User login
STEMI: Hospital destination policies improve time to first medical contact
Patients with ST-elevation myocardial infarction (STEMI) may get more timely treatment when state policies allow emergency medical services to steer patients to percutaneous coronary intervention (PCI)–capable hospitals, results of a registry study suggested.
Time to receipt of guideline-recommended therapy was significantly faster for states that had adopted STEMI hospital destination policies that permit bypassing closer facilities that are not PCI capable, according to results of the study, which was published in Circulation: Cardiovascular Interventions.
In addition, the mean door-to-balloon time was 48 minutes for patients in states with emergency medical services (EMS) destination policies, versus 52 minutes for patients in states with no destination policies.
These findings provide a compelling case for state-level policies to allow EMS to take patients directly to PCI-capable centers, according to lead study author Jacqueline Green, MD, MPH, a cardiologist at Piedmont Heart Institute in Fayetteville, Ga.
“A policy that improves access to timely care for even an additional 10% of patients could have a significant impact on a population level,” Dr. Green said in a statement.
The analysis by Dr. Green and her colleagues was based on 2013-2014 registry data for six states with bypass policies (Delaware, Iowa, Maryland, North Carolina, Pennsylvania, and Massachusetts) and six control states without bypass policies that were matched based on region, hospital density, and registry participation.
Time from first medical contact to treatment is a “critical determinant” of outcomes in patients with STEMI, Dr. Green and her colleagues wrote in their report.
“When a patient initially is taken to a non–PCI-capable hospital, considerable treatment delays are introduced as the patient must be evaluated, triaged, and wait for a second EMS transport to be called, arrive, and take the patient from the initial hospital to the PCI hospital,” they wrote.
However, whether reducing total ischemic time by “a few minutes” has clinical significance remains controversial, they acknowledged.
They noted that in one previous study, annual improvements in door-to-balloon times of about 16 minutes was not associated with significant reductions in mortality at the population level; however, a reanalysis of that data showed that effects at the individual lever were “important, even if modest at the population level,” they wrote.
In the present study, mean door-to-balloon times were “well within guideline-recommended time frames” for both the states with hospital destination policies and the states without them, Dr. Green and colleagues wrote.
“Many mitigating factors affecting STEMI mortality continue to exist when considering both population- and individual-level effects, and most cardiologists still agree that minimizing total ischemic time improves patient outcomes,” they said.
The project was funded by the American Heart Association’s Mission: Lifeline program, which aims to develop coordinated systems of care for time-sensitive, high-risk emergencies including heart attacks, stroke, and cardiac arrest.
One study coauthor reported serving as the Medical Director at ZOLL Medical. The others had no conflicts reported.
SOURCE: Green J et al. Circulation Cardiovasc Interven. 2018 May;11(5):e005706.
The important and comprehensive analysis by Jacqueline Green, MD, MPH, and her colleagues showed that care and outcomes of STEMI patients can be improved without increasing the number of PCI-capable facilities.
“The results indicate that simply living in a state which has a statewide prehospital plan for EMS [emergency medical services] transport is associated with improved treatment times for heart attack patients,” Daniel M. Kolansky, MD, and Paul N. Fiorilli, MD, wrote in an editorial.
Dr. Green and her colleagues did show that adopting statewide EMS policies which steer STEMI patients directly to PCI-capable hospitals was associated with significantly faster delivery of guideline-directed therapy.
However, the 4-minute improvement in mean door-to-balloon times for states with EMS destination policies versus those with no such policies is “modest,” according to the editorial authors.
“While it is difficult to be certain of the clinical significance of these findings, as the authors point out, it would seem that any action that shortens reperfusion time is an important step in the right direction,” they wrote.
Beyond prehospital EMS transport programs, there are many other aspects of care that could be improved to optimize timely delivery of care to STEMI patients.
Those aspects include routine use of prehospital ECG transmission, development of community outreach programs to help patients recognize symptoms, and more development of regionalized systems of care to reduce time from EMS activation to appropriate treatment.
“Although much work has already been accomplished to expedite the care of these patients, we need to continue to put together all the pieces of this puzzle to provide the best possible heart attack care for our patients,” Dr. Kolansky and Dr. Fiorilli concluded.
Dr. Kolansky and Dr. Fiorilli are with the cardiovascular medicine division at Hospital of the University of Pennsylvania in Philadelphia. These comments are derived from their editorial in Circulation: Cardiovascular Interventions . They had no disclosures.
The important and comprehensive analysis by Jacqueline Green, MD, MPH, and her colleagues showed that care and outcomes of STEMI patients can be improved without increasing the number of PCI-capable facilities.
“The results indicate that simply living in a state which has a statewide prehospital plan for EMS [emergency medical services] transport is associated with improved treatment times for heart attack patients,” Daniel M. Kolansky, MD, and Paul N. Fiorilli, MD, wrote in an editorial.
Dr. Green and her colleagues did show that adopting statewide EMS policies which steer STEMI patients directly to PCI-capable hospitals was associated with significantly faster delivery of guideline-directed therapy.
However, the 4-minute improvement in mean door-to-balloon times for states with EMS destination policies versus those with no such policies is “modest,” according to the editorial authors.
“While it is difficult to be certain of the clinical significance of these findings, as the authors point out, it would seem that any action that shortens reperfusion time is an important step in the right direction,” they wrote.
Beyond prehospital EMS transport programs, there are many other aspects of care that could be improved to optimize timely delivery of care to STEMI patients.
Those aspects include routine use of prehospital ECG transmission, development of community outreach programs to help patients recognize symptoms, and more development of regionalized systems of care to reduce time from EMS activation to appropriate treatment.
“Although much work has already been accomplished to expedite the care of these patients, we need to continue to put together all the pieces of this puzzle to provide the best possible heart attack care for our patients,” Dr. Kolansky and Dr. Fiorilli concluded.
Dr. Kolansky and Dr. Fiorilli are with the cardiovascular medicine division at Hospital of the University of Pennsylvania in Philadelphia. These comments are derived from their editorial in Circulation: Cardiovascular Interventions . They had no disclosures.
The important and comprehensive analysis by Jacqueline Green, MD, MPH, and her colleagues showed that care and outcomes of STEMI patients can be improved without increasing the number of PCI-capable facilities.
“The results indicate that simply living in a state which has a statewide prehospital plan for EMS [emergency medical services] transport is associated with improved treatment times for heart attack patients,” Daniel M. Kolansky, MD, and Paul N. Fiorilli, MD, wrote in an editorial.
Dr. Green and her colleagues did show that adopting statewide EMS policies which steer STEMI patients directly to PCI-capable hospitals was associated with significantly faster delivery of guideline-directed therapy.
However, the 4-minute improvement in mean door-to-balloon times for states with EMS destination policies versus those with no such policies is “modest,” according to the editorial authors.
“While it is difficult to be certain of the clinical significance of these findings, as the authors point out, it would seem that any action that shortens reperfusion time is an important step in the right direction,” they wrote.
Beyond prehospital EMS transport programs, there are many other aspects of care that could be improved to optimize timely delivery of care to STEMI patients.
Those aspects include routine use of prehospital ECG transmission, development of community outreach programs to help patients recognize symptoms, and more development of regionalized systems of care to reduce time from EMS activation to appropriate treatment.
“Although much work has already been accomplished to expedite the care of these patients, we need to continue to put together all the pieces of this puzzle to provide the best possible heart attack care for our patients,” Dr. Kolansky and Dr. Fiorilli concluded.
Dr. Kolansky and Dr. Fiorilli are with the cardiovascular medicine division at Hospital of the University of Pennsylvania in Philadelphia. These comments are derived from their editorial in Circulation: Cardiovascular Interventions . They had no disclosures.
Patients with ST-elevation myocardial infarction (STEMI) may get more timely treatment when state policies allow emergency medical services to steer patients to percutaneous coronary intervention (PCI)–capable hospitals, results of a registry study suggested.
Time to receipt of guideline-recommended therapy was significantly faster for states that had adopted STEMI hospital destination policies that permit bypassing closer facilities that are not PCI capable, according to results of the study, which was published in Circulation: Cardiovascular Interventions.
In addition, the mean door-to-balloon time was 48 minutes for patients in states with emergency medical services (EMS) destination policies, versus 52 minutes for patients in states with no destination policies.
These findings provide a compelling case for state-level policies to allow EMS to take patients directly to PCI-capable centers, according to lead study author Jacqueline Green, MD, MPH, a cardiologist at Piedmont Heart Institute in Fayetteville, Ga.
“A policy that improves access to timely care for even an additional 10% of patients could have a significant impact on a population level,” Dr. Green said in a statement.
The analysis by Dr. Green and her colleagues was based on 2013-2014 registry data for six states with bypass policies (Delaware, Iowa, Maryland, North Carolina, Pennsylvania, and Massachusetts) and six control states without bypass policies that were matched based on region, hospital density, and registry participation.
Time from first medical contact to treatment is a “critical determinant” of outcomes in patients with STEMI, Dr. Green and her colleagues wrote in their report.
“When a patient initially is taken to a non–PCI-capable hospital, considerable treatment delays are introduced as the patient must be evaluated, triaged, and wait for a second EMS transport to be called, arrive, and take the patient from the initial hospital to the PCI hospital,” they wrote.
However, whether reducing total ischemic time by “a few minutes” has clinical significance remains controversial, they acknowledged.
They noted that in one previous study, annual improvements in door-to-balloon times of about 16 minutes was not associated with significant reductions in mortality at the population level; however, a reanalysis of that data showed that effects at the individual lever were “important, even if modest at the population level,” they wrote.
In the present study, mean door-to-balloon times were “well within guideline-recommended time frames” for both the states with hospital destination policies and the states without them, Dr. Green and colleagues wrote.
“Many mitigating factors affecting STEMI mortality continue to exist when considering both population- and individual-level effects, and most cardiologists still agree that minimizing total ischemic time improves patient outcomes,” they said.
The project was funded by the American Heart Association’s Mission: Lifeline program, which aims to develop coordinated systems of care for time-sensitive, high-risk emergencies including heart attacks, stroke, and cardiac arrest.
One study coauthor reported serving as the Medical Director at ZOLL Medical. The others had no conflicts reported.
SOURCE: Green J et al. Circulation Cardiovasc Interven. 2018 May;11(5):e005706.
Patients with ST-elevation myocardial infarction (STEMI) may get more timely treatment when state policies allow emergency medical services to steer patients to percutaneous coronary intervention (PCI)–capable hospitals, results of a registry study suggested.
Time to receipt of guideline-recommended therapy was significantly faster for states that had adopted STEMI hospital destination policies that permit bypassing closer facilities that are not PCI capable, according to results of the study, which was published in Circulation: Cardiovascular Interventions.
In addition, the mean door-to-balloon time was 48 minutes for patients in states with emergency medical services (EMS) destination policies, versus 52 minutes for patients in states with no destination policies.
These findings provide a compelling case for state-level policies to allow EMS to take patients directly to PCI-capable centers, according to lead study author Jacqueline Green, MD, MPH, a cardiologist at Piedmont Heart Institute in Fayetteville, Ga.
“A policy that improves access to timely care for even an additional 10% of patients could have a significant impact on a population level,” Dr. Green said in a statement.
The analysis by Dr. Green and her colleagues was based on 2013-2014 registry data for six states with bypass policies (Delaware, Iowa, Maryland, North Carolina, Pennsylvania, and Massachusetts) and six control states without bypass policies that were matched based on region, hospital density, and registry participation.
Time from first medical contact to treatment is a “critical determinant” of outcomes in patients with STEMI, Dr. Green and her colleagues wrote in their report.
“When a patient initially is taken to a non–PCI-capable hospital, considerable treatment delays are introduced as the patient must be evaluated, triaged, and wait for a second EMS transport to be called, arrive, and take the patient from the initial hospital to the PCI hospital,” they wrote.
However, whether reducing total ischemic time by “a few minutes” has clinical significance remains controversial, they acknowledged.
They noted that in one previous study, annual improvements in door-to-balloon times of about 16 minutes was not associated with significant reductions in mortality at the population level; however, a reanalysis of that data showed that effects at the individual lever were “important, even if modest at the population level,” they wrote.
In the present study, mean door-to-balloon times were “well within guideline-recommended time frames” for both the states with hospital destination policies and the states without them, Dr. Green and colleagues wrote.
“Many mitigating factors affecting STEMI mortality continue to exist when considering both population- and individual-level effects, and most cardiologists still agree that minimizing total ischemic time improves patient outcomes,” they said.
The project was funded by the American Heart Association’s Mission: Lifeline program, which aims to develop coordinated systems of care for time-sensitive, high-risk emergencies including heart attacks, stroke, and cardiac arrest.
One study coauthor reported serving as the Medical Director at ZOLL Medical. The others had no conflicts reported.
SOURCE: Green J et al. Circulation Cardiovasc Interven. 2018 May;11(5):e005706.
FROM CIRCULATION: CARDIOVASCULAR INTERVENTIONS
Key clinical point: Significantly faster time to guideline-recommended treatment was seen in states that adopted STEMI hospital destination policies that allow EMS to bypass facilities that are not PCI capable.
Major finding: Primary PCI was delivered within the guideline-recommended time from first contact for 58% of patients living in states with hospital destination policies, compared with 48% of patients in states with no such policies.
Study details: A report from the American Heart Association’s Mission: Lifeline program that was based on analysis of 2013-2014 registry data for six states with bypass policies and six matched control states without bypass policies.
Disclosures: The AHA Mission: Lifeline program funded the project. One study coauthor reported serving as the medical director at ZOLL Medical. The others had no conflicts reported.
Source: Green J et al. Circ Cardiovasc Interv. 2018 May;11(5):e005706.
Retinal vessel diameter in children may offer window into future CVD risk
Body mass index, blood pressure, and physical activity all affect retinal vessel diameters, authors of the meta-analysis found.
The findings raise the possibility that, one day, clinicians could help “substantially counteract” the increasing burden of adult cardiovascular disease by diagnosing retinal microvascular impairments early in life, according to investigator Sabrina Köchli, MSc, and her colleagues with the department of sport, exercise, and health at the University of Basel (Switzerland).
“Regular retinal vessel screening may have the potential to be implemented in future medical examination programs to optimize therapy guidance in children and adolescents,” Ms. Köchli and her colleagues wrote in the journal Pediatrics.
The meta-analysis by Ms. Köchli and her coauthors included 11 studies looking at the association between BMI, blood pressure, or physical activity in children.
They found that higher BMI was associated with narrower retinal arteriolar diameters and wider venular diameters in 8 studies including a total of 5,003 participants. The pooled estimate effect size was –0.37 for the association between BMI and retinal arteriolar diameters and 0.35 for the association between BMI and retinal venular diameters, data showed.
Higher blood pressure likewise was associated with narrower retinal arteriolar diameters in 6 studies including 7,687 participants, with a pooled estimate of –0.63 for systolic blood pressure and –0.60 for diastolic blood pressure.
Several studies individually showed that physical activity was associated with retinal vessel diameters. For example, one German study of school children showing that physical inactivity was associated with a lower arteriolar-to-venular diameter. And an Australian study found that more time spent outdoors engaged in physical activity was tied to wider arteriolor diameters and that sedentary behavior was linked to narrower arteriolor diameters. However, because of differences among the studies in how physical activity or inactivity was measured, meta-analysis was not possible, the investigators said.
Retinal vessels, part of the cerebrovascular bed, have been described as a “window to the heart,” Ms. Köchli and her coauthors said in their report. Previous investigations have recently shown that retinal vessel diameters may predict cardiovascular outcomes in adults over the long term.
That suggests retinal vessel diameters are a “reproducible biomarker” that adds value in determining atherosclerotic cardiovascular disease risk, the authors commented.
“However, future researchers will have to ensure that retinal vessel imaging during childhood development has a prognostic value for the adult clinical outcome of pediatric populations,” they added.
No external funding was received for the study. Ms. Köchli and her coauthors had no financial disclosures or potential conflicts of interest to disclose.
SOURCE: Köchli S et al. Pediatrics. 2018;141(6):e20174090
Currently, retinal vessel diameters are not a strong contender for clinical risk prediction, Alan E. Simon, MD, and Matthew W. Gillman, MD, wrote in an editorial commenting on the study by Köchli et al.
However, the concept has “intuitive appeal because the eye provides the opportunity to view systemic arteries and veins directly,” wrote Dr. Simon and Dr. Gillman.
The association between childhood retinal vessel diameters and cardiovascular endpoints in adults has not been evaluated, understandably, because of the very long follow-up that would be required. And even though studies have suggested that there might be some small added benefit for women in adding adult retinal vessel diameters to other cardiovascular risk prediction scores, the same does not appear to be true for men.
Even if childhood retinal vessel diameters had strong predictive value, it’s still not clear whether the retinal vessel evaluation would be useful for pediatricians grouping patients into risk categories for strokes or heart attacks at some point in the future.
Likewise, using retinal vessel diameters as an outcome measure in clinical trials is not justified at present, although that could change in the future.
“As technological advances make this novel assessment more available to clinicians, we hope that such availability is accompanied by additional evidence for or against its usefulness among clinicians and researchers,” the authors said in the editorial.
Currently, retinal vessel diameters are not a strong contender for clinical risk prediction, Alan E. Simon, MD, and Matthew W. Gillman, MD, wrote in an editorial commenting on the study by Köchli et al.
However, the concept has “intuitive appeal because the eye provides the opportunity to view systemic arteries and veins directly,” wrote Dr. Simon and Dr. Gillman.
The association between childhood retinal vessel diameters and cardiovascular endpoints in adults has not been evaluated, understandably, because of the very long follow-up that would be required. And even though studies have suggested that there might be some small added benefit for women in adding adult retinal vessel diameters to other cardiovascular risk prediction scores, the same does not appear to be true for men.
Even if childhood retinal vessel diameters had strong predictive value, it’s still not clear whether the retinal vessel evaluation would be useful for pediatricians grouping patients into risk categories for strokes or heart attacks at some point in the future.
Likewise, using retinal vessel diameters as an outcome measure in clinical trials is not justified at present, although that could change in the future.
“As technological advances make this novel assessment more available to clinicians, we hope that such availability is accompanied by additional evidence for or against its usefulness among clinicians and researchers,” the authors said in the editorial.
Currently, retinal vessel diameters are not a strong contender for clinical risk prediction, Alan E. Simon, MD, and Matthew W. Gillman, MD, wrote in an editorial commenting on the study by Köchli et al.
However, the concept has “intuitive appeal because the eye provides the opportunity to view systemic arteries and veins directly,” wrote Dr. Simon and Dr. Gillman.
The association between childhood retinal vessel diameters and cardiovascular endpoints in adults has not been evaluated, understandably, because of the very long follow-up that would be required. And even though studies have suggested that there might be some small added benefit for women in adding adult retinal vessel diameters to other cardiovascular risk prediction scores, the same does not appear to be true for men.
Even if childhood retinal vessel diameters had strong predictive value, it’s still not clear whether the retinal vessel evaluation would be useful for pediatricians grouping patients into risk categories for strokes or heart attacks at some point in the future.
Likewise, using retinal vessel diameters as an outcome measure in clinical trials is not justified at present, although that could change in the future.
“As technological advances make this novel assessment more available to clinicians, we hope that such availability is accompanied by additional evidence for or against its usefulness among clinicians and researchers,” the authors said in the editorial.
Body mass index, blood pressure, and physical activity all affect retinal vessel diameters, authors of the meta-analysis found.
The findings raise the possibility that, one day, clinicians could help “substantially counteract” the increasing burden of adult cardiovascular disease by diagnosing retinal microvascular impairments early in life, according to investigator Sabrina Köchli, MSc, and her colleagues with the department of sport, exercise, and health at the University of Basel (Switzerland).
“Regular retinal vessel screening may have the potential to be implemented in future medical examination programs to optimize therapy guidance in children and adolescents,” Ms. Köchli and her colleagues wrote in the journal Pediatrics.
The meta-analysis by Ms. Köchli and her coauthors included 11 studies looking at the association between BMI, blood pressure, or physical activity in children.
They found that higher BMI was associated with narrower retinal arteriolar diameters and wider venular diameters in 8 studies including a total of 5,003 participants. The pooled estimate effect size was –0.37 for the association between BMI and retinal arteriolar diameters and 0.35 for the association between BMI and retinal venular diameters, data showed.
Higher blood pressure likewise was associated with narrower retinal arteriolar diameters in 6 studies including 7,687 participants, with a pooled estimate of –0.63 for systolic blood pressure and –0.60 for diastolic blood pressure.
Several studies individually showed that physical activity was associated with retinal vessel diameters. For example, one German study of school children showing that physical inactivity was associated with a lower arteriolar-to-venular diameter. And an Australian study found that more time spent outdoors engaged in physical activity was tied to wider arteriolor diameters and that sedentary behavior was linked to narrower arteriolor diameters. However, because of differences among the studies in how physical activity or inactivity was measured, meta-analysis was not possible, the investigators said.
Retinal vessels, part of the cerebrovascular bed, have been described as a “window to the heart,” Ms. Köchli and her coauthors said in their report. Previous investigations have recently shown that retinal vessel diameters may predict cardiovascular outcomes in adults over the long term.
That suggests retinal vessel diameters are a “reproducible biomarker” that adds value in determining atherosclerotic cardiovascular disease risk, the authors commented.
“However, future researchers will have to ensure that retinal vessel imaging during childhood development has a prognostic value for the adult clinical outcome of pediatric populations,” they added.
No external funding was received for the study. Ms. Köchli and her coauthors had no financial disclosures or potential conflicts of interest to disclose.
SOURCE: Köchli S et al. Pediatrics. 2018;141(6):e20174090
Body mass index, blood pressure, and physical activity all affect retinal vessel diameters, authors of the meta-analysis found.
The findings raise the possibility that, one day, clinicians could help “substantially counteract” the increasing burden of adult cardiovascular disease by diagnosing retinal microvascular impairments early in life, according to investigator Sabrina Köchli, MSc, and her colleagues with the department of sport, exercise, and health at the University of Basel (Switzerland).
“Regular retinal vessel screening may have the potential to be implemented in future medical examination programs to optimize therapy guidance in children and adolescents,” Ms. Köchli and her colleagues wrote in the journal Pediatrics.
The meta-analysis by Ms. Köchli and her coauthors included 11 studies looking at the association between BMI, blood pressure, or physical activity in children.
They found that higher BMI was associated with narrower retinal arteriolar diameters and wider venular diameters in 8 studies including a total of 5,003 participants. The pooled estimate effect size was –0.37 for the association between BMI and retinal arteriolar diameters and 0.35 for the association between BMI and retinal venular diameters, data showed.
Higher blood pressure likewise was associated with narrower retinal arteriolar diameters in 6 studies including 7,687 participants, with a pooled estimate of –0.63 for systolic blood pressure and –0.60 for diastolic blood pressure.
Several studies individually showed that physical activity was associated with retinal vessel diameters. For example, one German study of school children showing that physical inactivity was associated with a lower arteriolar-to-venular diameter. And an Australian study found that more time spent outdoors engaged in physical activity was tied to wider arteriolor diameters and that sedentary behavior was linked to narrower arteriolor diameters. However, because of differences among the studies in how physical activity or inactivity was measured, meta-analysis was not possible, the investigators said.
Retinal vessels, part of the cerebrovascular bed, have been described as a “window to the heart,” Ms. Köchli and her coauthors said in their report. Previous investigations have recently shown that retinal vessel diameters may predict cardiovascular outcomes in adults over the long term.
That suggests retinal vessel diameters are a “reproducible biomarker” that adds value in determining atherosclerotic cardiovascular disease risk, the authors commented.
“However, future researchers will have to ensure that retinal vessel imaging during childhood development has a prognostic value for the adult clinical outcome of pediatric populations,” they added.
No external funding was received for the study. Ms. Köchli and her coauthors had no financial disclosures or potential conflicts of interest to disclose.
SOURCE: Köchli S et al. Pediatrics. 2018;141(6):e20174090
Key clinical point: In children, retinal vessel diameters may be sensitive biomarkers for cardiovascular risk stratification.
Major finding: BMI was associated with retinal arteriolar and venular diameters; blood pressure also was associated with retinal arteriolar diameters.
Study details: A meta-analysis of 11 studies.
Disclosures: No external funding was received for the study. Authors reported that they had no financial disclosures or potential conflicts of interest.
Source: Köchli S et al. Pediatrics. 2018;141(6):e20174090.
Adding vasopressin in distributive shock may cut AF risk
In patients with distributive shock, the risk of atrial fibrillation may be lower when vasopressin is administered along with catecholamine vasopressors, results of a recent systematic review and meta-analysis suggest.
The relative risk of atrial fibrillation was reduced for the combination of vasopressin and catecholamines versus the current standard of care, which is catecholamines alone, according to study results published in JAMA.
Beyond atrial fibrillation, however, findings of the meta-analysis were consistent with regard to other endpoints, including mortality, according to William F. McIntyre, MD, of McMaster University, Hamilton, Ont., and his coinvestigators.
Mortality was lower with the combination approach when all studies were analyzed together. Yet, when the analysis was limited to the studies with the lowest risk of bias, the difference in mortality versus catecholamines alone was not statistically significant, investigators said.
Nevertheless, the meta-analysis does suggest that vasopressin may offer a clinical advantage regarding prevention of atrial fibrillation in patients with distributive shock, a frequently fatal condition most often seen in patients with sepsis.
Vasopressin is an endogenous peptide hormone that decreases stimulation of certain myocardial receptors associated with cardiac arrhythmia, the authors noted.
“This, among other mechanisms, may translate into a reduction in adverse events, including atrial fibrillation, injury to other organs, and death,” they said in their report.
Dr. McIntyre and his colleagues included 23 trials that had enrolled a total of 3,088 patients with distributive shock, a condition in which widespread vasodilation lowers vascular resistances and mean arterial pressure. Sepsis is its most common cause. The current study is one of the first to directly compare the combination of vasopressin and catecholamine to catecholamines alone, which is the current standard of care, the investigators wrote.
They found that the administration of vasopressin was associated with a significant 23% reduction in risk of atrial fibrillation.
“The absolute effect is that 68 fewer people per 1,000 patients will experience atrial fibrillation when vasopressin is added to catecholaminergic vasopressors,” Dr. McIntyre and his coauthors said of the results.
The atrial fibrillation finding was judged to be high-quality evidence, they said, noting that two separate sensitivity analyses confirmed the benefit.
Mortality data were less consistent, they said.
Pooled data showed administration of vasopressin along with catecholamines was associated an 11% relative reduction in mortality. In absolute terms, 45 lives would be saved for every 1,000 patients receiving vasopressin, they noted.
However, the mortality findings were different when the analysis was limited to the two studies with low risk of bias. That analysis yielded a relative risk of 0.96 and was not statistically significant.
Studies show patients with distributive shock have a relative vasopressin deficiency, providing a theoretical basis for vasopressin administration as part of care, investigators said.
The current Surviving Sepsis guidelines suggest either adding vasopressin to norepinephrine to help raise mean arterial pressure to target or adding vasopressin to decrease the dosage of norepinephrine. Those are considered weak recommendations based on moderate quality of evidence, Dr. McIntyre and colleagues noted in their report.
Authors of the study reported disclosures related to Tenax Therapeutics, Orion Pharma, Ferring Pharmaceuticals, GlaxoSmithKline, and Bristol-Myers Squibb, among other entities.
SOURCE: McIntyre WF et al. JAMA. 2018;319(18):1889-900.
In patients with distributive shock, the risk of atrial fibrillation may be lower when vasopressin is administered along with catecholamine vasopressors, results of a recent systematic review and meta-analysis suggest.
The relative risk of atrial fibrillation was reduced for the combination of vasopressin and catecholamines versus the current standard of care, which is catecholamines alone, according to study results published in JAMA.
Beyond atrial fibrillation, however, findings of the meta-analysis were consistent with regard to other endpoints, including mortality, according to William F. McIntyre, MD, of McMaster University, Hamilton, Ont., and his coinvestigators.
Mortality was lower with the combination approach when all studies were analyzed together. Yet, when the analysis was limited to the studies with the lowest risk of bias, the difference in mortality versus catecholamines alone was not statistically significant, investigators said.
Nevertheless, the meta-analysis does suggest that vasopressin may offer a clinical advantage regarding prevention of atrial fibrillation in patients with distributive shock, a frequently fatal condition most often seen in patients with sepsis.
Vasopressin is an endogenous peptide hormone that decreases stimulation of certain myocardial receptors associated with cardiac arrhythmia, the authors noted.
“This, among other mechanisms, may translate into a reduction in adverse events, including atrial fibrillation, injury to other organs, and death,” they said in their report.
Dr. McIntyre and his colleagues included 23 trials that had enrolled a total of 3,088 patients with distributive shock, a condition in which widespread vasodilation lowers vascular resistances and mean arterial pressure. Sepsis is its most common cause. The current study is one of the first to directly compare the combination of vasopressin and catecholamine to catecholamines alone, which is the current standard of care, the investigators wrote.
They found that the administration of vasopressin was associated with a significant 23% reduction in risk of atrial fibrillation.
“The absolute effect is that 68 fewer people per 1,000 patients will experience atrial fibrillation when vasopressin is added to catecholaminergic vasopressors,” Dr. McIntyre and his coauthors said of the results.
The atrial fibrillation finding was judged to be high-quality evidence, they said, noting that two separate sensitivity analyses confirmed the benefit.
Mortality data were less consistent, they said.
Pooled data showed administration of vasopressin along with catecholamines was associated an 11% relative reduction in mortality. In absolute terms, 45 lives would be saved for every 1,000 patients receiving vasopressin, they noted.
However, the mortality findings were different when the analysis was limited to the two studies with low risk of bias. That analysis yielded a relative risk of 0.96 and was not statistically significant.
Studies show patients with distributive shock have a relative vasopressin deficiency, providing a theoretical basis for vasopressin administration as part of care, investigators said.
The current Surviving Sepsis guidelines suggest either adding vasopressin to norepinephrine to help raise mean arterial pressure to target or adding vasopressin to decrease the dosage of norepinephrine. Those are considered weak recommendations based on moderate quality of evidence, Dr. McIntyre and colleagues noted in their report.
Authors of the study reported disclosures related to Tenax Therapeutics, Orion Pharma, Ferring Pharmaceuticals, GlaxoSmithKline, and Bristol-Myers Squibb, among other entities.
SOURCE: McIntyre WF et al. JAMA. 2018;319(18):1889-900.
In patients with distributive shock, the risk of atrial fibrillation may be lower when vasopressin is administered along with catecholamine vasopressors, results of a recent systematic review and meta-analysis suggest.
The relative risk of atrial fibrillation was reduced for the combination of vasopressin and catecholamines versus the current standard of care, which is catecholamines alone, according to study results published in JAMA.
Beyond atrial fibrillation, however, findings of the meta-analysis were consistent with regard to other endpoints, including mortality, according to William F. McIntyre, MD, of McMaster University, Hamilton, Ont., and his coinvestigators.
Mortality was lower with the combination approach when all studies were analyzed together. Yet, when the analysis was limited to the studies with the lowest risk of bias, the difference in mortality versus catecholamines alone was not statistically significant, investigators said.
Nevertheless, the meta-analysis does suggest that vasopressin may offer a clinical advantage regarding prevention of atrial fibrillation in patients with distributive shock, a frequently fatal condition most often seen in patients with sepsis.
Vasopressin is an endogenous peptide hormone that decreases stimulation of certain myocardial receptors associated with cardiac arrhythmia, the authors noted.
“This, among other mechanisms, may translate into a reduction in adverse events, including atrial fibrillation, injury to other organs, and death,” they said in their report.
Dr. McIntyre and his colleagues included 23 trials that had enrolled a total of 3,088 patients with distributive shock, a condition in which widespread vasodilation lowers vascular resistances and mean arterial pressure. Sepsis is its most common cause. The current study is one of the first to directly compare the combination of vasopressin and catecholamine to catecholamines alone, which is the current standard of care, the investigators wrote.
They found that the administration of vasopressin was associated with a significant 23% reduction in risk of atrial fibrillation.
“The absolute effect is that 68 fewer people per 1,000 patients will experience atrial fibrillation when vasopressin is added to catecholaminergic vasopressors,” Dr. McIntyre and his coauthors said of the results.
The atrial fibrillation finding was judged to be high-quality evidence, they said, noting that two separate sensitivity analyses confirmed the benefit.
Mortality data were less consistent, they said.
Pooled data showed administration of vasopressin along with catecholamines was associated an 11% relative reduction in mortality. In absolute terms, 45 lives would be saved for every 1,000 patients receiving vasopressin, they noted.
However, the mortality findings were different when the analysis was limited to the two studies with low risk of bias. That analysis yielded a relative risk of 0.96 and was not statistically significant.
Studies show patients with distributive shock have a relative vasopressin deficiency, providing a theoretical basis for vasopressin administration as part of care, investigators said.
The current Surviving Sepsis guidelines suggest either adding vasopressin to norepinephrine to help raise mean arterial pressure to target or adding vasopressin to decrease the dosage of norepinephrine. Those are considered weak recommendations based on moderate quality of evidence, Dr. McIntyre and colleagues noted in their report.
Authors of the study reported disclosures related to Tenax Therapeutics, Orion Pharma, Ferring Pharmaceuticals, GlaxoSmithKline, and Bristol-Myers Squibb, among other entities.
SOURCE: McIntyre WF et al. JAMA. 2018;319(18):1889-900.
FROM JAMA
Key clinical point: For patients with distributive shock, the addition of vasopressin to catecholamine vasopressors may reduce atrial fibrillation risk, compared with catecholamines alone.
Major finding: Vasopressin was associated with a 23% lower risk of atrial fibrillation.
Study details: A systematic review and meta-analysis including 23 randomized clinical trials enrolling a total of 3,088 patients.
Disclosures: Authors reported disclosures related to Tenax Therapeutics, Orion Pharma, Ferring Pharmaceuticals, GlaxoSmithKline, and Bristol-Myers Squibb, among other entities.
Source: McIntyre WF et al. JAMA. 2018;319(18):1889-900.
Even a year of increased water intake did not change CKD course
Coaching adults with stage 3 chronic kidney disease (CKD) to increase water intake did not significantly slow decline in kidney function, results of a randomized clinical trial show.
Compared with coaching to maintain water intake, coaching to increase water intake did in fact increase water intake but did not prevent a decrease in estimated glomerular filtration rate (eGFR) over 1 year, according to findings of the study, which was published in JAMA..
However, the study may have been underpowered to detect a clinically important difference in this primary endpoint, and certain secondary endpoints did suggest a favorable effect of the intervention, according to William F. Clark, MD, of the London (Ontario) Health Sciences Centre and his coauthors.
“The increased water intake achieved in this trial was sufficient to lower vasopressin secretion, as assessed by plasma copeptin concentrations,” Dr. Clark and his colleagues said in their report
An increasing number of studies suggest that drinking water may benefit the kidneys. In some human studies, water intake was associated with reduced risk of kidney stones and better kidney function.
However, it remains unknown whether increasing water intake would benefit patients with CKD. To evaluate this question, Dr. Clark and colleagues initiated CKD WIT (Chronic Kidney Disease Water Intake Trial), a randomized clinical trial conducted in 9 centers in Ontario.
The study included 631 patients with stage 3 CKD and a 24-hour urine volume below 3 L. Patients randomized to the hydration group were coached to increase water intake gradually to 1-1.5 L/day for 1 year, while those randomized to the control group were coached to maintain their usual water intake.
Patients in the hydration group were also given reusable drinking containers and 20 vouchers per month redeemable for 1.5 L of bottled water, investigators reported.
Urine volume did significantly increase in the hydration group versus controls, by 0.6 L per day (P less than .001). However, change in eGFR – the primary outcome – was not significantly different between groups. Mean change in eGFR was –2.2 mL/min per 1.73 m2 in patients coached to drink more water and –1.9 mL/min per 1.73 m2 in those coached to maintain water intake (P = .74).
Some secondary outcome measures demonstrated significant differences in favor of the hydration group. Plasma copeptin and creatinine clearance both showed significant differences in favor of the hydration group. In contrast, there were no significant differences between intervention arms in urine albumin or quality of health, according to analyses of secondary outcomes described in the study report.
There are several ways to interpret the finding that drinking more water had no effect on eGFR, investigators said. Increasing water intake may simply not be protective against kidney function decline. Perhaps follow-up longer than 1 year would be needed to see an effect, or perhaps there was an effect, but the study was underpowered to detect it.
It could also be that a greater volume of water would be needed to demonstrate a protective effect for the kidneys. Despite the coaching efforts of dietitians and research assistants, the mean urine volume increase in the hydration group relative to the control group was just 0.6 liter per day, or 2.4 cups.
“This highlights how difficult it would be to achieve a large sustained increase in water intake in routine practice,” Dr. Clark and colleagues said in their report.
Dr. Clark reported disclosures related to Danone Research. Thermo Fisher Scientific provided instrumentation, assay reagent, and disposables used in the study.
SOURCE: Clark WF et al. JAMA. 2018;319(18):1870-9.
Coaching adults with stage 3 chronic kidney disease (CKD) to increase water intake did not significantly slow decline in kidney function, results of a randomized clinical trial show.
Compared with coaching to maintain water intake, coaching to increase water intake did in fact increase water intake but did not prevent a decrease in estimated glomerular filtration rate (eGFR) over 1 year, according to findings of the study, which was published in JAMA..
However, the study may have been underpowered to detect a clinically important difference in this primary endpoint, and certain secondary endpoints did suggest a favorable effect of the intervention, according to William F. Clark, MD, of the London (Ontario) Health Sciences Centre and his coauthors.
“The increased water intake achieved in this trial was sufficient to lower vasopressin secretion, as assessed by plasma copeptin concentrations,” Dr. Clark and his colleagues said in their report
An increasing number of studies suggest that drinking water may benefit the kidneys. In some human studies, water intake was associated with reduced risk of kidney stones and better kidney function.
However, it remains unknown whether increasing water intake would benefit patients with CKD. To evaluate this question, Dr. Clark and colleagues initiated CKD WIT (Chronic Kidney Disease Water Intake Trial), a randomized clinical trial conducted in 9 centers in Ontario.
The study included 631 patients with stage 3 CKD and a 24-hour urine volume below 3 L. Patients randomized to the hydration group were coached to increase water intake gradually to 1-1.5 L/day for 1 year, while those randomized to the control group were coached to maintain their usual water intake.
Patients in the hydration group were also given reusable drinking containers and 20 vouchers per month redeemable for 1.5 L of bottled water, investigators reported.
Urine volume did significantly increase in the hydration group versus controls, by 0.6 L per day (P less than .001). However, change in eGFR – the primary outcome – was not significantly different between groups. Mean change in eGFR was –2.2 mL/min per 1.73 m2 in patients coached to drink more water and –1.9 mL/min per 1.73 m2 in those coached to maintain water intake (P = .74).
Some secondary outcome measures demonstrated significant differences in favor of the hydration group. Plasma copeptin and creatinine clearance both showed significant differences in favor of the hydration group. In contrast, there were no significant differences between intervention arms in urine albumin or quality of health, according to analyses of secondary outcomes described in the study report.
There are several ways to interpret the finding that drinking more water had no effect on eGFR, investigators said. Increasing water intake may simply not be protective against kidney function decline. Perhaps follow-up longer than 1 year would be needed to see an effect, or perhaps there was an effect, but the study was underpowered to detect it.
It could also be that a greater volume of water would be needed to demonstrate a protective effect for the kidneys. Despite the coaching efforts of dietitians and research assistants, the mean urine volume increase in the hydration group relative to the control group was just 0.6 liter per day, or 2.4 cups.
“This highlights how difficult it would be to achieve a large sustained increase in water intake in routine practice,” Dr. Clark and colleagues said in their report.
Dr. Clark reported disclosures related to Danone Research. Thermo Fisher Scientific provided instrumentation, assay reagent, and disposables used in the study.
SOURCE: Clark WF et al. JAMA. 2018;319(18):1870-9.
Coaching adults with stage 3 chronic kidney disease (CKD) to increase water intake did not significantly slow decline in kidney function, results of a randomized clinical trial show.
Compared with coaching to maintain water intake, coaching to increase water intake did in fact increase water intake but did not prevent a decrease in estimated glomerular filtration rate (eGFR) over 1 year, according to findings of the study, which was published in JAMA..
However, the study may have been underpowered to detect a clinically important difference in this primary endpoint, and certain secondary endpoints did suggest a favorable effect of the intervention, according to William F. Clark, MD, of the London (Ontario) Health Sciences Centre and his coauthors.
“The increased water intake achieved in this trial was sufficient to lower vasopressin secretion, as assessed by plasma copeptin concentrations,” Dr. Clark and his colleagues said in their report
An increasing number of studies suggest that drinking water may benefit the kidneys. In some human studies, water intake was associated with reduced risk of kidney stones and better kidney function.
However, it remains unknown whether increasing water intake would benefit patients with CKD. To evaluate this question, Dr. Clark and colleagues initiated CKD WIT (Chronic Kidney Disease Water Intake Trial), a randomized clinical trial conducted in 9 centers in Ontario.
The study included 631 patients with stage 3 CKD and a 24-hour urine volume below 3 L. Patients randomized to the hydration group were coached to increase water intake gradually to 1-1.5 L/day for 1 year, while those randomized to the control group were coached to maintain their usual water intake.
Patients in the hydration group were also given reusable drinking containers and 20 vouchers per month redeemable for 1.5 L of bottled water, investigators reported.
Urine volume did significantly increase in the hydration group versus controls, by 0.6 L per day (P less than .001). However, change in eGFR – the primary outcome – was not significantly different between groups. Mean change in eGFR was –2.2 mL/min per 1.73 m2 in patients coached to drink more water and –1.9 mL/min per 1.73 m2 in those coached to maintain water intake (P = .74).
Some secondary outcome measures demonstrated significant differences in favor of the hydration group. Plasma copeptin and creatinine clearance both showed significant differences in favor of the hydration group. In contrast, there were no significant differences between intervention arms in urine albumin or quality of health, according to analyses of secondary outcomes described in the study report.
There are several ways to interpret the finding that drinking more water had no effect on eGFR, investigators said. Increasing water intake may simply not be protective against kidney function decline. Perhaps follow-up longer than 1 year would be needed to see an effect, or perhaps there was an effect, but the study was underpowered to detect it.
It could also be that a greater volume of water would be needed to demonstrate a protective effect for the kidneys. Despite the coaching efforts of dietitians and research assistants, the mean urine volume increase in the hydration group relative to the control group was just 0.6 liter per day, or 2.4 cups.
“This highlights how difficult it would be to achieve a large sustained increase in water intake in routine practice,” Dr. Clark and colleagues said in their report.
Dr. Clark reported disclosures related to Danone Research. Thermo Fisher Scientific provided instrumentation, assay reagent, and disposables used in the study.
SOURCE: Clark WF et al. JAMA. 2018;319(18):1870-9.
Key clinical point: Adults with CKD were coached to increase water intake, but that intervention did not appear to slow their decline in kidney function.
Major finding: The 1-year change in eGFR was –2.2 mL/min per 1.73 m2 in patients coached to drink more water and –1.9 mL/min per 1.73 m2 in those coached to maintain water intake; the difference was not significant.
Study details: The CKD WIT (Chronic Kidney Disease Water Intake Trial), a randomized clinical trial was conducted in 9 centers in Ontario, Canada, from 2013 until 2017 and included 631 patients with stage 3 CKD and a 24-hour urine volume below 3.0 L.
Disclosures: Authors reported disclosures related to Danone Research and the ISN/Danone Hydration for Kidney Health Research Initiative. Thermo Fisher Scientific provided instrumentation, assay reagent, and disposables used in the study.
Source: Clark WF et al. JAMA. 2018;319(18):1870-9.
In PAH trials, clinical worsening risk rose with time
Current clinical trials evaluating combination therapy for pulmonary artery hypertension (PAH) may be longer than what is needed to demonstrate treatment benefit, results of a recent meta-analysis suggest.
, according to results of the study published in the May issue of the journal Chest®.
The meta-analysis by Dr. Lajoie and her colleagues included 3,801 patients enrolled in one of 15 previously published randomized clinical trials. Of those trials, four were long-term, event driven studies, with a mean duration of 87 weeks, while the remainder were shorter studies with a mean duration of 15 weeks.
For the long-term, event-driven trials, the mean number needed to treat (NNT) was 17.4 at week 16, gradually decreasing to 8.8 at 52 weeks of follow-up, remaining stable after that, according to investigators.
Consistent with that finding, the mean relative risk of clinical worsening was 0.38 at 16 weeks, and similarly, 0.41 at 26 weeks, investigators reported. After that, the relative risk progressively increased to 0.54 at 52 weeks and 0.68 at 104 weeks.
Looking at all trials combined, Dr. Lajoie and her colleagues observed that longer trial duration had a positive correlation with relative risk of clinical worsening (P = .0002).
Pragmatically, these results raise the possibility that PAH combination therapy trials could be shorter in duration. Some recent event-driven studies have lasted up to 6 years, with patients on treatment for about 2 of those years, investigators noted.
“In the context of an orphan disease with limited and competing recruitment for trials and the rapidly changing treatment paradigm in PAH, the optimal duration of future trials should be revisited,” Dr. Lajoie and her colleagues wrote in a discussion of their findings.
They also cautioned that NNT, a measure of how many patient treatments are needed to prevent one additional adverse event, could be “misleading” despite its value as a simple measure of treatment impact.
Likewise, relative risk can be misleading; for example, a treatment reducing event risk from 30% to 20% represents a relative risk reduction of approximately 35%, but so does a treatment reducing event risk from 3% to 2%, the researchers noted.
“Multiple factors, in addition to the efficacy of the therapy and the comparator, may directly influence the NNT and relative risk and should be taken into account in their interpretation,” they said in their report.
Dr. Lajoie had no disclosures related to the study. Her coauthors had disclosures related to Actelion Pharmaceuticals, Bayer, and GlaxoSmithKline, among others.
SOURCE: Lajoie AC et al. Chest. 2017 May;153(5):1142-52.
Key clinical point: A meta-analysis calls into question the need to perform pulmonary arterial hypertension (PAH) trials beyond 6 to 12 months of treatment in the future.
Major finding: The mean number needed to treat was stable at 52 weeks of follow-up and thereafter, while the mean relative risk of clinical worsening progressively increased from approximately 0.38 at week 16 to 0.68 at week 104.
Study details: A systematic review of 3,801 patients enrolled in 15 randomized clinical trials.
Disclosures: The authors reported disclosures related to Actelion Pharmaceuticals, Bayer, and GlaxoSmithKline, among other entities.
Source: Lajoie AC et al. Chest. 2017 May;153(5):1142-52.
Key clinical point: A meta-analysis calls into question the need to perform pulmonary arterial hypertension (PAH) trials beyond 6 to 12 months of treatment in the future.
Major finding: The mean number needed to treat was stable at 52 weeks of follow-up and thereafter, while the mean relative risk of clinical worsening progressively increased from approximately 0.38 at week 16 to 0.68 at week 104.
Study details: A systematic review of 3,801 patients enrolled in 15 randomized clinical trials.
Disclosures: The authors reported disclosures related to Actelion Pharmaceuticals, Bayer, and GlaxoSmithKline, among other entities.
Source: Lajoie AC et al. Chest. 2017 May;153(5):1142-52.
Key clinical point: A meta-analysis calls into question the need to perform pulmonary arterial hypertension (PAH) trials beyond 6 to 12 months of treatment in the future.
Major finding: The mean number needed to treat was stable at 52 weeks of follow-up and thereafter, while the mean relative risk of clinical worsening progressively increased from approximately 0.38 at week 16 to 0.68 at week 104.
Study details: A systematic review of 3,801 patients enrolled in 15 randomized clinical trials.
Disclosures: The authors reported disclosures related to Actelion Pharmaceuticals, Bayer, and GlaxoSmithKline, among other entities.
Source: Lajoie AC et al. Chest. 2017 May;153(5):1142-52.
Current clinical trials evaluating combination therapy for pulmonary artery hypertension (PAH) may be longer than what is needed to demonstrate treatment benefit, results of a recent meta-analysis suggest.
, according to results of the study published in the May issue of the journal Chest®.
The meta-analysis by Dr. Lajoie and her colleagues included 3,801 patients enrolled in one of 15 previously published randomized clinical trials. Of those trials, four were long-term, event driven studies, with a mean duration of 87 weeks, while the remainder were shorter studies with a mean duration of 15 weeks.
For the long-term, event-driven trials, the mean number needed to treat (NNT) was 17.4 at week 16, gradually decreasing to 8.8 at 52 weeks of follow-up, remaining stable after that, according to investigators.
Consistent with that finding, the mean relative risk of clinical worsening was 0.38 at 16 weeks, and similarly, 0.41 at 26 weeks, investigators reported. After that, the relative risk progressively increased to 0.54 at 52 weeks and 0.68 at 104 weeks.
Looking at all trials combined, Dr. Lajoie and her colleagues observed that longer trial duration had a positive correlation with relative risk of clinical worsening (P = .0002).
Pragmatically, these results raise the possibility that PAH combination therapy trials could be shorter in duration. Some recent event-driven studies have lasted up to 6 years, with patients on treatment for about 2 of those years, investigators noted.
“In the context of an orphan disease with limited and competing recruitment for trials and the rapidly changing treatment paradigm in PAH, the optimal duration of future trials should be revisited,” Dr. Lajoie and her colleagues wrote in a discussion of their findings.
They also cautioned that NNT, a measure of how many patient treatments are needed to prevent one additional adverse event, could be “misleading” despite its value as a simple measure of treatment impact.
Likewise, relative risk can be misleading; for example, a treatment reducing event risk from 30% to 20% represents a relative risk reduction of approximately 35%, but so does a treatment reducing event risk from 3% to 2%, the researchers noted.
“Multiple factors, in addition to the efficacy of the therapy and the comparator, may directly influence the NNT and relative risk and should be taken into account in their interpretation,” they said in their report.
Dr. Lajoie had no disclosures related to the study. Her coauthors had disclosures related to Actelion Pharmaceuticals, Bayer, and GlaxoSmithKline, among others.
SOURCE: Lajoie AC et al. Chest. 2017 May;153(5):1142-52.
Current clinical trials evaluating combination therapy for pulmonary artery hypertension (PAH) may be longer than what is needed to demonstrate treatment benefit, results of a recent meta-analysis suggest.
, according to results of the study published in the May issue of the journal Chest®.
The meta-analysis by Dr. Lajoie and her colleagues included 3,801 patients enrolled in one of 15 previously published randomized clinical trials. Of those trials, four were long-term, event driven studies, with a mean duration of 87 weeks, while the remainder were shorter studies with a mean duration of 15 weeks.
For the long-term, event-driven trials, the mean number needed to treat (NNT) was 17.4 at week 16, gradually decreasing to 8.8 at 52 weeks of follow-up, remaining stable after that, according to investigators.
Consistent with that finding, the mean relative risk of clinical worsening was 0.38 at 16 weeks, and similarly, 0.41 at 26 weeks, investigators reported. After that, the relative risk progressively increased to 0.54 at 52 weeks and 0.68 at 104 weeks.
Looking at all trials combined, Dr. Lajoie and her colleagues observed that longer trial duration had a positive correlation with relative risk of clinical worsening (P = .0002).
Pragmatically, these results raise the possibility that PAH combination therapy trials could be shorter in duration. Some recent event-driven studies have lasted up to 6 years, with patients on treatment for about 2 of those years, investigators noted.
“In the context of an orphan disease with limited and competing recruitment for trials and the rapidly changing treatment paradigm in PAH, the optimal duration of future trials should be revisited,” Dr. Lajoie and her colleagues wrote in a discussion of their findings.
They also cautioned that NNT, a measure of how many patient treatments are needed to prevent one additional adverse event, could be “misleading” despite its value as a simple measure of treatment impact.
Likewise, relative risk can be misleading; for example, a treatment reducing event risk from 30% to 20% represents a relative risk reduction of approximately 35%, but so does a treatment reducing event risk from 3% to 2%, the researchers noted.
“Multiple factors, in addition to the efficacy of the therapy and the comparator, may directly influence the NNT and relative risk and should be taken into account in their interpretation,” they said in their report.
Dr. Lajoie had no disclosures related to the study. Her coauthors had disclosures related to Actelion Pharmaceuticals, Bayer, and GlaxoSmithKline, among others.
SOURCE: Lajoie AC et al. Chest. 2017 May;153(5):1142-52.
FROM CHEST®
Celiac disease: Can biopsy be avoided?
LAS VEGAS – It may be only a matter of time before the “gold standard” small biopsy is no longer considered mandatory to make a diagnosis of celiac disease in adults, according to Joseph A. Murray, MD, consultant in the division of gastroenterology and hepatology and department of immunology, Mayo Clinic, Rochester, Minn.
“Right now, none of the adult societies support biopsy avoidance, but I predict that it will come to be,” Dr. Murray said at the inaugural Perspectives in Digestive Diseases meeting held by Global Academy for Medical Education.
Biopsy, already a tarnished standard because of issues such as interpretation, according to Dr. Murray, is being challenged in studies that examine alternate ways of making the diagnosis.
In one recently reported study, investigators at Royal Derby Hospital, England, suggested that clinicians could make a reliable diagnosis of celiac disease by looking at serum IgA-tissue transglutaminase antibody levels.
Those investigators retrospectively analyzed an unselected series of 270 adult patients and found that an IgA-tissue transglutaminase antibody cut-off of 45 U/mL, or 8 times the upper limit of normal, had a positive predictive value of 100%.
Biopsy avoidance remains controversial, however. In a published letter to the editor commenting on the Derby study, authors took issue with some of the statistical analysis and remarked that the study included some patients with Marsh 1 histology.
“Studies suggest that the majority of seropositive patients with Marsh 1 histology do not progress to develop villous atrophy while on a gluten-containing diet, raising the question whether all of them are truly celiac,” they wrote.
The first society to endorse skipping the biopsy was the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition.
In guidelines for the diagnosis of celiac disease, that group said a celiac diagnosis could be made based on symptoms, antibodies, and HLA in children with symptoms suggestive of the disease and high antibody levels (IgA anti-tissue transglutaminase type 2 antibody titers greater than 10 times the upper limit of normal).
“The data [are] now pretty good to support that approach in symptomatic children,” Dr. Murray said. “If we apply these to adult patients, it’s not bad, actually, partly because our biopsies aren’t perfect.”
However, not all adult gastroenterology specialists agreed with the recommendations of the pediatric society. Guidelines from the British Society of Gastroenterology have stated that serology cannot replace biopsy, which “remains essential” for celiac disease diagnosis.
Global Academy and this news organization are owned by the same parent company.Dr. Murray reported disclosures related to Ardent Mills, DBV Technologies, Evelo, GlaxoSmithKline, Johnson & Johnson, Immunogenix, Innovate, National Center for Complementary and Integrative Health, Takeda, Torax Medical, and UCB.
AGA offers celiac disease information for your patients in the GI Patient Center at http://ow.ly/VwJ130jN7Sx.
LAS VEGAS – It may be only a matter of time before the “gold standard” small biopsy is no longer considered mandatory to make a diagnosis of celiac disease in adults, according to Joseph A. Murray, MD, consultant in the division of gastroenterology and hepatology and department of immunology, Mayo Clinic, Rochester, Minn.
“Right now, none of the adult societies support biopsy avoidance, but I predict that it will come to be,” Dr. Murray said at the inaugural Perspectives in Digestive Diseases meeting held by Global Academy for Medical Education.
Biopsy, already a tarnished standard because of issues such as interpretation, according to Dr. Murray, is being challenged in studies that examine alternate ways of making the diagnosis.
In one recently reported study, investigators at Royal Derby Hospital, England, suggested that clinicians could make a reliable diagnosis of celiac disease by looking at serum IgA-tissue transglutaminase antibody levels.
Those investigators retrospectively analyzed an unselected series of 270 adult patients and found that an IgA-tissue transglutaminase antibody cut-off of 45 U/mL, or 8 times the upper limit of normal, had a positive predictive value of 100%.
Biopsy avoidance remains controversial, however. In a published letter to the editor commenting on the Derby study, authors took issue with some of the statistical analysis and remarked that the study included some patients with Marsh 1 histology.
“Studies suggest that the majority of seropositive patients with Marsh 1 histology do not progress to develop villous atrophy while on a gluten-containing diet, raising the question whether all of them are truly celiac,” they wrote.
The first society to endorse skipping the biopsy was the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition.
In guidelines for the diagnosis of celiac disease, that group said a celiac diagnosis could be made based on symptoms, antibodies, and HLA in children with symptoms suggestive of the disease and high antibody levels (IgA anti-tissue transglutaminase type 2 antibody titers greater than 10 times the upper limit of normal).
“The data [are] now pretty good to support that approach in symptomatic children,” Dr. Murray said. “If we apply these to adult patients, it’s not bad, actually, partly because our biopsies aren’t perfect.”
However, not all adult gastroenterology specialists agreed with the recommendations of the pediatric society. Guidelines from the British Society of Gastroenterology have stated that serology cannot replace biopsy, which “remains essential” for celiac disease diagnosis.
Global Academy and this news organization are owned by the same parent company.Dr. Murray reported disclosures related to Ardent Mills, DBV Technologies, Evelo, GlaxoSmithKline, Johnson & Johnson, Immunogenix, Innovate, National Center for Complementary and Integrative Health, Takeda, Torax Medical, and UCB.
AGA offers celiac disease information for your patients in the GI Patient Center at http://ow.ly/VwJ130jN7Sx.
LAS VEGAS – It may be only a matter of time before the “gold standard” small biopsy is no longer considered mandatory to make a diagnosis of celiac disease in adults, according to Joseph A. Murray, MD, consultant in the division of gastroenterology and hepatology and department of immunology, Mayo Clinic, Rochester, Minn.
“Right now, none of the adult societies support biopsy avoidance, but I predict that it will come to be,” Dr. Murray said at the inaugural Perspectives in Digestive Diseases meeting held by Global Academy for Medical Education.
Biopsy, already a tarnished standard because of issues such as interpretation, according to Dr. Murray, is being challenged in studies that examine alternate ways of making the diagnosis.
In one recently reported study, investigators at Royal Derby Hospital, England, suggested that clinicians could make a reliable diagnosis of celiac disease by looking at serum IgA-tissue transglutaminase antibody levels.
Those investigators retrospectively analyzed an unselected series of 270 adult patients and found that an IgA-tissue transglutaminase antibody cut-off of 45 U/mL, or 8 times the upper limit of normal, had a positive predictive value of 100%.
Biopsy avoidance remains controversial, however. In a published letter to the editor commenting on the Derby study, authors took issue with some of the statistical analysis and remarked that the study included some patients with Marsh 1 histology.
“Studies suggest that the majority of seropositive patients with Marsh 1 histology do not progress to develop villous atrophy while on a gluten-containing diet, raising the question whether all of them are truly celiac,” they wrote.
The first society to endorse skipping the biopsy was the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition.
In guidelines for the diagnosis of celiac disease, that group said a celiac diagnosis could be made based on symptoms, antibodies, and HLA in children with symptoms suggestive of the disease and high antibody levels (IgA anti-tissue transglutaminase type 2 antibody titers greater than 10 times the upper limit of normal).
“The data [are] now pretty good to support that approach in symptomatic children,” Dr. Murray said. “If we apply these to adult patients, it’s not bad, actually, partly because our biopsies aren’t perfect.”
However, not all adult gastroenterology specialists agreed with the recommendations of the pediatric society. Guidelines from the British Society of Gastroenterology have stated that serology cannot replace biopsy, which “remains essential” for celiac disease diagnosis.
Global Academy and this news organization are owned by the same parent company.Dr. Murray reported disclosures related to Ardent Mills, DBV Technologies, Evelo, GlaxoSmithKline, Johnson & Johnson, Immunogenix, Innovate, National Center for Complementary and Integrative Health, Takeda, Torax Medical, and UCB.
AGA offers celiac disease information for your patients in the GI Patient Center at http://ow.ly/VwJ130jN7Sx.
Relapse rate drives stem cell transplant failure in pediatric ALL patients
Relapse was the main impediment to successful hematopoietic stem cell transplant (HSCT) in high-risk pediatric acute lymphocytic leukemia (ALL), according to one of the largest single-center experiences reported to date.
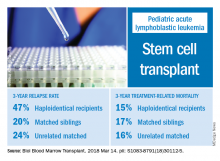
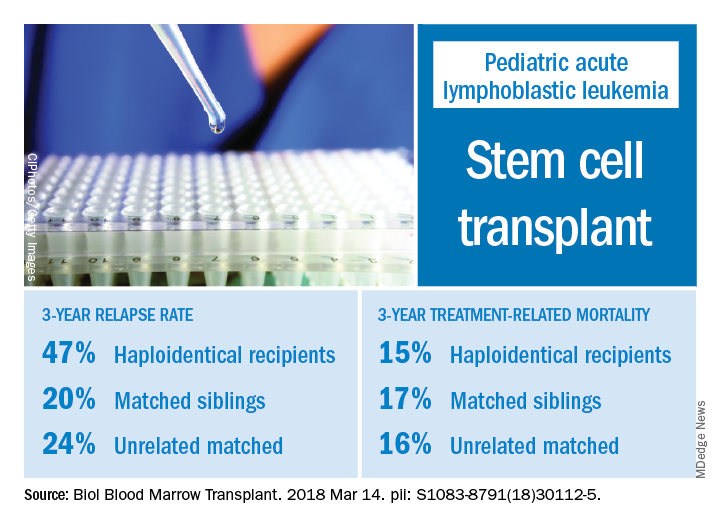
The effects of relapse were especially evident for patients with haploidentical donors; these patients had a 3-year cumulative relapse incidence of 47% and event-free survival rate of 35%, both significantly higher than what was seen in other transplant recipients treated at the same center.
The findings, recently published in the journal Biology of Blood and Marrow Transplantation, suggest a substantial unmet need in the treatment of high-risk patients in first remission.
“Newer methods to improve graft-versus-leukemia effect are being tested and will need to be incorporated into the management of high-risk patients,” Asaf D. Yanir, MD, and coauthors at Baylor College of Medicine in Houston said in the report.
Dr. Yanir and colleagues reported recent outcomes for 124 patients who had undergone HSCT for ALL at their center during 2008-2016. That group included 20 haploidentical transplant recipients, 48 patients with matched sibling donors, and 56 with unrelated matched donors.
The 3-year cumulative incidence of relapse was 47% for haploidentical recipients, compared with 20% for matched sibling donors recipients and 24% for unrelated matched donors recipients (P = .02), according to their findings.
The main cause of HSCT failure was relapse, occurring in 47% of haploidentical transplant recipients, compared with 20% for those with matched sibling donors and 24% for those with unrelated matched donors (P = .02).
Those findings are in line with other studies showing inferior outcomes following haploidentical donor HSCT. However, in contrast to those studies, Dr. Yanir and colleagues did find a rate of treatment-related mortality comparable with other transplant approaches. The 3-year incidence of treatment-related mortality was 15% for the haploidentical group and, similarly, 17% in the matched sibling donor group and 16% in the unrelated matched donor group.
That lower rate of treatment-related mortality in the haploidentical group may be caused by improvements in procedures and supportive care. “Unfortunately, the benefits gained by reducing treatment-related mortality were offset by the high rate of relapse, which remains the main obstacle to successful haploidentical donor HSCT,” Dr. Yanir and coauthors wrote in their report.
New strategies are being studied to retain the graft-versus-leukemia effect or enhance it in patients who’ve undergone haploidentical HSCT, such as selectively depleting alloreactive T cells while sparing other immune effectors, investigators wrote.
“Given evolving practices, it is important to continually evaluate the projected event-free survival for pediatric ALL following HSCT, based on the donor type used,” they wrote.
Dr. Yanir and coauthors had no financial disclosures or conflicts of interest to report.
SOURCE: Yanir AD et al. Biol Blood Marrow Transplant. 2018 Mar 14. doi: 10.1016/j.bbmt.2018.03.001.
Relapse was the main impediment to successful hematopoietic stem cell transplant (HSCT) in high-risk pediatric acute lymphocytic leukemia (ALL), according to one of the largest single-center experiences reported to date.
The effects of relapse were especially evident for patients with haploidentical donors; these patients had a 3-year cumulative relapse incidence of 47% and event-free survival rate of 35%, both significantly higher than what was seen in other transplant recipients treated at the same center.
The findings, recently published in the journal Biology of Blood and Marrow Transplantation, suggest a substantial unmet need in the treatment of high-risk patients in first remission.
“Newer methods to improve graft-versus-leukemia effect are being tested and will need to be incorporated into the management of high-risk patients,” Asaf D. Yanir, MD, and coauthors at Baylor College of Medicine in Houston said in the report.
Dr. Yanir and colleagues reported recent outcomes for 124 patients who had undergone HSCT for ALL at their center during 2008-2016. That group included 20 haploidentical transplant recipients, 48 patients with matched sibling donors, and 56 with unrelated matched donors.
The 3-year cumulative incidence of relapse was 47% for haploidentical recipients, compared with 20% for matched sibling donors recipients and 24% for unrelated matched donors recipients (P = .02), according to their findings.
The main cause of HSCT failure was relapse, occurring in 47% of haploidentical transplant recipients, compared with 20% for those with matched sibling donors and 24% for those with unrelated matched donors (P = .02).
Those findings are in line with other studies showing inferior outcomes following haploidentical donor HSCT. However, in contrast to those studies, Dr. Yanir and colleagues did find a rate of treatment-related mortality comparable with other transplant approaches. The 3-year incidence of treatment-related mortality was 15% for the haploidentical group and, similarly, 17% in the matched sibling donor group and 16% in the unrelated matched donor group.
That lower rate of treatment-related mortality in the haploidentical group may be caused by improvements in procedures and supportive care. “Unfortunately, the benefits gained by reducing treatment-related mortality were offset by the high rate of relapse, which remains the main obstacle to successful haploidentical donor HSCT,” Dr. Yanir and coauthors wrote in their report.
New strategies are being studied to retain the graft-versus-leukemia effect or enhance it in patients who’ve undergone haploidentical HSCT, such as selectively depleting alloreactive T cells while sparing other immune effectors, investigators wrote.
“Given evolving practices, it is important to continually evaluate the projected event-free survival for pediatric ALL following HSCT, based on the donor type used,” they wrote.
Dr. Yanir and coauthors had no financial disclosures or conflicts of interest to report.
SOURCE: Yanir AD et al. Biol Blood Marrow Transplant. 2018 Mar 14. doi: 10.1016/j.bbmt.2018.03.001.
Relapse was the main impediment to successful hematopoietic stem cell transplant (HSCT) in high-risk pediatric acute lymphocytic leukemia (ALL), according to one of the largest single-center experiences reported to date.
The effects of relapse were especially evident for patients with haploidentical donors; these patients had a 3-year cumulative relapse incidence of 47% and event-free survival rate of 35%, both significantly higher than what was seen in other transplant recipients treated at the same center.
The findings, recently published in the journal Biology of Blood and Marrow Transplantation, suggest a substantial unmet need in the treatment of high-risk patients in first remission.
“Newer methods to improve graft-versus-leukemia effect are being tested and will need to be incorporated into the management of high-risk patients,” Asaf D. Yanir, MD, and coauthors at Baylor College of Medicine in Houston said in the report.
Dr. Yanir and colleagues reported recent outcomes for 124 patients who had undergone HSCT for ALL at their center during 2008-2016. That group included 20 haploidentical transplant recipients, 48 patients with matched sibling donors, and 56 with unrelated matched donors.
The 3-year cumulative incidence of relapse was 47% for haploidentical recipients, compared with 20% for matched sibling donors recipients and 24% for unrelated matched donors recipients (P = .02), according to their findings.
The main cause of HSCT failure was relapse, occurring in 47% of haploidentical transplant recipients, compared with 20% for those with matched sibling donors and 24% for those with unrelated matched donors (P = .02).
Those findings are in line with other studies showing inferior outcomes following haploidentical donor HSCT. However, in contrast to those studies, Dr. Yanir and colleagues did find a rate of treatment-related mortality comparable with other transplant approaches. The 3-year incidence of treatment-related mortality was 15% for the haploidentical group and, similarly, 17% in the matched sibling donor group and 16% in the unrelated matched donor group.
That lower rate of treatment-related mortality in the haploidentical group may be caused by improvements in procedures and supportive care. “Unfortunately, the benefits gained by reducing treatment-related mortality were offset by the high rate of relapse, which remains the main obstacle to successful haploidentical donor HSCT,” Dr. Yanir and coauthors wrote in their report.
New strategies are being studied to retain the graft-versus-leukemia effect or enhance it in patients who’ve undergone haploidentical HSCT, such as selectively depleting alloreactive T cells while sparing other immune effectors, investigators wrote.
“Given evolving practices, it is important to continually evaluate the projected event-free survival for pediatric ALL following HSCT, based on the donor type used,” they wrote.
Dr. Yanir and coauthors had no financial disclosures or conflicts of interest to report.
SOURCE: Yanir AD et al. Biol Blood Marrow Transplant. 2018 Mar 14. doi: 10.1016/j.bbmt.2018.03.001.
FROM BIOLOGY OF BLOOD AND MARROW TRANSPLANTATION
Key clinical point: In pediatric acute lymphocytic leukemia (ALL) patients, relapse is the main barrier to successful hematopoietic stem cell transplant (HSCT), especially for those who receive haploidentical donor grafts.
Major finding: The main cause of HSCT failure was relapse, occurring in 47% of haploidentical transplant recipients, compared with 20% for those with matched sibling donors and 24% for those with unrelated matched donors (P = .02).
Study details: A retrospective analysis of 124 transplants performed at a single center during 2008-2016.
Disclosures: Authors had no financial disclosures or conflicts of interest to report.
Source: Yanir AD et al. Biol Blood Marrow Transplant. doi: 10.1016/j.bbmt.2018.03.001.
New drugs provide new options in HCC
PHILADELPHIA – Recent approvals and investigations of targeted and immune treatments for advanced hepatocellular carcinoma (HCC) are encouraging, Nikolaos Pyrsopoulos, MD, MBA, said at Digestive Diseases: New Advances, jointly provided by Rutgers and Global Academy for Medical Education.
“I am excited, because a few years ago, there was only one [Food and Drug Administration] approved medication,” Dr. Pyrsopoulos, division director for gastroenterology and hepatology at Rutgers New Jersey Medical School, Newark, said in an interview. “We are on the cusp where new compounds not only are being tested, but they are being approved.”
Median overall survival in CELESTIAL was 10.2 months for cabozantinib versus 8.0 for placebo (P = .0049), according to the published report, and investigators also reported significant improvements in progression-free survival and response versus placebo.
“It is very encouraging,” Dr. Pyrsopoulos said of the cabozantinib results in a presentation on advances in HCC that he gave at the conference.
For years, the only FDA-approved treatment for advanced HCC was sorafenib. In the randomized phase 3 SHARP trial, published in the New England Journal of Medicine in 2008, patients receiving the multikinase inhibitor had a median survival of 10.7 months, versus 7.9 months for placebo (P less than .001).
In April 2017, the FDA approved regorafenib for patients with HCC previously treated with sorafenib. In the randomized phase 3 RESORCE trial, published in The Lancet in 2017, median overall survival was 10.6 months for regorafenib-treated patients versus 7.8 months in the placebo group. Investigators reported that regorafenib improved overall survival with a hazard ratio of 0.63 (P less than .0001).
Dr. Pyrsopoulos noted that a strategy of sorafenib followed by regorafenib would combine two treatments, each of which in clinical trials had a median overall survival approaching 11 months.
“In essence, you have an approximate 2-year survival,” he said.
More agents are under investigation, including lenvatinib, another multikinase inhibitor. In results of a phase 3 randomized trial presented at the 2017 meeting of the American Society of Clinical Oncology, lenvatinib was noninferior to sorafenib in overall survival, with treatment-related adverse effects such as hypertension and diarrhea that were expected based on previous experience with the drug, investigators said.
Cancer immunotherapy is making inroads into HCC. Just a few months after approving regorafenib, the FDA granted approval to nivolumab, a PD-1 inhibitor, for patients with HCC previously treated with sorafenib. The September 2017 approval of this checkpoint inhibitor was based in part on data from the CheckMate-040 trial that included a 14.3% response rate in the 154-patient subgroup of patients who had progressive disease on sorafenib or were intolerant of the treatment.
Dr. Pyrsopoulos highlighted another checkpoint inhibitor, known as BGB-A317, or tislelizumab. In January, BeiGene announced the initiation of a global phase 3 trial of this anti-PD-1 antibody versus sorafenib as first-line treatment of patients with unresectable HCC.
Although cancer immunotherapy holds great promise for HCC and other cancers, the treatments are associated with unique immune-related adverse events (irAEs) including immune-related hepatitis that may require corticosteroid treatment, according to Dr. Pyrsopoulos.
Dr. Pyrsopoulos reported disclosures related to AbbVie, Bayer, Genfit, Gilead Sciences, Hologic, Merck, Prometheus, Shire, and Vital Therapies.
Global Academy for Medical Education and this news organization are owned by the same company.
PHILADELPHIA – Recent approvals and investigations of targeted and immune treatments for advanced hepatocellular carcinoma (HCC) are encouraging, Nikolaos Pyrsopoulos, MD, MBA, said at Digestive Diseases: New Advances, jointly provided by Rutgers and Global Academy for Medical Education.
“I am excited, because a few years ago, there was only one [Food and Drug Administration] approved medication,” Dr. Pyrsopoulos, division director for gastroenterology and hepatology at Rutgers New Jersey Medical School, Newark, said in an interview. “We are on the cusp where new compounds not only are being tested, but they are being approved.”
Median overall survival in CELESTIAL was 10.2 months for cabozantinib versus 8.0 for placebo (P = .0049), according to the published report, and investigators also reported significant improvements in progression-free survival and response versus placebo.
“It is very encouraging,” Dr. Pyrsopoulos said of the cabozantinib results in a presentation on advances in HCC that he gave at the conference.
For years, the only FDA-approved treatment for advanced HCC was sorafenib. In the randomized phase 3 SHARP trial, published in the New England Journal of Medicine in 2008, patients receiving the multikinase inhibitor had a median survival of 10.7 months, versus 7.9 months for placebo (P less than .001).
In April 2017, the FDA approved regorafenib for patients with HCC previously treated with sorafenib. In the randomized phase 3 RESORCE trial, published in The Lancet in 2017, median overall survival was 10.6 months for regorafenib-treated patients versus 7.8 months in the placebo group. Investigators reported that regorafenib improved overall survival with a hazard ratio of 0.63 (P less than .0001).
Dr. Pyrsopoulos noted that a strategy of sorafenib followed by regorafenib would combine two treatments, each of which in clinical trials had a median overall survival approaching 11 months.
“In essence, you have an approximate 2-year survival,” he said.
More agents are under investigation, including lenvatinib, another multikinase inhibitor. In results of a phase 3 randomized trial presented at the 2017 meeting of the American Society of Clinical Oncology, lenvatinib was noninferior to sorafenib in overall survival, with treatment-related adverse effects such as hypertension and diarrhea that were expected based on previous experience with the drug, investigators said.
Cancer immunotherapy is making inroads into HCC. Just a few months after approving regorafenib, the FDA granted approval to nivolumab, a PD-1 inhibitor, for patients with HCC previously treated with sorafenib. The September 2017 approval of this checkpoint inhibitor was based in part on data from the CheckMate-040 trial that included a 14.3% response rate in the 154-patient subgroup of patients who had progressive disease on sorafenib or were intolerant of the treatment.
Dr. Pyrsopoulos highlighted another checkpoint inhibitor, known as BGB-A317, or tislelizumab. In January, BeiGene announced the initiation of a global phase 3 trial of this anti-PD-1 antibody versus sorafenib as first-line treatment of patients with unresectable HCC.
Although cancer immunotherapy holds great promise for HCC and other cancers, the treatments are associated with unique immune-related adverse events (irAEs) including immune-related hepatitis that may require corticosteroid treatment, according to Dr. Pyrsopoulos.
Dr. Pyrsopoulos reported disclosures related to AbbVie, Bayer, Genfit, Gilead Sciences, Hologic, Merck, Prometheus, Shire, and Vital Therapies.
Global Academy for Medical Education and this news organization are owned by the same company.
PHILADELPHIA – Recent approvals and investigations of targeted and immune treatments for advanced hepatocellular carcinoma (HCC) are encouraging, Nikolaos Pyrsopoulos, MD, MBA, said at Digestive Diseases: New Advances, jointly provided by Rutgers and Global Academy for Medical Education.
“I am excited, because a few years ago, there was only one [Food and Drug Administration] approved medication,” Dr. Pyrsopoulos, division director for gastroenterology and hepatology at Rutgers New Jersey Medical School, Newark, said in an interview. “We are on the cusp where new compounds not only are being tested, but they are being approved.”
Median overall survival in CELESTIAL was 10.2 months for cabozantinib versus 8.0 for placebo (P = .0049), according to the published report, and investigators also reported significant improvements in progression-free survival and response versus placebo.
“It is very encouraging,” Dr. Pyrsopoulos said of the cabozantinib results in a presentation on advances in HCC that he gave at the conference.
For years, the only FDA-approved treatment for advanced HCC was sorafenib. In the randomized phase 3 SHARP trial, published in the New England Journal of Medicine in 2008, patients receiving the multikinase inhibitor had a median survival of 10.7 months, versus 7.9 months for placebo (P less than .001).
In April 2017, the FDA approved regorafenib for patients with HCC previously treated with sorafenib. In the randomized phase 3 RESORCE trial, published in The Lancet in 2017, median overall survival was 10.6 months for regorafenib-treated patients versus 7.8 months in the placebo group. Investigators reported that regorafenib improved overall survival with a hazard ratio of 0.63 (P less than .0001).
Dr. Pyrsopoulos noted that a strategy of sorafenib followed by regorafenib would combine two treatments, each of which in clinical trials had a median overall survival approaching 11 months.
“In essence, you have an approximate 2-year survival,” he said.
More agents are under investigation, including lenvatinib, another multikinase inhibitor. In results of a phase 3 randomized trial presented at the 2017 meeting of the American Society of Clinical Oncology, lenvatinib was noninferior to sorafenib in overall survival, with treatment-related adverse effects such as hypertension and diarrhea that were expected based on previous experience with the drug, investigators said.
Cancer immunotherapy is making inroads into HCC. Just a few months after approving regorafenib, the FDA granted approval to nivolumab, a PD-1 inhibitor, for patients with HCC previously treated with sorafenib. The September 2017 approval of this checkpoint inhibitor was based in part on data from the CheckMate-040 trial that included a 14.3% response rate in the 154-patient subgroup of patients who had progressive disease on sorafenib or were intolerant of the treatment.
Dr. Pyrsopoulos highlighted another checkpoint inhibitor, known as BGB-A317, or tislelizumab. In January, BeiGene announced the initiation of a global phase 3 trial of this anti-PD-1 antibody versus sorafenib as first-line treatment of patients with unresectable HCC.
Although cancer immunotherapy holds great promise for HCC and other cancers, the treatments are associated with unique immune-related adverse events (irAEs) including immune-related hepatitis that may require corticosteroid treatment, according to Dr. Pyrsopoulos.
Dr. Pyrsopoulos reported disclosures related to AbbVie, Bayer, Genfit, Gilead Sciences, Hologic, Merck, Prometheus, Shire, and Vital Therapies.
Global Academy for Medical Education and this news organization are owned by the same company.
REPORTING FROM DIGESTIVE DISEASES: NEW ADVANCES
In young MCL patients, optimal treatment may vary
, according to a recent review published in Best Practice & Research Clinical Haematology.
Use of high-dose cytarabine plus rituximab as frontline treatment is well established, with median overall survival now exceeding 10 years, said Rory McCulloch, MD, and Simon Rule, MD, of the department of Haematology, Derriford Hospital, Plymouth, England. However, there is no proven benefit to conventional therapy in patients with asymptomatic, non-bulky disease, making a watch-and-wait strategy appropriate for these patients, the authors said.
On the opposite end of the spectrum there is a subgroup of patients characterized by TP53 mutations and poor prognostic index scores that have poor outcomes in spite of conventional therapy.
These patients might have improved outcomes either with early allogeneic haematopoietic cell transplantation (allo-HCT), or, especially, clinical trials of novel agents in the upfront setting, the authors noted.
“There are a host of exciting novel agents, most prominently the BTK inhibitors, that are game changing with respect to their activity,” wrote Dr. McCulloch and Dr. Rule. “Based on the long-term results seen with conventional therapy, it is premature to be considering such new drugs in the frontline setting outside the context of a clinical trial, but it is hard to believe they will not become incorporated into treatment protocols in the future.”
Watch-and-wait treatment strategies for lower-risk patients are supported by the results of two single-center, retrospective studies published in 2009 that suggest the practice has no adverse impact on overall survival. More recent registry studies, published in 2016 and 2017, have shown that a significant proportion of patients can be managed according to the watch-and-wait strategy.
Although it’s been challenging to precisely define the group of patients for whom watch-and-wait is appropriate, enrollment criteria for studies have generally specified that patients be asymptomatic with non-bulky disease and non-blastoid morphology, they said.
For the minority of patients presenting with high-risk disease, allo-HCT may improve outcomes, according to Dr. McCulloch and Dr. Rule. One prospective study evaluating allogeneic transplants in frontline therapy showed favorable outcomes in younger patients, although few high-risk patients were enrolled.
However, a second prospective study of allo-HCT, involving 25 patients with untreated MCL in the United Kingdom, demonstrated a 2-year overall survival of 80%. “Although immature, the results are encouraging and provide data to support frontline allogeneic transplant for some patients,” Dr. McCulloch and Dr. Rule said in a comment on that study.
Novel agent studies have produced mixed results in treatment settings relevant to younger, high-risk MCL patients, though key trials are ongoing that could change practice.
One phase 2 study is evaluating obinituzumab, the fully humanized anti-CD20, as part of MCL induction and maintenance. Results of that study could challenge the role of rituximab in maintenance, the review authors noted. Likewise, the immune modulator lenalidomide has been evaluated as maintenance in an Italian phase 3 trial that recently closed to recruitment.
BTK inhibitors represent a “step change” in the management of MCL, according to the authors of this review.
“It has become clear that earlier use of ibrutinib leads to an improved outcome [in MCL] and it is logical to extend this into frontline treatment,” they wrote.
A randomized phase 3, multinational trial known as TRIANGLE, now open to recruitment, is designed to evaluate use of ibrutinib in both induction and maintenance. Investigators plan to enroll 870 patients into the three-arm study, which will also evaluate the use of ibrutinib as part of induction, but with no autologous stem cell transplant.
“The trial is the first to randomize to a non-ASCT arm since the introduction of rituximab and cytarabine to the induction regimen and the results have the potential to significantly reduce chemotherapy intensity and toxicity,” the authors said.
Dr. Rule reported consulting for Pharmacyclics, Napp, Sunesis, Acerta Pharma, Kite, AstraZeneca, Roche, Janssen, and Celgene, and research funding from Janssen, Celgene, and GSK. Dr. McCulloch reported having no financial disclosures.
SOURCE: McCulloch R et al. Best Pract Res Clin Haematol. 2018 Mar;31(1):90-8.
, according to a recent review published in Best Practice & Research Clinical Haematology.
Use of high-dose cytarabine plus rituximab as frontline treatment is well established, with median overall survival now exceeding 10 years, said Rory McCulloch, MD, and Simon Rule, MD, of the department of Haematology, Derriford Hospital, Plymouth, England. However, there is no proven benefit to conventional therapy in patients with asymptomatic, non-bulky disease, making a watch-and-wait strategy appropriate for these patients, the authors said.
On the opposite end of the spectrum there is a subgroup of patients characterized by TP53 mutations and poor prognostic index scores that have poor outcomes in spite of conventional therapy.
These patients might have improved outcomes either with early allogeneic haematopoietic cell transplantation (allo-HCT), or, especially, clinical trials of novel agents in the upfront setting, the authors noted.
“There are a host of exciting novel agents, most prominently the BTK inhibitors, that are game changing with respect to their activity,” wrote Dr. McCulloch and Dr. Rule. “Based on the long-term results seen with conventional therapy, it is premature to be considering such new drugs in the frontline setting outside the context of a clinical trial, but it is hard to believe they will not become incorporated into treatment protocols in the future.”
Watch-and-wait treatment strategies for lower-risk patients are supported by the results of two single-center, retrospective studies published in 2009 that suggest the practice has no adverse impact on overall survival. More recent registry studies, published in 2016 and 2017, have shown that a significant proportion of patients can be managed according to the watch-and-wait strategy.
Although it’s been challenging to precisely define the group of patients for whom watch-and-wait is appropriate, enrollment criteria for studies have generally specified that patients be asymptomatic with non-bulky disease and non-blastoid morphology, they said.
For the minority of patients presenting with high-risk disease, allo-HCT may improve outcomes, according to Dr. McCulloch and Dr. Rule. One prospective study evaluating allogeneic transplants in frontline therapy showed favorable outcomes in younger patients, although few high-risk patients were enrolled.
However, a second prospective study of allo-HCT, involving 25 patients with untreated MCL in the United Kingdom, demonstrated a 2-year overall survival of 80%. “Although immature, the results are encouraging and provide data to support frontline allogeneic transplant for some patients,” Dr. McCulloch and Dr. Rule said in a comment on that study.
Novel agent studies have produced mixed results in treatment settings relevant to younger, high-risk MCL patients, though key trials are ongoing that could change practice.
One phase 2 study is evaluating obinituzumab, the fully humanized anti-CD20, as part of MCL induction and maintenance. Results of that study could challenge the role of rituximab in maintenance, the review authors noted. Likewise, the immune modulator lenalidomide has been evaluated as maintenance in an Italian phase 3 trial that recently closed to recruitment.
BTK inhibitors represent a “step change” in the management of MCL, according to the authors of this review.
“It has become clear that earlier use of ibrutinib leads to an improved outcome [in MCL] and it is logical to extend this into frontline treatment,” they wrote.
A randomized phase 3, multinational trial known as TRIANGLE, now open to recruitment, is designed to evaluate use of ibrutinib in both induction and maintenance. Investigators plan to enroll 870 patients into the three-arm study, which will also evaluate the use of ibrutinib as part of induction, but with no autologous stem cell transplant.
“The trial is the first to randomize to a non-ASCT arm since the introduction of rituximab and cytarabine to the induction regimen and the results have the potential to significantly reduce chemotherapy intensity and toxicity,” the authors said.
Dr. Rule reported consulting for Pharmacyclics, Napp, Sunesis, Acerta Pharma, Kite, AstraZeneca, Roche, Janssen, and Celgene, and research funding from Janssen, Celgene, and GSK. Dr. McCulloch reported having no financial disclosures.
SOURCE: McCulloch R et al. Best Pract Res Clin Haematol. 2018 Mar;31(1):90-8.
, according to a recent review published in Best Practice & Research Clinical Haematology.
Use of high-dose cytarabine plus rituximab as frontline treatment is well established, with median overall survival now exceeding 10 years, said Rory McCulloch, MD, and Simon Rule, MD, of the department of Haematology, Derriford Hospital, Plymouth, England. However, there is no proven benefit to conventional therapy in patients with asymptomatic, non-bulky disease, making a watch-and-wait strategy appropriate for these patients, the authors said.
On the opposite end of the spectrum there is a subgroup of patients characterized by TP53 mutations and poor prognostic index scores that have poor outcomes in spite of conventional therapy.
These patients might have improved outcomes either with early allogeneic haematopoietic cell transplantation (allo-HCT), or, especially, clinical trials of novel agents in the upfront setting, the authors noted.
“There are a host of exciting novel agents, most prominently the BTK inhibitors, that are game changing with respect to their activity,” wrote Dr. McCulloch and Dr. Rule. “Based on the long-term results seen with conventional therapy, it is premature to be considering such new drugs in the frontline setting outside the context of a clinical trial, but it is hard to believe they will not become incorporated into treatment protocols in the future.”
Watch-and-wait treatment strategies for lower-risk patients are supported by the results of two single-center, retrospective studies published in 2009 that suggest the practice has no adverse impact on overall survival. More recent registry studies, published in 2016 and 2017, have shown that a significant proportion of patients can be managed according to the watch-and-wait strategy.
Although it’s been challenging to precisely define the group of patients for whom watch-and-wait is appropriate, enrollment criteria for studies have generally specified that patients be asymptomatic with non-bulky disease and non-blastoid morphology, they said.
For the minority of patients presenting with high-risk disease, allo-HCT may improve outcomes, according to Dr. McCulloch and Dr. Rule. One prospective study evaluating allogeneic transplants in frontline therapy showed favorable outcomes in younger patients, although few high-risk patients were enrolled.
However, a second prospective study of allo-HCT, involving 25 patients with untreated MCL in the United Kingdom, demonstrated a 2-year overall survival of 80%. “Although immature, the results are encouraging and provide data to support frontline allogeneic transplant for some patients,” Dr. McCulloch and Dr. Rule said in a comment on that study.
Novel agent studies have produced mixed results in treatment settings relevant to younger, high-risk MCL patients, though key trials are ongoing that could change practice.
One phase 2 study is evaluating obinituzumab, the fully humanized anti-CD20, as part of MCL induction and maintenance. Results of that study could challenge the role of rituximab in maintenance, the review authors noted. Likewise, the immune modulator lenalidomide has been evaluated as maintenance in an Italian phase 3 trial that recently closed to recruitment.
BTK inhibitors represent a “step change” in the management of MCL, according to the authors of this review.
“It has become clear that earlier use of ibrutinib leads to an improved outcome [in MCL] and it is logical to extend this into frontline treatment,” they wrote.
A randomized phase 3, multinational trial known as TRIANGLE, now open to recruitment, is designed to evaluate use of ibrutinib in both induction and maintenance. Investigators plan to enroll 870 patients into the three-arm study, which will also evaluate the use of ibrutinib as part of induction, but with no autologous stem cell transplant.
“The trial is the first to randomize to a non-ASCT arm since the introduction of rituximab and cytarabine to the induction regimen and the results have the potential to significantly reduce chemotherapy intensity and toxicity,” the authors said.
Dr. Rule reported consulting for Pharmacyclics, Napp, Sunesis, Acerta Pharma, Kite, AstraZeneca, Roche, Janssen, and Celgene, and research funding from Janssen, Celgene, and GSK. Dr. McCulloch reported having no financial disclosures.
SOURCE: McCulloch R et al. Best Pract Res Clin Haematol. 2018 Mar;31(1):90-8.
FROM BEST PRACTICE & RESEARCH CLINICAL HAEMATOLOGY
Hepatic adenoma assessment: Wait longer to avoid overtreatment?
LAS VEGAS – For women with larger hepatic adenomas, current guidelines suggest reassessment at 6 months after oral contraceptive withdrawal to determine whether resection is warranted.
However, emerging data show reassessing at that time point may lead to overtreatment, according to Laura M. Kulik, MD, professor of medicine (gastroenterology and hepatology), radiology and surgery (organ transplantation), Northwestern University, Chicago.
“There’s been some controversy that 6 months may be too short,” Dr. Kulik said at the inaugural Perspectives in Digestive Diseases meeting held by Global Academy for Medical Education.
Unlike other benign liver lesions, hepatic adenomas can hemorrhage and transform to hepatocellular carcinoma. Current guidelines from the European Association for the Study of the Liver state that larger lesions (i.e., 5 cm or greater on baseline imaging) are associated with a higher risk of complications. According to one systematic review cited in the document, almost all cases of hemorrhage or spontaneous rupture occur in lesions 5 cm or larger.
Oral contraceptive use has been associated with a 30- to 40-fold increase in hepatic adenoma incidence, according to the guidelines.
All men with hepatic adenomas should undergo resection or curative treatment, the guidelines say, since they have a significantly higher risk of hepatocellular carcinoma.
By contrast, women with hepatic adenomas larger than 5 cm should discontinue oral contraceptives – which may lead to tumor regression in some cases – and should be reassessed 6 months later with contrast-enhanced MRI; if the lesion is still greater than 5 cm at that time, they should be considered for resection or curative treatment, the guidelines say.
However, authors of a retrospective cohort study have challenged that advice, suggesting that a 6-month follow-up may not always be long enough to see adequate tumor regression (HPB 2017 Apr;19[Suppl 1]:S3).
In the study, researchers from Erasmus MC University Medical Center, Rotterdam, the Netherlands, reviewed records for patients who were diagnosed with a hepatic adenoma of at least 5 cm and followed for at least 6 months after oral contraceptives were stopped.
Of that group, 104 underwent surgical treatment for a lesion larger than 5 cm, while the remaining 86 were conservatively treated.
The researchers found that in the conservatively treated group, 61 lesions (71%) regressed below the 5-cm cutoff after a median of 85 weeks (95% confidence interval, 52-110 weeks), with larger lesions taking significantly longer to regress.
Based on those findings, the investigators said the 6-month cutoff may lead to overtreatment, and that for some patients with particularly large tumors, it may be justified to wait up to 24 months.
“The study does suggest that, potentially, 6 months may be too short,” Dr. Kulik told attendees at the meeting. “They do caution that beta-catenin mutated adenomas should probably be removed without waiting longer because of the risk of developing cancer.”
Dr. Kulik reported disclosures related to Bayer, BMS, BTG, and Eisai. Global Academy and this news organization are owned by the same parent company.
LAS VEGAS – For women with larger hepatic adenomas, current guidelines suggest reassessment at 6 months after oral contraceptive withdrawal to determine whether resection is warranted.
However, emerging data show reassessing at that time point may lead to overtreatment, according to Laura M. Kulik, MD, professor of medicine (gastroenterology and hepatology), radiology and surgery (organ transplantation), Northwestern University, Chicago.
“There’s been some controversy that 6 months may be too short,” Dr. Kulik said at the inaugural Perspectives in Digestive Diseases meeting held by Global Academy for Medical Education.
Unlike other benign liver lesions, hepatic adenomas can hemorrhage and transform to hepatocellular carcinoma. Current guidelines from the European Association for the Study of the Liver state that larger lesions (i.e., 5 cm or greater on baseline imaging) are associated with a higher risk of complications. According to one systematic review cited in the document, almost all cases of hemorrhage or spontaneous rupture occur in lesions 5 cm or larger.
Oral contraceptive use has been associated with a 30- to 40-fold increase in hepatic adenoma incidence, according to the guidelines.
All men with hepatic adenomas should undergo resection or curative treatment, the guidelines say, since they have a significantly higher risk of hepatocellular carcinoma.
By contrast, women with hepatic adenomas larger than 5 cm should discontinue oral contraceptives – which may lead to tumor regression in some cases – and should be reassessed 6 months later with contrast-enhanced MRI; if the lesion is still greater than 5 cm at that time, they should be considered for resection or curative treatment, the guidelines say.
However, authors of a retrospective cohort study have challenged that advice, suggesting that a 6-month follow-up may not always be long enough to see adequate tumor regression (HPB 2017 Apr;19[Suppl 1]:S3).
In the study, researchers from Erasmus MC University Medical Center, Rotterdam, the Netherlands, reviewed records for patients who were diagnosed with a hepatic adenoma of at least 5 cm and followed for at least 6 months after oral contraceptives were stopped.
Of that group, 104 underwent surgical treatment for a lesion larger than 5 cm, while the remaining 86 were conservatively treated.
The researchers found that in the conservatively treated group, 61 lesions (71%) regressed below the 5-cm cutoff after a median of 85 weeks (95% confidence interval, 52-110 weeks), with larger lesions taking significantly longer to regress.
Based on those findings, the investigators said the 6-month cutoff may lead to overtreatment, and that for some patients with particularly large tumors, it may be justified to wait up to 24 months.
“The study does suggest that, potentially, 6 months may be too short,” Dr. Kulik told attendees at the meeting. “They do caution that beta-catenin mutated adenomas should probably be removed without waiting longer because of the risk of developing cancer.”
Dr. Kulik reported disclosures related to Bayer, BMS, BTG, and Eisai. Global Academy and this news organization are owned by the same parent company.
LAS VEGAS – For women with larger hepatic adenomas, current guidelines suggest reassessment at 6 months after oral contraceptive withdrawal to determine whether resection is warranted.
However, emerging data show reassessing at that time point may lead to overtreatment, according to Laura M. Kulik, MD, professor of medicine (gastroenterology and hepatology), radiology and surgery (organ transplantation), Northwestern University, Chicago.
“There’s been some controversy that 6 months may be too short,” Dr. Kulik said at the inaugural Perspectives in Digestive Diseases meeting held by Global Academy for Medical Education.
Unlike other benign liver lesions, hepatic adenomas can hemorrhage and transform to hepatocellular carcinoma. Current guidelines from the European Association for the Study of the Liver state that larger lesions (i.e., 5 cm or greater on baseline imaging) are associated with a higher risk of complications. According to one systematic review cited in the document, almost all cases of hemorrhage or spontaneous rupture occur in lesions 5 cm or larger.
Oral contraceptive use has been associated with a 30- to 40-fold increase in hepatic adenoma incidence, according to the guidelines.
All men with hepatic adenomas should undergo resection or curative treatment, the guidelines say, since they have a significantly higher risk of hepatocellular carcinoma.
By contrast, women with hepatic adenomas larger than 5 cm should discontinue oral contraceptives – which may lead to tumor regression in some cases – and should be reassessed 6 months later with contrast-enhanced MRI; if the lesion is still greater than 5 cm at that time, they should be considered for resection or curative treatment, the guidelines say.
However, authors of a retrospective cohort study have challenged that advice, suggesting that a 6-month follow-up may not always be long enough to see adequate tumor regression (HPB 2017 Apr;19[Suppl 1]:S3).
In the study, researchers from Erasmus MC University Medical Center, Rotterdam, the Netherlands, reviewed records for patients who were diagnosed with a hepatic adenoma of at least 5 cm and followed for at least 6 months after oral contraceptives were stopped.
Of that group, 104 underwent surgical treatment for a lesion larger than 5 cm, while the remaining 86 were conservatively treated.
The researchers found that in the conservatively treated group, 61 lesions (71%) regressed below the 5-cm cutoff after a median of 85 weeks (95% confidence interval, 52-110 weeks), with larger lesions taking significantly longer to regress.
Based on those findings, the investigators said the 6-month cutoff may lead to overtreatment, and that for some patients with particularly large tumors, it may be justified to wait up to 24 months.
“The study does suggest that, potentially, 6 months may be too short,” Dr. Kulik told attendees at the meeting. “They do caution that beta-catenin mutated adenomas should probably be removed without waiting longer because of the risk of developing cancer.”
Dr. Kulik reported disclosures related to Bayer, BMS, BTG, and Eisai. Global Academy and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM PERSPECTIVES IN DIGESTIVE DISEASES