User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
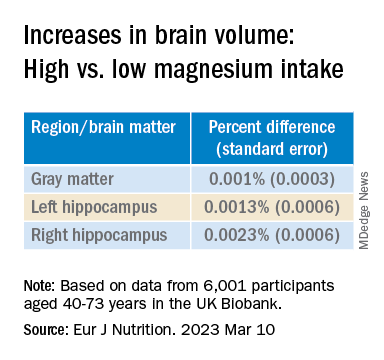
Antiamyloids linked to accelerated brain atrophy
a comprehensive meta-analysis of MRI data from clinical trials suggests.
Depending on the anti–amyloid-beta drug class, these agents can accelerate loss of whole brain and hippocampal volume and increase ventricular volume. This has been shown for some of the beta-secretase inhibitors and with several of the antiamyloid monoclonal antibodies, researchers noted.
“These data warrant concern, but we can’t make any firm conclusions yet. It is possible that the finding is not detrimental, but the usual interpretation of this finding is that volume changes are a surrogate for disease progression,” study investigator Scott Ayton, PhD, of the Florey Institute of Neuroscience and Mental Health, University of Melbourne, said in an interview.
“These data should be factored into the decisions by clinicians when they consider prescribing antiamyloid therapies. Like any side effect, clinicians should inform patients regarding the risk of brain atrophy. Patients should be actively monitored for this side effect,” Dr. Ayton said.
The study was published online in Neurology.
Earlier progression from MCI to AD?
Dr. Ayton and colleagues evaluated brain volume changes in 31 clinical trials of anti–amyloid-beta drugs that demonstrated a favorable change in at least one biomarker of pathological amyloid-beta and included detailed MRI data sufficient to assess the volumetric changes in at least one brain region.
A meta-analysis on the highest dose in each trial on the hippocampus, ventricles, and whole brain showed drug-induced acceleration of volume changes that varied by anti–amyloid-beta drug class.
Secretase inhibitors accelerated atrophy in the hippocampus (mean difference –37.1 mcL; –19.6% relative to change in placebo) and whole brain (mean difference –3.3 mL; –21.8% relative to change in placebo), but not ventricles.
Conversely, monoclonal antibodies caused accelerated ventricular enlargement (mean difference +1.3 mL; +23.8% relative to change in placebo), which was driven by the subset of monoclonal antibodies that induce amyloid-related imaging abnormalities (ARIA) (+2.1 mL; +38.7% relative to change in placebo). There was a “striking correlation between ventricular volume and ARIA frequency,” the investigators reported.
The effect of ARIA-inducing monoclonal antibodies on whole brain volume varied, with accelerated whole brain volume loss caused by donanemab (mean difference –4.6 mL; +23% relative to change in placebo) and lecanemab (–5.2 mL; +36.4% relative to change in placebo). This was not observed with aducanumab and bapineuzumab.
Monoclonal antibodies did not cause accelerated volume loss to the hippocampus regardless of whether they caused ARIA.
The researchers also modeled the effect of anti–amyloid-beta drugs on brain volume changes. In this analysis, participants with mild cognitive impairment (MCI) treated with anti–amyloid-beta drugs were projected to have a “material regression” toward brain volumes typical of AD roughly 8 months earlier than untreated peers.
The data, they note, “permit robust conclusions regarding the effect of [anti–amyloid-beta] drug classes on different brain structures, but the lack of individual patient data (which has yet to be released) limits the interpretations of our findings.”
“Questions like which brain regions are impacted by [anti–amyloid-beta] drugs and whether the volume changes are related to ARIA, plaque loss, cognitive/noncognitive outcomes, or clinical factors such as age, sex, and apoE4 genotype can and should be addressed with available data,” said Dr. Ayton.
Dr. Ayton and colleagues called on data safety monitoring boards (DSMBs) for current clinical trials of anti–amyloid-beta drugs to review volumetric data to determine if patient safety is at risk, particularly in patients who develop ARIA.
In addition, they noted ethics boards that approve trials for anti–amyloid-beta drugs “should request that volume changes be actively monitored. Long-term follow-up of brain volumes should be factored into the trial designs to determine if brain atrophy is progressive, particularly in patients who develop ARIA.”
Finally, they added that drug companies that have conducted trials of anti–amyloid-beta drugs should interrogate prior data on brain volume, report the findings, and release the data for researchers to investigate.
“I have been banging on about this for years,” said Dr. Ayton. “Unfortunately, my raising of this issue has not led to any response. The data are not available, and the basic questions haven’t been asked (publicly).”
Commendable research
In an accompanying editorial, Frederik Barkhof, MD, PhD, with Amsterdam University Medical Centers, and David Knopman, MD, with Mayo Clinic Alzheimer’s Disease Research Center, Rochester, Minn., wrote that the investigators should be “commended” for their analysis.
“The reality in 2023 is that the relevance of brain volume reductions in this therapeutic context remains uncertain,” they wrote.
“Longer periods of observation will be needed to know whether the brain volume losses continue at an accelerated rate or if they attenuate or disappear. Ultimately, it’s the clinical outcomes that matter, regardless of the MRI changes,” Barkhof and Knopman concluded.
The research was supported by funds from the Australian National Health & Medical Research Council. Dr. Ayton reported being a consultant for Eisai in the past 3 years. Dr. Barkhof reported serving on the data and safety monitoring board for Prothena and the A45-AHEAD studies; being a steering committee member for Merck, Bayer, and Biogen; and being a consultant for IXICO, Roche, Celltrion, Rewind Therapeutics, and Combinostics. Dr. Knopman reported serving on the DSMB for the Dominantly Inherited Alzheimer Network Treatment Unit study; serving on a DSMB for a tau therapeutic for Biogen; being an investigator for clinical trials sponsored by Biogen, Lilly Pharmaceuticals, and the University of Southern California. He reported consulting with Roche, Samus Therapeutics, Magellan Health, BioVie, and Alzeca Biosciences.
A version of this article first appeared on Medscape.com.
a comprehensive meta-analysis of MRI data from clinical trials suggests.
Depending on the anti–amyloid-beta drug class, these agents can accelerate loss of whole brain and hippocampal volume and increase ventricular volume. This has been shown for some of the beta-secretase inhibitors and with several of the antiamyloid monoclonal antibodies, researchers noted.
“These data warrant concern, but we can’t make any firm conclusions yet. It is possible that the finding is not detrimental, but the usual interpretation of this finding is that volume changes are a surrogate for disease progression,” study investigator Scott Ayton, PhD, of the Florey Institute of Neuroscience and Mental Health, University of Melbourne, said in an interview.
“These data should be factored into the decisions by clinicians when they consider prescribing antiamyloid therapies. Like any side effect, clinicians should inform patients regarding the risk of brain atrophy. Patients should be actively monitored for this side effect,” Dr. Ayton said.
The study was published online in Neurology.
Earlier progression from MCI to AD?
Dr. Ayton and colleagues evaluated brain volume changes in 31 clinical trials of anti–amyloid-beta drugs that demonstrated a favorable change in at least one biomarker of pathological amyloid-beta and included detailed MRI data sufficient to assess the volumetric changes in at least one brain region.
A meta-analysis on the highest dose in each trial on the hippocampus, ventricles, and whole brain showed drug-induced acceleration of volume changes that varied by anti–amyloid-beta drug class.
Secretase inhibitors accelerated atrophy in the hippocampus (mean difference –37.1 mcL; –19.6% relative to change in placebo) and whole brain (mean difference –3.3 mL; –21.8% relative to change in placebo), but not ventricles.
Conversely, monoclonal antibodies caused accelerated ventricular enlargement (mean difference +1.3 mL; +23.8% relative to change in placebo), which was driven by the subset of monoclonal antibodies that induce amyloid-related imaging abnormalities (ARIA) (+2.1 mL; +38.7% relative to change in placebo). There was a “striking correlation between ventricular volume and ARIA frequency,” the investigators reported.
The effect of ARIA-inducing monoclonal antibodies on whole brain volume varied, with accelerated whole brain volume loss caused by donanemab (mean difference –4.6 mL; +23% relative to change in placebo) and lecanemab (–5.2 mL; +36.4% relative to change in placebo). This was not observed with aducanumab and bapineuzumab.
Monoclonal antibodies did not cause accelerated volume loss to the hippocampus regardless of whether they caused ARIA.
The researchers also modeled the effect of anti–amyloid-beta drugs on brain volume changes. In this analysis, participants with mild cognitive impairment (MCI) treated with anti–amyloid-beta drugs were projected to have a “material regression” toward brain volumes typical of AD roughly 8 months earlier than untreated peers.
The data, they note, “permit robust conclusions regarding the effect of [anti–amyloid-beta] drug classes on different brain structures, but the lack of individual patient data (which has yet to be released) limits the interpretations of our findings.”
“Questions like which brain regions are impacted by [anti–amyloid-beta] drugs and whether the volume changes are related to ARIA, plaque loss, cognitive/noncognitive outcomes, or clinical factors such as age, sex, and apoE4 genotype can and should be addressed with available data,” said Dr. Ayton.
Dr. Ayton and colleagues called on data safety monitoring boards (DSMBs) for current clinical trials of anti–amyloid-beta drugs to review volumetric data to determine if patient safety is at risk, particularly in patients who develop ARIA.
In addition, they noted ethics boards that approve trials for anti–amyloid-beta drugs “should request that volume changes be actively monitored. Long-term follow-up of brain volumes should be factored into the trial designs to determine if brain atrophy is progressive, particularly in patients who develop ARIA.”
Finally, they added that drug companies that have conducted trials of anti–amyloid-beta drugs should interrogate prior data on brain volume, report the findings, and release the data for researchers to investigate.
“I have been banging on about this for years,” said Dr. Ayton. “Unfortunately, my raising of this issue has not led to any response. The data are not available, and the basic questions haven’t been asked (publicly).”
Commendable research
In an accompanying editorial, Frederik Barkhof, MD, PhD, with Amsterdam University Medical Centers, and David Knopman, MD, with Mayo Clinic Alzheimer’s Disease Research Center, Rochester, Minn., wrote that the investigators should be “commended” for their analysis.
“The reality in 2023 is that the relevance of brain volume reductions in this therapeutic context remains uncertain,” they wrote.
“Longer periods of observation will be needed to know whether the brain volume losses continue at an accelerated rate or if they attenuate or disappear. Ultimately, it’s the clinical outcomes that matter, regardless of the MRI changes,” Barkhof and Knopman concluded.
The research was supported by funds from the Australian National Health & Medical Research Council. Dr. Ayton reported being a consultant for Eisai in the past 3 years. Dr. Barkhof reported serving on the data and safety monitoring board for Prothena and the A45-AHEAD studies; being a steering committee member for Merck, Bayer, and Biogen; and being a consultant for IXICO, Roche, Celltrion, Rewind Therapeutics, and Combinostics. Dr. Knopman reported serving on the DSMB for the Dominantly Inherited Alzheimer Network Treatment Unit study; serving on a DSMB for a tau therapeutic for Biogen; being an investigator for clinical trials sponsored by Biogen, Lilly Pharmaceuticals, and the University of Southern California. He reported consulting with Roche, Samus Therapeutics, Magellan Health, BioVie, and Alzeca Biosciences.
A version of this article first appeared on Medscape.com.
a comprehensive meta-analysis of MRI data from clinical trials suggests.
Depending on the anti–amyloid-beta drug class, these agents can accelerate loss of whole brain and hippocampal volume and increase ventricular volume. This has been shown for some of the beta-secretase inhibitors and with several of the antiamyloid monoclonal antibodies, researchers noted.
“These data warrant concern, but we can’t make any firm conclusions yet. It is possible that the finding is not detrimental, but the usual interpretation of this finding is that volume changes are a surrogate for disease progression,” study investigator Scott Ayton, PhD, of the Florey Institute of Neuroscience and Mental Health, University of Melbourne, said in an interview.
“These data should be factored into the decisions by clinicians when they consider prescribing antiamyloid therapies. Like any side effect, clinicians should inform patients regarding the risk of brain atrophy. Patients should be actively monitored for this side effect,” Dr. Ayton said.
The study was published online in Neurology.
Earlier progression from MCI to AD?
Dr. Ayton and colleagues evaluated brain volume changes in 31 clinical trials of anti–amyloid-beta drugs that demonstrated a favorable change in at least one biomarker of pathological amyloid-beta and included detailed MRI data sufficient to assess the volumetric changes in at least one brain region.
A meta-analysis on the highest dose in each trial on the hippocampus, ventricles, and whole brain showed drug-induced acceleration of volume changes that varied by anti–amyloid-beta drug class.
Secretase inhibitors accelerated atrophy in the hippocampus (mean difference –37.1 mcL; –19.6% relative to change in placebo) and whole brain (mean difference –3.3 mL; –21.8% relative to change in placebo), but not ventricles.
Conversely, monoclonal antibodies caused accelerated ventricular enlargement (mean difference +1.3 mL; +23.8% relative to change in placebo), which was driven by the subset of monoclonal antibodies that induce amyloid-related imaging abnormalities (ARIA) (+2.1 mL; +38.7% relative to change in placebo). There was a “striking correlation between ventricular volume and ARIA frequency,” the investigators reported.
The effect of ARIA-inducing monoclonal antibodies on whole brain volume varied, with accelerated whole brain volume loss caused by donanemab (mean difference –4.6 mL; +23% relative to change in placebo) and lecanemab (–5.2 mL; +36.4% relative to change in placebo). This was not observed with aducanumab and bapineuzumab.
Monoclonal antibodies did not cause accelerated volume loss to the hippocampus regardless of whether they caused ARIA.
The researchers also modeled the effect of anti–amyloid-beta drugs on brain volume changes. In this analysis, participants with mild cognitive impairment (MCI) treated with anti–amyloid-beta drugs were projected to have a “material regression” toward brain volumes typical of AD roughly 8 months earlier than untreated peers.
The data, they note, “permit robust conclusions regarding the effect of [anti–amyloid-beta] drug classes on different brain structures, but the lack of individual patient data (which has yet to be released) limits the interpretations of our findings.”
“Questions like which brain regions are impacted by [anti–amyloid-beta] drugs and whether the volume changes are related to ARIA, plaque loss, cognitive/noncognitive outcomes, or clinical factors such as age, sex, and apoE4 genotype can and should be addressed with available data,” said Dr. Ayton.
Dr. Ayton and colleagues called on data safety monitoring boards (DSMBs) for current clinical trials of anti–amyloid-beta drugs to review volumetric data to determine if patient safety is at risk, particularly in patients who develop ARIA.
In addition, they noted ethics boards that approve trials for anti–amyloid-beta drugs “should request that volume changes be actively monitored. Long-term follow-up of brain volumes should be factored into the trial designs to determine if brain atrophy is progressive, particularly in patients who develop ARIA.”
Finally, they added that drug companies that have conducted trials of anti–amyloid-beta drugs should interrogate prior data on brain volume, report the findings, and release the data for researchers to investigate.
“I have been banging on about this for years,” said Dr. Ayton. “Unfortunately, my raising of this issue has not led to any response. The data are not available, and the basic questions haven’t been asked (publicly).”
Commendable research
In an accompanying editorial, Frederik Barkhof, MD, PhD, with Amsterdam University Medical Centers, and David Knopman, MD, with Mayo Clinic Alzheimer’s Disease Research Center, Rochester, Minn., wrote that the investigators should be “commended” for their analysis.
“The reality in 2023 is that the relevance of brain volume reductions in this therapeutic context remains uncertain,” they wrote.
“Longer periods of observation will be needed to know whether the brain volume losses continue at an accelerated rate or if they attenuate or disappear. Ultimately, it’s the clinical outcomes that matter, regardless of the MRI changes,” Barkhof and Knopman concluded.
The research was supported by funds from the Australian National Health & Medical Research Council. Dr. Ayton reported being a consultant for Eisai in the past 3 years. Dr. Barkhof reported serving on the data and safety monitoring board for Prothena and the A45-AHEAD studies; being a steering committee member for Merck, Bayer, and Biogen; and being a consultant for IXICO, Roche, Celltrion, Rewind Therapeutics, and Combinostics. Dr. Knopman reported serving on the DSMB for the Dominantly Inherited Alzheimer Network Treatment Unit study; serving on a DSMB for a tau therapeutic for Biogen; being an investigator for clinical trials sponsored by Biogen, Lilly Pharmaceuticals, and the University of Southern California. He reported consulting with Roche, Samus Therapeutics, Magellan Health, BioVie, and Alzeca Biosciences.
A version of this article first appeared on Medscape.com.
FROM NEUROLOGY
Malpractice risks for docs who oversee NPs or PAs
Even in states that have abolished requirements that NPs be physician-supervised, physicians may still be liable by virtue of employing the NP, according to William P. Sullivan, DO, an attorney and emergency physician in Frankfort, Ill.
Indeed, the vast majority of lawsuits against NPs and PAs name the supervising physician. According to a study of claims against NPs from 2011 to 2016, 82% of the cases also named the supervising physician.
Employed or contracted physicians assigned to supervise NPs or PAs are also affected, Dr. Sullivan said. “The employed physicians’ contract with a hospital or staffing company may require them to assist in the selection, supervision, and/or training of NPs or PAs,” he said. He added that supervisory duties may also be assigned through hospital bylaws.
“The physician is usually not paid anything extra for this work and may not be given extra time to perform it,” Dr. Sullivan said. But still, he said, that physician could be named in a lawsuit and wind up bearing some responsibility for an NP’s or PA’s mistake.
In addition to facing medical malpractice suits, Dr. Sullivan said, doctors are often sanctioned by state licensure boards for improperly supervising NPs and PAs. Licensure boards often require extensive protocols for supervision of NPs and PAs.
Yet more states are removing supervision requirements
With the addition of Kansas and New York in 2022 and California in 2023, 27 states no longer require supervision for all or most NPs. Sixteen of those states, including New York and California, have instituted progressive practice authority that requires temporary supervision of new NPs but then removes supervision after a period of 6 months to 4 years, depending on the state, for the rest of their career.
“When it comes to NP independence, the horse is already out of the barn,” Dr. Sullivan said. “It’s unlikely that states will repeal laws granting NPs independence, and in fact, more states are likely to pass them.”
*PAs, in contrast, are well behind NPs in achieving independence, but the American Academy of Physician Associates (AAPA) is calling to eliminate a mandated relationship with a specific physician. So far, Utah, North Dakota and Wyoming have ended physician supervision of PAs, while California and Hawaii have eliminated mandated chart review. Other states are considering eliminating physician supervision of PAs, according to the AAPA.
In states that have abolished oversight requirements for NPs, “liability can then shift to the NP when the NP is fully independent,” Cathy Klein, an advanced practice registered nurse who helped found the NP profession 50 years ago, told this news organization. “More NPs are starting their own practices, and in many cases, patients actually prefer to see an NP.”
As more NPs became more autonomous, the average payment that NPs incurred in professional liability lawsuits rose by 10.5% from 2017 to 2022, to $332,187, according to the Nurses Service Organization (NSO), a nursing malpractice insurer.
The number of malpractice judgments against autonomous NPs alone has also been rising. From 2012 to 2017, autonomous NPs’ share of all NP cases rose from 7% to 16.4%, the NSO reported.
The good news for physicians is that states’ removal of restrictions on NPs has reduced physicians’ liability to some extent. A 2017 study found that enacting less restrictive scope-of-practice laws for NPs decreased the number of payments made by physicians in NP cases by as much as 31%.
However, the top location for NP payouts remains the physician’s office, not the autonomous NP’s practice, according to the latter NSO report. Plaintiffs sue NPs’ and PAs’ supervising physicians on the basis of legal concepts, such as vicarious liability and respondeat superior. Even if the physician-employer never saw the patient, he or she can be held liable.
Court cases in which supervising physician was found liable
There are plenty of judgments against supervising or collaborating physicians when the NP or PA made the error. Typically, the doctor was faulted for paying little attention to the NP or PA he or she was supposed to supervise.
Dr. Sullivan points to a 2016 case in which a New York jury held a physician 40% liable for a $7 million judgment in a malpractice case involving a PA’s care of a patient in the emergency department. The case is Shajan v. South Nassau Community Hospital in New York.
“The patient presented with nontraumatic leg pain to his lower leg, was diagnosed by the PA with a muscle strain, and discharged without a physician evaluation,” Dr. Sullivan said. The next day, the patient visited an orthopedist who immediately diagnosed compartment syndrome, an emergent condition in which pressure builds up in an affected extremity, damaging the muscles and nerves. “The patient developed irreversible nerve damage and chronic regional pain syndrome,” he said.
A malpractice lawsuit named the PA and the emergency physician he was supposed to be reporting to. Even though the physician had never seen the patient, he had signed off on the PA’s note from a patient’s ED visit. “Testimony during the trial focused on hospital protocols that the supervising physician was supposed to take,” Dr. Sullivan said.
When doctors share fault, they frequently failed to follow the collaborative agreement with the NP or PA. In Collip v. Ratts, a 2015 Indiana case in which the patient died from a drug interaction, the doctor’s certified public accountant stated that the doctor was required to review at least 5% of the NP’s charts every week to evaluate her prescriptive practices.
The doctor admitted that he never reviewed the NP’s charts on a weekly basis. He did conduct some cursory reviews of some of the NP’s notes, and in them he noted concerns for her prescribing practices and suggested she attend a narcotics-prescribing seminar, but he did not follow up to make sure she had done this.
Sometimes the NP or PA who made the mistake may actually be dropped from the lawsuit, leaving the supervising physician fully liable. In these cases, courts reason that a fully engaged supervisor could have prevented the error. In the 2006 case of Husak v. Siegal, the Florida Supreme Court dropped the NP from the case, ruling that the NP had provided the supervising doctor all the information he needed in order to tell her what to do for the patient.
The court noted the physician had failed to look at the chart, even though he was required to do so under his supervisory agreement with the NP. The doctor “could have made the correct diagnosis or referral had he been attentive,” the court said. Therefore, there was “no evidence of independent negligence” by the NP, even though she was the one who had made the incorrect diagnosis that harmed the patient.
When states require an autonomous NP to have a supervisory relationship with a doctor, the supervisor may be unavailable and may fail to designate a substitute. In Texas in January 2019, a 7-year-old girl died of pneumonia after being treated by an NP in an urgent care clinic. The NP had told the parents that the child could safely go home and only needed ibuprofen. The parents brought the girl back home, and she died 15 hours later. The Wattenbargers sued the NP, and the doctor’s supervision was a topic in the trial.
The supervising physician for the NP was out of the country at the time. He said that he had found a substitute, but the substitute doctor testified she had no idea she was designated to be the substitute, according to Niran Al-Agba, MD, a family physician in Silverdale, Wash., who has written on the Texas case. Dr. Al-Agba told this news organization the case appears to have been settled confidentially.
Different standards for expert witnesses
In many states, courts do not allow physicians to testify as expert witnesses in malpractice cases against NPs, arguing that nurses have a different set of standards than doctors have, Dr. Sullivan reported.
These states include Arkansas, Illinois, North Carolina, and New York, according to a report by SEAK Inc., an expert witness training program. The report said most other states allow physician experts in these cases, but they may still require that they have experience with the nursing standard of care.
Dr. Sullivan said some courts are whittling away at the ban on physician experts, and the ban may eventually disappear. He reported that in Oklahoma, which normally upholds the ban, a judge recently allowed a physician-expert to testify in a case involving the death of a 19-year-old woman, Alexus Ochoa, in an ED staffed by an NP. The judge reasoned that Ms. Ochoa’s parents assumed the ED was staffed by physicians and would adhere to medical standards.
Supervision pointers from a physician
Physicians who supervise NPs or PAs say it is important to keep track of their skills and help them sharpen their expertise. Their scope of practice and physicians’ supervisory responsibilities are included in the collaborative agreement.
Arthur Apolinario, MD, a family physician in Clinton, N.C., says his 10-physician practice, which employs six NPs and one PA, works under a collaborative agreement. “The agreement defines each person’s scope of practice. They can’t do certain procedures, such as surgery, and they need extra training before doing certain tasks alone, such as joint injection.
“You have to always figure that if there is a lawsuit against one of them, you as the supervising physician would be named,” said Dr. Apolinario, who is also president of the North Carolina Medical Society. “We try to avert mistakes by meeting regularly with our NPs and PAs and making sure they keep up to date.”
Collaborating with autonomous NPs
Even when NPs operate independently in states that have abolished supervision, physicians may still have some liability if they give NPs advice, Dr. Al-Agba said.
At her Washington state practice, Dr. Al-Agba shares an office with an autonomous NP. “We share overhead and a front desk, but we have separate patients,” Dr. Al-Agba said. “This arrangement works very well for both of us.”
The NP sometimes asks her for advice. When this occurs, Dr. Al-Agba said she always makes sure to see the patient first. “If you don’t actually see the patient, there could be a misunderstanding that could lead to an error,” she said.
Conclusion
Even though NPs now have autonomy in most states, supervising physicians may still be liable for NP malpractice by virtue of being their employers, and physicians in the remaining states are liable for NPs through state law and for PAs in virtually all the states. To determine the supervising physician’s fault, courts often study whether the physician has met the terms of the collaborative agreement.
Physicians can reduce collaborating NPs’ and PAs’ liability by properly training them, by verifying their scope of practice, by making themselves easily available for consultation, and by occasionally seeing their patients. If their NPs and PAs do commit malpractice, supervising physicians may be able to protect themselves from liability by adhering to all requirements of the collaborative agreement.
*Correction, 4/19/2023: An earlier version of this story misstated the name of the AAPA and the states that have ended physician supervision of PAs.
A version of this article first appeared on Medscape.com.
Even in states that have abolished requirements that NPs be physician-supervised, physicians may still be liable by virtue of employing the NP, according to William P. Sullivan, DO, an attorney and emergency physician in Frankfort, Ill.
Indeed, the vast majority of lawsuits against NPs and PAs name the supervising physician. According to a study of claims against NPs from 2011 to 2016, 82% of the cases also named the supervising physician.
Employed or contracted physicians assigned to supervise NPs or PAs are also affected, Dr. Sullivan said. “The employed physicians’ contract with a hospital or staffing company may require them to assist in the selection, supervision, and/or training of NPs or PAs,” he said. He added that supervisory duties may also be assigned through hospital bylaws.
“The physician is usually not paid anything extra for this work and may not be given extra time to perform it,” Dr. Sullivan said. But still, he said, that physician could be named in a lawsuit and wind up bearing some responsibility for an NP’s or PA’s mistake.
In addition to facing medical malpractice suits, Dr. Sullivan said, doctors are often sanctioned by state licensure boards for improperly supervising NPs and PAs. Licensure boards often require extensive protocols for supervision of NPs and PAs.
Yet more states are removing supervision requirements
With the addition of Kansas and New York in 2022 and California in 2023, 27 states no longer require supervision for all or most NPs. Sixteen of those states, including New York and California, have instituted progressive practice authority that requires temporary supervision of new NPs but then removes supervision after a period of 6 months to 4 years, depending on the state, for the rest of their career.
“When it comes to NP independence, the horse is already out of the barn,” Dr. Sullivan said. “It’s unlikely that states will repeal laws granting NPs independence, and in fact, more states are likely to pass them.”
*PAs, in contrast, are well behind NPs in achieving independence, but the American Academy of Physician Associates (AAPA) is calling to eliminate a mandated relationship with a specific physician. So far, Utah, North Dakota and Wyoming have ended physician supervision of PAs, while California and Hawaii have eliminated mandated chart review. Other states are considering eliminating physician supervision of PAs, according to the AAPA.
In states that have abolished oversight requirements for NPs, “liability can then shift to the NP when the NP is fully independent,” Cathy Klein, an advanced practice registered nurse who helped found the NP profession 50 years ago, told this news organization. “More NPs are starting their own practices, and in many cases, patients actually prefer to see an NP.”
As more NPs became more autonomous, the average payment that NPs incurred in professional liability lawsuits rose by 10.5% from 2017 to 2022, to $332,187, according to the Nurses Service Organization (NSO), a nursing malpractice insurer.
The number of malpractice judgments against autonomous NPs alone has also been rising. From 2012 to 2017, autonomous NPs’ share of all NP cases rose from 7% to 16.4%, the NSO reported.
The good news for physicians is that states’ removal of restrictions on NPs has reduced physicians’ liability to some extent. A 2017 study found that enacting less restrictive scope-of-practice laws for NPs decreased the number of payments made by physicians in NP cases by as much as 31%.
However, the top location for NP payouts remains the physician’s office, not the autonomous NP’s practice, according to the latter NSO report. Plaintiffs sue NPs’ and PAs’ supervising physicians on the basis of legal concepts, such as vicarious liability and respondeat superior. Even if the physician-employer never saw the patient, he or she can be held liable.
Court cases in which supervising physician was found liable
There are plenty of judgments against supervising or collaborating physicians when the NP or PA made the error. Typically, the doctor was faulted for paying little attention to the NP or PA he or she was supposed to supervise.
Dr. Sullivan points to a 2016 case in which a New York jury held a physician 40% liable for a $7 million judgment in a malpractice case involving a PA’s care of a patient in the emergency department. The case is Shajan v. South Nassau Community Hospital in New York.
“The patient presented with nontraumatic leg pain to his lower leg, was diagnosed by the PA with a muscle strain, and discharged without a physician evaluation,” Dr. Sullivan said. The next day, the patient visited an orthopedist who immediately diagnosed compartment syndrome, an emergent condition in which pressure builds up in an affected extremity, damaging the muscles and nerves. “The patient developed irreversible nerve damage and chronic regional pain syndrome,” he said.
A malpractice lawsuit named the PA and the emergency physician he was supposed to be reporting to. Even though the physician had never seen the patient, he had signed off on the PA’s note from a patient’s ED visit. “Testimony during the trial focused on hospital protocols that the supervising physician was supposed to take,” Dr. Sullivan said.
When doctors share fault, they frequently failed to follow the collaborative agreement with the NP or PA. In Collip v. Ratts, a 2015 Indiana case in which the patient died from a drug interaction, the doctor’s certified public accountant stated that the doctor was required to review at least 5% of the NP’s charts every week to evaluate her prescriptive practices.
The doctor admitted that he never reviewed the NP’s charts on a weekly basis. He did conduct some cursory reviews of some of the NP’s notes, and in them he noted concerns for her prescribing practices and suggested she attend a narcotics-prescribing seminar, but he did not follow up to make sure she had done this.
Sometimes the NP or PA who made the mistake may actually be dropped from the lawsuit, leaving the supervising physician fully liable. In these cases, courts reason that a fully engaged supervisor could have prevented the error. In the 2006 case of Husak v. Siegal, the Florida Supreme Court dropped the NP from the case, ruling that the NP had provided the supervising doctor all the information he needed in order to tell her what to do for the patient.
The court noted the physician had failed to look at the chart, even though he was required to do so under his supervisory agreement with the NP. The doctor “could have made the correct diagnosis or referral had he been attentive,” the court said. Therefore, there was “no evidence of independent negligence” by the NP, even though she was the one who had made the incorrect diagnosis that harmed the patient.
When states require an autonomous NP to have a supervisory relationship with a doctor, the supervisor may be unavailable and may fail to designate a substitute. In Texas in January 2019, a 7-year-old girl died of pneumonia after being treated by an NP in an urgent care clinic. The NP had told the parents that the child could safely go home and only needed ibuprofen. The parents brought the girl back home, and she died 15 hours later. The Wattenbargers sued the NP, and the doctor’s supervision was a topic in the trial.
The supervising physician for the NP was out of the country at the time. He said that he had found a substitute, but the substitute doctor testified she had no idea she was designated to be the substitute, according to Niran Al-Agba, MD, a family physician in Silverdale, Wash., who has written on the Texas case. Dr. Al-Agba told this news organization the case appears to have been settled confidentially.
Different standards for expert witnesses
In many states, courts do not allow physicians to testify as expert witnesses in malpractice cases against NPs, arguing that nurses have a different set of standards than doctors have, Dr. Sullivan reported.
These states include Arkansas, Illinois, North Carolina, and New York, according to a report by SEAK Inc., an expert witness training program. The report said most other states allow physician experts in these cases, but they may still require that they have experience with the nursing standard of care.
Dr. Sullivan said some courts are whittling away at the ban on physician experts, and the ban may eventually disappear. He reported that in Oklahoma, which normally upholds the ban, a judge recently allowed a physician-expert to testify in a case involving the death of a 19-year-old woman, Alexus Ochoa, in an ED staffed by an NP. The judge reasoned that Ms. Ochoa’s parents assumed the ED was staffed by physicians and would adhere to medical standards.
Supervision pointers from a physician
Physicians who supervise NPs or PAs say it is important to keep track of their skills and help them sharpen their expertise. Their scope of practice and physicians’ supervisory responsibilities are included in the collaborative agreement.
Arthur Apolinario, MD, a family physician in Clinton, N.C., says his 10-physician practice, which employs six NPs and one PA, works under a collaborative agreement. “The agreement defines each person’s scope of practice. They can’t do certain procedures, such as surgery, and they need extra training before doing certain tasks alone, such as joint injection.
“You have to always figure that if there is a lawsuit against one of them, you as the supervising physician would be named,” said Dr. Apolinario, who is also president of the North Carolina Medical Society. “We try to avert mistakes by meeting regularly with our NPs and PAs and making sure they keep up to date.”
Collaborating with autonomous NPs
Even when NPs operate independently in states that have abolished supervision, physicians may still have some liability if they give NPs advice, Dr. Al-Agba said.
At her Washington state practice, Dr. Al-Agba shares an office with an autonomous NP. “We share overhead and a front desk, but we have separate patients,” Dr. Al-Agba said. “This arrangement works very well for both of us.”
The NP sometimes asks her for advice. When this occurs, Dr. Al-Agba said she always makes sure to see the patient first. “If you don’t actually see the patient, there could be a misunderstanding that could lead to an error,” she said.
Conclusion
Even though NPs now have autonomy in most states, supervising physicians may still be liable for NP malpractice by virtue of being their employers, and physicians in the remaining states are liable for NPs through state law and for PAs in virtually all the states. To determine the supervising physician’s fault, courts often study whether the physician has met the terms of the collaborative agreement.
Physicians can reduce collaborating NPs’ and PAs’ liability by properly training them, by verifying their scope of practice, by making themselves easily available for consultation, and by occasionally seeing their patients. If their NPs and PAs do commit malpractice, supervising physicians may be able to protect themselves from liability by adhering to all requirements of the collaborative agreement.
*Correction, 4/19/2023: An earlier version of this story misstated the name of the AAPA and the states that have ended physician supervision of PAs.
A version of this article first appeared on Medscape.com.
Even in states that have abolished requirements that NPs be physician-supervised, physicians may still be liable by virtue of employing the NP, according to William P. Sullivan, DO, an attorney and emergency physician in Frankfort, Ill.
Indeed, the vast majority of lawsuits against NPs and PAs name the supervising physician. According to a study of claims against NPs from 2011 to 2016, 82% of the cases also named the supervising physician.
Employed or contracted physicians assigned to supervise NPs or PAs are also affected, Dr. Sullivan said. “The employed physicians’ contract with a hospital or staffing company may require them to assist in the selection, supervision, and/or training of NPs or PAs,” he said. He added that supervisory duties may also be assigned through hospital bylaws.
“The physician is usually not paid anything extra for this work and may not be given extra time to perform it,” Dr. Sullivan said. But still, he said, that physician could be named in a lawsuit and wind up bearing some responsibility for an NP’s or PA’s mistake.
In addition to facing medical malpractice suits, Dr. Sullivan said, doctors are often sanctioned by state licensure boards for improperly supervising NPs and PAs. Licensure boards often require extensive protocols for supervision of NPs and PAs.
Yet more states are removing supervision requirements
With the addition of Kansas and New York in 2022 and California in 2023, 27 states no longer require supervision for all or most NPs. Sixteen of those states, including New York and California, have instituted progressive practice authority that requires temporary supervision of new NPs but then removes supervision after a period of 6 months to 4 years, depending on the state, for the rest of their career.
“When it comes to NP independence, the horse is already out of the barn,” Dr. Sullivan said. “It’s unlikely that states will repeal laws granting NPs independence, and in fact, more states are likely to pass them.”
*PAs, in contrast, are well behind NPs in achieving independence, but the American Academy of Physician Associates (AAPA) is calling to eliminate a mandated relationship with a specific physician. So far, Utah, North Dakota and Wyoming have ended physician supervision of PAs, while California and Hawaii have eliminated mandated chart review. Other states are considering eliminating physician supervision of PAs, according to the AAPA.
In states that have abolished oversight requirements for NPs, “liability can then shift to the NP when the NP is fully independent,” Cathy Klein, an advanced practice registered nurse who helped found the NP profession 50 years ago, told this news organization. “More NPs are starting their own practices, and in many cases, patients actually prefer to see an NP.”
As more NPs became more autonomous, the average payment that NPs incurred in professional liability lawsuits rose by 10.5% from 2017 to 2022, to $332,187, according to the Nurses Service Organization (NSO), a nursing malpractice insurer.
The number of malpractice judgments against autonomous NPs alone has also been rising. From 2012 to 2017, autonomous NPs’ share of all NP cases rose from 7% to 16.4%, the NSO reported.
The good news for physicians is that states’ removal of restrictions on NPs has reduced physicians’ liability to some extent. A 2017 study found that enacting less restrictive scope-of-practice laws for NPs decreased the number of payments made by physicians in NP cases by as much as 31%.
However, the top location for NP payouts remains the physician’s office, not the autonomous NP’s practice, according to the latter NSO report. Plaintiffs sue NPs’ and PAs’ supervising physicians on the basis of legal concepts, such as vicarious liability and respondeat superior. Even if the physician-employer never saw the patient, he or she can be held liable.
Court cases in which supervising physician was found liable
There are plenty of judgments against supervising or collaborating physicians when the NP or PA made the error. Typically, the doctor was faulted for paying little attention to the NP or PA he or she was supposed to supervise.
Dr. Sullivan points to a 2016 case in which a New York jury held a physician 40% liable for a $7 million judgment in a malpractice case involving a PA’s care of a patient in the emergency department. The case is Shajan v. South Nassau Community Hospital in New York.
“The patient presented with nontraumatic leg pain to his lower leg, was diagnosed by the PA with a muscle strain, and discharged without a physician evaluation,” Dr. Sullivan said. The next day, the patient visited an orthopedist who immediately diagnosed compartment syndrome, an emergent condition in which pressure builds up in an affected extremity, damaging the muscles and nerves. “The patient developed irreversible nerve damage and chronic regional pain syndrome,” he said.
A malpractice lawsuit named the PA and the emergency physician he was supposed to be reporting to. Even though the physician had never seen the patient, he had signed off on the PA’s note from a patient’s ED visit. “Testimony during the trial focused on hospital protocols that the supervising physician was supposed to take,” Dr. Sullivan said.
When doctors share fault, they frequently failed to follow the collaborative agreement with the NP or PA. In Collip v. Ratts, a 2015 Indiana case in which the patient died from a drug interaction, the doctor’s certified public accountant stated that the doctor was required to review at least 5% of the NP’s charts every week to evaluate her prescriptive practices.
The doctor admitted that he never reviewed the NP’s charts on a weekly basis. He did conduct some cursory reviews of some of the NP’s notes, and in them he noted concerns for her prescribing practices and suggested she attend a narcotics-prescribing seminar, but he did not follow up to make sure she had done this.
Sometimes the NP or PA who made the mistake may actually be dropped from the lawsuit, leaving the supervising physician fully liable. In these cases, courts reason that a fully engaged supervisor could have prevented the error. In the 2006 case of Husak v. Siegal, the Florida Supreme Court dropped the NP from the case, ruling that the NP had provided the supervising doctor all the information he needed in order to tell her what to do for the patient.
The court noted the physician had failed to look at the chart, even though he was required to do so under his supervisory agreement with the NP. The doctor “could have made the correct diagnosis or referral had he been attentive,” the court said. Therefore, there was “no evidence of independent negligence” by the NP, even though she was the one who had made the incorrect diagnosis that harmed the patient.
When states require an autonomous NP to have a supervisory relationship with a doctor, the supervisor may be unavailable and may fail to designate a substitute. In Texas in January 2019, a 7-year-old girl died of pneumonia after being treated by an NP in an urgent care clinic. The NP had told the parents that the child could safely go home and only needed ibuprofen. The parents brought the girl back home, and she died 15 hours later. The Wattenbargers sued the NP, and the doctor’s supervision was a topic in the trial.
The supervising physician for the NP was out of the country at the time. He said that he had found a substitute, but the substitute doctor testified she had no idea she was designated to be the substitute, according to Niran Al-Agba, MD, a family physician in Silverdale, Wash., who has written on the Texas case. Dr. Al-Agba told this news organization the case appears to have been settled confidentially.
Different standards for expert witnesses
In many states, courts do not allow physicians to testify as expert witnesses in malpractice cases against NPs, arguing that nurses have a different set of standards than doctors have, Dr. Sullivan reported.
These states include Arkansas, Illinois, North Carolina, and New York, according to a report by SEAK Inc., an expert witness training program. The report said most other states allow physician experts in these cases, but they may still require that they have experience with the nursing standard of care.
Dr. Sullivan said some courts are whittling away at the ban on physician experts, and the ban may eventually disappear. He reported that in Oklahoma, which normally upholds the ban, a judge recently allowed a physician-expert to testify in a case involving the death of a 19-year-old woman, Alexus Ochoa, in an ED staffed by an NP. The judge reasoned that Ms. Ochoa’s parents assumed the ED was staffed by physicians and would adhere to medical standards.
Supervision pointers from a physician
Physicians who supervise NPs or PAs say it is important to keep track of their skills and help them sharpen their expertise. Their scope of practice and physicians’ supervisory responsibilities are included in the collaborative agreement.
Arthur Apolinario, MD, a family physician in Clinton, N.C., says his 10-physician practice, which employs six NPs and one PA, works under a collaborative agreement. “The agreement defines each person’s scope of practice. They can’t do certain procedures, such as surgery, and they need extra training before doing certain tasks alone, such as joint injection.
“You have to always figure that if there is a lawsuit against one of them, you as the supervising physician would be named,” said Dr. Apolinario, who is also president of the North Carolina Medical Society. “We try to avert mistakes by meeting regularly with our NPs and PAs and making sure they keep up to date.”
Collaborating with autonomous NPs
Even when NPs operate independently in states that have abolished supervision, physicians may still have some liability if they give NPs advice, Dr. Al-Agba said.
At her Washington state practice, Dr. Al-Agba shares an office with an autonomous NP. “We share overhead and a front desk, but we have separate patients,” Dr. Al-Agba said. “This arrangement works very well for both of us.”
The NP sometimes asks her for advice. When this occurs, Dr. Al-Agba said she always makes sure to see the patient first. “If you don’t actually see the patient, there could be a misunderstanding that could lead to an error,” she said.
Conclusion
Even though NPs now have autonomy in most states, supervising physicians may still be liable for NP malpractice by virtue of being their employers, and physicians in the remaining states are liable for NPs through state law and for PAs in virtually all the states. To determine the supervising physician’s fault, courts often study whether the physician has met the terms of the collaborative agreement.
Physicians can reduce collaborating NPs’ and PAs’ liability by properly training them, by verifying their scope of practice, by making themselves easily available for consultation, and by occasionally seeing their patients. If their NPs and PAs do commit malpractice, supervising physicians may be able to protect themselves from liability by adhering to all requirements of the collaborative agreement.
*Correction, 4/19/2023: An earlier version of this story misstated the name of the AAPA and the states that have ended physician supervision of PAs.
A version of this article first appeared on Medscape.com.
Noisy incubators could stunt infant hearing
Incubators save the lives of many babies, but new data suggest that the ambient noise associated with the incubator experience could put babies’ hearing and language development skills at risk.
Previous studies have shown that the neonatal intensive care unit is a noisy environment, but specific data on levels of sound inside and outside incubators are limited, wrote Christoph Reuter, MA, a musicology professor at the University of Vienna, and colleagues.
“By the age of 3 years, deficits in language acquisition are detectable in nearly 50% of very preterm infants,” and high levels of NICU noise have been cited as possible contributors to this increased risk, the researchers say.
In a study published in Frontiers in Pediatrics, the researchers aimed to compare real-life NICU noise with previously reported levels to describe the sound characteristics and to identify resonance characteristics inside an incubator.
The study was conducted at the Pediatric Simulation Center at the Medical University of Vienna. The researchers placed a simulation mannequin with an ear microphone inside an incubator. They also placed microphones outside the incubator to collect measures of outside noise and activity involved in NICU care.
Data regarding sound were collected for 11 environmental noises and 12 incubator handlings using weighted and unweighted decibel levels. Specific environmental noises included starting the incubator engine; environmental noise with incubator off; environmental noise with incubator on; normal conversation; light conversation; laughter; telephone sounds; the infusion pump alarm; the monitor alarm (anomaly); the monitor alarm (emergency); and blood pressure measurement.
The 12 incubator handling noises included those associated with water flap, water pouring into the incubator, incubator doors opening properly, incubators doors closing properly, incubator doors closing improperly, hatch closing, hatch opening, incubator drawer, neighbor incubator doors closing (1.82 m distance), taking a stethoscope from the incubator wall, putting a stethoscope on the incubator, and suctioning tube. Noise from six levels of respiratory support was also measured.
The researchers reported that the incubator tended to dampen most sounds but also that some sounds resonated inside the incubator, which raised the interior noise level by as much as 28 decibels.
Most of the measures using both A-weighted decibels (dBA) and sound pressure level decibels (dBSPL) were above the 45-decibel level for neonatal sound exposure recommended by the American Academy of Pediatrics. The measurements (dBA) versus unweighted (dBSPL) are limited in that they are designed to measure low levels of sound and therefore might underestimate proportions of high and low frequencies at stronger levels, the researchers acknowledge.
Overall, most measures were clustered in the 55-75 decibel range, although some sound levels for incubator handling, while below levels previously reported in the literature, reached approximately 100 decibels.
The noise involved inside the incubator was not perceived as loud by those working with the incubator, the researchers note.
As for resonance inside the incubator, the researchers measured a low-frequency main resonance of 97 Hz, but they write that this resonance can be hard to capture in weighted measurements. However, the resonance means that “noises from the outside sound more tonal inside the incubator, booming and muffled as well as less rough or noisy,” and sounds inside the incubator are similarly affected, the researchers say.
“Most of the noise situations described in this manuscript far exceed not only the recommendation of the AAP but also international guidelines provided by the World Health Organization and the U.S. Environmental Protection Agency,” which recommend, respectively, maximum dBA levels of 35 dBA and 45 dBA for daytime and 30 dBA and 35 dBA for night, the researchers indicate.
Potential long-term implications are that babies who spend time in the NICU are at risk for hearing impairment, which could lead to delays in language acquisition, they say.
The findings were limited by several factors, including the variance among the incubators, which prevents generalizability, the researchers note. Other limitations include the use of a simulation room rather than everyday conditions, in which the environmental sounds would likely be even louder.
However, the results provide insights into the specifics of incubator and NICU noise and suggest that sound be a consideration in the development and promotion of incubators to help protect the hearing of the infants inside them, the researchers conclude.
A generalist’s take
“This is an interesting study looking at the level and character of the sound experienced by preterm infants inside an incubator and how it may compare to sounds experienced within the mother’s womb,” said Tim Joos, MD, a Seattle-based clinician with a combination internal medicine/pediatrics practice, in an interview.
In society at large, “there has been more focus lately on the general environment and its effect on health, and this study is a unique take on this concept,” he said. “Although in general the incubators work to dampen external sounds, low-frequency sounds may actually resonate more inside the incubators, and taps on the outside or inside of the incubator itself are amplified within the incubator,” he noted. “It is sad but not surprising that the decibel levels experienced by the infants in the incubators exceed the recommended levels recommended by AAP.”
As for additional research, “it would be interesting to see the results of trials looking at various short- or long-term outcomes experienced by infants exposed to a lower-level noise compared to the current levels,” Dr. Joos told this news organization.
A neonatologist’s perspective
“As the field of neonatology advances, we are caring for an ever-growing number of extremely preterm infants,” said Caitlin M. Drumm, MD, of Walter Reed National Military Medical Center, Bethesda, Md., in an interview.
“These infants will spend the first few months of their lives within an incubator in the neonatal intensive care unit, so it is important to understand the potential long-term implications of environmental effects on these vulnerable patients,” she said.
“As in prior studies, it was not surprising that essentially every environmental, handling, or respiratory intervention led to noise levels higher than the limit recommended by the American Academy of Pediatrics,” Dr. Drumm said. “What was surprising was just how high above the 45-dB recommended noise limit many environmental stimuli are. For example, the authors cite respiratory flow rates of 8 L/min or higher as risky for hearing health at 84.72 dBSPL, “ she said.
The key message for clinicians is to be aware of noise levels in the NICU, Dr. Drumm said. “Environmental stimuli as simple as putting a stethoscope on the incubator lead to noise levels well above the limit recommended by the American Academy of Pediatrics. The entire NICU care team has a role to play in minimizing environmental sound hazards for our most critically ill patients.”
Looking ahead, “future research should focus on providing more information correlating neonatal environmental sound exposure to long-term hearing and neurodevelopmental outcomes,” she said.
The study received no outside funding. The researchers report no relevant financial relationships. Dr. Joos serves on the editorial advisory board of Pediatric News. Dr. Drumm has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Incubators save the lives of many babies, but new data suggest that the ambient noise associated with the incubator experience could put babies’ hearing and language development skills at risk.
Previous studies have shown that the neonatal intensive care unit is a noisy environment, but specific data on levels of sound inside and outside incubators are limited, wrote Christoph Reuter, MA, a musicology professor at the University of Vienna, and colleagues.
“By the age of 3 years, deficits in language acquisition are detectable in nearly 50% of very preterm infants,” and high levels of NICU noise have been cited as possible contributors to this increased risk, the researchers say.
In a study published in Frontiers in Pediatrics, the researchers aimed to compare real-life NICU noise with previously reported levels to describe the sound characteristics and to identify resonance characteristics inside an incubator.
The study was conducted at the Pediatric Simulation Center at the Medical University of Vienna. The researchers placed a simulation mannequin with an ear microphone inside an incubator. They also placed microphones outside the incubator to collect measures of outside noise and activity involved in NICU care.
Data regarding sound were collected for 11 environmental noises and 12 incubator handlings using weighted and unweighted decibel levels. Specific environmental noises included starting the incubator engine; environmental noise with incubator off; environmental noise with incubator on; normal conversation; light conversation; laughter; telephone sounds; the infusion pump alarm; the monitor alarm (anomaly); the monitor alarm (emergency); and blood pressure measurement.
The 12 incubator handling noises included those associated with water flap, water pouring into the incubator, incubator doors opening properly, incubators doors closing properly, incubator doors closing improperly, hatch closing, hatch opening, incubator drawer, neighbor incubator doors closing (1.82 m distance), taking a stethoscope from the incubator wall, putting a stethoscope on the incubator, and suctioning tube. Noise from six levels of respiratory support was also measured.
The researchers reported that the incubator tended to dampen most sounds but also that some sounds resonated inside the incubator, which raised the interior noise level by as much as 28 decibels.
Most of the measures using both A-weighted decibels (dBA) and sound pressure level decibels (dBSPL) were above the 45-decibel level for neonatal sound exposure recommended by the American Academy of Pediatrics. The measurements (dBA) versus unweighted (dBSPL) are limited in that they are designed to measure low levels of sound and therefore might underestimate proportions of high and low frequencies at stronger levels, the researchers acknowledge.
Overall, most measures were clustered in the 55-75 decibel range, although some sound levels for incubator handling, while below levels previously reported in the literature, reached approximately 100 decibels.
The noise involved inside the incubator was not perceived as loud by those working with the incubator, the researchers note.
As for resonance inside the incubator, the researchers measured a low-frequency main resonance of 97 Hz, but they write that this resonance can be hard to capture in weighted measurements. However, the resonance means that “noises from the outside sound more tonal inside the incubator, booming and muffled as well as less rough or noisy,” and sounds inside the incubator are similarly affected, the researchers say.
“Most of the noise situations described in this manuscript far exceed not only the recommendation of the AAP but also international guidelines provided by the World Health Organization and the U.S. Environmental Protection Agency,” which recommend, respectively, maximum dBA levels of 35 dBA and 45 dBA for daytime and 30 dBA and 35 dBA for night, the researchers indicate.
Potential long-term implications are that babies who spend time in the NICU are at risk for hearing impairment, which could lead to delays in language acquisition, they say.
The findings were limited by several factors, including the variance among the incubators, which prevents generalizability, the researchers note. Other limitations include the use of a simulation room rather than everyday conditions, in which the environmental sounds would likely be even louder.
However, the results provide insights into the specifics of incubator and NICU noise and suggest that sound be a consideration in the development and promotion of incubators to help protect the hearing of the infants inside them, the researchers conclude.
A generalist’s take
“This is an interesting study looking at the level and character of the sound experienced by preterm infants inside an incubator and how it may compare to sounds experienced within the mother’s womb,” said Tim Joos, MD, a Seattle-based clinician with a combination internal medicine/pediatrics practice, in an interview.
In society at large, “there has been more focus lately on the general environment and its effect on health, and this study is a unique take on this concept,” he said. “Although in general the incubators work to dampen external sounds, low-frequency sounds may actually resonate more inside the incubators, and taps on the outside or inside of the incubator itself are amplified within the incubator,” he noted. “It is sad but not surprising that the decibel levels experienced by the infants in the incubators exceed the recommended levels recommended by AAP.”
As for additional research, “it would be interesting to see the results of trials looking at various short- or long-term outcomes experienced by infants exposed to a lower-level noise compared to the current levels,” Dr. Joos told this news organization.
A neonatologist’s perspective
“As the field of neonatology advances, we are caring for an ever-growing number of extremely preterm infants,” said Caitlin M. Drumm, MD, of Walter Reed National Military Medical Center, Bethesda, Md., in an interview.
“These infants will spend the first few months of their lives within an incubator in the neonatal intensive care unit, so it is important to understand the potential long-term implications of environmental effects on these vulnerable patients,” she said.
“As in prior studies, it was not surprising that essentially every environmental, handling, or respiratory intervention led to noise levels higher than the limit recommended by the American Academy of Pediatrics,” Dr. Drumm said. “What was surprising was just how high above the 45-dB recommended noise limit many environmental stimuli are. For example, the authors cite respiratory flow rates of 8 L/min or higher as risky for hearing health at 84.72 dBSPL, “ she said.
The key message for clinicians is to be aware of noise levels in the NICU, Dr. Drumm said. “Environmental stimuli as simple as putting a stethoscope on the incubator lead to noise levels well above the limit recommended by the American Academy of Pediatrics. The entire NICU care team has a role to play in minimizing environmental sound hazards for our most critically ill patients.”
Looking ahead, “future research should focus on providing more information correlating neonatal environmental sound exposure to long-term hearing and neurodevelopmental outcomes,” she said.
The study received no outside funding. The researchers report no relevant financial relationships. Dr. Joos serves on the editorial advisory board of Pediatric News. Dr. Drumm has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Incubators save the lives of many babies, but new data suggest that the ambient noise associated with the incubator experience could put babies’ hearing and language development skills at risk.
Previous studies have shown that the neonatal intensive care unit is a noisy environment, but specific data on levels of sound inside and outside incubators are limited, wrote Christoph Reuter, MA, a musicology professor at the University of Vienna, and colleagues.
“By the age of 3 years, deficits in language acquisition are detectable in nearly 50% of very preterm infants,” and high levels of NICU noise have been cited as possible contributors to this increased risk, the researchers say.
In a study published in Frontiers in Pediatrics, the researchers aimed to compare real-life NICU noise with previously reported levels to describe the sound characteristics and to identify resonance characteristics inside an incubator.
The study was conducted at the Pediatric Simulation Center at the Medical University of Vienna. The researchers placed a simulation mannequin with an ear microphone inside an incubator. They also placed microphones outside the incubator to collect measures of outside noise and activity involved in NICU care.
Data regarding sound were collected for 11 environmental noises and 12 incubator handlings using weighted and unweighted decibel levels. Specific environmental noises included starting the incubator engine; environmental noise with incubator off; environmental noise with incubator on; normal conversation; light conversation; laughter; telephone sounds; the infusion pump alarm; the monitor alarm (anomaly); the monitor alarm (emergency); and blood pressure measurement.
The 12 incubator handling noises included those associated with water flap, water pouring into the incubator, incubator doors opening properly, incubators doors closing properly, incubator doors closing improperly, hatch closing, hatch opening, incubator drawer, neighbor incubator doors closing (1.82 m distance), taking a stethoscope from the incubator wall, putting a stethoscope on the incubator, and suctioning tube. Noise from six levels of respiratory support was also measured.
The researchers reported that the incubator tended to dampen most sounds but also that some sounds resonated inside the incubator, which raised the interior noise level by as much as 28 decibels.
Most of the measures using both A-weighted decibels (dBA) and sound pressure level decibels (dBSPL) were above the 45-decibel level for neonatal sound exposure recommended by the American Academy of Pediatrics. The measurements (dBA) versus unweighted (dBSPL) are limited in that they are designed to measure low levels of sound and therefore might underestimate proportions of high and low frequencies at stronger levels, the researchers acknowledge.
Overall, most measures were clustered in the 55-75 decibel range, although some sound levels for incubator handling, while below levels previously reported in the literature, reached approximately 100 decibels.
The noise involved inside the incubator was not perceived as loud by those working with the incubator, the researchers note.
As for resonance inside the incubator, the researchers measured a low-frequency main resonance of 97 Hz, but they write that this resonance can be hard to capture in weighted measurements. However, the resonance means that “noises from the outside sound more tonal inside the incubator, booming and muffled as well as less rough or noisy,” and sounds inside the incubator are similarly affected, the researchers say.
“Most of the noise situations described in this manuscript far exceed not only the recommendation of the AAP but also international guidelines provided by the World Health Organization and the U.S. Environmental Protection Agency,” which recommend, respectively, maximum dBA levels of 35 dBA and 45 dBA for daytime and 30 dBA and 35 dBA for night, the researchers indicate.
Potential long-term implications are that babies who spend time in the NICU are at risk for hearing impairment, which could lead to delays in language acquisition, they say.
The findings were limited by several factors, including the variance among the incubators, which prevents generalizability, the researchers note. Other limitations include the use of a simulation room rather than everyday conditions, in which the environmental sounds would likely be even louder.
However, the results provide insights into the specifics of incubator and NICU noise and suggest that sound be a consideration in the development and promotion of incubators to help protect the hearing of the infants inside them, the researchers conclude.
A generalist’s take
“This is an interesting study looking at the level and character of the sound experienced by preterm infants inside an incubator and how it may compare to sounds experienced within the mother’s womb,” said Tim Joos, MD, a Seattle-based clinician with a combination internal medicine/pediatrics practice, in an interview.
In society at large, “there has been more focus lately on the general environment and its effect on health, and this study is a unique take on this concept,” he said. “Although in general the incubators work to dampen external sounds, low-frequency sounds may actually resonate more inside the incubators, and taps on the outside or inside of the incubator itself are amplified within the incubator,” he noted. “It is sad but not surprising that the decibel levels experienced by the infants in the incubators exceed the recommended levels recommended by AAP.”
As for additional research, “it would be interesting to see the results of trials looking at various short- or long-term outcomes experienced by infants exposed to a lower-level noise compared to the current levels,” Dr. Joos told this news organization.
A neonatologist’s perspective
“As the field of neonatology advances, we are caring for an ever-growing number of extremely preterm infants,” said Caitlin M. Drumm, MD, of Walter Reed National Military Medical Center, Bethesda, Md., in an interview.
“These infants will spend the first few months of their lives within an incubator in the neonatal intensive care unit, so it is important to understand the potential long-term implications of environmental effects on these vulnerable patients,” she said.
“As in prior studies, it was not surprising that essentially every environmental, handling, or respiratory intervention led to noise levels higher than the limit recommended by the American Academy of Pediatrics,” Dr. Drumm said. “What was surprising was just how high above the 45-dB recommended noise limit many environmental stimuli are. For example, the authors cite respiratory flow rates of 8 L/min or higher as risky for hearing health at 84.72 dBSPL, “ she said.
The key message for clinicians is to be aware of noise levels in the NICU, Dr. Drumm said. “Environmental stimuli as simple as putting a stethoscope on the incubator lead to noise levels well above the limit recommended by the American Academy of Pediatrics. The entire NICU care team has a role to play in minimizing environmental sound hazards for our most critically ill patients.”
Looking ahead, “future research should focus on providing more information correlating neonatal environmental sound exposure to long-term hearing and neurodevelopmental outcomes,” she said.
The study received no outside funding. The researchers report no relevant financial relationships. Dr. Joos serves on the editorial advisory board of Pediatric News. Dr. Drumm has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Four PTSD blood biomarkers identified
“More accurate means of predicting or screening for PTSD could help to overcome the disorder by identifying individuals at high risk of developing PTSD and providing them with early intervention or prevention strategies,” said study investigator Stacy-Ann Miller, MS.
She also noted that the biomarkers could be used to monitor treatment for PTSD, identify subtypes of PTSD, and lead to a new understanding of the mechanisms underlying PTSD.
The findings were presented at Discover BMB, the annual meeting of the American Society for Biochemistry and Molecular Biology.
Toward better clinical assessment
The findings originated from research conducted by the Department of Defense–initiated PTSD Systems Biology Consortium. The consortium’s goals include developing a reproducible panel of blood-based biomarkers with high sensitivity and specificity for PTSD diagnosis and is made up of about 45 researchers, led by Marti Jett, PhD, Charles Marmar, MD, and Francis J. Doyle III, PhD.
The researchers analyzed blood samples from 1,000 active-duty Army personnel from the 101st Airborne at Fort Campbell, Ky. Participants were assessed before and after deployment to Afghanistan in February 2014 and are referred to as the Fort Campbell Cohort (FCC). Participants’ age ranged from 25 to 30 and approximately 6% were female.
Investigators collected blood samples from the service members and looked for four biomarkers: glycolytic ratio, arginine, serotonin, and glutamate. The team then divided the participants into four groups – those with PTSD (PTSD Checklist score above 30), those who were subthreshold for PTSD (PTSD Checklist score 15-30), those who had high resilience, and those who had low levels of resilience.
The resilience groups were determined based on answers to the Generalized Anxiety Disorder Questionnaire, Patient Health Questionnaire, Pittsburgh Sleep Quality Index, Intensive Combat Exposure (DRRI-D), the number of deployments, whether they had moderate or severe traumatic brain injury, and scores on the Alcohol Use Disorders Identification Test.
Those who scored in the high range at current or prior time points or who were PTSD/subthreshold at prior time points were placed in the low resilience group.
Ms. Miller noted that those in the PTSD group had more severe symptoms than those in the PTSD subthreshold group based on the longitudinal clinical assessment at 3-6 months, 5 years, and longer post deployment. The low resilience group had a much higher rate of PTSD post deployment than the high resilience group.
Investigators found participants with PTSD or subthreshold PTSD had significantly higher glycolic ratios and lower arginine than those with high resilience. They also found that those with PTSD had significantly lower serotonin and higher glutamate levels versus those with high resilience. These associations were independent of factors such as sex, age, body mass index, smoking, and caffeine consumption.
Ms. Miller said that the study results require further validation by the consortium’s labs and third-party labs.
“We are also interested in determining the most appropriate time to screen soldiers for PTSD, as it has been noted that the time period where we see the most psychological issues is around 2-3 months post return from deployment and when the soldier is preparing for their next assignment, perhaps a next deployment,” she said.
She added that previous studies have identified several promising biomarkers of PTSD. “However, like much of the research data, the study sample was comprised mainly of combat-exposed males. With more women serving on the front lines, the military faces new challenges in how combat affects females in the military,” including sex-specific biomarkers that will improve clinical assessment for female soldiers.
Eventually, the team would also like to be able to apply their research to the civilian population experiencing PTSD.
“Our research is anticipated to be useful in helping the medical provider select appropriate therapeutic interventions,” Ms. Miller said.
A version of this article first appeared on Medscape.com.
“More accurate means of predicting or screening for PTSD could help to overcome the disorder by identifying individuals at high risk of developing PTSD and providing them with early intervention or prevention strategies,” said study investigator Stacy-Ann Miller, MS.
She also noted that the biomarkers could be used to monitor treatment for PTSD, identify subtypes of PTSD, and lead to a new understanding of the mechanisms underlying PTSD.
The findings were presented at Discover BMB, the annual meeting of the American Society for Biochemistry and Molecular Biology.
Toward better clinical assessment
The findings originated from research conducted by the Department of Defense–initiated PTSD Systems Biology Consortium. The consortium’s goals include developing a reproducible panel of blood-based biomarkers with high sensitivity and specificity for PTSD diagnosis and is made up of about 45 researchers, led by Marti Jett, PhD, Charles Marmar, MD, and Francis J. Doyle III, PhD.
The researchers analyzed blood samples from 1,000 active-duty Army personnel from the 101st Airborne at Fort Campbell, Ky. Participants were assessed before and after deployment to Afghanistan in February 2014 and are referred to as the Fort Campbell Cohort (FCC). Participants’ age ranged from 25 to 30 and approximately 6% were female.
Investigators collected blood samples from the service members and looked for four biomarkers: glycolytic ratio, arginine, serotonin, and glutamate. The team then divided the participants into four groups – those with PTSD (PTSD Checklist score above 30), those who were subthreshold for PTSD (PTSD Checklist score 15-30), those who had high resilience, and those who had low levels of resilience.
The resilience groups were determined based on answers to the Generalized Anxiety Disorder Questionnaire, Patient Health Questionnaire, Pittsburgh Sleep Quality Index, Intensive Combat Exposure (DRRI-D), the number of deployments, whether they had moderate or severe traumatic brain injury, and scores on the Alcohol Use Disorders Identification Test.
Those who scored in the high range at current or prior time points or who were PTSD/subthreshold at prior time points were placed in the low resilience group.
Ms. Miller noted that those in the PTSD group had more severe symptoms than those in the PTSD subthreshold group based on the longitudinal clinical assessment at 3-6 months, 5 years, and longer post deployment. The low resilience group had a much higher rate of PTSD post deployment than the high resilience group.
Investigators found participants with PTSD or subthreshold PTSD had significantly higher glycolic ratios and lower arginine than those with high resilience. They also found that those with PTSD had significantly lower serotonin and higher glutamate levels versus those with high resilience. These associations were independent of factors such as sex, age, body mass index, smoking, and caffeine consumption.
Ms. Miller said that the study results require further validation by the consortium’s labs and third-party labs.
“We are also interested in determining the most appropriate time to screen soldiers for PTSD, as it has been noted that the time period where we see the most psychological issues is around 2-3 months post return from deployment and when the soldier is preparing for their next assignment, perhaps a next deployment,” she said.
She added that previous studies have identified several promising biomarkers of PTSD. “However, like much of the research data, the study sample was comprised mainly of combat-exposed males. With more women serving on the front lines, the military faces new challenges in how combat affects females in the military,” including sex-specific biomarkers that will improve clinical assessment for female soldiers.
Eventually, the team would also like to be able to apply their research to the civilian population experiencing PTSD.
“Our research is anticipated to be useful in helping the medical provider select appropriate therapeutic interventions,” Ms. Miller said.
A version of this article first appeared on Medscape.com.
“More accurate means of predicting or screening for PTSD could help to overcome the disorder by identifying individuals at high risk of developing PTSD and providing them with early intervention or prevention strategies,” said study investigator Stacy-Ann Miller, MS.
She also noted that the biomarkers could be used to monitor treatment for PTSD, identify subtypes of PTSD, and lead to a new understanding of the mechanisms underlying PTSD.
The findings were presented at Discover BMB, the annual meeting of the American Society for Biochemistry and Molecular Biology.
Toward better clinical assessment
The findings originated from research conducted by the Department of Defense–initiated PTSD Systems Biology Consortium. The consortium’s goals include developing a reproducible panel of blood-based biomarkers with high sensitivity and specificity for PTSD diagnosis and is made up of about 45 researchers, led by Marti Jett, PhD, Charles Marmar, MD, and Francis J. Doyle III, PhD.
The researchers analyzed blood samples from 1,000 active-duty Army personnel from the 101st Airborne at Fort Campbell, Ky. Participants were assessed before and after deployment to Afghanistan in February 2014 and are referred to as the Fort Campbell Cohort (FCC). Participants’ age ranged from 25 to 30 and approximately 6% were female.
Investigators collected blood samples from the service members and looked for four biomarkers: glycolytic ratio, arginine, serotonin, and glutamate. The team then divided the participants into four groups – those with PTSD (PTSD Checklist score above 30), those who were subthreshold for PTSD (PTSD Checklist score 15-30), those who had high resilience, and those who had low levels of resilience.
The resilience groups were determined based on answers to the Generalized Anxiety Disorder Questionnaire, Patient Health Questionnaire, Pittsburgh Sleep Quality Index, Intensive Combat Exposure (DRRI-D), the number of deployments, whether they had moderate or severe traumatic brain injury, and scores on the Alcohol Use Disorders Identification Test.
Those who scored in the high range at current or prior time points or who were PTSD/subthreshold at prior time points were placed in the low resilience group.
Ms. Miller noted that those in the PTSD group had more severe symptoms than those in the PTSD subthreshold group based on the longitudinal clinical assessment at 3-6 months, 5 years, and longer post deployment. The low resilience group had a much higher rate of PTSD post deployment than the high resilience group.
Investigators found participants with PTSD or subthreshold PTSD had significantly higher glycolic ratios and lower arginine than those with high resilience. They also found that those with PTSD had significantly lower serotonin and higher glutamate levels versus those with high resilience. These associations were independent of factors such as sex, age, body mass index, smoking, and caffeine consumption.
Ms. Miller said that the study results require further validation by the consortium’s labs and third-party labs.
“We are also interested in determining the most appropriate time to screen soldiers for PTSD, as it has been noted that the time period where we see the most psychological issues is around 2-3 months post return from deployment and when the soldier is preparing for their next assignment, perhaps a next deployment,” she said.
She added that previous studies have identified several promising biomarkers of PTSD. “However, like much of the research data, the study sample was comprised mainly of combat-exposed males. With more women serving on the front lines, the military faces new challenges in how combat affects females in the military,” including sex-specific biomarkers that will improve clinical assessment for female soldiers.
Eventually, the team would also like to be able to apply their research to the civilian population experiencing PTSD.
“Our research is anticipated to be useful in helping the medical provider select appropriate therapeutic interventions,” Ms. Miller said.
A version of this article first appeared on Medscape.com.
FROM DISCOVER BMB
Lack of food for thought: Starve a bacterium, feed an infection
A whole new, tiny level of hangry
Ever been so hungry that everything just got on your nerves? Maybe you feel a little snappy right now? Like you’ll just lash out unless you get something to eat? Been there. And so have bacteria.
New research shows that some bacteria go into a full-on Hulk smash if they’re not getting the nutrients they need by releasing toxins into the body. Sounds like a bacterial temper tantrum.
Even though two cells may be genetically identical, they don’t always behave the same in a bacterial community. Some do their job and stay in line, but some evil twins rage out and make people sick by releasing toxins into the environment, Adam Rosenthal, PhD, of the University of North Carolina and his colleagues discovered.
To figure out why some cells were all business as usual while others were not, the investigators looked at Clostridium perfringens, a bacterium found in the intestines of humans and other vertebrates. When the C. perfringens cells were fed a little acetate to munch on, the hangry cells calmed down faster than a kid with a bag of fruit snacks, reducing toxin levels. Some cells even disappeared, falling in line with their model-citizen counterparts.
So what does this really mean? More research, duh. Now that we know nutrients play a role in toxicity, it may open the door to finding a way to fight against antibiotic resistance in humans and reduce antibiotic use in the food industry.
So think to yourself. Are you bothered for no reason? Getting a little testy with your friends and coworkers? Maybe you just haven’t eaten in a while. You’re literally not alone. Even a single-cell organism can behave based on its hunger levels.
Now go have a snack. Your bacteria are getting restless.
The very hangry iguana?
Imagine yourself on a warm, sunny tropical beach. You are enjoying a piece of cake as you take in the slow beat of the waves lapping against the shore. Life is as good as it could be.
Then you feel a presence nearby. Hostility. Hunger. A set of feral, covetous eyes in the nearby jungle. A reptilian beast stalks you, and its all-encompassing sweet tooth desires your cake.
Wait, hold on, what?
As an unfortunate 3-year-old on vacation in Costa Rica found out, there’s at least one iguana in the world out there with a taste for sugar (better than a taste for blood, we suppose).
While out on the beach, the lizard darted out of nowhere, bit the girl on the back of the hand, and stole her cake. Still not the worst party guest ever. The child was taken to a local clinic, where the wound was cleaned and a 5-day antibiotic treatment (lizards carry salmonella) was provided. Things seemed fine, and the girl returned home without incident.
But of course, that’s not the end of the story. Five months later, the girl’s parents noticed a red bump at the wound site. Over the next 3 months, the surrounding skin grew red and painful. A trip to the hospital in California revealed that she had a ganglion cyst and a discharge of pus. Turns out our cake-obsessed lizard friend did give the little girl a gift: the first known human case of Mycobacterium marinum infection following an iguana bite on record.
M. marinum, which causes a disease similar to tuberculosis, typically infects fish but can infect humans if skin wounds are exposed to contaminated water. It’s also resistant to most antibiotics, which is why the first round didn’t clear up the infection. A second round of more-potent antibiotics seems to be working well.
So, to sum up, this poor child got bitten by a lizard, had her cake stolen, and contracted a rare illness in exchange. For a 3-year-old, that’s gotta be in the top-10 worst days ever. Unless, of course, we’re actually living in the Marvel universe (sorry, multiverse at this point). Then we’re totally going to see the emergence of the new superhero Iguana Girl in 15 years or so. Keep your eyes open.
No allergies? Let them give up cake
Allergy season is already here – starting earlier every year, it seems – and many people are not happy about it. So unhappy, actually, that there’s a list of things they would be willing to give up for a year to get rid of their of allergies, according to a survey conducted by OnePoll on behalf of Flonase.
Nearly 40% of 2,000 respondents with allergies would go a year without eating cake or chocolate or playing video games in exchange for allergy-free status, the survey results show. Almost as many would forgo coffee (38%) or pizza (37%) for a year, while 36% would stay off social media and 31% would take a pay cut or give up their smartphones, the Independent reported.
More than half of the allergic Americans – 54%, to be exact – who were polled this past winter – Feb. 24 to March 1, to be exact – consider allergy symptoms to be the most frustrating part of the spring. Annoying things that were less frustrating to the group included mosquitoes (41%), filing tax returns (38%), and daylight savings time (37%).
The Trump arraignment circus, of course, occurred too late to make the list, as did the big “We’re going back to the office! No wait, we’re closing the office forever!” email extravaganza and emotional roller coaster. That second one, however, did not get nearly as much media coverage.
A whole new, tiny level of hangry
Ever been so hungry that everything just got on your nerves? Maybe you feel a little snappy right now? Like you’ll just lash out unless you get something to eat? Been there. And so have bacteria.
New research shows that some bacteria go into a full-on Hulk smash if they’re not getting the nutrients they need by releasing toxins into the body. Sounds like a bacterial temper tantrum.
Even though two cells may be genetically identical, they don’t always behave the same in a bacterial community. Some do their job and stay in line, but some evil twins rage out and make people sick by releasing toxins into the environment, Adam Rosenthal, PhD, of the University of North Carolina and his colleagues discovered.
To figure out why some cells were all business as usual while others were not, the investigators looked at Clostridium perfringens, a bacterium found in the intestines of humans and other vertebrates. When the C. perfringens cells were fed a little acetate to munch on, the hangry cells calmed down faster than a kid with a bag of fruit snacks, reducing toxin levels. Some cells even disappeared, falling in line with their model-citizen counterparts.
So what does this really mean? More research, duh. Now that we know nutrients play a role in toxicity, it may open the door to finding a way to fight against antibiotic resistance in humans and reduce antibiotic use in the food industry.
So think to yourself. Are you bothered for no reason? Getting a little testy with your friends and coworkers? Maybe you just haven’t eaten in a while. You’re literally not alone. Even a single-cell organism can behave based on its hunger levels.
Now go have a snack. Your bacteria are getting restless.
The very hangry iguana?
Imagine yourself on a warm, sunny tropical beach. You are enjoying a piece of cake as you take in the slow beat of the waves lapping against the shore. Life is as good as it could be.
Then you feel a presence nearby. Hostility. Hunger. A set of feral, covetous eyes in the nearby jungle. A reptilian beast stalks you, and its all-encompassing sweet tooth desires your cake.
Wait, hold on, what?
As an unfortunate 3-year-old on vacation in Costa Rica found out, there’s at least one iguana in the world out there with a taste for sugar (better than a taste for blood, we suppose).
While out on the beach, the lizard darted out of nowhere, bit the girl on the back of the hand, and stole her cake. Still not the worst party guest ever. The child was taken to a local clinic, where the wound was cleaned and a 5-day antibiotic treatment (lizards carry salmonella) was provided. Things seemed fine, and the girl returned home without incident.
But of course, that’s not the end of the story. Five months later, the girl’s parents noticed a red bump at the wound site. Over the next 3 months, the surrounding skin grew red and painful. A trip to the hospital in California revealed that she had a ganglion cyst and a discharge of pus. Turns out our cake-obsessed lizard friend did give the little girl a gift: the first known human case of Mycobacterium marinum infection following an iguana bite on record.
M. marinum, which causes a disease similar to tuberculosis, typically infects fish but can infect humans if skin wounds are exposed to contaminated water. It’s also resistant to most antibiotics, which is why the first round didn’t clear up the infection. A second round of more-potent antibiotics seems to be working well.
So, to sum up, this poor child got bitten by a lizard, had her cake stolen, and contracted a rare illness in exchange. For a 3-year-old, that’s gotta be in the top-10 worst days ever. Unless, of course, we’re actually living in the Marvel universe (sorry, multiverse at this point). Then we’re totally going to see the emergence of the new superhero Iguana Girl in 15 years or so. Keep your eyes open.
No allergies? Let them give up cake
Allergy season is already here – starting earlier every year, it seems – and many people are not happy about it. So unhappy, actually, that there’s a list of things they would be willing to give up for a year to get rid of their of allergies, according to a survey conducted by OnePoll on behalf of Flonase.
Nearly 40% of 2,000 respondents with allergies would go a year without eating cake or chocolate or playing video games in exchange for allergy-free status, the survey results show. Almost as many would forgo coffee (38%) or pizza (37%) for a year, while 36% would stay off social media and 31% would take a pay cut or give up their smartphones, the Independent reported.
More than half of the allergic Americans – 54%, to be exact – who were polled this past winter – Feb. 24 to March 1, to be exact – consider allergy symptoms to be the most frustrating part of the spring. Annoying things that were less frustrating to the group included mosquitoes (41%), filing tax returns (38%), and daylight savings time (37%).
The Trump arraignment circus, of course, occurred too late to make the list, as did the big “We’re going back to the office! No wait, we’re closing the office forever!” email extravaganza and emotional roller coaster. That second one, however, did not get nearly as much media coverage.
A whole new, tiny level of hangry
Ever been so hungry that everything just got on your nerves? Maybe you feel a little snappy right now? Like you’ll just lash out unless you get something to eat? Been there. And so have bacteria.
New research shows that some bacteria go into a full-on Hulk smash if they’re not getting the nutrients they need by releasing toxins into the body. Sounds like a bacterial temper tantrum.
Even though two cells may be genetically identical, they don’t always behave the same in a bacterial community. Some do their job and stay in line, but some evil twins rage out and make people sick by releasing toxins into the environment, Adam Rosenthal, PhD, of the University of North Carolina and his colleagues discovered.
To figure out why some cells were all business as usual while others were not, the investigators looked at Clostridium perfringens, a bacterium found in the intestines of humans and other vertebrates. When the C. perfringens cells were fed a little acetate to munch on, the hangry cells calmed down faster than a kid with a bag of fruit snacks, reducing toxin levels. Some cells even disappeared, falling in line with their model-citizen counterparts.
So what does this really mean? More research, duh. Now that we know nutrients play a role in toxicity, it may open the door to finding a way to fight against antibiotic resistance in humans and reduce antibiotic use in the food industry.
So think to yourself. Are you bothered for no reason? Getting a little testy with your friends and coworkers? Maybe you just haven’t eaten in a while. You’re literally not alone. Even a single-cell organism can behave based on its hunger levels.
Now go have a snack. Your bacteria are getting restless.
The very hangry iguana?
Imagine yourself on a warm, sunny tropical beach. You are enjoying a piece of cake as you take in the slow beat of the waves lapping against the shore. Life is as good as it could be.
Then you feel a presence nearby. Hostility. Hunger. A set of feral, covetous eyes in the nearby jungle. A reptilian beast stalks you, and its all-encompassing sweet tooth desires your cake.
Wait, hold on, what?
As an unfortunate 3-year-old on vacation in Costa Rica found out, there’s at least one iguana in the world out there with a taste for sugar (better than a taste for blood, we suppose).
While out on the beach, the lizard darted out of nowhere, bit the girl on the back of the hand, and stole her cake. Still not the worst party guest ever. The child was taken to a local clinic, where the wound was cleaned and a 5-day antibiotic treatment (lizards carry salmonella) was provided. Things seemed fine, and the girl returned home without incident.
But of course, that’s not the end of the story. Five months later, the girl’s parents noticed a red bump at the wound site. Over the next 3 months, the surrounding skin grew red and painful. A trip to the hospital in California revealed that she had a ganglion cyst and a discharge of pus. Turns out our cake-obsessed lizard friend did give the little girl a gift: the first known human case of Mycobacterium marinum infection following an iguana bite on record.
M. marinum, which causes a disease similar to tuberculosis, typically infects fish but can infect humans if skin wounds are exposed to contaminated water. It’s also resistant to most antibiotics, which is why the first round didn’t clear up the infection. A second round of more-potent antibiotics seems to be working well.
So, to sum up, this poor child got bitten by a lizard, had her cake stolen, and contracted a rare illness in exchange. For a 3-year-old, that’s gotta be in the top-10 worst days ever. Unless, of course, we’re actually living in the Marvel universe (sorry, multiverse at this point). Then we’re totally going to see the emergence of the new superhero Iguana Girl in 15 years or so. Keep your eyes open.
No allergies? Let them give up cake
Allergy season is already here – starting earlier every year, it seems – and many people are not happy about it. So unhappy, actually, that there’s a list of things they would be willing to give up for a year to get rid of their of allergies, according to a survey conducted by OnePoll on behalf of Flonase.
Nearly 40% of 2,000 respondents with allergies would go a year without eating cake or chocolate or playing video games in exchange for allergy-free status, the survey results show. Almost as many would forgo coffee (38%) or pizza (37%) for a year, while 36% would stay off social media and 31% would take a pay cut or give up their smartphones, the Independent reported.
More than half of the allergic Americans – 54%, to be exact – who were polled this past winter – Feb. 24 to March 1, to be exact – consider allergy symptoms to be the most frustrating part of the spring. Annoying things that were less frustrating to the group included mosquitoes (41%), filing tax returns (38%), and daylight savings time (37%).
The Trump arraignment circus, of course, occurred too late to make the list, as did the big “We’re going back to the office! No wait, we’re closing the office forever!” email extravaganza and emotional roller coaster. That second one, however, did not get nearly as much media coverage.
SARS-CoV-2 crosses placenta and infects brains of two infants: ‘This is a first’
, according to a study published online today in Pediatrics .
One of the infants died at 13 months and the other remained in hospice care at time of manuscript submission.
Lead author Merline Benny, MD, with the division of neonatology, department of pediatrics at University of Miami, and colleagues briefed reporters today ahead of the release.
“This is a first,” said senior author Shahnaz Duara, MD, medical director of the Neonatal Intensive Care Unit at Holtz Children’s Hospital, Miami, explaining it is the first study to confirm cross-placental SARS-CoV-2 transmission leading to brain injury in a newborn.
Both infants negative for the virus at birth
The two infants were admitted in the early days of the pandemic in the Delta wave to the neonatal ICU at Holtz Children’s Hospital at University of Miami/Jackson Memorial Medical Center.
Both infants tested negative for the virus at birth, but had significantly elevated SARS-CoV-2 antibodies in their blood, indicating that either antibodies crossed the placenta, or the virus crossed and the immune response was the baby’s.
Dr. Benny explained that the researchers have seen, to this point, more than 700 mother/infant pairs in whom the mother tested positive for COVID in Jackson hospital.
Most who tested positive for COVID were asymptomatic and most of the mothers and infants left the hospital without complications.
“However, (these) two babies had a very unusual clinical picture,” Dr. Benny said.
Those infants were born to mothers who became COVID positive in the second trimester and delivered a few weeks later.
Seizures started on day 1 of life
The babies began to seize from the first day of life. They had profound low tone (hypotonia) in their clinical exam, Dr. Benny explained.
“We had absolutely no good explanation for the early seizures and the degree of brain injury we saw,” Dr. Duara said.
Dr. Benny said that as their bodies grew, they had very small head circumference. Unlike some babies born with the Zika virus, these babies were not microcephalic at birth. Brain imaging on the two babies indicated significant brain atrophy, and neurodevelopment exams showed significant delay.
Discussions began with the center’s multidisciplinary team including neurologists, pathologists, neuroradiologists, and obstetricians who cared for both the mothers and the babies.
The experts examined the placentas and found some characteristic COVID changes and presence of the COVID virus. This was accompanied by increased markers for inflammation and a severe reduction in a hormone critical for placental health and brain development.
Examining the infant’s autopsy findings further raised suspicions of maternal transmission, something that had not been documented before.
Coauthor Ali G. Saad, MD, pediatric and perinatal pathology director at Miami, said, “I have seen literally thousands of brains in autopsies over the last 14 years, and this was the most dramatic case of leukoencephalopathy or loss of white matter in a patient with no significant reason. That’s what triggered the investigation.”
Mothers had very different presentations
Coauthor Michael J. Paidas, MD, with the department of obstetrics, gynecology, and reproductive sciences at Miami, pointed out that the circumstances of the two mothers, who were in their 20s, were very different.
One mother delivered at 32 weeks and had a very severe COVID presentation and spent a month in the intensive care unit. The team decided to deliver the child to save the mother, Dr. Paidas said.
In contrast, the other mother had asymptomatic COVID infection in the second trimester and delivered at full term.
He said one of the early suspicions in the babies’ presentations was hypoxic ischemic encephalopathy. “But it wasn’t lack of blood flow to the placenta that caused this,” he said. “As best we can tell, it was the viral infection.”
Instances are rare
The researchers emphasized that these instances are rare and have not been seen before or since the period of this study to their knowledge.
Dr. Duara said, “This is something we want to alert the medical community to more than the general public. We do not want the lay public to be panicked. We’re trying to understand what made these two pregnancies different, so we can direct research towards protecting vulnerable babies.”
Previous data have indicated a relatively benign status in infants who test negative for the COVID virus after birth. Dr. Benny added that COVID vaccination has been found safe in pregnancy and both vaccination and breastfeeding can help passage of antibodies to the infant and help protect the baby. Because these cases happened in the early days of the pandemic, no vaccines were available.
Dr. Paidas received funding from BioIncept to study hypoxic-ischemic encephalopathy with Preimplantation Factor, is a scientific advisory board member, and has stock options. Dr. Paidas and coauthor Dr. Jayakumar are coinventors of SPIKENET, University of Miami, patent pending 2023. The other authors have no conflicts of interest to disclose.
, according to a study published online today in Pediatrics .
One of the infants died at 13 months and the other remained in hospice care at time of manuscript submission.
Lead author Merline Benny, MD, with the division of neonatology, department of pediatrics at University of Miami, and colleagues briefed reporters today ahead of the release.
“This is a first,” said senior author Shahnaz Duara, MD, medical director of the Neonatal Intensive Care Unit at Holtz Children’s Hospital, Miami, explaining it is the first study to confirm cross-placental SARS-CoV-2 transmission leading to brain injury in a newborn.
Both infants negative for the virus at birth
The two infants were admitted in the early days of the pandemic in the Delta wave to the neonatal ICU at Holtz Children’s Hospital at University of Miami/Jackson Memorial Medical Center.
Both infants tested negative for the virus at birth, but had significantly elevated SARS-CoV-2 antibodies in their blood, indicating that either antibodies crossed the placenta, or the virus crossed and the immune response was the baby’s.
Dr. Benny explained that the researchers have seen, to this point, more than 700 mother/infant pairs in whom the mother tested positive for COVID in Jackson hospital.
Most who tested positive for COVID were asymptomatic and most of the mothers and infants left the hospital without complications.
“However, (these) two babies had a very unusual clinical picture,” Dr. Benny said.
Those infants were born to mothers who became COVID positive in the second trimester and delivered a few weeks later.
Seizures started on day 1 of life
The babies began to seize from the first day of life. They had profound low tone (hypotonia) in their clinical exam, Dr. Benny explained.
“We had absolutely no good explanation for the early seizures and the degree of brain injury we saw,” Dr. Duara said.
Dr. Benny said that as their bodies grew, they had very small head circumference. Unlike some babies born with the Zika virus, these babies were not microcephalic at birth. Brain imaging on the two babies indicated significant brain atrophy, and neurodevelopment exams showed significant delay.
Discussions began with the center’s multidisciplinary team including neurologists, pathologists, neuroradiologists, and obstetricians who cared for both the mothers and the babies.
The experts examined the placentas and found some characteristic COVID changes and presence of the COVID virus. This was accompanied by increased markers for inflammation and a severe reduction in a hormone critical for placental health and brain development.
Examining the infant’s autopsy findings further raised suspicions of maternal transmission, something that had not been documented before.
Coauthor Ali G. Saad, MD, pediatric and perinatal pathology director at Miami, said, “I have seen literally thousands of brains in autopsies over the last 14 years, and this was the most dramatic case of leukoencephalopathy or loss of white matter in a patient with no significant reason. That’s what triggered the investigation.”
Mothers had very different presentations
Coauthor Michael J. Paidas, MD, with the department of obstetrics, gynecology, and reproductive sciences at Miami, pointed out that the circumstances of the two mothers, who were in their 20s, were very different.
One mother delivered at 32 weeks and had a very severe COVID presentation and spent a month in the intensive care unit. The team decided to deliver the child to save the mother, Dr. Paidas said.
In contrast, the other mother had asymptomatic COVID infection in the second trimester and delivered at full term.
He said one of the early suspicions in the babies’ presentations was hypoxic ischemic encephalopathy. “But it wasn’t lack of blood flow to the placenta that caused this,” he said. “As best we can tell, it was the viral infection.”
Instances are rare
The researchers emphasized that these instances are rare and have not been seen before or since the period of this study to their knowledge.
Dr. Duara said, “This is something we want to alert the medical community to more than the general public. We do not want the lay public to be panicked. We’re trying to understand what made these two pregnancies different, so we can direct research towards protecting vulnerable babies.”
Previous data have indicated a relatively benign status in infants who test negative for the COVID virus after birth. Dr. Benny added that COVID vaccination has been found safe in pregnancy and both vaccination and breastfeeding can help passage of antibodies to the infant and help protect the baby. Because these cases happened in the early days of the pandemic, no vaccines were available.
Dr. Paidas received funding from BioIncept to study hypoxic-ischemic encephalopathy with Preimplantation Factor, is a scientific advisory board member, and has stock options. Dr. Paidas and coauthor Dr. Jayakumar are coinventors of SPIKENET, University of Miami, patent pending 2023. The other authors have no conflicts of interest to disclose.
, according to a study published online today in Pediatrics .
One of the infants died at 13 months and the other remained in hospice care at time of manuscript submission.
Lead author Merline Benny, MD, with the division of neonatology, department of pediatrics at University of Miami, and colleagues briefed reporters today ahead of the release.
“This is a first,” said senior author Shahnaz Duara, MD, medical director of the Neonatal Intensive Care Unit at Holtz Children’s Hospital, Miami, explaining it is the first study to confirm cross-placental SARS-CoV-2 transmission leading to brain injury in a newborn.
Both infants negative for the virus at birth
The two infants were admitted in the early days of the pandemic in the Delta wave to the neonatal ICU at Holtz Children’s Hospital at University of Miami/Jackson Memorial Medical Center.
Both infants tested negative for the virus at birth, but had significantly elevated SARS-CoV-2 antibodies in their blood, indicating that either antibodies crossed the placenta, or the virus crossed and the immune response was the baby’s.
Dr. Benny explained that the researchers have seen, to this point, more than 700 mother/infant pairs in whom the mother tested positive for COVID in Jackson hospital.
Most who tested positive for COVID were asymptomatic and most of the mothers and infants left the hospital without complications.
“However, (these) two babies had a very unusual clinical picture,” Dr. Benny said.
Those infants were born to mothers who became COVID positive in the second trimester and delivered a few weeks later.
Seizures started on day 1 of life
The babies began to seize from the first day of life. They had profound low tone (hypotonia) in their clinical exam, Dr. Benny explained.
“We had absolutely no good explanation for the early seizures and the degree of brain injury we saw,” Dr. Duara said.
Dr. Benny said that as their bodies grew, they had very small head circumference. Unlike some babies born with the Zika virus, these babies were not microcephalic at birth. Brain imaging on the two babies indicated significant brain atrophy, and neurodevelopment exams showed significant delay.
Discussions began with the center’s multidisciplinary team including neurologists, pathologists, neuroradiologists, and obstetricians who cared for both the mothers and the babies.
The experts examined the placentas and found some characteristic COVID changes and presence of the COVID virus. This was accompanied by increased markers for inflammation and a severe reduction in a hormone critical for placental health and brain development.
Examining the infant’s autopsy findings further raised suspicions of maternal transmission, something that had not been documented before.
Coauthor Ali G. Saad, MD, pediatric and perinatal pathology director at Miami, said, “I have seen literally thousands of brains in autopsies over the last 14 years, and this was the most dramatic case of leukoencephalopathy or loss of white matter in a patient with no significant reason. That’s what triggered the investigation.”
Mothers had very different presentations
Coauthor Michael J. Paidas, MD, with the department of obstetrics, gynecology, and reproductive sciences at Miami, pointed out that the circumstances of the two mothers, who were in their 20s, were very different.
One mother delivered at 32 weeks and had a very severe COVID presentation and spent a month in the intensive care unit. The team decided to deliver the child to save the mother, Dr. Paidas said.
In contrast, the other mother had asymptomatic COVID infection in the second trimester and delivered at full term.
He said one of the early suspicions in the babies’ presentations was hypoxic ischemic encephalopathy. “But it wasn’t lack of blood flow to the placenta that caused this,” he said. “As best we can tell, it was the viral infection.”
Instances are rare
The researchers emphasized that these instances are rare and have not been seen before or since the period of this study to their knowledge.
Dr. Duara said, “This is something we want to alert the medical community to more than the general public. We do not want the lay public to be panicked. We’re trying to understand what made these two pregnancies different, so we can direct research towards protecting vulnerable babies.”
Previous data have indicated a relatively benign status in infants who test negative for the COVID virus after birth. Dr. Benny added that COVID vaccination has been found safe in pregnancy and both vaccination and breastfeeding can help passage of antibodies to the infant and help protect the baby. Because these cases happened in the early days of the pandemic, no vaccines were available.
Dr. Paidas received funding from BioIncept to study hypoxic-ischemic encephalopathy with Preimplantation Factor, is a scientific advisory board member, and has stock options. Dr. Paidas and coauthor Dr. Jayakumar are coinventors of SPIKENET, University of Miami, patent pending 2023. The other authors have no conflicts of interest to disclose.
FROM PEDIATRICS
New update on left atrial appendage closure recommendations
An updated consensus statement on transcatheter left atrial appendage closure (LAAC) has put a newfound focus on patient selection for the procedure, specifically recommending that the procedure is appropriate for patients with nonvalvular atrial fibrillation who have risk for thromboembolism, aren’t well suited for direct oral anticoagulants (DOACs) and have a good chance of living for at least another year.
The statement, published online in the Journal of the Society for Cardiovascular Angiography & Interventions, also makes recommendations for how much experience operators should have, how many procedures they should perform to keep their skills up, and when and how to use imaging and prescribe DOACs, among other suggestions.
The statement represents the first updated guidance for LAAC since 2015. “Since then this field has really expanded and evolved,” writing group chair Jacqueline Saw, MD, said in an interview. “For instance, the indications are more matured and specific, and the procedural technical steps have matured. Imaging has also advanced, there’s more understanding about postprocedural care and there are also new devices that have been approved.”
Dr. Saw, an interventional cardiologist at Vancouver General Hospital and St. Paul’s Hospital, and a professor at the University of British Columbia in Vancouver, called the statement “a piece that puts everything together.”
“This document really summarizes the whole practice for doing transcatheter procedures,” she added, “so it’s all-in-one document in terms of recommendation of who we do the procedure for, how we should do it, how we should image and guide the procedure, and what complications to look out for and how to manage patients post procedure, be it with antithrombotic therapy and/or device surveillance.”
13 recommendations
In all, the statement carries 13 recommendations for LAAC. The Society for Cardiovascular Angiography & Interventions and the Heart Rhythm Society commissioned the writing group. The American College of Cardiology and Society of Cardiovascular Computed Tomography have endorsed the statement. The following are among the recommendations:
- Transcatheter LAAC is appropriate for patients with nonvalvular atrial fibrillation with high thromboembolic risk but for whom long-term oral anticoagulation may be contraindicated and who have at least 1 year’s life expectancy.
- Operators should have performed at least 50 prior left-sided ablations or structural procedures and at least 25 transseptal punctures (TSPs). Interventional-imaging physicians should have experience in guiding 25 or more TSPs before supporting LAAC procedures independently.
- To maintain skills, operators should do 25 or more TSPs and at least 12 LAACs over each 2-year period.
- On-site cardiovascular surgery backup should be available for new programs and for operators early in their learning curve.
- Baseline imaging with transesophageal echocardiography (TEE) or cardiac computed tomography should be performed before LAAC.
- Intraprocedural imaging guidance with TEE or intracardiac echocardiography.
- Follow labeling of each specific LAAC device for technical aspects of the procedure.
- Familiarity with avoiding, recognizing, and managing LAAC complications.
- Predischarge 2-dimensional TEE to rule out pericardial effusion and device embolization.
- Anticoagulation for device-related thrombus.
- Make all efforts to minimize peridevice leaks during implantation because their clinical impact and management isn’t well understood.
- Antithrombotic therapy with warfarin, DOAC, or dual-antiplatelet therapy after LAAC based on the studied regimen and instructions for each specific device, tailored to the bleeding risks for each patient.
- TEE or cardiac computed tomography at 45-90 days after LAAC for device surveillance to assess for peridevice leak and device-related thrombus.
The statement also includes precautionary recommendations. It advises against using routine closure of LAAC-associated iatrogenic atrial septal defects and states that combined procedures with LAAC, such as structural interventions and pulmonary vein isolation, should be avoided because randomized controlled trial data are pending.
“These recommendations are based upon data from updated publications and randomized trial data as well as large registries, including the National Cardiovascular Data Registry, so I think this is a very practical statement that puts all these pieces together for any budding interventionalist doing this procedure and even experienced operations,” Dr. Saw said.
Authors of an accompanying editorial agreed that the “rigorous standards” set out in the statement will help maintain “a high level of procedural safety in the setting of rapid expansion.”
The editorialists, Faisal M. Merchant, MD, of Emory University, Atlanta, and Mohamad Alkhouli, MD, professor of medicine at Mayo Clinic School of Medicine, Rochester, Minn., point out that the incidence of pericardial effusion has decreased from more than 5% in the pivotal Watchman trials to less than 1.5% in the most recent report from the National Cardiovascular Data Registry, which shows that more than 100,000 procedures have been performed in the United States.
But most important as the field moves forward, they stress, is patient selection. The recommendation of limiting patients to those with a life expectancy of 1 year “is a tacit recognition of the fact that the benefits of LAAC take time to accrue, and many older and frail patients are unlikely to derive meaningful benefit.”
Dr. Merchant and Dr. Alkhouli also note that there remains a conundrum in patient selection that remains from the original LAAC trials, which enrolled patients who were eligible for anticoagulation. “Somewhat paradoxically, after its approval, LAAC is mostly prescribed to patients who are not felt to be good anticoagulation candidates.” This leaves physicians “in the precarious position of extrapolating data to patients who were excluded from the original clinical trials.”
Therefore, the consensus statement “is right to put patient selection front and center in its recommendations, but as the field of LAAC comes of age, better evidence to support patient selection will be the real sign of maturity.”
Dr. Saw said she envisions another update over the next 2 years or so as ongoing clinical trials comparing DOAC and LAAC, namely the CHAMPION-AF and OPTION trials, report results.
Dr. Saw and Dr. Merchant, reported no conflicts of interest. Dr. Alkhouli has financial ties to Boston Scientific, Abbott, and Philips.
An updated consensus statement on transcatheter left atrial appendage closure (LAAC) has put a newfound focus on patient selection for the procedure, specifically recommending that the procedure is appropriate for patients with nonvalvular atrial fibrillation who have risk for thromboembolism, aren’t well suited for direct oral anticoagulants (DOACs) and have a good chance of living for at least another year.
The statement, published online in the Journal of the Society for Cardiovascular Angiography & Interventions, also makes recommendations for how much experience operators should have, how many procedures they should perform to keep their skills up, and when and how to use imaging and prescribe DOACs, among other suggestions.
The statement represents the first updated guidance for LAAC since 2015. “Since then this field has really expanded and evolved,” writing group chair Jacqueline Saw, MD, said in an interview. “For instance, the indications are more matured and specific, and the procedural technical steps have matured. Imaging has also advanced, there’s more understanding about postprocedural care and there are also new devices that have been approved.”
Dr. Saw, an interventional cardiologist at Vancouver General Hospital and St. Paul’s Hospital, and a professor at the University of British Columbia in Vancouver, called the statement “a piece that puts everything together.”
“This document really summarizes the whole practice for doing transcatheter procedures,” she added, “so it’s all-in-one document in terms of recommendation of who we do the procedure for, how we should do it, how we should image and guide the procedure, and what complications to look out for and how to manage patients post procedure, be it with antithrombotic therapy and/or device surveillance.”
13 recommendations
In all, the statement carries 13 recommendations for LAAC. The Society for Cardiovascular Angiography & Interventions and the Heart Rhythm Society commissioned the writing group. The American College of Cardiology and Society of Cardiovascular Computed Tomography have endorsed the statement. The following are among the recommendations:
- Transcatheter LAAC is appropriate for patients with nonvalvular atrial fibrillation with high thromboembolic risk but for whom long-term oral anticoagulation may be contraindicated and who have at least 1 year’s life expectancy.
- Operators should have performed at least 50 prior left-sided ablations or structural procedures and at least 25 transseptal punctures (TSPs). Interventional-imaging physicians should have experience in guiding 25 or more TSPs before supporting LAAC procedures independently.
- To maintain skills, operators should do 25 or more TSPs and at least 12 LAACs over each 2-year period.
- On-site cardiovascular surgery backup should be available for new programs and for operators early in their learning curve.
- Baseline imaging with transesophageal echocardiography (TEE) or cardiac computed tomography should be performed before LAAC.
- Intraprocedural imaging guidance with TEE or intracardiac echocardiography.
- Follow labeling of each specific LAAC device for technical aspects of the procedure.
- Familiarity with avoiding, recognizing, and managing LAAC complications.
- Predischarge 2-dimensional TEE to rule out pericardial effusion and device embolization.
- Anticoagulation for device-related thrombus.
- Make all efforts to minimize peridevice leaks during implantation because their clinical impact and management isn’t well understood.
- Antithrombotic therapy with warfarin, DOAC, or dual-antiplatelet therapy after LAAC based on the studied regimen and instructions for each specific device, tailored to the bleeding risks for each patient.
- TEE or cardiac computed tomography at 45-90 days after LAAC for device surveillance to assess for peridevice leak and device-related thrombus.
The statement also includes precautionary recommendations. It advises against using routine closure of LAAC-associated iatrogenic atrial septal defects and states that combined procedures with LAAC, such as structural interventions and pulmonary vein isolation, should be avoided because randomized controlled trial data are pending.
“These recommendations are based upon data from updated publications and randomized trial data as well as large registries, including the National Cardiovascular Data Registry, so I think this is a very practical statement that puts all these pieces together for any budding interventionalist doing this procedure and even experienced operations,” Dr. Saw said.
Authors of an accompanying editorial agreed that the “rigorous standards” set out in the statement will help maintain “a high level of procedural safety in the setting of rapid expansion.”
The editorialists, Faisal M. Merchant, MD, of Emory University, Atlanta, and Mohamad Alkhouli, MD, professor of medicine at Mayo Clinic School of Medicine, Rochester, Minn., point out that the incidence of pericardial effusion has decreased from more than 5% in the pivotal Watchman trials to less than 1.5% in the most recent report from the National Cardiovascular Data Registry, which shows that more than 100,000 procedures have been performed in the United States.
But most important as the field moves forward, they stress, is patient selection. The recommendation of limiting patients to those with a life expectancy of 1 year “is a tacit recognition of the fact that the benefits of LAAC take time to accrue, and many older and frail patients are unlikely to derive meaningful benefit.”
Dr. Merchant and Dr. Alkhouli also note that there remains a conundrum in patient selection that remains from the original LAAC trials, which enrolled patients who were eligible for anticoagulation. “Somewhat paradoxically, after its approval, LAAC is mostly prescribed to patients who are not felt to be good anticoagulation candidates.” This leaves physicians “in the precarious position of extrapolating data to patients who were excluded from the original clinical trials.”
Therefore, the consensus statement “is right to put patient selection front and center in its recommendations, but as the field of LAAC comes of age, better evidence to support patient selection will be the real sign of maturity.”
Dr. Saw said she envisions another update over the next 2 years or so as ongoing clinical trials comparing DOAC and LAAC, namely the CHAMPION-AF and OPTION trials, report results.
Dr. Saw and Dr. Merchant, reported no conflicts of interest. Dr. Alkhouli has financial ties to Boston Scientific, Abbott, and Philips.
An updated consensus statement on transcatheter left atrial appendage closure (LAAC) has put a newfound focus on patient selection for the procedure, specifically recommending that the procedure is appropriate for patients with nonvalvular atrial fibrillation who have risk for thromboembolism, aren’t well suited for direct oral anticoagulants (DOACs) and have a good chance of living for at least another year.
The statement, published online in the Journal of the Society for Cardiovascular Angiography & Interventions, also makes recommendations for how much experience operators should have, how many procedures they should perform to keep their skills up, and when and how to use imaging and prescribe DOACs, among other suggestions.
The statement represents the first updated guidance for LAAC since 2015. “Since then this field has really expanded and evolved,” writing group chair Jacqueline Saw, MD, said in an interview. “For instance, the indications are more matured and specific, and the procedural technical steps have matured. Imaging has also advanced, there’s more understanding about postprocedural care and there are also new devices that have been approved.”
Dr. Saw, an interventional cardiologist at Vancouver General Hospital and St. Paul’s Hospital, and a professor at the University of British Columbia in Vancouver, called the statement “a piece that puts everything together.”
“This document really summarizes the whole practice for doing transcatheter procedures,” she added, “so it’s all-in-one document in terms of recommendation of who we do the procedure for, how we should do it, how we should image and guide the procedure, and what complications to look out for and how to manage patients post procedure, be it with antithrombotic therapy and/or device surveillance.”
13 recommendations
In all, the statement carries 13 recommendations for LAAC. The Society for Cardiovascular Angiography & Interventions and the Heart Rhythm Society commissioned the writing group. The American College of Cardiology and Society of Cardiovascular Computed Tomography have endorsed the statement. The following are among the recommendations:
- Transcatheter LAAC is appropriate for patients with nonvalvular atrial fibrillation with high thromboembolic risk but for whom long-term oral anticoagulation may be contraindicated and who have at least 1 year’s life expectancy.
- Operators should have performed at least 50 prior left-sided ablations or structural procedures and at least 25 transseptal punctures (TSPs). Interventional-imaging physicians should have experience in guiding 25 or more TSPs before supporting LAAC procedures independently.
- To maintain skills, operators should do 25 or more TSPs and at least 12 LAACs over each 2-year period.
- On-site cardiovascular surgery backup should be available for new programs and for operators early in their learning curve.
- Baseline imaging with transesophageal echocardiography (TEE) or cardiac computed tomography should be performed before LAAC.
- Intraprocedural imaging guidance with TEE or intracardiac echocardiography.
- Follow labeling of each specific LAAC device for technical aspects of the procedure.
- Familiarity with avoiding, recognizing, and managing LAAC complications.
- Predischarge 2-dimensional TEE to rule out pericardial effusion and device embolization.
- Anticoagulation for device-related thrombus.
- Make all efforts to minimize peridevice leaks during implantation because their clinical impact and management isn’t well understood.
- Antithrombotic therapy with warfarin, DOAC, or dual-antiplatelet therapy after LAAC based on the studied regimen and instructions for each specific device, tailored to the bleeding risks for each patient.
- TEE or cardiac computed tomography at 45-90 days after LAAC for device surveillance to assess for peridevice leak and device-related thrombus.
The statement also includes precautionary recommendations. It advises against using routine closure of LAAC-associated iatrogenic atrial septal defects and states that combined procedures with LAAC, such as structural interventions and pulmonary vein isolation, should be avoided because randomized controlled trial data are pending.
“These recommendations are based upon data from updated publications and randomized trial data as well as large registries, including the National Cardiovascular Data Registry, so I think this is a very practical statement that puts all these pieces together for any budding interventionalist doing this procedure and even experienced operations,” Dr. Saw said.
Authors of an accompanying editorial agreed that the “rigorous standards” set out in the statement will help maintain “a high level of procedural safety in the setting of rapid expansion.”
The editorialists, Faisal M. Merchant, MD, of Emory University, Atlanta, and Mohamad Alkhouli, MD, professor of medicine at Mayo Clinic School of Medicine, Rochester, Minn., point out that the incidence of pericardial effusion has decreased from more than 5% in the pivotal Watchman trials to less than 1.5% in the most recent report from the National Cardiovascular Data Registry, which shows that more than 100,000 procedures have been performed in the United States.
But most important as the field moves forward, they stress, is patient selection. The recommendation of limiting patients to those with a life expectancy of 1 year “is a tacit recognition of the fact that the benefits of LAAC take time to accrue, and many older and frail patients are unlikely to derive meaningful benefit.”
Dr. Merchant and Dr. Alkhouli also note that there remains a conundrum in patient selection that remains from the original LAAC trials, which enrolled patients who were eligible for anticoagulation. “Somewhat paradoxically, after its approval, LAAC is mostly prescribed to patients who are not felt to be good anticoagulation candidates.” This leaves physicians “in the precarious position of extrapolating data to patients who were excluded from the original clinical trials.”
Therefore, the consensus statement “is right to put patient selection front and center in its recommendations, but as the field of LAAC comes of age, better evidence to support patient selection will be the real sign of maturity.”
Dr. Saw said she envisions another update over the next 2 years or so as ongoing clinical trials comparing DOAC and LAAC, namely the CHAMPION-AF and OPTION trials, report results.
Dr. Saw and Dr. Merchant, reported no conflicts of interest. Dr. Alkhouli has financial ties to Boston Scientific, Abbott, and Philips.
FROM THE JOURNAL OF THE SOCIETY FOR CARDIOVASCULAR ANGIOGRAPHY & INTERVENTIONS
Kidney disease skews Alzheimer’s biomarker testing
New research provides more evidence that
In a cross-sectional study of adults with and those without cognitive impairment, chronic kidney disease was associated with increased plasma concentrations of phosphorylated tau (p-tau) 217 and 181.
However, there were no associations between chronic kidney disease and the ratio of p-tau217 to unphosphorylated tau 217 (pT217/T217), and the associations with p-tau181 to unphosphorylated tau 181 (pT181/T181) were attenuated in patients with cognitive impairment.
“These novel findings suggest that plasma measures of the phosphorylated to unphosphorylated tau ratios are more accurate than p-tau forms alone as they correlate less with individual difference in glomerular filtration rate or impaired kidney function,” reported an investigative team led by Oskar Hansson, MD, PhD, with Lund University in Sweden.
“Thus, to mitigate the effects of non-Alzheimer’s–related comorbidities like chronic kidney disease on the performance of plasma Alzheimer’s disease biomarkers, certain tau ratios, and specifically pT217/T217, should be considered for implementation in clinical practice and drug trials,” they added.
The study was published online in JAMA Neurology to coincide with a presentation at the International Conference on Alzheimer’s and Parkinson’s Diseases in Gothenburg, Sweden.
Skewed tau levels
Plasma biomarkers of amyloid-beta (Abeta) and tau pathologies, and in particular variants in p-tau217 and p-tau181, have shown promise for use in Alzheimer’s disease diagnosis and prognosis. However, previous reports have suggested that chronic kidney disease might influence plasma p-tau217 and p-tau181 concentrations, potentially decreasing their usefulness in the diagnostic workup of dementia.
Researchers investigated associations of chronic kidney disease with plasma ratios of p-tau217 and p-tau181 to the corresponding unphosphorylated peptides in Alzheimer’s disease.
The data came from 473 older adults, with and without cognitive impairment, from the Swedish BioFinder-2 study who had plasma tau assessments and chronic kidney disease status established within 6 months of plasma collection.
The researchers found that lower estimated glomerular filtration rate levels (indicative of kidney dysfunction) were associated with higher plasma concentrations of phosphorylated and unphosphorylated tau peptides measured simultaneously using the tau immunoprecipitation mass spectrometry assay.
However, the correlations with estimated glomerular filtration rate were nonsignificant for the pT217/T217 ratio in individuals with cognitive impairment and were significantly attenuated for pT217/T217 in cognitively unimpaired individuals and for pT181/T181 in both cognitively unimpaired and impaired individuals.
“Importantly, we demonstrate that there were no significant associations between chronic kidney disease and the pT217/T217 ratio and changes in plasma pT181/T181 associated with chronic kidney disease were small or nonsignificant,” the researchers noted.
“Our results indicate that by using p-tau/tau ratios we may be able to reduce the variability in plasma p-tau levels driven by impaired kidney function and consequently such ratios are more robust measures of brain p-tau pathology in individuals with both early- and later-stage Alzheimer’s disease,” they added.
The researchers believe this is likely true for the ratios of other related proteins – a view that is supported by findings of attenuated associations of chronic kidney disease with Abeta42/40, compared with Abeta42 and Abeta40 in the current study and in previous studies.
Important clinical implications
Reached for comment, Rebecca Edelmayer, PhD, Alzheimer’s Association senior director of scientific engagement, noted that research continues to indicate that blood biomarkers have “promise for improving, and possibly even redefining, the clinical workup for Alzheimer’s disease.
“Many of the Alzheimer’s blood tests in development today have focused on core blood biomarkers associated with amyloid accumulation and tau tangle formation in the brain,” Dr. Edelmayer said.
“Before these tests can be used more broadly in the clinic, we need to understand all of the variables that may impact the results of various blood biomarkers, including differences that may be driven by race, ethnicity, sex, and underlying health conditions, such as chronic kidney disease.
“This study corroborates other research suggesting that some Alzheimer’s-associated markers can be affected by chronic kidney disease, but by using ratios of amyloid or tau markers, we may be able to minimize these differences in results caused by underlying disease,” Dr. Edelmayer said.
Howard Fillit, MD, cofounder and chief science officer at the Alzheimer’s Drug Discovery Foundation, said, “Using these ratios makes a lot of sense because the ratios wouldn’t change with kidney function. I think this is an important advance in clinical utilization of these tests.”
Dr. Fillit noted that older people often have declining kidney function, which can be easily measured by glomerular filtration rate. Changes in blood levels of these markers with declining kidney function are “not unexpected, but [it’s] important that it’s been demonstrated.
“As we go forward, maybe the future utilization of these tests will be not only recording the ratios but also reporting the ratios in the context of somebody’s glomerular filtration rate. You could imagine a scenario where when the test is done, it’s automatically done alongside of glomerular filtration rate,” Dr. Fillit said in an interview.
The study was supported by Coins for Alzheimer’s Research Trust, the Tracy Family Stable Isotope Labeling Quantitation Center, and the National Institute of Neurological Disorders and Stroke. Dr. Hansson has received support to his institution from ADx, AVID Radiopharmaceuticals, Biogen, Eli Lilly, Eisai, Fujirebio, GE Healthcare, Pfizer, and Roche; consultancy/speaker fees from Amylyx, Alzpath, Biogen, Cerveau, Fujirebio, Genentech, Novartis, Roche, and Siemens; and personal fees from Eli Lilly, Eisai, Bioarctic, and Biogen outside the submitted work. Dr. Edelmayer and Dr. Fillit have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
New research provides more evidence that
In a cross-sectional study of adults with and those without cognitive impairment, chronic kidney disease was associated with increased plasma concentrations of phosphorylated tau (p-tau) 217 and 181.
However, there were no associations between chronic kidney disease and the ratio of p-tau217 to unphosphorylated tau 217 (pT217/T217), and the associations with p-tau181 to unphosphorylated tau 181 (pT181/T181) were attenuated in patients with cognitive impairment.
“These novel findings suggest that plasma measures of the phosphorylated to unphosphorylated tau ratios are more accurate than p-tau forms alone as they correlate less with individual difference in glomerular filtration rate or impaired kidney function,” reported an investigative team led by Oskar Hansson, MD, PhD, with Lund University in Sweden.
“Thus, to mitigate the effects of non-Alzheimer’s–related comorbidities like chronic kidney disease on the performance of plasma Alzheimer’s disease biomarkers, certain tau ratios, and specifically pT217/T217, should be considered for implementation in clinical practice and drug trials,” they added.
The study was published online in JAMA Neurology to coincide with a presentation at the International Conference on Alzheimer’s and Parkinson’s Diseases in Gothenburg, Sweden.
Skewed tau levels
Plasma biomarkers of amyloid-beta (Abeta) and tau pathologies, and in particular variants in p-tau217 and p-tau181, have shown promise for use in Alzheimer’s disease diagnosis and prognosis. However, previous reports have suggested that chronic kidney disease might influence plasma p-tau217 and p-tau181 concentrations, potentially decreasing their usefulness in the diagnostic workup of dementia.
Researchers investigated associations of chronic kidney disease with plasma ratios of p-tau217 and p-tau181 to the corresponding unphosphorylated peptides in Alzheimer’s disease.
The data came from 473 older adults, with and without cognitive impairment, from the Swedish BioFinder-2 study who had plasma tau assessments and chronic kidney disease status established within 6 months of plasma collection.
The researchers found that lower estimated glomerular filtration rate levels (indicative of kidney dysfunction) were associated with higher plasma concentrations of phosphorylated and unphosphorylated tau peptides measured simultaneously using the tau immunoprecipitation mass spectrometry assay.
However, the correlations with estimated glomerular filtration rate were nonsignificant for the pT217/T217 ratio in individuals with cognitive impairment and were significantly attenuated for pT217/T217 in cognitively unimpaired individuals and for pT181/T181 in both cognitively unimpaired and impaired individuals.
“Importantly, we demonstrate that there were no significant associations between chronic kidney disease and the pT217/T217 ratio and changes in plasma pT181/T181 associated with chronic kidney disease were small or nonsignificant,” the researchers noted.
“Our results indicate that by using p-tau/tau ratios we may be able to reduce the variability in plasma p-tau levels driven by impaired kidney function and consequently such ratios are more robust measures of brain p-tau pathology in individuals with both early- and later-stage Alzheimer’s disease,” they added.
The researchers believe this is likely true for the ratios of other related proteins – a view that is supported by findings of attenuated associations of chronic kidney disease with Abeta42/40, compared with Abeta42 and Abeta40 in the current study and in previous studies.
Important clinical implications
Reached for comment, Rebecca Edelmayer, PhD, Alzheimer’s Association senior director of scientific engagement, noted that research continues to indicate that blood biomarkers have “promise for improving, and possibly even redefining, the clinical workup for Alzheimer’s disease.
“Many of the Alzheimer’s blood tests in development today have focused on core blood biomarkers associated with amyloid accumulation and tau tangle formation in the brain,” Dr. Edelmayer said.
“Before these tests can be used more broadly in the clinic, we need to understand all of the variables that may impact the results of various blood biomarkers, including differences that may be driven by race, ethnicity, sex, and underlying health conditions, such as chronic kidney disease.
“This study corroborates other research suggesting that some Alzheimer’s-associated markers can be affected by chronic kidney disease, but by using ratios of amyloid or tau markers, we may be able to minimize these differences in results caused by underlying disease,” Dr. Edelmayer said.
Howard Fillit, MD, cofounder and chief science officer at the Alzheimer’s Drug Discovery Foundation, said, “Using these ratios makes a lot of sense because the ratios wouldn’t change with kidney function. I think this is an important advance in clinical utilization of these tests.”
Dr. Fillit noted that older people often have declining kidney function, which can be easily measured by glomerular filtration rate. Changes in blood levels of these markers with declining kidney function are “not unexpected, but [it’s] important that it’s been demonstrated.
“As we go forward, maybe the future utilization of these tests will be not only recording the ratios but also reporting the ratios in the context of somebody’s glomerular filtration rate. You could imagine a scenario where when the test is done, it’s automatically done alongside of glomerular filtration rate,” Dr. Fillit said in an interview.
The study was supported by Coins for Alzheimer’s Research Trust, the Tracy Family Stable Isotope Labeling Quantitation Center, and the National Institute of Neurological Disorders and Stroke. Dr. Hansson has received support to his institution from ADx, AVID Radiopharmaceuticals, Biogen, Eli Lilly, Eisai, Fujirebio, GE Healthcare, Pfizer, and Roche; consultancy/speaker fees from Amylyx, Alzpath, Biogen, Cerveau, Fujirebio, Genentech, Novartis, Roche, and Siemens; and personal fees from Eli Lilly, Eisai, Bioarctic, and Biogen outside the submitted work. Dr. Edelmayer and Dr. Fillit have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
New research provides more evidence that
In a cross-sectional study of adults with and those without cognitive impairment, chronic kidney disease was associated with increased plasma concentrations of phosphorylated tau (p-tau) 217 and 181.
However, there were no associations between chronic kidney disease and the ratio of p-tau217 to unphosphorylated tau 217 (pT217/T217), and the associations with p-tau181 to unphosphorylated tau 181 (pT181/T181) were attenuated in patients with cognitive impairment.
“These novel findings suggest that plasma measures of the phosphorylated to unphosphorylated tau ratios are more accurate than p-tau forms alone as they correlate less with individual difference in glomerular filtration rate or impaired kidney function,” reported an investigative team led by Oskar Hansson, MD, PhD, with Lund University in Sweden.
“Thus, to mitigate the effects of non-Alzheimer’s–related comorbidities like chronic kidney disease on the performance of plasma Alzheimer’s disease biomarkers, certain tau ratios, and specifically pT217/T217, should be considered for implementation in clinical practice and drug trials,” they added.
The study was published online in JAMA Neurology to coincide with a presentation at the International Conference on Alzheimer’s and Parkinson’s Diseases in Gothenburg, Sweden.
Skewed tau levels
Plasma biomarkers of amyloid-beta (Abeta) and tau pathologies, and in particular variants in p-tau217 and p-tau181, have shown promise for use in Alzheimer’s disease diagnosis and prognosis. However, previous reports have suggested that chronic kidney disease might influence plasma p-tau217 and p-tau181 concentrations, potentially decreasing their usefulness in the diagnostic workup of dementia.
Researchers investigated associations of chronic kidney disease with plasma ratios of p-tau217 and p-tau181 to the corresponding unphosphorylated peptides in Alzheimer’s disease.
The data came from 473 older adults, with and without cognitive impairment, from the Swedish BioFinder-2 study who had plasma tau assessments and chronic kidney disease status established within 6 months of plasma collection.
The researchers found that lower estimated glomerular filtration rate levels (indicative of kidney dysfunction) were associated with higher plasma concentrations of phosphorylated and unphosphorylated tau peptides measured simultaneously using the tau immunoprecipitation mass spectrometry assay.
However, the correlations with estimated glomerular filtration rate were nonsignificant for the pT217/T217 ratio in individuals with cognitive impairment and were significantly attenuated for pT217/T217 in cognitively unimpaired individuals and for pT181/T181 in both cognitively unimpaired and impaired individuals.
“Importantly, we demonstrate that there were no significant associations between chronic kidney disease and the pT217/T217 ratio and changes in plasma pT181/T181 associated with chronic kidney disease were small or nonsignificant,” the researchers noted.
“Our results indicate that by using p-tau/tau ratios we may be able to reduce the variability in plasma p-tau levels driven by impaired kidney function and consequently such ratios are more robust measures of brain p-tau pathology in individuals with both early- and later-stage Alzheimer’s disease,” they added.
The researchers believe this is likely true for the ratios of other related proteins – a view that is supported by findings of attenuated associations of chronic kidney disease with Abeta42/40, compared with Abeta42 and Abeta40 in the current study and in previous studies.
Important clinical implications
Reached for comment, Rebecca Edelmayer, PhD, Alzheimer’s Association senior director of scientific engagement, noted that research continues to indicate that blood biomarkers have “promise for improving, and possibly even redefining, the clinical workup for Alzheimer’s disease.
“Many of the Alzheimer’s blood tests in development today have focused on core blood biomarkers associated with amyloid accumulation and tau tangle formation in the brain,” Dr. Edelmayer said.
“Before these tests can be used more broadly in the clinic, we need to understand all of the variables that may impact the results of various blood biomarkers, including differences that may be driven by race, ethnicity, sex, and underlying health conditions, such as chronic kidney disease.
“This study corroborates other research suggesting that some Alzheimer’s-associated markers can be affected by chronic kidney disease, but by using ratios of amyloid or tau markers, we may be able to minimize these differences in results caused by underlying disease,” Dr. Edelmayer said.
Howard Fillit, MD, cofounder and chief science officer at the Alzheimer’s Drug Discovery Foundation, said, “Using these ratios makes a lot of sense because the ratios wouldn’t change with kidney function. I think this is an important advance in clinical utilization of these tests.”
Dr. Fillit noted that older people often have declining kidney function, which can be easily measured by glomerular filtration rate. Changes in blood levels of these markers with declining kidney function are “not unexpected, but [it’s] important that it’s been demonstrated.
“As we go forward, maybe the future utilization of these tests will be not only recording the ratios but also reporting the ratios in the context of somebody’s glomerular filtration rate. You could imagine a scenario where when the test is done, it’s automatically done alongside of glomerular filtration rate,” Dr. Fillit said in an interview.
The study was supported by Coins for Alzheimer’s Research Trust, the Tracy Family Stable Isotope Labeling Quantitation Center, and the National Institute of Neurological Disorders and Stroke. Dr. Hansson has received support to his institution from ADx, AVID Radiopharmaceuticals, Biogen, Eli Lilly, Eisai, Fujirebio, GE Healthcare, Pfizer, and Roche; consultancy/speaker fees from Amylyx, Alzpath, Biogen, Cerveau, Fujirebio, Genentech, Novartis, Roche, and Siemens; and personal fees from Eli Lilly, Eisai, Bioarctic, and Biogen outside the submitted work. Dr. Edelmayer and Dr. Fillit have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM AD/PD 2023
Parkinson’s disease: What’s trauma got to do with it?
This transcript has been edited for clarity.
Kathrin LaFaver, MD: Hello. I’m happy to talk today to Dr. Indu Subramanian, clinical professor at University of California, Los Angeles, and director of the Parkinson’s Disease Research, Education and Clinical Center in Los Angeles. I am a neurologist in Saratoga Springs, New York, and we will be talking today about Indu’s new paper on childhood trauma and Parkinson’s disease. Welcome and thanks for taking the time.
Indu Subramanian, MD: Thank you so much for letting us highlight this important topic.
Dr. LaFaver: There are many papers published every month on Parkinson’s disease, but this topic stands out because it’s not a thing that has been commonly looked at. What gave you the idea to study this?
Neurology behind other specialties
Dr. Subramanian: Kathrin, you and I have been looking at things that can inform us about our patients – the person who’s standing in front of us when they come in and we’re giving them this diagnosis. I think that so much of what we’ve done [in the past] is a cookie cutter approach to giving everybody the standard treatment. [We’ve been assuming that] It doesn’t matter if they’re a man or woman. It doesn’t matter if they’re a veteran. It doesn’t matter if they may be from a minoritized population.
We’ve also been interested in approaches that are outside the box, right? We have this integrative medicine and lifestyle medicine background. I’ve been going to those meetings and really been struck by the mounting evidence on the importance of things like early adverse childhood events (ACEs), what zip code you live in, what your pollution index is, and how these things can affect people through their life and their health.
I think that it is high time neurologists pay attention to this. There’s been mounting evidence throughout many disease states, various types of cancers, and mental health. Cardiology is much more advanced, but we haven’t had much data in neurology. In fact, when we went to write this paper, there were just one or two papers that were looking at multiple sclerosis or general neurologic issues, but really nothing in Parkinson’s disease.
We know that Parkinson’s disease is not only a motor disease that affects mental health, but that it also affects nonmotor issues. Childhood adversity may affect how people progress or how quickly they may get a disease, and we were interested in how it may manifest in a disease like Parkinson’s disease.
That was the framework going to meetings. As we wrote this paper and were in various editing stages, there was a beautiful paper that came out by Nadine Burke Harris and team that really was a call to action for neurologists and caring about trauma.
Dr. LaFaver: I couldn’t agree more. It’s really an underrecognized issue. With my own background, being very interested in functional movement disorders, psychosomatic disorders, and so on, it becomes much more evident how common a trauma background is, not only for people we were traditionally asking about.
Why don’t you summarize your findings for us?
Adverse childhood events
Dr. Subramanian: This is a web-based survey, so obviously, these are patient self-reports of their disease. We have a large cohort of people that we’ve been following over 7 years. I’m looking at modifiable variables and what really impacts Parkinson’s disease. Some of our previous papers have looked at diet, exercise, and loneliness. This is the same cohort.
We ended up putting the ACEs questionnaire, which is 10 questions looking at whether you were exposed to certain things in your household below the age of 18. This is a relatively standard questionnaire that’s administered one time, and you get a score out of 10. This is something that has been pushed, at least in the state of California, as something that we should be checking more in all people coming in.
We introduced the survey, and we didn’t force everyone to take it. Unfortunately, there was 20% or so of our patients who chose not to answer these questions. One has to ask, who are those people that didn’t answer the questions? Are they the ones that may have had trauma and these questions were triggering? It was a gap. We didn’t add extra questions to explore why people didn’t answer those questions.
We have to also put this in context. We have a patient population that’s largely quite affluent, who are able to access web-based surveys through their computer, and largely Caucasian; there are not many minoritized populations in our cohort. We want to do better with that. We actually were able to gather a decent number of women. We represent women quite well in our survey. I think that’s because of this online approach and some of the things that we’re studying.
In our survey, we broke it down into people who had no ACEs, one to three ACEs, or four or more ACEs. This is a standard way to break down ACEs so that we’re able to categorize what to do with these patient populations.
What we saw – and it’s preliminary evidence – is that people who had higher ACE scores seemed to have more symptom severity when we controlled for things like years since diagnosis, age, and gender. They also seem to have a worse quality of life. There was some indication that there were more nonmotor issues in those populations, as you might expect, such as anxiety, depression, and things that presumably ACEs can affect separately.
There are some confounders, but I think we really want to use this as the first piece of evidence to hopefully pave the way for caring about trauma in Parkinson’s disease moving forward.
Dr. LaFaver: Thank you so much for that summary. You already mentioned the main methodology you used.
What is the next step for you? How do you see these findings informing our clinical care? Do you have suggestions for all of the neurologists listening in this regard?
PD not yet considered ACE-related
Dr. Subramanian: Dr. Burke Harris was the former surgeon general in California. She’s a woman of color and a brilliant speaker, and she had worked in inner cities, I think in San Francisco, with pediatric populations, seeing these effects of adversity in that time frame.
You see this population at risk, and then you’re following this cohort, which we knew from the Kaiser cohort determines earlier morbidity and mortality across a number of disease states. We’re seeing things like more heart attacks, more diabetes, and all kinds of things in these populations. This is not new news; we just have not been focusing on this.
In her paper, this call to action, they had talked about some ACE-related conditions that currently do not include Parkinson’s disease. There are three ACE-related neurologic conditions that people should be aware of. One is in the headache/pain universe. Another is in the stroke universe, and that’s understandable, given cardiovascular risk factors . Then the third is in this dementia risk category. I think Parkinson’s disease, as we know, can be associated with dementia. A large percentage of our patients get dementia, but we don’t have Parkinson’s disease called out in this framework.
What people are talking about is if you have no ACEs or are in this middle category of one to three ACEs and you don’t have an ACE-related diagnosis – which Parkinson’s disease is not currently – we just give some basic counseling about the importance of lifestyle. I think we would love to see that anyway. They’re talking about things like exercise, diet, sleep, social connection, getting out in nature, things like that, so just general counseling on the importance of that.
Then if you’re in this higher-risk category, and so with these ACE-related neurologic conditions, including dementia, headache, and stroke, if you had this middle range of one to three ACEs, they’re getting additional resources. Some of them may be referred for social work help or mental health support and things like that.
I’d really love to see that happening in Parkinson’s disease, because I think we have so many needs in our population. I’m always hoping to advocate for more mental health needs that are scarce and resources in the social support realm because I believe that social connection and social support is a huge buffer for this trauma.
ACEs are just one type of trauma. I take care of veterans in the Veterans [Affairs Department]. We have some information now coming out about posttraumatic stress disorder, predisposing to certain things in Parkinson’s disease, possibly head injury, and things like that. I think we have populations at risk that we can hopefully screen at intake, and I’m really pushing for that.
Maybe it’s not the neurologist that does this intake. It might be someone else on the team that can spend some time doing these questionnaires and understand if your patient has a high ACE score. Unless you ask, many patients don’t necessarily come forward to talk about this. I really am pushing for trying to screen and trying to advocate for more research in this area so that we can classify Parkinson’s disease as an ACE-related condition and thus give more resources from the mental health world, and also the social support world, to our patients.
Dr. LaFaver: Thank you. There are many important points, and I think it’s a very important thing to recognize that it may not be only trauma in childhood but also throughout life, as you said, and might really influence nonmotor symptoms of Parkinson’s disease in particular, including anxiety and pain, which are often difficult to treat.
I think there’s much more to do in research, advocacy, and education. We’re going to educate patients about this, and also educate other neurologists and providers. I think you mentioned that trauma-informed care is getting its spotlight in primary care and other specialties. I think we have catching up to do in neurology, and I think this is a really important work toward that goal.
Thank you so much for your work and for taking the time to share your thoughts. I hope to talk to you again soon.
Dr. Subramanian: Thank you so much, Kathrin.
Dr. LaFaver has disclosed no relevant financial relationships. Dr. Subramanian disclosed ties with Acorda Therapeutics.
A version of this article originally appeared on Medscape.com.
This transcript has been edited for clarity.
Kathrin LaFaver, MD: Hello. I’m happy to talk today to Dr. Indu Subramanian, clinical professor at University of California, Los Angeles, and director of the Parkinson’s Disease Research, Education and Clinical Center in Los Angeles. I am a neurologist in Saratoga Springs, New York, and we will be talking today about Indu’s new paper on childhood trauma and Parkinson’s disease. Welcome and thanks for taking the time.
Indu Subramanian, MD: Thank you so much for letting us highlight this important topic.
Dr. LaFaver: There are many papers published every month on Parkinson’s disease, but this topic stands out because it’s not a thing that has been commonly looked at. What gave you the idea to study this?
Neurology behind other specialties
Dr. Subramanian: Kathrin, you and I have been looking at things that can inform us about our patients – the person who’s standing in front of us when they come in and we’re giving them this diagnosis. I think that so much of what we’ve done [in the past] is a cookie cutter approach to giving everybody the standard treatment. [We’ve been assuming that] It doesn’t matter if they’re a man or woman. It doesn’t matter if they’re a veteran. It doesn’t matter if they may be from a minoritized population.
We’ve also been interested in approaches that are outside the box, right? We have this integrative medicine and lifestyle medicine background. I’ve been going to those meetings and really been struck by the mounting evidence on the importance of things like early adverse childhood events (ACEs), what zip code you live in, what your pollution index is, and how these things can affect people through their life and their health.
I think that it is high time neurologists pay attention to this. There’s been mounting evidence throughout many disease states, various types of cancers, and mental health. Cardiology is much more advanced, but we haven’t had much data in neurology. In fact, when we went to write this paper, there were just one or two papers that were looking at multiple sclerosis or general neurologic issues, but really nothing in Parkinson’s disease.
We know that Parkinson’s disease is not only a motor disease that affects mental health, but that it also affects nonmotor issues. Childhood adversity may affect how people progress or how quickly they may get a disease, and we were interested in how it may manifest in a disease like Parkinson’s disease.
That was the framework going to meetings. As we wrote this paper and were in various editing stages, there was a beautiful paper that came out by Nadine Burke Harris and team that really was a call to action for neurologists and caring about trauma.
Dr. LaFaver: I couldn’t agree more. It’s really an underrecognized issue. With my own background, being very interested in functional movement disorders, psychosomatic disorders, and so on, it becomes much more evident how common a trauma background is, not only for people we were traditionally asking about.
Why don’t you summarize your findings for us?
Adverse childhood events
Dr. Subramanian: This is a web-based survey, so obviously, these are patient self-reports of their disease. We have a large cohort of people that we’ve been following over 7 years. I’m looking at modifiable variables and what really impacts Parkinson’s disease. Some of our previous papers have looked at diet, exercise, and loneliness. This is the same cohort.
We ended up putting the ACEs questionnaire, which is 10 questions looking at whether you were exposed to certain things in your household below the age of 18. This is a relatively standard questionnaire that’s administered one time, and you get a score out of 10. This is something that has been pushed, at least in the state of California, as something that we should be checking more in all people coming in.
We introduced the survey, and we didn’t force everyone to take it. Unfortunately, there was 20% or so of our patients who chose not to answer these questions. One has to ask, who are those people that didn’t answer the questions? Are they the ones that may have had trauma and these questions were triggering? It was a gap. We didn’t add extra questions to explore why people didn’t answer those questions.
We have to also put this in context. We have a patient population that’s largely quite affluent, who are able to access web-based surveys through their computer, and largely Caucasian; there are not many minoritized populations in our cohort. We want to do better with that. We actually were able to gather a decent number of women. We represent women quite well in our survey. I think that’s because of this online approach and some of the things that we’re studying.
In our survey, we broke it down into people who had no ACEs, one to three ACEs, or four or more ACEs. This is a standard way to break down ACEs so that we’re able to categorize what to do with these patient populations.
What we saw – and it’s preliminary evidence – is that people who had higher ACE scores seemed to have more symptom severity when we controlled for things like years since diagnosis, age, and gender. They also seem to have a worse quality of life. There was some indication that there were more nonmotor issues in those populations, as you might expect, such as anxiety, depression, and things that presumably ACEs can affect separately.
There are some confounders, but I think we really want to use this as the first piece of evidence to hopefully pave the way for caring about trauma in Parkinson’s disease moving forward.
Dr. LaFaver: Thank you so much for that summary. You already mentioned the main methodology you used.
What is the next step for you? How do you see these findings informing our clinical care? Do you have suggestions for all of the neurologists listening in this regard?
PD not yet considered ACE-related
Dr. Subramanian: Dr. Burke Harris was the former surgeon general in California. She’s a woman of color and a brilliant speaker, and she had worked in inner cities, I think in San Francisco, with pediatric populations, seeing these effects of adversity in that time frame.
You see this population at risk, and then you’re following this cohort, which we knew from the Kaiser cohort determines earlier morbidity and mortality across a number of disease states. We’re seeing things like more heart attacks, more diabetes, and all kinds of things in these populations. This is not new news; we just have not been focusing on this.
In her paper, this call to action, they had talked about some ACE-related conditions that currently do not include Parkinson’s disease. There are three ACE-related neurologic conditions that people should be aware of. One is in the headache/pain universe. Another is in the stroke universe, and that’s understandable, given cardiovascular risk factors . Then the third is in this dementia risk category. I think Parkinson’s disease, as we know, can be associated with dementia. A large percentage of our patients get dementia, but we don’t have Parkinson’s disease called out in this framework.
What people are talking about is if you have no ACEs or are in this middle category of one to three ACEs and you don’t have an ACE-related diagnosis – which Parkinson’s disease is not currently – we just give some basic counseling about the importance of lifestyle. I think we would love to see that anyway. They’re talking about things like exercise, diet, sleep, social connection, getting out in nature, things like that, so just general counseling on the importance of that.
Then if you’re in this higher-risk category, and so with these ACE-related neurologic conditions, including dementia, headache, and stroke, if you had this middle range of one to three ACEs, they’re getting additional resources. Some of them may be referred for social work help or mental health support and things like that.
I’d really love to see that happening in Parkinson’s disease, because I think we have so many needs in our population. I’m always hoping to advocate for more mental health needs that are scarce and resources in the social support realm because I believe that social connection and social support is a huge buffer for this trauma.
ACEs are just one type of trauma. I take care of veterans in the Veterans [Affairs Department]. We have some information now coming out about posttraumatic stress disorder, predisposing to certain things in Parkinson’s disease, possibly head injury, and things like that. I think we have populations at risk that we can hopefully screen at intake, and I’m really pushing for that.
Maybe it’s not the neurologist that does this intake. It might be someone else on the team that can spend some time doing these questionnaires and understand if your patient has a high ACE score. Unless you ask, many patients don’t necessarily come forward to talk about this. I really am pushing for trying to screen and trying to advocate for more research in this area so that we can classify Parkinson’s disease as an ACE-related condition and thus give more resources from the mental health world, and also the social support world, to our patients.
Dr. LaFaver: Thank you. There are many important points, and I think it’s a very important thing to recognize that it may not be only trauma in childhood but also throughout life, as you said, and might really influence nonmotor symptoms of Parkinson’s disease in particular, including anxiety and pain, which are often difficult to treat.
I think there’s much more to do in research, advocacy, and education. We’re going to educate patients about this, and also educate other neurologists and providers. I think you mentioned that trauma-informed care is getting its spotlight in primary care and other specialties. I think we have catching up to do in neurology, and I think this is a really important work toward that goal.
Thank you so much for your work and for taking the time to share your thoughts. I hope to talk to you again soon.
Dr. Subramanian: Thank you so much, Kathrin.
Dr. LaFaver has disclosed no relevant financial relationships. Dr. Subramanian disclosed ties with Acorda Therapeutics.
A version of this article originally appeared on Medscape.com.
This transcript has been edited for clarity.
Kathrin LaFaver, MD: Hello. I’m happy to talk today to Dr. Indu Subramanian, clinical professor at University of California, Los Angeles, and director of the Parkinson’s Disease Research, Education and Clinical Center in Los Angeles. I am a neurologist in Saratoga Springs, New York, and we will be talking today about Indu’s new paper on childhood trauma and Parkinson’s disease. Welcome and thanks for taking the time.
Indu Subramanian, MD: Thank you so much for letting us highlight this important topic.
Dr. LaFaver: There are many papers published every month on Parkinson’s disease, but this topic stands out because it’s not a thing that has been commonly looked at. What gave you the idea to study this?
Neurology behind other specialties
Dr. Subramanian: Kathrin, you and I have been looking at things that can inform us about our patients – the person who’s standing in front of us when they come in and we’re giving them this diagnosis. I think that so much of what we’ve done [in the past] is a cookie cutter approach to giving everybody the standard treatment. [We’ve been assuming that] It doesn’t matter if they’re a man or woman. It doesn’t matter if they’re a veteran. It doesn’t matter if they may be from a minoritized population.
We’ve also been interested in approaches that are outside the box, right? We have this integrative medicine and lifestyle medicine background. I’ve been going to those meetings and really been struck by the mounting evidence on the importance of things like early adverse childhood events (ACEs), what zip code you live in, what your pollution index is, and how these things can affect people through their life and their health.
I think that it is high time neurologists pay attention to this. There’s been mounting evidence throughout many disease states, various types of cancers, and mental health. Cardiology is much more advanced, but we haven’t had much data in neurology. In fact, when we went to write this paper, there were just one or two papers that were looking at multiple sclerosis or general neurologic issues, but really nothing in Parkinson’s disease.
We know that Parkinson’s disease is not only a motor disease that affects mental health, but that it also affects nonmotor issues. Childhood adversity may affect how people progress or how quickly they may get a disease, and we were interested in how it may manifest in a disease like Parkinson’s disease.
That was the framework going to meetings. As we wrote this paper and were in various editing stages, there was a beautiful paper that came out by Nadine Burke Harris and team that really was a call to action for neurologists and caring about trauma.
Dr. LaFaver: I couldn’t agree more. It’s really an underrecognized issue. With my own background, being very interested in functional movement disorders, psychosomatic disorders, and so on, it becomes much more evident how common a trauma background is, not only for people we were traditionally asking about.
Why don’t you summarize your findings for us?
Adverse childhood events
Dr. Subramanian: This is a web-based survey, so obviously, these are patient self-reports of their disease. We have a large cohort of people that we’ve been following over 7 years. I’m looking at modifiable variables and what really impacts Parkinson’s disease. Some of our previous papers have looked at diet, exercise, and loneliness. This is the same cohort.
We ended up putting the ACEs questionnaire, which is 10 questions looking at whether you were exposed to certain things in your household below the age of 18. This is a relatively standard questionnaire that’s administered one time, and you get a score out of 10. This is something that has been pushed, at least in the state of California, as something that we should be checking more in all people coming in.
We introduced the survey, and we didn’t force everyone to take it. Unfortunately, there was 20% or so of our patients who chose not to answer these questions. One has to ask, who are those people that didn’t answer the questions? Are they the ones that may have had trauma and these questions were triggering? It was a gap. We didn’t add extra questions to explore why people didn’t answer those questions.
We have to also put this in context. We have a patient population that’s largely quite affluent, who are able to access web-based surveys through their computer, and largely Caucasian; there are not many minoritized populations in our cohort. We want to do better with that. We actually were able to gather a decent number of women. We represent women quite well in our survey. I think that’s because of this online approach and some of the things that we’re studying.
In our survey, we broke it down into people who had no ACEs, one to three ACEs, or four or more ACEs. This is a standard way to break down ACEs so that we’re able to categorize what to do with these patient populations.
What we saw – and it’s preliminary evidence – is that people who had higher ACE scores seemed to have more symptom severity when we controlled for things like years since diagnosis, age, and gender. They also seem to have a worse quality of life. There was some indication that there were more nonmotor issues in those populations, as you might expect, such as anxiety, depression, and things that presumably ACEs can affect separately.
There are some confounders, but I think we really want to use this as the first piece of evidence to hopefully pave the way for caring about trauma in Parkinson’s disease moving forward.
Dr. LaFaver: Thank you so much for that summary. You already mentioned the main methodology you used.
What is the next step for you? How do you see these findings informing our clinical care? Do you have suggestions for all of the neurologists listening in this regard?
PD not yet considered ACE-related
Dr. Subramanian: Dr. Burke Harris was the former surgeon general in California. She’s a woman of color and a brilliant speaker, and she had worked in inner cities, I think in San Francisco, with pediatric populations, seeing these effects of adversity in that time frame.
You see this population at risk, and then you’re following this cohort, which we knew from the Kaiser cohort determines earlier morbidity and mortality across a number of disease states. We’re seeing things like more heart attacks, more diabetes, and all kinds of things in these populations. This is not new news; we just have not been focusing on this.
In her paper, this call to action, they had talked about some ACE-related conditions that currently do not include Parkinson’s disease. There are three ACE-related neurologic conditions that people should be aware of. One is in the headache/pain universe. Another is in the stroke universe, and that’s understandable, given cardiovascular risk factors . Then the third is in this dementia risk category. I think Parkinson’s disease, as we know, can be associated with dementia. A large percentage of our patients get dementia, but we don’t have Parkinson’s disease called out in this framework.
What people are talking about is if you have no ACEs or are in this middle category of one to three ACEs and you don’t have an ACE-related diagnosis – which Parkinson’s disease is not currently – we just give some basic counseling about the importance of lifestyle. I think we would love to see that anyway. They’re talking about things like exercise, diet, sleep, social connection, getting out in nature, things like that, so just general counseling on the importance of that.
Then if you’re in this higher-risk category, and so with these ACE-related neurologic conditions, including dementia, headache, and stroke, if you had this middle range of one to three ACEs, they’re getting additional resources. Some of them may be referred for social work help or mental health support and things like that.
I’d really love to see that happening in Parkinson’s disease, because I think we have so many needs in our population. I’m always hoping to advocate for more mental health needs that are scarce and resources in the social support realm because I believe that social connection and social support is a huge buffer for this trauma.
ACEs are just one type of trauma. I take care of veterans in the Veterans [Affairs Department]. We have some information now coming out about posttraumatic stress disorder, predisposing to certain things in Parkinson’s disease, possibly head injury, and things like that. I think we have populations at risk that we can hopefully screen at intake, and I’m really pushing for that.
Maybe it’s not the neurologist that does this intake. It might be someone else on the team that can spend some time doing these questionnaires and understand if your patient has a high ACE score. Unless you ask, many patients don’t necessarily come forward to talk about this. I really am pushing for trying to screen and trying to advocate for more research in this area so that we can classify Parkinson’s disease as an ACE-related condition and thus give more resources from the mental health world, and also the social support world, to our patients.
Dr. LaFaver: Thank you. There are many important points, and I think it’s a very important thing to recognize that it may not be only trauma in childhood but also throughout life, as you said, and might really influence nonmotor symptoms of Parkinson’s disease in particular, including anxiety and pain, which are often difficult to treat.
I think there’s much more to do in research, advocacy, and education. We’re going to educate patients about this, and also educate other neurologists and providers. I think you mentioned that trauma-informed care is getting its spotlight in primary care and other specialties. I think we have catching up to do in neurology, and I think this is a really important work toward that goal.
Thank you so much for your work and for taking the time to share your thoughts. I hope to talk to you again soon.
Dr. Subramanian: Thank you so much, Kathrin.
Dr. LaFaver has disclosed no relevant financial relationships. Dr. Subramanian disclosed ties with Acorda Therapeutics.
A version of this article originally appeared on Medscape.com.
Magnesium-rich diet linked to lower dementia risk
Investigators studied more than 6,000 cognitively healthy individuals, aged 40-73, and found that those who consumed more than 550 mg of magnesium daily had a brain age approximately 1 year younger by age 55 years, compared with a person who consumed a normal magnesium intake (~360 mg per day).
“This research highlights the potential benefits of a diet high in magnesium and the role it plays in promoting good brain health,” lead author Khawlah Alateeq, a PhD candidate in neuroscience at Australian National University’s National Centre for Epidemiology and Population Health, said in an interview.
Clinicians “can use [the findings] to counsel patients on the benefits of increasing magnesium intake through a healthy diet and monitoring magnesium levels to prevent deficiencies,” she stated.
The study was published online in the European Journal of Nutrition.
Promising target
The researchers were motivated to conduct the study because of “the growing concern over the increasing prevalence of dementia,” Ms. Alateeq said.
“Since there is no cure for dementia, and the development of pharmacological treatment for dementia has been unsuccessful over the last 30 years, prevention has been suggested as an effective approach to address the issue,” she added.
Nutrition, Ms. Alateeq said, is a “modifiable risk factor that can influence brain health and is highly amenable to scalable and cost-effective interventions.” It represents “a promising target” for risk reduction at a population level.
Previous research shows individuals with lower magnesium levels are at higher risk for AD, while those with higher dietary magnesium intake may be at lower risk of progressing from normal aging to cognitive impairment.
Most previous studies, however, included participants older than age 60 years, and it’s “unclear when the neuroprotective effects of dietary magnesium become detectable,” the researchers note.
Moreover, dietary patterns change and fluctuate, potentially leading to changes in magnesium intake over time. These changes may have as much impact as absolute magnesium at any point in time.
In light of the “current lack of understanding of when and to what extent dietary magnesium exerts its protective effects on the brain,” the researchers examined the association between magnesium trajectories over time, brain matter, and white matter lesions.
They also examined the association between magnesium and several different blood pressure measures (mean arterial pressure, systolic blood pressure, diastolic blood pressure, and pulse pressure).
Since cardiovascular health, neurodegeneration, and brain shrinkage patterns differ between men and women, the researchers stratified their analyses by sex.
Brain volume differences
The researchers analyzed the dietary magnesium intake of 6,001 individuals (mean age, 55.3 years) selected from the UK Biobank – a prospective cohort study of participants aged 37-73 at baseline, who were assessed between 2005 and 2023.
For the current study, only participants with baseline DBP and SBP measurements and structural MRI scans were included. Participants were also required to be free of neurologic disorders and to have an available record of dietary magnesium intake.
Covariates included age, sex, education, health conditions, smoking status, body mass index, amount of physical activity, smoking status, and alcohol intake.
Over a 16-month period, participants completed an online questionnaire five times. Their responses were used to calculate daily magnesium intake. Foods of particular interest included leafy green vegetables, legumes, nuts, seeds, and whole grains, all of which are magnesium rich.
They used latent class analysis (LCA) to “identify mutually exclusive subgroup (classes) of magnesium intake trajectory separately for men and women.”
Men had a slightly higher prevalence of BP medication and diabetes, compared with women, and postmenopausal women had a higher prevalence of BP medication and diabetes, compared with premenopausal women.
Compared with lower baseline magnesium intake, higher baseline dietary intake of magnesium was associated with larger brain volumes in several regions in both men and women.
The latent class analysis identified three classes of magnesium intake:
In women in particular, the “high-decreasing” trajectory was significantly associated with larger brain volumes, compared with the “normal-stable” trajectory, while the “low-increasing” trajectory was associated with smaller brain volumes.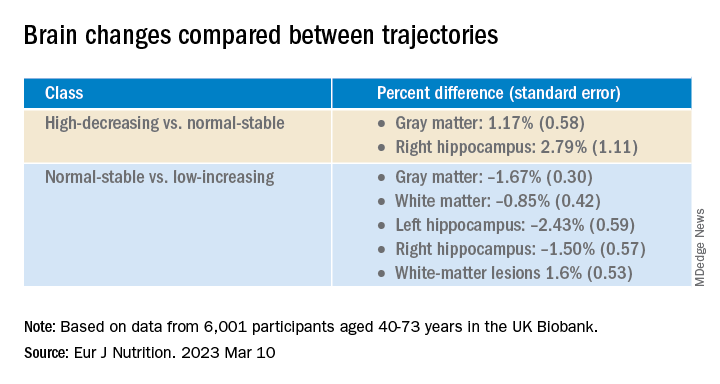
Even an increase of 1 mg of magnesium per day (above 350 mg/day) made a difference in brain volume, especially in women. The changes associated with every 1-mg increase are found in the table below: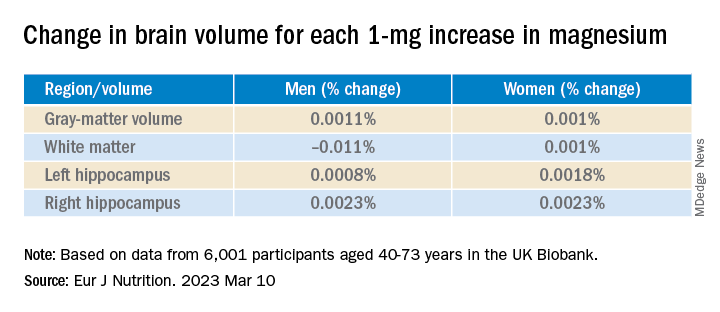
Associations between magnesium and BP measures were “mostly nonsignificant,” the researchers say, and the neuroprotective effect of higher magnesium intake in the high-decreasing trajectory was greater in postmenopausal versus premenopausal women.
“Our models indicate that compared to somebody with a normal magnesium intake (~350 mg per day), somebody in the top quartile of magnesium intake (≥ 550 mg per day) would be predicted to have a ~0.20% larger GM and ~0.46% larger RHC,” the authors summarize.
“In a population with an average age of 55 years, this effect corresponds to ~1 year of typical aging,” they note. “In other words, if this effect is generalizable to other populations, a 41% increase in magnesium intake may lead to significantly better brain health.”
Although the exact mechanisms underlying magnesium’s protective effects are “not yet clearly understood, there’s considerable evidence that magnesium levels are related to better cardiovascular health. Magnesium supplementation has been found to decrease blood pressure – and high blood pressure is a well-established risk factor for dementia,” said Ms. Alateeq.
Association, not causation
Yuko Hara, PhD, director of Aging and Prevention, Alzheimer’s Drug Discovery Foundation, noted that the study is observational and therefore shows an association, not causation.
“People eating a high-magnesium diet may also be eating a brain-healthy diet and getting high levels of nutrients/minerals other than magnesium alone,” suggested Dr. Hara, who was not involved with the study.
She noted that many foods are good sources of magnesium, including spinach, almonds, cashews, legumes, yogurt, brown rice, and avocados.
“Eating a brain-healthy diet (for example, the Mediterranean diet) is one of the Seven Steps to Protect Your Cognitive Vitality that ADDF’s Cognitive Vitality promotes,” she said.
Open Access funding was enabled and organized by the Council of Australian University Librarians and its Member Institutions. Ms. Alateeq, her co-authors, and Dr. Hara declare no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Investigators studied more than 6,000 cognitively healthy individuals, aged 40-73, and found that those who consumed more than 550 mg of magnesium daily had a brain age approximately 1 year younger by age 55 years, compared with a person who consumed a normal magnesium intake (~360 mg per day).
“This research highlights the potential benefits of a diet high in magnesium and the role it plays in promoting good brain health,” lead author Khawlah Alateeq, a PhD candidate in neuroscience at Australian National University’s National Centre for Epidemiology and Population Health, said in an interview.
Clinicians “can use [the findings] to counsel patients on the benefits of increasing magnesium intake through a healthy diet and monitoring magnesium levels to prevent deficiencies,” she stated.
The study was published online in the European Journal of Nutrition.
Promising target
The researchers were motivated to conduct the study because of “the growing concern over the increasing prevalence of dementia,” Ms. Alateeq said.
“Since there is no cure for dementia, and the development of pharmacological treatment for dementia has been unsuccessful over the last 30 years, prevention has been suggested as an effective approach to address the issue,” she added.
Nutrition, Ms. Alateeq said, is a “modifiable risk factor that can influence brain health and is highly amenable to scalable and cost-effective interventions.” It represents “a promising target” for risk reduction at a population level.
Previous research shows individuals with lower magnesium levels are at higher risk for AD, while those with higher dietary magnesium intake may be at lower risk of progressing from normal aging to cognitive impairment.
Most previous studies, however, included participants older than age 60 years, and it’s “unclear when the neuroprotective effects of dietary magnesium become detectable,” the researchers note.
Moreover, dietary patterns change and fluctuate, potentially leading to changes in magnesium intake over time. These changes may have as much impact as absolute magnesium at any point in time.
In light of the “current lack of understanding of when and to what extent dietary magnesium exerts its protective effects on the brain,” the researchers examined the association between magnesium trajectories over time, brain matter, and white matter lesions.
They also examined the association between magnesium and several different blood pressure measures (mean arterial pressure, systolic blood pressure, diastolic blood pressure, and pulse pressure).
Since cardiovascular health, neurodegeneration, and brain shrinkage patterns differ between men and women, the researchers stratified their analyses by sex.
Brain volume differences
The researchers analyzed the dietary magnesium intake of 6,001 individuals (mean age, 55.3 years) selected from the UK Biobank – a prospective cohort study of participants aged 37-73 at baseline, who were assessed between 2005 and 2023.
For the current study, only participants with baseline DBP and SBP measurements and structural MRI scans were included. Participants were also required to be free of neurologic disorders and to have an available record of dietary magnesium intake.
Covariates included age, sex, education, health conditions, smoking status, body mass index, amount of physical activity, smoking status, and alcohol intake.
Over a 16-month period, participants completed an online questionnaire five times. Their responses were used to calculate daily magnesium intake. Foods of particular interest included leafy green vegetables, legumes, nuts, seeds, and whole grains, all of which are magnesium rich.
They used latent class analysis (LCA) to “identify mutually exclusive subgroup (classes) of magnesium intake trajectory separately for men and women.”
Men had a slightly higher prevalence of BP medication and diabetes, compared with women, and postmenopausal women had a higher prevalence of BP medication and diabetes, compared with premenopausal women.
Compared with lower baseline magnesium intake, higher baseline dietary intake of magnesium was associated with larger brain volumes in several regions in both men and women.
The latent class analysis identified three classes of magnesium intake:
In women in particular, the “high-decreasing” trajectory was significantly associated with larger brain volumes, compared with the “normal-stable” trajectory, while the “low-increasing” trajectory was associated with smaller brain volumes.
Even an increase of 1 mg of magnesium per day (above 350 mg/day) made a difference in brain volume, especially in women. The changes associated with every 1-mg increase are found in the table below:
Associations between magnesium and BP measures were “mostly nonsignificant,” the researchers say, and the neuroprotective effect of higher magnesium intake in the high-decreasing trajectory was greater in postmenopausal versus premenopausal women.
“Our models indicate that compared to somebody with a normal magnesium intake (~350 mg per day), somebody in the top quartile of magnesium intake (≥ 550 mg per day) would be predicted to have a ~0.20% larger GM and ~0.46% larger RHC,” the authors summarize.
“In a population with an average age of 55 years, this effect corresponds to ~1 year of typical aging,” they note. “In other words, if this effect is generalizable to other populations, a 41% increase in magnesium intake may lead to significantly better brain health.”
Although the exact mechanisms underlying magnesium’s protective effects are “not yet clearly understood, there’s considerable evidence that magnesium levels are related to better cardiovascular health. Magnesium supplementation has been found to decrease blood pressure – and high blood pressure is a well-established risk factor for dementia,” said Ms. Alateeq.
Association, not causation
Yuko Hara, PhD, director of Aging and Prevention, Alzheimer’s Drug Discovery Foundation, noted that the study is observational and therefore shows an association, not causation.
“People eating a high-magnesium diet may also be eating a brain-healthy diet and getting high levels of nutrients/minerals other than magnesium alone,” suggested Dr. Hara, who was not involved with the study.
She noted that many foods are good sources of magnesium, including spinach, almonds, cashews, legumes, yogurt, brown rice, and avocados.
“Eating a brain-healthy diet (for example, the Mediterranean diet) is one of the Seven Steps to Protect Your Cognitive Vitality that ADDF’s Cognitive Vitality promotes,” she said.
Open Access funding was enabled and organized by the Council of Australian University Librarians and its Member Institutions. Ms. Alateeq, her co-authors, and Dr. Hara declare no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Investigators studied more than 6,000 cognitively healthy individuals, aged 40-73, and found that those who consumed more than 550 mg of magnesium daily had a brain age approximately 1 year younger by age 55 years, compared with a person who consumed a normal magnesium intake (~360 mg per day).
“This research highlights the potential benefits of a diet high in magnesium and the role it plays in promoting good brain health,” lead author Khawlah Alateeq, a PhD candidate in neuroscience at Australian National University’s National Centre for Epidemiology and Population Health, said in an interview.
Clinicians “can use [the findings] to counsel patients on the benefits of increasing magnesium intake through a healthy diet and monitoring magnesium levels to prevent deficiencies,” she stated.
The study was published online in the European Journal of Nutrition.
Promising target
The researchers were motivated to conduct the study because of “the growing concern over the increasing prevalence of dementia,” Ms. Alateeq said.
“Since there is no cure for dementia, and the development of pharmacological treatment for dementia has been unsuccessful over the last 30 years, prevention has been suggested as an effective approach to address the issue,” she added.
Nutrition, Ms. Alateeq said, is a “modifiable risk factor that can influence brain health and is highly amenable to scalable and cost-effective interventions.” It represents “a promising target” for risk reduction at a population level.
Previous research shows individuals with lower magnesium levels are at higher risk for AD, while those with higher dietary magnesium intake may be at lower risk of progressing from normal aging to cognitive impairment.
Most previous studies, however, included participants older than age 60 years, and it’s “unclear when the neuroprotective effects of dietary magnesium become detectable,” the researchers note.
Moreover, dietary patterns change and fluctuate, potentially leading to changes in magnesium intake over time. These changes may have as much impact as absolute magnesium at any point in time.
In light of the “current lack of understanding of when and to what extent dietary magnesium exerts its protective effects on the brain,” the researchers examined the association between magnesium trajectories over time, brain matter, and white matter lesions.
They also examined the association between magnesium and several different blood pressure measures (mean arterial pressure, systolic blood pressure, diastolic blood pressure, and pulse pressure).
Since cardiovascular health, neurodegeneration, and brain shrinkage patterns differ between men and women, the researchers stratified their analyses by sex.
Brain volume differences
The researchers analyzed the dietary magnesium intake of 6,001 individuals (mean age, 55.3 years) selected from the UK Biobank – a prospective cohort study of participants aged 37-73 at baseline, who were assessed between 2005 and 2023.
For the current study, only participants with baseline DBP and SBP measurements and structural MRI scans were included. Participants were also required to be free of neurologic disorders and to have an available record of dietary magnesium intake.
Covariates included age, sex, education, health conditions, smoking status, body mass index, amount of physical activity, smoking status, and alcohol intake.
Over a 16-month period, participants completed an online questionnaire five times. Their responses were used to calculate daily magnesium intake. Foods of particular interest included leafy green vegetables, legumes, nuts, seeds, and whole grains, all of which are magnesium rich.
They used latent class analysis (LCA) to “identify mutually exclusive subgroup (classes) of magnesium intake trajectory separately for men and women.”
Men had a slightly higher prevalence of BP medication and diabetes, compared with women, and postmenopausal women had a higher prevalence of BP medication and diabetes, compared with premenopausal women.
Compared with lower baseline magnesium intake, higher baseline dietary intake of magnesium was associated with larger brain volumes in several regions in both men and women.
The latent class analysis identified three classes of magnesium intake:
In women in particular, the “high-decreasing” trajectory was significantly associated with larger brain volumes, compared with the “normal-stable” trajectory, while the “low-increasing” trajectory was associated with smaller brain volumes.
Even an increase of 1 mg of magnesium per day (above 350 mg/day) made a difference in brain volume, especially in women. The changes associated with every 1-mg increase are found in the table below:
Associations between magnesium and BP measures were “mostly nonsignificant,” the researchers say, and the neuroprotective effect of higher magnesium intake in the high-decreasing trajectory was greater in postmenopausal versus premenopausal women.
“Our models indicate that compared to somebody with a normal magnesium intake (~350 mg per day), somebody in the top quartile of magnesium intake (≥ 550 mg per day) would be predicted to have a ~0.20% larger GM and ~0.46% larger RHC,” the authors summarize.
“In a population with an average age of 55 years, this effect corresponds to ~1 year of typical aging,” they note. “In other words, if this effect is generalizable to other populations, a 41% increase in magnesium intake may lead to significantly better brain health.”
Although the exact mechanisms underlying magnesium’s protective effects are “not yet clearly understood, there’s considerable evidence that magnesium levels are related to better cardiovascular health. Magnesium supplementation has been found to decrease blood pressure – and high blood pressure is a well-established risk factor for dementia,” said Ms. Alateeq.
Association, not causation
Yuko Hara, PhD, director of Aging and Prevention, Alzheimer’s Drug Discovery Foundation, noted that the study is observational and therefore shows an association, not causation.
“People eating a high-magnesium diet may also be eating a brain-healthy diet and getting high levels of nutrients/minerals other than magnesium alone,” suggested Dr. Hara, who was not involved with the study.
She noted that many foods are good sources of magnesium, including spinach, almonds, cashews, legumes, yogurt, brown rice, and avocados.
“Eating a brain-healthy diet (for example, the Mediterranean diet) is one of the Seven Steps to Protect Your Cognitive Vitality that ADDF’s Cognitive Vitality promotes,” she said.
Open Access funding was enabled and organized by the Council of Australian University Librarians and its Member Institutions. Ms. Alateeq, her co-authors, and Dr. Hara declare no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM EUROPEAN JOURNAL OF NUTRITION