User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Tackling Inflammatory and Infectious Nail Disorders in Children
Nail disorders are common among pediatric patients but often are underdiagnosed or misdiagnosed because of their unique disease manifestations. These conditions may severely impact quality of life. There are few nail disease clinical trials that include children. Consequently, most treatment recommendations are based on case series and expert consensus recommendations. We review inflammatory and infectious nail disorders in pediatric patients. By describing characteristics, clinical manifestations, and management approaches for these conditions, we aim to provide guidance to dermatologists in their diagnosis and treatment.
INFLAMMATORY NAIL DISORDERS
Nail Psoriasis
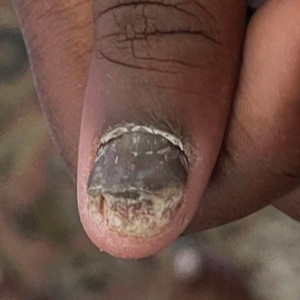
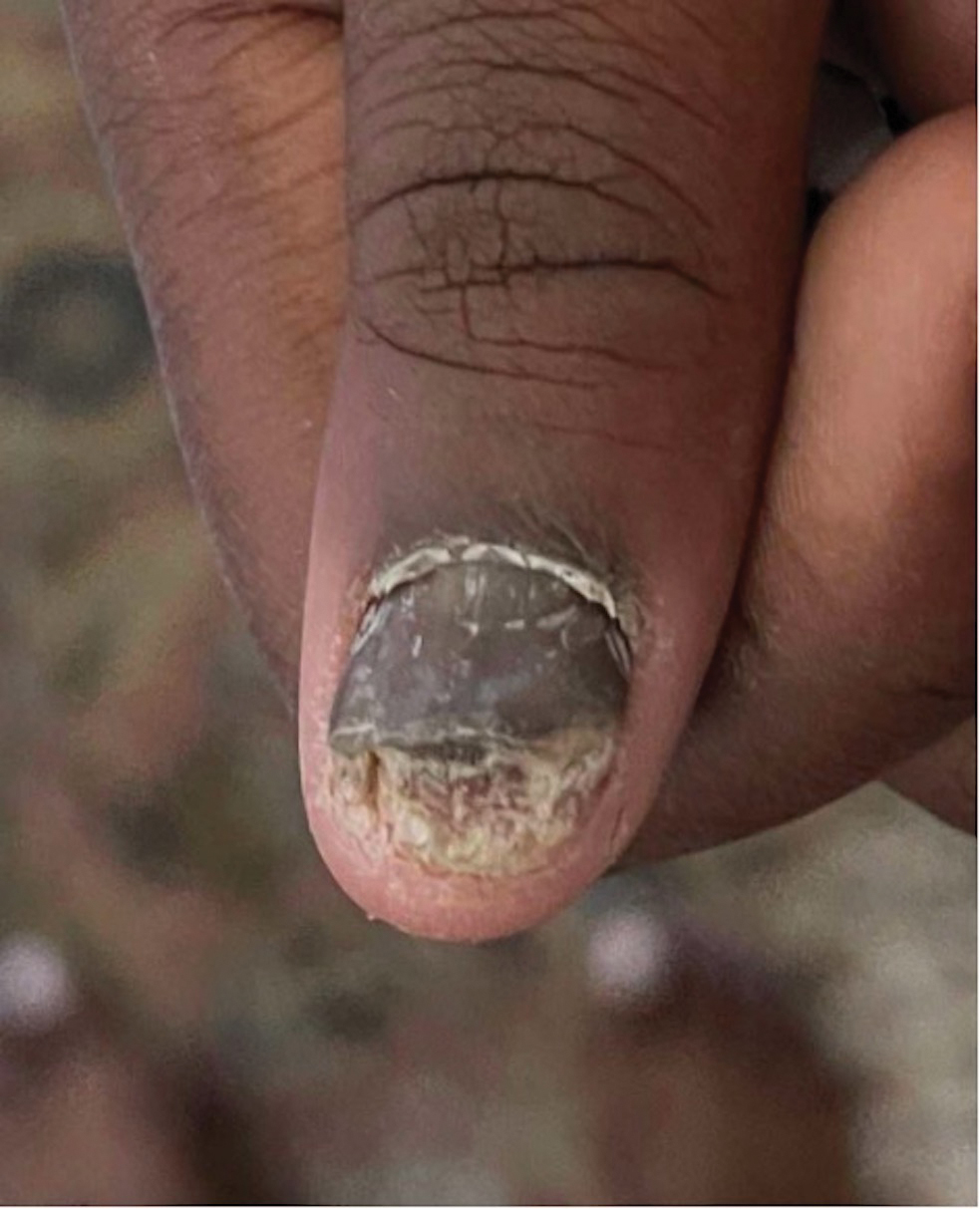
Nail involvement in children with psoriasis is common, with prevalence estimates ranging from 17% to 39.2%.1 Nail matrix psoriasis may manifest with pitting (large irregular pits) and leukonychia as well as chromonychia and nail plate crumbling. Onycholysis, oil drop spots (salmon patches), and subungual hyperkeratosis can be seen in nail bed psoriasis. Nail pitting is the most frequently observed clinical finding (Figure 1).2,3 In a cross-sectional multicenter study of 313 children with cutaneous psoriasis in France, nail findings were present in 101 patients (32.3%). There were associations between nail findings and presence of psoriatic arthritis (P=.03), palmoplantar psoriasis (P<.001), and severity of psoriatic disease, defined as use of systemic treatment with phototherapy (psoralen plus UVA, UVB), traditional systemic treatment (acitretin, methotrexate, cyclosporine), or a biologic (P=.003).4
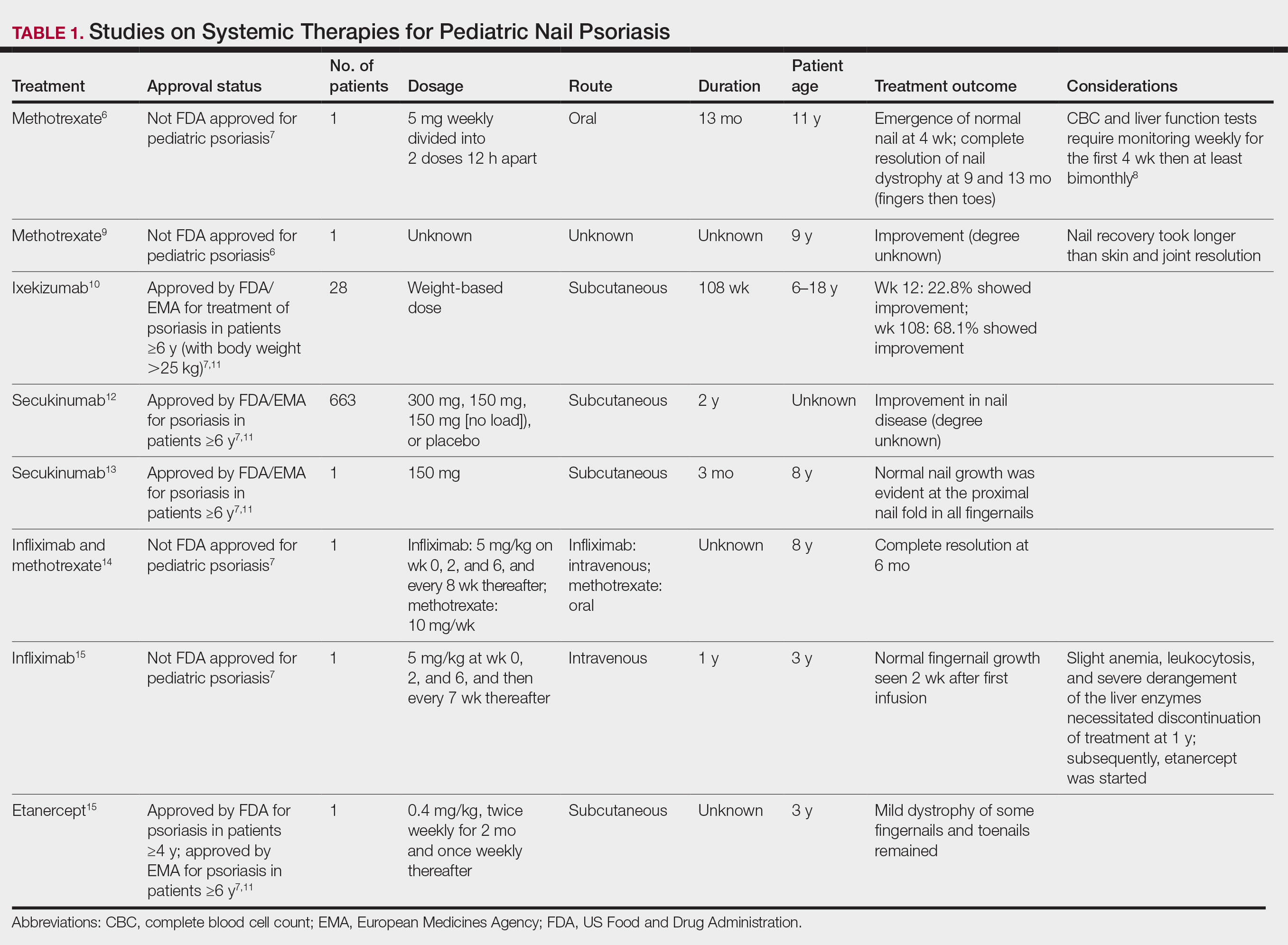
Topical steroids and vitamin D analogues may be used with or without occlusion and may be efficacious.5 Several case reports describe systemic treatments for psoriasis in children, including methotrexate, acitretin, and apremilast (approved for children 6 years and older for plaque psoriasis by the US Food and Drug Administration [FDA]).2 There are 5 biologic drugs currently approved for the treatment of pediatric psoriasis—adalimumab, etanercept, ustekinumab, secukinumab, ixekizumab—and 6 drugs currently undergoing phase 3 studies—brodalumab, guselkumab, risankizumab, tildrakizumab, certolizumab pegol, and deucravacitinib (Table 1).6-15 Adalimumab is specifically approved for moderate to severe nail psoriasis in adults 18 years and older.
Intralesional steroid injections are sometimes useful in the management of nail matrix psoriasis; however, appropriate patient selection is critical due to the pain associated with the procedure. In a prospective study of 16 children (age range, 9–17 years) with nail psoriasis treated with intralesional triamcinolone (ILTAC) 2.5 to 5 mg/mL every 4 to 8 weeks for a minimum of 3 to 6 months, 9 patients achieved resolution and 6 had improvement of clinical findings.16 Local adverse events were mild, including injection-site pain (66%), subungual hematoma (n=1), Beau lines (n=1), proximal nail fold hypopigmentation (n=2), and proximal nail fold atrophy (n=2). Because the proximal nail fold in children is thinner than in adults, there may be an increased risk for nail fold hypopigmentation and atrophy in children. Therefore, a maximum ILTAC concentration of 2.5 mg/mL with 0.2 mL maximum volume per nail per session is recommended for children younger than 15 years.16
Nail Lichen Planus
Nail lichen planus (NLP) is uncommon in children, with few biopsy-proven cases documented in the literature.17 Common clinical findings are onychorrhexis, nail plate thinning, fissuring, splitting, and atrophy with koilonychia.5 Although pterygium development (irreversible nail matrix scarring) is uncommon in pediatric patients, NLP can be progressive and may cause irreversible destruction of the nail matrix and subsequent nail loss, warranting therapeutic intervention.18
Treatment of NLP may be difficult, as there are no options that work in all patients. Current literature supports the use of systemic corticosteroids or ILTAC for the treatment of NLP; however, recurrence rates can be high. According to an expert consensus paper on NLP treatment, ILTAC may be injected in a concentration of 2.5, 5, or 10 mg/mL according to disease severity.19 In severe or resistant cases, intramuscular (IM) triamcinolone may be considered, especially if more than 3 nails are affected. A dosage of 0.5 to 1 mg/kg/mo for at least 3 to 6 months is recommended for both children and adults, with 1 mg/kg/mo recommended in the active treatment phase (first 2–3 months).19 In a retrospective review of 5 pediatric patients with NLP treated with IM triamcinolone 0.5 mg/kg/mo, 3 patients had resolution and 2 improved with treatment.20 In a prospective study of 10 children with NLP, IM triamcinolone at a dosage of 0.5 to 1 mg/kg every 30 days for 3 to 6 months resulted in resolution of nail findings in 9 patients.17 In a prospective study of 14 pediatric patients with NLP treated with 2.5 to 5 mg/mL of ILTAC, 10 achieved resolution and 3 improved.16
Intralesional triamcinolone injections may be better suited for teenagers compared to younger children who may be more apprehensive of needles. To minimize pain, it is recommended to inject ILTAC slowly at room temperature, with use of “talkesthesia” and vibration devices, 1% lidocaine, or ethyl chloride spray.18
Trachyonychia
Trachyonychia is characterized by the presence of sandpaperlike nails. It manifests with brittle thin nails with longitudinal ridging, onychoschizia, and thickened hyperkeratotic cuticles. Trachyonychia typically involves multiple nails, with a peak age of onset between 3 and 12 years.21,22 There are 2 variants: the opaque type with rough longitudinal ridging, and the shiny variant with opalescent nails and pits that reflect light. The opaque variant is more common and is associated with psoriasis, whereas the shiny variant is less common and is associated with alopecia areata.23 Although most cases are idiopathic, some are associated with psoriasis and alopecia areata, as previously noted, as well as atopic dermatitis (AD) and lichen planus.22,24
Fortunately, trachyonychia does not lead to permanent nail damage or pterygium, making treatment primarily focused on addressing functional and cosmetic concerns.24 Spontaneous resolution occurs in approximately 50% of patients. In a prospective study of 11 patients with idiopathic trachyonychia, there was partial improvement in 5 of 9 patients treated with topical steroids, 1 with only petrolatum, and 1 with vitamin supplements. Complete resolution was reported in 1 patient treated with topical steroids.25 Because trachyonychia often is self-resolving, no treatment is required and a conservative approach is strongly recommended.26 Treatment options include topical corticosteroids, tazarotene, and 5-fluorouracil. Intralesional triamcinolone, systemic cyclosporine, retinoids, systemic corticosteroids, and tofacitinib have been described in case reports, though none of these have been shown to be 100% efficacious.24
Nail Lichen Striatus
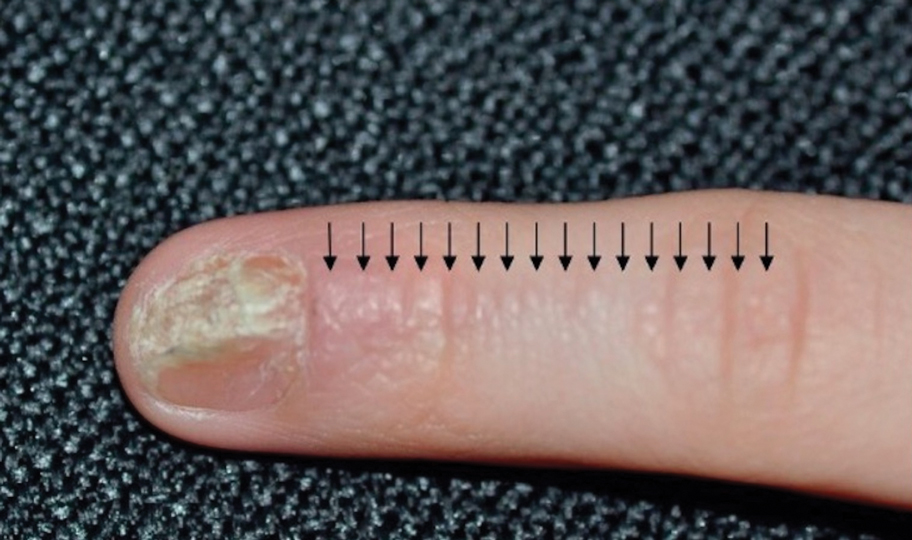
Lichen striatus involving the nail is uncommon and is characterized by onycholysis, longitudinal ridging, splitting, and fraying, as well as what appears to be a subungual tumor. It can encompass the entire nail or may be isolated to a portion of the nail (Figure 2). Usually, a Blaschko-linear array of flesh-colored papules on the more proximal digit directly adjacent to the nail dystrophy will be seen, though nail findings can occur in isolation.27-29 The underlying pathophysiology is not clear; however, one hypothesis is that a triggering event, such as trauma, induces the expression of antigens that elicit a self-limiting immune-mediated response by CD8 T lymphocytes.30
Generally, nail lichen striatus spontaneously resolves in 1 to 2 years without treatment. In a prospective study of 5 patients with nail lichen striatus, the median time to resolution was 22.6 months (range, 10–30 months).31 Topical steroids may be used for pruritus. In one case report, a 3-year-old boy with nail lichen striatus of 4 months’ duration was treated with tacrolimus ointment 0.03% daily for 3 months.28
Nail AD
Nail changes with AD may be more common in adults than children or are underreported. In a study of 777 adults with AD, nail dystrophy was present in 124 patients (16%), whereas in a study of 250 pediatric patients with AD (aged 0-2 years), nail dystrophy was present in only 4 patients.32,33
Periungual inflammation from AD causes the nail changes.34 In a cross-sectional study of 24 pediatric patients with nail dystrophy due to AD, transverse grooves (Beau lines) were present in 25% (6/24), nail pitting in 16.7% (4/24), koilonychia in 16.7% (4/24), trachyonychia in 12.5% (3/24), leukonychia in 12.5% (3/24), brachyonychia in 8.3% (2/24), melanonychia in 8.3% (2/24), onychomadesis in 8.3% (2/24), onychoschizia in 8.3% (2/24), and onycholysis in 8.3% (2/24). There was an association between disease severity and presence of toenail dystrophy (P=.03).35
Topical steroids with or without occlusion can be used to treat nail changes. Although there is limited literature describing the treatment of nail AD in children, a 61-year-old man with nail changes associated with AD achieved resolution with 3 months of treatment with dupilumab.36 Anecdotally, most patients will improve with usual cutaneous AD management.
INFECTIOUS NAIL DISORDERS
Viral Infections
Hand, Foot, and Mouth Disease—Hand, foot, and mouth disease (HFMD) is a common childhood viral infection caused by various enteroviruses, most commonly coxsackievirus A16, with the A6 variant causing more severe disease. Fever and painful vesicles involving the oral mucosa as well as palms and soles give the disease its name. Nail changes are common. In a prospective study involving 130 patients with laboratory-confirmed coxsackievirus CA6 serotype infection, 37% developed onychomadesis vs only 5% of 145 cases with non-CA6 enterovirus infection who developed nail findings. There was an association between CA6 infection and presence of nail changes (P<.001).37
Findings ranging from transverse grooves (Beau lines) to complete nail shedding (onychomadesis)(Figure 3) may be seen.38,39 Nail findings in HFMD are due to transient inhibition of nail growth and present approximately 3 to 6 weeks after infection.40 Onychomadesis is seen in 30% to 68% of patients with HFMD.37,41,42 Nail findings in HFMD spontaneously resolve with nail growth (2–3 mm per month for fingernails and 1 mm per month for toenails) and do not require specific treatment. Although the appearance of nail changes associated with HFMD can be disturbing, dermatologists can reassure children and their parents that the nails will resolve with the next cycle of growth.
Kawasaki Disease—Kawasaki disease (KD) is a vasculitis primarily affecting children and infants. Although the specific pathogen and pathophysiology is not entirely clear, clinical observations have suggested an infectious cause, most likely a virus.43 In Japan, more than 15,000 cases of KD are documented annually, while approximately 4200 cases are seen in the United States.44 In a prospective study from 1984 to 1990, 4 of 26 (15.4%) patients with KD presented with nail manifestations during the late acute phase or early convalescent phase of disease. There were no significant associations between nail dystrophy and severity of KD, such as coronary artery aneurysm.45
Nail changes reported in children with KD include onychomadesis, onycholysis, orange-brown chromonychia, splinter hemorrhages, Beau lines, and pincer nails. In a review of nail changes associated with KD from 1980 to 2021, orange-brown transverse chromonychia, which may evolve into transverse leukonychia, was the most common nail finding reported, occurring in 17 of 31 (54.8%) patients.44 It has been hypothesized that nail changes may result from blood flow disturbance due to the underlying vasculitis.46 Nail changes appear several weeks after the onset of fever and are self-limited. Resolution occurs with nail growth, with no treatment required.
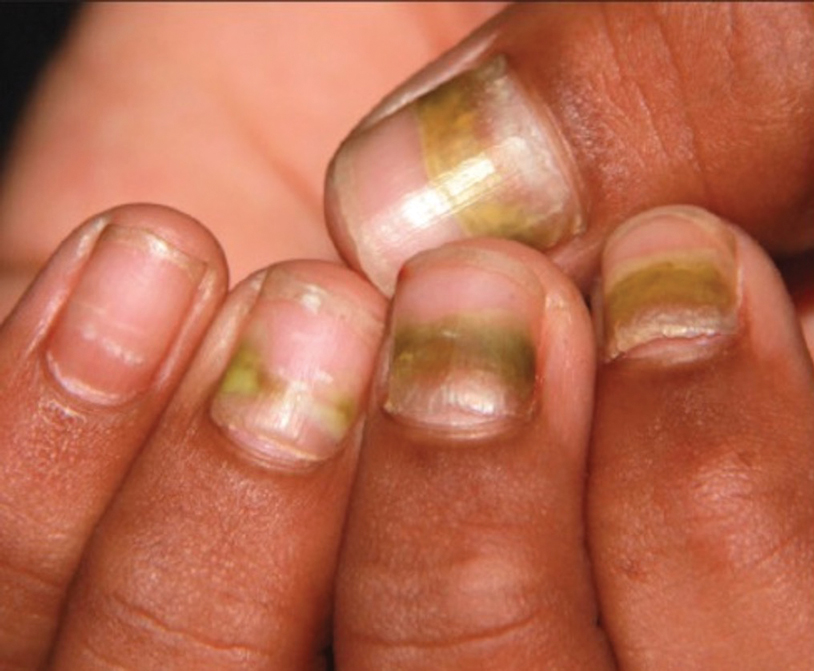
FUNGAL INFECTIONS
Onychomycosis
Onychomycosis is a fungal infection of the nails that occurs in 0.2% to 5.5% of pediatric patients, and its prevalence may be increasing, which may be due to environmental factors or increased rates of diabetes mellitus and obesity in the pediatric population.47 Onychomycosis represents 15.5% of nail dystrophies in pediatric patients.48 Some dermatologists treat presumptive onychomycosis without confirmation; however, we do not recommend that approach. Because the differential is broad and the duration of treatment is long, mycologic examination (potassium hydroxide preparation, fungal culture, polymerase chain reaction, and/or histopathology) should be obtained to confirm onychomycosis prior to initiation of antifungal management. Family members of affected individuals should be evaluated and treated, if indicated, for onychomycosis and tinea pedis, as household transmission is common.
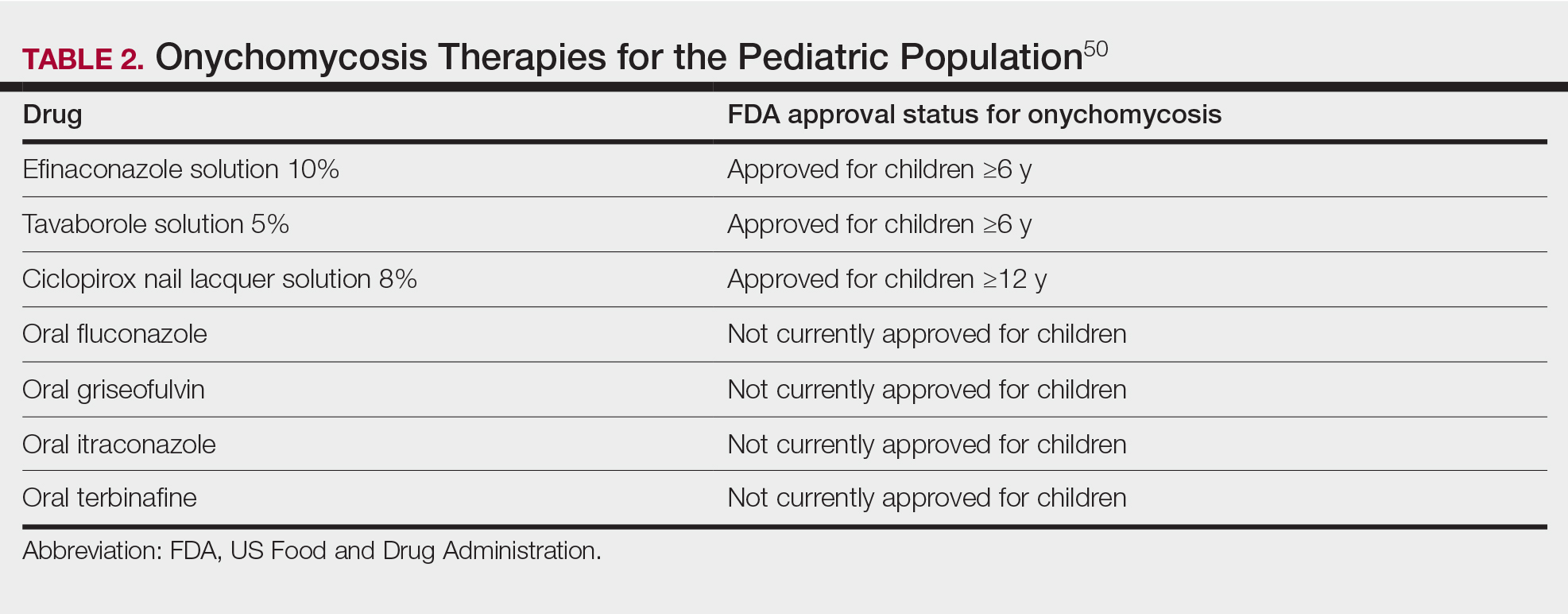
Currently, there are 2 topical FDA-approved treatments for pediatric onychomycosis in children 6 years and older (Table 2).49,50 There is a discussion of the need for confirmatory testing for onychomycosis in children, particularly when systemic treatment is prescribed. In a retrospective review of 269 pediatric patients with onychomycosis prescribed terbinafine, 53.5% (n=144) underwent laboratory monitoring of liver function and complete blood cell counts, and 12.5% had grade 1 laboratory abnormalities either prior to (12/144 [8.3%]) or during (6/144 [4.2%]) therapy.51 Baseline transaminase monitoring is recommended, though subsequent routine laboratory monitoring in healthy children may have limited utility with associated increased costs, incidental findings, and patient discomfort and likely is not needed.51
Pediatric onychomycosis responds better to topical therapy than adult disease, and pediatric patients do not always require systemic treatment.52 Ciclopirox is not FDA approved for the treatment of pediatric onychomycosis, but in a 32-week clinical trial of ciclopirox lacquer 8% use in 40 patients, 77% (27/35) of treated patients achieved mycologic cure. Overall, 71% of treated patients (25/35) vs 22% (2/9) of controls achieved efficacy (defined as investigator global assessment score of 2 or lower).52 In an open-label, single-arm clinical trial assessing tavaborole solution 5% applied once daily for 48 weeks for the treatment of toenail onychomycosis in pediatric patients (aged 6–17 years), 36.2% (20/55) of patients achieved mycologic cure, and 8.5% (5/55) achieved complete cure at week 52 with mild or minimal adverse effects.53 In an open-label, phase 4 study of the safety and efficacy of efinaconazole solution 10% applied once daily for 48 weeks in pediatric patients (aged 6 to 16 years) (n=60), 65% (35/60) achieved mycologic cure, 42% (25/60) achieved clinical cure, and 40% (24/60) achieved complete cure at 52 weeks. The most common adverse effects of efinaconazole were local and included ingrown toenail (1/60), application-site dermatitis (1/60), application-site vesicles (1/60), and application-site pain (1/60).54
In a systematic review of systemic antifungals for onychomycosis in 151 pediatric patients, itraconazole, fluconazole, griseofulvin, and terbinafine resulted in complete cure rates similar to those of the adult population, with excellent safety profiles.55 Depending on the situation, initiation of treatment with topical medications followed by addition of systemic antifungal agents only if needed may be an appropriate course of action.
BACTERIAL INFECTIONS
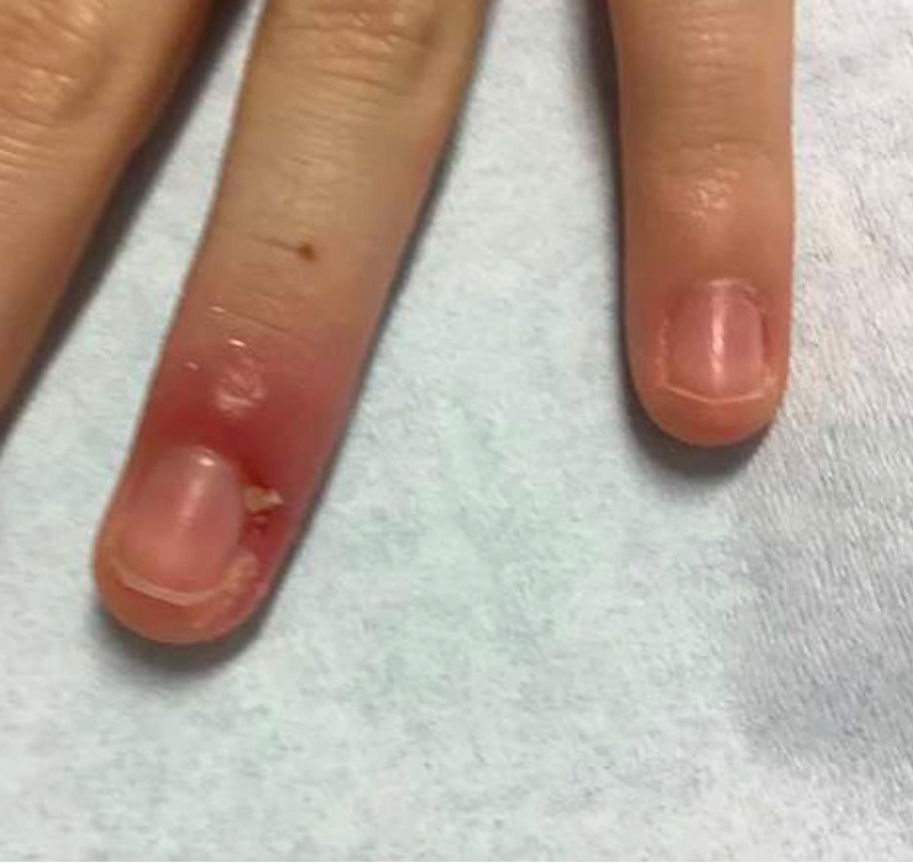
Acute Paronychia
Acute paronychia is a nail-fold infection that develops after the protective nail barrier has been compromised.56 In children, thumb-sucking, nail-biting, frequent oral manipulation of the digits, and poor skin hygiene are risk factors. Acute paronychia also may develop in association with congenital malalignment of the great toenails.57
Clinical manifestations include localized pain, erythema, and nail fold edema (Figure 4). Purulent material and abscess formation may ensue. Staphylococcus aureus as well as methicillin-resistant S aureus and Streptococcus pyogenes are classically the most common causes of acute paronychia. Treatment of paronychia is based on severity. In mild cases, warm soaks with topical antibiotics are indicated. Oral antibiotics should be prescribed for more severe presentations. If there is no improvement after 48 hours, surgical drainage is required to facilitate healing.56
FINAL THOUGHTS
Inflammatory and infectious nail disorders in children are relatively common and may impact the physical and emotional well-being of young patients. By understanding the distinctive features of these nail disorders in pediatric patients, dermatologists can provide anticipatory guidance and informed treatment options to children and their parents. Further research is needed to expand our understanding of pediatric nail disorders and create targeted therapeutic interventions, particularly for NLP and psoriasis.
- Uber M, Carvalho VO, Abagge KT, et al. Clinical features and nail clippings in 52 children with psoriasis. Pediatr Dermatol. 2018;35:202-207. doi:10.1111/pde.13402
- Plachouri KM, Mulita F, Georgiou S. Management of pediatric nail psoriasis. Cutis. 2021;108:292-294. doi:10.12788/cutis.0386
- Smith RJ, Rubin AI. Pediatric nail disorders: a review. Curr Opin Pediatr. 2020;32:506-515. doi:10.1097/mop.0000000000000921
- Pourchot D, Bodemer C, Phan A, et al. Nail psoriasis: a systematic evaluation in 313 children with psoriasis. Pediatr Dermatol. 2017;34:58-63. doi:10.1111/pde.13028
- Richert B, André J. Nail disorders in children: diagnosis and management. Am J Clin Dermatol. 2011;12:101-112. doi:10.2165/11537110-000000000-00000
- Lee JYY. Severe 20-nail psoriasis successfully treated by low dose methotrexate. Dermatol Online J. 2009;15:8.
- Nogueira M, Paller AS, Torres T. Targeted therapy for pediatric psoriasis. Paediatr Drugs. May 2021;23:203-212. doi:10.1007/s40272-021-00443-5
- Hanoodi M, Mittal M. Methotrexate. StatPearls [Internet]. Updated August 16, 2023. Accessed July 1, 2024. https://www.ncbi.nlm.nih.gov/books/NBK556114/
- Teran CG, Teran-Escalera CN, Balderrama C. A severe case of erythrodermic psoriasis associated with advanced nail and joint manifestations: a case report. J Med Case Rep. 2010;4:179. doi:10.1186/1752-1947-4-179
- Paller AS, Seyger MMB, Magariños GA, et al. Long-term efficacy and safety of up to 108 weeks of ixekizumab in pediatric patients with moderate to severe plaque psoriasis: the IXORA-PEDS randomized clinical trial. JAMA Dermatol. 2022;158:533-541. doi:10.1001/jamadermatol.2022.0655
- Diotallevi F, Simonetti O, Rizzetto G, et al. Biological treatments for pediatric psoriasis: state of the art and future perspectives. Int J Mol Sci. 2022;23:11128. doi:10.3390/ijms231911128
- Nash P, Mease PJ, Kirkham B, et al. Secukinumab provides sustained improvement in nail psoriasis, signs and symptoms of psoriatic arthritis and low rate of radiographic progression in patients with concomitant nail involvement: 2-year results from the Phase III FUTURE 5 study. Clin Exp Rheumatol. 2022;40:952-959. doi:10.55563/clinexprheumatol/3nuz51
- Wells LE, Evans T, Hilton R, et al. Use of secukinumab in a pediatric patient leads to significant improvement in nail psoriasis and psoriatic arthritis. Pediatr Dermatol. 2019;36:384-385. doi:10.1111/pde.13767
- Watabe D, Endoh K, Maeda F, et al. Childhood-onset psoriaticonycho-pachydermo-periostitis treated successfully with infliximab. Eur J Dermatol. 2015;25:506-508. doi:10.1684/ejd.2015.2616
- Pereira TM, Vieira AP, Fernandes JC, et al. Anti-TNF-alpha therapy in childhood pustular psoriasis. Dermatology. 2006;213:350-352. doi:10.1159/000096202
- Iorizzo M, Gioia Di Chiacchio N, Di Chiacchio N, et al. Intralesional steroid injections for inflammatory nail dystrophies in the pediatric population. Pediatr Dermatol. 2023;40:759-761. doi:10.1111/pde.15295
- Tosti A, Piraccini BM, Cambiaghi S, et al. Nail lichen planus in children: clinical features, response to treatment, and long-term follow-up. Arch Dermatol. 2001;137:1027-1032.
- Lipner SR. Nail lichen planus: a true nail emergency. J Am Acad Dermatol. 2019;80:e177-e178. doi:10.1016/j.jaad.2018.11.065
- Iorizzo M, Tosti A, Starace M, et al. Isolated nail lichen planus: an expert consensus on treatment of the classical form. J Am Acad Dermatol. 2020;83:1717-1723. doi:10.1016/j.jaad.2020.02.056
- Piraccini BM, Saccani E, Starace M, et al. Nail lichen planus: response to treatment and long term follow-up. Eur J Dermatol. 2010;20:489-496. doi:10.1684/ejd.2010.0952
- Mahajan R, Kaushik A, De D, et al. Pediatric trachyonychia- a retrospective study of 17 cases. Indian J Dermatol. 2021;66:689-690. doi:10.4103/ijd.ijd_42_21
- Leung AKC, Leong KF, Barankin B. Trachyonychia. J Pediatr. 2020;216:239-239.e1. doi:10.1016/j.jpeds.2019.08.034
- Haber JS, Chairatchaneeboon M, Rubin AI. Trachyonychia: review and update on clinical aspects, histology, and therapy. Skin Appendage Disord. 2017;2:109-115. doi:10.1159/000449063
- Jacobsen AA, Tosti A. Trachyonychia and twenty-nail dystrophy: a comprehensive review and discussion of diagnostic accuracy. Skin Appendage Disord. 2016;2:7-13. doi:10.1159/000445544
- Kumar MG, Ciliberto H, Bayliss SJ. Long-term follow-up of pediatric trachyonychia. Pediatr Dermatol. 2015;32:198-200. doi:10.1111/pde.12427
- Tosti A, Piraccini BM, Iorizzo M. Trachyonychia and related disorders: evaluation and treatment plans. Dermatolog Ther. 2002;15:121-125. doi:10.1046/j.1529-8019.2002.01511.x
- Leung AKC, Leong KF, Barankin B. Lichen striatus with nail involvement in a 6-year-old boy. Case Rep Pediatr. 2020;2020:1494760. doi:10.1155/2020/1494760
- Kim GW, Kim SH, Seo SH, et al. Lichen striatus with nail abnormality successfully treated with tacrolimus ointment. J Dermatol. 2009;36:616-617. doi:10.1111/j.1346-8138.2009.00720.x
- Iorizzo M, Rubin AI, Starace M. Nail lichen striatus: is dermoscopy useful for the diagnosis? Pediatr Dermatol. 2019;36:859-863. doi:10.1111/pde.13916
- Karp DL, Cohen BA. Onychodystrophy in lichen striatus. Pediatr Dermatol. 1993;10:359-361. doi:10.1111/j.1525-1470.1993.tb00399.x
- Tosti A, Peluso AM, Misciali C, et al. Nail lichen striatus: clinical features and long-term follow-up of five patients. J Am Acad Dermatol. 1997;36(6, pt 1):908-913. doi:10.1016/s0190-9622(97)80270-8
- Simpson EL, Thompson MM, Hanifin JM. Prevalence and morphology of hand eczema in patients with atopic dermatitis. Dermatitis. 2006;17:123-127. doi:10.2310/6620.2006.06005
- Sarifakioglu E, Yilmaz AE, Gorpelioglu C. Nail alterations in 250 infant patients: a clinical study. J Eur Acad Dermatol Venereol. 2008;22:741-744. doi:10.1111/j.1468-3083.2008.02592.x
- Milanesi N, D’Erme AM, Gola M. Nail improvement during alitretinoin treatment: three case reports and review of the literature. Clin Exp Dermatol. 2015;40:533-536. doi:10.1111/ced.12584
- Chung BY, Choi YW, Kim HO, et al. Nail dystrophy in patients with atopic dermatitis and its association with disease severity. Ann Dermatol. 2019;31:121-126. doi:10.5021/ad.2019.31.2.121
- Navarro-Triviño FJ, Vega-Castillo JJ, Ruiz-Villaverde R. Nail changes successfully treated with dupilumab in a patient with severe atopic dermatitis. Australas J Dermatol. 2021;62:e468-e469. doi:10.1111/ajd.13633
- Wei SH, Huang YP, Liu MC, et al. An outbreak of coxsackievirus A6 hand, foot, and mouth disease associated with onychomadesis in Taiwan, 2010. BMC Infect Dis. 2011;11:346. doi:10.1186/1471-2334-11-346
- Shin JY, Cho BK, Park HJ. A clinical study of nail changes occurring secondary to hand-foot-mouth disease: onychomadesis and Beau’s lines. Ann Dermatol. 2014;26:280-283. doi:10.5021/ad.2014.26.2.280
- Verma S, Singal A. Nail changes in hand-foot-and-mouth disease (HFMD). Indian Dermatol Online J. 2021;12:656-657. doi:10.4103 /idoj.IDOJ_271_20
- Giordano LMC, de la Fuente LA, Lorca JMB, et al. Onychomadesis secondary to hand-foot-mouth disease: a frequent manifestation and cause of concern for parents. Article in Spanish. Rev Chil Pediatr. 2018;89:380-383. doi:10.4067/s0370-41062018005000203
- Justino MCA, da SMD, Souza MF, et al. Atypical hand-foot-mouth disease in Belém, Amazon region, northern Brazil, with detection of coxsackievirus A6. J Clin Virol. 2020;126:104307. doi:10.1016/j.jcv.2020.104307
- Cheng FF, Zhang BB, Cao ML, et al. Clinical characteristics of 68 children with atypical hand, foot, and mouth disease caused by coxsackievirus A6: a single-center retrospective analysis. Transl Pediatr. 2022;11:1502-1509. doi:10.21037/tp-22-352
- Nagata S. Causes of Kawasaki disease-from past to present. Front Pediatr. 2019;7:18. doi:10.3389/fped.2019.00018
- Mitsuishi T, Miyata K, Ando A, et al. Characteristic nail lesions in Kawasaki disease: case series and literature review. J Dermatol. 2022;49:232-238. doi:10.1111/1346-8138.16276
- Lindsley CB. Nail-bed lines in Kawasaki disease. Am J Dis Child. 1992;146:659-660. doi:10.1001/archpedi.1992.02160180017005
- Matsumura O, Nakagishi Y. Pincer nails upon convalescence from Kawasaki disease. J Pediatr. 2022;246:279. doi:10.1016/j.jpeds.2022.03.002
- Solís-Arias MP, García-Romero MT. Onychomycosis in children. a review. Int J Dermatol. 2017;56:123-130. doi:10.1111/ijd.13392
- Gupta AK, Mays RR, Versteeg SG, et al. Onychomycosis in children: safety and efficacy of antifungal agents. Pediatr Dermatol. 2018;35:552-559. doi:10.1111/pde.13561
- 49. Gupta AK, Venkataraman M, Shear NH, et al. Labeled use of efinaconazole topical solution 10% in treating onychomycosis in children and a review of the management of pediatric onychomycosis. Dermatol Ther. 2020;33:e13613. doi:10.1111/dth.13613
- Falotico JM, Lipner SR. Updated perspectives on the diagnosis and management of onychomycosis. Clin Cosmet Investig Dermatol. 2022;15:1933-1957. doi:10.2147/ccid.S362635
- Patel D, Castelo-Soccio LA, Rubin AI, et al. Laboratory monitoring during systemic terbinafine therapy for pediatric onychomycosis. JAMA Dermatol. 2017;153:1326-1327. doi:10.1001/jamadermatol.2017.4483
- Friedlander SF, Chan YC, Chan YH, et al. Onychomycosis does not always require systemic treatment for cure: a trial using topical therapy. Pediatr Dermatol. 2013;30:316-322. doi:10.1111/pde.12064
- Rich P, Spellman M, Purohit V, et al. Tavaborole 5% topical solution for the treatment of toenail onychomycosis in pediatric patients: results from a phase 4 open-label study. J Drugs Dermatol. 2019;18:190-195.
- Gupta AK, Venkataraman M, Abramovits W, et al. JUBLIA (efinaconazole 10% solution) in the treatment of pediatric onychomycosis. Skinmed. 2021;19:206-210.
- Gupta AK, Paquet M. Systemic antifungals to treat onychomycosis in children: a systematic review. Pediatr Dermatol. 2013;30:294-302. doi:10.1111/pde.12048
- Leggit JC. Acute and chronic paronychia. Am Fam Physician. 2017;96:44-51.
- Lipner SR, Scher RK. Congenital malalignment of the great toenails with acute paronychia. Pediatr Dermatol. 2016;33:e288-e289.doi:10.1111/pde.12924
Nail disorders are common among pediatric patients but often are underdiagnosed or misdiagnosed because of their unique disease manifestations. These conditions may severely impact quality of life. There are few nail disease clinical trials that include children. Consequently, most treatment recommendations are based on case series and expert consensus recommendations. We review inflammatory and infectious nail disorders in pediatric patients. By describing characteristics, clinical manifestations, and management approaches for these conditions, we aim to provide guidance to dermatologists in their diagnosis and treatment.
INFLAMMATORY NAIL DISORDERS
Nail Psoriasis
Nail involvement in children with psoriasis is common, with prevalence estimates ranging from 17% to 39.2%.1 Nail matrix psoriasis may manifest with pitting (large irregular pits) and leukonychia as well as chromonychia and nail plate crumbling. Onycholysis, oil drop spots (salmon patches), and subungual hyperkeratosis can be seen in nail bed psoriasis. Nail pitting is the most frequently observed clinical finding (Figure 1).2,3 In a cross-sectional multicenter study of 313 children with cutaneous psoriasis in France, nail findings were present in 101 patients (32.3%). There were associations between nail findings and presence of psoriatic arthritis (P=.03), palmoplantar psoriasis (P<.001), and severity of psoriatic disease, defined as use of systemic treatment with phototherapy (psoralen plus UVA, UVB), traditional systemic treatment (acitretin, methotrexate, cyclosporine), or a biologic (P=.003).4
Topical steroids and vitamin D analogues may be used with or without occlusion and may be efficacious.5 Several case reports describe systemic treatments for psoriasis in children, including methotrexate, acitretin, and apremilast (approved for children 6 years and older for plaque psoriasis by the US Food and Drug Administration [FDA]).2 There are 5 biologic drugs currently approved for the treatment of pediatric psoriasis—adalimumab, etanercept, ustekinumab, secukinumab, ixekizumab—and 6 drugs currently undergoing phase 3 studies—brodalumab, guselkumab, risankizumab, tildrakizumab, certolizumab pegol, and deucravacitinib (Table 1).6-15 Adalimumab is specifically approved for moderate to severe nail psoriasis in adults 18 years and older.


Intralesional steroid injections are sometimes useful in the management of nail matrix psoriasis; however, appropriate patient selection is critical due to the pain associated with the procedure. In a prospective study of 16 children (age range, 9–17 years) with nail psoriasis treated with intralesional triamcinolone (ILTAC) 2.5 to 5 mg/mL every 4 to 8 weeks for a minimum of 3 to 6 months, 9 patients achieved resolution and 6 had improvement of clinical findings.16 Local adverse events were mild, including injection-site pain (66%), subungual hematoma (n=1), Beau lines (n=1), proximal nail fold hypopigmentation (n=2), and proximal nail fold atrophy (n=2). Because the proximal nail fold in children is thinner than in adults, there may be an increased risk for nail fold hypopigmentation and atrophy in children. Therefore, a maximum ILTAC concentration of 2.5 mg/mL with 0.2 mL maximum volume per nail per session is recommended for children younger than 15 years.16
Nail Lichen Planus
Nail lichen planus (NLP) is uncommon in children, with few biopsy-proven cases documented in the literature.17 Common clinical findings are onychorrhexis, nail plate thinning, fissuring, splitting, and atrophy with koilonychia.5 Although pterygium development (irreversible nail matrix scarring) is uncommon in pediatric patients, NLP can be progressive and may cause irreversible destruction of the nail matrix and subsequent nail loss, warranting therapeutic intervention.18
Treatment of NLP may be difficult, as there are no options that work in all patients. Current literature supports the use of systemic corticosteroids or ILTAC for the treatment of NLP; however, recurrence rates can be high. According to an expert consensus paper on NLP treatment, ILTAC may be injected in a concentration of 2.5, 5, or 10 mg/mL according to disease severity.19 In severe or resistant cases, intramuscular (IM) triamcinolone may be considered, especially if more than 3 nails are affected. A dosage of 0.5 to 1 mg/kg/mo for at least 3 to 6 months is recommended for both children and adults, with 1 mg/kg/mo recommended in the active treatment phase (first 2–3 months).19 In a retrospective review of 5 pediatric patients with NLP treated with IM triamcinolone 0.5 mg/kg/mo, 3 patients had resolution and 2 improved with treatment.20 In a prospective study of 10 children with NLP, IM triamcinolone at a dosage of 0.5 to 1 mg/kg every 30 days for 3 to 6 months resulted in resolution of nail findings in 9 patients.17 In a prospective study of 14 pediatric patients with NLP treated with 2.5 to 5 mg/mL of ILTAC, 10 achieved resolution and 3 improved.16
Intralesional triamcinolone injections may be better suited for teenagers compared to younger children who may be more apprehensive of needles. To minimize pain, it is recommended to inject ILTAC slowly at room temperature, with use of “talkesthesia” and vibration devices, 1% lidocaine, or ethyl chloride spray.18
Trachyonychia
Trachyonychia is characterized by the presence of sandpaperlike nails. It manifests with brittle thin nails with longitudinal ridging, onychoschizia, and thickened hyperkeratotic cuticles. Trachyonychia typically involves multiple nails, with a peak age of onset between 3 and 12 years.21,22 There are 2 variants: the opaque type with rough longitudinal ridging, and the shiny variant with opalescent nails and pits that reflect light. The opaque variant is more common and is associated with psoriasis, whereas the shiny variant is less common and is associated with alopecia areata.23 Although most cases are idiopathic, some are associated with psoriasis and alopecia areata, as previously noted, as well as atopic dermatitis (AD) and lichen planus.22,24
Fortunately, trachyonychia does not lead to permanent nail damage or pterygium, making treatment primarily focused on addressing functional and cosmetic concerns.24 Spontaneous resolution occurs in approximately 50% of patients. In a prospective study of 11 patients with idiopathic trachyonychia, there was partial improvement in 5 of 9 patients treated with topical steroids, 1 with only petrolatum, and 1 with vitamin supplements. Complete resolution was reported in 1 patient treated with topical steroids.25 Because trachyonychia often is self-resolving, no treatment is required and a conservative approach is strongly recommended.26 Treatment options include topical corticosteroids, tazarotene, and 5-fluorouracil. Intralesional triamcinolone, systemic cyclosporine, retinoids, systemic corticosteroids, and tofacitinib have been described in case reports, though none of these have been shown to be 100% efficacious.24
Nail Lichen Striatus
Lichen striatus involving the nail is uncommon and is characterized by onycholysis, longitudinal ridging, splitting, and fraying, as well as what appears to be a subungual tumor. It can encompass the entire nail or may be isolated to a portion of the nail (Figure 2). Usually, a Blaschko-linear array of flesh-colored papules on the more proximal digit directly adjacent to the nail dystrophy will be seen, though nail findings can occur in isolation.27-29 The underlying pathophysiology is not clear; however, one hypothesis is that a triggering event, such as trauma, induces the expression of antigens that elicit a self-limiting immune-mediated response by CD8 T lymphocytes.30

Generally, nail lichen striatus spontaneously resolves in 1 to 2 years without treatment. In a prospective study of 5 patients with nail lichen striatus, the median time to resolution was 22.6 months (range, 10–30 months).31 Topical steroids may be used for pruritus. In one case report, a 3-year-old boy with nail lichen striatus of 4 months’ duration was treated with tacrolimus ointment 0.03% daily for 3 months.28
Nail AD
Nail changes with AD may be more common in adults than children or are underreported. In a study of 777 adults with AD, nail dystrophy was present in 124 patients (16%), whereas in a study of 250 pediatric patients with AD (aged 0-2 years), nail dystrophy was present in only 4 patients.32,33
Periungual inflammation from AD causes the nail changes.34 In a cross-sectional study of 24 pediatric patients with nail dystrophy due to AD, transverse grooves (Beau lines) were present in 25% (6/24), nail pitting in 16.7% (4/24), koilonychia in 16.7% (4/24), trachyonychia in 12.5% (3/24), leukonychia in 12.5% (3/24), brachyonychia in 8.3% (2/24), melanonychia in 8.3% (2/24), onychomadesis in 8.3% (2/24), onychoschizia in 8.3% (2/24), and onycholysis in 8.3% (2/24). There was an association between disease severity and presence of toenail dystrophy (P=.03).35
Topical steroids with or without occlusion can be used to treat nail changes. Although there is limited literature describing the treatment of nail AD in children, a 61-year-old man with nail changes associated with AD achieved resolution with 3 months of treatment with dupilumab.36 Anecdotally, most patients will improve with usual cutaneous AD management.
INFECTIOUS NAIL DISORDERS
Viral Infections
Hand, Foot, and Mouth Disease—Hand, foot, and mouth disease (HFMD) is a common childhood viral infection caused by various enteroviruses, most commonly coxsackievirus A16, with the A6 variant causing more severe disease. Fever and painful vesicles involving the oral mucosa as well as palms and soles give the disease its name. Nail changes are common. In a prospective study involving 130 patients with laboratory-confirmed coxsackievirus CA6 serotype infection, 37% developed onychomadesis vs only 5% of 145 cases with non-CA6 enterovirus infection who developed nail findings. There was an association between CA6 infection and presence of nail changes (P<.001).37
Findings ranging from transverse grooves (Beau lines) to complete nail shedding (onychomadesis)(Figure 3) may be seen.38,39 Nail findings in HFMD are due to transient inhibition of nail growth and present approximately 3 to 6 weeks after infection.40 Onychomadesis is seen in 30% to 68% of patients with HFMD.37,41,42 Nail findings in HFMD spontaneously resolve with nail growth (2–3 mm per month for fingernails and 1 mm per month for toenails) and do not require specific treatment. Although the appearance of nail changes associated with HFMD can be disturbing, dermatologists can reassure children and their parents that the nails will resolve with the next cycle of growth.
Kawasaki Disease—Kawasaki disease (KD) is a vasculitis primarily affecting children and infants. Although the specific pathogen and pathophysiology is not entirely clear, clinical observations have suggested an infectious cause, most likely a virus.43 In Japan, more than 15,000 cases of KD are documented annually, while approximately 4200 cases are seen in the United States.44 In a prospective study from 1984 to 1990, 4 of 26 (15.4%) patients with KD presented with nail manifestations during the late acute phase or early convalescent phase of disease. There were no significant associations between nail dystrophy and severity of KD, such as coronary artery aneurysm.45
Nail changes reported in children with KD include onychomadesis, onycholysis, orange-brown chromonychia, splinter hemorrhages, Beau lines, and pincer nails. In a review of nail changes associated with KD from 1980 to 2021, orange-brown transverse chromonychia, which may evolve into transverse leukonychia, was the most common nail finding reported, occurring in 17 of 31 (54.8%) patients.44 It has been hypothesized that nail changes may result from blood flow disturbance due to the underlying vasculitis.46 Nail changes appear several weeks after the onset of fever and are self-limited. Resolution occurs with nail growth, with no treatment required.

FUNGAL INFECTIONS
Onychomycosis
Onychomycosis is a fungal infection of the nails that occurs in 0.2% to 5.5% of pediatric patients, and its prevalence may be increasing, which may be due to environmental factors or increased rates of diabetes mellitus and obesity in the pediatric population.47 Onychomycosis represents 15.5% of nail dystrophies in pediatric patients.48 Some dermatologists treat presumptive onychomycosis without confirmation; however, we do not recommend that approach. Because the differential is broad and the duration of treatment is long, mycologic examination (potassium hydroxide preparation, fungal culture, polymerase chain reaction, and/or histopathology) should be obtained to confirm onychomycosis prior to initiation of antifungal management. Family members of affected individuals should be evaluated and treated, if indicated, for onychomycosis and tinea pedis, as household transmission is common.
Currently, there are 2 topical FDA-approved treatments for pediatric onychomycosis in children 6 years and older (Table 2).49,50 There is a discussion of the need for confirmatory testing for onychomycosis in children, particularly when systemic treatment is prescribed. In a retrospective review of 269 pediatric patients with onychomycosis prescribed terbinafine, 53.5% (n=144) underwent laboratory monitoring of liver function and complete blood cell counts, and 12.5% had grade 1 laboratory abnormalities either prior to (12/144 [8.3%]) or during (6/144 [4.2%]) therapy.51 Baseline transaminase monitoring is recommended, though subsequent routine laboratory monitoring in healthy children may have limited utility with associated increased costs, incidental findings, and patient discomfort and likely is not needed.51
Pediatric onychomycosis responds better to topical therapy than adult disease, and pediatric patients do not always require systemic treatment.52 Ciclopirox is not FDA approved for the treatment of pediatric onychomycosis, but in a 32-week clinical trial of ciclopirox lacquer 8% use in 40 patients, 77% (27/35) of treated patients achieved mycologic cure. Overall, 71% of treated patients (25/35) vs 22% (2/9) of controls achieved efficacy (defined as investigator global assessment score of 2 or lower).52 In an open-label, single-arm clinical trial assessing tavaborole solution 5% applied once daily for 48 weeks for the treatment of toenail onychomycosis in pediatric patients (aged 6–17 years), 36.2% (20/55) of patients achieved mycologic cure, and 8.5% (5/55) achieved complete cure at week 52 with mild or minimal adverse effects.53 In an open-label, phase 4 study of the safety and efficacy of efinaconazole solution 10% applied once daily for 48 weeks in pediatric patients (aged 6 to 16 years) (n=60), 65% (35/60) achieved mycologic cure, 42% (25/60) achieved clinical cure, and 40% (24/60) achieved complete cure at 52 weeks. The most common adverse effects of efinaconazole were local and included ingrown toenail (1/60), application-site dermatitis (1/60), application-site vesicles (1/60), and application-site pain (1/60).54
In a systematic review of systemic antifungals for onychomycosis in 151 pediatric patients, itraconazole, fluconazole, griseofulvin, and terbinafine resulted in complete cure rates similar to those of the adult population, with excellent safety profiles.55 Depending on the situation, initiation of treatment with topical medications followed by addition of systemic antifungal agents only if needed may be an appropriate course of action.
BACTERIAL INFECTIONS
Acute Paronychia
Acute paronychia is a nail-fold infection that develops after the protective nail barrier has been compromised.56 In children, thumb-sucking, nail-biting, frequent oral manipulation of the digits, and poor skin hygiene are risk factors. Acute paronychia also may develop in association with congenital malalignment of the great toenails.57
Clinical manifestations include localized pain, erythema, and nail fold edema (Figure 4). Purulent material and abscess formation may ensue. Staphylococcus aureus as well as methicillin-resistant S aureus and Streptococcus pyogenes are classically the most common causes of acute paronychia. Treatment of paronychia is based on severity. In mild cases, warm soaks with topical antibiotics are indicated. Oral antibiotics should be prescribed for more severe presentations. If there is no improvement after 48 hours, surgical drainage is required to facilitate healing.56
FINAL THOUGHTS
Inflammatory and infectious nail disorders in children are relatively common and may impact the physical and emotional well-being of young patients. By understanding the distinctive features of these nail disorders in pediatric patients, dermatologists can provide anticipatory guidance and informed treatment options to children and their parents. Further research is needed to expand our understanding of pediatric nail disorders and create targeted therapeutic interventions, particularly for NLP and psoriasis.


Nail disorders are common among pediatric patients but often are underdiagnosed or misdiagnosed because of their unique disease manifestations. These conditions may severely impact quality of life. There are few nail disease clinical trials that include children. Consequently, most treatment recommendations are based on case series and expert consensus recommendations. We review inflammatory and infectious nail disorders in pediatric patients. By describing characteristics, clinical manifestations, and management approaches for these conditions, we aim to provide guidance to dermatologists in their diagnosis and treatment.
INFLAMMATORY NAIL DISORDERS
Nail Psoriasis
Nail involvement in children with psoriasis is common, with prevalence estimates ranging from 17% to 39.2%.1 Nail matrix psoriasis may manifest with pitting (large irregular pits) and leukonychia as well as chromonychia and nail plate crumbling. Onycholysis, oil drop spots (salmon patches), and subungual hyperkeratosis can be seen in nail bed psoriasis. Nail pitting is the most frequently observed clinical finding (Figure 1).2,3 In a cross-sectional multicenter study of 313 children with cutaneous psoriasis in France, nail findings were present in 101 patients (32.3%). There were associations between nail findings and presence of psoriatic arthritis (P=.03), palmoplantar psoriasis (P<.001), and severity of psoriatic disease, defined as use of systemic treatment with phototherapy (psoralen plus UVA, UVB), traditional systemic treatment (acitretin, methotrexate, cyclosporine), or a biologic (P=.003).4
Topical steroids and vitamin D analogues may be used with or without occlusion and may be efficacious.5 Several case reports describe systemic treatments for psoriasis in children, including methotrexate, acitretin, and apremilast (approved for children 6 years and older for plaque psoriasis by the US Food and Drug Administration [FDA]).2 There are 5 biologic drugs currently approved for the treatment of pediatric psoriasis—adalimumab, etanercept, ustekinumab, secukinumab, ixekizumab—and 6 drugs currently undergoing phase 3 studies—brodalumab, guselkumab, risankizumab, tildrakizumab, certolizumab pegol, and deucravacitinib (Table 1).6-15 Adalimumab is specifically approved for moderate to severe nail psoriasis in adults 18 years and older.


Intralesional steroid injections are sometimes useful in the management of nail matrix psoriasis; however, appropriate patient selection is critical due to the pain associated with the procedure. In a prospective study of 16 children (age range, 9–17 years) with nail psoriasis treated with intralesional triamcinolone (ILTAC) 2.5 to 5 mg/mL every 4 to 8 weeks for a minimum of 3 to 6 months, 9 patients achieved resolution and 6 had improvement of clinical findings.16 Local adverse events were mild, including injection-site pain (66%), subungual hematoma (n=1), Beau lines (n=1), proximal nail fold hypopigmentation (n=2), and proximal nail fold atrophy (n=2). Because the proximal nail fold in children is thinner than in adults, there may be an increased risk for nail fold hypopigmentation and atrophy in children. Therefore, a maximum ILTAC concentration of 2.5 mg/mL with 0.2 mL maximum volume per nail per session is recommended for children younger than 15 years.16
Nail Lichen Planus
Nail lichen planus (NLP) is uncommon in children, with few biopsy-proven cases documented in the literature.17 Common clinical findings are onychorrhexis, nail plate thinning, fissuring, splitting, and atrophy with koilonychia.5 Although pterygium development (irreversible nail matrix scarring) is uncommon in pediatric patients, NLP can be progressive and may cause irreversible destruction of the nail matrix and subsequent nail loss, warranting therapeutic intervention.18
Treatment of NLP may be difficult, as there are no options that work in all patients. Current literature supports the use of systemic corticosteroids or ILTAC for the treatment of NLP; however, recurrence rates can be high. According to an expert consensus paper on NLP treatment, ILTAC may be injected in a concentration of 2.5, 5, or 10 mg/mL according to disease severity.19 In severe or resistant cases, intramuscular (IM) triamcinolone may be considered, especially if more than 3 nails are affected. A dosage of 0.5 to 1 mg/kg/mo for at least 3 to 6 months is recommended for both children and adults, with 1 mg/kg/mo recommended in the active treatment phase (first 2–3 months).19 In a retrospective review of 5 pediatric patients with NLP treated with IM triamcinolone 0.5 mg/kg/mo, 3 patients had resolution and 2 improved with treatment.20 In a prospective study of 10 children with NLP, IM triamcinolone at a dosage of 0.5 to 1 mg/kg every 30 days for 3 to 6 months resulted in resolution of nail findings in 9 patients.17 In a prospective study of 14 pediatric patients with NLP treated with 2.5 to 5 mg/mL of ILTAC, 10 achieved resolution and 3 improved.16
Intralesional triamcinolone injections may be better suited for teenagers compared to younger children who may be more apprehensive of needles. To minimize pain, it is recommended to inject ILTAC slowly at room temperature, with use of “talkesthesia” and vibration devices, 1% lidocaine, or ethyl chloride spray.18
Trachyonychia
Trachyonychia is characterized by the presence of sandpaperlike nails. It manifests with brittle thin nails with longitudinal ridging, onychoschizia, and thickened hyperkeratotic cuticles. Trachyonychia typically involves multiple nails, with a peak age of onset between 3 and 12 years.21,22 There are 2 variants: the opaque type with rough longitudinal ridging, and the shiny variant with opalescent nails and pits that reflect light. The opaque variant is more common and is associated with psoriasis, whereas the shiny variant is less common and is associated with alopecia areata.23 Although most cases are idiopathic, some are associated with psoriasis and alopecia areata, as previously noted, as well as atopic dermatitis (AD) and lichen planus.22,24
Fortunately, trachyonychia does not lead to permanent nail damage or pterygium, making treatment primarily focused on addressing functional and cosmetic concerns.24 Spontaneous resolution occurs in approximately 50% of patients. In a prospective study of 11 patients with idiopathic trachyonychia, there was partial improvement in 5 of 9 patients treated with topical steroids, 1 with only petrolatum, and 1 with vitamin supplements. Complete resolution was reported in 1 patient treated with topical steroids.25 Because trachyonychia often is self-resolving, no treatment is required and a conservative approach is strongly recommended.26 Treatment options include topical corticosteroids, tazarotene, and 5-fluorouracil. Intralesional triamcinolone, systemic cyclosporine, retinoids, systemic corticosteroids, and tofacitinib have been described in case reports, though none of these have been shown to be 100% efficacious.24
Nail Lichen Striatus
Lichen striatus involving the nail is uncommon and is characterized by onycholysis, longitudinal ridging, splitting, and fraying, as well as what appears to be a subungual tumor. It can encompass the entire nail or may be isolated to a portion of the nail (Figure 2). Usually, a Blaschko-linear array of flesh-colored papules on the more proximal digit directly adjacent to the nail dystrophy will be seen, though nail findings can occur in isolation.27-29 The underlying pathophysiology is not clear; however, one hypothesis is that a triggering event, such as trauma, induces the expression of antigens that elicit a self-limiting immune-mediated response by CD8 T lymphocytes.30

Generally, nail lichen striatus spontaneously resolves in 1 to 2 years without treatment. In a prospective study of 5 patients with nail lichen striatus, the median time to resolution was 22.6 months (range, 10–30 months).31 Topical steroids may be used for pruritus. In one case report, a 3-year-old boy with nail lichen striatus of 4 months’ duration was treated with tacrolimus ointment 0.03% daily for 3 months.28
Nail AD
Nail changes with AD may be more common in adults than children or are underreported. In a study of 777 adults with AD, nail dystrophy was present in 124 patients (16%), whereas in a study of 250 pediatric patients with AD (aged 0-2 years), nail dystrophy was present in only 4 patients.32,33
Periungual inflammation from AD causes the nail changes.34 In a cross-sectional study of 24 pediatric patients with nail dystrophy due to AD, transverse grooves (Beau lines) were present in 25% (6/24), nail pitting in 16.7% (4/24), koilonychia in 16.7% (4/24), trachyonychia in 12.5% (3/24), leukonychia in 12.5% (3/24), brachyonychia in 8.3% (2/24), melanonychia in 8.3% (2/24), onychomadesis in 8.3% (2/24), onychoschizia in 8.3% (2/24), and onycholysis in 8.3% (2/24). There was an association between disease severity and presence of toenail dystrophy (P=.03).35
Topical steroids with or without occlusion can be used to treat nail changes. Although there is limited literature describing the treatment of nail AD in children, a 61-year-old man with nail changes associated with AD achieved resolution with 3 months of treatment with dupilumab.36 Anecdotally, most patients will improve with usual cutaneous AD management.
INFECTIOUS NAIL DISORDERS
Viral Infections
Hand, Foot, and Mouth Disease—Hand, foot, and mouth disease (HFMD) is a common childhood viral infection caused by various enteroviruses, most commonly coxsackievirus A16, with the A6 variant causing more severe disease. Fever and painful vesicles involving the oral mucosa as well as palms and soles give the disease its name. Nail changes are common. In a prospective study involving 130 patients with laboratory-confirmed coxsackievirus CA6 serotype infection, 37% developed onychomadesis vs only 5% of 145 cases with non-CA6 enterovirus infection who developed nail findings. There was an association between CA6 infection and presence of nail changes (P<.001).37
Findings ranging from transverse grooves (Beau lines) to complete nail shedding (onychomadesis)(Figure 3) may be seen.38,39 Nail findings in HFMD are due to transient inhibition of nail growth and present approximately 3 to 6 weeks after infection.40 Onychomadesis is seen in 30% to 68% of patients with HFMD.37,41,42 Nail findings in HFMD spontaneously resolve with nail growth (2–3 mm per month for fingernails and 1 mm per month for toenails) and do not require specific treatment. Although the appearance of nail changes associated with HFMD can be disturbing, dermatologists can reassure children and their parents that the nails will resolve with the next cycle of growth.
Kawasaki Disease—Kawasaki disease (KD) is a vasculitis primarily affecting children and infants. Although the specific pathogen and pathophysiology is not entirely clear, clinical observations have suggested an infectious cause, most likely a virus.43 In Japan, more than 15,000 cases of KD are documented annually, while approximately 4200 cases are seen in the United States.44 In a prospective study from 1984 to 1990, 4 of 26 (15.4%) patients with KD presented with nail manifestations during the late acute phase or early convalescent phase of disease. There were no significant associations between nail dystrophy and severity of KD, such as coronary artery aneurysm.45
Nail changes reported in children with KD include onychomadesis, onycholysis, orange-brown chromonychia, splinter hemorrhages, Beau lines, and pincer nails. In a review of nail changes associated with KD from 1980 to 2021, orange-brown transverse chromonychia, which may evolve into transverse leukonychia, was the most common nail finding reported, occurring in 17 of 31 (54.8%) patients.44 It has been hypothesized that nail changes may result from blood flow disturbance due to the underlying vasculitis.46 Nail changes appear several weeks after the onset of fever and are self-limited. Resolution occurs with nail growth, with no treatment required.

FUNGAL INFECTIONS
Onychomycosis
Onychomycosis is a fungal infection of the nails that occurs in 0.2% to 5.5% of pediatric patients, and its prevalence may be increasing, which may be due to environmental factors or increased rates of diabetes mellitus and obesity in the pediatric population.47 Onychomycosis represents 15.5% of nail dystrophies in pediatric patients.48 Some dermatologists treat presumptive onychomycosis without confirmation; however, we do not recommend that approach. Because the differential is broad and the duration of treatment is long, mycologic examination (potassium hydroxide preparation, fungal culture, polymerase chain reaction, and/or histopathology) should be obtained to confirm onychomycosis prior to initiation of antifungal management. Family members of affected individuals should be evaluated and treated, if indicated, for onychomycosis and tinea pedis, as household transmission is common.
Currently, there are 2 topical FDA-approved treatments for pediatric onychomycosis in children 6 years and older (Table 2).49,50 There is a discussion of the need for confirmatory testing for onychomycosis in children, particularly when systemic treatment is prescribed. In a retrospective review of 269 pediatric patients with onychomycosis prescribed terbinafine, 53.5% (n=144) underwent laboratory monitoring of liver function and complete blood cell counts, and 12.5% had grade 1 laboratory abnormalities either prior to (12/144 [8.3%]) or during (6/144 [4.2%]) therapy.51 Baseline transaminase monitoring is recommended, though subsequent routine laboratory monitoring in healthy children may have limited utility with associated increased costs, incidental findings, and patient discomfort and likely is not needed.51
Pediatric onychomycosis responds better to topical therapy than adult disease, and pediatric patients do not always require systemic treatment.52 Ciclopirox is not FDA approved for the treatment of pediatric onychomycosis, but in a 32-week clinical trial of ciclopirox lacquer 8% use in 40 patients, 77% (27/35) of treated patients achieved mycologic cure. Overall, 71% of treated patients (25/35) vs 22% (2/9) of controls achieved efficacy (defined as investigator global assessment score of 2 or lower).52 In an open-label, single-arm clinical trial assessing tavaborole solution 5% applied once daily for 48 weeks for the treatment of toenail onychomycosis in pediatric patients (aged 6–17 years), 36.2% (20/55) of patients achieved mycologic cure, and 8.5% (5/55) achieved complete cure at week 52 with mild or minimal adverse effects.53 In an open-label, phase 4 study of the safety and efficacy of efinaconazole solution 10% applied once daily for 48 weeks in pediatric patients (aged 6 to 16 years) (n=60), 65% (35/60) achieved mycologic cure, 42% (25/60) achieved clinical cure, and 40% (24/60) achieved complete cure at 52 weeks. The most common adverse effects of efinaconazole were local and included ingrown toenail (1/60), application-site dermatitis (1/60), application-site vesicles (1/60), and application-site pain (1/60).54
In a systematic review of systemic antifungals for onychomycosis in 151 pediatric patients, itraconazole, fluconazole, griseofulvin, and terbinafine resulted in complete cure rates similar to those of the adult population, with excellent safety profiles.55 Depending on the situation, initiation of treatment with topical medications followed by addition of systemic antifungal agents only if needed may be an appropriate course of action.
BACTERIAL INFECTIONS
Acute Paronychia
Acute paronychia is a nail-fold infection that develops after the protective nail barrier has been compromised.56 In children, thumb-sucking, nail-biting, frequent oral manipulation of the digits, and poor skin hygiene are risk factors. Acute paronychia also may develop in association with congenital malalignment of the great toenails.57
Clinical manifestations include localized pain, erythema, and nail fold edema (Figure 4). Purulent material and abscess formation may ensue. Staphylococcus aureus as well as methicillin-resistant S aureus and Streptococcus pyogenes are classically the most common causes of acute paronychia. Treatment of paronychia is based on severity. In mild cases, warm soaks with topical antibiotics are indicated. Oral antibiotics should be prescribed for more severe presentations. If there is no improvement after 48 hours, surgical drainage is required to facilitate healing.56
FINAL THOUGHTS
Inflammatory and infectious nail disorders in children are relatively common and may impact the physical and emotional well-being of young patients. By understanding the distinctive features of these nail disorders in pediatric patients, dermatologists can provide anticipatory guidance and informed treatment options to children and their parents. Further research is needed to expand our understanding of pediatric nail disorders and create targeted therapeutic interventions, particularly for NLP and psoriasis.


- Uber M, Carvalho VO, Abagge KT, et al. Clinical features and nail clippings in 52 children with psoriasis. Pediatr Dermatol. 2018;35:202-207. doi:10.1111/pde.13402
- Plachouri KM, Mulita F, Georgiou S. Management of pediatric nail psoriasis. Cutis. 2021;108:292-294. doi:10.12788/cutis.0386
- Smith RJ, Rubin AI. Pediatric nail disorders: a review. Curr Opin Pediatr. 2020;32:506-515. doi:10.1097/mop.0000000000000921
- Pourchot D, Bodemer C, Phan A, et al. Nail psoriasis: a systematic evaluation in 313 children with psoriasis. Pediatr Dermatol. 2017;34:58-63. doi:10.1111/pde.13028
- Richert B, André J. Nail disorders in children: diagnosis and management. Am J Clin Dermatol. 2011;12:101-112. doi:10.2165/11537110-000000000-00000
- Lee JYY. Severe 20-nail psoriasis successfully treated by low dose methotrexate. Dermatol Online J. 2009;15:8.
- Nogueira M, Paller AS, Torres T. Targeted therapy for pediatric psoriasis. Paediatr Drugs. May 2021;23:203-212. doi:10.1007/s40272-021-00443-5
- Hanoodi M, Mittal M. Methotrexate. StatPearls [Internet]. Updated August 16, 2023. Accessed July 1, 2024. https://www.ncbi.nlm.nih.gov/books/NBK556114/
- Teran CG, Teran-Escalera CN, Balderrama C. A severe case of erythrodermic psoriasis associated with advanced nail and joint manifestations: a case report. J Med Case Rep. 2010;4:179. doi:10.1186/1752-1947-4-179
- Paller AS, Seyger MMB, Magariños GA, et al. Long-term efficacy and safety of up to 108 weeks of ixekizumab in pediatric patients with moderate to severe plaque psoriasis: the IXORA-PEDS randomized clinical trial. JAMA Dermatol. 2022;158:533-541. doi:10.1001/jamadermatol.2022.0655
- Diotallevi F, Simonetti O, Rizzetto G, et al. Biological treatments for pediatric psoriasis: state of the art and future perspectives. Int J Mol Sci. 2022;23:11128. doi:10.3390/ijms231911128
- Nash P, Mease PJ, Kirkham B, et al. Secukinumab provides sustained improvement in nail psoriasis, signs and symptoms of psoriatic arthritis and low rate of radiographic progression in patients with concomitant nail involvement: 2-year results from the Phase III FUTURE 5 study. Clin Exp Rheumatol. 2022;40:952-959. doi:10.55563/clinexprheumatol/3nuz51
- Wells LE, Evans T, Hilton R, et al. Use of secukinumab in a pediatric patient leads to significant improvement in nail psoriasis and psoriatic arthritis. Pediatr Dermatol. 2019;36:384-385. doi:10.1111/pde.13767
- Watabe D, Endoh K, Maeda F, et al. Childhood-onset psoriaticonycho-pachydermo-periostitis treated successfully with infliximab. Eur J Dermatol. 2015;25:506-508. doi:10.1684/ejd.2015.2616
- Pereira TM, Vieira AP, Fernandes JC, et al. Anti-TNF-alpha therapy in childhood pustular psoriasis. Dermatology. 2006;213:350-352. doi:10.1159/000096202
- Iorizzo M, Gioia Di Chiacchio N, Di Chiacchio N, et al. Intralesional steroid injections for inflammatory nail dystrophies in the pediatric population. Pediatr Dermatol. 2023;40:759-761. doi:10.1111/pde.15295
- Tosti A, Piraccini BM, Cambiaghi S, et al. Nail lichen planus in children: clinical features, response to treatment, and long-term follow-up. Arch Dermatol. 2001;137:1027-1032.
- Lipner SR. Nail lichen planus: a true nail emergency. J Am Acad Dermatol. 2019;80:e177-e178. doi:10.1016/j.jaad.2018.11.065
- Iorizzo M, Tosti A, Starace M, et al. Isolated nail lichen planus: an expert consensus on treatment of the classical form. J Am Acad Dermatol. 2020;83:1717-1723. doi:10.1016/j.jaad.2020.02.056
- Piraccini BM, Saccani E, Starace M, et al. Nail lichen planus: response to treatment and long term follow-up. Eur J Dermatol. 2010;20:489-496. doi:10.1684/ejd.2010.0952
- Mahajan R, Kaushik A, De D, et al. Pediatric trachyonychia- a retrospective study of 17 cases. Indian J Dermatol. 2021;66:689-690. doi:10.4103/ijd.ijd_42_21
- Leung AKC, Leong KF, Barankin B. Trachyonychia. J Pediatr. 2020;216:239-239.e1. doi:10.1016/j.jpeds.2019.08.034
- Haber JS, Chairatchaneeboon M, Rubin AI. Trachyonychia: review and update on clinical aspects, histology, and therapy. Skin Appendage Disord. 2017;2:109-115. doi:10.1159/000449063
- Jacobsen AA, Tosti A. Trachyonychia and twenty-nail dystrophy: a comprehensive review and discussion of diagnostic accuracy. Skin Appendage Disord. 2016;2:7-13. doi:10.1159/000445544
- Kumar MG, Ciliberto H, Bayliss SJ. Long-term follow-up of pediatric trachyonychia. Pediatr Dermatol. 2015;32:198-200. doi:10.1111/pde.12427
- Tosti A, Piraccini BM, Iorizzo M. Trachyonychia and related disorders: evaluation and treatment plans. Dermatolog Ther. 2002;15:121-125. doi:10.1046/j.1529-8019.2002.01511.x
- Leung AKC, Leong KF, Barankin B. Lichen striatus with nail involvement in a 6-year-old boy. Case Rep Pediatr. 2020;2020:1494760. doi:10.1155/2020/1494760
- Kim GW, Kim SH, Seo SH, et al. Lichen striatus with nail abnormality successfully treated with tacrolimus ointment. J Dermatol. 2009;36:616-617. doi:10.1111/j.1346-8138.2009.00720.x
- Iorizzo M, Rubin AI, Starace M. Nail lichen striatus: is dermoscopy useful for the diagnosis? Pediatr Dermatol. 2019;36:859-863. doi:10.1111/pde.13916
- Karp DL, Cohen BA. Onychodystrophy in lichen striatus. Pediatr Dermatol. 1993;10:359-361. doi:10.1111/j.1525-1470.1993.tb00399.x
- Tosti A, Peluso AM, Misciali C, et al. Nail lichen striatus: clinical features and long-term follow-up of five patients. J Am Acad Dermatol. 1997;36(6, pt 1):908-913. doi:10.1016/s0190-9622(97)80270-8
- Simpson EL, Thompson MM, Hanifin JM. Prevalence and morphology of hand eczema in patients with atopic dermatitis. Dermatitis. 2006;17:123-127. doi:10.2310/6620.2006.06005
- Sarifakioglu E, Yilmaz AE, Gorpelioglu C. Nail alterations in 250 infant patients: a clinical study. J Eur Acad Dermatol Venereol. 2008;22:741-744. doi:10.1111/j.1468-3083.2008.02592.x
- Milanesi N, D’Erme AM, Gola M. Nail improvement during alitretinoin treatment: three case reports and review of the literature. Clin Exp Dermatol. 2015;40:533-536. doi:10.1111/ced.12584
- Chung BY, Choi YW, Kim HO, et al. Nail dystrophy in patients with atopic dermatitis and its association with disease severity. Ann Dermatol. 2019;31:121-126. doi:10.5021/ad.2019.31.2.121
- Navarro-Triviño FJ, Vega-Castillo JJ, Ruiz-Villaverde R. Nail changes successfully treated with dupilumab in a patient with severe atopic dermatitis. Australas J Dermatol. 2021;62:e468-e469. doi:10.1111/ajd.13633
- Wei SH, Huang YP, Liu MC, et al. An outbreak of coxsackievirus A6 hand, foot, and mouth disease associated with onychomadesis in Taiwan, 2010. BMC Infect Dis. 2011;11:346. doi:10.1186/1471-2334-11-346
- Shin JY, Cho BK, Park HJ. A clinical study of nail changes occurring secondary to hand-foot-mouth disease: onychomadesis and Beau’s lines. Ann Dermatol. 2014;26:280-283. doi:10.5021/ad.2014.26.2.280
- Verma S, Singal A. Nail changes in hand-foot-and-mouth disease (HFMD). Indian Dermatol Online J. 2021;12:656-657. doi:10.4103 /idoj.IDOJ_271_20
- Giordano LMC, de la Fuente LA, Lorca JMB, et al. Onychomadesis secondary to hand-foot-mouth disease: a frequent manifestation and cause of concern for parents. Article in Spanish. Rev Chil Pediatr. 2018;89:380-383. doi:10.4067/s0370-41062018005000203
- Justino MCA, da SMD, Souza MF, et al. Atypical hand-foot-mouth disease in Belém, Amazon region, northern Brazil, with detection of coxsackievirus A6. J Clin Virol. 2020;126:104307. doi:10.1016/j.jcv.2020.104307
- Cheng FF, Zhang BB, Cao ML, et al. Clinical characteristics of 68 children with atypical hand, foot, and mouth disease caused by coxsackievirus A6: a single-center retrospective analysis. Transl Pediatr. 2022;11:1502-1509. doi:10.21037/tp-22-352
- Nagata S. Causes of Kawasaki disease-from past to present. Front Pediatr. 2019;7:18. doi:10.3389/fped.2019.00018
- Mitsuishi T, Miyata K, Ando A, et al. Characteristic nail lesions in Kawasaki disease: case series and literature review. J Dermatol. 2022;49:232-238. doi:10.1111/1346-8138.16276
- Lindsley CB. Nail-bed lines in Kawasaki disease. Am J Dis Child. 1992;146:659-660. doi:10.1001/archpedi.1992.02160180017005
- Matsumura O, Nakagishi Y. Pincer nails upon convalescence from Kawasaki disease. J Pediatr. 2022;246:279. doi:10.1016/j.jpeds.2022.03.002
- Solís-Arias MP, García-Romero MT. Onychomycosis in children. a review. Int J Dermatol. 2017;56:123-130. doi:10.1111/ijd.13392
- Gupta AK, Mays RR, Versteeg SG, et al. Onychomycosis in children: safety and efficacy of antifungal agents. Pediatr Dermatol. 2018;35:552-559. doi:10.1111/pde.13561
- 49. Gupta AK, Venkataraman M, Shear NH, et al. Labeled use of efinaconazole topical solution 10% in treating onychomycosis in children and a review of the management of pediatric onychomycosis. Dermatol Ther. 2020;33:e13613. doi:10.1111/dth.13613
- Falotico JM, Lipner SR. Updated perspectives on the diagnosis and management of onychomycosis. Clin Cosmet Investig Dermatol. 2022;15:1933-1957. doi:10.2147/ccid.S362635
- Patel D, Castelo-Soccio LA, Rubin AI, et al. Laboratory monitoring during systemic terbinafine therapy for pediatric onychomycosis. JAMA Dermatol. 2017;153:1326-1327. doi:10.1001/jamadermatol.2017.4483
- Friedlander SF, Chan YC, Chan YH, et al. Onychomycosis does not always require systemic treatment for cure: a trial using topical therapy. Pediatr Dermatol. 2013;30:316-322. doi:10.1111/pde.12064
- Rich P, Spellman M, Purohit V, et al. Tavaborole 5% topical solution for the treatment of toenail onychomycosis in pediatric patients: results from a phase 4 open-label study. J Drugs Dermatol. 2019;18:190-195.
- Gupta AK, Venkataraman M, Abramovits W, et al. JUBLIA (efinaconazole 10% solution) in the treatment of pediatric onychomycosis. Skinmed. 2021;19:206-210.
- Gupta AK, Paquet M. Systemic antifungals to treat onychomycosis in children: a systematic review. Pediatr Dermatol. 2013;30:294-302. doi:10.1111/pde.12048
- Leggit JC. Acute and chronic paronychia. Am Fam Physician. 2017;96:44-51.
- Lipner SR, Scher RK. Congenital malalignment of the great toenails with acute paronychia. Pediatr Dermatol. 2016;33:e288-e289.doi:10.1111/pde.12924
- Uber M, Carvalho VO, Abagge KT, et al. Clinical features and nail clippings in 52 children with psoriasis. Pediatr Dermatol. 2018;35:202-207. doi:10.1111/pde.13402
- Plachouri KM, Mulita F, Georgiou S. Management of pediatric nail psoriasis. Cutis. 2021;108:292-294. doi:10.12788/cutis.0386
- Smith RJ, Rubin AI. Pediatric nail disorders: a review. Curr Opin Pediatr. 2020;32:506-515. doi:10.1097/mop.0000000000000921
- Pourchot D, Bodemer C, Phan A, et al. Nail psoriasis: a systematic evaluation in 313 children with psoriasis. Pediatr Dermatol. 2017;34:58-63. doi:10.1111/pde.13028
- Richert B, André J. Nail disorders in children: diagnosis and management. Am J Clin Dermatol. 2011;12:101-112. doi:10.2165/11537110-000000000-00000
- Lee JYY. Severe 20-nail psoriasis successfully treated by low dose methotrexate. Dermatol Online J. 2009;15:8.
- Nogueira M, Paller AS, Torres T. Targeted therapy for pediatric psoriasis. Paediatr Drugs. May 2021;23:203-212. doi:10.1007/s40272-021-00443-5
- Hanoodi M, Mittal M. Methotrexate. StatPearls [Internet]. Updated August 16, 2023. Accessed July 1, 2024. https://www.ncbi.nlm.nih.gov/books/NBK556114/
- Teran CG, Teran-Escalera CN, Balderrama C. A severe case of erythrodermic psoriasis associated with advanced nail and joint manifestations: a case report. J Med Case Rep. 2010;4:179. doi:10.1186/1752-1947-4-179
- Paller AS, Seyger MMB, Magariños GA, et al. Long-term efficacy and safety of up to 108 weeks of ixekizumab in pediatric patients with moderate to severe plaque psoriasis: the IXORA-PEDS randomized clinical trial. JAMA Dermatol. 2022;158:533-541. doi:10.1001/jamadermatol.2022.0655
- Diotallevi F, Simonetti O, Rizzetto G, et al. Biological treatments for pediatric psoriasis: state of the art and future perspectives. Int J Mol Sci. 2022;23:11128. doi:10.3390/ijms231911128
- Nash P, Mease PJ, Kirkham B, et al. Secukinumab provides sustained improvement in nail psoriasis, signs and symptoms of psoriatic arthritis and low rate of radiographic progression in patients with concomitant nail involvement: 2-year results from the Phase III FUTURE 5 study. Clin Exp Rheumatol. 2022;40:952-959. doi:10.55563/clinexprheumatol/3nuz51
- Wells LE, Evans T, Hilton R, et al. Use of secukinumab in a pediatric patient leads to significant improvement in nail psoriasis and psoriatic arthritis. Pediatr Dermatol. 2019;36:384-385. doi:10.1111/pde.13767
- Watabe D, Endoh K, Maeda F, et al. Childhood-onset psoriaticonycho-pachydermo-periostitis treated successfully with infliximab. Eur J Dermatol. 2015;25:506-508. doi:10.1684/ejd.2015.2616
- Pereira TM, Vieira AP, Fernandes JC, et al. Anti-TNF-alpha therapy in childhood pustular psoriasis. Dermatology. 2006;213:350-352. doi:10.1159/000096202
- Iorizzo M, Gioia Di Chiacchio N, Di Chiacchio N, et al. Intralesional steroid injections for inflammatory nail dystrophies in the pediatric population. Pediatr Dermatol. 2023;40:759-761. doi:10.1111/pde.15295
- Tosti A, Piraccini BM, Cambiaghi S, et al. Nail lichen planus in children: clinical features, response to treatment, and long-term follow-up. Arch Dermatol. 2001;137:1027-1032.
- Lipner SR. Nail lichen planus: a true nail emergency. J Am Acad Dermatol. 2019;80:e177-e178. doi:10.1016/j.jaad.2018.11.065
- Iorizzo M, Tosti A, Starace M, et al. Isolated nail lichen planus: an expert consensus on treatment of the classical form. J Am Acad Dermatol. 2020;83:1717-1723. doi:10.1016/j.jaad.2020.02.056
- Piraccini BM, Saccani E, Starace M, et al. Nail lichen planus: response to treatment and long term follow-up. Eur J Dermatol. 2010;20:489-496. doi:10.1684/ejd.2010.0952
- Mahajan R, Kaushik A, De D, et al. Pediatric trachyonychia- a retrospective study of 17 cases. Indian J Dermatol. 2021;66:689-690. doi:10.4103/ijd.ijd_42_21
- Leung AKC, Leong KF, Barankin B. Trachyonychia. J Pediatr. 2020;216:239-239.e1. doi:10.1016/j.jpeds.2019.08.034
- Haber JS, Chairatchaneeboon M, Rubin AI. Trachyonychia: review and update on clinical aspects, histology, and therapy. Skin Appendage Disord. 2017;2:109-115. doi:10.1159/000449063
- Jacobsen AA, Tosti A. Trachyonychia and twenty-nail dystrophy: a comprehensive review and discussion of diagnostic accuracy. Skin Appendage Disord. 2016;2:7-13. doi:10.1159/000445544
- Kumar MG, Ciliberto H, Bayliss SJ. Long-term follow-up of pediatric trachyonychia. Pediatr Dermatol. 2015;32:198-200. doi:10.1111/pde.12427
- Tosti A, Piraccini BM, Iorizzo M. Trachyonychia and related disorders: evaluation and treatment plans. Dermatolog Ther. 2002;15:121-125. doi:10.1046/j.1529-8019.2002.01511.x
- Leung AKC, Leong KF, Barankin B. Lichen striatus with nail involvement in a 6-year-old boy. Case Rep Pediatr. 2020;2020:1494760. doi:10.1155/2020/1494760
- Kim GW, Kim SH, Seo SH, et al. Lichen striatus with nail abnormality successfully treated with tacrolimus ointment. J Dermatol. 2009;36:616-617. doi:10.1111/j.1346-8138.2009.00720.x
- Iorizzo M, Rubin AI, Starace M. Nail lichen striatus: is dermoscopy useful for the diagnosis? Pediatr Dermatol. 2019;36:859-863. doi:10.1111/pde.13916
- Karp DL, Cohen BA. Onychodystrophy in lichen striatus. Pediatr Dermatol. 1993;10:359-361. doi:10.1111/j.1525-1470.1993.tb00399.x
- Tosti A, Peluso AM, Misciali C, et al. Nail lichen striatus: clinical features and long-term follow-up of five patients. J Am Acad Dermatol. 1997;36(6, pt 1):908-913. doi:10.1016/s0190-9622(97)80270-8
- Simpson EL, Thompson MM, Hanifin JM. Prevalence and morphology of hand eczema in patients with atopic dermatitis. Dermatitis. 2006;17:123-127. doi:10.2310/6620.2006.06005
- Sarifakioglu E, Yilmaz AE, Gorpelioglu C. Nail alterations in 250 infant patients: a clinical study. J Eur Acad Dermatol Venereol. 2008;22:741-744. doi:10.1111/j.1468-3083.2008.02592.x
- Milanesi N, D’Erme AM, Gola M. Nail improvement during alitretinoin treatment: three case reports and review of the literature. Clin Exp Dermatol. 2015;40:533-536. doi:10.1111/ced.12584
- Chung BY, Choi YW, Kim HO, et al. Nail dystrophy in patients with atopic dermatitis and its association with disease severity. Ann Dermatol. 2019;31:121-126. doi:10.5021/ad.2019.31.2.121
- Navarro-Triviño FJ, Vega-Castillo JJ, Ruiz-Villaverde R. Nail changes successfully treated with dupilumab in a patient with severe atopic dermatitis. Australas J Dermatol. 2021;62:e468-e469. doi:10.1111/ajd.13633
- Wei SH, Huang YP, Liu MC, et al. An outbreak of coxsackievirus A6 hand, foot, and mouth disease associated with onychomadesis in Taiwan, 2010. BMC Infect Dis. 2011;11:346. doi:10.1186/1471-2334-11-346
- Shin JY, Cho BK, Park HJ. A clinical study of nail changes occurring secondary to hand-foot-mouth disease: onychomadesis and Beau’s lines. Ann Dermatol. 2014;26:280-283. doi:10.5021/ad.2014.26.2.280
- Verma S, Singal A. Nail changes in hand-foot-and-mouth disease (HFMD). Indian Dermatol Online J. 2021;12:656-657. doi:10.4103 /idoj.IDOJ_271_20
- Giordano LMC, de la Fuente LA, Lorca JMB, et al. Onychomadesis secondary to hand-foot-mouth disease: a frequent manifestation and cause of concern for parents. Article in Spanish. Rev Chil Pediatr. 2018;89:380-383. doi:10.4067/s0370-41062018005000203
- Justino MCA, da SMD, Souza MF, et al. Atypical hand-foot-mouth disease in Belém, Amazon region, northern Brazil, with detection of coxsackievirus A6. J Clin Virol. 2020;126:104307. doi:10.1016/j.jcv.2020.104307
- Cheng FF, Zhang BB, Cao ML, et al. Clinical characteristics of 68 children with atypical hand, foot, and mouth disease caused by coxsackievirus A6: a single-center retrospective analysis. Transl Pediatr. 2022;11:1502-1509. doi:10.21037/tp-22-352
- Nagata S. Causes of Kawasaki disease-from past to present. Front Pediatr. 2019;7:18. doi:10.3389/fped.2019.00018
- Mitsuishi T, Miyata K, Ando A, et al. Characteristic nail lesions in Kawasaki disease: case series and literature review. J Dermatol. 2022;49:232-238. doi:10.1111/1346-8138.16276
- Lindsley CB. Nail-bed lines in Kawasaki disease. Am J Dis Child. 1992;146:659-660. doi:10.1001/archpedi.1992.02160180017005
- Matsumura O, Nakagishi Y. Pincer nails upon convalescence from Kawasaki disease. J Pediatr. 2022;246:279. doi:10.1016/j.jpeds.2022.03.002
- Solís-Arias MP, García-Romero MT. Onychomycosis in children. a review. Int J Dermatol. 2017;56:123-130. doi:10.1111/ijd.13392
- Gupta AK, Mays RR, Versteeg SG, et al. Onychomycosis in children: safety and efficacy of antifungal agents. Pediatr Dermatol. 2018;35:552-559. doi:10.1111/pde.13561
- 49. Gupta AK, Venkataraman M, Shear NH, et al. Labeled use of efinaconazole topical solution 10% in treating onychomycosis in children and a review of the management of pediatric onychomycosis. Dermatol Ther. 2020;33:e13613. doi:10.1111/dth.13613
- Falotico JM, Lipner SR. Updated perspectives on the diagnosis and management of onychomycosis. Clin Cosmet Investig Dermatol. 2022;15:1933-1957. doi:10.2147/ccid.S362635
- Patel D, Castelo-Soccio LA, Rubin AI, et al. Laboratory monitoring during systemic terbinafine therapy for pediatric onychomycosis. JAMA Dermatol. 2017;153:1326-1327. doi:10.1001/jamadermatol.2017.4483
- Friedlander SF, Chan YC, Chan YH, et al. Onychomycosis does not always require systemic treatment for cure: a trial using topical therapy. Pediatr Dermatol. 2013;30:316-322. doi:10.1111/pde.12064
- Rich P, Spellman M, Purohit V, et al. Tavaborole 5% topical solution for the treatment of toenail onychomycosis in pediatric patients: results from a phase 4 open-label study. J Drugs Dermatol. 2019;18:190-195.
- Gupta AK, Venkataraman M, Abramovits W, et al. JUBLIA (efinaconazole 10% solution) in the treatment of pediatric onychomycosis. Skinmed. 2021;19:206-210.
- Gupta AK, Paquet M. Systemic antifungals to treat onychomycosis in children: a systematic review. Pediatr Dermatol. 2013;30:294-302. doi:10.1111/pde.12048
- Leggit JC. Acute and chronic paronychia. Am Fam Physician. 2017;96:44-51.
- Lipner SR, Scher RK. Congenital malalignment of the great toenails with acute paronychia. Pediatr Dermatol. 2016;33:e288-e289.doi:10.1111/pde.12924
Practice Points
- Nail plate pitting is the most common clinical sign of nail psoriasis in children.
- Nail changes are common in hand, foot, and mouth disease, with the most frequent being onychomadesis.
- Because onychomycosis may resemble other nail disorders, mycologic confirmation is recommended to avoid misdiagnosis.
- Many nail conditions in children self-resolve but recognizing these manifestations is important in providing anticipatory guidance to patients and caregivers.
Medicare Rates in 2025 Would Cut Pay For Docs by 3%
Federal officials on July 11 proposed Medicare rates that effectively would cut physician pay by about 3% in 2025, touching off a fresh round of protests from medical associations.
, the Centers for Medicare & Medicaid Services said.
The American Medical Association (AMA), the American Academy of Family Physicians (AAFP) and other groups on July 10 reiterated calls on Congress to revise the law on Medicare payment for physicians and move away from short-term tweaks.
This proposed cut is mostly due to the 5-year freeze in the physician schedule base rate mandated by the 2015 Medicare Access and CHIP Reauthorization Act (MACRA). Congress designed MACRA with an aim of shifting clinicians toward programs that would peg pay increases to quality measures.
Lawmakers have since had to soften the blow of that freeze, acknowledging flaws in MACRA and inflation’s significant toll on medical practices. Yet lawmakers have made temporary fixes, such as a 2.93% increase in current payment that’s set to expire.
“Previous quick fixes have been insufficient — this situation requires a bold, substantial approach,” Bruce A. Scott, MD, the AMA president, said in a statement. “A Band-Aid goes only so far when the patient is in dire need.”
Dr. Scott noted that the Medicare Economic Index — a measure of practice cost inflation — is expected to rise by 3.6% in 2025.
“As a first step, Congress must enact an annual inflationary update to help physician payment rates keep pace with rising practice costs,” Steven P. Furr, MD, AAFP’s president, said in a statement released July 10. “Any payment reductions will threaten practices and exacerbate workforce shortages, preventing patients from accessing the primary care, behavioral health care, and other critical preventive services they need.”
Many medical groups, including the AMA, AAFP, and the Medical Group Management Association, are pressing Congress to pass a law that would tie the conversion factor of the physician fee schedule to inflation.
Influential advisory groups also have backed the idea of increasing the conversion factor. For example, the Medicare Payment Advisory Commission in March recommended to Congress that it increase the 2025 conversion factor, suggesting a bump of half of the projected increase in the Medicare Economic Index.
Congress seems unlikely to revamp the physician fee schedule this year, with members spending significant time away from Washington ahead of the November election.
That could make it likely that Congress’ next action on Medicare payment rates would be another short-term tweak — instead of long-lasting change.
A version of this article first appeared on Medscape.com.
Federal officials on July 11 proposed Medicare rates that effectively would cut physician pay by about 3% in 2025, touching off a fresh round of protests from medical associations.
, the Centers for Medicare & Medicaid Services said.
The American Medical Association (AMA), the American Academy of Family Physicians (AAFP) and other groups on July 10 reiterated calls on Congress to revise the law on Medicare payment for physicians and move away from short-term tweaks.
This proposed cut is mostly due to the 5-year freeze in the physician schedule base rate mandated by the 2015 Medicare Access and CHIP Reauthorization Act (MACRA). Congress designed MACRA with an aim of shifting clinicians toward programs that would peg pay increases to quality measures.
Lawmakers have since had to soften the blow of that freeze, acknowledging flaws in MACRA and inflation’s significant toll on medical practices. Yet lawmakers have made temporary fixes, such as a 2.93% increase in current payment that’s set to expire.
“Previous quick fixes have been insufficient — this situation requires a bold, substantial approach,” Bruce A. Scott, MD, the AMA president, said in a statement. “A Band-Aid goes only so far when the patient is in dire need.”
Dr. Scott noted that the Medicare Economic Index — a measure of practice cost inflation — is expected to rise by 3.6% in 2025.
“As a first step, Congress must enact an annual inflationary update to help physician payment rates keep pace with rising practice costs,” Steven P. Furr, MD, AAFP’s president, said in a statement released July 10. “Any payment reductions will threaten practices and exacerbate workforce shortages, preventing patients from accessing the primary care, behavioral health care, and other critical preventive services they need.”
Many medical groups, including the AMA, AAFP, and the Medical Group Management Association, are pressing Congress to pass a law that would tie the conversion factor of the physician fee schedule to inflation.
Influential advisory groups also have backed the idea of increasing the conversion factor. For example, the Medicare Payment Advisory Commission in March recommended to Congress that it increase the 2025 conversion factor, suggesting a bump of half of the projected increase in the Medicare Economic Index.
Congress seems unlikely to revamp the physician fee schedule this year, with members spending significant time away from Washington ahead of the November election.
That could make it likely that Congress’ next action on Medicare payment rates would be another short-term tweak — instead of long-lasting change.
A version of this article first appeared on Medscape.com.
Federal officials on July 11 proposed Medicare rates that effectively would cut physician pay by about 3% in 2025, touching off a fresh round of protests from medical associations.
, the Centers for Medicare & Medicaid Services said.
The American Medical Association (AMA), the American Academy of Family Physicians (AAFP) and other groups on July 10 reiterated calls on Congress to revise the law on Medicare payment for physicians and move away from short-term tweaks.
This proposed cut is mostly due to the 5-year freeze in the physician schedule base rate mandated by the 2015 Medicare Access and CHIP Reauthorization Act (MACRA). Congress designed MACRA with an aim of shifting clinicians toward programs that would peg pay increases to quality measures.
Lawmakers have since had to soften the blow of that freeze, acknowledging flaws in MACRA and inflation’s significant toll on medical practices. Yet lawmakers have made temporary fixes, such as a 2.93% increase in current payment that’s set to expire.
“Previous quick fixes have been insufficient — this situation requires a bold, substantial approach,” Bruce A. Scott, MD, the AMA president, said in a statement. “A Band-Aid goes only so far when the patient is in dire need.”
Dr. Scott noted that the Medicare Economic Index — a measure of practice cost inflation — is expected to rise by 3.6% in 2025.
“As a first step, Congress must enact an annual inflationary update to help physician payment rates keep pace with rising practice costs,” Steven P. Furr, MD, AAFP’s president, said in a statement released July 10. “Any payment reductions will threaten practices and exacerbate workforce shortages, preventing patients from accessing the primary care, behavioral health care, and other critical preventive services they need.”
Many medical groups, including the AMA, AAFP, and the Medical Group Management Association, are pressing Congress to pass a law that would tie the conversion factor of the physician fee schedule to inflation.
Influential advisory groups also have backed the idea of increasing the conversion factor. For example, the Medicare Payment Advisory Commission in March recommended to Congress that it increase the 2025 conversion factor, suggesting a bump of half of the projected increase in the Medicare Economic Index.
Congress seems unlikely to revamp the physician fee schedule this year, with members spending significant time away from Washington ahead of the November election.
That could make it likely that Congress’ next action on Medicare payment rates would be another short-term tweak — instead of long-lasting change.
A version of this article first appeared on Medscape.com.
Dupilumab Safe, Effective Over 5 Years in Moderate to Severe Atopic Dermatitis
TOPLINE:
Over 5 years, dupilumab demonstrated acceptable safety and sustained efficacy, with significant improvements in the signs and symptoms of AD, in the treatment of moderate to severe atopic dermatitis (AD).
METHODOLOGY:
- The phase 3 multinational LIBERTY AD open-label extension study evaluated the safety and efficacy of dupilumab in 2677 adults with moderate to severe AD who had previously participated in dupilumab trials over 5 years; 334 patients (12.5%) completed treatment up to 5 years.
- Patients started with subcutaneous dupilumab, initially dosed weekly after a loading dose, then every 2 weeks in 2019.
- The primary outcomes were the incidence and rate of treatment-emergent adverse events (TEAEs).
TAKEAWAY:
- Overall, 14,717 TEAEs were reported over 5 years. The exposure-adjusted incidence rate decreased over time and was 252.48 events per 100 patient-years.
- The most common TEAEs were nasopharyngitis (28.9%), worsening AD (16.7%), upper respiratory tract infection (13.6%), conjunctivitis (10.3%), allergic conjunctivitis (9%), headache (8.1%), oral herpes (7.5%), and injection-site reactions (5.2%).
- Serious and severe TEAE rates were 10.6% and 10.0%, respectively. Exposure-adjusted incidence rates were 6.66 and 6.71 events per 100 patient-years, respectively.
- At week 260, 67.5% of patients had achieved clear or almost clear skin according to the Investigator’s Global Assessment, and 88.9% experienced a 75% or greater improvement in the Eczema Area and Severity Index.
IN PRACTICE:
“Safety and efficacy results from up to 5 years of dupilumab treatment in the LIBERTY AD open-label extension study support dupilumab as a continuous long-term treatment for adults with moderate to severe AD,” the authors concluded.
SOURCE:
The study was led by Lisa A. Beck, MD, University of Rochester, Rochester, New York, and was published online in JAMA Dermatology.
LIMITATIONS:
Study limitations included the absence of a placebo arm and treatment interruptions stemming from protocol changes. The number of patients who received biweekly doses was small. The early conclusion of the trial by the sponsor because of regulatory approval also resulted in a lower number of patients at later stages.
DISCLOSURES:
This study was funded by dupilumab manufacturers Sanofi and Regeneron Pharmaceuticals. Several authors declared ties with various pharmaceutical companies including Sanofi and Regeneron, and several authors were employees of Sanofi or Regeneron. No disclosures were reported by other authors.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Over 5 years, dupilumab demonstrated acceptable safety and sustained efficacy, with significant improvements in the signs and symptoms of AD, in the treatment of moderate to severe atopic dermatitis (AD).
METHODOLOGY:
- The phase 3 multinational LIBERTY AD open-label extension study evaluated the safety and efficacy of dupilumab in 2677 adults with moderate to severe AD who had previously participated in dupilumab trials over 5 years; 334 patients (12.5%) completed treatment up to 5 years.
- Patients started with subcutaneous dupilumab, initially dosed weekly after a loading dose, then every 2 weeks in 2019.
- The primary outcomes were the incidence and rate of treatment-emergent adverse events (TEAEs).
TAKEAWAY:
- Overall, 14,717 TEAEs were reported over 5 years. The exposure-adjusted incidence rate decreased over time and was 252.48 events per 100 patient-years.
- The most common TEAEs were nasopharyngitis (28.9%), worsening AD (16.7%), upper respiratory tract infection (13.6%), conjunctivitis (10.3%), allergic conjunctivitis (9%), headache (8.1%), oral herpes (7.5%), and injection-site reactions (5.2%).
- Serious and severe TEAE rates were 10.6% and 10.0%, respectively. Exposure-adjusted incidence rates were 6.66 and 6.71 events per 100 patient-years, respectively.
- At week 260, 67.5% of patients had achieved clear or almost clear skin according to the Investigator’s Global Assessment, and 88.9% experienced a 75% or greater improvement in the Eczema Area and Severity Index.
IN PRACTICE:
“Safety and efficacy results from up to 5 years of dupilumab treatment in the LIBERTY AD open-label extension study support dupilumab as a continuous long-term treatment for adults with moderate to severe AD,” the authors concluded.
SOURCE:
The study was led by Lisa A. Beck, MD, University of Rochester, Rochester, New York, and was published online in JAMA Dermatology.
LIMITATIONS:
Study limitations included the absence of a placebo arm and treatment interruptions stemming from protocol changes. The number of patients who received biweekly doses was small. The early conclusion of the trial by the sponsor because of regulatory approval also resulted in a lower number of patients at later stages.
DISCLOSURES:
This study was funded by dupilumab manufacturers Sanofi and Regeneron Pharmaceuticals. Several authors declared ties with various pharmaceutical companies including Sanofi and Regeneron, and several authors were employees of Sanofi or Regeneron. No disclosures were reported by other authors.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Over 5 years, dupilumab demonstrated acceptable safety and sustained efficacy, with significant improvements in the signs and symptoms of AD, in the treatment of moderate to severe atopic dermatitis (AD).
METHODOLOGY:
- The phase 3 multinational LIBERTY AD open-label extension study evaluated the safety and efficacy of dupilumab in 2677 adults with moderate to severe AD who had previously participated in dupilumab trials over 5 years; 334 patients (12.5%) completed treatment up to 5 years.
- Patients started with subcutaneous dupilumab, initially dosed weekly after a loading dose, then every 2 weeks in 2019.
- The primary outcomes were the incidence and rate of treatment-emergent adverse events (TEAEs).
TAKEAWAY:
- Overall, 14,717 TEAEs were reported over 5 years. The exposure-adjusted incidence rate decreased over time and was 252.48 events per 100 patient-years.
- The most common TEAEs were nasopharyngitis (28.9%), worsening AD (16.7%), upper respiratory tract infection (13.6%), conjunctivitis (10.3%), allergic conjunctivitis (9%), headache (8.1%), oral herpes (7.5%), and injection-site reactions (5.2%).
- Serious and severe TEAE rates were 10.6% and 10.0%, respectively. Exposure-adjusted incidence rates were 6.66 and 6.71 events per 100 patient-years, respectively.
- At week 260, 67.5% of patients had achieved clear or almost clear skin according to the Investigator’s Global Assessment, and 88.9% experienced a 75% or greater improvement in the Eczema Area and Severity Index.
IN PRACTICE:
“Safety and efficacy results from up to 5 years of dupilumab treatment in the LIBERTY AD open-label extension study support dupilumab as a continuous long-term treatment for adults with moderate to severe AD,” the authors concluded.
SOURCE:
The study was led by Lisa A. Beck, MD, University of Rochester, Rochester, New York, and was published online in JAMA Dermatology.
LIMITATIONS:
Study limitations included the absence of a placebo arm and treatment interruptions stemming from protocol changes. The number of patients who received biweekly doses was small. The early conclusion of the trial by the sponsor because of regulatory approval also resulted in a lower number of patients at later stages.
DISCLOSURES:
This study was funded by dupilumab manufacturers Sanofi and Regeneron Pharmaceuticals. Several authors declared ties with various pharmaceutical companies including Sanofi and Regeneron, and several authors were employees of Sanofi or Regeneron. No disclosures were reported by other authors.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Women’s Risk for Lupus Rises With Greater Intake of Ultraprocessed Foods
TOPLINE:
A higher intake of ultraprocessed foods increases the risk for systemic lupus erythematosus (SLE) by over 50% in women. The risk doubled in those with anti–double-stranded DNA antibodies.
METHODOLOGY:
- Researchers assessed 204,175 women from two Nurses’ Health Study cohorts from 1984 to 2016.
- Participants completed semiquantitative food frequency questionnaires every 4 years for the assessment of dietary intake.
- Incident SLE cases were self-reported and confirmed using medical records, with 212 cases identified.
TAKEAWAY:
- A higher cumulative average daily intake of ultraprocessed foods was associated with a 56% increased risk for SLE (95% confidence interval [CI], 1.04-2.32).
- The risk for anti–double-stranded DNA antibody-positive SLE was more than doubled (hazard ratio, 2.05; 95% CI, 1.15-3.65).
- Sugar or artificially sweetened beverages were associated with a 45% increased risk for SLE (95% CI, 1.01-2.09).
- No significant interactions with body mass index were observed in the association between ultraprocessed food intake and SLE.
IN PRACTICE:
This study is too preliminary to have practical application.
SOURCE:
The study was led by Sinara Rossato, PhD, Harvard T.H. Chan School of Public Health, Boston. It was published online in Arthritis Care & Research.
LIMITATIONS:
The study’s generalizability is limited due to the predominantly White female population of registered nurses. The relatively high baseline age of participants may not fully capture the peak incidence age range for SLE. The observational nature of the study cannot establish causality between ultraprocessed food intake and SLE risk.
DISCLOSURES:
The study was supported by the National Institutes of Health. The authors did not declare any competing interests.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
A higher intake of ultraprocessed foods increases the risk for systemic lupus erythematosus (SLE) by over 50% in women. The risk doubled in those with anti–double-stranded DNA antibodies.
METHODOLOGY:
- Researchers assessed 204,175 women from two Nurses’ Health Study cohorts from 1984 to 2016.
- Participants completed semiquantitative food frequency questionnaires every 4 years for the assessment of dietary intake.
- Incident SLE cases were self-reported and confirmed using medical records, with 212 cases identified.
TAKEAWAY:
- A higher cumulative average daily intake of ultraprocessed foods was associated with a 56% increased risk for SLE (95% confidence interval [CI], 1.04-2.32).
- The risk for anti–double-stranded DNA antibody-positive SLE was more than doubled (hazard ratio, 2.05; 95% CI, 1.15-3.65).
- Sugar or artificially sweetened beverages were associated with a 45% increased risk for SLE (95% CI, 1.01-2.09).
- No significant interactions with body mass index were observed in the association between ultraprocessed food intake and SLE.
IN PRACTICE:
This study is too preliminary to have practical application.
SOURCE:
The study was led by Sinara Rossato, PhD, Harvard T.H. Chan School of Public Health, Boston. It was published online in Arthritis Care & Research.
LIMITATIONS:
The study’s generalizability is limited due to the predominantly White female population of registered nurses. The relatively high baseline age of participants may not fully capture the peak incidence age range for SLE. The observational nature of the study cannot establish causality between ultraprocessed food intake and SLE risk.
DISCLOSURES:
The study was supported by the National Institutes of Health. The authors did not declare any competing interests.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
A higher intake of ultraprocessed foods increases the risk for systemic lupus erythematosus (SLE) by over 50% in women. The risk doubled in those with anti–double-stranded DNA antibodies.
METHODOLOGY:
- Researchers assessed 204,175 women from two Nurses’ Health Study cohorts from 1984 to 2016.
- Participants completed semiquantitative food frequency questionnaires every 4 years for the assessment of dietary intake.
- Incident SLE cases were self-reported and confirmed using medical records, with 212 cases identified.
TAKEAWAY:
- A higher cumulative average daily intake of ultraprocessed foods was associated with a 56% increased risk for SLE (95% confidence interval [CI], 1.04-2.32).
- The risk for anti–double-stranded DNA antibody-positive SLE was more than doubled (hazard ratio, 2.05; 95% CI, 1.15-3.65).
- Sugar or artificially sweetened beverages were associated with a 45% increased risk for SLE (95% CI, 1.01-2.09).
- No significant interactions with body mass index were observed in the association between ultraprocessed food intake and SLE.
IN PRACTICE:
This study is too preliminary to have practical application.
SOURCE:
The study was led by Sinara Rossato, PhD, Harvard T.H. Chan School of Public Health, Boston. It was published online in Arthritis Care & Research.
LIMITATIONS:
The study’s generalizability is limited due to the predominantly White female population of registered nurses. The relatively high baseline age of participants may not fully capture the peak incidence age range for SLE. The observational nature of the study cannot establish causality between ultraprocessed food intake and SLE risk.
DISCLOSURES:
The study was supported by the National Institutes of Health. The authors did not declare any competing interests.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
Plastic Surgeon to Pay $5 Million for Restriction of Negative Reviews, Directing Fake Reviews
A Seattle plastic surgeon who illegally restricted patients from posting negative reviews about his practice and directed his staff to post fake positive reviews will pay $5 million for violating Washington state’s consumer protection law.
According to a July 1 consent decree, Javad Sajan, MD, and his practice Allure Esthetic must pay $1.5 million in restitution to 21,000 patients and $3.5 million to the state for manipulation of patient ratings.
In an April decision, US District Judge Ricardo S. Martinez sided with the state, ruling that Allure Esthetic’s actions violated the federal Consumer Review Fairness Act (CRFA).
“Writing a truthful review about a business should not subject you to threats or intimidation,” Mr. Ferguson said in a July 2 statement. “Consumers rely on reviews when determining who to trust, especially services that affect their health and safety. This resolution holds Allure accountable for brazenly violating that trust — and the law — and ensures the clinic stops its harmful conduct.”
In court documents, Dr. Sajan’s attorneys had argued that the agreements did not violate CRFA because patients had the opportunity to modify the language or decline signing them.
The surgeon’s practice is “pleased to have resolved its case with the Attorney General’s Office,” according to a statement provided by Dr. Sajan’s attorney. “The cooperative settlement, while not admitting fault and resolving claims asserted by both sides, allows Allure Esthetic to continue to focus on its core mission of providing compassionate care to patients and serving the community. The decision to settle was not an easy one, but it was necessary to allocate time and resources where they matter most — the patients.”
The dispute stemmed from NDAs that Dr. Sajan’s practice required patients to sign starting in 2017, according to Mr. Ferguson’s complaint. The terms instructed patients to contact the business directly if they had concerns rather than post a negative review.
If patients posted negative reviews, the clinic, in some cases, threatened litigation, according to the lawsuit. In other cases, patients were allegedly offered money and free services in exchange for taking the reviews down. Patients who accepted cash or services were required to sign a second agreement forbidding them from posting future negative reviews and imposing a $250,000 penalty for failure to comply, according to court documents.
In addition, Mr. Ferguson accused Dr. Sajan of creating fake positive accounts of patient experiences and buying fake followers on social media. State investigators found Dr. Sajan directed Allure Esthetic’s employees to create fake Gmail accounts to post the false reviews, many of which are still online today, according to the state’s complaint.
Mr. Ferguson also claimed Dr. Sajan and his practice manipulated social media to appear more popular by purchasing followers through an online vendor. The practice also allegedly used a social media bot tool to buy thousands of fake likes on Instagram, YouTube, and other social media.
After filing the lawsuit, Mr. Ferguson’s office uncovered further evidence of Dr. Sajan’s efforts to influence his professional reputation through fabrication, according to the July 2 release. Allure Esthetic “rigged” “best doctor” competitions hosted by local media outlets by paying staff and contractors to vote for Dr. Sajan as “best plastic surgeon” in the region, according to the release. The staff cast as many votes as websites allowed, despite not being patients of Allure Esthetic.
The practice also allegedly edited before-and-after photos of patients to make their results appear better and kept tens of thousands of dollars in rebates intended for its patients.
In addition to paying $5 million, the consent decree requires Dr. Sajan and his practice also:
- Stop posting or influencing consumer reviews; perform a full audit of all public reviews on Google, Yelp, and other third-party review platforms; and request removal of every review Allure Esthetic was involved in creating, posting, or shaping in any manner.
- Remove all misleading “before-and-after” photographs of plastic surgery procedures from its website and social media and stop altering photographs of future procedures.
- Cease use of and attempts to enforce all illegal NDAs and notify patients who previously signed them that they are released from the terms of those NDAs.
- Pay a third-party forensic accounting firm to perform a full, independent audit of Allure Esthetic’s consumer rebate program to identify consumers who are owed rebates that were unlawfully claimed by Allure Esthetic.
Additionally, the attorney general’s office will continue to monitor Allure Esthetic, and upon request, the practice must provide information that demonstrates its compliance with the consent decree for the next 10 years.
The practice must also develop internal policies and implement a training program to educate staff about nondeceptive advertising and compliance with consumer protection laws.
Dr. Sajan and his practice agreed to the terms of the consent decree, and the settlement is not considered an admission of liability.
A version of this article first appeared on Medscape.com.
A Seattle plastic surgeon who illegally restricted patients from posting negative reviews about his practice and directed his staff to post fake positive reviews will pay $5 million for violating Washington state’s consumer protection law.
According to a July 1 consent decree, Javad Sajan, MD, and his practice Allure Esthetic must pay $1.5 million in restitution to 21,000 patients and $3.5 million to the state for manipulation of patient ratings.
In an April decision, US District Judge Ricardo S. Martinez sided with the state, ruling that Allure Esthetic’s actions violated the federal Consumer Review Fairness Act (CRFA).
“Writing a truthful review about a business should not subject you to threats or intimidation,” Mr. Ferguson said in a July 2 statement. “Consumers rely on reviews when determining who to trust, especially services that affect their health and safety. This resolution holds Allure accountable for brazenly violating that trust — and the law — and ensures the clinic stops its harmful conduct.”
In court documents, Dr. Sajan’s attorneys had argued that the agreements did not violate CRFA because patients had the opportunity to modify the language or decline signing them.
The surgeon’s practice is “pleased to have resolved its case with the Attorney General’s Office,” according to a statement provided by Dr. Sajan’s attorney. “The cooperative settlement, while not admitting fault and resolving claims asserted by both sides, allows Allure Esthetic to continue to focus on its core mission of providing compassionate care to patients and serving the community. The decision to settle was not an easy one, but it was necessary to allocate time and resources where they matter most — the patients.”
The dispute stemmed from NDAs that Dr. Sajan’s practice required patients to sign starting in 2017, according to Mr. Ferguson’s complaint. The terms instructed patients to contact the business directly if they had concerns rather than post a negative review.
If patients posted negative reviews, the clinic, in some cases, threatened litigation, according to the lawsuit. In other cases, patients were allegedly offered money and free services in exchange for taking the reviews down. Patients who accepted cash or services were required to sign a second agreement forbidding them from posting future negative reviews and imposing a $250,000 penalty for failure to comply, according to court documents.
In addition, Mr. Ferguson accused Dr. Sajan of creating fake positive accounts of patient experiences and buying fake followers on social media. State investigators found Dr. Sajan directed Allure Esthetic’s employees to create fake Gmail accounts to post the false reviews, many of which are still online today, according to the state’s complaint.
Mr. Ferguson also claimed Dr. Sajan and his practice manipulated social media to appear more popular by purchasing followers through an online vendor. The practice also allegedly used a social media bot tool to buy thousands of fake likes on Instagram, YouTube, and other social media.
After filing the lawsuit, Mr. Ferguson’s office uncovered further evidence of Dr. Sajan’s efforts to influence his professional reputation through fabrication, according to the July 2 release. Allure Esthetic “rigged” “best doctor” competitions hosted by local media outlets by paying staff and contractors to vote for Dr. Sajan as “best plastic surgeon” in the region, according to the release. The staff cast as many votes as websites allowed, despite not being patients of Allure Esthetic.
The practice also allegedly edited before-and-after photos of patients to make their results appear better and kept tens of thousands of dollars in rebates intended for its patients.
In addition to paying $5 million, the consent decree requires Dr. Sajan and his practice also:
- Stop posting or influencing consumer reviews; perform a full audit of all public reviews on Google, Yelp, and other third-party review platforms; and request removal of every review Allure Esthetic was involved in creating, posting, or shaping in any manner.
- Remove all misleading “before-and-after” photographs of plastic surgery procedures from its website and social media and stop altering photographs of future procedures.
- Cease use of and attempts to enforce all illegal NDAs and notify patients who previously signed them that they are released from the terms of those NDAs.
- Pay a third-party forensic accounting firm to perform a full, independent audit of Allure Esthetic’s consumer rebate program to identify consumers who are owed rebates that were unlawfully claimed by Allure Esthetic.
Additionally, the attorney general’s office will continue to monitor Allure Esthetic, and upon request, the practice must provide information that demonstrates its compliance with the consent decree for the next 10 years.
The practice must also develop internal policies and implement a training program to educate staff about nondeceptive advertising and compliance with consumer protection laws.
Dr. Sajan and his practice agreed to the terms of the consent decree, and the settlement is not considered an admission of liability.
A version of this article first appeared on Medscape.com.
A Seattle plastic surgeon who illegally restricted patients from posting negative reviews about his practice and directed his staff to post fake positive reviews will pay $5 million for violating Washington state’s consumer protection law.
According to a July 1 consent decree, Javad Sajan, MD, and his practice Allure Esthetic must pay $1.5 million in restitution to 21,000 patients and $3.5 million to the state for manipulation of patient ratings.
In an April decision, US District Judge Ricardo S. Martinez sided with the state, ruling that Allure Esthetic’s actions violated the federal Consumer Review Fairness Act (CRFA).
“Writing a truthful review about a business should not subject you to threats or intimidation,” Mr. Ferguson said in a July 2 statement. “Consumers rely on reviews when determining who to trust, especially services that affect their health and safety. This resolution holds Allure accountable for brazenly violating that trust — and the law — and ensures the clinic stops its harmful conduct.”
In court documents, Dr. Sajan’s attorneys had argued that the agreements did not violate CRFA because patients had the opportunity to modify the language or decline signing them.
The surgeon’s practice is “pleased to have resolved its case with the Attorney General’s Office,” according to a statement provided by Dr. Sajan’s attorney. “The cooperative settlement, while not admitting fault and resolving claims asserted by both sides, allows Allure Esthetic to continue to focus on its core mission of providing compassionate care to patients and serving the community. The decision to settle was not an easy one, but it was necessary to allocate time and resources where they matter most — the patients.”
The dispute stemmed from NDAs that Dr. Sajan’s practice required patients to sign starting in 2017, according to Mr. Ferguson’s complaint. The terms instructed patients to contact the business directly if they had concerns rather than post a negative review.
If patients posted negative reviews, the clinic, in some cases, threatened litigation, according to the lawsuit. In other cases, patients were allegedly offered money and free services in exchange for taking the reviews down. Patients who accepted cash or services were required to sign a second agreement forbidding them from posting future negative reviews and imposing a $250,000 penalty for failure to comply, according to court documents.
In addition, Mr. Ferguson accused Dr. Sajan of creating fake positive accounts of patient experiences and buying fake followers on social media. State investigators found Dr. Sajan directed Allure Esthetic’s employees to create fake Gmail accounts to post the false reviews, many of which are still online today, according to the state’s complaint.
Mr. Ferguson also claimed Dr. Sajan and his practice manipulated social media to appear more popular by purchasing followers through an online vendor. The practice also allegedly used a social media bot tool to buy thousands of fake likes on Instagram, YouTube, and other social media.
After filing the lawsuit, Mr. Ferguson’s office uncovered further evidence of Dr. Sajan’s efforts to influence his professional reputation through fabrication, according to the July 2 release. Allure Esthetic “rigged” “best doctor” competitions hosted by local media outlets by paying staff and contractors to vote for Dr. Sajan as “best plastic surgeon” in the region, according to the release. The staff cast as many votes as websites allowed, despite not being patients of Allure Esthetic.
The practice also allegedly edited before-and-after photos of patients to make their results appear better and kept tens of thousands of dollars in rebates intended for its patients.
In addition to paying $5 million, the consent decree requires Dr. Sajan and his practice also:
- Stop posting or influencing consumer reviews; perform a full audit of all public reviews on Google, Yelp, and other third-party review platforms; and request removal of every review Allure Esthetic was involved in creating, posting, or shaping in any manner.
- Remove all misleading “before-and-after” photographs of plastic surgery procedures from its website and social media and stop altering photographs of future procedures.
- Cease use of and attempts to enforce all illegal NDAs and notify patients who previously signed them that they are released from the terms of those NDAs.
- Pay a third-party forensic accounting firm to perform a full, independent audit of Allure Esthetic’s consumer rebate program to identify consumers who are owed rebates that were unlawfully claimed by Allure Esthetic.
Additionally, the attorney general’s office will continue to monitor Allure Esthetic, and upon request, the practice must provide information that demonstrates its compliance with the consent decree for the next 10 years.
The practice must also develop internal policies and implement a training program to educate staff about nondeceptive advertising and compliance with consumer protection laws.
Dr. Sajan and his practice agreed to the terms of the consent decree, and the settlement is not considered an admission of liability.
A version of this article first appeared on Medscape.com.
A Doctor’s Guide to Relocation
Moving for any new opportunity in medicine can feel like starting a new life, not just a new job. This is especially true for residency or fellowships, as taking a step forward in your career is exciting. But in the process, you may be leaving family and friends for an unknown city or region where you will need to find a community. And the changes could be long-term. According to the Association of American Medical Colleges’ 2023 Report on Residents, 57.1% of the individuals who completed residency training between 2013 and 2022 are still practicing in the state where they completed their residency.
The process of planning out the right timeline; securing a comfortable, convenient, and affordable place to live; and meeting people while working long hours in an unfamiliar location can be overwhelming. And in the case of many residency programs and healthcare settings, financial assistance, relocation information, and other resources are scarce.
This news organization spoke to recent residents and medical school faculty members about how to navigate a medical move and set yourself up for success.
1. Find Relocation Resources
First things first. Find out what your program or hospital has to offer.
Some institutions help incoming residents by providing housing options or information. The Icahn School of Medicine at Mount Sinai’s Real Estate Division, for example, provides off-campus housing resources that guide new residents and faculty toward safe, convenient places to live in New York City. It also guarantees on-campus or block-leased housing offers to all incoming residents who apply.
Michael Leitman, MD, FACS, professor of surgery and medical education and dean for Graduate Medical Education at the Icahn School of Medicine at Mount Sinai in New York City, recommends connecting with colleagues at your program for guidance on navigating a new city and a new healthcare setting. He encourages incoming residents to use the contact information they receive during the interview and orientation processes to reach out to co-residents and faculty members.
Other residency programs offer partial reimbursement or need-based financial aid to help with the expense of relocation. But this is unlikely to cover all or even most of the cost of a cross-country move.
When Morgen Owens, MD, moved from Alabama to New York City for a physical medicine and rehabilitation residency at Mount Sinai in 2021, her program offered subsidized housing options. But there was little reimbursement for relocation. She paid around $3000 for a one-way rental truck, gas, one night in a hotel, and movers to unload her belongings. She says driving herself kept the price down because full-service movers would have cost her between $4000 and $6000.
If this will strain your finances, several banks offer loans specifically for medical school graduates to cover residency and internship expenses. But be aware that these loans tend to have higher interest rates than federal student loans because they are based on credit score rather than fixed.
2. Reach Out and Buddy Up
Reaching out to more senior residents is essential, and some programs facilitate a buddy system for relocation advice.
Family physician Mursal Sekandari, MD, known as “Dr. Mursi,” attended a residency program at St. Luke’s University Hospital–Bethlehem Campus, in Bethlehem, Pennsylvania. The program’s official buddy system paired her with a senior resident who advised her on the area and gave tips for her apartment search.
On the other hand, when America Revere, MD, moved from Texas to Georgia for a surgery residency, she found that her program offered little relocation assistance, financial or otherwise. She leaned on her co-residents, and especially senior ones, for support while she settled in.
Dr. Revere also discovered the importance of accepting invitations to events hosted by both her fellow residents and her program itself, especially in the early stages of residency. “Accepting social invitations is really the only way to get to know people,” she said. “Sure, you’ll meet people at work and get to know their ‘work’ personalities.” But Dr. Revere’s attendings also threw parties, which she says were a great way to connect with a wider group and build a community.
To meet people both within and beyond her own residency program, Dr. Owens joined a group chat for physical medicine and rehab residents in the New York City area. She suggests looking into GroupMe or WhatsApp groups specific to your specialty.
3. Play the ‘Doctor Card’
Finding a place to live in an unfamiliar and competitive housing market can be one of the biggest challenges of any move. Dr. Owens’ options were limited by owning a dog, which wouldn’t be allowed in her hospital’s subsidized housing. Instead, she opted to find her own apartment in New York City. Her strategy: Playing the “doctor card.”
“I explained my situation: ‘I’m a doctor moving from out of state,’ ” Owens said. “Own that! These companies and brokers will look at you as a student and think, ‘Oh, she has no money, she has no savings, she’s got all of these loans, how is she going to pay for this apartment?’ But you have to say, ‘I’m a doctor. I’m an incoming resident who has X amount of years of job security. I’m not going to lose my job while living here.’ ”
4. Move Early
Dr. Revere found it important to move into her new home 2 weeks before the start of her residency program. Moving in early allowed her to settle in, get to know her area, neighbors, and co-residents, and generally prepare for her first day. It also gave her time to put furniture together — her new vanity alone took 12 hours.
Having a larger window of time before residency can also benefit those who hire movers or have their furniture shipped. When it comes to a cross-country move, it can take a few days to a few weeks for the truck to arrive — which could translate to a few nights or a few weeks without a bed.
“When residency comes, it comes fast,” Dr. Revere said. “It’s very confusing, and the last thing you need is to have half of your stuff unpacked or have no idea where you are or know nobody around you.”
5. Make Your New Home Your Sanctuary
During the stress of residency, your home can be a source of peace, and finding that might require trade-offs.
Dr. Sekandari’s parents urged her to live with roommates to save money on rent, but she insisted that spending more for solitude would be worth it. For her first year of residency, she barely saw her apartment. But when she did, she felt grateful to be in such a tranquil place to ease some of the stress of studying. “If you feel uncomfortable while you’re dealing with something stressful, the stress just exponentially increases,” she said. Creating an environment where you can really relax “makes a difference in how you respond to everything else around you.”
Dr. Revere agrees, urging medical professionals — and particularly residents — to invest in the most comfortable mattresses and bedding they can. Whether you are working nights, she also recommends blackout curtains to help facilitate daytime naps or better sleep in general, especially among the bright lights of bigger cities.
“You’re going to need somewhere to decompress,” she said. “That will look different for everyone. But I would definitely invest in your apartment to make it a sanctuary away from work.”
6. Consider a ‘Live’ Stress Reliever
When it comes to crucial stress relief during residency, “I like mine live,” Dr. Revere said in a YouTube vlog while petting her cat, Calyx.
Taking on the added responsibility of a pet during residency or any medical role may seem counterintuitive. But Revere has zero regrets about bringing Calyx along on her journey. “Cats are very easy,” she said. “I have nothing but wonderful things to say about having a cat during my difficult surgical residency.”
Dr. Owens admits that moving to New York City with her dog was difficult during her first years of residency. She worked an average of 80 hours each week and had little time for walks. She made room in her budget for dog walkers. Thankfully, her hours have eased up as she has progressed through her program, and she can now take her dog on longer walks every day. “He definitely has a better life now that I work fewer hours,” she said.
Once you’ve prepared, made the move, and found your village, it’s time for the real work to begin. “The first couple of months are certainly a challenge of adjusting to a new hospital, a new electronic medical record, a new culture, and a new geographic location,” said Dr. Leitman, who has relocated several times. “But at the end of the day ... it’s you and the patient.” By minimizing stress and getting the support you need, it can even be “a fun process,” Dr. Mursi added, “so make it an exciting chapter in your life.”
A version of this article first appeared on Medscape.com.
Moving for any new opportunity in medicine can feel like starting a new life, not just a new job. This is especially true for residency or fellowships, as taking a step forward in your career is exciting. But in the process, you may be leaving family and friends for an unknown city or region where you will need to find a community. And the changes could be long-term. According to the Association of American Medical Colleges’ 2023 Report on Residents, 57.1% of the individuals who completed residency training between 2013 and 2022 are still practicing in the state where they completed their residency.
The process of planning out the right timeline; securing a comfortable, convenient, and affordable place to live; and meeting people while working long hours in an unfamiliar location can be overwhelming. And in the case of many residency programs and healthcare settings, financial assistance, relocation information, and other resources are scarce.
This news organization spoke to recent residents and medical school faculty members about how to navigate a medical move and set yourself up for success.
1. Find Relocation Resources
First things first. Find out what your program or hospital has to offer.
Some institutions help incoming residents by providing housing options or information. The Icahn School of Medicine at Mount Sinai’s Real Estate Division, for example, provides off-campus housing resources that guide new residents and faculty toward safe, convenient places to live in New York City. It also guarantees on-campus or block-leased housing offers to all incoming residents who apply.
Michael Leitman, MD, FACS, professor of surgery and medical education and dean for Graduate Medical Education at the Icahn School of Medicine at Mount Sinai in New York City, recommends connecting with colleagues at your program for guidance on navigating a new city and a new healthcare setting. He encourages incoming residents to use the contact information they receive during the interview and orientation processes to reach out to co-residents and faculty members.
Other residency programs offer partial reimbursement or need-based financial aid to help with the expense of relocation. But this is unlikely to cover all or even most of the cost of a cross-country move.
When Morgen Owens, MD, moved from Alabama to New York City for a physical medicine and rehabilitation residency at Mount Sinai in 2021, her program offered subsidized housing options. But there was little reimbursement for relocation. She paid around $3000 for a one-way rental truck, gas, one night in a hotel, and movers to unload her belongings. She says driving herself kept the price down because full-service movers would have cost her between $4000 and $6000.
If this will strain your finances, several banks offer loans specifically for medical school graduates to cover residency and internship expenses. But be aware that these loans tend to have higher interest rates than federal student loans because they are based on credit score rather than fixed.
2. Reach Out and Buddy Up
Reaching out to more senior residents is essential, and some programs facilitate a buddy system for relocation advice.
Family physician Mursal Sekandari, MD, known as “Dr. Mursi,” attended a residency program at St. Luke’s University Hospital–Bethlehem Campus, in Bethlehem, Pennsylvania. The program’s official buddy system paired her with a senior resident who advised her on the area and gave tips for her apartment search.
On the other hand, when America Revere, MD, moved from Texas to Georgia for a surgery residency, she found that her program offered little relocation assistance, financial or otherwise. She leaned on her co-residents, and especially senior ones, for support while she settled in.
Dr. Revere also discovered the importance of accepting invitations to events hosted by both her fellow residents and her program itself, especially in the early stages of residency. “Accepting social invitations is really the only way to get to know people,” she said. “Sure, you’ll meet people at work and get to know their ‘work’ personalities.” But Dr. Revere’s attendings also threw parties, which she says were a great way to connect with a wider group and build a community.
To meet people both within and beyond her own residency program, Dr. Owens joined a group chat for physical medicine and rehab residents in the New York City area. She suggests looking into GroupMe or WhatsApp groups specific to your specialty.
3. Play the ‘Doctor Card’
Finding a place to live in an unfamiliar and competitive housing market can be one of the biggest challenges of any move. Dr. Owens’ options were limited by owning a dog, which wouldn’t be allowed in her hospital’s subsidized housing. Instead, she opted to find her own apartment in New York City. Her strategy: Playing the “doctor card.”
“I explained my situation: ‘I’m a doctor moving from out of state,’ ” Owens said. “Own that! These companies and brokers will look at you as a student and think, ‘Oh, she has no money, she has no savings, she’s got all of these loans, how is she going to pay for this apartment?’ But you have to say, ‘I’m a doctor. I’m an incoming resident who has X amount of years of job security. I’m not going to lose my job while living here.’ ”
4. Move Early
Dr. Revere found it important to move into her new home 2 weeks before the start of her residency program. Moving in early allowed her to settle in, get to know her area, neighbors, and co-residents, and generally prepare for her first day. It also gave her time to put furniture together — her new vanity alone took 12 hours.
Having a larger window of time before residency can also benefit those who hire movers or have their furniture shipped. When it comes to a cross-country move, it can take a few days to a few weeks for the truck to arrive — which could translate to a few nights or a few weeks without a bed.
“When residency comes, it comes fast,” Dr. Revere said. “It’s very confusing, and the last thing you need is to have half of your stuff unpacked or have no idea where you are or know nobody around you.”
5. Make Your New Home Your Sanctuary
During the stress of residency, your home can be a source of peace, and finding that might require trade-offs.
Dr. Sekandari’s parents urged her to live with roommates to save money on rent, but she insisted that spending more for solitude would be worth it. For her first year of residency, she barely saw her apartment. But when she did, she felt grateful to be in such a tranquil place to ease some of the stress of studying. “If you feel uncomfortable while you’re dealing with something stressful, the stress just exponentially increases,” she said. Creating an environment where you can really relax “makes a difference in how you respond to everything else around you.”
Dr. Revere agrees, urging medical professionals — and particularly residents — to invest in the most comfortable mattresses and bedding they can. Whether you are working nights, she also recommends blackout curtains to help facilitate daytime naps or better sleep in general, especially among the bright lights of bigger cities.
“You’re going to need somewhere to decompress,” she said. “That will look different for everyone. But I would definitely invest in your apartment to make it a sanctuary away from work.”
6. Consider a ‘Live’ Stress Reliever
When it comes to crucial stress relief during residency, “I like mine live,” Dr. Revere said in a YouTube vlog while petting her cat, Calyx.
Taking on the added responsibility of a pet during residency or any medical role may seem counterintuitive. But Revere has zero regrets about bringing Calyx along on her journey. “Cats are very easy,” she said. “I have nothing but wonderful things to say about having a cat during my difficult surgical residency.”
Dr. Owens admits that moving to New York City with her dog was difficult during her first years of residency. She worked an average of 80 hours each week and had little time for walks. She made room in her budget for dog walkers. Thankfully, her hours have eased up as she has progressed through her program, and she can now take her dog on longer walks every day. “He definitely has a better life now that I work fewer hours,” she said.
Once you’ve prepared, made the move, and found your village, it’s time for the real work to begin. “The first couple of months are certainly a challenge of adjusting to a new hospital, a new electronic medical record, a new culture, and a new geographic location,” said Dr. Leitman, who has relocated several times. “But at the end of the day ... it’s you and the patient.” By minimizing stress and getting the support you need, it can even be “a fun process,” Dr. Mursi added, “so make it an exciting chapter in your life.”
A version of this article first appeared on Medscape.com.
Moving for any new opportunity in medicine can feel like starting a new life, not just a new job. This is especially true for residency or fellowships, as taking a step forward in your career is exciting. But in the process, you may be leaving family and friends for an unknown city or region where you will need to find a community. And the changes could be long-term. According to the Association of American Medical Colleges’ 2023 Report on Residents, 57.1% of the individuals who completed residency training between 2013 and 2022 are still practicing in the state where they completed their residency.
The process of planning out the right timeline; securing a comfortable, convenient, and affordable place to live; and meeting people while working long hours in an unfamiliar location can be overwhelming. And in the case of many residency programs and healthcare settings, financial assistance, relocation information, and other resources are scarce.
This news organization spoke to recent residents and medical school faculty members about how to navigate a medical move and set yourself up for success.
1. Find Relocation Resources
First things first. Find out what your program or hospital has to offer.
Some institutions help incoming residents by providing housing options or information. The Icahn School of Medicine at Mount Sinai’s Real Estate Division, for example, provides off-campus housing resources that guide new residents and faculty toward safe, convenient places to live in New York City. It also guarantees on-campus or block-leased housing offers to all incoming residents who apply.
Michael Leitman, MD, FACS, professor of surgery and medical education and dean for Graduate Medical Education at the Icahn School of Medicine at Mount Sinai in New York City, recommends connecting with colleagues at your program for guidance on navigating a new city and a new healthcare setting. He encourages incoming residents to use the contact information they receive during the interview and orientation processes to reach out to co-residents and faculty members.
Other residency programs offer partial reimbursement or need-based financial aid to help with the expense of relocation. But this is unlikely to cover all or even most of the cost of a cross-country move.
When Morgen Owens, MD, moved from Alabama to New York City for a physical medicine and rehabilitation residency at Mount Sinai in 2021, her program offered subsidized housing options. But there was little reimbursement for relocation. She paid around $3000 for a one-way rental truck, gas, one night in a hotel, and movers to unload her belongings. She says driving herself kept the price down because full-service movers would have cost her between $4000 and $6000.
If this will strain your finances, several banks offer loans specifically for medical school graduates to cover residency and internship expenses. But be aware that these loans tend to have higher interest rates than federal student loans because they are based on credit score rather than fixed.
2. Reach Out and Buddy Up
Reaching out to more senior residents is essential, and some programs facilitate a buddy system for relocation advice.
Family physician Mursal Sekandari, MD, known as “Dr. Mursi,” attended a residency program at St. Luke’s University Hospital–Bethlehem Campus, in Bethlehem, Pennsylvania. The program’s official buddy system paired her with a senior resident who advised her on the area and gave tips for her apartment search.
On the other hand, when America Revere, MD, moved from Texas to Georgia for a surgery residency, she found that her program offered little relocation assistance, financial or otherwise. She leaned on her co-residents, and especially senior ones, for support while she settled in.
Dr. Revere also discovered the importance of accepting invitations to events hosted by both her fellow residents and her program itself, especially in the early stages of residency. “Accepting social invitations is really the only way to get to know people,” she said. “Sure, you’ll meet people at work and get to know their ‘work’ personalities.” But Dr. Revere’s attendings also threw parties, which she says were a great way to connect with a wider group and build a community.
To meet people both within and beyond her own residency program, Dr. Owens joined a group chat for physical medicine and rehab residents in the New York City area. She suggests looking into GroupMe or WhatsApp groups specific to your specialty.
3. Play the ‘Doctor Card’
Finding a place to live in an unfamiliar and competitive housing market can be one of the biggest challenges of any move. Dr. Owens’ options were limited by owning a dog, which wouldn’t be allowed in her hospital’s subsidized housing. Instead, she opted to find her own apartment in New York City. Her strategy: Playing the “doctor card.”
“I explained my situation: ‘I’m a doctor moving from out of state,’ ” Owens said. “Own that! These companies and brokers will look at you as a student and think, ‘Oh, she has no money, she has no savings, she’s got all of these loans, how is she going to pay for this apartment?’ But you have to say, ‘I’m a doctor. I’m an incoming resident who has X amount of years of job security. I’m not going to lose my job while living here.’ ”
4. Move Early
Dr. Revere found it important to move into her new home 2 weeks before the start of her residency program. Moving in early allowed her to settle in, get to know her area, neighbors, and co-residents, and generally prepare for her first day. It also gave her time to put furniture together — her new vanity alone took 12 hours.
Having a larger window of time before residency can also benefit those who hire movers or have their furniture shipped. When it comes to a cross-country move, it can take a few days to a few weeks for the truck to arrive — which could translate to a few nights or a few weeks without a bed.
“When residency comes, it comes fast,” Dr. Revere said. “It’s very confusing, and the last thing you need is to have half of your stuff unpacked or have no idea where you are or know nobody around you.”
5. Make Your New Home Your Sanctuary
During the stress of residency, your home can be a source of peace, and finding that might require trade-offs.
Dr. Sekandari’s parents urged her to live with roommates to save money on rent, but she insisted that spending more for solitude would be worth it. For her first year of residency, she barely saw her apartment. But when she did, she felt grateful to be in such a tranquil place to ease some of the stress of studying. “If you feel uncomfortable while you’re dealing with something stressful, the stress just exponentially increases,” she said. Creating an environment where you can really relax “makes a difference in how you respond to everything else around you.”
Dr. Revere agrees, urging medical professionals — and particularly residents — to invest in the most comfortable mattresses and bedding they can. Whether you are working nights, she also recommends blackout curtains to help facilitate daytime naps or better sleep in general, especially among the bright lights of bigger cities.
“You’re going to need somewhere to decompress,” she said. “That will look different for everyone. But I would definitely invest in your apartment to make it a sanctuary away from work.”
6. Consider a ‘Live’ Stress Reliever
When it comes to crucial stress relief during residency, “I like mine live,” Dr. Revere said in a YouTube vlog while petting her cat, Calyx.
Taking on the added responsibility of a pet during residency or any medical role may seem counterintuitive. But Revere has zero regrets about bringing Calyx along on her journey. “Cats are very easy,” she said. “I have nothing but wonderful things to say about having a cat during my difficult surgical residency.”
Dr. Owens admits that moving to New York City with her dog was difficult during her first years of residency. She worked an average of 80 hours each week and had little time for walks. She made room in her budget for dog walkers. Thankfully, her hours have eased up as she has progressed through her program, and she can now take her dog on longer walks every day. “He definitely has a better life now that I work fewer hours,” she said.
Once you’ve prepared, made the move, and found your village, it’s time for the real work to begin. “The first couple of months are certainly a challenge of adjusting to a new hospital, a new electronic medical record, a new culture, and a new geographic location,” said Dr. Leitman, who has relocated several times. “But at the end of the day ... it’s you and the patient.” By minimizing stress and getting the support you need, it can even be “a fun process,” Dr. Mursi added, “so make it an exciting chapter in your life.”
A version of this article first appeared on Medscape.com.
Optimal JAK Inhibition for Severe Alopecia Areata
Alopecia areata (AA) is an autoimmune condition that affects children, adolescents, and adults. Severe AA often causes significant burdens, physical discomfort, and psychological stress. Yet, response to therapy is often unpredictable, running the gamut from being refractory to treatment to spontaneous remission.
Dermatologists Raj Chovatiya from Chicago Medical School and Jason Hawkes from the Pacific Skin Institute discuss how to assess AA severity and appropriate therapies, particularly the evolving landscape of JAK inhibitors for patients with severe AA.
The panelists begin by defining severe AA on the basis of the Severity of Alopecia Tool (SALT) score, which assesses AA severity by percentage of hair loss. A patient who has lost over 50% of scalp hair is considered to have severe AA.
Until recently, traditional therapy for severe AA has relied on injectable and systemic corticosteroids, both of which have drawbacks for patients with severe disease. The emergence of Janus kinase (JAK) inhibitors has offered another option for these patients. The panelists discuss recent clinical trials that have shown promising results from JAK inhibitors for treatment of severe AA.
--
Raj Chovatiya, MD, PhD, MSCI, Clinical Associate Professor, Chicago Medical School, Rosalind Franklin University of Medicine and Science, North Chicago, Illinois; Founder and Director, Center for Medical Dermatology and Immunology Research, Chicago, Illinois
Raj Chovatiya, MD, PhD, MSCI, has disclosed the following relevant financial relationships:
Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for: AbbVie; Amgen; Apogee Therapeutics; Arcutis; Argenx; ASLAN Pharmaceuticals; Beiersdorf; Boehringer Ingelheim; Bristol Myers Squibb; Cara Therapeutics; Dermavant; Eli Lilly
Serve(d) as a speaker or a member of a speakers bureau for: AbbVie; Arcutis; Beiersdorf; Boehringer Ingelheim; Bristol Myers Squibb; Dermavant; Eli Lilly and Company; Incyte; LEO Pharma
Alopecia areata (AA) is an autoimmune condition that affects children, adolescents, and adults. Severe AA often causes significant burdens, physical discomfort, and psychological stress. Yet, response to therapy is often unpredictable, running the gamut from being refractory to treatment to spontaneous remission.
Dermatologists Raj Chovatiya from Chicago Medical School and Jason Hawkes from the Pacific Skin Institute discuss how to assess AA severity and appropriate therapies, particularly the evolving landscape of JAK inhibitors for patients with severe AA.
The panelists begin by defining severe AA on the basis of the Severity of Alopecia Tool (SALT) score, which assesses AA severity by percentage of hair loss. A patient who has lost over 50% of scalp hair is considered to have severe AA.
Until recently, traditional therapy for severe AA has relied on injectable and systemic corticosteroids, both of which have drawbacks for patients with severe disease. The emergence of Janus kinase (JAK) inhibitors has offered another option for these patients. The panelists discuss recent clinical trials that have shown promising results from JAK inhibitors for treatment of severe AA.
--
Raj Chovatiya, MD, PhD, MSCI, Clinical Associate Professor, Chicago Medical School, Rosalind Franklin University of Medicine and Science, North Chicago, Illinois; Founder and Director, Center for Medical Dermatology and Immunology Research, Chicago, Illinois
Raj Chovatiya, MD, PhD, MSCI, has disclosed the following relevant financial relationships:
Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for: AbbVie; Amgen; Apogee Therapeutics; Arcutis; Argenx; ASLAN Pharmaceuticals; Beiersdorf; Boehringer Ingelheim; Bristol Myers Squibb; Cara Therapeutics; Dermavant; Eli Lilly
Serve(d) as a speaker or a member of a speakers bureau for: AbbVie; Arcutis; Beiersdorf; Boehringer Ingelheim; Bristol Myers Squibb; Dermavant; Eli Lilly and Company; Incyte; LEO Pharma
Alopecia areata (AA) is an autoimmune condition that affects children, adolescents, and adults. Severe AA often causes significant burdens, physical discomfort, and psychological stress. Yet, response to therapy is often unpredictable, running the gamut from being refractory to treatment to spontaneous remission.
Dermatologists Raj Chovatiya from Chicago Medical School and Jason Hawkes from the Pacific Skin Institute discuss how to assess AA severity and appropriate therapies, particularly the evolving landscape of JAK inhibitors for patients with severe AA.
The panelists begin by defining severe AA on the basis of the Severity of Alopecia Tool (SALT) score, which assesses AA severity by percentage of hair loss. A patient who has lost over 50% of scalp hair is considered to have severe AA.
Until recently, traditional therapy for severe AA has relied on injectable and systemic corticosteroids, both of which have drawbacks for patients with severe disease. The emergence of Janus kinase (JAK) inhibitors has offered another option for these patients. The panelists discuss recent clinical trials that have shown promising results from JAK inhibitors for treatment of severe AA.
--
Raj Chovatiya, MD, PhD, MSCI, Clinical Associate Professor, Chicago Medical School, Rosalind Franklin University of Medicine and Science, North Chicago, Illinois; Founder and Director, Center for Medical Dermatology and Immunology Research, Chicago, Illinois
Raj Chovatiya, MD, PhD, MSCI, has disclosed the following relevant financial relationships:
Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for: AbbVie; Amgen; Apogee Therapeutics; Arcutis; Argenx; ASLAN Pharmaceuticals; Beiersdorf; Boehringer Ingelheim; Bristol Myers Squibb; Cara Therapeutics; Dermavant; Eli Lilly
Serve(d) as a speaker or a member of a speakers bureau for: AbbVie; Arcutis; Beiersdorf; Boehringer Ingelheim; Bristol Myers Squibb; Dermavant; Eli Lilly and Company; Incyte; LEO Pharma

Should Cancer Trial Eligibility Become More Inclusive?
The study, published online in Clinical Cancer Research, highlighted the potential benefits of broadening eligibility criteria for clinical trials.
“It is well known that results in an ‘ideal’ population do not always translate to the real-world population,” senior author Hans Gelderblom, MD, chair of the Department of Medical Oncology at the Leiden University Medical Center, Leiden, the Netherlands, said in a press release. “Eligibility criteria are often too strict, and educated exemptions by experienced investigators can help individual patients, especially in a last-resort trial.”
Although experts have expressed interest in improving trial inclusivity, it’s unclear how doing so might impact treatment safety and efficacy.
In the Drug Rediscovery Protocol (DRUP), Dr. Gelderblom and colleagues examined the impact of broadening trial eligibility on patient outcomes. DRUP is an ongoing Dutch national, multicenter, pan-cancer, nonrandomized clinical trial in which patients are treated off-label with approved molecularly targeted or immunotherapies.
In the trial, 1019 patients with treatment-refractory disease were matched to one of the available study drugs based on their tumor molecular profile and enrolled in parallel cohorts. Cohorts were defined by tumor type, molecular profile, and study drug.
Among these patients, 82 patients — 8% of the cohort — were granted waivers to participate. Most waivers (45%) were granted as exceptions to general- or drug-related eligibility criteria, often because of out-of-range lab results. Other categories included treatment and testing exceptions, as well as out-of-window testing.
The researchers then compared safety and efficacy outcomes between the 82 participants granted waivers and the 937 who did not receive waivers.
Overall, Dr. Gelderblom’s team found that the rate of serious adverse events was similar between patients who received a waiver and those who did not: 39% vs 41%, respectively.
A relationship between waivers and serious adverse events was deemed “unlikely” for 86% of patients and “possible” for 14%. In two cases concerning a direct relationship, for instance, patients who received waivers for decreased hemoglobin levels developed anemia.
The rate of clinical benefit — defined as an objective response or stable disease for at least 16 weeks — was similar between the groups. Overall, 40% of patients who received a waiver (33 of 82) had a clinical benefit vs 33% of patients without a waiver (P = .43). Median overall survival for patients that received a waiver was also similar — 11 months in the waiver group and 8 months in the nonwaiver group (hazard ratio, 0.87; P = .33).
“Safety and clinical benefit were preserved in patients for whom a waiver was granted,” the authors concluded.
The study had several limitations. The diversity of cancer types, treatments, and reasons for protocol exemptions precluded subgroup analyses. In addition, because the decision to grant waivers depended in large part on the likelihood of clinical benefit, “it is possible that patients who received waivers were positively selected for clinical benefit compared with the general study population,” the authors wrote.
So, “although the clinical benefit rate of the patient group for whom a waiver was granted appears to be slightly higher, this difference might be explained by the selection process of the central study team, in which each waiver request was carefully considered, weighing the risks and potential benefits for the patient in question,” the authors explained.
Overall, “these findings advocate for a broader and more inclusive design when establishing novel trials, paving the way for a more effective and tailored application of cancer therapies in patients with advanced or refractory disease,” Dr. Gelderblom said.
Commenting on the study, Bishal Gyawali, MD, PhD, said that “relaxing eligibility criteria is important, and I support this. Trials should include patients that are more representative of the real-world, so that results are generalizable.”
However, “the paper overemphasized efficacy,” said Dr. Gyawali, from Queen’s University, Kingston, Ontario, Canada. The sample size of waiver-granted patients was small, plus “the clinical benefit rate is not a marker of efficacy.
“The response rate is somewhat better, but for a heterogeneous study with multiple targets and drugs, it is difficult to say much about treatment effects here,” Dr. Gyawali added. Overall, “we shouldn’t read too much into treatment benefits based on these numbers.”
Funding for the study was provided by the Stelvio for Life Foundation, the Dutch Cancer Society, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, pharma&, Eisai Co., Ipsen, Merck Sharp & Dohme, Novartis, Pfizer, and Roche. Dr. Gelderblom declared no conflicts of interest, and Dr. Gyawali declared no conflicts of interest related to his comment.
A version of this article appeared on Medscape.com.
The study, published online in Clinical Cancer Research, highlighted the potential benefits of broadening eligibility criteria for clinical trials.
“It is well known that results in an ‘ideal’ population do not always translate to the real-world population,” senior author Hans Gelderblom, MD, chair of the Department of Medical Oncology at the Leiden University Medical Center, Leiden, the Netherlands, said in a press release. “Eligibility criteria are often too strict, and educated exemptions by experienced investigators can help individual patients, especially in a last-resort trial.”
Although experts have expressed interest in improving trial inclusivity, it’s unclear how doing so might impact treatment safety and efficacy.
In the Drug Rediscovery Protocol (DRUP), Dr. Gelderblom and colleagues examined the impact of broadening trial eligibility on patient outcomes. DRUP is an ongoing Dutch national, multicenter, pan-cancer, nonrandomized clinical trial in which patients are treated off-label with approved molecularly targeted or immunotherapies.
In the trial, 1019 patients with treatment-refractory disease were matched to one of the available study drugs based on their tumor molecular profile and enrolled in parallel cohorts. Cohorts were defined by tumor type, molecular profile, and study drug.
Among these patients, 82 patients — 8% of the cohort — were granted waivers to participate. Most waivers (45%) were granted as exceptions to general- or drug-related eligibility criteria, often because of out-of-range lab results. Other categories included treatment and testing exceptions, as well as out-of-window testing.
The researchers then compared safety and efficacy outcomes between the 82 participants granted waivers and the 937 who did not receive waivers.
Overall, Dr. Gelderblom’s team found that the rate of serious adverse events was similar between patients who received a waiver and those who did not: 39% vs 41%, respectively.
A relationship between waivers and serious adverse events was deemed “unlikely” for 86% of patients and “possible” for 14%. In two cases concerning a direct relationship, for instance, patients who received waivers for decreased hemoglobin levels developed anemia.
The rate of clinical benefit — defined as an objective response or stable disease for at least 16 weeks — was similar between the groups. Overall, 40% of patients who received a waiver (33 of 82) had a clinical benefit vs 33% of patients without a waiver (P = .43). Median overall survival for patients that received a waiver was also similar — 11 months in the waiver group and 8 months in the nonwaiver group (hazard ratio, 0.87; P = .33).
“Safety and clinical benefit were preserved in patients for whom a waiver was granted,” the authors concluded.
The study had several limitations. The diversity of cancer types, treatments, and reasons for protocol exemptions precluded subgroup analyses. In addition, because the decision to grant waivers depended in large part on the likelihood of clinical benefit, “it is possible that patients who received waivers were positively selected for clinical benefit compared with the general study population,” the authors wrote.
So, “although the clinical benefit rate of the patient group for whom a waiver was granted appears to be slightly higher, this difference might be explained by the selection process of the central study team, in which each waiver request was carefully considered, weighing the risks and potential benefits for the patient in question,” the authors explained.
Overall, “these findings advocate for a broader and more inclusive design when establishing novel trials, paving the way for a more effective and tailored application of cancer therapies in patients with advanced or refractory disease,” Dr. Gelderblom said.
Commenting on the study, Bishal Gyawali, MD, PhD, said that “relaxing eligibility criteria is important, and I support this. Trials should include patients that are more representative of the real-world, so that results are generalizable.”
However, “the paper overemphasized efficacy,” said Dr. Gyawali, from Queen’s University, Kingston, Ontario, Canada. The sample size of waiver-granted patients was small, plus “the clinical benefit rate is not a marker of efficacy.
“The response rate is somewhat better, but for a heterogeneous study with multiple targets and drugs, it is difficult to say much about treatment effects here,” Dr. Gyawali added. Overall, “we shouldn’t read too much into treatment benefits based on these numbers.”
Funding for the study was provided by the Stelvio for Life Foundation, the Dutch Cancer Society, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, pharma&, Eisai Co., Ipsen, Merck Sharp & Dohme, Novartis, Pfizer, and Roche. Dr. Gelderblom declared no conflicts of interest, and Dr. Gyawali declared no conflicts of interest related to his comment.
A version of this article appeared on Medscape.com.
The study, published online in Clinical Cancer Research, highlighted the potential benefits of broadening eligibility criteria for clinical trials.
“It is well known that results in an ‘ideal’ population do not always translate to the real-world population,” senior author Hans Gelderblom, MD, chair of the Department of Medical Oncology at the Leiden University Medical Center, Leiden, the Netherlands, said in a press release. “Eligibility criteria are often too strict, and educated exemptions by experienced investigators can help individual patients, especially in a last-resort trial.”
Although experts have expressed interest in improving trial inclusivity, it’s unclear how doing so might impact treatment safety and efficacy.
In the Drug Rediscovery Protocol (DRUP), Dr. Gelderblom and colleagues examined the impact of broadening trial eligibility on patient outcomes. DRUP is an ongoing Dutch national, multicenter, pan-cancer, nonrandomized clinical trial in which patients are treated off-label with approved molecularly targeted or immunotherapies.
In the trial, 1019 patients with treatment-refractory disease were matched to one of the available study drugs based on their tumor molecular profile and enrolled in parallel cohorts. Cohorts were defined by tumor type, molecular profile, and study drug.
Among these patients, 82 patients — 8% of the cohort — were granted waivers to participate. Most waivers (45%) were granted as exceptions to general- or drug-related eligibility criteria, often because of out-of-range lab results. Other categories included treatment and testing exceptions, as well as out-of-window testing.
The researchers then compared safety and efficacy outcomes between the 82 participants granted waivers and the 937 who did not receive waivers.
Overall, Dr. Gelderblom’s team found that the rate of serious adverse events was similar between patients who received a waiver and those who did not: 39% vs 41%, respectively.
A relationship between waivers and serious adverse events was deemed “unlikely” for 86% of patients and “possible” for 14%. In two cases concerning a direct relationship, for instance, patients who received waivers for decreased hemoglobin levels developed anemia.
The rate of clinical benefit — defined as an objective response or stable disease for at least 16 weeks — was similar between the groups. Overall, 40% of patients who received a waiver (33 of 82) had a clinical benefit vs 33% of patients without a waiver (P = .43). Median overall survival for patients that received a waiver was also similar — 11 months in the waiver group and 8 months in the nonwaiver group (hazard ratio, 0.87; P = .33).
“Safety and clinical benefit were preserved in patients for whom a waiver was granted,” the authors concluded.
The study had several limitations. The diversity of cancer types, treatments, and reasons for protocol exemptions precluded subgroup analyses. In addition, because the decision to grant waivers depended in large part on the likelihood of clinical benefit, “it is possible that patients who received waivers were positively selected for clinical benefit compared with the general study population,” the authors wrote.
So, “although the clinical benefit rate of the patient group for whom a waiver was granted appears to be slightly higher, this difference might be explained by the selection process of the central study team, in which each waiver request was carefully considered, weighing the risks and potential benefits for the patient in question,” the authors explained.
Overall, “these findings advocate for a broader and more inclusive design when establishing novel trials, paving the way for a more effective and tailored application of cancer therapies in patients with advanced or refractory disease,” Dr. Gelderblom said.
Commenting on the study, Bishal Gyawali, MD, PhD, said that “relaxing eligibility criteria is important, and I support this. Trials should include patients that are more representative of the real-world, so that results are generalizable.”
However, “the paper overemphasized efficacy,” said Dr. Gyawali, from Queen’s University, Kingston, Ontario, Canada. The sample size of waiver-granted patients was small, plus “the clinical benefit rate is not a marker of efficacy.
“The response rate is somewhat better, but for a heterogeneous study with multiple targets and drugs, it is difficult to say much about treatment effects here,” Dr. Gyawali added. Overall, “we shouldn’t read too much into treatment benefits based on these numbers.”
Funding for the study was provided by the Stelvio for Life Foundation, the Dutch Cancer Society, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, pharma&, Eisai Co., Ipsen, Merck Sharp & Dohme, Novartis, Pfizer, and Roche. Dr. Gelderblom declared no conflicts of interest, and Dr. Gyawali declared no conflicts of interest related to his comment.
A version of this article appeared on Medscape.com.
CAR T-Cell Treatment Data Expands in Refractory Rheumatic Diseases, Demonstrating Consistent Efficacy
VIENNA — From a dozen or so studies and sessions devoted to the role of chimeric antigen receptor (CAR) T cells in rheumatic diseases at the annual European Congress of Rheumatology, the message was uniformly positive, supporting growing evidence that drugs in this class are heading toward a paradigm shift in refractory rheumatic diseases.
Of the reports, an update from a 15-patient case series with at least 1 year of follow-up provides “the first long-term evidence of safety and efficacy in multiple rheumatic diseases,” according to Georg Schett, MD, PhD, director of rheumatology and immunology, University of Erlangen-Nürnberg, Erlangen, Germany.
The report of high rates of activity and low relative risk of serious adverse events from the same series was published earlier this year in The New England Journal of Medicine when the median follow-up was 15 months. Almost all of the patients have now completed at least 1 year of follow-up and about a third have completed more than 2 years.
SLE Is Frequently Targeted in CAR T-Cell Studies
The three rheumatic diseases represented in this series of patients, all of whom had failed multiple previous immune suppressive treatments, were systemic lupus erythematosus (SLE), idiopathic inflammatory myositis (IIM), and systemic sclerosis (SSc). After the autologous T cells were harvested, they were expanded and transfected with the CD19 CAR. The proprietary investigational product, called MB-CART19.1 (Miltenyi Biotec), was administered in a single dose of one million cells per kg bodyweight.
The response rates have been, and continue to be, impressive. For the eight patients with SLE, all achieved the definition of remission in SLE criteria after one dose of treatment. Complete resolution of all major symptom types was achieved after 6 months of follow-up. So far, no patient has relapsed.
For the three patients with IIM, all reached the American College of Rheumatology–EULAR criteria for a major response. All creatine kinase levels had normalized by 3 months. In this group, there was one relapse, which occurred after 18 months of follow-up.
All four patients with SSc achieved a major response on the European Scleroderma Trials and Research (EUSTAR) group activity index. The median reduction from baseline in the EUSTAR score was 4.2 points, and this has been maintained in follow-up to date.
Remissions Have Persisted Off All Therapies
These remissions were achieved and maintained after a single dose of CAR T-cell therapy despite discontinuation of all immunosuppressive therapies. With the exception of the single relapse, all remissions have persisted through follow-up to date.
These responses were achieved with manageable side effects, according to Dr. Schett. The most serious adverse event was a grade 4 neutropenia that developed 4 months after receiving CAR T cells. It resolved with granulocyte colony-stimulating factor treatment. Cytokine release syndrome (CRS) has occurred in 10 patients, but it was grade 1 in eight patients and grade 2 in the others. There has been no neurotoxicity.
Almost all patients have experienced an infection during follow-up, but there has been no discernible pattern in relationship to the timing or types of infections. The most common have involved the upper respiratory tract and have been of mild severity, with cases disseminated similarly over early vs late follow-up. There was one case of pneumonia involving antibiotic treatment and a hospital stay, but it resolved.
Dr. Schett acknowledged that safety is a bigger concern in autoimmune diseases, which are often serious but rarely fatal, than in the hematologic malignancies for which CAR T cells were initially tested, but the low rates of serious adverse events in his and other early studies have supported the premise that the risks are not the same.
Asked specifically if CAR T cells can be considered a game changer in autoimmune rheumatic diseases, Dr. Schett was cautious. One reason is the CAR T cells are a complex therapy relative to biologic disease-modifying antirheumatic drugs. He thinks, therefore, that much more data are needed to confirm safety and efficacy. In addition, they are expensive, so it is not yet clear how they will be integrated with other options.
Yet, he thinks the evidence so far suggests a profound effect on the fundamental drivers of autoimmune disease. Their specific mechanism of benefit is still being evaluated, but he considers the clinical responses consistent with a “reset” hypothesis.
After a response, “we are seeing drug-free remissions in some patients as long as they have been followed,” Dr. Schett said. Based on the fact that disease control is being observed off all other therapies, “this only makes sense to me if there is some sort of immunologic reset.”
CAR T-Cell Studies in Autoimmune Diseases Are Proliferating
At last count, there were about 40 studies being performed with CAR T cells in various autoimmune diseases, most of which were rheumatologic disorders, according to Dr. Schett. He noted that funding is coming from multinational drug companies, small biotech startups, and investigator-initiated studies at academic centers.
At EULAR, beyond case studies and anecdotal reports, all of the clinical studies were still at the level of phase 1 or 1/2. Consistent with the data presented by Dr. Schett, the drugs have been nearly uniformly effective, with major responses persisting in patients off other therapies. Adverse events have been manageable.
Examples include a phase 1/2 multinational study with the investigational CAR T-cell therapy YTB323 (Novartis), which demonstrated acceptable safety and a strong signal of benefit in six patients with SLE. In this report, CRS was also common, but no case of CRS was more severe than grade 2. There was no neurotoxicity. Infections did occur but were of relatively mild grades and resolved with treatment.
For efficacy in the ongoing follow-up, SLE symptoms as measured with the SLE Disease Activity Index began to abate at about 14 days after the single-infusion treatment. Improvement on the Physician Global Assessment was also observed between 14 and 28 days. C3 and C4 complement levels started to rise at about 28 days. While the responses have correlated with the observed changes in biomarkers of immune function, they have endured through a median follow-up that now exceeds 6 months.
Complete B-Cell Depletion Is Followed by Full Recovery
“Pharmacokinetic and pharmacodynamic studies revealed peak expansion of CAR T cells approximately 13-21 days post infusion, which was accompanied by deep B-cell depletion followed by subsequent B-cell recovery,” reported Josefina Cortés-Hernández, MD, PhD, a senior lecturer at Vall d’Hebron Research Institute, Barcelona, Spain.
Dr. Schett had reported the same pattern of expansion followed by a rapid elimination of detectable CAR T cells despite the sustained clinical benefit.
Dr. Cortés-Hernández said that the signal of efficacy in the context of acceptable safety supports an expansion of clinical studies with this CAR T-cell product in SLE and perhaps other autoimmune disorders.
In another early-stage study, patients with SLE who had failed multiple prior lines of therapy have been enrolled in an ongoing study with a compound CAR (cCAR) T cell. This experimental proprietary product (iCAR Bio Therapeutics, Zhongshan, China) targets both the B-cell maturation antigen and CD19, according to Greg Deener, the chief executive officer of iCell Gene Therapeutics, New York City.
cCAR T-Cell Construct Targets Immune Reset
With this construct, the goal is to deplete long-lived plasma cells as well as B cells in order to achieve a more complete humoral reset. While preliminary data from the phase 1 trial were published earlier this year in Annals of the Rheumatic Diseases, Mr. Deener focused his presentation at EULAR 2024 on 12 patients with SLE and lupus nephritis, a severe form of SLE that threatens glomerular structures and can lead to end-stage liver disease.
B cells in the peripheral blood could not be detected within 10 days of the cCAR infusion, and the immunoglobulins IgM and IgA were undetectable by day 42.
However, after B-cell recovery by day 150, “flow cytometry and B-cell receptor sequencing confirmed full humoral reset was achieved,” Mr. Deener said.
The remission has been durable in 11 of the 12 patients after a mean follow-up of 458 days, Mr. Deener reported. He noted that an improvement in renal function has been observed in the majority of patients.
Like others, he reported that treatment has been relatively well tolerated. In this series of patients, there have been no cases of CRS more severe than grade 1.
Overall, the cCAR data in lupus nephritis support the hypothesis that CAR T cells are reprogramming the immune system, according to Mr. Deener.
Combined with a reasonable safety profile, the consistency of benefit from CAR T cells in autoimmune rheumatic diseases is good news, but all of the investigators who spoke at EULAR agreed that there are still many unanswered questions. Not least, it is unclear whether patients can be effectively and safely retreated when and if relapses occur. Even though Dr. Schett did report a response with retreatment following a relapse, he said that there is no conclusion to draw from a single patient.
Yet, the high rates of remissions in patients with disease refractory to other therapeutic options is highly encouraging, particularly with the manageable side effects now reported by multiple investigators using different CAR T-cell products.
“Roughly 100 patients with rheumatic diseases have been treated with CAR T-cells, and we have not seen a high-grade CRS or neurotoxicity,” he said.
Long-term efficacy is less clear. With the first clinical studies in autoimmune diseases initiated in 2021, few patients have been followed for more than 2 years. Even with the high rates of response that will certainly fuel efforts to rapidly bring these treatments forward, long-term data are now the missing piece.
Other Case Series Presented at EULAR
Several other abstracts reported on patients with SSc who were treated with CD19-targeting CAR T cells:
Three patients for whom autologous hematopoietic stem cell transplantation was contraindicated or unsuccessful were successfully and safely treated.
Six patients with diffuse and progressive disease achieved stable disease activity without additional immunosuppression for up to 1 year after treatment.
Dr. Schett reported no potential conflicts of interest, and the study he presented was not funded by industry. Dr. Cortés-Hernández reported a financial relationship with Novartis, which funded the study of the CAR T-cell therapy YTB323, as well as with GlaxoSmithKline, which was not involved in the study she presented. Mr. Deener is an employee of iCell Gene Therapeutics, which provided funding for the trial he presented.
August 7, 2024 — Editor's note: This article was updated with additional disclosure information for Dr. Josefina Cortés-Hernández.
A version of this article appeared on Medscape.com.
VIENNA — From a dozen or so studies and sessions devoted to the role of chimeric antigen receptor (CAR) T cells in rheumatic diseases at the annual European Congress of Rheumatology, the message was uniformly positive, supporting growing evidence that drugs in this class are heading toward a paradigm shift in refractory rheumatic diseases.
Of the reports, an update from a 15-patient case series with at least 1 year of follow-up provides “the first long-term evidence of safety and efficacy in multiple rheumatic diseases,” according to Georg Schett, MD, PhD, director of rheumatology and immunology, University of Erlangen-Nürnberg, Erlangen, Germany.
The report of high rates of activity and low relative risk of serious adverse events from the same series was published earlier this year in The New England Journal of Medicine when the median follow-up was 15 months. Almost all of the patients have now completed at least 1 year of follow-up and about a third have completed more than 2 years.
SLE Is Frequently Targeted in CAR T-Cell Studies
The three rheumatic diseases represented in this series of patients, all of whom had failed multiple previous immune suppressive treatments, were systemic lupus erythematosus (SLE), idiopathic inflammatory myositis (IIM), and systemic sclerosis (SSc). After the autologous T cells were harvested, they were expanded and transfected with the CD19 CAR. The proprietary investigational product, called MB-CART19.1 (Miltenyi Biotec), was administered in a single dose of one million cells per kg bodyweight.
The response rates have been, and continue to be, impressive. For the eight patients with SLE, all achieved the definition of remission in SLE criteria after one dose of treatment. Complete resolution of all major symptom types was achieved after 6 months of follow-up. So far, no patient has relapsed.
For the three patients with IIM, all reached the American College of Rheumatology–EULAR criteria for a major response. All creatine kinase levels had normalized by 3 months. In this group, there was one relapse, which occurred after 18 months of follow-up.
All four patients with SSc achieved a major response on the European Scleroderma Trials and Research (EUSTAR) group activity index. The median reduction from baseline in the EUSTAR score was 4.2 points, and this has been maintained in follow-up to date.
Remissions Have Persisted Off All Therapies
These remissions were achieved and maintained after a single dose of CAR T-cell therapy despite discontinuation of all immunosuppressive therapies. With the exception of the single relapse, all remissions have persisted through follow-up to date.
These responses were achieved with manageable side effects, according to Dr. Schett. The most serious adverse event was a grade 4 neutropenia that developed 4 months after receiving CAR T cells. It resolved with granulocyte colony-stimulating factor treatment. Cytokine release syndrome (CRS) has occurred in 10 patients, but it was grade 1 in eight patients and grade 2 in the others. There has been no neurotoxicity.
Almost all patients have experienced an infection during follow-up, but there has been no discernible pattern in relationship to the timing or types of infections. The most common have involved the upper respiratory tract and have been of mild severity, with cases disseminated similarly over early vs late follow-up. There was one case of pneumonia involving antibiotic treatment and a hospital stay, but it resolved.
Dr. Schett acknowledged that safety is a bigger concern in autoimmune diseases, which are often serious but rarely fatal, than in the hematologic malignancies for which CAR T cells were initially tested, but the low rates of serious adverse events in his and other early studies have supported the premise that the risks are not the same.
Asked specifically if CAR T cells can be considered a game changer in autoimmune rheumatic diseases, Dr. Schett was cautious. One reason is the CAR T cells are a complex therapy relative to biologic disease-modifying antirheumatic drugs. He thinks, therefore, that much more data are needed to confirm safety and efficacy. In addition, they are expensive, so it is not yet clear how they will be integrated with other options.
Yet, he thinks the evidence so far suggests a profound effect on the fundamental drivers of autoimmune disease. Their specific mechanism of benefit is still being evaluated, but he considers the clinical responses consistent with a “reset” hypothesis.
After a response, “we are seeing drug-free remissions in some patients as long as they have been followed,” Dr. Schett said. Based on the fact that disease control is being observed off all other therapies, “this only makes sense to me if there is some sort of immunologic reset.”
CAR T-Cell Studies in Autoimmune Diseases Are Proliferating
At last count, there were about 40 studies being performed with CAR T cells in various autoimmune diseases, most of which were rheumatologic disorders, according to Dr. Schett. He noted that funding is coming from multinational drug companies, small biotech startups, and investigator-initiated studies at academic centers.
At EULAR, beyond case studies and anecdotal reports, all of the clinical studies were still at the level of phase 1 or 1/2. Consistent with the data presented by Dr. Schett, the drugs have been nearly uniformly effective, with major responses persisting in patients off other therapies. Adverse events have been manageable.
Examples include a phase 1/2 multinational study with the investigational CAR T-cell therapy YTB323 (Novartis), which demonstrated acceptable safety and a strong signal of benefit in six patients with SLE. In this report, CRS was also common, but no case of CRS was more severe than grade 2. There was no neurotoxicity. Infections did occur but were of relatively mild grades and resolved with treatment.
For efficacy in the ongoing follow-up, SLE symptoms as measured with the SLE Disease Activity Index began to abate at about 14 days after the single-infusion treatment. Improvement on the Physician Global Assessment was also observed between 14 and 28 days. C3 and C4 complement levels started to rise at about 28 days. While the responses have correlated with the observed changes in biomarkers of immune function, they have endured through a median follow-up that now exceeds 6 months.
Complete B-Cell Depletion Is Followed by Full Recovery
“Pharmacokinetic and pharmacodynamic studies revealed peak expansion of CAR T cells approximately 13-21 days post infusion, which was accompanied by deep B-cell depletion followed by subsequent B-cell recovery,” reported Josefina Cortés-Hernández, MD, PhD, a senior lecturer at Vall d’Hebron Research Institute, Barcelona, Spain.
Dr. Schett had reported the same pattern of expansion followed by a rapid elimination of detectable CAR T cells despite the sustained clinical benefit.
Dr. Cortés-Hernández said that the signal of efficacy in the context of acceptable safety supports an expansion of clinical studies with this CAR T-cell product in SLE and perhaps other autoimmune disorders.
In another early-stage study, patients with SLE who had failed multiple prior lines of therapy have been enrolled in an ongoing study with a compound CAR (cCAR) T cell. This experimental proprietary product (iCAR Bio Therapeutics, Zhongshan, China) targets both the B-cell maturation antigen and CD19, according to Greg Deener, the chief executive officer of iCell Gene Therapeutics, New York City.
cCAR T-Cell Construct Targets Immune Reset
With this construct, the goal is to deplete long-lived plasma cells as well as B cells in order to achieve a more complete humoral reset. While preliminary data from the phase 1 trial were published earlier this year in Annals of the Rheumatic Diseases, Mr. Deener focused his presentation at EULAR 2024 on 12 patients with SLE and lupus nephritis, a severe form of SLE that threatens glomerular structures and can lead to end-stage liver disease.
B cells in the peripheral blood could not be detected within 10 days of the cCAR infusion, and the immunoglobulins IgM and IgA were undetectable by day 42.
However, after B-cell recovery by day 150, “flow cytometry and B-cell receptor sequencing confirmed full humoral reset was achieved,” Mr. Deener said.
The remission has been durable in 11 of the 12 patients after a mean follow-up of 458 days, Mr. Deener reported. He noted that an improvement in renal function has been observed in the majority of patients.
Like others, he reported that treatment has been relatively well tolerated. In this series of patients, there have been no cases of CRS more severe than grade 1.
Overall, the cCAR data in lupus nephritis support the hypothesis that CAR T cells are reprogramming the immune system, according to Mr. Deener.
Combined with a reasonable safety profile, the consistency of benefit from CAR T cells in autoimmune rheumatic diseases is good news, but all of the investigators who spoke at EULAR agreed that there are still many unanswered questions. Not least, it is unclear whether patients can be effectively and safely retreated when and if relapses occur. Even though Dr. Schett did report a response with retreatment following a relapse, he said that there is no conclusion to draw from a single patient.
Yet, the high rates of remissions in patients with disease refractory to other therapeutic options is highly encouraging, particularly with the manageable side effects now reported by multiple investigators using different CAR T-cell products.
“Roughly 100 patients with rheumatic diseases have been treated with CAR T-cells, and we have not seen a high-grade CRS or neurotoxicity,” he said.
Long-term efficacy is less clear. With the first clinical studies in autoimmune diseases initiated in 2021, few patients have been followed for more than 2 years. Even with the high rates of response that will certainly fuel efforts to rapidly bring these treatments forward, long-term data are now the missing piece.
Other Case Series Presented at EULAR
Several other abstracts reported on patients with SSc who were treated with CD19-targeting CAR T cells:
Three patients for whom autologous hematopoietic stem cell transplantation was contraindicated or unsuccessful were successfully and safely treated.
Six patients with diffuse and progressive disease achieved stable disease activity without additional immunosuppression for up to 1 year after treatment.
Dr. Schett reported no potential conflicts of interest, and the study he presented was not funded by industry. Dr. Cortés-Hernández reported a financial relationship with Novartis, which funded the study of the CAR T-cell therapy YTB323, as well as with GlaxoSmithKline, which was not involved in the study she presented. Mr. Deener is an employee of iCell Gene Therapeutics, which provided funding for the trial he presented.
August 7, 2024 — Editor's note: This article was updated with additional disclosure information for Dr. Josefina Cortés-Hernández.
A version of this article appeared on Medscape.com.
VIENNA — From a dozen or so studies and sessions devoted to the role of chimeric antigen receptor (CAR) T cells in rheumatic diseases at the annual European Congress of Rheumatology, the message was uniformly positive, supporting growing evidence that drugs in this class are heading toward a paradigm shift in refractory rheumatic diseases.
Of the reports, an update from a 15-patient case series with at least 1 year of follow-up provides “the first long-term evidence of safety and efficacy in multiple rheumatic diseases,” according to Georg Schett, MD, PhD, director of rheumatology and immunology, University of Erlangen-Nürnberg, Erlangen, Germany.
The report of high rates of activity and low relative risk of serious adverse events from the same series was published earlier this year in The New England Journal of Medicine when the median follow-up was 15 months. Almost all of the patients have now completed at least 1 year of follow-up and about a third have completed more than 2 years.
SLE Is Frequently Targeted in CAR T-Cell Studies
The three rheumatic diseases represented in this series of patients, all of whom had failed multiple previous immune suppressive treatments, were systemic lupus erythematosus (SLE), idiopathic inflammatory myositis (IIM), and systemic sclerosis (SSc). After the autologous T cells were harvested, they were expanded and transfected with the CD19 CAR. The proprietary investigational product, called MB-CART19.1 (Miltenyi Biotec), was administered in a single dose of one million cells per kg bodyweight.
The response rates have been, and continue to be, impressive. For the eight patients with SLE, all achieved the definition of remission in SLE criteria after one dose of treatment. Complete resolution of all major symptom types was achieved after 6 months of follow-up. So far, no patient has relapsed.
For the three patients with IIM, all reached the American College of Rheumatology–EULAR criteria for a major response. All creatine kinase levels had normalized by 3 months. In this group, there was one relapse, which occurred after 18 months of follow-up.
All four patients with SSc achieved a major response on the European Scleroderma Trials and Research (EUSTAR) group activity index. The median reduction from baseline in the EUSTAR score was 4.2 points, and this has been maintained in follow-up to date.
Remissions Have Persisted Off All Therapies
These remissions were achieved and maintained after a single dose of CAR T-cell therapy despite discontinuation of all immunosuppressive therapies. With the exception of the single relapse, all remissions have persisted through follow-up to date.
These responses were achieved with manageable side effects, according to Dr. Schett. The most serious adverse event was a grade 4 neutropenia that developed 4 months after receiving CAR T cells. It resolved with granulocyte colony-stimulating factor treatment. Cytokine release syndrome (CRS) has occurred in 10 patients, but it was grade 1 in eight patients and grade 2 in the others. There has been no neurotoxicity.
Almost all patients have experienced an infection during follow-up, but there has been no discernible pattern in relationship to the timing or types of infections. The most common have involved the upper respiratory tract and have been of mild severity, with cases disseminated similarly over early vs late follow-up. There was one case of pneumonia involving antibiotic treatment and a hospital stay, but it resolved.
Dr. Schett acknowledged that safety is a bigger concern in autoimmune diseases, which are often serious but rarely fatal, than in the hematologic malignancies for which CAR T cells were initially tested, but the low rates of serious adverse events in his and other early studies have supported the premise that the risks are not the same.
Asked specifically if CAR T cells can be considered a game changer in autoimmune rheumatic diseases, Dr. Schett was cautious. One reason is the CAR T cells are a complex therapy relative to biologic disease-modifying antirheumatic drugs. He thinks, therefore, that much more data are needed to confirm safety and efficacy. In addition, they are expensive, so it is not yet clear how they will be integrated with other options.
Yet, he thinks the evidence so far suggests a profound effect on the fundamental drivers of autoimmune disease. Their specific mechanism of benefit is still being evaluated, but he considers the clinical responses consistent with a “reset” hypothesis.
After a response, “we are seeing drug-free remissions in some patients as long as they have been followed,” Dr. Schett said. Based on the fact that disease control is being observed off all other therapies, “this only makes sense to me if there is some sort of immunologic reset.”
CAR T-Cell Studies in Autoimmune Diseases Are Proliferating
At last count, there were about 40 studies being performed with CAR T cells in various autoimmune diseases, most of which were rheumatologic disorders, according to Dr. Schett. He noted that funding is coming from multinational drug companies, small biotech startups, and investigator-initiated studies at academic centers.
At EULAR, beyond case studies and anecdotal reports, all of the clinical studies were still at the level of phase 1 or 1/2. Consistent with the data presented by Dr. Schett, the drugs have been nearly uniformly effective, with major responses persisting in patients off other therapies. Adverse events have been manageable.
Examples include a phase 1/2 multinational study with the investigational CAR T-cell therapy YTB323 (Novartis), which demonstrated acceptable safety and a strong signal of benefit in six patients with SLE. In this report, CRS was also common, but no case of CRS was more severe than grade 2. There was no neurotoxicity. Infections did occur but were of relatively mild grades and resolved with treatment.
For efficacy in the ongoing follow-up, SLE symptoms as measured with the SLE Disease Activity Index began to abate at about 14 days after the single-infusion treatment. Improvement on the Physician Global Assessment was also observed between 14 and 28 days. C3 and C4 complement levels started to rise at about 28 days. While the responses have correlated with the observed changes in biomarkers of immune function, they have endured through a median follow-up that now exceeds 6 months.
Complete B-Cell Depletion Is Followed by Full Recovery
“Pharmacokinetic and pharmacodynamic studies revealed peak expansion of CAR T cells approximately 13-21 days post infusion, which was accompanied by deep B-cell depletion followed by subsequent B-cell recovery,” reported Josefina Cortés-Hernández, MD, PhD, a senior lecturer at Vall d’Hebron Research Institute, Barcelona, Spain.
Dr. Schett had reported the same pattern of expansion followed by a rapid elimination of detectable CAR T cells despite the sustained clinical benefit.
Dr. Cortés-Hernández said that the signal of efficacy in the context of acceptable safety supports an expansion of clinical studies with this CAR T-cell product in SLE and perhaps other autoimmune disorders.
In another early-stage study, patients with SLE who had failed multiple prior lines of therapy have been enrolled in an ongoing study with a compound CAR (cCAR) T cell. This experimental proprietary product (iCAR Bio Therapeutics, Zhongshan, China) targets both the B-cell maturation antigen and CD19, according to Greg Deener, the chief executive officer of iCell Gene Therapeutics, New York City.
cCAR T-Cell Construct Targets Immune Reset
With this construct, the goal is to deplete long-lived plasma cells as well as B cells in order to achieve a more complete humoral reset. While preliminary data from the phase 1 trial were published earlier this year in Annals of the Rheumatic Diseases, Mr. Deener focused his presentation at EULAR 2024 on 12 patients with SLE and lupus nephritis, a severe form of SLE that threatens glomerular structures and can lead to end-stage liver disease.
B cells in the peripheral blood could not be detected within 10 days of the cCAR infusion, and the immunoglobulins IgM and IgA were undetectable by day 42.
However, after B-cell recovery by day 150, “flow cytometry and B-cell receptor sequencing confirmed full humoral reset was achieved,” Mr. Deener said.
The remission has been durable in 11 of the 12 patients after a mean follow-up of 458 days, Mr. Deener reported. He noted that an improvement in renal function has been observed in the majority of patients.
Like others, he reported that treatment has been relatively well tolerated. In this series of patients, there have been no cases of CRS more severe than grade 1.
Overall, the cCAR data in lupus nephritis support the hypothesis that CAR T cells are reprogramming the immune system, according to Mr. Deener.
Combined with a reasonable safety profile, the consistency of benefit from CAR T cells in autoimmune rheumatic diseases is good news, but all of the investigators who spoke at EULAR agreed that there are still many unanswered questions. Not least, it is unclear whether patients can be effectively and safely retreated when and if relapses occur. Even though Dr. Schett did report a response with retreatment following a relapse, he said that there is no conclusion to draw from a single patient.
Yet, the high rates of remissions in patients with disease refractory to other therapeutic options is highly encouraging, particularly with the manageable side effects now reported by multiple investigators using different CAR T-cell products.
“Roughly 100 patients with rheumatic diseases have been treated with CAR T-cells, and we have not seen a high-grade CRS or neurotoxicity,” he said.
Long-term efficacy is less clear. With the first clinical studies in autoimmune diseases initiated in 2021, few patients have been followed for more than 2 years. Even with the high rates of response that will certainly fuel efforts to rapidly bring these treatments forward, long-term data are now the missing piece.
Other Case Series Presented at EULAR
Several other abstracts reported on patients with SSc who were treated with CD19-targeting CAR T cells:
Three patients for whom autologous hematopoietic stem cell transplantation was contraindicated or unsuccessful were successfully and safely treated.
Six patients with diffuse and progressive disease achieved stable disease activity without additional immunosuppression for up to 1 year after treatment.
Dr. Schett reported no potential conflicts of interest, and the study he presented was not funded by industry. Dr. Cortés-Hernández reported a financial relationship with Novartis, which funded the study of the CAR T-cell therapy YTB323, as well as with GlaxoSmithKline, which was not involved in the study she presented. Mr. Deener is an employee of iCell Gene Therapeutics, which provided funding for the trial he presented.
August 7, 2024 — Editor's note: This article was updated with additional disclosure information for Dr. Josefina Cortés-Hernández.
A version of this article appeared on Medscape.com.
FROM EULAR 2024
How Dermatologists Can Safeguard Against Malpractice Claims
for liability. Dermatologists can protect themselves by understanding malpractice trends and taking preventive steps, such as making sure NPOs have appropriate training and using a rigorous informed consent process, according to a dermatology resident and a dermatologist who have researched recent trends in dermatology lawsuits.
“It’s really important that physicians recognize their responsibility when delegating procedures to nonphysician operators and the physician’s role in supervision of these procedures,” Scott Stratman, MD, MPH, a dermatology resident at the Icahn School of Medicine at Mount Sinai, New York City, told this news organization. He led a study recently published in the Journal of the American Academy of Dermatology, which found that the majority (52%) of malpractice cases for cutaneous energy-based device procedures in the LexisNexis database from 1985 to September 2023 involved NPOs. The study did not break the data down between different types of NPOs.
Trends in Dermatology Malpractice
This follows a similar trend reported in a 2014 study led by Mathew M. Avram, MD, JD, director of the MGH Dermatology Laser and Cosmetic Center at Massachusetts General Hospital, Boston. The study analyzed liability claims related to cutaneous laser surgery performed by nonphysicians from January 1999 to December 2012.
“With nonphysician litigation data, we saw trend lines beginning in 2008 where the proportion of cases began to increase,” Dr. Avram said at the American Society for Laser Medicine and Surgery (ASLMS) meeting on April 12, 2024. “Over a period of 2008-2012, it went from 36% of cases to about 78%,” he said.
About a quarter (23.4%) of those were in medical offices; 76.6% were in nontraditional settings such as medical spas, he added. The proportion of NPOs was similar in a 2022 study that looked at causes of litigation in cutaneous laser surgery from 2012 to 2020, Dr. Avram said. Again, neither study broke down cases involving NPOs by specific type, but the 2014 study reported that 64% of cases by NPOs occurred outside of a traditional medical setting.
“So it seems that the location and potentially the supervision are issues that are important to patient safety,” Dr. Avram said at the meeting. While state laws regarding laser delegation vary widely, “depending on where you practice, it’s incumbent upon you to know that.”
Dr. Avram and colleagues were also the authors of a study published in June in Dermatologic Surgery that looked at the reasons behind ligations involving dermatologists in a retrospective analysis of 48 state and federal cases between 2011 and 2022. The majority of cases — 54.2% — were for unexpected harm, followed by wrong or delayed diagnoses, which accounted for a third of litigations.
Dr. Stratman’s study found that laser hair removal was the most common procedure for malpractice claims in dermatology among cutaneous energy-based device procedures. Complications from energy-based devices included burns, scarring, and pigmentation changes.
The growth of malpractice suits involving NPOs could be because NPOs are performing a greater proportion of dermatologic procedures, “particularly those practicing without direct supervision, such as in the context of a medical spa,” Dr. Stratman said in the interview. “Again, this highlights a physician’s responsibility in delegating these kinds of procedures to NPOs.”
Training Is a Must — But Not Standardized
Comprehensive training for physicians, staff, NPOs, and physicians “is all necessary and paramount in order to diminish adverse outcomes and legal risk, and then, of course, all these practitioners, be it staff or [NPOs], and, of course, physicians, are all held to the same standard of care,” Dr. Stratman said.
However, he added, “There is really no standardized training to operate these devices. That being said, it’s really important to know that both providers and facility owners have a significant obligation to their patients to make sure that their staff in their centers are appropriately trained.”
Training not only involves protocols and procedures but also how to handle patient interactions, Dr. Stratman said.
The legal concept of respondeat superior applies when nonphysicians participate in a patient’s care, Dr. Avram said at the ASLMS meeting. The physician is held liable for a nonphysician’s “negligence provided he or she is an employee receiving a salary [and] benefits and is performing within the scope of his or her duty,” regardless of whether the physician saw the patient or not at that visit, he said. Again, supervision of nonphysician laser procedures varies from state to state, he added.
“So the take-home point is to provide excellent training and appropriate supervision, and if you’re the owner of that practice, you are liable in the event of negligence even though you never were part of the treatment,” Dr. Avram said.
Ins and Outs of Informed Consent
When a patient outcome is less than desirable, or at least less than what the patient expected, a transparent and thorough informed consent process can protect the practice and physician, Dr. Avram said at the meeting.
“Malpractice and consent have nothing to do with each other,” he said. “Consent is getting permission to do a procedure. It’s needed actually for any medical intervention that you perform. What you need to do is to provide information to enable the patient or guardian or to choose knowledgeably among reasonable medical alternatives. This places the patient in control of the course of their medical treatment.”
The information conveyed to the patient should include the diagnosis, the medical causes, the nature and purpose of the treatment, and the risks and alternatives of procedure, “particularly if they’re high risk,” Dr. Avram said.
“Failure to obtain informed consent constitutes a civil battery, and the physician is liable for civil damages,” he said. “The patient need only show that he or she was not informed of the medical nature of the medical touching; physical injury is not necessary.”
A battery could occur if a procedure extends beyond the scope or area of treatment the patient agreed to — for example, extending a liposuction to an area that wasn’t originally targeted, or extending a laser procedure to an area of the body as a presumed favor to the patient. “It does not require a standard of care or an expert witness,” Dr. Avram said. “One only needs to show nonconsensual touching.”
Informed consents should include plain language, he said. “The whole idea is the patient understands what the risks and benefits are,” Dr. Avram said. “You don’t need to use medical jargon.” As an example, he suggested using the term “blisters” instead of “bullae.” If the treatment involves an off-label procedure, include that too, he said.
He also advised avoiding blanket authorizations. “Courts disfavor them,” he noted. “They need more specificity. So those are not valid.”
Dr. Stratman added that providers should think about the setting in which they obtain informed consent. “It’s really important that providers are consenting their patients in private and quiet places, free from distractions, that they accommodate patients who might have disabilities or limitations in English proficiency, using a teach-back method to help patients understand or demonstrate their understanding of the procedure in order to gauge comprehension,” he said.
Both Dr. Avram and Dr. Stratman pointed out that another strategy to prevent malpractice is to build trusting patient-provider relationships. “The patient-provider relationship is paramount not only to the success of the procedure but to the clinical visit as a whole,” Dr. Stratman said.
That’s a two-way street, he added. Patients should be able to trust that their provider provides them with the best treatment based on their own history, and providers should also be able to trust that patients are providing them with an accurate history, asking relevant questions, or expressing any level of apprehension about the procedure or visit. “The patient-provider relationship is everything,” Dr. Stratman said.
Dr. Stratman and Dr. Avram had no relevant disclosures.
A version of this article appeared on Medscape.com.
for liability. Dermatologists can protect themselves by understanding malpractice trends and taking preventive steps, such as making sure NPOs have appropriate training and using a rigorous informed consent process, according to a dermatology resident and a dermatologist who have researched recent trends in dermatology lawsuits.
“It’s really important that physicians recognize their responsibility when delegating procedures to nonphysician operators and the physician’s role in supervision of these procedures,” Scott Stratman, MD, MPH, a dermatology resident at the Icahn School of Medicine at Mount Sinai, New York City, told this news organization. He led a study recently published in the Journal of the American Academy of Dermatology, which found that the majority (52%) of malpractice cases for cutaneous energy-based device procedures in the LexisNexis database from 1985 to September 2023 involved NPOs. The study did not break the data down between different types of NPOs.
Trends in Dermatology Malpractice
This follows a similar trend reported in a 2014 study led by Mathew M. Avram, MD, JD, director of the MGH Dermatology Laser and Cosmetic Center at Massachusetts General Hospital, Boston. The study analyzed liability claims related to cutaneous laser surgery performed by nonphysicians from January 1999 to December 2012.
“With nonphysician litigation data, we saw trend lines beginning in 2008 where the proportion of cases began to increase,” Dr. Avram said at the American Society for Laser Medicine and Surgery (ASLMS) meeting on April 12, 2024. “Over a period of 2008-2012, it went from 36% of cases to about 78%,” he said.
About a quarter (23.4%) of those were in medical offices; 76.6% were in nontraditional settings such as medical spas, he added. The proportion of NPOs was similar in a 2022 study that looked at causes of litigation in cutaneous laser surgery from 2012 to 2020, Dr. Avram said. Again, neither study broke down cases involving NPOs by specific type, but the 2014 study reported that 64% of cases by NPOs occurred outside of a traditional medical setting.
“So it seems that the location and potentially the supervision are issues that are important to patient safety,” Dr. Avram said at the meeting. While state laws regarding laser delegation vary widely, “depending on where you practice, it’s incumbent upon you to know that.”
Dr. Avram and colleagues were also the authors of a study published in June in Dermatologic Surgery that looked at the reasons behind ligations involving dermatologists in a retrospective analysis of 48 state and federal cases between 2011 and 2022. The majority of cases — 54.2% — were for unexpected harm, followed by wrong or delayed diagnoses, which accounted for a third of litigations.
Dr. Stratman’s study found that laser hair removal was the most common procedure for malpractice claims in dermatology among cutaneous energy-based device procedures. Complications from energy-based devices included burns, scarring, and pigmentation changes.
The growth of malpractice suits involving NPOs could be because NPOs are performing a greater proportion of dermatologic procedures, “particularly those practicing without direct supervision, such as in the context of a medical spa,” Dr. Stratman said in the interview. “Again, this highlights a physician’s responsibility in delegating these kinds of procedures to NPOs.”
Training Is a Must — But Not Standardized
Comprehensive training for physicians, staff, NPOs, and physicians “is all necessary and paramount in order to diminish adverse outcomes and legal risk, and then, of course, all these practitioners, be it staff or [NPOs], and, of course, physicians, are all held to the same standard of care,” Dr. Stratman said.
However, he added, “There is really no standardized training to operate these devices. That being said, it’s really important to know that both providers and facility owners have a significant obligation to their patients to make sure that their staff in their centers are appropriately trained.”
Training not only involves protocols and procedures but also how to handle patient interactions, Dr. Stratman said.
The legal concept of respondeat superior applies when nonphysicians participate in a patient’s care, Dr. Avram said at the ASLMS meeting. The physician is held liable for a nonphysician’s “negligence provided he or she is an employee receiving a salary [and] benefits and is performing within the scope of his or her duty,” regardless of whether the physician saw the patient or not at that visit, he said. Again, supervision of nonphysician laser procedures varies from state to state, he added.
“So the take-home point is to provide excellent training and appropriate supervision, and if you’re the owner of that practice, you are liable in the event of negligence even though you never were part of the treatment,” Dr. Avram said.
Ins and Outs of Informed Consent
When a patient outcome is less than desirable, or at least less than what the patient expected, a transparent and thorough informed consent process can protect the practice and physician, Dr. Avram said at the meeting.
“Malpractice and consent have nothing to do with each other,” he said. “Consent is getting permission to do a procedure. It’s needed actually for any medical intervention that you perform. What you need to do is to provide information to enable the patient or guardian or to choose knowledgeably among reasonable medical alternatives. This places the patient in control of the course of their medical treatment.”
The information conveyed to the patient should include the diagnosis, the medical causes, the nature and purpose of the treatment, and the risks and alternatives of procedure, “particularly if they’re high risk,” Dr. Avram said.
“Failure to obtain informed consent constitutes a civil battery, and the physician is liable for civil damages,” he said. “The patient need only show that he or she was not informed of the medical nature of the medical touching; physical injury is not necessary.”
A battery could occur if a procedure extends beyond the scope or area of treatment the patient agreed to — for example, extending a liposuction to an area that wasn’t originally targeted, or extending a laser procedure to an area of the body as a presumed favor to the patient. “It does not require a standard of care or an expert witness,” Dr. Avram said. “One only needs to show nonconsensual touching.”
Informed consents should include plain language, he said. “The whole idea is the patient understands what the risks and benefits are,” Dr. Avram said. “You don’t need to use medical jargon.” As an example, he suggested using the term “blisters” instead of “bullae.” If the treatment involves an off-label procedure, include that too, he said.
He also advised avoiding blanket authorizations. “Courts disfavor them,” he noted. “They need more specificity. So those are not valid.”
Dr. Stratman added that providers should think about the setting in which they obtain informed consent. “It’s really important that providers are consenting their patients in private and quiet places, free from distractions, that they accommodate patients who might have disabilities or limitations in English proficiency, using a teach-back method to help patients understand or demonstrate their understanding of the procedure in order to gauge comprehension,” he said.
Both Dr. Avram and Dr. Stratman pointed out that another strategy to prevent malpractice is to build trusting patient-provider relationships. “The patient-provider relationship is paramount not only to the success of the procedure but to the clinical visit as a whole,” Dr. Stratman said.
That’s a two-way street, he added. Patients should be able to trust that their provider provides them with the best treatment based on their own history, and providers should also be able to trust that patients are providing them with an accurate history, asking relevant questions, or expressing any level of apprehension about the procedure or visit. “The patient-provider relationship is everything,” Dr. Stratman said.
Dr. Stratman and Dr. Avram had no relevant disclosures.
A version of this article appeared on Medscape.com.
for liability. Dermatologists can protect themselves by understanding malpractice trends and taking preventive steps, such as making sure NPOs have appropriate training and using a rigorous informed consent process, according to a dermatology resident and a dermatologist who have researched recent trends in dermatology lawsuits.
“It’s really important that physicians recognize their responsibility when delegating procedures to nonphysician operators and the physician’s role in supervision of these procedures,” Scott Stratman, MD, MPH, a dermatology resident at the Icahn School of Medicine at Mount Sinai, New York City, told this news organization. He led a study recently published in the Journal of the American Academy of Dermatology, which found that the majority (52%) of malpractice cases for cutaneous energy-based device procedures in the LexisNexis database from 1985 to September 2023 involved NPOs. The study did not break the data down between different types of NPOs.
Trends in Dermatology Malpractice
This follows a similar trend reported in a 2014 study led by Mathew M. Avram, MD, JD, director of the MGH Dermatology Laser and Cosmetic Center at Massachusetts General Hospital, Boston. The study analyzed liability claims related to cutaneous laser surgery performed by nonphysicians from January 1999 to December 2012.
“With nonphysician litigation data, we saw trend lines beginning in 2008 where the proportion of cases began to increase,” Dr. Avram said at the American Society for Laser Medicine and Surgery (ASLMS) meeting on April 12, 2024. “Over a period of 2008-2012, it went from 36% of cases to about 78%,” he said.
About a quarter (23.4%) of those were in medical offices; 76.6% were in nontraditional settings such as medical spas, he added. The proportion of NPOs was similar in a 2022 study that looked at causes of litigation in cutaneous laser surgery from 2012 to 2020, Dr. Avram said. Again, neither study broke down cases involving NPOs by specific type, but the 2014 study reported that 64% of cases by NPOs occurred outside of a traditional medical setting.
“So it seems that the location and potentially the supervision are issues that are important to patient safety,” Dr. Avram said at the meeting. While state laws regarding laser delegation vary widely, “depending on where you practice, it’s incumbent upon you to know that.”
Dr. Avram and colleagues were also the authors of a study published in June in Dermatologic Surgery that looked at the reasons behind ligations involving dermatologists in a retrospective analysis of 48 state and federal cases between 2011 and 2022. The majority of cases — 54.2% — were for unexpected harm, followed by wrong or delayed diagnoses, which accounted for a third of litigations.
Dr. Stratman’s study found that laser hair removal was the most common procedure for malpractice claims in dermatology among cutaneous energy-based device procedures. Complications from energy-based devices included burns, scarring, and pigmentation changes.
The growth of malpractice suits involving NPOs could be because NPOs are performing a greater proportion of dermatologic procedures, “particularly those practicing without direct supervision, such as in the context of a medical spa,” Dr. Stratman said in the interview. “Again, this highlights a physician’s responsibility in delegating these kinds of procedures to NPOs.”
Training Is a Must — But Not Standardized
Comprehensive training for physicians, staff, NPOs, and physicians “is all necessary and paramount in order to diminish adverse outcomes and legal risk, and then, of course, all these practitioners, be it staff or [NPOs], and, of course, physicians, are all held to the same standard of care,” Dr. Stratman said.
However, he added, “There is really no standardized training to operate these devices. That being said, it’s really important to know that both providers and facility owners have a significant obligation to their patients to make sure that their staff in their centers are appropriately trained.”
Training not only involves protocols and procedures but also how to handle patient interactions, Dr. Stratman said.
The legal concept of respondeat superior applies when nonphysicians participate in a patient’s care, Dr. Avram said at the ASLMS meeting. The physician is held liable for a nonphysician’s “negligence provided he or she is an employee receiving a salary [and] benefits and is performing within the scope of his or her duty,” regardless of whether the physician saw the patient or not at that visit, he said. Again, supervision of nonphysician laser procedures varies from state to state, he added.
“So the take-home point is to provide excellent training and appropriate supervision, and if you’re the owner of that practice, you are liable in the event of negligence even though you never were part of the treatment,” Dr. Avram said.
Ins and Outs of Informed Consent
When a patient outcome is less than desirable, or at least less than what the patient expected, a transparent and thorough informed consent process can protect the practice and physician, Dr. Avram said at the meeting.
“Malpractice and consent have nothing to do with each other,” he said. “Consent is getting permission to do a procedure. It’s needed actually for any medical intervention that you perform. What you need to do is to provide information to enable the patient or guardian or to choose knowledgeably among reasonable medical alternatives. This places the patient in control of the course of their medical treatment.”
The information conveyed to the patient should include the diagnosis, the medical causes, the nature and purpose of the treatment, and the risks and alternatives of procedure, “particularly if they’re high risk,” Dr. Avram said.
“Failure to obtain informed consent constitutes a civil battery, and the physician is liable for civil damages,” he said. “The patient need only show that he or she was not informed of the medical nature of the medical touching; physical injury is not necessary.”
A battery could occur if a procedure extends beyond the scope or area of treatment the patient agreed to — for example, extending a liposuction to an area that wasn’t originally targeted, or extending a laser procedure to an area of the body as a presumed favor to the patient. “It does not require a standard of care or an expert witness,” Dr. Avram said. “One only needs to show nonconsensual touching.”
Informed consents should include plain language, he said. “The whole idea is the patient understands what the risks and benefits are,” Dr. Avram said. “You don’t need to use medical jargon.” As an example, he suggested using the term “blisters” instead of “bullae.” If the treatment involves an off-label procedure, include that too, he said.
He also advised avoiding blanket authorizations. “Courts disfavor them,” he noted. “They need more specificity. So those are not valid.”
Dr. Stratman added that providers should think about the setting in which they obtain informed consent. “It’s really important that providers are consenting their patients in private and quiet places, free from distractions, that they accommodate patients who might have disabilities or limitations in English proficiency, using a teach-back method to help patients understand or demonstrate their understanding of the procedure in order to gauge comprehension,” he said.
Both Dr. Avram and Dr. Stratman pointed out that another strategy to prevent malpractice is to build trusting patient-provider relationships. “The patient-provider relationship is paramount not only to the success of the procedure but to the clinical visit as a whole,” Dr. Stratman said.
That’s a two-way street, he added. Patients should be able to trust that their provider provides them with the best treatment based on their own history, and providers should also be able to trust that patients are providing them with an accurate history, asking relevant questions, or expressing any level of apprehension about the procedure or visit. “The patient-provider relationship is everything,” Dr. Stratman said.
Dr. Stratman and Dr. Avram had no relevant disclosures.
A version of this article appeared on Medscape.com.