSD and adherence to treatment. Exploring the connection between breast cancer, epigenetics, and specific military service-related exposures could be an area of analysis among this veteran population exhibiting increased breast cancer rates. VAMCs are situated in rural, suburban, and urban locations across the United States and offers a diverse socioeconomic and ethnic patient population for inclusion in clinical investigations. Women veterans make up a small subpopulation of women in the United States, but it is worth considering VA patients as an untapped resource for research collaboration.
Acknowledgements
The authors thank Steven Sanchez and Marissa Vallette, PhD, Breast Health Research Group. This research project was approved by the James J. Peters VA Medical Center Quality Executive Committee and the Washington, DC VA Medical Center Institutional Review Board. This work was supported by the US Department of Veterans Affairs. This work did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the US Government, or any of its agencies.
1. US Department of Veterans Affairs. National Center for Veterans Analysis and Statistics. The past, present and future of women veterans. Published February 2017. Accessed April 28, 2021. https://www.va.gov/vetdata/docs/specialreports/women_veterans_2015_final.pdf.
2. Frayne SM, Carney DV, Bastian L, et al. The VA Women’s Health Practice-Based Research Network: amplifying women veterans’ voices in VA research. J Gen Intern Med. 2013;28 Suppl 2(Suppl 2):S504-S509. doi:10.1007/s11606-013-2476-3
3. US Department of Veterans Affairs, Veterans Health Administration, Women’s Health Evaluation Initiative, Women Veterans Health Strategic Health Care Group. Sourcebook: women veterans in the Veterans Health Administration. Volume 1: Sociodemographic characteristics and use of VHA care. Published December 2010. Accessed April 12, 2021. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=2455
4. Bean-Mayberry B, Yano EM, Bayliss N, Navratil J, Weisman CS, Scholle SH. Federally funded comprehensive women’s health centers: leading innovation in women’s healthcare delivery. J Womens Health (Larchmt). 2007;16(9):1281-1290. doi:10.1089/jwh.2006.0284
5. US Department of Veterans Affairs. National Center for Veterans Analysis and Statistics.VA utilization profile FY 2016. Published November 2017. Accessed April 12, 2021. https://www.va.gov/vetdata/docs/QuickFacts/VA_Utilization_Profile.PDF
6. Ekenga CC, Parks CG, Sandler DP. Chemical exposures in the workplace and breast cancer risk: a prospective cohort study. Int J Cancer. 2015;137(7):1765-1774. doi:10.1002/ijc.29545
7. Rennix CP, Quinn MM, Amoroso PJ, Eisen EA, Wegman DH. Risk of breast cancer among enlisted Army women occupationally exposed to volatile organic compounds. Am J Ind Med. 2005;48(3):157-167. doi:10.1002/ajim.20201
8. Ritz B. Cancer mortality among workers exposed to chemicals during uranium processing. J Occup Environ Med. 1999;41(7):556-566. doi:10.1097/00043764-199907000-00004
9. Zhu K, Devesa SS, Wu H, et al. Cancer incidence in the U.S. military population: comparison with rates from the SEER program. Cancer Epidemiol Biomarkers Prev. 2009;18(6):1740-1745. doi:10.1158/1055-9965.EPI-09-0041
10. Freedman AN, Yu B, Gail MH, et al. Benefit/risk assessment for breast cancer chemoprevention with raloxifene or tamoxifen for women age 50 years or older [published correction appears in J Clin Oncol. 2013 Nov 10;31(32):4167]. J Clin Oncol. 2011;29(17):2327-2333. doi:10.1200/JCO.2010.33.0258
11. Greene, H. Cancer prevention, screening and early detection. In: Gobel BH, Triest-Robertson S, Vogel WH, eds. Advanced Oncology Nursing Certification Review and Resource Manual. 3rd ed. Oncology Nursing Society; 2016:1-34. https://www.ons.org/sites/default/files/publication_pdfs/2%20ADVPrac%20chapter%201.pdf
12. National Comprehensive Cancer Network. NCCN Breast Cancer Risk Reduction. Version 1.2021 NCCN Clinical Practice Guidelines in Oncology. Updated March 24, 2021 Accessed April 12, 2021. https://www.nccn.org/professionals/physician_gls/pdf/breast_risk.pdf
13. US Preventive Services Task Force. Breast cancer: Medications use to reduce risk. Updated September 3, 2019. Accessed April 12, 2021. https://www.uspreventiveservicestaskforce.org/uspstf/recommendation/breast-cancer-medications-for-risk-reduction
14. Moyer VA; U.S. Preventive Services Task Force. Medications to decrease the risk for breast cancer in women: recommendations from the U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2013;159(10):698-708. doi:10.7326/0003-4819-159-10-201311190-00717
15. Boucher JE. Chemoprevention: an overview of pharmacologic agents and nursing considerations. Clin J Oncol Nurs. 2018;22(3):350-353. doi:10.1188/18.CJON.350-353
16. Nichols HB, Stürmer T, Lee VS, et al. Breast cancer chemoprevention in an integrated health care setting. JCO Clin Cancer Inform. 2017;1:1-12. doi:10.1200/CCI.16.00059
17. Bevers TB, Helvie M, Bonaccio E, et al. Breast cancer screening and diagnosis, Version 3.2018, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2018;16(11):1362-1389. doi:10.6004/jnccn.2018.0083
18. Visvanathan K, Hurley P, Bantug E, et al. Use of pharmacologic interventions for breast cancer risk reduction: American Society of Clinical Oncology clinical practice guideline [published correction appears in J Clin Oncol. 2013 Dec 1;31(34):4383]. J Clin Oncol. 2013;31(23):2942-2962. doi:10.1200/JCO.2013.49.3122
19. Sealy-Jefferson S, Roseland ME, Cote ML, et al. rural-urban residence and stage at breast cancer diagnosis among postmenopausal women: The Women’s Health Initiative. J Womens Health (Larchmt). 2019;28(2):276-283. doi:10.1089/jwh.2017.6884
20. Holder KA. Veterans in rural America: 2011-2015. Published January 25, 2017. Accessed April 12, 2021. https://www.census.gov/library/publications/2017/acs/acs-36.html
21. Owens WL, Gallagher TJ, Kincheloe MJ, Ruetten VL. Implementation in a large health system of a program to identify women at high risk for breast cancer. J Oncol Pract. 2011;7(2):85-88. doi:10.1200/JOP.2010.000107
2. Pivot X, Viguier J, Touboul C, et al. Breast cancer screening controversy: too much or not enough?. Eur J Cancer Prev. 2015;24 Suppl:S73-S76. doi:10.1097/CEJ.0000000000000145
23. Bidassie B, Kovach A, Vallette MA, et al. Breast Cancer risk assessment and chemoprevention use among veterans affairs primary care providers: a national online survey. Mil Med. 2020;185(3-4):512-518. doi:10.1093/milmed/usz291
24. Brewster AM, Davidson NE, McCaskill-Stevens W. Chemoprevention for breast cancer: overcoming barriers to treatment. Am Soc Clin Oncol Educ Book. 2012;85-90. doi:10.14694/EdBook_AM.2012.32.152
25. Meyskens FL Jr, Curt GA, Brenner DE, et al. Regulatory approval of cancer risk-reducing (chemopreventive) drugs: moving what we have learned into the clinic. Cancer Prev Res (Phila). 2011;4(3):311-323. doi:10.1158/1940-6207.CAPR-09-0014
26. Tice JA, Kerlikowske K. Screening and prevention of breast cancer in primary care. Prim Care. 2009;36(3):533-558. doi:10.1016/j.pop.2009.04.003
27. Vogel VG. Selective estrogen receptor modulators and aromatase inhibitors for breast cancer chemoprevention. Curr Drug Targets. 2011;12(13):1874-1887. doi:10.2174/138945011798184164
28. Vogel VG, Costantino JP, Wickerham DL, et al. Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial [published correction appears in JAMA. 2006 Dec 27;296(24):2926] [published correction appears in JAMA. 2007 Sep 5;298(9):973]. JAMA. 2006;295(23):2727-2741. doi:10.1001/jama.295.23.joc60074
29. Pruthi S, Heisey RE, Bevers TB. Chemoprevention for breast cancer. Ann Surg Oncol. 2015;22(10):3230-3235. doi:10.1245/s10434-015-4715-9
30. Cuzick J, Sestak I, Forbes JF, et al. Anastrozole for prevention of breast cancer in high-risk postmenopausal women (IBIS-II): an international, double-blind, randomised placebo-controlled trial [published correction appears in Lancet. 2014 Mar 22;383(9922):1040] [published correction appears in Lancet. 2017 Mar 11;389(10073):1010]. Lancet. 2014;383(9922):1041-1048. doi:10.1016/S0140-6736(13)62292-8
31. Bozovic-Spasojevic I, Azambuja E, McCaskill-Stevens W, Dinh P, Cardoso F. Chemoprevention for breast cancer. Cancer Treat Rev. 2012;38(5):329-339. doi:10.1016/j.ctrv.2011.07.005
32. Gabriel EM, Jatoi I. Breast cancer chemoprevention. Expert Rev Anticancer Ther. 2012;12(2):223-228. doi:10.1586/era.11.206

33. Crew KD, Albain KS, Hershman DL, Unger JM, Lo SS. How do we increase uptake of tamoxifen and other anti-estrogens for breast cancer prevention?. NPJ Breast Cancer. 2017;3:20. Published 2017 May 19. doi:10.1038/s41523-017-0021-y
34. Ropka ME, Keim J, Philbrick JT. Patient decisions about breast cancer chemoprevention: a systematic review and meta-analysis. J Clin Oncol. 2010;28(18):3090-3095. doi:10.1200/JCO.2009.27.8077
35. Smith SG, Sestak I, Forster A, et al. Factors affecting uptake and adherence to breast cancer chemoprevention: a systematic review and meta-analysis. Ann Oncol. 2016;27(4):575-590. doi:10.1093/annonc/mdv590
36. Grann VR, Patel PR, Jacobson JS, et al. Comparative effectiveness of screening and prevention strategies among BRCA1/2-affected mutation carriers. Breast Cancer Res Treat. 2011 Feb;125(3):837-847. doi:10.1007/s10549-010-1043-4
37. Goss PE, Ingle JN, Alés-Martínez JE, et al. Exemestane for breast-cancer prevention in postmenopausal women [published correction appears in N Engl J Med. 2011 Oct 6;365(14):1361]. N Engl J Med. 2011;364(25):2381-2391. doi:10.1056/NEJMoa1103507
38. Kmietowicz Z. Five in six women reject drugs that could reduce their risk of breast cancer. BMJ. 2015;351:h6650. Published 2015 Dec 8. doi:10.1136/bmj.h6650
39. Nelson HD, Fu R, Griffin JC, Nygren P, Smith ME, Humphrey L. Systematic review: comparative effectiveness of medications to reduce risk for primary breast cancer. Ann Intern Med. 2009;151(10):703-235. doi:10.7326/0003-4819-151-10-200911170-00147
40. Dahabreh IJ, Wieland LS, Adam GP, Halladay C, Lau J, Trikalinos TA. Core needle and open surgery biopsy for diagnosis of breast lesions: an update to the 2009 report. Published September 2014. Accessed April 12, 2021. https://www.ncbi.nlm.nih.gov/books/NBK246878
41. National Cancer Institute. Genetics of breast and ovarian cancer (PDQ)—health profession version. Updated February 12, 2021. Accessed April 12, 2021. http://www.cancer.gov/cancertopics/pdq/genetics/breast-and-ovarian/HealthProfessional
42. US Department of Health and Human Services. National Institutes of Health, National Institute of Environmental Health Sciences The sister study. Accessed April 12, 2021. https://sisterstudy.niehs.nih.gov/english/NIEHS.htm
43. Tutt A, Ashworth A. Can genetic testing guide treatment in breast cancer?. Eur J Cancer. 2008;44(18):2774-2780. doi:10.1016/j.ejca.2008.10.009
44. Katz SJ, Ward KC, Hamilton AS, et al. Gaps in receipt of clinically indicated genetic counseling after diagnosis of breast cancer. J Clin Oncol. 2018;36(12):1218-1224. doi:10.1200/JCO.2017.76.2369
45. US Department of Veterans Affairs. PTSD: National Center for PTSD. How common is PTSD in adults? Updated October 17, 2019. Accessed April 12, 2021. https://www.ptsd.va.gov/understand/common/common_adults.asp
46. US Department of Veterans Affairs. PTSD: National Center for PTSD. How common is PTSD in women? Updated October 16, 2019. Accessed April 12, 2021. https://www.ptsd.va.gov/understand/common/common_women.asp
47. US Department of Veterans Affairs. PTSD: National Center for PTSD. How common is PTSD in veterans? Updated September 24, 2018. Accessed April 12, 2021. https://www.ptsd.va.gov/understand/common/common_veterans.asp
48. Lindberg NM, Wellisch D. Anxiety and compliance among women at high risk for breast cancer. Ann Behav Med. 2001;23(4):298-303. doi:10.1207/S15324796ABM2304_9
49. DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: meta-analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med. 2000;160(14):2101-2107. doi:10.1001/archinte.160.14.2101
50. Centers for Disease Control and Prevention. MMWR appendix: breast cancer rates among black women and white women. Updated October 13, 2016. Accessed April 12, 2021. https://www.cdc.gov/cancer/breast/statistics/trends_invasive.htm
51. Richardson LC, Henley SJ, Miller JW, Massetti G, Thomas CC. Patterns and trends in age-specific black-white differences in breast cancer incidence and mortality - United States, 1999-2014. MMWR Morb Mortal Wkly Rep. 2016;65(40):1093-1098. Published 2016 Oct 14. doi:10.15585/mmwr.mm6540a1
52. Brody JG, Moysich KB, Humblet O, Attfield KR, Beehler GP, Rudel RA. Environmental pollutants and breast cancer: epidemiologic studies. Cancer. 2007;109(12 Suppl):2667-2711. doi:10.1002/cncr.22655
53. Brophy JT, Keith MM, Watterson A, et al. Breast cancer risk in relation to occupations with exposure to carcinogens and endocrine disruptors: a Canadian case-control study. Environ Health. 2012;11:87. Published 2012 Nov 19. doi:10.1186/1476-069X-11-87
54. Labrèche F, Goldberg MS, Valois MF, Nadon L. Postmenopausal breast cancer and occupational exposures. Occup Environ Med. 2010;67(4):263-269. doi:10.1136/oem.2009.049817
55. National Institute of Environmental Health Sciences, Interagency Breast Cancer & Environmental Research Coordinating Committee. Breast cancer and the environment: prioritizing prevention. Updated March 8, 2013. Accessed April 12, 2021. https://www.niehs.nih.gov/about/boards/ibcercc/index.cfm
56. Gail MH, Costantino JP, Pee D, et al. Projecting individualized absolute invasive breast cancer risk in African American women [published correction appears in J Natl Cancer Inst. 2008 Aug 6;100(15):1118] [published correction appears in J Natl Cancer Inst. 2008 Mar 5;100(5):373]. J Natl Cancer Inst. 2007;99(23):1782-1792. doi:10.1093/jnci/djm223
57. Corbie-Smith G, Thomas SB, Williams MV, Moody-Ayers S. Attitudes and beliefs of African Americans toward participation in medical research. J Gen Intern Med. 1999;14(9):537-546. doi:10.1046/j.1525-1497.1999.07048.x
58. Braunstein JB, Sherber NS, Schulman SP, Ding EL, Powe NR. Race, medical researcher distrust, perceived harm, and willingness to participate in cardiovascular prevention trials. Medicine (Baltimore). 2008;87(1):1-9. doi:10.1097/MD.0b013e3181625d78
The number of women seeking care from the Veterans Health Administration (VHA) is increasing.1 In 2015, there were 2 million women veterans in the United States, which is 9.4% of the total veteran population. This group is expected to increase at an average of about 18,000 women per year for the next 10 years.2 The percentage of women veterans who are US Department of Veterans Affairs (VA) users aged 45 to 64 years rose 46% from 2000 to 2015.1,3-4 It is estimated that 15% of veterans who used VA services in 2020 were women.1 Nineteen percent of women veterans are Black.1 The median age of women veterans in 2015 was 50 years.5 Breast cancer is the leading cancer affecting female veterans, and data suggest they have an increased risk of breast cancer based on unique service-related exposures.1,6-9
In the US, about 10 million women are eligible for breast cancer preventive therapy, including, but not limited to, medications, surgery, or lifestyle changes.10 Secondary prevention options include change in surveillance that can reduce their risk or identify cancer at an earlier stage when treatment is more effective. The United States Preventive Services Task Force, the National Comprehensive Cancer Network, the American Society for Clinical Oncology, the National Institute for Health and Care Excellence, and the Oncology Nursing Society recommend screening women aged ≥ 35 years to assess breast cancer risk.11-18 If a woman is at increased risk, she may be a candidate for chemoprevention, prozphylactic surgery, and possibly an enhanced screening regimen.
Urban and minority women are an understudied population. Most veterans (75%) live in urban or suburban settings.19,20 Urban veteran women constitute an important potential study population.
Chemoprevention measures have been underused because of factors involving both women and their health care providers. A large proportion of women are unaware of their higher risk status due to lack of adequate screening and risk assessment.21,22 In addition to patient lack of awareness of their high-risk status, primary care physicians are also reluctant to prescribe chemopreventive agents due to a lack of comfort or familiarity with the risks and benefits.23-26 The STAR2015, BCPT2005, IBIS2014, MAP3 2011, IBIS-I 2014, and IBIS II 2014 studies clearly demonstrate a 49 to 62% reduction in risk for women using chemoprevention such as selective estrogen receptor modulators or aromatase inhibitors, respectively.27-32 Yet only 4 to 9% of high-risk women not enrolled in a clinical trial are using chemoprevention.33-39
The possibility of developing breast cancer also may be increased because of a positive family history or being a member of a family in which there is a known susceptibility gene mutation.40 Based on these risk factors, women may be eligible for tailored follow-up and genetic counseling.41-44
Nationally, 7 to 10% of the civilian US population will experience posttraumatic stress disorder (PTSD).45 The rates are remarkably higher for women veterans, with roughly 20% diagnosed with PTSD.46,47 Anxiety and PTSD have been implicated in poor adherence to medical advice.48,49
In 2014, a national VA multidisciplinary group focused on breast cancer prevention, detection, treatment, and research to address breast health in the growing population of women veterans. High-risk breast cancer screenings are not routinely carried out by the VA in primary care, women’s health, or oncology services. Furthermore, the recording of screening questionnaire results was not synchronized until a standard questionnaire was created and approved as a template by this group in the VA electronic medical record (EMR) in 2015.
Several prediction models can identify which women are at an increased risk of developing breast cancer. The most commonly used risk assessment model, the Gail breast cancer risk assessment tool (BCRAT), has been refined to include women of additional ethnicities (https://www.cancer.gov/bcrisktool).
This pilot project was launched to identify an effective manner to screen women veterans regarding their risk of developing breast cancer and refer them for chemoprevention education or genetic counseling as appropriate.
Methods
A high-risk breast cancer screening questionnaire based on the Gail BCRAT and including lifestyle questions was developed and included as a note template in the VA EMR. The James J. Peters VA Medical Center, Bronx, NY (JJPVAMC) and the Washington DC VA Medical Center (DCVAMC) ran a pilot study between 2015 and 2018 using this breast cancer screening questionnaire to collect data from women veterans. Quality Executive Committee and institutional review board approvals were granted respectively.
Eligibility criteria included women aged ≥ 35 years with no personal history of breast cancer. Most patients were self-referred, but participants also were recruited during VA Breast Cancer Awareness month events, health fairs, or at informational tables in the hospital lobbies. After completing the 20 multiple choice questionnaire with a study team member, either in person or over the phone, a 5-year and lifetime risk of invasive breast cancer was calculated using the Gail BCRAT. A woman is considered high risk and eligible for chemoprevention if her 5-year risk is > 1.66% or her lifetime risk is ≥ 20%. Eligibility for genetic counseling is based on the Breast Cancer Referral Screening Tool, which includes a personal or family history of breast or ovarian cancer and Jewish ancestry.
All patients were notified of their average or high risk status by a clinician. Those who were deemed to be average risk received a follow-up letter in the mail with instructions (eg, to follow-up with a yearly mammogram). Those who were deemed to be high risk for developing breast cancer were asked to come in for an appointment with the study principal investigator (a VA oncologist/breast cancer specialist) to discuss prevention options, further screening, or referrals to genetic counseling. Depending on a patient’s other health factors, a woman at high risk for developing breast cancer also may be a candidate for chemoprevention with tamoxifen, raloxifene, exemestane, anastrozole, or letrozole.
Data on the participant’s lifestyle, including exercise, diet, and smoking, were evaluated to determine whether these factors had an impact on risk status.
Results
The JJP and DC VAMCs screened 103 women veterans between 2015 and 2018. Four patients were excluded for nonveteran (spousal) status, leaving 99 women veterans with a mean age of 54 years. The most common self-reported races were Black (60%), non-Hispanic White (14%), and Hispanic or Latino (13%) (Table 1).
Women veterans in our study were nearly 3-times more likely than the general population were to receive a high-risk Gail Score/BCRAT (35% vs 13%, respectively).50,51 Of this subset, 46% had breast biopsies, and 86% had a positive family history. Thirty-one percent of Black women in our study were high risk, while nationally, 8.2 to 13.3% of Black women aged 50 to 59 years are considered high risk.50,51 Of the Black high-risk group with a high Gail/BCRAT score, 94% had a positive family history, and 33% had a history of breast biopsy (Table 2).
Of the 35 high-risk patients 26 (74%) patients accepted consultations for chemoprevention and 5 (19%) started chemoprevention. Of this high-risk group, 13 (37%) patients were referred for genetic counseling (Table 3).44 The prevalence of PTSD was present in 31% of high-risk women and 29% of the cohort (Figure).The lifestyle questions indicated that, among all participants, 79% had an overweight or obese body mass index; 58% exercised weekly; 51% consumed alcohol; 14% were smokers; and 21% consumed 3 to 4 servings of fruits/vegetables daily.
Discussion
Breast cancer is the most common cancer in women.52 The number of women with breast cancer in the VHA has more than tripled from 1995 to 2012.1 The lifetime risk of developing breast cancer in the general population is about 13%.50 This rate can be affected by risk factors including age, hormone exposure, family history, radiation exposure, and lifestyle factors, such as weight and alcohol use.6,52-56 In the United States, invasive breast cancer affects 1 in 8 women.50,52,57
Our screened population showed nearly 3 times as many women veterans were at an increased risk for breast cancer when compared with historical averages in US women. This difference may be based on a high rate of prior breast biopsies or positive family history, although a provocative study using the Surveillance, Epidemiology, and End Results database showed military women to have higher rates of breast cancer as well.9 Historically, Blacks are vastly understudied in clinical research with only 5% representation on a national level.5,58 The urban locations of both pilot sites (Washington, DC and Bronx, NY) allowed for the inclusion of minority patients in our study. We found that the rates of breast cancer in Black women veterans to be higher than seen nationally, possibly prompting further screening initiatives for this understudied population.
Our pilot study’s chemoprevention utilization (19%) was double the < 10% seen in the national population.33-35 The presence of a knowledgeable breast health practitioner to recruit study participants and offer personalized counseling to women veterans is a likely factor in overcoming barriers to chemopreventive acceptance. These participants may have been motivated to seek care for their high-risk status given a strong family history and prior breast biopsies.
Interestingly, a 3-fold higher PTSD rate was seen in this pilot population (29%) when compared with PTSD rates in the general female population (7-10%) and still one-third higher than the general population of women veterans (20%).45-47 Mental health, anxiety, and PTSD have been barriers to patients who sought treatment and have been implicated in poor adherence to medical advice.48,49 Cancer screening can induce anxiety in patients, and it may be amplified in patients with PTSD. It was remarkable that although adherence with screening recommendations is decreased when PTSD is present, our patient population demonstrated a higher rate of screening adherence.
Women who are seen at the VA often use multiple clinical specialties, and their EMR can be accessed across VA medical centers nationwide. Therefore, identifying women veterans who meet screening criteria is easily attainable within the VA.
When comparing high-risk with average risk women, the lifestyle results (BMI, smoking history, exercise and consumption of fruits, vegetables and alcohol) were essentially the same. Lifestyle factors were similar to national population rates and were unlikely to impact risk levels.
Limitations
Study limitations included a high number of self-referrals and the large percentage of patients with a family history of breast cancer, making them more likely to seek screening. The higher-than-average risk of breast cancer may be driven by a high rate of breast biopsies and a strong family history. Lifestyle metrics could not be accurately compared to other national assessments of lifestyle factors due to the difference in data points that we used or the format of our questions.
Conclusions
As the number of women veterans increases and the incidence of breast cancer in women veterans rise, chemoprevention options should follow national guidelines. To our knowledge, this is the only oncology study with 60% Black women veterans. This study had a higher participation rate for Black women veterans than is typically seen in national research studies and shows the VA to be a germane source for further understanding of an understudied population that may benefit from increased screening for breast cancer.
A team-based, multidisciplinary model that meets the unique healthcare needs of women veterans results in a patient-centric delivery of care for assessing breast cancer risk status and prevention options. This model can be replicated nationally by directing primary care physicians and women’s health practitioners to a risk-assessment questionnaire and referring high-risk women for appropriate preventative care. Given that these results show chemoprevention adherence rates doubled those seen nationally, perhaps techniques used within this VA pilot study may be adapted to decrease breast cancer incidence nationally.
Since the rate of PTSD among women veterans is triple the national average, we would expect adherence rates to be lower in our patient cohort. However, the multidisciplinary approach we used in this study (eg, 1:1 consultation with oncologist; genetic counseling referrals; mental health support available), may have improved adherence rates. Perhaps the high rates of PTSD seen in the VA patient population can be a useful way to explore patient adherence rates in those with mental illness and medical conditions.
Future research with a larger cohort may lead to greater insight into the correlation between PTSD and adherence to treatment. Exploring the connection between breast cancer, epigenetics, and specific military service-related exposures could be an area of analysis among this veteran population exhibiting increased breast cancer rates. VAMCs are situated in rural, suburban, and urban locations across the United States and offers a diverse socioeconomic and ethnic patient population for inclusion in clinical investigations. Women veterans make up a small subpopulation of women in the United States, but it is worth considering VA patients as an untapped resource for research collaboration.
Acknowledgements
The authors thank Steven Sanchez and Marissa Vallette, PhD, Breast Health Research Group. This research project was approved by the James J. Peters VA Medical Center Quality Executive Committee and the Washington, DC VA Medical Center Institutional Review Board. This work was supported by the US Department of Veterans Affairs. This work did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the US Government, or any of its agencies.
The number of women seeking care from the Veterans Health Administration (VHA) is increasing.1 In 2015, there were 2 million women veterans in the United States, which is 9.4% of the total veteran population. This group is expected to increase at an average of about 18,000 women per year for the next 10 years.2 The percentage of women veterans who are US Department of Veterans Affairs (VA) users aged 45 to 64 years rose 46% from 2000 to 2015.1,3-4 It is estimated that 15% of veterans who used VA services in 2020 were women.1 Nineteen percent of women veterans are Black.1 The median age of women veterans in 2015 was 50 years.5 Breast cancer is the leading cancer affecting female veterans, and data suggest they have an increased risk of breast cancer based on unique service-related exposures.1,6-9
In the US, about 10 million women are eligible for breast cancer preventive therapy, including, but not limited to, medications, surgery, or lifestyle changes.10 Secondary prevention options include change in surveillance that can reduce their risk or identify cancer at an earlier stage when treatment is more effective. The United States Preventive Services Task Force, the National Comprehensive Cancer Network, the American Society for Clinical Oncology, the National Institute for Health and Care Excellence, and the Oncology Nursing Society recommend screening women aged ≥ 35 years to assess breast cancer risk.11-18 If a woman is at increased risk, she may be a candidate for chemoprevention, prozphylactic surgery, and possibly an enhanced screening regimen.
Urban and minority women are an understudied population. Most veterans (75%) live in urban or suburban settings.19,20 Urban veteran women constitute an important potential study population.
Chemoprevention measures have been underused because of factors involving both women and their health care providers. A large proportion of women are unaware of their higher risk status due to lack of adequate screening and risk assessment.21,22 In addition to patient lack of awareness of their high-risk status, primary care physicians are also reluctant to prescribe chemopreventive agents due to a lack of comfort or familiarity with the risks and benefits.23-26 The STAR2015, BCPT2005, IBIS2014, MAP3 2011, IBIS-I 2014, and IBIS II 2014 studies clearly demonstrate a 49 to 62% reduction in risk for women using chemoprevention such as selective estrogen receptor modulators or aromatase inhibitors, respectively.27-32 Yet only 4 to 9% of high-risk women not enrolled in a clinical trial are using chemoprevention.33-39
The possibility of developing breast cancer also may be increased because of a positive family history or being a member of a family in which there is a known susceptibility gene mutation.40 Based on these risk factors, women may be eligible for tailored follow-up and genetic counseling.41-44
Nationally, 7 to 10% of the civilian US population will experience posttraumatic stress disorder (PTSD).45 The rates are remarkably higher for women veterans, with roughly 20% diagnosed with PTSD.46,47 Anxiety and PTSD have been implicated in poor adherence to medical advice.48,49
In 2014, a national VA multidisciplinary group focused on breast cancer prevention, detection, treatment, and research to address breast health in the growing population of women veterans. High-risk breast cancer screenings are not routinely carried out by the VA in primary care, women’s health, or oncology services. Furthermore, the recording of screening questionnaire results was not synchronized until a standard questionnaire was created and approved as a template by this group in the VA electronic medical record (EMR) in 2015.
Several prediction models can identify which women are at an increased risk of developing breast cancer. The most commonly used risk assessment model, the Gail breast cancer risk assessment tool (BCRAT), has been refined to include women of additional ethnicities (https://www.cancer.gov/bcrisktool).
This pilot project was launched to identify an effective manner to screen women veterans regarding their risk of developing breast cancer and refer them for chemoprevention education or genetic counseling as appropriate.
Methods
A high-risk breast cancer screening questionnaire based on the Gail BCRAT and including lifestyle questions was developed and included as a note template in the VA EMR. The James J. Peters VA Medical Center, Bronx, NY (JJPVAMC) and the Washington DC VA Medical Center (DCVAMC) ran a pilot study between 2015 and 2018 using this breast cancer screening questionnaire to collect data from women veterans. Quality Executive Committee and institutional review board approvals were granted respectively.
Eligibility criteria included women aged ≥ 35 years with no personal history of breast cancer. Most patients were self-referred, but participants also were recruited during VA Breast Cancer Awareness month events, health fairs, or at informational tables in the hospital lobbies. After completing the 20 multiple choice questionnaire with a study team member, either in person or over the phone, a 5-year and lifetime risk of invasive breast cancer was calculated using the Gail BCRAT. A woman is considered high risk and eligible for chemoprevention if her 5-year risk is > 1.66% or her lifetime risk is ≥ 20%. Eligibility for genetic counseling is based on the Breast Cancer Referral Screening Tool, which includes a personal or family history of breast or ovarian cancer and Jewish ancestry.
All patients were notified of their average or high risk status by a clinician. Those who were deemed to be average risk received a follow-up letter in the mail with instructions (eg, to follow-up with a yearly mammogram). Those who were deemed to be high risk for developing breast cancer were asked to come in for an appointment with the study principal investigator (a VA oncologist/breast cancer specialist) to discuss prevention options, further screening, or referrals to genetic counseling. Depending on a patient’s other health factors, a woman at high risk for developing breast cancer also may be a candidate for chemoprevention with tamoxifen, raloxifene, exemestane, anastrozole, or letrozole.
Data on the participant’s lifestyle, including exercise, diet, and smoking, were evaluated to determine whether these factors had an impact on risk status.
Results
The JJP and DC VAMCs screened 103 women veterans between 2015 and 2018. Four patients were excluded for nonveteran (spousal) status, leaving 99 women veterans with a mean age of 54 years. The most common self-reported races were Black (60%), non-Hispanic White (14%), and Hispanic or Latino (13%) (Table 1).
Women veterans in our study were nearly 3-times more likely than the general population were to receive a high-risk Gail Score/BCRAT (35% vs 13%, respectively).50,51 Of this subset, 46% had breast biopsies, and 86% had a positive family history. Thirty-one percent of Black women in our study were high risk, while nationally, 8.2 to 13.3% of Black women aged 50 to 59 years are considered high risk.50,51 Of the Black high-risk group with a high Gail/BCRAT score, 94% had a positive family history, and 33% had a history of breast biopsy (Table 2).
Of the 35 high-risk patients 26 (74%) patients accepted consultations for chemoprevention and 5 (19%) started chemoprevention. Of this high-risk group, 13 (37%) patients were referred for genetic counseling (Table 3).44 The prevalence of PTSD was present in 31% of high-risk women and 29% of the cohort (Figure).The lifestyle questions indicated that, among all participants, 79% had an overweight or obese body mass index; 58% exercised weekly; 51% consumed alcohol; 14% were smokers; and 21% consumed 3 to 4 servings of fruits/vegetables daily.
Discussion
Breast cancer is the most common cancer in women.52 The number of women with breast cancer in the VHA has more than tripled from 1995 to 2012.1 The lifetime risk of developing breast cancer in the general population is about 13%.50 This rate can be affected by risk factors including age, hormone exposure, family history, radiation exposure, and lifestyle factors, such as weight and alcohol use.6,52-56 In the United States, invasive breast cancer affects 1 in 8 women.50,52,57
Our screened population showed nearly 3 times as many women veterans were at an increased risk for breast cancer when compared with historical averages in US women. This difference may be based on a high rate of prior breast biopsies or positive family history, although a provocative study using the Surveillance, Epidemiology, and End Results database showed military women to have higher rates of breast cancer as well.9 Historically, Blacks are vastly understudied in clinical research with only 5% representation on a national level.5,58 The urban locations of both pilot sites (Washington, DC and Bronx, NY) allowed for the inclusion of minority patients in our study. We found that the rates of breast cancer in Black women veterans to be higher than seen nationally, possibly prompting further screening initiatives for this understudied population.
Our pilot study’s chemoprevention utilization (19%) was double the < 10% seen in the national population.33-35 The presence of a knowledgeable breast health practitioner to recruit study participants and offer personalized counseling to women veterans is a likely factor in overcoming barriers to chemopreventive acceptance. These participants may have been motivated to seek care for their high-risk status given a strong family history and prior breast biopsies.
Interestingly, a 3-fold higher PTSD rate was seen in this pilot population (29%) when compared with PTSD rates in the general female population (7-10%) and still one-third higher than the general population of women veterans (20%).45-47 Mental health, anxiety, and PTSD have been barriers to patients who sought treatment and have been implicated in poor adherence to medical advice.48,49 Cancer screening can induce anxiety in patients, and it may be amplified in patients with PTSD. It was remarkable that although adherence with screening recommendations is decreased when PTSD is present, our patient population demonstrated a higher rate of screening adherence.
Women who are seen at the VA often use multiple clinical specialties, and their EMR can be accessed across VA medical centers nationwide. Therefore, identifying women veterans who meet screening criteria is easily attainable within the VA.
When comparing high-risk with average risk women, the lifestyle results (BMI, smoking history, exercise and consumption of fruits, vegetables and alcohol) were essentially the same. Lifestyle factors were similar to national population rates and were unlikely to impact risk levels.
Limitations
Study limitations included a high number of self-referrals and the large percentage of patients with a family history of breast cancer, making them more likely to seek screening. The higher-than-average risk of breast cancer may be driven by a high rate of breast biopsies and a strong family history. Lifestyle metrics could not be accurately compared to other national assessments of lifestyle factors due to the difference in data points that we used or the format of our questions.
Conclusions
As the number of women veterans increases and the incidence of breast cancer in women veterans rise, chemoprevention options should follow national guidelines. To our knowledge, this is the only oncology study with 60% Black women veterans. This study had a higher participation rate for Black women veterans than is typically seen in national research studies and shows the VA to be a germane source for further understanding of an understudied population that may benefit from increased screening for breast cancer.
A team-based, multidisciplinary model that meets the unique healthcare needs of women veterans results in a patient-centric delivery of care for assessing breast cancer risk status and prevention options. This model can be replicated nationally by directing primary care physicians and women’s health practitioners to a risk-assessment questionnaire and referring high-risk women for appropriate preventative care. Given that these results show chemoprevention adherence rates doubled those seen nationally, perhaps techniques used within this VA pilot study may be adapted to decrease breast cancer incidence nationally.
Since the rate of PTSD among women veterans is triple the national average, we would expect adherence rates to be lower in our patient cohort. However, the multidisciplinary approach we used in this study (eg, 1:1 consultation with oncologist; genetic counseling referrals; mental health support available), may have improved adherence rates. Perhaps the high rates of PTSD seen in the VA patient population can be a useful way to explore patient adherence rates in those with mental illness and medical conditions.
Future research with a larger cohort may lead to greater insight into the correlation between PTSD and adherence to treatment. Exploring the connection between breast cancer, epigenetics, and specific military service-related exposures could be an area of analysis among this veteran population exhibiting increased breast cancer rates. VAMCs are situated in rural, suburban, and urban locations across the United States and offers a diverse socioeconomic and ethnic patient population for inclusion in clinical investigations. Women veterans make up a small subpopulation of women in the United States, but it is worth considering VA patients as an untapped resource for research collaboration.
Acknowledgements
The authors thank Steven Sanchez and Marissa Vallette, PhD, Breast Health Research Group. This research project was approved by the James J. Peters VA Medical Center Quality Executive Committee and the Washington, DC VA Medical Center Institutional Review Board. This work was supported by the US Department of Veterans Affairs. This work did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the US Government, or any of its agencies.
1. US Department of Veterans Affairs. National Center for Veterans Analysis and Statistics. The past, present and future of women veterans. Published February 2017. Accessed April 28, 2021. https://www.va.gov/vetdata/docs/specialreports/women_veterans_2015_final.pdf.
2. Frayne SM, Carney DV, Bastian L, et al. The VA Women’s Health Practice-Based Research Network: amplifying women veterans’ voices in VA research. J Gen Intern Med. 2013;28 Suppl 2(Suppl 2):S504-S509. doi:10.1007/s11606-013-2476-3
3. US Department of Veterans Affairs, Veterans Health Administration, Women’s Health Evaluation Initiative, Women Veterans Health Strategic Health Care Group. Sourcebook: women veterans in the Veterans Health Administration. Volume 1: Sociodemographic characteristics and use of VHA care. Published December 2010. Accessed April 12, 2021. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=2455
4. Bean-Mayberry B, Yano EM, Bayliss N, Navratil J, Weisman CS, Scholle SH. Federally funded comprehensive women’s health centers: leading innovation in women’s healthcare delivery. J Womens Health (Larchmt). 2007;16(9):1281-1290. doi:10.1089/jwh.2006.0284
5. US Department of Veterans Affairs. National Center for Veterans Analysis and Statistics.VA utilization profile FY 2016. Published November 2017. Accessed April 12, 2021. https://www.va.gov/vetdata/docs/QuickFacts/VA_Utilization_Profile.PDF
6. Ekenga CC, Parks CG, Sandler DP. Chemical exposures in the workplace and breast cancer risk: a prospective cohort study. Int J Cancer. 2015;137(7):1765-1774. doi:10.1002/ijc.29545
7. Rennix CP, Quinn MM, Amoroso PJ, Eisen EA, Wegman DH. Risk of breast cancer among enlisted Army women occupationally exposed to volatile organic compounds. Am J Ind Med. 2005;48(3):157-167. doi:10.1002/ajim.20201
8. Ritz B. Cancer mortality among workers exposed to chemicals during uranium processing. J Occup Environ Med. 1999;41(7):556-566. doi:10.1097/00043764-199907000-00004
9. Zhu K, Devesa SS, Wu H, et al. Cancer incidence in the U.S. military population: comparison with rates from the SEER program. Cancer Epidemiol Biomarkers Prev. 2009;18(6):1740-1745. doi:10.1158/1055-9965.EPI-09-0041
10. Freedman AN, Yu B, Gail MH, et al. Benefit/risk assessment for breast cancer chemoprevention with raloxifene or tamoxifen for women age 50 years or older [published correction appears in J Clin Oncol. 2013 Nov 10;31(32):4167]. J Clin Oncol. 2011;29(17):2327-2333. doi:10.1200/JCO.2010.33.0258
11. Greene, H. Cancer prevention, screening and early detection. In: Gobel BH, Triest-Robertson S, Vogel WH, eds. Advanced Oncology Nursing Certification Review and Resource Manual. 3rd ed. Oncology Nursing Society; 2016:1-34. https://www.ons.org/sites/default/files/publication_pdfs/2%20ADVPrac%20chapter%201.pdf
12. National Comprehensive Cancer Network. NCCN Breast Cancer Risk Reduction. Version 1.2021 NCCN Clinical Practice Guidelines in Oncology. Updated March 24, 2021 Accessed April 12, 2021. https://www.nccn.org/professionals/physician_gls/pdf/breast_risk.pdf
13. US Preventive Services Task Force. Breast cancer: Medications use to reduce risk. Updated September 3, 2019. Accessed April 12, 2021. https://www.uspreventiveservicestaskforce.org/uspstf/recommendation/breast-cancer-medications-for-risk-reduction
14. Moyer VA; U.S. Preventive Services Task Force. Medications to decrease the risk for breast cancer in women: recommendations from the U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2013;159(10):698-708. doi:10.7326/0003-4819-159-10-201311190-00717
15. Boucher JE. Chemoprevention: an overview of pharmacologic agents and nursing considerations. Clin J Oncol Nurs. 2018;22(3):350-353. doi:10.1188/18.CJON.350-353
16. Nichols HB, Stürmer T, Lee VS, et al. Breast cancer chemoprevention in an integrated health care setting. JCO Clin Cancer Inform. 2017;1:1-12. doi:10.1200/CCI.16.00059
17. Bevers TB, Helvie M, Bonaccio E, et al. Breast cancer screening and diagnosis, Version 3.2018, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2018;16(11):1362-1389. doi:10.6004/jnccn.2018.0083
18. Visvanathan K, Hurley P, Bantug E, et al. Use of pharmacologic interventions for breast cancer risk reduction: American Society of Clinical Oncology clinical practice guideline [published correction appears in J Clin Oncol. 2013 Dec 1;31(34):4383]. J Clin Oncol. 2013;31(23):2942-2962. doi:10.1200/JCO.2013.49.3122
19. Sealy-Jefferson S, Roseland ME, Cote ML, et al. rural-urban residence and stage at breast cancer diagnosis among postmenopausal women: The Women’s Health Initiative. J Womens Health (Larchmt). 2019;28(2):276-283. doi:10.1089/jwh.2017.6884
20. Holder KA. Veterans in rural America: 2011-2015. Published January 25, 2017. Accessed April 12, 2021. https://www.census.gov/library/publications/2017/acs/acs-36.html
21. Owens WL, Gallagher TJ, Kincheloe MJ, Ruetten VL. Implementation in a large health system of a program to identify women at high risk for breast cancer. J Oncol Pract. 2011;7(2):85-88. doi:10.1200/JOP.2010.000107
2. Pivot X, Viguier J, Touboul C, et al. Breast cancer screening controversy: too much or not enough?. Eur J Cancer Prev. 2015;24 Suppl:S73-S76. doi:10.1097/CEJ.0000000000000145
23. Bidassie B, Kovach A, Vallette MA, et al. Breast Cancer risk assessment and chemoprevention use among veterans affairs primary care providers: a national online survey. Mil Med. 2020;185(3-4):512-518. doi:10.1093/milmed/usz291
24. Brewster AM, Davidson NE, McCaskill-Stevens W. Chemoprevention for breast cancer: overcoming barriers to treatment. Am Soc Clin Oncol Educ Book. 2012;85-90. doi:10.14694/EdBook_AM.2012.32.152
25. Meyskens FL Jr, Curt GA, Brenner DE, et al. Regulatory approval of cancer risk-reducing (chemopreventive) drugs: moving what we have learned into the clinic. Cancer Prev Res (Phila). 2011;4(3):311-323. doi:10.1158/1940-6207.CAPR-09-0014
26. Tice JA, Kerlikowske K. Screening and prevention of breast cancer in primary care. Prim Care. 2009;36(3):533-558. doi:10.1016/j.pop.2009.04.003
27. Vogel VG. Selective estrogen receptor modulators and aromatase inhibitors for breast cancer chemoprevention. Curr Drug Targets. 2011;12(13):1874-1887. doi:10.2174/138945011798184164
28. Vogel VG, Costantino JP, Wickerham DL, et al. Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial [published correction appears in JAMA. 2006 Dec 27;296(24):2926] [published correction appears in JAMA. 2007 Sep 5;298(9):973]. JAMA. 2006;295(23):2727-2741. doi:10.1001/jama.295.23.joc60074
29. Pruthi S, Heisey RE, Bevers TB. Chemoprevention for breast cancer. Ann Surg Oncol. 2015;22(10):3230-3235. doi:10.1245/s10434-015-4715-9
30. Cuzick J, Sestak I, Forbes JF, et al. Anastrozole for prevention of breast cancer in high-risk postmenopausal women (IBIS-II): an international, double-blind, randomised placebo-controlled trial [published correction appears in Lancet. 2014 Mar 22;383(9922):1040] [published correction appears in Lancet. 2017 Mar 11;389(10073):1010]. Lancet. 2014;383(9922):1041-1048. doi:10.1016/S0140-6736(13)62292-8
31. Bozovic-Spasojevic I, Azambuja E, McCaskill-Stevens W, Dinh P, Cardoso F. Chemoprevention for breast cancer. Cancer Treat Rev. 2012;38(5):329-339. doi:10.1016/j.ctrv.2011.07.005
32. Gabriel EM, Jatoi I. Breast cancer chemoprevention. Expert Rev Anticancer Ther. 2012;12(2):223-228. doi:10.1586/era.11.206

33. Crew KD, Albain KS, Hershman DL, Unger JM, Lo SS. How do we increase uptake of tamoxifen and other anti-estrogens for breast cancer prevention?. NPJ Breast Cancer. 2017;3:20. Published 2017 May 19. doi:10.1038/s41523-017-0021-y
34. Ropka ME, Keim J, Philbrick JT. Patient decisions about breast cancer chemoprevention: a systematic review and meta-analysis. J Clin Oncol. 2010;28(18):3090-3095. doi:10.1200/JCO.2009.27.8077
35. Smith SG, Sestak I, Forster A, et al. Factors affecting uptake and adherence to breast cancer chemoprevention: a systematic review and meta-analysis. Ann Oncol. 2016;27(4):575-590. doi:10.1093/annonc/mdv590
36. Grann VR, Patel PR, Jacobson JS, et al. Comparative effectiveness of screening and prevention strategies among BRCA1/2-affected mutation carriers. Breast Cancer Res Treat. 2011 Feb;125(3):837-847. doi:10.1007/s10549-010-1043-4
37. Goss PE, Ingle JN, Alés-Martínez JE, et al. Exemestane for breast-cancer prevention in postmenopausal women [published correction appears in N Engl J Med. 2011 Oct 6;365(14):1361]. N Engl J Med. 2011;364(25):2381-2391. doi:10.1056/NEJMoa1103507
38. Kmietowicz Z. Five in six women reject drugs that could reduce their risk of breast cancer. BMJ. 2015;351:h6650. Published 2015 Dec 8. doi:10.1136/bmj.h6650
39. Nelson HD, Fu R, Griffin JC, Nygren P, Smith ME, Humphrey L. Systematic review: comparative effectiveness of medications to reduce risk for primary breast cancer. Ann Intern Med. 2009;151(10):703-235. doi:10.7326/0003-4819-151-10-200911170-00147
40. Dahabreh IJ, Wieland LS, Adam GP, Halladay C, Lau J, Trikalinos TA. Core needle and open surgery biopsy for diagnosis of breast lesions: an update to the 2009 report. Published September 2014. Accessed April 12, 2021. https://www.ncbi.nlm.nih.gov/books/NBK246878
41. National Cancer Institute. Genetics of breast and ovarian cancer (PDQ)—health profession version. Updated February 12, 2021. Accessed April 12, 2021. http://www.cancer.gov/cancertopics/pdq/genetics/breast-and-ovarian/HealthProfessional
42. US Department of Health and Human Services. National Institutes of Health, National Institute of Environmental Health Sciences The sister study. Accessed April 12, 2021. https://sisterstudy.niehs.nih.gov/english/NIEHS.htm
43. Tutt A, Ashworth A. Can genetic testing guide treatment in breast cancer?. Eur J Cancer. 2008;44(18):2774-2780. doi:10.1016/j.ejca.2008.10.009
44. Katz SJ, Ward KC, Hamilton AS, et al. Gaps in receipt of clinically indicated genetic counseling after diagnosis of breast cancer. J Clin Oncol. 2018;36(12):1218-1224. doi:10.1200/JCO.2017.76.2369
45. US Department of Veterans Affairs. PTSD: National Center for PTSD. How common is PTSD in adults? Updated October 17, 2019. Accessed April 12, 2021. https://www.ptsd.va.gov/understand/common/common_adults.asp
46. US Department of Veterans Affairs. PTSD: National Center for PTSD. How common is PTSD in women? Updated October 16, 2019. Accessed April 12, 2021. https://www.ptsd.va.gov/understand/common/common_women.asp
47. US Department of Veterans Affairs. PTSD: National Center for PTSD. How common is PTSD in veterans? Updated September 24, 2018. Accessed April 12, 2021. https://www.ptsd.va.gov/understand/common/common_veterans.asp
48. Lindberg NM, Wellisch D. Anxiety and compliance among women at high risk for breast cancer. Ann Behav Med. 2001;23(4):298-303. doi:10.1207/S15324796ABM2304_9
49. DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: meta-analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med. 2000;160(14):2101-2107. doi:10.1001/archinte.160.14.2101
50. Centers for Disease Control and Prevention. MMWR appendix: breast cancer rates among black women and white women. Updated October 13, 2016. Accessed April 12, 2021. https://www.cdc.gov/cancer/breast/statistics/trends_invasive.htm
51. Richardson LC, Henley SJ, Miller JW, Massetti G, Thomas CC. Patterns and trends in age-specific black-white differences in breast cancer incidence and mortality - United States, 1999-2014. MMWR Morb Mortal Wkly Rep. 2016;65(40):1093-1098. Published 2016 Oct 14. doi:10.15585/mmwr.mm6540a1
52. Brody JG, Moysich KB, Humblet O, Attfield KR, Beehler GP, Rudel RA. Environmental pollutants and breast cancer: epidemiologic studies. Cancer. 2007;109(12 Suppl):2667-2711. doi:10.1002/cncr.22655
53. Brophy JT, Keith MM, Watterson A, et al. Breast cancer risk in relation to occupations with exposure to carcinogens and endocrine disruptors: a Canadian case-control study. Environ Health. 2012;11:87. Published 2012 Nov 19. doi:10.1186/1476-069X-11-87
54. Labrèche F, Goldberg MS, Valois MF, Nadon L. Postmenopausal breast cancer and occupational exposures. Occup Environ Med. 2010;67(4):263-269. doi:10.1136/oem.2009.049817
55. National Institute of Environmental Health Sciences, Interagency Breast Cancer & Environmental Research Coordinating Committee. Breast cancer and the environment: prioritizing prevention. Updated March 8, 2013. Accessed April 12, 2021. https://www.niehs.nih.gov/about/boards/ibcercc/index.cfm
56. Gail MH, Costantino JP, Pee D, et al. Projecting individualized absolute invasive breast cancer risk in African American women [published correction appears in J Natl Cancer Inst. 2008 Aug 6;100(15):1118] [published correction appears in J Natl Cancer Inst. 2008 Mar 5;100(5):373]. J Natl Cancer Inst. 2007;99(23):1782-1792. doi:10.1093/jnci/djm223
57. Corbie-Smith G, Thomas SB, Williams MV, Moody-Ayers S. Attitudes and beliefs of African Americans toward participation in medical research. J Gen Intern Med. 1999;14(9):537-546. doi:10.1046/j.1525-1497.1999.07048.x
58. Braunstein JB, Sherber NS, Schulman SP, Ding EL, Powe NR. Race, medical researcher distrust, perceived harm, and willingness to participate in cardiovascular prevention trials. Medicine (Baltimore). 2008;87(1):1-9. doi:10.1097/MD.0b013e3181625d78
1. US Department of Veterans Affairs. National Center for Veterans Analysis and Statistics. The past, present and future of women veterans. Published February 2017. Accessed April 28, 2021. https://www.va.gov/vetdata/docs/specialreports/women_veterans_2015_final.pdf.
2. Frayne SM, Carney DV, Bastian L, et al. The VA Women’s Health Practice-Based Research Network: amplifying women veterans’ voices in VA research. J Gen Intern Med. 2013;28 Suppl 2(Suppl 2):S504-S509. doi:10.1007/s11606-013-2476-3
3. US Department of Veterans Affairs, Veterans Health Administration, Women’s Health Evaluation Initiative, Women Veterans Health Strategic Health Care Group. Sourcebook: women veterans in the Veterans Health Administration. Volume 1: Sociodemographic characteristics and use of VHA care. Published December 2010. Accessed April 12, 2021. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=2455
4. Bean-Mayberry B, Yano EM, Bayliss N, Navratil J, Weisman CS, Scholle SH. Federally funded comprehensive women’s health centers: leading innovation in women’s healthcare delivery. J Womens Health (Larchmt). 2007;16(9):1281-1290. doi:10.1089/jwh.2006.0284
5. US Department of Veterans Affairs. National Center for Veterans Analysis and Statistics.VA utilization profile FY 2016. Published November 2017. Accessed April 12, 2021. https://www.va.gov/vetdata/docs/QuickFacts/VA_Utilization_Profile.PDF
6. Ekenga CC, Parks CG, Sandler DP. Chemical exposures in the workplace and breast cancer risk: a prospective cohort study. Int J Cancer. 2015;137(7):1765-1774. doi:10.1002/ijc.29545
7. Rennix CP, Quinn MM, Amoroso PJ, Eisen EA, Wegman DH. Risk of breast cancer among enlisted Army women occupationally exposed to volatile organic compounds. Am J Ind Med. 2005;48(3):157-167. doi:10.1002/ajim.20201
8. Ritz B. Cancer mortality among workers exposed to chemicals during uranium processing. J Occup Environ Med. 1999;41(7):556-566. doi:10.1097/00043764-199907000-00004
9. Zhu K, Devesa SS, Wu H, et al. Cancer incidence in the U.S. military population: comparison with rates from the SEER program. Cancer Epidemiol Biomarkers Prev. 2009;18(6):1740-1745. doi:10.1158/1055-9965.EPI-09-0041
10. Freedman AN, Yu B, Gail MH, et al. Benefit/risk assessment for breast cancer chemoprevention with raloxifene or tamoxifen for women age 50 years or older [published correction appears in J Clin Oncol. 2013 Nov 10;31(32):4167]. J Clin Oncol. 2011;29(17):2327-2333. doi:10.1200/JCO.2010.33.0258
11. Greene, H. Cancer prevention, screening and early detection. In: Gobel BH, Triest-Robertson S, Vogel WH, eds. Advanced Oncology Nursing Certification Review and Resource Manual. 3rd ed. Oncology Nursing Society; 2016:1-34. https://www.ons.org/sites/default/files/publication_pdfs/2%20ADVPrac%20chapter%201.pdf
12. National Comprehensive Cancer Network. NCCN Breast Cancer Risk Reduction. Version 1.2021 NCCN Clinical Practice Guidelines in Oncology. Updated March 24, 2021 Accessed April 12, 2021. https://www.nccn.org/professionals/physician_gls/pdf/breast_risk.pdf
13. US Preventive Services Task Force. Breast cancer: Medications use to reduce risk. Updated September 3, 2019. Accessed April 12, 2021. https://www.uspreventiveservicestaskforce.org/uspstf/recommendation/breast-cancer-medications-for-risk-reduction
14. Moyer VA; U.S. Preventive Services Task Force. Medications to decrease the risk for breast cancer in women: recommendations from the U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2013;159(10):698-708. doi:10.7326/0003-4819-159-10-201311190-00717
15. Boucher JE. Chemoprevention: an overview of pharmacologic agents and nursing considerations. Clin J Oncol Nurs. 2018;22(3):350-353. doi:10.1188/18.CJON.350-353
16. Nichols HB, Stürmer T, Lee VS, et al. Breast cancer chemoprevention in an integrated health care setting. JCO Clin Cancer Inform. 2017;1:1-12. doi:10.1200/CCI.16.00059
17. Bevers TB, Helvie M, Bonaccio E, et al. Breast cancer screening and diagnosis, Version 3.2018, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2018;16(11):1362-1389. doi:10.6004/jnccn.2018.0083
18. Visvanathan K, Hurley P, Bantug E, et al. Use of pharmacologic interventions for breast cancer risk reduction: American Society of Clinical Oncology clinical practice guideline [published correction appears in J Clin Oncol. 2013 Dec 1;31(34):4383]. J Clin Oncol. 2013;31(23):2942-2962. doi:10.1200/JCO.2013.49.3122
19. Sealy-Jefferson S, Roseland ME, Cote ML, et al. rural-urban residence and stage at breast cancer diagnosis among postmenopausal women: The Women’s Health Initiative. J Womens Health (Larchmt). 2019;28(2):276-283. doi:10.1089/jwh.2017.6884
20. Holder KA. Veterans in rural America: 2011-2015. Published January 25, 2017. Accessed April 12, 2021. https://www.census.gov/library/publications/2017/acs/acs-36.html
21. Owens WL, Gallagher TJ, Kincheloe MJ, Ruetten VL. Implementation in a large health system of a program to identify women at high risk for breast cancer. J Oncol Pract. 2011;7(2):85-88. doi:10.1200/JOP.2010.000107
2. Pivot X, Viguier J, Touboul C, et al. Breast cancer screening controversy: too much or not enough?. Eur J Cancer Prev. 2015;24 Suppl:S73-S76. doi:10.1097/CEJ.0000000000000145
23. Bidassie B, Kovach A, Vallette MA, et al. Breast Cancer risk assessment and chemoprevention use among veterans affairs primary care providers: a national online survey. Mil Med. 2020;185(3-4):512-518. doi:10.1093/milmed/usz291
24. Brewster AM, Davidson NE, McCaskill-Stevens W. Chemoprevention for breast cancer: overcoming barriers to treatment. Am Soc Clin Oncol Educ Book. 2012;85-90. doi:10.14694/EdBook_AM.2012.32.152
25. Meyskens FL Jr, Curt GA, Brenner DE, et al. Regulatory approval of cancer risk-reducing (chemopreventive) drugs: moving what we have learned into the clinic. Cancer Prev Res (Phila). 2011;4(3):311-323. doi:10.1158/1940-6207.CAPR-09-0014
26. Tice JA, Kerlikowske K. Screening and prevention of breast cancer in primary care. Prim Care. 2009;36(3):533-558. doi:10.1016/j.pop.2009.04.003
27. Vogel VG. Selective estrogen receptor modulators and aromatase inhibitors for breast cancer chemoprevention. Curr Drug Targets. 2011;12(13):1874-1887. doi:10.2174/138945011798184164
28. Vogel VG, Costantino JP, Wickerham DL, et al. Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial [published correction appears in JAMA. 2006 Dec 27;296(24):2926] [published correction appears in JAMA. 2007 Sep 5;298(9):973]. JAMA. 2006;295(23):2727-2741. doi:10.1001/jama.295.23.joc60074
29. Pruthi S, Heisey RE, Bevers TB. Chemoprevention for breast cancer. Ann Surg Oncol. 2015;22(10):3230-3235. doi:10.1245/s10434-015-4715-9
30. Cuzick J, Sestak I, Forbes JF, et al. Anastrozole for prevention of breast cancer in high-risk postmenopausal women (IBIS-II): an international, double-blind, randomised placebo-controlled trial [published correction appears in Lancet. 2014 Mar 22;383(9922):1040] [published correction appears in Lancet. 2017 Mar 11;389(10073):1010]. Lancet. 2014;383(9922):1041-1048. doi:10.1016/S0140-6736(13)62292-8
31. Bozovic-Spasojevic I, Azambuja E, McCaskill-Stevens W, Dinh P, Cardoso F. Chemoprevention for breast cancer. Cancer Treat Rev. 2012;38(5):329-339. doi:10.1016/j.ctrv.2011.07.005
32. Gabriel EM, Jatoi I. Breast cancer chemoprevention. Expert Rev Anticancer Ther. 2012;12(2):223-228. doi:10.1586/era.11.206

33. Crew KD, Albain KS, Hershman DL, Unger JM, Lo SS. How do we increase uptake of tamoxifen and other anti-estrogens for breast cancer prevention?. NPJ Breast Cancer. 2017;3:20. Published 2017 May 19. doi:10.1038/s41523-017-0021-y
34. Ropka ME, Keim J, Philbrick JT. Patient decisions about breast cancer chemoprevention: a systematic review and meta-analysis. J Clin Oncol. 2010;28(18):3090-3095. doi:10.1200/JCO.2009.27.8077
35. Smith SG, Sestak I, Forster A, et al. Factors affecting uptake and adherence to breast cancer chemoprevention: a systematic review and meta-analysis. Ann Oncol. 2016;27(4):575-590. doi:10.1093/annonc/mdv590
36. Grann VR, Patel PR, Jacobson JS, et al. Comparative effectiveness of screening and prevention strategies among BRCA1/2-affected mutation carriers. Breast Cancer Res Treat. 2011 Feb;125(3):837-847. doi:10.1007/s10549-010-1043-4
37. Goss PE, Ingle JN, Alés-Martínez JE, et al. Exemestane for breast-cancer prevention in postmenopausal women [published correction appears in N Engl J Med. 2011 Oct 6;365(14):1361]. N Engl J Med. 2011;364(25):2381-2391. doi:10.1056/NEJMoa1103507
38. Kmietowicz Z. Five in six women reject drugs that could reduce their risk of breast cancer. BMJ. 2015;351:h6650. Published 2015 Dec 8. doi:10.1136/bmj.h6650
39. Nelson HD, Fu R, Griffin JC, Nygren P, Smith ME, Humphrey L. Systematic review: comparative effectiveness of medications to reduce risk for primary breast cancer. Ann Intern Med. 2009;151(10):703-235. doi:10.7326/0003-4819-151-10-200911170-00147
40. Dahabreh IJ, Wieland LS, Adam GP, Halladay C, Lau J, Trikalinos TA. Core needle and open surgery biopsy for diagnosis of breast lesions: an update to the 2009 report. Published September 2014. Accessed April 12, 2021. https://www.ncbi.nlm.nih.gov/books/NBK246878
41. National Cancer Institute. Genetics of breast and ovarian cancer (PDQ)—health profession version. Updated February 12, 2021. Accessed April 12, 2021. http://www.cancer.gov/cancertopics/pdq/genetics/breast-and-ovarian/HealthProfessional
42. US Department of Health and Human Services. National Institutes of Health, National Institute of Environmental Health Sciences The sister study. Accessed April 12, 2021. https://sisterstudy.niehs.nih.gov/english/NIEHS.htm
43. Tutt A, Ashworth A. Can genetic testing guide treatment in breast cancer?. Eur J Cancer. 2008;44(18):2774-2780. doi:10.1016/j.ejca.2008.10.009
44. Katz SJ, Ward KC, Hamilton AS, et al. Gaps in receipt of clinically indicated genetic counseling after diagnosis of breast cancer. J Clin Oncol. 2018;36(12):1218-1224. doi:10.1200/JCO.2017.76.2369
45. US Department of Veterans Affairs. PTSD: National Center for PTSD. How common is PTSD in adults? Updated October 17, 2019. Accessed April 12, 2021. https://www.ptsd.va.gov/understand/common/common_adults.asp
46. US Department of Veterans Affairs. PTSD: National Center for PTSD. How common is PTSD in women? Updated October 16, 2019. Accessed April 12, 2021. https://www.ptsd.va.gov/understand/common/common_women.asp
47. US Department of Veterans Affairs. PTSD: National Center for PTSD. How common is PTSD in veterans? Updated September 24, 2018. Accessed April 12, 2021. https://www.ptsd.va.gov/understand/common/common_veterans.asp
48. Lindberg NM, Wellisch D. Anxiety and compliance among women at high risk for breast cancer. Ann Behav Med. 2001;23(4):298-303. doi:10.1207/S15324796ABM2304_9
49. DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: meta-analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med. 2000;160(14):2101-2107. doi:10.1001/archinte.160.14.2101
50. Centers for Disease Control and Prevention. MMWR appendix: breast cancer rates among black women and white women. Updated October 13, 2016. Accessed April 12, 2021. https://www.cdc.gov/cancer/breast/statistics/trends_invasive.htm
51. Richardson LC, Henley SJ, Miller JW, Massetti G, Thomas CC. Patterns and trends in age-specific black-white differences in breast cancer incidence and mortality - United States, 1999-2014. MMWR Morb Mortal Wkly Rep. 2016;65(40):1093-1098. Published 2016 Oct 14. doi:10.15585/mmwr.mm6540a1
52. Brody JG, Moysich KB, Humblet O, Attfield KR, Beehler GP, Rudel RA. Environmental pollutants and breast cancer: epidemiologic studies. Cancer. 2007;109(12 Suppl):2667-2711. doi:10.1002/cncr.22655
53. Brophy JT, Keith MM, Watterson A, et al. Breast cancer risk in relation to occupations with exposure to carcinogens and endocrine disruptors: a Canadian case-control study. Environ Health. 2012;11:87. Published 2012 Nov 19. doi:10.1186/1476-069X-11-87
54. Labrèche F, Goldberg MS, Valois MF, Nadon L. Postmenopausal breast cancer and occupational exposures. Occup Environ Med. 2010;67(4):263-269. doi:10.1136/oem.2009.049817
55. National Institute of Environmental Health Sciences, Interagency Breast Cancer & Environmental Research Coordinating Committee. Breast cancer and the environment: prioritizing prevention. Updated March 8, 2013. Accessed April 12, 2021. https://www.niehs.nih.gov/about/boards/ibcercc/index.cfm
56. Gail MH, Costantino JP, Pee D, et al. Projecting individualized absolute invasive breast cancer risk in African American women [published correction appears in J Natl Cancer Inst. 2008 Aug 6;100(15):1118] [published correction appears in J Natl Cancer Inst. 2008 Mar 5;100(5):373]. J Natl Cancer Inst. 2007;99(23):1782-1792. doi:10.1093/jnci/djm223
57. Corbie-Smith G, Thomas SB, Williams MV, Moody-Ayers S. Attitudes and beliefs of African Americans toward participation in medical research. J Gen Intern Med. 1999;14(9):537-546. doi:10.1046/j.1525-1497.1999.07048.x
58. Braunstein JB, Sherber NS, Schulman SP, Ding EL, Powe NR. Race, medical researcher distrust, perceived harm, and willingness to participate in cardiovascular prevention trials. Medicine (Baltimore). 2008;87(1):1-9. doi:10.1097/MD.0b013e3181625d78
Carbon monoxide diffusion with COPD declines more in women
Single breath diffusion capacity for carbon monoxide shows greater decline over time in COPD patients compared with controls, but declines significantly more in women compared with men, according to data from 602 adults with a history of smoking.
In previous studies, diffusion capacity for carbon monoxide (DLco) has been associated with decreased exercise capacity and poor health status in patients with COPD, but its association as a measure of disease progression has not been well studied, wrote Ciro Casanova, MD, of Hospital Universitario La Candelaria, Spain, and colleagues.
In a study published in the journal CHEST®, the researchers identified 506 adult smokers with COPD and 96 adult smoker controls without COPD. Lung function based on single breath DLco was measured each year for 5 years. The study population was part of the COPH History Assessment in SpaiN (CHAIN), an ongoing observational study of adults with COPD. COPD was defined as a history of at least 10 pack-years of smoking and a post-bronchodilator FEV1/FVC greater than 0.7 after 400 micrograms of albuterol, the researchers said.
During the 5-year period, the average overall annual decline in DLco was 1.34% in COPD patients, compared with .04% in non-COPD controls (P = .004). Among COPD patients, age, body mass index, FEV1%, and active smoking were not associated with longitudinal change in DLco values, the researchers said.
Notably, women with COPD at baseline had lower baseline DLco values compared with men (11.37%) and a significantly steeper decline in DLco (.89%) compared with men (P = .039). “Being a woman was the only factor that related to the annual rate of change in DLco,” the researchers said.
In a subgroup analysis, the researchers identified 305 COPD patients and 69 non-COPD controls who had at least 3 DLco measurements over the 5-year study period. In this group, 16.4% patients with COPD and 4.3% smokers without COPD showed significant yearly declines in DLco of –4.139% and –4.440%, respectively. Among COPD patients, significantly more women than men showed significant DLco declines (26% vs. 14%, P = .005). No significant differences were observed in mortality or hospitalizations per patient-year for COPD patients with and without DLco decline, the researchers said.
The study findings were limited by several factors including the lack of annual measurements of DLco among some patients, potential variability in the instruments used to measure DLco, and the absence of computerized tomography data for the chest, the researchers noted. However, the results support the value of the test for COPD progression when conducted at 3- to 4-year intervals, given the slow pace of the decline, they said. More research is needed, but “women seem to have a different susceptibility to cigarette smoke in the alveolar or pulmonary vascular domains,” they added.
DLco remains a valuable marker
The study is important because the usual longitudinal decline of diffusion capacity, an important physiological parameter in patients with COPD, was unknown, Juan P. de Torres, MD, of Queen’s University, Kingston, Ont., said in an interview.
“The finding of a different longitudinal decline of DLco in women was a surprise,” said Dr. de Torres, who was a coauthor on the study. “We knew from previous works from our group that COPD has a different clinical and prognostic behavior in women with COPD, but this specific finding is novel and important,” he said.
“These results provide information about the testing frequency (3-4 years) needed to use DLco as a marker of COPD progression in clinical practice,” Dr. de Torres added.
“What is the driving cause of this sex difference is unknown. We speculate that different causes of low DLco in COPD such as degree of emphysema, interstitial lung abnormalities, and pulmonary hypertension, may have a different prevalence and progression in women with COPD,” he said.
Looking ahead, “Large studies including an adequate sample of women with COPD is urgently needed because they will be the main face of COPD in the near future,” said Dr. de Torres. “Sex difference in their physiological characteristics, the reason to explain those differences and how they behave longitudinally is also urgently needed,” he added.
The study was supported in part by AstraZeneca and by the COPD research program of the Spanish Respiratory Society. The researchers and Dr. de Torres had no financial conflicts to disclose.
Single breath diffusion capacity for carbon monoxide shows greater decline over time in COPD patients compared with controls, but declines significantly more in women compared with men, according to data from 602 adults with a history of smoking.
In previous studies, diffusion capacity for carbon monoxide (DLco) has been associated with decreased exercise capacity and poor health status in patients with COPD, but its association as a measure of disease progression has not been well studied, wrote Ciro Casanova, MD, of Hospital Universitario La Candelaria, Spain, and colleagues.
In a study published in the journal CHEST®, the researchers identified 506 adult smokers with COPD and 96 adult smoker controls without COPD. Lung function based on single breath DLco was measured each year for 5 years. The study population was part of the COPH History Assessment in SpaiN (CHAIN), an ongoing observational study of adults with COPD. COPD was defined as a history of at least 10 pack-years of smoking and a post-bronchodilator FEV1/FVC greater than 0.7 after 400 micrograms of albuterol, the researchers said.
During the 5-year period, the average overall annual decline in DLco was 1.34% in COPD patients, compared with .04% in non-COPD controls (P = .004). Among COPD patients, age, body mass index, FEV1%, and active smoking were not associated with longitudinal change in DLco values, the researchers said.
Notably, women with COPD at baseline had lower baseline DLco values compared with men (11.37%) and a significantly steeper decline in DLco (.89%) compared with men (P = .039). “Being a woman was the only factor that related to the annual rate of change in DLco,” the researchers said.
In a subgroup analysis, the researchers identified 305 COPD patients and 69 non-COPD controls who had at least 3 DLco measurements over the 5-year study period. In this group, 16.4% patients with COPD and 4.3% smokers without COPD showed significant yearly declines in DLco of –4.139% and –4.440%, respectively. Among COPD patients, significantly more women than men showed significant DLco declines (26% vs. 14%, P = .005). No significant differences were observed in mortality or hospitalizations per patient-year for COPD patients with and without DLco decline, the researchers said.
The study findings were limited by several factors including the lack of annual measurements of DLco among some patients, potential variability in the instruments used to measure DLco, and the absence of computerized tomography data for the chest, the researchers noted. However, the results support the value of the test for COPD progression when conducted at 3- to 4-year intervals, given the slow pace of the decline, they said. More research is needed, but “women seem to have a different susceptibility to cigarette smoke in the alveolar or pulmonary vascular domains,” they added.
DLco remains a valuable marker
The study is important because the usual longitudinal decline of diffusion capacity, an important physiological parameter in patients with COPD, was unknown, Juan P. de Torres, MD, of Queen’s University, Kingston, Ont., said in an interview.
“The finding of a different longitudinal decline of DLco in women was a surprise,” said Dr. de Torres, who was a coauthor on the study. “We knew from previous works from our group that COPD has a different clinical and prognostic behavior in women with COPD, but this specific finding is novel and important,” he said.
“These results provide information about the testing frequency (3-4 years) needed to use DLco as a marker of COPD progression in clinical practice,” Dr. de Torres added.
“What is the driving cause of this sex difference is unknown. We speculate that different causes of low DLco in COPD such as degree of emphysema, interstitial lung abnormalities, and pulmonary hypertension, may have a different prevalence and progression in women with COPD,” he said.
Looking ahead, “Large studies including an adequate sample of women with COPD is urgently needed because they will be the main face of COPD in the near future,” said Dr. de Torres. “Sex difference in their physiological characteristics, the reason to explain those differences and how they behave longitudinally is also urgently needed,” he added.
The study was supported in part by AstraZeneca and by the COPD research program of the Spanish Respiratory Society. The researchers and Dr. de Torres had no financial conflicts to disclose.
Single breath diffusion capacity for carbon monoxide shows greater decline over time in COPD patients compared with controls, but declines significantly more in women compared with men, according to data from 602 adults with a history of smoking.
In previous studies, diffusion capacity for carbon monoxide (DLco) has been associated with decreased exercise capacity and poor health status in patients with COPD, but its association as a measure of disease progression has not been well studied, wrote Ciro Casanova, MD, of Hospital Universitario La Candelaria, Spain, and colleagues.
In a study published in the journal CHEST®, the researchers identified 506 adult smokers with COPD and 96 adult smoker controls without COPD. Lung function based on single breath DLco was measured each year for 5 years. The study population was part of the COPH History Assessment in SpaiN (CHAIN), an ongoing observational study of adults with COPD. COPD was defined as a history of at least 10 pack-years of smoking and a post-bronchodilator FEV1/FVC greater than 0.7 after 400 micrograms of albuterol, the researchers said.
During the 5-year period, the average overall annual decline in DLco was 1.34% in COPD patients, compared with .04% in non-COPD controls (P = .004). Among COPD patients, age, body mass index, FEV1%, and active smoking were not associated with longitudinal change in DLco values, the researchers said.
Notably, women with COPD at baseline had lower baseline DLco values compared with men (11.37%) and a significantly steeper decline in DLco (.89%) compared with men (P = .039). “Being a woman was the only factor that related to the annual rate of change in DLco,” the researchers said.
In a subgroup analysis, the researchers identified 305 COPD patients and 69 non-COPD controls who had at least 3 DLco measurements over the 5-year study period. In this group, 16.4% patients with COPD and 4.3% smokers without COPD showed significant yearly declines in DLco of –4.139% and –4.440%, respectively. Among COPD patients, significantly more women than men showed significant DLco declines (26% vs. 14%, P = .005). No significant differences were observed in mortality or hospitalizations per patient-year for COPD patients with and without DLco decline, the researchers said.
The study findings were limited by several factors including the lack of annual measurements of DLco among some patients, potential variability in the instruments used to measure DLco, and the absence of computerized tomography data for the chest, the researchers noted. However, the results support the value of the test for COPD progression when conducted at 3- to 4-year intervals, given the slow pace of the decline, they said. More research is needed, but “women seem to have a different susceptibility to cigarette smoke in the alveolar or pulmonary vascular domains,” they added.
DLco remains a valuable marker
The study is important because the usual longitudinal decline of diffusion capacity, an important physiological parameter in patients with COPD, was unknown, Juan P. de Torres, MD, of Queen’s University, Kingston, Ont., said in an interview.
“The finding of a different longitudinal decline of DLco in women was a surprise,” said Dr. de Torres, who was a coauthor on the study. “We knew from previous works from our group that COPD has a different clinical and prognostic behavior in women with COPD, but this specific finding is novel and important,” he said.
“These results provide information about the testing frequency (3-4 years) needed to use DLco as a marker of COPD progression in clinical practice,” Dr. de Torres added.
“What is the driving cause of this sex difference is unknown. We speculate that different causes of low DLco in COPD such as degree of emphysema, interstitial lung abnormalities, and pulmonary hypertension, may have a different prevalence and progression in women with COPD,” he said.
Looking ahead, “Large studies including an adequate sample of women with COPD is urgently needed because they will be the main face of COPD in the near future,” said Dr. de Torres. “Sex difference in their physiological characteristics, the reason to explain those differences and how they behave longitudinally is also urgently needed,” he added.
The study was supported in part by AstraZeneca and by the COPD research program of the Spanish Respiratory Society. The researchers and Dr. de Torres had no financial conflicts to disclose.
FROM CHEST
Structural racism tied to psychosis risk in Black people
Social and economic disparities are linked to an increased risk for psychosis in Black and Latino communities, new research shows.
Results of a literature review of social and economic disparities in mental illness suggest that “structural racism” contributes to social and environmental conditions that affect psychosis risk.
“Black and Latino people suffer disproportionately from psychosis risk factors, at the neighborhood level and at the individual level, in large part as a result of structural racism,” study investigator Deidre M. Anglin, PhD, associate professor, department of psychology, City College of New York (N.Y.), told reporters attending a press briefing.
The social environment, which, for minorities, involves disadvantage and discrimination, may account for this increased psychosis risk, perhaps even more so than genetics, she said. Structural racism “is a critical public health threat,” Dr. Anglin added.
The findings were presented at the virtual American Psychiatric Association annual meeting and were simultaneously published online May 3 in The American Journal of Psychiatry.
Perpetual disadvantage
Dr. Anglin and colleagues examined U.S.-based evidence connecting characteristics of social environments with outcomes across the psychosis continuum – from psychotic experiences to schizophrenia.
Citing numerous studies, the researchers highlighted three key areas that reflect social and environmental conditions that may affect psychosis risk, and that disproportionately affect minorities. These were neighborhood factors, trauma in a U.S. context, and racial disparities during the prenatal and perinatal periods.
The data that were related to neighborhoods revealed “just how much racism has historically structured U.S. neighborhoods in ways that generationally perpetuate disadvantage for racially minoritized communities,” said Dr. Anglin.
“This happens through inequitable access to resources, such as health care, clean air, education, [and] employment, but also in terms of disproportionate exposure to environmental toxins and stressors,” she said.
These neighborhood factors are associated with cumulative stress that may be linked to heightened risk for psychosis, the investigators noted.
U.S. studies show that rates of adverse childhood experiences, such as abuse and emotional and physical neglect, are higher among racial and ethnic minorities.
Police victimization and gun violence disproportionately affect racial minorities and create what the investigators call “a unique type of collective trauma” in the United States. They note that Black men have a 1 in 1,000 chance of being victims of lethal force by police over their lifetimes. By comparison, White men have a 39 in 100,000 chance.
One study of a diverse sample from four large U.S. urban centers showed that those who self-reported different types of police victimization were more likely to report psychotic experiences. Another study showed that greater exposure to gun violence fatalities, regardless of police involvement, was positively associated with psychotic experiences.
Obstetric complications
A variety of obstetric complications, including infection, maternal inflammation, and stress, have been associated with increased risk for psychotic disorders in U.S. samples.
“What we saw emerge from the literature is that Black women in the U.S. are at substantially increased risk for many of these obstetrical complications compared to White women, and this is not necessarily explained by socioeconomic status,” said Dr. Anglin.
Neighborhood- and individual-level factors appear to affect the disparity in these outcomes. A recent study revealed that exposure to environmental contaminants such as air pollution is associated with higher rates of preterm birth and low birth weight differentially in Black mothers compared with other mothers, “possibly as a result of an interaction between prenatal stress and contaminants,” the investigators noted.
Research also indicates that Black women are more likely to have lower levels of cortisol during the second trimester of pregnancy compared with women of other racial and ethnic groups. Cortisol is essential for fetal growth. Evidence links lower cortisol levels in later stages of pregnancy with decreased fetal growth in individuals who develop schizophrenia.
, compared with White women of the same socioeconomic status.
Such findings “highlight a complex picture” involving maternal cortisol levels and other stress biomarkers, “potentially leading to poor birth outcomes and subsequent risk for psychotic disorders in adulthood,” the investigators noted.
The researchers call for the dismantling of structural racism and the social policies and norms it shapes. They also recommend changes in health care policy and in the approach to early intervention for psychosis among Black and other racially-minoritized groups.
“Altogether, the current evidence suggests the need to identify, address, and tackle the social determinants deeply ingrained in U.S. society, in tandem with empowering the most marginalized communities,” the researchers wrote.
“We recommend that the field of psychiatry devote considerably more effort to addressing structural racism and social determinants of psychosis in funding priorities, training, and intervention development,” they added.
Dr. Anglin suggests that mental health providers use what she called a “cultural formulation interview” that takes a person’s environmental and social context into consideration. Studies show that incorporating this into clinical practice helps reduce misdiagnosis of mental illness in Black populations, she said.
Call to action
Commenting on the findings in an interview, Ned H. Kalin, MD, editor of The American Journal of Psychiatry and professor and chair of the department of psychiatry, University of Wisconsin, Madison, said the study was well done and serves as a “call to action” to address the impact of structural racism on mental health issues and psychiatric diseases.
The article highlights the need for “collecting better data” on structural racism, said Dr. Kalin. “We know it’s a big issue, but we can’t even quantitate it, so we need some fundamental measures to use as a benchmark as we move forward, as we try to make change.”
He noted that racism “is so embedded in one’s experience and in our society that we sort of don’t even think about it as a trauma.”
In psychiatry, for example, trauma is often thought of as a loss or a traumatic event. “We don’t typically think of trauma as an experience that pervades one’s entire life,” but that needs to change, he said. “At the individual level and in the doctor’s office, being sensitive to and aware of these issues is absolutely critical.”
Dr. Anglin and Dr. Kalin have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Social and economic disparities are linked to an increased risk for psychosis in Black and Latino communities, new research shows.
Results of a literature review of social and economic disparities in mental illness suggest that “structural racism” contributes to social and environmental conditions that affect psychosis risk.
“Black and Latino people suffer disproportionately from psychosis risk factors, at the neighborhood level and at the individual level, in large part as a result of structural racism,” study investigator Deidre M. Anglin, PhD, associate professor, department of psychology, City College of New York (N.Y.), told reporters attending a press briefing.
The social environment, which, for minorities, involves disadvantage and discrimination, may account for this increased psychosis risk, perhaps even more so than genetics, she said. Structural racism “is a critical public health threat,” Dr. Anglin added.
The findings were presented at the virtual American Psychiatric Association annual meeting and were simultaneously published online May 3 in The American Journal of Psychiatry.
Perpetual disadvantage
Dr. Anglin and colleagues examined U.S.-based evidence connecting characteristics of social environments with outcomes across the psychosis continuum – from psychotic experiences to schizophrenia.
Citing numerous studies, the researchers highlighted three key areas that reflect social and environmental conditions that may affect psychosis risk, and that disproportionately affect minorities. These were neighborhood factors, trauma in a U.S. context, and racial disparities during the prenatal and perinatal periods.
The data that were related to neighborhoods revealed “just how much racism has historically structured U.S. neighborhoods in ways that generationally perpetuate disadvantage for racially minoritized communities,” said Dr. Anglin.
“This happens through inequitable access to resources, such as health care, clean air, education, [and] employment, but also in terms of disproportionate exposure to environmental toxins and stressors,” she said.
These neighborhood factors are associated with cumulative stress that may be linked to heightened risk for psychosis, the investigators noted.
U.S. studies show that rates of adverse childhood experiences, such as abuse and emotional and physical neglect, are higher among racial and ethnic minorities.
Police victimization and gun violence disproportionately affect racial minorities and create what the investigators call “a unique type of collective trauma” in the United States. They note that Black men have a 1 in 1,000 chance of being victims of lethal force by police over their lifetimes. By comparison, White men have a 39 in 100,000 chance.
One study of a diverse sample from four large U.S. urban centers showed that those who self-reported different types of police victimization were more likely to report psychotic experiences. Another study showed that greater exposure to gun violence fatalities, regardless of police involvement, was positively associated with psychotic experiences.
Obstetric complications
A variety of obstetric complications, including infection, maternal inflammation, and stress, have been associated with increased risk for psychotic disorders in U.S. samples.
“What we saw emerge from the literature is that Black women in the U.S. are at substantially increased risk for many of these obstetrical complications compared to White women, and this is not necessarily explained by socioeconomic status,” said Dr. Anglin.
Neighborhood- and individual-level factors appear to affect the disparity in these outcomes. A recent study revealed that exposure to environmental contaminants such as air pollution is associated with higher rates of preterm birth and low birth weight differentially in Black mothers compared with other mothers, “possibly as a result of an interaction between prenatal stress and contaminants,” the investigators noted.
Research also indicates that Black women are more likely to have lower levels of cortisol during the second trimester of pregnancy compared with women of other racial and ethnic groups. Cortisol is essential for fetal growth. Evidence links lower cortisol levels in later stages of pregnancy with decreased fetal growth in individuals who develop schizophrenia.
, compared with White women of the same socioeconomic status.
Such findings “highlight a complex picture” involving maternal cortisol levels and other stress biomarkers, “potentially leading to poor birth outcomes and subsequent risk for psychotic disorders in adulthood,” the investigators noted.
The researchers call for the dismantling of structural racism and the social policies and norms it shapes. They also recommend changes in health care policy and in the approach to early intervention for psychosis among Black and other racially-minoritized groups.
“Altogether, the current evidence suggests the need to identify, address, and tackle the social determinants deeply ingrained in U.S. society, in tandem with empowering the most marginalized communities,” the researchers wrote.
“We recommend that the field of psychiatry devote considerably more effort to addressing structural racism and social determinants of psychosis in funding priorities, training, and intervention development,” they added.
Dr. Anglin suggests that mental health providers use what she called a “cultural formulation interview” that takes a person’s environmental and social context into consideration. Studies show that incorporating this into clinical practice helps reduce misdiagnosis of mental illness in Black populations, she said.
Call to action
Commenting on the findings in an interview, Ned H. Kalin, MD, editor of The American Journal of Psychiatry and professor and chair of the department of psychiatry, University of Wisconsin, Madison, said the study was well done and serves as a “call to action” to address the impact of structural racism on mental health issues and psychiatric diseases.
The article highlights the need for “collecting better data” on structural racism, said Dr. Kalin. “We know it’s a big issue, but we can’t even quantitate it, so we need some fundamental measures to use as a benchmark as we move forward, as we try to make change.”
He noted that racism “is so embedded in one’s experience and in our society that we sort of don’t even think about it as a trauma.”
In psychiatry, for example, trauma is often thought of as a loss or a traumatic event. “We don’t typically think of trauma as an experience that pervades one’s entire life,” but that needs to change, he said. “At the individual level and in the doctor’s office, being sensitive to and aware of these issues is absolutely critical.”
Dr. Anglin and Dr. Kalin have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Social and economic disparities are linked to an increased risk for psychosis in Black and Latino communities, new research shows.
Results of a literature review of social and economic disparities in mental illness suggest that “structural racism” contributes to social and environmental conditions that affect psychosis risk.
“Black and Latino people suffer disproportionately from psychosis risk factors, at the neighborhood level and at the individual level, in large part as a result of structural racism,” study investigator Deidre M. Anglin, PhD, associate professor, department of psychology, City College of New York (N.Y.), told reporters attending a press briefing.
The social environment, which, for minorities, involves disadvantage and discrimination, may account for this increased psychosis risk, perhaps even more so than genetics, she said. Structural racism “is a critical public health threat,” Dr. Anglin added.
The findings were presented at the virtual American Psychiatric Association annual meeting and were simultaneously published online May 3 in The American Journal of Psychiatry.
Perpetual disadvantage
Dr. Anglin and colleagues examined U.S.-based evidence connecting characteristics of social environments with outcomes across the psychosis continuum – from psychotic experiences to schizophrenia.
Citing numerous studies, the researchers highlighted three key areas that reflect social and environmental conditions that may affect psychosis risk, and that disproportionately affect minorities. These were neighborhood factors, trauma in a U.S. context, and racial disparities during the prenatal and perinatal periods.
The data that were related to neighborhoods revealed “just how much racism has historically structured U.S. neighborhoods in ways that generationally perpetuate disadvantage for racially minoritized communities,” said Dr. Anglin.
“This happens through inequitable access to resources, such as health care, clean air, education, [and] employment, but also in terms of disproportionate exposure to environmental toxins and stressors,” she said.
These neighborhood factors are associated with cumulative stress that may be linked to heightened risk for psychosis, the investigators noted.
U.S. studies show that rates of adverse childhood experiences, such as abuse and emotional and physical neglect, are higher among racial and ethnic minorities.
Police victimization and gun violence disproportionately affect racial minorities and create what the investigators call “a unique type of collective trauma” in the United States. They note that Black men have a 1 in 1,000 chance of being victims of lethal force by police over their lifetimes. By comparison, White men have a 39 in 100,000 chance.
One study of a diverse sample from four large U.S. urban centers showed that those who self-reported different types of police victimization were more likely to report psychotic experiences. Another study showed that greater exposure to gun violence fatalities, regardless of police involvement, was positively associated with psychotic experiences.
Obstetric complications
A variety of obstetric complications, including infection, maternal inflammation, and stress, have been associated with increased risk for psychotic disorders in U.S. samples.
“What we saw emerge from the literature is that Black women in the U.S. are at substantially increased risk for many of these obstetrical complications compared to White women, and this is not necessarily explained by socioeconomic status,” said Dr. Anglin.
Neighborhood- and individual-level factors appear to affect the disparity in these outcomes. A recent study revealed that exposure to environmental contaminants such as air pollution is associated with higher rates of preterm birth and low birth weight differentially in Black mothers compared with other mothers, “possibly as a result of an interaction between prenatal stress and contaminants,” the investigators noted.
Research also indicates that Black women are more likely to have lower levels of cortisol during the second trimester of pregnancy compared with women of other racial and ethnic groups. Cortisol is essential for fetal growth. Evidence links lower cortisol levels in later stages of pregnancy with decreased fetal growth in individuals who develop schizophrenia.
, compared with White women of the same socioeconomic status.
Such findings “highlight a complex picture” involving maternal cortisol levels and other stress biomarkers, “potentially leading to poor birth outcomes and subsequent risk for psychotic disorders in adulthood,” the investigators noted.
The researchers call for the dismantling of structural racism and the social policies and norms it shapes. They also recommend changes in health care policy and in the approach to early intervention for psychosis among Black and other racially-minoritized groups.
“Altogether, the current evidence suggests the need to identify, address, and tackle the social determinants deeply ingrained in U.S. society, in tandem with empowering the most marginalized communities,” the researchers wrote.
“We recommend that the field of psychiatry devote considerably more effort to addressing structural racism and social determinants of psychosis in funding priorities, training, and intervention development,” they added.
Dr. Anglin suggests that mental health providers use what she called a “cultural formulation interview” that takes a person’s environmental and social context into consideration. Studies show that incorporating this into clinical practice helps reduce misdiagnosis of mental illness in Black populations, she said.
Call to action
Commenting on the findings in an interview, Ned H. Kalin, MD, editor of The American Journal of Psychiatry and professor and chair of the department of psychiatry, University of Wisconsin, Madison, said the study was well done and serves as a “call to action” to address the impact of structural racism on mental health issues and psychiatric diseases.
The article highlights the need for “collecting better data” on structural racism, said Dr. Kalin. “We know it’s a big issue, but we can’t even quantitate it, so we need some fundamental measures to use as a benchmark as we move forward, as we try to make change.”
He noted that racism “is so embedded in one’s experience and in our society that we sort of don’t even think about it as a trauma.”
In psychiatry, for example, trauma is often thought of as a loss or a traumatic event. “We don’t typically think of trauma as an experience that pervades one’s entire life,” but that needs to change, he said. “At the individual level and in the doctor’s office, being sensitive to and aware of these issues is absolutely critical.”
Dr. Anglin and Dr. Kalin have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
COVID-19 impact on breast cancer: Upfront endocrine Rx increased
The use of neoadjuvant endocrine therapy (NET) increased significantly during the first 8 months of the COVID-19 pandemic for women with estrogen receptor–positive (ER+) breast cancer. These patients would normally undergo surgery first, but because of operating room restrictions, those surgeries were delayed because of the pandemic, according to a new study.
“We hypothesized that by offering a nontoxic therapy, we would be able to ‘hold over’ patients until such time when personal protective equipment supplies were renewed and we could get into the operating room,” lead author Lee Wilke, MD, professor of surgery, University of Wisconsin, Madison, said in an interview.
“And while a small number of women with ER+ tumors get NET anyway, we found over one-third of patients with ER+ breast cancer were treated with NET due to COVID-19 during the first 8 months of last year,” she said.
“One year later, 31% of the same patient population is still getting NET,” she added.
The study was presented during the online annual meeting of the American Society of Breast Surgeons (ASBrS).
COVID-specific registry
Dr. Wilke believes that this study presents an accurate snapshot of changes in treatment caused by the pandemic.
She and her colleagues compared data collected in the ASBrS Mastery Program registry to data collected in an embedded but separate COVID-19 segment. The data were for the period from March 1 to Oct. 28, 2020.
Almost three-quarters of the surgeons who entered patients into the COVID-19 segment were from urban areas; 95% reported stopping mammographic screening during part of this period.
The preliminary analysis focused on data collected from 2,476 patients in the COVID-19 segment and 2,303 patients within the Mastery registry.
For patients with ER+/HER2- breast cancer, NET was described as a usual approach in 6.5% of patients in the COVID-19 registry. In the Mastery registry, 7.8% of patients received NET.
Compared with surgery first/usual practice, which served as the reference, older patients were more likely to receive NET first because of the COVID-19 pandemic than younger patients, and they were more likely to receive NET first if they lived in the Northeast or the Southeast compared to other regions of the United States. Dr. Wilke pointed out that the Northeast and the Southeast were hardest hit by COVID-19 early on in the pandemic.
Genomic testing was carried out in a small subgroup of patients; 24% of those patients underwent testing on the core biopsy specimen because of COVID-19, the investigators noted. Genomic testing on a core biopsy specimen helps determine whether it’s feasible to forgo chemotherapy and use NET instead or whether the patient should proceed directly to surgery. The authors noted that almost 11% of patients required a change in the usual surgical approach because of COVID-19. Such changes were made primarily to avoid hospitalizations during the early phase of the pandemic for patients who were to undergo mastectomy or reconstruction.
“Patients who needed standard approaches still got them,” Dr. Wilke emphasized in a statement. For example, women with aggressive triple-negative and HER2+ tumors were treated with neoadjuvant chemotherapy, she added. “However, NET is a very good approach for a moderate subset of patients, and we think we will see it being used more often in the U.S. now,” Dr. Wilke observed.
“But especially early during the pandemic, these revised treatments were necessary because access to hospital ORs was limited or unavailable, so our algorithmic-based treatment guidelines allowed us to offer high-quality, evidence-based care fine-tuned for a patient’s specific cancer profile,” she affirmed.
Dr. Wilke has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The use of neoadjuvant endocrine therapy (NET) increased significantly during the first 8 months of the COVID-19 pandemic for women with estrogen receptor–positive (ER+) breast cancer. These patients would normally undergo surgery first, but because of operating room restrictions, those surgeries were delayed because of the pandemic, according to a new study.
“We hypothesized that by offering a nontoxic therapy, we would be able to ‘hold over’ patients until such time when personal protective equipment supplies were renewed and we could get into the operating room,” lead author Lee Wilke, MD, professor of surgery, University of Wisconsin, Madison, said in an interview.
“And while a small number of women with ER+ tumors get NET anyway, we found over one-third of patients with ER+ breast cancer were treated with NET due to COVID-19 during the first 8 months of last year,” she said.
“One year later, 31% of the same patient population is still getting NET,” she added.
The study was presented during the online annual meeting of the American Society of Breast Surgeons (ASBrS).
COVID-specific registry
Dr. Wilke believes that this study presents an accurate snapshot of changes in treatment caused by the pandemic.
She and her colleagues compared data collected in the ASBrS Mastery Program registry to data collected in an embedded but separate COVID-19 segment. The data were for the period from March 1 to Oct. 28, 2020.
Almost three-quarters of the surgeons who entered patients into the COVID-19 segment were from urban areas; 95% reported stopping mammographic screening during part of this period.
The preliminary analysis focused on data collected from 2,476 patients in the COVID-19 segment and 2,303 patients within the Mastery registry.
For patients with ER+/HER2- breast cancer, NET was described as a usual approach in 6.5% of patients in the COVID-19 registry. In the Mastery registry, 7.8% of patients received NET.
Compared with surgery first/usual practice, which served as the reference, older patients were more likely to receive NET first because of the COVID-19 pandemic than younger patients, and they were more likely to receive NET first if they lived in the Northeast or the Southeast compared to other regions of the United States. Dr. Wilke pointed out that the Northeast and the Southeast were hardest hit by COVID-19 early on in the pandemic.
Genomic testing was carried out in a small subgroup of patients; 24% of those patients underwent testing on the core biopsy specimen because of COVID-19, the investigators noted. Genomic testing on a core biopsy specimen helps determine whether it’s feasible to forgo chemotherapy and use NET instead or whether the patient should proceed directly to surgery. The authors noted that almost 11% of patients required a change in the usual surgical approach because of COVID-19. Such changes were made primarily to avoid hospitalizations during the early phase of the pandemic for patients who were to undergo mastectomy or reconstruction.
“Patients who needed standard approaches still got them,” Dr. Wilke emphasized in a statement. For example, women with aggressive triple-negative and HER2+ tumors were treated with neoadjuvant chemotherapy, she added. “However, NET is a very good approach for a moderate subset of patients, and we think we will see it being used more often in the U.S. now,” Dr. Wilke observed.
“But especially early during the pandemic, these revised treatments were necessary because access to hospital ORs was limited or unavailable, so our algorithmic-based treatment guidelines allowed us to offer high-quality, evidence-based care fine-tuned for a patient’s specific cancer profile,” she affirmed.
Dr. Wilke has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The use of neoadjuvant endocrine therapy (NET) increased significantly during the first 8 months of the COVID-19 pandemic for women with estrogen receptor–positive (ER+) breast cancer. These patients would normally undergo surgery first, but because of operating room restrictions, those surgeries were delayed because of the pandemic, according to a new study.
“We hypothesized that by offering a nontoxic therapy, we would be able to ‘hold over’ patients until such time when personal protective equipment supplies were renewed and we could get into the operating room,” lead author Lee Wilke, MD, professor of surgery, University of Wisconsin, Madison, said in an interview.
“And while a small number of women with ER+ tumors get NET anyway, we found over one-third of patients with ER+ breast cancer were treated with NET due to COVID-19 during the first 8 months of last year,” she said.
“One year later, 31% of the same patient population is still getting NET,” she added.
The study was presented during the online annual meeting of the American Society of Breast Surgeons (ASBrS).
COVID-specific registry
Dr. Wilke believes that this study presents an accurate snapshot of changes in treatment caused by the pandemic.
She and her colleagues compared data collected in the ASBrS Mastery Program registry to data collected in an embedded but separate COVID-19 segment. The data were for the period from March 1 to Oct. 28, 2020.
Almost three-quarters of the surgeons who entered patients into the COVID-19 segment were from urban areas; 95% reported stopping mammographic screening during part of this period.
The preliminary analysis focused on data collected from 2,476 patients in the COVID-19 segment and 2,303 patients within the Mastery registry.
For patients with ER+/HER2- breast cancer, NET was described as a usual approach in 6.5% of patients in the COVID-19 registry. In the Mastery registry, 7.8% of patients received NET.
Compared with surgery first/usual practice, which served as the reference, older patients were more likely to receive NET first because of the COVID-19 pandemic than younger patients, and they were more likely to receive NET first if they lived in the Northeast or the Southeast compared to other regions of the United States. Dr. Wilke pointed out that the Northeast and the Southeast were hardest hit by COVID-19 early on in the pandemic.
Genomic testing was carried out in a small subgroup of patients; 24% of those patients underwent testing on the core biopsy specimen because of COVID-19, the investigators noted. Genomic testing on a core biopsy specimen helps determine whether it’s feasible to forgo chemotherapy and use NET instead or whether the patient should proceed directly to surgery. The authors noted that almost 11% of patients required a change in the usual surgical approach because of COVID-19. Such changes were made primarily to avoid hospitalizations during the early phase of the pandemic for patients who were to undergo mastectomy or reconstruction.
“Patients who needed standard approaches still got them,” Dr. Wilke emphasized in a statement. For example, women with aggressive triple-negative and HER2+ tumors were treated with neoadjuvant chemotherapy, she added. “However, NET is a very good approach for a moderate subset of patients, and we think we will see it being used more often in the U.S. now,” Dr. Wilke observed.
“But especially early during the pandemic, these revised treatments were necessary because access to hospital ORs was limited or unavailable, so our algorithmic-based treatment guidelines allowed us to offer high-quality, evidence-based care fine-tuned for a patient’s specific cancer profile,” she affirmed.
Dr. Wilke has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Checkpoint inhibitor skin side effects more common in women
of 235 patients at Dana Farber Cancer Center, Boston.
Overall, 62.4% of the 93 women in the review and 48.6% of the 142 men experienced confirmed skin reactions, for an odds ratio (OR) of 2.11 for women compared with men (P = .01).
“Clinicians should consider these results in counseling female patients regarding an elevated risk of dermatologic adverse events” when taking checkpoint inhibitors, said investigators led by Harvard University medical student Jordan Said, who presented the results at the American Academy of Dermatology Virtual Meeting Experience.
Autoimmune-like adverse events are common with checkpoint inhibitors. Dermatologic side effects occur in about half of people receiving monotherapy and more than that among patients receiving combination therapy.
Skin reactions can include psoriasiform dermatitis, lichenoid reactions, vitiligo, and bullous pemphigoid and may require hospitalization and prolonged steroid treatment.
Not much is known about risk factors for these reactions. A higher incidence among women has been previously reported. A 2019 study found a higher risk for pneumonitis and endocrinopathy, including hypophysitis, among women who underwent treatment for non–small cell lung cancer or metastatic melanoma.
The 2019 study found that the risk was higher among premenopausal women than postmenopausal women, which led some to suggest that estrogen may play a role.
The results of the Dana Farber review argue against that notion. In their review, the investigators found that the risk was similarly elevated among the 27 premenopausal women (OR, 1.97; P = .40) and the 66 postmenopausal women (OR, 2.17, P = .05). In the study, women who were aged 52 years or older at the start of treatment were considered to be postmenopausal.
“This suggests that factors beyond sex hormones are likely contributory” to the difference in risk between men and women. It’s known that women are at higher risk for autoimmune disease overall, which might be related to the increased odds of autoimmune-like reactions, and it may be that sex-related differences in innate and adoptive immunity are at work, Mr. Said noted.
When asked for comment, Douglas Johnson, MD, assistant professor of hematology/oncology at Vanderbilt University, Nashville, Tenn., said that although some studies have reported a greater risk for side effects among women, others have not. “Additional research is needed to determine the interactions between sex and effects of immune checkpoint inhibitors, as well as many other possible triggers of immune-related adverse events,” he said.
“Continued work in this area will be so important to help determine how to best counsel women and to ensure early recognition and intervention for dermatologic side effects,” said Bernice Kwong, MD, director of the Supportive Dermato-Oncology Program at Stanford (Calif.) University.
The patients in the review were treated from 2011 to 2016 and underwent at least monthly evaluations by their medical teams. They were taking either nivolumab, pembrolizumab, or ipilimumab or a nivolumab/ipilimumab combination.
The median age of the men in the study was 65 years; the median age of women was 60 years. Almost 98% of the participants were White. The majority received one to three infusions, most commonly with pembrolizumab monotherapy.
No funding for the study was reported. Mr. Said has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
of 235 patients at Dana Farber Cancer Center, Boston.
Overall, 62.4% of the 93 women in the review and 48.6% of the 142 men experienced confirmed skin reactions, for an odds ratio (OR) of 2.11 for women compared with men (P = .01).
“Clinicians should consider these results in counseling female patients regarding an elevated risk of dermatologic adverse events” when taking checkpoint inhibitors, said investigators led by Harvard University medical student Jordan Said, who presented the results at the American Academy of Dermatology Virtual Meeting Experience.
Autoimmune-like adverse events are common with checkpoint inhibitors. Dermatologic side effects occur in about half of people receiving monotherapy and more than that among patients receiving combination therapy.
Skin reactions can include psoriasiform dermatitis, lichenoid reactions, vitiligo, and bullous pemphigoid and may require hospitalization and prolonged steroid treatment.
Not much is known about risk factors for these reactions. A higher incidence among women has been previously reported. A 2019 study found a higher risk for pneumonitis and endocrinopathy, including hypophysitis, among women who underwent treatment for non–small cell lung cancer or metastatic melanoma.
The 2019 study found that the risk was higher among premenopausal women than postmenopausal women, which led some to suggest that estrogen may play a role.
The results of the Dana Farber review argue against that notion. In their review, the investigators found that the risk was similarly elevated among the 27 premenopausal women (OR, 1.97; P = .40) and the 66 postmenopausal women (OR, 2.17, P = .05). In the study, women who were aged 52 years or older at the start of treatment were considered to be postmenopausal.
“This suggests that factors beyond sex hormones are likely contributory” to the difference in risk between men and women. It’s known that women are at higher risk for autoimmune disease overall, which might be related to the increased odds of autoimmune-like reactions, and it may be that sex-related differences in innate and adoptive immunity are at work, Mr. Said noted.
When asked for comment, Douglas Johnson, MD, assistant professor of hematology/oncology at Vanderbilt University, Nashville, Tenn., said that although some studies have reported a greater risk for side effects among women, others have not. “Additional research is needed to determine the interactions between sex and effects of immune checkpoint inhibitors, as well as many other possible triggers of immune-related adverse events,” he said.
“Continued work in this area will be so important to help determine how to best counsel women and to ensure early recognition and intervention for dermatologic side effects,” said Bernice Kwong, MD, director of the Supportive Dermato-Oncology Program at Stanford (Calif.) University.
The patients in the review were treated from 2011 to 2016 and underwent at least monthly evaluations by their medical teams. They were taking either nivolumab, pembrolizumab, or ipilimumab or a nivolumab/ipilimumab combination.
The median age of the men in the study was 65 years; the median age of women was 60 years. Almost 98% of the participants were White. The majority received one to three infusions, most commonly with pembrolizumab monotherapy.
No funding for the study was reported. Mr. Said has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
of 235 patients at Dana Farber Cancer Center, Boston.
Overall, 62.4% of the 93 women in the review and 48.6% of the 142 men experienced confirmed skin reactions, for an odds ratio (OR) of 2.11 for women compared with men (P = .01).
“Clinicians should consider these results in counseling female patients regarding an elevated risk of dermatologic adverse events” when taking checkpoint inhibitors, said investigators led by Harvard University medical student Jordan Said, who presented the results at the American Academy of Dermatology Virtual Meeting Experience.
Autoimmune-like adverse events are common with checkpoint inhibitors. Dermatologic side effects occur in about half of people receiving monotherapy and more than that among patients receiving combination therapy.
Skin reactions can include psoriasiform dermatitis, lichenoid reactions, vitiligo, and bullous pemphigoid and may require hospitalization and prolonged steroid treatment.
Not much is known about risk factors for these reactions. A higher incidence among women has been previously reported. A 2019 study found a higher risk for pneumonitis and endocrinopathy, including hypophysitis, among women who underwent treatment for non–small cell lung cancer or metastatic melanoma.
The 2019 study found that the risk was higher among premenopausal women than postmenopausal women, which led some to suggest that estrogen may play a role.
The results of the Dana Farber review argue against that notion. In their review, the investigators found that the risk was similarly elevated among the 27 premenopausal women (OR, 1.97; P = .40) and the 66 postmenopausal women (OR, 2.17, P = .05). In the study, women who were aged 52 years or older at the start of treatment were considered to be postmenopausal.
“This suggests that factors beyond sex hormones are likely contributory” to the difference in risk between men and women. It’s known that women are at higher risk for autoimmune disease overall, which might be related to the increased odds of autoimmune-like reactions, and it may be that sex-related differences in innate and adoptive immunity are at work, Mr. Said noted.
When asked for comment, Douglas Johnson, MD, assistant professor of hematology/oncology at Vanderbilt University, Nashville, Tenn., said that although some studies have reported a greater risk for side effects among women, others have not. “Additional research is needed to determine the interactions between sex and effects of immune checkpoint inhibitors, as well as many other possible triggers of immune-related adverse events,” he said.
“Continued work in this area will be so important to help determine how to best counsel women and to ensure early recognition and intervention for dermatologic side effects,” said Bernice Kwong, MD, director of the Supportive Dermato-Oncology Program at Stanford (Calif.) University.
The patients in the review were treated from 2011 to 2016 and underwent at least monthly evaluations by their medical teams. They were taking either nivolumab, pembrolizumab, or ipilimumab or a nivolumab/ipilimumab combination.
The median age of the men in the study was 65 years; the median age of women was 60 years. Almost 98% of the participants were White. The majority received one to three infusions, most commonly with pembrolizumab monotherapy.
No funding for the study was reported. Mr. Said has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.