User login
Female Runner, 47, with Inguinal Lump
A 47-year-old woman was referred to the gynecology office by her primary care NP for surgical excision of an enlarging nodule on the right side of her mons pubis. Onset occurred about 6 months earlier. The patient reported that symptoms waxed and waned but had worsened progressively over the past 2 to 3 months, adding that the nodule hurt only occasionally. She noted that symptoms were exacerbated by exercise, specifically running. Further questioning prompted the observation that her symptoms were more noticeable at the time of menses.
The patient’s medical history was unremarkable, with no chronic conditions; her surgical history consisted of a wisdom tooth extraction. She had no known drug allergies. Her family history included cerebrovascular accident, hypertension, and arthritis. Reproductive history revealed that she was G1 P1, with a 38-week uncomplicated vaginal delivery. She experienced menarche at age 14, and her menses was regular at every 28 days. For the past 5 days, there had been no dysmenorrhea. The patient was married, exercised regularly, and did not use tobacco, alcohol, or illicit drugs.
On examination, the patient’s blood pressure was 123/73 mm Hg; heart rate, 77 beats/min; respiratory rate, 12 breaths/min; weight, 128 lb; height, 5 ft 7 in; O2 saturation, 99% on room air; and BMI, 20. The patient was alert and oriented to person, place, and time. She was thin, appeared physically fit, and exhibited no signs of distress. Her physical exam was unremarkable, apart from a firm, minimally tender, well-circumscribed, 3.5 × 3.5–cm nodule right of midline on the mons pubis.
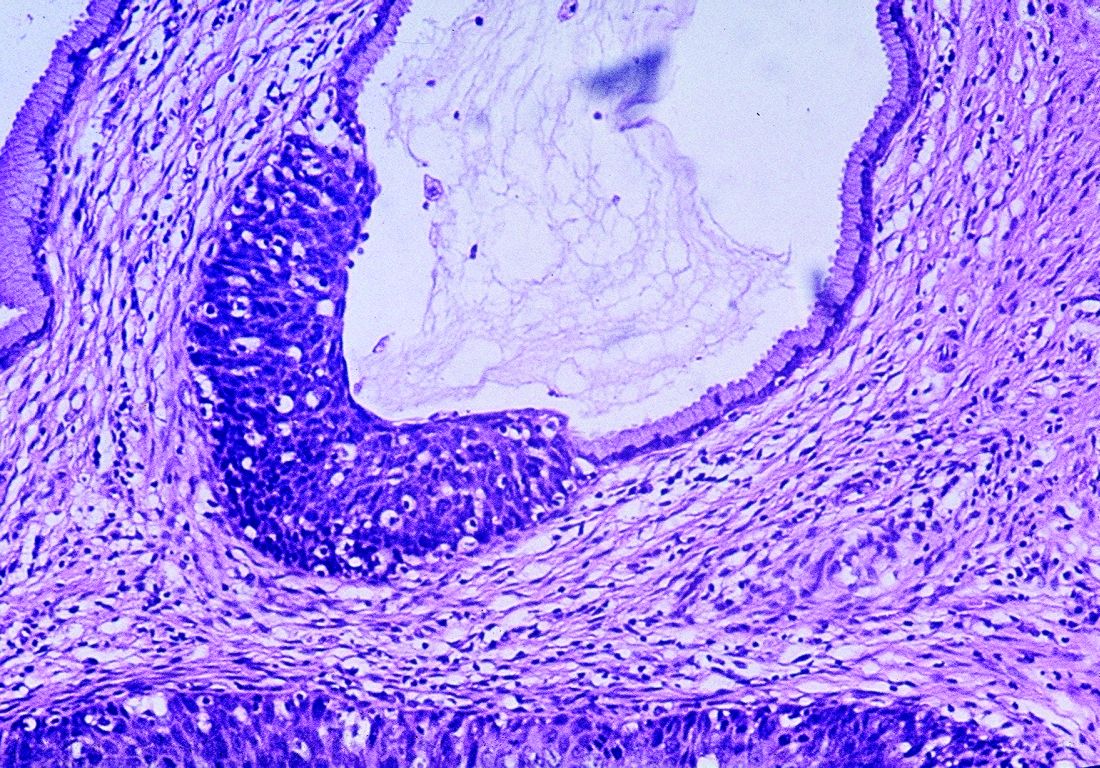
The patient was scheduled for outpatient surgical excision of a benign skin lesion (excluding skin tags) of the genitalia, 3.1 to 3.5 cm (CPT code 11424). During this procedure, it became evident that this was not a lipoma. The lesion was exceptionally hard, and it was difficult to discern if it was incorporated into the rectus abdominis near the point of attachment to the pubic symphysis. The lesion was unintentionally disrupted, revealing black powdery material within the capsule. The tissue was sent for a fast, frozen section that showed “soft tissue with extensive involvement by endometriosis.” The pathology report noted “[m]any endometrial glands in a background of stromal tissue. Necrosis was not a feature. No evidence of atypia.” The patient’s postoperative diagnosis was endometriosis.
DISCUSSION
Endometriosis occurs when endometrial or “endometrial-like” tissue is displaced to sites other than within the uterus. It is most frequently found on tissues close to the uterus, such as the ovaries or pelvic peritoneum. Estrogen is the driving force that feeds the endometrium, causing it to proliferate, whether inside or outside the uterus. Given this dependence on hormones, endometriosis occurs most often during a woman’s fertile years, although it can occur after menopause. Endometriosis is common, affecting at least 10% of premenopausal women; moreover, it is identified as the cause in 70% of all female chronic pelvic pain cases.1-4
Endometriosis has certain identifiable features, such as chronic pain, dyspareunia, infertility, and menstrual and gastrointestinal symptoms. However, it is seldom diagnosed quickly; studies indicate that diagnosis can be delayed by 5 to 10 years after a patient has first sought treatment for symptoms.2,4 Multiple factors contribute to a lag in diagnosis: Presentation is not always straightforward. There are no definitive lab values or biomarkers. Symptoms vary from patient to patient, as do clinical skills from one diagnostician to another.1
Unlike pelvic endometriosis, inguinal endometriosis is not common; disease in this location encompasses only 0.3% to 0.6% of all diagnosed cases.3,5-7 Since the discovery of the first known case of round ligament endometriosis in 1896, there have been only 70 cases reported in the medical literature.6,7
If the more common form of endometriosis is frequently missed, this rarely seen variant presents an even greater diagnostic challenge. The typical presentation of inguinal endometriosis includes a firm nodule in the groin, accompanied by tenderness and swelling. A careful history will allude to pain that occurs cyclically with menses.
Cause
Among several theories about the etiology of endometriosis, the most popular has been retrograde menstruation.1,4,5 According to this hypothesis, the flow of menstrual blood moves backward through the fallopian tubes, spilling into the pelvic cavity and carrying endometrial tissue with it. One theory purports that endometrial tissue is transplanted from the uterus to other areas of the body via the bloodstream or the lymphatics, much like a metastatic disease.1,4 Another theory states that cells outside the uterus, which line the peritoneum, transform into endometrial cells through metaplasia.4,5 Endometrial tissue can also be transplanted iatrogenically during surgery—for example, when endometrial tissue is displaced during a cesarean delivery, resulting in implants above the fascia and below the subcutaneous layers. Several other hypotheses concern stem-cell involvement, hormonal factors, immune system dysfunction, and genetics.4,5 Currently, there are no definitive answers.
Location
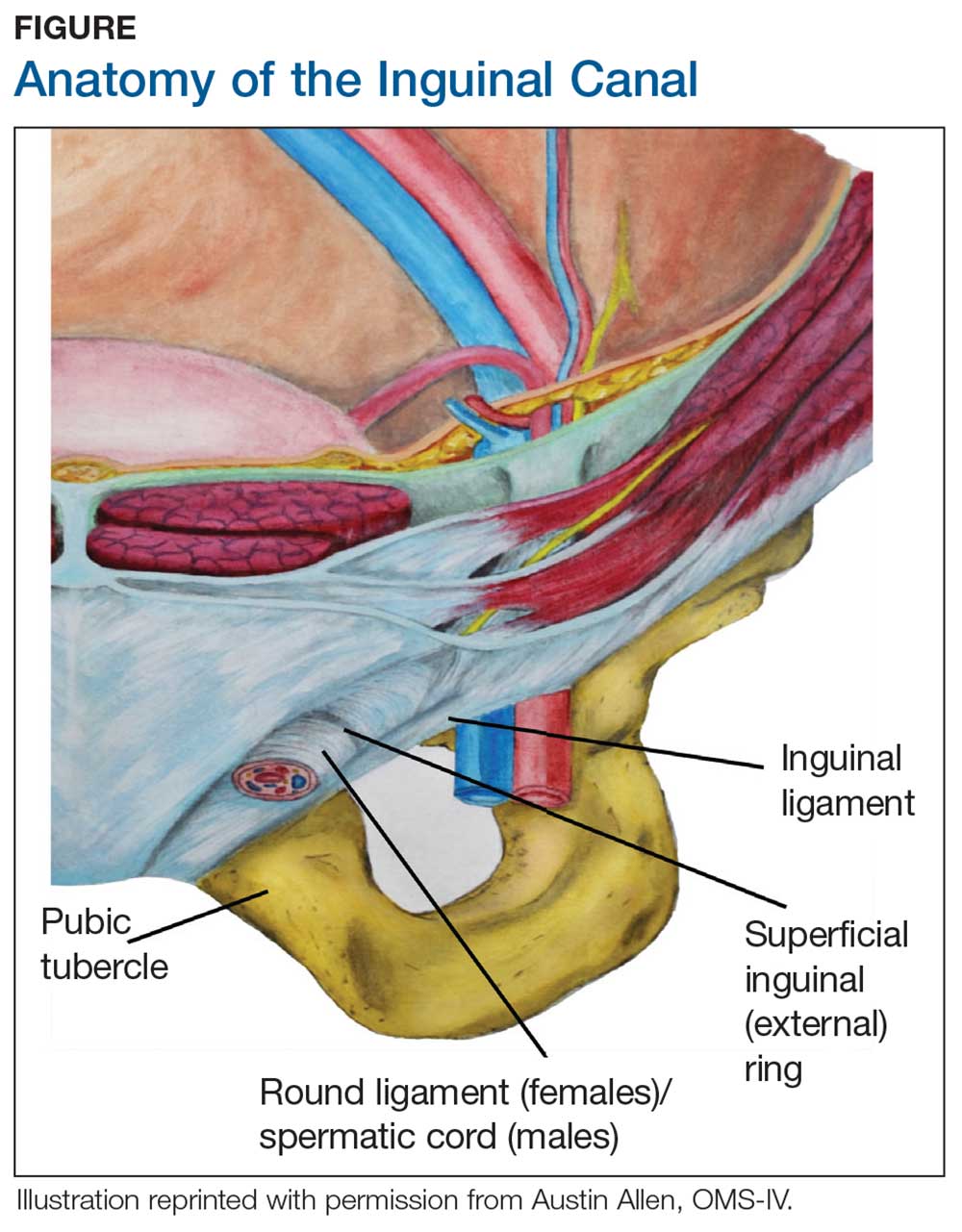
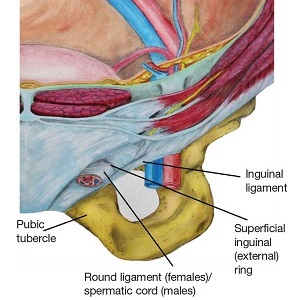
During maturation, the parietal peritoneum develops a pouch called the processus vaginalis, which serves as a passageway for the gubernaculum to transport the round ligament running from the uterus, through the inguinal canal, and ending at the labia. After these structures reach their destination, in normal development, the processus vaginalis degenerates, closing the inguinal canal. Occasionally the processus vaginalis fails to close, allowing for a communication pathway between the peritoneal cavity and the inguinal canal. This leaves the canal vulnerable to the contents of the pelvic cavity, such as a hernia or hydrocele, and provides a clear path for endometriosis.5-7 The implant found in the case patient was at the point where the external ring lies, just above the right pubic tubercle (see Figure 1).
Endometriosis implants can occur anywhere along the round ligament in either the intrapelvic or extrapelvic segments. Implants have also been found in the wall of a hernia sac, the wall of a Nuck canal hydrocele, or even in the subcutaneous tissue surrounding the inguinal canal.3 Interestingly, inguinal endometriosis occurs more often in the right side (up to 94% of cases) than in the left side, as was the case with our patient.5-7 The reason for this predominance has not been established, although there are several theories, including one that suggests the left side is afforded protection by the sigmoid colon.5-7
Laboratory diagnosis
Imaging, such as ultrasound and MRI, offers some diagnostic benefit, although its usefulness is most often realized in the pelvis. Pelvic ultrasound can be used to identify ovarian endometriomas.1 MRI can help rule out, locate, or sometimes determine the degree of deep infiltrating endometriosis, which is an indispensable tool for surgical planning.5,7 Unfortunately, the diagnostic accuracy for extra-pelvic lesions is variable; neither modality is particularly useful in identifying superficial lesions, which comprises most cases.
Ultrasound of the groin can be employed to evaluate for hernia; if a hernia has been excluded, histologic confirmation can be obtained via fine-needle aspiration of nodule contents.5,7 One caveat is that these tests are helpful only if the clinician suspects the diagnosis and orders them. The definitive diagnostic test remains direct visualization, which requires laparoscopy.1,5
Differential diagnosis
Lipoma was a favored diagnosis in this case because of the palpable, well-circumscribed borders, nontender on exam; intermittent, minimal tenderness; and no evidence of erythema or color change. A second possibility was an enlarged lymph node, which was less likely due to the location, large size, and sudden onset without any accompanying symptoms of infection or chronic illness. Finally, an inguinal hernia was least likely, again because of well-defined borders, no history of a lump in the area, a nodule that was not reducible, only minimal tenderness, and no color changes on the skin.
Management
Definitive treatment for inguinal endometriosis entails complete surgical excision.5-7 The provider should be prepared to repair a defect after the excision; there is potential for a substantial defect that might require mesh. Additionally, a herniorrhaphy may be indicated if there is a coexisting hernia.5 The risk for recurrent disease in the inguinal canal after treatment is uncommon, unless the excision was not complete.3
There is an association between inguinal and pelvic endometriosis but not a direct correlation. Data on concomitant pelvic and inguinal endometriosis have been variable. In one case series of 9 patients diagnosed with inguinal endometriosis, none had a history of pelvic endometriosis, and only 1 was subsequently diagnosed with pelvic endometriosis.7 An increased association was noted for patients with implants found on the proximal segment of the round ligament.7 However, implants on the extrapelvic segment were not likely to represent pelvic disease but rather isolated lesions in the canal.7 For those with pelvic endometriosis, complications and recurrence are likely, resulting in the need for long-term treatment.
There is some debate in the literature whether to proceed with laparoscopy once inguinal endometriosis has been identified. Diagnostic laparoscopy to evaluate the pelvis is indicated for symptomatic patients or for cases in which an indirect inguinal hernia is suspected.5 Laparoscopy can offer the benefit of both a diagnostic tool and a mechanism for treatment. However, this is an invasive procedure that also incurs risks. The medical provider, in discussion with the patient, must weigh the risks against the benefits of an invasive procedure before determining how to proceed.
OUTCOME FOR THE CASE PATIENT
The lesion was excised completely. Since the patient had been entirely asymptomatic until age 47, and the risks of a potentially unnecessary surgery outweighed the theoretical benefits, the decision was made not to perform a diagnostic laparoscopy to investigate for pelvic endometriosis. The patient made a complete and uneventful recovery. No further treatment was initiated. She continues to be asymptomatic, denying any menstrual complaints, dyspareunia, or further problems with the groin.
CONCLUSION
This case describes a satellite lesion of endometrial tissue found in an unusual location, in a patient with no history, no risk factors, and no symptoms. The final diagnosis had been omitted from the differential—perhaps because the patient initially associated her symptoms with exercise and mentioned the correlation to her menstrual cycle as an afterthought. Fortunately, the correct diagnosis was made and the appropriate treatment provided.
There are numerous presentations of endometriosis; extrapelvic lesions can have very different, often vague, presentations when compared to the familiar symptoms of pelvic disease. Unfortunately, diagnosis is often delayed. Obscure presentations, in unusual sites, can further impede both speed and accuracy of diagnosis. To date, there are no lab tests or biomarkers to aid diagnosis; imaging studies are inconsistent. Until more accurate diagnostic tools become available, the diagnosis remains dependent on history taking, physical exam, and the clinical judgment of the provider. The astute clinician will recognize the catamenial pattern and consider endometriosis as part of the differential.
1. Parasar P, Ozcan P, Terry KL. Endometriosis: epidemiology, diagnosis and clinical management. Curr Obstet Gynecol Rep. 2017;6(1):34-41.
2. Soliman AM, Fuldeore M, Snabes MC. Factors associated with time to endometriosis diagnosis in the United States. J Womens Health (Larchmt). 2017;26(7):788-797.
3. Niitsu H, Tsumura H, Kanehiro T, et al. Clinical characteristics and surgical treatment for inguinal endometriosis in young women of reproductive age. Dig Surg. 2019;36(2):166-172.
4. Mehedintu C, Plotogea MN, Ionescu S, Antonovici M. Endometriosis still a challenge. J Med Life. 2014;7(3):349-357.
5. Wolfhagen N, Simons NE, de Jong KH, et al. Inguinal endometriosis, a rare entity of which surgeons should be aware: clinical aspects and long-term follow-up of nine cases. Hernia. 2018;22(5):881-886.
6. Prabhu R, Krishna S, Shenoy R, Thangavelu S. Endometriosis of extra-pelvic round ligament, a diagnostic dilemma for physicians. BMJ Case Rep. 2013;2013.
7. Pandey D, Coondoo A, Shetty J, Mathew S. Jack in the box: inguinal endometriosis. BMJ Case Rep. 2015;2015.
A 47-year-old woman was referred to the gynecology office by her primary care NP for surgical excision of an enlarging nodule on the right side of her mons pubis. Onset occurred about 6 months earlier. The patient reported that symptoms waxed and waned but had worsened progressively over the past 2 to 3 months, adding that the nodule hurt only occasionally. She noted that symptoms were exacerbated by exercise, specifically running. Further questioning prompted the observation that her symptoms were more noticeable at the time of menses.
The patient’s medical history was unremarkable, with no chronic conditions; her surgical history consisted of a wisdom tooth extraction. She had no known drug allergies. Her family history included cerebrovascular accident, hypertension, and arthritis. Reproductive history revealed that she was G1 P1, with a 38-week uncomplicated vaginal delivery. She experienced menarche at age 14, and her menses was regular at every 28 days. For the past 5 days, there had been no dysmenorrhea. The patient was married, exercised regularly, and did not use tobacco, alcohol, or illicit drugs.
On examination, the patient’s blood pressure was 123/73 mm Hg; heart rate, 77 beats/min; respiratory rate, 12 breaths/min; weight, 128 lb; height, 5 ft 7 in; O2 saturation, 99% on room air; and BMI, 20. The patient was alert and oriented to person, place, and time. She was thin, appeared physically fit, and exhibited no signs of distress. Her physical exam was unremarkable, apart from a firm, minimally tender, well-circumscribed, 3.5 × 3.5–cm nodule right of midline on the mons pubis.
The patient was scheduled for outpatient surgical excision of a benign skin lesion (excluding skin tags) of the genitalia, 3.1 to 3.5 cm (CPT code 11424). During this procedure, it became evident that this was not a lipoma. The lesion was exceptionally hard, and it was difficult to discern if it was incorporated into the rectus abdominis near the point of attachment to the pubic symphysis. The lesion was unintentionally disrupted, revealing black powdery material within the capsule. The tissue was sent for a fast, frozen section that showed “soft tissue with extensive involvement by endometriosis.” The pathology report noted “[m]any endometrial glands in a background of stromal tissue. Necrosis was not a feature. No evidence of atypia.” The patient’s postoperative diagnosis was endometriosis.
DISCUSSION
Endometriosis occurs when endometrial or “endometrial-like” tissue is displaced to sites other than within the uterus. It is most frequently found on tissues close to the uterus, such as the ovaries or pelvic peritoneum. Estrogen is the driving force that feeds the endometrium, causing it to proliferate, whether inside or outside the uterus. Given this dependence on hormones, endometriosis occurs most often during a woman’s fertile years, although it can occur after menopause. Endometriosis is common, affecting at least 10% of premenopausal women; moreover, it is identified as the cause in 70% of all female chronic pelvic pain cases.1-4
Endometriosis has certain identifiable features, such as chronic pain, dyspareunia, infertility, and menstrual and gastrointestinal symptoms. However, it is seldom diagnosed quickly; studies indicate that diagnosis can be delayed by 5 to 10 years after a patient has first sought treatment for symptoms.2,4 Multiple factors contribute to a lag in diagnosis: Presentation is not always straightforward. There are no definitive lab values or biomarkers. Symptoms vary from patient to patient, as do clinical skills from one diagnostician to another.1
Unlike pelvic endometriosis, inguinal endometriosis is not common; disease in this location encompasses only 0.3% to 0.6% of all diagnosed cases.3,5-7 Since the discovery of the first known case of round ligament endometriosis in 1896, there have been only 70 cases reported in the medical literature.6,7
If the more common form of endometriosis is frequently missed, this rarely seen variant presents an even greater diagnostic challenge. The typical presentation of inguinal endometriosis includes a firm nodule in the groin, accompanied by tenderness and swelling. A careful history will allude to pain that occurs cyclically with menses.
Cause
Among several theories about the etiology of endometriosis, the most popular has been retrograde menstruation.1,4,5 According to this hypothesis, the flow of menstrual blood moves backward through the fallopian tubes, spilling into the pelvic cavity and carrying endometrial tissue with it. One theory purports that endometrial tissue is transplanted from the uterus to other areas of the body via the bloodstream or the lymphatics, much like a metastatic disease.1,4 Another theory states that cells outside the uterus, which line the peritoneum, transform into endometrial cells through metaplasia.4,5 Endometrial tissue can also be transplanted iatrogenically during surgery—for example, when endometrial tissue is displaced during a cesarean delivery, resulting in implants above the fascia and below the subcutaneous layers. Several other hypotheses concern stem-cell involvement, hormonal factors, immune system dysfunction, and genetics.4,5 Currently, there are no definitive answers.
Location
During maturation, the parietal peritoneum develops a pouch called the processus vaginalis, which serves as a passageway for the gubernaculum to transport the round ligament running from the uterus, through the inguinal canal, and ending at the labia. After these structures reach their destination, in normal development, the processus vaginalis degenerates, closing the inguinal canal. Occasionally the processus vaginalis fails to close, allowing for a communication pathway between the peritoneal cavity and the inguinal canal. This leaves the canal vulnerable to the contents of the pelvic cavity, such as a hernia or hydrocele, and provides a clear path for endometriosis.5-7 The implant found in the case patient was at the point where the external ring lies, just above the right pubic tubercle (see Figure 1).
Endometriosis implants can occur anywhere along the round ligament in either the intrapelvic or extrapelvic segments. Implants have also been found in the wall of a hernia sac, the wall of a Nuck canal hydrocele, or even in the subcutaneous tissue surrounding the inguinal canal.3 Interestingly, inguinal endometriosis occurs more often in the right side (up to 94% of cases) than in the left side, as was the case with our patient.5-7 The reason for this predominance has not been established, although there are several theories, including one that suggests the left side is afforded protection by the sigmoid colon.5-7
Laboratory diagnosis
Imaging, such as ultrasound and MRI, offers some diagnostic benefit, although its usefulness is most often realized in the pelvis. Pelvic ultrasound can be used to identify ovarian endometriomas.1 MRI can help rule out, locate, or sometimes determine the degree of deep infiltrating endometriosis, which is an indispensable tool for surgical planning.5,7 Unfortunately, the diagnostic accuracy for extra-pelvic lesions is variable; neither modality is particularly useful in identifying superficial lesions, which comprises most cases.
Ultrasound of the groin can be employed to evaluate for hernia; if a hernia has been excluded, histologic confirmation can be obtained via fine-needle aspiration of nodule contents.5,7 One caveat is that these tests are helpful only if the clinician suspects the diagnosis and orders them. The definitive diagnostic test remains direct visualization, which requires laparoscopy.1,5
Differential diagnosis
Lipoma was a favored diagnosis in this case because of the palpable, well-circumscribed borders, nontender on exam; intermittent, minimal tenderness; and no evidence of erythema or color change. A second possibility was an enlarged lymph node, which was less likely due to the location, large size, and sudden onset without any accompanying symptoms of infection or chronic illness. Finally, an inguinal hernia was least likely, again because of well-defined borders, no history of a lump in the area, a nodule that was not reducible, only minimal tenderness, and no color changes on the skin.
Management
Definitive treatment for inguinal endometriosis entails complete surgical excision.5-7 The provider should be prepared to repair a defect after the excision; there is potential for a substantial defect that might require mesh. Additionally, a herniorrhaphy may be indicated if there is a coexisting hernia.5 The risk for recurrent disease in the inguinal canal after treatment is uncommon, unless the excision was not complete.3
There is an association between inguinal and pelvic endometriosis but not a direct correlation. Data on concomitant pelvic and inguinal endometriosis have been variable. In one case series of 9 patients diagnosed with inguinal endometriosis, none had a history of pelvic endometriosis, and only 1 was subsequently diagnosed with pelvic endometriosis.7 An increased association was noted for patients with implants found on the proximal segment of the round ligament.7 However, implants on the extrapelvic segment were not likely to represent pelvic disease but rather isolated lesions in the canal.7 For those with pelvic endometriosis, complications and recurrence are likely, resulting in the need for long-term treatment.
There is some debate in the literature whether to proceed with laparoscopy once inguinal endometriosis has been identified. Diagnostic laparoscopy to evaluate the pelvis is indicated for symptomatic patients or for cases in which an indirect inguinal hernia is suspected.5 Laparoscopy can offer the benefit of both a diagnostic tool and a mechanism for treatment. However, this is an invasive procedure that also incurs risks. The medical provider, in discussion with the patient, must weigh the risks against the benefits of an invasive procedure before determining how to proceed.
OUTCOME FOR THE CASE PATIENT
The lesion was excised completely. Since the patient had been entirely asymptomatic until age 47, and the risks of a potentially unnecessary surgery outweighed the theoretical benefits, the decision was made not to perform a diagnostic laparoscopy to investigate for pelvic endometriosis. The patient made a complete and uneventful recovery. No further treatment was initiated. She continues to be asymptomatic, denying any menstrual complaints, dyspareunia, or further problems with the groin.
CONCLUSION
This case describes a satellite lesion of endometrial tissue found in an unusual location, in a patient with no history, no risk factors, and no symptoms. The final diagnosis had been omitted from the differential—perhaps because the patient initially associated her symptoms with exercise and mentioned the correlation to her menstrual cycle as an afterthought. Fortunately, the correct diagnosis was made and the appropriate treatment provided.
There are numerous presentations of endometriosis; extrapelvic lesions can have very different, often vague, presentations when compared to the familiar symptoms of pelvic disease. Unfortunately, diagnosis is often delayed. Obscure presentations, in unusual sites, can further impede both speed and accuracy of diagnosis. To date, there are no lab tests or biomarkers to aid diagnosis; imaging studies are inconsistent. Until more accurate diagnostic tools become available, the diagnosis remains dependent on history taking, physical exam, and the clinical judgment of the provider. The astute clinician will recognize the catamenial pattern and consider endometriosis as part of the differential.
A 47-year-old woman was referred to the gynecology office by her primary care NP for surgical excision of an enlarging nodule on the right side of her mons pubis. Onset occurred about 6 months earlier. The patient reported that symptoms waxed and waned but had worsened progressively over the past 2 to 3 months, adding that the nodule hurt only occasionally. She noted that symptoms were exacerbated by exercise, specifically running. Further questioning prompted the observation that her symptoms were more noticeable at the time of menses.
The patient’s medical history was unremarkable, with no chronic conditions; her surgical history consisted of a wisdom tooth extraction. She had no known drug allergies. Her family history included cerebrovascular accident, hypertension, and arthritis. Reproductive history revealed that she was G1 P1, with a 38-week uncomplicated vaginal delivery. She experienced menarche at age 14, and her menses was regular at every 28 days. For the past 5 days, there had been no dysmenorrhea. The patient was married, exercised regularly, and did not use tobacco, alcohol, or illicit drugs.
On examination, the patient’s blood pressure was 123/73 mm Hg; heart rate, 77 beats/min; respiratory rate, 12 breaths/min; weight, 128 lb; height, 5 ft 7 in; O2 saturation, 99% on room air; and BMI, 20. The patient was alert and oriented to person, place, and time. She was thin, appeared physically fit, and exhibited no signs of distress. Her physical exam was unremarkable, apart from a firm, minimally tender, well-circumscribed, 3.5 × 3.5–cm nodule right of midline on the mons pubis.
The patient was scheduled for outpatient surgical excision of a benign skin lesion (excluding skin tags) of the genitalia, 3.1 to 3.5 cm (CPT code 11424). During this procedure, it became evident that this was not a lipoma. The lesion was exceptionally hard, and it was difficult to discern if it was incorporated into the rectus abdominis near the point of attachment to the pubic symphysis. The lesion was unintentionally disrupted, revealing black powdery material within the capsule. The tissue was sent for a fast, frozen section that showed “soft tissue with extensive involvement by endometriosis.” The pathology report noted “[m]any endometrial glands in a background of stromal tissue. Necrosis was not a feature. No evidence of atypia.” The patient’s postoperative diagnosis was endometriosis.
DISCUSSION
Endometriosis occurs when endometrial or “endometrial-like” tissue is displaced to sites other than within the uterus. It is most frequently found on tissues close to the uterus, such as the ovaries or pelvic peritoneum. Estrogen is the driving force that feeds the endometrium, causing it to proliferate, whether inside or outside the uterus. Given this dependence on hormones, endometriosis occurs most often during a woman’s fertile years, although it can occur after menopause. Endometriosis is common, affecting at least 10% of premenopausal women; moreover, it is identified as the cause in 70% of all female chronic pelvic pain cases.1-4
Endometriosis has certain identifiable features, such as chronic pain, dyspareunia, infertility, and menstrual and gastrointestinal symptoms. However, it is seldom diagnosed quickly; studies indicate that diagnosis can be delayed by 5 to 10 years after a patient has first sought treatment for symptoms.2,4 Multiple factors contribute to a lag in diagnosis: Presentation is not always straightforward. There are no definitive lab values or biomarkers. Symptoms vary from patient to patient, as do clinical skills from one diagnostician to another.1
Unlike pelvic endometriosis, inguinal endometriosis is not common; disease in this location encompasses only 0.3% to 0.6% of all diagnosed cases.3,5-7 Since the discovery of the first known case of round ligament endometriosis in 1896, there have been only 70 cases reported in the medical literature.6,7
If the more common form of endometriosis is frequently missed, this rarely seen variant presents an even greater diagnostic challenge. The typical presentation of inguinal endometriosis includes a firm nodule in the groin, accompanied by tenderness and swelling. A careful history will allude to pain that occurs cyclically with menses.
Cause
Among several theories about the etiology of endometriosis, the most popular has been retrograde menstruation.1,4,5 According to this hypothesis, the flow of menstrual blood moves backward through the fallopian tubes, spilling into the pelvic cavity and carrying endometrial tissue with it. One theory purports that endometrial tissue is transplanted from the uterus to other areas of the body via the bloodstream or the lymphatics, much like a metastatic disease.1,4 Another theory states that cells outside the uterus, which line the peritoneum, transform into endometrial cells through metaplasia.4,5 Endometrial tissue can also be transplanted iatrogenically during surgery—for example, when endometrial tissue is displaced during a cesarean delivery, resulting in implants above the fascia and below the subcutaneous layers. Several other hypotheses concern stem-cell involvement, hormonal factors, immune system dysfunction, and genetics.4,5 Currently, there are no definitive answers.
Location
During maturation, the parietal peritoneum develops a pouch called the processus vaginalis, which serves as a passageway for the gubernaculum to transport the round ligament running from the uterus, through the inguinal canal, and ending at the labia. After these structures reach their destination, in normal development, the processus vaginalis degenerates, closing the inguinal canal. Occasionally the processus vaginalis fails to close, allowing for a communication pathway between the peritoneal cavity and the inguinal canal. This leaves the canal vulnerable to the contents of the pelvic cavity, such as a hernia or hydrocele, and provides a clear path for endometriosis.5-7 The implant found in the case patient was at the point where the external ring lies, just above the right pubic tubercle (see Figure 1).
Endometriosis implants can occur anywhere along the round ligament in either the intrapelvic or extrapelvic segments. Implants have also been found in the wall of a hernia sac, the wall of a Nuck canal hydrocele, or even in the subcutaneous tissue surrounding the inguinal canal.3 Interestingly, inguinal endometriosis occurs more often in the right side (up to 94% of cases) than in the left side, as was the case with our patient.5-7 The reason for this predominance has not been established, although there are several theories, including one that suggests the left side is afforded protection by the sigmoid colon.5-7
Laboratory diagnosis
Imaging, such as ultrasound and MRI, offers some diagnostic benefit, although its usefulness is most often realized in the pelvis. Pelvic ultrasound can be used to identify ovarian endometriomas.1 MRI can help rule out, locate, or sometimes determine the degree of deep infiltrating endometriosis, which is an indispensable tool for surgical planning.5,7 Unfortunately, the diagnostic accuracy for extra-pelvic lesions is variable; neither modality is particularly useful in identifying superficial lesions, which comprises most cases.
Ultrasound of the groin can be employed to evaluate for hernia; if a hernia has been excluded, histologic confirmation can be obtained via fine-needle aspiration of nodule contents.5,7 One caveat is that these tests are helpful only if the clinician suspects the diagnosis and orders them. The definitive diagnostic test remains direct visualization, which requires laparoscopy.1,5
Differential diagnosis
Lipoma was a favored diagnosis in this case because of the palpable, well-circumscribed borders, nontender on exam; intermittent, minimal tenderness; and no evidence of erythema or color change. A second possibility was an enlarged lymph node, which was less likely due to the location, large size, and sudden onset without any accompanying symptoms of infection or chronic illness. Finally, an inguinal hernia was least likely, again because of well-defined borders, no history of a lump in the area, a nodule that was not reducible, only minimal tenderness, and no color changes on the skin.
Management
Definitive treatment for inguinal endometriosis entails complete surgical excision.5-7 The provider should be prepared to repair a defect after the excision; there is potential for a substantial defect that might require mesh. Additionally, a herniorrhaphy may be indicated if there is a coexisting hernia.5 The risk for recurrent disease in the inguinal canal after treatment is uncommon, unless the excision was not complete.3
There is an association between inguinal and pelvic endometriosis but not a direct correlation. Data on concomitant pelvic and inguinal endometriosis have been variable. In one case series of 9 patients diagnosed with inguinal endometriosis, none had a history of pelvic endometriosis, and only 1 was subsequently diagnosed with pelvic endometriosis.7 An increased association was noted for patients with implants found on the proximal segment of the round ligament.7 However, implants on the extrapelvic segment were not likely to represent pelvic disease but rather isolated lesions in the canal.7 For those with pelvic endometriosis, complications and recurrence are likely, resulting in the need for long-term treatment.
There is some debate in the literature whether to proceed with laparoscopy once inguinal endometriosis has been identified. Diagnostic laparoscopy to evaluate the pelvis is indicated for symptomatic patients or for cases in which an indirect inguinal hernia is suspected.5 Laparoscopy can offer the benefit of both a diagnostic tool and a mechanism for treatment. However, this is an invasive procedure that also incurs risks. The medical provider, in discussion with the patient, must weigh the risks against the benefits of an invasive procedure before determining how to proceed.
OUTCOME FOR THE CASE PATIENT
The lesion was excised completely. Since the patient had been entirely asymptomatic until age 47, and the risks of a potentially unnecessary surgery outweighed the theoretical benefits, the decision was made not to perform a diagnostic laparoscopy to investigate for pelvic endometriosis. The patient made a complete and uneventful recovery. No further treatment was initiated. She continues to be asymptomatic, denying any menstrual complaints, dyspareunia, or further problems with the groin.
CONCLUSION
This case describes a satellite lesion of endometrial tissue found in an unusual location, in a patient with no history, no risk factors, and no symptoms. The final diagnosis had been omitted from the differential—perhaps because the patient initially associated her symptoms with exercise and mentioned the correlation to her menstrual cycle as an afterthought. Fortunately, the correct diagnosis was made and the appropriate treatment provided.
There are numerous presentations of endometriosis; extrapelvic lesions can have very different, often vague, presentations when compared to the familiar symptoms of pelvic disease. Unfortunately, diagnosis is often delayed. Obscure presentations, in unusual sites, can further impede both speed and accuracy of diagnosis. To date, there are no lab tests or biomarkers to aid diagnosis; imaging studies are inconsistent. Until more accurate diagnostic tools become available, the diagnosis remains dependent on history taking, physical exam, and the clinical judgment of the provider. The astute clinician will recognize the catamenial pattern and consider endometriosis as part of the differential.
1. Parasar P, Ozcan P, Terry KL. Endometriosis: epidemiology, diagnosis and clinical management. Curr Obstet Gynecol Rep. 2017;6(1):34-41.
2. Soliman AM, Fuldeore M, Snabes MC. Factors associated with time to endometriosis diagnosis in the United States. J Womens Health (Larchmt). 2017;26(7):788-797.
3. Niitsu H, Tsumura H, Kanehiro T, et al. Clinical characteristics and surgical treatment for inguinal endometriosis in young women of reproductive age. Dig Surg. 2019;36(2):166-172.
4. Mehedintu C, Plotogea MN, Ionescu S, Antonovici M. Endometriosis still a challenge. J Med Life. 2014;7(3):349-357.
5. Wolfhagen N, Simons NE, de Jong KH, et al. Inguinal endometriosis, a rare entity of which surgeons should be aware: clinical aspects and long-term follow-up of nine cases. Hernia. 2018;22(5):881-886.
6. Prabhu R, Krishna S, Shenoy R, Thangavelu S. Endometriosis of extra-pelvic round ligament, a diagnostic dilemma for physicians. BMJ Case Rep. 2013;2013.
7. Pandey D, Coondoo A, Shetty J, Mathew S. Jack in the box: inguinal endometriosis. BMJ Case Rep. 2015;2015.
1. Parasar P, Ozcan P, Terry KL. Endometriosis: epidemiology, diagnosis and clinical management. Curr Obstet Gynecol Rep. 2017;6(1):34-41.
2. Soliman AM, Fuldeore M, Snabes MC. Factors associated with time to endometriosis diagnosis in the United States. J Womens Health (Larchmt). 2017;26(7):788-797.
3. Niitsu H, Tsumura H, Kanehiro T, et al. Clinical characteristics and surgical treatment for inguinal endometriosis in young women of reproductive age. Dig Surg. 2019;36(2):166-172.
4. Mehedintu C, Plotogea MN, Ionescu S, Antonovici M. Endometriosis still a challenge. J Med Life. 2014;7(3):349-357.
5. Wolfhagen N, Simons NE, de Jong KH, et al. Inguinal endometriosis, a rare entity of which surgeons should be aware: clinical aspects and long-term follow-up of nine cases. Hernia. 2018;22(5):881-886.
6. Prabhu R, Krishna S, Shenoy R, Thangavelu S. Endometriosis of extra-pelvic round ligament, a diagnostic dilemma for physicians. BMJ Case Rep. 2013;2013.
7. Pandey D, Coondoo A, Shetty J, Mathew S. Jack in the box: inguinal endometriosis. BMJ Case Rep. 2015;2015.
FDA approves cefiderocol for multidrug-resistant, complicated urinary tract infections
The Food and Drug Administration announced that it has approved cefiderocol (Fetroja), an IV antibacterial drug to treat complicated urinary tract infections (cUTIs), including kidney infections, caused by multidrug-resistant gram-negative microorganisms in patients 18 years of age or older.
The safety and effectiveness of cefiderocol was demonstrated in a pivotal study of 448 patients with cUTIs. Published results indicated that 73% of patients had resolution of symptoms and eradication of the bacteria approximately 7 days after completing treatment, compared with 55% in patients who received an alternative antibiotic.
observed in comparison to patients treated with other antibiotics in a trial of critically ill patients having multidrug-resistant gram-negative bacterial infections (clinical trials. gov NCT02714595).
The cause of the increase in mortality has not been determined, according to the FDA. Some of the deaths in the study were attributable to worsening or complications of infection, or underlying comorbidities, in patients treated for hospital-acquired/ventilator-associated pneumonia (i.e., nosocomial pneumonia), bloodstream infections, or sepsis. Thus, safety and efficacy of cefiderocol has not been established for the treating these types of infections, according to the announcement.
Adverse reactions observed in patients treated with cefiderocol included diarrhea, constipation, nausea, vomiting, elevations in liver tests, rash, infusion-site reactions, and candidiasis. The FDA added that cefiderocol should not be used in persons known to have a severe hypersensitivity to beta-lactam antibacterial drugs.
“A key global challenge the FDA faces as a public health agency is addressing the threat of antimicrobial-resistant infections, like cUTIs. This approval represents another step forward in the FDA’s overall efforts to ensure safe and effective antimicrobial drugs are available to patients for treating infections,” John Farley, MD, acting director of the Office of Infectious Diseases in the FDA’s Center for Drug Evaluation and Research said in the FDA press statement.
Fetroja is a product of Shionogi.
The Food and Drug Administration announced that it has approved cefiderocol (Fetroja), an IV antibacterial drug to treat complicated urinary tract infections (cUTIs), including kidney infections, caused by multidrug-resistant gram-negative microorganisms in patients 18 years of age or older.
The safety and effectiveness of cefiderocol was demonstrated in a pivotal study of 448 patients with cUTIs. Published results indicated that 73% of patients had resolution of symptoms and eradication of the bacteria approximately 7 days after completing treatment, compared with 55% in patients who received an alternative antibiotic.
observed in comparison to patients treated with other antibiotics in a trial of critically ill patients having multidrug-resistant gram-negative bacterial infections (clinical trials. gov NCT02714595).
The cause of the increase in mortality has not been determined, according to the FDA. Some of the deaths in the study were attributable to worsening or complications of infection, or underlying comorbidities, in patients treated for hospital-acquired/ventilator-associated pneumonia (i.e., nosocomial pneumonia), bloodstream infections, or sepsis. Thus, safety and efficacy of cefiderocol has not been established for the treating these types of infections, according to the announcement.
Adverse reactions observed in patients treated with cefiderocol included diarrhea, constipation, nausea, vomiting, elevations in liver tests, rash, infusion-site reactions, and candidiasis. The FDA added that cefiderocol should not be used in persons known to have a severe hypersensitivity to beta-lactam antibacterial drugs.
“A key global challenge the FDA faces as a public health agency is addressing the threat of antimicrobial-resistant infections, like cUTIs. This approval represents another step forward in the FDA’s overall efforts to ensure safe and effective antimicrobial drugs are available to patients for treating infections,” John Farley, MD, acting director of the Office of Infectious Diseases in the FDA’s Center for Drug Evaluation and Research said in the FDA press statement.
Fetroja is a product of Shionogi.
The Food and Drug Administration announced that it has approved cefiderocol (Fetroja), an IV antibacterial drug to treat complicated urinary tract infections (cUTIs), including kidney infections, caused by multidrug-resistant gram-negative microorganisms in patients 18 years of age or older.
The safety and effectiveness of cefiderocol was demonstrated in a pivotal study of 448 patients with cUTIs. Published results indicated that 73% of patients had resolution of symptoms and eradication of the bacteria approximately 7 days after completing treatment, compared with 55% in patients who received an alternative antibiotic.
observed in comparison to patients treated with other antibiotics in a trial of critically ill patients having multidrug-resistant gram-negative bacterial infections (clinical trials. gov NCT02714595).
The cause of the increase in mortality has not been determined, according to the FDA. Some of the deaths in the study were attributable to worsening or complications of infection, or underlying comorbidities, in patients treated for hospital-acquired/ventilator-associated pneumonia (i.e., nosocomial pneumonia), bloodstream infections, or sepsis. Thus, safety and efficacy of cefiderocol has not been established for the treating these types of infections, according to the announcement.
Adverse reactions observed in patients treated with cefiderocol included diarrhea, constipation, nausea, vomiting, elevations in liver tests, rash, infusion-site reactions, and candidiasis. The FDA added that cefiderocol should not be used in persons known to have a severe hypersensitivity to beta-lactam antibacterial drugs.
“A key global challenge the FDA faces as a public health agency is addressing the threat of antimicrobial-resistant infections, like cUTIs. This approval represents another step forward in the FDA’s overall efforts to ensure safe and effective antimicrobial drugs are available to patients for treating infections,” John Farley, MD, acting director of the Office of Infectious Diseases in the FDA’s Center for Drug Evaluation and Research said in the FDA press statement.
Fetroja is a product of Shionogi.
FROM THE FDA
Opioid reduction works after minimally invasive gynecologic surgery
VANCOUVER – Two new randomized trials demonstrate that pain following minimally invasive gynecologic surgery can be successfully managed using reduced opioid prescriptions.
In each case, patients were randomized to receive higher or lower numbers of oxycodone tablets. In both trials, the lower amount was five 5-mg oxycodone tablets. The work should reassure surgeons who wish to change their prescribing patterns, but may worry about patient dissatisfaction, at least in the context of prolapse repair and benign minor gynecologic laparoscopy, which were the focus of the two studies.
The ob.gyn. literature cites rates of 4%-6% of persistent opioid use after surgery on opioid-naive patients, and that’s a risk that needs to be addressed. “If we look at this as a risk factor of our surgical process, this is much higher than any other risk in patients undergoing surgery, and it’s not something we routinely talk to patients about,” Kari Plewniak, MD, an ob.gyn. at Montefiore Medical Center, New York, said during her presentation on pain control during benign gynecologic laparoscopy at the meeting sponsored by AAGL.
The trials provide some welcome guidance. “They provide pretty concrete guidelines with strong evidence of safety, so this is really helpful,” said Sean Dowdy, MD, chair of gynecologic oncology at Mayo Clinic in Rochester, Minn., while speaking as a discussant for the presentations.
Emily Davidson, MD, and associates at the Cleveland Clinic conducted a single-institution, noninferiority trial of standard- versus reduced-prescription opioids in 116 women undergoing prolapse repair. Half were randomized to receive 28 tablets of 5 mg oxycodone (routine arm) and half were prescribed just 5 tablets (reduced arm). All patients also received multimodal pain therapy featuring acetaminophen and ibuprofen. The mean age of patients was 62 years, 91% were white, and 84% were post menopausal. The most common surgery was hysterectomy combined with native tissue repair (60.2%), followed by vaginal colpopexy (15.3%), hysteropexy (15.3%), and sacrocolpopexy (9.3%).
At their postsurgical visit, patients were asked about their satisfaction with their postoperative pain management; 93% in the reduced arm reported that they were very satisfied or somewhat satisfied, as did 93% in the routine arm, which met the standard for noninferiority with a 15% margin. About 15% of patients in the reduced arm used more opioids than originally prescribed, compared with 2% of patients in the routine arm (P less than .01). The reduced arm had an average of 4 unused opioid tablets, compared with 26 in the routine arm. On average, the reduced arm used one tablet, compared with three in the routine arm (P = .03).
The researchers suggested that clinicians should consider prescribing 5-10 tablets for most patients, and all patients should receive multimodal pain management.
The noninferiority nature of the design was welcome, according to Dr. Dowdy. “I think we need to do more noninferiority trial designs because it allows us to make more observations about other parts of the value equation, so if we have two interventions that are equivalent, we can pick the one that has the best patient experience and the lowest cost, so it simplifies a lot of our management.”
The other study, conducted at Montefiore Medical Center, set out to see if a similar regimen of 5 5-mg oxycodone tablets, combined with acetaminophen and ibuprofen, could adequately manage postoperative pain after minor benign gynecologic laparoscopy (excluding hysterectomy), compared with a 10-tablet regimen. All patients received 25 tablets of 600 mg ibuprofen (1 tablet every 6 hours or as needed), plus 50 tablets of 250 mg acetaminophen (1-2 tablets every 6 hours or as needed).
The median number of opioid tablets taken was 2.0 in the 5-tablet group and 2.5 in the 10-tablet group; 32% and 28% took no tablets, and 68% and 65% took three or fewer tablets in the respective groups. The median number of leftover opioid tablets was 3 in the 5-tablet group and 8 in the 10-tablet group, reported Dr. Plewniak.
The studies are a good first step, but more is needed, according to Dr. Dowdy. It’s important to begin looking at more-challenging patient groups, such as those who are not opioid naive, as well as patients taking buprenorphine. “That creates some unique challenges with postoperative pain management,” he said.
Dr. Dowdy, Dr. Davidson, and Dr. Plewniak have no relevant financial disclosures.*
* This article was updated 11/27/2019.
VANCOUVER – Two new randomized trials demonstrate that pain following minimally invasive gynecologic surgery can be successfully managed using reduced opioid prescriptions.
In each case, patients were randomized to receive higher or lower numbers of oxycodone tablets. In both trials, the lower amount was five 5-mg oxycodone tablets. The work should reassure surgeons who wish to change their prescribing patterns, but may worry about patient dissatisfaction, at least in the context of prolapse repair and benign minor gynecologic laparoscopy, which were the focus of the two studies.
The ob.gyn. literature cites rates of 4%-6% of persistent opioid use after surgery on opioid-naive patients, and that’s a risk that needs to be addressed. “If we look at this as a risk factor of our surgical process, this is much higher than any other risk in patients undergoing surgery, and it’s not something we routinely talk to patients about,” Kari Plewniak, MD, an ob.gyn. at Montefiore Medical Center, New York, said during her presentation on pain control during benign gynecologic laparoscopy at the meeting sponsored by AAGL.
The trials provide some welcome guidance. “They provide pretty concrete guidelines with strong evidence of safety, so this is really helpful,” said Sean Dowdy, MD, chair of gynecologic oncology at Mayo Clinic in Rochester, Minn., while speaking as a discussant for the presentations.
Emily Davidson, MD, and associates at the Cleveland Clinic conducted a single-institution, noninferiority trial of standard- versus reduced-prescription opioids in 116 women undergoing prolapse repair. Half were randomized to receive 28 tablets of 5 mg oxycodone (routine arm) and half were prescribed just 5 tablets (reduced arm). All patients also received multimodal pain therapy featuring acetaminophen and ibuprofen. The mean age of patients was 62 years, 91% were white, and 84% were post menopausal. The most common surgery was hysterectomy combined with native tissue repair (60.2%), followed by vaginal colpopexy (15.3%), hysteropexy (15.3%), and sacrocolpopexy (9.3%).
At their postsurgical visit, patients were asked about their satisfaction with their postoperative pain management; 93% in the reduced arm reported that they were very satisfied or somewhat satisfied, as did 93% in the routine arm, which met the standard for noninferiority with a 15% margin. About 15% of patients in the reduced arm used more opioids than originally prescribed, compared with 2% of patients in the routine arm (P less than .01). The reduced arm had an average of 4 unused opioid tablets, compared with 26 in the routine arm. On average, the reduced arm used one tablet, compared with three in the routine arm (P = .03).
The researchers suggested that clinicians should consider prescribing 5-10 tablets for most patients, and all patients should receive multimodal pain management.
The noninferiority nature of the design was welcome, according to Dr. Dowdy. “I think we need to do more noninferiority trial designs because it allows us to make more observations about other parts of the value equation, so if we have two interventions that are equivalent, we can pick the one that has the best patient experience and the lowest cost, so it simplifies a lot of our management.”
The other study, conducted at Montefiore Medical Center, set out to see if a similar regimen of 5 5-mg oxycodone tablets, combined with acetaminophen and ibuprofen, could adequately manage postoperative pain after minor benign gynecologic laparoscopy (excluding hysterectomy), compared with a 10-tablet regimen. All patients received 25 tablets of 600 mg ibuprofen (1 tablet every 6 hours or as needed), plus 50 tablets of 250 mg acetaminophen (1-2 tablets every 6 hours or as needed).
The median number of opioid tablets taken was 2.0 in the 5-tablet group and 2.5 in the 10-tablet group; 32% and 28% took no tablets, and 68% and 65% took three or fewer tablets in the respective groups. The median number of leftover opioid tablets was 3 in the 5-tablet group and 8 in the 10-tablet group, reported Dr. Plewniak.
The studies are a good first step, but more is needed, according to Dr. Dowdy. It’s important to begin looking at more-challenging patient groups, such as those who are not opioid naive, as well as patients taking buprenorphine. “That creates some unique challenges with postoperative pain management,” he said.
Dr. Dowdy, Dr. Davidson, and Dr. Plewniak have no relevant financial disclosures.*
* This article was updated 11/27/2019.
VANCOUVER – Two new randomized trials demonstrate that pain following minimally invasive gynecologic surgery can be successfully managed using reduced opioid prescriptions.
In each case, patients were randomized to receive higher or lower numbers of oxycodone tablets. In both trials, the lower amount was five 5-mg oxycodone tablets. The work should reassure surgeons who wish to change their prescribing patterns, but may worry about patient dissatisfaction, at least in the context of prolapse repair and benign minor gynecologic laparoscopy, which were the focus of the two studies.
The ob.gyn. literature cites rates of 4%-6% of persistent opioid use after surgery on opioid-naive patients, and that’s a risk that needs to be addressed. “If we look at this as a risk factor of our surgical process, this is much higher than any other risk in patients undergoing surgery, and it’s not something we routinely talk to patients about,” Kari Plewniak, MD, an ob.gyn. at Montefiore Medical Center, New York, said during her presentation on pain control during benign gynecologic laparoscopy at the meeting sponsored by AAGL.
The trials provide some welcome guidance. “They provide pretty concrete guidelines with strong evidence of safety, so this is really helpful,” said Sean Dowdy, MD, chair of gynecologic oncology at Mayo Clinic in Rochester, Minn., while speaking as a discussant for the presentations.
Emily Davidson, MD, and associates at the Cleveland Clinic conducted a single-institution, noninferiority trial of standard- versus reduced-prescription opioids in 116 women undergoing prolapse repair. Half were randomized to receive 28 tablets of 5 mg oxycodone (routine arm) and half were prescribed just 5 tablets (reduced arm). All patients also received multimodal pain therapy featuring acetaminophen and ibuprofen. The mean age of patients was 62 years, 91% were white, and 84% were post menopausal. The most common surgery was hysterectomy combined with native tissue repair (60.2%), followed by vaginal colpopexy (15.3%), hysteropexy (15.3%), and sacrocolpopexy (9.3%).
At their postsurgical visit, patients were asked about their satisfaction with their postoperative pain management; 93% in the reduced arm reported that they were very satisfied or somewhat satisfied, as did 93% in the routine arm, which met the standard for noninferiority with a 15% margin. About 15% of patients in the reduced arm used more opioids than originally prescribed, compared with 2% of patients in the routine arm (P less than .01). The reduced arm had an average of 4 unused opioid tablets, compared with 26 in the routine arm. On average, the reduced arm used one tablet, compared with three in the routine arm (P = .03).
The researchers suggested that clinicians should consider prescribing 5-10 tablets for most patients, and all patients should receive multimodal pain management.
The noninferiority nature of the design was welcome, according to Dr. Dowdy. “I think we need to do more noninferiority trial designs because it allows us to make more observations about other parts of the value equation, so if we have two interventions that are equivalent, we can pick the one that has the best patient experience and the lowest cost, so it simplifies a lot of our management.”
The other study, conducted at Montefiore Medical Center, set out to see if a similar regimen of 5 5-mg oxycodone tablets, combined with acetaminophen and ibuprofen, could adequately manage postoperative pain after minor benign gynecologic laparoscopy (excluding hysterectomy), compared with a 10-tablet regimen. All patients received 25 tablets of 600 mg ibuprofen (1 tablet every 6 hours or as needed), plus 50 tablets of 250 mg acetaminophen (1-2 tablets every 6 hours or as needed).
The median number of opioid tablets taken was 2.0 in the 5-tablet group and 2.5 in the 10-tablet group; 32% and 28% took no tablets, and 68% and 65% took three or fewer tablets in the respective groups. The median number of leftover opioid tablets was 3 in the 5-tablet group and 8 in the 10-tablet group, reported Dr. Plewniak.
The studies are a good first step, but more is needed, according to Dr. Dowdy. It’s important to begin looking at more-challenging patient groups, such as those who are not opioid naive, as well as patients taking buprenorphine. “That creates some unique challenges with postoperative pain management,” he said.
Dr. Dowdy, Dr. Davidson, and Dr. Plewniak have no relevant financial disclosures.*
* This article was updated 11/27/2019.
REPORTING FROM THE AAGL GLOBAL CONGRESS
Surgical staging improves cervical cancer outcomes
VANCOUVER – Follow-up oncologic data from the UTERUS-11 trial shows advantages to surgical staging over clinical staging in stage IIB-IVA cervical cancer, with little apparent risk.
Compared with clinical staging using CT, laparoscopic staging led to an improvement in cancer-specific survival, with no delays in treatment or increases in toxicity. It also prompted surgical up-staging and led to treatment changes in 33% of cases. There was no difference in overall survival, but progression-free survival trended towards better outcomes in the surgical-staging group.
The new study presents 5-year follow-up data from patients randomly assigned to surgical (n = 121) or clinical staging (n = 114). The original study, published in 2017 (Oncology. 2017;92[4]:213-20), reported that 33% of surgical-staging patients in the surgical staging were up-staged as a result, compared with 6% who were revealed to have positive paraaortic lymph nodes through a CT-guided core biopsy after suspicious CT results. After a median follow-up of 90 months in both arms, overall survival was similar between the two groups, and progression-free survival trended towards an improvement in the surgical-staging group (P = .088). Cancer-specific survival was better in the surgical-staging arm, compared with clinical staging (P=.028), Audrey Tsunoda, MD, PhD, reported.
Surgical staging didn’t impact the toxicity profile, said Dr. Tsunoda, a surgical oncologist focused in gynecologic cancer surgery who practices at Hospital Erasto Gaertner in Curitiba, Brazil.
The mean time to initiation of chemoradiotherapy following surgery was 14 days (range, 7-21 days) after surgery: 64% had intensity-modulated radiotherapy and 36% had three-dimensional radiotherapy. There were no grade 5 toxicities during chemoradiotherapy and both groups had similar gastrointestinal and genitourinary toxicity profiles. About 97% of the surgical staging procedures were conducted laparoscopically. Two patients had a blood loss of more than 500 cc, and two had a delay to primary chemoradiotherapy (4 days and 5 days). One patient had to be converted to an open approach because of obesity and severe adhesions, and there was no intraoperative mortality.
Previous retrospective studies examining surgical staging in these patients led to confusion and disagreements among guidelines. Surgical staging is clearly associated with increased up-staging, but the oncologic benefit is uncertain. The LiLACS study attempted to address the question with prospective data, but failed to accrue enough patients and was later abandoned. That leaves the UTERUS-11 study, the initial results of which were published in 2017, as the first prospective study to examine the benefit of surgical staging.
The new follow-up results suggest a benefit to surgical staging, but they leave an important question unanswered, according to Lois Ramondetta, MD, professor of gynecologic oncology at the University of Texas MD Anderson Cancer Center, Houston, who served as a discussant at the meeting sponsored by AAGL. “Paraaortic lymph node status does connect to clinical benefit, but the question is really [whether] the removal of the lymph nodes accounts for the benefit, or is the identification of them and the change in treatment plan responsible? [If the latter is the case], a PET scan would have done a better job,” said Dr. Ramondetta. “The question remains unanswered, but I think this was huge progress in trying to answer it. Future studies need to incorporate a PET scan.”
Dr. Tsunoda has received honoraria from AstraZeneca and Roche. Dr. Ramondetta has no relevant financial disclosures.
VANCOUVER – Follow-up oncologic data from the UTERUS-11 trial shows advantages to surgical staging over clinical staging in stage IIB-IVA cervical cancer, with little apparent risk.
Compared with clinical staging using CT, laparoscopic staging led to an improvement in cancer-specific survival, with no delays in treatment or increases in toxicity. It also prompted surgical up-staging and led to treatment changes in 33% of cases. There was no difference in overall survival, but progression-free survival trended towards better outcomes in the surgical-staging group.
The new study presents 5-year follow-up data from patients randomly assigned to surgical (n = 121) or clinical staging (n = 114). The original study, published in 2017 (Oncology. 2017;92[4]:213-20), reported that 33% of surgical-staging patients in the surgical staging were up-staged as a result, compared with 6% who were revealed to have positive paraaortic lymph nodes through a CT-guided core biopsy after suspicious CT results. After a median follow-up of 90 months in both arms, overall survival was similar between the two groups, and progression-free survival trended towards an improvement in the surgical-staging group (P = .088). Cancer-specific survival was better in the surgical-staging arm, compared with clinical staging (P=.028), Audrey Tsunoda, MD, PhD, reported.
Surgical staging didn’t impact the toxicity profile, said Dr. Tsunoda, a surgical oncologist focused in gynecologic cancer surgery who practices at Hospital Erasto Gaertner in Curitiba, Brazil.
The mean time to initiation of chemoradiotherapy following surgery was 14 days (range, 7-21 days) after surgery: 64% had intensity-modulated radiotherapy and 36% had three-dimensional radiotherapy. There were no grade 5 toxicities during chemoradiotherapy and both groups had similar gastrointestinal and genitourinary toxicity profiles. About 97% of the surgical staging procedures were conducted laparoscopically. Two patients had a blood loss of more than 500 cc, and two had a delay to primary chemoradiotherapy (4 days and 5 days). One patient had to be converted to an open approach because of obesity and severe adhesions, and there was no intraoperative mortality.
Previous retrospective studies examining surgical staging in these patients led to confusion and disagreements among guidelines. Surgical staging is clearly associated with increased up-staging, but the oncologic benefit is uncertain. The LiLACS study attempted to address the question with prospective data, but failed to accrue enough patients and was later abandoned. That leaves the UTERUS-11 study, the initial results of which were published in 2017, as the first prospective study to examine the benefit of surgical staging.
The new follow-up results suggest a benefit to surgical staging, but they leave an important question unanswered, according to Lois Ramondetta, MD, professor of gynecologic oncology at the University of Texas MD Anderson Cancer Center, Houston, who served as a discussant at the meeting sponsored by AAGL. “Paraaortic lymph node status does connect to clinical benefit, but the question is really [whether] the removal of the lymph nodes accounts for the benefit, or is the identification of them and the change in treatment plan responsible? [If the latter is the case], a PET scan would have done a better job,” said Dr. Ramondetta. “The question remains unanswered, but I think this was huge progress in trying to answer it. Future studies need to incorporate a PET scan.”
Dr. Tsunoda has received honoraria from AstraZeneca and Roche. Dr. Ramondetta has no relevant financial disclosures.
VANCOUVER – Follow-up oncologic data from the UTERUS-11 trial shows advantages to surgical staging over clinical staging in stage IIB-IVA cervical cancer, with little apparent risk.
Compared with clinical staging using CT, laparoscopic staging led to an improvement in cancer-specific survival, with no delays in treatment or increases in toxicity. It also prompted surgical up-staging and led to treatment changes in 33% of cases. There was no difference in overall survival, but progression-free survival trended towards better outcomes in the surgical-staging group.
The new study presents 5-year follow-up data from patients randomly assigned to surgical (n = 121) or clinical staging (n = 114). The original study, published in 2017 (Oncology. 2017;92[4]:213-20), reported that 33% of surgical-staging patients in the surgical staging were up-staged as a result, compared with 6% who were revealed to have positive paraaortic lymph nodes through a CT-guided core biopsy after suspicious CT results. After a median follow-up of 90 months in both arms, overall survival was similar between the two groups, and progression-free survival trended towards an improvement in the surgical-staging group (P = .088). Cancer-specific survival was better in the surgical-staging arm, compared with clinical staging (P=.028), Audrey Tsunoda, MD, PhD, reported.
Surgical staging didn’t impact the toxicity profile, said Dr. Tsunoda, a surgical oncologist focused in gynecologic cancer surgery who practices at Hospital Erasto Gaertner in Curitiba, Brazil.
The mean time to initiation of chemoradiotherapy following surgery was 14 days (range, 7-21 days) after surgery: 64% had intensity-modulated radiotherapy and 36% had three-dimensional radiotherapy. There were no grade 5 toxicities during chemoradiotherapy and both groups had similar gastrointestinal and genitourinary toxicity profiles. About 97% of the surgical staging procedures were conducted laparoscopically. Two patients had a blood loss of more than 500 cc, and two had a delay to primary chemoradiotherapy (4 days and 5 days). One patient had to be converted to an open approach because of obesity and severe adhesions, and there was no intraoperative mortality.
Previous retrospective studies examining surgical staging in these patients led to confusion and disagreements among guidelines. Surgical staging is clearly associated with increased up-staging, but the oncologic benefit is uncertain. The LiLACS study attempted to address the question with prospective data, but failed to accrue enough patients and was later abandoned. That leaves the UTERUS-11 study, the initial results of which were published in 2017, as the first prospective study to examine the benefit of surgical staging.
The new follow-up results suggest a benefit to surgical staging, but they leave an important question unanswered, according to Lois Ramondetta, MD, professor of gynecologic oncology at the University of Texas MD Anderson Cancer Center, Houston, who served as a discussant at the meeting sponsored by AAGL. “Paraaortic lymph node status does connect to clinical benefit, but the question is really [whether] the removal of the lymph nodes accounts for the benefit, or is the identification of them and the change in treatment plan responsible? [If the latter is the case], a PET scan would have done a better job,” said Dr. Ramondetta. “The question remains unanswered, but I think this was huge progress in trying to answer it. Future studies need to incorporate a PET scan.”
Dr. Tsunoda has received honoraria from AstraZeneca and Roche. Dr. Ramondetta has no relevant financial disclosures.
REPORTING FROM THE AAGL GLOBAL CONGRESS
Does tranexamic acid reduce mortality in women with postpartum hemorrhage?
EVIDENCE SUMMARY
A 2017 double-blind RCT that included 20,060 women with PPH from 21 countries (the WOMAN trial) found that the risk of maternal mortality was significantly lower among women who received tranexamic acid as part of their PPH treatment compared with placebo (1.5% [N = 155] vs 1.9% [N = 191]; P = .045; relative risk [RR] = 0.81; 95% confidence interval [CI], 0.65-1; number needed to treat [NNT] = 250).1
Inclusion criteria were age 16 years or older, postpartum course complicated by hemorrhage of known or unknown etiology, and a case in which the clinician considered using tranexamic acid in addition to the standard of care. PPH was defined as > 500 mL blood loss after vaginal delivery, > 1000 mL blood loss after cesarean section, or blood loss sufficient to produce hemodynamic compromise.
Researchers randomized 10,051 women to the tranexamic acid group and 10,009 to the placebo group. Women in the experimental group received a 1-g IV injection of tranexamic acid over 10 to 20 minutes. A second dose was given if bleeding restarted after 30 minutes and within 24 hours of the first dose.
To reduce mortality give tranexamic acid promptly
Tranexamic acid reduced mortality most effectively compared with placebo when given within 3 hours of delivery (1.2% [N = 89] vs 1.7% [N = 127]; P = .008; RR = 0.69; 95% CI 0.52-0.91; NNT = 200). After 3 hours, no significant decrease in mortality occurred. No significant difference in effect was noted between vaginal and cesarean deliveries nor between uterine atony as the primary cause of hemorrhage and other causes.
Administering tranexamic acid didn’t reduce the composite primary endpoint of hysterectomy or death from all causes. Nor did it reduce the secondary endpoints of intrauterine tamponade, embolization, manual placental extraction, arterial ligation, blood transfusions, or number of units of packed red blood cells. The tranexamic acid group showed a significant decrease in cases of laparotomy for PPH (0.8% vs 1.3%; P = .002; RR = 0.64; 95% CI, 0.49-0.85; NNT = 200).
Women who received tranexamic acid vs placebo showed no significant difference in mortality from pulmonary embolism (0.1% [N = 10] vs 0.1% [N = 11]; P = .82; RR = .9; 95% CI, 0.38-2.13), organ failure ure (0.3% [N = 25] vs 0.2% [N = 18]; P = .29; RR = 1.38; 95% CI, 0.75-2.53), sepsis (0.2% [N = 15] vs 0.1% [N = 8]; P = .15; RR = 1.87; 95% CI, 0.79-4.4), eclampsia (0.02% [N = 2] vs 0.1% [N = 8]; P = .057; RR = .25; 95% CI, 0.05-1.17), or other causes (0.2% [N = 20] vs 0.2% [N = 20]; P = .99; RR = 0.99; 95% CI, 0.54-1.85).
Tranexamic acid doesn’t increase the risk of thromboembolism
A 2018 Cochrane review sought more broadly to determine the general effectiveness and safety of antifibrinolytic drugs in treating primary PPH.2 Of 15 RCTs identified, only 3 met the inclusion criteria for the review, 1 of which was the WOMAN trial (which contributed most of the data in the review). The other trials were a study conducted in France that recruited 152 women and a study of 200 women in Iran that contributed only 1 primary outcome—estimated blood loss—to the review. The former study didn’t report any maternal deaths, and the latter study didn’t look at maternal deaths. The Cochrane review concluded, based on data from the WOMAN trial, that IV tranexamic acid, if given as early as possible, reduced mortality from bleeding in women with primary PPH after both vaginal and cesarean delivery and didn’t increase the risk of thromboembolic events.2
Continue to: RECOMMENDATIONS
RECOMMENDATIONS
The newest practice guidelines on the management of postpartum hemorrhage published by the American College of Obstetricians and Gynecologists recommends considering tranexamic acid as an additional agent in managing PPH when initial standard-of-care treatments fail.3
Editor’s takeaway
The large international double-blind, randomized placebo-controlled trial provides convincing evidence that tranexamic acid should be administered readily in cases of PPH.
1. WOMAN Trial Collaborators. Effect of early tranexamic acid administration on mortality, hysterectomy, and other morbidities in women with post-partum haemorrhage (WOMAN): an international, randomised, double-blind, placebo-controlled trial. Lancet. 2017;389:2105–2116.
2. Shakur H, Beaumont D, Pavord S, et al. Antifibrinolytic drugs for treating primary postpartum haemorrhage. Cochrane Database Syst Rev. 2018;2:CD012964.
3. Committee on Practice Bulletins-Obstetrics (American College of Obstetricians and Gynecologists). Practice Bulletin No. 183: Postpartum Hemorrhage. Obstet Gynecol. 2017;130:e168-e186.
EVIDENCE SUMMARY
A 2017 double-blind RCT that included 20,060 women with PPH from 21 countries (the WOMAN trial) found that the risk of maternal mortality was significantly lower among women who received tranexamic acid as part of their PPH treatment compared with placebo (1.5% [N = 155] vs 1.9% [N = 191]; P = .045; relative risk [RR] = 0.81; 95% confidence interval [CI], 0.65-1; number needed to treat [NNT] = 250).1
Inclusion criteria were age 16 years or older, postpartum course complicated by hemorrhage of known or unknown etiology, and a case in which the clinician considered using tranexamic acid in addition to the standard of care. PPH was defined as > 500 mL blood loss after vaginal delivery, > 1000 mL blood loss after cesarean section, or blood loss sufficient to produce hemodynamic compromise.
Researchers randomized 10,051 women to the tranexamic acid group and 10,009 to the placebo group. Women in the experimental group received a 1-g IV injection of tranexamic acid over 10 to 20 minutes. A second dose was given if bleeding restarted after 30 minutes and within 24 hours of the first dose.
To reduce mortality give tranexamic acid promptly
Tranexamic acid reduced mortality most effectively compared with placebo when given within 3 hours of delivery (1.2% [N = 89] vs 1.7% [N = 127]; P = .008; RR = 0.69; 95% CI 0.52-0.91; NNT = 200). After 3 hours, no significant decrease in mortality occurred. No significant difference in effect was noted between vaginal and cesarean deliveries nor between uterine atony as the primary cause of hemorrhage and other causes.
Administering tranexamic acid didn’t reduce the composite primary endpoint of hysterectomy or death from all causes. Nor did it reduce the secondary endpoints of intrauterine tamponade, embolization, manual placental extraction, arterial ligation, blood transfusions, or number of units of packed red blood cells. The tranexamic acid group showed a significant decrease in cases of laparotomy for PPH (0.8% vs 1.3%; P = .002; RR = 0.64; 95% CI, 0.49-0.85; NNT = 200).
Women who received tranexamic acid vs placebo showed no significant difference in mortality from pulmonary embolism (0.1% [N = 10] vs 0.1% [N = 11]; P = .82; RR = .9; 95% CI, 0.38-2.13), organ failure ure (0.3% [N = 25] vs 0.2% [N = 18]; P = .29; RR = 1.38; 95% CI, 0.75-2.53), sepsis (0.2% [N = 15] vs 0.1% [N = 8]; P = .15; RR = 1.87; 95% CI, 0.79-4.4), eclampsia (0.02% [N = 2] vs 0.1% [N = 8]; P = .057; RR = .25; 95% CI, 0.05-1.17), or other causes (0.2% [N = 20] vs 0.2% [N = 20]; P = .99; RR = 0.99; 95% CI, 0.54-1.85).
Tranexamic acid doesn’t increase the risk of thromboembolism
A 2018 Cochrane review sought more broadly to determine the general effectiveness and safety of antifibrinolytic drugs in treating primary PPH.2 Of 15 RCTs identified, only 3 met the inclusion criteria for the review, 1 of which was the WOMAN trial (which contributed most of the data in the review). The other trials were a study conducted in France that recruited 152 women and a study of 200 women in Iran that contributed only 1 primary outcome—estimated blood loss—to the review. The former study didn’t report any maternal deaths, and the latter study didn’t look at maternal deaths. The Cochrane review concluded, based on data from the WOMAN trial, that IV tranexamic acid, if given as early as possible, reduced mortality from bleeding in women with primary PPH after both vaginal and cesarean delivery and didn’t increase the risk of thromboembolic events.2
Continue to: RECOMMENDATIONS
RECOMMENDATIONS
The newest practice guidelines on the management of postpartum hemorrhage published by the American College of Obstetricians and Gynecologists recommends considering tranexamic acid as an additional agent in managing PPH when initial standard-of-care treatments fail.3
Editor’s takeaway
The large international double-blind, randomized placebo-controlled trial provides convincing evidence that tranexamic acid should be administered readily in cases of PPH.
EVIDENCE SUMMARY
A 2017 double-blind RCT that included 20,060 women with PPH from 21 countries (the WOMAN trial) found that the risk of maternal mortality was significantly lower among women who received tranexamic acid as part of their PPH treatment compared with placebo (1.5% [N = 155] vs 1.9% [N = 191]; P = .045; relative risk [RR] = 0.81; 95% confidence interval [CI], 0.65-1; number needed to treat [NNT] = 250).1
Inclusion criteria were age 16 years or older, postpartum course complicated by hemorrhage of known or unknown etiology, and a case in which the clinician considered using tranexamic acid in addition to the standard of care. PPH was defined as > 500 mL blood loss after vaginal delivery, > 1000 mL blood loss after cesarean section, or blood loss sufficient to produce hemodynamic compromise.
Researchers randomized 10,051 women to the tranexamic acid group and 10,009 to the placebo group. Women in the experimental group received a 1-g IV injection of tranexamic acid over 10 to 20 minutes. A second dose was given if bleeding restarted after 30 minutes and within 24 hours of the first dose.
To reduce mortality give tranexamic acid promptly
Tranexamic acid reduced mortality most effectively compared with placebo when given within 3 hours of delivery (1.2% [N = 89] vs 1.7% [N = 127]; P = .008; RR = 0.69; 95% CI 0.52-0.91; NNT = 200). After 3 hours, no significant decrease in mortality occurred. No significant difference in effect was noted between vaginal and cesarean deliveries nor between uterine atony as the primary cause of hemorrhage and other causes.
Administering tranexamic acid didn’t reduce the composite primary endpoint of hysterectomy or death from all causes. Nor did it reduce the secondary endpoints of intrauterine tamponade, embolization, manual placental extraction, arterial ligation, blood transfusions, or number of units of packed red blood cells. The tranexamic acid group showed a significant decrease in cases of laparotomy for PPH (0.8% vs 1.3%; P = .002; RR = 0.64; 95% CI, 0.49-0.85; NNT = 200).
Women who received tranexamic acid vs placebo showed no significant difference in mortality from pulmonary embolism (0.1% [N = 10] vs 0.1% [N = 11]; P = .82; RR = .9; 95% CI, 0.38-2.13), organ failure ure (0.3% [N = 25] vs 0.2% [N = 18]; P = .29; RR = 1.38; 95% CI, 0.75-2.53), sepsis (0.2% [N = 15] vs 0.1% [N = 8]; P = .15; RR = 1.87; 95% CI, 0.79-4.4), eclampsia (0.02% [N = 2] vs 0.1% [N = 8]; P = .057; RR = .25; 95% CI, 0.05-1.17), or other causes (0.2% [N = 20] vs 0.2% [N = 20]; P = .99; RR = 0.99; 95% CI, 0.54-1.85).
Tranexamic acid doesn’t increase the risk of thromboembolism
A 2018 Cochrane review sought more broadly to determine the general effectiveness and safety of antifibrinolytic drugs in treating primary PPH.2 Of 15 RCTs identified, only 3 met the inclusion criteria for the review, 1 of which was the WOMAN trial (which contributed most of the data in the review). The other trials were a study conducted in France that recruited 152 women and a study of 200 women in Iran that contributed only 1 primary outcome—estimated blood loss—to the review. The former study didn’t report any maternal deaths, and the latter study didn’t look at maternal deaths. The Cochrane review concluded, based on data from the WOMAN trial, that IV tranexamic acid, if given as early as possible, reduced mortality from bleeding in women with primary PPH after both vaginal and cesarean delivery and didn’t increase the risk of thromboembolic events.2
Continue to: RECOMMENDATIONS
RECOMMENDATIONS
The newest practice guidelines on the management of postpartum hemorrhage published by the American College of Obstetricians and Gynecologists recommends considering tranexamic acid as an additional agent in managing PPH when initial standard-of-care treatments fail.3
Editor’s takeaway
The large international double-blind, randomized placebo-controlled trial provides convincing evidence that tranexamic acid should be administered readily in cases of PPH.
1. WOMAN Trial Collaborators. Effect of early tranexamic acid administration on mortality, hysterectomy, and other morbidities in women with post-partum haemorrhage (WOMAN): an international, randomised, double-blind, placebo-controlled trial. Lancet. 2017;389:2105–2116.
2. Shakur H, Beaumont D, Pavord S, et al. Antifibrinolytic drugs for treating primary postpartum haemorrhage. Cochrane Database Syst Rev. 2018;2:CD012964.
3. Committee on Practice Bulletins-Obstetrics (American College of Obstetricians and Gynecologists). Practice Bulletin No. 183: Postpartum Hemorrhage. Obstet Gynecol. 2017;130:e168-e186.
1. WOMAN Trial Collaborators. Effect of early tranexamic acid administration on mortality, hysterectomy, and other morbidities in women with post-partum haemorrhage (WOMAN): an international, randomised, double-blind, placebo-controlled trial. Lancet. 2017;389:2105–2116.
2. Shakur H, Beaumont D, Pavord S, et al. Antifibrinolytic drugs for treating primary postpartum haemorrhage. Cochrane Database Syst Rev. 2018;2:CD012964.
3. Committee on Practice Bulletins-Obstetrics (American College of Obstetricians and Gynecologists). Practice Bulletin No. 183: Postpartum Hemorrhage. Obstet Gynecol. 2017;130:e168-e186.
EVIDENCE-BASED ANSWER:
Yes. When used in conjunction with the standard of care, 1 g intravenous (IV) tranexamic acid given 1 to 3 hours after delivery is associated with a significant reduction in maternal mortality from postpartum hemorrhage (PPH) (strength of recommendation: A, randomized controlled trial [RCT] and Cochrane review).
No known significant risks are associated with the use of tranexamic acid to treat PPH.
Multidisciplinary care could address fertility preservation in transgender youth
PHILADELPHIA – A multidisciplinary approach is needed to care for gender-diverse transgender adolescents interested in fertility preservation, Leena Nahata, MD, said at the annual meeting of the American Society for Reproductive Medicine.
especially in the absence of longitudinal data, said Dr. Nahata, medical director of the fertility and reproductive health program at Nationwide Children’s Hospital, Columbus, Ohio. “We’re trying to counsel these youth and their parents about long-term outcomes of hormone therapies. However, despite the lack of data, not treating them also is not a viable option.”
Another concern among transgender individuals, Dr. Nahata said, is a high risk of mental health issues. Approximately one-third of transgender individuals experience depression, and between one-third and one-half have suicidal ideation or attempted suicide.
“It’s important to realize that these risks are not inevitable,” she said. Support from parents, peers, and social groups; engaging with the health care system; and having access to puberty suppression, gender-affirming hormones, and surgery are protective outcomes for mental health concerns. “It’s because of this that so many of us feel obligated to move on with treatments even in a setting of a lack of data.”
According to 2017 guidelines from the Endocrine Society on gender-dysphoric and gender-incongruent persons, patients can begin gonadotropin-releasing hormone (GnRH) agonists at Tanner Stage 2 of puberty (J Clin Endocrinol Metab. 2017 Nov. doi: 10.1210/jc.2017-01658). Before starting treatment, a mental health provider should confirm gender dysphoria or incongruence, and determine whether the patient has “sufficient mental capacity” to understand the long-term consequences of treatment with gender-affirming hormones such as estrogen and testosterone because the effects are partially irreversible, including a potential loss of fertility. Most pediatric patients will have this ability by 16 years old, but some programs across the country begin treatment between 13.5 years and 14 years of age, said Dr. Nahata. One consideration of beginning GnRH agonists and then moving directly to gender-affirming hormone therapy, there may not be an opportunity to explore fertility preservation.
Dr. Nahata acknowledged the data for the long-term effects of testosterone and estrogen on fertility is “murky,” but despite a lack of data, the American Society for Reproductive Medicine released an ethics statement in 2015 affirming that transgender patients “have the same interests as other persons in having children and in accessing fertility services for fertility preservation and reproduction” and pediatric providers “should offer fertility preservation options to individuals before gender transition” (Fertil Steril. 2015 Sep 9. doi: 10.1016/j.fertnstert.2015.08.021).
There also is mixed evidence that transgender individuals take advantage of fertility preservation services, whether offered or not. Two studies from Belgium that surveyed transgender individuals on parenthood preferences found 54% of adult trans men had a desire for children and that 38% of adult trans men and 51% of adult trans women would consider fertility preservation if it was an option. However, Dr. Nahata said a retrospective study from her own group of 50 adolescent trans males and 23 adolescent trans females found 99% of the cohort was counseled on fertility preservation, but only 3% (2 patients) attempted fertility preservation, and both were trans females (J Adolesc Health. 2017 Jul. doi: 10.1016/j.jadohealth.2016.12.012).
Another study examining use of fertility preservation in trans females in the Netherlands by Brik et al. found a much higher use of fertility preservation, with 38% of patients attempting cryopreservation after counseling (J Adolesc Health. 2019 May. doi: 10.1016/j.jadohealth.2018.11.008). “It’s unclear whether this is a regional difference or whether things are actually shifting over a short period of time,” said Dr. Nahata.
Attitudes about fertility preservation among gender-diverse transgender youth also impact its use in this patient population. A survey of transgender youth found less than 40% preferred adoption to biological parenthood, but said their feelings might change as time passes. However, more than half wanted more information on their family-building options. For other transgender youth aged 12-19 years, having children was their “lowest life priority,” compared with having friends, their health, and other issues in their lives, said Dr. Nahata.
In a 24-item survey Dr. Nahata and her team administered to 44 trans nonbinary adolescents, the most common reasons for not seeking fertility preservation were feelings of being too young, not wanting to be a parent or have a biological child, not wanting to delay treatment, and not being able to afford the cost of fertility preservation.
“This just speaks to the complexities of counseling in this population, and the importance of having a multidisciplinary team to see these youth and families to do more comprehensive counseling,” she said.
Dr. Nahata reported no relevant conflicts of interest.
PHILADELPHIA – A multidisciplinary approach is needed to care for gender-diverse transgender adolescents interested in fertility preservation, Leena Nahata, MD, said at the annual meeting of the American Society for Reproductive Medicine.
especially in the absence of longitudinal data, said Dr. Nahata, medical director of the fertility and reproductive health program at Nationwide Children’s Hospital, Columbus, Ohio. “We’re trying to counsel these youth and their parents about long-term outcomes of hormone therapies. However, despite the lack of data, not treating them also is not a viable option.”
Another concern among transgender individuals, Dr. Nahata said, is a high risk of mental health issues. Approximately one-third of transgender individuals experience depression, and between one-third and one-half have suicidal ideation or attempted suicide.
“It’s important to realize that these risks are not inevitable,” she said. Support from parents, peers, and social groups; engaging with the health care system; and having access to puberty suppression, gender-affirming hormones, and surgery are protective outcomes for mental health concerns. “It’s because of this that so many of us feel obligated to move on with treatments even in a setting of a lack of data.”
According to 2017 guidelines from the Endocrine Society on gender-dysphoric and gender-incongruent persons, patients can begin gonadotropin-releasing hormone (GnRH) agonists at Tanner Stage 2 of puberty (J Clin Endocrinol Metab. 2017 Nov. doi: 10.1210/jc.2017-01658). Before starting treatment, a mental health provider should confirm gender dysphoria or incongruence, and determine whether the patient has “sufficient mental capacity” to understand the long-term consequences of treatment with gender-affirming hormones such as estrogen and testosterone because the effects are partially irreversible, including a potential loss of fertility. Most pediatric patients will have this ability by 16 years old, but some programs across the country begin treatment between 13.5 years and 14 years of age, said Dr. Nahata. One consideration of beginning GnRH agonists and then moving directly to gender-affirming hormone therapy, there may not be an opportunity to explore fertility preservation.
Dr. Nahata acknowledged the data for the long-term effects of testosterone and estrogen on fertility is “murky,” but despite a lack of data, the American Society for Reproductive Medicine released an ethics statement in 2015 affirming that transgender patients “have the same interests as other persons in having children and in accessing fertility services for fertility preservation and reproduction” and pediatric providers “should offer fertility preservation options to individuals before gender transition” (Fertil Steril. 2015 Sep 9. doi: 10.1016/j.fertnstert.2015.08.021).
There also is mixed evidence that transgender individuals take advantage of fertility preservation services, whether offered or not. Two studies from Belgium that surveyed transgender individuals on parenthood preferences found 54% of adult trans men had a desire for children and that 38% of adult trans men and 51% of adult trans women would consider fertility preservation if it was an option. However, Dr. Nahata said a retrospective study from her own group of 50 adolescent trans males and 23 adolescent trans females found 99% of the cohort was counseled on fertility preservation, but only 3% (2 patients) attempted fertility preservation, and both were trans females (J Adolesc Health. 2017 Jul. doi: 10.1016/j.jadohealth.2016.12.012).
Another study examining use of fertility preservation in trans females in the Netherlands by Brik et al. found a much higher use of fertility preservation, with 38% of patients attempting cryopreservation after counseling (J Adolesc Health. 2019 May. doi: 10.1016/j.jadohealth.2018.11.008). “It’s unclear whether this is a regional difference or whether things are actually shifting over a short period of time,” said Dr. Nahata.
Attitudes about fertility preservation among gender-diverse transgender youth also impact its use in this patient population. A survey of transgender youth found less than 40% preferred adoption to biological parenthood, but said their feelings might change as time passes. However, more than half wanted more information on their family-building options. For other transgender youth aged 12-19 years, having children was their “lowest life priority,” compared with having friends, their health, and other issues in their lives, said Dr. Nahata.
In a 24-item survey Dr. Nahata and her team administered to 44 trans nonbinary adolescents, the most common reasons for not seeking fertility preservation were feelings of being too young, not wanting to be a parent or have a biological child, not wanting to delay treatment, and not being able to afford the cost of fertility preservation.
“This just speaks to the complexities of counseling in this population, and the importance of having a multidisciplinary team to see these youth and families to do more comprehensive counseling,” she said.
Dr. Nahata reported no relevant conflicts of interest.
PHILADELPHIA – A multidisciplinary approach is needed to care for gender-diverse transgender adolescents interested in fertility preservation, Leena Nahata, MD, said at the annual meeting of the American Society for Reproductive Medicine.
especially in the absence of longitudinal data, said Dr. Nahata, medical director of the fertility and reproductive health program at Nationwide Children’s Hospital, Columbus, Ohio. “We’re trying to counsel these youth and their parents about long-term outcomes of hormone therapies. However, despite the lack of data, not treating them also is not a viable option.”
Another concern among transgender individuals, Dr. Nahata said, is a high risk of mental health issues. Approximately one-third of transgender individuals experience depression, and between one-third and one-half have suicidal ideation or attempted suicide.
“It’s important to realize that these risks are not inevitable,” she said. Support from parents, peers, and social groups; engaging with the health care system; and having access to puberty suppression, gender-affirming hormones, and surgery are protective outcomes for mental health concerns. “It’s because of this that so many of us feel obligated to move on with treatments even in a setting of a lack of data.”
According to 2017 guidelines from the Endocrine Society on gender-dysphoric and gender-incongruent persons, patients can begin gonadotropin-releasing hormone (GnRH) agonists at Tanner Stage 2 of puberty (J Clin Endocrinol Metab. 2017 Nov. doi: 10.1210/jc.2017-01658). Before starting treatment, a mental health provider should confirm gender dysphoria or incongruence, and determine whether the patient has “sufficient mental capacity” to understand the long-term consequences of treatment with gender-affirming hormones such as estrogen and testosterone because the effects are partially irreversible, including a potential loss of fertility. Most pediatric patients will have this ability by 16 years old, but some programs across the country begin treatment between 13.5 years and 14 years of age, said Dr. Nahata. One consideration of beginning GnRH agonists and then moving directly to gender-affirming hormone therapy, there may not be an opportunity to explore fertility preservation.
Dr. Nahata acknowledged the data for the long-term effects of testosterone and estrogen on fertility is “murky,” but despite a lack of data, the American Society for Reproductive Medicine released an ethics statement in 2015 affirming that transgender patients “have the same interests as other persons in having children and in accessing fertility services for fertility preservation and reproduction” and pediatric providers “should offer fertility preservation options to individuals before gender transition” (Fertil Steril. 2015 Sep 9. doi: 10.1016/j.fertnstert.2015.08.021).
There also is mixed evidence that transgender individuals take advantage of fertility preservation services, whether offered or not. Two studies from Belgium that surveyed transgender individuals on parenthood preferences found 54% of adult trans men had a desire for children and that 38% of adult trans men and 51% of adult trans women would consider fertility preservation if it was an option. However, Dr. Nahata said a retrospective study from her own group of 50 adolescent trans males and 23 adolescent trans females found 99% of the cohort was counseled on fertility preservation, but only 3% (2 patients) attempted fertility preservation, and both were trans females (J Adolesc Health. 2017 Jul. doi: 10.1016/j.jadohealth.2016.12.012).
Another study examining use of fertility preservation in trans females in the Netherlands by Brik et al. found a much higher use of fertility preservation, with 38% of patients attempting cryopreservation after counseling (J Adolesc Health. 2019 May. doi: 10.1016/j.jadohealth.2018.11.008). “It’s unclear whether this is a regional difference or whether things are actually shifting over a short period of time,” said Dr. Nahata.
Attitudes about fertility preservation among gender-diverse transgender youth also impact its use in this patient population. A survey of transgender youth found less than 40% preferred adoption to biological parenthood, but said their feelings might change as time passes. However, more than half wanted more information on their family-building options. For other transgender youth aged 12-19 years, having children was their “lowest life priority,” compared with having friends, their health, and other issues in their lives, said Dr. Nahata.
In a 24-item survey Dr. Nahata and her team administered to 44 trans nonbinary adolescents, the most common reasons for not seeking fertility preservation were feelings of being too young, not wanting to be a parent or have a biological child, not wanting to delay treatment, and not being able to afford the cost of fertility preservation.
“This just speaks to the complexities of counseling in this population, and the importance of having a multidisciplinary team to see these youth and families to do more comprehensive counseling,” she said.
Dr. Nahata reported no relevant conflicts of interest.
EXPERT ANALYSIS FROM ASRM 2019
Melanoma incidence continues to increase, yet mortality stabilizing
LAS VEGAS – The according to data from the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) program.
At the Skin Disease Education Foundation’s annual Las Vegas Dermatology Seminar, Laura Korb Ferris, MD, PhD, said that SEER data project 96,480 new cases of melanoma in 2019, as well as 7,230 deaths from the disease. In 2016, SEER projected 10,130 deaths from melanoma, “so we’re actually projecting a reduction in melanoma deaths,” said Dr. Ferris, director of clinical trials at the University of Pittsburgh Medical Center’s department of dermatology. She added that the death rate from melanoma in 2016 was 2.17 per 100,000 population, a reduction from 2.69 per 100,000 population in 2011, “so it looks like melanoma mortality may be stable,” or even reduced, despite an increase in melanoma incidence.
A study of SEER data between 1989 and 2009 found that melanoma incidence is increasing across all lesion thicknesses (J Natl Cancer Inst. 2015 Nov 12. doi: 10.1093/jnci/djv294). Specifically, the incidence increased most among thin lesions, but there was a smaller increased incidence of thick melanoma. “This suggests that the overall burden of disease is truly increasing, but it is primarily stemming from an increase in T1/T2 disease,” Dr. Ferris said. “This could be due in part to increased early detection.”
Improvements in melanoma-specific survival, she continued, are likely a combination of improved management of T4 disease, a shift toward detection of thinner T1/T2 melanoma, and increased detection of T1/T2 disease.
The SEER data also showed that the incidence of fatal cases of melanoma has decreased since 1989, but only in thick melanomas. This trend may indicate a modest improvement in the management of T4 tumors. “Optimistically, I think increased detection efforts are improving survival by early detection of thin but ultimately fatal melanomas,” Dr. Ferris said. “Hopefully we are finding disease earlier and we are preventing patients from progressing to these fatal T4 melanomas.”
Disparities in melanoma-specific survival also come into play. Men have poorer survival compared with women, whites have the highest survival, and non-Hispanic whites have a better survival than Hispanic whites, Dr. Ferris said, while lower rates of survival are seen in blacks and nonblack minorities, as well as among those in high poverty and those who are separated/nonmarried. Lesion type also matters. The highest survival is seen in those with superficial spreading melanoma, while lower survival is observed in those with nodular melanoma, and acral lentiginous melanoma.
Early detection of thin nodular melanomas has the potential to significantly impact melanoma mortality, “but we want to keep in mind that the majority of ultimately fatal melanomas are superficial spreading melanomas,” Dr. Ferris said. “That is because they are so much more prevalent. As a dermatologist, I think a lot about screening and early detection. Periodic screening is a good strategy for a slower-growing superficial spreading melanoma, but it’s not necessarily a good strategy for a rapidly growing nodular melanoma. That’s going to require better education and better access to health care.”
Self-detection of melanoma is another strategy to consider. According to Dr. Ferris, results from multiple studies suggest that about 50% of all melanomas are detected by patients, but the ones they find tend to be thicker than the ones that clinicians detect during office visits. “It would be great if we can get that number higher than 50%,” Dr. Ferris said. “If patients really understood what melanoma is, what it looks like, and when they needed to seek medical attention, perhaps we could get that over 50% and see self-detection of thinner melanomas. That’s a very low-cost intervention.”
Targeted screening efforts that stratify by risk factors and by age “makes screening more efficient and more cost-effective,” she added. She cited one analysis, which found that clinicians need to screen 606 people and conduct 25 biopsies in order to find one melanoma. “That’s very resource intensive,” she said. “However, if you only screened people 50 or older or 65 or older, the number needed to screen goes down, and because your pretest probability is higher, your number need to biopsy goes down as well. If you factor in things like a history of atypical nevi or a personal history of melanoma, those patients are at a higher risk of developing melanoma.”
Dr. Ferris closed her presentation by noting that Australia leads other countries in melanoma prevention efforts. There, the combined incidence of skin cancer is higher than the incidence of any other type of cancer. Four decades ago, Australian health officials launched SunSmart, a series of initiatives intended to reduce skin cancer. These include implementation of policies for hat wearing and shade provision in schools and at work, availability of more effective sunscreens, inclusion of sun protection items as a tax-deductible expense for outdoor workers, increased availability since the 1980s of long-sleeved sun protective swimwear, a ban on the use of indoor tanning since 2014, provision of UV forecasts in weather, and a comprehensive program of grants for community shade structures (PLoSMed. 2019 Oct 8;16[10]:e1002932).
“One approach to melanoma prevention won’t fit all,” she concluded. “We need to focus on prevention, public education to improve knowledge and self-detection.”
Dr. Ferris disclosed that she is a consultant to and an investigator for DermTech and Scibase. She is also an investigator for Castle Biosciences.
SDEF and this news organization are owned by the same parent company. Dr. Ferris spoke during a forum on cutaneous malignancies at the meeting.
LAS VEGAS – The according to data from the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) program.
At the Skin Disease Education Foundation’s annual Las Vegas Dermatology Seminar, Laura Korb Ferris, MD, PhD, said that SEER data project 96,480 new cases of melanoma in 2019, as well as 7,230 deaths from the disease. In 2016, SEER projected 10,130 deaths from melanoma, “so we’re actually projecting a reduction in melanoma deaths,” said Dr. Ferris, director of clinical trials at the University of Pittsburgh Medical Center’s department of dermatology. She added that the death rate from melanoma in 2016 was 2.17 per 100,000 population, a reduction from 2.69 per 100,000 population in 2011, “so it looks like melanoma mortality may be stable,” or even reduced, despite an increase in melanoma incidence.
A study of SEER data between 1989 and 2009 found that melanoma incidence is increasing across all lesion thicknesses (J Natl Cancer Inst. 2015 Nov 12. doi: 10.1093/jnci/djv294). Specifically, the incidence increased most among thin lesions, but there was a smaller increased incidence of thick melanoma. “This suggests that the overall burden of disease is truly increasing, but it is primarily stemming from an increase in T1/T2 disease,” Dr. Ferris said. “This could be due in part to increased early detection.”
Improvements in melanoma-specific survival, she continued, are likely a combination of improved management of T4 disease, a shift toward detection of thinner T1/T2 melanoma, and increased detection of T1/T2 disease.
The SEER data also showed that the incidence of fatal cases of melanoma has decreased since 1989, but only in thick melanomas. This trend may indicate a modest improvement in the management of T4 tumors. “Optimistically, I think increased detection efforts are improving survival by early detection of thin but ultimately fatal melanomas,” Dr. Ferris said. “Hopefully we are finding disease earlier and we are preventing patients from progressing to these fatal T4 melanomas.”
Disparities in melanoma-specific survival also come into play. Men have poorer survival compared with women, whites have the highest survival, and non-Hispanic whites have a better survival than Hispanic whites, Dr. Ferris said, while lower rates of survival are seen in blacks and nonblack minorities, as well as among those in high poverty and those who are separated/nonmarried. Lesion type also matters. The highest survival is seen in those with superficial spreading melanoma, while lower survival is observed in those with nodular melanoma, and acral lentiginous melanoma.
Early detection of thin nodular melanomas has the potential to significantly impact melanoma mortality, “but we want to keep in mind that the majority of ultimately fatal melanomas are superficial spreading melanomas,” Dr. Ferris said. “That is because they are so much more prevalent. As a dermatologist, I think a lot about screening and early detection. Periodic screening is a good strategy for a slower-growing superficial spreading melanoma, but it’s not necessarily a good strategy for a rapidly growing nodular melanoma. That’s going to require better education and better access to health care.”
Self-detection of melanoma is another strategy to consider. According to Dr. Ferris, results from multiple studies suggest that about 50% of all melanomas are detected by patients, but the ones they find tend to be thicker than the ones that clinicians detect during office visits. “It would be great if we can get that number higher than 50%,” Dr. Ferris said. “If patients really understood what melanoma is, what it looks like, and when they needed to seek medical attention, perhaps we could get that over 50% and see self-detection of thinner melanomas. That’s a very low-cost intervention.”
Targeted screening efforts that stratify by risk factors and by age “makes screening more efficient and more cost-effective,” she added. She cited one analysis, which found that clinicians need to screen 606 people and conduct 25 biopsies in order to find one melanoma. “That’s very resource intensive,” she said. “However, if you only screened people 50 or older or 65 or older, the number needed to screen goes down, and because your pretest probability is higher, your number need to biopsy goes down as well. If you factor in things like a history of atypical nevi or a personal history of melanoma, those patients are at a higher risk of developing melanoma.”
Dr. Ferris closed her presentation by noting that Australia leads other countries in melanoma prevention efforts. There, the combined incidence of skin cancer is higher than the incidence of any other type of cancer. Four decades ago, Australian health officials launched SunSmart, a series of initiatives intended to reduce skin cancer. These include implementation of policies for hat wearing and shade provision in schools and at work, availability of more effective sunscreens, inclusion of sun protection items as a tax-deductible expense for outdoor workers, increased availability since the 1980s of long-sleeved sun protective swimwear, a ban on the use of indoor tanning since 2014, provision of UV forecasts in weather, and a comprehensive program of grants for community shade structures (PLoSMed. 2019 Oct 8;16[10]:e1002932).
“One approach to melanoma prevention won’t fit all,” she concluded. “We need to focus on prevention, public education to improve knowledge and self-detection.”
Dr. Ferris disclosed that she is a consultant to and an investigator for DermTech and Scibase. She is also an investigator for Castle Biosciences.
SDEF and this news organization are owned by the same parent company. Dr. Ferris spoke during a forum on cutaneous malignancies at the meeting.
LAS VEGAS – The according to data from the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) program.
At the Skin Disease Education Foundation’s annual Las Vegas Dermatology Seminar, Laura Korb Ferris, MD, PhD, said that SEER data project 96,480 new cases of melanoma in 2019, as well as 7,230 deaths from the disease. In 2016, SEER projected 10,130 deaths from melanoma, “so we’re actually projecting a reduction in melanoma deaths,” said Dr. Ferris, director of clinical trials at the University of Pittsburgh Medical Center’s department of dermatology. She added that the death rate from melanoma in 2016 was 2.17 per 100,000 population, a reduction from 2.69 per 100,000 population in 2011, “so it looks like melanoma mortality may be stable,” or even reduced, despite an increase in melanoma incidence.
A study of SEER data between 1989 and 2009 found that melanoma incidence is increasing across all lesion thicknesses (J Natl Cancer Inst. 2015 Nov 12. doi: 10.1093/jnci/djv294). Specifically, the incidence increased most among thin lesions, but there was a smaller increased incidence of thick melanoma. “This suggests that the overall burden of disease is truly increasing, but it is primarily stemming from an increase in T1/T2 disease,” Dr. Ferris said. “This could be due in part to increased early detection.”
Improvements in melanoma-specific survival, she continued, are likely a combination of improved management of T4 disease, a shift toward detection of thinner T1/T2 melanoma, and increased detection of T1/T2 disease.
The SEER data also showed that the incidence of fatal cases of melanoma has decreased since 1989, but only in thick melanomas. This trend may indicate a modest improvement in the management of T4 tumors. “Optimistically, I think increased detection efforts are improving survival by early detection of thin but ultimately fatal melanomas,” Dr. Ferris said. “Hopefully we are finding disease earlier and we are preventing patients from progressing to these fatal T4 melanomas.”
Disparities in melanoma-specific survival also come into play. Men have poorer survival compared with women, whites have the highest survival, and non-Hispanic whites have a better survival than Hispanic whites, Dr. Ferris said, while lower rates of survival are seen in blacks and nonblack minorities, as well as among those in high poverty and those who are separated/nonmarried. Lesion type also matters. The highest survival is seen in those with superficial spreading melanoma, while lower survival is observed in those with nodular melanoma, and acral lentiginous melanoma.
Early detection of thin nodular melanomas has the potential to significantly impact melanoma mortality, “but we want to keep in mind that the majority of ultimately fatal melanomas are superficial spreading melanomas,” Dr. Ferris said. “That is because they are so much more prevalent. As a dermatologist, I think a lot about screening and early detection. Periodic screening is a good strategy for a slower-growing superficial spreading melanoma, but it’s not necessarily a good strategy for a rapidly growing nodular melanoma. That’s going to require better education and better access to health care.”
Self-detection of melanoma is another strategy to consider. According to Dr. Ferris, results from multiple studies suggest that about 50% of all melanomas are detected by patients, but the ones they find tend to be thicker than the ones that clinicians detect during office visits. “It would be great if we can get that number higher than 50%,” Dr. Ferris said. “If patients really understood what melanoma is, what it looks like, and when they needed to seek medical attention, perhaps we could get that over 50% and see self-detection of thinner melanomas. That’s a very low-cost intervention.”
Targeted screening efforts that stratify by risk factors and by age “makes screening more efficient and more cost-effective,” she added. She cited one analysis, which found that clinicians need to screen 606 people and conduct 25 biopsies in order to find one melanoma. “That’s very resource intensive,” she said. “However, if you only screened people 50 or older or 65 or older, the number needed to screen goes down, and because your pretest probability is higher, your number need to biopsy goes down as well. If you factor in things like a history of atypical nevi or a personal history of melanoma, those patients are at a higher risk of developing melanoma.”
Dr. Ferris closed her presentation by noting that Australia leads other countries in melanoma prevention efforts. There, the combined incidence of skin cancer is higher than the incidence of any other type of cancer. Four decades ago, Australian health officials launched SunSmart, a series of initiatives intended to reduce skin cancer. These include implementation of policies for hat wearing and shade provision in schools and at work, availability of more effective sunscreens, inclusion of sun protection items as a tax-deductible expense for outdoor workers, increased availability since the 1980s of long-sleeved sun protective swimwear, a ban on the use of indoor tanning since 2014, provision of UV forecasts in weather, and a comprehensive program of grants for community shade structures (PLoSMed. 2019 Oct 8;16[10]:e1002932).
“One approach to melanoma prevention won’t fit all,” she concluded. “We need to focus on prevention, public education to improve knowledge and self-detection.”
Dr. Ferris disclosed that she is a consultant to and an investigator for DermTech and Scibase. She is also an investigator for Castle Biosciences.
SDEF and this news organization are owned by the same parent company. Dr. Ferris spoke during a forum on cutaneous malignancies at the meeting.
EXPERT ANALYSIS FROM THE SDEF LAS VEGAS DERMATOLOGY SEMINAR
Probiotics with Lactobacillus reduce loss in spine BMD for postmenopausal women
, according to recent research published in The Lancet Rheumatology.
“The menopausal and early postmenopausal lumbar spine bone loss is substantial in women, and by using a prevention therapy with bacteria naturally occurring in the human gut microbiota we observed a close to complete protection against lumbar spine bone loss in healthy postmenopausal women,” Per-Anders Jansson, MD, chief physician at the University of Gothenburg (Sweden), and colleagues wrote in their study.
Dr. Jansson and colleagues performed a double-blind trial at four centers in Sweden in which 249 postmenopausal women were randomized during April-November 2016 to receive probiotics consisting of three Lactobacillus strains or placebo once per day for 12 months. Participants were healthy women, neither underweight nor overweight, and were postmenopausal, which was defined as being 2-12 years or less from last menstruation. The Lactobacillus strains, L. paracasei 8700:2 (DSM 13434), L. plantarum Heal 9 (DSM 15312), and L. plantarum Heal 19 (DSM 15313), were equally represented in a capsule at a dose of 1 x 1010 colony-forming unit per capsule. The researchers measured the lumbar spine bone mineral density (LS-BMD) at baseline and at 12 months, and also evaluated the safety profile of participants in both the probiotic and placebo groups.
Overall, 234 participants (94%) had data available for analysis at the end of the study. There was a significant reduction in LS-BMD loss for participants who received the probiotic treatment, compared with women in the control group (mean difference, 0.71%; 95% confidence interval, 0.06%-1.35%), while there was a significant loss in LS-BMD for participants in the placebo group (percentage change, –0.72%; 95% CI, –1.22% to –0.22%) compared with loss in the probiotic group (percentage change, –0.01%; 95% CI, –0.50% to 0.48%). Using analysis of covariance, the researchers found the probiotic group had reduced LS-BMD loss after adjustment for factors such as study site, age at baseline, BMD at baseline, and number of years from menopause (mean difference, 7.44 mg/cm2; 95% CI, 0.38 to 14.50).
In a subgroup analysis of women above and below the median time since menopause at baseline (6 years), participants in the probiotic group who were below the median time saw a significant protective effect of Lactobacillus treatment (mean difference, 1.08%; 95% CI, 0.20%-1.96%), compared with women above the median time (mean difference, 0.31%; 95% CI, –0.62% to 1.23%).
Researchers also examined the effects of probiotic treatment on total hip and femoral neck BMD as secondary endpoints. Lactobacillus treatment did not appear to affect total hip (–1.01%; 95% CI, –1.65% to –0.37%) or trochanter BMD (–1.13%; 95% CI, –2.27% to 0.20%), but femoral neck BMD was reduced in the probiotic group (–1.34%; 95% CI, –2.09% to –0.58%), compared with the placebo group (–0.88%; 95% CI, –1.64% to –0.13%).
Limitations of the study included examining only one dose of Lactobacillus treatment and no analysis of the effect of short-chain fatty acids on LS-BMD. The researchers noted that “recent studies have shown that short-chain fatty acids, which are generated by fermentation of complex carbohydrates by the gut microbiota, are important regulators of both bone formation and resorption.”
The researchers also acknowledged that the LS-BMD effect size for the probiotic treatment over the 12 months was a lower magnitude, compared with first-line treatments for osteoporosis in postmenopausal women using bisphosphonates. “Further long-term studies should be done to evaluate if the bone-protective effect becomes more pronounced with prolonged treatment with the Lactobacillus strains used in the present study,” they said.
In a related editorial, Shivani Sahni, PhD, of Harvard Medical School, Boston, and Connie M. Weaver, PhD, of Purdue University, West Lafayette, Ind., reiterated that the effect size of probiotics is “of far less magnitude” than such treatments as bisphosphonates and expressed concern about the reduction of femoral neck BMD in the probiotic group, which was not explained in the study (Lancet Rheumatol. 2019 Nov;1[3]:e135-e137. doi: 10.1016/S2665-9913(19)30073-6). There is a need to learn the optimum dose of probiotics as well as which Lactobacillus strains should be used in future studies, as the strains chosen by Jansson et al. were based on results in mice.
In the meantime, patients might be better off choosing dietary interventions with proven bone protection and no documented negative effects on the hip, such as prebiotics like soluble corn fiber and dried prunes, in tandem with drug therapies, Dr. Sahni and Dr. Weaver said.
“Although Jansson and colleagues’ results are important, more work is needed before such probiotics are ready for consumers,” they concluded.
This study was funded by Probi, which employs two of the study’s authors. Three authors reported being coinventors of a patent involving the effects of probiotics in osteoporosis treatment, and one author is listed as an inventor on a pending patent application on probiotic compositions and uses. Dr. Sahni reported receiving grants from Dairy Management. Dr. Weaver reported no relevant conflicts of interest.
SOURCE: Jansson P-A et al. Lancet Rheumatol. 2019 Nov;1(3):e154-e162. doi: 10.1016/S2665-9913(19)30068-2
, according to recent research published in The Lancet Rheumatology.
“The menopausal and early postmenopausal lumbar spine bone loss is substantial in women, and by using a prevention therapy with bacteria naturally occurring in the human gut microbiota we observed a close to complete protection against lumbar spine bone loss in healthy postmenopausal women,” Per-Anders Jansson, MD, chief physician at the University of Gothenburg (Sweden), and colleagues wrote in their study.
Dr. Jansson and colleagues performed a double-blind trial at four centers in Sweden in which 249 postmenopausal women were randomized during April-November 2016 to receive probiotics consisting of three Lactobacillus strains or placebo once per day for 12 months. Participants were healthy women, neither underweight nor overweight, and were postmenopausal, which was defined as being 2-12 years or less from last menstruation. The Lactobacillus strains, L. paracasei 8700:2 (DSM 13434), L. plantarum Heal 9 (DSM 15312), and L. plantarum Heal 19 (DSM 15313), were equally represented in a capsule at a dose of 1 x 1010 colony-forming unit per capsule. The researchers measured the lumbar spine bone mineral density (LS-BMD) at baseline and at 12 months, and also evaluated the safety profile of participants in both the probiotic and placebo groups.
Overall, 234 participants (94%) had data available for analysis at the end of the study. There was a significant reduction in LS-BMD loss for participants who received the probiotic treatment, compared with women in the control group (mean difference, 0.71%; 95% confidence interval, 0.06%-1.35%), while there was a significant loss in LS-BMD for participants in the placebo group (percentage change, –0.72%; 95% CI, –1.22% to –0.22%) compared with loss in the probiotic group (percentage change, –0.01%; 95% CI, –0.50% to 0.48%). Using analysis of covariance, the researchers found the probiotic group had reduced LS-BMD loss after adjustment for factors such as study site, age at baseline, BMD at baseline, and number of years from menopause (mean difference, 7.44 mg/cm2; 95% CI, 0.38 to 14.50).
In a subgroup analysis of women above and below the median time since menopause at baseline (6 years), participants in the probiotic group who were below the median time saw a significant protective effect of Lactobacillus treatment (mean difference, 1.08%; 95% CI, 0.20%-1.96%), compared with women above the median time (mean difference, 0.31%; 95% CI, –0.62% to 1.23%).
Researchers also examined the effects of probiotic treatment on total hip and femoral neck BMD as secondary endpoints. Lactobacillus treatment did not appear to affect total hip (–1.01%; 95% CI, –1.65% to –0.37%) or trochanter BMD (–1.13%; 95% CI, –2.27% to 0.20%), but femoral neck BMD was reduced in the probiotic group (–1.34%; 95% CI, –2.09% to –0.58%), compared with the placebo group (–0.88%; 95% CI, –1.64% to –0.13%).
Limitations of the study included examining only one dose of Lactobacillus treatment and no analysis of the effect of short-chain fatty acids on LS-BMD. The researchers noted that “recent studies have shown that short-chain fatty acids, which are generated by fermentation of complex carbohydrates by the gut microbiota, are important regulators of both bone formation and resorption.”
The researchers also acknowledged that the LS-BMD effect size for the probiotic treatment over the 12 months was a lower magnitude, compared with first-line treatments for osteoporosis in postmenopausal women using bisphosphonates. “Further long-term studies should be done to evaluate if the bone-protective effect becomes more pronounced with prolonged treatment with the Lactobacillus strains used in the present study,” they said.
In a related editorial, Shivani Sahni, PhD, of Harvard Medical School, Boston, and Connie M. Weaver, PhD, of Purdue University, West Lafayette, Ind., reiterated that the effect size of probiotics is “of far less magnitude” than such treatments as bisphosphonates and expressed concern about the reduction of femoral neck BMD in the probiotic group, which was not explained in the study (Lancet Rheumatol. 2019 Nov;1[3]:e135-e137. doi: 10.1016/S2665-9913(19)30073-6). There is a need to learn the optimum dose of probiotics as well as which Lactobacillus strains should be used in future studies, as the strains chosen by Jansson et al. were based on results in mice.
In the meantime, patients might be better off choosing dietary interventions with proven bone protection and no documented negative effects on the hip, such as prebiotics like soluble corn fiber and dried prunes, in tandem with drug therapies, Dr. Sahni and Dr. Weaver said.
“Although Jansson and colleagues’ results are important, more work is needed before such probiotics are ready for consumers,” they concluded.
This study was funded by Probi, which employs two of the study’s authors. Three authors reported being coinventors of a patent involving the effects of probiotics in osteoporosis treatment, and one author is listed as an inventor on a pending patent application on probiotic compositions and uses. Dr. Sahni reported receiving grants from Dairy Management. Dr. Weaver reported no relevant conflicts of interest.
SOURCE: Jansson P-A et al. Lancet Rheumatol. 2019 Nov;1(3):e154-e162. doi: 10.1016/S2665-9913(19)30068-2
, according to recent research published in The Lancet Rheumatology.
“The menopausal and early postmenopausal lumbar spine bone loss is substantial in women, and by using a prevention therapy with bacteria naturally occurring in the human gut microbiota we observed a close to complete protection against lumbar spine bone loss in healthy postmenopausal women,” Per-Anders Jansson, MD, chief physician at the University of Gothenburg (Sweden), and colleagues wrote in their study.
Dr. Jansson and colleagues performed a double-blind trial at four centers in Sweden in which 249 postmenopausal women were randomized during April-November 2016 to receive probiotics consisting of three Lactobacillus strains or placebo once per day for 12 months. Participants were healthy women, neither underweight nor overweight, and were postmenopausal, which was defined as being 2-12 years or less from last menstruation. The Lactobacillus strains, L. paracasei 8700:2 (DSM 13434), L. plantarum Heal 9 (DSM 15312), and L. plantarum Heal 19 (DSM 15313), were equally represented in a capsule at a dose of 1 x 1010 colony-forming unit per capsule. The researchers measured the lumbar spine bone mineral density (LS-BMD) at baseline and at 12 months, and also evaluated the safety profile of participants in both the probiotic and placebo groups.
Overall, 234 participants (94%) had data available for analysis at the end of the study. There was a significant reduction in LS-BMD loss for participants who received the probiotic treatment, compared with women in the control group (mean difference, 0.71%; 95% confidence interval, 0.06%-1.35%), while there was a significant loss in LS-BMD for participants in the placebo group (percentage change, –0.72%; 95% CI, –1.22% to –0.22%) compared with loss in the probiotic group (percentage change, –0.01%; 95% CI, –0.50% to 0.48%). Using analysis of covariance, the researchers found the probiotic group had reduced LS-BMD loss after adjustment for factors such as study site, age at baseline, BMD at baseline, and number of years from menopause (mean difference, 7.44 mg/cm2; 95% CI, 0.38 to 14.50).
In a subgroup analysis of women above and below the median time since menopause at baseline (6 years), participants in the probiotic group who were below the median time saw a significant protective effect of Lactobacillus treatment (mean difference, 1.08%; 95% CI, 0.20%-1.96%), compared with women above the median time (mean difference, 0.31%; 95% CI, –0.62% to 1.23%).
Researchers also examined the effects of probiotic treatment on total hip and femoral neck BMD as secondary endpoints. Lactobacillus treatment did not appear to affect total hip (–1.01%; 95% CI, –1.65% to –0.37%) or trochanter BMD (–1.13%; 95% CI, –2.27% to 0.20%), but femoral neck BMD was reduced in the probiotic group (–1.34%; 95% CI, –2.09% to –0.58%), compared with the placebo group (–0.88%; 95% CI, –1.64% to –0.13%).
Limitations of the study included examining only one dose of Lactobacillus treatment and no analysis of the effect of short-chain fatty acids on LS-BMD. The researchers noted that “recent studies have shown that short-chain fatty acids, which are generated by fermentation of complex carbohydrates by the gut microbiota, are important regulators of both bone formation and resorption.”
The researchers also acknowledged that the LS-BMD effect size for the probiotic treatment over the 12 months was a lower magnitude, compared with first-line treatments for osteoporosis in postmenopausal women using bisphosphonates. “Further long-term studies should be done to evaluate if the bone-protective effect becomes more pronounced with prolonged treatment with the Lactobacillus strains used in the present study,” they said.
In a related editorial, Shivani Sahni, PhD, of Harvard Medical School, Boston, and Connie M. Weaver, PhD, of Purdue University, West Lafayette, Ind., reiterated that the effect size of probiotics is “of far less magnitude” than such treatments as bisphosphonates and expressed concern about the reduction of femoral neck BMD in the probiotic group, which was not explained in the study (Lancet Rheumatol. 2019 Nov;1[3]:e135-e137. doi: 10.1016/S2665-9913(19)30073-6). There is a need to learn the optimum dose of probiotics as well as which Lactobacillus strains should be used in future studies, as the strains chosen by Jansson et al. were based on results in mice.
In the meantime, patients might be better off choosing dietary interventions with proven bone protection and no documented negative effects on the hip, such as prebiotics like soluble corn fiber and dried prunes, in tandem with drug therapies, Dr. Sahni and Dr. Weaver said.
“Although Jansson and colleagues’ results are important, more work is needed before such probiotics are ready for consumers,” they concluded.
This study was funded by Probi, which employs two of the study’s authors. Three authors reported being coinventors of a patent involving the effects of probiotics in osteoporosis treatment, and one author is listed as an inventor on a pending patent application on probiotic compositions and uses. Dr. Sahni reported receiving grants from Dairy Management. Dr. Weaver reported no relevant conflicts of interest.
SOURCE: Jansson P-A et al. Lancet Rheumatol. 2019 Nov;1(3):e154-e162. doi: 10.1016/S2665-9913(19)30068-2
FROM THE LANCET RHEUMATOLOGY
Ask about vaping and e-cigarette use
When we studied the knowledge and practice of e-cigarette use among pregnant women in one of our outpatient practices, we found that 43% of more than 300 survey participants believed e-cigarettes are less harmful to a fetus than traditional cigarettes. Just over half – 57% – believed that e-cigarettes contain nicotine.
This study from 5 years ago demonstrated the need for more patient education.1 Today, we have even more clarity that, while there may be health benefits of switching to noncombustible forms of nicotine consumption outside of pregnancy, these potential benefits do not extend to pregnancy. Both human and animal studies have demonstrated that nicotine itself is harmful to the developing fetus; the Centers for Disease Control and Prevention warns against the use of e-cigarettes in pregnancy for this reason.
A 2018 literature review on the use of e-cigarettes in pregnancy and the effects on perinatal/neonatal outcomes reported that the amount of nicotine consumed by e-cigarette users is similar to that of cigarette smokers and that most animal studies suggest a potential danger to the fetus, primarily because of the nicotine.2 Effects on the immune system, neural development, lung function, and cardiac function were all noted in the review. Other research has shown that e-cigarette fluid can contain formaldehyde and other harmful substances.
A new analysis of data from the 2014-2017 National Health Interview Survey shows a significantly lower prevalence of conventional cigarette use among pregnant women than in nonpregnant women, and an almost identical prevalence of e-cigarette use among pregnant and nonpregnant women of reproductive age.3 This discrepancy again suggests that women may not be aware of the potential harms of e-cigarettes in pregnancy, which is not surprising considering that prenatal care clinicians often are not appropriately screening or counseling regarding e-cigarette use.4
and counsel women that the use of e-cigarettes is not a safer alternative to cigarette smoking. I urge patients who have switched to e-cigarettes as a means of smoking cessation or as a choice they perceive to be safer to work together with me to find another way to reduce potential harm to their baby.
References
1. J Addict Med. 2015 Jul-Aug;9(4):266-72.
2. Obstet Gynecol Surv. 2018 Sep;73(9):544-9.
3. JAMA Pediatr. 2019 Jun 1;173(6):600-2.
4. Am J Obstet Gynecol. 2014 Dec;211(6):695.e1-7.
When we studied the knowledge and practice of e-cigarette use among pregnant women in one of our outpatient practices, we found that 43% of more than 300 survey participants believed e-cigarettes are less harmful to a fetus than traditional cigarettes. Just over half – 57% – believed that e-cigarettes contain nicotine.
This study from 5 years ago demonstrated the need for more patient education.1 Today, we have even more clarity that, while there may be health benefits of switching to noncombustible forms of nicotine consumption outside of pregnancy, these potential benefits do not extend to pregnancy. Both human and animal studies have demonstrated that nicotine itself is harmful to the developing fetus; the Centers for Disease Control and Prevention warns against the use of e-cigarettes in pregnancy for this reason.
A 2018 literature review on the use of e-cigarettes in pregnancy and the effects on perinatal/neonatal outcomes reported that the amount of nicotine consumed by e-cigarette users is similar to that of cigarette smokers and that most animal studies suggest a potential danger to the fetus, primarily because of the nicotine.2 Effects on the immune system, neural development, lung function, and cardiac function were all noted in the review. Other research has shown that e-cigarette fluid can contain formaldehyde and other harmful substances.
A new analysis of data from the 2014-2017 National Health Interview Survey shows a significantly lower prevalence of conventional cigarette use among pregnant women than in nonpregnant women, and an almost identical prevalence of e-cigarette use among pregnant and nonpregnant women of reproductive age.3 This discrepancy again suggests that women may not be aware of the potential harms of e-cigarettes in pregnancy, which is not surprising considering that prenatal care clinicians often are not appropriately screening or counseling regarding e-cigarette use.4
and counsel women that the use of e-cigarettes is not a safer alternative to cigarette smoking. I urge patients who have switched to e-cigarettes as a means of smoking cessation or as a choice they perceive to be safer to work together with me to find another way to reduce potential harm to their baby.
References
1. J Addict Med. 2015 Jul-Aug;9(4):266-72.
2. Obstet Gynecol Surv. 2018 Sep;73(9):544-9.
3. JAMA Pediatr. 2019 Jun 1;173(6):600-2.
4. Am J Obstet Gynecol. 2014 Dec;211(6):695.e1-7.
When we studied the knowledge and practice of e-cigarette use among pregnant women in one of our outpatient practices, we found that 43% of more than 300 survey participants believed e-cigarettes are less harmful to a fetus than traditional cigarettes. Just over half – 57% – believed that e-cigarettes contain nicotine.
This study from 5 years ago demonstrated the need for more patient education.1 Today, we have even more clarity that, while there may be health benefits of switching to noncombustible forms of nicotine consumption outside of pregnancy, these potential benefits do not extend to pregnancy. Both human and animal studies have demonstrated that nicotine itself is harmful to the developing fetus; the Centers for Disease Control and Prevention warns against the use of e-cigarettes in pregnancy for this reason.
A 2018 literature review on the use of e-cigarettes in pregnancy and the effects on perinatal/neonatal outcomes reported that the amount of nicotine consumed by e-cigarette users is similar to that of cigarette smokers and that most animal studies suggest a potential danger to the fetus, primarily because of the nicotine.2 Effects on the immune system, neural development, lung function, and cardiac function were all noted in the review. Other research has shown that e-cigarette fluid can contain formaldehyde and other harmful substances.
A new analysis of data from the 2014-2017 National Health Interview Survey shows a significantly lower prevalence of conventional cigarette use among pregnant women than in nonpregnant women, and an almost identical prevalence of e-cigarette use among pregnant and nonpregnant women of reproductive age.3 This discrepancy again suggests that women may not be aware of the potential harms of e-cigarettes in pregnancy, which is not surprising considering that prenatal care clinicians often are not appropriately screening or counseling regarding e-cigarette use.4
and counsel women that the use of e-cigarettes is not a safer alternative to cigarette smoking. I urge patients who have switched to e-cigarettes as a means of smoking cessation or as a choice they perceive to be safer to work together with me to find another way to reduce potential harm to their baby.
References
1. J Addict Med. 2015 Jul-Aug;9(4):266-72.
2. Obstet Gynecol Surv. 2018 Sep;73(9):544-9.
3. JAMA Pediatr. 2019 Jun 1;173(6):600-2.
4. Am J Obstet Gynecol. 2014 Dec;211(6):695.e1-7.
Cannabis and prenatal care
We know that the environment significantly impacts our health. People who live in areas prone to industrial waste, poor air or water quality, and crime have higher risks for cardiovascular disease, severe asthma, and stress-induced illnesses. Children who grow up under these conditions can experience a failure to thrive.
As ob.gyns., we also recognize that the intrauterine environment plays a key role in influencing embryonic and fetal development. For this reason, we counsel our pregnant patients to eat well-balanced diets, drink healthy amounts of water, get plenty of rest, and incorporate physical activity into their daily routines. Indeed, the seminal work by Sir David Barker demonstrated that the roots of chronic diseases – including hypertension, stroke, and type 2 diabetes – begin in utero. We truly are where we live – from before birth up through adulthood.
Because the womb environment, where we spend the first critical 9 months of life, dramatically affects our lifelong health, we advise against the use of certain medications and other substances during pregnancy. Some of these recommendations seem clear-cut: Don’t smoke and significantly reduce or abstain from alcohol consumption; illicit drugs – such as cocaine or heroin – should never be used. However, gray areas exist. For example, although anticonvulsants carry higher risks for congenital malformations, patients who experience seizures may need to continue taking antiepileptic drugs during pregnancy, especially those with long safety records.
One of the newer challenges the medical community in general must face is the broadened use and wider societal acceptance of cannabis. Currently legal in 33 U.S. states and Washington, D.C., medical marijuana now is viewed as another legitimate tool in the health care arsenal, rather than the off-limits, off-label substance it was less than a generation ago.
Although proponents may tout the health benefits of cannabis and related products like cannabidiol, it remains unclear what the long-term effects of routine use may have on development, especially fetal development. However, how we as ob.gyns. navigate conversations with our patients around substance use remains crucial to our delivery of the best possible prenatal care.
We have invited Katrina S. Mark, MD, associate professor of obstetrics, gynecology, and reproductive sciences at the University of Maryland School of Medicine, to examine use of cannabis in pregnancy and the need for maintaining trust in the patient-practitioner relationship when discussing substance use during prenatal counseling.
Dr. Reece, who specializes in maternal-fetal medicine, is executive vice president for medical affairs at the University of Maryland School of Medicine as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He is the medical editor of this column. He said he had no relevant financial disclosures. Contact him at obnews@mdedge.com.
We know that the environment significantly impacts our health. People who live in areas prone to industrial waste, poor air or water quality, and crime have higher risks for cardiovascular disease, severe asthma, and stress-induced illnesses. Children who grow up under these conditions can experience a failure to thrive.
As ob.gyns., we also recognize that the intrauterine environment plays a key role in influencing embryonic and fetal development. For this reason, we counsel our pregnant patients to eat well-balanced diets, drink healthy amounts of water, get plenty of rest, and incorporate physical activity into their daily routines. Indeed, the seminal work by Sir David Barker demonstrated that the roots of chronic diseases – including hypertension, stroke, and type 2 diabetes – begin in utero. We truly are where we live – from before birth up through adulthood.
Because the womb environment, where we spend the first critical 9 months of life, dramatically affects our lifelong health, we advise against the use of certain medications and other substances during pregnancy. Some of these recommendations seem clear-cut: Don’t smoke and significantly reduce or abstain from alcohol consumption; illicit drugs – such as cocaine or heroin – should never be used. However, gray areas exist. For example, although anticonvulsants carry higher risks for congenital malformations, patients who experience seizures may need to continue taking antiepileptic drugs during pregnancy, especially those with long safety records.
One of the newer challenges the medical community in general must face is the broadened use and wider societal acceptance of cannabis. Currently legal in 33 U.S. states and Washington, D.C., medical marijuana now is viewed as another legitimate tool in the health care arsenal, rather than the off-limits, off-label substance it was less than a generation ago.
Although proponents may tout the health benefits of cannabis and related products like cannabidiol, it remains unclear what the long-term effects of routine use may have on development, especially fetal development. However, how we as ob.gyns. navigate conversations with our patients around substance use remains crucial to our delivery of the best possible prenatal care.
We have invited Katrina S. Mark, MD, associate professor of obstetrics, gynecology, and reproductive sciences at the University of Maryland School of Medicine, to examine use of cannabis in pregnancy and the need for maintaining trust in the patient-practitioner relationship when discussing substance use during prenatal counseling.
Dr. Reece, who specializes in maternal-fetal medicine, is executive vice president for medical affairs at the University of Maryland School of Medicine as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He is the medical editor of this column. He said he had no relevant financial disclosures. Contact him at obnews@mdedge.com.
We know that the environment significantly impacts our health. People who live in areas prone to industrial waste, poor air or water quality, and crime have higher risks for cardiovascular disease, severe asthma, and stress-induced illnesses. Children who grow up under these conditions can experience a failure to thrive.
As ob.gyns., we also recognize that the intrauterine environment plays a key role in influencing embryonic and fetal development. For this reason, we counsel our pregnant patients to eat well-balanced diets, drink healthy amounts of water, get plenty of rest, and incorporate physical activity into their daily routines. Indeed, the seminal work by Sir David Barker demonstrated that the roots of chronic diseases – including hypertension, stroke, and type 2 diabetes – begin in utero. We truly are where we live – from before birth up through adulthood.
Because the womb environment, where we spend the first critical 9 months of life, dramatically affects our lifelong health, we advise against the use of certain medications and other substances during pregnancy. Some of these recommendations seem clear-cut: Don’t smoke and significantly reduce or abstain from alcohol consumption; illicit drugs – such as cocaine or heroin – should never be used. However, gray areas exist. For example, although anticonvulsants carry higher risks for congenital malformations, patients who experience seizures may need to continue taking antiepileptic drugs during pregnancy, especially those with long safety records.
One of the newer challenges the medical community in general must face is the broadened use and wider societal acceptance of cannabis. Currently legal in 33 U.S. states and Washington, D.C., medical marijuana now is viewed as another legitimate tool in the health care arsenal, rather than the off-limits, off-label substance it was less than a generation ago.
Although proponents may tout the health benefits of cannabis and related products like cannabidiol, it remains unclear what the long-term effects of routine use may have on development, especially fetal development. However, how we as ob.gyns. navigate conversations with our patients around substance use remains crucial to our delivery of the best possible prenatal care.
We have invited Katrina S. Mark, MD, associate professor of obstetrics, gynecology, and reproductive sciences at the University of Maryland School of Medicine, to examine use of cannabis in pregnancy and the need for maintaining trust in the patient-practitioner relationship when discussing substance use during prenatal counseling.
Dr. Reece, who specializes in maternal-fetal medicine, is executive vice president for medical affairs at the University of Maryland School of Medicine as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He is the medical editor of this column. He said he had no relevant financial disclosures. Contact him at obnews@mdedge.com.