User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Lupus may confer higher risk of death from COVID-19
There is a significantly increased risk for acute respiratory distress syndrome (ARDS)–related death from COVID-19 among people with systemic lupus erythematous (SLE), compared with the general population, according to data collected in Brazil in 2020.
“Special care is therefore necessary for these patients, as well as reinforcement of the importance of preventive measures during a pandemic for this population,” said Eloisa Bonfá, MD, PhD, at the 14th International Congress on Systemic Lupus Erythematosus, which was held together with the 6th International Congress on Controversies in Rheumatology and Autoimmunity.
“We know that lupus patients have an increased susceptibility to infections due to autoimmune dysregulation and use of immunosuppressive therapy,” explained Dr. Bonfá, who is clinical director of the largest tertiary referral center for autoimmune rheumatic diseases in Latin America, the University of São Paulo Faculty of Medicine Hospital Clinics.
“Our study demonstrates for the first time that lupus patients have an increased ARDS severity,” she added.
Prior to the meeting, the study was published in ACR Open Rheumatology.
Collating the evidence
Since the COVID-19 pandemic began, there have been more than 20 million confirmed cases of SARS-CoV-2 infection in Brazil and more than half a million deaths.
Dr. Bonfá presented the results of a cross-sectional study that was part of the country’s national Influenza Epidemiological Reporting Surveillance System. Data from 2020 were used, which included just over 252,000 individuals who had polymerase chain reaction–confirmed SARS-CoV-2 infection. Of these individuals, there were 319 consecutively recruited patients with SLE.
The aim was to look at the effect of being hospitalized for COVID-19–related ARDS on outcomes in people with SLE versus the general population.
ARDS was defined as a positive polymerase chain reaction test and accompanying flu-like symptoms with dyspnea, respiratory discomfort, persistent pressure in the chest, or desaturation less than 95% in room air or having a bluish tinge to the lips or face.
Other telling signs of a serious respiratory infection that were evaluated, but not mandatory for study eligibility, were loss of smell, impaired taste, typical CT findings, or having had contact with a confirmed COVID-19 case in the preceding 2 weeks.
Key findings
The risk for death from COVID-19–related ARDS was “more than double” in patients with SLE, compared with the general population, Dr. Bonfá reported. The relative risk in the fully adjusted, propensity-scored analysis was approximately 2.25.
That analysis did not account for other comorbidities but was fully adjusted for individuals’ age, sex, and region of Brazil where they lived. The latter was important, Dr. Bonfá said, because “we have a high disparity regarding health access and treatment among regions.”
Comorbidities considered as part of the analyses included arterial hypertension, diabetes, malignancies, neurologic disease, and diseases affecting the heart, lung, liver, and kidneys. Researchers also adjusted for smoking, alcohol intake, body weight, pregnancy, and transplantation.
SLE had a greater impact on individuals’ outcomes than all other comorbidities considered.
“We evaluated lupus as one comorbidity compared to all other comorbidities,” Dr. Bonfá explained.
SLE “more than doubled the chances” of dying from ARDS, she said. “This is [a] very impressive finding.”
They found that SLE was associated with an RR for death of 1.73, compared with non-SLE patients, when propensity-score matching without adjustment for comorbidities was used. The RR for death dropped to 1.40 but was still significant when researchers included comorbidities.
Dr. Bonfá and her team also looked at a combined endpoint of death, ICU admission, and need for mechanical ventilation. They found an increased risk in patients with SLE versus the general population in all their analyses, ranging from 1.70 if comorbidities were included in the model to 1.27 if they weren’t to 1.39 if propensity-score matching alone was used.
Got lupus? ‘Get vaccinated’
“The data we have are in nonvaccinated patients,” Dr. Bonfá said. “We didn’t have vaccines in 2020.”
Whether being vaccinated might make a different to the risks found in this study is an “interesting question,” and one that may be examined in the future.
Certainly, other work Dr. Bonfá has been involved in seems to point to a likely benefit of vaccination in patients with autoimmune diseases in terms of reducing mortality from COVID-19, even when rates of infection may be on the rise.
“There’s considerable vaccine hesitancy in SLE patients,” Chi-Chiu Mok, MD, of Tuen Mun Hospital in Hong Kong, observed in a separate presentation at the congress.
This may be for several reasons, such as worry that their disease may flare or the vaccine might compromise their drug treatment or result in uncommon complications.
However, “we should encourage our SLE patients to receive COVID-19 vaccination at a time of clinical remission or low disease activity state,” Dr. Mok advised.
“Physical distancing, protective masks, and personal hygiene [measures]” should also continue.
The bottom line for those with SLE is to get vaccinated, stressed Sandra Navarra, MD, of the University of Santo Tomas Hospital in Manila, the Philippines, during the discussion.
“There’s still so much out there that we do not know about,” she said. “Just get yourself vaccinated.”
The study had no outside funding. Dr. Bonfá, Dr. Mok, and Dr. Navarra reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
There is a significantly increased risk for acute respiratory distress syndrome (ARDS)–related death from COVID-19 among people with systemic lupus erythematous (SLE), compared with the general population, according to data collected in Brazil in 2020.
“Special care is therefore necessary for these patients, as well as reinforcement of the importance of preventive measures during a pandemic for this population,” said Eloisa Bonfá, MD, PhD, at the 14th International Congress on Systemic Lupus Erythematosus, which was held together with the 6th International Congress on Controversies in Rheumatology and Autoimmunity.
“We know that lupus patients have an increased susceptibility to infections due to autoimmune dysregulation and use of immunosuppressive therapy,” explained Dr. Bonfá, who is clinical director of the largest tertiary referral center for autoimmune rheumatic diseases in Latin America, the University of São Paulo Faculty of Medicine Hospital Clinics.
“Our study demonstrates for the first time that lupus patients have an increased ARDS severity,” she added.
Prior to the meeting, the study was published in ACR Open Rheumatology.
Collating the evidence
Since the COVID-19 pandemic began, there have been more than 20 million confirmed cases of SARS-CoV-2 infection in Brazil and more than half a million deaths.
Dr. Bonfá presented the results of a cross-sectional study that was part of the country’s national Influenza Epidemiological Reporting Surveillance System. Data from 2020 were used, which included just over 252,000 individuals who had polymerase chain reaction–confirmed SARS-CoV-2 infection. Of these individuals, there were 319 consecutively recruited patients with SLE.
The aim was to look at the effect of being hospitalized for COVID-19–related ARDS on outcomes in people with SLE versus the general population.
ARDS was defined as a positive polymerase chain reaction test and accompanying flu-like symptoms with dyspnea, respiratory discomfort, persistent pressure in the chest, or desaturation less than 95% in room air or having a bluish tinge to the lips or face.
Other telling signs of a serious respiratory infection that were evaluated, but not mandatory for study eligibility, were loss of smell, impaired taste, typical CT findings, or having had contact with a confirmed COVID-19 case in the preceding 2 weeks.
Key findings
The risk for death from COVID-19–related ARDS was “more than double” in patients with SLE, compared with the general population, Dr. Bonfá reported. The relative risk in the fully adjusted, propensity-scored analysis was approximately 2.25.
That analysis did not account for other comorbidities but was fully adjusted for individuals’ age, sex, and region of Brazil where they lived. The latter was important, Dr. Bonfá said, because “we have a high disparity regarding health access and treatment among regions.”
Comorbidities considered as part of the analyses included arterial hypertension, diabetes, malignancies, neurologic disease, and diseases affecting the heart, lung, liver, and kidneys. Researchers also adjusted for smoking, alcohol intake, body weight, pregnancy, and transplantation.
SLE had a greater impact on individuals’ outcomes than all other comorbidities considered.
“We evaluated lupus as one comorbidity compared to all other comorbidities,” Dr. Bonfá explained.
SLE “more than doubled the chances” of dying from ARDS, she said. “This is [a] very impressive finding.”
They found that SLE was associated with an RR for death of 1.73, compared with non-SLE patients, when propensity-score matching without adjustment for comorbidities was used. The RR for death dropped to 1.40 but was still significant when researchers included comorbidities.
Dr. Bonfá and her team also looked at a combined endpoint of death, ICU admission, and need for mechanical ventilation. They found an increased risk in patients with SLE versus the general population in all their analyses, ranging from 1.70 if comorbidities were included in the model to 1.27 if they weren’t to 1.39 if propensity-score matching alone was used.
Got lupus? ‘Get vaccinated’
“The data we have are in nonvaccinated patients,” Dr. Bonfá said. “We didn’t have vaccines in 2020.”
Whether being vaccinated might make a different to the risks found in this study is an “interesting question,” and one that may be examined in the future.
Certainly, other work Dr. Bonfá has been involved in seems to point to a likely benefit of vaccination in patients with autoimmune diseases in terms of reducing mortality from COVID-19, even when rates of infection may be on the rise.
“There’s considerable vaccine hesitancy in SLE patients,” Chi-Chiu Mok, MD, of Tuen Mun Hospital in Hong Kong, observed in a separate presentation at the congress.
This may be for several reasons, such as worry that their disease may flare or the vaccine might compromise their drug treatment or result in uncommon complications.
However, “we should encourage our SLE patients to receive COVID-19 vaccination at a time of clinical remission or low disease activity state,” Dr. Mok advised.
“Physical distancing, protective masks, and personal hygiene [measures]” should also continue.
The bottom line for those with SLE is to get vaccinated, stressed Sandra Navarra, MD, of the University of Santo Tomas Hospital in Manila, the Philippines, during the discussion.
“There’s still so much out there that we do not know about,” she said. “Just get yourself vaccinated.”
The study had no outside funding. Dr. Bonfá, Dr. Mok, and Dr. Navarra reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
There is a significantly increased risk for acute respiratory distress syndrome (ARDS)–related death from COVID-19 among people with systemic lupus erythematous (SLE), compared with the general population, according to data collected in Brazil in 2020.
“Special care is therefore necessary for these patients, as well as reinforcement of the importance of preventive measures during a pandemic for this population,” said Eloisa Bonfá, MD, PhD, at the 14th International Congress on Systemic Lupus Erythematosus, which was held together with the 6th International Congress on Controversies in Rheumatology and Autoimmunity.
“We know that lupus patients have an increased susceptibility to infections due to autoimmune dysregulation and use of immunosuppressive therapy,” explained Dr. Bonfá, who is clinical director of the largest tertiary referral center for autoimmune rheumatic diseases in Latin America, the University of São Paulo Faculty of Medicine Hospital Clinics.
“Our study demonstrates for the first time that lupus patients have an increased ARDS severity,” she added.
Prior to the meeting, the study was published in ACR Open Rheumatology.
Collating the evidence
Since the COVID-19 pandemic began, there have been more than 20 million confirmed cases of SARS-CoV-2 infection in Brazil and more than half a million deaths.
Dr. Bonfá presented the results of a cross-sectional study that was part of the country’s national Influenza Epidemiological Reporting Surveillance System. Data from 2020 were used, which included just over 252,000 individuals who had polymerase chain reaction–confirmed SARS-CoV-2 infection. Of these individuals, there were 319 consecutively recruited patients with SLE.
The aim was to look at the effect of being hospitalized for COVID-19–related ARDS on outcomes in people with SLE versus the general population.
ARDS was defined as a positive polymerase chain reaction test and accompanying flu-like symptoms with dyspnea, respiratory discomfort, persistent pressure in the chest, or desaturation less than 95% in room air or having a bluish tinge to the lips or face.
Other telling signs of a serious respiratory infection that were evaluated, but not mandatory for study eligibility, were loss of smell, impaired taste, typical CT findings, or having had contact with a confirmed COVID-19 case in the preceding 2 weeks.
Key findings
The risk for death from COVID-19–related ARDS was “more than double” in patients with SLE, compared with the general population, Dr. Bonfá reported. The relative risk in the fully adjusted, propensity-scored analysis was approximately 2.25.
That analysis did not account for other comorbidities but was fully adjusted for individuals’ age, sex, and region of Brazil where they lived. The latter was important, Dr. Bonfá said, because “we have a high disparity regarding health access and treatment among regions.”
Comorbidities considered as part of the analyses included arterial hypertension, diabetes, malignancies, neurologic disease, and diseases affecting the heart, lung, liver, and kidneys. Researchers also adjusted for smoking, alcohol intake, body weight, pregnancy, and transplantation.
SLE had a greater impact on individuals’ outcomes than all other comorbidities considered.
“We evaluated lupus as one comorbidity compared to all other comorbidities,” Dr. Bonfá explained.
SLE “more than doubled the chances” of dying from ARDS, she said. “This is [a] very impressive finding.”
They found that SLE was associated with an RR for death of 1.73, compared with non-SLE patients, when propensity-score matching without adjustment for comorbidities was used. The RR for death dropped to 1.40 but was still significant when researchers included comorbidities.
Dr. Bonfá and her team also looked at a combined endpoint of death, ICU admission, and need for mechanical ventilation. They found an increased risk in patients with SLE versus the general population in all their analyses, ranging from 1.70 if comorbidities were included in the model to 1.27 if they weren’t to 1.39 if propensity-score matching alone was used.
Got lupus? ‘Get vaccinated’
“The data we have are in nonvaccinated patients,” Dr. Bonfá said. “We didn’t have vaccines in 2020.”
Whether being vaccinated might make a different to the risks found in this study is an “interesting question,” and one that may be examined in the future.
Certainly, other work Dr. Bonfá has been involved in seems to point to a likely benefit of vaccination in patients with autoimmune diseases in terms of reducing mortality from COVID-19, even when rates of infection may be on the rise.
“There’s considerable vaccine hesitancy in SLE patients,” Chi-Chiu Mok, MD, of Tuen Mun Hospital in Hong Kong, observed in a separate presentation at the congress.
This may be for several reasons, such as worry that their disease may flare or the vaccine might compromise their drug treatment or result in uncommon complications.
However, “we should encourage our SLE patients to receive COVID-19 vaccination at a time of clinical remission or low disease activity state,” Dr. Mok advised.
“Physical distancing, protective masks, and personal hygiene [measures]” should also continue.
The bottom line for those with SLE is to get vaccinated, stressed Sandra Navarra, MD, of the University of Santo Tomas Hospital in Manila, the Philippines, during the discussion.
“There’s still so much out there that we do not know about,” she said. “Just get yourself vaccinated.”
The study had no outside funding. Dr. Bonfá, Dr. Mok, and Dr. Navarra reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
‘Baby-wearing’ poses serious injury risks for infants, ED data show
Baby-wearing – carrying a child against your body in a sling, soft carrier, or other device – is associated with benefits like reduced crying and increased breastfeeding, studies have shown.
But this practice also entails risks. Babies can fall out of carriers, or be injured when an adult carrying them falls, for example.
researchers estimated in a study presented at the annual meeting of the American Academy of Pediatrics.
To characterize the epidemiology of these injuries, Samantha J. Rowe, MD, chief resident physician at Walter Reed National Military Medical Center in Bethesda, Md., and colleagues analyzed data from the National Electronic Injury Surveillance System between 2011 and 2020.
They included in their analysis data from patients aged 5 years and younger who sustained an injury associated with a baby-wearing product. Baby harnesses, carriers, slings, framed baby carriers, and soft baby carriers were among the devices included in the study. The researchers used 601 cases to generate national estimates.
An estimated 14,024 patients presented to EDs because of baby-wearing injuries, and 52% of the injuries occurred when a patient fell from the product.
Most injuries (61%) occurred in children aged 5 months and younger; 19.3% of these infants required hospitalization, most often for head injuries.
The investigators found that about 22% of the injuries were associated with a caregiver falling, noted Rachel Y. Moon, MD, who was not involved in the study.
“Carrying a baby changes your center of gravity – and can also obscure your vision of where you’re walking, so adults who use these devices should be cognizant of this,” said Dr. Moon, with the University of Virginia, Charlottesville.
Dr. Rowe often practiced baby-wearing with her daughter, and found that it was beneficial. And studies have demonstrated various benefits of baby-wearing, including improved thermoregulation and glycemic control.
Still, the new analysis illustrates the potential for baby-wearing products “to cause serious injury, especially in infants 5 months and younger,” Dr. Rowe said. “We need to provide more education to caregivers on safe baby-wearing and continue to improve our safety standards for baby-wearing products.”
Study coauthor Patrick T. Reeves, MD, with the Naval Medical Center at San Diego, offered additional guidance in a news release: “Like when buying a new pair of shoes, parents must be educated on the proper sizing, selection, and wear of baby carriers to prevent injury to themselves and their child.”
Parents also need to ensure that the child’s nose and mouth are not obstructed, Dr. Moon
In a recent article discussing the possible benefits of baby-wearing in terms of helping with breastfeeding, Dr. Moon also pointed out further safety considerations: “No matter which carrier is used, for safety reasons, we need to remind parents that the baby should be positioned so that the head is upright and the nose and mouth are not obstructed.”
The researchers and Dr. Moon had no relevant financial disclosures.
Baby-wearing – carrying a child against your body in a sling, soft carrier, or other device – is associated with benefits like reduced crying and increased breastfeeding, studies have shown.
But this practice also entails risks. Babies can fall out of carriers, or be injured when an adult carrying them falls, for example.
researchers estimated in a study presented at the annual meeting of the American Academy of Pediatrics.
To characterize the epidemiology of these injuries, Samantha J. Rowe, MD, chief resident physician at Walter Reed National Military Medical Center in Bethesda, Md., and colleagues analyzed data from the National Electronic Injury Surveillance System between 2011 and 2020.
They included in their analysis data from patients aged 5 years and younger who sustained an injury associated with a baby-wearing product. Baby harnesses, carriers, slings, framed baby carriers, and soft baby carriers were among the devices included in the study. The researchers used 601 cases to generate national estimates.
An estimated 14,024 patients presented to EDs because of baby-wearing injuries, and 52% of the injuries occurred when a patient fell from the product.
Most injuries (61%) occurred in children aged 5 months and younger; 19.3% of these infants required hospitalization, most often for head injuries.
The investigators found that about 22% of the injuries were associated with a caregiver falling, noted Rachel Y. Moon, MD, who was not involved in the study.
“Carrying a baby changes your center of gravity – and can also obscure your vision of where you’re walking, so adults who use these devices should be cognizant of this,” said Dr. Moon, with the University of Virginia, Charlottesville.
Dr. Rowe often practiced baby-wearing with her daughter, and found that it was beneficial. And studies have demonstrated various benefits of baby-wearing, including improved thermoregulation and glycemic control.
Still, the new analysis illustrates the potential for baby-wearing products “to cause serious injury, especially in infants 5 months and younger,” Dr. Rowe said. “We need to provide more education to caregivers on safe baby-wearing and continue to improve our safety standards for baby-wearing products.”
Study coauthor Patrick T. Reeves, MD, with the Naval Medical Center at San Diego, offered additional guidance in a news release: “Like when buying a new pair of shoes, parents must be educated on the proper sizing, selection, and wear of baby carriers to prevent injury to themselves and their child.”
Parents also need to ensure that the child’s nose and mouth are not obstructed, Dr. Moon
In a recent article discussing the possible benefits of baby-wearing in terms of helping with breastfeeding, Dr. Moon also pointed out further safety considerations: “No matter which carrier is used, for safety reasons, we need to remind parents that the baby should be positioned so that the head is upright and the nose and mouth are not obstructed.”
The researchers and Dr. Moon had no relevant financial disclosures.
Baby-wearing – carrying a child against your body in a sling, soft carrier, or other device – is associated with benefits like reduced crying and increased breastfeeding, studies have shown.
But this practice also entails risks. Babies can fall out of carriers, or be injured when an adult carrying them falls, for example.
researchers estimated in a study presented at the annual meeting of the American Academy of Pediatrics.
To characterize the epidemiology of these injuries, Samantha J. Rowe, MD, chief resident physician at Walter Reed National Military Medical Center in Bethesda, Md., and colleagues analyzed data from the National Electronic Injury Surveillance System between 2011 and 2020.
They included in their analysis data from patients aged 5 years and younger who sustained an injury associated with a baby-wearing product. Baby harnesses, carriers, slings, framed baby carriers, and soft baby carriers were among the devices included in the study. The researchers used 601 cases to generate national estimates.
An estimated 14,024 patients presented to EDs because of baby-wearing injuries, and 52% of the injuries occurred when a patient fell from the product.
Most injuries (61%) occurred in children aged 5 months and younger; 19.3% of these infants required hospitalization, most often for head injuries.
The investigators found that about 22% of the injuries were associated with a caregiver falling, noted Rachel Y. Moon, MD, who was not involved in the study.
“Carrying a baby changes your center of gravity – and can also obscure your vision of where you’re walking, so adults who use these devices should be cognizant of this,” said Dr. Moon, with the University of Virginia, Charlottesville.
Dr. Rowe often practiced baby-wearing with her daughter, and found that it was beneficial. And studies have demonstrated various benefits of baby-wearing, including improved thermoregulation and glycemic control.
Still, the new analysis illustrates the potential for baby-wearing products “to cause serious injury, especially in infants 5 months and younger,” Dr. Rowe said. “We need to provide more education to caregivers on safe baby-wearing and continue to improve our safety standards for baby-wearing products.”
Study coauthor Patrick T. Reeves, MD, with the Naval Medical Center at San Diego, offered additional guidance in a news release: “Like when buying a new pair of shoes, parents must be educated on the proper sizing, selection, and wear of baby carriers to prevent injury to themselves and their child.”
Parents also need to ensure that the child’s nose and mouth are not obstructed, Dr. Moon
In a recent article discussing the possible benefits of baby-wearing in terms of helping with breastfeeding, Dr. Moon also pointed out further safety considerations: “No matter which carrier is used, for safety reasons, we need to remind parents that the baby should be positioned so that the head is upright and the nose and mouth are not obstructed.”
The researchers and Dr. Moon had no relevant financial disclosures.
FROM AAP 2021
USPSTF rules out aspirin for over 60s in primary CVD prevention
New draft recommendations from the U.S. Preventive Services Task Force (USPSTF) on the use of aspirin for the primary prevention of cardiovascular disease (CVD) have been released and appear to limit the population in which it should be considered.
“The USPSTF concludes with moderate certainty that aspirin use for the primary prevention of CVD events in adults ages 40 to 59 years who have a 10% or greater 10-year CVD risk has a small net benefit,” the recommendation notes. They conclude that for these patients, the decision to use aspirin “should be an individual one.”
“Persons who are not at increased risk for bleeding and are willing to take low-dose aspirin daily are more likely to benefit,” they note.
For older individuals, however, the task force concludes.
The new recommendations were posted online Oct. 12 and will be available for public comment until November 8. Once it is finalized, the recommendation will replace the 2016 USPSTF recommendation on aspirin use to prevent CVD and colorectal cancer (CRC), they note.
In that document, the task force recommended initiating low-dose aspirin for the primary prevention of both CVD and CRC in adults 50-59 years of age who had a 10% or greater 10-year CVD risk, were not at increased risk for bleeding, had a life expectancy of at least 10 years, and were willing to take daily low-dose aspirin for at least 10 years, with the decision to start being an individual one.
For older and younger patients, they found at that time that the evidence was “insufficient to assess the balance of benefits and harms of initiating aspirin use for the primary prevention of CVD and CRC in adults younger than age 50 years or adults aged 70 years or older.”
In the new draft document, “the USPSTF has changed the age ranges and grades of its recommendation on aspirin use.” Besides the recommendations for CVD prevention, they have also changed the previous recommendation of aspirin for the prevention of CRC given evidence generated from large primary CVD prevention trials.
“Based on new analyses of the evidence from primary CVD prevention populations, longer-term follow-up data from the Women’s Health Study (WHS) (JE Buring, personal communication, November 23, 2020), and new trial evidence, the USPSTF concluded that the evidence is inadequate that low-dose aspirin use reduces CRC incidence or mortality,” it states.
Optimum dose
On the optimum dose for primary CVD prevention, the task force says the benefit appears similar for a low dose (≤100 mg/d) and all doses that have been studied in CVD prevention trials (50 to 500 mg/d). “A pragmatic approach would be to use 81 mg/d, which is the most commonly prescribed dose in the United States,” it states.
The USPSTF recommends using the ACC/AHA Pooled Cohort Equations to estimate cardiovascular risk but it points out that these equations are imperfect for risk prediction at the individual level, and suggests using these risk estimates as a starting point to discuss with appropriate candidates their desire for daily aspirin use. The benefits of initiating aspirin use are greater for individuals at higher risk for CVD events (eg, those with >15% or >20% 10-year CVD risk), they note.
“Decisions about initiating aspirin use should be based on shared decision-making between clinicians and patients about the potential benefits and harms. Persons who place a higher value on the potential benefits than the potential harms may choose to initiate low-dose aspirin use. Persons who place a higher value on the potential harms or on the burden of taking a daily preventive medication than the potential benefits may choose not to initiate low-dose aspirin use,” the task force says.
It also points out that the risk for bleeding increases modestly with advancing age. “For persons who have initiated aspirin use, the net benefits continue to accrue over time in the absence of a bleeding event. The net benefits, however, become smaller with advancing age because of an increased risk for bleeding, so modeling data suggest that it may be reasonable to consider stopping aspirin use around age 75 years,” it states.
Systematic review
The updated draft recommendations are based on a new systematic review commissioned by the USPSTF on the effectiveness of aspirin to reduce the risk of CVD events (myocardial infarction and stroke), cardiovascular mortality, and all-cause mortality in persons without a history of CVD.
The systematic review also investigated the effect of aspirin use on CRC incidence and mortality in primary CVD prevention populations, as well as the harms, particularly bleeding harms, associated with aspirin use.
In addition to the systematic evidence review, the USPSTF commissioned a microsimulation modeling study to assess the net balance of benefits and harms from aspirin use for primary prevention of CVD and CRC, stratified by age, sex, and CVD risk level. Modeling study parameter inputs were informed by the results of the systematic review, and the primary outcomes were net benefits expressed as quality-adjusted life-years and life-years.
The USPSTF found 13 randomized clinical trials (RCTs) that reported on the benefits of aspirin use for the primary prevention of cardiovascular morbidity and mortality. The total number of participants was 161,680, and most trials used low-dose aspirin of 100 mg/d or less or aspirin every other day. The 13 primary prevention trials included a balanced number of male and female participants and included a broad distribution of ages, with mean age ranging from 53 years in the Physicians’ Health Study to 74 years in the ASPREE trial.
This body of evidence shows that aspirin use for primary prevention of CVD is associated with a decreased risk of myocardial infarction and stroke but not cardiovascular mortality or all-cause mortality. Results are quite similar when including studies using all doses of aspirin compared with studies using low-dose aspirin.
The USPSTF reviewed 14 RCTs in CVD primary prevention populations that reported on the bleeding harms of aspirin.
When looking at studies reporting on the harms of low-dose aspirin use (≤100 mg/d), which is most relevant to current practice, a pooled analysis of 10 trials showed that aspirin use was associated with a 58% increase in major gastrointestinal bleeding, and a pooled analysis of 11 trials showed a 31% increase in intracranial bleeds in the aspirin group compared with the control group. Low-dose aspirin use was not associated with a statistically significant increase in risk of fatal hemorrhagic stroke.
Data suggested that the increased risk of bleeding associated with aspirin use occurs relatively quickly after initiating aspirin, and data do not suggest that aspirin has a differential relative bleeding risk based on age, sex, presence of diabetes, level of CVD risk, or race or ethnicity. Although the increase in relative risk does not appear to differ based on age, the absolute risk of bleeding, and thus the magnitude of bleeding harm, does increase with age, and more so in adults age 60 years or older, they note.
The microsimulation model to estimate the magnitude of net benefit of low-dose aspirin use incorporated findings from the systematic review.
Modeling data demonstrated that aspirin use in both men and women ages 40-59 years with 10% or greater 10-year CVD risk generally provides a modest net benefit in both quality-adjusted life-years and life-years gained. Initiation of aspirin use in persons aged 60-69 years results in quality-adjusted life-years gained that range from slightly negative to slightly positive depending on CVD risk level, and life-years gained are generally negative.
In persons aged 70-79 years, initiation of aspirin use results in a loss of both quality-adjusted life-years and life-years at essentially all CVD risk levels modeled (ie, up to 20% 10-year CVD risk).
The USPSTF thus determined that aspirin use has a small net benefit in persons aged 40-59 years with 10% or greater 10-year CVD risk, and initiation of aspirin use has no net benefit in persons age 60 years or older.
When looking at net lifetime benefit of continuous aspirin use until stopping at age 65, 70, 75, 80, or 85 years, modeling data suggest that there is generally little incremental lifetime net benefit in continuing aspirin use beyond the age of 75-80 years.
The task force points out that the net benefit of continuing aspirin use by a person in their 60s or 70s is not the same as the net benefit of initiating aspirin use by a person in their 60s or 70s. This is because, in part, of the fact that CVD risk is heavily influenced by age. Persons who meet the eligibility criteria for aspirin use at a younger age (ie, ≥10% 10-year CVD risk in their 40s or 50s) typically have even higher CVD risk by their 60s or 70s compared with persons who first reach a 10% or greater 10-year CVD risk in their 60s or 70s, and may gain more benefit by continuing aspirin use than a person at lower risk might gain by initiating aspirin use, the USPSTF explains.
A version of this article first appeared on Medscape.com.
New draft recommendations from the U.S. Preventive Services Task Force (USPSTF) on the use of aspirin for the primary prevention of cardiovascular disease (CVD) have been released and appear to limit the population in which it should be considered.
“The USPSTF concludes with moderate certainty that aspirin use for the primary prevention of CVD events in adults ages 40 to 59 years who have a 10% or greater 10-year CVD risk has a small net benefit,” the recommendation notes. They conclude that for these patients, the decision to use aspirin “should be an individual one.”
“Persons who are not at increased risk for bleeding and are willing to take low-dose aspirin daily are more likely to benefit,” they note.
For older individuals, however, the task force concludes.
The new recommendations were posted online Oct. 12 and will be available for public comment until November 8. Once it is finalized, the recommendation will replace the 2016 USPSTF recommendation on aspirin use to prevent CVD and colorectal cancer (CRC), they note.
In that document, the task force recommended initiating low-dose aspirin for the primary prevention of both CVD and CRC in adults 50-59 years of age who had a 10% or greater 10-year CVD risk, were not at increased risk for bleeding, had a life expectancy of at least 10 years, and were willing to take daily low-dose aspirin for at least 10 years, with the decision to start being an individual one.
For older and younger patients, they found at that time that the evidence was “insufficient to assess the balance of benefits and harms of initiating aspirin use for the primary prevention of CVD and CRC in adults younger than age 50 years or adults aged 70 years or older.”
In the new draft document, “the USPSTF has changed the age ranges and grades of its recommendation on aspirin use.” Besides the recommendations for CVD prevention, they have also changed the previous recommendation of aspirin for the prevention of CRC given evidence generated from large primary CVD prevention trials.
“Based on new analyses of the evidence from primary CVD prevention populations, longer-term follow-up data from the Women’s Health Study (WHS) (JE Buring, personal communication, November 23, 2020), and new trial evidence, the USPSTF concluded that the evidence is inadequate that low-dose aspirin use reduces CRC incidence or mortality,” it states.
Optimum dose
On the optimum dose for primary CVD prevention, the task force says the benefit appears similar for a low dose (≤100 mg/d) and all doses that have been studied in CVD prevention trials (50 to 500 mg/d). “A pragmatic approach would be to use 81 mg/d, which is the most commonly prescribed dose in the United States,” it states.
The USPSTF recommends using the ACC/AHA Pooled Cohort Equations to estimate cardiovascular risk but it points out that these equations are imperfect for risk prediction at the individual level, and suggests using these risk estimates as a starting point to discuss with appropriate candidates their desire for daily aspirin use. The benefits of initiating aspirin use are greater for individuals at higher risk for CVD events (eg, those with >15% or >20% 10-year CVD risk), they note.
“Decisions about initiating aspirin use should be based on shared decision-making between clinicians and patients about the potential benefits and harms. Persons who place a higher value on the potential benefits than the potential harms may choose to initiate low-dose aspirin use. Persons who place a higher value on the potential harms or on the burden of taking a daily preventive medication than the potential benefits may choose not to initiate low-dose aspirin use,” the task force says.
It also points out that the risk for bleeding increases modestly with advancing age. “For persons who have initiated aspirin use, the net benefits continue to accrue over time in the absence of a bleeding event. The net benefits, however, become smaller with advancing age because of an increased risk for bleeding, so modeling data suggest that it may be reasonable to consider stopping aspirin use around age 75 years,” it states.
Systematic review
The updated draft recommendations are based on a new systematic review commissioned by the USPSTF on the effectiveness of aspirin to reduce the risk of CVD events (myocardial infarction and stroke), cardiovascular mortality, and all-cause mortality in persons without a history of CVD.
The systematic review also investigated the effect of aspirin use on CRC incidence and mortality in primary CVD prevention populations, as well as the harms, particularly bleeding harms, associated with aspirin use.
In addition to the systematic evidence review, the USPSTF commissioned a microsimulation modeling study to assess the net balance of benefits and harms from aspirin use for primary prevention of CVD and CRC, stratified by age, sex, and CVD risk level. Modeling study parameter inputs were informed by the results of the systematic review, and the primary outcomes were net benefits expressed as quality-adjusted life-years and life-years.
The USPSTF found 13 randomized clinical trials (RCTs) that reported on the benefits of aspirin use for the primary prevention of cardiovascular morbidity and mortality. The total number of participants was 161,680, and most trials used low-dose aspirin of 100 mg/d or less or aspirin every other day. The 13 primary prevention trials included a balanced number of male and female participants and included a broad distribution of ages, with mean age ranging from 53 years in the Physicians’ Health Study to 74 years in the ASPREE trial.
This body of evidence shows that aspirin use for primary prevention of CVD is associated with a decreased risk of myocardial infarction and stroke but not cardiovascular mortality or all-cause mortality. Results are quite similar when including studies using all doses of aspirin compared with studies using low-dose aspirin.
The USPSTF reviewed 14 RCTs in CVD primary prevention populations that reported on the bleeding harms of aspirin.
When looking at studies reporting on the harms of low-dose aspirin use (≤100 mg/d), which is most relevant to current practice, a pooled analysis of 10 trials showed that aspirin use was associated with a 58% increase in major gastrointestinal bleeding, and a pooled analysis of 11 trials showed a 31% increase in intracranial bleeds in the aspirin group compared with the control group. Low-dose aspirin use was not associated with a statistically significant increase in risk of fatal hemorrhagic stroke.
Data suggested that the increased risk of bleeding associated with aspirin use occurs relatively quickly after initiating aspirin, and data do not suggest that aspirin has a differential relative bleeding risk based on age, sex, presence of diabetes, level of CVD risk, or race or ethnicity. Although the increase in relative risk does not appear to differ based on age, the absolute risk of bleeding, and thus the magnitude of bleeding harm, does increase with age, and more so in adults age 60 years or older, they note.
The microsimulation model to estimate the magnitude of net benefit of low-dose aspirin use incorporated findings from the systematic review.
Modeling data demonstrated that aspirin use in both men and women ages 40-59 years with 10% or greater 10-year CVD risk generally provides a modest net benefit in both quality-adjusted life-years and life-years gained. Initiation of aspirin use in persons aged 60-69 years results in quality-adjusted life-years gained that range from slightly negative to slightly positive depending on CVD risk level, and life-years gained are generally negative.
In persons aged 70-79 years, initiation of aspirin use results in a loss of both quality-adjusted life-years and life-years at essentially all CVD risk levels modeled (ie, up to 20% 10-year CVD risk).
The USPSTF thus determined that aspirin use has a small net benefit in persons aged 40-59 years with 10% or greater 10-year CVD risk, and initiation of aspirin use has no net benefit in persons age 60 years or older.
When looking at net lifetime benefit of continuous aspirin use until stopping at age 65, 70, 75, 80, or 85 years, modeling data suggest that there is generally little incremental lifetime net benefit in continuing aspirin use beyond the age of 75-80 years.
The task force points out that the net benefit of continuing aspirin use by a person in their 60s or 70s is not the same as the net benefit of initiating aspirin use by a person in their 60s or 70s. This is because, in part, of the fact that CVD risk is heavily influenced by age. Persons who meet the eligibility criteria for aspirin use at a younger age (ie, ≥10% 10-year CVD risk in their 40s or 50s) typically have even higher CVD risk by their 60s or 70s compared with persons who first reach a 10% or greater 10-year CVD risk in their 60s or 70s, and may gain more benefit by continuing aspirin use than a person at lower risk might gain by initiating aspirin use, the USPSTF explains.
A version of this article first appeared on Medscape.com.
New draft recommendations from the U.S. Preventive Services Task Force (USPSTF) on the use of aspirin for the primary prevention of cardiovascular disease (CVD) have been released and appear to limit the population in which it should be considered.
“The USPSTF concludes with moderate certainty that aspirin use for the primary prevention of CVD events in adults ages 40 to 59 years who have a 10% or greater 10-year CVD risk has a small net benefit,” the recommendation notes. They conclude that for these patients, the decision to use aspirin “should be an individual one.”
“Persons who are not at increased risk for bleeding and are willing to take low-dose aspirin daily are more likely to benefit,” they note.
For older individuals, however, the task force concludes.
The new recommendations were posted online Oct. 12 and will be available for public comment until November 8. Once it is finalized, the recommendation will replace the 2016 USPSTF recommendation on aspirin use to prevent CVD and colorectal cancer (CRC), they note.
In that document, the task force recommended initiating low-dose aspirin for the primary prevention of both CVD and CRC in adults 50-59 years of age who had a 10% or greater 10-year CVD risk, were not at increased risk for bleeding, had a life expectancy of at least 10 years, and were willing to take daily low-dose aspirin for at least 10 years, with the decision to start being an individual one.
For older and younger patients, they found at that time that the evidence was “insufficient to assess the balance of benefits and harms of initiating aspirin use for the primary prevention of CVD and CRC in adults younger than age 50 years or adults aged 70 years or older.”
In the new draft document, “the USPSTF has changed the age ranges and grades of its recommendation on aspirin use.” Besides the recommendations for CVD prevention, they have also changed the previous recommendation of aspirin for the prevention of CRC given evidence generated from large primary CVD prevention trials.
“Based on new analyses of the evidence from primary CVD prevention populations, longer-term follow-up data from the Women’s Health Study (WHS) (JE Buring, personal communication, November 23, 2020), and new trial evidence, the USPSTF concluded that the evidence is inadequate that low-dose aspirin use reduces CRC incidence or mortality,” it states.
Optimum dose
On the optimum dose for primary CVD prevention, the task force says the benefit appears similar for a low dose (≤100 mg/d) and all doses that have been studied in CVD prevention trials (50 to 500 mg/d). “A pragmatic approach would be to use 81 mg/d, which is the most commonly prescribed dose in the United States,” it states.
The USPSTF recommends using the ACC/AHA Pooled Cohort Equations to estimate cardiovascular risk but it points out that these equations are imperfect for risk prediction at the individual level, and suggests using these risk estimates as a starting point to discuss with appropriate candidates their desire for daily aspirin use. The benefits of initiating aspirin use are greater for individuals at higher risk for CVD events (eg, those with >15% or >20% 10-year CVD risk), they note.
“Decisions about initiating aspirin use should be based on shared decision-making between clinicians and patients about the potential benefits and harms. Persons who place a higher value on the potential benefits than the potential harms may choose to initiate low-dose aspirin use. Persons who place a higher value on the potential harms or on the burden of taking a daily preventive medication than the potential benefits may choose not to initiate low-dose aspirin use,” the task force says.
It also points out that the risk for bleeding increases modestly with advancing age. “For persons who have initiated aspirin use, the net benefits continue to accrue over time in the absence of a bleeding event. The net benefits, however, become smaller with advancing age because of an increased risk for bleeding, so modeling data suggest that it may be reasonable to consider stopping aspirin use around age 75 years,” it states.
Systematic review
The updated draft recommendations are based on a new systematic review commissioned by the USPSTF on the effectiveness of aspirin to reduce the risk of CVD events (myocardial infarction and stroke), cardiovascular mortality, and all-cause mortality in persons without a history of CVD.
The systematic review also investigated the effect of aspirin use on CRC incidence and mortality in primary CVD prevention populations, as well as the harms, particularly bleeding harms, associated with aspirin use.
In addition to the systematic evidence review, the USPSTF commissioned a microsimulation modeling study to assess the net balance of benefits and harms from aspirin use for primary prevention of CVD and CRC, stratified by age, sex, and CVD risk level. Modeling study parameter inputs were informed by the results of the systematic review, and the primary outcomes were net benefits expressed as quality-adjusted life-years and life-years.
The USPSTF found 13 randomized clinical trials (RCTs) that reported on the benefits of aspirin use for the primary prevention of cardiovascular morbidity and mortality. The total number of participants was 161,680, and most trials used low-dose aspirin of 100 mg/d or less or aspirin every other day. The 13 primary prevention trials included a balanced number of male and female participants and included a broad distribution of ages, with mean age ranging from 53 years in the Physicians’ Health Study to 74 years in the ASPREE trial.
This body of evidence shows that aspirin use for primary prevention of CVD is associated with a decreased risk of myocardial infarction and stroke but not cardiovascular mortality or all-cause mortality. Results are quite similar when including studies using all doses of aspirin compared with studies using low-dose aspirin.
The USPSTF reviewed 14 RCTs in CVD primary prevention populations that reported on the bleeding harms of aspirin.
When looking at studies reporting on the harms of low-dose aspirin use (≤100 mg/d), which is most relevant to current practice, a pooled analysis of 10 trials showed that aspirin use was associated with a 58% increase in major gastrointestinal bleeding, and a pooled analysis of 11 trials showed a 31% increase in intracranial bleeds in the aspirin group compared with the control group. Low-dose aspirin use was not associated with a statistically significant increase in risk of fatal hemorrhagic stroke.
Data suggested that the increased risk of bleeding associated with aspirin use occurs relatively quickly after initiating aspirin, and data do not suggest that aspirin has a differential relative bleeding risk based on age, sex, presence of diabetes, level of CVD risk, or race or ethnicity. Although the increase in relative risk does not appear to differ based on age, the absolute risk of bleeding, and thus the magnitude of bleeding harm, does increase with age, and more so in adults age 60 years or older, they note.
The microsimulation model to estimate the magnitude of net benefit of low-dose aspirin use incorporated findings from the systematic review.
Modeling data demonstrated that aspirin use in both men and women ages 40-59 years with 10% or greater 10-year CVD risk generally provides a modest net benefit in both quality-adjusted life-years and life-years gained. Initiation of aspirin use in persons aged 60-69 years results in quality-adjusted life-years gained that range from slightly negative to slightly positive depending on CVD risk level, and life-years gained are generally negative.
In persons aged 70-79 years, initiation of aspirin use results in a loss of both quality-adjusted life-years and life-years at essentially all CVD risk levels modeled (ie, up to 20% 10-year CVD risk).
The USPSTF thus determined that aspirin use has a small net benefit in persons aged 40-59 years with 10% or greater 10-year CVD risk, and initiation of aspirin use has no net benefit in persons age 60 years or older.
When looking at net lifetime benefit of continuous aspirin use until stopping at age 65, 70, 75, 80, or 85 years, modeling data suggest that there is generally little incremental lifetime net benefit in continuing aspirin use beyond the age of 75-80 years.
The task force points out that the net benefit of continuing aspirin use by a person in their 60s or 70s is not the same as the net benefit of initiating aspirin use by a person in their 60s or 70s. This is because, in part, of the fact that CVD risk is heavily influenced by age. Persons who meet the eligibility criteria for aspirin use at a younger age (ie, ≥10% 10-year CVD risk in their 40s or 50s) typically have even higher CVD risk by their 60s or 70s compared with persons who first reach a 10% or greater 10-year CVD risk in their 60s or 70s, and may gain more benefit by continuing aspirin use than a person at lower risk might gain by initiating aspirin use, the USPSTF explains.
A version of this article first appeared on Medscape.com.
Pediatricians can effectively promote gun safety
When pediatricians and other pediatric providers are given training and resource materials, levels of firearm screenings and anticipatory guidance about firearm safety increase significantly, according to two new studies presented at the annual meeting of the American Academy of Pediatrics.
“With the rise in firearm sales and injuries during the COVID-19 pandemic, it is more important than ever that pediatricians address the firearm epidemic,” said Alexandra Byrne, MD, a pediatric resident at the University of Florida in Gainesville, who presented one of the studies.
There were 4.3 million more firearms purchased from March through July 2020 than expected, a recent study estimates, and 4,075 more firearm injuries than expected from April through July 2020.
In states with more excess purchases, firearm injuries related to domestic violence increased in April (rate ratio, 2.60; 95% CI, 1.32-5.93) and May (RR, 1.79; 95% CI, 1.19-2.91) 2020. However, excess gun purchases had no effect on rates of firearm violence outside the home.
In addition to the link between firearms in the home and domestic violence, they are also linked to a three- to fourfold greater risk for teen suicide, and both depression and suicidal thoughts have risen in teens during the pandemic.
“The data are pretty clear that if you have an unlocked, loaded weapon in your home, and you have a kid who’s depressed or anxious or dysregulated or doing maladaptive things for the pandemic, they’re much more likely to inadvertently take their own or someone else’s life by grabbing [a gun],” said Cora Breuner, MD, MPH, professor of pediatrics at Seattle Children’s Hospital.
However, there is no difference in gun ownership or gun-safety measures between homes with and without at-risk children, previous research shows.
Training, guidance, and locks
Previous research has also shown that there has been a reluctance by pediatricians to conduct firearm screenings and counsel parents about gun safety in the home.
For their two-step program, Dr. Byrne’s team used a plan-do-study-act approach. They started by providing training on firearm safety, evidence-based recommendations for firearm screening, and anticipatory guidance regarding safe firearm storage to members of the general pediatrics division at the University of Florida. And they supplied clinics with free firearm locks.
Next they supplied clinics with posters and educational cards from the Be SMART campaign, an initiative of the Everytown for Gun Safety Support Fund, which provides materials for anyone, including physicians, to use.
During their study, the researchers sent three anonymous six-question online surveys – at baseline and 3 to 4 months after each of the two steps – to pediatric residents, physician assistants, advanced practice registered nurses, and attendings to assess the project. There were 52 responses to the first survey, for a response rate of 58.4%, 42 responses to the second survey, for a response rate of 47.2%, and 23 responses to the third survey, for a rate of response 25.8%.
The program nearly doubled screenings during well-child visits and dramatically increased the proportion of families who received a firearm lock when they told providers they had a firearm at home.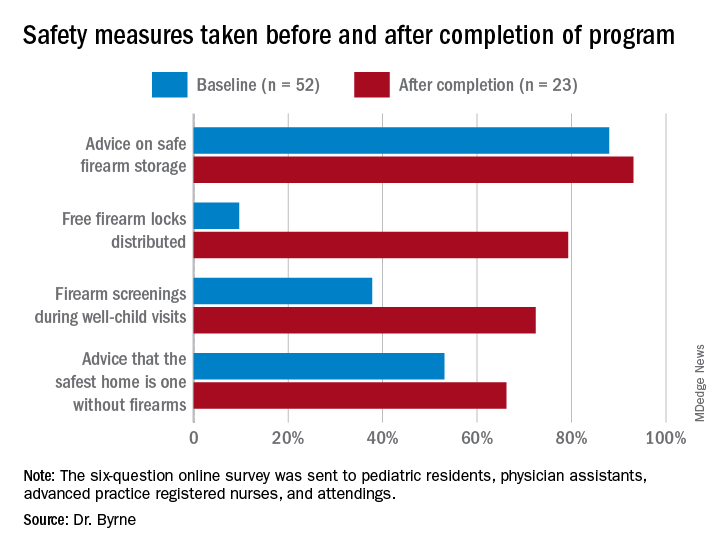
Previous research has shown “a significant increase in safe firearm storage when firearm locks were provided to families in clinic compared to verbal counseling alone,” Dr. Byrne said. “We know that safe firearm storage reduces injuries. Roughly one in three children in the United States lives in a home with a firearm. Individuals with a firearm are at two times the risk of homicide and three to four times the risk of suicide, so it is essential we further study how pediatricians can be most effective when it comes to firearm counseling.”
The difference in lock distribution as a result of the program is a “tremendous increase,” said Christopher S. Greeley, MD, MS, chief of the division of public health pediatrics at Texas Children’s Hospital and professor of pediatrics at Baylor College of Medicine in Houston, who was not involved in the research.
“Locks could go a long way to minimizing the risk,” he said in an interview, adding that nearly half of all teen suicide deaths that occurred over a decade in Houston involved a firearm.
Adding a social-history component
A program to increase firearm screening was also presented at the AAP conference.
After random review of medical records from 30 patients admitted to the hospital documented zero firearm screenings, Marjorie Farrington, MD, and Samantha Gunkelman, MD, from Akron Children’s Hospital in Ohio, implemented a program that they hope will increase firearm screenings during inpatient admissions to at least 50%.
They started their ongoing program in April 2020 by adding a social-history component to the history and physical (H&P) exam template and educating residents on how to screen and included guidance on safe firearm storage.
They also had physicians with firearm expertise give gun-safety lectures, and they plan to involve the Family Resource Center at their hospital in the creation of resources that can be incorporated into discharge instructions.
From April 2020 to June 2021, after the addition to the H&P template, 63% of the 5196 patients admitted to the hospital underwent a firearm screening. Of the 25% of patients who reported guns at home, 3% were not storing their firearms safely.
The pair used the “Store It Safe” Physician Handout provided by the Ohio chapter of the AAP.
Many pediatricians and pediatric trainees are not comfortable counseling on firearm safety, often a result of inadequate training on the topic.
The BulletPoints Project — developed by the Violence Prevention Research Program at the University of California, Davis — can also help physicians talk to patients about guns.
“Many pediatricians and pediatric trainees are not comfortable counseling on firearm safety, often a result of inadequate training on the topic,” Dr. Byrne said in an interview. “Additionally, it is a challenging topic that can often be met with resistance from patients and families. Lack of time during visits is also a huge barrier.”
Lack of training is an obstacle to greater firearm screenings, Dr. Greeley agreed, as are the feeling that guidance simply won’t make a difference and concerns about political pressure and divineness. The lack of research on firearm injuries and the impact of firearm screenings and anticipatory guidance is a challenge, he added, although that is starting to change.
Pediatricians need education on how to make a difference when it comes to firearm safety, and should follow AAP guidelines, Dr. Greeley said.
Counseling on firearm safety is in the same category as immunizations, seatbelts, substance use, helmets, and other public-health issues that are important to address at visits, regardless of how difficult it might be, Dr. Breuner told this news organization.
“It is our mission, as pediatricians, to provide every ounce of prevention in our well-child and anticipatory guidance visits,” she said. “It’s our job, so we shouldn’t shy away from it even though it’s hard.”
Doctors are more comfortable discussing firearm safety if they are firearm owners, previous research has shown, so she advises pediatricians who feel unqualified to discuss firearms to seek guidance from their peers on how to approach screenings and anticipatory guidance, she noted.
The firearm study being done in an academic center gives me great pause. The populations are often very different than private practice.
Both of these studies were conducted at single institutions and might not reflect what would work in private clinics.
“The firearm study being done in an academic center gives me great pause,” Dr. Greeley said. “The populations are often very different than private practice. I think that there is still a lot that remains unknown about decreasing household firearm injury and death.”
And the degree to which findings from these two gun-safety programs can be generalized to other academic centers or children’s hospitals is unclear.
“There are states where, I suspect, firearm screening is much more common. Some states have very pro-firearm cultures and others are anti-firearm,” Dr. Greeley said. “There are also likely differences within states,” particularly between urban and rural regions.
“Firearms are often a very personal issue for families, and pediatricians in ‘pro-firearm’ communities may have greater resistance to working on this,” he pointed out.
Nevertheless, Dr. Greeley said, “this is a promising strategy that could be part of a broad injury prevention initiative.”
Neither study noted any external funding. Dr. Byrne is a member of the Moms Demand Action Gainesville Chapter, which donated the firearm locks for the project. Dr. Breuner, Dr. Greeley, and Dr. Farrington have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
When pediatricians and other pediatric providers are given training and resource materials, levels of firearm screenings and anticipatory guidance about firearm safety increase significantly, according to two new studies presented at the annual meeting of the American Academy of Pediatrics.
“With the rise in firearm sales and injuries during the COVID-19 pandemic, it is more important than ever that pediatricians address the firearm epidemic,” said Alexandra Byrne, MD, a pediatric resident at the University of Florida in Gainesville, who presented one of the studies.
There were 4.3 million more firearms purchased from March through July 2020 than expected, a recent study estimates, and 4,075 more firearm injuries than expected from April through July 2020.
In states with more excess purchases, firearm injuries related to domestic violence increased in April (rate ratio, 2.60; 95% CI, 1.32-5.93) and May (RR, 1.79; 95% CI, 1.19-2.91) 2020. However, excess gun purchases had no effect on rates of firearm violence outside the home.
In addition to the link between firearms in the home and domestic violence, they are also linked to a three- to fourfold greater risk for teen suicide, and both depression and suicidal thoughts have risen in teens during the pandemic.
“The data are pretty clear that if you have an unlocked, loaded weapon in your home, and you have a kid who’s depressed or anxious or dysregulated or doing maladaptive things for the pandemic, they’re much more likely to inadvertently take their own or someone else’s life by grabbing [a gun],” said Cora Breuner, MD, MPH, professor of pediatrics at Seattle Children’s Hospital.
However, there is no difference in gun ownership or gun-safety measures between homes with and without at-risk children, previous research shows.
Training, guidance, and locks
Previous research has also shown that there has been a reluctance by pediatricians to conduct firearm screenings and counsel parents about gun safety in the home.
For their two-step program, Dr. Byrne’s team used a plan-do-study-act approach. They started by providing training on firearm safety, evidence-based recommendations for firearm screening, and anticipatory guidance regarding safe firearm storage to members of the general pediatrics division at the University of Florida. And they supplied clinics with free firearm locks.
Next they supplied clinics with posters and educational cards from the Be SMART campaign, an initiative of the Everytown for Gun Safety Support Fund, which provides materials for anyone, including physicians, to use.
During their study, the researchers sent three anonymous six-question online surveys – at baseline and 3 to 4 months after each of the two steps – to pediatric residents, physician assistants, advanced practice registered nurses, and attendings to assess the project. There were 52 responses to the first survey, for a response rate of 58.4%, 42 responses to the second survey, for a response rate of 47.2%, and 23 responses to the third survey, for a rate of response 25.8%.
The program nearly doubled screenings during well-child visits and dramatically increased the proportion of families who received a firearm lock when they told providers they had a firearm at home.
Previous research has shown “a significant increase in safe firearm storage when firearm locks were provided to families in clinic compared to verbal counseling alone,” Dr. Byrne said. “We know that safe firearm storage reduces injuries. Roughly one in three children in the United States lives in a home with a firearm. Individuals with a firearm are at two times the risk of homicide and three to four times the risk of suicide, so it is essential we further study how pediatricians can be most effective when it comes to firearm counseling.”
The difference in lock distribution as a result of the program is a “tremendous increase,” said Christopher S. Greeley, MD, MS, chief of the division of public health pediatrics at Texas Children’s Hospital and professor of pediatrics at Baylor College of Medicine in Houston, who was not involved in the research.
“Locks could go a long way to minimizing the risk,” he said in an interview, adding that nearly half of all teen suicide deaths that occurred over a decade in Houston involved a firearm.
Adding a social-history component
A program to increase firearm screening was also presented at the AAP conference.
After random review of medical records from 30 patients admitted to the hospital documented zero firearm screenings, Marjorie Farrington, MD, and Samantha Gunkelman, MD, from Akron Children’s Hospital in Ohio, implemented a program that they hope will increase firearm screenings during inpatient admissions to at least 50%.
They started their ongoing program in April 2020 by adding a social-history component to the history and physical (H&P) exam template and educating residents on how to screen and included guidance on safe firearm storage.
They also had physicians with firearm expertise give gun-safety lectures, and they plan to involve the Family Resource Center at their hospital in the creation of resources that can be incorporated into discharge instructions.
From April 2020 to June 2021, after the addition to the H&P template, 63% of the 5196 patients admitted to the hospital underwent a firearm screening. Of the 25% of patients who reported guns at home, 3% were not storing their firearms safely.
The pair used the “Store It Safe” Physician Handout provided by the Ohio chapter of the AAP.
Many pediatricians and pediatric trainees are not comfortable counseling on firearm safety, often a result of inadequate training on the topic.
The BulletPoints Project — developed by the Violence Prevention Research Program at the University of California, Davis — can also help physicians talk to patients about guns.
“Many pediatricians and pediatric trainees are not comfortable counseling on firearm safety, often a result of inadequate training on the topic,” Dr. Byrne said in an interview. “Additionally, it is a challenging topic that can often be met with resistance from patients and families. Lack of time during visits is also a huge barrier.”
Lack of training is an obstacle to greater firearm screenings, Dr. Greeley agreed, as are the feeling that guidance simply won’t make a difference and concerns about political pressure and divineness. The lack of research on firearm injuries and the impact of firearm screenings and anticipatory guidance is a challenge, he added, although that is starting to change.
Pediatricians need education on how to make a difference when it comes to firearm safety, and should follow AAP guidelines, Dr. Greeley said.
Counseling on firearm safety is in the same category as immunizations, seatbelts, substance use, helmets, and other public-health issues that are important to address at visits, regardless of how difficult it might be, Dr. Breuner told this news organization.
“It is our mission, as pediatricians, to provide every ounce of prevention in our well-child and anticipatory guidance visits,” she said. “It’s our job, so we shouldn’t shy away from it even though it’s hard.”
Doctors are more comfortable discussing firearm safety if they are firearm owners, previous research has shown, so she advises pediatricians who feel unqualified to discuss firearms to seek guidance from their peers on how to approach screenings and anticipatory guidance, she noted.
The firearm study being done in an academic center gives me great pause. The populations are often very different than private practice.
Both of these studies were conducted at single institutions and might not reflect what would work in private clinics.
“The firearm study being done in an academic center gives me great pause,” Dr. Greeley said. “The populations are often very different than private practice. I think that there is still a lot that remains unknown about decreasing household firearm injury and death.”
And the degree to which findings from these two gun-safety programs can be generalized to other academic centers or children’s hospitals is unclear.
“There are states where, I suspect, firearm screening is much more common. Some states have very pro-firearm cultures and others are anti-firearm,” Dr. Greeley said. “There are also likely differences within states,” particularly between urban and rural regions.
“Firearms are often a very personal issue for families, and pediatricians in ‘pro-firearm’ communities may have greater resistance to working on this,” he pointed out.
Nevertheless, Dr. Greeley said, “this is a promising strategy that could be part of a broad injury prevention initiative.”
Neither study noted any external funding. Dr. Byrne is a member of the Moms Demand Action Gainesville Chapter, which donated the firearm locks for the project. Dr. Breuner, Dr. Greeley, and Dr. Farrington have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
When pediatricians and other pediatric providers are given training and resource materials, levels of firearm screenings and anticipatory guidance about firearm safety increase significantly, according to two new studies presented at the annual meeting of the American Academy of Pediatrics.
“With the rise in firearm sales and injuries during the COVID-19 pandemic, it is more important than ever that pediatricians address the firearm epidemic,” said Alexandra Byrne, MD, a pediatric resident at the University of Florida in Gainesville, who presented one of the studies.
There were 4.3 million more firearms purchased from March through July 2020 than expected, a recent study estimates, and 4,075 more firearm injuries than expected from April through July 2020.
In states with more excess purchases, firearm injuries related to domestic violence increased in April (rate ratio, 2.60; 95% CI, 1.32-5.93) and May (RR, 1.79; 95% CI, 1.19-2.91) 2020. However, excess gun purchases had no effect on rates of firearm violence outside the home.
In addition to the link between firearms in the home and domestic violence, they are also linked to a three- to fourfold greater risk for teen suicide, and both depression and suicidal thoughts have risen in teens during the pandemic.
“The data are pretty clear that if you have an unlocked, loaded weapon in your home, and you have a kid who’s depressed or anxious or dysregulated or doing maladaptive things for the pandemic, they’re much more likely to inadvertently take their own or someone else’s life by grabbing [a gun],” said Cora Breuner, MD, MPH, professor of pediatrics at Seattle Children’s Hospital.
However, there is no difference in gun ownership or gun-safety measures between homes with and without at-risk children, previous research shows.
Training, guidance, and locks
Previous research has also shown that there has been a reluctance by pediatricians to conduct firearm screenings and counsel parents about gun safety in the home.
For their two-step program, Dr. Byrne’s team used a plan-do-study-act approach. They started by providing training on firearm safety, evidence-based recommendations for firearm screening, and anticipatory guidance regarding safe firearm storage to members of the general pediatrics division at the University of Florida. And they supplied clinics with free firearm locks.
Next they supplied clinics with posters and educational cards from the Be SMART campaign, an initiative of the Everytown for Gun Safety Support Fund, which provides materials for anyone, including physicians, to use.
During their study, the researchers sent three anonymous six-question online surveys – at baseline and 3 to 4 months after each of the two steps – to pediatric residents, physician assistants, advanced practice registered nurses, and attendings to assess the project. There were 52 responses to the first survey, for a response rate of 58.4%, 42 responses to the second survey, for a response rate of 47.2%, and 23 responses to the third survey, for a rate of response 25.8%.
The program nearly doubled screenings during well-child visits and dramatically increased the proportion of families who received a firearm lock when they told providers they had a firearm at home.
Previous research has shown “a significant increase in safe firearm storage when firearm locks were provided to families in clinic compared to verbal counseling alone,” Dr. Byrne said. “We know that safe firearm storage reduces injuries. Roughly one in three children in the United States lives in a home with a firearm. Individuals with a firearm are at two times the risk of homicide and three to four times the risk of suicide, so it is essential we further study how pediatricians can be most effective when it comes to firearm counseling.”
The difference in lock distribution as a result of the program is a “tremendous increase,” said Christopher S. Greeley, MD, MS, chief of the division of public health pediatrics at Texas Children’s Hospital and professor of pediatrics at Baylor College of Medicine in Houston, who was not involved in the research.
“Locks could go a long way to minimizing the risk,” he said in an interview, adding that nearly half of all teen suicide deaths that occurred over a decade in Houston involved a firearm.
Adding a social-history component
A program to increase firearm screening was also presented at the AAP conference.
After random review of medical records from 30 patients admitted to the hospital documented zero firearm screenings, Marjorie Farrington, MD, and Samantha Gunkelman, MD, from Akron Children’s Hospital in Ohio, implemented a program that they hope will increase firearm screenings during inpatient admissions to at least 50%.
They started their ongoing program in April 2020 by adding a social-history component to the history and physical (H&P) exam template and educating residents on how to screen and included guidance on safe firearm storage.
They also had physicians with firearm expertise give gun-safety lectures, and they plan to involve the Family Resource Center at their hospital in the creation of resources that can be incorporated into discharge instructions.
From April 2020 to June 2021, after the addition to the H&P template, 63% of the 5196 patients admitted to the hospital underwent a firearm screening. Of the 25% of patients who reported guns at home, 3% were not storing their firearms safely.
The pair used the “Store It Safe” Physician Handout provided by the Ohio chapter of the AAP.
Many pediatricians and pediatric trainees are not comfortable counseling on firearm safety, often a result of inadequate training on the topic.
The BulletPoints Project — developed by the Violence Prevention Research Program at the University of California, Davis — can also help physicians talk to patients about guns.
“Many pediatricians and pediatric trainees are not comfortable counseling on firearm safety, often a result of inadequate training on the topic,” Dr. Byrne said in an interview. “Additionally, it is a challenging topic that can often be met with resistance from patients and families. Lack of time during visits is also a huge barrier.”
Lack of training is an obstacle to greater firearm screenings, Dr. Greeley agreed, as are the feeling that guidance simply won’t make a difference and concerns about political pressure and divineness. The lack of research on firearm injuries and the impact of firearm screenings and anticipatory guidance is a challenge, he added, although that is starting to change.
Pediatricians need education on how to make a difference when it comes to firearm safety, and should follow AAP guidelines, Dr. Greeley said.
Counseling on firearm safety is in the same category as immunizations, seatbelts, substance use, helmets, and other public-health issues that are important to address at visits, regardless of how difficult it might be, Dr. Breuner told this news organization.
“It is our mission, as pediatricians, to provide every ounce of prevention in our well-child and anticipatory guidance visits,” she said. “It’s our job, so we shouldn’t shy away from it even though it’s hard.”
Doctors are more comfortable discussing firearm safety if they are firearm owners, previous research has shown, so she advises pediatricians who feel unqualified to discuss firearms to seek guidance from their peers on how to approach screenings and anticipatory guidance, she noted.
The firearm study being done in an academic center gives me great pause. The populations are often very different than private practice.
Both of these studies were conducted at single institutions and might not reflect what would work in private clinics.
“The firearm study being done in an academic center gives me great pause,” Dr. Greeley said. “The populations are often very different than private practice. I think that there is still a lot that remains unknown about decreasing household firearm injury and death.”
And the degree to which findings from these two gun-safety programs can be generalized to other academic centers or children’s hospitals is unclear.
“There are states where, I suspect, firearm screening is much more common. Some states have very pro-firearm cultures and others are anti-firearm,” Dr. Greeley said. “There are also likely differences within states,” particularly between urban and rural regions.
“Firearms are often a very personal issue for families, and pediatricians in ‘pro-firearm’ communities may have greater resistance to working on this,” he pointed out.
Nevertheless, Dr. Greeley said, “this is a promising strategy that could be part of a broad injury prevention initiative.”
Neither study noted any external funding. Dr. Byrne is a member of the Moms Demand Action Gainesville Chapter, which donated the firearm locks for the project. Dr. Breuner, Dr. Greeley, and Dr. Farrington have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM AAP 2021
Synthetic chemical in consumer products linked to early death, study says
Daily exposure to phthalates, which are synthetic chemicals founds in many consumer products, may lead to hundreds of thousands of early deaths each year among older adults in the United States, according to a new study published Oct. 12, 2021, in the peer-reviewed journal Environmental Pollution.
The chemicals are found in hundreds of types of products, including children’s toys, food storage containers, makeup, perfume, and shampoo. In the study, those with the highest levels of phthalates had a greater risk of death from any cause, especially heart disease.
“This study adds to the growing database on the impact of plastics on the human body and bolsters public health and business cases for reducing or eliminating the use of plastics,” Leonardo Trasande, MD, the lead author and a professor of environmental medicine and population health at New York University Langone Health, told CNN.
Dr. Trasande and colleagues measured the urine concentration of phthalates in more than 5,000 adults aged 55-64 and compared the levels with the risk of early death over an average of 10 years. The research team controlled for preexisting heart diseases, diabetes, cancer, poor eating habits, physical activity, body mass, and other known hormone disruptors such as bisphenol A, or BPA, an industrial chemical that’s been used since the 1950s to make certain plastics and resins, according to the Mayo Clinic
The research team found that phthalates could contribute to 91,000-107,000 premature deaths per year in the United States. These early deaths could cost the nation $40 billion to $47 billion each year in lost economic productivity.
Phthalates interrupt the body’s endocrine system and hormone production. Previous studies have found that the chemicals are linked with developmental, reproductive, and immune system problems, according to NYU Langone Health. They’ve also been linked with asthma, childhood obesity, heart issues, and cancer.
“These chemicals have a rap sheet,” Dr. Trasande told CNN. “And the fact of the matter is that when you look at the entire body of evidence, it provides a haunting pattern of concern.”
Phthalates are often called “everywhere chemicals” because they are so common, CNN reported. Also called “plasticizers,” they are added to products to make them more durable, including PVC plumbing, vinyl flooring, medical tubing, garden hoses, food packaging, detergents, clothing, furniture, and automotive materials.
People are often exposed when they breathe contaminated air or consume food that comes into contact with the chemical, according to the Centers for Disease Control and Prevention. Children may be exposed by touching plastic items and putting their hands in their mouth.
Dr. Trasande told CNN that it’s possible to lessen exposure to phthalates and other endocrine disruptors such as BPA by using unscented lotions, laundry detergents, and cleaning supplies, as well as substituting glass, stainless steel, ceramic, and wood for plastic food storage.
“First, avoid plastics as much as you can. Never put plastic containers in the microwave or dishwasher, where the heat can break down the linings so they might be absorbed more readily,” he said. “In addition, cooking at home and reducing your use of processed foods can reduce the levels of the chemical exposures you come in contact with.”
A version of this article first appeared on WebMD.com.
Daily exposure to phthalates, which are synthetic chemicals founds in many consumer products, may lead to hundreds of thousands of early deaths each year among older adults in the United States, according to a new study published Oct. 12, 2021, in the peer-reviewed journal Environmental Pollution.
The chemicals are found in hundreds of types of products, including children’s toys, food storage containers, makeup, perfume, and shampoo. In the study, those with the highest levels of phthalates had a greater risk of death from any cause, especially heart disease.
“This study adds to the growing database on the impact of plastics on the human body and bolsters public health and business cases for reducing or eliminating the use of plastics,” Leonardo Trasande, MD, the lead author and a professor of environmental medicine and population health at New York University Langone Health, told CNN.
Dr. Trasande and colleagues measured the urine concentration of phthalates in more than 5,000 adults aged 55-64 and compared the levels with the risk of early death over an average of 10 years. The research team controlled for preexisting heart diseases, diabetes, cancer, poor eating habits, physical activity, body mass, and other known hormone disruptors such as bisphenol A, or BPA, an industrial chemical that’s been used since the 1950s to make certain plastics and resins, according to the Mayo Clinic
The research team found that phthalates could contribute to 91,000-107,000 premature deaths per year in the United States. These early deaths could cost the nation $40 billion to $47 billion each year in lost economic productivity.
Phthalates interrupt the body’s endocrine system and hormone production. Previous studies have found that the chemicals are linked with developmental, reproductive, and immune system problems, according to NYU Langone Health. They’ve also been linked with asthma, childhood obesity, heart issues, and cancer.
“These chemicals have a rap sheet,” Dr. Trasande told CNN. “And the fact of the matter is that when you look at the entire body of evidence, it provides a haunting pattern of concern.”
Phthalates are often called “everywhere chemicals” because they are so common, CNN reported. Also called “plasticizers,” they are added to products to make them more durable, including PVC plumbing, vinyl flooring, medical tubing, garden hoses, food packaging, detergents, clothing, furniture, and automotive materials.
People are often exposed when they breathe contaminated air or consume food that comes into contact with the chemical, according to the Centers for Disease Control and Prevention. Children may be exposed by touching plastic items and putting their hands in their mouth.
Dr. Trasande told CNN that it’s possible to lessen exposure to phthalates and other endocrine disruptors such as BPA by using unscented lotions, laundry detergents, and cleaning supplies, as well as substituting glass, stainless steel, ceramic, and wood for plastic food storage.
“First, avoid plastics as much as you can. Never put plastic containers in the microwave or dishwasher, where the heat can break down the linings so they might be absorbed more readily,” he said. “In addition, cooking at home and reducing your use of processed foods can reduce the levels of the chemical exposures you come in contact with.”
A version of this article first appeared on WebMD.com.
Daily exposure to phthalates, which are synthetic chemicals founds in many consumer products, may lead to hundreds of thousands of early deaths each year among older adults in the United States, according to a new study published Oct. 12, 2021, in the peer-reviewed journal Environmental Pollution.
The chemicals are found in hundreds of types of products, including children’s toys, food storage containers, makeup, perfume, and shampoo. In the study, those with the highest levels of phthalates had a greater risk of death from any cause, especially heart disease.
“This study adds to the growing database on the impact of plastics on the human body and bolsters public health and business cases for reducing or eliminating the use of plastics,” Leonardo Trasande, MD, the lead author and a professor of environmental medicine and population health at New York University Langone Health, told CNN.
Dr. Trasande and colleagues measured the urine concentration of phthalates in more than 5,000 adults aged 55-64 and compared the levels with the risk of early death over an average of 10 years. The research team controlled for preexisting heart diseases, diabetes, cancer, poor eating habits, physical activity, body mass, and other known hormone disruptors such as bisphenol A, or BPA, an industrial chemical that’s been used since the 1950s to make certain plastics and resins, according to the Mayo Clinic
The research team found that phthalates could contribute to 91,000-107,000 premature deaths per year in the United States. These early deaths could cost the nation $40 billion to $47 billion each year in lost economic productivity.
Phthalates interrupt the body’s endocrine system and hormone production. Previous studies have found that the chemicals are linked with developmental, reproductive, and immune system problems, according to NYU Langone Health. They’ve also been linked with asthma, childhood obesity, heart issues, and cancer.
“These chemicals have a rap sheet,” Dr. Trasande told CNN. “And the fact of the matter is that when you look at the entire body of evidence, it provides a haunting pattern of concern.”
Phthalates are often called “everywhere chemicals” because they are so common, CNN reported. Also called “plasticizers,” they are added to products to make them more durable, including PVC plumbing, vinyl flooring, medical tubing, garden hoses, food packaging, detergents, clothing, furniture, and automotive materials.
People are often exposed when they breathe contaminated air or consume food that comes into contact with the chemical, according to the Centers for Disease Control and Prevention. Children may be exposed by touching plastic items and putting their hands in their mouth.
Dr. Trasande told CNN that it’s possible to lessen exposure to phthalates and other endocrine disruptors such as BPA by using unscented lotions, laundry detergents, and cleaning supplies, as well as substituting glass, stainless steel, ceramic, and wood for plastic food storage.
“First, avoid plastics as much as you can. Never put plastic containers in the microwave or dishwasher, where the heat can break down the linings so they might be absorbed more readily,” he said. “In addition, cooking at home and reducing your use of processed foods can reduce the levels of the chemical exposures you come in contact with.”
A version of this article first appeared on WebMD.com.
Children and COVID-19: U.S. adds latest million cases in record time
The United States just passed the 6-million mark in COVID-19 cases among children, with the last million cases taking less time to record than any of the first five, according to new data from the American Academy of Pediatrics and the Children’s Hospital Association.

The five-millionth case was reported during the week of Aug. 27 to Sept. 2, and case number 6 million came during the week of Oct. 1-7, just 5 weeks later, compared with the 6 weeks it took to go from 1 million to 2 million last November and December, the AAP and CHA said in their weekly COVID-19 report.
New cases continued to drop, however, and that weekly count was down by 14.6% from the previous week and by 41.1% from the peak of almost 252,000 reached in early September, the two groups said while also noting limitations to the data, such as three states (Alabama, Nebraska, and Texas) that are no longer updating their COVID-19 dashboards.
Other metrics show similar drops in recent weeks. Among children aged 0-11 years, emergency department visits involving a COVID-19 diagnosis dropped from 4.1% of all ED visits in late August to 1.4% of ED visits on Oct. 6. ED visits with a COVID-19 diagnosis fell from a peak of 8.5% on Aug. 22 to 1.5% on Oct. 6 for 12- to 15-year-olds and from 8.5% to 1.5% in those aged 16-17 years, according to data from the Centers for Disease Control and Prevention.
The rate of new hospital admissions for children aged 0-17 years was down to 0.26 per 100,000 population on Oct. 9 after reaching 0.51 per 100,000 on Sept. 4. Hospitalizations in children totaled just over 64,000 from Aug. 1, 2020, to Oct. 9, 2021, which is just over 2% of all COVID-19–related admissions over that time period, the CDC said on its COVID Data Tracker.
That pattern, unfortunately, also applies to vaccinations. “The number of children receiving their first COVID-19 vaccine this week [Sept. 30 to Oct. 6], about 156,000, was the lowest number since vaccines were available,” the AAP said in a separate report on vaccination trends, adding that “the number of children receiving their first dose has steadily declined from 8 weeks ago when 586,000 children received their initial dose the week ending Aug. 11.”
The United States just passed the 6-million mark in COVID-19 cases among children, with the last million cases taking less time to record than any of the first five, according to new data from the American Academy of Pediatrics and the Children’s Hospital Association.

The five-millionth case was reported during the week of Aug. 27 to Sept. 2, and case number 6 million came during the week of Oct. 1-7, just 5 weeks later, compared with the 6 weeks it took to go from 1 million to 2 million last November and December, the AAP and CHA said in their weekly COVID-19 report.
New cases continued to drop, however, and that weekly count was down by 14.6% from the previous week and by 41.1% from the peak of almost 252,000 reached in early September, the two groups said while also noting limitations to the data, such as three states (Alabama, Nebraska, and Texas) that are no longer updating their COVID-19 dashboards.
Other metrics show similar drops in recent weeks. Among children aged 0-11 years, emergency department visits involving a COVID-19 diagnosis dropped from 4.1% of all ED visits in late August to 1.4% of ED visits on Oct. 6. ED visits with a COVID-19 diagnosis fell from a peak of 8.5% on Aug. 22 to 1.5% on Oct. 6 for 12- to 15-year-olds and from 8.5% to 1.5% in those aged 16-17 years, according to data from the Centers for Disease Control and Prevention.
The rate of new hospital admissions for children aged 0-17 years was down to 0.26 per 100,000 population on Oct. 9 after reaching 0.51 per 100,000 on Sept. 4. Hospitalizations in children totaled just over 64,000 from Aug. 1, 2020, to Oct. 9, 2021, which is just over 2% of all COVID-19–related admissions over that time period, the CDC said on its COVID Data Tracker.
That pattern, unfortunately, also applies to vaccinations. “The number of children receiving their first COVID-19 vaccine this week [Sept. 30 to Oct. 6], about 156,000, was the lowest number since vaccines were available,” the AAP said in a separate report on vaccination trends, adding that “the number of children receiving their first dose has steadily declined from 8 weeks ago when 586,000 children received their initial dose the week ending Aug. 11.”
The United States just passed the 6-million mark in COVID-19 cases among children, with the last million cases taking less time to record than any of the first five, according to new data from the American Academy of Pediatrics and the Children’s Hospital Association.

The five-millionth case was reported during the week of Aug. 27 to Sept. 2, and case number 6 million came during the week of Oct. 1-7, just 5 weeks later, compared with the 6 weeks it took to go from 1 million to 2 million last November and December, the AAP and CHA said in their weekly COVID-19 report.
New cases continued to drop, however, and that weekly count was down by 14.6% from the previous week and by 41.1% from the peak of almost 252,000 reached in early September, the two groups said while also noting limitations to the data, such as three states (Alabama, Nebraska, and Texas) that are no longer updating their COVID-19 dashboards.
Other metrics show similar drops in recent weeks. Among children aged 0-11 years, emergency department visits involving a COVID-19 diagnosis dropped from 4.1% of all ED visits in late August to 1.4% of ED visits on Oct. 6. ED visits with a COVID-19 diagnosis fell from a peak of 8.5% on Aug. 22 to 1.5% on Oct. 6 for 12- to 15-year-olds and from 8.5% to 1.5% in those aged 16-17 years, according to data from the Centers for Disease Control and Prevention.
The rate of new hospital admissions for children aged 0-17 years was down to 0.26 per 100,000 population on Oct. 9 after reaching 0.51 per 100,000 on Sept. 4. Hospitalizations in children totaled just over 64,000 from Aug. 1, 2020, to Oct. 9, 2021, which is just over 2% of all COVID-19–related admissions over that time period, the CDC said on its COVID Data Tracker.
That pattern, unfortunately, also applies to vaccinations. “The number of children receiving their first COVID-19 vaccine this week [Sept. 30 to Oct. 6], about 156,000, was the lowest number since vaccines were available,” the AAP said in a separate report on vaccination trends, adding that “the number of children receiving their first dose has steadily declined from 8 weeks ago when 586,000 children received their initial dose the week ending Aug. 11.”
Bystander actions can reduce children’s risk of drowning
The likelihood that a child will survive a near-drowning without long-term damage is substantially greater if a bystander attempts a rescue, even if that person doesn’t perform cardiopulmonary resuscitation (CPR), according to new research presented October 10 at the American Academy of Pediatrics (AAP) 2021 National Conference.
“The extent to which bystander rescue is associated with reduced odds of unfavorable drowning outcomes was surprising,” said lead investigator Rohit P. Shenoi, MD, professor of pediatrics at Baylor College of Medicine and attending physician at Texas Children’s Hospital, Houston.
“While we do know that early rescue and resuscitation is helpful in preventing severe drowning injury, the degree of benefit from bystander rescue in all cases of pediatric drowning has not been described so far,” he told this news organization.
The fact that a bystander’s rescue attempt improves a child’s odds of a good outcome is not surprising on its own, but the magnitude of the finding really affirms the importance of bystander intervention, said Benjamin Hoffman, MD, professor of pediatrics at the Oregon Health & Science University School of Medicine and medical director of the Tom Sargent Safety Center at the Doernbecher Children’s Hospital, Portland.
“If an adult finds a child in the water, even if they don’t administer formal CPR, they’re going to be doing things” to try to help, Dr. Hoffman, who was not involved in this research but who specializes in child injury prevention, said in an interview. The act of intervening – whether it’s formal CPR or a CPR attempt or even just calling appropriate first responders – “likely impacts the duration of the submersion” and “clearly makes a difference.”
Drowning is the leading cause of death for children younger than 4 years, Dr. Hoffman noted, adding that the AAP recommends swimming lessons for children older than 1 year to reduce that risk.
In their cross-sectional study, Dr. Shenoi and his colleagues analyzed data on drownings and near-drownings in children and adolescents younger than 18 years using hospital, emergency medical services, and child fatality records from Harris County, Texas.
They analyzed 237 incidents from 2010 to 2013 in which the young person was submerged. Median age of the victims was 3.2 years, 60% were male, 64% were Black, Hispanic, or Native American, and 78% occurred in a swimming pool.
Unfavorable outcomes – defined as death or severe impairment after hospital discharge – were experienced by 38 victims (16%) and were significantly associated with being submerged for longer than 5 minutes (P < .001).
The odds of an unfavorable outcome dropped by 80% if a bystander attempted a rescue, whether or not they performed CPR (adjusted odds ratio, 0.2; P = .004). If the bystander performed CPR, the odds of an unfavorable outcome dropped by a similar amount, but the difference was not statistically significant (aOR, 0.22; P = .07).
However, previous research has shown a significant reduction in poor outcomes when CPR is administered to children who have been submerged, Dr. Hoffman explained.
The most important thing a bystander can do is simply get a submerged child out of the water. “Early rescue in drowning terminates what is initially a respiratory arrest from progressing to a full cardiopulmonary arrest with severe hypoxic brain injury and death,” Dr. Shenoi said.
“CPR is also very important, and rescue and resuscitation go hand in hand. We encourage all laypersons to be trained in CPR so that they can administer correct CPR techniques,” he added.
Both Dr. Shenoi and Dr. Hoffman emphasized the value of CPR training for adults, as the AAP recommends, and the importance of other precautions that reduce the risk of drowning.
“Drowning prevention should consist of multiple layers of prevention,” Dr. Shenoi said. These consist of “close, constant, and attentive supervision; isolation fencing for swimming pools; and water competency, including water-safety knowledge, basic swim skills, and the ability to recognize and respond to a swimmer in trouble, use of life jackets, and early bystander CPR.”
The relative importance of each of those layers depends on geography and circumstances, Dr. Hoffman said. Pools are the most common drowning sites in the United States overall, but they’re much more common in warmer states, such as California, Florida, and Texas, which have more pools. In contrast, drownings in Oregon are more likely to occur in rivers, so prevention is more about access to life jackets and increasing access to swim lessons.
The findings from this study drive home how important it is for physicians to provide anticipatory guidance to families on reducing the risk of drowning. Pediatricians should convey to families the need for different layers of protection, he added.
“If your family spends a lot of time around water, whether open water or swimming pools, the more layers you can provide, the better off you’re going to be,” Dr. Hoffman said.
Dr. Shenoi echoed this sentiment.
“The take-home message is to be observant if you are entrusted with the care of a child around water,” Dr. Shenoi said. “If you notice the child to be drowning, either attempt rescue yourself if it is safe to do so or enlist the help of others to save the victim as soon as possible. However, the rescuer should not place himself or herself in danger when attempting rescue.”
The five steps in the “drowning chain of survival” – preventing drowning, recognizing distress, providing flotation, removing the victim from the water, and providing care and CPR as needed – are key to reducing drowning deaths and injury, Dr. Shenoi emphasized.
Dr. Shenoi has disclosed no relevant financial relationships. Dr. Hoffman is a paid consultant on child drowning prevention for the nonprofit Anonymous Philanthropy.
A version of this article first appeared on Medscape.com.
The likelihood that a child will survive a near-drowning without long-term damage is substantially greater if a bystander attempts a rescue, even if that person doesn’t perform cardiopulmonary resuscitation (CPR), according to new research presented October 10 at the American Academy of Pediatrics (AAP) 2021 National Conference.
“The extent to which bystander rescue is associated with reduced odds of unfavorable drowning outcomes was surprising,” said lead investigator Rohit P. Shenoi, MD, professor of pediatrics at Baylor College of Medicine and attending physician at Texas Children’s Hospital, Houston.
“While we do know that early rescue and resuscitation is helpful in preventing severe drowning injury, the degree of benefit from bystander rescue in all cases of pediatric drowning has not been described so far,” he told this news organization.
The fact that a bystander’s rescue attempt improves a child’s odds of a good outcome is not surprising on its own, but the magnitude of the finding really affirms the importance of bystander intervention, said Benjamin Hoffman, MD, professor of pediatrics at the Oregon Health & Science University School of Medicine and medical director of the Tom Sargent Safety Center at the Doernbecher Children’s Hospital, Portland.
“If an adult finds a child in the water, even if they don’t administer formal CPR, they’re going to be doing things” to try to help, Dr. Hoffman, who was not involved in this research but who specializes in child injury prevention, said in an interview. The act of intervening – whether it’s formal CPR or a CPR attempt or even just calling appropriate first responders – “likely impacts the duration of the submersion” and “clearly makes a difference.”
Drowning is the leading cause of death for children younger than 4 years, Dr. Hoffman noted, adding that the AAP recommends swimming lessons for children older than 1 year to reduce that risk.
In their cross-sectional study, Dr. Shenoi and his colleagues analyzed data on drownings and near-drownings in children and adolescents younger than 18 years using hospital, emergency medical services, and child fatality records from Harris County, Texas.
They analyzed 237 incidents from 2010 to 2013 in which the young person was submerged. Median age of the victims was 3.2 years, 60% were male, 64% were Black, Hispanic, or Native American, and 78% occurred in a swimming pool.
Unfavorable outcomes – defined as death or severe impairment after hospital discharge – were experienced by 38 victims (16%) and were significantly associated with being submerged for longer than 5 minutes (P < .001).
The odds of an unfavorable outcome dropped by 80% if a bystander attempted a rescue, whether or not they performed CPR (adjusted odds ratio, 0.2; P = .004). If the bystander performed CPR, the odds of an unfavorable outcome dropped by a similar amount, but the difference was not statistically significant (aOR, 0.22; P = .07).
However, previous research has shown a significant reduction in poor outcomes when CPR is administered to children who have been submerged, Dr. Hoffman explained.
The most important thing a bystander can do is simply get a submerged child out of the water. “Early rescue in drowning terminates what is initially a respiratory arrest from progressing to a full cardiopulmonary arrest with severe hypoxic brain injury and death,” Dr. Shenoi said.
“CPR is also very important, and rescue and resuscitation go hand in hand. We encourage all laypersons to be trained in CPR so that they can administer correct CPR techniques,” he added.
Both Dr. Shenoi and Dr. Hoffman emphasized the value of CPR training for adults, as the AAP recommends, and the importance of other precautions that reduce the risk of drowning.
“Drowning prevention should consist of multiple layers of prevention,” Dr. Shenoi said. These consist of “close, constant, and attentive supervision; isolation fencing for swimming pools; and water competency, including water-safety knowledge, basic swim skills, and the ability to recognize and respond to a swimmer in trouble, use of life jackets, and early bystander CPR.”
The relative importance of each of those layers depends on geography and circumstances, Dr. Hoffman said. Pools are the most common drowning sites in the United States overall, but they’re much more common in warmer states, such as California, Florida, and Texas, which have more pools. In contrast, drownings in Oregon are more likely to occur in rivers, so prevention is more about access to life jackets and increasing access to swim lessons.
The findings from this study drive home how important it is for physicians to provide anticipatory guidance to families on reducing the risk of drowning. Pediatricians should convey to families the need for different layers of protection, he added.
“If your family spends a lot of time around water, whether open water or swimming pools, the more layers you can provide, the better off you’re going to be,” Dr. Hoffman said.
Dr. Shenoi echoed this sentiment.
“The take-home message is to be observant if you are entrusted with the care of a child around water,” Dr. Shenoi said. “If you notice the child to be drowning, either attempt rescue yourself if it is safe to do so or enlist the help of others to save the victim as soon as possible. However, the rescuer should not place himself or herself in danger when attempting rescue.”
The five steps in the “drowning chain of survival” – preventing drowning, recognizing distress, providing flotation, removing the victim from the water, and providing care and CPR as needed – are key to reducing drowning deaths and injury, Dr. Shenoi emphasized.
Dr. Shenoi has disclosed no relevant financial relationships. Dr. Hoffman is a paid consultant on child drowning prevention for the nonprofit Anonymous Philanthropy.
A version of this article first appeared on Medscape.com.
The likelihood that a child will survive a near-drowning without long-term damage is substantially greater if a bystander attempts a rescue, even if that person doesn’t perform cardiopulmonary resuscitation (CPR), according to new research presented October 10 at the American Academy of Pediatrics (AAP) 2021 National Conference.
“The extent to which bystander rescue is associated with reduced odds of unfavorable drowning outcomes was surprising,” said lead investigator Rohit P. Shenoi, MD, professor of pediatrics at Baylor College of Medicine and attending physician at Texas Children’s Hospital, Houston.
“While we do know that early rescue and resuscitation is helpful in preventing severe drowning injury, the degree of benefit from bystander rescue in all cases of pediatric drowning has not been described so far,” he told this news organization.
The fact that a bystander’s rescue attempt improves a child’s odds of a good outcome is not surprising on its own, but the magnitude of the finding really affirms the importance of bystander intervention, said Benjamin Hoffman, MD, professor of pediatrics at the Oregon Health & Science University School of Medicine and medical director of the Tom Sargent Safety Center at the Doernbecher Children’s Hospital, Portland.
“If an adult finds a child in the water, even if they don’t administer formal CPR, they’re going to be doing things” to try to help, Dr. Hoffman, who was not involved in this research but who specializes in child injury prevention, said in an interview. The act of intervening – whether it’s formal CPR or a CPR attempt or even just calling appropriate first responders – “likely impacts the duration of the submersion” and “clearly makes a difference.”
Drowning is the leading cause of death for children younger than 4 years, Dr. Hoffman noted, adding that the AAP recommends swimming lessons for children older than 1 year to reduce that risk.
In their cross-sectional study, Dr. Shenoi and his colleagues analyzed data on drownings and near-drownings in children and adolescents younger than 18 years using hospital, emergency medical services, and child fatality records from Harris County, Texas.
They analyzed 237 incidents from 2010 to 2013 in which the young person was submerged. Median age of the victims was 3.2 years, 60% were male, 64% were Black, Hispanic, or Native American, and 78% occurred in a swimming pool.
Unfavorable outcomes – defined as death or severe impairment after hospital discharge – were experienced by 38 victims (16%) and were significantly associated with being submerged for longer than 5 minutes (P < .001).
The odds of an unfavorable outcome dropped by 80% if a bystander attempted a rescue, whether or not they performed CPR (adjusted odds ratio, 0.2; P = .004). If the bystander performed CPR, the odds of an unfavorable outcome dropped by a similar amount, but the difference was not statistically significant (aOR, 0.22; P = .07).
However, previous research has shown a significant reduction in poor outcomes when CPR is administered to children who have been submerged, Dr. Hoffman explained.
The most important thing a bystander can do is simply get a submerged child out of the water. “Early rescue in drowning terminates what is initially a respiratory arrest from progressing to a full cardiopulmonary arrest with severe hypoxic brain injury and death,” Dr. Shenoi said.
“CPR is also very important, and rescue and resuscitation go hand in hand. We encourage all laypersons to be trained in CPR so that they can administer correct CPR techniques,” he added.
Both Dr. Shenoi and Dr. Hoffman emphasized the value of CPR training for adults, as the AAP recommends, and the importance of other precautions that reduce the risk of drowning.
“Drowning prevention should consist of multiple layers of prevention,” Dr. Shenoi said. These consist of “close, constant, and attentive supervision; isolation fencing for swimming pools; and water competency, including water-safety knowledge, basic swim skills, and the ability to recognize and respond to a swimmer in trouble, use of life jackets, and early bystander CPR.”
The relative importance of each of those layers depends on geography and circumstances, Dr. Hoffman said. Pools are the most common drowning sites in the United States overall, but they’re much more common in warmer states, such as California, Florida, and Texas, which have more pools. In contrast, drownings in Oregon are more likely to occur in rivers, so prevention is more about access to life jackets and increasing access to swim lessons.
The findings from this study drive home how important it is for physicians to provide anticipatory guidance to families on reducing the risk of drowning. Pediatricians should convey to families the need for different layers of protection, he added.
“If your family spends a lot of time around water, whether open water or swimming pools, the more layers you can provide, the better off you’re going to be,” Dr. Hoffman said.
Dr. Shenoi echoed this sentiment.
“The take-home message is to be observant if you are entrusted with the care of a child around water,” Dr. Shenoi said. “If you notice the child to be drowning, either attempt rescue yourself if it is safe to do so or enlist the help of others to save the victim as soon as possible. However, the rescuer should not place himself or herself in danger when attempting rescue.”
The five steps in the “drowning chain of survival” – preventing drowning, recognizing distress, providing flotation, removing the victim from the water, and providing care and CPR as needed – are key to reducing drowning deaths and injury, Dr. Shenoi emphasized.
Dr. Shenoi has disclosed no relevant financial relationships. Dr. Hoffman is a paid consultant on child drowning prevention for the nonprofit Anonymous Philanthropy.
A version of this article first appeared on Medscape.com.
New reports help nail down myocarditis risk with COVID-19 vaccine
Recent literature features new descriptions of myocarditis linked to the two available mRNA vaccines against SARS-CoV-2. They tell a story largely consistent with experience to date, and support what might be its most useful public health message: The associated myocarditis is usually mild and self-limiting, and is far less likely to occur than myocarditis or death in unvaccinated people with COVID-19.
In line with previous research, the new analyses suggest the myocarditis – with onset usually a few days to a week after injection – has an overall incidence that ranges from less than 1 to perhaps 3 per 100,000 people who received at least one of the full mRNA-vaccine regimen’s two injections. Also, as in earlier studies, the incidence climbed higher – sometimes sharply – in certain groups by age and sex, particularly in young men and older male teens.
The new studies “are confirmatory, in terms of the risk being low,” but underscore that clinicians still must be wary of myocarditis as a potential complication of the mRNA vaccines, Biykem Bozkurt, MD, PhD, Baylor College of Medicine, Houston, told this news organization.
Dr. Bozkurt, a leading heart failure specialist and researcher, did not contribute to any of the new reports but does study the myocarditis of COVID-19 and was lead author on a recent review of the potential vaccine complication’s features and possible mechanisms.
In the new myocarditis reports, she observed, more than 90% of the cases were mild and “resolved on their own without a major adverse outcome.” Dr. Bozkurt emphasized the need for perspective regarding the risk. For example, the myocarditis associated with SARS-CoV-2 infection is not only more likely than the vaccine-related myocarditis, but it’s also usually far more severe.
Dr. Bozkurt pointed to a recent study in which the mRNA vaccines, compared with no vaccination, appeared to escalate the myocarditis risk by a factor of 3, whereas the risk for myocarditis in SARS-CoV-2 infection was increased 18 times.
In contrast, she observed, the new myocarditis cases reported this week feature a few that are novel or are at least very rare, including the case of a patient who developed cardiogenic shock and another with fulminant myocarditis who died.
The Centers for Disease Control and Prevention in May publicly described the apparent link between myocarditis and the two available mRNA vaccines against SARS-CoV-2: BNT162b2 (Pfizer-BioNTech) and mRNA-1273 (Moderna). The next month, the Food and Drug Administration added a warning about the risk to the labeling.
Less than 1 case per 100,000
Fifteen confirmed cases of myocarditis were identified among about 2.4 million members of Kaiser Permanente Southern California aged 18 or older who received at least one injection of the Pfizer or Moderna mRNA vaccines between December 2020 and July 2021, in a report published in JAMA Internal Medicine. The study counted cases up to 10 days after the first or second injection, of which there were 2 and 13, respectively.
All eight patients who received the Pfizer BNT162b2 vaccine and the eight given the Moderna mRNA-1273 vaccine were male with a median age of 25 years (interquartile range, 20-32 years).
“The main takeaway messages from our study are that the incidence of myocarditis after COVID-19 mRNA vaccinations is very low, that this condition is primarily observed in young men within a few days after the second dose, and that most patients recover quickly,” senior author Mingsum Lee, MD, PhD, Kaiser Permanente Los Angeles Medical Center, told this news organization.
“The incidence of vaccine-related myocarditis was significantly lower than rates of COVID-19 hospitalization during the same period and population area,” she added.
The group saw a per-million incidence of 0.8 and 5.8 myocarditis cases in the 10 days after first and second injections, respectively. That made for an incidence of 0.58 per 100,000, or 1 case per 172,414 fully vaccinated adults.
The group also considered a cohort of 1,577,741 unvaccinated people with a median age of 39 years (interquartile range, 28-53 years) during the same period. Of the 75 cases of myocarditis, 52% were in men, they reported.
Comparing the vaccinated and unvaccinated cohorts, they saw a 10-day vaccine-associated myocarditis incidence rate ratio of 0.38 (95% confidence interval, 0.05-1.40; P = .15) after the first dose, and 2.7 (95% CI, 1.4-4.8; P = .004) after the second dose.
In a comparison of the vaccinated group with itself using data from a 10-day period in the previous year, the corresponding myocarditis IRRs were 1.0 (P > .99) and 3.3 (P = .03), respectively.
Dr. Lee said none of the 15 patients required admission to an intensive care unit. “All patients with myocarditis responded well to treatment and felt better quickly,” she noted.
Myocarditis after an mRNA vaccine injection is rare and, Dr. Lee said emphatically, and “the benefits of the COVID-19 vaccine greatly outweigh the risks.”
Sex- and age-stratified rates
In a separate analysis of 5,442,696 people given a first dose of the Pfizer BNT162b2 vaccine and 5,125,635 given a second dose, there were 142 cases of myocarditis with onset 21 days after dose 1 and 30 days after dose 2. Of those cases, 136 were documented as “definite or probable” in an Israeli Ministry of Health database that covered up to the end of May 2021.
There were also 40 cases among vaccinated people seen after the 30-day window, which were considered not related to the vaccination, and 101 cases among unvaccinated people; of the latter, 29 had confirmed diagnoses of COVID-19.
Of the 136 people with definite or probable cases, the myocarditis was “generally mild” in 129 and usually resolved on its own, notes the report on the study, published in the New England Journal of Medicine, with lead author Dror Mevorach, MD, Hadassah-Hebrew University Medical Center, Jerusalem.
The estimated myocarditis incidence after a second such vaccine dose across the entire Israeli population, based on the current study, was about one per 26,000 males and one per 218,000 females, the group writes. Those figures compare with one case per 10,857 among “the general unvaccinated population.”
Again, the risk was concentrated among younger men and male adolescents. In an analysis limited to vaccinated people aged 16-19 years, myocarditis in the 21 days after a second mRNA injection was seen in about one of 6,637 males and one of 99,853 females, the group reported.
The standardized incidence ratio of 5.34 (95% CI, 4.48-6.40) after a second injection, across all groups, “was driven mostly by the diagnosis of myocarditis in younger male recipients.” Among that male subgroup, the ratios by age group were 13.60 (95% CI, 9.30-19.20) for 16-19 years, 8.53 (95% CI, 5.57-12.50) for 20-24 years, and 6.96 (95% CI, 4.25-10.75) for 25-29 years.
Among people who received a second injection, compared with unvaccinated people, the 30-day rate ratio was 2.35 (95% CI, 1.10-5.02). Again, the effect was concentrated in males aged 16-19 years. Among them, the myocarditis rate ratios in the 30 days after a second mRNA vaccine injection were 8.96 (95% CI, 4.50-17.83) for the 16-19 years group, 6.13 (95% CI, 3.16-11.88) for the 20-24 group, and 3.58 (95% CI, 1.82-7.01) for 25-29 years.
Most of the patients with myocarditis showed “significant clinical improvement,” with a mean hospitalization time of only 3-4 days, the report notes. Treatment consisted of nonsteroidal anti-inflammatory drugs “with or without colchicine for presumed pericardial inflammation.”
However, seven patients (4.9%) developed important complications, including left-ventricular dysfunction, ventricular arrhythmias, and heart failure. Among them was a 22-year-old patient who died of fulminant myocarditis within 24 hours of diagnosis, the group wrote.
From an Israeli health care organization
Published by the same journal as the study by Dr. Menvorach and associates, an analysis of a separate database showed largely consistent findings among patients in the largest of Israel’s four health care organizations charged by the government to administer health services.
The report, with authors led by Guy Witberg, MD, Rabin Medical Center, Petah Tikva, Israel, focused on members of the health care organization aged 16 years or older who had received at least one Pfizer mRNA vaccine dose by the end of May 2021.
The cohorts from the two separate reports surely overlap substantially, as the Ministry of Health analysis from Dr. Mevorach and colleagues derived from a nationwide database, and – as Dr. Witberg and associates wrote – the health care organization providing their data covers 52% of the Israeli population.
Of 2,558,421 vaccinated people in the analysis, of whom 94% received two doses, 54 developed confirmed myocarditis in the 42 days after the first dose. Their median age was 27 years (interquartile range, 21-35 years) and all but three (94%) were male. Of those 54 cases, 41 were considered mild and 12 intermediate in severity, and one was fulminant with the patient in cardiogenic shock, the group writes. In addition, nonsustained ventricular tachycardia and atrial fibrillation developed in 5% and 3% of cases, respectively.
The estimated myocarditis incidence in the 42 days after administration of at least one mRNA vaccine dose was 2.13 per 100,000 vaccinated people. In that group, Dr. Witberg and colleagues note, the corresponding incidences per 100,000 were 4.12 and 0.23 for males and females, respectively.
Also in the current report, incidences per 100,000 vaccinated people aged 16-29 years, by sex, included 5.49 (95% CI, 3.59-7.39) overall, and 10.69 (95% CI, 6.93-14.46) for males (the highest rate in the report).
There was only one case in a female aged 16-29 years, and two cases in females 30 years or older.
Of note, some authors of the current study are also authors on the high-profile report from Noam Barda, MD, and colleagues published in the New England Journal of Medicine, that used the same database to arrive at an mRNA-vaccine-related incidence of myocarditis of 2.7 per 100,000. Eligibility criteria and follow-up time were different in that report, as were case ascertainment criteria.
The myocarditis risk associated with the two mRNA vaccines is small compared with “the morbidity and mortality of COVID-19 infection, in which up to 28% of hospitalized patients showed signs of myocardial injury,” wrote Vinay Guduguntla, MD, University of California, San Francisco, and Mitchell H. Katz, MD, NYC Health + Hospitals, New York, in an editorial accompanying the report from Dr. Lee and associates.
“Randomized clinical trials show that COVID-19 mRNA vaccines represent a safe and effective method of preventing infection,” they stated. “The identification of rare myocarditis does not change clinical decision-making.”
Dr. Bozkurt, who is immediate past president of the Heart Failure Society of America, has disclosed consulting for Bayer and scPharmaceuticals and serving on a clinical events committee for a trial supported by Abbott Pharmaceuticals and on a data and safety monitoring board for a trial supported by Liva Nova Pharmaceuticals. Dr. Lee and the report’s other authors had no disclosures. Dr. Mevorach discloses consulting for Enlivex Therapeutics; disclosures for the other authors are available at NEJM.org. Dr. Witberg said he has no interests to disclose; disclosures for the other authors are available at NEJM.org. Dr. Guduguntla is an editorial fellow and Dr. Katz a deputy editor at JAMA Internal Medicine; neither had disclosures.
A version of this article first appeared on Medscape.com.
Recent literature features new descriptions of myocarditis linked to the two available mRNA vaccines against SARS-CoV-2. They tell a story largely consistent with experience to date, and support what might be its most useful public health message: The associated myocarditis is usually mild and self-limiting, and is far less likely to occur than myocarditis or death in unvaccinated people with COVID-19.
In line with previous research, the new analyses suggest the myocarditis – with onset usually a few days to a week after injection – has an overall incidence that ranges from less than 1 to perhaps 3 per 100,000 people who received at least one of the full mRNA-vaccine regimen’s two injections. Also, as in earlier studies, the incidence climbed higher – sometimes sharply – in certain groups by age and sex, particularly in young men and older male teens.
The new studies “are confirmatory, in terms of the risk being low,” but underscore that clinicians still must be wary of myocarditis as a potential complication of the mRNA vaccines, Biykem Bozkurt, MD, PhD, Baylor College of Medicine, Houston, told this news organization.
Dr. Bozkurt, a leading heart failure specialist and researcher, did not contribute to any of the new reports but does study the myocarditis of COVID-19 and was lead author on a recent review of the potential vaccine complication’s features and possible mechanisms.
In the new myocarditis reports, she observed, more than 90% of the cases were mild and “resolved on their own without a major adverse outcome.” Dr. Bozkurt emphasized the need for perspective regarding the risk. For example, the myocarditis associated with SARS-CoV-2 infection is not only more likely than the vaccine-related myocarditis, but it’s also usually far more severe.
Dr. Bozkurt pointed to a recent study in which the mRNA vaccines, compared with no vaccination, appeared to escalate the myocarditis risk by a factor of 3, whereas the risk for myocarditis in SARS-CoV-2 infection was increased 18 times.
In contrast, she observed, the new myocarditis cases reported this week feature a few that are novel or are at least very rare, including the case of a patient who developed cardiogenic shock and another with fulminant myocarditis who died.
The Centers for Disease Control and Prevention in May publicly described the apparent link between myocarditis and the two available mRNA vaccines against SARS-CoV-2: BNT162b2 (Pfizer-BioNTech) and mRNA-1273 (Moderna). The next month, the Food and Drug Administration added a warning about the risk to the labeling.
Less than 1 case per 100,000
Fifteen confirmed cases of myocarditis were identified among about 2.4 million members of Kaiser Permanente Southern California aged 18 or older who received at least one injection of the Pfizer or Moderna mRNA vaccines between December 2020 and July 2021, in a report published in JAMA Internal Medicine. The study counted cases up to 10 days after the first or second injection, of which there were 2 and 13, respectively.
All eight patients who received the Pfizer BNT162b2 vaccine and the eight given the Moderna mRNA-1273 vaccine were male with a median age of 25 years (interquartile range, 20-32 years).
“The main takeaway messages from our study are that the incidence of myocarditis after COVID-19 mRNA vaccinations is very low, that this condition is primarily observed in young men within a few days after the second dose, and that most patients recover quickly,” senior author Mingsum Lee, MD, PhD, Kaiser Permanente Los Angeles Medical Center, told this news organization.
“The incidence of vaccine-related myocarditis was significantly lower than rates of COVID-19 hospitalization during the same period and population area,” she added.
The group saw a per-million incidence of 0.8 and 5.8 myocarditis cases in the 10 days after first and second injections, respectively. That made for an incidence of 0.58 per 100,000, or 1 case per 172,414 fully vaccinated adults.
The group also considered a cohort of 1,577,741 unvaccinated people with a median age of 39 years (interquartile range, 28-53 years) during the same period. Of the 75 cases of myocarditis, 52% were in men, they reported.
Comparing the vaccinated and unvaccinated cohorts, they saw a 10-day vaccine-associated myocarditis incidence rate ratio of 0.38 (95% confidence interval, 0.05-1.40; P = .15) after the first dose, and 2.7 (95% CI, 1.4-4.8; P = .004) after the second dose.
In a comparison of the vaccinated group with itself using data from a 10-day period in the previous year, the corresponding myocarditis IRRs were 1.0 (P > .99) and 3.3 (P = .03), respectively.
Dr. Lee said none of the 15 patients required admission to an intensive care unit. “All patients with myocarditis responded well to treatment and felt better quickly,” she noted.
Myocarditis after an mRNA vaccine injection is rare and, Dr. Lee said emphatically, and “the benefits of the COVID-19 vaccine greatly outweigh the risks.”
Sex- and age-stratified rates
In a separate analysis of 5,442,696 people given a first dose of the Pfizer BNT162b2 vaccine and 5,125,635 given a second dose, there were 142 cases of myocarditis with onset 21 days after dose 1 and 30 days after dose 2. Of those cases, 136 were documented as “definite or probable” in an Israeli Ministry of Health database that covered up to the end of May 2021.
There were also 40 cases among vaccinated people seen after the 30-day window, which were considered not related to the vaccination, and 101 cases among unvaccinated people; of the latter, 29 had confirmed diagnoses of COVID-19.
Of the 136 people with definite or probable cases, the myocarditis was “generally mild” in 129 and usually resolved on its own, notes the report on the study, published in the New England Journal of Medicine, with lead author Dror Mevorach, MD, Hadassah-Hebrew University Medical Center, Jerusalem.
The estimated myocarditis incidence after a second such vaccine dose across the entire Israeli population, based on the current study, was about one per 26,000 males and one per 218,000 females, the group writes. Those figures compare with one case per 10,857 among “the general unvaccinated population.”
Again, the risk was concentrated among younger men and male adolescents. In an analysis limited to vaccinated people aged 16-19 years, myocarditis in the 21 days after a second mRNA injection was seen in about one of 6,637 males and one of 99,853 females, the group reported.
The standardized incidence ratio of 5.34 (95% CI, 4.48-6.40) after a second injection, across all groups, “was driven mostly by the diagnosis of myocarditis in younger male recipients.” Among that male subgroup, the ratios by age group were 13.60 (95% CI, 9.30-19.20) for 16-19 years, 8.53 (95% CI, 5.57-12.50) for 20-24 years, and 6.96 (95% CI, 4.25-10.75) for 25-29 years.
Among people who received a second injection, compared with unvaccinated people, the 30-day rate ratio was 2.35 (95% CI, 1.10-5.02). Again, the effect was concentrated in males aged 16-19 years. Among them, the myocarditis rate ratios in the 30 days after a second mRNA vaccine injection were 8.96 (95% CI, 4.50-17.83) for the 16-19 years group, 6.13 (95% CI, 3.16-11.88) for the 20-24 group, and 3.58 (95% CI, 1.82-7.01) for 25-29 years.
Most of the patients with myocarditis showed “significant clinical improvement,” with a mean hospitalization time of only 3-4 days, the report notes. Treatment consisted of nonsteroidal anti-inflammatory drugs “with or without colchicine for presumed pericardial inflammation.”
However, seven patients (4.9%) developed important complications, including left-ventricular dysfunction, ventricular arrhythmias, and heart failure. Among them was a 22-year-old patient who died of fulminant myocarditis within 24 hours of diagnosis, the group wrote.
From an Israeli health care organization
Published by the same journal as the study by Dr. Menvorach and associates, an analysis of a separate database showed largely consistent findings among patients in the largest of Israel’s four health care organizations charged by the government to administer health services.
The report, with authors led by Guy Witberg, MD, Rabin Medical Center, Petah Tikva, Israel, focused on members of the health care organization aged 16 years or older who had received at least one Pfizer mRNA vaccine dose by the end of May 2021.
The cohorts from the two separate reports surely overlap substantially, as the Ministry of Health analysis from Dr. Mevorach and colleagues derived from a nationwide database, and – as Dr. Witberg and associates wrote – the health care organization providing their data covers 52% of the Israeli population.
Of 2,558,421 vaccinated people in the analysis, of whom 94% received two doses, 54 developed confirmed myocarditis in the 42 days after the first dose. Their median age was 27 years (interquartile range, 21-35 years) and all but three (94%) were male. Of those 54 cases, 41 were considered mild and 12 intermediate in severity, and one was fulminant with the patient in cardiogenic shock, the group writes. In addition, nonsustained ventricular tachycardia and atrial fibrillation developed in 5% and 3% of cases, respectively.
The estimated myocarditis incidence in the 42 days after administration of at least one mRNA vaccine dose was 2.13 per 100,000 vaccinated people. In that group, Dr. Witberg and colleagues note, the corresponding incidences per 100,000 were 4.12 and 0.23 for males and females, respectively.
Also in the current report, incidences per 100,000 vaccinated people aged 16-29 years, by sex, included 5.49 (95% CI, 3.59-7.39) overall, and 10.69 (95% CI, 6.93-14.46) for males (the highest rate in the report).
There was only one case in a female aged 16-29 years, and two cases in females 30 years or older.
Of note, some authors of the current study are also authors on the high-profile report from Noam Barda, MD, and colleagues published in the New England Journal of Medicine, that used the same database to arrive at an mRNA-vaccine-related incidence of myocarditis of 2.7 per 100,000. Eligibility criteria and follow-up time were different in that report, as were case ascertainment criteria.
The myocarditis risk associated with the two mRNA vaccines is small compared with “the morbidity and mortality of COVID-19 infection, in which up to 28% of hospitalized patients showed signs of myocardial injury,” wrote Vinay Guduguntla, MD, University of California, San Francisco, and Mitchell H. Katz, MD, NYC Health + Hospitals, New York, in an editorial accompanying the report from Dr. Lee and associates.
“Randomized clinical trials show that COVID-19 mRNA vaccines represent a safe and effective method of preventing infection,” they stated. “The identification of rare myocarditis does not change clinical decision-making.”
Dr. Bozkurt, who is immediate past president of the Heart Failure Society of America, has disclosed consulting for Bayer and scPharmaceuticals and serving on a clinical events committee for a trial supported by Abbott Pharmaceuticals and on a data and safety monitoring board for a trial supported by Liva Nova Pharmaceuticals. Dr. Lee and the report’s other authors had no disclosures. Dr. Mevorach discloses consulting for Enlivex Therapeutics; disclosures for the other authors are available at NEJM.org. Dr. Witberg said he has no interests to disclose; disclosures for the other authors are available at NEJM.org. Dr. Guduguntla is an editorial fellow and Dr. Katz a deputy editor at JAMA Internal Medicine; neither had disclosures.
A version of this article first appeared on Medscape.com.
Recent literature features new descriptions of myocarditis linked to the two available mRNA vaccines against SARS-CoV-2. They tell a story largely consistent with experience to date, and support what might be its most useful public health message: The associated myocarditis is usually mild and self-limiting, and is far less likely to occur than myocarditis or death in unvaccinated people with COVID-19.
In line with previous research, the new analyses suggest the myocarditis – with onset usually a few days to a week after injection – has an overall incidence that ranges from less than 1 to perhaps 3 per 100,000 people who received at least one of the full mRNA-vaccine regimen’s two injections. Also, as in earlier studies, the incidence climbed higher – sometimes sharply – in certain groups by age and sex, particularly in young men and older male teens.
The new studies “are confirmatory, in terms of the risk being low,” but underscore that clinicians still must be wary of myocarditis as a potential complication of the mRNA vaccines, Biykem Bozkurt, MD, PhD, Baylor College of Medicine, Houston, told this news organization.
Dr. Bozkurt, a leading heart failure specialist and researcher, did not contribute to any of the new reports but does study the myocarditis of COVID-19 and was lead author on a recent review of the potential vaccine complication’s features and possible mechanisms.
In the new myocarditis reports, she observed, more than 90% of the cases were mild and “resolved on their own without a major adverse outcome.” Dr. Bozkurt emphasized the need for perspective regarding the risk. For example, the myocarditis associated with SARS-CoV-2 infection is not only more likely than the vaccine-related myocarditis, but it’s also usually far more severe.
Dr. Bozkurt pointed to a recent study in which the mRNA vaccines, compared with no vaccination, appeared to escalate the myocarditis risk by a factor of 3, whereas the risk for myocarditis in SARS-CoV-2 infection was increased 18 times.
In contrast, she observed, the new myocarditis cases reported this week feature a few that are novel or are at least very rare, including the case of a patient who developed cardiogenic shock and another with fulminant myocarditis who died.
The Centers for Disease Control and Prevention in May publicly described the apparent link between myocarditis and the two available mRNA vaccines against SARS-CoV-2: BNT162b2 (Pfizer-BioNTech) and mRNA-1273 (Moderna). The next month, the Food and Drug Administration added a warning about the risk to the labeling.
Less than 1 case per 100,000
Fifteen confirmed cases of myocarditis were identified among about 2.4 million members of Kaiser Permanente Southern California aged 18 or older who received at least one injection of the Pfizer or Moderna mRNA vaccines between December 2020 and July 2021, in a report published in JAMA Internal Medicine. The study counted cases up to 10 days after the first or second injection, of which there were 2 and 13, respectively.
All eight patients who received the Pfizer BNT162b2 vaccine and the eight given the Moderna mRNA-1273 vaccine were male with a median age of 25 years (interquartile range, 20-32 years).
“The main takeaway messages from our study are that the incidence of myocarditis after COVID-19 mRNA vaccinations is very low, that this condition is primarily observed in young men within a few days after the second dose, and that most patients recover quickly,” senior author Mingsum Lee, MD, PhD, Kaiser Permanente Los Angeles Medical Center, told this news organization.
“The incidence of vaccine-related myocarditis was significantly lower than rates of COVID-19 hospitalization during the same period and population area,” she added.
The group saw a per-million incidence of 0.8 and 5.8 myocarditis cases in the 10 days after first and second injections, respectively. That made for an incidence of 0.58 per 100,000, or 1 case per 172,414 fully vaccinated adults.
The group also considered a cohort of 1,577,741 unvaccinated people with a median age of 39 years (interquartile range, 28-53 years) during the same period. Of the 75 cases of myocarditis, 52% were in men, they reported.
Comparing the vaccinated and unvaccinated cohorts, they saw a 10-day vaccine-associated myocarditis incidence rate ratio of 0.38 (95% confidence interval, 0.05-1.40; P = .15) after the first dose, and 2.7 (95% CI, 1.4-4.8; P = .004) after the second dose.
In a comparison of the vaccinated group with itself using data from a 10-day period in the previous year, the corresponding myocarditis IRRs were 1.0 (P > .99) and 3.3 (P = .03), respectively.
Dr. Lee said none of the 15 patients required admission to an intensive care unit. “All patients with myocarditis responded well to treatment and felt better quickly,” she noted.
Myocarditis after an mRNA vaccine injection is rare and, Dr. Lee said emphatically, and “the benefits of the COVID-19 vaccine greatly outweigh the risks.”
Sex- and age-stratified rates
In a separate analysis of 5,442,696 people given a first dose of the Pfizer BNT162b2 vaccine and 5,125,635 given a second dose, there were 142 cases of myocarditis with onset 21 days after dose 1 and 30 days after dose 2. Of those cases, 136 were documented as “definite or probable” in an Israeli Ministry of Health database that covered up to the end of May 2021.
There were also 40 cases among vaccinated people seen after the 30-day window, which were considered not related to the vaccination, and 101 cases among unvaccinated people; of the latter, 29 had confirmed diagnoses of COVID-19.
Of the 136 people with definite or probable cases, the myocarditis was “generally mild” in 129 and usually resolved on its own, notes the report on the study, published in the New England Journal of Medicine, with lead author Dror Mevorach, MD, Hadassah-Hebrew University Medical Center, Jerusalem.
The estimated myocarditis incidence after a second such vaccine dose across the entire Israeli population, based on the current study, was about one per 26,000 males and one per 218,000 females, the group writes. Those figures compare with one case per 10,857 among “the general unvaccinated population.”
Again, the risk was concentrated among younger men and male adolescents. In an analysis limited to vaccinated people aged 16-19 years, myocarditis in the 21 days after a second mRNA injection was seen in about one of 6,637 males and one of 99,853 females, the group reported.
The standardized incidence ratio of 5.34 (95% CI, 4.48-6.40) after a second injection, across all groups, “was driven mostly by the diagnosis of myocarditis in younger male recipients.” Among that male subgroup, the ratios by age group were 13.60 (95% CI, 9.30-19.20) for 16-19 years, 8.53 (95% CI, 5.57-12.50) for 20-24 years, and 6.96 (95% CI, 4.25-10.75) for 25-29 years.
Among people who received a second injection, compared with unvaccinated people, the 30-day rate ratio was 2.35 (95% CI, 1.10-5.02). Again, the effect was concentrated in males aged 16-19 years. Among them, the myocarditis rate ratios in the 30 days after a second mRNA vaccine injection were 8.96 (95% CI, 4.50-17.83) for the 16-19 years group, 6.13 (95% CI, 3.16-11.88) for the 20-24 group, and 3.58 (95% CI, 1.82-7.01) for 25-29 years.
Most of the patients with myocarditis showed “significant clinical improvement,” with a mean hospitalization time of only 3-4 days, the report notes. Treatment consisted of nonsteroidal anti-inflammatory drugs “with or without colchicine for presumed pericardial inflammation.”
However, seven patients (4.9%) developed important complications, including left-ventricular dysfunction, ventricular arrhythmias, and heart failure. Among them was a 22-year-old patient who died of fulminant myocarditis within 24 hours of diagnosis, the group wrote.
From an Israeli health care organization
Published by the same journal as the study by Dr. Menvorach and associates, an analysis of a separate database showed largely consistent findings among patients in the largest of Israel’s four health care organizations charged by the government to administer health services.
The report, with authors led by Guy Witberg, MD, Rabin Medical Center, Petah Tikva, Israel, focused on members of the health care organization aged 16 years or older who had received at least one Pfizer mRNA vaccine dose by the end of May 2021.
The cohorts from the two separate reports surely overlap substantially, as the Ministry of Health analysis from Dr. Mevorach and colleagues derived from a nationwide database, and – as Dr. Witberg and associates wrote – the health care organization providing their data covers 52% of the Israeli population.
Of 2,558,421 vaccinated people in the analysis, of whom 94% received two doses, 54 developed confirmed myocarditis in the 42 days after the first dose. Their median age was 27 years (interquartile range, 21-35 years) and all but three (94%) were male. Of those 54 cases, 41 were considered mild and 12 intermediate in severity, and one was fulminant with the patient in cardiogenic shock, the group writes. In addition, nonsustained ventricular tachycardia and atrial fibrillation developed in 5% and 3% of cases, respectively.
The estimated myocarditis incidence in the 42 days after administration of at least one mRNA vaccine dose was 2.13 per 100,000 vaccinated people. In that group, Dr. Witberg and colleagues note, the corresponding incidences per 100,000 were 4.12 and 0.23 for males and females, respectively.
Also in the current report, incidences per 100,000 vaccinated people aged 16-29 years, by sex, included 5.49 (95% CI, 3.59-7.39) overall, and 10.69 (95% CI, 6.93-14.46) for males (the highest rate in the report).
There was only one case in a female aged 16-29 years, and two cases in females 30 years or older.
Of note, some authors of the current study are also authors on the high-profile report from Noam Barda, MD, and colleagues published in the New England Journal of Medicine, that used the same database to arrive at an mRNA-vaccine-related incidence of myocarditis of 2.7 per 100,000. Eligibility criteria and follow-up time were different in that report, as were case ascertainment criteria.
The myocarditis risk associated with the two mRNA vaccines is small compared with “the morbidity and mortality of COVID-19 infection, in which up to 28% of hospitalized patients showed signs of myocardial injury,” wrote Vinay Guduguntla, MD, University of California, San Francisco, and Mitchell H. Katz, MD, NYC Health + Hospitals, New York, in an editorial accompanying the report from Dr. Lee and associates.
“Randomized clinical trials show that COVID-19 mRNA vaccines represent a safe and effective method of preventing infection,” they stated. “The identification of rare myocarditis does not change clinical decision-making.”
Dr. Bozkurt, who is immediate past president of the Heart Failure Society of America, has disclosed consulting for Bayer and scPharmaceuticals and serving on a clinical events committee for a trial supported by Abbott Pharmaceuticals and on a data and safety monitoring board for a trial supported by Liva Nova Pharmaceuticals. Dr. Lee and the report’s other authors had no disclosures. Dr. Mevorach discloses consulting for Enlivex Therapeutics; disclosures for the other authors are available at NEJM.org. Dr. Witberg said he has no interests to disclose; disclosures for the other authors are available at NEJM.org. Dr. Guduguntla is an editorial fellow and Dr. Katz a deputy editor at JAMA Internal Medicine; neither had disclosures.
A version of this article first appeared on Medscape.com.
Merck seeks FDA authorization for antiviral COVID-19 pill
, an experimental antiviral COVID-19 treatment.
If the FDA grants authorization, the drug would be the first oral antiviral treatment for COVID-19. The capsule, made by Merck and Ridgeback Biotherapeutics, is intended to treat mild to moderate COVID-19 in adults who are at risk of having severe COVID-19 or hospitalization.
“The extraordinary impact of this pandemic demands that we move with unprecedented urgency, and that is what our teams have done by submitting this application for molnupiravir to the FDA within 10 days of receiving the data,” Robert Davis, CEO and president of Merck, said in a statement. On Oct. 1, Merck and Ridgeback released interim data from its phase III clinical trial, which showed that molnupiravir reduced the risk of hospitalization or death by about 50%. About 7% of patients who received the drug were hospitalized within 30 days in the study, as compared with 14% of patients who took a placebo, the company said.
No deaths were reported in the group that received the drug, as compared with eight deaths in the group that received the placebo. None of the trial participants had been vaccinated.
“Medicines and vaccines are both essential to our collective efforts,” Mr. Davis said. “We look forward to working with the FDA on its review of our application, and to working with other regulatory agencies as we do everything we can to bring molnupiravir to patients around the world as quickly as possible.”
Merck has been producing molnupiravir in anticipation of the clinical trial results and FDA authorization. The company expects to produce 10 million courses of treatment by the end of the year, with more expected for 2022.
In June, Merck signed an agreement with the United States to supply 1.7 million courses of molnupiravir once the FDA authorizes the drug. The company has agreed to advance purchase agreements with other countries as well.
Earlier in the year, Merck also announced voluntary licensing agreements with several generics manufacturers in India to provide molnupiravir to more than 100 low- and middle-income countries after approval from local regulatory agencies.
Data from the company’s late-stage clinical trial has not yet been peer-reviewed or published.
Last week, Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, said the clinical trial results were “very encouraging” but noted that the FDA should closely scrutinize the drug, CNN reported.
“It is very important that this now must go through the usual process of careful examination of the data by the Food and Drug Administration, both for effectiveness but also for safety, because whenever you introduce a new compound, safety is very important,” Dr. Fauci said, adding that vaccines remain “our best tools against COVID-19.”
A version of this article firsts appeared on WebMD.com.
, an experimental antiviral COVID-19 treatment.
If the FDA grants authorization, the drug would be the first oral antiviral treatment for COVID-19. The capsule, made by Merck and Ridgeback Biotherapeutics, is intended to treat mild to moderate COVID-19 in adults who are at risk of having severe COVID-19 or hospitalization.
“The extraordinary impact of this pandemic demands that we move with unprecedented urgency, and that is what our teams have done by submitting this application for molnupiravir to the FDA within 10 days of receiving the data,” Robert Davis, CEO and president of Merck, said in a statement. On Oct. 1, Merck and Ridgeback released interim data from its phase III clinical trial, which showed that molnupiravir reduced the risk of hospitalization or death by about 50%. About 7% of patients who received the drug were hospitalized within 30 days in the study, as compared with 14% of patients who took a placebo, the company said.
No deaths were reported in the group that received the drug, as compared with eight deaths in the group that received the placebo. None of the trial participants had been vaccinated.
“Medicines and vaccines are both essential to our collective efforts,” Mr. Davis said. “We look forward to working with the FDA on its review of our application, and to working with other regulatory agencies as we do everything we can to bring molnupiravir to patients around the world as quickly as possible.”
Merck has been producing molnupiravir in anticipation of the clinical trial results and FDA authorization. The company expects to produce 10 million courses of treatment by the end of the year, with more expected for 2022.
In June, Merck signed an agreement with the United States to supply 1.7 million courses of molnupiravir once the FDA authorizes the drug. The company has agreed to advance purchase agreements with other countries as well.
Earlier in the year, Merck also announced voluntary licensing agreements with several generics manufacturers in India to provide molnupiravir to more than 100 low- and middle-income countries after approval from local regulatory agencies.
Data from the company’s late-stage clinical trial has not yet been peer-reviewed or published.
Last week, Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, said the clinical trial results were “very encouraging” but noted that the FDA should closely scrutinize the drug, CNN reported.
“It is very important that this now must go through the usual process of careful examination of the data by the Food and Drug Administration, both for effectiveness but also for safety, because whenever you introduce a new compound, safety is very important,” Dr. Fauci said, adding that vaccines remain “our best tools against COVID-19.”
A version of this article firsts appeared on WebMD.com.
, an experimental antiviral COVID-19 treatment.
If the FDA grants authorization, the drug would be the first oral antiviral treatment for COVID-19. The capsule, made by Merck and Ridgeback Biotherapeutics, is intended to treat mild to moderate COVID-19 in adults who are at risk of having severe COVID-19 or hospitalization.
“The extraordinary impact of this pandemic demands that we move with unprecedented urgency, and that is what our teams have done by submitting this application for molnupiravir to the FDA within 10 days of receiving the data,” Robert Davis, CEO and president of Merck, said in a statement. On Oct. 1, Merck and Ridgeback released interim data from its phase III clinical trial, which showed that molnupiravir reduced the risk of hospitalization or death by about 50%. About 7% of patients who received the drug were hospitalized within 30 days in the study, as compared with 14% of patients who took a placebo, the company said.
No deaths were reported in the group that received the drug, as compared with eight deaths in the group that received the placebo. None of the trial participants had been vaccinated.
“Medicines and vaccines are both essential to our collective efforts,” Mr. Davis said. “We look forward to working with the FDA on its review of our application, and to working with other regulatory agencies as we do everything we can to bring molnupiravir to patients around the world as quickly as possible.”
Merck has been producing molnupiravir in anticipation of the clinical trial results and FDA authorization. The company expects to produce 10 million courses of treatment by the end of the year, with more expected for 2022.
In June, Merck signed an agreement with the United States to supply 1.7 million courses of molnupiravir once the FDA authorizes the drug. The company has agreed to advance purchase agreements with other countries as well.
Earlier in the year, Merck also announced voluntary licensing agreements with several generics manufacturers in India to provide molnupiravir to more than 100 low- and middle-income countries after approval from local regulatory agencies.
Data from the company’s late-stage clinical trial has not yet been peer-reviewed or published.
Last week, Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, said the clinical trial results were “very encouraging” but noted that the FDA should closely scrutinize the drug, CNN reported.
“It is very important that this now must go through the usual process of careful examination of the data by the Food and Drug Administration, both for effectiveness but also for safety, because whenever you introduce a new compound, safety is very important,” Dr. Fauci said, adding that vaccines remain “our best tools against COVID-19.”
A version of this article firsts appeared on WebMD.com.
High-dose omega-3s tied to higher AFib risk
Taking high-doses of marine omega-3 fatty acids, more than 1 gram daily, may raise the risk for atrial fibrillation (AFib), according to a meta-analysis of relevant research.
However, the risk of developing AFib appears to be “relatively small” for those taking 1 gram or less of fish oil per day, Christine M. Albert, MD, chair of the department of cardiology at the Smidt Heart Institute at Cedars-Sinai, Los Angeles, told this news organization.
The study was published online Oct. 6 in the journal Circulation.
It’s estimated that 7.8% of U.S. adults – almost 19 million in all – take fish oil supplements, often unbeknownst to their health care providers, the researchers noted. Yet, the literature on the effects of omega-3 fatty acid supplementation on cardiovascular outcomes are mixed.
“Some, but not all” large-scale randomized controlled trials investigating the effects of marine omega-3 fatty acid supplements on cardiovascular outcomes have reported increased risks for AFib. The potential reasons for differing findings may be dose related, the authors note in their paper.
The goal of this meta-analysis was to “bring clarity, answers, and actionable information” to doctors and patients, said Dr. Albert. The results suggest, however, that there may not be a “straightforward answer” to whether fish oil is good or bad for AFib. Instead, the answer may depend on the dose, she added.
Pooled data
After screening 4,049 articles and abstracts, the researchers included in their analysis seven large-scale randomized controlled trials reporting cardiovascular outcomes of marine omega-3 fatty acids.
The trials reported results for AFib, either as prespecified outcome, adverse event, or a reason for hospitalization. Each had a minimum of 500 patients and a median follow-up of at least 1 year.
Trials examining the effects of omega-3 fatty acids on recurrent AFib in patients with established AFib or postoperative AFib were excluded.
The seven trials enrolled a total of 81,210 patients (mean age, 65 years; 39% women); 72.6% of participants were enrolled in clinical trials testing ≤1 gram of marine omega-3 fatty acids per day and 27.4% were enrolled in clinical trials testing >1 gram of the supplement per day. The weighted average follow-up was 4.9 years.
Overall, use of omega-3 fatty acids was associated with a 25% increased risk for AFib (hazard ratio, 1.25; 95% confidence interval, 1.07-1.46; P = .013).
In analyses stratified by dose, the risk for AFib was “significantly more pronounced” in trials testing high doses of marine omega-3 fatty acid supplements (>1 gram per day: HR, 1.49; 95% CI, 1.04-2.15; P = .042) compared with those testing lower doses (≤1 gram per day: HR, 1.12; 95% CI, 1.03-1.22; P = .024; P for interaction < .001).
In meta-regression, the HR for AFib increased per 1 gram increase in daily omega-3 fatty acid dose (HR. 1.11; 95% CI, 1.06-1.15; P = .001).
Risk-benefit balance
“This meta-analysis adds new evidence regarding the risk of AFib in patients taking marine omega-3 fatty acid supplements,” wrote Dr. Albert and colleagues.
“Since the benefit of omega-3 fatty acids also appears to be dose dependent, the associated risk of AFib should be balanced against the benefit on atherosclerotic cardiovascular outcomes,” they suggested.
They cautioned that the meta-analysis pooled aggregate-level trial data, not individual patient data. Therefore, subgroup analyses by age or other patient level characteristics were not possible.
The risk of developing AFib increases with advancing age and is more common in men than in women. Additional risk factors include elevated blood pressure, coronary artery disease, heart failure, heart valve defects, obesity, and diabetes.
The authors said the potential risk of developing AFib with high doses of omega-3 fatty acid supplements should be discussed with patients and they should know the signs and symptoms of the condition.
The study had no specific funding. Dr. Albert has received grants from St. Jude Medical, Abbott, and Roche Diagnostics.
A version of this article first appeared on Medscape.com.
Taking high-doses of marine omega-3 fatty acids, more than 1 gram daily, may raise the risk for atrial fibrillation (AFib), according to a meta-analysis of relevant research.
However, the risk of developing AFib appears to be “relatively small” for those taking 1 gram or less of fish oil per day, Christine M. Albert, MD, chair of the department of cardiology at the Smidt Heart Institute at Cedars-Sinai, Los Angeles, told this news organization.
The study was published online Oct. 6 in the journal Circulation.
It’s estimated that 7.8% of U.S. adults – almost 19 million in all – take fish oil supplements, often unbeknownst to their health care providers, the researchers noted. Yet, the literature on the effects of omega-3 fatty acid supplementation on cardiovascular outcomes are mixed.
“Some, but not all” large-scale randomized controlled trials investigating the effects of marine omega-3 fatty acid supplements on cardiovascular outcomes have reported increased risks for AFib. The potential reasons for differing findings may be dose related, the authors note in their paper.
The goal of this meta-analysis was to “bring clarity, answers, and actionable information” to doctors and patients, said Dr. Albert. The results suggest, however, that there may not be a “straightforward answer” to whether fish oil is good or bad for AFib. Instead, the answer may depend on the dose, she added.
Pooled data
After screening 4,049 articles and abstracts, the researchers included in their analysis seven large-scale randomized controlled trials reporting cardiovascular outcomes of marine omega-3 fatty acids.
The trials reported results for AFib, either as prespecified outcome, adverse event, or a reason for hospitalization. Each had a minimum of 500 patients and a median follow-up of at least 1 year.
Trials examining the effects of omega-3 fatty acids on recurrent AFib in patients with established AFib or postoperative AFib were excluded.
The seven trials enrolled a total of 81,210 patients (mean age, 65 years; 39% women); 72.6% of participants were enrolled in clinical trials testing ≤1 gram of marine omega-3 fatty acids per day and 27.4% were enrolled in clinical trials testing >1 gram of the supplement per day. The weighted average follow-up was 4.9 years.
Overall, use of omega-3 fatty acids was associated with a 25% increased risk for AFib (hazard ratio, 1.25; 95% confidence interval, 1.07-1.46; P = .013).
In analyses stratified by dose, the risk for AFib was “significantly more pronounced” in trials testing high doses of marine omega-3 fatty acid supplements (>1 gram per day: HR, 1.49; 95% CI, 1.04-2.15; P = .042) compared with those testing lower doses (≤1 gram per day: HR, 1.12; 95% CI, 1.03-1.22; P = .024; P for interaction < .001).
In meta-regression, the HR for AFib increased per 1 gram increase in daily omega-3 fatty acid dose (HR. 1.11; 95% CI, 1.06-1.15; P = .001).
Risk-benefit balance
“This meta-analysis adds new evidence regarding the risk of AFib in patients taking marine omega-3 fatty acid supplements,” wrote Dr. Albert and colleagues.
“Since the benefit of omega-3 fatty acids also appears to be dose dependent, the associated risk of AFib should be balanced against the benefit on atherosclerotic cardiovascular outcomes,” they suggested.
They cautioned that the meta-analysis pooled aggregate-level trial data, not individual patient data. Therefore, subgroup analyses by age or other patient level characteristics were not possible.
The risk of developing AFib increases with advancing age and is more common in men than in women. Additional risk factors include elevated blood pressure, coronary artery disease, heart failure, heart valve defects, obesity, and diabetes.
The authors said the potential risk of developing AFib with high doses of omega-3 fatty acid supplements should be discussed with patients and they should know the signs and symptoms of the condition.
The study had no specific funding. Dr. Albert has received grants from St. Jude Medical, Abbott, and Roche Diagnostics.
A version of this article first appeared on Medscape.com.
Taking high-doses of marine omega-3 fatty acids, more than 1 gram daily, may raise the risk for atrial fibrillation (AFib), according to a meta-analysis of relevant research.
However, the risk of developing AFib appears to be “relatively small” for those taking 1 gram or less of fish oil per day, Christine M. Albert, MD, chair of the department of cardiology at the Smidt Heart Institute at Cedars-Sinai, Los Angeles, told this news organization.
The study was published online Oct. 6 in the journal Circulation.
It’s estimated that 7.8% of U.S. adults – almost 19 million in all – take fish oil supplements, often unbeknownst to their health care providers, the researchers noted. Yet, the literature on the effects of omega-3 fatty acid supplementation on cardiovascular outcomes are mixed.
“Some, but not all” large-scale randomized controlled trials investigating the effects of marine omega-3 fatty acid supplements on cardiovascular outcomes have reported increased risks for AFib. The potential reasons for differing findings may be dose related, the authors note in their paper.
The goal of this meta-analysis was to “bring clarity, answers, and actionable information” to doctors and patients, said Dr. Albert. The results suggest, however, that there may not be a “straightforward answer” to whether fish oil is good or bad for AFib. Instead, the answer may depend on the dose, she added.
Pooled data
After screening 4,049 articles and abstracts, the researchers included in their analysis seven large-scale randomized controlled trials reporting cardiovascular outcomes of marine omega-3 fatty acids.
The trials reported results for AFib, either as prespecified outcome, adverse event, or a reason for hospitalization. Each had a minimum of 500 patients and a median follow-up of at least 1 year.
Trials examining the effects of omega-3 fatty acids on recurrent AFib in patients with established AFib or postoperative AFib were excluded.
The seven trials enrolled a total of 81,210 patients (mean age, 65 years; 39% women); 72.6% of participants were enrolled in clinical trials testing ≤1 gram of marine omega-3 fatty acids per day and 27.4% were enrolled in clinical trials testing >1 gram of the supplement per day. The weighted average follow-up was 4.9 years.
Overall, use of omega-3 fatty acids was associated with a 25% increased risk for AFib (hazard ratio, 1.25; 95% confidence interval, 1.07-1.46; P = .013).
In analyses stratified by dose, the risk for AFib was “significantly more pronounced” in trials testing high doses of marine omega-3 fatty acid supplements (>1 gram per day: HR, 1.49; 95% CI, 1.04-2.15; P = .042) compared with those testing lower doses (≤1 gram per day: HR, 1.12; 95% CI, 1.03-1.22; P = .024; P for interaction < .001).
In meta-regression, the HR for AFib increased per 1 gram increase in daily omega-3 fatty acid dose (HR. 1.11; 95% CI, 1.06-1.15; P = .001).
Risk-benefit balance
“This meta-analysis adds new evidence regarding the risk of AFib in patients taking marine omega-3 fatty acid supplements,” wrote Dr. Albert and colleagues.
“Since the benefit of omega-3 fatty acids also appears to be dose dependent, the associated risk of AFib should be balanced against the benefit on atherosclerotic cardiovascular outcomes,” they suggested.
They cautioned that the meta-analysis pooled aggregate-level trial data, not individual patient data. Therefore, subgroup analyses by age or other patient level characteristics were not possible.
The risk of developing AFib increases with advancing age and is more common in men than in women. Additional risk factors include elevated blood pressure, coronary artery disease, heart failure, heart valve defects, obesity, and diabetes.
The authors said the potential risk of developing AFib with high doses of omega-3 fatty acid supplements should be discussed with patients and they should know the signs and symptoms of the condition.
The study had no specific funding. Dr. Albert has received grants from St. Jude Medical, Abbott, and Roche Diagnostics.
A version of this article first appeared on Medscape.com.