User login
OSA raises risk of atrial fibrillation and stroke
compared with controls, based on data from 303 individuals.
OSA has become a common chronic disease, and cardiovascular diseases including AFib also are known independent risk factors associated with OSA, Anna Hojager, MD, of Zealand University Hospital, Roskilde, Denmark, and colleagues wrote. Previous studies have shown a significant increase in AFib risk in OSA patients with severe disease, but the prevalence of undiagnosed AFib in OSA patients has not been explored.
In a study published in Sleep Medicine, the researchers enrolled 238 adults with severe OSA (based on apnea-hypopnea index of 15 or higher) and 65 with mild or no OSA (based on an AHI of less than 15). The mean AHI across all participants was 34.2, and ranged from 0.2 to 115.8.
Participants underwent heart rhythm monitoring using a home system or standard ECG for 7 days; they were instructed to carry the device at all times except when showering or sweating heavily. The primary outcome was the detection of AFib, defined as at least one period of 30 seconds or longer with an irregular heart rhythm but without detectable evidence of another diagnosis. Sleep was assessed for one night using a portable sleep monitoring device. All participants were examined at baseline and measured for blood pressure, body mass index, waist-to-hip ratio, and ECG.
Overall, AFib occurred in 21 patients with moderate to severe OSA and 1 patient with mild/no OSA (8.8% vs. 1.5%, P = .045). The majority of patients across both groups had hypertension (66%) and dyslipidemia (77.6%), but the severe OSA group was more likely to be dysregulated and to have unknown prediabetes. Participants who were deemed candidates for anticoagulation therapy were referred for additional treatment. None of the 22 total patients with AFib had heart failure with reduced ejection fraction, and 68.2% had normal ejection fraction and ventricle function.
The researchers noted that no guidelines currently exist for systematic opportunistic screening for comorbidities in OSA patients, although the American Academy of Sleep Medicine recommends patient education as part of a multidisciplinary chronic disease management strategy. The high prevalence of AFib in OSA patients, as seen in the current study, “might warrant a recommendation of screening for paroxysmal [AFib] and could be valuable in the management of modifiable cardiovascular risk factors in patients with OSA,” they wrote.
The study findings were limited by several factors including the observational design and absence of polysomnography to assess OSA, the researchers noted. However, the study has the highest known prevalence of silent AFib in patients with moderate to severe OSA, and supports the value of screening and management for known comorbidities of OSA.
The study received no outside funding. The researchers had no financial conflicts to disclose.
compared with controls, based on data from 303 individuals.
OSA has become a common chronic disease, and cardiovascular diseases including AFib also are known independent risk factors associated with OSA, Anna Hojager, MD, of Zealand University Hospital, Roskilde, Denmark, and colleagues wrote. Previous studies have shown a significant increase in AFib risk in OSA patients with severe disease, but the prevalence of undiagnosed AFib in OSA patients has not been explored.
In a study published in Sleep Medicine, the researchers enrolled 238 adults with severe OSA (based on apnea-hypopnea index of 15 or higher) and 65 with mild or no OSA (based on an AHI of less than 15). The mean AHI across all participants was 34.2, and ranged from 0.2 to 115.8.
Participants underwent heart rhythm monitoring using a home system or standard ECG for 7 days; they were instructed to carry the device at all times except when showering or sweating heavily. The primary outcome was the detection of AFib, defined as at least one period of 30 seconds or longer with an irregular heart rhythm but without detectable evidence of another diagnosis. Sleep was assessed for one night using a portable sleep monitoring device. All participants were examined at baseline and measured for blood pressure, body mass index, waist-to-hip ratio, and ECG.
Overall, AFib occurred in 21 patients with moderate to severe OSA and 1 patient with mild/no OSA (8.8% vs. 1.5%, P = .045). The majority of patients across both groups had hypertension (66%) and dyslipidemia (77.6%), but the severe OSA group was more likely to be dysregulated and to have unknown prediabetes. Participants who were deemed candidates for anticoagulation therapy were referred for additional treatment. None of the 22 total patients with AFib had heart failure with reduced ejection fraction, and 68.2% had normal ejection fraction and ventricle function.
The researchers noted that no guidelines currently exist for systematic opportunistic screening for comorbidities in OSA patients, although the American Academy of Sleep Medicine recommends patient education as part of a multidisciplinary chronic disease management strategy. The high prevalence of AFib in OSA patients, as seen in the current study, “might warrant a recommendation of screening for paroxysmal [AFib] and could be valuable in the management of modifiable cardiovascular risk factors in patients with OSA,” they wrote.
The study findings were limited by several factors including the observational design and absence of polysomnography to assess OSA, the researchers noted. However, the study has the highest known prevalence of silent AFib in patients with moderate to severe OSA, and supports the value of screening and management for known comorbidities of OSA.
The study received no outside funding. The researchers had no financial conflicts to disclose.
compared with controls, based on data from 303 individuals.
OSA has become a common chronic disease, and cardiovascular diseases including AFib also are known independent risk factors associated with OSA, Anna Hojager, MD, of Zealand University Hospital, Roskilde, Denmark, and colleagues wrote. Previous studies have shown a significant increase in AFib risk in OSA patients with severe disease, but the prevalence of undiagnosed AFib in OSA patients has not been explored.
In a study published in Sleep Medicine, the researchers enrolled 238 adults with severe OSA (based on apnea-hypopnea index of 15 or higher) and 65 with mild or no OSA (based on an AHI of less than 15). The mean AHI across all participants was 34.2, and ranged from 0.2 to 115.8.
Participants underwent heart rhythm monitoring using a home system or standard ECG for 7 days; they were instructed to carry the device at all times except when showering or sweating heavily. The primary outcome was the detection of AFib, defined as at least one period of 30 seconds or longer with an irregular heart rhythm but without detectable evidence of another diagnosis. Sleep was assessed for one night using a portable sleep monitoring device. All participants were examined at baseline and measured for blood pressure, body mass index, waist-to-hip ratio, and ECG.
Overall, AFib occurred in 21 patients with moderate to severe OSA and 1 patient with mild/no OSA (8.8% vs. 1.5%, P = .045). The majority of patients across both groups had hypertension (66%) and dyslipidemia (77.6%), but the severe OSA group was more likely to be dysregulated and to have unknown prediabetes. Participants who were deemed candidates for anticoagulation therapy were referred for additional treatment. None of the 22 total patients with AFib had heart failure with reduced ejection fraction, and 68.2% had normal ejection fraction and ventricle function.
The researchers noted that no guidelines currently exist for systematic opportunistic screening for comorbidities in OSA patients, although the American Academy of Sleep Medicine recommends patient education as part of a multidisciplinary chronic disease management strategy. The high prevalence of AFib in OSA patients, as seen in the current study, “might warrant a recommendation of screening for paroxysmal [AFib] and could be valuable in the management of modifiable cardiovascular risk factors in patients with OSA,” they wrote.
The study findings were limited by several factors including the observational design and absence of polysomnography to assess OSA, the researchers noted. However, the study has the highest known prevalence of silent AFib in patients with moderate to severe OSA, and supports the value of screening and management for known comorbidities of OSA.
The study received no outside funding. The researchers had no financial conflicts to disclose.
FROM SLEEP MEDICINE
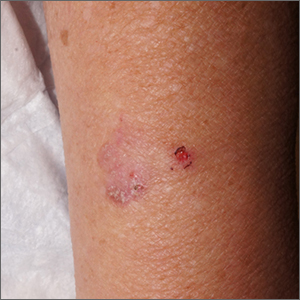
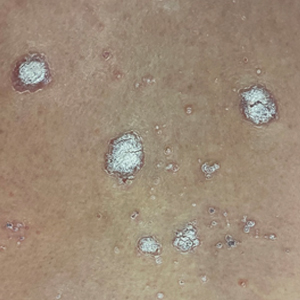
Scaly forearm plaque
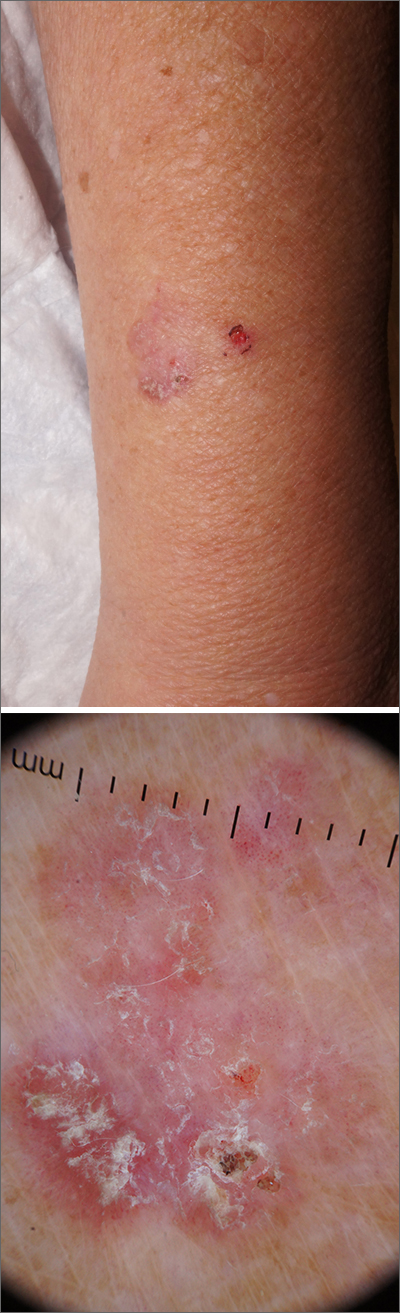
Dermoscopy revealed a keratotic, 2.5-cm scaly plaque with linearly arranged dotted vessels, ulceration, and shiny white lines. A shave biopsy was consistent with a squamous cell carcinoma in situ (SCC in situ)—a pre-invasive keratinocyte carcinoma.
SCC in situ, also known as Bowen’s disease, is a very common skin cancer that can be easily treated. Lesions may manifest anywhere on the skin but are most often found on sun-damaged areas. Actinic keratoses are a pre-malignant precursor of SCC in situ; both are characterized by a sandpapery rough surface on a pink or brown background. Histologically, SCC in situ has atypia of keratinocytes over the full thickness of the epidermis, while actinic keratoses have limited atypia of the upper epidermis only. With this in mind, suspect SCC in situ (over actinic keratosis) when a lesion is thicker than 1 mm, larger in diameter than 5 mm, or painful.1
Treatment options include surgical and nonsurgical modalities. Excision and electrodessication and curettage (EDC) are both effective surgical procedures, with cure rates greater than 90%.2 Nonsurgical options include cryotherapy, 5-fluorouracil (5FU), imiquimod, and photodynamic therapy. Treatment with 5FU or imiquimod involves the application of cream to the lesion for 4 to 6 weeks. Marked inflammation during treatment is to be expected.
In the case described here, the patient underwent EDC in the office and was counseled to continue with complete skin exams twice a year for the next 2 years.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
1. Mills KC, Kwatra SG, Feneran AN, et al. Itch and pain in nonmelanoma skin cancer: pain as an important feature of cutaneous squamous cell carcinoma. Arch Dermatol. 2012;148:1422-1423. doi: 10.1001/archdermatol.2012.3104
2. Reschly MJ, Shenefelt PD. Controversies in skin surgery: electrodessication and curettage versus excision for low-risk, small, well-differentiated squamous cell carcinomas. J Drugs Dermatol. 2010;9:773-776.

Dermoscopy revealed a keratotic, 2.5-cm scaly plaque with linearly arranged dotted vessels, ulceration, and shiny white lines. A shave biopsy was consistent with a squamous cell carcinoma in situ (SCC in situ)—a pre-invasive keratinocyte carcinoma.
SCC in situ, also known as Bowen’s disease, is a very common skin cancer that can be easily treated. Lesions may manifest anywhere on the skin but are most often found on sun-damaged areas. Actinic keratoses are a pre-malignant precursor of SCC in situ; both are characterized by a sandpapery rough surface on a pink or brown background. Histologically, SCC in situ has atypia of keratinocytes over the full thickness of the epidermis, while actinic keratoses have limited atypia of the upper epidermis only. With this in mind, suspect SCC in situ (over actinic keratosis) when a lesion is thicker than 1 mm, larger in diameter than 5 mm, or painful.1
Treatment options include surgical and nonsurgical modalities. Excision and electrodessication and curettage (EDC) are both effective surgical procedures, with cure rates greater than 90%.2 Nonsurgical options include cryotherapy, 5-fluorouracil (5FU), imiquimod, and photodynamic therapy. Treatment with 5FU or imiquimod involves the application of cream to the lesion for 4 to 6 weeks. Marked inflammation during treatment is to be expected.
In the case described here, the patient underwent EDC in the office and was counseled to continue with complete skin exams twice a year for the next 2 years.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.

Dermoscopy revealed a keratotic, 2.5-cm scaly plaque with linearly arranged dotted vessels, ulceration, and shiny white lines. A shave biopsy was consistent with a squamous cell carcinoma in situ (SCC in situ)—a pre-invasive keratinocyte carcinoma.
SCC in situ, also known as Bowen’s disease, is a very common skin cancer that can be easily treated. Lesions may manifest anywhere on the skin but are most often found on sun-damaged areas. Actinic keratoses are a pre-malignant precursor of SCC in situ; both are characterized by a sandpapery rough surface on a pink or brown background. Histologically, SCC in situ has atypia of keratinocytes over the full thickness of the epidermis, while actinic keratoses have limited atypia of the upper epidermis only. With this in mind, suspect SCC in situ (over actinic keratosis) when a lesion is thicker than 1 mm, larger in diameter than 5 mm, or painful.1
Treatment options include surgical and nonsurgical modalities. Excision and electrodessication and curettage (EDC) are both effective surgical procedures, with cure rates greater than 90%.2 Nonsurgical options include cryotherapy, 5-fluorouracil (5FU), imiquimod, and photodynamic therapy. Treatment with 5FU or imiquimod involves the application of cream to the lesion for 4 to 6 weeks. Marked inflammation during treatment is to be expected.
In the case described here, the patient underwent EDC in the office and was counseled to continue with complete skin exams twice a year for the next 2 years.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
1. Mills KC, Kwatra SG, Feneran AN, et al. Itch and pain in nonmelanoma skin cancer: pain as an important feature of cutaneous squamous cell carcinoma. Arch Dermatol. 2012;148:1422-1423. doi: 10.1001/archdermatol.2012.3104
2. Reschly MJ, Shenefelt PD. Controversies in skin surgery: electrodessication and curettage versus excision for low-risk, small, well-differentiated squamous cell carcinomas. J Drugs Dermatol. 2010;9:773-776.
1. Mills KC, Kwatra SG, Feneran AN, et al. Itch and pain in nonmelanoma skin cancer: pain as an important feature of cutaneous squamous cell carcinoma. Arch Dermatol. 2012;148:1422-1423. doi: 10.1001/archdermatol.2012.3104
2. Reschly MJ, Shenefelt PD. Controversies in skin surgery: electrodessication and curettage versus excision for low-risk, small, well-differentiated squamous cell carcinomas. J Drugs Dermatol. 2010;9:773-776.
New recommendations for hyperglycemia management
This transcript has been edited for clarity.
I’m Dr. Neil Skolnik. Today we’re going to talk about the consensus report by the American Diabetes Association and the European Association for the Study of Diabetes on the management of hyperglycemia.
After lifestyle modifications, metformin is no longer the go-to drug for every patient in the management of hyperglycemia. It is recommended that we assess each patient’s personal characteristics in deciding what medication to prescribe. For patients at high cardiorenal risk, refer to the left side of the algorithm and to the right side for all other patients.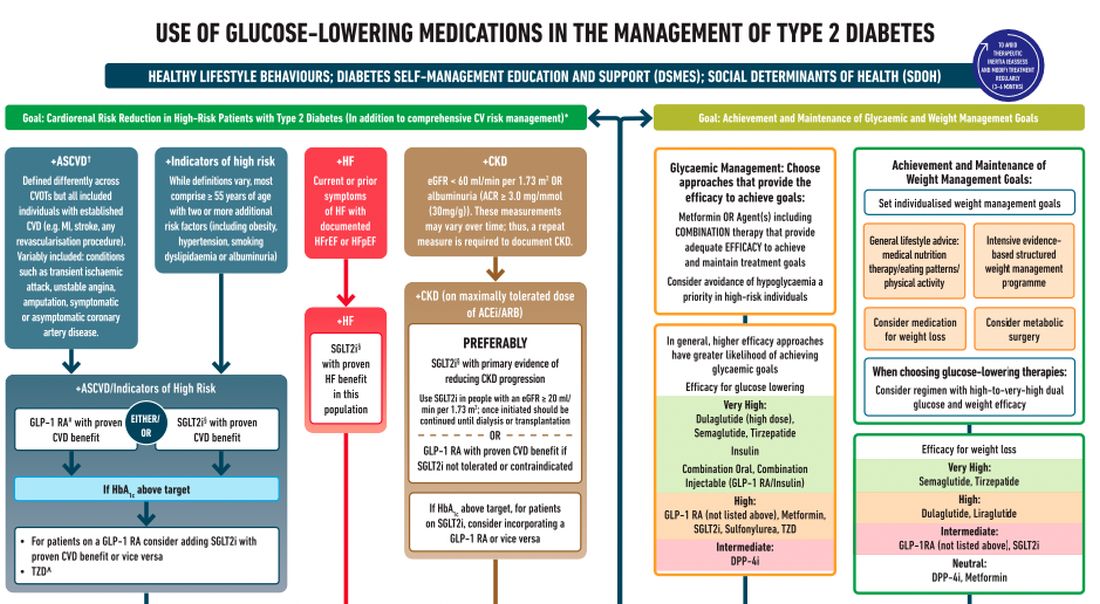
Cardiovascular disease. First, assess whether the patient is at high risk for atherosclerotic cardiovascular disease (ASCVD) or already has ASCVD. How is ASCVD defined? Either coronary artery disease (a history of a myocardial infarction [MI] or coronary disease), peripheral vascular disease, stroke, or transient ischemic attack.
What is high risk for ASCVD? Diabetes in someone older than 55 years with two or more additional risk factors. If the patient is at high risk for or has existing ASCVD then it is recommended to prescribe a glucagon-like peptide 1 (GLP-1) agonist with proven CVD benefit or an sodium-glucose cotransporter 2 (SGLT-2) inhibitor with proven CVD benefit.
For patients at very high risk for ASCVD, it might be reasonable to combine both agents. The recommendation to use these agents holds true whether the patients are at their A1c goals or not. The patient doesn’t need to be on metformin to benefit from these agents. The patient with reduced or preserved ejection fraction heart failure should be taking an SGLT-2 inhibitor.
Chronic kidney disease. Next up, chronic kidney disease (CKD). CKD is defined by an estimated glomerular filtration rate < 60 mL/min/1.73 m2 or a urine albumin to creatinine ratio > 30. In that case, the patient should be preferentially on an SGLT-2 inhibitor. Patients not able to take an SGLT-2 for some reason should be prescribed a GLP-1 receptor agonist.
If someone doesn’t fit into that high cardiorenal risk category, then we go to the right side of the algorithm. The goal then is achievement and maintenance of glycemic and weight management goals.
Glycemic management. In choosing medicine for glycemic management, metformin is a reasonable choice. You may need to add another agent to metformin to reach the patient’s glycemic goal. If the patient is far away from goal, then a medication with higher efficacy at lowering glucose might be chosen.
Efficacy is listed as:
- Very high efficacy for glucose lowering: dulaglutide at a high dose, semaglutide, tirzepatide, insulin, or combination injectable agents (GLP-1 receptor agonist/insulin combinations).
- High glucose-lowering efficacy: a GLP-1 receptor agonist not already mentioned, metformin, SGLT-2 inhibitors, sulfonylureas, thiazolidinediones.
- Intermediate glucose lowering efficacy: dipeptidyl peptidase 4 (DPP-4) inhibitors.
Weight management. For weight management, lifestyle modification (diet and exercise) is important. If lifestyle modification alone is insufficient, consider either a medication that specifically helps with weight management or metabolic surgery.
We particularly want to focus on weight management in patients who have complications from obesity. What would those complications be? Sleep apnea, hip or knee pain from arthritis, back pain – that is, biomechanical complications of obesity or nonalcoholic fatty liver disease. Medications for weight loss are listed by degree of efficacy:
- Very high efficacy for weight loss: semaglutide, tirzepatide.
- High efficacy for weight loss: dulaglutide and liraglutide.
- Intermediate for weight loss: GLP-1 receptor agonist (not listed above), SGLT-2 inhibitor.
- Neutral for weight loss: DPP-4 inhibitors and metformin.
Where does insulin fit in? If patients present with a very high A1c, if they are on other medications and their A1c is still not to goal, or if they are catabolic and losing weight because of their diabetes, then insulin has an important place in management.
These are incredibly important guidelines that provide a clear algorithm for a personalized approach to diabetes management.
Dr. Skolnik is professor, department of family medicine, Sidney Kimmel Medical College, Philadelphia, and associate director, department of family medicine, Abington (Pa.) Jefferson Health. He reported conflicts of interest with AstraZeneca, Teva, Eli Lilly, Boehringer Ingelheim, Sanofi, Sanofi Pasteur, GlaxoSmithKline, Merck, and Bayer. A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
I’m Dr. Neil Skolnik. Today we’re going to talk about the consensus report by the American Diabetes Association and the European Association for the Study of Diabetes on the management of hyperglycemia.
After lifestyle modifications, metformin is no longer the go-to drug for every patient in the management of hyperglycemia. It is recommended that we assess each patient’s personal characteristics in deciding what medication to prescribe. For patients at high cardiorenal risk, refer to the left side of the algorithm and to the right side for all other patients.
Cardiovascular disease. First, assess whether the patient is at high risk for atherosclerotic cardiovascular disease (ASCVD) or already has ASCVD. How is ASCVD defined? Either coronary artery disease (a history of a myocardial infarction [MI] or coronary disease), peripheral vascular disease, stroke, or transient ischemic attack.
What is high risk for ASCVD? Diabetes in someone older than 55 years with two or more additional risk factors. If the patient is at high risk for or has existing ASCVD then it is recommended to prescribe a glucagon-like peptide 1 (GLP-1) agonist with proven CVD benefit or an sodium-glucose cotransporter 2 (SGLT-2) inhibitor with proven CVD benefit.
For patients at very high risk for ASCVD, it might be reasonable to combine both agents. The recommendation to use these agents holds true whether the patients are at their A1c goals or not. The patient doesn’t need to be on metformin to benefit from these agents. The patient with reduced or preserved ejection fraction heart failure should be taking an SGLT-2 inhibitor.
Chronic kidney disease. Next up, chronic kidney disease (CKD). CKD is defined by an estimated glomerular filtration rate < 60 mL/min/1.73 m2 or a urine albumin to creatinine ratio > 30. In that case, the patient should be preferentially on an SGLT-2 inhibitor. Patients not able to take an SGLT-2 for some reason should be prescribed a GLP-1 receptor agonist.
If someone doesn’t fit into that high cardiorenal risk category, then we go to the right side of the algorithm. The goal then is achievement and maintenance of glycemic and weight management goals.
Glycemic management. In choosing medicine for glycemic management, metformin is a reasonable choice. You may need to add another agent to metformin to reach the patient’s glycemic goal. If the patient is far away from goal, then a medication with higher efficacy at lowering glucose might be chosen.
Efficacy is listed as:
- Very high efficacy for glucose lowering: dulaglutide at a high dose, semaglutide, tirzepatide, insulin, or combination injectable agents (GLP-1 receptor agonist/insulin combinations).
- High glucose-lowering efficacy: a GLP-1 receptor agonist not already mentioned, metformin, SGLT-2 inhibitors, sulfonylureas, thiazolidinediones.
- Intermediate glucose lowering efficacy: dipeptidyl peptidase 4 (DPP-4) inhibitors.
Weight management. For weight management, lifestyle modification (diet and exercise) is important. If lifestyle modification alone is insufficient, consider either a medication that specifically helps with weight management or metabolic surgery.
We particularly want to focus on weight management in patients who have complications from obesity. What would those complications be? Sleep apnea, hip or knee pain from arthritis, back pain – that is, biomechanical complications of obesity or nonalcoholic fatty liver disease. Medications for weight loss are listed by degree of efficacy:
- Very high efficacy for weight loss: semaglutide, tirzepatide.
- High efficacy for weight loss: dulaglutide and liraglutide.
- Intermediate for weight loss: GLP-1 receptor agonist (not listed above), SGLT-2 inhibitor.
- Neutral for weight loss: DPP-4 inhibitors and metformin.
Where does insulin fit in? If patients present with a very high A1c, if they are on other medications and their A1c is still not to goal, or if they are catabolic and losing weight because of their diabetes, then insulin has an important place in management.
These are incredibly important guidelines that provide a clear algorithm for a personalized approach to diabetes management.
Dr. Skolnik is professor, department of family medicine, Sidney Kimmel Medical College, Philadelphia, and associate director, department of family medicine, Abington (Pa.) Jefferson Health. He reported conflicts of interest with AstraZeneca, Teva, Eli Lilly, Boehringer Ingelheim, Sanofi, Sanofi Pasteur, GlaxoSmithKline, Merck, and Bayer. A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
I’m Dr. Neil Skolnik. Today we’re going to talk about the consensus report by the American Diabetes Association and the European Association for the Study of Diabetes on the management of hyperglycemia.
After lifestyle modifications, metformin is no longer the go-to drug for every patient in the management of hyperglycemia. It is recommended that we assess each patient’s personal characteristics in deciding what medication to prescribe. For patients at high cardiorenal risk, refer to the left side of the algorithm and to the right side for all other patients.
Cardiovascular disease. First, assess whether the patient is at high risk for atherosclerotic cardiovascular disease (ASCVD) or already has ASCVD. How is ASCVD defined? Either coronary artery disease (a history of a myocardial infarction [MI] or coronary disease), peripheral vascular disease, stroke, or transient ischemic attack.
What is high risk for ASCVD? Diabetes in someone older than 55 years with two or more additional risk factors. If the patient is at high risk for or has existing ASCVD then it is recommended to prescribe a glucagon-like peptide 1 (GLP-1) agonist with proven CVD benefit or an sodium-glucose cotransporter 2 (SGLT-2) inhibitor with proven CVD benefit.
For patients at very high risk for ASCVD, it might be reasonable to combine both agents. The recommendation to use these agents holds true whether the patients are at their A1c goals or not. The patient doesn’t need to be on metformin to benefit from these agents. The patient with reduced or preserved ejection fraction heart failure should be taking an SGLT-2 inhibitor.
Chronic kidney disease. Next up, chronic kidney disease (CKD). CKD is defined by an estimated glomerular filtration rate < 60 mL/min/1.73 m2 or a urine albumin to creatinine ratio > 30. In that case, the patient should be preferentially on an SGLT-2 inhibitor. Patients not able to take an SGLT-2 for some reason should be prescribed a GLP-1 receptor agonist.
If someone doesn’t fit into that high cardiorenal risk category, then we go to the right side of the algorithm. The goal then is achievement and maintenance of glycemic and weight management goals.
Glycemic management. In choosing medicine for glycemic management, metformin is a reasonable choice. You may need to add another agent to metformin to reach the patient’s glycemic goal. If the patient is far away from goal, then a medication with higher efficacy at lowering glucose might be chosen.
Efficacy is listed as:
- Very high efficacy for glucose lowering: dulaglutide at a high dose, semaglutide, tirzepatide, insulin, or combination injectable agents (GLP-1 receptor agonist/insulin combinations).
- High glucose-lowering efficacy: a GLP-1 receptor agonist not already mentioned, metformin, SGLT-2 inhibitors, sulfonylureas, thiazolidinediones.
- Intermediate glucose lowering efficacy: dipeptidyl peptidase 4 (DPP-4) inhibitors.
Weight management. For weight management, lifestyle modification (diet and exercise) is important. If lifestyle modification alone is insufficient, consider either a medication that specifically helps with weight management or metabolic surgery.
We particularly want to focus on weight management in patients who have complications from obesity. What would those complications be? Sleep apnea, hip or knee pain from arthritis, back pain – that is, biomechanical complications of obesity or nonalcoholic fatty liver disease. Medications for weight loss are listed by degree of efficacy:
- Very high efficacy for weight loss: semaglutide, tirzepatide.
- High efficacy for weight loss: dulaglutide and liraglutide.
- Intermediate for weight loss: GLP-1 receptor agonist (not listed above), SGLT-2 inhibitor.
- Neutral for weight loss: DPP-4 inhibitors and metformin.
Where does insulin fit in? If patients present with a very high A1c, if they are on other medications and their A1c is still not to goal, or if they are catabolic and losing weight because of their diabetes, then insulin has an important place in management.
These are incredibly important guidelines that provide a clear algorithm for a personalized approach to diabetes management.
Dr. Skolnik is professor, department of family medicine, Sidney Kimmel Medical College, Philadelphia, and associate director, department of family medicine, Abington (Pa.) Jefferson Health. He reported conflicts of interest with AstraZeneca, Teva, Eli Lilly, Boehringer Ingelheim, Sanofi, Sanofi Pasteur, GlaxoSmithKline, Merck, and Bayer. A version of this article first appeared on Medscape.com.
RSV causes 1 in 50 deaths in children under age 5: European study
But RSV – formally known as respiratory syncytial virus – is also a problem in high-income nations. In those countries, 1 in 56 otherwise healthy babies are hospitalized with RSV during their first year of life, said the study, which was published in the Lancet Respiratory Medicine.
Researchers looked at the health records of 9,154 infants born between July 1, 2017, and July 31, 2020, who were treated at health centers across Europe. Previous studies have concentrated on babies with preexisting conditions, but this one looked at otherwise healthy children, researchers said.
“This is the lowest-risk baby who is being hospitalized for this, so really, numbers are really much higher than I think some people would have guessed,” said study coauthor Louis Bont, MD, a professor of pediatric infectious diseases at Wilhelmina Children’s Hospital at University Medical Center Utrecht in the Netherlands, according to CNN. He is also chairman of the ReSViNET foundation, which aims to reduce RSV infection globally.
The study said more than 97% of deaths from RSV occur in low-income and middle-income countries. The study concluded that “maternal vaccination and passive [immunization] could have a profound impact on the RSV burden.”
In developed nations, children who get RSV usually survive because they have access to ventilators and other health care equipment. Still, just being treated for RSV can have long-range negative effects on a child’s health, Kristina Deeter, MD, chair of pediatrics at the University of Nevada, Reno, told CNN.
“Whether that is just traumatic psychosocial, emotional issues after hospitalization or even having more vulnerable lungs – you can develop asthma later on, for instance, if you’ve had a really severe infection at a young age – it can damage your lungs permanently,” she said of the study. “It’s still an important virus in our world and something that we really focus on.”
The Lancet study was published days after the CDC warned public health officials that respiratory viruses, including RSV, are surging among children across the country.
A version of this article first appeared on WebMD.com.
But RSV – formally known as respiratory syncytial virus – is also a problem in high-income nations. In those countries, 1 in 56 otherwise healthy babies are hospitalized with RSV during their first year of life, said the study, which was published in the Lancet Respiratory Medicine.
Researchers looked at the health records of 9,154 infants born between July 1, 2017, and July 31, 2020, who were treated at health centers across Europe. Previous studies have concentrated on babies with preexisting conditions, but this one looked at otherwise healthy children, researchers said.
“This is the lowest-risk baby who is being hospitalized for this, so really, numbers are really much higher than I think some people would have guessed,” said study coauthor Louis Bont, MD, a professor of pediatric infectious diseases at Wilhelmina Children’s Hospital at University Medical Center Utrecht in the Netherlands, according to CNN. He is also chairman of the ReSViNET foundation, which aims to reduce RSV infection globally.
The study said more than 97% of deaths from RSV occur in low-income and middle-income countries. The study concluded that “maternal vaccination and passive [immunization] could have a profound impact on the RSV burden.”
In developed nations, children who get RSV usually survive because they have access to ventilators and other health care equipment. Still, just being treated for RSV can have long-range negative effects on a child’s health, Kristina Deeter, MD, chair of pediatrics at the University of Nevada, Reno, told CNN.
“Whether that is just traumatic psychosocial, emotional issues after hospitalization or even having more vulnerable lungs – you can develop asthma later on, for instance, if you’ve had a really severe infection at a young age – it can damage your lungs permanently,” she said of the study. “It’s still an important virus in our world and something that we really focus on.”
The Lancet study was published days after the CDC warned public health officials that respiratory viruses, including RSV, are surging among children across the country.
A version of this article first appeared on WebMD.com.
But RSV – formally known as respiratory syncytial virus – is also a problem in high-income nations. In those countries, 1 in 56 otherwise healthy babies are hospitalized with RSV during their first year of life, said the study, which was published in the Lancet Respiratory Medicine.
Researchers looked at the health records of 9,154 infants born between July 1, 2017, and July 31, 2020, who were treated at health centers across Europe. Previous studies have concentrated on babies with preexisting conditions, but this one looked at otherwise healthy children, researchers said.
“This is the lowest-risk baby who is being hospitalized for this, so really, numbers are really much higher than I think some people would have guessed,” said study coauthor Louis Bont, MD, a professor of pediatric infectious diseases at Wilhelmina Children’s Hospital at University Medical Center Utrecht in the Netherlands, according to CNN. He is also chairman of the ReSViNET foundation, which aims to reduce RSV infection globally.
The study said more than 97% of deaths from RSV occur in low-income and middle-income countries. The study concluded that “maternal vaccination and passive [immunization] could have a profound impact on the RSV burden.”
In developed nations, children who get RSV usually survive because they have access to ventilators and other health care equipment. Still, just being treated for RSV can have long-range negative effects on a child’s health, Kristina Deeter, MD, chair of pediatrics at the University of Nevada, Reno, told CNN.
“Whether that is just traumatic psychosocial, emotional issues after hospitalization or even having more vulnerable lungs – you can develop asthma later on, for instance, if you’ve had a really severe infection at a young age – it can damage your lungs permanently,” she said of the study. “It’s still an important virus in our world and something that we really focus on.”
The Lancet study was published days after the CDC warned public health officials that respiratory viruses, including RSV, are surging among children across the country.
A version of this article first appeared on WebMD.com.
FROM LANCET RESPIRATORY MEDICINE
Meditation equal to first-line medication for anxiety
“I would encourage clinicians to list meditation training as one possible treatment option for patients who are diagnosed with anxiety disorders. Doctors should feel comfortable recommending in-person, group-based meditation classes,” study investigator Elizabeth A. Hoge, MD, director, Anxiety Disorders Research Program, Georgetown University Medical Center, Washington, told this news organization.
The findings were published online in JAMA Psychiatry.
Screening recommended
Anxiety disorders, including generalized anxiety, social anxiety, panic disorder, and agoraphobia, are the most common type of mental disorder, affecting an estimated 301 million people worldwide. Owing to their high prevalence, the United States Preventive Services Task Force recommends screening for anxiety disorders.
Effective treatments for anxiety disorders include medications and cognitive-behavioral therapy. However, not all patients have access to these interventions, respond to them, or are comfortable seeking care in a psychiatric setting.
Mindfulness meditation, which has risen in popularity in recent years, may help people experiencing intrusive, anxious thoughts. “By practicing mindfulness meditation, people learn not to be overwhelmed by those thoughts,” said Dr. Hoge.
The study included 276 adult patients with an anxiety disorder, mostly generalized anxiety or social anxiety. The mean age of the study population was 33 years; 75% were women, 59% were White, 15% were Black, and 20% were Asian.
Researchers randomly assigned 136 patients to receive MBSR and 140 to receive the selective serotonin reuptake inhibitor escitalopram, a first-line medication for treating anxiety disorders.
The MBSR intervention included a weekly 2.5-hour class and a day-long weekend class. Participants also completed daily 45-minute guided meditation sessions at home. They learned mindfulness meditation exercises, including breath awareness, body scanning, and mindful movement.
Those in the escitalopram group initially received 10 mg of the oral drug daily. The dose was increased to 20 mg daily at week 2 if well tolerated.
The primary outcome was the score on the Clinical Global Impression of Severity (CGI-S) scale for anxiety, assessed by clinicians blinded to treatment allocation. This instrument measures overall symptom severity on a scale from 1 (not at all ill) to 7 (most extremely ill) and can be used to assess different types of anxiety disorders, said Dr. Hoge.
Among the 208 participants who completed the study, the baseline mean CGI-S score was 4.44 for MBSR and 4.51 for escitalopram. At week 8, on the CGI-S scale, the MBSR group’s score improved by a mean of 1.35 points, and the escitalopram group’s score improved by 1.43 points (difference of –0.07; 95% CI, –0.38 to 0.23; P = .65).
The lower end of the confidence interval (–0.38) was smaller than the prespecified noninferiority margin of –0.495, indicating noninferiority of MBSR, compared with escitalopram.
Remarkable results
“What was remarkable was that the medication worked great, like it always does, but the meditation also worked great; we saw about a 30% drop in symptoms for both groups,” said Dr. Hoge. “That helps us know that meditation, and in particular mindfulness meditation, could be useful as a first-line treatment for patients with anxiety disorders.”
The patient-reported outcome of the Overall Anxiety Severity and Impairment Scale also showed no significant group differences. “It’s important to have the self-reports, because that gives us two ways to look at the information,” said Dr. Hoge.
Anecdotally, participants noted that the meditation helped with their personal relationships and with being “kinder to themselves,” said Dr. Hoge. “In meditation, there’s an implicit teaching to be accepting and nonjudgmental towards your own thoughts, and that teaches people to be more self-compassionate.”
Just over 78% of patients in the escitalopram group had at least one treatment-related adverse event (AE), which included sleep disturbances, nausea, fatigue, and headache, compared with 15.4% in the MBSR group.
The most common AE in the meditation group was anxiety, which is “counterintuitive” but represents “a momentary anxiety,” said Dr. Hoge. “People who are meditating have feelings come up that maybe they didn’t pay attention to before. This gives them an opportunity to process through those emotions.”
Fatigue was the next most common AE for meditators, which “makes sense,” since they’re putting away their phones and not being stimulated, said Dr. Hoge.
MBSR was delivered in person, which limits extrapolation to mindfulness apps or programs delivered over the internet. Dr. Hoge believes apps would likely be less effective because they don’t have the face-to-face component, instructors available for consultation, or fellow participants contributing group support.
But online classes might work if “the exact same class,” including all its components, is moved online, she said.
MBSR is available in all major U.S. cities, doesn’t require finding a therapist, and is available outside a mental health environment – for example, at yoga centers and some places of employment. Anyone can learn MBSR, although it takes time and commitment, said Dr. Hoge.
A time-tested intervention
Commenting on the study, psychiatrist Gregory Scott Brown, MD, affiliate faculty, University of Texas Dell Medical School, and author of “The Self-Healing Mind: An Essential Five-Step Practice for Overcoming Anxiety and Depression and Revitalizing Your Life,” said the results aren’t surprising inasmuch as mindfulness, including spirituality, breath work, and meditation, is a “time-tested and evidence-based” intervention.
“I’m encouraged by the fact studies like this are now being conducted and there’s more evidence that supports these mindfulness-based interventions, so they can start to make their way into standard-of-care treatments.” he said.
He noted that mindfulness can produce “long-term, sustainable improvements” and that the 45-minute daily home exercise included in the study “is not a huge time commitment when you talk about benefits you can potentially glean from incorporating that time.”
Because most study participants were women and “men are anxious too,” Dr. Brown said he would like to see the study replicated “with a more diverse pool of participants.”
The study was supported by the Patient-Centered Outcomes Research Institute. Dr. Hoge and Dr. Brown have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
“I would encourage clinicians to list meditation training as one possible treatment option for patients who are diagnosed with anxiety disorders. Doctors should feel comfortable recommending in-person, group-based meditation classes,” study investigator Elizabeth A. Hoge, MD, director, Anxiety Disorders Research Program, Georgetown University Medical Center, Washington, told this news organization.
The findings were published online in JAMA Psychiatry.
Screening recommended
Anxiety disorders, including generalized anxiety, social anxiety, panic disorder, and agoraphobia, are the most common type of mental disorder, affecting an estimated 301 million people worldwide. Owing to their high prevalence, the United States Preventive Services Task Force recommends screening for anxiety disorders.
Effective treatments for anxiety disorders include medications and cognitive-behavioral therapy. However, not all patients have access to these interventions, respond to them, or are comfortable seeking care in a psychiatric setting.
Mindfulness meditation, which has risen in popularity in recent years, may help people experiencing intrusive, anxious thoughts. “By practicing mindfulness meditation, people learn not to be overwhelmed by those thoughts,” said Dr. Hoge.
The study included 276 adult patients with an anxiety disorder, mostly generalized anxiety or social anxiety. The mean age of the study population was 33 years; 75% were women, 59% were White, 15% were Black, and 20% were Asian.
Researchers randomly assigned 136 patients to receive MBSR and 140 to receive the selective serotonin reuptake inhibitor escitalopram, a first-line medication for treating anxiety disorders.
The MBSR intervention included a weekly 2.5-hour class and a day-long weekend class. Participants also completed daily 45-minute guided meditation sessions at home. They learned mindfulness meditation exercises, including breath awareness, body scanning, and mindful movement.
Those in the escitalopram group initially received 10 mg of the oral drug daily. The dose was increased to 20 mg daily at week 2 if well tolerated.
The primary outcome was the score on the Clinical Global Impression of Severity (CGI-S) scale for anxiety, assessed by clinicians blinded to treatment allocation. This instrument measures overall symptom severity on a scale from 1 (not at all ill) to 7 (most extremely ill) and can be used to assess different types of anxiety disorders, said Dr. Hoge.
Among the 208 participants who completed the study, the baseline mean CGI-S score was 4.44 for MBSR and 4.51 for escitalopram. At week 8, on the CGI-S scale, the MBSR group’s score improved by a mean of 1.35 points, and the escitalopram group’s score improved by 1.43 points (difference of –0.07; 95% CI, –0.38 to 0.23; P = .65).
The lower end of the confidence interval (–0.38) was smaller than the prespecified noninferiority margin of –0.495, indicating noninferiority of MBSR, compared with escitalopram.
Remarkable results
“What was remarkable was that the medication worked great, like it always does, but the meditation also worked great; we saw about a 30% drop in symptoms for both groups,” said Dr. Hoge. “That helps us know that meditation, and in particular mindfulness meditation, could be useful as a first-line treatment for patients with anxiety disorders.”
The patient-reported outcome of the Overall Anxiety Severity and Impairment Scale also showed no significant group differences. “It’s important to have the self-reports, because that gives us two ways to look at the information,” said Dr. Hoge.
Anecdotally, participants noted that the meditation helped with their personal relationships and with being “kinder to themselves,” said Dr. Hoge. “In meditation, there’s an implicit teaching to be accepting and nonjudgmental towards your own thoughts, and that teaches people to be more self-compassionate.”
Just over 78% of patients in the escitalopram group had at least one treatment-related adverse event (AE), which included sleep disturbances, nausea, fatigue, and headache, compared with 15.4% in the MBSR group.
The most common AE in the meditation group was anxiety, which is “counterintuitive” but represents “a momentary anxiety,” said Dr. Hoge. “People who are meditating have feelings come up that maybe they didn’t pay attention to before. This gives them an opportunity to process through those emotions.”
Fatigue was the next most common AE for meditators, which “makes sense,” since they’re putting away their phones and not being stimulated, said Dr. Hoge.
MBSR was delivered in person, which limits extrapolation to mindfulness apps or programs delivered over the internet. Dr. Hoge believes apps would likely be less effective because they don’t have the face-to-face component, instructors available for consultation, or fellow participants contributing group support.
But online classes might work if “the exact same class,” including all its components, is moved online, she said.
MBSR is available in all major U.S. cities, doesn’t require finding a therapist, and is available outside a mental health environment – for example, at yoga centers and some places of employment. Anyone can learn MBSR, although it takes time and commitment, said Dr. Hoge.
A time-tested intervention
Commenting on the study, psychiatrist Gregory Scott Brown, MD, affiliate faculty, University of Texas Dell Medical School, and author of “The Self-Healing Mind: An Essential Five-Step Practice for Overcoming Anxiety and Depression and Revitalizing Your Life,” said the results aren’t surprising inasmuch as mindfulness, including spirituality, breath work, and meditation, is a “time-tested and evidence-based” intervention.
“I’m encouraged by the fact studies like this are now being conducted and there’s more evidence that supports these mindfulness-based interventions, so they can start to make their way into standard-of-care treatments.” he said.
He noted that mindfulness can produce “long-term, sustainable improvements” and that the 45-minute daily home exercise included in the study “is not a huge time commitment when you talk about benefits you can potentially glean from incorporating that time.”
Because most study participants were women and “men are anxious too,” Dr. Brown said he would like to see the study replicated “with a more diverse pool of participants.”
The study was supported by the Patient-Centered Outcomes Research Institute. Dr. Hoge and Dr. Brown have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
“I would encourage clinicians to list meditation training as one possible treatment option for patients who are diagnosed with anxiety disorders. Doctors should feel comfortable recommending in-person, group-based meditation classes,” study investigator Elizabeth A. Hoge, MD, director, Anxiety Disorders Research Program, Georgetown University Medical Center, Washington, told this news organization.
The findings were published online in JAMA Psychiatry.
Screening recommended
Anxiety disorders, including generalized anxiety, social anxiety, panic disorder, and agoraphobia, are the most common type of mental disorder, affecting an estimated 301 million people worldwide. Owing to their high prevalence, the United States Preventive Services Task Force recommends screening for anxiety disorders.
Effective treatments for anxiety disorders include medications and cognitive-behavioral therapy. However, not all patients have access to these interventions, respond to them, or are comfortable seeking care in a psychiatric setting.
Mindfulness meditation, which has risen in popularity in recent years, may help people experiencing intrusive, anxious thoughts. “By practicing mindfulness meditation, people learn not to be overwhelmed by those thoughts,” said Dr. Hoge.
The study included 276 adult patients with an anxiety disorder, mostly generalized anxiety or social anxiety. The mean age of the study population was 33 years; 75% were women, 59% were White, 15% were Black, and 20% were Asian.
Researchers randomly assigned 136 patients to receive MBSR and 140 to receive the selective serotonin reuptake inhibitor escitalopram, a first-line medication for treating anxiety disorders.
The MBSR intervention included a weekly 2.5-hour class and a day-long weekend class. Participants also completed daily 45-minute guided meditation sessions at home. They learned mindfulness meditation exercises, including breath awareness, body scanning, and mindful movement.
Those in the escitalopram group initially received 10 mg of the oral drug daily. The dose was increased to 20 mg daily at week 2 if well tolerated.
The primary outcome was the score on the Clinical Global Impression of Severity (CGI-S) scale for anxiety, assessed by clinicians blinded to treatment allocation. This instrument measures overall symptom severity on a scale from 1 (not at all ill) to 7 (most extremely ill) and can be used to assess different types of anxiety disorders, said Dr. Hoge.
Among the 208 participants who completed the study, the baseline mean CGI-S score was 4.44 for MBSR and 4.51 for escitalopram. At week 8, on the CGI-S scale, the MBSR group’s score improved by a mean of 1.35 points, and the escitalopram group’s score improved by 1.43 points (difference of –0.07; 95% CI, –0.38 to 0.23; P = .65).
The lower end of the confidence interval (–0.38) was smaller than the prespecified noninferiority margin of –0.495, indicating noninferiority of MBSR, compared with escitalopram.
Remarkable results
“What was remarkable was that the medication worked great, like it always does, but the meditation also worked great; we saw about a 30% drop in symptoms for both groups,” said Dr. Hoge. “That helps us know that meditation, and in particular mindfulness meditation, could be useful as a first-line treatment for patients with anxiety disorders.”
The patient-reported outcome of the Overall Anxiety Severity and Impairment Scale also showed no significant group differences. “It’s important to have the self-reports, because that gives us two ways to look at the information,” said Dr. Hoge.
Anecdotally, participants noted that the meditation helped with their personal relationships and with being “kinder to themselves,” said Dr. Hoge. “In meditation, there’s an implicit teaching to be accepting and nonjudgmental towards your own thoughts, and that teaches people to be more self-compassionate.”
Just over 78% of patients in the escitalopram group had at least one treatment-related adverse event (AE), which included sleep disturbances, nausea, fatigue, and headache, compared with 15.4% in the MBSR group.
The most common AE in the meditation group was anxiety, which is “counterintuitive” but represents “a momentary anxiety,” said Dr. Hoge. “People who are meditating have feelings come up that maybe they didn’t pay attention to before. This gives them an opportunity to process through those emotions.”
Fatigue was the next most common AE for meditators, which “makes sense,” since they’re putting away their phones and not being stimulated, said Dr. Hoge.
MBSR was delivered in person, which limits extrapolation to mindfulness apps or programs delivered over the internet. Dr. Hoge believes apps would likely be less effective because they don’t have the face-to-face component, instructors available for consultation, or fellow participants contributing group support.
But online classes might work if “the exact same class,” including all its components, is moved online, she said.
MBSR is available in all major U.S. cities, doesn’t require finding a therapist, and is available outside a mental health environment – for example, at yoga centers and some places of employment. Anyone can learn MBSR, although it takes time and commitment, said Dr. Hoge.
A time-tested intervention
Commenting on the study, psychiatrist Gregory Scott Brown, MD, affiliate faculty, University of Texas Dell Medical School, and author of “The Self-Healing Mind: An Essential Five-Step Practice for Overcoming Anxiety and Depression and Revitalizing Your Life,” said the results aren’t surprising inasmuch as mindfulness, including spirituality, breath work, and meditation, is a “time-tested and evidence-based” intervention.
“I’m encouraged by the fact studies like this are now being conducted and there’s more evidence that supports these mindfulness-based interventions, so they can start to make their way into standard-of-care treatments.” he said.
He noted that mindfulness can produce “long-term, sustainable improvements” and that the 45-minute daily home exercise included in the study “is not a huge time commitment when you talk about benefits you can potentially glean from incorporating that time.”
Because most study participants were women and “men are anxious too,” Dr. Brown said he would like to see the study replicated “with a more diverse pool of participants.”
The study was supported by the Patient-Centered Outcomes Research Institute. Dr. Hoge and Dr. Brown have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA PSYCHIATRY
Imaging IDs brain activity related to dissociative symptoms
Results from a neuroimaging study showed that different dissociative symptoms were linked to hyperconnectivity within several key regions of the brain, including the central executive, default, and salience networks as well as decreased connectivity of the central executive and salience networks with other brain areas.
Depersonalization/derealization showed a different brain signature than partially dissociated intrusions, and participants with posttraumatic stress disorder showed a different brain signature, compared with those who had dissociative identity disorder (DID).
“Dissociation is a complex, subjective set of symptoms that are largely experienced internally and, contrary to media portrayal, are not usually overtly observable,” lead author Lauren Lebois, PhD, director of the Dissociative Disorders and Trauma Research Program, McLean Hospital, Belmont, Mass., and assistant professor of psychiatry at Harvard Medical School, Boston, told this news organization.
“However, we have shown that you can objectively measure dissociation and link it to robust brain signatures. We hope these results will encourage clinicians to screen for dissociation and approach reports of these experiences seriously, empathetically, and with awareness that they can be treated effectively,” Dr. Lebois said.
The findings were published online in Neuropsychopharmacology.
Detachment, discontinuity
Pathological dissociation is “the experience of detachment from or discontinuity in one’s internal experience, sense of self, or surroundings” and is common in the aftermath of trauma, the investigators write.
Previous research into trauma-related pathological dissociation suggests it encompasses a range of experiences or “subtypes,” some of which frequently occur in PTSD and DID.
“Depersonalization and derealization involve feelings of detachment or disconnection from one’s sense of self, body, and environment,” the current researchers write. “Individuals report feeling like their body or surroundings are unreal or like they are in a movie.”
Dissociation also includes “experiences of self-alteration common in DID, in which people lose a sense of agency and ownership over their thoughts, emotions, actions, and body [and] experience some thoughts, emotions, etc. as partially dissociated intrusions,” Dr. Lebois said.
She added that dissociative symptoms are “common and disabling.” And dissociation and severe dissociative disorders such as DID “remain at best underappreciated and, at worst, frequently go undiagnosed or misdiagnosed,” with a high cost of stigmatization and misunderstanding preventing individuals from accessing effective treatment.
In addition, “given that DID disproportionately affects women, gender disparity is an important issue in this context,” Dr. Lebois noted.
Her team was motivated to conduct the study “to learn more about how different types of dissociation manifest in brain activity and to help combat the stigma around dissociation and DID.”
Filling the gap
The investigators drew on the “Triple Network” model of psychopathology, which “offers an integrative framework based in systems neuroscience for understanding cognitive and affective dysfunction across psychiatric conditions,” they write.
This model “implicates altered intrinsic organization and interactions between three large-scale brain networks across disorders,” they add.
The brain networks included in the study were the right-lateralized central executive network (rCEN), with the lateral frontoparietal brain region; the medial temporal subnetwork of the default network (tDN), with the medial frontoparietal brain region; and the cingulo-opercular subnetwork (cSN), with the midcingulo-insular brain region.
Previous neuroimaging research into dissociative disorders has implicated altered connectivity in these regions. However, although previous studies covered dissociation subtypes, they did not directly compare these subtypes. This study was designed to fill that gap, the investigators note.
They assessed 91 women with and without a history of childhood trauma, current PTSD, and with varying degrees of dissociation.
This included 19 with conventional PTSD (mean age, 33.4 years), 18 with PTSD dissociative subtype (mean age, 29.5 years), 26 with DID (mean age, 37.4 years), and 28 who acted as the healthy control group (mean age, 32 years).
Participants completed several scales regarding symptoms of PTSD, dissociation, and childhood trauma. They also underwent functional magnetic resonance imaging. Covariates included age, childhood maltreatment, and PTSD severity.
Connectivity alterations
Results showed the rCEN was “most impacted” by pathological dissociation, with 39 clusters linked to connectivity alterations.
Ten clusters within tDN exhibited within-network hyperconnectivity related to dissociation but only of the depersonalization/derealization subtype.
Eight clusters within cSN were linked to dissociation – specifically, within-network hyperconnectivity and decreased connectivity between regions in rCEN with cSN, with “no significant unique contributions of dissociation subtypes,” the researchers report.
“Depersonalization and derealization symptoms were associated with increased communication between a brain network involved in reasoning, attention, inhibition, and working memory and a brain region implicated in out-of-body experiences. This may, in part, contribute to depersonalization/derealization feelings of detachment, strangeness or unreality experienced with your body and surroundings,” Dr. Lebois said.
“In contrast, partially dissociated intrusion symptoms central to DID were linked to increased communication between a brain network involved in autobiographical memory and your sense of self and a brain network involved in reasoning, attention, inhibition, and working memory,” she added.
She noted that this matches how patients with DID describe their mental experiences: as sometimes feeling as if they lost a sense of ownership over their own thoughts and feelings, which can “intrude into their mental landscape.”
In the future, Dr. Lebois hopes that “we may be able to monitor dissociative brain signatures during psychotherapy to help assess recovery or relapse, or we could target brain activity directly with neurofeedback or neuromodulatory techniques as a dissociation treatment in and of itself.”
A first step?
Commenting on the study, Richard Loewenstein, MD, adjunct professor, department of psychiatry, University of Maryland School of Medicine, Baltimore, called the paper a “first step in more sophisticated studies of pathological dissociation using cutting-edge concepts of brain connectivity, methodology based on naturalistic, dimensional symptoms categories, and innovative statistical methods.”
Dr. Loewenstein, who was not involved with the current study, added that there is an “oversimplified conflation of hallucinations and other symptoms of dissociation with psychosis.” So studies may “incorrectly relate phenomena such as racism-based trauma to psychosis, rather than pathological dissociation and racism-based PTSD,” he said.
He noted that the implications are “profound, as pathological dissociation is not treatable with antipsychotic medications and requires treatment with psychotherapy specifically targeting symptoms of pathological dissociation.”
The study was funded by the Julia Kasparian Fund for Neuroscience Research and the National Institute of Mental Health. Dr. Lebois reported unpaid membership on the Scientific Committee for the International Society for the Study of Trauma and Dissociation, grant support from the NIMH and the Julia Kasparian Fund for Neuroscience Research, and spousal IP payments from Vanderbilt University for technology licensed to Acadia Pharmaceuticals unrelated to the present work. The other investigators’ disclosures are listed in the original paper. Dr. Loewenstein has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Results from a neuroimaging study showed that different dissociative symptoms were linked to hyperconnectivity within several key regions of the brain, including the central executive, default, and salience networks as well as decreased connectivity of the central executive and salience networks with other brain areas.
Depersonalization/derealization showed a different brain signature than partially dissociated intrusions, and participants with posttraumatic stress disorder showed a different brain signature, compared with those who had dissociative identity disorder (DID).
“Dissociation is a complex, subjective set of symptoms that are largely experienced internally and, contrary to media portrayal, are not usually overtly observable,” lead author Lauren Lebois, PhD, director of the Dissociative Disorders and Trauma Research Program, McLean Hospital, Belmont, Mass., and assistant professor of psychiatry at Harvard Medical School, Boston, told this news organization.
“However, we have shown that you can objectively measure dissociation and link it to robust brain signatures. We hope these results will encourage clinicians to screen for dissociation and approach reports of these experiences seriously, empathetically, and with awareness that they can be treated effectively,” Dr. Lebois said.
The findings were published online in Neuropsychopharmacology.
Detachment, discontinuity
Pathological dissociation is “the experience of detachment from or discontinuity in one’s internal experience, sense of self, or surroundings” and is common in the aftermath of trauma, the investigators write.
Previous research into trauma-related pathological dissociation suggests it encompasses a range of experiences or “subtypes,” some of which frequently occur in PTSD and DID.
“Depersonalization and derealization involve feelings of detachment or disconnection from one’s sense of self, body, and environment,” the current researchers write. “Individuals report feeling like their body or surroundings are unreal or like they are in a movie.”
Dissociation also includes “experiences of self-alteration common in DID, in which people lose a sense of agency and ownership over their thoughts, emotions, actions, and body [and] experience some thoughts, emotions, etc. as partially dissociated intrusions,” Dr. Lebois said.
She added that dissociative symptoms are “common and disabling.” And dissociation and severe dissociative disorders such as DID “remain at best underappreciated and, at worst, frequently go undiagnosed or misdiagnosed,” with a high cost of stigmatization and misunderstanding preventing individuals from accessing effective treatment.
In addition, “given that DID disproportionately affects women, gender disparity is an important issue in this context,” Dr. Lebois noted.
Her team was motivated to conduct the study “to learn more about how different types of dissociation manifest in brain activity and to help combat the stigma around dissociation and DID.”
Filling the gap
The investigators drew on the “Triple Network” model of psychopathology, which “offers an integrative framework based in systems neuroscience for understanding cognitive and affective dysfunction across psychiatric conditions,” they write.
This model “implicates altered intrinsic organization and interactions between three large-scale brain networks across disorders,” they add.
The brain networks included in the study were the right-lateralized central executive network (rCEN), with the lateral frontoparietal brain region; the medial temporal subnetwork of the default network (tDN), with the medial frontoparietal brain region; and the cingulo-opercular subnetwork (cSN), with the midcingulo-insular brain region.
Previous neuroimaging research into dissociative disorders has implicated altered connectivity in these regions. However, although previous studies covered dissociation subtypes, they did not directly compare these subtypes. This study was designed to fill that gap, the investigators note.
They assessed 91 women with and without a history of childhood trauma, current PTSD, and with varying degrees of dissociation.
This included 19 with conventional PTSD (mean age, 33.4 years), 18 with PTSD dissociative subtype (mean age, 29.5 years), 26 with DID (mean age, 37.4 years), and 28 who acted as the healthy control group (mean age, 32 years).
Participants completed several scales regarding symptoms of PTSD, dissociation, and childhood trauma. They also underwent functional magnetic resonance imaging. Covariates included age, childhood maltreatment, and PTSD severity.
Connectivity alterations
Results showed the rCEN was “most impacted” by pathological dissociation, with 39 clusters linked to connectivity alterations.
Ten clusters within tDN exhibited within-network hyperconnectivity related to dissociation but only of the depersonalization/derealization subtype.
Eight clusters within cSN were linked to dissociation – specifically, within-network hyperconnectivity and decreased connectivity between regions in rCEN with cSN, with “no significant unique contributions of dissociation subtypes,” the researchers report.
“Depersonalization and derealization symptoms were associated with increased communication between a brain network involved in reasoning, attention, inhibition, and working memory and a brain region implicated in out-of-body experiences. This may, in part, contribute to depersonalization/derealization feelings of detachment, strangeness or unreality experienced with your body and surroundings,” Dr. Lebois said.
“In contrast, partially dissociated intrusion symptoms central to DID were linked to increased communication between a brain network involved in autobiographical memory and your sense of self and a brain network involved in reasoning, attention, inhibition, and working memory,” she added.
She noted that this matches how patients with DID describe their mental experiences: as sometimes feeling as if they lost a sense of ownership over their own thoughts and feelings, which can “intrude into their mental landscape.”
In the future, Dr. Lebois hopes that “we may be able to monitor dissociative brain signatures during psychotherapy to help assess recovery or relapse, or we could target brain activity directly with neurofeedback or neuromodulatory techniques as a dissociation treatment in and of itself.”
A first step?
Commenting on the study, Richard Loewenstein, MD, adjunct professor, department of psychiatry, University of Maryland School of Medicine, Baltimore, called the paper a “first step in more sophisticated studies of pathological dissociation using cutting-edge concepts of brain connectivity, methodology based on naturalistic, dimensional symptoms categories, and innovative statistical methods.”
Dr. Loewenstein, who was not involved with the current study, added that there is an “oversimplified conflation of hallucinations and other symptoms of dissociation with psychosis.” So studies may “incorrectly relate phenomena such as racism-based trauma to psychosis, rather than pathological dissociation and racism-based PTSD,” he said.
He noted that the implications are “profound, as pathological dissociation is not treatable with antipsychotic medications and requires treatment with psychotherapy specifically targeting symptoms of pathological dissociation.”
The study was funded by the Julia Kasparian Fund for Neuroscience Research and the National Institute of Mental Health. Dr. Lebois reported unpaid membership on the Scientific Committee for the International Society for the Study of Trauma and Dissociation, grant support from the NIMH and the Julia Kasparian Fund for Neuroscience Research, and spousal IP payments from Vanderbilt University for technology licensed to Acadia Pharmaceuticals unrelated to the present work. The other investigators’ disclosures are listed in the original paper. Dr. Loewenstein has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Results from a neuroimaging study showed that different dissociative symptoms were linked to hyperconnectivity within several key regions of the brain, including the central executive, default, and salience networks as well as decreased connectivity of the central executive and salience networks with other brain areas.
Depersonalization/derealization showed a different brain signature than partially dissociated intrusions, and participants with posttraumatic stress disorder showed a different brain signature, compared with those who had dissociative identity disorder (DID).
“Dissociation is a complex, subjective set of symptoms that are largely experienced internally and, contrary to media portrayal, are not usually overtly observable,” lead author Lauren Lebois, PhD, director of the Dissociative Disorders and Trauma Research Program, McLean Hospital, Belmont, Mass., and assistant professor of psychiatry at Harvard Medical School, Boston, told this news organization.
“However, we have shown that you can objectively measure dissociation and link it to robust brain signatures. We hope these results will encourage clinicians to screen for dissociation and approach reports of these experiences seriously, empathetically, and with awareness that they can be treated effectively,” Dr. Lebois said.
The findings were published online in Neuropsychopharmacology.
Detachment, discontinuity
Pathological dissociation is “the experience of detachment from or discontinuity in one’s internal experience, sense of self, or surroundings” and is common in the aftermath of trauma, the investigators write.
Previous research into trauma-related pathological dissociation suggests it encompasses a range of experiences or “subtypes,” some of which frequently occur in PTSD and DID.
“Depersonalization and derealization involve feelings of detachment or disconnection from one’s sense of self, body, and environment,” the current researchers write. “Individuals report feeling like their body or surroundings are unreal or like they are in a movie.”
Dissociation also includes “experiences of self-alteration common in DID, in which people lose a sense of agency and ownership over their thoughts, emotions, actions, and body [and] experience some thoughts, emotions, etc. as partially dissociated intrusions,” Dr. Lebois said.
She added that dissociative symptoms are “common and disabling.” And dissociation and severe dissociative disorders such as DID “remain at best underappreciated and, at worst, frequently go undiagnosed or misdiagnosed,” with a high cost of stigmatization and misunderstanding preventing individuals from accessing effective treatment.
In addition, “given that DID disproportionately affects women, gender disparity is an important issue in this context,” Dr. Lebois noted.
Her team was motivated to conduct the study “to learn more about how different types of dissociation manifest in brain activity and to help combat the stigma around dissociation and DID.”
Filling the gap
The investigators drew on the “Triple Network” model of psychopathology, which “offers an integrative framework based in systems neuroscience for understanding cognitive and affective dysfunction across psychiatric conditions,” they write.
This model “implicates altered intrinsic organization and interactions between three large-scale brain networks across disorders,” they add.
The brain networks included in the study were the right-lateralized central executive network (rCEN), with the lateral frontoparietal brain region; the medial temporal subnetwork of the default network (tDN), with the medial frontoparietal brain region; and the cingulo-opercular subnetwork (cSN), with the midcingulo-insular brain region.
Previous neuroimaging research into dissociative disorders has implicated altered connectivity in these regions. However, although previous studies covered dissociation subtypes, they did not directly compare these subtypes. This study was designed to fill that gap, the investigators note.
They assessed 91 women with and without a history of childhood trauma, current PTSD, and with varying degrees of dissociation.
This included 19 with conventional PTSD (mean age, 33.4 years), 18 with PTSD dissociative subtype (mean age, 29.5 years), 26 with DID (mean age, 37.4 years), and 28 who acted as the healthy control group (mean age, 32 years).
Participants completed several scales regarding symptoms of PTSD, dissociation, and childhood trauma. They also underwent functional magnetic resonance imaging. Covariates included age, childhood maltreatment, and PTSD severity.
Connectivity alterations
Results showed the rCEN was “most impacted” by pathological dissociation, with 39 clusters linked to connectivity alterations.
Ten clusters within tDN exhibited within-network hyperconnectivity related to dissociation but only of the depersonalization/derealization subtype.
Eight clusters within cSN were linked to dissociation – specifically, within-network hyperconnectivity and decreased connectivity between regions in rCEN with cSN, with “no significant unique contributions of dissociation subtypes,” the researchers report.
“Depersonalization and derealization symptoms were associated with increased communication between a brain network involved in reasoning, attention, inhibition, and working memory and a brain region implicated in out-of-body experiences. This may, in part, contribute to depersonalization/derealization feelings of detachment, strangeness or unreality experienced with your body and surroundings,” Dr. Lebois said.
“In contrast, partially dissociated intrusion symptoms central to DID were linked to increased communication between a brain network involved in autobiographical memory and your sense of self and a brain network involved in reasoning, attention, inhibition, and working memory,” she added.
She noted that this matches how patients with DID describe their mental experiences: as sometimes feeling as if they lost a sense of ownership over their own thoughts and feelings, which can “intrude into their mental landscape.”
In the future, Dr. Lebois hopes that “we may be able to monitor dissociative brain signatures during psychotherapy to help assess recovery or relapse, or we could target brain activity directly with neurofeedback or neuromodulatory techniques as a dissociation treatment in and of itself.”
A first step?
Commenting on the study, Richard Loewenstein, MD, adjunct professor, department of psychiatry, University of Maryland School of Medicine, Baltimore, called the paper a “first step in more sophisticated studies of pathological dissociation using cutting-edge concepts of brain connectivity, methodology based on naturalistic, dimensional symptoms categories, and innovative statistical methods.”
Dr. Loewenstein, who was not involved with the current study, added that there is an “oversimplified conflation of hallucinations and other symptoms of dissociation with psychosis.” So studies may “incorrectly relate phenomena such as racism-based trauma to psychosis, rather than pathological dissociation and racism-based PTSD,” he said.
He noted that the implications are “profound, as pathological dissociation is not treatable with antipsychotic medications and requires treatment with psychotherapy specifically targeting symptoms of pathological dissociation.”
The study was funded by the Julia Kasparian Fund for Neuroscience Research and the National Institute of Mental Health. Dr. Lebois reported unpaid membership on the Scientific Committee for the International Society for the Study of Trauma and Dissociation, grant support from the NIMH and the Julia Kasparian Fund for Neuroscience Research, and spousal IP payments from Vanderbilt University for technology licensed to Acadia Pharmaceuticals unrelated to the present work. The other investigators’ disclosures are listed in the original paper. Dr. Loewenstein has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM NEUROPSYCHOPHARMACOLOGY
Psoriasiform Dermatitis Associated With the Moderna COVID-19 Messenger RNA Vaccine
To the Editor:
The Moderna COVID-19 messenger RNA (mRNA) vaccine was authorized for use on December 18, 2020, with the second dose beginning on January 15, 2021.1-3 Some individuals who received the Moderna vaccine experienced an intense rash known as “COVID arm,” a harmless but bothersome adverse effect that typically appears within a week and is a localized and transient immunogenic response.4 COVID arm differs from most vaccine adverse effects. The rash emerges not immediately but 5 to 9 days after the initial dose—on average, 1 week later. Apart from being itchy, the rash does not appear to be harmful and is not a reason to hesitate getting vaccinated.
Dermatologists and allergists have been studying this adverse effect, which has been formally termed delayed cutaneous hypersensitivity. Of potential clinical consequence is that the efficacy of the mRNA COVID-19 vaccine may be harmed if postvaccination dermal reactions necessitate systemic corticosteroid therapy. Because this vaccine stimulates an immune response as viral RNA integrates in cells secondary to production of the spike protein of the virus, the skin may be affected secondarily and manifestations of any underlying disease may be aggravated.5 We report a patient who developed a psoriasiform dermatitis after the first dose of the Moderna vaccine.

A 65-year-old woman presented to her primary care physician because of the severity of psoriasiform dermatitis that developed 5 days after she received the first dose of the Moderna COVID-19 mRNA vaccine. The patient had a medical history of Sjögren syndrome. Her medication history was negative, and her family history was negative for autoimmune disease. Physical examination by primary care revealed an erythematous scaly rash with plaques and papules on the neck and back (Figure 1). The patient presented again to primary care 2 days later with swollen, painful, discolored digits (Figure 2) and a stiff, sore neck.

Laboratory results were positive for anti–Sjögren syndrome–related antigens A and B. A complete blood cell count; comprehensive metabolic panel; erythrocyte sedimentation rate; and assays of rheumatoid factor, C-reactive protein, and anti–cyclic citrullinated peptide were within reference range. A biopsy of a lesion on the back showed psoriasiform dermatitis with confluent parakeratosis and scattered necrotic keratinocytes. There was superficial perivascular inflammation with rare eosinophils (Figure 3).

The patient was treated with a course of systemic corticosteroids. The rash resolved in 1 week. She did not receive the second dose due to the rash.
Two mRNA COVID-19 vaccines—Pfizer BioNTech and Moderna—have been granted emergency use authorization by the US Food and Drug Administration.6 The safety profile of the mRNA-1273 vaccine for the median 2-month follow-up showed no safety concerns.3 Minor localized adverse effects (eg, pain, redness, swelling) have been observed more frequently with the vaccines than with placebo. Systemic symptoms, such as fever, fatigue, headache, and muscle and joint pain, also were seen somewhat more often with the vaccines than with placebo; most such effects occurred 24 to 48 hours after vaccination.3,6,7 The frequency of unsolicited adverse events and serious adverse events reported during the 28-day period after vaccination generally was similar among participants in the vaccine and placebo groups.3
There are 2 types of reactions to COVID-19 vaccination: immediate and delayed. Immediate reactions usually are due to anaphylaxis, requiring prompt recognition and treatment with epinephrine to stop rapid progression of life-threatening symptoms. Delayed reactions include localized reactions, such as urticaria and benign exanthema; serum sickness and serum sickness–like reactions; fever; and rare skin, organ, and neurologic sequelae.1,6-8
Cutaneous manifestations, present in 16% to 50% of patients with Sjögren syndrome, are considered one of the most common extraglandular presentations of the syndrome. They are classified as nonvascular (eg, xerosis, angular cheilitis, eyelid dermatitis, annular erythema) and vascular (eg, Raynaud phenomenon, vasculitis).9-11 Our patient did not have any of those findings. She had not taken any medications before the rash appeared, thereby ruling out a drug reaction.
The differential for our patient included post–urinary tract infection immune-reactive arthritis and rash, which is not typical with Escherichia coli infection but is described with infection with Chlamydia species and Salmonella species. Moreover, post–urinary tract infection immune-reactive arthritis and rash appear mostly on the palms and soles. Systemic lupus erythematosus–like rashes have a different histology and appear on sun-exposed areas; our patient’s rash was found mainly on unexposed areas.12
Because our patient received the Moderna vaccine 5 days before the rash appeared and later developed swelling of the digits with morning stiffness, a delayed serum sickness–like reaction secondary to COVID-19 vaccination was possible.3,6
COVID-19 mRNA vaccines developed by Pfizer-BioNTech and Moderna incorporate a lipid-based nanoparticle carrier system that prevents rapid enzymatic degradation of mRNA and facilitates in vivo delivery of mRNA. This lipid-based nanoparticle carrier system is further stabilized by a polyethylene glycol 2000 lipid conjugate that provides a hydrophilic layer, thus prolonging half-life. The presence of lipid polyethylene glycol 2000 in mRNA vaccines has led to concern that this component could be implicated in anaphylaxis.6
COVID-19 antigens can give rise to varying clinical manifestations that are directly related to viral tissue damage or are indirectly induced by the antiviral immune response.13,14 Hyperactivation of the immune system to eradicate COVID-19 may trigger autoimmunity; several immune-mediated disorders have been described in individuals infected with SARS-CoV-2. Dermal manifestations include cutaneous rash and vasculitis.13-16 Crucial immunologic steps occur during SARS-CoV-2 infection that may link autoimmunity to COVID-19.13,14 In preliminary published data on the efficacy of the Moderna vaccine on 45 trial enrollees, 3 did not receive the second dose of vaccination, including 1 who developed urticaria on both legs 5 days after the first dose.1
Introduction of viral RNA can induce autoimmunity that can be explained by various phenomena, including epitope spreading, molecular mimicry, cryptic antigen, and bystander activation. Remarkably, more than one-third of immunogenic proteins in SARS-CoV-2 have potentially problematic homology to proteins that are key to the human adaptive immune system.5
Moreover, SARS-CoV-2 seems to induce organ injury through alternative mechanisms beyond direct viral infection, including immunologic injury. In some situations, hyperactivation of the immune response to SARS-CoV-2 RNA can result in autoimmune disease. COVID-19 has been associated with immune-mediated systemic or organ-selective manifestations, some of which fulfill the diagnostic or classification criteria of specific autoimmune diseases. It is unclear whether those medical disorders are the result of transitory postinfectious epiphenomena.5
A few studies have shown that patients with rheumatic disease have an incidence and prevalence of COVID-19 that is similar to the general population. A similar pattern has been detected in COVID-19 morbidity and mortality rates, even among patients with an autoimmune disease, such as rheumatoid arthritis and Sjögren syndrome.5,17 Furthermore, exacerbation of preexisting rheumatic symptoms may be due to hyperactivation of antiviral pathways in a person with an autoimmune disease.17-19 The findings in our patient suggested a direct role for the vaccine in skin manifestations, rather than for reactivation or development of new systemic autoimmune processes, such as systemic lupus erythematosus.
Exacerbation of psoriasis following COVID-19 vaccination has been described20; however, the case patient did not have a history of psoriasis. The mechanism(s) of such exacerbation remain unclear; COVID-19 vaccine–induced helper T cells (TH17) may play a role.21 Other skin manifestations encountered following COVID-19 vaccination include lichen planus, leukocytoclastic vasculitic rash, erythema multiforme–like rash, and pityriasis rosea–like rash.22-25 The immune mechanisms of these manifestations remain unclear.
The clinical presentation of delayed vaccination reactions can be attributed to the timing of symptoms and, in this case, the immune-mediated background of a psoriasiform reaction. Although adverse reactions to the SARS-CoV-2 mRNA vaccine are rare, more individuals should be studied after vaccination to confirm and better understand this phenomenon.
- Jackson LA, Anderson EJ, Rouphael NG, et al; . An mRNA vaccine against SARS-CoV-2—preliminary report. N Engl J Med. 2020;383:1920-1931. doi:10.1056/NEJMoa2022483
- Anderson EJ, Rouphael NG, Widge AT, et al; . Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adults. N Engl J Med. 2020;383:2427-2438. doi:10.1056/NEJMoa2028436
- Baden LR, El Sahly HM, Essink B, et al; COVE Study Group. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403-416. doi:10.1056/NEJMoa2035389
- Weise E. ‘COVID arm’ rash seen after Moderna vaccine annoying but harmless, doctors say. USA Today. January 27, 2021. Accessed September 4, 2022. https://www.usatoday.com/story/news/health/2021/01/27/covid-arm-moderna-vaccine-rash-harmless-side-effect-doctors-say/4277725001/
- Talotta R, Robertson E. Autoimmunity as the comet tail of COVID-19 pandemic. World J Clin Cases. 2020;8:3621-3644. doi:10.12998/wjcc.v8.i17.3621
- Castells MC, Phillips EJ. Maintaining safety with SARS-CoV-2 vaccines. N Engl J Med. 2021;384:643-649. doi:10.1056/NEJMra2035343
- Polack FP, Thomas SJ, Kitchin N, et al; . Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603-2615. doi:10.1056/NEJMoa2034577
- Dooling K, McClung N, Chamberland M, et al. The Advisory Committee on Immunization Practices’ interim recommendation for allocating initial supplies of COVID-19 vaccine—United States, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1857-1859. doi:10.15585/mmwr.mm6949e1
- Roguedas AM, Misery L, Sassolas B, et al. Cutaneous manifestations of primary Sjögren’s syndrome are underestimated. Clin Exp Rheumatol. 2004;22:632-636.
- Katayama I. Dry skin manifestations in Sjögren syndrome and atopic dermatitis related to aberrant sudomotor function in inflammatory allergic skin diseases. Allergol Int. 2018;67:448-454. doi:10.1016/j.alit.2018.07.001
- Generali E, Costanzo A, Mainetti C, et al. Cutaneous and mucosal manifestations of Sjögren’s syndrome. Clin Rev Allergy Immunol. 2017;53:357-370. doi:10.1007/s12016-017-8639-y
- Chanprapaph K, Tankunakorn J, Suchonwanit P, et al. Dermatologic manifestations, histologic features and disease progression among cutaneous lupus erythematosus subtypes: a prospective observational study in Asians. Dermatol Ther (Heidelb). 2021;11:131-147. doi:10.1007/s13555-020-00471-y
- Ortega-Quijano D, Jimenez-Cauhe J, Selda-Enriquez G, et al. Algorithm for the classification of COVID-19 rashes. J Am Acad Dermatol. 2020;83:e103-e104. doi:10.1016/j.jaad.2020.05.034
- Rahimi H, Tehranchinia Z. A comprehensive review of cutaneous manifestations associated with COVID-19. Biomed Res Int. 2020;2020:1236520. doi:10.1155/2020/1236520
- Sachdeva M, Gianotti R, Shah M, et al. Cutaneous manifestations of COVID-19: report of three cases and a review of literature. J Dermatol Sci. 2020;98:75-81. doi:10.1016/j.jdermsci.2020.04.011
- Landa N, Mendieta-Eckert M, Fonda-Pascual P, et al. Chilblain-like lesions on feet and hands during the COVID-19 pandemic. Int J Dermatol. 2020;59:739-743. doi:10.1111/ijd.14937
- Dellavance A, Coelho Andrade LE. Immunologic derangement preceding clinical autoimmunity. Lupus. 2014;23:1305-1308. doi:10.1177/0961203314531346
- Parodi A, Gasparini G, Cozzani E. Could antiphospholipid antibodies contribute to coagulopathy in COVID-19? J Am Acad Dermatol. 2020;83:e249. doi:10.1016/j.jaad.2020.06.003
- Zhou Y, Han T, Chen J, et al. Clinical and autoimmune characteristics of severe and critical cases of COVID-19. Clin Transl Sci. 2020;13:1077-1086. doi:10.1111/cts.12805
- Huang YW, Tsai TF. Exacerbation of psoriasis following COVID-19 vaccination: report from a single center. Front Med (Lausanne). 2021;8:812010. doi:10.3389/fmed.2021.812010
- Rouai M, Slimane MB, Sassi W, et al. Pustular rash triggered by Pfizer-BioNTech COVID-19 vaccination: a case report. Dermatol Ther. 2022:e15465. doi:10.1111/dth.15465
- Altun E, Kuzucular E. Leukocytoclastic vasculitis after COVID-19 vaccination. Dermatol Ther. 2022;35:e15279. doi:10.1111/dth.15279
- Buckley JE, Landis LN, Rapini RP. Pityriasis rosea-like rash after mRNA COVID-19 vaccination: a case report and review of the literature. JAAD Int. 2022;7:164-168. doi:10.1016/j.jdin.2022.01.009
- Gökçek GE, Öksüm Solak E, Çölgeçen E. Pityriasis rosea like eruption: a dermatological manifestation of Coronavac-COVID-19 vaccine. Dermatol Ther. 2022;35:e15256. doi:10.1111/dth.15256
- Kim MJ, Kim JW, Kim MS, et al. Generalized erythema multiforme-like skin rash following the first dose of COVID-19 vaccine (Pfizer-BioNTech). J Eur Acad Dermatol Venereol. 2022;36:e98-e100. doi:10.1111/jdv.17757
To the Editor:
The Moderna COVID-19 messenger RNA (mRNA) vaccine was authorized for use on December 18, 2020, with the second dose beginning on January 15, 2021.1-3 Some individuals who received the Moderna vaccine experienced an intense rash known as “COVID arm,” a harmless but bothersome adverse effect that typically appears within a week and is a localized and transient immunogenic response.4 COVID arm differs from most vaccine adverse effects. The rash emerges not immediately but 5 to 9 days after the initial dose—on average, 1 week later. Apart from being itchy, the rash does not appear to be harmful and is not a reason to hesitate getting vaccinated.
Dermatologists and allergists have been studying this adverse effect, which has been formally termed delayed cutaneous hypersensitivity. Of potential clinical consequence is that the efficacy of the mRNA COVID-19 vaccine may be harmed if postvaccination dermal reactions necessitate systemic corticosteroid therapy. Because this vaccine stimulates an immune response as viral RNA integrates in cells secondary to production of the spike protein of the virus, the skin may be affected secondarily and manifestations of any underlying disease may be aggravated.5 We report a patient who developed a psoriasiform dermatitis after the first dose of the Moderna vaccine.

A 65-year-old woman presented to her primary care physician because of the severity of psoriasiform dermatitis that developed 5 days after she received the first dose of the Moderna COVID-19 mRNA vaccine. The patient had a medical history of Sjögren syndrome. Her medication history was negative, and her family history was negative for autoimmune disease. Physical examination by primary care revealed an erythematous scaly rash with plaques and papules on the neck and back (Figure 1). The patient presented again to primary care 2 days later with swollen, painful, discolored digits (Figure 2) and a stiff, sore neck.

Laboratory results were positive for anti–Sjögren syndrome–related antigens A and B. A complete blood cell count; comprehensive metabolic panel; erythrocyte sedimentation rate; and assays of rheumatoid factor, C-reactive protein, and anti–cyclic citrullinated peptide were within reference range. A biopsy of a lesion on the back showed psoriasiform dermatitis with confluent parakeratosis and scattered necrotic keratinocytes. There was superficial perivascular inflammation with rare eosinophils (Figure 3).

The patient was treated with a course of systemic corticosteroids. The rash resolved in 1 week. She did not receive the second dose due to the rash.
Two mRNA COVID-19 vaccines—Pfizer BioNTech and Moderna—have been granted emergency use authorization by the US Food and Drug Administration.6 The safety profile of the mRNA-1273 vaccine for the median 2-month follow-up showed no safety concerns.3 Minor localized adverse effects (eg, pain, redness, swelling) have been observed more frequently with the vaccines than with placebo. Systemic symptoms, such as fever, fatigue, headache, and muscle and joint pain, also were seen somewhat more often with the vaccines than with placebo; most such effects occurred 24 to 48 hours after vaccination.3,6,7 The frequency of unsolicited adverse events and serious adverse events reported during the 28-day period after vaccination generally was similar among participants in the vaccine and placebo groups.3
There are 2 types of reactions to COVID-19 vaccination: immediate and delayed. Immediate reactions usually are due to anaphylaxis, requiring prompt recognition and treatment with epinephrine to stop rapid progression of life-threatening symptoms. Delayed reactions include localized reactions, such as urticaria and benign exanthema; serum sickness and serum sickness–like reactions; fever; and rare skin, organ, and neurologic sequelae.1,6-8
Cutaneous manifestations, present in 16% to 50% of patients with Sjögren syndrome, are considered one of the most common extraglandular presentations of the syndrome. They are classified as nonvascular (eg, xerosis, angular cheilitis, eyelid dermatitis, annular erythema) and vascular (eg, Raynaud phenomenon, vasculitis).9-11 Our patient did not have any of those findings. She had not taken any medications before the rash appeared, thereby ruling out a drug reaction.
The differential for our patient included post–urinary tract infection immune-reactive arthritis and rash, which is not typical with Escherichia coli infection but is described with infection with Chlamydia species and Salmonella species. Moreover, post–urinary tract infection immune-reactive arthritis and rash appear mostly on the palms and soles. Systemic lupus erythematosus–like rashes have a different histology and appear on sun-exposed areas; our patient’s rash was found mainly on unexposed areas.12
Because our patient received the Moderna vaccine 5 days before the rash appeared and later developed swelling of the digits with morning stiffness, a delayed serum sickness–like reaction secondary to COVID-19 vaccination was possible.3,6
COVID-19 mRNA vaccines developed by Pfizer-BioNTech and Moderna incorporate a lipid-based nanoparticle carrier system that prevents rapid enzymatic degradation of mRNA and facilitates in vivo delivery of mRNA. This lipid-based nanoparticle carrier system is further stabilized by a polyethylene glycol 2000 lipid conjugate that provides a hydrophilic layer, thus prolonging half-life. The presence of lipid polyethylene glycol 2000 in mRNA vaccines has led to concern that this component could be implicated in anaphylaxis.6
COVID-19 antigens can give rise to varying clinical manifestations that are directly related to viral tissue damage or are indirectly induced by the antiviral immune response.13,14 Hyperactivation of the immune system to eradicate COVID-19 may trigger autoimmunity; several immune-mediated disorders have been described in individuals infected with SARS-CoV-2. Dermal manifestations include cutaneous rash and vasculitis.13-16 Crucial immunologic steps occur during SARS-CoV-2 infection that may link autoimmunity to COVID-19.13,14 In preliminary published data on the efficacy of the Moderna vaccine on 45 trial enrollees, 3 did not receive the second dose of vaccination, including 1 who developed urticaria on both legs 5 days after the first dose.1
Introduction of viral RNA can induce autoimmunity that can be explained by various phenomena, including epitope spreading, molecular mimicry, cryptic antigen, and bystander activation. Remarkably, more than one-third of immunogenic proteins in SARS-CoV-2 have potentially problematic homology to proteins that are key to the human adaptive immune system.5
Moreover, SARS-CoV-2 seems to induce organ injury through alternative mechanisms beyond direct viral infection, including immunologic injury. In some situations, hyperactivation of the immune response to SARS-CoV-2 RNA can result in autoimmune disease. COVID-19 has been associated with immune-mediated systemic or organ-selective manifestations, some of which fulfill the diagnostic or classification criteria of specific autoimmune diseases. It is unclear whether those medical disorders are the result of transitory postinfectious epiphenomena.5
A few studies have shown that patients with rheumatic disease have an incidence and prevalence of COVID-19 that is similar to the general population. A similar pattern has been detected in COVID-19 morbidity and mortality rates, even among patients with an autoimmune disease, such as rheumatoid arthritis and Sjögren syndrome.5,17 Furthermore, exacerbation of preexisting rheumatic symptoms may be due to hyperactivation of antiviral pathways in a person with an autoimmune disease.17-19 The findings in our patient suggested a direct role for the vaccine in skin manifestations, rather than for reactivation or development of new systemic autoimmune processes, such as systemic lupus erythematosus.
Exacerbation of psoriasis following COVID-19 vaccination has been described20; however, the case patient did not have a history of psoriasis. The mechanism(s) of such exacerbation remain unclear; COVID-19 vaccine–induced helper T cells (TH17) may play a role.21 Other skin manifestations encountered following COVID-19 vaccination include lichen planus, leukocytoclastic vasculitic rash, erythema multiforme–like rash, and pityriasis rosea–like rash.22-25 The immune mechanisms of these manifestations remain unclear.
The clinical presentation of delayed vaccination reactions can be attributed to the timing of symptoms and, in this case, the immune-mediated background of a psoriasiform reaction. Although adverse reactions to the SARS-CoV-2 mRNA vaccine are rare, more individuals should be studied after vaccination to confirm and better understand this phenomenon.
To the Editor:
The Moderna COVID-19 messenger RNA (mRNA) vaccine was authorized for use on December 18, 2020, with the second dose beginning on January 15, 2021.1-3 Some individuals who received the Moderna vaccine experienced an intense rash known as “COVID arm,” a harmless but bothersome adverse effect that typically appears within a week and is a localized and transient immunogenic response.4 COVID arm differs from most vaccine adverse effects. The rash emerges not immediately but 5 to 9 days after the initial dose—on average, 1 week later. Apart from being itchy, the rash does not appear to be harmful and is not a reason to hesitate getting vaccinated.
Dermatologists and allergists have been studying this adverse effect, which has been formally termed delayed cutaneous hypersensitivity. Of potential clinical consequence is that the efficacy of the mRNA COVID-19 vaccine may be harmed if postvaccination dermal reactions necessitate systemic corticosteroid therapy. Because this vaccine stimulates an immune response as viral RNA integrates in cells secondary to production of the spike protein of the virus, the skin may be affected secondarily and manifestations of any underlying disease may be aggravated.5 We report a patient who developed a psoriasiform dermatitis after the first dose of the Moderna vaccine.

A 65-year-old woman presented to her primary care physician because of the severity of psoriasiform dermatitis that developed 5 days after she received the first dose of the Moderna COVID-19 mRNA vaccine. The patient had a medical history of Sjögren syndrome. Her medication history was negative, and her family history was negative for autoimmune disease. Physical examination by primary care revealed an erythematous scaly rash with plaques and papules on the neck and back (Figure 1). The patient presented again to primary care 2 days later with swollen, painful, discolored digits (Figure 2) and a stiff, sore neck.

Laboratory results were positive for anti–Sjögren syndrome–related antigens A and B. A complete blood cell count; comprehensive metabolic panel; erythrocyte sedimentation rate; and assays of rheumatoid factor, C-reactive protein, and anti–cyclic citrullinated peptide were within reference range. A biopsy of a lesion on the back showed psoriasiform dermatitis with confluent parakeratosis and scattered necrotic keratinocytes. There was superficial perivascular inflammation with rare eosinophils (Figure 3).

The patient was treated with a course of systemic corticosteroids. The rash resolved in 1 week. She did not receive the second dose due to the rash.
Two mRNA COVID-19 vaccines—Pfizer BioNTech and Moderna—have been granted emergency use authorization by the US Food and Drug Administration.6 The safety profile of the mRNA-1273 vaccine for the median 2-month follow-up showed no safety concerns.3 Minor localized adverse effects (eg, pain, redness, swelling) have been observed more frequently with the vaccines than with placebo. Systemic symptoms, such as fever, fatigue, headache, and muscle and joint pain, also were seen somewhat more often with the vaccines than with placebo; most such effects occurred 24 to 48 hours after vaccination.3,6,7 The frequency of unsolicited adverse events and serious adverse events reported during the 28-day period after vaccination generally was similar among participants in the vaccine and placebo groups.3
There are 2 types of reactions to COVID-19 vaccination: immediate and delayed. Immediate reactions usually are due to anaphylaxis, requiring prompt recognition and treatment with epinephrine to stop rapid progression of life-threatening symptoms. Delayed reactions include localized reactions, such as urticaria and benign exanthema; serum sickness and serum sickness–like reactions; fever; and rare skin, organ, and neurologic sequelae.1,6-8
Cutaneous manifestations, present in 16% to 50% of patients with Sjögren syndrome, are considered one of the most common extraglandular presentations of the syndrome. They are classified as nonvascular (eg, xerosis, angular cheilitis, eyelid dermatitis, annular erythema) and vascular (eg, Raynaud phenomenon, vasculitis).9-11 Our patient did not have any of those findings. She had not taken any medications before the rash appeared, thereby ruling out a drug reaction.
The differential for our patient included post–urinary tract infection immune-reactive arthritis and rash, which is not typical with Escherichia coli infection but is described with infection with Chlamydia species and Salmonella species. Moreover, post–urinary tract infection immune-reactive arthritis and rash appear mostly on the palms and soles. Systemic lupus erythematosus–like rashes have a different histology and appear on sun-exposed areas; our patient’s rash was found mainly on unexposed areas.12
Because our patient received the Moderna vaccine 5 days before the rash appeared and later developed swelling of the digits with morning stiffness, a delayed serum sickness–like reaction secondary to COVID-19 vaccination was possible.3,6
COVID-19 mRNA vaccines developed by Pfizer-BioNTech and Moderna incorporate a lipid-based nanoparticle carrier system that prevents rapid enzymatic degradation of mRNA and facilitates in vivo delivery of mRNA. This lipid-based nanoparticle carrier system is further stabilized by a polyethylene glycol 2000 lipid conjugate that provides a hydrophilic layer, thus prolonging half-life. The presence of lipid polyethylene glycol 2000 in mRNA vaccines has led to concern that this component could be implicated in anaphylaxis.6
COVID-19 antigens can give rise to varying clinical manifestations that are directly related to viral tissue damage or are indirectly induced by the antiviral immune response.13,14 Hyperactivation of the immune system to eradicate COVID-19 may trigger autoimmunity; several immune-mediated disorders have been described in individuals infected with SARS-CoV-2. Dermal manifestations include cutaneous rash and vasculitis.13-16 Crucial immunologic steps occur during SARS-CoV-2 infection that may link autoimmunity to COVID-19.13,14 In preliminary published data on the efficacy of the Moderna vaccine on 45 trial enrollees, 3 did not receive the second dose of vaccination, including 1 who developed urticaria on both legs 5 days after the first dose.1
Introduction of viral RNA can induce autoimmunity that can be explained by various phenomena, including epitope spreading, molecular mimicry, cryptic antigen, and bystander activation. Remarkably, more than one-third of immunogenic proteins in SARS-CoV-2 have potentially problematic homology to proteins that are key to the human adaptive immune system.5
Moreover, SARS-CoV-2 seems to induce organ injury through alternative mechanisms beyond direct viral infection, including immunologic injury. In some situations, hyperactivation of the immune response to SARS-CoV-2 RNA can result in autoimmune disease. COVID-19 has been associated with immune-mediated systemic or organ-selective manifestations, some of which fulfill the diagnostic or classification criteria of specific autoimmune diseases. It is unclear whether those medical disorders are the result of transitory postinfectious epiphenomena.5
A few studies have shown that patients with rheumatic disease have an incidence and prevalence of COVID-19 that is similar to the general population. A similar pattern has been detected in COVID-19 morbidity and mortality rates, even among patients with an autoimmune disease, such as rheumatoid arthritis and Sjögren syndrome.5,17 Furthermore, exacerbation of preexisting rheumatic symptoms may be due to hyperactivation of antiviral pathways in a person with an autoimmune disease.17-19 The findings in our patient suggested a direct role for the vaccine in skin manifestations, rather than for reactivation or development of new systemic autoimmune processes, such as systemic lupus erythematosus.
Exacerbation of psoriasis following COVID-19 vaccination has been described20; however, the case patient did not have a history of psoriasis. The mechanism(s) of such exacerbation remain unclear; COVID-19 vaccine–induced helper T cells (TH17) may play a role.21 Other skin manifestations encountered following COVID-19 vaccination include lichen planus, leukocytoclastic vasculitic rash, erythema multiforme–like rash, and pityriasis rosea–like rash.22-25 The immune mechanisms of these manifestations remain unclear.
The clinical presentation of delayed vaccination reactions can be attributed to the timing of symptoms and, in this case, the immune-mediated background of a psoriasiform reaction. Although adverse reactions to the SARS-CoV-2 mRNA vaccine are rare, more individuals should be studied after vaccination to confirm and better understand this phenomenon.
- Jackson LA, Anderson EJ, Rouphael NG, et al; . An mRNA vaccine against SARS-CoV-2—preliminary report. N Engl J Med. 2020;383:1920-1931. doi:10.1056/NEJMoa2022483
- Anderson EJ, Rouphael NG, Widge AT, et al; . Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adults. N Engl J Med. 2020;383:2427-2438. doi:10.1056/NEJMoa2028436
- Baden LR, El Sahly HM, Essink B, et al; COVE Study Group. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403-416. doi:10.1056/NEJMoa2035389
- Weise E. ‘COVID arm’ rash seen after Moderna vaccine annoying but harmless, doctors say. USA Today. January 27, 2021. Accessed September 4, 2022. https://www.usatoday.com/story/news/health/2021/01/27/covid-arm-moderna-vaccine-rash-harmless-side-effect-doctors-say/4277725001/
- Talotta R, Robertson E. Autoimmunity as the comet tail of COVID-19 pandemic. World J Clin Cases. 2020;8:3621-3644. doi:10.12998/wjcc.v8.i17.3621
- Castells MC, Phillips EJ. Maintaining safety with SARS-CoV-2 vaccines. N Engl J Med. 2021;384:643-649. doi:10.1056/NEJMra2035343
- Polack FP, Thomas SJ, Kitchin N, et al; . Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603-2615. doi:10.1056/NEJMoa2034577
- Dooling K, McClung N, Chamberland M, et al. The Advisory Committee on Immunization Practices’ interim recommendation for allocating initial supplies of COVID-19 vaccine—United States, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1857-1859. doi:10.15585/mmwr.mm6949e1
- Roguedas AM, Misery L, Sassolas B, et al. Cutaneous manifestations of primary Sjögren’s syndrome are underestimated. Clin Exp Rheumatol. 2004;22:632-636.
- Katayama I. Dry skin manifestations in Sjögren syndrome and atopic dermatitis related to aberrant sudomotor function in inflammatory allergic skin diseases. Allergol Int. 2018;67:448-454. doi:10.1016/j.alit.2018.07.001
- Generali E, Costanzo A, Mainetti C, et al. Cutaneous and mucosal manifestations of Sjögren’s syndrome. Clin Rev Allergy Immunol. 2017;53:357-370. doi:10.1007/s12016-017-8639-y
- Chanprapaph K, Tankunakorn J, Suchonwanit P, et al. Dermatologic manifestations, histologic features and disease progression among cutaneous lupus erythematosus subtypes: a prospective observational study in Asians. Dermatol Ther (Heidelb). 2021;11:131-147. doi:10.1007/s13555-020-00471-y
- Ortega-Quijano D, Jimenez-Cauhe J, Selda-Enriquez G, et al. Algorithm for the classification of COVID-19 rashes. J Am Acad Dermatol. 2020;83:e103-e104. doi:10.1016/j.jaad.2020.05.034
- Rahimi H, Tehranchinia Z. A comprehensive review of cutaneous manifestations associated with COVID-19. Biomed Res Int. 2020;2020:1236520. doi:10.1155/2020/1236520
- Sachdeva M, Gianotti R, Shah M, et al. Cutaneous manifestations of COVID-19: report of three cases and a review of literature. J Dermatol Sci. 2020;98:75-81. doi:10.1016/j.jdermsci.2020.04.011
- Landa N, Mendieta-Eckert M, Fonda-Pascual P, et al. Chilblain-like lesions on feet and hands during the COVID-19 pandemic. Int J Dermatol. 2020;59:739-743. doi:10.1111/ijd.14937
- Dellavance A, Coelho Andrade LE. Immunologic derangement preceding clinical autoimmunity. Lupus. 2014;23:1305-1308. doi:10.1177/0961203314531346
- Parodi A, Gasparini G, Cozzani E. Could antiphospholipid antibodies contribute to coagulopathy in COVID-19? J Am Acad Dermatol. 2020;83:e249. doi:10.1016/j.jaad.2020.06.003
- Zhou Y, Han T, Chen J, et al. Clinical and autoimmune characteristics of severe and critical cases of COVID-19. Clin Transl Sci. 2020;13:1077-1086. doi:10.1111/cts.12805
- Huang YW, Tsai TF. Exacerbation of psoriasis following COVID-19 vaccination: report from a single center. Front Med (Lausanne). 2021;8:812010. doi:10.3389/fmed.2021.812010
- Rouai M, Slimane MB, Sassi W, et al. Pustular rash triggered by Pfizer-BioNTech COVID-19 vaccination: a case report. Dermatol Ther. 2022:e15465. doi:10.1111/dth.15465
- Altun E, Kuzucular E. Leukocytoclastic vasculitis after COVID-19 vaccination. Dermatol Ther. 2022;35:e15279. doi:10.1111/dth.15279
- Buckley JE, Landis LN, Rapini RP. Pityriasis rosea-like rash after mRNA COVID-19 vaccination: a case report and review of the literature. JAAD Int. 2022;7:164-168. doi:10.1016/j.jdin.2022.01.009
- Gökçek GE, Öksüm Solak E, Çölgeçen E. Pityriasis rosea like eruption: a dermatological manifestation of Coronavac-COVID-19 vaccine. Dermatol Ther. 2022;35:e15256. doi:10.1111/dth.15256
- Kim MJ, Kim JW, Kim MS, et al. Generalized erythema multiforme-like skin rash following the first dose of COVID-19 vaccine (Pfizer-BioNTech). J Eur Acad Dermatol Venereol. 2022;36:e98-e100. doi:10.1111/jdv.17757
- Jackson LA, Anderson EJ, Rouphael NG, et al; . An mRNA vaccine against SARS-CoV-2—preliminary report. N Engl J Med. 2020;383:1920-1931. doi:10.1056/NEJMoa2022483
- Anderson EJ, Rouphael NG, Widge AT, et al; . Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adults. N Engl J Med. 2020;383:2427-2438. doi:10.1056/NEJMoa2028436
- Baden LR, El Sahly HM, Essink B, et al; COVE Study Group. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403-416. doi:10.1056/NEJMoa2035389
- Weise E. ‘COVID arm’ rash seen after Moderna vaccine annoying but harmless, doctors say. USA Today. January 27, 2021. Accessed September 4, 2022. https://www.usatoday.com/story/news/health/2021/01/27/covid-arm-moderna-vaccine-rash-harmless-side-effect-doctors-say/4277725001/
- Talotta R, Robertson E. Autoimmunity as the comet tail of COVID-19 pandemic. World J Clin Cases. 2020;8:3621-3644. doi:10.12998/wjcc.v8.i17.3621
- Castells MC, Phillips EJ. Maintaining safety with SARS-CoV-2 vaccines. N Engl J Med. 2021;384:643-649. doi:10.1056/NEJMra2035343
- Polack FP, Thomas SJ, Kitchin N, et al; . Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603-2615. doi:10.1056/NEJMoa2034577
- Dooling K, McClung N, Chamberland M, et al. The Advisory Committee on Immunization Practices’ interim recommendation for allocating initial supplies of COVID-19 vaccine—United States, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1857-1859. doi:10.15585/mmwr.mm6949e1
- Roguedas AM, Misery L, Sassolas B, et al. Cutaneous manifestations of primary Sjögren’s syndrome are underestimated. Clin Exp Rheumatol. 2004;22:632-636.
- Katayama I. Dry skin manifestations in Sjögren syndrome and atopic dermatitis related to aberrant sudomotor function in inflammatory allergic skin diseases. Allergol Int. 2018;67:448-454. doi:10.1016/j.alit.2018.07.001
- Generali E, Costanzo A, Mainetti C, et al. Cutaneous and mucosal manifestations of Sjögren’s syndrome. Clin Rev Allergy Immunol. 2017;53:357-370. doi:10.1007/s12016-017-8639-y
- Chanprapaph K, Tankunakorn J, Suchonwanit P, et al. Dermatologic manifestations, histologic features and disease progression among cutaneous lupus erythematosus subtypes: a prospective observational study in Asians. Dermatol Ther (Heidelb). 2021;11:131-147. doi:10.1007/s13555-020-00471-y
- Ortega-Quijano D, Jimenez-Cauhe J, Selda-Enriquez G, et al. Algorithm for the classification of COVID-19 rashes. J Am Acad Dermatol. 2020;83:e103-e104. doi:10.1016/j.jaad.2020.05.034
- Rahimi H, Tehranchinia Z. A comprehensive review of cutaneous manifestations associated with COVID-19. Biomed Res Int. 2020;2020:1236520. doi:10.1155/2020/1236520
- Sachdeva M, Gianotti R, Shah M, et al. Cutaneous manifestations of COVID-19: report of three cases and a review of literature. J Dermatol Sci. 2020;98:75-81. doi:10.1016/j.jdermsci.2020.04.011
- Landa N, Mendieta-Eckert M, Fonda-Pascual P, et al. Chilblain-like lesions on feet and hands during the COVID-19 pandemic. Int J Dermatol. 2020;59:739-743. doi:10.1111/ijd.14937
- Dellavance A, Coelho Andrade LE. Immunologic derangement preceding clinical autoimmunity. Lupus. 2014;23:1305-1308. doi:10.1177/0961203314531346
- Parodi A, Gasparini G, Cozzani E. Could antiphospholipid antibodies contribute to coagulopathy in COVID-19? J Am Acad Dermatol. 2020;83:e249. doi:10.1016/j.jaad.2020.06.003
- Zhou Y, Han T, Chen J, et al. Clinical and autoimmune characteristics of severe and critical cases of COVID-19. Clin Transl Sci. 2020;13:1077-1086. doi:10.1111/cts.12805
- Huang YW, Tsai TF. Exacerbation of psoriasis following COVID-19 vaccination: report from a single center. Front Med (Lausanne). 2021;8:812010. doi:10.3389/fmed.2021.812010
- Rouai M, Slimane MB, Sassi W, et al. Pustular rash triggered by Pfizer-BioNTech COVID-19 vaccination: a case report. Dermatol Ther. 2022:e15465. doi:10.1111/dth.15465
- Altun E, Kuzucular E. Leukocytoclastic vasculitis after COVID-19 vaccination. Dermatol Ther. 2022;35:e15279. doi:10.1111/dth.15279
- Buckley JE, Landis LN, Rapini RP. Pityriasis rosea-like rash after mRNA COVID-19 vaccination: a case report and review of the literature. JAAD Int. 2022;7:164-168. doi:10.1016/j.jdin.2022.01.009
- Gökçek GE, Öksüm Solak E, Çölgeçen E. Pityriasis rosea like eruption: a dermatological manifestation of Coronavac-COVID-19 vaccine. Dermatol Ther. 2022;35:e15256. doi:10.1111/dth.15256
- Kim MJ, Kim JW, Kim MS, et al. Generalized erythema multiforme-like skin rash following the first dose of COVID-19 vaccine (Pfizer-BioNTech). J Eur Acad Dermatol Venereol. 2022;36:e98-e100. doi:10.1111/jdv.17757
PRACTICE POINTS
- The differential diagnosis for a new-onset psoriasiform rash in an elderly patient should include a vaccine-related rash.
- A rash following vaccination that necessitates systemic corticosteroid therapy can decrease vaccine efficacy.
Microtox and Mesotox
The terms when they mention one of these terms.
Let’s settle the nomenclature confusion. In this column, I define and outline suggested terminology based on studies and my 15 years of experience using neuromodulators. If any readers or colleagues disagree, please write to me and we can discuss the alternatives in a subsequent article; if you agree, please also write to me so we can collaboratively correct the discrepancies in the literature accordingly.
The term mesotherapy, originating from the Greek “mesos” referring to the early embryonic mesoderm, was identified in the 1950’s by Dr. Michel Pistor, a French physician who administered drugs intradermally. The term was defined as a minimally invasive technique by which drugs or bioactive substances are given in small quantities through dermal micropunctures. Drugs administered intradermally diffuse very slowly and therefore, stay in the tissue longer than those administered intramuscularly.
Thus, Mesotox is defined not by the concentration of the neuromodulator or location, but by the depth of injection in the superficial dermis. It can be delivered through individual injections or through a microneedling pen.
Microtox refers to the dilution of the neuromodulator at concentrations below the proposed dilution guidelines of the manufacturer: Less than 2.5 U per 0.1 mL for onabotulinumtoxinA (OBA), incobotulinumtoxinA (IBA), and prabotulinumtoxinA (PBA); and less than 10 U per 0.1 mL for abobotulinumtoxinA (ABO), This method allows for the injection of superficial cutaneous muscles softening the dynamic rhytids without complete paralysis.
Mesotox is widely used off label for facial lifting, reduction in skin laxity or crepiness, flushing of rosacea, acne, hyperhidrosis of the face, keloids, seborrhea, neck rejuvenation, contouring of the mandibular border, and scalp oiliness. Based on a review of articles using this technique, dilution methods were less than 2.5 U per 1 mL (OBA, IBA) and less than 10 U per 0.1 mL (ABO) depth of injection was the superficial to mid-dermis with injection points 0.5 cm to 1 cm apart.
In a study by Atwa and colleagues, 25 patients with mild facial skin laxity received intradermal Botox-A on one side and saline on the other. This split face study showed a highly significant difference with facial lifting on the treated side. Mesotox injection points vary based on the clinical indication and area being treated.
The treatment of dynamic muscles using standard neuromodulator dosing protocols include the treatment of the glabella, crow’s feet, forehead lines, masseter hypertrophy, bunny lines, gummy smile, perioral lines, mentalis hypertonia, platysmal bands, and marionette lines.
However, hyperdilute neuromodulators or Microtox can effectively be used alone or in combination with standard dosing for the following off-label uses. Used in combination with standard dosing of the forehead lines, I use Microtox in the lateral brow to soften the frontalis muscle without dropping the brow in patients with a low-set brow or lid laxity. I also use it for the jelly roll of the eyes and to open the aperture of the eyes. Along the nose, Microtox can also be used to treat a sagging nasal tip, decrease the width of the ala, and treat overactive facial muscles adjacent to the nose resulting in an overactive nasolabial fold.
Similarly, Microtox can be used to treat lateral smile lines and downward extensions of the crow’s feet. In all of the aforementioned treatment areas, I recommend approximately 0.5-1 U of toxin in each area divided at 1-cm intervals.Mesotox and Microtox are both highly effective strategies to treat the aging face. However, the nomenclature is not interchangeable. I propose that the term Mesotox be used only to articulate or define the superficial injection of a neuromodulator for the improvement of the skin that does not involve the injection into or paralysis of a cutaneous muscle (“tox” being used generically for all neuromodulators). I also propose that the term Microtox should be used to define the dilution of a neuromodulator beyond the manufacturer-recommended dilution protocols – used for the paralysis of a cutaneous muscle. In addition, I recommend that the terms MicroBotox and MesoBotox no longer be used. These procedures all have risks, and adverse events associated with Microtox and Mesotox are similar to those of any neuromodulator injection at FDA-recommended maximum doses, and dilution and storage protocols and proper injection techniques need to be followed. Expertise and training is crucial and treatment by a board-certified dermatologist or plastic surgeon is imperative.
Dr. Talakoub and Naissan O. Wesley, MD, are cocontributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub. Write to her at dermnews@mdedge.com. Dr. Talakoub had no relevant disclosures.
References
Awaida CJ et al. Plast Reconstr Surg. 2018 Sep;142(3):640-9.
Calvani F et al. Plast Surg (Oakv). 2019 May;27(2):156-61.
Iranmanesh B et al. J Cosmet Dermatol. 2022 Oct;21(10):4160-70.
Kandhari R et al. J Cutan Aesthet Surg. 2022 Apr-Jun;15(2):101-7.
Lewandowski M et al. Molecules. 2022 May 13;27(10):3143.
Mammucari M et al. Eur Rev Med Pharmacol Sci. 2011 Jun;15(6):682-94.
Park KY et al. Ann Dermatol. 2018 Dec;30(6):688-93.
Pistor M. Chir Dent Fr. 1976;46:59-60.
Rho NK, Gil YC. Toxins (Basel). 2021 Nov 19;13(11):817.
Wu WTL. Plast Reconstr Surg. 2015 Nov;136(5 Suppl):92S-100S.
Zhang H et al. Clin Cosmet Investig Dermatol. 2021 Apr 30;14:407-17.
The terms when they mention one of these terms.
Let’s settle the nomenclature confusion. In this column, I define and outline suggested terminology based on studies and my 15 years of experience using neuromodulators. If any readers or colleagues disagree, please write to me and we can discuss the alternatives in a subsequent article; if you agree, please also write to me so we can collaboratively correct the discrepancies in the literature accordingly.
The term mesotherapy, originating from the Greek “mesos” referring to the early embryonic mesoderm, was identified in the 1950’s by Dr. Michel Pistor, a French physician who administered drugs intradermally. The term was defined as a minimally invasive technique by which drugs or bioactive substances are given in small quantities through dermal micropunctures. Drugs administered intradermally diffuse very slowly and therefore, stay in the tissue longer than those administered intramuscularly.
Thus, Mesotox is defined not by the concentration of the neuromodulator or location, but by the depth of injection in the superficial dermis. It can be delivered through individual injections or through a microneedling pen.
Microtox refers to the dilution of the neuromodulator at concentrations below the proposed dilution guidelines of the manufacturer: Less than 2.5 U per 0.1 mL for onabotulinumtoxinA (OBA), incobotulinumtoxinA (IBA), and prabotulinumtoxinA (PBA); and less than 10 U per 0.1 mL for abobotulinumtoxinA (ABO), This method allows for the injection of superficial cutaneous muscles softening the dynamic rhytids without complete paralysis.
Mesotox is widely used off label for facial lifting, reduction in skin laxity or crepiness, flushing of rosacea, acne, hyperhidrosis of the face, keloids, seborrhea, neck rejuvenation, contouring of the mandibular border, and scalp oiliness. Based on a review of articles using this technique, dilution methods were less than 2.5 U per 1 mL (OBA, IBA) and less than 10 U per 0.1 mL (ABO) depth of injection was the superficial to mid-dermis with injection points 0.5 cm to 1 cm apart.
In a study by Atwa and colleagues, 25 patients with mild facial skin laxity received intradermal Botox-A on one side and saline on the other. This split face study showed a highly significant difference with facial lifting on the treated side. Mesotox injection points vary based on the clinical indication and area being treated.
The treatment of dynamic muscles using standard neuromodulator dosing protocols include the treatment of the glabella, crow’s feet, forehead lines, masseter hypertrophy, bunny lines, gummy smile, perioral lines, mentalis hypertonia, platysmal bands, and marionette lines.
However, hyperdilute neuromodulators or Microtox can effectively be used alone or in combination with standard dosing for the following off-label uses. Used in combination with standard dosing of the forehead lines, I use Microtox in the lateral brow to soften the frontalis muscle without dropping the brow in patients with a low-set brow or lid laxity. I also use it for the jelly roll of the eyes and to open the aperture of the eyes. Along the nose, Microtox can also be used to treat a sagging nasal tip, decrease the width of the ala, and treat overactive facial muscles adjacent to the nose resulting in an overactive nasolabial fold.
Similarly, Microtox can be used to treat lateral smile lines and downward extensions of the crow’s feet. In all of the aforementioned treatment areas, I recommend approximately 0.5-1 U of toxin in each area divided at 1-cm intervals.Mesotox and Microtox are both highly effective strategies to treat the aging face. However, the nomenclature is not interchangeable. I propose that the term Mesotox be used only to articulate or define the superficial injection of a neuromodulator for the improvement of the skin that does not involve the injection into or paralysis of a cutaneous muscle (“tox” being used generically for all neuromodulators). I also propose that the term Microtox should be used to define the dilution of a neuromodulator beyond the manufacturer-recommended dilution protocols – used for the paralysis of a cutaneous muscle. In addition, I recommend that the terms MicroBotox and MesoBotox no longer be used. These procedures all have risks, and adverse events associated with Microtox and Mesotox are similar to those of any neuromodulator injection at FDA-recommended maximum doses, and dilution and storage protocols and proper injection techniques need to be followed. Expertise and training is crucial and treatment by a board-certified dermatologist or plastic surgeon is imperative.
Dr. Talakoub and Naissan O. Wesley, MD, are cocontributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub. Write to her at dermnews@mdedge.com. Dr. Talakoub had no relevant disclosures.
References
Awaida CJ et al. Plast Reconstr Surg. 2018 Sep;142(3):640-9.
Calvani F et al. Plast Surg (Oakv). 2019 May;27(2):156-61.
Iranmanesh B et al. J Cosmet Dermatol. 2022 Oct;21(10):4160-70.
Kandhari R et al. J Cutan Aesthet Surg. 2022 Apr-Jun;15(2):101-7.
Lewandowski M et al. Molecules. 2022 May 13;27(10):3143.
Mammucari M et al. Eur Rev Med Pharmacol Sci. 2011 Jun;15(6):682-94.
Park KY et al. Ann Dermatol. 2018 Dec;30(6):688-93.
Pistor M. Chir Dent Fr. 1976;46:59-60.
Rho NK, Gil YC. Toxins (Basel). 2021 Nov 19;13(11):817.
Wu WTL. Plast Reconstr Surg. 2015 Nov;136(5 Suppl):92S-100S.
Zhang H et al. Clin Cosmet Investig Dermatol. 2021 Apr 30;14:407-17.
The terms when they mention one of these terms.
Let’s settle the nomenclature confusion. In this column, I define and outline suggested terminology based on studies and my 15 years of experience using neuromodulators. If any readers or colleagues disagree, please write to me and we can discuss the alternatives in a subsequent article; if you agree, please also write to me so we can collaboratively correct the discrepancies in the literature accordingly.
The term mesotherapy, originating from the Greek “mesos” referring to the early embryonic mesoderm, was identified in the 1950’s by Dr. Michel Pistor, a French physician who administered drugs intradermally. The term was defined as a minimally invasive technique by which drugs or bioactive substances are given in small quantities through dermal micropunctures. Drugs administered intradermally diffuse very slowly and therefore, stay in the tissue longer than those administered intramuscularly.
Thus, Mesotox is defined not by the concentration of the neuromodulator or location, but by the depth of injection in the superficial dermis. It can be delivered through individual injections or through a microneedling pen.
Microtox refers to the dilution of the neuromodulator at concentrations below the proposed dilution guidelines of the manufacturer: Less than 2.5 U per 0.1 mL for onabotulinumtoxinA (OBA), incobotulinumtoxinA (IBA), and prabotulinumtoxinA (PBA); and less than 10 U per 0.1 mL for abobotulinumtoxinA (ABO), This method allows for the injection of superficial cutaneous muscles softening the dynamic rhytids without complete paralysis.
Mesotox is widely used off label for facial lifting, reduction in skin laxity or crepiness, flushing of rosacea, acne, hyperhidrosis of the face, keloids, seborrhea, neck rejuvenation, contouring of the mandibular border, and scalp oiliness. Based on a review of articles using this technique, dilution methods were less than 2.5 U per 1 mL (OBA, IBA) and less than 10 U per 0.1 mL (ABO) depth of injection was the superficial to mid-dermis with injection points 0.5 cm to 1 cm apart.
In a study by Atwa and colleagues, 25 patients with mild facial skin laxity received intradermal Botox-A on one side and saline on the other. This split face study showed a highly significant difference with facial lifting on the treated side. Mesotox injection points vary based on the clinical indication and area being treated.
The treatment of dynamic muscles using standard neuromodulator dosing protocols include the treatment of the glabella, crow’s feet, forehead lines, masseter hypertrophy, bunny lines, gummy smile, perioral lines, mentalis hypertonia, platysmal bands, and marionette lines.
However, hyperdilute neuromodulators or Microtox can effectively be used alone or in combination with standard dosing for the following off-label uses. Used in combination with standard dosing of the forehead lines, I use Microtox in the lateral brow to soften the frontalis muscle without dropping the brow in patients with a low-set brow or lid laxity. I also use it for the jelly roll of the eyes and to open the aperture of the eyes. Along the nose, Microtox can also be used to treat a sagging nasal tip, decrease the width of the ala, and treat overactive facial muscles adjacent to the nose resulting in an overactive nasolabial fold.
Similarly, Microtox can be used to treat lateral smile lines and downward extensions of the crow’s feet. In all of the aforementioned treatment areas, I recommend approximately 0.5-1 U of toxin in each area divided at 1-cm intervals.Mesotox and Microtox are both highly effective strategies to treat the aging face. However, the nomenclature is not interchangeable. I propose that the term Mesotox be used only to articulate or define the superficial injection of a neuromodulator for the improvement of the skin that does not involve the injection into or paralysis of a cutaneous muscle (“tox” being used generically for all neuromodulators). I also propose that the term Microtox should be used to define the dilution of a neuromodulator beyond the manufacturer-recommended dilution protocols – used for the paralysis of a cutaneous muscle. In addition, I recommend that the terms MicroBotox and MesoBotox no longer be used. These procedures all have risks, and adverse events associated with Microtox and Mesotox are similar to those of any neuromodulator injection at FDA-recommended maximum doses, and dilution and storage protocols and proper injection techniques need to be followed. Expertise and training is crucial and treatment by a board-certified dermatologist or plastic surgeon is imperative.
Dr. Talakoub and Naissan O. Wesley, MD, are cocontributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub. Write to her at dermnews@mdedge.com. Dr. Talakoub had no relevant disclosures.
References
Awaida CJ et al. Plast Reconstr Surg. 2018 Sep;142(3):640-9.
Calvani F et al. Plast Surg (Oakv). 2019 May;27(2):156-61.
Iranmanesh B et al. J Cosmet Dermatol. 2022 Oct;21(10):4160-70.
Kandhari R et al. J Cutan Aesthet Surg. 2022 Apr-Jun;15(2):101-7.
Lewandowski M et al. Molecules. 2022 May 13;27(10):3143.
Mammucari M et al. Eur Rev Med Pharmacol Sci. 2011 Jun;15(6):682-94.
Park KY et al. Ann Dermatol. 2018 Dec;30(6):688-93.
Pistor M. Chir Dent Fr. 1976;46:59-60.
Rho NK, Gil YC. Toxins (Basel). 2021 Nov 19;13(11):817.
Wu WTL. Plast Reconstr Surg. 2015 Nov;136(5 Suppl):92S-100S.
Zhang H et al. Clin Cosmet Investig Dermatol. 2021 Apr 30;14:407-17.
Ulmus davidiana root extract
Ulmus davidiana, commonly known as yugeunpi, has a long history of use in Korea in treating burns, eczema, frostbite, difficulties in urination, inflammation, and psoriasis,1 and has also been used in China for some of these indications, including skin inflammation.2,3 Currently, there are several areas in which the bioactivity of U. davidiana are under investigation, with numerous potential applications in dermatology. This column focuses briefly on the evidence supporting the traditional uses of the plant and potential new applications.
Anti-inflammatory activity
Eom and colleagues studied the potential of a polysaccharide extract from the root bark of U. davidiana to serve as a suitable cosmetic ingredient for conferring moisturizing, anti-inflammatory, and photoprotective activity. In this 2006 investigation, the composition of the polysaccharide extract was found to be primarily rhamnose, galactose, and glucose. The root extract exhibited a similar humectant moisturizing effect as hyaluronic acid, the researchers reported. The U. davidiana root extract was also found to dose-dependently suppress prostaglandin E2. The inhibition of the release of interleukin-6 and IL-8 was also reported to be significant. The use of the U. davidiana extract also stimulated the recovery of human fibroblasts (two times that of positive control) exposed to UVA irradiation. The researchers suggested that their overall results point to the viability of U. davidiana root extract as a cosmetic agent ingredient to protect skin from UV exposure and the inflammation that follows.2
In 2013, Choi and colleagues found that a methanol extract of the stem and root barks of U. davidiana revealed anti-inflammatory properties, with activity attributed to two trihydroxy acids [then-new trihydroxy fatty acid, 9,12,13-trihydroxyoctadeca-10(Z),15(Z)-dienoic acid, and pinellic acid], both of which blocked prostaglandin D₂ production.4
That same year, Lyu and colleagues studied the antiallergic and anti-inflammatory effects of U. davidiana using a 1-fluoro-2,4-dinitrofluorobenzene (DNFB)–induced contact dermatitis mouse model. They found that treatment at a dose of 10 mg/mL successfully prevented skin lesions caused by consistent DNFB application. Further, the researchers observed that topically applied U. davidiana suppressed spongiosis and reduced total serum immunoglobulin and IgG2a levels. Overall, they concluded that the botanical treatment improved contact dermatitis in mice.1
In 2019, So and colleagues studied the chemical components of U. davidiana root bark (isolating a chromane derivative and 22 known substances) and reported data supporting the traditional use of the root bark for gastroenteric and inflammatory indications.3
Bakuchiol [(1E,3S)-3-ethenyl-3,7-dimethyl-1,6-octadien-1-yl]phenol, a prenylated phenolic monoterpene found in the seeds and leaves of various plants, including U. davidiana, is used for its anti-inflammatory properties in traditional Korean medicine.5 Choi and colleagues determined that bakuchiol exhibited robust anti-inflammatory activity in a study of U. davidiana constituents, at least partially accounting for the anti-inflammatory functions of the plant.5
Antifungal activity
In 2021, Alishir and colleagues conducted a phytochemical analysis of the root bark extract of U. davidiana, resulting in the isolation of 10 substances including the novel coumarin glycoside derivative ulmusakidian. Some of the compounds exhibited antifungal activity against Cryptococcus neoformans, though none demonstrated antifungal activity against Candida albicans.6
Wound dressing
Park and colleagues demonstrated in 2020 that superabsorbing hydrogel wound dressings composed of U. davidiana root bark powders, which exhibit gelling activity, performed effectively in speeding up wound closure and cutaneous regeneration in skin-wound mice models. These dressings also displayed thermal stability and superior mechanical properties to pullulan-only gel films. The researchers concluded that gel films composed of U. davidiana have potential to surpass the effectiveness of current products.7
Anti–hair loss activity
Early in 2022, Kwon and colleagues investigated the anti–hair loss mechanism of U. davidiana and determined that supercritical extraction-residues of U. davidiana significantly hinder the secretion of transforming growth factor–beta but dose dependently salvage insulinlike growth factor 1, and substantially decrease dihydrotestosterone synthesis. They concluded that these U. davidiana supercritical fluid extract residues have the potential to halt the loss of human hair.8
Photoprotective potential
Late in 2020, Her and colleagues reported on their development and analysis of a new distillate derived from a fermented mixture of nine anti-inflammatory herbs including U. davidiana. The investigators assessed the effects of the topically applied distillate on UVB-induced skin damage in Institute of Cancer Research mice, finding significant improvements in the dorsal skin photodamage. Application of the distillate also ameliorated collagen production impairment and diminished proinflammatory cytokine levels of tumor necrosis factor (TNF)–alpha and IL-1B. The researchers concluded that this anti-inflammatory herbal distillate, which includes U. davidiana, displays the potential to serve as a photoprotective agent.9
Antiaging activity
In 2011, Yang and colleagues set out to identify constituent substances of the root bark of U. davidiana that have the capacity to suppress cellular senescence in human fibroblasts and human umbilical vein endothelial cells. They isolated 22 compounds, of which epifriedelanol, ssioriside, and catechin-7-O-beta-D-glucopyranoside impeded adriamycin-induced cellular senescence in human dermal fibroblasts and friedelin, epifriedelanol, and catechin-7-O-beta-apiofuranoside in the umbilical vein endothelial cells. Epifriedelanol was the most potent of the substances, leading the researchers to conclude that this U. davidiana component can diminish cellular senescence in human primary cells and has the potential as an oral and/or topical antiaging agent.10
Also that year, in a study on the protective effects of U. davidiana on UVB-irradiated hairless mice, the authors claimed that an ethanol extract of U. davidiana significantly suppressed wrinkle development in mice chronically exposed to UVB.11 This study showed that U. davidiana extract exerts antioxidant activity as evidenced by a decrease in MMP-1 activity. It also demonstrated antielastase activity. The treated mice showed a decrease in wrinkles as compared with water-treated mice.11 Although this is just one study in mice, it may demonstrate a protective effect on elastic fibers on skin exposed to UVB light.
Late in 2020, Lee and colleagues reported on their study of the possible antiaging effects on the skin of (-)-phenolic compounds isolated from the root bark of U. davidiana. The function of collagenase MMP-1 was found to be inhibited by the isolate (-)-catechin, which also halted collagen degradation caused by TNF-alpha in normal human dermal fibroblasts. Further, the investigators demonstrated that the U. davidiana isolate (-)-catechin reduced the expression of proinflammatory cytokines such as IL-1B and IL-6. They concluded that the U. davidiana isolate exhibits the potential to combat intrinsic as well as extrinsic cutaneous aging.12
These findings are particularly intriguing. There is much overlap between intrinsic and extrinsic aging. If U. davidiana can keep collagen intact and inhibit cellular senescence, it may serve as an early intervention toward slowing or preventing skin aging.
Summary
Of greatest interest now, perhaps, is its potential to impede cellular senescence. Senescent cells release a multitude of inflammatory and other factors that hasten intrinsic aging. Blocking cellular senescence is an important approach to the prevention and treatment of skin aging.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur in Miami. She founded the division of cosmetic dermatology at the University of Miami in 1997. The third edition of her bestselling textbook, “Cosmetic Dermatology,” was published in 2022. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Johnson & Johnson, and Burt’s Bees. She is the CEO of Skin Type Solutions, a SaaS company used to generate skin care routines in the office and as an ecommerce solution. Write to her at dermnews@mdedge.com.
References
1. Lyu J et al. J Pharmacopuncture. 2013 Jun;16(2):41-5.
2. Eom SY et al. J Cosmet Sci. 2006 Sep-Oct;57(5):355-67.
3. So HM et al. Bioorg Chem. 2019 Oct;91:103145.
4. Choi HG et al. Phytother Res. 2013 Sep;27(9):1376-80.
5. Choi SY et al. J Med Food. 2010 Aug;13(4):1019-23.
6. Alishir A et al. Bioorg Med Chem Lett. 2021 Mar 15;36:127828.
7. Park TH et al. Saudi Pharm J. 2020 Jul;28(7):791-802.
8. Kwon YE et al. Molecules. 2022 Feb 19;27(4):1419.
9. Her Y et al. Molecules. 2020 Dec 29;26(1):124.
10. Yang HH et al. Planta Med. 2011 Mar;77(5):441-9.
11. Kim YO et al. Korean Journal of Medicinal Crop Science. 2011;19(6):508-13.
12. Lee S et al. Antioxidants (Basel). 2020 Oct 13;9(10):981.
Ulmus davidiana, commonly known as yugeunpi, has a long history of use in Korea in treating burns, eczema, frostbite, difficulties in urination, inflammation, and psoriasis,1 and has also been used in China for some of these indications, including skin inflammation.2,3 Currently, there are several areas in which the bioactivity of U. davidiana are under investigation, with numerous potential applications in dermatology. This column focuses briefly on the evidence supporting the traditional uses of the plant and potential new applications.
Anti-inflammatory activity
Eom and colleagues studied the potential of a polysaccharide extract from the root bark of U. davidiana to serve as a suitable cosmetic ingredient for conferring moisturizing, anti-inflammatory, and photoprotective activity. In this 2006 investigation, the composition of the polysaccharide extract was found to be primarily rhamnose, galactose, and glucose. The root extract exhibited a similar humectant moisturizing effect as hyaluronic acid, the researchers reported. The U. davidiana root extract was also found to dose-dependently suppress prostaglandin E2. The inhibition of the release of interleukin-6 and IL-8 was also reported to be significant. The use of the U. davidiana extract also stimulated the recovery of human fibroblasts (two times that of positive control) exposed to UVA irradiation. The researchers suggested that their overall results point to the viability of U. davidiana root extract as a cosmetic agent ingredient to protect skin from UV exposure and the inflammation that follows.2
In 2013, Choi and colleagues found that a methanol extract of the stem and root barks of U. davidiana revealed anti-inflammatory properties, with activity attributed to two trihydroxy acids [then-new trihydroxy fatty acid, 9,12,13-trihydroxyoctadeca-10(Z),15(Z)-dienoic acid, and pinellic acid], both of which blocked prostaglandin D₂ production.4
That same year, Lyu and colleagues studied the antiallergic and anti-inflammatory effects of U. davidiana using a 1-fluoro-2,4-dinitrofluorobenzene (DNFB)–induced contact dermatitis mouse model. They found that treatment at a dose of 10 mg/mL successfully prevented skin lesions caused by consistent DNFB application. Further, the researchers observed that topically applied U. davidiana suppressed spongiosis and reduced total serum immunoglobulin and IgG2a levels. Overall, they concluded that the botanical treatment improved contact dermatitis in mice.1
In 2019, So and colleagues studied the chemical components of U. davidiana root bark (isolating a chromane derivative and 22 known substances) and reported data supporting the traditional use of the root bark for gastroenteric and inflammatory indications.3
Bakuchiol [(1E,3S)-3-ethenyl-3,7-dimethyl-1,6-octadien-1-yl]phenol, a prenylated phenolic monoterpene found in the seeds and leaves of various plants, including U. davidiana, is used for its anti-inflammatory properties in traditional Korean medicine.5 Choi and colleagues determined that bakuchiol exhibited robust anti-inflammatory activity in a study of U. davidiana constituents, at least partially accounting for the anti-inflammatory functions of the plant.5
Antifungal activity
In 2021, Alishir and colleagues conducted a phytochemical analysis of the root bark extract of U. davidiana, resulting in the isolation of 10 substances including the novel coumarin glycoside derivative ulmusakidian. Some of the compounds exhibited antifungal activity against Cryptococcus neoformans, though none demonstrated antifungal activity against Candida albicans.6
Wound dressing
Park and colleagues demonstrated in 2020 that superabsorbing hydrogel wound dressings composed of U. davidiana root bark powders, which exhibit gelling activity, performed effectively in speeding up wound closure and cutaneous regeneration in skin-wound mice models. These dressings also displayed thermal stability and superior mechanical properties to pullulan-only gel films. The researchers concluded that gel films composed of U. davidiana have potential to surpass the effectiveness of current products.7
Anti–hair loss activity
Early in 2022, Kwon and colleagues investigated the anti–hair loss mechanism of U. davidiana and determined that supercritical extraction-residues of U. davidiana significantly hinder the secretion of transforming growth factor–beta but dose dependently salvage insulinlike growth factor 1, and substantially decrease dihydrotestosterone synthesis. They concluded that these U. davidiana supercritical fluid extract residues have the potential to halt the loss of human hair.8
Photoprotective potential
Late in 2020, Her and colleagues reported on their development and analysis of a new distillate derived from a fermented mixture of nine anti-inflammatory herbs including U. davidiana. The investigators assessed the effects of the topically applied distillate on UVB-induced skin damage in Institute of Cancer Research mice, finding significant improvements in the dorsal skin photodamage. Application of the distillate also ameliorated collagen production impairment and diminished proinflammatory cytokine levels of tumor necrosis factor (TNF)–alpha and IL-1B. The researchers concluded that this anti-inflammatory herbal distillate, which includes U. davidiana, displays the potential to serve as a photoprotective agent.9
Antiaging activity
In 2011, Yang and colleagues set out to identify constituent substances of the root bark of U. davidiana that have the capacity to suppress cellular senescence in human fibroblasts and human umbilical vein endothelial cells. They isolated 22 compounds, of which epifriedelanol, ssioriside, and catechin-7-O-beta-D-glucopyranoside impeded adriamycin-induced cellular senescence in human dermal fibroblasts and friedelin, epifriedelanol, and catechin-7-O-beta-apiofuranoside in the umbilical vein endothelial cells. Epifriedelanol was the most potent of the substances, leading the researchers to conclude that this U. davidiana component can diminish cellular senescence in human primary cells and has the potential as an oral and/or topical antiaging agent.10
Also that year, in a study on the protective effects of U. davidiana on UVB-irradiated hairless mice, the authors claimed that an ethanol extract of U. davidiana significantly suppressed wrinkle development in mice chronically exposed to UVB.11 This study showed that U. davidiana extract exerts antioxidant activity as evidenced by a decrease in MMP-1 activity. It also demonstrated antielastase activity. The treated mice showed a decrease in wrinkles as compared with water-treated mice.11 Although this is just one study in mice, it may demonstrate a protective effect on elastic fibers on skin exposed to UVB light.
Late in 2020, Lee and colleagues reported on their study of the possible antiaging effects on the skin of (-)-phenolic compounds isolated from the root bark of U. davidiana. The function of collagenase MMP-1 was found to be inhibited by the isolate (-)-catechin, which also halted collagen degradation caused by TNF-alpha in normal human dermal fibroblasts. Further, the investigators demonstrated that the U. davidiana isolate (-)-catechin reduced the expression of proinflammatory cytokines such as IL-1B and IL-6. They concluded that the U. davidiana isolate exhibits the potential to combat intrinsic as well as extrinsic cutaneous aging.12
These findings are particularly intriguing. There is much overlap between intrinsic and extrinsic aging. If U. davidiana can keep collagen intact and inhibit cellular senescence, it may serve as an early intervention toward slowing or preventing skin aging.
Summary
Of greatest interest now, perhaps, is its potential to impede cellular senescence. Senescent cells release a multitude of inflammatory and other factors that hasten intrinsic aging. Blocking cellular senescence is an important approach to the prevention and treatment of skin aging.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur in Miami. She founded the division of cosmetic dermatology at the University of Miami in 1997. The third edition of her bestselling textbook, “Cosmetic Dermatology,” was published in 2022. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Johnson & Johnson, and Burt’s Bees. She is the CEO of Skin Type Solutions, a SaaS company used to generate skin care routines in the office and as an ecommerce solution. Write to her at dermnews@mdedge.com.
References
1. Lyu J et al. J Pharmacopuncture. 2013 Jun;16(2):41-5.
2. Eom SY et al. J Cosmet Sci. 2006 Sep-Oct;57(5):355-67.
3. So HM et al. Bioorg Chem. 2019 Oct;91:103145.
4. Choi HG et al. Phytother Res. 2013 Sep;27(9):1376-80.
5. Choi SY et al. J Med Food. 2010 Aug;13(4):1019-23.
6. Alishir A et al. Bioorg Med Chem Lett. 2021 Mar 15;36:127828.
7. Park TH et al. Saudi Pharm J. 2020 Jul;28(7):791-802.
8. Kwon YE et al. Molecules. 2022 Feb 19;27(4):1419.
9. Her Y et al. Molecules. 2020 Dec 29;26(1):124.
10. Yang HH et al. Planta Med. 2011 Mar;77(5):441-9.
11. Kim YO et al. Korean Journal of Medicinal Crop Science. 2011;19(6):508-13.
12. Lee S et al. Antioxidants (Basel). 2020 Oct 13;9(10):981.
Ulmus davidiana, commonly known as yugeunpi, has a long history of use in Korea in treating burns, eczema, frostbite, difficulties in urination, inflammation, and psoriasis,1 and has also been used in China for some of these indications, including skin inflammation.2,3 Currently, there are several areas in which the bioactivity of U. davidiana are under investigation, with numerous potential applications in dermatology. This column focuses briefly on the evidence supporting the traditional uses of the plant and potential new applications.
Anti-inflammatory activity
Eom and colleagues studied the potential of a polysaccharide extract from the root bark of U. davidiana to serve as a suitable cosmetic ingredient for conferring moisturizing, anti-inflammatory, and photoprotective activity. In this 2006 investigation, the composition of the polysaccharide extract was found to be primarily rhamnose, galactose, and glucose. The root extract exhibited a similar humectant moisturizing effect as hyaluronic acid, the researchers reported. The U. davidiana root extract was also found to dose-dependently suppress prostaglandin E2. The inhibition of the release of interleukin-6 and IL-8 was also reported to be significant. The use of the U. davidiana extract also stimulated the recovery of human fibroblasts (two times that of positive control) exposed to UVA irradiation. The researchers suggested that their overall results point to the viability of U. davidiana root extract as a cosmetic agent ingredient to protect skin from UV exposure and the inflammation that follows.2
In 2013, Choi and colleagues found that a methanol extract of the stem and root barks of U. davidiana revealed anti-inflammatory properties, with activity attributed to two trihydroxy acids [then-new trihydroxy fatty acid, 9,12,13-trihydroxyoctadeca-10(Z),15(Z)-dienoic acid, and pinellic acid], both of which blocked prostaglandin D₂ production.4
That same year, Lyu and colleagues studied the antiallergic and anti-inflammatory effects of U. davidiana using a 1-fluoro-2,4-dinitrofluorobenzene (DNFB)–induced contact dermatitis mouse model. They found that treatment at a dose of 10 mg/mL successfully prevented skin lesions caused by consistent DNFB application. Further, the researchers observed that topically applied U. davidiana suppressed spongiosis and reduced total serum immunoglobulin and IgG2a levels. Overall, they concluded that the botanical treatment improved contact dermatitis in mice.1
In 2019, So and colleagues studied the chemical components of U. davidiana root bark (isolating a chromane derivative and 22 known substances) and reported data supporting the traditional use of the root bark for gastroenteric and inflammatory indications.3
Bakuchiol [(1E,3S)-3-ethenyl-3,7-dimethyl-1,6-octadien-1-yl]phenol, a prenylated phenolic monoterpene found in the seeds and leaves of various plants, including U. davidiana, is used for its anti-inflammatory properties in traditional Korean medicine.5 Choi and colleagues determined that bakuchiol exhibited robust anti-inflammatory activity in a study of U. davidiana constituents, at least partially accounting for the anti-inflammatory functions of the plant.5
Antifungal activity
In 2021, Alishir and colleagues conducted a phytochemical analysis of the root bark extract of U. davidiana, resulting in the isolation of 10 substances including the novel coumarin glycoside derivative ulmusakidian. Some of the compounds exhibited antifungal activity against Cryptococcus neoformans, though none demonstrated antifungal activity against Candida albicans.6
Wound dressing
Park and colleagues demonstrated in 2020 that superabsorbing hydrogel wound dressings composed of U. davidiana root bark powders, which exhibit gelling activity, performed effectively in speeding up wound closure and cutaneous regeneration in skin-wound mice models. These dressings also displayed thermal stability and superior mechanical properties to pullulan-only gel films. The researchers concluded that gel films composed of U. davidiana have potential to surpass the effectiveness of current products.7
Anti–hair loss activity
Early in 2022, Kwon and colleagues investigated the anti–hair loss mechanism of U. davidiana and determined that supercritical extraction-residues of U. davidiana significantly hinder the secretion of transforming growth factor–beta but dose dependently salvage insulinlike growth factor 1, and substantially decrease dihydrotestosterone synthesis. They concluded that these U. davidiana supercritical fluid extract residues have the potential to halt the loss of human hair.8
Photoprotective potential
Late in 2020, Her and colleagues reported on their development and analysis of a new distillate derived from a fermented mixture of nine anti-inflammatory herbs including U. davidiana. The investigators assessed the effects of the topically applied distillate on UVB-induced skin damage in Institute of Cancer Research mice, finding significant improvements in the dorsal skin photodamage. Application of the distillate also ameliorated collagen production impairment and diminished proinflammatory cytokine levels of tumor necrosis factor (TNF)–alpha and IL-1B. The researchers concluded that this anti-inflammatory herbal distillate, which includes U. davidiana, displays the potential to serve as a photoprotective agent.9
Antiaging activity
In 2011, Yang and colleagues set out to identify constituent substances of the root bark of U. davidiana that have the capacity to suppress cellular senescence in human fibroblasts and human umbilical vein endothelial cells. They isolated 22 compounds, of which epifriedelanol, ssioriside, and catechin-7-O-beta-D-glucopyranoside impeded adriamycin-induced cellular senescence in human dermal fibroblasts and friedelin, epifriedelanol, and catechin-7-O-beta-apiofuranoside in the umbilical vein endothelial cells. Epifriedelanol was the most potent of the substances, leading the researchers to conclude that this U. davidiana component can diminish cellular senescence in human primary cells and has the potential as an oral and/or topical antiaging agent.10
Also that year, in a study on the protective effects of U. davidiana on UVB-irradiated hairless mice, the authors claimed that an ethanol extract of U. davidiana significantly suppressed wrinkle development in mice chronically exposed to UVB.11 This study showed that U. davidiana extract exerts antioxidant activity as evidenced by a decrease in MMP-1 activity. It also demonstrated antielastase activity. The treated mice showed a decrease in wrinkles as compared with water-treated mice.11 Although this is just one study in mice, it may demonstrate a protective effect on elastic fibers on skin exposed to UVB light.
Late in 2020, Lee and colleagues reported on their study of the possible antiaging effects on the skin of (-)-phenolic compounds isolated from the root bark of U. davidiana. The function of collagenase MMP-1 was found to be inhibited by the isolate (-)-catechin, which also halted collagen degradation caused by TNF-alpha in normal human dermal fibroblasts. Further, the investigators demonstrated that the U. davidiana isolate (-)-catechin reduced the expression of proinflammatory cytokines such as IL-1B and IL-6. They concluded that the U. davidiana isolate exhibits the potential to combat intrinsic as well as extrinsic cutaneous aging.12
These findings are particularly intriguing. There is much overlap between intrinsic and extrinsic aging. If U. davidiana can keep collagen intact and inhibit cellular senescence, it may serve as an early intervention toward slowing or preventing skin aging.
Summary
Of greatest interest now, perhaps, is its potential to impede cellular senescence. Senescent cells release a multitude of inflammatory and other factors that hasten intrinsic aging. Blocking cellular senescence is an important approach to the prevention and treatment of skin aging.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur in Miami. She founded the division of cosmetic dermatology at the University of Miami in 1997. The third edition of her bestselling textbook, “Cosmetic Dermatology,” was published in 2022. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Johnson & Johnson, and Burt’s Bees. She is the CEO of Skin Type Solutions, a SaaS company used to generate skin care routines in the office and as an ecommerce solution. Write to her at dermnews@mdedge.com.
References
1. Lyu J et al. J Pharmacopuncture. 2013 Jun;16(2):41-5.
2. Eom SY et al. J Cosmet Sci. 2006 Sep-Oct;57(5):355-67.
3. So HM et al. Bioorg Chem. 2019 Oct;91:103145.
4. Choi HG et al. Phytother Res. 2013 Sep;27(9):1376-80.
5. Choi SY et al. J Med Food. 2010 Aug;13(4):1019-23.
6. Alishir A et al. Bioorg Med Chem Lett. 2021 Mar 15;36:127828.
7. Park TH et al. Saudi Pharm J. 2020 Jul;28(7):791-802.
8. Kwon YE et al. Molecules. 2022 Feb 19;27(4):1419.
9. Her Y et al. Molecules. 2020 Dec 29;26(1):124.
10. Yang HH et al. Planta Med. 2011 Mar;77(5):441-9.
11. Kim YO et al. Korean Journal of Medicinal Crop Science. 2011;19(6):508-13.
12. Lee S et al. Antioxidants (Basel). 2020 Oct 13;9(10):981.
IBD and pregnancy: What to tell your patients
While many gastroenterologists may be comfortable with inflammatory bowel disease (IBD), most are not experts in women’s concerns about pregnancy. One study found that, although women with IBD may have concerns about the interplay of their disease and reproductive health, many have not had extensive conversations with their gastroenterologist about it. In fact, that same study found most women expect their gastroenterologist to initiate these conversations.
In this roundtable discussion, Uma Mahadevan, MD, professor of medicine and the director of the Colitis and Crohn’s Disease Center at the University of California, San Francisco; Marla C. Dubinsky, MD, professor of medicine at the Icahn School of Medicine at Mount Sinai, New York; and Sunanda V. Kane, MD, professor of medicine at Mayo Clinic in Rochester, Minn., share how they respond to these questions in their clinical practice.
What should a woman with IBD who is interested in having biological children in the future be thinking about now?
Dr. Mahadevan: Because active disease is associated with lower rates of conception and higher rates of pregnancy loss, women with IBD should first ensure they are in remission. I like to document endoscopic healing with a colonoscopy or sigmoidoscopy, but, if this has been done recently, a fecal calprotectin test can be helpful.
Women with IBD, particularly those with small bowel disease, are at risk for nutritional deficiencies, so prior to conception, I also check vitamin B-12, vitamin D, and iron, and repeat as needed. Zinc and folate can be considered. Those who are underweight should work with a nutritionist to ensure adequate caloric intake.
Dr. Dubinsky: I think it’s also important to stress the importance of taking their IBD medications because they can help patients achieve and maintain disease remission. Uncontrolled inflammation is a key risk factor for spontaneous abortion in the first trimester. Medication we would use in pregnancy is not putting them at risk for spontaneous abortion or congenital anomalies, which is what mothers to be are understandably most concerned about.
I am very honest and transparent with my patients: “About the only thing I need to take care of is you. If you are good, the baby is good.”
Dr. Kane: As Dr. Mahadevan mentioned, women with IBD are at higher risk for vitamin deficiencies, so those need to be corrected before conception. If they smoke, they should stop before conceiving.
There is no increased risk of infertility unless there has been a history of abdominal surgery.
Also, if women are not actively planning on getting pregnant, that would be important to share because some gastroenterologists will avoid certain effective medications if pregnancy is a possibility.
If a woman has had surgery for her IBD, could that make it harder for her to get pregnant?
Dr. Kane: Yes, it can because scar tissue may develop within the pelvis. However, if surgery is indicated to manage a patient’s IBD, then talk to the surgeon about ways that they might be able to reduce the risk of scar tissue formation.
Dr. Dubinsky: One thing to note is that almost all the data of infertility risk and scarring are based on open surgical techniques that involve dissection of the rectum. On the other hand, we don’t yet have enough prospective data on the impact of the modern era of laparoscopic surgery to suggest whether it affects fertility. More data is needed because providers may be giving women old information that is no longer relevant in the modern era.
If a woman is experiencing IBD symptoms, should she attempt to conceive?
Dr. Kane: Gastrointestinal symptoms in patients with IBD could be from active disease but also other things, so it’s important to have a thorough check-up to assess if there is active disease or not. Active disease can (but does not always) lead to a more complicated pregnancy, and conception is not recommended while a patient has active IBD.
Dr. Dubinsky: Although some patients feel an urgency to conceive regardless of disease activity, we need to do our due diligence and explain that we need to focus on getting them into the deepest remission possible, including endoscopic findings, biomarkers, and symptoms.
The most important gift you can give your future moms is to optimize the therapy they’re on before they conceive.
Is it important for someone who’s working with a gastroenterologist and an obstetrician to also work with a maternal-fetal medicine (MFM) specialist?
Dr. Kane: Having a diagnosis of IBD makes a woman’s pregnancy “high risk” because just having the diagnosis is associated with a higher risk of prematurity and small for gestational age – but importantly, not birth defects. A woman whose IBD is in remission should still have a discussion with an MFM specialist, just so everyone is on the same page.
Dr. Dubinsky: I refer to care with MFM specialists as “tighter monitoring.” I tell my patients that MFM specialists have managed many complex pregnancies and feel confident around the safety of their medications, understand the impact of when the baby may be exposed to certain medications, and will focus on following them more closely.
What are the risks of IBD medications during pregnancy and while breastfeeding? Should women stop their medications during pregnancy and breastfeeding?
Dr. Dubinsky: Organogenesis occurs in the first 10 weeks, so any medicines that cross the placenta during that time are up for discussion and debate. Methotrexate and the newer small molecules, such as Janus kinase (JAK) inhibitors and S1P receptor modulators, do cross the placenta during the first trimester and need to be discontinued before conception, sometimes as early as 3 months before conception.
However, biologics are very large proteins and do not cross the placenta until closer to week 27. We are not advocating stopping biologics in advance of conception, or during pregnancy, or during breastfeeding. There is more risk to stopping than continuing.
Dr. Mahadevan: Methotrexate should be stopped at least 3 months prior to conception and should not be taken during pregnancy.
There are limited antibiotic safety data in pregnancy for the longer periods of time used in IBD. I generally prefer amoxicillin/clavulanic acid over ciprofloxacin or metronidazole, but short term (less than 2 weeks) use of any of those three are not contraindicated.
Mesalamine agents and thiopurine monotherapy can be continued through pregnancy and breastfeeding.
Biologic agents, such as anti–tumor necrosis factor, anti-interleukin 23, anti-integrin, and biosimilars, can be continued through pregnancy and during breastfeeding. Given limited exposure in the first trimester, there is no evidence of increased risk of birth defects. As Dr. Dubinsky pointed out, there is active transfer, particularly in the third trimester and minimal transfer in breast milk, but this has not been associated with harm.
Lastly, small molecules, such as the JAK inhibitors tofacitinib and upadacitinib, as well as ozanimod, have virtually no human safety data during pregnancy, and animal data show harm. The use of these agents in pregnancy is not recommended.
Dr. Kane: As Dr. Dubinsky stated, most of the medications our patients take are low risk to continue through pregnancy if the patients are in remission. Although a woman “in remission” on steroids is not really in remission and should not get pregnant until she is on something else.
As far as breastfeeding goes, that should be stopped if the patient is on methotrexate, cyclosporine, or certain antibiotics. If she is on more than 20 mg of prednisone, this can pass to the infant, and a mother should not breastfeed.
Women should avoid fenugreek as a lactation aid, as that contains a compound that can promote bleeding. Lactation cookies are ok.
Otherwise, there are lots of potential benefits to breastfeeding, and I encourage it.
How is a flare treated if it occurs during pregnancy?
Dr. Dubinsky: A flare during pregnancy is treated the same as a flare outside of pregnancy. We want to use noninvasive ways to confirm it, but I think we don’t need to overly investigate in most of our women. If they’re already on a biologic, you may consider changing.
Some women may need corticosteroids. It’s not our favorite move, but there is an urgency to getting a flare under control during pregnancy because of possible complications.
Dr. Mahadevan: Some of this is contingent on when during pregnancy the flare occurs. A patient who has a flare at 38 weeks’ gestation will likely proceed with delivery and the flare will be dealt with separately. Someone at 8 weeks’ gestation is at high risk for pregnancy loss, so treatment should be quick and effective.
As does Dr. Dubinsky, I do try to avoid steroids if possible. For example, I would rather start an effective biologic right away than drag out steroids to see if they will respond.
Dr. Kane: I would add that, if a mother is losing weight, she might need to be hospitalized for additional nutritional support. If surgery is necessary, we usually try to time it for the second or third trimester.
What needs to be taken into consideration regarding mode of delivery? Also, if a woman has undergone prior surgeries, do they increase the risk of delivery complications?
For ulcerative colitis, mode of delivery is based on obstetric, not gastrointestinal, variables. For Crohn’s disease, if there is evidence of perianal disease, then a cesarean is appropriate.
If there is no history of perianal disease, then delivery is based on obstetric variables.
For a woman who has a J pouch, if possible, the surgeon who created it should be contacted to ask about the technical aspects of the pouch and how it lies in the pelvis.
What’s the risk of a postpartum flare if a woman’s IBD remains in clinical remission during pregnancy?
Dr. Mahadevan: There is no increased risk of postpartum flare if a woman continues her IBD medications after delivery. Many of the reports of flare are from stopping medications (mistakenly often) to breastfeed.
Dr. Kane: As Dr. Mahadevan said, the risk of a flare is usually because a woman stops taking her medications because she thinks that medication will be passed to the infant through breastfeeding, which in most cases is not true.
Otherwise, there is not an increased risk of a flare in a 12-month period. However, it is important to monitor for symptoms after delivery; the risk of a flare is not zero.
What symptoms should women watch out for after delivery that may indicate an uptick in disease activity?
Dr. Kane: The same symptoms as before they were pregnant. Diarrhea, abdominal pain, and rectal bleeding are not normal after delivery and should be considered signs of returning disease.
As a gastroenterologist, is there any additional advice you’d offer about conception, fertility, and pregnancy when treating women with IBD?
Dr. Mahadevan: Women with IBD should, when feasible, have a planned pregnancy when in documented remission and under the care of their gastroenterologists, obstetrician, and an MFM specialist. Life happens, and this is not always possible. That said, a woman with IBD has the same chance of getting pregnant as a woman of the same age without IBD, unless she has active disease or a history of pelvic surgery. Women with IBD in remission will generally have healthy pregnancies if they continue appropriate medications.
Dr. Kane: Agreed. The majority of women with IBD will have normal, healthy pregnancies. It is important for them to not stop their IBD therapy without talking to their gastroenterologist first. Well-intentioned but ignorant obstetricians or midwives may recommend stopping, but then panic when disease flares and the mother’s health is at risk. Active inflammation is the worst enemy to a pregnancy, not active therapy.
Dr. Dubinsky: One additional thing to consider is: How do we help women with IBD who have delivered meet the needs of their family and continue to stay on their meds and be in good inflammatory control?
For example, we can give the biologic in the hospital after they’ve had a cesarean or a vaginal delivery and before they leave. We know that that is safe, giving that to them before they leave the hospital is a huge value added.
Another thing is possibly changing their infusions to home infusions. That would be helpful for the moms as well.
Dr. Mahadevan reports being a consultant for AbbVie, Janssen, Pfizer, Gilead, Bristol-Myers Squibb, Takeda, Protagonist, Prometheus, and Boehringer Ingelheim. Dr. Dubinsky is a consultant for AbbVie, Arena, Bristol-Myers Squibb, Janssen, Eli Lilly, Takeda, and Prometheus BioSciences. She is a shareholder and CEO of a publicly traded company, Trellis Health. Dr. Kane is a consultant for Bristol-Myers Squibb, Boehringer Ingelheim, Gilead, Janssen, Takeda, Seres Therapeutics, TechLab, United Healthcare, Predicta-Med, and InveniAI, and is the editor for the IBD section of UptoDate.
AGA Resource
Planning for a family can be challenging, and if your patient has IBD, there are additional factors to consider. The AGA IBD Parenthood Project is the “go-to” resource for everything patients need to know about IBD and pregnancy throughout all stages of family planning.
While many gastroenterologists may be comfortable with inflammatory bowel disease (IBD), most are not experts in women’s concerns about pregnancy. One study found that, although women with IBD may have concerns about the interplay of their disease and reproductive health, many have not had extensive conversations with their gastroenterologist about it. In fact, that same study found most women expect their gastroenterologist to initiate these conversations.
In this roundtable discussion, Uma Mahadevan, MD, professor of medicine and the director of the Colitis and Crohn’s Disease Center at the University of California, San Francisco; Marla C. Dubinsky, MD, professor of medicine at the Icahn School of Medicine at Mount Sinai, New York; and Sunanda V. Kane, MD, professor of medicine at Mayo Clinic in Rochester, Minn., share how they respond to these questions in their clinical practice.
What should a woman with IBD who is interested in having biological children in the future be thinking about now?
Dr. Mahadevan: Because active disease is associated with lower rates of conception and higher rates of pregnancy loss, women with IBD should first ensure they are in remission. I like to document endoscopic healing with a colonoscopy or sigmoidoscopy, but, if this has been done recently, a fecal calprotectin test can be helpful.
Women with IBD, particularly those with small bowel disease, are at risk for nutritional deficiencies, so prior to conception, I also check vitamin B-12, vitamin D, and iron, and repeat as needed. Zinc and folate can be considered. Those who are underweight should work with a nutritionist to ensure adequate caloric intake.
Dr. Dubinsky: I think it’s also important to stress the importance of taking their IBD medications because they can help patients achieve and maintain disease remission. Uncontrolled inflammation is a key risk factor for spontaneous abortion in the first trimester. Medication we would use in pregnancy is not putting them at risk for spontaneous abortion or congenital anomalies, which is what mothers to be are understandably most concerned about.
I am very honest and transparent with my patients: “About the only thing I need to take care of is you. If you are good, the baby is good.”
Dr. Kane: As Dr. Mahadevan mentioned, women with IBD are at higher risk for vitamin deficiencies, so those need to be corrected before conception. If they smoke, they should stop before conceiving.
There is no increased risk of infertility unless there has been a history of abdominal surgery.
Also, if women are not actively planning on getting pregnant, that would be important to share because some gastroenterologists will avoid certain effective medications if pregnancy is a possibility.
If a woman has had surgery for her IBD, could that make it harder for her to get pregnant?
Dr. Kane: Yes, it can because scar tissue may develop within the pelvis. However, if surgery is indicated to manage a patient’s IBD, then talk to the surgeon about ways that they might be able to reduce the risk of scar tissue formation.
Dr. Dubinsky: One thing to note is that almost all the data of infertility risk and scarring are based on open surgical techniques that involve dissection of the rectum. On the other hand, we don’t yet have enough prospective data on the impact of the modern era of laparoscopic surgery to suggest whether it affects fertility. More data is needed because providers may be giving women old information that is no longer relevant in the modern era.
If a woman is experiencing IBD symptoms, should she attempt to conceive?
Dr. Kane: Gastrointestinal symptoms in patients with IBD could be from active disease but also other things, so it’s important to have a thorough check-up to assess if there is active disease or not. Active disease can (but does not always) lead to a more complicated pregnancy, and conception is not recommended while a patient has active IBD.
Dr. Dubinsky: Although some patients feel an urgency to conceive regardless of disease activity, we need to do our due diligence and explain that we need to focus on getting them into the deepest remission possible, including endoscopic findings, biomarkers, and symptoms.
The most important gift you can give your future moms is to optimize the therapy they’re on before they conceive.
Is it important for someone who’s working with a gastroenterologist and an obstetrician to also work with a maternal-fetal medicine (MFM) specialist?
Dr. Kane: Having a diagnosis of IBD makes a woman’s pregnancy “high risk” because just having the diagnosis is associated with a higher risk of prematurity and small for gestational age – but importantly, not birth defects. A woman whose IBD is in remission should still have a discussion with an MFM specialist, just so everyone is on the same page.
Dr. Dubinsky: I refer to care with MFM specialists as “tighter monitoring.” I tell my patients that MFM specialists have managed many complex pregnancies and feel confident around the safety of their medications, understand the impact of when the baby may be exposed to certain medications, and will focus on following them more closely.
What are the risks of IBD medications during pregnancy and while breastfeeding? Should women stop their medications during pregnancy and breastfeeding?
Dr. Dubinsky: Organogenesis occurs in the first 10 weeks, so any medicines that cross the placenta during that time are up for discussion and debate. Methotrexate and the newer small molecules, such as Janus kinase (JAK) inhibitors and S1P receptor modulators, do cross the placenta during the first trimester and need to be discontinued before conception, sometimes as early as 3 months before conception.
However, biologics are very large proteins and do not cross the placenta until closer to week 27. We are not advocating stopping biologics in advance of conception, or during pregnancy, or during breastfeeding. There is more risk to stopping than continuing.
Dr. Mahadevan: Methotrexate should be stopped at least 3 months prior to conception and should not be taken during pregnancy.
There are limited antibiotic safety data in pregnancy for the longer periods of time used in IBD. I generally prefer amoxicillin/clavulanic acid over ciprofloxacin or metronidazole, but short term (less than 2 weeks) use of any of those three are not contraindicated.
Mesalamine agents and thiopurine monotherapy can be continued through pregnancy and breastfeeding.
Biologic agents, such as anti–tumor necrosis factor, anti-interleukin 23, anti-integrin, and biosimilars, can be continued through pregnancy and during breastfeeding. Given limited exposure in the first trimester, there is no evidence of increased risk of birth defects. As Dr. Dubinsky pointed out, there is active transfer, particularly in the third trimester and minimal transfer in breast milk, but this has not been associated with harm.
Lastly, small molecules, such as the JAK inhibitors tofacitinib and upadacitinib, as well as ozanimod, have virtually no human safety data during pregnancy, and animal data show harm. The use of these agents in pregnancy is not recommended.
Dr. Kane: As Dr. Dubinsky stated, most of the medications our patients take are low risk to continue through pregnancy if the patients are in remission. Although a woman “in remission” on steroids is not really in remission and should not get pregnant until she is on something else.
As far as breastfeeding goes, that should be stopped if the patient is on methotrexate, cyclosporine, or certain antibiotics. If she is on more than 20 mg of prednisone, this can pass to the infant, and a mother should not breastfeed.
Women should avoid fenugreek as a lactation aid, as that contains a compound that can promote bleeding. Lactation cookies are ok.
Otherwise, there are lots of potential benefits to breastfeeding, and I encourage it.
How is a flare treated if it occurs during pregnancy?
Dr. Dubinsky: A flare during pregnancy is treated the same as a flare outside of pregnancy. We want to use noninvasive ways to confirm it, but I think we don’t need to overly investigate in most of our women. If they’re already on a biologic, you may consider changing.
Some women may need corticosteroids. It’s not our favorite move, but there is an urgency to getting a flare under control during pregnancy because of possible complications.
Dr. Mahadevan: Some of this is contingent on when during pregnancy the flare occurs. A patient who has a flare at 38 weeks’ gestation will likely proceed with delivery and the flare will be dealt with separately. Someone at 8 weeks’ gestation is at high risk for pregnancy loss, so treatment should be quick and effective.
As does Dr. Dubinsky, I do try to avoid steroids if possible. For example, I would rather start an effective biologic right away than drag out steroids to see if they will respond.
Dr. Kane: I would add that, if a mother is losing weight, she might need to be hospitalized for additional nutritional support. If surgery is necessary, we usually try to time it for the second or third trimester.
What needs to be taken into consideration regarding mode of delivery? Also, if a woman has undergone prior surgeries, do they increase the risk of delivery complications?
For ulcerative colitis, mode of delivery is based on obstetric, not gastrointestinal, variables. For Crohn’s disease, if there is evidence of perianal disease, then a cesarean is appropriate.
If there is no history of perianal disease, then delivery is based on obstetric variables.
For a woman who has a J pouch, if possible, the surgeon who created it should be contacted to ask about the technical aspects of the pouch and how it lies in the pelvis.
What’s the risk of a postpartum flare if a woman’s IBD remains in clinical remission during pregnancy?
Dr. Mahadevan: There is no increased risk of postpartum flare if a woman continues her IBD medications after delivery. Many of the reports of flare are from stopping medications (mistakenly often) to breastfeed.
Dr. Kane: As Dr. Mahadevan said, the risk of a flare is usually because a woman stops taking her medications because she thinks that medication will be passed to the infant through breastfeeding, which in most cases is not true.
Otherwise, there is not an increased risk of a flare in a 12-month period. However, it is important to monitor for symptoms after delivery; the risk of a flare is not zero.
What symptoms should women watch out for after delivery that may indicate an uptick in disease activity?
Dr. Kane: The same symptoms as before they were pregnant. Diarrhea, abdominal pain, and rectal bleeding are not normal after delivery and should be considered signs of returning disease.
As a gastroenterologist, is there any additional advice you’d offer about conception, fertility, and pregnancy when treating women with IBD?
Dr. Mahadevan: Women with IBD should, when feasible, have a planned pregnancy when in documented remission and under the care of their gastroenterologists, obstetrician, and an MFM specialist. Life happens, and this is not always possible. That said, a woman with IBD has the same chance of getting pregnant as a woman of the same age without IBD, unless she has active disease or a history of pelvic surgery. Women with IBD in remission will generally have healthy pregnancies if they continue appropriate medications.
Dr. Kane: Agreed. The majority of women with IBD will have normal, healthy pregnancies. It is important for them to not stop their IBD therapy without talking to their gastroenterologist first. Well-intentioned but ignorant obstetricians or midwives may recommend stopping, but then panic when disease flares and the mother’s health is at risk. Active inflammation is the worst enemy to a pregnancy, not active therapy.
Dr. Dubinsky: One additional thing to consider is: How do we help women with IBD who have delivered meet the needs of their family and continue to stay on their meds and be in good inflammatory control?
For example, we can give the biologic in the hospital after they’ve had a cesarean or a vaginal delivery and before they leave. We know that that is safe, giving that to them before they leave the hospital is a huge value added.
Another thing is possibly changing their infusions to home infusions. That would be helpful for the moms as well.
Dr. Mahadevan reports being a consultant for AbbVie, Janssen, Pfizer, Gilead, Bristol-Myers Squibb, Takeda, Protagonist, Prometheus, and Boehringer Ingelheim. Dr. Dubinsky is a consultant for AbbVie, Arena, Bristol-Myers Squibb, Janssen, Eli Lilly, Takeda, and Prometheus BioSciences. She is a shareholder and CEO of a publicly traded company, Trellis Health. Dr. Kane is a consultant for Bristol-Myers Squibb, Boehringer Ingelheim, Gilead, Janssen, Takeda, Seres Therapeutics, TechLab, United Healthcare, Predicta-Med, and InveniAI, and is the editor for the IBD section of UptoDate.
AGA Resource
Planning for a family can be challenging, and if your patient has IBD, there are additional factors to consider. The AGA IBD Parenthood Project is the “go-to” resource for everything patients need to know about IBD and pregnancy throughout all stages of family planning.
While many gastroenterologists may be comfortable with inflammatory bowel disease (IBD), most are not experts in women’s concerns about pregnancy. One study found that, although women with IBD may have concerns about the interplay of their disease and reproductive health, many have not had extensive conversations with their gastroenterologist about it. In fact, that same study found most women expect their gastroenterologist to initiate these conversations.
In this roundtable discussion, Uma Mahadevan, MD, professor of medicine and the director of the Colitis and Crohn’s Disease Center at the University of California, San Francisco; Marla C. Dubinsky, MD, professor of medicine at the Icahn School of Medicine at Mount Sinai, New York; and Sunanda V. Kane, MD, professor of medicine at Mayo Clinic in Rochester, Minn., share how they respond to these questions in their clinical practice.
What should a woman with IBD who is interested in having biological children in the future be thinking about now?
Dr. Mahadevan: Because active disease is associated with lower rates of conception and higher rates of pregnancy loss, women with IBD should first ensure they are in remission. I like to document endoscopic healing with a colonoscopy or sigmoidoscopy, but, if this has been done recently, a fecal calprotectin test can be helpful.
Women with IBD, particularly those with small bowel disease, are at risk for nutritional deficiencies, so prior to conception, I also check vitamin B-12, vitamin D, and iron, and repeat as needed. Zinc and folate can be considered. Those who are underweight should work with a nutritionist to ensure adequate caloric intake.
Dr. Dubinsky: I think it’s also important to stress the importance of taking their IBD medications because they can help patients achieve and maintain disease remission. Uncontrolled inflammation is a key risk factor for spontaneous abortion in the first trimester. Medication we would use in pregnancy is not putting them at risk for spontaneous abortion or congenital anomalies, which is what mothers to be are understandably most concerned about.
I am very honest and transparent with my patients: “About the only thing I need to take care of is you. If you are good, the baby is good.”
Dr. Kane: As Dr. Mahadevan mentioned, women with IBD are at higher risk for vitamin deficiencies, so those need to be corrected before conception. If they smoke, they should stop before conceiving.
There is no increased risk of infertility unless there has been a history of abdominal surgery.
Also, if women are not actively planning on getting pregnant, that would be important to share because some gastroenterologists will avoid certain effective medications if pregnancy is a possibility.
If a woman has had surgery for her IBD, could that make it harder for her to get pregnant?
Dr. Kane: Yes, it can because scar tissue may develop within the pelvis. However, if surgery is indicated to manage a patient’s IBD, then talk to the surgeon about ways that they might be able to reduce the risk of scar tissue formation.
Dr. Dubinsky: One thing to note is that almost all the data of infertility risk and scarring are based on open surgical techniques that involve dissection of the rectum. On the other hand, we don’t yet have enough prospective data on the impact of the modern era of laparoscopic surgery to suggest whether it affects fertility. More data is needed because providers may be giving women old information that is no longer relevant in the modern era.
If a woman is experiencing IBD symptoms, should she attempt to conceive?
Dr. Kane: Gastrointestinal symptoms in patients with IBD could be from active disease but also other things, so it’s important to have a thorough check-up to assess if there is active disease or not. Active disease can (but does not always) lead to a more complicated pregnancy, and conception is not recommended while a patient has active IBD.
Dr. Dubinsky: Although some patients feel an urgency to conceive regardless of disease activity, we need to do our due diligence and explain that we need to focus on getting them into the deepest remission possible, including endoscopic findings, biomarkers, and symptoms.
The most important gift you can give your future moms is to optimize the therapy they’re on before they conceive.
Is it important for someone who’s working with a gastroenterologist and an obstetrician to also work with a maternal-fetal medicine (MFM) specialist?
Dr. Kane: Having a diagnosis of IBD makes a woman’s pregnancy “high risk” because just having the diagnosis is associated with a higher risk of prematurity and small for gestational age – but importantly, not birth defects. A woman whose IBD is in remission should still have a discussion with an MFM specialist, just so everyone is on the same page.
Dr. Dubinsky: I refer to care with MFM specialists as “tighter monitoring.” I tell my patients that MFM specialists have managed many complex pregnancies and feel confident around the safety of their medications, understand the impact of when the baby may be exposed to certain medications, and will focus on following them more closely.
What are the risks of IBD medications during pregnancy and while breastfeeding? Should women stop their medications during pregnancy and breastfeeding?
Dr. Dubinsky: Organogenesis occurs in the first 10 weeks, so any medicines that cross the placenta during that time are up for discussion and debate. Methotrexate and the newer small molecules, such as Janus kinase (JAK) inhibitors and S1P receptor modulators, do cross the placenta during the first trimester and need to be discontinued before conception, sometimes as early as 3 months before conception.
However, biologics are very large proteins and do not cross the placenta until closer to week 27. We are not advocating stopping biologics in advance of conception, or during pregnancy, or during breastfeeding. There is more risk to stopping than continuing.
Dr. Mahadevan: Methotrexate should be stopped at least 3 months prior to conception and should not be taken during pregnancy.
There are limited antibiotic safety data in pregnancy for the longer periods of time used in IBD. I generally prefer amoxicillin/clavulanic acid over ciprofloxacin or metronidazole, but short term (less than 2 weeks) use of any of those three are not contraindicated.
Mesalamine agents and thiopurine monotherapy can be continued through pregnancy and breastfeeding.
Biologic agents, such as anti–tumor necrosis factor, anti-interleukin 23, anti-integrin, and biosimilars, can be continued through pregnancy and during breastfeeding. Given limited exposure in the first trimester, there is no evidence of increased risk of birth defects. As Dr. Dubinsky pointed out, there is active transfer, particularly in the third trimester and minimal transfer in breast milk, but this has not been associated with harm.
Lastly, small molecules, such as the JAK inhibitors tofacitinib and upadacitinib, as well as ozanimod, have virtually no human safety data during pregnancy, and animal data show harm. The use of these agents in pregnancy is not recommended.
Dr. Kane: As Dr. Dubinsky stated, most of the medications our patients take are low risk to continue through pregnancy if the patients are in remission. Although a woman “in remission” on steroids is not really in remission and should not get pregnant until she is on something else.
As far as breastfeeding goes, that should be stopped if the patient is on methotrexate, cyclosporine, or certain antibiotics. If she is on more than 20 mg of prednisone, this can pass to the infant, and a mother should not breastfeed.
Women should avoid fenugreek as a lactation aid, as that contains a compound that can promote bleeding. Lactation cookies are ok.
Otherwise, there are lots of potential benefits to breastfeeding, and I encourage it.
How is a flare treated if it occurs during pregnancy?
Dr. Dubinsky: A flare during pregnancy is treated the same as a flare outside of pregnancy. We want to use noninvasive ways to confirm it, but I think we don’t need to overly investigate in most of our women. If they’re already on a biologic, you may consider changing.
Some women may need corticosteroids. It’s not our favorite move, but there is an urgency to getting a flare under control during pregnancy because of possible complications.
Dr. Mahadevan: Some of this is contingent on when during pregnancy the flare occurs. A patient who has a flare at 38 weeks’ gestation will likely proceed with delivery and the flare will be dealt with separately. Someone at 8 weeks’ gestation is at high risk for pregnancy loss, so treatment should be quick and effective.
As does Dr. Dubinsky, I do try to avoid steroids if possible. For example, I would rather start an effective biologic right away than drag out steroids to see if they will respond.
Dr. Kane: I would add that, if a mother is losing weight, she might need to be hospitalized for additional nutritional support. If surgery is necessary, we usually try to time it for the second or third trimester.
What needs to be taken into consideration regarding mode of delivery? Also, if a woman has undergone prior surgeries, do they increase the risk of delivery complications?
For ulcerative colitis, mode of delivery is based on obstetric, not gastrointestinal, variables. For Crohn’s disease, if there is evidence of perianal disease, then a cesarean is appropriate.
If there is no history of perianal disease, then delivery is based on obstetric variables.
For a woman who has a J pouch, if possible, the surgeon who created it should be contacted to ask about the technical aspects of the pouch and how it lies in the pelvis.
What’s the risk of a postpartum flare if a woman’s IBD remains in clinical remission during pregnancy?
Dr. Mahadevan: There is no increased risk of postpartum flare if a woman continues her IBD medications after delivery. Many of the reports of flare are from stopping medications (mistakenly often) to breastfeed.
Dr. Kane: As Dr. Mahadevan said, the risk of a flare is usually because a woman stops taking her medications because she thinks that medication will be passed to the infant through breastfeeding, which in most cases is not true.
Otherwise, there is not an increased risk of a flare in a 12-month period. However, it is important to monitor for symptoms after delivery; the risk of a flare is not zero.
What symptoms should women watch out for after delivery that may indicate an uptick in disease activity?
Dr. Kane: The same symptoms as before they were pregnant. Diarrhea, abdominal pain, and rectal bleeding are not normal after delivery and should be considered signs of returning disease.
As a gastroenterologist, is there any additional advice you’d offer about conception, fertility, and pregnancy when treating women with IBD?
Dr. Mahadevan: Women with IBD should, when feasible, have a planned pregnancy when in documented remission and under the care of their gastroenterologists, obstetrician, and an MFM specialist. Life happens, and this is not always possible. That said, a woman with IBD has the same chance of getting pregnant as a woman of the same age without IBD, unless she has active disease or a history of pelvic surgery. Women with IBD in remission will generally have healthy pregnancies if they continue appropriate medications.
Dr. Kane: Agreed. The majority of women with IBD will have normal, healthy pregnancies. It is important for them to not stop their IBD therapy without talking to their gastroenterologist first. Well-intentioned but ignorant obstetricians or midwives may recommend stopping, but then panic when disease flares and the mother’s health is at risk. Active inflammation is the worst enemy to a pregnancy, not active therapy.
Dr. Dubinsky: One additional thing to consider is: How do we help women with IBD who have delivered meet the needs of their family and continue to stay on their meds and be in good inflammatory control?
For example, we can give the biologic in the hospital after they’ve had a cesarean or a vaginal delivery and before they leave. We know that that is safe, giving that to them before they leave the hospital is a huge value added.
Another thing is possibly changing their infusions to home infusions. That would be helpful for the moms as well.
Dr. Mahadevan reports being a consultant for AbbVie, Janssen, Pfizer, Gilead, Bristol-Myers Squibb, Takeda, Protagonist, Prometheus, and Boehringer Ingelheim. Dr. Dubinsky is a consultant for AbbVie, Arena, Bristol-Myers Squibb, Janssen, Eli Lilly, Takeda, and Prometheus BioSciences. She is a shareholder and CEO of a publicly traded company, Trellis Health. Dr. Kane is a consultant for Bristol-Myers Squibb, Boehringer Ingelheim, Gilead, Janssen, Takeda, Seres Therapeutics, TechLab, United Healthcare, Predicta-Med, and InveniAI, and is the editor for the IBD section of UptoDate.
AGA Resource
Planning for a family can be challenging, and if your patient has IBD, there are additional factors to consider. The AGA IBD Parenthood Project is the “go-to” resource for everything patients need to know about IBD and pregnancy throughout all stages of family planning.