User login
Pseudofolliculitis Barbae in the Military: Policy, Stigma, and Practical Solutions
Pseudofolliculitis Barbae in the Military: Policy, Stigma, and Practical Solutions
The impact of pseudofolliculitis barbae (PFB) on military service members and other uniformed professionals has been a topic of recent interest due to the announcement of the US Army’s new shaving rule in July 2025.1 The policy prohibits permanent shaving waivers, requires medical re-evaluation of shaving profiles within 90 days, and allows for administrative separation if a service member accumulates shaving exceptions totaling more than 12 months over a 24-month period.2 A common skin condition triggered or worsened by shaving, PFB causes painful bumps, pustules, and hyperpigmentation most often in the beard and cheek areas and negatively impacts quality of life. It disproportionately affects 45% to 83% of men in the United States, particularly those of African, Hispanic, or Middle Eastern descent.3,4 Genetic factors, particularly tightly coiled or coarse curly hair, can predispose individuals to PFB. The most successful treatment for PFB is to stop shaving, but this conflicts with military shaving standards and interferes with the use of protective equipment (eg, masks). Herein, we highlight the adverse impact of PFB on military career progression and provide context for clinicians who treat patients with PFB, especially as policies recently have shifted to allow nonmilitary clinicians to evaluate PFB in service members.5
Shaving Waivers and Advancement
Pseudofolliculitis barbae disproportionately prolongs the time to advancement of many service members, and those with PFB also are overburdened by policy changes related to shaving.6 In the US military, nearly 18% of the active-duty force is Black,7 a population that is more susceptible to PFB. Military personnel may request PFB-related accommodations, including medical shaving waivers that vary by branch. Through a formal documentation process, waivers allow service members to maintain facial hair up to one-quarter inch in length.5 Previously, waivers could be temporary (eg, up to 90 days) or permanent as subjectively determined based on clinician-documented disease severity. Almost 65% of US Air Force medical shaving waivers are held by Black men, and PFB is one of the most common reasons.6 Notably, the US Navy discontinued permanent shaving waivers in October 2019.8 A US Marine Corps policy issued in March 2025 now allows administrative separation of service members with PFB if symptoms do not improve after a 1-year medical shaving waiver due to “incompatibility with service.”9 This change reversed a 2022 policy that protected Marines from separation based on PFB.10 A Marine Corps spokesperson stated that this change aims to clarify how medical conditions can impact uniform compliance and standardize medical condition management while prioritizing compliance and duty readiness.1
Even in the absence of policy changes, obtaining a medical shaving waiver for PFB can be challenging. Service members may have little to no access to military dermatologists who specialize in management of PFB and experience long wait times for civilian network deferment. Service members seen in civilian clinics may have restricted treatment options due to limited insurance coverage for laser hair reduction, even in the most difficult-to-manage areas (eg, neck, jawline). Expanding access to military dermatologists, civilian dermatologists who are experienced with PFB and understand the impact and necessity of military waivers, and teledermatology services could help improve and streamline care. Other challenges include the subjective nature of documenting PFB disease severity, the need for validated assessment tools, a lack of standardized policies across military branches, and stigma. A standardized approach to documentation may reduce variability in how shaving waivers are evaluated across service branches, but at a minimum, clinicians should document the diagnosis, clinical findings, severity of PFB, and the treatment used. Having a waiver would help these service members focus on mastering critical skillsets and performing duties without the time pressures, angst, and expense dedicated to caring for and managing PFB.
Clinical and Policy Barriers
Unfortunately, service members with PFB or shaving waivers often face stigma that can hinder career advancement.6 In a recent analysis of 9339 US Air Force personnel, those with shaving waivers experienced longer times to promotion compared to those without waivers: in the waiver group, 94.47% were enlisted and 5.53% were officers; in the nonwaiver group, 72.11% were enlisted and 27.89% were officers (P=.0003).6 While delays in promotion were consistent across racial groups, most of the waiver holders identified as Black (64.8%), despite this demographic group representing only a small portion of the overall cohort (12.9%).6 Promotion delays may be linked to perceptions of unprofessionalism and exclusion from high-profile assignments, which notably require “the highest standards of military appearance and professional conduct.”11 The burden of career-limiting shaving policies falls disproportionately on military personnel with PFB who self-identify as Black. Perceptions about unprofessional appearance or job readiness often unintentionally introduce bias, unjustly restricting career advancement.6
Safety Equipment and Shaving Standards
Conditions that potentially affect the use of masks and chemical defense equipment extend beyond the military. Firefighters and law enforcement officers generally are required to maintain a clean-shaven face for proper fit of respirator masks; the standard is that no respirator fit test shall be conducted if hair—including stubble, beards, mustaches, or sideburns—grows between the skin and the facepiece sealing surface, and any apparel interfering with a proper seal must be altered or removed.12 This creates challenges for uniformed professionals with PFB who must manage their condition while adhering to safety requirements. Some endure long-term pain and scarring in order to comply, while others seek waivers to treat and prevent symptoms while also facing the stigma of doing so.13 One of the most effective treatments for PFB is to discontinue shaving,14 which may not be feasible for those in uniformed professions with strict grooming standards. Research on mask seal effectiveness in individuals with neatly trimmed beards or PFB remains limited.5 Studies evaluating mask fit across facial hair types and lengths are needed, along with the development of protective equipment that accommodates career-limiting conditions such as PFB, cystic acne, and acne keloidalis nuchae. This also may encourage development of equipment that does not induce such conditions (eg, mechanical acne from friction). These efforts would promote safety, scientific innovation for dermatologic follicular-based disorders, and overall quality of life for service members as well as increase their ability to serve without stigma. These developments also would positively impact other fields that require intermittent or full-time use of masks, including health care and some food service industries.
Final Thoughts
The disproportionate impact of PFB in the military highlights the need for improved access to treatment, culturally informed care, and policies that avoid penalizing service members with tightly coiled hair and a desire to serve. We discussed PFB management strategies, clinical features, and implications across various skin tones in a previous publication.14 It is important to consider insights from individuals with PFB who are serving in the military as well as the medical personnel who care for them. Ensuring or creating effective treatment options drives innovation, and evidence-based accommodation plans can help individuals in uniformed professions avoid choosing between PFB management and their career. Promoting awareness about the impact of PFB beyond the razor is key to reducing disparities and supporting excellence among those who serve and desire to continue to do so.
- Lawrence DF. Marines with skin condition affecting mostly black men could now be booted under new policy. Military.com. March 14, 2025. Accessed May 4, 2025. https://www.military.com/daily-news/2025/03/14/marines-can-now-be-kicked-out-skin-condition-affects-mostly-black-men.html
- Secretary of the Army. Army directive 2025-13 (facial hair grooming standards). Published July 7, 2025. Accessed September 19, 2025. https://lyster.tricare.mil/Portals/61/ARN44307-ARMY_DIR_2025-13-000.pdf
- Alexis A, Heath CR, Halder RM. Folliculitis keloidalis nuchae and pseudofolliculitis barbae: are prevention and effective treatment within reach? Dermatol Clin. 2014;32:183-191. doi:10.1016/j.det.2013.12.001
- Gray J, McMichael AJ. Pseudofolliculitis barbae: understanding the condition and the role of facial grooming. Int J Cosmet Sci. 2016;38:24-27. doi:10.1111/ics.12331
- Jung I, Lannan FM, Weiss A, et al. Treatment and current policies on pseudofolliculitis barbae in the US military. Cutis. 2023;112:299-302. doi:10.12788/cutis.0907
- Ritchie S, Park J, Banta J, et al. Shaving waivers in the United States Air Force and their impact on promotions of Black/African-American members. Mil Med. 2023;188:E242-E247. doi:10.1093/milmed/usab272
- Defense Manpower Data Center. Active-duty military personnel master file and reserve components common personnel data system. Military OneSource. September 2023. Accessed May 3, 2025. https://download.militaryonesource.mil/12038/MOS/Reports/2023-demographics-report.pdf
- Tshudy MT, Cho S. Pseudofolliculitis barbae in the US. Military, a review. Mil Med. 2021;186:E52-E57. doi:10.1093/milmed/usaa243
- US Marine Corps. Uniform and grooming standards for medical conditions (MARADMINS number: 124/25). Published March 13, 2025. Accessed September 19, 2025. https://www.marines.mil/News/Messages/Messages-Display/Article/4119098/uniform-and-grooming-standards-for-medical-conditions/
- US Marine Corps. Advance notification of change to MCO 6310.1C (Pseudofolliculitis Barbae), MCO 1900.16 CH2 (Marine Corps Retirement and Separation Manual), and MCO 1040.31 (Enlisted Retention and Career Development Program). Published January 21, 2022. Accessed September 19, 2025. https://www.marines.mil/News/Messages/Messages-Display/Article/2907104/advance-notification-of-change-to-mco-63101c-pseudofolliculitis-barbae-mco-1900/
- US Department of Defense. Special duty catalog (SPECAT). Published August 15, 2013. Accessed September 19, 2025. https://share.google/iuMrVMIASWx4EFLVN
- Occupational Safety and Health Administration. Appendix A to §1910.134—fit testing procedures (mandatory). Accessed September 19, 2025. https://www.osha.gov/laws-regs/regulations/standardnumber/1910/1910.134AppA
- Jiang YR. Reasonable accommodation and disparate impact: clean shave policy discrimination in today’s workplace. J Law Med Ethics. 2023;51:185-195. doi:10.1017/jme.2023.55
- Welch D, Usatine R, Heath C. Implications of PFB beyond the razor. Cutis. 2025;115:135-136. doi:10.12788/cutis.1194
The impact of pseudofolliculitis barbae (PFB) on military service members and other uniformed professionals has been a topic of recent interest due to the announcement of the US Army’s new shaving rule in July 2025.1 The policy prohibits permanent shaving waivers, requires medical re-evaluation of shaving profiles within 90 days, and allows for administrative separation if a service member accumulates shaving exceptions totaling more than 12 months over a 24-month period.2 A common skin condition triggered or worsened by shaving, PFB causes painful bumps, pustules, and hyperpigmentation most often in the beard and cheek areas and negatively impacts quality of life. It disproportionately affects 45% to 83% of men in the United States, particularly those of African, Hispanic, or Middle Eastern descent.3,4 Genetic factors, particularly tightly coiled or coarse curly hair, can predispose individuals to PFB. The most successful treatment for PFB is to stop shaving, but this conflicts with military shaving standards and interferes with the use of protective equipment (eg, masks). Herein, we highlight the adverse impact of PFB on military career progression and provide context for clinicians who treat patients with PFB, especially as policies recently have shifted to allow nonmilitary clinicians to evaluate PFB in service members.5
Shaving Waivers and Advancement
Pseudofolliculitis barbae disproportionately prolongs the time to advancement of many service members, and those with PFB also are overburdened by policy changes related to shaving.6 In the US military, nearly 18% of the active-duty force is Black,7 a population that is more susceptible to PFB. Military personnel may request PFB-related accommodations, including medical shaving waivers that vary by branch. Through a formal documentation process, waivers allow service members to maintain facial hair up to one-quarter inch in length.5 Previously, waivers could be temporary (eg, up to 90 days) or permanent as subjectively determined based on clinician-documented disease severity. Almost 65% of US Air Force medical shaving waivers are held by Black men, and PFB is one of the most common reasons.6 Notably, the US Navy discontinued permanent shaving waivers in October 2019.8 A US Marine Corps policy issued in March 2025 now allows administrative separation of service members with PFB if symptoms do not improve after a 1-year medical shaving waiver due to “incompatibility with service.”9 This change reversed a 2022 policy that protected Marines from separation based on PFB.10 A Marine Corps spokesperson stated that this change aims to clarify how medical conditions can impact uniform compliance and standardize medical condition management while prioritizing compliance and duty readiness.1
Even in the absence of policy changes, obtaining a medical shaving waiver for PFB can be challenging. Service members may have little to no access to military dermatologists who specialize in management of PFB and experience long wait times for civilian network deferment. Service members seen in civilian clinics may have restricted treatment options due to limited insurance coverage for laser hair reduction, even in the most difficult-to-manage areas (eg, neck, jawline). Expanding access to military dermatologists, civilian dermatologists who are experienced with PFB and understand the impact and necessity of military waivers, and teledermatology services could help improve and streamline care. Other challenges include the subjective nature of documenting PFB disease severity, the need for validated assessment tools, a lack of standardized policies across military branches, and stigma. A standardized approach to documentation may reduce variability in how shaving waivers are evaluated across service branches, but at a minimum, clinicians should document the diagnosis, clinical findings, severity of PFB, and the treatment used. Having a waiver would help these service members focus on mastering critical skillsets and performing duties without the time pressures, angst, and expense dedicated to caring for and managing PFB.
Clinical and Policy Barriers
Unfortunately, service members with PFB or shaving waivers often face stigma that can hinder career advancement.6 In a recent analysis of 9339 US Air Force personnel, those with shaving waivers experienced longer times to promotion compared to those without waivers: in the waiver group, 94.47% were enlisted and 5.53% were officers; in the nonwaiver group, 72.11% were enlisted and 27.89% were officers (P=.0003).6 While delays in promotion were consistent across racial groups, most of the waiver holders identified as Black (64.8%), despite this demographic group representing only a small portion of the overall cohort (12.9%).6 Promotion delays may be linked to perceptions of unprofessionalism and exclusion from high-profile assignments, which notably require “the highest standards of military appearance and professional conduct.”11 The burden of career-limiting shaving policies falls disproportionately on military personnel with PFB who self-identify as Black. Perceptions about unprofessional appearance or job readiness often unintentionally introduce bias, unjustly restricting career advancement.6
Safety Equipment and Shaving Standards
Conditions that potentially affect the use of masks and chemical defense equipment extend beyond the military. Firefighters and law enforcement officers generally are required to maintain a clean-shaven face for proper fit of respirator masks; the standard is that no respirator fit test shall be conducted if hair—including stubble, beards, mustaches, or sideburns—grows between the skin and the facepiece sealing surface, and any apparel interfering with a proper seal must be altered or removed.12 This creates challenges for uniformed professionals with PFB who must manage their condition while adhering to safety requirements. Some endure long-term pain and scarring in order to comply, while others seek waivers to treat and prevent symptoms while also facing the stigma of doing so.13 One of the most effective treatments for PFB is to discontinue shaving,14 which may not be feasible for those in uniformed professions with strict grooming standards. Research on mask seal effectiveness in individuals with neatly trimmed beards or PFB remains limited.5 Studies evaluating mask fit across facial hair types and lengths are needed, along with the development of protective equipment that accommodates career-limiting conditions such as PFB, cystic acne, and acne keloidalis nuchae. This also may encourage development of equipment that does not induce such conditions (eg, mechanical acne from friction). These efforts would promote safety, scientific innovation for dermatologic follicular-based disorders, and overall quality of life for service members as well as increase their ability to serve without stigma. These developments also would positively impact other fields that require intermittent or full-time use of masks, including health care and some food service industries.
Final Thoughts
The disproportionate impact of PFB in the military highlights the need for improved access to treatment, culturally informed care, and policies that avoid penalizing service members with tightly coiled hair and a desire to serve. We discussed PFB management strategies, clinical features, and implications across various skin tones in a previous publication.14 It is important to consider insights from individuals with PFB who are serving in the military as well as the medical personnel who care for them. Ensuring or creating effective treatment options drives innovation, and evidence-based accommodation plans can help individuals in uniformed professions avoid choosing between PFB management and their career. Promoting awareness about the impact of PFB beyond the razor is key to reducing disparities and supporting excellence among those who serve and desire to continue to do so.
The impact of pseudofolliculitis barbae (PFB) on military service members and other uniformed professionals has been a topic of recent interest due to the announcement of the US Army’s new shaving rule in July 2025.1 The policy prohibits permanent shaving waivers, requires medical re-evaluation of shaving profiles within 90 days, and allows for administrative separation if a service member accumulates shaving exceptions totaling more than 12 months over a 24-month period.2 A common skin condition triggered or worsened by shaving, PFB causes painful bumps, pustules, and hyperpigmentation most often in the beard and cheek areas and negatively impacts quality of life. It disproportionately affects 45% to 83% of men in the United States, particularly those of African, Hispanic, or Middle Eastern descent.3,4 Genetic factors, particularly tightly coiled or coarse curly hair, can predispose individuals to PFB. The most successful treatment for PFB is to stop shaving, but this conflicts with military shaving standards and interferes with the use of protective equipment (eg, masks). Herein, we highlight the adverse impact of PFB on military career progression and provide context for clinicians who treat patients with PFB, especially as policies recently have shifted to allow nonmilitary clinicians to evaluate PFB in service members.5
Shaving Waivers and Advancement
Pseudofolliculitis barbae disproportionately prolongs the time to advancement of many service members, and those with PFB also are overburdened by policy changes related to shaving.6 In the US military, nearly 18% of the active-duty force is Black,7 a population that is more susceptible to PFB. Military personnel may request PFB-related accommodations, including medical shaving waivers that vary by branch. Through a formal documentation process, waivers allow service members to maintain facial hair up to one-quarter inch in length.5 Previously, waivers could be temporary (eg, up to 90 days) or permanent as subjectively determined based on clinician-documented disease severity. Almost 65% of US Air Force medical shaving waivers are held by Black men, and PFB is one of the most common reasons.6 Notably, the US Navy discontinued permanent shaving waivers in October 2019.8 A US Marine Corps policy issued in March 2025 now allows administrative separation of service members with PFB if symptoms do not improve after a 1-year medical shaving waiver due to “incompatibility with service.”9 This change reversed a 2022 policy that protected Marines from separation based on PFB.10 A Marine Corps spokesperson stated that this change aims to clarify how medical conditions can impact uniform compliance and standardize medical condition management while prioritizing compliance and duty readiness.1
Even in the absence of policy changes, obtaining a medical shaving waiver for PFB can be challenging. Service members may have little to no access to military dermatologists who specialize in management of PFB and experience long wait times for civilian network deferment. Service members seen in civilian clinics may have restricted treatment options due to limited insurance coverage for laser hair reduction, even in the most difficult-to-manage areas (eg, neck, jawline). Expanding access to military dermatologists, civilian dermatologists who are experienced with PFB and understand the impact and necessity of military waivers, and teledermatology services could help improve and streamline care. Other challenges include the subjective nature of documenting PFB disease severity, the need for validated assessment tools, a lack of standardized policies across military branches, and stigma. A standardized approach to documentation may reduce variability in how shaving waivers are evaluated across service branches, but at a minimum, clinicians should document the diagnosis, clinical findings, severity of PFB, and the treatment used. Having a waiver would help these service members focus on mastering critical skillsets and performing duties without the time pressures, angst, and expense dedicated to caring for and managing PFB.
Clinical and Policy Barriers
Unfortunately, service members with PFB or shaving waivers often face stigma that can hinder career advancement.6 In a recent analysis of 9339 US Air Force personnel, those with shaving waivers experienced longer times to promotion compared to those without waivers: in the waiver group, 94.47% were enlisted and 5.53% were officers; in the nonwaiver group, 72.11% were enlisted and 27.89% were officers (P=.0003).6 While delays in promotion were consistent across racial groups, most of the waiver holders identified as Black (64.8%), despite this demographic group representing only a small portion of the overall cohort (12.9%).6 Promotion delays may be linked to perceptions of unprofessionalism and exclusion from high-profile assignments, which notably require “the highest standards of military appearance and professional conduct.”11 The burden of career-limiting shaving policies falls disproportionately on military personnel with PFB who self-identify as Black. Perceptions about unprofessional appearance or job readiness often unintentionally introduce bias, unjustly restricting career advancement.6
Safety Equipment and Shaving Standards
Conditions that potentially affect the use of masks and chemical defense equipment extend beyond the military. Firefighters and law enforcement officers generally are required to maintain a clean-shaven face for proper fit of respirator masks; the standard is that no respirator fit test shall be conducted if hair—including stubble, beards, mustaches, or sideburns—grows between the skin and the facepiece sealing surface, and any apparel interfering with a proper seal must be altered or removed.12 This creates challenges for uniformed professionals with PFB who must manage their condition while adhering to safety requirements. Some endure long-term pain and scarring in order to comply, while others seek waivers to treat and prevent symptoms while also facing the stigma of doing so.13 One of the most effective treatments for PFB is to discontinue shaving,14 which may not be feasible for those in uniformed professions with strict grooming standards. Research on mask seal effectiveness in individuals with neatly trimmed beards or PFB remains limited.5 Studies evaluating mask fit across facial hair types and lengths are needed, along with the development of protective equipment that accommodates career-limiting conditions such as PFB, cystic acne, and acne keloidalis nuchae. This also may encourage development of equipment that does not induce such conditions (eg, mechanical acne from friction). These efforts would promote safety, scientific innovation for dermatologic follicular-based disorders, and overall quality of life for service members as well as increase their ability to serve without stigma. These developments also would positively impact other fields that require intermittent or full-time use of masks, including health care and some food service industries.
Final Thoughts
The disproportionate impact of PFB in the military highlights the need for improved access to treatment, culturally informed care, and policies that avoid penalizing service members with tightly coiled hair and a desire to serve. We discussed PFB management strategies, clinical features, and implications across various skin tones in a previous publication.14 It is important to consider insights from individuals with PFB who are serving in the military as well as the medical personnel who care for them. Ensuring or creating effective treatment options drives innovation, and evidence-based accommodation plans can help individuals in uniformed professions avoid choosing between PFB management and their career. Promoting awareness about the impact of PFB beyond the razor is key to reducing disparities and supporting excellence among those who serve and desire to continue to do so.
- Lawrence DF. Marines with skin condition affecting mostly black men could now be booted under new policy. Military.com. March 14, 2025. Accessed May 4, 2025. https://www.military.com/daily-news/2025/03/14/marines-can-now-be-kicked-out-skin-condition-affects-mostly-black-men.html
- Secretary of the Army. Army directive 2025-13 (facial hair grooming standards). Published July 7, 2025. Accessed September 19, 2025. https://lyster.tricare.mil/Portals/61/ARN44307-ARMY_DIR_2025-13-000.pdf
- Alexis A, Heath CR, Halder RM. Folliculitis keloidalis nuchae and pseudofolliculitis barbae: are prevention and effective treatment within reach? Dermatol Clin. 2014;32:183-191. doi:10.1016/j.det.2013.12.001
- Gray J, McMichael AJ. Pseudofolliculitis barbae: understanding the condition and the role of facial grooming. Int J Cosmet Sci. 2016;38:24-27. doi:10.1111/ics.12331
- Jung I, Lannan FM, Weiss A, et al. Treatment and current policies on pseudofolliculitis barbae in the US military. Cutis. 2023;112:299-302. doi:10.12788/cutis.0907
- Ritchie S, Park J, Banta J, et al. Shaving waivers in the United States Air Force and their impact on promotions of Black/African-American members. Mil Med. 2023;188:E242-E247. doi:10.1093/milmed/usab272
- Defense Manpower Data Center. Active-duty military personnel master file and reserve components common personnel data system. Military OneSource. September 2023. Accessed May 3, 2025. https://download.militaryonesource.mil/12038/MOS/Reports/2023-demographics-report.pdf
- Tshudy MT, Cho S. Pseudofolliculitis barbae in the US. Military, a review. Mil Med. 2021;186:E52-E57. doi:10.1093/milmed/usaa243
- US Marine Corps. Uniform and grooming standards for medical conditions (MARADMINS number: 124/25). Published March 13, 2025. Accessed September 19, 2025. https://www.marines.mil/News/Messages/Messages-Display/Article/4119098/uniform-and-grooming-standards-for-medical-conditions/
- US Marine Corps. Advance notification of change to MCO 6310.1C (Pseudofolliculitis Barbae), MCO 1900.16 CH2 (Marine Corps Retirement and Separation Manual), and MCO 1040.31 (Enlisted Retention and Career Development Program). Published January 21, 2022. Accessed September 19, 2025. https://www.marines.mil/News/Messages/Messages-Display/Article/2907104/advance-notification-of-change-to-mco-63101c-pseudofolliculitis-barbae-mco-1900/
- US Department of Defense. Special duty catalog (SPECAT). Published August 15, 2013. Accessed September 19, 2025. https://share.google/iuMrVMIASWx4EFLVN
- Occupational Safety and Health Administration. Appendix A to §1910.134—fit testing procedures (mandatory). Accessed September 19, 2025. https://www.osha.gov/laws-regs/regulations/standardnumber/1910/1910.134AppA
- Jiang YR. Reasonable accommodation and disparate impact: clean shave policy discrimination in today’s workplace. J Law Med Ethics. 2023;51:185-195. doi:10.1017/jme.2023.55
- Welch D, Usatine R, Heath C. Implications of PFB beyond the razor. Cutis. 2025;115:135-136. doi:10.12788/cutis.1194
- Lawrence DF. Marines with skin condition affecting mostly black men could now be booted under new policy. Military.com. March 14, 2025. Accessed May 4, 2025. https://www.military.com/daily-news/2025/03/14/marines-can-now-be-kicked-out-skin-condition-affects-mostly-black-men.html
- Secretary of the Army. Army directive 2025-13 (facial hair grooming standards). Published July 7, 2025. Accessed September 19, 2025. https://lyster.tricare.mil/Portals/61/ARN44307-ARMY_DIR_2025-13-000.pdf
- Alexis A, Heath CR, Halder RM. Folliculitis keloidalis nuchae and pseudofolliculitis barbae: are prevention and effective treatment within reach? Dermatol Clin. 2014;32:183-191. doi:10.1016/j.det.2013.12.001
- Gray J, McMichael AJ. Pseudofolliculitis barbae: understanding the condition and the role of facial grooming. Int J Cosmet Sci. 2016;38:24-27. doi:10.1111/ics.12331
- Jung I, Lannan FM, Weiss A, et al. Treatment and current policies on pseudofolliculitis barbae in the US military. Cutis. 2023;112:299-302. doi:10.12788/cutis.0907
- Ritchie S, Park J, Banta J, et al. Shaving waivers in the United States Air Force and their impact on promotions of Black/African-American members. Mil Med. 2023;188:E242-E247. doi:10.1093/milmed/usab272
- Defense Manpower Data Center. Active-duty military personnel master file and reserve components common personnel data system. Military OneSource. September 2023. Accessed May 3, 2025. https://download.militaryonesource.mil/12038/MOS/Reports/2023-demographics-report.pdf
- Tshudy MT, Cho S. Pseudofolliculitis barbae in the US. Military, a review. Mil Med. 2021;186:E52-E57. doi:10.1093/milmed/usaa243
- US Marine Corps. Uniform and grooming standards for medical conditions (MARADMINS number: 124/25). Published March 13, 2025. Accessed September 19, 2025. https://www.marines.mil/News/Messages/Messages-Display/Article/4119098/uniform-and-grooming-standards-for-medical-conditions/
- US Marine Corps. Advance notification of change to MCO 6310.1C (Pseudofolliculitis Barbae), MCO 1900.16 CH2 (Marine Corps Retirement and Separation Manual), and MCO 1040.31 (Enlisted Retention and Career Development Program). Published January 21, 2022. Accessed September 19, 2025. https://www.marines.mil/News/Messages/Messages-Display/Article/2907104/advance-notification-of-change-to-mco-63101c-pseudofolliculitis-barbae-mco-1900/
- US Department of Defense. Special duty catalog (SPECAT). Published August 15, 2013. Accessed September 19, 2025. https://share.google/iuMrVMIASWx4EFLVN
- Occupational Safety and Health Administration. Appendix A to §1910.134—fit testing procedures (mandatory). Accessed September 19, 2025. https://www.osha.gov/laws-regs/regulations/standardnumber/1910/1910.134AppA
- Jiang YR. Reasonable accommodation and disparate impact: clean shave policy discrimination in today’s workplace. J Law Med Ethics. 2023;51:185-195. doi:10.1017/jme.2023.55
- Welch D, Usatine R, Heath C. Implications of PFB beyond the razor. Cutis. 2025;115:135-136. doi:10.12788/cutis.1194
Pseudofolliculitis Barbae in the Military: Policy, Stigma, and Practical Solutions
Pseudofolliculitis Barbae in the Military: Policy, Stigma, and Practical Solutions
Tender Nodule on the Umbilicus
Tender Nodule on the Umbilicus
THE DIAGNOSIS: Villar Nodule
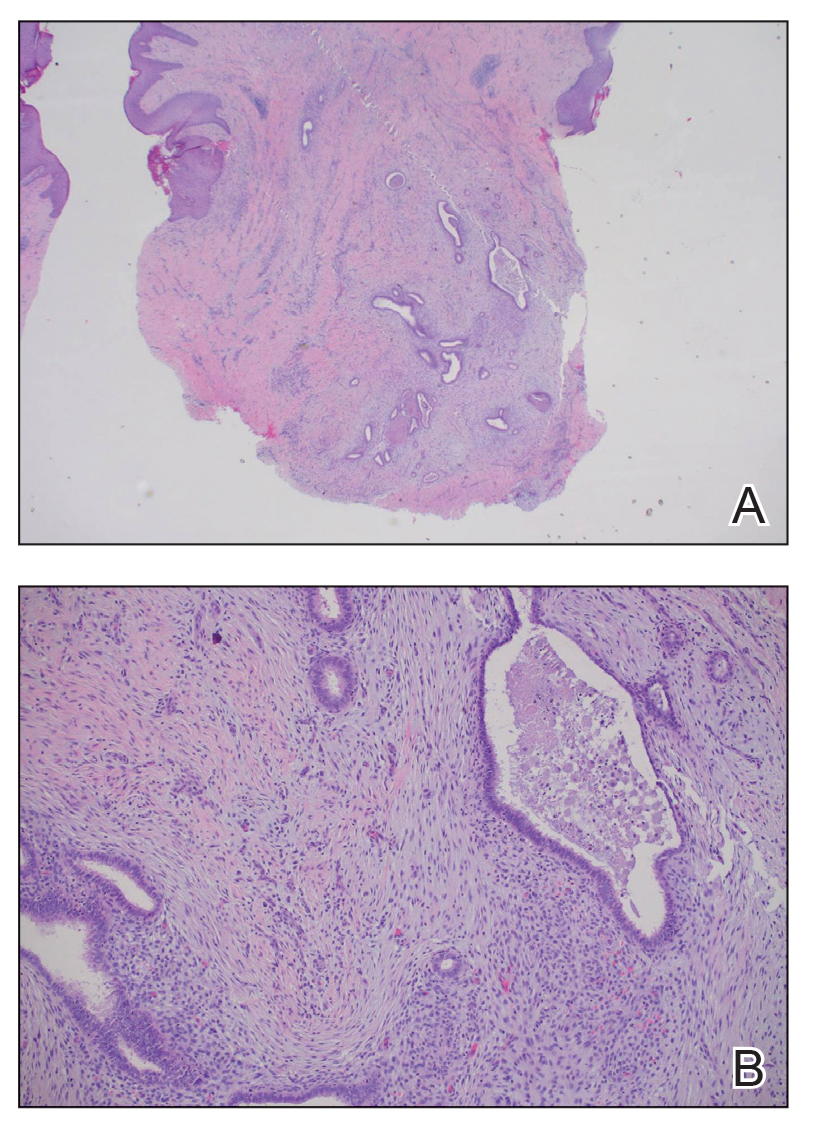
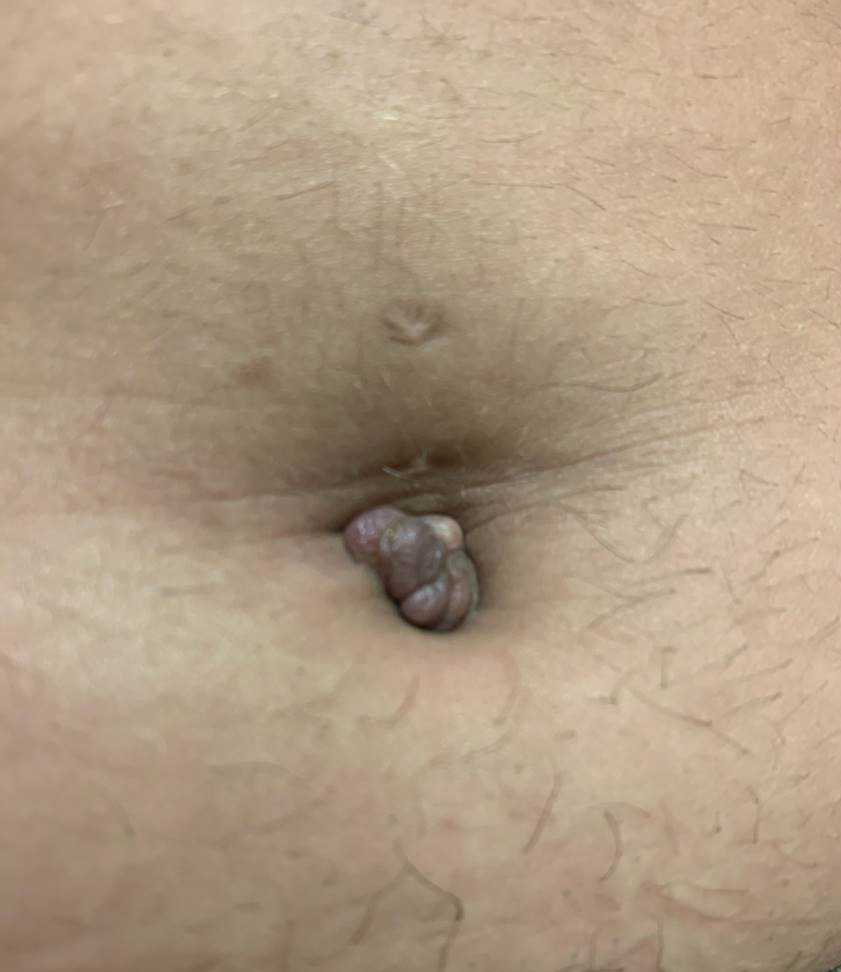
The biopsy revealed features consistent with cutaneous endometriosis in the setting of a painful, tender, multilobulated nodule with a cyclical bleeding pattern (Figure 1). The bleeding pattern of the nodule during menses and lack of surgical history supported the diagnosis of primary cutaneous endometriosis in our patient. She was diagnosed with endometriosis by gynecology, and her primary care physician started her on an oral contraceptive based on this diagnosis. She also was referred to gynecology and plastic surgery for a joint surgical consultation to remove the nodule. She initially decided to do a trial of the oral contraceptive but subsequently underwent umbilical endometrioma excision with neo-umbilicus creation with no evidence of recurrence.
Primary cutaneous endometriosis should be considered in young females who present with tender umbilical nodules. Endometriosis refers to the presence of an endometriumlike epithelium outside the endometrium and myometrium.1 The condition affects 10% to 15% of reproductive-aged (ie, 18-49 years) women in the United States and typically involves tissues within the pelvis, such as the ovaries, pouch of Douglas, or pelvic ligaments.2 Cutaneous endometriosis is the growth of endometrial tissue in the skin and is rare, accounting for less than 5.5% of cases of extrapelvic endometriosis worldwide, affecting primarily the umbilicus, abdominal wall, and vulva.3,4
The 2 main types of cutaneous endometriosis are primary (spontaneous) and secondary. Primary lesions develop in patients without prior surgical history, and secondary lesions occur within previous surgical incision sites, often scars from cesarean delivery.5 Less than 30% of cases of cutaneous endometriosis are primary disease.6 Primary cutaneous endometriosis of the umbilicus, known as Villar nodule, was first described in 1886.3,7 Up to 40% of patients with extrapelvic endometriosis worldwide presented with Villar nodules in a systematic literature review.6 The prevalence of these nodules is unknown, but the incidence is less than 1% of cases of extragenital endometriosis.4
There are 2 leading theories of primary cutaneous endometriosis pathogenesis. The first is the transportation theory, in which endometrial cells are transported outside the uterus via the lymphatic system.8 The second is the metaplasia theory, which proposed that endometrial cells develop in the coelomic mesothelium in the presence of high estrogen levels.8,9
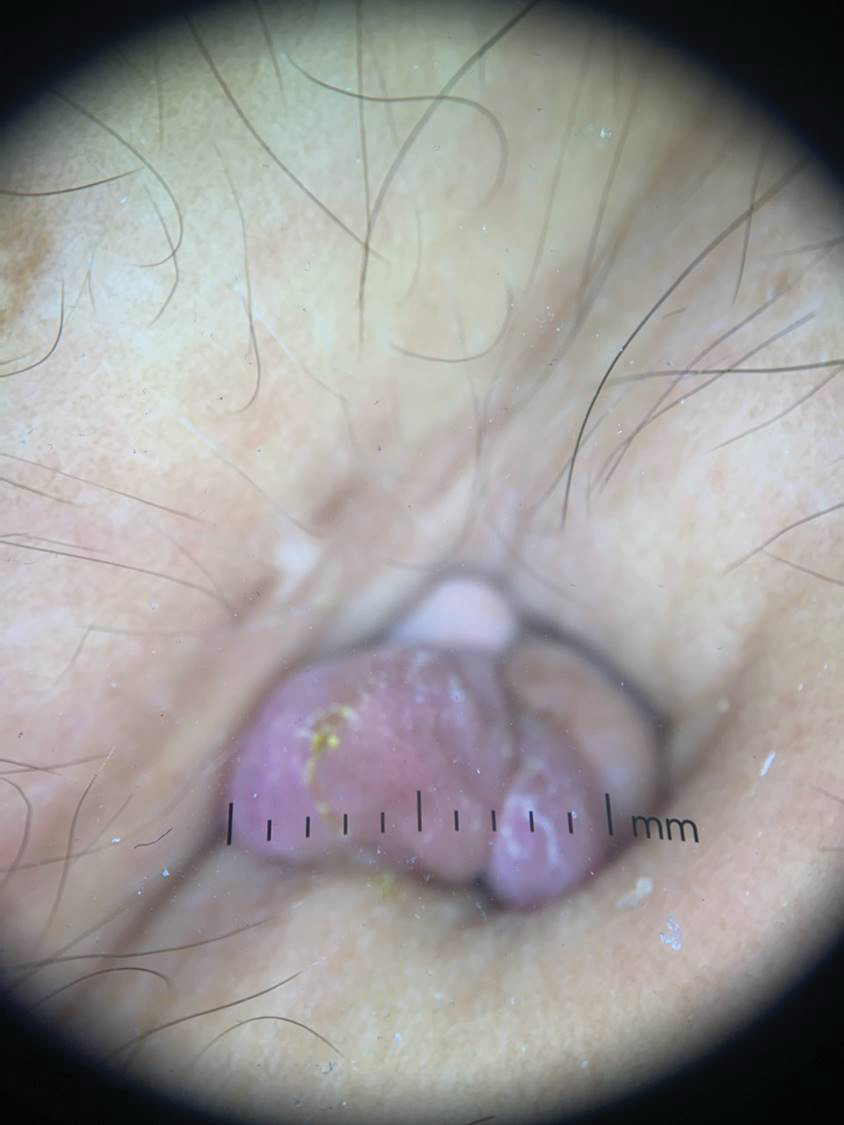
Secondary cutaneous endometriosis, also known as scar endometriosis, is suspected to be caused by an iatrogenic implantation of endometrial cells at the scar of a prior surgical site.9 Although our patient had an existing umbilicus scar from a piercing, it was improbable for that to have been the nidus, as the keloid scar was superficial and did not have contact with the abdominal cavity for iatrogenic implantation. Clinical diagnosis for secondary cutaneous endometriosis often is made based on a triad of features: a nonmalignant abdominal mass, recurring pain and bleeding of the lesion with menses, and prior history of abdominal surgery.9,10 On clinical examination, these features typically manifest as a palpable subcutaneous mass that is black, blue, brown, or red. Often, the lesions enlarge and bleed during the menstrual cycle, causing pain, tenderness, or pruritus.3 Dermoscopic features of secondary cutaneous endometriosis are erythematous umbilical nodules with a homogeneous vascular pattern that appears red with a brownish hue (Figure 2).9,11 Dermoscopic features may vary with the hormone cycle; for example, the follicular phase (correlating with day 7 of menses) demonstrates polypoid projections, erythematous violaceous color, dark-brown spots, and active bleeding of the lesion.12 Clinical and dermoscopic examination are useful tools in this diagnosis.
Imaging such as ultrasonography, computed tomography, or magnetic resonance imaging may be useful in identifying abdominal endometriomas.8,13,14 Pelvic involvement of endometriosis was found in approximately 15% of patients in a case series,4 with concurrent primary umbilical endometriosis. Imaging studies may assist evaluation for fistula formation, presence of malignancies, and the extent of endometriosis within the abdominal cavity.
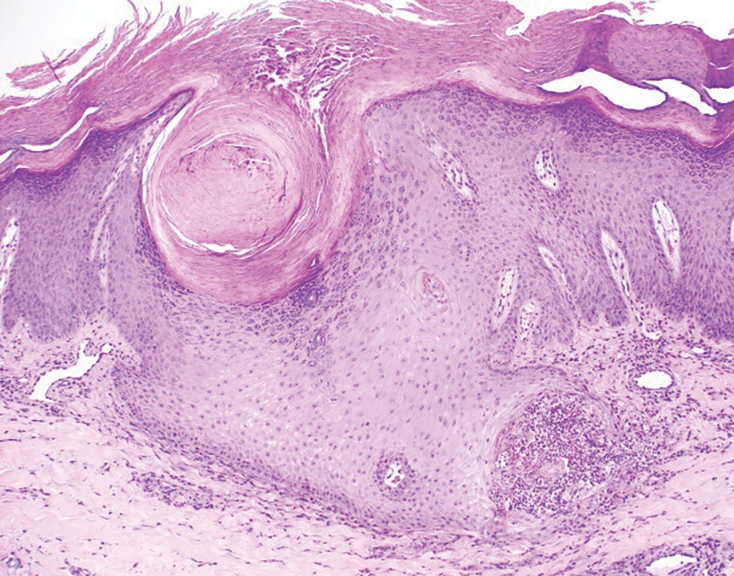
Histopathology is key to confirming cutaneous endometriosis and shows multiple bland-appearing glands of varying sizes with loose, concentric, edematous, or fibromyxoid stroma (Figure 1).3 Red blood cells sometimes are found with hemosiderin within the stroma. Immunohistochemical staining with estrogen receptors may aid in identifying the endometriumlike epithelial cells.13
Standard treatment involves surgical excision with 1-cm margins and umbilical preservation, which results in a recurrence rate of less than 10%.4,10 Medical therapy, such as aromatase inhibitors, progestogens, antiprogestogens, combined oral contraceptives, or gonadotropin-releasing hormone agonists or antagonists may help manage pain or reduce the size of the nodule.4,15 Simple observation also is a potential course for patients who decline treatment options.
Differential diagnoses include lobular capillary hemangioma, also known as pyogenic granuloma; Sister Mary Joseph nodule; umbilical hernia; and dermatofibrosarcoma protuberans. Lobular capillary hemangiomas commonly are acquired benign vascular proliferations of the skin that are friable and tend to ulcerate.16 These lesions typically grow rapidly and often are located on the face, lips, mucosae, and fingers. Histopathologic examination may show an exophytic lesion with lobules of proliferating capillaries within an edematous matrix, superficial ulceration, and an epithelial collarette.17 Treatment includes surgical excision, cauterization, laser treatments, sclerotherapy, injectable medications, and topical medications, but recurrence is possible with any of these interventions.18
Cutaneous metastasis of an internal solid organ cancer, commonly known as a Sister Mary Joseph nodule, typically manifests as an erythematous, irregularly shaped nodule that may protrude from the umbilicus.14 Gastrointestinal symptoms such as change in bowel habits or obstructive symptoms in the setting of a progressive malignancy are common.14 Clinical features include a firm fixed lesion, oozing, and ulceration.19 On dermoscopy, polymorphous vascular patterns, milky red structureless areas, and white lines typically are present.11 Although dermoscopic features may differentiate this entity from cutaneous endometriosis, tissue sampling and histologic examination are crucial diagnostic tools to identify malignant vs benign lesions.
An umbilical hernia is a protrusion of omentum, bowel, or other intra-abdominal organs in an abdominal wall defect. Clinical presentation includes a soft protrusion that may be reduced on palpation if nonstrangulated.20 Treatment includes watchful waiting or surgical repair. The reducibility and presence of an abdominal wall defect may point to this diagnosis. Imaging also may aid in the diagnosis if the history and physical examination are unclear.
Dermatofibrosarcoma protuberans is a slow-developing, low- to intermediate-grade, soft-tissue sarcoma that occurs in less than 0.1% of all cancers in the United States.21 Lesions often manifest as small, firm, slow-growing, painless, flesh-colored dermal plaques; subcutaneous thickening; or atrophic nonprotuberant lesions typically involving the trunk.21 Histopathologically, they are composed of uniform spindle-cell proliferation growing in a storiform pattern and subcutaneous fat trapping that has strong and diffuse CD34 immunoreactivity.21,22 Pathologic examination typically distinguishes this diagnosis from cutaneous endometriosis. Treatment includes tumor resection that may or may not involve radiotherapy and targeted therapy, as recurrence and metastases are possible.
Primary cutaneous endometriosis is a rare but important diagnosis for dermatologists to consider when evaluating umbilical nodules. Clinical features may include bleeding masses during menses in females of reproductive age. Dermoscopic examination aids in workup, and histopathologic testing can confirm the diagnosis and rule out malignancies. Surgical excision is the treatment of choice with a low rate of recurrence.
- International Working Group of AAGL, ESGE, ESHRE and WES; Tomassetti C, Johnson NP, et al. An international terminology for endometriosis, 2021. Hum Reprod Open. 2021;2021:hoab029. doi:10.1093/hropen/hoab029
- Batista M, Alves F, Cardoso J, et al. Cutaneous endometriosis: a differential diagnosis of umbilical nodule. Acta Med Port. 2020; 33:282-284. doi:10.20344/amp.10966
- Brown ME, Osswald S, Biediger T. Cutaneous endometriosis of the umbilicus (Villar’s nodule). Int J Womens Dermatol. 2020;6:214-215. doi:10.1016/j.ijwd.2020.01.001
- Bindra V, Sampurna S, Kade S, et al. Primary umbilical endometriosis - case series and review of clinical presentation, diagnosis and management. Int J Surg Case Rep. 2022;94:107134. doi:10.1016/j.ijscr.2022.107134
- Loh SH, Lew BL, Sim WY. Primary cutaneous endometriosis of umbilicus. Ann Dermatol. 2017;29:621-625. doi:10.5021/ad.2017.29.5.621
- Victory R, Diamond MP, Johns DA. Villar’s nodule: a case report and systematic literature review of endometriosis externa of the umbilicus. J Minim Invasive Gynecol. 2007;14:23-32. doi:10.1016/j.jmig.2006.07.01
- Van den Nouland D, Kaur M. Primary umbilical endometriosis: a case report. Facts Views Vis Obgyn. 2017;9:115-119.
- Machairiotis N, Stylianaki A, Dryllis G, et al. Extrapelvic endometriosis: a rare entity or an under diagnosed condition? Diagn Pathol. 2013;8:194. doi:10.1186/1746-1596-8-194
- Huang QF, Jiang B, Yang X, et al. Primary versus secondary cutaneous endometriosis: literature review and case study. Heliyon. 2023;9:E20094. doi:10.1016/j.heliyon.2023.e20094
- Gonzalez RH, Singh MS, Hamza SA. Cutaneous endometriosis: a case report and review of the literature. Am J Case Rep. 2021;22:E932493. doi:10.12659/AJCR.932493
- Buljan M, Arzberger E, Šitum M, et al. The use of dermoscopy in differentiating Sister Mary Joseph nodule and cutaneous endometriosis. Australas J Dermatol. 2019;60:E233-E235. doi:10.1111/ajd.12980
- Costa IM, Gomes CM, Morais OO, et al. Cutaneous endometriosis: dermoscopic findings related to phases of the female hormonal cycle. Int J Dermatol. 2014;53:E130-E132. doi:10.1111 /j.1365-4632.2012.05854.x
- Mohaghegh F, Hatami P, Rajabi P, et al. Coexistence of cutaneous endometriosis and ovarian endometrioma: a case report. J Med Case Rep. 2022;16:256. doi:10.1186/s13256-022-03483-8
- Raffi L, Suresh R, McCalmont TH, et al. Cutaneous endometriosis. Int J Womens Dermatol. 2019;5:384-386. doi:10.1016 /j.ijwd.2019.06.025
- Saunders PTK, Horne AW. Endometriosis: etiology, pathobiology, and therapeutic prospects. Cell. 2021;184:2807-2824. doi:10.1016 /j.cell.2021.04.041
- Habif TP. Clinical Dermatology a Color Guide to Diagnosis and Therapy. St. Louis, Mo. Elsevier; 2016.
- Patrice SJ, Wiss K, Mulliken JB. Pyogenic granuloma (lobular capillary hemangioma): a clinicopathologic study of 178 cases. Pediatr Dermatol. 1991;8:267-276. doi:10.1111/j.15251470.1991.tb00931.x
- Kaleeny JD, Janis JE. Pyogenic granuloma diagnosis and management: a practical review. Plast Reconstr Surg Glob Open. 2024;12:E6160. doi:10.1097/GOX.0000000000006160
- Ha DL, Yang MY, Shin JO, et al. Benign umbilical tumors resembling Sister Mary Joseph nodule. Clin Med Insights Oncol. 2021;15:1179554921995022. doi:10.1177/1179554921995022
- Lawrence PF, Smeds M, Jessica Beth O’connell. Essentials of General Surgery and Surgical Specialties. Wolters Kluwer Health; 2019.
- Hao X, Billings SD, Wu F, et al. Dermatofibrosarcoma protuberans: update on the diagnosis and treatment. J Clin Med. 2020;9:1752. doi:10.3390/jcm9061752
- Allen A, Ahn C, Sangüeza OP. Dermatofibrosarcoma protuberans. Dermatol Clin. 2019;37:483-488. doi:10.1016/j.det.2019.05.006
THE DIAGNOSIS: Villar Nodule
The biopsy revealed features consistent with cutaneous endometriosis in the setting of a painful, tender, multilobulated nodule with a cyclical bleeding pattern (Figure 1). The bleeding pattern of the nodule during menses and lack of surgical history supported the diagnosis of primary cutaneous endometriosis in our patient. She was diagnosed with endometriosis by gynecology, and her primary care physician started her on an oral contraceptive based on this diagnosis. She also was referred to gynecology and plastic surgery for a joint surgical consultation to remove the nodule. She initially decided to do a trial of the oral contraceptive but subsequently underwent umbilical endometrioma excision with neo-umbilicus creation with no evidence of recurrence.

Primary cutaneous endometriosis should be considered in young females who present with tender umbilical nodules. Endometriosis refers to the presence of an endometriumlike epithelium outside the endometrium and myometrium.1 The condition affects 10% to 15% of reproductive-aged (ie, 18-49 years) women in the United States and typically involves tissues within the pelvis, such as the ovaries, pouch of Douglas, or pelvic ligaments.2 Cutaneous endometriosis is the growth of endometrial tissue in the skin and is rare, accounting for less than 5.5% of cases of extrapelvic endometriosis worldwide, affecting primarily the umbilicus, abdominal wall, and vulva.3,4
The 2 main types of cutaneous endometriosis are primary (spontaneous) and secondary. Primary lesions develop in patients without prior surgical history, and secondary lesions occur within previous surgical incision sites, often scars from cesarean delivery.5 Less than 30% of cases of cutaneous endometriosis are primary disease.6 Primary cutaneous endometriosis of the umbilicus, known as Villar nodule, was first described in 1886.3,7 Up to 40% of patients with extrapelvic endometriosis worldwide presented with Villar nodules in a systematic literature review.6 The prevalence of these nodules is unknown, but the incidence is less than 1% of cases of extragenital endometriosis.4
There are 2 leading theories of primary cutaneous endometriosis pathogenesis. The first is the transportation theory, in which endometrial cells are transported outside the uterus via the lymphatic system.8 The second is the metaplasia theory, which proposed that endometrial cells develop in the coelomic mesothelium in the presence of high estrogen levels.8,9
Secondary cutaneous endometriosis, also known as scar endometriosis, is suspected to be caused by an iatrogenic implantation of endometrial cells at the scar of a prior surgical site.9 Although our patient had an existing umbilicus scar from a piercing, it was improbable for that to have been the nidus, as the keloid scar was superficial and did not have contact with the abdominal cavity for iatrogenic implantation. Clinical diagnosis for secondary cutaneous endometriosis often is made based on a triad of features: a nonmalignant abdominal mass, recurring pain and bleeding of the lesion with menses, and prior history of abdominal surgery.9,10 On clinical examination, these features typically manifest as a palpable subcutaneous mass that is black, blue, brown, or red. Often, the lesions enlarge and bleed during the menstrual cycle, causing pain, tenderness, or pruritus.3 Dermoscopic features of secondary cutaneous endometriosis are erythematous umbilical nodules with a homogeneous vascular pattern that appears red with a brownish hue (Figure 2).9,11 Dermoscopic features may vary with the hormone cycle; for example, the follicular phase (correlating with day 7 of menses) demonstrates polypoid projections, erythematous violaceous color, dark-brown spots, and active bleeding of the lesion.12 Clinical and dermoscopic examination are useful tools in this diagnosis.

Imaging such as ultrasonography, computed tomography, or magnetic resonance imaging may be useful in identifying abdominal endometriomas.8,13,14 Pelvic involvement of endometriosis was found in approximately 15% of patients in a case series,4 with concurrent primary umbilical endometriosis. Imaging studies may assist evaluation for fistula formation, presence of malignancies, and the extent of endometriosis within the abdominal cavity.
Histopathology is key to confirming cutaneous endometriosis and shows multiple bland-appearing glands of varying sizes with loose, concentric, edematous, or fibromyxoid stroma (Figure 1).3 Red blood cells sometimes are found with hemosiderin within the stroma. Immunohistochemical staining with estrogen receptors may aid in identifying the endometriumlike epithelial cells.13
Standard treatment involves surgical excision with 1-cm margins and umbilical preservation, which results in a recurrence rate of less than 10%.4,10 Medical therapy, such as aromatase inhibitors, progestogens, antiprogestogens, combined oral contraceptives, or gonadotropin-releasing hormone agonists or antagonists may help manage pain or reduce the size of the nodule.4,15 Simple observation also is a potential course for patients who decline treatment options.
Differential diagnoses include lobular capillary hemangioma, also known as pyogenic granuloma; Sister Mary Joseph nodule; umbilical hernia; and dermatofibrosarcoma protuberans. Lobular capillary hemangiomas commonly are acquired benign vascular proliferations of the skin that are friable and tend to ulcerate.16 These lesions typically grow rapidly and often are located on the face, lips, mucosae, and fingers. Histopathologic examination may show an exophytic lesion with lobules of proliferating capillaries within an edematous matrix, superficial ulceration, and an epithelial collarette.17 Treatment includes surgical excision, cauterization, laser treatments, sclerotherapy, injectable medications, and topical medications, but recurrence is possible with any of these interventions.18
Cutaneous metastasis of an internal solid organ cancer, commonly known as a Sister Mary Joseph nodule, typically manifests as an erythematous, irregularly shaped nodule that may protrude from the umbilicus.14 Gastrointestinal symptoms such as change in bowel habits or obstructive symptoms in the setting of a progressive malignancy are common.14 Clinical features include a firm fixed lesion, oozing, and ulceration.19 On dermoscopy, polymorphous vascular patterns, milky red structureless areas, and white lines typically are present.11 Although dermoscopic features may differentiate this entity from cutaneous endometriosis, tissue sampling and histologic examination are crucial diagnostic tools to identify malignant vs benign lesions.
An umbilical hernia is a protrusion of omentum, bowel, or other intra-abdominal organs in an abdominal wall defect. Clinical presentation includes a soft protrusion that may be reduced on palpation if nonstrangulated.20 Treatment includes watchful waiting or surgical repair. The reducibility and presence of an abdominal wall defect may point to this diagnosis. Imaging also may aid in the diagnosis if the history and physical examination are unclear.
Dermatofibrosarcoma protuberans is a slow-developing, low- to intermediate-grade, soft-tissue sarcoma that occurs in less than 0.1% of all cancers in the United States.21 Lesions often manifest as small, firm, slow-growing, painless, flesh-colored dermal plaques; subcutaneous thickening; or atrophic nonprotuberant lesions typically involving the trunk.21 Histopathologically, they are composed of uniform spindle-cell proliferation growing in a storiform pattern and subcutaneous fat trapping that has strong and diffuse CD34 immunoreactivity.21,22 Pathologic examination typically distinguishes this diagnosis from cutaneous endometriosis. Treatment includes tumor resection that may or may not involve radiotherapy and targeted therapy, as recurrence and metastases are possible.
Primary cutaneous endometriosis is a rare but important diagnosis for dermatologists to consider when evaluating umbilical nodules. Clinical features may include bleeding masses during menses in females of reproductive age. Dermoscopic examination aids in workup, and histopathologic testing can confirm the diagnosis and rule out malignancies. Surgical excision is the treatment of choice with a low rate of recurrence.
THE DIAGNOSIS: Villar Nodule
The biopsy revealed features consistent with cutaneous endometriosis in the setting of a painful, tender, multilobulated nodule with a cyclical bleeding pattern (Figure 1). The bleeding pattern of the nodule during menses and lack of surgical history supported the diagnosis of primary cutaneous endometriosis in our patient. She was diagnosed with endometriosis by gynecology, and her primary care physician started her on an oral contraceptive based on this diagnosis. She also was referred to gynecology and plastic surgery for a joint surgical consultation to remove the nodule. She initially decided to do a trial of the oral contraceptive but subsequently underwent umbilical endometrioma excision with neo-umbilicus creation with no evidence of recurrence.

Primary cutaneous endometriosis should be considered in young females who present with tender umbilical nodules. Endometriosis refers to the presence of an endometriumlike epithelium outside the endometrium and myometrium.1 The condition affects 10% to 15% of reproductive-aged (ie, 18-49 years) women in the United States and typically involves tissues within the pelvis, such as the ovaries, pouch of Douglas, or pelvic ligaments.2 Cutaneous endometriosis is the growth of endometrial tissue in the skin and is rare, accounting for less than 5.5% of cases of extrapelvic endometriosis worldwide, affecting primarily the umbilicus, abdominal wall, and vulva.3,4
The 2 main types of cutaneous endometriosis are primary (spontaneous) and secondary. Primary lesions develop in patients without prior surgical history, and secondary lesions occur within previous surgical incision sites, often scars from cesarean delivery.5 Less than 30% of cases of cutaneous endometriosis are primary disease.6 Primary cutaneous endometriosis of the umbilicus, known as Villar nodule, was first described in 1886.3,7 Up to 40% of patients with extrapelvic endometriosis worldwide presented with Villar nodules in a systematic literature review.6 The prevalence of these nodules is unknown, but the incidence is less than 1% of cases of extragenital endometriosis.4
There are 2 leading theories of primary cutaneous endometriosis pathogenesis. The first is the transportation theory, in which endometrial cells are transported outside the uterus via the lymphatic system.8 The second is the metaplasia theory, which proposed that endometrial cells develop in the coelomic mesothelium in the presence of high estrogen levels.8,9
Secondary cutaneous endometriosis, also known as scar endometriosis, is suspected to be caused by an iatrogenic implantation of endometrial cells at the scar of a prior surgical site.9 Although our patient had an existing umbilicus scar from a piercing, it was improbable for that to have been the nidus, as the keloid scar was superficial and did not have contact with the abdominal cavity for iatrogenic implantation. Clinical diagnosis for secondary cutaneous endometriosis often is made based on a triad of features: a nonmalignant abdominal mass, recurring pain and bleeding of the lesion with menses, and prior history of abdominal surgery.9,10 On clinical examination, these features typically manifest as a palpable subcutaneous mass that is black, blue, brown, or red. Often, the lesions enlarge and bleed during the menstrual cycle, causing pain, tenderness, or pruritus.3 Dermoscopic features of secondary cutaneous endometriosis are erythematous umbilical nodules with a homogeneous vascular pattern that appears red with a brownish hue (Figure 2).9,11 Dermoscopic features may vary with the hormone cycle; for example, the follicular phase (correlating with day 7 of menses) demonstrates polypoid projections, erythematous violaceous color, dark-brown spots, and active bleeding of the lesion.12 Clinical and dermoscopic examination are useful tools in this diagnosis.

Imaging such as ultrasonography, computed tomography, or magnetic resonance imaging may be useful in identifying abdominal endometriomas.8,13,14 Pelvic involvement of endometriosis was found in approximately 15% of patients in a case series,4 with concurrent primary umbilical endometriosis. Imaging studies may assist evaluation for fistula formation, presence of malignancies, and the extent of endometriosis within the abdominal cavity.
Histopathology is key to confirming cutaneous endometriosis and shows multiple bland-appearing glands of varying sizes with loose, concentric, edematous, or fibromyxoid stroma (Figure 1).3 Red blood cells sometimes are found with hemosiderin within the stroma. Immunohistochemical staining with estrogen receptors may aid in identifying the endometriumlike epithelial cells.13
Standard treatment involves surgical excision with 1-cm margins and umbilical preservation, which results in a recurrence rate of less than 10%.4,10 Medical therapy, such as aromatase inhibitors, progestogens, antiprogestogens, combined oral contraceptives, or gonadotropin-releasing hormone agonists or antagonists may help manage pain or reduce the size of the nodule.4,15 Simple observation also is a potential course for patients who decline treatment options.
Differential diagnoses include lobular capillary hemangioma, also known as pyogenic granuloma; Sister Mary Joseph nodule; umbilical hernia; and dermatofibrosarcoma protuberans. Lobular capillary hemangiomas commonly are acquired benign vascular proliferations of the skin that are friable and tend to ulcerate.16 These lesions typically grow rapidly and often are located on the face, lips, mucosae, and fingers. Histopathologic examination may show an exophytic lesion with lobules of proliferating capillaries within an edematous matrix, superficial ulceration, and an epithelial collarette.17 Treatment includes surgical excision, cauterization, laser treatments, sclerotherapy, injectable medications, and topical medications, but recurrence is possible with any of these interventions.18
Cutaneous metastasis of an internal solid organ cancer, commonly known as a Sister Mary Joseph nodule, typically manifests as an erythematous, irregularly shaped nodule that may protrude from the umbilicus.14 Gastrointestinal symptoms such as change in bowel habits or obstructive symptoms in the setting of a progressive malignancy are common.14 Clinical features include a firm fixed lesion, oozing, and ulceration.19 On dermoscopy, polymorphous vascular patterns, milky red structureless areas, and white lines typically are present.11 Although dermoscopic features may differentiate this entity from cutaneous endometriosis, tissue sampling and histologic examination are crucial diagnostic tools to identify malignant vs benign lesions.
An umbilical hernia is a protrusion of omentum, bowel, or other intra-abdominal organs in an abdominal wall defect. Clinical presentation includes a soft protrusion that may be reduced on palpation if nonstrangulated.20 Treatment includes watchful waiting or surgical repair. The reducibility and presence of an abdominal wall defect may point to this diagnosis. Imaging also may aid in the diagnosis if the history and physical examination are unclear.
Dermatofibrosarcoma protuberans is a slow-developing, low- to intermediate-grade, soft-tissue sarcoma that occurs in less than 0.1% of all cancers in the United States.21 Lesions often manifest as small, firm, slow-growing, painless, flesh-colored dermal plaques; subcutaneous thickening; or atrophic nonprotuberant lesions typically involving the trunk.21 Histopathologically, they are composed of uniform spindle-cell proliferation growing in a storiform pattern and subcutaneous fat trapping that has strong and diffuse CD34 immunoreactivity.21,22 Pathologic examination typically distinguishes this diagnosis from cutaneous endometriosis. Treatment includes tumor resection that may or may not involve radiotherapy and targeted therapy, as recurrence and metastases are possible.
Primary cutaneous endometriosis is a rare but important diagnosis for dermatologists to consider when evaluating umbilical nodules. Clinical features may include bleeding masses during menses in females of reproductive age. Dermoscopic examination aids in workup, and histopathologic testing can confirm the diagnosis and rule out malignancies. Surgical excision is the treatment of choice with a low rate of recurrence.
- International Working Group of AAGL, ESGE, ESHRE and WES; Tomassetti C, Johnson NP, et al. An international terminology for endometriosis, 2021. Hum Reprod Open. 2021;2021:hoab029. doi:10.1093/hropen/hoab029
- Batista M, Alves F, Cardoso J, et al. Cutaneous endometriosis: a differential diagnosis of umbilical nodule. Acta Med Port. 2020; 33:282-284. doi:10.20344/amp.10966
- Brown ME, Osswald S, Biediger T. Cutaneous endometriosis of the umbilicus (Villar’s nodule). Int J Womens Dermatol. 2020;6:214-215. doi:10.1016/j.ijwd.2020.01.001
- Bindra V, Sampurna S, Kade S, et al. Primary umbilical endometriosis - case series and review of clinical presentation, diagnosis and management. Int J Surg Case Rep. 2022;94:107134. doi:10.1016/j.ijscr.2022.107134
- Loh SH, Lew BL, Sim WY. Primary cutaneous endometriosis of umbilicus. Ann Dermatol. 2017;29:621-625. doi:10.5021/ad.2017.29.5.621
- Victory R, Diamond MP, Johns DA. Villar’s nodule: a case report and systematic literature review of endometriosis externa of the umbilicus. J Minim Invasive Gynecol. 2007;14:23-32. doi:10.1016/j.jmig.2006.07.01
- Van den Nouland D, Kaur M. Primary umbilical endometriosis: a case report. Facts Views Vis Obgyn. 2017;9:115-119.
- Machairiotis N, Stylianaki A, Dryllis G, et al. Extrapelvic endometriosis: a rare entity or an under diagnosed condition? Diagn Pathol. 2013;8:194. doi:10.1186/1746-1596-8-194
- Huang QF, Jiang B, Yang X, et al. Primary versus secondary cutaneous endometriosis: literature review and case study. Heliyon. 2023;9:E20094. doi:10.1016/j.heliyon.2023.e20094
- Gonzalez RH, Singh MS, Hamza SA. Cutaneous endometriosis: a case report and review of the literature. Am J Case Rep. 2021;22:E932493. doi:10.12659/AJCR.932493
- Buljan M, Arzberger E, Šitum M, et al. The use of dermoscopy in differentiating Sister Mary Joseph nodule and cutaneous endometriosis. Australas J Dermatol. 2019;60:E233-E235. doi:10.1111/ajd.12980
- Costa IM, Gomes CM, Morais OO, et al. Cutaneous endometriosis: dermoscopic findings related to phases of the female hormonal cycle. Int J Dermatol. 2014;53:E130-E132. doi:10.1111 /j.1365-4632.2012.05854.x
- Mohaghegh F, Hatami P, Rajabi P, et al. Coexistence of cutaneous endometriosis and ovarian endometrioma: a case report. J Med Case Rep. 2022;16:256. doi:10.1186/s13256-022-03483-8
- Raffi L, Suresh R, McCalmont TH, et al. Cutaneous endometriosis. Int J Womens Dermatol. 2019;5:384-386. doi:10.1016 /j.ijwd.2019.06.025
- Saunders PTK, Horne AW. Endometriosis: etiology, pathobiology, and therapeutic prospects. Cell. 2021;184:2807-2824. doi:10.1016 /j.cell.2021.04.041
- Habif TP. Clinical Dermatology a Color Guide to Diagnosis and Therapy. St. Louis, Mo. Elsevier; 2016.
- Patrice SJ, Wiss K, Mulliken JB. Pyogenic granuloma (lobular capillary hemangioma): a clinicopathologic study of 178 cases. Pediatr Dermatol. 1991;8:267-276. doi:10.1111/j.15251470.1991.tb00931.x
- Kaleeny JD, Janis JE. Pyogenic granuloma diagnosis and management: a practical review. Plast Reconstr Surg Glob Open. 2024;12:E6160. doi:10.1097/GOX.0000000000006160
- Ha DL, Yang MY, Shin JO, et al. Benign umbilical tumors resembling Sister Mary Joseph nodule. Clin Med Insights Oncol. 2021;15:1179554921995022. doi:10.1177/1179554921995022
- Lawrence PF, Smeds M, Jessica Beth O’connell. Essentials of General Surgery and Surgical Specialties. Wolters Kluwer Health; 2019.
- Hao X, Billings SD, Wu F, et al. Dermatofibrosarcoma protuberans: update on the diagnosis and treatment. J Clin Med. 2020;9:1752. doi:10.3390/jcm9061752
- Allen A, Ahn C, Sangüeza OP. Dermatofibrosarcoma protuberans. Dermatol Clin. 2019;37:483-488. doi:10.1016/j.det.2019.05.006
- International Working Group of AAGL, ESGE, ESHRE and WES; Tomassetti C, Johnson NP, et al. An international terminology for endometriosis, 2021. Hum Reprod Open. 2021;2021:hoab029. doi:10.1093/hropen/hoab029
- Batista M, Alves F, Cardoso J, et al. Cutaneous endometriosis: a differential diagnosis of umbilical nodule. Acta Med Port. 2020; 33:282-284. doi:10.20344/amp.10966
- Brown ME, Osswald S, Biediger T. Cutaneous endometriosis of the umbilicus (Villar’s nodule). Int J Womens Dermatol. 2020;6:214-215. doi:10.1016/j.ijwd.2020.01.001
- Bindra V, Sampurna S, Kade S, et al. Primary umbilical endometriosis - case series and review of clinical presentation, diagnosis and management. Int J Surg Case Rep. 2022;94:107134. doi:10.1016/j.ijscr.2022.107134
- Loh SH, Lew BL, Sim WY. Primary cutaneous endometriosis of umbilicus. Ann Dermatol. 2017;29:621-625. doi:10.5021/ad.2017.29.5.621
- Victory R, Diamond MP, Johns DA. Villar’s nodule: a case report and systematic literature review of endometriosis externa of the umbilicus. J Minim Invasive Gynecol. 2007;14:23-32. doi:10.1016/j.jmig.2006.07.01
- Van den Nouland D, Kaur M. Primary umbilical endometriosis: a case report. Facts Views Vis Obgyn. 2017;9:115-119.
- Machairiotis N, Stylianaki A, Dryllis G, et al. Extrapelvic endometriosis: a rare entity or an under diagnosed condition? Diagn Pathol. 2013;8:194. doi:10.1186/1746-1596-8-194
- Huang QF, Jiang B, Yang X, et al. Primary versus secondary cutaneous endometriosis: literature review and case study. Heliyon. 2023;9:E20094. doi:10.1016/j.heliyon.2023.e20094
- Gonzalez RH, Singh MS, Hamza SA. Cutaneous endometriosis: a case report and review of the literature. Am J Case Rep. 2021;22:E932493. doi:10.12659/AJCR.932493
- Buljan M, Arzberger E, Šitum M, et al. The use of dermoscopy in differentiating Sister Mary Joseph nodule and cutaneous endometriosis. Australas J Dermatol. 2019;60:E233-E235. doi:10.1111/ajd.12980
- Costa IM, Gomes CM, Morais OO, et al. Cutaneous endometriosis: dermoscopic findings related to phases of the female hormonal cycle. Int J Dermatol. 2014;53:E130-E132. doi:10.1111 /j.1365-4632.2012.05854.x
- Mohaghegh F, Hatami P, Rajabi P, et al. Coexistence of cutaneous endometriosis and ovarian endometrioma: a case report. J Med Case Rep. 2022;16:256. doi:10.1186/s13256-022-03483-8
- Raffi L, Suresh R, McCalmont TH, et al. Cutaneous endometriosis. Int J Womens Dermatol. 2019;5:384-386. doi:10.1016 /j.ijwd.2019.06.025
- Saunders PTK, Horne AW. Endometriosis: etiology, pathobiology, and therapeutic prospects. Cell. 2021;184:2807-2824. doi:10.1016 /j.cell.2021.04.041
- Habif TP. Clinical Dermatology a Color Guide to Diagnosis and Therapy. St. Louis, Mo. Elsevier; 2016.
- Patrice SJ, Wiss K, Mulliken JB. Pyogenic granuloma (lobular capillary hemangioma): a clinicopathologic study of 178 cases. Pediatr Dermatol. 1991;8:267-276. doi:10.1111/j.15251470.1991.tb00931.x
- Kaleeny JD, Janis JE. Pyogenic granuloma diagnosis and management: a practical review. Plast Reconstr Surg Glob Open. 2024;12:E6160. doi:10.1097/GOX.0000000000006160
- Ha DL, Yang MY, Shin JO, et al. Benign umbilical tumors resembling Sister Mary Joseph nodule. Clin Med Insights Oncol. 2021;15:1179554921995022. doi:10.1177/1179554921995022
- Lawrence PF, Smeds M, Jessica Beth O’connell. Essentials of General Surgery and Surgical Specialties. Wolters Kluwer Health; 2019.
- Hao X, Billings SD, Wu F, et al. Dermatofibrosarcoma protuberans: update on the diagnosis and treatment. J Clin Med. 2020;9:1752. doi:10.3390/jcm9061752
- Allen A, Ahn C, Sangüeza OP. Dermatofibrosarcoma protuberans. Dermatol Clin. 2019;37:483-488. doi:10.1016/j.det.2019.05.006
Tender Nodule on the Umbilicus
Tender Nodule on the Umbilicus
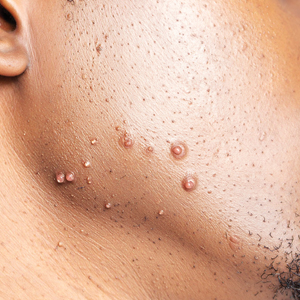
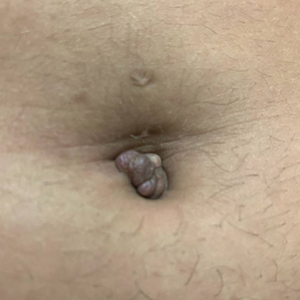
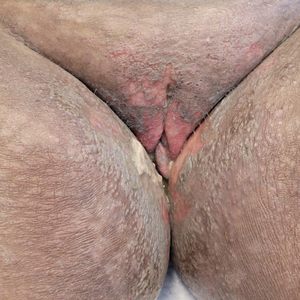
A 25-year-old woman was referred to the dermatology clinic by her primary care provider for evaluation of a tender nodule on the inferior umbilicus of 2 years' duration at the site of a preexisting keloid scar. The patient reported that the lesion caused occasional pain and tenderness. A few weeks prior to the current presentation, a dark-red bloody discharge developed at the superior aspect of the lesion that subsequently crusted over. The patient denied any use of oral contraceptives or history of abdominal surgery.
The original keloid scar had been treated successfully by an outside physician with intralesional steroid injections, and the patient was interested in a similar procedure for the current nodule. She also had a history of a hyperpigmented hypertrophic scar on the superior periumbilical area from a previous piercing that had resolved several years prior to presentation.
Physical examination of the lesion revealed a 1.2-cm, soft, tender, violaceous nodule with scant yellow crust along the superior surface of the umbilicus. There was no palpable abdominal wall defect, and the nodule was not reducible into the abdominal cavity. An interval history revealed bleeding of the lesion during the patient's menstrual cycle with persistent pain and tenderness. A punch biopsy was performed.
Diffuse Pruritic Keratotic Papules
Diffuse Pruritic Keratotic Papules
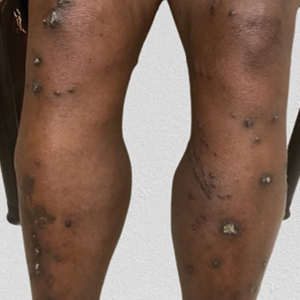
THE DIAGNOSIS: Reactive Perforating Collagenosis
Histopathology revealed invagination of the epidermis with hyperkeratosis; prominent epidermal hyperplasia; and a central basophilic plug of keratin, collagen, and inflammatory debris. Transepidermal elimination of bright eosinophilic altered collagen fibers was seen (Figure). The findings were consistent with a diagnosis of reactive perforating collagenosis (RPC).
Reactive perforating collagenosis, a subtype of perforating dermatosis, is a rare skin condition in which altered collagen is eliminated through the epidermis.1 There are 2 forms of RPC: the inherited form, which is very rare and manifests in childhood, and the acquired form, which manifests in adulthood and is associated with systemic diseases, most notably diabetes and/or chronic renal failure, both of which our patient had been diagnosed with.1,2 The clinical presentation of RPC includes erythematous papules or nodules that evolve into umbilicated 4- to 10-mm craterlike ulcerations with a central keratotic plug. The lesions favor a linear distribution along the extensor surfaces of the arms and legs, trunk, and gluteal area. Involvement of the head, neck, and scalp has been reported less commonly, which makes our case particularly unique.3 Histopathologically, RPC is characterized by a cup-shaped depression of the epidermis with an overlying keratin plug containing inflammatory cells, keratinous debris, and collagen fibers. Vertically oriented collagen fibers are seen extruded through the epidermis.4,5
While the pathogenesis of RPC remains unknown, it is believed that superficial trauma due to chronic scratching results in transepithelial elimination of collagen. Due to the association of acquired RPC (ARPC) with diabetes, it also has been proposed that scratching can cause microtrauma and necrosis of the dermal structures, potentially due to diabetic microangiopathy.3 Additionally, RPC is associated with overexpression of transforming growth factor beta 3 in lesional skin, suggesting that transforming growth factor beta 3 is involved with tissue repair and extracellular remodeling in this condition.6
Treatment of ARPC should include the management of underlying disease. While no definitive treatment has been reported to date, topical corticosteroids, retinoids, keratolytics, emollients, antihistamines, narrow-band UVB phototherapy, and psoralen plus UVA phototherapy have been used with varying degrees of improvement. Typically, the lesions self-resolve within 6 to 8 weeks; however, they often recur and usually leave scarring with or without hyperpigmentation.2,7-10
Acquired RPC can be misdiagnosed initially, as it mimics several other conditions and commonly is associated with systemic diseases. While biopsy is necessary for diagnosis, if it cannot be performed or the results are indeterminate, dermoscopy can serve as a helpful diagnostic tool. The most common dermoscopic patterns seen in RPC include a yellow-brown structureless area in the center of the lesion with a peripheral surface crust and surrounding white rim—thought to represent epidermal invagination or keratinous debris. Additionally, inflammation with visible vessels both centrally and peripherally is represented by an outer pink circle on dermoscopy.5,11
The differential diagnoses for RPC include perforating folliculitis (PF), elastosis perforans serpiginosa (EPS), prurigo nodularis, and keratoacanthomas. The primary perforating dermatoses (PF, EPS, and RPC) are similarly characterized by elimination of altered dermal material through the epidermis. As these conditions manifest with similar features on clinical examination, differentiation is made by the type of epidermal damage and the features of elimination material, making histopathologic examination paramount for definitive diagnosis.
Perforating folliculitis manifests as erythematous, follicular papules with a small central keratotic core or a central hair. Histopathologically, PF reveals a widely dilated follicle containing keratin, necrotic debris, and degenerated inflammatory cells. Elastosis perforans serpiginosa manifests clinically as hyperkeratotic papules in serpiginous patterns rather than the linear pattern commonly seen with ARPC. Histopathologically, EPS reveals thickened elastic fibers, rather than collagen fibers as seen in ARPC, extruded through the epidermis. Prurigo nodularis manifests clinically as dome-shaped papules with possible excoriation and crusting. Histopathologic examination reveals epidermal hyperplasia and hyperkeratosis; however, the characteristic features of transepithelial elimination of collagen and invaginations of epidermis differentiate ARPC from prurigo nodularis.12,13 Keratoacanthomas manifest clinically as an eruption of small, round, pink papules that rapidly grow and evolve into 1- to 2-cm dome-shaped nodules with central keratinaceous plugs, mimicking a crateriform appearance. Histopathologic examination reveals a circumscribed proliferation of well-differentiated keratinocytes. Multilobular exophytic or endophytic cystlike invaginations of the epidermis also are noted. The expulsion of collagen from the epidermis is more consistent with ARPC.14
- Cohen RW, Auerbach R. Acquired reactive perforating collagenosis. J Am Acad Dermatol. 1989;20(2 pt 1):287-289. doi:10.1016/s0190 -9622(89)80059-3
- Bejjanki H, Siroy AE, Koratala A. Reactive perforating collagenosis in end-stage renal disease: not all that itches is uremic pruritis! Am J Med. 2019;132:E658-E660. doi:10.1016/j.amjmed.2019.03.015
- Gontijo JRV, Júnior FF, Pereira LB, et al. Trauma-induced acquired reactive perforating collagenosis. An Bras Dermatol. 2021;96:392-393. doi:10.1016/j.abd.2020.06.022
- Ambalathinkal JJ, Phiske MM, Someshwar SJ. Acquired reactive perforating collagenosis, a rare entity at uncommon site. Indian J Pathol Microbiol. 2022;65:895-897. doi:10.4103/ijpm.ijpm_333_21
- Ormerod E, Atwan A, Intzedy L, et al. Dermoscopy features of acquired reactive perforating collagenosis: a case series. Dermatol Pract Concept. 2018;8:303-305. doi:10.5826/dpc.0804a11
- Fei C, Wang Y, Gong Y, et al. Acquired reactive perforating collagenosis: a report of a typical case. Medicine (Baltimore). 2016;95:E4305. doi:10.1097/md.0000000000004305
- Bartling SJ, Naff JL, Canevari MM, et al. Pruritic rash in an elderly patient with uncontrolled diabetes mellitus. AACE Clin Case Rep. 2018;5:E146-E149. doi:10.4158/ACCR-2018-0388
- Kollipara H, Satya RS, Rao GR, et al. Acquired reactive perforating collagenosis: case series. Indian Dermatol Online J. 2023;14:72-76. doi:10.4103/idoj.idoj_373_22
- Wang C, Liu YH, Wang YX, et al. Acquired reactive perforating collagenosis. Chin Med J (Engl). 2020;133:2119-2120. doi:10.1097 /cm9.0000000000000906
- Harbaoui S, Litaiem N. Acquired perforating dermatosis. StatPearls [Internet]. Updated February 13, 2023. Accessed August 13, 2025. https://www.ncbi.nlm.nih.gov/books/NBK539715/
- Elmas ÖF, Kilitci A, Uyar B. Dermoscopic patterns of acquired reactive perforating collagenosis. Dermatol Pract Concept. 2021;11:E2020085. doi:10.5826/dpc.1101a85
- Patterson JW. The perforating disorders. J Am Acad Dermatol. 1984;10:561-581. doi:10.1016/s0190-9622(84)80259-5
- Huang AH, Williams KA, Kwatra SG. Prurigo nodularis: epidemiology and clinical features. J Am Acad Dermatol. 2020;83:1559-1565. doi:10.1016/j.jaad.2020.04.183
- Zito PM, Scharf R. Keratoacanthoma. StatPearls [Internet]. Updated August 8, 2023. Accessed August 13, 2025. https://www.ncbi.nlm.nih.gov/books/NBK499931/
THE DIAGNOSIS: Reactive Perforating Collagenosis
Histopathology revealed invagination of the epidermis with hyperkeratosis; prominent epidermal hyperplasia; and a central basophilic plug of keratin, collagen, and inflammatory debris. Transepidermal elimination of bright eosinophilic altered collagen fibers was seen (Figure). The findings were consistent with a diagnosis of reactive perforating collagenosis (RPC).

Reactive perforating collagenosis, a subtype of perforating dermatosis, is a rare skin condition in which altered collagen is eliminated through the epidermis.1 There are 2 forms of RPC: the inherited form, which is very rare and manifests in childhood, and the acquired form, which manifests in adulthood and is associated with systemic diseases, most notably diabetes and/or chronic renal failure, both of which our patient had been diagnosed with.1,2 The clinical presentation of RPC includes erythematous papules or nodules that evolve into umbilicated 4- to 10-mm craterlike ulcerations with a central keratotic plug. The lesions favor a linear distribution along the extensor surfaces of the arms and legs, trunk, and gluteal area. Involvement of the head, neck, and scalp has been reported less commonly, which makes our case particularly unique.3 Histopathologically, RPC is characterized by a cup-shaped depression of the epidermis with an overlying keratin plug containing inflammatory cells, keratinous debris, and collagen fibers. Vertically oriented collagen fibers are seen extruded through the epidermis.4,5
While the pathogenesis of RPC remains unknown, it is believed that superficial trauma due to chronic scratching results in transepithelial elimination of collagen. Due to the association of acquired RPC (ARPC) with diabetes, it also has been proposed that scratching can cause microtrauma and necrosis of the dermal structures, potentially due to diabetic microangiopathy.3 Additionally, RPC is associated with overexpression of transforming growth factor beta 3 in lesional skin, suggesting that transforming growth factor beta 3 is involved with tissue repair and extracellular remodeling in this condition.6
Treatment of ARPC should include the management of underlying disease. While no definitive treatment has been reported to date, topical corticosteroids, retinoids, keratolytics, emollients, antihistamines, narrow-band UVB phototherapy, and psoralen plus UVA phototherapy have been used with varying degrees of improvement. Typically, the lesions self-resolve within 6 to 8 weeks; however, they often recur and usually leave scarring with or without hyperpigmentation.2,7-10
Acquired RPC can be misdiagnosed initially, as it mimics several other conditions and commonly is associated with systemic diseases. While biopsy is necessary for diagnosis, if it cannot be performed or the results are indeterminate, dermoscopy can serve as a helpful diagnostic tool. The most common dermoscopic patterns seen in RPC include a yellow-brown structureless area in the center of the lesion with a peripheral surface crust and surrounding white rim—thought to represent epidermal invagination or keratinous debris. Additionally, inflammation with visible vessels both centrally and peripherally is represented by an outer pink circle on dermoscopy.5,11
The differential diagnoses for RPC include perforating folliculitis (PF), elastosis perforans serpiginosa (EPS), prurigo nodularis, and keratoacanthomas. The primary perforating dermatoses (PF, EPS, and RPC) are similarly characterized by elimination of altered dermal material through the epidermis. As these conditions manifest with similar features on clinical examination, differentiation is made by the type of epidermal damage and the features of elimination material, making histopathologic examination paramount for definitive diagnosis.
Perforating folliculitis manifests as erythematous, follicular papules with a small central keratotic core or a central hair. Histopathologically, PF reveals a widely dilated follicle containing keratin, necrotic debris, and degenerated inflammatory cells. Elastosis perforans serpiginosa manifests clinically as hyperkeratotic papules in serpiginous patterns rather than the linear pattern commonly seen with ARPC. Histopathologically, EPS reveals thickened elastic fibers, rather than collagen fibers as seen in ARPC, extruded through the epidermis. Prurigo nodularis manifests clinically as dome-shaped papules with possible excoriation and crusting. Histopathologic examination reveals epidermal hyperplasia and hyperkeratosis; however, the characteristic features of transepithelial elimination of collagen and invaginations of epidermis differentiate ARPC from prurigo nodularis.12,13 Keratoacanthomas manifest clinically as an eruption of small, round, pink papules that rapidly grow and evolve into 1- to 2-cm dome-shaped nodules with central keratinaceous plugs, mimicking a crateriform appearance. Histopathologic examination reveals a circumscribed proliferation of well-differentiated keratinocytes. Multilobular exophytic or endophytic cystlike invaginations of the epidermis also are noted. The expulsion of collagen from the epidermis is more consistent with ARPC.14
THE DIAGNOSIS: Reactive Perforating Collagenosis
Histopathology revealed invagination of the epidermis with hyperkeratosis; prominent epidermal hyperplasia; and a central basophilic plug of keratin, collagen, and inflammatory debris. Transepidermal elimination of bright eosinophilic altered collagen fibers was seen (Figure). The findings were consistent with a diagnosis of reactive perforating collagenosis (RPC).

Reactive perforating collagenosis, a subtype of perforating dermatosis, is a rare skin condition in which altered collagen is eliminated through the epidermis.1 There are 2 forms of RPC: the inherited form, which is very rare and manifests in childhood, and the acquired form, which manifests in adulthood and is associated with systemic diseases, most notably diabetes and/or chronic renal failure, both of which our patient had been diagnosed with.1,2 The clinical presentation of RPC includes erythematous papules or nodules that evolve into umbilicated 4- to 10-mm craterlike ulcerations with a central keratotic plug. The lesions favor a linear distribution along the extensor surfaces of the arms and legs, trunk, and gluteal area. Involvement of the head, neck, and scalp has been reported less commonly, which makes our case particularly unique.3 Histopathologically, RPC is characterized by a cup-shaped depression of the epidermis with an overlying keratin plug containing inflammatory cells, keratinous debris, and collagen fibers. Vertically oriented collagen fibers are seen extruded through the epidermis.4,5
While the pathogenesis of RPC remains unknown, it is believed that superficial trauma due to chronic scratching results in transepithelial elimination of collagen. Due to the association of acquired RPC (ARPC) with diabetes, it also has been proposed that scratching can cause microtrauma and necrosis of the dermal structures, potentially due to diabetic microangiopathy.3 Additionally, RPC is associated with overexpression of transforming growth factor beta 3 in lesional skin, suggesting that transforming growth factor beta 3 is involved with tissue repair and extracellular remodeling in this condition.6
Treatment of ARPC should include the management of underlying disease. While no definitive treatment has been reported to date, topical corticosteroids, retinoids, keratolytics, emollients, antihistamines, narrow-band UVB phototherapy, and psoralen plus UVA phototherapy have been used with varying degrees of improvement. Typically, the lesions self-resolve within 6 to 8 weeks; however, they often recur and usually leave scarring with or without hyperpigmentation.2,7-10
Acquired RPC can be misdiagnosed initially, as it mimics several other conditions and commonly is associated with systemic diseases. While biopsy is necessary for diagnosis, if it cannot be performed or the results are indeterminate, dermoscopy can serve as a helpful diagnostic tool. The most common dermoscopic patterns seen in RPC include a yellow-brown structureless area in the center of the lesion with a peripheral surface crust and surrounding white rim—thought to represent epidermal invagination or keratinous debris. Additionally, inflammation with visible vessels both centrally and peripherally is represented by an outer pink circle on dermoscopy.5,11
The differential diagnoses for RPC include perforating folliculitis (PF), elastosis perforans serpiginosa (EPS), prurigo nodularis, and keratoacanthomas. The primary perforating dermatoses (PF, EPS, and RPC) are similarly characterized by elimination of altered dermal material through the epidermis. As these conditions manifest with similar features on clinical examination, differentiation is made by the type of epidermal damage and the features of elimination material, making histopathologic examination paramount for definitive diagnosis.
Perforating folliculitis manifests as erythematous, follicular papules with a small central keratotic core or a central hair. Histopathologically, PF reveals a widely dilated follicle containing keratin, necrotic debris, and degenerated inflammatory cells. Elastosis perforans serpiginosa manifests clinically as hyperkeratotic papules in serpiginous patterns rather than the linear pattern commonly seen with ARPC. Histopathologically, EPS reveals thickened elastic fibers, rather than collagen fibers as seen in ARPC, extruded through the epidermis. Prurigo nodularis manifests clinically as dome-shaped papules with possible excoriation and crusting. Histopathologic examination reveals epidermal hyperplasia and hyperkeratosis; however, the characteristic features of transepithelial elimination of collagen and invaginations of epidermis differentiate ARPC from prurigo nodularis.12,13 Keratoacanthomas manifest clinically as an eruption of small, round, pink papules that rapidly grow and evolve into 1- to 2-cm dome-shaped nodules with central keratinaceous plugs, mimicking a crateriform appearance. Histopathologic examination reveals a circumscribed proliferation of well-differentiated keratinocytes. Multilobular exophytic or endophytic cystlike invaginations of the epidermis also are noted. The expulsion of collagen from the epidermis is more consistent with ARPC.14
- Cohen RW, Auerbach R. Acquired reactive perforating collagenosis. J Am Acad Dermatol. 1989;20(2 pt 1):287-289. doi:10.1016/s0190 -9622(89)80059-3
- Bejjanki H, Siroy AE, Koratala A. Reactive perforating collagenosis in end-stage renal disease: not all that itches is uremic pruritis! Am J Med. 2019;132:E658-E660. doi:10.1016/j.amjmed.2019.03.015
- Gontijo JRV, Júnior FF, Pereira LB, et al. Trauma-induced acquired reactive perforating collagenosis. An Bras Dermatol. 2021;96:392-393. doi:10.1016/j.abd.2020.06.022
- Ambalathinkal JJ, Phiske MM, Someshwar SJ. Acquired reactive perforating collagenosis, a rare entity at uncommon site. Indian J Pathol Microbiol. 2022;65:895-897. doi:10.4103/ijpm.ijpm_333_21
- Ormerod E, Atwan A, Intzedy L, et al. Dermoscopy features of acquired reactive perforating collagenosis: a case series. Dermatol Pract Concept. 2018;8:303-305. doi:10.5826/dpc.0804a11
- Fei C, Wang Y, Gong Y, et al. Acquired reactive perforating collagenosis: a report of a typical case. Medicine (Baltimore). 2016;95:E4305. doi:10.1097/md.0000000000004305
- Bartling SJ, Naff JL, Canevari MM, et al. Pruritic rash in an elderly patient with uncontrolled diabetes mellitus. AACE Clin Case Rep. 2018;5:E146-E149. doi:10.4158/ACCR-2018-0388
- Kollipara H, Satya RS, Rao GR, et al. Acquired reactive perforating collagenosis: case series. Indian Dermatol Online J. 2023;14:72-76. doi:10.4103/idoj.idoj_373_22
- Wang C, Liu YH, Wang YX, et al. Acquired reactive perforating collagenosis. Chin Med J (Engl). 2020;133:2119-2120. doi:10.1097 /cm9.0000000000000906
- Harbaoui S, Litaiem N. Acquired perforating dermatosis. StatPearls [Internet]. Updated February 13, 2023. Accessed August 13, 2025. https://www.ncbi.nlm.nih.gov/books/NBK539715/
- Elmas ÖF, Kilitci A, Uyar B. Dermoscopic patterns of acquired reactive perforating collagenosis. Dermatol Pract Concept. 2021;11:E2020085. doi:10.5826/dpc.1101a85
- Patterson JW. The perforating disorders. J Am Acad Dermatol. 1984;10:561-581. doi:10.1016/s0190-9622(84)80259-5
- Huang AH, Williams KA, Kwatra SG. Prurigo nodularis: epidemiology and clinical features. J Am Acad Dermatol. 2020;83:1559-1565. doi:10.1016/j.jaad.2020.04.183
- Zito PM, Scharf R. Keratoacanthoma. StatPearls [Internet]. Updated August 8, 2023. Accessed August 13, 2025. https://www.ncbi.nlm.nih.gov/books/NBK499931/
- Cohen RW, Auerbach R. Acquired reactive perforating collagenosis. J Am Acad Dermatol. 1989;20(2 pt 1):287-289. doi:10.1016/s0190 -9622(89)80059-3
- Bejjanki H, Siroy AE, Koratala A. Reactive perforating collagenosis in end-stage renal disease: not all that itches is uremic pruritis! Am J Med. 2019;132:E658-E660. doi:10.1016/j.amjmed.2019.03.015
- Gontijo JRV, Júnior FF, Pereira LB, et al. Trauma-induced acquired reactive perforating collagenosis. An Bras Dermatol. 2021;96:392-393. doi:10.1016/j.abd.2020.06.022
- Ambalathinkal JJ, Phiske MM, Someshwar SJ. Acquired reactive perforating collagenosis, a rare entity at uncommon site. Indian J Pathol Microbiol. 2022;65:895-897. doi:10.4103/ijpm.ijpm_333_21
- Ormerod E, Atwan A, Intzedy L, et al. Dermoscopy features of acquired reactive perforating collagenosis: a case series. Dermatol Pract Concept. 2018;8:303-305. doi:10.5826/dpc.0804a11
- Fei C, Wang Y, Gong Y, et al. Acquired reactive perforating collagenosis: a report of a typical case. Medicine (Baltimore). 2016;95:E4305. doi:10.1097/md.0000000000004305
- Bartling SJ, Naff JL, Canevari MM, et al. Pruritic rash in an elderly patient with uncontrolled diabetes mellitus. AACE Clin Case Rep. 2018;5:E146-E149. doi:10.4158/ACCR-2018-0388
- Kollipara H, Satya RS, Rao GR, et al. Acquired reactive perforating collagenosis: case series. Indian Dermatol Online J. 2023;14:72-76. doi:10.4103/idoj.idoj_373_22
- Wang C, Liu YH, Wang YX, et al. Acquired reactive perforating collagenosis. Chin Med J (Engl). 2020;133:2119-2120. doi:10.1097 /cm9.0000000000000906
- Harbaoui S, Litaiem N. Acquired perforating dermatosis. StatPearls [Internet]. Updated February 13, 2023. Accessed August 13, 2025. https://www.ncbi.nlm.nih.gov/books/NBK539715/
- Elmas ÖF, Kilitci A, Uyar B. Dermoscopic patterns of acquired reactive perforating collagenosis. Dermatol Pract Concept. 2021;11:E2020085. doi:10.5826/dpc.1101a85
- Patterson JW. The perforating disorders. J Am Acad Dermatol. 1984;10:561-581. doi:10.1016/s0190-9622(84)80259-5
- Huang AH, Williams KA, Kwatra SG. Prurigo nodularis: epidemiology and clinical features. J Am Acad Dermatol. 2020;83:1559-1565. doi:10.1016/j.jaad.2020.04.183
- Zito PM, Scharf R. Keratoacanthoma. StatPearls [Internet]. Updated August 8, 2023. Accessed August 13, 2025. https://www.ncbi.nlm.nih.gov/books/NBK499931/
Diffuse Pruritic Keratotic Papules
Diffuse Pruritic Keratotic Papules
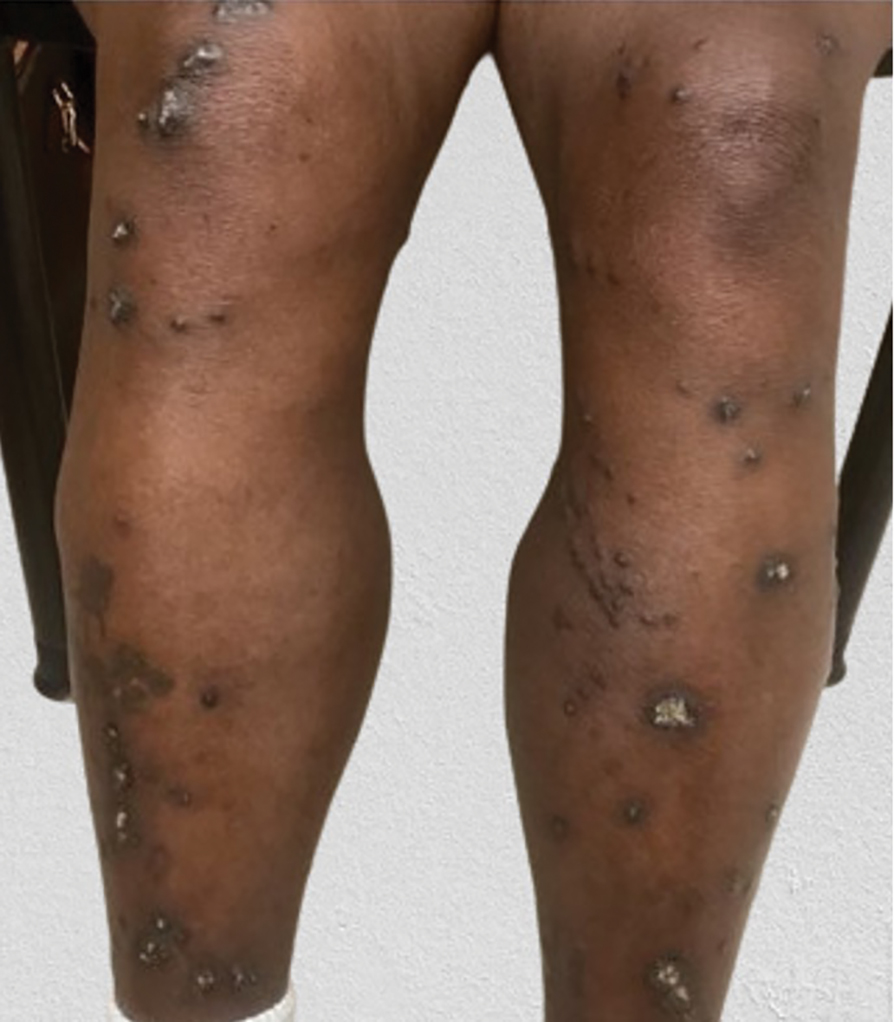
A 65-year-old woman presented to dermatology with an intensely pruritic rash on the arms, legs, neck, and face of several months’ duration. The patient reported scratching the lesions but denied any recent trauma to the affected areas. She previously had been evaluated by her primary care provider, who prescribed cephalexin with no improvement. Her medical history was remarkable for chronic renal failure on dialysis, diabetes, hypertension, and congestive heart failure. Physical examination of the skin revealed hard white cutaneous nodules distributed on the proximal posterior upper arms, bilateral proximal pretibial regions, right elbow, and left knee. Two shave biopsies from the right elbow and left knee were obtained for histopathology.
Advantages and Disadvantages of Private vs Academic Dermatology Practices
Advantages and Disadvantages of Private vs Academic Dermatology Practices
Dermatology is a rapidly growing, highly competitive specialty with patients that can be served via private practice, academic medicine, hybrid settings, and rural health clinics. Medical residents’ choice of a career path has been rapidly evolving alongside shifts in health care policy, increasing demand for dermatologic services, stagnant fees falling behind inflation for more than a decade, and payment methods that no longer reflect the traditional fee-for-service model. This places a lot of pressure on young dermatologists to evaluate which practice structure best fits their career goals. A nuanced understanding of the strengths and limitations of each practice model is essential for dermatologists to make informed career decisions that are aligned with their values.
While there are many health care practice models, the first decision dermatology residents must make is whether they would prefer working in the private sector or an academic practice. Of course, it is not uncommon for academic dermatologists to embark on a midcareer segue into private practice and, less commonly, for private dermatologists to culminate their careers with a move to academics. The private sector includes private practice, private equity (PE)–owned group practices that often are single-specialty focused, and hospital-owned group practices that usually are multispecialty. Traditionally, private practices are health care businesses owned by one physician (solo practice) or a group of physicians (group practice) operated independently from hospitals, health systems, or private investors. Financially, these practices rely heavily on volume-based services, especially clinic visits and cosmetic procedures, which provide higher reimbursement rates and usually cash payments at the time of service.1 Roughly 35% of dermatologists in the United States work in private practice, and a dwindling 15% work in solo practice.2,3
Medical practices that are not self-owned by physicians vary widely, and they include hospital- or medical center–owned, private equity, and university-based academic practices. Private equity practices typically are characterized as profit driven. Hospital-owned practices shoulder business decisions and administrative duties for the physician at the cost of provider autonomy. Academic medicine is the most different from the other practice types. In contrast to private practice dermatologists, university-based dermatologists practice at academic medical centers (AMCs) with the core goals of patient care, education, and research. Compensation generally is based on the relative value unit (RVU), which is supplemented by government support and research grants.
As evidenced in this brief discussion, health care practice models are complex, and choosing the right model to align with professional goals can pose a major challenge for many physicians. The advantages and disadvantages of various practice models will be reviewed, highlighting trends and emerging models.
Solo or Small-Group Single-Specialty Private Practice
Private practice offers dermatologists the advantage of higher income potential but with greater economic risk; it often requires physicians to be more involved in the business aspects of dermatologic practice. In the early 1990s, a survey of private practice dermatologists revealed that income was the first or second most important factor that contributed to their career choice of private vs academic practice.4 Earning potential in private practice largely is driven by the autonomy afforded in this setting. Physicians have the liberty of choosing their practice location, structure, schedule, and staff in addition to tailoring services toward profitability; this typically leads to a higher volume of cosmetic and procedural visits, which may be attractive to providers wishing to focus on aesthetics. Private practice dermatologists also are not subject to institutional requirements that may include the preparation of grant submissions, research productivity targets, and devotion of time to teaching. Many private dermatologists find satisfaction in tailoring their work environments to align with personal values and goals and in cultivating long-term relationships with patients in a more personal and less bureaucratic context.
There also are drawbacks to private practice. The profitability often can be attributed to the higher patient load and more hours devoted to practice.5 A 2006 study found that academics saw 32% to 41% fewer patients per week than private practice dermatologists.6 Along with the opportunity for financial gain is the risk of financial ruin. Cost is the largest hurdle for establishing a practice, and most practices do not turn a profit for the first few years.1,5 The financial burden of running a practice includes pressure from the federal government to adopt expensive electronic health record systems to achieve maximum Medicare payment through the Merit-Based Incentive Payment System, liability insurance, health insurance, and staff salaries.7 These challenges require strong business acumen, including managing overhead costs, navigating insurance negotiations, marketing a practice, and maintaining compliance with evolving health care regulations. The purchase of a $100,000 laser could be a boon or bust, requiring the development of a business plan that ensures a positive return on investment. Additionally, private practice profitability has the potential to dwindle as governmental reimbursements fail to match inflation rates. Securing business advisors or even obtaining a Master of Business Administration degree can be helpful.
Insurance and government agencies also are infringing upon some of the autonomy of private practice dermatologists, as evidenced by a 2017 survey of dermatologists that found that more than half of respondents altered treatment plans based on insurance coverage more than 20% of the time.2 Private equity firms also could infringe on private practice autonomy, as providers are beholden to the firm’s restrictions—from which company’s product will be stocked to which partner will be on call. Lastly, private practice is less conducive to consistent referral patterns and strong relationships with specialists when compared to academic practice. Additionally, reliance on high patient throughput or cosmetic services for financial sustainability can shift focus away from complex medical dermatology, which often is referred to AMCs.
Academic Medicine
Academic dermatology offers a stimulating and collaborative environment with opportunities to advance the field through research and education. Often, the opportunity to teach medical students, residents, and peers is the deciding factor for academic dermatologists, as supported by a 2016 survey that found teaching opportunities are a major influence on career decision.8 The mixture of patient care, education, and research roles can be satisfying when compared to the grind of seeing large numbers of patients every day. Because they typically are salaried with an RVU-based income, academic dermatologists often are less concerned with the costs associated with medical treatment, and they typically treat more medically complex patients and underserved populations.9 The salary structure of academic roles also provides the benefit of a stable and predictable income. Physicians in this setting often are considered experts in their field, positioning them to have a strong built-in referral system along with frequent participation in multidisciplinary care alongside colleagues in rheumatology, oncology, and infectious diseases. The benefits of downstream income from dermatopathology, Mohs surgery, and other ancillary testing can provide great financial advantages for an academic or large group practice.10 Academic medical centers also afford the benefit of resources, such as research offices, clinical trial units, and institutional support for scholarly publication.
Despite its benefits, academic dermatology is not without unique demands. The resources afforded by research work come with grant application deadlines and the pressure to maintain research productivity as measured by grant dollars. Academic providers also must navigate institutional political dynamics and deal with limits on autonomy. Additionally, the administrative burden associated with committee work, mentorship obligations, and publishing requirements further limit clinical time and contribute to burnout. According to Loo et al,5 92% of 89 dermatology department chairmen responding to a poll believed that the lower compensation was the primary factor preventing more residents from pursuing academia.
The adoption of RVU-based and incentive compensation models at many AMCs, along with dwindling government funds available for research, also have created pressure to increase patient volume, sometimes at the expense of teaching and research. Of those academic dermatologists spending more than half their time seeing patients, a majority reported that they lack the time to also conduct research, teach, and mentor students and resident physicians.6 A survey of academic dermatologists suggested that, for those already serving in academic positions, salary was less of a concern than the lack of protected academic time.4 While competing demands can erode the appeal of academic dermatology, academia continues to offer a meaningful and fulfilling career path for those motivated by scholarship, mentorship, teaching opportunities, and systemic impact.
Hybrid and Emerging Models
To reconcile the trade-offs inherent in private and academic models, hybrid roles are becoming increasingly common. In these arrangements, dermatologists split their time between private practice and academic appointments settings, allowing for participation in resident education and research while also benefiting from the operational and financial structure of a private office. In some cases, private groups formally affiliate with academic institutions, creating academic-private practices that host trainees and produce scholarly work while operating financially outside of traditional hospital systems. Individual dermatologists also may choose to accept part-time academic roles that allow residents and medical students to rotate in their offices. Hybrid roles may be of most interest to individuals who feel that they are missing out on the mentorship and teaching opportunities afforded at AMCs.
Government-funded systems such as Veterans Affairs (VA) hospitals offer another alternative. Dermatologists at VA hospitals often hold faculty appointments, treat a wide range of conditions in a population with great need, and engage in teaching without the intensity of productivity requirements seen at AMCs. These roles can be attractive to physicians who value public service, work-life balance, and minimal malpractice risk, as well as dermatologists who wish to introduce variety in their practice through an additional clinical setting. Notably, these roles are limited, as roughly 80% of VA hospitals employ part-time dermatologists and 72% reported being understaffed.11 Despite the challenges of limited resources and increased bureaucracy, the VA is the largest health care delivery system in the United States, offering the benefits of protection from most malpractice risk and participation in medical education at 80% of VA hospitals.12 A VA-based practice may be most attractive to physicians with prior military service or those looking for a stable practice that serves the underserved and the mission of medical education.
Similarly, rural health clinics are private practices with special subsidies from the federal government that bring Medicaid payments up to the level of Medicare.13 Rural dermatology also mirrors that of a VA-based practice by offering the opportunity to treat an array of conditions in a population of great need, as rural patients often are in care deserts and would otherwise need to travel for miles to receive dermatologic care. There is a shortage of dermatologists working in rural areas, and rural dermatologists are more likely than those in suburban or urban areas to practice alone.2 Although potentially more physically isolating, rural dermatology offers providers the opportunity to establish a lucrative practice with minimal competition and development of meaningful patient relationships.
The most rapidly increasing practice model emerging in dermatology over the past decade is the private equity (PE) group. Rajabi-Estarabadi et al14 estimated that at least 184 dermatology practices have been acquired by PE groups between 2010 and 2019. An estimated 15% of all PE acquisitions in health care have been within the field of dermatology.9 Private equity firms typically acquire 1 or more practices, then consolidate the operations with the short-term goals of reducing costs and maximizing profits and longer-term goals of selling the practice for further profit in 3 to 7 years.9 They often rely heavily on a dermatologist supervising a number of nurse practitioners.15 While PE acquisition may provide additional financial stability and income, providers have less autonomy and potentially risk a shift in their focus from patient care to profit.
The blurred lines between practice settings reflect a broader shift in the profession. Dermatologists have increasingly crafted flexible, individualized careers that align with their goals and values while drawing from both academic and private models. Hybrid roles may prove critical in preserving the educational and research missions of dermatology while adapting to economic and institutional realities.
Gender Trends, Career Satisfaction, and Other Factors Influencing Career Choice
The gender demographics of dermatology have changed greatly in recent decades. In the years 2010 to 2021, the percentage of women in the field rose from 41% to 52.2%, mirroring the rise in female medical students.16 Despite this, gender disparities persist through differences in pay, promotion rates, leadership opportunities, and research productivity.17 Women who are academic dermatologists are less likely to have protected research time and often shoulder a disproportionate share of mentorship and administrative responsibilities, which frequently are undervalued in promotion and compensation structures. Similarly, women physicians are less likely to own their own private practice.18 Notably, women physicians work part-time more often than their male counterparts, which likely impacts their income.19 Interestingly, no differences were noted in job satisfaction between men and women in academic or private practice settings, suggesting that dermatology is a fulfilling field for female physicians.16 Similar data were observed in the field of dermatopathology; in fact, there is no difference in job satisfaction when comparing providers in academics vs private practice.20
Geographic factors also influence career decisions. Some dermatologists may choose private practice to remain close to family or serve a rural area, while some choose academic centers typically located in major metropolitan areas. Others are drawn to AMCs due to their reputation, resources, or opportunities for specialization. The number of practicing dermatologists in an area also may be considered, as areas with fewer providers likely have more individuals seeking a provider and thus more earning potential.
In summary, career satisfaction is influenced by many factors, including practice setting, colleagues, institutional leadership, work environment, and professional goals. For individuals who are seeking intellectual stimulation and teaching opportunities, academic dermatology may be a great career option. Academic or large group practices may come with a large group of clinical dermatologists to provide a steady stream of specimens. Private practice appeals to those seeking autonomy, reduced bureaucracy, and higher earning potential. Tierney et al21 found that the greatest predictor of a future career in academics among Mohs surgeons was the number of publications a fellow had before and during fellowship training. These data suggest that personal interests greatly influence career decisions.
The Role of Mentorship in Career Decision-Making
Just as personal preferences guide career decisions, so too do interpersonal interactions. Mentorship plays a large role in career success, and the involvement of faculty mentors in society meetings and editorial boards has been shown to positively correlate with the number of residents pursuing academia.14 Similarly, negative interactions have strong impacts, as the top cited reason for Mohs surgeons leaving academia was lack of support from their academic chair.21 While many academic dermatologists report fulfillment from the collegial environment, retention remains an issue. Tierney et al21 found that, among 455 academic Mohs surgeons, only 28% of those who began in academia remained in those roles over the long term, and this trend of low retention holds true across the field of academic dermatology. Lack of autonomy, insufficient institutional support, and more lucrative private practice opportunities were all cited as reasons for leaving. For dermatologists seeking separation from academics but continued research opportunities, data suggest that private practice allows for continued research and publications, indicating that scholarly engagement is not exclusive to academic settings. These trends point to the increasing viability of hybrid or academic-private models that combine academic productivity with greater flexibility and financial stability.
Final Thoughts
Academic and private practice dermatology each offer compelling advantages and distinct challenges (Table). The growing popularity of hybrid models reflects a desire among dermatologists to balance the intellectual fulfillment associated with academic medicine with professional sustainability and autonomy of private practice. Whether through part-time academic appointments, rural health clinics, VA employment, or affiliations between private groups and academic institutions, these emerging roles offer a flexible and adaptive approach to career development.
Ultimately, the ideal practice model is one that aligns with a physician’s personal values, long-term goals, and lifestyle preferences. No single path fits all, but thoughtful career planning supported by mentorship and institutional transparency can help dermatologists thrive in a rapidly evolving health care landscape.
- Kaplan J. Part I: private practice versus academic medicine. BoardVitals Blog. June 5, 2018. Accessed August 5, 2025. https://www.boardvitals.com/blog/private-practice-academic-medicine/
- Ehrlich A, Kostecki J, Olkaba H. Trends in dermatology practices and the implications for the workforce. J Am Acad Dermatol. 2017;77:746-752. doi:10.1016/j.jaad.2017.06.030
- Parthasarathy V, Pollock JR, McNeely GL, et al. A cross-sectional analysis of trends in dermatology practice size in the United States from 2012 to 2020. Arch Dermatol Res. 2022;315:223-229. doi:10.1007/s00403-022-02344-0
- Bergstresser PR. Perceptions of the academic environment: a national survey. J Am Acad Dermatol. 1991;25:1092-1096. doi:10.1016/0190-9622(91)70311-o
- Loo DS, Liu CL, Geller AC, et al. Academic dermatology manpower: issues of recruitment and retention. Arch Dermatol. 2007;143:341-347. doi:10.1001/archderm.143.3.341
- Resneck JS, Tierney EP, Kimball AB. Challenges facing academic dermatology: survey data on the faculty workforce. J Am Acad Dermatol. 2006;54:211-216. doi:10.1016/j.jaad.2005.10.013
- Salmen N, Brodell R, Brodell Dolohanty L. The electronic health record: should small practices adopt this technology? J of Skin. 2024;8:1269-1273. doi:10.25251/skin.8.1.8
- Morales-Pico BM, Cotton CC, Morrell DS. Factors correlated with residents’ decisions to enter academic dermatology. Dermatol Online J. 2016;22:13030/qt7295783b.
- DeWane ME, Mostow E, Grant-Kels JM. The corporatization of care in academic dermatology. Clin Dermatol. 2020;38:289-295. doi:10.1016/j.clindermatol.2020.02.003
- Pearlman RL, Nahar VK, Sisson WT, et al. Understanding downstream service profitability generated by dermatology faculty in an academic medical center: a key driver to promotion of access-to-care. Arch Dermatol Res. 2023;315:1425-1427. doi:10.1007/s00403-022-02406-3
- Huang WW, Tsoukas MM, Bhutani T, et al. Benchmarking U.S. Department of Veterans Affairs dermatologic services: a nationwide survey of VA dermatologists. J Am Acad Dermatol. 2011;65:50-54. doi:10.1016/j.jaad.2010.04.035
- 20 reasons doctors like working for the Veterans Health Administration. US Department of Veterans Affairs. August 2016. Accessed August 5, 2025. https://www.va.gov/HEALTH/docs/20ReasonsVHA_508_IB10935.pdf
- Rural health clinics (RHCs). Rural Health Information Hub. Updated April 7, 2025. Accessed August 5, 2025. https://www .ruralhealthinfo.org/topics/rural-health-clinics
- Rajabi-Estarabadi A, Jones VA, Zheng C, et al. Dermatologist transitions: academics into private practices and vice versa. Clin Dermatol. 2020;38:541-546. doi:10.1016/j.clindermatol.2020.05.012
- Bruch JD, Foot C, Singh Y, et al. Workforce composition in private equity–acquired versus non–private equity–acquired physician practices. Health Affairs. 2023;42:121-129. doi:10.1377/hlthaff.2022.00308
- Zlakishvili B, Horev A. Gender disparities in high-quality dermatology research over the past 15 years. Int J Womens Dermatol. 2024;10:e160. doi:10.1097/JW9.0000000000000160
- Jambusaria-Pahlajani A, Crow LD, Levender MM, et al. Practice patterns and job satisfaction of Mohs surgeons: a gender-based survey. J Drugs Dermatol. 2017;16:1103-1108. https://pubmed.ncbi.nlm.nih.gov/29140863/
- Kane CK. Policy Research Perspectives. Recent changes in physician practice arrangements: shifts away from private practice and towards larger practice size continue through 2022. American Medical Association website. 2023. Accessed August 5, 2025. https://www.ama-assn.org/system/files/2022-prp-practice-arrangement.pdf
- Frank E, Zhao Z, Sen S, et al. Gender disparities in work and parental status among early career physicians. JAMA Netw Open. 2019;2:e198340. doi:10.1001/jamanetworkopen.2019.8340
- Boyd AS, Fang F. A survey-based evaluation of dermatopathology in the United States. Am J Dermatopathol. 2011;33:173-176. doi:10.1097/dad.0b013e3181f0ed84
- Tierney EP, Hanke CW, Kimball AB. Career trajectory and job satisfaction trends in Mohs micrographic surgeons. Dermatol Surg. 2011;37:1229-1238. doi:10.1111/j.1524-4725.2011.02076.x
Dermatology is a rapidly growing, highly competitive specialty with patients that can be served via private practice, academic medicine, hybrid settings, and rural health clinics. Medical residents’ choice of a career path has been rapidly evolving alongside shifts in health care policy, increasing demand for dermatologic services, stagnant fees falling behind inflation for more than a decade, and payment methods that no longer reflect the traditional fee-for-service model. This places a lot of pressure on young dermatologists to evaluate which practice structure best fits their career goals. A nuanced understanding of the strengths and limitations of each practice model is essential for dermatologists to make informed career decisions that are aligned with their values.
While there are many health care practice models, the first decision dermatology residents must make is whether they would prefer working in the private sector or an academic practice. Of course, it is not uncommon for academic dermatologists to embark on a midcareer segue into private practice and, less commonly, for private dermatologists to culminate their careers with a move to academics. The private sector includes private practice, private equity (PE)–owned group practices that often are single-specialty focused, and hospital-owned group practices that usually are multispecialty. Traditionally, private practices are health care businesses owned by one physician (solo practice) or a group of physicians (group practice) operated independently from hospitals, health systems, or private investors. Financially, these practices rely heavily on volume-based services, especially clinic visits and cosmetic procedures, which provide higher reimbursement rates and usually cash payments at the time of service.1 Roughly 35% of dermatologists in the United States work in private practice, and a dwindling 15% work in solo practice.2,3
Medical practices that are not self-owned by physicians vary widely, and they include hospital- or medical center–owned, private equity, and university-based academic practices. Private equity practices typically are characterized as profit driven. Hospital-owned practices shoulder business decisions and administrative duties for the physician at the cost of provider autonomy. Academic medicine is the most different from the other practice types. In contrast to private practice dermatologists, university-based dermatologists practice at academic medical centers (AMCs) with the core goals of patient care, education, and research. Compensation generally is based on the relative value unit (RVU), which is supplemented by government support and research grants.
As evidenced in this brief discussion, health care practice models are complex, and choosing the right model to align with professional goals can pose a major challenge for many physicians. The advantages and disadvantages of various practice models will be reviewed, highlighting trends and emerging models.
Solo or Small-Group Single-Specialty Private Practice
Private practice offers dermatologists the advantage of higher income potential but with greater economic risk; it often requires physicians to be more involved in the business aspects of dermatologic practice. In the early 1990s, a survey of private practice dermatologists revealed that income was the first or second most important factor that contributed to their career choice of private vs academic practice.4 Earning potential in private practice largely is driven by the autonomy afforded in this setting. Physicians have the liberty of choosing their practice location, structure, schedule, and staff in addition to tailoring services toward profitability; this typically leads to a higher volume of cosmetic and procedural visits, which may be attractive to providers wishing to focus on aesthetics. Private practice dermatologists also are not subject to institutional requirements that may include the preparation of grant submissions, research productivity targets, and devotion of time to teaching. Many private dermatologists find satisfaction in tailoring their work environments to align with personal values and goals and in cultivating long-term relationships with patients in a more personal and less bureaucratic context.
There also are drawbacks to private practice. The profitability often can be attributed to the higher patient load and more hours devoted to practice.5 A 2006 study found that academics saw 32% to 41% fewer patients per week than private practice dermatologists.6 Along with the opportunity for financial gain is the risk of financial ruin. Cost is the largest hurdle for establishing a practice, and most practices do not turn a profit for the first few years.1,5 The financial burden of running a practice includes pressure from the federal government to adopt expensive electronic health record systems to achieve maximum Medicare payment through the Merit-Based Incentive Payment System, liability insurance, health insurance, and staff salaries.7 These challenges require strong business acumen, including managing overhead costs, navigating insurance negotiations, marketing a practice, and maintaining compliance with evolving health care regulations. The purchase of a $100,000 laser could be a boon or bust, requiring the development of a business plan that ensures a positive return on investment. Additionally, private practice profitability has the potential to dwindle as governmental reimbursements fail to match inflation rates. Securing business advisors or even obtaining a Master of Business Administration degree can be helpful.
Insurance and government agencies also are infringing upon some of the autonomy of private practice dermatologists, as evidenced by a 2017 survey of dermatologists that found that more than half of respondents altered treatment plans based on insurance coverage more than 20% of the time.2 Private equity firms also could infringe on private practice autonomy, as providers are beholden to the firm’s restrictions—from which company’s product will be stocked to which partner will be on call. Lastly, private practice is less conducive to consistent referral patterns and strong relationships with specialists when compared to academic practice. Additionally, reliance on high patient throughput or cosmetic services for financial sustainability can shift focus away from complex medical dermatology, which often is referred to AMCs.
Academic Medicine
Academic dermatology offers a stimulating and collaborative environment with opportunities to advance the field through research and education. Often, the opportunity to teach medical students, residents, and peers is the deciding factor for academic dermatologists, as supported by a 2016 survey that found teaching opportunities are a major influence on career decision.8 The mixture of patient care, education, and research roles can be satisfying when compared to the grind of seeing large numbers of patients every day. Because they typically are salaried with an RVU-based income, academic dermatologists often are less concerned with the costs associated with medical treatment, and they typically treat more medically complex patients and underserved populations.9 The salary structure of academic roles also provides the benefit of a stable and predictable income. Physicians in this setting often are considered experts in their field, positioning them to have a strong built-in referral system along with frequent participation in multidisciplinary care alongside colleagues in rheumatology, oncology, and infectious diseases. The benefits of downstream income from dermatopathology, Mohs surgery, and other ancillary testing can provide great financial advantages for an academic or large group practice.10 Academic medical centers also afford the benefit of resources, such as research offices, clinical trial units, and institutional support for scholarly publication.
Despite its benefits, academic dermatology is not without unique demands. The resources afforded by research work come with grant application deadlines and the pressure to maintain research productivity as measured by grant dollars. Academic providers also must navigate institutional political dynamics and deal with limits on autonomy. Additionally, the administrative burden associated with committee work, mentorship obligations, and publishing requirements further limit clinical time and contribute to burnout. According to Loo et al,5 92% of 89 dermatology department chairmen responding to a poll believed that the lower compensation was the primary factor preventing more residents from pursuing academia.
The adoption of RVU-based and incentive compensation models at many AMCs, along with dwindling government funds available for research, also have created pressure to increase patient volume, sometimes at the expense of teaching and research. Of those academic dermatologists spending more than half their time seeing patients, a majority reported that they lack the time to also conduct research, teach, and mentor students and resident physicians.6 A survey of academic dermatologists suggested that, for those already serving in academic positions, salary was less of a concern than the lack of protected academic time.4 While competing demands can erode the appeal of academic dermatology, academia continues to offer a meaningful and fulfilling career path for those motivated by scholarship, mentorship, teaching opportunities, and systemic impact.
Hybrid and Emerging Models
To reconcile the trade-offs inherent in private and academic models, hybrid roles are becoming increasingly common. In these arrangements, dermatologists split their time between private practice and academic appointments settings, allowing for participation in resident education and research while also benefiting from the operational and financial structure of a private office. In some cases, private groups formally affiliate with academic institutions, creating academic-private practices that host trainees and produce scholarly work while operating financially outside of traditional hospital systems. Individual dermatologists also may choose to accept part-time academic roles that allow residents and medical students to rotate in their offices. Hybrid roles may be of most interest to individuals who feel that they are missing out on the mentorship and teaching opportunities afforded at AMCs.
Government-funded systems such as Veterans Affairs (VA) hospitals offer another alternative. Dermatologists at VA hospitals often hold faculty appointments, treat a wide range of conditions in a population with great need, and engage in teaching without the intensity of productivity requirements seen at AMCs. These roles can be attractive to physicians who value public service, work-life balance, and minimal malpractice risk, as well as dermatologists who wish to introduce variety in their practice through an additional clinical setting. Notably, these roles are limited, as roughly 80% of VA hospitals employ part-time dermatologists and 72% reported being understaffed.11 Despite the challenges of limited resources and increased bureaucracy, the VA is the largest health care delivery system in the United States, offering the benefits of protection from most malpractice risk and participation in medical education at 80% of VA hospitals.12 A VA-based practice may be most attractive to physicians with prior military service or those looking for a stable practice that serves the underserved and the mission of medical education.
Similarly, rural health clinics are private practices with special subsidies from the federal government that bring Medicaid payments up to the level of Medicare.13 Rural dermatology also mirrors that of a VA-based practice by offering the opportunity to treat an array of conditions in a population of great need, as rural patients often are in care deserts and would otherwise need to travel for miles to receive dermatologic care. There is a shortage of dermatologists working in rural areas, and rural dermatologists are more likely than those in suburban or urban areas to practice alone.2 Although potentially more physically isolating, rural dermatology offers providers the opportunity to establish a lucrative practice with minimal competition and development of meaningful patient relationships.
The most rapidly increasing practice model emerging in dermatology over the past decade is the private equity (PE) group. Rajabi-Estarabadi et al14 estimated that at least 184 dermatology practices have been acquired by PE groups between 2010 and 2019. An estimated 15% of all PE acquisitions in health care have been within the field of dermatology.9 Private equity firms typically acquire 1 or more practices, then consolidate the operations with the short-term goals of reducing costs and maximizing profits and longer-term goals of selling the practice for further profit in 3 to 7 years.9 They often rely heavily on a dermatologist supervising a number of nurse practitioners.15 While PE acquisition may provide additional financial stability and income, providers have less autonomy and potentially risk a shift in their focus from patient care to profit.
The blurred lines between practice settings reflect a broader shift in the profession. Dermatologists have increasingly crafted flexible, individualized careers that align with their goals and values while drawing from both academic and private models. Hybrid roles may prove critical in preserving the educational and research missions of dermatology while adapting to economic and institutional realities.
Gender Trends, Career Satisfaction, and Other Factors Influencing Career Choice
The gender demographics of dermatology have changed greatly in recent decades. In the years 2010 to 2021, the percentage of women in the field rose from 41% to 52.2%, mirroring the rise in female medical students.16 Despite this, gender disparities persist through differences in pay, promotion rates, leadership opportunities, and research productivity.17 Women who are academic dermatologists are less likely to have protected research time and often shoulder a disproportionate share of mentorship and administrative responsibilities, which frequently are undervalued in promotion and compensation structures. Similarly, women physicians are less likely to own their own private practice.18 Notably, women physicians work part-time more often than their male counterparts, which likely impacts their income.19 Interestingly, no differences were noted in job satisfaction between men and women in academic or private practice settings, suggesting that dermatology is a fulfilling field for female physicians.16 Similar data were observed in the field of dermatopathology; in fact, there is no difference in job satisfaction when comparing providers in academics vs private practice.20
Geographic factors also influence career decisions. Some dermatologists may choose private practice to remain close to family or serve a rural area, while some choose academic centers typically located in major metropolitan areas. Others are drawn to AMCs due to their reputation, resources, or opportunities for specialization. The number of practicing dermatologists in an area also may be considered, as areas with fewer providers likely have more individuals seeking a provider and thus more earning potential.
In summary, career satisfaction is influenced by many factors, including practice setting, colleagues, institutional leadership, work environment, and professional goals. For individuals who are seeking intellectual stimulation and teaching opportunities, academic dermatology may be a great career option. Academic or large group practices may come with a large group of clinical dermatologists to provide a steady stream of specimens. Private practice appeals to those seeking autonomy, reduced bureaucracy, and higher earning potential. Tierney et al21 found that the greatest predictor of a future career in academics among Mohs surgeons was the number of publications a fellow had before and during fellowship training. These data suggest that personal interests greatly influence career decisions.
The Role of Mentorship in Career Decision-Making
Just as personal preferences guide career decisions, so too do interpersonal interactions. Mentorship plays a large role in career success, and the involvement of faculty mentors in society meetings and editorial boards has been shown to positively correlate with the number of residents pursuing academia.14 Similarly, negative interactions have strong impacts, as the top cited reason for Mohs surgeons leaving academia was lack of support from their academic chair.21 While many academic dermatologists report fulfillment from the collegial environment, retention remains an issue. Tierney et al21 found that, among 455 academic Mohs surgeons, only 28% of those who began in academia remained in those roles over the long term, and this trend of low retention holds true across the field of academic dermatology. Lack of autonomy, insufficient institutional support, and more lucrative private practice opportunities were all cited as reasons for leaving. For dermatologists seeking separation from academics but continued research opportunities, data suggest that private practice allows for continued research and publications, indicating that scholarly engagement is not exclusive to academic settings. These trends point to the increasing viability of hybrid or academic-private models that combine academic productivity with greater flexibility and financial stability.
Final Thoughts
Academic and private practice dermatology each offer compelling advantages and distinct challenges (Table). The growing popularity of hybrid models reflects a desire among dermatologists to balance the intellectual fulfillment associated with academic medicine with professional sustainability and autonomy of private practice. Whether through part-time academic appointments, rural health clinics, VA employment, or affiliations between private groups and academic institutions, these emerging roles offer a flexible and adaptive approach to career development.

Ultimately, the ideal practice model is one that aligns with a physician’s personal values, long-term goals, and lifestyle preferences. No single path fits all, but thoughtful career planning supported by mentorship and institutional transparency can help dermatologists thrive in a rapidly evolving health care landscape.
Dermatology is a rapidly growing, highly competitive specialty with patients that can be served via private practice, academic medicine, hybrid settings, and rural health clinics. Medical residents’ choice of a career path has been rapidly evolving alongside shifts in health care policy, increasing demand for dermatologic services, stagnant fees falling behind inflation for more than a decade, and payment methods that no longer reflect the traditional fee-for-service model. This places a lot of pressure on young dermatologists to evaluate which practice structure best fits their career goals. A nuanced understanding of the strengths and limitations of each practice model is essential for dermatologists to make informed career decisions that are aligned with their values.
While there are many health care practice models, the first decision dermatology residents must make is whether they would prefer working in the private sector or an academic practice. Of course, it is not uncommon for academic dermatologists to embark on a midcareer segue into private practice and, less commonly, for private dermatologists to culminate their careers with a move to academics. The private sector includes private practice, private equity (PE)–owned group practices that often are single-specialty focused, and hospital-owned group practices that usually are multispecialty. Traditionally, private practices are health care businesses owned by one physician (solo practice) or a group of physicians (group practice) operated independently from hospitals, health systems, or private investors. Financially, these practices rely heavily on volume-based services, especially clinic visits and cosmetic procedures, which provide higher reimbursement rates and usually cash payments at the time of service.1 Roughly 35% of dermatologists in the United States work in private practice, and a dwindling 15% work in solo practice.2,3
Medical practices that are not self-owned by physicians vary widely, and they include hospital- or medical center–owned, private equity, and university-based academic practices. Private equity practices typically are characterized as profit driven. Hospital-owned practices shoulder business decisions and administrative duties for the physician at the cost of provider autonomy. Academic medicine is the most different from the other practice types. In contrast to private practice dermatologists, university-based dermatologists practice at academic medical centers (AMCs) with the core goals of patient care, education, and research. Compensation generally is based on the relative value unit (RVU), which is supplemented by government support and research grants.
As evidenced in this brief discussion, health care practice models are complex, and choosing the right model to align with professional goals can pose a major challenge for many physicians. The advantages and disadvantages of various practice models will be reviewed, highlighting trends and emerging models.
Solo or Small-Group Single-Specialty Private Practice
Private practice offers dermatologists the advantage of higher income potential but with greater economic risk; it often requires physicians to be more involved in the business aspects of dermatologic practice. In the early 1990s, a survey of private practice dermatologists revealed that income was the first or second most important factor that contributed to their career choice of private vs academic practice.4 Earning potential in private practice largely is driven by the autonomy afforded in this setting. Physicians have the liberty of choosing their practice location, structure, schedule, and staff in addition to tailoring services toward profitability; this typically leads to a higher volume of cosmetic and procedural visits, which may be attractive to providers wishing to focus on aesthetics. Private practice dermatologists also are not subject to institutional requirements that may include the preparation of grant submissions, research productivity targets, and devotion of time to teaching. Many private dermatologists find satisfaction in tailoring their work environments to align with personal values and goals and in cultivating long-term relationships with patients in a more personal and less bureaucratic context.
There also are drawbacks to private practice. The profitability often can be attributed to the higher patient load and more hours devoted to practice.5 A 2006 study found that academics saw 32% to 41% fewer patients per week than private practice dermatologists.6 Along with the opportunity for financial gain is the risk of financial ruin. Cost is the largest hurdle for establishing a practice, and most practices do not turn a profit for the first few years.1,5 The financial burden of running a practice includes pressure from the federal government to adopt expensive electronic health record systems to achieve maximum Medicare payment through the Merit-Based Incentive Payment System, liability insurance, health insurance, and staff salaries.7 These challenges require strong business acumen, including managing overhead costs, navigating insurance negotiations, marketing a practice, and maintaining compliance with evolving health care regulations. The purchase of a $100,000 laser could be a boon or bust, requiring the development of a business plan that ensures a positive return on investment. Additionally, private practice profitability has the potential to dwindle as governmental reimbursements fail to match inflation rates. Securing business advisors or even obtaining a Master of Business Administration degree can be helpful.
Insurance and government agencies also are infringing upon some of the autonomy of private practice dermatologists, as evidenced by a 2017 survey of dermatologists that found that more than half of respondents altered treatment plans based on insurance coverage more than 20% of the time.2 Private equity firms also could infringe on private practice autonomy, as providers are beholden to the firm’s restrictions—from which company’s product will be stocked to which partner will be on call. Lastly, private practice is less conducive to consistent referral patterns and strong relationships with specialists when compared to academic practice. Additionally, reliance on high patient throughput or cosmetic services for financial sustainability can shift focus away from complex medical dermatology, which often is referred to AMCs.
Academic Medicine
Academic dermatology offers a stimulating and collaborative environment with opportunities to advance the field through research and education. Often, the opportunity to teach medical students, residents, and peers is the deciding factor for academic dermatologists, as supported by a 2016 survey that found teaching opportunities are a major influence on career decision.8 The mixture of patient care, education, and research roles can be satisfying when compared to the grind of seeing large numbers of patients every day. Because they typically are salaried with an RVU-based income, academic dermatologists often are less concerned with the costs associated with medical treatment, and they typically treat more medically complex patients and underserved populations.9 The salary structure of academic roles also provides the benefit of a stable and predictable income. Physicians in this setting often are considered experts in their field, positioning them to have a strong built-in referral system along with frequent participation in multidisciplinary care alongside colleagues in rheumatology, oncology, and infectious diseases. The benefits of downstream income from dermatopathology, Mohs surgery, and other ancillary testing can provide great financial advantages for an academic or large group practice.10 Academic medical centers also afford the benefit of resources, such as research offices, clinical trial units, and institutional support for scholarly publication.
Despite its benefits, academic dermatology is not without unique demands. The resources afforded by research work come with grant application deadlines and the pressure to maintain research productivity as measured by grant dollars. Academic providers also must navigate institutional political dynamics and deal with limits on autonomy. Additionally, the administrative burden associated with committee work, mentorship obligations, and publishing requirements further limit clinical time and contribute to burnout. According to Loo et al,5 92% of 89 dermatology department chairmen responding to a poll believed that the lower compensation was the primary factor preventing more residents from pursuing academia.
The adoption of RVU-based and incentive compensation models at many AMCs, along with dwindling government funds available for research, also have created pressure to increase patient volume, sometimes at the expense of teaching and research. Of those academic dermatologists spending more than half their time seeing patients, a majority reported that they lack the time to also conduct research, teach, and mentor students and resident physicians.6 A survey of academic dermatologists suggested that, for those already serving in academic positions, salary was less of a concern than the lack of protected academic time.4 While competing demands can erode the appeal of academic dermatology, academia continues to offer a meaningful and fulfilling career path for those motivated by scholarship, mentorship, teaching opportunities, and systemic impact.
Hybrid and Emerging Models
To reconcile the trade-offs inherent in private and academic models, hybrid roles are becoming increasingly common. In these arrangements, dermatologists split their time between private practice and academic appointments settings, allowing for participation in resident education and research while also benefiting from the operational and financial structure of a private office. In some cases, private groups formally affiliate with academic institutions, creating academic-private practices that host trainees and produce scholarly work while operating financially outside of traditional hospital systems. Individual dermatologists also may choose to accept part-time academic roles that allow residents and medical students to rotate in their offices. Hybrid roles may be of most interest to individuals who feel that they are missing out on the mentorship and teaching opportunities afforded at AMCs.
Government-funded systems such as Veterans Affairs (VA) hospitals offer another alternative. Dermatologists at VA hospitals often hold faculty appointments, treat a wide range of conditions in a population with great need, and engage in teaching without the intensity of productivity requirements seen at AMCs. These roles can be attractive to physicians who value public service, work-life balance, and minimal malpractice risk, as well as dermatologists who wish to introduce variety in their practice through an additional clinical setting. Notably, these roles are limited, as roughly 80% of VA hospitals employ part-time dermatologists and 72% reported being understaffed.11 Despite the challenges of limited resources and increased bureaucracy, the VA is the largest health care delivery system in the United States, offering the benefits of protection from most malpractice risk and participation in medical education at 80% of VA hospitals.12 A VA-based practice may be most attractive to physicians with prior military service or those looking for a stable practice that serves the underserved and the mission of medical education.
Similarly, rural health clinics are private practices with special subsidies from the federal government that bring Medicaid payments up to the level of Medicare.13 Rural dermatology also mirrors that of a VA-based practice by offering the opportunity to treat an array of conditions in a population of great need, as rural patients often are in care deserts and would otherwise need to travel for miles to receive dermatologic care. There is a shortage of dermatologists working in rural areas, and rural dermatologists are more likely than those in suburban or urban areas to practice alone.2 Although potentially more physically isolating, rural dermatology offers providers the opportunity to establish a lucrative practice with minimal competition and development of meaningful patient relationships.
The most rapidly increasing practice model emerging in dermatology over the past decade is the private equity (PE) group. Rajabi-Estarabadi et al14 estimated that at least 184 dermatology practices have been acquired by PE groups between 2010 and 2019. An estimated 15% of all PE acquisitions in health care have been within the field of dermatology.9 Private equity firms typically acquire 1 or more practices, then consolidate the operations with the short-term goals of reducing costs and maximizing profits and longer-term goals of selling the practice for further profit in 3 to 7 years.9 They often rely heavily on a dermatologist supervising a number of nurse practitioners.15 While PE acquisition may provide additional financial stability and income, providers have less autonomy and potentially risk a shift in their focus from patient care to profit.
The blurred lines between practice settings reflect a broader shift in the profession. Dermatologists have increasingly crafted flexible, individualized careers that align with their goals and values while drawing from both academic and private models. Hybrid roles may prove critical in preserving the educational and research missions of dermatology while adapting to economic and institutional realities.
Gender Trends, Career Satisfaction, and Other Factors Influencing Career Choice
The gender demographics of dermatology have changed greatly in recent decades. In the years 2010 to 2021, the percentage of women in the field rose from 41% to 52.2%, mirroring the rise in female medical students.16 Despite this, gender disparities persist through differences in pay, promotion rates, leadership opportunities, and research productivity.17 Women who are academic dermatologists are less likely to have protected research time and often shoulder a disproportionate share of mentorship and administrative responsibilities, which frequently are undervalued in promotion and compensation structures. Similarly, women physicians are less likely to own their own private practice.18 Notably, women physicians work part-time more often than their male counterparts, which likely impacts their income.19 Interestingly, no differences were noted in job satisfaction between men and women in academic or private practice settings, suggesting that dermatology is a fulfilling field for female physicians.16 Similar data were observed in the field of dermatopathology; in fact, there is no difference in job satisfaction when comparing providers in academics vs private practice.20
Geographic factors also influence career decisions. Some dermatologists may choose private practice to remain close to family or serve a rural area, while some choose academic centers typically located in major metropolitan areas. Others are drawn to AMCs due to their reputation, resources, or opportunities for specialization. The number of practicing dermatologists in an area also may be considered, as areas with fewer providers likely have more individuals seeking a provider and thus more earning potential.
In summary, career satisfaction is influenced by many factors, including practice setting, colleagues, institutional leadership, work environment, and professional goals. For individuals who are seeking intellectual stimulation and teaching opportunities, academic dermatology may be a great career option. Academic or large group practices may come with a large group of clinical dermatologists to provide a steady stream of specimens. Private practice appeals to those seeking autonomy, reduced bureaucracy, and higher earning potential. Tierney et al21 found that the greatest predictor of a future career in academics among Mohs surgeons was the number of publications a fellow had before and during fellowship training. These data suggest that personal interests greatly influence career decisions.
The Role of Mentorship in Career Decision-Making
Just as personal preferences guide career decisions, so too do interpersonal interactions. Mentorship plays a large role in career success, and the involvement of faculty mentors in society meetings and editorial boards has been shown to positively correlate with the number of residents pursuing academia.14 Similarly, negative interactions have strong impacts, as the top cited reason for Mohs surgeons leaving academia was lack of support from their academic chair.21 While many academic dermatologists report fulfillment from the collegial environment, retention remains an issue. Tierney et al21 found that, among 455 academic Mohs surgeons, only 28% of those who began in academia remained in those roles over the long term, and this trend of low retention holds true across the field of academic dermatology. Lack of autonomy, insufficient institutional support, and more lucrative private practice opportunities were all cited as reasons for leaving. For dermatologists seeking separation from academics but continued research opportunities, data suggest that private practice allows for continued research and publications, indicating that scholarly engagement is not exclusive to academic settings. These trends point to the increasing viability of hybrid or academic-private models that combine academic productivity with greater flexibility and financial stability.
Final Thoughts
Academic and private practice dermatology each offer compelling advantages and distinct challenges (Table). The growing popularity of hybrid models reflects a desire among dermatologists to balance the intellectual fulfillment associated with academic medicine with professional sustainability and autonomy of private practice. Whether through part-time academic appointments, rural health clinics, VA employment, or affiliations between private groups and academic institutions, these emerging roles offer a flexible and adaptive approach to career development.

Ultimately, the ideal practice model is one that aligns with a physician’s personal values, long-term goals, and lifestyle preferences. No single path fits all, but thoughtful career planning supported by mentorship and institutional transparency can help dermatologists thrive in a rapidly evolving health care landscape.
- Kaplan J. Part I: private practice versus academic medicine. BoardVitals Blog. June 5, 2018. Accessed August 5, 2025. https://www.boardvitals.com/blog/private-practice-academic-medicine/
- Ehrlich A, Kostecki J, Olkaba H. Trends in dermatology practices and the implications for the workforce. J Am Acad Dermatol. 2017;77:746-752. doi:10.1016/j.jaad.2017.06.030
- Parthasarathy V, Pollock JR, McNeely GL, et al. A cross-sectional analysis of trends in dermatology practice size in the United States from 2012 to 2020. Arch Dermatol Res. 2022;315:223-229. doi:10.1007/s00403-022-02344-0
- Bergstresser PR. Perceptions of the academic environment: a national survey. J Am Acad Dermatol. 1991;25:1092-1096. doi:10.1016/0190-9622(91)70311-o
- Loo DS, Liu CL, Geller AC, et al. Academic dermatology manpower: issues of recruitment and retention. Arch Dermatol. 2007;143:341-347. doi:10.1001/archderm.143.3.341
- Resneck JS, Tierney EP, Kimball AB. Challenges facing academic dermatology: survey data on the faculty workforce. J Am Acad Dermatol. 2006;54:211-216. doi:10.1016/j.jaad.2005.10.013
- Salmen N, Brodell R, Brodell Dolohanty L. The electronic health record: should small practices adopt this technology? J of Skin. 2024;8:1269-1273. doi:10.25251/skin.8.1.8
- Morales-Pico BM, Cotton CC, Morrell DS. Factors correlated with residents’ decisions to enter academic dermatology. Dermatol Online J. 2016;22:13030/qt7295783b.
- DeWane ME, Mostow E, Grant-Kels JM. The corporatization of care in academic dermatology. Clin Dermatol. 2020;38:289-295. doi:10.1016/j.clindermatol.2020.02.003
- Pearlman RL, Nahar VK, Sisson WT, et al. Understanding downstream service profitability generated by dermatology faculty in an academic medical center: a key driver to promotion of access-to-care. Arch Dermatol Res. 2023;315:1425-1427. doi:10.1007/s00403-022-02406-3
- Huang WW, Tsoukas MM, Bhutani T, et al. Benchmarking U.S. Department of Veterans Affairs dermatologic services: a nationwide survey of VA dermatologists. J Am Acad Dermatol. 2011;65:50-54. doi:10.1016/j.jaad.2010.04.035
- 20 reasons doctors like working for the Veterans Health Administration. US Department of Veterans Affairs. August 2016. Accessed August 5, 2025. https://www.va.gov/HEALTH/docs/20ReasonsVHA_508_IB10935.pdf
- Rural health clinics (RHCs). Rural Health Information Hub. Updated April 7, 2025. Accessed August 5, 2025. https://www .ruralhealthinfo.org/topics/rural-health-clinics
- Rajabi-Estarabadi A, Jones VA, Zheng C, et al. Dermatologist transitions: academics into private practices and vice versa. Clin Dermatol. 2020;38:541-546. doi:10.1016/j.clindermatol.2020.05.012
- Bruch JD, Foot C, Singh Y, et al. Workforce composition in private equity–acquired versus non–private equity–acquired physician practices. Health Affairs. 2023;42:121-129. doi:10.1377/hlthaff.2022.00308
- Zlakishvili B, Horev A. Gender disparities in high-quality dermatology research over the past 15 years. Int J Womens Dermatol. 2024;10:e160. doi:10.1097/JW9.0000000000000160
- Jambusaria-Pahlajani A, Crow LD, Levender MM, et al. Practice patterns and job satisfaction of Mohs surgeons: a gender-based survey. J Drugs Dermatol. 2017;16:1103-1108. https://pubmed.ncbi.nlm.nih.gov/29140863/
- Kane CK. Policy Research Perspectives. Recent changes in physician practice arrangements: shifts away from private practice and towards larger practice size continue through 2022. American Medical Association website. 2023. Accessed August 5, 2025. https://www.ama-assn.org/system/files/2022-prp-practice-arrangement.pdf
- Frank E, Zhao Z, Sen S, et al. Gender disparities in work and parental status among early career physicians. JAMA Netw Open. 2019;2:e198340. doi:10.1001/jamanetworkopen.2019.8340
- Boyd AS, Fang F. A survey-based evaluation of dermatopathology in the United States. Am J Dermatopathol. 2011;33:173-176. doi:10.1097/dad.0b013e3181f0ed84
- Tierney EP, Hanke CW, Kimball AB. Career trajectory and job satisfaction trends in Mohs micrographic surgeons. Dermatol Surg. 2011;37:1229-1238. doi:10.1111/j.1524-4725.2011.02076.x
- Kaplan J. Part I: private practice versus academic medicine. BoardVitals Blog. June 5, 2018. Accessed August 5, 2025. https://www.boardvitals.com/blog/private-practice-academic-medicine/
- Ehrlich A, Kostecki J, Olkaba H. Trends in dermatology practices and the implications for the workforce. J Am Acad Dermatol. 2017;77:746-752. doi:10.1016/j.jaad.2017.06.030
- Parthasarathy V, Pollock JR, McNeely GL, et al. A cross-sectional analysis of trends in dermatology practice size in the United States from 2012 to 2020. Arch Dermatol Res. 2022;315:223-229. doi:10.1007/s00403-022-02344-0
- Bergstresser PR. Perceptions of the academic environment: a national survey. J Am Acad Dermatol. 1991;25:1092-1096. doi:10.1016/0190-9622(91)70311-o
- Loo DS, Liu CL, Geller AC, et al. Academic dermatology manpower: issues of recruitment and retention. Arch Dermatol. 2007;143:341-347. doi:10.1001/archderm.143.3.341
- Resneck JS, Tierney EP, Kimball AB. Challenges facing academic dermatology: survey data on the faculty workforce. J Am Acad Dermatol. 2006;54:211-216. doi:10.1016/j.jaad.2005.10.013
- Salmen N, Brodell R, Brodell Dolohanty L. The electronic health record: should small practices adopt this technology? J of Skin. 2024;8:1269-1273. doi:10.25251/skin.8.1.8
- Morales-Pico BM, Cotton CC, Morrell DS. Factors correlated with residents’ decisions to enter academic dermatology. Dermatol Online J. 2016;22:13030/qt7295783b.
- DeWane ME, Mostow E, Grant-Kels JM. The corporatization of care in academic dermatology. Clin Dermatol. 2020;38:289-295. doi:10.1016/j.clindermatol.2020.02.003
- Pearlman RL, Nahar VK, Sisson WT, et al. Understanding downstream service profitability generated by dermatology faculty in an academic medical center: a key driver to promotion of access-to-care. Arch Dermatol Res. 2023;315:1425-1427. doi:10.1007/s00403-022-02406-3
- Huang WW, Tsoukas MM, Bhutani T, et al. Benchmarking U.S. Department of Veterans Affairs dermatologic services: a nationwide survey of VA dermatologists. J Am Acad Dermatol. 2011;65:50-54. doi:10.1016/j.jaad.2010.04.035
- 20 reasons doctors like working for the Veterans Health Administration. US Department of Veterans Affairs. August 2016. Accessed August 5, 2025. https://www.va.gov/HEALTH/docs/20ReasonsVHA_508_IB10935.pdf
- Rural health clinics (RHCs). Rural Health Information Hub. Updated April 7, 2025. Accessed August 5, 2025. https://www .ruralhealthinfo.org/topics/rural-health-clinics
- Rajabi-Estarabadi A, Jones VA, Zheng C, et al. Dermatologist transitions: academics into private practices and vice versa. Clin Dermatol. 2020;38:541-546. doi:10.1016/j.clindermatol.2020.05.012
- Bruch JD, Foot C, Singh Y, et al. Workforce composition in private equity–acquired versus non–private equity–acquired physician practices. Health Affairs. 2023;42:121-129. doi:10.1377/hlthaff.2022.00308
- Zlakishvili B, Horev A. Gender disparities in high-quality dermatology research over the past 15 years. Int J Womens Dermatol. 2024;10:e160. doi:10.1097/JW9.0000000000000160
- Jambusaria-Pahlajani A, Crow LD, Levender MM, et al. Practice patterns and job satisfaction of Mohs surgeons: a gender-based survey. J Drugs Dermatol. 2017;16:1103-1108. https://pubmed.ncbi.nlm.nih.gov/29140863/
- Kane CK. Policy Research Perspectives. Recent changes in physician practice arrangements: shifts away from private practice and towards larger practice size continue through 2022. American Medical Association website. 2023. Accessed August 5, 2025. https://www.ama-assn.org/system/files/2022-prp-practice-arrangement.pdf
- Frank E, Zhao Z, Sen S, et al. Gender disparities in work and parental status among early career physicians. JAMA Netw Open. 2019;2:e198340. doi:10.1001/jamanetworkopen.2019.8340
- Boyd AS, Fang F. A survey-based evaluation of dermatopathology in the United States. Am J Dermatopathol. 2011;33:173-176. doi:10.1097/dad.0b013e3181f0ed84
- Tierney EP, Hanke CW, Kimball AB. Career trajectory and job satisfaction trends in Mohs micrographic surgeons. Dermatol Surg. 2011;37:1229-1238. doi:10.1111/j.1524-4725.2011.02076.x
Advantages and Disadvantages of Private vs Academic Dermatology Practices
Advantages and Disadvantages of Private vs Academic Dermatology Practices
PRACTICE POINTS
- In the field of dermatology, solo and small-group single-specialty private practices are shrinking while academic medicine is growing.
- Hybrid models reflect a desire among some dermatologists to balance the intellectual fulfillment and sustainability associated with academic medicine and the professional autonomy of private practice.
Quality of Life for Males With Abdominal Aortic Aneurysm
Quality of Life for Males With Abdominal Aortic Aneurysm
Abdominal aortic aneurysm (AAA) is a public health threat, with a global prevalence of 4.8% and a prevalence in males that increases with age, from 1.3% between ages 45 and 54 years to 12.5% between ages 75 and 84 years.1 AAA is often asymptomatic until it ruptures and can become life-threatening, with mortality rates near 90% in the event of rupture with survival rates of about 50% to 70% for individuals with rupture who require urgent surgical intervention.2,3 Males experience AAA at 4 times the rate of females.4
Previous research has found that the awareness of having an AAA causes anxiety that some have described as “living with a ticking time bomb.”5 Others reported worries and concerns about life’s fragility and mortality due to an AAA diagnosis.6 However, the psychological impact on the individuals’ quality of life (QoL) remains unclear, especially for individuals with a small AAA (< 5.5 cm).7 Factors such as age, male sex, smoking, family history, hypertension, carotid artery disease, and hypercholesterolemia have been strongly associated with increased growth rate and the risk of small AAA ruptures.8,9
Most patients with a small AAA enter surveillance awaiting future repair and not only have the anxiety of living with an AAA despite the low risk of rupture, but also a worse QoL than those who have undergone repair.10,11 However, data are sparse regarding the effects on QoL of knowing they have an AAA, whether repaired or not. This study sought to examine the impact an AAA diagnosis had on male QoL at the initial investigation and after 12 months.
Methods
This prospective study was examined and approved by the Veterans Affairs Northern California Health Care System (NCHCS) Institutional Review Board. It was conducted at the Sacramento US Department of Veterans Affairs (VA) Medical Center from January 1, 2019, to February 28, 2022. Patients were identified through the vascular clinic. One hundred sixteen patients with AAA were eligible and agreed to participate. Of these, 91 (78%) completed the survey at baseline and 12 months later. Participation was voluntary; written informed consent was obtained from every patient before completing the survey. This study included only male patients due to their higher prevalence than female patients.4 Patients were also eligible if they were aged > 18 years and had a previously known AAA that was being followed with a recorded clinical imaging study in the NCHCS vascular clinic. Patients were excluded if they were unable to return for their 12-month follow-up investigation, were incapable of giving informed consent, were unable to complete the 12-item short form health survey version 2 (SF-12v2), had a documented history of psychiatric illness, or refused to participate. The SF-12v2, an abbreviated version of the 36-item short form health survey (SF-36), is a generic health-related quality-of-life survey that measures 8 domains of general health status: general health (GH), physical functioning (PF), role limitations due to physical problems (RP), bodily pain (BP), vitality (VT), social functioning (SF), role emotional (RE), and mental health (MH). A higher number on the QoL scale indicates better QoL. The GH, PF, RP, and BP scales yield a physical component score (PCS), and the VT, SF, RE, and MH scales generate a mental component score (MCS). Although SF-12v2 has not been validated for patients with AAA, it has been widely used and validated to measure health-related QoL in cohorts of healthy and chronically ill individuals.12,13
Analysis
Descriptive statistics, including means, SDs, frequency, percentages, 95% CIs, and correlations were calculated. The t test was used to analyze differences in mean scores. For continuous variables, such as SF-12v2 domains, PCS, and MCS, mean, SD, 95% CI, and range were determined. Comparisons were performed using X2 or t test. P < .05 was considered statistically significant. Clinical risk factors, including age, race, body mass index (BMI), diabetes, hypertension, hyperlipidemia, coronary artery disease, cerebrovascular accident, myocardial infarction, and smoking status, were also recorded.
Results
Between January 1, 2019, and February 28, 2022, 91 patients were diagnosed with an AAA and completed the survey at the initial and 12-month investigations. Patients had a mean (SD) age of 76.0 (5.6) years (range, 64-93) and BMI of 29.7 (6.4). Comorbid diabetes was present in 31% of patients, hypertension in 75%, hyperlipidemia 66%, and coronary artery disease in 12% (Table 1). Most patients smoked tobacco: 71% indicated previous use and 22% were current users.
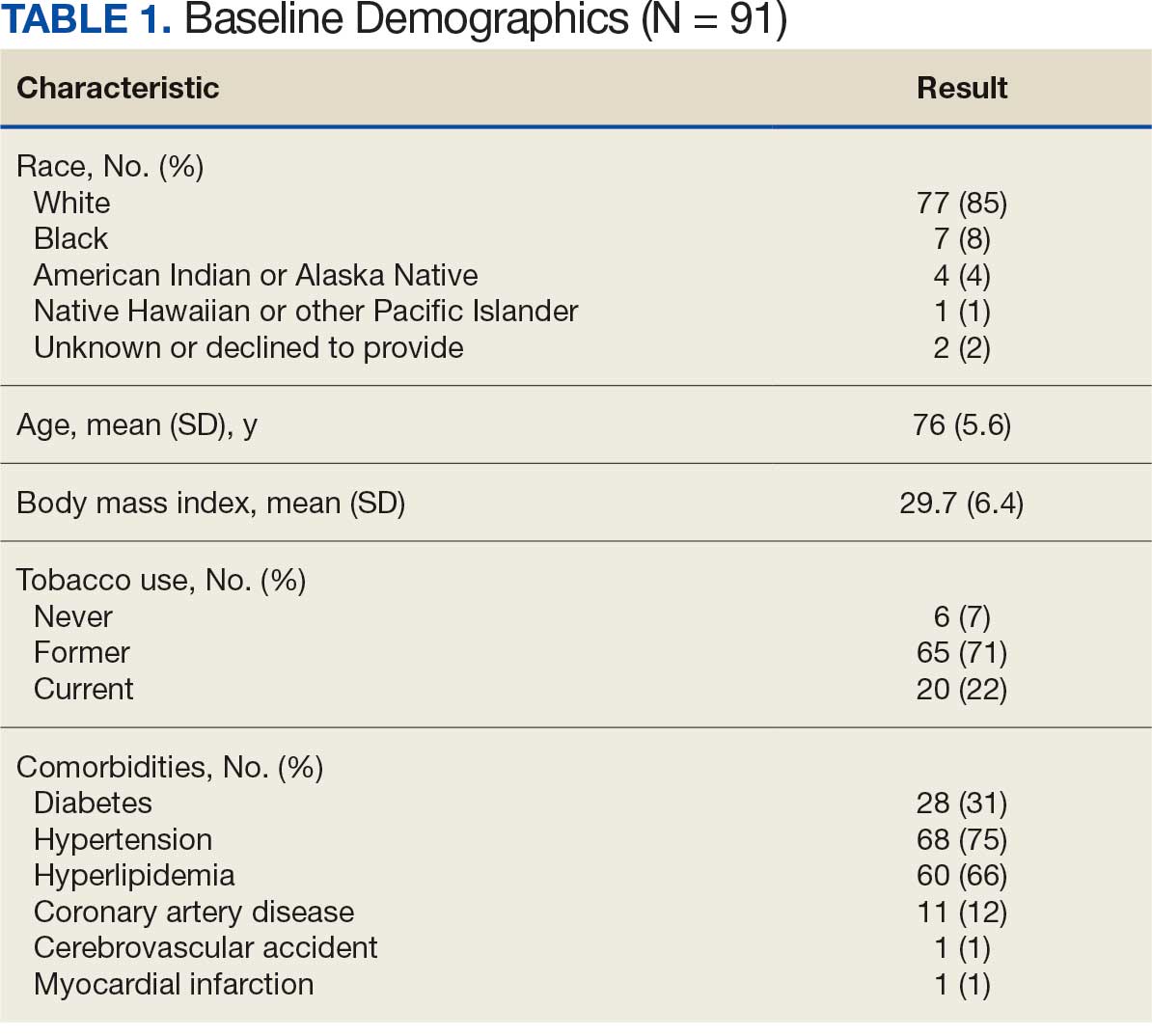
When comparing baseline vs 12-month follow-up, patients indicated a higher QoL in GH (3.2 vs 3.5, respectively; P < .05) and BP (3.1 vs 3.6, respectively; P < .05). No statistically significant difference was seen PF, RP, VT, SF, RE, MH, as well as PCS and MCS between baseline and follow-up with respect to QoL (P < .05). However, the 5 domains of SF-12v2: PF, RP, SF, RE, MH, and PCS had lower QoL scores at the 12-month follow-up when compared with baseline, but with no statistically significant difference between both investigations (Table 2).
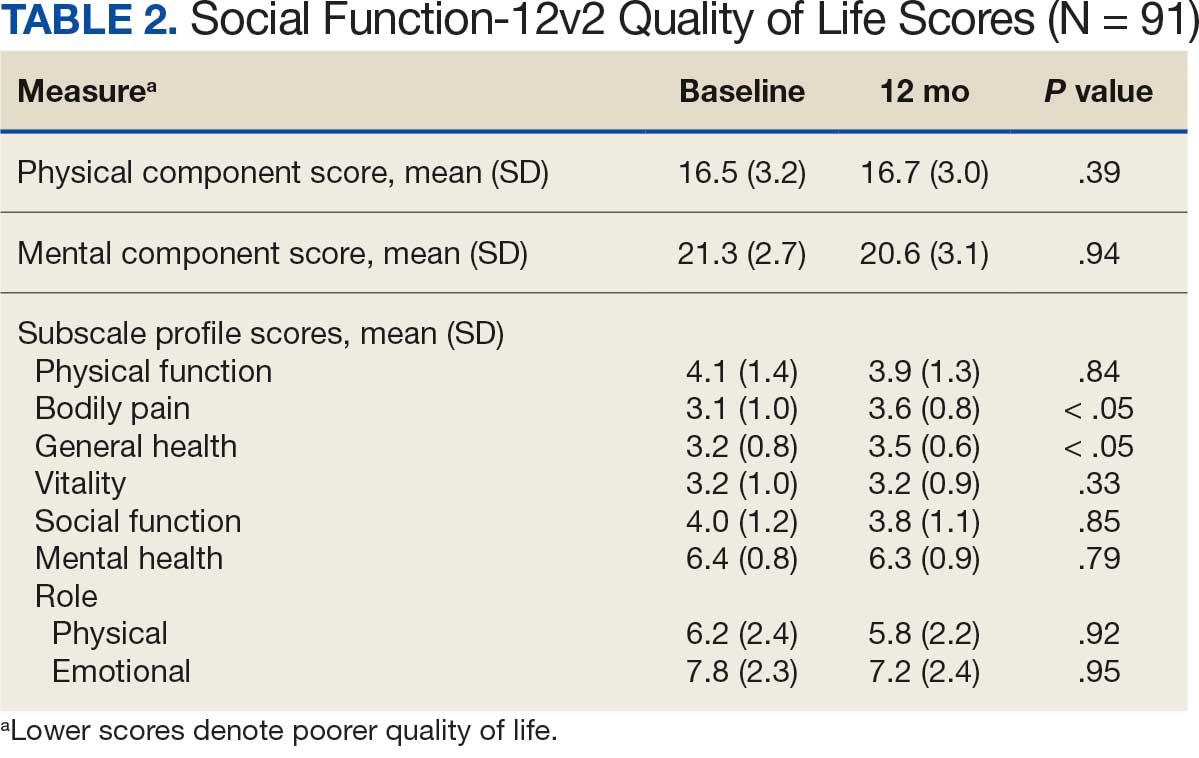
Discussion
Previous studies have characterized the results of QoL measures as subjective because they are based on patient perceptions of their physical and psychological condition.14,15 However, SF-36 and SF-12v2 responses provide a multifaceted account that encompasses the physical, psychological, and social aspects of QoL. Despite being the most widely used generic instrument in many fields of medicine, SF-36 is time consuming for clinicians who may prefer simpler and more time-efficient instruments.16-18 The SF-12v2 not only imposes less burden on respondents but also generates accurate summary scores for patients physical and mental health.19
The replicability of SF-12v2 PCS and MCS scores has been demonstrated. In the United Kingdom, Jenkinson and Layte constructed SF-12v2 summary measures from a large scale dataset by sending the SF-36 and other questions on health and lifestyles to 9332 individuals and compared the results of the SF-36 and SF-12v2 across diverse patient groups (eg, Parkinson disease, congestive heart failure, sleep apnea, benign prostatic hypertrophy). Results from SF-36 PCS, SF-36 MCS, and PCS-12v2 (ρ, 0.94; P < .001) and SF-12v2 MCS (ρ, 0.96; P < .001) were found to be highly correlated, and also produced similar results, both in the community sample and across a variety of disease-specific groups.20
The aim of this longitudinal observational study was to measure the QoL of males with an AAA ≥ 3.0 cm at baseline and 12 months later. The mean age of participants was 76 years, which aligns with previous research that found the prevalence of AAAs increased with age.1 Study participants had a mean BMI of 29.7, which also supports previous research that indicated that obesity is independently associated with an AAA.21 Patients with an AAA and a history of smoking (former or current), hypertension, or hyperlipidemia had lower mean scores for 3 of 8 SF-12v2 domains at the 12-month follow-up.
These findings support previous research that indicated smoking is not only a very strong risk factor for the presence of an AAA but also associated with increased rates of expansion and the risk of rupture in patients with an AAA.22 Bath et al found that patients with an AAA compared to patients without an AAA were older (age 72.6 vs 69.8 years; P < .001), had a higher BMI (28.1 vs 27.0; P < .001), were more likely to be a current smoker (15.1% vs 5.2%; P < .001), and were more likely to have diabetes (18.8% vs 10.0%; P < .001), ischemic heart disease (12.2% vs 4.4%; P < .001), high cholesterol (53.2% vs 30.8%; P <. 001), previous stroke (6.1% vs 2.9%; P < .001), and a previous myocardial infarction (21.1% vs 5.8%; P < .001).23 Lesjak et al found that men with AAA reported significantly lower scores in the domains of social functioning, pain, and general health 6 months after ultrasound compared with men without AAA.24
Previous research indicates that patients with an AAA have a higher risk of cardiovascular diseases and comorbidities that may impact their perceived QoL. In a study assessing cardiovascular risk in 2323 patients with a small AAA, Bath et al found a high prevalence of coronary artery disease (44.9%), myocardial infarction (26.8%), heart failure (4.4%) and cerebrovascular accident (14.0%) which may have contributed to the decreased level of self-perceived QoL in these patients.25
This aligned with a study by Golledge et al, who found that participants diagnosed with an AAA and peripheral artery disease not only had significantly poorer QoL scores in 5 SF-36 domains (PF, RP, GH, VT, and PCS)when compared with participants diagnosed with an AAA alone. They also had significantly poorer QoL scores in 7 domains of the SF-36 (PF, RP, GH, VT, SF, RE, and PCS) when compared with controls without an AAA.26
Our analysis found that males with an AAA had a rise in SF-12v2 QoL scores from baseline to 12-month follow-up in the GH and BP domains. There was no statistically significant difference in QoL in the other 6 domains (PF, RP, VT, SF, RE, and MH) between the initial and 12-month investigations. Bath et al also found that men with an AAA had a transient reduction in mental QoL during the first year after the initial screening but returned to baseline.23
Strengths and Limitations
This study is notable for its sample of patients who previously had a diagnosed AAA that were followed with a recorded clinical imaging study and the use of a validated QoL measure (SF-12v2) that provided virtually identical summary scores (PCS and MCS) as the SF-36.27 However, this study was limited by the brevity of the SF-12v2 instrument which made it difficult to extract sufficient reliable information for the 8 domains.28 Subjective perception of patients is another limitation inherent to any QoL study. QoL scores were not available before the initial investigation. Measuring QoL at baseline and 12 months later does not capture the potential fluctuations and changes in QoL that the patient may experience some months later. Another limitation arises from the fact that the AAA patient population in the study included patients under surveillance and patients who had undergone repair.
Fourteen patients (15%) had received AAA repair: 10 had endovascular reconstruction and 4 had open surgical repair. Including patients with a previous AAA repair may have influenced reported QoL levels. Suckow et al performed a 2-phase study on 1008 patients, 351 (35%) were under surveillance and 657 (65%) had undergone repair. In that study, patients under AAA surveillance had worse emotional impact scores compared with patients with repair (22 vs 13; P < .001).11 Additionally, the size of the abdominal aorta at the time of survey was not addressed in the study, which could constitute explanatory variables.
Conclusions
This study found higher QoL at 12-month follow-up compared to baseline in both the GH and BP domains of the SF-12v2 health survey for male veterans with an AAA. Periodic QoL assessments for patients with an AAA may be helpful in tracking QoL course, minimizing their physical and psychological concerns, and improving overall care and support. However, further research is necessary to assess the QoL of patients with an AAA who are under surveillance compared with those who had an aneurysm repair to accurately measure the impact of an AAA on QoL.
- Altobelli E, Rapacchietta L, Profeta VF, et al. Risk factors for abdominal aortic aneurysm in population- based studies: a systematic review and meta-analysis. Int J Environ Res Public Health. 2018;15:2805. doi:10.3390/ijerph15122805
- Chaikof EL, Dalman RL, Eskandari MK, et al. The society for vascular surgery practice guidelines on the care of patients with an abdominal aortic aneurysm. J Vasc Surg. 2018;67:2-77.e2. doi:10.1016/j.jvs.2017.10.044
- Kent KC. Abdominal aortic aneurysms. N Engl J Med. 2014;371:2101-2108. doi:10.1056/NEJMcp1401430
- Harthun NL. Current issues in the treatment of women with abdominal aortic aneurysm. Gend Med. 2008;5:36-43.
- Aoki H. Taking control of the time bomb in abdominal aortic aneurysm. Circ J. 2016;80:314-315. doi:10.1253/circj.CJ-15-1350
- Damhus CS, Siersma V, Hansson A, Bang CW, Brodersen J. Psychosocial consequences of screeningdetected abdominal aortic aneurisms: a cross-sectional study. Scand J Prim Health Care. 2021;39:459-465. doi:10.1080/02813432.2021.2004713
- Ericsson A, Kumlien C, Ching S, Carlson E, Molassiotis A. Impact on quality of life of men with screening-detected abdominal aortic aneurysms attending regular follow ups: a narrative literature review. Eur J Vasc Endovasc Surg. 2019;57:589-596. doi:10.1016/j.ejvs.2018.10.012
- Galyfos G, Voulalas G, Stamatatos I, et al. Small abdominal aortic aneurysms: should we wait? Vasc Dis Manag. 2015;12:E152-E159.
- Kristensen KL, Dahl M, Rasmussen LM, et al. Glycated hemoglobin is associated with the growth rate of abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol. 2017;37:730-736. doi:10.1161/ATVBAHA.116.308874
- Xiao-Yan L, Yu-Kui M, Li-Hui L. Risk factors for preoperative anxiety and depression in patients scheduled for abdominal aortic aneurysm repair. Chine Med J. 2018;131:1951-1957. doi:10.4103/0366-6999.238154
- Suckow BD, Schanzer AS, Hoel AW, et al. A novel quality of life instrument for patients with an abdominal aortic aneurysm. Eur J Vasc Endovasc Surg. 2019;57:809-815. doi:10.1016/j.ejvs.2019.01.018
- Flatz A, Casillas A, Stringhini S, et al. Association between education and quality of diabetes care in Switzerland. Int J Gen Med. 2015;8:87-92. doi:10.2147/IJGM.S77139
- Christensen AV, Bjorner JB, Ekholm O, et al. Increased risk of mortality and readmission associated with lower SF-12 scores in cardiac patients: Results from the national DenHeart study. Eur J Cardiovasc Nurs. 2020;19:330-338. doi:10.1177/1474515119885480
- Hamming JF, De Vries J. Measuring quality of life. Br J Surg. 2007;94:923-924. doi:10.1002/bjs.5948
- Urbach DR. Measuring quality of life after surgery. Surg Innov. 2005;12:161-165. doi:10.1177/ 155335060501200216
- Gandek B, Sinclair SJ, Kosinski M, et al. Psychometric evaluation of the SF-36® health survey in medicare managed care. Health Care Financ Rev. 2004;25:5.
- Ware JE, Sherbourne CD. The MOS 36-item short form health survey (SF-36). Med Care. 1992;30:473-483. doi:10.1097/00005650-199206000-00002
- Takayoshi K, Mototsugu T, Tomohiro T, et al. Health-related quality of life prospectively evaluated by the 8-item short form after endovascular repair versus open surgery for abdominal aortic aneurysms. Heart Vessels. 2017;32:960- 968. doi:10.1007/s00380-017-0956-9
- Pickard AS, Johnson JA, Penn A, et al. Replicability of SF-36 summary scores by the SF-12 in stroke patients. Stroke. 1999;30:1213-1217. doi:10.1161/01.str.30.6.1213
- Jenkinson C, Layte R. The development and testing of the UK SF-12. J Health Serv Res Policy. 1997;2:14-18. doi:10.1177/135581969700200105
- Golledge J, Clancy P, Jamrozik K, et al. Obesity, adipokines, and abdominal aortic aneurysm: Health in Men study. Circulation. 2007;116:2275-2279. doi:10.1161/CIRCULATIONAHA.107.717926
- Norman PE, Curci JA. Understanding the effects of tobacco smoke on the pathogenesis of aortic aneurysm. Arterioscler Thromb Vasc Biol. 2013;33:1473-1477. doi:10.1161/ATVBAHA.112.300158
- Bath MF, Sidloff D, Saratzis A, et al. Impact of abdominal aortic aneurysm screening on quality of life. BJS. 2018;105:203-208. doi:10.1002/bjs.10721
- Lesjak M, Boreland F, Lyle D, Sidford J, Flecknoe-Brown S, Fletcher J. Screening for abdominal aortic aneurysm: does it affect men’s quality of life? Aust J Prim Health. 2012;18:284-288. doi:10.1071/PY11131
- Bath MF, Gokani VJ, Sidloff DA, et al. Systematic review of cardiovascular disease and cardiovascular death in patients with a small abdominal aortic aneurysm. Br J Surg. 2015;102:866-872. doi:10.1002/bjs.9837
- Golledge J, Pinchbeck J, Rowbotham SE, et al. Health-related quality of life amongst people diagnosed with abdominal aortic aneurysm and peripheral artery disease and the effect of fenofibrate. Sci Rep. 2020;10:14583. doi:10.1038/s41598-020-71454-4
- Jenkinson C, Layte R, Jenkinson D. A shorter form health survey: can the SF-12 replicate results from the SF-36 in longitudinal studies? J Public Health Med. 1997;19:179- 186. doi:10.1093/oxfordjournals.pubmed.a024606
- White MK, Maher SM, Rizio AA, et al. A meta-analytic review of measurement equivalence study findings of the SF-36® and SF-12® Health Surveys across electronic modes compared to paper administration. Qual Life Res. 2018;27:1757-1767. doi:10.1007/s11136-018-1851-2
Abdominal aortic aneurysm (AAA) is a public health threat, with a global prevalence of 4.8% and a prevalence in males that increases with age, from 1.3% between ages 45 and 54 years to 12.5% between ages 75 and 84 years.1 AAA is often asymptomatic until it ruptures and can become life-threatening, with mortality rates near 90% in the event of rupture with survival rates of about 50% to 70% for individuals with rupture who require urgent surgical intervention.2,3 Males experience AAA at 4 times the rate of females.4
Previous research has found that the awareness of having an AAA causes anxiety that some have described as “living with a ticking time bomb.”5 Others reported worries and concerns about life’s fragility and mortality due to an AAA diagnosis.6 However, the psychological impact on the individuals’ quality of life (QoL) remains unclear, especially for individuals with a small AAA (< 5.5 cm).7 Factors such as age, male sex, smoking, family history, hypertension, carotid artery disease, and hypercholesterolemia have been strongly associated with increased growth rate and the risk of small AAA ruptures.8,9
Most patients with a small AAA enter surveillance awaiting future repair and not only have the anxiety of living with an AAA despite the low risk of rupture, but also a worse QoL than those who have undergone repair.10,11 However, data are sparse regarding the effects on QoL of knowing they have an AAA, whether repaired or not. This study sought to examine the impact an AAA diagnosis had on male QoL at the initial investigation and after 12 months.
Methods
This prospective study was examined and approved by the Veterans Affairs Northern California Health Care System (NCHCS) Institutional Review Board. It was conducted at the Sacramento US Department of Veterans Affairs (VA) Medical Center from January 1, 2019, to February 28, 2022. Patients were identified through the vascular clinic. One hundred sixteen patients with AAA were eligible and agreed to participate. Of these, 91 (78%) completed the survey at baseline and 12 months later. Participation was voluntary; written informed consent was obtained from every patient before completing the survey. This study included only male patients due to their higher prevalence than female patients.4 Patients were also eligible if they were aged > 18 years and had a previously known AAA that was being followed with a recorded clinical imaging study in the NCHCS vascular clinic. Patients were excluded if they were unable to return for their 12-month follow-up investigation, were incapable of giving informed consent, were unable to complete the 12-item short form health survey version 2 (SF-12v2), had a documented history of psychiatric illness, or refused to participate. The SF-12v2, an abbreviated version of the 36-item short form health survey (SF-36), is a generic health-related quality-of-life survey that measures 8 domains of general health status: general health (GH), physical functioning (PF), role limitations due to physical problems (RP), bodily pain (BP), vitality (VT), social functioning (SF), role emotional (RE), and mental health (MH). A higher number on the QoL scale indicates better QoL. The GH, PF, RP, and BP scales yield a physical component score (PCS), and the VT, SF, RE, and MH scales generate a mental component score (MCS). Although SF-12v2 has not been validated for patients with AAA, it has been widely used and validated to measure health-related QoL in cohorts of healthy and chronically ill individuals.12,13
Analysis
Descriptive statistics, including means, SDs, frequency, percentages, 95% CIs, and correlations were calculated. The t test was used to analyze differences in mean scores. For continuous variables, such as SF-12v2 domains, PCS, and MCS, mean, SD, 95% CI, and range were determined. Comparisons were performed using X2 or t test. P < .05 was considered statistically significant. Clinical risk factors, including age, race, body mass index (BMI), diabetes, hypertension, hyperlipidemia, coronary artery disease, cerebrovascular accident, myocardial infarction, and smoking status, were also recorded.
Results
Between January 1, 2019, and February 28, 2022, 91 patients were diagnosed with an AAA and completed the survey at the initial and 12-month investigations. Patients had a mean (SD) age of 76.0 (5.6) years (range, 64-93) and BMI of 29.7 (6.4). Comorbid diabetes was present in 31% of patients, hypertension in 75%, hyperlipidemia 66%, and coronary artery disease in 12% (Table 1). Most patients smoked tobacco: 71% indicated previous use and 22% were current users.

When comparing baseline vs 12-month follow-up, patients indicated a higher QoL in GH (3.2 vs 3.5, respectively; P < .05) and BP (3.1 vs 3.6, respectively; P < .05). No statistically significant difference was seen PF, RP, VT, SF, RE, MH, as well as PCS and MCS between baseline and follow-up with respect to QoL (P < .05). However, the 5 domains of SF-12v2: PF, RP, SF, RE, MH, and PCS had lower QoL scores at the 12-month follow-up when compared with baseline, but with no statistically significant difference between both investigations (Table 2).

Discussion
Previous studies have characterized the results of QoL measures as subjective because they are based on patient perceptions of their physical and psychological condition.14,15 However, SF-36 and SF-12v2 responses provide a multifaceted account that encompasses the physical, psychological, and social aspects of QoL. Despite being the most widely used generic instrument in many fields of medicine, SF-36 is time consuming for clinicians who may prefer simpler and more time-efficient instruments.16-18 The SF-12v2 not only imposes less burden on respondents but also generates accurate summary scores for patients physical and mental health.19
The replicability of SF-12v2 PCS and MCS scores has been demonstrated. In the United Kingdom, Jenkinson and Layte constructed SF-12v2 summary measures from a large scale dataset by sending the SF-36 and other questions on health and lifestyles to 9332 individuals and compared the results of the SF-36 and SF-12v2 across diverse patient groups (eg, Parkinson disease, congestive heart failure, sleep apnea, benign prostatic hypertrophy). Results from SF-36 PCS, SF-36 MCS, and PCS-12v2 (ρ, 0.94; P < .001) and SF-12v2 MCS (ρ, 0.96; P < .001) were found to be highly correlated, and also produced similar results, both in the community sample and across a variety of disease-specific groups.20
The aim of this longitudinal observational study was to measure the QoL of males with an AAA ≥ 3.0 cm at baseline and 12 months later. The mean age of participants was 76 years, which aligns with previous research that found the prevalence of AAAs increased with age.1 Study participants had a mean BMI of 29.7, which also supports previous research that indicated that obesity is independently associated with an AAA.21 Patients with an AAA and a history of smoking (former or current), hypertension, or hyperlipidemia had lower mean scores for 3 of 8 SF-12v2 domains at the 12-month follow-up.
These findings support previous research that indicated smoking is not only a very strong risk factor for the presence of an AAA but also associated with increased rates of expansion and the risk of rupture in patients with an AAA.22 Bath et al found that patients with an AAA compared to patients without an AAA were older (age 72.6 vs 69.8 years; P < .001), had a higher BMI (28.1 vs 27.0; P < .001), were more likely to be a current smoker (15.1% vs 5.2%; P < .001), and were more likely to have diabetes (18.8% vs 10.0%; P < .001), ischemic heart disease (12.2% vs 4.4%; P < .001), high cholesterol (53.2% vs 30.8%; P <. 001), previous stroke (6.1% vs 2.9%; P < .001), and a previous myocardial infarction (21.1% vs 5.8%; P < .001).23 Lesjak et al found that men with AAA reported significantly lower scores in the domains of social functioning, pain, and general health 6 months after ultrasound compared with men without AAA.24
Previous research indicates that patients with an AAA have a higher risk of cardiovascular diseases and comorbidities that may impact their perceived QoL. In a study assessing cardiovascular risk in 2323 patients with a small AAA, Bath et al found a high prevalence of coronary artery disease (44.9%), myocardial infarction (26.8%), heart failure (4.4%) and cerebrovascular accident (14.0%) which may have contributed to the decreased level of self-perceived QoL in these patients.25
This aligned with a study by Golledge et al, who found that participants diagnosed with an AAA and peripheral artery disease not only had significantly poorer QoL scores in 5 SF-36 domains (PF, RP, GH, VT, and PCS)when compared with participants diagnosed with an AAA alone. They also had significantly poorer QoL scores in 7 domains of the SF-36 (PF, RP, GH, VT, SF, RE, and PCS) when compared with controls without an AAA.26
Our analysis found that males with an AAA had a rise in SF-12v2 QoL scores from baseline to 12-month follow-up in the GH and BP domains. There was no statistically significant difference in QoL in the other 6 domains (PF, RP, VT, SF, RE, and MH) between the initial and 12-month investigations. Bath et al also found that men with an AAA had a transient reduction in mental QoL during the first year after the initial screening but returned to baseline.23
Strengths and Limitations
This study is notable for its sample of patients who previously had a diagnosed AAA that were followed with a recorded clinical imaging study and the use of a validated QoL measure (SF-12v2) that provided virtually identical summary scores (PCS and MCS) as the SF-36.27 However, this study was limited by the brevity of the SF-12v2 instrument which made it difficult to extract sufficient reliable information for the 8 domains.28 Subjective perception of patients is another limitation inherent to any QoL study. QoL scores were not available before the initial investigation. Measuring QoL at baseline and 12 months later does not capture the potential fluctuations and changes in QoL that the patient may experience some months later. Another limitation arises from the fact that the AAA patient population in the study included patients under surveillance and patients who had undergone repair.
Fourteen patients (15%) had received AAA repair: 10 had endovascular reconstruction and 4 had open surgical repair. Including patients with a previous AAA repair may have influenced reported QoL levels. Suckow et al performed a 2-phase study on 1008 patients, 351 (35%) were under surveillance and 657 (65%) had undergone repair. In that study, patients under AAA surveillance had worse emotional impact scores compared with patients with repair (22 vs 13; P < .001).11 Additionally, the size of the abdominal aorta at the time of survey was not addressed in the study, which could constitute explanatory variables.
Conclusions
This study found higher QoL at 12-month follow-up compared to baseline in both the GH and BP domains of the SF-12v2 health survey for male veterans with an AAA. Periodic QoL assessments for patients with an AAA may be helpful in tracking QoL course, minimizing their physical and psychological concerns, and improving overall care and support. However, further research is necessary to assess the QoL of patients with an AAA who are under surveillance compared with those who had an aneurysm repair to accurately measure the impact of an AAA on QoL.
Abdominal aortic aneurysm (AAA) is a public health threat, with a global prevalence of 4.8% and a prevalence in males that increases with age, from 1.3% between ages 45 and 54 years to 12.5% between ages 75 and 84 years.1 AAA is often asymptomatic until it ruptures and can become life-threatening, with mortality rates near 90% in the event of rupture with survival rates of about 50% to 70% for individuals with rupture who require urgent surgical intervention.2,3 Males experience AAA at 4 times the rate of females.4
Previous research has found that the awareness of having an AAA causes anxiety that some have described as “living with a ticking time bomb.”5 Others reported worries and concerns about life’s fragility and mortality due to an AAA diagnosis.6 However, the psychological impact on the individuals’ quality of life (QoL) remains unclear, especially for individuals with a small AAA (< 5.5 cm).7 Factors such as age, male sex, smoking, family history, hypertension, carotid artery disease, and hypercholesterolemia have been strongly associated with increased growth rate and the risk of small AAA ruptures.8,9
Most patients with a small AAA enter surveillance awaiting future repair and not only have the anxiety of living with an AAA despite the low risk of rupture, but also a worse QoL than those who have undergone repair.10,11 However, data are sparse regarding the effects on QoL of knowing they have an AAA, whether repaired or not. This study sought to examine the impact an AAA diagnosis had on male QoL at the initial investigation and after 12 months.
Methods
This prospective study was examined and approved by the Veterans Affairs Northern California Health Care System (NCHCS) Institutional Review Board. It was conducted at the Sacramento US Department of Veterans Affairs (VA) Medical Center from January 1, 2019, to February 28, 2022. Patients were identified through the vascular clinic. One hundred sixteen patients with AAA were eligible and agreed to participate. Of these, 91 (78%) completed the survey at baseline and 12 months later. Participation was voluntary; written informed consent was obtained from every patient before completing the survey. This study included only male patients due to their higher prevalence than female patients.4 Patients were also eligible if they were aged > 18 years and had a previously known AAA that was being followed with a recorded clinical imaging study in the NCHCS vascular clinic. Patients were excluded if they were unable to return for their 12-month follow-up investigation, were incapable of giving informed consent, were unable to complete the 12-item short form health survey version 2 (SF-12v2), had a documented history of psychiatric illness, or refused to participate. The SF-12v2, an abbreviated version of the 36-item short form health survey (SF-36), is a generic health-related quality-of-life survey that measures 8 domains of general health status: general health (GH), physical functioning (PF), role limitations due to physical problems (RP), bodily pain (BP), vitality (VT), social functioning (SF), role emotional (RE), and mental health (MH). A higher number on the QoL scale indicates better QoL. The GH, PF, RP, and BP scales yield a physical component score (PCS), and the VT, SF, RE, and MH scales generate a mental component score (MCS). Although SF-12v2 has not been validated for patients with AAA, it has been widely used and validated to measure health-related QoL in cohorts of healthy and chronically ill individuals.12,13
Analysis
Descriptive statistics, including means, SDs, frequency, percentages, 95% CIs, and correlations were calculated. The t test was used to analyze differences in mean scores. For continuous variables, such as SF-12v2 domains, PCS, and MCS, mean, SD, 95% CI, and range were determined. Comparisons were performed using X2 or t test. P < .05 was considered statistically significant. Clinical risk factors, including age, race, body mass index (BMI), diabetes, hypertension, hyperlipidemia, coronary artery disease, cerebrovascular accident, myocardial infarction, and smoking status, were also recorded.
Results
Between January 1, 2019, and February 28, 2022, 91 patients were diagnosed with an AAA and completed the survey at the initial and 12-month investigations. Patients had a mean (SD) age of 76.0 (5.6) years (range, 64-93) and BMI of 29.7 (6.4). Comorbid diabetes was present in 31% of patients, hypertension in 75%, hyperlipidemia 66%, and coronary artery disease in 12% (Table 1). Most patients smoked tobacco: 71% indicated previous use and 22% were current users.

When comparing baseline vs 12-month follow-up, patients indicated a higher QoL in GH (3.2 vs 3.5, respectively; P < .05) and BP (3.1 vs 3.6, respectively; P < .05). No statistically significant difference was seen PF, RP, VT, SF, RE, MH, as well as PCS and MCS between baseline and follow-up with respect to QoL (P < .05). However, the 5 domains of SF-12v2: PF, RP, SF, RE, MH, and PCS had lower QoL scores at the 12-month follow-up when compared with baseline, but with no statistically significant difference between both investigations (Table 2).

Discussion
Previous studies have characterized the results of QoL measures as subjective because they are based on patient perceptions of their physical and psychological condition.14,15 However, SF-36 and SF-12v2 responses provide a multifaceted account that encompasses the physical, psychological, and social aspects of QoL. Despite being the most widely used generic instrument in many fields of medicine, SF-36 is time consuming for clinicians who may prefer simpler and more time-efficient instruments.16-18 The SF-12v2 not only imposes less burden on respondents but also generates accurate summary scores for patients physical and mental health.19
The replicability of SF-12v2 PCS and MCS scores has been demonstrated. In the United Kingdom, Jenkinson and Layte constructed SF-12v2 summary measures from a large scale dataset by sending the SF-36 and other questions on health and lifestyles to 9332 individuals and compared the results of the SF-36 and SF-12v2 across diverse patient groups (eg, Parkinson disease, congestive heart failure, sleep apnea, benign prostatic hypertrophy). Results from SF-36 PCS, SF-36 MCS, and PCS-12v2 (ρ, 0.94; P < .001) and SF-12v2 MCS (ρ, 0.96; P < .001) were found to be highly correlated, and also produced similar results, both in the community sample and across a variety of disease-specific groups.20
The aim of this longitudinal observational study was to measure the QoL of males with an AAA ≥ 3.0 cm at baseline and 12 months later. The mean age of participants was 76 years, which aligns with previous research that found the prevalence of AAAs increased with age.1 Study participants had a mean BMI of 29.7, which also supports previous research that indicated that obesity is independently associated with an AAA.21 Patients with an AAA and a history of smoking (former or current), hypertension, or hyperlipidemia had lower mean scores for 3 of 8 SF-12v2 domains at the 12-month follow-up.
These findings support previous research that indicated smoking is not only a very strong risk factor for the presence of an AAA but also associated with increased rates of expansion and the risk of rupture in patients with an AAA.22 Bath et al found that patients with an AAA compared to patients without an AAA were older (age 72.6 vs 69.8 years; P < .001), had a higher BMI (28.1 vs 27.0; P < .001), were more likely to be a current smoker (15.1% vs 5.2%; P < .001), and were more likely to have diabetes (18.8% vs 10.0%; P < .001), ischemic heart disease (12.2% vs 4.4%; P < .001), high cholesterol (53.2% vs 30.8%; P <. 001), previous stroke (6.1% vs 2.9%; P < .001), and a previous myocardial infarction (21.1% vs 5.8%; P < .001).23 Lesjak et al found that men with AAA reported significantly lower scores in the domains of social functioning, pain, and general health 6 months after ultrasound compared with men without AAA.24
Previous research indicates that patients with an AAA have a higher risk of cardiovascular diseases and comorbidities that may impact their perceived QoL. In a study assessing cardiovascular risk in 2323 patients with a small AAA, Bath et al found a high prevalence of coronary artery disease (44.9%), myocardial infarction (26.8%), heart failure (4.4%) and cerebrovascular accident (14.0%) which may have contributed to the decreased level of self-perceived QoL in these patients.25
This aligned with a study by Golledge et al, who found that participants diagnosed with an AAA and peripheral artery disease not only had significantly poorer QoL scores in 5 SF-36 domains (PF, RP, GH, VT, and PCS)when compared with participants diagnosed with an AAA alone. They also had significantly poorer QoL scores in 7 domains of the SF-36 (PF, RP, GH, VT, SF, RE, and PCS) when compared with controls without an AAA.26
Our analysis found that males with an AAA had a rise in SF-12v2 QoL scores from baseline to 12-month follow-up in the GH and BP domains. There was no statistically significant difference in QoL in the other 6 domains (PF, RP, VT, SF, RE, and MH) between the initial and 12-month investigations. Bath et al also found that men with an AAA had a transient reduction in mental QoL during the first year after the initial screening but returned to baseline.23
Strengths and Limitations
This study is notable for its sample of patients who previously had a diagnosed AAA that were followed with a recorded clinical imaging study and the use of a validated QoL measure (SF-12v2) that provided virtually identical summary scores (PCS and MCS) as the SF-36.27 However, this study was limited by the brevity of the SF-12v2 instrument which made it difficult to extract sufficient reliable information for the 8 domains.28 Subjective perception of patients is another limitation inherent to any QoL study. QoL scores were not available before the initial investigation. Measuring QoL at baseline and 12 months later does not capture the potential fluctuations and changes in QoL that the patient may experience some months later. Another limitation arises from the fact that the AAA patient population in the study included patients under surveillance and patients who had undergone repair.
Fourteen patients (15%) had received AAA repair: 10 had endovascular reconstruction and 4 had open surgical repair. Including patients with a previous AAA repair may have influenced reported QoL levels. Suckow et al performed a 2-phase study on 1008 patients, 351 (35%) were under surveillance and 657 (65%) had undergone repair. In that study, patients under AAA surveillance had worse emotional impact scores compared with patients with repair (22 vs 13; P < .001).11 Additionally, the size of the abdominal aorta at the time of survey was not addressed in the study, which could constitute explanatory variables.
Conclusions
This study found higher QoL at 12-month follow-up compared to baseline in both the GH and BP domains of the SF-12v2 health survey for male veterans with an AAA. Periodic QoL assessments for patients with an AAA may be helpful in tracking QoL course, minimizing their physical and psychological concerns, and improving overall care and support. However, further research is necessary to assess the QoL of patients with an AAA who are under surveillance compared with those who had an aneurysm repair to accurately measure the impact of an AAA on QoL.
- Altobelli E, Rapacchietta L, Profeta VF, et al. Risk factors for abdominal aortic aneurysm in population- based studies: a systematic review and meta-analysis. Int J Environ Res Public Health. 2018;15:2805. doi:10.3390/ijerph15122805
- Chaikof EL, Dalman RL, Eskandari MK, et al. The society for vascular surgery practice guidelines on the care of patients with an abdominal aortic aneurysm. J Vasc Surg. 2018;67:2-77.e2. doi:10.1016/j.jvs.2017.10.044
- Kent KC. Abdominal aortic aneurysms. N Engl J Med. 2014;371:2101-2108. doi:10.1056/NEJMcp1401430
- Harthun NL. Current issues in the treatment of women with abdominal aortic aneurysm. Gend Med. 2008;5:36-43.
- Aoki H. Taking control of the time bomb in abdominal aortic aneurysm. Circ J. 2016;80:314-315. doi:10.1253/circj.CJ-15-1350
- Damhus CS, Siersma V, Hansson A, Bang CW, Brodersen J. Psychosocial consequences of screeningdetected abdominal aortic aneurisms: a cross-sectional study. Scand J Prim Health Care. 2021;39:459-465. doi:10.1080/02813432.2021.2004713
- Ericsson A, Kumlien C, Ching S, Carlson E, Molassiotis A. Impact on quality of life of men with screening-detected abdominal aortic aneurysms attending regular follow ups: a narrative literature review. Eur J Vasc Endovasc Surg. 2019;57:589-596. doi:10.1016/j.ejvs.2018.10.012
- Galyfos G, Voulalas G, Stamatatos I, et al. Small abdominal aortic aneurysms: should we wait? Vasc Dis Manag. 2015;12:E152-E159.
- Kristensen KL, Dahl M, Rasmussen LM, et al. Glycated hemoglobin is associated with the growth rate of abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol. 2017;37:730-736. doi:10.1161/ATVBAHA.116.308874
- Xiao-Yan L, Yu-Kui M, Li-Hui L. Risk factors for preoperative anxiety and depression in patients scheduled for abdominal aortic aneurysm repair. Chine Med J. 2018;131:1951-1957. doi:10.4103/0366-6999.238154
- Suckow BD, Schanzer AS, Hoel AW, et al. A novel quality of life instrument for patients with an abdominal aortic aneurysm. Eur J Vasc Endovasc Surg. 2019;57:809-815. doi:10.1016/j.ejvs.2019.01.018
- Flatz A, Casillas A, Stringhini S, et al. Association between education and quality of diabetes care in Switzerland. Int J Gen Med. 2015;8:87-92. doi:10.2147/IJGM.S77139
- Christensen AV, Bjorner JB, Ekholm O, et al. Increased risk of mortality and readmission associated with lower SF-12 scores in cardiac patients: Results from the national DenHeart study. Eur J Cardiovasc Nurs. 2020;19:330-338. doi:10.1177/1474515119885480
- Hamming JF, De Vries J. Measuring quality of life. Br J Surg. 2007;94:923-924. doi:10.1002/bjs.5948
- Urbach DR. Measuring quality of life after surgery. Surg Innov. 2005;12:161-165. doi:10.1177/ 155335060501200216
- Gandek B, Sinclair SJ, Kosinski M, et al. Psychometric evaluation of the SF-36® health survey in medicare managed care. Health Care Financ Rev. 2004;25:5.
- Ware JE, Sherbourne CD. The MOS 36-item short form health survey (SF-36). Med Care. 1992;30:473-483. doi:10.1097/00005650-199206000-00002
- Takayoshi K, Mototsugu T, Tomohiro T, et al. Health-related quality of life prospectively evaluated by the 8-item short form after endovascular repair versus open surgery for abdominal aortic aneurysms. Heart Vessels. 2017;32:960- 968. doi:10.1007/s00380-017-0956-9
- Pickard AS, Johnson JA, Penn A, et al. Replicability of SF-36 summary scores by the SF-12 in stroke patients. Stroke. 1999;30:1213-1217. doi:10.1161/01.str.30.6.1213
- Jenkinson C, Layte R. The development and testing of the UK SF-12. J Health Serv Res Policy. 1997;2:14-18. doi:10.1177/135581969700200105
- Golledge J, Clancy P, Jamrozik K, et al. Obesity, adipokines, and abdominal aortic aneurysm: Health in Men study. Circulation. 2007;116:2275-2279. doi:10.1161/CIRCULATIONAHA.107.717926
- Norman PE, Curci JA. Understanding the effects of tobacco smoke on the pathogenesis of aortic aneurysm. Arterioscler Thromb Vasc Biol. 2013;33:1473-1477. doi:10.1161/ATVBAHA.112.300158
- Bath MF, Sidloff D, Saratzis A, et al. Impact of abdominal aortic aneurysm screening on quality of life. BJS. 2018;105:203-208. doi:10.1002/bjs.10721
- Lesjak M, Boreland F, Lyle D, Sidford J, Flecknoe-Brown S, Fletcher J. Screening for abdominal aortic aneurysm: does it affect men’s quality of life? Aust J Prim Health. 2012;18:284-288. doi:10.1071/PY11131
- Bath MF, Gokani VJ, Sidloff DA, et al. Systematic review of cardiovascular disease and cardiovascular death in patients with a small abdominal aortic aneurysm. Br J Surg. 2015;102:866-872. doi:10.1002/bjs.9837
- Golledge J, Pinchbeck J, Rowbotham SE, et al. Health-related quality of life amongst people diagnosed with abdominal aortic aneurysm and peripheral artery disease and the effect of fenofibrate. Sci Rep. 2020;10:14583. doi:10.1038/s41598-020-71454-4
- Jenkinson C, Layte R, Jenkinson D. A shorter form health survey: can the SF-12 replicate results from the SF-36 in longitudinal studies? J Public Health Med. 1997;19:179- 186. doi:10.1093/oxfordjournals.pubmed.a024606
- White MK, Maher SM, Rizio AA, et al. A meta-analytic review of measurement equivalence study findings of the SF-36® and SF-12® Health Surveys across electronic modes compared to paper administration. Qual Life Res. 2018;27:1757-1767. doi:10.1007/s11136-018-1851-2
- Altobelli E, Rapacchietta L, Profeta VF, et al. Risk factors for abdominal aortic aneurysm in population- based studies: a systematic review and meta-analysis. Int J Environ Res Public Health. 2018;15:2805. doi:10.3390/ijerph15122805
- Chaikof EL, Dalman RL, Eskandari MK, et al. The society for vascular surgery practice guidelines on the care of patients with an abdominal aortic aneurysm. J Vasc Surg. 2018;67:2-77.e2. doi:10.1016/j.jvs.2017.10.044
- Kent KC. Abdominal aortic aneurysms. N Engl J Med. 2014;371:2101-2108. doi:10.1056/NEJMcp1401430
- Harthun NL. Current issues in the treatment of women with abdominal aortic aneurysm. Gend Med. 2008;5:36-43.
- Aoki H. Taking control of the time bomb in abdominal aortic aneurysm. Circ J. 2016;80:314-315. doi:10.1253/circj.CJ-15-1350
- Damhus CS, Siersma V, Hansson A, Bang CW, Brodersen J. Psychosocial consequences of screeningdetected abdominal aortic aneurisms: a cross-sectional study. Scand J Prim Health Care. 2021;39:459-465. doi:10.1080/02813432.2021.2004713
- Ericsson A, Kumlien C, Ching S, Carlson E, Molassiotis A. Impact on quality of life of men with screening-detected abdominal aortic aneurysms attending regular follow ups: a narrative literature review. Eur J Vasc Endovasc Surg. 2019;57:589-596. doi:10.1016/j.ejvs.2018.10.012
- Galyfos G, Voulalas G, Stamatatos I, et al. Small abdominal aortic aneurysms: should we wait? Vasc Dis Manag. 2015;12:E152-E159.
- Kristensen KL, Dahl M, Rasmussen LM, et al. Glycated hemoglobin is associated with the growth rate of abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol. 2017;37:730-736. doi:10.1161/ATVBAHA.116.308874
- Xiao-Yan L, Yu-Kui M, Li-Hui L. Risk factors for preoperative anxiety and depression in patients scheduled for abdominal aortic aneurysm repair. Chine Med J. 2018;131:1951-1957. doi:10.4103/0366-6999.238154
- Suckow BD, Schanzer AS, Hoel AW, et al. A novel quality of life instrument for patients with an abdominal aortic aneurysm. Eur J Vasc Endovasc Surg. 2019;57:809-815. doi:10.1016/j.ejvs.2019.01.018
- Flatz A, Casillas A, Stringhini S, et al. Association between education and quality of diabetes care in Switzerland. Int J Gen Med. 2015;8:87-92. doi:10.2147/IJGM.S77139
- Christensen AV, Bjorner JB, Ekholm O, et al. Increased risk of mortality and readmission associated with lower SF-12 scores in cardiac patients: Results from the national DenHeart study. Eur J Cardiovasc Nurs. 2020;19:330-338. doi:10.1177/1474515119885480
- Hamming JF, De Vries J. Measuring quality of life. Br J Surg. 2007;94:923-924. doi:10.1002/bjs.5948
- Urbach DR. Measuring quality of life after surgery. Surg Innov. 2005;12:161-165. doi:10.1177/ 155335060501200216
- Gandek B, Sinclair SJ, Kosinski M, et al. Psychometric evaluation of the SF-36® health survey in medicare managed care. Health Care Financ Rev. 2004;25:5.
- Ware JE, Sherbourne CD. The MOS 36-item short form health survey (SF-36). Med Care. 1992;30:473-483. doi:10.1097/00005650-199206000-00002
- Takayoshi K, Mototsugu T, Tomohiro T, et al. Health-related quality of life prospectively evaluated by the 8-item short form after endovascular repair versus open surgery for abdominal aortic aneurysms. Heart Vessels. 2017;32:960- 968. doi:10.1007/s00380-017-0956-9
- Pickard AS, Johnson JA, Penn A, et al. Replicability of SF-36 summary scores by the SF-12 in stroke patients. Stroke. 1999;30:1213-1217. doi:10.1161/01.str.30.6.1213
- Jenkinson C, Layte R. The development and testing of the UK SF-12. J Health Serv Res Policy. 1997;2:14-18. doi:10.1177/135581969700200105
- Golledge J, Clancy P, Jamrozik K, et al. Obesity, adipokines, and abdominal aortic aneurysm: Health in Men study. Circulation. 2007;116:2275-2279. doi:10.1161/CIRCULATIONAHA.107.717926
- Norman PE, Curci JA. Understanding the effects of tobacco smoke on the pathogenesis of aortic aneurysm. Arterioscler Thromb Vasc Biol. 2013;33:1473-1477. doi:10.1161/ATVBAHA.112.300158
- Bath MF, Sidloff D, Saratzis A, et al. Impact of abdominal aortic aneurysm screening on quality of life. BJS. 2018;105:203-208. doi:10.1002/bjs.10721
- Lesjak M, Boreland F, Lyle D, Sidford J, Flecknoe-Brown S, Fletcher J. Screening for abdominal aortic aneurysm: does it affect men’s quality of life? Aust J Prim Health. 2012;18:284-288. doi:10.1071/PY11131
- Bath MF, Gokani VJ, Sidloff DA, et al. Systematic review of cardiovascular disease and cardiovascular death in patients with a small abdominal aortic aneurysm. Br J Surg. 2015;102:866-872. doi:10.1002/bjs.9837
- Golledge J, Pinchbeck J, Rowbotham SE, et al. Health-related quality of life amongst people diagnosed with abdominal aortic aneurysm and peripheral artery disease and the effect of fenofibrate. Sci Rep. 2020;10:14583. doi:10.1038/s41598-020-71454-4
- Jenkinson C, Layte R, Jenkinson D. A shorter form health survey: can the SF-12 replicate results from the SF-36 in longitudinal studies? J Public Health Med. 1997;19:179- 186. doi:10.1093/oxfordjournals.pubmed.a024606
- White MK, Maher SM, Rizio AA, et al. A meta-analytic review of measurement equivalence study findings of the SF-36® and SF-12® Health Surveys across electronic modes compared to paper administration. Qual Life Res. 2018;27:1757-1767. doi:10.1007/s11136-018-1851-2
Quality of Life for Males With Abdominal Aortic Aneurysm
Quality of Life for Males With Abdominal Aortic Aneurysm
VIP Boot Camp: Expanding the Impact of VA Primary Care Mental Health With a Transdiagnostic Modular Group Program
VIP Boot Camp: Expanding the Impact of VA Primary Care Mental Health With a Transdiagnostic Modular Group Program
Since 2007, Primary Care Mental Health Integration (PCMHI) at the Veterans Health Administration (VHA) has improved access to mental health care services for veterans by directly embedding mental health care professionals (HCPs) within primary care teams.1 Veterans referred to PCMHI often have co-occurring physical and mental health disorders.2 Untreated chronic physical and mental comorbidities can diminish the effectiveness of medical and mental health interventions. Growing evidence suggests that treatment of mental health conditions can improve physical health outcomes and management of physical conditions can improve mental health outcomes.2,3
Chronic pain and sleep disorders are common reasons patients present to primary care, and often coexist together with mental health comorbidities.4 Sleep disorders affect 50% to 88% of patients with chronic pain, and 40% of patients with sleep disorders report chronic pain.4 Research has found that chronic pain and sleep disorders increase the risk of suicide attempts and deaths by suicide. Addressing suicide prevention simultaneously with treating chronic pain and insomnia is encouraged.5
Background
PCMHI treats physical and mental health comorbidities with a collaborative framework and a biopsychosocial integrative model.6 PCMHI staff provide mental health services as members of primary care teams. An interdisciplinary PCMHI team can include, but is not limited to, psychologists, mental health social workers, psychiatrists, nurse practitioners, clinical pharmacists, and mental health nurses. Quality of care within this model is elevated, as mental and physical health are recognized as interconnected. Collaboration between primary care and mental health benefits veterans and the VHA by increasing access to mental health care, decreasing stigma associated with mental health treatment, improving health outcomes, and enhancing the likelihood of recovery, resulting in high patient satisfaction.6-8
In the existing PCMHI model, HCPs are encouraged to use short-term, evidence-based psychotherapies (EBPs).9 Veterans referred to PCMHI from primary care are typically able to attend 1 to 6 brief sessions of mental health treatment, often 20 to 30 minutes long. Most EBPs in PCMHI are disorder- specific, providing interventions focused on a single presenting problem (eg, insomnia, chronic pain, or posttraumatic stress disorder [PTSD]). For veterans with a single issue, this model can be very effective. 1,10 However, the high rate of co-occurrence of mental and physical health issues can make it difficult to fully treat interrelated problems if the focus is on 1 specific diagnosis. Veterans with a need for additional (more comprehensive or intensive) mental health treatment are frequently referred to a higher, more resource-intensive level of mental health care, either in the VHA or the community. Examples of higher levels of mental health care include the longer term behavioral health interdisciplinary program (BHIP), sometimes called a mental health clinic (MHC), or a specialty mental health program such as a PTSD clinic.
As PCMHI continues to grow, new challenges have emerged related to staffing shortages and gaps in the clinical delivery of mental health treatment within the VHA. At the same time, demand for VHA mental health treatment has increased. However, a mental health professional shortage severely limits the ability of the VHA to meet this demand. In many systems, this shortage may result in more referrals being made to a higher level of mental health care because of fewer resources to provide comprehensive treatment in a less intensive PCMHI setting.8,10,11 This referral pattern can overburden higher level care, often with long wait times for treatment and lengthy lag times between appointments. Furthermore, these gaps in the clinical delivery of care cannot be effectively addressed by hiring additional mental health professionals. This strain on resources can impede access to care and negatively affect outcomes.10
Recent congressional reports highlight these issues, noting that demand for mental health services continues to outpace the capacity of both PCMHI and higher levels of mental health care, leading to delays in treatment that may negatively affect outcomes.8,10,11 These delays can be particularly detrimental for individuals with conditions requiring timely intervention.8,11 Some veterans are willing to engage with PCMHI in a primary care setting but may be reluctant to engage in general mental health treatment. These veterans might not receive the mental health care they need without PCMHI.
Group Psychotherapy
A group psychotherapy format can address gaps in care delivery and provide advantages for patients, mental health professionals, and the VHA. Group psychotherapy aligns with the US Department of Veterans Affairs (VA) 2018 Blueprint for Excellence and 2018 to 2024 strategic plan, underscoring the need for more timely and efficient mental health services.12,13
Benefits of group psychotherapy include reductions in symptoms, decreased feelings of isolation, increased social support, decreased emotional suppression, and enhanced satisfaction with overall quality of life.14-17 Studies of veterans with PTSD have found less attrition among those who chose group therapy compared with individual therapy.14,18 Group psychotherapy improves access to care by enabling delivery to more patients.14 When compared with individual therapy, the group format allows for a large number of patients to be treated simultaneously, maximizing resources and reducing costs.3,19-21
VISN 9 CRH Innovation
The VA provides care to veterans through regionally distinct administrative systems known as Veterans Integrated Service Networks (VISNs). Clinical resource hubs (CRH) are VISN-based programs created to cover VA staffing shortages by virtually deploying HCPs into local VA systems until vacancies are filled. The national CRH vision of effectively using resources and innovative technologies to meet veterans’ health care needs, along with the above-referenced clinical gaps in the delivery of care, inspired the development of VIP Boot Camp within the VISN 9 CRH.22
Program Description
VIP Boot Camp is an evidence-informed group psychotherapy program designed to provide timely, brief, and comprehensive mental health treatment for veterans. VIP Boot Camp was developed to address the needs of veterans accessing PCMHI services who experience ≥ 1 of the often overlapping problems of anxiety/emotion regulation/stress, sleep difficulties, and chronic pain (Figure). VIP Boot Camp uses an integrative approach to highlight interconnections and similarities among these difficulties and their treatment. A primary vision of the program is to provide this comprehensive treatment within PCMHI (upstream) so additional referrals to higher levels of mental health care (downstream) may not be needed.
This design is intentional because it increases the number of individuals who can be treated upstream with comprehensive, preventive, and proactive care within PCMHI which, over time, frees up resources in the BHIP for individuals requiring higher levels of care. This approach also aligns with the importance of early treatment for chronic pain and sleep disturbances, which are linked to increased risk of suicide attempts and deaths by suicide for veterans.5 National interest for VIP Boot Camp grew during fiscal year 2024 after it received the Gold Medal Recognition for Most Adoptable and Greatest Potential for Impact during VHA National Access Sprint Wave 3—Mental Health Call of Champions.
History
VIP Boot Camp began in August 2021 at VISN 9 as a 6-week virtual group for veterans with chronic pain. It was established to assist a large VA medical center experiencing PCMHI staffing shortages and lacking available PCMHI groups. Many veterans in the chronic pain group discussed co-occurring issues such as sleep disturbances, anxiety, and stress. The CRH team considered launching 2 separate groups to address these additional PCMHI-level issues; however, in developing the group material which drew from multiple clinical approaches, the team recognized significant overlapping and interconnected themes.
The team discussed EBPs within the VHA and how certain interventions within these treatments could be helpful across many other co-occurring disorders. Integrated tactics (clinical interventions) were drawn from cognitive-behavioral therapy (for depression, insomnia, or chronic pain), acceptance and commitment therapy, prolonged exposure, cognitive processing therapy, dialectical behavior therapy, unified protocol, pain reprocessing therapy, emotional awareness and expression therapy, interpersonal neurobiology, and mindfulness. We collaborated with veterans during VIP Boot Camp groups to determine how to present and discuss complex interventions in ways that were clinically accurate, understandable, relatable, and relevant to their experiences.
To address accessibility issues, the chronic pain group was reduced to 4 weeks. A second 4-week module for anxiety, emotion regulation, and stress was developed, mirroring the tactics, language, and integrative approach of the revised chronic pain module. A similar integrative approach led to the development of the third and final 4-week module for sleep disturbances.
Current Program
The VIP Boot Camp consists of three 4-week integrated modules, each highlighting a critical area: sleep disturbances (Improving Sleep), chronic pain difficulties (Outsmarting Chronic Pain), and emotion regulation difficulties (Rewiring Your Brain). VIP Boot Camp is designed for veterans who are at the PCMHI level of care. Referrals are accepted for patients receiving treatment from primary care or PCMHI.
Guidelines for participation in VIP Boot Camp may differ across sites or VISNs. For example, a veteran who has been referred to the BHIP for medication management only or to a specialty MHC such as a pain clinic or PTSD clinic might also be appropriate and eligible for VIP Boot Camp.
Given the interconnectedness of foundational themes, elements, and practices across the VIP Boot Camp modules, the modules are offered in a rolling format with a veteran-centric “choose your own adventure” approach. Tactics are presented in the modules in a way that allows patients to begin with any 1 of the 3 modules and receive treatment that will help in the other areas. Participants choose their core module and initial treatment focus based on their values, needs, and goals. Individuals who complete a core module can end their VIP Boot Camp experience or continue to the next 4-week module for up to 3 modules.
The group is open to new individuals at the start of any 4-week module and closed for the remainder of its 4-week duration. This innovative rolling modular approach combines elements of open- and closed-group format, allowing for the flexibility and accessibility of an open group with the stability and peer support of a closed group.
Given the complicated and overlapping nature of chronic pain, emotion regulation/ stress, and sleep disturbances, VIP Boot Camp acknowledges that everything is interconnected and difficulties in 1 area may impact other areas. The 3 interconnected modules with repeating themes provide coherence and consistency. Veterans learn how interconnections across difficulties can be leveraged so that tactics learned and practiced in 1 area can assist in other areas, changing the cycle of suffering into a cycle of growth.
VIP Boot Camp sessions are 90 minutes long, once weekly for 4 weeks, with 2 mental health professionals trained to lead a dynamic group psychotherapy experience that aims to be fun for participants. VIP Boot Camp synthesizes evidence-based and evidence-informed interventions, as well as techniques from VHA complementary and integrative health programs, psychoeducation, and interpersonal interventions that model connection, playfulness, and healthy boundaries. These varied strategies combine to equip veterans with practical tactics for self-management outside of sessions, a process described as “finding puzzle pieces.” VIP Boot Camp is built on the idea that people are more likely to adopt and practice any tactic after being taught why that tactic is important, and how it fits into their larger interconnected puzzle. After each session, participants are provided with additional asynchronous educational material to help reinforce their learnings and practices.
Although individuals may hesitate to participate in a group setting, they often find the experience of community enhances and accelerates their treatment and gains. This involvement is highlighted in a core aspect of a VIP Boot Camp session called wins, during which participants learn how others on their Boot Camp team are implementing new skills and moving toward their personal values and objectives in a stepwise manner. Through these shared experiences, veterans discover how tactics working for others may serve as a model for their own personal objectives and plans for practice. The sense of relief described by many upon realizing they are not alone in their experiences, along with the satisfaction felt in discovering their ability to support others in Boot Camp, is described by many participants as deeply meaningful and in line with their personal values.
While developed as a fully virtual group program, VIP Boot Camp can also be conducted in person. The virtual program has been successful and continues to spread across VISN 9. There are 8 virtual VIP Boot Camps running in VISN 9, with plans for continued expansion. In the VISN 9 CRH, Boot Camps typically have 10 to 12 participants. Additionally, as VIP Boot Camp grows within a location there are frequently sufficient referrals to support a second rolling group, which enables staggering of the module offerings to allow for even more timely treatment.
Training Program
VISN 9 CRH also developed a VIP Boot Camp 3-day intensive training program for PCMHI HCPs that consists of learning and practicing VIP Boot Camp material for chronic pain, emotion regulation/ stress, sleep disturbances, mindfulness, and guided imagery, along with gaining experience as a VIP Boot Camp coleader. Feedback received from PCMHI HCPs who completed training has been positive. There is also a private Microsoft Teams channel for HCPs, which allows for resource sharing and community building among coleaders. More than 75 PCMHI HCPs have completed VIP Boot Camp training and > 25 VIP Boot Camps have been established at 4 additional VISNs.
The VISN 9 CRH VIP Boot Camp program initiated an implementation and effectiveness project with the Michael E. DeBakey VA Medical Center and the South Central Mental Illness Research, Education and Clinical Center. The focus of this collaboration is support for implementation and treatment effectiveness research with reports, articles, and a white paper on findings and best practices, alongside continued dissemination of the VIP Boot Camp program and training.
Conclusions
VIP Boot Camp is a PCMHI group program offering readily available, comprehensive, and integrative group psychotherapy services to veterans experiencing . 1 of the following: chronic pain, emotion regulation/ stress, and sleep disturbances. It was launched at the VISN 9 CRH with a goal of addressing clinical gaps in the delivery of mental health care, by increasing the number of patients treated within PCMHI. The VIP Boot Camp model provides veterans the opportunity to transform cycles of suffering into cycles of growth through a single approach that can address multiple presenting and interconnected issues.
A 3-day VIP Boot Camp training program provides a quick and effective path for a PCMHI program to train HCPs to launch a VIP Boot Camp. The VISN 9 CRH will continue to champion VIP Boot Camp as a model for the successful provision of comprehensive and integrative mental health treatment within PCMHI at the VA. Through readily available access to comprehensive mental health treatment in an environment that promotes participant empowerment and social engagement, VIP Boot Camp represents an integrative and innovative model of mental health treatment that offers benefits to veteran participants, HCPs, and the VHA.
- Leung LB, Yoon J, Escarce JJ, et al. Primary care-mental health integration in the VA: shifting mental health services for common mental illnesses to primary care. Psychiatr Serv. 2018;69:403-409. doi:10.1176/appi.ps.201700190
- Zhang A, Park S, Sullivan JE, et al. The effectiveness of problem-solving therapy for primary care patients’ depressive and/or anxiety disorders: a systematic review and meta-analysis. J Am Board Fam Med. 2018;31:139-150. doi:10.3122/jabfm.2018.01.170270
- Hundt NE, Barrera TL, Robinson A, et al. A systematic review of cognitive behavioral therapy for depression in veterans. Mil Med. 2014;179:942-949. doi:10.7205/milmed-d-14-00128
- Jank R, Gallee A, Boeckle M, et al. Chronic pain and sleep disorders in primary care. Pain Res Treat. 2017;2017:1-9. doi:10.1155/2017/9081802
- Ashrafioun L, Bishop TM, Pigeon WR. The relationship between pain severity, insomnia, and suicide attempts among a national veteran sample initiating pain care. Psychosom Med. 2021;83:733- 738. doi:10.1097/psy.0000000000000975
- Ramanuj P, Ferenchik E, Docherty M, et al. Evolving models of integrated behavioral health and primary care. Curr Psychiatry Rep. 2019;21:1. doi:10.1007/s11920-019-0985-4
- Post EP, Metzger M, Dumas P, et al. Integrating mental health into primary care within the Veterans Health Administration. Fam Syst Health. 2010;28:83-90. doi:10.1037/a0020130
- Smith TL, Kim B, Benzer JK, et al. FLOW: early results from a clinical demonstration project to improve the transition of patients with mental health disorders back to primary care. Psychol Serv. 2021;18:23-32. doi:10.1037/ser0000336
- Kearney LK, Post EP, Pomerantz AS, et al. Applying the interprofessional patient aligned care team in the department of veterans affairs transforming primary care. Am Psychol. 2014;69(4):399-408. doi:10.1037/a0035909
- US Government Accountability Office. Veterans health care: staffing challenges persist for fully integrating mental health and primary care services. December 15, 2022. Accessed July 9, 2025. https://www.gao.gov/products/gao-23-105372
- National Academies of Science and Engineering. Evaluation of the Department of Veterans Affairs Mental Health Services. National Academies Press; 2018. Accessed July 9, 2025. https://nap.nationalacademies.org/catalog/24915/evaluation-of-the-department-of-veterans-affairs-mental-health-services
- US Department of Veterans Affairs. Blueprint for excellence: achieving veterans’ excellence. October 6, 2014. Accessed July 9, 2025. https://www.volunteer.va.gov/docs/blueprintforexcellence_factsheet.PDF
- US Department of Veterans Affairs. Department of Veterans Affairs FY 2018-2024 strategic plan. Accessed July 9, 2025. https://www.calvet.ca.gov/Regulations/USDVA%20Strategic%20Plan%202018-2024.pdf
- Sripada RK, Bohnert KM, Ganoczy D, et al. Initial group versus individual therapy for posttraumatic stress disorder and subsequent follow-up treatment adequacy. Psychol Serv. 2016;13:349-355. doi:10.1037/ser0000077
- Burnett-Zeigler IE, Pfeiffer P, Zivin K, et al. Psychotherapy utilization for acute depression within the Veterans Affairs health care system. Psychol Serv. 2012;9:325-335. doi:10.1037/a0027957
- Kim JS, Prins A, Hirschhorn EW, et al. Preliminary investigation into the effectiveness of group webSTAIR for trauma-exposed veterans in primary care. Mil Med. 2024;189:e1403-e1408. doi:10.1093/milmed/usae052
- Jakupcak M, Blais RK, Grossbard J, et al. “Toughness” in association with mental health symptoms among Iraq and Afghanistan war veterans seeking Veterans Affairs health care. Psychol Men Masc. 2014;15:100-104. doi:10.1037/a0031508
- Stoycos SA, Berzenski SR, Beck JG, et al. Predictors of treatment completion in group psychotherapy for male veterans with posttraumatic stress disorder. J Trauma Stress. 2023;36:346-358. doi:10.1002/jts.22915
- Possemato K. The current state of intervention research for posttraumatic stress disorder within the primary care setting. J Clin Psychol Med Settings. 2011;18:268-280. doi:10.1007/s10880-011-9237-4
- Hunt MG, Rosenheck RA. Psychotherapy in mental health clinics of the Department of Veterans Affairs. J Clin Psychol. 2011;67:561-573. doi:10.1002/jclp.20788
- Khatri N, Marziali E, Tchernikov I, et al. Comparing telehealth-based and clinic-based group cognitive behavioral therapy for adults with depression and anxiety: a pilot study. Clin Interv Aging. 2014;9:765. doi:10.2147/cia.s57832
- Dangel J. Clinical resource hub increases veterans' access to care. VA News. January 12, 2025. Accessed September 3, 2025. https://news.va.gov/137439/clinical-resource-hub-increases-access-to-care/
Since 2007, Primary Care Mental Health Integration (PCMHI) at the Veterans Health Administration (VHA) has improved access to mental health care services for veterans by directly embedding mental health care professionals (HCPs) within primary care teams.1 Veterans referred to PCMHI often have co-occurring physical and mental health disorders.2 Untreated chronic physical and mental comorbidities can diminish the effectiveness of medical and mental health interventions. Growing evidence suggests that treatment of mental health conditions can improve physical health outcomes and management of physical conditions can improve mental health outcomes.2,3
Chronic pain and sleep disorders are common reasons patients present to primary care, and often coexist together with mental health comorbidities.4 Sleep disorders affect 50% to 88% of patients with chronic pain, and 40% of patients with sleep disorders report chronic pain.4 Research has found that chronic pain and sleep disorders increase the risk of suicide attempts and deaths by suicide. Addressing suicide prevention simultaneously with treating chronic pain and insomnia is encouraged.5
Background
PCMHI treats physical and mental health comorbidities with a collaborative framework and a biopsychosocial integrative model.6 PCMHI staff provide mental health services as members of primary care teams. An interdisciplinary PCMHI team can include, but is not limited to, psychologists, mental health social workers, psychiatrists, nurse practitioners, clinical pharmacists, and mental health nurses. Quality of care within this model is elevated, as mental and physical health are recognized as interconnected. Collaboration between primary care and mental health benefits veterans and the VHA by increasing access to mental health care, decreasing stigma associated with mental health treatment, improving health outcomes, and enhancing the likelihood of recovery, resulting in high patient satisfaction.6-8
In the existing PCMHI model, HCPs are encouraged to use short-term, evidence-based psychotherapies (EBPs).9 Veterans referred to PCMHI from primary care are typically able to attend 1 to 6 brief sessions of mental health treatment, often 20 to 30 minutes long. Most EBPs in PCMHI are disorder- specific, providing interventions focused on a single presenting problem (eg, insomnia, chronic pain, or posttraumatic stress disorder [PTSD]). For veterans with a single issue, this model can be very effective. 1,10 However, the high rate of co-occurrence of mental and physical health issues can make it difficult to fully treat interrelated problems if the focus is on 1 specific diagnosis. Veterans with a need for additional (more comprehensive or intensive) mental health treatment are frequently referred to a higher, more resource-intensive level of mental health care, either in the VHA or the community. Examples of higher levels of mental health care include the longer term behavioral health interdisciplinary program (BHIP), sometimes called a mental health clinic (MHC), or a specialty mental health program such as a PTSD clinic.
As PCMHI continues to grow, new challenges have emerged related to staffing shortages and gaps in the clinical delivery of mental health treatment within the VHA. At the same time, demand for VHA mental health treatment has increased. However, a mental health professional shortage severely limits the ability of the VHA to meet this demand. In many systems, this shortage may result in more referrals being made to a higher level of mental health care because of fewer resources to provide comprehensive treatment in a less intensive PCMHI setting.8,10,11 This referral pattern can overburden higher level care, often with long wait times for treatment and lengthy lag times between appointments. Furthermore, these gaps in the clinical delivery of care cannot be effectively addressed by hiring additional mental health professionals. This strain on resources can impede access to care and negatively affect outcomes.10
Recent congressional reports highlight these issues, noting that demand for mental health services continues to outpace the capacity of both PCMHI and higher levels of mental health care, leading to delays in treatment that may negatively affect outcomes.8,10,11 These delays can be particularly detrimental for individuals with conditions requiring timely intervention.8,11 Some veterans are willing to engage with PCMHI in a primary care setting but may be reluctant to engage in general mental health treatment. These veterans might not receive the mental health care they need without PCMHI.
Group Psychotherapy
A group psychotherapy format can address gaps in care delivery and provide advantages for patients, mental health professionals, and the VHA. Group psychotherapy aligns with the US Department of Veterans Affairs (VA) 2018 Blueprint for Excellence and 2018 to 2024 strategic plan, underscoring the need for more timely and efficient mental health services.12,13
Benefits of group psychotherapy include reductions in symptoms, decreased feelings of isolation, increased social support, decreased emotional suppression, and enhanced satisfaction with overall quality of life.14-17 Studies of veterans with PTSD have found less attrition among those who chose group therapy compared with individual therapy.14,18 Group psychotherapy improves access to care by enabling delivery to more patients.14 When compared with individual therapy, the group format allows for a large number of patients to be treated simultaneously, maximizing resources and reducing costs.3,19-21
VISN 9 CRH Innovation
The VA provides care to veterans through regionally distinct administrative systems known as Veterans Integrated Service Networks (VISNs). Clinical resource hubs (CRH) are VISN-based programs created to cover VA staffing shortages by virtually deploying HCPs into local VA systems until vacancies are filled. The national CRH vision of effectively using resources and innovative technologies to meet veterans’ health care needs, along with the above-referenced clinical gaps in the delivery of care, inspired the development of VIP Boot Camp within the VISN 9 CRH.22
Program Description
VIP Boot Camp is an evidence-informed group psychotherapy program designed to provide timely, brief, and comprehensive mental health treatment for veterans. VIP Boot Camp was developed to address the needs of veterans accessing PCMHI services who experience ≥ 1 of the often overlapping problems of anxiety/emotion regulation/stress, sleep difficulties, and chronic pain (Figure). VIP Boot Camp uses an integrative approach to highlight interconnections and similarities among these difficulties and their treatment. A primary vision of the program is to provide this comprehensive treatment within PCMHI (upstream) so additional referrals to higher levels of mental health care (downstream) may not be needed.

This design is intentional because it increases the number of individuals who can be treated upstream with comprehensive, preventive, and proactive care within PCMHI which, over time, frees up resources in the BHIP for individuals requiring higher levels of care. This approach also aligns with the importance of early treatment for chronic pain and sleep disturbances, which are linked to increased risk of suicide attempts and deaths by suicide for veterans.5 National interest for VIP Boot Camp grew during fiscal year 2024 after it received the Gold Medal Recognition for Most Adoptable and Greatest Potential for Impact during VHA National Access Sprint Wave 3—Mental Health Call of Champions.
History
VIP Boot Camp began in August 2021 at VISN 9 as a 6-week virtual group for veterans with chronic pain. It was established to assist a large VA medical center experiencing PCMHI staffing shortages and lacking available PCMHI groups. Many veterans in the chronic pain group discussed co-occurring issues such as sleep disturbances, anxiety, and stress. The CRH team considered launching 2 separate groups to address these additional PCMHI-level issues; however, in developing the group material which drew from multiple clinical approaches, the team recognized significant overlapping and interconnected themes.
The team discussed EBPs within the VHA and how certain interventions within these treatments could be helpful across many other co-occurring disorders. Integrated tactics (clinical interventions) were drawn from cognitive-behavioral therapy (for depression, insomnia, or chronic pain), acceptance and commitment therapy, prolonged exposure, cognitive processing therapy, dialectical behavior therapy, unified protocol, pain reprocessing therapy, emotional awareness and expression therapy, interpersonal neurobiology, and mindfulness. We collaborated with veterans during VIP Boot Camp groups to determine how to present and discuss complex interventions in ways that were clinically accurate, understandable, relatable, and relevant to their experiences.
To address accessibility issues, the chronic pain group was reduced to 4 weeks. A second 4-week module for anxiety, emotion regulation, and stress was developed, mirroring the tactics, language, and integrative approach of the revised chronic pain module. A similar integrative approach led to the development of the third and final 4-week module for sleep disturbances.
Current Program
The VIP Boot Camp consists of three 4-week integrated modules, each highlighting a critical area: sleep disturbances (Improving Sleep), chronic pain difficulties (Outsmarting Chronic Pain), and emotion regulation difficulties (Rewiring Your Brain). VIP Boot Camp is designed for veterans who are at the PCMHI level of care. Referrals are accepted for patients receiving treatment from primary care or PCMHI.
Guidelines for participation in VIP Boot Camp may differ across sites or VISNs. For example, a veteran who has been referred to the BHIP for medication management only or to a specialty MHC such as a pain clinic or PTSD clinic might also be appropriate and eligible for VIP Boot Camp.
Given the interconnectedness of foundational themes, elements, and practices across the VIP Boot Camp modules, the modules are offered in a rolling format with a veteran-centric “choose your own adventure” approach. Tactics are presented in the modules in a way that allows patients to begin with any 1 of the 3 modules and receive treatment that will help in the other areas. Participants choose their core module and initial treatment focus based on their values, needs, and goals. Individuals who complete a core module can end their VIP Boot Camp experience or continue to the next 4-week module for up to 3 modules.
The group is open to new individuals at the start of any 4-week module and closed for the remainder of its 4-week duration. This innovative rolling modular approach combines elements of open- and closed-group format, allowing for the flexibility and accessibility of an open group with the stability and peer support of a closed group.
Given the complicated and overlapping nature of chronic pain, emotion regulation/ stress, and sleep disturbances, VIP Boot Camp acknowledges that everything is interconnected and difficulties in 1 area may impact other areas. The 3 interconnected modules with repeating themes provide coherence and consistency. Veterans learn how interconnections across difficulties can be leveraged so that tactics learned and practiced in 1 area can assist in other areas, changing the cycle of suffering into a cycle of growth.
VIP Boot Camp sessions are 90 minutes long, once weekly for 4 weeks, with 2 mental health professionals trained to lead a dynamic group psychotherapy experience that aims to be fun for participants. VIP Boot Camp synthesizes evidence-based and evidence-informed interventions, as well as techniques from VHA complementary and integrative health programs, psychoeducation, and interpersonal interventions that model connection, playfulness, and healthy boundaries. These varied strategies combine to equip veterans with practical tactics for self-management outside of sessions, a process described as “finding puzzle pieces.” VIP Boot Camp is built on the idea that people are more likely to adopt and practice any tactic after being taught why that tactic is important, and how it fits into their larger interconnected puzzle. After each session, participants are provided with additional asynchronous educational material to help reinforce their learnings and practices.
Although individuals may hesitate to participate in a group setting, they often find the experience of community enhances and accelerates their treatment and gains. This involvement is highlighted in a core aspect of a VIP Boot Camp session called wins, during which participants learn how others on their Boot Camp team are implementing new skills and moving toward their personal values and objectives in a stepwise manner. Through these shared experiences, veterans discover how tactics working for others may serve as a model for their own personal objectives and plans for practice. The sense of relief described by many upon realizing they are not alone in their experiences, along with the satisfaction felt in discovering their ability to support others in Boot Camp, is described by many participants as deeply meaningful and in line with their personal values.
While developed as a fully virtual group program, VIP Boot Camp can also be conducted in person. The virtual program has been successful and continues to spread across VISN 9. There are 8 virtual VIP Boot Camps running in VISN 9, with plans for continued expansion. In the VISN 9 CRH, Boot Camps typically have 10 to 12 participants. Additionally, as VIP Boot Camp grows within a location there are frequently sufficient referrals to support a second rolling group, which enables staggering of the module offerings to allow for even more timely treatment.
Training Program
VISN 9 CRH also developed a VIP Boot Camp 3-day intensive training program for PCMHI HCPs that consists of learning and practicing VIP Boot Camp material for chronic pain, emotion regulation/ stress, sleep disturbances, mindfulness, and guided imagery, along with gaining experience as a VIP Boot Camp coleader. Feedback received from PCMHI HCPs who completed training has been positive. There is also a private Microsoft Teams channel for HCPs, which allows for resource sharing and community building among coleaders. More than 75 PCMHI HCPs have completed VIP Boot Camp training and > 25 VIP Boot Camps have been established at 4 additional VISNs.
The VISN 9 CRH VIP Boot Camp program initiated an implementation and effectiveness project with the Michael E. DeBakey VA Medical Center and the South Central Mental Illness Research, Education and Clinical Center. The focus of this collaboration is support for implementation and treatment effectiveness research with reports, articles, and a white paper on findings and best practices, alongside continued dissemination of the VIP Boot Camp program and training.
Conclusions
VIP Boot Camp is a PCMHI group program offering readily available, comprehensive, and integrative group psychotherapy services to veterans experiencing . 1 of the following: chronic pain, emotion regulation/ stress, and sleep disturbances. It was launched at the VISN 9 CRH with a goal of addressing clinical gaps in the delivery of mental health care, by increasing the number of patients treated within PCMHI. The VIP Boot Camp model provides veterans the opportunity to transform cycles of suffering into cycles of growth through a single approach that can address multiple presenting and interconnected issues.
A 3-day VIP Boot Camp training program provides a quick and effective path for a PCMHI program to train HCPs to launch a VIP Boot Camp. The VISN 9 CRH will continue to champion VIP Boot Camp as a model for the successful provision of comprehensive and integrative mental health treatment within PCMHI at the VA. Through readily available access to comprehensive mental health treatment in an environment that promotes participant empowerment and social engagement, VIP Boot Camp represents an integrative and innovative model of mental health treatment that offers benefits to veteran participants, HCPs, and the VHA.
Since 2007, Primary Care Mental Health Integration (PCMHI) at the Veterans Health Administration (VHA) has improved access to mental health care services for veterans by directly embedding mental health care professionals (HCPs) within primary care teams.1 Veterans referred to PCMHI often have co-occurring physical and mental health disorders.2 Untreated chronic physical and mental comorbidities can diminish the effectiveness of medical and mental health interventions. Growing evidence suggests that treatment of mental health conditions can improve physical health outcomes and management of physical conditions can improve mental health outcomes.2,3
Chronic pain and sleep disorders are common reasons patients present to primary care, and often coexist together with mental health comorbidities.4 Sleep disorders affect 50% to 88% of patients with chronic pain, and 40% of patients with sleep disorders report chronic pain.4 Research has found that chronic pain and sleep disorders increase the risk of suicide attempts and deaths by suicide. Addressing suicide prevention simultaneously with treating chronic pain and insomnia is encouraged.5
Background
PCMHI treats physical and mental health comorbidities with a collaborative framework and a biopsychosocial integrative model.6 PCMHI staff provide mental health services as members of primary care teams. An interdisciplinary PCMHI team can include, but is not limited to, psychologists, mental health social workers, psychiatrists, nurse practitioners, clinical pharmacists, and mental health nurses. Quality of care within this model is elevated, as mental and physical health are recognized as interconnected. Collaboration between primary care and mental health benefits veterans and the VHA by increasing access to mental health care, decreasing stigma associated with mental health treatment, improving health outcomes, and enhancing the likelihood of recovery, resulting in high patient satisfaction.6-8
In the existing PCMHI model, HCPs are encouraged to use short-term, evidence-based psychotherapies (EBPs).9 Veterans referred to PCMHI from primary care are typically able to attend 1 to 6 brief sessions of mental health treatment, often 20 to 30 minutes long. Most EBPs in PCMHI are disorder- specific, providing interventions focused on a single presenting problem (eg, insomnia, chronic pain, or posttraumatic stress disorder [PTSD]). For veterans with a single issue, this model can be very effective. 1,10 However, the high rate of co-occurrence of mental and physical health issues can make it difficult to fully treat interrelated problems if the focus is on 1 specific diagnosis. Veterans with a need for additional (more comprehensive or intensive) mental health treatment are frequently referred to a higher, more resource-intensive level of mental health care, either in the VHA or the community. Examples of higher levels of mental health care include the longer term behavioral health interdisciplinary program (BHIP), sometimes called a mental health clinic (MHC), or a specialty mental health program such as a PTSD clinic.
As PCMHI continues to grow, new challenges have emerged related to staffing shortages and gaps in the clinical delivery of mental health treatment within the VHA. At the same time, demand for VHA mental health treatment has increased. However, a mental health professional shortage severely limits the ability of the VHA to meet this demand. In many systems, this shortage may result in more referrals being made to a higher level of mental health care because of fewer resources to provide comprehensive treatment in a less intensive PCMHI setting.8,10,11 This referral pattern can overburden higher level care, often with long wait times for treatment and lengthy lag times between appointments. Furthermore, these gaps in the clinical delivery of care cannot be effectively addressed by hiring additional mental health professionals. This strain on resources can impede access to care and negatively affect outcomes.10
Recent congressional reports highlight these issues, noting that demand for mental health services continues to outpace the capacity of both PCMHI and higher levels of mental health care, leading to delays in treatment that may negatively affect outcomes.8,10,11 These delays can be particularly detrimental for individuals with conditions requiring timely intervention.8,11 Some veterans are willing to engage with PCMHI in a primary care setting but may be reluctant to engage in general mental health treatment. These veterans might not receive the mental health care they need without PCMHI.
Group Psychotherapy
A group psychotherapy format can address gaps in care delivery and provide advantages for patients, mental health professionals, and the VHA. Group psychotherapy aligns with the US Department of Veterans Affairs (VA) 2018 Blueprint for Excellence and 2018 to 2024 strategic plan, underscoring the need for more timely and efficient mental health services.12,13
Benefits of group psychotherapy include reductions in symptoms, decreased feelings of isolation, increased social support, decreased emotional suppression, and enhanced satisfaction with overall quality of life.14-17 Studies of veterans with PTSD have found less attrition among those who chose group therapy compared with individual therapy.14,18 Group psychotherapy improves access to care by enabling delivery to more patients.14 When compared with individual therapy, the group format allows for a large number of patients to be treated simultaneously, maximizing resources and reducing costs.3,19-21
VISN 9 CRH Innovation
The VA provides care to veterans through regionally distinct administrative systems known as Veterans Integrated Service Networks (VISNs). Clinical resource hubs (CRH) are VISN-based programs created to cover VA staffing shortages by virtually deploying HCPs into local VA systems until vacancies are filled. The national CRH vision of effectively using resources and innovative technologies to meet veterans’ health care needs, along with the above-referenced clinical gaps in the delivery of care, inspired the development of VIP Boot Camp within the VISN 9 CRH.22
Program Description
VIP Boot Camp is an evidence-informed group psychotherapy program designed to provide timely, brief, and comprehensive mental health treatment for veterans. VIP Boot Camp was developed to address the needs of veterans accessing PCMHI services who experience ≥ 1 of the often overlapping problems of anxiety/emotion regulation/stress, sleep difficulties, and chronic pain (Figure). VIP Boot Camp uses an integrative approach to highlight interconnections and similarities among these difficulties and their treatment. A primary vision of the program is to provide this comprehensive treatment within PCMHI (upstream) so additional referrals to higher levels of mental health care (downstream) may not be needed.

This design is intentional because it increases the number of individuals who can be treated upstream with comprehensive, preventive, and proactive care within PCMHI which, over time, frees up resources in the BHIP for individuals requiring higher levels of care. This approach also aligns with the importance of early treatment for chronic pain and sleep disturbances, which are linked to increased risk of suicide attempts and deaths by suicide for veterans.5 National interest for VIP Boot Camp grew during fiscal year 2024 after it received the Gold Medal Recognition for Most Adoptable and Greatest Potential for Impact during VHA National Access Sprint Wave 3—Mental Health Call of Champions.
History
VIP Boot Camp began in August 2021 at VISN 9 as a 6-week virtual group for veterans with chronic pain. It was established to assist a large VA medical center experiencing PCMHI staffing shortages and lacking available PCMHI groups. Many veterans in the chronic pain group discussed co-occurring issues such as sleep disturbances, anxiety, and stress. The CRH team considered launching 2 separate groups to address these additional PCMHI-level issues; however, in developing the group material which drew from multiple clinical approaches, the team recognized significant overlapping and interconnected themes.
The team discussed EBPs within the VHA and how certain interventions within these treatments could be helpful across many other co-occurring disorders. Integrated tactics (clinical interventions) were drawn from cognitive-behavioral therapy (for depression, insomnia, or chronic pain), acceptance and commitment therapy, prolonged exposure, cognitive processing therapy, dialectical behavior therapy, unified protocol, pain reprocessing therapy, emotional awareness and expression therapy, interpersonal neurobiology, and mindfulness. We collaborated with veterans during VIP Boot Camp groups to determine how to present and discuss complex interventions in ways that were clinically accurate, understandable, relatable, and relevant to their experiences.
To address accessibility issues, the chronic pain group was reduced to 4 weeks. A second 4-week module for anxiety, emotion regulation, and stress was developed, mirroring the tactics, language, and integrative approach of the revised chronic pain module. A similar integrative approach led to the development of the third and final 4-week module for sleep disturbances.
Current Program
The VIP Boot Camp consists of three 4-week integrated modules, each highlighting a critical area: sleep disturbances (Improving Sleep), chronic pain difficulties (Outsmarting Chronic Pain), and emotion regulation difficulties (Rewiring Your Brain). VIP Boot Camp is designed for veterans who are at the PCMHI level of care. Referrals are accepted for patients receiving treatment from primary care or PCMHI.
Guidelines for participation in VIP Boot Camp may differ across sites or VISNs. For example, a veteran who has been referred to the BHIP for medication management only or to a specialty MHC such as a pain clinic or PTSD clinic might also be appropriate and eligible for VIP Boot Camp.
Given the interconnectedness of foundational themes, elements, and practices across the VIP Boot Camp modules, the modules are offered in a rolling format with a veteran-centric “choose your own adventure” approach. Tactics are presented in the modules in a way that allows patients to begin with any 1 of the 3 modules and receive treatment that will help in the other areas. Participants choose their core module and initial treatment focus based on their values, needs, and goals. Individuals who complete a core module can end their VIP Boot Camp experience or continue to the next 4-week module for up to 3 modules.
The group is open to new individuals at the start of any 4-week module and closed for the remainder of its 4-week duration. This innovative rolling modular approach combines elements of open- and closed-group format, allowing for the flexibility and accessibility of an open group with the stability and peer support of a closed group.
Given the complicated and overlapping nature of chronic pain, emotion regulation/ stress, and sleep disturbances, VIP Boot Camp acknowledges that everything is interconnected and difficulties in 1 area may impact other areas. The 3 interconnected modules with repeating themes provide coherence and consistency. Veterans learn how interconnections across difficulties can be leveraged so that tactics learned and practiced in 1 area can assist in other areas, changing the cycle of suffering into a cycle of growth.
VIP Boot Camp sessions are 90 minutes long, once weekly for 4 weeks, with 2 mental health professionals trained to lead a dynamic group psychotherapy experience that aims to be fun for participants. VIP Boot Camp synthesizes evidence-based and evidence-informed interventions, as well as techniques from VHA complementary and integrative health programs, psychoeducation, and interpersonal interventions that model connection, playfulness, and healthy boundaries. These varied strategies combine to equip veterans with practical tactics for self-management outside of sessions, a process described as “finding puzzle pieces.” VIP Boot Camp is built on the idea that people are more likely to adopt and practice any tactic after being taught why that tactic is important, and how it fits into their larger interconnected puzzle. After each session, participants are provided with additional asynchronous educational material to help reinforce their learnings and practices.
Although individuals may hesitate to participate in a group setting, they often find the experience of community enhances and accelerates their treatment and gains. This involvement is highlighted in a core aspect of a VIP Boot Camp session called wins, during which participants learn how others on their Boot Camp team are implementing new skills and moving toward their personal values and objectives in a stepwise manner. Through these shared experiences, veterans discover how tactics working for others may serve as a model for their own personal objectives and plans for practice. The sense of relief described by many upon realizing they are not alone in their experiences, along with the satisfaction felt in discovering their ability to support others in Boot Camp, is described by many participants as deeply meaningful and in line with their personal values.
While developed as a fully virtual group program, VIP Boot Camp can also be conducted in person. The virtual program has been successful and continues to spread across VISN 9. There are 8 virtual VIP Boot Camps running in VISN 9, with plans for continued expansion. In the VISN 9 CRH, Boot Camps typically have 10 to 12 participants. Additionally, as VIP Boot Camp grows within a location there are frequently sufficient referrals to support a second rolling group, which enables staggering of the module offerings to allow for even more timely treatment.
Training Program
VISN 9 CRH also developed a VIP Boot Camp 3-day intensive training program for PCMHI HCPs that consists of learning and practicing VIP Boot Camp material for chronic pain, emotion regulation/ stress, sleep disturbances, mindfulness, and guided imagery, along with gaining experience as a VIP Boot Camp coleader. Feedback received from PCMHI HCPs who completed training has been positive. There is also a private Microsoft Teams channel for HCPs, which allows for resource sharing and community building among coleaders. More than 75 PCMHI HCPs have completed VIP Boot Camp training and > 25 VIP Boot Camps have been established at 4 additional VISNs.
The VISN 9 CRH VIP Boot Camp program initiated an implementation and effectiveness project with the Michael E. DeBakey VA Medical Center and the South Central Mental Illness Research, Education and Clinical Center. The focus of this collaboration is support for implementation and treatment effectiveness research with reports, articles, and a white paper on findings and best practices, alongside continued dissemination of the VIP Boot Camp program and training.
Conclusions
VIP Boot Camp is a PCMHI group program offering readily available, comprehensive, and integrative group psychotherapy services to veterans experiencing . 1 of the following: chronic pain, emotion regulation/ stress, and sleep disturbances. It was launched at the VISN 9 CRH with a goal of addressing clinical gaps in the delivery of mental health care, by increasing the number of patients treated within PCMHI. The VIP Boot Camp model provides veterans the opportunity to transform cycles of suffering into cycles of growth through a single approach that can address multiple presenting and interconnected issues.
A 3-day VIP Boot Camp training program provides a quick and effective path for a PCMHI program to train HCPs to launch a VIP Boot Camp. The VISN 9 CRH will continue to champion VIP Boot Camp as a model for the successful provision of comprehensive and integrative mental health treatment within PCMHI at the VA. Through readily available access to comprehensive mental health treatment in an environment that promotes participant empowerment and social engagement, VIP Boot Camp represents an integrative and innovative model of mental health treatment that offers benefits to veteran participants, HCPs, and the VHA.
- Leung LB, Yoon J, Escarce JJ, et al. Primary care-mental health integration in the VA: shifting mental health services for common mental illnesses to primary care. Psychiatr Serv. 2018;69:403-409. doi:10.1176/appi.ps.201700190
- Zhang A, Park S, Sullivan JE, et al. The effectiveness of problem-solving therapy for primary care patients’ depressive and/or anxiety disorders: a systematic review and meta-analysis. J Am Board Fam Med. 2018;31:139-150. doi:10.3122/jabfm.2018.01.170270
- Hundt NE, Barrera TL, Robinson A, et al. A systematic review of cognitive behavioral therapy for depression in veterans. Mil Med. 2014;179:942-949. doi:10.7205/milmed-d-14-00128
- Jank R, Gallee A, Boeckle M, et al. Chronic pain and sleep disorders in primary care. Pain Res Treat. 2017;2017:1-9. doi:10.1155/2017/9081802
- Ashrafioun L, Bishop TM, Pigeon WR. The relationship between pain severity, insomnia, and suicide attempts among a national veteran sample initiating pain care. Psychosom Med. 2021;83:733- 738. doi:10.1097/psy.0000000000000975
- Ramanuj P, Ferenchik E, Docherty M, et al. Evolving models of integrated behavioral health and primary care. Curr Psychiatry Rep. 2019;21:1. doi:10.1007/s11920-019-0985-4
- Post EP, Metzger M, Dumas P, et al. Integrating mental health into primary care within the Veterans Health Administration. Fam Syst Health. 2010;28:83-90. doi:10.1037/a0020130
- Smith TL, Kim B, Benzer JK, et al. FLOW: early results from a clinical demonstration project to improve the transition of patients with mental health disorders back to primary care. Psychol Serv. 2021;18:23-32. doi:10.1037/ser0000336
- Kearney LK, Post EP, Pomerantz AS, et al. Applying the interprofessional patient aligned care team in the department of veterans affairs transforming primary care. Am Psychol. 2014;69(4):399-408. doi:10.1037/a0035909
- US Government Accountability Office. Veterans health care: staffing challenges persist for fully integrating mental health and primary care services. December 15, 2022. Accessed July 9, 2025. https://www.gao.gov/products/gao-23-105372
- National Academies of Science and Engineering. Evaluation of the Department of Veterans Affairs Mental Health Services. National Academies Press; 2018. Accessed July 9, 2025. https://nap.nationalacademies.org/catalog/24915/evaluation-of-the-department-of-veterans-affairs-mental-health-services
- US Department of Veterans Affairs. Blueprint for excellence: achieving veterans’ excellence. October 6, 2014. Accessed July 9, 2025. https://www.volunteer.va.gov/docs/blueprintforexcellence_factsheet.PDF
- US Department of Veterans Affairs. Department of Veterans Affairs FY 2018-2024 strategic plan. Accessed July 9, 2025. https://www.calvet.ca.gov/Regulations/USDVA%20Strategic%20Plan%202018-2024.pdf
- Sripada RK, Bohnert KM, Ganoczy D, et al. Initial group versus individual therapy for posttraumatic stress disorder and subsequent follow-up treatment adequacy. Psychol Serv. 2016;13:349-355. doi:10.1037/ser0000077
- Burnett-Zeigler IE, Pfeiffer P, Zivin K, et al. Psychotherapy utilization for acute depression within the Veterans Affairs health care system. Psychol Serv. 2012;9:325-335. doi:10.1037/a0027957
- Kim JS, Prins A, Hirschhorn EW, et al. Preliminary investigation into the effectiveness of group webSTAIR for trauma-exposed veterans in primary care. Mil Med. 2024;189:e1403-e1408. doi:10.1093/milmed/usae052
- Jakupcak M, Blais RK, Grossbard J, et al. “Toughness” in association with mental health symptoms among Iraq and Afghanistan war veterans seeking Veterans Affairs health care. Psychol Men Masc. 2014;15:100-104. doi:10.1037/a0031508
- Stoycos SA, Berzenski SR, Beck JG, et al. Predictors of treatment completion in group psychotherapy for male veterans with posttraumatic stress disorder. J Trauma Stress. 2023;36:346-358. doi:10.1002/jts.22915
- Possemato K. The current state of intervention research for posttraumatic stress disorder within the primary care setting. J Clin Psychol Med Settings. 2011;18:268-280. doi:10.1007/s10880-011-9237-4
- Hunt MG, Rosenheck RA. Psychotherapy in mental health clinics of the Department of Veterans Affairs. J Clin Psychol. 2011;67:561-573. doi:10.1002/jclp.20788
- Khatri N, Marziali E, Tchernikov I, et al. Comparing telehealth-based and clinic-based group cognitive behavioral therapy for adults with depression and anxiety: a pilot study. Clin Interv Aging. 2014;9:765. doi:10.2147/cia.s57832
- Dangel J. Clinical resource hub increases veterans' access to care. VA News. January 12, 2025. Accessed September 3, 2025. https://news.va.gov/137439/clinical-resource-hub-increases-access-to-care/
- Leung LB, Yoon J, Escarce JJ, et al. Primary care-mental health integration in the VA: shifting mental health services for common mental illnesses to primary care. Psychiatr Serv. 2018;69:403-409. doi:10.1176/appi.ps.201700190
- Zhang A, Park S, Sullivan JE, et al. The effectiveness of problem-solving therapy for primary care patients’ depressive and/or anxiety disorders: a systematic review and meta-analysis. J Am Board Fam Med. 2018;31:139-150. doi:10.3122/jabfm.2018.01.170270
- Hundt NE, Barrera TL, Robinson A, et al. A systematic review of cognitive behavioral therapy for depression in veterans. Mil Med. 2014;179:942-949. doi:10.7205/milmed-d-14-00128
- Jank R, Gallee A, Boeckle M, et al. Chronic pain and sleep disorders in primary care. Pain Res Treat. 2017;2017:1-9. doi:10.1155/2017/9081802
- Ashrafioun L, Bishop TM, Pigeon WR. The relationship between pain severity, insomnia, and suicide attempts among a national veteran sample initiating pain care. Psychosom Med. 2021;83:733- 738. doi:10.1097/psy.0000000000000975
- Ramanuj P, Ferenchik E, Docherty M, et al. Evolving models of integrated behavioral health and primary care. Curr Psychiatry Rep. 2019;21:1. doi:10.1007/s11920-019-0985-4
- Post EP, Metzger M, Dumas P, et al. Integrating mental health into primary care within the Veterans Health Administration. Fam Syst Health. 2010;28:83-90. doi:10.1037/a0020130
- Smith TL, Kim B, Benzer JK, et al. FLOW: early results from a clinical demonstration project to improve the transition of patients with mental health disorders back to primary care. Psychol Serv. 2021;18:23-32. doi:10.1037/ser0000336
- Kearney LK, Post EP, Pomerantz AS, et al. Applying the interprofessional patient aligned care team in the department of veterans affairs transforming primary care. Am Psychol. 2014;69(4):399-408. doi:10.1037/a0035909
- US Government Accountability Office. Veterans health care: staffing challenges persist for fully integrating mental health and primary care services. December 15, 2022. Accessed July 9, 2025. https://www.gao.gov/products/gao-23-105372
- National Academies of Science and Engineering. Evaluation of the Department of Veterans Affairs Mental Health Services. National Academies Press; 2018. Accessed July 9, 2025. https://nap.nationalacademies.org/catalog/24915/evaluation-of-the-department-of-veterans-affairs-mental-health-services
- US Department of Veterans Affairs. Blueprint for excellence: achieving veterans’ excellence. October 6, 2014. Accessed July 9, 2025. https://www.volunteer.va.gov/docs/blueprintforexcellence_factsheet.PDF
- US Department of Veterans Affairs. Department of Veterans Affairs FY 2018-2024 strategic plan. Accessed July 9, 2025. https://www.calvet.ca.gov/Regulations/USDVA%20Strategic%20Plan%202018-2024.pdf
- Sripada RK, Bohnert KM, Ganoczy D, et al. Initial group versus individual therapy for posttraumatic stress disorder and subsequent follow-up treatment adequacy. Psychol Serv. 2016;13:349-355. doi:10.1037/ser0000077
- Burnett-Zeigler IE, Pfeiffer P, Zivin K, et al. Psychotherapy utilization for acute depression within the Veterans Affairs health care system. Psychol Serv. 2012;9:325-335. doi:10.1037/a0027957
- Kim JS, Prins A, Hirschhorn EW, et al. Preliminary investigation into the effectiveness of group webSTAIR for trauma-exposed veterans in primary care. Mil Med. 2024;189:e1403-e1408. doi:10.1093/milmed/usae052
- Jakupcak M, Blais RK, Grossbard J, et al. “Toughness” in association with mental health symptoms among Iraq and Afghanistan war veterans seeking Veterans Affairs health care. Psychol Men Masc. 2014;15:100-104. doi:10.1037/a0031508
- Stoycos SA, Berzenski SR, Beck JG, et al. Predictors of treatment completion in group psychotherapy for male veterans with posttraumatic stress disorder. J Trauma Stress. 2023;36:346-358. doi:10.1002/jts.22915
- Possemato K. The current state of intervention research for posttraumatic stress disorder within the primary care setting. J Clin Psychol Med Settings. 2011;18:268-280. doi:10.1007/s10880-011-9237-4
- Hunt MG, Rosenheck RA. Psychotherapy in mental health clinics of the Department of Veterans Affairs. J Clin Psychol. 2011;67:561-573. doi:10.1002/jclp.20788
- Khatri N, Marziali E, Tchernikov I, et al. Comparing telehealth-based and clinic-based group cognitive behavioral therapy for adults with depression and anxiety: a pilot study. Clin Interv Aging. 2014;9:765. doi:10.2147/cia.s57832
- Dangel J. Clinical resource hub increases veterans' access to care. VA News. January 12, 2025. Accessed September 3, 2025. https://news.va.gov/137439/clinical-resource-hub-increases-access-to-care/
VIP Boot Camp: Expanding the Impact of VA Primary Care Mental Health With a Transdiagnostic Modular Group Program
VIP Boot Camp: Expanding the Impact of VA Primary Care Mental Health With a Transdiagnostic Modular Group Program
Painful Edematous Labial Erosions
Painful Edematous Labial Erosions
THE DIAGNOSIS: Kaposi Varicelliform Eruption
Genital erosions tested positive for herpes simplex virus (HSV) 2 via PCR, confirming a Kaposi varicelliform eruption (KVE) in a patient with mycosis fungoides. The medical team began antiviral therapy with intravenous (IV) acyclovir; however, susceptibility testing during the hospital admission confirmed acyclovir resistance, requiring a transition to cidofovir cream 1% and IV foscarnet.1 Subsequent concerns by the care team about chemical burns, dysuria, and renal impairment led to discontinuation of both the cidofovir and foscarnet, considerably narrowing the treatment options.1 The patient’s condition was complicated by polymicrobial bacteremia. Additionally, worsening acidosis and acute kidney injury required initiation of continuous renal replacement therapy.1 Considering these conditions, the patient was enrolled in a promising clinical trial for pritelivir, a novel antiviral medication; however, due to the development of oliguria and the progression of renal failure, this course of treatment had to be discontinued. Faced with potential viral encephalitis, the infectious disease team concluded that, despite previous adverse reactions, resumption of IV foscarnet treatment would present more benefits than risks, given the patient’s critical situation.1
Mycosis fungoides (MF) is a slowly progressive cutaneous T-cell lymphoma of CD4+ cells that primarily affects the skin. Clinically, it often is characterized by pruritic scaly patches or plaques with sharply demarcated borders, the enduring nature of which consistently poses a therapeutic challenge due to their noted resistance to preliminary lines of treatment. Presently, potential cures are limited to allogeneic stem cell transplantation and unilesional radiotherapy for advanced MF; however, no treatment has been found to notably improve survival rates.1 Mycosis fungoides can result in various complications including diffuse spread of a skin infection caused by HSV, known as KVE.1 Kaposi varicelliform eruption usually manifests clinically with painful skin vesicles that often are accompanied by systemic signs such as fever and malaise. The vesicles rapidly progress into pustules or erosions, predominantly affecting regions such as the head, neck, groin, and upper torso (Figure 1).2 Kaposi varicelliform eruption is considered a dermatologic emergency due to its potential to precipitate serious complications such as life-threatening secondary bacterial infection, HSV viremia, and multiorgan involvement; it also carries the risk of instigating ocular complications, such as keratitis, conjunctivitis, blepharitis, uveitis, and potential vision loss.2
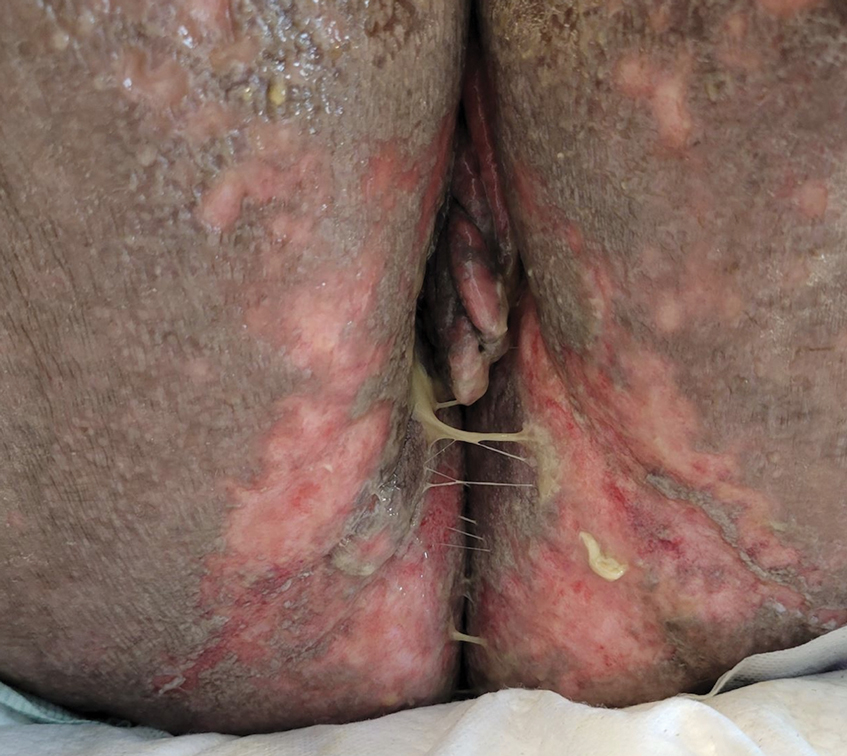
Kaposi varicelliform eruption usually is diagnosed through clinical examination supported by polymerase chain reaction, viral culture, histopathology, HSV serology, and Tzanck smear.2 The differential diagnosis includes varicella, atypical varicella, herpes genitalis, herpes zoster, allergic or irritant contact dermatitis, or MF, which may result in painful skin ulcers.2-4 If an HSV superinfection is suspected, a polymerase chain reaction test ideally should be conducted within the first 72 hours of symptom onset.2 Herpes simplex virus infection may be reinforced by histologic features such as intraepidermal blistering, acantholysis, keratinocyte ballooning degeneration, and multinuclear giant cells with intranuclear inclusions. Given its severe nature, immediate empiric antiviral treatment for KVE is essential, even while awaiting confirmatory tests. The recommended treatment protocol involves acyclovir (400 mg orally 3 times daily or 10 mg/kg IV) or valacyclovir (500 mg orally twice daily), continued until KVE resolves.2
Herpes genitalis caused by HSV-2 is estimated to affect approximately 45 million adults in the United States.2 First-line treatment for HSV-2 includes acyclovir and its derivatives, which are viral nucleoside analogs that inhibit viral DNA polymerases.5,6 However, over the past 2 decades, increasing HSV resistance to acyclovir and its derivatives has been noted among immunocompromised patients.5,6 Second-line agents, such as IV foscarnet and cidofovir, require close laboratory monitoring for nephrotoxicity and are contraindicated in those with renal insufficiency, thus limiting their use.5 To combat acyclovir resistance, novel antivirals such as pritelivir are being developed. Pritelivir targets the HSV helicase-primase complex and has been shown to outperform acyclovir in in-vitro animal models.7 Due to its unique mechanism of action (Figure 2), pritelivir is effective against acyclovir-resistant HSV strains, and clinical trials suggest its serum half-life may allow for daily dosing. A phase 2 study showed pritelivir reduced viral shedding days, sped up genital lesion healing in adults infected with HSV-2, and exhibited a good safety profile.7 Our patient participated in ongoing open-label trials of pritelivir that aimed to assess its efficacy and safety in immunocompromised patients. Given the limited alternative treatments for acyclovir-resistant HSV-2, clinicians need to stay updated on antiviral agents under development.
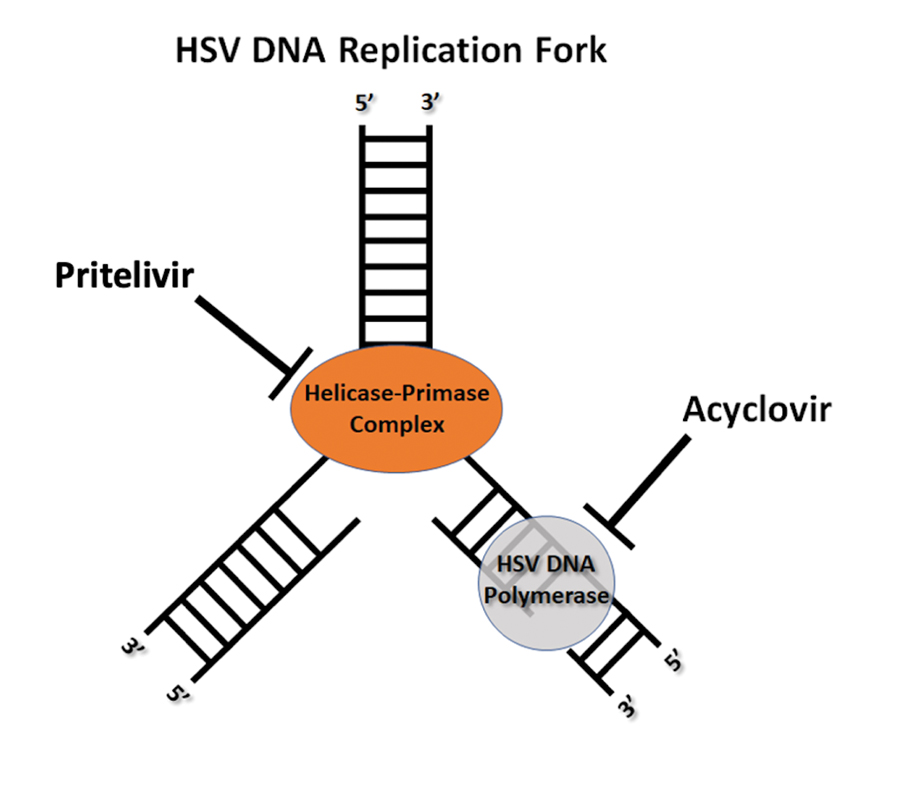
- García-Díaz N, Piris MÁ, Ortiz-Romero PL, et al. Mycosis fungoides and Sézary syndrome: an integrative review of the pathophysiology, molecular drivers, and targeted therapy. Cancers. 2021;13:1931. doi:10.3390/cancers13081931
- Baaniya B, Agrawal S. Kaposi varicelliform eruption in a patient with pemphigus vulgaris: a case report and review of the literature. Case Rep Dermatol Med. 2020;2020:6695342. doi:10.1155/2020/6695342
- Shin D, Lee MS, Kim DY, et al. Increased large unstained cells value in varicella patients: a valuable parameter to aid rapid diagnosis of varicella infection. J Dermatol. 2015;42:795-799. doi:10.1111
- Joshi A, Sah SP, Agrawal S. Kaposi’s varicelliform eruption or atypical chickenpox in a normal individual. Australas J Dermatol. 2000;41:126-127.
- Groves MJ. Genital herpes: a review. Am Fam Physician. 2016; 93:928-934.
- Fleming DT, Leone P, Esposito D, et al. Herpes virus type 2 infection and genital symptoms in primary care patients. Sex Transm Dis. 2006;33:416-421. doi:10.1097/01.olq.0000200578.86276.0b
- Poole CL, James SH. Antiviral therapies for herpesviruses: current agents and new directions. Clin Ther. 2018;40:1282-1298. doi:10.1016 /j.clinthera.2018.07.006.
THE DIAGNOSIS: Kaposi Varicelliform Eruption
Genital erosions tested positive for herpes simplex virus (HSV) 2 via PCR, confirming a Kaposi varicelliform eruption (KVE) in a patient with mycosis fungoides. The medical team began antiviral therapy with intravenous (IV) acyclovir; however, susceptibility testing during the hospital admission confirmed acyclovir resistance, requiring a transition to cidofovir cream 1% and IV foscarnet.1 Subsequent concerns by the care team about chemical burns, dysuria, and renal impairment led to discontinuation of both the cidofovir and foscarnet, considerably narrowing the treatment options.1 The patient’s condition was complicated by polymicrobial bacteremia. Additionally, worsening acidosis and acute kidney injury required initiation of continuous renal replacement therapy.1 Considering these conditions, the patient was enrolled in a promising clinical trial for pritelivir, a novel antiviral medication; however, due to the development of oliguria and the progression of renal failure, this course of treatment had to be discontinued. Faced with potential viral encephalitis, the infectious disease team concluded that, despite previous adverse reactions, resumption of IV foscarnet treatment would present more benefits than risks, given the patient’s critical situation.1
Mycosis fungoides (MF) is a slowly progressive cutaneous T-cell lymphoma of CD4+ cells that primarily affects the skin. Clinically, it often is characterized by pruritic scaly patches or plaques with sharply demarcated borders, the enduring nature of which consistently poses a therapeutic challenge due to their noted resistance to preliminary lines of treatment. Presently, potential cures are limited to allogeneic stem cell transplantation and unilesional radiotherapy for advanced MF; however, no treatment has been found to notably improve survival rates.1 Mycosis fungoides can result in various complications including diffuse spread of a skin infection caused by HSV, known as KVE.1 Kaposi varicelliform eruption usually manifests clinically with painful skin vesicles that often are accompanied by systemic signs such as fever and malaise. The vesicles rapidly progress into pustules or erosions, predominantly affecting regions such as the head, neck, groin, and upper torso (Figure 1).2 Kaposi varicelliform eruption is considered a dermatologic emergency due to its potential to precipitate serious complications such as life-threatening secondary bacterial infection, HSV viremia, and multiorgan involvement; it also carries the risk of instigating ocular complications, such as keratitis, conjunctivitis, blepharitis, uveitis, and potential vision loss.2

Kaposi varicelliform eruption usually is diagnosed through clinical examination supported by polymerase chain reaction, viral culture, histopathology, HSV serology, and Tzanck smear.2 The differential diagnosis includes varicella, atypical varicella, herpes genitalis, herpes zoster, allergic or irritant contact dermatitis, or MF, which may result in painful skin ulcers.2-4 If an HSV superinfection is suspected, a polymerase chain reaction test ideally should be conducted within the first 72 hours of symptom onset.2 Herpes simplex virus infection may be reinforced by histologic features such as intraepidermal blistering, acantholysis, keratinocyte ballooning degeneration, and multinuclear giant cells with intranuclear inclusions. Given its severe nature, immediate empiric antiviral treatment for KVE is essential, even while awaiting confirmatory tests. The recommended treatment protocol involves acyclovir (400 mg orally 3 times daily or 10 mg/kg IV) or valacyclovir (500 mg orally twice daily), continued until KVE resolves.2
Herpes genitalis caused by HSV-2 is estimated to affect approximately 45 million adults in the United States.2 First-line treatment for HSV-2 includes acyclovir and its derivatives, which are viral nucleoside analogs that inhibit viral DNA polymerases.5,6 However, over the past 2 decades, increasing HSV resistance to acyclovir and its derivatives has been noted among immunocompromised patients.5,6 Second-line agents, such as IV foscarnet and cidofovir, require close laboratory monitoring for nephrotoxicity and are contraindicated in those with renal insufficiency, thus limiting their use.5 To combat acyclovir resistance, novel antivirals such as pritelivir are being developed. Pritelivir targets the HSV helicase-primase complex and has been shown to outperform acyclovir in in-vitro animal models.7 Due to its unique mechanism of action (Figure 2), pritelivir is effective against acyclovir-resistant HSV strains, and clinical trials suggest its serum half-life may allow for daily dosing. A phase 2 study showed pritelivir reduced viral shedding days, sped up genital lesion healing in adults infected with HSV-2, and exhibited a good safety profile.7 Our patient participated in ongoing open-label trials of pritelivir that aimed to assess its efficacy and safety in immunocompromised patients. Given the limited alternative treatments for acyclovir-resistant HSV-2, clinicians need to stay updated on antiviral agents under development.

THE DIAGNOSIS: Kaposi Varicelliform Eruption
Genital erosions tested positive for herpes simplex virus (HSV) 2 via PCR, confirming a Kaposi varicelliform eruption (KVE) in a patient with mycosis fungoides. The medical team began antiviral therapy with intravenous (IV) acyclovir; however, susceptibility testing during the hospital admission confirmed acyclovir resistance, requiring a transition to cidofovir cream 1% and IV foscarnet.1 Subsequent concerns by the care team about chemical burns, dysuria, and renal impairment led to discontinuation of both the cidofovir and foscarnet, considerably narrowing the treatment options.1 The patient’s condition was complicated by polymicrobial bacteremia. Additionally, worsening acidosis and acute kidney injury required initiation of continuous renal replacement therapy.1 Considering these conditions, the patient was enrolled in a promising clinical trial for pritelivir, a novel antiviral medication; however, due to the development of oliguria and the progression of renal failure, this course of treatment had to be discontinued. Faced with potential viral encephalitis, the infectious disease team concluded that, despite previous adverse reactions, resumption of IV foscarnet treatment would present more benefits than risks, given the patient’s critical situation.1
Mycosis fungoides (MF) is a slowly progressive cutaneous T-cell lymphoma of CD4+ cells that primarily affects the skin. Clinically, it often is characterized by pruritic scaly patches or plaques with sharply demarcated borders, the enduring nature of which consistently poses a therapeutic challenge due to their noted resistance to preliminary lines of treatment. Presently, potential cures are limited to allogeneic stem cell transplantation and unilesional radiotherapy for advanced MF; however, no treatment has been found to notably improve survival rates.1 Mycosis fungoides can result in various complications including diffuse spread of a skin infection caused by HSV, known as KVE.1 Kaposi varicelliform eruption usually manifests clinically with painful skin vesicles that often are accompanied by systemic signs such as fever and malaise. The vesicles rapidly progress into pustules or erosions, predominantly affecting regions such as the head, neck, groin, and upper torso (Figure 1).2 Kaposi varicelliform eruption is considered a dermatologic emergency due to its potential to precipitate serious complications such as life-threatening secondary bacterial infection, HSV viremia, and multiorgan involvement; it also carries the risk of instigating ocular complications, such as keratitis, conjunctivitis, blepharitis, uveitis, and potential vision loss.2

Kaposi varicelliform eruption usually is diagnosed through clinical examination supported by polymerase chain reaction, viral culture, histopathology, HSV serology, and Tzanck smear.2 The differential diagnosis includes varicella, atypical varicella, herpes genitalis, herpes zoster, allergic or irritant contact dermatitis, or MF, which may result in painful skin ulcers.2-4 If an HSV superinfection is suspected, a polymerase chain reaction test ideally should be conducted within the first 72 hours of symptom onset.2 Herpes simplex virus infection may be reinforced by histologic features such as intraepidermal blistering, acantholysis, keratinocyte ballooning degeneration, and multinuclear giant cells with intranuclear inclusions. Given its severe nature, immediate empiric antiviral treatment for KVE is essential, even while awaiting confirmatory tests. The recommended treatment protocol involves acyclovir (400 mg orally 3 times daily or 10 mg/kg IV) or valacyclovir (500 mg orally twice daily), continued until KVE resolves.2
Herpes genitalis caused by HSV-2 is estimated to affect approximately 45 million adults in the United States.2 First-line treatment for HSV-2 includes acyclovir and its derivatives, which are viral nucleoside analogs that inhibit viral DNA polymerases.5,6 However, over the past 2 decades, increasing HSV resistance to acyclovir and its derivatives has been noted among immunocompromised patients.5,6 Second-line agents, such as IV foscarnet and cidofovir, require close laboratory monitoring for nephrotoxicity and are contraindicated in those with renal insufficiency, thus limiting their use.5 To combat acyclovir resistance, novel antivirals such as pritelivir are being developed. Pritelivir targets the HSV helicase-primase complex and has been shown to outperform acyclovir in in-vitro animal models.7 Due to its unique mechanism of action (Figure 2), pritelivir is effective against acyclovir-resistant HSV strains, and clinical trials suggest its serum half-life may allow for daily dosing. A phase 2 study showed pritelivir reduced viral shedding days, sped up genital lesion healing in adults infected with HSV-2, and exhibited a good safety profile.7 Our patient participated in ongoing open-label trials of pritelivir that aimed to assess its efficacy and safety in immunocompromised patients. Given the limited alternative treatments for acyclovir-resistant HSV-2, clinicians need to stay updated on antiviral agents under development.

- García-Díaz N, Piris MÁ, Ortiz-Romero PL, et al. Mycosis fungoides and Sézary syndrome: an integrative review of the pathophysiology, molecular drivers, and targeted therapy. Cancers. 2021;13:1931. doi:10.3390/cancers13081931
- Baaniya B, Agrawal S. Kaposi varicelliform eruption in a patient with pemphigus vulgaris: a case report and review of the literature. Case Rep Dermatol Med. 2020;2020:6695342. doi:10.1155/2020/6695342
- Shin D, Lee MS, Kim DY, et al. Increased large unstained cells value in varicella patients: a valuable parameter to aid rapid diagnosis of varicella infection. J Dermatol. 2015;42:795-799. doi:10.1111
- Joshi A, Sah SP, Agrawal S. Kaposi’s varicelliform eruption or atypical chickenpox in a normal individual. Australas J Dermatol. 2000;41:126-127.
- Groves MJ. Genital herpes: a review. Am Fam Physician. 2016; 93:928-934.
- Fleming DT, Leone P, Esposito D, et al. Herpes virus type 2 infection and genital symptoms in primary care patients. Sex Transm Dis. 2006;33:416-421. doi:10.1097/01.olq.0000200578.86276.0b
- Poole CL, James SH. Antiviral therapies for herpesviruses: current agents and new directions. Clin Ther. 2018;40:1282-1298. doi:10.1016 /j.clinthera.2018.07.006.
- García-Díaz N, Piris MÁ, Ortiz-Romero PL, et al. Mycosis fungoides and Sézary syndrome: an integrative review of the pathophysiology, molecular drivers, and targeted therapy. Cancers. 2021;13:1931. doi:10.3390/cancers13081931
- Baaniya B, Agrawal S. Kaposi varicelliform eruption in a patient with pemphigus vulgaris: a case report and review of the literature. Case Rep Dermatol Med. 2020;2020:6695342. doi:10.1155/2020/6695342
- Shin D, Lee MS, Kim DY, et al. Increased large unstained cells value in varicella patients: a valuable parameter to aid rapid diagnosis of varicella infection. J Dermatol. 2015;42:795-799. doi:10.1111
- Joshi A, Sah SP, Agrawal S. Kaposi’s varicelliform eruption or atypical chickenpox in a normal individual. Australas J Dermatol. 2000;41:126-127.
- Groves MJ. Genital herpes: a review. Am Fam Physician. 2016; 93:928-934.
- Fleming DT, Leone P, Esposito D, et al. Herpes virus type 2 infection and genital symptoms in primary care patients. Sex Transm Dis. 2006;33:416-421. doi:10.1097/01.olq.0000200578.86276.0b
- Poole CL, James SH. Antiviral therapies for herpesviruses: current agents and new directions. Clin Ther. 2018;40:1282-1298. doi:10.1016 /j.clinthera.2018.07.006.
Painful Edematous Labial Erosions
Painful Edematous Labial Erosions
A 40-year-old woman presented to the emergency department with painful, well-defined, edematous labial erosions of several weeks’ duration. The patient reported a medical history of stage IIIA (T4N0M0B0) mycosis fungoides. She had been hospitalized 2 months prior for sepsis that was attributed to a polymicrobial bacteremia involving Acinetobacter baumannii and Staphylococcus epidermidis. During that admission, a polymerase chain reaction test conducted on a skin swab from a lesion on the labia majora confirmed the presence of herpes simplex virus type 2. At the current presentation, physical examination by dermatology also revealed discrete, coalescing, erythematous erosions on the buttocks, groin, and proximal thighs with a punched-out appearance. These areas also exhibited skin sloughing and were covered with a gray-brown exudate. No other mucosal surfaces were involved.
Clinical Characteristics and Outcomes of Tall Cell Carcinoma with Reversed Polarity
Background
Tall cell carcinoma with reversed polarity (TCCRP) is a rare and distinct subtype of invasive breast carcinoma, defined by tall columnar cells with eosinophilic cytoplasm and reversed nuclear polarity. TCCRP remains poorly characterized in the literature, with limited population-level evidence to guide management and prognostication. This study uses the National Cancer Database (NCDB) to examine the epidemiology, clinical features, and outcomes of this neoplasm.
Methods
A retrospective cohort analysis included 951 patients diagnosed with TCCRP (ICD-O-3 code 8509) from 2018–2020 using the NCDB. Demographic and treatment variables were analyzed using descriptive statistics. Incidence trends were assessed using linear regression, and overall survival was evaluated using Kaplan-Meier methods.
Results
Most patients were female (98.1%) with a mean age of 69.1 years. The majority were White (82.0%), followed by Black (9.0%) and Hispanic (8.7%). Primary tumor sites included overlapping breast lesions (28.5%) and the upper-inner quadrant (27.0%). Incidence remained stable (R2 = 0.0). Most patients were diagnosed at Stage I (58.4%) and had a Charlson-Deyo score of 0 (76.2%). Socioeconomically, 41.8% lived in the highest income quartile (≥$74,063), and most had Medicare (64.7%). The most common treatment settings were comprehensive community cancer programs (40.3%). Surgery was performed in 95.6% of cases, with negative margins in 91.1%. Radiation therapy (46.6%) and hormone therapy (44.3%) were frequently used. Mortality was 1.1% at 30 days and 1.7% at 90 days. Survival was 98.9% at 2 years, 97.3% at 5 years, and 94.5% at 10 years, with a mean survival of 46.4 months.
Conclusions
This is the first NCDB-based study of TCCRP, highlighting favorable outcomes and distinct clinicodemographic features. Patients were predominantly older, White, and Medicare-insured, often receiving care at community cancer programs. These findings suggest that socioeconomic factors may influence access and treatment. Results may inform strategies to promote equitable care delivery across health systems and guide further research on clinical management and survivorship in TCCRP, particularly for rare cancers within community-based settings such as the VHA.
Background
Tall cell carcinoma with reversed polarity (TCCRP) is a rare and distinct subtype of invasive breast carcinoma, defined by tall columnar cells with eosinophilic cytoplasm and reversed nuclear polarity. TCCRP remains poorly characterized in the literature, with limited population-level evidence to guide management and prognostication. This study uses the National Cancer Database (NCDB) to examine the epidemiology, clinical features, and outcomes of this neoplasm.
Methods
A retrospective cohort analysis included 951 patients diagnosed with TCCRP (ICD-O-3 code 8509) from 2018–2020 using the NCDB. Demographic and treatment variables were analyzed using descriptive statistics. Incidence trends were assessed using linear regression, and overall survival was evaluated using Kaplan-Meier methods.
Results
Most patients were female (98.1%) with a mean age of 69.1 years. The majority were White (82.0%), followed by Black (9.0%) and Hispanic (8.7%). Primary tumor sites included overlapping breast lesions (28.5%) and the upper-inner quadrant (27.0%). Incidence remained stable (R2 = 0.0). Most patients were diagnosed at Stage I (58.4%) and had a Charlson-Deyo score of 0 (76.2%). Socioeconomically, 41.8% lived in the highest income quartile (≥$74,063), and most had Medicare (64.7%). The most common treatment settings were comprehensive community cancer programs (40.3%). Surgery was performed in 95.6% of cases, with negative margins in 91.1%. Radiation therapy (46.6%) and hormone therapy (44.3%) were frequently used. Mortality was 1.1% at 30 days and 1.7% at 90 days. Survival was 98.9% at 2 years, 97.3% at 5 years, and 94.5% at 10 years, with a mean survival of 46.4 months.
Conclusions
This is the first NCDB-based study of TCCRP, highlighting favorable outcomes and distinct clinicodemographic features. Patients were predominantly older, White, and Medicare-insured, often receiving care at community cancer programs. These findings suggest that socioeconomic factors may influence access and treatment. Results may inform strategies to promote equitable care delivery across health systems and guide further research on clinical management and survivorship in TCCRP, particularly for rare cancers within community-based settings such as the VHA.
Background
Tall cell carcinoma with reversed polarity (TCCRP) is a rare and distinct subtype of invasive breast carcinoma, defined by tall columnar cells with eosinophilic cytoplasm and reversed nuclear polarity. TCCRP remains poorly characterized in the literature, with limited population-level evidence to guide management and prognostication. This study uses the National Cancer Database (NCDB) to examine the epidemiology, clinical features, and outcomes of this neoplasm.
Methods
A retrospective cohort analysis included 951 patients diagnosed with TCCRP (ICD-O-3 code 8509) from 2018–2020 using the NCDB. Demographic and treatment variables were analyzed using descriptive statistics. Incidence trends were assessed using linear regression, and overall survival was evaluated using Kaplan-Meier methods.
Results
Most patients were female (98.1%) with a mean age of 69.1 years. The majority were White (82.0%), followed by Black (9.0%) and Hispanic (8.7%). Primary tumor sites included overlapping breast lesions (28.5%) and the upper-inner quadrant (27.0%). Incidence remained stable (R2 = 0.0). Most patients were diagnosed at Stage I (58.4%) and had a Charlson-Deyo score of 0 (76.2%). Socioeconomically, 41.8% lived in the highest income quartile (≥$74,063), and most had Medicare (64.7%). The most common treatment settings were comprehensive community cancer programs (40.3%). Surgery was performed in 95.6% of cases, with negative margins in 91.1%. Radiation therapy (46.6%) and hormone therapy (44.3%) were frequently used. Mortality was 1.1% at 30 days and 1.7% at 90 days. Survival was 98.9% at 2 years, 97.3% at 5 years, and 94.5% at 10 years, with a mean survival of 46.4 months.
Conclusions
This is the first NCDB-based study of TCCRP, highlighting favorable outcomes and distinct clinicodemographic features. Patients were predominantly older, White, and Medicare-insured, often receiving care at community cancer programs. These findings suggest that socioeconomic factors may influence access and treatment. Results may inform strategies to promote equitable care delivery across health systems and guide further research on clinical management and survivorship in TCCRP, particularly for rare cancers within community-based settings such as the VHA.
ERCC2, KDM6A, and TERT as Key Prognostic Factors in Bladder Cancer: Insights from the AACR Project GENIE Database
Background
Urothelial carcinoma (UC) is among the top 10 frequently diagnosed cancers in the world. Mutations in FGFR3, ARID1A, and TP53 are well documented as being some of the most frequent mutations found in UC. Despite advances in treatment, survival outcomes remain poor, especially in advanced stages. To promote future pharmacotherapeutic development, the molecular understanding of UC needs to be continually updated using more recently available databases.
Methods
This study utilizes the AACR Project GENIE database from the American Association for Cancer Research to explore the mutational profiles of patients with UC. Gene mutation frequencies were calculated, and two Kaplan-Meier curves were drawn for each gene, showing one curve for patients with the mutation and one for those without. Log-Rank tests were calculated with subsequent FDR (Benjamini–Hochberg) correction applied to account for multiple hypothesis testing. Data was analyzed using R 4.4.2 and statistical significance was set at α = 0.05.
Results
In this study, 4525 patients had histology consistent with UC. The 5 most common mutations were TERT (n = 1714, 37.9%), TP53 (n = 1689, 37.3%), KDM6A (n = 1091, 24.1%), ARID1A (n = 872, 19.3%), and FGFR3 (n = 762, 16.8%). Mutations associated with differential survival outcomes included ERCC2 (mutated n = 387, wild type n = 3751, p < 0.0001), KDM6A (mutated n = 1091, wild type n = 3047, p < 0.0001), TERT (mutated n = 1714, wild type n = 2424), and TP53 (mutated n = 1689, wild type n = 2449, p < 0.0001).
Conclusions
Interestingly, while mutations in TP53 and ERCC2 were associated with shorter median survival, mutations in KDM6A and TERT were associated with longer median survival.
Background
Urothelial carcinoma (UC) is among the top 10 frequently diagnosed cancers in the world. Mutations in FGFR3, ARID1A, and TP53 are well documented as being some of the most frequent mutations found in UC. Despite advances in treatment, survival outcomes remain poor, especially in advanced stages. To promote future pharmacotherapeutic development, the molecular understanding of UC needs to be continually updated using more recently available databases.
Methods
This study utilizes the AACR Project GENIE database from the American Association for Cancer Research to explore the mutational profiles of patients with UC. Gene mutation frequencies were calculated, and two Kaplan-Meier curves were drawn for each gene, showing one curve for patients with the mutation and one for those without. Log-Rank tests were calculated with subsequent FDR (Benjamini–Hochberg) correction applied to account for multiple hypothesis testing. Data was analyzed using R 4.4.2 and statistical significance was set at α = 0.05.
Results
In this study, 4525 patients had histology consistent with UC. The 5 most common mutations were TERT (n = 1714, 37.9%), TP53 (n = 1689, 37.3%), KDM6A (n = 1091, 24.1%), ARID1A (n = 872, 19.3%), and FGFR3 (n = 762, 16.8%). Mutations associated with differential survival outcomes included ERCC2 (mutated n = 387, wild type n = 3751, p < 0.0001), KDM6A (mutated n = 1091, wild type n = 3047, p < 0.0001), TERT (mutated n = 1714, wild type n = 2424), and TP53 (mutated n = 1689, wild type n = 2449, p < 0.0001).
Conclusions
Interestingly, while mutations in TP53 and ERCC2 were associated with shorter median survival, mutations in KDM6A and TERT were associated with longer median survival.
Background
Urothelial carcinoma (UC) is among the top 10 frequently diagnosed cancers in the world. Mutations in FGFR3, ARID1A, and TP53 are well documented as being some of the most frequent mutations found in UC. Despite advances in treatment, survival outcomes remain poor, especially in advanced stages. To promote future pharmacotherapeutic development, the molecular understanding of UC needs to be continually updated using more recently available databases.
Methods
This study utilizes the AACR Project GENIE database from the American Association for Cancer Research to explore the mutational profiles of patients with UC. Gene mutation frequencies were calculated, and two Kaplan-Meier curves were drawn for each gene, showing one curve for patients with the mutation and one for those without. Log-Rank tests were calculated with subsequent FDR (Benjamini–Hochberg) correction applied to account for multiple hypothesis testing. Data was analyzed using R 4.4.2 and statistical significance was set at α = 0.05.
Results
In this study, 4525 patients had histology consistent with UC. The 5 most common mutations were TERT (n = 1714, 37.9%), TP53 (n = 1689, 37.3%), KDM6A (n = 1091, 24.1%), ARID1A (n = 872, 19.3%), and FGFR3 (n = 762, 16.8%). Mutations associated with differential survival outcomes included ERCC2 (mutated n = 387, wild type n = 3751, p < 0.0001), KDM6A (mutated n = 1091, wild type n = 3047, p < 0.0001), TERT (mutated n = 1714, wild type n = 2424), and TP53 (mutated n = 1689, wild type n = 2449, p < 0.0001).
Conclusions
Interestingly, while mutations in TP53 and ERCC2 were associated with shorter median survival, mutations in KDM6A and TERT were associated with longer median survival.