User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Studies validate IL-17 as hidradenitis suppurativa drug target
NEW ORLEANS – In two phase 3 trials, bimekizumab, a monoclonal antibody targeting two types of interleukin-17 — IL-17A and IL-17F — reduced the abscess and inflammatory nodule count better than placebo in the chronic inflammatory skin condition hidradenitis suppurativa (HS), according to results presented together during a late-breaker session at the annual meeting of the American Academy of Dermatology.
“We are very excited to add this data to what we already have around IL-17 inhibition. This clearly validates this target for the control of HS,” reported lead investigator Alexa B. Kimball, MD, MPH, professor of dermatology at Harvard Medical School and Beth Israel Deaconess Medical Center, both in Boston.
The trials, called BE HEARD I and BE HEARD II, enrolled 505 and 509 patients with HS, respectively. About 50% of patients in BE HEARD I and 60% of patients in BE HEARD II had Hurley stage 3 disease, which is the most severe of the three stratifications. The remainder were in Hurley stage 2. The mean duration of HS was 8.3 and 7.1 years, respectively.
Patients in both studies were randomized to one of four groups – either to a dosing regimen of 320 mg of bimekizumab administered by subcutaneous injection or to a placebo group. Both trials comprised double-blind 16-week initial and 32-week maintenance treatment periods.
In one experimental group, bimekizumab was given once every 2 weeks for the full course of the 48-week study (Q2W/Q2W). In another, patients started on the every-2-week schedule for 16 weeks and then were switched to every-4-week dosing (Q2W/Q4W). In the third group, patients started and remained on the every-4-week schedule (Q4W/Q4W). Patients in a fourth group started on placebo and switched at 16 weeks to the every-2-week bimekizumab schedule (placebo/Q2W).
Results at primary endpoint
The primary endpoint was HiSCR50, signifying a 50% reduction from baseline in abscess and inflammatory nodule count on the Hidradenitis Suppurativa Clinical Response (HiSCR) assessment tool. At 16 weeks, the initial Q2W dose in two of the groups outperformed the placebo in both BE HEARD I (47.8% vs. 28.7%) and BE HEARD II (52.0% vs. 32.2%). The response rates in the Q4W arm in BE HEARD I (45.3%) and BE HEARD II (53.8%) were also higher than the placebo, but the difference was only significant in BE HEARD II.
At 48 weeks, the proportion of patients with an HiSCR50 response climbed in all groups in both trials. The patterns were generally the same with slightly higher numerical responses among the groups that received the every-2-week dosing schedule relative to the every-4-week schedule.
In BE HEARD I at 48 weeks, the HiSCR50 response rate was about 60% for those who started and remained on every-2-week bimekizumab (Q2W/Q2W) or were switched at 16 weeks to every-4-week bimekizumab (Q2W/Q4W). For those who started and remained on every-4-week bimekizumab and the group started on placebo and switched to every-2-week bimekizumab, the response rates were 52.7% and 45.3%, respectively.
In BE HEARD II, the HiSCR50 response rates were higher in all groups, including the placebo, and the patterns of response were similar at 48 weeks. Most patients reached the HiSCR50 response – 79.8% (Q2W/Q2W), 78.4% (Q2W/Q4W), 76.7% (Q4W/Q4W), and 65.9 % (placebo/Q2W) of patients.
It is notable that, although there was rapid increase in the proportion of placebo patients reaching HiSCR50 after the switch at 16 weeks, there appeared to be an advantage at 48 weeks for starting on full-dose bimekizumab over starting on placebo.
In this trial, patients were listed as nonresponders if they received antibiotics at any time and for any reason after randomization. This might have concealed an even greater benefit of bimekizumab, Dr. Kimball said, but the study design element was considered necessary to isolate the activity of the study drug.
“In future HS trials, it will be helpful to address the difficulty of handling the impact of antibiotics and pain medications [in assessing results],” Dr. Kimball said.
Clinically meaningful secondary endpoint
For HS patients, the secondary endpoint of HiSCR75 might be considered the most meaningful, according to Dr. Kimball. She said that this higher bar not only documents a higher level of efficacy but correlates with meaningful improvement in quality of life. In the two trials combined, more than 55% of patients on continuous bimekizumab achieved HiSCR75 at week 48 in the observed case analysis, according to a news release from biopharmaceutical company UCB, developer of bimekizumab.
In BE HEARD I, the HiSCR75 rates were 33.4% and 24.7% for the every-2-week and every-4-week bimekizumab doses, respectively. The 33.4% response was statistically superior to placebo (18.4%). In BE HEARD II, both the every-2-week dose (35.7%) and the every-4-week dose (33.7%) were superior to the 15.6% response in placebo patients.
The improvements in quality of life as measured with the Dermatology Life Quality Index (DLQI), reflected the changes in disease activity. Relative to about a 3-point reduction from baseline in the placebo groups of the two trials, the 5-point reduction for either the 2-week or 4-week bimekizumab groups in each clinical trial were highly significant, Dr. Kimball said.
Bimekizumab was relatively well tolerated, although it shares the increased risk for candidiasis observed with this agent when used in psoriasis and with other IL-17 inhibitors, such as secukinumab (Cosentyx), in general. The risk of candidiasis appeared to be dose related, but cases were generally mild and easily managed, according to Dr. Kimball. She noted that only three patients discontinued treatment for this reason. Discontinuations for a treatment-related adverse event overall was less than 4% at 16 weeks.
This is only the third phase 3 trial ever completed in patients with HS. In fact, Dr. Kimball has led all of the phase 3 trials so far, including clinical studies of adalimumab (Humira), published in 2016, and of secukinumab, published earlier this year. All were positive studies.
“This is amazing news for our patients,” Dr. Kimball said. HS remains a challenging disease, even with a growing number of options showing benefit in large studies, she said, and the high rate of response, particularly at the level of HiSCR75, “is a huge milestone for what we can achieve.”
Multiple treatment options important
Her assessment was echoed by other experts, including Christopher J. Sayed, MD, an associate professor of dermatology at the University of North Carolina at Chapel Hill, who publishes frequently about this disease.
“It is incredibly exciting to see the strong phase 3 data on bimekizumab, particularly the deep responses at the HiSCR75 in a majority of patients after the first year,” he said.
Importantly, he does not see the growing array of treatment options as necessarily competitive for a disease with heterogeneous manifestations and variable responses to any one agent.
“While this may be a major step forward, it will still be critical to see more drugs come along for those who do not respond fully enough or have comorbidities that prevent the use of IL-17 and TNF [tumor necrosis factor] antagonists,” he said.
Bimekizumab is not approved for any indication in the United States; it is approved for treating moderate to severe plaque psoriasis in adults who are candidates for systemic therapy in the EU/EEA, where it is marketed as Bimzelx, according to UCB. Dr. Kimball reports financial relationships with AbbVie, Janssen, Kymera, Lilly, Novartis, Pfizer, and UCB. Dr. Sayed reports financial relationships with AbbVie, InflaRx, and UCB.
A version of this article first appeared on Medscape.com.
NEW ORLEANS – In two phase 3 trials, bimekizumab, a monoclonal antibody targeting two types of interleukin-17 — IL-17A and IL-17F — reduced the abscess and inflammatory nodule count better than placebo in the chronic inflammatory skin condition hidradenitis suppurativa (HS), according to results presented together during a late-breaker session at the annual meeting of the American Academy of Dermatology.
“We are very excited to add this data to what we already have around IL-17 inhibition. This clearly validates this target for the control of HS,” reported lead investigator Alexa B. Kimball, MD, MPH, professor of dermatology at Harvard Medical School and Beth Israel Deaconess Medical Center, both in Boston.
The trials, called BE HEARD I and BE HEARD II, enrolled 505 and 509 patients with HS, respectively. About 50% of patients in BE HEARD I and 60% of patients in BE HEARD II had Hurley stage 3 disease, which is the most severe of the three stratifications. The remainder were in Hurley stage 2. The mean duration of HS was 8.3 and 7.1 years, respectively.
Patients in both studies were randomized to one of four groups – either to a dosing regimen of 320 mg of bimekizumab administered by subcutaneous injection or to a placebo group. Both trials comprised double-blind 16-week initial and 32-week maintenance treatment periods.
In one experimental group, bimekizumab was given once every 2 weeks for the full course of the 48-week study (Q2W/Q2W). In another, patients started on the every-2-week schedule for 16 weeks and then were switched to every-4-week dosing (Q2W/Q4W). In the third group, patients started and remained on the every-4-week schedule (Q4W/Q4W). Patients in a fourth group started on placebo and switched at 16 weeks to the every-2-week bimekizumab schedule (placebo/Q2W).
Results at primary endpoint
The primary endpoint was HiSCR50, signifying a 50% reduction from baseline in abscess and inflammatory nodule count on the Hidradenitis Suppurativa Clinical Response (HiSCR) assessment tool. At 16 weeks, the initial Q2W dose in two of the groups outperformed the placebo in both BE HEARD I (47.8% vs. 28.7%) and BE HEARD II (52.0% vs. 32.2%). The response rates in the Q4W arm in BE HEARD I (45.3%) and BE HEARD II (53.8%) were also higher than the placebo, but the difference was only significant in BE HEARD II.
At 48 weeks, the proportion of patients with an HiSCR50 response climbed in all groups in both trials. The patterns were generally the same with slightly higher numerical responses among the groups that received the every-2-week dosing schedule relative to the every-4-week schedule.
In BE HEARD I at 48 weeks, the HiSCR50 response rate was about 60% for those who started and remained on every-2-week bimekizumab (Q2W/Q2W) or were switched at 16 weeks to every-4-week bimekizumab (Q2W/Q4W). For those who started and remained on every-4-week bimekizumab and the group started on placebo and switched to every-2-week bimekizumab, the response rates were 52.7% and 45.3%, respectively.
In BE HEARD II, the HiSCR50 response rates were higher in all groups, including the placebo, and the patterns of response were similar at 48 weeks. Most patients reached the HiSCR50 response – 79.8% (Q2W/Q2W), 78.4% (Q2W/Q4W), 76.7% (Q4W/Q4W), and 65.9 % (placebo/Q2W) of patients.
It is notable that, although there was rapid increase in the proportion of placebo patients reaching HiSCR50 after the switch at 16 weeks, there appeared to be an advantage at 48 weeks for starting on full-dose bimekizumab over starting on placebo.
In this trial, patients were listed as nonresponders if they received antibiotics at any time and for any reason after randomization. This might have concealed an even greater benefit of bimekizumab, Dr. Kimball said, but the study design element was considered necessary to isolate the activity of the study drug.
“In future HS trials, it will be helpful to address the difficulty of handling the impact of antibiotics and pain medications [in assessing results],” Dr. Kimball said.
Clinically meaningful secondary endpoint
For HS patients, the secondary endpoint of HiSCR75 might be considered the most meaningful, according to Dr. Kimball. She said that this higher bar not only documents a higher level of efficacy but correlates with meaningful improvement in quality of life. In the two trials combined, more than 55% of patients on continuous bimekizumab achieved HiSCR75 at week 48 in the observed case analysis, according to a news release from biopharmaceutical company UCB, developer of bimekizumab.
In BE HEARD I, the HiSCR75 rates were 33.4% and 24.7% for the every-2-week and every-4-week bimekizumab doses, respectively. The 33.4% response was statistically superior to placebo (18.4%). In BE HEARD II, both the every-2-week dose (35.7%) and the every-4-week dose (33.7%) were superior to the 15.6% response in placebo patients.
The improvements in quality of life as measured with the Dermatology Life Quality Index (DLQI), reflected the changes in disease activity. Relative to about a 3-point reduction from baseline in the placebo groups of the two trials, the 5-point reduction for either the 2-week or 4-week bimekizumab groups in each clinical trial were highly significant, Dr. Kimball said.
Bimekizumab was relatively well tolerated, although it shares the increased risk for candidiasis observed with this agent when used in psoriasis and with other IL-17 inhibitors, such as secukinumab (Cosentyx), in general. The risk of candidiasis appeared to be dose related, but cases were generally mild and easily managed, according to Dr. Kimball. She noted that only three patients discontinued treatment for this reason. Discontinuations for a treatment-related adverse event overall was less than 4% at 16 weeks.
This is only the third phase 3 trial ever completed in patients with HS. In fact, Dr. Kimball has led all of the phase 3 trials so far, including clinical studies of adalimumab (Humira), published in 2016, and of secukinumab, published earlier this year. All were positive studies.
“This is amazing news for our patients,” Dr. Kimball said. HS remains a challenging disease, even with a growing number of options showing benefit in large studies, she said, and the high rate of response, particularly at the level of HiSCR75, “is a huge milestone for what we can achieve.”
Multiple treatment options important
Her assessment was echoed by other experts, including Christopher J. Sayed, MD, an associate professor of dermatology at the University of North Carolina at Chapel Hill, who publishes frequently about this disease.
“It is incredibly exciting to see the strong phase 3 data on bimekizumab, particularly the deep responses at the HiSCR75 in a majority of patients after the first year,” he said.
Importantly, he does not see the growing array of treatment options as necessarily competitive for a disease with heterogeneous manifestations and variable responses to any one agent.
“While this may be a major step forward, it will still be critical to see more drugs come along for those who do not respond fully enough or have comorbidities that prevent the use of IL-17 and TNF [tumor necrosis factor] antagonists,” he said.
Bimekizumab is not approved for any indication in the United States; it is approved for treating moderate to severe plaque psoriasis in adults who are candidates for systemic therapy in the EU/EEA, where it is marketed as Bimzelx, according to UCB. Dr. Kimball reports financial relationships with AbbVie, Janssen, Kymera, Lilly, Novartis, Pfizer, and UCB. Dr. Sayed reports financial relationships with AbbVie, InflaRx, and UCB.
A version of this article first appeared on Medscape.com.
NEW ORLEANS – In two phase 3 trials, bimekizumab, a monoclonal antibody targeting two types of interleukin-17 — IL-17A and IL-17F — reduced the abscess and inflammatory nodule count better than placebo in the chronic inflammatory skin condition hidradenitis suppurativa (HS), according to results presented together during a late-breaker session at the annual meeting of the American Academy of Dermatology.
“We are very excited to add this data to what we already have around IL-17 inhibition. This clearly validates this target for the control of HS,” reported lead investigator Alexa B. Kimball, MD, MPH, professor of dermatology at Harvard Medical School and Beth Israel Deaconess Medical Center, both in Boston.
The trials, called BE HEARD I and BE HEARD II, enrolled 505 and 509 patients with HS, respectively. About 50% of patients in BE HEARD I and 60% of patients in BE HEARD II had Hurley stage 3 disease, which is the most severe of the three stratifications. The remainder were in Hurley stage 2. The mean duration of HS was 8.3 and 7.1 years, respectively.
Patients in both studies were randomized to one of four groups – either to a dosing regimen of 320 mg of bimekizumab administered by subcutaneous injection or to a placebo group. Both trials comprised double-blind 16-week initial and 32-week maintenance treatment periods.
In one experimental group, bimekizumab was given once every 2 weeks for the full course of the 48-week study (Q2W/Q2W). In another, patients started on the every-2-week schedule for 16 weeks and then were switched to every-4-week dosing (Q2W/Q4W). In the third group, patients started and remained on the every-4-week schedule (Q4W/Q4W). Patients in a fourth group started on placebo and switched at 16 weeks to the every-2-week bimekizumab schedule (placebo/Q2W).
Results at primary endpoint
The primary endpoint was HiSCR50, signifying a 50% reduction from baseline in abscess and inflammatory nodule count on the Hidradenitis Suppurativa Clinical Response (HiSCR) assessment tool. At 16 weeks, the initial Q2W dose in two of the groups outperformed the placebo in both BE HEARD I (47.8% vs. 28.7%) and BE HEARD II (52.0% vs. 32.2%). The response rates in the Q4W arm in BE HEARD I (45.3%) and BE HEARD II (53.8%) were also higher than the placebo, but the difference was only significant in BE HEARD II.
At 48 weeks, the proportion of patients with an HiSCR50 response climbed in all groups in both trials. The patterns were generally the same with slightly higher numerical responses among the groups that received the every-2-week dosing schedule relative to the every-4-week schedule.
In BE HEARD I at 48 weeks, the HiSCR50 response rate was about 60% for those who started and remained on every-2-week bimekizumab (Q2W/Q2W) or were switched at 16 weeks to every-4-week bimekizumab (Q2W/Q4W). For those who started and remained on every-4-week bimekizumab and the group started on placebo and switched to every-2-week bimekizumab, the response rates were 52.7% and 45.3%, respectively.
In BE HEARD II, the HiSCR50 response rates were higher in all groups, including the placebo, and the patterns of response were similar at 48 weeks. Most patients reached the HiSCR50 response – 79.8% (Q2W/Q2W), 78.4% (Q2W/Q4W), 76.7% (Q4W/Q4W), and 65.9 % (placebo/Q2W) of patients.
It is notable that, although there was rapid increase in the proportion of placebo patients reaching HiSCR50 after the switch at 16 weeks, there appeared to be an advantage at 48 weeks for starting on full-dose bimekizumab over starting on placebo.
In this trial, patients were listed as nonresponders if they received antibiotics at any time and for any reason after randomization. This might have concealed an even greater benefit of bimekizumab, Dr. Kimball said, but the study design element was considered necessary to isolate the activity of the study drug.
“In future HS trials, it will be helpful to address the difficulty of handling the impact of antibiotics and pain medications [in assessing results],” Dr. Kimball said.
Clinically meaningful secondary endpoint
For HS patients, the secondary endpoint of HiSCR75 might be considered the most meaningful, according to Dr. Kimball. She said that this higher bar not only documents a higher level of efficacy but correlates with meaningful improvement in quality of life. In the two trials combined, more than 55% of patients on continuous bimekizumab achieved HiSCR75 at week 48 in the observed case analysis, according to a news release from biopharmaceutical company UCB, developer of bimekizumab.
In BE HEARD I, the HiSCR75 rates were 33.4% and 24.7% for the every-2-week and every-4-week bimekizumab doses, respectively. The 33.4% response was statistically superior to placebo (18.4%). In BE HEARD II, both the every-2-week dose (35.7%) and the every-4-week dose (33.7%) were superior to the 15.6% response in placebo patients.
The improvements in quality of life as measured with the Dermatology Life Quality Index (DLQI), reflected the changes in disease activity. Relative to about a 3-point reduction from baseline in the placebo groups of the two trials, the 5-point reduction for either the 2-week or 4-week bimekizumab groups in each clinical trial were highly significant, Dr. Kimball said.
Bimekizumab was relatively well tolerated, although it shares the increased risk for candidiasis observed with this agent when used in psoriasis and with other IL-17 inhibitors, such as secukinumab (Cosentyx), in general. The risk of candidiasis appeared to be dose related, but cases were generally mild and easily managed, according to Dr. Kimball. She noted that only three patients discontinued treatment for this reason. Discontinuations for a treatment-related adverse event overall was less than 4% at 16 weeks.
This is only the third phase 3 trial ever completed in patients with HS. In fact, Dr. Kimball has led all of the phase 3 trials so far, including clinical studies of adalimumab (Humira), published in 2016, and of secukinumab, published earlier this year. All were positive studies.
“This is amazing news for our patients,” Dr. Kimball said. HS remains a challenging disease, even with a growing number of options showing benefit in large studies, she said, and the high rate of response, particularly at the level of HiSCR75, “is a huge milestone for what we can achieve.”
Multiple treatment options important
Her assessment was echoed by other experts, including Christopher J. Sayed, MD, an associate professor of dermatology at the University of North Carolina at Chapel Hill, who publishes frequently about this disease.
“It is incredibly exciting to see the strong phase 3 data on bimekizumab, particularly the deep responses at the HiSCR75 in a majority of patients after the first year,” he said.
Importantly, he does not see the growing array of treatment options as necessarily competitive for a disease with heterogeneous manifestations and variable responses to any one agent.
“While this may be a major step forward, it will still be critical to see more drugs come along for those who do not respond fully enough or have comorbidities that prevent the use of IL-17 and TNF [tumor necrosis factor] antagonists,” he said.
Bimekizumab is not approved for any indication in the United States; it is approved for treating moderate to severe plaque psoriasis in adults who are candidates for systemic therapy in the EU/EEA, where it is marketed as Bimzelx, according to UCB. Dr. Kimball reports financial relationships with AbbVie, Janssen, Kymera, Lilly, Novartis, Pfizer, and UCB. Dr. Sayed reports financial relationships with AbbVie, InflaRx, and UCB.
A version of this article first appeared on Medscape.com.
AT AAD 2023
Fat Necrosis of the Breast Mimicking Breast Cancer in a Male Patient Following Wax Hair Removal
To the Editor:
Fat necrosis of the breast is a benign inflammatory disease of adipose tissue commonly observed after trauma in the female breast during the perimenopausal period.1 Fat necrosis of the male breast is rare, first described by Silverstone2 in 1949; the condition usually presents with unilateral, painful or asymptomatic, firm nodules, which in rare cases are observed as skin retraction and thickening, ecchymosis, erythematous plaque–like cellulitis, local depression, and/or discoloration of the breast skin.3-5
Diagnosis of fat necrosis of the male breast may need to be confirmed via biopsy in conjunction with clinical and radiologic findings because the condition can mimic breast cancer.1 We report a case of bilateral fat necrosis of the breast mimicking breast cancer following wax hair removal.
A 42-year-old man presented to our outpatient dermatology clinic for evaluation of redness, swelling, and hardness of the skin of both breasts of 3 weeks’ duration. The patient had a history of wax hair removal of the entire anterior aspect of the body. He reported an erythematous, edematous, warm plaque that developed on the breasts 2 days after waxing. The plaque did not respond to antibiotics. The swelling and induration progressed over the 2 weeks after the patient was waxed. The patient had no family history of breast cancer. He had a standing diagnosis of gynecomastia. He denied any history of fat or filler injection in the affected area.
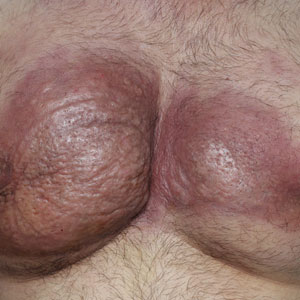
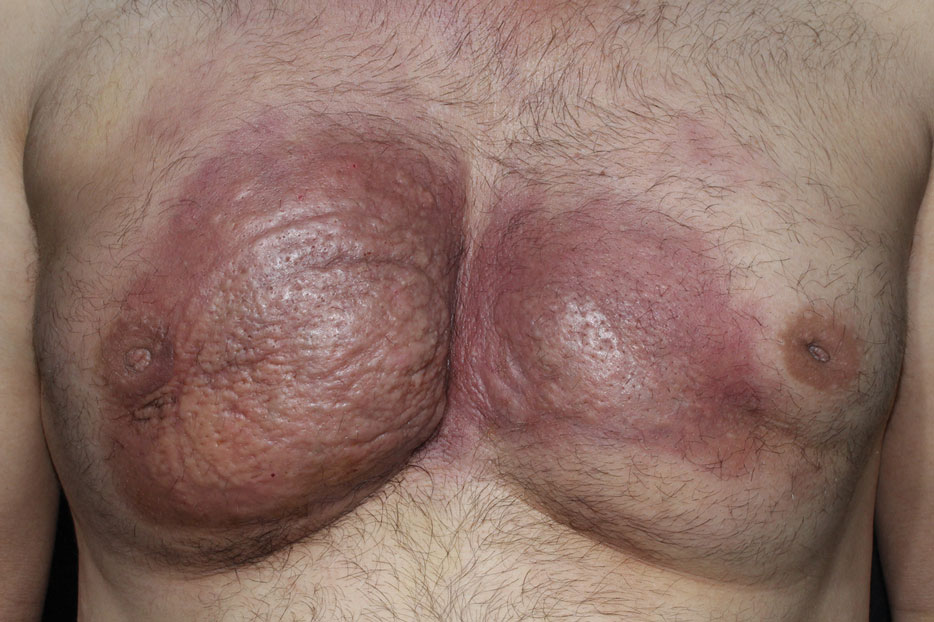
Dermatologic examination revealed erythematous, edematous, indurated, asymptomatic plaques with a peau d’orange appearance on the bilateral pectoral and presternal region. Minimal retraction of the right areola was noted (Figure 1). The bilateral axillary lymph nodes were palpable.
Laboratory results including erythrocyte sedimentation rate (108 mm/h [reference range, 2–20 mm/h]), C-reactive protein (9.2 mg/dL [reference range, >0.5 mg/dL]), and ferritin levels (645
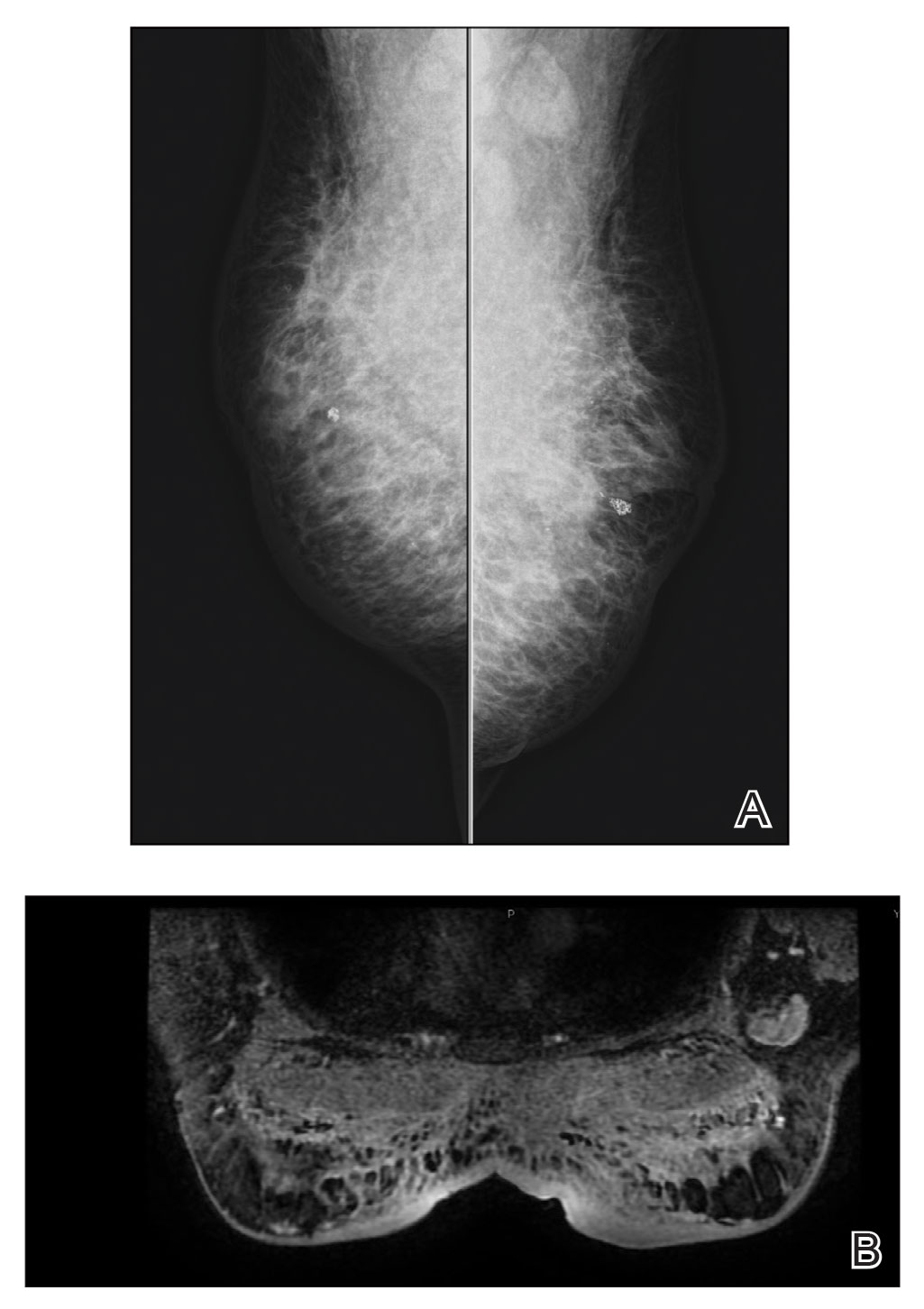
Mammography of both breasts revealed a Breast Imaging Reporting and Data System (BI-RADS) score of 4 with a suspicious abnormality (ie, diffuse edema of the breast, multiple calcifications in a nonspecific pattern, oil cysts with calcifications, and bilateral axillary lymphadenopathy with a diameter of 2.5 cm and a thick and irregular cortex)(Figure 2A). Ultrasonography of both breasts revealed an inflammatory breast. Magnetic resonance imaging showed similar findings with diffuse edema and a heterogeneous appearance. Contrast-enhanced magnetic resonance imaging showed diffuse contrast enhancement in both breasts extending to the pectoral muscles and axillary regions, consistent with inflammatory changes (Figure 2B).
Because of difficulty differentiating inflammation and an infiltrating tumor, histopathologic examination was recommended by radiology. Results from a 5-mm punch biopsy from the right breast yielded the following differential diagnoses: cellulitis, panniculitis, inflammatory breast cancer, subcutaneous fat necrosis, and paraffinoma. Histopathologic examination of the skin revealed a normal epidermis and a dense inflammatory cell infiltrate comprising lymphocytes and monocytes in the dermis and subcutaneous tissue. Marked fibrosis also was noted in the dermis and subcutaneous tissue. Lipophagic fat necrosis accompanied by a variable inflammatory cell infiltrate consisted of histiocytes and neutrophils (Figure 3A). Pankeratin immunostaining was negative. Fat necrosis was present in a biopsy specimen obtained from the right breast; no signs of malignancy were present (Figure 3B). Fine-needle aspiration of the axillary lymph nodes was benign. Given these histopathologic findings, malignancy was excluded from the differential diagnosis. Paraffinoma also was ruled out because the patient insistently denied any history of fat or filler injection.
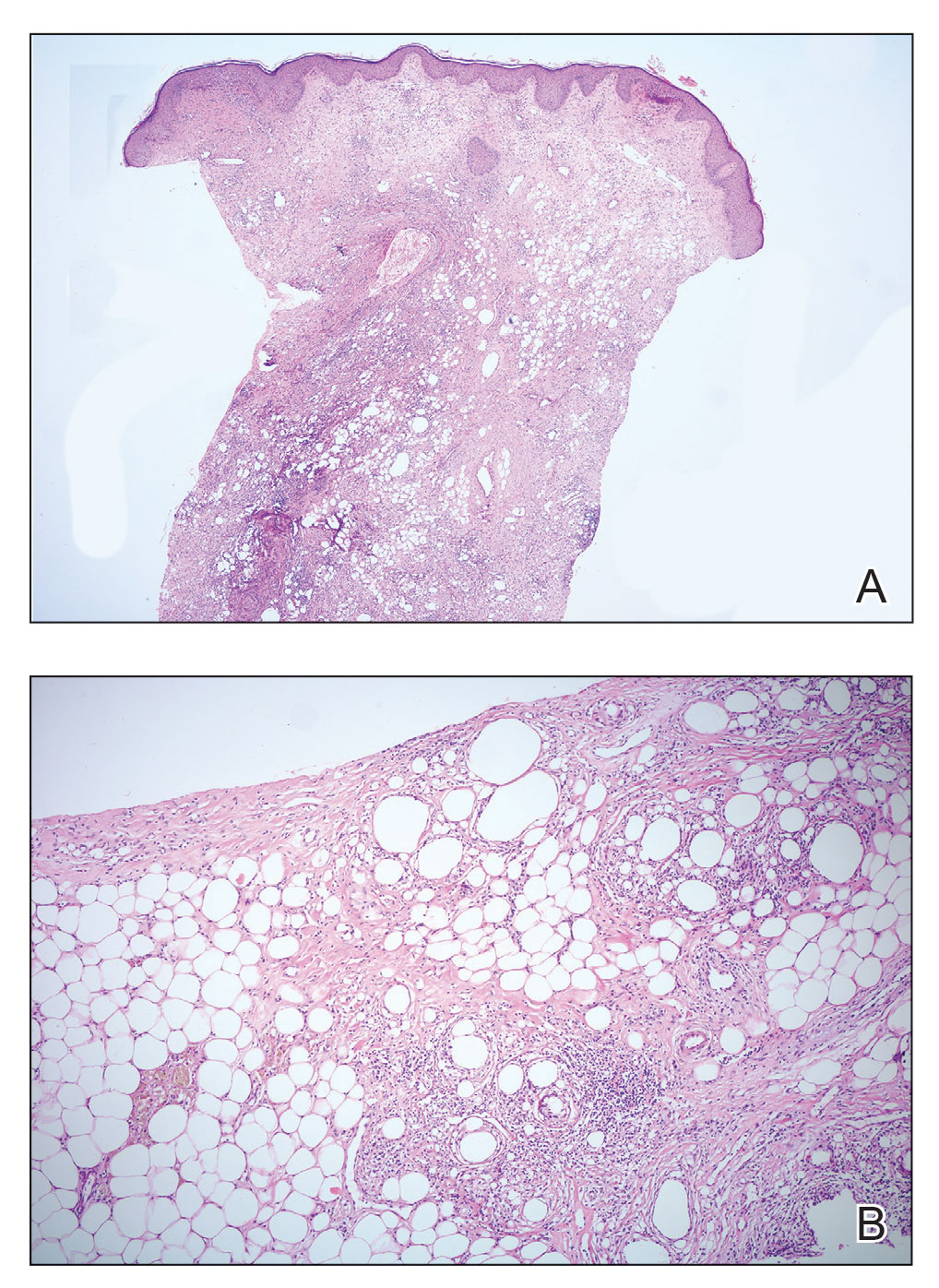
Based on the clinical, histopathologic, and radiologic findings, as well as the history of minor trauma due to wax hair removal, a diagnosis of fat necrosis of the breast was made. Intervention was not recommended by the plastic surgeons who subsequently evaluated the patient, because the additional trauma may aggravate the lesion. He was treated with nonsteroidal anti-inflammatory drugs.
At 6-month follow-up, there was marked reduction in the erythema and edema but no notable improvement of the induration. A potent topical steroid was added to the treatment, but only slight regression of the induration was observed.
The normal male breast is comprised of fat and a few secretory ducts.6 Gynecomastia and breast cancer are the 2 most common conditions of the male breast; fat necrosis of the male breast is rare. In a study of 236 male patients with breast disease, only 5 had fat necrosis.7
Fat necrosis of the breast can be observed with various clinical and radiological presentations. Subcutaneous nodules, skin retraction and thickening, local skin depression, and ecchymosis are the more common presentations of fat necrosis.3-5 In our case, the first symptoms of disease were similar to those seen in cellulitis. The presentation of fat necrosis–like cellulitis has been described only rarely in the medical literature. Haikin et al5 reported a case of fat necrosis of the leg in a child that presented with cellulitis followed by induration, which did not respond to antibiotics, as was the case with our patient.5
Blunt trauma, breast reduction surgery, and breast augmentation surgery can cause fat necrosis of the breast1,4; in some cases, the cause cannot be determined.8 The only pertinent history in our patient was wax hair removal. Fat necrosis was an unexpected complication, but hair removal can be considered minor trauma; however, this is not commonly reported in the literature following hair removal with wax. In a study that reviewed diseases of the male breast, the investigators observed that all male patients with fat necrosis had pseudogynecomastia (adipomastia).7 Although our patient’s entire anterior trunk was epilated, only the breast was affected. This situation might be explained by underlying gynecomastia because fat necrosis is common in areas of the body where subcutaneous fat tissue is dense.
Fat necrosis of the breast can be mistaken—both clinically and radiologically—for malignancy, such as in our case. Diagnosis of fat necrosis of the breast should be a diagnosis of exclusion; therefore, histopathologic confirmation of the lesion is imperative.9
In conclusion, fat necrosis of the male breast is rare. The condition can present as cellulitis. Hair removal with wax might be a cause of fat necrosis. Because breast cancer and fat necrosis can exhibit clinical and radiologic similarities, the diagnosis of fat necrosis should be confirmed by histopathologic analysis in conjunction with clinical and radiologic findings.
- Tan PH, Lai LM, Carrington EV, et al. Fat necrosis of the breast—a review. Breast. 2006;15:313-318. doi:10.1016/j.breast.2005.07.003
- Silverstone M. Fat necrosis of the breast with report of a case in a male. Br J Surg. 1949;37:49-52. doi:10.1002/bjs.18003714508
- Akyol M, Kayali A, Yildirim N. Traumatic fat necrosis of male breast. Clin Imaging. 2013;37:954-956. doi:10.1016/j.clinimag.2013.05.009
- Crawford EA, King JJ, Fox EJ, et al. Symptomatic fat necrosis and lipoatrophy of the posterior pelvis following trauma. Orthopedics. 2009;32:444. doi:10.3928/01477447-20090511-25
- Haikin Herzberger E, Aviner S, Cherniavsky E. Posttraumatic fat necrosis presented as cellulitis of the leg. Case Rep Pediatr. 2012;2012:672397. doi:10.1155/2012/672397
- Michels LG, Gold RH, Arndt RD. Radiography of gynecomastia and other disorders of the male breast. Radiology. 1977;122:117-122. doi:10.1148/122.1.117
- Günhan-Bilgen I, Bozkaya H, Ustün E, et al. Male breast disease: clinical, mammographic, and ultrasonographic features. Eur J Radiol. 2002;43:246-255. doi:10.1016/s0720-048x(01)00483-1
- Chala LF, de Barros N, de Camargo Moraes P, et al. Fat necrosis of the breast: mammographic, sonographic, computed tomography, and magnetic resonance imaging findings. Curr Probl Diagn Radiol. 2004;33:106-126. doi:10.1067/j.cpradiol.2004.01.001
- Pullyblank AM, Davies JD, Basten J, et al. Fat necrosis of the female breast—Hadfield re-visited. Breast. 2001;10:388-391. doi:10.1054/brst.2000.0287
To the Editor:
Fat necrosis of the breast is a benign inflammatory disease of adipose tissue commonly observed after trauma in the female breast during the perimenopausal period.1 Fat necrosis of the male breast is rare, first described by Silverstone2 in 1949; the condition usually presents with unilateral, painful or asymptomatic, firm nodules, which in rare cases are observed as skin retraction and thickening, ecchymosis, erythematous plaque–like cellulitis, local depression, and/or discoloration of the breast skin.3-5
Diagnosis of fat necrosis of the male breast may need to be confirmed via biopsy in conjunction with clinical and radiologic findings because the condition can mimic breast cancer.1 We report a case of bilateral fat necrosis of the breast mimicking breast cancer following wax hair removal.
A 42-year-old man presented to our outpatient dermatology clinic for evaluation of redness, swelling, and hardness of the skin of both breasts of 3 weeks’ duration. The patient had a history of wax hair removal of the entire anterior aspect of the body. He reported an erythematous, edematous, warm plaque that developed on the breasts 2 days after waxing. The plaque did not respond to antibiotics. The swelling and induration progressed over the 2 weeks after the patient was waxed. The patient had no family history of breast cancer. He had a standing diagnosis of gynecomastia. He denied any history of fat or filler injection in the affected area.
Dermatologic examination revealed erythematous, edematous, indurated, asymptomatic plaques with a peau d’orange appearance on the bilateral pectoral and presternal region. Minimal retraction of the right areola was noted (Figure 1). The bilateral axillary lymph nodes were palpable.

Laboratory results including erythrocyte sedimentation rate (108 mm/h [reference range, 2–20 mm/h]), C-reactive protein (9.2 mg/dL [reference range, >0.5 mg/dL]), and ferritin levels (645
Mammography of both breasts revealed a Breast Imaging Reporting and Data System (BI-RADS) score of 4 with a suspicious abnormality (ie, diffuse edema of the breast, multiple calcifications in a nonspecific pattern, oil cysts with calcifications, and bilateral axillary lymphadenopathy with a diameter of 2.5 cm and a thick and irregular cortex)(Figure 2A). Ultrasonography of both breasts revealed an inflammatory breast. Magnetic resonance imaging showed similar findings with diffuse edema and a heterogeneous appearance. Contrast-enhanced magnetic resonance imaging showed diffuse contrast enhancement in both breasts extending to the pectoral muscles and axillary regions, consistent with inflammatory changes (Figure 2B).

Because of difficulty differentiating inflammation and an infiltrating tumor, histopathologic examination was recommended by radiology. Results from a 5-mm punch biopsy from the right breast yielded the following differential diagnoses: cellulitis, panniculitis, inflammatory breast cancer, subcutaneous fat necrosis, and paraffinoma. Histopathologic examination of the skin revealed a normal epidermis and a dense inflammatory cell infiltrate comprising lymphocytes and monocytes in the dermis and subcutaneous tissue. Marked fibrosis also was noted in the dermis and subcutaneous tissue. Lipophagic fat necrosis accompanied by a variable inflammatory cell infiltrate consisted of histiocytes and neutrophils (Figure 3A). Pankeratin immunostaining was negative. Fat necrosis was present in a biopsy specimen obtained from the right breast; no signs of malignancy were present (Figure 3B). Fine-needle aspiration of the axillary lymph nodes was benign. Given these histopathologic findings, malignancy was excluded from the differential diagnosis. Paraffinoma also was ruled out because the patient insistently denied any history of fat or filler injection.

Based on the clinical, histopathologic, and radiologic findings, as well as the history of minor trauma due to wax hair removal, a diagnosis of fat necrosis of the breast was made. Intervention was not recommended by the plastic surgeons who subsequently evaluated the patient, because the additional trauma may aggravate the lesion. He was treated with nonsteroidal anti-inflammatory drugs.
At 6-month follow-up, there was marked reduction in the erythema and edema but no notable improvement of the induration. A potent topical steroid was added to the treatment, but only slight regression of the induration was observed.
The normal male breast is comprised of fat and a few secretory ducts.6 Gynecomastia and breast cancer are the 2 most common conditions of the male breast; fat necrosis of the male breast is rare. In a study of 236 male patients with breast disease, only 5 had fat necrosis.7
Fat necrosis of the breast can be observed with various clinical and radiological presentations. Subcutaneous nodules, skin retraction and thickening, local skin depression, and ecchymosis are the more common presentations of fat necrosis.3-5 In our case, the first symptoms of disease were similar to those seen in cellulitis. The presentation of fat necrosis–like cellulitis has been described only rarely in the medical literature. Haikin et al5 reported a case of fat necrosis of the leg in a child that presented with cellulitis followed by induration, which did not respond to antibiotics, as was the case with our patient.5
Blunt trauma, breast reduction surgery, and breast augmentation surgery can cause fat necrosis of the breast1,4; in some cases, the cause cannot be determined.8 The only pertinent history in our patient was wax hair removal. Fat necrosis was an unexpected complication, but hair removal can be considered minor trauma; however, this is not commonly reported in the literature following hair removal with wax. In a study that reviewed diseases of the male breast, the investigators observed that all male patients with fat necrosis had pseudogynecomastia (adipomastia).7 Although our patient’s entire anterior trunk was epilated, only the breast was affected. This situation might be explained by underlying gynecomastia because fat necrosis is common in areas of the body where subcutaneous fat tissue is dense.
Fat necrosis of the breast can be mistaken—both clinically and radiologically—for malignancy, such as in our case. Diagnosis of fat necrosis of the breast should be a diagnosis of exclusion; therefore, histopathologic confirmation of the lesion is imperative.9
In conclusion, fat necrosis of the male breast is rare. The condition can present as cellulitis. Hair removal with wax might be a cause of fat necrosis. Because breast cancer and fat necrosis can exhibit clinical and radiologic similarities, the diagnosis of fat necrosis should be confirmed by histopathologic analysis in conjunction with clinical and radiologic findings.
To the Editor:
Fat necrosis of the breast is a benign inflammatory disease of adipose tissue commonly observed after trauma in the female breast during the perimenopausal period.1 Fat necrosis of the male breast is rare, first described by Silverstone2 in 1949; the condition usually presents with unilateral, painful or asymptomatic, firm nodules, which in rare cases are observed as skin retraction and thickening, ecchymosis, erythematous plaque–like cellulitis, local depression, and/or discoloration of the breast skin.3-5
Diagnosis of fat necrosis of the male breast may need to be confirmed via biopsy in conjunction with clinical and radiologic findings because the condition can mimic breast cancer.1 We report a case of bilateral fat necrosis of the breast mimicking breast cancer following wax hair removal.
A 42-year-old man presented to our outpatient dermatology clinic for evaluation of redness, swelling, and hardness of the skin of both breasts of 3 weeks’ duration. The patient had a history of wax hair removal of the entire anterior aspect of the body. He reported an erythematous, edematous, warm plaque that developed on the breasts 2 days after waxing. The plaque did not respond to antibiotics. The swelling and induration progressed over the 2 weeks after the patient was waxed. The patient had no family history of breast cancer. He had a standing diagnosis of gynecomastia. He denied any history of fat or filler injection in the affected area.
Dermatologic examination revealed erythematous, edematous, indurated, asymptomatic plaques with a peau d’orange appearance on the bilateral pectoral and presternal region. Minimal retraction of the right areola was noted (Figure 1). The bilateral axillary lymph nodes were palpable.

Laboratory results including erythrocyte sedimentation rate (108 mm/h [reference range, 2–20 mm/h]), C-reactive protein (9.2 mg/dL [reference range, >0.5 mg/dL]), and ferritin levels (645
Mammography of both breasts revealed a Breast Imaging Reporting and Data System (BI-RADS) score of 4 with a suspicious abnormality (ie, diffuse edema of the breast, multiple calcifications in a nonspecific pattern, oil cysts with calcifications, and bilateral axillary lymphadenopathy with a diameter of 2.5 cm and a thick and irregular cortex)(Figure 2A). Ultrasonography of both breasts revealed an inflammatory breast. Magnetic resonance imaging showed similar findings with diffuse edema and a heterogeneous appearance. Contrast-enhanced magnetic resonance imaging showed diffuse contrast enhancement in both breasts extending to the pectoral muscles and axillary regions, consistent with inflammatory changes (Figure 2B).

Because of difficulty differentiating inflammation and an infiltrating tumor, histopathologic examination was recommended by radiology. Results from a 5-mm punch biopsy from the right breast yielded the following differential diagnoses: cellulitis, panniculitis, inflammatory breast cancer, subcutaneous fat necrosis, and paraffinoma. Histopathologic examination of the skin revealed a normal epidermis and a dense inflammatory cell infiltrate comprising lymphocytes and monocytes in the dermis and subcutaneous tissue. Marked fibrosis also was noted in the dermis and subcutaneous tissue. Lipophagic fat necrosis accompanied by a variable inflammatory cell infiltrate consisted of histiocytes and neutrophils (Figure 3A). Pankeratin immunostaining was negative. Fat necrosis was present in a biopsy specimen obtained from the right breast; no signs of malignancy were present (Figure 3B). Fine-needle aspiration of the axillary lymph nodes was benign. Given these histopathologic findings, malignancy was excluded from the differential diagnosis. Paraffinoma also was ruled out because the patient insistently denied any history of fat or filler injection.

Based on the clinical, histopathologic, and radiologic findings, as well as the history of minor trauma due to wax hair removal, a diagnosis of fat necrosis of the breast was made. Intervention was not recommended by the plastic surgeons who subsequently evaluated the patient, because the additional trauma may aggravate the lesion. He was treated with nonsteroidal anti-inflammatory drugs.
At 6-month follow-up, there was marked reduction in the erythema and edema but no notable improvement of the induration. A potent topical steroid was added to the treatment, but only slight regression of the induration was observed.
The normal male breast is comprised of fat and a few secretory ducts.6 Gynecomastia and breast cancer are the 2 most common conditions of the male breast; fat necrosis of the male breast is rare. In a study of 236 male patients with breast disease, only 5 had fat necrosis.7
Fat necrosis of the breast can be observed with various clinical and radiological presentations. Subcutaneous nodules, skin retraction and thickening, local skin depression, and ecchymosis are the more common presentations of fat necrosis.3-5 In our case, the first symptoms of disease were similar to those seen in cellulitis. The presentation of fat necrosis–like cellulitis has been described only rarely in the medical literature. Haikin et al5 reported a case of fat necrosis of the leg in a child that presented with cellulitis followed by induration, which did not respond to antibiotics, as was the case with our patient.5
Blunt trauma, breast reduction surgery, and breast augmentation surgery can cause fat necrosis of the breast1,4; in some cases, the cause cannot be determined.8 The only pertinent history in our patient was wax hair removal. Fat necrosis was an unexpected complication, but hair removal can be considered minor trauma; however, this is not commonly reported in the literature following hair removal with wax. In a study that reviewed diseases of the male breast, the investigators observed that all male patients with fat necrosis had pseudogynecomastia (adipomastia).7 Although our patient’s entire anterior trunk was epilated, only the breast was affected. This situation might be explained by underlying gynecomastia because fat necrosis is common in areas of the body where subcutaneous fat tissue is dense.
Fat necrosis of the breast can be mistaken—both clinically and radiologically—for malignancy, such as in our case. Diagnosis of fat necrosis of the breast should be a diagnosis of exclusion; therefore, histopathologic confirmation of the lesion is imperative.9
In conclusion, fat necrosis of the male breast is rare. The condition can present as cellulitis. Hair removal with wax might be a cause of fat necrosis. Because breast cancer and fat necrosis can exhibit clinical and radiologic similarities, the diagnosis of fat necrosis should be confirmed by histopathologic analysis in conjunction with clinical and radiologic findings.
- Tan PH, Lai LM, Carrington EV, et al. Fat necrosis of the breast—a review. Breast. 2006;15:313-318. doi:10.1016/j.breast.2005.07.003
- Silverstone M. Fat necrosis of the breast with report of a case in a male. Br J Surg. 1949;37:49-52. doi:10.1002/bjs.18003714508
- Akyol M, Kayali A, Yildirim N. Traumatic fat necrosis of male breast. Clin Imaging. 2013;37:954-956. doi:10.1016/j.clinimag.2013.05.009
- Crawford EA, King JJ, Fox EJ, et al. Symptomatic fat necrosis and lipoatrophy of the posterior pelvis following trauma. Orthopedics. 2009;32:444. doi:10.3928/01477447-20090511-25
- Haikin Herzberger E, Aviner S, Cherniavsky E. Posttraumatic fat necrosis presented as cellulitis of the leg. Case Rep Pediatr. 2012;2012:672397. doi:10.1155/2012/672397
- Michels LG, Gold RH, Arndt RD. Radiography of gynecomastia and other disorders of the male breast. Radiology. 1977;122:117-122. doi:10.1148/122.1.117
- Günhan-Bilgen I, Bozkaya H, Ustün E, et al. Male breast disease: clinical, mammographic, and ultrasonographic features. Eur J Radiol. 2002;43:246-255. doi:10.1016/s0720-048x(01)00483-1
- Chala LF, de Barros N, de Camargo Moraes P, et al. Fat necrosis of the breast: mammographic, sonographic, computed tomography, and magnetic resonance imaging findings. Curr Probl Diagn Radiol. 2004;33:106-126. doi:10.1067/j.cpradiol.2004.01.001
- Pullyblank AM, Davies JD, Basten J, et al. Fat necrosis of the female breast—Hadfield re-visited. Breast. 2001;10:388-391. doi:10.1054/brst.2000.0287
- Tan PH, Lai LM, Carrington EV, et al. Fat necrosis of the breast—a review. Breast. 2006;15:313-318. doi:10.1016/j.breast.2005.07.003
- Silverstone M. Fat necrosis of the breast with report of a case in a male. Br J Surg. 1949;37:49-52. doi:10.1002/bjs.18003714508
- Akyol M, Kayali A, Yildirim N. Traumatic fat necrosis of male breast. Clin Imaging. 2013;37:954-956. doi:10.1016/j.clinimag.2013.05.009
- Crawford EA, King JJ, Fox EJ, et al. Symptomatic fat necrosis and lipoatrophy of the posterior pelvis following trauma. Orthopedics. 2009;32:444. doi:10.3928/01477447-20090511-25
- Haikin Herzberger E, Aviner S, Cherniavsky E. Posttraumatic fat necrosis presented as cellulitis of the leg. Case Rep Pediatr. 2012;2012:672397. doi:10.1155/2012/672397
- Michels LG, Gold RH, Arndt RD. Radiography of gynecomastia and other disorders of the male breast. Radiology. 1977;122:117-122. doi:10.1148/122.1.117
- Günhan-Bilgen I, Bozkaya H, Ustün E, et al. Male breast disease: clinical, mammographic, and ultrasonographic features. Eur J Radiol. 2002;43:246-255. doi:10.1016/s0720-048x(01)00483-1
- Chala LF, de Barros N, de Camargo Moraes P, et al. Fat necrosis of the breast: mammographic, sonographic, computed tomography, and magnetic resonance imaging findings. Curr Probl Diagn Radiol. 2004;33:106-126. doi:10.1067/j.cpradiol.2004.01.001
- Pullyblank AM, Davies JD, Basten J, et al. Fat necrosis of the female breast—Hadfield re-visited. Breast. 2001;10:388-391. doi:10.1054/brst.2000.0287
Practice Points
- Fat necrosis of the breast can be mistaken—both clinically and radiologically—for malignancy; therefore, diagnosis should be confirmed by histopathology in conjunction with clinical and radiologic findings.
- Fat necrosis of the male breast is rare, and hair removal with wax may be a rare cause of the disease.
Match Day: Record number of residencies offered
Baily Nagle, vice president of her graduating class at Harvard Medical School, Boston, celebrated “the luck of the Irish” on St. Patrick’s Day that allowed her to match into her chosen specialty and top choice of residency programs: anesthesia at Brigham and Women’s Hospital.
“I am feeling very excited and relieved – I matched,” she said in an interview upon hearing her good fortune on Match Monday, March 13. She had a similar reaction on Match Day, March 17. “After a lot of long nights and hard work, happy to have it pay off.”
Ms. Nagle was so determined to match into her specialty that she didn’t have any other specialties in mind as a backup.
The annual process of matching medical school graduates with compatible residency programs is an emotional roller coaster for all applicants, their personal March Madness, so to speak. But Ms. Nagle was one of the more fortunate applicants. She didn’t have to confront the heartbreak other applicants felt when the National Resident Matching Program (NRMP) announced results of the main residency match and the Supplemental Offer and Acceptance Program (SOAP), which offers alternate programs for unfilled positions or unmatched applicants.
During the 2023 Match process, this news organization has been following a handful of students, checking in with them periodically for updates on their progress. Most of them matched successfully, but at least one international medical graduate (IMG) did not. What the others have in common is that their hearts were set on a chosen specialty. Like Ms. Nagle, another student banked on landing his chosen specialty without a backup plan, whereas another said that she’d continue through the SOAP if she didn’t match successfully.
Overall, Match Day resulted in a record number of residency positions offered, most notably in primary care, which “hit an all-time high,” according to NRMP President and CEO Donna L. Lamb, DHSc, MBA, BSN. The number of positions has “consistently increased over the past 5 years, and most importantly the fill rate for primary care has remained steady,” Dr.. Lamb noted in the NRMP release of Match Day results. The release coincided with students learning through emails at noon Eastern Time to which residency or supplemental programs they were matched.
Though more applicants registered for the Match in 2023 than in 2022 – driven primarily by non-U.S. IMGs – the NRMP stated that it was surprised by the decrease in U.S. MD senior applicants.
U.S. MD seniors had a nearly 94% Match rate, a small increase over 2022. U.S. citizen IMGs saw a nearly 68% Match rate, which NRMP reported as an “all-time high” and about six percentage points over in 2022, whereas non-U.S. IMGs had a nearly 60% Match rate, a 1.3 percentage point increase over 2022.
Among the specialties that filled all available positions in 2023 were orthopedic surgery, plastic surgery (integrated), and radiology – diagnostic and thoracic surgery.
Not everyone matches
On March 13, the American College of Emergency Physicians issued a joint statement with other emergency medicine (EM) organizations about a high rate of unfilled EM positions expected in 2023.
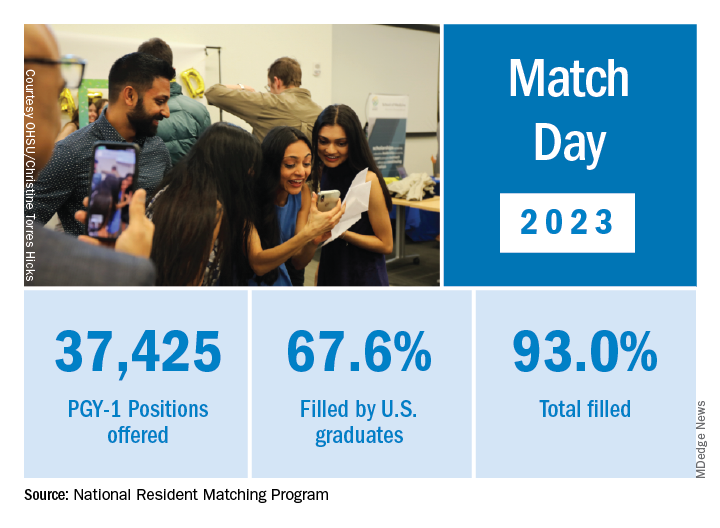
NRMP acknowledged March 17 that 554 positions remained unfilled, an increase of 335 more unfilled positions than 2022. NRMP attributed the increase in unfilled positions in part to a decrease in the number of U.S. MD and U.S. DO seniors who submitted ranks for the specialty, which “could reflect changing applicant interests or projections about workforce opportunities post residency.”
Applicants who didn’t match usually try to obtain an unfilled position through SOAP. In 2023, 2,685 positions were unfilled after the matching algorithm was processed, an increase of nearly 19% over 2022. The vast majority of those positions were placed in SOAP, an increase of 17.5% over 2022.
Asim Ansari was one of the unlucky ones. Mr. Ansari was trying to match for the fifth time. He was unsuccessful in doing so again in 2023 in the Match and SOAP. Still, he was offered and accepted a child and adolescent psychiatry fellowship at Kansas University Medical Center in Kansas City. Psychiatry was his chosen specialty, so he was “feeling good. It’s a nice place to go to do the next 2 years.”
Mr. Ansari, who started the #MatchMadness support group for unmatched doctors on Twitter Spaces, was quick to cheer on his fellow matching peers on March 13 while revealing his own fate: “Congratulations to everyone who matched!!! Y’all are amazing. So proud of each one of you!!! I didn’t.”
Soon after the results, #MatchMadness held a #Soap2023 support session, and Mr. Ansari sought advice for those willing to review SOAP applications. Elsewhere on Twitter Match Day threads, a few doctors offered their support to those who planned to SOAP, students announced their matches, and others either congratulated or encouraged those still trying to match.
Couples match
Not everyone who matched considered the alternative. Before March 13, William Boyer said that he hadn’t given much thought to what would happen if he didn’t match because he was “optimistically confident” he would match into his chosen EM specialty. But he did and got his top choice of programs: Yale New Haven (Conn.) Hospital.
“I feel great,” he said in an interview. “I was definitely nervous opening the envelope” that revealed his residency program, “but there was a rush of relief” when he saw he landed Yale.
Earlier in the match cycle, he said in an interview that he “interviewed at a few ‘reach’ programs, so I hope I don’t match lower than expected on my rank list.”
Mr. Boyer considers himself “a mature applicant,” entering the University of South Carolina, Columbia, after 4 years as an insurance broker.
“I am celebrating today by playing pickleball with a few close medical friends who also matched this morning,” Mr. Boyer said on March 13. “I definitely had periods of nervousness leading up to this morning though that quickly turned into joy and relief” after learning he matched.
Mr. Boyer believes that his professional experience in the insurance industry and health care lobbying efforts with the National Association of Health Underwriters set him apart from other applicants.
“I changed careers to pursue this aspiration, which demonstrates my full dedication to the medical profession.”
He applied to 48 programs and was offered interviews to nearly half. Mr. Boyer visited the majority of those virtually. He said he targeted programs close to where his and his partner’s families are located: Massachusetts, North Carolina, and Texas. “My partner, who I met in medical school, matched into ortho as well so the whole household is very happy,” Mr. Boyer said.
She matched into her top choice as well on March 17, though a distance away at UT Health in San Antonio, he said. “We are both ecstatic. We both got our no. 1 choice. That was the plan going into it. We will make it work. I have 4 weeks of vacation.”
In his program choices, Mr. Boyer prioritized access to nature, minimal leadership turnover, a mix of clinical training sites, and adequate elective rotations and fellowship opportunities, such as in wilderness medicine and health policy.
NRMP reported that there were 1,239 couples participating in the Match; 1,095 had both partners match, and 114 had one partner match to residency training programs for a match rate of 93%.
Like Mr. Boyer, Hannah Hedriana matched into EM, one of the more popular despite the reported unfilled positions. In the past few years, it has consistently been one of the fastest-growing specialties, according to the NRMP.
Still Ms. Hedriana had a fall-back plan. “If I don’t match, then I do plan on going through SOAP. With the number of EM spots that were unfilled in 2022, there’s a chance I could still be an EM physician, but if not, then that’s okay with me.”
Her reaction on March 13, after learning she matched? “Super excited, celebrating with my friends right now.” On Match Day, she said she was “ecstatic” to be matched into Lakeland (Fla.) Regional Health. “This was my first choice so now I can stay close to family and friends,” she said in an interview soon after the results were released.
A first-generation, Filipino American student from the University of South Florida, Tampa, Ms. Hedriana comes from a family of health care professionals. Her father is a respiratory therapist turned physical therapist; her mother a registered nurse. Her sister is a patient care technician applying to nursing school.
Ms. Hedriana applied to 70 programs and interviewed mostly online with 24. Her goal was to stay on the East Coast.
“My partner is a licensed dentist in the state of Florida, and so for his career it would be more practical to stay in state, rather than get relicensed in another state, which could take months,” she said earlier in the matching cycle. “However, when we discussed choosing a residency program, he ultimately left it up to me and wanted me to pick where I thought I’d flourish best,” Ms. Hedriana said, adding that her family lives in Florida, too.
She said she sought a residency program that values family and teamwork.
“A program gets more points in my book if they have sites at nonprofit hospitals or has residents that regularly volunteer throughout their communities or participate in DEI [diversity, equity, and inclusion] initiatives.”
Ms. Hedriana noted that some specialties exclusively offered virtual interviews in 2023, whereas other specialties favored in-person interviews. “This year, many of my classmates were able to do multiple away rotations, which they saw as a positive regarding their chances of matching.” During COVID, in-person visits were limited.
“However, I’ve noticed that many of my classmates are not fond of the signaling aspect that was present for this year’s cycle,” she said. Signaling is a relatively new process that allows applicants to indicate interest in a limited number of residency programs. Not all residencies participate, but it’s growing in popularity among specialties, according to the American Medical Association.
‘Extremely competitive’
Ms. Nagle, a second lieutenant in the U.S. Air Force, applied to 12 programs and interviewed with half of them online. She said that she wasn’t targeting any specific type of program through the match.
“I believe you can get phenomenal training anywhere where you mesh with the residents and leadership. My ultimate priority is to (1) be near good people, (2) be near good food (Indian and Thai are a must), and (3) be near an international airport so I can flee the country during breaks.”
Meanwhile, she said that she found the application process, in which students have to articulate their entire medical school experience, extremely competitive. “I think this process is so easy to get wound up in and the anxiety can be palpable,” Ms. Nagle said. “People around you match your energy. So if you are a ball of anxiety then so are your attendings and residents – and that doesn’t bode well for passing the ‘do I want to be on call with them’ test.”
Looking back at medical school, Ms. Nagle recalled having a baby named after her during her first anesthesia rotation and being featured on The Kelly Clarkson Show. Ms. Nagle said that she had walked into the delivery room where new parents had been debating names of babies beginning with the letter B. “And when I introduced myself, they looked at each other and said, ‘Yep, that’s the one.’”
Mr. Boyer recounted how the majority of his medical school experience involved online education. “Roughly two-thirds of my first year was in-person prior to the pandemic. However, from spring break first year to in-person clinical rotations at the beginning of third year, we were all virtual. While I missed interacting with my classmates, I benefited from the virtual learning environment as I learn more efficiently from reading and visual aids than auditory lectures.”
Ms. Hedriana cited the friends and memories she made while learning to be a doctor. “Medical school was hard, but I wouldn’t have changed a thing.”
A version of this article first appeared on Medscape.com.
Baily Nagle, vice president of her graduating class at Harvard Medical School, Boston, celebrated “the luck of the Irish” on St. Patrick’s Day that allowed her to match into her chosen specialty and top choice of residency programs: anesthesia at Brigham and Women’s Hospital.
“I am feeling very excited and relieved – I matched,” she said in an interview upon hearing her good fortune on Match Monday, March 13. She had a similar reaction on Match Day, March 17. “After a lot of long nights and hard work, happy to have it pay off.”
Ms. Nagle was so determined to match into her specialty that she didn’t have any other specialties in mind as a backup.
The annual process of matching medical school graduates with compatible residency programs is an emotional roller coaster for all applicants, their personal March Madness, so to speak. But Ms. Nagle was one of the more fortunate applicants. She didn’t have to confront the heartbreak other applicants felt when the National Resident Matching Program (NRMP) announced results of the main residency match and the Supplemental Offer and Acceptance Program (SOAP), which offers alternate programs for unfilled positions or unmatched applicants.
During the 2023 Match process, this news organization has been following a handful of students, checking in with them periodically for updates on their progress. Most of them matched successfully, but at least one international medical graduate (IMG) did not. What the others have in common is that their hearts were set on a chosen specialty. Like Ms. Nagle, another student banked on landing his chosen specialty without a backup plan, whereas another said that she’d continue through the SOAP if she didn’t match successfully.
Overall, Match Day resulted in a record number of residency positions offered, most notably in primary care, which “hit an all-time high,” according to NRMP President and CEO Donna L. Lamb, DHSc, MBA, BSN. The number of positions has “consistently increased over the past 5 years, and most importantly the fill rate for primary care has remained steady,” Dr.. Lamb noted in the NRMP release of Match Day results. The release coincided with students learning through emails at noon Eastern Time to which residency or supplemental programs they were matched.
Though more applicants registered for the Match in 2023 than in 2022 – driven primarily by non-U.S. IMGs – the NRMP stated that it was surprised by the decrease in U.S. MD senior applicants.
U.S. MD seniors had a nearly 94% Match rate, a small increase over 2022. U.S. citizen IMGs saw a nearly 68% Match rate, which NRMP reported as an “all-time high” and about six percentage points over in 2022, whereas non-U.S. IMGs had a nearly 60% Match rate, a 1.3 percentage point increase over 2022.
Among the specialties that filled all available positions in 2023 were orthopedic surgery, plastic surgery (integrated), and radiology – diagnostic and thoracic surgery.
Not everyone matches
On March 13, the American College of Emergency Physicians issued a joint statement with other emergency medicine (EM) organizations about a high rate of unfilled EM positions expected in 2023.

NRMP acknowledged March 17 that 554 positions remained unfilled, an increase of 335 more unfilled positions than 2022. NRMP attributed the increase in unfilled positions in part to a decrease in the number of U.S. MD and U.S. DO seniors who submitted ranks for the specialty, which “could reflect changing applicant interests or projections about workforce opportunities post residency.”
Applicants who didn’t match usually try to obtain an unfilled position through SOAP. In 2023, 2,685 positions were unfilled after the matching algorithm was processed, an increase of nearly 19% over 2022. The vast majority of those positions were placed in SOAP, an increase of 17.5% over 2022.
Asim Ansari was one of the unlucky ones. Mr. Ansari was trying to match for the fifth time. He was unsuccessful in doing so again in 2023 in the Match and SOAP. Still, he was offered and accepted a child and adolescent psychiatry fellowship at Kansas University Medical Center in Kansas City. Psychiatry was his chosen specialty, so he was “feeling good. It’s a nice place to go to do the next 2 years.”
Mr. Ansari, who started the #MatchMadness support group for unmatched doctors on Twitter Spaces, was quick to cheer on his fellow matching peers on March 13 while revealing his own fate: “Congratulations to everyone who matched!!! Y’all are amazing. So proud of each one of you!!! I didn’t.”
Soon after the results, #MatchMadness held a #Soap2023 support session, and Mr. Ansari sought advice for those willing to review SOAP applications. Elsewhere on Twitter Match Day threads, a few doctors offered their support to those who planned to SOAP, students announced their matches, and others either congratulated or encouraged those still trying to match.
Couples match
Not everyone who matched considered the alternative. Before March 13, William Boyer said that he hadn’t given much thought to what would happen if he didn’t match because he was “optimistically confident” he would match into his chosen EM specialty. But he did and got his top choice of programs: Yale New Haven (Conn.) Hospital.
“I feel great,” he said in an interview. “I was definitely nervous opening the envelope” that revealed his residency program, “but there was a rush of relief” when he saw he landed Yale.
Earlier in the match cycle, he said in an interview that he “interviewed at a few ‘reach’ programs, so I hope I don’t match lower than expected on my rank list.”
Mr. Boyer considers himself “a mature applicant,” entering the University of South Carolina, Columbia, after 4 years as an insurance broker.
“I am celebrating today by playing pickleball with a few close medical friends who also matched this morning,” Mr. Boyer said on March 13. “I definitely had periods of nervousness leading up to this morning though that quickly turned into joy and relief” after learning he matched.
Mr. Boyer believes that his professional experience in the insurance industry and health care lobbying efforts with the National Association of Health Underwriters set him apart from other applicants.
“I changed careers to pursue this aspiration, which demonstrates my full dedication to the medical profession.”
He applied to 48 programs and was offered interviews to nearly half. Mr. Boyer visited the majority of those virtually. He said he targeted programs close to where his and his partner’s families are located: Massachusetts, North Carolina, and Texas. “My partner, who I met in medical school, matched into ortho as well so the whole household is very happy,” Mr. Boyer said.
She matched into her top choice as well on March 17, though a distance away at UT Health in San Antonio, he said. “We are both ecstatic. We both got our no. 1 choice. That was the plan going into it. We will make it work. I have 4 weeks of vacation.”
In his program choices, Mr. Boyer prioritized access to nature, minimal leadership turnover, a mix of clinical training sites, and adequate elective rotations and fellowship opportunities, such as in wilderness medicine and health policy.
NRMP reported that there were 1,239 couples participating in the Match; 1,095 had both partners match, and 114 had one partner match to residency training programs for a match rate of 93%.
Like Mr. Boyer, Hannah Hedriana matched into EM, one of the more popular despite the reported unfilled positions. In the past few years, it has consistently been one of the fastest-growing specialties, according to the NRMP.
Still Ms. Hedriana had a fall-back plan. “If I don’t match, then I do plan on going through SOAP. With the number of EM spots that were unfilled in 2022, there’s a chance I could still be an EM physician, but if not, then that’s okay with me.”
Her reaction on March 13, after learning she matched? “Super excited, celebrating with my friends right now.” On Match Day, she said she was “ecstatic” to be matched into Lakeland (Fla.) Regional Health. “This was my first choice so now I can stay close to family and friends,” she said in an interview soon after the results were released.
A first-generation, Filipino American student from the University of South Florida, Tampa, Ms. Hedriana comes from a family of health care professionals. Her father is a respiratory therapist turned physical therapist; her mother a registered nurse. Her sister is a patient care technician applying to nursing school.
Ms. Hedriana applied to 70 programs and interviewed mostly online with 24. Her goal was to stay on the East Coast.
“My partner is a licensed dentist in the state of Florida, and so for his career it would be more practical to stay in state, rather than get relicensed in another state, which could take months,” she said earlier in the matching cycle. “However, when we discussed choosing a residency program, he ultimately left it up to me and wanted me to pick where I thought I’d flourish best,” Ms. Hedriana said, adding that her family lives in Florida, too.
She said she sought a residency program that values family and teamwork.
“A program gets more points in my book if they have sites at nonprofit hospitals or has residents that regularly volunteer throughout their communities or participate in DEI [diversity, equity, and inclusion] initiatives.”
Ms. Hedriana noted that some specialties exclusively offered virtual interviews in 2023, whereas other specialties favored in-person interviews. “This year, many of my classmates were able to do multiple away rotations, which they saw as a positive regarding their chances of matching.” During COVID, in-person visits were limited.
“However, I’ve noticed that many of my classmates are not fond of the signaling aspect that was present for this year’s cycle,” she said. Signaling is a relatively new process that allows applicants to indicate interest in a limited number of residency programs. Not all residencies participate, but it’s growing in popularity among specialties, according to the American Medical Association.
‘Extremely competitive’
Ms. Nagle, a second lieutenant in the U.S. Air Force, applied to 12 programs and interviewed with half of them online. She said that she wasn’t targeting any specific type of program through the match.
“I believe you can get phenomenal training anywhere where you mesh with the residents and leadership. My ultimate priority is to (1) be near good people, (2) be near good food (Indian and Thai are a must), and (3) be near an international airport so I can flee the country during breaks.”
Meanwhile, she said that she found the application process, in which students have to articulate their entire medical school experience, extremely competitive. “I think this process is so easy to get wound up in and the anxiety can be palpable,” Ms. Nagle said. “People around you match your energy. So if you are a ball of anxiety then so are your attendings and residents – and that doesn’t bode well for passing the ‘do I want to be on call with them’ test.”
Looking back at medical school, Ms. Nagle recalled having a baby named after her during her first anesthesia rotation and being featured on The Kelly Clarkson Show. Ms. Nagle said that she had walked into the delivery room where new parents had been debating names of babies beginning with the letter B. “And when I introduced myself, they looked at each other and said, ‘Yep, that’s the one.’”
Mr. Boyer recounted how the majority of his medical school experience involved online education. “Roughly two-thirds of my first year was in-person prior to the pandemic. However, from spring break first year to in-person clinical rotations at the beginning of third year, we were all virtual. While I missed interacting with my classmates, I benefited from the virtual learning environment as I learn more efficiently from reading and visual aids than auditory lectures.”
Ms. Hedriana cited the friends and memories she made while learning to be a doctor. “Medical school was hard, but I wouldn’t have changed a thing.”
A version of this article first appeared on Medscape.com.
Baily Nagle, vice president of her graduating class at Harvard Medical School, Boston, celebrated “the luck of the Irish” on St. Patrick’s Day that allowed her to match into her chosen specialty and top choice of residency programs: anesthesia at Brigham and Women’s Hospital.
“I am feeling very excited and relieved – I matched,” she said in an interview upon hearing her good fortune on Match Monday, March 13. She had a similar reaction on Match Day, March 17. “After a lot of long nights and hard work, happy to have it pay off.”
Ms. Nagle was so determined to match into her specialty that she didn’t have any other specialties in mind as a backup.
The annual process of matching medical school graduates with compatible residency programs is an emotional roller coaster for all applicants, their personal March Madness, so to speak. But Ms. Nagle was one of the more fortunate applicants. She didn’t have to confront the heartbreak other applicants felt when the National Resident Matching Program (NRMP) announced results of the main residency match and the Supplemental Offer and Acceptance Program (SOAP), which offers alternate programs for unfilled positions or unmatched applicants.
During the 2023 Match process, this news organization has been following a handful of students, checking in with them periodically for updates on their progress. Most of them matched successfully, but at least one international medical graduate (IMG) did not. What the others have in common is that their hearts were set on a chosen specialty. Like Ms. Nagle, another student banked on landing his chosen specialty without a backup plan, whereas another said that she’d continue through the SOAP if she didn’t match successfully.
Overall, Match Day resulted in a record number of residency positions offered, most notably in primary care, which “hit an all-time high,” according to NRMP President and CEO Donna L. Lamb, DHSc, MBA, BSN. The number of positions has “consistently increased over the past 5 years, and most importantly the fill rate for primary care has remained steady,” Dr.. Lamb noted in the NRMP release of Match Day results. The release coincided with students learning through emails at noon Eastern Time to which residency or supplemental programs they were matched.
Though more applicants registered for the Match in 2023 than in 2022 – driven primarily by non-U.S. IMGs – the NRMP stated that it was surprised by the decrease in U.S. MD senior applicants.
U.S. MD seniors had a nearly 94% Match rate, a small increase over 2022. U.S. citizen IMGs saw a nearly 68% Match rate, which NRMP reported as an “all-time high” and about six percentage points over in 2022, whereas non-U.S. IMGs had a nearly 60% Match rate, a 1.3 percentage point increase over 2022.
Among the specialties that filled all available positions in 2023 were orthopedic surgery, plastic surgery (integrated), and radiology – diagnostic and thoracic surgery.
Not everyone matches
On March 13, the American College of Emergency Physicians issued a joint statement with other emergency medicine (EM) organizations about a high rate of unfilled EM positions expected in 2023.

NRMP acknowledged March 17 that 554 positions remained unfilled, an increase of 335 more unfilled positions than 2022. NRMP attributed the increase in unfilled positions in part to a decrease in the number of U.S. MD and U.S. DO seniors who submitted ranks for the specialty, which “could reflect changing applicant interests or projections about workforce opportunities post residency.”
Applicants who didn’t match usually try to obtain an unfilled position through SOAP. In 2023, 2,685 positions were unfilled after the matching algorithm was processed, an increase of nearly 19% over 2022. The vast majority of those positions were placed in SOAP, an increase of 17.5% over 2022.
Asim Ansari was one of the unlucky ones. Mr. Ansari was trying to match for the fifth time. He was unsuccessful in doing so again in 2023 in the Match and SOAP. Still, he was offered and accepted a child and adolescent psychiatry fellowship at Kansas University Medical Center in Kansas City. Psychiatry was his chosen specialty, so he was “feeling good. It’s a nice place to go to do the next 2 years.”
Mr. Ansari, who started the #MatchMadness support group for unmatched doctors on Twitter Spaces, was quick to cheer on his fellow matching peers on March 13 while revealing his own fate: “Congratulations to everyone who matched!!! Y’all are amazing. So proud of each one of you!!! I didn’t.”
Soon after the results, #MatchMadness held a #Soap2023 support session, and Mr. Ansari sought advice for those willing to review SOAP applications. Elsewhere on Twitter Match Day threads, a few doctors offered their support to those who planned to SOAP, students announced their matches, and others either congratulated or encouraged those still trying to match.
Couples match
Not everyone who matched considered the alternative. Before March 13, William Boyer said that he hadn’t given much thought to what would happen if he didn’t match because he was “optimistically confident” he would match into his chosen EM specialty. But he did and got his top choice of programs: Yale New Haven (Conn.) Hospital.
“I feel great,” he said in an interview. “I was definitely nervous opening the envelope” that revealed his residency program, “but there was a rush of relief” when he saw he landed Yale.
Earlier in the match cycle, he said in an interview that he “interviewed at a few ‘reach’ programs, so I hope I don’t match lower than expected on my rank list.”
Mr. Boyer considers himself “a mature applicant,” entering the University of South Carolina, Columbia, after 4 years as an insurance broker.
“I am celebrating today by playing pickleball with a few close medical friends who also matched this morning,” Mr. Boyer said on March 13. “I definitely had periods of nervousness leading up to this morning though that quickly turned into joy and relief” after learning he matched.
Mr. Boyer believes that his professional experience in the insurance industry and health care lobbying efforts with the National Association of Health Underwriters set him apart from other applicants.
“I changed careers to pursue this aspiration, which demonstrates my full dedication to the medical profession.”
He applied to 48 programs and was offered interviews to nearly half. Mr. Boyer visited the majority of those virtually. He said he targeted programs close to where his and his partner’s families are located: Massachusetts, North Carolina, and Texas. “My partner, who I met in medical school, matched into ortho as well so the whole household is very happy,” Mr. Boyer said.
She matched into her top choice as well on March 17, though a distance away at UT Health in San Antonio, he said. “We are both ecstatic. We both got our no. 1 choice. That was the plan going into it. We will make it work. I have 4 weeks of vacation.”
In his program choices, Mr. Boyer prioritized access to nature, minimal leadership turnover, a mix of clinical training sites, and adequate elective rotations and fellowship opportunities, such as in wilderness medicine and health policy.
NRMP reported that there were 1,239 couples participating in the Match; 1,095 had both partners match, and 114 had one partner match to residency training programs for a match rate of 93%.
Like Mr. Boyer, Hannah Hedriana matched into EM, one of the more popular despite the reported unfilled positions. In the past few years, it has consistently been one of the fastest-growing specialties, according to the NRMP.
Still Ms. Hedriana had a fall-back plan. “If I don’t match, then I do plan on going through SOAP. With the number of EM spots that were unfilled in 2022, there’s a chance I could still be an EM physician, but if not, then that’s okay with me.”
Her reaction on March 13, after learning she matched? “Super excited, celebrating with my friends right now.” On Match Day, she said she was “ecstatic” to be matched into Lakeland (Fla.) Regional Health. “This was my first choice so now I can stay close to family and friends,” she said in an interview soon after the results were released.
A first-generation, Filipino American student from the University of South Florida, Tampa, Ms. Hedriana comes from a family of health care professionals. Her father is a respiratory therapist turned physical therapist; her mother a registered nurse. Her sister is a patient care technician applying to nursing school.
Ms. Hedriana applied to 70 programs and interviewed mostly online with 24. Her goal was to stay on the East Coast.
“My partner is a licensed dentist in the state of Florida, and so for his career it would be more practical to stay in state, rather than get relicensed in another state, which could take months,” she said earlier in the matching cycle. “However, when we discussed choosing a residency program, he ultimately left it up to me and wanted me to pick where I thought I’d flourish best,” Ms. Hedriana said, adding that her family lives in Florida, too.
She said she sought a residency program that values family and teamwork.
“A program gets more points in my book if they have sites at nonprofit hospitals or has residents that regularly volunteer throughout their communities or participate in DEI [diversity, equity, and inclusion] initiatives.”
Ms. Hedriana noted that some specialties exclusively offered virtual interviews in 2023, whereas other specialties favored in-person interviews. “This year, many of my classmates were able to do multiple away rotations, which they saw as a positive regarding their chances of matching.” During COVID, in-person visits were limited.
“However, I’ve noticed that many of my classmates are not fond of the signaling aspect that was present for this year’s cycle,” she said. Signaling is a relatively new process that allows applicants to indicate interest in a limited number of residency programs. Not all residencies participate, but it’s growing in popularity among specialties, according to the American Medical Association.
‘Extremely competitive’
Ms. Nagle, a second lieutenant in the U.S. Air Force, applied to 12 programs and interviewed with half of them online. She said that she wasn’t targeting any specific type of program through the match.
“I believe you can get phenomenal training anywhere where you mesh with the residents and leadership. My ultimate priority is to (1) be near good people, (2) be near good food (Indian and Thai are a must), and (3) be near an international airport so I can flee the country during breaks.”
Meanwhile, she said that she found the application process, in which students have to articulate their entire medical school experience, extremely competitive. “I think this process is so easy to get wound up in and the anxiety can be palpable,” Ms. Nagle said. “People around you match your energy. So if you are a ball of anxiety then so are your attendings and residents – and that doesn’t bode well for passing the ‘do I want to be on call with them’ test.”
Looking back at medical school, Ms. Nagle recalled having a baby named after her during her first anesthesia rotation and being featured on The Kelly Clarkson Show. Ms. Nagle said that she had walked into the delivery room where new parents had been debating names of babies beginning with the letter B. “And when I introduced myself, they looked at each other and said, ‘Yep, that’s the one.’”
Mr. Boyer recounted how the majority of his medical school experience involved online education. “Roughly two-thirds of my first year was in-person prior to the pandemic. However, from spring break first year to in-person clinical rotations at the beginning of third year, we were all virtual. While I missed interacting with my classmates, I benefited from the virtual learning environment as I learn more efficiently from reading and visual aids than auditory lectures.”
Ms. Hedriana cited the friends and memories she made while learning to be a doctor. “Medical school was hard, but I wouldn’t have changed a thing.”
A version of this article first appeared on Medscape.com.
Increased cancer in military pilots and ground crew: Pentagon
“Military aircrew and ground crew were overall more likely to be diagnosed with cancer, but less likely to die from cancer compared to the U.S. population,” the report concludes.
The study involved 156,050 aircrew and 737,891 ground crew. Participants were followed between 1992 and 2017. Both groups were predominantly male and non-Hispanic.
Data on cancer incidence and mortality for these two groups were compared with data from groups of similar age in the general population through use of the Surveillance, Epidemiology, and End Results (SEER) Database of the National Cancer Institute.
For aircrew, the study found an 87% higher rate of melanoma, a 39% higher rate of thyroid cancer, a 16% higher rate of prostate cancer, and a 24% higher rate of cancer for all sites combined.
A higher rate of melanoma and prostate cancer among aircrew has been reported previously, but the increased rate of thyroid cancer is a new finding, the authors note.
The uptick in melanoma has also been reported in studies of civilian pilots and cabin crew. It has been attributed to exposure to hazardous ultraviolet and cosmic radiation.
For ground crew members, the analysis found a 19% higher rate of cancers of the brain and nervous system, a 15% higher rate of thyroid cancer, a 9% higher rate of melanoma and of kidney and renal pelvis cancers, and a 3% higher rate of cancer for all sites combined.
There is little to compare these findings with: This is the first time that cancer risk has been evaluated in such a large population of military ground crew.
Lower rates of cancer mortality
In contrast to the increase in cancer incidence, the report found a decrease in cancer mortality.
When compared with a demographically similar U.S. population, the mortality rate among aircrew was 56% lower for all cancer sites; for ground crew, the mortality rate was 35% lower.
However, the report authors emphasize that “it is important to note that the military study population was relatively young.”
The median age at the end of follow-up for the cancer incidence analysis was 41 years for aircrew and 26 years for ground crew. The median age at the end of follow-up for the cancer mortality analysis was 48 years for aircrew and 41 years for ground crew.
“Results may have differed if additional older former Service members had been included in the study, since cancer risk and mortality rates increase with age,” the authors comment.
Other studies have found an increase in deaths from melanoma as well as an increase in the incidence of melanoma. A meta-analysis published in 2019 in the British Journal of Dermatology found that airline pilots and cabin crew have about twice the risk of melanoma and other skin cancers than the general population. Pilots are also more likely to die from melanoma.
Further study underway
The findings on military air and ground crew come from phase 1 of a study that was required by Congress in the 2021 defense bill. Because the investigators found an increase in the incidence of cancer, phase 2 of the study is now necessary.
The report authors explain that phase 2 will consist of identifying the carcinogenic toxicants or hazardous materials associated with military flight operations; identifying operating environments that could be associated with increased amounts of ionizing and nonionizing radiation; identifying specific duties, dates of service, and types of aircraft flown that could have increased the risk for cancer; identifying duty locations associated with a higher incidence of cancers; identifying potential exposures through military service that are not related to aviation; and determining the appropriate age to begin screening military aircrew and ground crew for cancers.
A version of this article first appeared on Medscape.com.
“Military aircrew and ground crew were overall more likely to be diagnosed with cancer, but less likely to die from cancer compared to the U.S. population,” the report concludes.
The study involved 156,050 aircrew and 737,891 ground crew. Participants were followed between 1992 and 2017. Both groups were predominantly male and non-Hispanic.
Data on cancer incidence and mortality for these two groups were compared with data from groups of similar age in the general population through use of the Surveillance, Epidemiology, and End Results (SEER) Database of the National Cancer Institute.
For aircrew, the study found an 87% higher rate of melanoma, a 39% higher rate of thyroid cancer, a 16% higher rate of prostate cancer, and a 24% higher rate of cancer for all sites combined.
A higher rate of melanoma and prostate cancer among aircrew has been reported previously, but the increased rate of thyroid cancer is a new finding, the authors note.
The uptick in melanoma has also been reported in studies of civilian pilots and cabin crew. It has been attributed to exposure to hazardous ultraviolet and cosmic radiation.
For ground crew members, the analysis found a 19% higher rate of cancers of the brain and nervous system, a 15% higher rate of thyroid cancer, a 9% higher rate of melanoma and of kidney and renal pelvis cancers, and a 3% higher rate of cancer for all sites combined.
There is little to compare these findings with: This is the first time that cancer risk has been evaluated in such a large population of military ground crew.
Lower rates of cancer mortality
In contrast to the increase in cancer incidence, the report found a decrease in cancer mortality.
When compared with a demographically similar U.S. population, the mortality rate among aircrew was 56% lower for all cancer sites; for ground crew, the mortality rate was 35% lower.
However, the report authors emphasize that “it is important to note that the military study population was relatively young.”
The median age at the end of follow-up for the cancer incidence analysis was 41 years for aircrew and 26 years for ground crew. The median age at the end of follow-up for the cancer mortality analysis was 48 years for aircrew and 41 years for ground crew.
“Results may have differed if additional older former Service members had been included in the study, since cancer risk and mortality rates increase with age,” the authors comment.
Other studies have found an increase in deaths from melanoma as well as an increase in the incidence of melanoma. A meta-analysis published in 2019 in the British Journal of Dermatology found that airline pilots and cabin crew have about twice the risk of melanoma and other skin cancers than the general population. Pilots are also more likely to die from melanoma.
Further study underway
The findings on military air and ground crew come from phase 1 of a study that was required by Congress in the 2021 defense bill. Because the investigators found an increase in the incidence of cancer, phase 2 of the study is now necessary.
The report authors explain that phase 2 will consist of identifying the carcinogenic toxicants or hazardous materials associated with military flight operations; identifying operating environments that could be associated with increased amounts of ionizing and nonionizing radiation; identifying specific duties, dates of service, and types of aircraft flown that could have increased the risk for cancer; identifying duty locations associated with a higher incidence of cancers; identifying potential exposures through military service that are not related to aviation; and determining the appropriate age to begin screening military aircrew and ground crew for cancers.
A version of this article first appeared on Medscape.com.
“Military aircrew and ground crew were overall more likely to be diagnosed with cancer, but less likely to die from cancer compared to the U.S. population,” the report concludes.
The study involved 156,050 aircrew and 737,891 ground crew. Participants were followed between 1992 and 2017. Both groups were predominantly male and non-Hispanic.
Data on cancer incidence and mortality for these two groups were compared with data from groups of similar age in the general population through use of the Surveillance, Epidemiology, and End Results (SEER) Database of the National Cancer Institute.
For aircrew, the study found an 87% higher rate of melanoma, a 39% higher rate of thyroid cancer, a 16% higher rate of prostate cancer, and a 24% higher rate of cancer for all sites combined.
A higher rate of melanoma and prostate cancer among aircrew has been reported previously, but the increased rate of thyroid cancer is a new finding, the authors note.
The uptick in melanoma has also been reported in studies of civilian pilots and cabin crew. It has been attributed to exposure to hazardous ultraviolet and cosmic radiation.
For ground crew members, the analysis found a 19% higher rate of cancers of the brain and nervous system, a 15% higher rate of thyroid cancer, a 9% higher rate of melanoma and of kidney and renal pelvis cancers, and a 3% higher rate of cancer for all sites combined.
There is little to compare these findings with: This is the first time that cancer risk has been evaluated in such a large population of military ground crew.
Lower rates of cancer mortality
In contrast to the increase in cancer incidence, the report found a decrease in cancer mortality.
When compared with a demographically similar U.S. population, the mortality rate among aircrew was 56% lower for all cancer sites; for ground crew, the mortality rate was 35% lower.
However, the report authors emphasize that “it is important to note that the military study population was relatively young.”
The median age at the end of follow-up for the cancer incidence analysis was 41 years for aircrew and 26 years for ground crew. The median age at the end of follow-up for the cancer mortality analysis was 48 years for aircrew and 41 years for ground crew.
“Results may have differed if additional older former Service members had been included in the study, since cancer risk and mortality rates increase with age,” the authors comment.
Other studies have found an increase in deaths from melanoma as well as an increase in the incidence of melanoma. A meta-analysis published in 2019 in the British Journal of Dermatology found that airline pilots and cabin crew have about twice the risk of melanoma and other skin cancers than the general population. Pilots are also more likely to die from melanoma.
Further study underway
The findings on military air and ground crew come from phase 1 of a study that was required by Congress in the 2021 defense bill. Because the investigators found an increase in the incidence of cancer, phase 2 of the study is now necessary.
The report authors explain that phase 2 will consist of identifying the carcinogenic toxicants or hazardous materials associated with military flight operations; identifying operating environments that could be associated with increased amounts of ionizing and nonionizing radiation; identifying specific duties, dates of service, and types of aircraft flown that could have increased the risk for cancer; identifying duty locations associated with a higher incidence of cancers; identifying potential exposures through military service that are not related to aviation; and determining the appropriate age to begin screening military aircrew and ground crew for cancers.
A version of this article first appeared on Medscape.com.
Melanoma screening: Consensus statement offers greater clarity
That is why a group of expert panelists evaluated the existing evidence and a range of clinical scenarios to help clarify the optimal strategies for early detection and assessment of cutaneous melanoma.
Overall, the panelists agreed that a risk-stratified approach is likely the most appropriate strategy for melanoma screening and follow-up and supported the use of visual and dermoscopic examination. However, the panelists did not reach consensus on the role for gene expression profile (GEP) testing in clinical decision-making, citing the need for these assays to be validated in large randomized clinical trials.
In an accompanying editorial, two experts highlighted the importance of carefully evaluating the role of diagnostic tests.
“Diagnostic tests such as GEP must face critical scrutiny; if not, there are immediate concerns for patient care, such as the patient being erroneously informed that they do not have cancer or told that they do have cancer when they do not,” write Alan C. Geller, MPH, RN, from the Harvard T.H. Chan School of Public Health, Boston, and Marvin A. Weinstock, MD, PhD, from Brown University, Providence, R.I.
The consensus statement was published online in JAMA Dermatology.
The need for guidance
Although focusing melanoma screening on higher-risk populations may be cost effective, compared with population-based screening, the major guidelines lack consistent guidance to support a risk-stratified approach to skin cancer screening and best practices on diagnosing cutaneous melanoma.
In the prebiopsy setting, the appropriate use of diagnostic tools for evaluating the need for biopsy remain poorly defined, and, in the post-biopsy setting, questions remain concerning the diagnostic accuracy of molecular techniques, diagnostic GEP testing, next-generation sequencing, and immunohistochemical assessment for various markers of melanoma.
To provide consensus recommendations on optimal screening practices, prebiopsy and postbiopsy diagnostics, and prognostic assessment of cutaneous melanoma, a group of 42 panelists voted on hypothetical scenarios via an emailed survey. The panel then came together for a consensus conference, which included 51 experts who discussed their approach to the various clinical case scenarios. Most attendees (45 of the 51) answered a follow-up survey for their final recommendations.
The panelists reached a consensus, with 70% agreement, to support a risk-stratified approach to melanoma screening in clinical settings and public screening events. The experts agreed that higher-risk individuals (those with a relative risk of 5 or greater) could be appropriately screened by a general dermatologist or pigmented lesion evaluation. Higher-risk individuals included those with severe skin damage from the sun, systemic immunosuppression, or a personal history of nonmelanoma or melanoma skin cancer.
Panelists agreed that those at general or lower risk (RR < 2) could be screened by a primary care provider or through regular self- or partner examinations, whereas those at moderate risk could be screened by their primary care clinician or general dermatologist. The experts observed “a shift in acceptance” of primary care physicians screening the general population, and an acknowledgement of the importance of self- and partner examinations as screening adjuncts for all populations.
In the prebiopsy setting, panelists reached consensus that visual and dermoscopic examination was appropriate for evaluating patients with “no new, changing, or unusual skin lesions or with a new lesion that is not visually concerning.”
The panelists also reached consensus that lesions deemed clinically suspicious for cancer or showing features of cancer on reflectance confocal microscopy should be biopsied. Although most respondents (86%) did not currently use epidermal tape stripping routinely, they agreed that, in a hypothetical situation where epidermal tape stripping was used, that lesions positive for PRAME or LINC should be biopsied.
In the postbiopsy setting, views on the use of GEP scores varied. Although panelists agreed that a low-risk prognostic GEP score should not outweigh concerning histologic features when patients are selected to undergo sentinel lymph node biopsy (SLNB), they did not reach consensus for imaging recommendations in the setting of a high-risk prognostic GEP score and low-risk histology and/or negative nodal status.
“The panelists await future, well-designed prospective studies to determine if use of these and newer technologies improves the care of patients with melanoma,” the panelists write.
In the editorial, Mr. Geller and Dr. Weinstock highlighted concerns about the cost and potential access issues associated with these newer technologies, given that the current cost of GEP testing exceeds $7,000.
The editorialists also emphasize that “going forward, the field should be advanced by tackling one of the more pressing, common, potentially morbid, and costly procedures – the prognostic use of sentinel lymph node biopsy.”
Of critical importance is “whether GEP can reduce morbidity and cost by safely reducing the number of SLNBs performed,” Mr. Geller and Dr. Weinstock write.
The funding for the administration and facilitation of the consensus development conference and the development of the manuscript was provided by Dermtech, in an unrestricted award overseen by the Melanoma Research Foundation and managed and executed at UPMC by the principal investigator. Several of the coauthors disclosed relationships with industry. Mr. Geller is a contributor to UptoDate for which he receives royalties. Dr. Weinstock receives consulting fees from AbbVie.
A version of this article first appeared on Medscape.com.
That is why a group of expert panelists evaluated the existing evidence and a range of clinical scenarios to help clarify the optimal strategies for early detection and assessment of cutaneous melanoma.
Overall, the panelists agreed that a risk-stratified approach is likely the most appropriate strategy for melanoma screening and follow-up and supported the use of visual and dermoscopic examination. However, the panelists did not reach consensus on the role for gene expression profile (GEP) testing in clinical decision-making, citing the need for these assays to be validated in large randomized clinical trials.
In an accompanying editorial, two experts highlighted the importance of carefully evaluating the role of diagnostic tests.
“Diagnostic tests such as GEP must face critical scrutiny; if not, there are immediate concerns for patient care, such as the patient being erroneously informed that they do not have cancer or told that they do have cancer when they do not,” write Alan C. Geller, MPH, RN, from the Harvard T.H. Chan School of Public Health, Boston, and Marvin A. Weinstock, MD, PhD, from Brown University, Providence, R.I.
The consensus statement was published online in JAMA Dermatology.
The need for guidance
Although focusing melanoma screening on higher-risk populations may be cost effective, compared with population-based screening, the major guidelines lack consistent guidance to support a risk-stratified approach to skin cancer screening and best practices on diagnosing cutaneous melanoma.
In the prebiopsy setting, the appropriate use of diagnostic tools for evaluating the need for biopsy remain poorly defined, and, in the post-biopsy setting, questions remain concerning the diagnostic accuracy of molecular techniques, diagnostic GEP testing, next-generation sequencing, and immunohistochemical assessment for various markers of melanoma.
To provide consensus recommendations on optimal screening practices, prebiopsy and postbiopsy diagnostics, and prognostic assessment of cutaneous melanoma, a group of 42 panelists voted on hypothetical scenarios via an emailed survey. The panel then came together for a consensus conference, which included 51 experts who discussed their approach to the various clinical case scenarios. Most attendees (45 of the 51) answered a follow-up survey for their final recommendations.
The panelists reached a consensus, with 70% agreement, to support a risk-stratified approach to melanoma screening in clinical settings and public screening events. The experts agreed that higher-risk individuals (those with a relative risk of 5 or greater) could be appropriately screened by a general dermatologist or pigmented lesion evaluation. Higher-risk individuals included those with severe skin damage from the sun, systemic immunosuppression, or a personal history of nonmelanoma or melanoma skin cancer.
Panelists agreed that those at general or lower risk (RR < 2) could be screened by a primary care provider or through regular self- or partner examinations, whereas those at moderate risk could be screened by their primary care clinician or general dermatologist. The experts observed “a shift in acceptance” of primary care physicians screening the general population, and an acknowledgement of the importance of self- and partner examinations as screening adjuncts for all populations.
In the prebiopsy setting, panelists reached consensus that visual and dermoscopic examination was appropriate for evaluating patients with “no new, changing, or unusual skin lesions or with a new lesion that is not visually concerning.”
The panelists also reached consensus that lesions deemed clinically suspicious for cancer or showing features of cancer on reflectance confocal microscopy should be biopsied. Although most respondents (86%) did not currently use epidermal tape stripping routinely, they agreed that, in a hypothetical situation where epidermal tape stripping was used, that lesions positive for PRAME or LINC should be biopsied.
In the postbiopsy setting, views on the use of GEP scores varied. Although panelists agreed that a low-risk prognostic GEP score should not outweigh concerning histologic features when patients are selected to undergo sentinel lymph node biopsy (SLNB), they did not reach consensus for imaging recommendations in the setting of a high-risk prognostic GEP score and low-risk histology and/or negative nodal status.
“The panelists await future, well-designed prospective studies to determine if use of these and newer technologies improves the care of patients with melanoma,” the panelists write.
In the editorial, Mr. Geller and Dr. Weinstock highlighted concerns about the cost and potential access issues associated with these newer technologies, given that the current cost of GEP testing exceeds $7,000.
The editorialists also emphasize that “going forward, the field should be advanced by tackling one of the more pressing, common, potentially morbid, and costly procedures – the prognostic use of sentinel lymph node biopsy.”
Of critical importance is “whether GEP can reduce morbidity and cost by safely reducing the number of SLNBs performed,” Mr. Geller and Dr. Weinstock write.
The funding for the administration and facilitation of the consensus development conference and the development of the manuscript was provided by Dermtech, in an unrestricted award overseen by the Melanoma Research Foundation and managed and executed at UPMC by the principal investigator. Several of the coauthors disclosed relationships with industry. Mr. Geller is a contributor to UptoDate for which he receives royalties. Dr. Weinstock receives consulting fees from AbbVie.
A version of this article first appeared on Medscape.com.
That is why a group of expert panelists evaluated the existing evidence and a range of clinical scenarios to help clarify the optimal strategies for early detection and assessment of cutaneous melanoma.
Overall, the panelists agreed that a risk-stratified approach is likely the most appropriate strategy for melanoma screening and follow-up and supported the use of visual and dermoscopic examination. However, the panelists did not reach consensus on the role for gene expression profile (GEP) testing in clinical decision-making, citing the need for these assays to be validated in large randomized clinical trials.
In an accompanying editorial, two experts highlighted the importance of carefully evaluating the role of diagnostic tests.
“Diagnostic tests such as GEP must face critical scrutiny; if not, there are immediate concerns for patient care, such as the patient being erroneously informed that they do not have cancer or told that they do have cancer when they do not,” write Alan C. Geller, MPH, RN, from the Harvard T.H. Chan School of Public Health, Boston, and Marvin A. Weinstock, MD, PhD, from Brown University, Providence, R.I.
The consensus statement was published online in JAMA Dermatology.
The need for guidance
Although focusing melanoma screening on higher-risk populations may be cost effective, compared with population-based screening, the major guidelines lack consistent guidance to support a risk-stratified approach to skin cancer screening and best practices on diagnosing cutaneous melanoma.
In the prebiopsy setting, the appropriate use of diagnostic tools for evaluating the need for biopsy remain poorly defined, and, in the post-biopsy setting, questions remain concerning the diagnostic accuracy of molecular techniques, diagnostic GEP testing, next-generation sequencing, and immunohistochemical assessment for various markers of melanoma.
To provide consensus recommendations on optimal screening practices, prebiopsy and postbiopsy diagnostics, and prognostic assessment of cutaneous melanoma, a group of 42 panelists voted on hypothetical scenarios via an emailed survey. The panel then came together for a consensus conference, which included 51 experts who discussed their approach to the various clinical case scenarios. Most attendees (45 of the 51) answered a follow-up survey for their final recommendations.
The panelists reached a consensus, with 70% agreement, to support a risk-stratified approach to melanoma screening in clinical settings and public screening events. The experts agreed that higher-risk individuals (those with a relative risk of 5 or greater) could be appropriately screened by a general dermatologist or pigmented lesion evaluation. Higher-risk individuals included those with severe skin damage from the sun, systemic immunosuppression, or a personal history of nonmelanoma or melanoma skin cancer.
Panelists agreed that those at general or lower risk (RR < 2) could be screened by a primary care provider or through regular self- or partner examinations, whereas those at moderate risk could be screened by their primary care clinician or general dermatologist. The experts observed “a shift in acceptance” of primary care physicians screening the general population, and an acknowledgement of the importance of self- and partner examinations as screening adjuncts for all populations.
In the prebiopsy setting, panelists reached consensus that visual and dermoscopic examination was appropriate for evaluating patients with “no new, changing, or unusual skin lesions or with a new lesion that is not visually concerning.”
The panelists also reached consensus that lesions deemed clinically suspicious for cancer or showing features of cancer on reflectance confocal microscopy should be biopsied. Although most respondents (86%) did not currently use epidermal tape stripping routinely, they agreed that, in a hypothetical situation where epidermal tape stripping was used, that lesions positive for PRAME or LINC should be biopsied.
In the postbiopsy setting, views on the use of GEP scores varied. Although panelists agreed that a low-risk prognostic GEP score should not outweigh concerning histologic features when patients are selected to undergo sentinel lymph node biopsy (SLNB), they did not reach consensus for imaging recommendations in the setting of a high-risk prognostic GEP score and low-risk histology and/or negative nodal status.
“The panelists await future, well-designed prospective studies to determine if use of these and newer technologies improves the care of patients with melanoma,” the panelists write.
In the editorial, Mr. Geller and Dr. Weinstock highlighted concerns about the cost and potential access issues associated with these newer technologies, given that the current cost of GEP testing exceeds $7,000.
The editorialists also emphasize that “going forward, the field should be advanced by tackling one of the more pressing, common, potentially morbid, and costly procedures – the prognostic use of sentinel lymph node biopsy.”
Of critical importance is “whether GEP can reduce morbidity and cost by safely reducing the number of SLNBs performed,” Mr. Geller and Dr. Weinstock write.
The funding for the administration and facilitation of the consensus development conference and the development of the manuscript was provided by Dermtech, in an unrestricted award overseen by the Melanoma Research Foundation and managed and executed at UPMC by the principal investigator. Several of the coauthors disclosed relationships with industry. Mr. Geller is a contributor to UptoDate for which he receives royalties. Dr. Weinstock receives consulting fees from AbbVie.
A version of this article first appeared on Medscape.com.
FROM JAMA DERMATOLOGY
Novel single-use patch shows promise for primary axillary hyperhidrosis
NEW ORLEANS – , results from a pivotal randomized trial showed.
“This is a new kind of device that is going to be a nice tool to have for treating patients who have hyperhidrosis of the axilla,” the study’s lead investigator, David M. Pariser, MD, who practices dermatology in Norfolk, Va., said during a late-breaking abstract session at the annual meeting of the American Academy of Dermatology.
In a study known as SAHARA, investigators at 11 sites evaluated the efficacy of the targeted alkali thermolysis (TAT) patch, a single-use disposable device. The patch consists of a thin sodium layer on an adhesive overlay. It’s applied to the dry axilla, and as the patient sweats during treatment, the sweat reacts with the sodium. According to Dr. Pariser, this interaction generates precisely targeted thermal energy that targets sweat glands, leading to a reduction in excessive sweat production for up to three months.
The researchers enrolled 110 individuals with Hyperhidrosis Disease Severity Scale (HDSS) scores of 3 or 4 and randomized them to either an active TAT or a sham patch for up to 3 minutes. Their mean age was about 33 years, and slightly more than half were women. “If significant discomfort or pain was noted, [the patch] treatment was halted; otherwise, it was left on for 3 minutes,” said Dr. Pariser, professor of dermatology at Eastern Virginia Medical School, Norfolk. “The treated area was thoroughly cleaned after treatment, and the TAT patch was deactivated. This process was repeated on the other axilla.”
The HDSS, Gravimetric Sweat Production (GSP), and quality of life assessments for bother and impact were measured through 12 weeks. The quality of life assessments were an exploratory endpoint and scored from 0 to 4, with 4 being extremely bothered or impacted and 0 not being bothered or impacted at all. The primary efficacy endpoint was the proportion of treated patients achieving a 1 or 2 on the HDSS at week 4, compared with sham treatment.
Secondary endpoints included the proportion of patients with an improvement of at least 2 grades from baseline to 4 weeks in HDSS by treatment group; mean improvement in the quality of life scale bother by treatment group; mean improvement in the quality of life scale impact by treatment group; and the proportion of subjects with at least 50% improvement in GSP from baseline to 4 weeks in the active patch group only.
Adverse events (AEs) were divided into 3 categories: AEs at the treatment site (or skin reactions within the treated part of the axilla); procedure-related AEs (those that are the result of treatment, but not in the treated part of the axilla), and non-axillary AEs.
Dr. Pariser reported that at 4 weeks, 63.6% of patients in the active patch group versus 44.2% of those in the sham group improved to an HDSS score of 1 or 2 (P = .0332) and that 43.2% of those in the active patch group versus 16.3% of those in the sham group (P = .0107) achieved a 2-point or greater HDSS improvement. In addition, 9.1% of those in the active patch group achieved a 3-point improvement on the HDSS, compared with none in the sham group. “That’s an amazing improvement; you’re basically going from moderate or severe to none,” he commented.
In other findings, 60.5% of patients in the active patch group showed at least a 50% reduction in GSP, compared with 32.6% of those in the sham group (P = .0102), with mean reductions of 57.3 mg/5min and 18.2 mg/5min, respectively (P = .0036). As for quality-of-life outcome scores, bother associated with hyperhidrosis was reduced by 1.52 points in active versus 0.61 in sham subjects (P = .0005), while impact was reduced by 1.44 in active versus 0.57 in sham subjects (P = .0004).
Adverse events
A total of 13 patients in the active patch group experienced AEs at the treatment site, including six with erythema; four with erosion; two with burning, itching or stinging; and one with underarm odor. “The two procedure-related AEs in the TAT-treated group were compensatory sweating and irritant contact dermatitis due to the adhesive,” said Dr. Pariser said.
Most adverse events resolved in fewer than 2 weeks, and all were mild to moderate. No serious adverse events occurred. Only five adverse events occurred in the sham group.
The TAT patch is currently undergoing review by the Food and Drug Administration, and according to Dr. Pariser, no other body sites have been treated with the device.
Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, who was asked to comment on the study, characterized hyperhidrosis as “an exceedingly common medical condition that is commonly overlooked even though it has a tremendous burden on quality of life. I should know, as both someone who manages a large cohort of these patients but also as someone who suffers from it.”
Treatment options “have historically been limited, many of which are off-label and some which are difficult to access due to cost and/or duration/frequency of treatment,” added Dr. Friedman, who was not involved with the study. “The TAT patch offers a new, targeted, in-office, practical procedure-based approach to treat primary axillary hyperhidrosis. Innovation is certainly welcomed and needed, and I am curious to see how this technology is employed in practice once approved.”
The device is being developed by Candesant Biomedical. Dr. Pariser disclosed that he is a consultant or investigator for Bickel Biotechnology, Biofrontera AG, Bristol Myers Squibb, Celgene Corporation, Novartis Pharmaceuticals, Pfizer, Regeneron, and Sanofi.
Dr. Friedman reported having no relevant disclosures.
NEW ORLEANS – , results from a pivotal randomized trial showed.
“This is a new kind of device that is going to be a nice tool to have for treating patients who have hyperhidrosis of the axilla,” the study’s lead investigator, David M. Pariser, MD, who practices dermatology in Norfolk, Va., said during a late-breaking abstract session at the annual meeting of the American Academy of Dermatology.
In a study known as SAHARA, investigators at 11 sites evaluated the efficacy of the targeted alkali thermolysis (TAT) patch, a single-use disposable device. The patch consists of a thin sodium layer on an adhesive overlay. It’s applied to the dry axilla, and as the patient sweats during treatment, the sweat reacts with the sodium. According to Dr. Pariser, this interaction generates precisely targeted thermal energy that targets sweat glands, leading to a reduction in excessive sweat production for up to three months.
The researchers enrolled 110 individuals with Hyperhidrosis Disease Severity Scale (HDSS) scores of 3 or 4 and randomized them to either an active TAT or a sham patch for up to 3 minutes. Their mean age was about 33 years, and slightly more than half were women. “If significant discomfort or pain was noted, [the patch] treatment was halted; otherwise, it was left on for 3 minutes,” said Dr. Pariser, professor of dermatology at Eastern Virginia Medical School, Norfolk. “The treated area was thoroughly cleaned after treatment, and the TAT patch was deactivated. This process was repeated on the other axilla.”
The HDSS, Gravimetric Sweat Production (GSP), and quality of life assessments for bother and impact were measured through 12 weeks. The quality of life assessments were an exploratory endpoint and scored from 0 to 4, with 4 being extremely bothered or impacted and 0 not being bothered or impacted at all. The primary efficacy endpoint was the proportion of treated patients achieving a 1 or 2 on the HDSS at week 4, compared with sham treatment.
Secondary endpoints included the proportion of patients with an improvement of at least 2 grades from baseline to 4 weeks in HDSS by treatment group; mean improvement in the quality of life scale bother by treatment group; mean improvement in the quality of life scale impact by treatment group; and the proportion of subjects with at least 50% improvement in GSP from baseline to 4 weeks in the active patch group only.
Adverse events (AEs) were divided into 3 categories: AEs at the treatment site (or skin reactions within the treated part of the axilla); procedure-related AEs (those that are the result of treatment, but not in the treated part of the axilla), and non-axillary AEs.
Dr. Pariser reported that at 4 weeks, 63.6% of patients in the active patch group versus 44.2% of those in the sham group improved to an HDSS score of 1 or 2 (P = .0332) and that 43.2% of those in the active patch group versus 16.3% of those in the sham group (P = .0107) achieved a 2-point or greater HDSS improvement. In addition, 9.1% of those in the active patch group achieved a 3-point improvement on the HDSS, compared with none in the sham group. “That’s an amazing improvement; you’re basically going from moderate or severe to none,” he commented.
In other findings, 60.5% of patients in the active patch group showed at least a 50% reduction in GSP, compared with 32.6% of those in the sham group (P = .0102), with mean reductions of 57.3 mg/5min and 18.2 mg/5min, respectively (P = .0036). As for quality-of-life outcome scores, bother associated with hyperhidrosis was reduced by 1.52 points in active versus 0.61 in sham subjects (P = .0005), while impact was reduced by 1.44 in active versus 0.57 in sham subjects (P = .0004).
Adverse events
A total of 13 patients in the active patch group experienced AEs at the treatment site, including six with erythema; four with erosion; two with burning, itching or stinging; and one with underarm odor. “The two procedure-related AEs in the TAT-treated group were compensatory sweating and irritant contact dermatitis due to the adhesive,” said Dr. Pariser said.
Most adverse events resolved in fewer than 2 weeks, and all were mild to moderate. No serious adverse events occurred. Only five adverse events occurred in the sham group.
The TAT patch is currently undergoing review by the Food and Drug Administration, and according to Dr. Pariser, no other body sites have been treated with the device.
Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, who was asked to comment on the study, characterized hyperhidrosis as “an exceedingly common medical condition that is commonly overlooked even though it has a tremendous burden on quality of life. I should know, as both someone who manages a large cohort of these patients but also as someone who suffers from it.”
Treatment options “have historically been limited, many of which are off-label and some which are difficult to access due to cost and/or duration/frequency of treatment,” added Dr. Friedman, who was not involved with the study. “The TAT patch offers a new, targeted, in-office, practical procedure-based approach to treat primary axillary hyperhidrosis. Innovation is certainly welcomed and needed, and I am curious to see how this technology is employed in practice once approved.”
The device is being developed by Candesant Biomedical. Dr. Pariser disclosed that he is a consultant or investigator for Bickel Biotechnology, Biofrontera AG, Bristol Myers Squibb, Celgene Corporation, Novartis Pharmaceuticals, Pfizer, Regeneron, and Sanofi.
Dr. Friedman reported having no relevant disclosures.
NEW ORLEANS – , results from a pivotal randomized trial showed.
“This is a new kind of device that is going to be a nice tool to have for treating patients who have hyperhidrosis of the axilla,” the study’s lead investigator, David M. Pariser, MD, who practices dermatology in Norfolk, Va., said during a late-breaking abstract session at the annual meeting of the American Academy of Dermatology.
In a study known as SAHARA, investigators at 11 sites evaluated the efficacy of the targeted alkali thermolysis (TAT) patch, a single-use disposable device. The patch consists of a thin sodium layer on an adhesive overlay. It’s applied to the dry axilla, and as the patient sweats during treatment, the sweat reacts with the sodium. According to Dr. Pariser, this interaction generates precisely targeted thermal energy that targets sweat glands, leading to a reduction in excessive sweat production for up to three months.
The researchers enrolled 110 individuals with Hyperhidrosis Disease Severity Scale (HDSS) scores of 3 or 4 and randomized them to either an active TAT or a sham patch for up to 3 minutes. Their mean age was about 33 years, and slightly more than half were women. “If significant discomfort or pain was noted, [the patch] treatment was halted; otherwise, it was left on for 3 minutes,” said Dr. Pariser, professor of dermatology at Eastern Virginia Medical School, Norfolk. “The treated area was thoroughly cleaned after treatment, and the TAT patch was deactivated. This process was repeated on the other axilla.”
The HDSS, Gravimetric Sweat Production (GSP), and quality of life assessments for bother and impact were measured through 12 weeks. The quality of life assessments were an exploratory endpoint and scored from 0 to 4, with 4 being extremely bothered or impacted and 0 not being bothered or impacted at all. The primary efficacy endpoint was the proportion of treated patients achieving a 1 or 2 on the HDSS at week 4, compared with sham treatment.
Secondary endpoints included the proportion of patients with an improvement of at least 2 grades from baseline to 4 weeks in HDSS by treatment group; mean improvement in the quality of life scale bother by treatment group; mean improvement in the quality of life scale impact by treatment group; and the proportion of subjects with at least 50% improvement in GSP from baseline to 4 weeks in the active patch group only.
Adverse events (AEs) were divided into 3 categories: AEs at the treatment site (or skin reactions within the treated part of the axilla); procedure-related AEs (those that are the result of treatment, but not in the treated part of the axilla), and non-axillary AEs.
Dr. Pariser reported that at 4 weeks, 63.6% of patients in the active patch group versus 44.2% of those in the sham group improved to an HDSS score of 1 or 2 (P = .0332) and that 43.2% of those in the active patch group versus 16.3% of those in the sham group (P = .0107) achieved a 2-point or greater HDSS improvement. In addition, 9.1% of those in the active patch group achieved a 3-point improvement on the HDSS, compared with none in the sham group. “That’s an amazing improvement; you’re basically going from moderate or severe to none,” he commented.
In other findings, 60.5% of patients in the active patch group showed at least a 50% reduction in GSP, compared with 32.6% of those in the sham group (P = .0102), with mean reductions of 57.3 mg/5min and 18.2 mg/5min, respectively (P = .0036). As for quality-of-life outcome scores, bother associated with hyperhidrosis was reduced by 1.52 points in active versus 0.61 in sham subjects (P = .0005), while impact was reduced by 1.44 in active versus 0.57 in sham subjects (P = .0004).
Adverse events
A total of 13 patients in the active patch group experienced AEs at the treatment site, including six with erythema; four with erosion; two with burning, itching or stinging; and one with underarm odor. “The two procedure-related AEs in the TAT-treated group were compensatory sweating and irritant contact dermatitis due to the adhesive,” said Dr. Pariser said.
Most adverse events resolved in fewer than 2 weeks, and all were mild to moderate. No serious adverse events occurred. Only five adverse events occurred in the sham group.
The TAT patch is currently undergoing review by the Food and Drug Administration, and according to Dr. Pariser, no other body sites have been treated with the device.
Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, who was asked to comment on the study, characterized hyperhidrosis as “an exceedingly common medical condition that is commonly overlooked even though it has a tremendous burden on quality of life. I should know, as both someone who manages a large cohort of these patients but also as someone who suffers from it.”
Treatment options “have historically been limited, many of which are off-label and some which are difficult to access due to cost and/or duration/frequency of treatment,” added Dr. Friedman, who was not involved with the study. “The TAT patch offers a new, targeted, in-office, practical procedure-based approach to treat primary axillary hyperhidrosis. Innovation is certainly welcomed and needed, and I am curious to see how this technology is employed in practice once approved.”
The device is being developed by Candesant Biomedical. Dr. Pariser disclosed that he is a consultant or investigator for Bickel Biotechnology, Biofrontera AG, Bristol Myers Squibb, Celgene Corporation, Novartis Pharmaceuticals, Pfizer, Regeneron, and Sanofi.
Dr. Friedman reported having no relevant disclosures.
AT AAD 2023
CSU in children: Study identifies biomarkers associated with responses to different treatments
NEW ORLEANS – , results from a single-center prospective study showed.
“Given that the majority of CSU cases in adults are due to autoimmunity and there being very [few] studies on biomarkers for CSU in children, our study furthers our current understanding of the role of different biomarkers in treatment response,” lead study author Alex Nguyen, MsC, said in an interview at the annual meeting of the American Academy of Dermatology, where the study was presented during a poster session.
To identify biomarkers with treatment and disease resolution in children with CSU, Mr. Nguyen, a 4-year medical student at McGill University, Montreal, and colleagues prospectively recruited 109 children from the Montreal Children’s Hospital Allergy and Immunology Clinic who reported hives for at least 6 weeks from 2013 to 2022. They obtained levels of thyroid stimulating hormone (TSH), anti-thyroxine peroxidase (anti-TPO), total immunoglobulin E (IgE), CD63, tryptase, eosinophils, MPV, and platelets; the weekly urticaria activity score (UAS7) was recorded at study entry.
Levels of treatment included antihistamines at standard dose, four times the standard dose, omalizumab, and resolution of treatment. The researchers used univariate and multivariate logistic regressions to determine factors associated with different treatment levels and resolution.
Slightly more than half of the study participants (55%) were female, and their mean age was 9 years. Mr. Nguyen and colleagues observed that elevated MPV was associated with the four times increased dose of antihistamines treatment level (odds ratio = 1.052, 95% confidence interval = 1.004-1.103). Lower age was associated with disease resolution (OR = 0.982, 95% CI = 0.965-0.999).
After adjustment for age, sex, TSH, anti-TPO, total IgE, CD63, eosinophils, MPV, and platelets, elevated tryptase was associated with the antihistamine use at standard dose level (OR = 1.152, 95% CI = 1.019-1.302) and lower tryptase levels with disease resolution (OR = .861, 95% CI = 0.777-0.955).
“We were fascinated when we found that tryptase levels in patients with chronic spontaneous urticaria were associated with standard dose of antihistamines and even disease resolution,” Mr. Nguyen said. “Higher tryptase levels were associated with standard dose antihistamines, which potentially could imply an increase in mast cell activation. Furthermore, we saw that lower tryptase levels were associated with disease resolution likely given if the disease may not have been as severe.”
He acknowledged certain limitations of the study, including a limited sample size and an unbalanced sample size among treatment groups. In the future, he and his colleagues plan to increase the sample size and to include other biomarkers such as interleukin (IL)-6, D-dimer, vitamin D, and matrix mettaloproteinase-9.
“Much as the name suggests, CSU often arises without a clear trigger,” said Raj Chovatiya, MD, PhD, assistant professor in the department of dermatology at Northwestern University, Chicago, who was asked to comment on the study. “Particularly in children, little is known about potential biomarkers that may guide treatment or disease resolution. While a larger, prospective analysis would better characterize temporal trends in serum biomarkers in relation to disease activity, these data suggest that underlying mechanisms of tryptase may be worth an in-depth look in children with CSU.”
The study was recognized as the second-best poster at the meeting. The researchers reported having no financial disclosures. The other study coauthors were Michelle Le MD, Sofianne Gabrielli MSc, Elena Netchiporouk, MD, MSc, and Moshe Ben-Shoshan, MD, MSc. Dr. Chovatiya disclosed that he is a consultant to, a speaker for, and/or a member of the advisory board for several pharmaceutical companies.
NEW ORLEANS – , results from a single-center prospective study showed.
“Given that the majority of CSU cases in adults are due to autoimmunity and there being very [few] studies on biomarkers for CSU in children, our study furthers our current understanding of the role of different biomarkers in treatment response,” lead study author Alex Nguyen, MsC, said in an interview at the annual meeting of the American Academy of Dermatology, where the study was presented during a poster session.
To identify biomarkers with treatment and disease resolution in children with CSU, Mr. Nguyen, a 4-year medical student at McGill University, Montreal, and colleagues prospectively recruited 109 children from the Montreal Children’s Hospital Allergy and Immunology Clinic who reported hives for at least 6 weeks from 2013 to 2022. They obtained levels of thyroid stimulating hormone (TSH), anti-thyroxine peroxidase (anti-TPO), total immunoglobulin E (IgE), CD63, tryptase, eosinophils, MPV, and platelets; the weekly urticaria activity score (UAS7) was recorded at study entry.
Levels of treatment included antihistamines at standard dose, four times the standard dose, omalizumab, and resolution of treatment. The researchers used univariate and multivariate logistic regressions to determine factors associated with different treatment levels and resolution.
Slightly more than half of the study participants (55%) were female, and their mean age was 9 years. Mr. Nguyen and colleagues observed that elevated MPV was associated with the four times increased dose of antihistamines treatment level (odds ratio = 1.052, 95% confidence interval = 1.004-1.103). Lower age was associated with disease resolution (OR = 0.982, 95% CI = 0.965-0.999).
After adjustment for age, sex, TSH, anti-TPO, total IgE, CD63, eosinophils, MPV, and platelets, elevated tryptase was associated with the antihistamine use at standard dose level (OR = 1.152, 95% CI = 1.019-1.302) and lower tryptase levels with disease resolution (OR = .861, 95% CI = 0.777-0.955).
“We were fascinated when we found that tryptase levels in patients with chronic spontaneous urticaria were associated with standard dose of antihistamines and even disease resolution,” Mr. Nguyen said. “Higher tryptase levels were associated with standard dose antihistamines, which potentially could imply an increase in mast cell activation. Furthermore, we saw that lower tryptase levels were associated with disease resolution likely given if the disease may not have been as severe.”
He acknowledged certain limitations of the study, including a limited sample size and an unbalanced sample size among treatment groups. In the future, he and his colleagues plan to increase the sample size and to include other biomarkers such as interleukin (IL)-6, D-dimer, vitamin D, and matrix mettaloproteinase-9.
“Much as the name suggests, CSU often arises without a clear trigger,” said Raj Chovatiya, MD, PhD, assistant professor in the department of dermatology at Northwestern University, Chicago, who was asked to comment on the study. “Particularly in children, little is known about potential biomarkers that may guide treatment or disease resolution. While a larger, prospective analysis would better characterize temporal trends in serum biomarkers in relation to disease activity, these data suggest that underlying mechanisms of tryptase may be worth an in-depth look in children with CSU.”
The study was recognized as the second-best poster at the meeting. The researchers reported having no financial disclosures. The other study coauthors were Michelle Le MD, Sofianne Gabrielli MSc, Elena Netchiporouk, MD, MSc, and Moshe Ben-Shoshan, MD, MSc. Dr. Chovatiya disclosed that he is a consultant to, a speaker for, and/or a member of the advisory board for several pharmaceutical companies.
NEW ORLEANS – , results from a single-center prospective study showed.
“Given that the majority of CSU cases in adults are due to autoimmunity and there being very [few] studies on biomarkers for CSU in children, our study furthers our current understanding of the role of different biomarkers in treatment response,” lead study author Alex Nguyen, MsC, said in an interview at the annual meeting of the American Academy of Dermatology, where the study was presented during a poster session.
To identify biomarkers with treatment and disease resolution in children with CSU, Mr. Nguyen, a 4-year medical student at McGill University, Montreal, and colleagues prospectively recruited 109 children from the Montreal Children’s Hospital Allergy and Immunology Clinic who reported hives for at least 6 weeks from 2013 to 2022. They obtained levels of thyroid stimulating hormone (TSH), anti-thyroxine peroxidase (anti-TPO), total immunoglobulin E (IgE), CD63, tryptase, eosinophils, MPV, and platelets; the weekly urticaria activity score (UAS7) was recorded at study entry.
Levels of treatment included antihistamines at standard dose, four times the standard dose, omalizumab, and resolution of treatment. The researchers used univariate and multivariate logistic regressions to determine factors associated with different treatment levels and resolution.
Slightly more than half of the study participants (55%) were female, and their mean age was 9 years. Mr. Nguyen and colleagues observed that elevated MPV was associated with the four times increased dose of antihistamines treatment level (odds ratio = 1.052, 95% confidence interval = 1.004-1.103). Lower age was associated with disease resolution (OR = 0.982, 95% CI = 0.965-0.999).
After adjustment for age, sex, TSH, anti-TPO, total IgE, CD63, eosinophils, MPV, and platelets, elevated tryptase was associated with the antihistamine use at standard dose level (OR = 1.152, 95% CI = 1.019-1.302) and lower tryptase levels with disease resolution (OR = .861, 95% CI = 0.777-0.955).
“We were fascinated when we found that tryptase levels in patients with chronic spontaneous urticaria were associated with standard dose of antihistamines and even disease resolution,” Mr. Nguyen said. “Higher tryptase levels were associated with standard dose antihistamines, which potentially could imply an increase in mast cell activation. Furthermore, we saw that lower tryptase levels were associated with disease resolution likely given if the disease may not have been as severe.”
He acknowledged certain limitations of the study, including a limited sample size and an unbalanced sample size among treatment groups. In the future, he and his colleagues plan to increase the sample size and to include other biomarkers such as interleukin (IL)-6, D-dimer, vitamin D, and matrix mettaloproteinase-9.
“Much as the name suggests, CSU often arises without a clear trigger,” said Raj Chovatiya, MD, PhD, assistant professor in the department of dermatology at Northwestern University, Chicago, who was asked to comment on the study. “Particularly in children, little is known about potential biomarkers that may guide treatment or disease resolution. While a larger, prospective analysis would better characterize temporal trends in serum biomarkers in relation to disease activity, these data suggest that underlying mechanisms of tryptase may be worth an in-depth look in children with CSU.”
The study was recognized as the second-best poster at the meeting. The researchers reported having no financial disclosures. The other study coauthors were Michelle Le MD, Sofianne Gabrielli MSc, Elena Netchiporouk, MD, MSc, and Moshe Ben-Shoshan, MD, MSc. Dr. Chovatiya disclosed that he is a consultant to, a speaker for, and/or a member of the advisory board for several pharmaceutical companies.
AT AAD 2023
JAK inhibitor safety warnings drawn from rheumatologic data may be misleading in dermatology
NEW ORLEANS – , even though the basis for all the risks is a rheumatoid arthritis study, according to a critical review at the annual meeting of the American Academy of Dermatology.
Given the fact that the postmarketing RA study was specifically enriched with high-risk patients by requiring an age at enrollment of at least 50 years and the presence of at least one cardiovascular risk factor, the extrapolation of these risks to dermatologic indications is “not necessarily data-driven,” said Brett A. King, MD, PhD, associate professor of dermatology, Yale University, New Haven, Conn.
The recently approved deucravacitinib is the only JAK inhibitor that has so far been exempt from these warnings. Instead, based on the ORAL Surveillance study, published in the New England Journal of Medicine, the Food and Drug Administration requires a boxed warning in nearly identical language for all the other JAK inhibitors. Relative to tofacitinib, the JAK inhibitor tested in ORAL Surveillance, many of these drugs differ by JAK selectivity and other characteristics that are likely relevant to risk of adverse events, Dr. King said. The same language has even been applied to topical ruxolitinib cream.
Basis of boxed warnings
In ORAL Surveillance, about 4,300 high-risk patients with RA were randomized to one of two doses of tofacitinib (5 mg or 10 mg) twice daily or a tumor necrosis factor (TNF) inhibitor. All patients in the trial were taking methotrexate, and almost 60% were taking concomitant corticosteroids. The average body mass index of the study population was about 30 kg/m2.
After a median 4 years of follow-up (about 5,000 patient-years), the incidence of many of the adverse events tracked in the study were higher in the tofacitinib groups, including serious infections, MACE, thromboembolic events, and cancer. Dr. King did not challenge the importance of these data, but he questioned whether they are reasonably extrapolated to dermatologic indications, particularly as many of those treated are younger than those common to an RA population.
In fact, despite a study enriched for a higher risk of many events tracked, most adverse events were only slightly elevated, Dr. King pointed out. For example, the incidence of MACE over the 4 years of follow-up was 3.4% among those taking any dose of tofacitinib versus 2.5% of those randomized to TNF inhibitor. Rates of cancer were 4.2% versus 2.9%, respectively. There were also absolute increases in the number of serious infections and thromboembolic events for tofacitinib relative to TNF inhibitor.
Dr. King acknowledged that the numbers in ORAL Surveillance associated tofacitinib with a higher risk of serious events than TNF inhibitor in patients with RA, but he believes that “JAK inhibitor safety is almost certainly not the same in dermatology as it is in rheumatology patients.”
Evidence of difference in dermatology
There is some evidence to back this up. Dr. King cited a recently published study in RMD Open that evaluated the safety profile of the JAK inhibitor upadacitinib in nearly 7,000 patients over 15,000 patient-years of follow-up. Drug safety data were evaluated with up to 5.5 years of follow-up from 12 clinical trials of the four diseases for which upadacitinib is now indicated. Three were rheumatologic (RA, psoriatic arthritis, and ankylosing spondylitis), and the fourth was atopic dermatitis (AD). Fourteen outcomes, including numerous types of infection, MACE, hepatic complications, and malignancy, were compared with methotrexate and the TNF inhibitor adalimumab.
For the RA diseases, upadacitinib was associated with a greater risk than comparators for several outcomes, including serious infections. But in AD, there was a smaller increased risk of adverse outcomes for the JAK inhibitor relative to comparators.
When evaluated by risk of adverse events across indications, for MACE, the exposure-adjusted event rates for upadacitinib were less than 0.1 in patients treated for AD over the observation period versus 0.3 and 0.4 for RA and psoriatic arthritis, respectively. Similarly, for venous thromboembolism, the rates for upadacitinib were again less than 0.1 in patients with AD versus 0.4 and 0.2 in RA and psoriatic arthritis, respectively.
Referring back to the postmarketing study, Dr. King emphasized that it is essential to consider how the boxed warning for JAK inhibitors was generated before applying them to dermatologic indications.
“Is a 30-year-old patient with a dermatologic disorder possibly at the same risk as the patients in the study from which we got the boxed warning? The answer is simply no,” he said.
Like the tofacitinib data in the ORAL Surveillance study, the upadacitinib clinical trial data are not necessarily relevant to other JAK inhibitors. In fact, Dr. King pointed out that the safety profiles of the available JAK inhibitors are not identical, an observation that is consistent with differences in JAK inhibitor selectivity that has implications for off-target events.
Dr. King does not dismiss the potential risks outlined in the current regulatory cautions about the use of JAK inhibitors, but he believes that dermatologists should be cognizant of “where the black box warning comes from.”
“We need to think carefully about the risk-to-benefit ratio in older patients or patients with risk factors, such as obesity and diabetes,” he said. But the safety profile of JAK inhibitors “is almost certainly better” than the profile suggested in boxed warnings applied to JAK inhibitors for dermatologic indications, he advised.
Risk-benefit considerations in dermatology
This position was supported by numerous other experts when asked for their perspectives. “I fully agree,” said Emma Guttman-Yassky, MD, PhD, system chair of dermatology and immunology, Icahn School of Medicine, Mount Sinai, New York.
Like Dr. King, Dr. Guttman-Yassky did not dismiss the potential risks of JAK inhibitors when treating dermatologic diseases.
“While JAK inhibitors need monitoring as advised, adopting a boxed warning from an RA study for patients who are older [is problematic],” she commented. A study with the nonselective tofacitinib in this population “cannot be compared to more selective inhibitors in a much younger population, such as those treated [for] alopecia areata or atopic dermatitis.”
George Z. Han, MD, PhD, an associate professor of dermatology, Zucker School of Medicine, Hofstra, Northwell Medical Center, New Hyde Park, New York, also agreed but added some caveats.
“The comments about the ORAL Surveillance study are salient,” he said in an interview. “This kind of data should not directly be extrapolated to other patient types or to other medications.” However, one of Dr. Han’s most important caveats involves long-term use.
“JAK inhibitors are still relatively narrow-therapeutic-window drugs that in a dose-dependent fashion could lead to negative effects, including thromboembolic events, abnormalities in red blood cells, white blood cells, platelets, and lipids,” he said. While doses used in dermatology “are generally below the level of any major concern,” Dr. Han cautioned that “we lack definitive data” on long-term use, and this is important for understanding “any potential small risk of rare events, such as malignancy or thromboembolism.”
Saakshi Khattri, MD, a colleague of Dr. Guttman-Yassky at Mount Sinai, said the risks of JAK inhibitors should not be underestimated, but she also agreed that risk “needs to be delivered in the right context.” Dr. Khattri, who is board certified in both dermatology and rheumatology, noted the safety profiles of available JAK inhibitors differ and that extrapolating safety from an RA study to dermatologic indications does not make sense. “Different diseases, different age groups,” she said.
Dr. King has reported financial relationships with more than 15 pharmaceutical companies, including companies that make JAK inhibitors. Dr. Guttman-Yassky has reported financial relationships with more than 20 pharmaceutical companies, including companies that make JAK inhibitors. Dr. Han reports financial relationships with Amgen, Athenex, Boehringer Ingelheim, Bond Avillion, Bristol-Myers Squibb, Celgene, Janssen, Lilly, Novartis, PellePharm, Pfizer, and UCB. Dr. Khattri has reported financial relationships with AbbVie, Arcutis, Bristol-Myers Squibb, Janssen, Leo, Lilly, Novartis, Pfizer, and UCB.
A version of this article originally appeared on Medscape.com.
NEW ORLEANS – , even though the basis for all the risks is a rheumatoid arthritis study, according to a critical review at the annual meeting of the American Academy of Dermatology.
Given the fact that the postmarketing RA study was specifically enriched with high-risk patients by requiring an age at enrollment of at least 50 years and the presence of at least one cardiovascular risk factor, the extrapolation of these risks to dermatologic indications is “not necessarily data-driven,” said Brett A. King, MD, PhD, associate professor of dermatology, Yale University, New Haven, Conn.
The recently approved deucravacitinib is the only JAK inhibitor that has so far been exempt from these warnings. Instead, based on the ORAL Surveillance study, published in the New England Journal of Medicine, the Food and Drug Administration requires a boxed warning in nearly identical language for all the other JAK inhibitors. Relative to tofacitinib, the JAK inhibitor tested in ORAL Surveillance, many of these drugs differ by JAK selectivity and other characteristics that are likely relevant to risk of adverse events, Dr. King said. The same language has even been applied to topical ruxolitinib cream.
Basis of boxed warnings
In ORAL Surveillance, about 4,300 high-risk patients with RA were randomized to one of two doses of tofacitinib (5 mg or 10 mg) twice daily or a tumor necrosis factor (TNF) inhibitor. All patients in the trial were taking methotrexate, and almost 60% were taking concomitant corticosteroids. The average body mass index of the study population was about 30 kg/m2.
After a median 4 years of follow-up (about 5,000 patient-years), the incidence of many of the adverse events tracked in the study were higher in the tofacitinib groups, including serious infections, MACE, thromboembolic events, and cancer. Dr. King did not challenge the importance of these data, but he questioned whether they are reasonably extrapolated to dermatologic indications, particularly as many of those treated are younger than those common to an RA population.
In fact, despite a study enriched for a higher risk of many events tracked, most adverse events were only slightly elevated, Dr. King pointed out. For example, the incidence of MACE over the 4 years of follow-up was 3.4% among those taking any dose of tofacitinib versus 2.5% of those randomized to TNF inhibitor. Rates of cancer were 4.2% versus 2.9%, respectively. There were also absolute increases in the number of serious infections and thromboembolic events for tofacitinib relative to TNF inhibitor.
Dr. King acknowledged that the numbers in ORAL Surveillance associated tofacitinib with a higher risk of serious events than TNF inhibitor in patients with RA, but he believes that “JAK inhibitor safety is almost certainly not the same in dermatology as it is in rheumatology patients.”
Evidence of difference in dermatology
There is some evidence to back this up. Dr. King cited a recently published study in RMD Open that evaluated the safety profile of the JAK inhibitor upadacitinib in nearly 7,000 patients over 15,000 patient-years of follow-up. Drug safety data were evaluated with up to 5.5 years of follow-up from 12 clinical trials of the four diseases for which upadacitinib is now indicated. Three were rheumatologic (RA, psoriatic arthritis, and ankylosing spondylitis), and the fourth was atopic dermatitis (AD). Fourteen outcomes, including numerous types of infection, MACE, hepatic complications, and malignancy, were compared with methotrexate and the TNF inhibitor adalimumab.
For the RA diseases, upadacitinib was associated with a greater risk than comparators for several outcomes, including serious infections. But in AD, there was a smaller increased risk of adverse outcomes for the JAK inhibitor relative to comparators.
When evaluated by risk of adverse events across indications, for MACE, the exposure-adjusted event rates for upadacitinib were less than 0.1 in patients treated for AD over the observation period versus 0.3 and 0.4 for RA and psoriatic arthritis, respectively. Similarly, for venous thromboembolism, the rates for upadacitinib were again less than 0.1 in patients with AD versus 0.4 and 0.2 in RA and psoriatic arthritis, respectively.
Referring back to the postmarketing study, Dr. King emphasized that it is essential to consider how the boxed warning for JAK inhibitors was generated before applying them to dermatologic indications.
“Is a 30-year-old patient with a dermatologic disorder possibly at the same risk as the patients in the study from which we got the boxed warning? The answer is simply no,” he said.
Like the tofacitinib data in the ORAL Surveillance study, the upadacitinib clinical trial data are not necessarily relevant to other JAK inhibitors. In fact, Dr. King pointed out that the safety profiles of the available JAK inhibitors are not identical, an observation that is consistent with differences in JAK inhibitor selectivity that has implications for off-target events.
Dr. King does not dismiss the potential risks outlined in the current regulatory cautions about the use of JAK inhibitors, but he believes that dermatologists should be cognizant of “where the black box warning comes from.”
“We need to think carefully about the risk-to-benefit ratio in older patients or patients with risk factors, such as obesity and diabetes,” he said. But the safety profile of JAK inhibitors “is almost certainly better” than the profile suggested in boxed warnings applied to JAK inhibitors for dermatologic indications, he advised.
Risk-benefit considerations in dermatology
This position was supported by numerous other experts when asked for their perspectives. “I fully agree,” said Emma Guttman-Yassky, MD, PhD, system chair of dermatology and immunology, Icahn School of Medicine, Mount Sinai, New York.
Like Dr. King, Dr. Guttman-Yassky did not dismiss the potential risks of JAK inhibitors when treating dermatologic diseases.
“While JAK inhibitors need monitoring as advised, adopting a boxed warning from an RA study for patients who are older [is problematic],” she commented. A study with the nonselective tofacitinib in this population “cannot be compared to more selective inhibitors in a much younger population, such as those treated [for] alopecia areata or atopic dermatitis.”
George Z. Han, MD, PhD, an associate professor of dermatology, Zucker School of Medicine, Hofstra, Northwell Medical Center, New Hyde Park, New York, also agreed but added some caveats.
“The comments about the ORAL Surveillance study are salient,” he said in an interview. “This kind of data should not directly be extrapolated to other patient types or to other medications.” However, one of Dr. Han’s most important caveats involves long-term use.
“JAK inhibitors are still relatively narrow-therapeutic-window drugs that in a dose-dependent fashion could lead to negative effects, including thromboembolic events, abnormalities in red blood cells, white blood cells, platelets, and lipids,” he said. While doses used in dermatology “are generally below the level of any major concern,” Dr. Han cautioned that “we lack definitive data” on long-term use, and this is important for understanding “any potential small risk of rare events, such as malignancy or thromboembolism.”
Saakshi Khattri, MD, a colleague of Dr. Guttman-Yassky at Mount Sinai, said the risks of JAK inhibitors should not be underestimated, but she also agreed that risk “needs to be delivered in the right context.” Dr. Khattri, who is board certified in both dermatology and rheumatology, noted the safety profiles of available JAK inhibitors differ and that extrapolating safety from an RA study to dermatologic indications does not make sense. “Different diseases, different age groups,” she said.
Dr. King has reported financial relationships with more than 15 pharmaceutical companies, including companies that make JAK inhibitors. Dr. Guttman-Yassky has reported financial relationships with more than 20 pharmaceutical companies, including companies that make JAK inhibitors. Dr. Han reports financial relationships with Amgen, Athenex, Boehringer Ingelheim, Bond Avillion, Bristol-Myers Squibb, Celgene, Janssen, Lilly, Novartis, PellePharm, Pfizer, and UCB. Dr. Khattri has reported financial relationships with AbbVie, Arcutis, Bristol-Myers Squibb, Janssen, Leo, Lilly, Novartis, Pfizer, and UCB.
A version of this article originally appeared on Medscape.com.
NEW ORLEANS – , even though the basis for all the risks is a rheumatoid arthritis study, according to a critical review at the annual meeting of the American Academy of Dermatology.
Given the fact that the postmarketing RA study was specifically enriched with high-risk patients by requiring an age at enrollment of at least 50 years and the presence of at least one cardiovascular risk factor, the extrapolation of these risks to dermatologic indications is “not necessarily data-driven,” said Brett A. King, MD, PhD, associate professor of dermatology, Yale University, New Haven, Conn.
The recently approved deucravacitinib is the only JAK inhibitor that has so far been exempt from these warnings. Instead, based on the ORAL Surveillance study, published in the New England Journal of Medicine, the Food and Drug Administration requires a boxed warning in nearly identical language for all the other JAK inhibitors. Relative to tofacitinib, the JAK inhibitor tested in ORAL Surveillance, many of these drugs differ by JAK selectivity and other characteristics that are likely relevant to risk of adverse events, Dr. King said. The same language has even been applied to topical ruxolitinib cream.
Basis of boxed warnings
In ORAL Surveillance, about 4,300 high-risk patients with RA were randomized to one of two doses of tofacitinib (5 mg or 10 mg) twice daily or a tumor necrosis factor (TNF) inhibitor. All patients in the trial were taking methotrexate, and almost 60% were taking concomitant corticosteroids. The average body mass index of the study population was about 30 kg/m2.
After a median 4 years of follow-up (about 5,000 patient-years), the incidence of many of the adverse events tracked in the study were higher in the tofacitinib groups, including serious infections, MACE, thromboembolic events, and cancer. Dr. King did not challenge the importance of these data, but he questioned whether they are reasonably extrapolated to dermatologic indications, particularly as many of those treated are younger than those common to an RA population.
In fact, despite a study enriched for a higher risk of many events tracked, most adverse events were only slightly elevated, Dr. King pointed out. For example, the incidence of MACE over the 4 years of follow-up was 3.4% among those taking any dose of tofacitinib versus 2.5% of those randomized to TNF inhibitor. Rates of cancer were 4.2% versus 2.9%, respectively. There were also absolute increases in the number of serious infections and thromboembolic events for tofacitinib relative to TNF inhibitor.
Dr. King acknowledged that the numbers in ORAL Surveillance associated tofacitinib with a higher risk of serious events than TNF inhibitor in patients with RA, but he believes that “JAK inhibitor safety is almost certainly not the same in dermatology as it is in rheumatology patients.”
Evidence of difference in dermatology
There is some evidence to back this up. Dr. King cited a recently published study in RMD Open that evaluated the safety profile of the JAK inhibitor upadacitinib in nearly 7,000 patients over 15,000 patient-years of follow-up. Drug safety data were evaluated with up to 5.5 years of follow-up from 12 clinical trials of the four diseases for which upadacitinib is now indicated. Three were rheumatologic (RA, psoriatic arthritis, and ankylosing spondylitis), and the fourth was atopic dermatitis (AD). Fourteen outcomes, including numerous types of infection, MACE, hepatic complications, and malignancy, were compared with methotrexate and the TNF inhibitor adalimumab.
For the RA diseases, upadacitinib was associated with a greater risk than comparators for several outcomes, including serious infections. But in AD, there was a smaller increased risk of adverse outcomes for the JAK inhibitor relative to comparators.
When evaluated by risk of adverse events across indications, for MACE, the exposure-adjusted event rates for upadacitinib were less than 0.1 in patients treated for AD over the observation period versus 0.3 and 0.4 for RA and psoriatic arthritis, respectively. Similarly, for venous thromboembolism, the rates for upadacitinib were again less than 0.1 in patients with AD versus 0.4 and 0.2 in RA and psoriatic arthritis, respectively.
Referring back to the postmarketing study, Dr. King emphasized that it is essential to consider how the boxed warning for JAK inhibitors was generated before applying them to dermatologic indications.
“Is a 30-year-old patient with a dermatologic disorder possibly at the same risk as the patients in the study from which we got the boxed warning? The answer is simply no,” he said.
Like the tofacitinib data in the ORAL Surveillance study, the upadacitinib clinical trial data are not necessarily relevant to other JAK inhibitors. In fact, Dr. King pointed out that the safety profiles of the available JAK inhibitors are not identical, an observation that is consistent with differences in JAK inhibitor selectivity that has implications for off-target events.
Dr. King does not dismiss the potential risks outlined in the current regulatory cautions about the use of JAK inhibitors, but he believes that dermatologists should be cognizant of “where the black box warning comes from.”
“We need to think carefully about the risk-to-benefit ratio in older patients or patients with risk factors, such as obesity and diabetes,” he said. But the safety profile of JAK inhibitors “is almost certainly better” than the profile suggested in boxed warnings applied to JAK inhibitors for dermatologic indications, he advised.
Risk-benefit considerations in dermatology
This position was supported by numerous other experts when asked for their perspectives. “I fully agree,” said Emma Guttman-Yassky, MD, PhD, system chair of dermatology and immunology, Icahn School of Medicine, Mount Sinai, New York.
Like Dr. King, Dr. Guttman-Yassky did not dismiss the potential risks of JAK inhibitors when treating dermatologic diseases.
“While JAK inhibitors need monitoring as advised, adopting a boxed warning from an RA study for patients who are older [is problematic],” she commented. A study with the nonselective tofacitinib in this population “cannot be compared to more selective inhibitors in a much younger population, such as those treated [for] alopecia areata or atopic dermatitis.”
George Z. Han, MD, PhD, an associate professor of dermatology, Zucker School of Medicine, Hofstra, Northwell Medical Center, New Hyde Park, New York, also agreed but added some caveats.
“The comments about the ORAL Surveillance study are salient,” he said in an interview. “This kind of data should not directly be extrapolated to other patient types or to other medications.” However, one of Dr. Han’s most important caveats involves long-term use.
“JAK inhibitors are still relatively narrow-therapeutic-window drugs that in a dose-dependent fashion could lead to negative effects, including thromboembolic events, abnormalities in red blood cells, white blood cells, platelets, and lipids,” he said. While doses used in dermatology “are generally below the level of any major concern,” Dr. Han cautioned that “we lack definitive data” on long-term use, and this is important for understanding “any potential small risk of rare events, such as malignancy or thromboembolism.”
Saakshi Khattri, MD, a colleague of Dr. Guttman-Yassky at Mount Sinai, said the risks of JAK inhibitors should not be underestimated, but she also agreed that risk “needs to be delivered in the right context.” Dr. Khattri, who is board certified in both dermatology and rheumatology, noted the safety profiles of available JAK inhibitors differ and that extrapolating safety from an RA study to dermatologic indications does not make sense. “Different diseases, different age groups,” she said.
Dr. King has reported financial relationships with more than 15 pharmaceutical companies, including companies that make JAK inhibitors. Dr. Guttman-Yassky has reported financial relationships with more than 20 pharmaceutical companies, including companies that make JAK inhibitors. Dr. Han reports financial relationships with Amgen, Athenex, Boehringer Ingelheim, Bond Avillion, Bristol-Myers Squibb, Celgene, Janssen, Lilly, Novartis, PellePharm, Pfizer, and UCB. Dr. Khattri has reported financial relationships with AbbVie, Arcutis, Bristol-Myers Squibb, Janssen, Leo, Lilly, Novartis, Pfizer, and UCB.
A version of this article originally appeared on Medscape.com.
AT AAD 2023
How to become wise
The only true wisdom is in knowing you know nothing. – Socrates
At what age is one supposed to be wise? I feel like I’m falling behind. I’ve crossed the middle of life and can check the prerequisite experiences: Joy, tragedy, love, adventure, love again. I lived a jetsetter life with an overnight bag always packed. I’ve sported the “Dad AF” tee with a fully loaded dad-pack. I’ve seen the 50 states and had my picture wrapped on a city bus (super-weird when you pull up next to one). Yet, when a moment arrives to pop in pithy advice for a resident or drop a few reassuring lines for a grieving friend, I’m often unable to find the words. If life were a video game, I’ve not earned the wisdom level yet.
Who are the wise men and women in your life? It’s difficult to list them. This is because it’s a complex attribute and hard to explain. It’s also because the wise who walk among us are rare. Wise is more than being brilliant at bullous diseases or knowing how to sleep train a baby. Nor is wise the buddy who purchased $1,000 of Bitcoin in 2010 (although stay close with him, he probably owns a jet). Neither content experts nor lucky friends rise to the appellation. Both experience and empathy.
The ancients considered wisdom to be one of the vital virtues. It was personified in high-profile gods like Apollo and Athena. It’s rare and important enough to be seen as spiritual. It features heavily in the Bible, the Bhagavad Gita, the Meditations of Marcus Aurelius. In some cultures the wise are called elders or sages. In all cultures they are helpful, respected, sought after, appreciated. We need more wise people in this game of life. I want to be one. But there’s no Coursera for it.
To become wise you have to pass through many levels, put in a lot of reps, suffer through many sleepless nights. Like the third molar, also known as the wisdom tooth, it takes years. You also have to emerge stronger and smarter through those experiences. FDR would not have become one of the wisest presidents in history had it not been for his trials, and victories, over polio. Osler missed Cushing syndrome multiple times before he got it right. It seems you have to go to the mountain, like Batman, and fight a few battles to realize your full wisdom potential.
You must also reflect on your experiences and hone your insight. The management sage Peter Drucker would write what he expected to happen after a decision. Then he’d return to it to hone his intuition and judgment.
Lastly, you have to use your powers for good. Using insight to win your NCAA bracket pool isn’t wisdom. Helping a friend whose marriage is falling apart or colleague whose patient is suing them or a resident whose excision hit an arteriole surely is.
I’ve got a ways to go before anyone puts me on their wise friend list. I’m working on it though. Perhaps you will too – we are desperately short-staffed in this area. For now, I can start with writing better condolences.
“Who maintains that it is not a heavy blow? But it is part of being human.” – Seneca
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at dermnews@mdedge.com.
The only true wisdom is in knowing you know nothing. – Socrates
At what age is one supposed to be wise? I feel like I’m falling behind. I’ve crossed the middle of life and can check the prerequisite experiences: Joy, tragedy, love, adventure, love again. I lived a jetsetter life with an overnight bag always packed. I’ve sported the “Dad AF” tee with a fully loaded dad-pack. I’ve seen the 50 states and had my picture wrapped on a city bus (super-weird when you pull up next to one). Yet, when a moment arrives to pop in pithy advice for a resident or drop a few reassuring lines for a grieving friend, I’m often unable to find the words. If life were a video game, I’ve not earned the wisdom level yet.
Who are the wise men and women in your life? It’s difficult to list them. This is because it’s a complex attribute and hard to explain. It’s also because the wise who walk among us are rare. Wise is more than being brilliant at bullous diseases or knowing how to sleep train a baby. Nor is wise the buddy who purchased $1,000 of Bitcoin in 2010 (although stay close with him, he probably owns a jet). Neither content experts nor lucky friends rise to the appellation. Both experience and empathy.
The ancients considered wisdom to be one of the vital virtues. It was personified in high-profile gods like Apollo and Athena. It’s rare and important enough to be seen as spiritual. It features heavily in the Bible, the Bhagavad Gita, the Meditations of Marcus Aurelius. In some cultures the wise are called elders or sages. In all cultures they are helpful, respected, sought after, appreciated. We need more wise people in this game of life. I want to be one. But there’s no Coursera for it.
To become wise you have to pass through many levels, put in a lot of reps, suffer through many sleepless nights. Like the third molar, also known as the wisdom tooth, it takes years. You also have to emerge stronger and smarter through those experiences. FDR would not have become one of the wisest presidents in history had it not been for his trials, and victories, over polio. Osler missed Cushing syndrome multiple times before he got it right. It seems you have to go to the mountain, like Batman, and fight a few battles to realize your full wisdom potential.
You must also reflect on your experiences and hone your insight. The management sage Peter Drucker would write what he expected to happen after a decision. Then he’d return to it to hone his intuition and judgment.
Lastly, you have to use your powers for good. Using insight to win your NCAA bracket pool isn’t wisdom. Helping a friend whose marriage is falling apart or colleague whose patient is suing them or a resident whose excision hit an arteriole surely is.
I’ve got a ways to go before anyone puts me on their wise friend list. I’m working on it though. Perhaps you will too – we are desperately short-staffed in this area. For now, I can start with writing better condolences.
“Who maintains that it is not a heavy blow? But it is part of being human.” – Seneca
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at dermnews@mdedge.com.
The only true wisdom is in knowing you know nothing. – Socrates
At what age is one supposed to be wise? I feel like I’m falling behind. I’ve crossed the middle of life and can check the prerequisite experiences: Joy, tragedy, love, adventure, love again. I lived a jetsetter life with an overnight bag always packed. I’ve sported the “Dad AF” tee with a fully loaded dad-pack. I’ve seen the 50 states and had my picture wrapped on a city bus (super-weird when you pull up next to one). Yet, when a moment arrives to pop in pithy advice for a resident or drop a few reassuring lines for a grieving friend, I’m often unable to find the words. If life were a video game, I’ve not earned the wisdom level yet.
Who are the wise men and women in your life? It’s difficult to list them. This is because it’s a complex attribute and hard to explain. It’s also because the wise who walk among us are rare. Wise is more than being brilliant at bullous diseases or knowing how to sleep train a baby. Nor is wise the buddy who purchased $1,000 of Bitcoin in 2010 (although stay close with him, he probably owns a jet). Neither content experts nor lucky friends rise to the appellation. Both experience and empathy.
The ancients considered wisdom to be one of the vital virtues. It was personified in high-profile gods like Apollo and Athena. It’s rare and important enough to be seen as spiritual. It features heavily in the Bible, the Bhagavad Gita, the Meditations of Marcus Aurelius. In some cultures the wise are called elders or sages. In all cultures they are helpful, respected, sought after, appreciated. We need more wise people in this game of life. I want to be one. But there’s no Coursera for it.
To become wise you have to pass through many levels, put in a lot of reps, suffer through many sleepless nights. Like the third molar, also known as the wisdom tooth, it takes years. You also have to emerge stronger and smarter through those experiences. FDR would not have become one of the wisest presidents in history had it not been for his trials, and victories, over polio. Osler missed Cushing syndrome multiple times before he got it right. It seems you have to go to the mountain, like Batman, and fight a few battles to realize your full wisdom potential.
You must also reflect on your experiences and hone your insight. The management sage Peter Drucker would write what he expected to happen after a decision. Then he’d return to it to hone his intuition and judgment.
Lastly, you have to use your powers for good. Using insight to win your NCAA bracket pool isn’t wisdom. Helping a friend whose marriage is falling apart or colleague whose patient is suing them or a resident whose excision hit an arteriole surely is.
I’ve got a ways to go before anyone puts me on their wise friend list. I’m working on it though. Perhaps you will too – we are desperately short-staffed in this area. For now, I can start with writing better condolences.
“Who maintains that it is not a heavy blow? But it is part of being human.” – Seneca
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at dermnews@mdedge.com.
Lanolin gets nod for Allergen of the Year
Lanolin is a complex and varying mixture of high molecular weight esters, aliphatic alcohols, sterols, fatty acids, and hydrocarbons, but the allergic components are mainly the free lanolin alcohols, especially alkanediols, said Donald V. Belsito, MD, professor of dermatology, Columbia University, New York, who announced the Allergen of the Year at the society’s annual meeting.
Criteria for selection can include a known allergen with a new twist or increasing frequency or a newly reported allergen with mini-epidemics that may have been missed for years, Dr. Belsito said.
“The prevalence and severity of allergy to ‘lanolin’ have been hotly debated” since a potential case was first reported in the 1920s, wrote Dr. Belsito and Blair A. Jenkins, MD, PhD, a dermatology resident at New York–Presbyterian Hospital, Columbia Campus, in a review published in Dermatitis.
“ ‘Lanolin’ is indeed a paradox allergen,” wrote Dr. Jenkins and Dr. Belsito. “The most appropriate patch test preparation(s) for detecting allergy remain disputed. Detection of lanolin-induced contact dermatitis in diseased skin by patch testing on normal skin may lead to false negative results.”
And those who test positive for a lanolin allergy on diseased skin may be able to use lanolin products on normal skin, they wrote.
“From my perspective, this was a timely year to think about lanolin, as there is significant ongoing controversy about whether it is allergenic,” Dr. Jenkins said in an interview. “Numerous companies market lanolin-containing topicals as safe and effective emollients,” she said.
Medical grade and highly purified anhydrous lanolin, which contain less than 2.5% and less than 1.5% of free alcohols, respectively, can still elicit or induce a contact allergy, Dr. Belsito said in his presentation. Hydrogenated lanolin has shown more allergenicity than lanolin alcohol, while lanolin wax, lanolin acid, and lanolin esters possess lower allergenicity than lanolin alcohol, he said.
Notably, modern wool textiles do not contain lanolin, and lanolin-allergic patients need not avoid wool, Dr. Belsito added.
Amerchol L-101, a common trade name on products containing lanolin, contains 10% wool wax alcohols obtained from the hydrolysis of wool fat dissolved in mineral oil at a 1:1 ratio, said Dr. Belsito. He recommended testing lanolin alcohols (in 30% petrolatum) and Amerchol L-101 (in 50% petrolatum) simultaneously with or without other lanolin derivatives and/or the patient’s products in cases of possible allergy, he said.
Consider high-risk groups
Current evidence suggests that the prevalence of contact allergy in the western European population is 0.4%, wrote Dr. Jenkins and Dr. Belsito.
Although the frequency of lanolin allergy is relatively low, certain conditions convey greater risk, such as stasis dermatitis, leg ulcers, perianal/genital dermatitis, and atopic dermatitis, they wrote. Older adults and children are at increased risk because they are more likely to have these conditions. Demographic data also suggest that lanolin allergy is more common in non-Hispanic Whites than in non-Hispanic Blacks, they wrote.
Looking ahead, “I think further exploration of allergy across different skin types and ethnicities is warranted,” Dr. Jenkins said. “Further investigation of ideal [lanolin] allergens for patch testing is also needed.”
Dr. Jenkins and Dr. Belsito said they had no relevant financial conflicts to disclose.
Lanolin is a complex and varying mixture of high molecular weight esters, aliphatic alcohols, sterols, fatty acids, and hydrocarbons, but the allergic components are mainly the free lanolin alcohols, especially alkanediols, said Donald V. Belsito, MD, professor of dermatology, Columbia University, New York, who announced the Allergen of the Year at the society’s annual meeting.
Criteria for selection can include a known allergen with a new twist or increasing frequency or a newly reported allergen with mini-epidemics that may have been missed for years, Dr. Belsito said.
“The prevalence and severity of allergy to ‘lanolin’ have been hotly debated” since a potential case was first reported in the 1920s, wrote Dr. Belsito and Blair A. Jenkins, MD, PhD, a dermatology resident at New York–Presbyterian Hospital, Columbia Campus, in a review published in Dermatitis.
“ ‘Lanolin’ is indeed a paradox allergen,” wrote Dr. Jenkins and Dr. Belsito. “The most appropriate patch test preparation(s) for detecting allergy remain disputed. Detection of lanolin-induced contact dermatitis in diseased skin by patch testing on normal skin may lead to false negative results.”
And those who test positive for a lanolin allergy on diseased skin may be able to use lanolin products on normal skin, they wrote.
“From my perspective, this was a timely year to think about lanolin, as there is significant ongoing controversy about whether it is allergenic,” Dr. Jenkins said in an interview. “Numerous companies market lanolin-containing topicals as safe and effective emollients,” she said.
Medical grade and highly purified anhydrous lanolin, which contain less than 2.5% and less than 1.5% of free alcohols, respectively, can still elicit or induce a contact allergy, Dr. Belsito said in his presentation. Hydrogenated lanolin has shown more allergenicity than lanolin alcohol, while lanolin wax, lanolin acid, and lanolin esters possess lower allergenicity than lanolin alcohol, he said.
Notably, modern wool textiles do not contain lanolin, and lanolin-allergic patients need not avoid wool, Dr. Belsito added.
Amerchol L-101, a common trade name on products containing lanolin, contains 10% wool wax alcohols obtained from the hydrolysis of wool fat dissolved in mineral oil at a 1:1 ratio, said Dr. Belsito. He recommended testing lanolin alcohols (in 30% petrolatum) and Amerchol L-101 (in 50% petrolatum) simultaneously with or without other lanolin derivatives and/or the patient’s products in cases of possible allergy, he said.
Consider high-risk groups
Current evidence suggests that the prevalence of contact allergy in the western European population is 0.4%, wrote Dr. Jenkins and Dr. Belsito.
Although the frequency of lanolin allergy is relatively low, certain conditions convey greater risk, such as stasis dermatitis, leg ulcers, perianal/genital dermatitis, and atopic dermatitis, they wrote. Older adults and children are at increased risk because they are more likely to have these conditions. Demographic data also suggest that lanolin allergy is more common in non-Hispanic Whites than in non-Hispanic Blacks, they wrote.
Looking ahead, “I think further exploration of allergy across different skin types and ethnicities is warranted,” Dr. Jenkins said. “Further investigation of ideal [lanolin] allergens for patch testing is also needed.”
Dr. Jenkins and Dr. Belsito said they had no relevant financial conflicts to disclose.
Lanolin is a complex and varying mixture of high molecular weight esters, aliphatic alcohols, sterols, fatty acids, and hydrocarbons, but the allergic components are mainly the free lanolin alcohols, especially alkanediols, said Donald V. Belsito, MD, professor of dermatology, Columbia University, New York, who announced the Allergen of the Year at the society’s annual meeting.
Criteria for selection can include a known allergen with a new twist or increasing frequency or a newly reported allergen with mini-epidemics that may have been missed for years, Dr. Belsito said.
“The prevalence and severity of allergy to ‘lanolin’ have been hotly debated” since a potential case was first reported in the 1920s, wrote Dr. Belsito and Blair A. Jenkins, MD, PhD, a dermatology resident at New York–Presbyterian Hospital, Columbia Campus, in a review published in Dermatitis.
“ ‘Lanolin’ is indeed a paradox allergen,” wrote Dr. Jenkins and Dr. Belsito. “The most appropriate patch test preparation(s) for detecting allergy remain disputed. Detection of lanolin-induced contact dermatitis in diseased skin by patch testing on normal skin may lead to false negative results.”
And those who test positive for a lanolin allergy on diseased skin may be able to use lanolin products on normal skin, they wrote.
“From my perspective, this was a timely year to think about lanolin, as there is significant ongoing controversy about whether it is allergenic,” Dr. Jenkins said in an interview. “Numerous companies market lanolin-containing topicals as safe and effective emollients,” she said.
Medical grade and highly purified anhydrous lanolin, which contain less than 2.5% and less than 1.5% of free alcohols, respectively, can still elicit or induce a contact allergy, Dr. Belsito said in his presentation. Hydrogenated lanolin has shown more allergenicity than lanolin alcohol, while lanolin wax, lanolin acid, and lanolin esters possess lower allergenicity than lanolin alcohol, he said.
Notably, modern wool textiles do not contain lanolin, and lanolin-allergic patients need not avoid wool, Dr. Belsito added.
Amerchol L-101, a common trade name on products containing lanolin, contains 10% wool wax alcohols obtained from the hydrolysis of wool fat dissolved in mineral oil at a 1:1 ratio, said Dr. Belsito. He recommended testing lanolin alcohols (in 30% petrolatum) and Amerchol L-101 (in 50% petrolatum) simultaneously with or without other lanolin derivatives and/or the patient’s products in cases of possible allergy, he said.
Consider high-risk groups
Current evidence suggests that the prevalence of contact allergy in the western European population is 0.4%, wrote Dr. Jenkins and Dr. Belsito.
Although the frequency of lanolin allergy is relatively low, certain conditions convey greater risk, such as stasis dermatitis, leg ulcers, perianal/genital dermatitis, and atopic dermatitis, they wrote. Older adults and children are at increased risk because they are more likely to have these conditions. Demographic data also suggest that lanolin allergy is more common in non-Hispanic Whites than in non-Hispanic Blacks, they wrote.
Looking ahead, “I think further exploration of allergy across different skin types and ethnicities is warranted,” Dr. Jenkins said. “Further investigation of ideal [lanolin] allergens for patch testing is also needed.”
Dr. Jenkins and Dr. Belsito said they had no relevant financial conflicts to disclose.
FROM ACDS 2023