User login
Formerly Skin & Allergy News
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')]
The leading independent newspaper covering dermatology news and commentary.
Use your court awareness to go faster in practice
Have you ever had a nightmare you’re running late? Recently I dreamt I was seeing patients on a ship, a little cruiser like the ones that give you tours of Boston Harbor, with low ceilings and narrow iron stairs. My nurse stood where what would have been the coffee and danish window. My first patient was a newborn (this was a nightmare, in case you forgot) who was enormous. She had a big belly and spindly legs that hung off the table. Uniform, umbilicated papules and pustules covered her body. At the sight of her, terror ripped through me – no clue. I rushed to the doctor lounge (nice the ship had one) and flipped channels on a little TV mounted on the ceiling. Suddenly, my nurse burst in, she was frantic because dozens of angry adults and crying children were crammed in the hallway. Apparently, I had been watching TV for hours and my whole clinic was now backed up.
Running-late dreams are common and usually relate to real life. For us, the clinic has been busy lately. Vaccinated patients are returning after a year with their skin cancers that have flourished and psoriasis covering them like kudzu. In particular, they “see the floor” better than other docs and therefore make continual adjustments to stay on pace. At its essence, they are using super-powers of observation to make decisions. It reminded me of a podcast about court awareness and great passers in basketball like the Charlotte Hornets’ LaMelo Ball and NBA great, Bill Bradley.
Bradley had an extraordinary ability to know where all the players were, and where they would be, at any given moment. He spent years honing this skill, noticing details in store windows as he stared straight ahead walking down a street. It’s reported his peripheral vision extended 5%-15% wider than average and he used it to gather more information and to process it more quickly. As a result he made outstanding decisions and fast, ultimately earning a spot in the Hall of Fame in Springfield.
Hall of Fame clinicians similarly take in a wider view than others and process that information quickly. They know how much time they have spent in the room, sense the emotional needs of the patient and anticipate the complexity of the problem. They quickly get to the critical questions and examinations that will make the diagnosis. They know the experience and skill of their medical assistant. They know the level of difficulty and even the temperament of patients who lie ahead on the schedule. All this is processed and used in moment-to-moment decision making. Do I sit down or stand up now? Can I excise this today, or reschedule? Do I ask another question? Do I step out of this room and see another in parallel while this biopsy is set up? And always, do I dare ask about grandkids or do I politely move on?
By broadening out their vision, they optimize their clinic, providing the best possible service, whether the day is busy or slow. I found their economy of motion also means they are less exhausted at the end of the day. I bet if when they dream of being on a ship, they’re sipping a Mai Tai, lounging on the deck.
For more on Bill Bradley and becoming more observant about your surroundings, you might appreciate the following:
www.newyorker.com/magazine/1965/01/23/a-sense-of-where-you-are and freakonomics.com/podcast/nsq-mindfulness/
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at dermnews@mdedge.com.
Have you ever had a nightmare you’re running late? Recently I dreamt I was seeing patients on a ship, a little cruiser like the ones that give you tours of Boston Harbor, with low ceilings and narrow iron stairs. My nurse stood where what would have been the coffee and danish window. My first patient was a newborn (this was a nightmare, in case you forgot) who was enormous. She had a big belly and spindly legs that hung off the table. Uniform, umbilicated papules and pustules covered her body. At the sight of her, terror ripped through me – no clue. I rushed to the doctor lounge (nice the ship had one) and flipped channels on a little TV mounted on the ceiling. Suddenly, my nurse burst in, she was frantic because dozens of angry adults and crying children were crammed in the hallway. Apparently, I had been watching TV for hours and my whole clinic was now backed up.
Running-late dreams are common and usually relate to real life. For us, the clinic has been busy lately. Vaccinated patients are returning after a year with their skin cancers that have flourished and psoriasis covering them like kudzu. In particular, they “see the floor” better than other docs and therefore make continual adjustments to stay on pace. At its essence, they are using super-powers of observation to make decisions. It reminded me of a podcast about court awareness and great passers in basketball like the Charlotte Hornets’ LaMelo Ball and NBA great, Bill Bradley.
Bradley had an extraordinary ability to know where all the players were, and where they would be, at any given moment. He spent years honing this skill, noticing details in store windows as he stared straight ahead walking down a street. It’s reported his peripheral vision extended 5%-15% wider than average and he used it to gather more information and to process it more quickly. As a result he made outstanding decisions and fast, ultimately earning a spot in the Hall of Fame in Springfield.
Hall of Fame clinicians similarly take in a wider view than others and process that information quickly. They know how much time they have spent in the room, sense the emotional needs of the patient and anticipate the complexity of the problem. They quickly get to the critical questions and examinations that will make the diagnosis. They know the experience and skill of their medical assistant. They know the level of difficulty and even the temperament of patients who lie ahead on the schedule. All this is processed and used in moment-to-moment decision making. Do I sit down or stand up now? Can I excise this today, or reschedule? Do I ask another question? Do I step out of this room and see another in parallel while this biopsy is set up? And always, do I dare ask about grandkids or do I politely move on?
By broadening out their vision, they optimize their clinic, providing the best possible service, whether the day is busy or slow. I found their economy of motion also means they are less exhausted at the end of the day. I bet if when they dream of being on a ship, they’re sipping a Mai Tai, lounging on the deck.
For more on Bill Bradley and becoming more observant about your surroundings, you might appreciate the following:
www.newyorker.com/magazine/1965/01/23/a-sense-of-where-you-are and freakonomics.com/podcast/nsq-mindfulness/
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at dermnews@mdedge.com.
Have you ever had a nightmare you’re running late? Recently I dreamt I was seeing patients on a ship, a little cruiser like the ones that give you tours of Boston Harbor, with low ceilings and narrow iron stairs. My nurse stood where what would have been the coffee and danish window. My first patient was a newborn (this was a nightmare, in case you forgot) who was enormous. She had a big belly and spindly legs that hung off the table. Uniform, umbilicated papules and pustules covered her body. At the sight of her, terror ripped through me – no clue. I rushed to the doctor lounge (nice the ship had one) and flipped channels on a little TV mounted on the ceiling. Suddenly, my nurse burst in, she was frantic because dozens of angry adults and crying children were crammed in the hallway. Apparently, I had been watching TV for hours and my whole clinic was now backed up.
Running-late dreams are common and usually relate to real life. For us, the clinic has been busy lately. Vaccinated patients are returning after a year with their skin cancers that have flourished and psoriasis covering them like kudzu. In particular, they “see the floor” better than other docs and therefore make continual adjustments to stay on pace. At its essence, they are using super-powers of observation to make decisions. It reminded me of a podcast about court awareness and great passers in basketball like the Charlotte Hornets’ LaMelo Ball and NBA great, Bill Bradley.
Bradley had an extraordinary ability to know where all the players were, and where they would be, at any given moment. He spent years honing this skill, noticing details in store windows as he stared straight ahead walking down a street. It’s reported his peripheral vision extended 5%-15% wider than average and he used it to gather more information and to process it more quickly. As a result he made outstanding decisions and fast, ultimately earning a spot in the Hall of Fame in Springfield.
Hall of Fame clinicians similarly take in a wider view than others and process that information quickly. They know how much time they have spent in the room, sense the emotional needs of the patient and anticipate the complexity of the problem. They quickly get to the critical questions and examinations that will make the diagnosis. They know the experience and skill of their medical assistant. They know the level of difficulty and even the temperament of patients who lie ahead on the schedule. All this is processed and used in moment-to-moment decision making. Do I sit down or stand up now? Can I excise this today, or reschedule? Do I ask another question? Do I step out of this room and see another in parallel while this biopsy is set up? And always, do I dare ask about grandkids or do I politely move on?
By broadening out their vision, they optimize their clinic, providing the best possible service, whether the day is busy or slow. I found their economy of motion also means they are less exhausted at the end of the day. I bet if when they dream of being on a ship, they’re sipping a Mai Tai, lounging on the deck.
For more on Bill Bradley and becoming more observant about your surroundings, you might appreciate the following:
www.newyorker.com/magazine/1965/01/23/a-sense-of-where-you-are and freakonomics.com/podcast/nsq-mindfulness/
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at dermnews@mdedge.com.
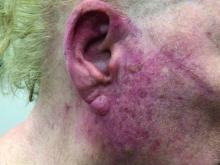
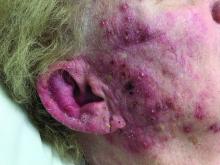
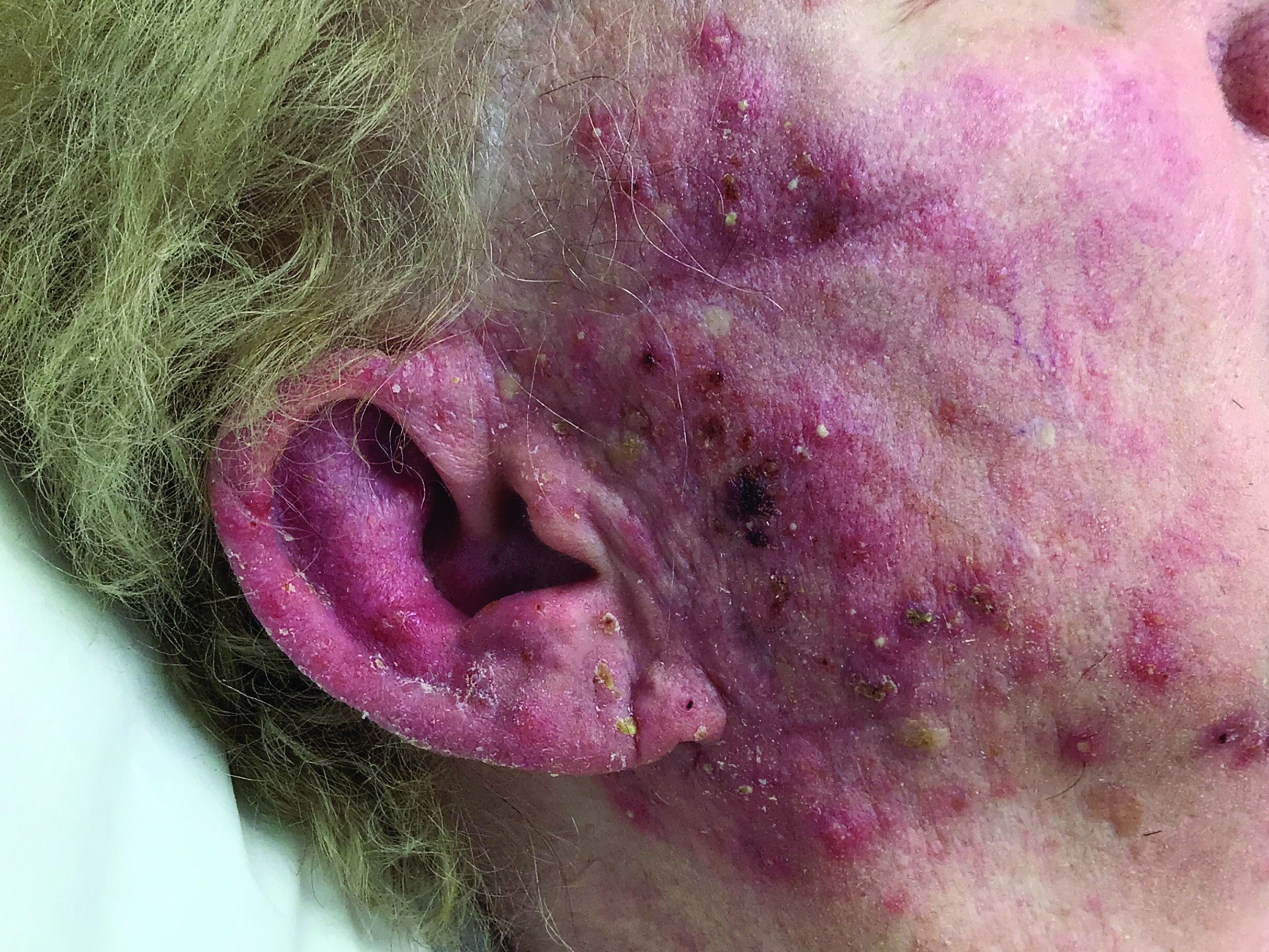
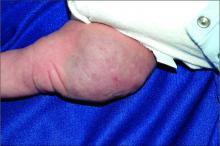
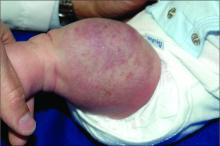
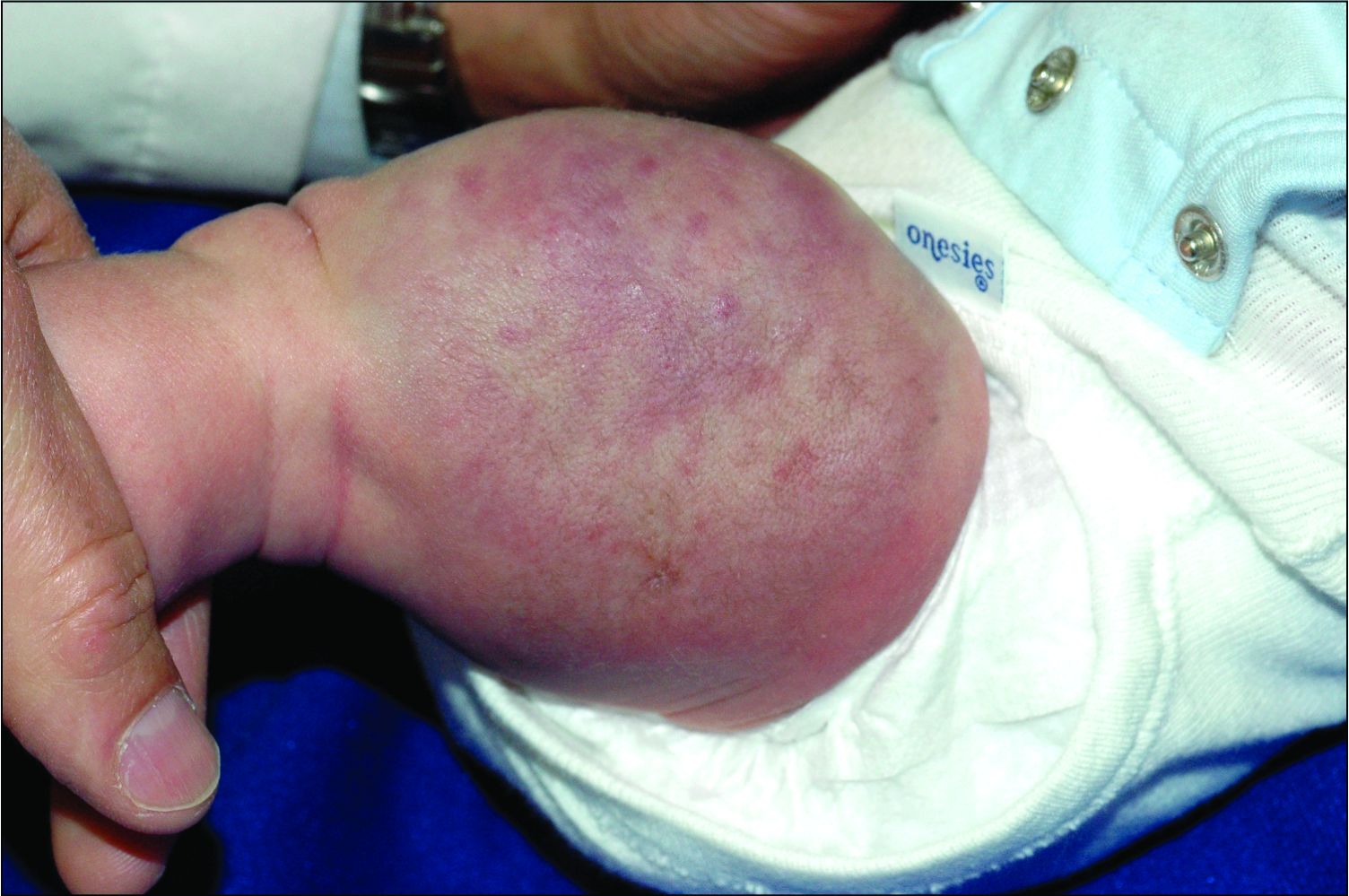
A woman with scaling, and painful, crusted, erythematous papules and pustules on her face
Biopsy for this patient revealed folliculitis with Demodex mites visualized on histology. Direct immunofluorescence was negative. A KOH preparation was performed and was positive for large numbers of Demodex. Bacterial cultures were negative. The patient was started on a course of submicrobial doxycycline and ivermectin and showed marked improvement 1 month following treatment.
Demodex folliculorum and Demodex brevis (collectively referred to as Demodex) are microscopic parasitic mites that commonly live on human skin.1 Typically, the mite remains asymptomatic. However, in higher numbers, the infestation may cause dermatoses, called demodicosis. Lesions often present as itchy papules, pustules, and erythematous scaling on the face, ears, and scalp. Blepharitis may be present. Demodex folliculitis is more common in immunocompromised patients.2
Demodex may have a causative role in rosacea and present similarly, with a key difference being that Demodex-type rosacea is more scaly/dry and pustular than common rosacea.1 In Demodex folliculitis, bacterial cultures are often negative. A skin scraping for KOH will reveal increased mite colonization. The Demodex mite may also be seen in histologic slides.
Treatment of Demodex folliculitis includes crotamiton cream, permethrin cream, oral tetracyclines, topical or systemic metronidazole, and topical or oral ivermectin.
This case and photos were submitted by Susannah McClain, MD, Three Rivers Dermatology, Pittsburgh.
References
1. Rather PA and Hassan I. Indian J Dermatol. 2014 Jan;59(1):60-6.
2. Bachmeyer C and Moreno-Sabater A. CMAJ. 2017 Jun 26;189(25):E865.
Biopsy for this patient revealed folliculitis with Demodex mites visualized on histology. Direct immunofluorescence was negative. A KOH preparation was performed and was positive for large numbers of Demodex. Bacterial cultures were negative. The patient was started on a course of submicrobial doxycycline and ivermectin and showed marked improvement 1 month following treatment.
Demodex folliculorum and Demodex brevis (collectively referred to as Demodex) are microscopic parasitic mites that commonly live on human skin.1 Typically, the mite remains asymptomatic. However, in higher numbers, the infestation may cause dermatoses, called demodicosis. Lesions often present as itchy papules, pustules, and erythematous scaling on the face, ears, and scalp. Blepharitis may be present. Demodex folliculitis is more common in immunocompromised patients.2
Demodex may have a causative role in rosacea and present similarly, with a key difference being that Demodex-type rosacea is more scaly/dry and pustular than common rosacea.1 In Demodex folliculitis, bacterial cultures are often negative. A skin scraping for KOH will reveal increased mite colonization. The Demodex mite may also be seen in histologic slides.
Treatment of Demodex folliculitis includes crotamiton cream, permethrin cream, oral tetracyclines, topical or systemic metronidazole, and topical or oral ivermectin.
This case and photos were submitted by Susannah McClain, MD, Three Rivers Dermatology, Pittsburgh.
References
1. Rather PA and Hassan I. Indian J Dermatol. 2014 Jan;59(1):60-6.
2. Bachmeyer C and Moreno-Sabater A. CMAJ. 2017 Jun 26;189(25):E865.
Biopsy for this patient revealed folliculitis with Demodex mites visualized on histology. Direct immunofluorescence was negative. A KOH preparation was performed and was positive for large numbers of Demodex. Bacterial cultures were negative. The patient was started on a course of submicrobial doxycycline and ivermectin and showed marked improvement 1 month following treatment.
Demodex folliculorum and Demodex brevis (collectively referred to as Demodex) are microscopic parasitic mites that commonly live on human skin.1 Typically, the mite remains asymptomatic. However, in higher numbers, the infestation may cause dermatoses, called demodicosis. Lesions often present as itchy papules, pustules, and erythematous scaling on the face, ears, and scalp. Blepharitis may be present. Demodex folliculitis is more common in immunocompromised patients.2
Demodex may have a causative role in rosacea and present similarly, with a key difference being that Demodex-type rosacea is more scaly/dry and pustular than common rosacea.1 In Demodex folliculitis, bacterial cultures are often negative. A skin scraping for KOH will reveal increased mite colonization. The Demodex mite may also be seen in histologic slides.
Treatment of Demodex folliculitis includes crotamiton cream, permethrin cream, oral tetracyclines, topical or systemic metronidazole, and topical or oral ivermectin.
This case and photos were submitted by Susannah McClain, MD, Three Rivers Dermatology, Pittsburgh.
References
1. Rather PA and Hassan I. Indian J Dermatol. 2014 Jan;59(1):60-6.
2. Bachmeyer C and Moreno-Sabater A. CMAJ. 2017 Jun 26;189(25):E865.
Dr. Fauci: Extraordinary challenges, scientific triumphs with COVID-19
“Vaccines have been the bright light of this extraordinary challenge that we’ve gone through,” said Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases.
In an address for the opening ceremony of the American Thoracic Society’s virtual international conference, Dr. Fauci emphasized the role of basic and clinical research and government support for science in helping turn the tide of the COVID-19 pandemic.
“A few weeks ago, I wrote an editorial in Science, because there was some misunderstanding about how and why we were able to go from a realization of a new pathogen in January of 2020, to getting doses of vaccines in the arms of individuals – a highly efficacious vaccine – 11 months later. Truly, an unprecedented accomplishment,” he said.
“But as I said in the editorial, the speed and efficiency with which these highly efficacious vaccines were developed, and their potential for saving millions of lives, are due to an extraordinary multidisciplinary effort, involving basic, preclinical, and clinical science that had been underway – out of the spotlight – for decades and decades before the unfolding of the COVID-19 pandemic, a fact that very few people really appreciate: namely, the importance of investment in biomedical research.”
The general addresses the troops
Perhaps no other audience is so well suited to receive Dr. Fauci’s speech as those who are currently attending (virtually) the ATS conference, including researchers who scrutinize the virus from every angle to describe its workings and identify its vulnerabilities, epidemiologists who study viral transmission and look for ways to thwart it, public health workers who fan out to communities across the country to push vaccine acceptance, and clinicians who specialize in critical care and pulmonary medicine, many of whom staff the respiratory floors and intensive care units where the most severely ill patients are treated.
Speaking about the lessons learned and challenges remaining from the COVID-19 pandemic, Dr. Fauci briefly reviewed the epidemiology, virology and transmission, diagnostics, and clinical course of SARS-CoV-2 infections and the therapeutics and vaccines for COVID-19.
Epidemiology
The pandemic began in December 2019 with recognition of a novel type of pneumonia in the Wuhan District of Central China, Dr. Fauci noted.
“Very quickly thereafter, in the first week of January 2020, the Chinese identified a new strain of coronavirus as [the] source of the outbreak. Fast forward to where we are right now: We have experienced and are experiencing the most devastating pandemic of a respiratory illness in the last 102 years, with already approximately 160 million individuals having been infected – and this is clearly a gross undercounting – and also 3.3 million deaths, again, very likely an undercounting,” he said.
According to the Centers for Disease Control and Prevention, as of May 9, 2021, there were approximately 32.5 million cases of COVID-19 and 578,520 deaths in the United States. Those cases and deaths occurred largely in three surges in the United States, in early spring, early summer, and late fall of 2020.
Virology and transmission
SARS-CoV-2 is a beta-coronavirus in the same subgenus as SARS-CoV-1 and some bat coronaviruses, Dr. Fauci explained. The viral genome is large, about 30,000 kilobases, and it has four structural proteins, most importantly the S or “spike” protein that allows the virus to attach to and fuse with cell membranes by binding to the ACE2 receptor on tissues in the upper and lower respiratory tract, gastrointestinal tract, cardiovascular system, and other organ systems.
The virus is transmitted mainly through exposure to respiratory droplets within 6 feet of an infected person, or sometimes through droplets or particles that remain in the air over time and various distances.
Contact with contaminated surfaces, once feared as a means of transmission, is now understood to be less common.
The virus has been detected in stool, blood, semen, and ocular secretions, although the role of transmission through these sources is still unknown.
“Some very interesting characteristics of this virus, really quite unique compared to other viruses, certainly other respiratory viruses, is [that] about a third to 40% of people who are infected never develop any symptoms,” Dr. Fauci said. “Importantly, and very problematic to what we do to contain it – particularly with regard to identification, isolation, and contract tracing – between 50% and 60% of the transmissions occur either from someone who will never develop symptoms, or someone in the presymptomatic phase of disease.”
The fundamentals of preventing acquisition and transmission are as familiar to most Americans now as the Pledge of Allegiance: universal mask wearing, physical distancing, avoiding crowds and congregate settings, preference for outdoor over indoor settings, and frequent hand washing, he noted.
Diagnostics
Tests for SARS-CoV-2 infection fall into three basic categories: molecular tests such as polymerase chain reaction (PCR) that are highly specific and highly sensitive for actual infections, antigen tests that detect the viral protein rather than the nucleic acids, and antibody tests to detect serum proteins made in response to viral infection.
Antigen testing is used largely for broader surveillance of groups of individuals to detect viral penetrance within that group, Dr. Fauci noted.
Clinical course
The clinical course of COVID-19 has some interesting characteristics but is not substantially different from a flu-like syndrome, Dr. Fauci said.
Symptoms and signs common to both types of infections include fever, cough, fatigue, anorexia, dyspnea, and myalgias, but the loss of smell and/or taste preceding the onset of respiratory symptoms is a unique feature of COVID-19.
Dr. Fauci cited data on more than 44,000 individuals with confirmed COVID-19 in China that showed that a large majority (81%) of cases were mild or moderate in nature, but 14% of patients experienced severe disease, and 5% were critically ill. The case-fatality rate in this study was 2.3%.
People at increased risk for severe disease include older adults and those of any age with certain comorbidities.
Manifestations of severe COVID-19 infections in adults can include neurological disorders, hyperinflammation, acute respiratory distress syndrome, cardiac dysfunction, hypercoagulability, and acute kidney injury.
In children, COVID-19 has been associated with a multisystem inflammatory syndrome (MIS-C) similar to Kawasaki disease.
In a substantial number of cases, the effects of COVID-19 can linger for 6 months or longer, Dr. Fauci said, pointing to a study from the University of Washington in Seattle.
Investigators there found that approximately 30% of patients enrolled at their center reported persistent symptoms for as long as 9 months after the initial illness, with fatigue as the most commonly reported symptom. One-third of outpatients with mild disease also reported persistent symptoms.
Therapeutics
Therapeutics that are either approved by the Food and Drug Administration, have emergency use authorization, or are in clinical trials for early or moderate disease include remdesivir (Veklury, Gilead Sciences), monoclonal antibodies, convalescent plasma, antiviral agents, hyperimmune globulin, anticoagulants, and immunomodulators.
Options for moderate to severe to advanced disease include dexamethasone, baricitinib (Olumiant, Eli Lilly and Company) plus remdesivir, and immunomodulators such as infliximab (Remicade, Janssen Biotech), and biosimilars.
Vaccines
Finally, Dr. Fauci reviewed the current state of vaccines, including the three with emergency use authorization from the FDA as of this writing: two nucleic acid, messenger RNA-based (mRNA) vaccines from Moderna and Pfizer/BioNTech, and an adenoviral vector-based vaccine from Johnson & Johnson.
Other vaccines in development or in use elsewhere in the world include recombinant protein and adjuvant approaches by GlaxoSmithKline and Sanofi (in a phase 2 clinical trial launched in February 2021) and by Novavax.
The three vaccines in use in the United States were highly efficacious in both clinical trials, with efficacy of about 95% for the mRNA vaccines and 67% for the Johnson & Johnson vaccine.
The real-world performance of these vaccines has been even more impressive, however.
For example, the Johnson & Johnson vaccine had 72% efficacy at preventing moderate to severe COVID 19 in the United States, 68% in Brazil, and 64% in South Africa, and 85% efficacy against severe disease across all regions studied, Dr. Fauci said.
He cited a study of 22,234 employees of the University of Texas Southwestern Medical Center in Dallas who were vaccinated under a program started on Dec. 15, 2020. The COVID-19 infection rate among these vaccinated employees was 0.05%.
Dr. Fauci recounted the experience in Israel, where the highly transmissible B.1.1.7 strain of SARS-CoV-2 is predominant. A chart of the progress shows clearly that as the vaccine doses delivered steadily increased, the number of COVID-19 cases began a precipitous decline.
Horse race
Fittingly for a speech presented on the day that the Preakness Stakes – the second leg in thoroughbred racing’s Triple Crown – was run, Dr. Fauci closed with a cartoon showing two racehorses, labeled “SARS-CoV-2” and “Vaccines,” nearly neck-and-neck, but with vaccines having a slight lead.
“We are in a race against the virus. The vaccines, and the virus: If we vaccinate the overwhelming proportion of our population, we will without a doubt be able to crush the outbreak in the same way as we have done with other viral-borne diseases like measles, smallpox, and polio.
“So, the message is: Get vaccinated,” he concluded.
“Vaccines have been the bright light of this extraordinary challenge that we’ve gone through,” said Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases.
In an address for the opening ceremony of the American Thoracic Society’s virtual international conference, Dr. Fauci emphasized the role of basic and clinical research and government support for science in helping turn the tide of the COVID-19 pandemic.
“A few weeks ago, I wrote an editorial in Science, because there was some misunderstanding about how and why we were able to go from a realization of a new pathogen in January of 2020, to getting doses of vaccines in the arms of individuals – a highly efficacious vaccine – 11 months later. Truly, an unprecedented accomplishment,” he said.
“But as I said in the editorial, the speed and efficiency with which these highly efficacious vaccines were developed, and their potential for saving millions of lives, are due to an extraordinary multidisciplinary effort, involving basic, preclinical, and clinical science that had been underway – out of the spotlight – for decades and decades before the unfolding of the COVID-19 pandemic, a fact that very few people really appreciate: namely, the importance of investment in biomedical research.”
The general addresses the troops
Perhaps no other audience is so well suited to receive Dr. Fauci’s speech as those who are currently attending (virtually) the ATS conference, including researchers who scrutinize the virus from every angle to describe its workings and identify its vulnerabilities, epidemiologists who study viral transmission and look for ways to thwart it, public health workers who fan out to communities across the country to push vaccine acceptance, and clinicians who specialize in critical care and pulmonary medicine, many of whom staff the respiratory floors and intensive care units where the most severely ill patients are treated.
Speaking about the lessons learned and challenges remaining from the COVID-19 pandemic, Dr. Fauci briefly reviewed the epidemiology, virology and transmission, diagnostics, and clinical course of SARS-CoV-2 infections and the therapeutics and vaccines for COVID-19.
Epidemiology
The pandemic began in December 2019 with recognition of a novel type of pneumonia in the Wuhan District of Central China, Dr. Fauci noted.
“Very quickly thereafter, in the first week of January 2020, the Chinese identified a new strain of coronavirus as [the] source of the outbreak. Fast forward to where we are right now: We have experienced and are experiencing the most devastating pandemic of a respiratory illness in the last 102 years, with already approximately 160 million individuals having been infected – and this is clearly a gross undercounting – and also 3.3 million deaths, again, very likely an undercounting,” he said.
According to the Centers for Disease Control and Prevention, as of May 9, 2021, there were approximately 32.5 million cases of COVID-19 and 578,520 deaths in the United States. Those cases and deaths occurred largely in three surges in the United States, in early spring, early summer, and late fall of 2020.
Virology and transmission
SARS-CoV-2 is a beta-coronavirus in the same subgenus as SARS-CoV-1 and some bat coronaviruses, Dr. Fauci explained. The viral genome is large, about 30,000 kilobases, and it has four structural proteins, most importantly the S or “spike” protein that allows the virus to attach to and fuse with cell membranes by binding to the ACE2 receptor on tissues in the upper and lower respiratory tract, gastrointestinal tract, cardiovascular system, and other organ systems.
The virus is transmitted mainly through exposure to respiratory droplets within 6 feet of an infected person, or sometimes through droplets or particles that remain in the air over time and various distances.
Contact with contaminated surfaces, once feared as a means of transmission, is now understood to be less common.
The virus has been detected in stool, blood, semen, and ocular secretions, although the role of transmission through these sources is still unknown.
“Some very interesting characteristics of this virus, really quite unique compared to other viruses, certainly other respiratory viruses, is [that] about a third to 40% of people who are infected never develop any symptoms,” Dr. Fauci said. “Importantly, and very problematic to what we do to contain it – particularly with regard to identification, isolation, and contract tracing – between 50% and 60% of the transmissions occur either from someone who will never develop symptoms, or someone in the presymptomatic phase of disease.”
The fundamentals of preventing acquisition and transmission are as familiar to most Americans now as the Pledge of Allegiance: universal mask wearing, physical distancing, avoiding crowds and congregate settings, preference for outdoor over indoor settings, and frequent hand washing, he noted.
Diagnostics
Tests for SARS-CoV-2 infection fall into three basic categories: molecular tests such as polymerase chain reaction (PCR) that are highly specific and highly sensitive for actual infections, antigen tests that detect the viral protein rather than the nucleic acids, and antibody tests to detect serum proteins made in response to viral infection.
Antigen testing is used largely for broader surveillance of groups of individuals to detect viral penetrance within that group, Dr. Fauci noted.
Clinical course
The clinical course of COVID-19 has some interesting characteristics but is not substantially different from a flu-like syndrome, Dr. Fauci said.
Symptoms and signs common to both types of infections include fever, cough, fatigue, anorexia, dyspnea, and myalgias, but the loss of smell and/or taste preceding the onset of respiratory symptoms is a unique feature of COVID-19.
Dr. Fauci cited data on more than 44,000 individuals with confirmed COVID-19 in China that showed that a large majority (81%) of cases were mild or moderate in nature, but 14% of patients experienced severe disease, and 5% were critically ill. The case-fatality rate in this study was 2.3%.
People at increased risk for severe disease include older adults and those of any age with certain comorbidities.
Manifestations of severe COVID-19 infections in adults can include neurological disorders, hyperinflammation, acute respiratory distress syndrome, cardiac dysfunction, hypercoagulability, and acute kidney injury.
In children, COVID-19 has been associated with a multisystem inflammatory syndrome (MIS-C) similar to Kawasaki disease.
In a substantial number of cases, the effects of COVID-19 can linger for 6 months or longer, Dr. Fauci said, pointing to a study from the University of Washington in Seattle.
Investigators there found that approximately 30% of patients enrolled at their center reported persistent symptoms for as long as 9 months after the initial illness, with fatigue as the most commonly reported symptom. One-third of outpatients with mild disease also reported persistent symptoms.
Therapeutics
Therapeutics that are either approved by the Food and Drug Administration, have emergency use authorization, or are in clinical trials for early or moderate disease include remdesivir (Veklury, Gilead Sciences), monoclonal antibodies, convalescent plasma, antiviral agents, hyperimmune globulin, anticoagulants, and immunomodulators.
Options for moderate to severe to advanced disease include dexamethasone, baricitinib (Olumiant, Eli Lilly and Company) plus remdesivir, and immunomodulators such as infliximab (Remicade, Janssen Biotech), and biosimilars.
Vaccines
Finally, Dr. Fauci reviewed the current state of vaccines, including the three with emergency use authorization from the FDA as of this writing: two nucleic acid, messenger RNA-based (mRNA) vaccines from Moderna and Pfizer/BioNTech, and an adenoviral vector-based vaccine from Johnson & Johnson.
Other vaccines in development or in use elsewhere in the world include recombinant protein and adjuvant approaches by GlaxoSmithKline and Sanofi (in a phase 2 clinical trial launched in February 2021) and by Novavax.
The three vaccines in use in the United States were highly efficacious in both clinical trials, with efficacy of about 95% for the mRNA vaccines and 67% for the Johnson & Johnson vaccine.
The real-world performance of these vaccines has been even more impressive, however.
For example, the Johnson & Johnson vaccine had 72% efficacy at preventing moderate to severe COVID 19 in the United States, 68% in Brazil, and 64% in South Africa, and 85% efficacy against severe disease across all regions studied, Dr. Fauci said.
He cited a study of 22,234 employees of the University of Texas Southwestern Medical Center in Dallas who were vaccinated under a program started on Dec. 15, 2020. The COVID-19 infection rate among these vaccinated employees was 0.05%.
Dr. Fauci recounted the experience in Israel, where the highly transmissible B.1.1.7 strain of SARS-CoV-2 is predominant. A chart of the progress shows clearly that as the vaccine doses delivered steadily increased, the number of COVID-19 cases began a precipitous decline.
Horse race
Fittingly for a speech presented on the day that the Preakness Stakes – the second leg in thoroughbred racing’s Triple Crown – was run, Dr. Fauci closed with a cartoon showing two racehorses, labeled “SARS-CoV-2” and “Vaccines,” nearly neck-and-neck, but with vaccines having a slight lead.
“We are in a race against the virus. The vaccines, and the virus: If we vaccinate the overwhelming proportion of our population, we will without a doubt be able to crush the outbreak in the same way as we have done with other viral-borne diseases like measles, smallpox, and polio.
“So, the message is: Get vaccinated,” he concluded.
“Vaccines have been the bright light of this extraordinary challenge that we’ve gone through,” said Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases.
In an address for the opening ceremony of the American Thoracic Society’s virtual international conference, Dr. Fauci emphasized the role of basic and clinical research and government support for science in helping turn the tide of the COVID-19 pandemic.
“A few weeks ago, I wrote an editorial in Science, because there was some misunderstanding about how and why we were able to go from a realization of a new pathogen in January of 2020, to getting doses of vaccines in the arms of individuals – a highly efficacious vaccine – 11 months later. Truly, an unprecedented accomplishment,” he said.
“But as I said in the editorial, the speed and efficiency with which these highly efficacious vaccines were developed, and their potential for saving millions of lives, are due to an extraordinary multidisciplinary effort, involving basic, preclinical, and clinical science that had been underway – out of the spotlight – for decades and decades before the unfolding of the COVID-19 pandemic, a fact that very few people really appreciate: namely, the importance of investment in biomedical research.”
The general addresses the troops
Perhaps no other audience is so well suited to receive Dr. Fauci’s speech as those who are currently attending (virtually) the ATS conference, including researchers who scrutinize the virus from every angle to describe its workings and identify its vulnerabilities, epidemiologists who study viral transmission and look for ways to thwart it, public health workers who fan out to communities across the country to push vaccine acceptance, and clinicians who specialize in critical care and pulmonary medicine, many of whom staff the respiratory floors and intensive care units where the most severely ill patients are treated.
Speaking about the lessons learned and challenges remaining from the COVID-19 pandemic, Dr. Fauci briefly reviewed the epidemiology, virology and transmission, diagnostics, and clinical course of SARS-CoV-2 infections and the therapeutics and vaccines for COVID-19.
Epidemiology
The pandemic began in December 2019 with recognition of a novel type of pneumonia in the Wuhan District of Central China, Dr. Fauci noted.
“Very quickly thereafter, in the first week of January 2020, the Chinese identified a new strain of coronavirus as [the] source of the outbreak. Fast forward to where we are right now: We have experienced and are experiencing the most devastating pandemic of a respiratory illness in the last 102 years, with already approximately 160 million individuals having been infected – and this is clearly a gross undercounting – and also 3.3 million deaths, again, very likely an undercounting,” he said.
According to the Centers for Disease Control and Prevention, as of May 9, 2021, there were approximately 32.5 million cases of COVID-19 and 578,520 deaths in the United States. Those cases and deaths occurred largely in three surges in the United States, in early spring, early summer, and late fall of 2020.
Virology and transmission
SARS-CoV-2 is a beta-coronavirus in the same subgenus as SARS-CoV-1 and some bat coronaviruses, Dr. Fauci explained. The viral genome is large, about 30,000 kilobases, and it has four structural proteins, most importantly the S or “spike” protein that allows the virus to attach to and fuse with cell membranes by binding to the ACE2 receptor on tissues in the upper and lower respiratory tract, gastrointestinal tract, cardiovascular system, and other organ systems.
The virus is transmitted mainly through exposure to respiratory droplets within 6 feet of an infected person, or sometimes through droplets or particles that remain in the air over time and various distances.
Contact with contaminated surfaces, once feared as a means of transmission, is now understood to be less common.
The virus has been detected in stool, blood, semen, and ocular secretions, although the role of transmission through these sources is still unknown.
“Some very interesting characteristics of this virus, really quite unique compared to other viruses, certainly other respiratory viruses, is [that] about a third to 40% of people who are infected never develop any symptoms,” Dr. Fauci said. “Importantly, and very problematic to what we do to contain it – particularly with regard to identification, isolation, and contract tracing – between 50% and 60% of the transmissions occur either from someone who will never develop symptoms, or someone in the presymptomatic phase of disease.”
The fundamentals of preventing acquisition and transmission are as familiar to most Americans now as the Pledge of Allegiance: universal mask wearing, physical distancing, avoiding crowds and congregate settings, preference for outdoor over indoor settings, and frequent hand washing, he noted.
Diagnostics
Tests for SARS-CoV-2 infection fall into three basic categories: molecular tests such as polymerase chain reaction (PCR) that are highly specific and highly sensitive for actual infections, antigen tests that detect the viral protein rather than the nucleic acids, and antibody tests to detect serum proteins made in response to viral infection.
Antigen testing is used largely for broader surveillance of groups of individuals to detect viral penetrance within that group, Dr. Fauci noted.
Clinical course
The clinical course of COVID-19 has some interesting characteristics but is not substantially different from a flu-like syndrome, Dr. Fauci said.
Symptoms and signs common to both types of infections include fever, cough, fatigue, anorexia, dyspnea, and myalgias, but the loss of smell and/or taste preceding the onset of respiratory symptoms is a unique feature of COVID-19.
Dr. Fauci cited data on more than 44,000 individuals with confirmed COVID-19 in China that showed that a large majority (81%) of cases were mild or moderate in nature, but 14% of patients experienced severe disease, and 5% were critically ill. The case-fatality rate in this study was 2.3%.
People at increased risk for severe disease include older adults and those of any age with certain comorbidities.
Manifestations of severe COVID-19 infections in adults can include neurological disorders, hyperinflammation, acute respiratory distress syndrome, cardiac dysfunction, hypercoagulability, and acute kidney injury.
In children, COVID-19 has been associated with a multisystem inflammatory syndrome (MIS-C) similar to Kawasaki disease.
In a substantial number of cases, the effects of COVID-19 can linger for 6 months or longer, Dr. Fauci said, pointing to a study from the University of Washington in Seattle.
Investigators there found that approximately 30% of patients enrolled at their center reported persistent symptoms for as long as 9 months after the initial illness, with fatigue as the most commonly reported symptom. One-third of outpatients with mild disease also reported persistent symptoms.
Therapeutics
Therapeutics that are either approved by the Food and Drug Administration, have emergency use authorization, or are in clinical trials for early or moderate disease include remdesivir (Veklury, Gilead Sciences), monoclonal antibodies, convalescent plasma, antiviral agents, hyperimmune globulin, anticoagulants, and immunomodulators.
Options for moderate to severe to advanced disease include dexamethasone, baricitinib (Olumiant, Eli Lilly and Company) plus remdesivir, and immunomodulators such as infliximab (Remicade, Janssen Biotech), and biosimilars.
Vaccines
Finally, Dr. Fauci reviewed the current state of vaccines, including the three with emergency use authorization from the FDA as of this writing: two nucleic acid, messenger RNA-based (mRNA) vaccines from Moderna and Pfizer/BioNTech, and an adenoviral vector-based vaccine from Johnson & Johnson.
Other vaccines in development or in use elsewhere in the world include recombinant protein and adjuvant approaches by GlaxoSmithKline and Sanofi (in a phase 2 clinical trial launched in February 2021) and by Novavax.
The three vaccines in use in the United States were highly efficacious in both clinical trials, with efficacy of about 95% for the mRNA vaccines and 67% for the Johnson & Johnson vaccine.
The real-world performance of these vaccines has been even more impressive, however.
For example, the Johnson & Johnson vaccine had 72% efficacy at preventing moderate to severe COVID 19 in the United States, 68% in Brazil, and 64% in South Africa, and 85% efficacy against severe disease across all regions studied, Dr. Fauci said.
He cited a study of 22,234 employees of the University of Texas Southwestern Medical Center in Dallas who were vaccinated under a program started on Dec. 15, 2020. The COVID-19 infection rate among these vaccinated employees was 0.05%.
Dr. Fauci recounted the experience in Israel, where the highly transmissible B.1.1.7 strain of SARS-CoV-2 is predominant. A chart of the progress shows clearly that as the vaccine doses delivered steadily increased, the number of COVID-19 cases began a precipitous decline.
Horse race
Fittingly for a speech presented on the day that the Preakness Stakes – the second leg in thoroughbred racing’s Triple Crown – was run, Dr. Fauci closed with a cartoon showing two racehorses, labeled “SARS-CoV-2” and “Vaccines,” nearly neck-and-neck, but with vaccines having a slight lead.
“We are in a race against the virus. The vaccines, and the virus: If we vaccinate the overwhelming proportion of our population, we will without a doubt be able to crush the outbreak in the same way as we have done with other viral-borne diseases like measles, smallpox, and polio.
“So, the message is: Get vaccinated,” he concluded.
Seaweed and other marine-derived products in skin care, Part II: Cosmetic formulations, fucoidan, and salmon eggs
The use of bioactive ingredients culled from the marine environment has increased significantly in recent years for use in skin care because of the reputed antioxidant and anti-aging activity of these substances.1-3
In the last couple of decades, secondary metabolites with bioactive properties have been identified in seaweeds. Among these substances, phlorotannins have been isolated from brown seaweeds and demonstrated to exhibit anti-allergic, anti-inflammatory, antioxidant, anticancer, and antiwrinkling activity, as well as some capacity to promote hair growth.4 Sanjeewa et al. suggest that phlorotannins, or marine polyphenols, derived from brown seaweed are well suited for use in cosmeceutical formulations and appear to exhibit skin whitening and antiwrinkling properties in particular.4 This column will discuss recent findings regarding the use of marine ingredients in cosmetic formulations, with a particular focus on substances such as fucoidan, as well as emerging evidence regarding the benefits to human skin derived from salmon eggs.
Recent studies of marine products in cosmetic formulations
In 2017, Fabrowska et al. showed in two groups of 10 volunteers each (one ranging from 20 to 30 years old and one from 40 to 50 years old) that the freshwater alga Cladophora glomerate is an effective ingredient for use as a cosmetic agent intended to moisturize and firm the skin.5
The next year, Thu et al. reported on the preparation of a cream mask composed of Vietnamese seaweeds (Caulerpa lentillifera, Sargassum crassifolium, Ulva reticulata, and Kappaphycus alvarezii), which they found to be abundant in proteins, polysaccharides, carotenoids, and other vitamins and to have potent antibacterial, cell proliferation, moisture retention, and tyrosinase inhibitory properties. The authors added that the seaweed cream mask was safe, provoked no irritation, and appeared to be effective in delivering anti-aging and moisturizing benefits.6
In 2019, Jesumani et al., in reviewing the potential cutaneous benefits of bioactive substances in seaweed, noted a significant increase in the use of ingredients found in macroalgae or seaweed in cosmetic formulations, also noting the range of reputed bioactivity (i.e., antioxidant, antitumor, anti-inflammatory, antilipidemic, antimicrobial, and anti-allergic).7 Seaweeds are a significant source of vitamins A, B, C, D, and E, and green, red, and brown algae contain pigments that protect against UV irradiation.7,8
Also that year, Hameury et al. conducted an ex vivo assessment to predict the cutaneous anti-aging benefits of an aqueous gel containing 6.1% marine ingredients (amino acid-enriched giant kelp extract, trace element-enriched seawater, and dedifferentiated sea fennel cells) topically applied on human skin explants. The investigators found that 64 proteins were significantly regulated by the gel when marine ingredients were compared with untreated skin explants, with the ingredients shown to act on the epidermis and dermis. These proteins are involved in multiple functions including gene expression, inflammatory processes, dermal extracellular matrix production, and melanogenesis and keratinocyte proliferation, suggesting, according to the authors, that marine ingredients could play a role in preventing cutaneous aging and contributing to the health of the epidermis and dermis.9
Early in 2020, Poulose et al. reported on the first use of a photoprotective cosmetic cream combining nanomelanin and seaweed that exerts antioxidant, antibacterial, and wound healing activity.10
The skin-lightening potential of fucoidan
In 2017, Wang et al. investigated the antimelanogenic activity of fucoidan – a complex sulfated polysaccharide extracted from brown seaweed known to possess a broad array of biologic functions – on B16 murine melanoma cells. Their in vitro studies revealed that fucoidan suppresses B16 melanoma cell proliferation and cellular tyrosinase activity and has potential as a skin-whitening cosmeceutical agent.11
Two years later, Jesumani et al. investigated the polysaccharides extracted from the seaweed species Sargassum vachellianum, S. horneri, and S. hemiphyllum. Found to be abundant in fucose, all of the evaluated polysaccharides demonstrated dose-dependent antioxidant activity and effectiveness in hindering tyrosinase and elastase. The researchers concluded that all of the tested species display potential as key ingredients in cosmeceutical agents intended to treat wrinkles or lighten skin.12
More recently, a comparative study by the same team revealed that both fucoidan-rich polysaccharide extract and polyphenol-rich extract from the seaweed S. vachellianum delivered significant protective activity. Both protected the skin from UV harm: The fucoidan-rich extract showed superior free radical scavenging and antimicrobial activity, while the polyphenol extract performed better at absorbing UV radiation. The investigators suggested that both extracts could provide a balanced approach to skin protection when featured in skin care products.13
In addition, it is worth noting that a key monomeric component of red macroalgae (Rhodophyta), 3,6-anhydro-l-galactose, has been found in vitro to display skin-whitening activity.14
Salmon eggs
In a 2013 double-blind, randomized clinical trial with 66 patients, Lønne et al. reported that subjects treated topically with salmon egg extract experienced significant amelioration of photoaging, including wrinkles, pigmentation, erythema, and xerosis, yielding global skin appearance improvement.3,15
A pilot study by Mekas et al., which was reported 2 years later and included 75 patients, revealed that skin tone and evenness were improved by a topical exfoliative cream featuring hydrolyzed roe proteins, based on subjective and objective measures comparing 4% glycolic acid.3,16
In 2016, Yoshino et al. showed that human dermal fibroblasts incubated with salmon egg extract upregulated the expression of collagen type I genes and several oxidative genes.3,17 The topical application of hydrolyzed salmon roe proteins to human skin has also been demonstrated to eliminate cell-to-cell adhesions thus ameliorating the appearance of photodamaged skin.1,3,16
More recently, a comprehensive PubMed search on the bioactive ingredients used in Korean cosmeceuticals reported early in 2020 that there is increased interest in salmon eggs because they provide a copious supply of unsaturated fatty acids, proteins, vitamins, and minerals known to nurture cutaneous health.3,15
Conclusion
. Research into the numerous bioactive properties of these multitudinous species has ramped up in recent years and is yielding evidence regarding the efficacy and potential broader uses of such ingredients in cutaneous health care. As we build on our understanding of just how dynamic a source of treatment options may lie under the sea, we become increasingly aware, ironically, of the damage that human industrialization exerts on the planet, as well as these precious marine resources (including the possibly deleterious effects of chemical sunscreens like those that are now banned for sale in Hawai‘i). Humanity will need to become much better stewards of the Earth if we are to enhance our future opportunities and possibly harness the potent marine ingredients still available with the potential to enhance skin health and appearance.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at dermnews@mdedge.com.
References
1. Kim SK. J Cosmet Dermatol. 2014;13(1):56-67.
2. Venkatesan J et al. Mar Drugs. 2017;15(5):1-18.
3. Nguyen JK et al. J Cosmet Dermatol 2020 Jul;19(7):1555-69.
4. Sanjeewa KKA et al. J Photochem Photobiol B. 2016 Sep;162:100-5.
5. Fabrowska J et al. Acta Pol Pharm. 2017 Mar;74(2):633-41.
6. Thu NTH et al. J Cosmet Sci. Nov/Dec 2018;69(6):447-62.
7. Jesumani V et al. Mar Drugs. 2019 Dec 6;17(12):688.
8. Kim MS et al. Photochem Photobiol. Jul-Aug 2013;89(4):911-8.
9. Hameury S et al. J Cosmet Dermatol. 2019 Feb;18(1):355-70.
10. Poulose N et al. J Photochem Photobiol B. 2020 Apr;205:111816.
11. Wang ZJ et al. Afr J Tradit Complement Altern Med. 2017 Jun 5;14(4);149-55.
12. Jesumani V et al. Int J Biol Macromol. 2019 Nov 1;140:216-24.
13. Jesumani V et al. PLoS One. 2020 Jan 7;15(1):e0227308.
14. Kim JH et al. Mar Drugs. 2017 Oct 20;15(10):321.
15. Lønne GK et al. Int J Cosmet Sci. 2013 Oct;35(5):515-22.
16. Mekas M et al. J Drugs Dermatol. 2015 Nov;14(11):1306-19.
17. Yoshino A et al. Clin Interv Aging. 2016;11:1159-68.
The use of bioactive ingredients culled from the marine environment has increased significantly in recent years for use in skin care because of the reputed antioxidant and anti-aging activity of these substances.1-3
In the last couple of decades, secondary metabolites with bioactive properties have been identified in seaweeds. Among these substances, phlorotannins have been isolated from brown seaweeds and demonstrated to exhibit anti-allergic, anti-inflammatory, antioxidant, anticancer, and antiwrinkling activity, as well as some capacity to promote hair growth.4 Sanjeewa et al. suggest that phlorotannins, or marine polyphenols, derived from brown seaweed are well suited for use in cosmeceutical formulations and appear to exhibit skin whitening and antiwrinkling properties in particular.4 This column will discuss recent findings regarding the use of marine ingredients in cosmetic formulations, with a particular focus on substances such as fucoidan, as well as emerging evidence regarding the benefits to human skin derived from salmon eggs.
Recent studies of marine products in cosmetic formulations
In 2017, Fabrowska et al. showed in two groups of 10 volunteers each (one ranging from 20 to 30 years old and one from 40 to 50 years old) that the freshwater alga Cladophora glomerate is an effective ingredient for use as a cosmetic agent intended to moisturize and firm the skin.5
The next year, Thu et al. reported on the preparation of a cream mask composed of Vietnamese seaweeds (Caulerpa lentillifera, Sargassum crassifolium, Ulva reticulata, and Kappaphycus alvarezii), which they found to be abundant in proteins, polysaccharides, carotenoids, and other vitamins and to have potent antibacterial, cell proliferation, moisture retention, and tyrosinase inhibitory properties. The authors added that the seaweed cream mask was safe, provoked no irritation, and appeared to be effective in delivering anti-aging and moisturizing benefits.6
In 2019, Jesumani et al., in reviewing the potential cutaneous benefits of bioactive substances in seaweed, noted a significant increase in the use of ingredients found in macroalgae or seaweed in cosmetic formulations, also noting the range of reputed bioactivity (i.e., antioxidant, antitumor, anti-inflammatory, antilipidemic, antimicrobial, and anti-allergic).7 Seaweeds are a significant source of vitamins A, B, C, D, and E, and green, red, and brown algae contain pigments that protect against UV irradiation.7,8
Also that year, Hameury et al. conducted an ex vivo assessment to predict the cutaneous anti-aging benefits of an aqueous gel containing 6.1% marine ingredients (amino acid-enriched giant kelp extract, trace element-enriched seawater, and dedifferentiated sea fennel cells) topically applied on human skin explants. The investigators found that 64 proteins were significantly regulated by the gel when marine ingredients were compared with untreated skin explants, with the ingredients shown to act on the epidermis and dermis. These proteins are involved in multiple functions including gene expression, inflammatory processes, dermal extracellular matrix production, and melanogenesis and keratinocyte proliferation, suggesting, according to the authors, that marine ingredients could play a role in preventing cutaneous aging and contributing to the health of the epidermis and dermis.9
Early in 2020, Poulose et al. reported on the first use of a photoprotective cosmetic cream combining nanomelanin and seaweed that exerts antioxidant, antibacterial, and wound healing activity.10
The skin-lightening potential of fucoidan
In 2017, Wang et al. investigated the antimelanogenic activity of fucoidan – a complex sulfated polysaccharide extracted from brown seaweed known to possess a broad array of biologic functions – on B16 murine melanoma cells. Their in vitro studies revealed that fucoidan suppresses B16 melanoma cell proliferation and cellular tyrosinase activity and has potential as a skin-whitening cosmeceutical agent.11
Two years later, Jesumani et al. investigated the polysaccharides extracted from the seaweed species Sargassum vachellianum, S. horneri, and S. hemiphyllum. Found to be abundant in fucose, all of the evaluated polysaccharides demonstrated dose-dependent antioxidant activity and effectiveness in hindering tyrosinase and elastase. The researchers concluded that all of the tested species display potential as key ingredients in cosmeceutical agents intended to treat wrinkles or lighten skin.12
More recently, a comparative study by the same team revealed that both fucoidan-rich polysaccharide extract and polyphenol-rich extract from the seaweed S. vachellianum delivered significant protective activity. Both protected the skin from UV harm: The fucoidan-rich extract showed superior free radical scavenging and antimicrobial activity, while the polyphenol extract performed better at absorbing UV radiation. The investigators suggested that both extracts could provide a balanced approach to skin protection when featured in skin care products.13
In addition, it is worth noting that a key monomeric component of red macroalgae (Rhodophyta), 3,6-anhydro-l-galactose, has been found in vitro to display skin-whitening activity.14
Salmon eggs
In a 2013 double-blind, randomized clinical trial with 66 patients, Lønne et al. reported that subjects treated topically with salmon egg extract experienced significant amelioration of photoaging, including wrinkles, pigmentation, erythema, and xerosis, yielding global skin appearance improvement.3,15
A pilot study by Mekas et al., which was reported 2 years later and included 75 patients, revealed that skin tone and evenness were improved by a topical exfoliative cream featuring hydrolyzed roe proteins, based on subjective and objective measures comparing 4% glycolic acid.3,16
In 2016, Yoshino et al. showed that human dermal fibroblasts incubated with salmon egg extract upregulated the expression of collagen type I genes and several oxidative genes.3,17 The topical application of hydrolyzed salmon roe proteins to human skin has also been demonstrated to eliminate cell-to-cell adhesions thus ameliorating the appearance of photodamaged skin.1,3,16
More recently, a comprehensive PubMed search on the bioactive ingredients used in Korean cosmeceuticals reported early in 2020 that there is increased interest in salmon eggs because they provide a copious supply of unsaturated fatty acids, proteins, vitamins, and minerals known to nurture cutaneous health.3,15
Conclusion
. Research into the numerous bioactive properties of these multitudinous species has ramped up in recent years and is yielding evidence regarding the efficacy and potential broader uses of such ingredients in cutaneous health care. As we build on our understanding of just how dynamic a source of treatment options may lie under the sea, we become increasingly aware, ironically, of the damage that human industrialization exerts on the planet, as well as these precious marine resources (including the possibly deleterious effects of chemical sunscreens like those that are now banned for sale in Hawai‘i). Humanity will need to become much better stewards of the Earth if we are to enhance our future opportunities and possibly harness the potent marine ingredients still available with the potential to enhance skin health and appearance.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at dermnews@mdedge.com.
References
1. Kim SK. J Cosmet Dermatol. 2014;13(1):56-67.
2. Venkatesan J et al. Mar Drugs. 2017;15(5):1-18.
3. Nguyen JK et al. J Cosmet Dermatol 2020 Jul;19(7):1555-69.
4. Sanjeewa KKA et al. J Photochem Photobiol B. 2016 Sep;162:100-5.
5. Fabrowska J et al. Acta Pol Pharm. 2017 Mar;74(2):633-41.
6. Thu NTH et al. J Cosmet Sci. Nov/Dec 2018;69(6):447-62.
7. Jesumani V et al. Mar Drugs. 2019 Dec 6;17(12):688.
8. Kim MS et al. Photochem Photobiol. Jul-Aug 2013;89(4):911-8.
9. Hameury S et al. J Cosmet Dermatol. 2019 Feb;18(1):355-70.
10. Poulose N et al. J Photochem Photobiol B. 2020 Apr;205:111816.
11. Wang ZJ et al. Afr J Tradit Complement Altern Med. 2017 Jun 5;14(4);149-55.
12. Jesumani V et al. Int J Biol Macromol. 2019 Nov 1;140:216-24.
13. Jesumani V et al. PLoS One. 2020 Jan 7;15(1):e0227308.
14. Kim JH et al. Mar Drugs. 2017 Oct 20;15(10):321.
15. Lønne GK et al. Int J Cosmet Sci. 2013 Oct;35(5):515-22.
16. Mekas M et al. J Drugs Dermatol. 2015 Nov;14(11):1306-19.
17. Yoshino A et al. Clin Interv Aging. 2016;11:1159-68.
The use of bioactive ingredients culled from the marine environment has increased significantly in recent years for use in skin care because of the reputed antioxidant and anti-aging activity of these substances.1-3
In the last couple of decades, secondary metabolites with bioactive properties have been identified in seaweeds. Among these substances, phlorotannins have been isolated from brown seaweeds and demonstrated to exhibit anti-allergic, anti-inflammatory, antioxidant, anticancer, and antiwrinkling activity, as well as some capacity to promote hair growth.4 Sanjeewa et al. suggest that phlorotannins, or marine polyphenols, derived from brown seaweed are well suited for use in cosmeceutical formulations and appear to exhibit skin whitening and antiwrinkling properties in particular.4 This column will discuss recent findings regarding the use of marine ingredients in cosmetic formulations, with a particular focus on substances such as fucoidan, as well as emerging evidence regarding the benefits to human skin derived from salmon eggs.
Recent studies of marine products in cosmetic formulations
In 2017, Fabrowska et al. showed in two groups of 10 volunteers each (one ranging from 20 to 30 years old and one from 40 to 50 years old) that the freshwater alga Cladophora glomerate is an effective ingredient for use as a cosmetic agent intended to moisturize and firm the skin.5
The next year, Thu et al. reported on the preparation of a cream mask composed of Vietnamese seaweeds (Caulerpa lentillifera, Sargassum crassifolium, Ulva reticulata, and Kappaphycus alvarezii), which they found to be abundant in proteins, polysaccharides, carotenoids, and other vitamins and to have potent antibacterial, cell proliferation, moisture retention, and tyrosinase inhibitory properties. The authors added that the seaweed cream mask was safe, provoked no irritation, and appeared to be effective in delivering anti-aging and moisturizing benefits.6
In 2019, Jesumani et al., in reviewing the potential cutaneous benefits of bioactive substances in seaweed, noted a significant increase in the use of ingredients found in macroalgae or seaweed in cosmetic formulations, also noting the range of reputed bioactivity (i.e., antioxidant, antitumor, anti-inflammatory, antilipidemic, antimicrobial, and anti-allergic).7 Seaweeds are a significant source of vitamins A, B, C, D, and E, and green, red, and brown algae contain pigments that protect against UV irradiation.7,8
Also that year, Hameury et al. conducted an ex vivo assessment to predict the cutaneous anti-aging benefits of an aqueous gel containing 6.1% marine ingredients (amino acid-enriched giant kelp extract, trace element-enriched seawater, and dedifferentiated sea fennel cells) topically applied on human skin explants. The investigators found that 64 proteins were significantly regulated by the gel when marine ingredients were compared with untreated skin explants, with the ingredients shown to act on the epidermis and dermis. These proteins are involved in multiple functions including gene expression, inflammatory processes, dermal extracellular matrix production, and melanogenesis and keratinocyte proliferation, suggesting, according to the authors, that marine ingredients could play a role in preventing cutaneous aging and contributing to the health of the epidermis and dermis.9
Early in 2020, Poulose et al. reported on the first use of a photoprotective cosmetic cream combining nanomelanin and seaweed that exerts antioxidant, antibacterial, and wound healing activity.10
The skin-lightening potential of fucoidan
In 2017, Wang et al. investigated the antimelanogenic activity of fucoidan – a complex sulfated polysaccharide extracted from brown seaweed known to possess a broad array of biologic functions – on B16 murine melanoma cells. Their in vitro studies revealed that fucoidan suppresses B16 melanoma cell proliferation and cellular tyrosinase activity and has potential as a skin-whitening cosmeceutical agent.11
Two years later, Jesumani et al. investigated the polysaccharides extracted from the seaweed species Sargassum vachellianum, S. horneri, and S. hemiphyllum. Found to be abundant in fucose, all of the evaluated polysaccharides demonstrated dose-dependent antioxidant activity and effectiveness in hindering tyrosinase and elastase. The researchers concluded that all of the tested species display potential as key ingredients in cosmeceutical agents intended to treat wrinkles or lighten skin.12
More recently, a comparative study by the same team revealed that both fucoidan-rich polysaccharide extract and polyphenol-rich extract from the seaweed S. vachellianum delivered significant protective activity. Both protected the skin from UV harm: The fucoidan-rich extract showed superior free radical scavenging and antimicrobial activity, while the polyphenol extract performed better at absorbing UV radiation. The investigators suggested that both extracts could provide a balanced approach to skin protection when featured in skin care products.13
In addition, it is worth noting that a key monomeric component of red macroalgae (Rhodophyta), 3,6-anhydro-l-galactose, has been found in vitro to display skin-whitening activity.14
Salmon eggs
In a 2013 double-blind, randomized clinical trial with 66 patients, Lønne et al. reported that subjects treated topically with salmon egg extract experienced significant amelioration of photoaging, including wrinkles, pigmentation, erythema, and xerosis, yielding global skin appearance improvement.3,15
A pilot study by Mekas et al., which was reported 2 years later and included 75 patients, revealed that skin tone and evenness were improved by a topical exfoliative cream featuring hydrolyzed roe proteins, based on subjective and objective measures comparing 4% glycolic acid.3,16
In 2016, Yoshino et al. showed that human dermal fibroblasts incubated with salmon egg extract upregulated the expression of collagen type I genes and several oxidative genes.3,17 The topical application of hydrolyzed salmon roe proteins to human skin has also been demonstrated to eliminate cell-to-cell adhesions thus ameliorating the appearance of photodamaged skin.1,3,16
More recently, a comprehensive PubMed search on the bioactive ingredients used in Korean cosmeceuticals reported early in 2020 that there is increased interest in salmon eggs because they provide a copious supply of unsaturated fatty acids, proteins, vitamins, and minerals known to nurture cutaneous health.3,15
Conclusion
. Research into the numerous bioactive properties of these multitudinous species has ramped up in recent years and is yielding evidence regarding the efficacy and potential broader uses of such ingredients in cutaneous health care. As we build on our understanding of just how dynamic a source of treatment options may lie under the sea, we become increasingly aware, ironically, of the damage that human industrialization exerts on the planet, as well as these precious marine resources (including the possibly deleterious effects of chemical sunscreens like those that are now banned for sale in Hawai‘i). Humanity will need to become much better stewards of the Earth if we are to enhance our future opportunities and possibly harness the potent marine ingredients still available with the potential to enhance skin health and appearance.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at dermnews@mdedge.com.
References
1. Kim SK. J Cosmet Dermatol. 2014;13(1):56-67.
2. Venkatesan J et al. Mar Drugs. 2017;15(5):1-18.
3. Nguyen JK et al. J Cosmet Dermatol 2020 Jul;19(7):1555-69.
4. Sanjeewa KKA et al. J Photochem Photobiol B. 2016 Sep;162:100-5.
5. Fabrowska J et al. Acta Pol Pharm. 2017 Mar;74(2):633-41.
6. Thu NTH et al. J Cosmet Sci. Nov/Dec 2018;69(6):447-62.
7. Jesumani V et al. Mar Drugs. 2019 Dec 6;17(12):688.
8. Kim MS et al. Photochem Photobiol. Jul-Aug 2013;89(4):911-8.
9. Hameury S et al. J Cosmet Dermatol. 2019 Feb;18(1):355-70.
10. Poulose N et al. J Photochem Photobiol B. 2020 Apr;205:111816.
11. Wang ZJ et al. Afr J Tradit Complement Altern Med. 2017 Jun 5;14(4);149-55.
12. Jesumani V et al. Int J Biol Macromol. 2019 Nov 1;140:216-24.
13. Jesumani V et al. PLoS One. 2020 Jan 7;15(1):e0227308.
14. Kim JH et al. Mar Drugs. 2017 Oct 20;15(10):321.
15. Lønne GK et al. Int J Cosmet Sci. 2013 Oct;35(5):515-22.
16. Mekas M et al. J Drugs Dermatol. 2015 Nov;14(11):1306-19.
17. Yoshino A et al. Clin Interv Aging. 2016;11:1159-68.
FDA preparing an environmental impact statement for 2 sunscreen ingredients
The Food and Drug Administration is launching a process to prepare an environmental impact statement (EIS) regarding the use oxybenzone and octinoxate in over-the-counter sunscreen products.
According to the “Intent to Prepare an Environmental Impact Statement for Certain Sunscreen Drug Products for Over-The-Counter Use,” which was published in the Federal Register on May 13, 2021, the FDA will prepare an EIS “when data or information in an environmental assessment or otherwise available to the Agency leads to a finding that the proposed agency action may significantly affect the quality of the human environment.” The first step in this effort involves a “public scoping process” to evaluate any potential environmental impacts associated with the use of oxybenzone and octinoxate in sunscreens so that an EIS, if required, “can be completed prior to issuance of a final sunscreen order addressing sunscreens containing these ingredients.”
The American Academy of Dermatology Association weighed in on the FDA’s announcement, noting that it “appreciates the efforts of the agency to thoroughly examine all relevant science before issuing a final sunscreen order on these ingredients,” according to a statement released by the AADA on May 13, 2021.
The statement added: “Skin cancer is the most common cancer in the U.S., and unprotected exposure to the sun’s harmful ultraviolet rays is a major risk factor. The AADA continues to focus on encouraging members of the public to protect themselves by seeking shade, wearing protective clothing – including a lightweight and long-sleeved shirt, pants, a wide-brimmed hat and sunglasses – and applying a broad-spectrum sunscreen with an SPF of 30 or higher to all exposed skin.”
According to the FDA document, a series of developments regarding oxybenzone and octinoxate prompted the agency to take this step, including comments the agency received in response to the 2019 proposed rule titled “Sunscreen Drug Products for Over-The-Counter Human Use,” which raised concern about the potential effects of the two ingredients on coral and/or coral reefs, as well as research efforts by the National Oceanic and Atmospheric Administration Coral Reef Conservation Programs on the potential impacts of sunscreen products that include oxybenzone and octinoxate on coral reefs and other aquatic systems. Hawaii’s 2018 state law prohibiting the sale, offer of sale, and distribution of sunscreens that contain oxybenzone and/or octinoxate also influenced the agency’s decision to further evaluate the topic.
“The purpose of the public scoping process is to determine relevant issues that will influence the scope of the environmental analysis, including potential alternatives and the extent to which those issues and impacts will be analyzed,” the FDA document states. “At this initial stage of the scoping process, we have identified the following four alternatives: FDA will conclude that the inclusion of oxybenzone and octinoxate in sunscreens marketed without an NDA [new drug application] is impermissible; FDA will conclude that the inclusion of oxybenzone and octinoxate in sunscreens marketed without an NDA is permissible; FDA will conclude that inclusion of oxybenzone in sunscreens marketed without an NDA is permissible but that the inclusion of octinoxate in sunscreens marketed without an NDA is impermissible; or FDA will conclude that inclusion of octinoxate in sunscreens marketed without an NDA is permissible but that the inclusion of oxybenzone in sunscreens marketed without an NDA is impermissible.”
Until June 14, the FDA is accepting comments from the public electronically via the Federal eRulemaking Portal at www.regulations.gov (search for Docket No. FDA-2021-N-0352) or by mail to: Dockets Management Staff (HFA-305), Food and Drug Administration, 5630 Fishers Lane, Rm. 1061, Rockville, Md., 20852. Refer to Docket No. FDA-2021-N-0352.
The Food and Drug Administration is launching a process to prepare an environmental impact statement (EIS) regarding the use oxybenzone and octinoxate in over-the-counter sunscreen products.
According to the “Intent to Prepare an Environmental Impact Statement for Certain Sunscreen Drug Products for Over-The-Counter Use,” which was published in the Federal Register on May 13, 2021, the FDA will prepare an EIS “when data or information in an environmental assessment or otherwise available to the Agency leads to a finding that the proposed agency action may significantly affect the quality of the human environment.” The first step in this effort involves a “public scoping process” to evaluate any potential environmental impacts associated with the use of oxybenzone and octinoxate in sunscreens so that an EIS, if required, “can be completed prior to issuance of a final sunscreen order addressing sunscreens containing these ingredients.”
The American Academy of Dermatology Association weighed in on the FDA’s announcement, noting that it “appreciates the efforts of the agency to thoroughly examine all relevant science before issuing a final sunscreen order on these ingredients,” according to a statement released by the AADA on May 13, 2021.
The statement added: “Skin cancer is the most common cancer in the U.S., and unprotected exposure to the sun’s harmful ultraviolet rays is a major risk factor. The AADA continues to focus on encouraging members of the public to protect themselves by seeking shade, wearing protective clothing – including a lightweight and long-sleeved shirt, pants, a wide-brimmed hat and sunglasses – and applying a broad-spectrum sunscreen with an SPF of 30 or higher to all exposed skin.”
According to the FDA document, a series of developments regarding oxybenzone and octinoxate prompted the agency to take this step, including comments the agency received in response to the 2019 proposed rule titled “Sunscreen Drug Products for Over-The-Counter Human Use,” which raised concern about the potential effects of the two ingredients on coral and/or coral reefs, as well as research efforts by the National Oceanic and Atmospheric Administration Coral Reef Conservation Programs on the potential impacts of sunscreen products that include oxybenzone and octinoxate on coral reefs and other aquatic systems. Hawaii’s 2018 state law prohibiting the sale, offer of sale, and distribution of sunscreens that contain oxybenzone and/or octinoxate also influenced the agency’s decision to further evaluate the topic.
“The purpose of the public scoping process is to determine relevant issues that will influence the scope of the environmental analysis, including potential alternatives and the extent to which those issues and impacts will be analyzed,” the FDA document states. “At this initial stage of the scoping process, we have identified the following four alternatives: FDA will conclude that the inclusion of oxybenzone and octinoxate in sunscreens marketed without an NDA [new drug application] is impermissible; FDA will conclude that the inclusion of oxybenzone and octinoxate in sunscreens marketed without an NDA is permissible; FDA will conclude that inclusion of oxybenzone in sunscreens marketed without an NDA is permissible but that the inclusion of octinoxate in sunscreens marketed without an NDA is impermissible; or FDA will conclude that inclusion of octinoxate in sunscreens marketed without an NDA is permissible but that the inclusion of oxybenzone in sunscreens marketed without an NDA is impermissible.”
Until June 14, the FDA is accepting comments from the public electronically via the Federal eRulemaking Portal at www.regulations.gov (search for Docket No. FDA-2021-N-0352) or by mail to: Dockets Management Staff (HFA-305), Food and Drug Administration, 5630 Fishers Lane, Rm. 1061, Rockville, Md., 20852. Refer to Docket No. FDA-2021-N-0352.
The Food and Drug Administration is launching a process to prepare an environmental impact statement (EIS) regarding the use oxybenzone and octinoxate in over-the-counter sunscreen products.
According to the “Intent to Prepare an Environmental Impact Statement for Certain Sunscreen Drug Products for Over-The-Counter Use,” which was published in the Federal Register on May 13, 2021, the FDA will prepare an EIS “when data or information in an environmental assessment or otherwise available to the Agency leads to a finding that the proposed agency action may significantly affect the quality of the human environment.” The first step in this effort involves a “public scoping process” to evaluate any potential environmental impacts associated with the use of oxybenzone and octinoxate in sunscreens so that an EIS, if required, “can be completed prior to issuance of a final sunscreen order addressing sunscreens containing these ingredients.”
The American Academy of Dermatology Association weighed in on the FDA’s announcement, noting that it “appreciates the efforts of the agency to thoroughly examine all relevant science before issuing a final sunscreen order on these ingredients,” according to a statement released by the AADA on May 13, 2021.
The statement added: “Skin cancer is the most common cancer in the U.S., and unprotected exposure to the sun’s harmful ultraviolet rays is a major risk factor. The AADA continues to focus on encouraging members of the public to protect themselves by seeking shade, wearing protective clothing – including a lightweight and long-sleeved shirt, pants, a wide-brimmed hat and sunglasses – and applying a broad-spectrum sunscreen with an SPF of 30 or higher to all exposed skin.”
According to the FDA document, a series of developments regarding oxybenzone and octinoxate prompted the agency to take this step, including comments the agency received in response to the 2019 proposed rule titled “Sunscreen Drug Products for Over-The-Counter Human Use,” which raised concern about the potential effects of the two ingredients on coral and/or coral reefs, as well as research efforts by the National Oceanic and Atmospheric Administration Coral Reef Conservation Programs on the potential impacts of sunscreen products that include oxybenzone and octinoxate on coral reefs and other aquatic systems. Hawaii’s 2018 state law prohibiting the sale, offer of sale, and distribution of sunscreens that contain oxybenzone and/or octinoxate also influenced the agency’s decision to further evaluate the topic.
“The purpose of the public scoping process is to determine relevant issues that will influence the scope of the environmental analysis, including potential alternatives and the extent to which those issues and impacts will be analyzed,” the FDA document states. “At this initial stage of the scoping process, we have identified the following four alternatives: FDA will conclude that the inclusion of oxybenzone and octinoxate in sunscreens marketed without an NDA [new drug application] is impermissible; FDA will conclude that the inclusion of oxybenzone and octinoxate in sunscreens marketed without an NDA is permissible; FDA will conclude that inclusion of oxybenzone in sunscreens marketed without an NDA is permissible but that the inclusion of octinoxate in sunscreens marketed without an NDA is impermissible; or FDA will conclude that inclusion of octinoxate in sunscreens marketed without an NDA is permissible but that the inclusion of oxybenzone in sunscreens marketed without an NDA is impermissible.”
Until June 14, the FDA is accepting comments from the public electronically via the Federal eRulemaking Portal at www.regulations.gov (search for Docket No. FDA-2021-N-0352) or by mail to: Dockets Management Staff (HFA-305), Food and Drug Administration, 5630 Fishers Lane, Rm. 1061, Rockville, Md., 20852. Refer to Docket No. FDA-2021-N-0352.
Telemedicine is popular among Mohs surgeons – for now
A majority of
A variety of factors combine to make it “very difficult for surgeons to make long-term plans for implementing telemedicine in their practices,” said Mario Maruthur, MD, who presented the findings at the annual meeting of the American College of Mohs Surgery. “Telemedicine likely has a role in Mohs practices, particularly with postop follow-up visits. However, postpandemic reimbursement and regulatory issues need to be formally laid out before Mohs surgeons are able to incorporate it into their permanent work flow.”
Dr. Maruthur, a Mohs surgery and dermatologic oncology fellow at Memorial Sloan Kettering Cancer Center, New York, and colleagues sent a survey to ACMS members in September and October 2020. “We saw first-hand in our surgical practice that telemedicine quickly became an important tool when the pandemic surged in the spring of 2020,” he said. Considering that surgical practices are highly dependent on in-person visits, the impetus for this study was to assess to what degree Mohs practices from across the spectrum, including academic and private practices, embraced telemedicine during the pandemic, and “what these surgical practices used telemedicine for, how it was received by their patients, which telemedicine platforms were most often utilized, and lastly, what are their plans if any for incorporating telemedicine into their surgical practices after the pandemic subsides.”
The researchers received responses from 115 surgeons representing all regions of the country (40% Northeast, 21% South, 21% Midwest, and 18% West). Half practiced in urban areas (37%) and large cities (13%), and 40% were in an academic setting versus 36% in a single-specialty private practice.
More than 70% of the respondents said their case load fell by at least 75% during the initial surge of the pandemic; 80% turned to telemedicine, compared with just 23% who relied on the technology prior to the pandemic. The most commonly used telemedicine technologies were FaceTime, Zoom, Doximity, and Epic.
Mohs surgeons reported most commonly using telemedicine for postsurgery management (77% of the total 115 responses). “Telemedicine is a great fit for this category of visits as they allow the surgeon to view the surgical site and answer any questions they patient may have,” Dr. Maruthur said. “If the surgeon does suspect a postop infection or other concern based on a patient’s signs or symptoms, they can easily schedule the patient for an in-person assessment. We suspect that postop follow-up visits may be the best candidate for long-term use of telemedicine in Mohs surgery practices.”
Surgeons also reported using telemedicine for “spot checks” (61%) and surgical consultations (59%).
However, Dr. Maruther noted that preoperative assessments and spot checks can be difficult to perform using telemedicine. “The quality of the video image is not always great, patients can have a difficult time pointing the camera at the right spot and at the right distance. Even appreciating the actual size of the lesion are all difficult over a video encounter. And there is a lot of information gleaned from in-person physical examination, such as whether the lesion is fixed to a deeper structure and whether there are any nearby scars or other suspicious lesions.”
Nearly three-quarters of the surgeons using the technology said most or all patients were receptive to telemedicine.
However, the surgeons reported multiple barriers to the use of telemedicine: Limitations when compared with physical exams (88%), fitting it into the work flow (58%), patient response and training (57%), reimbursement concerns (50%), implementation of the technology (37%), regulations such as HIPAA (24%), training of staff (17%), and licensing (8%).
In an interview, Sumaira Z. Aasi, MD, director of Mohs and dermatologic surgery, Stanford University, agreed that there are many obstacles to routine use of telemedicine by Mohs surgeons. “As surgeons, we rely on the physical and tactile exam to get a sense of the size and extent of the cancer and characteristics such as the laxity of the surrounding tissue whether the tumor is fixed,” she said. “It is very difficult to access this on a telemedicine visit.”
In addition, she said, “many of our patients are in the elderly population, and some may not be comfortable using this technology. Also, it’s not a work flow that we are comfortable or familiar with. And I think that the technology has to improve to allow for better resolution of images as we ‘examine’ patients through a telemedicine visit.”
She added that “another con is there is a reliance on having the patient point out lesions of concern. Many cancers are picked by a careful in-person examination by a qualified physician/dermatologist/Mohs surgeon when the lesion is quite small or subtle and not even noticed by the patient themselves. This approach invariably leads to earlier biopsies and earlier treatments that can prevent morbidity and save health care money.”
On the other hand, she said, telemedicine “may save patients some time and money in terms of the effort and cost of transportation to come in for simpler postoperative medical visits that are often short in their very nature, such as postop check-ups.”
Most of the surgeons surveyed (69%) said telemedicine probably or definitely deserves a place in the practice Mohs surgery, but only 50% said they’d like to or would definitely pursue giving telemedicine a role in their practices once the pandemic is over.
“At the start of the pandemic, many regulations in areas such as HIPAA were eased, and reimbursements were increased, which allowed telemedicine to be quickly adopted,” Dr. Maruther said. “The government and payers have yet to decide which regulations and reimbursements will be in place after the pandemic. That makes it very difficult for surgeons to make long-term plans for implementing telemedicine in their practices.”
Dr. Aasi predicted that telemedicine will become more appealing to patients and physicians as it its technology and usability improves. More familiarity with its use will also be helpful, she said, and surgeons will be more receptive as it’s incorporated into efficient daily work flow.
The study was funded in part by the National Institutes of Health.
A majority of
A variety of factors combine to make it “very difficult for surgeons to make long-term plans for implementing telemedicine in their practices,” said Mario Maruthur, MD, who presented the findings at the annual meeting of the American College of Mohs Surgery. “Telemedicine likely has a role in Mohs practices, particularly with postop follow-up visits. However, postpandemic reimbursement and regulatory issues need to be formally laid out before Mohs surgeons are able to incorporate it into their permanent work flow.”
Dr. Maruthur, a Mohs surgery and dermatologic oncology fellow at Memorial Sloan Kettering Cancer Center, New York, and colleagues sent a survey to ACMS members in September and October 2020. “We saw first-hand in our surgical practice that telemedicine quickly became an important tool when the pandemic surged in the spring of 2020,” he said. Considering that surgical practices are highly dependent on in-person visits, the impetus for this study was to assess to what degree Mohs practices from across the spectrum, including academic and private practices, embraced telemedicine during the pandemic, and “what these surgical practices used telemedicine for, how it was received by their patients, which telemedicine platforms were most often utilized, and lastly, what are their plans if any for incorporating telemedicine into their surgical practices after the pandemic subsides.”
The researchers received responses from 115 surgeons representing all regions of the country (40% Northeast, 21% South, 21% Midwest, and 18% West). Half practiced in urban areas (37%) and large cities (13%), and 40% were in an academic setting versus 36% in a single-specialty private practice.
More than 70% of the respondents said their case load fell by at least 75% during the initial surge of the pandemic; 80% turned to telemedicine, compared with just 23% who relied on the technology prior to the pandemic. The most commonly used telemedicine technologies were FaceTime, Zoom, Doximity, and Epic.
Mohs surgeons reported most commonly using telemedicine for postsurgery management (77% of the total 115 responses). “Telemedicine is a great fit for this category of visits as they allow the surgeon to view the surgical site and answer any questions they patient may have,” Dr. Maruthur said. “If the surgeon does suspect a postop infection or other concern based on a patient’s signs or symptoms, they can easily schedule the patient for an in-person assessment. We suspect that postop follow-up visits may be the best candidate for long-term use of telemedicine in Mohs surgery practices.”
Surgeons also reported using telemedicine for “spot checks” (61%) and surgical consultations (59%).
However, Dr. Maruther noted that preoperative assessments and spot checks can be difficult to perform using telemedicine. “The quality of the video image is not always great, patients can have a difficult time pointing the camera at the right spot and at the right distance. Even appreciating the actual size of the lesion are all difficult over a video encounter. And there is a lot of information gleaned from in-person physical examination, such as whether the lesion is fixed to a deeper structure and whether there are any nearby scars or other suspicious lesions.”
Nearly three-quarters of the surgeons using the technology said most or all patients were receptive to telemedicine.
However, the surgeons reported multiple barriers to the use of telemedicine: Limitations when compared with physical exams (88%), fitting it into the work flow (58%), patient response and training (57%), reimbursement concerns (50%), implementation of the technology (37%), regulations such as HIPAA (24%), training of staff (17%), and licensing (8%).
In an interview, Sumaira Z. Aasi, MD, director of Mohs and dermatologic surgery, Stanford University, agreed that there are many obstacles to routine use of telemedicine by Mohs surgeons. “As surgeons, we rely on the physical and tactile exam to get a sense of the size and extent of the cancer and characteristics such as the laxity of the surrounding tissue whether the tumor is fixed,” she said. “It is very difficult to access this on a telemedicine visit.”
In addition, she said, “many of our patients are in the elderly population, and some may not be comfortable using this technology. Also, it’s not a work flow that we are comfortable or familiar with. And I think that the technology has to improve to allow for better resolution of images as we ‘examine’ patients through a telemedicine visit.”
She added that “another con is there is a reliance on having the patient point out lesions of concern. Many cancers are picked by a careful in-person examination by a qualified physician/dermatologist/Mohs surgeon when the lesion is quite small or subtle and not even noticed by the patient themselves. This approach invariably leads to earlier biopsies and earlier treatments that can prevent morbidity and save health care money.”
On the other hand, she said, telemedicine “may save patients some time and money in terms of the effort and cost of transportation to come in for simpler postoperative medical visits that are often short in their very nature, such as postop check-ups.”
Most of the surgeons surveyed (69%) said telemedicine probably or definitely deserves a place in the practice Mohs surgery, but only 50% said they’d like to or would definitely pursue giving telemedicine a role in their practices once the pandemic is over.
“At the start of the pandemic, many regulations in areas such as HIPAA were eased, and reimbursements were increased, which allowed telemedicine to be quickly adopted,” Dr. Maruther said. “The government and payers have yet to decide which regulations and reimbursements will be in place after the pandemic. That makes it very difficult for surgeons to make long-term plans for implementing telemedicine in their practices.”
Dr. Aasi predicted that telemedicine will become more appealing to patients and physicians as it its technology and usability improves. More familiarity with its use will also be helpful, she said, and surgeons will be more receptive as it’s incorporated into efficient daily work flow.
The study was funded in part by the National Institutes of Health.
A majority of
A variety of factors combine to make it “very difficult for surgeons to make long-term plans for implementing telemedicine in their practices,” said Mario Maruthur, MD, who presented the findings at the annual meeting of the American College of Mohs Surgery. “Telemedicine likely has a role in Mohs practices, particularly with postop follow-up visits. However, postpandemic reimbursement and regulatory issues need to be formally laid out before Mohs surgeons are able to incorporate it into their permanent work flow.”
Dr. Maruthur, a Mohs surgery and dermatologic oncology fellow at Memorial Sloan Kettering Cancer Center, New York, and colleagues sent a survey to ACMS members in September and October 2020. “We saw first-hand in our surgical practice that telemedicine quickly became an important tool when the pandemic surged in the spring of 2020,” he said. Considering that surgical practices are highly dependent on in-person visits, the impetus for this study was to assess to what degree Mohs practices from across the spectrum, including academic and private practices, embraced telemedicine during the pandemic, and “what these surgical practices used telemedicine for, how it was received by their patients, which telemedicine platforms were most often utilized, and lastly, what are their plans if any for incorporating telemedicine into their surgical practices after the pandemic subsides.”
The researchers received responses from 115 surgeons representing all regions of the country (40% Northeast, 21% South, 21% Midwest, and 18% West). Half practiced in urban areas (37%) and large cities (13%), and 40% were in an academic setting versus 36% in a single-specialty private practice.
More than 70% of the respondents said their case load fell by at least 75% during the initial surge of the pandemic; 80% turned to telemedicine, compared with just 23% who relied on the technology prior to the pandemic. The most commonly used telemedicine technologies were FaceTime, Zoom, Doximity, and Epic.
Mohs surgeons reported most commonly using telemedicine for postsurgery management (77% of the total 115 responses). “Telemedicine is a great fit for this category of visits as they allow the surgeon to view the surgical site and answer any questions they patient may have,” Dr. Maruthur said. “If the surgeon does suspect a postop infection or other concern based on a patient’s signs or symptoms, they can easily schedule the patient for an in-person assessment. We suspect that postop follow-up visits may be the best candidate for long-term use of telemedicine in Mohs surgery practices.”
Surgeons also reported using telemedicine for “spot checks” (61%) and surgical consultations (59%).
However, Dr. Maruther noted that preoperative assessments and spot checks can be difficult to perform using telemedicine. “The quality of the video image is not always great, patients can have a difficult time pointing the camera at the right spot and at the right distance. Even appreciating the actual size of the lesion are all difficult over a video encounter. And there is a lot of information gleaned from in-person physical examination, such as whether the lesion is fixed to a deeper structure and whether there are any nearby scars or other suspicious lesions.”
Nearly three-quarters of the surgeons using the technology said most or all patients were receptive to telemedicine.
However, the surgeons reported multiple barriers to the use of telemedicine: Limitations when compared with physical exams (88%), fitting it into the work flow (58%), patient response and training (57%), reimbursement concerns (50%), implementation of the technology (37%), regulations such as HIPAA (24%), training of staff (17%), and licensing (8%).
In an interview, Sumaira Z. Aasi, MD, director of Mohs and dermatologic surgery, Stanford University, agreed that there are many obstacles to routine use of telemedicine by Mohs surgeons. “As surgeons, we rely on the physical and tactile exam to get a sense of the size and extent of the cancer and characteristics such as the laxity of the surrounding tissue whether the tumor is fixed,” she said. “It is very difficult to access this on a telemedicine visit.”
In addition, she said, “many of our patients are in the elderly population, and some may not be comfortable using this technology. Also, it’s not a work flow that we are comfortable or familiar with. And I think that the technology has to improve to allow for better resolution of images as we ‘examine’ patients through a telemedicine visit.”
She added that “another con is there is a reliance on having the patient point out lesions of concern. Many cancers are picked by a careful in-person examination by a qualified physician/dermatologist/Mohs surgeon when the lesion is quite small or subtle and not even noticed by the patient themselves. This approach invariably leads to earlier biopsies and earlier treatments that can prevent morbidity and save health care money.”
On the other hand, she said, telemedicine “may save patients some time and money in terms of the effort and cost of transportation to come in for simpler postoperative medical visits that are often short in their very nature, such as postop check-ups.”
Most of the surgeons surveyed (69%) said telemedicine probably or definitely deserves a place in the practice Mohs surgery, but only 50% said they’d like to or would definitely pursue giving telemedicine a role in their practices once the pandemic is over.
“At the start of the pandemic, many regulations in areas such as HIPAA were eased, and reimbursements were increased, which allowed telemedicine to be quickly adopted,” Dr. Maruther said. “The government and payers have yet to decide which regulations and reimbursements will be in place after the pandemic. That makes it very difficult for surgeons to make long-term plans for implementing telemedicine in their practices.”
Dr. Aasi predicted that telemedicine will become more appealing to patients and physicians as it its technology and usability improves. More familiarity with its use will also be helpful, she said, and surgeons will be more receptive as it’s incorporated into efficient daily work flow.
The study was funded in part by the National Institutes of Health.
FROM THE ACMS ANNUAL MEETING
Online patient reviews and HIPAA
In 2013, a California hospital paid $275,000 to settle claims that it violated the HIPAA privacy rule when it disclosed a patient’s health information in response to a negative online review. More recently, a Texas dental practice paid a substantial fine to the Department of Health & Human Services, which enforces HIPAA, after it responded to unfavorable Yelp reviews with patient names and details of their health conditions, treatment plans, and cost information. In addition to the fine, the practice agreed to 2 years of monitoring by HHS for compliance with HIPAA rules.
Most physicians have had the unpleasant experience of finding a negative online review from a disgruntled patient or family member. Some are justified, many are not; either way, your first impulse will often be to post a response – but that is almost always a bad idea. “Social media is not the place for providers to discuss a patient’s care,” an HHS official said in a statement issued about the dental practice case in 2016. “Doctors and dentists must think carefully about patient privacy before responding to online reviews.”
Any information that could be used to identify a patient is a HIPAA breach. This is true even if the patient has already disclosed information, because doing so does not nullify their HIPAA rights, and HIPAA provides no exceptions for responses. Even acknowledging that the reviewer was in fact your patient could, in some cases, be considered a violation.
Responding to good reviews can get you in trouble too, for the same reasons. In 2016, a physical therapy practice paid a $25,000 fine after it posted patient testimonials, “including full names and full-face photographic images to its website without obtaining valid, HIPAA-compliant authorizations.”
And by the way, most malpractice policies specifically exclude disciplinary fines and settlements from coverage.
All of that said,
- Ignore them. This is your best choice most of the time. Most negative reviews have minimal impact and simply do not deserve a response; responding may pour fuel on the fire. Besides, an occasional negative review actually lends credibility to a reviewing site and to the positive reviews posted on that site. Polls show that readers are suspicious of sites that contain only rave reviews. They assume such reviews have been “whitewashed” – or just fabricated.
- Solicit more reviews to that site. The more you can obtain, the less impact any complaints will have, since you know the overwhelming majority of your patients are happy with your care and will post a positive review if asked. Solicit them on your website, on social media, or in your email reminders. To be clear, you must encourage reviews from all patients, whether they have had a positive experience or not. If you invite only the satisfied ones, you are “filtering,” which can be perceived as false or deceptive advertising. (Google calls it “review-gating,” and according to their guidelines, if they catch you doing it they will remove all of your reviews.)
- Respond politely. In those rare cases where you feel you must respond, do so without acknowledging that the individual was a patient, or disclosing any information that may be linked to the patient. For example, you can say that you provide excellent and appropriate care, or describe your general policies. Be polite, professional, and sensitive to the patient’s position. Readers tend to respect and sympathize with a doctor who responds in a professional, respectful manner and does not trash the complainant in retaliation.
- Take the discussion offline. Sometimes the person posting the review is just frustrated and wants to be heard. In those cases, consider contacting the patient and offering to discuss their concerns privately. If you cannot resolve your differences, try to get the patient’s written permission to post a response to their review. If they refuse, you can explain that, thereby capturing the moral high ground.
If the review contains false or defamatory content, that’s a different situation entirely; you will probably need to consult your attorney.
Regardless of how you handle negative reviews, be sure to learn from them. Your critics, as the song goes, are not always evil – and not always wrong. Complaints give you a chance to review your office policies and procedures and your own conduct, identify weaknesses, and make changes as necessary. At the very least, the exercise will help you to avoid similar complaints in the future. Don’t let valuable opportunities like that pass you by.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at dermnews@frontlinemedcom.com.
In 2013, a California hospital paid $275,000 to settle claims that it violated the HIPAA privacy rule when it disclosed a patient’s health information in response to a negative online review. More recently, a Texas dental practice paid a substantial fine to the Department of Health & Human Services, which enforces HIPAA, after it responded to unfavorable Yelp reviews with patient names and details of their health conditions, treatment plans, and cost information. In addition to the fine, the practice agreed to 2 years of monitoring by HHS for compliance with HIPAA rules.
Most physicians have had the unpleasant experience of finding a negative online review from a disgruntled patient or family member. Some are justified, many are not; either way, your first impulse will often be to post a response – but that is almost always a bad idea. “Social media is not the place for providers to discuss a patient’s care,” an HHS official said in a statement issued about the dental practice case in 2016. “Doctors and dentists must think carefully about patient privacy before responding to online reviews.”
Any information that could be used to identify a patient is a HIPAA breach. This is true even if the patient has already disclosed information, because doing so does not nullify their HIPAA rights, and HIPAA provides no exceptions for responses. Even acknowledging that the reviewer was in fact your patient could, in some cases, be considered a violation.
Responding to good reviews can get you in trouble too, for the same reasons. In 2016, a physical therapy practice paid a $25,000 fine after it posted patient testimonials, “including full names and full-face photographic images to its website without obtaining valid, HIPAA-compliant authorizations.”
And by the way, most malpractice policies specifically exclude disciplinary fines and settlements from coverage.
All of that said,
- Ignore them. This is your best choice most of the time. Most negative reviews have minimal impact and simply do not deserve a response; responding may pour fuel on the fire. Besides, an occasional negative review actually lends credibility to a reviewing site and to the positive reviews posted on that site. Polls show that readers are suspicious of sites that contain only rave reviews. They assume such reviews have been “whitewashed” – or just fabricated.
- Solicit more reviews to that site. The more you can obtain, the less impact any complaints will have, since you know the overwhelming majority of your patients are happy with your care and will post a positive review if asked. Solicit them on your website, on social media, or in your email reminders. To be clear, you must encourage reviews from all patients, whether they have had a positive experience or not. If you invite only the satisfied ones, you are “filtering,” which can be perceived as false or deceptive advertising. (Google calls it “review-gating,” and according to their guidelines, if they catch you doing it they will remove all of your reviews.)
- Respond politely. In those rare cases where you feel you must respond, do so without acknowledging that the individual was a patient, or disclosing any information that may be linked to the patient. For example, you can say that you provide excellent and appropriate care, or describe your general policies. Be polite, professional, and sensitive to the patient’s position. Readers tend to respect and sympathize with a doctor who responds in a professional, respectful manner and does not trash the complainant in retaliation.
- Take the discussion offline. Sometimes the person posting the review is just frustrated and wants to be heard. In those cases, consider contacting the patient and offering to discuss their concerns privately. If you cannot resolve your differences, try to get the patient’s written permission to post a response to their review. If they refuse, you can explain that, thereby capturing the moral high ground.
If the review contains false or defamatory content, that’s a different situation entirely; you will probably need to consult your attorney.
Regardless of how you handle negative reviews, be sure to learn from them. Your critics, as the song goes, are not always evil – and not always wrong. Complaints give you a chance to review your office policies and procedures and your own conduct, identify weaknesses, and make changes as necessary. At the very least, the exercise will help you to avoid similar complaints in the future. Don’t let valuable opportunities like that pass you by.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at dermnews@frontlinemedcom.com.
In 2013, a California hospital paid $275,000 to settle claims that it violated the HIPAA privacy rule when it disclosed a patient’s health information in response to a negative online review. More recently, a Texas dental practice paid a substantial fine to the Department of Health & Human Services, which enforces HIPAA, after it responded to unfavorable Yelp reviews with patient names and details of their health conditions, treatment plans, and cost information. In addition to the fine, the practice agreed to 2 years of monitoring by HHS for compliance with HIPAA rules.
Most physicians have had the unpleasant experience of finding a negative online review from a disgruntled patient or family member. Some are justified, many are not; either way, your first impulse will often be to post a response – but that is almost always a bad idea. “Social media is not the place for providers to discuss a patient’s care,” an HHS official said in a statement issued about the dental practice case in 2016. “Doctors and dentists must think carefully about patient privacy before responding to online reviews.”
Any information that could be used to identify a patient is a HIPAA breach. This is true even if the patient has already disclosed information, because doing so does not nullify their HIPAA rights, and HIPAA provides no exceptions for responses. Even acknowledging that the reviewer was in fact your patient could, in some cases, be considered a violation.
Responding to good reviews can get you in trouble too, for the same reasons. In 2016, a physical therapy practice paid a $25,000 fine after it posted patient testimonials, “including full names and full-face photographic images to its website without obtaining valid, HIPAA-compliant authorizations.”
And by the way, most malpractice policies specifically exclude disciplinary fines and settlements from coverage.
All of that said,
- Ignore them. This is your best choice most of the time. Most negative reviews have minimal impact and simply do not deserve a response; responding may pour fuel on the fire. Besides, an occasional negative review actually lends credibility to a reviewing site and to the positive reviews posted on that site. Polls show that readers are suspicious of sites that contain only rave reviews. They assume such reviews have been “whitewashed” – or just fabricated.
- Solicit more reviews to that site. The more you can obtain, the less impact any complaints will have, since you know the overwhelming majority of your patients are happy with your care and will post a positive review if asked. Solicit them on your website, on social media, or in your email reminders. To be clear, you must encourage reviews from all patients, whether they have had a positive experience or not. If you invite only the satisfied ones, you are “filtering,” which can be perceived as false or deceptive advertising. (Google calls it “review-gating,” and according to their guidelines, if they catch you doing it they will remove all of your reviews.)
- Respond politely. In those rare cases where you feel you must respond, do so without acknowledging that the individual was a patient, or disclosing any information that may be linked to the patient. For example, you can say that you provide excellent and appropriate care, or describe your general policies. Be polite, professional, and sensitive to the patient’s position. Readers tend to respect and sympathize with a doctor who responds in a professional, respectful manner and does not trash the complainant in retaliation.
- Take the discussion offline. Sometimes the person posting the review is just frustrated and wants to be heard. In those cases, consider contacting the patient and offering to discuss their concerns privately. If you cannot resolve your differences, try to get the patient’s written permission to post a response to their review. If they refuse, you can explain that, thereby capturing the moral high ground.
If the review contains false or defamatory content, that’s a different situation entirely; you will probably need to consult your attorney.
Regardless of how you handle negative reviews, be sure to learn from them. Your critics, as the song goes, are not always evil – and not always wrong. Complaints give you a chance to review your office policies and procedures and your own conduct, identify weaknesses, and make changes as necessary. At the very least, the exercise will help you to avoid similar complaints in the future. Don’t let valuable opportunities like that pass you by.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at dermnews@frontlinemedcom.com.
CDC: Vaccinated? You don’t need a mask indoors
the CDC announced on May 13.
“Anyone who is fully vaccinated can participate in indoor and outdoor activities, large or small, without wearing a mask or physically distancing,” CDC director Rochelle Walensky, MD, said at a press briefing. “We have all longed for this moment when we can get back to some sense of normalcy.
“This is an exciting and powerful moment,” she added, “It could only happen because of the work from so many who made sure we had the rapid administration of three safe and effective vaccines.”
Dr. Walensky cited three large studies on the effectiveness of COVID-19 vaccines against the original virus and its variants. One study from Israel found the vaccine to be 97% effective against symptomatic infection.
Those who are symptomatic should still wear masks, Dr. Walensky said, and those who are immunocompromised should talk to their doctors for further guidance. The CDC still advises travelers to wear masks while on airplanes or trains.
The COVID-19 death rates are now the lowest they have been since April 2020.
A version of this article first appeared on Medscape.com.
the CDC announced on May 13.
“Anyone who is fully vaccinated can participate in indoor and outdoor activities, large or small, without wearing a mask or physically distancing,” CDC director Rochelle Walensky, MD, said at a press briefing. “We have all longed for this moment when we can get back to some sense of normalcy.
“This is an exciting and powerful moment,” she added, “It could only happen because of the work from so many who made sure we had the rapid administration of three safe and effective vaccines.”
Dr. Walensky cited three large studies on the effectiveness of COVID-19 vaccines against the original virus and its variants. One study from Israel found the vaccine to be 97% effective against symptomatic infection.
Those who are symptomatic should still wear masks, Dr. Walensky said, and those who are immunocompromised should talk to their doctors for further guidance. The CDC still advises travelers to wear masks while on airplanes or trains.
The COVID-19 death rates are now the lowest they have been since April 2020.
A version of this article first appeared on Medscape.com.
the CDC announced on May 13.
“Anyone who is fully vaccinated can participate in indoor and outdoor activities, large or small, without wearing a mask or physically distancing,” CDC director Rochelle Walensky, MD, said at a press briefing. “We have all longed for this moment when we can get back to some sense of normalcy.
“This is an exciting and powerful moment,” she added, “It could only happen because of the work from so many who made sure we had the rapid administration of three safe and effective vaccines.”
Dr. Walensky cited three large studies on the effectiveness of COVID-19 vaccines against the original virus and its variants. One study from Israel found the vaccine to be 97% effective against symptomatic infection.
Those who are symptomatic should still wear masks, Dr. Walensky said, and those who are immunocompromised should talk to their doctors for further guidance. The CDC still advises travelers to wear masks while on airplanes or trains.
The COVID-19 death rates are now the lowest they have been since April 2020.
A version of this article first appeared on Medscape.com.
An infant girl presents with a growing pink-red leg nodule
The history of a brownish to pink patch with color change and rapid growth within the first year combined with the exam findings, are suggestive of a tufted angioma, though the findings presented may be nonspecific.
A tufted angioma is a rare vascular tumor of infancy or early childhood, that is present at birth in approximately half of cases. It may initially present as a faint pink to brown plaque, but develops as a firm, red to violaceous nodule or plaque, usually with “lumpiness” or nodularity.1-3 Lesions usually are infiltrative with indistinct borders. They are named for their histologic appearance, with lobules of capillaries which appear as “tufts” in the dermis and subdermis with “cannonball” appearance, and are considered to be on a spectrum with another vascular tumor called kaposiform hemangioendothelioma (KHE).4 These vascular tumors can trigger Kasabach-Merritt syndrome, a disease process in which vascular tumors trap platelets and clotting factors, resulting in a life-threatening thrombocytopenia and consumptive coagulopathy with a high risk of bleeding and high-output heart failure.5
What’s the differential diagnosis?
The differential diagnosis of tufted angioma includes other potentially large vascular lesions including infantile hemangioma, congenital hemangioma, port-wine birth marks (capillary malformations), hemangioendotheliomas, and rhabdomyosarcomas.
Infantile hemangiomas (IH) are common vascular tumors of infancy seen in 4%-5% of infants that are characterized by a growth and involution phase. Classically, lesions can be absent or minimally evident at birth, becoming noticeable within the first months of life with a rapid growth phase and typical progression to bright red papules, nodules, or plaques. Deeper hemangiomas may appear more skin colored on the surface with a bluish coloration underneath. They are usually more discreet, with relatively defined borders. Diagnosis is typically clinical and many IHs self-resolve, albeit with residual findings including skin atrophy, scarring, and telangiectasia. Observation or topical timolol are first-line treatment options for more superficial lesions while systemic propranolol is the treatment of choice for deeper IHs or those resulting in possible airway or vision compromise.
Congenital hemangiomas (CH) are another type of vascular growth characterized by a solitary erythematous to violaceous plaque or nodule present at birth with overlying telangiectasia. CHs can be subdivided into categories including rapidly involuting (RICH), partially involuting (PICH), and noninvoluting (NICH). Diagnosis is usually clinical and, depending on the subtype, treatment can involve watchful waiting (for RICHs) or more active intervention such as pulse dye laser or surgical resection (for PICHs or NICHs). The growing nature of this patient’s mass makes a diagnosis of CH unlikely.
Port-wine birth mark, also known as nevus flammeus, is a vascular malformation that appears at birth as a nonpalpable irregular erythematous to violaceous macular plaque. Port-wine stains may be isolated birthmarks, or associated with Sturge-Weber syndrome, complex vascular malformations, or soft-tissue overgrowth. Klippel-Trenauny syndrome (KTS) describes capillary-venous malformations with limb overgrowth, with or without lymphatic malformations, and many are associated with somatic mutations in the PIK3CA gene. While KTS could be considered in this patient, the nodular appearance with lumpy texture and rapid growth makes a vascular tumor more likely.
Rhabdomyosarcoma is a malignancy of skeletal muscle lineage and the most common soft tissue tumor in pediatrics. Cutaneous rhabdomyosarcomas present as erythematous nodules, markedly firm, often “fixed” to deep tissue. A rapidly growing atypical, firm tumor of infancy should raise the consideration of rhabdomyosarcoma and imaging and biopsy are appropriate for evaluation.
What should the evaluation and management of this patient be?
Initial workup should include a complete blood count with platelet count as well as coagulation studies including D-dimer, fibrinogen, prothrombin time, and activated partial thromboplastin time, to assess for any thrombocytopenia or coagulopathy.6 Ultrasound and/or MRI may also be performed to determine lesion extent. While typical MRI findings might be suggestive of a tufted angioma or hemangioendothelioma, biopsy for histologic examination is usually the approach to diagnosis, which will demonstrate stereotypic round lobules of capillaries in a “tufted” distribution.2,7 Biopsy may be performed by a surgeon or dermatologist but bleeding at time of biopsy needs to be considered before moving forward with the procedure.
Tufted angiomas of early life may regress spontaneously, though lesions with symptoms, with functional significance, or associated with KHE may require therapy. Surgical excision is one option, but it may be difficult to execute given that these lesions often have poorly defined margins.1 Other treatment choices include but are not limited to aspirin, systemic corticosteroids, vincristine, interferon-alpha, embolization, and sirolimus.8 No specific expert-directed consensus guidelines exist for these lesions, and suspicion of this lesion should prompt urgent referral to a pediatric dermatologist. Concern for Kasabach-Merritt syndrome should trigger immediate referral for rapid evaluation and management.
Complete blood count with platelet count and coagulation studies were normal in our patient. This infant underwent biopsy to confirm the diagnosis of tufted angioma and MRI to determine lesion extent. The lesion slowly involuted spontaneously without recurrence.
Mr. Haft is a pediatric dermatology research associate in the division of pediatric and adolescent dermatology at the University of California, San Diego, and Rady Children’s Hospital, San Diego. He is MS4 at the University of Rochester, N.Y. Dr. Eichenfield is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego, and Rady Children’s Hospital, San Diego. Neither Mr. Haft nor Dr. Eichenfield have any relevant financial disclosures.
References
1. Herron MD et al. Pediatr Dermatol. 2002;19(5):394-401.
2. Jones EW and Orkin M. J Am Acad Dermatol. 1989;20(2 Pt 1):214-25.
3. Wong SN and Tay YK. Pediatr Dermatol. 2002;19(5):388-93.
4. Croteau SE and Gupta D. Semin Cutan Med Surg. 2016;35(3):147-52.
5. Kelly M. Pediatr Clin North Am. 2010;57(5):1085-9.
6. Osio A et al. Arch Dermatol. 2010;146(7):758-63.
7. Padilla RS et al. Am J Dermatopathol. 1987;9(4):292-300.
8. Liu XH et al. Int J Cancer. 2016;139(7):1658-66.
The history of a brownish to pink patch with color change and rapid growth within the first year combined with the exam findings, are suggestive of a tufted angioma, though the findings presented may be nonspecific.
A tufted angioma is a rare vascular tumor of infancy or early childhood, that is present at birth in approximately half of cases. It may initially present as a faint pink to brown plaque, but develops as a firm, red to violaceous nodule or plaque, usually with “lumpiness” or nodularity.1-3 Lesions usually are infiltrative with indistinct borders. They are named for their histologic appearance, with lobules of capillaries which appear as “tufts” in the dermis and subdermis with “cannonball” appearance, and are considered to be on a spectrum with another vascular tumor called kaposiform hemangioendothelioma (KHE).4 These vascular tumors can trigger Kasabach-Merritt syndrome, a disease process in which vascular tumors trap platelets and clotting factors, resulting in a life-threatening thrombocytopenia and consumptive coagulopathy with a high risk of bleeding and high-output heart failure.5
What’s the differential diagnosis?
The differential diagnosis of tufted angioma includes other potentially large vascular lesions including infantile hemangioma, congenital hemangioma, port-wine birth marks (capillary malformations), hemangioendotheliomas, and rhabdomyosarcomas.
Infantile hemangiomas (IH) are common vascular tumors of infancy seen in 4%-5% of infants that are characterized by a growth and involution phase. Classically, lesions can be absent or minimally evident at birth, becoming noticeable within the first months of life with a rapid growth phase and typical progression to bright red papules, nodules, or plaques. Deeper hemangiomas may appear more skin colored on the surface with a bluish coloration underneath. They are usually more discreet, with relatively defined borders. Diagnosis is typically clinical and many IHs self-resolve, albeit with residual findings including skin atrophy, scarring, and telangiectasia. Observation or topical timolol are first-line treatment options for more superficial lesions while systemic propranolol is the treatment of choice for deeper IHs or those resulting in possible airway or vision compromise.
Congenital hemangiomas (CH) are another type of vascular growth characterized by a solitary erythematous to violaceous plaque or nodule present at birth with overlying telangiectasia. CHs can be subdivided into categories including rapidly involuting (RICH), partially involuting (PICH), and noninvoluting (NICH). Diagnosis is usually clinical and, depending on the subtype, treatment can involve watchful waiting (for RICHs) or more active intervention such as pulse dye laser or surgical resection (for PICHs or NICHs). The growing nature of this patient’s mass makes a diagnosis of CH unlikely.
Port-wine birth mark, also known as nevus flammeus, is a vascular malformation that appears at birth as a nonpalpable irregular erythematous to violaceous macular plaque. Port-wine stains may be isolated birthmarks, or associated with Sturge-Weber syndrome, complex vascular malformations, or soft-tissue overgrowth. Klippel-Trenauny syndrome (KTS) describes capillary-venous malformations with limb overgrowth, with or without lymphatic malformations, and many are associated with somatic mutations in the PIK3CA gene. While KTS could be considered in this patient, the nodular appearance with lumpy texture and rapid growth makes a vascular tumor more likely.
Rhabdomyosarcoma is a malignancy of skeletal muscle lineage and the most common soft tissue tumor in pediatrics. Cutaneous rhabdomyosarcomas present as erythematous nodules, markedly firm, often “fixed” to deep tissue. A rapidly growing atypical, firm tumor of infancy should raise the consideration of rhabdomyosarcoma and imaging and biopsy are appropriate for evaluation.
What should the evaluation and management of this patient be?
Initial workup should include a complete blood count with platelet count as well as coagulation studies including D-dimer, fibrinogen, prothrombin time, and activated partial thromboplastin time, to assess for any thrombocytopenia or coagulopathy.6 Ultrasound and/or MRI may also be performed to determine lesion extent. While typical MRI findings might be suggestive of a tufted angioma or hemangioendothelioma, biopsy for histologic examination is usually the approach to diagnosis, which will demonstrate stereotypic round lobules of capillaries in a “tufted” distribution.2,7 Biopsy may be performed by a surgeon or dermatologist but bleeding at time of biopsy needs to be considered before moving forward with the procedure.
Tufted angiomas of early life may regress spontaneously, though lesions with symptoms, with functional significance, or associated with KHE may require therapy. Surgical excision is one option, but it may be difficult to execute given that these lesions often have poorly defined margins.1 Other treatment choices include but are not limited to aspirin, systemic corticosteroids, vincristine, interferon-alpha, embolization, and sirolimus.8 No specific expert-directed consensus guidelines exist for these lesions, and suspicion of this lesion should prompt urgent referral to a pediatric dermatologist. Concern for Kasabach-Merritt syndrome should trigger immediate referral for rapid evaluation and management.
Complete blood count with platelet count and coagulation studies were normal in our patient. This infant underwent biopsy to confirm the diagnosis of tufted angioma and MRI to determine lesion extent. The lesion slowly involuted spontaneously without recurrence.
Mr. Haft is a pediatric dermatology research associate in the division of pediatric and adolescent dermatology at the University of California, San Diego, and Rady Children’s Hospital, San Diego. He is MS4 at the University of Rochester, N.Y. Dr. Eichenfield is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego, and Rady Children’s Hospital, San Diego. Neither Mr. Haft nor Dr. Eichenfield have any relevant financial disclosures.
References
1. Herron MD et al. Pediatr Dermatol. 2002;19(5):394-401.
2. Jones EW and Orkin M. J Am Acad Dermatol. 1989;20(2 Pt 1):214-25.
3. Wong SN and Tay YK. Pediatr Dermatol. 2002;19(5):388-93.
4. Croteau SE and Gupta D. Semin Cutan Med Surg. 2016;35(3):147-52.
5. Kelly M. Pediatr Clin North Am. 2010;57(5):1085-9.
6. Osio A et al. Arch Dermatol. 2010;146(7):758-63.
7. Padilla RS et al. Am J Dermatopathol. 1987;9(4):292-300.
8. Liu XH et al. Int J Cancer. 2016;139(7):1658-66.
The history of a brownish to pink patch with color change and rapid growth within the first year combined with the exam findings, are suggestive of a tufted angioma, though the findings presented may be nonspecific.
A tufted angioma is a rare vascular tumor of infancy or early childhood, that is present at birth in approximately half of cases. It may initially present as a faint pink to brown plaque, but develops as a firm, red to violaceous nodule or plaque, usually with “lumpiness” or nodularity.1-3 Lesions usually are infiltrative with indistinct borders. They are named for their histologic appearance, with lobules of capillaries which appear as “tufts” in the dermis and subdermis with “cannonball” appearance, and are considered to be on a spectrum with another vascular tumor called kaposiform hemangioendothelioma (KHE).4 These vascular tumors can trigger Kasabach-Merritt syndrome, a disease process in which vascular tumors trap platelets and clotting factors, resulting in a life-threatening thrombocytopenia and consumptive coagulopathy with a high risk of bleeding and high-output heart failure.5
What’s the differential diagnosis?
The differential diagnosis of tufted angioma includes other potentially large vascular lesions including infantile hemangioma, congenital hemangioma, port-wine birth marks (capillary malformations), hemangioendotheliomas, and rhabdomyosarcomas.
Infantile hemangiomas (IH) are common vascular tumors of infancy seen in 4%-5% of infants that are characterized by a growth and involution phase. Classically, lesions can be absent or minimally evident at birth, becoming noticeable within the first months of life with a rapid growth phase and typical progression to bright red papules, nodules, or plaques. Deeper hemangiomas may appear more skin colored on the surface with a bluish coloration underneath. They are usually more discreet, with relatively defined borders. Diagnosis is typically clinical and many IHs self-resolve, albeit with residual findings including skin atrophy, scarring, and telangiectasia. Observation or topical timolol are first-line treatment options for more superficial lesions while systemic propranolol is the treatment of choice for deeper IHs or those resulting in possible airway or vision compromise.
Congenital hemangiomas (CH) are another type of vascular growth characterized by a solitary erythematous to violaceous plaque or nodule present at birth with overlying telangiectasia. CHs can be subdivided into categories including rapidly involuting (RICH), partially involuting (PICH), and noninvoluting (NICH). Diagnosis is usually clinical and, depending on the subtype, treatment can involve watchful waiting (for RICHs) or more active intervention such as pulse dye laser or surgical resection (for PICHs or NICHs). The growing nature of this patient’s mass makes a diagnosis of CH unlikely.
Port-wine birth mark, also known as nevus flammeus, is a vascular malformation that appears at birth as a nonpalpable irregular erythematous to violaceous macular plaque. Port-wine stains may be isolated birthmarks, or associated with Sturge-Weber syndrome, complex vascular malformations, or soft-tissue overgrowth. Klippel-Trenauny syndrome (KTS) describes capillary-venous malformations with limb overgrowth, with or without lymphatic malformations, and many are associated with somatic mutations in the PIK3CA gene. While KTS could be considered in this patient, the nodular appearance with lumpy texture and rapid growth makes a vascular tumor more likely.
Rhabdomyosarcoma is a malignancy of skeletal muscle lineage and the most common soft tissue tumor in pediatrics. Cutaneous rhabdomyosarcomas present as erythematous nodules, markedly firm, often “fixed” to deep tissue. A rapidly growing atypical, firm tumor of infancy should raise the consideration of rhabdomyosarcoma and imaging and biopsy are appropriate for evaluation.
What should the evaluation and management of this patient be?
Initial workup should include a complete blood count with platelet count as well as coagulation studies including D-dimer, fibrinogen, prothrombin time, and activated partial thromboplastin time, to assess for any thrombocytopenia or coagulopathy.6 Ultrasound and/or MRI may also be performed to determine lesion extent. While typical MRI findings might be suggestive of a tufted angioma or hemangioendothelioma, biopsy for histologic examination is usually the approach to diagnosis, which will demonstrate stereotypic round lobules of capillaries in a “tufted” distribution.2,7 Biopsy may be performed by a surgeon or dermatologist but bleeding at time of biopsy needs to be considered before moving forward with the procedure.
Tufted angiomas of early life may regress spontaneously, though lesions with symptoms, with functional significance, or associated with KHE may require therapy. Surgical excision is one option, but it may be difficult to execute given that these lesions often have poorly defined margins.1 Other treatment choices include but are not limited to aspirin, systemic corticosteroids, vincristine, interferon-alpha, embolization, and sirolimus.8 No specific expert-directed consensus guidelines exist for these lesions, and suspicion of this lesion should prompt urgent referral to a pediatric dermatologist. Concern for Kasabach-Merritt syndrome should trigger immediate referral for rapid evaluation and management.
Complete blood count with platelet count and coagulation studies were normal in our patient. This infant underwent biopsy to confirm the diagnosis of tufted angioma and MRI to determine lesion extent. The lesion slowly involuted spontaneously without recurrence.
Mr. Haft is a pediatric dermatology research associate in the division of pediatric and adolescent dermatology at the University of California, San Diego, and Rady Children’s Hospital, San Diego. He is MS4 at the University of Rochester, N.Y. Dr. Eichenfield is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego, and Rady Children’s Hospital, San Diego. Neither Mr. Haft nor Dr. Eichenfield have any relevant financial disclosures.
References
1. Herron MD et al. Pediatr Dermatol. 2002;19(5):394-401.
2. Jones EW and Orkin M. J Am Acad Dermatol. 1989;20(2 Pt 1):214-25.
3. Wong SN and Tay YK. Pediatr Dermatol. 2002;19(5):388-93.
4. Croteau SE and Gupta D. Semin Cutan Med Surg. 2016;35(3):147-52.
5. Kelly M. Pediatr Clin North Am. 2010;57(5):1085-9.
6. Osio A et al. Arch Dermatol. 2010;146(7):758-63.
7. Padilla RS et al. Am J Dermatopathol. 1987;9(4):292-300.
8. Liu XH et al. Int J Cancer. 2016;139(7):1658-66.
On physical exam, you see an infant with a mass of the left lower extremity. Close examination reveals an approximately 7 cm x 8 cm poorly defined mass with overlying central erythematous to violaceous color of the left anterior upper leg with a lumpy texture. The lesion is moderately firm and mildly tender on palpation.
New guideline provides recommendations on reconstruction after skin cancer resection
You’ve successfully resected a skin cancer lesion, leaving clear margins. Now what?
That’s
The guideline – a joint effort of the American Society of Plastic Surgeons, American Society for Dermatologic Surgery, American Academy of Dermatology, American Academy of Facial Plastic and Reconstructive Surgery, American Academy of Otolaryngology – Head and Neck Surgery Foundation, American College of Mohs Surgery, American Society for Mohs Surgery, and American Society of Ophthalmic Plastic and Reconstructive Surgery – was published online in the Journal of the American Academy of Dermatology.
From the outset, the panel members realized that to keep the guideline manageable they had to limit recommendations to the practice of reconstruction defined as “cutaneous closure that requires a flap, graft, or tissue rearrangement.”
Other wound closure methods, such as secondary intention healing; simple closures; and complex closures that do not involve flaps, grafts, muscle, or bone, were not covered in the recommendations.
As with similar guidelines, the developers selected seven clinical questions to be addressed, and attempted to find consensus through literature searches, appraisal of the evidence, grading of recommendations, peer review, and public comment.
“We had a very heterogeneous set of things that we were trying to comment on, so we had to keep things somewhat generic,” lead author Andrew Chen, MD, chief of the division of plastic surgery, at the University of Connecticut Health Center, Farmington, said in an interview.
“Skin cancer and reconstruction affect different body areas and areas of different sizes. When we were creating the guidelines, we had to tailor the questions we could ask based on things that would make sense to answer, because obviously we couldn’t ask a question such as: ‘What’s better, a skin graft or a flap?’ Well, there are some things you can’t put a skin graft on – it won’t last, so we couldn’t ask that kind of question,” Dr. Chen said.
Curtis Cetrulo, MD, a plastic and reconstructive surgeon at Massachusetts General Hospital, Boston, who was not involved in the guideline process, said in an interview that the broad recommendations are in keeping with his practice and experience. He also acknowledged, however, the difficulty in creating a guideline that covers the complexity and heterogeneity of reconstructive surgery.
“These are generally good recommendations, but they’re recommendations only, with generally weak levels of evidence. What we really need are clinical trials that can give us definitive answers to some of these questions,” he said.
Recommendations
The seven key recommendations, based on the clinical questions raised, are summarized below:
- Delayed (asynchronous) reconstruction is acceptable. Although the quality of the evidence is low and the recommendations are listed as an option, the guideline authors said that depending on the situation, reconstruction can be performed either immediately after resection or delayed by days, weeks, “or even months.”
- Systemic antibiotics should not be routinely prescribed in the interim between resection and reconstruction in adults. Here too, the evidence is low and the recommendation strength is weak, but in “the absence of data showing convincing benefits, systemic antibiotic therapy does not appear necessary or desirable in most cases when there is an interval between cancer resection and reconstruction,” the work group wrote.
- Clinicians may administer perioperative systemic antibiotics in a facility-based setting for adults undergoing reconstruction (3a), but antibiotics should not be routinely prescribed in an office-based setting (3b). The rationale for these recommendations, supported by a moderate level of evidence, is that the risk of surgical-site infection is generally higher in facilities, compared with an office-based setting. Patients who undergo reconstruction in hospitals or surgical centers are more likely to have complex reconstructions or have risks that may make them suitable candidates for antibiotics, but patients in office-based setting may often be spared from the additional costs, side effects, and possible drug interactions from antibiotic use. “There is no evidence in either setting that long-term antibiotic prophylaxis provides infection risk reduction, compared with short-term prophylaxis,” the guideline working group wrote.
- Continue anticoagulant, antithrombotic, and antiplatelet medications for adult patients undergoing reconstruction after skin cancer resection in the office-based setting (4a), and in the facility-based setting should coordinate with the physician managing anticoagulation before modifying the medication prior to surgery (4b). Evidence quality and recommendation strength are both moderate.
- The guideline authors recommend against routine prescription of narcotics as first-line treatment for pain in adults undergoing skin reconstruction (5a), favoring instead acetaminophen and NSAIDs as first-line therapy (5b). Evidence quality and recommendation strength are both moderate.
- In the absence of standardized protocols for the management of pain medications, oral antibiotics, and/or anticoagulants in the perioperative period, clinicians should discuss possible approaches with adult patients. “Educating patients about their perioperative treatment through discussion of treatment strategies may help alleviate anxiety, improve communication, increase patient satisfaction, and maximize patient compliance with the postoperative orders,” the guideline authors wrote.
- The authors suggest that adult patients may be offered follow-up assessments to discuss functional and cosmetic outcomes. “The return of the patient for follow-up visits is an excellent opportunity to better understand and measure these outcomes, improve patient-physician communication, and foster quality improvement. Postoperative follow-up can lead to increased communication between the patient and physician, thereby empowering patients to comment on satisfaction and other important outcomes measures,” they wrote.
What’s next
The guideline developers acknowledged that data are limited regarding reconstructive surgery following skin cancer resection, and that higher-quality studies would help to improve future guidelines. Dr. Chen said that greater use of prospective surgical databases and more systematic collection of patient-reported outcomes could inform further efforts.
The guideline development process was supported by the various groups represented. Dr. Chen and Dr. Cetrulo reported no relevant disclosures.
You’ve successfully resected a skin cancer lesion, leaving clear margins. Now what?
That’s
The guideline – a joint effort of the American Society of Plastic Surgeons, American Society for Dermatologic Surgery, American Academy of Dermatology, American Academy of Facial Plastic and Reconstructive Surgery, American Academy of Otolaryngology – Head and Neck Surgery Foundation, American College of Mohs Surgery, American Society for Mohs Surgery, and American Society of Ophthalmic Plastic and Reconstructive Surgery – was published online in the Journal of the American Academy of Dermatology.
From the outset, the panel members realized that to keep the guideline manageable they had to limit recommendations to the practice of reconstruction defined as “cutaneous closure that requires a flap, graft, or tissue rearrangement.”
Other wound closure methods, such as secondary intention healing; simple closures; and complex closures that do not involve flaps, grafts, muscle, or bone, were not covered in the recommendations.
As with similar guidelines, the developers selected seven clinical questions to be addressed, and attempted to find consensus through literature searches, appraisal of the evidence, grading of recommendations, peer review, and public comment.
“We had a very heterogeneous set of things that we were trying to comment on, so we had to keep things somewhat generic,” lead author Andrew Chen, MD, chief of the division of plastic surgery, at the University of Connecticut Health Center, Farmington, said in an interview.
“Skin cancer and reconstruction affect different body areas and areas of different sizes. When we were creating the guidelines, we had to tailor the questions we could ask based on things that would make sense to answer, because obviously we couldn’t ask a question such as: ‘What’s better, a skin graft or a flap?’ Well, there are some things you can’t put a skin graft on – it won’t last, so we couldn’t ask that kind of question,” Dr. Chen said.
Curtis Cetrulo, MD, a plastic and reconstructive surgeon at Massachusetts General Hospital, Boston, who was not involved in the guideline process, said in an interview that the broad recommendations are in keeping with his practice and experience. He also acknowledged, however, the difficulty in creating a guideline that covers the complexity and heterogeneity of reconstructive surgery.
“These are generally good recommendations, but they’re recommendations only, with generally weak levels of evidence. What we really need are clinical trials that can give us definitive answers to some of these questions,” he said.
Recommendations
The seven key recommendations, based on the clinical questions raised, are summarized below:
- Delayed (asynchronous) reconstruction is acceptable. Although the quality of the evidence is low and the recommendations are listed as an option, the guideline authors said that depending on the situation, reconstruction can be performed either immediately after resection or delayed by days, weeks, “or even months.”
- Systemic antibiotics should not be routinely prescribed in the interim between resection and reconstruction in adults. Here too, the evidence is low and the recommendation strength is weak, but in “the absence of data showing convincing benefits, systemic antibiotic therapy does not appear necessary or desirable in most cases when there is an interval between cancer resection and reconstruction,” the work group wrote.
- Clinicians may administer perioperative systemic antibiotics in a facility-based setting for adults undergoing reconstruction (3a), but antibiotics should not be routinely prescribed in an office-based setting (3b). The rationale for these recommendations, supported by a moderate level of evidence, is that the risk of surgical-site infection is generally higher in facilities, compared with an office-based setting. Patients who undergo reconstruction in hospitals or surgical centers are more likely to have complex reconstructions or have risks that may make them suitable candidates for antibiotics, but patients in office-based setting may often be spared from the additional costs, side effects, and possible drug interactions from antibiotic use. “There is no evidence in either setting that long-term antibiotic prophylaxis provides infection risk reduction, compared with short-term prophylaxis,” the guideline working group wrote.
- Continue anticoagulant, antithrombotic, and antiplatelet medications for adult patients undergoing reconstruction after skin cancer resection in the office-based setting (4a), and in the facility-based setting should coordinate with the physician managing anticoagulation before modifying the medication prior to surgery (4b). Evidence quality and recommendation strength are both moderate.
- The guideline authors recommend against routine prescription of narcotics as first-line treatment for pain in adults undergoing skin reconstruction (5a), favoring instead acetaminophen and NSAIDs as first-line therapy (5b). Evidence quality and recommendation strength are both moderate.
- In the absence of standardized protocols for the management of pain medications, oral antibiotics, and/or anticoagulants in the perioperative period, clinicians should discuss possible approaches with adult patients. “Educating patients about their perioperative treatment through discussion of treatment strategies may help alleviate anxiety, improve communication, increase patient satisfaction, and maximize patient compliance with the postoperative orders,” the guideline authors wrote.
- The authors suggest that adult patients may be offered follow-up assessments to discuss functional and cosmetic outcomes. “The return of the patient for follow-up visits is an excellent opportunity to better understand and measure these outcomes, improve patient-physician communication, and foster quality improvement. Postoperative follow-up can lead to increased communication between the patient and physician, thereby empowering patients to comment on satisfaction and other important outcomes measures,” they wrote.
What’s next
The guideline developers acknowledged that data are limited regarding reconstructive surgery following skin cancer resection, and that higher-quality studies would help to improve future guidelines. Dr. Chen said that greater use of prospective surgical databases and more systematic collection of patient-reported outcomes could inform further efforts.
The guideline development process was supported by the various groups represented. Dr. Chen and Dr. Cetrulo reported no relevant disclosures.
You’ve successfully resected a skin cancer lesion, leaving clear margins. Now what?
That’s
The guideline – a joint effort of the American Society of Plastic Surgeons, American Society for Dermatologic Surgery, American Academy of Dermatology, American Academy of Facial Plastic and Reconstructive Surgery, American Academy of Otolaryngology – Head and Neck Surgery Foundation, American College of Mohs Surgery, American Society for Mohs Surgery, and American Society of Ophthalmic Plastic and Reconstructive Surgery – was published online in the Journal of the American Academy of Dermatology.
From the outset, the panel members realized that to keep the guideline manageable they had to limit recommendations to the practice of reconstruction defined as “cutaneous closure that requires a flap, graft, or tissue rearrangement.”
Other wound closure methods, such as secondary intention healing; simple closures; and complex closures that do not involve flaps, grafts, muscle, or bone, were not covered in the recommendations.
As with similar guidelines, the developers selected seven clinical questions to be addressed, and attempted to find consensus through literature searches, appraisal of the evidence, grading of recommendations, peer review, and public comment.
“We had a very heterogeneous set of things that we were trying to comment on, so we had to keep things somewhat generic,” lead author Andrew Chen, MD, chief of the division of plastic surgery, at the University of Connecticut Health Center, Farmington, said in an interview.
“Skin cancer and reconstruction affect different body areas and areas of different sizes. When we were creating the guidelines, we had to tailor the questions we could ask based on things that would make sense to answer, because obviously we couldn’t ask a question such as: ‘What’s better, a skin graft or a flap?’ Well, there are some things you can’t put a skin graft on – it won’t last, so we couldn’t ask that kind of question,” Dr. Chen said.
Curtis Cetrulo, MD, a plastic and reconstructive surgeon at Massachusetts General Hospital, Boston, who was not involved in the guideline process, said in an interview that the broad recommendations are in keeping with his practice and experience. He also acknowledged, however, the difficulty in creating a guideline that covers the complexity and heterogeneity of reconstructive surgery.
“These are generally good recommendations, but they’re recommendations only, with generally weak levels of evidence. What we really need are clinical trials that can give us definitive answers to some of these questions,” he said.
Recommendations
The seven key recommendations, based on the clinical questions raised, are summarized below:
- Delayed (asynchronous) reconstruction is acceptable. Although the quality of the evidence is low and the recommendations are listed as an option, the guideline authors said that depending on the situation, reconstruction can be performed either immediately after resection or delayed by days, weeks, “or even months.”
- Systemic antibiotics should not be routinely prescribed in the interim between resection and reconstruction in adults. Here too, the evidence is low and the recommendation strength is weak, but in “the absence of data showing convincing benefits, systemic antibiotic therapy does not appear necessary or desirable in most cases when there is an interval between cancer resection and reconstruction,” the work group wrote.
- Clinicians may administer perioperative systemic antibiotics in a facility-based setting for adults undergoing reconstruction (3a), but antibiotics should not be routinely prescribed in an office-based setting (3b). The rationale for these recommendations, supported by a moderate level of evidence, is that the risk of surgical-site infection is generally higher in facilities, compared with an office-based setting. Patients who undergo reconstruction in hospitals or surgical centers are more likely to have complex reconstructions or have risks that may make them suitable candidates for antibiotics, but patients in office-based setting may often be spared from the additional costs, side effects, and possible drug interactions from antibiotic use. “There is no evidence in either setting that long-term antibiotic prophylaxis provides infection risk reduction, compared with short-term prophylaxis,” the guideline working group wrote.
- Continue anticoagulant, antithrombotic, and antiplatelet medications for adult patients undergoing reconstruction after skin cancer resection in the office-based setting (4a), and in the facility-based setting should coordinate with the physician managing anticoagulation before modifying the medication prior to surgery (4b). Evidence quality and recommendation strength are both moderate.
- The guideline authors recommend against routine prescription of narcotics as first-line treatment for pain in adults undergoing skin reconstruction (5a), favoring instead acetaminophen and NSAIDs as first-line therapy (5b). Evidence quality and recommendation strength are both moderate.
- In the absence of standardized protocols for the management of pain medications, oral antibiotics, and/or anticoagulants in the perioperative period, clinicians should discuss possible approaches with adult patients. “Educating patients about their perioperative treatment through discussion of treatment strategies may help alleviate anxiety, improve communication, increase patient satisfaction, and maximize patient compliance with the postoperative orders,” the guideline authors wrote.
- The authors suggest that adult patients may be offered follow-up assessments to discuss functional and cosmetic outcomes. “The return of the patient for follow-up visits is an excellent opportunity to better understand and measure these outcomes, improve patient-physician communication, and foster quality improvement. Postoperative follow-up can lead to increased communication between the patient and physician, thereby empowering patients to comment on satisfaction and other important outcomes measures,” they wrote.
What’s next
The guideline developers acknowledged that data are limited regarding reconstructive surgery following skin cancer resection, and that higher-quality studies would help to improve future guidelines. Dr. Chen said that greater use of prospective surgical databases and more systematic collection of patient-reported outcomes could inform further efforts.
The guideline development process was supported by the various groups represented. Dr. Chen and Dr. Cetrulo reported no relevant disclosures.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY