User login
Restrict J&J COVID vaccine in women under 50?
Use of mRNA COVID-19 vaccines should be considered as the preferable option in the United States rather than Johnson & Johnson’s (J&J) Janssen COVID-19 vaccine in women aged under 50 years, according to one group of experts.
The group made their recommendation in an editorial in JAMA published online April 30, 2021, accompanying a paper describing details of 12 case reports of cerebral venous sinus thrombosis (CVST) with thrombocytopenia following the J&J COVID-19 vaccine, also known as the Ad26.COV2.S vaccine.
The editorialists are Ruth A. Karron, MD, professor of international health at Johns Hopkins University, Baltimore; Nigel S. Key, MD, professor of hematology at the University of North Carolina at Chapel Hill; and Joshua M. Sharfstein, MD, associate dean for public health practice at Johns Hopkins
They noted that, after an initial pause following reports of thrombosis with thrombocytopenia syndrome (TTS) linked to the J&J vaccine, and on the recommendation of the Advisory Committee on Immunization Practices, the United States has permitted the use of the J&J vaccine in all adults with information on the risk of TTS added to educational materials.
The editorialists pointed out that no cases of TTS have been confirmed following administration of more than 180 million doses of the mRNA vaccines in the United States.
They said that, while the J&J vaccine will still be needed for individuals with allergies to components of the mRNA vaccines and for those who live in remote locations where the cold chain for transport and storage of mRNA vaccines cannot be maintained, “U.S. public health agencies and clinicians should consider recommending mRNA vaccines as safer options for those who may be at substantially higher risk for TTS after Ad26.COV2.S vaccination, currently women younger than 50 years.”
In the main JAMA paper, a group led by Isaac See, MD, Centers for Disease Control and Prevention COVID-19 Response Team, reported full details of 12 cases of CVST with thrombocytopenia following the J&J COVID-19 vaccine reported to the U.S. Vaccine Adverse Event Reporting System (VAERS).
The 12 U.S. case reports, 3 of which were fatal, show many similarities to cases described in Europe after the AstraZeneca vaccine.
The authors noted that, by April 12, approximately 7 million doses of the J&J vaccine had been given in the United States. The 12 cases of CVST and thrombocytopenia following receipt of the vaccine were reported to VAERS between March 2 and April 21. All 12 cases were in White women, 11 of whom were aged under 50 years.
As of April 25, a further two cases have been confirmed and reported to VAERS; one in a man younger than 40 years, the other in a woman aged between 40 and 59 years.
In the 12 cases reported in detail, symptoms started between 6 and 15 days post vaccination.
At least one risk factor for CVST was identified in seven patients (obesity in six, hypothyroidism in one, and use of combined oral contraceptives in one). None of the patients was pregnant or within 12 weeks post partum, had prior thrombosis, a personal or family history of thrombophilia, or documented prior exposure to heparin.
In addition to CVST, seven patients had intracerebral hemorrhage and eight had non-CVST thromboses.
One patient reported a history of SARS-CoV-2 infection approximately 4 months prior to vaccination. Of the other 11 patients, 4 had negative serologic tests and 7 were not tested.
All 12 patients were hospitalized and 10 were admitted to an ICU. At the time of the last follow-up, three patients had died (all of whom had intraparenchymal hemorrhage), three remained in the ICU, two were still hospitalized but not in an ICU, and four had been discharged home.
The authors pointed out that the U.S. cases of CVST with thrombocytopenia following the J&J vaccine have many similarities to those reported in Europe after the AstraZeneca vaccine, occurring primarily in women younger than 40 years and in patients without diagnosed thrombophilia. Both European and U.S. patients had a median platelet nadir count of 19 x 103/mcL and several also had non-CVST large-vessel thrombosis.
In the European cases of CVST with thrombocytopenia, 50% of patients died, compared with 25% of U.S. patients.
Like the European cases, the U.S. cases had positive heparin-PF4 HIT antibody enzyme-linked immunosorbent assay tests in the absence of prior exposure to heparin, as would be seen in autoimmune HIT.
However, in the initial European CVST reports, 88% of patients tested with functional platelet HIT antibody tests had positive results, compared with only 11% of the U.S. cases. But the authors noted that lack of standardization in functional platelet HIT antibody assays may lead to differences in results by different laboratories.
“It may be important to notify testing laboratories that postvaccination TTS is being evaluated, so that testing methods can be adjusted if needed,” they said.
They concluded that these case reports suggest that the pathogenesis of TTS may be similar to autoimmune HIT, triggered by the formation of antibodies directed against PF4, a constituent of platelet alpha granules released during platelet activation. In contrast to classic HIT in which exogenous heparin triggers antibody formation, in autoimmune HIT, an endogenous polyanion triggers PF4 antibody formation.
They noted that the precise mechanism of TTS in relation to COVID-19 vaccination has not yet been established. The Global Advisory Committee on Vaccine Safety has stated that a platform-specific mechanism related to adenovirus vector vaccines cannot be excluded. Both the J&J and AstraZeneca vaccines use an adenoviral vector, but they are different; J&J uses a human vector, while AstraZeneca uses a chimpanzee vector.
They also pointed out that CVST and thrombocytopenia following SARS-CoV-2 infection has been reported in at least two cases, but HIT testing was not done in these cases. There have not so far been any reports to VAERS of CVST with thrombocytopenia following mRNA COVID-19 vaccines.
The authors said these findings have important clinical and public health implications, noting that the CDC has updated its interim clinical considerations for use of authorized COVID-19 vaccines to indicate that women aged 18-49 years should be aware of the increased risk of TTS after receipt of the J&J vaccine, and to use a nonheparin anticoagulant in suspected cases.
They noted that a subacute presentation of headache is present in 90% of patients with typical CVST. While headache is a common symptom after the J&J vaccination, most headaches begin and resolve within 2 days. Whereas in the U.S. cases of CVST after vaccination, headache symptoms began at least 6 days after vaccination and persisted for at least a week for most.
“Urgent consultation with a neurologist is prudent when a patient is suspected or confirmed to have CVST. In addition, since the median time from symptom onset to hospitalization was 7 days in the U.S. CVST case series, patient and clinician education might shorten the time to clinical evaluation and therefore treatment,” they stated.
The authors also note that VAERS is a passive surveillance system, so cases of CVST with thrombocytopenia may be underreported.
In their accompanying editorial, Dr. Karron and colleagues pointed out that, in addition to the 12 patients with CVST with thrombocytopenia described in this case series, at least three patients without CVST but meeting diagnostic criteria for TTS have been reported to VAERS (as of April 21), all in women aged 18-59 years (median age, 37 years).
The editorialists reported that the rate of CVST with thrombocytopenia after the J&J vaccine is approximately 5 per million women aged 18-50 years. This is compared with a background rate of approximately 0.05-0.13 per million per month.
They said that the availability of an interim standardized case definition of this adverse effect will facilitate prospective case ascertainment through review of large linked databases and active case finding.
This will also permit greater understanding of whether individuals who are otherwise at increased risk for hypercoagulation in general and for CVST in particular (for example, women taking hormonal contraceptive medications or who are pregnant) are also at increased risk for TTS.
Obtaining this information will support dynamic country-specific assessments of the risks of each vaccine, compared with the risk of COVID-19 disease for their populations and subpopulations, they added.
A version of this article first appeared on Medscape.com.
Use of mRNA COVID-19 vaccines should be considered as the preferable option in the United States rather than Johnson & Johnson’s (J&J) Janssen COVID-19 vaccine in women aged under 50 years, according to one group of experts.
The group made their recommendation in an editorial in JAMA published online April 30, 2021, accompanying a paper describing details of 12 case reports of cerebral venous sinus thrombosis (CVST) with thrombocytopenia following the J&J COVID-19 vaccine, also known as the Ad26.COV2.S vaccine.
The editorialists are Ruth A. Karron, MD, professor of international health at Johns Hopkins University, Baltimore; Nigel S. Key, MD, professor of hematology at the University of North Carolina at Chapel Hill; and Joshua M. Sharfstein, MD, associate dean for public health practice at Johns Hopkins
They noted that, after an initial pause following reports of thrombosis with thrombocytopenia syndrome (TTS) linked to the J&J vaccine, and on the recommendation of the Advisory Committee on Immunization Practices, the United States has permitted the use of the J&J vaccine in all adults with information on the risk of TTS added to educational materials.
The editorialists pointed out that no cases of TTS have been confirmed following administration of more than 180 million doses of the mRNA vaccines in the United States.
They said that, while the J&J vaccine will still be needed for individuals with allergies to components of the mRNA vaccines and for those who live in remote locations where the cold chain for transport and storage of mRNA vaccines cannot be maintained, “U.S. public health agencies and clinicians should consider recommending mRNA vaccines as safer options for those who may be at substantially higher risk for TTS after Ad26.COV2.S vaccination, currently women younger than 50 years.”
In the main JAMA paper, a group led by Isaac See, MD, Centers for Disease Control and Prevention COVID-19 Response Team, reported full details of 12 cases of CVST with thrombocytopenia following the J&J COVID-19 vaccine reported to the U.S. Vaccine Adverse Event Reporting System (VAERS).
The 12 U.S. case reports, 3 of which were fatal, show many similarities to cases described in Europe after the AstraZeneca vaccine.
The authors noted that, by April 12, approximately 7 million doses of the J&J vaccine had been given in the United States. The 12 cases of CVST and thrombocytopenia following receipt of the vaccine were reported to VAERS between March 2 and April 21. All 12 cases were in White women, 11 of whom were aged under 50 years.
As of April 25, a further two cases have been confirmed and reported to VAERS; one in a man younger than 40 years, the other in a woman aged between 40 and 59 years.
In the 12 cases reported in detail, symptoms started between 6 and 15 days post vaccination.
At least one risk factor for CVST was identified in seven patients (obesity in six, hypothyroidism in one, and use of combined oral contraceptives in one). None of the patients was pregnant or within 12 weeks post partum, had prior thrombosis, a personal or family history of thrombophilia, or documented prior exposure to heparin.
In addition to CVST, seven patients had intracerebral hemorrhage and eight had non-CVST thromboses.
One patient reported a history of SARS-CoV-2 infection approximately 4 months prior to vaccination. Of the other 11 patients, 4 had negative serologic tests and 7 were not tested.
All 12 patients were hospitalized and 10 were admitted to an ICU. At the time of the last follow-up, three patients had died (all of whom had intraparenchymal hemorrhage), three remained in the ICU, two were still hospitalized but not in an ICU, and four had been discharged home.
The authors pointed out that the U.S. cases of CVST with thrombocytopenia following the J&J vaccine have many similarities to those reported in Europe after the AstraZeneca vaccine, occurring primarily in women younger than 40 years and in patients without diagnosed thrombophilia. Both European and U.S. patients had a median platelet nadir count of 19 x 103/mcL and several also had non-CVST large-vessel thrombosis.
In the European cases of CVST with thrombocytopenia, 50% of patients died, compared with 25% of U.S. patients.
Like the European cases, the U.S. cases had positive heparin-PF4 HIT antibody enzyme-linked immunosorbent assay tests in the absence of prior exposure to heparin, as would be seen in autoimmune HIT.
However, in the initial European CVST reports, 88% of patients tested with functional platelet HIT antibody tests had positive results, compared with only 11% of the U.S. cases. But the authors noted that lack of standardization in functional platelet HIT antibody assays may lead to differences in results by different laboratories.
“It may be important to notify testing laboratories that postvaccination TTS is being evaluated, so that testing methods can be adjusted if needed,” they said.
They concluded that these case reports suggest that the pathogenesis of TTS may be similar to autoimmune HIT, triggered by the formation of antibodies directed against PF4, a constituent of platelet alpha granules released during platelet activation. In contrast to classic HIT in which exogenous heparin triggers antibody formation, in autoimmune HIT, an endogenous polyanion triggers PF4 antibody formation.
They noted that the precise mechanism of TTS in relation to COVID-19 vaccination has not yet been established. The Global Advisory Committee on Vaccine Safety has stated that a platform-specific mechanism related to adenovirus vector vaccines cannot be excluded. Both the J&J and AstraZeneca vaccines use an adenoviral vector, but they are different; J&J uses a human vector, while AstraZeneca uses a chimpanzee vector.
They also pointed out that CVST and thrombocytopenia following SARS-CoV-2 infection has been reported in at least two cases, but HIT testing was not done in these cases. There have not so far been any reports to VAERS of CVST with thrombocytopenia following mRNA COVID-19 vaccines.
The authors said these findings have important clinical and public health implications, noting that the CDC has updated its interim clinical considerations for use of authorized COVID-19 vaccines to indicate that women aged 18-49 years should be aware of the increased risk of TTS after receipt of the J&J vaccine, and to use a nonheparin anticoagulant in suspected cases.
They noted that a subacute presentation of headache is present in 90% of patients with typical CVST. While headache is a common symptom after the J&J vaccination, most headaches begin and resolve within 2 days. Whereas in the U.S. cases of CVST after vaccination, headache symptoms began at least 6 days after vaccination and persisted for at least a week for most.
“Urgent consultation with a neurologist is prudent when a patient is suspected or confirmed to have CVST. In addition, since the median time from symptom onset to hospitalization was 7 days in the U.S. CVST case series, patient and clinician education might shorten the time to clinical evaluation and therefore treatment,” they stated.
The authors also note that VAERS is a passive surveillance system, so cases of CVST with thrombocytopenia may be underreported.
In their accompanying editorial, Dr. Karron and colleagues pointed out that, in addition to the 12 patients with CVST with thrombocytopenia described in this case series, at least three patients without CVST but meeting diagnostic criteria for TTS have been reported to VAERS (as of April 21), all in women aged 18-59 years (median age, 37 years).
The editorialists reported that the rate of CVST with thrombocytopenia after the J&J vaccine is approximately 5 per million women aged 18-50 years. This is compared with a background rate of approximately 0.05-0.13 per million per month.
They said that the availability of an interim standardized case definition of this adverse effect will facilitate prospective case ascertainment through review of large linked databases and active case finding.
This will also permit greater understanding of whether individuals who are otherwise at increased risk for hypercoagulation in general and for CVST in particular (for example, women taking hormonal contraceptive medications or who are pregnant) are also at increased risk for TTS.
Obtaining this information will support dynamic country-specific assessments of the risks of each vaccine, compared with the risk of COVID-19 disease for their populations and subpopulations, they added.
A version of this article first appeared on Medscape.com.
Use of mRNA COVID-19 vaccines should be considered as the preferable option in the United States rather than Johnson & Johnson’s (J&J) Janssen COVID-19 vaccine in women aged under 50 years, according to one group of experts.
The group made their recommendation in an editorial in JAMA published online April 30, 2021, accompanying a paper describing details of 12 case reports of cerebral venous sinus thrombosis (CVST) with thrombocytopenia following the J&J COVID-19 vaccine, also known as the Ad26.COV2.S vaccine.
The editorialists are Ruth A. Karron, MD, professor of international health at Johns Hopkins University, Baltimore; Nigel S. Key, MD, professor of hematology at the University of North Carolina at Chapel Hill; and Joshua M. Sharfstein, MD, associate dean for public health practice at Johns Hopkins
They noted that, after an initial pause following reports of thrombosis with thrombocytopenia syndrome (TTS) linked to the J&J vaccine, and on the recommendation of the Advisory Committee on Immunization Practices, the United States has permitted the use of the J&J vaccine in all adults with information on the risk of TTS added to educational materials.
The editorialists pointed out that no cases of TTS have been confirmed following administration of more than 180 million doses of the mRNA vaccines in the United States.
They said that, while the J&J vaccine will still be needed for individuals with allergies to components of the mRNA vaccines and for those who live in remote locations where the cold chain for transport and storage of mRNA vaccines cannot be maintained, “U.S. public health agencies and clinicians should consider recommending mRNA vaccines as safer options for those who may be at substantially higher risk for TTS after Ad26.COV2.S vaccination, currently women younger than 50 years.”
In the main JAMA paper, a group led by Isaac See, MD, Centers for Disease Control and Prevention COVID-19 Response Team, reported full details of 12 cases of CVST with thrombocytopenia following the J&J COVID-19 vaccine reported to the U.S. Vaccine Adverse Event Reporting System (VAERS).
The 12 U.S. case reports, 3 of which were fatal, show many similarities to cases described in Europe after the AstraZeneca vaccine.
The authors noted that, by April 12, approximately 7 million doses of the J&J vaccine had been given in the United States. The 12 cases of CVST and thrombocytopenia following receipt of the vaccine were reported to VAERS between March 2 and April 21. All 12 cases were in White women, 11 of whom were aged under 50 years.
As of April 25, a further two cases have been confirmed and reported to VAERS; one in a man younger than 40 years, the other in a woman aged between 40 and 59 years.
In the 12 cases reported in detail, symptoms started between 6 and 15 days post vaccination.
At least one risk factor for CVST was identified in seven patients (obesity in six, hypothyroidism in one, and use of combined oral contraceptives in one). None of the patients was pregnant or within 12 weeks post partum, had prior thrombosis, a personal or family history of thrombophilia, or documented prior exposure to heparin.
In addition to CVST, seven patients had intracerebral hemorrhage and eight had non-CVST thromboses.
One patient reported a history of SARS-CoV-2 infection approximately 4 months prior to vaccination. Of the other 11 patients, 4 had negative serologic tests and 7 were not tested.
All 12 patients were hospitalized and 10 were admitted to an ICU. At the time of the last follow-up, three patients had died (all of whom had intraparenchymal hemorrhage), three remained in the ICU, two were still hospitalized but not in an ICU, and four had been discharged home.
The authors pointed out that the U.S. cases of CVST with thrombocytopenia following the J&J vaccine have many similarities to those reported in Europe after the AstraZeneca vaccine, occurring primarily in women younger than 40 years and in patients without diagnosed thrombophilia. Both European and U.S. patients had a median platelet nadir count of 19 x 103/mcL and several also had non-CVST large-vessel thrombosis.
In the European cases of CVST with thrombocytopenia, 50% of patients died, compared with 25% of U.S. patients.
Like the European cases, the U.S. cases had positive heparin-PF4 HIT antibody enzyme-linked immunosorbent assay tests in the absence of prior exposure to heparin, as would be seen in autoimmune HIT.
However, in the initial European CVST reports, 88% of patients tested with functional platelet HIT antibody tests had positive results, compared with only 11% of the U.S. cases. But the authors noted that lack of standardization in functional platelet HIT antibody assays may lead to differences in results by different laboratories.
“It may be important to notify testing laboratories that postvaccination TTS is being evaluated, so that testing methods can be adjusted if needed,” they said.
They concluded that these case reports suggest that the pathogenesis of TTS may be similar to autoimmune HIT, triggered by the formation of antibodies directed against PF4, a constituent of platelet alpha granules released during platelet activation. In contrast to classic HIT in which exogenous heparin triggers antibody formation, in autoimmune HIT, an endogenous polyanion triggers PF4 antibody formation.
They noted that the precise mechanism of TTS in relation to COVID-19 vaccination has not yet been established. The Global Advisory Committee on Vaccine Safety has stated that a platform-specific mechanism related to adenovirus vector vaccines cannot be excluded. Both the J&J and AstraZeneca vaccines use an adenoviral vector, but they are different; J&J uses a human vector, while AstraZeneca uses a chimpanzee vector.
They also pointed out that CVST and thrombocytopenia following SARS-CoV-2 infection has been reported in at least two cases, but HIT testing was not done in these cases. There have not so far been any reports to VAERS of CVST with thrombocytopenia following mRNA COVID-19 vaccines.
The authors said these findings have important clinical and public health implications, noting that the CDC has updated its interim clinical considerations for use of authorized COVID-19 vaccines to indicate that women aged 18-49 years should be aware of the increased risk of TTS after receipt of the J&J vaccine, and to use a nonheparin anticoagulant in suspected cases.
They noted that a subacute presentation of headache is present in 90% of patients with typical CVST. While headache is a common symptom after the J&J vaccination, most headaches begin and resolve within 2 days. Whereas in the U.S. cases of CVST after vaccination, headache symptoms began at least 6 days after vaccination and persisted for at least a week for most.
“Urgent consultation with a neurologist is prudent when a patient is suspected or confirmed to have CVST. In addition, since the median time from symptom onset to hospitalization was 7 days in the U.S. CVST case series, patient and clinician education might shorten the time to clinical evaluation and therefore treatment,” they stated.
The authors also note that VAERS is a passive surveillance system, so cases of CVST with thrombocytopenia may be underreported.
In their accompanying editorial, Dr. Karron and colleagues pointed out that, in addition to the 12 patients with CVST with thrombocytopenia described in this case series, at least three patients without CVST but meeting diagnostic criteria for TTS have been reported to VAERS (as of April 21), all in women aged 18-59 years (median age, 37 years).
The editorialists reported that the rate of CVST with thrombocytopenia after the J&J vaccine is approximately 5 per million women aged 18-50 years. This is compared with a background rate of approximately 0.05-0.13 per million per month.
They said that the availability of an interim standardized case definition of this adverse effect will facilitate prospective case ascertainment through review of large linked databases and active case finding.
This will also permit greater understanding of whether individuals who are otherwise at increased risk for hypercoagulation in general and for CVST in particular (for example, women taking hormonal contraceptive medications or who are pregnant) are also at increased risk for TTS.
Obtaining this information will support dynamic country-specific assessments of the risks of each vaccine, compared with the risk of COVID-19 disease for their populations and subpopulations, they added.
A version of this article first appeared on Medscape.com.
Breast cancer survivors have specific gynecological needs
Sexual dysfunction is a common problem among breast cancer survivors, but it’s also an issue inadequately addressed by either ob.gyns. or hematologists and oncologists, according to Erin Keyser, MD, the program director of the San Antonio Uniformed Services Health Education Consortium. Dr. Keyser discussed management of sexual dysfunction and a variety of other issues frequently faced by women who have survived breast cancer at the at the 2021 virtual meeting of the American College of Obstetricians and Gynecologists.
“Despite the fact that no specialty is better qualified to render care for this consequence of cancer treatments, many obstetrician-gynecologists feel uncomfortable or ill-equipped to address sexual pain in women affected by cancer,” Dr. Keyser quoted from a 2016 article in Obstetrics & Gynecology about the sexual health of women affected by cancer. As a breast cancer survivor herself, Dr. Keyser said hematologists and oncologists are even less equipped to discuss sexual health, “so oftentimes patients get punted between their hem-onc and their gyn,” with each telling the patient to ask the other specialist.
“There’s plenty of data in chronic health disease that maintaining sexual function for women is an indicator of the overall quality of life and that many women really don’t want to bring this up,” Dr. Keyser told attendees, so the onus is on the ob.gyn. to bring it up.
The effects of breast cancer treatment can impact women’s body image, fertility, menopause, sexual function, osteoporosis, and cardiovascular disease, but the bulk of Dr. Keyser’s talk focused on sexual health and bilateral salpingo-oophorectomy (BSO).
Lauren Streicher, MD, a clinical professor of obstetrics and gynecology at Northwestern University, Chicago, thought Dr. Keyser’s talk was useful for the general gynecologist but had some concerns about a few parts.
“She gave a very thoughtful analysis of whether someone should have their ovaries removed or not in a breast cancer diagnosis, ” Dr. Streicher said in an interview. “I would have liked to hear more about the consequences of an early menopause in women in terms of heart health, bone health, and cognitive function.”
Dr. Keyser noted that her talk pertained mostly to survivors of estrogen receptor (ER)–positive breast cancer since that population tends to struggle most with side effects of treatment. The most common medications used in this population are tamoxifen and aromatase inhibitors – such as anastrazole, letrozole, and exemestane – and these medications can affect management of different concerns.
Current guidance on ovarian removal
For women with a BRCA mutation, ACOG clinical guidance already exists regarding BSO. For other women, the complementary TEXT and SOFT trials changed the management of breast cancer treatment in premenopausal women, Dr. Keyser said.
Before these trials, postmenopausal hormone receptor–positive women began aromatase inhibitors and premenopausal HR-positive women began tamoxifen. These trials found that premenopausal women with HR-positive early breast cancer were less likely to experience recurrence when receiving adjuvant treatment with exemestane plus ovarian suppression compared to tamoxifen plus ovarian suppression. Ovarian suppression was achieved by either GnRH agonist injections, surgical removal of the ovaries, or radiation therapy to the ovaries.
The side effects of these treatments included hot flushes (92%), depression (87%), musculoskeletal symptoms (89%), vaginal dryness (52%), decreased libido (45%), dyspareunia (31%), osteoporosis (39%), insomnia (58%), and fatigue (61%). These are all quality of life concerns, Dr. Keyser said, and these findings raise questions about the consequences of long-term ovarian suppression. Findings from the Nurses’ Health Study showed that BSO before age 47.5 years resulted in lower mortality from ovarian cancer and breast cancer but was linked in women under 50 to increased all-cause mortality and mortality from coronary heart disease, lung cancer, and colorectal cancer, compared with ovarian conservation. Further, 74% of women who undergo risk-reducing BSO experience sexual dysfunction.
The bottom line, Dr. Keyser said, is that “premature removal of ovaries is not completely benign.” Her own recommendation is to follow ACOG guidance for women with BRCA mutations and, for women aged under 35 years, use ovarian suppression for 5-10 years, after which ovarian function may resume along with improved quality of life. In women aged over 40, remove ovaries since, after 5-10 years of treatment, there’s likely no benefit of retaining ovaries.
Addressing sexual health
Dyspareunia affects up to 45% of cancer survivors, Dr. Keyser said, and multiple treatment options exist for breast cancer survivors. The therapies she discussed included lubricants, moisturizers, local vaginal estrogen, DHEA, ospemifene, and CO2 laser therapy.
Though Dr. Keyser briefly touched on vaginal lubricants and moisturizers, Dr. Streicher was disappointed that Dr. Keyser did not clearly define and differentiate between lubricants and moisturizers or mention hyaluronic acid products. Dr. Streicher also disagreed with the way Dr. Keyser represented the benefits of coconut oil as a lubricant. “Oils are not condom compatible and are known to potentially increase the risk of infection, and not just from poor handwashing,” Dr. Streicher said.
Small retrospective studies support the safety of topical vaginal estrogen in breast cancer survivors, Dr. Keyser said, and the 10-mcg Vagifem tablet and vaginal estradiol ring appear to have the lowest systemic absorption. ACOG guidance recommends that women taking aromatase inhibitors who don’t respond to nonhormonal approaches may benefit from switching temporarily to tamoxifen with vaginal estrogen and then returning to aromatase inhibitors. However, Dr. Keyser said there’s plenty of data to support using vaginal estrogen in patients taking aromatase inhibitors.
“I do feel that it’s safe for patients, whether they’re on tamoxifen or aromatase inhibitors, to take vaginal estrogen,” Dr. Keyser said. “I usually stick with the estradiol vaginal ring or the estradiol tablet, and I base that on a patient’s comfort with placing and removing a ring.” She also, instead of asking the patient’s hematologist-oncologist, simply notifies them of the treatment since most hematologist-oncologists are less familiar with the data.
Another effective option is vaginal DHEA/prasterone, which can significantly improve sexual desire, arousal, pain, and overall sexual function. Although breast cancer patients were included in early studies on DHEA, Intrarosa manufacturers excluded breast cancer patients in their Food and Drug Administration application, resulting in a package stating that “use of exogenous estrogen is contraindicated in women with a known or suspected history of breast cancer” and that “Intrarosa has not been studied in women with a history of breast cancer.” While that’s true for Intrarosa specifically, DHEA has been studied in breast cancer patients, Dr. Keyser said, so she expects to see more research in this area.
Ospemifene is another option for improving vulvovaginal atrophy but cannot be taken at the same time as tamoxifen. It has similar chemopreventive effects as tamoxifen in rat studies, but it’s not as effective. It’s a reasonable option in women with refractory genitourinary syndrome of menopause (GSM) who have completed their 5-10 years of adjuvant therapy and have no history of venous thromboembolism.
Dr. Keyser said CO2 laser therapy is still being studied for treating GSM, and current data have shown benefits for dyspareunia and vaginal dryness without documented harms. There have now been randomized, controlled trials; however, since it’s not FDA approved, it’s not covered by insurance and costs approximately $5,000 for three treatments.
Dr. Streicher was glad to see Dr. Keyser’s discussion of the safety and types of local vaginal estrogen, “although she neglected to mention the 4-mcg vaginal suppository, Imvexxy, which has the lowest systemic absorption,” Dr. Streicher said. Dr. Streicher also felt the inclusion of DHEA/prasterone and ospemifene were also important, especially since the latter is “underutilized in breast cancer patients.”
The information provided on CO2 laser therapy, however, was problematic, Dr. Streicher said, given that long-term and randomized, controlled studies have now been published. Dr. Streicher also noted that two of the devices listed on the presentation slide, Thermiva and Voltiva, are radiofrequency, not laser devices.
Aside from these treatment options, the most consistent predictor of satisfying sexual experiences in women with breast cancer is the quality of their relationships, Dr. Keyser said, so couples counseling is recommended, and treatments in general are more effective with regularly sexual activity.
In discussing nonhormonal options for treating vasomotor symptoms, Dr. Keyser recommended venlafaxine, gabapentin, and low-dose paroxetine (though SSRIs and tamoxifen are contraindicated since they may reduce tamoxifen’s efficacy).
These are all off label, Dr. Streicher said it was important to note, and she would have liked to have seen a mention of the development of KNdy neurokinin disrupters along with a more in-depth discussion about which lifestyle modifications and botanicals have been shown in randomized, controlled trials to mitigate vasomotor symptoms.
Dr. Keyser wrapped up with a few additional notes and takeaways:
- The only safe reversible long-term option for contraception in HR-positive breast cancer survivors is the Paraguard IUD.
- It’s important to discuss fertility with breast cancer patients and survivors since a majority report unmet needs in this area.
- Patients taking tamoxifen need to be sure to report any vaginal spotting or bleeding since it increases risk of endometrial cancer in postmenopausal women.
- Screen for depression and anxiety.
- Ask women about sexual health and hot flashes.
- Ensure that they’re getting bone screening.
- A recommended resource is Living Beyond Breast Cancer.
Dr. Keyser had no disclosures. Dr. Streicher has consulted for Astellas Pharma and Church & Dwight, and she owns investments in InControl Medical and Sermonix Pharmaceuticals.
Sexual dysfunction is a common problem among breast cancer survivors, but it’s also an issue inadequately addressed by either ob.gyns. or hematologists and oncologists, according to Erin Keyser, MD, the program director of the San Antonio Uniformed Services Health Education Consortium. Dr. Keyser discussed management of sexual dysfunction and a variety of other issues frequently faced by women who have survived breast cancer at the at the 2021 virtual meeting of the American College of Obstetricians and Gynecologists.
“Despite the fact that no specialty is better qualified to render care for this consequence of cancer treatments, many obstetrician-gynecologists feel uncomfortable or ill-equipped to address sexual pain in women affected by cancer,” Dr. Keyser quoted from a 2016 article in Obstetrics & Gynecology about the sexual health of women affected by cancer. As a breast cancer survivor herself, Dr. Keyser said hematologists and oncologists are even less equipped to discuss sexual health, “so oftentimes patients get punted between their hem-onc and their gyn,” with each telling the patient to ask the other specialist.
“There’s plenty of data in chronic health disease that maintaining sexual function for women is an indicator of the overall quality of life and that many women really don’t want to bring this up,” Dr. Keyser told attendees, so the onus is on the ob.gyn. to bring it up.
The effects of breast cancer treatment can impact women’s body image, fertility, menopause, sexual function, osteoporosis, and cardiovascular disease, but the bulk of Dr. Keyser’s talk focused on sexual health and bilateral salpingo-oophorectomy (BSO).
Lauren Streicher, MD, a clinical professor of obstetrics and gynecology at Northwestern University, Chicago, thought Dr. Keyser’s talk was useful for the general gynecologist but had some concerns about a few parts.
“She gave a very thoughtful analysis of whether someone should have their ovaries removed or not in a breast cancer diagnosis, ” Dr. Streicher said in an interview. “I would have liked to hear more about the consequences of an early menopause in women in terms of heart health, bone health, and cognitive function.”
Dr. Keyser noted that her talk pertained mostly to survivors of estrogen receptor (ER)–positive breast cancer since that population tends to struggle most with side effects of treatment. The most common medications used in this population are tamoxifen and aromatase inhibitors – such as anastrazole, letrozole, and exemestane – and these medications can affect management of different concerns.
Current guidance on ovarian removal
For women with a BRCA mutation, ACOG clinical guidance already exists regarding BSO. For other women, the complementary TEXT and SOFT trials changed the management of breast cancer treatment in premenopausal women, Dr. Keyser said.
Before these trials, postmenopausal hormone receptor–positive women began aromatase inhibitors and premenopausal HR-positive women began tamoxifen. These trials found that premenopausal women with HR-positive early breast cancer were less likely to experience recurrence when receiving adjuvant treatment with exemestane plus ovarian suppression compared to tamoxifen plus ovarian suppression. Ovarian suppression was achieved by either GnRH agonist injections, surgical removal of the ovaries, or radiation therapy to the ovaries.
The side effects of these treatments included hot flushes (92%), depression (87%), musculoskeletal symptoms (89%), vaginal dryness (52%), decreased libido (45%), dyspareunia (31%), osteoporosis (39%), insomnia (58%), and fatigue (61%). These are all quality of life concerns, Dr. Keyser said, and these findings raise questions about the consequences of long-term ovarian suppression. Findings from the Nurses’ Health Study showed that BSO before age 47.5 years resulted in lower mortality from ovarian cancer and breast cancer but was linked in women under 50 to increased all-cause mortality and mortality from coronary heart disease, lung cancer, and colorectal cancer, compared with ovarian conservation. Further, 74% of women who undergo risk-reducing BSO experience sexual dysfunction.
The bottom line, Dr. Keyser said, is that “premature removal of ovaries is not completely benign.” Her own recommendation is to follow ACOG guidance for women with BRCA mutations and, for women aged under 35 years, use ovarian suppression for 5-10 years, after which ovarian function may resume along with improved quality of life. In women aged over 40, remove ovaries since, after 5-10 years of treatment, there’s likely no benefit of retaining ovaries.
Addressing sexual health
Dyspareunia affects up to 45% of cancer survivors, Dr. Keyser said, and multiple treatment options exist for breast cancer survivors. The therapies she discussed included lubricants, moisturizers, local vaginal estrogen, DHEA, ospemifene, and CO2 laser therapy.
Though Dr. Keyser briefly touched on vaginal lubricants and moisturizers, Dr. Streicher was disappointed that Dr. Keyser did not clearly define and differentiate between lubricants and moisturizers or mention hyaluronic acid products. Dr. Streicher also disagreed with the way Dr. Keyser represented the benefits of coconut oil as a lubricant. “Oils are not condom compatible and are known to potentially increase the risk of infection, and not just from poor handwashing,” Dr. Streicher said.
Small retrospective studies support the safety of topical vaginal estrogen in breast cancer survivors, Dr. Keyser said, and the 10-mcg Vagifem tablet and vaginal estradiol ring appear to have the lowest systemic absorption. ACOG guidance recommends that women taking aromatase inhibitors who don’t respond to nonhormonal approaches may benefit from switching temporarily to tamoxifen with vaginal estrogen and then returning to aromatase inhibitors. However, Dr. Keyser said there’s plenty of data to support using vaginal estrogen in patients taking aromatase inhibitors.
“I do feel that it’s safe for patients, whether they’re on tamoxifen or aromatase inhibitors, to take vaginal estrogen,” Dr. Keyser said. “I usually stick with the estradiol vaginal ring or the estradiol tablet, and I base that on a patient’s comfort with placing and removing a ring.” She also, instead of asking the patient’s hematologist-oncologist, simply notifies them of the treatment since most hematologist-oncologists are less familiar with the data.
Another effective option is vaginal DHEA/prasterone, which can significantly improve sexual desire, arousal, pain, and overall sexual function. Although breast cancer patients were included in early studies on DHEA, Intrarosa manufacturers excluded breast cancer patients in their Food and Drug Administration application, resulting in a package stating that “use of exogenous estrogen is contraindicated in women with a known or suspected history of breast cancer” and that “Intrarosa has not been studied in women with a history of breast cancer.” While that’s true for Intrarosa specifically, DHEA has been studied in breast cancer patients, Dr. Keyser said, so she expects to see more research in this area.
Ospemifene is another option for improving vulvovaginal atrophy but cannot be taken at the same time as tamoxifen. It has similar chemopreventive effects as tamoxifen in rat studies, but it’s not as effective. It’s a reasonable option in women with refractory genitourinary syndrome of menopause (GSM) who have completed their 5-10 years of adjuvant therapy and have no history of venous thromboembolism.
Dr. Keyser said CO2 laser therapy is still being studied for treating GSM, and current data have shown benefits for dyspareunia and vaginal dryness without documented harms. There have now been randomized, controlled trials; however, since it’s not FDA approved, it’s not covered by insurance and costs approximately $5,000 for three treatments.
Dr. Streicher was glad to see Dr. Keyser’s discussion of the safety and types of local vaginal estrogen, “although she neglected to mention the 4-mcg vaginal suppository, Imvexxy, which has the lowest systemic absorption,” Dr. Streicher said. Dr. Streicher also felt the inclusion of DHEA/prasterone and ospemifene were also important, especially since the latter is “underutilized in breast cancer patients.”
The information provided on CO2 laser therapy, however, was problematic, Dr. Streicher said, given that long-term and randomized, controlled studies have now been published. Dr. Streicher also noted that two of the devices listed on the presentation slide, Thermiva and Voltiva, are radiofrequency, not laser devices.
Aside from these treatment options, the most consistent predictor of satisfying sexual experiences in women with breast cancer is the quality of their relationships, Dr. Keyser said, so couples counseling is recommended, and treatments in general are more effective with regularly sexual activity.
In discussing nonhormonal options for treating vasomotor symptoms, Dr. Keyser recommended venlafaxine, gabapentin, and low-dose paroxetine (though SSRIs and tamoxifen are contraindicated since they may reduce tamoxifen’s efficacy).
These are all off label, Dr. Streicher said it was important to note, and she would have liked to have seen a mention of the development of KNdy neurokinin disrupters along with a more in-depth discussion about which lifestyle modifications and botanicals have been shown in randomized, controlled trials to mitigate vasomotor symptoms.
Dr. Keyser wrapped up with a few additional notes and takeaways:
- The only safe reversible long-term option for contraception in HR-positive breast cancer survivors is the Paraguard IUD.
- It’s important to discuss fertility with breast cancer patients and survivors since a majority report unmet needs in this area.
- Patients taking tamoxifen need to be sure to report any vaginal spotting or bleeding since it increases risk of endometrial cancer in postmenopausal women.
- Screen for depression and anxiety.
- Ask women about sexual health and hot flashes.
- Ensure that they’re getting bone screening.
- A recommended resource is Living Beyond Breast Cancer.
Dr. Keyser had no disclosures. Dr. Streicher has consulted for Astellas Pharma and Church & Dwight, and she owns investments in InControl Medical and Sermonix Pharmaceuticals.
Sexual dysfunction is a common problem among breast cancer survivors, but it’s also an issue inadequately addressed by either ob.gyns. or hematologists and oncologists, according to Erin Keyser, MD, the program director of the San Antonio Uniformed Services Health Education Consortium. Dr. Keyser discussed management of sexual dysfunction and a variety of other issues frequently faced by women who have survived breast cancer at the at the 2021 virtual meeting of the American College of Obstetricians and Gynecologists.
“Despite the fact that no specialty is better qualified to render care for this consequence of cancer treatments, many obstetrician-gynecologists feel uncomfortable or ill-equipped to address sexual pain in women affected by cancer,” Dr. Keyser quoted from a 2016 article in Obstetrics & Gynecology about the sexual health of women affected by cancer. As a breast cancer survivor herself, Dr. Keyser said hematologists and oncologists are even less equipped to discuss sexual health, “so oftentimes patients get punted between their hem-onc and their gyn,” with each telling the patient to ask the other specialist.
“There’s plenty of data in chronic health disease that maintaining sexual function for women is an indicator of the overall quality of life and that many women really don’t want to bring this up,” Dr. Keyser told attendees, so the onus is on the ob.gyn. to bring it up.
The effects of breast cancer treatment can impact women’s body image, fertility, menopause, sexual function, osteoporosis, and cardiovascular disease, but the bulk of Dr. Keyser’s talk focused on sexual health and bilateral salpingo-oophorectomy (BSO).
Lauren Streicher, MD, a clinical professor of obstetrics and gynecology at Northwestern University, Chicago, thought Dr. Keyser’s talk was useful for the general gynecologist but had some concerns about a few parts.
“She gave a very thoughtful analysis of whether someone should have their ovaries removed or not in a breast cancer diagnosis, ” Dr. Streicher said in an interview. “I would have liked to hear more about the consequences of an early menopause in women in terms of heart health, bone health, and cognitive function.”
Dr. Keyser noted that her talk pertained mostly to survivors of estrogen receptor (ER)–positive breast cancer since that population tends to struggle most with side effects of treatment. The most common medications used in this population are tamoxifen and aromatase inhibitors – such as anastrazole, letrozole, and exemestane – and these medications can affect management of different concerns.
Current guidance on ovarian removal
For women with a BRCA mutation, ACOG clinical guidance already exists regarding BSO. For other women, the complementary TEXT and SOFT trials changed the management of breast cancer treatment in premenopausal women, Dr. Keyser said.
Before these trials, postmenopausal hormone receptor–positive women began aromatase inhibitors and premenopausal HR-positive women began tamoxifen. These trials found that premenopausal women with HR-positive early breast cancer were less likely to experience recurrence when receiving adjuvant treatment with exemestane plus ovarian suppression compared to tamoxifen plus ovarian suppression. Ovarian suppression was achieved by either GnRH agonist injections, surgical removal of the ovaries, or radiation therapy to the ovaries.
The side effects of these treatments included hot flushes (92%), depression (87%), musculoskeletal symptoms (89%), vaginal dryness (52%), decreased libido (45%), dyspareunia (31%), osteoporosis (39%), insomnia (58%), and fatigue (61%). These are all quality of life concerns, Dr. Keyser said, and these findings raise questions about the consequences of long-term ovarian suppression. Findings from the Nurses’ Health Study showed that BSO before age 47.5 years resulted in lower mortality from ovarian cancer and breast cancer but was linked in women under 50 to increased all-cause mortality and mortality from coronary heart disease, lung cancer, and colorectal cancer, compared with ovarian conservation. Further, 74% of women who undergo risk-reducing BSO experience sexual dysfunction.
The bottom line, Dr. Keyser said, is that “premature removal of ovaries is not completely benign.” Her own recommendation is to follow ACOG guidance for women with BRCA mutations and, for women aged under 35 years, use ovarian suppression for 5-10 years, after which ovarian function may resume along with improved quality of life. In women aged over 40, remove ovaries since, after 5-10 years of treatment, there’s likely no benefit of retaining ovaries.
Addressing sexual health
Dyspareunia affects up to 45% of cancer survivors, Dr. Keyser said, and multiple treatment options exist for breast cancer survivors. The therapies she discussed included lubricants, moisturizers, local vaginal estrogen, DHEA, ospemifene, and CO2 laser therapy.
Though Dr. Keyser briefly touched on vaginal lubricants and moisturizers, Dr. Streicher was disappointed that Dr. Keyser did not clearly define and differentiate between lubricants and moisturizers or mention hyaluronic acid products. Dr. Streicher also disagreed with the way Dr. Keyser represented the benefits of coconut oil as a lubricant. “Oils are not condom compatible and are known to potentially increase the risk of infection, and not just from poor handwashing,” Dr. Streicher said.
Small retrospective studies support the safety of topical vaginal estrogen in breast cancer survivors, Dr. Keyser said, and the 10-mcg Vagifem tablet and vaginal estradiol ring appear to have the lowest systemic absorption. ACOG guidance recommends that women taking aromatase inhibitors who don’t respond to nonhormonal approaches may benefit from switching temporarily to tamoxifen with vaginal estrogen and then returning to aromatase inhibitors. However, Dr. Keyser said there’s plenty of data to support using vaginal estrogen in patients taking aromatase inhibitors.
“I do feel that it’s safe for patients, whether they’re on tamoxifen or aromatase inhibitors, to take vaginal estrogen,” Dr. Keyser said. “I usually stick with the estradiol vaginal ring or the estradiol tablet, and I base that on a patient’s comfort with placing and removing a ring.” She also, instead of asking the patient’s hematologist-oncologist, simply notifies them of the treatment since most hematologist-oncologists are less familiar with the data.
Another effective option is vaginal DHEA/prasterone, which can significantly improve sexual desire, arousal, pain, and overall sexual function. Although breast cancer patients were included in early studies on DHEA, Intrarosa manufacturers excluded breast cancer patients in their Food and Drug Administration application, resulting in a package stating that “use of exogenous estrogen is contraindicated in women with a known or suspected history of breast cancer” and that “Intrarosa has not been studied in women with a history of breast cancer.” While that’s true for Intrarosa specifically, DHEA has been studied in breast cancer patients, Dr. Keyser said, so she expects to see more research in this area.
Ospemifene is another option for improving vulvovaginal atrophy but cannot be taken at the same time as tamoxifen. It has similar chemopreventive effects as tamoxifen in rat studies, but it’s not as effective. It’s a reasonable option in women with refractory genitourinary syndrome of menopause (GSM) who have completed their 5-10 years of adjuvant therapy and have no history of venous thromboembolism.
Dr. Keyser said CO2 laser therapy is still being studied for treating GSM, and current data have shown benefits for dyspareunia and vaginal dryness without documented harms. There have now been randomized, controlled trials; however, since it’s not FDA approved, it’s not covered by insurance and costs approximately $5,000 for three treatments.
Dr. Streicher was glad to see Dr. Keyser’s discussion of the safety and types of local vaginal estrogen, “although she neglected to mention the 4-mcg vaginal suppository, Imvexxy, which has the lowest systemic absorption,” Dr. Streicher said. Dr. Streicher also felt the inclusion of DHEA/prasterone and ospemifene were also important, especially since the latter is “underutilized in breast cancer patients.”
The information provided on CO2 laser therapy, however, was problematic, Dr. Streicher said, given that long-term and randomized, controlled studies have now been published. Dr. Streicher also noted that two of the devices listed on the presentation slide, Thermiva and Voltiva, are radiofrequency, not laser devices.
Aside from these treatment options, the most consistent predictor of satisfying sexual experiences in women with breast cancer is the quality of their relationships, Dr. Keyser said, so couples counseling is recommended, and treatments in general are more effective with regularly sexual activity.
In discussing nonhormonal options for treating vasomotor symptoms, Dr. Keyser recommended venlafaxine, gabapentin, and low-dose paroxetine (though SSRIs and tamoxifen are contraindicated since they may reduce tamoxifen’s efficacy).
These are all off label, Dr. Streicher said it was important to note, and she would have liked to have seen a mention of the development of KNdy neurokinin disrupters along with a more in-depth discussion about which lifestyle modifications and botanicals have been shown in randomized, controlled trials to mitigate vasomotor symptoms.
Dr. Keyser wrapped up with a few additional notes and takeaways:
- The only safe reversible long-term option for contraception in HR-positive breast cancer survivors is the Paraguard IUD.
- It’s important to discuss fertility with breast cancer patients and survivors since a majority report unmet needs in this area.
- Patients taking tamoxifen need to be sure to report any vaginal spotting or bleeding since it increases risk of endometrial cancer in postmenopausal women.
- Screen for depression and anxiety.
- Ask women about sexual health and hot flashes.
- Ensure that they’re getting bone screening.
- A recommended resource is Living Beyond Breast Cancer.
Dr. Keyser had no disclosures. Dr. Streicher has consulted for Astellas Pharma and Church & Dwight, and she owns investments in InControl Medical and Sermonix Pharmaceuticals.
FROM ACOG 2021
For cervical cancer screening, any strategy is acceptable
Cytology testing every 3 years, cytology/human papillomavirus cotesting every 5 years, and primary HPV testing every 5 years are similarly effective at reducing cervical cancer risk, said Rachel P. Brook, MD, of the University of California, Los Angeles Health Iris Cantor Women’s Health Center, during a presentation at the annual meeting of the American College of Physicians.
“The most important thing a primary care provider can do is to screen with whatever test is most accessible,” Dr. Brook said in an interview. She also noted that access to screening remains a pressing concern, particularly among underrepresented groups and women in rural areas. Even when women can access testing, follow-up after abnormal results can be inadequate, leading to increased risk of cervical cancer mortality.
To address some of these shortcomings, Dr. Brook provided an overview of current guidelines and appropriate responses to abnormal test results.
First, during her presentation, she noted that guideline recommendations do not apply to patients with additional risk factors, including a compromised immune system, HIV infection, previous treatment of cervical cancer or a high-grade cancerous lesion, or in utero exposure to diethylstilbestrol.
“This is very important,” Dr. Brook said during her presentation. “They should receive individualized care due to their above average risk of cervical cancer.”
Among women with average risk, both the USPSTF 2018 guideline and the ACS 2020 guideline recommend against screening women aged less than 21 years.
In a major change to the most recent ACS guideline, screening women aged 21-24 years is no longer recommended, in contrast with the USPSTF guideline, which still calls for cytology every 3 years for this age group. This recommendation by the USPSTF extends to women aged 25-29 years, a group for which the ACS recommends primary HPV testing every 5 years, cytology/HPV cotesting every 5 years, or cytology testing every 3 years. For both organizations, any of these three testing methods is recommended for women aged 30-65 years, followed by discontinuation of testing after 65 years, given adequate prior screening.
“For all these recommendations and guidelines, they’re pertinent to patients regardless of HPV vaccination status,” Dr. Brook said. But she added that increased rates of HPV vaccination may affect future screening guidelines, as vaccinated patients are more likely to have false positive cytology results because of low-risk HPV strains. This trend may steer future recommendations toward primary HPV testing, Dr. Brook said.
Presently, for applicable age groups, the ACS guideline favors HPV testing alone over cytology alone or cotesting, whereas the USPSTF guideline offers no preference between the three testing strategies.
Primary HPV vs. cytology testing
Dr. Brook said a single negative HPV test provides more than 95% assurance that a patient will not develop cervical cancer or a cancer precursor within the next 5 years. One negative HPV test offers similar reliability to about 3 negative cytology tests.
Switching to a 5-year testing cycle may be unsettling for patients who are used to getting a Pap test every year, but having a conversation about test accuracy can help assuage patient concerns, she said.
Still, Dr. Brook emphasized that any of the three testing strategies is ultimately acceptable.
“The take-home message here is – truly – that any of the recommended screening options will greatly reduce cervical cancer risk,” Dr. Brook said. “So, screen. And if there is any confusion or concern with your patients about which [screening strategy to use], just help them decide on any of the three. But please screen.”
Self-swabbing could improve screening in certain groups
To improve screening rates, particularly for women with poor access and those averse to a speculum exam, Dr. Brook highlighted self-swabbing primary HPV tests, which may soon be available. While no self-swabbing HPV tests are yet approved by the Food and Drug Administration, they offer a 76% sensitivity rate for cervical intraepithelial neoplasia grade 2, and a rate of 85% for CIN3, compared with 91% for physician-collected samples.
Regardless of the exact HPV test, Dr. Brook advised appropriate reflex testing.
“We need to make sure all primary HPV screening tests positive for types other than HPV-16 or -18 will require additional reflex triage testing with cytology,” Dr. Brook said in interview. “If not – if a woman has a primary HPV screening test that is positive and I cannot perform reflex cytology – I have to bring her back for an additional test and speculum exam to get cytology, which is an unnecessary burden to the patient, and also increases testing.”
Kathy L. MacLaughlin, MD, associate professor of family medicine at Mayo Clinic, Rochester, Minn., said this is one drawback to self-swabbing tests in an interview.
“If there is a positive HPV result [with a self-swabbing test], the patient will need to have a clinic appointment for Pap collection [if one of the ‘other’ 12 HPV types are identified], or be referred for a colposcopy [if HPV types 16 or 18 are identified],” Dr. MacLaughlin said. “There need to be plans in place for access to those services.”
Incidentally, it may be women who face barriers to access that need self-swabbing HPV tests the most, according to Dr. MacLaughlin.
“I think there is significant potential to improve screening rates among never-screened and underscreened women and those are the groups for whom this makes the most sense,” she said. “I don’t think anyone is suggesting that women who have the means and interest in scheduling a face-to-face visit for clinician-collected screening switch to self-screening, but it is a promising option [once FDA approved] for reaching other women and reducing disparities in screening rates.”
Dr. MacLaughlin suggested that self-screening programs could operate outside of normal business hours in a variety of settings, such as homes, community centers, and churches.
Until self-screening is an option, Dr. MacLaughlin agreed with Dr. Brook that any of the three testing strategies is suitable for screening, and recommended that primary care providers seize the opportunities presented to them.
“Individual primary care providers can improve screening rates by offering to update cervical cancer screening at a clinic appointment even if that was not the primary indication for the visit, especially for women who are long overdue,” Dr. MacLaughlin said. “If there is just no time to fit in the screening or the patient declines, then order a return visit and have the patient stop at the appointment desk as they leave.”
“I recognize we are asked to fit in more and more in less time, but I’ve found this to be effective when I have capacity in the clinic day to offer it,” she added.
Dr. Brook and Dr. MacLaughlin reported no conflicts of interest.
Cytology testing every 3 years, cytology/human papillomavirus cotesting every 5 years, and primary HPV testing every 5 years are similarly effective at reducing cervical cancer risk, said Rachel P. Brook, MD, of the University of California, Los Angeles Health Iris Cantor Women’s Health Center, during a presentation at the annual meeting of the American College of Physicians.
“The most important thing a primary care provider can do is to screen with whatever test is most accessible,” Dr. Brook said in an interview. She also noted that access to screening remains a pressing concern, particularly among underrepresented groups and women in rural areas. Even when women can access testing, follow-up after abnormal results can be inadequate, leading to increased risk of cervical cancer mortality.
To address some of these shortcomings, Dr. Brook provided an overview of current guidelines and appropriate responses to abnormal test results.
First, during her presentation, she noted that guideline recommendations do not apply to patients with additional risk factors, including a compromised immune system, HIV infection, previous treatment of cervical cancer or a high-grade cancerous lesion, or in utero exposure to diethylstilbestrol.
“This is very important,” Dr. Brook said during her presentation. “They should receive individualized care due to their above average risk of cervical cancer.”
Among women with average risk, both the USPSTF 2018 guideline and the ACS 2020 guideline recommend against screening women aged less than 21 years.
In a major change to the most recent ACS guideline, screening women aged 21-24 years is no longer recommended, in contrast with the USPSTF guideline, which still calls for cytology every 3 years for this age group. This recommendation by the USPSTF extends to women aged 25-29 years, a group for which the ACS recommends primary HPV testing every 5 years, cytology/HPV cotesting every 5 years, or cytology testing every 3 years. For both organizations, any of these three testing methods is recommended for women aged 30-65 years, followed by discontinuation of testing after 65 years, given adequate prior screening.
“For all these recommendations and guidelines, they’re pertinent to patients regardless of HPV vaccination status,” Dr. Brook said. But she added that increased rates of HPV vaccination may affect future screening guidelines, as vaccinated patients are more likely to have false positive cytology results because of low-risk HPV strains. This trend may steer future recommendations toward primary HPV testing, Dr. Brook said.
Presently, for applicable age groups, the ACS guideline favors HPV testing alone over cytology alone or cotesting, whereas the USPSTF guideline offers no preference between the three testing strategies.
Primary HPV vs. cytology testing
Dr. Brook said a single negative HPV test provides more than 95% assurance that a patient will not develop cervical cancer or a cancer precursor within the next 5 years. One negative HPV test offers similar reliability to about 3 negative cytology tests.
Switching to a 5-year testing cycle may be unsettling for patients who are used to getting a Pap test every year, but having a conversation about test accuracy can help assuage patient concerns, she said.
Still, Dr. Brook emphasized that any of the three testing strategies is ultimately acceptable.
“The take-home message here is – truly – that any of the recommended screening options will greatly reduce cervical cancer risk,” Dr. Brook said. “So, screen. And if there is any confusion or concern with your patients about which [screening strategy to use], just help them decide on any of the three. But please screen.”
Self-swabbing could improve screening in certain groups
To improve screening rates, particularly for women with poor access and those averse to a speculum exam, Dr. Brook highlighted self-swabbing primary HPV tests, which may soon be available. While no self-swabbing HPV tests are yet approved by the Food and Drug Administration, they offer a 76% sensitivity rate for cervical intraepithelial neoplasia grade 2, and a rate of 85% for CIN3, compared with 91% for physician-collected samples.
Regardless of the exact HPV test, Dr. Brook advised appropriate reflex testing.
“We need to make sure all primary HPV screening tests positive for types other than HPV-16 or -18 will require additional reflex triage testing with cytology,” Dr. Brook said in interview. “If not – if a woman has a primary HPV screening test that is positive and I cannot perform reflex cytology – I have to bring her back for an additional test and speculum exam to get cytology, which is an unnecessary burden to the patient, and also increases testing.”
Kathy L. MacLaughlin, MD, associate professor of family medicine at Mayo Clinic, Rochester, Minn., said this is one drawback to self-swabbing tests in an interview.
“If there is a positive HPV result [with a self-swabbing test], the patient will need to have a clinic appointment for Pap collection [if one of the ‘other’ 12 HPV types are identified], or be referred for a colposcopy [if HPV types 16 or 18 are identified],” Dr. MacLaughlin said. “There need to be plans in place for access to those services.”
Incidentally, it may be women who face barriers to access that need self-swabbing HPV tests the most, according to Dr. MacLaughlin.
“I think there is significant potential to improve screening rates among never-screened and underscreened women and those are the groups for whom this makes the most sense,” she said. “I don’t think anyone is suggesting that women who have the means and interest in scheduling a face-to-face visit for clinician-collected screening switch to self-screening, but it is a promising option [once FDA approved] for reaching other women and reducing disparities in screening rates.”
Dr. MacLaughlin suggested that self-screening programs could operate outside of normal business hours in a variety of settings, such as homes, community centers, and churches.
Until self-screening is an option, Dr. MacLaughlin agreed with Dr. Brook that any of the three testing strategies is suitable for screening, and recommended that primary care providers seize the opportunities presented to them.
“Individual primary care providers can improve screening rates by offering to update cervical cancer screening at a clinic appointment even if that was not the primary indication for the visit, especially for women who are long overdue,” Dr. MacLaughlin said. “If there is just no time to fit in the screening or the patient declines, then order a return visit and have the patient stop at the appointment desk as they leave.”
“I recognize we are asked to fit in more and more in less time, but I’ve found this to be effective when I have capacity in the clinic day to offer it,” she added.
Dr. Brook and Dr. MacLaughlin reported no conflicts of interest.
Cytology testing every 3 years, cytology/human papillomavirus cotesting every 5 years, and primary HPV testing every 5 years are similarly effective at reducing cervical cancer risk, said Rachel P. Brook, MD, of the University of California, Los Angeles Health Iris Cantor Women’s Health Center, during a presentation at the annual meeting of the American College of Physicians.
“The most important thing a primary care provider can do is to screen with whatever test is most accessible,” Dr. Brook said in an interview. She also noted that access to screening remains a pressing concern, particularly among underrepresented groups and women in rural areas. Even when women can access testing, follow-up after abnormal results can be inadequate, leading to increased risk of cervical cancer mortality.
To address some of these shortcomings, Dr. Brook provided an overview of current guidelines and appropriate responses to abnormal test results.
First, during her presentation, she noted that guideline recommendations do not apply to patients with additional risk factors, including a compromised immune system, HIV infection, previous treatment of cervical cancer or a high-grade cancerous lesion, or in utero exposure to diethylstilbestrol.
“This is very important,” Dr. Brook said during her presentation. “They should receive individualized care due to their above average risk of cervical cancer.”
Among women with average risk, both the USPSTF 2018 guideline and the ACS 2020 guideline recommend against screening women aged less than 21 years.
In a major change to the most recent ACS guideline, screening women aged 21-24 years is no longer recommended, in contrast with the USPSTF guideline, which still calls for cytology every 3 years for this age group. This recommendation by the USPSTF extends to women aged 25-29 years, a group for which the ACS recommends primary HPV testing every 5 years, cytology/HPV cotesting every 5 years, or cytology testing every 3 years. For both organizations, any of these three testing methods is recommended for women aged 30-65 years, followed by discontinuation of testing after 65 years, given adequate prior screening.
“For all these recommendations and guidelines, they’re pertinent to patients regardless of HPV vaccination status,” Dr. Brook said. But she added that increased rates of HPV vaccination may affect future screening guidelines, as vaccinated patients are more likely to have false positive cytology results because of low-risk HPV strains. This trend may steer future recommendations toward primary HPV testing, Dr. Brook said.
Presently, for applicable age groups, the ACS guideline favors HPV testing alone over cytology alone or cotesting, whereas the USPSTF guideline offers no preference between the three testing strategies.
Primary HPV vs. cytology testing
Dr. Brook said a single negative HPV test provides more than 95% assurance that a patient will not develop cervical cancer or a cancer precursor within the next 5 years. One negative HPV test offers similar reliability to about 3 negative cytology tests.
Switching to a 5-year testing cycle may be unsettling for patients who are used to getting a Pap test every year, but having a conversation about test accuracy can help assuage patient concerns, she said.
Still, Dr. Brook emphasized that any of the three testing strategies is ultimately acceptable.
“The take-home message here is – truly – that any of the recommended screening options will greatly reduce cervical cancer risk,” Dr. Brook said. “So, screen. And if there is any confusion or concern with your patients about which [screening strategy to use], just help them decide on any of the three. But please screen.”
Self-swabbing could improve screening in certain groups
To improve screening rates, particularly for women with poor access and those averse to a speculum exam, Dr. Brook highlighted self-swabbing primary HPV tests, which may soon be available. While no self-swabbing HPV tests are yet approved by the Food and Drug Administration, they offer a 76% sensitivity rate for cervical intraepithelial neoplasia grade 2, and a rate of 85% for CIN3, compared with 91% for physician-collected samples.
Regardless of the exact HPV test, Dr. Brook advised appropriate reflex testing.
“We need to make sure all primary HPV screening tests positive for types other than HPV-16 or -18 will require additional reflex triage testing with cytology,” Dr. Brook said in interview. “If not – if a woman has a primary HPV screening test that is positive and I cannot perform reflex cytology – I have to bring her back for an additional test and speculum exam to get cytology, which is an unnecessary burden to the patient, and also increases testing.”
Kathy L. MacLaughlin, MD, associate professor of family medicine at Mayo Clinic, Rochester, Minn., said this is one drawback to self-swabbing tests in an interview.
“If there is a positive HPV result [with a self-swabbing test], the patient will need to have a clinic appointment for Pap collection [if one of the ‘other’ 12 HPV types are identified], or be referred for a colposcopy [if HPV types 16 or 18 are identified],” Dr. MacLaughlin said. “There need to be plans in place for access to those services.”
Incidentally, it may be women who face barriers to access that need self-swabbing HPV tests the most, according to Dr. MacLaughlin.
“I think there is significant potential to improve screening rates among never-screened and underscreened women and those are the groups for whom this makes the most sense,” she said. “I don’t think anyone is suggesting that women who have the means and interest in scheduling a face-to-face visit for clinician-collected screening switch to self-screening, but it is a promising option [once FDA approved] for reaching other women and reducing disparities in screening rates.”
Dr. MacLaughlin suggested that self-screening programs could operate outside of normal business hours in a variety of settings, such as homes, community centers, and churches.
Until self-screening is an option, Dr. MacLaughlin agreed with Dr. Brook that any of the three testing strategies is suitable for screening, and recommended that primary care providers seize the opportunities presented to them.
“Individual primary care providers can improve screening rates by offering to update cervical cancer screening at a clinic appointment even if that was not the primary indication for the visit, especially for women who are long overdue,” Dr. MacLaughlin said. “If there is just no time to fit in the screening or the patient declines, then order a return visit and have the patient stop at the appointment desk as they leave.”
“I recognize we are asked to fit in more and more in less time, but I’ve found this to be effective when I have capacity in the clinic day to offer it,” she added.
Dr. Brook and Dr. MacLaughlin reported no conflicts of interest.
FROM INTERNAL MEDICINE 2021
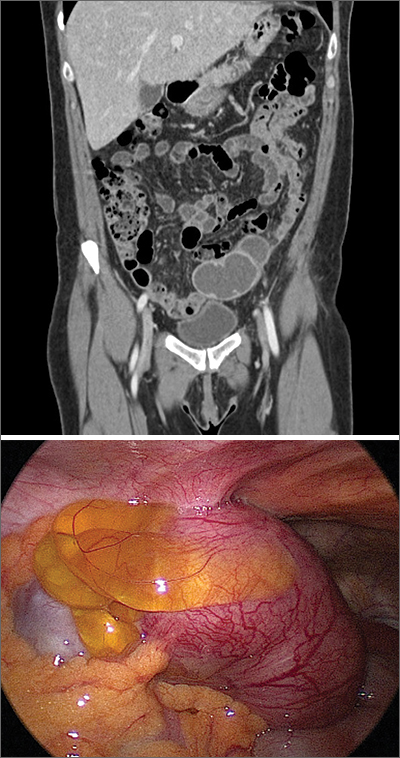
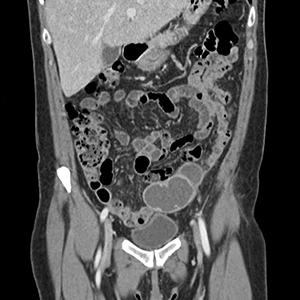
Perimenopausal woman with adnexal mass
The presence and location of this mass, paired with the patient’s symptoms, led to the diagnosis of pelvic inflammatory disease (PID).
PID is an acute infection of the upper genital tract in women that is thought to be due to an ascending infection from the lower genital tract. Diagnosis of PID in middle-aged women is a challenge, given the broad differential diagnosis of nonspecific presenting symptoms, lower index of suspicion in this age group, and unknown exact incidence of PID in postmenopausal women. Delay in diagnosis in postmenopausal women can pose serious potential complications such as tubo-ovarian abscess (TOA)—as was seen with this patient—and concurrent gynecologic malignancy found on pathology of TOA specimens.
Risk factors for PID in the postmenopausal population include recent uterine instrumentation, history of prior PID, and structural abnormalities such as cervical stenosis, uterine anatomic abnormalities, or tubal disease.
The Centers for Disease Control and Prevention (CDC) 2015 Sexually Transmitted Diseases Treatment Guidelines recommend presumptive treatment for PID in women with pelvic or lower abdominal pain with 1 or more of the following clinical criteria: cervical motion tenderness, uterine tenderness, or adnexal tenderness. The CDC also suggests that the most specific criteria for PID include endometrial biopsy consistent with endometritis, imaging (transvaginal ultrasound or magnetic resonance imaging) demonstrating fluid-filled tubes, or laparoscopic findings consistent with PID.
Due to the polymicrobial nature of PID, antibiotics should cover not only gonorrhea and chlamydia but also anaerobic pathogens. CDC guidelines recommend the following treatment:
- intravenous (IV) cefotetan (2 g bid) plus doxycycline (100 mg PO or IV bid),
- IV cefoxitin (2 g qid) plus doxycycline (100 mg PO or IV bid), or
- IV clindamycin (900 mg tid) plus IV or intramuscular gentamicin loading dose (2 mg/kg) followed by a maintenance dose (1.5 mg/kg tid).
Due to the increased risk of malignancy in postmenopausal women with TOA, surgical intervention may be needed.
This patient underwent diagnostic laparoscopy, hysterectomy, left salpingo-oophorectomy, and right salpingectomy (with her right ovary left in place due to her perimenopausal status). Intraoperatively, she was found to have cervical stenosis. Postoperatively, she improved on IV cefoxitin (2 g qid) and IV doxycycline (100 mg bid), which was eventually transitioned to oral doxycycline (100 mg bid) and metronidazole (500 mg bid) on discharge. She made a full recovery and is doing well.
This case was adapted from: Khoo CP. Fever, abdominal pain, and adnexal mass. J Fam Pract. 2020;69:101-103
The presence and location of this mass, paired with the patient’s symptoms, led to the diagnosis of pelvic inflammatory disease (PID).
PID is an acute infection of the upper genital tract in women that is thought to be due to an ascending infection from the lower genital tract. Diagnosis of PID in middle-aged women is a challenge, given the broad differential diagnosis of nonspecific presenting symptoms, lower index of suspicion in this age group, and unknown exact incidence of PID in postmenopausal women. Delay in diagnosis in postmenopausal women can pose serious potential complications such as tubo-ovarian abscess (TOA)—as was seen with this patient—and concurrent gynecologic malignancy found on pathology of TOA specimens.
Risk factors for PID in the postmenopausal population include recent uterine instrumentation, history of prior PID, and structural abnormalities such as cervical stenosis, uterine anatomic abnormalities, or tubal disease.
The Centers for Disease Control and Prevention (CDC) 2015 Sexually Transmitted Diseases Treatment Guidelines recommend presumptive treatment for PID in women with pelvic or lower abdominal pain with 1 or more of the following clinical criteria: cervical motion tenderness, uterine tenderness, or adnexal tenderness. The CDC also suggests that the most specific criteria for PID include endometrial biopsy consistent with endometritis, imaging (transvaginal ultrasound or magnetic resonance imaging) demonstrating fluid-filled tubes, or laparoscopic findings consistent with PID.
Due to the polymicrobial nature of PID, antibiotics should cover not only gonorrhea and chlamydia but also anaerobic pathogens. CDC guidelines recommend the following treatment:
- intravenous (IV) cefotetan (2 g bid) plus doxycycline (100 mg PO or IV bid),
- IV cefoxitin (2 g qid) plus doxycycline (100 mg PO or IV bid), or
- IV clindamycin (900 mg tid) plus IV or intramuscular gentamicin loading dose (2 mg/kg) followed by a maintenance dose (1.5 mg/kg tid).
Due to the increased risk of malignancy in postmenopausal women with TOA, surgical intervention may be needed.
This patient underwent diagnostic laparoscopy, hysterectomy, left salpingo-oophorectomy, and right salpingectomy (with her right ovary left in place due to her perimenopausal status). Intraoperatively, she was found to have cervical stenosis. Postoperatively, she improved on IV cefoxitin (2 g qid) and IV doxycycline (100 mg bid), which was eventually transitioned to oral doxycycline (100 mg bid) and metronidazole (500 mg bid) on discharge. She made a full recovery and is doing well.
This case was adapted from: Khoo CP. Fever, abdominal pain, and adnexal mass. J Fam Pract. 2020;69:101-103
The presence and location of this mass, paired with the patient’s symptoms, led to the diagnosis of pelvic inflammatory disease (PID).
PID is an acute infection of the upper genital tract in women that is thought to be due to an ascending infection from the lower genital tract. Diagnosis of PID in middle-aged women is a challenge, given the broad differential diagnosis of nonspecific presenting symptoms, lower index of suspicion in this age group, and unknown exact incidence of PID in postmenopausal women. Delay in diagnosis in postmenopausal women can pose serious potential complications such as tubo-ovarian abscess (TOA)—as was seen with this patient—and concurrent gynecologic malignancy found on pathology of TOA specimens.
Risk factors for PID in the postmenopausal population include recent uterine instrumentation, history of prior PID, and structural abnormalities such as cervical stenosis, uterine anatomic abnormalities, or tubal disease.
The Centers for Disease Control and Prevention (CDC) 2015 Sexually Transmitted Diseases Treatment Guidelines recommend presumptive treatment for PID in women with pelvic or lower abdominal pain with 1 or more of the following clinical criteria: cervical motion tenderness, uterine tenderness, or adnexal tenderness. The CDC also suggests that the most specific criteria for PID include endometrial biopsy consistent with endometritis, imaging (transvaginal ultrasound or magnetic resonance imaging) demonstrating fluid-filled tubes, or laparoscopic findings consistent with PID.
Due to the polymicrobial nature of PID, antibiotics should cover not only gonorrhea and chlamydia but also anaerobic pathogens. CDC guidelines recommend the following treatment:
- intravenous (IV) cefotetan (2 g bid) plus doxycycline (100 mg PO or IV bid),
- IV cefoxitin (2 g qid) plus doxycycline (100 mg PO or IV bid), or
- IV clindamycin (900 mg tid) plus IV or intramuscular gentamicin loading dose (2 mg/kg) followed by a maintenance dose (1.5 mg/kg tid).
Due to the increased risk of malignancy in postmenopausal women with TOA, surgical intervention may be needed.
This patient underwent diagnostic laparoscopy, hysterectomy, left salpingo-oophorectomy, and right salpingectomy (with her right ovary left in place due to her perimenopausal status). Intraoperatively, she was found to have cervical stenosis. Postoperatively, she improved on IV cefoxitin (2 g qid) and IV doxycycline (100 mg bid), which was eventually transitioned to oral doxycycline (100 mg bid) and metronidazole (500 mg bid) on discharge. She made a full recovery and is doing well.
This case was adapted from: Khoo CP. Fever, abdominal pain, and adnexal mass. J Fam Pract. 2020;69:101-103
Pregnancy increases risk for symptomatic kidney stones
Pregnancy increases the risk for first-time symptomatic kidney stone formation which peaks close to the time of delivery but can persist even a year later, a population-based, case-controlled study suggests.
“We suspected the risk of a kidney stone event would be high during pregnancy, but we were surprised that the risk remained high for up to a year after delivery,” senior author Andrew Rule, MD, a nephrologist at Mayo Clinic, Rochester, Minn, said in a statement from his institution.
“[So] while most kidney stones that form during pregnancy are detected early by painful passage, some may remain stable in the kidney undetected for a longer period before dislodging and [again] resulting in a painful passage,” he added.
The study was published online April 15, 2021, in the American Journal of Kidney Diseases by Charat Thongprayoon, MD, also of the Mayo Clinic, and colleagues.
“The results of this study indicate that prenatal counseling regarding kidney stones may be warranted, especially for women with other risk factors for kidney stones, such as obesity,” he noted.
First-time stone formers
The observational study included 945 first-time symptomatic kidney stone formers aged between 15 and 45 years who were compared with 1,890 age-matched female controls from the Rochester Epidemiology Project. The latter is a medical record linkage system for almost all medical care administered in Olmsted County in Minnesota.
Compared with nonpregnant women, the odds of a symptomatic kidney stone forming in a pregnant woman was similar in the first trimester (odds ratio, 0.92; P = .8), began to increase during the second trimester (OR, 2.00; P = .007), further increased during the third trimester (OR, 2.69; P = .001), and peaked at 0-3 months after delivery (OR, 3.53; P < .001). The risk returned to baseline by 1 year after delivery.
These associations persisted after adjustment for age and race or for diabetes, hypertension, and obesity. These results did not significantly differ by age, race, time period, or number of prior pregnancies.
The risk of a pregnant woman developing a symptomatic kidney stone was higher in women with obesity, compared with those of normal weight (P = .01).
And compared with women who had not been pregnant before, one prior pregnancy also increased the risk of having a symptomatic kidney stone by approximately 30% (OR, 1.29; P = .03), although two or more prior pregnancies did not significantly increase symptomatic kidney stone risk.
Thus, “it can be inferred that the odds of a symptomatic kidney stone peak around the time of delivery,” the authors emphasized. “The odds of a first-time symptomatic kidney stone then decreased over time and were fully attenuated and no longer statistically significant by 12 months after delivery.”
Dr. Thongprayoon said there are several physiologic reasons why pregnancy might contribute to kidney stone formation.
During pregnancy, ureteral compression and ureteral relaxation caused by elevated progesterone levels can cause urinary stasis.
Furthermore, increased urinary calcium excretion and elevated urine pH during pregnancy can promote calcium phosphate stone formation. It is noteworthy that almost all pregnant, first-time stone formers had calcium phosphate stones.
“During pregnancy, a kidney stone may contribute to serious complications,” Dr. Thongprayoon explained.
General dietary recommendations for preventing kidney stones include drinking abundant fluids and consuming a low-salt diet.
The study was supported by the Mayo Clinic O’Brien Urology Research Center and a grant from the National Institute of Diabetes and Digestive and Kidney Diseases. The authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Pregnancy increases the risk for first-time symptomatic kidney stone formation which peaks close to the time of delivery but can persist even a year later, a population-based, case-controlled study suggests.
“We suspected the risk of a kidney stone event would be high during pregnancy, but we were surprised that the risk remained high for up to a year after delivery,” senior author Andrew Rule, MD, a nephrologist at Mayo Clinic, Rochester, Minn, said in a statement from his institution.
“[So] while most kidney stones that form during pregnancy are detected early by painful passage, some may remain stable in the kidney undetected for a longer period before dislodging and [again] resulting in a painful passage,” he added.
The study was published online April 15, 2021, in the American Journal of Kidney Diseases by Charat Thongprayoon, MD, also of the Mayo Clinic, and colleagues.
“The results of this study indicate that prenatal counseling regarding kidney stones may be warranted, especially for women with other risk factors for kidney stones, such as obesity,” he noted.
First-time stone formers
The observational study included 945 first-time symptomatic kidney stone formers aged between 15 and 45 years who were compared with 1,890 age-matched female controls from the Rochester Epidemiology Project. The latter is a medical record linkage system for almost all medical care administered in Olmsted County in Minnesota.
Compared with nonpregnant women, the odds of a symptomatic kidney stone forming in a pregnant woman was similar in the first trimester (odds ratio, 0.92; P = .8), began to increase during the second trimester (OR, 2.00; P = .007), further increased during the third trimester (OR, 2.69; P = .001), and peaked at 0-3 months after delivery (OR, 3.53; P < .001). The risk returned to baseline by 1 year after delivery.
These associations persisted after adjustment for age and race or for diabetes, hypertension, and obesity. These results did not significantly differ by age, race, time period, or number of prior pregnancies.
The risk of a pregnant woman developing a symptomatic kidney stone was higher in women with obesity, compared with those of normal weight (P = .01).
And compared with women who had not been pregnant before, one prior pregnancy also increased the risk of having a symptomatic kidney stone by approximately 30% (OR, 1.29; P = .03), although two or more prior pregnancies did not significantly increase symptomatic kidney stone risk.
Thus, “it can be inferred that the odds of a symptomatic kidney stone peak around the time of delivery,” the authors emphasized. “The odds of a first-time symptomatic kidney stone then decreased over time and were fully attenuated and no longer statistically significant by 12 months after delivery.”
Dr. Thongprayoon said there are several physiologic reasons why pregnancy might contribute to kidney stone formation.
During pregnancy, ureteral compression and ureteral relaxation caused by elevated progesterone levels can cause urinary stasis.
Furthermore, increased urinary calcium excretion and elevated urine pH during pregnancy can promote calcium phosphate stone formation. It is noteworthy that almost all pregnant, first-time stone formers had calcium phosphate stones.
“During pregnancy, a kidney stone may contribute to serious complications,” Dr. Thongprayoon explained.
General dietary recommendations for preventing kidney stones include drinking abundant fluids and consuming a low-salt diet.
The study was supported by the Mayo Clinic O’Brien Urology Research Center and a grant from the National Institute of Diabetes and Digestive and Kidney Diseases. The authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Pregnancy increases the risk for first-time symptomatic kidney stone formation which peaks close to the time of delivery but can persist even a year later, a population-based, case-controlled study suggests.
“We suspected the risk of a kidney stone event would be high during pregnancy, but we were surprised that the risk remained high for up to a year after delivery,” senior author Andrew Rule, MD, a nephrologist at Mayo Clinic, Rochester, Minn, said in a statement from his institution.
“[So] while most kidney stones that form during pregnancy are detected early by painful passage, some may remain stable in the kidney undetected for a longer period before dislodging and [again] resulting in a painful passage,” he added.
The study was published online April 15, 2021, in the American Journal of Kidney Diseases by Charat Thongprayoon, MD, also of the Mayo Clinic, and colleagues.
“The results of this study indicate that prenatal counseling regarding kidney stones may be warranted, especially for women with other risk factors for kidney stones, such as obesity,” he noted.
First-time stone formers
The observational study included 945 first-time symptomatic kidney stone formers aged between 15 and 45 years who were compared with 1,890 age-matched female controls from the Rochester Epidemiology Project. The latter is a medical record linkage system for almost all medical care administered in Olmsted County in Minnesota.
Compared with nonpregnant women, the odds of a symptomatic kidney stone forming in a pregnant woman was similar in the first trimester (odds ratio, 0.92; P = .8), began to increase during the second trimester (OR, 2.00; P = .007), further increased during the third trimester (OR, 2.69; P = .001), and peaked at 0-3 months after delivery (OR, 3.53; P < .001). The risk returned to baseline by 1 year after delivery.
These associations persisted after adjustment for age and race or for diabetes, hypertension, and obesity. These results did not significantly differ by age, race, time period, or number of prior pregnancies.
The risk of a pregnant woman developing a symptomatic kidney stone was higher in women with obesity, compared with those of normal weight (P = .01).
And compared with women who had not been pregnant before, one prior pregnancy also increased the risk of having a symptomatic kidney stone by approximately 30% (OR, 1.29; P = .03), although two or more prior pregnancies did not significantly increase symptomatic kidney stone risk.
Thus, “it can be inferred that the odds of a symptomatic kidney stone peak around the time of delivery,” the authors emphasized. “The odds of a first-time symptomatic kidney stone then decreased over time and were fully attenuated and no longer statistically significant by 12 months after delivery.”
Dr. Thongprayoon said there are several physiologic reasons why pregnancy might contribute to kidney stone formation.
During pregnancy, ureteral compression and ureteral relaxation caused by elevated progesterone levels can cause urinary stasis.
Furthermore, increased urinary calcium excretion and elevated urine pH during pregnancy can promote calcium phosphate stone formation. It is noteworthy that almost all pregnant, first-time stone formers had calcium phosphate stones.
“During pregnancy, a kidney stone may contribute to serious complications,” Dr. Thongprayoon explained.
General dietary recommendations for preventing kidney stones include drinking abundant fluids and consuming a low-salt diet.
The study was supported by the Mayo Clinic O’Brien Urology Research Center and a grant from the National Institute of Diabetes and Digestive and Kidney Diseases. The authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Pfizer and Moderna vaccines appear safe, effective during pregnancy
The Pfizer and Moderna COVID-19 vaccines appear to be safe in pregnant patients, according to preliminary findings published in the New England Journal of Medicine.
The Centers for Disease Control and Prevention have said pregnant people have an increased risk of being severely ill from COVID-19; however, this group was excluded from major clinical trials that led up to the current vaccine approvals.
But based on the new findings, Rochelle Walensky, MD, director of the CDC, announced during a White House COVID-19 briefing that the CDC recommends that pregnant people receive the COVID-19 vaccine.
The new study, which analyzed data between Dec. 14, 2020, and Feb. 28, 2021, from three federal databases, adds to a pool of limited data about the safety and efficacy of the vaccine in pregnant persons. Researchers did not include people who received the Johnson & Johnson vaccine because it received emergency use authorization on Feb. 27, just 1 day before they study’s cutoff.
“Our hope is that these initial data will be reassuring to pregnant people and their health care providers as well as the public, and contribute to increasing vaccination rates,” study author Christine Olson, MD, said in an interview. “While the data are preliminary and will continue to be analyzed as more reports become available, our findings are reassuring.”
For the study, Dr. Olson and colleagues analyzed v-safe survey data, data from those enrolled in the v-safe pregnancy registry, and Vaccine Adverse Event Reporting System (VAERS) reports.
Researchers found that 86% of pregnancies resulted in a live birth, 12.6 % resulted in spontaneous abortions, and 0.1% resulted in stillbirth. They also found that, among the live births, 9.4% were preterm, 3.2% of babies were small for their gestational age, and 2.2% had congenital anomalies.
Researchers also found that injection-site pain, fatigue, and headaches were reported more frequently in pregnant patients than among those who were not pregnant. Among VAERS reports, they found that 70% of adverse events were nonpregnancy specific. Nearly 30% involved pregnancy- or neonatal-specific adverse events. The most frequently reported pregnancy-related events were spontaneous abortions, followed by stillbirths, premature rupture of membranes and vaginal bleeding.
“I think the results are actually quite reassuring as the proportion of the pregnancy outcomes, such as pregnancy loss and health effects to the newborns, are really quite consistent with what we’d expect in the background rate of the population,” Dr. Walensky said in a podcast accompanying the study. “So this study adds to growing evidence confirming that pregnant people develop a robust immune response to COVID-19 vaccination without so far seeing any adverse events to the mom or the fetus.”
Researchers said limitations of the study include the accuracy of self-reported data, and there being limited information on other potential risk factors for adverse pregnancies and neonatal outcomes. They acknowledged that continuous monitoring is needed to look at maternal safety and pregnancy outcomes in earlier stages of pregnancy and during the preconception period.
David Jaspan, DO, chair of the department of obstetrics and gynecology at Einstein Medical Center, Philadelphia, who was not involved with the study, said in an interview that, despite the limitations, the study provides much-needed insight on the vaccine’s safety and efficacy in pregnant patients.
“In December we had no data for any pregnant patient,” Dr. Jaspan said. “And now just 4 short months later, this paper [has data from] at least had 35,000 people. We can’t answer every question, but we have more answers today than we had just 4 months ago.”
Dr. Olson hopes the present data is enough to help inform decision-making of pregnant patients and their health care providers when it comes to deciding to get the COVID-19 vaccination.
The study author and experts interviewed disclosed no relevant financial relationships.
The Pfizer and Moderna COVID-19 vaccines appear to be safe in pregnant patients, according to preliminary findings published in the New England Journal of Medicine.
The Centers for Disease Control and Prevention have said pregnant people have an increased risk of being severely ill from COVID-19; however, this group was excluded from major clinical trials that led up to the current vaccine approvals.
But based on the new findings, Rochelle Walensky, MD, director of the CDC, announced during a White House COVID-19 briefing that the CDC recommends that pregnant people receive the COVID-19 vaccine.
The new study, which analyzed data between Dec. 14, 2020, and Feb. 28, 2021, from three federal databases, adds to a pool of limited data about the safety and efficacy of the vaccine in pregnant persons. Researchers did not include people who received the Johnson & Johnson vaccine because it received emergency use authorization on Feb. 27, just 1 day before they study’s cutoff.
“Our hope is that these initial data will be reassuring to pregnant people and their health care providers as well as the public, and contribute to increasing vaccination rates,” study author Christine Olson, MD, said in an interview. “While the data are preliminary and will continue to be analyzed as more reports become available, our findings are reassuring.”
For the study, Dr. Olson and colleagues analyzed v-safe survey data, data from those enrolled in the v-safe pregnancy registry, and Vaccine Adverse Event Reporting System (VAERS) reports.
Researchers found that 86% of pregnancies resulted in a live birth, 12.6 % resulted in spontaneous abortions, and 0.1% resulted in stillbirth. They also found that, among the live births, 9.4% were preterm, 3.2% of babies were small for their gestational age, and 2.2% had congenital anomalies.
Researchers also found that injection-site pain, fatigue, and headaches were reported more frequently in pregnant patients than among those who were not pregnant. Among VAERS reports, they found that 70% of adverse events were nonpregnancy specific. Nearly 30% involved pregnancy- or neonatal-specific adverse events. The most frequently reported pregnancy-related events were spontaneous abortions, followed by stillbirths, premature rupture of membranes and vaginal bleeding.
“I think the results are actually quite reassuring as the proportion of the pregnancy outcomes, such as pregnancy loss and health effects to the newborns, are really quite consistent with what we’d expect in the background rate of the population,” Dr. Walensky said in a podcast accompanying the study. “So this study adds to growing evidence confirming that pregnant people develop a robust immune response to COVID-19 vaccination without so far seeing any adverse events to the mom or the fetus.”
Researchers said limitations of the study include the accuracy of self-reported data, and there being limited information on other potential risk factors for adverse pregnancies and neonatal outcomes. They acknowledged that continuous monitoring is needed to look at maternal safety and pregnancy outcomes in earlier stages of pregnancy and during the preconception period.
David Jaspan, DO, chair of the department of obstetrics and gynecology at Einstein Medical Center, Philadelphia, who was not involved with the study, said in an interview that, despite the limitations, the study provides much-needed insight on the vaccine’s safety and efficacy in pregnant patients.
“In December we had no data for any pregnant patient,” Dr. Jaspan said. “And now just 4 short months later, this paper [has data from] at least had 35,000 people. We can’t answer every question, but we have more answers today than we had just 4 months ago.”
Dr. Olson hopes the present data is enough to help inform decision-making of pregnant patients and their health care providers when it comes to deciding to get the COVID-19 vaccination.
The study author and experts interviewed disclosed no relevant financial relationships.
The Pfizer and Moderna COVID-19 vaccines appear to be safe in pregnant patients, according to preliminary findings published in the New England Journal of Medicine.
The Centers for Disease Control and Prevention have said pregnant people have an increased risk of being severely ill from COVID-19; however, this group was excluded from major clinical trials that led up to the current vaccine approvals.
But based on the new findings, Rochelle Walensky, MD, director of the CDC, announced during a White House COVID-19 briefing that the CDC recommends that pregnant people receive the COVID-19 vaccine.
The new study, which analyzed data between Dec. 14, 2020, and Feb. 28, 2021, from three federal databases, adds to a pool of limited data about the safety and efficacy of the vaccine in pregnant persons. Researchers did not include people who received the Johnson & Johnson vaccine because it received emergency use authorization on Feb. 27, just 1 day before they study’s cutoff.
“Our hope is that these initial data will be reassuring to pregnant people and their health care providers as well as the public, and contribute to increasing vaccination rates,” study author Christine Olson, MD, said in an interview. “While the data are preliminary and will continue to be analyzed as more reports become available, our findings are reassuring.”
For the study, Dr. Olson and colleagues analyzed v-safe survey data, data from those enrolled in the v-safe pregnancy registry, and Vaccine Adverse Event Reporting System (VAERS) reports.
Researchers found that 86% of pregnancies resulted in a live birth, 12.6 % resulted in spontaneous abortions, and 0.1% resulted in stillbirth. They also found that, among the live births, 9.4% were preterm, 3.2% of babies were small for their gestational age, and 2.2% had congenital anomalies.
Researchers also found that injection-site pain, fatigue, and headaches were reported more frequently in pregnant patients than among those who were not pregnant. Among VAERS reports, they found that 70% of adverse events were nonpregnancy specific. Nearly 30% involved pregnancy- or neonatal-specific adverse events. The most frequently reported pregnancy-related events were spontaneous abortions, followed by stillbirths, premature rupture of membranes and vaginal bleeding.
“I think the results are actually quite reassuring as the proportion of the pregnancy outcomes, such as pregnancy loss and health effects to the newborns, are really quite consistent with what we’d expect in the background rate of the population,” Dr. Walensky said in a podcast accompanying the study. “So this study adds to growing evidence confirming that pregnant people develop a robust immune response to COVID-19 vaccination without so far seeing any adverse events to the mom or the fetus.”
Researchers said limitations of the study include the accuracy of self-reported data, and there being limited information on other potential risk factors for adverse pregnancies and neonatal outcomes. They acknowledged that continuous monitoring is needed to look at maternal safety and pregnancy outcomes in earlier stages of pregnancy and during the preconception period.
David Jaspan, DO, chair of the department of obstetrics and gynecology at Einstein Medical Center, Philadelphia, who was not involved with the study, said in an interview that, despite the limitations, the study provides much-needed insight on the vaccine’s safety and efficacy in pregnant patients.
“In December we had no data for any pregnant patient,” Dr. Jaspan said. “And now just 4 short months later, this paper [has data from] at least had 35,000 people. We can’t answer every question, but we have more answers today than we had just 4 months ago.”
Dr. Olson hopes the present data is enough to help inform decision-making of pregnant patients and their health care providers when it comes to deciding to get the COVID-19 vaccination.
The study author and experts interviewed disclosed no relevant financial relationships.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
More signs COVID shots are safe for pregnant women
As the U.S. races to vaccinate millions of people against the coronavirus, pregnant women face the extra challenge of not knowing whether the vaccines are safe for them or their unborn babies.
None of the recent COVID-19 vaccine trials, including those for Pfizer, Moderna, and Johnson & Johnson, enrolled pregnant or breastfeeding women because they consider them a high-risk group.
That was despite the Society for Maternal-Fetal Medicine and the American College of Obstetricians and Gynecologists asking that pregnant and breastfeeding women be included in trials. The Food and Drug Administration even included pregnant women in the COVID-19 vaccine emergency use authorization (EUA) because of their higher risk of having a more severe disease.
Despite that lack of clinical trial data, more and more smaller studies are suggesting that the vaccines are safe for both mother and child.
Pfizer is now studying its two-dose vaccine in 4,000 pregnant and breastfeeding women to see how safe, tolerated, and robust their immune response is. Researchers will also look at how safe the vaccine is for infants and whether mothers pass along antibodies to children. But the preliminary results won’t be available until the end of the year, a Pfizer spokesperson says.
Without that information, pregnant women are less likely to get vaccinated, according to a large international survey. Less than 45% of pregnant women in the United States said they intended to get vaccinated even when they were told the vaccine was safe and 90% effective. That figure rises to 52% of pregnant women in 16 countries, including the United States, compared with 74% of nonpregnant women willing to be vaccinated. The findings were published online March 1, 2021, in the European Journal of Epidemiology.
The vaccine-hesitant pregnant women in the international study were most concerned that the COVID-19 vaccine could harm their developing fetuses, a worry related to the lack of clinical evidence in pregnant women, said lead researcher Julia Wu, ScD, an epidemiologist at the Harvard School of Public Health’s Human Immunomics Initiative in Boston.
The information vacuum also increases the chances that “people will fall victim to misinformation campaigns like the one on social media that claims that the COVID-19 vaccine causes infertility,” Dr. Wu said. This unfounded claim has deterred some women of childbearing age from getting the vaccine.
Deciding to get vaccinated
Frontline health care professionals were in the first group eligible to receive the vaccine in December 2020. “All of us who were pregnant ... had to decide whether to wait for the data, because we don’t know what the risks are, or go ahead and get it [the vaccine]. We had been dealing with the pandemic for months and were afraid of being exposed to the virus and infecting family members,” said Jacqueline Parchem, MD, a maternal-fetal medicine specialist at the University of Texas Health Science Center, Houston.
Given the lack of safety data, the CDC guidance to pregnant women has been to consult with their doctors and that it’s a personal choice. The Center for Disease Control and Prevention’s latest vaccine guidance said that “there is no evidence that antibodies formed from COVID-19 vaccination cause any problem with pregnancy, including the development of the placenta.”
The CDC is monitoring vaccinated people through its v-safe program and reported on April 12 that more than 86,000 v-safe participants said they were pregnant when they were vaccinated.
Health care workers who were nursing their infants when they were eligible for the vaccine faced a similar dilemma as pregnant women – they lacked the data on them to make a truly informed decision.
“I was nervous about the vaccine side effects for myself and whether my son Bennett, who was about a year old, would experience any of these himself,” said Christa Carrig, a labor and delivery nurse at Massachusetts General Hospital in Boston, who was breastfeeding at the time.
She and Dr. Parchem know that pregnant women with COVID-19 are more likely to have severe illness and complications such as high blood pressure and preterm delivery. “Pregnancy takes a toll on the body. When a woman gets COVID-19 and that insult is added, women who were otherwise young and healthy get much sicker than you would expect,” said Ms. Carrig.
“As a high-risk pregnancy specialist, I know that, with COVID, that babies don’t do well when moms are sick,” said Dr. Parchem.
Pregnant women accounted for more than 84,629 cases of COVID-19 and 95 deaths in the United States between Jan. 22 last year and April 12 this year, according to the CDC COVID data tracker.
Dr. Parchem and Ms. Carrig decided to get vaccinated because of their high risk of exposure to COVID-19 at work. After the second dose, Ms. Carrig reported chills but Bennett had no side effects from breastfeeding. Dr. Parchem, who delivered a healthy baby boy in February, reported no side effects other than a sore arm.
“There’s also a psychological benefit to returning to some sense of normalcy,” said Dr. Parchem. “My mother was finally able to visit us to see the new baby after we were all vaccinated. This was the first visit in more than a year.”
New study results
Ms. Carrig was one of 131 vaccinated hospital workers in the Boston area who took part in the first study to profile the immune response in pregnant and breastfeeding women and compare it with both nonpregnant and pregnant women who had COVID-19.
The study was not designed to evaluate the safety of the vaccines or whether they prevent COVID-19 illness and hospitalizations. That is the role of the large vaccine trials, the authors said.
The participants were aged 18-45 years and received both doses of either Pfizer or Moderna vaccines during one of their trimesters. They provided blood and/or breast milk samples after each vaccine dose, 2-6 weeks after the last dose, and at delivery for the 10 who gave birth during the study.
The vaccines produced a similar strong antibody response among the pregnant/breastfeeding women and nonpregnant women. Their antibody levels were much higher than those found in the pregnant women who had COVID-19, the researchers reported on March 25, 2021, in the American Journal of Obstetrics and Gynecology.
“This is important because a lot of people tend to think once they’ve had COVID-19, they are protected from the virus. This finding suggests that the vaccines produce a stronger antibody response than the infection itself, and this might be important for long-lasting protection against COVID-19,” said Dr. Parchem.
The study also addressed whether newborns benefit from the antibodies produced by their mothers. “In the 10 women who delivered, we detected antibodies in their umbilical cords and breast milk,” says Andrea Edlow, MD, lead researcher and a maternal-fetal medicine specialist at Massachusetts General Hospital.
Newborns are particularly vulnerable to respiratory infections because they have small airways and their immune systems are underdeveloped. These infections can be lethal early in life.
“The public health strategy is to vaccinate mothers against respiratory viruses, bacteria, and parasites that neonates up to 6 months are exposed to. Influenza and pertussis (whooping cough) are two examples of vaccines that we give mothers that we know transfer [antibodies] across the umbilical cord,” said Dr. Edlow.
But this “passive transfer immunity” is different from active immunity, when the body produces its own antibody immune response, she explains.
A different study, also published in March, confirmed that antibodies were transferred from 27 vaccinated pregnant mothers to their infants when they delivered. A new finding was that the women who were vaccinated with both doses and earlier in their third semester passed on more antibodies than the women who were vaccinated later or with only one dose.
Impact of the studies
The Society for Maternal-Fetal Medicine updated its guidance on counseling pregnant and lactating patients about the COVID-19 vaccines to include Dr. Edlow’s study.
“We were struck by how much pregnant and breastfeeding women want to participate in research and to help others in the same situation make decisions. I hope this will be an example to drug companies doing research on new vaccines in the future – that they should not be left behind and can make decisions themselves whether to participate after weighing the risks and benefits,” said Dr. Edlow.
She continues to enroll more vaccinated women in her study in the Boston area, including non–health care workers who have asked to take part.
“It was worth getting vaccinated and participating in the study. I know that I have antibodies and it worked and that I passed them on to Bennett. Also, I know that all the information is available for other women who are questioning whether to get vaccinated or not,” said Ms. Carrig.
Dr. Parchem is also taking part in the CDC’s v-safe pregnancy registry, which is collecting health and safety data on vaccinated pregnant women.
Before she was vaccinated, Dr. Parchem said, “my advice was very measured because we lacked data either saying that it definitely works or showing that it was unsafe. Now that we have this data supporting the benefits, I feel more confident in recommending the vaccines.”
A version of this article first appeared on Medscape.com.
As the U.S. races to vaccinate millions of people against the coronavirus, pregnant women face the extra challenge of not knowing whether the vaccines are safe for them or their unborn babies.
None of the recent COVID-19 vaccine trials, including those for Pfizer, Moderna, and Johnson & Johnson, enrolled pregnant or breastfeeding women because they consider them a high-risk group.
That was despite the Society for Maternal-Fetal Medicine and the American College of Obstetricians and Gynecologists asking that pregnant and breastfeeding women be included in trials. The Food and Drug Administration even included pregnant women in the COVID-19 vaccine emergency use authorization (EUA) because of their higher risk of having a more severe disease.
Despite that lack of clinical trial data, more and more smaller studies are suggesting that the vaccines are safe for both mother and child.
Pfizer is now studying its two-dose vaccine in 4,000 pregnant and breastfeeding women to see how safe, tolerated, and robust their immune response is. Researchers will also look at how safe the vaccine is for infants and whether mothers pass along antibodies to children. But the preliminary results won’t be available until the end of the year, a Pfizer spokesperson says.
Without that information, pregnant women are less likely to get vaccinated, according to a large international survey. Less than 45% of pregnant women in the United States said they intended to get vaccinated even when they were told the vaccine was safe and 90% effective. That figure rises to 52% of pregnant women in 16 countries, including the United States, compared with 74% of nonpregnant women willing to be vaccinated. The findings were published online March 1, 2021, in the European Journal of Epidemiology.
The vaccine-hesitant pregnant women in the international study were most concerned that the COVID-19 vaccine could harm their developing fetuses, a worry related to the lack of clinical evidence in pregnant women, said lead researcher Julia Wu, ScD, an epidemiologist at the Harvard School of Public Health’s Human Immunomics Initiative in Boston.
The information vacuum also increases the chances that “people will fall victim to misinformation campaigns like the one on social media that claims that the COVID-19 vaccine causes infertility,” Dr. Wu said. This unfounded claim has deterred some women of childbearing age from getting the vaccine.
Deciding to get vaccinated
Frontline health care professionals were in the first group eligible to receive the vaccine in December 2020. “All of us who were pregnant ... had to decide whether to wait for the data, because we don’t know what the risks are, or go ahead and get it [the vaccine]. We had been dealing with the pandemic for months and were afraid of being exposed to the virus and infecting family members,” said Jacqueline Parchem, MD, a maternal-fetal medicine specialist at the University of Texas Health Science Center, Houston.
Given the lack of safety data, the CDC guidance to pregnant women has been to consult with their doctors and that it’s a personal choice. The Center for Disease Control and Prevention’s latest vaccine guidance said that “there is no evidence that antibodies formed from COVID-19 vaccination cause any problem with pregnancy, including the development of the placenta.”
The CDC is monitoring vaccinated people through its v-safe program and reported on April 12 that more than 86,000 v-safe participants said they were pregnant when they were vaccinated.
Health care workers who were nursing their infants when they were eligible for the vaccine faced a similar dilemma as pregnant women – they lacked the data on them to make a truly informed decision.
“I was nervous about the vaccine side effects for myself and whether my son Bennett, who was about a year old, would experience any of these himself,” said Christa Carrig, a labor and delivery nurse at Massachusetts General Hospital in Boston, who was breastfeeding at the time.
She and Dr. Parchem know that pregnant women with COVID-19 are more likely to have severe illness and complications such as high blood pressure and preterm delivery. “Pregnancy takes a toll on the body. When a woman gets COVID-19 and that insult is added, women who were otherwise young and healthy get much sicker than you would expect,” said Ms. Carrig.
“As a high-risk pregnancy specialist, I know that, with COVID, that babies don’t do well when moms are sick,” said Dr. Parchem.
Pregnant women accounted for more than 84,629 cases of COVID-19 and 95 deaths in the United States between Jan. 22 last year and April 12 this year, according to the CDC COVID data tracker.
Dr. Parchem and Ms. Carrig decided to get vaccinated because of their high risk of exposure to COVID-19 at work. After the second dose, Ms. Carrig reported chills but Bennett had no side effects from breastfeeding. Dr. Parchem, who delivered a healthy baby boy in February, reported no side effects other than a sore arm.
“There’s also a psychological benefit to returning to some sense of normalcy,” said Dr. Parchem. “My mother was finally able to visit us to see the new baby after we were all vaccinated. This was the first visit in more than a year.”
New study results
Ms. Carrig was one of 131 vaccinated hospital workers in the Boston area who took part in the first study to profile the immune response in pregnant and breastfeeding women and compare it with both nonpregnant and pregnant women who had COVID-19.
The study was not designed to evaluate the safety of the vaccines or whether they prevent COVID-19 illness and hospitalizations. That is the role of the large vaccine trials, the authors said.
The participants were aged 18-45 years and received both doses of either Pfizer or Moderna vaccines during one of their trimesters. They provided blood and/or breast milk samples after each vaccine dose, 2-6 weeks after the last dose, and at delivery for the 10 who gave birth during the study.
The vaccines produced a similar strong antibody response among the pregnant/breastfeeding women and nonpregnant women. Their antibody levels were much higher than those found in the pregnant women who had COVID-19, the researchers reported on March 25, 2021, in the American Journal of Obstetrics and Gynecology.
“This is important because a lot of people tend to think once they’ve had COVID-19, they are protected from the virus. This finding suggests that the vaccines produce a stronger antibody response than the infection itself, and this might be important for long-lasting protection against COVID-19,” said Dr. Parchem.
The study also addressed whether newborns benefit from the antibodies produced by their mothers. “In the 10 women who delivered, we detected antibodies in their umbilical cords and breast milk,” says Andrea Edlow, MD, lead researcher and a maternal-fetal medicine specialist at Massachusetts General Hospital.
Newborns are particularly vulnerable to respiratory infections because they have small airways and their immune systems are underdeveloped. These infections can be lethal early in life.
“The public health strategy is to vaccinate mothers against respiratory viruses, bacteria, and parasites that neonates up to 6 months are exposed to. Influenza and pertussis (whooping cough) are two examples of vaccines that we give mothers that we know transfer [antibodies] across the umbilical cord,” said Dr. Edlow.
But this “passive transfer immunity” is different from active immunity, when the body produces its own antibody immune response, she explains.
A different study, also published in March, confirmed that antibodies were transferred from 27 vaccinated pregnant mothers to their infants when they delivered. A new finding was that the women who were vaccinated with both doses and earlier in their third semester passed on more antibodies than the women who were vaccinated later or with only one dose.
Impact of the studies
The Society for Maternal-Fetal Medicine updated its guidance on counseling pregnant and lactating patients about the COVID-19 vaccines to include Dr. Edlow’s study.
“We were struck by how much pregnant and breastfeeding women want to participate in research and to help others in the same situation make decisions. I hope this will be an example to drug companies doing research on new vaccines in the future – that they should not be left behind and can make decisions themselves whether to participate after weighing the risks and benefits,” said Dr. Edlow.
She continues to enroll more vaccinated women in her study in the Boston area, including non–health care workers who have asked to take part.
“It was worth getting vaccinated and participating in the study. I know that I have antibodies and it worked and that I passed them on to Bennett. Also, I know that all the information is available for other women who are questioning whether to get vaccinated or not,” said Ms. Carrig.
Dr. Parchem is also taking part in the CDC’s v-safe pregnancy registry, which is collecting health and safety data on vaccinated pregnant women.
Before she was vaccinated, Dr. Parchem said, “my advice was very measured because we lacked data either saying that it definitely works or showing that it was unsafe. Now that we have this data supporting the benefits, I feel more confident in recommending the vaccines.”
A version of this article first appeared on Medscape.com.
As the U.S. races to vaccinate millions of people against the coronavirus, pregnant women face the extra challenge of not knowing whether the vaccines are safe for them or their unborn babies.
None of the recent COVID-19 vaccine trials, including those for Pfizer, Moderna, and Johnson & Johnson, enrolled pregnant or breastfeeding women because they consider them a high-risk group.
That was despite the Society for Maternal-Fetal Medicine and the American College of Obstetricians and Gynecologists asking that pregnant and breastfeeding women be included in trials. The Food and Drug Administration even included pregnant women in the COVID-19 vaccine emergency use authorization (EUA) because of their higher risk of having a more severe disease.
Despite that lack of clinical trial data, more and more smaller studies are suggesting that the vaccines are safe for both mother and child.
Pfizer is now studying its two-dose vaccine in 4,000 pregnant and breastfeeding women to see how safe, tolerated, and robust their immune response is. Researchers will also look at how safe the vaccine is for infants and whether mothers pass along antibodies to children. But the preliminary results won’t be available until the end of the year, a Pfizer spokesperson says.
Without that information, pregnant women are less likely to get vaccinated, according to a large international survey. Less than 45% of pregnant women in the United States said they intended to get vaccinated even when they were told the vaccine was safe and 90% effective. That figure rises to 52% of pregnant women in 16 countries, including the United States, compared with 74% of nonpregnant women willing to be vaccinated. The findings were published online March 1, 2021, in the European Journal of Epidemiology.
The vaccine-hesitant pregnant women in the international study were most concerned that the COVID-19 vaccine could harm their developing fetuses, a worry related to the lack of clinical evidence in pregnant women, said lead researcher Julia Wu, ScD, an epidemiologist at the Harvard School of Public Health’s Human Immunomics Initiative in Boston.
The information vacuum also increases the chances that “people will fall victim to misinformation campaigns like the one on social media that claims that the COVID-19 vaccine causes infertility,” Dr. Wu said. This unfounded claim has deterred some women of childbearing age from getting the vaccine.
Deciding to get vaccinated
Frontline health care professionals were in the first group eligible to receive the vaccine in December 2020. “All of us who were pregnant ... had to decide whether to wait for the data, because we don’t know what the risks are, or go ahead and get it [the vaccine]. We had been dealing with the pandemic for months and were afraid of being exposed to the virus and infecting family members,” said Jacqueline Parchem, MD, a maternal-fetal medicine specialist at the University of Texas Health Science Center, Houston.
Given the lack of safety data, the CDC guidance to pregnant women has been to consult with their doctors and that it’s a personal choice. The Center for Disease Control and Prevention’s latest vaccine guidance said that “there is no evidence that antibodies formed from COVID-19 vaccination cause any problem with pregnancy, including the development of the placenta.”
The CDC is monitoring vaccinated people through its v-safe program and reported on April 12 that more than 86,000 v-safe participants said they were pregnant when they were vaccinated.
Health care workers who were nursing their infants when they were eligible for the vaccine faced a similar dilemma as pregnant women – they lacked the data on them to make a truly informed decision.
“I was nervous about the vaccine side effects for myself and whether my son Bennett, who was about a year old, would experience any of these himself,” said Christa Carrig, a labor and delivery nurse at Massachusetts General Hospital in Boston, who was breastfeeding at the time.
She and Dr. Parchem know that pregnant women with COVID-19 are more likely to have severe illness and complications such as high blood pressure and preterm delivery. “Pregnancy takes a toll on the body. When a woman gets COVID-19 and that insult is added, women who were otherwise young and healthy get much sicker than you would expect,” said Ms. Carrig.
“As a high-risk pregnancy specialist, I know that, with COVID, that babies don’t do well when moms are sick,” said Dr. Parchem.
Pregnant women accounted for more than 84,629 cases of COVID-19 and 95 deaths in the United States between Jan. 22 last year and April 12 this year, according to the CDC COVID data tracker.
Dr. Parchem and Ms. Carrig decided to get vaccinated because of their high risk of exposure to COVID-19 at work. After the second dose, Ms. Carrig reported chills but Bennett had no side effects from breastfeeding. Dr. Parchem, who delivered a healthy baby boy in February, reported no side effects other than a sore arm.
“There’s also a psychological benefit to returning to some sense of normalcy,” said Dr. Parchem. “My mother was finally able to visit us to see the new baby after we were all vaccinated. This was the first visit in more than a year.”
New study results
Ms. Carrig was one of 131 vaccinated hospital workers in the Boston area who took part in the first study to profile the immune response in pregnant and breastfeeding women and compare it with both nonpregnant and pregnant women who had COVID-19.
The study was not designed to evaluate the safety of the vaccines or whether they prevent COVID-19 illness and hospitalizations. That is the role of the large vaccine trials, the authors said.
The participants were aged 18-45 years and received both doses of either Pfizer or Moderna vaccines during one of their trimesters. They provided blood and/or breast milk samples after each vaccine dose, 2-6 weeks after the last dose, and at delivery for the 10 who gave birth during the study.
The vaccines produced a similar strong antibody response among the pregnant/breastfeeding women and nonpregnant women. Their antibody levels were much higher than those found in the pregnant women who had COVID-19, the researchers reported on March 25, 2021, in the American Journal of Obstetrics and Gynecology.
“This is important because a lot of people tend to think once they’ve had COVID-19, they are protected from the virus. This finding suggests that the vaccines produce a stronger antibody response than the infection itself, and this might be important for long-lasting protection against COVID-19,” said Dr. Parchem.
The study also addressed whether newborns benefit from the antibodies produced by their mothers. “In the 10 women who delivered, we detected antibodies in their umbilical cords and breast milk,” says Andrea Edlow, MD, lead researcher and a maternal-fetal medicine specialist at Massachusetts General Hospital.
Newborns are particularly vulnerable to respiratory infections because they have small airways and their immune systems are underdeveloped. These infections can be lethal early in life.
“The public health strategy is to vaccinate mothers against respiratory viruses, bacteria, and parasites that neonates up to 6 months are exposed to. Influenza and pertussis (whooping cough) are two examples of vaccines that we give mothers that we know transfer [antibodies] across the umbilical cord,” said Dr. Edlow.
But this “passive transfer immunity” is different from active immunity, when the body produces its own antibody immune response, she explains.
A different study, also published in March, confirmed that antibodies were transferred from 27 vaccinated pregnant mothers to their infants when they delivered. A new finding was that the women who were vaccinated with both doses and earlier in their third semester passed on more antibodies than the women who were vaccinated later or with only one dose.
Impact of the studies
The Society for Maternal-Fetal Medicine updated its guidance on counseling pregnant and lactating patients about the COVID-19 vaccines to include Dr. Edlow’s study.
“We were struck by how much pregnant and breastfeeding women want to participate in research and to help others in the same situation make decisions. I hope this will be an example to drug companies doing research on new vaccines in the future – that they should not be left behind and can make decisions themselves whether to participate after weighing the risks and benefits,” said Dr. Edlow.
She continues to enroll more vaccinated women in her study in the Boston area, including non–health care workers who have asked to take part.
“It was worth getting vaccinated and participating in the study. I know that I have antibodies and it worked and that I passed them on to Bennett. Also, I know that all the information is available for other women who are questioning whether to get vaccinated or not,” said Ms. Carrig.
Dr. Parchem is also taking part in the CDC’s v-safe pregnancy registry, which is collecting health and safety data on vaccinated pregnant women.
Before she was vaccinated, Dr. Parchem said, “my advice was very measured because we lacked data either saying that it definitely works or showing that it was unsafe. Now that we have this data supporting the benefits, I feel more confident in recommending the vaccines.”
A version of this article first appeared on Medscape.com.
PTSD linked to ischemic heart disease
A study using data from Veterans Health Administration (VHA) electronic medical records shows a significant association between posttraumatic stress disorder (PTSD) among female veterans and an increased risk for incident ischemic heart disease (IHD).
The increased risk for IHD was highest among women younger than 40 with PTSD, and among racial and ethnic minorities.
“These women have been emerging as important targets for cardiovascular prevention, and our study suggests that PTSD may be an important psychosocial risk factor for IHD in these individuals,” wrote the researchers, led by Ramin Ebrahimi, MD, department of medicine, cardiology section, Veterans Affairs Greater Los Angeles Health Care System. “With the number of women veterans growing, it is critical to appreciate the health care needs of this relatively young and diverse patient population.”
The study results also have “important implications for earlier and more aggressive IHD risk assessment, monitoring and management in vulnerable women veterans,” they added. “Indeed, our findings support recent calls for cardiovascular risk screening in younger individuals and for the need to harness a broad range of clinicians who routinely treat younger women to maximize prevention efforts.”
The article was published online in JAMA Cardiology on March 17.
Increasing number of VHA users
“As an interventional cardiologist and the director of the cardiac catheterization laboratory, I noticed a significant number of the patients referred to the cath lab carried a diagnosis of posttraumatic stress disorder,” Dr. Ebrahimi said in an interview. “This intrigued me and started my journey into trying to understand how psychiatric disorders in general, and PTSD, may impact/interact with cardiovascular disorders,” he added.
The number of female veterans in the military has been increasing, and they now make up about 10% of the 20 million American veterans; that number is projected to exceed 2.2 million in the next 20 years, the authors wrote. Female veterans are also the fastest growing group of users of the VHA, they added.
IHD is the leading cause of death in women in the United States, despite the advancements in prevention and treatment. Although women are twice as likely to develop PTSD as are men, and it is even more likely in female veterans, much of the research has predominately been on male veterans, the authors wrote.
For this retrospective study, which used data from the VHA Corporate Data Warehouse, the authors examined a cohort of female veterans who were 18 years or older who had used the VHA health care system between Jan. 1, 2000, and Dec. 31, 2017.
Of the 828,997 female veterans, 151,030 had PTSD. Women excluded from the study were those who did not have any clinical encounters after their index visit, participants who had a diagnosis of IHD at or before the index visit, and those with incident IHD within 90 days of the index visit, allowing time between a PTSD diagnosis and IHD.
Propensity score matching on age at index visit, the number of previous visits, and the presence of traditional and female-specific cardiovascular risk factors, as well as mental and physical health conditions, was conducted to identify female veterans ever diagnosed with PTSD, who were matched in a 1:2 ratio to those never diagnosed with PTSD. In all, 132,923 women with PTSD and 265,846 women without PTSD were included, and data were analyzed for the period of Oct. 1, 2018, to Oct. 30, 2020.
IHD was defined as new-onset coronary artery disease, angina, or myocardial infarction–based ICD-9 and ICD-10 diagnostic codes. Age, race, and ethnicity were self-reported.
The analytic sample consisted of relatively young female veterans (mean [SD] age at baseline, 40.1 [12.2] years) of various races (White, 57.6%; Black, 29.8%) and ethnicities, the authors reported.
Of the 9,940 women who experienced incident IHD during follow-up, 5,559 did not have PTSD (2.1% of the overall population examined) and 4,381 had PTSD (3.3%). PTSD was significantly associated with an increased risk for IHD. Over the median follow-up of 4.9 years, female veterans with PTSD had a 44% higher rate of developing incident IHD compared with the female veterans without PTSD (hazard ratio [HR], 1.44; 95% confidence interval [CI], 1.38-1.50).
In addition, those with PTSD who developed IHD were younger at diagnosis (mean [SD] age, 55.5 [9.7]) than were patients without PTSD (mean [SD] age, 57.8 [10.7]). Effect sizes were largest in the group younger than 40 years (HR, 1.72; 95% CI, 1.55-1.90) and decreased for older participants (HR for those ≥60 years, 1.24; 95% CI, 1.12-1.38)
The authors found a 49% to 66% increase in risk for IHD associated with PTSD in Black women (HR, 1.49; 95% CI, 1.38-1.62) and those identified as non-White and non-Black (HR, 1.66; 95%, 1.33-2.08).
Women of all ethnic groups with PTSD were at higher risk of developing IHD, but this was especially true for Hispanic/Latina women (HR, 1.50; 95% CI 1.22-1.84), they noted.
The authors reported some limitations to their findings. The analytic sample could result in a lower ascertainment of certain conditions, such as psychiatric disorders, they wrote. Substance disorders were low in this study, possibly because of the younger age of female veterans in the sample. Because this study used VHA electronic medical records data, medical care outside of the VHA that was not paid for by the VHA could not be considered.
In addition, although this study used a large sample of female veterans, the findings cannot be generalized to female veterans outside of the VHA system, nonveteran women, or men, the researchers wrote.
A call to action
In an accompanying comment, Beth E. Cohen, MD, of the University of California, San Francisco, and the San Francisco Veterans Affairs Health Care System, points out that the physical implications for psychosocial conditions, including depression and PTSD, have been recognized for quite some time. For example, results of the INTERHEART case-control study of 30,000 people showed stress, depression, and stressful life events accounted for one-third the population-attributable risk for myocardial infarction.
As was also noted by Dr. Ebrahimi and colleagues, much of the current research has been on male veterans, yet types of trauma differ among genders; women experience higher rates of military sexual trauma but lower rates of combat trauma, Dr. Cohen wrote. The PTSD symptoms, trajectory, and biological effects can differ for women and men, as can the pathogenesis, presentation, and outcomes of cardiovascular disease (CVD).
These findings, she said, “are an important extension of the prior literature and represent the largest study in female veterans to date. Although methods differ across studies, the magnitude of risk associated with PTSD was consistent with that found in prior studies of male veterans and nonveteran samples.”
The assessment of age-specific risk is also a strength of the study, “and has implications for clinical practice, because PTSD-associated risk was greatest in a younger group in whom CVD may be overlooked.”
Dr. Cohen addressed the limitations outlined by the authors, including ascertainment bias, severity of PTSD symptoms, and their chronicity, but added that “even in the context of these limitations, this study illustrates the importance of PTSD to the health of women veterans and the additional work needed to reduce their CVD risk.”
Clinical questions remain, she added. Screens for PTSD are widely used in the VHA, yet no studies have examined whether screening or early detection decrease CVD risk. In addition, no evidence suggests that screening for or treatment of PTSD improves cardiovascular outcomes.
“Given the challenges of answering these questions in observational studies, it will be important to incorporate measures of CVD risk and outcomes in trials of behavioral and medical therapies for patients with PTSD,” she wrote.
She added that collaborations among multidisciplinary patient care teams will be important. “The findings of this study represent a call to action for this important work to understand the cardiovascular effects of PTSD and improve the health and well-being of women veterans,” Dr. Cohen concluded.
This research was supported by Investigator-Initiated Research Award from the Department of Defense U.S. Army Medical Research and Material Command Congressionally Directed Medical Research Programs (Dr. Ebrahimi) and in part by grants from the VA Informatics and Computing Infrastructure and the Offices of Research and Development at the Northport, Durham, and Greater Los Angeles Veterans Affairs medical centers. Dr. Ebrahimi reported receiving grants from the Department of Defense during the conduct of the study. Disclosures for other authors are available in the paper. Dr. Cohen reports no disclosures.
A version of this article first appeared on Medscape.com.
A study using data from Veterans Health Administration (VHA) electronic medical records shows a significant association between posttraumatic stress disorder (PTSD) among female veterans and an increased risk for incident ischemic heart disease (IHD).
The increased risk for IHD was highest among women younger than 40 with PTSD, and among racial and ethnic minorities.
“These women have been emerging as important targets for cardiovascular prevention, and our study suggests that PTSD may be an important psychosocial risk factor for IHD in these individuals,” wrote the researchers, led by Ramin Ebrahimi, MD, department of medicine, cardiology section, Veterans Affairs Greater Los Angeles Health Care System. “With the number of women veterans growing, it is critical to appreciate the health care needs of this relatively young and diverse patient population.”
The study results also have “important implications for earlier and more aggressive IHD risk assessment, monitoring and management in vulnerable women veterans,” they added. “Indeed, our findings support recent calls for cardiovascular risk screening in younger individuals and for the need to harness a broad range of clinicians who routinely treat younger women to maximize prevention efforts.”
The article was published online in JAMA Cardiology on March 17.
Increasing number of VHA users
“As an interventional cardiologist and the director of the cardiac catheterization laboratory, I noticed a significant number of the patients referred to the cath lab carried a diagnosis of posttraumatic stress disorder,” Dr. Ebrahimi said in an interview. “This intrigued me and started my journey into trying to understand how psychiatric disorders in general, and PTSD, may impact/interact with cardiovascular disorders,” he added.
The number of female veterans in the military has been increasing, and they now make up about 10% of the 20 million American veterans; that number is projected to exceed 2.2 million in the next 20 years, the authors wrote. Female veterans are also the fastest growing group of users of the VHA, they added.
IHD is the leading cause of death in women in the United States, despite the advancements in prevention and treatment. Although women are twice as likely to develop PTSD as are men, and it is even more likely in female veterans, much of the research has predominately been on male veterans, the authors wrote.
For this retrospective study, which used data from the VHA Corporate Data Warehouse, the authors examined a cohort of female veterans who were 18 years or older who had used the VHA health care system between Jan. 1, 2000, and Dec. 31, 2017.
Of the 828,997 female veterans, 151,030 had PTSD. Women excluded from the study were those who did not have any clinical encounters after their index visit, participants who had a diagnosis of IHD at or before the index visit, and those with incident IHD within 90 days of the index visit, allowing time between a PTSD diagnosis and IHD.
Propensity score matching on age at index visit, the number of previous visits, and the presence of traditional and female-specific cardiovascular risk factors, as well as mental and physical health conditions, was conducted to identify female veterans ever diagnosed with PTSD, who were matched in a 1:2 ratio to those never diagnosed with PTSD. In all, 132,923 women with PTSD and 265,846 women without PTSD were included, and data were analyzed for the period of Oct. 1, 2018, to Oct. 30, 2020.
IHD was defined as new-onset coronary artery disease, angina, or myocardial infarction–based ICD-9 and ICD-10 diagnostic codes. Age, race, and ethnicity were self-reported.
The analytic sample consisted of relatively young female veterans (mean [SD] age at baseline, 40.1 [12.2] years) of various races (White, 57.6%; Black, 29.8%) and ethnicities, the authors reported.
Of the 9,940 women who experienced incident IHD during follow-up, 5,559 did not have PTSD (2.1% of the overall population examined) and 4,381 had PTSD (3.3%). PTSD was significantly associated with an increased risk for IHD. Over the median follow-up of 4.9 years, female veterans with PTSD had a 44% higher rate of developing incident IHD compared with the female veterans without PTSD (hazard ratio [HR], 1.44; 95% confidence interval [CI], 1.38-1.50).
In addition, those with PTSD who developed IHD were younger at diagnosis (mean [SD] age, 55.5 [9.7]) than were patients without PTSD (mean [SD] age, 57.8 [10.7]). Effect sizes were largest in the group younger than 40 years (HR, 1.72; 95% CI, 1.55-1.90) and decreased for older participants (HR for those ≥60 years, 1.24; 95% CI, 1.12-1.38)
The authors found a 49% to 66% increase in risk for IHD associated with PTSD in Black women (HR, 1.49; 95% CI, 1.38-1.62) and those identified as non-White and non-Black (HR, 1.66; 95%, 1.33-2.08).
Women of all ethnic groups with PTSD were at higher risk of developing IHD, but this was especially true for Hispanic/Latina women (HR, 1.50; 95% CI 1.22-1.84), they noted.
The authors reported some limitations to their findings. The analytic sample could result in a lower ascertainment of certain conditions, such as psychiatric disorders, they wrote. Substance disorders were low in this study, possibly because of the younger age of female veterans in the sample. Because this study used VHA electronic medical records data, medical care outside of the VHA that was not paid for by the VHA could not be considered.
In addition, although this study used a large sample of female veterans, the findings cannot be generalized to female veterans outside of the VHA system, nonveteran women, or men, the researchers wrote.
A call to action
In an accompanying comment, Beth E. Cohen, MD, of the University of California, San Francisco, and the San Francisco Veterans Affairs Health Care System, points out that the physical implications for psychosocial conditions, including depression and PTSD, have been recognized for quite some time. For example, results of the INTERHEART case-control study of 30,000 people showed stress, depression, and stressful life events accounted for one-third the population-attributable risk for myocardial infarction.
As was also noted by Dr. Ebrahimi and colleagues, much of the current research has been on male veterans, yet types of trauma differ among genders; women experience higher rates of military sexual trauma but lower rates of combat trauma, Dr. Cohen wrote. The PTSD symptoms, trajectory, and biological effects can differ for women and men, as can the pathogenesis, presentation, and outcomes of cardiovascular disease (CVD).
These findings, she said, “are an important extension of the prior literature and represent the largest study in female veterans to date. Although methods differ across studies, the magnitude of risk associated with PTSD was consistent with that found in prior studies of male veterans and nonveteran samples.”
The assessment of age-specific risk is also a strength of the study, “and has implications for clinical practice, because PTSD-associated risk was greatest in a younger group in whom CVD may be overlooked.”
Dr. Cohen addressed the limitations outlined by the authors, including ascertainment bias, severity of PTSD symptoms, and their chronicity, but added that “even in the context of these limitations, this study illustrates the importance of PTSD to the health of women veterans and the additional work needed to reduce their CVD risk.”
Clinical questions remain, she added. Screens for PTSD are widely used in the VHA, yet no studies have examined whether screening or early detection decrease CVD risk. In addition, no evidence suggests that screening for or treatment of PTSD improves cardiovascular outcomes.
“Given the challenges of answering these questions in observational studies, it will be important to incorporate measures of CVD risk and outcomes in trials of behavioral and medical therapies for patients with PTSD,” she wrote.
She added that collaborations among multidisciplinary patient care teams will be important. “The findings of this study represent a call to action for this important work to understand the cardiovascular effects of PTSD and improve the health and well-being of women veterans,” Dr. Cohen concluded.
This research was supported by Investigator-Initiated Research Award from the Department of Defense U.S. Army Medical Research and Material Command Congressionally Directed Medical Research Programs (Dr. Ebrahimi) and in part by grants from the VA Informatics and Computing Infrastructure and the Offices of Research and Development at the Northport, Durham, and Greater Los Angeles Veterans Affairs medical centers. Dr. Ebrahimi reported receiving grants from the Department of Defense during the conduct of the study. Disclosures for other authors are available in the paper. Dr. Cohen reports no disclosures.
A version of this article first appeared on Medscape.com.
A study using data from Veterans Health Administration (VHA) electronic medical records shows a significant association between posttraumatic stress disorder (PTSD) among female veterans and an increased risk for incident ischemic heart disease (IHD).
The increased risk for IHD was highest among women younger than 40 with PTSD, and among racial and ethnic minorities.
“These women have been emerging as important targets for cardiovascular prevention, and our study suggests that PTSD may be an important psychosocial risk factor for IHD in these individuals,” wrote the researchers, led by Ramin Ebrahimi, MD, department of medicine, cardiology section, Veterans Affairs Greater Los Angeles Health Care System. “With the number of women veterans growing, it is critical to appreciate the health care needs of this relatively young and diverse patient population.”
The study results also have “important implications for earlier and more aggressive IHD risk assessment, monitoring and management in vulnerable women veterans,” they added. “Indeed, our findings support recent calls for cardiovascular risk screening in younger individuals and for the need to harness a broad range of clinicians who routinely treat younger women to maximize prevention efforts.”
The article was published online in JAMA Cardiology on March 17.
Increasing number of VHA users
“As an interventional cardiologist and the director of the cardiac catheterization laboratory, I noticed a significant number of the patients referred to the cath lab carried a diagnosis of posttraumatic stress disorder,” Dr. Ebrahimi said in an interview. “This intrigued me and started my journey into trying to understand how psychiatric disorders in general, and PTSD, may impact/interact with cardiovascular disorders,” he added.
The number of female veterans in the military has been increasing, and they now make up about 10% of the 20 million American veterans; that number is projected to exceed 2.2 million in the next 20 years, the authors wrote. Female veterans are also the fastest growing group of users of the VHA, they added.
IHD is the leading cause of death in women in the United States, despite the advancements in prevention and treatment. Although women are twice as likely to develop PTSD as are men, and it is even more likely in female veterans, much of the research has predominately been on male veterans, the authors wrote.
For this retrospective study, which used data from the VHA Corporate Data Warehouse, the authors examined a cohort of female veterans who were 18 years or older who had used the VHA health care system between Jan. 1, 2000, and Dec. 31, 2017.
Of the 828,997 female veterans, 151,030 had PTSD. Women excluded from the study were those who did not have any clinical encounters after their index visit, participants who had a diagnosis of IHD at or before the index visit, and those with incident IHD within 90 days of the index visit, allowing time between a PTSD diagnosis and IHD.
Propensity score matching on age at index visit, the number of previous visits, and the presence of traditional and female-specific cardiovascular risk factors, as well as mental and physical health conditions, was conducted to identify female veterans ever diagnosed with PTSD, who were matched in a 1:2 ratio to those never diagnosed with PTSD. In all, 132,923 women with PTSD and 265,846 women without PTSD were included, and data were analyzed for the period of Oct. 1, 2018, to Oct. 30, 2020.
IHD was defined as new-onset coronary artery disease, angina, or myocardial infarction–based ICD-9 and ICD-10 diagnostic codes. Age, race, and ethnicity were self-reported.
The analytic sample consisted of relatively young female veterans (mean [SD] age at baseline, 40.1 [12.2] years) of various races (White, 57.6%; Black, 29.8%) and ethnicities, the authors reported.
Of the 9,940 women who experienced incident IHD during follow-up, 5,559 did not have PTSD (2.1% of the overall population examined) and 4,381 had PTSD (3.3%). PTSD was significantly associated with an increased risk for IHD. Over the median follow-up of 4.9 years, female veterans with PTSD had a 44% higher rate of developing incident IHD compared with the female veterans without PTSD (hazard ratio [HR], 1.44; 95% confidence interval [CI], 1.38-1.50).
In addition, those with PTSD who developed IHD were younger at diagnosis (mean [SD] age, 55.5 [9.7]) than were patients without PTSD (mean [SD] age, 57.8 [10.7]). Effect sizes were largest in the group younger than 40 years (HR, 1.72; 95% CI, 1.55-1.90) and decreased for older participants (HR for those ≥60 years, 1.24; 95% CI, 1.12-1.38)
The authors found a 49% to 66% increase in risk for IHD associated with PTSD in Black women (HR, 1.49; 95% CI, 1.38-1.62) and those identified as non-White and non-Black (HR, 1.66; 95%, 1.33-2.08).
Women of all ethnic groups with PTSD were at higher risk of developing IHD, but this was especially true for Hispanic/Latina women (HR, 1.50; 95% CI 1.22-1.84), they noted.
The authors reported some limitations to their findings. The analytic sample could result in a lower ascertainment of certain conditions, such as psychiatric disorders, they wrote. Substance disorders were low in this study, possibly because of the younger age of female veterans in the sample. Because this study used VHA electronic medical records data, medical care outside of the VHA that was not paid for by the VHA could not be considered.
In addition, although this study used a large sample of female veterans, the findings cannot be generalized to female veterans outside of the VHA system, nonveteran women, or men, the researchers wrote.
A call to action
In an accompanying comment, Beth E. Cohen, MD, of the University of California, San Francisco, and the San Francisco Veterans Affairs Health Care System, points out that the physical implications for psychosocial conditions, including depression and PTSD, have been recognized for quite some time. For example, results of the INTERHEART case-control study of 30,000 people showed stress, depression, and stressful life events accounted for one-third the population-attributable risk for myocardial infarction.
As was also noted by Dr. Ebrahimi and colleagues, much of the current research has been on male veterans, yet types of trauma differ among genders; women experience higher rates of military sexual trauma but lower rates of combat trauma, Dr. Cohen wrote. The PTSD symptoms, trajectory, and biological effects can differ for women and men, as can the pathogenesis, presentation, and outcomes of cardiovascular disease (CVD).
These findings, she said, “are an important extension of the prior literature and represent the largest study in female veterans to date. Although methods differ across studies, the magnitude of risk associated with PTSD was consistent with that found in prior studies of male veterans and nonveteran samples.”
The assessment of age-specific risk is also a strength of the study, “and has implications for clinical practice, because PTSD-associated risk was greatest in a younger group in whom CVD may be overlooked.”
Dr. Cohen addressed the limitations outlined by the authors, including ascertainment bias, severity of PTSD symptoms, and their chronicity, but added that “even in the context of these limitations, this study illustrates the importance of PTSD to the health of women veterans and the additional work needed to reduce their CVD risk.”
Clinical questions remain, she added. Screens for PTSD are widely used in the VHA, yet no studies have examined whether screening or early detection decrease CVD risk. In addition, no evidence suggests that screening for or treatment of PTSD improves cardiovascular outcomes.
“Given the challenges of answering these questions in observational studies, it will be important to incorporate measures of CVD risk and outcomes in trials of behavioral and medical therapies for patients with PTSD,” she wrote.
She added that collaborations among multidisciplinary patient care teams will be important. “The findings of this study represent a call to action for this important work to understand the cardiovascular effects of PTSD and improve the health and well-being of women veterans,” Dr. Cohen concluded.
This research was supported by Investigator-Initiated Research Award from the Department of Defense U.S. Army Medical Research and Material Command Congressionally Directed Medical Research Programs (Dr. Ebrahimi) and in part by grants from the VA Informatics and Computing Infrastructure and the Offices of Research and Development at the Northport, Durham, and Greater Los Angeles Veterans Affairs medical centers. Dr. Ebrahimi reported receiving grants from the Department of Defense during the conduct of the study. Disclosures for other authors are available in the paper. Dr. Cohen reports no disclosures.
A version of this article first appeared on Medscape.com.
Oral contraceptive with new estrogen earns approval
The Food and Drug Administration has approved a new estrogen for the first time in more than 50 years.
The novel combined oral contraceptive, marketed as Nextstellis, contains 3 mg drospirenone (DRSP) and 14.2 mg of estetrol (E4) in tablet form. Estetrol is an estrogen that is naturally produced during pregnancy, but will now be produced from a plant source; it has not previously been used in oral contraceptives.
Approval of the unique estetrol/drospirenone combination was based on data from a pair of phase 3 clinical trials including 3,725 women. Overall, Nextstellis was safe and effective while meeting its primary endpoint of pregnancy prevention, according to a company press release. Participants also reported favorable results on secondary endpoints including cycle control, bleeding profile, safety, and tolerability.
Although many women take short-acting contraceptives containing estrogen and progestin, concerns persist about side effects, said Mitchell Creinin, MD, of the University of California, in the press release. In addition to providing effective contraception, the drug showed minimal impact on specific markers of concern, including triglycerides, cholesterol, and glucose, as well as weight and endocrine markers, Dr. Creinin said.
Nextstellis was developed by the Belgian biotech company Mithra Pharmaceuticals, and the drug is licensed for distribution in Australia and the United States by Mayne Pharma, with an expected launch at the end of June 2021.
The Food and Drug Administration has approved a new estrogen for the first time in more than 50 years.
The novel combined oral contraceptive, marketed as Nextstellis, contains 3 mg drospirenone (DRSP) and 14.2 mg of estetrol (E4) in tablet form. Estetrol is an estrogen that is naturally produced during pregnancy, but will now be produced from a plant source; it has not previously been used in oral contraceptives.
Approval of the unique estetrol/drospirenone combination was based on data from a pair of phase 3 clinical trials including 3,725 women. Overall, Nextstellis was safe and effective while meeting its primary endpoint of pregnancy prevention, according to a company press release. Participants also reported favorable results on secondary endpoints including cycle control, bleeding profile, safety, and tolerability.
Although many women take short-acting contraceptives containing estrogen and progestin, concerns persist about side effects, said Mitchell Creinin, MD, of the University of California, in the press release. In addition to providing effective contraception, the drug showed minimal impact on specific markers of concern, including triglycerides, cholesterol, and glucose, as well as weight and endocrine markers, Dr. Creinin said.
Nextstellis was developed by the Belgian biotech company Mithra Pharmaceuticals, and the drug is licensed for distribution in Australia and the United States by Mayne Pharma, with an expected launch at the end of June 2021.
The Food and Drug Administration has approved a new estrogen for the first time in more than 50 years.
The novel combined oral contraceptive, marketed as Nextstellis, contains 3 mg drospirenone (DRSP) and 14.2 mg of estetrol (E4) in tablet form. Estetrol is an estrogen that is naturally produced during pregnancy, but will now be produced from a plant source; it has not previously been used in oral contraceptives.
Approval of the unique estetrol/drospirenone combination was based on data from a pair of phase 3 clinical trials including 3,725 women. Overall, Nextstellis was safe and effective while meeting its primary endpoint of pregnancy prevention, according to a company press release. Participants also reported favorable results on secondary endpoints including cycle control, bleeding profile, safety, and tolerability.
Although many women take short-acting contraceptives containing estrogen and progestin, concerns persist about side effects, said Mitchell Creinin, MD, of the University of California, in the press release. In addition to providing effective contraception, the drug showed minimal impact on specific markers of concern, including triglycerides, cholesterol, and glucose, as well as weight and endocrine markers, Dr. Creinin said.
Nextstellis was developed by the Belgian biotech company Mithra Pharmaceuticals, and the drug is licensed for distribution in Australia and the United States by Mayne Pharma, with an expected launch at the end of June 2021.
Addressing women’s concerns about the J&J vaccine
A rare form of venous thromboembolism (VTE) has developed in premenopausal women who have received the Johnson & Johnson (J&J) SARS-CoV-2 vaccine.
This week we learned that of the more than 6.8 million individuals in the United States who received the single-dose J&J vaccine, six women aged 18-48 years have been diagnosed with cerebral venous sinus thrombosis, and all had thrombocytopenia. In each case, symptoms were first noted 1-2 weeks after vaccination. The Food and Drug Administration and Centers for Disease Control and Prevention have recommended a pause in the administration of this vaccine.
Women’s health clinicians are already hearing from concerned patients, who understandably have questions about what this news means for them.
If they have already received the J&J vaccine within the past 3 weeks, I advise them that, although risks for any vaccine-related problems are extremely low, they should be mindful of new-onset leg or abdominal pain, or an unusual or severe headache. Such patients should contact their physician as soon as possible, and if they cannot be seen quickly, it would be appropriate to visit a hospital ED. When seeking medical care, patients should specify details of their vaccination history. Depending on the individual issues present, women with suggestive symptoms should receive blood work, Doppler venous studies (if there is a suspicion of lower-extremity deep vein thrombosis), and appropriate imaging (if there is concern for cerebral venous sinus thrombosis or pulmonary embolism).
As physicians and scientists at the CDC and FDA dig into this issue, I assume they are asking questions to determine whether the affected women have any factors that might increase their baseline risk for VTE, such as:
- A body mass index of at least 30 kg/m2
- Use of combination estrogen-progestin contraceptives (pill, ring, or patch)
- Known or suspected chronic inflammatory conditions such as rheumatoid arthritis, systemic lupus erythematosus, or
- Known familial or other thrombophilic conditions or chronic
- Recent prolonged immobility, such as a long airplane or automobile trip, which might increase risk for VTE
Experts say that the risk for a serious adverse event following receipt of the J&J vaccine is outweighed by the benefits of vaccination against COVID disease. However, that may not be enough to allay concerns among some premenopausal women.
Even if the “pause” in the administration of the vaccine is lifted, some women may be asking whether they should receive J&J’s viral vector vaccine or request one of the messenger RNA vaccines. I will be looking to the expert opinions of Anthony S. Fauci, MD, and advice from the CDC and FDA for guidance here. However, it may be reasonable to steer high-risk reproductive-age women away from the J&J vaccine in favor of the Moderna and Pfizer vaccines, if these options are available.
A version of this article first appeared on Medscape.com.
A rare form of venous thromboembolism (VTE) has developed in premenopausal women who have received the Johnson & Johnson (J&J) SARS-CoV-2 vaccine.
This week we learned that of the more than 6.8 million individuals in the United States who received the single-dose J&J vaccine, six women aged 18-48 years have been diagnosed with cerebral venous sinus thrombosis, and all had thrombocytopenia. In each case, symptoms were first noted 1-2 weeks after vaccination. The Food and Drug Administration and Centers for Disease Control and Prevention have recommended a pause in the administration of this vaccine.
Women’s health clinicians are already hearing from concerned patients, who understandably have questions about what this news means for them.
If they have already received the J&J vaccine within the past 3 weeks, I advise them that, although risks for any vaccine-related problems are extremely low, they should be mindful of new-onset leg or abdominal pain, or an unusual or severe headache. Such patients should contact their physician as soon as possible, and if they cannot be seen quickly, it would be appropriate to visit a hospital ED. When seeking medical care, patients should specify details of their vaccination history. Depending on the individual issues present, women with suggestive symptoms should receive blood work, Doppler venous studies (if there is a suspicion of lower-extremity deep vein thrombosis), and appropriate imaging (if there is concern for cerebral venous sinus thrombosis or pulmonary embolism).
As physicians and scientists at the CDC and FDA dig into this issue, I assume they are asking questions to determine whether the affected women have any factors that might increase their baseline risk for VTE, such as:
- A body mass index of at least 30 kg/m2
- Use of combination estrogen-progestin contraceptives (pill, ring, or patch)
- Known or suspected chronic inflammatory conditions such as rheumatoid arthritis, systemic lupus erythematosus, or
- Known familial or other thrombophilic conditions or chronic
- Recent prolonged immobility, such as a long airplane or automobile trip, which might increase risk for VTE
Experts say that the risk for a serious adverse event following receipt of the J&J vaccine is outweighed by the benefits of vaccination against COVID disease. However, that may not be enough to allay concerns among some premenopausal women.
Even if the “pause” in the administration of the vaccine is lifted, some women may be asking whether they should receive J&J’s viral vector vaccine or request one of the messenger RNA vaccines. I will be looking to the expert opinions of Anthony S. Fauci, MD, and advice from the CDC and FDA for guidance here. However, it may be reasonable to steer high-risk reproductive-age women away from the J&J vaccine in favor of the Moderna and Pfizer vaccines, if these options are available.
A version of this article first appeared on Medscape.com.
A rare form of venous thromboembolism (VTE) has developed in premenopausal women who have received the Johnson & Johnson (J&J) SARS-CoV-2 vaccine.
This week we learned that of the more than 6.8 million individuals in the United States who received the single-dose J&J vaccine, six women aged 18-48 years have been diagnosed with cerebral venous sinus thrombosis, and all had thrombocytopenia. In each case, symptoms were first noted 1-2 weeks after vaccination. The Food and Drug Administration and Centers for Disease Control and Prevention have recommended a pause in the administration of this vaccine.
Women’s health clinicians are already hearing from concerned patients, who understandably have questions about what this news means for them.
If they have already received the J&J vaccine within the past 3 weeks, I advise them that, although risks for any vaccine-related problems are extremely low, they should be mindful of new-onset leg or abdominal pain, or an unusual or severe headache. Such patients should contact their physician as soon as possible, and if they cannot be seen quickly, it would be appropriate to visit a hospital ED. When seeking medical care, patients should specify details of their vaccination history. Depending on the individual issues present, women with suggestive symptoms should receive blood work, Doppler venous studies (if there is a suspicion of lower-extremity deep vein thrombosis), and appropriate imaging (if there is concern for cerebral venous sinus thrombosis or pulmonary embolism).
As physicians and scientists at the CDC and FDA dig into this issue, I assume they are asking questions to determine whether the affected women have any factors that might increase their baseline risk for VTE, such as:
- A body mass index of at least 30 kg/m2
- Use of combination estrogen-progestin contraceptives (pill, ring, or patch)
- Known or suspected chronic inflammatory conditions such as rheumatoid arthritis, systemic lupus erythematosus, or
- Known familial or other thrombophilic conditions or chronic
- Recent prolonged immobility, such as a long airplane or automobile trip, which might increase risk for VTE
Experts say that the risk for a serious adverse event following receipt of the J&J vaccine is outweighed by the benefits of vaccination against COVID disease. However, that may not be enough to allay concerns among some premenopausal women.
Even if the “pause” in the administration of the vaccine is lifted, some women may be asking whether they should receive J&J’s viral vector vaccine or request one of the messenger RNA vaccines. I will be looking to the expert opinions of Anthony S. Fauci, MD, and advice from the CDC and FDA for guidance here. However, it may be reasonable to steer high-risk reproductive-age women away from the J&J vaccine in favor of the Moderna and Pfizer vaccines, if these options are available.
A version of this article first appeared on Medscape.com.