User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
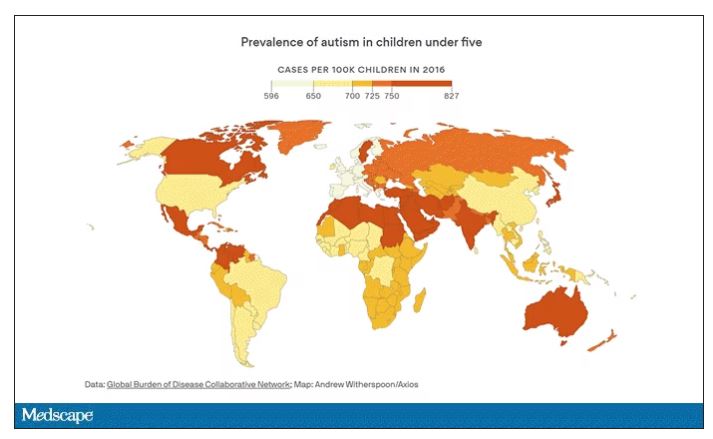
Autism: Is it in the water?
This transcript has been edited for clarity.
Few diseases have stymied explanation like autism spectrum disorder (ASD). We know that the prevalence has been increasing dramatically, but we aren’t quite sure whether that is because of more screening and awareness or more fundamental changes. We know that much of the risk appears to be genetic, but there may be 1,000 genes involved in the syndrome. We know that certain environmental exposures, like pollution, might increase the risk – perhaps on a susceptible genetic background – but we’re not really sure which exposures are most harmful.
So, the search continues, across all domains of inquiry from cell culture to large epidemiologic analyses. And this week, a new player enters the field, and, as they say, it’s something in the water.
We’re talking about this paper, by Zeyan Liew and colleagues, appearing in JAMA Pediatrics.
Using the incredibly robust health data infrastructure in Denmark, the researchers were able to identify 8,842 children born between 2000 and 2013 with ASD and matched each one to five control kids of the same sex and age without autism.
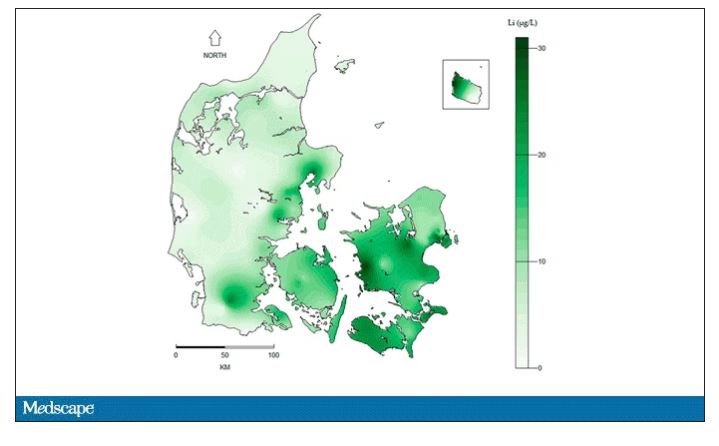
They then mapped the location the mothers of these kids lived while they were pregnant – down to 5 meters resolution, actually – to groundwater lithium levels.
Once that was done, the analysis was straightforward. Would moms who were pregnant in areas with higher groundwater lithium levels be more likely to have kids with ASD?
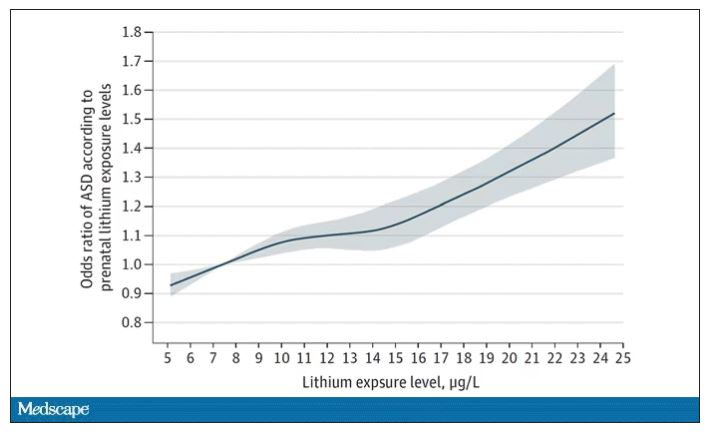
The results show a rather steady and consistent association between higher lithium levels in groundwater and the prevalence of ASD in children.
We’re not talking huge numbers, but moms who lived in the areas of the highest quartile of lithium were about 46% more likely to have a child with ASD. That’s a relative risk, of course – this would be like an increase from 1 in 100 kids to 1.5 in 100 kids. But still, it’s intriguing.
But the case is far from closed here.
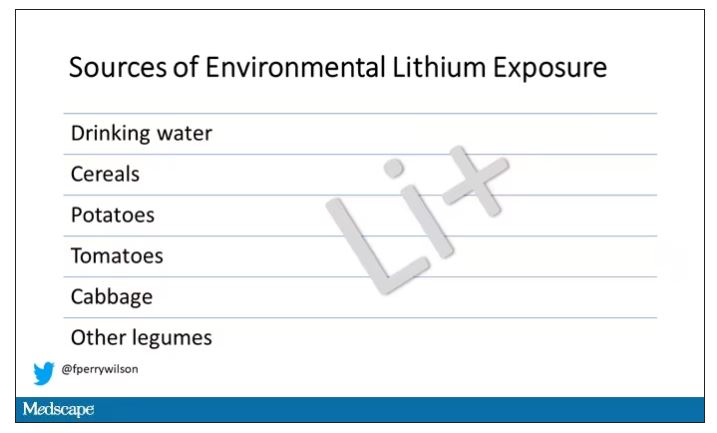
Groundwater concentration of lithium and the amount of lithium a pregnant mother ingests are not the same thing. It does turn out that virtually all drinking water in Denmark comes from groundwater sources – but not all lithium comes from drinking water. There are plenty of dietary sources of lithium as well. And, of course, there is medical lithium, but we’ll get to that in a second.
First, let’s talk about those lithium measurements. They were taken in 2013 – after all these kids were born. The authors acknowledge this limitation but show a high correlation between measured levels in 2013 and earlier measured levels from prior studies, suggesting that lithium levels in a given area are quite constant over time. That’s great – but if lithium levels are constant over time, this study does nothing to shed light on why autism diagnoses seem to be increasing.
Let’s put some numbers to the lithium concentrations the authors examined. The average was about 12 mcg/L.
As a reminder, a standard therapeutic dose of lithium used for bipolar disorder is like 600 mg. That means you’d need to drink more than 2,500 of those 5-gallon jugs that sit on your water cooler, per day, to approximate the dose you’d get from a lithium tablet. Of course, small doses can still cause toxicity – but I wanted to put this in perspective.
Also, we have some data on pregnant women who take medical lithium. An analysis of nine studies showed that first-trimester lithium use may be associated with congenital malformations – particularly some specific heart malformations – and some birth complications. But three of four separate studies looking at longer-term neurodevelopmental outcomes did not find any effect on development, attainment of milestones, or IQ. One study of 15 kids exposed to medical lithium in utero did note minor neurologic dysfunction in one child and a low verbal IQ in another – but that’s a very small study.
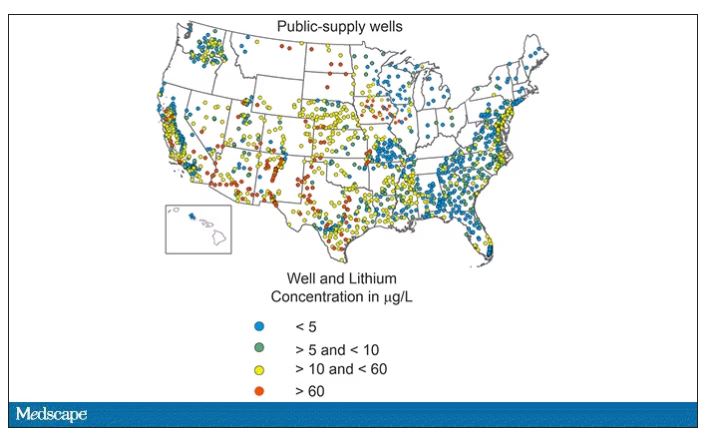
Of course, lithium levels vary around the world as well. The U.S. Geological Survey examined lithium content in groundwater in the United States, as you can see here.
Our numbers are pretty similar to Denmark’s – in the 0-60 range. But an area in the Argentine Andes has levels as high as 1,600 mcg/L. A study of 194 babies from that area found higher lithium exposure was associated with lower fetal size, but I haven’t seen follow-up on neurodevelopmental outcomes.
The point is that there is a lot of variability here. It would be really interesting to map groundwater lithium levels to autism rates around the world. As a teaser, I will point out that, if you look at worldwide autism rates, you may be able to convince yourself that they are higher in more arid climates, and arid climates tend to have more groundwater lithium. But I’m really reaching here. More work needs to be done.
And I hope it is done quickly. Lithium is in the midst of becoming a very important commodity thanks to the shift to electric vehicles. While we can hope that recycling will claim most of those batteries at the end of their life, some will escape reclamation and potentially put more lithium into the drinking water. I’d like to know how risky that is before it happens.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale’s Clinical and Translational Research Accelerator. He has disclosed no relevant financial relationships. His science communication work can be found in the Huffington Post, on NPR, and here on Medscape. He tweets @fperrywilson and his new book, “How Medicine Works and When It Doesn’t”, is available now.
A version of this article originally appeared on Medscape.com.
This transcript has been edited for clarity.
Few diseases have stymied explanation like autism spectrum disorder (ASD). We know that the prevalence has been increasing dramatically, but we aren’t quite sure whether that is because of more screening and awareness or more fundamental changes. We know that much of the risk appears to be genetic, but there may be 1,000 genes involved in the syndrome. We know that certain environmental exposures, like pollution, might increase the risk – perhaps on a susceptible genetic background – but we’re not really sure which exposures are most harmful.
So, the search continues, across all domains of inquiry from cell culture to large epidemiologic analyses. And this week, a new player enters the field, and, as they say, it’s something in the water.
We’re talking about this paper, by Zeyan Liew and colleagues, appearing in JAMA Pediatrics.
Using the incredibly robust health data infrastructure in Denmark, the researchers were able to identify 8,842 children born between 2000 and 2013 with ASD and matched each one to five control kids of the same sex and age without autism.
They then mapped the location the mothers of these kids lived while they were pregnant – down to 5 meters resolution, actually – to groundwater lithium levels.
Once that was done, the analysis was straightforward. Would moms who were pregnant in areas with higher groundwater lithium levels be more likely to have kids with ASD?
The results show a rather steady and consistent association between higher lithium levels in groundwater and the prevalence of ASD in children.
We’re not talking huge numbers, but moms who lived in the areas of the highest quartile of lithium were about 46% more likely to have a child with ASD. That’s a relative risk, of course – this would be like an increase from 1 in 100 kids to 1.5 in 100 kids. But still, it’s intriguing.
But the case is far from closed here.
Groundwater concentration of lithium and the amount of lithium a pregnant mother ingests are not the same thing. It does turn out that virtually all drinking water in Denmark comes from groundwater sources – but not all lithium comes from drinking water. There are plenty of dietary sources of lithium as well. And, of course, there is medical lithium, but we’ll get to that in a second.
First, let’s talk about those lithium measurements. They were taken in 2013 – after all these kids were born. The authors acknowledge this limitation but show a high correlation between measured levels in 2013 and earlier measured levels from prior studies, suggesting that lithium levels in a given area are quite constant over time. That’s great – but if lithium levels are constant over time, this study does nothing to shed light on why autism diagnoses seem to be increasing.
Let’s put some numbers to the lithium concentrations the authors examined. The average was about 12 mcg/L.
As a reminder, a standard therapeutic dose of lithium used for bipolar disorder is like 600 mg. That means you’d need to drink more than 2,500 of those 5-gallon jugs that sit on your water cooler, per day, to approximate the dose you’d get from a lithium tablet. Of course, small doses can still cause toxicity – but I wanted to put this in perspective.
Also, we have some data on pregnant women who take medical lithium. An analysis of nine studies showed that first-trimester lithium use may be associated with congenital malformations – particularly some specific heart malformations – and some birth complications. But three of four separate studies looking at longer-term neurodevelopmental outcomes did not find any effect on development, attainment of milestones, or IQ. One study of 15 kids exposed to medical lithium in utero did note minor neurologic dysfunction in one child and a low verbal IQ in another – but that’s a very small study.
Of course, lithium levels vary around the world as well. The U.S. Geological Survey examined lithium content in groundwater in the United States, as you can see here.
Our numbers are pretty similar to Denmark’s – in the 0-60 range. But an area in the Argentine Andes has levels as high as 1,600 mcg/L. A study of 194 babies from that area found higher lithium exposure was associated with lower fetal size, but I haven’t seen follow-up on neurodevelopmental outcomes.
The point is that there is a lot of variability here. It would be really interesting to map groundwater lithium levels to autism rates around the world. As a teaser, I will point out that, if you look at worldwide autism rates, you may be able to convince yourself that they are higher in more arid climates, and arid climates tend to have more groundwater lithium. But I’m really reaching here. More work needs to be done.
And I hope it is done quickly. Lithium is in the midst of becoming a very important commodity thanks to the shift to electric vehicles. While we can hope that recycling will claim most of those batteries at the end of their life, some will escape reclamation and potentially put more lithium into the drinking water. I’d like to know how risky that is before it happens.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale’s Clinical and Translational Research Accelerator. He has disclosed no relevant financial relationships. His science communication work can be found in the Huffington Post, on NPR, and here on Medscape. He tweets @fperrywilson and his new book, “How Medicine Works and When It Doesn’t”, is available now.
A version of this article originally appeared on Medscape.com.
This transcript has been edited for clarity.
Few diseases have stymied explanation like autism spectrum disorder (ASD). We know that the prevalence has been increasing dramatically, but we aren’t quite sure whether that is because of more screening and awareness or more fundamental changes. We know that much of the risk appears to be genetic, but there may be 1,000 genes involved in the syndrome. We know that certain environmental exposures, like pollution, might increase the risk – perhaps on a susceptible genetic background – but we’re not really sure which exposures are most harmful.
So, the search continues, across all domains of inquiry from cell culture to large epidemiologic analyses. And this week, a new player enters the field, and, as they say, it’s something in the water.
We’re talking about this paper, by Zeyan Liew and colleagues, appearing in JAMA Pediatrics.
Using the incredibly robust health data infrastructure in Denmark, the researchers were able to identify 8,842 children born between 2000 and 2013 with ASD and matched each one to five control kids of the same sex and age without autism.
They then mapped the location the mothers of these kids lived while they were pregnant – down to 5 meters resolution, actually – to groundwater lithium levels.
Once that was done, the analysis was straightforward. Would moms who were pregnant in areas with higher groundwater lithium levels be more likely to have kids with ASD?
The results show a rather steady and consistent association between higher lithium levels in groundwater and the prevalence of ASD in children.
We’re not talking huge numbers, but moms who lived in the areas of the highest quartile of lithium were about 46% more likely to have a child with ASD. That’s a relative risk, of course – this would be like an increase from 1 in 100 kids to 1.5 in 100 kids. But still, it’s intriguing.
But the case is far from closed here.
Groundwater concentration of lithium and the amount of lithium a pregnant mother ingests are not the same thing. It does turn out that virtually all drinking water in Denmark comes from groundwater sources – but not all lithium comes from drinking water. There are plenty of dietary sources of lithium as well. And, of course, there is medical lithium, but we’ll get to that in a second.
First, let’s talk about those lithium measurements. They were taken in 2013 – after all these kids were born. The authors acknowledge this limitation but show a high correlation between measured levels in 2013 and earlier measured levels from prior studies, suggesting that lithium levels in a given area are quite constant over time. That’s great – but if lithium levels are constant over time, this study does nothing to shed light on why autism diagnoses seem to be increasing.
Let’s put some numbers to the lithium concentrations the authors examined. The average was about 12 mcg/L.
As a reminder, a standard therapeutic dose of lithium used for bipolar disorder is like 600 mg. That means you’d need to drink more than 2,500 of those 5-gallon jugs that sit on your water cooler, per day, to approximate the dose you’d get from a lithium tablet. Of course, small doses can still cause toxicity – but I wanted to put this in perspective.
Also, we have some data on pregnant women who take medical lithium. An analysis of nine studies showed that first-trimester lithium use may be associated with congenital malformations – particularly some specific heart malformations – and some birth complications. But three of four separate studies looking at longer-term neurodevelopmental outcomes did not find any effect on development, attainment of milestones, or IQ. One study of 15 kids exposed to medical lithium in utero did note minor neurologic dysfunction in one child and a low verbal IQ in another – but that’s a very small study.
Of course, lithium levels vary around the world as well. The U.S. Geological Survey examined lithium content in groundwater in the United States, as you can see here.
Our numbers are pretty similar to Denmark’s – in the 0-60 range. But an area in the Argentine Andes has levels as high as 1,600 mcg/L. A study of 194 babies from that area found higher lithium exposure was associated with lower fetal size, but I haven’t seen follow-up on neurodevelopmental outcomes.
The point is that there is a lot of variability here. It would be really interesting to map groundwater lithium levels to autism rates around the world. As a teaser, I will point out that, if you look at worldwide autism rates, you may be able to convince yourself that they are higher in more arid climates, and arid climates tend to have more groundwater lithium. But I’m really reaching here. More work needs to be done.
And I hope it is done quickly. Lithium is in the midst of becoming a very important commodity thanks to the shift to electric vehicles. While we can hope that recycling will claim most of those batteries at the end of their life, some will escape reclamation and potentially put more lithium into the drinking water. I’d like to know how risky that is before it happens.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale’s Clinical and Translational Research Accelerator. He has disclosed no relevant financial relationships. His science communication work can be found in the Huffington Post, on NPR, and here on Medscape. He tweets @fperrywilson and his new book, “How Medicine Works and When It Doesn’t”, is available now.
A version of this article originally appeared on Medscape.com.
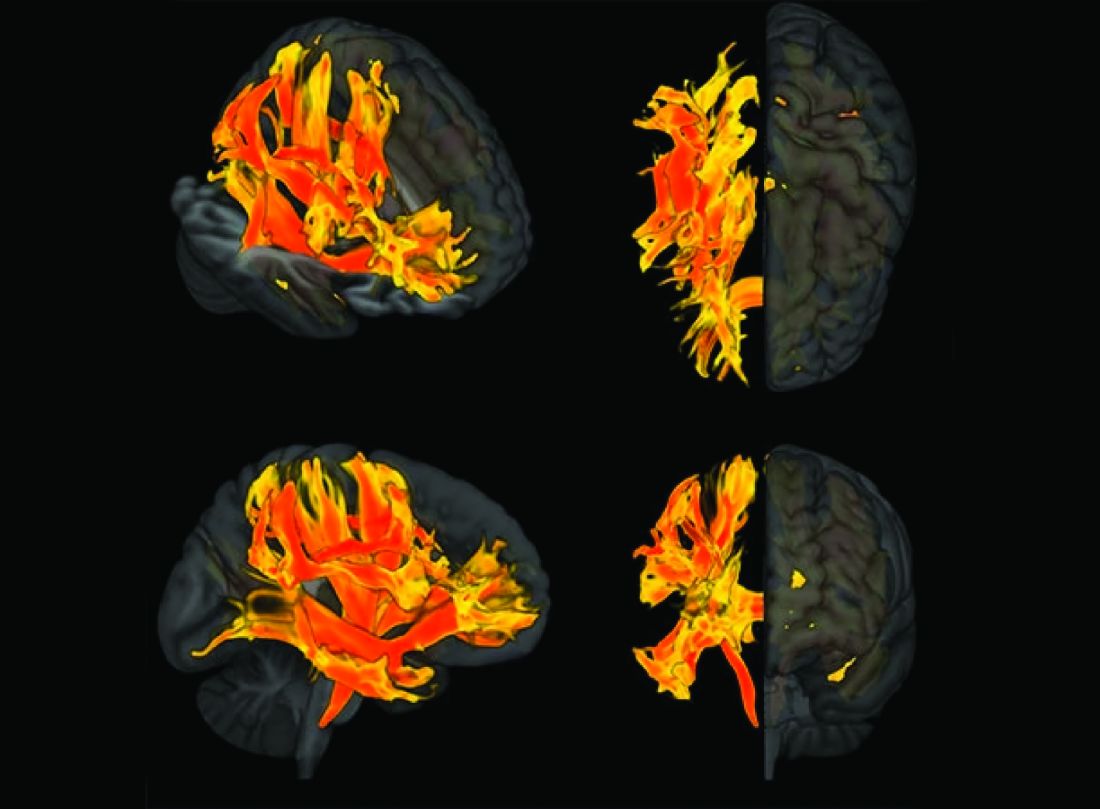
Specific brain damage links hypertension to cognitive impairment
Researchers have identified specific regions of the brain that appear to be damaged by high blood pressure. The finding may explain the link between hypertension and cognitive impairment.
They used genetic information from genome-wide association studies (GWASs) and MRI scans of the brain to study the relationship between hypertension, changes in brain structures, and cognitive impairment. Using Mendelian randomization techniques, they identified nine brain structures related to cognitive impairment that are affected by blood pressure.
“We knew before that raised blood pressure was related to changes in the brain, but our research has narrowed down the changes to those that appear to be potentially causally related to cognitive impairment,” senior author Tomasz Guzik, professor of cardiovascular medicine, at the University of Edinburgh and of the Jagiellonian University, Krakow, Poland, told this news organization.
“Our study confirms a potentially causal relationship between raised blood pressure and cognitive impairment, emphasizing the importance of preventing and treating hypertension,” Prof. Guzik noted.
“But it also identifies the brain culprits of this relationship,” he added.
In the future, it may be possible to assess these nine brain structures in people with high blood pressure to identify those at increased risk of developing cognitive impairment, he said. “These patients may need more intensive care for their blood pressure. We can also investigate these brain structures for potential signaling pathways and molecular changes to see if we can find new targets for treatment to prevent cognitive impairment.”
For this report, the investigators married together different research datasets to identify brain structures potentially responsible for the effects of blood pressure on cognitive function, using results from previous GWASs and observational data from 39,000 people in the UK Biobank registry for whom brain MRI data were available.
First, they mapped brain structures potentially influenced by blood pressure in midlife using MRI scans from people in the UK Biobank registry. Then they examined the relationship between blood pressure and cognitive function in the UK Biobank registry. Next, of the brain structures affected by blood pressure, they identified those that are causally linked to cognitive impairment.
This was possible thanks to genetic markers coding for increased blood pressure, brain structure imaging phenotypes, and those coding for cognitive impairment that could be used in Mendelian randomization studies.
“We looked at 3935 brain magnetic resonance imaging–derived phenotypes in the brain and cognitive function defined by fluid intelligence score to identify genetically predicted causal relationships,” Prof. Guzik said.
They identified 200 brain structures that were causally affected by systolic blood pressure. Of these, nine were also causally related to cognitive impairment. The results were validated in a second prospective cohort of patients with hypertension.
“Some of these structures, including putamen and the white matter regions spanning between the anterior corona radiata, anterior thalamic radiation, and anterior limb of the internal capsule, may represent the target brain regions at which systolic blood pressure acts on cognitive function,” the authors comment.
In an accompanying editorial, Ernesto Schiffrin, MD, and James Engert, PhD, McGill University, Montreal, say that further mechanistic studies of the effects of blood pressure on cognitive function are required to determine precise causal pathways and the roles of relevant brain regions.
“Eventually, biomarkers could be developed to inform antihypertensive trials. Whether clinical trials targeting the specific brain structures will be feasible or if specific antihypertensives could be found that target specific structures remains to be demonstrated,” they write.
“Thus, these new studies could lead to an understanding of the signaling pathways that explain how these structures relate vascular damage to cognitive impairment in hypertension, and contribute to the development of novel interventions to more successfully address the scourge of cognitive decline and dementia in the future,” the editorialists conclude.
The study was funded by the European Research Council, the British Heart Foundation, and the Italian Ministry of Health.
A version of this article first appeared on Medscape.com.
Researchers have identified specific regions of the brain that appear to be damaged by high blood pressure. The finding may explain the link between hypertension and cognitive impairment.
They used genetic information from genome-wide association studies (GWASs) and MRI scans of the brain to study the relationship between hypertension, changes in brain structures, and cognitive impairment. Using Mendelian randomization techniques, they identified nine brain structures related to cognitive impairment that are affected by blood pressure.
“We knew before that raised blood pressure was related to changes in the brain, but our research has narrowed down the changes to those that appear to be potentially causally related to cognitive impairment,” senior author Tomasz Guzik, professor of cardiovascular medicine, at the University of Edinburgh and of the Jagiellonian University, Krakow, Poland, told this news organization.
“Our study confirms a potentially causal relationship between raised blood pressure and cognitive impairment, emphasizing the importance of preventing and treating hypertension,” Prof. Guzik noted.
“But it also identifies the brain culprits of this relationship,” he added.
In the future, it may be possible to assess these nine brain structures in people with high blood pressure to identify those at increased risk of developing cognitive impairment, he said. “These patients may need more intensive care for their blood pressure. We can also investigate these brain structures for potential signaling pathways and molecular changes to see if we can find new targets for treatment to prevent cognitive impairment.”
For this report, the investigators married together different research datasets to identify brain structures potentially responsible for the effects of blood pressure on cognitive function, using results from previous GWASs and observational data from 39,000 people in the UK Biobank registry for whom brain MRI data were available.
First, they mapped brain structures potentially influenced by blood pressure in midlife using MRI scans from people in the UK Biobank registry. Then they examined the relationship between blood pressure and cognitive function in the UK Biobank registry. Next, of the brain structures affected by blood pressure, they identified those that are causally linked to cognitive impairment.
This was possible thanks to genetic markers coding for increased blood pressure, brain structure imaging phenotypes, and those coding for cognitive impairment that could be used in Mendelian randomization studies.
“We looked at 3935 brain magnetic resonance imaging–derived phenotypes in the brain and cognitive function defined by fluid intelligence score to identify genetically predicted causal relationships,” Prof. Guzik said.
They identified 200 brain structures that were causally affected by systolic blood pressure. Of these, nine were also causally related to cognitive impairment. The results were validated in a second prospective cohort of patients with hypertension.
“Some of these structures, including putamen and the white matter regions spanning between the anterior corona radiata, anterior thalamic radiation, and anterior limb of the internal capsule, may represent the target brain regions at which systolic blood pressure acts on cognitive function,” the authors comment.
In an accompanying editorial, Ernesto Schiffrin, MD, and James Engert, PhD, McGill University, Montreal, say that further mechanistic studies of the effects of blood pressure on cognitive function are required to determine precise causal pathways and the roles of relevant brain regions.
“Eventually, biomarkers could be developed to inform antihypertensive trials. Whether clinical trials targeting the specific brain structures will be feasible or if specific antihypertensives could be found that target specific structures remains to be demonstrated,” they write.
“Thus, these new studies could lead to an understanding of the signaling pathways that explain how these structures relate vascular damage to cognitive impairment in hypertension, and contribute to the development of novel interventions to more successfully address the scourge of cognitive decline and dementia in the future,” the editorialists conclude.
The study was funded by the European Research Council, the British Heart Foundation, and the Italian Ministry of Health.
A version of this article first appeared on Medscape.com.
Researchers have identified specific regions of the brain that appear to be damaged by high blood pressure. The finding may explain the link between hypertension and cognitive impairment.
They used genetic information from genome-wide association studies (GWASs) and MRI scans of the brain to study the relationship between hypertension, changes in brain structures, and cognitive impairment. Using Mendelian randomization techniques, they identified nine brain structures related to cognitive impairment that are affected by blood pressure.
“We knew before that raised blood pressure was related to changes in the brain, but our research has narrowed down the changes to those that appear to be potentially causally related to cognitive impairment,” senior author Tomasz Guzik, professor of cardiovascular medicine, at the University of Edinburgh and of the Jagiellonian University, Krakow, Poland, told this news organization.
“Our study confirms a potentially causal relationship between raised blood pressure and cognitive impairment, emphasizing the importance of preventing and treating hypertension,” Prof. Guzik noted.
“But it also identifies the brain culprits of this relationship,” he added.
In the future, it may be possible to assess these nine brain structures in people with high blood pressure to identify those at increased risk of developing cognitive impairment, he said. “These patients may need more intensive care for their blood pressure. We can also investigate these brain structures for potential signaling pathways and molecular changes to see if we can find new targets for treatment to prevent cognitive impairment.”
For this report, the investigators married together different research datasets to identify brain structures potentially responsible for the effects of blood pressure on cognitive function, using results from previous GWASs and observational data from 39,000 people in the UK Biobank registry for whom brain MRI data were available.
First, they mapped brain structures potentially influenced by blood pressure in midlife using MRI scans from people in the UK Biobank registry. Then they examined the relationship between blood pressure and cognitive function in the UK Biobank registry. Next, of the brain structures affected by blood pressure, they identified those that are causally linked to cognitive impairment.
This was possible thanks to genetic markers coding for increased blood pressure, brain structure imaging phenotypes, and those coding for cognitive impairment that could be used in Mendelian randomization studies.
“We looked at 3935 brain magnetic resonance imaging–derived phenotypes in the brain and cognitive function defined by fluid intelligence score to identify genetically predicted causal relationships,” Prof. Guzik said.
They identified 200 brain structures that were causally affected by systolic blood pressure. Of these, nine were also causally related to cognitive impairment. The results were validated in a second prospective cohort of patients with hypertension.
“Some of these structures, including putamen and the white matter regions spanning between the anterior corona radiata, anterior thalamic radiation, and anterior limb of the internal capsule, may represent the target brain regions at which systolic blood pressure acts on cognitive function,” the authors comment.
In an accompanying editorial, Ernesto Schiffrin, MD, and James Engert, PhD, McGill University, Montreal, say that further mechanistic studies of the effects of blood pressure on cognitive function are required to determine precise causal pathways and the roles of relevant brain regions.
“Eventually, biomarkers could be developed to inform antihypertensive trials. Whether clinical trials targeting the specific brain structures will be feasible or if specific antihypertensives could be found that target specific structures remains to be demonstrated,” they write.
“Thus, these new studies could lead to an understanding of the signaling pathways that explain how these structures relate vascular damage to cognitive impairment in hypertension, and contribute to the development of novel interventions to more successfully address the scourge of cognitive decline and dementia in the future,” the editorialists conclude.
The study was funded by the European Research Council, the British Heart Foundation, and the Italian Ministry of Health.
A version of this article first appeared on Medscape.com.
Cancer risk elevated after stroke in younger people
In young people, stroke might be the first manifestation of an underlying cancer, according to the investigators, led by Jamie Verhoeven, MD, PhD, with the department of neurology, Radboud University Medical Centre, Nijmegen, the Netherlands.
The new study can be viewed as a “stepping stone for future studies investigating the usefulness of screening for cancer after stroke,” the researchers say.
The study was published online in JAMA Network Open.
Currently, the diagnostic workup for young people with stroke includes searching for rare clotting disorders, although screening for cancer is not regularly performed.
Some research suggests that stroke and cancer are linked, but the literature is limited. In prior studies among people of all ages, cancer incidence after stroke has been variable – from 1% to 5% at 1 year and from 11% to 30% after 10 years.
To the team’s knowledge, only two studies have described the incidence of cancer after stroke among younger patients. One put the risk at 0.5% for people aged 18-50 years in the first year after stroke; the other described a cumulative risk of 17.3% in the 10 years after stroke for patients aged 18-55 years.
Using Dutch data, Dr. Verhoeven and colleagues identified 27,616 young stroke patients (age, 15-49 years; median age, 45 years) and 362,782 older stroke patients (median age, 76 years).
The cumulative incidence of any new cancer at 10 years was 3.7% among the younger stroke patients and 8.5% among the older stroke patients.
The incidence of a new cancer after stroke among younger patients was higher among women than men, while the opposite was true for older stroke patients.
Compared with the general population, younger stroke patients had a more than 2.5-fold greater likelihood of being diagnosed with a new cancer in the first year after ischemic stroke (standardized incidence ratio, 2.6). The risk was highest for lung cancer (SIR, 6.9), followed by hematologic cancers (SIR, 5.2).
Compared with the general population, younger stroke patients had nearly a 5.5-fold greater likelihood of being diagnosed with a new cancer in the first year after intracerebral hemorrhage (SIR, 5.4), and the risk was highest for hematologic cancers (SIR, 14.2).
In younger patients, the cumulative incidence of any cancer decreased over the years but remained significantly higher for 8 years following a stroke.
For patients aged 50 years or older, the 1-year risk for any new cancer after either ischemic stroke or intracerebral hemorrhage was 1.2 times higher, compared with the general population.
“We typically think of occult cancer as being a cause of stroke in an older population, given that the incidence of cancer increases over time [but] what this study shows is that we probably do need to consider occult cancer as an underlying cause of stroke even in a younger population,” said Laura Gioia, MD, stroke neurologist at the University of Montreal, who was not involved in the research.
Dr. Verhoeven and colleagues conclude that their finding supports the hypothesis of a causal link between cancer and stroke. Given the timing between stroke and cancer diagnosis, cancer may have been present when the stroke occurred and possibly played a role in causing it, the authors note. However, conclusions on causal mechanisms cannot be drawn from the current study.
The question of whether young stroke patients should be screened for cancer is a tough one, Dr. Gioia noted. “Cancer represents a small percentage of causes of stroke. That means you would have to screen a lot of people with a benefit that is still uncertain for the moment,” Dr. Gioia said in an interview.
“I think we need to keep cancer in mind as a cause of stroke in our young patients, and that should probably guide our history-taking with the patient and consider imaging when it’s appropriate and when we think that there could be an underlying occult cancer,” Dr. Gioia suggested.
The study was funded in part through unrestricted funding by Stryker, Medtronic, and Cerenovus. Dr. Verhoeven and Dr. Gioia have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In young people, stroke might be the first manifestation of an underlying cancer, according to the investigators, led by Jamie Verhoeven, MD, PhD, with the department of neurology, Radboud University Medical Centre, Nijmegen, the Netherlands.
The new study can be viewed as a “stepping stone for future studies investigating the usefulness of screening for cancer after stroke,” the researchers say.
The study was published online in JAMA Network Open.
Currently, the diagnostic workup for young people with stroke includes searching for rare clotting disorders, although screening for cancer is not regularly performed.
Some research suggests that stroke and cancer are linked, but the literature is limited. In prior studies among people of all ages, cancer incidence after stroke has been variable – from 1% to 5% at 1 year and from 11% to 30% after 10 years.
To the team’s knowledge, only two studies have described the incidence of cancer after stroke among younger patients. One put the risk at 0.5% for people aged 18-50 years in the first year after stroke; the other described a cumulative risk of 17.3% in the 10 years after stroke for patients aged 18-55 years.
Using Dutch data, Dr. Verhoeven and colleagues identified 27,616 young stroke patients (age, 15-49 years; median age, 45 years) and 362,782 older stroke patients (median age, 76 years).
The cumulative incidence of any new cancer at 10 years was 3.7% among the younger stroke patients and 8.5% among the older stroke patients.
The incidence of a new cancer after stroke among younger patients was higher among women than men, while the opposite was true for older stroke patients.
Compared with the general population, younger stroke patients had a more than 2.5-fold greater likelihood of being diagnosed with a new cancer in the first year after ischemic stroke (standardized incidence ratio, 2.6). The risk was highest for lung cancer (SIR, 6.9), followed by hematologic cancers (SIR, 5.2).
Compared with the general population, younger stroke patients had nearly a 5.5-fold greater likelihood of being diagnosed with a new cancer in the first year after intracerebral hemorrhage (SIR, 5.4), and the risk was highest for hematologic cancers (SIR, 14.2).
In younger patients, the cumulative incidence of any cancer decreased over the years but remained significantly higher for 8 years following a stroke.
For patients aged 50 years or older, the 1-year risk for any new cancer after either ischemic stroke or intracerebral hemorrhage was 1.2 times higher, compared with the general population.
“We typically think of occult cancer as being a cause of stroke in an older population, given that the incidence of cancer increases over time [but] what this study shows is that we probably do need to consider occult cancer as an underlying cause of stroke even in a younger population,” said Laura Gioia, MD, stroke neurologist at the University of Montreal, who was not involved in the research.
Dr. Verhoeven and colleagues conclude that their finding supports the hypothesis of a causal link between cancer and stroke. Given the timing between stroke and cancer diagnosis, cancer may have been present when the stroke occurred and possibly played a role in causing it, the authors note. However, conclusions on causal mechanisms cannot be drawn from the current study.
The question of whether young stroke patients should be screened for cancer is a tough one, Dr. Gioia noted. “Cancer represents a small percentage of causes of stroke. That means you would have to screen a lot of people with a benefit that is still uncertain for the moment,” Dr. Gioia said in an interview.
“I think we need to keep cancer in mind as a cause of stroke in our young patients, and that should probably guide our history-taking with the patient and consider imaging when it’s appropriate and when we think that there could be an underlying occult cancer,” Dr. Gioia suggested.
The study was funded in part through unrestricted funding by Stryker, Medtronic, and Cerenovus. Dr. Verhoeven and Dr. Gioia have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In young people, stroke might be the first manifestation of an underlying cancer, according to the investigators, led by Jamie Verhoeven, MD, PhD, with the department of neurology, Radboud University Medical Centre, Nijmegen, the Netherlands.
The new study can be viewed as a “stepping stone for future studies investigating the usefulness of screening for cancer after stroke,” the researchers say.
The study was published online in JAMA Network Open.
Currently, the diagnostic workup for young people with stroke includes searching for rare clotting disorders, although screening for cancer is not regularly performed.
Some research suggests that stroke and cancer are linked, but the literature is limited. In prior studies among people of all ages, cancer incidence after stroke has been variable – from 1% to 5% at 1 year and from 11% to 30% after 10 years.
To the team’s knowledge, only two studies have described the incidence of cancer after stroke among younger patients. One put the risk at 0.5% for people aged 18-50 years in the first year after stroke; the other described a cumulative risk of 17.3% in the 10 years after stroke for patients aged 18-55 years.
Using Dutch data, Dr. Verhoeven and colleagues identified 27,616 young stroke patients (age, 15-49 years; median age, 45 years) and 362,782 older stroke patients (median age, 76 years).
The cumulative incidence of any new cancer at 10 years was 3.7% among the younger stroke patients and 8.5% among the older stroke patients.
The incidence of a new cancer after stroke among younger patients was higher among women than men, while the opposite was true for older stroke patients.
Compared with the general population, younger stroke patients had a more than 2.5-fold greater likelihood of being diagnosed with a new cancer in the first year after ischemic stroke (standardized incidence ratio, 2.6). The risk was highest for lung cancer (SIR, 6.9), followed by hematologic cancers (SIR, 5.2).
Compared with the general population, younger stroke patients had nearly a 5.5-fold greater likelihood of being diagnosed with a new cancer in the first year after intracerebral hemorrhage (SIR, 5.4), and the risk was highest for hematologic cancers (SIR, 14.2).
In younger patients, the cumulative incidence of any cancer decreased over the years but remained significantly higher for 8 years following a stroke.
For patients aged 50 years or older, the 1-year risk for any new cancer after either ischemic stroke or intracerebral hemorrhage was 1.2 times higher, compared with the general population.
“We typically think of occult cancer as being a cause of stroke in an older population, given that the incidence of cancer increases over time [but] what this study shows is that we probably do need to consider occult cancer as an underlying cause of stroke even in a younger population,” said Laura Gioia, MD, stroke neurologist at the University of Montreal, who was not involved in the research.
Dr. Verhoeven and colleagues conclude that their finding supports the hypothesis of a causal link between cancer and stroke. Given the timing between stroke and cancer diagnosis, cancer may have been present when the stroke occurred and possibly played a role in causing it, the authors note. However, conclusions on causal mechanisms cannot be drawn from the current study.
The question of whether young stroke patients should be screened for cancer is a tough one, Dr. Gioia noted. “Cancer represents a small percentage of causes of stroke. That means you would have to screen a lot of people with a benefit that is still uncertain for the moment,” Dr. Gioia said in an interview.
“I think we need to keep cancer in mind as a cause of stroke in our young patients, and that should probably guide our history-taking with the patient and consider imaging when it’s appropriate and when we think that there could be an underlying occult cancer,” Dr. Gioia suggested.
The study was funded in part through unrestricted funding by Stryker, Medtronic, and Cerenovus. Dr. Verhoeven and Dr. Gioia have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
Cluster, migraine headache strongly linked to circadian rhythm
A meta-analysis of 16 studies showed a circadian pattern in 71% of cluster headache attacks (3,490 of 4,953), with a clear circadian peak between 9:00 p.m. and 3:00 a.m.
Migraine was also associated with a circadian pattern in 50% of cases (2,698 of 5,385) across eight studies, with a clear circadian trough between 11:00 p.m. and 7:00 a.m.
Seasonal peaks were also evident for cluster headache (spring and autumn) and migraine (April to October).
“In the short term, these findings help us explain the timing to patients – for example, it is possible that a headache at 8 a.m. is due to their internal body clock instead of their pillow, or breakfast food, or morning medications,” lead investigator Mark Burish, MD, PhD, associate professor, department of neurosurgery, at University of Texas Health Houston, told this news organization.
“In the long term, these findings do suggest that medications that target the circadian system could be effective in migraine and headache patients,” Dr. Burish added.
The study was published online in Neurology.
Treatment implications?
Across studies, chronotype was “highly variable” for both cluster headache and migraine, the investigators report.
Cluster headache was associated with lower melatonin and higher cortisol levels, compared with non–cluster headache controls.
On a genetic level, cluster headache was associated with two core circadian genes (CLOCK and REV-ERB–alpha), and five of the nine genes that increase the likelihood of having cluster headache are genes with a circadian pattern of expression.
Migraine headache was associated with lower urinary melatonin levels and with the core circadian genes, CK1-delta and ROR-alpha, and 110 of the 168 genes associated with migraine were clock-controlled genes.
“The data suggest that both of these headache disorders are highly circadian at multiple levels, especially cluster headache,” Dr. Burish said in a release.
“This reinforces the importance of the hypothalamus – the area of the brain that houses the primary biological clock – and its role in cluster headache and migraine. It also raises the question of the genetics of triggers such as sleep changes that are known triggers for migraine and are cues for the body’s circadian rhythm,” Dr. Burish said.
“We hope that future research will look into circadian medications as a new treatment option for migraine and cluster headache patients,” Dr. Burish told this news organization.
Importance of sleep regulation
The authors of an accompanying editorial note that even though the study doesn’t have immediate clinical implications, it offers a better understanding of the way chronobiologic factors may influence treatment.
“At a minimum, interventions known to regulate and improve sleep (e.g., melatonin, cognitive behavioral therapy), and which are safe and straightforward to introduce, may be useful in some individuals susceptible to circadian misalignment or sleep disorders,” write Heidi Sutherland, PhD, and Lyn Griffiths, PhD, with Queensland University of Technology, Brisbane, Australia.
“Treatment of comorbidities (e.g., insomnia) that result in sleep disturbances may also help headache management. Furthermore, chronobiological aspects of any pharmacological interventions should be considered, as some frequently used headache and migraine drugs can modulate circadian cycles and influence the expression of circadian genes (e.g., verapamil), or have sleep-related side effects,” they add.
A limitation of the study was the lack of information on factors that could influence the circadian cycle, such as medications; other disorders, such as bipolar disorder; or circadian rhythm issues, such as night-shift work.
The study was supported by grants from the Japan Society for the Promotion of Science, the National Institutes of Health, The Welch Foundation, and The Will Erwin Headache Research Foundation. Dr. Burish is an unpaid member of the medical advisory board of Clusterbusters, and a site investigator for a cluster headache clinical trial funded by Lundbeck. Dr. Sutherland has received grant funding from the U.S. Migraine Research Foundation, and received institute support from Queensland University of Technology for genetics research. Dr. Griffiths has received grant funding from the Australian NHMRC, U.S. Department of Defense, and the U.S. Migraine Research Foundation, and consultancy funding from TEVA.
A version of this article first appeared on Medscape.com.
A meta-analysis of 16 studies showed a circadian pattern in 71% of cluster headache attacks (3,490 of 4,953), with a clear circadian peak between 9:00 p.m. and 3:00 a.m.
Migraine was also associated with a circadian pattern in 50% of cases (2,698 of 5,385) across eight studies, with a clear circadian trough between 11:00 p.m. and 7:00 a.m.
Seasonal peaks were also evident for cluster headache (spring and autumn) and migraine (April to October).
“In the short term, these findings help us explain the timing to patients – for example, it is possible that a headache at 8 a.m. is due to their internal body clock instead of their pillow, or breakfast food, or morning medications,” lead investigator Mark Burish, MD, PhD, associate professor, department of neurosurgery, at University of Texas Health Houston, told this news organization.
“In the long term, these findings do suggest that medications that target the circadian system could be effective in migraine and headache patients,” Dr. Burish added.
The study was published online in Neurology.
Treatment implications?
Across studies, chronotype was “highly variable” for both cluster headache and migraine, the investigators report.
Cluster headache was associated with lower melatonin and higher cortisol levels, compared with non–cluster headache controls.
On a genetic level, cluster headache was associated with two core circadian genes (CLOCK and REV-ERB–alpha), and five of the nine genes that increase the likelihood of having cluster headache are genes with a circadian pattern of expression.
Migraine headache was associated with lower urinary melatonin levels and with the core circadian genes, CK1-delta and ROR-alpha, and 110 of the 168 genes associated with migraine were clock-controlled genes.
“The data suggest that both of these headache disorders are highly circadian at multiple levels, especially cluster headache,” Dr. Burish said in a release.
“This reinforces the importance of the hypothalamus – the area of the brain that houses the primary biological clock – and its role in cluster headache and migraine. It also raises the question of the genetics of triggers such as sleep changes that are known triggers for migraine and are cues for the body’s circadian rhythm,” Dr. Burish said.
“We hope that future research will look into circadian medications as a new treatment option for migraine and cluster headache patients,” Dr. Burish told this news organization.
Importance of sleep regulation
The authors of an accompanying editorial note that even though the study doesn’t have immediate clinical implications, it offers a better understanding of the way chronobiologic factors may influence treatment.
“At a minimum, interventions known to regulate and improve sleep (e.g., melatonin, cognitive behavioral therapy), and which are safe and straightforward to introduce, may be useful in some individuals susceptible to circadian misalignment or sleep disorders,” write Heidi Sutherland, PhD, and Lyn Griffiths, PhD, with Queensland University of Technology, Brisbane, Australia.
“Treatment of comorbidities (e.g., insomnia) that result in sleep disturbances may also help headache management. Furthermore, chronobiological aspects of any pharmacological interventions should be considered, as some frequently used headache and migraine drugs can modulate circadian cycles and influence the expression of circadian genes (e.g., verapamil), or have sleep-related side effects,” they add.
A limitation of the study was the lack of information on factors that could influence the circadian cycle, such as medications; other disorders, such as bipolar disorder; or circadian rhythm issues, such as night-shift work.
The study was supported by grants from the Japan Society for the Promotion of Science, the National Institutes of Health, The Welch Foundation, and The Will Erwin Headache Research Foundation. Dr. Burish is an unpaid member of the medical advisory board of Clusterbusters, and a site investigator for a cluster headache clinical trial funded by Lundbeck. Dr. Sutherland has received grant funding from the U.S. Migraine Research Foundation, and received institute support from Queensland University of Technology for genetics research. Dr. Griffiths has received grant funding from the Australian NHMRC, U.S. Department of Defense, and the U.S. Migraine Research Foundation, and consultancy funding from TEVA.
A version of this article first appeared on Medscape.com.
A meta-analysis of 16 studies showed a circadian pattern in 71% of cluster headache attacks (3,490 of 4,953), with a clear circadian peak between 9:00 p.m. and 3:00 a.m.
Migraine was also associated with a circadian pattern in 50% of cases (2,698 of 5,385) across eight studies, with a clear circadian trough between 11:00 p.m. and 7:00 a.m.
Seasonal peaks were also evident for cluster headache (spring and autumn) and migraine (April to October).
“In the short term, these findings help us explain the timing to patients – for example, it is possible that a headache at 8 a.m. is due to their internal body clock instead of their pillow, or breakfast food, or morning medications,” lead investigator Mark Burish, MD, PhD, associate professor, department of neurosurgery, at University of Texas Health Houston, told this news organization.
“In the long term, these findings do suggest that medications that target the circadian system could be effective in migraine and headache patients,” Dr. Burish added.
The study was published online in Neurology.
Treatment implications?
Across studies, chronotype was “highly variable” for both cluster headache and migraine, the investigators report.
Cluster headache was associated with lower melatonin and higher cortisol levels, compared with non–cluster headache controls.
On a genetic level, cluster headache was associated with two core circadian genes (CLOCK and REV-ERB–alpha), and five of the nine genes that increase the likelihood of having cluster headache are genes with a circadian pattern of expression.
Migraine headache was associated with lower urinary melatonin levels and with the core circadian genes, CK1-delta and ROR-alpha, and 110 of the 168 genes associated with migraine were clock-controlled genes.
“The data suggest that both of these headache disorders are highly circadian at multiple levels, especially cluster headache,” Dr. Burish said in a release.
“This reinforces the importance of the hypothalamus – the area of the brain that houses the primary biological clock – and its role in cluster headache and migraine. It also raises the question of the genetics of triggers such as sleep changes that are known triggers for migraine and are cues for the body’s circadian rhythm,” Dr. Burish said.
“We hope that future research will look into circadian medications as a new treatment option for migraine and cluster headache patients,” Dr. Burish told this news organization.
Importance of sleep regulation
The authors of an accompanying editorial note that even though the study doesn’t have immediate clinical implications, it offers a better understanding of the way chronobiologic factors may influence treatment.
“At a minimum, interventions known to regulate and improve sleep (e.g., melatonin, cognitive behavioral therapy), and which are safe and straightforward to introduce, may be useful in some individuals susceptible to circadian misalignment or sleep disorders,” write Heidi Sutherland, PhD, and Lyn Griffiths, PhD, with Queensland University of Technology, Brisbane, Australia.
“Treatment of comorbidities (e.g., insomnia) that result in sleep disturbances may also help headache management. Furthermore, chronobiological aspects of any pharmacological interventions should be considered, as some frequently used headache and migraine drugs can modulate circadian cycles and influence the expression of circadian genes (e.g., verapamil), or have sleep-related side effects,” they add.
A limitation of the study was the lack of information on factors that could influence the circadian cycle, such as medications; other disorders, such as bipolar disorder; or circadian rhythm issues, such as night-shift work.
The study was supported by grants from the Japan Society for the Promotion of Science, the National Institutes of Health, The Welch Foundation, and The Will Erwin Headache Research Foundation. Dr. Burish is an unpaid member of the medical advisory board of Clusterbusters, and a site investigator for a cluster headache clinical trial funded by Lundbeck. Dr. Sutherland has received grant funding from the U.S. Migraine Research Foundation, and received institute support from Queensland University of Technology for genetics research. Dr. Griffiths has received grant funding from the Australian NHMRC, U.S. Department of Defense, and the U.S. Migraine Research Foundation, and consultancy funding from TEVA.
A version of this article first appeared on Medscape.com.
FROM NEUROLOGY
Song stuck in your head? What earworms reveal about health
If Miley Cyrus has planted “Flowers” in your head, rest assured you’re not alone.
An earworm – a bit of music you can’t shake from your brain – happens to almost everyone.
The culprit is typically a song you’ve heard repeatedly with a strong rhythm and melody (like Miley’s No. 1 hit this year).
It pops into your head and stays there, unbidden and often unwanted. As you fish for something new on Spotify, there’s always a chance that a catchy hook holds an earworm.
“A catchy tune or melody is the part of a song most likely to get stuck in a person’s head, often a bit from the chorus,” said Elizabeth H. Margulis, PhD, a professor at Princeton (N.J.) University and director of its music cognition lab. The phenomenon, which has been studied since 1885 (way before earbuds), goes by such names as stuck song syndrome, sticky music, musical imagery repetition, intrusive musical imagery, or the semi-official term, involuntary musical imagery, or INMI.
Research confirms how common it is. A 2020 study of American college students found that 97% had experienced an earworm in the past month, similar to findings from a larger Finnish survey done more than 10 years ago.
One in five people had experienced an earworm more than once a day, the study found. The typical length was 10-30 minutes, though 8.5% said theirs lasted more than 3 hours. Levels of “distress and interference” that earworms caused was mostly “mild to moderate.”
Some 86% said they tried to stop it – most frequently by distraction, like talking to a friend or listening to another song.
If music is important to you, your earworms are more likely to last longer and be harder to control, earlier research found. And women are thought to be more likely to have them.
“Very musical people may have more earworms because it’s easy for them to conjure up a certain tune,” says David Silbersweig, MD, chairman of the department of psychiatry and codirector of the Institute for the Neurosciences at Brigham and Women’s Hospital in Boston.
Moreover, people who lack “psychological flexibility” may find earworms more bothersome. The more they try to avoid or control intrusive thoughts (or songs), the more persistent those thoughts become.
“This is consistent with OCD (obsessive-compulsive disorder) research on the paradoxical effect of thought suppression,” the authors of the 2020 study wrote. In fact, people who report very annoying or stressful earworms are more likely to have obsessive-compulsive symptoms.
That makes them worth a closer look.
Digging for the source of earworms
Scientists trace earworms to the auditory cortex in the temporal lobe of the brain, which controls how you perceive music, as well as deep temporal lobe areas that are responsible for retrieving memories. Your amygdala and ventral striatum, parts of your brain that involve emotion, also tie into the making of an earworm.
MRI experiments found that “INMI is a common internal experience recruiting brain networks involved in perception, emotions, memory and spontaneous thoughts,” a 2015 paper in Consciousness and Cognition reported.
These brain networks work in tandem if you connect a song to an emotional memory – that’s when you’re more likely to experience it as an earworm. The “loop” of music you’ll hear in your head is usually a 20-second snippet.
Think of it as a “cognitive itch,” as researchers from the Netherlands put it. An earworm can be triggered by associating a song with a specific situation or emotion. Trying to suppress it just reminds you it’s there, “scratching” the itch and making it worse. “The more one tries to suppress the songs, the more their impetus increases, a mental process known as ironic process theory,” they wrote.
“It’s also worth pointing out that earworms don’t always occur right after a song ends,” said Michael K. Scullin, PhD, an associate professor of psychology and neuroscience at Baylor University in Waco, Tex. “Sometimes they only occur many hours later, and sometimes the earworm isn’t the song you were most recently listening to.”
These processes aren’t fully understood, he said, “but they likely represent memory consolidation mechanisms; that is, the brain trying to reactivate and stabilize musical memories.” Kind of like switching “radio stations” in your head.
When to worry
Earworms are most often harmless. “They’re part of a healthy brain,” said Dr. Silbersweig. But in rare cases, they indicate certain medical conditions. People with OCD, for example, have been shown to have earworms during times of stress. If this is the case, cognitive-behavioral therapy as well as some antidepressants may help.
Take an earworm seriously if it’s linked to other symptoms, said Elaine Jones, MD, a neurologist in Hilton Head, S.C., and a fellow of the American Academy of Neurology. Those symptoms could include “loss of consciousness or confusion, visual loss or changes, speech arrest, tremors of arms or legs,” she said.
“Most worrisome would be a seizure, but other causes could include a migraine aura. In a younger person, less than 20 years old, this kind of earworm could indicate a psychiatric condition like schizophrenia.” Drug toxicity or brain damage can also present with earworms.
Her bottom line: “If an earworm is persistent for more than 24 hours, or if it is associated with the other symptoms mentioned above, it would be important to reach out to your primary care doctor to ensure that nothing more serious is going on,” said Dr. Jones. With no other symptoms, “it is more likely to be just an earworm.”
Japanese research also indicates that an earworm that lasts for several hours in a day can be linked to depression. If a person has symptoms such as low mood, insomnia, and loss of appetite, along with earworms that last several hours a day, treatment may help.
There’s another category called “musical hallucinations” – where the person thinks they are actually hearing music, which could be a symptom of depression, although scientists don’t know for sure. The drug vortioxetine, which may help boost serotonin in the brain, has shown some promise in reducing earworms.
Some research has shown that diseases that damage the auditory pathway in the brain have a link to musical hallucinations.
How to stop a simple earworm
Here are six easy ways to make it stop:
- Mix up your playlist. “Listening to songs repeatedly does increase the likelihood that they’ll get stuck,” said Dr. Margulis.
- Take breaks from your tunes throughout the day. “Longer listening durations are more likely to lead to earworms,” Dr. Scullin said.
- Use your feet. than the beat of your earworm. This will interrupt your memory of the tempo and can help chase away the earworm.
- Stick with that song. “Listen to a song all the way through,” said Dr. Silbersweig. If you only listen to snippets of a song, the can take hold. That’s the brain’s tendency to remember things that are interrupted more easily than completed things.
- Distract yourself. Lose yourself in a book, a movie, your work, or a hobby that requires concentration. “Redirecting attention to an absorbing task can be an effective way to dislodge an earworm,” said Dr. Margulis.
- Chew gum. shows that the action of doing so interferes with repetitive memories and stops your mind from “scanning” a song. Then enjoy the sound of silence!
A version of this article first appeared on WebMD.com.
If Miley Cyrus has planted “Flowers” in your head, rest assured you’re not alone.
An earworm – a bit of music you can’t shake from your brain – happens to almost everyone.
The culprit is typically a song you’ve heard repeatedly with a strong rhythm and melody (like Miley’s No. 1 hit this year).
It pops into your head and stays there, unbidden and often unwanted. As you fish for something new on Spotify, there’s always a chance that a catchy hook holds an earworm.
“A catchy tune or melody is the part of a song most likely to get stuck in a person’s head, often a bit from the chorus,” said Elizabeth H. Margulis, PhD, a professor at Princeton (N.J.) University and director of its music cognition lab. The phenomenon, which has been studied since 1885 (way before earbuds), goes by such names as stuck song syndrome, sticky music, musical imagery repetition, intrusive musical imagery, or the semi-official term, involuntary musical imagery, or INMI.
Research confirms how common it is. A 2020 study of American college students found that 97% had experienced an earworm in the past month, similar to findings from a larger Finnish survey done more than 10 years ago.
One in five people had experienced an earworm more than once a day, the study found. The typical length was 10-30 minutes, though 8.5% said theirs lasted more than 3 hours. Levels of “distress and interference” that earworms caused was mostly “mild to moderate.”
Some 86% said they tried to stop it – most frequently by distraction, like talking to a friend or listening to another song.
If music is important to you, your earworms are more likely to last longer and be harder to control, earlier research found. And women are thought to be more likely to have them.
“Very musical people may have more earworms because it’s easy for them to conjure up a certain tune,” says David Silbersweig, MD, chairman of the department of psychiatry and codirector of the Institute for the Neurosciences at Brigham and Women’s Hospital in Boston.
Moreover, people who lack “psychological flexibility” may find earworms more bothersome. The more they try to avoid or control intrusive thoughts (or songs), the more persistent those thoughts become.
“This is consistent with OCD (obsessive-compulsive disorder) research on the paradoxical effect of thought suppression,” the authors of the 2020 study wrote. In fact, people who report very annoying or stressful earworms are more likely to have obsessive-compulsive symptoms.
That makes them worth a closer look.
Digging for the source of earworms
Scientists trace earworms to the auditory cortex in the temporal lobe of the brain, which controls how you perceive music, as well as deep temporal lobe areas that are responsible for retrieving memories. Your amygdala and ventral striatum, parts of your brain that involve emotion, also tie into the making of an earworm.
MRI experiments found that “INMI is a common internal experience recruiting brain networks involved in perception, emotions, memory and spontaneous thoughts,” a 2015 paper in Consciousness and Cognition reported.
These brain networks work in tandem if you connect a song to an emotional memory – that’s when you’re more likely to experience it as an earworm. The “loop” of music you’ll hear in your head is usually a 20-second snippet.
Think of it as a “cognitive itch,” as researchers from the Netherlands put it. An earworm can be triggered by associating a song with a specific situation or emotion. Trying to suppress it just reminds you it’s there, “scratching” the itch and making it worse. “The more one tries to suppress the songs, the more their impetus increases, a mental process known as ironic process theory,” they wrote.
“It’s also worth pointing out that earworms don’t always occur right after a song ends,” said Michael K. Scullin, PhD, an associate professor of psychology and neuroscience at Baylor University in Waco, Tex. “Sometimes they only occur many hours later, and sometimes the earworm isn’t the song you were most recently listening to.”
These processes aren’t fully understood, he said, “but they likely represent memory consolidation mechanisms; that is, the brain trying to reactivate and stabilize musical memories.” Kind of like switching “radio stations” in your head.
When to worry
Earworms are most often harmless. “They’re part of a healthy brain,” said Dr. Silbersweig. But in rare cases, they indicate certain medical conditions. People with OCD, for example, have been shown to have earworms during times of stress. If this is the case, cognitive-behavioral therapy as well as some antidepressants may help.
Take an earworm seriously if it’s linked to other symptoms, said Elaine Jones, MD, a neurologist in Hilton Head, S.C., and a fellow of the American Academy of Neurology. Those symptoms could include “loss of consciousness or confusion, visual loss or changes, speech arrest, tremors of arms or legs,” she said.
“Most worrisome would be a seizure, but other causes could include a migraine aura. In a younger person, less than 20 years old, this kind of earworm could indicate a psychiatric condition like schizophrenia.” Drug toxicity or brain damage can also present with earworms.
Her bottom line: “If an earworm is persistent for more than 24 hours, or if it is associated with the other symptoms mentioned above, it would be important to reach out to your primary care doctor to ensure that nothing more serious is going on,” said Dr. Jones. With no other symptoms, “it is more likely to be just an earworm.”
Japanese research also indicates that an earworm that lasts for several hours in a day can be linked to depression. If a person has symptoms such as low mood, insomnia, and loss of appetite, along with earworms that last several hours a day, treatment may help.
There’s another category called “musical hallucinations” – where the person thinks they are actually hearing music, which could be a symptom of depression, although scientists don’t know for sure. The drug vortioxetine, which may help boost serotonin in the brain, has shown some promise in reducing earworms.
Some research has shown that diseases that damage the auditory pathway in the brain have a link to musical hallucinations.
How to stop a simple earworm
Here are six easy ways to make it stop:
- Mix up your playlist. “Listening to songs repeatedly does increase the likelihood that they’ll get stuck,” said Dr. Margulis.
- Take breaks from your tunes throughout the day. “Longer listening durations are more likely to lead to earworms,” Dr. Scullin said.
- Use your feet. than the beat of your earworm. This will interrupt your memory of the tempo and can help chase away the earworm.
- Stick with that song. “Listen to a song all the way through,” said Dr. Silbersweig. If you only listen to snippets of a song, the can take hold. That’s the brain’s tendency to remember things that are interrupted more easily than completed things.
- Distract yourself. Lose yourself in a book, a movie, your work, or a hobby that requires concentration. “Redirecting attention to an absorbing task can be an effective way to dislodge an earworm,” said Dr. Margulis.
- Chew gum. shows that the action of doing so interferes with repetitive memories and stops your mind from “scanning” a song. Then enjoy the sound of silence!
A version of this article first appeared on WebMD.com.
If Miley Cyrus has planted “Flowers” in your head, rest assured you’re not alone.
An earworm – a bit of music you can’t shake from your brain – happens to almost everyone.
The culprit is typically a song you’ve heard repeatedly with a strong rhythm and melody (like Miley’s No. 1 hit this year).
It pops into your head and stays there, unbidden and often unwanted. As you fish for something new on Spotify, there’s always a chance that a catchy hook holds an earworm.
“A catchy tune or melody is the part of a song most likely to get stuck in a person’s head, often a bit from the chorus,” said Elizabeth H. Margulis, PhD, a professor at Princeton (N.J.) University and director of its music cognition lab. The phenomenon, which has been studied since 1885 (way before earbuds), goes by such names as stuck song syndrome, sticky music, musical imagery repetition, intrusive musical imagery, or the semi-official term, involuntary musical imagery, or INMI.
Research confirms how common it is. A 2020 study of American college students found that 97% had experienced an earworm in the past month, similar to findings from a larger Finnish survey done more than 10 years ago.
One in five people had experienced an earworm more than once a day, the study found. The typical length was 10-30 minutes, though 8.5% said theirs lasted more than 3 hours. Levels of “distress and interference” that earworms caused was mostly “mild to moderate.”
Some 86% said they tried to stop it – most frequently by distraction, like talking to a friend or listening to another song.
If music is important to you, your earworms are more likely to last longer and be harder to control, earlier research found. And women are thought to be more likely to have them.
“Very musical people may have more earworms because it’s easy for them to conjure up a certain tune,” says David Silbersweig, MD, chairman of the department of psychiatry and codirector of the Institute for the Neurosciences at Brigham and Women’s Hospital in Boston.
Moreover, people who lack “psychological flexibility” may find earworms more bothersome. The more they try to avoid or control intrusive thoughts (or songs), the more persistent those thoughts become.
“This is consistent with OCD (obsessive-compulsive disorder) research on the paradoxical effect of thought suppression,” the authors of the 2020 study wrote. In fact, people who report very annoying or stressful earworms are more likely to have obsessive-compulsive symptoms.
That makes them worth a closer look.
Digging for the source of earworms
Scientists trace earworms to the auditory cortex in the temporal lobe of the brain, which controls how you perceive music, as well as deep temporal lobe areas that are responsible for retrieving memories. Your amygdala and ventral striatum, parts of your brain that involve emotion, also tie into the making of an earworm.
MRI experiments found that “INMI is a common internal experience recruiting brain networks involved in perception, emotions, memory and spontaneous thoughts,” a 2015 paper in Consciousness and Cognition reported.
These brain networks work in tandem if you connect a song to an emotional memory – that’s when you’re more likely to experience it as an earworm. The “loop” of music you’ll hear in your head is usually a 20-second snippet.
Think of it as a “cognitive itch,” as researchers from the Netherlands put it. An earworm can be triggered by associating a song with a specific situation or emotion. Trying to suppress it just reminds you it’s there, “scratching” the itch and making it worse. “The more one tries to suppress the songs, the more their impetus increases, a mental process known as ironic process theory,” they wrote.
“It’s also worth pointing out that earworms don’t always occur right after a song ends,” said Michael K. Scullin, PhD, an associate professor of psychology and neuroscience at Baylor University in Waco, Tex. “Sometimes they only occur many hours later, and sometimes the earworm isn’t the song you were most recently listening to.”
These processes aren’t fully understood, he said, “but they likely represent memory consolidation mechanisms; that is, the brain trying to reactivate and stabilize musical memories.” Kind of like switching “radio stations” in your head.
When to worry
Earworms are most often harmless. “They’re part of a healthy brain,” said Dr. Silbersweig. But in rare cases, they indicate certain medical conditions. People with OCD, for example, have been shown to have earworms during times of stress. If this is the case, cognitive-behavioral therapy as well as some antidepressants may help.
Take an earworm seriously if it’s linked to other symptoms, said Elaine Jones, MD, a neurologist in Hilton Head, S.C., and a fellow of the American Academy of Neurology. Those symptoms could include “loss of consciousness or confusion, visual loss or changes, speech arrest, tremors of arms or legs,” she said.
“Most worrisome would be a seizure, but other causes could include a migraine aura. In a younger person, less than 20 years old, this kind of earworm could indicate a psychiatric condition like schizophrenia.” Drug toxicity or brain damage can also present with earworms.
Her bottom line: “If an earworm is persistent for more than 24 hours, or if it is associated with the other symptoms mentioned above, it would be important to reach out to your primary care doctor to ensure that nothing more serious is going on,” said Dr. Jones. With no other symptoms, “it is more likely to be just an earworm.”
Japanese research also indicates that an earworm that lasts for several hours in a day can be linked to depression. If a person has symptoms such as low mood, insomnia, and loss of appetite, along with earworms that last several hours a day, treatment may help.
There’s another category called “musical hallucinations” – where the person thinks they are actually hearing music, which could be a symptom of depression, although scientists don’t know for sure. The drug vortioxetine, which may help boost serotonin in the brain, has shown some promise in reducing earworms.
Some research has shown that diseases that damage the auditory pathway in the brain have a link to musical hallucinations.
How to stop a simple earworm
Here are six easy ways to make it stop:
- Mix up your playlist. “Listening to songs repeatedly does increase the likelihood that they’ll get stuck,” said Dr. Margulis.
- Take breaks from your tunes throughout the day. “Longer listening durations are more likely to lead to earworms,” Dr. Scullin said.
- Use your feet. than the beat of your earworm. This will interrupt your memory of the tempo and can help chase away the earworm.
- Stick with that song. “Listen to a song all the way through,” said Dr. Silbersweig. If you only listen to snippets of a song, the can take hold. That’s the brain’s tendency to remember things that are interrupted more easily than completed things.
- Distract yourself. Lose yourself in a book, a movie, your work, or a hobby that requires concentration. “Redirecting attention to an absorbing task can be an effective way to dislodge an earworm,” said Dr. Margulis.
- Chew gum. shows that the action of doing so interferes with repetitive memories and stops your mind from “scanning” a song. Then enjoy the sound of silence!
A version of this article first appeared on WebMD.com.
Take time to relax and enjoy the ride
This past weekend was one of my least-favorite parts of the annual cycle: I shut off and drained my hot tub.
I’ve always loved sitting in hot tubs, as far back as I can remember. Growing up on family vacations I preferred them to the pool. So when I was grown up and could afford one, I got it for my house.
I spend my winter weekend afternoons relaxing in it with a can of beer, some bottles of iced tea, and a pile of journals or a book. I put instrumental jazz on my phone and spend a few pleasant hours there, catching up on my reading.
But, as the Phoenix weather swings back to summer temps, it’s time to turn it off until next November.
It’s interesting the ways we mark the passage of time in our lives. The traditional standards are New Year’s, major holidays, and birthdays. Some may mark it by their favorite sports seasons starting.
In medicine we may mark it by patient ages, or a drug that we thought just came to market now going generic, or realizing our state or DEA license is up for renewal.
It doesn’t really matter how you mark the time – it’s going to happen whether you do or don’t. The person you see in the mirror is the same one there since you were tall enough to see over the bathroom countertop. Isn’t it just the ones around us who change?
As Phoenix moves back to a summer footing, and as someone who’s been through 56 of them, it’s hard not to think about it. Summer vacations growing up, summer classes in college, summer elective rotations in medical school. Now I work year-round and watch the same cycle play out with my kids in college.
You often hear the phrase “a hundred years from now it won’t make a difference.” Probably true. In 2123 the time I spent relaxing in my hot tub won’t mean anything, or be remembered by anyone.
But I’m not sitting in it to think about that. I’m in it because I have what I have now, and none of us will ever have that again. And part of that, to me, is enjoying some time in the hot tub.
Because That may not matter in one hundred years, but it matters to me today. And that’s what’s really important.
To all of us.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
This past weekend was one of my least-favorite parts of the annual cycle: I shut off and drained my hot tub.
I’ve always loved sitting in hot tubs, as far back as I can remember. Growing up on family vacations I preferred them to the pool. So when I was grown up and could afford one, I got it for my house.
I spend my winter weekend afternoons relaxing in it with a can of beer, some bottles of iced tea, and a pile of journals or a book. I put instrumental jazz on my phone and spend a few pleasant hours there, catching up on my reading.
But, as the Phoenix weather swings back to summer temps, it’s time to turn it off until next November.
It’s interesting the ways we mark the passage of time in our lives. The traditional standards are New Year’s, major holidays, and birthdays. Some may mark it by their favorite sports seasons starting.
In medicine we may mark it by patient ages, or a drug that we thought just came to market now going generic, or realizing our state or DEA license is up for renewal.
It doesn’t really matter how you mark the time – it’s going to happen whether you do or don’t. The person you see in the mirror is the same one there since you were tall enough to see over the bathroom countertop. Isn’t it just the ones around us who change?
As Phoenix moves back to a summer footing, and as someone who’s been through 56 of them, it’s hard not to think about it. Summer vacations growing up, summer classes in college, summer elective rotations in medical school. Now I work year-round and watch the same cycle play out with my kids in college.
You often hear the phrase “a hundred years from now it won’t make a difference.” Probably true. In 2123 the time I spent relaxing in my hot tub won’t mean anything, or be remembered by anyone.
But I’m not sitting in it to think about that. I’m in it because I have what I have now, and none of us will ever have that again. And part of that, to me, is enjoying some time in the hot tub.
Because That may not matter in one hundred years, but it matters to me today. And that’s what’s really important.
To all of us.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
This past weekend was one of my least-favorite parts of the annual cycle: I shut off and drained my hot tub.
I’ve always loved sitting in hot tubs, as far back as I can remember. Growing up on family vacations I preferred them to the pool. So when I was grown up and could afford one, I got it for my house.
I spend my winter weekend afternoons relaxing in it with a can of beer, some bottles of iced tea, and a pile of journals or a book. I put instrumental jazz on my phone and spend a few pleasant hours there, catching up on my reading.
But, as the Phoenix weather swings back to summer temps, it’s time to turn it off until next November.
It’s interesting the ways we mark the passage of time in our lives. The traditional standards are New Year’s, major holidays, and birthdays. Some may mark it by their favorite sports seasons starting.
In medicine we may mark it by patient ages, or a drug that we thought just came to market now going generic, or realizing our state or DEA license is up for renewal.
It doesn’t really matter how you mark the time – it’s going to happen whether you do or don’t. The person you see in the mirror is the same one there since you were tall enough to see over the bathroom countertop. Isn’t it just the ones around us who change?
As Phoenix moves back to a summer footing, and as someone who’s been through 56 of them, it’s hard not to think about it. Summer vacations growing up, summer classes in college, summer elective rotations in medical school. Now I work year-round and watch the same cycle play out with my kids in college.
You often hear the phrase “a hundred years from now it won’t make a difference.” Probably true. In 2123 the time I spent relaxing in my hot tub won’t mean anything, or be remembered by anyone.
But I’m not sitting in it to think about that. I’m in it because I have what I have now, and none of us will ever have that again. And part of that, to me, is enjoying some time in the hot tub.
Because That may not matter in one hundred years, but it matters to me today. And that’s what’s really important.
To all of us.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Do B vitamins reduce Parkinson’s risk?
Though there was some evidence that vitamin B12 early in life was associated with decreased PD risk, the findings were inconsistent and were observed only in people whose daily intake was 10 times the recommended level.
“The results of this large prospective study do not support the hypothesis that increasing folate or vitamin B6 intakes above the current levels would reduce PD risk in this population of mostly White U.S. health professionals,” lead investigator Mario H. Flores-Torres, MD, PhD, a research scientist in the department of nutrition at the Harvard T.H. Chan School of Public Health, Boston, said in an interview.
However, he added, the study “leaves open the possibility that in some individuals the intake of vitamin B12 contributes to PD risk – a finding that warrants further research.”
The findings were published online in Movement Disorders.
Mixed findings
Previous studies have suggested B vitamins – including folate, B6 and B12 – might affect PD risk, but results have been mixed.
The new study included 80,965 women from the Nurses’ Health Study (1984-2016) and 48,837 men from the Health Professionals Follow-up Study (1986-2016). The average age at baseline was 50 years in women and 54 years in men, and participants were followed for about 30 years.
Participants completed questionnaires about diet at the beginning of the study and again every 4 years.
To account for the possibility of reverse causation due to the long prodromal phase of PD, investigators conducted lagged analyses at 8, 12, 16, and 20 years.
During the follow-up period, 1,426 incident cases of PD were diagnosed (687 in women and 739 in men).
Researchers found no link between reduced PD risk and intake of vitamin B6 or folate.
Though the total cumulative average intake of vitamin B12 was not associated with PD risk, investigators noted a modest decrease in risk between those with highest baseline of B12 and participants with the lowest baseline levels (hazard ratio, 0.80; P = .01).
Individuals in the highest quintile of B12 intake at baseline had an average intake of 21-22 mcg/d, close to 10 times the recommended daily intake of 2.4 mcg/d.
“Although some of our results suggest that a higher intake of vitamin B12 may decrease the risk of PD in a population of U.S. health professionals, the associations we observed were modest and not entirely consistent,” Dr. Flores-Torres said.
“Additional studies need to confirm our findings to better understand whether people who take higher amounts of B12 younger in life may have a protective benefit against PD,” he added.
The whole picture?
Commenting on the findings for this article, Rebecca Gilbert, MD, PhD, chief scientific officer of the American Parkinson Disease Association, New York, noted that checking B vitamin levels is a fairly standard practice for most clinicians. In that regard, this study highlights why this is important.
“Neurologists will often test B12 levels and recommend a supplement if your level is below the normal range,” she said. “No one is questioning the value of B12 for nerves and recommend that B12 is in the normal to high normal range.”
But understanding how B vitamins may or may not affect PD risk might require a different kind of study.
“This analysis, much like many others, is trying so hard to figure out what is it in diets that affects Parkinson’s disease risk,” Dr. Gilbert said. “But we have yet to say these are the nutrients that prevent Parkinson’s or increase the risk.”
One reason for the conflicting results in studies such as this could be that the explanation for the link between diet and PD risk may not be in specific minerals consumed but rather in the diet as a whole.
“Focusing on specific elements of a diet may not give us the answer,” Dr. Gilbert said. “We should be analyzing diet as a complete holistic picture because it’s not just the elements but how everything in what we eat works together.”
The study was funded by the National Institutes of Health and the Parkinson’s Foundation. Dr. Flores-Torres and Dr. Gilbert report no relevant conflicts.
A version of this article originally appeared on Medscape.com.
Though there was some evidence that vitamin B12 early in life was associated with decreased PD risk, the findings were inconsistent and were observed only in people whose daily intake was 10 times the recommended level.
“The results of this large prospective study do not support the hypothesis that increasing folate or vitamin B6 intakes above the current levels would reduce PD risk in this population of mostly White U.S. health professionals,” lead investigator Mario H. Flores-Torres, MD, PhD, a research scientist in the department of nutrition at the Harvard T.H. Chan School of Public Health, Boston, said in an interview.
However, he added, the study “leaves open the possibility that in some individuals the intake of vitamin B12 contributes to PD risk – a finding that warrants further research.”
The findings were published online in Movement Disorders.
Mixed findings
Previous studies have suggested B vitamins – including folate, B6 and B12 – might affect PD risk, but results have been mixed.
The new study included 80,965 women from the Nurses’ Health Study (1984-2016) and 48,837 men from the Health Professionals Follow-up Study (1986-2016). The average age at baseline was 50 years in women and 54 years in men, and participants were followed for about 30 years.
Participants completed questionnaires about diet at the beginning of the study and again every 4 years.
To account for the possibility of reverse causation due to the long prodromal phase of PD, investigators conducted lagged analyses at 8, 12, 16, and 20 years.
During the follow-up period, 1,426 incident cases of PD were diagnosed (687 in women and 739 in men).
Researchers found no link between reduced PD risk and intake of vitamin B6 or folate.
Though the total cumulative average intake of vitamin B12 was not associated with PD risk, investigators noted a modest decrease in risk between those with highest baseline of B12 and participants with the lowest baseline levels (hazard ratio, 0.80; P = .01).
Individuals in the highest quintile of B12 intake at baseline had an average intake of 21-22 mcg/d, close to 10 times the recommended daily intake of 2.4 mcg/d.
“Although some of our results suggest that a higher intake of vitamin B12 may decrease the risk of PD in a population of U.S. health professionals, the associations we observed were modest and not entirely consistent,” Dr. Flores-Torres said.
“Additional studies need to confirm our findings to better understand whether people who take higher amounts of B12 younger in life may have a protective benefit against PD,” he added.
The whole picture?
Commenting on the findings for this article, Rebecca Gilbert, MD, PhD, chief scientific officer of the American Parkinson Disease Association, New York, noted that checking B vitamin levels is a fairly standard practice for most clinicians. In that regard, this study highlights why this is important.
“Neurologists will often test B12 levels and recommend a supplement if your level is below the normal range,” she said. “No one is questioning the value of B12 for nerves and recommend that B12 is in the normal to high normal range.”
But understanding how B vitamins may or may not affect PD risk might require a different kind of study.
“This analysis, much like many others, is trying so hard to figure out what is it in diets that affects Parkinson’s disease risk,” Dr. Gilbert said. “But we have yet to say these are the nutrients that prevent Parkinson’s or increase the risk.”
One reason for the conflicting results in studies such as this could be that the explanation for the link between diet and PD risk may not be in specific minerals consumed but rather in the diet as a whole.
“Focusing on specific elements of a diet may not give us the answer,” Dr. Gilbert said. “We should be analyzing diet as a complete holistic picture because it’s not just the elements but how everything in what we eat works together.”
The study was funded by the National Institutes of Health and the Parkinson’s Foundation. Dr. Flores-Torres and Dr. Gilbert report no relevant conflicts.
A version of this article originally appeared on Medscape.com.
Though there was some evidence that vitamin B12 early in life was associated with decreased PD risk, the findings were inconsistent and were observed only in people whose daily intake was 10 times the recommended level.
“The results of this large prospective study do not support the hypothesis that increasing folate or vitamin B6 intakes above the current levels would reduce PD risk in this population of mostly White U.S. health professionals,” lead investigator Mario H. Flores-Torres, MD, PhD, a research scientist in the department of nutrition at the Harvard T.H. Chan School of Public Health, Boston, said in an interview.
However, he added, the study “leaves open the possibility that in some individuals the intake of vitamin B12 contributes to PD risk – a finding that warrants further research.”
The findings were published online in Movement Disorders.
Mixed findings
Previous studies have suggested B vitamins – including folate, B6 and B12 – might affect PD risk, but results have been mixed.
The new study included 80,965 women from the Nurses’ Health Study (1984-2016) and 48,837 men from the Health Professionals Follow-up Study (1986-2016). The average age at baseline was 50 years in women and 54 years in men, and participants were followed for about 30 years.
Participants completed questionnaires about diet at the beginning of the study and again every 4 years.
To account for the possibility of reverse causation due to the long prodromal phase of PD, investigators conducted lagged analyses at 8, 12, 16, and 20 years.
During the follow-up period, 1,426 incident cases of PD were diagnosed (687 in women and 739 in men).
Researchers found no link between reduced PD risk and intake of vitamin B6 or folate.
Though the total cumulative average intake of vitamin B12 was not associated with PD risk, investigators noted a modest decrease in risk between those with highest baseline of B12 and participants with the lowest baseline levels (hazard ratio, 0.80; P = .01).
Individuals in the highest quintile of B12 intake at baseline had an average intake of 21-22 mcg/d, close to 10 times the recommended daily intake of 2.4 mcg/d.
“Although some of our results suggest that a higher intake of vitamin B12 may decrease the risk of PD in a population of U.S. health professionals, the associations we observed were modest and not entirely consistent,” Dr. Flores-Torres said.
“Additional studies need to confirm our findings to better understand whether people who take higher amounts of B12 younger in life may have a protective benefit against PD,” he added.
The whole picture?
Commenting on the findings for this article, Rebecca Gilbert, MD, PhD, chief scientific officer of the American Parkinson Disease Association, New York, noted that checking B vitamin levels is a fairly standard practice for most clinicians. In that regard, this study highlights why this is important.
“Neurologists will often test B12 levels and recommend a supplement if your level is below the normal range,” she said. “No one is questioning the value of B12 for nerves and recommend that B12 is in the normal to high normal range.”
But understanding how B vitamins may or may not affect PD risk might require a different kind of study.
“This analysis, much like many others, is trying so hard to figure out what is it in diets that affects Parkinson’s disease risk,” Dr. Gilbert said. “But we have yet to say these are the nutrients that prevent Parkinson’s or increase the risk.”
One reason for the conflicting results in studies such as this could be that the explanation for the link between diet and PD risk may not be in specific minerals consumed but rather in the diet as a whole.
“Focusing on specific elements of a diet may not give us the answer,” Dr. Gilbert said. “We should be analyzing diet as a complete holistic picture because it’s not just the elements but how everything in what we eat works together.”
The study was funded by the National Institutes of Health and the Parkinson’s Foundation. Dr. Flores-Torres and Dr. Gilbert report no relevant conflicts.
A version of this article originally appeared on Medscape.com.
FROM MOVEMENT DISORDERS
‘Excess’ deaths surging, but why?
This transcript has been edited for clarity.
“Excess deaths.” You’ve heard the phrase countless times by now. It is one of the myriad of previously esoteric epidemiology terms that the pandemic brought squarely into the zeitgeist.
As a sort of standard candle of the performance of a state or a region or a country in terms of health care, it has a lot of utility – if for nothing more than Monday-morning quarterbacking. But this week, I want to dig in on the concept a bit because, according to a new study, the excess death gap between the United States and Western Europe has never been higher.
You might imagine that the best way to figure this out is for some group of intelligent people to review each death and decide, somehow, whether it was expected or not. But aside from being impractical, this would end up being somewhat subjective. That older person who died from pneumonia – was that an expected death? Could it have been avoided?
Rather, the calculation of excess mortality relies on large numbers and statistical inference to compare an expected number of deaths with those that are observed.
The difference is excess mortality, even if you can never be sure whether any particular death was expected or not.
As always, however, the devil is in the details. What data do you use to define the expected number of deaths?
There are options here. Probably the most straightforward analysis uses past data from the country of interest. You look at annual deaths over some historical period of time and compare those numbers with the rates today. Two issues need to be accounted for here: population growth – a larger population will have more deaths, so you need to adjust the historical population with current levels, and demographic shifts – an older or more male population will have more deaths, so you need to adjust for that as well.
But provided you take care of those factors, you can estimate fairly well how many deaths you can expect to see in any given period of time.
Still, you should see right away that excess mortality is a relative concept. If you think that, just perhaps, the United States has some systematic failure to deliver care that has been stable and persistent over time, you wouldn’t capture that failing in an excess mortality calculation that uses U.S. historical data as the baseline.
The best way to get around that is to use data from other countries, and that’s just what this article – a rare single-author piece by Patrick Heuveline – does, calculating excess deaths in the United States by standardizing our mortality rates to the five largest Western European countries: the United Kingdom, France, Germany, Italy, and Spain.
Controlling for the differences in the demographics of that European population, here is the expected number of deaths in the United States over the past 5 years.
Note that there is a small uptick in expected deaths in 2020, reflecting the pandemic, which returns to baseline levels by 2021. This is because that’s what happened in Europe; by 2021, the excess mortality due to COVID-19 was quite low.
Here are the actual deaths in the US during that time.
Highlighted here in green, then, is the excess mortality over time in the United States.
There are some fascinating and concerning findings here.
First of all, you can see that even before the pandemic, the United States has an excess mortality problem. This is not entirely a surprise; we’ve known that so-called “deaths of despair,” those due to alcohol abuse, drug overdoses, and suicide, are at an all-time high and tend to affect a “prime of life” population that would not otherwise be expected to die. In fact, fully 50% of the excess deaths in the United States occur in those between ages 15 and 64.
Excess deaths are also a concerning percentage of total deaths. In 2017, 17% of total deaths in the United States could be considered “excess.” In 2021, that number had doubled to 35%. Nearly 900,000 individuals in the United States died in 2021 who perhaps didn’t need to.
The obvious culprit to blame here is COVID, but COVID-associated excess deaths only explain about 50% of the excess we see in 2021. The rest reflect something even more concerning: a worsening of the failures of the past, perhaps exacerbated by the pandemic but not due to the virus itself.
Of course, we started this discussion acknowledging that the calculation of excess mortality is exquisitely dependent on how you model the expected number of deaths, and I’m sure some will take issue with the use of European numbers when applied to Americans. After all, Europe has, by and large, a robust public health service, socialized medicine, and healthcare that does not run the risk of bankrupting its citizens. How can we compare our outcomes to a place like that?
How indeed.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale University’s Clinical and Translational Research Accelerator in New Haven,Conn. He reported no relevant conflicts of interest.
A version of this article originally appeared on Medscape.com.
This transcript has been edited for clarity.
“Excess deaths.” You’ve heard the phrase countless times by now. It is one of the myriad of previously esoteric epidemiology terms that the pandemic brought squarely into the zeitgeist.
As a sort of standard candle of the performance of a state or a region or a country in terms of health care, it has a lot of utility – if for nothing more than Monday-morning quarterbacking. But this week, I want to dig in on the concept a bit because, according to a new study, the excess death gap between the United States and Western Europe has never been higher.
You might imagine that the best way to figure this out is for some group of intelligent people to review each death and decide, somehow, whether it was expected or not. But aside from being impractical, this would end up being somewhat subjective. That older person who died from pneumonia – was that an expected death? Could it have been avoided?
Rather, the calculation of excess mortality relies on large numbers and statistical inference to compare an expected number of deaths with those that are observed.
The difference is excess mortality, even if you can never be sure whether any particular death was expected or not.
As always, however, the devil is in the details. What data do you use to define the expected number of deaths?
There are options here. Probably the most straightforward analysis uses past data from the country of interest. You look at annual deaths over some historical period of time and compare those numbers with the rates today. Two issues need to be accounted for here: population growth – a larger population will have more deaths, so you need to adjust the historical population with current levels, and demographic shifts – an older or more male population will have more deaths, so you need to adjust for that as well.
But provided you take care of those factors, you can estimate fairly well how many deaths you can expect to see in any given period of time.
Still, you should see right away that excess mortality is a relative concept. If you think that, just perhaps, the United States has some systematic failure to deliver care that has been stable and persistent over time, you wouldn’t capture that failing in an excess mortality calculation that uses U.S. historical data as the baseline.
The best way to get around that is to use data from other countries, and that’s just what this article – a rare single-author piece by Patrick Heuveline – does, calculating excess deaths in the United States by standardizing our mortality rates to the five largest Western European countries: the United Kingdom, France, Germany, Italy, and Spain.
Controlling for the differences in the demographics of that European population, here is the expected number of deaths in the United States over the past 5 years.
Note that there is a small uptick in expected deaths in 2020, reflecting the pandemic, which returns to baseline levels by 2021. This is because that’s what happened in Europe; by 2021, the excess mortality due to COVID-19 was quite low.
Here are the actual deaths in the US during that time.
Highlighted here in green, then, is the excess mortality over time in the United States.
There are some fascinating and concerning findings here.
First of all, you can see that even before the pandemic, the United States has an excess mortality problem. This is not entirely a surprise; we’ve known that so-called “deaths of despair,” those due to alcohol abuse, drug overdoses, and suicide, are at an all-time high and tend to affect a “prime of life” population that would not otherwise be expected to die. In fact, fully 50% of the excess deaths in the United States occur in those between ages 15 and 64.
Excess deaths are also a concerning percentage of total deaths. In 2017, 17% of total deaths in the United States could be considered “excess.” In 2021, that number had doubled to 35%. Nearly 900,000 individuals in the United States died in 2021 who perhaps didn’t need to.
The obvious culprit to blame here is COVID, but COVID-associated excess deaths only explain about 50% of the excess we see in 2021. The rest reflect something even more concerning: a worsening of the failures of the past, perhaps exacerbated by the pandemic but not due to the virus itself.
Of course, we started this discussion acknowledging that the calculation of excess mortality is exquisitely dependent on how you model the expected number of deaths, and I’m sure some will take issue with the use of European numbers when applied to Americans. After all, Europe has, by and large, a robust public health service, socialized medicine, and healthcare that does not run the risk of bankrupting its citizens. How can we compare our outcomes to a place like that?
How indeed.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale University’s Clinical and Translational Research Accelerator in New Haven,Conn. He reported no relevant conflicts of interest.
A version of this article originally appeared on Medscape.com.
This transcript has been edited for clarity.
“Excess deaths.” You’ve heard the phrase countless times by now. It is one of the myriad of previously esoteric epidemiology terms that the pandemic brought squarely into the zeitgeist.
As a sort of standard candle of the performance of a state or a region or a country in terms of health care, it has a lot of utility – if for nothing more than Monday-morning quarterbacking. But this week, I want to dig in on the concept a bit because, according to a new study, the excess death gap between the United States and Western Europe has never been higher.
You might imagine that the best way to figure this out is for some group of intelligent people to review each death and decide, somehow, whether it was expected or not. But aside from being impractical, this would end up being somewhat subjective. That older person who died from pneumonia – was that an expected death? Could it have been avoided?
Rather, the calculation of excess mortality relies on large numbers and statistical inference to compare an expected number of deaths with those that are observed.
The difference is excess mortality, even if you can never be sure whether any particular death was expected or not.
As always, however, the devil is in the details. What data do you use to define the expected number of deaths?
There are options here. Probably the most straightforward analysis uses past data from the country of interest. You look at annual deaths over some historical period of time and compare those numbers with the rates today. Two issues need to be accounted for here: population growth – a larger population will have more deaths, so you need to adjust the historical population with current levels, and demographic shifts – an older or more male population will have more deaths, so you need to adjust for that as well.
But provided you take care of those factors, you can estimate fairly well how many deaths you can expect to see in any given period of time.
Still, you should see right away that excess mortality is a relative concept. If you think that, just perhaps, the United States has some systematic failure to deliver care that has been stable and persistent over time, you wouldn’t capture that failing in an excess mortality calculation that uses U.S. historical data as the baseline.
The best way to get around that is to use data from other countries, and that’s just what this article – a rare single-author piece by Patrick Heuveline – does, calculating excess deaths in the United States by standardizing our mortality rates to the five largest Western European countries: the United Kingdom, France, Germany, Italy, and Spain.
Controlling for the differences in the demographics of that European population, here is the expected number of deaths in the United States over the past 5 years.
Note that there is a small uptick in expected deaths in 2020, reflecting the pandemic, which returns to baseline levels by 2021. This is because that’s what happened in Europe; by 2021, the excess mortality due to COVID-19 was quite low.
Here are the actual deaths in the US during that time.
Highlighted here in green, then, is the excess mortality over time in the United States.
There are some fascinating and concerning findings here.
First of all, you can see that even before the pandemic, the United States has an excess mortality problem. This is not entirely a surprise; we’ve known that so-called “deaths of despair,” those due to alcohol abuse, drug overdoses, and suicide, are at an all-time high and tend to affect a “prime of life” population that would not otherwise be expected to die. In fact, fully 50% of the excess deaths in the United States occur in those between ages 15 and 64.
Excess deaths are also a concerning percentage of total deaths. In 2017, 17% of total deaths in the United States could be considered “excess.” In 2021, that number had doubled to 35%. Nearly 900,000 individuals in the United States died in 2021 who perhaps didn’t need to.
The obvious culprit to blame here is COVID, but COVID-associated excess deaths only explain about 50% of the excess we see in 2021. The rest reflect something even more concerning: a worsening of the failures of the past, perhaps exacerbated by the pandemic but not due to the virus itself.
Of course, we started this discussion acknowledging that the calculation of excess mortality is exquisitely dependent on how you model the expected number of deaths, and I’m sure some will take issue with the use of European numbers when applied to Americans. After all, Europe has, by and large, a robust public health service, socialized medicine, and healthcare that does not run the risk of bankrupting its citizens. How can we compare our outcomes to a place like that?
How indeed.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale University’s Clinical and Translational Research Accelerator in New Haven,Conn. He reported no relevant conflicts of interest.
A version of this article originally appeared on Medscape.com.
Risk for MS in children often missed
Imaging tests may miss early signs of multiple sclerosis (MS) in children who have no symptoms of the disease, according to a recent study that points to the need for a change in diagnostic criteria for the neuromuscular condition.
The findings suggest that children, unlike adults, may not need to meet the current clinical standard criteria to be considered at risk for MS.
“This is an important study confirming that some children who have no symptoms of demyelinating disease may nonetheless have MRI findings suggestive of demyelination detected on brain imaging,” said Naila Makhani, MD, associate professor of pediatrics and of neurology at Yale University and director of the Yale Pediatric Neuroimmunology Program, New Haven, Conn. Dr. Makhani was not affiliated with the study.
Researchers reviewed the MRI scans of 38 children aged 7-17 years who had radiologically isolated syndrome (RIS), a possible precursor to MS.
Like MS, RIS is characterized by destruction of the myelin. However, RIS is generally asymptomatic.
While RIS has been linked to MS, a diagnosis of RIS does not mean someone will be diagnosed with MS. Previous studies have shown that at least 3% of MS cases begin before age 16.
The children in the study likely received an MRI because of complaints of headaches or after having been diagnosed with a concussion, according to the researchers. The participants also did not show physical symptoms for MS, nor did they meet the McDonald or Barkohf criteria, which are clinical standards used to diagnose the condition in adults and children.
Within an average of 3 years following the imaging and RIS diagnosis, almost 36% of the children experienced a clinical attack, which led to an MS diagnosis. Almost three-fourths of the children developed additional brain and spinal cord lesions in the myelin that were evident on MRI.
MS often is diagnosed after a patient has had a clinical attack, such as vision impairment, loss of balance, inflammation, or severe fatigue. Identifying the potential for the disease earlier may allow clinicians to treat sooner, according to Leslie Benson, MD, assistant director of pediatric neuroimmunology at Massachusetts General Hospital, Boston, and one of the study authors.
“The field is leaning toward [the question of], ‘Should we treat presymptomatic MS?’ ” said Dr. Benson. “If we have the opportunity to prevent disability and improve long-term outcomes with safe medications, then we would like to do so.”
The findings were published in the journal Multiple Sclerosis and Related Disorders.
According to Dr. Benson and her colleagues, adjustments to the McDonald or Barkohf criteria for children may help in the detection of RIS and may allow earlier identification of MS.
“We don’t really know when MS first starts,” Dr. Benson said. “Unless you happen to have an MRI or symptoms, there’s no way to know how long the lesions have been evolving and how long the disease progression that led to those lesions has been there.”
MRI images showing lesions in the brain stem and spinal cord of children appeared to be different from those typically seen in adults, according to Tanuja Chitnis, MD, director of the Mass General Brigham Pediatric MS Center in Boston, who is one of the study’s coauthors.
“The concern of many practitioners is whether we should be treating at the first sign of MS,” Dr. Chitnis said. “We need to understand it better in children, and in teenagers especially, when these probably start biologically.”
Dr. Benson said current criteria for diagnosing MS in children require meeting a high threshold, which may limit diagnoses to those whose condition has progressed.
“This may miss patients at risk for MS,” Dr. Benson said. “That idea of who do you diagnose RIS and what criteria work to accurately diagnose RIS is really important.”
For now, the challenge remains of investigating characteristics of patients with RIS who will later have a clinical attack.
“We need a better understanding of what criteria do need to be met and how we can best risk-stratify our patients,” Dr. Benson said. “If it is recommended to treat presymptomatic cases, that we can best stratify those at risk and not overtreat those not at risk.”
Dr. Makhani receives funding from the National Institutes of Health, the Charles H. Hood Foundation, and the Multiple Sclerosis Society.
A version of this article originally appeared on Medscape.com.
Imaging tests may miss early signs of multiple sclerosis (MS) in children who have no symptoms of the disease, according to a recent study that points to the need for a change in diagnostic criteria for the neuromuscular condition.
The findings suggest that children, unlike adults, may not need to meet the current clinical standard criteria to be considered at risk for MS.
“This is an important study confirming that some children who have no symptoms of demyelinating disease may nonetheless have MRI findings suggestive of demyelination detected on brain imaging,” said Naila Makhani, MD, associate professor of pediatrics and of neurology at Yale University and director of the Yale Pediatric Neuroimmunology Program, New Haven, Conn. Dr. Makhani was not affiliated with the study.
Researchers reviewed the MRI scans of 38 children aged 7-17 years who had radiologically isolated syndrome (RIS), a possible precursor to MS.
Like MS, RIS is characterized by destruction of the myelin. However, RIS is generally asymptomatic.
While RIS has been linked to MS, a diagnosis of RIS does not mean someone will be diagnosed with MS. Previous studies have shown that at least 3% of MS cases begin before age 16.
The children in the study likely received an MRI because of complaints of headaches or after having been diagnosed with a concussion, according to the researchers. The participants also did not show physical symptoms for MS, nor did they meet the McDonald or Barkohf criteria, which are clinical standards used to diagnose the condition in adults and children.
Within an average of 3 years following the imaging and RIS diagnosis, almost 36% of the children experienced a clinical attack, which led to an MS diagnosis. Almost three-fourths of the children developed additional brain and spinal cord lesions in the myelin that were evident on MRI.
MS often is diagnosed after a patient has had a clinical attack, such as vision impairment, loss of balance, inflammation, or severe fatigue. Identifying the potential for the disease earlier may allow clinicians to treat sooner, according to Leslie Benson, MD, assistant director of pediatric neuroimmunology at Massachusetts General Hospital, Boston, and one of the study authors.
“The field is leaning toward [the question of], ‘Should we treat presymptomatic MS?’ ” said Dr. Benson. “If we have the opportunity to prevent disability and improve long-term outcomes with safe medications, then we would like to do so.”
The findings were published in the journal Multiple Sclerosis and Related Disorders.
According to Dr. Benson and her colleagues, adjustments to the McDonald or Barkohf criteria for children may help in the detection of RIS and may allow earlier identification of MS.
“We don’t really know when MS first starts,” Dr. Benson said. “Unless you happen to have an MRI or symptoms, there’s no way to know how long the lesions have been evolving and how long the disease progression that led to those lesions has been there.”
MRI images showing lesions in the brain stem and spinal cord of children appeared to be different from those typically seen in adults, according to Tanuja Chitnis, MD, director of the Mass General Brigham Pediatric MS Center in Boston, who is one of the study’s coauthors.
“The concern of many practitioners is whether we should be treating at the first sign of MS,” Dr. Chitnis said. “We need to understand it better in children, and in teenagers especially, when these probably start biologically.”
Dr. Benson said current criteria for diagnosing MS in children require meeting a high threshold, which may limit diagnoses to those whose condition has progressed.
“This may miss patients at risk for MS,” Dr. Benson said. “That idea of who do you diagnose RIS and what criteria work to accurately diagnose RIS is really important.”
For now, the challenge remains of investigating characteristics of patients with RIS who will later have a clinical attack.
“We need a better understanding of what criteria do need to be met and how we can best risk-stratify our patients,” Dr. Benson said. “If it is recommended to treat presymptomatic cases, that we can best stratify those at risk and not overtreat those not at risk.”
Dr. Makhani receives funding from the National Institutes of Health, the Charles H. Hood Foundation, and the Multiple Sclerosis Society.
A version of this article originally appeared on Medscape.com.
Imaging tests may miss early signs of multiple sclerosis (MS) in children who have no symptoms of the disease, according to a recent study that points to the need for a change in diagnostic criteria for the neuromuscular condition.
The findings suggest that children, unlike adults, may not need to meet the current clinical standard criteria to be considered at risk for MS.
“This is an important study confirming that some children who have no symptoms of demyelinating disease may nonetheless have MRI findings suggestive of demyelination detected on brain imaging,” said Naila Makhani, MD, associate professor of pediatrics and of neurology at Yale University and director of the Yale Pediatric Neuroimmunology Program, New Haven, Conn. Dr. Makhani was not affiliated with the study.
Researchers reviewed the MRI scans of 38 children aged 7-17 years who had radiologically isolated syndrome (RIS), a possible precursor to MS.
Like MS, RIS is characterized by destruction of the myelin. However, RIS is generally asymptomatic.
While RIS has been linked to MS, a diagnosis of RIS does not mean someone will be diagnosed with MS. Previous studies have shown that at least 3% of MS cases begin before age 16.
The children in the study likely received an MRI because of complaints of headaches or after having been diagnosed with a concussion, according to the researchers. The participants also did not show physical symptoms for MS, nor did they meet the McDonald or Barkohf criteria, which are clinical standards used to diagnose the condition in adults and children.
Within an average of 3 years following the imaging and RIS diagnosis, almost 36% of the children experienced a clinical attack, which led to an MS diagnosis. Almost three-fourths of the children developed additional brain and spinal cord lesions in the myelin that were evident on MRI.
MS often is diagnosed after a patient has had a clinical attack, such as vision impairment, loss of balance, inflammation, or severe fatigue. Identifying the potential for the disease earlier may allow clinicians to treat sooner, according to Leslie Benson, MD, assistant director of pediatric neuroimmunology at Massachusetts General Hospital, Boston, and one of the study authors.
“The field is leaning toward [the question of], ‘Should we treat presymptomatic MS?’ ” said Dr. Benson. “If we have the opportunity to prevent disability and improve long-term outcomes with safe medications, then we would like to do so.”
The findings were published in the journal Multiple Sclerosis and Related Disorders.
According to Dr. Benson and her colleagues, adjustments to the McDonald or Barkohf criteria for children may help in the detection of RIS and may allow earlier identification of MS.
“We don’t really know when MS first starts,” Dr. Benson said. “Unless you happen to have an MRI or symptoms, there’s no way to know how long the lesions have been evolving and how long the disease progression that led to those lesions has been there.”
MRI images showing lesions in the brain stem and spinal cord of children appeared to be different from those typically seen in adults, according to Tanuja Chitnis, MD, director of the Mass General Brigham Pediatric MS Center in Boston, who is one of the study’s coauthors.
“The concern of many practitioners is whether we should be treating at the first sign of MS,” Dr. Chitnis said. “We need to understand it better in children, and in teenagers especially, when these probably start biologically.”
Dr. Benson said current criteria for diagnosing MS in children require meeting a high threshold, which may limit diagnoses to those whose condition has progressed.
“This may miss patients at risk for MS,” Dr. Benson said. “That idea of who do you diagnose RIS and what criteria work to accurately diagnose RIS is really important.”
For now, the challenge remains of investigating characteristics of patients with RIS who will later have a clinical attack.
“We need a better understanding of what criteria do need to be met and how we can best risk-stratify our patients,” Dr. Benson said. “If it is recommended to treat presymptomatic cases, that we can best stratify those at risk and not overtreat those not at risk.”
Dr. Makhani receives funding from the National Institutes of Health, the Charles H. Hood Foundation, and the Multiple Sclerosis Society.
A version of this article originally appeared on Medscape.com.
Exercise tied to reduced Parkinson’s motor symptoms and increased well-being
A systematic review of 156 clinical trials involving 8,000 patients with Parkinson’s disease showed dancing and aquatic exercise, in particular, were most likely to improve motor symptoms, while swimming, endurance training, and mind-body training were most likely to benefit quality of life.
“For most types of exercise we studied, we observed positive effects on both the severity of motor signs and quality of life. These results highlight the importance of exercise in general, as they suggest people with Parkinson’s disease can benefit from a variety of exercises,” said study investigator Moritz Ernst, MSc, deputy head of the working group on evidence-based medicine at the University Hospital Cologne (Germany).
“Clinicians and people with Parkinson’s disease may have several options of exercise programs to choose from when establishing an individual training routine,” he added, emphasizing that overall those with Parkinson’s disease should seek professional advice, including assessment of motor and nonmotor symptoms, to develop a training agenda based on their individual needs.
The study was published online in the Cochrane Database of Systematic Reviews.
May I have this dance?
The investigators analyzed data from randomized, controlled trials comparing different types of exercise and no exercise and the subsequent effect on Parkinson’s disease symptoms. Exercise included dance, strength-resistance training, mind-body training such as tai chi and yoga, water-based training, resistance training, gait/balance/functional training, and endurance training.
The average age of study participants ranged from 60 to 74 years, and most of the studies included patients with mild to moderate Parkinson’s disease. The mean length of the various interventions was 12 weeks.
When the researchers examined the effect of exercise on motor symptoms, they found that dance (P = .88), aqua-based training (P = .69), and gait/balance/functional training (P = .67) were most likely to reduce symptom severity.
Aqua-based training (P = .95), endurance training (P = .77), and mind-body training (P = .75) were most were most likely to benefit quality of life, although the investigators caution that these findings were at risk of bias because quality of life was self-reported.
The investigators noted other study limitations including the fact that most of the studies included in the review had small sample sizes and their study only included patients with mild to moderate versus severe Parkinson’s disease.
The authors said that future research should include larger samples, report intent-to-treat analyses, and involve participants with more advanced forms of Parkinson’s disease who may also have cognitive difficulties.
Prescribe exercise
“We should be giving our patients, no matter where they are in their disease stage, a ‘prescription’ to exercise,” said Mitra Afshari, MD, MPH. Dr. Afshari was not involved in the study but leads her own research on Parkinson’s disease and exercise as the site principal investigator on the National Institutes of Health–funded SPARX3 Study in Parkinson’s Disease and Exercise at Rush University in Chicago. She said that, based on her experience caring for patients with Parkinson’s disease at all disease stages, “patients who have been physically active their whole lives and can maintain that activity despite their diagnosis fare the best.”
However, she added, those who initiate physical exercise after diagnosis can also do very well and reap benefits, including improved motor symptoms.
The study was funded by University Hospital of Cologne, Faculty of Medicine and University Hospital, University of Cologne, and the German Ministry of Education and Research. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A systematic review of 156 clinical trials involving 8,000 patients with Parkinson’s disease showed dancing and aquatic exercise, in particular, were most likely to improve motor symptoms, while swimming, endurance training, and mind-body training were most likely to benefit quality of life.
“For most types of exercise we studied, we observed positive effects on both the severity of motor signs and quality of life. These results highlight the importance of exercise in general, as they suggest people with Parkinson’s disease can benefit from a variety of exercises,” said study investigator Moritz Ernst, MSc, deputy head of the working group on evidence-based medicine at the University Hospital Cologne (Germany).
“Clinicians and people with Parkinson’s disease may have several options of exercise programs to choose from when establishing an individual training routine,” he added, emphasizing that overall those with Parkinson’s disease should seek professional advice, including assessment of motor and nonmotor symptoms, to develop a training agenda based on their individual needs.
The study was published online in the Cochrane Database of Systematic Reviews.
May I have this dance?
The investigators analyzed data from randomized, controlled trials comparing different types of exercise and no exercise and the subsequent effect on Parkinson’s disease symptoms. Exercise included dance, strength-resistance training, mind-body training such as tai chi and yoga, water-based training, resistance training, gait/balance/functional training, and endurance training.
The average age of study participants ranged from 60 to 74 years, and most of the studies included patients with mild to moderate Parkinson’s disease. The mean length of the various interventions was 12 weeks.
When the researchers examined the effect of exercise on motor symptoms, they found that dance (P = .88), aqua-based training (P = .69), and gait/balance/functional training (P = .67) were most likely to reduce symptom severity.
Aqua-based training (P = .95), endurance training (P = .77), and mind-body training (P = .75) were most were most likely to benefit quality of life, although the investigators caution that these findings were at risk of bias because quality of life was self-reported.
The investigators noted other study limitations including the fact that most of the studies included in the review had small sample sizes and their study only included patients with mild to moderate versus severe Parkinson’s disease.
The authors said that future research should include larger samples, report intent-to-treat analyses, and involve participants with more advanced forms of Parkinson’s disease who may also have cognitive difficulties.
Prescribe exercise
“We should be giving our patients, no matter where they are in their disease stage, a ‘prescription’ to exercise,” said Mitra Afshari, MD, MPH. Dr. Afshari was not involved in the study but leads her own research on Parkinson’s disease and exercise as the site principal investigator on the National Institutes of Health–funded SPARX3 Study in Parkinson’s Disease and Exercise at Rush University in Chicago. She said that, based on her experience caring for patients with Parkinson’s disease at all disease stages, “patients who have been physically active their whole lives and can maintain that activity despite their diagnosis fare the best.”
However, she added, those who initiate physical exercise after diagnosis can also do very well and reap benefits, including improved motor symptoms.
The study was funded by University Hospital of Cologne, Faculty of Medicine and University Hospital, University of Cologne, and the German Ministry of Education and Research. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A systematic review of 156 clinical trials involving 8,000 patients with Parkinson’s disease showed dancing and aquatic exercise, in particular, were most likely to improve motor symptoms, while swimming, endurance training, and mind-body training were most likely to benefit quality of life.
“For most types of exercise we studied, we observed positive effects on both the severity of motor signs and quality of life. These results highlight the importance of exercise in general, as they suggest people with Parkinson’s disease can benefit from a variety of exercises,” said study investigator Moritz Ernst, MSc, deputy head of the working group on evidence-based medicine at the University Hospital Cologne (Germany).
“Clinicians and people with Parkinson’s disease may have several options of exercise programs to choose from when establishing an individual training routine,” he added, emphasizing that overall those with Parkinson’s disease should seek professional advice, including assessment of motor and nonmotor symptoms, to develop a training agenda based on their individual needs.
The study was published online in the Cochrane Database of Systematic Reviews.
May I have this dance?
The investigators analyzed data from randomized, controlled trials comparing different types of exercise and no exercise and the subsequent effect on Parkinson’s disease symptoms. Exercise included dance, strength-resistance training, mind-body training such as tai chi and yoga, water-based training, resistance training, gait/balance/functional training, and endurance training.
The average age of study participants ranged from 60 to 74 years, and most of the studies included patients with mild to moderate Parkinson’s disease. The mean length of the various interventions was 12 weeks.
When the researchers examined the effect of exercise on motor symptoms, they found that dance (P = .88), aqua-based training (P = .69), and gait/balance/functional training (P = .67) were most likely to reduce symptom severity.
Aqua-based training (P = .95), endurance training (P = .77), and mind-body training (P = .75) were most were most likely to benefit quality of life, although the investigators caution that these findings were at risk of bias because quality of life was self-reported.
The investigators noted other study limitations including the fact that most of the studies included in the review had small sample sizes and their study only included patients with mild to moderate versus severe Parkinson’s disease.
The authors said that future research should include larger samples, report intent-to-treat analyses, and involve participants with more advanced forms of Parkinson’s disease who may also have cognitive difficulties.
Prescribe exercise
“We should be giving our patients, no matter where they are in their disease stage, a ‘prescription’ to exercise,” said Mitra Afshari, MD, MPH. Dr. Afshari was not involved in the study but leads her own research on Parkinson’s disease and exercise as the site principal investigator on the National Institutes of Health–funded SPARX3 Study in Parkinson’s Disease and Exercise at Rush University in Chicago. She said that, based on her experience caring for patients with Parkinson’s disease at all disease stages, “patients who have been physically active their whole lives and can maintain that activity despite their diagnosis fare the best.”
However, she added, those who initiate physical exercise after diagnosis can also do very well and reap benefits, including improved motor symptoms.
The study was funded by University Hospital of Cologne, Faculty of Medicine and University Hospital, University of Cologne, and the German Ministry of Education and Research. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE COCHRANE DATABASE OF SYSTEMATIC REVIEWS