User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
Methylphenidate Linked to Small Increase in CV Event Risk
TOPLINE:
Methylphenidate was associated with a small increased risk for cardiovascular events in individuals taking the drug for more than 6 months in a new cohort study.
METHODOLOGY:
- The retrospective, population-based cohort study was based on national Swedish registry data and included 26,710 patients with attention-deficit/hyperactivity disorder (ADHD) aged 12-60 years (median age 20) who had been prescribed methylphenidate between 2007 and 2012. They were each matched on birth date, sex, and county with up to 10 nonusers without ADHD (a total of 225,672 controls).
- Rates of cardiovascular events, including ischemic heart disease, venous thromboembolism, heart failure, or tachyarrhythmias 1 year before methylphenidate treatment and 6 months after treatment initiation were compared between individuals receiving methylphenidate and matched controls using a Bayesian within-individual design.
TAKEAWAY:
- The overall incidence of cardiovascular events was 1.51 per 10,000 person-weeks for individuals receiving methylphenidate and 0.77 for the matched controls.
- Individuals taking methylphenidate had a 70% posterior probability for a greater than 10% increased risk for cardiovascular events than controls and a 49% posterior probability for an increased risk larger than 20%.
- No difference was found in this risk between individuals with and without a history of cardiovascular disease.
IN PRACTICE:
The researchers concluded that these results support a small (10%) increased risk for cardiovascular events in individuals receiving methylphenidate compared with matched controls after 6 months of treatment. The probability of finding a difference in risk between users and nonusers decreased when considering risk for 20% or larger, with no evidence of differences between those with and without a history of cardiovascular disease. They said the findings suggest the decision to initiate methylphenidate should incorporate considerations of potential adverse cardiovascular effects among the broader benefits and risks for treatment for individual patients.
SOURCE:
The study, led by Miguel Garcia-Argibay, PhD, Örebro University, Örebro, Sweden, was published online in JAMA Network Open on March 6.
LIMITATIONS:
The data were observational, and thus, causality could not be inferred. Lack of information on methylphenidate dose meant that it was not possible to assess a dose effect. Compliance with the medication was also not known, and the association may therefore have been underestimated. The findings of this study were based on data collected from a Swedish population, which may not be representative of other populations.
DISCLOSURES:
The study received funding from the European Union’s Horizon 2020 research and innovation program and the Swedish Research Council for Health, Working Life, and Welfare.
A version of this article appeared on Medscape.com.
TOPLINE:
Methylphenidate was associated with a small increased risk for cardiovascular events in individuals taking the drug for more than 6 months in a new cohort study.
METHODOLOGY:
- The retrospective, population-based cohort study was based on national Swedish registry data and included 26,710 patients with attention-deficit/hyperactivity disorder (ADHD) aged 12-60 years (median age 20) who had been prescribed methylphenidate between 2007 and 2012. They were each matched on birth date, sex, and county with up to 10 nonusers without ADHD (a total of 225,672 controls).
- Rates of cardiovascular events, including ischemic heart disease, venous thromboembolism, heart failure, or tachyarrhythmias 1 year before methylphenidate treatment and 6 months after treatment initiation were compared between individuals receiving methylphenidate and matched controls using a Bayesian within-individual design.
TAKEAWAY:
- The overall incidence of cardiovascular events was 1.51 per 10,000 person-weeks for individuals receiving methylphenidate and 0.77 for the matched controls.
- Individuals taking methylphenidate had a 70% posterior probability for a greater than 10% increased risk for cardiovascular events than controls and a 49% posterior probability for an increased risk larger than 20%.
- No difference was found in this risk between individuals with and without a history of cardiovascular disease.
IN PRACTICE:
The researchers concluded that these results support a small (10%) increased risk for cardiovascular events in individuals receiving methylphenidate compared with matched controls after 6 months of treatment. The probability of finding a difference in risk between users and nonusers decreased when considering risk for 20% or larger, with no evidence of differences between those with and without a history of cardiovascular disease. They said the findings suggest the decision to initiate methylphenidate should incorporate considerations of potential adverse cardiovascular effects among the broader benefits and risks for treatment for individual patients.
SOURCE:
The study, led by Miguel Garcia-Argibay, PhD, Örebro University, Örebro, Sweden, was published online in JAMA Network Open on March 6.
LIMITATIONS:
The data were observational, and thus, causality could not be inferred. Lack of information on methylphenidate dose meant that it was not possible to assess a dose effect. Compliance with the medication was also not known, and the association may therefore have been underestimated. The findings of this study were based on data collected from a Swedish population, which may not be representative of other populations.
DISCLOSURES:
The study received funding from the European Union’s Horizon 2020 research and innovation program and the Swedish Research Council for Health, Working Life, and Welfare.
A version of this article appeared on Medscape.com.
TOPLINE:
Methylphenidate was associated with a small increased risk for cardiovascular events in individuals taking the drug for more than 6 months in a new cohort study.
METHODOLOGY:
- The retrospective, population-based cohort study was based on national Swedish registry data and included 26,710 patients with attention-deficit/hyperactivity disorder (ADHD) aged 12-60 years (median age 20) who had been prescribed methylphenidate between 2007 and 2012. They were each matched on birth date, sex, and county with up to 10 nonusers without ADHD (a total of 225,672 controls).
- Rates of cardiovascular events, including ischemic heart disease, venous thromboembolism, heart failure, or tachyarrhythmias 1 year before methylphenidate treatment and 6 months after treatment initiation were compared between individuals receiving methylphenidate and matched controls using a Bayesian within-individual design.
TAKEAWAY:
- The overall incidence of cardiovascular events was 1.51 per 10,000 person-weeks for individuals receiving methylphenidate and 0.77 for the matched controls.
- Individuals taking methylphenidate had a 70% posterior probability for a greater than 10% increased risk for cardiovascular events than controls and a 49% posterior probability for an increased risk larger than 20%.
- No difference was found in this risk between individuals with and without a history of cardiovascular disease.
IN PRACTICE:
The researchers concluded that these results support a small (10%) increased risk for cardiovascular events in individuals receiving methylphenidate compared with matched controls after 6 months of treatment. The probability of finding a difference in risk between users and nonusers decreased when considering risk for 20% or larger, with no evidence of differences between those with and without a history of cardiovascular disease. They said the findings suggest the decision to initiate methylphenidate should incorporate considerations of potential adverse cardiovascular effects among the broader benefits and risks for treatment for individual patients.
SOURCE:
The study, led by Miguel Garcia-Argibay, PhD, Örebro University, Örebro, Sweden, was published online in JAMA Network Open on March 6.
LIMITATIONS:
The data were observational, and thus, causality could not be inferred. Lack of information on methylphenidate dose meant that it was not possible to assess a dose effect. Compliance with the medication was also not known, and the association may therefore have been underestimated. The findings of this study were based on data collected from a Swedish population, which may not be representative of other populations.
DISCLOSURES:
The study received funding from the European Union’s Horizon 2020 research and innovation program and the Swedish Research Council for Health, Working Life, and Welfare.
A version of this article appeared on Medscape.com.
Help Patients Avoid Weight Gain After Stopping GLP-1s
Weight loss drugs have surged in popularity — in part because they work. Patients on glucagon-like peptide 1 (GLP-1) agonists like liraglutide, semaglutide, and tirzepatide (which is technically also a glucose-dependent insulinotropic polypeptide agonist) can lose 10%, 20%, or even 25% of their body weight.
But if those patients stop taking GLP-1s, they tend to regain most of that weight within a year, studies showed.
“These drugs work inside the person from a biologic point of view to alter appetite,” said Robert Kushner, MD, an endocrinologist and professor at Northwestern University Feinberg School of Medicine, Chicago, Illinois, who specializes in obesity medicine. “And when the drug is gone, that disease comes back.”
Often, “patients are told by their insurers that they are no longer going to cover a GLP-1 for obesity,” said Carolyn Bramante, MD, MPH, an assistant professor at the University of Minnesota Medical School, Minneapolis, Minnesota, who sees patients at the M Health Fairview weight management clinic.
Other barriers include side effects like nausea, diarrhea, stomach pain, and vomiting. Some patients simply don’t want to take a medication forever, instead choosing to take their chances keeping the weight off sans drug.
If your patient must stop GLP-1s, or really wants to, here’s how to help.
Find out why the patient wants to go off the GLP-1. Ask them to help you understand, suggested Jaime Almandoz, MD, associate professor of internal medicine and medical director of the University of Texas Southwestern Medical Center’s Weight Wellness Program. Sometimes, the patient or family members worry about safety, Dr. Almandoz said. “They may be concerned about the risks and may not have had an opportunity to ask questions.” Dr. Almandoz reviews the drug safety data and tells patients that studies show, on average, people gain back two-thirds of the weight they’ve lost within a year. You’re not trying to persuade them, only to equip them to make a well-informed choice.
Don’t let bias affect treatment decisions. Patients on GLP-1s often ask: How long will I have to take this? The reason: “We’re biased to believe that this is not a disease state, that this is a character flaw,” said Sean Wharton, MD, PharmD, medical director of the Wharton Medical Clinic for weight management in Burlington, Ontario, Canada. Remind your patient that obesity is not a personal failure but rather a complex mix of genetic and biological factors.
Give patients a primer on the biology of obesity. Science shows that when we lose weight, our bodies fight back, trying to return to our highest-ever fat mass. Changes in neurohormones, gut hormones, satiety mechanisms, metabolism, and muscle function all converge to promote weight recurrence, Dr. Almandoz said. To explain this to patients, Dr. Almandoz compares gaining fat to depositing money in a savings account. “When we try to lose weight, it isn’t as simple as withdrawing this money,” he’ll tell them. “It is almost like the money that we put into the savings account is now tied up in investments that we can’t liquidate easily.”
Prepare patients for an uptick in appetite. When patients stop GLP-1s, their hunger and food cravings tend to increase. “I explain that GLP-1 medications mimic a hormone that is released from our intestines when they sense we have eaten,” said Dr. Almandoz. This signals the brain and body that food is on board, decreasing appetite and cravings. Ask patients what hungry and full feel like on the medication, Dr. Almandoz suggested. “Many will report that their hunger and cravings are low, that they now have an indifference to foods,” said Dr. Almandoz. Such probing questions can help patients be more aware of the medication’s effects. “This positions a more informed conversation if medications are to be discontinued,” Dr. Almandoz said.
Help their body adjust. “Slowly wean down on the dose, if possible, to avoid a big rebound in hunger,” said Dr. Bramante. If your patient has the time — say, they received a letter from their insurance that coverage will end in 3 months — use it to taper the dose as low as possible before stopping. The slower and more gradual, the better. Dr. Almandoz checks in with patients every 4-8 weeks. If they›re maintaining weight well, he considers decreasing the dose again and repeating with follow-up visits.
Substitute one intervention for another. In general, maintaining weight loss requires some intervention, Dr. Wharton said. “But that intervention does not need to be the same as the intervention that got the weight down.” If the patient can›t continue a GLP-1, consider an alternate medication, cognitive behavioral therapy, or a combination of the two. When patients lose coverage for GLP-1s, Dr. Bramante sometimes prescribes an older, less-expensive weight loss drug, such as phentermine, topiramate, or metformin. And sometimes, insurers that don’t cover GLP-1s (like Medicare), do cover bariatric surgery, a potential option depending on the patient›s body mass index, overall health, and comorbidities, said Dr. Almandoz.
Create a habit template. Dr. Kushner asks patients who have successfully lost weight to take an inventory of everything they’re doing to support their efforts. He’ll have them describe how they plan their diet, what types of food they’re eating, how much they eat, and when they eat it. He’ll also ask about physical activity, exercise patterns, and sleep. He logs all the habits into a bulleted list in the patient’s after-visit summary and hands them a printout before they leave. “That’s your template,” he’ll tell them. “That’s what you’re going to try to maintain to the best of your ability because it’s working for you.”
Prescribe exercise. “Increasing exercise is not usually effective for initial weight loss, but it is important for maintaining weight loss,” said Dr. Bramante. Tell patients to start right away, ideally while they’re still on the drug. In a study published last month, patients on liraglutide (Saxenda) who exercised 4 days a week were much more likely to keep weight off after stopping the drug than those who didn’t work out. (The study was partially funded by Novo Nordisk Foundation, the charitable arm of Saxenda’s maker, also the maker of semaglutide meds Ozempic and Wegovy.) By establishing strong exercise habits while on the medication, they were able to sustain higher physical activity levels after they stopped. Ask your patient to identify someone or something to help them stick to their plan, “whether it’s seeing a personal trainer or being accountable to a friend or family member or to themselves through record keeping,” said Dr. Kushner. Learn more about how to prescribe exercise to patients here.
Help them create a “microenvironment” for success. Dr. Kushner asks patients which of the recommended dietary habits for weight loss are hardest to follow: Eating more plant-based foods? Cutting back on ultra-processed foods, fatty foods, fast foods, and/or sugary beverages? Depending on the patient’s answers, he tries to recommend strategies — maybe going meatless a few days a week or keeping tempting foods out of the house. “If you go off medication, food may become more enticing, and you may not feel as content eating less,” Dr. Kushner said. “Make sure your own what we call microenvironment, your home environment, is filled with healthy foods.”
Rely on multidisciplinary expertise. Obesity is a complex, multifactorial disease, so call in reinforcements. “When I see someone, I’m always evaluating what other team members they would benefit from,” said Dr. Kushner. If the patient lacks nutrition knowledge, he refers them to a registered dietitian. If they struggle with self-blame, low self-esteem, and emotional eating, he’ll refer them to a psychologist. It can make a difference: A 2023 study showed that people who lost weight and received support from professionals like trainers, dietitians, and mental health therapists regained less weight over 2 years than those who did not receive the same help.
Reassure patients you will help them no matter what. Ask patients to follow-up within the first month of quitting medication or to call back sooner if they gain 5 pounds. People who stop taking GLP-1s often report less satisfaction with eating, or that they think about food more. That’s when Dr. Kushner asks whether they want to go back on the medication or focus on other strategies. Sometimes, patients who gain weight feel embarrassed and delay their follow-up visits. If that happens, welcome them back and let them know that all chronic conditions ebb and flow. “I constantly remind them that I am here to help you, and there are many tools or resources that will help you,” Dr. Kushner said. “And dispel the notion that it’s somehow your fault.”
Dr. Kushner reported participation on the medical advisory board or consultancy with Novo Nordisk, WeightWatchers, Eli Lilly and Company, Boehringer Ingelheim, Structure Therapeutics, and Altimmune. He added he does not own stock or participate in any speaker’s bureau. Dr. Almandoz reported participation on advisory boards with Novo Nordisk, Boehringer Ingelheim, and Eli Lilly and Company. Dr. Wharton reported participation on advisory boards and honoraria for academic talks and clinical research with Novo Nordisk, Eli Lilly and Company, Boehringer Ingelheim, Amgen, Regeneron, and BioHaven.
A version of this article appeared on Medscape.com.
Weight loss drugs have surged in popularity — in part because they work. Patients on glucagon-like peptide 1 (GLP-1) agonists like liraglutide, semaglutide, and tirzepatide (which is technically also a glucose-dependent insulinotropic polypeptide agonist) can lose 10%, 20%, or even 25% of their body weight.
But if those patients stop taking GLP-1s, they tend to regain most of that weight within a year, studies showed.
“These drugs work inside the person from a biologic point of view to alter appetite,” said Robert Kushner, MD, an endocrinologist and professor at Northwestern University Feinberg School of Medicine, Chicago, Illinois, who specializes in obesity medicine. “And when the drug is gone, that disease comes back.”
Often, “patients are told by their insurers that they are no longer going to cover a GLP-1 for obesity,” said Carolyn Bramante, MD, MPH, an assistant professor at the University of Minnesota Medical School, Minneapolis, Minnesota, who sees patients at the M Health Fairview weight management clinic.
Other barriers include side effects like nausea, diarrhea, stomach pain, and vomiting. Some patients simply don’t want to take a medication forever, instead choosing to take their chances keeping the weight off sans drug.
If your patient must stop GLP-1s, or really wants to, here’s how to help.
Find out why the patient wants to go off the GLP-1. Ask them to help you understand, suggested Jaime Almandoz, MD, associate professor of internal medicine and medical director of the University of Texas Southwestern Medical Center’s Weight Wellness Program. Sometimes, the patient or family members worry about safety, Dr. Almandoz said. “They may be concerned about the risks and may not have had an opportunity to ask questions.” Dr. Almandoz reviews the drug safety data and tells patients that studies show, on average, people gain back two-thirds of the weight they’ve lost within a year. You’re not trying to persuade them, only to equip them to make a well-informed choice.
Don’t let bias affect treatment decisions. Patients on GLP-1s often ask: How long will I have to take this? The reason: “We’re biased to believe that this is not a disease state, that this is a character flaw,” said Sean Wharton, MD, PharmD, medical director of the Wharton Medical Clinic for weight management in Burlington, Ontario, Canada. Remind your patient that obesity is not a personal failure but rather a complex mix of genetic and biological factors.
Give patients a primer on the biology of obesity. Science shows that when we lose weight, our bodies fight back, trying to return to our highest-ever fat mass. Changes in neurohormones, gut hormones, satiety mechanisms, metabolism, and muscle function all converge to promote weight recurrence, Dr. Almandoz said. To explain this to patients, Dr. Almandoz compares gaining fat to depositing money in a savings account. “When we try to lose weight, it isn’t as simple as withdrawing this money,” he’ll tell them. “It is almost like the money that we put into the savings account is now tied up in investments that we can’t liquidate easily.”
Prepare patients for an uptick in appetite. When patients stop GLP-1s, their hunger and food cravings tend to increase. “I explain that GLP-1 medications mimic a hormone that is released from our intestines when they sense we have eaten,” said Dr. Almandoz. This signals the brain and body that food is on board, decreasing appetite and cravings. Ask patients what hungry and full feel like on the medication, Dr. Almandoz suggested. “Many will report that their hunger and cravings are low, that they now have an indifference to foods,” said Dr. Almandoz. Such probing questions can help patients be more aware of the medication’s effects. “This positions a more informed conversation if medications are to be discontinued,” Dr. Almandoz said.
Help their body adjust. “Slowly wean down on the dose, if possible, to avoid a big rebound in hunger,” said Dr. Bramante. If your patient has the time — say, they received a letter from their insurance that coverage will end in 3 months — use it to taper the dose as low as possible before stopping. The slower and more gradual, the better. Dr. Almandoz checks in with patients every 4-8 weeks. If they›re maintaining weight well, he considers decreasing the dose again and repeating with follow-up visits.
Substitute one intervention for another. In general, maintaining weight loss requires some intervention, Dr. Wharton said. “But that intervention does not need to be the same as the intervention that got the weight down.” If the patient can›t continue a GLP-1, consider an alternate medication, cognitive behavioral therapy, or a combination of the two. When patients lose coverage for GLP-1s, Dr. Bramante sometimes prescribes an older, less-expensive weight loss drug, such as phentermine, topiramate, or metformin. And sometimes, insurers that don’t cover GLP-1s (like Medicare), do cover bariatric surgery, a potential option depending on the patient›s body mass index, overall health, and comorbidities, said Dr. Almandoz.
Create a habit template. Dr. Kushner asks patients who have successfully lost weight to take an inventory of everything they’re doing to support their efforts. He’ll have them describe how they plan their diet, what types of food they’re eating, how much they eat, and when they eat it. He’ll also ask about physical activity, exercise patterns, and sleep. He logs all the habits into a bulleted list in the patient’s after-visit summary and hands them a printout before they leave. “That’s your template,” he’ll tell them. “That’s what you’re going to try to maintain to the best of your ability because it’s working for you.”
Prescribe exercise. “Increasing exercise is not usually effective for initial weight loss, but it is important for maintaining weight loss,” said Dr. Bramante. Tell patients to start right away, ideally while they’re still on the drug. In a study published last month, patients on liraglutide (Saxenda) who exercised 4 days a week were much more likely to keep weight off after stopping the drug than those who didn’t work out. (The study was partially funded by Novo Nordisk Foundation, the charitable arm of Saxenda’s maker, also the maker of semaglutide meds Ozempic and Wegovy.) By establishing strong exercise habits while on the medication, they were able to sustain higher physical activity levels after they stopped. Ask your patient to identify someone or something to help them stick to their plan, “whether it’s seeing a personal trainer or being accountable to a friend or family member or to themselves through record keeping,” said Dr. Kushner. Learn more about how to prescribe exercise to patients here.
Help them create a “microenvironment” for success. Dr. Kushner asks patients which of the recommended dietary habits for weight loss are hardest to follow: Eating more plant-based foods? Cutting back on ultra-processed foods, fatty foods, fast foods, and/or sugary beverages? Depending on the patient’s answers, he tries to recommend strategies — maybe going meatless a few days a week or keeping tempting foods out of the house. “If you go off medication, food may become more enticing, and you may not feel as content eating less,” Dr. Kushner said. “Make sure your own what we call microenvironment, your home environment, is filled with healthy foods.”
Rely on multidisciplinary expertise. Obesity is a complex, multifactorial disease, so call in reinforcements. “When I see someone, I’m always evaluating what other team members they would benefit from,” said Dr. Kushner. If the patient lacks nutrition knowledge, he refers them to a registered dietitian. If they struggle with self-blame, low self-esteem, and emotional eating, he’ll refer them to a psychologist. It can make a difference: A 2023 study showed that people who lost weight and received support from professionals like trainers, dietitians, and mental health therapists regained less weight over 2 years than those who did not receive the same help.
Reassure patients you will help them no matter what. Ask patients to follow-up within the first month of quitting medication or to call back sooner if they gain 5 pounds. People who stop taking GLP-1s often report less satisfaction with eating, or that they think about food more. That’s when Dr. Kushner asks whether they want to go back on the medication or focus on other strategies. Sometimes, patients who gain weight feel embarrassed and delay their follow-up visits. If that happens, welcome them back and let them know that all chronic conditions ebb and flow. “I constantly remind them that I am here to help you, and there are many tools or resources that will help you,” Dr. Kushner said. “And dispel the notion that it’s somehow your fault.”
Dr. Kushner reported participation on the medical advisory board or consultancy with Novo Nordisk, WeightWatchers, Eli Lilly and Company, Boehringer Ingelheim, Structure Therapeutics, and Altimmune. He added he does not own stock or participate in any speaker’s bureau. Dr. Almandoz reported participation on advisory boards with Novo Nordisk, Boehringer Ingelheim, and Eli Lilly and Company. Dr. Wharton reported participation on advisory boards and honoraria for academic talks and clinical research with Novo Nordisk, Eli Lilly and Company, Boehringer Ingelheim, Amgen, Regeneron, and BioHaven.
A version of this article appeared on Medscape.com.
Weight loss drugs have surged in popularity — in part because they work. Patients on glucagon-like peptide 1 (GLP-1) agonists like liraglutide, semaglutide, and tirzepatide (which is technically also a glucose-dependent insulinotropic polypeptide agonist) can lose 10%, 20%, or even 25% of their body weight.
But if those patients stop taking GLP-1s, they tend to regain most of that weight within a year, studies showed.
“These drugs work inside the person from a biologic point of view to alter appetite,” said Robert Kushner, MD, an endocrinologist and professor at Northwestern University Feinberg School of Medicine, Chicago, Illinois, who specializes in obesity medicine. “And when the drug is gone, that disease comes back.”
Often, “patients are told by their insurers that they are no longer going to cover a GLP-1 for obesity,” said Carolyn Bramante, MD, MPH, an assistant professor at the University of Minnesota Medical School, Minneapolis, Minnesota, who sees patients at the M Health Fairview weight management clinic.
Other barriers include side effects like nausea, diarrhea, stomach pain, and vomiting. Some patients simply don’t want to take a medication forever, instead choosing to take their chances keeping the weight off sans drug.
If your patient must stop GLP-1s, or really wants to, here’s how to help.
Find out why the patient wants to go off the GLP-1. Ask them to help you understand, suggested Jaime Almandoz, MD, associate professor of internal medicine and medical director of the University of Texas Southwestern Medical Center’s Weight Wellness Program. Sometimes, the patient or family members worry about safety, Dr. Almandoz said. “They may be concerned about the risks and may not have had an opportunity to ask questions.” Dr. Almandoz reviews the drug safety data and tells patients that studies show, on average, people gain back two-thirds of the weight they’ve lost within a year. You’re not trying to persuade them, only to equip them to make a well-informed choice.
Don’t let bias affect treatment decisions. Patients on GLP-1s often ask: How long will I have to take this? The reason: “We’re biased to believe that this is not a disease state, that this is a character flaw,” said Sean Wharton, MD, PharmD, medical director of the Wharton Medical Clinic for weight management in Burlington, Ontario, Canada. Remind your patient that obesity is not a personal failure but rather a complex mix of genetic and biological factors.
Give patients a primer on the biology of obesity. Science shows that when we lose weight, our bodies fight back, trying to return to our highest-ever fat mass. Changes in neurohormones, gut hormones, satiety mechanisms, metabolism, and muscle function all converge to promote weight recurrence, Dr. Almandoz said. To explain this to patients, Dr. Almandoz compares gaining fat to depositing money in a savings account. “When we try to lose weight, it isn’t as simple as withdrawing this money,” he’ll tell them. “It is almost like the money that we put into the savings account is now tied up in investments that we can’t liquidate easily.”
Prepare patients for an uptick in appetite. When patients stop GLP-1s, their hunger and food cravings tend to increase. “I explain that GLP-1 medications mimic a hormone that is released from our intestines when they sense we have eaten,” said Dr. Almandoz. This signals the brain and body that food is on board, decreasing appetite and cravings. Ask patients what hungry and full feel like on the medication, Dr. Almandoz suggested. “Many will report that their hunger and cravings are low, that they now have an indifference to foods,” said Dr. Almandoz. Such probing questions can help patients be more aware of the medication’s effects. “This positions a more informed conversation if medications are to be discontinued,” Dr. Almandoz said.
Help their body adjust. “Slowly wean down on the dose, if possible, to avoid a big rebound in hunger,” said Dr. Bramante. If your patient has the time — say, they received a letter from their insurance that coverage will end in 3 months — use it to taper the dose as low as possible before stopping. The slower and more gradual, the better. Dr. Almandoz checks in with patients every 4-8 weeks. If they›re maintaining weight well, he considers decreasing the dose again and repeating with follow-up visits.
Substitute one intervention for another. In general, maintaining weight loss requires some intervention, Dr. Wharton said. “But that intervention does not need to be the same as the intervention that got the weight down.” If the patient can›t continue a GLP-1, consider an alternate medication, cognitive behavioral therapy, or a combination of the two. When patients lose coverage for GLP-1s, Dr. Bramante sometimes prescribes an older, less-expensive weight loss drug, such as phentermine, topiramate, or metformin. And sometimes, insurers that don’t cover GLP-1s (like Medicare), do cover bariatric surgery, a potential option depending on the patient›s body mass index, overall health, and comorbidities, said Dr. Almandoz.
Create a habit template. Dr. Kushner asks patients who have successfully lost weight to take an inventory of everything they’re doing to support their efforts. He’ll have them describe how they plan their diet, what types of food they’re eating, how much they eat, and when they eat it. He’ll also ask about physical activity, exercise patterns, and sleep. He logs all the habits into a bulleted list in the patient’s after-visit summary and hands them a printout before they leave. “That’s your template,” he’ll tell them. “That’s what you’re going to try to maintain to the best of your ability because it’s working for you.”
Prescribe exercise. “Increasing exercise is not usually effective for initial weight loss, but it is important for maintaining weight loss,” said Dr. Bramante. Tell patients to start right away, ideally while they’re still on the drug. In a study published last month, patients on liraglutide (Saxenda) who exercised 4 days a week were much more likely to keep weight off after stopping the drug than those who didn’t work out. (The study was partially funded by Novo Nordisk Foundation, the charitable arm of Saxenda’s maker, also the maker of semaglutide meds Ozempic and Wegovy.) By establishing strong exercise habits while on the medication, they were able to sustain higher physical activity levels after they stopped. Ask your patient to identify someone or something to help them stick to their plan, “whether it’s seeing a personal trainer or being accountable to a friend or family member or to themselves through record keeping,” said Dr. Kushner. Learn more about how to prescribe exercise to patients here.
Help them create a “microenvironment” for success. Dr. Kushner asks patients which of the recommended dietary habits for weight loss are hardest to follow: Eating more plant-based foods? Cutting back on ultra-processed foods, fatty foods, fast foods, and/or sugary beverages? Depending on the patient’s answers, he tries to recommend strategies — maybe going meatless a few days a week or keeping tempting foods out of the house. “If you go off medication, food may become more enticing, and you may not feel as content eating less,” Dr. Kushner said. “Make sure your own what we call microenvironment, your home environment, is filled with healthy foods.”
Rely on multidisciplinary expertise. Obesity is a complex, multifactorial disease, so call in reinforcements. “When I see someone, I’m always evaluating what other team members they would benefit from,” said Dr. Kushner. If the patient lacks nutrition knowledge, he refers them to a registered dietitian. If they struggle with self-blame, low self-esteem, and emotional eating, he’ll refer them to a psychologist. It can make a difference: A 2023 study showed that people who lost weight and received support from professionals like trainers, dietitians, and mental health therapists regained less weight over 2 years than those who did not receive the same help.
Reassure patients you will help them no matter what. Ask patients to follow-up within the first month of quitting medication or to call back sooner if they gain 5 pounds. People who stop taking GLP-1s often report less satisfaction with eating, or that they think about food more. That’s when Dr. Kushner asks whether they want to go back on the medication or focus on other strategies. Sometimes, patients who gain weight feel embarrassed and delay their follow-up visits. If that happens, welcome them back and let them know that all chronic conditions ebb and flow. “I constantly remind them that I am here to help you, and there are many tools or resources that will help you,” Dr. Kushner said. “And dispel the notion that it’s somehow your fault.”
Dr. Kushner reported participation on the medical advisory board or consultancy with Novo Nordisk, WeightWatchers, Eli Lilly and Company, Boehringer Ingelheim, Structure Therapeutics, and Altimmune. He added he does not own stock or participate in any speaker’s bureau. Dr. Almandoz reported participation on advisory boards with Novo Nordisk, Boehringer Ingelheim, and Eli Lilly and Company. Dr. Wharton reported participation on advisory boards and honoraria for academic talks and clinical research with Novo Nordisk, Eli Lilly and Company, Boehringer Ingelheim, Amgen, Regeneron, and BioHaven.
A version of this article appeared on Medscape.com.
Ginger, Cinnamon, Cumin Improve Glycemic Control
TOPLINE:
The spices and aromatic herbs of the Mediterranean diet with significant benefits in improving glycemic health in type 2 diabetes are limited to ginger, cinnamon, black cumin, turmeric, and saffron, with ginger, black cumin, and cinnamon having the strongest effects on fasting glucose, according to a systematic review and meta-analysis of research.
The meta-analysis also evaluated clove, thyme, turmeric, and various other spices and herbs common in the diet but showed no other correlations with glycemic benefits.
METHODOLOGY:
- In the analysis of 77 studies, 45, involving 3050 participants, were included in the meta-analysis and 32 studies in the systematic review.
- The studies’ inclusion criteria included adult patients with type 2 diabetes, with data on fasting glucose and/or A1c and/or , and involving any supplementation with black cumin, clove, , saffron, thyme, ginger, black pepper, , curcumin, cinnamon, basil, and/or oregano.
- The number of studies involving clove, parsley, thyme, black pepper, rosemary, basil, or oregano and their association with glycemic factors in people with type 2 diabetes was insufficient, hence the analysis primarily focused on the remaining five ingredients of cinnamon, curcumin, ginger, black cumin, saffron, and rosemary.
TAKEAWAY:
- However, the most significant decreases in fasting glucose, between 17 mg/dL and 27 mg/dL, occurred after supplementation with black cumin, followed by cinnamon and ginger.
- Notably, only ginger and black cumin were associated with a significant improvement in A1c.
- Only cinnamon and ginger were associated with a significant decrease in insulin values.
- Of the 11 studies including cinnamon in the meta-analysis, 6 reported significant differences in fasting glucose, while 4 had differences in A1c after the supplementation.
- However, ginger was the only component associated with a significant decrease in each of the 3 outcomes examined of fasting glucose, A1c, and insulin.
IN PRACTICE:
“The Mediterranean Diet is the dietary pattern par excellence for managing and preventing metabolic diseases, such as type 2 diabetes,” the authors reported.
“As far as we are aware, this is the first systematic review and meta-analysis aiming to evaluate the effect of aromatic herbs and spices included in the Mediterranean Diet, such as black cumin, clove [and others], on the glycemic profile of individuals with type 2 diabetes,” they added.
“When focusing on HbA1c, only ginger and black cumin demonstrated therapeutic effects,” the authors noted. “However, our meta-analysis highlights ginger as an herb with substantial translational potential for diabetes treatment, impacting all three glycemic parameters.”
“Regarding clove, parsley, thyme, black pepper, rosemary, basil, and oregano, more studies are needed to analyze the effect of these herbs on the glycemic profile in type 2 diabetes subjects,” the authors concluded.
SOURCE:
The study was published on March 7, 2024, in Nutrients. The first author was Maria Carmen Garza, PhD, of the Department of Human Anatomy and Histology, School Medicine, University of Zaragoza, Zaragoza, Spain.
LIMITATIONS:
Despite the results, a variety of other factors can affect fasting glucose levels, including changes in body weight or body mass index, as well as the combination of spice or aromatic herb supplementation with physical activity or lifestyle changes, the authors noted.
Due to the studies’ differences, the determination of effective dosages of the herbs and spices was not possible.
Furthermore, the studies had wide variations in quality, with few studies including adequate statistical analysis.
DISCLOSURES:
The authors had no disclosures to report.
A version of this article appeared on Medscape.com.
TOPLINE:
The spices and aromatic herbs of the Mediterranean diet with significant benefits in improving glycemic health in type 2 diabetes are limited to ginger, cinnamon, black cumin, turmeric, and saffron, with ginger, black cumin, and cinnamon having the strongest effects on fasting glucose, according to a systematic review and meta-analysis of research.
The meta-analysis also evaluated clove, thyme, turmeric, and various other spices and herbs common in the diet but showed no other correlations with glycemic benefits.
METHODOLOGY:
- In the analysis of 77 studies, 45, involving 3050 participants, were included in the meta-analysis and 32 studies in the systematic review.
- The studies’ inclusion criteria included adult patients with type 2 diabetes, with data on fasting glucose and/or A1c and/or , and involving any supplementation with black cumin, clove, , saffron, thyme, ginger, black pepper, , curcumin, cinnamon, basil, and/or oregano.
- The number of studies involving clove, parsley, thyme, black pepper, rosemary, basil, or oregano and their association with glycemic factors in people with type 2 diabetes was insufficient, hence the analysis primarily focused on the remaining five ingredients of cinnamon, curcumin, ginger, black cumin, saffron, and rosemary.
TAKEAWAY:
- However, the most significant decreases in fasting glucose, between 17 mg/dL and 27 mg/dL, occurred after supplementation with black cumin, followed by cinnamon and ginger.
- Notably, only ginger and black cumin were associated with a significant improvement in A1c.
- Only cinnamon and ginger were associated with a significant decrease in insulin values.
- Of the 11 studies including cinnamon in the meta-analysis, 6 reported significant differences in fasting glucose, while 4 had differences in A1c after the supplementation.
- However, ginger was the only component associated with a significant decrease in each of the 3 outcomes examined of fasting glucose, A1c, and insulin.
IN PRACTICE:
“The Mediterranean Diet is the dietary pattern par excellence for managing and preventing metabolic diseases, such as type 2 diabetes,” the authors reported.
“As far as we are aware, this is the first systematic review and meta-analysis aiming to evaluate the effect of aromatic herbs and spices included in the Mediterranean Diet, such as black cumin, clove [and others], on the glycemic profile of individuals with type 2 diabetes,” they added.
“When focusing on HbA1c, only ginger and black cumin demonstrated therapeutic effects,” the authors noted. “However, our meta-analysis highlights ginger as an herb with substantial translational potential for diabetes treatment, impacting all three glycemic parameters.”
“Regarding clove, parsley, thyme, black pepper, rosemary, basil, and oregano, more studies are needed to analyze the effect of these herbs on the glycemic profile in type 2 diabetes subjects,” the authors concluded.
SOURCE:
The study was published on March 7, 2024, in Nutrients. The first author was Maria Carmen Garza, PhD, of the Department of Human Anatomy and Histology, School Medicine, University of Zaragoza, Zaragoza, Spain.
LIMITATIONS:
Despite the results, a variety of other factors can affect fasting glucose levels, including changes in body weight or body mass index, as well as the combination of spice or aromatic herb supplementation with physical activity or lifestyle changes, the authors noted.
Due to the studies’ differences, the determination of effective dosages of the herbs and spices was not possible.
Furthermore, the studies had wide variations in quality, with few studies including adequate statistical analysis.
DISCLOSURES:
The authors had no disclosures to report.
A version of this article appeared on Medscape.com.
TOPLINE:
The spices and aromatic herbs of the Mediterranean diet with significant benefits in improving glycemic health in type 2 diabetes are limited to ginger, cinnamon, black cumin, turmeric, and saffron, with ginger, black cumin, and cinnamon having the strongest effects on fasting glucose, according to a systematic review and meta-analysis of research.
The meta-analysis also evaluated clove, thyme, turmeric, and various other spices and herbs common in the diet but showed no other correlations with glycemic benefits.
METHODOLOGY:
- In the analysis of 77 studies, 45, involving 3050 participants, were included in the meta-analysis and 32 studies in the systematic review.
- The studies’ inclusion criteria included adult patients with type 2 diabetes, with data on fasting glucose and/or A1c and/or , and involving any supplementation with black cumin, clove, , saffron, thyme, ginger, black pepper, , curcumin, cinnamon, basil, and/or oregano.
- The number of studies involving clove, parsley, thyme, black pepper, rosemary, basil, or oregano and their association with glycemic factors in people with type 2 diabetes was insufficient, hence the analysis primarily focused on the remaining five ingredients of cinnamon, curcumin, ginger, black cumin, saffron, and rosemary.
TAKEAWAY:
- However, the most significant decreases in fasting glucose, between 17 mg/dL and 27 mg/dL, occurred after supplementation with black cumin, followed by cinnamon and ginger.
- Notably, only ginger and black cumin were associated with a significant improvement in A1c.
- Only cinnamon and ginger were associated with a significant decrease in insulin values.
- Of the 11 studies including cinnamon in the meta-analysis, 6 reported significant differences in fasting glucose, while 4 had differences in A1c after the supplementation.
- However, ginger was the only component associated with a significant decrease in each of the 3 outcomes examined of fasting glucose, A1c, and insulin.
IN PRACTICE:
“The Mediterranean Diet is the dietary pattern par excellence for managing and preventing metabolic diseases, such as type 2 diabetes,” the authors reported.
“As far as we are aware, this is the first systematic review and meta-analysis aiming to evaluate the effect of aromatic herbs and spices included in the Mediterranean Diet, such as black cumin, clove [and others], on the glycemic profile of individuals with type 2 diabetes,” they added.
“When focusing on HbA1c, only ginger and black cumin demonstrated therapeutic effects,” the authors noted. “However, our meta-analysis highlights ginger as an herb with substantial translational potential for diabetes treatment, impacting all three glycemic parameters.”
“Regarding clove, parsley, thyme, black pepper, rosemary, basil, and oregano, more studies are needed to analyze the effect of these herbs on the glycemic profile in type 2 diabetes subjects,” the authors concluded.
SOURCE:
The study was published on March 7, 2024, in Nutrients. The first author was Maria Carmen Garza, PhD, of the Department of Human Anatomy and Histology, School Medicine, University of Zaragoza, Zaragoza, Spain.
LIMITATIONS:
Despite the results, a variety of other factors can affect fasting glucose levels, including changes in body weight or body mass index, as well as the combination of spice or aromatic herb supplementation with physical activity or lifestyle changes, the authors noted.
Due to the studies’ differences, the determination of effective dosages of the herbs and spices was not possible.
Furthermore, the studies had wide variations in quality, with few studies including adequate statistical analysis.
DISCLOSURES:
The authors had no disclosures to report.
A version of this article appeared on Medscape.com.
Can Treating Depression Mitigate CVD Risk?
TOPLINE:
Depression is linked to a significantly increased risk for cardiovascular disease (CVD), particularly in women, new data from a large retrospective cohort study show.
METHODOLOGY:
- Researchers analyzed health insurance claims from more than 4 million Japanese patients filed between 2005 and 2022.
- Participants were 18-75 (median age, 44) without a history of CVD or stroke, heart failure, or atrial fibrillation.
- Investigators followed participants for a mean period of 2.5-3.5 years to observe the number of CVD events in those who had a diagnosis of depression.
- During the follow-up period, there were 119,000 CVD events in men (14 per 10,000 person-years) and 61,800 CVD events in women (111 per 10,000 person-years).
TAKEAWAY:
- Compared with women without depression, those with depression had a 64% higher risk for CVD (hazard ratio [HR], 1.64), while men with depression had a 39% higher risk for CVD vs their counterparts without depression (HR, 1.39; P < .001).
- This association was significant even after controlling for various factors such as body mass index, diabetes, smoking, alcohol consumption, and physical inactivity.
- Investigators offered several theories about the increased risk for CVD in women with depression, including how depression during hormonal shifts can contribute to a greater impact on cardiovascular health.
IN PRACTICE:
“Healthcare professionals must recognize the important role of depression in the development of CVD and emphasize the importance of a comprehensive, patient-centered approach to its prevention and management,” study author Hidehiro Kaneko, MD, said in a press release. “Assessing the risk of CVD in depressed patients and treating and preventing depression may lead to a decrease of CVD cases.”
SOURCE:
Keitaro Senoo, MD, of the Kyoto Prefectural University of Medicine, Kyoto, Japan, led the study, which was published online on March 12 in JACC: Asia.
LIMITATIONS:
The study is observational, so causality between depression and subsequent CVD events cannot be established. In addition, depression severity is unknown.
DISCLOSURES:
The study was funded by the Ministry of Health, Labour, and Welfare, Japan, and the Ministry of Education, Culture, Sports, Science, and Technology, Japan. There were no disclosures reported.
A version of this article appeared on Medscape.com.
TOPLINE:
Depression is linked to a significantly increased risk for cardiovascular disease (CVD), particularly in women, new data from a large retrospective cohort study show.
METHODOLOGY:
- Researchers analyzed health insurance claims from more than 4 million Japanese patients filed between 2005 and 2022.
- Participants were 18-75 (median age, 44) without a history of CVD or stroke, heart failure, or atrial fibrillation.
- Investigators followed participants for a mean period of 2.5-3.5 years to observe the number of CVD events in those who had a diagnosis of depression.
- During the follow-up period, there were 119,000 CVD events in men (14 per 10,000 person-years) and 61,800 CVD events in women (111 per 10,000 person-years).
TAKEAWAY:
- Compared with women without depression, those with depression had a 64% higher risk for CVD (hazard ratio [HR], 1.64), while men with depression had a 39% higher risk for CVD vs their counterparts without depression (HR, 1.39; P < .001).
- This association was significant even after controlling for various factors such as body mass index, diabetes, smoking, alcohol consumption, and physical inactivity.
- Investigators offered several theories about the increased risk for CVD in women with depression, including how depression during hormonal shifts can contribute to a greater impact on cardiovascular health.
IN PRACTICE:
“Healthcare professionals must recognize the important role of depression in the development of CVD and emphasize the importance of a comprehensive, patient-centered approach to its prevention and management,” study author Hidehiro Kaneko, MD, said in a press release. “Assessing the risk of CVD in depressed patients and treating and preventing depression may lead to a decrease of CVD cases.”
SOURCE:
Keitaro Senoo, MD, of the Kyoto Prefectural University of Medicine, Kyoto, Japan, led the study, which was published online on March 12 in JACC: Asia.
LIMITATIONS:
The study is observational, so causality between depression and subsequent CVD events cannot be established. In addition, depression severity is unknown.
DISCLOSURES:
The study was funded by the Ministry of Health, Labour, and Welfare, Japan, and the Ministry of Education, Culture, Sports, Science, and Technology, Japan. There were no disclosures reported.
A version of this article appeared on Medscape.com.
TOPLINE:
Depression is linked to a significantly increased risk for cardiovascular disease (CVD), particularly in women, new data from a large retrospective cohort study show.
METHODOLOGY:
- Researchers analyzed health insurance claims from more than 4 million Japanese patients filed between 2005 and 2022.
- Participants were 18-75 (median age, 44) without a history of CVD or stroke, heart failure, or atrial fibrillation.
- Investigators followed participants for a mean period of 2.5-3.5 years to observe the number of CVD events in those who had a diagnosis of depression.
- During the follow-up period, there were 119,000 CVD events in men (14 per 10,000 person-years) and 61,800 CVD events in women (111 per 10,000 person-years).
TAKEAWAY:
- Compared with women without depression, those with depression had a 64% higher risk for CVD (hazard ratio [HR], 1.64), while men with depression had a 39% higher risk for CVD vs their counterparts without depression (HR, 1.39; P < .001).
- This association was significant even after controlling for various factors such as body mass index, diabetes, smoking, alcohol consumption, and physical inactivity.
- Investigators offered several theories about the increased risk for CVD in women with depression, including how depression during hormonal shifts can contribute to a greater impact on cardiovascular health.
IN PRACTICE:
“Healthcare professionals must recognize the important role of depression in the development of CVD and emphasize the importance of a comprehensive, patient-centered approach to its prevention and management,” study author Hidehiro Kaneko, MD, said in a press release. “Assessing the risk of CVD in depressed patients and treating and preventing depression may lead to a decrease of CVD cases.”
SOURCE:
Keitaro Senoo, MD, of the Kyoto Prefectural University of Medicine, Kyoto, Japan, led the study, which was published online on March 12 in JACC: Asia.
LIMITATIONS:
The study is observational, so causality between depression and subsequent CVD events cannot be established. In addition, depression severity is unknown.
DISCLOSURES:
The study was funded by the Ministry of Health, Labour, and Welfare, Japan, and the Ministry of Education, Culture, Sports, Science, and Technology, Japan. There were no disclosures reported.
A version of this article appeared on Medscape.com.
Topical Roflumilast Effective in 4 Weeks for Atopic Dermatitis in Young Children
SAN DIEGO — Treatment with (AD), according to the results of a phase 3 study reported at the annual meeting of the American Academy of Dermatology.
Among patients treated with roflumilast cream, 0.05%, 25.4% reached the primary endpoint of “clear” or “almost clear” plus a two-grade improvement from baseline at week 4 vs 10.7% among those in the vehicle group (P < .0001) in a phase 3 randomized controlled trial of children. The findings were released in a late-breaker session at the meeting.
Roflumilast cream, 0.3% (Zoryve), is approved by the Food and Drug Administration (FDA) for treating psoriasis in patients 6 years and older, and lower doses are being evaluated for AD: 0.15% for adults and children ages 6 and older, and 0.05% for ages 2-5. Roflumilast is a phosphodiesterase-4 inhibitor. In 2023, the FDA accepted a supplemental drug application from the manufacturer, Arcutis, for roflumilast, 0.15%, for treating AD in patients ages 6 and older, based on the results from two recently published phase 3 trials, INTEGUMENT-1 and INTEGUMENT-2.
The study of younger children, INTEGUMENT-PED, recruited 652 patients aged 2-5 with mild to moderate AD, with a Validated Investigator Global Assessment scale for AD (vlGA-AD) score of 2 or 3, a mean body surface area of 22% overall (range, 3%-82%), and an Eczema Area and Severity Index (EASI) score of at least 5. Of the patients enrolled, 437 were assigned to 0.05% roflumilast cream, applied once a day for 4 weeks (mean age, 3.3 years; 51.6% male; 67.4% White; 15.6% Black; 8.5% Asian; 8.5% other or more than one race; 80.5% not Latino/Hispanic). The remaining 215 children were assigned to vehicle cream and had similar characteristics.
About 52% of the patients in both groups had an inadequate response, intolerance, or contraindications to topical corticosteroids (and about 17% for topical calcineurin inhibitors and about 9% for crisaborole).
The proportions of patients who reached “clear” (0) or “almost clear” (1) on the vlGA-AD scale were 35.4% and 14.6%, respectively, at week 4 (P < .0001) for roflumilast and vehicle, respectively, according to the lead author of the study, Lawrence M. Eichenfield, MD, professor of dermatology and pediatrics at the University of California, San Diego, who presented the results at the meeting. In addition, 39.4% and 20.6% achieved an EASI-75 (a secondary endpoint), respectively (P < .0001), and itch also improved within 24 hours of starting treatment.
With regard to safety, 29.7% of patients taking roflumilast had treatment-emergent adverse effects (including upper respiratory tract infections in 4.1%) vs 21.9% of those in the vehicle arm (including upper respiratory tract infections in 1.4%). Reports of pain at the administration site were low (1.6% for roflumilast vs 1.9% for vehicle). Only one patient, a 2-year-old girl, had a treatment-emergent serious adverse event. The child, who was in the roflumilast group, had cellulitis involving noneczematous skin and was treated with antibiotics in the hospital for 3 days. The event was not attributed to roflumilast, which was stopped for 5 days, according to Dr. Eichenfield.
In an interview, Fairfield, Connecticut–based dermatologist Brittany Craiglow, MD, who was not involved in the study, said topical roflumilast would be an “important” new treatment because there are still few nonsteroidal options for the treatment of AD in children under 12. “The excellent local tolerability combined with early improvements in itch and skin clearance will make this a particularly attractive option, if approved,” she said.
Dr. Eichenfield disclosed multiple relationships with various drugmakers. He and several other study authors are investigators and/or consultants for Arcutis and received grants/research funding and/or honoraria. Two authors are Arcutis employees. Other disclosure information for the authors was not immediately available. Dr. Craiglow had no disclosures.
A version of this article appeared on Medscape.com .
SAN DIEGO — Treatment with (AD), according to the results of a phase 3 study reported at the annual meeting of the American Academy of Dermatology.
Among patients treated with roflumilast cream, 0.05%, 25.4% reached the primary endpoint of “clear” or “almost clear” plus a two-grade improvement from baseline at week 4 vs 10.7% among those in the vehicle group (P < .0001) in a phase 3 randomized controlled trial of children. The findings were released in a late-breaker session at the meeting.
Roflumilast cream, 0.3% (Zoryve), is approved by the Food and Drug Administration (FDA) for treating psoriasis in patients 6 years and older, and lower doses are being evaluated for AD: 0.15% for adults and children ages 6 and older, and 0.05% for ages 2-5. Roflumilast is a phosphodiesterase-4 inhibitor. In 2023, the FDA accepted a supplemental drug application from the manufacturer, Arcutis, for roflumilast, 0.15%, for treating AD in patients ages 6 and older, based on the results from two recently published phase 3 trials, INTEGUMENT-1 and INTEGUMENT-2.
The study of younger children, INTEGUMENT-PED, recruited 652 patients aged 2-5 with mild to moderate AD, with a Validated Investigator Global Assessment scale for AD (vlGA-AD) score of 2 or 3, a mean body surface area of 22% overall (range, 3%-82%), and an Eczema Area and Severity Index (EASI) score of at least 5. Of the patients enrolled, 437 were assigned to 0.05% roflumilast cream, applied once a day for 4 weeks (mean age, 3.3 years; 51.6% male; 67.4% White; 15.6% Black; 8.5% Asian; 8.5% other or more than one race; 80.5% not Latino/Hispanic). The remaining 215 children were assigned to vehicle cream and had similar characteristics.
About 52% of the patients in both groups had an inadequate response, intolerance, or contraindications to topical corticosteroids (and about 17% for topical calcineurin inhibitors and about 9% for crisaborole).
The proportions of patients who reached “clear” (0) or “almost clear” (1) on the vlGA-AD scale were 35.4% and 14.6%, respectively, at week 4 (P < .0001) for roflumilast and vehicle, respectively, according to the lead author of the study, Lawrence M. Eichenfield, MD, professor of dermatology and pediatrics at the University of California, San Diego, who presented the results at the meeting. In addition, 39.4% and 20.6% achieved an EASI-75 (a secondary endpoint), respectively (P < .0001), and itch also improved within 24 hours of starting treatment.
With regard to safety, 29.7% of patients taking roflumilast had treatment-emergent adverse effects (including upper respiratory tract infections in 4.1%) vs 21.9% of those in the vehicle arm (including upper respiratory tract infections in 1.4%). Reports of pain at the administration site were low (1.6% for roflumilast vs 1.9% for vehicle). Only one patient, a 2-year-old girl, had a treatment-emergent serious adverse event. The child, who was in the roflumilast group, had cellulitis involving noneczematous skin and was treated with antibiotics in the hospital for 3 days. The event was not attributed to roflumilast, which was stopped for 5 days, according to Dr. Eichenfield.
In an interview, Fairfield, Connecticut–based dermatologist Brittany Craiglow, MD, who was not involved in the study, said topical roflumilast would be an “important” new treatment because there are still few nonsteroidal options for the treatment of AD in children under 12. “The excellent local tolerability combined with early improvements in itch and skin clearance will make this a particularly attractive option, if approved,” she said.
Dr. Eichenfield disclosed multiple relationships with various drugmakers. He and several other study authors are investigators and/or consultants for Arcutis and received grants/research funding and/or honoraria. Two authors are Arcutis employees. Other disclosure information for the authors was not immediately available. Dr. Craiglow had no disclosures.
A version of this article appeared on Medscape.com .
SAN DIEGO — Treatment with (AD), according to the results of a phase 3 study reported at the annual meeting of the American Academy of Dermatology.
Among patients treated with roflumilast cream, 0.05%, 25.4% reached the primary endpoint of “clear” or “almost clear” plus a two-grade improvement from baseline at week 4 vs 10.7% among those in the vehicle group (P < .0001) in a phase 3 randomized controlled trial of children. The findings were released in a late-breaker session at the meeting.
Roflumilast cream, 0.3% (Zoryve), is approved by the Food and Drug Administration (FDA) for treating psoriasis in patients 6 years and older, and lower doses are being evaluated for AD: 0.15% for adults and children ages 6 and older, and 0.05% for ages 2-5. Roflumilast is a phosphodiesterase-4 inhibitor. In 2023, the FDA accepted a supplemental drug application from the manufacturer, Arcutis, for roflumilast, 0.15%, for treating AD in patients ages 6 and older, based on the results from two recently published phase 3 trials, INTEGUMENT-1 and INTEGUMENT-2.
The study of younger children, INTEGUMENT-PED, recruited 652 patients aged 2-5 with mild to moderate AD, with a Validated Investigator Global Assessment scale for AD (vlGA-AD) score of 2 or 3, a mean body surface area of 22% overall (range, 3%-82%), and an Eczema Area and Severity Index (EASI) score of at least 5. Of the patients enrolled, 437 were assigned to 0.05% roflumilast cream, applied once a day for 4 weeks (mean age, 3.3 years; 51.6% male; 67.4% White; 15.6% Black; 8.5% Asian; 8.5% other or more than one race; 80.5% not Latino/Hispanic). The remaining 215 children were assigned to vehicle cream and had similar characteristics.
About 52% of the patients in both groups had an inadequate response, intolerance, or contraindications to topical corticosteroids (and about 17% for topical calcineurin inhibitors and about 9% for crisaborole).
The proportions of patients who reached “clear” (0) or “almost clear” (1) on the vlGA-AD scale were 35.4% and 14.6%, respectively, at week 4 (P < .0001) for roflumilast and vehicle, respectively, according to the lead author of the study, Lawrence M. Eichenfield, MD, professor of dermatology and pediatrics at the University of California, San Diego, who presented the results at the meeting. In addition, 39.4% and 20.6% achieved an EASI-75 (a secondary endpoint), respectively (P < .0001), and itch also improved within 24 hours of starting treatment.
With regard to safety, 29.7% of patients taking roflumilast had treatment-emergent adverse effects (including upper respiratory tract infections in 4.1%) vs 21.9% of those in the vehicle arm (including upper respiratory tract infections in 1.4%). Reports of pain at the administration site were low (1.6% for roflumilast vs 1.9% for vehicle). Only one patient, a 2-year-old girl, had a treatment-emergent serious adverse event. The child, who was in the roflumilast group, had cellulitis involving noneczematous skin and was treated with antibiotics in the hospital for 3 days. The event was not attributed to roflumilast, which was stopped for 5 days, according to Dr. Eichenfield.
In an interview, Fairfield, Connecticut–based dermatologist Brittany Craiglow, MD, who was not involved in the study, said topical roflumilast would be an “important” new treatment because there are still few nonsteroidal options for the treatment of AD in children under 12. “The excellent local tolerability combined with early improvements in itch and skin clearance will make this a particularly attractive option, if approved,” she said.
Dr. Eichenfield disclosed multiple relationships with various drugmakers. He and several other study authors are investigators and/or consultants for Arcutis and received grants/research funding and/or honoraria. Two authors are Arcutis employees. Other disclosure information for the authors was not immediately available. Dr. Craiglow had no disclosures.
A version of this article appeared on Medscape.com .
FROM AAD 2024
New Research Dissects Transgenerational Obesity and Diabetes
FAIRFAX, VIRGINIA — Nearly 30 years ago, in a 1995 paper, the British physician-epidemiologist David Barker, MD, PhD, wrote about his fetal origins hypothesis — the idea that programs to address fetal undernutrition and low birth weight produced later coronary heart disease (BMJ 1995;311:171-4).
His hypothesis and subsequent research led to the concept of adult diseases of fetal origins, which today extends beyond low birth weight and implicates the in utero environment as a significant determinant of risk for adverse childhood and adult metabolic outcomes and for major chronic diseases, including diabetes and obesity. Studies have shown that the offspring of pregnant mothers with diabetes have a higher risk of developing obesity and diabetes themselves.
“It’s a whole discipline [of research],” E. Albert Reece, MD, PhD, MBA, of the University of Maryland School of Medicine (UMSOM), said in an interview. “But what we’ve never quite understood is the ‘how’ and ‘why’? What are the mechanisms driving the fetal origins of such adverse outcomes in offspring?
At the biennial meeting of the Diabetes in Pregnancy Study Group of North America (DPSG), investigators described studies underway that are digging deeper into the associations between the intrauterine milieu and longer-term offspring health — and that are searching for biological and molecular processes that may be involved.
The studies are like “branches of the Barker hypothesis,” said Dr. Reece, former dean of UMSOM and current director of the UMSOM Center for Advanced Research Training and Innovation, who co-organized the DPSG meeting. “They’re taking the hypothesis and dissecting it by asking, for instance, it is possible that transgenerational obesity may align with the Barker hypothesis? Is it possible that it involves epigenetics regulation? Could we find biomarkers?”
The need for a better understanding of the fetal origins framework — and its subsequent transgenerational impact — is urgent. From 2000 to 2018, the prevalence of childhood obesity increased from 14.7% to 19.2% (a 31% increase) and the prevalence of severe childhood obesity rose from 3.9% to 6.1% (a 56% increase), according to data from the U.S. National Health and Nutrition Examination Survey (Obes Facts. 2022;15[4]:560-9).
Children aged 2-5 years have had an especially sharp increase in obesity (Pediatrics 2018;141[3]:e20173459), Christine Wey Hockett, PhD, of the University of South Dakota School of Medicine, said at the DPSG meeting (Figure 1). 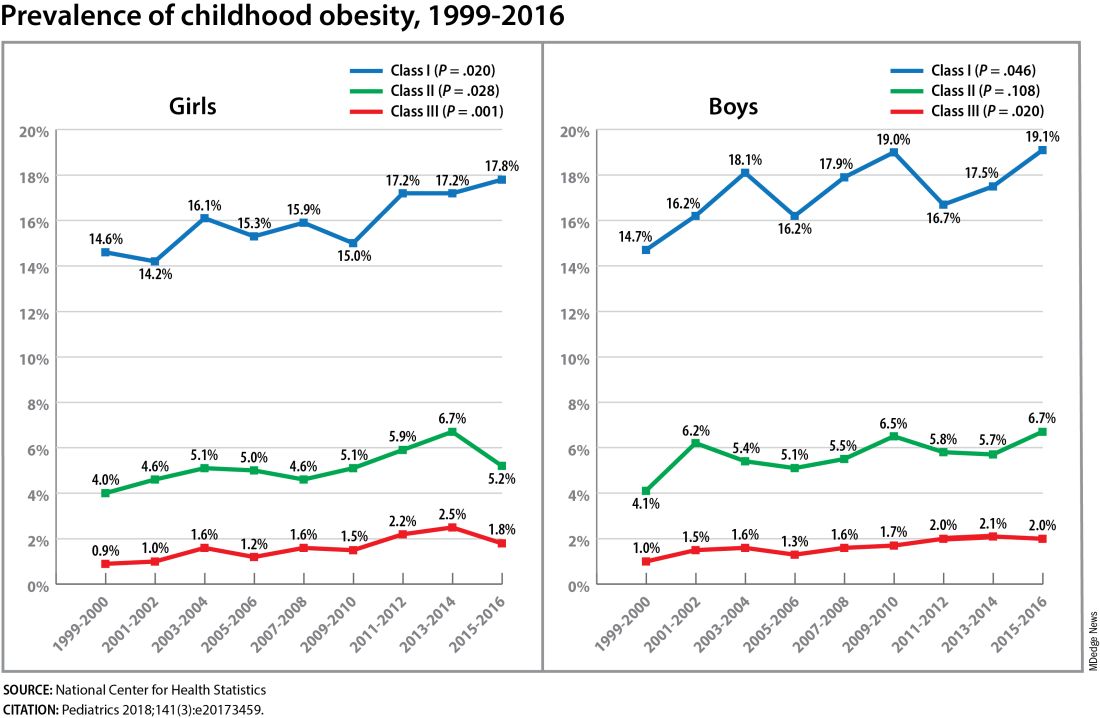
Also notable, she said, is that one-quarter of today’s pediatric diabetes cases are type 2 diabetes, which “is significant as there is a higher prevalence of early complications and comorbidities in youth with type 2 diabetes compared to type 1 diabetes.”
Moreover, recent projections estimate that 57% of today’s children will be obese at 35 years of age (N Engl J Med. 2017;377[22]:2145-53) and that 45% will have diabetes or prediabetes by 2030 (Popul Health Manag. 2017;20[1]:6-12), said Dr. Hockett, assistant professor in the university’s department of pediatrics. An investigator of the Exploring Perinatal Outcomes Among Children (EPOCH) study, which looked at gestational diabetes (GDM) and offspring cardiometabolic risks, she said more chronic disease “at increasingly younger ages [points toward] prebirth influences.”
She noted that there are critical periods postnatally — such as infancy and puberty — that can “impact or further shift the trajectory of chronic disease.” The developmental origins theory posits that life events and biological and environmental processes during the lifespan can modify the effects of intrauterine exposures.
The transgenerational implications “are clear,” she said. “As the number of reproductive-aged individuals with chronic diseases rises, the number of exposed offspring also rises ... It leads to a vicious cycle.”
Deeper Dives Into Associations, Potential Mechanisms
The EPOCH prospective cohort study with which Dr. Hockett was involved gave her a front-seat view of the transgenerational adverse effects of in utero exposure to hyperglycemia. The study recruited ethnically diverse maternal/child dyads from the Kaiser Permanente of Colorado perinatal database from 1992 to 2002 and assessed 418 offspring at two points — a mean age of 10.5 years and 16.5 years — for fasting blood glucose, adiposity, and diet and physical activity. The second visit also involved an oral glucose tolerance test.
The 77 offspring who had been exposed in utero to GDM had a homeostatic model assessment of insulin resistance (HOMA-IR) that was 18% higher, a 19% lower Matsuda index, and a 9% greater HOMA of β-cell function (HOMA-β) than the 341 offspring whose mothers did not have diabetes. Each 5-kg/m2 increase in prepregnancy body mass index predicted increased insulin resistance, but there was no combined effect of both maternal obesity and diabetes in utero.
Exposed offspring had a higher BMI and increased adiposity, but when BMI was controlled for in the analysis of metabolic outcomes, maternal diabetes was still associated with 12% higher HOMA-IR and a 17% lower Matsuda index. “So [the metabolic outcomes] are a direct effect of maternal diabetes,” Dr. Hockett said at the DPSG meeting, noting the fetal overnutrition hypothesis in which maternal glucose, but not maternal insulin, freely passes through the placenta, promoting growth and adiposity in the fetus.
[The EPOCH results on metabolic outcomes and offspring adiposity were published in 2017 and 2019, respectively (Diabet Med. 2017;34:1392-9; Diabetologia. 2019;62:2017-24). In 2020, EPOCH researchers reported sex-specific effects on cardiovascular outcomes, with GDM exposure associated with higher total and LDL cholesterol in girls and higher systolic blood pressure in boys (Pediatr Obes. 2020;15[5]:e12611).]
Now, a new longitudinal cohort study underway in Phoenix, is taking a deeper dive, trying to pinpoint what exactly influences childhood obesity and metabolic risk by following Hispanic and American Indian maternal/child dyads from pregnancy until 18 years postpartum. Researchers are looking not only at associations between maternal risk factors (pregnancy BMI, gestational weight gain, and diabetes in pregnancy) and offspring BMI, adiposity, and growth patterns, but also how various factors during pregnancy — clinical, genetic, lifestyle, biochemical — ”may mediate the associations,” said lead investigator Madhumita Sinha, MD.
“We need a better understanding at the molecular level of the biological processes that lead to obesity in children and that cause metabolic dysfunction,” said Dr. Sinha, who heads the Diabetes Epidemiology and Clinical Research Section of the of the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) branch in Phoenix.
The populations being enrolled in the ETCHED study (for Early Tracking of Childhood Health Determinants) are at especially high risk of childhood obesity and metabolic dysfunction. Research conducted decades ago by the NIDDK in Phoenix showed that approximately 50% of Pima Indian children from diabetic pregnancies develop type 2 diabetes by age 25 (N Engl J Med. 1983;308:242-5). Years later, to tease out possible genetic factors, researchers compared siblings born before and after their mother was found to have type 2 diabetes, and found significantly higher rates of diabetes in those born after the mother’s diagnosis, affirming the role of in utero toxicity (Diabetes 2000;49:2208-11).
In the new study, the researchers will look at adipokines and inflammatory biomarkers in the mothers and offspring in addition to traditional anthropometric and glycemic measures. They’ll analyze placental tissue, breast milk, and the gut microbiome longitudinally, and they’ll lean heavily on genomics/epigenomics, proteomics, and metabolomics. “There’s potential,” Dr. Sinha said, “to develop a more accurate predictive and prognostic model of childhood obesity.”
The researchers also will study the role of family, socioeconomics, and environmental factors in influencing child growth patterns and they’ll look at neurodevelopment in infancy and childhood. As of October 2023, almost 80 pregnant women, most with obesity and almost one-third with type 2 diabetes, had enrolled in the study. Over the next several years, the study aims to enroll 750 dyads.
The Timing of In Utero Exposure
Shelley Ehrlich, MD, ScD, MPH, of the University of Cincinnati and Cincinnati Children’s Hospital Medical Center, is aiming, meanwhile, to learn how the timing of in utero exposure to hyperglycemia predicts specific metabolic and cardiovascular morbidities in the adult offspring of diabetic mothers.
“While we know that exposure to maternal diabetes, regardless of type, increases the risk of obesity, insulin resistance, diabetes, renal compromise, and cardiovascular disease in the offspring, there is little known about the level and timing of hyperglycemic exposure during fetal development that triggers these adverse outcomes,” said Dr. Ehrlich. A goal, she said, is to identify gestational profiles that predict phenotypes of offspring at risk for morbidity in later life.
She and other investigators with the TEAM (Transgenerational Effect on Adult Morbidity) study have recruited over 170 offspring of mothers who participated in the Diabetes in Pregnancy Program Project Grant (PPG) at the University of Cincinnati Medical Center from 1978 to 1995 — a landmark study that demonstrated the effect of strict glucose control in reducing major congenital malformations.
The women in the PPG study had frequent glucose monitoring (up to 6-8 times a day) throughout their pregnancies, and now, their recruited offspring, who are up to 43 years of age, are being assessed for obesity, diabetes/metabolic health, cardiovascular disease/cardiac and peripheral vascular structure and function, and other outcomes including those that may be amenable to secondary prevention (J Diabetes Res. Nov 1;2021:6590431).
Preliminary findings from over 170 offspring recruited between 2017 and 2022 suggest that in utero exposure to dysglycemia (as measured by standard deviations of glycohemoglobin) in the third trimester appears to increase the risk of morbid obesity in adulthood, while exposure to dysglycemia in the first trimester increases the risk of impaired glucose tolerance. The risk of B-cell dysfunction, meanwhile, appears to be linked to dysglycemia in the first and third trimesters — particularly the first — Dr. Ehrlich reported.
Cognitive outcomes in offspring have also been assessed and here it appears that dysglycemia in the third trimester is linked to worse scores on the Wechsler Abbreviated Scale of Intelligence (WASI-II), said Katherine Bowers, PhD, MPH, a TEAM study coinvestigator, also of Cincinnati Children’s Hospital Medical Center.
“We’ve already observed [an association between] diabetes in pregnancy and cognition in early childhood and through adolescence, but [the question has been] does this association persist into adulthood?” she said.
Preliminary analyses of 104 offspring show no statistically significant associations between maternal dysglycemia in the first or second trimesters and offspring cognition, but “consistent inverse associations between maternal glycohemoglobin in the third trimester across two [WASI-II] subscales and composite measures of cognition,” Dr. Bowers said.
Their analysis adjusted for a variety of factors, including maternal age, prepregnancy and first trimester BMI, race, family history of diabetes, and diabetes severity/macrovascular complications.
Back In The Laboratory
At the other end of the research spectrum, basic research scientists are also investigating the mechanisms and sequelae of in utero hyperglycemia and other injuries, including congenital malformations, placental adaptive responses and fetal programming. Researchers are asking, for instance, what does placental metabolic reprogramming entail? What role do placental extracellular vesicles play in GDM? Can we alter the in utero environment and thus improve the short and long-term fetal/infant outcomes?
Animal research done at the UMSOM Center for Birth Defects Research, led by Dr. Reece and Peixin Yang, PhD, suggests that “a good portion of in utero injury is due to epigenetics,” Dr. Reece said in the interview. “We’ve shown that under conditions of hyperglycemia, for example, genetic regulation and genetic function can be altered.”
Through in vivo research, they have also shown that antioxidants or membrane stabilizers such as arachidonic acid or myo-inositol, or experimental inhibitors to certain pro-apoptotic intermediates, can individually or collectively result in reduced malformations. “It is highly likely that understanding the biological impact of various altered in utero environments, and then modifying or reversing those environments, will result in short and long-term outcome improvements similar to those shown with congenital malformations,” Dr. Reece said.
FAIRFAX, VIRGINIA — Nearly 30 years ago, in a 1995 paper, the British physician-epidemiologist David Barker, MD, PhD, wrote about his fetal origins hypothesis — the idea that programs to address fetal undernutrition and low birth weight produced later coronary heart disease (BMJ 1995;311:171-4).
His hypothesis and subsequent research led to the concept of adult diseases of fetal origins, which today extends beyond low birth weight and implicates the in utero environment as a significant determinant of risk for adverse childhood and adult metabolic outcomes and for major chronic diseases, including diabetes and obesity. Studies have shown that the offspring of pregnant mothers with diabetes have a higher risk of developing obesity and diabetes themselves.
“It’s a whole discipline [of research],” E. Albert Reece, MD, PhD, MBA, of the University of Maryland School of Medicine (UMSOM), said in an interview. “But what we’ve never quite understood is the ‘how’ and ‘why’? What are the mechanisms driving the fetal origins of such adverse outcomes in offspring?
At the biennial meeting of the Diabetes in Pregnancy Study Group of North America (DPSG), investigators described studies underway that are digging deeper into the associations between the intrauterine milieu and longer-term offspring health — and that are searching for biological and molecular processes that may be involved.
The studies are like “branches of the Barker hypothesis,” said Dr. Reece, former dean of UMSOM and current director of the UMSOM Center for Advanced Research Training and Innovation, who co-organized the DPSG meeting. “They’re taking the hypothesis and dissecting it by asking, for instance, it is possible that transgenerational obesity may align with the Barker hypothesis? Is it possible that it involves epigenetics regulation? Could we find biomarkers?”
The need for a better understanding of the fetal origins framework — and its subsequent transgenerational impact — is urgent. From 2000 to 2018, the prevalence of childhood obesity increased from 14.7% to 19.2% (a 31% increase) and the prevalence of severe childhood obesity rose from 3.9% to 6.1% (a 56% increase), according to data from the U.S. National Health and Nutrition Examination Survey (Obes Facts. 2022;15[4]:560-9).
Children aged 2-5 years have had an especially sharp increase in obesity (Pediatrics 2018;141[3]:e20173459), Christine Wey Hockett, PhD, of the University of South Dakota School of Medicine, said at the DPSG meeting (Figure 1). 
Also notable, she said, is that one-quarter of today’s pediatric diabetes cases are type 2 diabetes, which “is significant as there is a higher prevalence of early complications and comorbidities in youth with type 2 diabetes compared to type 1 diabetes.”
Moreover, recent projections estimate that 57% of today’s children will be obese at 35 years of age (N Engl J Med. 2017;377[22]:2145-53) and that 45% will have diabetes or prediabetes by 2030 (Popul Health Manag. 2017;20[1]:6-12), said Dr. Hockett, assistant professor in the university’s department of pediatrics. An investigator of the Exploring Perinatal Outcomes Among Children (EPOCH) study, which looked at gestational diabetes (GDM) and offspring cardiometabolic risks, she said more chronic disease “at increasingly younger ages [points toward] prebirth influences.”
She noted that there are critical periods postnatally — such as infancy and puberty — that can “impact or further shift the trajectory of chronic disease.” The developmental origins theory posits that life events and biological and environmental processes during the lifespan can modify the effects of intrauterine exposures.
The transgenerational implications “are clear,” she said. “As the number of reproductive-aged individuals with chronic diseases rises, the number of exposed offspring also rises ... It leads to a vicious cycle.”
Deeper Dives Into Associations, Potential Mechanisms
The EPOCH prospective cohort study with which Dr. Hockett was involved gave her a front-seat view of the transgenerational adverse effects of in utero exposure to hyperglycemia. The study recruited ethnically diverse maternal/child dyads from the Kaiser Permanente of Colorado perinatal database from 1992 to 2002 and assessed 418 offspring at two points — a mean age of 10.5 years and 16.5 years — for fasting blood glucose, adiposity, and diet and physical activity. The second visit also involved an oral glucose tolerance test.
The 77 offspring who had been exposed in utero to GDM had a homeostatic model assessment of insulin resistance (HOMA-IR) that was 18% higher, a 19% lower Matsuda index, and a 9% greater HOMA of β-cell function (HOMA-β) than the 341 offspring whose mothers did not have diabetes. Each 5-kg/m2 increase in prepregnancy body mass index predicted increased insulin resistance, but there was no combined effect of both maternal obesity and diabetes in utero.
Exposed offspring had a higher BMI and increased adiposity, but when BMI was controlled for in the analysis of metabolic outcomes, maternal diabetes was still associated with 12% higher HOMA-IR and a 17% lower Matsuda index. “So [the metabolic outcomes] are a direct effect of maternal diabetes,” Dr. Hockett said at the DPSG meeting, noting the fetal overnutrition hypothesis in which maternal glucose, but not maternal insulin, freely passes through the placenta, promoting growth and adiposity in the fetus.
[The EPOCH results on metabolic outcomes and offspring adiposity were published in 2017 and 2019, respectively (Diabet Med. 2017;34:1392-9; Diabetologia. 2019;62:2017-24). In 2020, EPOCH researchers reported sex-specific effects on cardiovascular outcomes, with GDM exposure associated with higher total and LDL cholesterol in girls and higher systolic blood pressure in boys (Pediatr Obes. 2020;15[5]:e12611).]
Now, a new longitudinal cohort study underway in Phoenix, is taking a deeper dive, trying to pinpoint what exactly influences childhood obesity and metabolic risk by following Hispanic and American Indian maternal/child dyads from pregnancy until 18 years postpartum. Researchers are looking not only at associations between maternal risk factors (pregnancy BMI, gestational weight gain, and diabetes in pregnancy) and offspring BMI, adiposity, and growth patterns, but also how various factors during pregnancy — clinical, genetic, lifestyle, biochemical — ”may mediate the associations,” said lead investigator Madhumita Sinha, MD.
“We need a better understanding at the molecular level of the biological processes that lead to obesity in children and that cause metabolic dysfunction,” said Dr. Sinha, who heads the Diabetes Epidemiology and Clinical Research Section of the of the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) branch in Phoenix.
The populations being enrolled in the ETCHED study (for Early Tracking of Childhood Health Determinants) are at especially high risk of childhood obesity and metabolic dysfunction. Research conducted decades ago by the NIDDK in Phoenix showed that approximately 50% of Pima Indian children from diabetic pregnancies develop type 2 diabetes by age 25 (N Engl J Med. 1983;308:242-5). Years later, to tease out possible genetic factors, researchers compared siblings born before and after their mother was found to have type 2 diabetes, and found significantly higher rates of diabetes in those born after the mother’s diagnosis, affirming the role of in utero toxicity (Diabetes 2000;49:2208-11).
In the new study, the researchers will look at adipokines and inflammatory biomarkers in the mothers and offspring in addition to traditional anthropometric and glycemic measures. They’ll analyze placental tissue, breast milk, and the gut microbiome longitudinally, and they’ll lean heavily on genomics/epigenomics, proteomics, and metabolomics. “There’s potential,” Dr. Sinha said, “to develop a more accurate predictive and prognostic model of childhood obesity.”
The researchers also will study the role of family, socioeconomics, and environmental factors in influencing child growth patterns and they’ll look at neurodevelopment in infancy and childhood. As of October 2023, almost 80 pregnant women, most with obesity and almost one-third with type 2 diabetes, had enrolled in the study. Over the next several years, the study aims to enroll 750 dyads.
The Timing of In Utero Exposure
Shelley Ehrlich, MD, ScD, MPH, of the University of Cincinnati and Cincinnati Children’s Hospital Medical Center, is aiming, meanwhile, to learn how the timing of in utero exposure to hyperglycemia predicts specific metabolic and cardiovascular morbidities in the adult offspring of diabetic mothers.
“While we know that exposure to maternal diabetes, regardless of type, increases the risk of obesity, insulin resistance, diabetes, renal compromise, and cardiovascular disease in the offspring, there is little known about the level and timing of hyperglycemic exposure during fetal development that triggers these adverse outcomes,” said Dr. Ehrlich. A goal, she said, is to identify gestational profiles that predict phenotypes of offspring at risk for morbidity in later life.
She and other investigators with the TEAM (Transgenerational Effect on Adult Morbidity) study have recruited over 170 offspring of mothers who participated in the Diabetes in Pregnancy Program Project Grant (PPG) at the University of Cincinnati Medical Center from 1978 to 1995 — a landmark study that demonstrated the effect of strict glucose control in reducing major congenital malformations.
The women in the PPG study had frequent glucose monitoring (up to 6-8 times a day) throughout their pregnancies, and now, their recruited offspring, who are up to 43 years of age, are being assessed for obesity, diabetes/metabolic health, cardiovascular disease/cardiac and peripheral vascular structure and function, and other outcomes including those that may be amenable to secondary prevention (J Diabetes Res. Nov 1;2021:6590431).
Preliminary findings from over 170 offspring recruited between 2017 and 2022 suggest that in utero exposure to dysglycemia (as measured by standard deviations of glycohemoglobin) in the third trimester appears to increase the risk of morbid obesity in adulthood, while exposure to dysglycemia in the first trimester increases the risk of impaired glucose tolerance. The risk of B-cell dysfunction, meanwhile, appears to be linked to dysglycemia in the first and third trimesters — particularly the first — Dr. Ehrlich reported.
Cognitive outcomes in offspring have also been assessed and here it appears that dysglycemia in the third trimester is linked to worse scores on the Wechsler Abbreviated Scale of Intelligence (WASI-II), said Katherine Bowers, PhD, MPH, a TEAM study coinvestigator, also of Cincinnati Children’s Hospital Medical Center.
“We’ve already observed [an association between] diabetes in pregnancy and cognition in early childhood and through adolescence, but [the question has been] does this association persist into adulthood?” she said.
Preliminary analyses of 104 offspring show no statistically significant associations between maternal dysglycemia in the first or second trimesters and offspring cognition, but “consistent inverse associations between maternal glycohemoglobin in the third trimester across two [WASI-II] subscales and composite measures of cognition,” Dr. Bowers said.
Their analysis adjusted for a variety of factors, including maternal age, prepregnancy and first trimester BMI, race, family history of diabetes, and diabetes severity/macrovascular complications.
Back In The Laboratory
At the other end of the research spectrum, basic research scientists are also investigating the mechanisms and sequelae of in utero hyperglycemia and other injuries, including congenital malformations, placental adaptive responses and fetal programming. Researchers are asking, for instance, what does placental metabolic reprogramming entail? What role do placental extracellular vesicles play in GDM? Can we alter the in utero environment and thus improve the short and long-term fetal/infant outcomes?
Animal research done at the UMSOM Center for Birth Defects Research, led by Dr. Reece and Peixin Yang, PhD, suggests that “a good portion of in utero injury is due to epigenetics,” Dr. Reece said in the interview. “We’ve shown that under conditions of hyperglycemia, for example, genetic regulation and genetic function can be altered.”
Through in vivo research, they have also shown that antioxidants or membrane stabilizers such as arachidonic acid or myo-inositol, or experimental inhibitors to certain pro-apoptotic intermediates, can individually or collectively result in reduced malformations. “It is highly likely that understanding the biological impact of various altered in utero environments, and then modifying or reversing those environments, will result in short and long-term outcome improvements similar to those shown with congenital malformations,” Dr. Reece said.
FAIRFAX, VIRGINIA — Nearly 30 years ago, in a 1995 paper, the British physician-epidemiologist David Barker, MD, PhD, wrote about his fetal origins hypothesis — the idea that programs to address fetal undernutrition and low birth weight produced later coronary heart disease (BMJ 1995;311:171-4).
His hypothesis and subsequent research led to the concept of adult diseases of fetal origins, which today extends beyond low birth weight and implicates the in utero environment as a significant determinant of risk for adverse childhood and adult metabolic outcomes and for major chronic diseases, including diabetes and obesity. Studies have shown that the offspring of pregnant mothers with diabetes have a higher risk of developing obesity and diabetes themselves.
“It’s a whole discipline [of research],” E. Albert Reece, MD, PhD, MBA, of the University of Maryland School of Medicine (UMSOM), said in an interview. “But what we’ve never quite understood is the ‘how’ and ‘why’? What are the mechanisms driving the fetal origins of such adverse outcomes in offspring?
At the biennial meeting of the Diabetes in Pregnancy Study Group of North America (DPSG), investigators described studies underway that are digging deeper into the associations between the intrauterine milieu and longer-term offspring health — and that are searching for biological and molecular processes that may be involved.
The studies are like “branches of the Barker hypothesis,” said Dr. Reece, former dean of UMSOM and current director of the UMSOM Center for Advanced Research Training and Innovation, who co-organized the DPSG meeting. “They’re taking the hypothesis and dissecting it by asking, for instance, it is possible that transgenerational obesity may align with the Barker hypothesis? Is it possible that it involves epigenetics regulation? Could we find biomarkers?”
The need for a better understanding of the fetal origins framework — and its subsequent transgenerational impact — is urgent. From 2000 to 2018, the prevalence of childhood obesity increased from 14.7% to 19.2% (a 31% increase) and the prevalence of severe childhood obesity rose from 3.9% to 6.1% (a 56% increase), according to data from the U.S. National Health and Nutrition Examination Survey (Obes Facts. 2022;15[4]:560-9).
Children aged 2-5 years have had an especially sharp increase in obesity (Pediatrics 2018;141[3]:e20173459), Christine Wey Hockett, PhD, of the University of South Dakota School of Medicine, said at the DPSG meeting (Figure 1). 
Also notable, she said, is that one-quarter of today’s pediatric diabetes cases are type 2 diabetes, which “is significant as there is a higher prevalence of early complications and comorbidities in youth with type 2 diabetes compared to type 1 diabetes.”
Moreover, recent projections estimate that 57% of today’s children will be obese at 35 years of age (N Engl J Med. 2017;377[22]:2145-53) and that 45% will have diabetes or prediabetes by 2030 (Popul Health Manag. 2017;20[1]:6-12), said Dr. Hockett, assistant professor in the university’s department of pediatrics. An investigator of the Exploring Perinatal Outcomes Among Children (EPOCH) study, which looked at gestational diabetes (GDM) and offspring cardiometabolic risks, she said more chronic disease “at increasingly younger ages [points toward] prebirth influences.”
She noted that there are critical periods postnatally — such as infancy and puberty — that can “impact or further shift the trajectory of chronic disease.” The developmental origins theory posits that life events and biological and environmental processes during the lifespan can modify the effects of intrauterine exposures.
The transgenerational implications “are clear,” she said. “As the number of reproductive-aged individuals with chronic diseases rises, the number of exposed offspring also rises ... It leads to a vicious cycle.”
Deeper Dives Into Associations, Potential Mechanisms
The EPOCH prospective cohort study with which Dr. Hockett was involved gave her a front-seat view of the transgenerational adverse effects of in utero exposure to hyperglycemia. The study recruited ethnically diverse maternal/child dyads from the Kaiser Permanente of Colorado perinatal database from 1992 to 2002 and assessed 418 offspring at two points — a mean age of 10.5 years and 16.5 years — for fasting blood glucose, adiposity, and diet and physical activity. The second visit also involved an oral glucose tolerance test.
The 77 offspring who had been exposed in utero to GDM had a homeostatic model assessment of insulin resistance (HOMA-IR) that was 18% higher, a 19% lower Matsuda index, and a 9% greater HOMA of β-cell function (HOMA-β) than the 341 offspring whose mothers did not have diabetes. Each 5-kg/m2 increase in prepregnancy body mass index predicted increased insulin resistance, but there was no combined effect of both maternal obesity and diabetes in utero.
Exposed offspring had a higher BMI and increased adiposity, but when BMI was controlled for in the analysis of metabolic outcomes, maternal diabetes was still associated with 12% higher HOMA-IR and a 17% lower Matsuda index. “So [the metabolic outcomes] are a direct effect of maternal diabetes,” Dr. Hockett said at the DPSG meeting, noting the fetal overnutrition hypothesis in which maternal glucose, but not maternal insulin, freely passes through the placenta, promoting growth and adiposity in the fetus.
[The EPOCH results on metabolic outcomes and offspring adiposity were published in 2017 and 2019, respectively (Diabet Med. 2017;34:1392-9; Diabetologia. 2019;62:2017-24). In 2020, EPOCH researchers reported sex-specific effects on cardiovascular outcomes, with GDM exposure associated with higher total and LDL cholesterol in girls and higher systolic blood pressure in boys (Pediatr Obes. 2020;15[5]:e12611).]
Now, a new longitudinal cohort study underway in Phoenix, is taking a deeper dive, trying to pinpoint what exactly influences childhood obesity and metabolic risk by following Hispanic and American Indian maternal/child dyads from pregnancy until 18 years postpartum. Researchers are looking not only at associations between maternal risk factors (pregnancy BMI, gestational weight gain, and diabetes in pregnancy) and offspring BMI, adiposity, and growth patterns, but also how various factors during pregnancy — clinical, genetic, lifestyle, biochemical — ”may mediate the associations,” said lead investigator Madhumita Sinha, MD.
“We need a better understanding at the molecular level of the biological processes that lead to obesity in children and that cause metabolic dysfunction,” said Dr. Sinha, who heads the Diabetes Epidemiology and Clinical Research Section of the of the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) branch in Phoenix.
The populations being enrolled in the ETCHED study (for Early Tracking of Childhood Health Determinants) are at especially high risk of childhood obesity and metabolic dysfunction. Research conducted decades ago by the NIDDK in Phoenix showed that approximately 50% of Pima Indian children from diabetic pregnancies develop type 2 diabetes by age 25 (N Engl J Med. 1983;308:242-5). Years later, to tease out possible genetic factors, researchers compared siblings born before and after their mother was found to have type 2 diabetes, and found significantly higher rates of diabetes in those born after the mother’s diagnosis, affirming the role of in utero toxicity (Diabetes 2000;49:2208-11).
In the new study, the researchers will look at adipokines and inflammatory biomarkers in the mothers and offspring in addition to traditional anthropometric and glycemic measures. They’ll analyze placental tissue, breast milk, and the gut microbiome longitudinally, and they’ll lean heavily on genomics/epigenomics, proteomics, and metabolomics. “There’s potential,” Dr. Sinha said, “to develop a more accurate predictive and prognostic model of childhood obesity.”
The researchers also will study the role of family, socioeconomics, and environmental factors in influencing child growth patterns and they’ll look at neurodevelopment in infancy and childhood. As of October 2023, almost 80 pregnant women, most with obesity and almost one-third with type 2 diabetes, had enrolled in the study. Over the next several years, the study aims to enroll 750 dyads.
The Timing of In Utero Exposure
Shelley Ehrlich, MD, ScD, MPH, of the University of Cincinnati and Cincinnati Children’s Hospital Medical Center, is aiming, meanwhile, to learn how the timing of in utero exposure to hyperglycemia predicts specific metabolic and cardiovascular morbidities in the adult offspring of diabetic mothers.
“While we know that exposure to maternal diabetes, regardless of type, increases the risk of obesity, insulin resistance, diabetes, renal compromise, and cardiovascular disease in the offspring, there is little known about the level and timing of hyperglycemic exposure during fetal development that triggers these adverse outcomes,” said Dr. Ehrlich. A goal, she said, is to identify gestational profiles that predict phenotypes of offspring at risk for morbidity in later life.
She and other investigators with the TEAM (Transgenerational Effect on Adult Morbidity) study have recruited over 170 offspring of mothers who participated in the Diabetes in Pregnancy Program Project Grant (PPG) at the University of Cincinnati Medical Center from 1978 to 1995 — a landmark study that demonstrated the effect of strict glucose control in reducing major congenital malformations.
The women in the PPG study had frequent glucose monitoring (up to 6-8 times a day) throughout their pregnancies, and now, their recruited offspring, who are up to 43 years of age, are being assessed for obesity, diabetes/metabolic health, cardiovascular disease/cardiac and peripheral vascular structure and function, and other outcomes including those that may be amenable to secondary prevention (J Diabetes Res. Nov 1;2021:6590431).
Preliminary findings from over 170 offspring recruited between 2017 and 2022 suggest that in utero exposure to dysglycemia (as measured by standard deviations of glycohemoglobin) in the third trimester appears to increase the risk of morbid obesity in adulthood, while exposure to dysglycemia in the first trimester increases the risk of impaired glucose tolerance. The risk of B-cell dysfunction, meanwhile, appears to be linked to dysglycemia in the first and third trimesters — particularly the first — Dr. Ehrlich reported.
Cognitive outcomes in offspring have also been assessed and here it appears that dysglycemia in the third trimester is linked to worse scores on the Wechsler Abbreviated Scale of Intelligence (WASI-II), said Katherine Bowers, PhD, MPH, a TEAM study coinvestigator, also of Cincinnati Children’s Hospital Medical Center.
“We’ve already observed [an association between] diabetes in pregnancy and cognition in early childhood and through adolescence, but [the question has been] does this association persist into adulthood?” she said.
Preliminary analyses of 104 offspring show no statistically significant associations between maternal dysglycemia in the first or second trimesters and offspring cognition, but “consistent inverse associations between maternal glycohemoglobin in the third trimester across two [WASI-II] subscales and composite measures of cognition,” Dr. Bowers said.
Their analysis adjusted for a variety of factors, including maternal age, prepregnancy and first trimester BMI, race, family history of diabetes, and diabetes severity/macrovascular complications.
Back In The Laboratory
At the other end of the research spectrum, basic research scientists are also investigating the mechanisms and sequelae of in utero hyperglycemia and other injuries, including congenital malformations, placental adaptive responses and fetal programming. Researchers are asking, for instance, what does placental metabolic reprogramming entail? What role do placental extracellular vesicles play in GDM? Can we alter the in utero environment and thus improve the short and long-term fetal/infant outcomes?
Animal research done at the UMSOM Center for Birth Defects Research, led by Dr. Reece and Peixin Yang, PhD, suggests that “a good portion of in utero injury is due to epigenetics,” Dr. Reece said in the interview. “We’ve shown that under conditions of hyperglycemia, for example, genetic regulation and genetic function can be altered.”
Through in vivo research, they have also shown that antioxidants or membrane stabilizers such as arachidonic acid or myo-inositol, or experimental inhibitors to certain pro-apoptotic intermediates, can individually or collectively result in reduced malformations. “It is highly likely that understanding the biological impact of various altered in utero environments, and then modifying or reversing those environments, will result in short and long-term outcome improvements similar to those shown with congenital malformations,” Dr. Reece said.
FROM DPSG-NA 2023
Hormones and Viruses Influence Each Other: Consider These Connections in Your Patients
Stefan Bornstein, MD, PhD, professor, made it clear during a press conference at the 67th Congress of the German Society of Endocrinology (DGE) that there is more than one interaction between them. Nowadays, one can almost speak of an “endocrine virology and even of the virome as an additional, hormonally metabolically active gland,” said Dr. Bornstein, who will receive the Berthold Medal from the DGE in 2024.
Many questions remain unanswered: “We need a better understanding of the interaction of hormone systems with infectious agents — from basics to therapeutic applications,” emphasized the director of the Medical Clinic and Polyclinic III and the Center for Internal Medicine at the Carl Gustav Carus University Hospital, Dresden, Germany.
If infectious diseases could trigger diabetes and other metabolic diseases, this means that “through vaccination programs, we may be able to prevent the occurrence of common metabolic diseases such as diabetes,” said Dr. Bornstein. He highlighted that many people who experienced severe COVID-19 during the pandemic, or died from it, exhibited diabetes or a pre-metabolic syndrome.
“SARS-CoV-2 has utilized an endocrine signaling pathway to invade our cells and cause damage in the organ systems and inflammation,” said Dr. Bornstein. Conversely, it is now known that infections with coronaviruses or other infectious agents like influenza can significantly worsen metabolic status, diabetes, and other endocrine diseases.
SARS-CoV-2 Infects the Beta Cells
Data from the COVID-19 pandemic showed that the likelihood of developing type 1 diabetes significantly increases with a SARS-CoV-2 infection. Researchers led by Dr. Bornstein demonstrated in 2021 that SARS-CoV-2 can infect the insulin-producing cells of the organ. They examined pancreatic tissue from 20 patients who died from COVID-19 using immunofluorescence, immunohistochemistry, RNA in situ hybridization, and electron microscopy.
They found viral SARS-CoV-2 infiltration of the beta cells in all patients. In 11 patients with COVID-19, the expression of ACE2, TMPRSS, and other receptors and factors like DPP4, HMBG1, and NRP1 that can facilitate virus entry was examined. They found that even in the absence of manifest newly onset diabetes, necroptotic cell death, immune cell infiltration, and SARS-CoV-2 infection of the pancreas beta cells can contribute to varying degrees of metabolic disturbance in patients with COVID-19.
In a report published in October 2020, Tim Hollstein, MD, from the Institute for Diabetology and Clinical Metabolic Research at UKSH in Kiel, Germany, and colleagues described the case of a 19-year-old man who developed symptoms of insulin-dependent diabetes after a SARS-CoV-2 infection, without the presence of autoantibodies typical for type 1 diabetes.
The man presented to the emergency department with diabetic ketoacidosis, a C-peptide level of 0.62 µg/L, a blood glucose concentration of 30.6 mmol/L (552 mg/dL), and an A1c level of 16.8%. The patient’s history revealed a probable SARS-CoV-2 infection 5-7 weeks before admission, based on a positive antibody test against SARS-CoV-2.
Some Viruses Produce Insulin-Like Proteins
Recent studies have shown that some viruses can produce insulin-like proteins or hormones that interfere with the metabolism of the affected organism, reported Dr. Bornstein. In addition to metabolic regulation, these “viral hormones” also seem to influence cell turnover and cell death.
Dr. Bornstein pointed out that antiviral medications can delay the onset of type 1 diabetes by preserving the function of insulin-producing beta cells. It has also been shown that conventional medications used to treat hormonal disorders can reduce the susceptibility of the organism to infections — such as antidiabetic preparations like DPP-4 inhibitors or metformin.
In a review published in 2023, Nikolaos Perakakis, MD, professor, research group leader at the Paul Langerhans Institute Dresden, Dresden, Germany, Dr. Bornstein, and colleagues discussed scientific evidence for a close mutual dependence between various virus infections and metabolic diseases. They discussed how viruses can lead to the development or progression of metabolic diseases and vice versa and how metabolic diseases can increase the severity of a virus infection.
Viruses Favor Metabolic Diseases...
Viruses can favor metabolic diseases by, for example, influencing the regulation of cell survival and specific signaling pathways relevant for cell death, proliferation, or dedifferentiation in important endocrine and metabolic organs. Viruses are also capable of controlling cellular glucose metabolism by modulating glucose transporters, altering glucose uptake, regulating signaling pathways, and stimulating glycolysis in infected cells.
Due to the destruction of beta cells, enteroviruses, but also the mumps virus, parainfluenza virus, or human herpes virus 6, are associated with the development of diabetes. The timing of infection often precedes or coincides with the peak of development of islet autoantibodies. The fact that only a small proportion of patients actually develop type 1 diabetes suggests that genetic background, and likely the timing of infection, play an important role.
...And Metabolic Diseases Influence the Course of Infection
Infection with hepatitis C virus (HCV), on the other hand, is associated with an increased risk for type 2 diabetes, with the risk being higher for older individuals with a family history of diabetes. The negative effects of HCV on glucose balance are mainly attributed to increased insulin resistance in the liver. HCV reduces hepatic glucose uptake by downregulating the expression of glucose transporters and additionally impairs insulin signal transduction by inhibiting the PI3K/Akt signaling pathway.
People with obesity, diabetes, or insulin resistance show significant changes in the innate and adaptive functions of the immune system. Regarding the innate immune system, impaired chemotaxis and phagocytosis of neutrophils have been observed in patients with type 2 diabetes.
In the case of obesity, the number of natural killer T cells in adipose tissue decreases, whereas B cells accumulate in adipose tissue and secrete more proinflammatory cytokines. Longitudinal multiomics analyses of various biopsies from individuals with insulin resistance showed a delayed immune response to respiratory virus infections compared with individuals with normal insulin sensitivity.
This story was translated from Medscape Germany using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Stefan Bornstein, MD, PhD, professor, made it clear during a press conference at the 67th Congress of the German Society of Endocrinology (DGE) that there is more than one interaction between them. Nowadays, one can almost speak of an “endocrine virology and even of the virome as an additional, hormonally metabolically active gland,” said Dr. Bornstein, who will receive the Berthold Medal from the DGE in 2024.
Many questions remain unanswered: “We need a better understanding of the interaction of hormone systems with infectious agents — from basics to therapeutic applications,” emphasized the director of the Medical Clinic and Polyclinic III and the Center for Internal Medicine at the Carl Gustav Carus University Hospital, Dresden, Germany.
If infectious diseases could trigger diabetes and other metabolic diseases, this means that “through vaccination programs, we may be able to prevent the occurrence of common metabolic diseases such as diabetes,” said Dr. Bornstein. He highlighted that many people who experienced severe COVID-19 during the pandemic, or died from it, exhibited diabetes or a pre-metabolic syndrome.
“SARS-CoV-2 has utilized an endocrine signaling pathway to invade our cells and cause damage in the organ systems and inflammation,” said Dr. Bornstein. Conversely, it is now known that infections with coronaviruses or other infectious agents like influenza can significantly worsen metabolic status, diabetes, and other endocrine diseases.
SARS-CoV-2 Infects the Beta Cells
Data from the COVID-19 pandemic showed that the likelihood of developing type 1 diabetes significantly increases with a SARS-CoV-2 infection. Researchers led by Dr. Bornstein demonstrated in 2021 that SARS-CoV-2 can infect the insulin-producing cells of the organ. They examined pancreatic tissue from 20 patients who died from COVID-19 using immunofluorescence, immunohistochemistry, RNA in situ hybridization, and electron microscopy.
They found viral SARS-CoV-2 infiltration of the beta cells in all patients. In 11 patients with COVID-19, the expression of ACE2, TMPRSS, and other receptors and factors like DPP4, HMBG1, and NRP1 that can facilitate virus entry was examined. They found that even in the absence of manifest newly onset diabetes, necroptotic cell death, immune cell infiltration, and SARS-CoV-2 infection of the pancreas beta cells can contribute to varying degrees of metabolic disturbance in patients with COVID-19.
In a report published in October 2020, Tim Hollstein, MD, from the Institute for Diabetology and Clinical Metabolic Research at UKSH in Kiel, Germany, and colleagues described the case of a 19-year-old man who developed symptoms of insulin-dependent diabetes after a SARS-CoV-2 infection, without the presence of autoantibodies typical for type 1 diabetes.
The man presented to the emergency department with diabetic ketoacidosis, a C-peptide level of 0.62 µg/L, a blood glucose concentration of 30.6 mmol/L (552 mg/dL), and an A1c level of 16.8%. The patient’s history revealed a probable SARS-CoV-2 infection 5-7 weeks before admission, based on a positive antibody test against SARS-CoV-2.
Some Viruses Produce Insulin-Like Proteins
Recent studies have shown that some viruses can produce insulin-like proteins or hormones that interfere with the metabolism of the affected organism, reported Dr. Bornstein. In addition to metabolic regulation, these “viral hormones” also seem to influence cell turnover and cell death.
Dr. Bornstein pointed out that antiviral medications can delay the onset of type 1 diabetes by preserving the function of insulin-producing beta cells. It has also been shown that conventional medications used to treat hormonal disorders can reduce the susceptibility of the organism to infections — such as antidiabetic preparations like DPP-4 inhibitors or metformin.
In a review published in 2023, Nikolaos Perakakis, MD, professor, research group leader at the Paul Langerhans Institute Dresden, Dresden, Germany, Dr. Bornstein, and colleagues discussed scientific evidence for a close mutual dependence between various virus infections and metabolic diseases. They discussed how viruses can lead to the development or progression of metabolic diseases and vice versa and how metabolic diseases can increase the severity of a virus infection.
Viruses Favor Metabolic Diseases...
Viruses can favor metabolic diseases by, for example, influencing the regulation of cell survival and specific signaling pathways relevant for cell death, proliferation, or dedifferentiation in important endocrine and metabolic organs. Viruses are also capable of controlling cellular glucose metabolism by modulating glucose transporters, altering glucose uptake, regulating signaling pathways, and stimulating glycolysis in infected cells.
Due to the destruction of beta cells, enteroviruses, but also the mumps virus, parainfluenza virus, or human herpes virus 6, are associated with the development of diabetes. The timing of infection often precedes or coincides with the peak of development of islet autoantibodies. The fact that only a small proportion of patients actually develop type 1 diabetes suggests that genetic background, and likely the timing of infection, play an important role.
...And Metabolic Diseases Influence the Course of Infection
Infection with hepatitis C virus (HCV), on the other hand, is associated with an increased risk for type 2 diabetes, with the risk being higher for older individuals with a family history of diabetes. The negative effects of HCV on glucose balance are mainly attributed to increased insulin resistance in the liver. HCV reduces hepatic glucose uptake by downregulating the expression of glucose transporters and additionally impairs insulin signal transduction by inhibiting the PI3K/Akt signaling pathway.
People with obesity, diabetes, or insulin resistance show significant changes in the innate and adaptive functions of the immune system. Regarding the innate immune system, impaired chemotaxis and phagocytosis of neutrophils have been observed in patients with type 2 diabetes.
In the case of obesity, the number of natural killer T cells in adipose tissue decreases, whereas B cells accumulate in adipose tissue and secrete more proinflammatory cytokines. Longitudinal multiomics analyses of various biopsies from individuals with insulin resistance showed a delayed immune response to respiratory virus infections compared with individuals with normal insulin sensitivity.
This story was translated from Medscape Germany using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Stefan Bornstein, MD, PhD, professor, made it clear during a press conference at the 67th Congress of the German Society of Endocrinology (DGE) that there is more than one interaction between them. Nowadays, one can almost speak of an “endocrine virology and even of the virome as an additional, hormonally metabolically active gland,” said Dr. Bornstein, who will receive the Berthold Medal from the DGE in 2024.
Many questions remain unanswered: “We need a better understanding of the interaction of hormone systems with infectious agents — from basics to therapeutic applications,” emphasized the director of the Medical Clinic and Polyclinic III and the Center for Internal Medicine at the Carl Gustav Carus University Hospital, Dresden, Germany.
If infectious diseases could trigger diabetes and other metabolic diseases, this means that “through vaccination programs, we may be able to prevent the occurrence of common metabolic diseases such as diabetes,” said Dr. Bornstein. He highlighted that many people who experienced severe COVID-19 during the pandemic, or died from it, exhibited diabetes or a pre-metabolic syndrome.
“SARS-CoV-2 has utilized an endocrine signaling pathway to invade our cells and cause damage in the organ systems and inflammation,” said Dr. Bornstein. Conversely, it is now known that infections with coronaviruses or other infectious agents like influenza can significantly worsen metabolic status, diabetes, and other endocrine diseases.
SARS-CoV-2 Infects the Beta Cells
Data from the COVID-19 pandemic showed that the likelihood of developing type 1 diabetes significantly increases with a SARS-CoV-2 infection. Researchers led by Dr. Bornstein demonstrated in 2021 that SARS-CoV-2 can infect the insulin-producing cells of the organ. They examined pancreatic tissue from 20 patients who died from COVID-19 using immunofluorescence, immunohistochemistry, RNA in situ hybridization, and electron microscopy.
They found viral SARS-CoV-2 infiltration of the beta cells in all patients. In 11 patients with COVID-19, the expression of ACE2, TMPRSS, and other receptors and factors like DPP4, HMBG1, and NRP1 that can facilitate virus entry was examined. They found that even in the absence of manifest newly onset diabetes, necroptotic cell death, immune cell infiltration, and SARS-CoV-2 infection of the pancreas beta cells can contribute to varying degrees of metabolic disturbance in patients with COVID-19.
In a report published in October 2020, Tim Hollstein, MD, from the Institute for Diabetology and Clinical Metabolic Research at UKSH in Kiel, Germany, and colleagues described the case of a 19-year-old man who developed symptoms of insulin-dependent diabetes after a SARS-CoV-2 infection, without the presence of autoantibodies typical for type 1 diabetes.
The man presented to the emergency department with diabetic ketoacidosis, a C-peptide level of 0.62 µg/L, a blood glucose concentration of 30.6 mmol/L (552 mg/dL), and an A1c level of 16.8%. The patient’s history revealed a probable SARS-CoV-2 infection 5-7 weeks before admission, based on a positive antibody test against SARS-CoV-2.
Some Viruses Produce Insulin-Like Proteins
Recent studies have shown that some viruses can produce insulin-like proteins or hormones that interfere with the metabolism of the affected organism, reported Dr. Bornstein. In addition to metabolic regulation, these “viral hormones” also seem to influence cell turnover and cell death.
Dr. Bornstein pointed out that antiviral medications can delay the onset of type 1 diabetes by preserving the function of insulin-producing beta cells. It has also been shown that conventional medications used to treat hormonal disorders can reduce the susceptibility of the organism to infections — such as antidiabetic preparations like DPP-4 inhibitors or metformin.
In a review published in 2023, Nikolaos Perakakis, MD, professor, research group leader at the Paul Langerhans Institute Dresden, Dresden, Germany, Dr. Bornstein, and colleagues discussed scientific evidence for a close mutual dependence between various virus infections and metabolic diseases. They discussed how viruses can lead to the development or progression of metabolic diseases and vice versa and how metabolic diseases can increase the severity of a virus infection.
Viruses Favor Metabolic Diseases...
Viruses can favor metabolic diseases by, for example, influencing the regulation of cell survival and specific signaling pathways relevant for cell death, proliferation, or dedifferentiation in important endocrine and metabolic organs. Viruses are also capable of controlling cellular glucose metabolism by modulating glucose transporters, altering glucose uptake, regulating signaling pathways, and stimulating glycolysis in infected cells.
Due to the destruction of beta cells, enteroviruses, but also the mumps virus, parainfluenza virus, or human herpes virus 6, are associated with the development of diabetes. The timing of infection often precedes or coincides with the peak of development of islet autoantibodies. The fact that only a small proportion of patients actually develop type 1 diabetes suggests that genetic background, and likely the timing of infection, play an important role.
...And Metabolic Diseases Influence the Course of Infection
Infection with hepatitis C virus (HCV), on the other hand, is associated with an increased risk for type 2 diabetes, with the risk being higher for older individuals with a family history of diabetes. The negative effects of HCV on glucose balance are mainly attributed to increased insulin resistance in the liver. HCV reduces hepatic glucose uptake by downregulating the expression of glucose transporters and additionally impairs insulin signal transduction by inhibiting the PI3K/Akt signaling pathway.
People with obesity, diabetes, or insulin resistance show significant changes in the innate and adaptive functions of the immune system. Regarding the innate immune system, impaired chemotaxis and phagocytosis of neutrophils have been observed in patients with type 2 diabetes.
In the case of obesity, the number of natural killer T cells in adipose tissue decreases, whereas B cells accumulate in adipose tissue and secrete more proinflammatory cytokines. Longitudinal multiomics analyses of various biopsies from individuals with insulin resistance showed a delayed immune response to respiratory virus infections compared with individuals with normal insulin sensitivity.
This story was translated from Medscape Germany using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Sustained Control Reported for Anti–IL-17, Anti–IL-23 Psoriasis Treatments
SAN DIEGO — , but late-breaker data presented at the annual meeting of the American Academy of Dermatology show that these types of responses are sustained for as long as patients have remained on therapy.
Of the two, the longer follow up is with the IL-17 inhibitor bimekizumab (Bimzelx). In a 4-year open-label extension study, the Psoriasis Area and Severity Index (PASI) 90 rate was approximately 85% in treated patients, according to Mark Lebwohl, MD, professor and chairman emeritus of the Department of Dermatology, Icahn School of Medicine at Mount Sinai in New York City
A PASI 90 score signifies that 90% of skin surface area is cleared. The proportion of patients who achieved a PASI 100 score, signifying total clearance, approached 70% at 4 years in the group with the greatest response. PASI 90 and PASI 100 rates at this point were only modestly lower than those reported at the end of the double-blind phase 3 trial when evaluated 3 years earlier.
Follow-up with a novel oral anti-IL-23 inhibitor JNJ-2113 (JNJ-77242113) was only 52 weeks, far shorter. But again, the response for the most effective dose at the end of this period was essentially unchanged from that at 16 weeks. Among those on the highest and most effective test dose of once-daily 100 mg, the PASI 90 at 1 year was 64.3%, a rate that was essentially unchanged from week 16.
No Apparent Loss of Benefit Over Time
“We can really look at those dose-response curves and see that there is, overall, a maintenance of response,” reported Laura K. Ferris, MD, PhD, professor and director of clinical trials, Department of Dermatology, University of Pittsburgh Medical Center, Pittsburgh, Pennsylvania. In her presentation of the data, she showed similar sustained control for the most effective doses of JNJ-2113 for multiple clinical outcomes, including an investigator’s global assessment (IGA) score of 0 or 1, also signifying clear or near clear skin.
Bimekizumab, a monoclonal antibody that inhibits both IL-17A and IL-17F, is already approved for the treatment of plaque psoriasis. The 52-week BE SURE trial, which provided the 478 patients who entered into the BE BRIGHT open label extension study, was published in The New England Journal of Medicine in July 2021.
In the 4-year data reported by Dr. Lebwohl, three groups were compared: Those initially randomized to an every-4-week dosing schedule of bimekizumab over the course of the 52-week BE SURE trial; those randomized to an every-4-week bimekizumab schedule who were then subsequently switched to an every-8-week schedule; and those initiated on the TNF-inhibitor adalimumab (Humira) and were then switched at week 24 to every-4-week bimekizumab.
The PASI 90 responses at 52 weeks in these three groups, respectively, were 91.2%, 89.3%, and 95.2%. At 4 years, this almost clear response was observed in 82.4%, 83.2%, and 87.6%, respectively. At 52 weeks, the PASI 100 responses in these three groups, respectively, were 75.3%, 74.2%, and 72.9%. At 4 years, 61.9%, 58.5%, and 69.5% still had complete skin clearance.
Bimekizumab was well tolerated during the randomized trial, reported Dr. Lebwohl. The rates of nasopharyngitis and oral candidiasis, which were observed in approximately 12% and 8%, respectively, of treated patients during the randomized phase remained at about the same level in the long-term follow up. There were no new safety signals, he said.
JNJ-2113 Is First Potential Oral IL-23 Inhibitor
JNJ-2113 is a first-in-class oral peptide that binds to the IL-23 receptor, blocking the IL-23 signaling pathway. If approved, it would be the first oral therapy targeting IL-23. The 16-week outcomes of the dose-finding FRONTIER 1 phase 2b trial were published in The New England Journal of Medicine earlier this year. The primary endpoint was PASI 75, achieved by 79% of those on the 100 mg twice daily dose at week 16, vs 9% on placebo, and at 52 weeks, was 76%.
“The proportion of patients achieving the FRONTIER 1 primary endpoint was maintained from week 16 to the end of week 52 in the extension study,” Dr. Ferris said, but further pointed out that rates of near or complete clearance achieved at week 16 were also essentially unchanged at week 52. This was true of PASI scores and IGA.
Clearance of psoriatic lesions on the scalp was particularly impressive. By scalp-specific IGA, rates of clear or near clear (0/1) were not just maintained but improved over the course of follow-up, reaching 75.1% at 52 weeks in the highest dose group, she said.
JNJ-2113 was well tolerated in FRONTIER 1 and remained so during long-term follow-up, in the FRONTIER 2 extension study, according to Dr. Ferris. The most common complaints with JNJ-2113, such as nasopharyngitis (18.1% vs 25.7% in placebo), did not appear to differ significantly from placebo and the treatment remained well tolerated over the course of the extended follow-up.
There are limited direct comparisons of different biologics active in the treatment of plaque psoriasis for efficacy and safety, but these data appear to show a depth and durability of benefit for psoriasis that is exceptional, Dr. Lebwohl told this news organization. “The PASI 100 scores achieved by bimekizumab exceed anything we have seen to date,” he said. “And the durability of those exceedingly high scores is remarkable.”
Dr. Lebwohl reports financial relationships with approximately 40 pharmaceutical companies, including UCB Pharma, which developed bimekizumab. Dr. Ferris reports financial relationships with more than 20 pharmaceutical companies, including Janssen, which is developing JNJ-2113.
A version of this article appeared on Medscape.com.
SAN DIEGO — , but late-breaker data presented at the annual meeting of the American Academy of Dermatology show that these types of responses are sustained for as long as patients have remained on therapy.
Of the two, the longer follow up is with the IL-17 inhibitor bimekizumab (Bimzelx). In a 4-year open-label extension study, the Psoriasis Area and Severity Index (PASI) 90 rate was approximately 85% in treated patients, according to Mark Lebwohl, MD, professor and chairman emeritus of the Department of Dermatology, Icahn School of Medicine at Mount Sinai in New York City
A PASI 90 score signifies that 90% of skin surface area is cleared. The proportion of patients who achieved a PASI 100 score, signifying total clearance, approached 70% at 4 years in the group with the greatest response. PASI 90 and PASI 100 rates at this point were only modestly lower than those reported at the end of the double-blind phase 3 trial when evaluated 3 years earlier.
Follow-up with a novel oral anti-IL-23 inhibitor JNJ-2113 (JNJ-77242113) was only 52 weeks, far shorter. But again, the response for the most effective dose at the end of this period was essentially unchanged from that at 16 weeks. Among those on the highest and most effective test dose of once-daily 100 mg, the PASI 90 at 1 year was 64.3%, a rate that was essentially unchanged from week 16.
No Apparent Loss of Benefit Over Time
“We can really look at those dose-response curves and see that there is, overall, a maintenance of response,” reported Laura K. Ferris, MD, PhD, professor and director of clinical trials, Department of Dermatology, University of Pittsburgh Medical Center, Pittsburgh, Pennsylvania. In her presentation of the data, she showed similar sustained control for the most effective doses of JNJ-2113 for multiple clinical outcomes, including an investigator’s global assessment (IGA) score of 0 or 1, also signifying clear or near clear skin.
Bimekizumab, a monoclonal antibody that inhibits both IL-17A and IL-17F, is already approved for the treatment of plaque psoriasis. The 52-week BE SURE trial, which provided the 478 patients who entered into the BE BRIGHT open label extension study, was published in The New England Journal of Medicine in July 2021.
In the 4-year data reported by Dr. Lebwohl, three groups were compared: Those initially randomized to an every-4-week dosing schedule of bimekizumab over the course of the 52-week BE SURE trial; those randomized to an every-4-week bimekizumab schedule who were then subsequently switched to an every-8-week schedule; and those initiated on the TNF-inhibitor adalimumab (Humira) and were then switched at week 24 to every-4-week bimekizumab.
The PASI 90 responses at 52 weeks in these three groups, respectively, were 91.2%, 89.3%, and 95.2%. At 4 years, this almost clear response was observed in 82.4%, 83.2%, and 87.6%, respectively. At 52 weeks, the PASI 100 responses in these three groups, respectively, were 75.3%, 74.2%, and 72.9%. At 4 years, 61.9%, 58.5%, and 69.5% still had complete skin clearance.
Bimekizumab was well tolerated during the randomized trial, reported Dr. Lebwohl. The rates of nasopharyngitis and oral candidiasis, which were observed in approximately 12% and 8%, respectively, of treated patients during the randomized phase remained at about the same level in the long-term follow up. There were no new safety signals, he said.
JNJ-2113 Is First Potential Oral IL-23 Inhibitor
JNJ-2113 is a first-in-class oral peptide that binds to the IL-23 receptor, blocking the IL-23 signaling pathway. If approved, it would be the first oral therapy targeting IL-23. The 16-week outcomes of the dose-finding FRONTIER 1 phase 2b trial were published in The New England Journal of Medicine earlier this year. The primary endpoint was PASI 75, achieved by 79% of those on the 100 mg twice daily dose at week 16, vs 9% on placebo, and at 52 weeks, was 76%.
“The proportion of patients achieving the FRONTIER 1 primary endpoint was maintained from week 16 to the end of week 52 in the extension study,” Dr. Ferris said, but further pointed out that rates of near or complete clearance achieved at week 16 were also essentially unchanged at week 52. This was true of PASI scores and IGA.
Clearance of psoriatic lesions on the scalp was particularly impressive. By scalp-specific IGA, rates of clear or near clear (0/1) were not just maintained but improved over the course of follow-up, reaching 75.1% at 52 weeks in the highest dose group, she said.
JNJ-2113 was well tolerated in FRONTIER 1 and remained so during long-term follow-up, in the FRONTIER 2 extension study, according to Dr. Ferris. The most common complaints with JNJ-2113, such as nasopharyngitis (18.1% vs 25.7% in placebo), did not appear to differ significantly from placebo and the treatment remained well tolerated over the course of the extended follow-up.
There are limited direct comparisons of different biologics active in the treatment of plaque psoriasis for efficacy and safety, but these data appear to show a depth and durability of benefit for psoriasis that is exceptional, Dr. Lebwohl told this news organization. “The PASI 100 scores achieved by bimekizumab exceed anything we have seen to date,” he said. “And the durability of those exceedingly high scores is remarkable.”
Dr. Lebwohl reports financial relationships with approximately 40 pharmaceutical companies, including UCB Pharma, which developed bimekizumab. Dr. Ferris reports financial relationships with more than 20 pharmaceutical companies, including Janssen, which is developing JNJ-2113.
A version of this article appeared on Medscape.com.
SAN DIEGO — , but late-breaker data presented at the annual meeting of the American Academy of Dermatology show that these types of responses are sustained for as long as patients have remained on therapy.
Of the two, the longer follow up is with the IL-17 inhibitor bimekizumab (Bimzelx). In a 4-year open-label extension study, the Psoriasis Area and Severity Index (PASI) 90 rate was approximately 85% in treated patients, according to Mark Lebwohl, MD, professor and chairman emeritus of the Department of Dermatology, Icahn School of Medicine at Mount Sinai in New York City
A PASI 90 score signifies that 90% of skin surface area is cleared. The proportion of patients who achieved a PASI 100 score, signifying total clearance, approached 70% at 4 years in the group with the greatest response. PASI 90 and PASI 100 rates at this point were only modestly lower than those reported at the end of the double-blind phase 3 trial when evaluated 3 years earlier.
Follow-up with a novel oral anti-IL-23 inhibitor JNJ-2113 (JNJ-77242113) was only 52 weeks, far shorter. But again, the response for the most effective dose at the end of this period was essentially unchanged from that at 16 weeks. Among those on the highest and most effective test dose of once-daily 100 mg, the PASI 90 at 1 year was 64.3%, a rate that was essentially unchanged from week 16.
No Apparent Loss of Benefit Over Time
“We can really look at those dose-response curves and see that there is, overall, a maintenance of response,” reported Laura K. Ferris, MD, PhD, professor and director of clinical trials, Department of Dermatology, University of Pittsburgh Medical Center, Pittsburgh, Pennsylvania. In her presentation of the data, she showed similar sustained control for the most effective doses of JNJ-2113 for multiple clinical outcomes, including an investigator’s global assessment (IGA) score of 0 or 1, also signifying clear or near clear skin.
Bimekizumab, a monoclonal antibody that inhibits both IL-17A and IL-17F, is already approved for the treatment of plaque psoriasis. The 52-week BE SURE trial, which provided the 478 patients who entered into the BE BRIGHT open label extension study, was published in The New England Journal of Medicine in July 2021.
In the 4-year data reported by Dr. Lebwohl, three groups were compared: Those initially randomized to an every-4-week dosing schedule of bimekizumab over the course of the 52-week BE SURE trial; those randomized to an every-4-week bimekizumab schedule who were then subsequently switched to an every-8-week schedule; and those initiated on the TNF-inhibitor adalimumab (Humira) and were then switched at week 24 to every-4-week bimekizumab.
The PASI 90 responses at 52 weeks in these three groups, respectively, were 91.2%, 89.3%, and 95.2%. At 4 years, this almost clear response was observed in 82.4%, 83.2%, and 87.6%, respectively. At 52 weeks, the PASI 100 responses in these three groups, respectively, were 75.3%, 74.2%, and 72.9%. At 4 years, 61.9%, 58.5%, and 69.5% still had complete skin clearance.
Bimekizumab was well tolerated during the randomized trial, reported Dr. Lebwohl. The rates of nasopharyngitis and oral candidiasis, which were observed in approximately 12% and 8%, respectively, of treated patients during the randomized phase remained at about the same level in the long-term follow up. There were no new safety signals, he said.
JNJ-2113 Is First Potential Oral IL-23 Inhibitor
JNJ-2113 is a first-in-class oral peptide that binds to the IL-23 receptor, blocking the IL-23 signaling pathway. If approved, it would be the first oral therapy targeting IL-23. The 16-week outcomes of the dose-finding FRONTIER 1 phase 2b trial were published in The New England Journal of Medicine earlier this year. The primary endpoint was PASI 75, achieved by 79% of those on the 100 mg twice daily dose at week 16, vs 9% on placebo, and at 52 weeks, was 76%.
“The proportion of patients achieving the FRONTIER 1 primary endpoint was maintained from week 16 to the end of week 52 in the extension study,” Dr. Ferris said, but further pointed out that rates of near or complete clearance achieved at week 16 were also essentially unchanged at week 52. This was true of PASI scores and IGA.
Clearance of psoriatic lesions on the scalp was particularly impressive. By scalp-specific IGA, rates of clear or near clear (0/1) were not just maintained but improved over the course of follow-up, reaching 75.1% at 52 weeks in the highest dose group, she said.
JNJ-2113 was well tolerated in FRONTIER 1 and remained so during long-term follow-up, in the FRONTIER 2 extension study, according to Dr. Ferris. The most common complaints with JNJ-2113, such as nasopharyngitis (18.1% vs 25.7% in placebo), did not appear to differ significantly from placebo and the treatment remained well tolerated over the course of the extended follow-up.
There are limited direct comparisons of different biologics active in the treatment of plaque psoriasis for efficacy and safety, but these data appear to show a depth and durability of benefit for psoriasis that is exceptional, Dr. Lebwohl told this news organization. “The PASI 100 scores achieved by bimekizumab exceed anything we have seen to date,” he said. “And the durability of those exceedingly high scores is remarkable.”
Dr. Lebwohl reports financial relationships with approximately 40 pharmaceutical companies, including UCB Pharma, which developed bimekizumab. Dr. Ferris reports financial relationships with more than 20 pharmaceutical companies, including Janssen, which is developing JNJ-2113.
A version of this article appeared on Medscape.com.
FROM AAD 2024
Phase 2 Results: Zerlasiran siRNA Drug Lowers Lp(a) by 90%
Silence Therapeutics shared positive topline 36-week data from its ongoing phase 2 study of zerlasiran, a long-acting agent directed at lowering Lp(a) levels.
In a statement, the company said the study shows a highly significant reduction from baseline in Lp(a) levels with zerlasiran compared with placebo at 36 weeks, the primary endpoint.
Zerlasiran (formerly known as SLN360), is a short interfering RNA (siRNA) agent, or “ gene silencing” therapy. It binds to and temporarily blocks the action of the LPA gene which encodes for apolipoprotein(a), a dominant and a rate-limiting component in the hepatic synthesis of the Lp(a) particle.
A previous phase 1 study showed that single subcutaneous doses of the drug, ranging from 30 mg to 600 mg, produced a dose-dependent reduction in Lp(a) plasma levels at 45-60 days.
The current double-blind placebo-controlled phase 2 trial — known as ALPACAR-360 — enrolled 178 patients at high risk for atherosclerotic cardiovascular events who had elevated levels of Lp(a), ie, ≥ 125 nmol/L (median baseline Lp(a) was approximately 215 nmol/L). They were randomized to zerlasiran or placebo.
Zerlasiran was administered at 300 mg subcutaneously every 16 or 24 weeks or at 450 mg every 24 weeks.
The 60-week study is ongoing, and secondary endpoints, including change in Lp(a) from baseline to 48 weeks (end of treatment period) and 60 weeks (end of study) and potential effects on other lipids/lipoproteins, will be evaluated.
Silence says it plans to report topline 48-week data from the ALPACAR-360 study in the second quarter of this year.
Elevated levels of Lp(a) represent a genetic risk factor for cardiovascular disease, which is believed to affect approximately 20% of the population. Although there are currently no approved Lp(a)-lowering therapies, several drug candidates are in late-stage clinical testing.
A version of this article appeared on Medscape.com.
Silence Therapeutics shared positive topline 36-week data from its ongoing phase 2 study of zerlasiran, a long-acting agent directed at lowering Lp(a) levels.
In a statement, the company said the study shows a highly significant reduction from baseline in Lp(a) levels with zerlasiran compared with placebo at 36 weeks, the primary endpoint.
Zerlasiran (formerly known as SLN360), is a short interfering RNA (siRNA) agent, or “ gene silencing” therapy. It binds to and temporarily blocks the action of the LPA gene which encodes for apolipoprotein(a), a dominant and a rate-limiting component in the hepatic synthesis of the Lp(a) particle.
A previous phase 1 study showed that single subcutaneous doses of the drug, ranging from 30 mg to 600 mg, produced a dose-dependent reduction in Lp(a) plasma levels at 45-60 days.
The current double-blind placebo-controlled phase 2 trial — known as ALPACAR-360 — enrolled 178 patients at high risk for atherosclerotic cardiovascular events who had elevated levels of Lp(a), ie, ≥ 125 nmol/L (median baseline Lp(a) was approximately 215 nmol/L). They were randomized to zerlasiran or placebo.
Zerlasiran was administered at 300 mg subcutaneously every 16 or 24 weeks or at 450 mg every 24 weeks.
The 60-week study is ongoing, and secondary endpoints, including change in Lp(a) from baseline to 48 weeks (end of treatment period) and 60 weeks (end of study) and potential effects on other lipids/lipoproteins, will be evaluated.
Silence says it plans to report topline 48-week data from the ALPACAR-360 study in the second quarter of this year.
Elevated levels of Lp(a) represent a genetic risk factor for cardiovascular disease, which is believed to affect approximately 20% of the population. Although there are currently no approved Lp(a)-lowering therapies, several drug candidates are in late-stage clinical testing.
A version of this article appeared on Medscape.com.
Silence Therapeutics shared positive topline 36-week data from its ongoing phase 2 study of zerlasiran, a long-acting agent directed at lowering Lp(a) levels.
In a statement, the company said the study shows a highly significant reduction from baseline in Lp(a) levels with zerlasiran compared with placebo at 36 weeks, the primary endpoint.
Zerlasiran (formerly known as SLN360), is a short interfering RNA (siRNA) agent, or “ gene silencing” therapy. It binds to and temporarily blocks the action of the LPA gene which encodes for apolipoprotein(a), a dominant and a rate-limiting component in the hepatic synthesis of the Lp(a) particle.
A previous phase 1 study showed that single subcutaneous doses of the drug, ranging from 30 mg to 600 mg, produced a dose-dependent reduction in Lp(a) plasma levels at 45-60 days.
The current double-blind placebo-controlled phase 2 trial — known as ALPACAR-360 — enrolled 178 patients at high risk for atherosclerotic cardiovascular events who had elevated levels of Lp(a), ie, ≥ 125 nmol/L (median baseline Lp(a) was approximately 215 nmol/L). They were randomized to zerlasiran or placebo.
Zerlasiran was administered at 300 mg subcutaneously every 16 or 24 weeks or at 450 mg every 24 weeks.
The 60-week study is ongoing, and secondary endpoints, including change in Lp(a) from baseline to 48 weeks (end of treatment period) and 60 weeks (end of study) and potential effects on other lipids/lipoproteins, will be evaluated.
Silence says it plans to report topline 48-week data from the ALPACAR-360 study in the second quarter of this year.
Elevated levels of Lp(a) represent a genetic risk factor for cardiovascular disease, which is believed to affect approximately 20% of the population. Although there are currently no approved Lp(a)-lowering therapies, several drug candidates are in late-stage clinical testing.
A version of this article appeared on Medscape.com.
Tirzepatide Weight Loss Consistent Regardless of BMI
Tirzepatide (Zepbound for weight loss; Mounjaro for type 2 diabetes; Eli Lilly) consistently reduced body weight regardless of pretreatment body mass index (BMI) and reduced body weight and waist circumference regardless of duration of overweight or obesity.
The analyses — firstly of the impact of baseline BMI and secondly investigating the impact of the duration of overweight/obesity — are drawn from combined findings from the SURMOUNT 1-4 studies that examined the efficacy and safety of tirzepatide vs placebo. They are scheduled to be presented at May’s European Congress on Obesity (ECO) by Carel Le Roux, MD, University College Dublin, Ireland, and Giovanna Dr. Muscogiuri, MD, endocrinologist from the University of Naples Federico II, Naples, Italy, respectively.
The first analysis of tirzepatide, a dual glucose-dependent insulinotropic polypeptide and glucagon-like peptide 1 receptor agonist, aimed to analyze the impact of baseline BMI category on weight reduction across the series of phase 3 trials.
More participants on tirzepatide than on placebo achieved the body weight reduction targets of 5%, 10%, and 15%. “Across the SURMOUNT 1-4 trials, treatment with tirzepatide, along with a reduced-calorie diet and increased physical activity, consistently resulted in clinically significant weight reductions of 5% or more, 10% or more, or 15% or more, as compared to placebo, regardless of baseline BMI subgroup, in adults with obesity or overweight (BMI of 27 and above),” said obesity specialist, Louis J. Aronne, MD, from the Comprehensive Weight Control Center, Weill Cornell Medicine, New York City, and coauthor of the BMI-related analysis.
Dr. Muscogiuri, who is first author of the second analysis that looked at the impact of duration of adiposity, and her coauthors concluded that, “Tirzepatide consistently reduced body weight and waist circumference in people living with obesity or overweight with weight-related comorbidities regardless of the duration of disease. These results are consistent with the overall findings from each study in the SURMOUNT program.”
Weight Loss Consistent Regardless of BMI
The SURMOUNT series of trials involved people with a BMI of 30 kg/m2 and above, or 27 kg/m2 with at least one weight-related comorbidity without type 2 diabetes (SURMOUNT-1, 72 weeks), with type 2 diabetes (SURMOUNT-2, 72 weeks), and without type 2 diabetes after a 12-week intensive lifestyle intervention (SURMOUNT-3, 72 weeks from randomization) or after an 88 week intervention (SURMOUNT-4, 36-week open label tirzepatide lead-in and 52 weeks following randomization).
BMI subgroups were defined by 27-30 (overweight), 30-35 (obesity class I), 35-40 (obesity class II), and 40 kg/m2 and above (obesity class III). Percentage change in body weight from randomization to week 72 (SURMOUNT-1, -2, and -3) or to week 52 (SURMOUNT-4) was determined, as well as the proportions of participants achieving the weight reduction targets of 5%, 10%, and 15%. The per protocol analyses included all participants who received at least one dose of tirzepatide or placebo.
Across these BMI levels, up to 100% of tirzepatide-treated participants achieved weight reduction of 5% or more compared with 30% on placebo in SURMOUNT-1, up to 93% vs 43% in SURMOUNT-2, and up to 97% vs 15%, respectively, in SURMOUNT-3.
At least 10% weight reduction was achieved by up to 93% vs 16%, respectively, in SURMOUNT-1, up to 76% vs 14% in SURMOUNT-2, and up to 92% vs 8% in SURMOUNT-3.
Weight reduction of 15% was achieved by up to 85% compared with 7% of patients on tirzepatide and placebo, respectively, in SURMOUNT-1; up to 60% vs 3%, respectively, in SURMOUNT-2; and up to 78% vs 4% in SURMOUNT-3.
In SURMOUNT-4, during the 36-week open-label tirzepatide treatment, the mean body weight % or more reduction was 21%. Following this lead-in period, further weight reductions of 5% or more, 10%, and 15% or more were achieved by up to 70%, 39%, and 22%, respectively, of participants treated with tirzepatide compared with 2%, 2%, and 0% of patients on placebo.
Body Weight and Waist Circumference Reduced Regardless of Disease Duration
In this second presentation, participants were categorized based on duration with overweight/obesity at baseline (10 years or less, between 10 and 20 years, and above 20 years). Percentage body weight change; the proportions achieving weight loss targets of 5%, 10%, 15%, 20%, and 25%; and the change in waist circumference were analyzed.
Greater weight reductions were found in participants who took tirzepatide than in those who took placebo across the SURMOUNT 1-4 study endpoints, including weight reduction targets of 5%, 10%, 15%, 20%, and 25% compared with placebo-treated participants, regardless of disease duration, reported the authors in an early press release from ECO. The magnitude of weight reductions was generally similar across the disease duration categories.
For example, in the SURMOUNT-1 trial, for patients given 10-mg dose of tirzepatide, those with disease duration under 10 years lost 21% of their weight after 72 weeks compared with 20% body weight loss for those with 10-20 years disease duration and 23% for those with over 20 years disease duration.
In the SURMOUNT-2 trial (where all participants were also living with type 2 diabetes), for patients given the 10-mg dose of tirzepatide, those with disease duration under 10 years lost 12.6% of their body weight, while those with disease duration of 10-20 years lost 12.5%; in people living with overweight or obesity for over 20 years, 14.4% of body weight was lost.
Waist circumference also reduced to a greater extent than placebo for each disease duration category across the four studies, and again, these reductions were consistent across disease duration subgroups.
A difference between patients with and without type 2 diabetes was evident and requires further analysis to explore and understand why patients with type 2 diabetes have less weight loss in these trials than those without type 2 diabetes.
Asked to comment on the findings, Jens Juul Holst, MD, from the Department of Biomedical Sciences and Novo Nordisk Foundation Center for Basic Metabolic Research, University of Copenhagen, Copenhagen, Denmark, said that the results were as expected.
“The first abstract is said to show that there is the same effect regardless of the baseline BMI, but this is the expected outcome — nothing exciting there,” he told this news organization. “The second deals with the effects in people with different duration of adiposity. Again, it was equally effective in all groups and that was also the expected outcome, although important.”
“One question is whether one should treat people with BMI < 30 at all, and that depends on preexisting comorbidities — in particular metabolic syndrome, where treatment could be lifesaving and prevent complications,” added Dr. Holst.
This news organization also asked Jason Halford, ECO president, for his view on the findings. He remarked that with these weight loss drugs overall, “Usually weight loss tends to be proportional and actually greater in the lower BMI categories. This is partly because dosing is not done by body weight, and everyone gets the same doses irrespective of how they weigh. There is an argument that doses should be adjusted. The data suggests these drugs are so potent this does not occur for some reason.”
Dr. Holst added that, “In principle, for a given reduction in food intake, one would expect a similar reduction in body mass, and these agents should be dosed according to the size of the individual — since energy expenditure depends linearly on body weight, this is probably a reasonable measure. But what actually happens is dosing is according to the occurrence of side effects, which is a good pragmatic principle.”
Dr. Holst pointed out that the interesting question here is whether the very obese would somehow be resistant to the GLP-1 RAs (like leptin) — “they are not,” he noted.
He added that to his knowledge, the question around the role played by duration of the adiposity had not been explicitly looked at before. “However, the many individuals with obesity studied after GLP-1 RA treatment have varied widely with respect to duration and weight loss has not previously been known to depend on this, but there is no known physiological mechanism underpinning this.”
Tirzepatide (Mounjaro) was approved by the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) for the treatment of type 2 diabetes in 2022. In November 2023, the FDA approved tirzepatide (Zepbound) for chronic weight management in adults with BMI ≥ 30 kg/m2 or BMI ≥ 27 kg/m2 with at least one weight-related comorbidity. Also in November 2023, the EMA Committee for Medicinal Products for Human Use offered a positive opinion on extension of the Mounjaro label to include weight management in adults with BMI ≥ 30 kg/m2 or BMI ≥ 27 kg/m2 and at least one weight-related comorbid condition.
Dr. Holst had no conflicting interest with Eli Lilly but is a member of advisory boards for Novo Nordisk. This work (abstract 014) was funded by Eli Lilly and Company. Dr. Le Roux reported grants from the Irish Research Council, Science Foundation Ireland, Anabio, and the Health Research Board. He served on advisory boards and speaker panels of Novo Nordisk, Herbalife, GI Dynamics, Eli Lilly, Johnson & Johnson, Glia, Irish Life Health, Boehringer Ingelheim, Currax, Zealand Pharma, and Rhythm Pharma. Dr. Le Roux is a member of the Irish Society for Nutrition and Metabolism outside the area of work commented on here. He was the chief medical officer and director of the Medical Device Division of Keyron in 2021. Both of these are unremunerated positions. Dr. Le Roux was a previous investor in Keyron, which develops endoscopically implantable medical devices intended to mimic the surgical procedures of sleeve gastrectomy and gastric bypass. No patients have been included in any of Keyron’s studies, and they are not listed on the stock market. Dr. Le Roux was gifted stock holdings in September 2021 and divested all stock holdings in Keyron in September 2021. He continues to provide scientific advice to Keyron for no remuneration. Dr. Le Roux provides obesity clinical care in the Beyond BMI clinic and is a shareholder in the clinic. Dr. Aronne reported receiving grants or personal fees from Altimmune, AstraZeneca, Boehringer Ingelheim, Eli Lilly, ERX, Gelesis, Intellihealth, Jamieson Wellness, Janssen, Novo Nordisk, Optum, Pfizer, Senda Biosciences, and Versanis and being a shareholder of Allurion, ERX Pharmaceuticals, Gelesis, Intellihealth, and Jamieson Wellness. FJ, TF, MM, LG, and LN are employees and shareholders of Eli Lilly and Company.
A version of this article appeared on Medscape.com.
Tirzepatide (Zepbound for weight loss; Mounjaro for type 2 diabetes; Eli Lilly) consistently reduced body weight regardless of pretreatment body mass index (BMI) and reduced body weight and waist circumference regardless of duration of overweight or obesity.
The analyses — firstly of the impact of baseline BMI and secondly investigating the impact of the duration of overweight/obesity — are drawn from combined findings from the SURMOUNT 1-4 studies that examined the efficacy and safety of tirzepatide vs placebo. They are scheduled to be presented at May’s European Congress on Obesity (ECO) by Carel Le Roux, MD, University College Dublin, Ireland, and Giovanna Dr. Muscogiuri, MD, endocrinologist from the University of Naples Federico II, Naples, Italy, respectively.
The first analysis of tirzepatide, a dual glucose-dependent insulinotropic polypeptide and glucagon-like peptide 1 receptor agonist, aimed to analyze the impact of baseline BMI category on weight reduction across the series of phase 3 trials.
More participants on tirzepatide than on placebo achieved the body weight reduction targets of 5%, 10%, and 15%. “Across the SURMOUNT 1-4 trials, treatment with tirzepatide, along with a reduced-calorie diet and increased physical activity, consistently resulted in clinically significant weight reductions of 5% or more, 10% or more, or 15% or more, as compared to placebo, regardless of baseline BMI subgroup, in adults with obesity or overweight (BMI of 27 and above),” said obesity specialist, Louis J. Aronne, MD, from the Comprehensive Weight Control Center, Weill Cornell Medicine, New York City, and coauthor of the BMI-related analysis.
Dr. Muscogiuri, who is first author of the second analysis that looked at the impact of duration of adiposity, and her coauthors concluded that, “Tirzepatide consistently reduced body weight and waist circumference in people living with obesity or overweight with weight-related comorbidities regardless of the duration of disease. These results are consistent with the overall findings from each study in the SURMOUNT program.”
Weight Loss Consistent Regardless of BMI
The SURMOUNT series of trials involved people with a BMI of 30 kg/m2 and above, or 27 kg/m2 with at least one weight-related comorbidity without type 2 diabetes (SURMOUNT-1, 72 weeks), with type 2 diabetes (SURMOUNT-2, 72 weeks), and without type 2 diabetes after a 12-week intensive lifestyle intervention (SURMOUNT-3, 72 weeks from randomization) or after an 88 week intervention (SURMOUNT-4, 36-week open label tirzepatide lead-in and 52 weeks following randomization).
BMI subgroups were defined by 27-30 (overweight), 30-35 (obesity class I), 35-40 (obesity class II), and 40 kg/m2 and above (obesity class III). Percentage change in body weight from randomization to week 72 (SURMOUNT-1, -2, and -3) or to week 52 (SURMOUNT-4) was determined, as well as the proportions of participants achieving the weight reduction targets of 5%, 10%, and 15%. The per protocol analyses included all participants who received at least one dose of tirzepatide or placebo.
Across these BMI levels, up to 100% of tirzepatide-treated participants achieved weight reduction of 5% or more compared with 30% on placebo in SURMOUNT-1, up to 93% vs 43% in SURMOUNT-2, and up to 97% vs 15%, respectively, in SURMOUNT-3.
At least 10% weight reduction was achieved by up to 93% vs 16%, respectively, in SURMOUNT-1, up to 76% vs 14% in SURMOUNT-2, and up to 92% vs 8% in SURMOUNT-3.
Weight reduction of 15% was achieved by up to 85% compared with 7% of patients on tirzepatide and placebo, respectively, in SURMOUNT-1; up to 60% vs 3%, respectively, in SURMOUNT-2; and up to 78% vs 4% in SURMOUNT-3.
In SURMOUNT-4, during the 36-week open-label tirzepatide treatment, the mean body weight % or more reduction was 21%. Following this lead-in period, further weight reductions of 5% or more, 10%, and 15% or more were achieved by up to 70%, 39%, and 22%, respectively, of participants treated with tirzepatide compared with 2%, 2%, and 0% of patients on placebo.
Body Weight and Waist Circumference Reduced Regardless of Disease Duration
In this second presentation, participants were categorized based on duration with overweight/obesity at baseline (10 years or less, between 10 and 20 years, and above 20 years). Percentage body weight change; the proportions achieving weight loss targets of 5%, 10%, 15%, 20%, and 25%; and the change in waist circumference were analyzed.
Greater weight reductions were found in participants who took tirzepatide than in those who took placebo across the SURMOUNT 1-4 study endpoints, including weight reduction targets of 5%, 10%, 15%, 20%, and 25% compared with placebo-treated participants, regardless of disease duration, reported the authors in an early press release from ECO. The magnitude of weight reductions was generally similar across the disease duration categories.
For example, in the SURMOUNT-1 trial, for patients given 10-mg dose of tirzepatide, those with disease duration under 10 years lost 21% of their weight after 72 weeks compared with 20% body weight loss for those with 10-20 years disease duration and 23% for those with over 20 years disease duration.
In the SURMOUNT-2 trial (where all participants were also living with type 2 diabetes), for patients given the 10-mg dose of tirzepatide, those with disease duration under 10 years lost 12.6% of their body weight, while those with disease duration of 10-20 years lost 12.5%; in people living with overweight or obesity for over 20 years, 14.4% of body weight was lost.
Waist circumference also reduced to a greater extent than placebo for each disease duration category across the four studies, and again, these reductions were consistent across disease duration subgroups.
A difference between patients with and without type 2 diabetes was evident and requires further analysis to explore and understand why patients with type 2 diabetes have less weight loss in these trials than those without type 2 diabetes.
Asked to comment on the findings, Jens Juul Holst, MD, from the Department of Biomedical Sciences and Novo Nordisk Foundation Center for Basic Metabolic Research, University of Copenhagen, Copenhagen, Denmark, said that the results were as expected.
“The first abstract is said to show that there is the same effect regardless of the baseline BMI, but this is the expected outcome — nothing exciting there,” he told this news organization. “The second deals with the effects in people with different duration of adiposity. Again, it was equally effective in all groups and that was also the expected outcome, although important.”
“One question is whether one should treat people with BMI < 30 at all, and that depends on preexisting comorbidities — in particular metabolic syndrome, where treatment could be lifesaving and prevent complications,” added Dr. Holst.
This news organization also asked Jason Halford, ECO president, for his view on the findings. He remarked that with these weight loss drugs overall, “Usually weight loss tends to be proportional and actually greater in the lower BMI categories. This is partly because dosing is not done by body weight, and everyone gets the same doses irrespective of how they weigh. There is an argument that doses should be adjusted. The data suggests these drugs are so potent this does not occur for some reason.”
Dr. Holst added that, “In principle, for a given reduction in food intake, one would expect a similar reduction in body mass, and these agents should be dosed according to the size of the individual — since energy expenditure depends linearly on body weight, this is probably a reasonable measure. But what actually happens is dosing is according to the occurrence of side effects, which is a good pragmatic principle.”
Dr. Holst pointed out that the interesting question here is whether the very obese would somehow be resistant to the GLP-1 RAs (like leptin) — “they are not,” he noted.
He added that to his knowledge, the question around the role played by duration of the adiposity had not been explicitly looked at before. “However, the many individuals with obesity studied after GLP-1 RA treatment have varied widely with respect to duration and weight loss has not previously been known to depend on this, but there is no known physiological mechanism underpinning this.”
Tirzepatide (Mounjaro) was approved by the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) for the treatment of type 2 diabetes in 2022. In November 2023, the FDA approved tirzepatide (Zepbound) for chronic weight management in adults with BMI ≥ 30 kg/m2 or BMI ≥ 27 kg/m2 with at least one weight-related comorbidity. Also in November 2023, the EMA Committee for Medicinal Products for Human Use offered a positive opinion on extension of the Mounjaro label to include weight management in adults with BMI ≥ 30 kg/m2 or BMI ≥ 27 kg/m2 and at least one weight-related comorbid condition.
Dr. Holst had no conflicting interest with Eli Lilly but is a member of advisory boards for Novo Nordisk. This work (abstract 014) was funded by Eli Lilly and Company. Dr. Le Roux reported grants from the Irish Research Council, Science Foundation Ireland, Anabio, and the Health Research Board. He served on advisory boards and speaker panels of Novo Nordisk, Herbalife, GI Dynamics, Eli Lilly, Johnson & Johnson, Glia, Irish Life Health, Boehringer Ingelheim, Currax, Zealand Pharma, and Rhythm Pharma. Dr. Le Roux is a member of the Irish Society for Nutrition and Metabolism outside the area of work commented on here. He was the chief medical officer and director of the Medical Device Division of Keyron in 2021. Both of these are unremunerated positions. Dr. Le Roux was a previous investor in Keyron, which develops endoscopically implantable medical devices intended to mimic the surgical procedures of sleeve gastrectomy and gastric bypass. No patients have been included in any of Keyron’s studies, and they are not listed on the stock market. Dr. Le Roux was gifted stock holdings in September 2021 and divested all stock holdings in Keyron in September 2021. He continues to provide scientific advice to Keyron for no remuneration. Dr. Le Roux provides obesity clinical care in the Beyond BMI clinic and is a shareholder in the clinic. Dr. Aronne reported receiving grants or personal fees from Altimmune, AstraZeneca, Boehringer Ingelheim, Eli Lilly, ERX, Gelesis, Intellihealth, Jamieson Wellness, Janssen, Novo Nordisk, Optum, Pfizer, Senda Biosciences, and Versanis and being a shareholder of Allurion, ERX Pharmaceuticals, Gelesis, Intellihealth, and Jamieson Wellness. FJ, TF, MM, LG, and LN are employees and shareholders of Eli Lilly and Company.
A version of this article appeared on Medscape.com.
Tirzepatide (Zepbound for weight loss; Mounjaro for type 2 diabetes; Eli Lilly) consistently reduced body weight regardless of pretreatment body mass index (BMI) and reduced body weight and waist circumference regardless of duration of overweight or obesity.
The analyses — firstly of the impact of baseline BMI and secondly investigating the impact of the duration of overweight/obesity — are drawn from combined findings from the SURMOUNT 1-4 studies that examined the efficacy and safety of tirzepatide vs placebo. They are scheduled to be presented at May’s European Congress on Obesity (ECO) by Carel Le Roux, MD, University College Dublin, Ireland, and Giovanna Dr. Muscogiuri, MD, endocrinologist from the University of Naples Federico II, Naples, Italy, respectively.
The first analysis of tirzepatide, a dual glucose-dependent insulinotropic polypeptide and glucagon-like peptide 1 receptor agonist, aimed to analyze the impact of baseline BMI category on weight reduction across the series of phase 3 trials.
More participants on tirzepatide than on placebo achieved the body weight reduction targets of 5%, 10%, and 15%. “Across the SURMOUNT 1-4 trials, treatment with tirzepatide, along with a reduced-calorie diet and increased physical activity, consistently resulted in clinically significant weight reductions of 5% or more, 10% or more, or 15% or more, as compared to placebo, regardless of baseline BMI subgroup, in adults with obesity or overweight (BMI of 27 and above),” said obesity specialist, Louis J. Aronne, MD, from the Comprehensive Weight Control Center, Weill Cornell Medicine, New York City, and coauthor of the BMI-related analysis.
Dr. Muscogiuri, who is first author of the second analysis that looked at the impact of duration of adiposity, and her coauthors concluded that, “Tirzepatide consistently reduced body weight and waist circumference in people living with obesity or overweight with weight-related comorbidities regardless of the duration of disease. These results are consistent with the overall findings from each study in the SURMOUNT program.”
Weight Loss Consistent Regardless of BMI
The SURMOUNT series of trials involved people with a BMI of 30 kg/m2 and above, or 27 kg/m2 with at least one weight-related comorbidity without type 2 diabetes (SURMOUNT-1, 72 weeks), with type 2 diabetes (SURMOUNT-2, 72 weeks), and without type 2 diabetes after a 12-week intensive lifestyle intervention (SURMOUNT-3, 72 weeks from randomization) or after an 88 week intervention (SURMOUNT-4, 36-week open label tirzepatide lead-in and 52 weeks following randomization).
BMI subgroups were defined by 27-30 (overweight), 30-35 (obesity class I), 35-40 (obesity class II), and 40 kg/m2 and above (obesity class III). Percentage change in body weight from randomization to week 72 (SURMOUNT-1, -2, and -3) or to week 52 (SURMOUNT-4) was determined, as well as the proportions of participants achieving the weight reduction targets of 5%, 10%, and 15%. The per protocol analyses included all participants who received at least one dose of tirzepatide or placebo.
Across these BMI levels, up to 100% of tirzepatide-treated participants achieved weight reduction of 5% or more compared with 30% on placebo in SURMOUNT-1, up to 93% vs 43% in SURMOUNT-2, and up to 97% vs 15%, respectively, in SURMOUNT-3.
At least 10% weight reduction was achieved by up to 93% vs 16%, respectively, in SURMOUNT-1, up to 76% vs 14% in SURMOUNT-2, and up to 92% vs 8% in SURMOUNT-3.
Weight reduction of 15% was achieved by up to 85% compared with 7% of patients on tirzepatide and placebo, respectively, in SURMOUNT-1; up to 60% vs 3%, respectively, in SURMOUNT-2; and up to 78% vs 4% in SURMOUNT-3.
In SURMOUNT-4, during the 36-week open-label tirzepatide treatment, the mean body weight % or more reduction was 21%. Following this lead-in period, further weight reductions of 5% or more, 10%, and 15% or more were achieved by up to 70%, 39%, and 22%, respectively, of participants treated with tirzepatide compared with 2%, 2%, and 0% of patients on placebo.
Body Weight and Waist Circumference Reduced Regardless of Disease Duration
In this second presentation, participants were categorized based on duration with overweight/obesity at baseline (10 years or less, between 10 and 20 years, and above 20 years). Percentage body weight change; the proportions achieving weight loss targets of 5%, 10%, 15%, 20%, and 25%; and the change in waist circumference were analyzed.
Greater weight reductions were found in participants who took tirzepatide than in those who took placebo across the SURMOUNT 1-4 study endpoints, including weight reduction targets of 5%, 10%, 15%, 20%, and 25% compared with placebo-treated participants, regardless of disease duration, reported the authors in an early press release from ECO. The magnitude of weight reductions was generally similar across the disease duration categories.
For example, in the SURMOUNT-1 trial, for patients given 10-mg dose of tirzepatide, those with disease duration under 10 years lost 21% of their weight after 72 weeks compared with 20% body weight loss for those with 10-20 years disease duration and 23% for those with over 20 years disease duration.
In the SURMOUNT-2 trial (where all participants were also living with type 2 diabetes), for patients given the 10-mg dose of tirzepatide, those with disease duration under 10 years lost 12.6% of their body weight, while those with disease duration of 10-20 years lost 12.5%; in people living with overweight or obesity for over 20 years, 14.4% of body weight was lost.
Waist circumference also reduced to a greater extent than placebo for each disease duration category across the four studies, and again, these reductions were consistent across disease duration subgroups.
A difference between patients with and without type 2 diabetes was evident and requires further analysis to explore and understand why patients with type 2 diabetes have less weight loss in these trials than those without type 2 diabetes.
Asked to comment on the findings, Jens Juul Holst, MD, from the Department of Biomedical Sciences and Novo Nordisk Foundation Center for Basic Metabolic Research, University of Copenhagen, Copenhagen, Denmark, said that the results were as expected.
“The first abstract is said to show that there is the same effect regardless of the baseline BMI, but this is the expected outcome — nothing exciting there,” he told this news organization. “The second deals with the effects in people with different duration of adiposity. Again, it was equally effective in all groups and that was also the expected outcome, although important.”
“One question is whether one should treat people with BMI < 30 at all, and that depends on preexisting comorbidities — in particular metabolic syndrome, where treatment could be lifesaving and prevent complications,” added Dr. Holst.
This news organization also asked Jason Halford, ECO president, for his view on the findings. He remarked that with these weight loss drugs overall, “Usually weight loss tends to be proportional and actually greater in the lower BMI categories. This is partly because dosing is not done by body weight, and everyone gets the same doses irrespective of how they weigh. There is an argument that doses should be adjusted. The data suggests these drugs are so potent this does not occur for some reason.”
Dr. Holst added that, “In principle, for a given reduction in food intake, one would expect a similar reduction in body mass, and these agents should be dosed according to the size of the individual — since energy expenditure depends linearly on body weight, this is probably a reasonable measure. But what actually happens is dosing is according to the occurrence of side effects, which is a good pragmatic principle.”
Dr. Holst pointed out that the interesting question here is whether the very obese would somehow be resistant to the GLP-1 RAs (like leptin) — “they are not,” he noted.
He added that to his knowledge, the question around the role played by duration of the adiposity had not been explicitly looked at before. “However, the many individuals with obesity studied after GLP-1 RA treatment have varied widely with respect to duration and weight loss has not previously been known to depend on this, but there is no known physiological mechanism underpinning this.”
Tirzepatide (Mounjaro) was approved by the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) for the treatment of type 2 diabetes in 2022. In November 2023, the FDA approved tirzepatide (Zepbound) for chronic weight management in adults with BMI ≥ 30 kg/m2 or BMI ≥ 27 kg/m2 with at least one weight-related comorbidity. Also in November 2023, the EMA Committee for Medicinal Products for Human Use offered a positive opinion on extension of the Mounjaro label to include weight management in adults with BMI ≥ 30 kg/m2 or BMI ≥ 27 kg/m2 and at least one weight-related comorbid condition.
Dr. Holst had no conflicting interest with Eli Lilly but is a member of advisory boards for Novo Nordisk. This work (abstract 014) was funded by Eli Lilly and Company. Dr. Le Roux reported grants from the Irish Research Council, Science Foundation Ireland, Anabio, and the Health Research Board. He served on advisory boards and speaker panels of Novo Nordisk, Herbalife, GI Dynamics, Eli Lilly, Johnson & Johnson, Glia, Irish Life Health, Boehringer Ingelheim, Currax, Zealand Pharma, and Rhythm Pharma. Dr. Le Roux is a member of the Irish Society for Nutrition and Metabolism outside the area of work commented on here. He was the chief medical officer and director of the Medical Device Division of Keyron in 2021. Both of these are unremunerated positions. Dr. Le Roux was a previous investor in Keyron, which develops endoscopically implantable medical devices intended to mimic the surgical procedures of sleeve gastrectomy and gastric bypass. No patients have been included in any of Keyron’s studies, and they are not listed on the stock market. Dr. Le Roux was gifted stock holdings in September 2021 and divested all stock holdings in Keyron in September 2021. He continues to provide scientific advice to Keyron for no remuneration. Dr. Le Roux provides obesity clinical care in the Beyond BMI clinic and is a shareholder in the clinic. Dr. Aronne reported receiving grants or personal fees from Altimmune, AstraZeneca, Boehringer Ingelheim, Eli Lilly, ERX, Gelesis, Intellihealth, Jamieson Wellness, Janssen, Novo Nordisk, Optum, Pfizer, Senda Biosciences, and Versanis and being a shareholder of Allurion, ERX Pharmaceuticals, Gelesis, Intellihealth, and Jamieson Wellness. FJ, TF, MM, LG, and LN are employees and shareholders of Eli Lilly and Company.
A version of this article appeared on Medscape.com.