User login
Formerly Skin & Allergy News
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')]
The leading independent newspaper covering dermatology news and commentary.
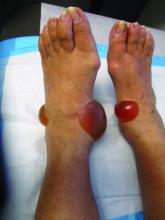
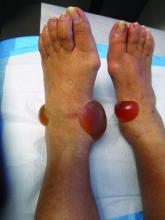
A 67-year-old White woman presented with 2 weeks of bullae on her lower feet
Bullous arthropod assault
Insect-bite reactions are commonly seen in dermatology practice. Most often, they present as pruritic papules. Vesicles and bullae can be seen as well but are less common. Flea bites are the most likely to cause blisters.1 Lesions may be grouped or in a linear pattern. Children tend to have more severe reactions than adults. Body temperature and odor may make some people more susceptible than others to bites. Of note, patients with chronic lymphocytic leukemia tend to have more severe, bullous reactions.2 The differential diagnosis includes bullous pemphigoid, bullous impetigo, bullous tinea, bullous fixed drug, and bullous diabeticorum.
In general, bullous arthropod reactions begin as intraepidermal vesicles that can progress to subepidermal blisters. Eosinophils can be present. Flame figures are often seen in patients with chronic lymphocytic leukemia.3 Histopathology in this patient revealed a subepidermal vesicular dermatitis with minimal inflammation. Periodic acid–Schiff (PAS) stain was negative. Direct immunofluorescence was negative for IgG, C3, IgA, IgM, and fibrinogen. Of note, systemic steroids may alter histologic and immunologic findings.
Bullous pemphigoid is an autoimmune blistering disorder where patients develop widespread tense bullae. Histopathology revealed a subepidermal blister with numerous eosinophils. Direct immunofluorescence study of perilesional skin showed linear IgG and C3 deposits at the basal membrane level. Systemic steroids, tetracyclines, and immunosuppressive medications are a mainstay of treatment. In bullous impetigo, the toxin of Staphylococcus aureus causes blister formation. It is treated with antistaphylococcal antibiotics. Bullous tinea reveals hyphae with PAS staining. Topical or systemic antifungals are used for treatment.
In severe cases, systemic steroids can be used as well. Bacterial culture was negative in this patient. The patient was treated with 1 week of oral prednisone prior to biopsy and topical betamethasone ointment. Her lesions subsequently resolved with no recurrence.
This case and photo were submitted by Brooke Resh Sateesh, MD, San Diego Family Dermatology.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
References
1-3. “Dermatology” 2nd ed. (Maryland Heights, Mo.: Mosby, 2008).
Bullous arthropod assault
Insect-bite reactions are commonly seen in dermatology practice. Most often, they present as pruritic papules. Vesicles and bullae can be seen as well but are less common. Flea bites are the most likely to cause blisters.1 Lesions may be grouped or in a linear pattern. Children tend to have more severe reactions than adults. Body temperature and odor may make some people more susceptible than others to bites. Of note, patients with chronic lymphocytic leukemia tend to have more severe, bullous reactions.2 The differential diagnosis includes bullous pemphigoid, bullous impetigo, bullous tinea, bullous fixed drug, and bullous diabeticorum.
In general, bullous arthropod reactions begin as intraepidermal vesicles that can progress to subepidermal blisters. Eosinophils can be present. Flame figures are often seen in patients with chronic lymphocytic leukemia.3 Histopathology in this patient revealed a subepidermal vesicular dermatitis with minimal inflammation. Periodic acid–Schiff (PAS) stain was negative. Direct immunofluorescence was negative for IgG, C3, IgA, IgM, and fibrinogen. Of note, systemic steroids may alter histologic and immunologic findings.
Bullous pemphigoid is an autoimmune blistering disorder where patients develop widespread tense bullae. Histopathology revealed a subepidermal blister with numerous eosinophils. Direct immunofluorescence study of perilesional skin showed linear IgG and C3 deposits at the basal membrane level. Systemic steroids, tetracyclines, and immunosuppressive medications are a mainstay of treatment. In bullous impetigo, the toxin of Staphylococcus aureus causes blister formation. It is treated with antistaphylococcal antibiotics. Bullous tinea reveals hyphae with PAS staining. Topical or systemic antifungals are used for treatment.
In severe cases, systemic steroids can be used as well. Bacterial culture was negative in this patient. The patient was treated with 1 week of oral prednisone prior to biopsy and topical betamethasone ointment. Her lesions subsequently resolved with no recurrence.
This case and photo were submitted by Brooke Resh Sateesh, MD, San Diego Family Dermatology.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
References
1-3. “Dermatology” 2nd ed. (Maryland Heights, Mo.: Mosby, 2008).
Bullous arthropod assault
Insect-bite reactions are commonly seen in dermatology practice. Most often, they present as pruritic papules. Vesicles and bullae can be seen as well but are less common. Flea bites are the most likely to cause blisters.1 Lesions may be grouped or in a linear pattern. Children tend to have more severe reactions than adults. Body temperature and odor may make some people more susceptible than others to bites. Of note, patients with chronic lymphocytic leukemia tend to have more severe, bullous reactions.2 The differential diagnosis includes bullous pemphigoid, bullous impetigo, bullous tinea, bullous fixed drug, and bullous diabeticorum.
In general, bullous arthropod reactions begin as intraepidermal vesicles that can progress to subepidermal blisters. Eosinophils can be present. Flame figures are often seen in patients with chronic lymphocytic leukemia.3 Histopathology in this patient revealed a subepidermal vesicular dermatitis with minimal inflammation. Periodic acid–Schiff (PAS) stain was negative. Direct immunofluorescence was negative for IgG, C3, IgA, IgM, and fibrinogen. Of note, systemic steroids may alter histologic and immunologic findings.
Bullous pemphigoid is an autoimmune blistering disorder where patients develop widespread tense bullae. Histopathology revealed a subepidermal blister with numerous eosinophils. Direct immunofluorescence study of perilesional skin showed linear IgG and C3 deposits at the basal membrane level. Systemic steroids, tetracyclines, and immunosuppressive medications are a mainstay of treatment. In bullous impetigo, the toxin of Staphylococcus aureus causes blister formation. It is treated with antistaphylococcal antibiotics. Bullous tinea reveals hyphae with PAS staining. Topical or systemic antifungals are used for treatment.
In severe cases, systemic steroids can be used as well. Bacterial culture was negative in this patient. The patient was treated with 1 week of oral prednisone prior to biopsy and topical betamethasone ointment. Her lesions subsequently resolved with no recurrence.
This case and photo were submitted by Brooke Resh Sateesh, MD, San Diego Family Dermatology.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
References
1-3. “Dermatology” 2nd ed. (Maryland Heights, Mo.: Mosby, 2008).
Feds to states: Give COVID-19 vaccine to 65+ and those with comorbidities
Federal health officials are urging states to vaccinate all Americans over age 65 and those aged 16-64 who have a documented underlying health condition that makes them more vulnerable to COVID-19.
U.S. Department of Health and Human Services (HHS) Secretary Alex Azar and Centers for Disease Control and Prevention Director Robert Redfield, MD, made the recommendation in a briefing with reporters on Jan. 12, saying that the current vaccine supply was sufficient to meet demand for the next phase of immunization as recommended by the CDC’s Advisory Committee on Immunization Practices.
“We are ready for a transition that we outlined last September in the playbook we sent to states,” Mr. Azar said. Both he and U.S. Army General Gustave F. Perna, chief operations officer for Operation Warp Speed, said that confidence in the distribution system had led to the decision to urge wider access.
The federal government will also increase the number of sites eligible to receive vaccine – including some 13,000 federally qualified community health centers – and will not keep doses in reserve as insurance against issues that might prevent people from receiving a second dose on a timely basis.
“We don’t need to hold back reserve doses,” Mr. Azar said, noting that if there were any “glitches in production” the federal government would move to fulfill obligations for second doses first and delay initial doses.
Azar: Use it or lose it
In a move that is sure to generate pushback, Mr. Azar said that states that don’t quickly administer vaccines will receive fewer doses in the future. That policy will not go into effect until later in February, which leaves open the possibility that it could be reversed by the incoming Biden administration.
“We have too much vaccine sitting in freezers at hospitals with hospitals not using it,” said Mr. Azar, who also blamed the slow administration process on a reporting lag and states being what he called “overly prescriptive” in who has been eligible to receive a shot.
“I would rather have people working to get appointments to get vaccinated than having vaccine going to waste sitting in freezers,” he told reporters.
Mr. Azar had already been pushing for broader vaccination, telling states to do so in an Operation Warp Speed briefing on Jan. 6. At that briefing, he also said that the federal government would be stepping up vaccination through an “early launch” of a federal partnership with 19 pharmacy chains, which will let states allocate vaccines directly to some 40,000 pharmacy sites.
Gen. Perna said during the Jan. 12 briefing that the aim is to further expand that to some 70,000 locations total.
The CDC reported that as of Jan. 11 some 25.4 million doses have been distributed, with 8.9 million administered. An additional 4.2 million doses were distributed to long-term care facilities, and 937,000 residents and staff have received a dose.
“Pace of administration”
Alaska, Connecticut, North Dakota, South Dakota, the District of Columbia, West Virginia, and the Northern Mariana Islands have administered the most vaccines per capita, according to the CDC. But even these locations have immunized only 4%-5% of their populations, the New York Times reports. At the bottom: Alabama, Arizona, Arkansas, Georgia, Mississippi, and South Carolina.
The federal government can encourage but not require states to move on to new phases of vaccination.
“States ultimately determine how they will proceed with vaccination,” said Marcus Plescia, MD, MPH, chief medical officer for the Association of State and Territorial Health Officials. “Most will be cautious about assuring there are doses for those needing a second dose,” he said in an interview.
Dr. Plescia said that ensuring a second dose is available is especially important for health care workers “who need to be confident that they are protected and not inadvertently transmitting the disease themselves.”
He added that “once we reach a steady state of supply and administration, the rate-limiting factor will be supply of vaccine.”
That supply could now be threatened if states don’t comply with a just-announced federal action that will change how doses are allocated.
Beginning in late February, vaccine allocations to states will be based on “the pace of administration reported by states,” and the size of the 65-and-older population, said Mr. Azar, who has previously criticized New York Governor Andrew Cuomo for fining hospitals that didn’t use up vaccine supply within a week.
“This new system gives states a strong incentive to ensure that all vaccinations are being promptly reported, which they currently are not,” he said.
Currently, allocations are based on a state’s or territory’s population.
Prepandemic, states were required to report vaccinations within 30 days. Since COVID-19 vaccines became available, the CDC has required reporting of shots within 72 hours.
Dr. Redfield said the requirement has caused some difficulty, and that the CDC is investigating why some states have reported using only 15% of doses while others have used 80%.
States have been scrambling to ramp up vaccinations.
Just ahead of the federal briefing, Gov. Cuomo tweeted that New York would be opening up vaccinations to anyone older than 65.
The Associated Press is reporting that some states have started mass vaccination sites.
Arizona has begun operating a 24/7 appointment-only vaccination program at State Farm Stadium outside of Phoenix, with the aim of immunizing 6,000 people each day, according to local radio station KJZZ.
California and Florida have also taken steps to use stadiums, while Michigan, New Jersey, New York, and Texas will use convention centers and fairgrounds, Axios has reported.
In Florida, Palm Beach County Health Director Alina Alonso, MD, told county commissioners on Jan. 12 that there isn’t enough vaccine to meet demand, WPTV reported. “We need to realize that there’s a shortage of vaccine. So it’s not the plan, it’s not our ability to do it. It’s simply supply and demand at this point,” Dr. Alonso said, according to the TV station report.
A version of this article first appeared on Medscape.com.
Federal health officials are urging states to vaccinate all Americans over age 65 and those aged 16-64 who have a documented underlying health condition that makes them more vulnerable to COVID-19.
U.S. Department of Health and Human Services (HHS) Secretary Alex Azar and Centers for Disease Control and Prevention Director Robert Redfield, MD, made the recommendation in a briefing with reporters on Jan. 12, saying that the current vaccine supply was sufficient to meet demand for the next phase of immunization as recommended by the CDC’s Advisory Committee on Immunization Practices.
“We are ready for a transition that we outlined last September in the playbook we sent to states,” Mr. Azar said. Both he and U.S. Army General Gustave F. Perna, chief operations officer for Operation Warp Speed, said that confidence in the distribution system had led to the decision to urge wider access.
The federal government will also increase the number of sites eligible to receive vaccine – including some 13,000 federally qualified community health centers – and will not keep doses in reserve as insurance against issues that might prevent people from receiving a second dose on a timely basis.
“We don’t need to hold back reserve doses,” Mr. Azar said, noting that if there were any “glitches in production” the federal government would move to fulfill obligations for second doses first and delay initial doses.
Azar: Use it or lose it
In a move that is sure to generate pushback, Mr. Azar said that states that don’t quickly administer vaccines will receive fewer doses in the future. That policy will not go into effect until later in February, which leaves open the possibility that it could be reversed by the incoming Biden administration.
“We have too much vaccine sitting in freezers at hospitals with hospitals not using it,” said Mr. Azar, who also blamed the slow administration process on a reporting lag and states being what he called “overly prescriptive” in who has been eligible to receive a shot.
“I would rather have people working to get appointments to get vaccinated than having vaccine going to waste sitting in freezers,” he told reporters.
Mr. Azar had already been pushing for broader vaccination, telling states to do so in an Operation Warp Speed briefing on Jan. 6. At that briefing, he also said that the federal government would be stepping up vaccination through an “early launch” of a federal partnership with 19 pharmacy chains, which will let states allocate vaccines directly to some 40,000 pharmacy sites.
Gen. Perna said during the Jan. 12 briefing that the aim is to further expand that to some 70,000 locations total.
The CDC reported that as of Jan. 11 some 25.4 million doses have been distributed, with 8.9 million administered. An additional 4.2 million doses were distributed to long-term care facilities, and 937,000 residents and staff have received a dose.
“Pace of administration”
Alaska, Connecticut, North Dakota, South Dakota, the District of Columbia, West Virginia, and the Northern Mariana Islands have administered the most vaccines per capita, according to the CDC. But even these locations have immunized only 4%-5% of their populations, the New York Times reports. At the bottom: Alabama, Arizona, Arkansas, Georgia, Mississippi, and South Carolina.
The federal government can encourage but not require states to move on to new phases of vaccination.
“States ultimately determine how they will proceed with vaccination,” said Marcus Plescia, MD, MPH, chief medical officer for the Association of State and Territorial Health Officials. “Most will be cautious about assuring there are doses for those needing a second dose,” he said in an interview.
Dr. Plescia said that ensuring a second dose is available is especially important for health care workers “who need to be confident that they are protected and not inadvertently transmitting the disease themselves.”
He added that “once we reach a steady state of supply and administration, the rate-limiting factor will be supply of vaccine.”
That supply could now be threatened if states don’t comply with a just-announced federal action that will change how doses are allocated.
Beginning in late February, vaccine allocations to states will be based on “the pace of administration reported by states,” and the size of the 65-and-older population, said Mr. Azar, who has previously criticized New York Governor Andrew Cuomo for fining hospitals that didn’t use up vaccine supply within a week.
“This new system gives states a strong incentive to ensure that all vaccinations are being promptly reported, which they currently are not,” he said.
Currently, allocations are based on a state’s or territory’s population.
Prepandemic, states were required to report vaccinations within 30 days. Since COVID-19 vaccines became available, the CDC has required reporting of shots within 72 hours.
Dr. Redfield said the requirement has caused some difficulty, and that the CDC is investigating why some states have reported using only 15% of doses while others have used 80%.
States have been scrambling to ramp up vaccinations.
Just ahead of the federal briefing, Gov. Cuomo tweeted that New York would be opening up vaccinations to anyone older than 65.
The Associated Press is reporting that some states have started mass vaccination sites.
Arizona has begun operating a 24/7 appointment-only vaccination program at State Farm Stadium outside of Phoenix, with the aim of immunizing 6,000 people each day, according to local radio station KJZZ.
California and Florida have also taken steps to use stadiums, while Michigan, New Jersey, New York, and Texas will use convention centers and fairgrounds, Axios has reported.
In Florida, Palm Beach County Health Director Alina Alonso, MD, told county commissioners on Jan. 12 that there isn’t enough vaccine to meet demand, WPTV reported. “We need to realize that there’s a shortage of vaccine. So it’s not the plan, it’s not our ability to do it. It’s simply supply and demand at this point,” Dr. Alonso said, according to the TV station report.
A version of this article first appeared on Medscape.com.
Federal health officials are urging states to vaccinate all Americans over age 65 and those aged 16-64 who have a documented underlying health condition that makes them more vulnerable to COVID-19.
U.S. Department of Health and Human Services (HHS) Secretary Alex Azar and Centers for Disease Control and Prevention Director Robert Redfield, MD, made the recommendation in a briefing with reporters on Jan. 12, saying that the current vaccine supply was sufficient to meet demand for the next phase of immunization as recommended by the CDC’s Advisory Committee on Immunization Practices.
“We are ready for a transition that we outlined last September in the playbook we sent to states,” Mr. Azar said. Both he and U.S. Army General Gustave F. Perna, chief operations officer for Operation Warp Speed, said that confidence in the distribution system had led to the decision to urge wider access.
The federal government will also increase the number of sites eligible to receive vaccine – including some 13,000 federally qualified community health centers – and will not keep doses in reserve as insurance against issues that might prevent people from receiving a second dose on a timely basis.
“We don’t need to hold back reserve doses,” Mr. Azar said, noting that if there were any “glitches in production” the federal government would move to fulfill obligations for second doses first and delay initial doses.
Azar: Use it or lose it
In a move that is sure to generate pushback, Mr. Azar said that states that don’t quickly administer vaccines will receive fewer doses in the future. That policy will not go into effect until later in February, which leaves open the possibility that it could be reversed by the incoming Biden administration.
“We have too much vaccine sitting in freezers at hospitals with hospitals not using it,” said Mr. Azar, who also blamed the slow administration process on a reporting lag and states being what he called “overly prescriptive” in who has been eligible to receive a shot.
“I would rather have people working to get appointments to get vaccinated than having vaccine going to waste sitting in freezers,” he told reporters.
Mr. Azar had already been pushing for broader vaccination, telling states to do so in an Operation Warp Speed briefing on Jan. 6. At that briefing, he also said that the federal government would be stepping up vaccination through an “early launch” of a federal partnership with 19 pharmacy chains, which will let states allocate vaccines directly to some 40,000 pharmacy sites.
Gen. Perna said during the Jan. 12 briefing that the aim is to further expand that to some 70,000 locations total.
The CDC reported that as of Jan. 11 some 25.4 million doses have been distributed, with 8.9 million administered. An additional 4.2 million doses were distributed to long-term care facilities, and 937,000 residents and staff have received a dose.
“Pace of administration”
Alaska, Connecticut, North Dakota, South Dakota, the District of Columbia, West Virginia, and the Northern Mariana Islands have administered the most vaccines per capita, according to the CDC. But even these locations have immunized only 4%-5% of their populations, the New York Times reports. At the bottom: Alabama, Arizona, Arkansas, Georgia, Mississippi, and South Carolina.
The federal government can encourage but not require states to move on to new phases of vaccination.
“States ultimately determine how they will proceed with vaccination,” said Marcus Plescia, MD, MPH, chief medical officer for the Association of State and Territorial Health Officials. “Most will be cautious about assuring there are doses for those needing a second dose,” he said in an interview.
Dr. Plescia said that ensuring a second dose is available is especially important for health care workers “who need to be confident that they are protected and not inadvertently transmitting the disease themselves.”
He added that “once we reach a steady state of supply and administration, the rate-limiting factor will be supply of vaccine.”
That supply could now be threatened if states don’t comply with a just-announced federal action that will change how doses are allocated.
Beginning in late February, vaccine allocations to states will be based on “the pace of administration reported by states,” and the size of the 65-and-older population, said Mr. Azar, who has previously criticized New York Governor Andrew Cuomo for fining hospitals that didn’t use up vaccine supply within a week.
“This new system gives states a strong incentive to ensure that all vaccinations are being promptly reported, which they currently are not,” he said.
Currently, allocations are based on a state’s or territory’s population.
Prepandemic, states were required to report vaccinations within 30 days. Since COVID-19 vaccines became available, the CDC has required reporting of shots within 72 hours.
Dr. Redfield said the requirement has caused some difficulty, and that the CDC is investigating why some states have reported using only 15% of doses while others have used 80%.
States have been scrambling to ramp up vaccinations.
Just ahead of the federal briefing, Gov. Cuomo tweeted that New York would be opening up vaccinations to anyone older than 65.
The Associated Press is reporting that some states have started mass vaccination sites.
Arizona has begun operating a 24/7 appointment-only vaccination program at State Farm Stadium outside of Phoenix, with the aim of immunizing 6,000 people each day, according to local radio station KJZZ.
California and Florida have also taken steps to use stadiums, while Michigan, New Jersey, New York, and Texas will use convention centers and fairgrounds, Axios has reported.
In Florida, Palm Beach County Health Director Alina Alonso, MD, told county commissioners on Jan. 12 that there isn’t enough vaccine to meet demand, WPTV reported. “We need to realize that there’s a shortage of vaccine. So it’s not the plan, it’s not our ability to do it. It’s simply supply and demand at this point,” Dr. Alonso said, according to the TV station report.
A version of this article first appeared on Medscape.com.
Arguing with doctors
They think people should accept what experts advise. After all, experts work hard to learn accurate facts to promote the public’s best interests.
Those who disagree and justify their reluctance – to be vaccinated against COVID-19, for instance – are unrepentant. First of all, they are not so sure experts are public spirited. Perhaps doctors have something to gain from illness and approve vaccines for political reasons, or assign certain diagnoses to get higher reimbursement.
In this contentious climate, peculiar treatments and unproved “cures” are claimed to deserve more respect than so-called experts are willing to grant them: hydroxychloroquine, bleach, and so on.
From my perspective, what is notable about such public disputes with medical experts is not that they exist but that they are public. In private, people have always argued with doctors. Most of those arguments don’t reach public notice. They are not interesting enough.
For instance, as I think back over the years, I can recall:
- A man who preferred to treat his eczema using topical yogurt. And not just any yogurt: only low-fat, plain Market Basket. He had tried them all.
- The woman with perioral scarring. She had let an unlicensed practitioner apply a painful acid on her face – he never told her what the acid was, and she hadn’t asked – as she lay on a neighbor’s living room floor to have her “skin cancer” treated.
- The man with an obvious melanoma on his chest. He did not want to treat it, because his faith healer in Milwaukee, whom he had never met in person, assured him that “it’s all taken care of.”
I could go on.
I cite these examples only because they are striking. They are far from unique.
People argue with doctors for the same reason they argue with anybody – because they think they know better. They may have heard otherwise from a friend, a magazine article, a blog, a different kind of practitioner.
Many such disagreements are never spoken out loud, because people who expect to argue usually don’t show up at their doctors’ offices. They either stay home or see a different kind of healer. If they do visit a doctor whose point of view differs from their own, most keep disagreements to themselves, because few people relish in-person confrontation. Instead they go home and ignore medical advice there.
Even when overt disagreements do erupt at a medical visit, the doctor can often find a way to convince the patient to reconsider, or somehow deflect the clash. The physician has to at least try to convince a patient who thinks his melanoma has “been taken care of” to have it removed. Whereas if someone really prefers low-fat yogurt to topical steroids, there is no need to win the argument. If the patient decides at some point that his eczema is out of control, he can call and request a prescription. He usually won’t.
For dermatologists, medical arguments rarely involve stakes high enough to force the doctor to try changing patients’ minds or discharging them from the practice. Had I stayed in my original field of pediatrics, I would have confronted patients who refused to vaccinate their children. I would have had to negotiate a compromise – vaccinate “more slowly” – or else part ways with the family.
I always advised medical students, when they found themselves argued with, to separate patients’ needs from their own egos. Being challenged in a small room can be challenging. Still, what matters is how the patient fares, not how the doctor feels.
Public disputes with scientists during the COVID-19 pandemic strike me as being motivated by the same factors behind private disputes in physicians’ offices: skepticism, resentment, suspicion, and – often underlying all these – fear.
Public disputes carried out over social media allow for posturing and aggression. A tweet is a better medium behind which to cloak opinions in the mantle of a noble cause, such as personal freedom. It is also easier to express derision and hostility toward opponents, expert or otherwise, from behind the screen of a Twitter handle.
Fortunately, in everyday medical practice, in-your-face disputes don’t happen very often.
You do remember them, though.
Dr. Rockoff, who wrote the Dermatology News column “Under My Skin,” is now semiretired, after 40 years of practice in Brookline, Mass. He served on the clinical faculty at Tufts University, Boston, and taught senior medical students and other trainees for 30 years. His second book, “Act Like a Doctor, Think Like a Patient,” is available at online. Write to him at dermnews@mdedge.com.
They think people should accept what experts advise. After all, experts work hard to learn accurate facts to promote the public’s best interests.
Those who disagree and justify their reluctance – to be vaccinated against COVID-19, for instance – are unrepentant. First of all, they are not so sure experts are public spirited. Perhaps doctors have something to gain from illness and approve vaccines for political reasons, or assign certain diagnoses to get higher reimbursement.
In this contentious climate, peculiar treatments and unproved “cures” are claimed to deserve more respect than so-called experts are willing to grant them: hydroxychloroquine, bleach, and so on.
From my perspective, what is notable about such public disputes with medical experts is not that they exist but that they are public. In private, people have always argued with doctors. Most of those arguments don’t reach public notice. They are not interesting enough.
For instance, as I think back over the years, I can recall:
- A man who preferred to treat his eczema using topical yogurt. And not just any yogurt: only low-fat, plain Market Basket. He had tried them all.
- The woman with perioral scarring. She had let an unlicensed practitioner apply a painful acid on her face – he never told her what the acid was, and she hadn’t asked – as she lay on a neighbor’s living room floor to have her “skin cancer” treated.
- The man with an obvious melanoma on his chest. He did not want to treat it, because his faith healer in Milwaukee, whom he had never met in person, assured him that “it’s all taken care of.”
I could go on.
I cite these examples only because they are striking. They are far from unique.
People argue with doctors for the same reason they argue with anybody – because they think they know better. They may have heard otherwise from a friend, a magazine article, a blog, a different kind of practitioner.
Many such disagreements are never spoken out loud, because people who expect to argue usually don’t show up at their doctors’ offices. They either stay home or see a different kind of healer. If they do visit a doctor whose point of view differs from their own, most keep disagreements to themselves, because few people relish in-person confrontation. Instead they go home and ignore medical advice there.
Even when overt disagreements do erupt at a medical visit, the doctor can often find a way to convince the patient to reconsider, or somehow deflect the clash. The physician has to at least try to convince a patient who thinks his melanoma has “been taken care of” to have it removed. Whereas if someone really prefers low-fat yogurt to topical steroids, there is no need to win the argument. If the patient decides at some point that his eczema is out of control, he can call and request a prescription. He usually won’t.
For dermatologists, medical arguments rarely involve stakes high enough to force the doctor to try changing patients’ minds or discharging them from the practice. Had I stayed in my original field of pediatrics, I would have confronted patients who refused to vaccinate their children. I would have had to negotiate a compromise – vaccinate “more slowly” – or else part ways with the family.
I always advised medical students, when they found themselves argued with, to separate patients’ needs from their own egos. Being challenged in a small room can be challenging. Still, what matters is how the patient fares, not how the doctor feels.
Public disputes with scientists during the COVID-19 pandemic strike me as being motivated by the same factors behind private disputes in physicians’ offices: skepticism, resentment, suspicion, and – often underlying all these – fear.
Public disputes carried out over social media allow for posturing and aggression. A tweet is a better medium behind which to cloak opinions in the mantle of a noble cause, such as personal freedom. It is also easier to express derision and hostility toward opponents, expert or otherwise, from behind the screen of a Twitter handle.
Fortunately, in everyday medical practice, in-your-face disputes don’t happen very often.
You do remember them, though.
Dr. Rockoff, who wrote the Dermatology News column “Under My Skin,” is now semiretired, after 40 years of practice in Brookline, Mass. He served on the clinical faculty at Tufts University, Boston, and taught senior medical students and other trainees for 30 years. His second book, “Act Like a Doctor, Think Like a Patient,” is available at online. Write to him at dermnews@mdedge.com.
They think people should accept what experts advise. After all, experts work hard to learn accurate facts to promote the public’s best interests.
Those who disagree and justify their reluctance – to be vaccinated against COVID-19, for instance – are unrepentant. First of all, they are not so sure experts are public spirited. Perhaps doctors have something to gain from illness and approve vaccines for political reasons, or assign certain diagnoses to get higher reimbursement.
In this contentious climate, peculiar treatments and unproved “cures” are claimed to deserve more respect than so-called experts are willing to grant them: hydroxychloroquine, bleach, and so on.
From my perspective, what is notable about such public disputes with medical experts is not that they exist but that they are public. In private, people have always argued with doctors. Most of those arguments don’t reach public notice. They are not interesting enough.
For instance, as I think back over the years, I can recall:
- A man who preferred to treat his eczema using topical yogurt. And not just any yogurt: only low-fat, plain Market Basket. He had tried them all.
- The woman with perioral scarring. She had let an unlicensed practitioner apply a painful acid on her face – he never told her what the acid was, and she hadn’t asked – as she lay on a neighbor’s living room floor to have her “skin cancer” treated.
- The man with an obvious melanoma on his chest. He did not want to treat it, because his faith healer in Milwaukee, whom he had never met in person, assured him that “it’s all taken care of.”
I could go on.
I cite these examples only because they are striking. They are far from unique.
People argue with doctors for the same reason they argue with anybody – because they think they know better. They may have heard otherwise from a friend, a magazine article, a blog, a different kind of practitioner.
Many such disagreements are never spoken out loud, because people who expect to argue usually don’t show up at their doctors’ offices. They either stay home or see a different kind of healer. If they do visit a doctor whose point of view differs from their own, most keep disagreements to themselves, because few people relish in-person confrontation. Instead they go home and ignore medical advice there.
Even when overt disagreements do erupt at a medical visit, the doctor can often find a way to convince the patient to reconsider, or somehow deflect the clash. The physician has to at least try to convince a patient who thinks his melanoma has “been taken care of” to have it removed. Whereas if someone really prefers low-fat yogurt to topical steroids, there is no need to win the argument. If the patient decides at some point that his eczema is out of control, he can call and request a prescription. He usually won’t.
For dermatologists, medical arguments rarely involve stakes high enough to force the doctor to try changing patients’ minds or discharging them from the practice. Had I stayed in my original field of pediatrics, I would have confronted patients who refused to vaccinate their children. I would have had to negotiate a compromise – vaccinate “more slowly” – or else part ways with the family.
I always advised medical students, when they found themselves argued with, to separate patients’ needs from their own egos. Being challenged in a small room can be challenging. Still, what matters is how the patient fares, not how the doctor feels.
Public disputes with scientists during the COVID-19 pandemic strike me as being motivated by the same factors behind private disputes in physicians’ offices: skepticism, resentment, suspicion, and – often underlying all these – fear.
Public disputes carried out over social media allow for posturing and aggression. A tweet is a better medium behind which to cloak opinions in the mantle of a noble cause, such as personal freedom. It is also easier to express derision and hostility toward opponents, expert or otherwise, from behind the screen of a Twitter handle.
Fortunately, in everyday medical practice, in-your-face disputes don’t happen very often.
You do remember them, though.
Dr. Rockoff, who wrote the Dermatology News column “Under My Skin,” is now semiretired, after 40 years of practice in Brookline, Mass. He served on the clinical faculty at Tufts University, Boston, and taught senior medical students and other trainees for 30 years. His second book, “Act Like a Doctor, Think Like a Patient,” is available at online. Write to him at dermnews@mdedge.com.
Cloth masks provide inferior protection vs. medical masks, suggests evidence review
according to an evidence review published Jan. 11 in Annals of Family Medicine.
Nevertheless, cloth masks may provide some degree of protection, filtration studies indicate. If clinicians use cloth masks, they should take into account the fit, material, and number of layers, the review authors wrote.
And if cloth masks are used as a last resort, such as during shortages of personal protective equipment (PPE), additional measures may help, such as pairing cloth masks with plastic face shields.
“We recommend frequent cloth mask changes to reduce the risk of moisture retention and washing according to hospital laundry standards to decrease the risk of ineffective cleaning,” review author Ariel Kiyomi Daoud, a researcher at the University of Colorado at Denver, Aurora, and colleagues wrote.
The investigators identified and analyzed nine studies related to cloth masks’ ability to prevent respiratory viral infections among health care clinicians. The studies generally were not specific to SARS-CoV-2. They focused on four nonrandomized trials, three laboratory efficacy studies, one single-case experiment, and one randomized controlled trial.
Filtration and fit
“Seven publications addressed the filtration efficacy of commercial cloth masks or materials used to create homemade masks ... in a laboratory setting,” the researchers wrote. These studies found that cloth materials prevent some level of penetration, but generally have “lesser filtration efficiency and greater variability than medical masks” do.
One study found that the materials with the greatest filtration efficacy – vacuum bags and tea towels – had low airflow, which limits their use.
Two studies found that additional layers may increase the viral filtration efficacy of cloth masks.
Several studies that assessed mask fit and airflow found that cloth masks “have worse fit and a greater level of particle leakage, compared to medical masks,” the authors reported. Most studies did not examine cloth masks’ ability to protect wearers from respiratory droplets or contact, which the World Health Organization consider the primary means of SARS-CoV-2 spread, with aerosols playing a smaller role. “Thus, we must interpret these results with caution in the context of COVID-19,” the authors wrote. “For a primary care clinician without access to medical masks, our qualitative synthesis of the literature suggests that it is better to wear a cloth mask than no mask,” as long as other protective measures are considered along with cloth mask use.
Generally consistent guidance
Agencies and researchers have shared similar recommendations about the use of cloth masks in health care settings.
“Health care workers are at the frontline and they need to be protected,” said Abrar Ahmad Chughtai, MBBS, MPH, PhD, an epidemiologist at University of New South Wales, Sydney, in an interview. “Many studies show that respirators are more effective, compared to medical masks, and medical masks are more effective, compared to cloth masks. So ideally, all frontline health care workers should use respirators. If respirators are not available, then medical masks should be used. Cloth masks are not as effective as medical masks and ideally should not be used in health care settings.”
Dr. Chughtai has written about cloth masks for protection against SARS-CoV-2 and was an investigator for a 2015 randomized trial that compared medical masks and cloth masks in health care workers.
In that trial, which was considered in the review, greater rates of influenza-like illness occurred in the cloth mask arm, compared with the medical mask arm.
“Studies show that three or more layers of cloth may reduce the spread of droplets and aerosols from the wearers,” Dr. Chughtai said. “So, cloth masks may be used in community settings to prevent spread of infections from the sick, particularly asymptomatic, people.”
In addition, cloth masks “may be used by health care workers as a last resort, if no other option is available,” he said. In that case, they should have at least three layers, fit to the face, and be washed regularly.
Not considered PPE
According to routine infection prevention and control recommendations for health care personnel from the Centers for Disease Control and Prevention, face masks – often referred to as surgical masks or procedure masks – should be worn by workers “at all times while they are in the healthcare facility, including in break rooms or other spaces where they might encounter coworkers.”
Unlike cloth masks, face masks offer “protection for the wearer against exposure to splashes and sprays of infectious material from others,” as well as source control, the agency says. Health care personnel “should remove their respirator or face mask, perform hand hygiene, and put on their cloth mask when leaving the facility at the end of their shift,” according to the CDC.
“Cloth masks are NOT PPE and should not be worn for the care of patients with suspected or confirmed COVID-19 or other situations where use of a respirator or face mask is recommended,” the agency notes.
When respirators or face masks are unavailable, health care personnel “might use cloth masks as a last resort for care of patients with suspected or confirmed diagnosis for which face mask or respirator use is normally recommended,” according to CDC guidance.
In that scenario, cloth masks “should ideally be used in combination with a face shield that covers the entire front (that extends to the chin or below) and sides of the face,” the CDC says.
Limited data for comparisons
A Dec. 29, 2020, update in Annals of Internal Medicine about masks for prevention of respiratory virus infections highlighted two recent studies in the United States that reported on mask use in health care settings. A study of more than 16,000 health care workers and first responders found that those who used an N95 or surgical mask all of the time were less likely to have SARS-CoV-2 antibodies, compared with workers who did not wear masks all the time. The adjusted odds ratio with consistent N95 use was 0.83, and the aOR with consistent surgical mask use was 0.86.
In the second study, which included more than 20,000 asymptomatic health care workers, risk for infection was reduced with any mask use versus no mask use (OR, 0.58). An N95 mask was associated with decreased risk versus a surgical mask (OR, 0.76). The studies had methodological limitations, however, and “evidence for various comparisons about mask use in health care settings and risk for SARS-CoV-2 remains insufficient,” the authors of the update wrote.
The Annals of Family Medicine review authors had no relevant disclosures. Dr. Chughtai has tested filtration of 3M masks and worked with CleanSpace Technology to research fit testing of respirators, and the 2015 randomized trial was funded by an Australian Research Council Linkage Grant with 3M as a partner on the grant. The Dec. 29, 2020, update was of a review that originally was supported by grants from the Agency for Healthcare Research Quality.
SOURCE: Daoud AK et al. Ann Fam Med. 2020 Jan 11. doi: 10.1370/afm.2640.
according to an evidence review published Jan. 11 in Annals of Family Medicine.
Nevertheless, cloth masks may provide some degree of protection, filtration studies indicate. If clinicians use cloth masks, they should take into account the fit, material, and number of layers, the review authors wrote.
And if cloth masks are used as a last resort, such as during shortages of personal protective equipment (PPE), additional measures may help, such as pairing cloth masks with plastic face shields.
“We recommend frequent cloth mask changes to reduce the risk of moisture retention and washing according to hospital laundry standards to decrease the risk of ineffective cleaning,” review author Ariel Kiyomi Daoud, a researcher at the University of Colorado at Denver, Aurora, and colleagues wrote.
The investigators identified and analyzed nine studies related to cloth masks’ ability to prevent respiratory viral infections among health care clinicians. The studies generally were not specific to SARS-CoV-2. They focused on four nonrandomized trials, three laboratory efficacy studies, one single-case experiment, and one randomized controlled trial.
Filtration and fit
“Seven publications addressed the filtration efficacy of commercial cloth masks or materials used to create homemade masks ... in a laboratory setting,” the researchers wrote. These studies found that cloth materials prevent some level of penetration, but generally have “lesser filtration efficiency and greater variability than medical masks” do.
One study found that the materials with the greatest filtration efficacy – vacuum bags and tea towels – had low airflow, which limits their use.
Two studies found that additional layers may increase the viral filtration efficacy of cloth masks.
Several studies that assessed mask fit and airflow found that cloth masks “have worse fit and a greater level of particle leakage, compared to medical masks,” the authors reported. Most studies did not examine cloth masks’ ability to protect wearers from respiratory droplets or contact, which the World Health Organization consider the primary means of SARS-CoV-2 spread, with aerosols playing a smaller role. “Thus, we must interpret these results with caution in the context of COVID-19,” the authors wrote. “For a primary care clinician without access to medical masks, our qualitative synthesis of the literature suggests that it is better to wear a cloth mask than no mask,” as long as other protective measures are considered along with cloth mask use.
Generally consistent guidance
Agencies and researchers have shared similar recommendations about the use of cloth masks in health care settings.
“Health care workers are at the frontline and they need to be protected,” said Abrar Ahmad Chughtai, MBBS, MPH, PhD, an epidemiologist at University of New South Wales, Sydney, in an interview. “Many studies show that respirators are more effective, compared to medical masks, and medical masks are more effective, compared to cloth masks. So ideally, all frontline health care workers should use respirators. If respirators are not available, then medical masks should be used. Cloth masks are not as effective as medical masks and ideally should not be used in health care settings.”
Dr. Chughtai has written about cloth masks for protection against SARS-CoV-2 and was an investigator for a 2015 randomized trial that compared medical masks and cloth masks in health care workers.
In that trial, which was considered in the review, greater rates of influenza-like illness occurred in the cloth mask arm, compared with the medical mask arm.
“Studies show that three or more layers of cloth may reduce the spread of droplets and aerosols from the wearers,” Dr. Chughtai said. “So, cloth masks may be used in community settings to prevent spread of infections from the sick, particularly asymptomatic, people.”
In addition, cloth masks “may be used by health care workers as a last resort, if no other option is available,” he said. In that case, they should have at least three layers, fit to the face, and be washed regularly.
Not considered PPE
According to routine infection prevention and control recommendations for health care personnel from the Centers for Disease Control and Prevention, face masks – often referred to as surgical masks or procedure masks – should be worn by workers “at all times while they are in the healthcare facility, including in break rooms or other spaces where they might encounter coworkers.”
Unlike cloth masks, face masks offer “protection for the wearer against exposure to splashes and sprays of infectious material from others,” as well as source control, the agency says. Health care personnel “should remove their respirator or face mask, perform hand hygiene, and put on their cloth mask when leaving the facility at the end of their shift,” according to the CDC.
“Cloth masks are NOT PPE and should not be worn for the care of patients with suspected or confirmed COVID-19 or other situations where use of a respirator or face mask is recommended,” the agency notes.
When respirators or face masks are unavailable, health care personnel “might use cloth masks as a last resort for care of patients with suspected or confirmed diagnosis for which face mask or respirator use is normally recommended,” according to CDC guidance.
In that scenario, cloth masks “should ideally be used in combination with a face shield that covers the entire front (that extends to the chin or below) and sides of the face,” the CDC says.
Limited data for comparisons
A Dec. 29, 2020, update in Annals of Internal Medicine about masks for prevention of respiratory virus infections highlighted two recent studies in the United States that reported on mask use in health care settings. A study of more than 16,000 health care workers and first responders found that those who used an N95 or surgical mask all of the time were less likely to have SARS-CoV-2 antibodies, compared with workers who did not wear masks all the time. The adjusted odds ratio with consistent N95 use was 0.83, and the aOR with consistent surgical mask use was 0.86.
In the second study, which included more than 20,000 asymptomatic health care workers, risk for infection was reduced with any mask use versus no mask use (OR, 0.58). An N95 mask was associated with decreased risk versus a surgical mask (OR, 0.76). The studies had methodological limitations, however, and “evidence for various comparisons about mask use in health care settings and risk for SARS-CoV-2 remains insufficient,” the authors of the update wrote.
The Annals of Family Medicine review authors had no relevant disclosures. Dr. Chughtai has tested filtration of 3M masks and worked with CleanSpace Technology to research fit testing of respirators, and the 2015 randomized trial was funded by an Australian Research Council Linkage Grant with 3M as a partner on the grant. The Dec. 29, 2020, update was of a review that originally was supported by grants from the Agency for Healthcare Research Quality.
SOURCE: Daoud AK et al. Ann Fam Med. 2020 Jan 11. doi: 10.1370/afm.2640.
according to an evidence review published Jan. 11 in Annals of Family Medicine.
Nevertheless, cloth masks may provide some degree of protection, filtration studies indicate. If clinicians use cloth masks, they should take into account the fit, material, and number of layers, the review authors wrote.
And if cloth masks are used as a last resort, such as during shortages of personal protective equipment (PPE), additional measures may help, such as pairing cloth masks with plastic face shields.
“We recommend frequent cloth mask changes to reduce the risk of moisture retention and washing according to hospital laundry standards to decrease the risk of ineffective cleaning,” review author Ariel Kiyomi Daoud, a researcher at the University of Colorado at Denver, Aurora, and colleagues wrote.
The investigators identified and analyzed nine studies related to cloth masks’ ability to prevent respiratory viral infections among health care clinicians. The studies generally were not specific to SARS-CoV-2. They focused on four nonrandomized trials, three laboratory efficacy studies, one single-case experiment, and one randomized controlled trial.
Filtration and fit
“Seven publications addressed the filtration efficacy of commercial cloth masks or materials used to create homemade masks ... in a laboratory setting,” the researchers wrote. These studies found that cloth materials prevent some level of penetration, but generally have “lesser filtration efficiency and greater variability than medical masks” do.
One study found that the materials with the greatest filtration efficacy – vacuum bags and tea towels – had low airflow, which limits their use.
Two studies found that additional layers may increase the viral filtration efficacy of cloth masks.
Several studies that assessed mask fit and airflow found that cloth masks “have worse fit and a greater level of particle leakage, compared to medical masks,” the authors reported. Most studies did not examine cloth masks’ ability to protect wearers from respiratory droplets or contact, which the World Health Organization consider the primary means of SARS-CoV-2 spread, with aerosols playing a smaller role. “Thus, we must interpret these results with caution in the context of COVID-19,” the authors wrote. “For a primary care clinician without access to medical masks, our qualitative synthesis of the literature suggests that it is better to wear a cloth mask than no mask,” as long as other protective measures are considered along with cloth mask use.
Generally consistent guidance
Agencies and researchers have shared similar recommendations about the use of cloth masks in health care settings.
“Health care workers are at the frontline and they need to be protected,” said Abrar Ahmad Chughtai, MBBS, MPH, PhD, an epidemiologist at University of New South Wales, Sydney, in an interview. “Many studies show that respirators are more effective, compared to medical masks, and medical masks are more effective, compared to cloth masks. So ideally, all frontline health care workers should use respirators. If respirators are not available, then medical masks should be used. Cloth masks are not as effective as medical masks and ideally should not be used in health care settings.”
Dr. Chughtai has written about cloth masks for protection against SARS-CoV-2 and was an investigator for a 2015 randomized trial that compared medical masks and cloth masks in health care workers.
In that trial, which was considered in the review, greater rates of influenza-like illness occurred in the cloth mask arm, compared with the medical mask arm.
“Studies show that three or more layers of cloth may reduce the spread of droplets and aerosols from the wearers,” Dr. Chughtai said. “So, cloth masks may be used in community settings to prevent spread of infections from the sick, particularly asymptomatic, people.”
In addition, cloth masks “may be used by health care workers as a last resort, if no other option is available,” he said. In that case, they should have at least three layers, fit to the face, and be washed regularly.
Not considered PPE
According to routine infection prevention and control recommendations for health care personnel from the Centers for Disease Control and Prevention, face masks – often referred to as surgical masks or procedure masks – should be worn by workers “at all times while they are in the healthcare facility, including in break rooms or other spaces where they might encounter coworkers.”
Unlike cloth masks, face masks offer “protection for the wearer against exposure to splashes and sprays of infectious material from others,” as well as source control, the agency says. Health care personnel “should remove their respirator or face mask, perform hand hygiene, and put on their cloth mask when leaving the facility at the end of their shift,” according to the CDC.
“Cloth masks are NOT PPE and should not be worn for the care of patients with suspected or confirmed COVID-19 or other situations where use of a respirator or face mask is recommended,” the agency notes.
When respirators or face masks are unavailable, health care personnel “might use cloth masks as a last resort for care of patients with suspected or confirmed diagnosis for which face mask or respirator use is normally recommended,” according to CDC guidance.
In that scenario, cloth masks “should ideally be used in combination with a face shield that covers the entire front (that extends to the chin or below) and sides of the face,” the CDC says.
Limited data for comparisons
A Dec. 29, 2020, update in Annals of Internal Medicine about masks for prevention of respiratory virus infections highlighted two recent studies in the United States that reported on mask use in health care settings. A study of more than 16,000 health care workers and first responders found that those who used an N95 or surgical mask all of the time were less likely to have SARS-CoV-2 antibodies, compared with workers who did not wear masks all the time. The adjusted odds ratio with consistent N95 use was 0.83, and the aOR with consistent surgical mask use was 0.86.
In the second study, which included more than 20,000 asymptomatic health care workers, risk for infection was reduced with any mask use versus no mask use (OR, 0.58). An N95 mask was associated with decreased risk versus a surgical mask (OR, 0.76). The studies had methodological limitations, however, and “evidence for various comparisons about mask use in health care settings and risk for SARS-CoV-2 remains insufficient,” the authors of the update wrote.
The Annals of Family Medicine review authors had no relevant disclosures. Dr. Chughtai has tested filtration of 3M masks and worked with CleanSpace Technology to research fit testing of respirators, and the 2015 randomized trial was funded by an Australian Research Council Linkage Grant with 3M as a partner on the grant. The Dec. 29, 2020, update was of a review that originally was supported by grants from the Agency for Healthcare Research Quality.
SOURCE: Daoud AK et al. Ann Fam Med. 2020 Jan 11. doi: 10.1370/afm.2640.
FROM ANNALS OF FAMILY MEDICINE
Feds authorize $3 billion to boost vaccine rollout
The CDC will send $3 billion to the states to boost a lagging national COVID-19 vaccination program.
The Department of Health and Human Services announced the new funding as only 30% of the more than 22 million doses of vaccine distributed in the U.S. has been injected into Americans’ arms.
Along with the $3 billion, HHS said another $19 billion is headed to states and jurisdictions to boost COVID-19 testing programs. The amount each state will receive will be determined by population.
The news comes days after President-elect Joe Biden said he planned to release all available doses of vaccine after he takes office on Jan. 20. The Trump administration has been holding back millions of doses to ensure supply of vaccine to provide the necessary second dose for those who received the first shot.
“This funding is another timely investment that will strengthen our nation’s efforts to stop the COVID-19 pandemic in America,” CDC Director Robert Redfield, MD, said in a statement. “Particularly now, it is crucial that states and communities have the resources they need to conduct testing, and to distribute and administer safe, high-quality COVID-19 vaccines safely and equitably.”
Federal officials and public health experts, however, expressed concerns this weekend about Biden’s plan.
Outgoing Trump administration officials and others said they worry that doing so will leave providers without enough second doses for people getting the two-shot vaccines.
If Biden releases all available doses and the vaccine-making process has an issue, they said, that could pose a supply risk.
“We have product that is going through QC right now – quality control – for sterility, identity check that we have tens and tens of millions of product. We always will. But batches fail. Sterility fails ... and then you don’t have a product for that second dose,” Alex Azar, secretary of health and human services, told the American Hospital Association on Jan. 8, according to CNN.
“And frankly, talking about that or encouraging that can really undermine a critical public health need, which is that people come back for their second vaccine,” he said.
One of the main roadblocks in the vaccine rollout has been administering the doses that have already been distributed. The U.S. has shipped 22.1 million doses, and 6.6 million first shots have been given, according to the latest CDC data updated Jan. 8. Mr. Azar and other federal health officials have encouraged states to use their current supply and expand vaccine access to more priority groups.
“We would be delighted to learn that jurisdictions have actually administered many more doses than they are presently reporting,” a spokesman for the U.S. Department of Health and Human Services told CNN. “We are encouraging jurisdictions to expand their priority groups as needed to ensure no vaccine is sitting on the shelf after having been delivered to the jurisdiction-directed locations.”
Releasing more vaccines for first doses could create ethical concerns as well, since people getting vaccines expect to get a second dose in the proper amount of time, according to The Week. Biden’s transition team said on Jan. 8 that he won’t delay the second dose but, instead, plans to ramp up production to stay on track.
To do this well, the federal government should create a coordinated vaccine strategy that sets expectations for an around-the-clock operation and help state and local vaccination programs meet their goals, Leana Wen, MD, a professor at George Washington University, wrote in an editorial for The Washington Post.
“The Biden team’s urgency around vaccinations is commendable,” she added in a Twitter post on Jan. 11. “I’d like to see a guarantee that every 1st dose given will be followed with a timely 2nd dose. Otherwise, there are ethical concerns that could add to vaccine hesitancy.”
Biden has pledged that 100 million doses will be administered in his first 100 days in office. He has grown frustrated as concerns grow that his administration could fall short of the promise, according to Politico. His coronavirus response team has noted several challenges, including what they say is a lack of long-term planning by the Trump administration and an initial refusal to share key information.
“We’re uncovering new information each day, and we’re unearthing – of course – more work to be done,” Vivek Murthy, MD, Biden’s nominee for surgeon general, told Politico.
The team has uncovered staffing shortages, technology problems, and issues with health care insurance coverage. The incoming Biden team has developed several initiatives, such as mobile vaccination units and new federal sites to give shots. It could take weeks to get the vaccine rollout on track, the news outlet reported.
“Will this be challenging? Absolutely,” Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases and Biden’s incoming chief medical adviser on the coronavirus, told Politico. “This is an unprecedented effort to vaccinate the entire country over a period of time that’s fighting against people dying at record numbers. To say it’s not a challenge would be unrealistic. Do I think it can be done? Yes.”
A version of this article first appeared on WebMD.com.
The CDC will send $3 billion to the states to boost a lagging national COVID-19 vaccination program.
The Department of Health and Human Services announced the new funding as only 30% of the more than 22 million doses of vaccine distributed in the U.S. has been injected into Americans’ arms.
Along with the $3 billion, HHS said another $19 billion is headed to states and jurisdictions to boost COVID-19 testing programs. The amount each state will receive will be determined by population.
The news comes days after President-elect Joe Biden said he planned to release all available doses of vaccine after he takes office on Jan. 20. The Trump administration has been holding back millions of doses to ensure supply of vaccine to provide the necessary second dose for those who received the first shot.
“This funding is another timely investment that will strengthen our nation’s efforts to stop the COVID-19 pandemic in America,” CDC Director Robert Redfield, MD, said in a statement. “Particularly now, it is crucial that states and communities have the resources they need to conduct testing, and to distribute and administer safe, high-quality COVID-19 vaccines safely and equitably.”
Federal officials and public health experts, however, expressed concerns this weekend about Biden’s plan.
Outgoing Trump administration officials and others said they worry that doing so will leave providers without enough second doses for people getting the two-shot vaccines.
If Biden releases all available doses and the vaccine-making process has an issue, they said, that could pose a supply risk.
“We have product that is going through QC right now – quality control – for sterility, identity check that we have tens and tens of millions of product. We always will. But batches fail. Sterility fails ... and then you don’t have a product for that second dose,” Alex Azar, secretary of health and human services, told the American Hospital Association on Jan. 8, according to CNN.
“And frankly, talking about that or encouraging that can really undermine a critical public health need, which is that people come back for their second vaccine,” he said.
One of the main roadblocks in the vaccine rollout has been administering the doses that have already been distributed. The U.S. has shipped 22.1 million doses, and 6.6 million first shots have been given, according to the latest CDC data updated Jan. 8. Mr. Azar and other federal health officials have encouraged states to use their current supply and expand vaccine access to more priority groups.
“We would be delighted to learn that jurisdictions have actually administered many more doses than they are presently reporting,” a spokesman for the U.S. Department of Health and Human Services told CNN. “We are encouraging jurisdictions to expand their priority groups as needed to ensure no vaccine is sitting on the shelf after having been delivered to the jurisdiction-directed locations.”
Releasing more vaccines for first doses could create ethical concerns as well, since people getting vaccines expect to get a second dose in the proper amount of time, according to The Week. Biden’s transition team said on Jan. 8 that he won’t delay the second dose but, instead, plans to ramp up production to stay on track.
To do this well, the federal government should create a coordinated vaccine strategy that sets expectations for an around-the-clock operation and help state and local vaccination programs meet their goals, Leana Wen, MD, a professor at George Washington University, wrote in an editorial for The Washington Post.
“The Biden team’s urgency around vaccinations is commendable,” she added in a Twitter post on Jan. 11. “I’d like to see a guarantee that every 1st dose given will be followed with a timely 2nd dose. Otherwise, there are ethical concerns that could add to vaccine hesitancy.”
Biden has pledged that 100 million doses will be administered in his first 100 days in office. He has grown frustrated as concerns grow that his administration could fall short of the promise, according to Politico. His coronavirus response team has noted several challenges, including what they say is a lack of long-term planning by the Trump administration and an initial refusal to share key information.
“We’re uncovering new information each day, and we’re unearthing – of course – more work to be done,” Vivek Murthy, MD, Biden’s nominee for surgeon general, told Politico.
The team has uncovered staffing shortages, technology problems, and issues with health care insurance coverage. The incoming Biden team has developed several initiatives, such as mobile vaccination units and new federal sites to give shots. It could take weeks to get the vaccine rollout on track, the news outlet reported.
“Will this be challenging? Absolutely,” Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases and Biden’s incoming chief medical adviser on the coronavirus, told Politico. “This is an unprecedented effort to vaccinate the entire country over a period of time that’s fighting against people dying at record numbers. To say it’s not a challenge would be unrealistic. Do I think it can be done? Yes.”
A version of this article first appeared on WebMD.com.
The CDC will send $3 billion to the states to boost a lagging national COVID-19 vaccination program.
The Department of Health and Human Services announced the new funding as only 30% of the more than 22 million doses of vaccine distributed in the U.S. has been injected into Americans’ arms.
Along with the $3 billion, HHS said another $19 billion is headed to states and jurisdictions to boost COVID-19 testing programs. The amount each state will receive will be determined by population.
The news comes days after President-elect Joe Biden said he planned to release all available doses of vaccine after he takes office on Jan. 20. The Trump administration has been holding back millions of doses to ensure supply of vaccine to provide the necessary second dose for those who received the first shot.
“This funding is another timely investment that will strengthen our nation’s efforts to stop the COVID-19 pandemic in America,” CDC Director Robert Redfield, MD, said in a statement. “Particularly now, it is crucial that states and communities have the resources they need to conduct testing, and to distribute and administer safe, high-quality COVID-19 vaccines safely and equitably.”
Federal officials and public health experts, however, expressed concerns this weekend about Biden’s plan.
Outgoing Trump administration officials and others said they worry that doing so will leave providers without enough second doses for people getting the two-shot vaccines.
If Biden releases all available doses and the vaccine-making process has an issue, they said, that could pose a supply risk.
“We have product that is going through QC right now – quality control – for sterility, identity check that we have tens and tens of millions of product. We always will. But batches fail. Sterility fails ... and then you don’t have a product for that second dose,” Alex Azar, secretary of health and human services, told the American Hospital Association on Jan. 8, according to CNN.
“And frankly, talking about that or encouraging that can really undermine a critical public health need, which is that people come back for their second vaccine,” he said.
One of the main roadblocks in the vaccine rollout has been administering the doses that have already been distributed. The U.S. has shipped 22.1 million doses, and 6.6 million first shots have been given, according to the latest CDC data updated Jan. 8. Mr. Azar and other federal health officials have encouraged states to use their current supply and expand vaccine access to more priority groups.
“We would be delighted to learn that jurisdictions have actually administered many more doses than they are presently reporting,” a spokesman for the U.S. Department of Health and Human Services told CNN. “We are encouraging jurisdictions to expand their priority groups as needed to ensure no vaccine is sitting on the shelf after having been delivered to the jurisdiction-directed locations.”
Releasing more vaccines for first doses could create ethical concerns as well, since people getting vaccines expect to get a second dose in the proper amount of time, according to The Week. Biden’s transition team said on Jan. 8 that he won’t delay the second dose but, instead, plans to ramp up production to stay on track.
To do this well, the federal government should create a coordinated vaccine strategy that sets expectations for an around-the-clock operation and help state and local vaccination programs meet their goals, Leana Wen, MD, a professor at George Washington University, wrote in an editorial for The Washington Post.
“The Biden team’s urgency around vaccinations is commendable,” she added in a Twitter post on Jan. 11. “I’d like to see a guarantee that every 1st dose given will be followed with a timely 2nd dose. Otherwise, there are ethical concerns that could add to vaccine hesitancy.”
Biden has pledged that 100 million doses will be administered in his first 100 days in office. He has grown frustrated as concerns grow that his administration could fall short of the promise, according to Politico. His coronavirus response team has noted several challenges, including what they say is a lack of long-term planning by the Trump administration and an initial refusal to share key information.
“We’re uncovering new information each day, and we’re unearthing – of course – more work to be done,” Vivek Murthy, MD, Biden’s nominee for surgeon general, told Politico.
The team has uncovered staffing shortages, technology problems, and issues with health care insurance coverage. The incoming Biden team has developed several initiatives, such as mobile vaccination units and new federal sites to give shots. It could take weeks to get the vaccine rollout on track, the news outlet reported.
“Will this be challenging? Absolutely,” Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases and Biden’s incoming chief medical adviser on the coronavirus, told Politico. “This is an unprecedented effort to vaccinate the entire country over a period of time that’s fighting against people dying at record numbers. To say it’s not a challenge would be unrealistic. Do I think it can be done? Yes.”
A version of this article first appeared on WebMD.com.
Skin rejuvenation: Full-field ablative resurfacing remains a gold standard
according to Brian S. Biesman, MD.
“When performing laser skin resurfacing, our goal is to match the degree of injury to the needs of the patient we’re treating,” Dr. Biesman, an oculofacial plastic surgeon who practices in Nashville, Tenn., said during a virtual course on laser and aesthetic skin therapy.
“If we’re treating a 35-year-old with minimal photoaging, we don’t need to use full-field resurfacing. By the same token, a 60-year-old who’s never had anything but sun exposure is not going to do well with nonablative fractional resurfacing or other modalities that produce only modest changes,” he noted. “Full ablative resurfacing is a useful tool that can be used to treat a variety of patients. We can tailor each treatment to the individual patient. We can simply dial the energy up or down and adjust the density.”
Full-field laser ablation removes the epidermis as well as a part of the dermis, and the degree of dermal injury varies depending on the relative aggressiveness of the treatment. “We can treat very superficially in the dermis or we can do deep dermal treatments,” he said at the meeting, which was sponsored by Harvard Medical School, Massachusetts General Hospital, and the Wellman Center for Photomedicine.
“The residual thermal injury will vary to some degree, depending on our treatment parameters. It does cause immediate collagen contracture. It also stimulates a process of neocollagenesis.”
Two main lasers used for full-field treatments are the erbium:YAG laser and the CO2 laser at wavelengths of 2,940 nm and 10,600 nm, respectively. “The erbium:YAG is far more highly absorbed by water, by a factor of about 13,” he said. “But both of these wavelengths can be used successfully as long as you understand the physics behind them.”
The short-pulsed erbium:YAG laser ablates effectively, producing about a 10 mcm zone of thermal injury. “That’s not going to induce much by the way of remodeling, but it will be effective in removing tissue from the superficial layers of the skin,” said Dr. Biesman, who is a past president of the American Society for Laser Medicine and Surgery. “There’s also an absorption peak for collagen, so if you’re treating a scar, this laser can be highly effective.”
The CO2 laser creates more residual thermal injury during full-field resurfacing, compared with the short-pulsed erbium:YAG laser. The long-pulsed erbium:YAG laser can be used in both short- and long-pulsed modes and is more ablative than the CO2 laser when used in short-pulsed mode. When used in long-pulse mode, it makes it possible to produce results “very similar to CO2 in terms of the thermal injury profile,” he said. “It’s a versatile device. So, the CO2 in its native mode produces more thermal injury, while the erbium:YAG laser is more ablative. Both can be used effectively for facial skin rejuvenation.”
Full-field laser resurfacing requires local infiltration with lidocaine 1% or 2% with epinephrine or general anesthesia. “This is not a treatment that you can do comfortably under topical anesthesia, even if you’re using cold air unless you are doing treatments essentially confined to the epidermis and superficial dermis,” Dr. Biesman said. “When working around the eyes or on the face you need to use ocular protection with metal ocular shields. There’s no two ways about it. There is no scenario in which you’re doing an ablative resurfacing around the eye where you don’t use metal corneal shields.”
Energy and density levels can be fine-tuned in order to optimize treatment. For deep rhytides in the perioral region or on the forehead or lateral cheeks, clinicians may choose to treat at a higher density, while rhytides located in other areas may respond well to treatments at a lower density. Relative danger zones include the eyelids in general, especially the medial lower eyelid, as well as the upper lip. “These are the areas that are most prone to developing scarring,” he said.
For the upper eyelids, Dr. Biesman treats from the lashes to the upper brow. “It’s important to protect the lashes and treat from the lower-lid margin all the way down to the orbital rim. I debride relatively aggressively. I want to debride all the eschar created by the first pass and come back with a second pass. I sometimes will decrease the density on the second pass, depending on the type of tissue response that I see. If I see a dramatic response on the second pass I will definitely decrease the density.” He uses Aquaphor to protect the eyebrows. “It’s difficult to do that on the lashes. For the lashes, I usually use a wet tongue blade and keep the lid on stretch as I do my treatments.”
Dr. Biesman recommends feathering to blend full-field treatments with the neck. This means bringing treatments below the mandible. “There are times when we want to conservatively treat the neck,” he said. “The neck does not recover nearly as well after ablative resurfacing as the face does due to the fact that there’s probably about 90% fewer sebaceous glands and hair follicles in the neck relative to the face.”
In Dr. Biesman’s opinion, the important perioperative preparation is counseling the patient, including setting realistic expectations and devising a plan for wound care. “They can expect 7-10 days to heal, depending on the area we’re treating and the relative aggressiveness of the planned treatment,” he said. For patients with a history of herpes simplex virus type 1, he recommends antiviral treatment prior to the procedure. “If you do encounter a herpetic infection postoperatively, you may not see typical clinical signs of blistering as the epidermis has been removed.”
Dr. Biesman uses both antiviral and antibiotic prophylaxis prior to full-field treatments. “The literature by and large says that antibiotic/antiviral prophylaxis is not required prior to full-face ablation,” he said. “The reason I choose to is that I have had some issues with community-acquired MRSA infections. Because it’s so ubiquitous these days, I typically do prescribe an agent that gives good MRSA coverage.”
As for wound care, the literature differs on open versus closed techniques. Dr. Biesman favors using Aquaphor for the first week or so and seeing patients back on posttreatment day 2, “who by that time are usually beyond the inflammatory phase of wound healing,” he said. “A lot of the initial oozing has stopped by then. We clean that off any dried exudate in the office very carefully. We debride gently with warm-water soaks and I like to use PRP [platelet-rich plasma]. There is literature to support the role of PRP in wound healing.”
Even in the most experienced hands, complications can occur from full-field laser resurfacing, including bacterial, viral, or fungal infections. Other potential complications include persistent erythema, hypopigmentation, hyperpigmentation, scarring, and ectropion. “Knowledge of treatment parameters, endpoints, and wound healing is required for safe and successful outcomes,” Dr. Biesman said.
He reported having no relevant disclosures related to his presentation.
according to Brian S. Biesman, MD.
“When performing laser skin resurfacing, our goal is to match the degree of injury to the needs of the patient we’re treating,” Dr. Biesman, an oculofacial plastic surgeon who practices in Nashville, Tenn., said during a virtual course on laser and aesthetic skin therapy.
“If we’re treating a 35-year-old with minimal photoaging, we don’t need to use full-field resurfacing. By the same token, a 60-year-old who’s never had anything but sun exposure is not going to do well with nonablative fractional resurfacing or other modalities that produce only modest changes,” he noted. “Full ablative resurfacing is a useful tool that can be used to treat a variety of patients. We can tailor each treatment to the individual patient. We can simply dial the energy up or down and adjust the density.”
Full-field laser ablation removes the epidermis as well as a part of the dermis, and the degree of dermal injury varies depending on the relative aggressiveness of the treatment. “We can treat very superficially in the dermis or we can do deep dermal treatments,” he said at the meeting, which was sponsored by Harvard Medical School, Massachusetts General Hospital, and the Wellman Center for Photomedicine.
“The residual thermal injury will vary to some degree, depending on our treatment parameters. It does cause immediate collagen contracture. It also stimulates a process of neocollagenesis.”
Two main lasers used for full-field treatments are the erbium:YAG laser and the CO2 laser at wavelengths of 2,940 nm and 10,600 nm, respectively. “The erbium:YAG is far more highly absorbed by water, by a factor of about 13,” he said. “But both of these wavelengths can be used successfully as long as you understand the physics behind them.”
The short-pulsed erbium:YAG laser ablates effectively, producing about a 10 mcm zone of thermal injury. “That’s not going to induce much by the way of remodeling, but it will be effective in removing tissue from the superficial layers of the skin,” said Dr. Biesman, who is a past president of the American Society for Laser Medicine and Surgery. “There’s also an absorption peak for collagen, so if you’re treating a scar, this laser can be highly effective.”
The CO2 laser creates more residual thermal injury during full-field resurfacing, compared with the short-pulsed erbium:YAG laser. The long-pulsed erbium:YAG laser can be used in both short- and long-pulsed modes and is more ablative than the CO2 laser when used in short-pulsed mode. When used in long-pulse mode, it makes it possible to produce results “very similar to CO2 in terms of the thermal injury profile,” he said. “It’s a versatile device. So, the CO2 in its native mode produces more thermal injury, while the erbium:YAG laser is more ablative. Both can be used effectively for facial skin rejuvenation.”
Full-field laser resurfacing requires local infiltration with lidocaine 1% or 2% with epinephrine or general anesthesia. “This is not a treatment that you can do comfortably under topical anesthesia, even if you’re using cold air unless you are doing treatments essentially confined to the epidermis and superficial dermis,” Dr. Biesman said. “When working around the eyes or on the face you need to use ocular protection with metal ocular shields. There’s no two ways about it. There is no scenario in which you’re doing an ablative resurfacing around the eye where you don’t use metal corneal shields.”
Energy and density levels can be fine-tuned in order to optimize treatment. For deep rhytides in the perioral region or on the forehead or lateral cheeks, clinicians may choose to treat at a higher density, while rhytides located in other areas may respond well to treatments at a lower density. Relative danger zones include the eyelids in general, especially the medial lower eyelid, as well as the upper lip. “These are the areas that are most prone to developing scarring,” he said.
For the upper eyelids, Dr. Biesman treats from the lashes to the upper brow. “It’s important to protect the lashes and treat from the lower-lid margin all the way down to the orbital rim. I debride relatively aggressively. I want to debride all the eschar created by the first pass and come back with a second pass. I sometimes will decrease the density on the second pass, depending on the type of tissue response that I see. If I see a dramatic response on the second pass I will definitely decrease the density.” He uses Aquaphor to protect the eyebrows. “It’s difficult to do that on the lashes. For the lashes, I usually use a wet tongue blade and keep the lid on stretch as I do my treatments.”
Dr. Biesman recommends feathering to blend full-field treatments with the neck. This means bringing treatments below the mandible. “There are times when we want to conservatively treat the neck,” he said. “The neck does not recover nearly as well after ablative resurfacing as the face does due to the fact that there’s probably about 90% fewer sebaceous glands and hair follicles in the neck relative to the face.”
In Dr. Biesman’s opinion, the important perioperative preparation is counseling the patient, including setting realistic expectations and devising a plan for wound care. “They can expect 7-10 days to heal, depending on the area we’re treating and the relative aggressiveness of the planned treatment,” he said. For patients with a history of herpes simplex virus type 1, he recommends antiviral treatment prior to the procedure. “If you do encounter a herpetic infection postoperatively, you may not see typical clinical signs of blistering as the epidermis has been removed.”
Dr. Biesman uses both antiviral and antibiotic prophylaxis prior to full-field treatments. “The literature by and large says that antibiotic/antiviral prophylaxis is not required prior to full-face ablation,” he said. “The reason I choose to is that I have had some issues with community-acquired MRSA infections. Because it’s so ubiquitous these days, I typically do prescribe an agent that gives good MRSA coverage.”
As for wound care, the literature differs on open versus closed techniques. Dr. Biesman favors using Aquaphor for the first week or so and seeing patients back on posttreatment day 2, “who by that time are usually beyond the inflammatory phase of wound healing,” he said. “A lot of the initial oozing has stopped by then. We clean that off any dried exudate in the office very carefully. We debride gently with warm-water soaks and I like to use PRP [platelet-rich plasma]. There is literature to support the role of PRP in wound healing.”
Even in the most experienced hands, complications can occur from full-field laser resurfacing, including bacterial, viral, or fungal infections. Other potential complications include persistent erythema, hypopigmentation, hyperpigmentation, scarring, and ectropion. “Knowledge of treatment parameters, endpoints, and wound healing is required for safe and successful outcomes,” Dr. Biesman said.
He reported having no relevant disclosures related to his presentation.
according to Brian S. Biesman, MD.
“When performing laser skin resurfacing, our goal is to match the degree of injury to the needs of the patient we’re treating,” Dr. Biesman, an oculofacial plastic surgeon who practices in Nashville, Tenn., said during a virtual course on laser and aesthetic skin therapy.
“If we’re treating a 35-year-old with minimal photoaging, we don’t need to use full-field resurfacing. By the same token, a 60-year-old who’s never had anything but sun exposure is not going to do well with nonablative fractional resurfacing or other modalities that produce only modest changes,” he noted. “Full ablative resurfacing is a useful tool that can be used to treat a variety of patients. We can tailor each treatment to the individual patient. We can simply dial the energy up or down and adjust the density.”
Full-field laser ablation removes the epidermis as well as a part of the dermis, and the degree of dermal injury varies depending on the relative aggressiveness of the treatment. “We can treat very superficially in the dermis or we can do deep dermal treatments,” he said at the meeting, which was sponsored by Harvard Medical School, Massachusetts General Hospital, and the Wellman Center for Photomedicine.
“The residual thermal injury will vary to some degree, depending on our treatment parameters. It does cause immediate collagen contracture. It also stimulates a process of neocollagenesis.”
Two main lasers used for full-field treatments are the erbium:YAG laser and the CO2 laser at wavelengths of 2,940 nm and 10,600 nm, respectively. “The erbium:YAG is far more highly absorbed by water, by a factor of about 13,” he said. “But both of these wavelengths can be used successfully as long as you understand the physics behind them.”
The short-pulsed erbium:YAG laser ablates effectively, producing about a 10 mcm zone of thermal injury. “That’s not going to induce much by the way of remodeling, but it will be effective in removing tissue from the superficial layers of the skin,” said Dr. Biesman, who is a past president of the American Society for Laser Medicine and Surgery. “There’s also an absorption peak for collagen, so if you’re treating a scar, this laser can be highly effective.”
The CO2 laser creates more residual thermal injury during full-field resurfacing, compared with the short-pulsed erbium:YAG laser. The long-pulsed erbium:YAG laser can be used in both short- and long-pulsed modes and is more ablative than the CO2 laser when used in short-pulsed mode. When used in long-pulse mode, it makes it possible to produce results “very similar to CO2 in terms of the thermal injury profile,” he said. “It’s a versatile device. So, the CO2 in its native mode produces more thermal injury, while the erbium:YAG laser is more ablative. Both can be used effectively for facial skin rejuvenation.”
Full-field laser resurfacing requires local infiltration with lidocaine 1% or 2% with epinephrine or general anesthesia. “This is not a treatment that you can do comfortably under topical anesthesia, even if you’re using cold air unless you are doing treatments essentially confined to the epidermis and superficial dermis,” Dr. Biesman said. “When working around the eyes or on the face you need to use ocular protection with metal ocular shields. There’s no two ways about it. There is no scenario in which you’re doing an ablative resurfacing around the eye where you don’t use metal corneal shields.”
Energy and density levels can be fine-tuned in order to optimize treatment. For deep rhytides in the perioral region or on the forehead or lateral cheeks, clinicians may choose to treat at a higher density, while rhytides located in other areas may respond well to treatments at a lower density. Relative danger zones include the eyelids in general, especially the medial lower eyelid, as well as the upper lip. “These are the areas that are most prone to developing scarring,” he said.
For the upper eyelids, Dr. Biesman treats from the lashes to the upper brow. “It’s important to protect the lashes and treat from the lower-lid margin all the way down to the orbital rim. I debride relatively aggressively. I want to debride all the eschar created by the first pass and come back with a second pass. I sometimes will decrease the density on the second pass, depending on the type of tissue response that I see. If I see a dramatic response on the second pass I will definitely decrease the density.” He uses Aquaphor to protect the eyebrows. “It’s difficult to do that on the lashes. For the lashes, I usually use a wet tongue blade and keep the lid on stretch as I do my treatments.”
Dr. Biesman recommends feathering to blend full-field treatments with the neck. This means bringing treatments below the mandible. “There are times when we want to conservatively treat the neck,” he said. “The neck does not recover nearly as well after ablative resurfacing as the face does due to the fact that there’s probably about 90% fewer sebaceous glands and hair follicles in the neck relative to the face.”
In Dr. Biesman’s opinion, the important perioperative preparation is counseling the patient, including setting realistic expectations and devising a plan for wound care. “They can expect 7-10 days to heal, depending on the area we’re treating and the relative aggressiveness of the planned treatment,” he said. For patients with a history of herpes simplex virus type 1, he recommends antiviral treatment prior to the procedure. “If you do encounter a herpetic infection postoperatively, you may not see typical clinical signs of blistering as the epidermis has been removed.”
Dr. Biesman uses both antiviral and antibiotic prophylaxis prior to full-field treatments. “The literature by and large says that antibiotic/antiviral prophylaxis is not required prior to full-face ablation,” he said. “The reason I choose to is that I have had some issues with community-acquired MRSA infections. Because it’s so ubiquitous these days, I typically do prescribe an agent that gives good MRSA coverage.”
As for wound care, the literature differs on open versus closed techniques. Dr. Biesman favors using Aquaphor for the first week or so and seeing patients back on posttreatment day 2, “who by that time are usually beyond the inflammatory phase of wound healing,” he said. “A lot of the initial oozing has stopped by then. We clean that off any dried exudate in the office very carefully. We debride gently with warm-water soaks and I like to use PRP [platelet-rich plasma]. There is literature to support the role of PRP in wound healing.”
Even in the most experienced hands, complications can occur from full-field laser resurfacing, including bacterial, viral, or fungal infections. Other potential complications include persistent erythema, hypopigmentation, hyperpigmentation, scarring, and ectropion. “Knowledge of treatment parameters, endpoints, and wound healing is required for safe and successful outcomes,” Dr. Biesman said.
He reported having no relevant disclosures related to his presentation.
FROM A LASER & AESTHETIC SKIN THERAPY COURSE
Deucravacitinib offers biologic-like psoriasis efficacy in oral form
and a range of other chronic inflammatory diseases, Bruce E. Strober, MD, PhD, said at MedscapeLive’s annual Las Vegas Dermatology Seminar, held virtually this year.
Deucravacitinib solely blocks tyrosine kinase 2 (TYK2) signaling without touching Janus kinase (JAK) 1, 2, or 3. In so doing, it inhibits several cytokines important for inflammation: interleukin-12, IL-13, and interferon-alpha and -beta. Yet it doesn’t affect the numerous pathways mediated by JAKs 1-3, many of which relate to growth and development of cell lineages, including production of erythropoietin, thrombopoietin, granulocyte-macrophage colony-stimulating factor, prolactin, growth hormone, and leptin. These deucravacitinib characteristics should translate into fewer off-target side effects than with oral JAK inhibitors.
“The promise of TYK2 inhibition that’s brought to you by deucravacitinib is there will be no laboratory monitoring and the effects will be narrow in blocking inflammation,” said Dr. Strober, a dermatologist at Yale University, New Haven, Conn., and in private practice in Cromwell, Conn.
He highlighted the positive results of a randomized, phase 2, dose-ranging study conducted in 267 patients with moderate or severe plaque psoriasis. Participants had an average baseline Psoriasis Area and Severity Index (PASI) score of 19, with a Dermatology Life Quality Index score of about 12. At the top dose of 12 mg once daily, 75% of patients achieved a PASI 75 response at week 12, and 44% reached a PASI 90, as did 69% and 44%, respectively, who were on deucravacitinib at 3 mg twice daily. Those are collective efficacy numbers similar to adalimumab (Humira) or ustekinumab (Stelara).
Deucravacitinib may provide efficacy “like one of our second-tier biological therapies, yet it will be oral,” Dr. Strober commented.
Importantly, no laboratory abnormalities were detected in this trial. Only mild side effects were documented, most prominently acne, which occurred in dose-dependent fashion in 2% of patients on 3 mg of deucravacitinib twice daily and 4% at 12 mg once daily.
“The treatment of the acne that is elicited by this drug is yet to be fully described, but I’m sure we’ll learn the best approaches, given that acne is in our wheel house,” the dermatologist added.
Bristol-Myers Squibb has announced positive results from the pivotal phase 3 POETYK PSO-1 trial. Deucravacitinib at 6 mg once daily met both of its coprimary efficacy endpoints in the study, which included 666 patients with moderate to severe psoriasis. The TYK 2 inhibitor demonstrated superiority to both placebo and oral apremilast (Otezla) at week 16. The company said the safety profile was consistent with the phase 2 results, and that the full details of the phase 3 trial will be presented next year at a major medical meeting.
In addition, positive phase 2 results were reported for deucravacitinib in the treatment of psoriatic arthritis in a randomized trial presented at the fall 2020 meeting of the American College of Rheumatology. Deucravacitinib is also under study for lupus and inflammatory bowel disease.
Dr. Strober, an active clinical trialist, reported serving as a consultant to more than two dozen pharmaceutical companies, including Bristol-Myers Squibb.
MedscapeLive and this news organization are owned by the same parent company.
and a range of other chronic inflammatory diseases, Bruce E. Strober, MD, PhD, said at MedscapeLive’s annual Las Vegas Dermatology Seminar, held virtually this year.
Deucravacitinib solely blocks tyrosine kinase 2 (TYK2) signaling without touching Janus kinase (JAK) 1, 2, or 3. In so doing, it inhibits several cytokines important for inflammation: interleukin-12, IL-13, and interferon-alpha and -beta. Yet it doesn’t affect the numerous pathways mediated by JAKs 1-3, many of which relate to growth and development of cell lineages, including production of erythropoietin, thrombopoietin, granulocyte-macrophage colony-stimulating factor, prolactin, growth hormone, and leptin. These deucravacitinib characteristics should translate into fewer off-target side effects than with oral JAK inhibitors.
“The promise of TYK2 inhibition that’s brought to you by deucravacitinib is there will be no laboratory monitoring and the effects will be narrow in blocking inflammation,” said Dr. Strober, a dermatologist at Yale University, New Haven, Conn., and in private practice in Cromwell, Conn.
He highlighted the positive results of a randomized, phase 2, dose-ranging study conducted in 267 patients with moderate or severe plaque psoriasis. Participants had an average baseline Psoriasis Area and Severity Index (PASI) score of 19, with a Dermatology Life Quality Index score of about 12. At the top dose of 12 mg once daily, 75% of patients achieved a PASI 75 response at week 12, and 44% reached a PASI 90, as did 69% and 44%, respectively, who were on deucravacitinib at 3 mg twice daily. Those are collective efficacy numbers similar to adalimumab (Humira) or ustekinumab (Stelara).
Deucravacitinib may provide efficacy “like one of our second-tier biological therapies, yet it will be oral,” Dr. Strober commented.
Importantly, no laboratory abnormalities were detected in this trial. Only mild side effects were documented, most prominently acne, which occurred in dose-dependent fashion in 2% of patients on 3 mg of deucravacitinib twice daily and 4% at 12 mg once daily.
“The treatment of the acne that is elicited by this drug is yet to be fully described, but I’m sure we’ll learn the best approaches, given that acne is in our wheel house,” the dermatologist added.
Bristol-Myers Squibb has announced positive results from the pivotal phase 3 POETYK PSO-1 trial. Deucravacitinib at 6 mg once daily met both of its coprimary efficacy endpoints in the study, which included 666 patients with moderate to severe psoriasis. The TYK 2 inhibitor demonstrated superiority to both placebo and oral apremilast (Otezla) at week 16. The company said the safety profile was consistent with the phase 2 results, and that the full details of the phase 3 trial will be presented next year at a major medical meeting.
In addition, positive phase 2 results were reported for deucravacitinib in the treatment of psoriatic arthritis in a randomized trial presented at the fall 2020 meeting of the American College of Rheumatology. Deucravacitinib is also under study for lupus and inflammatory bowel disease.
Dr. Strober, an active clinical trialist, reported serving as a consultant to more than two dozen pharmaceutical companies, including Bristol-Myers Squibb.
MedscapeLive and this news organization are owned by the same parent company.
and a range of other chronic inflammatory diseases, Bruce E. Strober, MD, PhD, said at MedscapeLive’s annual Las Vegas Dermatology Seminar, held virtually this year.
Deucravacitinib solely blocks tyrosine kinase 2 (TYK2) signaling without touching Janus kinase (JAK) 1, 2, or 3. In so doing, it inhibits several cytokines important for inflammation: interleukin-12, IL-13, and interferon-alpha and -beta. Yet it doesn’t affect the numerous pathways mediated by JAKs 1-3, many of which relate to growth and development of cell lineages, including production of erythropoietin, thrombopoietin, granulocyte-macrophage colony-stimulating factor, prolactin, growth hormone, and leptin. These deucravacitinib characteristics should translate into fewer off-target side effects than with oral JAK inhibitors.
“The promise of TYK2 inhibition that’s brought to you by deucravacitinib is there will be no laboratory monitoring and the effects will be narrow in blocking inflammation,” said Dr. Strober, a dermatologist at Yale University, New Haven, Conn., and in private practice in Cromwell, Conn.
He highlighted the positive results of a randomized, phase 2, dose-ranging study conducted in 267 patients with moderate or severe plaque psoriasis. Participants had an average baseline Psoriasis Area and Severity Index (PASI) score of 19, with a Dermatology Life Quality Index score of about 12. At the top dose of 12 mg once daily, 75% of patients achieved a PASI 75 response at week 12, and 44% reached a PASI 90, as did 69% and 44%, respectively, who were on deucravacitinib at 3 mg twice daily. Those are collective efficacy numbers similar to adalimumab (Humira) or ustekinumab (Stelara).
Deucravacitinib may provide efficacy “like one of our second-tier biological therapies, yet it will be oral,” Dr. Strober commented.
Importantly, no laboratory abnormalities were detected in this trial. Only mild side effects were documented, most prominently acne, which occurred in dose-dependent fashion in 2% of patients on 3 mg of deucravacitinib twice daily and 4% at 12 mg once daily.
“The treatment of the acne that is elicited by this drug is yet to be fully described, but I’m sure we’ll learn the best approaches, given that acne is in our wheel house,” the dermatologist added.
Bristol-Myers Squibb has announced positive results from the pivotal phase 3 POETYK PSO-1 trial. Deucravacitinib at 6 mg once daily met both of its coprimary efficacy endpoints in the study, which included 666 patients with moderate to severe psoriasis. The TYK 2 inhibitor demonstrated superiority to both placebo and oral apremilast (Otezla) at week 16. The company said the safety profile was consistent with the phase 2 results, and that the full details of the phase 3 trial will be presented next year at a major medical meeting.
In addition, positive phase 2 results were reported for deucravacitinib in the treatment of psoriatic arthritis in a randomized trial presented at the fall 2020 meeting of the American College of Rheumatology. Deucravacitinib is also under study for lupus and inflammatory bowel disease.
Dr. Strober, an active clinical trialist, reported serving as a consultant to more than two dozen pharmaceutical companies, including Bristol-Myers Squibb.
MedscapeLive and this news organization are owned by the same parent company.
FROM MEDSCAPELIVE LAS VEGAS DERMATOLOGY SEMINAR
Baseline body surface area may drive optimal baricitinib responses
results from an analysis of phase 3 data showed.
“This proposed clinical tailoring approach for baricitinib 2 mg allows for treatment of patients who are more likely to respond to therapy and rapid decision on discontinuation of treatment for those who are not likely to benefit from baricitinib 2 mg,” Eric L. Simpson, MD, said during a late-breaking abstract session at the Revolutionizing Atopic Dermatitis virtual symposium.
Baricitinib is an oral, reversible and selective Janus kinase 1/JAK2 inhibitor that is approved in Europe for the treatment of moderate to severe AD in adults who are candidates for systemic therapy. In the United States, it is approved for treating rheumatoid arthritis, and is currently under Food and Drug Administration review in the United States for AD.
For the current analysis, Dr. Simpson, professor of dermatology at Oregon Health & Science University, Portland, and colleagues set out to identify responders to baricitinib 2 mg using a tailored approach based on baseline BSA affected and early clinical improvement in the phase 3 monotherapy trial BREEZE-AD5. The trial enrolled 440 patients: 147 to placebo, 147 to baricitinib 1 mg once daily, and 146 to baricitinib 2 mg once daily. The primary endpoint was Eczema Area and Severity Index (EASI)–75 at week 16.
“Understanding which patients can benefit most from this treatment was our goal,” Dr. Simpson said. “By tailoring your therapy, you can significantly improve the patient experience, increase the cost-effectiveness of a therapy, and you can ensure that only patients who are likely to benefit are exposed to a drug.”
The researchers used a classification and regression tree algorithm that identified baseline BSA as the strongest predictor of EASI-75 response at week 16. A BSA cutoff of 50% was established as the optimal cutoff for sensitivity and negative predictive value. Results for EASI-75 and Validated Investigator Global Assessment for Atopic Dermatitis (vIGA-AD) scores of 0 or 1 were confirmed using a BSA of 10%-50% at baseline to predict response, compared with a BSA or greater than 50% at baseline.
Sensitivity analyses revealed that about 90% of patients with an EASI-75 response were in the BSA 10%-50% group. Conversely, among patients with a BSA greater than 50%, the negative predictive value was greater than 90%, “so there’s a 90% chance you’re not going to hit that EASI-75 at week 16 if your BSA is greater than 50%,” Dr. Simpson explained. “The same holds true for vIGA-AD, so that 50% cutoff is important for understanding whether someone is going to respond or not.”
On the EASI-75, 38% of patients in the BSA 10%-50% group responded to baricitinib at week 16, compared with 10% in the BSA greater than 50% group. A similar association was observed on the vIGA-AD, where 32% of patients in the BSA 10%-50% group responded to baricitinib at week 16, compared with 5% in the BSA greater than 50% group.
When stratified by early response assessed at week 4, based on a 4-point improvement or greater on the Itch Numeric Rating Scale, 55% of those patients became EASI-75 responders, compared with 17% who were not. A similar association was observed by early response assessed at week 8.
“Due to the rapid onset of response, clinical assessment of patients after 4-8 weeks of initiation of baricitinib 2 mg treatment provided a positive feedback to patients who are likely to benefit from long-term therapy,” Dr. Simpson said. “This analysis may allow for a precision-medicine approach to therapy in moderate to severe AD.”
The study was supported by Eli Lilly, and was under license from Incyte. Dr. Simpson reported serving as an investigator for and consultant to numerous pharmaceutical companies.
results from an analysis of phase 3 data showed.
“This proposed clinical tailoring approach for baricitinib 2 mg allows for treatment of patients who are more likely to respond to therapy and rapid decision on discontinuation of treatment for those who are not likely to benefit from baricitinib 2 mg,” Eric L. Simpson, MD, said during a late-breaking abstract session at the Revolutionizing Atopic Dermatitis virtual symposium.
Baricitinib is an oral, reversible and selective Janus kinase 1/JAK2 inhibitor that is approved in Europe for the treatment of moderate to severe AD in adults who are candidates for systemic therapy. In the United States, it is approved for treating rheumatoid arthritis, and is currently under Food and Drug Administration review in the United States for AD.
For the current analysis, Dr. Simpson, professor of dermatology at Oregon Health & Science University, Portland, and colleagues set out to identify responders to baricitinib 2 mg using a tailored approach based on baseline BSA affected and early clinical improvement in the phase 3 monotherapy trial BREEZE-AD5. The trial enrolled 440 patients: 147 to placebo, 147 to baricitinib 1 mg once daily, and 146 to baricitinib 2 mg once daily. The primary endpoint was Eczema Area and Severity Index (EASI)–75 at week 16.
“Understanding which patients can benefit most from this treatment was our goal,” Dr. Simpson said. “By tailoring your therapy, you can significantly improve the patient experience, increase the cost-effectiveness of a therapy, and you can ensure that only patients who are likely to benefit are exposed to a drug.”
The researchers used a classification and regression tree algorithm that identified baseline BSA as the strongest predictor of EASI-75 response at week 16. A BSA cutoff of 50% was established as the optimal cutoff for sensitivity and negative predictive value. Results for EASI-75 and Validated Investigator Global Assessment for Atopic Dermatitis (vIGA-AD) scores of 0 or 1 were confirmed using a BSA of 10%-50% at baseline to predict response, compared with a BSA or greater than 50% at baseline.
Sensitivity analyses revealed that about 90% of patients with an EASI-75 response were in the BSA 10%-50% group. Conversely, among patients with a BSA greater than 50%, the negative predictive value was greater than 90%, “so there’s a 90% chance you’re not going to hit that EASI-75 at week 16 if your BSA is greater than 50%,” Dr. Simpson explained. “The same holds true for vIGA-AD, so that 50% cutoff is important for understanding whether someone is going to respond or not.”
On the EASI-75, 38% of patients in the BSA 10%-50% group responded to baricitinib at week 16, compared with 10% in the BSA greater than 50% group. A similar association was observed on the vIGA-AD, where 32% of patients in the BSA 10%-50% group responded to baricitinib at week 16, compared with 5% in the BSA greater than 50% group.
When stratified by early response assessed at week 4, based on a 4-point improvement or greater on the Itch Numeric Rating Scale, 55% of those patients became EASI-75 responders, compared with 17% who were not. A similar association was observed by early response assessed at week 8.
“Due to the rapid onset of response, clinical assessment of patients after 4-8 weeks of initiation of baricitinib 2 mg treatment provided a positive feedback to patients who are likely to benefit from long-term therapy,” Dr. Simpson said. “This analysis may allow for a precision-medicine approach to therapy in moderate to severe AD.”
The study was supported by Eli Lilly, and was under license from Incyte. Dr. Simpson reported serving as an investigator for and consultant to numerous pharmaceutical companies.
results from an analysis of phase 3 data showed.
“This proposed clinical tailoring approach for baricitinib 2 mg allows for treatment of patients who are more likely to respond to therapy and rapid decision on discontinuation of treatment for those who are not likely to benefit from baricitinib 2 mg,” Eric L. Simpson, MD, said during a late-breaking abstract session at the Revolutionizing Atopic Dermatitis virtual symposium.
Baricitinib is an oral, reversible and selective Janus kinase 1/JAK2 inhibitor that is approved in Europe for the treatment of moderate to severe AD in adults who are candidates for systemic therapy. In the United States, it is approved for treating rheumatoid arthritis, and is currently under Food and Drug Administration review in the United States for AD.
For the current analysis, Dr. Simpson, professor of dermatology at Oregon Health & Science University, Portland, and colleagues set out to identify responders to baricitinib 2 mg using a tailored approach based on baseline BSA affected and early clinical improvement in the phase 3 monotherapy trial BREEZE-AD5. The trial enrolled 440 patients: 147 to placebo, 147 to baricitinib 1 mg once daily, and 146 to baricitinib 2 mg once daily. The primary endpoint was Eczema Area and Severity Index (EASI)–75 at week 16.
“Understanding which patients can benefit most from this treatment was our goal,” Dr. Simpson said. “By tailoring your therapy, you can significantly improve the patient experience, increase the cost-effectiveness of a therapy, and you can ensure that only patients who are likely to benefit are exposed to a drug.”
The researchers used a classification and regression tree algorithm that identified baseline BSA as the strongest predictor of EASI-75 response at week 16. A BSA cutoff of 50% was established as the optimal cutoff for sensitivity and negative predictive value. Results for EASI-75 and Validated Investigator Global Assessment for Atopic Dermatitis (vIGA-AD) scores of 0 or 1 were confirmed using a BSA of 10%-50% at baseline to predict response, compared with a BSA or greater than 50% at baseline.
Sensitivity analyses revealed that about 90% of patients with an EASI-75 response were in the BSA 10%-50% group. Conversely, among patients with a BSA greater than 50%, the negative predictive value was greater than 90%, “so there’s a 90% chance you’re not going to hit that EASI-75 at week 16 if your BSA is greater than 50%,” Dr. Simpson explained. “The same holds true for vIGA-AD, so that 50% cutoff is important for understanding whether someone is going to respond or not.”
On the EASI-75, 38% of patients in the BSA 10%-50% group responded to baricitinib at week 16, compared with 10% in the BSA greater than 50% group. A similar association was observed on the vIGA-AD, where 32% of patients in the BSA 10%-50% group responded to baricitinib at week 16, compared with 5% in the BSA greater than 50% group.
When stratified by early response assessed at week 4, based on a 4-point improvement or greater on the Itch Numeric Rating Scale, 55% of those patients became EASI-75 responders, compared with 17% who were not. A similar association was observed by early response assessed at week 8.
“Due to the rapid onset of response, clinical assessment of patients after 4-8 weeks of initiation of baricitinib 2 mg treatment provided a positive feedback to patients who are likely to benefit from long-term therapy,” Dr. Simpson said. “This analysis may allow for a precision-medicine approach to therapy in moderate to severe AD.”
The study was supported by Eli Lilly, and was under license from Incyte. Dr. Simpson reported serving as an investigator for and consultant to numerous pharmaceutical companies.
FROM REVOLUTIONIZING AD 2020
Avoiding atopic dermatitis triggers easier said than done
“Guidelines on trigger avoidance are written as if it’s easy to do,” Jonathan I. Silverberg, MD, PhD, MPH, said during the Revolutionizing Atopic Dermatitis virtual symposium. “It turns out that trigger avoidance is really complicated.”
He and his colleagues conducted a study of most common triggers for itch based on a prospective dermatology practice–based study of 587 adults with AD . About two-thirds (65%) reported one or more itch trigger in the past week and 36% had three or more itch triggers in the past week. The two most common triggers were stress (35%) and sweat (31%).
“To me, this is provocative, because this is not how I was trained in residency,” said Dr. Silverberg, director of clinical research in the division of dermatology at George Washington University, Washington. “I was trained that it’s all about excess showering, dry air, or cold temperature. Those are important, but the most common triggers are stress and sweat.”
AD triggers are also commonly linked to seasonality. “If you ask patients when their AD is worse, sometimes it’s winter,” he said. “Sometimes it’s spring. Sometimes it’s summer. It turns out that there is a distinct set of triggers that are associated with AD seasonality.” Wintertime worsening of disease is associated with cold temperature and weather change, he continued, while springtime worsening of disease is often linked to weather change and dry air. Common summertime triggers for flares include hot temperature, heat, sweat, weather change, sunlight, humid air, and dry air. “In the fall, the weather change again comes up as a trigger. Humid air does as well.”
In their prospective study, Dr. Silverberg and colleagues found that 90% of those who had at least three itch triggers reported 3 months or less of AD remission in the past year, “meaning that 90% are reporting persistent disease when they have multiple itch triggers,” he said. In addition, 78% reported two or more flares per year and 61% reported that AD is worse during certain seasons.
Potential mitigation strategies for stress include stress management, biofeedback, meditation, relaxation training, and mindfulness. “These don’t necessarily require expensive psychotherapy,” he said. Freely available iPhone apps can be incorporated into daily practice, such as Calm, Relax with Andrew Johnson, Nature Sounds Relax and Sleep, Breathe2Relax, and Headspace.
Many AD patients are sedentary and avoid vigorous physical activity owing to heat and sweat as triggers. Simple solutions include exercising in a cooler temperature environment, “not just using fans,” he said. “Take a quick shower right after working out and consider pre- and/or post treatment with topical medication.”
High temperature and sweating can be problematic at bedtime, he continued. Even if the indoor temperature is 70° F, that might jump to 85° F or 90° F under a thick blanket. “That heat can trigger itch and may cause sweating, which can trigger itch,” said Dr. Silverberg, who has AD and is director of patch testing at George Washington University. Potential solutions include using a lighter blanket, lowering the indoor temperature, and wearing breathable pajamas.
Dryness, another common AD trigger, can be secondary to a combination of low outdoor and/or indoor humidity. “Lower outdoor humidity is a particular problem in the wintertime, because cold air doesn’t hold moisture as well,” he said. “That’s why the air feels much dryer in the wintertime. There’s also a problem of indoor heating and cooling. Sometimes central air systems can lower humidity to the point where it’s bone dry.”
In an effort to determine the impact of specific climatic factors on the U.S. prevalence of AD, Dr. Silverberg and colleagues conducted a study using a merged analysis of the 2007 National Survey of Children’s Health from a representative sample of 91,642 children aged 0-17 years and 2006-2007 measurements from the National Climate Data Center and Weather Service. They found that childhood AD prevalence was increased in geographical areas that use more indoor heat and cooling and had lower outdoor humidity. “So, we see that there’s a direct correlate of this dryness issue that is leading to more AD throughout the U.S.,” he said.
Practical solutions to mitigate the effect of dry air on AD include opening windows to allow entry of moist air, “which can be particularly helpful in residences that are overheated,” he said. “I deal with this a lot in patients who live in dormitories. Use humidifiers to add moisture back into the air. Aim for 40%-50% indoor humidity to avoid mold and dust mites. It’s better to use demineralized water to reduce bacterial growth. This can be helpful for aeroallergies. Of note, there are really no well-done studies that have examined the efficacy of humidifiers in AD, but based on our anecdotal experience, this is a good way to go.”
Cold temperatures and trigger intense itch, even in the setting of high humidity. “For me personally, this is one of my most brutal triggers,” Dr. Silverberg said. “When I’m in a place with extremes of cold, I get a rapid onset of itch, a mix of itch and pain, particularly on the dorsal hands. For solutions, you can encourage patients to avoid extremely low temperatures, to bundle up, and to potentially use hand warmers or other heating devices.”
Clothing can be a trigger as well, especially tight-fitting clothes, hot and nonbreathable clothes, and large-diameter wool, which has been shown to induce itching and irritation. Mitigation strategies include wearing loose-fitting, lightweight, nonirritating fabric. “Traditional cotton and silk fabrics have mixed evidence in improving AD but are generally safe,” he said. “Ultra- or superfine merino wool has been shown to be nonpruritic. There is sparse evidence to support chemically treated/coated clothing for AD, but this may be an emerging area.”
Dr. Silverberg pointed out variability of cultural perspectives and preferences for bathing practices, including temperature, duration, frequency, optimal bathing products, and the use of loofahs and other scrubbing products. “This stems from different perceptions of what it means to be clean, and how dry our skin should feel after a shower,” he said. “Many clinicians and patients were taught that regular bathing is harmful in AD. It turns out that’s not true.”
In a recently published systematic review and meta-analysis of 13 studies, he and his colleagues examined efficacy outcomes of different bathing/showering regimens in AD. All 13 studies showed numerically reduced AD severity with any bathing regimen in at least one time point. Numerical decreases over time were observed for body surface area (BSA), Eczema Area and Severity Index (EASI), and/or SCORAD measures for daily and less than daily bathing, with or without application of emollients or topical corticosteroids. In random effects regression models, taking baths more than or less than seven times per week were not associated with significant differences of Cohen’s D scores for EASI, SCORAD, or BSA. “The take-home message here is, let your AD patients bathe,” Dr. Silverberg said. “Bathing is good. It can be channeled to help the eczema, but it has to be done the right way.”
Patients should be counseled to use nonirritating cleansers and shampoos, avoid excessively long baths/showers, avoid excessively hot baths/showers, avoid excessive rubbing or scrubbing of skin, and to apply emollients and/or topical corticosteroids immediately after the bath/shower.
PROMIS Itch-Triggers is a simple and feasible checklist to screen for the most common itch triggers in AD in clinical practice (patients are asked to check off which of the following have caused their itch in the previous 7 days: cold temperature, hot temperature, heat, sweat, tight clothing, fragrances, boredom, talking about itch, stress, weather change, sunlight, humid air, dry air). “It takes less than 1 minute to complete,” he said. “Additional testing with skin patch and/or prick testing may be warranted to identify allergenic triggers.”
Dr. Silverberg reported that he is a consultant to and/or an advisory board member for several pharmaceutical companies. He is also a speaker for Regeneron and Sanofi and has received a grant from Galderma.
“Guidelines on trigger avoidance are written as if it’s easy to do,” Jonathan I. Silverberg, MD, PhD, MPH, said during the Revolutionizing Atopic Dermatitis virtual symposium. “It turns out that trigger avoidance is really complicated.”
He and his colleagues conducted a study of most common triggers for itch based on a prospective dermatology practice–based study of 587 adults with AD . About two-thirds (65%) reported one or more itch trigger in the past week and 36% had three or more itch triggers in the past week. The two most common triggers were stress (35%) and sweat (31%).
“To me, this is provocative, because this is not how I was trained in residency,” said Dr. Silverberg, director of clinical research in the division of dermatology at George Washington University, Washington. “I was trained that it’s all about excess showering, dry air, or cold temperature. Those are important, but the most common triggers are stress and sweat.”
AD triggers are also commonly linked to seasonality. “If you ask patients when their AD is worse, sometimes it’s winter,” he said. “Sometimes it’s spring. Sometimes it’s summer. It turns out that there is a distinct set of triggers that are associated with AD seasonality.” Wintertime worsening of disease is associated with cold temperature and weather change, he continued, while springtime worsening of disease is often linked to weather change and dry air. Common summertime triggers for flares include hot temperature, heat, sweat, weather change, sunlight, humid air, and dry air. “In the fall, the weather change again comes up as a trigger. Humid air does as well.”
In their prospective study, Dr. Silverberg and colleagues found that 90% of those who had at least three itch triggers reported 3 months or less of AD remission in the past year, “meaning that 90% are reporting persistent disease when they have multiple itch triggers,” he said. In addition, 78% reported two or more flares per year and 61% reported that AD is worse during certain seasons.
Potential mitigation strategies for stress include stress management, biofeedback, meditation, relaxation training, and mindfulness. “These don’t necessarily require expensive psychotherapy,” he said. Freely available iPhone apps can be incorporated into daily practice, such as Calm, Relax with Andrew Johnson, Nature Sounds Relax and Sleep, Breathe2Relax, and Headspace.
Many AD patients are sedentary and avoid vigorous physical activity owing to heat and sweat as triggers. Simple solutions include exercising in a cooler temperature environment, “not just using fans,” he said. “Take a quick shower right after working out and consider pre- and/or post treatment with topical medication.”
High temperature and sweating can be problematic at bedtime, he continued. Even if the indoor temperature is 70° F, that might jump to 85° F or 90° F under a thick blanket. “That heat can trigger itch and may cause sweating, which can trigger itch,” said Dr. Silverberg, who has AD and is director of patch testing at George Washington University. Potential solutions include using a lighter blanket, lowering the indoor temperature, and wearing breathable pajamas.
Dryness, another common AD trigger, can be secondary to a combination of low outdoor and/or indoor humidity. “Lower outdoor humidity is a particular problem in the wintertime, because cold air doesn’t hold moisture as well,” he said. “That’s why the air feels much dryer in the wintertime. There’s also a problem of indoor heating and cooling. Sometimes central air systems can lower humidity to the point where it’s bone dry.”
In an effort to determine the impact of specific climatic factors on the U.S. prevalence of AD, Dr. Silverberg and colleagues conducted a study using a merged analysis of the 2007 National Survey of Children’s Health from a representative sample of 91,642 children aged 0-17 years and 2006-2007 measurements from the National Climate Data Center and Weather Service. They found that childhood AD prevalence was increased in geographical areas that use more indoor heat and cooling and had lower outdoor humidity. “So, we see that there’s a direct correlate of this dryness issue that is leading to more AD throughout the U.S.,” he said.
Practical solutions to mitigate the effect of dry air on AD include opening windows to allow entry of moist air, “which can be particularly helpful in residences that are overheated,” he said. “I deal with this a lot in patients who live in dormitories. Use humidifiers to add moisture back into the air. Aim for 40%-50% indoor humidity to avoid mold and dust mites. It’s better to use demineralized water to reduce bacterial growth. This can be helpful for aeroallergies. Of note, there are really no well-done studies that have examined the efficacy of humidifiers in AD, but based on our anecdotal experience, this is a good way to go.”
Cold temperatures and trigger intense itch, even in the setting of high humidity. “For me personally, this is one of my most brutal triggers,” Dr. Silverberg said. “When I’m in a place with extremes of cold, I get a rapid onset of itch, a mix of itch and pain, particularly on the dorsal hands. For solutions, you can encourage patients to avoid extremely low temperatures, to bundle up, and to potentially use hand warmers or other heating devices.”
Clothing can be a trigger as well, especially tight-fitting clothes, hot and nonbreathable clothes, and large-diameter wool, which has been shown to induce itching and irritation. Mitigation strategies include wearing loose-fitting, lightweight, nonirritating fabric. “Traditional cotton and silk fabrics have mixed evidence in improving AD but are generally safe,” he said. “Ultra- or superfine merino wool has been shown to be nonpruritic. There is sparse evidence to support chemically treated/coated clothing for AD, but this may be an emerging area.”
Dr. Silverberg pointed out variability of cultural perspectives and preferences for bathing practices, including temperature, duration, frequency, optimal bathing products, and the use of loofahs and other scrubbing products. “This stems from different perceptions of what it means to be clean, and how dry our skin should feel after a shower,” he said. “Many clinicians and patients were taught that regular bathing is harmful in AD. It turns out that’s not true.”
In a recently published systematic review and meta-analysis of 13 studies, he and his colleagues examined efficacy outcomes of different bathing/showering regimens in AD. All 13 studies showed numerically reduced AD severity with any bathing regimen in at least one time point. Numerical decreases over time were observed for body surface area (BSA), Eczema Area and Severity Index (EASI), and/or SCORAD measures for daily and less than daily bathing, with or without application of emollients or topical corticosteroids. In random effects regression models, taking baths more than or less than seven times per week were not associated with significant differences of Cohen’s D scores for EASI, SCORAD, or BSA. “The take-home message here is, let your AD patients bathe,” Dr. Silverberg said. “Bathing is good. It can be channeled to help the eczema, but it has to be done the right way.”
Patients should be counseled to use nonirritating cleansers and shampoos, avoid excessively long baths/showers, avoid excessively hot baths/showers, avoid excessive rubbing or scrubbing of skin, and to apply emollients and/or topical corticosteroids immediately after the bath/shower.
PROMIS Itch-Triggers is a simple and feasible checklist to screen for the most common itch triggers in AD in clinical practice (patients are asked to check off which of the following have caused their itch in the previous 7 days: cold temperature, hot temperature, heat, sweat, tight clothing, fragrances, boredom, talking about itch, stress, weather change, sunlight, humid air, dry air). “It takes less than 1 minute to complete,” he said. “Additional testing with skin patch and/or prick testing may be warranted to identify allergenic triggers.”
Dr. Silverberg reported that he is a consultant to and/or an advisory board member for several pharmaceutical companies. He is also a speaker for Regeneron and Sanofi and has received a grant from Galderma.
“Guidelines on trigger avoidance are written as if it’s easy to do,” Jonathan I. Silverberg, MD, PhD, MPH, said during the Revolutionizing Atopic Dermatitis virtual symposium. “It turns out that trigger avoidance is really complicated.”
He and his colleagues conducted a study of most common triggers for itch based on a prospective dermatology practice–based study of 587 adults with AD . About two-thirds (65%) reported one or more itch trigger in the past week and 36% had three or more itch triggers in the past week. The two most common triggers were stress (35%) and sweat (31%).
“To me, this is provocative, because this is not how I was trained in residency,” said Dr. Silverberg, director of clinical research in the division of dermatology at George Washington University, Washington. “I was trained that it’s all about excess showering, dry air, or cold temperature. Those are important, but the most common triggers are stress and sweat.”
AD triggers are also commonly linked to seasonality. “If you ask patients when their AD is worse, sometimes it’s winter,” he said. “Sometimes it’s spring. Sometimes it’s summer. It turns out that there is a distinct set of triggers that are associated with AD seasonality.” Wintertime worsening of disease is associated with cold temperature and weather change, he continued, while springtime worsening of disease is often linked to weather change and dry air. Common summertime triggers for flares include hot temperature, heat, sweat, weather change, sunlight, humid air, and dry air. “In the fall, the weather change again comes up as a trigger. Humid air does as well.”
In their prospective study, Dr. Silverberg and colleagues found that 90% of those who had at least three itch triggers reported 3 months or less of AD remission in the past year, “meaning that 90% are reporting persistent disease when they have multiple itch triggers,” he said. In addition, 78% reported two or more flares per year and 61% reported that AD is worse during certain seasons.
Potential mitigation strategies for stress include stress management, biofeedback, meditation, relaxation training, and mindfulness. “These don’t necessarily require expensive psychotherapy,” he said. Freely available iPhone apps can be incorporated into daily practice, such as Calm, Relax with Andrew Johnson, Nature Sounds Relax and Sleep, Breathe2Relax, and Headspace.
Many AD patients are sedentary and avoid vigorous physical activity owing to heat and sweat as triggers. Simple solutions include exercising in a cooler temperature environment, “not just using fans,” he said. “Take a quick shower right after working out and consider pre- and/or post treatment with topical medication.”
High temperature and sweating can be problematic at bedtime, he continued. Even if the indoor temperature is 70° F, that might jump to 85° F or 90° F under a thick blanket. “That heat can trigger itch and may cause sweating, which can trigger itch,” said Dr. Silverberg, who has AD and is director of patch testing at George Washington University. Potential solutions include using a lighter blanket, lowering the indoor temperature, and wearing breathable pajamas.
Dryness, another common AD trigger, can be secondary to a combination of low outdoor and/or indoor humidity. “Lower outdoor humidity is a particular problem in the wintertime, because cold air doesn’t hold moisture as well,” he said. “That’s why the air feels much dryer in the wintertime. There’s also a problem of indoor heating and cooling. Sometimes central air systems can lower humidity to the point where it’s bone dry.”
In an effort to determine the impact of specific climatic factors on the U.S. prevalence of AD, Dr. Silverberg and colleagues conducted a study using a merged analysis of the 2007 National Survey of Children’s Health from a representative sample of 91,642 children aged 0-17 years and 2006-2007 measurements from the National Climate Data Center and Weather Service. They found that childhood AD prevalence was increased in geographical areas that use more indoor heat and cooling and had lower outdoor humidity. “So, we see that there’s a direct correlate of this dryness issue that is leading to more AD throughout the U.S.,” he said.
Practical solutions to mitigate the effect of dry air on AD include opening windows to allow entry of moist air, “which can be particularly helpful in residences that are overheated,” he said. “I deal with this a lot in patients who live in dormitories. Use humidifiers to add moisture back into the air. Aim for 40%-50% indoor humidity to avoid mold and dust mites. It’s better to use demineralized water to reduce bacterial growth. This can be helpful for aeroallergies. Of note, there are really no well-done studies that have examined the efficacy of humidifiers in AD, but based on our anecdotal experience, this is a good way to go.”
Cold temperatures and trigger intense itch, even in the setting of high humidity. “For me personally, this is one of my most brutal triggers,” Dr. Silverberg said. “When I’m in a place with extremes of cold, I get a rapid onset of itch, a mix of itch and pain, particularly on the dorsal hands. For solutions, you can encourage patients to avoid extremely low temperatures, to bundle up, and to potentially use hand warmers or other heating devices.”
Clothing can be a trigger as well, especially tight-fitting clothes, hot and nonbreathable clothes, and large-diameter wool, which has been shown to induce itching and irritation. Mitigation strategies include wearing loose-fitting, lightweight, nonirritating fabric. “Traditional cotton and silk fabrics have mixed evidence in improving AD but are generally safe,” he said. “Ultra- or superfine merino wool has been shown to be nonpruritic. There is sparse evidence to support chemically treated/coated clothing for AD, but this may be an emerging area.”
Dr. Silverberg pointed out variability of cultural perspectives and preferences for bathing practices, including temperature, duration, frequency, optimal bathing products, and the use of loofahs and other scrubbing products. “This stems from different perceptions of what it means to be clean, and how dry our skin should feel after a shower,” he said. “Many clinicians and patients were taught that regular bathing is harmful in AD. It turns out that’s not true.”
In a recently published systematic review and meta-analysis of 13 studies, he and his colleagues examined efficacy outcomes of different bathing/showering regimens in AD. All 13 studies showed numerically reduced AD severity with any bathing regimen in at least one time point. Numerical decreases over time were observed for body surface area (BSA), Eczema Area and Severity Index (EASI), and/or SCORAD measures for daily and less than daily bathing, with or without application of emollients or topical corticosteroids. In random effects regression models, taking baths more than or less than seven times per week were not associated with significant differences of Cohen’s D scores for EASI, SCORAD, or BSA. “The take-home message here is, let your AD patients bathe,” Dr. Silverberg said. “Bathing is good. It can be channeled to help the eczema, but it has to be done the right way.”
Patients should be counseled to use nonirritating cleansers and shampoos, avoid excessively long baths/showers, avoid excessively hot baths/showers, avoid excessive rubbing or scrubbing of skin, and to apply emollients and/or topical corticosteroids immediately after the bath/shower.
PROMIS Itch-Triggers is a simple and feasible checklist to screen for the most common itch triggers in AD in clinical practice (patients are asked to check off which of the following have caused their itch in the previous 7 days: cold temperature, hot temperature, heat, sweat, tight clothing, fragrances, boredom, talking about itch, stress, weather change, sunlight, humid air, dry air). “It takes less than 1 minute to complete,” he said. “Additional testing with skin patch and/or prick testing may be warranted to identify allergenic triggers.”
Dr. Silverberg reported that he is a consultant to and/or an advisory board member for several pharmaceutical companies. He is also a speaker for Regeneron and Sanofi and has received a grant from Galderma.
FROM REVOLUTIONIZING AD 2020
Anaphylaxis cases after COVID-19 vaccine rising but still rare: CDC
Health care providers should be ready to treat rare cases of anaphylaxis following administration of COVID-19 vaccines, federal medical officials have urged. The officials also stressed the importance of continuing vaccinations, despite reports of the rare side effect.
There have been 29 cases of anaphylaxis to date following administration of a COVID-19 vaccine, officials from the Centers for Disease Control and Prevention said in a call with reporters on Jan. 6.
The severe allergic reaction, which appears to be rare, can happen with either the Pfizer-BioNTech vaccine or the rival Moderna product. The Food and Drug Administration granted emergency use authorizations for these two vaccines in December.
Even with the cases seen to date, the COVID-19 vaccines remain a “good value proposition,” Nancy Messonnier, MD, director of the CDC’s National Center for Immunization, said in the call.
There have been about 11.1 cases of anaphylaxis per million doses with the Pfizer-BioNTech COVID-19 vaccine, which is higher than the estimated 1.3 cases per million doses with influenza vaccines, she said. But the low risk of anaphylaxis must be balanced against the threat of COVID-19, which currently claims about 2,000 lives a day in the United States, she said. In addition, many people are reporting long-term complications with COVID-19 even if they recover.
Kept in context, the data on anaphylaxis should not scare people away from getting a COVID-19 vaccine, she added.
“Their risk from COVID and poor outcomes is still more than the risk of a severe outcome from the vaccine,” Dr. Messonnier said. “And fortunately, we know how to treat anaphylaxis.”
Dr. Messonnier urged health care workers administering COVID-19 vaccines to be prepared.
“Anybody administering vaccines needs not just to have the EpiPen available, but frankly, to know how to use it,” Dr. Messonnier said.
MMWR details
The CDC on Jan. 6 also provided an update on anaphylaxis in Morbidity and Mortality Weekly Report (MMWR).
The information included in the report was based on cases reported with the Pfizer-BioNTech vaccine – the first to get emergency use authorization from the FDA. On the call with reporters, CDC officials confirmed there have been additional reports since then and anaphylaxis has been reported with both the Pfizer-BioNTech and Moderna vaccines. CDC officials said they could not give a breakdown of how many cases were linked to each of these products at this time.
Between Dec. 14 and 23, 2020, monitoring by the Vaccine Adverse Event Reporting System detected 21 cases of anaphylaxis after administration of a reported 1,893,360 first doses of the Pfizer-BioNTech COVID-19 vaccine. Most reactions – 71% – occurred within 15 minutes of vaccination.
A version of this article first appeared on Medscape.com.
Health care providers should be ready to treat rare cases of anaphylaxis following administration of COVID-19 vaccines, federal medical officials have urged. The officials also stressed the importance of continuing vaccinations, despite reports of the rare side effect.
There have been 29 cases of anaphylaxis to date following administration of a COVID-19 vaccine, officials from the Centers for Disease Control and Prevention said in a call with reporters on Jan. 6.
The severe allergic reaction, which appears to be rare, can happen with either the Pfizer-BioNTech vaccine or the rival Moderna product. The Food and Drug Administration granted emergency use authorizations for these two vaccines in December.
Even with the cases seen to date, the COVID-19 vaccines remain a “good value proposition,” Nancy Messonnier, MD, director of the CDC’s National Center for Immunization, said in the call.
There have been about 11.1 cases of anaphylaxis per million doses with the Pfizer-BioNTech COVID-19 vaccine, which is higher than the estimated 1.3 cases per million doses with influenza vaccines, she said. But the low risk of anaphylaxis must be balanced against the threat of COVID-19, which currently claims about 2,000 lives a day in the United States, she said. In addition, many people are reporting long-term complications with COVID-19 even if they recover.
Kept in context, the data on anaphylaxis should not scare people away from getting a COVID-19 vaccine, she added.
“Their risk from COVID and poor outcomes is still more than the risk of a severe outcome from the vaccine,” Dr. Messonnier said. “And fortunately, we know how to treat anaphylaxis.”
Dr. Messonnier urged health care workers administering COVID-19 vaccines to be prepared.
“Anybody administering vaccines needs not just to have the EpiPen available, but frankly, to know how to use it,” Dr. Messonnier said.
MMWR details
The CDC on Jan. 6 also provided an update on anaphylaxis in Morbidity and Mortality Weekly Report (MMWR).
The information included in the report was based on cases reported with the Pfizer-BioNTech vaccine – the first to get emergency use authorization from the FDA. On the call with reporters, CDC officials confirmed there have been additional reports since then and anaphylaxis has been reported with both the Pfizer-BioNTech and Moderna vaccines. CDC officials said they could not give a breakdown of how many cases were linked to each of these products at this time.
Between Dec. 14 and 23, 2020, monitoring by the Vaccine Adverse Event Reporting System detected 21 cases of anaphylaxis after administration of a reported 1,893,360 first doses of the Pfizer-BioNTech COVID-19 vaccine. Most reactions – 71% – occurred within 15 minutes of vaccination.
A version of this article first appeared on Medscape.com.
Health care providers should be ready to treat rare cases of anaphylaxis following administration of COVID-19 vaccines, federal medical officials have urged. The officials also stressed the importance of continuing vaccinations, despite reports of the rare side effect.
There have been 29 cases of anaphylaxis to date following administration of a COVID-19 vaccine, officials from the Centers for Disease Control and Prevention said in a call with reporters on Jan. 6.
The severe allergic reaction, which appears to be rare, can happen with either the Pfizer-BioNTech vaccine or the rival Moderna product. The Food and Drug Administration granted emergency use authorizations for these two vaccines in December.
Even with the cases seen to date, the COVID-19 vaccines remain a “good value proposition,” Nancy Messonnier, MD, director of the CDC’s National Center for Immunization, said in the call.
There have been about 11.1 cases of anaphylaxis per million doses with the Pfizer-BioNTech COVID-19 vaccine, which is higher than the estimated 1.3 cases per million doses with influenza vaccines, she said. But the low risk of anaphylaxis must be balanced against the threat of COVID-19, which currently claims about 2,000 lives a day in the United States, she said. In addition, many people are reporting long-term complications with COVID-19 even if they recover.
Kept in context, the data on anaphylaxis should not scare people away from getting a COVID-19 vaccine, she added.
“Their risk from COVID and poor outcomes is still more than the risk of a severe outcome from the vaccine,” Dr. Messonnier said. “And fortunately, we know how to treat anaphylaxis.”
Dr. Messonnier urged health care workers administering COVID-19 vaccines to be prepared.
“Anybody administering vaccines needs not just to have the EpiPen available, but frankly, to know how to use it,” Dr. Messonnier said.
MMWR details
The CDC on Jan. 6 also provided an update on anaphylaxis in Morbidity and Mortality Weekly Report (MMWR).
The information included in the report was based on cases reported with the Pfizer-BioNTech vaccine – the first to get emergency use authorization from the FDA. On the call with reporters, CDC officials confirmed there have been additional reports since then and anaphylaxis has been reported with both the Pfizer-BioNTech and Moderna vaccines. CDC officials said they could not give a breakdown of how many cases were linked to each of these products at this time.
Between Dec. 14 and 23, 2020, monitoring by the Vaccine Adverse Event Reporting System detected 21 cases of anaphylaxis after administration of a reported 1,893,360 first doses of the Pfizer-BioNTech COVID-19 vaccine. Most reactions – 71% – occurred within 15 minutes of vaccination.
A version of this article first appeared on Medscape.com.