User login
Formerly Skin & Allergy News
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')]
The leading independent newspaper covering dermatology news and commentary.
Survey: Many Mohs surgeons are struggling on the job
.
In a measurement of well-being, 40% of members of the American College of Mohs
Surgery (ACMS) who responded to the survey – and 52% of women – scored at a level considered “at-risk” for adverse outcomes, such as poor quality of life.
“I didn’t think the numbers were going to be that high,” said study author Kemi O. Awe, MD, PhD, a dermatology resident at the University of Alabama at Birmingham, especially in light of Mohs surgery’s reputation as being an especially desirable field in dermatology. She presented the findings at the annual meeting of the ACMS.
Dr. Awe, who hopes to become a Mohs surgeon herself, said in an interview that she launched the study in part to understand how colleagues are faring. “Dermatology is known as a specialty that has a good lifestyle and less stress, but the rate of burnout is actually going up.”
For the study, Dr. Awe and colleagues sent a survey to ACMS members between October and December 2020. The 91 respondents had an average age of 46, and 58% were male. Most practiced in academic facilities (56%), while the rest worked in private practice (39%) or multispecialty (4%) practices. Almost all (89%) were married or in partnerships.
The survey calculated scores on the expanded Physician Well Being Index, a validated tool for measuring physician distress. Forty percent of 68 respondents to this part of the survey got a score of 3 or higher, which the study describes as “a threshold for respondents who are ‘at-risk’ of adverse outcomes such as poor quality of life, depression, and a high level of fatigue.”
Women were more likely to be considered at risk (52%) than men (28%). “This isn’t different than what’s already out there: Female physicians are more likely to be burned out compared to men,” Dr. Awe said.
Compared with their male counterparts, female Mohs surgeons were more likely to say that time at work, malpractice concerns, insurance reimbursement, and compensation structure negatively affected their well-being (P ≤ .05).
It’s unclear whether there’s a well-being gender gap among dermatologists overall, however. Dr. Awe highlighted a 2019 survey of 108 dermatologists that found no significant difference in overall burnout between men and women – about 42% of both genders reported symptoms. But the survey did find that “dermatologists with children living at home had significantly higher levels of burnout,” with a P value of .03.
Dr. Awe said the findings offer insight into what to look out for when pursuing a career as a Mohs surgeon. “There’s potentially excess stress about being a Mohs surgeon,” she said, although the field also has a reputation as being fulfilling and rewarding.
In an interview, Stanford (Calif.) University dermatologist Zakia Rahman, MD, praised the study and said it “certainly provides a framework to address professional fulfillment amongst Mohs surgeons.”
It was especially surprising, she said, that female surgeons didn’t rate their compensation structure as positively as did their male colleagues. “It is possible that there is still a significant amount of gender-based difference in compensation between male and female Mohs surgeons. This is an area that can be further explored.”
Moving forward, she said, “our professional dermatology societies must examine the increase in burnout within our specialty. Further funding and research in this area is needed.”
For now, dermatologists can focus on strategies that can reduce burnout in the field, Sailesh Konda, MD, a Mohs surgeon at the Univeristy of Florida, Gainesville, said in an interview. Dr. Konda highlighted a report published in 2020 that, he said, "recommended focusing on incremental changes that help restore autonomy and control over work, connecting with colleagues within dermatology and the broader medical community, developing self-awareness and recognition of a perfectionist mindset, and restoring meaning and joy to patient care.”*
No funding is reported for the study. Dr. Awe, Dr. Rahman, and Dr. Konda have no relevant disclosures.
*This story was updated on June 2 for clarity.
.
In a measurement of well-being, 40% of members of the American College of Mohs
Surgery (ACMS) who responded to the survey – and 52% of women – scored at a level considered “at-risk” for adverse outcomes, such as poor quality of life.
“I didn’t think the numbers were going to be that high,” said study author Kemi O. Awe, MD, PhD, a dermatology resident at the University of Alabama at Birmingham, especially in light of Mohs surgery’s reputation as being an especially desirable field in dermatology. She presented the findings at the annual meeting of the ACMS.
Dr. Awe, who hopes to become a Mohs surgeon herself, said in an interview that she launched the study in part to understand how colleagues are faring. “Dermatology is known as a specialty that has a good lifestyle and less stress, but the rate of burnout is actually going up.”
For the study, Dr. Awe and colleagues sent a survey to ACMS members between October and December 2020. The 91 respondents had an average age of 46, and 58% were male. Most practiced in academic facilities (56%), while the rest worked in private practice (39%) or multispecialty (4%) practices. Almost all (89%) were married or in partnerships.
The survey calculated scores on the expanded Physician Well Being Index, a validated tool for measuring physician distress. Forty percent of 68 respondents to this part of the survey got a score of 3 or higher, which the study describes as “a threshold for respondents who are ‘at-risk’ of adverse outcomes such as poor quality of life, depression, and a high level of fatigue.”
Women were more likely to be considered at risk (52%) than men (28%). “This isn’t different than what’s already out there: Female physicians are more likely to be burned out compared to men,” Dr. Awe said.
Compared with their male counterparts, female Mohs surgeons were more likely to say that time at work, malpractice concerns, insurance reimbursement, and compensation structure negatively affected their well-being (P ≤ .05).
It’s unclear whether there’s a well-being gender gap among dermatologists overall, however. Dr. Awe highlighted a 2019 survey of 108 dermatologists that found no significant difference in overall burnout between men and women – about 42% of both genders reported symptoms. But the survey did find that “dermatologists with children living at home had significantly higher levels of burnout,” with a P value of .03.
Dr. Awe said the findings offer insight into what to look out for when pursuing a career as a Mohs surgeon. “There’s potentially excess stress about being a Mohs surgeon,” she said, although the field also has a reputation as being fulfilling and rewarding.
In an interview, Stanford (Calif.) University dermatologist Zakia Rahman, MD, praised the study and said it “certainly provides a framework to address professional fulfillment amongst Mohs surgeons.”
It was especially surprising, she said, that female surgeons didn’t rate their compensation structure as positively as did their male colleagues. “It is possible that there is still a significant amount of gender-based difference in compensation between male and female Mohs surgeons. This is an area that can be further explored.”
Moving forward, she said, “our professional dermatology societies must examine the increase in burnout within our specialty. Further funding and research in this area is needed.”
For now, dermatologists can focus on strategies that can reduce burnout in the field, Sailesh Konda, MD, a Mohs surgeon at the Univeristy of Florida, Gainesville, said in an interview. Dr. Konda highlighted a report published in 2020 that, he said, "recommended focusing on incremental changes that help restore autonomy and control over work, connecting with colleagues within dermatology and the broader medical community, developing self-awareness and recognition of a perfectionist mindset, and restoring meaning and joy to patient care.”*
No funding is reported for the study. Dr. Awe, Dr. Rahman, and Dr. Konda have no relevant disclosures.
*This story was updated on June 2 for clarity.
.
In a measurement of well-being, 40% of members of the American College of Mohs
Surgery (ACMS) who responded to the survey – and 52% of women – scored at a level considered “at-risk” for adverse outcomes, such as poor quality of life.
“I didn’t think the numbers were going to be that high,” said study author Kemi O. Awe, MD, PhD, a dermatology resident at the University of Alabama at Birmingham, especially in light of Mohs surgery’s reputation as being an especially desirable field in dermatology. She presented the findings at the annual meeting of the ACMS.
Dr. Awe, who hopes to become a Mohs surgeon herself, said in an interview that she launched the study in part to understand how colleagues are faring. “Dermatology is known as a specialty that has a good lifestyle and less stress, but the rate of burnout is actually going up.”
For the study, Dr. Awe and colleagues sent a survey to ACMS members between October and December 2020. The 91 respondents had an average age of 46, and 58% were male. Most practiced in academic facilities (56%), while the rest worked in private practice (39%) or multispecialty (4%) practices. Almost all (89%) were married or in partnerships.
The survey calculated scores on the expanded Physician Well Being Index, a validated tool for measuring physician distress. Forty percent of 68 respondents to this part of the survey got a score of 3 or higher, which the study describes as “a threshold for respondents who are ‘at-risk’ of adverse outcomes such as poor quality of life, depression, and a high level of fatigue.”
Women were more likely to be considered at risk (52%) than men (28%). “This isn’t different than what’s already out there: Female physicians are more likely to be burned out compared to men,” Dr. Awe said.
Compared with their male counterparts, female Mohs surgeons were more likely to say that time at work, malpractice concerns, insurance reimbursement, and compensation structure negatively affected their well-being (P ≤ .05).
It’s unclear whether there’s a well-being gender gap among dermatologists overall, however. Dr. Awe highlighted a 2019 survey of 108 dermatologists that found no significant difference in overall burnout between men and women – about 42% of both genders reported symptoms. But the survey did find that “dermatologists with children living at home had significantly higher levels of burnout,” with a P value of .03.
Dr. Awe said the findings offer insight into what to look out for when pursuing a career as a Mohs surgeon. “There’s potentially excess stress about being a Mohs surgeon,” she said, although the field also has a reputation as being fulfilling and rewarding.
In an interview, Stanford (Calif.) University dermatologist Zakia Rahman, MD, praised the study and said it “certainly provides a framework to address professional fulfillment amongst Mohs surgeons.”
It was especially surprising, she said, that female surgeons didn’t rate their compensation structure as positively as did their male colleagues. “It is possible that there is still a significant amount of gender-based difference in compensation between male and female Mohs surgeons. This is an area that can be further explored.”
Moving forward, she said, “our professional dermatology societies must examine the increase in burnout within our specialty. Further funding and research in this area is needed.”
For now, dermatologists can focus on strategies that can reduce burnout in the field, Sailesh Konda, MD, a Mohs surgeon at the Univeristy of Florida, Gainesville, said in an interview. Dr. Konda highlighted a report published in 2020 that, he said, "recommended focusing on incremental changes that help restore autonomy and control over work, connecting with colleagues within dermatology and the broader medical community, developing self-awareness and recognition of a perfectionist mindset, and restoring meaning and joy to patient care.”*
No funding is reported for the study. Dr. Awe, Dr. Rahman, and Dr. Konda have no relevant disclosures.
*This story was updated on June 2 for clarity.
FROM THE ACMS ANNUAL MEETING
Physicians’ trust in health care leadership drops in pandemic
according to a survey conducted by NORC at the University of Chicago on behalf of the American Board of Internal Medicine Foundation.
Survey results, released May 21, indicate that 30% of physicians say their trust in the U.S. health care system and health care leadership has decreased during the pandemic. Only 18% reported an increase in trust.
Physicians, however, have great trust in their fellow clinicians.
In the survey of 600 physicians, 94% said they trust doctors within their practice; 85% trusted doctors outside of their practice; and 89% trusted nurses. That trust increased during the pandemic, with 41% saying their trust in fellow physicians rose and 37% saying their trust in nurses did.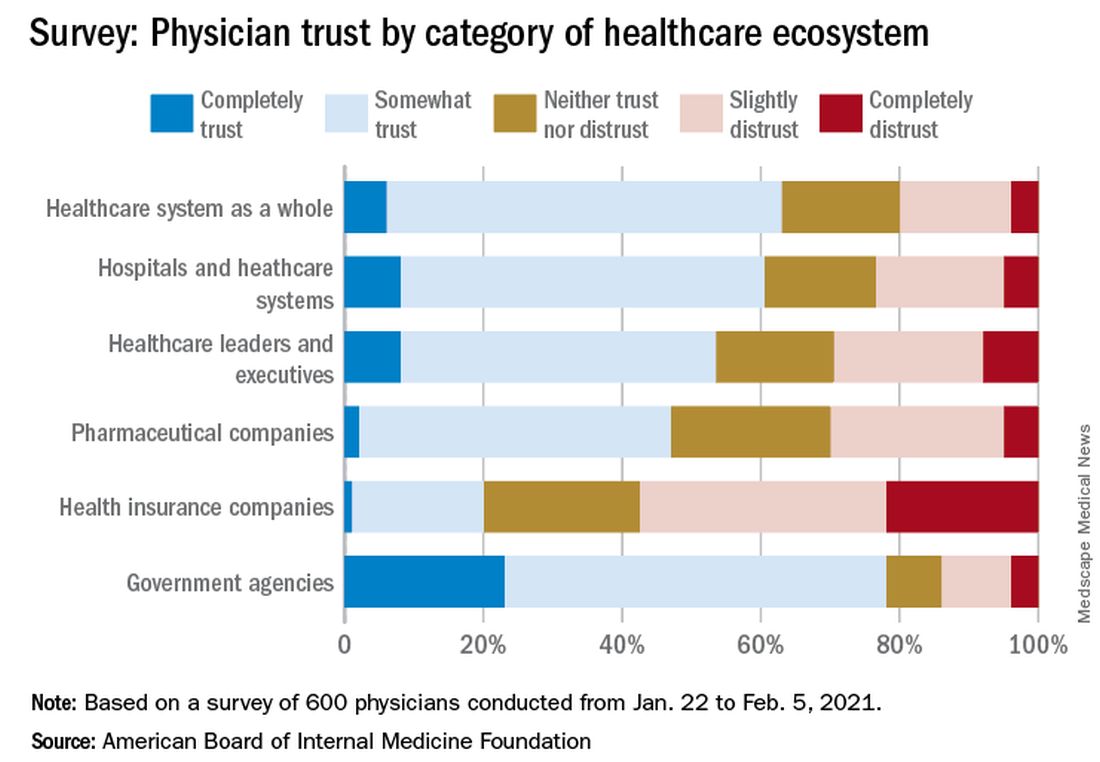
In a separate survey, NORC asked patients about their trust in various aspects of health care. Among 2,069 respondents, a wide majority reported that they trust doctors (84%) and nurses (85%), but only 64% trusted the health care system as a whole. One in three consumers (32%) said their trust in the health care system decreased during the pandemic, compared with 11% who said their trust increased.
The ABIM Foundation released the research findings on May 21 as part of Building Trust, a national campaign that aims to boost trust among patients, clinicians, system leaders, researchers, and others.
Richard J. Baron, MD, president and chief executive officer of the ABIM Foundation, said in an interview, “Clearly there’s lower trust in health care organization leaders and executives, and that’s troubling.
“Science by itself is not enough,” he said. “Becoming trustworthy has to be a core project of everybody in health care.”
Deterioration in physicians’ trust during the pandemic comes in part from failed promises of adequate personal protective equipment and some physicians’ loss of income as a result of the crisis, Dr. Baron said.
He added that the vaccine rollout was very uneven and that policies as to which elective procedures could be performed were handled differently in different parts of the country.
He also noted that, early on, transparency was lacking as to how many COVID patients hospitals were treating, which may have contributed to the decrease in trust in the system.
Fear of being known as ‘the COVID hospital’
Hospitals were afraid of being known as “the COVID hospital” and losing patients who were afraid to come there, Dr. Baron said.
He said the COVID-19 epidemic exacerbated problems regarding trust, but that trust has been declining for some time. The Building Trust campaign will focus on solutions in breaches of trust as physicians move increasingly toward being employees of huge systems, according to Dr. Baron.
However, trust works both ways, Dr. Baron notes. Physicians can be champions for their health care system or “throw the system under the bus,” he said.
For example, if a patient complains about the appointment system, clinicians who trust their institutions may say the system usually works and that they will try to make sure the patient has a better experience next time. Clinicians without trust may say they agree that the health care system doesn’t know what it is doing, and patients may further lose confidence when physicians validate their complaint, and patients may then go elsewhere.
78% of patients trust primary care doctor
When asked whether they trust their primary care physician, 78% of patients said yes. However, trust in doctors was higher among people who were older (90%), White (82%), or had high income (89%). Among people reporting lower trust, 25% said their physician spends too little time with them, and 14% said their doctor does not know or listen to them.
The survey shows that government agencies have work to do to earn trust. Responses indicate that 43% of physicians said they have “complete trust” in government health care agencies, such as the U.S. Food and Drug Administration and the Centers for Disease Control and Prevention, which is substantially higher than other parts of the health care system. However, trust in agencies declined for 43% of physician respondents and increased for 21%.
Dhruv Khullar, MD, MPP, of the department of health policy and economics at Weill Cornell Medical College in New York, told this news organization the survey results match what he sees anecdotally in medicine – that physicians have been losing trust in the system but not in their colleagues.
He said the sample size of 600 is enough to be influential, though he said he would like to know the response rate, which was not calculated for this survey.
He added that, in large part, physicians’ lack of trust in their systems may come from generally being asked to see more patients and to meet more metrics during the same or shorter periods.
Physicians’ lack of trust in the system can have significant consequences, he said. It can lead to burnout, which has been linked with poorer quality of care and physician turnover, he noted.
COVID-19 led some physicians to wonder whether their system had their best interests at heart, insofar as access to adequate medicines and supplies as well as emotional support were inconsistent, Dr. Khullar said.
He said that to regain trust health care systems need to ask themselves questions in three areas. The first is whether their goals are focused on the best interest of the organization or the best interest of the patient.
“Next is competency,” Dr. Khullar said. “Maybe your motives are right, but are you able to deliver? Are you delivering a good product, whether clinical services or something else?”
The third area is transparency, he said. “Are you going to be honest and forthright in what we’re doing and where we’re going?”
Caroline Pearson, senior vice president of health care strategy for NORC, said the emailed survey was conducted between Dec. 29, 2020, and Feb. 5, 2021, with a health care survey partner that maintains a nationwide panel of physicians across specialties.
She said this report is fairly novel insofar as surveys are more typically conducted regarding patients’ trust of their doctors or of the health care system.
Ms. Pearson said because health care is delivered in teams, understanding the level of trust among the entities helps ensure that care will be delivered effectively and seamlessly with high quality.
“We want our patients to trust our doctors, but we really want doctors to trust each other and trust the hospitals and systems in which they’re working,” she said.
Dr. Baron, Ms. Pearson, and Dr. Khullar report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
according to a survey conducted by NORC at the University of Chicago on behalf of the American Board of Internal Medicine Foundation.
Survey results, released May 21, indicate that 30% of physicians say their trust in the U.S. health care system and health care leadership has decreased during the pandemic. Only 18% reported an increase in trust.
Physicians, however, have great trust in their fellow clinicians.
In the survey of 600 physicians, 94% said they trust doctors within their practice; 85% trusted doctors outside of their practice; and 89% trusted nurses. That trust increased during the pandemic, with 41% saying their trust in fellow physicians rose and 37% saying their trust in nurses did.
In a separate survey, NORC asked patients about their trust in various aspects of health care. Among 2,069 respondents, a wide majority reported that they trust doctors (84%) and nurses (85%), but only 64% trusted the health care system as a whole. One in three consumers (32%) said their trust in the health care system decreased during the pandemic, compared with 11% who said their trust increased.
The ABIM Foundation released the research findings on May 21 as part of Building Trust, a national campaign that aims to boost trust among patients, clinicians, system leaders, researchers, and others.
Richard J. Baron, MD, president and chief executive officer of the ABIM Foundation, said in an interview, “Clearly there’s lower trust in health care organization leaders and executives, and that’s troubling.
“Science by itself is not enough,” he said. “Becoming trustworthy has to be a core project of everybody in health care.”
Deterioration in physicians’ trust during the pandemic comes in part from failed promises of adequate personal protective equipment and some physicians’ loss of income as a result of the crisis, Dr. Baron said.
He added that the vaccine rollout was very uneven and that policies as to which elective procedures could be performed were handled differently in different parts of the country.
He also noted that, early on, transparency was lacking as to how many COVID patients hospitals were treating, which may have contributed to the decrease in trust in the system.
Fear of being known as ‘the COVID hospital’
Hospitals were afraid of being known as “the COVID hospital” and losing patients who were afraid to come there, Dr. Baron said.
He said the COVID-19 epidemic exacerbated problems regarding trust, but that trust has been declining for some time. The Building Trust campaign will focus on solutions in breaches of trust as physicians move increasingly toward being employees of huge systems, according to Dr. Baron.
However, trust works both ways, Dr. Baron notes. Physicians can be champions for their health care system or “throw the system under the bus,” he said.
For example, if a patient complains about the appointment system, clinicians who trust their institutions may say the system usually works and that they will try to make sure the patient has a better experience next time. Clinicians without trust may say they agree that the health care system doesn’t know what it is doing, and patients may further lose confidence when physicians validate their complaint, and patients may then go elsewhere.
78% of patients trust primary care doctor
When asked whether they trust their primary care physician, 78% of patients said yes. However, trust in doctors was higher among people who were older (90%), White (82%), or had high income (89%). Among people reporting lower trust, 25% said their physician spends too little time with them, and 14% said their doctor does not know or listen to them.
The survey shows that government agencies have work to do to earn trust. Responses indicate that 43% of physicians said they have “complete trust” in government health care agencies, such as the U.S. Food and Drug Administration and the Centers for Disease Control and Prevention, which is substantially higher than other parts of the health care system. However, trust in agencies declined for 43% of physician respondents and increased for 21%.
Dhruv Khullar, MD, MPP, of the department of health policy and economics at Weill Cornell Medical College in New York, told this news organization the survey results match what he sees anecdotally in medicine – that physicians have been losing trust in the system but not in their colleagues.
He said the sample size of 600 is enough to be influential, though he said he would like to know the response rate, which was not calculated for this survey.
He added that, in large part, physicians’ lack of trust in their systems may come from generally being asked to see more patients and to meet more metrics during the same or shorter periods.
Physicians’ lack of trust in the system can have significant consequences, he said. It can lead to burnout, which has been linked with poorer quality of care and physician turnover, he noted.
COVID-19 led some physicians to wonder whether their system had their best interests at heart, insofar as access to adequate medicines and supplies as well as emotional support were inconsistent, Dr. Khullar said.
He said that to regain trust health care systems need to ask themselves questions in three areas. The first is whether their goals are focused on the best interest of the organization or the best interest of the patient.
“Next is competency,” Dr. Khullar said. “Maybe your motives are right, but are you able to deliver? Are you delivering a good product, whether clinical services or something else?”
The third area is transparency, he said. “Are you going to be honest and forthright in what we’re doing and where we’re going?”
Caroline Pearson, senior vice president of health care strategy for NORC, said the emailed survey was conducted between Dec. 29, 2020, and Feb. 5, 2021, with a health care survey partner that maintains a nationwide panel of physicians across specialties.
She said this report is fairly novel insofar as surveys are more typically conducted regarding patients’ trust of their doctors or of the health care system.
Ms. Pearson said because health care is delivered in teams, understanding the level of trust among the entities helps ensure that care will be delivered effectively and seamlessly with high quality.
“We want our patients to trust our doctors, but we really want doctors to trust each other and trust the hospitals and systems in which they’re working,” she said.
Dr. Baron, Ms. Pearson, and Dr. Khullar report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
according to a survey conducted by NORC at the University of Chicago on behalf of the American Board of Internal Medicine Foundation.
Survey results, released May 21, indicate that 30% of physicians say their trust in the U.S. health care system and health care leadership has decreased during the pandemic. Only 18% reported an increase in trust.
Physicians, however, have great trust in their fellow clinicians.
In the survey of 600 physicians, 94% said they trust doctors within their practice; 85% trusted doctors outside of their practice; and 89% trusted nurses. That trust increased during the pandemic, with 41% saying their trust in fellow physicians rose and 37% saying their trust in nurses did.
In a separate survey, NORC asked patients about their trust in various aspects of health care. Among 2,069 respondents, a wide majority reported that they trust doctors (84%) and nurses (85%), but only 64% trusted the health care system as a whole. One in three consumers (32%) said their trust in the health care system decreased during the pandemic, compared with 11% who said their trust increased.
The ABIM Foundation released the research findings on May 21 as part of Building Trust, a national campaign that aims to boost trust among patients, clinicians, system leaders, researchers, and others.
Richard J. Baron, MD, president and chief executive officer of the ABIM Foundation, said in an interview, “Clearly there’s lower trust in health care organization leaders and executives, and that’s troubling.
“Science by itself is not enough,” he said. “Becoming trustworthy has to be a core project of everybody in health care.”
Deterioration in physicians’ trust during the pandemic comes in part from failed promises of adequate personal protective equipment and some physicians’ loss of income as a result of the crisis, Dr. Baron said.
He added that the vaccine rollout was very uneven and that policies as to which elective procedures could be performed were handled differently in different parts of the country.
He also noted that, early on, transparency was lacking as to how many COVID patients hospitals were treating, which may have contributed to the decrease in trust in the system.
Fear of being known as ‘the COVID hospital’
Hospitals were afraid of being known as “the COVID hospital” and losing patients who were afraid to come there, Dr. Baron said.
He said the COVID-19 epidemic exacerbated problems regarding trust, but that trust has been declining for some time. The Building Trust campaign will focus on solutions in breaches of trust as physicians move increasingly toward being employees of huge systems, according to Dr. Baron.
However, trust works both ways, Dr. Baron notes. Physicians can be champions for their health care system or “throw the system under the bus,” he said.
For example, if a patient complains about the appointment system, clinicians who trust their institutions may say the system usually works and that they will try to make sure the patient has a better experience next time. Clinicians without trust may say they agree that the health care system doesn’t know what it is doing, and patients may further lose confidence when physicians validate their complaint, and patients may then go elsewhere.
78% of patients trust primary care doctor
When asked whether they trust their primary care physician, 78% of patients said yes. However, trust in doctors was higher among people who were older (90%), White (82%), or had high income (89%). Among people reporting lower trust, 25% said their physician spends too little time with them, and 14% said their doctor does not know or listen to them.
The survey shows that government agencies have work to do to earn trust. Responses indicate that 43% of physicians said they have “complete trust” in government health care agencies, such as the U.S. Food and Drug Administration and the Centers for Disease Control and Prevention, which is substantially higher than other parts of the health care system. However, trust in agencies declined for 43% of physician respondents and increased for 21%.
Dhruv Khullar, MD, MPP, of the department of health policy and economics at Weill Cornell Medical College in New York, told this news organization the survey results match what he sees anecdotally in medicine – that physicians have been losing trust in the system but not in their colleagues.
He said the sample size of 600 is enough to be influential, though he said he would like to know the response rate, which was not calculated for this survey.
He added that, in large part, physicians’ lack of trust in their systems may come from generally being asked to see more patients and to meet more metrics during the same or shorter periods.
Physicians’ lack of trust in the system can have significant consequences, he said. It can lead to burnout, which has been linked with poorer quality of care and physician turnover, he noted.
COVID-19 led some physicians to wonder whether their system had their best interests at heart, insofar as access to adequate medicines and supplies as well as emotional support were inconsistent, Dr. Khullar said.
He said that to regain trust health care systems need to ask themselves questions in three areas. The first is whether their goals are focused on the best interest of the organization or the best interest of the patient.
“Next is competency,” Dr. Khullar said. “Maybe your motives are right, but are you able to deliver? Are you delivering a good product, whether clinical services or something else?”
The third area is transparency, he said. “Are you going to be honest and forthright in what we’re doing and where we’re going?”
Caroline Pearson, senior vice president of health care strategy for NORC, said the emailed survey was conducted between Dec. 29, 2020, and Feb. 5, 2021, with a health care survey partner that maintains a nationwide panel of physicians across specialties.
She said this report is fairly novel insofar as surveys are more typically conducted regarding patients’ trust of their doctors or of the health care system.
Ms. Pearson said because health care is delivered in teams, understanding the level of trust among the entities helps ensure that care will be delivered effectively and seamlessly with high quality.
“We want our patients to trust our doctors, but we really want doctors to trust each other and trust the hospitals and systems in which they’re working,” she said.
Dr. Baron, Ms. Pearson, and Dr. Khullar report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Rituximab superior to mycophenolate mofetil in pemphigus vulgaris study
Mycophenolate mofetil, commonly used as a first-line corticosteroid-sparing agent for moderate to severe cases of the autoimmune blistering skin condition pemphigus vulgaris, has been found to be inferior to the biologic agent rituximab.
Mycophenolate mofetil is widely accepted as a first-in-line corticosteroid-sparing agent for pemphigus vulgaris, but few studies have compared the effectiveness of the two treatments for pemphigus vulgaris. The European Academy of Dermatology and Venereology recommends rituximab (Rituxan), a CD20 inhibitor, as first-line treatment for patients with new-onset cases of moderate to severe intensity or for patients who fail to achieve clinical remission with systemic corticosteroids with or without other immunosuppressive treatments.
In the current study, published online on May 19, 2021, in the New England Journal of Medicine, researchers led by Victoria P. Werth, MD, professor of dermatology at the University of Pennsylvania, Philadelphia, conducted a randomized, controlled trial of 135 patients (mean age, 48 years; 53% women) with moderate to severe pemphigus vulgaris with 67 receiving rituximab and 68 receiving mycophenolate mofetil (99% of patients in the rituximab group and 85% of patients in the mycophenolate mofetil group completed the trial).
Patients in the rituximab group received 1,000 mg of IV rituximab on days 1, 15, 168, and 182 of the study, plus twice-daily oral placebo. Intravenous methylprednisolone at 100 mg was administered before each rituximab infusion to reduce infusion-related reactions. Patients in the second group were given mycophenolate mofetil orally twice daily, starting at 1 g/day in divided doses and adjusted to 2 g/day in divided doses by week 2. They also received placebo infusions on days 1, 15, 168, and 182 of the study.
Patients in both groups received oral glucocorticoids throughout the course of the trial: an average of 3,545 mg for the rituximab treatment group and a cumulative dose of 5,140 mg for the group treated with mycophenolate mofetil, a statistically significant difference (P < .001). Outcomes based on 62 patients treated with rituximab and 63 on MMF, a modified intention-to-treat group.
By week 52, 25 patients (40%) who were treated with rituximab experienced complete sustained remission (the primary endpoint), compared with 6 patients (10%) in the mycophenolate mofetil group (95% confidence interval, 15-45, P < .001).
Only six patients in the rituximab group experienced a disease flare as compared with 44 patients in the mycophenolate mofetil group (adjusted rate ratio, 0.12; 95% CI, 0.05-0.29; P < .001). Serious adverse events occurred in 15 of 67 patients (22%) in the rituximab group and in 10 of 68 (15%) in the mycophenolate mofetil group with 3 patients in the rituximab group and 26 in the mycophenolate mofetil receiving rescue therapy.
Second to remission, the goal of treatment for pemphigus vulgaris is to reduce the use of glucocorticoids, Dr. Werth and colleagues wrote, adding: “The results of this trial showed that rituximab was superior to mycophenolate mofetil in producing sustained complete remission over 52 weeks among patients with moderate to severe pemphigus vulgaris. Rituximab had a greater glucocorticoid-sparing effect than mycophenolate mofetil, but more patients in this group had serious adverse events.”
Most adverse events in the rituximab group were limited to infusion-related reactions, but serious adverse events occurred in 15 patients (including pneumonia and upper respiratory tract infection, cellulitis and acute pyelonephritis, viral pneumonia, and skin infection). Ten patients in the mycophenolate mofetil group experienced serious adverse events (pneumonia and influenza, cellulitis and sepsis, herpes zoster, and pyelonephritis).
The current study had several limitations, primarily its small size. Plus, the authors noted a short follow-up period after glucocorticoids were stopped.
Mycophenolate mofetil, along with immunosuppressants, is approved in the United States as a treatment for organ rejection in patients who have received kidney, heart or liver transplants. But it is also used off label for pemphigus vulgaris and in rheumatology as a treatment for lupus, rheumatoid arthritis, vasculitis, inflammatory bowel disease (Crohn’s disease), inflammatory eye disease (uveitis) as well as kidney and skin disorders.
In the 2018 treatment guidelines for pemphigus by the European Dermatology Forum and the EADV, mycophenolate mofetil is recommended as a first-line corticosteroid sparing agent for pemphigus vulgaris.
Rituximab was approved in 2018 as the first biologic therapy for patients with pemphigus vulgaris and is currently recommended as a treatment for patients with pemphigus. But how well it works in comparison with the long-established mycophenolate mofetil hasn’t been extensively studied.
Other smaller studies show that mycophenolate mofetil has a treatment effect, but those studies were small. The Ritux 3 trial, published in The Lancet showed that rituximab plus glucocorticoids as opposed to glucocorticoids alone was beneficial in treating pemphigus.
“Rituximab has moved toward first-line therapy for moderate to severe pemphigus as recommended by an international panel of experts,” Dr. Werth said in an interview.
In her practice, Dr. Werth said that she has observed similar outcomes in clinical practice for patients prescribed oral mycophenolate mofetil. “Patients take a long time to get to remission and frequently end up staying on prednisone and long-term mycophenolate mofetil,” she said. She uses mycophenolate mofetil less often since rituximab has been shown to be effective for many patients, but mycophenolate mofetil “still has a place for patients who don’t want, or can’t tolerate, rituximab, or for cases in which rituximab doesn’t work.”
This study was supported by a grant from Hoffmann–La Roche. Dr. Werth disclosed having served as a consultant to Genentech on pemphigus, and that the University of Pennsylvania has received a grant/contract to perform a rituximab–mycophenolate mofetil trial for pemphigus vulgaris.
Mycophenolate mofetil, commonly used as a first-line corticosteroid-sparing agent for moderate to severe cases of the autoimmune blistering skin condition pemphigus vulgaris, has been found to be inferior to the biologic agent rituximab.
Mycophenolate mofetil is widely accepted as a first-in-line corticosteroid-sparing agent for pemphigus vulgaris, but few studies have compared the effectiveness of the two treatments for pemphigus vulgaris. The European Academy of Dermatology and Venereology recommends rituximab (Rituxan), a CD20 inhibitor, as first-line treatment for patients with new-onset cases of moderate to severe intensity or for patients who fail to achieve clinical remission with systemic corticosteroids with or without other immunosuppressive treatments.
In the current study, published online on May 19, 2021, in the New England Journal of Medicine, researchers led by Victoria P. Werth, MD, professor of dermatology at the University of Pennsylvania, Philadelphia, conducted a randomized, controlled trial of 135 patients (mean age, 48 years; 53% women) with moderate to severe pemphigus vulgaris with 67 receiving rituximab and 68 receiving mycophenolate mofetil (99% of patients in the rituximab group and 85% of patients in the mycophenolate mofetil group completed the trial).
Patients in the rituximab group received 1,000 mg of IV rituximab on days 1, 15, 168, and 182 of the study, plus twice-daily oral placebo. Intravenous methylprednisolone at 100 mg was administered before each rituximab infusion to reduce infusion-related reactions. Patients in the second group were given mycophenolate mofetil orally twice daily, starting at 1 g/day in divided doses and adjusted to 2 g/day in divided doses by week 2. They also received placebo infusions on days 1, 15, 168, and 182 of the study.
Patients in both groups received oral glucocorticoids throughout the course of the trial: an average of 3,545 mg for the rituximab treatment group and a cumulative dose of 5,140 mg for the group treated with mycophenolate mofetil, a statistically significant difference (P < .001). Outcomes based on 62 patients treated with rituximab and 63 on MMF, a modified intention-to-treat group.
By week 52, 25 patients (40%) who were treated with rituximab experienced complete sustained remission (the primary endpoint), compared with 6 patients (10%) in the mycophenolate mofetil group (95% confidence interval, 15-45, P < .001).
Only six patients in the rituximab group experienced a disease flare as compared with 44 patients in the mycophenolate mofetil group (adjusted rate ratio, 0.12; 95% CI, 0.05-0.29; P < .001). Serious adverse events occurred in 15 of 67 patients (22%) in the rituximab group and in 10 of 68 (15%) in the mycophenolate mofetil group with 3 patients in the rituximab group and 26 in the mycophenolate mofetil receiving rescue therapy.
Second to remission, the goal of treatment for pemphigus vulgaris is to reduce the use of glucocorticoids, Dr. Werth and colleagues wrote, adding: “The results of this trial showed that rituximab was superior to mycophenolate mofetil in producing sustained complete remission over 52 weeks among patients with moderate to severe pemphigus vulgaris. Rituximab had a greater glucocorticoid-sparing effect than mycophenolate mofetil, but more patients in this group had serious adverse events.”
Most adverse events in the rituximab group were limited to infusion-related reactions, but serious adverse events occurred in 15 patients (including pneumonia and upper respiratory tract infection, cellulitis and acute pyelonephritis, viral pneumonia, and skin infection). Ten patients in the mycophenolate mofetil group experienced serious adverse events (pneumonia and influenza, cellulitis and sepsis, herpes zoster, and pyelonephritis).
The current study had several limitations, primarily its small size. Plus, the authors noted a short follow-up period after glucocorticoids were stopped.
Mycophenolate mofetil, along with immunosuppressants, is approved in the United States as a treatment for organ rejection in patients who have received kidney, heart or liver transplants. But it is also used off label for pemphigus vulgaris and in rheumatology as a treatment for lupus, rheumatoid arthritis, vasculitis, inflammatory bowel disease (Crohn’s disease), inflammatory eye disease (uveitis) as well as kidney and skin disorders.
In the 2018 treatment guidelines for pemphigus by the European Dermatology Forum and the EADV, mycophenolate mofetil is recommended as a first-line corticosteroid sparing agent for pemphigus vulgaris.
Rituximab was approved in 2018 as the first biologic therapy for patients with pemphigus vulgaris and is currently recommended as a treatment for patients with pemphigus. But how well it works in comparison with the long-established mycophenolate mofetil hasn’t been extensively studied.
Other smaller studies show that mycophenolate mofetil has a treatment effect, but those studies were small. The Ritux 3 trial, published in The Lancet showed that rituximab plus glucocorticoids as opposed to glucocorticoids alone was beneficial in treating pemphigus.
“Rituximab has moved toward first-line therapy for moderate to severe pemphigus as recommended by an international panel of experts,” Dr. Werth said in an interview.
In her practice, Dr. Werth said that she has observed similar outcomes in clinical practice for patients prescribed oral mycophenolate mofetil. “Patients take a long time to get to remission and frequently end up staying on prednisone and long-term mycophenolate mofetil,” she said. She uses mycophenolate mofetil less often since rituximab has been shown to be effective for many patients, but mycophenolate mofetil “still has a place for patients who don’t want, or can’t tolerate, rituximab, or for cases in which rituximab doesn’t work.”
This study was supported by a grant from Hoffmann–La Roche. Dr. Werth disclosed having served as a consultant to Genentech on pemphigus, and that the University of Pennsylvania has received a grant/contract to perform a rituximab–mycophenolate mofetil trial for pemphigus vulgaris.
Mycophenolate mofetil, commonly used as a first-line corticosteroid-sparing agent for moderate to severe cases of the autoimmune blistering skin condition pemphigus vulgaris, has been found to be inferior to the biologic agent rituximab.
Mycophenolate mofetil is widely accepted as a first-in-line corticosteroid-sparing agent for pemphigus vulgaris, but few studies have compared the effectiveness of the two treatments for pemphigus vulgaris. The European Academy of Dermatology and Venereology recommends rituximab (Rituxan), a CD20 inhibitor, as first-line treatment for patients with new-onset cases of moderate to severe intensity or for patients who fail to achieve clinical remission with systemic corticosteroids with or without other immunosuppressive treatments.
In the current study, published online on May 19, 2021, in the New England Journal of Medicine, researchers led by Victoria P. Werth, MD, professor of dermatology at the University of Pennsylvania, Philadelphia, conducted a randomized, controlled trial of 135 patients (mean age, 48 years; 53% women) with moderate to severe pemphigus vulgaris with 67 receiving rituximab and 68 receiving mycophenolate mofetil (99% of patients in the rituximab group and 85% of patients in the mycophenolate mofetil group completed the trial).
Patients in the rituximab group received 1,000 mg of IV rituximab on days 1, 15, 168, and 182 of the study, plus twice-daily oral placebo. Intravenous methylprednisolone at 100 mg was administered before each rituximab infusion to reduce infusion-related reactions. Patients in the second group were given mycophenolate mofetil orally twice daily, starting at 1 g/day in divided doses and adjusted to 2 g/day in divided doses by week 2. They also received placebo infusions on days 1, 15, 168, and 182 of the study.
Patients in both groups received oral glucocorticoids throughout the course of the trial: an average of 3,545 mg for the rituximab treatment group and a cumulative dose of 5,140 mg for the group treated with mycophenolate mofetil, a statistically significant difference (P < .001). Outcomes based on 62 patients treated with rituximab and 63 on MMF, a modified intention-to-treat group.
By week 52, 25 patients (40%) who were treated with rituximab experienced complete sustained remission (the primary endpoint), compared with 6 patients (10%) in the mycophenolate mofetil group (95% confidence interval, 15-45, P < .001).
Only six patients in the rituximab group experienced a disease flare as compared with 44 patients in the mycophenolate mofetil group (adjusted rate ratio, 0.12; 95% CI, 0.05-0.29; P < .001). Serious adverse events occurred in 15 of 67 patients (22%) in the rituximab group and in 10 of 68 (15%) in the mycophenolate mofetil group with 3 patients in the rituximab group and 26 in the mycophenolate mofetil receiving rescue therapy.
Second to remission, the goal of treatment for pemphigus vulgaris is to reduce the use of glucocorticoids, Dr. Werth and colleagues wrote, adding: “The results of this trial showed that rituximab was superior to mycophenolate mofetil in producing sustained complete remission over 52 weeks among patients with moderate to severe pemphigus vulgaris. Rituximab had a greater glucocorticoid-sparing effect than mycophenolate mofetil, but more patients in this group had serious adverse events.”
Most adverse events in the rituximab group were limited to infusion-related reactions, but serious adverse events occurred in 15 patients (including pneumonia and upper respiratory tract infection, cellulitis and acute pyelonephritis, viral pneumonia, and skin infection). Ten patients in the mycophenolate mofetil group experienced serious adverse events (pneumonia and influenza, cellulitis and sepsis, herpes zoster, and pyelonephritis).
The current study had several limitations, primarily its small size. Plus, the authors noted a short follow-up period after glucocorticoids were stopped.
Mycophenolate mofetil, along with immunosuppressants, is approved in the United States as a treatment for organ rejection in patients who have received kidney, heart or liver transplants. But it is also used off label for pemphigus vulgaris and in rheumatology as a treatment for lupus, rheumatoid arthritis, vasculitis, inflammatory bowel disease (Crohn’s disease), inflammatory eye disease (uveitis) as well as kidney and skin disorders.
In the 2018 treatment guidelines for pemphigus by the European Dermatology Forum and the EADV, mycophenolate mofetil is recommended as a first-line corticosteroid sparing agent for pemphigus vulgaris.
Rituximab was approved in 2018 as the first biologic therapy for patients with pemphigus vulgaris and is currently recommended as a treatment for patients with pemphigus. But how well it works in comparison with the long-established mycophenolate mofetil hasn’t been extensively studied.
Other smaller studies show that mycophenolate mofetil has a treatment effect, but those studies were small. The Ritux 3 trial, published in The Lancet showed that rituximab plus glucocorticoids as opposed to glucocorticoids alone was beneficial in treating pemphigus.
“Rituximab has moved toward first-line therapy for moderate to severe pemphigus as recommended by an international panel of experts,” Dr. Werth said in an interview.
In her practice, Dr. Werth said that she has observed similar outcomes in clinical practice for patients prescribed oral mycophenolate mofetil. “Patients take a long time to get to remission and frequently end up staying on prednisone and long-term mycophenolate mofetil,” she said. She uses mycophenolate mofetil less often since rituximab has been shown to be effective for many patients, but mycophenolate mofetil “still has a place for patients who don’t want, or can’t tolerate, rituximab, or for cases in which rituximab doesn’t work.”
This study was supported by a grant from Hoffmann–La Roche. Dr. Werth disclosed having served as a consultant to Genentech on pemphigus, and that the University of Pennsylvania has received a grant/contract to perform a rituximab–mycophenolate mofetil trial for pemphigus vulgaris.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Novel immunotherapy relatlimab in advanced melanoma
Adding the novel immune checkpoint inhibitor relatlimab to the more established nivolumab (Opdivo) significantly extended the progression-free survival (PFS) of patients with previously untreated advanced melanoma in comparison with nivolumab alone in the phase 3 RELATIVITY-047 trial.
Both drugs are from Bristol-Myers Squibb, which funded the study.
“Our findings demonstrate that relatlimab plus nivolumab is a potential novel treatment option for this patient population,” said lead researcher Evan J. Lipson, MD, Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins University, Baltimore.
Relatlimab has a different mechanism of action from currently available immune checkpoint inhibitors, such as nivolumab and similar agents, which act as inhibitors of the programmed cell death protein–1 (PD-1) or programmed cell death–ligand-1 (PD-L1). In contrast, relatlimab acts as an antibody that targets lymphocyte-activation gene 3 (LAG-3), which inhibits T cells and thus helps cancer cells evade immune attack.
“This is the first phase 3 study to validate inhibition of the LAG-3 immune checkpoint as a therapeutic strategy for patients with cancer, and it establishes the LAG-3 pathway as the third immune checkpoint pathway in history, after CLTA-4 and PD-1, for which blockade appears to have clinical benefit,” Dr. Lipson said at a press briefing ahead of the annual meeting of the American Society of Clinical Oncology (ASCO), where this study will be presented (abstract 9503).
Commenting for ASCO, Julie R. Gralow, MD, chief medical officer and executive vice president, agreed that “these results provide validation of the LAG-3 immune checkpoint as a therapeutic target ... and they also support combination treatment with immunotherapies that act on different parts of the immune system.”
When Dr. Lipson was asked whether he would recommend the combination of relatlimab plus nivolumab as a first-line treatment for this patient population, he said that “for many patients,” the first-line treatment choice is made on a “case-by-case” basis.
“We are fortunate in melanoma that we have an ever-expanding list of seemingly effective options, and I think we’ll find at some point this will be added to that list,” he said. “Whether this is the first-line choice for any given patient really depends on a lot of factors,” he added.
Dr. Gralow added a note of caution. “The combination was clearly more toxic, and so I think there will be a lot of discussion” as to when it would be used and for which patients, she said.
In the absence of head-to-head comparisons, “I’m not sure that we have one answer” as to which treatment to choose, she added. With the ever-increasing number of options available in melanoma, the individual treatment choice is “getting more complicated,” she said.
Study details
The global RELATIVITY-047 study was conducted in 714 patients with previously untreated unresectable or metastatic melanoma. The participants were randomly assigned to receive either relatlimab plus nivolumab or nivolumab alone.
Dr. Lipson explained that the treatments were given as a fixed-dosed combination, meaning the preparation of relatlimab and nivolumab was given in the “same medication phial and administered as a single intravenous infusion in order to reduce preparation and infusion times and minimize the risk of administration errors.”
PFS, as determined on blinded independent central review, was significantly longer with the combination therapy than with nivolumab alone, at a median of 10.12 months vs. 4.63 months (hazard ratio, 0.75; P = .0055).
At 12 months, the PFS rate among patients given relatlimab plus nivolumab was 47.7%, versus 36.0% among those given nivolumab alone.
“This significant improvement meant that the study met its primary endpoint,” Dr. Lipson said, adding that the PFS benefit “appeared relatively early in the course of therapy.” The curves separated at 12 weeks, and benefit was “sustained” over the course of follow-up.
He added that the performance of nivolumab alone was “in the range” of that seen in previous studies, although he underlined that cross-trial comparison is difficult, given the differences in study design.
“In general, treatment-related adverse events” associated with the combination therapy were “manageable and reflected the safety profile that we typically see with immune checkpoint inhibitors,” he noted.
The results showed that 40.3% of patients who received the combination therapy experienced a grade 3-4 adverse event, compared with 33.4% of those given nivolumab alone. Grade 3-4 treatment-related adverse events leading to discontinuation occurred in 8.5% and 3.1% of patients, respectively.
Three treatment-related deaths occurred in the relatlimab and nivolumab arm. Two such deaths occurred in the nivolumab-alone group.
The study was funded by Bristol Myers Squibb. Dr. Lipson has relationships with Array BioPharma, Bristol Myers Squibb, EMD Serono, Genentech, Macrogenics, Merck, Millennium, Novartis, Sanofi/Regeneron, and Sysmex (inst). Dr. Gralow has relationships with AstraZeneca, Genentech, Sandoz, and Immunomedics.
A version of this article first appeared on Medscape.com.
Adding the novel immune checkpoint inhibitor relatlimab to the more established nivolumab (Opdivo) significantly extended the progression-free survival (PFS) of patients with previously untreated advanced melanoma in comparison with nivolumab alone in the phase 3 RELATIVITY-047 trial.
Both drugs are from Bristol-Myers Squibb, which funded the study.
“Our findings demonstrate that relatlimab plus nivolumab is a potential novel treatment option for this patient population,” said lead researcher Evan J. Lipson, MD, Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins University, Baltimore.
Relatlimab has a different mechanism of action from currently available immune checkpoint inhibitors, such as nivolumab and similar agents, which act as inhibitors of the programmed cell death protein–1 (PD-1) or programmed cell death–ligand-1 (PD-L1). In contrast, relatlimab acts as an antibody that targets lymphocyte-activation gene 3 (LAG-3), which inhibits T cells and thus helps cancer cells evade immune attack.
“This is the first phase 3 study to validate inhibition of the LAG-3 immune checkpoint as a therapeutic strategy for patients with cancer, and it establishes the LAG-3 pathway as the third immune checkpoint pathway in history, after CLTA-4 and PD-1, for which blockade appears to have clinical benefit,” Dr. Lipson said at a press briefing ahead of the annual meeting of the American Society of Clinical Oncology (ASCO), where this study will be presented (abstract 9503).
Commenting for ASCO, Julie R. Gralow, MD, chief medical officer and executive vice president, agreed that “these results provide validation of the LAG-3 immune checkpoint as a therapeutic target ... and they also support combination treatment with immunotherapies that act on different parts of the immune system.”
When Dr. Lipson was asked whether he would recommend the combination of relatlimab plus nivolumab as a first-line treatment for this patient population, he said that “for many patients,” the first-line treatment choice is made on a “case-by-case” basis.
“We are fortunate in melanoma that we have an ever-expanding list of seemingly effective options, and I think we’ll find at some point this will be added to that list,” he said. “Whether this is the first-line choice for any given patient really depends on a lot of factors,” he added.
Dr. Gralow added a note of caution. “The combination was clearly more toxic, and so I think there will be a lot of discussion” as to when it would be used and for which patients, she said.
In the absence of head-to-head comparisons, “I’m not sure that we have one answer” as to which treatment to choose, she added. With the ever-increasing number of options available in melanoma, the individual treatment choice is “getting more complicated,” she said.
Study details
The global RELATIVITY-047 study was conducted in 714 patients with previously untreated unresectable or metastatic melanoma. The participants were randomly assigned to receive either relatlimab plus nivolumab or nivolumab alone.
Dr. Lipson explained that the treatments were given as a fixed-dosed combination, meaning the preparation of relatlimab and nivolumab was given in the “same medication phial and administered as a single intravenous infusion in order to reduce preparation and infusion times and minimize the risk of administration errors.”
PFS, as determined on blinded independent central review, was significantly longer with the combination therapy than with nivolumab alone, at a median of 10.12 months vs. 4.63 months (hazard ratio, 0.75; P = .0055).
At 12 months, the PFS rate among patients given relatlimab plus nivolumab was 47.7%, versus 36.0% among those given nivolumab alone.
“This significant improvement meant that the study met its primary endpoint,” Dr. Lipson said, adding that the PFS benefit “appeared relatively early in the course of therapy.” The curves separated at 12 weeks, and benefit was “sustained” over the course of follow-up.
He added that the performance of nivolumab alone was “in the range” of that seen in previous studies, although he underlined that cross-trial comparison is difficult, given the differences in study design.
“In general, treatment-related adverse events” associated with the combination therapy were “manageable and reflected the safety profile that we typically see with immune checkpoint inhibitors,” he noted.
The results showed that 40.3% of patients who received the combination therapy experienced a grade 3-4 adverse event, compared with 33.4% of those given nivolumab alone. Grade 3-4 treatment-related adverse events leading to discontinuation occurred in 8.5% and 3.1% of patients, respectively.
Three treatment-related deaths occurred in the relatlimab and nivolumab arm. Two such deaths occurred in the nivolumab-alone group.
The study was funded by Bristol Myers Squibb. Dr. Lipson has relationships with Array BioPharma, Bristol Myers Squibb, EMD Serono, Genentech, Macrogenics, Merck, Millennium, Novartis, Sanofi/Regeneron, and Sysmex (inst). Dr. Gralow has relationships with AstraZeneca, Genentech, Sandoz, and Immunomedics.
A version of this article first appeared on Medscape.com.
Adding the novel immune checkpoint inhibitor relatlimab to the more established nivolumab (Opdivo) significantly extended the progression-free survival (PFS) of patients with previously untreated advanced melanoma in comparison with nivolumab alone in the phase 3 RELATIVITY-047 trial.
Both drugs are from Bristol-Myers Squibb, which funded the study.
“Our findings demonstrate that relatlimab plus nivolumab is a potential novel treatment option for this patient population,” said lead researcher Evan J. Lipson, MD, Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins University, Baltimore.
Relatlimab has a different mechanism of action from currently available immune checkpoint inhibitors, such as nivolumab and similar agents, which act as inhibitors of the programmed cell death protein–1 (PD-1) or programmed cell death–ligand-1 (PD-L1). In contrast, relatlimab acts as an antibody that targets lymphocyte-activation gene 3 (LAG-3), which inhibits T cells and thus helps cancer cells evade immune attack.
“This is the first phase 3 study to validate inhibition of the LAG-3 immune checkpoint as a therapeutic strategy for patients with cancer, and it establishes the LAG-3 pathway as the third immune checkpoint pathway in history, after CLTA-4 and PD-1, for which blockade appears to have clinical benefit,” Dr. Lipson said at a press briefing ahead of the annual meeting of the American Society of Clinical Oncology (ASCO), where this study will be presented (abstract 9503).
Commenting for ASCO, Julie R. Gralow, MD, chief medical officer and executive vice president, agreed that “these results provide validation of the LAG-3 immune checkpoint as a therapeutic target ... and they also support combination treatment with immunotherapies that act on different parts of the immune system.”
When Dr. Lipson was asked whether he would recommend the combination of relatlimab plus nivolumab as a first-line treatment for this patient population, he said that “for many patients,” the first-line treatment choice is made on a “case-by-case” basis.
“We are fortunate in melanoma that we have an ever-expanding list of seemingly effective options, and I think we’ll find at some point this will be added to that list,” he said. “Whether this is the first-line choice for any given patient really depends on a lot of factors,” he added.
Dr. Gralow added a note of caution. “The combination was clearly more toxic, and so I think there will be a lot of discussion” as to when it would be used and for which patients, she said.
In the absence of head-to-head comparisons, “I’m not sure that we have one answer” as to which treatment to choose, she added. With the ever-increasing number of options available in melanoma, the individual treatment choice is “getting more complicated,” she said.
Study details
The global RELATIVITY-047 study was conducted in 714 patients with previously untreated unresectable or metastatic melanoma. The participants were randomly assigned to receive either relatlimab plus nivolumab or nivolumab alone.
Dr. Lipson explained that the treatments were given as a fixed-dosed combination, meaning the preparation of relatlimab and nivolumab was given in the “same medication phial and administered as a single intravenous infusion in order to reduce preparation and infusion times and minimize the risk of administration errors.”
PFS, as determined on blinded independent central review, was significantly longer with the combination therapy than with nivolumab alone, at a median of 10.12 months vs. 4.63 months (hazard ratio, 0.75; P = .0055).
At 12 months, the PFS rate among patients given relatlimab plus nivolumab was 47.7%, versus 36.0% among those given nivolumab alone.
“This significant improvement meant that the study met its primary endpoint,” Dr. Lipson said, adding that the PFS benefit “appeared relatively early in the course of therapy.” The curves separated at 12 weeks, and benefit was “sustained” over the course of follow-up.
He added that the performance of nivolumab alone was “in the range” of that seen in previous studies, although he underlined that cross-trial comparison is difficult, given the differences in study design.
“In general, treatment-related adverse events” associated with the combination therapy were “manageable and reflected the safety profile that we typically see with immune checkpoint inhibitors,” he noted.
The results showed that 40.3% of patients who received the combination therapy experienced a grade 3-4 adverse event, compared with 33.4% of those given nivolumab alone. Grade 3-4 treatment-related adverse events leading to discontinuation occurred in 8.5% and 3.1% of patients, respectively.
Three treatment-related deaths occurred in the relatlimab and nivolumab arm. Two such deaths occurred in the nivolumab-alone group.
The study was funded by Bristol Myers Squibb. Dr. Lipson has relationships with Array BioPharma, Bristol Myers Squibb, EMD Serono, Genentech, Macrogenics, Merck, Millennium, Novartis, Sanofi/Regeneron, and Sysmex (inst). Dr. Gralow has relationships with AstraZeneca, Genentech, Sandoz, and Immunomedics.
A version of this article first appeared on Medscape.com.
ID experts dole out practical advice to help with mask confusion
The Centers for Disease Control and Prevention’s latest guidance on what fully vaccinated people can do safely – including not socially distancing and not wearing a mask indoors or outdoors unless other regulations require it – has been widely misinterpreted and caused confusion, two infectious disease experts said at a briefing on May 20 hosted by the Infectious Diseases Society of America (IDSA).
The CDC did not ‘’lift” the mask mandate, but rather supplied guidance for those who are fully vaccinated. However, many questions and gray areas remain, and the experts addressed those. ‘’The CDC guidance is really directed at people who are fully vaccinated and who we know are likely to have a really solid response to the vaccine,” said Jeanne Marrazzo, MD, MPH, director of infectious diseases at the University of Alabama at Birmingham and an IDSA board member.
That message was largely lost, said Dr. Marrazzo and Jeffrey Duchin, MD, health officer of public health for Seattle and King County, Washington, and also an IDSA board member. Dr. Duchin said many people mistakenly regarded the new guidance as a message that the pandemic is over.
Among their practical tips on how to interpret the guidance:
To mask or not?
To make the decision, people need to think about not only the numbers of vaccinated versus unvaccinated individuals in their community but the local rates of disease, the experts said. And they need to know that the CDC guidance doesn’t apply if regulations by federal or state authorities or businesses and workplace are in conflict.
Deciding on mask use sometimes depends on where you are going. What about going into grocery stores or large bin stores without a mask? “If you are fully vaccinated and have no other conditions that compromise your immune system, and the rates of COVID are relatively low where you live, and the vaccination rates are high, I would be 100% fine” without a mask, Dr. Marrazzo said. But it’s important to think of all these factors in calculating your risk.
“I’m still wearing a mask when I go anywhere in public,” she said, citing vaccination rates that have not yet reached 50% in her area.
If that rate reached 80%, the typical percentage talked about for herd immunity, and new cases were low, Dr. Marrazzo said she might shed the mask.
The CDC also continues to recommend masks on mass transit for all.
One population that also must be considered, and who must evaluate their risk, even if vaccinated, are the immunocompromised, Dr. Marrazzo said. While people think of the immunocompromised as those with HIV or organ transplants, the numbers are actually much larger.
“A study a couple of years ago indicated up to 3% of Americans may actually have been told by their physician they have some of level of being immunocompromised,” she said. Among the examples are those who are on dialysis, on chemotherapy, or those taking any of the medications that modify the immune system.
“Millions of people fit this bill, and we have [very] little data on whether the vaccine works in them. We think it does,” Dr. Marrazzo said.
Still, she said, it’s a reason for these people to be cautious. For some other vaccines, the dose is modified for those who are immunocompromised. What’s not known yet is whether additional doses of the COVID vaccines might boost protection for those who are immunocompromised.
Many people, even after vaccination, may choose to keep wearing a mask especially in indoor, crowded settings, Dr. Duchin said. “We need to expect, accept, and respect continued mask wearing by anyone at any time.”
In most outdoor settings, he said, “I think masks are probably not necessary, vaccinated or not, regardless of age.” One exception: close face-to-face contact, such as in certain sports.
How to protect toddlers and infants
With masks not practical or recommended for infants and toddlers under 2 years old, Dr. Marrazzo said adults should remember that ‘’those very little kids don’t do poorly at all [even if infected], although there is not a ton of data.”
Adults should still treat young children as vulnerable, especially newborns. Adults not yet vaccinated should wear a mask when around them, she said.
J & J vaccine recipients
With less ‘’real world” data on the Johnson & Johnson vaccine, should those who got it think of themselves in a different risk group than those who got Moderna or Pfizer and adjust their behavior accordingly?
“The J&J vaccine, based on everything we know, does provide a great deal of protection,” Dr. Marrazzo said. ‘’We don’t know as much about prevention of transmission in the asymptomatic cases in the J&J.”
Most of that data, she said, is from the mRNA vaccines Pfizer and Moderna. “I think it’s an important area to study and learn about.” But all three vaccines, overall, provide a high level of protection, she said.
A version of this article first appeared on Medscape.com.
The Centers for Disease Control and Prevention’s latest guidance on what fully vaccinated people can do safely – including not socially distancing and not wearing a mask indoors or outdoors unless other regulations require it – has been widely misinterpreted and caused confusion, two infectious disease experts said at a briefing on May 20 hosted by the Infectious Diseases Society of America (IDSA).
The CDC did not ‘’lift” the mask mandate, but rather supplied guidance for those who are fully vaccinated. However, many questions and gray areas remain, and the experts addressed those. ‘’The CDC guidance is really directed at people who are fully vaccinated and who we know are likely to have a really solid response to the vaccine,” said Jeanne Marrazzo, MD, MPH, director of infectious diseases at the University of Alabama at Birmingham and an IDSA board member.
That message was largely lost, said Dr. Marrazzo and Jeffrey Duchin, MD, health officer of public health for Seattle and King County, Washington, and also an IDSA board member. Dr. Duchin said many people mistakenly regarded the new guidance as a message that the pandemic is over.
Among their practical tips on how to interpret the guidance:
To mask or not?
To make the decision, people need to think about not only the numbers of vaccinated versus unvaccinated individuals in their community but the local rates of disease, the experts said. And they need to know that the CDC guidance doesn’t apply if regulations by federal or state authorities or businesses and workplace are in conflict.
Deciding on mask use sometimes depends on where you are going. What about going into grocery stores or large bin stores without a mask? “If you are fully vaccinated and have no other conditions that compromise your immune system, and the rates of COVID are relatively low where you live, and the vaccination rates are high, I would be 100% fine” without a mask, Dr. Marrazzo said. But it’s important to think of all these factors in calculating your risk.
“I’m still wearing a mask when I go anywhere in public,” she said, citing vaccination rates that have not yet reached 50% in her area.
If that rate reached 80%, the typical percentage talked about for herd immunity, and new cases were low, Dr. Marrazzo said she might shed the mask.
The CDC also continues to recommend masks on mass transit for all.
One population that also must be considered, and who must evaluate their risk, even if vaccinated, are the immunocompromised, Dr. Marrazzo said. While people think of the immunocompromised as those with HIV or organ transplants, the numbers are actually much larger.
“A study a couple of years ago indicated up to 3% of Americans may actually have been told by their physician they have some of level of being immunocompromised,” she said. Among the examples are those who are on dialysis, on chemotherapy, or those taking any of the medications that modify the immune system.
“Millions of people fit this bill, and we have [very] little data on whether the vaccine works in them. We think it does,” Dr. Marrazzo said.
Still, she said, it’s a reason for these people to be cautious. For some other vaccines, the dose is modified for those who are immunocompromised. What’s not known yet is whether additional doses of the COVID vaccines might boost protection for those who are immunocompromised.
Many people, even after vaccination, may choose to keep wearing a mask especially in indoor, crowded settings, Dr. Duchin said. “We need to expect, accept, and respect continued mask wearing by anyone at any time.”
In most outdoor settings, he said, “I think masks are probably not necessary, vaccinated or not, regardless of age.” One exception: close face-to-face contact, such as in certain sports.
How to protect toddlers and infants
With masks not practical or recommended for infants and toddlers under 2 years old, Dr. Marrazzo said adults should remember that ‘’those very little kids don’t do poorly at all [even if infected], although there is not a ton of data.”
Adults should still treat young children as vulnerable, especially newborns. Adults not yet vaccinated should wear a mask when around them, she said.
J & J vaccine recipients
With less ‘’real world” data on the Johnson & Johnson vaccine, should those who got it think of themselves in a different risk group than those who got Moderna or Pfizer and adjust their behavior accordingly?
“The J&J vaccine, based on everything we know, does provide a great deal of protection,” Dr. Marrazzo said. ‘’We don’t know as much about prevention of transmission in the asymptomatic cases in the J&J.”
Most of that data, she said, is from the mRNA vaccines Pfizer and Moderna. “I think it’s an important area to study and learn about.” But all three vaccines, overall, provide a high level of protection, she said.
A version of this article first appeared on Medscape.com.
The Centers for Disease Control and Prevention’s latest guidance on what fully vaccinated people can do safely – including not socially distancing and not wearing a mask indoors or outdoors unless other regulations require it – has been widely misinterpreted and caused confusion, two infectious disease experts said at a briefing on May 20 hosted by the Infectious Diseases Society of America (IDSA).
The CDC did not ‘’lift” the mask mandate, but rather supplied guidance for those who are fully vaccinated. However, many questions and gray areas remain, and the experts addressed those. ‘’The CDC guidance is really directed at people who are fully vaccinated and who we know are likely to have a really solid response to the vaccine,” said Jeanne Marrazzo, MD, MPH, director of infectious diseases at the University of Alabama at Birmingham and an IDSA board member.
That message was largely lost, said Dr. Marrazzo and Jeffrey Duchin, MD, health officer of public health for Seattle and King County, Washington, and also an IDSA board member. Dr. Duchin said many people mistakenly regarded the new guidance as a message that the pandemic is over.
Among their practical tips on how to interpret the guidance:
To mask or not?
To make the decision, people need to think about not only the numbers of vaccinated versus unvaccinated individuals in their community but the local rates of disease, the experts said. And they need to know that the CDC guidance doesn’t apply if regulations by federal or state authorities or businesses and workplace are in conflict.
Deciding on mask use sometimes depends on where you are going. What about going into grocery stores or large bin stores without a mask? “If you are fully vaccinated and have no other conditions that compromise your immune system, and the rates of COVID are relatively low where you live, and the vaccination rates are high, I would be 100% fine” without a mask, Dr. Marrazzo said. But it’s important to think of all these factors in calculating your risk.
“I’m still wearing a mask when I go anywhere in public,” she said, citing vaccination rates that have not yet reached 50% in her area.
If that rate reached 80%, the typical percentage talked about for herd immunity, and new cases were low, Dr. Marrazzo said she might shed the mask.
The CDC also continues to recommend masks on mass transit for all.
One population that also must be considered, and who must evaluate their risk, even if vaccinated, are the immunocompromised, Dr. Marrazzo said. While people think of the immunocompromised as those with HIV or organ transplants, the numbers are actually much larger.
“A study a couple of years ago indicated up to 3% of Americans may actually have been told by their physician they have some of level of being immunocompromised,” she said. Among the examples are those who are on dialysis, on chemotherapy, or those taking any of the medications that modify the immune system.
“Millions of people fit this bill, and we have [very] little data on whether the vaccine works in them. We think it does,” Dr. Marrazzo said.
Still, she said, it’s a reason for these people to be cautious. For some other vaccines, the dose is modified for those who are immunocompromised. What’s not known yet is whether additional doses of the COVID vaccines might boost protection for those who are immunocompromised.
Many people, even after vaccination, may choose to keep wearing a mask especially in indoor, crowded settings, Dr. Duchin said. “We need to expect, accept, and respect continued mask wearing by anyone at any time.”
In most outdoor settings, he said, “I think masks are probably not necessary, vaccinated or not, regardless of age.” One exception: close face-to-face contact, such as in certain sports.
How to protect toddlers and infants
With masks not practical or recommended for infants and toddlers under 2 years old, Dr. Marrazzo said adults should remember that ‘’those very little kids don’t do poorly at all [even if infected], although there is not a ton of data.”
Adults should still treat young children as vulnerable, especially newborns. Adults not yet vaccinated should wear a mask when around them, she said.
J & J vaccine recipients
With less ‘’real world” data on the Johnson & Johnson vaccine, should those who got it think of themselves in a different risk group than those who got Moderna or Pfizer and adjust their behavior accordingly?
“The J&J vaccine, based on everything we know, does provide a great deal of protection,” Dr. Marrazzo said. ‘’We don’t know as much about prevention of transmission in the asymptomatic cases in the J&J.”
Most of that data, she said, is from the mRNA vaccines Pfizer and Moderna. “I think it’s an important area to study and learn about.” But all three vaccines, overall, provide a high level of protection, she said.
A version of this article first appeared on Medscape.com.
Combined imaging methods found to enhance detection of squamous cell carcinoma
and distinguishing SCC in-situ and actinic keratosis (AK) from invasive SCC, results from a small prospective study demonstrated.
“A solitary scaly papule or plaque could represent an inflammatory or neoplastic process, and when neoplastic, it could be benign, premalignant, malignant in situ, or invasive malignant,” lead study author Abdullah Aleisa, MD, said in an interview during the annual conference of the American Society for Laser Medicine and Surgery. Noninvasive imaging devices, such as reflectance confocal microscopy (RCM) and optical coherence tomography (OCT), “have been used to help in the diagnosis of those clinically suspicious lesions, however each device has its own limitation.”
RCM images are horizontal sections of the skin with high cellular resolution but limited to 250 mcm of depth in skin, he said, while OCT images are vertical sections of the skin with low cellular resolution, but image up to 1,000-2,000 mcm of depth in skin.
“Combined RCM-OCT enables high cellular resolution and deep tissue evaluation,” said Dr. Aleisa, a micrographic surgery and dermatologic oncology fellow at Memorial Sloan Kettering Cancer Center, New York. “The value of combined RCM-OCT has been shown in the detection and depth assessment of basal cell carcinoma, but it has never been studied in SCC. Our objective is to combine RCM and OCT simultaneously to detect SCC and assess the depth of invasion.”
Between September and December 2020, Dr. Aleisa and colleagues prospectively imaged 36 lesions suspicious of SCC, SCC in situ, or AK between September 2020 and December 2020. The mean age of the cohort was 68 years and 63% were male. Using a prototype device from Andover, Mass.–based Caliber I.D., the investigators performed handheld RCM-OCT imaging at the center of clinically suspected lesions before biopsy and to previously diagnosed lesions before Mohs micrographic surgery (to check for residual tumor) and correlated RCM-OCT findings with histopathology results. A total of 36 lesions were treated.
Dr. Aleisa reported that most common RCM-OCT feature for invasive SCC was presence of vertical blood vessels (in 89% of lesions), while for SCC in situ/AK, it was acanthosis and parakeratosis without vertical blood vessels (in 84% of lesions). For the detection of invasive SCC, RCM-OCT had a sensitivity of 82%, a specificity of 92%, a negative predictive value of 92%, and a positive predictive value of 82%. For the detection of SCC in situ/AK, RCM-OCT had a sensitivity of 86%, a specificity of 100%, a negative predictive value of 92%, and a positive predictive value of 100%. The OCT depth measurement correlated well with histopathology with a concordance correlation coefficient of r2 = 0.9.
“Using RCM’s high-resolution pictures allowed us to easily spot the vertical ‘buttonhole’ vessels associated with SCC,” Dr. Aleisa said. “However, given the depth limitation of RCM, the distinction between SCC in situ and invasive SCC could not be accomplished using RCM alone. Therefore, having simultaneous OCT live feedback to the RCM images in the combined RCM-OCT device enabled us to assess the depth of those vertical ‘buttonholes’ and distinguish between SCC in situ and invasive SCC.”
He acknowledged certain limitations of the approach, including that it requires approximately 20 minutes per imaging session, there is a steep learning curve for interpreting images, and certain anatomical sites are challenging to image, especially the nose, periocular area, and lip.
The study won a “best of session” emerging technologies abstract award from the ASLMS.
Milind Rajadhyaksha, PhD, of Memorial Sloan Kettering Cancer Center helped to develop the prototype device. Dr. Aleisa reported having no relevant financial disclosures.
and distinguishing SCC in-situ and actinic keratosis (AK) from invasive SCC, results from a small prospective study demonstrated.
“A solitary scaly papule or plaque could represent an inflammatory or neoplastic process, and when neoplastic, it could be benign, premalignant, malignant in situ, or invasive malignant,” lead study author Abdullah Aleisa, MD, said in an interview during the annual conference of the American Society for Laser Medicine and Surgery. Noninvasive imaging devices, such as reflectance confocal microscopy (RCM) and optical coherence tomography (OCT), “have been used to help in the diagnosis of those clinically suspicious lesions, however each device has its own limitation.”
RCM images are horizontal sections of the skin with high cellular resolution but limited to 250 mcm of depth in skin, he said, while OCT images are vertical sections of the skin with low cellular resolution, but image up to 1,000-2,000 mcm of depth in skin.
“Combined RCM-OCT enables high cellular resolution and deep tissue evaluation,” said Dr. Aleisa, a micrographic surgery and dermatologic oncology fellow at Memorial Sloan Kettering Cancer Center, New York. “The value of combined RCM-OCT has been shown in the detection and depth assessment of basal cell carcinoma, but it has never been studied in SCC. Our objective is to combine RCM and OCT simultaneously to detect SCC and assess the depth of invasion.”
Between September and December 2020, Dr. Aleisa and colleagues prospectively imaged 36 lesions suspicious of SCC, SCC in situ, or AK between September 2020 and December 2020. The mean age of the cohort was 68 years and 63% were male. Using a prototype device from Andover, Mass.–based Caliber I.D., the investigators performed handheld RCM-OCT imaging at the center of clinically suspected lesions before biopsy and to previously diagnosed lesions before Mohs micrographic surgery (to check for residual tumor) and correlated RCM-OCT findings with histopathology results. A total of 36 lesions were treated.
Dr. Aleisa reported that most common RCM-OCT feature for invasive SCC was presence of vertical blood vessels (in 89% of lesions), while for SCC in situ/AK, it was acanthosis and parakeratosis without vertical blood vessels (in 84% of lesions). For the detection of invasive SCC, RCM-OCT had a sensitivity of 82%, a specificity of 92%, a negative predictive value of 92%, and a positive predictive value of 82%. For the detection of SCC in situ/AK, RCM-OCT had a sensitivity of 86%, a specificity of 100%, a negative predictive value of 92%, and a positive predictive value of 100%. The OCT depth measurement correlated well with histopathology with a concordance correlation coefficient of r2 = 0.9.
“Using RCM’s high-resolution pictures allowed us to easily spot the vertical ‘buttonhole’ vessels associated with SCC,” Dr. Aleisa said. “However, given the depth limitation of RCM, the distinction between SCC in situ and invasive SCC could not be accomplished using RCM alone. Therefore, having simultaneous OCT live feedback to the RCM images in the combined RCM-OCT device enabled us to assess the depth of those vertical ‘buttonholes’ and distinguish between SCC in situ and invasive SCC.”
He acknowledged certain limitations of the approach, including that it requires approximately 20 minutes per imaging session, there is a steep learning curve for interpreting images, and certain anatomical sites are challenging to image, especially the nose, periocular area, and lip.
The study won a “best of session” emerging technologies abstract award from the ASLMS.
Milind Rajadhyaksha, PhD, of Memorial Sloan Kettering Cancer Center helped to develop the prototype device. Dr. Aleisa reported having no relevant financial disclosures.
and distinguishing SCC in-situ and actinic keratosis (AK) from invasive SCC, results from a small prospective study demonstrated.
“A solitary scaly papule or plaque could represent an inflammatory or neoplastic process, and when neoplastic, it could be benign, premalignant, malignant in situ, or invasive malignant,” lead study author Abdullah Aleisa, MD, said in an interview during the annual conference of the American Society for Laser Medicine and Surgery. Noninvasive imaging devices, such as reflectance confocal microscopy (RCM) and optical coherence tomography (OCT), “have been used to help in the diagnosis of those clinically suspicious lesions, however each device has its own limitation.”
RCM images are horizontal sections of the skin with high cellular resolution but limited to 250 mcm of depth in skin, he said, while OCT images are vertical sections of the skin with low cellular resolution, but image up to 1,000-2,000 mcm of depth in skin.
“Combined RCM-OCT enables high cellular resolution and deep tissue evaluation,” said Dr. Aleisa, a micrographic surgery and dermatologic oncology fellow at Memorial Sloan Kettering Cancer Center, New York. “The value of combined RCM-OCT has been shown in the detection and depth assessment of basal cell carcinoma, but it has never been studied in SCC. Our objective is to combine RCM and OCT simultaneously to detect SCC and assess the depth of invasion.”
Between September and December 2020, Dr. Aleisa and colleagues prospectively imaged 36 lesions suspicious of SCC, SCC in situ, or AK between September 2020 and December 2020. The mean age of the cohort was 68 years and 63% were male. Using a prototype device from Andover, Mass.–based Caliber I.D., the investigators performed handheld RCM-OCT imaging at the center of clinically suspected lesions before biopsy and to previously diagnosed lesions before Mohs micrographic surgery (to check for residual tumor) and correlated RCM-OCT findings with histopathology results. A total of 36 lesions were treated.
Dr. Aleisa reported that most common RCM-OCT feature for invasive SCC was presence of vertical blood vessels (in 89% of lesions), while for SCC in situ/AK, it was acanthosis and parakeratosis without vertical blood vessels (in 84% of lesions). For the detection of invasive SCC, RCM-OCT had a sensitivity of 82%, a specificity of 92%, a negative predictive value of 92%, and a positive predictive value of 82%. For the detection of SCC in situ/AK, RCM-OCT had a sensitivity of 86%, a specificity of 100%, a negative predictive value of 92%, and a positive predictive value of 100%. The OCT depth measurement correlated well with histopathology with a concordance correlation coefficient of r2 = 0.9.
“Using RCM’s high-resolution pictures allowed us to easily spot the vertical ‘buttonhole’ vessels associated with SCC,” Dr. Aleisa said. “However, given the depth limitation of RCM, the distinction between SCC in situ and invasive SCC could not be accomplished using RCM alone. Therefore, having simultaneous OCT live feedback to the RCM images in the combined RCM-OCT device enabled us to assess the depth of those vertical ‘buttonholes’ and distinguish between SCC in situ and invasive SCC.”
He acknowledged certain limitations of the approach, including that it requires approximately 20 minutes per imaging session, there is a steep learning curve for interpreting images, and certain anatomical sites are challenging to image, especially the nose, periocular area, and lip.
The study won a “best of session” emerging technologies abstract award from the ASLMS.
Milind Rajadhyaksha, PhD, of Memorial Sloan Kettering Cancer Center helped to develop the prototype device. Dr. Aleisa reported having no relevant financial disclosures.
FROM ASLMS 2021
Systematic review of radiofrequency microneedling studies unveiled
Of the according to results from a new systematic review.
“Most devices for aesthetic purposes induce denaturation and remodeling of collagen, elastin, and other dermal structures through tissue injury and stimulating the body’s wound-healing response,” lead study author Marcus G. Tan, MD, told this news organization during the annual conference of the American Society for Laser Medicine and Surgery. “Radiofrequency microneedling is no exception in this regard. RFMN creates perforations in the skin and delivers radiofrequency-generated thermal energy into the underlying tissue. However, RFMN is unique in that thermal energy is delivered in a fashion that produces a reverse temperature gradient to most ablative lasers.”
When using ablative lasers, which target water as its chromophore through selective photothermolysis, the temperature gradient is highest at the epidermis and papillary dermis, and decreases as it penetrates the deeper structures of the skin. In RFMN, radiofrequency energy is delivered directly to the target depth through the microneedle electrodes, thus creating a temperature gradient that is highest in the deep, target structures and cooler at the superficial structures. “This results in less unwanted epidermal heating and reduces the risk of postinflammatory hyperpigmentation,” explained Dr. Tan, a resident in the division of dermatology at the University of Ottawa.
“Because RFMN is unaffected by skin chromophores, it is essentially a ‘color-blind’ technology and safe for use in patients of all skin phototypes. In comparison to lasers, radiofrequency energy can also be delivered to deeper structures of the skin by increasing the length of microneedle electrodes. Despite these advantages of RFMN, this technology remains utilized less frequently compared to ablative lasers for its skin rejuvenating effects.”
To review high-quality medical literature related to RFMN, Dr. Tan and colleagues searched EMBASE and MEDLINE from inception to May 13, 2020, by using the terms “radiofrequency microneedling,” “fractional radiofrequency,” “radiofrequency needling,” or “radiofrequency percutaneous collagen induction.” They limited the analysis to dermatology-related randomized, split-body, or blinded studies with original data in humans. Of the 42 studies included in the final analysis, there were 14 studies of skin rejuvenation, 7 of acne scars, 6 of acne vulgaris, 5 each of striae and axillary hyperhidrosis, 2 of melasma, and 1 each of rosacea, cellulite, and androgenetic alopecia.
After reviewing the 42 studies, the study authors proposed that a strong recommendation for RFMN be made for skin rejuvenation, acne vulgaris, acne scars, and axillary hyperhidrosis, and a weak recommendation for the technology to be used for papulopustular rosacea, striae, and male-pattern androgenetic alopecia when used in conjunction with topical 5% minoxidil. There was insufficient evidence to make recommendations for its use in cellulite and melasma.
One finding that Dr. Tan described as “interesting” was the observation that RFMN was superior to Er:YAG fractional ablative lasers for treatment of rhytides on the lower face (i.e., the nasolabial, perioral, jawline and neck regions). “Secondly, we observed that one session of RFMN was able to achieve 37% efficacy of a surgical face-lift, but without any adverse effects,” Dr. Tan said. “Two-thirds of the patients who received surgical face-lift developed hypertrophic scarring requiring further scar management, compared to none of the patients receiving RFMN.”
Based on their review, Dr. Tan and colleagues recommend that RFMN be offered as one of the therapeutic options for patients seeking treatment for skin rejuvenation, acne vulgaris, acne scars, and axillary hyperhidrosis. “It is usually tolerable with just topical anesthesia applied 30-60 minutes before treatment, and its side effects are transient and usually resolve after 5 days,” he said. “Patients should be counseled that the benefits of RFMN may have a slower onset, compared to other treatments, but it is progressive, durable, and can be used repeatedly and safely in all skin types including darker-skin phenotypes with minimal risk of adverse events.”
One of the abstract section chairs, Fernanda H. Sakamoto, MD, PhD, said that RFMN devices have become increasingly popular in recent years. “The paper presented by Tan et al. is very relevant, as it compares clinical indications, parameters, and results in search for evidence of efficacy and appropriate settings,” said Dr. Sakamoto, a dermatologist at the Wellman Center for Photomedicine at Massachusetts General Hospital, Boston, told this news organization. “The paper provides long-needed guidelines to clinicians and helps manage patients’ expectations.”
Dr. Tan acknowledged certain limitations of the study, including the lack of head-to-head studies comparing specific RFMN devices. “There are many RFMN devices available commercially, each with different capabilities and degrees of effectiveness,” he said. “With more research and technological advancements since the first radiofrequency device was approved in 2002, RFMN has made significant improvements. In general, the newer generation devices produce markedly better results.”
Dr. Tan reported having no financial disclosures. Dr. Sakamoto disclosed that she holds intellectual property rights with Accure Acne, Massachusetts General Hospital, and Lightwater Biosciences.
Of the according to results from a new systematic review.
“Most devices for aesthetic purposes induce denaturation and remodeling of collagen, elastin, and other dermal structures through tissue injury and stimulating the body’s wound-healing response,” lead study author Marcus G. Tan, MD, told this news organization during the annual conference of the American Society for Laser Medicine and Surgery. “Radiofrequency microneedling is no exception in this regard. RFMN creates perforations in the skin and delivers radiofrequency-generated thermal energy into the underlying tissue. However, RFMN is unique in that thermal energy is delivered in a fashion that produces a reverse temperature gradient to most ablative lasers.”
When using ablative lasers, which target water as its chromophore through selective photothermolysis, the temperature gradient is highest at the epidermis and papillary dermis, and decreases as it penetrates the deeper structures of the skin. In RFMN, radiofrequency energy is delivered directly to the target depth through the microneedle electrodes, thus creating a temperature gradient that is highest in the deep, target structures and cooler at the superficial structures. “This results in less unwanted epidermal heating and reduces the risk of postinflammatory hyperpigmentation,” explained Dr. Tan, a resident in the division of dermatology at the University of Ottawa.
“Because RFMN is unaffected by skin chromophores, it is essentially a ‘color-blind’ technology and safe for use in patients of all skin phototypes. In comparison to lasers, radiofrequency energy can also be delivered to deeper structures of the skin by increasing the length of microneedle electrodes. Despite these advantages of RFMN, this technology remains utilized less frequently compared to ablative lasers for its skin rejuvenating effects.”
To review high-quality medical literature related to RFMN, Dr. Tan and colleagues searched EMBASE and MEDLINE from inception to May 13, 2020, by using the terms “radiofrequency microneedling,” “fractional radiofrequency,” “radiofrequency needling,” or “radiofrequency percutaneous collagen induction.” They limited the analysis to dermatology-related randomized, split-body, or blinded studies with original data in humans. Of the 42 studies included in the final analysis, there were 14 studies of skin rejuvenation, 7 of acne scars, 6 of acne vulgaris, 5 each of striae and axillary hyperhidrosis, 2 of melasma, and 1 each of rosacea, cellulite, and androgenetic alopecia.
After reviewing the 42 studies, the study authors proposed that a strong recommendation for RFMN be made for skin rejuvenation, acne vulgaris, acne scars, and axillary hyperhidrosis, and a weak recommendation for the technology to be used for papulopustular rosacea, striae, and male-pattern androgenetic alopecia when used in conjunction with topical 5% minoxidil. There was insufficient evidence to make recommendations for its use in cellulite and melasma.
One finding that Dr. Tan described as “interesting” was the observation that RFMN was superior to Er:YAG fractional ablative lasers for treatment of rhytides on the lower face (i.e., the nasolabial, perioral, jawline and neck regions). “Secondly, we observed that one session of RFMN was able to achieve 37% efficacy of a surgical face-lift, but without any adverse effects,” Dr. Tan said. “Two-thirds of the patients who received surgical face-lift developed hypertrophic scarring requiring further scar management, compared to none of the patients receiving RFMN.”
Based on their review, Dr. Tan and colleagues recommend that RFMN be offered as one of the therapeutic options for patients seeking treatment for skin rejuvenation, acne vulgaris, acne scars, and axillary hyperhidrosis. “It is usually tolerable with just topical anesthesia applied 30-60 minutes before treatment, and its side effects are transient and usually resolve after 5 days,” he said. “Patients should be counseled that the benefits of RFMN may have a slower onset, compared to other treatments, but it is progressive, durable, and can be used repeatedly and safely in all skin types including darker-skin phenotypes with minimal risk of adverse events.”
One of the abstract section chairs, Fernanda H. Sakamoto, MD, PhD, said that RFMN devices have become increasingly popular in recent years. “The paper presented by Tan et al. is very relevant, as it compares clinical indications, parameters, and results in search for evidence of efficacy and appropriate settings,” said Dr. Sakamoto, a dermatologist at the Wellman Center for Photomedicine at Massachusetts General Hospital, Boston, told this news organization. “The paper provides long-needed guidelines to clinicians and helps manage patients’ expectations.”
Dr. Tan acknowledged certain limitations of the study, including the lack of head-to-head studies comparing specific RFMN devices. “There are many RFMN devices available commercially, each with different capabilities and degrees of effectiveness,” he said. “With more research and technological advancements since the first radiofrequency device was approved in 2002, RFMN has made significant improvements. In general, the newer generation devices produce markedly better results.”
Dr. Tan reported having no financial disclosures. Dr. Sakamoto disclosed that she holds intellectual property rights with Accure Acne, Massachusetts General Hospital, and Lightwater Biosciences.
Of the according to results from a new systematic review.
“Most devices for aesthetic purposes induce denaturation and remodeling of collagen, elastin, and other dermal structures through tissue injury and stimulating the body’s wound-healing response,” lead study author Marcus G. Tan, MD, told this news organization during the annual conference of the American Society for Laser Medicine and Surgery. “Radiofrequency microneedling is no exception in this regard. RFMN creates perforations in the skin and delivers radiofrequency-generated thermal energy into the underlying tissue. However, RFMN is unique in that thermal energy is delivered in a fashion that produces a reverse temperature gradient to most ablative lasers.”
When using ablative lasers, which target water as its chromophore through selective photothermolysis, the temperature gradient is highest at the epidermis and papillary dermis, and decreases as it penetrates the deeper structures of the skin. In RFMN, radiofrequency energy is delivered directly to the target depth through the microneedle electrodes, thus creating a temperature gradient that is highest in the deep, target structures and cooler at the superficial structures. “This results in less unwanted epidermal heating and reduces the risk of postinflammatory hyperpigmentation,” explained Dr. Tan, a resident in the division of dermatology at the University of Ottawa.
“Because RFMN is unaffected by skin chromophores, it is essentially a ‘color-blind’ technology and safe for use in patients of all skin phototypes. In comparison to lasers, radiofrequency energy can also be delivered to deeper structures of the skin by increasing the length of microneedle electrodes. Despite these advantages of RFMN, this technology remains utilized less frequently compared to ablative lasers for its skin rejuvenating effects.”
To review high-quality medical literature related to RFMN, Dr. Tan and colleagues searched EMBASE and MEDLINE from inception to May 13, 2020, by using the terms “radiofrequency microneedling,” “fractional radiofrequency,” “radiofrequency needling,” or “radiofrequency percutaneous collagen induction.” They limited the analysis to dermatology-related randomized, split-body, or blinded studies with original data in humans. Of the 42 studies included in the final analysis, there were 14 studies of skin rejuvenation, 7 of acne scars, 6 of acne vulgaris, 5 each of striae and axillary hyperhidrosis, 2 of melasma, and 1 each of rosacea, cellulite, and androgenetic alopecia.
After reviewing the 42 studies, the study authors proposed that a strong recommendation for RFMN be made for skin rejuvenation, acne vulgaris, acne scars, and axillary hyperhidrosis, and a weak recommendation for the technology to be used for papulopustular rosacea, striae, and male-pattern androgenetic alopecia when used in conjunction with topical 5% minoxidil. There was insufficient evidence to make recommendations for its use in cellulite and melasma.
One finding that Dr. Tan described as “interesting” was the observation that RFMN was superior to Er:YAG fractional ablative lasers for treatment of rhytides on the lower face (i.e., the nasolabial, perioral, jawline and neck regions). “Secondly, we observed that one session of RFMN was able to achieve 37% efficacy of a surgical face-lift, but without any adverse effects,” Dr. Tan said. “Two-thirds of the patients who received surgical face-lift developed hypertrophic scarring requiring further scar management, compared to none of the patients receiving RFMN.”
Based on their review, Dr. Tan and colleagues recommend that RFMN be offered as one of the therapeutic options for patients seeking treatment for skin rejuvenation, acne vulgaris, acne scars, and axillary hyperhidrosis. “It is usually tolerable with just topical anesthesia applied 30-60 minutes before treatment, and its side effects are transient and usually resolve after 5 days,” he said. “Patients should be counseled that the benefits of RFMN may have a slower onset, compared to other treatments, but it is progressive, durable, and can be used repeatedly and safely in all skin types including darker-skin phenotypes with minimal risk of adverse events.”
One of the abstract section chairs, Fernanda H. Sakamoto, MD, PhD, said that RFMN devices have become increasingly popular in recent years. “The paper presented by Tan et al. is very relevant, as it compares clinical indications, parameters, and results in search for evidence of efficacy and appropriate settings,” said Dr. Sakamoto, a dermatologist at the Wellman Center for Photomedicine at Massachusetts General Hospital, Boston, told this news organization. “The paper provides long-needed guidelines to clinicians and helps manage patients’ expectations.”
Dr. Tan acknowledged certain limitations of the study, including the lack of head-to-head studies comparing specific RFMN devices. “There are many RFMN devices available commercially, each with different capabilities and degrees of effectiveness,” he said. “With more research and technological advancements since the first radiofrequency device was approved in 2002, RFMN has made significant improvements. In general, the newer generation devices produce markedly better results.”
Dr. Tan reported having no financial disclosures. Dr. Sakamoto disclosed that she holds intellectual property rights with Accure Acne, Massachusetts General Hospital, and Lightwater Biosciences.
FROM ASLMS 2021
Photobiomodulation reduced acute radiodermatitis severity in head and neck cancer patients
The delivery of , according to results from the first randomized study of its kind.
“The use of light therapy-based applications for cancer therapy-related adverse events has steadily increased in the past 40 years,” lead study author Jolien Robijns, MSc, PhD, told this news organization during the annual conference of the American Society for Laser Medicine and Surgery. “The most well-known and studied indication of photobiomodulation therapy in supportive cancer care is oral mucositis,” she said, referring to a recent systematic review, which found that based on the available evidence, PBMT is an effective therapy for the prevention of oral mucositis, using well-defined PBM parameters in specific patient populations. “Various internationally well-recognized health organizations in oncology recommend PBMT to prevent and manage oral mucositis,” she added.
Based on the wound-healing and anti-inflammatory properties of PBMT, several studies have investigated its use for the prevention and management of acute radiodermatitis (ARD) since the 1990s, said Dr. Robijns, a postdoctoral researcher at Limburg Clinical Research Center in Hasselt, Belgium. Under the supervision of Jeroen Mebis, MD, PhD, at the Limburg Oncologic Laser Institute, she and her colleagues have been conducting clinical research on PBMT and ARD since 2014, with successful results. In 2020 they published a narrative review, which showed that based on nine clinical trials, PBMT could effectively reduce the incidence of severe ARD, decrease accompanying pain, and improve patients’ quality of life.
For the current study, known as the DERMISHEAD trial and published online March 9, 2021, in Radiotherapy and Oncology, investigators at Limburg Oncology Center at Jessa Hospital in Hasselt, and Hasselt University, recruited head and neck cancer patients who underwent bilateral radiotherapy with or without chemotherapy, for a total dose of 30-35 x 2 Gy . All patients received standard skin care combined with two PBMT or sham sessions twice per week during the complete course of RT, which resulted in 14 total sessions.
As described in the Radiotherapy and Oncology study, the commercially available device used for PBMT “consists of two laser diodes with different wavelengths (808-905 nm), peak powers (1.1-25 W), and emission modes (continuous and pulsed). Both diodes work simultaneously and synchronously with coincident propagation axes (average radiant power 3.3 W). The energy density (fluence) was set at 4 J/cm2 based on earlier recommendations and on our clinical experience.” A blinded study nurse used Radiation Therapy Oncology Group criteria to evaluate the skin reactions.
After 303 patients were initially assessed for eligibility, 46 patients were enrolled in DERMISHEAD (18 in the placebo group and 28 in the PBMT group). At the end of radiotherapy, 77.8% of patients in the placebo group had a grade 2 or 3 skin reaction, compared with 28.6% of patients in the PBMT group (P = .001).
“The DERMISHEAD trial proved that PBMT significantly reduces the severity of ARD,” Dr. Robijns said. “Thereby, it improves the patients’ quality of life during their radiotherapy course. The trial supports the further implementation of PBM in the supportive care of cancer patients undergoing radiotherapy.”
The results are similar to those in the TRANSDERMIS trial, in which Dr. Robijns and her colleagues used PMBT to treat breast cancer patients.
“However, an interesting difference is that the percentage decrease in severe ARD was higher in the DERMISHEAD trial than in the TRANSDERMIS trial: 49% vs. 23%, respectively,” she noted. “This difference can be rationalized because in total, more control head and neck cancer patients developed grade 3 ARD than did control breast cancer patients (17% vs. 5%). A possible explanation of this finding can be related to the difference in treatment regimens and radiotherapy parameters between the two trials.”
Christine Ko, MD, professor of dermatology and pathology at Yale University, New Haven, Conn., who was asked to comment on the study, said that acute radiation dermatitis “can be very painful and distressing to patients, and over time, the skin changes can create long-term problems. Prevention of acute and chronic radiation dermatitis is worthwhile, particularly for patients at risk.”
This study, she added, “shows a benefit of photobiomodulation therapy as a potential preventative treatment. Notably, patients did not always follow up appropriately for the therapy, and the authors said that it is yet another thing that patients need to keep track of, in addition to their cancer therapy visits. Thus, optimally, it would be useful to have a biomarker of which patients would most benefit from treatments that prevent/potentiate radiation dermatitis.”
Dr. Robijns acknowledged certain limitations of the trial, including its small sample size and the scarcity of clinical trials on PBM and acute radiation dermatitis. “More studies are needed,” she said. “Future studies should focus on randomized controlled study designs with well-described and complete PBMT parameters in a larger and more diverse patient population. This would enable the implementation of PBM in the field of ARD and supportive cancer care, which would enhance wound care management and improve the patient’s quality of life.”
This work won a “best of clinical applications” abstract award from the ASLMS.
The research is part of the Limburg Clinical Research Center UHasselt-ZOL-Jessa, financially supported by the foundation Limburg Sterk Merk, province of Limburg, Flemish Government, Hasselt University, Ziekenhuis Oost-Limburg, and Jessa Hospital. The research is also funded by Kom op tegen Kanker (Stand up to Cancer), the Flemish Cancer Society, Limburgs Kankerfonds, and ASA Srl. Dr. Robijns reported having no financial disclosures.
The delivery of , according to results from the first randomized study of its kind.
“The use of light therapy-based applications for cancer therapy-related adverse events has steadily increased in the past 40 years,” lead study author Jolien Robijns, MSc, PhD, told this news organization during the annual conference of the American Society for Laser Medicine and Surgery. “The most well-known and studied indication of photobiomodulation therapy in supportive cancer care is oral mucositis,” she said, referring to a recent systematic review, which found that based on the available evidence, PBMT is an effective therapy for the prevention of oral mucositis, using well-defined PBM parameters in specific patient populations. “Various internationally well-recognized health organizations in oncology recommend PBMT to prevent and manage oral mucositis,” she added.
Based on the wound-healing and anti-inflammatory properties of PBMT, several studies have investigated its use for the prevention and management of acute radiodermatitis (ARD) since the 1990s, said Dr. Robijns, a postdoctoral researcher at Limburg Clinical Research Center in Hasselt, Belgium. Under the supervision of Jeroen Mebis, MD, PhD, at the Limburg Oncologic Laser Institute, she and her colleagues have been conducting clinical research on PBMT and ARD since 2014, with successful results. In 2020 they published a narrative review, which showed that based on nine clinical trials, PBMT could effectively reduce the incidence of severe ARD, decrease accompanying pain, and improve patients’ quality of life.
For the current study, known as the DERMISHEAD trial and published online March 9, 2021, in Radiotherapy and Oncology, investigators at Limburg Oncology Center at Jessa Hospital in Hasselt, and Hasselt University, recruited head and neck cancer patients who underwent bilateral radiotherapy with or without chemotherapy, for a total dose of 30-35 x 2 Gy . All patients received standard skin care combined with two PBMT or sham sessions twice per week during the complete course of RT, which resulted in 14 total sessions.
As described in the Radiotherapy and Oncology study, the commercially available device used for PBMT “consists of two laser diodes with different wavelengths (808-905 nm), peak powers (1.1-25 W), and emission modes (continuous and pulsed). Both diodes work simultaneously and synchronously with coincident propagation axes (average radiant power 3.3 W). The energy density (fluence) was set at 4 J/cm2 based on earlier recommendations and on our clinical experience.” A blinded study nurse used Radiation Therapy Oncology Group criteria to evaluate the skin reactions.
After 303 patients were initially assessed for eligibility, 46 patients were enrolled in DERMISHEAD (18 in the placebo group and 28 in the PBMT group). At the end of radiotherapy, 77.8% of patients in the placebo group had a grade 2 or 3 skin reaction, compared with 28.6% of patients in the PBMT group (P = .001).
“The DERMISHEAD trial proved that PBMT significantly reduces the severity of ARD,” Dr. Robijns said. “Thereby, it improves the patients’ quality of life during their radiotherapy course. The trial supports the further implementation of PBM in the supportive care of cancer patients undergoing radiotherapy.”
The results are similar to those in the TRANSDERMIS trial, in which Dr. Robijns and her colleagues used PMBT to treat breast cancer patients.
“However, an interesting difference is that the percentage decrease in severe ARD was higher in the DERMISHEAD trial than in the TRANSDERMIS trial: 49% vs. 23%, respectively,” she noted. “This difference can be rationalized because in total, more control head and neck cancer patients developed grade 3 ARD than did control breast cancer patients (17% vs. 5%). A possible explanation of this finding can be related to the difference in treatment regimens and radiotherapy parameters between the two trials.”
Christine Ko, MD, professor of dermatology and pathology at Yale University, New Haven, Conn., who was asked to comment on the study, said that acute radiation dermatitis “can be very painful and distressing to patients, and over time, the skin changes can create long-term problems. Prevention of acute and chronic radiation dermatitis is worthwhile, particularly for patients at risk.”
This study, she added, “shows a benefit of photobiomodulation therapy as a potential preventative treatment. Notably, patients did not always follow up appropriately for the therapy, and the authors said that it is yet another thing that patients need to keep track of, in addition to their cancer therapy visits. Thus, optimally, it would be useful to have a biomarker of which patients would most benefit from treatments that prevent/potentiate radiation dermatitis.”
Dr. Robijns acknowledged certain limitations of the trial, including its small sample size and the scarcity of clinical trials on PBM and acute radiation dermatitis. “More studies are needed,” she said. “Future studies should focus on randomized controlled study designs with well-described and complete PBMT parameters in a larger and more diverse patient population. This would enable the implementation of PBM in the field of ARD and supportive cancer care, which would enhance wound care management and improve the patient’s quality of life.”
This work won a “best of clinical applications” abstract award from the ASLMS.
The research is part of the Limburg Clinical Research Center UHasselt-ZOL-Jessa, financially supported by the foundation Limburg Sterk Merk, province of Limburg, Flemish Government, Hasselt University, Ziekenhuis Oost-Limburg, and Jessa Hospital. The research is also funded by Kom op tegen Kanker (Stand up to Cancer), the Flemish Cancer Society, Limburgs Kankerfonds, and ASA Srl. Dr. Robijns reported having no financial disclosures.
The delivery of , according to results from the first randomized study of its kind.
“The use of light therapy-based applications for cancer therapy-related adverse events has steadily increased in the past 40 years,” lead study author Jolien Robijns, MSc, PhD, told this news organization during the annual conference of the American Society for Laser Medicine and Surgery. “The most well-known and studied indication of photobiomodulation therapy in supportive cancer care is oral mucositis,” she said, referring to a recent systematic review, which found that based on the available evidence, PBMT is an effective therapy for the prevention of oral mucositis, using well-defined PBM parameters in specific patient populations. “Various internationally well-recognized health organizations in oncology recommend PBMT to prevent and manage oral mucositis,” she added.
Based on the wound-healing and anti-inflammatory properties of PBMT, several studies have investigated its use for the prevention and management of acute radiodermatitis (ARD) since the 1990s, said Dr. Robijns, a postdoctoral researcher at Limburg Clinical Research Center in Hasselt, Belgium. Under the supervision of Jeroen Mebis, MD, PhD, at the Limburg Oncologic Laser Institute, she and her colleagues have been conducting clinical research on PBMT and ARD since 2014, with successful results. In 2020 they published a narrative review, which showed that based on nine clinical trials, PBMT could effectively reduce the incidence of severe ARD, decrease accompanying pain, and improve patients’ quality of life.
For the current study, known as the DERMISHEAD trial and published online March 9, 2021, in Radiotherapy and Oncology, investigators at Limburg Oncology Center at Jessa Hospital in Hasselt, and Hasselt University, recruited head and neck cancer patients who underwent bilateral radiotherapy with or without chemotherapy, for a total dose of 30-35 x 2 Gy . All patients received standard skin care combined with two PBMT or sham sessions twice per week during the complete course of RT, which resulted in 14 total sessions.
As described in the Radiotherapy and Oncology study, the commercially available device used for PBMT “consists of two laser diodes with different wavelengths (808-905 nm), peak powers (1.1-25 W), and emission modes (continuous and pulsed). Both diodes work simultaneously and synchronously with coincident propagation axes (average radiant power 3.3 W). The energy density (fluence) was set at 4 J/cm2 based on earlier recommendations and on our clinical experience.” A blinded study nurse used Radiation Therapy Oncology Group criteria to evaluate the skin reactions.
After 303 patients were initially assessed for eligibility, 46 patients were enrolled in DERMISHEAD (18 in the placebo group and 28 in the PBMT group). At the end of radiotherapy, 77.8% of patients in the placebo group had a grade 2 or 3 skin reaction, compared with 28.6% of patients in the PBMT group (P = .001).
“The DERMISHEAD trial proved that PBMT significantly reduces the severity of ARD,” Dr. Robijns said. “Thereby, it improves the patients’ quality of life during their radiotherapy course. The trial supports the further implementation of PBM in the supportive care of cancer patients undergoing radiotherapy.”
The results are similar to those in the TRANSDERMIS trial, in which Dr. Robijns and her colleagues used PMBT to treat breast cancer patients.
“However, an interesting difference is that the percentage decrease in severe ARD was higher in the DERMISHEAD trial than in the TRANSDERMIS trial: 49% vs. 23%, respectively,” she noted. “This difference can be rationalized because in total, more control head and neck cancer patients developed grade 3 ARD than did control breast cancer patients (17% vs. 5%). A possible explanation of this finding can be related to the difference in treatment regimens and radiotherapy parameters between the two trials.”
Christine Ko, MD, professor of dermatology and pathology at Yale University, New Haven, Conn., who was asked to comment on the study, said that acute radiation dermatitis “can be very painful and distressing to patients, and over time, the skin changes can create long-term problems. Prevention of acute and chronic radiation dermatitis is worthwhile, particularly for patients at risk.”
This study, she added, “shows a benefit of photobiomodulation therapy as a potential preventative treatment. Notably, patients did not always follow up appropriately for the therapy, and the authors said that it is yet another thing that patients need to keep track of, in addition to their cancer therapy visits. Thus, optimally, it would be useful to have a biomarker of which patients would most benefit from treatments that prevent/potentiate radiation dermatitis.”
Dr. Robijns acknowledged certain limitations of the trial, including its small sample size and the scarcity of clinical trials on PBM and acute radiation dermatitis. “More studies are needed,” she said. “Future studies should focus on randomized controlled study designs with well-described and complete PBMT parameters in a larger and more diverse patient population. This would enable the implementation of PBM in the field of ARD and supportive cancer care, which would enhance wound care management and improve the patient’s quality of life.”
This work won a “best of clinical applications” abstract award from the ASLMS.
The research is part of the Limburg Clinical Research Center UHasselt-ZOL-Jessa, financially supported by the foundation Limburg Sterk Merk, province of Limburg, Flemish Government, Hasselt University, Ziekenhuis Oost-Limburg, and Jessa Hospital. The research is also funded by Kom op tegen Kanker (Stand up to Cancer), the Flemish Cancer Society, Limburgs Kankerfonds, and ASA Srl. Dr. Robijns reported having no financial disclosures.
FROM ASLMS 2021
Botulinum toxin and depression
. But confounding factors, such as medications, injection/acupuncture effect, physician interaction or touch, or other life scenarios, have made it difficult to discern botulinum toxin type A’s true effect on mood or psychiatric diagnosis. Now a systematic review and meta-analysis of randomized controlled trials examining botulinum toxin versus placebo provides evidence that botulinum toxin type A (BTX-A) injections are associated with statistically significant improvement in depressive symptoms.
Qian et al. analyzed all randomized controlled trials that investigated the efficacy and safety of facial BTX-A injections on patients with a diagnosis of major depressive disorder in PubMed and Web of Science from inception to June 17, 2020. A meta-analysis of the changes in depressive symptoms 6 weeks after BTX-A injections compared with placebo were the primary outcome of the report, while the safety of injections were also assessed.
A total of 417 patients from five randomized controlled trials (189 patients who received BTX-A injections and 228 in the placebo group) were deemed eligible. There was a statistically significant improvement in depressive symptoms in the BTX-A injections compared with placebo (Hedges’ g, –0.82; 95% confidence interval, –1.38 to 0.27). BTX-A injections were well tolerated with mild and temporary adverse events (headache, eyelid ptosis, and upper respiratory tract infection) reported in three of the five studies.
Limitations to the analysis include publication bias due to the limited number of studies in the analysis, the difficulty of being able to reliably blind participants because of potential noticeable cosmetic effects of BTX-A treatment, and the heterogeneity of symptom severity associated with major depressive disorder.
The authors referred to the Global Burden of Disease Study, which estimated that approximately 216 million people experienced major depressive disorder in 2015, the latest data available. MDD symptoms of sadness, fatigue, and loss of interest or pleasure, “incur a tremendous burden on health and finances,” they wrote. According to the Department of Health and Human Services, it is estimated that about 60% of people who commit suicide have had a mood disorder (major depression, bipolar disorder, dysthymia). The high rate of suicide associated with severe depression is also a serious public health concern. While further analysis is clearly warranted, cosmetic BTX-A injections may provide an alternative option in the treatment of depression.
Dr. Wesley and Dr. Lily Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. Write to them at dermnews@mdedge.com. They had no relevant disclosures.
. But confounding factors, such as medications, injection/acupuncture effect, physician interaction or touch, or other life scenarios, have made it difficult to discern botulinum toxin type A’s true effect on mood or psychiatric diagnosis. Now a systematic review and meta-analysis of randomized controlled trials examining botulinum toxin versus placebo provides evidence that botulinum toxin type A (BTX-A) injections are associated with statistically significant improvement in depressive symptoms.
Qian et al. analyzed all randomized controlled trials that investigated the efficacy and safety of facial BTX-A injections on patients with a diagnosis of major depressive disorder in PubMed and Web of Science from inception to June 17, 2020. A meta-analysis of the changes in depressive symptoms 6 weeks after BTX-A injections compared with placebo were the primary outcome of the report, while the safety of injections were also assessed.
A total of 417 patients from five randomized controlled trials (189 patients who received BTX-A injections and 228 in the placebo group) were deemed eligible. There was a statistically significant improvement in depressive symptoms in the BTX-A injections compared with placebo (Hedges’ g, –0.82; 95% confidence interval, –1.38 to 0.27). BTX-A injections were well tolerated with mild and temporary adverse events (headache, eyelid ptosis, and upper respiratory tract infection) reported in three of the five studies.
Limitations to the analysis include publication bias due to the limited number of studies in the analysis, the difficulty of being able to reliably blind participants because of potential noticeable cosmetic effects of BTX-A treatment, and the heterogeneity of symptom severity associated with major depressive disorder.
The authors referred to the Global Burden of Disease Study, which estimated that approximately 216 million people experienced major depressive disorder in 2015, the latest data available. MDD symptoms of sadness, fatigue, and loss of interest or pleasure, “incur a tremendous burden on health and finances,” they wrote. According to the Department of Health and Human Services, it is estimated that about 60% of people who commit suicide have had a mood disorder (major depression, bipolar disorder, dysthymia). The high rate of suicide associated with severe depression is also a serious public health concern. While further analysis is clearly warranted, cosmetic BTX-A injections may provide an alternative option in the treatment of depression.
Dr. Wesley and Dr. Lily Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. Write to them at dermnews@mdedge.com. They had no relevant disclosures.
. But confounding factors, such as medications, injection/acupuncture effect, physician interaction or touch, or other life scenarios, have made it difficult to discern botulinum toxin type A’s true effect on mood or psychiatric diagnosis. Now a systematic review and meta-analysis of randomized controlled trials examining botulinum toxin versus placebo provides evidence that botulinum toxin type A (BTX-A) injections are associated with statistically significant improvement in depressive symptoms.
Qian et al. analyzed all randomized controlled trials that investigated the efficacy and safety of facial BTX-A injections on patients with a diagnosis of major depressive disorder in PubMed and Web of Science from inception to June 17, 2020. A meta-analysis of the changes in depressive symptoms 6 weeks after BTX-A injections compared with placebo were the primary outcome of the report, while the safety of injections were also assessed.
A total of 417 patients from five randomized controlled trials (189 patients who received BTX-A injections and 228 in the placebo group) were deemed eligible. There was a statistically significant improvement in depressive symptoms in the BTX-A injections compared with placebo (Hedges’ g, –0.82; 95% confidence interval, –1.38 to 0.27). BTX-A injections were well tolerated with mild and temporary adverse events (headache, eyelid ptosis, and upper respiratory tract infection) reported in three of the five studies.
Limitations to the analysis include publication bias due to the limited number of studies in the analysis, the difficulty of being able to reliably blind participants because of potential noticeable cosmetic effects of BTX-A treatment, and the heterogeneity of symptom severity associated with major depressive disorder.
The authors referred to the Global Burden of Disease Study, which estimated that approximately 216 million people experienced major depressive disorder in 2015, the latest data available. MDD symptoms of sadness, fatigue, and loss of interest or pleasure, “incur a tremendous burden on health and finances,” they wrote. According to the Department of Health and Human Services, it is estimated that about 60% of people who commit suicide have had a mood disorder (major depression, bipolar disorder, dysthymia). The high rate of suicide associated with severe depression is also a serious public health concern. While further analysis is clearly warranted, cosmetic BTX-A injections may provide an alternative option in the treatment of depression.
Dr. Wesley and Dr. Lily Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. Write to them at dermnews@mdedge.com. They had no relevant disclosures.
New guidance for those fully vaccinated against COVID-19
As has been dominating the headlines, the Centers for Disease Control and Prevention recently released updated public health guidance for those who are fully vaccinated against COVID-19.
This new guidance applies to those who are fully vaccinated as indicated by 2 weeks after the second dose in a 2-dose series or 2 weeks after a single-dose vaccine. Those who meet these criteria no longer need to wear a mask or physically distance themselves from others in both indoor and outdoor settings. For those not fully vaccinated, masking and social distancing should continue to be practiced.
The new guidance indicates that quarantine after a known exposure is no longer necessary.
Unless required by local, state, or territorial health authorities, testing is no longer required following domestic travel for fully vaccinated individuals. A negative test is still required prior to boarding an international flight to the United States and testing 3-5 days after arrival is still recommended. Self-quarantine is no longer required after international travel for fully vaccinated individuals.
The new guidance recommends that individuals who are fully vaccinated not participate in routine screening programs when feasible. Finally, if an individual has tested positive for COVID-19, regardless of vaccination status, that person should isolate and not visit public or private settings for a minimum of ten days.1
Updated guidance for health care facilities
In addition to changes for the general public in all settings, the CDC updated guidance for health care facilities on April 27, 2021. These updated guidelines allow for communal dining and visitation for fully vaccinated patients and their visitors. The guidelines indicate that fully vaccinated health care personnel (HCP) do not require quarantine after exposure to patients who have tested positive for COVID-19 as long as the HCP remains asymptomatic. They should, however, continue to utilize personal protective equipment as previously recommended. HCPs are able to be in break and meeting rooms unmasked if all HCPs are vaccinated.2
There are some important caveats to these updated guidelines. They do not apply to those who have immunocompromising conditions, including those using immunosuppressant agents. They also do not apply to locations subject to federal, state, local, tribal, or territorial laws, rules, and regulations, including local business and workplace guidance.
Those who work or reside in correction or detention facilities and homeless shelters are also still required to test after known exposures. Masking is still required by all travelers on all forms of public transportation into and within the United States.
Most importantly, the guidelines apply only to those who are fully vaccinated. Finally, no vaccine is perfect. As such, anyone who experiences symptoms indicative of COVID-19, regardless of vaccination status, should obtain viral testing and isolate themselves from others.1,2
Pros and cons to new guidance
Both sets of updated guidelines are a great example of public health guidance that is changing as the evidence is gathered and changes. This guidance is also a welcome encouragement that the vaccines are effective at decreasing transmission of this virus that has upended our world.
These guidelines leave room for change as evidence is gathered on emerging novel variants. There are, however, a few remaining concerns.
My first concern is for those who are not yet able to be vaccinated, including children under the age of 12. For families with members who are not fully vaccinated, they may have first heard the headlines of “you do not have to mask” to then read the fine print that remains. When truly following these guidelines, many social situations in both the public and private setting should still include both masking and social distancing.
There is no clarity on how these guidelines are enforced. Within the guidance, it is clear that individuals’ privacy is of utmost importance. In the absence of knowledge, that means that the assumption should be that all are not yet vaccinated. Unless there is a way to reliably demonstrate vaccination status, it would likely still be safer to assume that there are individuals who are not fully vaccinated within the setting.
Finally, although this is great news surrounding the efficacy of the vaccine, some are concerned that local mask mandates that have already started to be lifted will be completely removed. As there is still a large portion of the population not yet fully vaccinated, it seems premature for local, state, and territorial authorities to lift these mandates.
How to continue exercising caution
With the outstanding concerns, I will continue to mask in settings, particularly indoors, where I do not definitely know that everyone is vaccinated. I will continue to do this to protect my children and my patients who are not yet vaccinated, and my patients who are immunosuppressed for whom we do not yet have enough information.
I will continue to advise my patients to be thoughtful about the risk for themselves and their families as well.
There has been more benefit to these public health measures then just decreased transmission of COVID-19. I hope that this year has reinforced within us the benefits of masking and self-isolation in the cases of any contagious illnesses.
Although I am looking forward to the opportunities to interact in person with more colleagues and friends, I think we should continue to do this with caution and thoughtfulness. We must be prepared for the possibility of vaccines having decreased efficacy against novel variants as well as eventually the possibility of waning immunity. If these should occur, we need to be prepared for additional recommendation changes and tightening of restrictions.
Dr. Wheat is a family physician at Erie Family Health Center in Chicago. She is program director of Northwestern’s McGaw Family Medicine residency program at Humboldt Park, Chicago. Dr. Wheat serves on the editorial advisory board of Family Practice News. You can contact her at fpnews@mdedge.com.
References
1. Centers for Disease Control and Prevention. Interim Public Health Recommendations for Fully Vaccinated People. U.S. Department of Health & Human Services, May 13, 2021.
2. Centers for Disease Control and Prevention. Updated Healthcare Infection Prevention and Control Recommendations in Response to COVID-19 Vaccination. U.S. Department of Health and Human Services, April 27, 2021.
As has been dominating the headlines, the Centers for Disease Control and Prevention recently released updated public health guidance for those who are fully vaccinated against COVID-19.
This new guidance applies to those who are fully vaccinated as indicated by 2 weeks after the second dose in a 2-dose series or 2 weeks after a single-dose vaccine. Those who meet these criteria no longer need to wear a mask or physically distance themselves from others in both indoor and outdoor settings. For those not fully vaccinated, masking and social distancing should continue to be practiced.
The new guidance indicates that quarantine after a known exposure is no longer necessary.
Unless required by local, state, or territorial health authorities, testing is no longer required following domestic travel for fully vaccinated individuals. A negative test is still required prior to boarding an international flight to the United States and testing 3-5 days after arrival is still recommended. Self-quarantine is no longer required after international travel for fully vaccinated individuals.
The new guidance recommends that individuals who are fully vaccinated not participate in routine screening programs when feasible. Finally, if an individual has tested positive for COVID-19, regardless of vaccination status, that person should isolate and not visit public or private settings for a minimum of ten days.1
Updated guidance for health care facilities
In addition to changes for the general public in all settings, the CDC updated guidance for health care facilities on April 27, 2021. These updated guidelines allow for communal dining and visitation for fully vaccinated patients and their visitors. The guidelines indicate that fully vaccinated health care personnel (HCP) do not require quarantine after exposure to patients who have tested positive for COVID-19 as long as the HCP remains asymptomatic. They should, however, continue to utilize personal protective equipment as previously recommended. HCPs are able to be in break and meeting rooms unmasked if all HCPs are vaccinated.2
There are some important caveats to these updated guidelines. They do not apply to those who have immunocompromising conditions, including those using immunosuppressant agents. They also do not apply to locations subject to federal, state, local, tribal, or territorial laws, rules, and regulations, including local business and workplace guidance.
Those who work or reside in correction or detention facilities and homeless shelters are also still required to test after known exposures. Masking is still required by all travelers on all forms of public transportation into and within the United States.
Most importantly, the guidelines apply only to those who are fully vaccinated. Finally, no vaccine is perfect. As such, anyone who experiences symptoms indicative of COVID-19, regardless of vaccination status, should obtain viral testing and isolate themselves from others.1,2
Pros and cons to new guidance
Both sets of updated guidelines are a great example of public health guidance that is changing as the evidence is gathered and changes. This guidance is also a welcome encouragement that the vaccines are effective at decreasing transmission of this virus that has upended our world.
These guidelines leave room for change as evidence is gathered on emerging novel variants. There are, however, a few remaining concerns.
My first concern is for those who are not yet able to be vaccinated, including children under the age of 12. For families with members who are not fully vaccinated, they may have first heard the headlines of “you do not have to mask” to then read the fine print that remains. When truly following these guidelines, many social situations in both the public and private setting should still include both masking and social distancing.
There is no clarity on how these guidelines are enforced. Within the guidance, it is clear that individuals’ privacy is of utmost importance. In the absence of knowledge, that means that the assumption should be that all are not yet vaccinated. Unless there is a way to reliably demonstrate vaccination status, it would likely still be safer to assume that there are individuals who are not fully vaccinated within the setting.
Finally, although this is great news surrounding the efficacy of the vaccine, some are concerned that local mask mandates that have already started to be lifted will be completely removed. As there is still a large portion of the population not yet fully vaccinated, it seems premature for local, state, and territorial authorities to lift these mandates.
How to continue exercising caution
With the outstanding concerns, I will continue to mask in settings, particularly indoors, where I do not definitely know that everyone is vaccinated. I will continue to do this to protect my children and my patients who are not yet vaccinated, and my patients who are immunosuppressed for whom we do not yet have enough information.
I will continue to advise my patients to be thoughtful about the risk for themselves and their families as well.
There has been more benefit to these public health measures then just decreased transmission of COVID-19. I hope that this year has reinforced within us the benefits of masking and self-isolation in the cases of any contagious illnesses.
Although I am looking forward to the opportunities to interact in person with more colleagues and friends, I think we should continue to do this with caution and thoughtfulness. We must be prepared for the possibility of vaccines having decreased efficacy against novel variants as well as eventually the possibility of waning immunity. If these should occur, we need to be prepared for additional recommendation changes and tightening of restrictions.
Dr. Wheat is a family physician at Erie Family Health Center in Chicago. She is program director of Northwestern’s McGaw Family Medicine residency program at Humboldt Park, Chicago. Dr. Wheat serves on the editorial advisory board of Family Practice News. You can contact her at fpnews@mdedge.com.
References
1. Centers for Disease Control and Prevention. Interim Public Health Recommendations for Fully Vaccinated People. U.S. Department of Health & Human Services, May 13, 2021.
2. Centers for Disease Control and Prevention. Updated Healthcare Infection Prevention and Control Recommendations in Response to COVID-19 Vaccination. U.S. Department of Health and Human Services, April 27, 2021.
As has been dominating the headlines, the Centers for Disease Control and Prevention recently released updated public health guidance for those who are fully vaccinated against COVID-19.
This new guidance applies to those who are fully vaccinated as indicated by 2 weeks after the second dose in a 2-dose series or 2 weeks after a single-dose vaccine. Those who meet these criteria no longer need to wear a mask or physically distance themselves from others in both indoor and outdoor settings. For those not fully vaccinated, masking and social distancing should continue to be practiced.
The new guidance indicates that quarantine after a known exposure is no longer necessary.
Unless required by local, state, or territorial health authorities, testing is no longer required following domestic travel for fully vaccinated individuals. A negative test is still required prior to boarding an international flight to the United States and testing 3-5 days after arrival is still recommended. Self-quarantine is no longer required after international travel for fully vaccinated individuals.
The new guidance recommends that individuals who are fully vaccinated not participate in routine screening programs when feasible. Finally, if an individual has tested positive for COVID-19, regardless of vaccination status, that person should isolate and not visit public or private settings for a minimum of ten days.1
Updated guidance for health care facilities
In addition to changes for the general public in all settings, the CDC updated guidance for health care facilities on April 27, 2021. These updated guidelines allow for communal dining and visitation for fully vaccinated patients and their visitors. The guidelines indicate that fully vaccinated health care personnel (HCP) do not require quarantine after exposure to patients who have tested positive for COVID-19 as long as the HCP remains asymptomatic. They should, however, continue to utilize personal protective equipment as previously recommended. HCPs are able to be in break and meeting rooms unmasked if all HCPs are vaccinated.2
There are some important caveats to these updated guidelines. They do not apply to those who have immunocompromising conditions, including those using immunosuppressant agents. They also do not apply to locations subject to federal, state, local, tribal, or territorial laws, rules, and regulations, including local business and workplace guidance.
Those who work or reside in correction or detention facilities and homeless shelters are also still required to test after known exposures. Masking is still required by all travelers on all forms of public transportation into and within the United States.
Most importantly, the guidelines apply only to those who are fully vaccinated. Finally, no vaccine is perfect. As such, anyone who experiences symptoms indicative of COVID-19, regardless of vaccination status, should obtain viral testing and isolate themselves from others.1,2
Pros and cons to new guidance
Both sets of updated guidelines are a great example of public health guidance that is changing as the evidence is gathered and changes. This guidance is also a welcome encouragement that the vaccines are effective at decreasing transmission of this virus that has upended our world.
These guidelines leave room for change as evidence is gathered on emerging novel variants. There are, however, a few remaining concerns.
My first concern is for those who are not yet able to be vaccinated, including children under the age of 12. For families with members who are not fully vaccinated, they may have first heard the headlines of “you do not have to mask” to then read the fine print that remains. When truly following these guidelines, many social situations in both the public and private setting should still include both masking and social distancing.
There is no clarity on how these guidelines are enforced. Within the guidance, it is clear that individuals’ privacy is of utmost importance. In the absence of knowledge, that means that the assumption should be that all are not yet vaccinated. Unless there is a way to reliably demonstrate vaccination status, it would likely still be safer to assume that there are individuals who are not fully vaccinated within the setting.
Finally, although this is great news surrounding the efficacy of the vaccine, some are concerned that local mask mandates that have already started to be lifted will be completely removed. As there is still a large portion of the population not yet fully vaccinated, it seems premature for local, state, and territorial authorities to lift these mandates.
How to continue exercising caution
With the outstanding concerns, I will continue to mask in settings, particularly indoors, where I do not definitely know that everyone is vaccinated. I will continue to do this to protect my children and my patients who are not yet vaccinated, and my patients who are immunosuppressed for whom we do not yet have enough information.
I will continue to advise my patients to be thoughtful about the risk for themselves and their families as well.
There has been more benefit to these public health measures then just decreased transmission of COVID-19. I hope that this year has reinforced within us the benefits of masking and self-isolation in the cases of any contagious illnesses.
Although I am looking forward to the opportunities to interact in person with more colleagues and friends, I think we should continue to do this with caution and thoughtfulness. We must be prepared for the possibility of vaccines having decreased efficacy against novel variants as well as eventually the possibility of waning immunity. If these should occur, we need to be prepared for additional recommendation changes and tightening of restrictions.
Dr. Wheat is a family physician at Erie Family Health Center in Chicago. She is program director of Northwestern’s McGaw Family Medicine residency program at Humboldt Park, Chicago. Dr. Wheat serves on the editorial advisory board of Family Practice News. You can contact her at fpnews@mdedge.com.
References
1. Centers for Disease Control and Prevention. Interim Public Health Recommendations for Fully Vaccinated People. U.S. Department of Health & Human Services, May 13, 2021.
2. Centers for Disease Control and Prevention. Updated Healthcare Infection Prevention and Control Recommendations in Response to COVID-19 Vaccination. U.S. Department of Health and Human Services, April 27, 2021.

















