User login
Large study finds no link between gluten, IBD risk
Among women without celiac disease, dietary gluten intake was not associated with the risk of developing either Crohn’s disease or ulcerative colitis, investigators reported.
The findings spanned subgroups stratified by age, body mass index, smoking status, and whether individuals primarily consumed refined or whole grains, said Emily Walsh Lopes, MD, gastroenterology clinical and research fellow at Massachusetts General Hospital in Boston. She and associates reported the combined analysis of the prospective Nurses’ Health Study and Nurses’ Health Study II in an abstract released as part of the annual Digestive Disease Week.®
“Avoidance of dietary gluten is common, and many patients attribute gastrointestinal symptoms to gluten intake,” Dr. Lopes said in an interview. “Though our findings warrant further study, the results suggest to patients and providers that eating gluten does not increase a person’s chance of getting diagnosed with inflammatory bowel disease.”
Prior studies have found that many individuals with inflammatory bowel disease avoid gluten and report subsequent improvements in gastrointestinal symptoms, even if they do not have celiac disease. However, it remains unclear whether dietary gluten is a risk factor for new-onset inflammatory bowel disease.
To address this question, Dr. Lopes and associates analyzed data collected from 165,327 women who took part in the Nurses’ Health Study (1986 to 2016) or the Nurses’ Health Study II (1991 through 2017). None of the women had a preexisting diagnosis of celiac disease or inflammatory bowel disease. Dietary gluten intake was estimated based on food frequency questionnaires completed by the women at baseline and every 4 years. The researchers also reviewed medical records to confirm self-reported cases of new-onset ulcerative colitis and Crohn’s disease.
Over 4.02 million person-years of follow-up, 277 women developed Crohn’s disease and 359 developed ulcerative colitis. Gluten intake was not associated with the risk of either type of inflammatory bowel disease, even after the researchers controlled for multiple demographic and clinical risk factors.
After submitting their abstract, Dr. Lopes and coinvestigators expanded the dataset to include a large cohort of men from the prospective Health Professionals Follow-up Study. The final pooled cohort included more than 208,000 women and men followed for more than 20 years. Through the end of follow-up, the researchers documented 337 cases of Crohn’s disease and 446 cases of ulcerative colitis. “Inclusion of the male cohort in the pooled analysis did not materially change our estimates,” Dr. Lopes told MDedge. “That is, no association was seen between gluten intake and risk of either Crohn’s disease or ulcerative colitis in the final cohort.”
She noted that the findings cannot be extrapolated to individuals who are already diagnosed with inflammatory bowel disease. “It is possible that different mechanisms exist to explain how gluten intake impacts those already diagnosed with IBD, and this topic warrants further study,” she said. Also, because the three cohort studies were observational, they are subject to bias. “While we tried to account for this in our analyses, residual bias may still exist.”
Dr. Lopes reported having no conflicts of interest.
SOURCE: Walsh Lopes E et al. DDW 2020, abstract 847.
Among women without celiac disease, dietary gluten intake was not associated with the risk of developing either Crohn’s disease or ulcerative colitis, investigators reported.
The findings spanned subgroups stratified by age, body mass index, smoking status, and whether individuals primarily consumed refined or whole grains, said Emily Walsh Lopes, MD, gastroenterology clinical and research fellow at Massachusetts General Hospital in Boston. She and associates reported the combined analysis of the prospective Nurses’ Health Study and Nurses’ Health Study II in an abstract released as part of the annual Digestive Disease Week.®
“Avoidance of dietary gluten is common, and many patients attribute gastrointestinal symptoms to gluten intake,” Dr. Lopes said in an interview. “Though our findings warrant further study, the results suggest to patients and providers that eating gluten does not increase a person’s chance of getting diagnosed with inflammatory bowel disease.”
Prior studies have found that many individuals with inflammatory bowel disease avoid gluten and report subsequent improvements in gastrointestinal symptoms, even if they do not have celiac disease. However, it remains unclear whether dietary gluten is a risk factor for new-onset inflammatory bowel disease.
To address this question, Dr. Lopes and associates analyzed data collected from 165,327 women who took part in the Nurses’ Health Study (1986 to 2016) or the Nurses’ Health Study II (1991 through 2017). None of the women had a preexisting diagnosis of celiac disease or inflammatory bowel disease. Dietary gluten intake was estimated based on food frequency questionnaires completed by the women at baseline and every 4 years. The researchers also reviewed medical records to confirm self-reported cases of new-onset ulcerative colitis and Crohn’s disease.
Over 4.02 million person-years of follow-up, 277 women developed Crohn’s disease and 359 developed ulcerative colitis. Gluten intake was not associated with the risk of either type of inflammatory bowel disease, even after the researchers controlled for multiple demographic and clinical risk factors.
After submitting their abstract, Dr. Lopes and coinvestigators expanded the dataset to include a large cohort of men from the prospective Health Professionals Follow-up Study. The final pooled cohort included more than 208,000 women and men followed for more than 20 years. Through the end of follow-up, the researchers documented 337 cases of Crohn’s disease and 446 cases of ulcerative colitis. “Inclusion of the male cohort in the pooled analysis did not materially change our estimates,” Dr. Lopes told MDedge. “That is, no association was seen between gluten intake and risk of either Crohn’s disease or ulcerative colitis in the final cohort.”
She noted that the findings cannot be extrapolated to individuals who are already diagnosed with inflammatory bowel disease. “It is possible that different mechanisms exist to explain how gluten intake impacts those already diagnosed with IBD, and this topic warrants further study,” she said. Also, because the three cohort studies were observational, they are subject to bias. “While we tried to account for this in our analyses, residual bias may still exist.”
Dr. Lopes reported having no conflicts of interest.
SOURCE: Walsh Lopes E et al. DDW 2020, abstract 847.
Among women without celiac disease, dietary gluten intake was not associated with the risk of developing either Crohn’s disease or ulcerative colitis, investigators reported.
The findings spanned subgroups stratified by age, body mass index, smoking status, and whether individuals primarily consumed refined or whole grains, said Emily Walsh Lopes, MD, gastroenterology clinical and research fellow at Massachusetts General Hospital in Boston. She and associates reported the combined analysis of the prospective Nurses’ Health Study and Nurses’ Health Study II in an abstract released as part of the annual Digestive Disease Week.®
“Avoidance of dietary gluten is common, and many patients attribute gastrointestinal symptoms to gluten intake,” Dr. Lopes said in an interview. “Though our findings warrant further study, the results suggest to patients and providers that eating gluten does not increase a person’s chance of getting diagnosed with inflammatory bowel disease.”
Prior studies have found that many individuals with inflammatory bowel disease avoid gluten and report subsequent improvements in gastrointestinal symptoms, even if they do not have celiac disease. However, it remains unclear whether dietary gluten is a risk factor for new-onset inflammatory bowel disease.
To address this question, Dr. Lopes and associates analyzed data collected from 165,327 women who took part in the Nurses’ Health Study (1986 to 2016) or the Nurses’ Health Study II (1991 through 2017). None of the women had a preexisting diagnosis of celiac disease or inflammatory bowel disease. Dietary gluten intake was estimated based on food frequency questionnaires completed by the women at baseline and every 4 years. The researchers also reviewed medical records to confirm self-reported cases of new-onset ulcerative colitis and Crohn’s disease.
Over 4.02 million person-years of follow-up, 277 women developed Crohn’s disease and 359 developed ulcerative colitis. Gluten intake was not associated with the risk of either type of inflammatory bowel disease, even after the researchers controlled for multiple demographic and clinical risk factors.
After submitting their abstract, Dr. Lopes and coinvestigators expanded the dataset to include a large cohort of men from the prospective Health Professionals Follow-up Study. The final pooled cohort included more than 208,000 women and men followed for more than 20 years. Through the end of follow-up, the researchers documented 337 cases of Crohn’s disease and 446 cases of ulcerative colitis. “Inclusion of the male cohort in the pooled analysis did not materially change our estimates,” Dr. Lopes told MDedge. “That is, no association was seen between gluten intake and risk of either Crohn’s disease or ulcerative colitis in the final cohort.”
She noted that the findings cannot be extrapolated to individuals who are already diagnosed with inflammatory bowel disease. “It is possible that different mechanisms exist to explain how gluten intake impacts those already diagnosed with IBD, and this topic warrants further study,” she said. Also, because the three cohort studies were observational, they are subject to bias. “While we tried to account for this in our analyses, residual bias may still exist.”
Dr. Lopes reported having no conflicts of interest.
SOURCE: Walsh Lopes E et al. DDW 2020, abstract 847.
FROM DDW 2020
High ‘forever chemicals’ in blood linked to earlier menopause
In a national sample of U.S. women in their mid-40s to mid-50s, those with high serum levels of per- and polyfluoroalkyl substances (PFAS) were likely to enter menopause 2 years earlier than those with low levels of these chemicals.
That is, the median age of natural menopause was 52.8 years versus 50.8 years in women with high versus low serum levels of these chemicals in an analysis of data from more than 1,100 women in the Study of Women’s Health Across the Nation (SWAN) Multi-Pollutant Study, which excluded women with premature menopause (before age 40) or early menopause (before age 45).
“This study suggests that select PFAS serum concentrations are associated with earlier natural menopause, a risk factor for adverse health outcomes in later life,” Ning Ding, PhD, MPH, University of Michigan, Ann Arbor, and colleagues concluded in their article, published online June 3 in the Journal of Clinical Endocrinology & Metabolism.
“Even menopause a few years earlier than usual could have a significant impact on cardiovascular and bone health, quality of life, and overall health in general among women,” senior author Sung Kyun Park, ScD, MPH, from the same institution, added in a statement.
PFAS don’t break down in the body, build up with time
PFAS have been widely used in many consumer and industrial products such as nonstick cookware, stain-repellent carpets, waterproof rain gear, microwave popcorn bags, and firefighting foam, the authors explained.
These have been dubbed “forever chemicals” because they do not degrade. Household water for an estimated 110 million Americans (one in three) may be contaminated with these chemicals, according to an Endocrine Society press release.
“PFAS are everywhere. Once they enter the body, they don’t break down and [they] build up over time,” said Dr. Ding. “Because of their persistence in humans and potentially detrimental effects on ovarian function, it is important to raise awareness of this issue and reduce exposure to these chemicals.”
Environmental exposure and accelerated ovarian aging
Earlier menopause has been associated with an increased risk of cardiovascular disease, osteoporosis, and earlier cardiovascular and overall mortality, and environmental exposure may accelerate ovarian aging, the authors wrote.
PFAS, especially the most studied types – perfluorooctanoic acid (PFOA) and perfluorooctane sulfonic acid (PFOS) – are plausible endocrine-disrupting chemicals, but findings so far have been inconsistent.
A study of people in Ohio exposed to contaminated water found that women with earlier natural menopause had higher serum PFOA and PFOS levels (J Clin Endocriniol Metab. 2011;96:1747-53).
But in research based on National Health and Nutrition Survey Examination data, higher PFOA, PFOS, or perfluorononanoic acid (PFNA) levels were not linked to earlier menopause, although higher levels of perfluorohexane sulfonic acid (PFHxS) were (Environ Health Perspect. 2014;122:145-50).
There may have been reverse causation, where postmenopausal women had higher PFAS levels because they were not excreting these chemicals in menstrual blood.
In a third study, PFOA exposure was not linked with age at menopause onset, but this was based on recall from 10 years earlier (Environ Res. 2016;146:323-30).
The current analysis examined data from 1,120 premenopausal women who were aged 45-56 years from 1999 to 2000.
The women were seen at five sites (Boston; Detroit; Los Angeles; Oakland, Calif.; and Pittsburgh) and were ethnically diverse (577 white, 235 black, 142 Chinese, and 166 Japanese).
Baseline serum PFAS levels were measured using high performance liquid chromatography-mass spectrometry. The women were followed up to 2017 and incident menopause (12 consecutive months with no menstruation) was determined from annual interviews.
Of the 1,120 women and 5,466 person-years of follow-up, 578 women had a known date of natural incident menopause and were included in the analysis. The remaining 542 women were excluded mainly because their date of final menstruation was unknown because of hormone therapy (451) or they had a hysterectomy, or did not enter menopause during the study.
Compared with women in the lowest tertile of PFOS levels, women in the highest tertile had a significant 26%-27% greater risk of incident menopause – after adjusting for age, body mass index, and prior hormone use, race/ethnicity, study site, education, physical activity, smoking status, and parity.
Higher PFOA and PFNA levels but not higher PFHxS levels were also associated with increased risk.
Compared with women with a low overall PFAS level, those with a high level had a 63% increased risk of incident menopause (hazard ratio, 1.63; 95% confidence interval, 1.08-2.45), equivalent to having menopause a median of 2 years earlier.
Although production and use of some types of PFAS in the United States are declining, Dr. Ding and colleagues wrote, exposure continues, along with associated potential hazards to human reproductive health.
“Due to PFAS widespread use and environmental persistence, their potential adverse effects remain a public health concern,” they concluded.
SWAN was supported by the National Institutes of Health, Department of Health & Human Services through the National Institute on Aging, National Institute of Nursing Research, NIH Office of Research on Women’s Health, and the SWAN repository. The current article was supported by the National Center for Research Resources and National Center for Advancing Translational Sciences, NIH, National Institute of Environmental Health Sciences, and Centers for Disease Control and Prevention/National Institute for Occupational Safety and Health. The authors have reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
In a national sample of U.S. women in their mid-40s to mid-50s, those with high serum levels of per- and polyfluoroalkyl substances (PFAS) were likely to enter menopause 2 years earlier than those with low levels of these chemicals.
That is, the median age of natural menopause was 52.8 years versus 50.8 years in women with high versus low serum levels of these chemicals in an analysis of data from more than 1,100 women in the Study of Women’s Health Across the Nation (SWAN) Multi-Pollutant Study, which excluded women with premature menopause (before age 40) or early menopause (before age 45).
“This study suggests that select PFAS serum concentrations are associated with earlier natural menopause, a risk factor for adverse health outcomes in later life,” Ning Ding, PhD, MPH, University of Michigan, Ann Arbor, and colleagues concluded in their article, published online June 3 in the Journal of Clinical Endocrinology & Metabolism.
“Even menopause a few years earlier than usual could have a significant impact on cardiovascular and bone health, quality of life, and overall health in general among women,” senior author Sung Kyun Park, ScD, MPH, from the same institution, added in a statement.
PFAS don’t break down in the body, build up with time
PFAS have been widely used in many consumer and industrial products such as nonstick cookware, stain-repellent carpets, waterproof rain gear, microwave popcorn bags, and firefighting foam, the authors explained.
These have been dubbed “forever chemicals” because they do not degrade. Household water for an estimated 110 million Americans (one in three) may be contaminated with these chemicals, according to an Endocrine Society press release.
“PFAS are everywhere. Once they enter the body, they don’t break down and [they] build up over time,” said Dr. Ding. “Because of their persistence in humans and potentially detrimental effects on ovarian function, it is important to raise awareness of this issue and reduce exposure to these chemicals.”
Environmental exposure and accelerated ovarian aging
Earlier menopause has been associated with an increased risk of cardiovascular disease, osteoporosis, and earlier cardiovascular and overall mortality, and environmental exposure may accelerate ovarian aging, the authors wrote.
PFAS, especially the most studied types – perfluorooctanoic acid (PFOA) and perfluorooctane sulfonic acid (PFOS) – are plausible endocrine-disrupting chemicals, but findings so far have been inconsistent.
A study of people in Ohio exposed to contaminated water found that women with earlier natural menopause had higher serum PFOA and PFOS levels (J Clin Endocriniol Metab. 2011;96:1747-53).
But in research based on National Health and Nutrition Survey Examination data, higher PFOA, PFOS, or perfluorononanoic acid (PFNA) levels were not linked to earlier menopause, although higher levels of perfluorohexane sulfonic acid (PFHxS) were (Environ Health Perspect. 2014;122:145-50).
There may have been reverse causation, where postmenopausal women had higher PFAS levels because they were not excreting these chemicals in menstrual blood.
In a third study, PFOA exposure was not linked with age at menopause onset, but this was based on recall from 10 years earlier (Environ Res. 2016;146:323-30).
The current analysis examined data from 1,120 premenopausal women who were aged 45-56 years from 1999 to 2000.
The women were seen at five sites (Boston; Detroit; Los Angeles; Oakland, Calif.; and Pittsburgh) and were ethnically diverse (577 white, 235 black, 142 Chinese, and 166 Japanese).
Baseline serum PFAS levels were measured using high performance liquid chromatography-mass spectrometry. The women were followed up to 2017 and incident menopause (12 consecutive months with no menstruation) was determined from annual interviews.
Of the 1,120 women and 5,466 person-years of follow-up, 578 women had a known date of natural incident menopause and were included in the analysis. The remaining 542 women were excluded mainly because their date of final menstruation was unknown because of hormone therapy (451) or they had a hysterectomy, or did not enter menopause during the study.
Compared with women in the lowest tertile of PFOS levels, women in the highest tertile had a significant 26%-27% greater risk of incident menopause – after adjusting for age, body mass index, and prior hormone use, race/ethnicity, study site, education, physical activity, smoking status, and parity.
Higher PFOA and PFNA levels but not higher PFHxS levels were also associated with increased risk.
Compared with women with a low overall PFAS level, those with a high level had a 63% increased risk of incident menopause (hazard ratio, 1.63; 95% confidence interval, 1.08-2.45), equivalent to having menopause a median of 2 years earlier.
Although production and use of some types of PFAS in the United States are declining, Dr. Ding and colleagues wrote, exposure continues, along with associated potential hazards to human reproductive health.
“Due to PFAS widespread use and environmental persistence, their potential adverse effects remain a public health concern,” they concluded.
SWAN was supported by the National Institutes of Health, Department of Health & Human Services through the National Institute on Aging, National Institute of Nursing Research, NIH Office of Research on Women’s Health, and the SWAN repository. The current article was supported by the National Center for Research Resources and National Center for Advancing Translational Sciences, NIH, National Institute of Environmental Health Sciences, and Centers for Disease Control and Prevention/National Institute for Occupational Safety and Health. The authors have reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
In a national sample of U.S. women in their mid-40s to mid-50s, those with high serum levels of per- and polyfluoroalkyl substances (PFAS) were likely to enter menopause 2 years earlier than those with low levels of these chemicals.
That is, the median age of natural menopause was 52.8 years versus 50.8 years in women with high versus low serum levels of these chemicals in an analysis of data from more than 1,100 women in the Study of Women’s Health Across the Nation (SWAN) Multi-Pollutant Study, which excluded women with premature menopause (before age 40) or early menopause (before age 45).
“This study suggests that select PFAS serum concentrations are associated with earlier natural menopause, a risk factor for adverse health outcomes in later life,” Ning Ding, PhD, MPH, University of Michigan, Ann Arbor, and colleagues concluded in their article, published online June 3 in the Journal of Clinical Endocrinology & Metabolism.
“Even menopause a few years earlier than usual could have a significant impact on cardiovascular and bone health, quality of life, and overall health in general among women,” senior author Sung Kyun Park, ScD, MPH, from the same institution, added in a statement.
PFAS don’t break down in the body, build up with time
PFAS have been widely used in many consumer and industrial products such as nonstick cookware, stain-repellent carpets, waterproof rain gear, microwave popcorn bags, and firefighting foam, the authors explained.
These have been dubbed “forever chemicals” because they do not degrade. Household water for an estimated 110 million Americans (one in three) may be contaminated with these chemicals, according to an Endocrine Society press release.
“PFAS are everywhere. Once they enter the body, they don’t break down and [they] build up over time,” said Dr. Ding. “Because of their persistence in humans and potentially detrimental effects on ovarian function, it is important to raise awareness of this issue and reduce exposure to these chemicals.”
Environmental exposure and accelerated ovarian aging
Earlier menopause has been associated with an increased risk of cardiovascular disease, osteoporosis, and earlier cardiovascular and overall mortality, and environmental exposure may accelerate ovarian aging, the authors wrote.
PFAS, especially the most studied types – perfluorooctanoic acid (PFOA) and perfluorooctane sulfonic acid (PFOS) – are plausible endocrine-disrupting chemicals, but findings so far have been inconsistent.
A study of people in Ohio exposed to contaminated water found that women with earlier natural menopause had higher serum PFOA and PFOS levels (J Clin Endocriniol Metab. 2011;96:1747-53).
But in research based on National Health and Nutrition Survey Examination data, higher PFOA, PFOS, or perfluorononanoic acid (PFNA) levels were not linked to earlier menopause, although higher levels of perfluorohexane sulfonic acid (PFHxS) were (Environ Health Perspect. 2014;122:145-50).
There may have been reverse causation, where postmenopausal women had higher PFAS levels because they were not excreting these chemicals in menstrual blood.
In a third study, PFOA exposure was not linked with age at menopause onset, but this was based on recall from 10 years earlier (Environ Res. 2016;146:323-30).
The current analysis examined data from 1,120 premenopausal women who were aged 45-56 years from 1999 to 2000.
The women were seen at five sites (Boston; Detroit; Los Angeles; Oakland, Calif.; and Pittsburgh) and were ethnically diverse (577 white, 235 black, 142 Chinese, and 166 Japanese).
Baseline serum PFAS levels were measured using high performance liquid chromatography-mass spectrometry. The women were followed up to 2017 and incident menopause (12 consecutive months with no menstruation) was determined from annual interviews.
Of the 1,120 women and 5,466 person-years of follow-up, 578 women had a known date of natural incident menopause and were included in the analysis. The remaining 542 women were excluded mainly because their date of final menstruation was unknown because of hormone therapy (451) or they had a hysterectomy, or did not enter menopause during the study.
Compared with women in the lowest tertile of PFOS levels, women in the highest tertile had a significant 26%-27% greater risk of incident menopause – after adjusting for age, body mass index, and prior hormone use, race/ethnicity, study site, education, physical activity, smoking status, and parity.
Higher PFOA and PFNA levels but not higher PFHxS levels were also associated with increased risk.
Compared with women with a low overall PFAS level, those with a high level had a 63% increased risk of incident menopause (hazard ratio, 1.63; 95% confidence interval, 1.08-2.45), equivalent to having menopause a median of 2 years earlier.
Although production and use of some types of PFAS in the United States are declining, Dr. Ding and colleagues wrote, exposure continues, along with associated potential hazards to human reproductive health.
“Due to PFAS widespread use and environmental persistence, their potential adverse effects remain a public health concern,” they concluded.
SWAN was supported by the National Institutes of Health, Department of Health & Human Services through the National Institute on Aging, National Institute of Nursing Research, NIH Office of Research on Women’s Health, and the SWAN repository. The current article was supported by the National Center for Research Resources and National Center for Advancing Translational Sciences, NIH, National Institute of Environmental Health Sciences, and Centers for Disease Control and Prevention/National Institute for Occupational Safety and Health. The authors have reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Managing a woman with BRCA mutations? Shared decision-making is key
CASE
Sara T* recently moved back to the area to be closer to her family. The 34-year-old patient visited our office to discuss the benefits and potential risks of genetic counseling. She explained that her aunt had just died at age 64 of ovarian cancer. Also, her maternal cousin had been diagnosed at age 42 with breast cancer, and her maternal grandmother had died at age 45 of an unknown “female cancer.” She was scared to find out if she had high-risk genes because she felt it would change her life forever. However, if she ignored the issue, she thought she might worry too much.
We discussed the implications of a positive result, such as having to live with the knowledge and to make decisions about potential screening and risk-reducing surgery. On the other hand, not knowing could allow for the undetected growth of cancer that might otherwise be mitigated to some degree if she knew her risk status and pursued an aggressive screening program.
We worked with Ms. T to map out her next steps.
*The patient’s name has been changed to protect her identity.
Breast cancer is the most commonly diagnosed cancer in women worldwide, representing nearly one-quarter of all female cancer diagnoses in 2018.1 It is the second-leading cause of cancer death in women in developed nations and the leading cause of cancer death in women in developing nations.1 In the United States, 1 in 8 women will develop breast cancer in her lifetime.2 By comparison, the rate of ovarian cancer is much lower, with a lifetime prevalence of 1 in 70 to 80 women.3,4 Although ovarian cancer is less common than breast cancer, its associated mortality is high, and most cases are discovered at advanced stages.
The outsized threat of BRCA mutations. It is estimated that 5% to 10% of all breast cancers are hereditary, with 80% of these attributable to BRCA1 (45%) and BRCA2 (35%).5 These autosomal dominant mutations occur at the germline level, within the egg or sperm, and are therefore incorporated into the DNA of every cell and passed from one generation to the next. Families with BRCA mutations have much higher lifetime rates of cancer. The lifetime risk of breast cancer due to BRCA mutations is estimated at > 80% (BRCA1) and 45% (BRCA2).5BRCA mutations account for between
Male BRCA carriers have a lifetime breast cancer risk of 1% to 5% with BRCA1 and 5% to 10% with BRCA2,7,8 compared with about 1:1000 lifetime incidence in the unselected male population. Male carriers are also at risk for more aggressive prostate cancers.7,8
Continue to: Certain populatiosn carry undue burden of BRCA-related disease
Certain populations carry undue burden of BRCA-related disease due to specific founder mutations. While the estimated global prevalence of BRCA mutations is 0.2% to 1%, for those of Ashkenazi Jewish descent the range is 2% to 3%, representing a relative risk up to 15 times that of the general population.9 Hispanic Americans also appear to have higher rates of BRCA-related cancers.10 Ongoing genetics research continues to identify founder mutations worldwide,10 which may inform future screening guidelines.
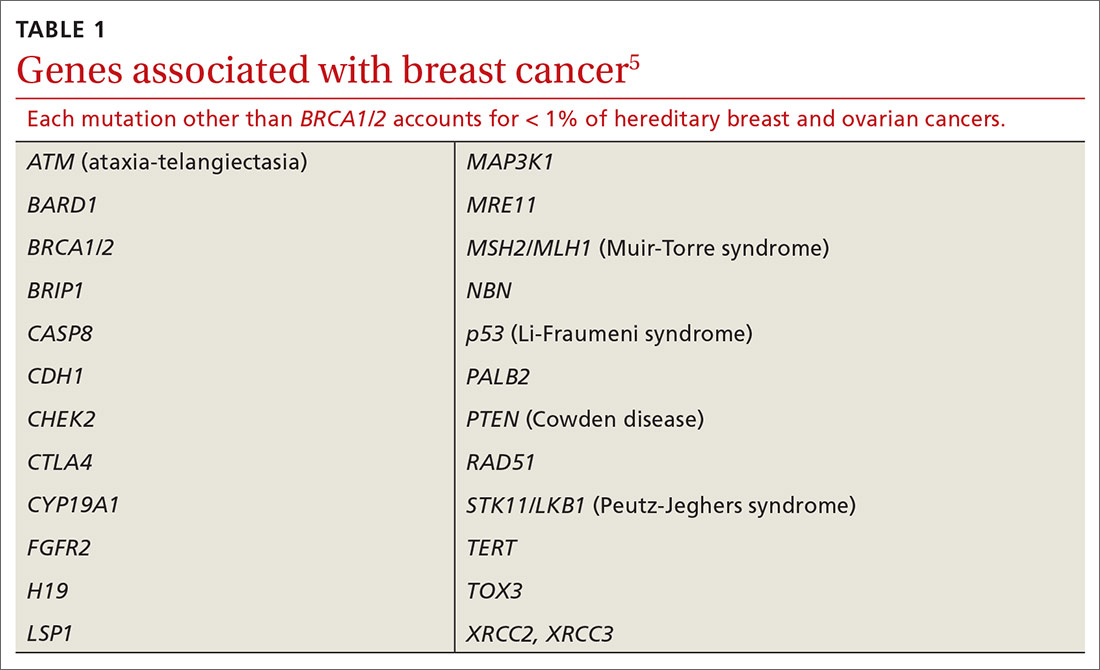
In addition to BRCA mutations, there are other, less common mutations (TABLE 15) known to cause hereditary breast and ovarian cancer.
Identifying BRCA genes enables treatment planning. Compared with sporadic cancers, BRCA-related breast cancers
Similarly, BRCA-related ovarian cancer is more likely to be high-grade and endometrioid or serous subtype.14
Shared decision-making helps give clarity to the way forward
Shared decision-making is a process of communication whereby the clinician and the patient identify a decision to be made, review data relevant to clinical options, discuss patient perspectives and preferences regarding each option, and arrive at the decision together.17 Shared decision-making is important when treating women with BRCA mutations because there is no single correct plan. Individual values and competing medical issues may strongly guide each woman’s decisions about screening and cancer prevention treatment decisions.
Continue to: Shared decision-making in this situation...
Shared decision-making in this situation is a strategy to use the evidence of risk along with patient preferences around fertility issues to help come to a decision that is the right one for the patient. Primary care clinicians aware of the general risks and benefits of each available option can refer women at high risk for breast or ovarian cancer to a specialist multidisciplinary clinic that can provide tailored risk assessment and risk reduction counseling as needed.18-20
Genetic screening recommendations
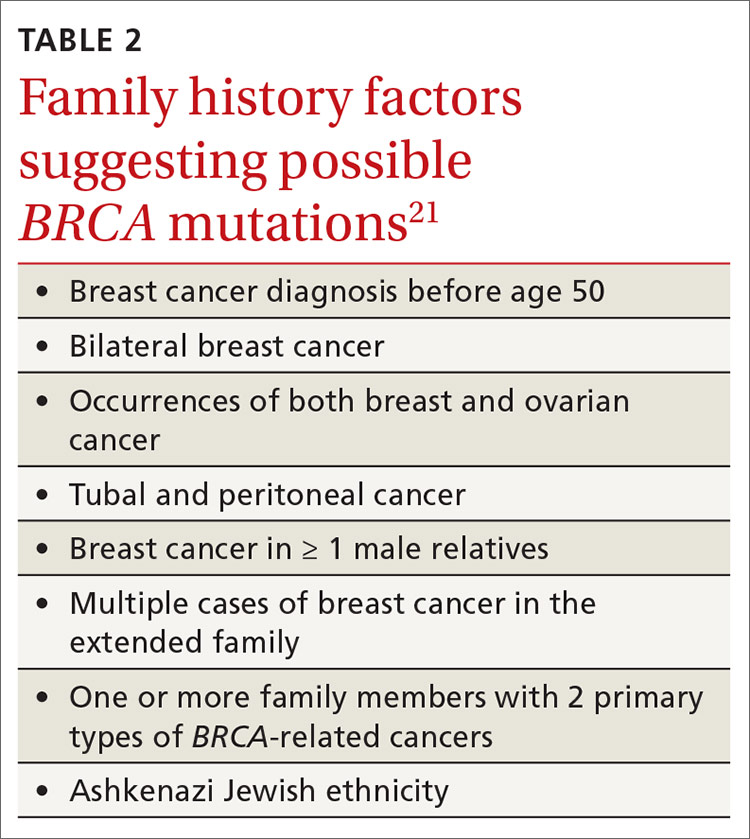
Screening is recommended for women who have any 1 of several family risk factors (TABLE 221). A number of risk assessment tools are available for primary care clinicians to determine which patients are at high enough risk for a hereditary breast or ovarian cancer to warrant referral to a genetic counselor.22-25 If screening suggests high risk, the US Preventive Services Task Force (USPSTF) recommends (Grade B) referral for genetic counseling.21
Explain to patients who are candidates for further investigation that a genetic counselor will review their family history and recommend testing for the specific mutations that increase cancer risk. Discuss potential benefits and harms of genetic testing. A benefit of genetic testing is that aggressive screening may suggest preventive procedures to reduce the risk of future cancer. Most tests come back definitively positive or negative, but an indeterminate result may cause harm. A small minority of results may indicate a genetic variant of unknown significance. The ramifications of this variant may not be known. Some women will experience anxiety about nonspecific test results and will be afraid to share them with family members. There is also some concern about privacy issues, potential insurance bias, and coverage of any preventive strategies.26
CASE
Based on Ms. T’s family history and her desire to know more, we referred her to a genetic counselor and she decided to undergo genetic testing. She screened positive for BRCA1. Ms. T was in a serious relationship and thought she would like to have children at some point. She returned to our office after receiving the positive genetic test results, wondering about screening for breast and ovarian cancer.
Breast cancer screening and risk-reduction strategies
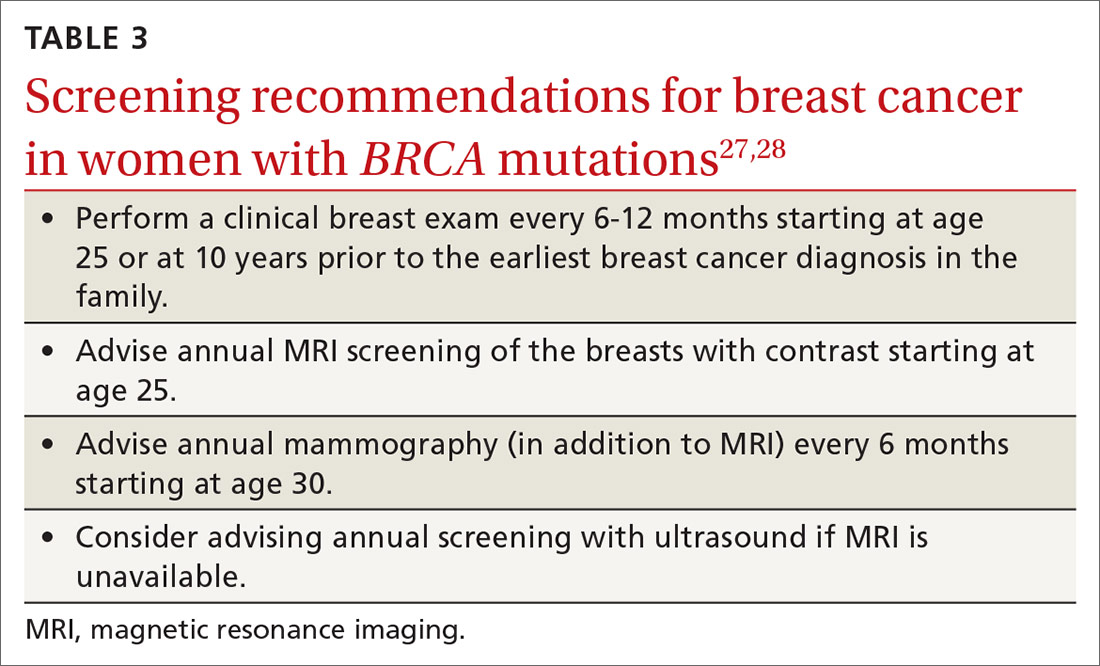
Screening. Because the risk of breast cancer is high in women with BRCA mutations, and because cancer in these women is more likely to be advanced at diagnosis, starting a screening program at an early age is prudent. Observational studies suggest that breastfeeding reduces the risk of breast and ovarian cancer in women with BRCA mutations, as it does for women in the general population.27 Women should return for a clinical breast exam every 6 to 12 months starting at age 25; they should start radiologic screening with magnetic resonance imaging at age 25 and mammography at age 30 (TABLE 327,28).
Continue to: Risk-reduction strategies
Risk-reduction strategies. There is weak evidence to support the use of tamoxifen or other synthetic estrogen reuptake modulators (SERMs) to reduce breast cancer risk in women with BRCA mutations. Many of these cancers do not express estrogen receptors, which may explain the lack of efficacy in certain cases. Several observational studies have shown that tamoxifen can reduce the risk of contralateral breast cancer in women with BRCA mutations who have already been diagnosed with cancer in the other breast.29-31 However, tamoxifen does not reduce a patient’s risk of ovarian cancer, and it may increase her risk of uterine cancer.
Prophylactic bilateral mastectomy is the mainstay of breast cancer prevention in this population. Data from a systematic review suggest that this surgery may prevent the incidence of breast cancer in women with BRCA mutations by 90% to 95%.32 However, this review did not demonstrate a reduction in mortality from breast cancer, likely due to poor data quality.32 The National Comprehensive Cancer Network (NCCN) recommends discussing prophylactic mastectomy with all women who have BRCA mutations.28 Further conversations are important to review the risk of tissue left behind and quality-of-life issues, including the inability to breastfeed if the woman wants more children and the cosmetic changes with reconstruction.
Ovarian cancer screening and risk-reduction strategies
Screening. No effective screening strategy has been endorsed for ovarian cancer, as most previous studies have shown screening to be ineffective.26,33 Recently, studies both in the United Kingdom and the United States have investigated a screening strategy using the risk-of-ovarian-cancer algorithm (ROCA), which calculates an individual’s risk based on serum levels of cancer antigen 125 (CA-125).34,35 These studies measured CA-125 levels every 3 to 4 months followed by transvaginal ultrasound if CA-125 increased substantially (as determined by ROCA). Absent an abnormal increase in CA-125, transvaginal ultrasound was performed annually. These screening strategies showed improved specificity over annual screening programs, and the cancers detected were more likely to be diagnosed at an early stage (stage II vs stage III) and had higher rates of zero residual disease after surgery compared with those detected 1 year after screening ended.34,35 However, survival data are not yet available. More research is needed to determine if more frequent screening approaches could improve survival in high-risk women.
NCCN and the American College of Obstetricians and Gynecologists (ACOG) do not endorse routine screening with transvaginal ultrasound and serum CA-125 for high-risk women, as the benefits are uncertain. However, they do advise that these screens may be considered as a short-term strategy for women ages 30 to 35 who defer risk-reducing surgery.26,36 The USPSTF does not make a recommendation regarding ovarian cancer screening in high-risk women.37
Risk-reduction strategies. Risk-reducing bilateral salpingo-oophorectomy (RRSO) is the only recommended technique for reducing the risk of ovarian cancer in women at high risk.26,33,36 Meta-analyses have shown an 80% reduction in ovarian cancer risk16 and 68% reduction in all-cause mortality with this approach.38 The NCCN recommends RRSO for women with a known BRCA1 mutation between the ages of 35 and 40 who have completed childbearing.36 Since the onset of ovarian cancer tends to be later in women with BRCA2 mutations, it is reasonable to delay RRSO until age 40 to 45 in this population if they have taken other steps to maximize breast cancer prevention (ie, bilateral mastectomy).36
Continue to: Adverse effects of RRSO...
Adverse effects of RRSO include surgery complications (wound infection, small bowel obstruction, bladder perforation) and effects of early menopause (vasomotor symptoms, decreased sexual functioning, and increased risk of osteoporosis, cardiovascular disease, and all-cause mortality).39-41 In the absence of contraindications, ACOG recommends using hormone therapy in women undergoing RRSO until the natural age of menopause,42 particularly if their breast tissue has been removed.
Salpingectomy as an alternative. In an attempt to reduce these adverse effects of early menopause, and because a large proportion of high-grade serous tumors originate in the fallopian tube,43 interest has increased in the use of risk-reducing salpingectomy (removal of fallopian tubes) and delayed oophorectomy in women at high risk of ovarian cancer.42 Studies have shown this may be a cost-effective approach and an acceptable alternative in BRCA mutation carriers who are unwilling to undergo RRSO.44,45 A clinical trial investigating this approach in women with BRCA mutations is currently underway in the United States.46 Many centers offer salpingectomy to high-risk patients < 40 years old, understanding that ovary removal is an eventuality for these patients.
When oral contraceptive pills might be beneficial. In younger women with BRCA mutations, there may also be a role for oral contraceptive pills (OCPs) as a risk-reducing strategy. Meta-analyses have shown an approximately 50% reduction in the risk of ovarian cancer among women with BRCA mutations who use OCPs.47-49
ACOG advises that it is appropriate for women with BRCA mutations to use oral contraceptives if indicated (for pregnancy prevention or menstrual cycle regulation), and that it is reasonable to use them for cancer prevention.26 NCCN does not make a formal recommendation, although it does state OCPs may reduce the risk of ovarian cancer in women with a BRCA mutation.36 Case-control studies have produced conflicting data on the association between OCP use and breast cancer risk in BRCA mutation carriers,50-53 although 2 meta-analyses found no significant association in this population.47,48
Decision aids for women with BRCA mutations
Decision aids are visual displays of risk that help patients work through complex decisions. Most decision aids are in print or digital format and include information about the decision to be made as well as pictorial examples of possible outcomes. Pictographs are especially helpful in communicating information. Some decision aids for women with BRCA mutations can be complicated with multiple outcomes (ie, breast cancer and ovarian cancer) and multiple potential interventions (risk-reducing surgery, enhanced screening options).54
Continue to: A Cochrane review...
A Cochrane review found that decision aids increased patients’ knowledge, helped patients clarify their values, and may improve value-concordant decisions.55 Two papers describing the use of decision aids for women with BRCA mutations56,57 documented decreased decisional conflict and increased satisfaction.
CASE
Ms. T underwent the recommended mammogram and MRI screening for breast cancer, as well as testing with serum CA-125 and ultrasound examinations for ovarian cancer. Her initial mammogram and MRI revealed early stage, triple-negative right breast cancer. She chose to undergo bilateral mastectomy and reconstruction. She has now completed treatment and continues to work closely with her oncology team for appropriate breast follow-up.
One year after her initial diagnosis, at the age of 35, she returned to discuss fertility. She was recently married, and she and her husband wanted to start having children. She was concerned about a safe timeline for her to pursue pregnancy, saying she felt “like a ticking time-bomb” given her prior cancer and carrier status. She wanted to discuss the risks and benefits of pregnancy and when she should consider prophylactic oophorectomy. She had a few options. She could have a baby and then undergo an RRSO, or she could talk to her gynecologist about having a salpingectomy to reduce her risk now and use assisted reproductive technology to get pregnant. She could also freeze eggs or embryos, have an RRSO, and then use a surrogate to get pregnant. We informed her that pregnancy would not affect her risk of ovarian cancer and discussed the options for pre-implantation genetic testing to assure that her children would not carry the genetic mutation.58
We provided Ms. T and her husband with a decision aid to help them navigate the decision. They are currently evaluating the options and said they would let us know when they made a decision.
CORRESPONDENCE
Sarina Schrager, MD, MS, Northeast Family Medicine Center, 3209 Dryden Drive, Madison, WI, 53704; sbschrag@wisc.edu.
1. Ferlay J, Colombet M, Soerjomataram I, et al. Estimating the global cancer incidence in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019;144:1941-1953.
2. SEER Cancer Statistics Review, 1975-2016. Cancer of the female breast. [Table 4.1] National Cancer Institute. Bethesda, MD. https://seer.cancer.gov/archive/csr/1975_2016/results_merged/sect_04_breast.pdf. Accessed May 27, 2020.
3. SEER Cancer Statistics Review, 1975-2016. Cancer of the ovary. [Table 21.10] National Cancer Institute. Bethesda, MD. https://seer.cancer.gov/archive/csr/1975_2016/results_merged/sect_21_ovary.pdf. Accessed May 22, 2020.
4. Torre LA, Trabert B, DeSantis C, et al. Ovarian cancer statistics, 2018. CA Cancer J Clin. 2018;68:284-296.
5. Narod SA, Foulkes WD. BRCA1 and BRCA2: 1994 and beyond. Nat Rev Cancer. 2004;4:665-676.
6. Pal T, Permuth-Wey J, Betts JA, et al. BRCA1 and BRCA2 mutations account for a large proportion of ovarian carcinoma cases. Cancer. 2005;104:2807-2816.
7. Tai YC, Domchek S, Parmigiani G, et al. Breast cancer risk among male BRCA1 and BRCA2 mutation carriers. J Natl Cancer Inst. 2007;99:1811-1814.
8. Evans DG, Susnerwala I, Dawson J, et al. Risk of breast cancer in male BRCA2 carriers. J Med Genet. 2010;47:710-711.
9. CDC. Jewish women and BRCA gene mutations. www.cdc.gov/cancer/breast/young_women/bringyourbrave/hereditary_breast_cancer/jewish_women_brca.htm. Accessed May 22, 2020.
10. Rebbeck TR, Friebel TM, Friedman E, et al. Mutational spectrum in a worldwide study of 29,700 families with BRCA1 or BRCA2 mutations. Hum Mutat. 2018;39:593-620.
11. Anders CK, Hsu DS, Broadwater G, et al. Young age at diagnosis correlates with worse prognosis and defines a subset of breast cancers with shared patterns of gene expression. J Clin Oncol. 2008;26:3324–3330.
12. Wang YA, Jian JW, Hung CF, et al. Germline breast cancer susceptibility gene mutations and breast cancer outcomes. BMC Cancer. 2018;18:315.
13. Baretta Z, Mocellin S, Goldin E, et al. Effect of BRCA germline mutations on breast cancer prognosis: a systematic review and meta-analysis. Medicine. 2016;95:e4975.
14. Lakhani SR, Manek S, Penault-Llorca F, et al. Pathology of ovarian cancers in BRCA1 and BRCA2 carriers. Clin Cancer Res. 2004;10:2473-2481.
15. Kurian AW. BRCA1 and BRCA2 mutations across race and ethnicity: distribution and clinical implications. Curr Opin Obstet Gynecol. 2010;22:72-78.
16. Rebbeck TR, Kauff ND, Domchek SM. Meta-analysis of risk reduction estimates associated with risk-reducing salpingo-oophorectomy in BRCA1 or BRCA2 mutation carriers. J Natl Cancer Inst. 2009;101:80-87.
17. Elwyn G, Frosch D, Thomson R, et al. Shared decision making: a model for clinical practice. J Gen Int Med. 2012;27:1361-1367.
18. Ardern-Jones A, Eeles R. Developments in clinical practice: follow up clinic for BRCA mutation carriers: a case study highlighting the “virtual clinic.” Hered Cancer Clin Pract. 2004;2:77-79.
19. Yerushalmi R, Rizel S, Zoref D, et al. A dedicated follow-up clinic for BRCA mutation carriers. Isr Med Assoc J. 2016;18:549-552.
20. Pichert G, Jacobs C, Jacobs I, et al. Novel one-stop multidisciplinary follow-up clinic significantly improves cancer risk management in BRCA1/2 carriers. Fam Cancer. 2010;9:313-319.
21. Owens DK, Davidson KW, Krist AH, et al; US Preventive Services Task Force. Risk assessment, genetic counseling, and genetic testing for BRCA-related cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2019;322:652-665.
22. Evans D, Eccles D, Rahman N, et al. A new scoring system for the chances of identifying a BRCA1/2 mutation outperforms existing models including BRCAPRO. J Med Genet. 2004;41:474-480.
23. Bellcross CA, Lemke AA, Pape LS, et al. Evaluation of a breast/ovarian cancer genetics referral screening tool in a mammography population. Genet Med. 2009;11:783-789.
24. Hoskins KF, Zwaagstra A, Ranz M. Validation of a tool for identifying women at high risk for hereditary breast cancer in population based screening. Cancer. 2006;107:1769-1776.
25. Gilpin CA, Carson N, Hunter AG. A preliminary validation of a family history assessment form to select women at risk for breast or ovarian cancer for referral to a genetics center. Clin Genet. 2000;58:299-308.
26. Committee on Practice Bulletins-Gynecology. ACOG Practice Bulletin No 182: Hereditary Breast and Ovarian Cancer Syndrome. Obstet Gynecol. 2017;130:e110-e126.
27. Paluch-Shimon S, Cardoso F, Sessa C, et al. Prevention and screening in BRCA mutation carriers and other breast/ovarian hereditary cancer syndromes: ESMO Clinical Practice Guidelines for cancer prevention and screening. Ann Oncol. 2016;27(suppl 5):v103-v110.
28. National Comprehensive Cancer Network. Genetic/familial high-risk assessment: breast and ovarian. 2019. NCCN Clinical Practice Guidelines in Oncology. www2.tri-kobe.org/nccn/guideline/gynecological/english/genetic_familial.pdf. Accessed May 22, 2020.
29. Phillips KA, Milne RL, Rookus MA, et al. Tamoxifen and risk of contralateral breast cancer for BRCA1 and BRCA2 mutation carriers. J Clin Oncol. 2013;31:3091-3099.
30. Foulkes WD, Goffin J, Brunet JS, et al. Tamoxifen may be an effective adjuvant treatment for BRCA1-related breast cancer irrespective of estrogen receptor status. J Natl Cancer Inst. 2002;94:1504-1506.
31. Gronwald J, Tung N, Foulkes WD, et al. Tamoxifen and contralateral breast cancer in BRCA1 and BRCA2 carriers: an update. Int J Cancer. 2006;118:2281-2284.
32. Ludwig KK, Neuner J, Butler A, et al. Risk reduction and survival benefit of prophylactic surgery in BRCA mutation carriers, a systematic review. Am J Surgery. 2016;212:660-669.
33. Bougie O, Weberpals JI. Clinical considerations of BRCA1- and BRCA2-mutation carriers: a review. Int J Surg Oncol. 2011;2011:374012.
34. Rosenthal AN, Fraser LSM, Philpott S, et al. Evidence of stage shift in women diagnosed with ovarian cancer during phase II of the United Kingdom Familial Ovarian Cancer Screening Study. J Clin Oncol. 2017;35:1411-1420.
35. Skates SJ, Greene MH, Buys SS, et al. Early detection of ovarian cancer using the Risk of Ovarian Cancer Algorithm with frequent CA125 testing in women at increased familial risk—combined results from two screening trials. Clin Cancer Res. 2017;23:3628-3637.
36. Daly MB, Pilarski R, Berry M, et al. NCCN guidelines insights: genetic/familial high-risk assessment: breast and ovarian, version 2.2017. J Natl Compr Canc Netw. 2017;15:9-20.
37. Grossman DC, Curry SJ, Owens DK, et al. Screening for ovarian cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018;319:588-594.
38. Marchetti C, De Felice F, Palaia I, et al. Risk-reducing salpingo-oophorectomy: a meta-analysis on impact on ovarian cancer risk and all cause mortality in BRCA 1 and BRCA 2 mutation carriers. BMC Womens Health. 2014;14:150.
39. Nelson HD, Pappas M, Zakher B, et al. Risk assessment, genetic counseling, and genetic testing for BRCA-related cancer in women: a systematic review to update the US Preventive Services Task Force recommendation. Ann Intern Med. 2014;160:255-266.
40. Parker WH, Feskanich D, Broder MS, et al. Long-term mortality associated with oophorectomy compared with ovarian conservation in the nurses’ health study. Obstet Gynecol. 2013;121:709-716.
41. Faubion SS, Kuhle CL, Shuster LT, et al. Long-term health consequences of premature or early menopause and considerations for management. Climacteric. 2015;18:483-491.
42. Menon U, Karpinskyj C, Gentry-Maharaj A. Ovarian cancer prevention and screening. Obstet Gynecol. 2018;131:909-927.
43. Crum CP, Drapkin R, Miron A, et al. The distal fallopian tube: a new model for pelvic serous carcinogenesis. Curr Opin Obstet Gynecol. 2007;19:3-9.
44. Kwon JS, Tinker A, Pansegrau G, et al. Prophylactic salpingectomy and delayed oophorectomy as an alternative for BRCA mutation carriers. Obstet Gynecol. 2013;121:14-24.
45. Holman LL, Friedman S, Daniels MS, et al. Acceptability of prophylactic salpingectomy with delayed oophorectomy as risk-reducing surgery among BRCA mutation carriers. Gynecol Oncol. 2014;133:283-286.
46. MD Anderson Cancer Center. Prophylactic salpingectomy with delayed oophorectomy, risk-reducing salpingo-oophorectomy, and ovarian cancer screening among BRCA mutation carriers: a proof-of-concept study. www.mdanderson.org/patients-family/diagnosis-treatment/clinical-trials/clinical-trials-index/clinical-trials-detail.ID2013-0340.html. Accessed May 22, 2020.
47. Iodice S, Barile M, Rotmensz N, et al. Oral contraceptive use and breast or ovarian cancer risk in BRCA1/2 carriers: a meta-analysis. Eur J Cancer. 2010;46:2275-2284.
48. Moorman PG, Havrilesky LJ, Gierisch JM, et al. Oral contraceptives and risk of ovarian cancer and breast cancer among high-risk women: a systematic review and meta-analysis. J Clin Oncol. 2013;31:4188-4198.
49. Friebel TM, Domchek SM, Rebbeck TR. Modifiers of cancer risk in BRCA1 and BRCA2 mutation carriers: systematic review and meta-analysis. J Natl Cancer Inst. 2014;106:dju091.
50. Haile RW, Thomas DC, McGuire V, et al. BRCA1 and BRCA2 mutation carriers, oral contraceptive use, and breast cancer before age 50. Cancer Epidemiol Biomarkers Prev. 2006;15:1863-1870.
51. Lee E, Ma H, McKean-Cowdin R, et al. Effect of reproductive factors and oral contraceptives on breast cancer risk in BRCA1/2 mutation carriers and noncarriers: results from a population-based study. Cancer Epidemiol Biomarkers Prev. 2008;17:3170-3178.
52. Narod SA, Dubé MP, Klijn J, et al. Oral contraceptives and the risk of breast cancer in BRCA1 and BRCA2 mutation carriers. J Natl Cancer Inst. 2002;94:1773-1779.
53. Milne RL, Knight JA, John EM, et al. Oral contraceptive use and risk of early-onset breast cancer in carriers and noncarriers of BRCA1 and BRCA2 mutations. Cancer Epidemiol Biomarkers Prev. 2005;14:350-356.
54. Culver JO, MacDonald DJ, Thornton AA, et al. Development and evaluation of a decision aid for BRCA carriers with breast cancer. J Genet Couns. 2011;20:294-307.
55. Stacey D, Légaré F, Lewis K, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database of Syst Rev. 2017;4:CD001431.
56. Schwartz MD, Valdimarsdottir HB, DeMarco TA, et al. Randomized trial of a decision aid for BRCA1/BRCA2 mutation carriers: impact on measures of decision making and satisfaction. Health Psychol. 2009;28:11-19.
57. Metcalfe KA, Dennis CL, Poll A, et al. Effect of decision aid for breast cancer prevention on decisional conflict in women with a BRCA1 or BRCA2 mutation: a multisite, randomized, controlled trial. Gen Med. 2017;19:330-336.
58. Friedman LC, Kramer RM. Reproductive issues for women with BRCA mutations. J Natl Cancer Inst Monogr. 2005;34:83-86.
CASE
Sara T* recently moved back to the area to be closer to her family. The 34-year-old patient visited our office to discuss the benefits and potential risks of genetic counseling. She explained that her aunt had just died at age 64 of ovarian cancer. Also, her maternal cousin had been diagnosed at age 42 with breast cancer, and her maternal grandmother had died at age 45 of an unknown “female cancer.” She was scared to find out if she had high-risk genes because she felt it would change her life forever. However, if she ignored the issue, she thought she might worry too much.
We discussed the implications of a positive result, such as having to live with the knowledge and to make decisions about potential screening and risk-reducing surgery. On the other hand, not knowing could allow for the undetected growth of cancer that might otherwise be mitigated to some degree if she knew her risk status and pursued an aggressive screening program.
We worked with Ms. T to map out her next steps.
*The patient’s name has been changed to protect her identity.
Breast cancer is the most commonly diagnosed cancer in women worldwide, representing nearly one-quarter of all female cancer diagnoses in 2018.1 It is the second-leading cause of cancer death in women in developed nations and the leading cause of cancer death in women in developing nations.1 In the United States, 1 in 8 women will develop breast cancer in her lifetime.2 By comparison, the rate of ovarian cancer is much lower, with a lifetime prevalence of 1 in 70 to 80 women.3,4 Although ovarian cancer is less common than breast cancer, its associated mortality is high, and most cases are discovered at advanced stages.
The outsized threat of BRCA mutations. It is estimated that 5% to 10% of all breast cancers are hereditary, with 80% of these attributable to BRCA1 (45%) and BRCA2 (35%).5 These autosomal dominant mutations occur at the germline level, within the egg or sperm, and are therefore incorporated into the DNA of every cell and passed from one generation to the next. Families with BRCA mutations have much higher lifetime rates of cancer. The lifetime risk of breast cancer due to BRCA mutations is estimated at > 80% (BRCA1) and 45% (BRCA2).5BRCA mutations account for between
Male BRCA carriers have a lifetime breast cancer risk of 1% to 5% with BRCA1 and 5% to 10% with BRCA2,7,8 compared with about 1:1000 lifetime incidence in the unselected male population. Male carriers are also at risk for more aggressive prostate cancers.7,8
Continue to: Certain populatiosn carry undue burden of BRCA-related disease
Certain populations carry undue burden of BRCA-related disease due to specific founder mutations. While the estimated global prevalence of BRCA mutations is 0.2% to 1%, for those of Ashkenazi Jewish descent the range is 2% to 3%, representing a relative risk up to 15 times that of the general population.9 Hispanic Americans also appear to have higher rates of BRCA-related cancers.10 Ongoing genetics research continues to identify founder mutations worldwide,10 which may inform future screening guidelines.
In addition to BRCA mutations, there are other, less common mutations (TABLE 15) known to cause hereditary breast and ovarian cancer.
Identifying BRCA genes enables treatment planning. Compared with sporadic cancers, BRCA-related breast cancers
Similarly, BRCA-related ovarian cancer is more likely to be high-grade and endometrioid or serous subtype.14
Shared decision-making helps give clarity to the way forward
Shared decision-making is a process of communication whereby the clinician and the patient identify a decision to be made, review data relevant to clinical options, discuss patient perspectives and preferences regarding each option, and arrive at the decision together.17 Shared decision-making is important when treating women with BRCA mutations because there is no single correct plan. Individual values and competing medical issues may strongly guide each woman’s decisions about screening and cancer prevention treatment decisions.
Continue to: Shared decision-making in this situation...
Shared decision-making in this situation is a strategy to use the evidence of risk along with patient preferences around fertility issues to help come to a decision that is the right one for the patient. Primary care clinicians aware of the general risks and benefits of each available option can refer women at high risk for breast or ovarian cancer to a specialist multidisciplinary clinic that can provide tailored risk assessment and risk reduction counseling as needed.18-20
Genetic screening recommendations
Screening is recommended for women who have any 1 of several family risk factors (TABLE 221). A number of risk assessment tools are available for primary care clinicians to determine which patients are at high enough risk for a hereditary breast or ovarian cancer to warrant referral to a genetic counselor.22-25 If screening suggests high risk, the US Preventive Services Task Force (USPSTF) recommends (Grade B) referral for genetic counseling.21
Explain to patients who are candidates for further investigation that a genetic counselor will review their family history and recommend testing for the specific mutations that increase cancer risk. Discuss potential benefits and harms of genetic testing. A benefit of genetic testing is that aggressive screening may suggest preventive procedures to reduce the risk of future cancer. Most tests come back definitively positive or negative, but an indeterminate result may cause harm. A small minority of results may indicate a genetic variant of unknown significance. The ramifications of this variant may not be known. Some women will experience anxiety about nonspecific test results and will be afraid to share them with family members. There is also some concern about privacy issues, potential insurance bias, and coverage of any preventive strategies.26
CASE
Based on Ms. T’s family history and her desire to know more, we referred her to a genetic counselor and she decided to undergo genetic testing. She screened positive for BRCA1. Ms. T was in a serious relationship and thought she would like to have children at some point. She returned to our office after receiving the positive genetic test results, wondering about screening for breast and ovarian cancer.
Breast cancer screening and risk-reduction strategies
Screening. Because the risk of breast cancer is high in women with BRCA mutations, and because cancer in these women is more likely to be advanced at diagnosis, starting a screening program at an early age is prudent. Observational studies suggest that breastfeeding reduces the risk of breast and ovarian cancer in women with BRCA mutations, as it does for women in the general population.27 Women should return for a clinical breast exam every 6 to 12 months starting at age 25; they should start radiologic screening with magnetic resonance imaging at age 25 and mammography at age 30 (TABLE 327,28).
Continue to: Risk-reduction strategies
Risk-reduction strategies. There is weak evidence to support the use of tamoxifen or other synthetic estrogen reuptake modulators (SERMs) to reduce breast cancer risk in women with BRCA mutations. Many of these cancers do not express estrogen receptors, which may explain the lack of efficacy in certain cases. Several observational studies have shown that tamoxifen can reduce the risk of contralateral breast cancer in women with BRCA mutations who have already been diagnosed with cancer in the other breast.29-31 However, tamoxifen does not reduce a patient’s risk of ovarian cancer, and it may increase her risk of uterine cancer.
Prophylactic bilateral mastectomy is the mainstay of breast cancer prevention in this population. Data from a systematic review suggest that this surgery may prevent the incidence of breast cancer in women with BRCA mutations by 90% to 95%.32 However, this review did not demonstrate a reduction in mortality from breast cancer, likely due to poor data quality.32 The National Comprehensive Cancer Network (NCCN) recommends discussing prophylactic mastectomy with all women who have BRCA mutations.28 Further conversations are important to review the risk of tissue left behind and quality-of-life issues, including the inability to breastfeed if the woman wants more children and the cosmetic changes with reconstruction.
Ovarian cancer screening and risk-reduction strategies
Screening. No effective screening strategy has been endorsed for ovarian cancer, as most previous studies have shown screening to be ineffective.26,33 Recently, studies both in the United Kingdom and the United States have investigated a screening strategy using the risk-of-ovarian-cancer algorithm (ROCA), which calculates an individual’s risk based on serum levels of cancer antigen 125 (CA-125).34,35 These studies measured CA-125 levels every 3 to 4 months followed by transvaginal ultrasound if CA-125 increased substantially (as determined by ROCA). Absent an abnormal increase in CA-125, transvaginal ultrasound was performed annually. These screening strategies showed improved specificity over annual screening programs, and the cancers detected were more likely to be diagnosed at an early stage (stage II vs stage III) and had higher rates of zero residual disease after surgery compared with those detected 1 year after screening ended.34,35 However, survival data are not yet available. More research is needed to determine if more frequent screening approaches could improve survival in high-risk women.
NCCN and the American College of Obstetricians and Gynecologists (ACOG) do not endorse routine screening with transvaginal ultrasound and serum CA-125 for high-risk women, as the benefits are uncertain. However, they do advise that these screens may be considered as a short-term strategy for women ages 30 to 35 who defer risk-reducing surgery.26,36 The USPSTF does not make a recommendation regarding ovarian cancer screening in high-risk women.37
Risk-reduction strategies. Risk-reducing bilateral salpingo-oophorectomy (RRSO) is the only recommended technique for reducing the risk of ovarian cancer in women at high risk.26,33,36 Meta-analyses have shown an 80% reduction in ovarian cancer risk16 and 68% reduction in all-cause mortality with this approach.38 The NCCN recommends RRSO for women with a known BRCA1 mutation between the ages of 35 and 40 who have completed childbearing.36 Since the onset of ovarian cancer tends to be later in women with BRCA2 mutations, it is reasonable to delay RRSO until age 40 to 45 in this population if they have taken other steps to maximize breast cancer prevention (ie, bilateral mastectomy).36
Continue to: Adverse effects of RRSO...
Adverse effects of RRSO include surgery complications (wound infection, small bowel obstruction, bladder perforation) and effects of early menopause (vasomotor symptoms, decreased sexual functioning, and increased risk of osteoporosis, cardiovascular disease, and all-cause mortality).39-41 In the absence of contraindications, ACOG recommends using hormone therapy in women undergoing RRSO until the natural age of menopause,42 particularly if their breast tissue has been removed.
Salpingectomy as an alternative. In an attempt to reduce these adverse effects of early menopause, and because a large proportion of high-grade serous tumors originate in the fallopian tube,43 interest has increased in the use of risk-reducing salpingectomy (removal of fallopian tubes) and delayed oophorectomy in women at high risk of ovarian cancer.42 Studies have shown this may be a cost-effective approach and an acceptable alternative in BRCA mutation carriers who are unwilling to undergo RRSO.44,45 A clinical trial investigating this approach in women with BRCA mutations is currently underway in the United States.46 Many centers offer salpingectomy to high-risk patients < 40 years old, understanding that ovary removal is an eventuality for these patients.
When oral contraceptive pills might be beneficial. In younger women with BRCA mutations, there may also be a role for oral contraceptive pills (OCPs) as a risk-reducing strategy. Meta-analyses have shown an approximately 50% reduction in the risk of ovarian cancer among women with BRCA mutations who use OCPs.47-49
ACOG advises that it is appropriate for women with BRCA mutations to use oral contraceptives if indicated (for pregnancy prevention or menstrual cycle regulation), and that it is reasonable to use them for cancer prevention.26 NCCN does not make a formal recommendation, although it does state OCPs may reduce the risk of ovarian cancer in women with a BRCA mutation.36 Case-control studies have produced conflicting data on the association between OCP use and breast cancer risk in BRCA mutation carriers,50-53 although 2 meta-analyses found no significant association in this population.47,48
Decision aids for women with BRCA mutations
Decision aids are visual displays of risk that help patients work through complex decisions. Most decision aids are in print or digital format and include information about the decision to be made as well as pictorial examples of possible outcomes. Pictographs are especially helpful in communicating information. Some decision aids for women with BRCA mutations can be complicated with multiple outcomes (ie, breast cancer and ovarian cancer) and multiple potential interventions (risk-reducing surgery, enhanced screening options).54
Continue to: A Cochrane review...
A Cochrane review found that decision aids increased patients’ knowledge, helped patients clarify their values, and may improve value-concordant decisions.55 Two papers describing the use of decision aids for women with BRCA mutations56,57 documented decreased decisional conflict and increased satisfaction.
CASE
Ms. T underwent the recommended mammogram and MRI screening for breast cancer, as well as testing with serum CA-125 and ultrasound examinations for ovarian cancer. Her initial mammogram and MRI revealed early stage, triple-negative right breast cancer. She chose to undergo bilateral mastectomy and reconstruction. She has now completed treatment and continues to work closely with her oncology team for appropriate breast follow-up.
One year after her initial diagnosis, at the age of 35, she returned to discuss fertility. She was recently married, and she and her husband wanted to start having children. She was concerned about a safe timeline for her to pursue pregnancy, saying she felt “like a ticking time-bomb” given her prior cancer and carrier status. She wanted to discuss the risks and benefits of pregnancy and when she should consider prophylactic oophorectomy. She had a few options. She could have a baby and then undergo an RRSO, or she could talk to her gynecologist about having a salpingectomy to reduce her risk now and use assisted reproductive technology to get pregnant. She could also freeze eggs or embryos, have an RRSO, and then use a surrogate to get pregnant. We informed her that pregnancy would not affect her risk of ovarian cancer and discussed the options for pre-implantation genetic testing to assure that her children would not carry the genetic mutation.58
We provided Ms. T and her husband with a decision aid to help them navigate the decision. They are currently evaluating the options and said they would let us know when they made a decision.
CORRESPONDENCE
Sarina Schrager, MD, MS, Northeast Family Medicine Center, 3209 Dryden Drive, Madison, WI, 53704; sbschrag@wisc.edu.
CASE
Sara T* recently moved back to the area to be closer to her family. The 34-year-old patient visited our office to discuss the benefits and potential risks of genetic counseling. She explained that her aunt had just died at age 64 of ovarian cancer. Also, her maternal cousin had been diagnosed at age 42 with breast cancer, and her maternal grandmother had died at age 45 of an unknown “female cancer.” She was scared to find out if she had high-risk genes because she felt it would change her life forever. However, if she ignored the issue, she thought she might worry too much.
We discussed the implications of a positive result, such as having to live with the knowledge and to make decisions about potential screening and risk-reducing surgery. On the other hand, not knowing could allow for the undetected growth of cancer that might otherwise be mitigated to some degree if she knew her risk status and pursued an aggressive screening program.
We worked with Ms. T to map out her next steps.
*The patient’s name has been changed to protect her identity.
Breast cancer is the most commonly diagnosed cancer in women worldwide, representing nearly one-quarter of all female cancer diagnoses in 2018.1 It is the second-leading cause of cancer death in women in developed nations and the leading cause of cancer death in women in developing nations.1 In the United States, 1 in 8 women will develop breast cancer in her lifetime.2 By comparison, the rate of ovarian cancer is much lower, with a lifetime prevalence of 1 in 70 to 80 women.3,4 Although ovarian cancer is less common than breast cancer, its associated mortality is high, and most cases are discovered at advanced stages.
The outsized threat of BRCA mutations. It is estimated that 5% to 10% of all breast cancers are hereditary, with 80% of these attributable to BRCA1 (45%) and BRCA2 (35%).5 These autosomal dominant mutations occur at the germline level, within the egg or sperm, and are therefore incorporated into the DNA of every cell and passed from one generation to the next. Families with BRCA mutations have much higher lifetime rates of cancer. The lifetime risk of breast cancer due to BRCA mutations is estimated at > 80% (BRCA1) and 45% (BRCA2).5BRCA mutations account for between
Male BRCA carriers have a lifetime breast cancer risk of 1% to 5% with BRCA1 and 5% to 10% with BRCA2,7,8 compared with about 1:1000 lifetime incidence in the unselected male population. Male carriers are also at risk for more aggressive prostate cancers.7,8
Continue to: Certain populatiosn carry undue burden of BRCA-related disease
Certain populations carry undue burden of BRCA-related disease due to specific founder mutations. While the estimated global prevalence of BRCA mutations is 0.2% to 1%, for those of Ashkenazi Jewish descent the range is 2% to 3%, representing a relative risk up to 15 times that of the general population.9 Hispanic Americans also appear to have higher rates of BRCA-related cancers.10 Ongoing genetics research continues to identify founder mutations worldwide,10 which may inform future screening guidelines.
In addition to BRCA mutations, there are other, less common mutations (TABLE 15) known to cause hereditary breast and ovarian cancer.
Identifying BRCA genes enables treatment planning. Compared with sporadic cancers, BRCA-related breast cancers
Similarly, BRCA-related ovarian cancer is more likely to be high-grade and endometrioid or serous subtype.14
Shared decision-making helps give clarity to the way forward
Shared decision-making is a process of communication whereby the clinician and the patient identify a decision to be made, review data relevant to clinical options, discuss patient perspectives and preferences regarding each option, and arrive at the decision together.17 Shared decision-making is important when treating women with BRCA mutations because there is no single correct plan. Individual values and competing medical issues may strongly guide each woman’s decisions about screening and cancer prevention treatment decisions.
Continue to: Shared decision-making in this situation...
Shared decision-making in this situation is a strategy to use the evidence of risk along with patient preferences around fertility issues to help come to a decision that is the right one for the patient. Primary care clinicians aware of the general risks and benefits of each available option can refer women at high risk for breast or ovarian cancer to a specialist multidisciplinary clinic that can provide tailored risk assessment and risk reduction counseling as needed.18-20
Genetic screening recommendations
Screening is recommended for women who have any 1 of several family risk factors (TABLE 221). A number of risk assessment tools are available for primary care clinicians to determine which patients are at high enough risk for a hereditary breast or ovarian cancer to warrant referral to a genetic counselor.22-25 If screening suggests high risk, the US Preventive Services Task Force (USPSTF) recommends (Grade B) referral for genetic counseling.21
Explain to patients who are candidates for further investigation that a genetic counselor will review their family history and recommend testing for the specific mutations that increase cancer risk. Discuss potential benefits and harms of genetic testing. A benefit of genetic testing is that aggressive screening may suggest preventive procedures to reduce the risk of future cancer. Most tests come back definitively positive or negative, but an indeterminate result may cause harm. A small minority of results may indicate a genetic variant of unknown significance. The ramifications of this variant may not be known. Some women will experience anxiety about nonspecific test results and will be afraid to share them with family members. There is also some concern about privacy issues, potential insurance bias, and coverage of any preventive strategies.26
CASE
Based on Ms. T’s family history and her desire to know more, we referred her to a genetic counselor and she decided to undergo genetic testing. She screened positive for BRCA1. Ms. T was in a serious relationship and thought she would like to have children at some point. She returned to our office after receiving the positive genetic test results, wondering about screening for breast and ovarian cancer.
Breast cancer screening and risk-reduction strategies
Screening. Because the risk of breast cancer is high in women with BRCA mutations, and because cancer in these women is more likely to be advanced at diagnosis, starting a screening program at an early age is prudent. Observational studies suggest that breastfeeding reduces the risk of breast and ovarian cancer in women with BRCA mutations, as it does for women in the general population.27 Women should return for a clinical breast exam every 6 to 12 months starting at age 25; they should start radiologic screening with magnetic resonance imaging at age 25 and mammography at age 30 (TABLE 327,28).
Continue to: Risk-reduction strategies
Risk-reduction strategies. There is weak evidence to support the use of tamoxifen or other synthetic estrogen reuptake modulators (SERMs) to reduce breast cancer risk in women with BRCA mutations. Many of these cancers do not express estrogen receptors, which may explain the lack of efficacy in certain cases. Several observational studies have shown that tamoxifen can reduce the risk of contralateral breast cancer in women with BRCA mutations who have already been diagnosed with cancer in the other breast.29-31 However, tamoxifen does not reduce a patient’s risk of ovarian cancer, and it may increase her risk of uterine cancer.
Prophylactic bilateral mastectomy is the mainstay of breast cancer prevention in this population. Data from a systematic review suggest that this surgery may prevent the incidence of breast cancer in women with BRCA mutations by 90% to 95%.32 However, this review did not demonstrate a reduction in mortality from breast cancer, likely due to poor data quality.32 The National Comprehensive Cancer Network (NCCN) recommends discussing prophylactic mastectomy with all women who have BRCA mutations.28 Further conversations are important to review the risk of tissue left behind and quality-of-life issues, including the inability to breastfeed if the woman wants more children and the cosmetic changes with reconstruction.
Ovarian cancer screening and risk-reduction strategies
Screening. No effective screening strategy has been endorsed for ovarian cancer, as most previous studies have shown screening to be ineffective.26,33 Recently, studies both in the United Kingdom and the United States have investigated a screening strategy using the risk-of-ovarian-cancer algorithm (ROCA), which calculates an individual’s risk based on serum levels of cancer antigen 125 (CA-125).34,35 These studies measured CA-125 levels every 3 to 4 months followed by transvaginal ultrasound if CA-125 increased substantially (as determined by ROCA). Absent an abnormal increase in CA-125, transvaginal ultrasound was performed annually. These screening strategies showed improved specificity over annual screening programs, and the cancers detected were more likely to be diagnosed at an early stage (stage II vs stage III) and had higher rates of zero residual disease after surgery compared with those detected 1 year after screening ended.34,35 However, survival data are not yet available. More research is needed to determine if more frequent screening approaches could improve survival in high-risk women.
NCCN and the American College of Obstetricians and Gynecologists (ACOG) do not endorse routine screening with transvaginal ultrasound and serum CA-125 for high-risk women, as the benefits are uncertain. However, they do advise that these screens may be considered as a short-term strategy for women ages 30 to 35 who defer risk-reducing surgery.26,36 The USPSTF does not make a recommendation regarding ovarian cancer screening in high-risk women.37
Risk-reduction strategies. Risk-reducing bilateral salpingo-oophorectomy (RRSO) is the only recommended technique for reducing the risk of ovarian cancer in women at high risk.26,33,36 Meta-analyses have shown an 80% reduction in ovarian cancer risk16 and 68% reduction in all-cause mortality with this approach.38 The NCCN recommends RRSO for women with a known BRCA1 mutation between the ages of 35 and 40 who have completed childbearing.36 Since the onset of ovarian cancer tends to be later in women with BRCA2 mutations, it is reasonable to delay RRSO until age 40 to 45 in this population if they have taken other steps to maximize breast cancer prevention (ie, bilateral mastectomy).36
Continue to: Adverse effects of RRSO...
Adverse effects of RRSO include surgery complications (wound infection, small bowel obstruction, bladder perforation) and effects of early menopause (vasomotor symptoms, decreased sexual functioning, and increased risk of osteoporosis, cardiovascular disease, and all-cause mortality).39-41 In the absence of contraindications, ACOG recommends using hormone therapy in women undergoing RRSO until the natural age of menopause,42 particularly if their breast tissue has been removed.
Salpingectomy as an alternative. In an attempt to reduce these adverse effects of early menopause, and because a large proportion of high-grade serous tumors originate in the fallopian tube,43 interest has increased in the use of risk-reducing salpingectomy (removal of fallopian tubes) and delayed oophorectomy in women at high risk of ovarian cancer.42 Studies have shown this may be a cost-effective approach and an acceptable alternative in BRCA mutation carriers who are unwilling to undergo RRSO.44,45 A clinical trial investigating this approach in women with BRCA mutations is currently underway in the United States.46 Many centers offer salpingectomy to high-risk patients < 40 years old, understanding that ovary removal is an eventuality for these patients.
When oral contraceptive pills might be beneficial. In younger women with BRCA mutations, there may also be a role for oral contraceptive pills (OCPs) as a risk-reducing strategy. Meta-analyses have shown an approximately 50% reduction in the risk of ovarian cancer among women with BRCA mutations who use OCPs.47-49
ACOG advises that it is appropriate for women with BRCA mutations to use oral contraceptives if indicated (for pregnancy prevention or menstrual cycle regulation), and that it is reasonable to use them for cancer prevention.26 NCCN does not make a formal recommendation, although it does state OCPs may reduce the risk of ovarian cancer in women with a BRCA mutation.36 Case-control studies have produced conflicting data on the association between OCP use and breast cancer risk in BRCA mutation carriers,50-53 although 2 meta-analyses found no significant association in this population.47,48
Decision aids for women with BRCA mutations
Decision aids are visual displays of risk that help patients work through complex decisions. Most decision aids are in print or digital format and include information about the decision to be made as well as pictorial examples of possible outcomes. Pictographs are especially helpful in communicating information. Some decision aids for women with BRCA mutations can be complicated with multiple outcomes (ie, breast cancer and ovarian cancer) and multiple potential interventions (risk-reducing surgery, enhanced screening options).54
Continue to: A Cochrane review...
A Cochrane review found that decision aids increased patients’ knowledge, helped patients clarify their values, and may improve value-concordant decisions.55 Two papers describing the use of decision aids for women with BRCA mutations56,57 documented decreased decisional conflict and increased satisfaction.
CASE
Ms. T underwent the recommended mammogram and MRI screening for breast cancer, as well as testing with serum CA-125 and ultrasound examinations for ovarian cancer. Her initial mammogram and MRI revealed early stage, triple-negative right breast cancer. She chose to undergo bilateral mastectomy and reconstruction. She has now completed treatment and continues to work closely with her oncology team for appropriate breast follow-up.
One year after her initial diagnosis, at the age of 35, she returned to discuss fertility. She was recently married, and she and her husband wanted to start having children. She was concerned about a safe timeline for her to pursue pregnancy, saying she felt “like a ticking time-bomb” given her prior cancer and carrier status. She wanted to discuss the risks and benefits of pregnancy and when she should consider prophylactic oophorectomy. She had a few options. She could have a baby and then undergo an RRSO, or she could talk to her gynecologist about having a salpingectomy to reduce her risk now and use assisted reproductive technology to get pregnant. She could also freeze eggs or embryos, have an RRSO, and then use a surrogate to get pregnant. We informed her that pregnancy would not affect her risk of ovarian cancer and discussed the options for pre-implantation genetic testing to assure that her children would not carry the genetic mutation.58
We provided Ms. T and her husband with a decision aid to help them navigate the decision. They are currently evaluating the options and said they would let us know when they made a decision.
CORRESPONDENCE
Sarina Schrager, MD, MS, Northeast Family Medicine Center, 3209 Dryden Drive, Madison, WI, 53704; sbschrag@wisc.edu.
1. Ferlay J, Colombet M, Soerjomataram I, et al. Estimating the global cancer incidence in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019;144:1941-1953.
2. SEER Cancer Statistics Review, 1975-2016. Cancer of the female breast. [Table 4.1] National Cancer Institute. Bethesda, MD. https://seer.cancer.gov/archive/csr/1975_2016/results_merged/sect_04_breast.pdf. Accessed May 27, 2020.
3. SEER Cancer Statistics Review, 1975-2016. Cancer of the ovary. [Table 21.10] National Cancer Institute. Bethesda, MD. https://seer.cancer.gov/archive/csr/1975_2016/results_merged/sect_21_ovary.pdf. Accessed May 22, 2020.
4. Torre LA, Trabert B, DeSantis C, et al. Ovarian cancer statistics, 2018. CA Cancer J Clin. 2018;68:284-296.
5. Narod SA, Foulkes WD. BRCA1 and BRCA2: 1994 and beyond. Nat Rev Cancer. 2004;4:665-676.
6. Pal T, Permuth-Wey J, Betts JA, et al. BRCA1 and BRCA2 mutations account for a large proportion of ovarian carcinoma cases. Cancer. 2005;104:2807-2816.
7. Tai YC, Domchek S, Parmigiani G, et al. Breast cancer risk among male BRCA1 and BRCA2 mutation carriers. J Natl Cancer Inst. 2007;99:1811-1814.
8. Evans DG, Susnerwala I, Dawson J, et al. Risk of breast cancer in male BRCA2 carriers. J Med Genet. 2010;47:710-711.
9. CDC. Jewish women and BRCA gene mutations. www.cdc.gov/cancer/breast/young_women/bringyourbrave/hereditary_breast_cancer/jewish_women_brca.htm. Accessed May 22, 2020.
10. Rebbeck TR, Friebel TM, Friedman E, et al. Mutational spectrum in a worldwide study of 29,700 families with BRCA1 or BRCA2 mutations. Hum Mutat. 2018;39:593-620.
11. Anders CK, Hsu DS, Broadwater G, et al. Young age at diagnosis correlates with worse prognosis and defines a subset of breast cancers with shared patterns of gene expression. J Clin Oncol. 2008;26:3324–3330.
12. Wang YA, Jian JW, Hung CF, et al. Germline breast cancer susceptibility gene mutations and breast cancer outcomes. BMC Cancer. 2018;18:315.
13. Baretta Z, Mocellin S, Goldin E, et al. Effect of BRCA germline mutations on breast cancer prognosis: a systematic review and meta-analysis. Medicine. 2016;95:e4975.
14. Lakhani SR, Manek S, Penault-Llorca F, et al. Pathology of ovarian cancers in BRCA1 and BRCA2 carriers. Clin Cancer Res. 2004;10:2473-2481.
15. Kurian AW. BRCA1 and BRCA2 mutations across race and ethnicity: distribution and clinical implications. Curr Opin Obstet Gynecol. 2010;22:72-78.
16. Rebbeck TR, Kauff ND, Domchek SM. Meta-analysis of risk reduction estimates associated with risk-reducing salpingo-oophorectomy in BRCA1 or BRCA2 mutation carriers. J Natl Cancer Inst. 2009;101:80-87.
17. Elwyn G, Frosch D, Thomson R, et al. Shared decision making: a model for clinical practice. J Gen Int Med. 2012;27:1361-1367.
18. Ardern-Jones A, Eeles R. Developments in clinical practice: follow up clinic for BRCA mutation carriers: a case study highlighting the “virtual clinic.” Hered Cancer Clin Pract. 2004;2:77-79.
19. Yerushalmi R, Rizel S, Zoref D, et al. A dedicated follow-up clinic for BRCA mutation carriers. Isr Med Assoc J. 2016;18:549-552.
20. Pichert G, Jacobs C, Jacobs I, et al. Novel one-stop multidisciplinary follow-up clinic significantly improves cancer risk management in BRCA1/2 carriers. Fam Cancer. 2010;9:313-319.
21. Owens DK, Davidson KW, Krist AH, et al; US Preventive Services Task Force. Risk assessment, genetic counseling, and genetic testing for BRCA-related cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2019;322:652-665.
22. Evans D, Eccles D, Rahman N, et al. A new scoring system for the chances of identifying a BRCA1/2 mutation outperforms existing models including BRCAPRO. J Med Genet. 2004;41:474-480.
23. Bellcross CA, Lemke AA, Pape LS, et al. Evaluation of a breast/ovarian cancer genetics referral screening tool in a mammography population. Genet Med. 2009;11:783-789.
24. Hoskins KF, Zwaagstra A, Ranz M. Validation of a tool for identifying women at high risk for hereditary breast cancer in population based screening. Cancer. 2006;107:1769-1776.
25. Gilpin CA, Carson N, Hunter AG. A preliminary validation of a family history assessment form to select women at risk for breast or ovarian cancer for referral to a genetics center. Clin Genet. 2000;58:299-308.
26. Committee on Practice Bulletins-Gynecology. ACOG Practice Bulletin No 182: Hereditary Breast and Ovarian Cancer Syndrome. Obstet Gynecol. 2017;130:e110-e126.
27. Paluch-Shimon S, Cardoso F, Sessa C, et al. Prevention and screening in BRCA mutation carriers and other breast/ovarian hereditary cancer syndromes: ESMO Clinical Practice Guidelines for cancer prevention and screening. Ann Oncol. 2016;27(suppl 5):v103-v110.
28. National Comprehensive Cancer Network. Genetic/familial high-risk assessment: breast and ovarian. 2019. NCCN Clinical Practice Guidelines in Oncology. www2.tri-kobe.org/nccn/guideline/gynecological/english/genetic_familial.pdf. Accessed May 22, 2020.
29. Phillips KA, Milne RL, Rookus MA, et al. Tamoxifen and risk of contralateral breast cancer for BRCA1 and BRCA2 mutation carriers. J Clin Oncol. 2013;31:3091-3099.
30. Foulkes WD, Goffin J, Brunet JS, et al. Tamoxifen may be an effective adjuvant treatment for BRCA1-related breast cancer irrespective of estrogen receptor status. J Natl Cancer Inst. 2002;94:1504-1506.
31. Gronwald J, Tung N, Foulkes WD, et al. Tamoxifen and contralateral breast cancer in BRCA1 and BRCA2 carriers: an update. Int J Cancer. 2006;118:2281-2284.
32. Ludwig KK, Neuner J, Butler A, et al. Risk reduction and survival benefit of prophylactic surgery in BRCA mutation carriers, a systematic review. Am J Surgery. 2016;212:660-669.
33. Bougie O, Weberpals JI. Clinical considerations of BRCA1- and BRCA2-mutation carriers: a review. Int J Surg Oncol. 2011;2011:374012.
34. Rosenthal AN, Fraser LSM, Philpott S, et al. Evidence of stage shift in women diagnosed with ovarian cancer during phase II of the United Kingdom Familial Ovarian Cancer Screening Study. J Clin Oncol. 2017;35:1411-1420.
35. Skates SJ, Greene MH, Buys SS, et al. Early detection of ovarian cancer using the Risk of Ovarian Cancer Algorithm with frequent CA125 testing in women at increased familial risk—combined results from two screening trials. Clin Cancer Res. 2017;23:3628-3637.
36. Daly MB, Pilarski R, Berry M, et al. NCCN guidelines insights: genetic/familial high-risk assessment: breast and ovarian, version 2.2017. J Natl Compr Canc Netw. 2017;15:9-20.
37. Grossman DC, Curry SJ, Owens DK, et al. Screening for ovarian cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018;319:588-594.
38. Marchetti C, De Felice F, Palaia I, et al. Risk-reducing salpingo-oophorectomy: a meta-analysis on impact on ovarian cancer risk and all cause mortality in BRCA 1 and BRCA 2 mutation carriers. BMC Womens Health. 2014;14:150.
39. Nelson HD, Pappas M, Zakher B, et al. Risk assessment, genetic counseling, and genetic testing for BRCA-related cancer in women: a systematic review to update the US Preventive Services Task Force recommendation. Ann Intern Med. 2014;160:255-266.
40. Parker WH, Feskanich D, Broder MS, et al. Long-term mortality associated with oophorectomy compared with ovarian conservation in the nurses’ health study. Obstet Gynecol. 2013;121:709-716.
41. Faubion SS, Kuhle CL, Shuster LT, et al. Long-term health consequences of premature or early menopause and considerations for management. Climacteric. 2015;18:483-491.
42. Menon U, Karpinskyj C, Gentry-Maharaj A. Ovarian cancer prevention and screening. Obstet Gynecol. 2018;131:909-927.
43. Crum CP, Drapkin R, Miron A, et al. The distal fallopian tube: a new model for pelvic serous carcinogenesis. Curr Opin Obstet Gynecol. 2007;19:3-9.
44. Kwon JS, Tinker A, Pansegrau G, et al. Prophylactic salpingectomy and delayed oophorectomy as an alternative for BRCA mutation carriers. Obstet Gynecol. 2013;121:14-24.
45. Holman LL, Friedman S, Daniels MS, et al. Acceptability of prophylactic salpingectomy with delayed oophorectomy as risk-reducing surgery among BRCA mutation carriers. Gynecol Oncol. 2014;133:283-286.
46. MD Anderson Cancer Center. Prophylactic salpingectomy with delayed oophorectomy, risk-reducing salpingo-oophorectomy, and ovarian cancer screening among BRCA mutation carriers: a proof-of-concept study. www.mdanderson.org/patients-family/diagnosis-treatment/clinical-trials/clinical-trials-index/clinical-trials-detail.ID2013-0340.html. Accessed May 22, 2020.
47. Iodice S, Barile M, Rotmensz N, et al. Oral contraceptive use and breast or ovarian cancer risk in BRCA1/2 carriers: a meta-analysis. Eur J Cancer. 2010;46:2275-2284.
48. Moorman PG, Havrilesky LJ, Gierisch JM, et al. Oral contraceptives and risk of ovarian cancer and breast cancer among high-risk women: a systematic review and meta-analysis. J Clin Oncol. 2013;31:4188-4198.
49. Friebel TM, Domchek SM, Rebbeck TR. Modifiers of cancer risk in BRCA1 and BRCA2 mutation carriers: systematic review and meta-analysis. J Natl Cancer Inst. 2014;106:dju091.
50. Haile RW, Thomas DC, McGuire V, et al. BRCA1 and BRCA2 mutation carriers, oral contraceptive use, and breast cancer before age 50. Cancer Epidemiol Biomarkers Prev. 2006;15:1863-1870.
51. Lee E, Ma H, McKean-Cowdin R, et al. Effect of reproductive factors and oral contraceptives on breast cancer risk in BRCA1/2 mutation carriers and noncarriers: results from a population-based study. Cancer Epidemiol Biomarkers Prev. 2008;17:3170-3178.
52. Narod SA, Dubé MP, Klijn J, et al. Oral contraceptives and the risk of breast cancer in BRCA1 and BRCA2 mutation carriers. J Natl Cancer Inst. 2002;94:1773-1779.
53. Milne RL, Knight JA, John EM, et al. Oral contraceptive use and risk of early-onset breast cancer in carriers and noncarriers of BRCA1 and BRCA2 mutations. Cancer Epidemiol Biomarkers Prev. 2005;14:350-356.
54. Culver JO, MacDonald DJ, Thornton AA, et al. Development and evaluation of a decision aid for BRCA carriers with breast cancer. J Genet Couns. 2011;20:294-307.
55. Stacey D, Légaré F, Lewis K, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database of Syst Rev. 2017;4:CD001431.
56. Schwartz MD, Valdimarsdottir HB, DeMarco TA, et al. Randomized trial of a decision aid for BRCA1/BRCA2 mutation carriers: impact on measures of decision making and satisfaction. Health Psychol. 2009;28:11-19.
57. Metcalfe KA, Dennis CL, Poll A, et al. Effect of decision aid for breast cancer prevention on decisional conflict in women with a BRCA1 or BRCA2 mutation: a multisite, randomized, controlled trial. Gen Med. 2017;19:330-336.
58. Friedman LC, Kramer RM. Reproductive issues for women with BRCA mutations. J Natl Cancer Inst Monogr. 2005;34:83-86.
1. Ferlay J, Colombet M, Soerjomataram I, et al. Estimating the global cancer incidence in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019;144:1941-1953.
2. SEER Cancer Statistics Review, 1975-2016. Cancer of the female breast. [Table 4.1] National Cancer Institute. Bethesda, MD. https://seer.cancer.gov/archive/csr/1975_2016/results_merged/sect_04_breast.pdf. Accessed May 27, 2020.
3. SEER Cancer Statistics Review, 1975-2016. Cancer of the ovary. [Table 21.10] National Cancer Institute. Bethesda, MD. https://seer.cancer.gov/archive/csr/1975_2016/results_merged/sect_21_ovary.pdf. Accessed May 22, 2020.
4. Torre LA, Trabert B, DeSantis C, et al. Ovarian cancer statistics, 2018. CA Cancer J Clin. 2018;68:284-296.
5. Narod SA, Foulkes WD. BRCA1 and BRCA2: 1994 and beyond. Nat Rev Cancer. 2004;4:665-676.
6. Pal T, Permuth-Wey J, Betts JA, et al. BRCA1 and BRCA2 mutations account for a large proportion of ovarian carcinoma cases. Cancer. 2005;104:2807-2816.
7. Tai YC, Domchek S, Parmigiani G, et al. Breast cancer risk among male BRCA1 and BRCA2 mutation carriers. J Natl Cancer Inst. 2007;99:1811-1814.
8. Evans DG, Susnerwala I, Dawson J, et al. Risk of breast cancer in male BRCA2 carriers. J Med Genet. 2010;47:710-711.
9. CDC. Jewish women and BRCA gene mutations. www.cdc.gov/cancer/breast/young_women/bringyourbrave/hereditary_breast_cancer/jewish_women_brca.htm. Accessed May 22, 2020.
10. Rebbeck TR, Friebel TM, Friedman E, et al. Mutational spectrum in a worldwide study of 29,700 families with BRCA1 or BRCA2 mutations. Hum Mutat. 2018;39:593-620.
11. Anders CK, Hsu DS, Broadwater G, et al. Young age at diagnosis correlates with worse prognosis and defines a subset of breast cancers with shared patterns of gene expression. J Clin Oncol. 2008;26:3324–3330.
12. Wang YA, Jian JW, Hung CF, et al. Germline breast cancer susceptibility gene mutations and breast cancer outcomes. BMC Cancer. 2018;18:315.
13. Baretta Z, Mocellin S, Goldin E, et al. Effect of BRCA germline mutations on breast cancer prognosis: a systematic review and meta-analysis. Medicine. 2016;95:e4975.
14. Lakhani SR, Manek S, Penault-Llorca F, et al. Pathology of ovarian cancers in BRCA1 and BRCA2 carriers. Clin Cancer Res. 2004;10:2473-2481.
15. Kurian AW. BRCA1 and BRCA2 mutations across race and ethnicity: distribution and clinical implications. Curr Opin Obstet Gynecol. 2010;22:72-78.
16. Rebbeck TR, Kauff ND, Domchek SM. Meta-analysis of risk reduction estimates associated with risk-reducing salpingo-oophorectomy in BRCA1 or BRCA2 mutation carriers. J Natl Cancer Inst. 2009;101:80-87.
17. Elwyn G, Frosch D, Thomson R, et al. Shared decision making: a model for clinical practice. J Gen Int Med. 2012;27:1361-1367.
18. Ardern-Jones A, Eeles R. Developments in clinical practice: follow up clinic for BRCA mutation carriers: a case study highlighting the “virtual clinic.” Hered Cancer Clin Pract. 2004;2:77-79.
19. Yerushalmi R, Rizel S, Zoref D, et al. A dedicated follow-up clinic for BRCA mutation carriers. Isr Med Assoc J. 2016;18:549-552.
20. Pichert G, Jacobs C, Jacobs I, et al. Novel one-stop multidisciplinary follow-up clinic significantly improves cancer risk management in BRCA1/2 carriers. Fam Cancer. 2010;9:313-319.
21. Owens DK, Davidson KW, Krist AH, et al; US Preventive Services Task Force. Risk assessment, genetic counseling, and genetic testing for BRCA-related cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2019;322:652-665.
22. Evans D, Eccles D, Rahman N, et al. A new scoring system for the chances of identifying a BRCA1/2 mutation outperforms existing models including BRCAPRO. J Med Genet. 2004;41:474-480.
23. Bellcross CA, Lemke AA, Pape LS, et al. Evaluation of a breast/ovarian cancer genetics referral screening tool in a mammography population. Genet Med. 2009;11:783-789.
24. Hoskins KF, Zwaagstra A, Ranz M. Validation of a tool for identifying women at high risk for hereditary breast cancer in population based screening. Cancer. 2006;107:1769-1776.
25. Gilpin CA, Carson N, Hunter AG. A preliminary validation of a family history assessment form to select women at risk for breast or ovarian cancer for referral to a genetics center. Clin Genet. 2000;58:299-308.
26. Committee on Practice Bulletins-Gynecology. ACOG Practice Bulletin No 182: Hereditary Breast and Ovarian Cancer Syndrome. Obstet Gynecol. 2017;130:e110-e126.
27. Paluch-Shimon S, Cardoso F, Sessa C, et al. Prevention and screening in BRCA mutation carriers and other breast/ovarian hereditary cancer syndromes: ESMO Clinical Practice Guidelines for cancer prevention and screening. Ann Oncol. 2016;27(suppl 5):v103-v110.
28. National Comprehensive Cancer Network. Genetic/familial high-risk assessment: breast and ovarian. 2019. NCCN Clinical Practice Guidelines in Oncology. www2.tri-kobe.org/nccn/guideline/gynecological/english/genetic_familial.pdf. Accessed May 22, 2020.
29. Phillips KA, Milne RL, Rookus MA, et al. Tamoxifen and risk of contralateral breast cancer for BRCA1 and BRCA2 mutation carriers. J Clin Oncol. 2013;31:3091-3099.
30. Foulkes WD, Goffin J, Brunet JS, et al. Tamoxifen may be an effective adjuvant treatment for BRCA1-related breast cancer irrespective of estrogen receptor status. J Natl Cancer Inst. 2002;94:1504-1506.
31. Gronwald J, Tung N, Foulkes WD, et al. Tamoxifen and contralateral breast cancer in BRCA1 and BRCA2 carriers: an update. Int J Cancer. 2006;118:2281-2284.
32. Ludwig KK, Neuner J, Butler A, et al. Risk reduction and survival benefit of prophylactic surgery in BRCA mutation carriers, a systematic review. Am J Surgery. 2016;212:660-669.
33. Bougie O, Weberpals JI. Clinical considerations of BRCA1- and BRCA2-mutation carriers: a review. Int J Surg Oncol. 2011;2011:374012.
34. Rosenthal AN, Fraser LSM, Philpott S, et al. Evidence of stage shift in women diagnosed with ovarian cancer during phase II of the United Kingdom Familial Ovarian Cancer Screening Study. J Clin Oncol. 2017;35:1411-1420.
35. Skates SJ, Greene MH, Buys SS, et al. Early detection of ovarian cancer using the Risk of Ovarian Cancer Algorithm with frequent CA125 testing in women at increased familial risk—combined results from two screening trials. Clin Cancer Res. 2017;23:3628-3637.
36. Daly MB, Pilarski R, Berry M, et al. NCCN guidelines insights: genetic/familial high-risk assessment: breast and ovarian, version 2.2017. J Natl Compr Canc Netw. 2017;15:9-20.
37. Grossman DC, Curry SJ, Owens DK, et al. Screening for ovarian cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018;319:588-594.
38. Marchetti C, De Felice F, Palaia I, et al. Risk-reducing salpingo-oophorectomy: a meta-analysis on impact on ovarian cancer risk and all cause mortality in BRCA 1 and BRCA 2 mutation carriers. BMC Womens Health. 2014;14:150.
39. Nelson HD, Pappas M, Zakher B, et al. Risk assessment, genetic counseling, and genetic testing for BRCA-related cancer in women: a systematic review to update the US Preventive Services Task Force recommendation. Ann Intern Med. 2014;160:255-266.
40. Parker WH, Feskanich D, Broder MS, et al. Long-term mortality associated with oophorectomy compared with ovarian conservation in the nurses’ health study. Obstet Gynecol. 2013;121:709-716.
41. Faubion SS, Kuhle CL, Shuster LT, et al. Long-term health consequences of premature or early menopause and considerations for management. Climacteric. 2015;18:483-491.
42. Menon U, Karpinskyj C, Gentry-Maharaj A. Ovarian cancer prevention and screening. Obstet Gynecol. 2018;131:909-927.
43. Crum CP, Drapkin R, Miron A, et al. The distal fallopian tube: a new model for pelvic serous carcinogenesis. Curr Opin Obstet Gynecol. 2007;19:3-9.
44. Kwon JS, Tinker A, Pansegrau G, et al. Prophylactic salpingectomy and delayed oophorectomy as an alternative for BRCA mutation carriers. Obstet Gynecol. 2013;121:14-24.
45. Holman LL, Friedman S, Daniels MS, et al. Acceptability of prophylactic salpingectomy with delayed oophorectomy as risk-reducing surgery among BRCA mutation carriers. Gynecol Oncol. 2014;133:283-286.
46. MD Anderson Cancer Center. Prophylactic salpingectomy with delayed oophorectomy, risk-reducing salpingo-oophorectomy, and ovarian cancer screening among BRCA mutation carriers: a proof-of-concept study. www.mdanderson.org/patients-family/diagnosis-treatment/clinical-trials/clinical-trials-index/clinical-trials-detail.ID2013-0340.html. Accessed May 22, 2020.
47. Iodice S, Barile M, Rotmensz N, et al. Oral contraceptive use and breast or ovarian cancer risk in BRCA1/2 carriers: a meta-analysis. Eur J Cancer. 2010;46:2275-2284.
48. Moorman PG, Havrilesky LJ, Gierisch JM, et al. Oral contraceptives and risk of ovarian cancer and breast cancer among high-risk women: a systematic review and meta-analysis. J Clin Oncol. 2013;31:4188-4198.
49. Friebel TM, Domchek SM, Rebbeck TR. Modifiers of cancer risk in BRCA1 and BRCA2 mutation carriers: systematic review and meta-analysis. J Natl Cancer Inst. 2014;106:dju091.
50. Haile RW, Thomas DC, McGuire V, et al. BRCA1 and BRCA2 mutation carriers, oral contraceptive use, and breast cancer before age 50. Cancer Epidemiol Biomarkers Prev. 2006;15:1863-1870.
51. Lee E, Ma H, McKean-Cowdin R, et al. Effect of reproductive factors and oral contraceptives on breast cancer risk in BRCA1/2 mutation carriers and noncarriers: results from a population-based study. Cancer Epidemiol Biomarkers Prev. 2008;17:3170-3178.
52. Narod SA, Dubé MP, Klijn J, et al. Oral contraceptives and the risk of breast cancer in BRCA1 and BRCA2 mutation carriers. J Natl Cancer Inst. 2002;94:1773-1779.
53. Milne RL, Knight JA, John EM, et al. Oral contraceptive use and risk of early-onset breast cancer in carriers and noncarriers of BRCA1 and BRCA2 mutations. Cancer Epidemiol Biomarkers Prev. 2005;14:350-356.
54. Culver JO, MacDonald DJ, Thornton AA, et al. Development and evaluation of a decision aid for BRCA carriers with breast cancer. J Genet Couns. 2011;20:294-307.
55. Stacey D, Légaré F, Lewis K, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database of Syst Rev. 2017;4:CD001431.
56. Schwartz MD, Valdimarsdottir HB, DeMarco TA, et al. Randomized trial of a decision aid for BRCA1/BRCA2 mutation carriers: impact on measures of decision making and satisfaction. Health Psychol. 2009;28:11-19.
57. Metcalfe KA, Dennis CL, Poll A, et al. Effect of decision aid for breast cancer prevention on decisional conflict in women with a BRCA1 or BRCA2 mutation: a multisite, randomized, controlled trial. Gen Med. 2017;19:330-336.
58. Friedman LC, Kramer RM. Reproductive issues for women with BRCA mutations. J Natl Cancer Inst Monogr. 2005;34:83-86.
PRACTICE RECOMMENDATIONS
› Recommend genetic screening for the BRCA mutation if a patient’s family history includes a breast cancer diagnosis before age 50, occurrences of both breast and ovarian cancers, or other suggestive features. C
› Advise women with the BRCA gene to return for a clinical breast exam every 6 to 12 months starting at age 25, and to start radiologic screening at age 30. C
› Consider recommending bilateral salpingo-oophorectomy to prevent ovarian cancer in women 35 to 40 years of age with a BRCA1 mutation who have completed childbearing. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
‘A good and peaceful death’: Cancer hospice during the pandemic
Lillie Shockney, RN, MAS, a two-time breast cancer survivor and adjunct professor at Johns Hopkins School of Nursing in Baltimore, Maryland, mourns the many losses that her patients with advanced cancer now face in the midst of the COVID-19 pandemic. But in the void of the usual support networks and treatment plans, she sees the resurgence of something that has recently been crowded out: hospice.
The pandemic has forced patients and their physicians to reassess the risk/benefit balance of continuing or embarking on yet another cancer treatment.
“It’s one of the pearls that we will get out of this nightmare,” said Ms. Shockney, who recently retired as administrative director of the cancer survivorship programs at the Sidney Kimmel Comprehensive Cancer Center.
“Physicians have been taught to treat the disease – so as long as there’s a treatment they give another treatment,” she told Medscape Medical News during a Zoom call from her home. “But for some patients with advanced disease, those treatments were making them very sick, so they were trading longevity over quality of life.”
Of course, longevity has never been a guarantee with cancer treatment, and even less so now, with the risk of COVID-19.
“This is going to bring them to some hard discussions,” says Brenda Nevidjon, RN, MSN, chief executive officer at the Oncology Nursing Society.
“We’ve known for a long time that there are patients who are on third- and fourth-round treatment options that have very little evidence of prolonging life or quality of life,” she told Medscape Medical News. “Do we bring these people out of their home to a setting where there could be a fair number of COVID-positive patients? Do we expose them to that?”
Across the world, these dilemmas are pushing cancer specialists to initiate discussions of hospice sooner with patients who have advanced disease, and with more clarity than before.
One of the reasons such conversations have often been avoided is that the concept of hospice is generally misunderstood, said Ms. Shockney.
“Patients think ‘you’re giving up on me, you’ve abandoned me’, but hospice is all about preserving the remainder of their quality of life and letting them have time with family and time to fulfill those elements of experiencing a good and peaceful death,” she said.
Indeed, hospice is “a benefit meant for somebody with at least a 6-month horizon,” agrees Ms. Nevidjon. Yet the average length of hospice in the United States is just 5 days. “It’s at the very, very end, and yet for some of these patients the 6 months they could get in hospice might be a better quality of life than the 4 months on another whole plan of chemotherapy. I can’t imagine that on the backside of this pandemic we will not have learned and we won’t start to change practices around initiating more of these conversations.”
Silver lining of this pandemic?
It’s too early into the pandemic to have hard data on whether hospice uptake has increased, but “it’s encouraging to hear that hospice is being discussed and offered sooner as an alternative to that third- or fourth-round chemo,” said Lori Bishop, MHA, RN, vice president of palliative and advanced care at the National Hospice and Palliative Care Organization.
“I agree that improving informed-decision discussions and timely access to hospice is a silver lining of the pandemic,” she told Medscape Medical News.
But she points out that today’s hospice looks quite different than it did before the pandemic, with the immediate and very obvious difference being telehealth, which was not widely utilized previously.
In March, the Centers for Medicare & Medicaid Services expanded telehealth options for hospice providers, something that Ms. Bishop and other hospice providers hope will remain in place after the pandemic passes.
“Telehealth visits are offered to replace some in-home visits both to minimize risk of exposure to COVID-19 and reduce the drain on personal protective equipment,” Bishop explained.
“In-patient hospice programs are also finding unique ways to provide support and connect patients to their loved ones: visitors are allowed but limited to one or two. Music and pet therapy are being provided through the window or virtually and devices such as iPads are being used to help patients connect with loved ones,” she said.
Telehealth links patients out of loneliness, but the one thing it cannot do is provide the comfort of touch – an important part of any hospice program.
“Hand-holding ... I miss that a lot,” says Ms. Shockney, her eyes filling with tears. “When you take somebody’s hand, you don’t even have to speak; that connection, and eye contact, is all you need to help that person emotionally heal.”
This article first appeared on Medscape.com.
Lillie Shockney, RN, MAS, a two-time breast cancer survivor and adjunct professor at Johns Hopkins School of Nursing in Baltimore, Maryland, mourns the many losses that her patients with advanced cancer now face in the midst of the COVID-19 pandemic. But in the void of the usual support networks and treatment plans, she sees the resurgence of something that has recently been crowded out: hospice.
The pandemic has forced patients and their physicians to reassess the risk/benefit balance of continuing or embarking on yet another cancer treatment.
“It’s one of the pearls that we will get out of this nightmare,” said Ms. Shockney, who recently retired as administrative director of the cancer survivorship programs at the Sidney Kimmel Comprehensive Cancer Center.
“Physicians have been taught to treat the disease – so as long as there’s a treatment they give another treatment,” she told Medscape Medical News during a Zoom call from her home. “But for some patients with advanced disease, those treatments were making them very sick, so they were trading longevity over quality of life.”
Of course, longevity has never been a guarantee with cancer treatment, and even less so now, with the risk of COVID-19.
“This is going to bring them to some hard discussions,” says Brenda Nevidjon, RN, MSN, chief executive officer at the Oncology Nursing Society.
“We’ve known for a long time that there are patients who are on third- and fourth-round treatment options that have very little evidence of prolonging life or quality of life,” she told Medscape Medical News. “Do we bring these people out of their home to a setting where there could be a fair number of COVID-positive patients? Do we expose them to that?”
Across the world, these dilemmas are pushing cancer specialists to initiate discussions of hospice sooner with patients who have advanced disease, and with more clarity than before.
One of the reasons such conversations have often been avoided is that the concept of hospice is generally misunderstood, said Ms. Shockney.
“Patients think ‘you’re giving up on me, you’ve abandoned me’, but hospice is all about preserving the remainder of their quality of life and letting them have time with family and time to fulfill those elements of experiencing a good and peaceful death,” she said.
Indeed, hospice is “a benefit meant for somebody with at least a 6-month horizon,” agrees Ms. Nevidjon. Yet the average length of hospice in the United States is just 5 days. “It’s at the very, very end, and yet for some of these patients the 6 months they could get in hospice might be a better quality of life than the 4 months on another whole plan of chemotherapy. I can’t imagine that on the backside of this pandemic we will not have learned and we won’t start to change practices around initiating more of these conversations.”
Silver lining of this pandemic?
It’s too early into the pandemic to have hard data on whether hospice uptake has increased, but “it’s encouraging to hear that hospice is being discussed and offered sooner as an alternative to that third- or fourth-round chemo,” said Lori Bishop, MHA, RN, vice president of palliative and advanced care at the National Hospice and Palliative Care Organization.
“I agree that improving informed-decision discussions and timely access to hospice is a silver lining of the pandemic,” she told Medscape Medical News.
But she points out that today’s hospice looks quite different than it did before the pandemic, with the immediate and very obvious difference being telehealth, which was not widely utilized previously.
In March, the Centers for Medicare & Medicaid Services expanded telehealth options for hospice providers, something that Ms. Bishop and other hospice providers hope will remain in place after the pandemic passes.
“Telehealth visits are offered to replace some in-home visits both to minimize risk of exposure to COVID-19 and reduce the drain on personal protective equipment,” Bishop explained.
“In-patient hospice programs are also finding unique ways to provide support and connect patients to their loved ones: visitors are allowed but limited to one or two. Music and pet therapy are being provided through the window or virtually and devices such as iPads are being used to help patients connect with loved ones,” she said.
Telehealth links patients out of loneliness, but the one thing it cannot do is provide the comfort of touch – an important part of any hospice program.
“Hand-holding ... I miss that a lot,” says Ms. Shockney, her eyes filling with tears. “When you take somebody’s hand, you don’t even have to speak; that connection, and eye contact, is all you need to help that person emotionally heal.”
This article first appeared on Medscape.com.
Lillie Shockney, RN, MAS, a two-time breast cancer survivor and adjunct professor at Johns Hopkins School of Nursing in Baltimore, Maryland, mourns the many losses that her patients with advanced cancer now face in the midst of the COVID-19 pandemic. But in the void of the usual support networks and treatment plans, she sees the resurgence of something that has recently been crowded out: hospice.
The pandemic has forced patients and their physicians to reassess the risk/benefit balance of continuing or embarking on yet another cancer treatment.
“It’s one of the pearls that we will get out of this nightmare,” said Ms. Shockney, who recently retired as administrative director of the cancer survivorship programs at the Sidney Kimmel Comprehensive Cancer Center.
“Physicians have been taught to treat the disease – so as long as there’s a treatment they give another treatment,” she told Medscape Medical News during a Zoom call from her home. “But for some patients with advanced disease, those treatments were making them very sick, so they were trading longevity over quality of life.”
Of course, longevity has never been a guarantee with cancer treatment, and even less so now, with the risk of COVID-19.
“This is going to bring them to some hard discussions,” says Brenda Nevidjon, RN, MSN, chief executive officer at the Oncology Nursing Society.
“We’ve known for a long time that there are patients who are on third- and fourth-round treatment options that have very little evidence of prolonging life or quality of life,” she told Medscape Medical News. “Do we bring these people out of their home to a setting where there could be a fair number of COVID-positive patients? Do we expose them to that?”
Across the world, these dilemmas are pushing cancer specialists to initiate discussions of hospice sooner with patients who have advanced disease, and with more clarity than before.
One of the reasons such conversations have often been avoided is that the concept of hospice is generally misunderstood, said Ms. Shockney.
“Patients think ‘you’re giving up on me, you’ve abandoned me’, but hospice is all about preserving the remainder of their quality of life and letting them have time with family and time to fulfill those elements of experiencing a good and peaceful death,” she said.
Indeed, hospice is “a benefit meant for somebody with at least a 6-month horizon,” agrees Ms. Nevidjon. Yet the average length of hospice in the United States is just 5 days. “It’s at the very, very end, and yet for some of these patients the 6 months they could get in hospice might be a better quality of life than the 4 months on another whole plan of chemotherapy. I can’t imagine that on the backside of this pandemic we will not have learned and we won’t start to change practices around initiating more of these conversations.”
Silver lining of this pandemic?
It’s too early into the pandemic to have hard data on whether hospice uptake has increased, but “it’s encouraging to hear that hospice is being discussed and offered sooner as an alternative to that third- or fourth-round chemo,” said Lori Bishop, MHA, RN, vice president of palliative and advanced care at the National Hospice and Palliative Care Organization.
“I agree that improving informed-decision discussions and timely access to hospice is a silver lining of the pandemic,” she told Medscape Medical News.
But she points out that today’s hospice looks quite different than it did before the pandemic, with the immediate and very obvious difference being telehealth, which was not widely utilized previously.
In March, the Centers for Medicare & Medicaid Services expanded telehealth options for hospice providers, something that Ms. Bishop and other hospice providers hope will remain in place after the pandemic passes.
“Telehealth visits are offered to replace some in-home visits both to minimize risk of exposure to COVID-19 and reduce the drain on personal protective equipment,” Bishop explained.
“In-patient hospice programs are also finding unique ways to provide support and connect patients to their loved ones: visitors are allowed but limited to one or two. Music and pet therapy are being provided through the window or virtually and devices such as iPads are being used to help patients connect with loved ones,” she said.
Telehealth links patients out of loneliness, but the one thing it cannot do is provide the comfort of touch – an important part of any hospice program.
“Hand-holding ... I miss that a lot,” says Ms. Shockney, her eyes filling with tears. “When you take somebody’s hand, you don’t even have to speak; that connection, and eye contact, is all you need to help that person emotionally heal.”
This article first appeared on Medscape.com.
Antenatal corticosteroids may increase risk for mental and behavioral disorders
according to a Finnish population-based study published in JAMA. The findings may lead to changes in clinical practice, particularly for infants who may be born full term.
After adjustment for variables such as maternal age, smoking during pregnancy, any lifetime mental disorder diagnosis, and gestational age at birth, exposure to maternal antenatal corticosteroid treatment was significantly associated with mental and behavioral disorders in children, compared with nonexposure, with a hazard ratio of 1.33. Among children born at term, the adjusted hazard ratio was 1.47. Among preterm children, the hazard ratio was not significant.
“Although benefits of this therapy outweigh risks in the most vulnerable infants, this may not be true for all infants,” wrote Sara B. DeMauro, MD, an attending neonatologist and program director of the neonatal follow-up program at Children’s Hospital of Philadelphia, in an editorial also published in JAMA. “Recommendations to administer this therapy to broader populations of pregnant women may need to be reexamined until sufficient safety data, particularly among more mature infants, are available.”
Corticosteroid treatment to accelerate fetal maturation is standard care before 34 weeks’ gestation when there is a likelihood of delivery within 7 days, and studies have found that providing this therapy reduces the risk for respiratory problems when administered beyond 34 weeks. In 2016, updates to U.S. guidelines allowed for the use of corticosteroid treatment between 34 weeks and 36 weeks 6 days when women are at risk for preterm delivery within 7 days and have not received a previous course of antenatal corticosteroids.
The data from Finland indicate that “a significant number of very preterm children who might have benefited from this treatment did not receive it,” Dr. DeMauro wrote. At the same time, “45% of steroid-exposed infants were delivered at term. In these infants, minor short-term benefit may have been outweighed by significant longer-term risks. These data elucidate both the continuing struggle to accurately predict preterm birth and the incomplete uptake of an effective therapy that is beneficial when administered to the correct patients.”
Pause expanded use?
“Since the recommendations came out to expand the use of corticosteroids for preterm labor up until 37 weeks gestational age, my practice has incorporated these guidelines,” said Santina Wheat, MD, assistant professor of family and community medicine at Northwestern University in Chicago. “We have incorporated the guidelines though with the understanding that the benefits outweigh the risk. This article indicates that we may have been wrong in that understanding.” Although the association does not establish that the treatment causes mental and behavioral disorders, it “raises the question of whether we should halt this practice until additional information can be gathered,” noted Dr. Wheat, who also serves on the editorial advisory board of Family Practice News.
When administered before delivery of a very premature infant, corticosteroid therapy accelerates fetal lung maturation and helps prevent neonatal mortality, respiratory distress syndrome, and brain injury. Investigators demonstrated the benefits of antenatal corticosteroids in 1972, and the treatment – “one of the most important advances in perinatal care” – became widely used in the 1990s, Dr. DeMauro said.
To examine whether treatment exposure is associated with a risk of childhood mental and behavioral disorders and whether the risk is similar in infants born at term and preterm, Katri Räikkönen, PhD, a researcher at the University of Helsinki, and colleagues conducted a population-based retrospective study of more than 670,000 children.
The researchers identified all singleton pregnancies ending in a live birth in Finland during Jan. 1, 2006–Dec.31, 2017. In addition, they identified all consecutive maternal sibling pairs born at term, including sibling pairs discordant for maternal antenatal corticosteroid treatment exposure and sibling pairs concordant for treatment exposure or nonexposure. The investigators identified diagnoses of childhood mental and behavioral disorders using the Finnish Care Register for Health Care using ICD-10 codes on hospital inpatient and outpatient treatments by physicians in specialized medical care.
A range of disorders
In all, 670,097 infants with a median follow-up duration of 5.8 years were included in the analysis, and 14,868 (2.22%) were exposed to antenatal corticosteroids. Of the treatment-exposed children, about 45% were born at term. Of the nonexposed children, approximately 97% were born at term. Cumulative incidence rates for any mental and behavioral disorder were significantly higher for treatment-exposed children, compared with nonexposed children, in the entire cohort (12.01% vs. 6.45%; P less than .001) and in term-born children (8.89% vs. 6.31%; P less than .001).
In preterm children, the incidence rate of any mental and behavioral disorder was significantly higher among those with treatment exposure (14.59% vs. 10.71%; P less than .001). Associations persisted when the investigators focused on 241,621 sibling pairs, “suggesting that unmeasured familial confounding did not explain these associations,” the authors said.
“[In] the entire cohort and term-born children, treatment exposure ... was significantly associated with psychological development disorders; attention-deficit/hyperactivity or conduct disorders; mixed disorders of conduct and emotions, emotional disorders, disorders of social functioning or tic disorders; other behavioral or emotional disorders; and sleep disorders,” Dr. Räikkönen and colleagues reported. Among preterm-born, treatment-exposed children, the adjusted hazard ratio was significantly lower for intellectual disability and higher for sleep disorders.
Dr. DeMauro noted potential confounders in this observational study, including abnormal pregnancy events that lead clinicians to administer steroids. Such events “predispose the exposed children to adverse cognitive outcomes,” suggests some research. “Alternately, after a pregnancy at high risk for preterm delivery, families may perceive their children as vulnerable and therefore may be more likely to seek care and earlier diagnosis of mental or behavioral disorders,” Dr. DeMauro said.
The study was funded by the Academy of Finland, European Commission, Foundation for Pediatric Research, the Signe and Ane Gyllenberg Foundation, the Novo Nordisk Foundation, the Sigrid Juselius Foundation, and the Juho Vainio Foundation. The investigators and Dr. DeMauro had no conflict of interest disclosures.
SOURCE: Räikkönen K et al. JAMA. 2020;323(19):1924-33. doi: 10.1001/jama.2020.3937.
according to a Finnish population-based study published in JAMA. The findings may lead to changes in clinical practice, particularly for infants who may be born full term.
After adjustment for variables such as maternal age, smoking during pregnancy, any lifetime mental disorder diagnosis, and gestational age at birth, exposure to maternal antenatal corticosteroid treatment was significantly associated with mental and behavioral disorders in children, compared with nonexposure, with a hazard ratio of 1.33. Among children born at term, the adjusted hazard ratio was 1.47. Among preterm children, the hazard ratio was not significant.
“Although benefits of this therapy outweigh risks in the most vulnerable infants, this may not be true for all infants,” wrote Sara B. DeMauro, MD, an attending neonatologist and program director of the neonatal follow-up program at Children’s Hospital of Philadelphia, in an editorial also published in JAMA. “Recommendations to administer this therapy to broader populations of pregnant women may need to be reexamined until sufficient safety data, particularly among more mature infants, are available.”
Corticosteroid treatment to accelerate fetal maturation is standard care before 34 weeks’ gestation when there is a likelihood of delivery within 7 days, and studies have found that providing this therapy reduces the risk for respiratory problems when administered beyond 34 weeks. In 2016, updates to U.S. guidelines allowed for the use of corticosteroid treatment between 34 weeks and 36 weeks 6 days when women are at risk for preterm delivery within 7 days and have not received a previous course of antenatal corticosteroids.
The data from Finland indicate that “a significant number of very preterm children who might have benefited from this treatment did not receive it,” Dr. DeMauro wrote. At the same time, “45% of steroid-exposed infants were delivered at term. In these infants, minor short-term benefit may have been outweighed by significant longer-term risks. These data elucidate both the continuing struggle to accurately predict preterm birth and the incomplete uptake of an effective therapy that is beneficial when administered to the correct patients.”
Pause expanded use?
“Since the recommendations came out to expand the use of corticosteroids for preterm labor up until 37 weeks gestational age, my practice has incorporated these guidelines,” said Santina Wheat, MD, assistant professor of family and community medicine at Northwestern University in Chicago. “We have incorporated the guidelines though with the understanding that the benefits outweigh the risk. This article indicates that we may have been wrong in that understanding.” Although the association does not establish that the treatment causes mental and behavioral disorders, it “raises the question of whether we should halt this practice until additional information can be gathered,” noted Dr. Wheat, who also serves on the editorial advisory board of Family Practice News.
When administered before delivery of a very premature infant, corticosteroid therapy accelerates fetal lung maturation and helps prevent neonatal mortality, respiratory distress syndrome, and brain injury. Investigators demonstrated the benefits of antenatal corticosteroids in 1972, and the treatment – “one of the most important advances in perinatal care” – became widely used in the 1990s, Dr. DeMauro said.
To examine whether treatment exposure is associated with a risk of childhood mental and behavioral disorders and whether the risk is similar in infants born at term and preterm, Katri Räikkönen, PhD, a researcher at the University of Helsinki, and colleagues conducted a population-based retrospective study of more than 670,000 children.
The researchers identified all singleton pregnancies ending in a live birth in Finland during Jan. 1, 2006–Dec.31, 2017. In addition, they identified all consecutive maternal sibling pairs born at term, including sibling pairs discordant for maternal antenatal corticosteroid treatment exposure and sibling pairs concordant for treatment exposure or nonexposure. The investigators identified diagnoses of childhood mental and behavioral disorders using the Finnish Care Register for Health Care using ICD-10 codes on hospital inpatient and outpatient treatments by physicians in specialized medical care.
A range of disorders
In all, 670,097 infants with a median follow-up duration of 5.8 years were included in the analysis, and 14,868 (2.22%) were exposed to antenatal corticosteroids. Of the treatment-exposed children, about 45% were born at term. Of the nonexposed children, approximately 97% were born at term. Cumulative incidence rates for any mental and behavioral disorder were significantly higher for treatment-exposed children, compared with nonexposed children, in the entire cohort (12.01% vs. 6.45%; P less than .001) and in term-born children (8.89% vs. 6.31%; P less than .001).
In preterm children, the incidence rate of any mental and behavioral disorder was significantly higher among those with treatment exposure (14.59% vs. 10.71%; P less than .001). Associations persisted when the investigators focused on 241,621 sibling pairs, “suggesting that unmeasured familial confounding did not explain these associations,” the authors said.
“[In] the entire cohort and term-born children, treatment exposure ... was significantly associated with psychological development disorders; attention-deficit/hyperactivity or conduct disorders; mixed disorders of conduct and emotions, emotional disorders, disorders of social functioning or tic disorders; other behavioral or emotional disorders; and sleep disorders,” Dr. Räikkönen and colleagues reported. Among preterm-born, treatment-exposed children, the adjusted hazard ratio was significantly lower for intellectual disability and higher for sleep disorders.
Dr. DeMauro noted potential confounders in this observational study, including abnormal pregnancy events that lead clinicians to administer steroids. Such events “predispose the exposed children to adverse cognitive outcomes,” suggests some research. “Alternately, after a pregnancy at high risk for preterm delivery, families may perceive their children as vulnerable and therefore may be more likely to seek care and earlier diagnosis of mental or behavioral disorders,” Dr. DeMauro said.
The study was funded by the Academy of Finland, European Commission, Foundation for Pediatric Research, the Signe and Ane Gyllenberg Foundation, the Novo Nordisk Foundation, the Sigrid Juselius Foundation, and the Juho Vainio Foundation. The investigators and Dr. DeMauro had no conflict of interest disclosures.
SOURCE: Räikkönen K et al. JAMA. 2020;323(19):1924-33. doi: 10.1001/jama.2020.3937.
according to a Finnish population-based study published in JAMA. The findings may lead to changes in clinical practice, particularly for infants who may be born full term.
After adjustment for variables such as maternal age, smoking during pregnancy, any lifetime mental disorder diagnosis, and gestational age at birth, exposure to maternal antenatal corticosteroid treatment was significantly associated with mental and behavioral disorders in children, compared with nonexposure, with a hazard ratio of 1.33. Among children born at term, the adjusted hazard ratio was 1.47. Among preterm children, the hazard ratio was not significant.
“Although benefits of this therapy outweigh risks in the most vulnerable infants, this may not be true for all infants,” wrote Sara B. DeMauro, MD, an attending neonatologist and program director of the neonatal follow-up program at Children’s Hospital of Philadelphia, in an editorial also published in JAMA. “Recommendations to administer this therapy to broader populations of pregnant women may need to be reexamined until sufficient safety data, particularly among more mature infants, are available.”
Corticosteroid treatment to accelerate fetal maturation is standard care before 34 weeks’ gestation when there is a likelihood of delivery within 7 days, and studies have found that providing this therapy reduces the risk for respiratory problems when administered beyond 34 weeks. In 2016, updates to U.S. guidelines allowed for the use of corticosteroid treatment between 34 weeks and 36 weeks 6 days when women are at risk for preterm delivery within 7 days and have not received a previous course of antenatal corticosteroids.
The data from Finland indicate that “a significant number of very preterm children who might have benefited from this treatment did not receive it,” Dr. DeMauro wrote. At the same time, “45% of steroid-exposed infants were delivered at term. In these infants, minor short-term benefit may have been outweighed by significant longer-term risks. These data elucidate both the continuing struggle to accurately predict preterm birth and the incomplete uptake of an effective therapy that is beneficial when administered to the correct patients.”
Pause expanded use?
“Since the recommendations came out to expand the use of corticosteroids for preterm labor up until 37 weeks gestational age, my practice has incorporated these guidelines,” said Santina Wheat, MD, assistant professor of family and community medicine at Northwestern University in Chicago. “We have incorporated the guidelines though with the understanding that the benefits outweigh the risk. This article indicates that we may have been wrong in that understanding.” Although the association does not establish that the treatment causes mental and behavioral disorders, it “raises the question of whether we should halt this practice until additional information can be gathered,” noted Dr. Wheat, who also serves on the editorial advisory board of Family Practice News.
When administered before delivery of a very premature infant, corticosteroid therapy accelerates fetal lung maturation and helps prevent neonatal mortality, respiratory distress syndrome, and brain injury. Investigators demonstrated the benefits of antenatal corticosteroids in 1972, and the treatment – “one of the most important advances in perinatal care” – became widely used in the 1990s, Dr. DeMauro said.
To examine whether treatment exposure is associated with a risk of childhood mental and behavioral disorders and whether the risk is similar in infants born at term and preterm, Katri Räikkönen, PhD, a researcher at the University of Helsinki, and colleagues conducted a population-based retrospective study of more than 670,000 children.
The researchers identified all singleton pregnancies ending in a live birth in Finland during Jan. 1, 2006–Dec.31, 2017. In addition, they identified all consecutive maternal sibling pairs born at term, including sibling pairs discordant for maternal antenatal corticosteroid treatment exposure and sibling pairs concordant for treatment exposure or nonexposure. The investigators identified diagnoses of childhood mental and behavioral disorders using the Finnish Care Register for Health Care using ICD-10 codes on hospital inpatient and outpatient treatments by physicians in specialized medical care.
A range of disorders
In all, 670,097 infants with a median follow-up duration of 5.8 years were included in the analysis, and 14,868 (2.22%) were exposed to antenatal corticosteroids. Of the treatment-exposed children, about 45% were born at term. Of the nonexposed children, approximately 97% were born at term. Cumulative incidence rates for any mental and behavioral disorder were significantly higher for treatment-exposed children, compared with nonexposed children, in the entire cohort (12.01% vs. 6.45%; P less than .001) and in term-born children (8.89% vs. 6.31%; P less than .001).
In preterm children, the incidence rate of any mental and behavioral disorder was significantly higher among those with treatment exposure (14.59% vs. 10.71%; P less than .001). Associations persisted when the investigators focused on 241,621 sibling pairs, “suggesting that unmeasured familial confounding did not explain these associations,” the authors said.
“[In] the entire cohort and term-born children, treatment exposure ... was significantly associated with psychological development disorders; attention-deficit/hyperactivity or conduct disorders; mixed disorders of conduct and emotions, emotional disorders, disorders of social functioning or tic disorders; other behavioral or emotional disorders; and sleep disorders,” Dr. Räikkönen and colleagues reported. Among preterm-born, treatment-exposed children, the adjusted hazard ratio was significantly lower for intellectual disability and higher for sleep disorders.
Dr. DeMauro noted potential confounders in this observational study, including abnormal pregnancy events that lead clinicians to administer steroids. Such events “predispose the exposed children to adverse cognitive outcomes,” suggests some research. “Alternately, after a pregnancy at high risk for preterm delivery, families may perceive their children as vulnerable and therefore may be more likely to seek care and earlier diagnosis of mental or behavioral disorders,” Dr. DeMauro said.
The study was funded by the Academy of Finland, European Commission, Foundation for Pediatric Research, the Signe and Ane Gyllenberg Foundation, the Novo Nordisk Foundation, the Sigrid Juselius Foundation, and the Juho Vainio Foundation. The investigators and Dr. DeMauro had no conflict of interest disclosures.
SOURCE: Räikkönen K et al. JAMA. 2020;323(19):1924-33. doi: 10.1001/jama.2020.3937.
FROM JAMA
Key clinical point: Exposure to maternal antenatal corticosteroid treatment is significantly associated with mental and behavioral disorders in children, compared with nonexposure.
Major finding: After adjustment for such variables as maternal age, smoking during pregnancy, any lifetime mental disorder diagnosis, and gestational age at birth, exposure to maternal antenatal corticosteroid treatment was significantly associated with mental and behavioral disorders in children, compared with nonexposure (HR, 1.33). Among children born at term, the adjusted HR was 1.47.
Study details: A population-based retrospective cohort study that included 670,097 children in Finland.
Disclosures: The study was funded by the Academy of Finland, European Commission, Foundation for Pediatric Research, the Signe and Ane Gyllenberg Foundation, the Novo Nordisk Foundation, the Sigrid Juselius Foundation, and the Juho Vainio Foundation. The authors had no conflict of interest disclosures.
Source: Räikkönen K et al. JAMA. 2020;323(19):1924-33. doi: 10.1001/jama.2020.3937.
Addressing racism, bias in the American maternal mortality crisis
This is the second of a two-part article on the role of racism and bias in the U.S. maternal mortality crisis and part of an ongoing Ob.Gyn. News feature series on the crisis. Part one of the story explored existing data, societal factors, and patient experiences related to structural racism, overt racism, and implicit bias as factors contributing to racial disparities in maternal outcomes. Here we explore potential solutions for addressing the inequities as proposed by thought leaders and key stakeholders.
The emerging racial disparities in COVID-19 incidence and outcomes in the United States are on a collision course with long-standing racial disparities in U.S. maternal care and mortality.
Maternal health advocates are bracing for the impact, but in the spotlight that the pandemic is training on the inequities and the health system changes taking shape in its wake, some also see hope for a shift in at least one important driver of the racial health disparities: access to care.
Non-Hispanic black women are at least three times more likely than Hispanic women and non-Hispanic white women to experience pregnancy-related death, and indigenous women are more than twice as likely, according to the latest data from the Centers for Disease Control and Prevention’s National Center for Health Statistics. and to exacerbate racial disparities, panelists agreed during a recent National Maternal Health Patient Centered Outcomes Research Network webinar entitled “The Impact of COVID-19 on Black, Brown, and Native Pregnant People.”
“The saying is that ‘the virus doesn’t discriminate,’ but it understands our biases, right? So, the virus takes advantage of the weaknesses in our system,” said panelist Joia A. Crear-Perry, MD, an ob.gyn. and founder and president of the National Birth Equity Collaborative (NBEC), a New Orleans–based research, training, and advocacy organization working to optimize black maternal and infant health.
Hope for solutions from the ashes of a pandemic
The weaknesses in the system that Dr. Crear-Perry spoke of are in many ways a product of structural racism as described in a conceptual report in The Lancet, titled “America: Equity and Equality in Health,” which dug into the entrenched and tangled historical roots of racist sociological and political factors that formed a foundation for health inequity over time.
Today, people of color remain more likely to be excluded from access to health insurance and adequate health care. The authors defined structural racism as “the totality of ways in which societies foster racial discrimination through mutually reinforcing systems of housing, education, employment, earnings, benefits, credit, media, health care, and criminal justice.” Today, largely as a result of these “reinforcing systems,” people of color remain more likely to be excluded from access to health insurance and adequate health care. At the same time, and for the same reasons, they are more likely to work in the service industry, be essential workers, and use mass transit, each of which increases the risk of exposure to COVID-19, Dr. Crear-Perry explained.
“It’s important for us to know that, for maternal mortality, it’s the same thing that happens,” she said. That means the focus on COVID-19–related disparities helps magnify and elevate the conversation regarding similar disparities in maternal outcomes.
It also means that some of the care delivery solutions embraced and facilitated amid the pandemic, such as extension of Medicaid coverage for up to a year after giving birth and broader use and insurance coverage of telemedicine, could finally gain traction; those are solutions long-sought by advocates like Dr. Crear-Perry and others as a means for alleviating racial disparities in maternal outcomes and addressing the maternal mortality crisis.
Therein lies the hope, she explained in an interview. “Some of the policies that we know would have been helpful prior to COVID-19 now are being seen as really important.”
Solution: Extending coverage
During a May 7 virtual Congressional hearing on “America’s Two Public Health Crises: The Impact of COVID-19 on Racial Inequities and Maternal Mortality in the U.S.,” cosponsored by the American College of Obstetricians and Gynecologists, the March of Dimes, and the NBEC, Dr. Crear-Perry further explained the importance of extended coverage and care access.
Asked what Congress could do immediately to “ensure that the pandemic does not compound the nation’s maternal mortality crisis, including unacceptable rates among black women,” she didn’t hesitate.
“Well, it would be amazing if we could get Medicaid extended for 12 months post delivery,” she said. “As you can imagine right now, we have moms who are birthing in hospitals where they have to worry about, 2 months later, not having coverage for themselves.”
If that mom is exposed to COVID-19 and has no insurance coverage and a newborn at home, the likelihood that she will call a provider if she develops symptoms is low, Dr. Crear-Perry said. “This is a great opportunity for us to really rethink some of those policies that we know are barriers, that we have created for people to be able to thrive after they have a baby and during child birth.”
Current policies are centered around an arbitrary cutoff of about 6 weeks for postpartum care, but the CDC reports that a third of all postpartum deaths occur between 1 week and 12 months after birth.
“We need our policies to reflect the current knowledge and the science,” she said. “Just like babies have automatic insurance coverage for a year later, mothers should have the same.”
Medicaid finances nearly half of all births in the United States, according to a 2019 Kaiser Family Foundation brief, which explained that federal law requires Medicaid coverage for only 60 days post partum for women who are eligible. Decisions regarding coverage after 60 days are determined by individual states; those that expanded Medicaid under the Affordable Care Act typically allow extended coverage – but only with reapplication at 60 days.
Many women in nonexpansion states become uninsured after pregnancy-related coverage ends, as do some in expansion states for whom reapplying is a hurdle too high to clear with a newborn baby to care for at home, Dr. Crear-Perry said.
Addressing these coverage gaps is key to improving access, and it is a core component of the Black Maternal Health Momnibus Act of 2020, a nine-bill package introduced in March by Rep. Lauren Underwood (D-Ill.), Rep. Alma Adams (D-N.C.), Sen. Kamala Harris (D-Calif.), and members of the Black Maternal Health Caucus to “fill gaps in existing legislation to comprehensively address every dimension of the Black maternal health crisis in America.”
One bill in the package addresses extended coverage with a goal to “promote innovative payment models to incentivize high-quality maternity care and continuity of health insurance coverage from pregnancy through labor and delivery and up to 1 year post partum.” Another focuses on promoting alternative ways to access care, such as through telemedicine.
Solution: Expanding care access
“There is a need for the democratization of care,” Dr. Crear-Perry said. “There is a need for people to have more ways to get care. This idea that the only way you can get prenatal care is you have to come to me at my office, has been a burden for working people for a long, long time.”
The COVID-19 pandemic necessitates increased use of telemedicine, but building blocks to allow patients to use it effectively must be put in place, she said. That means expanding broadband access, providing patients with blood pressure cuffs and other tools for use remotely, and expanding reimbursement to include not just video, but also phone calls.
Heart Safe Motherhood, a University of Pennsylvania text-based intervention developed to address postpartum hypertension – a leading cause of maternal morbidity and mortality, and at the start of the program, the leading cause of 7-day readmissions among obstetric patients, demonstrated the value of such approaches to care.
The program involves remote blood pressure monitoring using a digital monitor provided to at-risk patients at discharge. Text-based monitoring reminders encourage patients to check their blood pressure twice daily for the first 7 days.
“In our randomized, controlled trial, we saw our ability to meet ACOG guidelines on postpartum blood monitoring leap from 0% to 82%, compared to in-person office visits and 7-day readmissions from hypertension drop from 3% to 0%,” an update at the program website states.
Rebekah Gee, MD, an ob.gyn. and director of the Louisiana State University Health System in New Orleans, also noted the importance of finding ways to deliver care “that are outside the traditional norm.
“Telemedicine, home visiting ... I think there are a wide variety of ways,” she said, noting that these kind of approaches not only help circumvent roadblocks to care, such as lack of transportation, but also can feel more personal and approachable for some women.
Solution: Measuring, investing, diversifying, respecting
The aims of other bills in the Momnibus Act also mirror several solutions proposed by maternal health advocates interviewed for this article. Among them are:
- Development of improved data collection processes and quality measures to better understand the factors that contribute to the crisis overall and among special populations, and to inform solutions for addressing them.
- Investments in social determinants of health that influence maternal health outcomes, like housing, transportation, and nutrition.
- Commitment to the growth and diversification of the perinatal workforce to ensure that every mom receives maternity care and support from people she can trust to provide quality care and treat her with respect.
The latter is one that Dr. Gee, Dr. Crear-Perry, and others particularly emphasized.
“We need patient advocates like doulas, midwives and others who are better listeners and better able to advocate for patients,” Dr. Gee said. This would better allow for women’s desires in the childbirth experience to be addressed appropriately, she said, adding that this is something that “frankly, a lot of doctors do not have the time to do.”
That’s why the efforts to address maternal mortality have to focus on the health care system, not just on doctors’ behavior with respect to bias, she said.
Dr. Gee also said there is a need for culturally appropriate literacy and numeracy communications “that respect how people seek and understand information.” This varies by population, which is why it’s important to provide the same approach to care “no matter what the patient looks like,” while also understanding that different patients communicate in different ways.
A 2019 study published in Social Science & Medicine underscored how communication differences can affect outcomes; using a national sample of women who gave birth in U.S. hospitals, the authors found that those who had declined care for themselves or their infant during their childbirth hospitalization were more likely to report receiving poor treatment based on race or ethnicity. They concluded that, in the context of childbirth care, women – particularly black women – pay a penalty for what is perceived as uncooperative behavior.
This is another area where doulas and other patient advocates can help, Dr. Gee said.
Doulas have long been an integral part of the birthing process for many women, particularly women of color, and evidence suggests the supportive care they provide helps to improve outcomes. In fact, several states – including Oregon, Minnesota, and New York, among others – have expanded or have proposed expanding Medicaid coverage to include doula services for pregnant beneficiaries, a move cheered by doula associations and other maternal health advocates.
In many ways, it’s about “respectful maternity care,” which is something Dr. Crear-Perry has been working to promote through the NBEC in partnership with ACOG and the Robert Wood Johnson Foundation. It’s also something the World Health Organization has promoted by establishing global standards for such care.
“We’re hoping to socialize that as a norm in United States ... to really see what it would look like to value what birthing people want and to see them as partners in their birth,” she said.
However, the 2019 Giving Voice to Mothers study demonstrating consistently higher rates of mistreatment during obstetrical care for women of color than for comparable white women shows that the United States is falling short of those standards. The national study of 2,700 women examined how race, ethnicity, and place of birth interact with the experience of receiving maternity care in the United States, and showed that 1 in 6 experienced one or more types of mistreatment – with consistently higher rates among women of color, even after adjusting for interactions between race and other maternal characteristics, Saraswathi Veda, MD, of the Birth Place Lab and professor of midwifery at the University of British Columbia, Vancouver, and colleagues reported in Reproductive Health.
Solution: Listening, learning, reflecting, partnering
Timoria McQueen Saba, birth trauma survivor and maternal health advocate, has described experiencing instances of mistreatment throughout her obstetric care, and like Dr. Crear-Perry, she said trust and collaboration in care is imperative for improving outcomes.
“I think the most important thing you can do is really consider a patient a partner in the care you give them,” she said during a panel discussion at the 2019 ACOG annual meeting. “You’re not experts in their lived experience ... center a patient’s voice or the voice of a patient’s family. Incorporate that into your learning.”
During a virtual workshop held May 19-20 by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, Judette Louis, MD, chair of the department of obstetrics and gynecology at the University of South Florida, Tampa, and president of the Society of Maternal-Fetal Medicine, provided practical guidance for addressing racism and implicit bias in practice and in research to reduce disparities in outcomes.
In an interview, she summarized her key points, reiterating solutions proposed by Dr. Gee and Dr. Crear-Perry and addressed in the Momnibus Act, and also offering a few others:
First, put aside the notion that disparities are genetically driven. For a variety of reasons, that just doesn’t make sense. For one thing, not all blacks are African American.
“My family is from the Caribbean,” she said. “Is it really conceivable that we’re all so similar?”
Look also at the disparities among Native Americans, she said. “How can you take 500 distinct tribes that live across a wide geographic area and lump them into one group and assume that they are similar?”
The problem is racism, not race. “When you keep saying ‘it’s about race, it’s about race, it’s about race’ – that sends a message to the person who is of that race that there is something inherently broken about [them],” she said.
Recognize that the roots of the problem run deep. Learn about and support efforts to address the underlying structural factors that contribute to the problem, Dr. Louis emphasized, and recognize your own bias. “We all have it. The key is to recognize [biases] and mitigate them when taking care of patients.”
That’s easier said than done, at least judging by one survey of maternal-fetal medicine specialists in which 84% of respondents agreed that disparities impact practice, but only 29% agreed their own personal biases affect how they care for patients, she noted.
Tools are available to help individuals identify implicit bias, and training programs for health care providers can help, as well, she said. Implicit bias tests and training programs that help to identify and address bias and racism on individual and organizational levels are increasingly available through academic centers, health systems, and advocacy organizations.
Hope for solutions: Progress and promise
Like Dr. Crear-Perry, Dr. Louis sees hope for reducing disparities and improving maternal outcomes.
In another survey of SMFM members to identify the practice issues most important to them, racial disparities ranked in the top three.
“It says a lot that our [maternal-fetal medicine specialists] really see this as a problem and they want it fixed,” she said. “And I think it says that a lot of people need to work on this, not just us.”
Indeed, many are engaged in that work. Veronica Gillispie, MD, medical director of the Louisiana Perinatal Quality Collaborative and Pregnancy-Associated Mortality Review, has been instrumental in recent initiatives to improve maternal outcomes in Louisiana, and she too said she feels optimistic.
“I am hopeful and I do see signs of hope,” she said in an interview.
Teams that she works with and trains seem invested, institutions are increasingly implementing faculty training on racism and bias, and Oschner Health, where Dr. Gillispie practices as an ob.gyn., appointed its first chief diversity officer in February.
Medical students she works with are attuned to the issues of racism, bias, and disparities, and they show a desire to enact change, she said. “They already get it, and they are working to make it better.”
Dr. Crear-Perry also predicts practice-changing results from studies looking at the delivery of obstetrical care and the role of supportive care, and she pointed out another aspect of the COVID-19 crisis that provides an important lesson for health care providers who care for birthing people: the scarcity of personal protective equipment amid the pandemic.
“My friends who are ob.gyns., who are now not getting access to the things they need to stay safe to practice medicine and who are feeling very marginalized at this moment, feeling not valued – that’s how birthing people [of color] feel,” she said. “I’m hoping that builds a sense of empathy.
”I’m hoping at the end of this crisis, that those ob.gyns. will think of patients as allies in fighting for more access to health for everybody and for more resources to do their work,” Dr. Crear-Perry said. “We’re all in this together.”
This is the second of a two-part article on the role of racism and bias in the U.S. maternal mortality crisis and part of an ongoing Ob.Gyn. News feature series on the crisis. Part one of the story explored existing data, societal factors, and patient experiences related to structural racism, overt racism, and implicit bias as factors contributing to racial disparities in maternal outcomes. Here we explore potential solutions for addressing the inequities as proposed by thought leaders and key stakeholders.
The emerging racial disparities in COVID-19 incidence and outcomes in the United States are on a collision course with long-standing racial disparities in U.S. maternal care and mortality.
Maternal health advocates are bracing for the impact, but in the spotlight that the pandemic is training on the inequities and the health system changes taking shape in its wake, some also see hope for a shift in at least one important driver of the racial health disparities: access to care.
Non-Hispanic black women are at least three times more likely than Hispanic women and non-Hispanic white women to experience pregnancy-related death, and indigenous women are more than twice as likely, according to the latest data from the Centers for Disease Control and Prevention’s National Center for Health Statistics. and to exacerbate racial disparities, panelists agreed during a recent National Maternal Health Patient Centered Outcomes Research Network webinar entitled “The Impact of COVID-19 on Black, Brown, and Native Pregnant People.”
“The saying is that ‘the virus doesn’t discriminate,’ but it understands our biases, right? So, the virus takes advantage of the weaknesses in our system,” said panelist Joia A. Crear-Perry, MD, an ob.gyn. and founder and president of the National Birth Equity Collaborative (NBEC), a New Orleans–based research, training, and advocacy organization working to optimize black maternal and infant health.
Hope for solutions from the ashes of a pandemic
The weaknesses in the system that Dr. Crear-Perry spoke of are in many ways a product of structural racism as described in a conceptual report in The Lancet, titled “America: Equity and Equality in Health,” which dug into the entrenched and tangled historical roots of racist sociological and political factors that formed a foundation for health inequity over time.
Today, people of color remain more likely to be excluded from access to health insurance and adequate health care. The authors defined structural racism as “the totality of ways in which societies foster racial discrimination through mutually reinforcing systems of housing, education, employment, earnings, benefits, credit, media, health care, and criminal justice.” Today, largely as a result of these “reinforcing systems,” people of color remain more likely to be excluded from access to health insurance and adequate health care. At the same time, and for the same reasons, they are more likely to work in the service industry, be essential workers, and use mass transit, each of which increases the risk of exposure to COVID-19, Dr. Crear-Perry explained.
“It’s important for us to know that, for maternal mortality, it’s the same thing that happens,” she said. That means the focus on COVID-19–related disparities helps magnify and elevate the conversation regarding similar disparities in maternal outcomes.
It also means that some of the care delivery solutions embraced and facilitated amid the pandemic, such as extension of Medicaid coverage for up to a year after giving birth and broader use and insurance coverage of telemedicine, could finally gain traction; those are solutions long-sought by advocates like Dr. Crear-Perry and others as a means for alleviating racial disparities in maternal outcomes and addressing the maternal mortality crisis.
Therein lies the hope, she explained in an interview. “Some of the policies that we know would have been helpful prior to COVID-19 now are being seen as really important.”
Solution: Extending coverage
During a May 7 virtual Congressional hearing on “America’s Two Public Health Crises: The Impact of COVID-19 on Racial Inequities and Maternal Mortality in the U.S.,” cosponsored by the American College of Obstetricians and Gynecologists, the March of Dimes, and the NBEC, Dr. Crear-Perry further explained the importance of extended coverage and care access.
Asked what Congress could do immediately to “ensure that the pandemic does not compound the nation’s maternal mortality crisis, including unacceptable rates among black women,” she didn’t hesitate.
“Well, it would be amazing if we could get Medicaid extended for 12 months post delivery,” she said. “As you can imagine right now, we have moms who are birthing in hospitals where they have to worry about, 2 months later, not having coverage for themselves.”
If that mom is exposed to COVID-19 and has no insurance coverage and a newborn at home, the likelihood that she will call a provider if she develops symptoms is low, Dr. Crear-Perry said. “This is a great opportunity for us to really rethink some of those policies that we know are barriers, that we have created for people to be able to thrive after they have a baby and during child birth.”
Current policies are centered around an arbitrary cutoff of about 6 weeks for postpartum care, but the CDC reports that a third of all postpartum deaths occur between 1 week and 12 months after birth.
“We need our policies to reflect the current knowledge and the science,” she said. “Just like babies have automatic insurance coverage for a year later, mothers should have the same.”
Medicaid finances nearly half of all births in the United States, according to a 2019 Kaiser Family Foundation brief, which explained that federal law requires Medicaid coverage for only 60 days post partum for women who are eligible. Decisions regarding coverage after 60 days are determined by individual states; those that expanded Medicaid under the Affordable Care Act typically allow extended coverage – but only with reapplication at 60 days.
Many women in nonexpansion states become uninsured after pregnancy-related coverage ends, as do some in expansion states for whom reapplying is a hurdle too high to clear with a newborn baby to care for at home, Dr. Crear-Perry said.
Addressing these coverage gaps is key to improving access, and it is a core component of the Black Maternal Health Momnibus Act of 2020, a nine-bill package introduced in March by Rep. Lauren Underwood (D-Ill.), Rep. Alma Adams (D-N.C.), Sen. Kamala Harris (D-Calif.), and members of the Black Maternal Health Caucus to “fill gaps in existing legislation to comprehensively address every dimension of the Black maternal health crisis in America.”
One bill in the package addresses extended coverage with a goal to “promote innovative payment models to incentivize high-quality maternity care and continuity of health insurance coverage from pregnancy through labor and delivery and up to 1 year post partum.” Another focuses on promoting alternative ways to access care, such as through telemedicine.
Solution: Expanding care access
“There is a need for the democratization of care,” Dr. Crear-Perry said. “There is a need for people to have more ways to get care. This idea that the only way you can get prenatal care is you have to come to me at my office, has been a burden for working people for a long, long time.”
The COVID-19 pandemic necessitates increased use of telemedicine, but building blocks to allow patients to use it effectively must be put in place, she said. That means expanding broadband access, providing patients with blood pressure cuffs and other tools for use remotely, and expanding reimbursement to include not just video, but also phone calls.
Heart Safe Motherhood, a University of Pennsylvania text-based intervention developed to address postpartum hypertension – a leading cause of maternal morbidity and mortality, and at the start of the program, the leading cause of 7-day readmissions among obstetric patients, demonstrated the value of such approaches to care.
The program involves remote blood pressure monitoring using a digital monitor provided to at-risk patients at discharge. Text-based monitoring reminders encourage patients to check their blood pressure twice daily for the first 7 days.
“In our randomized, controlled trial, we saw our ability to meet ACOG guidelines on postpartum blood monitoring leap from 0% to 82%, compared to in-person office visits and 7-day readmissions from hypertension drop from 3% to 0%,” an update at the program website states.
Rebekah Gee, MD, an ob.gyn. and director of the Louisiana State University Health System in New Orleans, also noted the importance of finding ways to deliver care “that are outside the traditional norm.
“Telemedicine, home visiting ... I think there are a wide variety of ways,” she said, noting that these kind of approaches not only help circumvent roadblocks to care, such as lack of transportation, but also can feel more personal and approachable for some women.
Solution: Measuring, investing, diversifying, respecting
The aims of other bills in the Momnibus Act also mirror several solutions proposed by maternal health advocates interviewed for this article. Among them are:
- Development of improved data collection processes and quality measures to better understand the factors that contribute to the crisis overall and among special populations, and to inform solutions for addressing them.
- Investments in social determinants of health that influence maternal health outcomes, like housing, transportation, and nutrition.
- Commitment to the growth and diversification of the perinatal workforce to ensure that every mom receives maternity care and support from people she can trust to provide quality care and treat her with respect.
The latter is one that Dr. Gee, Dr. Crear-Perry, and others particularly emphasized.
“We need patient advocates like doulas, midwives and others who are better listeners and better able to advocate for patients,” Dr. Gee said. This would better allow for women’s desires in the childbirth experience to be addressed appropriately, she said, adding that this is something that “frankly, a lot of doctors do not have the time to do.”
That’s why the efforts to address maternal mortality have to focus on the health care system, not just on doctors’ behavior with respect to bias, she said.
Dr. Gee also said there is a need for culturally appropriate literacy and numeracy communications “that respect how people seek and understand information.” This varies by population, which is why it’s important to provide the same approach to care “no matter what the patient looks like,” while also understanding that different patients communicate in different ways.
A 2019 study published in Social Science & Medicine underscored how communication differences can affect outcomes; using a national sample of women who gave birth in U.S. hospitals, the authors found that those who had declined care for themselves or their infant during their childbirth hospitalization were more likely to report receiving poor treatment based on race or ethnicity. They concluded that, in the context of childbirth care, women – particularly black women – pay a penalty for what is perceived as uncooperative behavior.
This is another area where doulas and other patient advocates can help, Dr. Gee said.
Doulas have long been an integral part of the birthing process for many women, particularly women of color, and evidence suggests the supportive care they provide helps to improve outcomes. In fact, several states – including Oregon, Minnesota, and New York, among others – have expanded or have proposed expanding Medicaid coverage to include doula services for pregnant beneficiaries, a move cheered by doula associations and other maternal health advocates.
In many ways, it’s about “respectful maternity care,” which is something Dr. Crear-Perry has been working to promote through the NBEC in partnership with ACOG and the Robert Wood Johnson Foundation. It’s also something the World Health Organization has promoted by establishing global standards for such care.
“We’re hoping to socialize that as a norm in United States ... to really see what it would look like to value what birthing people want and to see them as partners in their birth,” she said.
However, the 2019 Giving Voice to Mothers study demonstrating consistently higher rates of mistreatment during obstetrical care for women of color than for comparable white women shows that the United States is falling short of those standards. The national study of 2,700 women examined how race, ethnicity, and place of birth interact with the experience of receiving maternity care in the United States, and showed that 1 in 6 experienced one or more types of mistreatment – with consistently higher rates among women of color, even after adjusting for interactions between race and other maternal characteristics, Saraswathi Veda, MD, of the Birth Place Lab and professor of midwifery at the University of British Columbia, Vancouver, and colleagues reported in Reproductive Health.
Solution: Listening, learning, reflecting, partnering
Timoria McQueen Saba, birth trauma survivor and maternal health advocate, has described experiencing instances of mistreatment throughout her obstetric care, and like Dr. Crear-Perry, she said trust and collaboration in care is imperative for improving outcomes.
“I think the most important thing you can do is really consider a patient a partner in the care you give them,” she said during a panel discussion at the 2019 ACOG annual meeting. “You’re not experts in their lived experience ... center a patient’s voice or the voice of a patient’s family. Incorporate that into your learning.”
During a virtual workshop held May 19-20 by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, Judette Louis, MD, chair of the department of obstetrics and gynecology at the University of South Florida, Tampa, and president of the Society of Maternal-Fetal Medicine, provided practical guidance for addressing racism and implicit bias in practice and in research to reduce disparities in outcomes.
In an interview, she summarized her key points, reiterating solutions proposed by Dr. Gee and Dr. Crear-Perry and addressed in the Momnibus Act, and also offering a few others:
First, put aside the notion that disparities are genetically driven. For a variety of reasons, that just doesn’t make sense. For one thing, not all blacks are African American.
“My family is from the Caribbean,” she said. “Is it really conceivable that we’re all so similar?”
Look also at the disparities among Native Americans, she said. “How can you take 500 distinct tribes that live across a wide geographic area and lump them into one group and assume that they are similar?”
The problem is racism, not race. “When you keep saying ‘it’s about race, it’s about race, it’s about race’ – that sends a message to the person who is of that race that there is something inherently broken about [them],” she said.
Recognize that the roots of the problem run deep. Learn about and support efforts to address the underlying structural factors that contribute to the problem, Dr. Louis emphasized, and recognize your own bias. “We all have it. The key is to recognize [biases] and mitigate them when taking care of patients.”
That’s easier said than done, at least judging by one survey of maternal-fetal medicine specialists in which 84% of respondents agreed that disparities impact practice, but only 29% agreed their own personal biases affect how they care for patients, she noted.
Tools are available to help individuals identify implicit bias, and training programs for health care providers can help, as well, she said. Implicit bias tests and training programs that help to identify and address bias and racism on individual and organizational levels are increasingly available through academic centers, health systems, and advocacy organizations.
Hope for solutions: Progress and promise
Like Dr. Crear-Perry, Dr. Louis sees hope for reducing disparities and improving maternal outcomes.
In another survey of SMFM members to identify the practice issues most important to them, racial disparities ranked in the top three.
“It says a lot that our [maternal-fetal medicine specialists] really see this as a problem and they want it fixed,” she said. “And I think it says that a lot of people need to work on this, not just us.”
Indeed, many are engaged in that work. Veronica Gillispie, MD, medical director of the Louisiana Perinatal Quality Collaborative and Pregnancy-Associated Mortality Review, has been instrumental in recent initiatives to improve maternal outcomes in Louisiana, and she too said she feels optimistic.
“I am hopeful and I do see signs of hope,” she said in an interview.
Teams that she works with and trains seem invested, institutions are increasingly implementing faculty training on racism and bias, and Oschner Health, where Dr. Gillispie practices as an ob.gyn., appointed its first chief diversity officer in February.
Medical students she works with are attuned to the issues of racism, bias, and disparities, and they show a desire to enact change, she said. “They already get it, and they are working to make it better.”
Dr. Crear-Perry also predicts practice-changing results from studies looking at the delivery of obstetrical care and the role of supportive care, and she pointed out another aspect of the COVID-19 crisis that provides an important lesson for health care providers who care for birthing people: the scarcity of personal protective equipment amid the pandemic.
“My friends who are ob.gyns., who are now not getting access to the things they need to stay safe to practice medicine and who are feeling very marginalized at this moment, feeling not valued – that’s how birthing people [of color] feel,” she said. “I’m hoping that builds a sense of empathy.
”I’m hoping at the end of this crisis, that those ob.gyns. will think of patients as allies in fighting for more access to health for everybody and for more resources to do their work,” Dr. Crear-Perry said. “We’re all in this together.”
This is the second of a two-part article on the role of racism and bias in the U.S. maternal mortality crisis and part of an ongoing Ob.Gyn. News feature series on the crisis. Part one of the story explored existing data, societal factors, and patient experiences related to structural racism, overt racism, and implicit bias as factors contributing to racial disparities in maternal outcomes. Here we explore potential solutions for addressing the inequities as proposed by thought leaders and key stakeholders.
The emerging racial disparities in COVID-19 incidence and outcomes in the United States are on a collision course with long-standing racial disparities in U.S. maternal care and mortality.
Maternal health advocates are bracing for the impact, but in the spotlight that the pandemic is training on the inequities and the health system changes taking shape in its wake, some also see hope for a shift in at least one important driver of the racial health disparities: access to care.
Non-Hispanic black women are at least three times more likely than Hispanic women and non-Hispanic white women to experience pregnancy-related death, and indigenous women are more than twice as likely, according to the latest data from the Centers for Disease Control and Prevention’s National Center for Health Statistics. and to exacerbate racial disparities, panelists agreed during a recent National Maternal Health Patient Centered Outcomes Research Network webinar entitled “The Impact of COVID-19 on Black, Brown, and Native Pregnant People.”
“The saying is that ‘the virus doesn’t discriminate,’ but it understands our biases, right? So, the virus takes advantage of the weaknesses in our system,” said panelist Joia A. Crear-Perry, MD, an ob.gyn. and founder and president of the National Birth Equity Collaborative (NBEC), a New Orleans–based research, training, and advocacy organization working to optimize black maternal and infant health.
Hope for solutions from the ashes of a pandemic
The weaknesses in the system that Dr. Crear-Perry spoke of are in many ways a product of structural racism as described in a conceptual report in The Lancet, titled “America: Equity and Equality in Health,” which dug into the entrenched and tangled historical roots of racist sociological and political factors that formed a foundation for health inequity over time.
Today, people of color remain more likely to be excluded from access to health insurance and adequate health care. The authors defined structural racism as “the totality of ways in which societies foster racial discrimination through mutually reinforcing systems of housing, education, employment, earnings, benefits, credit, media, health care, and criminal justice.” Today, largely as a result of these “reinforcing systems,” people of color remain more likely to be excluded from access to health insurance and adequate health care. At the same time, and for the same reasons, they are more likely to work in the service industry, be essential workers, and use mass transit, each of which increases the risk of exposure to COVID-19, Dr. Crear-Perry explained.
“It’s important for us to know that, for maternal mortality, it’s the same thing that happens,” she said. That means the focus on COVID-19–related disparities helps magnify and elevate the conversation regarding similar disparities in maternal outcomes.
It also means that some of the care delivery solutions embraced and facilitated amid the pandemic, such as extension of Medicaid coverage for up to a year after giving birth and broader use and insurance coverage of telemedicine, could finally gain traction; those are solutions long-sought by advocates like Dr. Crear-Perry and others as a means for alleviating racial disparities in maternal outcomes and addressing the maternal mortality crisis.
Therein lies the hope, she explained in an interview. “Some of the policies that we know would have been helpful prior to COVID-19 now are being seen as really important.”
Solution: Extending coverage
During a May 7 virtual Congressional hearing on “America’s Two Public Health Crises: The Impact of COVID-19 on Racial Inequities and Maternal Mortality in the U.S.,” cosponsored by the American College of Obstetricians and Gynecologists, the March of Dimes, and the NBEC, Dr. Crear-Perry further explained the importance of extended coverage and care access.
Asked what Congress could do immediately to “ensure that the pandemic does not compound the nation’s maternal mortality crisis, including unacceptable rates among black women,” she didn’t hesitate.
“Well, it would be amazing if we could get Medicaid extended for 12 months post delivery,” she said. “As you can imagine right now, we have moms who are birthing in hospitals where they have to worry about, 2 months later, not having coverage for themselves.”
If that mom is exposed to COVID-19 and has no insurance coverage and a newborn at home, the likelihood that she will call a provider if she develops symptoms is low, Dr. Crear-Perry said. “This is a great opportunity for us to really rethink some of those policies that we know are barriers, that we have created for people to be able to thrive after they have a baby and during child birth.”
Current policies are centered around an arbitrary cutoff of about 6 weeks for postpartum care, but the CDC reports that a third of all postpartum deaths occur between 1 week and 12 months after birth.
“We need our policies to reflect the current knowledge and the science,” she said. “Just like babies have automatic insurance coverage for a year later, mothers should have the same.”
Medicaid finances nearly half of all births in the United States, according to a 2019 Kaiser Family Foundation brief, which explained that federal law requires Medicaid coverage for only 60 days post partum for women who are eligible. Decisions regarding coverage after 60 days are determined by individual states; those that expanded Medicaid under the Affordable Care Act typically allow extended coverage – but only with reapplication at 60 days.
Many women in nonexpansion states become uninsured after pregnancy-related coverage ends, as do some in expansion states for whom reapplying is a hurdle too high to clear with a newborn baby to care for at home, Dr. Crear-Perry said.
Addressing these coverage gaps is key to improving access, and it is a core component of the Black Maternal Health Momnibus Act of 2020, a nine-bill package introduced in March by Rep. Lauren Underwood (D-Ill.), Rep. Alma Adams (D-N.C.), Sen. Kamala Harris (D-Calif.), and members of the Black Maternal Health Caucus to “fill gaps in existing legislation to comprehensively address every dimension of the Black maternal health crisis in America.”
One bill in the package addresses extended coverage with a goal to “promote innovative payment models to incentivize high-quality maternity care and continuity of health insurance coverage from pregnancy through labor and delivery and up to 1 year post partum.” Another focuses on promoting alternative ways to access care, such as through telemedicine.
Solution: Expanding care access
“There is a need for the democratization of care,” Dr. Crear-Perry said. “There is a need for people to have more ways to get care. This idea that the only way you can get prenatal care is you have to come to me at my office, has been a burden for working people for a long, long time.”
The COVID-19 pandemic necessitates increased use of telemedicine, but building blocks to allow patients to use it effectively must be put in place, she said. That means expanding broadband access, providing patients with blood pressure cuffs and other tools for use remotely, and expanding reimbursement to include not just video, but also phone calls.
Heart Safe Motherhood, a University of Pennsylvania text-based intervention developed to address postpartum hypertension – a leading cause of maternal morbidity and mortality, and at the start of the program, the leading cause of 7-day readmissions among obstetric patients, demonstrated the value of such approaches to care.
The program involves remote blood pressure monitoring using a digital monitor provided to at-risk patients at discharge. Text-based monitoring reminders encourage patients to check their blood pressure twice daily for the first 7 days.
“In our randomized, controlled trial, we saw our ability to meet ACOG guidelines on postpartum blood monitoring leap from 0% to 82%, compared to in-person office visits and 7-day readmissions from hypertension drop from 3% to 0%,” an update at the program website states.
Rebekah Gee, MD, an ob.gyn. and director of the Louisiana State University Health System in New Orleans, also noted the importance of finding ways to deliver care “that are outside the traditional norm.
“Telemedicine, home visiting ... I think there are a wide variety of ways,” she said, noting that these kind of approaches not only help circumvent roadblocks to care, such as lack of transportation, but also can feel more personal and approachable for some women.
Solution: Measuring, investing, diversifying, respecting
The aims of other bills in the Momnibus Act also mirror several solutions proposed by maternal health advocates interviewed for this article. Among them are:
- Development of improved data collection processes and quality measures to better understand the factors that contribute to the crisis overall and among special populations, and to inform solutions for addressing them.
- Investments in social determinants of health that influence maternal health outcomes, like housing, transportation, and nutrition.
- Commitment to the growth and diversification of the perinatal workforce to ensure that every mom receives maternity care and support from people she can trust to provide quality care and treat her with respect.
The latter is one that Dr. Gee, Dr. Crear-Perry, and others particularly emphasized.
“We need patient advocates like doulas, midwives and others who are better listeners and better able to advocate for patients,” Dr. Gee said. This would better allow for women’s desires in the childbirth experience to be addressed appropriately, she said, adding that this is something that “frankly, a lot of doctors do not have the time to do.”
That’s why the efforts to address maternal mortality have to focus on the health care system, not just on doctors’ behavior with respect to bias, she said.
Dr. Gee also said there is a need for culturally appropriate literacy and numeracy communications “that respect how people seek and understand information.” This varies by population, which is why it’s important to provide the same approach to care “no matter what the patient looks like,” while also understanding that different patients communicate in different ways.
A 2019 study published in Social Science & Medicine underscored how communication differences can affect outcomes; using a national sample of women who gave birth in U.S. hospitals, the authors found that those who had declined care for themselves or their infant during their childbirth hospitalization were more likely to report receiving poor treatment based on race or ethnicity. They concluded that, in the context of childbirth care, women – particularly black women – pay a penalty for what is perceived as uncooperative behavior.
This is another area where doulas and other patient advocates can help, Dr. Gee said.
Doulas have long been an integral part of the birthing process for many women, particularly women of color, and evidence suggests the supportive care they provide helps to improve outcomes. In fact, several states – including Oregon, Minnesota, and New York, among others – have expanded or have proposed expanding Medicaid coverage to include doula services for pregnant beneficiaries, a move cheered by doula associations and other maternal health advocates.
In many ways, it’s about “respectful maternity care,” which is something Dr. Crear-Perry has been working to promote through the NBEC in partnership with ACOG and the Robert Wood Johnson Foundation. It’s also something the World Health Organization has promoted by establishing global standards for such care.
“We’re hoping to socialize that as a norm in United States ... to really see what it would look like to value what birthing people want and to see them as partners in their birth,” she said.
However, the 2019 Giving Voice to Mothers study demonstrating consistently higher rates of mistreatment during obstetrical care for women of color than for comparable white women shows that the United States is falling short of those standards. The national study of 2,700 women examined how race, ethnicity, and place of birth interact with the experience of receiving maternity care in the United States, and showed that 1 in 6 experienced one or more types of mistreatment – with consistently higher rates among women of color, even after adjusting for interactions between race and other maternal characteristics, Saraswathi Veda, MD, of the Birth Place Lab and professor of midwifery at the University of British Columbia, Vancouver, and colleagues reported in Reproductive Health.
Solution: Listening, learning, reflecting, partnering
Timoria McQueen Saba, birth trauma survivor and maternal health advocate, has described experiencing instances of mistreatment throughout her obstetric care, and like Dr. Crear-Perry, she said trust and collaboration in care is imperative for improving outcomes.
“I think the most important thing you can do is really consider a patient a partner in the care you give them,” she said during a panel discussion at the 2019 ACOG annual meeting. “You’re not experts in their lived experience ... center a patient’s voice or the voice of a patient’s family. Incorporate that into your learning.”
During a virtual workshop held May 19-20 by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, Judette Louis, MD, chair of the department of obstetrics and gynecology at the University of South Florida, Tampa, and president of the Society of Maternal-Fetal Medicine, provided practical guidance for addressing racism and implicit bias in practice and in research to reduce disparities in outcomes.
In an interview, she summarized her key points, reiterating solutions proposed by Dr. Gee and Dr. Crear-Perry and addressed in the Momnibus Act, and also offering a few others:
First, put aside the notion that disparities are genetically driven. For a variety of reasons, that just doesn’t make sense. For one thing, not all blacks are African American.
“My family is from the Caribbean,” she said. “Is it really conceivable that we’re all so similar?”
Look also at the disparities among Native Americans, she said. “How can you take 500 distinct tribes that live across a wide geographic area and lump them into one group and assume that they are similar?”
The problem is racism, not race. “When you keep saying ‘it’s about race, it’s about race, it’s about race’ – that sends a message to the person who is of that race that there is something inherently broken about [them],” she said.
Recognize that the roots of the problem run deep. Learn about and support efforts to address the underlying structural factors that contribute to the problem, Dr. Louis emphasized, and recognize your own bias. “We all have it. The key is to recognize [biases] and mitigate them when taking care of patients.”
That’s easier said than done, at least judging by one survey of maternal-fetal medicine specialists in which 84% of respondents agreed that disparities impact practice, but only 29% agreed their own personal biases affect how they care for patients, she noted.
Tools are available to help individuals identify implicit bias, and training programs for health care providers can help, as well, she said. Implicit bias tests and training programs that help to identify and address bias and racism on individual and organizational levels are increasingly available through academic centers, health systems, and advocacy organizations.
Hope for solutions: Progress and promise
Like Dr. Crear-Perry, Dr. Louis sees hope for reducing disparities and improving maternal outcomes.
In another survey of SMFM members to identify the practice issues most important to them, racial disparities ranked in the top three.
“It says a lot that our [maternal-fetal medicine specialists] really see this as a problem and they want it fixed,” she said. “And I think it says that a lot of people need to work on this, not just us.”
Indeed, many are engaged in that work. Veronica Gillispie, MD, medical director of the Louisiana Perinatal Quality Collaborative and Pregnancy-Associated Mortality Review, has been instrumental in recent initiatives to improve maternal outcomes in Louisiana, and she too said she feels optimistic.
“I am hopeful and I do see signs of hope,” she said in an interview.
Teams that she works with and trains seem invested, institutions are increasingly implementing faculty training on racism and bias, and Oschner Health, where Dr. Gillispie practices as an ob.gyn., appointed its first chief diversity officer in February.
Medical students she works with are attuned to the issues of racism, bias, and disparities, and they show a desire to enact change, she said. “They already get it, and they are working to make it better.”
Dr. Crear-Perry also predicts practice-changing results from studies looking at the delivery of obstetrical care and the role of supportive care, and she pointed out another aspect of the COVID-19 crisis that provides an important lesson for health care providers who care for birthing people: the scarcity of personal protective equipment amid the pandemic.
“My friends who are ob.gyns., who are now not getting access to the things they need to stay safe to practice medicine and who are feeling very marginalized at this moment, feeling not valued – that’s how birthing people [of color] feel,” she said. “I’m hoping that builds a sense of empathy.
”I’m hoping at the end of this crisis, that those ob.gyns. will think of patients as allies in fighting for more access to health for everybody and for more resources to do their work,” Dr. Crear-Perry said. “We’re all in this together.”
FDA approves medication to treat heavy menstrual bleeding related to fibroids
The medication, marketed as Oriahnn, is an estrogen and progestin combination product that consists of elagolix, estradiol, and norethindrone acetate capsules packaged together for oral use, according to an FDA announcement.
“Uterine fibroids are the most common benign tumors affecting premenopausal women, and one of the most common symptoms from fibroids is heavy menstrual bleeding,” Christine P. Nguyen, MD, acting director of the division of urology, obstetrics, and gynecology in the FDA’s Center for Drug Evaluation and Research, said in a news release. “Although surgical treatments, such as hysterectomy, are available, patients may not qualify for surgery or want the procedure. Various nonsurgical therapies are used to treat fibroid-related heavy menstrual bleeding, but none have been FDA approved specifically for this use. Today’s approval provides an FDA-approved medical treatment option for these patients.”
Fibroids, which occur most commonly in women aged 35-49 years, typically resolve after menopause but are a leading reason for hysterectomy in the United States, according to the release.
Researchers established the efficacy of the treatment in two clinical trials that included 591 premenopausal women with heavy menstrual bleeding. Participants received the drug or placebo for 6 months. The investigators defined heavy menstrual bleeding as at least two menstrual cycles with greater than 80 mL of menstrual blood loss. The primary endpoint was the proportion of women who achieved menstrual blood loss less than 80 mL at the final month and 50% or greater reduction in menstrual blood loss volume from baseline to the final month. In one trial, 69% of patients who received Oriahnn met this endpoint, compared with 9% of patients who received placebo. In the second study, 77% of patients who received the drug achieved this endpoint, compared with 11% of patients who received placebo.
Oriahnn may cause bone loss that may not be completely recovered after stopping treatment, so women should not take the medication for more than 24 months, according to the FDA announcement. Health care professionals may recommend bone density scans before and during treatment.
The most common side effects included hot flushes, headache, fatigue, and irregular vaginal bleeding. The drug’s label includes a boxed warning about a risk of strokes and blood clots, especially in women at increased risk for these events. Contraindications include osteoporosis, a history of breast cancer or other hormonally sensitive cancer, liver disease, and abnormal uterine bleeding. Oriahnn does not prevent pregnancy and may increase blood pressure, according to the press release. AbbVie markets the drug.
The medication, marketed as Oriahnn, is an estrogen and progestin combination product that consists of elagolix, estradiol, and norethindrone acetate capsules packaged together for oral use, according to an FDA announcement.
“Uterine fibroids are the most common benign tumors affecting premenopausal women, and one of the most common symptoms from fibroids is heavy menstrual bleeding,” Christine P. Nguyen, MD, acting director of the division of urology, obstetrics, and gynecology in the FDA’s Center for Drug Evaluation and Research, said in a news release. “Although surgical treatments, such as hysterectomy, are available, patients may not qualify for surgery or want the procedure. Various nonsurgical therapies are used to treat fibroid-related heavy menstrual bleeding, but none have been FDA approved specifically for this use. Today’s approval provides an FDA-approved medical treatment option for these patients.”
Fibroids, which occur most commonly in women aged 35-49 years, typically resolve after menopause but are a leading reason for hysterectomy in the United States, according to the release.
Researchers established the efficacy of the treatment in two clinical trials that included 591 premenopausal women with heavy menstrual bleeding. Participants received the drug or placebo for 6 months. The investigators defined heavy menstrual bleeding as at least two menstrual cycles with greater than 80 mL of menstrual blood loss. The primary endpoint was the proportion of women who achieved menstrual blood loss less than 80 mL at the final month and 50% or greater reduction in menstrual blood loss volume from baseline to the final month. In one trial, 69% of patients who received Oriahnn met this endpoint, compared with 9% of patients who received placebo. In the second study, 77% of patients who received the drug achieved this endpoint, compared with 11% of patients who received placebo.
Oriahnn may cause bone loss that may not be completely recovered after stopping treatment, so women should not take the medication for more than 24 months, according to the FDA announcement. Health care professionals may recommend bone density scans before and during treatment.
The most common side effects included hot flushes, headache, fatigue, and irregular vaginal bleeding. The drug’s label includes a boxed warning about a risk of strokes and blood clots, especially in women at increased risk for these events. Contraindications include osteoporosis, a history of breast cancer or other hormonally sensitive cancer, liver disease, and abnormal uterine bleeding. Oriahnn does not prevent pregnancy and may increase blood pressure, according to the press release. AbbVie markets the drug.
The medication, marketed as Oriahnn, is an estrogen and progestin combination product that consists of elagolix, estradiol, and norethindrone acetate capsules packaged together for oral use, according to an FDA announcement.
“Uterine fibroids are the most common benign tumors affecting premenopausal women, and one of the most common symptoms from fibroids is heavy menstrual bleeding,” Christine P. Nguyen, MD, acting director of the division of urology, obstetrics, and gynecology in the FDA’s Center for Drug Evaluation and Research, said in a news release. “Although surgical treatments, such as hysterectomy, are available, patients may not qualify for surgery or want the procedure. Various nonsurgical therapies are used to treat fibroid-related heavy menstrual bleeding, but none have been FDA approved specifically for this use. Today’s approval provides an FDA-approved medical treatment option for these patients.”
Fibroids, which occur most commonly in women aged 35-49 years, typically resolve after menopause but are a leading reason for hysterectomy in the United States, according to the release.
Researchers established the efficacy of the treatment in two clinical trials that included 591 premenopausal women with heavy menstrual bleeding. Participants received the drug or placebo for 6 months. The investigators defined heavy menstrual bleeding as at least two menstrual cycles with greater than 80 mL of menstrual blood loss. The primary endpoint was the proportion of women who achieved menstrual blood loss less than 80 mL at the final month and 50% or greater reduction in menstrual blood loss volume from baseline to the final month. In one trial, 69% of patients who received Oriahnn met this endpoint, compared with 9% of patients who received placebo. In the second study, 77% of patients who received the drug achieved this endpoint, compared with 11% of patients who received placebo.
Oriahnn may cause bone loss that may not be completely recovered after stopping treatment, so women should not take the medication for more than 24 months, according to the FDA announcement. Health care professionals may recommend bone density scans before and during treatment.
The most common side effects included hot flushes, headache, fatigue, and irregular vaginal bleeding. The drug’s label includes a boxed warning about a risk of strokes and blood clots, especially in women at increased risk for these events. Contraindications include osteoporosis, a history of breast cancer or other hormonally sensitive cancer, liver disease, and abnormal uterine bleeding. Oriahnn does not prevent pregnancy and may increase blood pressure, according to the press release. AbbVie markets the drug.
Improving care for women who have experienced stillbirth
Think of the current standard of care and do the opposite
One of hardest parts of being an obstetrician is taking care of patients who experience a stillbirth. I am very comfortable with the care of a grieving patient and I always have been, although I am not sure why. I have a model of care that I have evolved in my 16 years since medical school graduation. This model is not based on formal instruction because I received none, but on my natural instincts of what a grieving mom and her family need to hear and receive in the worst moments of their lives. All obstetrics providers grieve the loss of the baby, but often not with the patient but on our own. We may do this because we want to respect the patient’s privacy or because we are not sure of the words to say. I hope I can provide some guidance for those who struggle with what to do.
I delivered my first stillborn baby as a third-year medical student. My mentor, a chief resident, saw something in me and encouraged me to care for this mother. She had twins and one baby was still living, but the prognosis was poor since this was the surviving twin from a monochorionic diamniotic pregnancy. On the day of the mom’s induction, I just pulled up a chair and talked with her. We talked about her life, the loss of the first baby several weeks before, and her hope that her surviving son would be okay. She felt so bonded to me that she refused to push until I was there. Her delivery is still firm in my mind. I still remember 17 years later the room she delivered in.
My first loss (stillbirth) as a resident was my intern year – a beautiful baby named Jude, who was stillborn at 39 weeks. After delivering Jude, I asked the family about the funeral arrangements. Three days later, I attended his funeral. I looked all around for the mother’s attending doctors but none of them were there. I remember thinking then that it was a given that they would be there, but now I know that it is rare. I also learned a lot about the grief a stillborn baby brings while listening to Jude’s father’s eulogy. He talked about how Jude would never bake cookies with Aunt Jane, ride the slide with cousin Chris, or put on a yellow backpack and ride the bus on the first day of school. Because of this eulogy, I understood this unique kind of grief that these losses bring early in my training.
I delivered many losses in my residency. The attendings left soon after the birth and I stayed behind with the family. I sat and counseled the families, and I helped them make memories. I realized that to care for these patients, I would need to trust my instincts because there was no formal and little informal training on how to care for families who lost their babies.
Once I completed residency, I was really able to do my “thing.” At loss deliveries, I was able to model for residents my method of care. I showed them that families want and need attention, support, and guidance. I modeled for them how to deliver and greet the baby. That it is not necessary to leave the room right after the birth, and it is okay to grieve and help families meet their babies. I modeled commenting on the baby’s features, on who they looked like. I showed how laughing about how the baby has grandma’s nose is okay. I showed them that it is okay to ask to hold and get a picture taken with the baby. I showed them that these are the only moments these families will get with their babies, and it is our job to help them do this. The family will have many moments alone in the days and weeks to come. They need our support and guidance. It is a part of being an ob.gyn. to care for families after stillbirths, and we do not want our patients to feel abandoned during this time by those they entrust to care for them.
I also was able to create a model for aftercare. I call my families often after they go home. Sometimes I catch them in the anger stage of the grief process and I let them vent. I work through this with them, and I answer their hardest and sometimes accusatory questions regarding care leading up to the diagnosis. I am not saying this is easy for me or for them. I think fear of these tough conversations is a barrier to giving the emotional support that these families need. I work through this with them in an honest and open manner. I also call to check on the patients as much as possible, especially on anniversaries. I am not saying that all providers must follow this model. This is my passion and is natural for me, but data clearly show that the standard of emotional care we provide is not what patients need at this time. Thankfully, there is an amazing resource of grief counselors, social workers, online resources, and support groups for these families to help them get through the tragedy. These resources, however, are not the provider who spent this precious time with them and their beloved baby, and our emotional support is invaluable.
This past year has been very eventful for me. One of my patients delivered a new baby, after a prior loss, and asked if we could teach together. I had mentioned that everything I do with stillbirths is not based on my residency education, but on my experience and instinctual feeling of what families need. She knew from friends in bereavement circles that they felt that their care was different. We started teaching last summer and have done 10 training sessions to date; hopefully we will continue to teach new groups of nurses, residents, medical students, doulas, and physician assistant students each year.
This year also was eventful because I discovered the Star Legacy Foundation, a national not-for-profit organization with the goal of spreading awareness, education, and prevention regarding stillbirth. I attended their 2019 Summit in Minnesota. I thought I would meet many more doctors and midwives like myself, and I would learn even more about care for bereaved patients. However, that summer I learned preventing stillbirth may be possible from the then chief medical officer of Scotland, Catherine Calderwood, MB ChB. She talked about the preventive protocol she had created that had reduced the stillbirth rate by 23%. Because I was one of only five ob.gyn. nonspeaker attendees in a room of 400, I realized I had a real opportunity to try to bring some model for prevention to the United States. I brought the U.K. protocol to my practice and we have been doing it now for 9 months. (See “Decreased fetal movement: Time to educate patients and ourselves” at mdedge.com/pediatrics.)
I have had a year to think about why the U.S. stillbirth rate is higher than that of many high-income nations and why we have the lowest annual rate of reduction in the 2016 Lancet series among high resource nations.1 I think it is due to lack of education and training for providers in stillbirth prevention and care, which has led to further marginalization and stigmatization of bereaved moms. This has pushed them further into the shadows and makes it taboo to share their stories. It is providers being fearful to even mention to patients that stillbirth still happens. It is the lack of any protocol on how to educate patients and providers about fetal movement, and what to do if pregnant women complain about a decrease or change in fetal movement. I think a lot of this stems from an innate discomfort that obstetric providers have in the care of these patients. That if women felt cared for and empowered to tell their stories, there would be more efforts at stillbirth education and prevention.
I often think of an experience that the founder of Star Legacy, Lindsey Wimmer, experienced when she lost her son, Garrett, 16 years ago. She told a story in the documentary, “Don’t talk about the baby.” She tells that on the first night of the induction, the nurse came in and told her that the attending wanted to turn off the oxytocin so “she could get her rest.” I heard this and immediately knew the attending’s true reason for turning off the oxytocin. Lindsey then said she knew it was because the attending did not want to wake up to deliver a dead baby. I wrote Lindsey that day and told her I completely agreed and apologized on behalf of my profession for that care. She wrote me back that she had waited 16 years to have a provider validate her feelings about this. I told her I think her doctor was fearful and uncomfortable with this birth and was avoiding it, but I believe with better education and training this can change. I want to deliver babies like Garrett during my shift, because it is giving this vital care that reminds me why I became a doctor in the first place.
I know there are many providers out there who follow a similar model, but I want more providers to do so, and so does the bereavement community. In one study of 20 parents, all but 2 were frustrated about how the ob.gyn. and staff handled their deliveries.2 I truly believe that every person who delivers babies does it because they love it. Part of doing this job we love is realizing there will be times of great sadness. I also believe if this model of care is attempted by wary providers, they will quickly realize that this is what patients and their families need. With this care, stillbirth may become less of a taboo subject, and our stillbirth rate may fall.
Dr. Florescue is an ob.gyn. in private practice at Women Gynecology and Childbirth Associates in Rochester, N.Y. She delivers babies at Highland Hospital in Rochester. She has no relevant financial disclosures. Email her a obnews@mdedge.com.
References
1. “Stillbirths 2016: ending preventable stillbirths.” Series from The Lancet journals. Published: Jan. 20, 2016.
2. BMC Pregnancy Childbirth. 2012 Nov 27. doi: 10.1186/1471-2393-12-137.
Think of the current standard of care and do the opposite
Think of the current standard of care and do the opposite
One of hardest parts of being an obstetrician is taking care of patients who experience a stillbirth. I am very comfortable with the care of a grieving patient and I always have been, although I am not sure why. I have a model of care that I have evolved in my 16 years since medical school graduation. This model is not based on formal instruction because I received none, but on my natural instincts of what a grieving mom and her family need to hear and receive in the worst moments of their lives. All obstetrics providers grieve the loss of the baby, but often not with the patient but on our own. We may do this because we want to respect the patient’s privacy or because we are not sure of the words to say. I hope I can provide some guidance for those who struggle with what to do.
I delivered my first stillborn baby as a third-year medical student. My mentor, a chief resident, saw something in me and encouraged me to care for this mother. She had twins and one baby was still living, but the prognosis was poor since this was the surviving twin from a monochorionic diamniotic pregnancy. On the day of the mom’s induction, I just pulled up a chair and talked with her. We talked about her life, the loss of the first baby several weeks before, and her hope that her surviving son would be okay. She felt so bonded to me that she refused to push until I was there. Her delivery is still firm in my mind. I still remember 17 years later the room she delivered in.
My first loss (stillbirth) as a resident was my intern year – a beautiful baby named Jude, who was stillborn at 39 weeks. After delivering Jude, I asked the family about the funeral arrangements. Three days later, I attended his funeral. I looked all around for the mother’s attending doctors but none of them were there. I remember thinking then that it was a given that they would be there, but now I know that it is rare. I also learned a lot about the grief a stillborn baby brings while listening to Jude’s father’s eulogy. He talked about how Jude would never bake cookies with Aunt Jane, ride the slide with cousin Chris, or put on a yellow backpack and ride the bus on the first day of school. Because of this eulogy, I understood this unique kind of grief that these losses bring early in my training.
I delivered many losses in my residency. The attendings left soon after the birth and I stayed behind with the family. I sat and counseled the families, and I helped them make memories. I realized that to care for these patients, I would need to trust my instincts because there was no formal and little informal training on how to care for families who lost their babies.
Once I completed residency, I was really able to do my “thing.” At loss deliveries, I was able to model for residents my method of care. I showed them that families want and need attention, support, and guidance. I modeled for them how to deliver and greet the baby. That it is not necessary to leave the room right after the birth, and it is okay to grieve and help families meet their babies. I modeled commenting on the baby’s features, on who they looked like. I showed how laughing about how the baby has grandma’s nose is okay. I showed them that it is okay to ask to hold and get a picture taken with the baby. I showed them that these are the only moments these families will get with their babies, and it is our job to help them do this. The family will have many moments alone in the days and weeks to come. They need our support and guidance. It is a part of being an ob.gyn. to care for families after stillbirths, and we do not want our patients to feel abandoned during this time by those they entrust to care for them.
I also was able to create a model for aftercare. I call my families often after they go home. Sometimes I catch them in the anger stage of the grief process and I let them vent. I work through this with them, and I answer their hardest and sometimes accusatory questions regarding care leading up to the diagnosis. I am not saying this is easy for me or for them. I think fear of these tough conversations is a barrier to giving the emotional support that these families need. I work through this with them in an honest and open manner. I also call to check on the patients as much as possible, especially on anniversaries. I am not saying that all providers must follow this model. This is my passion and is natural for me, but data clearly show that the standard of emotional care we provide is not what patients need at this time. Thankfully, there is an amazing resource of grief counselors, social workers, online resources, and support groups for these families to help them get through the tragedy. These resources, however, are not the provider who spent this precious time with them and their beloved baby, and our emotional support is invaluable.
This past year has been very eventful for me. One of my patients delivered a new baby, after a prior loss, and asked if we could teach together. I had mentioned that everything I do with stillbirths is not based on my residency education, but on my experience and instinctual feeling of what families need. She knew from friends in bereavement circles that they felt that their care was different. We started teaching last summer and have done 10 training sessions to date; hopefully we will continue to teach new groups of nurses, residents, medical students, doulas, and physician assistant students each year.
This year also was eventful because I discovered the Star Legacy Foundation, a national not-for-profit organization with the goal of spreading awareness, education, and prevention regarding stillbirth. I attended their 2019 Summit in Minnesota. I thought I would meet many more doctors and midwives like myself, and I would learn even more about care for bereaved patients. However, that summer I learned preventing stillbirth may be possible from the then chief medical officer of Scotland, Catherine Calderwood, MB ChB. She talked about the preventive protocol she had created that had reduced the stillbirth rate by 23%. Because I was one of only five ob.gyn. nonspeaker attendees in a room of 400, I realized I had a real opportunity to try to bring some model for prevention to the United States. I brought the U.K. protocol to my practice and we have been doing it now for 9 months. (See “Decreased fetal movement: Time to educate patients and ourselves” at mdedge.com/pediatrics.)
I have had a year to think about why the U.S. stillbirth rate is higher than that of many high-income nations and why we have the lowest annual rate of reduction in the 2016 Lancet series among high resource nations.1 I think it is due to lack of education and training for providers in stillbirth prevention and care, which has led to further marginalization and stigmatization of bereaved moms. This has pushed them further into the shadows and makes it taboo to share their stories. It is providers being fearful to even mention to patients that stillbirth still happens. It is the lack of any protocol on how to educate patients and providers about fetal movement, and what to do if pregnant women complain about a decrease or change in fetal movement. I think a lot of this stems from an innate discomfort that obstetric providers have in the care of these patients. That if women felt cared for and empowered to tell their stories, there would be more efforts at stillbirth education and prevention.
I often think of an experience that the founder of Star Legacy, Lindsey Wimmer, experienced when she lost her son, Garrett, 16 years ago. She told a story in the documentary, “Don’t talk about the baby.” She tells that on the first night of the induction, the nurse came in and told her that the attending wanted to turn off the oxytocin so “she could get her rest.” I heard this and immediately knew the attending’s true reason for turning off the oxytocin. Lindsey then said she knew it was because the attending did not want to wake up to deliver a dead baby. I wrote Lindsey that day and told her I completely agreed and apologized on behalf of my profession for that care. She wrote me back that she had waited 16 years to have a provider validate her feelings about this. I told her I think her doctor was fearful and uncomfortable with this birth and was avoiding it, but I believe with better education and training this can change. I want to deliver babies like Garrett during my shift, because it is giving this vital care that reminds me why I became a doctor in the first place.
I know there are many providers out there who follow a similar model, but I want more providers to do so, and so does the bereavement community. In one study of 20 parents, all but 2 were frustrated about how the ob.gyn. and staff handled their deliveries.2 I truly believe that every person who delivers babies does it because they love it. Part of doing this job we love is realizing there will be times of great sadness. I also believe if this model of care is attempted by wary providers, they will quickly realize that this is what patients and their families need. With this care, stillbirth may become less of a taboo subject, and our stillbirth rate may fall.
Dr. Florescue is an ob.gyn. in private practice at Women Gynecology and Childbirth Associates in Rochester, N.Y. She delivers babies at Highland Hospital in Rochester. She has no relevant financial disclosures. Email her a obnews@mdedge.com.
References
1. “Stillbirths 2016: ending preventable stillbirths.” Series from The Lancet journals. Published: Jan. 20, 2016.
2. BMC Pregnancy Childbirth. 2012 Nov 27. doi: 10.1186/1471-2393-12-137.
One of hardest parts of being an obstetrician is taking care of patients who experience a stillbirth. I am very comfortable with the care of a grieving patient and I always have been, although I am not sure why. I have a model of care that I have evolved in my 16 years since medical school graduation. This model is not based on formal instruction because I received none, but on my natural instincts of what a grieving mom and her family need to hear and receive in the worst moments of their lives. All obstetrics providers grieve the loss of the baby, but often not with the patient but on our own. We may do this because we want to respect the patient’s privacy or because we are not sure of the words to say. I hope I can provide some guidance for those who struggle with what to do.
I delivered my first stillborn baby as a third-year medical student. My mentor, a chief resident, saw something in me and encouraged me to care for this mother. She had twins and one baby was still living, but the prognosis was poor since this was the surviving twin from a monochorionic diamniotic pregnancy. On the day of the mom’s induction, I just pulled up a chair and talked with her. We talked about her life, the loss of the first baby several weeks before, and her hope that her surviving son would be okay. She felt so bonded to me that she refused to push until I was there. Her delivery is still firm in my mind. I still remember 17 years later the room she delivered in.
My first loss (stillbirth) as a resident was my intern year – a beautiful baby named Jude, who was stillborn at 39 weeks. After delivering Jude, I asked the family about the funeral arrangements. Three days later, I attended his funeral. I looked all around for the mother’s attending doctors but none of them were there. I remember thinking then that it was a given that they would be there, but now I know that it is rare. I also learned a lot about the grief a stillborn baby brings while listening to Jude’s father’s eulogy. He talked about how Jude would never bake cookies with Aunt Jane, ride the slide with cousin Chris, or put on a yellow backpack and ride the bus on the first day of school. Because of this eulogy, I understood this unique kind of grief that these losses bring early in my training.
I delivered many losses in my residency. The attendings left soon after the birth and I stayed behind with the family. I sat and counseled the families, and I helped them make memories. I realized that to care for these patients, I would need to trust my instincts because there was no formal and little informal training on how to care for families who lost their babies.
Once I completed residency, I was really able to do my “thing.” At loss deliveries, I was able to model for residents my method of care. I showed them that families want and need attention, support, and guidance. I modeled for them how to deliver and greet the baby. That it is not necessary to leave the room right after the birth, and it is okay to grieve and help families meet their babies. I modeled commenting on the baby’s features, on who they looked like. I showed how laughing about how the baby has grandma’s nose is okay. I showed them that it is okay to ask to hold and get a picture taken with the baby. I showed them that these are the only moments these families will get with their babies, and it is our job to help them do this. The family will have many moments alone in the days and weeks to come. They need our support and guidance. It is a part of being an ob.gyn. to care for families after stillbirths, and we do not want our patients to feel abandoned during this time by those they entrust to care for them.
I also was able to create a model for aftercare. I call my families often after they go home. Sometimes I catch them in the anger stage of the grief process and I let them vent. I work through this with them, and I answer their hardest and sometimes accusatory questions regarding care leading up to the diagnosis. I am not saying this is easy for me or for them. I think fear of these tough conversations is a barrier to giving the emotional support that these families need. I work through this with them in an honest and open manner. I also call to check on the patients as much as possible, especially on anniversaries. I am not saying that all providers must follow this model. This is my passion and is natural for me, but data clearly show that the standard of emotional care we provide is not what patients need at this time. Thankfully, there is an amazing resource of grief counselors, social workers, online resources, and support groups for these families to help them get through the tragedy. These resources, however, are not the provider who spent this precious time with them and their beloved baby, and our emotional support is invaluable.
This past year has been very eventful for me. One of my patients delivered a new baby, after a prior loss, and asked if we could teach together. I had mentioned that everything I do with stillbirths is not based on my residency education, but on my experience and instinctual feeling of what families need. She knew from friends in bereavement circles that they felt that their care was different. We started teaching last summer and have done 10 training sessions to date; hopefully we will continue to teach new groups of nurses, residents, medical students, doulas, and physician assistant students each year.
This year also was eventful because I discovered the Star Legacy Foundation, a national not-for-profit organization with the goal of spreading awareness, education, and prevention regarding stillbirth. I attended their 2019 Summit in Minnesota. I thought I would meet many more doctors and midwives like myself, and I would learn even more about care for bereaved patients. However, that summer I learned preventing stillbirth may be possible from the then chief medical officer of Scotland, Catherine Calderwood, MB ChB. She talked about the preventive protocol she had created that had reduced the stillbirth rate by 23%. Because I was one of only five ob.gyn. nonspeaker attendees in a room of 400, I realized I had a real opportunity to try to bring some model for prevention to the United States. I brought the U.K. protocol to my practice and we have been doing it now for 9 months. (See “Decreased fetal movement: Time to educate patients and ourselves” at mdedge.com/pediatrics.)
I have had a year to think about why the U.S. stillbirth rate is higher than that of many high-income nations and why we have the lowest annual rate of reduction in the 2016 Lancet series among high resource nations.1 I think it is due to lack of education and training for providers in stillbirth prevention and care, which has led to further marginalization and stigmatization of bereaved moms. This has pushed them further into the shadows and makes it taboo to share their stories. It is providers being fearful to even mention to patients that stillbirth still happens. It is the lack of any protocol on how to educate patients and providers about fetal movement, and what to do if pregnant women complain about a decrease or change in fetal movement. I think a lot of this stems from an innate discomfort that obstetric providers have in the care of these patients. That if women felt cared for and empowered to tell their stories, there would be more efforts at stillbirth education and prevention.
I often think of an experience that the founder of Star Legacy, Lindsey Wimmer, experienced when she lost her son, Garrett, 16 years ago. She told a story in the documentary, “Don’t talk about the baby.” She tells that on the first night of the induction, the nurse came in and told her that the attending wanted to turn off the oxytocin so “she could get her rest.” I heard this and immediately knew the attending’s true reason for turning off the oxytocin. Lindsey then said she knew it was because the attending did not want to wake up to deliver a dead baby. I wrote Lindsey that day and told her I completely agreed and apologized on behalf of my profession for that care. She wrote me back that she had waited 16 years to have a provider validate her feelings about this. I told her I think her doctor was fearful and uncomfortable with this birth and was avoiding it, but I believe with better education and training this can change. I want to deliver babies like Garrett during my shift, because it is giving this vital care that reminds me why I became a doctor in the first place.
I know there are many providers out there who follow a similar model, but I want more providers to do so, and so does the bereavement community. In one study of 20 parents, all but 2 were frustrated about how the ob.gyn. and staff handled their deliveries.2 I truly believe that every person who delivers babies does it because they love it. Part of doing this job we love is realizing there will be times of great sadness. I also believe if this model of care is attempted by wary providers, they will quickly realize that this is what patients and their families need. With this care, stillbirth may become less of a taboo subject, and our stillbirth rate may fall.
Dr. Florescue is an ob.gyn. in private practice at Women Gynecology and Childbirth Associates in Rochester, N.Y. She delivers babies at Highland Hospital in Rochester. She has no relevant financial disclosures. Email her a obnews@mdedge.com.
References
1. “Stillbirths 2016: ending preventable stillbirths.” Series from The Lancet journals. Published: Jan. 20, 2016.
2. BMC Pregnancy Childbirth. 2012 Nov 27. doi: 10.1186/1471-2393-12-137.
Placental injury reported in women with COVID-19
Neonates appear healthy so far
Maternal vascular malperfusion and intervillous thrombi were more common in the placentas of women infected with SARS-CoV-2, compared with historic controls, report researchers who conducted the first-of-its-kind case series in the English literature. Nevertheless, the neonates in the report appear to be healthy so far and all tested negative for the virus.
Although the series examining placentas from 16 women is small, it carries a larger implication – that increased antenatal surveillance for pregnant women infected with SARS-CoV-2 may be indicated, the researchers noted.
Furthermore, the results could align with other reports of coagulation and vascular abnormalities among people with COVID-19. “I would say that our findings fit into that larger picture of vascular injury. This is developing, and there are some significant ways that these feeder vessels to the placenta are different, but if this is the emerging paradigm, our findings can fit into it,” Jeffrey A. Goldstein, MD, PhD, assistant professor of pathology at Northwestern University, Chicago, said in an interview.
The research was published in the American Journal of Clinical Pathology.
Prior case series reported in Wuhan, China, do not currently suggest that pregnant women are more likely to experience severe COVID-19, in contrast to observations during severe acute respiratory syndrome and Middle East respiratory syndrome outbreaks. “However,” the researchers noted, “adverse perinatal outcomes have been reported, including increased risks of miscarriage, preeclampsia, preterm birth, and stillbirth.”
To learn more, Dr. Goldstein, lead author Elisheva D. Shanes, MD, and colleagues examined the histology of placentas from women with COVID-19 giving birth between March 18 and May 5, 2020. They compared these placentas with over 17,000 historic controls and 215 women who had their placentas evaluated as part of a melanoma history study.
A total of 10 women were diagnosed with COVID-19 upon presentation to labor and delivery, 4 others were diagnosed approximately 1 month before delivery and the remaining 2 within 1 week of delivery. Ten of the patients were symptomatic and two required oxygen. None of the patients received intubation or died. A total of 14 patients delivered at term, 1 delivered at 34 weeks, and the remaining case experienced a 16-week intrauterine fetal demise (IUFD). The IUFD was excluded from subsequent statistical analysis.
The neonates each had a 5-minute Apgar score of 9. Most infants were discharged on the first or second day of life, and there were no neonatal deaths.
Key findings
Of the 15 placentas, 12 featured maternal vascular malperfusion. This rate was significantly higher than historic controls (P = .046) and melanoma study controls (P = .001).
Specific features varied between groups, with decidual arteriopathy, atherosis and fibrinoid necrosis of maternal vessels, and mural hypertrophy of membrane arterioles observed more often in COVID-19 cases than in all historical controls. In addition, peripheral infarctions, decidual arteriopathy, atherosis, and fibrinoid necrosis, and mural hypertrophy being more common in COVID-19 cases than in placentas of women with a history of melanoma.
In contrast, features of fetal vascular malperfusion were observed in 12 of 15 cases, but not at rates significantly different from the control groups. Chorangiosis, villous edema, and intervillous thrombi also were more common in the COVID-19 cohort.
Dr. Goldstein was surprised they did not observe much acute or chronic inflammation. “We see chronic inflammation in the placenta in response to many viruses, such as cytomegalovirus, so you might expect similar findings, but we didn’t see any increase above the controls.”
There are a couple of case reports of histiocytic intervillositis – a particularly severe form of chronic inflammation – associated with COVID-19, “but we didn’t see that in our study,” he added.
Clinical implications
The healthy neonatal outcomes reported in the study occurred despite the placental injury, which may be caused by the redundancy built into placentas for delivering oxygen and nutrients and for removing waste.
The negative COVID-19 test results in all infants also supports existing evidence that vertical transmission of the virus is uncommon. The finding also suggests that any damage to the placenta is likely related to maternal infection.
Only one mother in the COVID-19 cohort was hypertensive, which surprised the researchers because intervillous thrombi have been associated with maternal high blood pressure. “In the context of research suggesting an increase of thrombotic and thromboembolic disorders in COVID-19,” the researchers noted, “these may represent placental formation or deposition of thrombi in response to the virus.”
One of the priorities for the researchers going forward is to monitor the longer-term outcomes of the infants, Dr. Goldstein said. “We know the people in utero during the 1918-1919 flu pandemic had higher rates of heart disease and other long-term problems, so we want to be on the lookout for something similar.”
Valuable insight
“This is a comprehensive case series of this topic, with findings worth noting and sharing in a timely fashion,” Karen Mestan, MD, associate professor of pediatrics within the division of neonatology at Northwestern University, said when asked to comment on the study.
“The information is valuable to neonatologists as the short- and long-term effects of COVID-19 exposure on newborn infants are still largely unknown,” she added. “Details of placental pathology provide emerging insight and may help us understand mother-baby vertical transmission during the current pandemic.”
Dr. Goldstein and Dr. Mestan had no relevant financial disclosures.
SOURCE: Shanes ED et al. Am J Clin Pathol. 2020 May 22. doi: 10.1093/ajcp/aqaa089.
Neonates appear healthy so far
Neonates appear healthy so far
Maternal vascular malperfusion and intervillous thrombi were more common in the placentas of women infected with SARS-CoV-2, compared with historic controls, report researchers who conducted the first-of-its-kind case series in the English literature. Nevertheless, the neonates in the report appear to be healthy so far and all tested negative for the virus.
Although the series examining placentas from 16 women is small, it carries a larger implication – that increased antenatal surveillance for pregnant women infected with SARS-CoV-2 may be indicated, the researchers noted.
Furthermore, the results could align with other reports of coagulation and vascular abnormalities among people with COVID-19. “I would say that our findings fit into that larger picture of vascular injury. This is developing, and there are some significant ways that these feeder vessels to the placenta are different, but if this is the emerging paradigm, our findings can fit into it,” Jeffrey A. Goldstein, MD, PhD, assistant professor of pathology at Northwestern University, Chicago, said in an interview.
The research was published in the American Journal of Clinical Pathology.
Prior case series reported in Wuhan, China, do not currently suggest that pregnant women are more likely to experience severe COVID-19, in contrast to observations during severe acute respiratory syndrome and Middle East respiratory syndrome outbreaks. “However,” the researchers noted, “adverse perinatal outcomes have been reported, including increased risks of miscarriage, preeclampsia, preterm birth, and stillbirth.”
To learn more, Dr. Goldstein, lead author Elisheva D. Shanes, MD, and colleagues examined the histology of placentas from women with COVID-19 giving birth between March 18 and May 5, 2020. They compared these placentas with over 17,000 historic controls and 215 women who had their placentas evaluated as part of a melanoma history study.
A total of 10 women were diagnosed with COVID-19 upon presentation to labor and delivery, 4 others were diagnosed approximately 1 month before delivery and the remaining 2 within 1 week of delivery. Ten of the patients were symptomatic and two required oxygen. None of the patients received intubation or died. A total of 14 patients delivered at term, 1 delivered at 34 weeks, and the remaining case experienced a 16-week intrauterine fetal demise (IUFD). The IUFD was excluded from subsequent statistical analysis.
The neonates each had a 5-minute Apgar score of 9. Most infants were discharged on the first or second day of life, and there were no neonatal deaths.
Key findings
Of the 15 placentas, 12 featured maternal vascular malperfusion. This rate was significantly higher than historic controls (P = .046) and melanoma study controls (P = .001).
Specific features varied between groups, with decidual arteriopathy, atherosis and fibrinoid necrosis of maternal vessels, and mural hypertrophy of membrane arterioles observed more often in COVID-19 cases than in all historical controls. In addition, peripheral infarctions, decidual arteriopathy, atherosis, and fibrinoid necrosis, and mural hypertrophy being more common in COVID-19 cases than in placentas of women with a history of melanoma.
In contrast, features of fetal vascular malperfusion were observed in 12 of 15 cases, but not at rates significantly different from the control groups. Chorangiosis, villous edema, and intervillous thrombi also were more common in the COVID-19 cohort.
Dr. Goldstein was surprised they did not observe much acute or chronic inflammation. “We see chronic inflammation in the placenta in response to many viruses, such as cytomegalovirus, so you might expect similar findings, but we didn’t see any increase above the controls.”
There are a couple of case reports of histiocytic intervillositis – a particularly severe form of chronic inflammation – associated with COVID-19, “but we didn’t see that in our study,” he added.
Clinical implications
The healthy neonatal outcomes reported in the study occurred despite the placental injury, which may be caused by the redundancy built into placentas for delivering oxygen and nutrients and for removing waste.
The negative COVID-19 test results in all infants also supports existing evidence that vertical transmission of the virus is uncommon. The finding also suggests that any damage to the placenta is likely related to maternal infection.
Only one mother in the COVID-19 cohort was hypertensive, which surprised the researchers because intervillous thrombi have been associated with maternal high blood pressure. “In the context of research suggesting an increase of thrombotic and thromboembolic disorders in COVID-19,” the researchers noted, “these may represent placental formation or deposition of thrombi in response to the virus.”
One of the priorities for the researchers going forward is to monitor the longer-term outcomes of the infants, Dr. Goldstein said. “We know the people in utero during the 1918-1919 flu pandemic had higher rates of heart disease and other long-term problems, so we want to be on the lookout for something similar.”
Valuable insight
“This is a comprehensive case series of this topic, with findings worth noting and sharing in a timely fashion,” Karen Mestan, MD, associate professor of pediatrics within the division of neonatology at Northwestern University, said when asked to comment on the study.
“The information is valuable to neonatologists as the short- and long-term effects of COVID-19 exposure on newborn infants are still largely unknown,” she added. “Details of placental pathology provide emerging insight and may help us understand mother-baby vertical transmission during the current pandemic.”
Dr. Goldstein and Dr. Mestan had no relevant financial disclosures.
SOURCE: Shanes ED et al. Am J Clin Pathol. 2020 May 22. doi: 10.1093/ajcp/aqaa089.
Maternal vascular malperfusion and intervillous thrombi were more common in the placentas of women infected with SARS-CoV-2, compared with historic controls, report researchers who conducted the first-of-its-kind case series in the English literature. Nevertheless, the neonates in the report appear to be healthy so far and all tested negative for the virus.
Although the series examining placentas from 16 women is small, it carries a larger implication – that increased antenatal surveillance for pregnant women infected with SARS-CoV-2 may be indicated, the researchers noted.
Furthermore, the results could align with other reports of coagulation and vascular abnormalities among people with COVID-19. “I would say that our findings fit into that larger picture of vascular injury. This is developing, and there are some significant ways that these feeder vessels to the placenta are different, but if this is the emerging paradigm, our findings can fit into it,” Jeffrey A. Goldstein, MD, PhD, assistant professor of pathology at Northwestern University, Chicago, said in an interview.
The research was published in the American Journal of Clinical Pathology.
Prior case series reported in Wuhan, China, do not currently suggest that pregnant women are more likely to experience severe COVID-19, in contrast to observations during severe acute respiratory syndrome and Middle East respiratory syndrome outbreaks. “However,” the researchers noted, “adverse perinatal outcomes have been reported, including increased risks of miscarriage, preeclampsia, preterm birth, and stillbirth.”
To learn more, Dr. Goldstein, lead author Elisheva D. Shanes, MD, and colleagues examined the histology of placentas from women with COVID-19 giving birth between March 18 and May 5, 2020. They compared these placentas with over 17,000 historic controls and 215 women who had their placentas evaluated as part of a melanoma history study.
A total of 10 women were diagnosed with COVID-19 upon presentation to labor and delivery, 4 others were diagnosed approximately 1 month before delivery and the remaining 2 within 1 week of delivery. Ten of the patients were symptomatic and two required oxygen. None of the patients received intubation or died. A total of 14 patients delivered at term, 1 delivered at 34 weeks, and the remaining case experienced a 16-week intrauterine fetal demise (IUFD). The IUFD was excluded from subsequent statistical analysis.
The neonates each had a 5-minute Apgar score of 9. Most infants were discharged on the first or second day of life, and there were no neonatal deaths.
Key findings
Of the 15 placentas, 12 featured maternal vascular malperfusion. This rate was significantly higher than historic controls (P = .046) and melanoma study controls (P = .001).
Specific features varied between groups, with decidual arteriopathy, atherosis and fibrinoid necrosis of maternal vessels, and mural hypertrophy of membrane arterioles observed more often in COVID-19 cases than in all historical controls. In addition, peripheral infarctions, decidual arteriopathy, atherosis, and fibrinoid necrosis, and mural hypertrophy being more common in COVID-19 cases than in placentas of women with a history of melanoma.
In contrast, features of fetal vascular malperfusion were observed in 12 of 15 cases, but not at rates significantly different from the control groups. Chorangiosis, villous edema, and intervillous thrombi also were more common in the COVID-19 cohort.
Dr. Goldstein was surprised they did not observe much acute or chronic inflammation. “We see chronic inflammation in the placenta in response to many viruses, such as cytomegalovirus, so you might expect similar findings, but we didn’t see any increase above the controls.”
There are a couple of case reports of histiocytic intervillositis – a particularly severe form of chronic inflammation – associated with COVID-19, “but we didn’t see that in our study,” he added.
Clinical implications
The healthy neonatal outcomes reported in the study occurred despite the placental injury, which may be caused by the redundancy built into placentas for delivering oxygen and nutrients and for removing waste.
The negative COVID-19 test results in all infants also supports existing evidence that vertical transmission of the virus is uncommon. The finding also suggests that any damage to the placenta is likely related to maternal infection.
Only one mother in the COVID-19 cohort was hypertensive, which surprised the researchers because intervillous thrombi have been associated with maternal high blood pressure. “In the context of research suggesting an increase of thrombotic and thromboembolic disorders in COVID-19,” the researchers noted, “these may represent placental formation or deposition of thrombi in response to the virus.”
One of the priorities for the researchers going forward is to monitor the longer-term outcomes of the infants, Dr. Goldstein said. “We know the people in utero during the 1918-1919 flu pandemic had higher rates of heart disease and other long-term problems, so we want to be on the lookout for something similar.”
Valuable insight
“This is a comprehensive case series of this topic, with findings worth noting and sharing in a timely fashion,” Karen Mestan, MD, associate professor of pediatrics within the division of neonatology at Northwestern University, said when asked to comment on the study.
“The information is valuable to neonatologists as the short- and long-term effects of COVID-19 exposure on newborn infants are still largely unknown,” she added. “Details of placental pathology provide emerging insight and may help us understand mother-baby vertical transmission during the current pandemic.”
Dr. Goldstein and Dr. Mestan had no relevant financial disclosures.
SOURCE: Shanes ED et al. Am J Clin Pathol. 2020 May 22. doi: 10.1093/ajcp/aqaa089.
FROM THE AMERICAN JOURNAL OF CLINICAL PATHOLOGY
SARS-CoV-2 infection rate 16% in asymptomatic pregnant women at delivery
Among women with a planned delivery in a New York City health system during the first half of April, the rate of asymptomatic SARS-CoV-2 infection was 16%, according to a study published in Obstetrics & Gynecology. Among the patients’ designated support persons, the asymptomatic carrier rate was 10%.
“If universal testing of pregnant patients in a high prevalence area is not performed, health care workers will be inadvertently exposed to COVID-19, unless universal precautions with personal protective equipment are taken,” wrote the researchers affiliated with the department of obstetrics, gynecology, and reproductive medicine at Icahn School of Medicine at Mount Sinai, New York.
Angela Bianco, MD, and colleagues conducted an observational study of women who were scheduled for a planned delivery within the Mount Sinai Health System between April 4 and April 15, 2020. Patients and their designated support person completed a telephone screen and underwent COVID-19 testing the day before a scheduled delivery. If support persons screened positive during the telephone interview about COVID-19 symptoms, they could not attend the birth, and patients could contact a different support person to be screened and tested. “All patients and their support persons were informed of their SARS-CoV-2 test results before admission,” the investigators wrote. “Those who tested positive were counseled regarding symptomatology that should prompt medical attention.”
In all, researchers screened 158 patients with a planned delivery, and 155 agreed to undergo COVID-19 testing. Of the 155 women tested, 24 (16%) tested positive for SARS CoV-2 infection. Among 146 support persons who had a negative interview screen and underwent SARS-CoV-2 testing, 14 (10%) tested positive for SARS-CoV-2 infection.
Test results were substantially concordant among patient and support person pairs. “Among patients who tested positive for COVID-19 infection and had a support person present, 11 of 19 (58%) support persons also tested positive for COVID-19 infection,” the authors reported. “Among patients who tested negative for COVID-19 infection and had a support person present, only 3 of 127 (2.4%) support persons tested positive for COVID-19 infection.”
Telephone screening did not identify any of the COVID-19–positive cases. Of the 24 patients with SARS-CoV-2 infection, none of their newborns tested positive at birth.
“Universal testing ... provides a mechanism for more accurate counseling of patients regarding issues such as newborn skin-to-skin contact and breastfeeding,” noted Dr. Bianco and colleagues. At their institution, parents with COVID-19 are instructed to wear a mask and practice proper hand hygiene when caring for their newborns.
Kristina Adams Waldorf, MD, said in an interview that the study by Bianco et al. underscores the high rate of asymptomatic or mildly symptomatic COVID-19 infections detected with universal screening in a hospital at the U.S. epicenter of the pandemic. “Each state and hospital will need to evaluate their own data to determine the value of universal screening for their patient population. In rural parts of America that have yet to see cases, universal screening may not make sense, but these areas are likely to be few and far between. The rest of America will need to quickly get on board with universal screening to protect their labor and delivery staff.”
Testing the partner was a strength of the study. “It is reassuring that when a pregnant woman tested negative for SARS-CoV-2, the rate was very, very low (2.4%) that her partner would test positive. However, it was disconcerting that telephone screening for common symptoms associated with COVID-19 was not very helpful in identifying cases,” said Dr. Waldorf, a professor of obstetrics and gynecology at the University of Washington, Seattle. She was not involved in the study by Bianco et al.
One study author receives payment from the American Board of Obstetrics and Gynecology for serving as a board examiner, receives payment from UpToDate, and serves as an expert witness in malpractice and products liability cases. The other authors did not report any potential conflicts of interest. Dr. Waldorf said she had no relevant financial disclosures.
SOURCE: Bianco A et al. Obstet Gynecol. 2020 May 19. doi: 10.1097/AOG.0000000000003985.
Among women with a planned delivery in a New York City health system during the first half of April, the rate of asymptomatic SARS-CoV-2 infection was 16%, according to a study published in Obstetrics & Gynecology. Among the patients’ designated support persons, the asymptomatic carrier rate was 10%.
“If universal testing of pregnant patients in a high prevalence area is not performed, health care workers will be inadvertently exposed to COVID-19, unless universal precautions with personal protective equipment are taken,” wrote the researchers affiliated with the department of obstetrics, gynecology, and reproductive medicine at Icahn School of Medicine at Mount Sinai, New York.
Angela Bianco, MD, and colleagues conducted an observational study of women who were scheduled for a planned delivery within the Mount Sinai Health System between April 4 and April 15, 2020. Patients and their designated support person completed a telephone screen and underwent COVID-19 testing the day before a scheduled delivery. If support persons screened positive during the telephone interview about COVID-19 symptoms, they could not attend the birth, and patients could contact a different support person to be screened and tested. “All patients and their support persons were informed of their SARS-CoV-2 test results before admission,” the investigators wrote. “Those who tested positive were counseled regarding symptomatology that should prompt medical attention.”
In all, researchers screened 158 patients with a planned delivery, and 155 agreed to undergo COVID-19 testing. Of the 155 women tested, 24 (16%) tested positive for SARS CoV-2 infection. Among 146 support persons who had a negative interview screen and underwent SARS-CoV-2 testing, 14 (10%) tested positive for SARS-CoV-2 infection.
Test results were substantially concordant among patient and support person pairs. “Among patients who tested positive for COVID-19 infection and had a support person present, 11 of 19 (58%) support persons also tested positive for COVID-19 infection,” the authors reported. “Among patients who tested negative for COVID-19 infection and had a support person present, only 3 of 127 (2.4%) support persons tested positive for COVID-19 infection.”
Telephone screening did not identify any of the COVID-19–positive cases. Of the 24 patients with SARS-CoV-2 infection, none of their newborns tested positive at birth.
“Universal testing ... provides a mechanism for more accurate counseling of patients regarding issues such as newborn skin-to-skin contact and breastfeeding,” noted Dr. Bianco and colleagues. At their institution, parents with COVID-19 are instructed to wear a mask and practice proper hand hygiene when caring for their newborns.
Kristina Adams Waldorf, MD, said in an interview that the study by Bianco et al. underscores the high rate of asymptomatic or mildly symptomatic COVID-19 infections detected with universal screening in a hospital at the U.S. epicenter of the pandemic. “Each state and hospital will need to evaluate their own data to determine the value of universal screening for their patient population. In rural parts of America that have yet to see cases, universal screening may not make sense, but these areas are likely to be few and far between. The rest of America will need to quickly get on board with universal screening to protect their labor and delivery staff.”
Testing the partner was a strength of the study. “It is reassuring that when a pregnant woman tested negative for SARS-CoV-2, the rate was very, very low (2.4%) that her partner would test positive. However, it was disconcerting that telephone screening for common symptoms associated with COVID-19 was not very helpful in identifying cases,” said Dr. Waldorf, a professor of obstetrics and gynecology at the University of Washington, Seattle. She was not involved in the study by Bianco et al.
One study author receives payment from the American Board of Obstetrics and Gynecology for serving as a board examiner, receives payment from UpToDate, and serves as an expert witness in malpractice and products liability cases. The other authors did not report any potential conflicts of interest. Dr. Waldorf said she had no relevant financial disclosures.
SOURCE: Bianco A et al. Obstet Gynecol. 2020 May 19. doi: 10.1097/AOG.0000000000003985.
Among women with a planned delivery in a New York City health system during the first half of April, the rate of asymptomatic SARS-CoV-2 infection was 16%, according to a study published in Obstetrics & Gynecology. Among the patients’ designated support persons, the asymptomatic carrier rate was 10%.
“If universal testing of pregnant patients in a high prevalence area is not performed, health care workers will be inadvertently exposed to COVID-19, unless universal precautions with personal protective equipment are taken,” wrote the researchers affiliated with the department of obstetrics, gynecology, and reproductive medicine at Icahn School of Medicine at Mount Sinai, New York.
Angela Bianco, MD, and colleagues conducted an observational study of women who were scheduled for a planned delivery within the Mount Sinai Health System between April 4 and April 15, 2020. Patients and their designated support person completed a telephone screen and underwent COVID-19 testing the day before a scheduled delivery. If support persons screened positive during the telephone interview about COVID-19 symptoms, they could not attend the birth, and patients could contact a different support person to be screened and tested. “All patients and their support persons were informed of their SARS-CoV-2 test results before admission,” the investigators wrote. “Those who tested positive were counseled regarding symptomatology that should prompt medical attention.”
In all, researchers screened 158 patients with a planned delivery, and 155 agreed to undergo COVID-19 testing. Of the 155 women tested, 24 (16%) tested positive for SARS CoV-2 infection. Among 146 support persons who had a negative interview screen and underwent SARS-CoV-2 testing, 14 (10%) tested positive for SARS-CoV-2 infection.
Test results were substantially concordant among patient and support person pairs. “Among patients who tested positive for COVID-19 infection and had a support person present, 11 of 19 (58%) support persons also tested positive for COVID-19 infection,” the authors reported. “Among patients who tested negative for COVID-19 infection and had a support person present, only 3 of 127 (2.4%) support persons tested positive for COVID-19 infection.”
Telephone screening did not identify any of the COVID-19–positive cases. Of the 24 patients with SARS-CoV-2 infection, none of their newborns tested positive at birth.
“Universal testing ... provides a mechanism for more accurate counseling of patients regarding issues such as newborn skin-to-skin contact and breastfeeding,” noted Dr. Bianco and colleagues. At their institution, parents with COVID-19 are instructed to wear a mask and practice proper hand hygiene when caring for their newborns.
Kristina Adams Waldorf, MD, said in an interview that the study by Bianco et al. underscores the high rate of asymptomatic or mildly symptomatic COVID-19 infections detected with universal screening in a hospital at the U.S. epicenter of the pandemic. “Each state and hospital will need to evaluate their own data to determine the value of universal screening for their patient population. In rural parts of America that have yet to see cases, universal screening may not make sense, but these areas are likely to be few and far between. The rest of America will need to quickly get on board with universal screening to protect their labor and delivery staff.”
Testing the partner was a strength of the study. “It is reassuring that when a pregnant woman tested negative for SARS-CoV-2, the rate was very, very low (2.4%) that her partner would test positive. However, it was disconcerting that telephone screening for common symptoms associated with COVID-19 was not very helpful in identifying cases,” said Dr. Waldorf, a professor of obstetrics and gynecology at the University of Washington, Seattle. She was not involved in the study by Bianco et al.
One study author receives payment from the American Board of Obstetrics and Gynecology for serving as a board examiner, receives payment from UpToDate, and serves as an expert witness in malpractice and products liability cases. The other authors did not report any potential conflicts of interest. Dr. Waldorf said she had no relevant financial disclosures.
SOURCE: Bianco A et al. Obstet Gynecol. 2020 May 19. doi: 10.1097/AOG.0000000000003985.
FROM OBSTETRICS & GYNECOLOGY