User login
Big missed opportunities for BP control in premenopausal women
A new report shows considerable gaps in the awareness, treatment, and control of hypertension in premenopausal women in the United States, with a key driver being regular access to health care.
In a nationally representative sample of women ages 35-54 with no prior cardiovascular disease, the prevalence of hypertension increased 8% from an estimated 15.2 million women between 2011 and 2014 to 16.4 million women between 2015 and 2018.
What’s more, the percentage of women with controlled hypertension dropped over the two time periods from 55% to 50%, which is well below the government’s Million Hearts target of 70%.
Missed opportunities for hypertension control in these premenopausal women were a lack of awareness of their hypertension in 23%, ineffective treatment in 34%, and a lack of health care access in 43%; increasing to 51% in non-Hispanic Black patients and 56% in Hispanic patients.
Notably, lack of health care access affected an estimated 3.1 million women (45%) in 2011-2014 and 3.5 million women (43%) in 2015-2018.
Equally stubborn over the two time periods was the lack of effective treatment, affecting 2.1 million (31%) versus 2.8 million (34%) women, and lack of awareness, affecting 1.6 million (24%) versus 1.9 million (23%) women.
“There’s been no improvement over the past decade, and there is evidence of race/ethnic disparities,” study author Susan Hennessy, PhD, said at the recent Epidemiology, Prevention/Lifestyle & Cardiometabolic Health (EPI|Lifestyle) 2022 conference sponsored by the American Heart Association.
The prevalence of uncontrolled hypertension among non-Hispanic Whites was less than that of the U.S. population, at 44%, and most of the missed opportunities were due to uncontrolled blood pressure (BP), noted Dr. Hennessy, a researcher with the University of California, San Francisco School of Medicine.
However, the uncontrolled prevalence was 54% in non-Hispanic Black women and 66% in Hispanic women. “In both of these subgroups, over half of the missed opportunities occur because these women have no regular access to health care,” she said.
In women who identified as “other,” which includes non-Hispanic Asian and mixed-race populations, the uncontrolled prevalence reached 70%, and the biggest missed opportunity was in those who were untreated.
Raising awareness, empowering women, and delivery of guideline-concordant care will help premenopausal women gain control of their blood pressure, Dr. Hennessy said. “But underpinning all of this is ensuring equitable health care access, because if we fail to get women into the system, then we have no opportunity to help them lower their blood pressure.”
She reminded the audience that cardiovascular disease (CVD) is the number one killer of women in the United States and that CVD risk, mediated through hypertension, increases after menopause. Thus, managing hypertension prior to this life event is an important element of primary prevention of CVD and should be a priority.
Session moderator Sadiya S. Khan, MD, Northwestern University Feinberg School of Medicine, Chicago, told this news organization that the findings should raise “alarm and concern that hypertension is not just a disease of the old but very prevalent in younger women, particularly around the time of pregnancy. And this is a clear driver of maternal morbidity and mortality as well.”
“This idea that patients should ‘Know Your Numbers’ is really important, and we talk a lot about that for hypertension, but if you don’t have a doctor, if you don’t have someone to go to, it’s very hard to know or understand what your numbers mean,” she said. “I think that’s really the main message.”
Speaking to this news organization, Dr. Hennessy said there’s no simple solution to the problem, given that some women are not even in the system, whereas others are not being treated effectively, but that increasing opportunities to screen BP would be a start. That could be through community programs, similar to the Barbershop Hypertension trial, or by making BP devices available for home monitoring.
“Again, this is about empowering ourselves to take some level of control, but, as a system, we have to be able to make it equitable for everyone and make sure they have the right equipment, the right cuff size,” she said. “The disparities arise because of the social determinants of health, so if these women are struggling to put food on the table, they aren’t going to be able to afford a blood pressure cuff.”
During a discussion of the findings, audience members noted that the National Health and Nutrition Examination Survey (NHANES) data used for the analysis were somewhat dated. Dr. Hennessy also pointed out that NHANES blood pressure is measured up to three times during a single visit, which differs from clinical practice, and that responses were based on self-report and thus subject to recall bias.
The sample included 3,343 women aged 35-54 years with no prior cardiovascular disease, representing an estimated 31.6 million American women. Hypertension was defined by a systolic BP of at least 140 mm Hg or a diastolic BP of at least 90 mm Hg or current BP medication use.
The authors and Dr. Khan report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A new report shows considerable gaps in the awareness, treatment, and control of hypertension in premenopausal women in the United States, with a key driver being regular access to health care.
In a nationally representative sample of women ages 35-54 with no prior cardiovascular disease, the prevalence of hypertension increased 8% from an estimated 15.2 million women between 2011 and 2014 to 16.4 million women between 2015 and 2018.
What’s more, the percentage of women with controlled hypertension dropped over the two time periods from 55% to 50%, which is well below the government’s Million Hearts target of 70%.
Missed opportunities for hypertension control in these premenopausal women were a lack of awareness of their hypertension in 23%, ineffective treatment in 34%, and a lack of health care access in 43%; increasing to 51% in non-Hispanic Black patients and 56% in Hispanic patients.
Notably, lack of health care access affected an estimated 3.1 million women (45%) in 2011-2014 and 3.5 million women (43%) in 2015-2018.
Equally stubborn over the two time periods was the lack of effective treatment, affecting 2.1 million (31%) versus 2.8 million (34%) women, and lack of awareness, affecting 1.6 million (24%) versus 1.9 million (23%) women.
“There’s been no improvement over the past decade, and there is evidence of race/ethnic disparities,” study author Susan Hennessy, PhD, said at the recent Epidemiology, Prevention/Lifestyle & Cardiometabolic Health (EPI|Lifestyle) 2022 conference sponsored by the American Heart Association.
The prevalence of uncontrolled hypertension among non-Hispanic Whites was less than that of the U.S. population, at 44%, and most of the missed opportunities were due to uncontrolled blood pressure (BP), noted Dr. Hennessy, a researcher with the University of California, San Francisco School of Medicine.
However, the uncontrolled prevalence was 54% in non-Hispanic Black women and 66% in Hispanic women. “In both of these subgroups, over half of the missed opportunities occur because these women have no regular access to health care,” she said.
In women who identified as “other,” which includes non-Hispanic Asian and mixed-race populations, the uncontrolled prevalence reached 70%, and the biggest missed opportunity was in those who were untreated.
Raising awareness, empowering women, and delivery of guideline-concordant care will help premenopausal women gain control of their blood pressure, Dr. Hennessy said. “But underpinning all of this is ensuring equitable health care access, because if we fail to get women into the system, then we have no opportunity to help them lower their blood pressure.”
She reminded the audience that cardiovascular disease (CVD) is the number one killer of women in the United States and that CVD risk, mediated through hypertension, increases after menopause. Thus, managing hypertension prior to this life event is an important element of primary prevention of CVD and should be a priority.
Session moderator Sadiya S. Khan, MD, Northwestern University Feinberg School of Medicine, Chicago, told this news organization that the findings should raise “alarm and concern that hypertension is not just a disease of the old but very prevalent in younger women, particularly around the time of pregnancy. And this is a clear driver of maternal morbidity and mortality as well.”
“This idea that patients should ‘Know Your Numbers’ is really important, and we talk a lot about that for hypertension, but if you don’t have a doctor, if you don’t have someone to go to, it’s very hard to know or understand what your numbers mean,” she said. “I think that’s really the main message.”
Speaking to this news organization, Dr. Hennessy said there’s no simple solution to the problem, given that some women are not even in the system, whereas others are not being treated effectively, but that increasing opportunities to screen BP would be a start. That could be through community programs, similar to the Barbershop Hypertension trial, or by making BP devices available for home monitoring.
“Again, this is about empowering ourselves to take some level of control, but, as a system, we have to be able to make it equitable for everyone and make sure they have the right equipment, the right cuff size,” she said. “The disparities arise because of the social determinants of health, so if these women are struggling to put food on the table, they aren’t going to be able to afford a blood pressure cuff.”
During a discussion of the findings, audience members noted that the National Health and Nutrition Examination Survey (NHANES) data used for the analysis were somewhat dated. Dr. Hennessy also pointed out that NHANES blood pressure is measured up to three times during a single visit, which differs from clinical practice, and that responses were based on self-report and thus subject to recall bias.
The sample included 3,343 women aged 35-54 years with no prior cardiovascular disease, representing an estimated 31.6 million American women. Hypertension was defined by a systolic BP of at least 140 mm Hg or a diastolic BP of at least 90 mm Hg or current BP medication use.
The authors and Dr. Khan report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A new report shows considerable gaps in the awareness, treatment, and control of hypertension in premenopausal women in the United States, with a key driver being regular access to health care.
In a nationally representative sample of women ages 35-54 with no prior cardiovascular disease, the prevalence of hypertension increased 8% from an estimated 15.2 million women between 2011 and 2014 to 16.4 million women between 2015 and 2018.
What’s more, the percentage of women with controlled hypertension dropped over the two time periods from 55% to 50%, which is well below the government’s Million Hearts target of 70%.
Missed opportunities for hypertension control in these premenopausal women were a lack of awareness of their hypertension in 23%, ineffective treatment in 34%, and a lack of health care access in 43%; increasing to 51% in non-Hispanic Black patients and 56% in Hispanic patients.
Notably, lack of health care access affected an estimated 3.1 million women (45%) in 2011-2014 and 3.5 million women (43%) in 2015-2018.
Equally stubborn over the two time periods was the lack of effective treatment, affecting 2.1 million (31%) versus 2.8 million (34%) women, and lack of awareness, affecting 1.6 million (24%) versus 1.9 million (23%) women.
“There’s been no improvement over the past decade, and there is evidence of race/ethnic disparities,” study author Susan Hennessy, PhD, said at the recent Epidemiology, Prevention/Lifestyle & Cardiometabolic Health (EPI|Lifestyle) 2022 conference sponsored by the American Heart Association.
The prevalence of uncontrolled hypertension among non-Hispanic Whites was less than that of the U.S. population, at 44%, and most of the missed opportunities were due to uncontrolled blood pressure (BP), noted Dr. Hennessy, a researcher with the University of California, San Francisco School of Medicine.
However, the uncontrolled prevalence was 54% in non-Hispanic Black women and 66% in Hispanic women. “In both of these subgroups, over half of the missed opportunities occur because these women have no regular access to health care,” she said.
In women who identified as “other,” which includes non-Hispanic Asian and mixed-race populations, the uncontrolled prevalence reached 70%, and the biggest missed opportunity was in those who were untreated.
Raising awareness, empowering women, and delivery of guideline-concordant care will help premenopausal women gain control of their blood pressure, Dr. Hennessy said. “But underpinning all of this is ensuring equitable health care access, because if we fail to get women into the system, then we have no opportunity to help them lower their blood pressure.”
She reminded the audience that cardiovascular disease (CVD) is the number one killer of women in the United States and that CVD risk, mediated through hypertension, increases after menopause. Thus, managing hypertension prior to this life event is an important element of primary prevention of CVD and should be a priority.
Session moderator Sadiya S. Khan, MD, Northwestern University Feinberg School of Medicine, Chicago, told this news organization that the findings should raise “alarm and concern that hypertension is not just a disease of the old but very prevalent in younger women, particularly around the time of pregnancy. And this is a clear driver of maternal morbidity and mortality as well.”
“This idea that patients should ‘Know Your Numbers’ is really important, and we talk a lot about that for hypertension, but if you don’t have a doctor, if you don’t have someone to go to, it’s very hard to know or understand what your numbers mean,” she said. “I think that’s really the main message.”
Speaking to this news organization, Dr. Hennessy said there’s no simple solution to the problem, given that some women are not even in the system, whereas others are not being treated effectively, but that increasing opportunities to screen BP would be a start. That could be through community programs, similar to the Barbershop Hypertension trial, or by making BP devices available for home monitoring.
“Again, this is about empowering ourselves to take some level of control, but, as a system, we have to be able to make it equitable for everyone and make sure they have the right equipment, the right cuff size,” she said. “The disparities arise because of the social determinants of health, so if these women are struggling to put food on the table, they aren’t going to be able to afford a blood pressure cuff.”
During a discussion of the findings, audience members noted that the National Health and Nutrition Examination Survey (NHANES) data used for the analysis were somewhat dated. Dr. Hennessy also pointed out that NHANES blood pressure is measured up to three times during a single visit, which differs from clinical practice, and that responses were based on self-report and thus subject to recall bias.
The sample included 3,343 women aged 35-54 years with no prior cardiovascular disease, representing an estimated 31.6 million American women. Hypertension was defined by a systolic BP of at least 140 mm Hg or a diastolic BP of at least 90 mm Hg or current BP medication use.
The authors and Dr. Khan report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Antiseptic as good as antibiotics for preventing recurrent UTI
The antiseptic methenamine hippurate (MH) is known to sterilize urine and has been suggested to be of use in preventing urinary tract infections (UTIs), but firm evidence has so far been lacking. Now researchers led by clinicians and scientists from Newcastle-upon-Tyne, England, have provided the ALTAR trial (Alternative to Prophylactic Antibiotics for the Treatment of Recurrent UTIs in Women).
Daily low-dose antibiotics as recommended by current guidelines for prophylactic treatment of recurrent UTI have been linked to antibiotic resistance. Using MH as an alternative could play an important role in helping to tackle the global problem of increasing antibiotic resistance, the team said.
Study details
They recruited 240 women aged 18 or over with recurrent UTIs requiring prophylactic treatment from eight secondary care urology and urogynecology centers in the United Kingdom from June 2016 to June 2018. Women were randomized to receive MH or daily low-dose antibiotics for 12 months, with follow up for a further 6 months beyond that.
Before trial entry the women had experienced an average of more than six UTI episodes per year. During the 12-month treatment period, in the modified intention-to-treat population, there were 90 symptomatic, antibiotic-treated UTI episodes reported over 101 person-years of follow-up in the antibiotic group, and 141 episodes over 102 person-years in the MH group.
This yielded a UTI rate of 0.89 episodes per person-year in the antibiotic group, compared with 1.38 in the MH group, an absolute difference of 0.49 episodes per person-year. In the 6-month posttreatment follow-up period, the UTI incidence rate was 1.19 episodes per person-year in the antibiotic prophylaxis group versus 1.72 in the MH group, an absolute difference of 0.53.
Before the trial, a patient and public involvement group had predefined the noninferiority margin as one episode of UTI per person-year. The small difference between the two groups was less than this, confirming noninferiority of MH to antibiotic prophylaxis in this setting. This finding was consistent across the modified intention-to-treat, strict intention-to-treat, per protocol, and modified per protocol (post hoc) analyses.
Thus the ALTAR results showed that MH was no worse than antibiotics at preventing UTIs, and MH was also associated with reduced antibiotic consumption.
The vast majority of participants were over 90% adherent with the allocated treatment. Patient satisfaction was generally high and rates of adverse events and adverse reactions generally low, and both were comparable between treatment groups. Adverse reactions were reported by 34/142 (24%) in the antibiotic group and 35/127 (28%) in the MH group, and most reactions were mild. In the antibiotic group there were two serious adverse reactions (severe abdominal pain and raised alanine transaminase), whereas six participants in the MH group reported an episode of febrile UTI and four were admitted to hospital because of UTI.
Substantial global health care problem
At least 50% and up to 80% of all women have at least one acute UTI in their lifetime, most often uncomplicated acute cystitis. About a quarter of them go on to suffer recurrent infection, defined as three or more repeat infections in the past year, or two infections in the preceding 6 months. Frequent recurrences thus represent “a substantial global health care problem,” the authors say.
Guidelines from the United Kingdom, Europe, and the United States acknowledge the need for preventive strategies and strongly recommend the use of daily, low-dose antibiotics as standard prophylactic treatment. However, the United Kingdom’s antimicrobial resistance strategy recommends a “strong focus on infection prevention,” and aims to reduce antimicrobial use in humans by 15% before 2024.
“To achieve that, exploration of nonantibiotic preventive treatments in common conditions such as UTI is essential,” the team said.
MH is one such nonantibiotic treatment. It is bactericidal and works by denaturing bacterial proteins and nucleic acids. Although previous Cochrane systematic reviews had concluded that it could be effective for preventing UTI, further large trials were needed.
“This trial adds to the evidence base for the use of MH for prophylactic treatment in adult women with recurrent UTI. Although the MH group had a 55% higher rate of UTI episodes than the antibiotics group, the absolute difference was just 0.49 UTI episodes per year, which has limited clinical consequence,” the team concluded.
Results could ‘support a change in practice’
In older patients, particularly, the risks of long-term antibiotic prophylaxis might outweigh the benefits, and the authors said that their results “could support a change in practice in terms of preventive treatments for recurrent UTI and provide patients and clinicians with a credible alternative to daily antibiotics, giving them the confidence to pursue strategies that avoid long-term antibiotic use.”
They acknowledged limitations of the study, including that treatment allocation was not masked, crossover between arms was allowed, and differences in antibiotics prescribed may have affected the results. In addition, data regarding long-term safety of MH are scarce.
However, they said that the trial accurately represented the broad range of women with recurrent UTI, and that its results “might encourage patients and clinicians to consider MH as a first line treatment for UTI prevention in women.”
In a linked editorial, scientists from the Institute for Evidence-Based Healthcare at Bond University in Queensland, Australia, commented: “Although the results need cautious interpretation, they align with others, and this new research increases the confidence with which MH can be offered as an option to women needing prophylaxis against recurrent urinary tract infection.”
References
Harding C et al. Alternative to prophylactic antibiotics for the treatment of recurrent urinary tract infections in women: multicentre, open label, randomised, noninferiority trial. BMJ 2022 Mar 9;376:e068229.
Hoffmann TC et al. Methenamine hippurate for recurrent urinary tract infections. BMJ 2022 Mar 9;376:o533.
A version of this article first appeared on Medscape.co.uk.
The antiseptic methenamine hippurate (MH) is known to sterilize urine and has been suggested to be of use in preventing urinary tract infections (UTIs), but firm evidence has so far been lacking. Now researchers led by clinicians and scientists from Newcastle-upon-Tyne, England, have provided the ALTAR trial (Alternative to Prophylactic Antibiotics for the Treatment of Recurrent UTIs in Women).
Daily low-dose antibiotics as recommended by current guidelines for prophylactic treatment of recurrent UTI have been linked to antibiotic resistance. Using MH as an alternative could play an important role in helping to tackle the global problem of increasing antibiotic resistance, the team said.
Study details
They recruited 240 women aged 18 or over with recurrent UTIs requiring prophylactic treatment from eight secondary care urology and urogynecology centers in the United Kingdom from June 2016 to June 2018. Women were randomized to receive MH or daily low-dose antibiotics for 12 months, with follow up for a further 6 months beyond that.
Before trial entry the women had experienced an average of more than six UTI episodes per year. During the 12-month treatment period, in the modified intention-to-treat population, there were 90 symptomatic, antibiotic-treated UTI episodes reported over 101 person-years of follow-up in the antibiotic group, and 141 episodes over 102 person-years in the MH group.
This yielded a UTI rate of 0.89 episodes per person-year in the antibiotic group, compared with 1.38 in the MH group, an absolute difference of 0.49 episodes per person-year. In the 6-month posttreatment follow-up period, the UTI incidence rate was 1.19 episodes per person-year in the antibiotic prophylaxis group versus 1.72 in the MH group, an absolute difference of 0.53.
Before the trial, a patient and public involvement group had predefined the noninferiority margin as one episode of UTI per person-year. The small difference between the two groups was less than this, confirming noninferiority of MH to antibiotic prophylaxis in this setting. This finding was consistent across the modified intention-to-treat, strict intention-to-treat, per protocol, and modified per protocol (post hoc) analyses.
Thus the ALTAR results showed that MH was no worse than antibiotics at preventing UTIs, and MH was also associated with reduced antibiotic consumption.
The vast majority of participants were over 90% adherent with the allocated treatment. Patient satisfaction was generally high and rates of adverse events and adverse reactions generally low, and both were comparable between treatment groups. Adverse reactions were reported by 34/142 (24%) in the antibiotic group and 35/127 (28%) in the MH group, and most reactions were mild. In the antibiotic group there were two serious adverse reactions (severe abdominal pain and raised alanine transaminase), whereas six participants in the MH group reported an episode of febrile UTI and four were admitted to hospital because of UTI.
Substantial global health care problem
At least 50% and up to 80% of all women have at least one acute UTI in their lifetime, most often uncomplicated acute cystitis. About a quarter of them go on to suffer recurrent infection, defined as three or more repeat infections in the past year, or two infections in the preceding 6 months. Frequent recurrences thus represent “a substantial global health care problem,” the authors say.
Guidelines from the United Kingdom, Europe, and the United States acknowledge the need for preventive strategies and strongly recommend the use of daily, low-dose antibiotics as standard prophylactic treatment. However, the United Kingdom’s antimicrobial resistance strategy recommends a “strong focus on infection prevention,” and aims to reduce antimicrobial use in humans by 15% before 2024.
“To achieve that, exploration of nonantibiotic preventive treatments in common conditions such as UTI is essential,” the team said.
MH is one such nonantibiotic treatment. It is bactericidal and works by denaturing bacterial proteins and nucleic acids. Although previous Cochrane systematic reviews had concluded that it could be effective for preventing UTI, further large trials were needed.
“This trial adds to the evidence base for the use of MH for prophylactic treatment in adult women with recurrent UTI. Although the MH group had a 55% higher rate of UTI episodes than the antibiotics group, the absolute difference was just 0.49 UTI episodes per year, which has limited clinical consequence,” the team concluded.
Results could ‘support a change in practice’
In older patients, particularly, the risks of long-term antibiotic prophylaxis might outweigh the benefits, and the authors said that their results “could support a change in practice in terms of preventive treatments for recurrent UTI and provide patients and clinicians with a credible alternative to daily antibiotics, giving them the confidence to pursue strategies that avoid long-term antibiotic use.”
They acknowledged limitations of the study, including that treatment allocation was not masked, crossover between arms was allowed, and differences in antibiotics prescribed may have affected the results. In addition, data regarding long-term safety of MH are scarce.
However, they said that the trial accurately represented the broad range of women with recurrent UTI, and that its results “might encourage patients and clinicians to consider MH as a first line treatment for UTI prevention in women.”
In a linked editorial, scientists from the Institute for Evidence-Based Healthcare at Bond University in Queensland, Australia, commented: “Although the results need cautious interpretation, they align with others, and this new research increases the confidence with which MH can be offered as an option to women needing prophylaxis against recurrent urinary tract infection.”
References
Harding C et al. Alternative to prophylactic antibiotics for the treatment of recurrent urinary tract infections in women: multicentre, open label, randomised, noninferiority trial. BMJ 2022 Mar 9;376:e068229.
Hoffmann TC et al. Methenamine hippurate for recurrent urinary tract infections. BMJ 2022 Mar 9;376:o533.
A version of this article first appeared on Medscape.co.uk.
The antiseptic methenamine hippurate (MH) is known to sterilize urine and has been suggested to be of use in preventing urinary tract infections (UTIs), but firm evidence has so far been lacking. Now researchers led by clinicians and scientists from Newcastle-upon-Tyne, England, have provided the ALTAR trial (Alternative to Prophylactic Antibiotics for the Treatment of Recurrent UTIs in Women).
Daily low-dose antibiotics as recommended by current guidelines for prophylactic treatment of recurrent UTI have been linked to antibiotic resistance. Using MH as an alternative could play an important role in helping to tackle the global problem of increasing antibiotic resistance, the team said.
Study details
They recruited 240 women aged 18 or over with recurrent UTIs requiring prophylactic treatment from eight secondary care urology and urogynecology centers in the United Kingdom from June 2016 to June 2018. Women were randomized to receive MH or daily low-dose antibiotics for 12 months, with follow up for a further 6 months beyond that.
Before trial entry the women had experienced an average of more than six UTI episodes per year. During the 12-month treatment period, in the modified intention-to-treat population, there were 90 symptomatic, antibiotic-treated UTI episodes reported over 101 person-years of follow-up in the antibiotic group, and 141 episodes over 102 person-years in the MH group.
This yielded a UTI rate of 0.89 episodes per person-year in the antibiotic group, compared with 1.38 in the MH group, an absolute difference of 0.49 episodes per person-year. In the 6-month posttreatment follow-up period, the UTI incidence rate was 1.19 episodes per person-year in the antibiotic prophylaxis group versus 1.72 in the MH group, an absolute difference of 0.53.
Before the trial, a patient and public involvement group had predefined the noninferiority margin as one episode of UTI per person-year. The small difference between the two groups was less than this, confirming noninferiority of MH to antibiotic prophylaxis in this setting. This finding was consistent across the modified intention-to-treat, strict intention-to-treat, per protocol, and modified per protocol (post hoc) analyses.
Thus the ALTAR results showed that MH was no worse than antibiotics at preventing UTIs, and MH was also associated with reduced antibiotic consumption.
The vast majority of participants were over 90% adherent with the allocated treatment. Patient satisfaction was generally high and rates of adverse events and adverse reactions generally low, and both were comparable between treatment groups. Adverse reactions were reported by 34/142 (24%) in the antibiotic group and 35/127 (28%) in the MH group, and most reactions were mild. In the antibiotic group there were two serious adverse reactions (severe abdominal pain and raised alanine transaminase), whereas six participants in the MH group reported an episode of febrile UTI and four were admitted to hospital because of UTI.
Substantial global health care problem
At least 50% and up to 80% of all women have at least one acute UTI in their lifetime, most often uncomplicated acute cystitis. About a quarter of them go on to suffer recurrent infection, defined as three or more repeat infections in the past year, or two infections in the preceding 6 months. Frequent recurrences thus represent “a substantial global health care problem,” the authors say.
Guidelines from the United Kingdom, Europe, and the United States acknowledge the need for preventive strategies and strongly recommend the use of daily, low-dose antibiotics as standard prophylactic treatment. However, the United Kingdom’s antimicrobial resistance strategy recommends a “strong focus on infection prevention,” and aims to reduce antimicrobial use in humans by 15% before 2024.
“To achieve that, exploration of nonantibiotic preventive treatments in common conditions such as UTI is essential,” the team said.
MH is one such nonantibiotic treatment. It is bactericidal and works by denaturing bacterial proteins and nucleic acids. Although previous Cochrane systematic reviews had concluded that it could be effective for preventing UTI, further large trials were needed.
“This trial adds to the evidence base for the use of MH for prophylactic treatment in adult women with recurrent UTI. Although the MH group had a 55% higher rate of UTI episodes than the antibiotics group, the absolute difference was just 0.49 UTI episodes per year, which has limited clinical consequence,” the team concluded.
Results could ‘support a change in practice’
In older patients, particularly, the risks of long-term antibiotic prophylaxis might outweigh the benefits, and the authors said that their results “could support a change in practice in terms of preventive treatments for recurrent UTI and provide patients and clinicians with a credible alternative to daily antibiotics, giving them the confidence to pursue strategies that avoid long-term antibiotic use.”
They acknowledged limitations of the study, including that treatment allocation was not masked, crossover between arms was allowed, and differences in antibiotics prescribed may have affected the results. In addition, data regarding long-term safety of MH are scarce.
However, they said that the trial accurately represented the broad range of women with recurrent UTI, and that its results “might encourage patients and clinicians to consider MH as a first line treatment for UTI prevention in women.”
In a linked editorial, scientists from the Institute for Evidence-Based Healthcare at Bond University in Queensland, Australia, commented: “Although the results need cautious interpretation, they align with others, and this new research increases the confidence with which MH can be offered as an option to women needing prophylaxis against recurrent urinary tract infection.”
References
Harding C et al. Alternative to prophylactic antibiotics for the treatment of recurrent urinary tract infections in women: multicentre, open label, randomised, noninferiority trial. BMJ 2022 Mar 9;376:e068229.
Hoffmann TC et al. Methenamine hippurate for recurrent urinary tract infections. BMJ 2022 Mar 9;376:o533.
A version of this article first appeared on Medscape.co.uk.
‘Baby-friendly’ steps help women meet prenatal breastfeeding goals
A first-ever study of the effect of evidence-based maternity care practices on prenatal breastfeeding intentions in women from low-income U.S. households shows that the use of “baby-friendly steps” during birth hospitalization made it possible for almost half to breastfeed exclusively for 1 month.
Analyses of national data from a longitudinal study of 1,080 women enrolled in the Special Supplemental Nutrition Program for Women, Infants, and Children (WIC) revealed that 47% were able to meet their prenatal intention to breastfeed without formula or other milk for at least 30 days.
The odds of meeting prenatal breastfeeding intentions more than quadrupled when babies received only breast milk (risk ratio, 4.4; 95% confidence interval, 3.4-5.7), the study showed. Breastfeeding within 1 hour of birth was also associated with greater likelihood of breastfeeding success (RR, 1.3; 95% CI, 1.0-1.6).
The study, led by Heather C. Hamner, PhD, MS, MPH, of the National Center for Chronic Disease Prevention and Health Promotion, , Atlanta, was reported online in Pediatrics.
“This study confirms the relationship between experiencing maternity care practices supportive of breastfeeding and meeting one’s breastfeeding intentions, and adds evidence specifically among low-income women, who are known to be at higher risk of not breastfeeding,” the study authors wrote.
Women from low-income households often face additional barriers to meeting their breastfeeding goals, including lack of access to professional lactation services, Dr. Hamner said in an interview. “We want physicians to know how important maternity care practices supportive of breastfeeding are to helping all women achieve their breastfeeding goals. Physicians can be champions for implementation of evidence-based maternity care practices in the hospitals and practices in which they work.”
Dr. Hamner emphasized that physicians need to discuss the importance of breastfeeding with patients and their families, brief them on what to expect in the maternity care setting, and ensure women are connected to lactation resources. The American Academy of Pediatrics is working to increase physician capacity to support breastfeeding through the Physician Engagement and Training Focused on Breastfeeding project.
For the study, Dr. Hamner and colleagues analyzed data from the longitudinal WIC Infant and Toddler Feeding Practices Study-2 (ITFPS-2), which assessed the impact of 6 steps from a 10-step maternity care protocol known as The Ten Steps To Successful Breastfeeding. These steps are part of the worldwide Baby-Friendly Hospital Initiative (BFHI), which has been shown to improve rates of breastfeeding initiation, duration, and exclusivity.
After adjusting for sociodemographic and other factors, the study authors estimated risk ratios for associations between each of six maternity care practices assessed in ITFPS-2 and the success of women who reported an intention to breastfeed exclusively for 1 month. The six steps included initiation of breastfeeding within 1 hour of birth (step 4), showing moms how to breastfeed and maintain lactation (step 5), giving no food or drink other than breast milk unless medically indicated (step 6), rooming-in (step 7), breastfeeding on demand (step 8), and giving no pacifiers (step 9).
The analyses showed that only steps 4 and 6 – initiating breastfeeding at birth and giving only breast milk – remained significantly associated with meeting breastfeeding intentions. The results also revealed a dose-response relationship between the number of baby steps experienced during birth hospitalization and the likelihood of meeting breastfeeding goals, a finding in keeping with previous studies. In women who experienced all six steps, for example, 76% were breastfeeding exclusively at 1 month, compared with 16% of those who experienced zero to two steps.
Although the dose-response relationship did not appear to differ significantly by race or ethnicity, it was driven primarily by a hospital policy of providing infant formula or other supplementation, the study authors found. Notably, 44% of women reported that their infant had been fed something other than breast milk while in the hospital, and about 60% said they stopped breastfeeding earlier than intended.
“This finding reiterates the importance of limiting in-hospital formula or other supplementation of breastfed infants to only those with medical necessity,” Dr. Hamner and colleagues said.
Despite improvements in maternity care practices that promote breastfeeding, including an increase in the number of births occurring in U.S. hospitals with a baby-friendly designation, many women continue to experience significant barriers to breastfeeding, the investigators pointed out. Currently, there are 592 baby-friendly hospitals in the United States, representing 28.29% of annual births.
“I think more hospitals becoming baby friendly would really help,” Mary Franklin, DNP, CNM, assistant professor at Case Western Reserve University, Cleveland, said in an interview. More needs to be done to support women during birth hospitalization and after they return home, so they can continue to breastfeed for “longer than the initial 6 weeks,” added Dr. Franklin, who is also director of the nurse midwifery and women’s health NP program.
The AAP recommends exclusive breastfeeding for about 6 months followed by complementary food introduction and continued breastfeeding through 12 months or beyond.
Like Dr. Hamner, Dr. Franklin emphasized that physicians have an important role to play in the initiation, duration, and exclusivity of breastfeeding. This includes promoting enrichment of the pregnancy experience with prenatal education and increased support from health care providers and peers. At delivery, obstetricians can delay cord clamping to facilitate early breastfeeding. They can also support the elimination of the central nursery in hospitals so that mother and baby stay together from birth. In addition, prescriptions can be written for breast pumps, which are covered by Medicaid.
The study received no outside funding. Dr. Hamner and coauthors disclosed having no potential financial conflicts of interest. Dr. Franklin also disclosed having no financial conflicts of interest.
A first-ever study of the effect of evidence-based maternity care practices on prenatal breastfeeding intentions in women from low-income U.S. households shows that the use of “baby-friendly steps” during birth hospitalization made it possible for almost half to breastfeed exclusively for 1 month.
Analyses of national data from a longitudinal study of 1,080 women enrolled in the Special Supplemental Nutrition Program for Women, Infants, and Children (WIC) revealed that 47% were able to meet their prenatal intention to breastfeed without formula or other milk for at least 30 days.
The odds of meeting prenatal breastfeeding intentions more than quadrupled when babies received only breast milk (risk ratio, 4.4; 95% confidence interval, 3.4-5.7), the study showed. Breastfeeding within 1 hour of birth was also associated with greater likelihood of breastfeeding success (RR, 1.3; 95% CI, 1.0-1.6).
The study, led by Heather C. Hamner, PhD, MS, MPH, of the National Center for Chronic Disease Prevention and Health Promotion, , Atlanta, was reported online in Pediatrics.
“This study confirms the relationship between experiencing maternity care practices supportive of breastfeeding and meeting one’s breastfeeding intentions, and adds evidence specifically among low-income women, who are known to be at higher risk of not breastfeeding,” the study authors wrote.
Women from low-income households often face additional barriers to meeting their breastfeeding goals, including lack of access to professional lactation services, Dr. Hamner said in an interview. “We want physicians to know how important maternity care practices supportive of breastfeeding are to helping all women achieve their breastfeeding goals. Physicians can be champions for implementation of evidence-based maternity care practices in the hospitals and practices in which they work.”
Dr. Hamner emphasized that physicians need to discuss the importance of breastfeeding with patients and their families, brief them on what to expect in the maternity care setting, and ensure women are connected to lactation resources. The American Academy of Pediatrics is working to increase physician capacity to support breastfeeding through the Physician Engagement and Training Focused on Breastfeeding project.
For the study, Dr. Hamner and colleagues analyzed data from the longitudinal WIC Infant and Toddler Feeding Practices Study-2 (ITFPS-2), which assessed the impact of 6 steps from a 10-step maternity care protocol known as The Ten Steps To Successful Breastfeeding. These steps are part of the worldwide Baby-Friendly Hospital Initiative (BFHI), which has been shown to improve rates of breastfeeding initiation, duration, and exclusivity.
After adjusting for sociodemographic and other factors, the study authors estimated risk ratios for associations between each of six maternity care practices assessed in ITFPS-2 and the success of women who reported an intention to breastfeed exclusively for 1 month. The six steps included initiation of breastfeeding within 1 hour of birth (step 4), showing moms how to breastfeed and maintain lactation (step 5), giving no food or drink other than breast milk unless medically indicated (step 6), rooming-in (step 7), breastfeeding on demand (step 8), and giving no pacifiers (step 9).
The analyses showed that only steps 4 and 6 – initiating breastfeeding at birth and giving only breast milk – remained significantly associated with meeting breastfeeding intentions. The results also revealed a dose-response relationship between the number of baby steps experienced during birth hospitalization and the likelihood of meeting breastfeeding goals, a finding in keeping with previous studies. In women who experienced all six steps, for example, 76% were breastfeeding exclusively at 1 month, compared with 16% of those who experienced zero to two steps.
Although the dose-response relationship did not appear to differ significantly by race or ethnicity, it was driven primarily by a hospital policy of providing infant formula or other supplementation, the study authors found. Notably, 44% of women reported that their infant had been fed something other than breast milk while in the hospital, and about 60% said they stopped breastfeeding earlier than intended.
“This finding reiterates the importance of limiting in-hospital formula or other supplementation of breastfed infants to only those with medical necessity,” Dr. Hamner and colleagues said.
Despite improvements in maternity care practices that promote breastfeeding, including an increase in the number of births occurring in U.S. hospitals with a baby-friendly designation, many women continue to experience significant barriers to breastfeeding, the investigators pointed out. Currently, there are 592 baby-friendly hospitals in the United States, representing 28.29% of annual births.
“I think more hospitals becoming baby friendly would really help,” Mary Franklin, DNP, CNM, assistant professor at Case Western Reserve University, Cleveland, said in an interview. More needs to be done to support women during birth hospitalization and after they return home, so they can continue to breastfeed for “longer than the initial 6 weeks,” added Dr. Franklin, who is also director of the nurse midwifery and women’s health NP program.
The AAP recommends exclusive breastfeeding for about 6 months followed by complementary food introduction and continued breastfeeding through 12 months or beyond.
Like Dr. Hamner, Dr. Franklin emphasized that physicians have an important role to play in the initiation, duration, and exclusivity of breastfeeding. This includes promoting enrichment of the pregnancy experience with prenatal education and increased support from health care providers and peers. At delivery, obstetricians can delay cord clamping to facilitate early breastfeeding. They can also support the elimination of the central nursery in hospitals so that mother and baby stay together from birth. In addition, prescriptions can be written for breast pumps, which are covered by Medicaid.
The study received no outside funding. Dr. Hamner and coauthors disclosed having no potential financial conflicts of interest. Dr. Franklin also disclosed having no financial conflicts of interest.
A first-ever study of the effect of evidence-based maternity care practices on prenatal breastfeeding intentions in women from low-income U.S. households shows that the use of “baby-friendly steps” during birth hospitalization made it possible for almost half to breastfeed exclusively for 1 month.
Analyses of national data from a longitudinal study of 1,080 women enrolled in the Special Supplemental Nutrition Program for Women, Infants, and Children (WIC) revealed that 47% were able to meet their prenatal intention to breastfeed without formula or other milk for at least 30 days.
The odds of meeting prenatal breastfeeding intentions more than quadrupled when babies received only breast milk (risk ratio, 4.4; 95% confidence interval, 3.4-5.7), the study showed. Breastfeeding within 1 hour of birth was also associated with greater likelihood of breastfeeding success (RR, 1.3; 95% CI, 1.0-1.6).
The study, led by Heather C. Hamner, PhD, MS, MPH, of the National Center for Chronic Disease Prevention and Health Promotion, , Atlanta, was reported online in Pediatrics.
“This study confirms the relationship between experiencing maternity care practices supportive of breastfeeding and meeting one’s breastfeeding intentions, and adds evidence specifically among low-income women, who are known to be at higher risk of not breastfeeding,” the study authors wrote.
Women from low-income households often face additional barriers to meeting their breastfeeding goals, including lack of access to professional lactation services, Dr. Hamner said in an interview. “We want physicians to know how important maternity care practices supportive of breastfeeding are to helping all women achieve their breastfeeding goals. Physicians can be champions for implementation of evidence-based maternity care practices in the hospitals and practices in which they work.”
Dr. Hamner emphasized that physicians need to discuss the importance of breastfeeding with patients and their families, brief them on what to expect in the maternity care setting, and ensure women are connected to lactation resources. The American Academy of Pediatrics is working to increase physician capacity to support breastfeeding through the Physician Engagement and Training Focused on Breastfeeding project.
For the study, Dr. Hamner and colleagues analyzed data from the longitudinal WIC Infant and Toddler Feeding Practices Study-2 (ITFPS-2), which assessed the impact of 6 steps from a 10-step maternity care protocol known as The Ten Steps To Successful Breastfeeding. These steps are part of the worldwide Baby-Friendly Hospital Initiative (BFHI), which has been shown to improve rates of breastfeeding initiation, duration, and exclusivity.
After adjusting for sociodemographic and other factors, the study authors estimated risk ratios for associations between each of six maternity care practices assessed in ITFPS-2 and the success of women who reported an intention to breastfeed exclusively for 1 month. The six steps included initiation of breastfeeding within 1 hour of birth (step 4), showing moms how to breastfeed and maintain lactation (step 5), giving no food or drink other than breast milk unless medically indicated (step 6), rooming-in (step 7), breastfeeding on demand (step 8), and giving no pacifiers (step 9).
The analyses showed that only steps 4 and 6 – initiating breastfeeding at birth and giving only breast milk – remained significantly associated with meeting breastfeeding intentions. The results also revealed a dose-response relationship between the number of baby steps experienced during birth hospitalization and the likelihood of meeting breastfeeding goals, a finding in keeping with previous studies. In women who experienced all six steps, for example, 76% were breastfeeding exclusively at 1 month, compared with 16% of those who experienced zero to two steps.
Although the dose-response relationship did not appear to differ significantly by race or ethnicity, it was driven primarily by a hospital policy of providing infant formula or other supplementation, the study authors found. Notably, 44% of women reported that their infant had been fed something other than breast milk while in the hospital, and about 60% said they stopped breastfeeding earlier than intended.
“This finding reiterates the importance of limiting in-hospital formula or other supplementation of breastfed infants to only those with medical necessity,” Dr. Hamner and colleagues said.
Despite improvements in maternity care practices that promote breastfeeding, including an increase in the number of births occurring in U.S. hospitals with a baby-friendly designation, many women continue to experience significant barriers to breastfeeding, the investigators pointed out. Currently, there are 592 baby-friendly hospitals in the United States, representing 28.29% of annual births.
“I think more hospitals becoming baby friendly would really help,” Mary Franklin, DNP, CNM, assistant professor at Case Western Reserve University, Cleveland, said in an interview. More needs to be done to support women during birth hospitalization and after they return home, so they can continue to breastfeed for “longer than the initial 6 weeks,” added Dr. Franklin, who is also director of the nurse midwifery and women’s health NP program.
The AAP recommends exclusive breastfeeding for about 6 months followed by complementary food introduction and continued breastfeeding through 12 months or beyond.
Like Dr. Hamner, Dr. Franklin emphasized that physicians have an important role to play in the initiation, duration, and exclusivity of breastfeeding. This includes promoting enrichment of the pregnancy experience with prenatal education and increased support from health care providers and peers. At delivery, obstetricians can delay cord clamping to facilitate early breastfeeding. They can also support the elimination of the central nursery in hospitals so that mother and baby stay together from birth. In addition, prescriptions can be written for breast pumps, which are covered by Medicaid.
The study received no outside funding. Dr. Hamner and coauthors disclosed having no potential financial conflicts of interest. Dr. Franklin also disclosed having no financial conflicts of interest.
FROM PEDIATRICS
Does adjunctive oxytocin infusion during balloon cervical ripening improve labor induction?
Evidence summary
Time to delivery is shortened with combined therapy
Two recent high-quality meta-analyses investigated the effect of adding oxytocin to transcervical Foley balloon placement for cervical dilation. A network meta-analysis, including 30 RCTs (with 6465 pregnant patients), examined the efficacy of multiple combinations of cervical ripening methods.1 A subset of 7 trials (n = 1313) compared oxytocin infusion with transcervical Foley (inflated to 30-60 mL) to Foley alone. Patients were at > 24 weeks’ gestation with a live fetus and undergoing elective or medical induction of labor; exclusion criteria were standard contraindications to vaginal delivery.
Compared to Foley alone, Foley plus oxytocin reduced both the time to the primary outcome of vaginal delivery (mean duration [MD] = –4.2 h; 95% CI, –1.9 to –6.5) and the time to overall (vaginal and cesarean) delivery (MD = –3.1 h; 95% CI, –1.5 to –4.6). There were no differences in rates of cesarean section, chorioamnionitis, epidural use, or neonatal intensive care unit admission. This analysis did not stratify by parity.1
In a standard meta-analysis, researchers identified 6 RCTs (N = 1133) comparing transcervical Foley balloon and oxytocin to Foley balloon alone for cervical ripening in pregnant patients at > 23 weeks’ gestation (1 trial was limited to patients at > 37 weeks’ gestation).2 Foley balloons were inflated with 30 to 60 mL saline, and oxytocin infusions started at 1 to 2 mU/min and were titrated up to 10 to 40 mU/min. Balloon time was usually 12 hours, but not always stated.
The authors found no statistically significant difference in cesarean rates (the primary outcome) between Foley plus oxytocin vs Foley alone (relative risk [RR] = 0.91; 95% CI, 0.76-1.1). Overall delivery within 12 hours was more likely with combined therapy (RR of remaining pregnant = 0.46; 95% CI, 0.34-0.63), but delivery at 24 hours was not (RR = 0.94; 95% CI, 0.92-1.05). However, in a sub-analysis by parity, nulliparous women who received combined therapy had higher overall delivery rates in 24 hours than did multiparous women (RR = 0.77; 95% CI, 0.62-0.97).2
Adding oxytocin may allow shorter transcervical balloon times
One recent RCT (N = 177) compared labor induction with oxytocin and a single trans-cervical balloon (Cook catheter with only the intrauterine balloon inflated) removed at either 6 or 12 hours.3 Patients were pregnant women (mean age, 31 years) with a term singleton vertex pregnancy, a Bishop score ≤ 6, and no contraindications to vaginal delivery. All patients received a balloon inflated to 60 mL with an oxytocin infusion (2-30 mU/min). The intervention group had the balloon removed at 6 hours, while the control group had it removed at 12 hours.
The mean Bishop score changed by 6 points in each group. Time to overall delivery (the primary outcome) was significantly shorter with 6 hours of balloon time than with 12 hours (19.2 vs 24.3 h; P < .04). Overall delivery within 24 hours was also significantly more likely in the 6-hour group (67.4% vs 47.4%; P < .01), although vaginal delivery in 24 hours did not change (74% vs 59%; P = .07). No differences were seen in cesarean delivery rates or maternal or neonatal morbidity rates.
A look at fixed-dose vs titrated oxytocin
Another RCT (N = 116) examined the effectiveness of cervical ripening using a Foley balloon plus either fixed-dose or titrated low-dose oxytocin.4 Patients (mean age, 26 years) had singleton pregnancies at ≥ 37 weeks’ gestation with a Bishop score < 6 and presented for induction of labor. Foley balloons were inflated to 30 mL, and patients received either a fixed oxytocin infusion of 2 mU/min or a titrated infusion starting at 1 mU/min, increasing by 2 mU/min every 30 minutes to a maximum of 20 mU/min.
Continue to: Thre was no statistically...
There was no statistically significant difference in median time from Foley placement to overall delivery (the primary outcome) between the fixed low-dose and incremental low-dose groups in either nulliparous women (24 vs 19 h; P = .18) or multiparous women (16 vs 12 h; P = .68). The authors acknowledged the study may have been underpowered to detect a true difference.
Recommendations from others
A 2009 Practice Bulletin from the American College of Obstetricians and Gynecologists (ACOG) recommended the Foley catheter as a reasonable and effective alternative to prostaglandins for cervical ripening and the induction of labor (based on good-quality evidence).5 The guideline stated that Foley catheter placement before oxytocin induction reduced both the duration of labor and risk of cesarean delivery, but that the use of oxytocin along with a Foley catheter did not appear to shorten the time to delivery.
Editor’s takeaway
High-quality evidence shows us that the addition of oxytocin to balloon cervical ripening shortens the time to delivery. This newer evidence may prompt an update to the 2009 ACOG statement.
1. Orr L, Reisinger-Kindle K, Roy A, et al. Combination of Foley and prostaglandins versus Foley and oxytocin for cervical ripening: a network meta-analysis. Am J Obstet Gynecol. 2020;223:743.e1-743.e17. doi: 10.1016/j.ajog.2020.05.007
2. Gallagher LT, Gardner B, Rahman M, et al. Cervical ripening using Foley balloon with or without oxytocin: a systematic review and meta-analysis. Am J Perinatol. 2019;36:406-421. doi: 10.1055/s-0038-1668577
3. Lassey SC, Haber HR, Kanbergs A, et al. Six vs twelve hours of single balloon catheter placement with oxytocin administration for labor induction: a randomized controlled trial. Am J Obstet Gynecol. 2021:S0002-9378(21)00185-X. doi: 10.1016/j.ajog.2021.03.021
4. Fitzpatrick CB, Grotegut CA, Bishop TS, et al. Cervical ripening with Foley balloon plus fixed versus incremental low-dose oxytocin: a randomized controlled trial. J Matern Fetal Neonatal Med. 2012;25:1006-1010. doi: 10.3109/14767058.2011.607522
5. ACOG Practice Bulletin No. 107: Induction of labor. Obstet Gynecol. 2009;114(2 pt 1):386-397. doi: 10.1097/AOG.0b013e3181b48ef5
Evidence summary
Time to delivery is shortened with combined therapy
Two recent high-quality meta-analyses investigated the effect of adding oxytocin to transcervical Foley balloon placement for cervical dilation. A network meta-analysis, including 30 RCTs (with 6465 pregnant patients), examined the efficacy of multiple combinations of cervical ripening methods.1 A subset of 7 trials (n = 1313) compared oxytocin infusion with transcervical Foley (inflated to 30-60 mL) to Foley alone. Patients were at > 24 weeks’ gestation with a live fetus and undergoing elective or medical induction of labor; exclusion criteria were standard contraindications to vaginal delivery.
Compared to Foley alone, Foley plus oxytocin reduced both the time to the primary outcome of vaginal delivery (mean duration [MD] = –4.2 h; 95% CI, –1.9 to –6.5) and the time to overall (vaginal and cesarean) delivery (MD = –3.1 h; 95% CI, –1.5 to –4.6). There were no differences in rates of cesarean section, chorioamnionitis, epidural use, or neonatal intensive care unit admission. This analysis did not stratify by parity.1
In a standard meta-analysis, researchers identified 6 RCTs (N = 1133) comparing transcervical Foley balloon and oxytocin to Foley balloon alone for cervical ripening in pregnant patients at > 23 weeks’ gestation (1 trial was limited to patients at > 37 weeks’ gestation).2 Foley balloons were inflated with 30 to 60 mL saline, and oxytocin infusions started at 1 to 2 mU/min and were titrated up to 10 to 40 mU/min. Balloon time was usually 12 hours, but not always stated.
The authors found no statistically significant difference in cesarean rates (the primary outcome) between Foley plus oxytocin vs Foley alone (relative risk [RR] = 0.91; 95% CI, 0.76-1.1). Overall delivery within 12 hours was more likely with combined therapy (RR of remaining pregnant = 0.46; 95% CI, 0.34-0.63), but delivery at 24 hours was not (RR = 0.94; 95% CI, 0.92-1.05). However, in a sub-analysis by parity, nulliparous women who received combined therapy had higher overall delivery rates in 24 hours than did multiparous women (RR = 0.77; 95% CI, 0.62-0.97).2
Adding oxytocin may allow shorter transcervical balloon times
One recent RCT (N = 177) compared labor induction with oxytocin and a single trans-cervical balloon (Cook catheter with only the intrauterine balloon inflated) removed at either 6 or 12 hours.3 Patients were pregnant women (mean age, 31 years) with a term singleton vertex pregnancy, a Bishop score ≤ 6, and no contraindications to vaginal delivery. All patients received a balloon inflated to 60 mL with an oxytocin infusion (2-30 mU/min). The intervention group had the balloon removed at 6 hours, while the control group had it removed at 12 hours.
The mean Bishop score changed by 6 points in each group. Time to overall delivery (the primary outcome) was significantly shorter with 6 hours of balloon time than with 12 hours (19.2 vs 24.3 h; P < .04). Overall delivery within 24 hours was also significantly more likely in the 6-hour group (67.4% vs 47.4%; P < .01), although vaginal delivery in 24 hours did not change (74% vs 59%; P = .07). No differences were seen in cesarean delivery rates or maternal or neonatal morbidity rates.
A look at fixed-dose vs titrated oxytocin
Another RCT (N = 116) examined the effectiveness of cervical ripening using a Foley balloon plus either fixed-dose or titrated low-dose oxytocin.4 Patients (mean age, 26 years) had singleton pregnancies at ≥ 37 weeks’ gestation with a Bishop score < 6 and presented for induction of labor. Foley balloons were inflated to 30 mL, and patients received either a fixed oxytocin infusion of 2 mU/min or a titrated infusion starting at 1 mU/min, increasing by 2 mU/min every 30 minutes to a maximum of 20 mU/min.
Continue to: Thre was no statistically...
There was no statistically significant difference in median time from Foley placement to overall delivery (the primary outcome) between the fixed low-dose and incremental low-dose groups in either nulliparous women (24 vs 19 h; P = .18) or multiparous women (16 vs 12 h; P = .68). The authors acknowledged the study may have been underpowered to detect a true difference.
Recommendations from others
A 2009 Practice Bulletin from the American College of Obstetricians and Gynecologists (ACOG) recommended the Foley catheter as a reasonable and effective alternative to prostaglandins for cervical ripening and the induction of labor (based on good-quality evidence).5 The guideline stated that Foley catheter placement before oxytocin induction reduced both the duration of labor and risk of cesarean delivery, but that the use of oxytocin along with a Foley catheter did not appear to shorten the time to delivery.
Editor’s takeaway
High-quality evidence shows us that the addition of oxytocin to balloon cervical ripening shortens the time to delivery. This newer evidence may prompt an update to the 2009 ACOG statement.
Evidence summary
Time to delivery is shortened with combined therapy
Two recent high-quality meta-analyses investigated the effect of adding oxytocin to transcervical Foley balloon placement for cervical dilation. A network meta-analysis, including 30 RCTs (with 6465 pregnant patients), examined the efficacy of multiple combinations of cervical ripening methods.1 A subset of 7 trials (n = 1313) compared oxytocin infusion with transcervical Foley (inflated to 30-60 mL) to Foley alone. Patients were at > 24 weeks’ gestation with a live fetus and undergoing elective or medical induction of labor; exclusion criteria were standard contraindications to vaginal delivery.
Compared to Foley alone, Foley plus oxytocin reduced both the time to the primary outcome of vaginal delivery (mean duration [MD] = –4.2 h; 95% CI, –1.9 to –6.5) and the time to overall (vaginal and cesarean) delivery (MD = –3.1 h; 95% CI, –1.5 to –4.6). There were no differences in rates of cesarean section, chorioamnionitis, epidural use, or neonatal intensive care unit admission. This analysis did not stratify by parity.1
In a standard meta-analysis, researchers identified 6 RCTs (N = 1133) comparing transcervical Foley balloon and oxytocin to Foley balloon alone for cervical ripening in pregnant patients at > 23 weeks’ gestation (1 trial was limited to patients at > 37 weeks’ gestation).2 Foley balloons were inflated with 30 to 60 mL saline, and oxytocin infusions started at 1 to 2 mU/min and were titrated up to 10 to 40 mU/min. Balloon time was usually 12 hours, but not always stated.
The authors found no statistically significant difference in cesarean rates (the primary outcome) between Foley plus oxytocin vs Foley alone (relative risk [RR] = 0.91; 95% CI, 0.76-1.1). Overall delivery within 12 hours was more likely with combined therapy (RR of remaining pregnant = 0.46; 95% CI, 0.34-0.63), but delivery at 24 hours was not (RR = 0.94; 95% CI, 0.92-1.05). However, in a sub-analysis by parity, nulliparous women who received combined therapy had higher overall delivery rates in 24 hours than did multiparous women (RR = 0.77; 95% CI, 0.62-0.97).2
Adding oxytocin may allow shorter transcervical balloon times
One recent RCT (N = 177) compared labor induction with oxytocin and a single trans-cervical balloon (Cook catheter with only the intrauterine balloon inflated) removed at either 6 or 12 hours.3 Patients were pregnant women (mean age, 31 years) with a term singleton vertex pregnancy, a Bishop score ≤ 6, and no contraindications to vaginal delivery. All patients received a balloon inflated to 60 mL with an oxytocin infusion (2-30 mU/min). The intervention group had the balloon removed at 6 hours, while the control group had it removed at 12 hours.
The mean Bishop score changed by 6 points in each group. Time to overall delivery (the primary outcome) was significantly shorter with 6 hours of balloon time than with 12 hours (19.2 vs 24.3 h; P < .04). Overall delivery within 24 hours was also significantly more likely in the 6-hour group (67.4% vs 47.4%; P < .01), although vaginal delivery in 24 hours did not change (74% vs 59%; P = .07). No differences were seen in cesarean delivery rates or maternal or neonatal morbidity rates.
A look at fixed-dose vs titrated oxytocin
Another RCT (N = 116) examined the effectiveness of cervical ripening using a Foley balloon plus either fixed-dose or titrated low-dose oxytocin.4 Patients (mean age, 26 years) had singleton pregnancies at ≥ 37 weeks’ gestation with a Bishop score < 6 and presented for induction of labor. Foley balloons were inflated to 30 mL, and patients received either a fixed oxytocin infusion of 2 mU/min or a titrated infusion starting at 1 mU/min, increasing by 2 mU/min every 30 minutes to a maximum of 20 mU/min.
Continue to: Thre was no statistically...
There was no statistically significant difference in median time from Foley placement to overall delivery (the primary outcome) between the fixed low-dose and incremental low-dose groups in either nulliparous women (24 vs 19 h; P = .18) or multiparous women (16 vs 12 h; P = .68). The authors acknowledged the study may have been underpowered to detect a true difference.
Recommendations from others
A 2009 Practice Bulletin from the American College of Obstetricians and Gynecologists (ACOG) recommended the Foley catheter as a reasonable and effective alternative to prostaglandins for cervical ripening and the induction of labor (based on good-quality evidence).5 The guideline stated that Foley catheter placement before oxytocin induction reduced both the duration of labor and risk of cesarean delivery, but that the use of oxytocin along with a Foley catheter did not appear to shorten the time to delivery.
Editor’s takeaway
High-quality evidence shows us that the addition of oxytocin to balloon cervical ripening shortens the time to delivery. This newer evidence may prompt an update to the 2009 ACOG statement.
1. Orr L, Reisinger-Kindle K, Roy A, et al. Combination of Foley and prostaglandins versus Foley and oxytocin for cervical ripening: a network meta-analysis. Am J Obstet Gynecol. 2020;223:743.e1-743.e17. doi: 10.1016/j.ajog.2020.05.007
2. Gallagher LT, Gardner B, Rahman M, et al. Cervical ripening using Foley balloon with or without oxytocin: a systematic review and meta-analysis. Am J Perinatol. 2019;36:406-421. doi: 10.1055/s-0038-1668577
3. Lassey SC, Haber HR, Kanbergs A, et al. Six vs twelve hours of single balloon catheter placement with oxytocin administration for labor induction: a randomized controlled trial. Am J Obstet Gynecol. 2021:S0002-9378(21)00185-X. doi: 10.1016/j.ajog.2021.03.021
4. Fitzpatrick CB, Grotegut CA, Bishop TS, et al. Cervical ripening with Foley balloon plus fixed versus incremental low-dose oxytocin: a randomized controlled trial. J Matern Fetal Neonatal Med. 2012;25:1006-1010. doi: 10.3109/14767058.2011.607522
5. ACOG Practice Bulletin No. 107: Induction of labor. Obstet Gynecol. 2009;114(2 pt 1):386-397. doi: 10.1097/AOG.0b013e3181b48ef5
1. Orr L, Reisinger-Kindle K, Roy A, et al. Combination of Foley and prostaglandins versus Foley and oxytocin for cervical ripening: a network meta-analysis. Am J Obstet Gynecol. 2020;223:743.e1-743.e17. doi: 10.1016/j.ajog.2020.05.007
2. Gallagher LT, Gardner B, Rahman M, et al. Cervical ripening using Foley balloon with or without oxytocin: a systematic review and meta-analysis. Am J Perinatol. 2019;36:406-421. doi: 10.1055/s-0038-1668577
3. Lassey SC, Haber HR, Kanbergs A, et al. Six vs twelve hours of single balloon catheter placement with oxytocin administration for labor induction: a randomized controlled trial. Am J Obstet Gynecol. 2021:S0002-9378(21)00185-X. doi: 10.1016/j.ajog.2021.03.021
4. Fitzpatrick CB, Grotegut CA, Bishop TS, et al. Cervical ripening with Foley balloon plus fixed versus incremental low-dose oxytocin: a randomized controlled trial. J Matern Fetal Neonatal Med. 2012;25:1006-1010. doi: 10.3109/14767058.2011.607522
5. ACOG Practice Bulletin No. 107: Induction of labor. Obstet Gynecol. 2009;114(2 pt 1):386-397. doi: 10.1097/AOG.0b013e3181b48ef5
EVIDENCE-BASED ANSWER:
YES. Compared to the use of a transcervical balloon alone, combined cervical ripening with a balloon catheter and oxytocin shortens the time to overall delivery by 3 hours and the time to vaginal delivery by 4 hours, without altering the rate of cesarean section (strength of recommendation [SOR]: A, network meta-analysis). The effect is more pronounced in nulliparous patients (SOR: A, meta-analysis).
When combined therapy is used, 6 hours of balloon time may result in faster delivery than 12 hours (SOR: B, single randomized controlled trial [RCT]). Fixed-dose oxytocin and titrated oxytocin appear to have similar effect when combined with a cervical ripening balloon (SOR: C, underpowered RCT).
Dyspareunia: Keys to biopsychosocial evaluation and treatment planning
Dyspareunia is persistent or recurrent pain before, during, or after sexual contact and is not limited to cisgender individuals or vaginal intercourse.1-3 With a prevalence as high as 45% in the United States,2-5 it is one of the most common complaints in gynecologic practices.5,6
Causes and contributing factors
There are many possible causes of dyspareunia.2,4,6 While some patients have a single cause, most cases are complex, with multiple overlapping causes and maintaining factors.4,6 Identifying each contributing factor can help you appropriately address all components.
Physical conditions. The range of physical contributors to dyspareunia includes inflammatory processes, structural abnormalities, musculoskeletal dysfunctions, pelvic organ disorders, injuries, iatrogenic effects, infections, allergic reactions, sensitization, hormonal changes, medication effects, adhesions, autoimmune disorders, and other pain syndromes (TABLE 12-4,6-11).

Inadequate arousal. One of the primary causes of pain during vaginal penetration is inadequate arousal and lubrication.1,2,9-11 Arousal is the phase of the sexual response cycle that leads to genital tumescence and prepares the genitals for sexual contact through penile/clitoral erection, vaginal engorgement, and lubrication, which prevents pain and enhances pleasurable sensation.9-11
While some physical conditions can lead to an inability to lubricate, the most common causes of inadequate lubrication are psychosocial-behavioral, wherein patients have the same physical ability to lubricate as patients without genital pain but do not progress through the arousal phase.9-11 Behavioral factors such as inadequate or ineffective foreplay can fail to produce engorgement and lubrication, while psychosocial factors such as low attraction to partner, relationship stressors, anxiety, or low self-esteem can have an inhibitory effect on sexual arousal.1,2,9-11 Psychosocial and behavioral factors may also be maintaining factors or consequences of dyspareunia, and need to be assessed and treated.1,2,9-11
Psychological trauma. Exposure to psychological traumas and the development of posttraumatic stress disorder (PTSD) have been linked with the development of pain disorders in general and dyspareunia specifically. Most patients seeking treatment for chronic pain disorders have a history of physical or sexual abuse.12 Changes in physiologic processes (eg, neurochemical, endocrine) that occur with PTSD interfere with the sexual response cycle, and sexual traumas specifically have been linked with pelvic floor dysfunction.13,14 Additionally, when PTSD is caused by a sexual trauma, even consensual sexual encounters can trigger flashbacks, intrusive memories, hyperarousal, and muscle tension that interfere with the sexual response cycle and contribute to genital pain.13
Vaginismus is both a physiologic and psychological contributor to dyspareunia.1,2,4 Patients experiencing pain can develop anxiety about repeated pain and involuntarily contract their pelvic muscles, thereby creating more pain, increasing anxiety, decreasing lubrication, and causing pelvic floor dysfunction.1-4,6 Consequently, all patients with dyspareunia should be assessed and continually monitored for symptoms of vaginismus.
Continue to: Anxiety
Anxiety. As with other pain disorders, anxiety develops around pain triggers.10,15 When expecting sexual activity, patients can experience extreme worry and panic attacks.10,15,16 The distress of sexual encounters can interfere with physiologic arousal and sexual desire, impacting all phases of the sexual response cycle.1,2
Relationship issues. Difficulty engaging in or avoidance of sexual activity can interfere with romantic relationships.2,10,16 Severe pain or vaginismus contractions can prevent penetration, leading to unconsummated marriages and an inability to conceive through intercourse.10 The distress surrounding sexual encounters can precipitate erectile dysfunction in male partners, or partners may continue to demand sexual encounters despite the patient’s pain, further impacting the relationship and heightening sexual distress.10 These stressors have led to relationships ending, patients reluctantly agreeing to nonmonogamy to appease their partners, and patients avoiding relationships altogether.10,16
Devalued self-image. Difficulties with sexuality and relationships impact the self-image of patients with dyspareunia. Diminished self-image may include feeling “inadequate” as a woman and as a sexual partner, or feeling like a “failure.”16 Women with dyspareunia often have more distress related to their body image, physical appearance, and genital self-image than do women without genital pain.17 Feeling resentment toward their body, or feeling “ugly,” embarrassed, shamed, “broken,” and “useless” also contribute to increased depressive symptoms found in patients with dyspareunia.16,18
Making the diagnosis
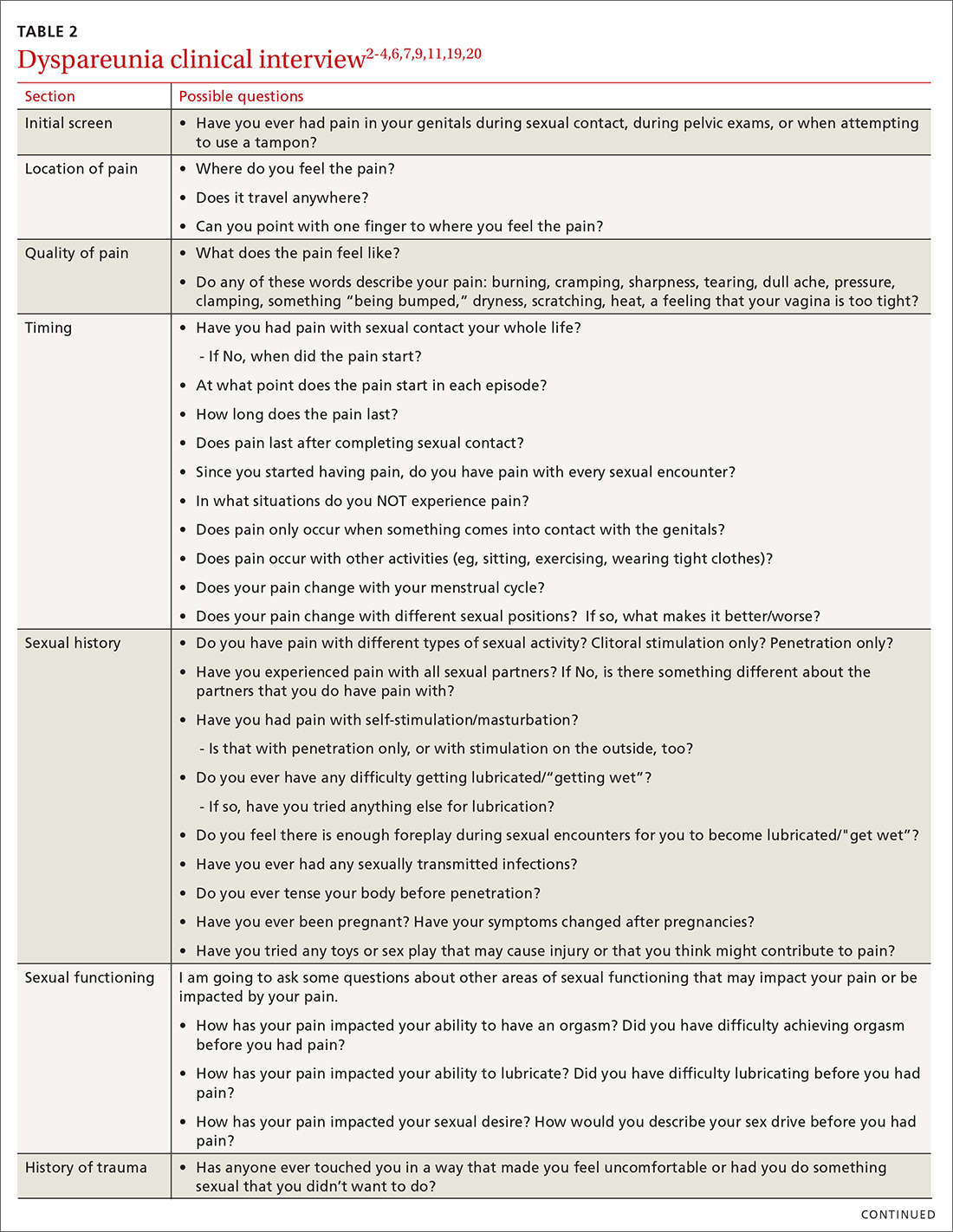
Most patients do not report symptoms unless directly asked2,7; therefore, it is recommended that all patients be screened as a part of an initial intake and before any genital exam (TABLE 22-4,6,7,9,11,19,20).4,7,21 If this screen is positive, a separate appointment may be needed for a thorough evaluation and before any attempt is made at a genital exam.4,7
Items to include in the clinical interview
Given the range of possible causes of dyspareunia and its contributing factors and symptoms, a thorough clinical interview is essential. Begin with a review of the patient’s complete medical and surgical history to identify possible known contributors to genital pain.4 Pregnancy history is of particular importance as the prevalence of postpartum dyspareunia is 35%, with risk being greater for patients who experienced dyspareunia symptoms before pregnancy.22

Knowing the location and quality of pain is important for differentiating between possible diagnoses, as is specifying dyspareunia as lifelong or acquired, superficial or deep, and primary or secondary.1-4,6 Confirm the specific location(s) of pain—eg, at the introitus, in the vestibule, on the labia, in the perineum, or near the clitoris.2,4,6 A diagram or model may be needed to help patients to localize pain.4
To help narrow the differential, include the following elements in your assessment: pain quality, timing (eg, initial onset, episode onset, episode duration, situational triggers), alleviating factors, symptoms in surrounding structures (eg, bladder, bowel, muscles, bones), sexual history, other areas of sexual functioning, history of psychological trauma, relationship effects, and mental health (TABLE 22-4,6,7,9,11,19,20 and Table 323-28). Screening for a history of sexual trauma is particularly important, as a recent systematic review and meta-analysis found that women with a history of sexual assault had a 42% higher risk of gynecologic problems overall, a 74% higher risk of dyspareunia, and a 71% higher risk of vaginismus than women without a history of sexual assault.29 Using measures such as the Female Sexual Function Index or the McGill Pain Questionnaire can help patients more thoroughly describe their symptoms (TABLE 323-28).3
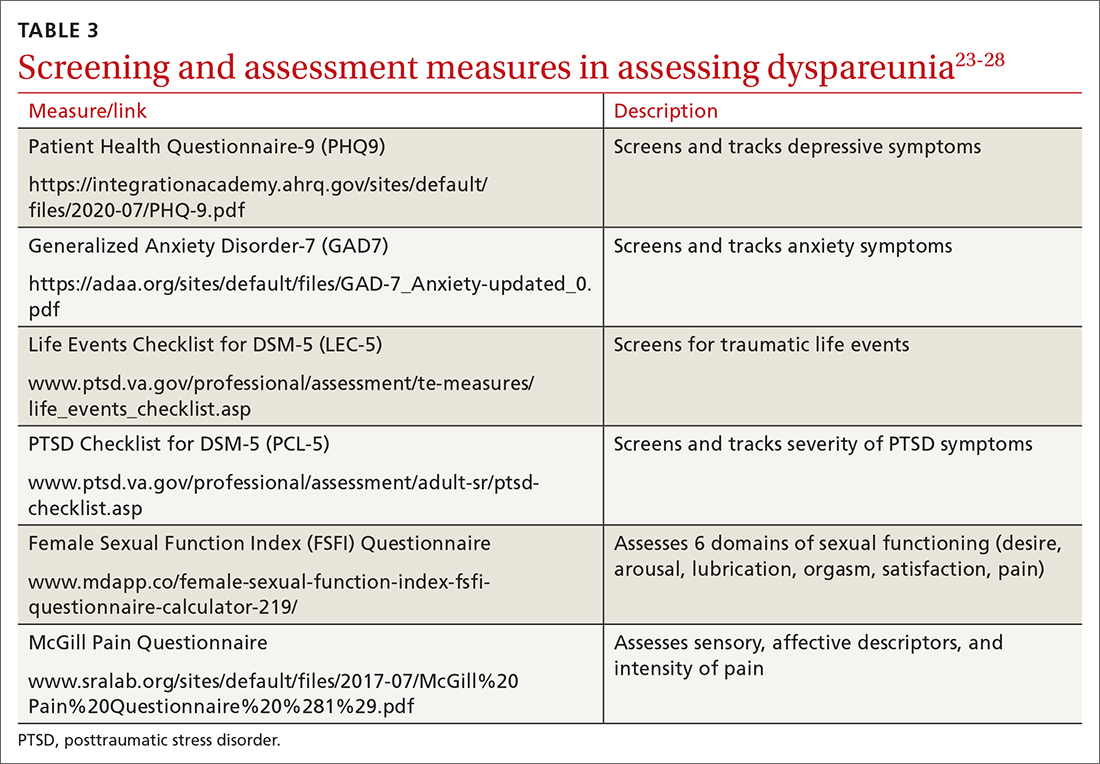
Continue to: Guidelines for the physical exam
Guidelines for the physical exam
Before the exam, ensure the patient has not used any topical genital treatment in the past 2 weeks that may interfere with sensitivity to the exam.4 To decrease patients’ anxiety about the exam, remind them that they can stop the exam at any time.7 Also consider offering the use of a mirror to better pinpoint the location of pain, and to possibly help the patient learn more about her anatomy.2,7
Begin the exam by palpating surrounding areas that may be involved in pain, including the abdomen and musculoskeletal features.3,6,19 Next visually inspect the external genitalia for lesions, abrasions, discoloration, erythema, or other abnormal findings.2,3,6 Ask the patient for permission before contacting the genitals. Because the labia may be a site of pain, apply gentle pressure in retracting it to fully examine the vestibule.6,7 Contraction of the pelvic floor muscles during approach or initial palpation could signal possible vaginismus.4
After visual inspection of external genitalia, use a cotton swab to map the vulva and vestibule in a clockwise fashion to precisely identify any painful locations.2-4,6 If the patient’s history of pain has been intermittent, it’s possible that the cotton swab will not elicit pain on the day of the initial exam, but it may on other days.4
Begin the internal exam by inserting a single finger into the first inch of the vagina and have the patient squeeze and release to assess tenderness, muscle tightness, and control.2,6 Advance the finger further into the vagina and palpate clockwise, examining the levator muscles, obturator muscles, rectum, urethra, and bladder for abnormal tightness or reproduction of pain.2,4,6 Complete a bimanual exam to evaluate the pelvic organs and adnexa.2,4 If indicated, a more thorough evaluation of pelvic floor musculature can be performed by a physical therapist or gynecologist who specializes in pelvic pain.2-4
If the patient consents to further evaluation, consider using a small speculum, advanced slowly, for further internal examination, noting any lesions, abrasions, discharge, ectropion, or tenderness.2-4,7 A rectal exam may also be needed in cases of deep dyspareunia.6 Initial work-up may include a potassium hydroxide wet prep, sexually transmitted infection testing, and pelvic ultrasound.2,4 In some cases, laparoscopy or biopsy may be needed.2,4
Treatments for common causes
Treatment often begins with education about anatomy, to help patients communicate about symptoms and engage more fully in their care.3 Additional education may be needed on genital functioning and the necessity of adequate stimulation and lubrication prior to penetration.1,2,9-11 A discussion of treatments for the wide range of possible causes of dyspareunia is outside the scope of this article. However, some basic behavioral changes may help patients address some of the more common contributing factors.
For example, if vaginal infection is suspected, advise patients to discontinue the use of harsh soaps, known vaginal irritants (eg, perfumed products, bath additives), and douches.3 Recommend using only preservative- and alcohol-free lubricants for sexual contact, and avoiding lubricants with added functions (eg, warming).3 It’s worth noting that avoidance of tight clothing and thong underwear due to possible risk for infections may not be necessary. A recent study found that women who frequently wore thong underwear (more than half of the time) were no more likely to develop urinary tract infections, yeast vaginitis, or bacterial vaginosis than those who avoid such items.30 However, noncotton underwear fabric, rather than tightness, was associated with yeast vaginitis30; therefore, patients may want to consider using only breathable underwear.3
Continue to: Medication
Medication. Medication may be used to treat the underlying contributing conditions or the symptom of pain directly. Some common options are particularly important for patients whose dyspareunia does not have an identifiable cause. These medications include anti-inflammatory agents, topical anesthetics, tricyclic antidepressants, and hormonal treatments.2-4 Since effectiveness varies based on subtypes of pain, select a medication according to the location, timing, and hypothesized mechanism of pain.3,31,32
Medication for deep pain. A meta-analysis and systematic review found that patients with some types of chronic pelvic pain with pain deep in the vagina or pelvis experienced greater than 50% reduction in pain using medroxyprogesterone acetate compared with placebo.33 Other treatments for deep pain depend on physical exam findings.
Medication for superficial pain. Many remedies have been tried, with at least 26 different treatments for vulvodynia pain alone.16 Only some of these treatments have supporting evidence. For patients with vulvar pain, an intent-to-treat RCT found that patients using a topical steroid experienced a 23% reduction in pain from pre-treatment to 6-month follow-up.32
Surgery is also effective for vulvar pain.34,35 For provoked vestibulodynia (in which pain is localized to the vestibule and triggered by contact with the vulva), or vulvar vestibulitis, RCTs have found that vestibulectomy has stronger effects on pain than other treatments,31,35 with a 53% reduction in pain during intercourse and a 70% reduction in vestibular pain overall.35 However, while vestibulectomy is effective for provoked vestibulodynia, it is not recommended for generalized vulvodynia, in which pain is diffuse across the vulva and occurs without vulvar contact.34
Unsupported treatments. A number of other treatments have not yet been found effective. Although lidocaine for vulvar pain is often used, RCTs have not found any significant reduction in symptoms, and a double-blind RCT found that lidocaine ointment actually performed worse than placebo.31,34 Similarly, oral tricyclics have not been found to decrease vulvar pain more than placebo in double-blind studies.31,34 Furthermore, a meta-analysis of RCTs comparing treatments with placebo for vestibular pain found no significant decrease in dyspareunia for topical conjugated estrogen, topical lidocaine, oral desipramine, oral desipramine with topical lidocaine, laser therapy, or transcranial direct current.32
Tx risks to consider. Risks and benefits of dyspareunia treatment options should be thoroughly weighed and discussed with the patient.2-4 Vestibulectomy, despite reducing pain for many patients, has led to increased pain for 9% of patients who underwent the procedure.35 Topical treatments may lead to allergic reactions, inflammation, and worsening of symptoms,4 and hormonal treatments have been found to increase the risk of weight gain and bloating and are not appropriate for patients trying to conceive.33
Coordinate care with other providers
While medications and surgery can reduce pain, they have not been shown to improve other aspects of sexual functioning such as sexual satisfaction, frequency of sexual intercourse, or overall sense of sexual functioning.35 Additionally, pain reduction does not address muscle tension, anxiety, self-esteem, and relationship problems. As a result, a multidisciplinary approach is generally needed.3,4,32,33
Continue to: Physical therapists
Physical therapists. Pelvic floor physical therapists are often members of the dyspareunia treatment team and can provide a thorough evaluation and treatment of pelvic floor disorders.2-4 An RCT with intent-to-treat analysis found that pain was reduced by 71% following pelvic floor physical therapy.36 Another RCT found that 90% of patients reported a clinically meaningful decrease in pain with pelvic floor physical therapy.37 In addition to addressing pain, pelvic floor physical therapy has also been found to improve sexual functioning, sexual satisfaction, distress, and patient perception of improvement.34,36,37
Behavioral health specialists. Psychotherapists, especially those trained in sex therapy, couples therapy, or cognitive behavioral therapy (CBT), are also typically on the treatment team. Multiple RCTs have found evidence of CBT’s effectiveness in the direct treatment of dyspareunia pain. Bergeron et al35 found a 37.5% reduction in vulvar vestibulitis pain intensity during intercourse after patients completed group CBT. Another intent-to-treat RCT found that patients receiving CBT experienced more pain reduction (~ 30%) than patients who were treated with a topical steroid.38
In addition to having a direct impact on pain, CBT has also been found to have a clinically and statistically significant positive impact on other aspects of sexual experience, such as overall sexuality, self-efficacy, overall sexual functioning, frequency of intercourse, and catastrophizing.34,38 A recent meta-analysis of RCTs found that about 80% of vaginismus patients were able to achieve penetrative intercourse after treatment with behavioral sex therapy or CBT.39 This success rate was not exceeded by physical or surgical treatments.39
When PTSD is thought to be a contributing factor, trauma therapy will likely be needed in addition to treatments for dyspareunia. First-line treatments for PTSD include cognitive processing therapy, prolonged exposure, trauma-focused CBT, and cognitive therapy.40
Psychotherapists can also help patients reduce anxiety, reintroduce sexual contact without triggering pain or anxiety, address emotional and self-esteem effects of dyspareunia, address relationship issues, and refocus sexual encounters on pleasure rather than pain avoidance.2-4 Despite patient reports of high treatment satisfaction following therapy,38 many patients may initially lack confidence in psychotherapy as a treatment for pain35 and may need to be educated on its effectiveness and multidimensional benefits.
Gynecologists. Often a gynecologist with specialization in pelvic pain is an essential member of the team for diagnostic clarification, recommendation of treatment options, and performance of more advanced treatments.2,3 If pain has become chronic, the patient may also benefit from a pain management team and support groups.2,3
Follow-up steps
Patients who screen negative for dyspareunia should be re-screened periodically. Continue to assess patients diagnosed with dyspareunia for vaginismus symptoms (if they are not initially present) to ensure that the treatment plan is appropriately adjusted. Once treatment has begun, ask about adverse effects and confidence in the treatment plan to minimize negative impacts on treatment adherence and to anticipate a need for a change in the treatment approach.31,35 In addition to tracking treatment effects on pain, continue to assess for patient-centered outcomes such as emotional functioning, self-esteem, and sexual and relationship satisfaction.34 The Female Sexual Function Index can be a useful tool to track symptoms.27,34
Finally, patients who do not experience sufficient improvement in symptoms and functioning with initial treatment may need continued support and encouragement. Given the broad range of contributing factors and the high number of potential treatments, patients may find hope in learning that multiple other treatment options may be available.
CORRESPONDENCE
Adrienne A. Williams, PhD, Department of Family and Community Medicine, University of Illinois at Chicago College of Medicine, 1919 W Taylor Street, MC 663, Chicago, IL 60612; awms@uic.edu
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th Ed. American Psychiatric Publishing; 2013.
2. Seehusen DA, Baird DC, Bode DV. Dyspareunia in women. Am Fam Phys. 2014;90:465-470.
3. Sorensen J, Bautista KE, Lamvu G, et al. Evaluation and treatment of female sexual pain: a clinical review. Cureus. 2018;10:e2379.
4. MacNeill C. Dyspareunia. Obstet Gynecol Clin North Am. 2006;33:565-77.
5. Latthe P, Latthe M, Say L, et al. WHO systematic review of prevalence of chronic pelvic pain: a neglected reproductive health morbidity. BMC Public Health. 2006;6:177.
6. Steege JF, Zolnoun DA. Evaluation and treatment of dyspareunia. Obstet Gynecol. 2009;113:1124-1136.
7. Williams AA, Williams M. A guide to performing pelvic speculum exams: a patient-centered approach to reducing iatrogenic effects. Teach Learn Med. 2013;25:383-391.
8. Ünlü Z, Yentur A, Çakil N. Pudendal nerve neuropathy: An unknown-rare cause of pelvic pain. Arch Rheumatol. 2016;31:102-103.
9. Dewitte M, Borg C, Lowenstein L. A psychosocial approach to female genital pain. Nat Rev Urol. 2018;15:25-41.
10. Masters WH, Johnson VE. Human Sexual Inadequacy. 1st ed. Little, Brown; 1970.
11. Rathus SA, Nevid JS, Fichner-Rathus L. Human Sexuality in a World of Diversity. 5th ed. Allyn and Bacon; 2002.
12. Bailey BE, Freedenfeld RN, Kiser RS, et al. Lifetime physical and sexual abuse in chronic pain patients: psychosocial correlates and treatment outcomes. Disabil Rehabil. 2003;25:331-342.
13. Yehuda R, Lehrner A, Rosenbaum TY. PTSD and sexual dysfunction in men and women. J Sex Med. 2015;12:1107-1119.
14. Postma R, Bicanic I, van der Vaart H, et al. Pelvic floor muscle problems mediate sexual problems in young adult rape victims. J Sex Med. 2013;10:1978-1987.
15. Binik YM, Bergeron S, Khalifé S. Dyspareunia and vaginismus: so-called sexual pain. In: Leiblum SR, ed. 4th ed. Principles and Practice of Sex Therapy. The Guilford Press; 2007:124-156.
16. Ayling K, Ussher JM. “If sex hurts, am I still a woman?” The subjective experience of vulvodynia in hetero-sexual women. Arch Sex Behav. 2008;37:294-304.
17. Pazmany E, Bergeron S, Van Oudenhove L, et al. Body image and genital self-image in pre-menopausal women with dyspareunia. Arch Sex Behav. 2013;42:999-1010.
18. Maillé DL, Bergeron S, Lambert B. Body image in women with primary and secondary provoked vestibulodynia: a controlled study. J Sex Med. 2015;12:505-515.
19. Ryan L, Hawton K. Female dyspareunia. BMJ. 2004;328:1357.
20. Waldura JF, Arora I, Randall AM, et al. Fifty shades of stigma: exploring the health care experiences of kink-oriented patients. J Sex Med. 2016;13:1918-1929.
21. Hinchliff S, Gott M. Seeking medical help for sexual concerns in mid- and later life: a review of the literature. J Sex Res. 2011;48:106-117.
22. Banaei M, Kariman N, Ozgoli G, et al. Prevalence of postpartum dyspareunia: a systematic review and meta-analysis. Int J Gynaecol Obstet. 2021;153:14-24.
23. Kroenke K, Spitzer RL. The PHQ-9: A new depression diagnostic and severity measure. Psychiatr Ann. 2002;32:509-515.
24. Spitzer RL, Kroenke K, Williams JB, et al. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166:1092-1097.
25. U.S. Department of Veterans Affairs. PTSD: National Center for PTSD. Life events checklist for DSM-5 (LEC-5). Accessed February 3, 2022. www.ptsd.va.gov/professional/assessment/te-measures/life_events_checklist.asp
26. Weathers FW, Litz BT, Keane TM, et al. The PTSD checklist for DSM-5 (PCL-5). 2013. Accessed February 3, 2022. www.ptsd.va.gov/professional/assessment/adult-sr/ptsd-checklist.asp
27. Rosen R, Brown C, Heiman J, et al. The female sexual function index (FSFI): a multidimensional self-report instrument for the assessment of female sexual function. J Sex Marital Ther. 2000;26:191-208.
28. Melzack R. The short-form McGill Pain Questionnaire. Pain. 1987;30:191-197.
29. Hassam T, Kelso E, Chowdary P, et al. Sexual assault as a risk factor for gynaecological morbidity: an exploratory systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2020;255:222-230.
30. Hamlin AA, Sheeder J, Muffly TM. Brief versus thong hygiene in obstetrics and gynecology (B-THONG): a survey study. J Obstet Gynaecol Res. 2019;45:1190-1196.
31. Foster DC, Kotok MB, Huang LS, et al. Oral desipramine and topical lidocaine for vulvodynia: a randomized controlled trial. Obstet Gynecol. 2010;116:583-593.
32. Pérez-López FR, Bueno-Notivol J, Hernandez AV, et al. Systematic review and meta-analysis of the effects of treatment modalities for vestibulodynia in women. Eur J Contracept Reprod Health Care. 2019;24:337-346.
33. Cheong YC, Smotra G, Williams AC. Non-surgical interventions for the management of chronic pelvic pain. Cochrane Database Syst Rev. 2014;(3):CD008797.
34. Goldstein AT, Pukall CF, Brown C, et al. Vulvodynia: assessment and treatment. J Sex Med. 2016;13:572-590.
35. Bergeron S, Binik YM, Khalifé S, et al. A randomized comparison of group cognitive-behavioral therapy, surface electromyographic biofeedback, and vestibulectomy in the treatment of dyspareunia resulting from vulvar vestibulitis. Pain. 2001;91:297-306.
36. Schvartzman R, Schvartzman L, Ferreira CF, et al. Physical therapy intervention for women with dyspareunia: a randomized clinical trial. J Sex Marital Ther. 2019;45:378-394.
37. Morin M, Dumoulin C, Bergeron S, et al. Multimodal physical therapy versus topical lidocaine for provoked vestibulodynia: a multicenter, randomized trial. Am J Obstet Gynecol. 2021;224:189.e1-189.e12.
38. Bergeron S, Khalifé S, Dupuis M-J, et al. A randomized clinical trial comparing group cognitive-behavioral therapy and a topical steroid for women with dyspareunia. J Consult Clin Psychol. 2016;84:259-268.
39. Maseroli E, Scavello I, Rastrelli G, et al. Outcome of medical and psychosexual interventions for vaginismus: a systematic review and meta-analysis. J Sex Med. 2018;15:1752-1764.
40. American Psychological Association. Clinical practice guideline for the treatment of posttraumatic stress disorder (PTSD) in adults. 2017. Accessed February 3, 2022. www.apa.org/ptsd-guideline/ptsd.pdf
Dyspareunia is persistent or recurrent pain before, during, or after sexual contact and is not limited to cisgender individuals or vaginal intercourse.1-3 With a prevalence as high as 45% in the United States,2-5 it is one of the most common complaints in gynecologic practices.5,6
Causes and contributing factors
There are many possible causes of dyspareunia.2,4,6 While some patients have a single cause, most cases are complex, with multiple overlapping causes and maintaining factors.4,6 Identifying each contributing factor can help you appropriately address all components.
Physical conditions. The range of physical contributors to dyspareunia includes inflammatory processes, structural abnormalities, musculoskeletal dysfunctions, pelvic organ disorders, injuries, iatrogenic effects, infections, allergic reactions, sensitization, hormonal changes, medication effects, adhesions, autoimmune disorders, and other pain syndromes (TABLE 12-4,6-11).

Inadequate arousal. One of the primary causes of pain during vaginal penetration is inadequate arousal and lubrication.1,2,9-11 Arousal is the phase of the sexual response cycle that leads to genital tumescence and prepares the genitals for sexual contact through penile/clitoral erection, vaginal engorgement, and lubrication, which prevents pain and enhances pleasurable sensation.9-11
While some physical conditions can lead to an inability to lubricate, the most common causes of inadequate lubrication are psychosocial-behavioral, wherein patients have the same physical ability to lubricate as patients without genital pain but do not progress through the arousal phase.9-11 Behavioral factors such as inadequate or ineffective foreplay can fail to produce engorgement and lubrication, while psychosocial factors such as low attraction to partner, relationship stressors, anxiety, or low self-esteem can have an inhibitory effect on sexual arousal.1,2,9-11 Psychosocial and behavioral factors may also be maintaining factors or consequences of dyspareunia, and need to be assessed and treated.1,2,9-11
Psychological trauma. Exposure to psychological traumas and the development of posttraumatic stress disorder (PTSD) have been linked with the development of pain disorders in general and dyspareunia specifically. Most patients seeking treatment for chronic pain disorders have a history of physical or sexual abuse.12 Changes in physiologic processes (eg, neurochemical, endocrine) that occur with PTSD interfere with the sexual response cycle, and sexual traumas specifically have been linked with pelvic floor dysfunction.13,14 Additionally, when PTSD is caused by a sexual trauma, even consensual sexual encounters can trigger flashbacks, intrusive memories, hyperarousal, and muscle tension that interfere with the sexual response cycle and contribute to genital pain.13
Vaginismus is both a physiologic and psychological contributor to dyspareunia.1,2,4 Patients experiencing pain can develop anxiety about repeated pain and involuntarily contract their pelvic muscles, thereby creating more pain, increasing anxiety, decreasing lubrication, and causing pelvic floor dysfunction.1-4,6 Consequently, all patients with dyspareunia should be assessed and continually monitored for symptoms of vaginismus.
Continue to: Anxiety
Anxiety. As with other pain disorders, anxiety develops around pain triggers.10,15 When expecting sexual activity, patients can experience extreme worry and panic attacks.10,15,16 The distress of sexual encounters can interfere with physiologic arousal and sexual desire, impacting all phases of the sexual response cycle.1,2
Relationship issues. Difficulty engaging in or avoidance of sexual activity can interfere with romantic relationships.2,10,16 Severe pain or vaginismus contractions can prevent penetration, leading to unconsummated marriages and an inability to conceive through intercourse.10 The distress surrounding sexual encounters can precipitate erectile dysfunction in male partners, or partners may continue to demand sexual encounters despite the patient’s pain, further impacting the relationship and heightening sexual distress.10 These stressors have led to relationships ending, patients reluctantly agreeing to nonmonogamy to appease their partners, and patients avoiding relationships altogether.10,16
Devalued self-image. Difficulties with sexuality and relationships impact the self-image of patients with dyspareunia. Diminished self-image may include feeling “inadequate” as a woman and as a sexual partner, or feeling like a “failure.”16 Women with dyspareunia often have more distress related to their body image, physical appearance, and genital self-image than do women without genital pain.17 Feeling resentment toward their body, or feeling “ugly,” embarrassed, shamed, “broken,” and “useless” also contribute to increased depressive symptoms found in patients with dyspareunia.16,18
Making the diagnosis
Most patients do not report symptoms unless directly asked2,7; therefore, it is recommended that all patients be screened as a part of an initial intake and before any genital exam (TABLE 22-4,6,7,9,11,19,20).4,7,21 If this screen is positive, a separate appointment may be needed for a thorough evaluation and before any attempt is made at a genital exam.4,7

Items to include in the clinical interview
Given the range of possible causes of dyspareunia and its contributing factors and symptoms, a thorough clinical interview is essential. Begin with a review of the patient’s complete medical and surgical history to identify possible known contributors to genital pain.4 Pregnancy history is of particular importance as the prevalence of postpartum dyspareunia is 35%, with risk being greater for patients who experienced dyspareunia symptoms before pregnancy.22

Knowing the location and quality of pain is important for differentiating between possible diagnoses, as is specifying dyspareunia as lifelong or acquired, superficial or deep, and primary or secondary.1-4,6 Confirm the specific location(s) of pain—eg, at the introitus, in the vestibule, on the labia, in the perineum, or near the clitoris.2,4,6 A diagram or model may be needed to help patients to localize pain.4
To help narrow the differential, include the following elements in your assessment: pain quality, timing (eg, initial onset, episode onset, episode duration, situational triggers), alleviating factors, symptoms in surrounding structures (eg, bladder, bowel, muscles, bones), sexual history, other areas of sexual functioning, history of psychological trauma, relationship effects, and mental health (TABLE 22-4,6,7,9,11,19,20 and Table 323-28). Screening for a history of sexual trauma is particularly important, as a recent systematic review and meta-analysis found that women with a history of sexual assault had a 42% higher risk of gynecologic problems overall, a 74% higher risk of dyspareunia, and a 71% higher risk of vaginismus than women without a history of sexual assault.29 Using measures such as the Female Sexual Function Index or the McGill Pain Questionnaire can help patients more thoroughly describe their symptoms (TABLE 323-28).3

Continue to: Guidelines for the physical exam
Guidelines for the physical exam
Before the exam, ensure the patient has not used any topical genital treatment in the past 2 weeks that may interfere with sensitivity to the exam.4 To decrease patients’ anxiety about the exam, remind them that they can stop the exam at any time.7 Also consider offering the use of a mirror to better pinpoint the location of pain, and to possibly help the patient learn more about her anatomy.2,7
Begin the exam by palpating surrounding areas that may be involved in pain, including the abdomen and musculoskeletal features.3,6,19 Next visually inspect the external genitalia for lesions, abrasions, discoloration, erythema, or other abnormal findings.2,3,6 Ask the patient for permission before contacting the genitals. Because the labia may be a site of pain, apply gentle pressure in retracting it to fully examine the vestibule.6,7 Contraction of the pelvic floor muscles during approach or initial palpation could signal possible vaginismus.4
After visual inspection of external genitalia, use a cotton swab to map the vulva and vestibule in a clockwise fashion to precisely identify any painful locations.2-4,6 If the patient’s history of pain has been intermittent, it’s possible that the cotton swab will not elicit pain on the day of the initial exam, but it may on other days.4
Begin the internal exam by inserting a single finger into the first inch of the vagina and have the patient squeeze and release to assess tenderness, muscle tightness, and control.2,6 Advance the finger further into the vagina and palpate clockwise, examining the levator muscles, obturator muscles, rectum, urethra, and bladder for abnormal tightness or reproduction of pain.2,4,6 Complete a bimanual exam to evaluate the pelvic organs and adnexa.2,4 If indicated, a more thorough evaluation of pelvic floor musculature can be performed by a physical therapist or gynecologist who specializes in pelvic pain.2-4
If the patient consents to further evaluation, consider using a small speculum, advanced slowly, for further internal examination, noting any lesions, abrasions, discharge, ectropion, or tenderness.2-4,7 A rectal exam may also be needed in cases of deep dyspareunia.6 Initial work-up may include a potassium hydroxide wet prep, sexually transmitted infection testing, and pelvic ultrasound.2,4 In some cases, laparoscopy or biopsy may be needed.2,4
Treatments for common causes
Treatment often begins with education about anatomy, to help patients communicate about symptoms and engage more fully in their care.3 Additional education may be needed on genital functioning and the necessity of adequate stimulation and lubrication prior to penetration.1,2,9-11 A discussion of treatments for the wide range of possible causes of dyspareunia is outside the scope of this article. However, some basic behavioral changes may help patients address some of the more common contributing factors.
For example, if vaginal infection is suspected, advise patients to discontinue the use of harsh soaps, known vaginal irritants (eg, perfumed products, bath additives), and douches.3 Recommend using only preservative- and alcohol-free lubricants for sexual contact, and avoiding lubricants with added functions (eg, warming).3 It’s worth noting that avoidance of tight clothing and thong underwear due to possible risk for infections may not be necessary. A recent study found that women who frequently wore thong underwear (more than half of the time) were no more likely to develop urinary tract infections, yeast vaginitis, or bacterial vaginosis than those who avoid such items.30 However, noncotton underwear fabric, rather than tightness, was associated with yeast vaginitis30; therefore, patients may want to consider using only breathable underwear.3
Continue to: Medication
Medication. Medication may be used to treat the underlying contributing conditions or the symptom of pain directly. Some common options are particularly important for patients whose dyspareunia does not have an identifiable cause. These medications include anti-inflammatory agents, topical anesthetics, tricyclic antidepressants, and hormonal treatments.2-4 Since effectiveness varies based on subtypes of pain, select a medication according to the location, timing, and hypothesized mechanism of pain.3,31,32
Medication for deep pain. A meta-analysis and systematic review found that patients with some types of chronic pelvic pain with pain deep in the vagina or pelvis experienced greater than 50% reduction in pain using medroxyprogesterone acetate compared with placebo.33 Other treatments for deep pain depend on physical exam findings.
Medication for superficial pain. Many remedies have been tried, with at least 26 different treatments for vulvodynia pain alone.16 Only some of these treatments have supporting evidence. For patients with vulvar pain, an intent-to-treat RCT found that patients using a topical steroid experienced a 23% reduction in pain from pre-treatment to 6-month follow-up.32
Surgery is also effective for vulvar pain.34,35 For provoked vestibulodynia (in which pain is localized to the vestibule and triggered by contact with the vulva), or vulvar vestibulitis, RCTs have found that vestibulectomy has stronger effects on pain than other treatments,31,35 with a 53% reduction in pain during intercourse and a 70% reduction in vestibular pain overall.35 However, while vestibulectomy is effective for provoked vestibulodynia, it is not recommended for generalized vulvodynia, in which pain is diffuse across the vulva and occurs without vulvar contact.34
Unsupported treatments. A number of other treatments have not yet been found effective. Although lidocaine for vulvar pain is often used, RCTs have not found any significant reduction in symptoms, and a double-blind RCT found that lidocaine ointment actually performed worse than placebo.31,34 Similarly, oral tricyclics have not been found to decrease vulvar pain more than placebo in double-blind studies.31,34 Furthermore, a meta-analysis of RCTs comparing treatments with placebo for vestibular pain found no significant decrease in dyspareunia for topical conjugated estrogen, topical lidocaine, oral desipramine, oral desipramine with topical lidocaine, laser therapy, or transcranial direct current.32
Tx risks to consider. Risks and benefits of dyspareunia treatment options should be thoroughly weighed and discussed with the patient.2-4 Vestibulectomy, despite reducing pain for many patients, has led to increased pain for 9% of patients who underwent the procedure.35 Topical treatments may lead to allergic reactions, inflammation, and worsening of symptoms,4 and hormonal treatments have been found to increase the risk of weight gain and bloating and are not appropriate for patients trying to conceive.33
Coordinate care with other providers
While medications and surgery can reduce pain, they have not been shown to improve other aspects of sexual functioning such as sexual satisfaction, frequency of sexual intercourse, or overall sense of sexual functioning.35 Additionally, pain reduction does not address muscle tension, anxiety, self-esteem, and relationship problems. As a result, a multidisciplinary approach is generally needed.3,4,32,33
Continue to: Physical therapists
Physical therapists. Pelvic floor physical therapists are often members of the dyspareunia treatment team and can provide a thorough evaluation and treatment of pelvic floor disorders.2-4 An RCT with intent-to-treat analysis found that pain was reduced by 71% following pelvic floor physical therapy.36 Another RCT found that 90% of patients reported a clinically meaningful decrease in pain with pelvic floor physical therapy.37 In addition to addressing pain, pelvic floor physical therapy has also been found to improve sexual functioning, sexual satisfaction, distress, and patient perception of improvement.34,36,37
Behavioral health specialists. Psychotherapists, especially those trained in sex therapy, couples therapy, or cognitive behavioral therapy (CBT), are also typically on the treatment team. Multiple RCTs have found evidence of CBT’s effectiveness in the direct treatment of dyspareunia pain. Bergeron et al35 found a 37.5% reduction in vulvar vestibulitis pain intensity during intercourse after patients completed group CBT. Another intent-to-treat RCT found that patients receiving CBT experienced more pain reduction (~ 30%) than patients who were treated with a topical steroid.38
In addition to having a direct impact on pain, CBT has also been found to have a clinically and statistically significant positive impact on other aspects of sexual experience, such as overall sexuality, self-efficacy, overall sexual functioning, frequency of intercourse, and catastrophizing.34,38 A recent meta-analysis of RCTs found that about 80% of vaginismus patients were able to achieve penetrative intercourse after treatment with behavioral sex therapy or CBT.39 This success rate was not exceeded by physical or surgical treatments.39
When PTSD is thought to be a contributing factor, trauma therapy will likely be needed in addition to treatments for dyspareunia. First-line treatments for PTSD include cognitive processing therapy, prolonged exposure, trauma-focused CBT, and cognitive therapy.40
Psychotherapists can also help patients reduce anxiety, reintroduce sexual contact without triggering pain or anxiety, address emotional and self-esteem effects of dyspareunia, address relationship issues, and refocus sexual encounters on pleasure rather than pain avoidance.2-4 Despite patient reports of high treatment satisfaction following therapy,38 many patients may initially lack confidence in psychotherapy as a treatment for pain35 and may need to be educated on its effectiveness and multidimensional benefits.
Gynecologists. Often a gynecologist with specialization in pelvic pain is an essential member of the team for diagnostic clarification, recommendation of treatment options, and performance of more advanced treatments.2,3 If pain has become chronic, the patient may also benefit from a pain management team and support groups.2,3
Follow-up steps
Patients who screen negative for dyspareunia should be re-screened periodically. Continue to assess patients diagnosed with dyspareunia for vaginismus symptoms (if they are not initially present) to ensure that the treatment plan is appropriately adjusted. Once treatment has begun, ask about adverse effects and confidence in the treatment plan to minimize negative impacts on treatment adherence and to anticipate a need for a change in the treatment approach.31,35 In addition to tracking treatment effects on pain, continue to assess for patient-centered outcomes such as emotional functioning, self-esteem, and sexual and relationship satisfaction.34 The Female Sexual Function Index can be a useful tool to track symptoms.27,34
Finally, patients who do not experience sufficient improvement in symptoms and functioning with initial treatment may need continued support and encouragement. Given the broad range of contributing factors and the high number of potential treatments, patients may find hope in learning that multiple other treatment options may be available.
CORRESPONDENCE
Adrienne A. Williams, PhD, Department of Family and Community Medicine, University of Illinois at Chicago College of Medicine, 1919 W Taylor Street, MC 663, Chicago, IL 60612; awms@uic.edu
Dyspareunia is persistent or recurrent pain before, during, or after sexual contact and is not limited to cisgender individuals or vaginal intercourse.1-3 With a prevalence as high as 45% in the United States,2-5 it is one of the most common complaints in gynecologic practices.5,6
Causes and contributing factors
There are many possible causes of dyspareunia.2,4,6 While some patients have a single cause, most cases are complex, with multiple overlapping causes and maintaining factors.4,6 Identifying each contributing factor can help you appropriately address all components.
Physical conditions. The range of physical contributors to dyspareunia includes inflammatory processes, structural abnormalities, musculoskeletal dysfunctions, pelvic organ disorders, injuries, iatrogenic effects, infections, allergic reactions, sensitization, hormonal changes, medication effects, adhesions, autoimmune disorders, and other pain syndromes (TABLE 12-4,6-11).

Inadequate arousal. One of the primary causes of pain during vaginal penetration is inadequate arousal and lubrication.1,2,9-11 Arousal is the phase of the sexual response cycle that leads to genital tumescence and prepares the genitals for sexual contact through penile/clitoral erection, vaginal engorgement, and lubrication, which prevents pain and enhances pleasurable sensation.9-11
While some physical conditions can lead to an inability to lubricate, the most common causes of inadequate lubrication are psychosocial-behavioral, wherein patients have the same physical ability to lubricate as patients without genital pain but do not progress through the arousal phase.9-11 Behavioral factors such as inadequate or ineffective foreplay can fail to produce engorgement and lubrication, while psychosocial factors such as low attraction to partner, relationship stressors, anxiety, or low self-esteem can have an inhibitory effect on sexual arousal.1,2,9-11 Psychosocial and behavioral factors may also be maintaining factors or consequences of dyspareunia, and need to be assessed and treated.1,2,9-11
Psychological trauma. Exposure to psychological traumas and the development of posttraumatic stress disorder (PTSD) have been linked with the development of pain disorders in general and dyspareunia specifically. Most patients seeking treatment for chronic pain disorders have a history of physical or sexual abuse.12 Changes in physiologic processes (eg, neurochemical, endocrine) that occur with PTSD interfere with the sexual response cycle, and sexual traumas specifically have been linked with pelvic floor dysfunction.13,14 Additionally, when PTSD is caused by a sexual trauma, even consensual sexual encounters can trigger flashbacks, intrusive memories, hyperarousal, and muscle tension that interfere with the sexual response cycle and contribute to genital pain.13
Vaginismus is both a physiologic and psychological contributor to dyspareunia.1,2,4 Patients experiencing pain can develop anxiety about repeated pain and involuntarily contract their pelvic muscles, thereby creating more pain, increasing anxiety, decreasing lubrication, and causing pelvic floor dysfunction.1-4,6 Consequently, all patients with dyspareunia should be assessed and continually monitored for symptoms of vaginismus.
Continue to: Anxiety
Anxiety. As with other pain disorders, anxiety develops around pain triggers.10,15 When expecting sexual activity, patients can experience extreme worry and panic attacks.10,15,16 The distress of sexual encounters can interfere with physiologic arousal and sexual desire, impacting all phases of the sexual response cycle.1,2
Relationship issues. Difficulty engaging in or avoidance of sexual activity can interfere with romantic relationships.2,10,16 Severe pain or vaginismus contractions can prevent penetration, leading to unconsummated marriages and an inability to conceive through intercourse.10 The distress surrounding sexual encounters can precipitate erectile dysfunction in male partners, or partners may continue to demand sexual encounters despite the patient’s pain, further impacting the relationship and heightening sexual distress.10 These stressors have led to relationships ending, patients reluctantly agreeing to nonmonogamy to appease their partners, and patients avoiding relationships altogether.10,16
Devalued self-image. Difficulties with sexuality and relationships impact the self-image of patients with dyspareunia. Diminished self-image may include feeling “inadequate” as a woman and as a sexual partner, or feeling like a “failure.”16 Women with dyspareunia often have more distress related to their body image, physical appearance, and genital self-image than do women without genital pain.17 Feeling resentment toward their body, or feeling “ugly,” embarrassed, shamed, “broken,” and “useless” also contribute to increased depressive symptoms found in patients with dyspareunia.16,18
Making the diagnosis
Most patients do not report symptoms unless directly asked2,7; therefore, it is recommended that all patients be screened as a part of an initial intake and before any genital exam (TABLE 22-4,6,7,9,11,19,20).4,7,21 If this screen is positive, a separate appointment may be needed for a thorough evaluation and before any attempt is made at a genital exam.4,7

Items to include in the clinical interview
Given the range of possible causes of dyspareunia and its contributing factors and symptoms, a thorough clinical interview is essential. Begin with a review of the patient’s complete medical and surgical history to identify possible known contributors to genital pain.4 Pregnancy history is of particular importance as the prevalence of postpartum dyspareunia is 35%, with risk being greater for patients who experienced dyspareunia symptoms before pregnancy.22

Knowing the location and quality of pain is important for differentiating between possible diagnoses, as is specifying dyspareunia as lifelong or acquired, superficial or deep, and primary or secondary.1-4,6 Confirm the specific location(s) of pain—eg, at the introitus, in the vestibule, on the labia, in the perineum, or near the clitoris.2,4,6 A diagram or model may be needed to help patients to localize pain.4
To help narrow the differential, include the following elements in your assessment: pain quality, timing (eg, initial onset, episode onset, episode duration, situational triggers), alleviating factors, symptoms in surrounding structures (eg, bladder, bowel, muscles, bones), sexual history, other areas of sexual functioning, history of psychological trauma, relationship effects, and mental health (TABLE 22-4,6,7,9,11,19,20 and Table 323-28). Screening for a history of sexual trauma is particularly important, as a recent systematic review and meta-analysis found that women with a history of sexual assault had a 42% higher risk of gynecologic problems overall, a 74% higher risk of dyspareunia, and a 71% higher risk of vaginismus than women without a history of sexual assault.29 Using measures such as the Female Sexual Function Index or the McGill Pain Questionnaire can help patients more thoroughly describe their symptoms (TABLE 323-28).3

Continue to: Guidelines for the physical exam
Guidelines for the physical exam
Before the exam, ensure the patient has not used any topical genital treatment in the past 2 weeks that may interfere with sensitivity to the exam.4 To decrease patients’ anxiety about the exam, remind them that they can stop the exam at any time.7 Also consider offering the use of a mirror to better pinpoint the location of pain, and to possibly help the patient learn more about her anatomy.2,7
Begin the exam by palpating surrounding areas that may be involved in pain, including the abdomen and musculoskeletal features.3,6,19 Next visually inspect the external genitalia for lesions, abrasions, discoloration, erythema, or other abnormal findings.2,3,6 Ask the patient for permission before contacting the genitals. Because the labia may be a site of pain, apply gentle pressure in retracting it to fully examine the vestibule.6,7 Contraction of the pelvic floor muscles during approach or initial palpation could signal possible vaginismus.4
After visual inspection of external genitalia, use a cotton swab to map the vulva and vestibule in a clockwise fashion to precisely identify any painful locations.2-4,6 If the patient’s history of pain has been intermittent, it’s possible that the cotton swab will not elicit pain on the day of the initial exam, but it may on other days.4
Begin the internal exam by inserting a single finger into the first inch of the vagina and have the patient squeeze and release to assess tenderness, muscle tightness, and control.2,6 Advance the finger further into the vagina and palpate clockwise, examining the levator muscles, obturator muscles, rectum, urethra, and bladder for abnormal tightness or reproduction of pain.2,4,6 Complete a bimanual exam to evaluate the pelvic organs and adnexa.2,4 If indicated, a more thorough evaluation of pelvic floor musculature can be performed by a physical therapist or gynecologist who specializes in pelvic pain.2-4
If the patient consents to further evaluation, consider using a small speculum, advanced slowly, for further internal examination, noting any lesions, abrasions, discharge, ectropion, or tenderness.2-4,7 A rectal exam may also be needed in cases of deep dyspareunia.6 Initial work-up may include a potassium hydroxide wet prep, sexually transmitted infection testing, and pelvic ultrasound.2,4 In some cases, laparoscopy or biopsy may be needed.2,4
Treatments for common causes
Treatment often begins with education about anatomy, to help patients communicate about symptoms and engage more fully in their care.3 Additional education may be needed on genital functioning and the necessity of adequate stimulation and lubrication prior to penetration.1,2,9-11 A discussion of treatments for the wide range of possible causes of dyspareunia is outside the scope of this article. However, some basic behavioral changes may help patients address some of the more common contributing factors.
For example, if vaginal infection is suspected, advise patients to discontinue the use of harsh soaps, known vaginal irritants (eg, perfumed products, bath additives), and douches.3 Recommend using only preservative- and alcohol-free lubricants for sexual contact, and avoiding lubricants with added functions (eg, warming).3 It’s worth noting that avoidance of tight clothing and thong underwear due to possible risk for infections may not be necessary. A recent study found that women who frequently wore thong underwear (more than half of the time) were no more likely to develop urinary tract infections, yeast vaginitis, or bacterial vaginosis than those who avoid such items.30 However, noncotton underwear fabric, rather than tightness, was associated with yeast vaginitis30; therefore, patients may want to consider using only breathable underwear.3
Continue to: Medication
Medication. Medication may be used to treat the underlying contributing conditions or the symptom of pain directly. Some common options are particularly important for patients whose dyspareunia does not have an identifiable cause. These medications include anti-inflammatory agents, topical anesthetics, tricyclic antidepressants, and hormonal treatments.2-4 Since effectiveness varies based on subtypes of pain, select a medication according to the location, timing, and hypothesized mechanism of pain.3,31,32
Medication for deep pain. A meta-analysis and systematic review found that patients with some types of chronic pelvic pain with pain deep in the vagina or pelvis experienced greater than 50% reduction in pain using medroxyprogesterone acetate compared with placebo.33 Other treatments for deep pain depend on physical exam findings.
Medication for superficial pain. Many remedies have been tried, with at least 26 different treatments for vulvodynia pain alone.16 Only some of these treatments have supporting evidence. For patients with vulvar pain, an intent-to-treat RCT found that patients using a topical steroid experienced a 23% reduction in pain from pre-treatment to 6-month follow-up.32
Surgery is also effective for vulvar pain.34,35 For provoked vestibulodynia (in which pain is localized to the vestibule and triggered by contact with the vulva), or vulvar vestibulitis, RCTs have found that vestibulectomy has stronger effects on pain than other treatments,31,35 with a 53% reduction in pain during intercourse and a 70% reduction in vestibular pain overall.35 However, while vestibulectomy is effective for provoked vestibulodynia, it is not recommended for generalized vulvodynia, in which pain is diffuse across the vulva and occurs without vulvar contact.34
Unsupported treatments. A number of other treatments have not yet been found effective. Although lidocaine for vulvar pain is often used, RCTs have not found any significant reduction in symptoms, and a double-blind RCT found that lidocaine ointment actually performed worse than placebo.31,34 Similarly, oral tricyclics have not been found to decrease vulvar pain more than placebo in double-blind studies.31,34 Furthermore, a meta-analysis of RCTs comparing treatments with placebo for vestibular pain found no significant decrease in dyspareunia for topical conjugated estrogen, topical lidocaine, oral desipramine, oral desipramine with topical lidocaine, laser therapy, or transcranial direct current.32
Tx risks to consider. Risks and benefits of dyspareunia treatment options should be thoroughly weighed and discussed with the patient.2-4 Vestibulectomy, despite reducing pain for many patients, has led to increased pain for 9% of patients who underwent the procedure.35 Topical treatments may lead to allergic reactions, inflammation, and worsening of symptoms,4 and hormonal treatments have been found to increase the risk of weight gain and bloating and are not appropriate for patients trying to conceive.33
Coordinate care with other providers
While medications and surgery can reduce pain, they have not been shown to improve other aspects of sexual functioning such as sexual satisfaction, frequency of sexual intercourse, or overall sense of sexual functioning.35 Additionally, pain reduction does not address muscle tension, anxiety, self-esteem, and relationship problems. As a result, a multidisciplinary approach is generally needed.3,4,32,33
Continue to: Physical therapists
Physical therapists. Pelvic floor physical therapists are often members of the dyspareunia treatment team and can provide a thorough evaluation and treatment of pelvic floor disorders.2-4 An RCT with intent-to-treat analysis found that pain was reduced by 71% following pelvic floor physical therapy.36 Another RCT found that 90% of patients reported a clinically meaningful decrease in pain with pelvic floor physical therapy.37 In addition to addressing pain, pelvic floor physical therapy has also been found to improve sexual functioning, sexual satisfaction, distress, and patient perception of improvement.34,36,37
Behavioral health specialists. Psychotherapists, especially those trained in sex therapy, couples therapy, or cognitive behavioral therapy (CBT), are also typically on the treatment team. Multiple RCTs have found evidence of CBT’s effectiveness in the direct treatment of dyspareunia pain. Bergeron et al35 found a 37.5% reduction in vulvar vestibulitis pain intensity during intercourse after patients completed group CBT. Another intent-to-treat RCT found that patients receiving CBT experienced more pain reduction (~ 30%) than patients who were treated with a topical steroid.38
In addition to having a direct impact on pain, CBT has also been found to have a clinically and statistically significant positive impact on other aspects of sexual experience, such as overall sexuality, self-efficacy, overall sexual functioning, frequency of intercourse, and catastrophizing.34,38 A recent meta-analysis of RCTs found that about 80% of vaginismus patients were able to achieve penetrative intercourse after treatment with behavioral sex therapy or CBT.39 This success rate was not exceeded by physical or surgical treatments.39
When PTSD is thought to be a contributing factor, trauma therapy will likely be needed in addition to treatments for dyspareunia. First-line treatments for PTSD include cognitive processing therapy, prolonged exposure, trauma-focused CBT, and cognitive therapy.40
Psychotherapists can also help patients reduce anxiety, reintroduce sexual contact without triggering pain or anxiety, address emotional and self-esteem effects of dyspareunia, address relationship issues, and refocus sexual encounters on pleasure rather than pain avoidance.2-4 Despite patient reports of high treatment satisfaction following therapy,38 many patients may initially lack confidence in psychotherapy as a treatment for pain35 and may need to be educated on its effectiveness and multidimensional benefits.
Gynecologists. Often a gynecologist with specialization in pelvic pain is an essential member of the team for diagnostic clarification, recommendation of treatment options, and performance of more advanced treatments.2,3 If pain has become chronic, the patient may also benefit from a pain management team and support groups.2,3
Follow-up steps
Patients who screen negative for dyspareunia should be re-screened periodically. Continue to assess patients diagnosed with dyspareunia for vaginismus symptoms (if they are not initially present) to ensure that the treatment plan is appropriately adjusted. Once treatment has begun, ask about adverse effects and confidence in the treatment plan to minimize negative impacts on treatment adherence and to anticipate a need for a change in the treatment approach.31,35 In addition to tracking treatment effects on pain, continue to assess for patient-centered outcomes such as emotional functioning, self-esteem, and sexual and relationship satisfaction.34 The Female Sexual Function Index can be a useful tool to track symptoms.27,34
Finally, patients who do not experience sufficient improvement in symptoms and functioning with initial treatment may need continued support and encouragement. Given the broad range of contributing factors and the high number of potential treatments, patients may find hope in learning that multiple other treatment options may be available.
CORRESPONDENCE
Adrienne A. Williams, PhD, Department of Family and Community Medicine, University of Illinois at Chicago College of Medicine, 1919 W Taylor Street, MC 663, Chicago, IL 60612; awms@uic.edu
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th Ed. American Psychiatric Publishing; 2013.
2. Seehusen DA, Baird DC, Bode DV. Dyspareunia in women. Am Fam Phys. 2014;90:465-470.
3. Sorensen J, Bautista KE, Lamvu G, et al. Evaluation and treatment of female sexual pain: a clinical review. Cureus. 2018;10:e2379.
4. MacNeill C. Dyspareunia. Obstet Gynecol Clin North Am. 2006;33:565-77.
5. Latthe P, Latthe M, Say L, et al. WHO systematic review of prevalence of chronic pelvic pain: a neglected reproductive health morbidity. BMC Public Health. 2006;6:177.
6. Steege JF, Zolnoun DA. Evaluation and treatment of dyspareunia. Obstet Gynecol. 2009;113:1124-1136.
7. Williams AA, Williams M. A guide to performing pelvic speculum exams: a patient-centered approach to reducing iatrogenic effects. Teach Learn Med. 2013;25:383-391.
8. Ünlü Z, Yentur A, Çakil N. Pudendal nerve neuropathy: An unknown-rare cause of pelvic pain. Arch Rheumatol. 2016;31:102-103.
9. Dewitte M, Borg C, Lowenstein L. A psychosocial approach to female genital pain. Nat Rev Urol. 2018;15:25-41.
10. Masters WH, Johnson VE. Human Sexual Inadequacy. 1st ed. Little, Brown; 1970.
11. Rathus SA, Nevid JS, Fichner-Rathus L. Human Sexuality in a World of Diversity. 5th ed. Allyn and Bacon; 2002.
12. Bailey BE, Freedenfeld RN, Kiser RS, et al. Lifetime physical and sexual abuse in chronic pain patients: psychosocial correlates and treatment outcomes. Disabil Rehabil. 2003;25:331-342.
13. Yehuda R, Lehrner A, Rosenbaum TY. PTSD and sexual dysfunction in men and women. J Sex Med. 2015;12:1107-1119.
14. Postma R, Bicanic I, van der Vaart H, et al. Pelvic floor muscle problems mediate sexual problems in young adult rape victims. J Sex Med. 2013;10:1978-1987.
15. Binik YM, Bergeron S, Khalifé S. Dyspareunia and vaginismus: so-called sexual pain. In: Leiblum SR, ed. 4th ed. Principles and Practice of Sex Therapy. The Guilford Press; 2007:124-156.
16. Ayling K, Ussher JM. “If sex hurts, am I still a woman?” The subjective experience of vulvodynia in hetero-sexual women. Arch Sex Behav. 2008;37:294-304.
17. Pazmany E, Bergeron S, Van Oudenhove L, et al. Body image and genital self-image in pre-menopausal women with dyspareunia. Arch Sex Behav. 2013;42:999-1010.
18. Maillé DL, Bergeron S, Lambert B. Body image in women with primary and secondary provoked vestibulodynia: a controlled study. J Sex Med. 2015;12:505-515.
19. Ryan L, Hawton K. Female dyspareunia. BMJ. 2004;328:1357.
20. Waldura JF, Arora I, Randall AM, et al. Fifty shades of stigma: exploring the health care experiences of kink-oriented patients. J Sex Med. 2016;13:1918-1929.
21. Hinchliff S, Gott M. Seeking medical help for sexual concerns in mid- and later life: a review of the literature. J Sex Res. 2011;48:106-117.
22. Banaei M, Kariman N, Ozgoli G, et al. Prevalence of postpartum dyspareunia: a systematic review and meta-analysis. Int J Gynaecol Obstet. 2021;153:14-24.
23. Kroenke K, Spitzer RL. The PHQ-9: A new depression diagnostic and severity measure. Psychiatr Ann. 2002;32:509-515.
24. Spitzer RL, Kroenke K, Williams JB, et al. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166:1092-1097.
25. U.S. Department of Veterans Affairs. PTSD: National Center for PTSD. Life events checklist for DSM-5 (LEC-5). Accessed February 3, 2022. www.ptsd.va.gov/professional/assessment/te-measures/life_events_checklist.asp
26. Weathers FW, Litz BT, Keane TM, et al. The PTSD checklist for DSM-5 (PCL-5). 2013. Accessed February 3, 2022. www.ptsd.va.gov/professional/assessment/adult-sr/ptsd-checklist.asp
27. Rosen R, Brown C, Heiman J, et al. The female sexual function index (FSFI): a multidimensional self-report instrument for the assessment of female sexual function. J Sex Marital Ther. 2000;26:191-208.
28. Melzack R. The short-form McGill Pain Questionnaire. Pain. 1987;30:191-197.
29. Hassam T, Kelso E, Chowdary P, et al. Sexual assault as a risk factor for gynaecological morbidity: an exploratory systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2020;255:222-230.
30. Hamlin AA, Sheeder J, Muffly TM. Brief versus thong hygiene in obstetrics and gynecology (B-THONG): a survey study. J Obstet Gynaecol Res. 2019;45:1190-1196.
31. Foster DC, Kotok MB, Huang LS, et al. Oral desipramine and topical lidocaine for vulvodynia: a randomized controlled trial. Obstet Gynecol. 2010;116:583-593.
32. Pérez-López FR, Bueno-Notivol J, Hernandez AV, et al. Systematic review and meta-analysis of the effects of treatment modalities for vestibulodynia in women. Eur J Contracept Reprod Health Care. 2019;24:337-346.
33. Cheong YC, Smotra G, Williams AC. Non-surgical interventions for the management of chronic pelvic pain. Cochrane Database Syst Rev. 2014;(3):CD008797.
34. Goldstein AT, Pukall CF, Brown C, et al. Vulvodynia: assessment and treatment. J Sex Med. 2016;13:572-590.
35. Bergeron S, Binik YM, Khalifé S, et al. A randomized comparison of group cognitive-behavioral therapy, surface electromyographic biofeedback, and vestibulectomy in the treatment of dyspareunia resulting from vulvar vestibulitis. Pain. 2001;91:297-306.
36. Schvartzman R, Schvartzman L, Ferreira CF, et al. Physical therapy intervention for women with dyspareunia: a randomized clinical trial. J Sex Marital Ther. 2019;45:378-394.
37. Morin M, Dumoulin C, Bergeron S, et al. Multimodal physical therapy versus topical lidocaine for provoked vestibulodynia: a multicenter, randomized trial. Am J Obstet Gynecol. 2021;224:189.e1-189.e12.
38. Bergeron S, Khalifé S, Dupuis M-J, et al. A randomized clinical trial comparing group cognitive-behavioral therapy and a topical steroid for women with dyspareunia. J Consult Clin Psychol. 2016;84:259-268.
39. Maseroli E, Scavello I, Rastrelli G, et al. Outcome of medical and psychosexual interventions for vaginismus: a systematic review and meta-analysis. J Sex Med. 2018;15:1752-1764.
40. American Psychological Association. Clinical practice guideline for the treatment of posttraumatic stress disorder (PTSD) in adults. 2017. Accessed February 3, 2022. www.apa.org/ptsd-guideline/ptsd.pdf
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th Ed. American Psychiatric Publishing; 2013.
2. Seehusen DA, Baird DC, Bode DV. Dyspareunia in women. Am Fam Phys. 2014;90:465-470.
3. Sorensen J, Bautista KE, Lamvu G, et al. Evaluation and treatment of female sexual pain: a clinical review. Cureus. 2018;10:e2379.
4. MacNeill C. Dyspareunia. Obstet Gynecol Clin North Am. 2006;33:565-77.
5. Latthe P, Latthe M, Say L, et al. WHO systematic review of prevalence of chronic pelvic pain: a neglected reproductive health morbidity. BMC Public Health. 2006;6:177.
6. Steege JF, Zolnoun DA. Evaluation and treatment of dyspareunia. Obstet Gynecol. 2009;113:1124-1136.
7. Williams AA, Williams M. A guide to performing pelvic speculum exams: a patient-centered approach to reducing iatrogenic effects. Teach Learn Med. 2013;25:383-391.
8. Ünlü Z, Yentur A, Çakil N. Pudendal nerve neuropathy: An unknown-rare cause of pelvic pain. Arch Rheumatol. 2016;31:102-103.
9. Dewitte M, Borg C, Lowenstein L. A psychosocial approach to female genital pain. Nat Rev Urol. 2018;15:25-41.
10. Masters WH, Johnson VE. Human Sexual Inadequacy. 1st ed. Little, Brown; 1970.
11. Rathus SA, Nevid JS, Fichner-Rathus L. Human Sexuality in a World of Diversity. 5th ed. Allyn and Bacon; 2002.
12. Bailey BE, Freedenfeld RN, Kiser RS, et al. Lifetime physical and sexual abuse in chronic pain patients: psychosocial correlates and treatment outcomes. Disabil Rehabil. 2003;25:331-342.
13. Yehuda R, Lehrner A, Rosenbaum TY. PTSD and sexual dysfunction in men and women. J Sex Med. 2015;12:1107-1119.
14. Postma R, Bicanic I, van der Vaart H, et al. Pelvic floor muscle problems mediate sexual problems in young adult rape victims. J Sex Med. 2013;10:1978-1987.
15. Binik YM, Bergeron S, Khalifé S. Dyspareunia and vaginismus: so-called sexual pain. In: Leiblum SR, ed. 4th ed. Principles and Practice of Sex Therapy. The Guilford Press; 2007:124-156.
16. Ayling K, Ussher JM. “If sex hurts, am I still a woman?” The subjective experience of vulvodynia in hetero-sexual women. Arch Sex Behav. 2008;37:294-304.
17. Pazmany E, Bergeron S, Van Oudenhove L, et al. Body image and genital self-image in pre-menopausal women with dyspareunia. Arch Sex Behav. 2013;42:999-1010.
18. Maillé DL, Bergeron S, Lambert B. Body image in women with primary and secondary provoked vestibulodynia: a controlled study. J Sex Med. 2015;12:505-515.
19. Ryan L, Hawton K. Female dyspareunia. BMJ. 2004;328:1357.
20. Waldura JF, Arora I, Randall AM, et al. Fifty shades of stigma: exploring the health care experiences of kink-oriented patients. J Sex Med. 2016;13:1918-1929.
21. Hinchliff S, Gott M. Seeking medical help for sexual concerns in mid- and later life: a review of the literature. J Sex Res. 2011;48:106-117.
22. Banaei M, Kariman N, Ozgoli G, et al. Prevalence of postpartum dyspareunia: a systematic review and meta-analysis. Int J Gynaecol Obstet. 2021;153:14-24.
23. Kroenke K, Spitzer RL. The PHQ-9: A new depression diagnostic and severity measure. Psychiatr Ann. 2002;32:509-515.
24. Spitzer RL, Kroenke K, Williams JB, et al. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166:1092-1097.
25. U.S. Department of Veterans Affairs. PTSD: National Center for PTSD. Life events checklist for DSM-5 (LEC-5). Accessed February 3, 2022. www.ptsd.va.gov/professional/assessment/te-measures/life_events_checklist.asp
26. Weathers FW, Litz BT, Keane TM, et al. The PTSD checklist for DSM-5 (PCL-5). 2013. Accessed February 3, 2022. www.ptsd.va.gov/professional/assessment/adult-sr/ptsd-checklist.asp
27. Rosen R, Brown C, Heiman J, et al. The female sexual function index (FSFI): a multidimensional self-report instrument for the assessment of female sexual function. J Sex Marital Ther. 2000;26:191-208.
28. Melzack R. The short-form McGill Pain Questionnaire. Pain. 1987;30:191-197.
29. Hassam T, Kelso E, Chowdary P, et al. Sexual assault as a risk factor for gynaecological morbidity: an exploratory systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2020;255:222-230.
30. Hamlin AA, Sheeder J, Muffly TM. Brief versus thong hygiene in obstetrics and gynecology (B-THONG): a survey study. J Obstet Gynaecol Res. 2019;45:1190-1196.
31. Foster DC, Kotok MB, Huang LS, et al. Oral desipramine and topical lidocaine for vulvodynia: a randomized controlled trial. Obstet Gynecol. 2010;116:583-593.
32. Pérez-López FR, Bueno-Notivol J, Hernandez AV, et al. Systematic review and meta-analysis of the effects of treatment modalities for vestibulodynia in women. Eur J Contracept Reprod Health Care. 2019;24:337-346.
33. Cheong YC, Smotra G, Williams AC. Non-surgical interventions for the management of chronic pelvic pain. Cochrane Database Syst Rev. 2014;(3):CD008797.
34. Goldstein AT, Pukall CF, Brown C, et al. Vulvodynia: assessment and treatment. J Sex Med. 2016;13:572-590.
35. Bergeron S, Binik YM, Khalifé S, et al. A randomized comparison of group cognitive-behavioral therapy, surface electromyographic biofeedback, and vestibulectomy in the treatment of dyspareunia resulting from vulvar vestibulitis. Pain. 2001;91:297-306.
36. Schvartzman R, Schvartzman L, Ferreira CF, et al. Physical therapy intervention for women with dyspareunia: a randomized clinical trial. J Sex Marital Ther. 2019;45:378-394.
37. Morin M, Dumoulin C, Bergeron S, et al. Multimodal physical therapy versus topical lidocaine for provoked vestibulodynia: a multicenter, randomized trial. Am J Obstet Gynecol. 2021;224:189.e1-189.e12.
38. Bergeron S, Khalifé S, Dupuis M-J, et al. A randomized clinical trial comparing group cognitive-behavioral therapy and a topical steroid for women with dyspareunia. J Consult Clin Psychol. 2016;84:259-268.
39. Maseroli E, Scavello I, Rastrelli G, et al. Outcome of medical and psychosexual interventions for vaginismus: a systematic review and meta-analysis. J Sex Med. 2018;15:1752-1764.
40. American Psychological Association. Clinical practice guideline for the treatment of posttraumatic stress disorder (PTSD) in adults. 2017. Accessed February 3, 2022. www.apa.org/ptsd-guideline/ptsd.pdf
PRACTICE RECOMMENDATIONS
› Screen all patients for sexual dysfunctions, as patients often do not report symptoms on their own. B
› Refer patients with dyspareunia for psychotherapy to address both pain and psychosocial causes and sequela of dyspareunia. A
› Refer patients with dyspareunia for pelvic floor physical therapy to address pain and sexual functioning. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Hair loss affects more than half of postmenopausal women
Female-pattern hair loss (FPHL) was identified in 52% of postmenopausal women, and 4% of these cases involved extensive baldness, based on data from 178 individuals.
FPHL can develop at any time from teenage years through and beyond menopause, wrote Sukanya Chaikittisilpa, MD, of Chulalongkorn University, Bangkok, and colleagues.
The cause of FPHL remains uncertain, but the presence of estrogen receptors in hair follicles suggests that the hormone changes of menopause may affect hair growth, the researchers said.
In a study published in Menopause, the researchers evaluated 178 postmenopausal women aged 50-65 years for FPHL. FPLH was determined based on photographs and on measures of hormone levels, hair density, and hair diameter.
The overall prevalence of FPHL was 52.2%. The hair loss was divided into three categories indicating mild, moderate, and severe (Ludwig grades I, II, and III) with prevalence of 73.2%, 22.6%, and 4.3%, respectively. The prevalence of FPHL also increased with age and time since menopause. In a simple logistic regression analysis, age 56 years and older and more than 6 years since menopause were significantly associated with FPHL (odds ratios, 3.41 and 1.98, respectively).
However, after adjustment for multiple variables, only a body mass index of 25 kg/m2 or higher also was associated with increased prevalence of FPHL (adjusted OR, 2.65).
A total of 60% of the study participants met criteria for low self-esteem, including all the women in the severe hair loss category.
“The postmenopausal women with FPHL in our cohort had lower total hair density, terminal hair density, hair thickness, hair unit density, and average hair per unit than those with normal hair patterns,” although vellus hair density was higher in women with FPHL, the researchers wrote in their discussion of the findings. This distinction may be caused in part by the shortened hair cycle and reduced anagen phase of velluslike follicles, they said.
The study findings were limited by several factors, including the cross-sectional design and the inclusion of only women from a single menopause clinic, which may not reflect FPHL in the general population, as well as the reliance on patients’ recall, the researchers noted. Another limitation was the inability to assess postmenopausal hormone levels, they added.
However, “This study may be the first FPHL study conducted in a menopause clinic that targeted only healthy postmenopausal women,” they wrote. More research is needed to determine the potential role of estrogen and testosterone on FPHL in postmenopausal women, and whether a history of polycystic ovarian syndrome has an effect, they said. Meanwhile, current study results may help clinicians and patients determine the most appropriate menopausal hormone therapies for postmenopausal women with FPHL, they concluded.
Consider lifestyle and self-esteem issues
The current study is important at this time because a larger proportion of women are either reaching menopause or are menopausal, said Constance Bohon, MD, a gynecologist in private practice in Washington, in an interview.
“Whatever we in the medical community can do to help women transition into the menopausal years with the least anxiety is important,” including helping women feel comfortable about their appearance, she said.
“For women in the peri- and postmenopausal years, hair loss is a relatively common concern,” Dr. Bohon said. However, in the current study, “I was surprised that it was associated with low self-esteem and obesity,” she noted. “For these women, it would be interesting to know whether they also had concerns about the appearance of their bodies, or just their hair loss,” she said. The question is whether the hair loss in and of itself caused low self-esteem in the study population, or whether it exacerbated their already poor self-assessment, Dr. Bohon said. “Another consideration is that perhaps these women were already feeling the effects of aging and were trying to change their appearance by using hair dyes, and now they find themselves losing hair as well,” she noted.
The takeaway message for clinicians is that discussions with perimenopausal and postmenopausal women should include the topic of hair loss along with hot flashes and night sweats, said Dr. Bohon.
Women who are experiencing hair loss or concerned about the possibility of hair loss should ask their doctors about possible interventions that may mitigate or prevent further hair loss, she said.
As for additional research, “the most important issue is to determine the factors that are associated with hair loss in the perimenopausal and postmenopausal years,” Dr. Bohon said. Research questions should include impact of dyeing or straightening hair on the likelihood of hair loss, and whether women with more severe hot flashes/night sweats and/or sleeplessness have more hair loss than women who do not experience any of the symptoms as they go through menopause, she emphasized.
Other considerations are whether certain diets or foods are more common among women who have more hair loss, and whether weight loss into a normal range or weight gain into a body mass index greater than 25 kg/m2 affects hair loss, said Dr. Bohon. Also, don’t discount the impact of stress, and whether women who have lost hair identify certain stressful times that preceded their hair loss, as well as what medications could be associated with hair loss, and whether hormone therapy might prevent hair loss, she said.
The study was supported by the Ratchadapiseksompotch Fund, Faculty of Medicine, Chulalongkorn University. The researchers had no financial conflicts to disclose. Dr. Bohon had no financial conflicts to disclose and serves on the Editorial Advisory Board of Ob.Gyn. News.
Female-pattern hair loss (FPHL) was identified in 52% of postmenopausal women, and 4% of these cases involved extensive baldness, based on data from 178 individuals.
FPHL can develop at any time from teenage years through and beyond menopause, wrote Sukanya Chaikittisilpa, MD, of Chulalongkorn University, Bangkok, and colleagues.
The cause of FPHL remains uncertain, but the presence of estrogen receptors in hair follicles suggests that the hormone changes of menopause may affect hair growth, the researchers said.
In a study published in Menopause, the researchers evaluated 178 postmenopausal women aged 50-65 years for FPHL. FPLH was determined based on photographs and on measures of hormone levels, hair density, and hair diameter.
The overall prevalence of FPHL was 52.2%. The hair loss was divided into three categories indicating mild, moderate, and severe (Ludwig grades I, II, and III) with prevalence of 73.2%, 22.6%, and 4.3%, respectively. The prevalence of FPHL also increased with age and time since menopause. In a simple logistic regression analysis, age 56 years and older and more than 6 years since menopause were significantly associated with FPHL (odds ratios, 3.41 and 1.98, respectively).
However, after adjustment for multiple variables, only a body mass index of 25 kg/m2 or higher also was associated with increased prevalence of FPHL (adjusted OR, 2.65).
A total of 60% of the study participants met criteria for low self-esteem, including all the women in the severe hair loss category.
“The postmenopausal women with FPHL in our cohort had lower total hair density, terminal hair density, hair thickness, hair unit density, and average hair per unit than those with normal hair patterns,” although vellus hair density was higher in women with FPHL, the researchers wrote in their discussion of the findings. This distinction may be caused in part by the shortened hair cycle and reduced anagen phase of velluslike follicles, they said.
The study findings were limited by several factors, including the cross-sectional design and the inclusion of only women from a single menopause clinic, which may not reflect FPHL in the general population, as well as the reliance on patients’ recall, the researchers noted. Another limitation was the inability to assess postmenopausal hormone levels, they added.
However, “This study may be the first FPHL study conducted in a menopause clinic that targeted only healthy postmenopausal women,” they wrote. More research is needed to determine the potential role of estrogen and testosterone on FPHL in postmenopausal women, and whether a history of polycystic ovarian syndrome has an effect, they said. Meanwhile, current study results may help clinicians and patients determine the most appropriate menopausal hormone therapies for postmenopausal women with FPHL, they concluded.
Consider lifestyle and self-esteem issues
The current study is important at this time because a larger proportion of women are either reaching menopause or are menopausal, said Constance Bohon, MD, a gynecologist in private practice in Washington, in an interview.
“Whatever we in the medical community can do to help women transition into the menopausal years with the least anxiety is important,” including helping women feel comfortable about their appearance, she said.
“For women in the peri- and postmenopausal years, hair loss is a relatively common concern,” Dr. Bohon said. However, in the current study, “I was surprised that it was associated with low self-esteem and obesity,” she noted. “For these women, it would be interesting to know whether they also had concerns about the appearance of their bodies, or just their hair loss,” she said. The question is whether the hair loss in and of itself caused low self-esteem in the study population, or whether it exacerbated their already poor self-assessment, Dr. Bohon said. “Another consideration is that perhaps these women were already feeling the effects of aging and were trying to change their appearance by using hair dyes, and now they find themselves losing hair as well,” she noted.
The takeaway message for clinicians is that discussions with perimenopausal and postmenopausal women should include the topic of hair loss along with hot flashes and night sweats, said Dr. Bohon.
Women who are experiencing hair loss or concerned about the possibility of hair loss should ask their doctors about possible interventions that may mitigate or prevent further hair loss, she said.
As for additional research, “the most important issue is to determine the factors that are associated with hair loss in the perimenopausal and postmenopausal years,” Dr. Bohon said. Research questions should include impact of dyeing or straightening hair on the likelihood of hair loss, and whether women with more severe hot flashes/night sweats and/or sleeplessness have more hair loss than women who do not experience any of the symptoms as they go through menopause, she emphasized.
Other considerations are whether certain diets or foods are more common among women who have more hair loss, and whether weight loss into a normal range or weight gain into a body mass index greater than 25 kg/m2 affects hair loss, said Dr. Bohon. Also, don’t discount the impact of stress, and whether women who have lost hair identify certain stressful times that preceded their hair loss, as well as what medications could be associated with hair loss, and whether hormone therapy might prevent hair loss, she said.
The study was supported by the Ratchadapiseksompotch Fund, Faculty of Medicine, Chulalongkorn University. The researchers had no financial conflicts to disclose. Dr. Bohon had no financial conflicts to disclose and serves on the Editorial Advisory Board of Ob.Gyn. News.
Female-pattern hair loss (FPHL) was identified in 52% of postmenopausal women, and 4% of these cases involved extensive baldness, based on data from 178 individuals.
FPHL can develop at any time from teenage years through and beyond menopause, wrote Sukanya Chaikittisilpa, MD, of Chulalongkorn University, Bangkok, and colleagues.
The cause of FPHL remains uncertain, but the presence of estrogen receptors in hair follicles suggests that the hormone changes of menopause may affect hair growth, the researchers said.
In a study published in Menopause, the researchers evaluated 178 postmenopausal women aged 50-65 years for FPHL. FPLH was determined based on photographs and on measures of hormone levels, hair density, and hair diameter.
The overall prevalence of FPHL was 52.2%. The hair loss was divided into three categories indicating mild, moderate, and severe (Ludwig grades I, II, and III) with prevalence of 73.2%, 22.6%, and 4.3%, respectively. The prevalence of FPHL also increased with age and time since menopause. In a simple logistic regression analysis, age 56 years and older and more than 6 years since menopause were significantly associated with FPHL (odds ratios, 3.41 and 1.98, respectively).
However, after adjustment for multiple variables, only a body mass index of 25 kg/m2 or higher also was associated with increased prevalence of FPHL (adjusted OR, 2.65).
A total of 60% of the study participants met criteria for low self-esteem, including all the women in the severe hair loss category.
“The postmenopausal women with FPHL in our cohort had lower total hair density, terminal hair density, hair thickness, hair unit density, and average hair per unit than those with normal hair patterns,” although vellus hair density was higher in women with FPHL, the researchers wrote in their discussion of the findings. This distinction may be caused in part by the shortened hair cycle and reduced anagen phase of velluslike follicles, they said.
The study findings were limited by several factors, including the cross-sectional design and the inclusion of only women from a single menopause clinic, which may not reflect FPHL in the general population, as well as the reliance on patients’ recall, the researchers noted. Another limitation was the inability to assess postmenopausal hormone levels, they added.
However, “This study may be the first FPHL study conducted in a menopause clinic that targeted only healthy postmenopausal women,” they wrote. More research is needed to determine the potential role of estrogen and testosterone on FPHL in postmenopausal women, and whether a history of polycystic ovarian syndrome has an effect, they said. Meanwhile, current study results may help clinicians and patients determine the most appropriate menopausal hormone therapies for postmenopausal women with FPHL, they concluded.
Consider lifestyle and self-esteem issues
The current study is important at this time because a larger proportion of women are either reaching menopause or are menopausal, said Constance Bohon, MD, a gynecologist in private practice in Washington, in an interview.
“Whatever we in the medical community can do to help women transition into the menopausal years with the least anxiety is important,” including helping women feel comfortable about their appearance, she said.
“For women in the peri- and postmenopausal years, hair loss is a relatively common concern,” Dr. Bohon said. However, in the current study, “I was surprised that it was associated with low self-esteem and obesity,” she noted. “For these women, it would be interesting to know whether they also had concerns about the appearance of their bodies, or just their hair loss,” she said. The question is whether the hair loss in and of itself caused low self-esteem in the study population, or whether it exacerbated their already poor self-assessment, Dr. Bohon said. “Another consideration is that perhaps these women were already feeling the effects of aging and were trying to change their appearance by using hair dyes, and now they find themselves losing hair as well,” she noted.
The takeaway message for clinicians is that discussions with perimenopausal and postmenopausal women should include the topic of hair loss along with hot flashes and night sweats, said Dr. Bohon.
Women who are experiencing hair loss or concerned about the possibility of hair loss should ask their doctors about possible interventions that may mitigate or prevent further hair loss, she said.
As for additional research, “the most important issue is to determine the factors that are associated with hair loss in the perimenopausal and postmenopausal years,” Dr. Bohon said. Research questions should include impact of dyeing or straightening hair on the likelihood of hair loss, and whether women with more severe hot flashes/night sweats and/or sleeplessness have more hair loss than women who do not experience any of the symptoms as they go through menopause, she emphasized.
Other considerations are whether certain diets or foods are more common among women who have more hair loss, and whether weight loss into a normal range or weight gain into a body mass index greater than 25 kg/m2 affects hair loss, said Dr. Bohon. Also, don’t discount the impact of stress, and whether women who have lost hair identify certain stressful times that preceded their hair loss, as well as what medications could be associated with hair loss, and whether hormone therapy might prevent hair loss, she said.
The study was supported by the Ratchadapiseksompotch Fund, Faculty of Medicine, Chulalongkorn University. The researchers had no financial conflicts to disclose. Dr. Bohon had no financial conflicts to disclose and serves on the Editorial Advisory Board of Ob.Gyn. News.
FROM MENOPAUSE
Past spontaneous abortion raises risk for gestational diabetes
Pregnant women with a history of spontaneous abortion had a significantly increased risk of gestational diabetes in subsequent pregnancies, based on data from more than 100,000 women.
Gestational diabetes is associated not only with adverse perinatal outcomes, but also with an increased risk of long-term cardiovascular and metabolic health issues in mothers and children, wrote Yan Zhao, PhD, of Tongji University, Shanghai, and colleagues.
Previous studies also have shown that spontaneous abortion (SAB) is associated with later maternal risk of cardiovascular disease and venous thromboembolism, the researchers said. The same mechanisms might contribute to the development of gestational diabetes, but the association between abortion history and gestational diabetes risk in subsequent pregnancies remains unclear, they added.
In a study published in JAMA Network Open, the researchers identified 102,259 pregnant women seen for routine prenatal care at a single hospital in Shanghai between January 2014 and December 2019. The mean age of the women was 29.8 years.
During the study period, 14,579 women experienced SAB (14.3%), 17,935 experienced induced abortion (17.5%), and 4,017 experienced both (11.9%).
In all, 12,153 cases of gestational diabetes were identified, for a prevalence of 11.9%. The relative risk of gestational diabetes was 1.25 for women who experienced SAB and 1.15 for those who experienced both SAB and induced abortion, and the association between SAB and gestational diabetes increased in a number-dependent manner, the researchers said. The increase in relative risk for gestational diabetes in pregnant women with one SAB, two SABs, and three or more SABs was 18%, 41%, and 43%, compared to pregnant women with no SAB history.
However, no association appeared between a history of induced abortion and gestational diabetes, the researchers said. “To date, no study has reported the association of prior induced abortion with gestational diabetes,” they wrote.
The study findings were limited by several factors including the reliance on self-reports for history of SAB and therefore possible underreporting, the researchers noted. Other limitations included the lack of data on the timing of SABs; therefore, the time between SAB and gestational diabetes diagnosis could not be included in the analysis, they said. Unknown variables and the inclusion only of women from a single city in China might limit the generalizability of the results, they added.
More research is needed to understand the biological mechanisms behind the association between SAB and gestational diabetes, an association that has potential public health implications, they noted. However, the results suggest that “pregnant women with a history of SAB, especially those with a history of recurrent SAB, should attend more antenatal visits to monitor their blood glucose and implement early prevention and intervention,” such as healthful eating and regular exercise, they wrote.
Findings confirm, not surprise
The diagnosis of gestational diabetes in the current study “was made with a slightly different test than we typically use in the United States – a 1-hour nonfasting glucola followed by a confirmatory 3-hour fasting glucola,” Sarah W. Prager, MD, of the University of Washington, Seattle, said in an interview. The current study of both SAB and gestational diabetes is important because both conditions are very common and have been the focus of increased attention in the popular media and in scientific study, she said.
Dr. Prager said she was not surprised by the findings of a link between a history of gestational diabetes and a history of SAB, “but the association is likely that people at risk for gestational diabetes or who have undiagnosed diabetes/glucose intolerance are more likely to experience SAB,” she noted. “I would be surprised if the direction of the association is that SAB puts people at risk for gestational diabetes; more likely undiagnosed diabetes is a risk factor for SAB,” she added. “Perhaps we should be screening for glucose intolerance and other metabolic disorders more frequently in people who have especially recurrent SAB, as the more miscarriages someone had, the more likely they were in this study to be diagnosed with gestational diabetes;” or perhaps those with a history of SAB/recurrent SAB should be screened closer to 24 weeks’ than 28 weeks’ gestation to enable earlier intervention in those more likely to have gestational diabetes, Dr. Prager said.
The study was supported by the Key Program of the National Natural Science Foundation of China, the National Natural Science Foundation of China, the National Key Research and Development Program of China, the Shanghai Municipal Medical and Health Discipline Construction Projects, and the Shanghai Rising-Star Program. The researchers and Dr. Prager had no financial conflicts to disclose. Dr. Prager serves on the editorial advisory board of Ob.Gyn. News.
Pregnant women with a history of spontaneous abortion had a significantly increased risk of gestational diabetes in subsequent pregnancies, based on data from more than 100,000 women.
Gestational diabetes is associated not only with adverse perinatal outcomes, but also with an increased risk of long-term cardiovascular and metabolic health issues in mothers and children, wrote Yan Zhao, PhD, of Tongji University, Shanghai, and colleagues.
Previous studies also have shown that spontaneous abortion (SAB) is associated with later maternal risk of cardiovascular disease and venous thromboembolism, the researchers said. The same mechanisms might contribute to the development of gestational diabetes, but the association between abortion history and gestational diabetes risk in subsequent pregnancies remains unclear, they added.
In a study published in JAMA Network Open, the researchers identified 102,259 pregnant women seen for routine prenatal care at a single hospital in Shanghai between January 2014 and December 2019. The mean age of the women was 29.8 years.
During the study period, 14,579 women experienced SAB (14.3%), 17,935 experienced induced abortion (17.5%), and 4,017 experienced both (11.9%).
In all, 12,153 cases of gestational diabetes were identified, for a prevalence of 11.9%. The relative risk of gestational diabetes was 1.25 for women who experienced SAB and 1.15 for those who experienced both SAB and induced abortion, and the association between SAB and gestational diabetes increased in a number-dependent manner, the researchers said. The increase in relative risk for gestational diabetes in pregnant women with one SAB, two SABs, and three or more SABs was 18%, 41%, and 43%, compared to pregnant women with no SAB history.
However, no association appeared between a history of induced abortion and gestational diabetes, the researchers said. “To date, no study has reported the association of prior induced abortion with gestational diabetes,” they wrote.
The study findings were limited by several factors including the reliance on self-reports for history of SAB and therefore possible underreporting, the researchers noted. Other limitations included the lack of data on the timing of SABs; therefore, the time between SAB and gestational diabetes diagnosis could not be included in the analysis, they said. Unknown variables and the inclusion only of women from a single city in China might limit the generalizability of the results, they added.
More research is needed to understand the biological mechanisms behind the association between SAB and gestational diabetes, an association that has potential public health implications, they noted. However, the results suggest that “pregnant women with a history of SAB, especially those with a history of recurrent SAB, should attend more antenatal visits to monitor their blood glucose and implement early prevention and intervention,” such as healthful eating and regular exercise, they wrote.
Findings confirm, not surprise
The diagnosis of gestational diabetes in the current study “was made with a slightly different test than we typically use in the United States – a 1-hour nonfasting glucola followed by a confirmatory 3-hour fasting glucola,” Sarah W. Prager, MD, of the University of Washington, Seattle, said in an interview. The current study of both SAB and gestational diabetes is important because both conditions are very common and have been the focus of increased attention in the popular media and in scientific study, she said.
Dr. Prager said she was not surprised by the findings of a link between a history of gestational diabetes and a history of SAB, “but the association is likely that people at risk for gestational diabetes or who have undiagnosed diabetes/glucose intolerance are more likely to experience SAB,” she noted. “I would be surprised if the direction of the association is that SAB puts people at risk for gestational diabetes; more likely undiagnosed diabetes is a risk factor for SAB,” she added. “Perhaps we should be screening for glucose intolerance and other metabolic disorders more frequently in people who have especially recurrent SAB, as the more miscarriages someone had, the more likely they were in this study to be diagnosed with gestational diabetes;” or perhaps those with a history of SAB/recurrent SAB should be screened closer to 24 weeks’ than 28 weeks’ gestation to enable earlier intervention in those more likely to have gestational diabetes, Dr. Prager said.
The study was supported by the Key Program of the National Natural Science Foundation of China, the National Natural Science Foundation of China, the National Key Research and Development Program of China, the Shanghai Municipal Medical and Health Discipline Construction Projects, and the Shanghai Rising-Star Program. The researchers and Dr. Prager had no financial conflicts to disclose. Dr. Prager serves on the editorial advisory board of Ob.Gyn. News.
Pregnant women with a history of spontaneous abortion had a significantly increased risk of gestational diabetes in subsequent pregnancies, based on data from more than 100,000 women.
Gestational diabetes is associated not only with adverse perinatal outcomes, but also with an increased risk of long-term cardiovascular and metabolic health issues in mothers and children, wrote Yan Zhao, PhD, of Tongji University, Shanghai, and colleagues.
Previous studies also have shown that spontaneous abortion (SAB) is associated with later maternal risk of cardiovascular disease and venous thromboembolism, the researchers said. The same mechanisms might contribute to the development of gestational diabetes, but the association between abortion history and gestational diabetes risk in subsequent pregnancies remains unclear, they added.
In a study published in JAMA Network Open, the researchers identified 102,259 pregnant women seen for routine prenatal care at a single hospital in Shanghai between January 2014 and December 2019. The mean age of the women was 29.8 years.
During the study period, 14,579 women experienced SAB (14.3%), 17,935 experienced induced abortion (17.5%), and 4,017 experienced both (11.9%).
In all, 12,153 cases of gestational diabetes were identified, for a prevalence of 11.9%. The relative risk of gestational diabetes was 1.25 for women who experienced SAB and 1.15 for those who experienced both SAB and induced abortion, and the association between SAB and gestational diabetes increased in a number-dependent manner, the researchers said. The increase in relative risk for gestational diabetes in pregnant women with one SAB, two SABs, and three or more SABs was 18%, 41%, and 43%, compared to pregnant women with no SAB history.
However, no association appeared between a history of induced abortion and gestational diabetes, the researchers said. “To date, no study has reported the association of prior induced abortion with gestational diabetes,” they wrote.
The study findings were limited by several factors including the reliance on self-reports for history of SAB and therefore possible underreporting, the researchers noted. Other limitations included the lack of data on the timing of SABs; therefore, the time between SAB and gestational diabetes diagnosis could not be included in the analysis, they said. Unknown variables and the inclusion only of women from a single city in China might limit the generalizability of the results, they added.
More research is needed to understand the biological mechanisms behind the association between SAB and gestational diabetes, an association that has potential public health implications, they noted. However, the results suggest that “pregnant women with a history of SAB, especially those with a history of recurrent SAB, should attend more antenatal visits to monitor their blood glucose and implement early prevention and intervention,” such as healthful eating and regular exercise, they wrote.
Findings confirm, not surprise
The diagnosis of gestational diabetes in the current study “was made with a slightly different test than we typically use in the United States – a 1-hour nonfasting glucola followed by a confirmatory 3-hour fasting glucola,” Sarah W. Prager, MD, of the University of Washington, Seattle, said in an interview. The current study of both SAB and gestational diabetes is important because both conditions are very common and have been the focus of increased attention in the popular media and in scientific study, she said.
Dr. Prager said she was not surprised by the findings of a link between a history of gestational diabetes and a history of SAB, “but the association is likely that people at risk for gestational diabetes or who have undiagnosed diabetes/glucose intolerance are more likely to experience SAB,” she noted. “I would be surprised if the direction of the association is that SAB puts people at risk for gestational diabetes; more likely undiagnosed diabetes is a risk factor for SAB,” she added. “Perhaps we should be screening for glucose intolerance and other metabolic disorders more frequently in people who have especially recurrent SAB, as the more miscarriages someone had, the more likely they were in this study to be diagnosed with gestational diabetes;” or perhaps those with a history of SAB/recurrent SAB should be screened closer to 24 weeks’ than 28 weeks’ gestation to enable earlier intervention in those more likely to have gestational diabetes, Dr. Prager said.
The study was supported by the Key Program of the National Natural Science Foundation of China, the National Natural Science Foundation of China, the National Key Research and Development Program of China, the Shanghai Municipal Medical and Health Discipline Construction Projects, and the Shanghai Rising-Star Program. The researchers and Dr. Prager had no financial conflicts to disclose. Dr. Prager serves on the editorial advisory board of Ob.Gyn. News.
FROM JAMA NETWORK OPEN
15th Report on Carcinogens Adds to Its List
From environmental tobacco smoke to ultraviolet (UV) radiation, diesel exhaust particulates, lead, and now, chronic infection with Helicobacter pylori (H pylori)—the Report on Carcinogens has regularly updated the list of substances known or “reasonably anticipated” to cause cancer.
The 15th report, which is prepared by the National Toxicology Program (NTP) for the Department of Health and Human Services, has 8 new entries, bringing the number of human carcinogens (eg, metals, pesticides, and drugs) on the list to 256. (The first report, released in 1980, listed 26.) In addition to H pylori infection, this edition adds the flame-retardant chemical antimony trioxide, and 6 haloacetic acids found as water disinfection byproducts.
In 1971, then President Nixon declared “war on cancer” (the second leading cause of death in the US) and signed the National Cancer Act. In 1978, Congress ordered the Report on Carcinogens, to educate the public and health professionals on potential environmental carcinogenic hazards.
Perhaps disheartening to know that even with 256 entries, the list probably understates the number of carcinogens humans and other creatures are exposed to. But things can change with time. Each list goes through a rigorous round of reviews. Sometimes substances are “delisted” after, for instance, litigation or new research. Saccharin, for example, was removed from the ninth edition. It was listed as “reasonably anticipated” in 1981, based on “sufficient evidence of carcinogenicity in experimental animals.” It was removed, however, after extensive review of decades of saccharin use determined that the data were not sufficient to meet current criteria. Further research had revealed, also, that the observed bladder tumors in rats arose from a mechanism not relevant to humans.
Other entries, such as the controversial listing of the cancer drug tamoxifen, walk a fine line between risk and benefit. Tamoxifen, first listed in the ninth report (and still in the 15th report), was included because studies revealed that it could increase the risk of uterine cancer in women. But there also was conclusive evidence that it may prevent or delay breast cancer in women who are at high risk.
Ultimately, the report’s authors make it clear that it is for informative value and guidance, not necessarily a dictate. As one report put it: “Personal decisions concerning voluntary exposures to carcinogenic agents need to be based on additional information that is beyond the scope” of the report.
“As the identification of carcinogens is a key step in cancer prevention,” said Rick Woychik, PhD, director of the National Institute of Environmental Health Sciences and NTP, “publication of the report represents an important government activity towards improving public health.”
From environmental tobacco smoke to ultraviolet (UV) radiation, diesel exhaust particulates, lead, and now, chronic infection with Helicobacter pylori (H pylori)—the Report on Carcinogens has regularly updated the list of substances known or “reasonably anticipated” to cause cancer.
The 15th report, which is prepared by the National Toxicology Program (NTP) for the Department of Health and Human Services, has 8 new entries, bringing the number of human carcinogens (eg, metals, pesticides, and drugs) on the list to 256. (The first report, released in 1980, listed 26.) In addition to H pylori infection, this edition adds the flame-retardant chemical antimony trioxide, and 6 haloacetic acids found as water disinfection byproducts.
In 1971, then President Nixon declared “war on cancer” (the second leading cause of death in the US) and signed the National Cancer Act. In 1978, Congress ordered the Report on Carcinogens, to educate the public and health professionals on potential environmental carcinogenic hazards.
Perhaps disheartening to know that even with 256 entries, the list probably understates the number of carcinogens humans and other creatures are exposed to. But things can change with time. Each list goes through a rigorous round of reviews. Sometimes substances are “delisted” after, for instance, litigation or new research. Saccharin, for example, was removed from the ninth edition. It was listed as “reasonably anticipated” in 1981, based on “sufficient evidence of carcinogenicity in experimental animals.” It was removed, however, after extensive review of decades of saccharin use determined that the data were not sufficient to meet current criteria. Further research had revealed, also, that the observed bladder tumors in rats arose from a mechanism not relevant to humans.
Other entries, such as the controversial listing of the cancer drug tamoxifen, walk a fine line between risk and benefit. Tamoxifen, first listed in the ninth report (and still in the 15th report), was included because studies revealed that it could increase the risk of uterine cancer in women. But there also was conclusive evidence that it may prevent or delay breast cancer in women who are at high risk.
Ultimately, the report’s authors make it clear that it is for informative value and guidance, not necessarily a dictate. As one report put it: “Personal decisions concerning voluntary exposures to carcinogenic agents need to be based on additional information that is beyond the scope” of the report.
“As the identification of carcinogens is a key step in cancer prevention,” said Rick Woychik, PhD, director of the National Institute of Environmental Health Sciences and NTP, “publication of the report represents an important government activity towards improving public health.”
From environmental tobacco smoke to ultraviolet (UV) radiation, diesel exhaust particulates, lead, and now, chronic infection with Helicobacter pylori (H pylori)—the Report on Carcinogens has regularly updated the list of substances known or “reasonably anticipated” to cause cancer.
The 15th report, which is prepared by the National Toxicology Program (NTP) for the Department of Health and Human Services, has 8 new entries, bringing the number of human carcinogens (eg, metals, pesticides, and drugs) on the list to 256. (The first report, released in 1980, listed 26.) In addition to H pylori infection, this edition adds the flame-retardant chemical antimony trioxide, and 6 haloacetic acids found as water disinfection byproducts.
In 1971, then President Nixon declared “war on cancer” (the second leading cause of death in the US) and signed the National Cancer Act. In 1978, Congress ordered the Report on Carcinogens, to educate the public and health professionals on potential environmental carcinogenic hazards.
Perhaps disheartening to know that even with 256 entries, the list probably understates the number of carcinogens humans and other creatures are exposed to. But things can change with time. Each list goes through a rigorous round of reviews. Sometimes substances are “delisted” after, for instance, litigation or new research. Saccharin, for example, was removed from the ninth edition. It was listed as “reasonably anticipated” in 1981, based on “sufficient evidence of carcinogenicity in experimental animals.” It was removed, however, after extensive review of decades of saccharin use determined that the data were not sufficient to meet current criteria. Further research had revealed, also, that the observed bladder tumors in rats arose from a mechanism not relevant to humans.
Other entries, such as the controversial listing of the cancer drug tamoxifen, walk a fine line between risk and benefit. Tamoxifen, first listed in the ninth report (and still in the 15th report), was included because studies revealed that it could increase the risk of uterine cancer in women. But there also was conclusive evidence that it may prevent or delay breast cancer in women who are at high risk.
Ultimately, the report’s authors make it clear that it is for informative value and guidance, not necessarily a dictate. As one report put it: “Personal decisions concerning voluntary exposures to carcinogenic agents need to be based on additional information that is beyond the scope” of the report.
“As the identification of carcinogens is a key step in cancer prevention,” said Rick Woychik, PhD, director of the National Institute of Environmental Health Sciences and NTP, “publication of the report represents an important government activity towards improving public health.”
Early menopause, early dementia risk, study suggests
Earlier menopause appears to be associated with a higher risk of dementia, and earlier onset of dementia, compared with menopause at normal age or later, according to a large study.
“Being aware of this increased risk can help women practice strategies to prevent dementia and to work with their physicians to closely monitor their cognitive status as they age,” study investigator Wenting Hao, MD, with Shandong University, Jinan, China, says in a news release.
The findings were presented in an e-poster March 1 at the Epidemiology, Prevention, Lifestyle & Cardiometabolic Health (EPI|Lifestyle) 2022 conference sponsored by the American Heart Association.
UK Biobank data
Dr. Hao and colleagues examined health data for 153,291 women who were 60 years old on average when they became participants in the UK Biobank.
Age at menopause was categorized as premature (younger than age 40), early (40 to 44 years), reference (45 to 51), 52 to 55 years, and 55+ years.
Compared with women who entered menopause around age 50 years (reference), women who experienced premature menopause were 35% more likely to develop some type of dementia later in life (hazard ratio, 1.35; 95% confidence interval, 1.22 to 1.91).
Women with early menopause were also more likely to develop early-onset dementia, that is, before age 65 (HR, 1.31; 95% confidence interval, 1.07 to 1.72).
Women who entered menopause later (at age 52+) had dementia risk similar to women who entered menopause at the average age of 50 to 51 years.
The results were adjusted for relevant cofactors, including age at last exam, race, educational level, cigarette and alcohol use, body mass index, cardiovascular disease, diabetes, income, and leisure and physical activities.
Blame it on estrogen?
Reduced estrogen levels may be a factor in the possible connection between early menopause and dementia, Dr. Hao and her colleagues say.
Estradiol plays a key role in a range of neurological functions, so the reduction of endogenous estrogen at menopause may aggravate brain changes related to neurodegenerative disease and speed up progression of dementia, they explain.
“We know that the lack of estrogen over the long term enhances oxidative stress, which may increase brain aging and lead to cognitive impairment,” Dr. Hao adds.
Limitations of the study include reliance on self-reported information about age at menopause onset.
Also, the researchers did not evaluate dementia rates in women who had a naturally occurring early menopause separate from the women with surgery-induced menopause, which may affect the results.
Finally, the data used for this study included mostly White women living in the U.K. and may not generalize to other populations.
Supportive evidence, critical area of research
The U.K. study supports results of a previously reported Kaiser Permanente study, which showed women who entered menopause at age 45 or younger were at 28% greater dementia risk, compared with women who experienced menopause after age 45.
Reached for comment, Heather Snyder, PhD, Alzheimer’s Association vice president of medical and scientific relations, noted that nearly two-thirds of Americans with Alzheimer’s are women.
“We know Alzheimer’s and other dementias impact a greater number of women than men, but we don’t know why,” she told this news organization.
“Lifelong differences in women may affect their risk or affect what is contributing to their underlying biology of the disease, and we need more research to better understand what may be these contributing factors,” said Dr. Snyder.
“Reproductive history is one critical area being studied. The physical and hormonal changes that occur during menopause – as well as other hormonal changes throughout life – are considerable, and it’s important to understand what impact, if any, these changes may have on the brain,” Dr. Snyder added.
“The potential link between reproduction history and brain health is intriguing, but much more research in this area is needed to understand these links,” she said.
The study was funded by the Start-up Foundation for Scientific Research at Shandong University. Dr. Hao and Dr. Snyder have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Earlier menopause appears to be associated with a higher risk of dementia, and earlier onset of dementia, compared with menopause at normal age or later, according to a large study.
“Being aware of this increased risk can help women practice strategies to prevent dementia and to work with their physicians to closely monitor their cognitive status as they age,” study investigator Wenting Hao, MD, with Shandong University, Jinan, China, says in a news release.
The findings were presented in an e-poster March 1 at the Epidemiology, Prevention, Lifestyle & Cardiometabolic Health (EPI|Lifestyle) 2022 conference sponsored by the American Heart Association.
UK Biobank data
Dr. Hao and colleagues examined health data for 153,291 women who were 60 years old on average when they became participants in the UK Biobank.
Age at menopause was categorized as premature (younger than age 40), early (40 to 44 years), reference (45 to 51), 52 to 55 years, and 55+ years.
Compared with women who entered menopause around age 50 years (reference), women who experienced premature menopause were 35% more likely to develop some type of dementia later in life (hazard ratio, 1.35; 95% confidence interval, 1.22 to 1.91).
Women with early menopause were also more likely to develop early-onset dementia, that is, before age 65 (HR, 1.31; 95% confidence interval, 1.07 to 1.72).
Women who entered menopause later (at age 52+) had dementia risk similar to women who entered menopause at the average age of 50 to 51 years.
The results were adjusted for relevant cofactors, including age at last exam, race, educational level, cigarette and alcohol use, body mass index, cardiovascular disease, diabetes, income, and leisure and physical activities.
Blame it on estrogen?
Reduced estrogen levels may be a factor in the possible connection between early menopause and dementia, Dr. Hao and her colleagues say.
Estradiol plays a key role in a range of neurological functions, so the reduction of endogenous estrogen at menopause may aggravate brain changes related to neurodegenerative disease and speed up progression of dementia, they explain.
“We know that the lack of estrogen over the long term enhances oxidative stress, which may increase brain aging and lead to cognitive impairment,” Dr. Hao adds.
Limitations of the study include reliance on self-reported information about age at menopause onset.
Also, the researchers did not evaluate dementia rates in women who had a naturally occurring early menopause separate from the women with surgery-induced menopause, which may affect the results.
Finally, the data used for this study included mostly White women living in the U.K. and may not generalize to other populations.
Supportive evidence, critical area of research
The U.K. study supports results of a previously reported Kaiser Permanente study, which showed women who entered menopause at age 45 or younger were at 28% greater dementia risk, compared with women who experienced menopause after age 45.
Reached for comment, Heather Snyder, PhD, Alzheimer’s Association vice president of medical and scientific relations, noted that nearly two-thirds of Americans with Alzheimer’s are women.
“We know Alzheimer’s and other dementias impact a greater number of women than men, but we don’t know why,” she told this news organization.
“Lifelong differences in women may affect their risk or affect what is contributing to their underlying biology of the disease, and we need more research to better understand what may be these contributing factors,” said Dr. Snyder.
“Reproductive history is one critical area being studied. The physical and hormonal changes that occur during menopause – as well as other hormonal changes throughout life – are considerable, and it’s important to understand what impact, if any, these changes may have on the brain,” Dr. Snyder added.
“The potential link between reproduction history and brain health is intriguing, but much more research in this area is needed to understand these links,” she said.
The study was funded by the Start-up Foundation for Scientific Research at Shandong University. Dr. Hao and Dr. Snyder have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Earlier menopause appears to be associated with a higher risk of dementia, and earlier onset of dementia, compared with menopause at normal age or later, according to a large study.
“Being aware of this increased risk can help women practice strategies to prevent dementia and to work with their physicians to closely monitor their cognitive status as they age,” study investigator Wenting Hao, MD, with Shandong University, Jinan, China, says in a news release.
The findings were presented in an e-poster March 1 at the Epidemiology, Prevention, Lifestyle & Cardiometabolic Health (EPI|Lifestyle) 2022 conference sponsored by the American Heart Association.
UK Biobank data
Dr. Hao and colleagues examined health data for 153,291 women who were 60 years old on average when they became participants in the UK Biobank.
Age at menopause was categorized as premature (younger than age 40), early (40 to 44 years), reference (45 to 51), 52 to 55 years, and 55+ years.
Compared with women who entered menopause around age 50 years (reference), women who experienced premature menopause were 35% more likely to develop some type of dementia later in life (hazard ratio, 1.35; 95% confidence interval, 1.22 to 1.91).
Women with early menopause were also more likely to develop early-onset dementia, that is, before age 65 (HR, 1.31; 95% confidence interval, 1.07 to 1.72).
Women who entered menopause later (at age 52+) had dementia risk similar to women who entered menopause at the average age of 50 to 51 years.
The results were adjusted for relevant cofactors, including age at last exam, race, educational level, cigarette and alcohol use, body mass index, cardiovascular disease, diabetes, income, and leisure and physical activities.
Blame it on estrogen?
Reduced estrogen levels may be a factor in the possible connection between early menopause and dementia, Dr. Hao and her colleagues say.
Estradiol plays a key role in a range of neurological functions, so the reduction of endogenous estrogen at menopause may aggravate brain changes related to neurodegenerative disease and speed up progression of dementia, they explain.
“We know that the lack of estrogen over the long term enhances oxidative stress, which may increase brain aging and lead to cognitive impairment,” Dr. Hao adds.
Limitations of the study include reliance on self-reported information about age at menopause onset.
Also, the researchers did not evaluate dementia rates in women who had a naturally occurring early menopause separate from the women with surgery-induced menopause, which may affect the results.
Finally, the data used for this study included mostly White women living in the U.K. and may not generalize to other populations.
Supportive evidence, critical area of research
The U.K. study supports results of a previously reported Kaiser Permanente study, which showed women who entered menopause at age 45 or younger were at 28% greater dementia risk, compared with women who experienced menopause after age 45.
Reached for comment, Heather Snyder, PhD, Alzheimer’s Association vice president of medical and scientific relations, noted that nearly two-thirds of Americans with Alzheimer’s are women.
“We know Alzheimer’s and other dementias impact a greater number of women than men, but we don’t know why,” she told this news organization.
“Lifelong differences in women may affect their risk or affect what is contributing to their underlying biology of the disease, and we need more research to better understand what may be these contributing factors,” said Dr. Snyder.
“Reproductive history is one critical area being studied. The physical and hormonal changes that occur during menopause – as well as other hormonal changes throughout life – are considerable, and it’s important to understand what impact, if any, these changes may have on the brain,” Dr. Snyder added.
“The potential link between reproduction history and brain health is intriguing, but much more research in this area is needed to understand these links,” she said.
The study was funded by the Start-up Foundation for Scientific Research at Shandong University. Dr. Hao and Dr. Snyder have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Gestational diabetes: Optimizing Dx and management in primary care
Gestational diabetes mellitus (GDM), defined as new-onset hyperglycemia detected in a pregnant woman after 24 weeks of gestation, affects 4% to 10% of pregnancies in the United States annually1 and is a major challenge for health care professionals.2 During pregnancy, the body’s physiologic responses are altered to support the growing fetus. One of these changes is an increase in insulin resistance, which suggests that pregnancy alone increases the patient’s risk for type 2 diabetes (T2D). However, several other factors also increase this risk, including maternal age, social barriers to care, obesity, poor weight control, and family history.
If not controlled, GDM results in poor health outcomes for the mother, such as preeclampsia, preterm labor, and maternal T2D.3-5 For the infant, intrauterine exposure to persistent hyperglycemia is correlated with neonatal macrosomia, hypoglycemia, perinatal complications (eg, preterm delivery, fetal demise), and obesity and insulin resistance later in life.4
Primary care physicians (PCPs) are the patient’s main point of contact prior to pregnancy. This relationship makes PCPs a resource for the patient and specialists during and after pregnancy. In this article, we discuss risk factors and how to screen for GDM, provide an update on practice recommendations for treatment and management of GDM in primary care, and describe the effects of uncontrolled GDM.
Know the key risk factors
Prevention begins with identifying the major risk factors that contribute to the development of GDM. These include maternal age, social barriers to care, family history of prediabetes, and obesity and poor weight control.
Older age. A meta-analysis of 24 studies noted strong positive correlation between GDM risk and maternal age.6 One of the population-based cohort studies in the meta-analysis examined relationships between maternal age and pregnancy outcomes in women living in British Columbia, Canada (n = 203,414). Data suggested that the relative risk of GDM increased linearly with maternal age to 3.2, 4.2, and 4.4 among women ages ≥ 35, ≥ 40, and ≥ 45 years, respectively.7
Social barriers to care. Although the prevalence of GDM has increased over the past few decades,1 from 2011 to 2019 the increase in GDM in individuals at first live birth was significantly higher in non-Hispanic Asian and Hispanic/Latina women than in non-Hispanic White women.8 Data from the Centers for Disease Control and Prevention further suggest that diabetes was more prevalent among individuals with a lower socioeconomic status as indicated by their level of education.9 Ogunwole et al10 suggest that racism is the root cause of these disparities and leads to long-term barriers to care (eg, socioeconomic deprivation, lack of health insurance, limited access to care, and poor health literacy), which ultimately contribute to the development of GDM and progression of diabetes. It is important for PCPs and all health professionals to be aware of these barriers so that they may practice mindfulness and deliver culturally sensitive care to patients from marginalized communities.
Family history of prediabetes. In a population-based cohort study (n = 7020), women with prediabetes (A1C, 5.7%-6.4%) were 2.8 times more likely to develop GDM compared with women with normal A1C (< 5.7%).11 Similar results were seen in a retrospective cohort study (n = 2812), in which women with prediabetes were more likely than women with a normal first trimester A1C to have GDM (29.1% vs 13.7%, respectively; adjusted relative risk = 1.48; 95% CI, 1.15-1.89).12 In both studies, prediabetes was not associated with a higher risk for adverse maternal or neonatal outcomes.11,12
Continue to: While there are no current...
While there are no current guidelines for treating prediabetes in pregnancy, women diagnosed with prediabetes in 1 study were found to have significantly less weight gain during pregnancy compared with patients with normal A1C,12 suggesting there may be a benefit in early identification and intervention, although further research is needed.11 In a separate case-control study (n = 345 women with GDM; n = 800 control), high rates of gestational weight gain (> 0.41 kg/wk) were associated with an increased risk of GDM (odds ratio [OR] = 1.74; 95% CI, 1.16-2.60) compared with women with the lowest rate of gestational weight gain (0.27-0.4 kg/wk [OR = 1.43; 95% CI, 0.96-2.14]).13 Thus, it is helpful to have proactive conversations about family planning and adequate weight and glycemic control with high-risk patients to prepare for a healthy pregnancy.
Obesity and weight management. Patients who are overweight (body mass index [BMI], 25-29.9) or obese (BMI > 30) have a substantially increased risk of GDM (adjusted OR = 1.44; 95% CI, 1.04-1.81), as seen in a retrospective cohort study of 1951 pregnant Malaysian women.14 Several factors have been found to contribute to successful weight control, including calorie prescription, a structured meal plan, high physical activity goals (60-90 min/d), daily weighing and monitoring of food intake, behavior therapy, and continued patient–provider contact.15
The safety, efficacy, and sustainability of weight loss with various dietary plans have been studied in individuals who are overweight and obese.16 Ultimately, energy expenditure must be greater than energy intake to promote weight loss. Conventional diets with continuous energy restriction (ie, low-fat, low-carbohydrate, and high-protein diets) have proven to be effective for short-term weight loss but data on long-term weight maintenance are limited.16 The Mediterranean diet, which is comprised mostly of vegetables, fruits, legumes, fish, and grains—with a lower intake of meat and dairy—may reduce gestational weight gain and risk of GDM as suggested by a randomized controlled trial (RCT; n = 1252).17 Although the choice of diet is up to the patient, it is important to be aware of different diets or refer the patient to a registered dietician who can help the patient if needed.
Reduce risk with adequate weight and glycemic control
Prevention of GDM during pregnancy should focus on weight maintenance and optimal glycemic control. Two systematic reviews, one with 8 RCTs (n = 1792) and another with 5 studies (n = 539), assessed the efficacy and safety of energy-restricted dietary intervention on GDM prevention.18 The first review found a significant reduction in gestational weight gain and improved glycemic control without increased risk of adverse maternal and fetal outcomes.18 The second review showed no clear difference between energy-restricted and non–energy-restricted diets on outcomes such as preeclampsia, gestational weight gain, large for gestational age, and macrosomia.18 These data suggest that while energy-restricted dietary interventions made no difference on maternal and fetal complications, they may still be safely used in pregnancy to reduce gestational weight gain and improve glycemic control.18
Once a woman is pregnant, it becomes difficult to lose weight because additional calories are needed to support a growing fetus. It is recommended that patients with healthy pregestational BMI consume an extra 200 to 300 calories/d after the first trimester. However, extra caloric intake in a woman with obesity who is pregnant leads to metabolic impairment and increased risk of diabetes for both the mother and fetus.19 Therefore, it is recommended that patients with obese pregestational BMI not consume additional calories because excess maternal fat is sufficient to support the energy needs of the growing fetus.19
Continue to: Ultimately, earlier intervention...
Ultimately, earlier intervention—prior to conception—helps patients prepare for a healthier pregnancy, resulting in better long-term outcomes. It is helpful to be familiar with the advantages and disadvantages of common approaches to weight management and to be able to refer patients to nutritionists for optimal planning. When establishing a dietary plan, consider patient-specific factors, such as cultural diets, financial and time constraints, and the patient’s readiness to make and maintain these changes. Consistent follow-up and behavioral therapy are necessary to maintain successful weight control.
There are many screening tools, but 1 is preferred in pregnancy
There are several ways to diagnose diabetes in patients who are not pregnant, including A1C, a fasting glucose test, an oral glucose tolerance test (OGTT), or random glucose testing (plus symptoms). However, the preferred method for diagnosing GDM is OGTT because it has a higher sensitivity.20 A1C, while a good measure of hyperglycemic stability, does not register hyperglycemia early enough to diagnose GDM and fasting glucose testing is less sensitive because for most women with GDM, that abnormal postprandial glucose level is the first glycemic abnormality.21
When to screen. Blood glucose levels should be checked in all pregnant women as part of their metabolic panel at the first prenatal visit. A reflex A1C for high glucose levels can be ordered based on the physician’s preference. This may help you to identify patients with prediabetes who are at risk for GDM and implement early behavioral and lifestyle changes. However, further research is needed to determine if intervention early in pregnancy can truly reduce the risk of GDM.11
Screening for GDM should be completed at 24 to 28 weeks of gestation20 because it is likely that this is when the hormonal effects of the placenta that contribute to insulin resistance set the woman up for postprandial hyperglycemia. Currently, there are no evidence-based guidelines for the use of continuous glucose monitoring prior to 24 weeks of gestation to identify GDM.20 If persistent hyperglycemia is present before 24 weeks of gestation, it is considered evidence of a pre-existing metabolic abnormality and is diagnosed as “pregestational diabetes.” Treatment should follow guidelines established for women who had diabetes prior to pregnancy.
How to screen? There is ongoing discussion about what is the optimal screening method for GDM: a 1-step strategy with a fasting 75-g OGTT only, or a 2-step strategy with a 50-g non-fasting glucose load test followed by a fasting 100-g OGTT in women who do not meet the plasma glucose cutoff (TABLE 1).22-24 Hillier et al25 compared the effectiveness of these strategies in diagnosing GDM and identifying pregnancy complications for the mother and infant. They found that while the 1-step strategy resulted in a 2-fold increase in the diagnosis of GDM, it did not lead to better outcomes for mothers and infants when compared with the 2-step method.25 Currently, the majority of obstetricians (95%) prefer to use the 2-step method.24
Continue to: Manage lifestyle, monitor glucose
Manage lifestyle, monitor glucose
Management of GDM in most women starts with diabetes self-management education and support for therapeutic lifestyle changes, such as nutritional interventions that reduce hyperglycemia and contribute to healthy weight gain during pregnancy.20 This may include medical nutrition therapy that focuses on adequate nutrition for the mother and fetus. Currently, the recommended dietary intake for women who are pregnant (regardless of diabetes) includes a minimum of 175 g of carbohydrates, 71 g of daily protein, and at least 28 g of fiber. Further refinement of dietary intake, including carbohydrate restriction, should be done with guidance from a registered dietitian.20 If the obstetrics team does not include a registered dietitian, a referral to one may be necessary. Regular physical activity should be continued throughout pregnancy as tolerated. Social support, stress reduction, and good sleep hygiene should be encouraged as much as possible.
For successful outcomes, therapeutic lifestyle changes should be coupled with glucose monitoring. The Fifth International Workshop-Conference on Gestational Diabetes Mellitus recommends that women with GDM monitor fasting blood glucose and typically 1-hour postprandial glucose. The glucose goals in GDM are as follows26:
- Fasting glucose < 95 mg/dL (5.3 mmol/L), and either
- 1-hour postprandial glucose < 140 mg/dL (7.8 mmol/L), or
- 2-hour postprandial glucose < 120 mg/dL (6.7 mmol/L).
Importantly, in the second and third trimester, the A1C goal for women with GDM is 6.0%. This is lower than the more traditional A1C goal for 2 reasons: (1) increases in A1C, even within the normal range, increase adverse outcomes; and (2) pregnant women will have an increased red blood cell count turnover, which can lower the A1C.27 In a historical cohort study (n = 27,213), Abell et al28 found that women who have an A1C < 6.0% in the second and third trimester have the lowest risk of giving birth to large-for-gestational-age infants and for having preeclampsia.
Add insulin if glucose targets are not met
Most women who engage in therapeutic lifestyle change (70%-85%) can achieve an A1C < 6% and will not need to take medication to manage GDM.29 If pharmacotherapy is needed to manage glucose, insulin is the preferred treatment for all women with GDM.20 Treatment should be individualized based on the glucose trends the woman is experiencing. Common treatments include bedtime NPH if fasting hyperglycemia is most prominent and analogue insulin at mealtimes for women with prominent postprandial hyperglycemia.
Noninsulin agents such as metformin and sulfonylureas are not currently recommended by the American College of Obstetricians and Gynecologists or the American Diabetes Association for use in GDM.20,24 Despite being used for years in women with pregestational diabetes, metabolic syndrome, and polycystic ovary syndrome, there is evidence that metformin crosses the placenta and fetal safety has not yet been established in RCTs. The Metformin in Gestational Diabetes: The Offspring Follow-Up (MiG TOFU) study was a longitudinal follow-up study that evaluated body composition and metabolic outcomes in children (ages 7-9 years) of women with GDM who had received metformin or insulin while pregnant.30 At age 9 years, children who were exposed to metformin weighed more and had a higher waist-to-height ratio and waist circumference than those exposed to insulin.30
Continue to: Sulfonylureas are no longer recommended...
Sulfonylureas are no longer recommended because of the risk of maternal and fetal hypoglycemia and concerns about this medication crossing the placenta.24,31,32 Specifically, in a 2015 meta-analysis and systematic review of 15 articles (n = 2509), glyburide had a higher risk of neonatal hypoglycemia and macrosomia than insulin or metformin.33 For women who cannot manage their glucose with therapeutic lifestyle changes and cannot take insulin, oral therapies may be considered if the risk-benefit ratio is balanced for that person.34
Watch for effects of poor glycemic control on mother, infant
Preeclampsia is defined as new-onset hypertension and proteinuria after 20 weeks of gestation. The correlation between GDM and preeclampsia has partly been explained by their shared overlapping risk factors, including maternal obesity, excessive gestational weight gain, and persistent hyperglycemia.35 On a biochemical level, these risk factors contribute to oxidative stress and systemic vascular dysfunction, which have been hypothesized as the underlying pathophysiology for the development of preeclampsia.35
Neonatal macrosomia, defined as a birth weight ≥ 4000 g, is a common complication that develops in 15% to 45% of infants of mothers with GDM.36 Placental transfer of glucose in mothers with hyperglycemia stimulates the secretion of neonatal insulin and the ultimate storage of the excess glucose as body fat. After delivery, the abrupt discontinuation of placental transfer of glucose to an infant who is actively secreting insulin leads to neonatal hypoglycemia, which if not detected or managed, can lead to long-term neurologic deficits, including recurrent seizures and developmental delays.37 Therefore, it is essential to screen for neonatal hypoglycemia immediately after birth and serially up to 12 hours.38
Postpartum T2D. Poor glycemic control increases the risk of increasing insulin resistance developing into T2D postpartum for mothers.39 It also increases the risk of obesity and insulin resistance later in life for the infant.40 A retrospective cohort study (n = 461) found a positive correlation between exposure to maternal GDM and elevated BMI in children ages 6 to 13 years.41 Kamana et al36 further discussed this correlation and suggested that exposure to maternal hyperglycemia in utero contributes to fetal programming of later adipose deposition. Children may develop without a notable increase in BMI until after puberty.42
Partner with specialists to improve outcomes
Although most women with GDM are managed by specialists (obstetricians, endocrinologists, and maternal-fetal medicine specialists),43 these patients are still seeking care from their family physicians for other complaints. These visits provide key touchpoints during pregnancy and are opportunities for PCPs to identify a pregnancy-related complication or provide additional education or referral to the obstetrician.
Continue to: Also, if you work in an area...
Also, if you work in an area where specialists are less accessible, you may be the clinician providing the majority of care to a patient with GDM. If this is the case, you’ll want to watch for the following risk factors, which should prompt a referral to specialty care:
- a previous pregnancy with GDM20
- a previous birth of an infant weighing > 4000 g44
- baseline history of hypertension45
- evidence of insulin resistance or polycystic ovary syndrome46,47
- a history of cardiovascular disease20
- a need to treat GDM with pharmacotherapy.48
Ensuring a smooth transition after the birth
Optimal communication and hand-offs throughout pregnancy and after delivery will benefit everyone. When the pregnant patient’s care has been managed by an obstetrician, it is important to address the following issues during the hand-off:
- baseline medical problems
- medical screenings and treatments in pregnancy (retinopathy and nephropathy screening)
- aspirin initiation, if indicated
- management of thyroid abnormalities
- management of mental health conditions
- postpartum glucose management and T2D screening postpartum
- management of complications identified during pregnancy (retinopathy and nephropathy).
Timing and other elements of postpartum care. The first postpartum screen should occur at 4 to 12 weeks postpartum. OGTT is recommended instead of A1C at this time because A1C may still be lowered by the increased red blood cell turnover related to pregnancy and blood loss at delivery. Because women with GDM have a 50% to 75% lifetime risk of T2D,20 patients with normal test results should be re-tested every 1 to 3 years using any of the standard screening methods (A1C, fasting glucose, or OGTT).20
After delivery it may be difficult for women to follow-up with their own personal health care because they are focused on the care of their baby. The increased use of telehealth may make postpartum follow-up visits easier to attend.
Visits present opportunities. Postpartum visits present another opportunity for PCPs to screen for diabetes and other postpartum complications, including depression and thyroid abnormalities. Visits are also an opportunity to discuss timely contraception so as to prevent an early, unplanned pregnancy. Other important aspects of postpartum care are outlined in TABLE 2.20,49
CORRESPONDENCE
Connie L. Ha, BS, OMS IV, Department of Primary Care, 1310 Club Drive, Touro University California, Vallejo, CA 94592; connie.ha@tu.edu
1. Sheiner E. Gestational diabetes mellitus: long-term consequences for the mother and child grand challenge: how to move on towards secondary prevention? Front Clin Diabetes Healthc. 2020. doi: 10.3389/fcdhc.2020.546256
2. Angueira AR, Ludvik AE, Reddy TE, et al. New insights into gestational glucose metabolism: lessons learned from 21st century approaches. Diabetes. 2015;64:327-334. doi: 10.2337/db14-0877
3. Shou C, Wei Y-M, Wang C, et al. Updates in long-term maternal and fetal adverse effects of gestational diabetes mellitus. Maternal-Fetal Med. 2019;1:91-94. doi: 10.1097/FM9.0000000000000019
4. Plows JF, Stanley JL, Baker PN, et al. The pathophysiology of gestational diabetes mellitus. Int J Mol Sci. 2018;19:3342. doi: 10.3390/ijms19113342
5. Kulshrestha V, Agarwal N. Maternal complications in pregnancy with diabetes. J Pak Med Assoc. 2016;66(9 suppl 1):S74-S77.
6. Li Y, Ren X, He L, et al. Maternal age and the risk of gestational diabetes mellitus: a systematic review and meta-analysis of over 120 million participants. Diabetes Res Clin Pract. 2020;162:108044. doi: 10.1016/j.diabres.2020.108044
7. Schummers L, Hutcheon JA, Hacker MR, et al. Absolute risks of obstetric outcomes by maternal age at first birth: a population-based cohort. Epidemiology. 2018;29:379-387. doi: 10.1097/EDE.0000000000000818
8. Shah NS, Wang MC, Freaney PM, et al. Trends in gestational diabetes at first live birth by race and ethnicity in the US, 2011-2019. JAMA. 2021;326:660-669. doi: 10.1001/jama.2021.7217
9. Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2020. Atlanta, GA: Centers for Disease Control and Prevention, U.S. Department of Health and Human Services; 2020. Accessed February 2, 2022. www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf
10. Ogunwole SM, Golden SH. Social determinants of health and structural inequities—root causes of diabetes disparities. Diabetes Care. 2021;44:11-13. doi: 10.2337/dci20-0060
11. Chen L, Pocobelli G, Yu O, et al. Early pregnancy hemoglobin A1C and pregnancy outcomes: a population-based study. Am J Perinatol. 2019;36:1045-1053. doi: 10.1055/s-0038-1675619
12. Osmundson S, Zhao BS, Kunz L, et al. First trimester hemoglobin A1C prediction of gestational diabetes. Am J Perinatol. 2016;33:977-982. doi: 10.1055/s-0036-1581055
13. Hedderson MM, Gunderson EP, Ferrara A. Gestational weight gain and risk of gestational diabetes mellitus [published correction appears in Obstet Gynecol. 2010;115:1092]. Obstet Gynecol. 2010;115:597-604. doi: 10.1097/AOG.0b013e3181cfce4f
14. Yong HY, Mohd Shariff Z, Mohd Yusof BN, et al. Independent and combined effects of age, body mass index and gestational weight gain on the risk of gestational diabetes mellitus. Sci Rep. 2020;10:8486. doi: 10.1038/s41598-020-65251-2
15. Phelan S. Windows of opportunity for lifestyle interventions to prevent gestational diabetes mellitus. Am J Perinatol. 2016;33:1291-1299. doi: 10.1055/s-0036-1586504
16. Koliaki C, Spinos T, Spinou M, et al. Defining the optimal dietary approach for safe, effective and sustainable weight loss in overweight and obese adults. Healthcare (Basel). 2018;6:73. doi: 10.3390/healthcare6030073
17. Al Wattar BH, Dodds J, Placzek A, et al. Mediterranean-style diet in pregnant women with metabolic risk factors (ESTEEM): a pragmatic multicentre randomised trial. PLOS Med. 2019;16:e1002857. doi: 10.1371/journal.pmed.1002857
18. Zarogiannis S. Are novel lifestyle approaches to management of type 2 diabetes applicable to prevention and treatment of women with gestational diabetes mellitus? Global Diabetes Open Access J. 2019;1:1-14.
19. Most J, Amant MS, Hsia DS, et al. Evidence-based recommendations for energy intake in pregnant women with obesity. J Clin Invest. 2019;129:4682-4690. doi: 10.1172/JCI130341
20. American Diabetes Association. 14. Management of diabetes in pregnancy: Standards of Medical Care in Diabetes—2021. Diabetes Care. 2021;44(suppl 1):S200-S210. doi: 10.2337/dc21-S014
21. McIntyre HD, Sacks DA, Barbour LA, et al. Issues with the diagnosis and classification of hyperglycemia in early pregnancy. Diabetes Care. 2016;39:53-54. doi: 10.2337/dc15-1887
22. American Diabetes Association. 2. Classification and diagnosis of diabetes: Standards of Medical Care in Diabetes—2022. Diabetes Care. 2022;45(suppl 1):S17-S38. doi: 10.2337/dc22-S002
23. Carpenter MW, Coustan DR. Criteria for screening tests for gestational diabetes. Am J Obstet Gynecol. 1982;144:768-773. doi: 10.1016/0002-9378(82)90349-0
24. ACOG Practice Bulletin No. 190: gestational diabetes mellitus. Obstet Gynecol. 2018;131:e49-e64. doi: 10.1097/AOG.0000000000002501
25. Hillier TA, Pedula KL, Ogasawara KK, et al. A pragmatic, randomized clinical trial of gestational diabetes screening. N Engl J Med. 2021;384:895-904. doi: 10.1056/NEJMoa2026028
26. Metzger BE, Buchanan TA, Coustan DR, et al. Summary and recommendations of the Fifth International Workshop-Conference on Gestational Diabetes Mellitus. Diabetes Care. 2007;30(suppl 2):S251-S260. doi: 10.2337/dc07-s225
27. Nielsen LR, Ekbom P, Damm P, et al. HbA1c levels are significantly lower in early and late pregnancy. Diabetes Care. 2004;27:1200-1201. doi: 10.2337/diacare.27.5.1200
28. Abell SK, Boyle JA, de Courten B, et al. Impact of type 2 diabetes, obesity and glycaemic control on pregnancy outcomes. Aust N Z J Obstet Gynaecol. 2017;57:308-314. doi: 10.1111/ajo.12521
29. Viana LV, Gross JL, Azevedo MJ. Dietary intervention in patients with gestational diabetes mellitus: a systematic review and meta-analysis of randomized clinical trials on maternal and newborn outcomes. Diabetes Care. 2014;37:3345-3355. doi: 10.2337/dc14-1530
30. Rowan JA, Rush EC, Plank LD, et al. Metformin in gestational diabetes: the offspring follow-up (MiG TOFU): body composition and metabolic outcomes at 7-9 years of age. BMJ Open Diabetes Res Care. 2018;6:e000456. doi: 10.1136/bmjdrc-2017-000456
31. Hebert MF, Ma X, Naraharisetti SB, et al. Are we optimizing gestational diabetes treatment with glyburide? The pharmacologic basis for better clinical practice. Clin Pharmacol Ther. 2009;85:607-614. doi: 10.1038/clpt.2009.5
32. Malek R, Davis SN. Pharmacokinetics, efficacy and safety of glyburide for treatment of gestational diabetes mellitus. Expert Opin Drug Metab Toxicol. 2016;12:691-699. doi: 10.1080/17425255.2016.1187131
33. Balsells M, García-Patterson A, Solà I, et al. Glibenclamide, metformin, and insulin for the treatment of gestational diabetes: a systematic review and meta-analysis. BMJ. 2015;350:h102. doi: 10.1136/bmj.h102
34. Kavitha N, De S, Kanagasabai S. Oral hypoglycemic agents in pregnancy: an update. J Obstet Gynaecol India. 2013;63:82-87. doi: 10.1007/s13224-012-0312-z
35. Weissgerber TL, Mudd LM. Preeclampsia and diabetes. Curr Diab Rep. 2015;15:9. doi: 10.1007/s11892-015-0579-4
36. Kamana KC, Shakya S, Zhang H. Gestational diabetes mellitus and macrosomia: a literature review. Ann Nutr Metab. 2015;66(suppl 2):14-20. doi: 10.1159/000371628
37. Mitanchez D, Yzydorczyk C, Simeoni U. What neonatal complications should the pediatrician be aware of in case of maternal gestational diabetes? World J Diabetes. 2015;6:734-743. doi: 10.4239/wjd.v6.i5.734
38. Stanescu A, Stoicescu SM. Neonatal hypoglycemia screening in newborns from diabetic mothers—arguments and controversies. J Med Life. 2014;7(spec iss 3):51-52.
39. Kim C. Maternal outcomes and follow-up after gestational diabetes mellitus. Diabet Med. 2014;31:292-301. doi: 10.1111/dme.12382
40. Stewart A, Malhotra A. Gestational diabetes and the neonate: challenges and solutions. Res Rep Neonatol. 2015;5:31-39. doi: 10.2147/RRN.S30971
41. Crume TL, Ogden L, West NA, et al. Association of exposure to diabetes in utero with adiposity and fat distribution in a multiethnic population of youth: the Exploring Perinatal Outcomes among Children (EPOCH) Study. Diabetologia. 2011;54:87-92. doi: 10.1007/s00125-010-1925-3
42. Crume TL, Ogden L, Daniels S, et al. The impact of in utero exposure to diabetes on childhood body mass index growth trajectories: the EPOCH study. J Pediatr. 2011;158:941-946. doi: 10.1016/j.jpeds.2010.12.007
43. Levels of maternal care. Obstetric Care Consensus No. 9. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2019;134:e41-e55. doi: 10.1097/AOG.0000000000003383
44. Caughey AB, Cheng YW, Stotland NE, et al. Maternal and paternal race/ethnicity are both associated with gestational diabetes. Am J Obstet Gynecol. 2010;202:616.e1-e5. doi: 10.1016/j.ajog.2010.01.082
45. Yogev Y, Xenakis EM, Langer O. The association between preeclampsia and severity of gestational diabetes: the impact of glycemic control. Am J Obstet Gynecol. 2004;191:1655-1660. doi: 10.1016/j.ajog.2004.03.074
46. Brown J, Alwan NA, West J, et al. Lifestyle interventions for the treatment of women with gestational diabetes. Cochrane Database Syst Rev. 2017;5:CD011970. doi: 10.1002/14651858.CD011970.pub2
47. Ceysens G, Rouiller D, Boulvain M. Exercise for the diabetic pregnant woman. Cochrane Database Syst Rev. 2006;3:CD004225. doi: 10.1002/14651858.CD004225.pub2
48. Chawla R, Mukherjee JJ, Chawla M, et al. Expert group recommendations on the effective use of bolus insulin in the management of type 2 diabetes mellitus. Med Sci (Basel). 2021;9:38. doi: 10.3390/medsci9020038
49. American Diabetes Association. Introduction: Standards of Medical Care in Diabetes—2021. Diabetes Care. 2021;44(suppl 1):S1-S2. doi: 10.2337/dc22-Sint
Gestational diabetes mellitus (GDM), defined as new-onset hyperglycemia detected in a pregnant woman after 24 weeks of gestation, affects 4% to 10% of pregnancies in the United States annually1 and is a major challenge for health care professionals.2 During pregnancy, the body’s physiologic responses are altered to support the growing fetus. One of these changes is an increase in insulin resistance, which suggests that pregnancy alone increases the patient’s risk for type 2 diabetes (T2D). However, several other factors also increase this risk, including maternal age, social barriers to care, obesity, poor weight control, and family history.
If not controlled, GDM results in poor health outcomes for the mother, such as preeclampsia, preterm labor, and maternal T2D.3-5 For the infant, intrauterine exposure to persistent hyperglycemia is correlated with neonatal macrosomia, hypoglycemia, perinatal complications (eg, preterm delivery, fetal demise), and obesity and insulin resistance later in life.4
Primary care physicians (PCPs) are the patient’s main point of contact prior to pregnancy. This relationship makes PCPs a resource for the patient and specialists during and after pregnancy. In this article, we discuss risk factors and how to screen for GDM, provide an update on practice recommendations for treatment and management of GDM in primary care, and describe the effects of uncontrolled GDM.
Know the key risk factors
Prevention begins with identifying the major risk factors that contribute to the development of GDM. These include maternal age, social barriers to care, family history of prediabetes, and obesity and poor weight control.
Older age. A meta-analysis of 24 studies noted strong positive correlation between GDM risk and maternal age.6 One of the population-based cohort studies in the meta-analysis examined relationships between maternal age and pregnancy outcomes in women living in British Columbia, Canada (n = 203,414). Data suggested that the relative risk of GDM increased linearly with maternal age to 3.2, 4.2, and 4.4 among women ages ≥ 35, ≥ 40, and ≥ 45 years, respectively.7
Social barriers to care. Although the prevalence of GDM has increased over the past few decades,1 from 2011 to 2019 the increase in GDM in individuals at first live birth was significantly higher in non-Hispanic Asian and Hispanic/Latina women than in non-Hispanic White women.8 Data from the Centers for Disease Control and Prevention further suggest that diabetes was more prevalent among individuals with a lower socioeconomic status as indicated by their level of education.9 Ogunwole et al10 suggest that racism is the root cause of these disparities and leads to long-term barriers to care (eg, socioeconomic deprivation, lack of health insurance, limited access to care, and poor health literacy), which ultimately contribute to the development of GDM and progression of diabetes. It is important for PCPs and all health professionals to be aware of these barriers so that they may practice mindfulness and deliver culturally sensitive care to patients from marginalized communities.
Family history of prediabetes. In a population-based cohort study (n = 7020), women with prediabetes (A1C, 5.7%-6.4%) were 2.8 times more likely to develop GDM compared with women with normal A1C (< 5.7%).11 Similar results were seen in a retrospective cohort study (n = 2812), in which women with prediabetes were more likely than women with a normal first trimester A1C to have GDM (29.1% vs 13.7%, respectively; adjusted relative risk = 1.48; 95% CI, 1.15-1.89).12 In both studies, prediabetes was not associated with a higher risk for adverse maternal or neonatal outcomes.11,12
Continue to: While there are no current...
While there are no current guidelines for treating prediabetes in pregnancy, women diagnosed with prediabetes in 1 study were found to have significantly less weight gain during pregnancy compared with patients with normal A1C,12 suggesting there may be a benefit in early identification and intervention, although further research is needed.11 In a separate case-control study (n = 345 women with GDM; n = 800 control), high rates of gestational weight gain (> 0.41 kg/wk) were associated with an increased risk of GDM (odds ratio [OR] = 1.74; 95% CI, 1.16-2.60) compared with women with the lowest rate of gestational weight gain (0.27-0.4 kg/wk [OR = 1.43; 95% CI, 0.96-2.14]).13 Thus, it is helpful to have proactive conversations about family planning and adequate weight and glycemic control with high-risk patients to prepare for a healthy pregnancy.
Obesity and weight management. Patients who are overweight (body mass index [BMI], 25-29.9) or obese (BMI > 30) have a substantially increased risk of GDM (adjusted OR = 1.44; 95% CI, 1.04-1.81), as seen in a retrospective cohort study of 1951 pregnant Malaysian women.14 Several factors have been found to contribute to successful weight control, including calorie prescription, a structured meal plan, high physical activity goals (60-90 min/d), daily weighing and monitoring of food intake, behavior therapy, and continued patient–provider contact.15
The safety, efficacy, and sustainability of weight loss with various dietary plans have been studied in individuals who are overweight and obese.16 Ultimately, energy expenditure must be greater than energy intake to promote weight loss. Conventional diets with continuous energy restriction (ie, low-fat, low-carbohydrate, and high-protein diets) have proven to be effective for short-term weight loss but data on long-term weight maintenance are limited.16 The Mediterranean diet, which is comprised mostly of vegetables, fruits, legumes, fish, and grains—with a lower intake of meat and dairy—may reduce gestational weight gain and risk of GDM as suggested by a randomized controlled trial (RCT; n = 1252).17 Although the choice of diet is up to the patient, it is important to be aware of different diets or refer the patient to a registered dietician who can help the patient if needed.
Reduce risk with adequate weight and glycemic control
Prevention of GDM during pregnancy should focus on weight maintenance and optimal glycemic control. Two systematic reviews, one with 8 RCTs (n = 1792) and another with 5 studies (n = 539), assessed the efficacy and safety of energy-restricted dietary intervention on GDM prevention.18 The first review found a significant reduction in gestational weight gain and improved glycemic control without increased risk of adverse maternal and fetal outcomes.18 The second review showed no clear difference between energy-restricted and non–energy-restricted diets on outcomes such as preeclampsia, gestational weight gain, large for gestational age, and macrosomia.18 These data suggest that while energy-restricted dietary interventions made no difference on maternal and fetal complications, they may still be safely used in pregnancy to reduce gestational weight gain and improve glycemic control.18
Once a woman is pregnant, it becomes difficult to lose weight because additional calories are needed to support a growing fetus. It is recommended that patients with healthy pregestational BMI consume an extra 200 to 300 calories/d after the first trimester. However, extra caloric intake in a woman with obesity who is pregnant leads to metabolic impairment and increased risk of diabetes for both the mother and fetus.19 Therefore, it is recommended that patients with obese pregestational BMI not consume additional calories because excess maternal fat is sufficient to support the energy needs of the growing fetus.19
Continue to: Ultimately, earlier intervention...
Ultimately, earlier intervention—prior to conception—helps patients prepare for a healthier pregnancy, resulting in better long-term outcomes. It is helpful to be familiar with the advantages and disadvantages of common approaches to weight management and to be able to refer patients to nutritionists for optimal planning. When establishing a dietary plan, consider patient-specific factors, such as cultural diets, financial and time constraints, and the patient’s readiness to make and maintain these changes. Consistent follow-up and behavioral therapy are necessary to maintain successful weight control.
There are many screening tools, but 1 is preferred in pregnancy
There are several ways to diagnose diabetes in patients who are not pregnant, including A1C, a fasting glucose test, an oral glucose tolerance test (OGTT), or random glucose testing (plus symptoms). However, the preferred method for diagnosing GDM is OGTT because it has a higher sensitivity.20 A1C, while a good measure of hyperglycemic stability, does not register hyperglycemia early enough to diagnose GDM and fasting glucose testing is less sensitive because for most women with GDM, that abnormal postprandial glucose level is the first glycemic abnormality.21
When to screen. Blood glucose levels should be checked in all pregnant women as part of their metabolic panel at the first prenatal visit. A reflex A1C for high glucose levels can be ordered based on the physician’s preference. This may help you to identify patients with prediabetes who are at risk for GDM and implement early behavioral and lifestyle changes. However, further research is needed to determine if intervention early in pregnancy can truly reduce the risk of GDM.11
Screening for GDM should be completed at 24 to 28 weeks of gestation20 because it is likely that this is when the hormonal effects of the placenta that contribute to insulin resistance set the woman up for postprandial hyperglycemia. Currently, there are no evidence-based guidelines for the use of continuous glucose monitoring prior to 24 weeks of gestation to identify GDM.20 If persistent hyperglycemia is present before 24 weeks of gestation, it is considered evidence of a pre-existing metabolic abnormality and is diagnosed as “pregestational diabetes.” Treatment should follow guidelines established for women who had diabetes prior to pregnancy.
How to screen? There is ongoing discussion about what is the optimal screening method for GDM: a 1-step strategy with a fasting 75-g OGTT only, or a 2-step strategy with a 50-g non-fasting glucose load test followed by a fasting 100-g OGTT in women who do not meet the plasma glucose cutoff (TABLE 1).22-24 Hillier et al25 compared the effectiveness of these strategies in diagnosing GDM and identifying pregnancy complications for the mother and infant. They found that while the 1-step strategy resulted in a 2-fold increase in the diagnosis of GDM, it did not lead to better outcomes for mothers and infants when compared with the 2-step method.25 Currently, the majority of obstetricians (95%) prefer to use the 2-step method.24
Continue to: Manage lifestyle, monitor glucose
Manage lifestyle, monitor glucose
Management of GDM in most women starts with diabetes self-management education and support for therapeutic lifestyle changes, such as nutritional interventions that reduce hyperglycemia and contribute to healthy weight gain during pregnancy.20 This may include medical nutrition therapy that focuses on adequate nutrition for the mother and fetus. Currently, the recommended dietary intake for women who are pregnant (regardless of diabetes) includes a minimum of 175 g of carbohydrates, 71 g of daily protein, and at least 28 g of fiber. Further refinement of dietary intake, including carbohydrate restriction, should be done with guidance from a registered dietitian.20 If the obstetrics team does not include a registered dietitian, a referral to one may be necessary. Regular physical activity should be continued throughout pregnancy as tolerated. Social support, stress reduction, and good sleep hygiene should be encouraged as much as possible.
For successful outcomes, therapeutic lifestyle changes should be coupled with glucose monitoring. The Fifth International Workshop-Conference on Gestational Diabetes Mellitus recommends that women with GDM monitor fasting blood glucose and typically 1-hour postprandial glucose. The glucose goals in GDM are as follows26:
- Fasting glucose < 95 mg/dL (5.3 mmol/L), and either
- 1-hour postprandial glucose < 140 mg/dL (7.8 mmol/L), or
- 2-hour postprandial glucose < 120 mg/dL (6.7 mmol/L).
Importantly, in the second and third trimester, the A1C goal for women with GDM is 6.0%. This is lower than the more traditional A1C goal for 2 reasons: (1) increases in A1C, even within the normal range, increase adverse outcomes; and (2) pregnant women will have an increased red blood cell count turnover, which can lower the A1C.27 In a historical cohort study (n = 27,213), Abell et al28 found that women who have an A1C < 6.0% in the second and third trimester have the lowest risk of giving birth to large-for-gestational-age infants and for having preeclampsia.
Add insulin if glucose targets are not met
Most women who engage in therapeutic lifestyle change (70%-85%) can achieve an A1C < 6% and will not need to take medication to manage GDM.29 If pharmacotherapy is needed to manage glucose, insulin is the preferred treatment for all women with GDM.20 Treatment should be individualized based on the glucose trends the woman is experiencing. Common treatments include bedtime NPH if fasting hyperglycemia is most prominent and analogue insulin at mealtimes for women with prominent postprandial hyperglycemia.
Noninsulin agents such as metformin and sulfonylureas are not currently recommended by the American College of Obstetricians and Gynecologists or the American Diabetes Association for use in GDM.20,24 Despite being used for years in women with pregestational diabetes, metabolic syndrome, and polycystic ovary syndrome, there is evidence that metformin crosses the placenta and fetal safety has not yet been established in RCTs. The Metformin in Gestational Diabetes: The Offspring Follow-Up (MiG TOFU) study was a longitudinal follow-up study that evaluated body composition and metabolic outcomes in children (ages 7-9 years) of women with GDM who had received metformin or insulin while pregnant.30 At age 9 years, children who were exposed to metformin weighed more and had a higher waist-to-height ratio and waist circumference than those exposed to insulin.30
Continue to: Sulfonylureas are no longer recommended...
Sulfonylureas are no longer recommended because of the risk of maternal and fetal hypoglycemia and concerns about this medication crossing the placenta.24,31,32 Specifically, in a 2015 meta-analysis and systematic review of 15 articles (n = 2509), glyburide had a higher risk of neonatal hypoglycemia and macrosomia than insulin or metformin.33 For women who cannot manage their glucose with therapeutic lifestyle changes and cannot take insulin, oral therapies may be considered if the risk-benefit ratio is balanced for that person.34
Watch for effects of poor glycemic control on mother, infant
Preeclampsia is defined as new-onset hypertension and proteinuria after 20 weeks of gestation. The correlation between GDM and preeclampsia has partly been explained by their shared overlapping risk factors, including maternal obesity, excessive gestational weight gain, and persistent hyperglycemia.35 On a biochemical level, these risk factors contribute to oxidative stress and systemic vascular dysfunction, which have been hypothesized as the underlying pathophysiology for the development of preeclampsia.35
Neonatal macrosomia, defined as a birth weight ≥ 4000 g, is a common complication that develops in 15% to 45% of infants of mothers with GDM.36 Placental transfer of glucose in mothers with hyperglycemia stimulates the secretion of neonatal insulin and the ultimate storage of the excess glucose as body fat. After delivery, the abrupt discontinuation of placental transfer of glucose to an infant who is actively secreting insulin leads to neonatal hypoglycemia, which if not detected or managed, can lead to long-term neurologic deficits, including recurrent seizures and developmental delays.37 Therefore, it is essential to screen for neonatal hypoglycemia immediately after birth and serially up to 12 hours.38
Postpartum T2D. Poor glycemic control increases the risk of increasing insulin resistance developing into T2D postpartum for mothers.39 It also increases the risk of obesity and insulin resistance later in life for the infant.40 A retrospective cohort study (n = 461) found a positive correlation between exposure to maternal GDM and elevated BMI in children ages 6 to 13 years.41 Kamana et al36 further discussed this correlation and suggested that exposure to maternal hyperglycemia in utero contributes to fetal programming of later adipose deposition. Children may develop without a notable increase in BMI until after puberty.42
Partner with specialists to improve outcomes
Although most women with GDM are managed by specialists (obstetricians, endocrinologists, and maternal-fetal medicine specialists),43 these patients are still seeking care from their family physicians for other complaints. These visits provide key touchpoints during pregnancy and are opportunities for PCPs to identify a pregnancy-related complication or provide additional education or referral to the obstetrician.
Continue to: Also, if you work in an area...
Also, if you work in an area where specialists are less accessible, you may be the clinician providing the majority of care to a patient with GDM. If this is the case, you’ll want to watch for the following risk factors, which should prompt a referral to specialty care:
- a previous pregnancy with GDM20
- a previous birth of an infant weighing > 4000 g44
- baseline history of hypertension45
- evidence of insulin resistance or polycystic ovary syndrome46,47
- a history of cardiovascular disease20
- a need to treat GDM with pharmacotherapy.48
Ensuring a smooth transition after the birth
Optimal communication and hand-offs throughout pregnancy and after delivery will benefit everyone. When the pregnant patient’s care has been managed by an obstetrician, it is important to address the following issues during the hand-off:
- baseline medical problems
- medical screenings and treatments in pregnancy (retinopathy and nephropathy screening)
- aspirin initiation, if indicated
- management of thyroid abnormalities
- management of mental health conditions
- postpartum glucose management and T2D screening postpartum
- management of complications identified during pregnancy (retinopathy and nephropathy).
Timing and other elements of postpartum care. The first postpartum screen should occur at 4 to 12 weeks postpartum. OGTT is recommended instead of A1C at this time because A1C may still be lowered by the increased red blood cell turnover related to pregnancy and blood loss at delivery. Because women with GDM have a 50% to 75% lifetime risk of T2D,20 patients with normal test results should be re-tested every 1 to 3 years using any of the standard screening methods (A1C, fasting glucose, or OGTT).20
After delivery it may be difficult for women to follow-up with their own personal health care because they are focused on the care of their baby. The increased use of telehealth may make postpartum follow-up visits easier to attend.
Visits present opportunities. Postpartum visits present another opportunity for PCPs to screen for diabetes and other postpartum complications, including depression and thyroid abnormalities. Visits are also an opportunity to discuss timely contraception so as to prevent an early, unplanned pregnancy. Other important aspects of postpartum care are outlined in TABLE 2.20,49
CORRESPONDENCE
Connie L. Ha, BS, OMS IV, Department of Primary Care, 1310 Club Drive, Touro University California, Vallejo, CA 94592; connie.ha@tu.edu
Gestational diabetes mellitus (GDM), defined as new-onset hyperglycemia detected in a pregnant woman after 24 weeks of gestation, affects 4% to 10% of pregnancies in the United States annually1 and is a major challenge for health care professionals.2 During pregnancy, the body’s physiologic responses are altered to support the growing fetus. One of these changes is an increase in insulin resistance, which suggests that pregnancy alone increases the patient’s risk for type 2 diabetes (T2D). However, several other factors also increase this risk, including maternal age, social barriers to care, obesity, poor weight control, and family history.
If not controlled, GDM results in poor health outcomes for the mother, such as preeclampsia, preterm labor, and maternal T2D.3-5 For the infant, intrauterine exposure to persistent hyperglycemia is correlated with neonatal macrosomia, hypoglycemia, perinatal complications (eg, preterm delivery, fetal demise), and obesity and insulin resistance later in life.4
Primary care physicians (PCPs) are the patient’s main point of contact prior to pregnancy. This relationship makes PCPs a resource for the patient and specialists during and after pregnancy. In this article, we discuss risk factors and how to screen for GDM, provide an update on practice recommendations for treatment and management of GDM in primary care, and describe the effects of uncontrolled GDM.
Know the key risk factors
Prevention begins with identifying the major risk factors that contribute to the development of GDM. These include maternal age, social barriers to care, family history of prediabetes, and obesity and poor weight control.
Older age. A meta-analysis of 24 studies noted strong positive correlation between GDM risk and maternal age.6 One of the population-based cohort studies in the meta-analysis examined relationships between maternal age and pregnancy outcomes in women living in British Columbia, Canada (n = 203,414). Data suggested that the relative risk of GDM increased linearly with maternal age to 3.2, 4.2, and 4.4 among women ages ≥ 35, ≥ 40, and ≥ 45 years, respectively.7
Social barriers to care. Although the prevalence of GDM has increased over the past few decades,1 from 2011 to 2019 the increase in GDM in individuals at first live birth was significantly higher in non-Hispanic Asian and Hispanic/Latina women than in non-Hispanic White women.8 Data from the Centers for Disease Control and Prevention further suggest that diabetes was more prevalent among individuals with a lower socioeconomic status as indicated by their level of education.9 Ogunwole et al10 suggest that racism is the root cause of these disparities and leads to long-term barriers to care (eg, socioeconomic deprivation, lack of health insurance, limited access to care, and poor health literacy), which ultimately contribute to the development of GDM and progression of diabetes. It is important for PCPs and all health professionals to be aware of these barriers so that they may practice mindfulness and deliver culturally sensitive care to patients from marginalized communities.
Family history of prediabetes. In a population-based cohort study (n = 7020), women with prediabetes (A1C, 5.7%-6.4%) were 2.8 times more likely to develop GDM compared with women with normal A1C (< 5.7%).11 Similar results were seen in a retrospective cohort study (n = 2812), in which women with prediabetes were more likely than women with a normal first trimester A1C to have GDM (29.1% vs 13.7%, respectively; adjusted relative risk = 1.48; 95% CI, 1.15-1.89).12 In both studies, prediabetes was not associated with a higher risk for adverse maternal or neonatal outcomes.11,12
Continue to: While there are no current...
While there are no current guidelines for treating prediabetes in pregnancy, women diagnosed with prediabetes in 1 study were found to have significantly less weight gain during pregnancy compared with patients with normal A1C,12 suggesting there may be a benefit in early identification and intervention, although further research is needed.11 In a separate case-control study (n = 345 women with GDM; n = 800 control), high rates of gestational weight gain (> 0.41 kg/wk) were associated with an increased risk of GDM (odds ratio [OR] = 1.74; 95% CI, 1.16-2.60) compared with women with the lowest rate of gestational weight gain (0.27-0.4 kg/wk [OR = 1.43; 95% CI, 0.96-2.14]).13 Thus, it is helpful to have proactive conversations about family planning and adequate weight and glycemic control with high-risk patients to prepare for a healthy pregnancy.
Obesity and weight management. Patients who are overweight (body mass index [BMI], 25-29.9) or obese (BMI > 30) have a substantially increased risk of GDM (adjusted OR = 1.44; 95% CI, 1.04-1.81), as seen in a retrospective cohort study of 1951 pregnant Malaysian women.14 Several factors have been found to contribute to successful weight control, including calorie prescription, a structured meal plan, high physical activity goals (60-90 min/d), daily weighing and monitoring of food intake, behavior therapy, and continued patient–provider contact.15
The safety, efficacy, and sustainability of weight loss with various dietary plans have been studied in individuals who are overweight and obese.16 Ultimately, energy expenditure must be greater than energy intake to promote weight loss. Conventional diets with continuous energy restriction (ie, low-fat, low-carbohydrate, and high-protein diets) have proven to be effective for short-term weight loss but data on long-term weight maintenance are limited.16 The Mediterranean diet, which is comprised mostly of vegetables, fruits, legumes, fish, and grains—with a lower intake of meat and dairy—may reduce gestational weight gain and risk of GDM as suggested by a randomized controlled trial (RCT; n = 1252).17 Although the choice of diet is up to the patient, it is important to be aware of different diets or refer the patient to a registered dietician who can help the patient if needed.
Reduce risk with adequate weight and glycemic control
Prevention of GDM during pregnancy should focus on weight maintenance and optimal glycemic control. Two systematic reviews, one with 8 RCTs (n = 1792) and another with 5 studies (n = 539), assessed the efficacy and safety of energy-restricted dietary intervention on GDM prevention.18 The first review found a significant reduction in gestational weight gain and improved glycemic control without increased risk of adverse maternal and fetal outcomes.18 The second review showed no clear difference between energy-restricted and non–energy-restricted diets on outcomes such as preeclampsia, gestational weight gain, large for gestational age, and macrosomia.18 These data suggest that while energy-restricted dietary interventions made no difference on maternal and fetal complications, they may still be safely used in pregnancy to reduce gestational weight gain and improve glycemic control.18
Once a woman is pregnant, it becomes difficult to lose weight because additional calories are needed to support a growing fetus. It is recommended that patients with healthy pregestational BMI consume an extra 200 to 300 calories/d after the first trimester. However, extra caloric intake in a woman with obesity who is pregnant leads to metabolic impairment and increased risk of diabetes for both the mother and fetus.19 Therefore, it is recommended that patients with obese pregestational BMI not consume additional calories because excess maternal fat is sufficient to support the energy needs of the growing fetus.19
Continue to: Ultimately, earlier intervention...
Ultimately, earlier intervention—prior to conception—helps patients prepare for a healthier pregnancy, resulting in better long-term outcomes. It is helpful to be familiar with the advantages and disadvantages of common approaches to weight management and to be able to refer patients to nutritionists for optimal planning. When establishing a dietary plan, consider patient-specific factors, such as cultural diets, financial and time constraints, and the patient’s readiness to make and maintain these changes. Consistent follow-up and behavioral therapy are necessary to maintain successful weight control.
There are many screening tools, but 1 is preferred in pregnancy
There are several ways to diagnose diabetes in patients who are not pregnant, including A1C, a fasting glucose test, an oral glucose tolerance test (OGTT), or random glucose testing (plus symptoms). However, the preferred method for diagnosing GDM is OGTT because it has a higher sensitivity.20 A1C, while a good measure of hyperglycemic stability, does not register hyperglycemia early enough to diagnose GDM and fasting glucose testing is less sensitive because for most women with GDM, that abnormal postprandial glucose level is the first glycemic abnormality.21
When to screen. Blood glucose levels should be checked in all pregnant women as part of their metabolic panel at the first prenatal visit. A reflex A1C for high glucose levels can be ordered based on the physician’s preference. This may help you to identify patients with prediabetes who are at risk for GDM and implement early behavioral and lifestyle changes. However, further research is needed to determine if intervention early in pregnancy can truly reduce the risk of GDM.11
Screening for GDM should be completed at 24 to 28 weeks of gestation20 because it is likely that this is when the hormonal effects of the placenta that contribute to insulin resistance set the woman up for postprandial hyperglycemia. Currently, there are no evidence-based guidelines for the use of continuous glucose monitoring prior to 24 weeks of gestation to identify GDM.20 If persistent hyperglycemia is present before 24 weeks of gestation, it is considered evidence of a pre-existing metabolic abnormality and is diagnosed as “pregestational diabetes.” Treatment should follow guidelines established for women who had diabetes prior to pregnancy.
How to screen? There is ongoing discussion about what is the optimal screening method for GDM: a 1-step strategy with a fasting 75-g OGTT only, or a 2-step strategy with a 50-g non-fasting glucose load test followed by a fasting 100-g OGTT in women who do not meet the plasma glucose cutoff (TABLE 1).22-24 Hillier et al25 compared the effectiveness of these strategies in diagnosing GDM and identifying pregnancy complications for the mother and infant. They found that while the 1-step strategy resulted in a 2-fold increase in the diagnosis of GDM, it did not lead to better outcomes for mothers and infants when compared with the 2-step method.25 Currently, the majority of obstetricians (95%) prefer to use the 2-step method.24
Continue to: Manage lifestyle, monitor glucose
Manage lifestyle, monitor glucose
Management of GDM in most women starts with diabetes self-management education and support for therapeutic lifestyle changes, such as nutritional interventions that reduce hyperglycemia and contribute to healthy weight gain during pregnancy.20 This may include medical nutrition therapy that focuses on adequate nutrition for the mother and fetus. Currently, the recommended dietary intake for women who are pregnant (regardless of diabetes) includes a minimum of 175 g of carbohydrates, 71 g of daily protein, and at least 28 g of fiber. Further refinement of dietary intake, including carbohydrate restriction, should be done with guidance from a registered dietitian.20 If the obstetrics team does not include a registered dietitian, a referral to one may be necessary. Regular physical activity should be continued throughout pregnancy as tolerated. Social support, stress reduction, and good sleep hygiene should be encouraged as much as possible.
For successful outcomes, therapeutic lifestyle changes should be coupled with glucose monitoring. The Fifth International Workshop-Conference on Gestational Diabetes Mellitus recommends that women with GDM monitor fasting blood glucose and typically 1-hour postprandial glucose. The glucose goals in GDM are as follows26:
- Fasting glucose < 95 mg/dL (5.3 mmol/L), and either
- 1-hour postprandial glucose < 140 mg/dL (7.8 mmol/L), or
- 2-hour postprandial glucose < 120 mg/dL (6.7 mmol/L).
Importantly, in the second and third trimester, the A1C goal for women with GDM is 6.0%. This is lower than the more traditional A1C goal for 2 reasons: (1) increases in A1C, even within the normal range, increase adverse outcomes; and (2) pregnant women will have an increased red blood cell count turnover, which can lower the A1C.27 In a historical cohort study (n = 27,213), Abell et al28 found that women who have an A1C < 6.0% in the second and third trimester have the lowest risk of giving birth to large-for-gestational-age infants and for having preeclampsia.
Add insulin if glucose targets are not met
Most women who engage in therapeutic lifestyle change (70%-85%) can achieve an A1C < 6% and will not need to take medication to manage GDM.29 If pharmacotherapy is needed to manage glucose, insulin is the preferred treatment for all women with GDM.20 Treatment should be individualized based on the glucose trends the woman is experiencing. Common treatments include bedtime NPH if fasting hyperglycemia is most prominent and analogue insulin at mealtimes for women with prominent postprandial hyperglycemia.
Noninsulin agents such as metformin and sulfonylureas are not currently recommended by the American College of Obstetricians and Gynecologists or the American Diabetes Association for use in GDM.20,24 Despite being used for years in women with pregestational diabetes, metabolic syndrome, and polycystic ovary syndrome, there is evidence that metformin crosses the placenta and fetal safety has not yet been established in RCTs. The Metformin in Gestational Diabetes: The Offspring Follow-Up (MiG TOFU) study was a longitudinal follow-up study that evaluated body composition and metabolic outcomes in children (ages 7-9 years) of women with GDM who had received metformin or insulin while pregnant.30 At age 9 years, children who were exposed to metformin weighed more and had a higher waist-to-height ratio and waist circumference than those exposed to insulin.30
Continue to: Sulfonylureas are no longer recommended...
Sulfonylureas are no longer recommended because of the risk of maternal and fetal hypoglycemia and concerns about this medication crossing the placenta.24,31,32 Specifically, in a 2015 meta-analysis and systematic review of 15 articles (n = 2509), glyburide had a higher risk of neonatal hypoglycemia and macrosomia than insulin or metformin.33 For women who cannot manage their glucose with therapeutic lifestyle changes and cannot take insulin, oral therapies may be considered if the risk-benefit ratio is balanced for that person.34
Watch for effects of poor glycemic control on mother, infant
Preeclampsia is defined as new-onset hypertension and proteinuria after 20 weeks of gestation. The correlation between GDM and preeclampsia has partly been explained by their shared overlapping risk factors, including maternal obesity, excessive gestational weight gain, and persistent hyperglycemia.35 On a biochemical level, these risk factors contribute to oxidative stress and systemic vascular dysfunction, which have been hypothesized as the underlying pathophysiology for the development of preeclampsia.35
Neonatal macrosomia, defined as a birth weight ≥ 4000 g, is a common complication that develops in 15% to 45% of infants of mothers with GDM.36 Placental transfer of glucose in mothers with hyperglycemia stimulates the secretion of neonatal insulin and the ultimate storage of the excess glucose as body fat. After delivery, the abrupt discontinuation of placental transfer of glucose to an infant who is actively secreting insulin leads to neonatal hypoglycemia, which if not detected or managed, can lead to long-term neurologic deficits, including recurrent seizures and developmental delays.37 Therefore, it is essential to screen for neonatal hypoglycemia immediately after birth and serially up to 12 hours.38
Postpartum T2D. Poor glycemic control increases the risk of increasing insulin resistance developing into T2D postpartum for mothers.39 It also increases the risk of obesity and insulin resistance later in life for the infant.40 A retrospective cohort study (n = 461) found a positive correlation between exposure to maternal GDM and elevated BMI in children ages 6 to 13 years.41 Kamana et al36 further discussed this correlation and suggested that exposure to maternal hyperglycemia in utero contributes to fetal programming of later adipose deposition. Children may develop without a notable increase in BMI until after puberty.42
Partner with specialists to improve outcomes
Although most women with GDM are managed by specialists (obstetricians, endocrinologists, and maternal-fetal medicine specialists),43 these patients are still seeking care from their family physicians for other complaints. These visits provide key touchpoints during pregnancy and are opportunities for PCPs to identify a pregnancy-related complication or provide additional education or referral to the obstetrician.
Continue to: Also, if you work in an area...
Also, if you work in an area where specialists are less accessible, you may be the clinician providing the majority of care to a patient with GDM. If this is the case, you’ll want to watch for the following risk factors, which should prompt a referral to specialty care:
- a previous pregnancy with GDM20
- a previous birth of an infant weighing > 4000 g44
- baseline history of hypertension45
- evidence of insulin resistance or polycystic ovary syndrome46,47
- a history of cardiovascular disease20
- a need to treat GDM with pharmacotherapy.48
Ensuring a smooth transition after the birth
Optimal communication and hand-offs throughout pregnancy and after delivery will benefit everyone. When the pregnant patient’s care has been managed by an obstetrician, it is important to address the following issues during the hand-off:
- baseline medical problems
- medical screenings and treatments in pregnancy (retinopathy and nephropathy screening)
- aspirin initiation, if indicated
- management of thyroid abnormalities
- management of mental health conditions
- postpartum glucose management and T2D screening postpartum
- management of complications identified during pregnancy (retinopathy and nephropathy).
Timing and other elements of postpartum care. The first postpartum screen should occur at 4 to 12 weeks postpartum. OGTT is recommended instead of A1C at this time because A1C may still be lowered by the increased red blood cell turnover related to pregnancy and blood loss at delivery. Because women with GDM have a 50% to 75% lifetime risk of T2D,20 patients with normal test results should be re-tested every 1 to 3 years using any of the standard screening methods (A1C, fasting glucose, or OGTT).20
After delivery it may be difficult for women to follow-up with their own personal health care because they are focused on the care of their baby. The increased use of telehealth may make postpartum follow-up visits easier to attend.
Visits present opportunities. Postpartum visits present another opportunity for PCPs to screen for diabetes and other postpartum complications, including depression and thyroid abnormalities. Visits are also an opportunity to discuss timely contraception so as to prevent an early, unplanned pregnancy. Other important aspects of postpartum care are outlined in TABLE 2.20,49
CORRESPONDENCE
Connie L. Ha, BS, OMS IV, Department of Primary Care, 1310 Club Drive, Touro University California, Vallejo, CA 94592; connie.ha@tu.edu
1. Sheiner E. Gestational diabetes mellitus: long-term consequences for the mother and child grand challenge: how to move on towards secondary prevention? Front Clin Diabetes Healthc. 2020. doi: 10.3389/fcdhc.2020.546256
2. Angueira AR, Ludvik AE, Reddy TE, et al. New insights into gestational glucose metabolism: lessons learned from 21st century approaches. Diabetes. 2015;64:327-334. doi: 10.2337/db14-0877
3. Shou C, Wei Y-M, Wang C, et al. Updates in long-term maternal and fetal adverse effects of gestational diabetes mellitus. Maternal-Fetal Med. 2019;1:91-94. doi: 10.1097/FM9.0000000000000019
4. Plows JF, Stanley JL, Baker PN, et al. The pathophysiology of gestational diabetes mellitus. Int J Mol Sci. 2018;19:3342. doi: 10.3390/ijms19113342
5. Kulshrestha V, Agarwal N. Maternal complications in pregnancy with diabetes. J Pak Med Assoc. 2016;66(9 suppl 1):S74-S77.
6. Li Y, Ren X, He L, et al. Maternal age and the risk of gestational diabetes mellitus: a systematic review and meta-analysis of over 120 million participants. Diabetes Res Clin Pract. 2020;162:108044. doi: 10.1016/j.diabres.2020.108044
7. Schummers L, Hutcheon JA, Hacker MR, et al. Absolute risks of obstetric outcomes by maternal age at first birth: a population-based cohort. Epidemiology. 2018;29:379-387. doi: 10.1097/EDE.0000000000000818
8. Shah NS, Wang MC, Freaney PM, et al. Trends in gestational diabetes at first live birth by race and ethnicity in the US, 2011-2019. JAMA. 2021;326:660-669. doi: 10.1001/jama.2021.7217
9. Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2020. Atlanta, GA: Centers for Disease Control and Prevention, U.S. Department of Health and Human Services; 2020. Accessed February 2, 2022. www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf
10. Ogunwole SM, Golden SH. Social determinants of health and structural inequities—root causes of diabetes disparities. Diabetes Care. 2021;44:11-13. doi: 10.2337/dci20-0060
11. Chen L, Pocobelli G, Yu O, et al. Early pregnancy hemoglobin A1C and pregnancy outcomes: a population-based study. Am J Perinatol. 2019;36:1045-1053. doi: 10.1055/s-0038-1675619
12. Osmundson S, Zhao BS, Kunz L, et al. First trimester hemoglobin A1C prediction of gestational diabetes. Am J Perinatol. 2016;33:977-982. doi: 10.1055/s-0036-1581055
13. Hedderson MM, Gunderson EP, Ferrara A. Gestational weight gain and risk of gestational diabetes mellitus [published correction appears in Obstet Gynecol. 2010;115:1092]. Obstet Gynecol. 2010;115:597-604. doi: 10.1097/AOG.0b013e3181cfce4f
14. Yong HY, Mohd Shariff Z, Mohd Yusof BN, et al. Independent and combined effects of age, body mass index and gestational weight gain on the risk of gestational diabetes mellitus. Sci Rep. 2020;10:8486. doi: 10.1038/s41598-020-65251-2
15. Phelan S. Windows of opportunity for lifestyle interventions to prevent gestational diabetes mellitus. Am J Perinatol. 2016;33:1291-1299. doi: 10.1055/s-0036-1586504
16. Koliaki C, Spinos T, Spinou M, et al. Defining the optimal dietary approach for safe, effective and sustainable weight loss in overweight and obese adults. Healthcare (Basel). 2018;6:73. doi: 10.3390/healthcare6030073
17. Al Wattar BH, Dodds J, Placzek A, et al. Mediterranean-style diet in pregnant women with metabolic risk factors (ESTEEM): a pragmatic multicentre randomised trial. PLOS Med. 2019;16:e1002857. doi: 10.1371/journal.pmed.1002857
18. Zarogiannis S. Are novel lifestyle approaches to management of type 2 diabetes applicable to prevention and treatment of women with gestational diabetes mellitus? Global Diabetes Open Access J. 2019;1:1-14.
19. Most J, Amant MS, Hsia DS, et al. Evidence-based recommendations for energy intake in pregnant women with obesity. J Clin Invest. 2019;129:4682-4690. doi: 10.1172/JCI130341
20. American Diabetes Association. 14. Management of diabetes in pregnancy: Standards of Medical Care in Diabetes—2021. Diabetes Care. 2021;44(suppl 1):S200-S210. doi: 10.2337/dc21-S014
21. McIntyre HD, Sacks DA, Barbour LA, et al. Issues with the diagnosis and classification of hyperglycemia in early pregnancy. Diabetes Care. 2016;39:53-54. doi: 10.2337/dc15-1887
22. American Diabetes Association. 2. Classification and diagnosis of diabetes: Standards of Medical Care in Diabetes—2022. Diabetes Care. 2022;45(suppl 1):S17-S38. doi: 10.2337/dc22-S002
23. Carpenter MW, Coustan DR. Criteria for screening tests for gestational diabetes. Am J Obstet Gynecol. 1982;144:768-773. doi: 10.1016/0002-9378(82)90349-0
24. ACOG Practice Bulletin No. 190: gestational diabetes mellitus. Obstet Gynecol. 2018;131:e49-e64. doi: 10.1097/AOG.0000000000002501
25. Hillier TA, Pedula KL, Ogasawara KK, et al. A pragmatic, randomized clinical trial of gestational diabetes screening. N Engl J Med. 2021;384:895-904. doi: 10.1056/NEJMoa2026028
26. Metzger BE, Buchanan TA, Coustan DR, et al. Summary and recommendations of the Fifth International Workshop-Conference on Gestational Diabetes Mellitus. Diabetes Care. 2007;30(suppl 2):S251-S260. doi: 10.2337/dc07-s225
27. Nielsen LR, Ekbom P, Damm P, et al. HbA1c levels are significantly lower in early and late pregnancy. Diabetes Care. 2004;27:1200-1201. doi: 10.2337/diacare.27.5.1200
28. Abell SK, Boyle JA, de Courten B, et al. Impact of type 2 diabetes, obesity and glycaemic control on pregnancy outcomes. Aust N Z J Obstet Gynaecol. 2017;57:308-314. doi: 10.1111/ajo.12521
29. Viana LV, Gross JL, Azevedo MJ. Dietary intervention in patients with gestational diabetes mellitus: a systematic review and meta-analysis of randomized clinical trials on maternal and newborn outcomes. Diabetes Care. 2014;37:3345-3355. doi: 10.2337/dc14-1530
30. Rowan JA, Rush EC, Plank LD, et al. Metformin in gestational diabetes: the offspring follow-up (MiG TOFU): body composition and metabolic outcomes at 7-9 years of age. BMJ Open Diabetes Res Care. 2018;6:e000456. doi: 10.1136/bmjdrc-2017-000456
31. Hebert MF, Ma X, Naraharisetti SB, et al. Are we optimizing gestational diabetes treatment with glyburide? The pharmacologic basis for better clinical practice. Clin Pharmacol Ther. 2009;85:607-614. doi: 10.1038/clpt.2009.5
32. Malek R, Davis SN. Pharmacokinetics, efficacy and safety of glyburide for treatment of gestational diabetes mellitus. Expert Opin Drug Metab Toxicol. 2016;12:691-699. doi: 10.1080/17425255.2016.1187131
33. Balsells M, García-Patterson A, Solà I, et al. Glibenclamide, metformin, and insulin for the treatment of gestational diabetes: a systematic review and meta-analysis. BMJ. 2015;350:h102. doi: 10.1136/bmj.h102
34. Kavitha N, De S, Kanagasabai S. Oral hypoglycemic agents in pregnancy: an update. J Obstet Gynaecol India. 2013;63:82-87. doi: 10.1007/s13224-012-0312-z
35. Weissgerber TL, Mudd LM. Preeclampsia and diabetes. Curr Diab Rep. 2015;15:9. doi: 10.1007/s11892-015-0579-4
36. Kamana KC, Shakya S, Zhang H. Gestational diabetes mellitus and macrosomia: a literature review. Ann Nutr Metab. 2015;66(suppl 2):14-20. doi: 10.1159/000371628
37. Mitanchez D, Yzydorczyk C, Simeoni U. What neonatal complications should the pediatrician be aware of in case of maternal gestational diabetes? World J Diabetes. 2015;6:734-743. doi: 10.4239/wjd.v6.i5.734
38. Stanescu A, Stoicescu SM. Neonatal hypoglycemia screening in newborns from diabetic mothers—arguments and controversies. J Med Life. 2014;7(spec iss 3):51-52.
39. Kim C. Maternal outcomes and follow-up after gestational diabetes mellitus. Diabet Med. 2014;31:292-301. doi: 10.1111/dme.12382
40. Stewart A, Malhotra A. Gestational diabetes and the neonate: challenges and solutions. Res Rep Neonatol. 2015;5:31-39. doi: 10.2147/RRN.S30971
41. Crume TL, Ogden L, West NA, et al. Association of exposure to diabetes in utero with adiposity and fat distribution in a multiethnic population of youth: the Exploring Perinatal Outcomes among Children (EPOCH) Study. Diabetologia. 2011;54:87-92. doi: 10.1007/s00125-010-1925-3
42. Crume TL, Ogden L, Daniels S, et al. The impact of in utero exposure to diabetes on childhood body mass index growth trajectories: the EPOCH study. J Pediatr. 2011;158:941-946. doi: 10.1016/j.jpeds.2010.12.007
43. Levels of maternal care. Obstetric Care Consensus No. 9. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2019;134:e41-e55. doi: 10.1097/AOG.0000000000003383
44. Caughey AB, Cheng YW, Stotland NE, et al. Maternal and paternal race/ethnicity are both associated with gestational diabetes. Am J Obstet Gynecol. 2010;202:616.e1-e5. doi: 10.1016/j.ajog.2010.01.082
45. Yogev Y, Xenakis EM, Langer O. The association between preeclampsia and severity of gestational diabetes: the impact of glycemic control. Am J Obstet Gynecol. 2004;191:1655-1660. doi: 10.1016/j.ajog.2004.03.074
46. Brown J, Alwan NA, West J, et al. Lifestyle interventions for the treatment of women with gestational diabetes. Cochrane Database Syst Rev. 2017;5:CD011970. doi: 10.1002/14651858.CD011970.pub2
47. Ceysens G, Rouiller D, Boulvain M. Exercise for the diabetic pregnant woman. Cochrane Database Syst Rev. 2006;3:CD004225. doi: 10.1002/14651858.CD004225.pub2
48. Chawla R, Mukherjee JJ, Chawla M, et al. Expert group recommendations on the effective use of bolus insulin in the management of type 2 diabetes mellitus. Med Sci (Basel). 2021;9:38. doi: 10.3390/medsci9020038
49. American Diabetes Association. Introduction: Standards of Medical Care in Diabetes—2021. Diabetes Care. 2021;44(suppl 1):S1-S2. doi: 10.2337/dc22-Sint
1. Sheiner E. Gestational diabetes mellitus: long-term consequences for the mother and child grand challenge: how to move on towards secondary prevention? Front Clin Diabetes Healthc. 2020. doi: 10.3389/fcdhc.2020.546256
2. Angueira AR, Ludvik AE, Reddy TE, et al. New insights into gestational glucose metabolism: lessons learned from 21st century approaches. Diabetes. 2015;64:327-334. doi: 10.2337/db14-0877
3. Shou C, Wei Y-M, Wang C, et al. Updates in long-term maternal and fetal adverse effects of gestational diabetes mellitus. Maternal-Fetal Med. 2019;1:91-94. doi: 10.1097/FM9.0000000000000019
4. Plows JF, Stanley JL, Baker PN, et al. The pathophysiology of gestational diabetes mellitus. Int J Mol Sci. 2018;19:3342. doi: 10.3390/ijms19113342
5. Kulshrestha V, Agarwal N. Maternal complications in pregnancy with diabetes. J Pak Med Assoc. 2016;66(9 suppl 1):S74-S77.
6. Li Y, Ren X, He L, et al. Maternal age and the risk of gestational diabetes mellitus: a systematic review and meta-analysis of over 120 million participants. Diabetes Res Clin Pract. 2020;162:108044. doi: 10.1016/j.diabres.2020.108044
7. Schummers L, Hutcheon JA, Hacker MR, et al. Absolute risks of obstetric outcomes by maternal age at first birth: a population-based cohort. Epidemiology. 2018;29:379-387. doi: 10.1097/EDE.0000000000000818
8. Shah NS, Wang MC, Freaney PM, et al. Trends in gestational diabetes at first live birth by race and ethnicity in the US, 2011-2019. JAMA. 2021;326:660-669. doi: 10.1001/jama.2021.7217
9. Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2020. Atlanta, GA: Centers for Disease Control and Prevention, U.S. Department of Health and Human Services; 2020. Accessed February 2, 2022. www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf
10. Ogunwole SM, Golden SH. Social determinants of health and structural inequities—root causes of diabetes disparities. Diabetes Care. 2021;44:11-13. doi: 10.2337/dci20-0060
11. Chen L, Pocobelli G, Yu O, et al. Early pregnancy hemoglobin A1C and pregnancy outcomes: a population-based study. Am J Perinatol. 2019;36:1045-1053. doi: 10.1055/s-0038-1675619
12. Osmundson S, Zhao BS, Kunz L, et al. First trimester hemoglobin A1C prediction of gestational diabetes. Am J Perinatol. 2016;33:977-982. doi: 10.1055/s-0036-1581055
13. Hedderson MM, Gunderson EP, Ferrara A. Gestational weight gain and risk of gestational diabetes mellitus [published correction appears in Obstet Gynecol. 2010;115:1092]. Obstet Gynecol. 2010;115:597-604. doi: 10.1097/AOG.0b013e3181cfce4f
14. Yong HY, Mohd Shariff Z, Mohd Yusof BN, et al. Independent and combined effects of age, body mass index and gestational weight gain on the risk of gestational diabetes mellitus. Sci Rep. 2020;10:8486. doi: 10.1038/s41598-020-65251-2
15. Phelan S. Windows of opportunity for lifestyle interventions to prevent gestational diabetes mellitus. Am J Perinatol. 2016;33:1291-1299. doi: 10.1055/s-0036-1586504
16. Koliaki C, Spinos T, Spinou M, et al. Defining the optimal dietary approach for safe, effective and sustainable weight loss in overweight and obese adults. Healthcare (Basel). 2018;6:73. doi: 10.3390/healthcare6030073
17. Al Wattar BH, Dodds J, Placzek A, et al. Mediterranean-style diet in pregnant women with metabolic risk factors (ESTEEM): a pragmatic multicentre randomised trial. PLOS Med. 2019;16:e1002857. doi: 10.1371/journal.pmed.1002857
18. Zarogiannis S. Are novel lifestyle approaches to management of type 2 diabetes applicable to prevention and treatment of women with gestational diabetes mellitus? Global Diabetes Open Access J. 2019;1:1-14.
19. Most J, Amant MS, Hsia DS, et al. Evidence-based recommendations for energy intake in pregnant women with obesity. J Clin Invest. 2019;129:4682-4690. doi: 10.1172/JCI130341
20. American Diabetes Association. 14. Management of diabetes in pregnancy: Standards of Medical Care in Diabetes—2021. Diabetes Care. 2021;44(suppl 1):S200-S210. doi: 10.2337/dc21-S014
21. McIntyre HD, Sacks DA, Barbour LA, et al. Issues with the diagnosis and classification of hyperglycemia in early pregnancy. Diabetes Care. 2016;39:53-54. doi: 10.2337/dc15-1887
22. American Diabetes Association. 2. Classification and diagnosis of diabetes: Standards of Medical Care in Diabetes—2022. Diabetes Care. 2022;45(suppl 1):S17-S38. doi: 10.2337/dc22-S002
23. Carpenter MW, Coustan DR. Criteria for screening tests for gestational diabetes. Am J Obstet Gynecol. 1982;144:768-773. doi: 10.1016/0002-9378(82)90349-0
24. ACOG Practice Bulletin No. 190: gestational diabetes mellitus. Obstet Gynecol. 2018;131:e49-e64. doi: 10.1097/AOG.0000000000002501
25. Hillier TA, Pedula KL, Ogasawara KK, et al. A pragmatic, randomized clinical trial of gestational diabetes screening. N Engl J Med. 2021;384:895-904. doi: 10.1056/NEJMoa2026028
26. Metzger BE, Buchanan TA, Coustan DR, et al. Summary and recommendations of the Fifth International Workshop-Conference on Gestational Diabetes Mellitus. Diabetes Care. 2007;30(suppl 2):S251-S260. doi: 10.2337/dc07-s225
27. Nielsen LR, Ekbom P, Damm P, et al. HbA1c levels are significantly lower in early and late pregnancy. Diabetes Care. 2004;27:1200-1201. doi: 10.2337/diacare.27.5.1200
28. Abell SK, Boyle JA, de Courten B, et al. Impact of type 2 diabetes, obesity and glycaemic control on pregnancy outcomes. Aust N Z J Obstet Gynaecol. 2017;57:308-314. doi: 10.1111/ajo.12521
29. Viana LV, Gross JL, Azevedo MJ. Dietary intervention in patients with gestational diabetes mellitus: a systematic review and meta-analysis of randomized clinical trials on maternal and newborn outcomes. Diabetes Care. 2014;37:3345-3355. doi: 10.2337/dc14-1530
30. Rowan JA, Rush EC, Plank LD, et al. Metformin in gestational diabetes: the offspring follow-up (MiG TOFU): body composition and metabolic outcomes at 7-9 years of age. BMJ Open Diabetes Res Care. 2018;6:e000456. doi: 10.1136/bmjdrc-2017-000456
31. Hebert MF, Ma X, Naraharisetti SB, et al. Are we optimizing gestational diabetes treatment with glyburide? The pharmacologic basis for better clinical practice. Clin Pharmacol Ther. 2009;85:607-614. doi: 10.1038/clpt.2009.5
32. Malek R, Davis SN. Pharmacokinetics, efficacy and safety of glyburide for treatment of gestational diabetes mellitus. Expert Opin Drug Metab Toxicol. 2016;12:691-699. doi: 10.1080/17425255.2016.1187131
33. Balsells M, García-Patterson A, Solà I, et al. Glibenclamide, metformin, and insulin for the treatment of gestational diabetes: a systematic review and meta-analysis. BMJ. 2015;350:h102. doi: 10.1136/bmj.h102
34. Kavitha N, De S, Kanagasabai S. Oral hypoglycemic agents in pregnancy: an update. J Obstet Gynaecol India. 2013;63:82-87. doi: 10.1007/s13224-012-0312-z
35. Weissgerber TL, Mudd LM. Preeclampsia and diabetes. Curr Diab Rep. 2015;15:9. doi: 10.1007/s11892-015-0579-4
36. Kamana KC, Shakya S, Zhang H. Gestational diabetes mellitus and macrosomia: a literature review. Ann Nutr Metab. 2015;66(suppl 2):14-20. doi: 10.1159/000371628
37. Mitanchez D, Yzydorczyk C, Simeoni U. What neonatal complications should the pediatrician be aware of in case of maternal gestational diabetes? World J Diabetes. 2015;6:734-743. doi: 10.4239/wjd.v6.i5.734
38. Stanescu A, Stoicescu SM. Neonatal hypoglycemia screening in newborns from diabetic mothers—arguments and controversies. J Med Life. 2014;7(spec iss 3):51-52.
39. Kim C. Maternal outcomes and follow-up after gestational diabetes mellitus. Diabet Med. 2014;31:292-301. doi: 10.1111/dme.12382
40. Stewart A, Malhotra A. Gestational diabetes and the neonate: challenges and solutions. Res Rep Neonatol. 2015;5:31-39. doi: 10.2147/RRN.S30971
41. Crume TL, Ogden L, West NA, et al. Association of exposure to diabetes in utero with adiposity and fat distribution in a multiethnic population of youth: the Exploring Perinatal Outcomes among Children (EPOCH) Study. Diabetologia. 2011;54:87-92. doi: 10.1007/s00125-010-1925-3
42. Crume TL, Ogden L, Daniels S, et al. The impact of in utero exposure to diabetes on childhood body mass index growth trajectories: the EPOCH study. J Pediatr. 2011;158:941-946. doi: 10.1016/j.jpeds.2010.12.007
43. Levels of maternal care. Obstetric Care Consensus No. 9. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2019;134:e41-e55. doi: 10.1097/AOG.0000000000003383
44. Caughey AB, Cheng YW, Stotland NE, et al. Maternal and paternal race/ethnicity are both associated with gestational diabetes. Am J Obstet Gynecol. 2010;202:616.e1-e5. doi: 10.1016/j.ajog.2010.01.082
45. Yogev Y, Xenakis EM, Langer O. The association between preeclampsia and severity of gestational diabetes: the impact of glycemic control. Am J Obstet Gynecol. 2004;191:1655-1660. doi: 10.1016/j.ajog.2004.03.074
46. Brown J, Alwan NA, West J, et al. Lifestyle interventions for the treatment of women with gestational diabetes. Cochrane Database Syst Rev. 2017;5:CD011970. doi: 10.1002/14651858.CD011970.pub2
47. Ceysens G, Rouiller D, Boulvain M. Exercise for the diabetic pregnant woman. Cochrane Database Syst Rev. 2006;3:CD004225. doi: 10.1002/14651858.CD004225.pub2
48. Chawla R, Mukherjee JJ, Chawla M, et al. Expert group recommendations on the effective use of bolus insulin in the management of type 2 diabetes mellitus. Med Sci (Basel). 2021;9:38. doi: 10.3390/medsci9020038
49. American Diabetes Association. Introduction: Standards of Medical Care in Diabetes—2021. Diabetes Care. 2021;44(suppl 1):S1-S2. doi: 10.2337/dc22-Sint
PRACTICE RECOMMENDATIONS
› Manage gestational diabetes mellitus (GDM) with lifestyle behavior changes first and add insulin as a secondary treatment only if glycemic targets are not being met. A
› Treat hyperglycemia in GDM with insulin, not metformin or glyburide; these agents cross the placenta to the fetus. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series