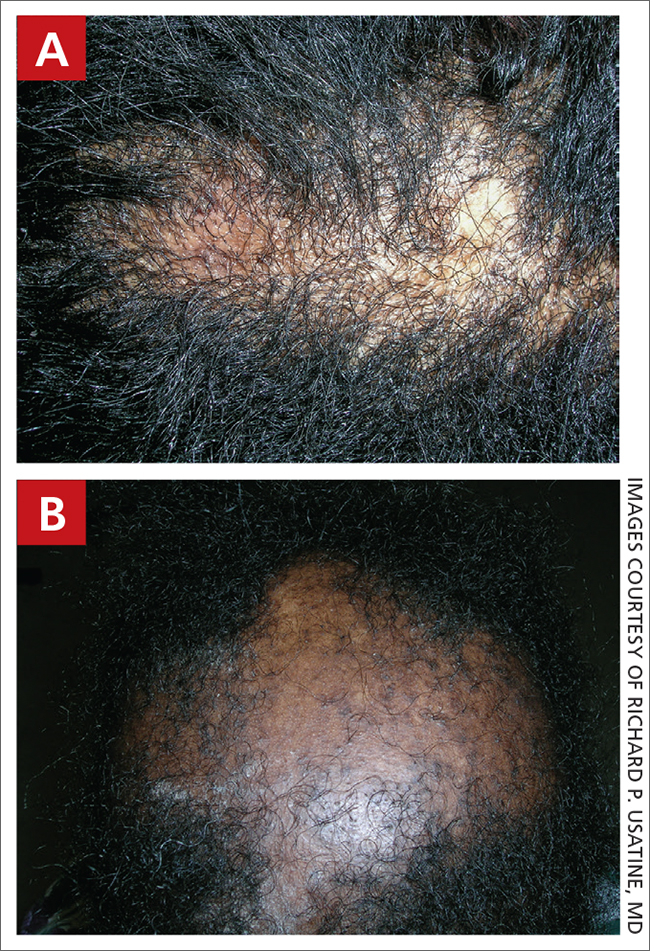
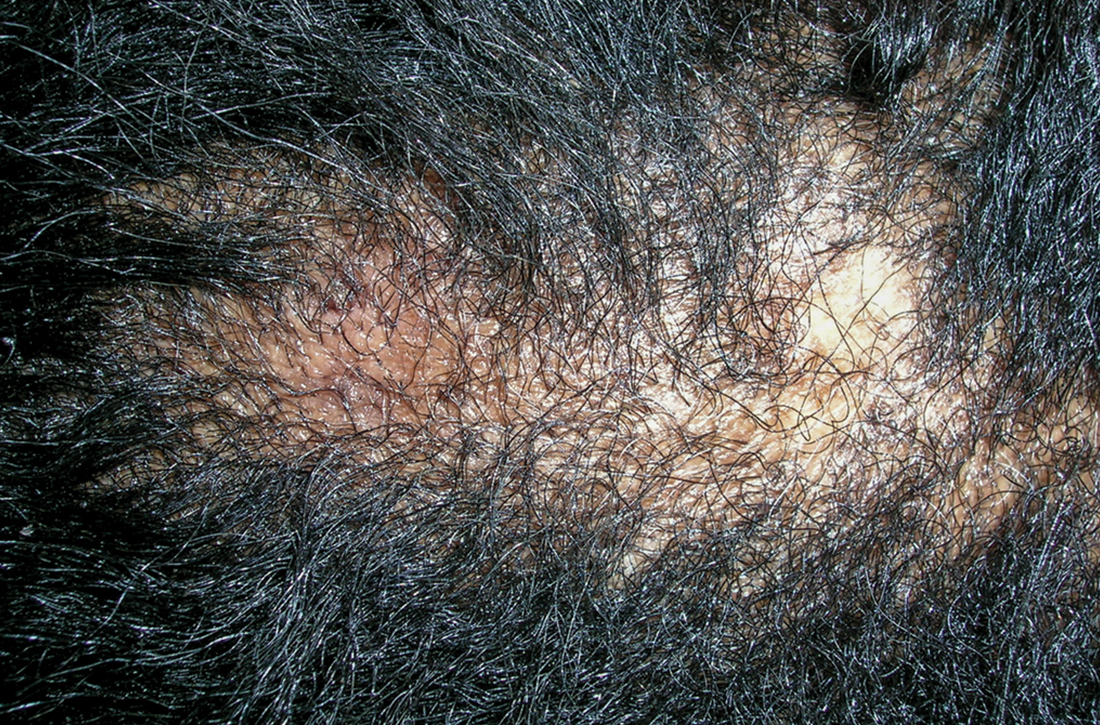
User login
Impact of Clinical Pharmacists on Access to Care in an Epilepsy Clinic
Epilepsy affects about 1% of the world population and is one of the most burdensome in terms of disability-adjusted life-years.1,2 Veterans are at increased risk of developing epilepsy when compared with the general population due to a variety of factors, including a higher frequency of traumatic brain injuries.3 A recent study from the US Centers for Disease Control and Prevention found that veterans who developed epilepsy during their service not only had a higher rate of mental and physical comorbidities, but also were 2.6 times more likely to die compared with veterans without epilepsy.4
Oral antiseizure medications (ASM) remain the mainstay of outpatient epilepsy treatment. Patterns of ASM use are complex within the US Department of Veterans Affairs (VA) patient population, particularly within patients at the Epilepsy Centers of Excellence (ECoE). For example, many patients are transitioned from older ASMs with greater adverse effects (AEs) to better tolerated newer generation ASMs or polytherapy regimens with complex pharmacokinetic profiles and drug interactions.5 Multiple factors are considered when choosing an ASM, including age, sex, epilepsy/seizure type, comorbidities, past medication trials, AEs, and drug interactions. The complex pharmacologic profile of both older and newer ASMs can confound the optimal management of epilepsy, and suboptimal management can lead to neurologic, psychological, physical, and social consequences, including sudden unexplained death in epilepsy.6,7 Psychiatric and behavioral problems are seen in up to 30% of patients with newly diagnosed epilepsy and 50% in those with pharmacoresistant epilepsy.8 Early screening, detection, and treatment for psychiatric comorbidities are an integral part of evidence-based care in epilepsy.
Being familiar with ASM AEs and comorbid conditions such as anxiety and depression can allow for quick identification and intervention to improve safety and quality of life. A 2007 population-based study found that measures of suicidality had a strong association with epilepsy, and performing mental health screenings, such as the Patient Health Questionnaire (PHQ-9), Generalized Anxiety Disorder Screener (GAD-7), and the Brief Irritability Test (BITe), can assist in identifying those patients at risk.9
During the COVID-19 pandemic, it has become increasingly clear that the health care sector is facing increasing pressure. The combination of patient acuity as well as critical health care professional (HCP) shortages may be of particular concern in certain specialty clinics where access to practitioners may already be limited. While this is a multifaceted problem, a pragmatic approach would be to increase the use of clinicians, such as clinical pharmacist practitioners (CPPs).
The William S. Middleton Memorial Veterans Hospital (WSMVH) in Madison, Wisconsin, is 1 of 17 VA ECoE sites. The VA ECoE provides high-quality, comprehensive epilepsy evaluation and care to veterans. In fiscal year (FY) 2020, the 17 sites provided care to 5544 veterans.10 The WSMVH epilepsy clinic sees about 400 veterans each year, receiving referrals from other VA medical centers, and prescribes ASMs, neuromodulation devices, and resective surgeries for epilepsy. The multidisciplinary team consists of an epileptologist, neurophysiology fellow, psychiatrist, nurse practitioner, CPP, and neurology residents. The WSMVH epilepsy clinic has employed CPPs at their highest level of clinical practice authority since 1991.
The WSMVH epilepsy clinic is open 4 hours once weekly. The clinic offers fourteen 30-minute appointment slots either in person or via telehealth. The epileptologist reviews patient charts prior to clinic and assigns each patient to the appropriate HCP. When making the determination to assign a patient to a CPP or pharmacy resident, the epileptologist considers current treatment response, mental health issues as well as medication-related concerns (eg, potential pharmacokinetic/pharmacodynamic interactions, AEs, adherence). The CPP can independently lead routine follow-up appointments and address acute as well as ongoing ASM therapy needs. Pharmacy residents are fully integrated into the clinic workflow, seeing assigned patients independently when appropriate but ensuring that each patient has access to either the epileptologist, CPP, or psychiatrist prior to finalizing the treatment plan. The epilepsy clinic rotation is required for first-year pharmacy residents and is an elective rotation in the second year.
While this level of service has been in place at WSMVH for more than 3 decades, a systematic evaluation on workload and clinical impact has not been conducted.11 The purpose of this analysis is to evaluate and quantify the breadth and impact of CPPs in this specialty setting. The WSMVH/University of Wisconsin-Madison institutional review board deemed this quality improvement study exempt from review.
Methods
This study was a single-center, retrospective, quality improvement project evaluating the impact of a CPP and clinical pharmacy resident have within the WSMVH epilepsy clinic on access to epilepsy care and medication management. The secondary outcomes were the types of interventions made by the CPP and mental health screening performed.
Between October 2019 and May 2021, 591 appointments were scheduled at the epilepsy clinic for medical, psychiatry, neurosurgery, and pharmacy residents; the epileptologist; CPP; psychiatrist; epilepsy fellow; or nurse practitioner. A retrospective chart review of the 446 patients seen by either a CPP or clinical pharmacy resident from October 2017 to June 2021 assessed pharmacist-led interventions made during each appointment. The following treatment interventions were assessed: medication initiations/discontinuations, dose changes, and nonpharmacologic interventions, including education. Additionally, any mental health screenings completed, consultations to other specialties placed, or laboratory tests ordered were documented.
Results
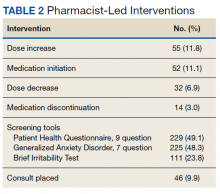
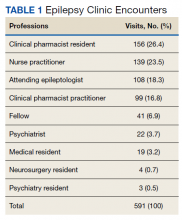
In the epilepsy clinic, 591 appointments were completed from October 1, 2019, to May 31, 2021. Of those appointments, 255 (43.2%) were led by pharmacists; 156 (26.4%) by pharmacy residents and 99 (16.8%) by CPPs (16.8%) (Table 1). Appointments held by other HCPs included 139 (23.5%) by nurse practitioner, 108 (18.3%) by the attending epileptologist, 41 (6.9%) by fellows, 22 (3.7%) by psychiatrists, 19 (3.2) by medical residents, 4 (0.7%) by neurosurgery residents, and 3 (0.5%) by psychiatry residents. Medication interventions included 55 (11.8%) dose increases, 52 (11.1%) medication initiations, and 32 (6.9%) dose decreases (Table 2). Mental health screening was conducted for 229 (49.1%) patients with PHQ-9, 225 (48.3%) with GAD-7, and 111 (23.8) with BITe. Some veterans received multiple screeners at a clinic visit, and others received none (most commonly during telephone follow-up appointments). The mean time spent with each patient was 27 minutes.
Discussion
Within the private sector, access to a neurologist or epileptologist is limited, and the US Health Resources and Services Administration National Center for Workforce Analysis projected that the demand for these specialists would exceed supply by 2025.12 In 2017, Kobau and colleagues found that only 1 in 10 adults with epilepsy saw a neurologist within the year, similar to previous years. As demand for specialty care exceeds capacity, additional members of the health care team are needed to ensure timely, effective, and safe care for patients with epilepsy.
One way to increase health care access is to use an interdisciplinary model of care, integrating pharmacists in the management of epilepsy in collaboration with other HCPs, a strategy that has been endorsed by the American Epilepsy Society (AES).13 As experts in pharmacotherapy, pharmacists can uniquely provide medication management for this complex disease as ASMs continue to remain the first-line treatment.14
In addition to increased demand for specialty services, there also is an increase in health care spending with a push to limit additional spending. In 2016, despite similar health care use in other high-income countries, health care costs are approximately twice as much in the US, mostly driven by prices of pharmaceuticals and administrative costs.15 Bond and colleagues evaluated 9380 Medicare patients with epilepsy or seizure disorders throughout US hospitals in 1998.16 They found that hospitals without pharmacist-managed ASM therapy had Medicare charges that were 11.2% higher than hospitals with pharmacist-managed therapy. Many factors contribute to the rise in cost, including an increase in laboratory charges for serum drug assays, legal litigations related to drug AEs, and an increase in hospital length of stay (about 14 additional days). Similar to pharmacist-managed anticoagulation, vancomycin, and aminoglycoside therapy, direct involvement of pharmacists with ASM management decreases health care costs.14
The American Academy of Neurology (AAN) developed 8 epilepsy quality measures: seizure type and frequency, etiology or epilepsy syndrome, review of electroencephalogram and imaging findings, counseling of ASM AEs, consideration of surgical treatment of intractable epilepsy, epilepsy-specific safety issues, and counseling for women of childbearing potential on contraception and pregnancy. These measures serve as a guide for evidence-based therapy and standardization of epilepsy care.17 Additionally, bone health, depression, and awareness of sudden unexplained death in epilepsy are increasing in importance when providing quality epilepsy care. Wasade and colleagues surveyed Michigan neurologists and found that only 37% of the respondents addressed ASM AEs at every clinic visit. They also found that just 26% of responding neurologists inquire about depression at every clinic visit, and 17% inquire only once a year. In our practice, screening for depression, suicidality, and counseling on ASM AEs are routinely provided by CPPs during each clinic visit.
Within the VA, CPPs are granted a scope of practice that allows them to perform comprehensive medication management, including but not limited to, prescribing medication regimens, ordering laboratory tests and diagnostic studies, and performing physical assessments. In our practice, the most common interventions made by CPPs were patient-focused counseling, bone health screening, mental health triage and referral, and ASM regimen adjustments. Assessment of ASM adherence also was noted to be an active area of CPP-patient engagement. These most common interventions align well with the AAN quality measures. It is now well recognized that nonadherence in patients with epilepsy not only can lead to loss of seizure control, but injury and death as well.18,19 Malek and colleagues found that patients with epilepsy who are nonadherent to their ASM regimens have a 3-times greater risk of mortality compared with those who were adherent.20 Adherence to the appropriate medication regimen in epilepsy can result in seizure-freedom in 70% of patients; therefore, exploring nonadherence in this population is crucial.21
The COVID-19 pandemic precipitated changes to the health care industry, including the heavy reliance on telehealth. Following the Wisconsin stay-at-home order on March 25, 2020, all nonessential face-to-face appointments at the WSMVH halted. The epilepsy clinic transitioned the majority of appointments to either telephone or VA Video Connect (VVC), which is a program on the veteran’s computer, tablet, or mobile device upon which the appointment is held. Although it became more challenging to obtain a mental health screening during virtual appointments and the frequency did decrease, patients were asked for a subjective report of their mood during each telephone or video appointment. The AES has since put forth a statement of support for the continuation of telehealth following the COVID-19 pandemic due to the flexibility that telehealth provides people with epilepsy. Additionally, the AES taskforce provided suggestions for continued pharmacist engagement within the epilepsy care team, including the triaging of patients, management of ASMs, and involvement in the delivery of telehealth.
Limitations
There is limited research available on the impact that a CPP has on medication management and access to care within an epilepsy clinic, especially those with a scope of practice. One limitation of this retrospective chart review is that the appropriateness of each medication intervention was not assessed; therefore, the impact of each intervention was not captured. Additionally, this single-site study of veterans may not reflect the general population. However, we believe that this model could be adapted to nonspecialty neurology practices. Of note the scope of this study did not include a comparison of medication interventions for the other specialties within the clinic.
Conclusions
The integration of a CPP and pharmacy residents into the WSMVH epilepsy clinic has allowed for greater and more timely access to care, managing 43.2% of all patients within the clinic during the study. Pharmacy scope of practice allows for collaborative autonomy with ASM adjustments and for the epileptologist time to focus on higher acuity cases. In settings where pharmacists do not have prescriptive status, medication management services, such as comprehensive medication reviews, identifying drug-drug and drug-disease interactions, recognizing adherence barriers, and medication safety surveillance, can still be performed to improve management of epilepsy.
Acknowledgments
Ellina S. Seckel, PharmD, BCACP, DPLA; Anita Kashyap, PharmD, BCACP; Brooke Keenan, NP; Leigh Heffner, PharmD
1. Stafstrom CE, Carmant L. Seizures and epilepsy: an overview for neuroscientists. Cold Spring Harb Perspect Med. 2015;5(6):a022426. doi:10.1101/cshperspect.a022426
2. GBD 2017 US Neurological Disorders Collaborators, Feigin VL, Vos T, et al. Burden of neurological disorders across the US from 1990-2017: a global burden of disease study. JAMA Neurol. 2021;78(2):165-176. doi:10.1001/jamaneurol.2020.4152
3. Rehman R, Kelly PR, Husain AM, Tran TT. Characteristics of veterans diagnosed with seizures within Veterans Health Administration. J Rehabil Res Dev. 2015;52(7):751-762. doi:10.1682/JRRD.2014.10.0241
4. Pugh MJ, Van Cott AC, Amuan M, et al. Epilepsy among Iraq and Afghanistan War veterans - United States, 2002-2015. MMWR Morb Mortal Wkly Rep. 2016;65(44):1224-1227. doi:10.15585/mmwr.mm6544a5
5. Rohde NN, Baca CB, Van Cott AC, Parko KL, Amuan ME, Pugh MJ. Antiepileptic drug prescribing patterns in Iraq and Afghanistan war veterans with epilepsy. Epilepsy Behav. 2015;46:133-139. doi:10.1016/j.yebeh.2015.03.027
6. Laxer KD, Trinka E, Hirsch LJ, et al. The consequences of refractory epilepsy and its treatment. Epilepsy Behav. 2014;37:59-70. doi:10.1016/j.yebeh.2014.05.031
7. Devinsky O, Hesdorffer DC, Thurman DJ, Lhatoo S, Richerson G. Sudden unexpected death in epilepsy: epidemiology, mechanisms, and prevention. Lancet Neurol. 2016;15(10):1075-1088. doi:10.1016/S1474-4422(16)30158-2
8. Tolchin B, Hirsch LJ, LaFrance WC Jr. Neuropsychiatric aspects of epilepsy. Psychiatr Clin North Am. 2020;43(2):275-290. doi:10.1016/j.psc.2020.02.002
9. Rai D, Kerr MP, McManus S, Jordanova V, Lewis G, Brugha TS. Epilepsy and psychiatric comorbidity: a nationally representative population-based study. Epilepsia. 2012;53(6):1095-1103. doi:10.1111/j.1528-1167.2012.03500.x
10. US Department of Veterans Affairs. Epilepsy Centers of Excellence. Annual report fiscal year 2020. Accessed March 11, 2022. https://www.epilepsy.va.gov/docs/ECoENational_AnnualReportFY20_web_508c.pdf
11. Fogg A, Staufenberg EF, Small I, Bhattacharya D. An exploratory study of primary care pharmacist-led epilepsy consultations. Int J Pharm Pract. 2012;20(5):294-302. doi:10.1111/j.2042-7174.2012.00207.x
12. Kobau R, Sapkota S, Pennell PB, Croft JB. Epilepsy by the numbers - from the US Centers for Disease Control and Prevention: six in 10 adults with active epilepsy saw a neurologist or epilepsy specialist in the past year, United States, 2017. Epilepsy Behav. 2020;112:107348. doi:10.1016/j.yebeh.2020.107348
13. Shawahna R. Development of key performance indicators to capture in measuring the impact of pharmacists in caring for patients with epilepsy in primary healthcare: A Delphi consensual study. Epilepsy Behav. 2019;98(pt A):129-138. doi:10.1016/j.yebeh.2019.07.034
14. Asadi-Pooya AA, Beniczky S, Rubboli G, Sperling MR, Rampp S, Perucca E. A pragmatic algorithm to select appropriate antiseizure medications in patients with epilepsy. Epilepsia. 2020;61(8):1668-1677. doi:10.1111/epi.16610
15. Papanicolas I, Woskie LR, Jha AK. Health Care Spending in the United States and Other High-Income Countries. JAMA. 2018;319(10):1024-1039. doi:10.1001/jama.2018.1150
16. Bond CA, Raehl CL. Clinical and economic outcomes of pharmacist-managed aminoglycoside or vancomycin therapy. Am J Health Syst Pharm. 2005;62(15):1596-1605. doi:10.2146/ajhp040555
17. Wasade VS, Spanaki M, Iyengar R, Barkley GL, Schultz L. AAN Epilepsy Quality Measures in clinical practice: a survey of neurologists. Epilepsy Behav. 2012;24(4):468-473. doi:10.1016/j.yebeh.2012.05.017
18. Hovinga CA, Asato MR, Manjunath R, et al. Association of non-adherence to antiepileptic drugs and seizures, quality of life, and productivity: survey of patients with epilepsy and physicians. Epilepsy Behav. 2008;13(2):316-322. doi:10.1016/j.yebeh.2008.03.009
19. Faught RE, Weiner JR, Guérin A, Cunnington MC, Duh MS. Impact of nonadherence to antiepileptic drugs on health care utilization and costs: findings from the RANSOM study. Epilepsia. 2009;50(3):501-509. doi:10.1111/j.1528-1167.2008.01794.x
20. Malek N, Heath CA, Greene J. A review of medication adherence in people with epilepsy. Acta Neurol Scand. 2017;135(5):507-515. doi:10.1111/ane.12703
21. O’ Rourke G, O’ Brien JJ. Identifying the barriers to antiepileptic drug adherence among adults with epilepsy. Seizure. 2017;45:160-168. doi:10.1016/j.seizure.2016.12.006
Epilepsy affects about 1% of the world population and is one of the most burdensome in terms of disability-adjusted life-years.1,2 Veterans are at increased risk of developing epilepsy when compared with the general population due to a variety of factors, including a higher frequency of traumatic brain injuries.3 A recent study from the US Centers for Disease Control and Prevention found that veterans who developed epilepsy during their service not only had a higher rate of mental and physical comorbidities, but also were 2.6 times more likely to die compared with veterans without epilepsy.4
Oral antiseizure medications (ASM) remain the mainstay of outpatient epilepsy treatment. Patterns of ASM use are complex within the US Department of Veterans Affairs (VA) patient population, particularly within patients at the Epilepsy Centers of Excellence (ECoE). For example, many patients are transitioned from older ASMs with greater adverse effects (AEs) to better tolerated newer generation ASMs or polytherapy regimens with complex pharmacokinetic profiles and drug interactions.5 Multiple factors are considered when choosing an ASM, including age, sex, epilepsy/seizure type, comorbidities, past medication trials, AEs, and drug interactions. The complex pharmacologic profile of both older and newer ASMs can confound the optimal management of epilepsy, and suboptimal management can lead to neurologic, psychological, physical, and social consequences, including sudden unexplained death in epilepsy.6,7 Psychiatric and behavioral problems are seen in up to 30% of patients with newly diagnosed epilepsy and 50% in those with pharmacoresistant epilepsy.8 Early screening, detection, and treatment for psychiatric comorbidities are an integral part of evidence-based care in epilepsy.
Being familiar with ASM AEs and comorbid conditions such as anxiety and depression can allow for quick identification and intervention to improve safety and quality of life. A 2007 population-based study found that measures of suicidality had a strong association with epilepsy, and performing mental health screenings, such as the Patient Health Questionnaire (PHQ-9), Generalized Anxiety Disorder Screener (GAD-7), and the Brief Irritability Test (BITe), can assist in identifying those patients at risk.9
During the COVID-19 pandemic, it has become increasingly clear that the health care sector is facing increasing pressure. The combination of patient acuity as well as critical health care professional (HCP) shortages may be of particular concern in certain specialty clinics where access to practitioners may already be limited. While this is a multifaceted problem, a pragmatic approach would be to increase the use of clinicians, such as clinical pharmacist practitioners (CPPs).
The William S. Middleton Memorial Veterans Hospital (WSMVH) in Madison, Wisconsin, is 1 of 17 VA ECoE sites. The VA ECoE provides high-quality, comprehensive epilepsy evaluation and care to veterans. In fiscal year (FY) 2020, the 17 sites provided care to 5544 veterans.10 The WSMVH epilepsy clinic sees about 400 veterans each year, receiving referrals from other VA medical centers, and prescribes ASMs, neuromodulation devices, and resective surgeries for epilepsy. The multidisciplinary team consists of an epileptologist, neurophysiology fellow, psychiatrist, nurse practitioner, CPP, and neurology residents. The WSMVH epilepsy clinic has employed CPPs at their highest level of clinical practice authority since 1991.
The WSMVH epilepsy clinic is open 4 hours once weekly. The clinic offers fourteen 30-minute appointment slots either in person or via telehealth. The epileptologist reviews patient charts prior to clinic and assigns each patient to the appropriate HCP. When making the determination to assign a patient to a CPP or pharmacy resident, the epileptologist considers current treatment response, mental health issues as well as medication-related concerns (eg, potential pharmacokinetic/pharmacodynamic interactions, AEs, adherence). The CPP can independently lead routine follow-up appointments and address acute as well as ongoing ASM therapy needs. Pharmacy residents are fully integrated into the clinic workflow, seeing assigned patients independently when appropriate but ensuring that each patient has access to either the epileptologist, CPP, or psychiatrist prior to finalizing the treatment plan. The epilepsy clinic rotation is required for first-year pharmacy residents and is an elective rotation in the second year.
While this level of service has been in place at WSMVH for more than 3 decades, a systematic evaluation on workload and clinical impact has not been conducted.11 The purpose of this analysis is to evaluate and quantify the breadth and impact of CPPs in this specialty setting. The WSMVH/University of Wisconsin-Madison institutional review board deemed this quality improvement study exempt from review.
Methods
This study was a single-center, retrospective, quality improvement project evaluating the impact of a CPP and clinical pharmacy resident have within the WSMVH epilepsy clinic on access to epilepsy care and medication management. The secondary outcomes were the types of interventions made by the CPP and mental health screening performed.
Between October 2019 and May 2021, 591 appointments were scheduled at the epilepsy clinic for medical, psychiatry, neurosurgery, and pharmacy residents; the epileptologist; CPP; psychiatrist; epilepsy fellow; or nurse practitioner. A retrospective chart review of the 446 patients seen by either a CPP or clinical pharmacy resident from October 2017 to June 2021 assessed pharmacist-led interventions made during each appointment. The following treatment interventions were assessed: medication initiations/discontinuations, dose changes, and nonpharmacologic interventions, including education. Additionally, any mental health screenings completed, consultations to other specialties placed, or laboratory tests ordered were documented.
Results
In the epilepsy clinic, 591 appointments were completed from October 1, 2019, to May 31, 2021. Of those appointments, 255 (43.2%) were led by pharmacists; 156 (26.4%) by pharmacy residents and 99 (16.8%) by CPPs (16.8%) (Table 1). Appointments held by other HCPs included 139 (23.5%) by nurse practitioner, 108 (18.3%) by the attending epileptologist, 41 (6.9%) by fellows, 22 (3.7%) by psychiatrists, 19 (3.2) by medical residents, 4 (0.7%) by neurosurgery residents, and 3 (0.5%) by psychiatry residents. Medication interventions included 55 (11.8%) dose increases, 52 (11.1%) medication initiations, and 32 (6.9%) dose decreases (Table 2). Mental health screening was conducted for 229 (49.1%) patients with PHQ-9, 225 (48.3%) with GAD-7, and 111 (23.8) with BITe. Some veterans received multiple screeners at a clinic visit, and others received none (most commonly during telephone follow-up appointments). The mean time spent with each patient was 27 minutes.
Discussion
Within the private sector, access to a neurologist or epileptologist is limited, and the US Health Resources and Services Administration National Center for Workforce Analysis projected that the demand for these specialists would exceed supply by 2025.12 In 2017, Kobau and colleagues found that only 1 in 10 adults with epilepsy saw a neurologist within the year, similar to previous years. As demand for specialty care exceeds capacity, additional members of the health care team are needed to ensure timely, effective, and safe care for patients with epilepsy.
One way to increase health care access is to use an interdisciplinary model of care, integrating pharmacists in the management of epilepsy in collaboration with other HCPs, a strategy that has been endorsed by the American Epilepsy Society (AES).13 As experts in pharmacotherapy, pharmacists can uniquely provide medication management for this complex disease as ASMs continue to remain the first-line treatment.14
In addition to increased demand for specialty services, there also is an increase in health care spending with a push to limit additional spending. In 2016, despite similar health care use in other high-income countries, health care costs are approximately twice as much in the US, mostly driven by prices of pharmaceuticals and administrative costs.15 Bond and colleagues evaluated 9380 Medicare patients with epilepsy or seizure disorders throughout US hospitals in 1998.16 They found that hospitals without pharmacist-managed ASM therapy had Medicare charges that were 11.2% higher than hospitals with pharmacist-managed therapy. Many factors contribute to the rise in cost, including an increase in laboratory charges for serum drug assays, legal litigations related to drug AEs, and an increase in hospital length of stay (about 14 additional days). Similar to pharmacist-managed anticoagulation, vancomycin, and aminoglycoside therapy, direct involvement of pharmacists with ASM management decreases health care costs.14
The American Academy of Neurology (AAN) developed 8 epilepsy quality measures: seizure type and frequency, etiology or epilepsy syndrome, review of electroencephalogram and imaging findings, counseling of ASM AEs, consideration of surgical treatment of intractable epilepsy, epilepsy-specific safety issues, and counseling for women of childbearing potential on contraception and pregnancy. These measures serve as a guide for evidence-based therapy and standardization of epilepsy care.17 Additionally, bone health, depression, and awareness of sudden unexplained death in epilepsy are increasing in importance when providing quality epilepsy care. Wasade and colleagues surveyed Michigan neurologists and found that only 37% of the respondents addressed ASM AEs at every clinic visit. They also found that just 26% of responding neurologists inquire about depression at every clinic visit, and 17% inquire only once a year. In our practice, screening for depression, suicidality, and counseling on ASM AEs are routinely provided by CPPs during each clinic visit.
Within the VA, CPPs are granted a scope of practice that allows them to perform comprehensive medication management, including but not limited to, prescribing medication regimens, ordering laboratory tests and diagnostic studies, and performing physical assessments. In our practice, the most common interventions made by CPPs were patient-focused counseling, bone health screening, mental health triage and referral, and ASM regimen adjustments. Assessment of ASM adherence also was noted to be an active area of CPP-patient engagement. These most common interventions align well with the AAN quality measures. It is now well recognized that nonadherence in patients with epilepsy not only can lead to loss of seizure control, but injury and death as well.18,19 Malek and colleagues found that patients with epilepsy who are nonadherent to their ASM regimens have a 3-times greater risk of mortality compared with those who were adherent.20 Adherence to the appropriate medication regimen in epilepsy can result in seizure-freedom in 70% of patients; therefore, exploring nonadherence in this population is crucial.21
The COVID-19 pandemic precipitated changes to the health care industry, including the heavy reliance on telehealth. Following the Wisconsin stay-at-home order on March 25, 2020, all nonessential face-to-face appointments at the WSMVH halted. The epilepsy clinic transitioned the majority of appointments to either telephone or VA Video Connect (VVC), which is a program on the veteran’s computer, tablet, or mobile device upon which the appointment is held. Although it became more challenging to obtain a mental health screening during virtual appointments and the frequency did decrease, patients were asked for a subjective report of their mood during each telephone or video appointment. The AES has since put forth a statement of support for the continuation of telehealth following the COVID-19 pandemic due to the flexibility that telehealth provides people with epilepsy. Additionally, the AES taskforce provided suggestions for continued pharmacist engagement within the epilepsy care team, including the triaging of patients, management of ASMs, and involvement in the delivery of telehealth.
Limitations
There is limited research available on the impact that a CPP has on medication management and access to care within an epilepsy clinic, especially those with a scope of practice. One limitation of this retrospective chart review is that the appropriateness of each medication intervention was not assessed; therefore, the impact of each intervention was not captured. Additionally, this single-site study of veterans may not reflect the general population. However, we believe that this model could be adapted to nonspecialty neurology practices. Of note the scope of this study did not include a comparison of medication interventions for the other specialties within the clinic.
Conclusions
The integration of a CPP and pharmacy residents into the WSMVH epilepsy clinic has allowed for greater and more timely access to care, managing 43.2% of all patients within the clinic during the study. Pharmacy scope of practice allows for collaborative autonomy with ASM adjustments and for the epileptologist time to focus on higher acuity cases. In settings where pharmacists do not have prescriptive status, medication management services, such as comprehensive medication reviews, identifying drug-drug and drug-disease interactions, recognizing adherence barriers, and medication safety surveillance, can still be performed to improve management of epilepsy.
Acknowledgments
Ellina S. Seckel, PharmD, BCACP, DPLA; Anita Kashyap, PharmD, BCACP; Brooke Keenan, NP; Leigh Heffner, PharmD
Epilepsy affects about 1% of the world population and is one of the most burdensome in terms of disability-adjusted life-years.1,2 Veterans are at increased risk of developing epilepsy when compared with the general population due to a variety of factors, including a higher frequency of traumatic brain injuries.3 A recent study from the US Centers for Disease Control and Prevention found that veterans who developed epilepsy during their service not only had a higher rate of mental and physical comorbidities, but also were 2.6 times more likely to die compared with veterans without epilepsy.4
Oral antiseizure medications (ASM) remain the mainstay of outpatient epilepsy treatment. Patterns of ASM use are complex within the US Department of Veterans Affairs (VA) patient population, particularly within patients at the Epilepsy Centers of Excellence (ECoE). For example, many patients are transitioned from older ASMs with greater adverse effects (AEs) to better tolerated newer generation ASMs or polytherapy regimens with complex pharmacokinetic profiles and drug interactions.5 Multiple factors are considered when choosing an ASM, including age, sex, epilepsy/seizure type, comorbidities, past medication trials, AEs, and drug interactions. The complex pharmacologic profile of both older and newer ASMs can confound the optimal management of epilepsy, and suboptimal management can lead to neurologic, psychological, physical, and social consequences, including sudden unexplained death in epilepsy.6,7 Psychiatric and behavioral problems are seen in up to 30% of patients with newly diagnosed epilepsy and 50% in those with pharmacoresistant epilepsy.8 Early screening, detection, and treatment for psychiatric comorbidities are an integral part of evidence-based care in epilepsy.
Being familiar with ASM AEs and comorbid conditions such as anxiety and depression can allow for quick identification and intervention to improve safety and quality of life. A 2007 population-based study found that measures of suicidality had a strong association with epilepsy, and performing mental health screenings, such as the Patient Health Questionnaire (PHQ-9), Generalized Anxiety Disorder Screener (GAD-7), and the Brief Irritability Test (BITe), can assist in identifying those patients at risk.9
During the COVID-19 pandemic, it has become increasingly clear that the health care sector is facing increasing pressure. The combination of patient acuity as well as critical health care professional (HCP) shortages may be of particular concern in certain specialty clinics where access to practitioners may already be limited. While this is a multifaceted problem, a pragmatic approach would be to increase the use of clinicians, such as clinical pharmacist practitioners (CPPs).
The William S. Middleton Memorial Veterans Hospital (WSMVH) in Madison, Wisconsin, is 1 of 17 VA ECoE sites. The VA ECoE provides high-quality, comprehensive epilepsy evaluation and care to veterans. In fiscal year (FY) 2020, the 17 sites provided care to 5544 veterans.10 The WSMVH epilepsy clinic sees about 400 veterans each year, receiving referrals from other VA medical centers, and prescribes ASMs, neuromodulation devices, and resective surgeries for epilepsy. The multidisciplinary team consists of an epileptologist, neurophysiology fellow, psychiatrist, nurse practitioner, CPP, and neurology residents. The WSMVH epilepsy clinic has employed CPPs at their highest level of clinical practice authority since 1991.
The WSMVH epilepsy clinic is open 4 hours once weekly. The clinic offers fourteen 30-minute appointment slots either in person or via telehealth. The epileptologist reviews patient charts prior to clinic and assigns each patient to the appropriate HCP. When making the determination to assign a patient to a CPP or pharmacy resident, the epileptologist considers current treatment response, mental health issues as well as medication-related concerns (eg, potential pharmacokinetic/pharmacodynamic interactions, AEs, adherence). The CPP can independently lead routine follow-up appointments and address acute as well as ongoing ASM therapy needs. Pharmacy residents are fully integrated into the clinic workflow, seeing assigned patients independently when appropriate but ensuring that each patient has access to either the epileptologist, CPP, or psychiatrist prior to finalizing the treatment plan. The epilepsy clinic rotation is required for first-year pharmacy residents and is an elective rotation in the second year.
While this level of service has been in place at WSMVH for more than 3 decades, a systematic evaluation on workload and clinical impact has not been conducted.11 The purpose of this analysis is to evaluate and quantify the breadth and impact of CPPs in this specialty setting. The WSMVH/University of Wisconsin-Madison institutional review board deemed this quality improvement study exempt from review.
Methods
This study was a single-center, retrospective, quality improvement project evaluating the impact of a CPP and clinical pharmacy resident have within the WSMVH epilepsy clinic on access to epilepsy care and medication management. The secondary outcomes were the types of interventions made by the CPP and mental health screening performed.
Between October 2019 and May 2021, 591 appointments were scheduled at the epilepsy clinic for medical, psychiatry, neurosurgery, and pharmacy residents; the epileptologist; CPP; psychiatrist; epilepsy fellow; or nurse practitioner. A retrospective chart review of the 446 patients seen by either a CPP or clinical pharmacy resident from October 2017 to June 2021 assessed pharmacist-led interventions made during each appointment. The following treatment interventions were assessed: medication initiations/discontinuations, dose changes, and nonpharmacologic interventions, including education. Additionally, any mental health screenings completed, consultations to other specialties placed, or laboratory tests ordered were documented.
Results
In the epilepsy clinic, 591 appointments were completed from October 1, 2019, to May 31, 2021. Of those appointments, 255 (43.2%) were led by pharmacists; 156 (26.4%) by pharmacy residents and 99 (16.8%) by CPPs (16.8%) (Table 1). Appointments held by other HCPs included 139 (23.5%) by nurse practitioner, 108 (18.3%) by the attending epileptologist, 41 (6.9%) by fellows, 22 (3.7%) by psychiatrists, 19 (3.2) by medical residents, 4 (0.7%) by neurosurgery residents, and 3 (0.5%) by psychiatry residents. Medication interventions included 55 (11.8%) dose increases, 52 (11.1%) medication initiations, and 32 (6.9%) dose decreases (Table 2). Mental health screening was conducted for 229 (49.1%) patients with PHQ-9, 225 (48.3%) with GAD-7, and 111 (23.8) with BITe. Some veterans received multiple screeners at a clinic visit, and others received none (most commonly during telephone follow-up appointments). The mean time spent with each patient was 27 minutes.
Discussion
Within the private sector, access to a neurologist or epileptologist is limited, and the US Health Resources and Services Administration National Center for Workforce Analysis projected that the demand for these specialists would exceed supply by 2025.12 In 2017, Kobau and colleagues found that only 1 in 10 adults with epilepsy saw a neurologist within the year, similar to previous years. As demand for specialty care exceeds capacity, additional members of the health care team are needed to ensure timely, effective, and safe care for patients with epilepsy.
One way to increase health care access is to use an interdisciplinary model of care, integrating pharmacists in the management of epilepsy in collaboration with other HCPs, a strategy that has been endorsed by the American Epilepsy Society (AES).13 As experts in pharmacotherapy, pharmacists can uniquely provide medication management for this complex disease as ASMs continue to remain the first-line treatment.14
In addition to increased demand for specialty services, there also is an increase in health care spending with a push to limit additional spending. In 2016, despite similar health care use in other high-income countries, health care costs are approximately twice as much in the US, mostly driven by prices of pharmaceuticals and administrative costs.15 Bond and colleagues evaluated 9380 Medicare patients with epilepsy or seizure disorders throughout US hospitals in 1998.16 They found that hospitals without pharmacist-managed ASM therapy had Medicare charges that were 11.2% higher than hospitals with pharmacist-managed therapy. Many factors contribute to the rise in cost, including an increase in laboratory charges for serum drug assays, legal litigations related to drug AEs, and an increase in hospital length of stay (about 14 additional days). Similar to pharmacist-managed anticoagulation, vancomycin, and aminoglycoside therapy, direct involvement of pharmacists with ASM management decreases health care costs.14
The American Academy of Neurology (AAN) developed 8 epilepsy quality measures: seizure type and frequency, etiology or epilepsy syndrome, review of electroencephalogram and imaging findings, counseling of ASM AEs, consideration of surgical treatment of intractable epilepsy, epilepsy-specific safety issues, and counseling for women of childbearing potential on contraception and pregnancy. These measures serve as a guide for evidence-based therapy and standardization of epilepsy care.17 Additionally, bone health, depression, and awareness of sudden unexplained death in epilepsy are increasing in importance when providing quality epilepsy care. Wasade and colleagues surveyed Michigan neurologists and found that only 37% of the respondents addressed ASM AEs at every clinic visit. They also found that just 26% of responding neurologists inquire about depression at every clinic visit, and 17% inquire only once a year. In our practice, screening for depression, suicidality, and counseling on ASM AEs are routinely provided by CPPs during each clinic visit.
Within the VA, CPPs are granted a scope of practice that allows them to perform comprehensive medication management, including but not limited to, prescribing medication regimens, ordering laboratory tests and diagnostic studies, and performing physical assessments. In our practice, the most common interventions made by CPPs were patient-focused counseling, bone health screening, mental health triage and referral, and ASM regimen adjustments. Assessment of ASM adherence also was noted to be an active area of CPP-patient engagement. These most common interventions align well with the AAN quality measures. It is now well recognized that nonadherence in patients with epilepsy not only can lead to loss of seizure control, but injury and death as well.18,19 Malek and colleagues found that patients with epilepsy who are nonadherent to their ASM regimens have a 3-times greater risk of mortality compared with those who were adherent.20 Adherence to the appropriate medication regimen in epilepsy can result in seizure-freedom in 70% of patients; therefore, exploring nonadherence in this population is crucial.21
The COVID-19 pandemic precipitated changes to the health care industry, including the heavy reliance on telehealth. Following the Wisconsin stay-at-home order on March 25, 2020, all nonessential face-to-face appointments at the WSMVH halted. The epilepsy clinic transitioned the majority of appointments to either telephone or VA Video Connect (VVC), which is a program on the veteran’s computer, tablet, or mobile device upon which the appointment is held. Although it became more challenging to obtain a mental health screening during virtual appointments and the frequency did decrease, patients were asked for a subjective report of their mood during each telephone or video appointment. The AES has since put forth a statement of support for the continuation of telehealth following the COVID-19 pandemic due to the flexibility that telehealth provides people with epilepsy. Additionally, the AES taskforce provided suggestions for continued pharmacist engagement within the epilepsy care team, including the triaging of patients, management of ASMs, and involvement in the delivery of telehealth.
Limitations
There is limited research available on the impact that a CPP has on medication management and access to care within an epilepsy clinic, especially those with a scope of practice. One limitation of this retrospective chart review is that the appropriateness of each medication intervention was not assessed; therefore, the impact of each intervention was not captured. Additionally, this single-site study of veterans may not reflect the general population. However, we believe that this model could be adapted to nonspecialty neurology practices. Of note the scope of this study did not include a comparison of medication interventions for the other specialties within the clinic.
Conclusions
The integration of a CPP and pharmacy residents into the WSMVH epilepsy clinic has allowed for greater and more timely access to care, managing 43.2% of all patients within the clinic during the study. Pharmacy scope of practice allows for collaborative autonomy with ASM adjustments and for the epileptologist time to focus on higher acuity cases. In settings where pharmacists do not have prescriptive status, medication management services, such as comprehensive medication reviews, identifying drug-drug and drug-disease interactions, recognizing adherence barriers, and medication safety surveillance, can still be performed to improve management of epilepsy.
Acknowledgments
Ellina S. Seckel, PharmD, BCACP, DPLA; Anita Kashyap, PharmD, BCACP; Brooke Keenan, NP; Leigh Heffner, PharmD
1. Stafstrom CE, Carmant L. Seizures and epilepsy: an overview for neuroscientists. Cold Spring Harb Perspect Med. 2015;5(6):a022426. doi:10.1101/cshperspect.a022426
2. GBD 2017 US Neurological Disorders Collaborators, Feigin VL, Vos T, et al. Burden of neurological disorders across the US from 1990-2017: a global burden of disease study. JAMA Neurol. 2021;78(2):165-176. doi:10.1001/jamaneurol.2020.4152
3. Rehman R, Kelly PR, Husain AM, Tran TT. Characteristics of veterans diagnosed with seizures within Veterans Health Administration. J Rehabil Res Dev. 2015;52(7):751-762. doi:10.1682/JRRD.2014.10.0241
4. Pugh MJ, Van Cott AC, Amuan M, et al. Epilepsy among Iraq and Afghanistan War veterans - United States, 2002-2015. MMWR Morb Mortal Wkly Rep. 2016;65(44):1224-1227. doi:10.15585/mmwr.mm6544a5
5. Rohde NN, Baca CB, Van Cott AC, Parko KL, Amuan ME, Pugh MJ. Antiepileptic drug prescribing patterns in Iraq and Afghanistan war veterans with epilepsy. Epilepsy Behav. 2015;46:133-139. doi:10.1016/j.yebeh.2015.03.027
6. Laxer KD, Trinka E, Hirsch LJ, et al. The consequences of refractory epilepsy and its treatment. Epilepsy Behav. 2014;37:59-70. doi:10.1016/j.yebeh.2014.05.031
7. Devinsky O, Hesdorffer DC, Thurman DJ, Lhatoo S, Richerson G. Sudden unexpected death in epilepsy: epidemiology, mechanisms, and prevention. Lancet Neurol. 2016;15(10):1075-1088. doi:10.1016/S1474-4422(16)30158-2
8. Tolchin B, Hirsch LJ, LaFrance WC Jr. Neuropsychiatric aspects of epilepsy. Psychiatr Clin North Am. 2020;43(2):275-290. doi:10.1016/j.psc.2020.02.002
9. Rai D, Kerr MP, McManus S, Jordanova V, Lewis G, Brugha TS. Epilepsy and psychiatric comorbidity: a nationally representative population-based study. Epilepsia. 2012;53(6):1095-1103. doi:10.1111/j.1528-1167.2012.03500.x
10. US Department of Veterans Affairs. Epilepsy Centers of Excellence. Annual report fiscal year 2020. Accessed March 11, 2022. https://www.epilepsy.va.gov/docs/ECoENational_AnnualReportFY20_web_508c.pdf
11. Fogg A, Staufenberg EF, Small I, Bhattacharya D. An exploratory study of primary care pharmacist-led epilepsy consultations. Int J Pharm Pract. 2012;20(5):294-302. doi:10.1111/j.2042-7174.2012.00207.x
12. Kobau R, Sapkota S, Pennell PB, Croft JB. Epilepsy by the numbers - from the US Centers for Disease Control and Prevention: six in 10 adults with active epilepsy saw a neurologist or epilepsy specialist in the past year, United States, 2017. Epilepsy Behav. 2020;112:107348. doi:10.1016/j.yebeh.2020.107348
13. Shawahna R. Development of key performance indicators to capture in measuring the impact of pharmacists in caring for patients with epilepsy in primary healthcare: A Delphi consensual study. Epilepsy Behav. 2019;98(pt A):129-138. doi:10.1016/j.yebeh.2019.07.034
14. Asadi-Pooya AA, Beniczky S, Rubboli G, Sperling MR, Rampp S, Perucca E. A pragmatic algorithm to select appropriate antiseizure medications in patients with epilepsy. Epilepsia. 2020;61(8):1668-1677. doi:10.1111/epi.16610
15. Papanicolas I, Woskie LR, Jha AK. Health Care Spending in the United States and Other High-Income Countries. JAMA. 2018;319(10):1024-1039. doi:10.1001/jama.2018.1150
16. Bond CA, Raehl CL. Clinical and economic outcomes of pharmacist-managed aminoglycoside or vancomycin therapy. Am J Health Syst Pharm. 2005;62(15):1596-1605. doi:10.2146/ajhp040555
17. Wasade VS, Spanaki M, Iyengar R, Barkley GL, Schultz L. AAN Epilepsy Quality Measures in clinical practice: a survey of neurologists. Epilepsy Behav. 2012;24(4):468-473. doi:10.1016/j.yebeh.2012.05.017
18. Hovinga CA, Asato MR, Manjunath R, et al. Association of non-adherence to antiepileptic drugs and seizures, quality of life, and productivity: survey of patients with epilepsy and physicians. Epilepsy Behav. 2008;13(2):316-322. doi:10.1016/j.yebeh.2008.03.009
19. Faught RE, Weiner JR, Guérin A, Cunnington MC, Duh MS. Impact of nonadherence to antiepileptic drugs on health care utilization and costs: findings from the RANSOM study. Epilepsia. 2009;50(3):501-509. doi:10.1111/j.1528-1167.2008.01794.x
20. Malek N, Heath CA, Greene J. A review of medication adherence in people with epilepsy. Acta Neurol Scand. 2017;135(5):507-515. doi:10.1111/ane.12703
21. O’ Rourke G, O’ Brien JJ. Identifying the barriers to antiepileptic drug adherence among adults with epilepsy. Seizure. 2017;45:160-168. doi:10.1016/j.seizure.2016.12.006
1. Stafstrom CE, Carmant L. Seizures and epilepsy: an overview for neuroscientists. Cold Spring Harb Perspect Med. 2015;5(6):a022426. doi:10.1101/cshperspect.a022426
2. GBD 2017 US Neurological Disorders Collaborators, Feigin VL, Vos T, et al. Burden of neurological disorders across the US from 1990-2017: a global burden of disease study. JAMA Neurol. 2021;78(2):165-176. doi:10.1001/jamaneurol.2020.4152
3. Rehman R, Kelly PR, Husain AM, Tran TT. Characteristics of veterans diagnosed with seizures within Veterans Health Administration. J Rehabil Res Dev. 2015;52(7):751-762. doi:10.1682/JRRD.2014.10.0241
4. Pugh MJ, Van Cott AC, Amuan M, et al. Epilepsy among Iraq and Afghanistan War veterans - United States, 2002-2015. MMWR Morb Mortal Wkly Rep. 2016;65(44):1224-1227. doi:10.15585/mmwr.mm6544a5
5. Rohde NN, Baca CB, Van Cott AC, Parko KL, Amuan ME, Pugh MJ. Antiepileptic drug prescribing patterns in Iraq and Afghanistan war veterans with epilepsy. Epilepsy Behav. 2015;46:133-139. doi:10.1016/j.yebeh.2015.03.027
6. Laxer KD, Trinka E, Hirsch LJ, et al. The consequences of refractory epilepsy and its treatment. Epilepsy Behav. 2014;37:59-70. doi:10.1016/j.yebeh.2014.05.031
7. Devinsky O, Hesdorffer DC, Thurman DJ, Lhatoo S, Richerson G. Sudden unexpected death in epilepsy: epidemiology, mechanisms, and prevention. Lancet Neurol. 2016;15(10):1075-1088. doi:10.1016/S1474-4422(16)30158-2
8. Tolchin B, Hirsch LJ, LaFrance WC Jr. Neuropsychiatric aspects of epilepsy. Psychiatr Clin North Am. 2020;43(2):275-290. doi:10.1016/j.psc.2020.02.002
9. Rai D, Kerr MP, McManus S, Jordanova V, Lewis G, Brugha TS. Epilepsy and psychiatric comorbidity: a nationally representative population-based study. Epilepsia. 2012;53(6):1095-1103. doi:10.1111/j.1528-1167.2012.03500.x
10. US Department of Veterans Affairs. Epilepsy Centers of Excellence. Annual report fiscal year 2020. Accessed March 11, 2022. https://www.epilepsy.va.gov/docs/ECoENational_AnnualReportFY20_web_508c.pdf
11. Fogg A, Staufenberg EF, Small I, Bhattacharya D. An exploratory study of primary care pharmacist-led epilepsy consultations. Int J Pharm Pract. 2012;20(5):294-302. doi:10.1111/j.2042-7174.2012.00207.x
12. Kobau R, Sapkota S, Pennell PB, Croft JB. Epilepsy by the numbers - from the US Centers for Disease Control and Prevention: six in 10 adults with active epilepsy saw a neurologist or epilepsy specialist in the past year, United States, 2017. Epilepsy Behav. 2020;112:107348. doi:10.1016/j.yebeh.2020.107348
13. Shawahna R. Development of key performance indicators to capture in measuring the impact of pharmacists in caring for patients with epilepsy in primary healthcare: A Delphi consensual study. Epilepsy Behav. 2019;98(pt A):129-138. doi:10.1016/j.yebeh.2019.07.034
14. Asadi-Pooya AA, Beniczky S, Rubboli G, Sperling MR, Rampp S, Perucca E. A pragmatic algorithm to select appropriate antiseizure medications in patients with epilepsy. Epilepsia. 2020;61(8):1668-1677. doi:10.1111/epi.16610
15. Papanicolas I, Woskie LR, Jha AK. Health Care Spending in the United States and Other High-Income Countries. JAMA. 2018;319(10):1024-1039. doi:10.1001/jama.2018.1150
16. Bond CA, Raehl CL. Clinical and economic outcomes of pharmacist-managed aminoglycoside or vancomycin therapy. Am J Health Syst Pharm. 2005;62(15):1596-1605. doi:10.2146/ajhp040555
17. Wasade VS, Spanaki M, Iyengar R, Barkley GL, Schultz L. AAN Epilepsy Quality Measures in clinical practice: a survey of neurologists. Epilepsy Behav. 2012;24(4):468-473. doi:10.1016/j.yebeh.2012.05.017
18. Hovinga CA, Asato MR, Manjunath R, et al. Association of non-adherence to antiepileptic drugs and seizures, quality of life, and productivity: survey of patients with epilepsy and physicians. Epilepsy Behav. 2008;13(2):316-322. doi:10.1016/j.yebeh.2008.03.009
19. Faught RE, Weiner JR, Guérin A, Cunnington MC, Duh MS. Impact of nonadherence to antiepileptic drugs on health care utilization and costs: findings from the RANSOM study. Epilepsia. 2009;50(3):501-509. doi:10.1111/j.1528-1167.2008.01794.x
20. Malek N, Heath CA, Greene J. A review of medication adherence in people with epilepsy. Acta Neurol Scand. 2017;135(5):507-515. doi:10.1111/ane.12703
21. O’ Rourke G, O’ Brien JJ. Identifying the barriers to antiepileptic drug adherence among adults with epilepsy. Seizure. 2017;45:160-168. doi:10.1016/j.seizure.2016.12.006
Neuroimaging in the Era of Artificial Intelligence: Current Applications
Artificial intelligence (AI) in medicine has shown significant promise, particularly in neuroimaging. AI refers to computer systems designed to perform tasks that normally require human intelligence.1 Machine learning (ML), a field in which computers learn from data without being specifically programmed, is the AI subset responsible for its success in matching or even surpassing humans in certain tasks.2
Supervised learning, a subset of ML, uses an algorithm with annotated data from which to learn.3 The program will use the characteristics of a training data set to predict a specific outcome or target when exposed to a sample data set of the same type. Unsupervised learning finds naturally occurring patterns or groupings within the data.4 With deep learning (DL) algorithms, computers learn the features that optimally represent the data for the problem at hand.5 Both ML and DL are meant to emulate neural networks in the brain, giving rise to artificial neural networks composed of nodes structured within input, hidden, and output layers.
The DL neural network differs from a conventional one by having many hidden layers instead of just 1 layer that extracts patterns within the data.6 Convolutional neural networks (CNNs) are the most prevalent DL architecture used in medical imaging. CNN’s hidden layers apply convolution and pooling operations to break down an image into features containing the most valuable information. The connecting layer applies high-level reasoning before the output layer provides predictions for the image. This framework has applications within radiology, such as predicting a lesion category or condition from an image, determining whether a specific pixel belongs to background or a target class, and predicting the location of lesions.1
AI promises to increase efficiency and reduces errors. With increased data processing and image interpretation, AI technology may help radiologists improve the quality of patient care.6 This article discusses the current applications and future integration of AI in neuroradiology.
Neuroimaging Applications
AI can improve the quality of neuroimaging and reduce the clinical and systemic loads of other imaging modalities. AI can predict patient wait times for computed tomography (CT), magnetic resonance imaging (MRI), ultrasound, and X-ray imaging.7 A ML-based AI has detected the variables that most affected patient wait times, including proximity to federal holidays and severity of the patient’s condition, and calculated how long patients would be delayed after their scheduled appointment time. This AI modality could allow more efficient patient scheduling and reveal areas of patient processing that could be changed, potentially improving patient satisfaction and outcomes for time-sensitive neurologic conditions.
AI can save patient and health care practitioner time for repeat MRIs. An estimated 20% of MRI scans require a repeat series—a massive loss of time and funds for both patients and the health care system.8 A DL approach can determine whether an MRI is usable clinically or unclear enough to require repetition.9 This initial screening measure can prevent patients from making return visits and neuroradiologists from reading inconclusive images. AI offers the opportunity to reduce time and costs incurred by optimizing the health care process before imaging is obtained.
Speeding Up Neuroimaging
AI can reduce the time spent performing imaging. Because MRIs consume time and resources, compressed sensing (CS) is commonly used. CS preferentially maintains in-plane resolution at the expense of through-plane resolution to produce a scan with a single, usable viewpoint that preserves signal-to-noise ratio (SNR). CS, however, limits interpretation to single directions and can create aliasing artifacts. An AI algorithm known as synthetic multi-orientation resolution enhancement works in real time to reduce aliasing and improve resolution in these compressed scans.10 This AI improved resolution of white matter lesions in patients with multiple sclerosis (MS) on FLAIR (fluid-attenuated inversion recovery) images, and permitted multiview reconstruction from these limited scans.
Tasks of reconstructing and anti-aliasing come with high computational costs that vary inversely with the extent of scanning compression, potentially negating the time and resource savings of CS. DL AI modalities have been developed to reduce operational loads and further improve image resolution in several directions from CS. One such deep residual learning AI was trained with compressed MRIs and used the framelet method to create a CNN that could rapidly remove global and deeply coherent aliasing artifacts.11 This system, compared with synthetic multi-orientation resolution enhancement, uses a pretrained, pretested AI that does not require additional time during scanning for computational analysis, thereby multiplying the time benefit of CS while retaining the benefits of multidirectional reconstruction and increased resolution. This methodology suffers from inherent degradation of perceptual image quality in its reconstructions because of the L2 loss function the CNN uses to reduce mean squared error, which causes blurring by averaging all possible outcomes of signal distribution during reconstruction. To combat this, researchers have developed another AI to reduce reconstruction times that uses a different loss function in a generative adversarial network to retain image quality, while offering reconstruction times several hundred times faster than current CS-MRI structures.12 So-called sparse-coding methods promise further reduction in reconstruction times, with the possibility of processing completed online with a lightweight architecture rather than on a local system.13
Neuroimaging of acute cases benefits most directly from these technologies because MRIs and their high resolution and SNR begin to approach CT imaging time scales. This could have important implications in clinical care, particularly for stroke imaging and evaluating spinal cord compression. CS-MRI optimization represents one of the greatest areas of neuroimaging cost savings and neurologic care improvement in the modern radiology era.
Reducing Contrast and Radiation Doses
AI has the ability to read CT, MRI, and positron emission tomography (PET) with reduced or without contrast without significant loss in sensitivity for detecting lesions. With MRI, gadolinium-based contrast can cause injection site reactions, allergic reactions, metal deposition throughout the body, and nephrogenic systemic fibrosis in the most severe instances.14 DL has been applied to brain MRIs performed with 10% of a full dose of contrast without significant degradation of image quality. Neuroradiologists did not rate the AI-synthesized images for several MRI indications lower than their full-dose counterparts.15 Low-dose contrast imaging, regardless of modality, generates greater noise with a significantly reduced signal. However, with AI applied, researchers found that the software suppressed motion and aliasing artifacts and improved image quality, perhaps evidence that this low-dose modality is less vulnerable to the most common pitfalls of MRI.
Recently, low-dose MRI moved into the spotlight when Subtle Medical SubtleGAD software received a National Institutes of Health grant and an expedited pathway to phase 2 clinical trials.16 SubtleGAD, a DL AI that enables low-dose MRI interpretation, might allow contrast MRI for patients with advanced kidney disease or contrast allergies. At some point, contrast with MRI might not be necessary because DL AI applied to noncontrast MRs for detecting MS lesions was found to be preliminarily effective with 78% lesion detection sensitivity.17
PET-MRI combines simultaneous PET and MRI and has been used to evaluate neurologic disorders. PET-MRI can detect amyloid plaques in Alzheimer disease 10 to 20 years before clinical signs of dementia emerge.18 PET-MRI has sparked DL AI development to decrease the dose of the IV radioactive tracer 18F-florbetaben used in imaging to reduce radiation exposure and imaging costs.This reduction is critical if PET-MRI is to become used widely.19-21
An initial CNN could reconstruct low-dose amyloid scans to full-dose resolution, albeit with a greater susceptibility to some artifacts and motion blurring.22 Similar to the synthetic multi-orientation resolution enhancement CNN, this program showed signal blurring from the L2 loss function, which was corrected in a later AI that used a generative adversarial network to minimize perceptual loss.23 This new AI demonstrated greater image resolution, feature preservation, and radiologist rating over the previous AI and was capable of reconstructing low-dose PET scans to full-dose resolution without an accompanying MRI. Applications of this algorithm are far-reaching, potentially allowing neuroimaging of brain tumors at more frequent intervals with higher resolution and lower total radiation exposure.
AI also has been applied to neurologic CT to reduce radiation exposure.24 Because it is critical to abide by the principles of ALARA (as low as reasonably achievable), the ability of AI to reduce radiation exposure holds significant promise. A CNN has been used to transform low-dose CTs of anthropomorphic models with calcium inserts and cardiac patients to normal-dose CTs, with the goal of improving the SNR.25 By training a noise-discriminating CNN and a noise-generating CNN together in a generative adversarial network, the AI improved image feature preservation during transformation. This algorithm has a direct application in imaging cerebral vasculature, including calcification that can explain lacunar infarcts and tracking systemic atherosclerosis.26
Another CNN has been applied to remove more complex noise patterns from the phenomena of beam hardening and photon starvation common in low-dose CT. This algorithm extracts the directional components of artifacts and compares them to known artifact patterns, allowing for highly specific suppression of unwanted signals.27 In June 2019, the US Food and Drug Administration (FDA) approved ClariPi, a deep CNN program for advanced denoising and resolution improvement of low- and ultra low-dose CTs.28 Aside from only low-dose settings, this AI could reduce artifacts in all CT imaging modalities and improve therapeutic value of procedures, including cerebral angiograms and emergency cranial scans. As the average CT radiation dose decreased from 12 mSv in 2009 to 1.5 mSv in 2014 and continues to fall, these algorithms will become increasingly necessary to retain the high resolution and diagnostic power expected of neurologic CTs.29,30
Downstream Applications
Downstream applications refer to AI use after a radiologic study is acquired, mostly image interpretation. More than 70% of FDA-approved AI medical devices are in radiology, and many of these relate to image analysis.6,31 Although AI is not limited to black-and-white image interpretation, it is hypothesized that one of the reasons radiology is inviting to AI is because gray-scale images lend themselves to standardization.3 Moreover, most radiology departments already use AI-friendly picture archiving and communication systems.31,32
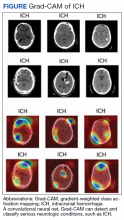
AI has been applied to a range of radiologic modalities, including MRI, CT, ultrasonography, PET, and mammography.32-38 AI also has been specifically applied to radiography, including the interpretation of tuberculosis, pneumonia, lung lesions, and COVID-19.33,39-45 AI also can assist triage, patient screening, providing a “second opinion” rapidly, shortening the time needed for attaining a diagnosis, monitoring disease progression, and predicting prognosis.37-39,43,45-47 Downstream applications of AI in neuroradiology and neurology include using CT to aid in detecting hemorrhage or ischemic stroke; using MRI to automatically segment lesions, such as tumors or MS lesions; assisting in early diagnosis and predicting prognosis in MS; assisting in treating paralysis, including from spinal cord injury; determining seizure type and localizing area of seizure onset; and using cameras, wearable devices, and smartphone applications to diagnose and assess treatment response in neurodegenerative disorders, such as Parkinson or Alzheimer diseases (Figure).37,48-56
Several AI tools have been deployed in the clinical setting, particularly triaging intracranial hemorrhage and moving these studies to the top of the radiologist’s worklist. In 2020 the Centers for Medicare and Medicaid Services (CMS) began reimbursing Viz.ai software’s AI-based Viz ContaCT (Viz LVO) with a new International Statistical Classification of Diseases, Tenth Revision procedure code.57
Viz LVO automatically detects large vessel occlusions, flags the occlusion on CT angiogram, alerts the stroke team (interventional radiologist, neuroradiologist, and neurologist), and transmits images through a secure application to the stroke team members’ mobile devices—all in less than 6 minutes from study acquisition to alarm notification.48 Additional software can quantify and measure perfusion in affected brain areas.48 This could have implications for quantifying and targeting areas of ischemic penumbra that could be salvaged after a stroke and then using that information to plan targeted treatment and/or intervention. Because many trials (DAWN/DEFUSE3) have shown benefits in stroke outcome by extending the therapeutic window for the endovascular thrombectomy, the ability to identify appropriate candidates is essential.58,59 Development of AI tools in assessing ischemic penumbra with quantitative parameters (mean transit time, cerebral blood volume, cerebral blood flow, mismatch ratio) using AI has benefited image interpretation. Medtronic RAPID software can provide quantitative assessment of CT perfusion. AI tools could be used to provide an automatic ASPECT score, which provides a quantitative measure for assessing potential ischemic zones and aids in assessing appropriate candidates for thrombectomy.
Several FDA-approved AI tools help quantify brain structures in neuroradiology, including quantitative analysis through MRI for analysis of anatomy and PET for analysis of functional uptake, assisting in more accurate and more objective detection and monitoring of conditions such as atrophy, dementia, trauma, seizure disorders, and MS.48 The growing number of FDA-approved AI technologies and the recent CMS-approved reimbursement for an AI tool indicate a changing landscape that is more accepting of downstream applications of AI in neuroradiology. As AI continues to integrate into medical regulation and finance, we predict AI will continue to play a prominent role in neuroradiology.
Practical and Ethical Considerations
In any discussion of the benefits of AI, it is prudent to address its shortcomings. Chief among these is overfitting, which occurs when an AI is too closely aligned with its training dataset and prone to error when applied to novel cases. Often this is a byproduct of a small training set.60 Neuroradiology, particularly with uncommon, advanced imaging methods, has a smaller number of available studies.61 Even with more prevalent imaging modalities, such as head CT, the work of collecting training scans from patients with the prerequisite disease processes, particularly if these processes are rare, can limit the number of datapoints collected. Neuroradiologists should understand how an AI tool was generated, including the size and variety of the training dataset used, to best gauge the clinical applicability and fitness of the system.
Another point of concern for AI clinical decision support tools’ implementation is automation bias—the tendency for clinicians to favor machine-generated decisions and ignore contrary data or conflicting human decisions.62 This situation often arises when radiologists experience overwhelming patient loads or are in underresourced settings, where there is little ability to review every AI-based diagnosis. Although AI might be of benefit in such conditions by reducing physician workload and streamlining the diagnostic process, there is the propensity to improperly rely on a tool meant to augment, not replace, a radiologist’s judgment. Such cases have led to adverse outcomes for patients, and legal precedence shows that this constitutes negligence.63 Maintaining awareness of each tool’s limitations and proper application is the only remedy for such situations.
Ethically, we must consider the opaqueness of ML-developed neuroimaging AIs. For many systems, the specific process by which an AI arrives at its conclusions is unknown. This AI “black box” can conceal potential errors and biases that are masked by overall positive performance metrics. The lack of understanding about how a tool functions in the zero-failure clinical setting understandably gives radiologists pause. The question must be asked: Is it ethical to use a system that is a relatively unknown quantity? Entities, including state governments, Canada, and the European Union, have produced an answer. Each of these governments have implemented policies requiring that health care AIs use some method to display to end users the process by which they arrive at conclusions.64-68
The 21st Century Cures Act declares that to attain approval, clinical AIs must demonstrate this explainability to clinicians and patients.69 The response has been an explosion in the development of explainable AI. Systems that visualize the areas where AI attention most often rests with heatmaps, generate labels for the most heavily weighted features of radiographic images, and create full diagnostic reports to justify AI conclusions aim to meet the goal of transparency and inspiring confidence in clinical end users.70 The ability to understand the “thought process” of a system proves useful for error correction and retooling. A trend toward under- or overdetecting conditions, flagging seemingly irrelevant image regions, or low reproducibility can be better addressed when it is clear how the AI is drawing its false conclusions. With an iterative process of testing and redesigning, false positive and negative rates can be reduced, the need for human intervention can be lowered to an appropriate minimum, and patient outcomes can be improved.71
Data collection raises another ethical concern. To train functional clinical decision support tools, massive amounts of patient demographic, laboratory, and imaging data are required. With incentives to develop the most powerful AI systems, record collection can venture down a path where patient autonomy and privacy are threatened. Radiologists have a duty to ensure data mining serves patients and improves the practice of radiology while protecting patients’ personal information.62 Policies have placed similar limits on the access to and use of patient records.64-69 Patients have the right to request explanation of the AI systems their data have been used to train. Approval for data acquisition requires the use of explainable AI, standardized data security protocol implementation, and adequate proof of communal benefit from the clinical decision support tool. Establishment of state-mandated protections bodes well for a future when developers can access enormous caches of data while patients and health care professionals are assured that no identifying information has escaped a well-regulated space. On the level of the individual radiologist, the knowledge that each datum represents a human life. These are people who has made themselves vulnerable by seeking relief for what ails them, which should serve as a lasting reminder to operate with utmost care when handling sensitive information.
Conclusions
The demonstrated applications of AI in neuroimaging are numerous and varied, and it is reasonable to assume that its implementation will increase as the technology matures. AI use for detecting important neurologic conditions holds promise in combatting ever greater imaging volumes and providing timely diagnoses. As medicine witnesses the continuing adoption of AI, it is important that practitioners possess an understanding of its current and emerging uses.
1. Chartrand G, Cheng PM, Vorontsov E, et al. Deep learning: a primer for radiologists. Radiographics. 2017;37(7):2113-2131. doi:10.1148/rg.2017170077
2. King BF Jr. Guest editorial: discovery and artificial intelligence. AJR Am J Roentgenol. 2017;209(6):1189-1190. doi:10.2214/AJR.17.19178
3. Syed AB, Zoga AC. Artificial intelligence in radiology: current technology and future directions. Semin Musculoskelet Radiol. 2018;22(5):540-545. doi:10.1055/s-0038-1673383
4. Deo RC. Machine learning in medicine. Circulation. 2015;132(20):1920-1930. doi:10.1161/CIRCULATIONAHA.115.001593 5. Litjens G, Kooi T, Bejnordi BE, et al. A survey on deep learning in medical image analysis. Med Image Anal. 2017;42:60-88. doi:10.1016/j.media.2017.07.005
6. Pesapane F, Codari M, Sardanelli F. Artificial intelligence in medical imaging: threat or opportunity? Radiologists again at the forefront of innovation in medicine. Eur Radiol Exp. 2018;2(1):35. doi:10.1186/s41747-018-0061-6
7. Curtis C, Liu C, Bollerman TJ, Pianykh OS. Machine learning for predicting patient wait times and appointment delays. J Am Coll Radiol. 2018;15(9):1310-1316. doi:10.1016/j.jacr.2017.08.021
8. Andre JB, Bresnahan BW, Mossa-Basha M, et al. Toward quantifying the prevalence, severity, and cost associated with patient motion during clinical MR examinations. J Am Coll Radiol. 2015;12(7):689-695. doi:10.1016/j.jacr.2015.03.007
9. Sreekumari A, Shanbhag D, Yeo D, et al. A deep learning-based approach to reduce rescan and recall rates in clinical MRI examinations. AJNR Am J Neuroradiol. 2019;40(2):217-223. doi:10.3174/ajnr.A5926
10. Zhao C, Shao M, Carass A, et al. Applications of a deep learning method for anti-aliasing and super-resolution in MRI. Magn Reson Imaging. 2019;64:132-141. doi:10.1016/j.mri.2019.05.038
11. Lee D, Yoo J, Tak S, Ye JC. Deep residual learning for accelerated MRI using magnitude and phase networks. IEEE Trans Biomed Eng. 2018;65(9):1985-1995. doi:10.1109/TBME.2018.2821699
12. Mardani M, Gong E, Cheng JY, et al. Deep generative adversarial neural networks for compressive sensing MRI. IEEE Trans Med Imaging. 2019;38(1):167-179. doi:10.1109/TMI.2018.2858752
13. Dong C, Loy CC, He K, Tang X. Image super-resolution using deep convolutional networks. IEEE Trans Pattern Anal Mach Intell. 2016;38(2):295-307. doi:10.1109/TPAMI.2015.2439281
14. Sammet S. Magnetic resonance safety. Abdom Radiol (NY). 2016;41(3):444-451. doi:10.1007/s00261-016-0680-4
15. Gong E, Pauly JM, Wintermark M, Zaharchuk G. Deep learning enables reduced gadolinium dose for contrast-enhanced brain MRI. J Magn Reson Imaging. 2018;48(2):330-340. doi:10.1002/jmri.25970
16. Subtle Medical NIH awards Subtle Medical, Inc. $1.6 million grant to improve safety of MRI exams by reducing gadolinium dose using AI. Press release. September 18, 2019. Accessed March 14, 2022. https://www.biospace.com/article/releases/nih-awards-subtle-medical-inc-1-6-million-grant-to-improve-safety-of-mri-exams-by-reducing-gadolinium-dose-using-ai
17. Narayana PA, Coronado I, Sujit SJ, Wolinsky JS, Lublin FD, Gabr RE. Deep learning for predicting enhancing lesions in multiple sclerosis from noncontrast MRI. Radiology. 2020;294(2):398-404. doi:10.1148/radiol.2019191061
18. Jack CR Jr, Knopman DS, Jagust WJ, et al. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol. 2010;9(1):119-128. doi:10.1016/S1474-4422(09)70299-6
19. Gatidis S, Würslin C, Seith F, et al. Towards tracer dose reduction in PET studies: simulation of dose reduction by retrospective randomized undersampling of list-mode data. Hell J Nucl Med. 2016;19(1):15-18. doi:10.1967/s002449910333
20. Kaplan S, Zhu YM. Full-dose PET image estimation from low-dose PET image using deep learning: a pilot study. J Digit Imaging. 2019;32(5):773-778. doi:10.1007/s10278-018-0150-3
21. Xu J, Gong E, Pauly J, Zaharchuk G. 200x low-dose PET reconstruction using deep learning. arXiv: 1712.04119. Accessed 2/16/2022. https://arxiv.org/pdf/1712.04119.pdf
22. Chen KT, Gong E, de Carvalho Macruz FB, et al. Ultra-low-dose 18F-florbetaben amyloid PET imaging using deep learning with multi-contrast MRI inputs. Radiology. 2019;290(3):649-656. doi:10.1148/radiol.2018180940
23. Ouyang J, Chen KT, Gong E, Pauly J, Zaharchuk G. Ultra-low-dose PET reconstruction using generative adversarial network with feature matching and task-specific perceptual loss. Med Phys. 2019;46(8):3555-3564. doi:10.1002/mp.13626
24. Brenner DJ, Hall EJ. Computed tomography—an increasing source of radiation exposure. N Engl J Med. 2007;357(22):2277-2284. doi:10.1056/NEJMra072149
25. Wolterink JM, Leiner T, Viergever MA, Isgum I. Generative adversarial networks for noise reduction in low-dose CT. IEEE Trans Med Imaging. 2017;36(12):2536-2545. doi:10.1109/TMI.2017.2708987
26. Sohn YH, Cheon HY, Jeon P, Kang SY. Clinical implication of cerebral artery calcification on brain CT. Cerebrovasc Dis. 2004;18(4):332-337. doi:10.1159/000080772
27. Kang E, Min J, Ye JC. A deep convolutional neural network using directional wavelets for low-dose X-ray CT reconstruction. Med Phys. 2017;44(10):e360-e375. doi:10.1002/mp.12344
28. ClariPi gets FDA clearance for AI-powered CT image denoising solution. Published June 24, 2019. Accessed February 16, 2022. https://www.itnonline.com/content/claripi-gets-fda-clearance-ai-powered-ct-image-denoising-solution
29. Hausleiter J, Meyer T, Hermann F, et al. Estimated radiation dose associated with cardiac CT angiography. JAMA. 2009;301(5):500-507. doi:10.1001/jama.2009.54
30. Al-Mallah M, Aljizeeri A, Alharthi M, Alsaileek A. Routine low-radiation-dose coronary computed tomography angiography. Eur Heart J Suppl. 2014;16(suppl B):B12-B16. doi:10.1093/eurheartj/suu024
31. Benjamens S, Dhunnoo P, Meskó B. The state of artificial intelligence-based FDA-approved medical devices and algorithms: an online database. NPJ Digit Med. 2020;3:118. doi:10.1038/s41746-020-00324-0
32. Talebi-Liasi F, Markowitz O. Is artificial intelligence going to replace dermatologists? Cutis. 2020;105(1):28-31.
33. Khan O, Bebb G, Alimohamed NA. Artificial intelligence in medicine: what oncologists need to know about its potential—and its limitations. Oncology Exchange. 2017;16(4):8-13. http://www.oncologyex.com/pdf/vol16_no4/feature_khan-ai.pdf
34. Liu X, Faes L, Kale AU, et al. A comparison of deep learning performance against health-care professionals in detecting diseases from medical imaging: a systematic review and meta-analysis. Lancet Digit Health. 2019;1(6):e271-e297. doi:10.1016/S2589-7500(19)30123-2
35. Topol EJ. High-performance medicine: the convergence of human and artificial intelligence. Nat Med. 2019;25(1):44-56. doi:10.1038/s41591-018-0300-7
36. Salim M, Wåhlin E, Dembrower K, et al. External evaluation of 3 commercial artificial intelligence algorithms for independent assessment of screening mammograms. JAMA Oncol. 2020;6(10):1581-1588. doi:10.1001/jamaoncol.2020.3321
37. Arbabshirani MR, Fornwalt BK, Mongelluzzo GJ, et al. Advanced machine learning in action: identification of intracranial hemorrhage on computed tomography scans of the head with clinical workflow integration. NPJ Digit Med. 2018;1(1):1-7. doi:10.1038/s41746-017-0015-z
38. Sheth D, Giger ML. Artificial intelligence in the interpretation of breast cancer on MRI. J Magn Reson Imaging. 2020;51(5):1310-1324. doi:10.1002/jmri.26878
39. Borkowski AA, Viswanadhan NA, Thomas LB, Guzman RD, Deland LA, Mastorides SM. Using artificial intelligence for COVID-19 chest X-ray diagnosis. Fed Pract. 2020;37(9):398-404. doi:10.12788/fp.0045
40. Kermany DS, Goldbaum M, Cai W, et al. Identifying medical diagnoses and treatable diseases by image-based deep learning. Cell. 2018;172(5):1122-1131.e9. doi:10.1016/j.cell.2018.02.010
41. Nam JG, Park S, Hwang EJ, et al. Development and validation of deep learning-based automatic detection algorithm for malignant pulmonary nodules on chest radiographs. Radiology. 2019;290(1):218-228. doi:10.1148/radiol.2018180237
42. Zech JR, Badgeley MA, Liu M, Costa AB, Titano JJ, Oermann EK. Variable generalization performance of a deep learning model to detect pneumonia in chest radiographs: a cross-sectional study. PLoS Med. 2018;15(11):e1002683. doi:10.1371/journal.pmed.1002683
43. Lakhani P, Sundaram B. Deep learning at chest radiography: automated classification of pulmonary tuberculosis by using convolutional neural networks. Radiology. 2017;284(2):574-582. doi:10.1148/radiol.2017162326
44. Rajpurkar P, Joshi A, Pareek A, et al. CheXpedition: investigating generalization challenges for translation of chest X-Ray algorithms to the clinical setting. arXiv preprint arXiv:200211379. Accessed February 16, 2022. https://arxiv.org/pdf/2002.11379.pdf
45. He J, Baxter SL, Xu J, Xu J, Zhou X, Zhang K. The practical implementation of artificial intelligence technologies in medicine. Nat Med. 2019;25(1):30-36. doi:10.1038/s41591-018-0307-0
46. Meyer-Bäse A, Morra L, Meyer-Bäse U, Pinker K. Current status and future perspectives of artificial intelligence in magnetic resonance breast imaging. Contrast Media Mol Imaging. 2020;2020:6805710. doi:10.1155/2020/6805710
47. Booth AL, Abels E, McCaffrey P. Development of a prognostic model for mortality in COVID-19 infection using machine learning. Mod Pathol. 2020;4(3):522-531. doi:10.1038/s41379-020-00700-x
48. Bash S. Enhancing neuroimaging with artificial intelligence. Applied Radiology. 2020;49(1):20-21.
49. Jiang F, Jiang Y, Zhi H, et al. Artificial intelligence in healthcare: past, present and future. Stroke Vasc Neurol. 2017;2(4):230-243. doi:10.1136/svn-2017-000101
50. Valliani AA, Ranti D, Oermann EK. Deep learning and neurology: a systematic review. Neurol Ther. 2019;8(2):351-365. doi:10.1007/s40120-019-00153-8
51. Gupta R, Krishnam SP, Schaefer PW, Lev MH, Gonzalez RG. An east coast perspective on artificial intelligence and machine learning: part 2: ischemic stroke imaging and triage. Neuroimaging Clin N Am. 2020;30(4):467-478. doi:10.1016/j.nic.2020.08.002
52. Belić M, Bobić V, Badža M, Šolaja N, Đurić-Jovičić M, Kostić VS. Artificial intelligence for assisting diagnostics and assessment of Parkinson’s disease-A review. Clin Neurol Neurosurg. 2019;184:105442. doi:10.1016/j.clineuro.2019.105442
53. An S, Kang C, Lee HW. Artificial intelligence and computational approaches for epilepsy. J Epilepsy Res. 2020;10(1):8-17. doi:10.14581/jer.20003
54. Pavel AM, Rennie JM, de Vries LS, et al. A machine-learning algorithm for neonatal seizure recognition: a multicentre, randomised, controlled trial. Lancet Child Adolesc Health. 2020;4(10):740-749. doi:10.1016/S2352-4642(20)30239-X
55. Afzal HMR, Luo S, Ramadan S, Lechner-Scott J. The emerging role of artificial intelligence in multiple sclerosis imaging. Mult Scler. 2020;1352458520966298. doi:10.1177/1352458520966298
56. Bouton CE. Restoring movement in paralysis with a bioelectronic neural bypass approach: current state and future directions. Cold Spring Harb Perspect Med. 2019;9(11):a034306. doi:10.1101/cshperspect.a034306
57. Hassan AE. New technology add-on payment (NTAP) for Viz LVO: a win for stroke care. J Neurointerv Surg. 2020;neurintsurg-2020-016897. doi:10.1136/neurintsurg-2020-016897
58. Nogueira RG , Jadhav AP , Haussen DC , et al; DAWN Trial Investigators. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med. 2018;378:11–21. doi:10.1056/NEJMoa1706442
59. Albers GW , Marks MP , Kemp S , et al; DEFUSE 3 Investigators. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med. 2018;378:708–18. doi:10.1056/NEJMoa1713973
60. Bi WL, Hosny A, Schabath MB, et al. Artificial intelligence in cancer imaging: clinical challenges and applications. CA Cancer J Clin. 2019;69(2):127-157. doi:10.3322/caac.21552
61. Wagner MW, Namdar K, Biswas A, Monah S, Khalvati F, Ertl-Wagner BB. Radiomics, machine learning, and artificial intelligence-what the neuroradiologist needs to know. Neuroradiology. 2021;63(12):1957-1967. doi:10.1007/s00234-021-02813-9
62. Geis JR, Brady AP, Wu CC, et al. Ethics of artificial intelligence in radiology: summary of the Joint European and North American Multisociety Statement. J Am Coll Radiol. 2019;16(11):1516-1521. doi:10.1016/j.jacr.2019.07.028
63. Kingston J. Artificial intelligence and legal liability. arXiv:1802.07782. https://arxiv.org/ftp/arxiv/papers/1802/1802.07782.pdf
64. Council of the European Union, General Data Protection Regulation. Official Journal of the European Union. Accessed February 16, 2022. https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32016R0679
65. Consumer Privacy Protection Act of 2017, HR 4081, 115th Cong (2017). Accessed February 10, 2022. https://www.congress.gov/bill/115th-congress/house-bill/4081
66. Cal. Civ. Code § 1798.198(a) (2018). California Consumer Privacy Act of 2018.
67. Va. Code Ann. § 59.1 (2021). Consumer Data Protection Act. Accessed February 10, 2022. https://lis.virginia.gov/cgi-bin/legp604.exe?212+ful+SB1392ER+pdf
68. Colo. Rev. Stat. § 6-1-1301 (2021). Colorado Privacy Act. Accessed February 10, 2022. https://leg.colorado.gov/sites/default/files/2021a_190_signed.pdf
69. 21st Century Cures Act, Pub L No. 114-255 (2016). Accessed February 10, 2022. https://www.govinfo.gov/content/pkg/PLAW-114publ255/html/PLAW-114publ255.htm
70. Huff DT, Weisman AJ, Jeraj R. Interpretation and visualization techniques for deep learning models in medical imaging. Phys Med Biol. 2021;66(4):04TR01. doi:10.1088/1361-6560/abcd17
71. Thrall JH, Li X, Li Q, et al. Artificial intelligence and machine learning in radiology: opportunities, challenges, pitfalls, and criteria for success. J Am Coll Radiol. 2018;15(3, pt B):504-508. doi:10.1016/j.jacr.2017.12.026
Artificial intelligence (AI) in medicine has shown significant promise, particularly in neuroimaging. AI refers to computer systems designed to perform tasks that normally require human intelligence.1 Machine learning (ML), a field in which computers learn from data without being specifically programmed, is the AI subset responsible for its success in matching or even surpassing humans in certain tasks.2
Supervised learning, a subset of ML, uses an algorithm with annotated data from which to learn.3 The program will use the characteristics of a training data set to predict a specific outcome or target when exposed to a sample data set of the same type. Unsupervised learning finds naturally occurring patterns or groupings within the data.4 With deep learning (DL) algorithms, computers learn the features that optimally represent the data for the problem at hand.5 Both ML and DL are meant to emulate neural networks in the brain, giving rise to artificial neural networks composed of nodes structured within input, hidden, and output layers.
The DL neural network differs from a conventional one by having many hidden layers instead of just 1 layer that extracts patterns within the data.6 Convolutional neural networks (CNNs) are the most prevalent DL architecture used in medical imaging. CNN’s hidden layers apply convolution and pooling operations to break down an image into features containing the most valuable information. The connecting layer applies high-level reasoning before the output layer provides predictions for the image. This framework has applications within radiology, such as predicting a lesion category or condition from an image, determining whether a specific pixel belongs to background or a target class, and predicting the location of lesions.1
AI promises to increase efficiency and reduces errors. With increased data processing and image interpretation, AI technology may help radiologists improve the quality of patient care.6 This article discusses the current applications and future integration of AI in neuroradiology.
Neuroimaging Applications
AI can improve the quality of neuroimaging and reduce the clinical and systemic loads of other imaging modalities. AI can predict patient wait times for computed tomography (CT), magnetic resonance imaging (MRI), ultrasound, and X-ray imaging.7 A ML-based AI has detected the variables that most affected patient wait times, including proximity to federal holidays and severity of the patient’s condition, and calculated how long patients would be delayed after their scheduled appointment time. This AI modality could allow more efficient patient scheduling and reveal areas of patient processing that could be changed, potentially improving patient satisfaction and outcomes for time-sensitive neurologic conditions.
AI can save patient and health care practitioner time for repeat MRIs. An estimated 20% of MRI scans require a repeat series—a massive loss of time and funds for both patients and the health care system.8 A DL approach can determine whether an MRI is usable clinically or unclear enough to require repetition.9 This initial screening measure can prevent patients from making return visits and neuroradiologists from reading inconclusive images. AI offers the opportunity to reduce time and costs incurred by optimizing the health care process before imaging is obtained.
Speeding Up Neuroimaging
AI can reduce the time spent performing imaging. Because MRIs consume time and resources, compressed sensing (CS) is commonly used. CS preferentially maintains in-plane resolution at the expense of through-plane resolution to produce a scan with a single, usable viewpoint that preserves signal-to-noise ratio (SNR). CS, however, limits interpretation to single directions and can create aliasing artifacts. An AI algorithm known as synthetic multi-orientation resolution enhancement works in real time to reduce aliasing and improve resolution in these compressed scans.10 This AI improved resolution of white matter lesions in patients with multiple sclerosis (MS) on FLAIR (fluid-attenuated inversion recovery) images, and permitted multiview reconstruction from these limited scans.
Tasks of reconstructing and anti-aliasing come with high computational costs that vary inversely with the extent of scanning compression, potentially negating the time and resource savings of CS. DL AI modalities have been developed to reduce operational loads and further improve image resolution in several directions from CS. One such deep residual learning AI was trained with compressed MRIs and used the framelet method to create a CNN that could rapidly remove global and deeply coherent aliasing artifacts.11 This system, compared with synthetic multi-orientation resolution enhancement, uses a pretrained, pretested AI that does not require additional time during scanning for computational analysis, thereby multiplying the time benefit of CS while retaining the benefits of multidirectional reconstruction and increased resolution. This methodology suffers from inherent degradation of perceptual image quality in its reconstructions because of the L2 loss function the CNN uses to reduce mean squared error, which causes blurring by averaging all possible outcomes of signal distribution during reconstruction. To combat this, researchers have developed another AI to reduce reconstruction times that uses a different loss function in a generative adversarial network to retain image quality, while offering reconstruction times several hundred times faster than current CS-MRI structures.12 So-called sparse-coding methods promise further reduction in reconstruction times, with the possibility of processing completed online with a lightweight architecture rather than on a local system.13
Neuroimaging of acute cases benefits most directly from these technologies because MRIs and their high resolution and SNR begin to approach CT imaging time scales. This could have important implications in clinical care, particularly for stroke imaging and evaluating spinal cord compression. CS-MRI optimization represents one of the greatest areas of neuroimaging cost savings and neurologic care improvement in the modern radiology era.
Reducing Contrast and Radiation Doses
AI has the ability to read CT, MRI, and positron emission tomography (PET) with reduced or without contrast without significant loss in sensitivity for detecting lesions. With MRI, gadolinium-based contrast can cause injection site reactions, allergic reactions, metal deposition throughout the body, and nephrogenic systemic fibrosis in the most severe instances.14 DL has been applied to brain MRIs performed with 10% of a full dose of contrast without significant degradation of image quality. Neuroradiologists did not rate the AI-synthesized images for several MRI indications lower than their full-dose counterparts.15 Low-dose contrast imaging, regardless of modality, generates greater noise with a significantly reduced signal. However, with AI applied, researchers found that the software suppressed motion and aliasing artifacts and improved image quality, perhaps evidence that this low-dose modality is less vulnerable to the most common pitfalls of MRI.
Recently, low-dose MRI moved into the spotlight when Subtle Medical SubtleGAD software received a National Institutes of Health grant and an expedited pathway to phase 2 clinical trials.16 SubtleGAD, a DL AI that enables low-dose MRI interpretation, might allow contrast MRI for patients with advanced kidney disease or contrast allergies. At some point, contrast with MRI might not be necessary because DL AI applied to noncontrast MRs for detecting MS lesions was found to be preliminarily effective with 78% lesion detection sensitivity.17
PET-MRI combines simultaneous PET and MRI and has been used to evaluate neurologic disorders. PET-MRI can detect amyloid plaques in Alzheimer disease 10 to 20 years before clinical signs of dementia emerge.18 PET-MRI has sparked DL AI development to decrease the dose of the IV radioactive tracer 18F-florbetaben used in imaging to reduce radiation exposure and imaging costs.This reduction is critical if PET-MRI is to become used widely.19-21
An initial CNN could reconstruct low-dose amyloid scans to full-dose resolution, albeit with a greater susceptibility to some artifacts and motion blurring.22 Similar to the synthetic multi-orientation resolution enhancement CNN, this program showed signal blurring from the L2 loss function, which was corrected in a later AI that used a generative adversarial network to minimize perceptual loss.23 This new AI demonstrated greater image resolution, feature preservation, and radiologist rating over the previous AI and was capable of reconstructing low-dose PET scans to full-dose resolution without an accompanying MRI. Applications of this algorithm are far-reaching, potentially allowing neuroimaging of brain tumors at more frequent intervals with higher resolution and lower total radiation exposure.
AI also has been applied to neurologic CT to reduce radiation exposure.24 Because it is critical to abide by the principles of ALARA (as low as reasonably achievable), the ability of AI to reduce radiation exposure holds significant promise. A CNN has been used to transform low-dose CTs of anthropomorphic models with calcium inserts and cardiac patients to normal-dose CTs, with the goal of improving the SNR.25 By training a noise-discriminating CNN and a noise-generating CNN together in a generative adversarial network, the AI improved image feature preservation during transformation. This algorithm has a direct application in imaging cerebral vasculature, including calcification that can explain lacunar infarcts and tracking systemic atherosclerosis.26
Another CNN has been applied to remove more complex noise patterns from the phenomena of beam hardening and photon starvation common in low-dose CT. This algorithm extracts the directional components of artifacts and compares them to known artifact patterns, allowing for highly specific suppression of unwanted signals.27 In June 2019, the US Food and Drug Administration (FDA) approved ClariPi, a deep CNN program for advanced denoising and resolution improvement of low- and ultra low-dose CTs.28 Aside from only low-dose settings, this AI could reduce artifacts in all CT imaging modalities and improve therapeutic value of procedures, including cerebral angiograms and emergency cranial scans. As the average CT radiation dose decreased from 12 mSv in 2009 to 1.5 mSv in 2014 and continues to fall, these algorithms will become increasingly necessary to retain the high resolution and diagnostic power expected of neurologic CTs.29,30
Downstream Applications
Downstream applications refer to AI use after a radiologic study is acquired, mostly image interpretation. More than 70% of FDA-approved AI medical devices are in radiology, and many of these relate to image analysis.6,31 Although AI is not limited to black-and-white image interpretation, it is hypothesized that one of the reasons radiology is inviting to AI is because gray-scale images lend themselves to standardization.3 Moreover, most radiology departments already use AI-friendly picture archiving and communication systems.31,32
AI has been applied to a range of radiologic modalities, including MRI, CT, ultrasonography, PET, and mammography.32-38 AI also has been specifically applied to radiography, including the interpretation of tuberculosis, pneumonia, lung lesions, and COVID-19.33,39-45 AI also can assist triage, patient screening, providing a “second opinion” rapidly, shortening the time needed for attaining a diagnosis, monitoring disease progression, and predicting prognosis.37-39,43,45-47 Downstream applications of AI in neuroradiology and neurology include using CT to aid in detecting hemorrhage or ischemic stroke; using MRI to automatically segment lesions, such as tumors or MS lesions; assisting in early diagnosis and predicting prognosis in MS; assisting in treating paralysis, including from spinal cord injury; determining seizure type and localizing area of seizure onset; and using cameras, wearable devices, and smartphone applications to diagnose and assess treatment response in neurodegenerative disorders, such as Parkinson or Alzheimer diseases (Figure).37,48-56
Several AI tools have been deployed in the clinical setting, particularly triaging intracranial hemorrhage and moving these studies to the top of the radiologist’s worklist. In 2020 the Centers for Medicare and Medicaid Services (CMS) began reimbursing Viz.ai software’s AI-based Viz ContaCT (Viz LVO) with a new International Statistical Classification of Diseases, Tenth Revision procedure code.57
Viz LVO automatically detects large vessel occlusions, flags the occlusion on CT angiogram, alerts the stroke team (interventional radiologist, neuroradiologist, and neurologist), and transmits images through a secure application to the stroke team members’ mobile devices—all in less than 6 minutes from study acquisition to alarm notification.48 Additional software can quantify and measure perfusion in affected brain areas.48 This could have implications for quantifying and targeting areas of ischemic penumbra that could be salvaged after a stroke and then using that information to plan targeted treatment and/or intervention. Because many trials (DAWN/DEFUSE3) have shown benefits in stroke outcome by extending the therapeutic window for the endovascular thrombectomy, the ability to identify appropriate candidates is essential.58,59 Development of AI tools in assessing ischemic penumbra with quantitative parameters (mean transit time, cerebral blood volume, cerebral blood flow, mismatch ratio) using AI has benefited image interpretation. Medtronic RAPID software can provide quantitative assessment of CT perfusion. AI tools could be used to provide an automatic ASPECT score, which provides a quantitative measure for assessing potential ischemic zones and aids in assessing appropriate candidates for thrombectomy.
Several FDA-approved AI tools help quantify brain structures in neuroradiology, including quantitative analysis through MRI for analysis of anatomy and PET for analysis of functional uptake, assisting in more accurate and more objective detection and monitoring of conditions such as atrophy, dementia, trauma, seizure disorders, and MS.48 The growing number of FDA-approved AI technologies and the recent CMS-approved reimbursement for an AI tool indicate a changing landscape that is more accepting of downstream applications of AI in neuroradiology. As AI continues to integrate into medical regulation and finance, we predict AI will continue to play a prominent role in neuroradiology.
Practical and Ethical Considerations
In any discussion of the benefits of AI, it is prudent to address its shortcomings. Chief among these is overfitting, which occurs when an AI is too closely aligned with its training dataset and prone to error when applied to novel cases. Often this is a byproduct of a small training set.60 Neuroradiology, particularly with uncommon, advanced imaging methods, has a smaller number of available studies.61 Even with more prevalent imaging modalities, such as head CT, the work of collecting training scans from patients with the prerequisite disease processes, particularly if these processes are rare, can limit the number of datapoints collected. Neuroradiologists should understand how an AI tool was generated, including the size and variety of the training dataset used, to best gauge the clinical applicability and fitness of the system.
Another point of concern for AI clinical decision support tools’ implementation is automation bias—the tendency for clinicians to favor machine-generated decisions and ignore contrary data or conflicting human decisions.62 This situation often arises when radiologists experience overwhelming patient loads or are in underresourced settings, where there is little ability to review every AI-based diagnosis. Although AI might be of benefit in such conditions by reducing physician workload and streamlining the diagnostic process, there is the propensity to improperly rely on a tool meant to augment, not replace, a radiologist’s judgment. Such cases have led to adverse outcomes for patients, and legal precedence shows that this constitutes negligence.63 Maintaining awareness of each tool’s limitations and proper application is the only remedy for such situations.
Ethically, we must consider the opaqueness of ML-developed neuroimaging AIs. For many systems, the specific process by which an AI arrives at its conclusions is unknown. This AI “black box” can conceal potential errors and biases that are masked by overall positive performance metrics. The lack of understanding about how a tool functions in the zero-failure clinical setting understandably gives radiologists pause. The question must be asked: Is it ethical to use a system that is a relatively unknown quantity? Entities, including state governments, Canada, and the European Union, have produced an answer. Each of these governments have implemented policies requiring that health care AIs use some method to display to end users the process by which they arrive at conclusions.64-68
The 21st Century Cures Act declares that to attain approval, clinical AIs must demonstrate this explainability to clinicians and patients.69 The response has been an explosion in the development of explainable AI. Systems that visualize the areas where AI attention most often rests with heatmaps, generate labels for the most heavily weighted features of radiographic images, and create full diagnostic reports to justify AI conclusions aim to meet the goal of transparency and inspiring confidence in clinical end users.70 The ability to understand the “thought process” of a system proves useful for error correction and retooling. A trend toward under- or overdetecting conditions, flagging seemingly irrelevant image regions, or low reproducibility can be better addressed when it is clear how the AI is drawing its false conclusions. With an iterative process of testing and redesigning, false positive and negative rates can be reduced, the need for human intervention can be lowered to an appropriate minimum, and patient outcomes can be improved.71
Data collection raises another ethical concern. To train functional clinical decision support tools, massive amounts of patient demographic, laboratory, and imaging data are required. With incentives to develop the most powerful AI systems, record collection can venture down a path where patient autonomy and privacy are threatened. Radiologists have a duty to ensure data mining serves patients and improves the practice of radiology while protecting patients’ personal information.62 Policies have placed similar limits on the access to and use of patient records.64-69 Patients have the right to request explanation of the AI systems their data have been used to train. Approval for data acquisition requires the use of explainable AI, standardized data security protocol implementation, and adequate proof of communal benefit from the clinical decision support tool. Establishment of state-mandated protections bodes well for a future when developers can access enormous caches of data while patients and health care professionals are assured that no identifying information has escaped a well-regulated space. On the level of the individual radiologist, the knowledge that each datum represents a human life. These are people who has made themselves vulnerable by seeking relief for what ails them, which should serve as a lasting reminder to operate with utmost care when handling sensitive information.
Conclusions
The demonstrated applications of AI in neuroimaging are numerous and varied, and it is reasonable to assume that its implementation will increase as the technology matures. AI use for detecting important neurologic conditions holds promise in combatting ever greater imaging volumes and providing timely diagnoses. As medicine witnesses the continuing adoption of AI, it is important that practitioners possess an understanding of its current and emerging uses.
Artificial intelligence (AI) in medicine has shown significant promise, particularly in neuroimaging. AI refers to computer systems designed to perform tasks that normally require human intelligence.1 Machine learning (ML), a field in which computers learn from data without being specifically programmed, is the AI subset responsible for its success in matching or even surpassing humans in certain tasks.2
Supervised learning, a subset of ML, uses an algorithm with annotated data from which to learn.3 The program will use the characteristics of a training data set to predict a specific outcome or target when exposed to a sample data set of the same type. Unsupervised learning finds naturally occurring patterns or groupings within the data.4 With deep learning (DL) algorithms, computers learn the features that optimally represent the data for the problem at hand.5 Both ML and DL are meant to emulate neural networks in the brain, giving rise to artificial neural networks composed of nodes structured within input, hidden, and output layers.
The DL neural network differs from a conventional one by having many hidden layers instead of just 1 layer that extracts patterns within the data.6 Convolutional neural networks (CNNs) are the most prevalent DL architecture used in medical imaging. CNN’s hidden layers apply convolution and pooling operations to break down an image into features containing the most valuable information. The connecting layer applies high-level reasoning before the output layer provides predictions for the image. This framework has applications within radiology, such as predicting a lesion category or condition from an image, determining whether a specific pixel belongs to background or a target class, and predicting the location of lesions.1
AI promises to increase efficiency and reduces errors. With increased data processing and image interpretation, AI technology may help radiologists improve the quality of patient care.6 This article discusses the current applications and future integration of AI in neuroradiology.
Neuroimaging Applications
AI can improve the quality of neuroimaging and reduce the clinical and systemic loads of other imaging modalities. AI can predict patient wait times for computed tomography (CT), magnetic resonance imaging (MRI), ultrasound, and X-ray imaging.7 A ML-based AI has detected the variables that most affected patient wait times, including proximity to federal holidays and severity of the patient’s condition, and calculated how long patients would be delayed after their scheduled appointment time. This AI modality could allow more efficient patient scheduling and reveal areas of patient processing that could be changed, potentially improving patient satisfaction and outcomes for time-sensitive neurologic conditions.
AI can save patient and health care practitioner time for repeat MRIs. An estimated 20% of MRI scans require a repeat series—a massive loss of time and funds for both patients and the health care system.8 A DL approach can determine whether an MRI is usable clinically or unclear enough to require repetition.9 This initial screening measure can prevent patients from making return visits and neuroradiologists from reading inconclusive images. AI offers the opportunity to reduce time and costs incurred by optimizing the health care process before imaging is obtained.
Speeding Up Neuroimaging
AI can reduce the time spent performing imaging. Because MRIs consume time and resources, compressed sensing (CS) is commonly used. CS preferentially maintains in-plane resolution at the expense of through-plane resolution to produce a scan with a single, usable viewpoint that preserves signal-to-noise ratio (SNR). CS, however, limits interpretation to single directions and can create aliasing artifacts. An AI algorithm known as synthetic multi-orientation resolution enhancement works in real time to reduce aliasing and improve resolution in these compressed scans.10 This AI improved resolution of white matter lesions in patients with multiple sclerosis (MS) on FLAIR (fluid-attenuated inversion recovery) images, and permitted multiview reconstruction from these limited scans.
Tasks of reconstructing and anti-aliasing come with high computational costs that vary inversely with the extent of scanning compression, potentially negating the time and resource savings of CS. DL AI modalities have been developed to reduce operational loads and further improve image resolution in several directions from CS. One such deep residual learning AI was trained with compressed MRIs and used the framelet method to create a CNN that could rapidly remove global and deeply coherent aliasing artifacts.11 This system, compared with synthetic multi-orientation resolution enhancement, uses a pretrained, pretested AI that does not require additional time during scanning for computational analysis, thereby multiplying the time benefit of CS while retaining the benefits of multidirectional reconstruction and increased resolution. This methodology suffers from inherent degradation of perceptual image quality in its reconstructions because of the L2 loss function the CNN uses to reduce mean squared error, which causes blurring by averaging all possible outcomes of signal distribution during reconstruction. To combat this, researchers have developed another AI to reduce reconstruction times that uses a different loss function in a generative adversarial network to retain image quality, while offering reconstruction times several hundred times faster than current CS-MRI structures.12 So-called sparse-coding methods promise further reduction in reconstruction times, with the possibility of processing completed online with a lightweight architecture rather than on a local system.13
Neuroimaging of acute cases benefits most directly from these technologies because MRIs and their high resolution and SNR begin to approach CT imaging time scales. This could have important implications in clinical care, particularly for stroke imaging and evaluating spinal cord compression. CS-MRI optimization represents one of the greatest areas of neuroimaging cost savings and neurologic care improvement in the modern radiology era.
Reducing Contrast and Radiation Doses
AI has the ability to read CT, MRI, and positron emission tomography (PET) with reduced or without contrast without significant loss in sensitivity for detecting lesions. With MRI, gadolinium-based contrast can cause injection site reactions, allergic reactions, metal deposition throughout the body, and nephrogenic systemic fibrosis in the most severe instances.14 DL has been applied to brain MRIs performed with 10% of a full dose of contrast without significant degradation of image quality. Neuroradiologists did not rate the AI-synthesized images for several MRI indications lower than their full-dose counterparts.15 Low-dose contrast imaging, regardless of modality, generates greater noise with a significantly reduced signal. However, with AI applied, researchers found that the software suppressed motion and aliasing artifacts and improved image quality, perhaps evidence that this low-dose modality is less vulnerable to the most common pitfalls of MRI.
Recently, low-dose MRI moved into the spotlight when Subtle Medical SubtleGAD software received a National Institutes of Health grant and an expedited pathway to phase 2 clinical trials.16 SubtleGAD, a DL AI that enables low-dose MRI interpretation, might allow contrast MRI for patients with advanced kidney disease or contrast allergies. At some point, contrast with MRI might not be necessary because DL AI applied to noncontrast MRs for detecting MS lesions was found to be preliminarily effective with 78% lesion detection sensitivity.17
PET-MRI combines simultaneous PET and MRI and has been used to evaluate neurologic disorders. PET-MRI can detect amyloid plaques in Alzheimer disease 10 to 20 years before clinical signs of dementia emerge.18 PET-MRI has sparked DL AI development to decrease the dose of the IV radioactive tracer 18F-florbetaben used in imaging to reduce radiation exposure and imaging costs.This reduction is critical if PET-MRI is to become used widely.19-21
An initial CNN could reconstruct low-dose amyloid scans to full-dose resolution, albeit with a greater susceptibility to some artifacts and motion blurring.22 Similar to the synthetic multi-orientation resolution enhancement CNN, this program showed signal blurring from the L2 loss function, which was corrected in a later AI that used a generative adversarial network to minimize perceptual loss.23 This new AI demonstrated greater image resolution, feature preservation, and radiologist rating over the previous AI and was capable of reconstructing low-dose PET scans to full-dose resolution without an accompanying MRI. Applications of this algorithm are far-reaching, potentially allowing neuroimaging of brain tumors at more frequent intervals with higher resolution and lower total radiation exposure.
AI also has been applied to neurologic CT to reduce radiation exposure.24 Because it is critical to abide by the principles of ALARA (as low as reasonably achievable), the ability of AI to reduce radiation exposure holds significant promise. A CNN has been used to transform low-dose CTs of anthropomorphic models with calcium inserts and cardiac patients to normal-dose CTs, with the goal of improving the SNR.25 By training a noise-discriminating CNN and a noise-generating CNN together in a generative adversarial network, the AI improved image feature preservation during transformation. This algorithm has a direct application in imaging cerebral vasculature, including calcification that can explain lacunar infarcts and tracking systemic atherosclerosis.26
Another CNN has been applied to remove more complex noise patterns from the phenomena of beam hardening and photon starvation common in low-dose CT. This algorithm extracts the directional components of artifacts and compares them to known artifact patterns, allowing for highly specific suppression of unwanted signals.27 In June 2019, the US Food and Drug Administration (FDA) approved ClariPi, a deep CNN program for advanced denoising and resolution improvement of low- and ultra low-dose CTs.28 Aside from only low-dose settings, this AI could reduce artifacts in all CT imaging modalities and improve therapeutic value of procedures, including cerebral angiograms and emergency cranial scans. As the average CT radiation dose decreased from 12 mSv in 2009 to 1.5 mSv in 2014 and continues to fall, these algorithms will become increasingly necessary to retain the high resolution and diagnostic power expected of neurologic CTs.29,30
Downstream Applications
Downstream applications refer to AI use after a radiologic study is acquired, mostly image interpretation. More than 70% of FDA-approved AI medical devices are in radiology, and many of these relate to image analysis.6,31 Although AI is not limited to black-and-white image interpretation, it is hypothesized that one of the reasons radiology is inviting to AI is because gray-scale images lend themselves to standardization.3 Moreover, most radiology departments already use AI-friendly picture archiving and communication systems.31,32
AI has been applied to a range of radiologic modalities, including MRI, CT, ultrasonography, PET, and mammography.32-38 AI also has been specifically applied to radiography, including the interpretation of tuberculosis, pneumonia, lung lesions, and COVID-19.33,39-45 AI also can assist triage, patient screening, providing a “second opinion” rapidly, shortening the time needed for attaining a diagnosis, monitoring disease progression, and predicting prognosis.37-39,43,45-47 Downstream applications of AI in neuroradiology and neurology include using CT to aid in detecting hemorrhage or ischemic stroke; using MRI to automatically segment lesions, such as tumors or MS lesions; assisting in early diagnosis and predicting prognosis in MS; assisting in treating paralysis, including from spinal cord injury; determining seizure type and localizing area of seizure onset; and using cameras, wearable devices, and smartphone applications to diagnose and assess treatment response in neurodegenerative disorders, such as Parkinson or Alzheimer diseases (Figure).37,48-56
Several AI tools have been deployed in the clinical setting, particularly triaging intracranial hemorrhage and moving these studies to the top of the radiologist’s worklist. In 2020 the Centers for Medicare and Medicaid Services (CMS) began reimbursing Viz.ai software’s AI-based Viz ContaCT (Viz LVO) with a new International Statistical Classification of Diseases, Tenth Revision procedure code.57
Viz LVO automatically detects large vessel occlusions, flags the occlusion on CT angiogram, alerts the stroke team (interventional radiologist, neuroradiologist, and neurologist), and transmits images through a secure application to the stroke team members’ mobile devices—all in less than 6 minutes from study acquisition to alarm notification.48 Additional software can quantify and measure perfusion in affected brain areas.48 This could have implications for quantifying and targeting areas of ischemic penumbra that could be salvaged after a stroke and then using that information to plan targeted treatment and/or intervention. Because many trials (DAWN/DEFUSE3) have shown benefits in stroke outcome by extending the therapeutic window for the endovascular thrombectomy, the ability to identify appropriate candidates is essential.58,59 Development of AI tools in assessing ischemic penumbra with quantitative parameters (mean transit time, cerebral blood volume, cerebral blood flow, mismatch ratio) using AI has benefited image interpretation. Medtronic RAPID software can provide quantitative assessment of CT perfusion. AI tools could be used to provide an automatic ASPECT score, which provides a quantitative measure for assessing potential ischemic zones and aids in assessing appropriate candidates for thrombectomy.
Several FDA-approved AI tools help quantify brain structures in neuroradiology, including quantitative analysis through MRI for analysis of anatomy and PET for analysis of functional uptake, assisting in more accurate and more objective detection and monitoring of conditions such as atrophy, dementia, trauma, seizure disorders, and MS.48 The growing number of FDA-approved AI technologies and the recent CMS-approved reimbursement for an AI tool indicate a changing landscape that is more accepting of downstream applications of AI in neuroradiology. As AI continues to integrate into medical regulation and finance, we predict AI will continue to play a prominent role in neuroradiology.
Practical and Ethical Considerations
In any discussion of the benefits of AI, it is prudent to address its shortcomings. Chief among these is overfitting, which occurs when an AI is too closely aligned with its training dataset and prone to error when applied to novel cases. Often this is a byproduct of a small training set.60 Neuroradiology, particularly with uncommon, advanced imaging methods, has a smaller number of available studies.61 Even with more prevalent imaging modalities, such as head CT, the work of collecting training scans from patients with the prerequisite disease processes, particularly if these processes are rare, can limit the number of datapoints collected. Neuroradiologists should understand how an AI tool was generated, including the size and variety of the training dataset used, to best gauge the clinical applicability and fitness of the system.
Another point of concern for AI clinical decision support tools’ implementation is automation bias—the tendency for clinicians to favor machine-generated decisions and ignore contrary data or conflicting human decisions.62 This situation often arises when radiologists experience overwhelming patient loads or are in underresourced settings, where there is little ability to review every AI-based diagnosis. Although AI might be of benefit in such conditions by reducing physician workload and streamlining the diagnostic process, there is the propensity to improperly rely on a tool meant to augment, not replace, a radiologist’s judgment. Such cases have led to adverse outcomes for patients, and legal precedence shows that this constitutes negligence.63 Maintaining awareness of each tool’s limitations and proper application is the only remedy for such situations.
Ethically, we must consider the opaqueness of ML-developed neuroimaging AIs. For many systems, the specific process by which an AI arrives at its conclusions is unknown. This AI “black box” can conceal potential errors and biases that are masked by overall positive performance metrics. The lack of understanding about how a tool functions in the zero-failure clinical setting understandably gives radiologists pause. The question must be asked: Is it ethical to use a system that is a relatively unknown quantity? Entities, including state governments, Canada, and the European Union, have produced an answer. Each of these governments have implemented policies requiring that health care AIs use some method to display to end users the process by which they arrive at conclusions.64-68
The 21st Century Cures Act declares that to attain approval, clinical AIs must demonstrate this explainability to clinicians and patients.69 The response has been an explosion in the development of explainable AI. Systems that visualize the areas where AI attention most often rests with heatmaps, generate labels for the most heavily weighted features of radiographic images, and create full diagnostic reports to justify AI conclusions aim to meet the goal of transparency and inspiring confidence in clinical end users.70 The ability to understand the “thought process” of a system proves useful for error correction and retooling. A trend toward under- or overdetecting conditions, flagging seemingly irrelevant image regions, or low reproducibility can be better addressed when it is clear how the AI is drawing its false conclusions. With an iterative process of testing and redesigning, false positive and negative rates can be reduced, the need for human intervention can be lowered to an appropriate minimum, and patient outcomes can be improved.71
Data collection raises another ethical concern. To train functional clinical decision support tools, massive amounts of patient demographic, laboratory, and imaging data are required. With incentives to develop the most powerful AI systems, record collection can venture down a path where patient autonomy and privacy are threatened. Radiologists have a duty to ensure data mining serves patients and improves the practice of radiology while protecting patients’ personal information.62 Policies have placed similar limits on the access to and use of patient records.64-69 Patients have the right to request explanation of the AI systems their data have been used to train. Approval for data acquisition requires the use of explainable AI, standardized data security protocol implementation, and adequate proof of communal benefit from the clinical decision support tool. Establishment of state-mandated protections bodes well for a future when developers can access enormous caches of data while patients and health care professionals are assured that no identifying information has escaped a well-regulated space. On the level of the individual radiologist, the knowledge that each datum represents a human life. These are people who has made themselves vulnerable by seeking relief for what ails them, which should serve as a lasting reminder to operate with utmost care when handling sensitive information.
Conclusions
The demonstrated applications of AI in neuroimaging are numerous and varied, and it is reasonable to assume that its implementation will increase as the technology matures. AI use for detecting important neurologic conditions holds promise in combatting ever greater imaging volumes and providing timely diagnoses. As medicine witnesses the continuing adoption of AI, it is important that practitioners possess an understanding of its current and emerging uses.
1. Chartrand G, Cheng PM, Vorontsov E, et al. Deep learning: a primer for radiologists. Radiographics. 2017;37(7):2113-2131. doi:10.1148/rg.2017170077
2. King BF Jr. Guest editorial: discovery and artificial intelligence. AJR Am J Roentgenol. 2017;209(6):1189-1190. doi:10.2214/AJR.17.19178
3. Syed AB, Zoga AC. Artificial intelligence in radiology: current technology and future directions. Semin Musculoskelet Radiol. 2018;22(5):540-545. doi:10.1055/s-0038-1673383
4. Deo RC. Machine learning in medicine. Circulation. 2015;132(20):1920-1930. doi:10.1161/CIRCULATIONAHA.115.001593 5. Litjens G, Kooi T, Bejnordi BE, et al. A survey on deep learning in medical image analysis. Med Image Anal. 2017;42:60-88. doi:10.1016/j.media.2017.07.005
6. Pesapane F, Codari M, Sardanelli F. Artificial intelligence in medical imaging: threat or opportunity? Radiologists again at the forefront of innovation in medicine. Eur Radiol Exp. 2018;2(1):35. doi:10.1186/s41747-018-0061-6
7. Curtis C, Liu C, Bollerman TJ, Pianykh OS. Machine learning for predicting patient wait times and appointment delays. J Am Coll Radiol. 2018;15(9):1310-1316. doi:10.1016/j.jacr.2017.08.021
8. Andre JB, Bresnahan BW, Mossa-Basha M, et al. Toward quantifying the prevalence, severity, and cost associated with patient motion during clinical MR examinations. J Am Coll Radiol. 2015;12(7):689-695. doi:10.1016/j.jacr.2015.03.007
9. Sreekumari A, Shanbhag D, Yeo D, et al. A deep learning-based approach to reduce rescan and recall rates in clinical MRI examinations. AJNR Am J Neuroradiol. 2019;40(2):217-223. doi:10.3174/ajnr.A5926
10. Zhao C, Shao M, Carass A, et al. Applications of a deep learning method for anti-aliasing and super-resolution in MRI. Magn Reson Imaging. 2019;64:132-141. doi:10.1016/j.mri.2019.05.038
11. Lee D, Yoo J, Tak S, Ye JC. Deep residual learning for accelerated MRI using magnitude and phase networks. IEEE Trans Biomed Eng. 2018;65(9):1985-1995. doi:10.1109/TBME.2018.2821699
12. Mardani M, Gong E, Cheng JY, et al. Deep generative adversarial neural networks for compressive sensing MRI. IEEE Trans Med Imaging. 2019;38(1):167-179. doi:10.1109/TMI.2018.2858752
13. Dong C, Loy CC, He K, Tang X. Image super-resolution using deep convolutional networks. IEEE Trans Pattern Anal Mach Intell. 2016;38(2):295-307. doi:10.1109/TPAMI.2015.2439281
14. Sammet S. Magnetic resonance safety. Abdom Radiol (NY). 2016;41(3):444-451. doi:10.1007/s00261-016-0680-4
15. Gong E, Pauly JM, Wintermark M, Zaharchuk G. Deep learning enables reduced gadolinium dose for contrast-enhanced brain MRI. J Magn Reson Imaging. 2018;48(2):330-340. doi:10.1002/jmri.25970
16. Subtle Medical NIH awards Subtle Medical, Inc. $1.6 million grant to improve safety of MRI exams by reducing gadolinium dose using AI. Press release. September 18, 2019. Accessed March 14, 2022. https://www.biospace.com/article/releases/nih-awards-subtle-medical-inc-1-6-million-grant-to-improve-safety-of-mri-exams-by-reducing-gadolinium-dose-using-ai
17. Narayana PA, Coronado I, Sujit SJ, Wolinsky JS, Lublin FD, Gabr RE. Deep learning for predicting enhancing lesions in multiple sclerosis from noncontrast MRI. Radiology. 2020;294(2):398-404. doi:10.1148/radiol.2019191061
18. Jack CR Jr, Knopman DS, Jagust WJ, et al. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol. 2010;9(1):119-128. doi:10.1016/S1474-4422(09)70299-6
19. Gatidis S, Würslin C, Seith F, et al. Towards tracer dose reduction in PET studies: simulation of dose reduction by retrospective randomized undersampling of list-mode data. Hell J Nucl Med. 2016;19(1):15-18. doi:10.1967/s002449910333
20. Kaplan S, Zhu YM. Full-dose PET image estimation from low-dose PET image using deep learning: a pilot study. J Digit Imaging. 2019;32(5):773-778. doi:10.1007/s10278-018-0150-3
21. Xu J, Gong E, Pauly J, Zaharchuk G. 200x low-dose PET reconstruction using deep learning. arXiv: 1712.04119. Accessed 2/16/2022. https://arxiv.org/pdf/1712.04119.pdf
22. Chen KT, Gong E, de Carvalho Macruz FB, et al. Ultra-low-dose 18F-florbetaben amyloid PET imaging using deep learning with multi-contrast MRI inputs. Radiology. 2019;290(3):649-656. doi:10.1148/radiol.2018180940
23. Ouyang J, Chen KT, Gong E, Pauly J, Zaharchuk G. Ultra-low-dose PET reconstruction using generative adversarial network with feature matching and task-specific perceptual loss. Med Phys. 2019;46(8):3555-3564. doi:10.1002/mp.13626
24. Brenner DJ, Hall EJ. Computed tomography—an increasing source of radiation exposure. N Engl J Med. 2007;357(22):2277-2284. doi:10.1056/NEJMra072149
25. Wolterink JM, Leiner T, Viergever MA, Isgum I. Generative adversarial networks for noise reduction in low-dose CT. IEEE Trans Med Imaging. 2017;36(12):2536-2545. doi:10.1109/TMI.2017.2708987
26. Sohn YH, Cheon HY, Jeon P, Kang SY. Clinical implication of cerebral artery calcification on brain CT. Cerebrovasc Dis. 2004;18(4):332-337. doi:10.1159/000080772
27. Kang E, Min J, Ye JC. A deep convolutional neural network using directional wavelets for low-dose X-ray CT reconstruction. Med Phys. 2017;44(10):e360-e375. doi:10.1002/mp.12344
28. ClariPi gets FDA clearance for AI-powered CT image denoising solution. Published June 24, 2019. Accessed February 16, 2022. https://www.itnonline.com/content/claripi-gets-fda-clearance-ai-powered-ct-image-denoising-solution
29. Hausleiter J, Meyer T, Hermann F, et al. Estimated radiation dose associated with cardiac CT angiography. JAMA. 2009;301(5):500-507. doi:10.1001/jama.2009.54
30. Al-Mallah M, Aljizeeri A, Alharthi M, Alsaileek A. Routine low-radiation-dose coronary computed tomography angiography. Eur Heart J Suppl. 2014;16(suppl B):B12-B16. doi:10.1093/eurheartj/suu024
31. Benjamens S, Dhunnoo P, Meskó B. The state of artificial intelligence-based FDA-approved medical devices and algorithms: an online database. NPJ Digit Med. 2020;3:118. doi:10.1038/s41746-020-00324-0
32. Talebi-Liasi F, Markowitz O. Is artificial intelligence going to replace dermatologists? Cutis. 2020;105(1):28-31.
33. Khan O, Bebb G, Alimohamed NA. Artificial intelligence in medicine: what oncologists need to know about its potential—and its limitations. Oncology Exchange. 2017;16(4):8-13. http://www.oncologyex.com/pdf/vol16_no4/feature_khan-ai.pdf
34. Liu X, Faes L, Kale AU, et al. A comparison of deep learning performance against health-care professionals in detecting diseases from medical imaging: a systematic review and meta-analysis. Lancet Digit Health. 2019;1(6):e271-e297. doi:10.1016/S2589-7500(19)30123-2
35. Topol EJ. High-performance medicine: the convergence of human and artificial intelligence. Nat Med. 2019;25(1):44-56. doi:10.1038/s41591-018-0300-7
36. Salim M, Wåhlin E, Dembrower K, et al. External evaluation of 3 commercial artificial intelligence algorithms for independent assessment of screening mammograms. JAMA Oncol. 2020;6(10):1581-1588. doi:10.1001/jamaoncol.2020.3321
37. Arbabshirani MR, Fornwalt BK, Mongelluzzo GJ, et al. Advanced machine learning in action: identification of intracranial hemorrhage on computed tomography scans of the head with clinical workflow integration. NPJ Digit Med. 2018;1(1):1-7. doi:10.1038/s41746-017-0015-z
38. Sheth D, Giger ML. Artificial intelligence in the interpretation of breast cancer on MRI. J Magn Reson Imaging. 2020;51(5):1310-1324. doi:10.1002/jmri.26878
39. Borkowski AA, Viswanadhan NA, Thomas LB, Guzman RD, Deland LA, Mastorides SM. Using artificial intelligence for COVID-19 chest X-ray diagnosis. Fed Pract. 2020;37(9):398-404. doi:10.12788/fp.0045
40. Kermany DS, Goldbaum M, Cai W, et al. Identifying medical diagnoses and treatable diseases by image-based deep learning. Cell. 2018;172(5):1122-1131.e9. doi:10.1016/j.cell.2018.02.010
41. Nam JG, Park S, Hwang EJ, et al. Development and validation of deep learning-based automatic detection algorithm for malignant pulmonary nodules on chest radiographs. Radiology. 2019;290(1):218-228. doi:10.1148/radiol.2018180237
42. Zech JR, Badgeley MA, Liu M, Costa AB, Titano JJ, Oermann EK. Variable generalization performance of a deep learning model to detect pneumonia in chest radiographs: a cross-sectional study. PLoS Med. 2018;15(11):e1002683. doi:10.1371/journal.pmed.1002683
43. Lakhani P, Sundaram B. Deep learning at chest radiography: automated classification of pulmonary tuberculosis by using convolutional neural networks. Radiology. 2017;284(2):574-582. doi:10.1148/radiol.2017162326
44. Rajpurkar P, Joshi A, Pareek A, et al. CheXpedition: investigating generalization challenges for translation of chest X-Ray algorithms to the clinical setting. arXiv preprint arXiv:200211379. Accessed February 16, 2022. https://arxiv.org/pdf/2002.11379.pdf
45. He J, Baxter SL, Xu J, Xu J, Zhou X, Zhang K. The practical implementation of artificial intelligence technologies in medicine. Nat Med. 2019;25(1):30-36. doi:10.1038/s41591-018-0307-0
46. Meyer-Bäse A, Morra L, Meyer-Bäse U, Pinker K. Current status and future perspectives of artificial intelligence in magnetic resonance breast imaging. Contrast Media Mol Imaging. 2020;2020:6805710. doi:10.1155/2020/6805710
47. Booth AL, Abels E, McCaffrey P. Development of a prognostic model for mortality in COVID-19 infection using machine learning. Mod Pathol. 2020;4(3):522-531. doi:10.1038/s41379-020-00700-x
48. Bash S. Enhancing neuroimaging with artificial intelligence. Applied Radiology. 2020;49(1):20-21.
49. Jiang F, Jiang Y, Zhi H, et al. Artificial intelligence in healthcare: past, present and future. Stroke Vasc Neurol. 2017;2(4):230-243. doi:10.1136/svn-2017-000101
50. Valliani AA, Ranti D, Oermann EK. Deep learning and neurology: a systematic review. Neurol Ther. 2019;8(2):351-365. doi:10.1007/s40120-019-00153-8
51. Gupta R, Krishnam SP, Schaefer PW, Lev MH, Gonzalez RG. An east coast perspective on artificial intelligence and machine learning: part 2: ischemic stroke imaging and triage. Neuroimaging Clin N Am. 2020;30(4):467-478. doi:10.1016/j.nic.2020.08.002
52. Belić M, Bobić V, Badža M, Šolaja N, Đurić-Jovičić M, Kostić VS. Artificial intelligence for assisting diagnostics and assessment of Parkinson’s disease-A review. Clin Neurol Neurosurg. 2019;184:105442. doi:10.1016/j.clineuro.2019.105442
53. An S, Kang C, Lee HW. Artificial intelligence and computational approaches for epilepsy. J Epilepsy Res. 2020;10(1):8-17. doi:10.14581/jer.20003
54. Pavel AM, Rennie JM, de Vries LS, et al. A machine-learning algorithm for neonatal seizure recognition: a multicentre, randomised, controlled trial. Lancet Child Adolesc Health. 2020;4(10):740-749. doi:10.1016/S2352-4642(20)30239-X
55. Afzal HMR, Luo S, Ramadan S, Lechner-Scott J. The emerging role of artificial intelligence in multiple sclerosis imaging. Mult Scler. 2020;1352458520966298. doi:10.1177/1352458520966298
56. Bouton CE. Restoring movement in paralysis with a bioelectronic neural bypass approach: current state and future directions. Cold Spring Harb Perspect Med. 2019;9(11):a034306. doi:10.1101/cshperspect.a034306
57. Hassan AE. New technology add-on payment (NTAP) for Viz LVO: a win for stroke care. J Neurointerv Surg. 2020;neurintsurg-2020-016897. doi:10.1136/neurintsurg-2020-016897
58. Nogueira RG , Jadhav AP , Haussen DC , et al; DAWN Trial Investigators. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med. 2018;378:11–21. doi:10.1056/NEJMoa1706442
59. Albers GW , Marks MP , Kemp S , et al; DEFUSE 3 Investigators. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med. 2018;378:708–18. doi:10.1056/NEJMoa1713973
60. Bi WL, Hosny A, Schabath MB, et al. Artificial intelligence in cancer imaging: clinical challenges and applications. CA Cancer J Clin. 2019;69(2):127-157. doi:10.3322/caac.21552
61. Wagner MW, Namdar K, Biswas A, Monah S, Khalvati F, Ertl-Wagner BB. Radiomics, machine learning, and artificial intelligence-what the neuroradiologist needs to know. Neuroradiology. 2021;63(12):1957-1967. doi:10.1007/s00234-021-02813-9
62. Geis JR, Brady AP, Wu CC, et al. Ethics of artificial intelligence in radiology: summary of the Joint European and North American Multisociety Statement. J Am Coll Radiol. 2019;16(11):1516-1521. doi:10.1016/j.jacr.2019.07.028
63. Kingston J. Artificial intelligence and legal liability. arXiv:1802.07782. https://arxiv.org/ftp/arxiv/papers/1802/1802.07782.pdf
64. Council of the European Union, General Data Protection Regulation. Official Journal of the European Union. Accessed February 16, 2022. https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32016R0679
65. Consumer Privacy Protection Act of 2017, HR 4081, 115th Cong (2017). Accessed February 10, 2022. https://www.congress.gov/bill/115th-congress/house-bill/4081
66. Cal. Civ. Code § 1798.198(a) (2018). California Consumer Privacy Act of 2018.
67. Va. Code Ann. § 59.1 (2021). Consumer Data Protection Act. Accessed February 10, 2022. https://lis.virginia.gov/cgi-bin/legp604.exe?212+ful+SB1392ER+pdf
68. Colo. Rev. Stat. § 6-1-1301 (2021). Colorado Privacy Act. Accessed February 10, 2022. https://leg.colorado.gov/sites/default/files/2021a_190_signed.pdf
69. 21st Century Cures Act, Pub L No. 114-255 (2016). Accessed February 10, 2022. https://www.govinfo.gov/content/pkg/PLAW-114publ255/html/PLAW-114publ255.htm
70. Huff DT, Weisman AJ, Jeraj R. Interpretation and visualization techniques for deep learning models in medical imaging. Phys Med Biol. 2021;66(4):04TR01. doi:10.1088/1361-6560/abcd17
71. Thrall JH, Li X, Li Q, et al. Artificial intelligence and machine learning in radiology: opportunities, challenges, pitfalls, and criteria for success. J Am Coll Radiol. 2018;15(3, pt B):504-508. doi:10.1016/j.jacr.2017.12.026
1. Chartrand G, Cheng PM, Vorontsov E, et al. Deep learning: a primer for radiologists. Radiographics. 2017;37(7):2113-2131. doi:10.1148/rg.2017170077
2. King BF Jr. Guest editorial: discovery and artificial intelligence. AJR Am J Roentgenol. 2017;209(6):1189-1190. doi:10.2214/AJR.17.19178
3. Syed AB, Zoga AC. Artificial intelligence in radiology: current technology and future directions. Semin Musculoskelet Radiol. 2018;22(5):540-545. doi:10.1055/s-0038-1673383
4. Deo RC. Machine learning in medicine. Circulation. 2015;132(20):1920-1930. doi:10.1161/CIRCULATIONAHA.115.001593 5. Litjens G, Kooi T, Bejnordi BE, et al. A survey on deep learning in medical image analysis. Med Image Anal. 2017;42:60-88. doi:10.1016/j.media.2017.07.005
6. Pesapane F, Codari M, Sardanelli F. Artificial intelligence in medical imaging: threat or opportunity? Radiologists again at the forefront of innovation in medicine. Eur Radiol Exp. 2018;2(1):35. doi:10.1186/s41747-018-0061-6
7. Curtis C, Liu C, Bollerman TJ, Pianykh OS. Machine learning for predicting patient wait times and appointment delays. J Am Coll Radiol. 2018;15(9):1310-1316. doi:10.1016/j.jacr.2017.08.021
8. Andre JB, Bresnahan BW, Mossa-Basha M, et al. Toward quantifying the prevalence, severity, and cost associated with patient motion during clinical MR examinations. J Am Coll Radiol. 2015;12(7):689-695. doi:10.1016/j.jacr.2015.03.007
9. Sreekumari A, Shanbhag D, Yeo D, et al. A deep learning-based approach to reduce rescan and recall rates in clinical MRI examinations. AJNR Am J Neuroradiol. 2019;40(2):217-223. doi:10.3174/ajnr.A5926
10. Zhao C, Shao M, Carass A, et al. Applications of a deep learning method for anti-aliasing and super-resolution in MRI. Magn Reson Imaging. 2019;64:132-141. doi:10.1016/j.mri.2019.05.038
11. Lee D, Yoo J, Tak S, Ye JC. Deep residual learning for accelerated MRI using magnitude and phase networks. IEEE Trans Biomed Eng. 2018;65(9):1985-1995. doi:10.1109/TBME.2018.2821699
12. Mardani M, Gong E, Cheng JY, et al. Deep generative adversarial neural networks for compressive sensing MRI. IEEE Trans Med Imaging. 2019;38(1):167-179. doi:10.1109/TMI.2018.2858752
13. Dong C, Loy CC, He K, Tang X. Image super-resolution using deep convolutional networks. IEEE Trans Pattern Anal Mach Intell. 2016;38(2):295-307. doi:10.1109/TPAMI.2015.2439281
14. Sammet S. Magnetic resonance safety. Abdom Radiol (NY). 2016;41(3):444-451. doi:10.1007/s00261-016-0680-4
15. Gong E, Pauly JM, Wintermark M, Zaharchuk G. Deep learning enables reduced gadolinium dose for contrast-enhanced brain MRI. J Magn Reson Imaging. 2018;48(2):330-340. doi:10.1002/jmri.25970
16. Subtle Medical NIH awards Subtle Medical, Inc. $1.6 million grant to improve safety of MRI exams by reducing gadolinium dose using AI. Press release. September 18, 2019. Accessed March 14, 2022. https://www.biospace.com/article/releases/nih-awards-subtle-medical-inc-1-6-million-grant-to-improve-safety-of-mri-exams-by-reducing-gadolinium-dose-using-ai
17. Narayana PA, Coronado I, Sujit SJ, Wolinsky JS, Lublin FD, Gabr RE. Deep learning for predicting enhancing lesions in multiple sclerosis from noncontrast MRI. Radiology. 2020;294(2):398-404. doi:10.1148/radiol.2019191061
18. Jack CR Jr, Knopman DS, Jagust WJ, et al. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol. 2010;9(1):119-128. doi:10.1016/S1474-4422(09)70299-6
19. Gatidis S, Würslin C, Seith F, et al. Towards tracer dose reduction in PET studies: simulation of dose reduction by retrospective randomized undersampling of list-mode data. Hell J Nucl Med. 2016;19(1):15-18. doi:10.1967/s002449910333
20. Kaplan S, Zhu YM. Full-dose PET image estimation from low-dose PET image using deep learning: a pilot study. J Digit Imaging. 2019;32(5):773-778. doi:10.1007/s10278-018-0150-3
21. Xu J, Gong E, Pauly J, Zaharchuk G. 200x low-dose PET reconstruction using deep learning. arXiv: 1712.04119. Accessed 2/16/2022. https://arxiv.org/pdf/1712.04119.pdf
22. Chen KT, Gong E, de Carvalho Macruz FB, et al. Ultra-low-dose 18F-florbetaben amyloid PET imaging using deep learning with multi-contrast MRI inputs. Radiology. 2019;290(3):649-656. doi:10.1148/radiol.2018180940
23. Ouyang J, Chen KT, Gong E, Pauly J, Zaharchuk G. Ultra-low-dose PET reconstruction using generative adversarial network with feature matching and task-specific perceptual loss. Med Phys. 2019;46(8):3555-3564. doi:10.1002/mp.13626
24. Brenner DJ, Hall EJ. Computed tomography—an increasing source of radiation exposure. N Engl J Med. 2007;357(22):2277-2284. doi:10.1056/NEJMra072149
25. Wolterink JM, Leiner T, Viergever MA, Isgum I. Generative adversarial networks for noise reduction in low-dose CT. IEEE Trans Med Imaging. 2017;36(12):2536-2545. doi:10.1109/TMI.2017.2708987
26. Sohn YH, Cheon HY, Jeon P, Kang SY. Clinical implication of cerebral artery calcification on brain CT. Cerebrovasc Dis. 2004;18(4):332-337. doi:10.1159/000080772
27. Kang E, Min J, Ye JC. A deep convolutional neural network using directional wavelets for low-dose X-ray CT reconstruction. Med Phys. 2017;44(10):e360-e375. doi:10.1002/mp.12344
28. ClariPi gets FDA clearance for AI-powered CT image denoising solution. Published June 24, 2019. Accessed February 16, 2022. https://www.itnonline.com/content/claripi-gets-fda-clearance-ai-powered-ct-image-denoising-solution
29. Hausleiter J, Meyer T, Hermann F, et al. Estimated radiation dose associated with cardiac CT angiography. JAMA. 2009;301(5):500-507. doi:10.1001/jama.2009.54
30. Al-Mallah M, Aljizeeri A, Alharthi M, Alsaileek A. Routine low-radiation-dose coronary computed tomography angiography. Eur Heart J Suppl. 2014;16(suppl B):B12-B16. doi:10.1093/eurheartj/suu024
31. Benjamens S, Dhunnoo P, Meskó B. The state of artificial intelligence-based FDA-approved medical devices and algorithms: an online database. NPJ Digit Med. 2020;3:118. doi:10.1038/s41746-020-00324-0
32. Talebi-Liasi F, Markowitz O. Is artificial intelligence going to replace dermatologists? Cutis. 2020;105(1):28-31.
33. Khan O, Bebb G, Alimohamed NA. Artificial intelligence in medicine: what oncologists need to know about its potential—and its limitations. Oncology Exchange. 2017;16(4):8-13. http://www.oncologyex.com/pdf/vol16_no4/feature_khan-ai.pdf
34. Liu X, Faes L, Kale AU, et al. A comparison of deep learning performance against health-care professionals in detecting diseases from medical imaging: a systematic review and meta-analysis. Lancet Digit Health. 2019;1(6):e271-e297. doi:10.1016/S2589-7500(19)30123-2
35. Topol EJ. High-performance medicine: the convergence of human and artificial intelligence. Nat Med. 2019;25(1):44-56. doi:10.1038/s41591-018-0300-7
36. Salim M, Wåhlin E, Dembrower K, et al. External evaluation of 3 commercial artificial intelligence algorithms for independent assessment of screening mammograms. JAMA Oncol. 2020;6(10):1581-1588. doi:10.1001/jamaoncol.2020.3321
37. Arbabshirani MR, Fornwalt BK, Mongelluzzo GJ, et al. Advanced machine learning in action: identification of intracranial hemorrhage on computed tomography scans of the head with clinical workflow integration. NPJ Digit Med. 2018;1(1):1-7. doi:10.1038/s41746-017-0015-z
38. Sheth D, Giger ML. Artificial intelligence in the interpretation of breast cancer on MRI. J Magn Reson Imaging. 2020;51(5):1310-1324. doi:10.1002/jmri.26878
39. Borkowski AA, Viswanadhan NA, Thomas LB, Guzman RD, Deland LA, Mastorides SM. Using artificial intelligence for COVID-19 chest X-ray diagnosis. Fed Pract. 2020;37(9):398-404. doi:10.12788/fp.0045
40. Kermany DS, Goldbaum M, Cai W, et al. Identifying medical diagnoses and treatable diseases by image-based deep learning. Cell. 2018;172(5):1122-1131.e9. doi:10.1016/j.cell.2018.02.010
41. Nam JG, Park S, Hwang EJ, et al. Development and validation of deep learning-based automatic detection algorithm for malignant pulmonary nodules on chest radiographs. Radiology. 2019;290(1):218-228. doi:10.1148/radiol.2018180237
42. Zech JR, Badgeley MA, Liu M, Costa AB, Titano JJ, Oermann EK. Variable generalization performance of a deep learning model to detect pneumonia in chest radiographs: a cross-sectional study. PLoS Med. 2018;15(11):e1002683. doi:10.1371/journal.pmed.1002683
43. Lakhani P, Sundaram B. Deep learning at chest radiography: automated classification of pulmonary tuberculosis by using convolutional neural networks. Radiology. 2017;284(2):574-582. doi:10.1148/radiol.2017162326
44. Rajpurkar P, Joshi A, Pareek A, et al. CheXpedition: investigating generalization challenges for translation of chest X-Ray algorithms to the clinical setting. arXiv preprint arXiv:200211379. Accessed February 16, 2022. https://arxiv.org/pdf/2002.11379.pdf
45. He J, Baxter SL, Xu J, Xu J, Zhou X, Zhang K. The practical implementation of artificial intelligence technologies in medicine. Nat Med. 2019;25(1):30-36. doi:10.1038/s41591-018-0307-0
46. Meyer-Bäse A, Morra L, Meyer-Bäse U, Pinker K. Current status and future perspectives of artificial intelligence in magnetic resonance breast imaging. Contrast Media Mol Imaging. 2020;2020:6805710. doi:10.1155/2020/6805710
47. Booth AL, Abels E, McCaffrey P. Development of a prognostic model for mortality in COVID-19 infection using machine learning. Mod Pathol. 2020;4(3):522-531. doi:10.1038/s41379-020-00700-x
48. Bash S. Enhancing neuroimaging with artificial intelligence. Applied Radiology. 2020;49(1):20-21.
49. Jiang F, Jiang Y, Zhi H, et al. Artificial intelligence in healthcare: past, present and future. Stroke Vasc Neurol. 2017;2(4):230-243. doi:10.1136/svn-2017-000101
50. Valliani AA, Ranti D, Oermann EK. Deep learning and neurology: a systematic review. Neurol Ther. 2019;8(2):351-365. doi:10.1007/s40120-019-00153-8
51. Gupta R, Krishnam SP, Schaefer PW, Lev MH, Gonzalez RG. An east coast perspective on artificial intelligence and machine learning: part 2: ischemic stroke imaging and triage. Neuroimaging Clin N Am. 2020;30(4):467-478. doi:10.1016/j.nic.2020.08.002
52. Belić M, Bobić V, Badža M, Šolaja N, Đurić-Jovičić M, Kostić VS. Artificial intelligence for assisting diagnostics and assessment of Parkinson’s disease-A review. Clin Neurol Neurosurg. 2019;184:105442. doi:10.1016/j.clineuro.2019.105442
53. An S, Kang C, Lee HW. Artificial intelligence and computational approaches for epilepsy. J Epilepsy Res. 2020;10(1):8-17. doi:10.14581/jer.20003
54. Pavel AM, Rennie JM, de Vries LS, et al. A machine-learning algorithm for neonatal seizure recognition: a multicentre, randomised, controlled trial. Lancet Child Adolesc Health. 2020;4(10):740-749. doi:10.1016/S2352-4642(20)30239-X
55. Afzal HMR, Luo S, Ramadan S, Lechner-Scott J. The emerging role of artificial intelligence in multiple sclerosis imaging. Mult Scler. 2020;1352458520966298. doi:10.1177/1352458520966298
56. Bouton CE. Restoring movement in paralysis with a bioelectronic neural bypass approach: current state and future directions. Cold Spring Harb Perspect Med. 2019;9(11):a034306. doi:10.1101/cshperspect.a034306
57. Hassan AE. New technology add-on payment (NTAP) for Viz LVO: a win for stroke care. J Neurointerv Surg. 2020;neurintsurg-2020-016897. doi:10.1136/neurintsurg-2020-016897
58. Nogueira RG , Jadhav AP , Haussen DC , et al; DAWN Trial Investigators. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med. 2018;378:11–21. doi:10.1056/NEJMoa1706442
59. Albers GW , Marks MP , Kemp S , et al; DEFUSE 3 Investigators. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med. 2018;378:708–18. doi:10.1056/NEJMoa1713973
60. Bi WL, Hosny A, Schabath MB, et al. Artificial intelligence in cancer imaging: clinical challenges and applications. CA Cancer J Clin. 2019;69(2):127-157. doi:10.3322/caac.21552
61. Wagner MW, Namdar K, Biswas A, Monah S, Khalvati F, Ertl-Wagner BB. Radiomics, machine learning, and artificial intelligence-what the neuroradiologist needs to know. Neuroradiology. 2021;63(12):1957-1967. doi:10.1007/s00234-021-02813-9
62. Geis JR, Brady AP, Wu CC, et al. Ethics of artificial intelligence in radiology: summary of the Joint European and North American Multisociety Statement. J Am Coll Radiol. 2019;16(11):1516-1521. doi:10.1016/j.jacr.2019.07.028
63. Kingston J. Artificial intelligence and legal liability. arXiv:1802.07782. https://arxiv.org/ftp/arxiv/papers/1802/1802.07782.pdf
64. Council of the European Union, General Data Protection Regulation. Official Journal of the European Union. Accessed February 16, 2022. https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32016R0679
65. Consumer Privacy Protection Act of 2017, HR 4081, 115th Cong (2017). Accessed February 10, 2022. https://www.congress.gov/bill/115th-congress/house-bill/4081
66. Cal. Civ. Code § 1798.198(a) (2018). California Consumer Privacy Act of 2018.
67. Va. Code Ann. § 59.1 (2021). Consumer Data Protection Act. Accessed February 10, 2022. https://lis.virginia.gov/cgi-bin/legp604.exe?212+ful+SB1392ER+pdf
68. Colo. Rev. Stat. § 6-1-1301 (2021). Colorado Privacy Act. Accessed February 10, 2022. https://leg.colorado.gov/sites/default/files/2021a_190_signed.pdf
69. 21st Century Cures Act, Pub L No. 114-255 (2016). Accessed February 10, 2022. https://www.govinfo.gov/content/pkg/PLAW-114publ255/html/PLAW-114publ255.htm
70. Huff DT, Weisman AJ, Jeraj R. Interpretation and visualization techniques for deep learning models in medical imaging. Phys Med Biol. 2021;66(4):04TR01. doi:10.1088/1361-6560/abcd17
71. Thrall JH, Li X, Li Q, et al. Artificial intelligence and machine learning in radiology: opportunities, challenges, pitfalls, and criteria for success. J Am Coll Radiol. 2018;15(3, pt B):504-508. doi:10.1016/j.jacr.2017.12.026
Autonomic Dysfunction in the Setting of CADASIL Syndrome
Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) syndrome is the most common monogenic inherited cause of stroke. CADASIL syndrome is a nonsclerotic angiopathy resulting from a mutation of the NOTCH3 gene on chromosome 19p13, encoding a receptor expressed by vascular smooth muscle cells.1 This mutation results in migraine, recurrent ischemic stroke, affective disorders, and dementia, with migraine often manifesting earliest.2,3
The onset of stroke symptoms occurs typically in ages ≥ 60 years with some patients experiencing stroke as early as in their 30s.1,4 Presentation varies among patients even within the same family.5 CADASIL syndrome is frequently mistaken for other more common neurologic conditions due to the low prevalence of CADASIL syndrome, reported to be between 2 and 5 per 100,000.3,6 The cumulative nature of multiple ischemic episodes seen in 85% of symptomatic individuals leads to disability. Dementia is often hallmarked as one of the features of end-stage CADASIL syndrome.7 Extent and severity of brain tissue damage are shown to be the most critical factors of clinical symptoms.8 There is no specific treatment for CADASIL syndrome other than addressing risk factors.9
Symptoms are traditionally described to be limited to the central nervous system (CNS); however, reports of other organ system effects exist. Twenty-six percent of premature mortality relating to CADASIL syndrome is sudden unexpected death, which several authors have postulated could be attributed to cardiac events.10,11
The NOTCH3 gene encodes a protein expressed during gastrulation and in the CNS during embryological development. The expression of this protein decreases with time and has limited expression in adulthood.12 The pathophysiology of CADASIL syndrome includes myriad changes, including cerebral vessels narrowed by intimal thickening due to expansion of the extracellular matrix, degeneration of smooth muscle cells of the cerebral vessel walls, and osmiophilic material deposition in patients with CADASIL syndrome.13 Granular osmiophilic material in the vascular basal lamina can be observed on electron microscopy of patients with CADASIL syndrome and are used for diagnostic purposes.14
CADASIL syndrome often presents a diagnostic dilemma for physicians and is easy to misdiagnose in the early stages. The diagnostic dilemma arises given the subacute onset of CADASIL syndrome with vague early presenting symptoms, such as headache, prior to more specific findings (ie, multiple early strokes or transient ischemic attacks [TIA]). Patients presenting with CADASIL syndrome may be misdiagnosed with other neurologic conditions, including migraine or multiple sclerosis (MS).15 Especially in the case of MS, lesions visible on magnetic resonance imaging (MRI) may be differentiated by the higher rates of temporo polar lesions seen in CADASIL syndrome in comparison with those in MS.3
It is important to consider CADASIL syndrome in patients presenting at a young age with stroke due to the compounding effects of multiple ischemic episodes and subsequent motor/sensory and neuropsychologic deficits. This necessitates increasing awareness of CADASIL syndrome in the neurologic and radiologic community and the importance of educating families of patients on the importance of being evaluated. This diagnostic dilemma can lead to delay in appropriate therapy and control of related modifiable risk factors, including hypertension, hyperlipidemia, etc. Delays in initiation of anti-stroke pharmacotherapy can lead to additional morbidity and mortality in these patients.
The radiology of CADASIL syndrome is unique and particularly important due to the possible confusion with MS. MRI is an important tool in the evaluation of the cerebral pathology of CADASIL syndrome, revealing white matter and microangiopathic signal abnormalities, indicative of ischemic infarcts, lacunar strokes, and diffuse leukoencephalopathy.13,16 MRI lesions are often seen in the basal ganglia, thalamus, external capsule, and pons.7 The lesions also are seen in the periventricular region, explaining its misperception as MS.17 In addition, cerebral microhemorrhages have been seen. To further differentiate these lesions, the anterior temporal lobe should be observed for gliosis or hyperintensities, which correlates with CADASIL syndrome.18 Location of hyperintensity in the temporal lobes, relative sparing of the occipital/orbitofrontal white matter, corpus callosum, subcortical u-fibers, and cortex is helpful in differentiating from other etiologies, such as microvascular white matter ischemic disease, MS, and mitochondrial encephalopathy with lactic acidosis and strokelike symptoms (MELAS).
Case Presentation
A patient aged > 50 years presented to the emergency department (ED) due to numbness of the right perioral area, gait difficulties, difficulty speaking, and increasing right lower extremity weakness with no numbness or paresthesia. The patient’s medical history is relevant for CADASIL syndrome, hypertension, prior cerebrovascular accident, recurrent TIAs, multinodular goiter with a history of radioactive iodine treatment, and neurogenic bladder controlled with oxybutynin since age 30 years. The patient had a significant stroke history: the first stroke occurred at age 36 years and 3 more strokes at ages 38, 44, and 53 years and 4 TIAs over that period. This patient reported no recent headache or memory changes and had no history of smoking, alcohol, or recreational drug use. Family history was pertinent for the mother’s death secondary to stroke, with a history of multiple strokes beginning at a young, undetermined age and no major motor, sensory, or neuropsychologic deficits prior to her death. A sister and first cousin had been diagnosed with MS.
On triage in the ED, stroke alert was called but tissue plasminogen activator was not given due to time eligibility. The patient’s numbness and weakness were improved within 7 hours, but she continued to have difficulty with dysarthric speech and unsteady gait following this incident. Antihypertensive medications were discontinued on admission to allow for permissive hypertension to improve cerebral blood flow. A brain MRI revealed bilateral increased T2 fluid-attenuated inversion recovery (FLAIR) signal in the anterior temporal lobes, confluent increased T2 FLAIR signal in the periventricular/deep white matter, bilateral basal ganglia chronic lacunar infarcts, and several chronic microbleeds (Figure 1). There was no evidence for an acute infarct on the MRI. Recrudescence of prior stroke symptoms secondary to CADASIL syndrome was suspected as a primary diagnosis with a differential of TIA.
Starting the second day of admission, the patient had intermittent sinus bradycardia with the lowest heart rate (HR) in the range of 40 beats per minute (bpm) while awake with an unchanged neurologic examination. Each episode was transient, lasting less than an hour per staff documentation. The electrocardiogram (ECG) on admission demonstrated normal sinus rhythm in the range of 70 to 80 bpm.
The patient was asymptomatic and normotensive during the episodes of bradycardia. The patient had not yet resumed any antihypertensives. An echocardiogram was unremarkable with a left ventricular ejection fraction of 55 to 60%, normal anatomy, and no significant pericardial effusion. Carotid artery duplex examination demonstrated patent vessels with anterograde vertebral flow bilaterally. Due to the unknown cause of the bradycardia, the patient was discharged with a 14-day ambulatory cardiac monitor, advised to continue statin, aspirin, and lisinopril, and given a referral to continue with outpatient physical therapy and occupational therapy.
The patient’s ambulatory cardiac monitoring showed dominant sinus rhythm, with the HR in the range of 40 to 170 bpm with an overall average 70 to 80 bpm. The patient’s HR spent 5% of the recording time under 50 bpm and 14% of the time > 100. There was no evidence of heart block. No symptoms were recorded per the patient’s symptom diary during the entire 2 weeks of monitoring. Further follow-up showed that the patient presented to a primary care practitioner 1 month later with similar symptoms and was sent to the ED of an outside hospital without admission. The ECG was again unremarkable, demonstrating only sinus bradycardia with normal T waves, QT interval, without ST elevations or depressions. About 3 weeks later, the patient presented to the ED again with chest pain and was discharged with a diagnosis of atypical chest pain possibly related to anxiety without findings consistent with acute coronary syndrome (ACS).
Discussion
This patient with CADASIL syndrome and significant stroke history with cardiac symptoms demonstrates 3 important discussion points: the difficulty of early diagnosis, high rates of morbidity/mortality, and the need for further research into the cardiac effects of CADASIL syndrome. Due to this patient’s bradycardic episodes while being monitored on telemetry, it is possible that the cause of the strokelike symptoms was a TIA, secondary to decreased perfusion pressure, explaining the lack of acute ischemia on imaging. With regards to the history of thyroid dysfunction, this particular episode of bradycardia was unlikely to be related as the thyroid-stimulating hormone was reflective of subclinical hyperthyroidism with T4 levels within normal limits.
This case demonstrates a potential link between CADASIL syndrome and autonomic dysfunction. Similar to general stroke patients, patients with CADASIL syndrome are at an increased risk of hypoperfusion injury secondary to cardiovascular and autonomic dysfunction. This raises a question of initial and surveillance screening tests on diagnosis of CADASIL syndrome. It may be appropriate to obtain routine echocardiogram and ECG and other arrhythmia screening tests in these patients, especially during or following an ischemic episode. However, more evidence is required to support creation of a formal recommendation.
In a study of cardiac rhythm abnormalities in a half-million adults, 1.57% of women aged 55 to 64 years were found to have rhythm abnormality with 0.27% having a bradyarrhythmia.19 In the setting of neurologic disease, ECG changes such as arrhythmias and repolarization changes are regularly noted.20 However, it is unlikely that the bradycardia would be causing the brain lesions. In CADASIL syndrome, there is relative sparing of the occipital, orbitofrontal subcortical white matter, subcortical fibers, and cortex. Specifically, within CADASIL syndrome, a study of 23 patients showed no ECG changes regarding infarction/ischemia, conduction disturbances, or arrhythmias compared with that of controls.21
Further research into the cardiac effects of CADASIL syndrome is needed. As CADASIL syndrome is primarily a disorder of the vasculature, the disease has potential to affect the heart in addition to the brain.1 This theory is well supported by the embryologic effects of the NOTCH3 receptor pathways, which are responsible for the development of the cardiovascular system.22 Anecdotal evidence supports this theory as few case reports have been published that describe various cardiac abnormalities in patients with CADASIL syndrome, including myocardial infarction (MI), conduction abnormalities, and arrhythmias.2, 23-25
There have only been 2 published studies regarding investigations into CADASIL syndrome and cardiac disease. The first paper was a case-control study that investigated ECG changes in the setting of CADASIL syndrome. The study found no evidence for MI, ischemia, conduction disorder, or arrhythmias in patients with CADASIL syndrome.21 Unfortunately, this study was underpowered and limited in scope, only investigating a single ECG recording from 23 patients with CADASIL syndrome in a single clinic.21 Other cardiac markers, such as echocardiogram, stress test, and contractility, and longitudinal cardiac outcomes were not investigated in this study.21 The second paper was also a case-control study by Rufa and colleagues that investigated HR variability and other ECG changes during a 10-minute rest recording on 23 patients with CADASIL syndrome and compared the results to 22 age- and gender-matched patients in good health.11
This study found reduced HR variability and an increased ratio of low-frequency to high-frequency variability, which the authors claimed demonstrates autonomic dysfunction in patients with CADASIL syndrome.11 Rufa and colleagues concluded that patients with CADASIL syndrome are at higher risk for cardiac arrhythmias.11 This study also found no evidence for MI, ischemia, conduction disorder, or arrhythmias in the patients with CADASIL syndrome compared with that of age-matched controls.11 Similar to the first paper, this study is underpowered, only looks at a single timepoint recording, and uses incomplete and indirect measurements of cardiac function.
There is a need for a longitudinal review of cardiac outcomes in the CADASIL syndrome population to determine whether these patients require additional surveillance or prophylaxis. While the variability in HR of our patient cannot be definitively attributed solely to CADASIL syndrome, the subsequent admissions demonstrate that long-term monitoring may be warranted.
Conclusions
CADASIL syndrome is an autosomal dominant NOTCH3 signaling disease that affects the small vessel vasculature and leads to early ischemic events, headache, dementia, and death. CADASIL syndrome is frequently misdiagnosed due to insidious onset and vague presenting symptoms. Delay in diagnosis often results in nonoptimized medical management. Current guidelines recommend following poststroke protocol and minimizing individual risk factors by using antiplatelet, antihypertensive, and dyslipidemia medications. This case demonstrates a classic presentation of CADASIL syndrome with lesser described cardiac symptoms. Few cases of unusual cardiac symptoms in the setting of CADASIL syndrome have been reported. The relationship between cardiovascular disease and CADASIL syndrome is not well described. Further research is needed to elucidate any links between CADASIL syndrome and cardiovascular disease and to optimize management for these patients.
1. Moreton FC, Razvi SS, Davidson R, Muir KW. Changing clinical patterns and increasing prevalence in CADASIL. Acta Neurol Scand. 2014;130(3):197-203. doi:10.1111/ane.12266
2. Lesnik Oberstein SA, Jukema JW, Van Duinen SG, Macfarlane PW, van Houwelingen HC, Breuning MH, et al. Myocardial infarction in cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL). Medicine (Baltimore). 2003;82(4):251-256. doi:10.1097/01.md.0000085054.63483.40
3. Di Donato I, Bianchi S, De Stefano N, Dichgans M, Dotti MT, Duering M, et al. Cerebral Autosomal Dominant Arteriopathy with Subcortical Infarcts and Leukoencephalopathy (CADASIL) as a model of small vessel disease: update on clinical, diagnostic, and management aspects. BMC Med. 2017;15(1):41. doi:10.1186/s12916-017-0778-8
4. Dunphy L, Rani A, Duodu Y, Behnam Y. Cerebral autosomal dominant arteriopathy with subcortical infarcts and leucoencephalopathy (CADASIL) presenting with stroke in a young man. BMJ Case Rep. 2019 ;12(7):e229609. doi:10.1136/bcr-2019-229609
5. Bianchi S, Zicari E, Carluccio A, Di Donato I, Pescini F, Nannucci S, et al. CADASIL in central Italy: a retrospective clinical and genetic study in 229 patients. J Neurol. 2015;262(1):134-141. doi:10.1007/s00415-014-7533-2
6. Phillips CD, Zuckerman SJ, Medical Education Commission. CADASIL can mimic multiple sclerosis. J La State Med Soc. 2010 May-Jun;162(3):174.
7. Hervé D, Chabriat H. CADASIL. J Geriatr Psychiatry Neurol. 2010;23(4):269-276. doi:10.1177/0891988710383570
8. Yamamoto Y, Hase Y, Ihara M, Khundakar A, Roeber S, Duering M, et al. Neuronal densities and vascular pathology in the hippocampal formation in CADASIL. Neurobiol Aging. 2021;97:33-40. doi:10.1016/j.neurobiolaging.2020.09.016
9. Ferrante EA, Cudrici CD, Boehm M. CADASIL: new advances in basic science and clinical perspectives. Curr Opin Hematol. 2019;26(3):193-198. doi:10.1097/MOH.0000000000000497
10. Opherk C, Peters N, Herzog J, Luedtke R, Dichgans M. Long-term prognosis and causes of death in CADASIL: a retrospective study in 411 patients. Brain. 2004;127(pt 11):2533-2539.
11. Rufa A, Guideri F, Acampa M, Cevenini G, Bianchi S, De Stefano N, et al. Cardiac autonomic nervous system and risk of arrhythmias in cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL). Stroke. 2007 Feb;38(2):276-280. doi:10.1093/brain/awh282
12. Joutel A, Corpechot C, Ducros A, Vahedi K, Chabriat H, Mouton P, et al. Notch3 mutations in CADASIL, a hereditary adult-onset condition causing stroke and dementia. Nature. 1996;383(6602):707-710. doi:10.1038/383707a0
13. Kalaria RN, Viitanen M, Kalimo H, Dichgans M, Tabira T, CASASIL Group of Vas-Cog. The pathogenesis of CADASIL: an update. J Neurol Sci. 2004;226(1-2):35-39. doi:10.1016/j.jns.2004.09.008
14. Reddy SPK, Vishnu VY, Goyal V, Singh MB, Arora S, Garg A, et al. CADASIL syndrome and stroke in young people. QJM. 2020 Feb 1;113(2):118-119. doi:10.1093/qjmed/hcz243
15. Carone DA. CADASIL and multiple sclerosis: A case report of prolonged misdiagnosis. Applied neuropsychology Adult. 2017;24(3):294-297. doi:10.1080/23279095.2016.1214132
16. Zhu S, Nahas SJ. CADASIL: Imaging characteristics and clinical correlation. Curr Pain Headache Rep. 2016;20(10):57. doi:10.1007/s11916-016-0584-6
17. Kalaria RN, Low WC, Oakley AE, Slade JY, Ince PG, Morris CM, et al. CADASIL and genetics of cerebral ischaemia. J Neural Transm Suppl. 2002;(63):75-90. doi:10.1007/978-3-7091-6137-1_5
18. O’Sullivan M, Jarosz JM, Martin RJ, Deasy N, Powell JF, Markus HS. MRI hyperintensities of the temporal lobe and external capsule in patients with CADASIL. Neurology. 2001;56(5):628-634. doi:10.1212/wnl.56.5.628
19. Khurshid S, Choi SH, Weng L-C, Wang EY, Trinquart L, Benjamin EJ, et al. Frequency of cardiac rhythm abnormalities in a half million adults. Circ ArrhythmElectrophysiol. 2018;11(7):e006273. doi:10.1161/CIRCEP.118.006273
20. Samuels MA. The brain–heart connection. Circulation. 2007;116(1):77-84. doi:10.1161/CIRCULATIONAHA. 106.678995
21. Cumurciuc R, Henry P, Gobron C, Vicaut E, Bousser MG, Chabriat H, et al. Electrocardiogram in cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy patients without any clinical evidence of coronary artery disease: a case-control study. Stroke. 2006;37(4):1100-1102. doi:10.1161/01.STR.0000209242.68844.20
22. Luxán G, D’Amato G, MacGrogan D, de la Pompa JL. Endocardial notch signaling in cardiac development and disease. Circ Res. 2016;118(1):e1-e18. doi:10.1161/CIRCRESAHA.115.305350
23. Rubin CB, Hahn V, Kobayashi T, Litwack A. A report of accelerated coronary artery disease associated with cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy. Case Rep Cardiol. 2015;2015:167513. doi:10.1155/2015/167513
24. Langer C, Adukauskaite A, Plank F, Feuchtner G, Cartes-Zumelzu F. Cerebral autosomal dominant arteriopathy (CADASIL) with cardiac involvement (ANOCA) and subcortical leukencephalopathy. J Cardiovasc Comput Tomogr. 2020;14(5):e1-e6. doi:10.1016/j.jcct.2018.08.005
25. Pettersen JA, Keith J, Gao F, Spence JD, Black SE. CADASIL accelerated by acute hypotension: Arterial and venous contribution to leukoaraiosis. Neurology. 2017;88(11):1077-1080. doi:10.1212/WNL.0000000000003717
Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) syndrome is the most common monogenic inherited cause of stroke. CADASIL syndrome is a nonsclerotic angiopathy resulting from a mutation of the NOTCH3 gene on chromosome 19p13, encoding a receptor expressed by vascular smooth muscle cells.1 This mutation results in migraine, recurrent ischemic stroke, affective disorders, and dementia, with migraine often manifesting earliest.2,3
The onset of stroke symptoms occurs typically in ages ≥ 60 years with some patients experiencing stroke as early as in their 30s.1,4 Presentation varies among patients even within the same family.5 CADASIL syndrome is frequently mistaken for other more common neurologic conditions due to the low prevalence of CADASIL syndrome, reported to be between 2 and 5 per 100,000.3,6 The cumulative nature of multiple ischemic episodes seen in 85% of symptomatic individuals leads to disability. Dementia is often hallmarked as one of the features of end-stage CADASIL syndrome.7 Extent and severity of brain tissue damage are shown to be the most critical factors of clinical symptoms.8 There is no specific treatment for CADASIL syndrome other than addressing risk factors.9
Symptoms are traditionally described to be limited to the central nervous system (CNS); however, reports of other organ system effects exist. Twenty-six percent of premature mortality relating to CADASIL syndrome is sudden unexpected death, which several authors have postulated could be attributed to cardiac events.10,11
The NOTCH3 gene encodes a protein expressed during gastrulation and in the CNS during embryological development. The expression of this protein decreases with time and has limited expression in adulthood.12 The pathophysiology of CADASIL syndrome includes myriad changes, including cerebral vessels narrowed by intimal thickening due to expansion of the extracellular matrix, degeneration of smooth muscle cells of the cerebral vessel walls, and osmiophilic material deposition in patients with CADASIL syndrome.13 Granular osmiophilic material in the vascular basal lamina can be observed on electron microscopy of patients with CADASIL syndrome and are used for diagnostic purposes.14
CADASIL syndrome often presents a diagnostic dilemma for physicians and is easy to misdiagnose in the early stages. The diagnostic dilemma arises given the subacute onset of CADASIL syndrome with vague early presenting symptoms, such as headache, prior to more specific findings (ie, multiple early strokes or transient ischemic attacks [TIA]). Patients presenting with CADASIL syndrome may be misdiagnosed with other neurologic conditions, including migraine or multiple sclerosis (MS).15 Especially in the case of MS, lesions visible on magnetic resonance imaging (MRI) may be differentiated by the higher rates of temporo polar lesions seen in CADASIL syndrome in comparison with those in MS.3
It is important to consider CADASIL syndrome in patients presenting at a young age with stroke due to the compounding effects of multiple ischemic episodes and subsequent motor/sensory and neuropsychologic deficits. This necessitates increasing awareness of CADASIL syndrome in the neurologic and radiologic community and the importance of educating families of patients on the importance of being evaluated. This diagnostic dilemma can lead to delay in appropriate therapy and control of related modifiable risk factors, including hypertension, hyperlipidemia, etc. Delays in initiation of anti-stroke pharmacotherapy can lead to additional morbidity and mortality in these patients.
The radiology of CADASIL syndrome is unique and particularly important due to the possible confusion with MS. MRI is an important tool in the evaluation of the cerebral pathology of CADASIL syndrome, revealing white matter and microangiopathic signal abnormalities, indicative of ischemic infarcts, lacunar strokes, and diffuse leukoencephalopathy.13,16 MRI lesions are often seen in the basal ganglia, thalamus, external capsule, and pons.7 The lesions also are seen in the periventricular region, explaining its misperception as MS.17 In addition, cerebral microhemorrhages have been seen. To further differentiate these lesions, the anterior temporal lobe should be observed for gliosis or hyperintensities, which correlates with CADASIL syndrome.18 Location of hyperintensity in the temporal lobes, relative sparing of the occipital/orbitofrontal white matter, corpus callosum, subcortical u-fibers, and cortex is helpful in differentiating from other etiologies, such as microvascular white matter ischemic disease, MS, and mitochondrial encephalopathy with lactic acidosis and strokelike symptoms (MELAS).
Case Presentation
A patient aged > 50 years presented to the emergency department (ED) due to numbness of the right perioral area, gait difficulties, difficulty speaking, and increasing right lower extremity weakness with no numbness or paresthesia. The patient’s medical history is relevant for CADASIL syndrome, hypertension, prior cerebrovascular accident, recurrent TIAs, multinodular goiter with a history of radioactive iodine treatment, and neurogenic bladder controlled with oxybutynin since age 30 years. The patient had a significant stroke history: the first stroke occurred at age 36 years and 3 more strokes at ages 38, 44, and 53 years and 4 TIAs over that period. This patient reported no recent headache or memory changes and had no history of smoking, alcohol, or recreational drug use. Family history was pertinent for the mother’s death secondary to stroke, with a history of multiple strokes beginning at a young, undetermined age and no major motor, sensory, or neuropsychologic deficits prior to her death. A sister and first cousin had been diagnosed with MS.
On triage in the ED, stroke alert was called but tissue plasminogen activator was not given due to time eligibility. The patient’s numbness and weakness were improved within 7 hours, but she continued to have difficulty with dysarthric speech and unsteady gait following this incident. Antihypertensive medications were discontinued on admission to allow for permissive hypertension to improve cerebral blood flow. A brain MRI revealed bilateral increased T2 fluid-attenuated inversion recovery (FLAIR) signal in the anterior temporal lobes, confluent increased T2 FLAIR signal in the periventricular/deep white matter, bilateral basal ganglia chronic lacunar infarcts, and several chronic microbleeds (Figure 1). There was no evidence for an acute infarct on the MRI. Recrudescence of prior stroke symptoms secondary to CADASIL syndrome was suspected as a primary diagnosis with a differential of TIA.
Starting the second day of admission, the patient had intermittent sinus bradycardia with the lowest heart rate (HR) in the range of 40 beats per minute (bpm) while awake with an unchanged neurologic examination. Each episode was transient, lasting less than an hour per staff documentation. The electrocardiogram (ECG) on admission demonstrated normal sinus rhythm in the range of 70 to 80 bpm.
The patient was asymptomatic and normotensive during the episodes of bradycardia. The patient had not yet resumed any antihypertensives. An echocardiogram was unremarkable with a left ventricular ejection fraction of 55 to 60%, normal anatomy, and no significant pericardial effusion. Carotid artery duplex examination demonstrated patent vessels with anterograde vertebral flow bilaterally. Due to the unknown cause of the bradycardia, the patient was discharged with a 14-day ambulatory cardiac monitor, advised to continue statin, aspirin, and lisinopril, and given a referral to continue with outpatient physical therapy and occupational therapy.
The patient’s ambulatory cardiac monitoring showed dominant sinus rhythm, with the HR in the range of 40 to 170 bpm with an overall average 70 to 80 bpm. The patient’s HR spent 5% of the recording time under 50 bpm and 14% of the time > 100. There was no evidence of heart block. No symptoms were recorded per the patient’s symptom diary during the entire 2 weeks of monitoring. Further follow-up showed that the patient presented to a primary care practitioner 1 month later with similar symptoms and was sent to the ED of an outside hospital without admission. The ECG was again unremarkable, demonstrating only sinus bradycardia with normal T waves, QT interval, without ST elevations or depressions. About 3 weeks later, the patient presented to the ED again with chest pain and was discharged with a diagnosis of atypical chest pain possibly related to anxiety without findings consistent with acute coronary syndrome (ACS).
Discussion
This patient with CADASIL syndrome and significant stroke history with cardiac symptoms demonstrates 3 important discussion points: the difficulty of early diagnosis, high rates of morbidity/mortality, and the need for further research into the cardiac effects of CADASIL syndrome. Due to this patient’s bradycardic episodes while being monitored on telemetry, it is possible that the cause of the strokelike symptoms was a TIA, secondary to decreased perfusion pressure, explaining the lack of acute ischemia on imaging. With regards to the history of thyroid dysfunction, this particular episode of bradycardia was unlikely to be related as the thyroid-stimulating hormone was reflective of subclinical hyperthyroidism with T4 levels within normal limits.
This case demonstrates a potential link between CADASIL syndrome and autonomic dysfunction. Similar to general stroke patients, patients with CADASIL syndrome are at an increased risk of hypoperfusion injury secondary to cardiovascular and autonomic dysfunction. This raises a question of initial and surveillance screening tests on diagnosis of CADASIL syndrome. It may be appropriate to obtain routine echocardiogram and ECG and other arrhythmia screening tests in these patients, especially during or following an ischemic episode. However, more evidence is required to support creation of a formal recommendation.
In a study of cardiac rhythm abnormalities in a half-million adults, 1.57% of women aged 55 to 64 years were found to have rhythm abnormality with 0.27% having a bradyarrhythmia.19 In the setting of neurologic disease, ECG changes such as arrhythmias and repolarization changes are regularly noted.20 However, it is unlikely that the bradycardia would be causing the brain lesions. In CADASIL syndrome, there is relative sparing of the occipital, orbitofrontal subcortical white matter, subcortical fibers, and cortex. Specifically, within CADASIL syndrome, a study of 23 patients showed no ECG changes regarding infarction/ischemia, conduction disturbances, or arrhythmias compared with that of controls.21
Further research into the cardiac effects of CADASIL syndrome is needed. As CADASIL syndrome is primarily a disorder of the vasculature, the disease has potential to affect the heart in addition to the brain.1 This theory is well supported by the embryologic effects of the NOTCH3 receptor pathways, which are responsible for the development of the cardiovascular system.22 Anecdotal evidence supports this theory as few case reports have been published that describe various cardiac abnormalities in patients with CADASIL syndrome, including myocardial infarction (MI), conduction abnormalities, and arrhythmias.2, 23-25
There have only been 2 published studies regarding investigations into CADASIL syndrome and cardiac disease. The first paper was a case-control study that investigated ECG changes in the setting of CADASIL syndrome. The study found no evidence for MI, ischemia, conduction disorder, or arrhythmias in patients with CADASIL syndrome.21 Unfortunately, this study was underpowered and limited in scope, only investigating a single ECG recording from 23 patients with CADASIL syndrome in a single clinic.21 Other cardiac markers, such as echocardiogram, stress test, and contractility, and longitudinal cardiac outcomes were not investigated in this study.21 The second paper was also a case-control study by Rufa and colleagues that investigated HR variability and other ECG changes during a 10-minute rest recording on 23 patients with CADASIL syndrome and compared the results to 22 age- and gender-matched patients in good health.11
This study found reduced HR variability and an increased ratio of low-frequency to high-frequency variability, which the authors claimed demonstrates autonomic dysfunction in patients with CADASIL syndrome.11 Rufa and colleagues concluded that patients with CADASIL syndrome are at higher risk for cardiac arrhythmias.11 This study also found no evidence for MI, ischemia, conduction disorder, or arrhythmias in the patients with CADASIL syndrome compared with that of age-matched controls.11 Similar to the first paper, this study is underpowered, only looks at a single timepoint recording, and uses incomplete and indirect measurements of cardiac function.
There is a need for a longitudinal review of cardiac outcomes in the CADASIL syndrome population to determine whether these patients require additional surveillance or prophylaxis. While the variability in HR of our patient cannot be definitively attributed solely to CADASIL syndrome, the subsequent admissions demonstrate that long-term monitoring may be warranted.
Conclusions
CADASIL syndrome is an autosomal dominant NOTCH3 signaling disease that affects the small vessel vasculature and leads to early ischemic events, headache, dementia, and death. CADASIL syndrome is frequently misdiagnosed due to insidious onset and vague presenting symptoms. Delay in diagnosis often results in nonoptimized medical management. Current guidelines recommend following poststroke protocol and minimizing individual risk factors by using antiplatelet, antihypertensive, and dyslipidemia medications. This case demonstrates a classic presentation of CADASIL syndrome with lesser described cardiac symptoms. Few cases of unusual cardiac symptoms in the setting of CADASIL syndrome have been reported. The relationship between cardiovascular disease and CADASIL syndrome is not well described. Further research is needed to elucidate any links between CADASIL syndrome and cardiovascular disease and to optimize management for these patients.
Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) syndrome is the most common monogenic inherited cause of stroke. CADASIL syndrome is a nonsclerotic angiopathy resulting from a mutation of the NOTCH3 gene on chromosome 19p13, encoding a receptor expressed by vascular smooth muscle cells.1 This mutation results in migraine, recurrent ischemic stroke, affective disorders, and dementia, with migraine often manifesting earliest.2,3
The onset of stroke symptoms occurs typically in ages ≥ 60 years with some patients experiencing stroke as early as in their 30s.1,4 Presentation varies among patients even within the same family.5 CADASIL syndrome is frequently mistaken for other more common neurologic conditions due to the low prevalence of CADASIL syndrome, reported to be between 2 and 5 per 100,000.3,6 The cumulative nature of multiple ischemic episodes seen in 85% of symptomatic individuals leads to disability. Dementia is often hallmarked as one of the features of end-stage CADASIL syndrome.7 Extent and severity of brain tissue damage are shown to be the most critical factors of clinical symptoms.8 There is no specific treatment for CADASIL syndrome other than addressing risk factors.9
Symptoms are traditionally described to be limited to the central nervous system (CNS); however, reports of other organ system effects exist. Twenty-six percent of premature mortality relating to CADASIL syndrome is sudden unexpected death, which several authors have postulated could be attributed to cardiac events.10,11
The NOTCH3 gene encodes a protein expressed during gastrulation and in the CNS during embryological development. The expression of this protein decreases with time and has limited expression in adulthood.12 The pathophysiology of CADASIL syndrome includes myriad changes, including cerebral vessels narrowed by intimal thickening due to expansion of the extracellular matrix, degeneration of smooth muscle cells of the cerebral vessel walls, and osmiophilic material deposition in patients with CADASIL syndrome.13 Granular osmiophilic material in the vascular basal lamina can be observed on electron microscopy of patients with CADASIL syndrome and are used for diagnostic purposes.14
CADASIL syndrome often presents a diagnostic dilemma for physicians and is easy to misdiagnose in the early stages. The diagnostic dilemma arises given the subacute onset of CADASIL syndrome with vague early presenting symptoms, such as headache, prior to more specific findings (ie, multiple early strokes or transient ischemic attacks [TIA]). Patients presenting with CADASIL syndrome may be misdiagnosed with other neurologic conditions, including migraine or multiple sclerosis (MS).15 Especially in the case of MS, lesions visible on magnetic resonance imaging (MRI) may be differentiated by the higher rates of temporo polar lesions seen in CADASIL syndrome in comparison with those in MS.3
It is important to consider CADASIL syndrome in patients presenting at a young age with stroke due to the compounding effects of multiple ischemic episodes and subsequent motor/sensory and neuropsychologic deficits. This necessitates increasing awareness of CADASIL syndrome in the neurologic and radiologic community and the importance of educating families of patients on the importance of being evaluated. This diagnostic dilemma can lead to delay in appropriate therapy and control of related modifiable risk factors, including hypertension, hyperlipidemia, etc. Delays in initiation of anti-stroke pharmacotherapy can lead to additional morbidity and mortality in these patients.
The radiology of CADASIL syndrome is unique and particularly important due to the possible confusion with MS. MRI is an important tool in the evaluation of the cerebral pathology of CADASIL syndrome, revealing white matter and microangiopathic signal abnormalities, indicative of ischemic infarcts, lacunar strokes, and diffuse leukoencephalopathy.13,16 MRI lesions are often seen in the basal ganglia, thalamus, external capsule, and pons.7 The lesions also are seen in the periventricular region, explaining its misperception as MS.17 In addition, cerebral microhemorrhages have been seen. To further differentiate these lesions, the anterior temporal lobe should be observed for gliosis or hyperintensities, which correlates with CADASIL syndrome.18 Location of hyperintensity in the temporal lobes, relative sparing of the occipital/orbitofrontal white matter, corpus callosum, subcortical u-fibers, and cortex is helpful in differentiating from other etiologies, such as microvascular white matter ischemic disease, MS, and mitochondrial encephalopathy with lactic acidosis and strokelike symptoms (MELAS).
Case Presentation
A patient aged > 50 years presented to the emergency department (ED) due to numbness of the right perioral area, gait difficulties, difficulty speaking, and increasing right lower extremity weakness with no numbness or paresthesia. The patient’s medical history is relevant for CADASIL syndrome, hypertension, prior cerebrovascular accident, recurrent TIAs, multinodular goiter with a history of radioactive iodine treatment, and neurogenic bladder controlled with oxybutynin since age 30 years. The patient had a significant stroke history: the first stroke occurred at age 36 years and 3 more strokes at ages 38, 44, and 53 years and 4 TIAs over that period. This patient reported no recent headache or memory changes and had no history of smoking, alcohol, or recreational drug use. Family history was pertinent for the mother’s death secondary to stroke, with a history of multiple strokes beginning at a young, undetermined age and no major motor, sensory, or neuropsychologic deficits prior to her death. A sister and first cousin had been diagnosed with MS.
On triage in the ED, stroke alert was called but tissue plasminogen activator was not given due to time eligibility. The patient’s numbness and weakness were improved within 7 hours, but she continued to have difficulty with dysarthric speech and unsteady gait following this incident. Antihypertensive medications were discontinued on admission to allow for permissive hypertension to improve cerebral blood flow. A brain MRI revealed bilateral increased T2 fluid-attenuated inversion recovery (FLAIR) signal in the anterior temporal lobes, confluent increased T2 FLAIR signal in the periventricular/deep white matter, bilateral basal ganglia chronic lacunar infarcts, and several chronic microbleeds (Figure 1). There was no evidence for an acute infarct on the MRI. Recrudescence of prior stroke symptoms secondary to CADASIL syndrome was suspected as a primary diagnosis with a differential of TIA.
Starting the second day of admission, the patient had intermittent sinus bradycardia with the lowest heart rate (HR) in the range of 40 beats per minute (bpm) while awake with an unchanged neurologic examination. Each episode was transient, lasting less than an hour per staff documentation. The electrocardiogram (ECG) on admission demonstrated normal sinus rhythm in the range of 70 to 80 bpm.
The patient was asymptomatic and normotensive during the episodes of bradycardia. The patient had not yet resumed any antihypertensives. An echocardiogram was unremarkable with a left ventricular ejection fraction of 55 to 60%, normal anatomy, and no significant pericardial effusion. Carotid artery duplex examination demonstrated patent vessels with anterograde vertebral flow bilaterally. Due to the unknown cause of the bradycardia, the patient was discharged with a 14-day ambulatory cardiac monitor, advised to continue statin, aspirin, and lisinopril, and given a referral to continue with outpatient physical therapy and occupational therapy.
The patient’s ambulatory cardiac monitoring showed dominant sinus rhythm, with the HR in the range of 40 to 170 bpm with an overall average 70 to 80 bpm. The patient’s HR spent 5% of the recording time under 50 bpm and 14% of the time > 100. There was no evidence of heart block. No symptoms were recorded per the patient’s symptom diary during the entire 2 weeks of monitoring. Further follow-up showed that the patient presented to a primary care practitioner 1 month later with similar symptoms and was sent to the ED of an outside hospital without admission. The ECG was again unremarkable, demonstrating only sinus bradycardia with normal T waves, QT interval, without ST elevations or depressions. About 3 weeks later, the patient presented to the ED again with chest pain and was discharged with a diagnosis of atypical chest pain possibly related to anxiety without findings consistent with acute coronary syndrome (ACS).
Discussion
This patient with CADASIL syndrome and significant stroke history with cardiac symptoms demonstrates 3 important discussion points: the difficulty of early diagnosis, high rates of morbidity/mortality, and the need for further research into the cardiac effects of CADASIL syndrome. Due to this patient’s bradycardic episodes while being monitored on telemetry, it is possible that the cause of the strokelike symptoms was a TIA, secondary to decreased perfusion pressure, explaining the lack of acute ischemia on imaging. With regards to the history of thyroid dysfunction, this particular episode of bradycardia was unlikely to be related as the thyroid-stimulating hormone was reflective of subclinical hyperthyroidism with T4 levels within normal limits.
This case demonstrates a potential link between CADASIL syndrome and autonomic dysfunction. Similar to general stroke patients, patients with CADASIL syndrome are at an increased risk of hypoperfusion injury secondary to cardiovascular and autonomic dysfunction. This raises a question of initial and surveillance screening tests on diagnosis of CADASIL syndrome. It may be appropriate to obtain routine echocardiogram and ECG and other arrhythmia screening tests in these patients, especially during or following an ischemic episode. However, more evidence is required to support creation of a formal recommendation.
In a study of cardiac rhythm abnormalities in a half-million adults, 1.57% of women aged 55 to 64 years were found to have rhythm abnormality with 0.27% having a bradyarrhythmia.19 In the setting of neurologic disease, ECG changes such as arrhythmias and repolarization changes are regularly noted.20 However, it is unlikely that the bradycardia would be causing the brain lesions. In CADASIL syndrome, there is relative sparing of the occipital, orbitofrontal subcortical white matter, subcortical fibers, and cortex. Specifically, within CADASIL syndrome, a study of 23 patients showed no ECG changes regarding infarction/ischemia, conduction disturbances, or arrhythmias compared with that of controls.21
Further research into the cardiac effects of CADASIL syndrome is needed. As CADASIL syndrome is primarily a disorder of the vasculature, the disease has potential to affect the heart in addition to the brain.1 This theory is well supported by the embryologic effects of the NOTCH3 receptor pathways, which are responsible for the development of the cardiovascular system.22 Anecdotal evidence supports this theory as few case reports have been published that describe various cardiac abnormalities in patients with CADASIL syndrome, including myocardial infarction (MI), conduction abnormalities, and arrhythmias.2, 23-25
There have only been 2 published studies regarding investigations into CADASIL syndrome and cardiac disease. The first paper was a case-control study that investigated ECG changes in the setting of CADASIL syndrome. The study found no evidence for MI, ischemia, conduction disorder, or arrhythmias in patients with CADASIL syndrome.21 Unfortunately, this study was underpowered and limited in scope, only investigating a single ECG recording from 23 patients with CADASIL syndrome in a single clinic.21 Other cardiac markers, such as echocardiogram, stress test, and contractility, and longitudinal cardiac outcomes were not investigated in this study.21 The second paper was also a case-control study by Rufa and colleagues that investigated HR variability and other ECG changes during a 10-minute rest recording on 23 patients with CADASIL syndrome and compared the results to 22 age- and gender-matched patients in good health.11
This study found reduced HR variability and an increased ratio of low-frequency to high-frequency variability, which the authors claimed demonstrates autonomic dysfunction in patients with CADASIL syndrome.11 Rufa and colleagues concluded that patients with CADASIL syndrome are at higher risk for cardiac arrhythmias.11 This study also found no evidence for MI, ischemia, conduction disorder, or arrhythmias in the patients with CADASIL syndrome compared with that of age-matched controls.11 Similar to the first paper, this study is underpowered, only looks at a single timepoint recording, and uses incomplete and indirect measurements of cardiac function.
There is a need for a longitudinal review of cardiac outcomes in the CADASIL syndrome population to determine whether these patients require additional surveillance or prophylaxis. While the variability in HR of our patient cannot be definitively attributed solely to CADASIL syndrome, the subsequent admissions demonstrate that long-term monitoring may be warranted.
Conclusions
CADASIL syndrome is an autosomal dominant NOTCH3 signaling disease that affects the small vessel vasculature and leads to early ischemic events, headache, dementia, and death. CADASIL syndrome is frequently misdiagnosed due to insidious onset and vague presenting symptoms. Delay in diagnosis often results in nonoptimized medical management. Current guidelines recommend following poststroke protocol and minimizing individual risk factors by using antiplatelet, antihypertensive, and dyslipidemia medications. This case demonstrates a classic presentation of CADASIL syndrome with lesser described cardiac symptoms. Few cases of unusual cardiac symptoms in the setting of CADASIL syndrome have been reported. The relationship between cardiovascular disease and CADASIL syndrome is not well described. Further research is needed to elucidate any links between CADASIL syndrome and cardiovascular disease and to optimize management for these patients.
1. Moreton FC, Razvi SS, Davidson R, Muir KW. Changing clinical patterns and increasing prevalence in CADASIL. Acta Neurol Scand. 2014;130(3):197-203. doi:10.1111/ane.12266
2. Lesnik Oberstein SA, Jukema JW, Van Duinen SG, Macfarlane PW, van Houwelingen HC, Breuning MH, et al. Myocardial infarction in cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL). Medicine (Baltimore). 2003;82(4):251-256. doi:10.1097/01.md.0000085054.63483.40
3. Di Donato I, Bianchi S, De Stefano N, Dichgans M, Dotti MT, Duering M, et al. Cerebral Autosomal Dominant Arteriopathy with Subcortical Infarcts and Leukoencephalopathy (CADASIL) as a model of small vessel disease: update on clinical, diagnostic, and management aspects. BMC Med. 2017;15(1):41. doi:10.1186/s12916-017-0778-8
4. Dunphy L, Rani A, Duodu Y, Behnam Y. Cerebral autosomal dominant arteriopathy with subcortical infarcts and leucoencephalopathy (CADASIL) presenting with stroke in a young man. BMJ Case Rep. 2019 ;12(7):e229609. doi:10.1136/bcr-2019-229609
5. Bianchi S, Zicari E, Carluccio A, Di Donato I, Pescini F, Nannucci S, et al. CADASIL in central Italy: a retrospective clinical and genetic study in 229 patients. J Neurol. 2015;262(1):134-141. doi:10.1007/s00415-014-7533-2
6. Phillips CD, Zuckerman SJ, Medical Education Commission. CADASIL can mimic multiple sclerosis. J La State Med Soc. 2010 May-Jun;162(3):174.
7. Hervé D, Chabriat H. CADASIL. J Geriatr Psychiatry Neurol. 2010;23(4):269-276. doi:10.1177/0891988710383570
8. Yamamoto Y, Hase Y, Ihara M, Khundakar A, Roeber S, Duering M, et al. Neuronal densities and vascular pathology in the hippocampal formation in CADASIL. Neurobiol Aging. 2021;97:33-40. doi:10.1016/j.neurobiolaging.2020.09.016
9. Ferrante EA, Cudrici CD, Boehm M. CADASIL: new advances in basic science and clinical perspectives. Curr Opin Hematol. 2019;26(3):193-198. doi:10.1097/MOH.0000000000000497
10. Opherk C, Peters N, Herzog J, Luedtke R, Dichgans M. Long-term prognosis and causes of death in CADASIL: a retrospective study in 411 patients. Brain. 2004;127(pt 11):2533-2539.
11. Rufa A, Guideri F, Acampa M, Cevenini G, Bianchi S, De Stefano N, et al. Cardiac autonomic nervous system and risk of arrhythmias in cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL). Stroke. 2007 Feb;38(2):276-280. doi:10.1093/brain/awh282
12. Joutel A, Corpechot C, Ducros A, Vahedi K, Chabriat H, Mouton P, et al. Notch3 mutations in CADASIL, a hereditary adult-onset condition causing stroke and dementia. Nature. 1996;383(6602):707-710. doi:10.1038/383707a0
13. Kalaria RN, Viitanen M, Kalimo H, Dichgans M, Tabira T, CASASIL Group of Vas-Cog. The pathogenesis of CADASIL: an update. J Neurol Sci. 2004;226(1-2):35-39. doi:10.1016/j.jns.2004.09.008
14. Reddy SPK, Vishnu VY, Goyal V, Singh MB, Arora S, Garg A, et al. CADASIL syndrome and stroke in young people. QJM. 2020 Feb 1;113(2):118-119. doi:10.1093/qjmed/hcz243
15. Carone DA. CADASIL and multiple sclerosis: A case report of prolonged misdiagnosis. Applied neuropsychology Adult. 2017;24(3):294-297. doi:10.1080/23279095.2016.1214132
16. Zhu S, Nahas SJ. CADASIL: Imaging characteristics and clinical correlation. Curr Pain Headache Rep. 2016;20(10):57. doi:10.1007/s11916-016-0584-6
17. Kalaria RN, Low WC, Oakley AE, Slade JY, Ince PG, Morris CM, et al. CADASIL and genetics of cerebral ischaemia. J Neural Transm Suppl. 2002;(63):75-90. doi:10.1007/978-3-7091-6137-1_5
18. O’Sullivan M, Jarosz JM, Martin RJ, Deasy N, Powell JF, Markus HS. MRI hyperintensities of the temporal lobe and external capsule in patients with CADASIL. Neurology. 2001;56(5):628-634. doi:10.1212/wnl.56.5.628
19. Khurshid S, Choi SH, Weng L-C, Wang EY, Trinquart L, Benjamin EJ, et al. Frequency of cardiac rhythm abnormalities in a half million adults. Circ ArrhythmElectrophysiol. 2018;11(7):e006273. doi:10.1161/CIRCEP.118.006273
20. Samuels MA. The brain–heart connection. Circulation. 2007;116(1):77-84. doi:10.1161/CIRCULATIONAHA. 106.678995
21. Cumurciuc R, Henry P, Gobron C, Vicaut E, Bousser MG, Chabriat H, et al. Electrocardiogram in cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy patients without any clinical evidence of coronary artery disease: a case-control study. Stroke. 2006;37(4):1100-1102. doi:10.1161/01.STR.0000209242.68844.20
22. Luxán G, D’Amato G, MacGrogan D, de la Pompa JL. Endocardial notch signaling in cardiac development and disease. Circ Res. 2016;118(1):e1-e18. doi:10.1161/CIRCRESAHA.115.305350
23. Rubin CB, Hahn V, Kobayashi T, Litwack A. A report of accelerated coronary artery disease associated with cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy. Case Rep Cardiol. 2015;2015:167513. doi:10.1155/2015/167513
24. Langer C, Adukauskaite A, Plank F, Feuchtner G, Cartes-Zumelzu F. Cerebral autosomal dominant arteriopathy (CADASIL) with cardiac involvement (ANOCA) and subcortical leukencephalopathy. J Cardiovasc Comput Tomogr. 2020;14(5):e1-e6. doi:10.1016/j.jcct.2018.08.005
25. Pettersen JA, Keith J, Gao F, Spence JD, Black SE. CADASIL accelerated by acute hypotension: Arterial and venous contribution to leukoaraiosis. Neurology. 2017;88(11):1077-1080. doi:10.1212/WNL.0000000000003717
1. Moreton FC, Razvi SS, Davidson R, Muir KW. Changing clinical patterns and increasing prevalence in CADASIL. Acta Neurol Scand. 2014;130(3):197-203. doi:10.1111/ane.12266
2. Lesnik Oberstein SA, Jukema JW, Van Duinen SG, Macfarlane PW, van Houwelingen HC, Breuning MH, et al. Myocardial infarction in cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL). Medicine (Baltimore). 2003;82(4):251-256. doi:10.1097/01.md.0000085054.63483.40
3. Di Donato I, Bianchi S, De Stefano N, Dichgans M, Dotti MT, Duering M, et al. Cerebral Autosomal Dominant Arteriopathy with Subcortical Infarcts and Leukoencephalopathy (CADASIL) as a model of small vessel disease: update on clinical, diagnostic, and management aspects. BMC Med. 2017;15(1):41. doi:10.1186/s12916-017-0778-8
4. Dunphy L, Rani A, Duodu Y, Behnam Y. Cerebral autosomal dominant arteriopathy with subcortical infarcts and leucoencephalopathy (CADASIL) presenting with stroke in a young man. BMJ Case Rep. 2019 ;12(7):e229609. doi:10.1136/bcr-2019-229609
5. Bianchi S, Zicari E, Carluccio A, Di Donato I, Pescini F, Nannucci S, et al. CADASIL in central Italy: a retrospective clinical and genetic study in 229 patients. J Neurol. 2015;262(1):134-141. doi:10.1007/s00415-014-7533-2
6. Phillips CD, Zuckerman SJ, Medical Education Commission. CADASIL can mimic multiple sclerosis. J La State Med Soc. 2010 May-Jun;162(3):174.
7. Hervé D, Chabriat H. CADASIL. J Geriatr Psychiatry Neurol. 2010;23(4):269-276. doi:10.1177/0891988710383570
8. Yamamoto Y, Hase Y, Ihara M, Khundakar A, Roeber S, Duering M, et al. Neuronal densities and vascular pathology in the hippocampal formation in CADASIL. Neurobiol Aging. 2021;97:33-40. doi:10.1016/j.neurobiolaging.2020.09.016
9. Ferrante EA, Cudrici CD, Boehm M. CADASIL: new advances in basic science and clinical perspectives. Curr Opin Hematol. 2019;26(3):193-198. doi:10.1097/MOH.0000000000000497
10. Opherk C, Peters N, Herzog J, Luedtke R, Dichgans M. Long-term prognosis and causes of death in CADASIL: a retrospective study in 411 patients. Brain. 2004;127(pt 11):2533-2539.
11. Rufa A, Guideri F, Acampa M, Cevenini G, Bianchi S, De Stefano N, et al. Cardiac autonomic nervous system and risk of arrhythmias in cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL). Stroke. 2007 Feb;38(2):276-280. doi:10.1093/brain/awh282
12. Joutel A, Corpechot C, Ducros A, Vahedi K, Chabriat H, Mouton P, et al. Notch3 mutations in CADASIL, a hereditary adult-onset condition causing stroke and dementia. Nature. 1996;383(6602):707-710. doi:10.1038/383707a0
13. Kalaria RN, Viitanen M, Kalimo H, Dichgans M, Tabira T, CASASIL Group of Vas-Cog. The pathogenesis of CADASIL: an update. J Neurol Sci. 2004;226(1-2):35-39. doi:10.1016/j.jns.2004.09.008
14. Reddy SPK, Vishnu VY, Goyal V, Singh MB, Arora S, Garg A, et al. CADASIL syndrome and stroke in young people. QJM. 2020 Feb 1;113(2):118-119. doi:10.1093/qjmed/hcz243
15. Carone DA. CADASIL and multiple sclerosis: A case report of prolonged misdiagnosis. Applied neuropsychology Adult. 2017;24(3):294-297. doi:10.1080/23279095.2016.1214132
16. Zhu S, Nahas SJ. CADASIL: Imaging characteristics and clinical correlation. Curr Pain Headache Rep. 2016;20(10):57. doi:10.1007/s11916-016-0584-6
17. Kalaria RN, Low WC, Oakley AE, Slade JY, Ince PG, Morris CM, et al. CADASIL and genetics of cerebral ischaemia. J Neural Transm Suppl. 2002;(63):75-90. doi:10.1007/978-3-7091-6137-1_5
18. O’Sullivan M, Jarosz JM, Martin RJ, Deasy N, Powell JF, Markus HS. MRI hyperintensities of the temporal lobe and external capsule in patients with CADASIL. Neurology. 2001;56(5):628-634. doi:10.1212/wnl.56.5.628
19. Khurshid S, Choi SH, Weng L-C, Wang EY, Trinquart L, Benjamin EJ, et al. Frequency of cardiac rhythm abnormalities in a half million adults. Circ ArrhythmElectrophysiol. 2018;11(7):e006273. doi:10.1161/CIRCEP.118.006273
20. Samuels MA. The brain–heart connection. Circulation. 2007;116(1):77-84. doi:10.1161/CIRCULATIONAHA. 106.678995
21. Cumurciuc R, Henry P, Gobron C, Vicaut E, Bousser MG, Chabriat H, et al. Electrocardiogram in cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy patients without any clinical evidence of coronary artery disease: a case-control study. Stroke. 2006;37(4):1100-1102. doi:10.1161/01.STR.0000209242.68844.20
22. Luxán G, D’Amato G, MacGrogan D, de la Pompa JL. Endocardial notch signaling in cardiac development and disease. Circ Res. 2016;118(1):e1-e18. doi:10.1161/CIRCRESAHA.115.305350
23. Rubin CB, Hahn V, Kobayashi T, Litwack A. A report of accelerated coronary artery disease associated with cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy. Case Rep Cardiol. 2015;2015:167513. doi:10.1155/2015/167513
24. Langer C, Adukauskaite A, Plank F, Feuchtner G, Cartes-Zumelzu F. Cerebral autosomal dominant arteriopathy (CADASIL) with cardiac involvement (ANOCA) and subcortical leukencephalopathy. J Cardiovasc Comput Tomogr. 2020;14(5):e1-e6. doi:10.1016/j.jcct.2018.08.005
25. Pettersen JA, Keith J, Gao F, Spence JD, Black SE. CADASIL accelerated by acute hypotension: Arterial and venous contribution to leukoaraiosis. Neurology. 2017;88(11):1077-1080. doi:10.1212/WNL.0000000000003717
Commentary: Babies die as congenital syphilis continues a decade-long surge across the U.S.
The data are shocking: Almost 35,000 U.S. syphilis cases by mid-July 2022 with the highest rates per/100,000 population in Nevada (n = 21), California (n = 19), and Mississippi (n = 16). Excluding Nevada, California, and Oklahoma, rates over 12/100,000 were concentrated in the southernmost U.S. states. Overall, the 2,268 congenital syphilis cases in U.S. children born in 2021 was a 6% increase over 2020, and a 680% increase over 2012. (Note: All 2021 data are not yet available because of public health STI resources being diverted to COVID-19 control.) A telling number is the 166 congenital syphilis deaths in babies born in 2021 – a 1,000% increase over 2012. Another concern is that 50% of U.S. counties reported at least one congenital syphilis case in 2019 – the last time frame from which county-specific data are available.
Syphilis afflicts the underserved and underprivileged more than other demographic groups, particularly when public health budgets are not adequate (funding for public health STI prevention/treatment efforts has lagged for more than a decade), and/or when public health emergencies such as the pandemic divert public health resources away from STI prevention/treatment efforts.
As pediatric care providers, we can help by heightening our vigilance and appropriately testing for and treating syphilis, particularly in newborns/infants, regardless of where we work. And we can advocate for increased public health STI funding allocation whenever possible. It is a smart economic move because it costs nearly 1,000 times more to manage congenital syphilis and its sequelae than to prevent or treat it.
Christopher J. Harrison, MD, is professor, University of Missouri Kansas City School of Medicine, department of medicine, infectious diseases section, Kansas City. He has no financial conflicts of interest.
The data are shocking: Almost 35,000 U.S. syphilis cases by mid-July 2022 with the highest rates per/100,000 population in Nevada (n = 21), California (n = 19), and Mississippi (n = 16). Excluding Nevada, California, and Oklahoma, rates over 12/100,000 were concentrated in the southernmost U.S. states. Overall, the 2,268 congenital syphilis cases in U.S. children born in 2021 was a 6% increase over 2020, and a 680% increase over 2012. (Note: All 2021 data are not yet available because of public health STI resources being diverted to COVID-19 control.) A telling number is the 166 congenital syphilis deaths in babies born in 2021 – a 1,000% increase over 2012. Another concern is that 50% of U.S. counties reported at least one congenital syphilis case in 2019 – the last time frame from which county-specific data are available.
Syphilis afflicts the underserved and underprivileged more than other demographic groups, particularly when public health budgets are not adequate (funding for public health STI prevention/treatment efforts has lagged for more than a decade), and/or when public health emergencies such as the pandemic divert public health resources away from STI prevention/treatment efforts.
As pediatric care providers, we can help by heightening our vigilance and appropriately testing for and treating syphilis, particularly in newborns/infants, regardless of where we work. And we can advocate for increased public health STI funding allocation whenever possible. It is a smart economic move because it costs nearly 1,000 times more to manage congenital syphilis and its sequelae than to prevent or treat it.
Christopher J. Harrison, MD, is professor, University of Missouri Kansas City School of Medicine, department of medicine, infectious diseases section, Kansas City. He has no financial conflicts of interest.
The data are shocking: Almost 35,000 U.S. syphilis cases by mid-July 2022 with the highest rates per/100,000 population in Nevada (n = 21), California (n = 19), and Mississippi (n = 16). Excluding Nevada, California, and Oklahoma, rates over 12/100,000 were concentrated in the southernmost U.S. states. Overall, the 2,268 congenital syphilis cases in U.S. children born in 2021 was a 6% increase over 2020, and a 680% increase over 2012. (Note: All 2021 data are not yet available because of public health STI resources being diverted to COVID-19 control.) A telling number is the 166 congenital syphilis deaths in babies born in 2021 – a 1,000% increase over 2012. Another concern is that 50% of U.S. counties reported at least one congenital syphilis case in 2019 – the last time frame from which county-specific data are available.
Syphilis afflicts the underserved and underprivileged more than other demographic groups, particularly when public health budgets are not adequate (funding for public health STI prevention/treatment efforts has lagged for more than a decade), and/or when public health emergencies such as the pandemic divert public health resources away from STI prevention/treatment efforts.
As pediatric care providers, we can help by heightening our vigilance and appropriately testing for and treating syphilis, particularly in newborns/infants, regardless of where we work. And we can advocate for increased public health STI funding allocation whenever possible. It is a smart economic move because it costs nearly 1,000 times more to manage congenital syphilis and its sequelae than to prevent or treat it.
Christopher J. Harrison, MD, is professor, University of Missouri Kansas City School of Medicine, department of medicine, infectious diseases section, Kansas City. He has no financial conflicts of interest.
The Enigma of MS Etiology: Find an Answer, Ask More Questions
Dr. Obeidat is an Assistant Professor in the Department of Neurology,
Neuroimmunology and Multiple Sclerosis and is the Founding Director of the Neuroimmunology and MS Fellowship Program at The Medical College of Wisconsin in Milwaukee, WI.
Dr. Obeidat reports having consulted with/spoken for/conducted clinical trials for AbbVie, Alexion, Atara Biotherapeutics, Biogen, Bristol-Myers Squibb, Central, Celgene, EMD Serono, GW Pharmaceuticals, Genentech, Horizon, Jazz Pharma, Novartis, Sanofi/Genzyme, TG Therapeutics, and Viela Bio. Dr. Obeidat serves on the editorial board of the International Journal of MS Care, the advisory board of Americas Committee for Treatment and Research in Multiple Sclerosis (ACTRIMS®), and the Board of Governors of the Consortium of Multiple Sclerosis Centers.
“Could multiple sclerosis be the direct result of a yet-to-be identified infection?” asked John Kurtzke, MD, of his audience during his Grand Rounds entitled “Epidemiology and the Cause of Multiple Sclerosis” at the National Institute of Health (NIH) in 2015.1 As a pioneer of neuroepidemiology, Dr Kurtzke had long considered that infection was a key step in the development of multiple sclerosis (MS), the most disabling nontraumatic neurologic disease in young adults. He and others, from the 1970s onwards, described disease outbreaks and patterns of disease distribution in various countries during periods of immigration and even wartime.1,2
A half century later and Dr Kurtzke’s question has a possible answer: The Epstein-Barr virus (EBV), a gamma herpes virus responsible for mononucleosis that has been long suspected as a link to the development of MS,3 is now more than a virus of interest. A longitudinal study pinpointed the virus’ almost universal presence in patients with MS.4 Not everyone who develops mononucleosis from EBV develops MS, but most people become infected with EBV at some point in their lives. EBV is highly prevalent in the general population, with some studies suggesting that more than 90% of people worldwide are infected with EBV.5 While the discovery raises many questions about MS etiology and disease progression, it also allows discussion on more therapeutic possibilities.
MS Numbers
With nearly 1 million people in the United States living with MS, and over 2.5 million people worldwide, MS has been the subject of numerous investigations.2 Its complexity and heterogeneity have gained significant interest from the scientific community, including from Dr. Kurtzke, who passed away the same year as his NIH presentation.1
Several investigators over the years have attempted to link viral infections to MS,3 especially EBV. In February 2022, a longitudinal study spanning 20 years shed additional light on this longstanding, controversial, heavily researched potential association.4 The collaborative group of investigators used a database of serial blood samples from more than 10 million active US military personnel to investigate the association between EBV and MS and to learn whether EBV infection preceded the development of MS.
Out of 801 persons with a documented diagnosis of MS in this study, only 1 case occurred in a person who tested negative for EBV infection.4 At baseline, 35 people with MS tested negative for EBV infection, but after receiving their MS diagnosis, they tested positive for the virus, suggesting a causal relationship between EBV and MS. The study also showed that the levels of serum neurofilament light (sNfL), a nonspecific biomarker indicative of neuroaxonal injury or degeneration, increased post-EBV infection in the sera of initially EBV-negative patients with MS.4 This raises the question again: Why do only a small subset of people with EBV develop MS?
Facts and Questions
MS is a complex, heterogeneous disease whose development would require more than a human gamma herpesvirus to directly trigger its life-long, unrelenting immune dysregulation in select people. The complexity, which has been reviewed in detail, 6 suggests a role for interaction between host genetics, vitamin D levels, vitamin D receptors, and a specific protein of EBV, called Epstein-Barr nuclear antigen 1 (EBNA1).6 A recent publication described the potential for molecular mimicry (also known as cross-reactivity) between (EBNA1)6 and a specific cell adhesion molecule expressed in glial cells of the central nervous system (GlialCAM).7
But this molecular mimicry is not sufficient to explain the EBV/MS relationship. Even in monozygotic twins, the concordance rate is around 25%, leaving three-fourths of the risk of MS to the environment and genetics-environment interaction.8 The chances for monozygotic twins to both be infected with EBV are estimated at much more than 25%, given the epidemiology of EBV. Thus, EBV infection combined with specific genetic susceptibility remains insufficient to explain the observed epidemiology of MS.
More Factors
Several investigators have reported on the association between low vitamin D levels and MS. Low vitamin D is thought to affect both disease development and inflammatory activity.9 So, does MS result from the interaction between EBV, genetics, and low vitamin D? This interaction is plausible and is supported by several lines of evidence.6 However, even the interaction between these 3 factors remains insufficient to explain the complexity of MS pathogenesis.
An Unknown Mechanism
The triggering mechanism from EBV into MS remains an open question, and further research is needed. Nevertheless, if infection by EBV is a necessary, yet insufficient, step for MS to occur, can we prevent MS simply by preventing the primary EBV infection via vaccination? If so, what considerations must we make? For example, if EBV infection triggers MS via the transformation of infected memory B cells, thereby triggering an autoreactive immune response, then a vaccine capable of preventing the primary EBV infection could reduce the number of new MS cases, or ambitiously eradicate the disease itself. On the other hand, if molecular mimicry is the leading mechanism by which EBV infection triggers MS, then an EBV vaccine may have detrimental effects and theoretically trigger MS in susceptible individuals. Thus, it is of utmost importance to clearly understand how EBV infection contributes to MS pathogenesis to evaluate potential EBV vaccine candidates.
Treatment Possibilities
What are some possible clinical implications for the EBV-MS story for people living with MS? An important consideration is whether latent EBV infection contributes to the disease process over time, or if the infection is just an initial step that triggers numerous events that then operate independently from the virus. Suppose latent EBV infection contributes to the ongoing inflammatory and neurodegenerative changes in MS. In that case, some may consider using antiviral therapies as possible therapeutics for MS (possibly as an add-on, in combination with existing or future classes of disease-modifying therapies). Other interventions targeted at infected, transformed, or autoreactive B cells may bring us closer to precision medicine in MS. On the other hand, if the role of EBV is mainly to kick off MS, then further interventions targeted at the virus may not prove to be clinically effective.
Finally, the recent evidence of possible molecular mimicry to support causality between EBV infection and MS needs further investigation to elucidate how a common, ubiquitous infection kicks off MS in selected individuals. Additionally, the complex interactions between EBV, the human immune system, and genetics, as well as with other factors such as emotional stress,10 low sun exposure,11 and other, yet-to-be-identified environmental factors, may add more pieces to the complex etiology puzzle of MS and perhaps allow for effective interventions to help reduce the incidence of MS and even modulate disease progression.
References
1. Obeidat AZ. John F. Kurtzke, MD (1926-2015). Neuroepidemiology. 2016;46(2):118-119.
2. Nathanson N, Miller A. Epidemiology of multiple sclerosis: critique of the evidence for a viral etiology. Am J Epidemiol. 1978;107(6):451-461.
3. Donati D. Viral infections and multiple sclerosis. Drug Discov Today Dis Models. 2020;32:27-33.
4. Bjornevik K, Cortese M, Healy BC, et al. Longitudinal analysis reveals high prevalence of Epstein-Barr virus associated with multiple sclerosis. Science. 2022;375(6578):296-301.
5. Smatti MK, Al-Sadeq DW, Ali NH, Pintus G, Abou-Saleh H, Nasrallah GK. Epstein-Barr virus epidemiology, serology, and genetic variability of LMP-1 oncogene among healthy population: an update. Front Oncol. 2018;8:211.
6. Marcucci SB, Obeidat AZ. EBNA1, EBNA2, and EBNA3 link Epstein-Barr virus and hypovitaminosis D in multiple sclerosis pathogenesis. J Neuroimmunol. 2020;339:577116.
7. Lanz, TV, Brewer RC, Ho PP, et al. Clonally expanded B cells in multiple sclerosis bind EBV EBNA1 and GlialCAM. Nature. 2022;603(7900):321-327.
8. Mumford CJ, Wood NW, Kellar-Wood H, Thorpe JW, Miller DH, Compston DA. The British Isles survey of multiple sclerosis in twins. Neurology. 1994;44(1):11-15.
9. Fitzgerald KC, Munger KL, Köchert K, et al. Association of vitamin D levels with multiple sclerosis activity and progression in patients receiving interferon beta-1b. JAMA Neurol. 2015;72(12):1458-1465.
10. Mohr DC, Hart SL, Julian L, Cox D, Pelletier D. Association between stressful life events and exacerbation in multiple sclerosis: a meta-analysis. BMJ. 2004;328(7442):731.
11. Hedström AK, Huang J, Brenner N, et al. Low sun exposure acts synergistically with high Epstein-Barr nuclear antigen 1 (EBNA-1) antibody levels in multiple sclerosis etiology. Eur J Neurol. 2021;28(12):4146-4152.
Dr. Obeidat is an Assistant Professor in the Department of Neurology,
Neuroimmunology and Multiple Sclerosis and is the Founding Director of the Neuroimmunology and MS Fellowship Program at The Medical College of Wisconsin in Milwaukee, WI.
Dr. Obeidat reports having consulted with/spoken for/conducted clinical trials for AbbVie, Alexion, Atara Biotherapeutics, Biogen, Bristol-Myers Squibb, Central, Celgene, EMD Serono, GW Pharmaceuticals, Genentech, Horizon, Jazz Pharma, Novartis, Sanofi/Genzyme, TG Therapeutics, and Viela Bio. Dr. Obeidat serves on the editorial board of the International Journal of MS Care, the advisory board of Americas Committee for Treatment and Research in Multiple Sclerosis (ACTRIMS®), and the Board of Governors of the Consortium of Multiple Sclerosis Centers.
“Could multiple sclerosis be the direct result of a yet-to-be identified infection?” asked John Kurtzke, MD, of his audience during his Grand Rounds entitled “Epidemiology and the Cause of Multiple Sclerosis” at the National Institute of Health (NIH) in 2015.1 As a pioneer of neuroepidemiology, Dr Kurtzke had long considered that infection was a key step in the development of multiple sclerosis (MS), the most disabling nontraumatic neurologic disease in young adults. He and others, from the 1970s onwards, described disease outbreaks and patterns of disease distribution in various countries during periods of immigration and even wartime.1,2
A half century later and Dr Kurtzke’s question has a possible answer: The Epstein-Barr virus (EBV), a gamma herpes virus responsible for mononucleosis that has been long suspected as a link to the development of MS,3 is now more than a virus of interest. A longitudinal study pinpointed the virus’ almost universal presence in patients with MS.4 Not everyone who develops mononucleosis from EBV develops MS, but most people become infected with EBV at some point in their lives. EBV is highly prevalent in the general population, with some studies suggesting that more than 90% of people worldwide are infected with EBV.5 While the discovery raises many questions about MS etiology and disease progression, it also allows discussion on more therapeutic possibilities.
MS Numbers
With nearly 1 million people in the United States living with MS, and over 2.5 million people worldwide, MS has been the subject of numerous investigations.2 Its complexity and heterogeneity have gained significant interest from the scientific community, including from Dr. Kurtzke, who passed away the same year as his NIH presentation.1
Several investigators over the years have attempted to link viral infections to MS,3 especially EBV. In February 2022, a longitudinal study spanning 20 years shed additional light on this longstanding, controversial, heavily researched potential association.4 The collaborative group of investigators used a database of serial blood samples from more than 10 million active US military personnel to investigate the association between EBV and MS and to learn whether EBV infection preceded the development of MS.
Out of 801 persons with a documented diagnosis of MS in this study, only 1 case occurred in a person who tested negative for EBV infection.4 At baseline, 35 people with MS tested negative for EBV infection, but after receiving their MS diagnosis, they tested positive for the virus, suggesting a causal relationship between EBV and MS. The study also showed that the levels of serum neurofilament light (sNfL), a nonspecific biomarker indicative of neuroaxonal injury or degeneration, increased post-EBV infection in the sera of initially EBV-negative patients with MS.4 This raises the question again: Why do only a small subset of people with EBV develop MS?
Facts and Questions
MS is a complex, heterogeneous disease whose development would require more than a human gamma herpesvirus to directly trigger its life-long, unrelenting immune dysregulation in select people. The complexity, which has been reviewed in detail, 6 suggests a role for interaction between host genetics, vitamin D levels, vitamin D receptors, and a specific protein of EBV, called Epstein-Barr nuclear antigen 1 (EBNA1).6 A recent publication described the potential for molecular mimicry (also known as cross-reactivity) between (EBNA1)6 and a specific cell adhesion molecule expressed in glial cells of the central nervous system (GlialCAM).7
But this molecular mimicry is not sufficient to explain the EBV/MS relationship. Even in monozygotic twins, the concordance rate is around 25%, leaving three-fourths of the risk of MS to the environment and genetics-environment interaction.8 The chances for monozygotic twins to both be infected with EBV are estimated at much more than 25%, given the epidemiology of EBV. Thus, EBV infection combined with specific genetic susceptibility remains insufficient to explain the observed epidemiology of MS.
More Factors
Several investigators have reported on the association between low vitamin D levels and MS. Low vitamin D is thought to affect both disease development and inflammatory activity.9 So, does MS result from the interaction between EBV, genetics, and low vitamin D? This interaction is plausible and is supported by several lines of evidence.6 However, even the interaction between these 3 factors remains insufficient to explain the complexity of MS pathogenesis.
An Unknown Mechanism
The triggering mechanism from EBV into MS remains an open question, and further research is needed. Nevertheless, if infection by EBV is a necessary, yet insufficient, step for MS to occur, can we prevent MS simply by preventing the primary EBV infection via vaccination? If so, what considerations must we make? For example, if EBV infection triggers MS via the transformation of infected memory B cells, thereby triggering an autoreactive immune response, then a vaccine capable of preventing the primary EBV infection could reduce the number of new MS cases, or ambitiously eradicate the disease itself. On the other hand, if molecular mimicry is the leading mechanism by which EBV infection triggers MS, then an EBV vaccine may have detrimental effects and theoretically trigger MS in susceptible individuals. Thus, it is of utmost importance to clearly understand how EBV infection contributes to MS pathogenesis to evaluate potential EBV vaccine candidates.
Treatment Possibilities
What are some possible clinical implications for the EBV-MS story for people living with MS? An important consideration is whether latent EBV infection contributes to the disease process over time, or if the infection is just an initial step that triggers numerous events that then operate independently from the virus. Suppose latent EBV infection contributes to the ongoing inflammatory and neurodegenerative changes in MS. In that case, some may consider using antiviral therapies as possible therapeutics for MS (possibly as an add-on, in combination with existing or future classes of disease-modifying therapies). Other interventions targeted at infected, transformed, or autoreactive B cells may bring us closer to precision medicine in MS. On the other hand, if the role of EBV is mainly to kick off MS, then further interventions targeted at the virus may not prove to be clinically effective.
Finally, the recent evidence of possible molecular mimicry to support causality between EBV infection and MS needs further investigation to elucidate how a common, ubiquitous infection kicks off MS in selected individuals. Additionally, the complex interactions between EBV, the human immune system, and genetics, as well as with other factors such as emotional stress,10 low sun exposure,11 and other, yet-to-be-identified environmental factors, may add more pieces to the complex etiology puzzle of MS and perhaps allow for effective interventions to help reduce the incidence of MS and even modulate disease progression.
Dr. Obeidat is an Assistant Professor in the Department of Neurology,
Neuroimmunology and Multiple Sclerosis and is the Founding Director of the Neuroimmunology and MS Fellowship Program at The Medical College of Wisconsin in Milwaukee, WI.
Dr. Obeidat reports having consulted with/spoken for/conducted clinical trials for AbbVie, Alexion, Atara Biotherapeutics, Biogen, Bristol-Myers Squibb, Central, Celgene, EMD Serono, GW Pharmaceuticals, Genentech, Horizon, Jazz Pharma, Novartis, Sanofi/Genzyme, TG Therapeutics, and Viela Bio. Dr. Obeidat serves on the editorial board of the International Journal of MS Care, the advisory board of Americas Committee for Treatment and Research in Multiple Sclerosis (ACTRIMS®), and the Board of Governors of the Consortium of Multiple Sclerosis Centers.
“Could multiple sclerosis be the direct result of a yet-to-be identified infection?” asked John Kurtzke, MD, of his audience during his Grand Rounds entitled “Epidemiology and the Cause of Multiple Sclerosis” at the National Institute of Health (NIH) in 2015.1 As a pioneer of neuroepidemiology, Dr Kurtzke had long considered that infection was a key step in the development of multiple sclerosis (MS), the most disabling nontraumatic neurologic disease in young adults. He and others, from the 1970s onwards, described disease outbreaks and patterns of disease distribution in various countries during periods of immigration and even wartime.1,2
A half century later and Dr Kurtzke’s question has a possible answer: The Epstein-Barr virus (EBV), a gamma herpes virus responsible for mononucleosis that has been long suspected as a link to the development of MS,3 is now more than a virus of interest. A longitudinal study pinpointed the virus’ almost universal presence in patients with MS.4 Not everyone who develops mononucleosis from EBV develops MS, but most people become infected with EBV at some point in their lives. EBV is highly prevalent in the general population, with some studies suggesting that more than 90% of people worldwide are infected with EBV.5 While the discovery raises many questions about MS etiology and disease progression, it also allows discussion on more therapeutic possibilities.
MS Numbers
With nearly 1 million people in the United States living with MS, and over 2.5 million people worldwide, MS has been the subject of numerous investigations.2 Its complexity and heterogeneity have gained significant interest from the scientific community, including from Dr. Kurtzke, who passed away the same year as his NIH presentation.1
Several investigators over the years have attempted to link viral infections to MS,3 especially EBV. In February 2022, a longitudinal study spanning 20 years shed additional light on this longstanding, controversial, heavily researched potential association.4 The collaborative group of investigators used a database of serial blood samples from more than 10 million active US military personnel to investigate the association between EBV and MS and to learn whether EBV infection preceded the development of MS.
Out of 801 persons with a documented diagnosis of MS in this study, only 1 case occurred in a person who tested negative for EBV infection.4 At baseline, 35 people with MS tested negative for EBV infection, but after receiving their MS diagnosis, they tested positive for the virus, suggesting a causal relationship between EBV and MS. The study also showed that the levels of serum neurofilament light (sNfL), a nonspecific biomarker indicative of neuroaxonal injury or degeneration, increased post-EBV infection in the sera of initially EBV-negative patients with MS.4 This raises the question again: Why do only a small subset of people with EBV develop MS?
Facts and Questions
MS is a complex, heterogeneous disease whose development would require more than a human gamma herpesvirus to directly trigger its life-long, unrelenting immune dysregulation in select people. The complexity, which has been reviewed in detail, 6 suggests a role for interaction between host genetics, vitamin D levels, vitamin D receptors, and a specific protein of EBV, called Epstein-Barr nuclear antigen 1 (EBNA1).6 A recent publication described the potential for molecular mimicry (also known as cross-reactivity) between (EBNA1)6 and a specific cell adhesion molecule expressed in glial cells of the central nervous system (GlialCAM).7
But this molecular mimicry is not sufficient to explain the EBV/MS relationship. Even in monozygotic twins, the concordance rate is around 25%, leaving three-fourths of the risk of MS to the environment and genetics-environment interaction.8 The chances for monozygotic twins to both be infected with EBV are estimated at much more than 25%, given the epidemiology of EBV. Thus, EBV infection combined with specific genetic susceptibility remains insufficient to explain the observed epidemiology of MS.
More Factors
Several investigators have reported on the association between low vitamin D levels and MS. Low vitamin D is thought to affect both disease development and inflammatory activity.9 So, does MS result from the interaction between EBV, genetics, and low vitamin D? This interaction is plausible and is supported by several lines of evidence.6 However, even the interaction between these 3 factors remains insufficient to explain the complexity of MS pathogenesis.
An Unknown Mechanism
The triggering mechanism from EBV into MS remains an open question, and further research is needed. Nevertheless, if infection by EBV is a necessary, yet insufficient, step for MS to occur, can we prevent MS simply by preventing the primary EBV infection via vaccination? If so, what considerations must we make? For example, if EBV infection triggers MS via the transformation of infected memory B cells, thereby triggering an autoreactive immune response, then a vaccine capable of preventing the primary EBV infection could reduce the number of new MS cases, or ambitiously eradicate the disease itself. On the other hand, if molecular mimicry is the leading mechanism by which EBV infection triggers MS, then an EBV vaccine may have detrimental effects and theoretically trigger MS in susceptible individuals. Thus, it is of utmost importance to clearly understand how EBV infection contributes to MS pathogenesis to evaluate potential EBV vaccine candidates.
Treatment Possibilities
What are some possible clinical implications for the EBV-MS story for people living with MS? An important consideration is whether latent EBV infection contributes to the disease process over time, or if the infection is just an initial step that triggers numerous events that then operate independently from the virus. Suppose latent EBV infection contributes to the ongoing inflammatory and neurodegenerative changes in MS. In that case, some may consider using antiviral therapies as possible therapeutics for MS (possibly as an add-on, in combination with existing or future classes of disease-modifying therapies). Other interventions targeted at infected, transformed, or autoreactive B cells may bring us closer to precision medicine in MS. On the other hand, if the role of EBV is mainly to kick off MS, then further interventions targeted at the virus may not prove to be clinically effective.
Finally, the recent evidence of possible molecular mimicry to support causality between EBV infection and MS needs further investigation to elucidate how a common, ubiquitous infection kicks off MS in selected individuals. Additionally, the complex interactions between EBV, the human immune system, and genetics, as well as with other factors such as emotional stress,10 low sun exposure,11 and other, yet-to-be-identified environmental factors, may add more pieces to the complex etiology puzzle of MS and perhaps allow for effective interventions to help reduce the incidence of MS and even modulate disease progression.
References
1. Obeidat AZ. John F. Kurtzke, MD (1926-2015). Neuroepidemiology. 2016;46(2):118-119.
2. Nathanson N, Miller A. Epidemiology of multiple sclerosis: critique of the evidence for a viral etiology. Am J Epidemiol. 1978;107(6):451-461.
3. Donati D. Viral infections and multiple sclerosis. Drug Discov Today Dis Models. 2020;32:27-33.
4. Bjornevik K, Cortese M, Healy BC, et al. Longitudinal analysis reveals high prevalence of Epstein-Barr virus associated with multiple sclerosis. Science. 2022;375(6578):296-301.
5. Smatti MK, Al-Sadeq DW, Ali NH, Pintus G, Abou-Saleh H, Nasrallah GK. Epstein-Barr virus epidemiology, serology, and genetic variability of LMP-1 oncogene among healthy population: an update. Front Oncol. 2018;8:211.
6. Marcucci SB, Obeidat AZ. EBNA1, EBNA2, and EBNA3 link Epstein-Barr virus and hypovitaminosis D in multiple sclerosis pathogenesis. J Neuroimmunol. 2020;339:577116.
7. Lanz, TV, Brewer RC, Ho PP, et al. Clonally expanded B cells in multiple sclerosis bind EBV EBNA1 and GlialCAM. Nature. 2022;603(7900):321-327.
8. Mumford CJ, Wood NW, Kellar-Wood H, Thorpe JW, Miller DH, Compston DA. The British Isles survey of multiple sclerosis in twins. Neurology. 1994;44(1):11-15.
9. Fitzgerald KC, Munger KL, Köchert K, et al. Association of vitamin D levels with multiple sclerosis activity and progression in patients receiving interferon beta-1b. JAMA Neurol. 2015;72(12):1458-1465.
10. Mohr DC, Hart SL, Julian L, Cox D, Pelletier D. Association between stressful life events and exacerbation in multiple sclerosis: a meta-analysis. BMJ. 2004;328(7442):731.
11. Hedström AK, Huang J, Brenner N, et al. Low sun exposure acts synergistically with high Epstein-Barr nuclear antigen 1 (EBNA-1) antibody levels in multiple sclerosis etiology. Eur J Neurol. 2021;28(12):4146-4152.
References
1. Obeidat AZ. John F. Kurtzke, MD (1926-2015). Neuroepidemiology. 2016;46(2):118-119.
2. Nathanson N, Miller A. Epidemiology of multiple sclerosis: critique of the evidence for a viral etiology. Am J Epidemiol. 1978;107(6):451-461.
3. Donati D. Viral infections and multiple sclerosis. Drug Discov Today Dis Models. 2020;32:27-33.
4. Bjornevik K, Cortese M, Healy BC, et al. Longitudinal analysis reveals high prevalence of Epstein-Barr virus associated with multiple sclerosis. Science. 2022;375(6578):296-301.
5. Smatti MK, Al-Sadeq DW, Ali NH, Pintus G, Abou-Saleh H, Nasrallah GK. Epstein-Barr virus epidemiology, serology, and genetic variability of LMP-1 oncogene among healthy population: an update. Front Oncol. 2018;8:211.
6. Marcucci SB, Obeidat AZ. EBNA1, EBNA2, and EBNA3 link Epstein-Barr virus and hypovitaminosis D in multiple sclerosis pathogenesis. J Neuroimmunol. 2020;339:577116.
7. Lanz, TV, Brewer RC, Ho PP, et al. Clonally expanded B cells in multiple sclerosis bind EBV EBNA1 and GlialCAM. Nature. 2022;603(7900):321-327.
8. Mumford CJ, Wood NW, Kellar-Wood H, Thorpe JW, Miller DH, Compston DA. The British Isles survey of multiple sclerosis in twins. Neurology. 1994;44(1):11-15.
9. Fitzgerald KC, Munger KL, Köchert K, et al. Association of vitamin D levels with multiple sclerosis activity and progression in patients receiving interferon beta-1b. JAMA Neurol. 2015;72(12):1458-1465.
10. Mohr DC, Hart SL, Julian L, Cox D, Pelletier D. Association between stressful life events and exacerbation in multiple sclerosis: a meta-analysis. BMJ. 2004;328(7442):731.
11. Hedström AK, Huang J, Brenner N, et al. Low sun exposure acts synergistically with high Epstein-Barr nuclear antigen 1 (EBNA-1) antibody levels in multiple sclerosis etiology. Eur J Neurol. 2021;28(12):4146-4152.
Global incidence, deaths from CRC doubled over past 3 decades
, according to a new analysis of 204 countries.
However, trends in CRC incidence and deaths varied by age, region, and sex. Investigators expressed particular concern over the rising incidence rates among people younger than 50 and those living in low and middle sociodemographic index countries in Asia and Africa.
“These results provide comprehensive and comparable estimates that can inform efforts for equitable colorectal cancer control worldwide,” the authors write. However, “further research is required to understand the causes of the colorectal cancer burden in younger adults (aged less than 50 years) and the main risk factors, including obesity, physical inactivity, alcohol consumption, smoking, and an altered gut microbiome, that might have led to the rise in the colorectal cancer burden.”
The study was published online in the Lancet Gastroenterology and Hepatology.
CRC is the third leading cause of cancer deaths worldwide, but data on incidence and mortality by location, age, and sex remain less clear.
In the current Global Burden of Diseases, Injuries and Risk Factors Study, researchers evaluated age, sex, and geography-level estimates of CRC incidence, deaths, and disability-adjusted life-years (DALYs) from 204 countries between 1990 and 2019.
The authors found that cases of CRC increased by almost 2.6-fold over the 30-year study period, from 842,098 to 2.17 million. Deaths from CRC increased at a similar but slightly lower rate – rising 2.1-fold over the same period, from 518,126 to 1.09 million. DALYs also nearly doubled, going from 12.4 million in 1990 to 24.3 million in 2019.
In addition, the global age-standardized incidence rate increased from 22.2 to 26.7 per 100,000.
Overall, the age-standardized mortality rate decreased slightly, from 14.3 to 13.7 per 100,000; however, only high-middle and high sociodemographic index regions experienced a decrease; low and middle sociodemographic index regions experienced an increase. The age-standardized DALY rate also declined overall, from 308.5 per 100,000 in 1900 to 295.5 per 100,000 in 2019.
The authors further broke down CRC incidence and deaths by age, region, and sex.
Over the study period, males demonstrated greater increases in CRC incidence, deaths, and DALYs, compared with females. In 2019, the age-standardized CRC incidence rate was 1.5 times higher in males (33.1 vs 21.2 per 100,000), as was the age-standardized mortality rate (16.6 vs. 11.2 per 100,000). The age-standardized DALY rate showed a similar trend by sex – 360 versus 238 per 100,000 in males versus females.
Trends varied by age as well. CRC incidence rates increased the most in people aged 85 and older, followed by those between 20 and 49 years, while rates decreased for those between 50 and 80 years in high sociodemographic index countries.
Geography mattered too. China, the United States, and Japan demonstrated the highest number of new CRC cases across all ages and for both sexes in 2019 – 607,900 in China, 227,242 in the United States, and 160,211 in Japan.
In terms of mortality, China, the United States, and India had the highest CRC death counts: 261,777 in China, 84,026 in the United States, and 79,098 in India.
When it comes to age-standardized incidence rates, Taiwan, Monaco, and Andorra were at the top: Taiwan with 62 per 100,000 cases, Monaco with 60.7 per 100,000, and Andorra with 56.6 per 100,000.
On the other hand, Somalia, Niger, and Bangladesh had the lowest age-standardized incidence rates, 5 per 100,000 in Somalia and 5.6 per 100,000 in Niger and Bangladesh.
The highest age-standardized mortality rates occurred in Greenland, Brunei, and Hungary: 31.4 per 100,000 in Greenland, 30.3 per 100,000 in Brunei, and 28.6 per 100,000 in Hungary.
The relative contribution of different risk factors also varied by region. For example, in sub-Saharan Africa and lower-income countries in Asia, diets low in calcium and milk were the main CRC risk factors. In contrast, smoking and alcohol consumption were the main risk factors driving CRC in high-income regions.
Still, the reasons underlying some of these trends – such as the increasing incidence of CRC in patients under the age of 50 – remain uncertain. One possible explanation for this trend, the researchers point out, is the birth cohort effect, which suggests that those born in the second half of the 20th century are increasingly exposed to potentially modifiable risk factors, such as an unhealthy diet, obesity, and sedentary lifestyles.
Overall, the authors note that the data generated in this study provide an important resource for both patients and oncologists about current trends in incidence and mortality and where gaps in preventive measures may exist.
In particular, the authors conclude that “public health interventions for colorectal cancer awareness, screening, and prevention through containment of modifiable risk factors such as alcohol, smoking, unhealthy diet ... and obesity are key to stemming the tide of colorectal cancer worldwide.”
The study was funded by the Bill & Melinda Gates Foundation. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, according to a new analysis of 204 countries.
However, trends in CRC incidence and deaths varied by age, region, and sex. Investigators expressed particular concern over the rising incidence rates among people younger than 50 and those living in low and middle sociodemographic index countries in Asia and Africa.
“These results provide comprehensive and comparable estimates that can inform efforts for equitable colorectal cancer control worldwide,” the authors write. However, “further research is required to understand the causes of the colorectal cancer burden in younger adults (aged less than 50 years) and the main risk factors, including obesity, physical inactivity, alcohol consumption, smoking, and an altered gut microbiome, that might have led to the rise in the colorectal cancer burden.”
The study was published online in the Lancet Gastroenterology and Hepatology.
CRC is the third leading cause of cancer deaths worldwide, but data on incidence and mortality by location, age, and sex remain less clear.
In the current Global Burden of Diseases, Injuries and Risk Factors Study, researchers evaluated age, sex, and geography-level estimates of CRC incidence, deaths, and disability-adjusted life-years (DALYs) from 204 countries between 1990 and 2019.
The authors found that cases of CRC increased by almost 2.6-fold over the 30-year study period, from 842,098 to 2.17 million. Deaths from CRC increased at a similar but slightly lower rate – rising 2.1-fold over the same period, from 518,126 to 1.09 million. DALYs also nearly doubled, going from 12.4 million in 1990 to 24.3 million in 2019.
In addition, the global age-standardized incidence rate increased from 22.2 to 26.7 per 100,000.
Overall, the age-standardized mortality rate decreased slightly, from 14.3 to 13.7 per 100,000; however, only high-middle and high sociodemographic index regions experienced a decrease; low and middle sociodemographic index regions experienced an increase. The age-standardized DALY rate also declined overall, from 308.5 per 100,000 in 1900 to 295.5 per 100,000 in 2019.
The authors further broke down CRC incidence and deaths by age, region, and sex.
Over the study period, males demonstrated greater increases in CRC incidence, deaths, and DALYs, compared with females. In 2019, the age-standardized CRC incidence rate was 1.5 times higher in males (33.1 vs 21.2 per 100,000), as was the age-standardized mortality rate (16.6 vs. 11.2 per 100,000). The age-standardized DALY rate showed a similar trend by sex – 360 versus 238 per 100,000 in males versus females.
Trends varied by age as well. CRC incidence rates increased the most in people aged 85 and older, followed by those between 20 and 49 years, while rates decreased for those between 50 and 80 years in high sociodemographic index countries.
Geography mattered too. China, the United States, and Japan demonstrated the highest number of new CRC cases across all ages and for both sexes in 2019 – 607,900 in China, 227,242 in the United States, and 160,211 in Japan.
In terms of mortality, China, the United States, and India had the highest CRC death counts: 261,777 in China, 84,026 in the United States, and 79,098 in India.
When it comes to age-standardized incidence rates, Taiwan, Monaco, and Andorra were at the top: Taiwan with 62 per 100,000 cases, Monaco with 60.7 per 100,000, and Andorra with 56.6 per 100,000.
On the other hand, Somalia, Niger, and Bangladesh had the lowest age-standardized incidence rates, 5 per 100,000 in Somalia and 5.6 per 100,000 in Niger and Bangladesh.
The highest age-standardized mortality rates occurred in Greenland, Brunei, and Hungary: 31.4 per 100,000 in Greenland, 30.3 per 100,000 in Brunei, and 28.6 per 100,000 in Hungary.
The relative contribution of different risk factors also varied by region. For example, in sub-Saharan Africa and lower-income countries in Asia, diets low in calcium and milk were the main CRC risk factors. In contrast, smoking and alcohol consumption were the main risk factors driving CRC in high-income regions.
Still, the reasons underlying some of these trends – such as the increasing incidence of CRC in patients under the age of 50 – remain uncertain. One possible explanation for this trend, the researchers point out, is the birth cohort effect, which suggests that those born in the second half of the 20th century are increasingly exposed to potentially modifiable risk factors, such as an unhealthy diet, obesity, and sedentary lifestyles.
Overall, the authors note that the data generated in this study provide an important resource for both patients and oncologists about current trends in incidence and mortality and where gaps in preventive measures may exist.
In particular, the authors conclude that “public health interventions for colorectal cancer awareness, screening, and prevention through containment of modifiable risk factors such as alcohol, smoking, unhealthy diet ... and obesity are key to stemming the tide of colorectal cancer worldwide.”
The study was funded by the Bill & Melinda Gates Foundation. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, according to a new analysis of 204 countries.
However, trends in CRC incidence and deaths varied by age, region, and sex. Investigators expressed particular concern over the rising incidence rates among people younger than 50 and those living in low and middle sociodemographic index countries in Asia and Africa.
“These results provide comprehensive and comparable estimates that can inform efforts for equitable colorectal cancer control worldwide,” the authors write. However, “further research is required to understand the causes of the colorectal cancer burden in younger adults (aged less than 50 years) and the main risk factors, including obesity, physical inactivity, alcohol consumption, smoking, and an altered gut microbiome, that might have led to the rise in the colorectal cancer burden.”
The study was published online in the Lancet Gastroenterology and Hepatology.
CRC is the third leading cause of cancer deaths worldwide, but data on incidence and mortality by location, age, and sex remain less clear.
In the current Global Burden of Diseases, Injuries and Risk Factors Study, researchers evaluated age, sex, and geography-level estimates of CRC incidence, deaths, and disability-adjusted life-years (DALYs) from 204 countries between 1990 and 2019.
The authors found that cases of CRC increased by almost 2.6-fold over the 30-year study period, from 842,098 to 2.17 million. Deaths from CRC increased at a similar but slightly lower rate – rising 2.1-fold over the same period, from 518,126 to 1.09 million. DALYs also nearly doubled, going from 12.4 million in 1990 to 24.3 million in 2019.
In addition, the global age-standardized incidence rate increased from 22.2 to 26.7 per 100,000.
Overall, the age-standardized mortality rate decreased slightly, from 14.3 to 13.7 per 100,000; however, only high-middle and high sociodemographic index regions experienced a decrease; low and middle sociodemographic index regions experienced an increase. The age-standardized DALY rate also declined overall, from 308.5 per 100,000 in 1900 to 295.5 per 100,000 in 2019.
The authors further broke down CRC incidence and deaths by age, region, and sex.
Over the study period, males demonstrated greater increases in CRC incidence, deaths, and DALYs, compared with females. In 2019, the age-standardized CRC incidence rate was 1.5 times higher in males (33.1 vs 21.2 per 100,000), as was the age-standardized mortality rate (16.6 vs. 11.2 per 100,000). The age-standardized DALY rate showed a similar trend by sex – 360 versus 238 per 100,000 in males versus females.
Trends varied by age as well. CRC incidence rates increased the most in people aged 85 and older, followed by those between 20 and 49 years, while rates decreased for those between 50 and 80 years in high sociodemographic index countries.
Geography mattered too. China, the United States, and Japan demonstrated the highest number of new CRC cases across all ages and for both sexes in 2019 – 607,900 in China, 227,242 in the United States, and 160,211 in Japan.
In terms of mortality, China, the United States, and India had the highest CRC death counts: 261,777 in China, 84,026 in the United States, and 79,098 in India.
When it comes to age-standardized incidence rates, Taiwan, Monaco, and Andorra were at the top: Taiwan with 62 per 100,000 cases, Monaco with 60.7 per 100,000, and Andorra with 56.6 per 100,000.
On the other hand, Somalia, Niger, and Bangladesh had the lowest age-standardized incidence rates, 5 per 100,000 in Somalia and 5.6 per 100,000 in Niger and Bangladesh.
The highest age-standardized mortality rates occurred in Greenland, Brunei, and Hungary: 31.4 per 100,000 in Greenland, 30.3 per 100,000 in Brunei, and 28.6 per 100,000 in Hungary.
The relative contribution of different risk factors also varied by region. For example, in sub-Saharan Africa and lower-income countries in Asia, diets low in calcium and milk were the main CRC risk factors. In contrast, smoking and alcohol consumption were the main risk factors driving CRC in high-income regions.
Still, the reasons underlying some of these trends – such as the increasing incidence of CRC in patients under the age of 50 – remain uncertain. One possible explanation for this trend, the researchers point out, is the birth cohort effect, which suggests that those born in the second half of the 20th century are increasingly exposed to potentially modifiable risk factors, such as an unhealthy diet, obesity, and sedentary lifestyles.
Overall, the authors note that the data generated in this study provide an important resource for both patients and oncologists about current trends in incidence and mortality and where gaps in preventive measures may exist.
In particular, the authors conclude that “public health interventions for colorectal cancer awareness, screening, and prevention through containment of modifiable risk factors such as alcohol, smoking, unhealthy diet ... and obesity are key to stemming the tide of colorectal cancer worldwide.”
The study was funded by the Bill & Melinda Gates Foundation. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE LANCET GASTROENTEROLOGY AND HEPATOLOGY
USPSTF recommends for the first time that kids 8 and older get screened for anxiety
The U.S. Preventive Services Task Force on Apr. 12 posted draft recommendations on screening for depression and anxiety in children and adolescents.
For the first time, the USPSTF is recommending screening children ages 8 and older for anxiety.
It also recommended screening children ages 12 and older for depression, which was consistent with the USPSTF’s prior recommendations on the topic.
These B-grade draft recommendations are for children and teens who are not showing signs or symptoms of these conditions. The task force emphasized that anyone who has concerns about or shows signs of these conditions should be connected to care.
Task force member Martha Kubik, PhD, RN, a professor with George Mason University, Fairfax, Va, said in a statement: “Fortunately, we found that screening older children for anxiety and depression is effective in identifying these conditions so children and teens can be connected to the support they need.”
The group cited in its recommendation on anxiety the 2018-2019 National Survey of Children’s Health, which found that 7.8% of children and adolescents ages 3-17 years had a current anxiety disorder. It also noted that the National Survey on LGBTQ Youth Mental Health found that 72% of LGBTQ youth and 77% of transgender and nonbinary youth described general anxiety disorder symptoms.
“Anxiety disorders in childhood and adolescence are associated with an increased likelihood of a future anxiety disorder or depression,” the task force authors wrote.
They highlighted that “the prevalence of anxiety in Black youth may be evolving.” Previously, studies had suggested that young Black people may have had lower rates of mental health disorders, compared with their White counterparts.
“However, recent cohorts of Black children or adolescents have reported a higher prevalence of anxiety disorders than in the past,” the authors wrote.
Joanna Quigley, MD, clinical associate professor and associate medical director for child & adolescent services at the University of Michigan, Ann Arbor, said in an interview she was not surprised the USPSTF recommended screening for anxiety starting at age 8.
That’s when parents and providers see anxiety disorders begin to present or become more problematic, she said.
“It’s also acknowledging the importance of prevention,” she said. “The sooner we can identify these challenges for kids, the sooner we can intervene and have better outcomes for that child across their lifespan.”
Screening gets providers and families in the habit of thinking about these concerns when a child or adolescent comes in for another kind of visit, Dr. Quigley said. Chest pains in a well-child check, for example, may trigger thoughts to consider anxiety later if the child is brought in for a cardiac check for chest pains.
“It creates a culture of awareness that is important as well,” Dr. Quigley said. “I think part of what the task force is trying to do is saying that identifying anxiety can be a precursor to what could turn out to be related to depression or related to ADHD and factors we think about when we think about suicide risk as well.
“We’re seeing an increase in suicide in the younger age group as well, which is a huge concern, “ she noted.
Dr. Quigley said, if these recommendations are adopted after the comment period, pediatricians and family practice providers will likely be doing most of the screening for anxiety, but there may also be a role for the screening in pediatric subspecialty care, such as those treating children with chronic illness and in specialized mental health care.
She added: “This builds on the national conversation going on about the mental health crisis, declared a national emergency in the fall. This deserves attention in continuing the momentum.”
Factors that may signal higher risk for depression
While the USPSTF recommends screening for major depressive disorder in all adolescents aged 12 years and older, the USPSTF notes that several risk factors might help identify those at higher risk.
Markers for higher risk include a combination of factors such as a family history of depression, prior episode of depression, and other mental health or behavioral problems.
“Other psychosocial risk factors include childhood abuse or neglect, exposure to traumatic events, bullying (either as perpetrators or as victims), adverse life events, early exposure to stress, maltreatment, and an insecure parental relationship,” the task force authors wrote.
There was limited evidence, however, on the benefits and harms of screening children younger than 8 for anxiety and screening kids younger than 12 for depression.
Not enough evidence for suicide risk screening
The authors of the recommendations acknowledged that, while suicide is a leading cause of death for older children and teens, evidence is still too sparse to make recommendations regarding screening for suicide risk in those without signs or symptoms at any age.
They also explained that evidence is lacking and inconsistent on the effectiveness of treatment (psychotherapy, pharmacotherapy, or collaborative care) for suicide risk in improving outcomes in children and adolescents.
Comments on the USPSTF recommendations may be submitted until May 9, 2022. The USPSTF topic leads review all comments, revise the draft recommendations, put them to a vote by the full task force, and then post the final versions to the website.
The task force authors and Dr. Quigley reported no financial disclosures.
The U.S. Preventive Services Task Force on Apr. 12 posted draft recommendations on screening for depression and anxiety in children and adolescents.
For the first time, the USPSTF is recommending screening children ages 8 and older for anxiety.
It also recommended screening children ages 12 and older for depression, which was consistent with the USPSTF’s prior recommendations on the topic.
These B-grade draft recommendations are for children and teens who are not showing signs or symptoms of these conditions. The task force emphasized that anyone who has concerns about or shows signs of these conditions should be connected to care.
Task force member Martha Kubik, PhD, RN, a professor with George Mason University, Fairfax, Va, said in a statement: “Fortunately, we found that screening older children for anxiety and depression is effective in identifying these conditions so children and teens can be connected to the support they need.”
The group cited in its recommendation on anxiety the 2018-2019 National Survey of Children’s Health, which found that 7.8% of children and adolescents ages 3-17 years had a current anxiety disorder. It also noted that the National Survey on LGBTQ Youth Mental Health found that 72% of LGBTQ youth and 77% of transgender and nonbinary youth described general anxiety disorder symptoms.
“Anxiety disorders in childhood and adolescence are associated with an increased likelihood of a future anxiety disorder or depression,” the task force authors wrote.
They highlighted that “the prevalence of anxiety in Black youth may be evolving.” Previously, studies had suggested that young Black people may have had lower rates of mental health disorders, compared with their White counterparts.
“However, recent cohorts of Black children or adolescents have reported a higher prevalence of anxiety disorders than in the past,” the authors wrote.
Joanna Quigley, MD, clinical associate professor and associate medical director for child & adolescent services at the University of Michigan, Ann Arbor, said in an interview she was not surprised the USPSTF recommended screening for anxiety starting at age 8.
That’s when parents and providers see anxiety disorders begin to present or become more problematic, she said.
“It’s also acknowledging the importance of prevention,” she said. “The sooner we can identify these challenges for kids, the sooner we can intervene and have better outcomes for that child across their lifespan.”
Screening gets providers and families in the habit of thinking about these concerns when a child or adolescent comes in for another kind of visit, Dr. Quigley said. Chest pains in a well-child check, for example, may trigger thoughts to consider anxiety later if the child is brought in for a cardiac check for chest pains.
“It creates a culture of awareness that is important as well,” Dr. Quigley said. “I think part of what the task force is trying to do is saying that identifying anxiety can be a precursor to what could turn out to be related to depression or related to ADHD and factors we think about when we think about suicide risk as well.
“We’re seeing an increase in suicide in the younger age group as well, which is a huge concern, “ she noted.
Dr. Quigley said, if these recommendations are adopted after the comment period, pediatricians and family practice providers will likely be doing most of the screening for anxiety, but there may also be a role for the screening in pediatric subspecialty care, such as those treating children with chronic illness and in specialized mental health care.
She added: “This builds on the national conversation going on about the mental health crisis, declared a national emergency in the fall. This deserves attention in continuing the momentum.”
Factors that may signal higher risk for depression
While the USPSTF recommends screening for major depressive disorder in all adolescents aged 12 years and older, the USPSTF notes that several risk factors might help identify those at higher risk.
Markers for higher risk include a combination of factors such as a family history of depression, prior episode of depression, and other mental health or behavioral problems.
“Other psychosocial risk factors include childhood abuse or neglect, exposure to traumatic events, bullying (either as perpetrators or as victims), adverse life events, early exposure to stress, maltreatment, and an insecure parental relationship,” the task force authors wrote.
There was limited evidence, however, on the benefits and harms of screening children younger than 8 for anxiety and screening kids younger than 12 for depression.
Not enough evidence for suicide risk screening
The authors of the recommendations acknowledged that, while suicide is a leading cause of death for older children and teens, evidence is still too sparse to make recommendations regarding screening for suicide risk in those without signs or symptoms at any age.
They also explained that evidence is lacking and inconsistent on the effectiveness of treatment (psychotherapy, pharmacotherapy, or collaborative care) for suicide risk in improving outcomes in children and adolescents.
Comments on the USPSTF recommendations may be submitted until May 9, 2022. The USPSTF topic leads review all comments, revise the draft recommendations, put them to a vote by the full task force, and then post the final versions to the website.
The task force authors and Dr. Quigley reported no financial disclosures.
The U.S. Preventive Services Task Force on Apr. 12 posted draft recommendations on screening for depression and anxiety in children and adolescents.
For the first time, the USPSTF is recommending screening children ages 8 and older for anxiety.
It also recommended screening children ages 12 and older for depression, which was consistent with the USPSTF’s prior recommendations on the topic.
These B-grade draft recommendations are for children and teens who are not showing signs or symptoms of these conditions. The task force emphasized that anyone who has concerns about or shows signs of these conditions should be connected to care.
Task force member Martha Kubik, PhD, RN, a professor with George Mason University, Fairfax, Va, said in a statement: “Fortunately, we found that screening older children for anxiety and depression is effective in identifying these conditions so children and teens can be connected to the support they need.”
The group cited in its recommendation on anxiety the 2018-2019 National Survey of Children’s Health, which found that 7.8% of children and adolescents ages 3-17 years had a current anxiety disorder. It also noted that the National Survey on LGBTQ Youth Mental Health found that 72% of LGBTQ youth and 77% of transgender and nonbinary youth described general anxiety disorder symptoms.
“Anxiety disorders in childhood and adolescence are associated with an increased likelihood of a future anxiety disorder or depression,” the task force authors wrote.
They highlighted that “the prevalence of anxiety in Black youth may be evolving.” Previously, studies had suggested that young Black people may have had lower rates of mental health disorders, compared with their White counterparts.
“However, recent cohorts of Black children or adolescents have reported a higher prevalence of anxiety disorders than in the past,” the authors wrote.
Joanna Quigley, MD, clinical associate professor and associate medical director for child & adolescent services at the University of Michigan, Ann Arbor, said in an interview she was not surprised the USPSTF recommended screening for anxiety starting at age 8.
That’s when parents and providers see anxiety disorders begin to present or become more problematic, she said.
“It’s also acknowledging the importance of prevention,” she said. “The sooner we can identify these challenges for kids, the sooner we can intervene and have better outcomes for that child across their lifespan.”
Screening gets providers and families in the habit of thinking about these concerns when a child or adolescent comes in for another kind of visit, Dr. Quigley said. Chest pains in a well-child check, for example, may trigger thoughts to consider anxiety later if the child is brought in for a cardiac check for chest pains.
“It creates a culture of awareness that is important as well,” Dr. Quigley said. “I think part of what the task force is trying to do is saying that identifying anxiety can be a precursor to what could turn out to be related to depression or related to ADHD and factors we think about when we think about suicide risk as well.
“We’re seeing an increase in suicide in the younger age group as well, which is a huge concern, “ she noted.
Dr. Quigley said, if these recommendations are adopted after the comment period, pediatricians and family practice providers will likely be doing most of the screening for anxiety, but there may also be a role for the screening in pediatric subspecialty care, such as those treating children with chronic illness and in specialized mental health care.
She added: “This builds on the national conversation going on about the mental health crisis, declared a national emergency in the fall. This deserves attention in continuing the momentum.”
Factors that may signal higher risk for depression
While the USPSTF recommends screening for major depressive disorder in all adolescents aged 12 years and older, the USPSTF notes that several risk factors might help identify those at higher risk.
Markers for higher risk include a combination of factors such as a family history of depression, prior episode of depression, and other mental health or behavioral problems.
“Other psychosocial risk factors include childhood abuse or neglect, exposure to traumatic events, bullying (either as perpetrators or as victims), adverse life events, early exposure to stress, maltreatment, and an insecure parental relationship,” the task force authors wrote.
There was limited evidence, however, on the benefits and harms of screening children younger than 8 for anxiety and screening kids younger than 12 for depression.
Not enough evidence for suicide risk screening
The authors of the recommendations acknowledged that, while suicide is a leading cause of death for older children and teens, evidence is still too sparse to make recommendations regarding screening for suicide risk in those without signs or symptoms at any age.
They also explained that evidence is lacking and inconsistent on the effectiveness of treatment (psychotherapy, pharmacotherapy, or collaborative care) for suicide risk in improving outcomes in children and adolescents.
Comments on the USPSTF recommendations may be submitted until May 9, 2022. The USPSTF topic leads review all comments, revise the draft recommendations, put them to a vote by the full task force, and then post the final versions to the website.
The task force authors and Dr. Quigley reported no financial disclosures.
A new target in schizophrenia treatment: Brain gamma oscillations
In a randomized, double-blind study that included two dozen men with schizophrenia, AUT00206, compared with placebo, increased the power of gamma oscillations, which were in turn associated with positive symptom scores.
The investigators note that targeting a potassium channel linked to brain gamma oscillations may offer a novel way to treat schizophrenia.
In addition, lead author Charles Large, PhD, chief executive officer, Autifony Therapeutics, Stevenage, United Kingdom, told this news organization that it may be “important” to study patients relatively early in their disease course. Participants in the current study were diagnosed less than 5 years previously.
Many previous trials in this area have failed, “and some of the questions were maybe the patients were sort of beyond the point in which you can actually make a difference,” Dr. Large said.
The findings were presented at the Congress of the Schizophrenia International Research Society (SIRS) 2022.
‘Opportunity to intervene’
Dr. Large noted that patients with chronic, long-term symptoms of schizophrenia have been treated with antipsychotics for decades, “and the pathology of that stage is then different.”
For the current study, the investigators hypothesized that the brain may be more “plastic” earlier on in the disease course, and so “maybe there’s an opportunity to intervene and make a change,” said Dr. Large.
In addition, patients with schizophrenia have abnormalities in both their resting state and induced and evolved gamma oscillations, which can include increased resting state power – and reduced power and “phase locking” to cyclical stimuli – the researchers note.
Previous studies have suggested such abnormalities are associated with dysfunction in parvalbumin-expressing interneurons (PVINs) found in cortical and subcortical circuits.
Moreover, Kv3.1 potassium channels expressed on PVINs are integral to establishing and maintaining fast-firing activity and to network synchronization across the brain. They may, therefore, offer a “potential therapeutic approach” for countering PVIN dysfunction, the investigators write.
To examine the impact of AUT00206, a novel Kv3.1/Kv3.2 positive neuromodulator, on resting state and induced gamma oscillations, they conducted a randomized, double-blind study in 24 men with schizophrenia who were aged 18-50 years.
Participants had been diagnosed less than 5 years previously and were stable on a maximum of two antipsychotic medications. They were randomly assigned 2:1 to a loading 2,000-mg dose of AUT00206 on day 1 and then 800 mg twice daily for 27 days or to placebo.
At baseline/day 1, and on a further 3 days over the treatment period, the men underwent resting-state electroencephalography, 40-Hz auditory steady-state response stimulation, and deviant and standard stimulation in an auditory oddball paradigm to assess resting state, induced, and evoked oscillations, respectively.
Positive associations
Results showed that early auditory gamma responses were increased at day 28 in patients who received AUT00206 but not in those who received placebo. The active drug was also associated with increases in the power of gamma oscillations from Day 5 in response to stimuli but not in phase locking.
There was also a significant positive association between frontal resting gamma power and baseline Positive and Negative Syndrome Scale (PANSS) positive symptom severity scores (r = 0.675; P < .001).
Moreover, changes in PANSS positive scores were significantly correlated with a decrease in frontal resting gamma power in patients treated with AUT00206 (r = 0.532; P = .05).
While a similar correlation was not found with placebo, the investigators note this “may be in part due to the low number” of individuals in the group.
They add that a larger study is now needed to confirm their findings and to “explore efficacy versus clinical symptoms.”
However, Dr. Large noted that participants in their next study will have fragile X syndrome.
He added the reason for this is “not because we’ve given up on schizophrenia – we feel that schizophrenia is a massive opportunity.”
Patients with schizophrenia are heterogeneous, both in terms of their clinical course and prior treatment. So it is “impossible” for a company of their size to take all of that into account in a single study, Dr. Large said.
In contrast, fragile X is “genetically homogenous,” and so it is possible to focus on the deficit and then translate the findings out into a “broader population.”
Preliminary but worth pursuing?
Commenting on the study, James M. McNally, PhD, assistant professor of psychiatry, Harvard Medical School, Boston, said the findings are “quite preliminary” and that the investigators provided “limited information as to how their findings were derived.”
Nevertheless, it is “nice to see that they observed a significant correlation between resting gamma and positive symptom severity at baseline [and] that the observed change in gamma correlates with change in PANSS scores,” said Dr. McNally, who was not involved with the research.
He added that the “idea of targeting Kv3.1 function to restore PV neuron/gamma activity is very interesting and worth pursuing.”
The study was funded by Autifony Therapeutics, of which Dr. Large is an employee.
A version of this article first appeared on Medscape.com.
In a randomized, double-blind study that included two dozen men with schizophrenia, AUT00206, compared with placebo, increased the power of gamma oscillations, which were in turn associated with positive symptom scores.
The investigators note that targeting a potassium channel linked to brain gamma oscillations may offer a novel way to treat schizophrenia.
In addition, lead author Charles Large, PhD, chief executive officer, Autifony Therapeutics, Stevenage, United Kingdom, told this news organization that it may be “important” to study patients relatively early in their disease course. Participants in the current study were diagnosed less than 5 years previously.
Many previous trials in this area have failed, “and some of the questions were maybe the patients were sort of beyond the point in which you can actually make a difference,” Dr. Large said.
The findings were presented at the Congress of the Schizophrenia International Research Society (SIRS) 2022.
‘Opportunity to intervene’
Dr. Large noted that patients with chronic, long-term symptoms of schizophrenia have been treated with antipsychotics for decades, “and the pathology of that stage is then different.”
For the current study, the investigators hypothesized that the brain may be more “plastic” earlier on in the disease course, and so “maybe there’s an opportunity to intervene and make a change,” said Dr. Large.
In addition, patients with schizophrenia have abnormalities in both their resting state and induced and evolved gamma oscillations, which can include increased resting state power – and reduced power and “phase locking” to cyclical stimuli – the researchers note.
Previous studies have suggested such abnormalities are associated with dysfunction in parvalbumin-expressing interneurons (PVINs) found in cortical and subcortical circuits.
Moreover, Kv3.1 potassium channels expressed on PVINs are integral to establishing and maintaining fast-firing activity and to network synchronization across the brain. They may, therefore, offer a “potential therapeutic approach” for countering PVIN dysfunction, the investigators write.
To examine the impact of AUT00206, a novel Kv3.1/Kv3.2 positive neuromodulator, on resting state and induced gamma oscillations, they conducted a randomized, double-blind study in 24 men with schizophrenia who were aged 18-50 years.
Participants had been diagnosed less than 5 years previously and were stable on a maximum of two antipsychotic medications. They were randomly assigned 2:1 to a loading 2,000-mg dose of AUT00206 on day 1 and then 800 mg twice daily for 27 days or to placebo.
At baseline/day 1, and on a further 3 days over the treatment period, the men underwent resting-state electroencephalography, 40-Hz auditory steady-state response stimulation, and deviant and standard stimulation in an auditory oddball paradigm to assess resting state, induced, and evoked oscillations, respectively.
Positive associations
Results showed that early auditory gamma responses were increased at day 28 in patients who received AUT00206 but not in those who received placebo. The active drug was also associated with increases in the power of gamma oscillations from Day 5 in response to stimuli but not in phase locking.
There was also a significant positive association between frontal resting gamma power and baseline Positive and Negative Syndrome Scale (PANSS) positive symptom severity scores (r = 0.675; P < .001).
Moreover, changes in PANSS positive scores were significantly correlated with a decrease in frontal resting gamma power in patients treated with AUT00206 (r = 0.532; P = .05).
While a similar correlation was not found with placebo, the investigators note this “may be in part due to the low number” of individuals in the group.
They add that a larger study is now needed to confirm their findings and to “explore efficacy versus clinical symptoms.”
However, Dr. Large noted that participants in their next study will have fragile X syndrome.
He added the reason for this is “not because we’ve given up on schizophrenia – we feel that schizophrenia is a massive opportunity.”
Patients with schizophrenia are heterogeneous, both in terms of their clinical course and prior treatment. So it is “impossible” for a company of their size to take all of that into account in a single study, Dr. Large said.
In contrast, fragile X is “genetically homogenous,” and so it is possible to focus on the deficit and then translate the findings out into a “broader population.”
Preliminary but worth pursuing?
Commenting on the study, James M. McNally, PhD, assistant professor of psychiatry, Harvard Medical School, Boston, said the findings are “quite preliminary” and that the investigators provided “limited information as to how their findings were derived.”
Nevertheless, it is “nice to see that they observed a significant correlation between resting gamma and positive symptom severity at baseline [and] that the observed change in gamma correlates with change in PANSS scores,” said Dr. McNally, who was not involved with the research.
He added that the “idea of targeting Kv3.1 function to restore PV neuron/gamma activity is very interesting and worth pursuing.”
The study was funded by Autifony Therapeutics, of which Dr. Large is an employee.
A version of this article first appeared on Medscape.com.
In a randomized, double-blind study that included two dozen men with schizophrenia, AUT00206, compared with placebo, increased the power of gamma oscillations, which were in turn associated with positive symptom scores.
The investigators note that targeting a potassium channel linked to brain gamma oscillations may offer a novel way to treat schizophrenia.
In addition, lead author Charles Large, PhD, chief executive officer, Autifony Therapeutics, Stevenage, United Kingdom, told this news organization that it may be “important” to study patients relatively early in their disease course. Participants in the current study were diagnosed less than 5 years previously.
Many previous trials in this area have failed, “and some of the questions were maybe the patients were sort of beyond the point in which you can actually make a difference,” Dr. Large said.
The findings were presented at the Congress of the Schizophrenia International Research Society (SIRS) 2022.
‘Opportunity to intervene’
Dr. Large noted that patients with chronic, long-term symptoms of schizophrenia have been treated with antipsychotics for decades, “and the pathology of that stage is then different.”
For the current study, the investigators hypothesized that the brain may be more “plastic” earlier on in the disease course, and so “maybe there’s an opportunity to intervene and make a change,” said Dr. Large.
In addition, patients with schizophrenia have abnormalities in both their resting state and induced and evolved gamma oscillations, which can include increased resting state power – and reduced power and “phase locking” to cyclical stimuli – the researchers note.
Previous studies have suggested such abnormalities are associated with dysfunction in parvalbumin-expressing interneurons (PVINs) found in cortical and subcortical circuits.
Moreover, Kv3.1 potassium channels expressed on PVINs are integral to establishing and maintaining fast-firing activity and to network synchronization across the brain. They may, therefore, offer a “potential therapeutic approach” for countering PVIN dysfunction, the investigators write.
To examine the impact of AUT00206, a novel Kv3.1/Kv3.2 positive neuromodulator, on resting state and induced gamma oscillations, they conducted a randomized, double-blind study in 24 men with schizophrenia who were aged 18-50 years.
Participants had been diagnosed less than 5 years previously and were stable on a maximum of two antipsychotic medications. They were randomly assigned 2:1 to a loading 2,000-mg dose of AUT00206 on day 1 and then 800 mg twice daily for 27 days or to placebo.
At baseline/day 1, and on a further 3 days over the treatment period, the men underwent resting-state electroencephalography, 40-Hz auditory steady-state response stimulation, and deviant and standard stimulation in an auditory oddball paradigm to assess resting state, induced, and evoked oscillations, respectively.
Positive associations
Results showed that early auditory gamma responses were increased at day 28 in patients who received AUT00206 but not in those who received placebo. The active drug was also associated with increases in the power of gamma oscillations from Day 5 in response to stimuli but not in phase locking.
There was also a significant positive association between frontal resting gamma power and baseline Positive and Negative Syndrome Scale (PANSS) positive symptom severity scores (r = 0.675; P < .001).
Moreover, changes in PANSS positive scores were significantly correlated with a decrease in frontal resting gamma power in patients treated with AUT00206 (r = 0.532; P = .05).
While a similar correlation was not found with placebo, the investigators note this “may be in part due to the low number” of individuals in the group.
They add that a larger study is now needed to confirm their findings and to “explore efficacy versus clinical symptoms.”
However, Dr. Large noted that participants in their next study will have fragile X syndrome.
He added the reason for this is “not because we’ve given up on schizophrenia – we feel that schizophrenia is a massive opportunity.”
Patients with schizophrenia are heterogeneous, both in terms of their clinical course and prior treatment. So it is “impossible” for a company of their size to take all of that into account in a single study, Dr. Large said.
In contrast, fragile X is “genetically homogenous,” and so it is possible to focus on the deficit and then translate the findings out into a “broader population.”
Preliminary but worth pursuing?
Commenting on the study, James M. McNally, PhD, assistant professor of psychiatry, Harvard Medical School, Boston, said the findings are “quite preliminary” and that the investigators provided “limited information as to how their findings were derived.”
Nevertheless, it is “nice to see that they observed a significant correlation between resting gamma and positive symptom severity at baseline [and] that the observed change in gamma correlates with change in PANSS scores,” said Dr. McNally, who was not involved with the research.
He added that the “idea of targeting Kv3.1 function to restore PV neuron/gamma activity is very interesting and worth pursuing.”
The study was funded by Autifony Therapeutics, of which Dr. Large is an employee.
A version of this article first appeared on Medscape.com.
FROM SIRS 2022
COVID-19 cardiovascular complications in children: AHA statement
Cardiovascular complications are uncommon for children and young adults after COVID-19 disease or SARS-CoV-2 infection, according to a new scientific statement from the American Heart Association.
However, the infection can cause some children and young people to experience arrhythmias, myocarditis, pericarditis, or multisystem inflammatory syndrome (MIS-C), a new condition identified during the pandemic, it notes.
The statement details what has been learned about how to treat, manage, and prevent cardiovascular complications associated with COVID-19 in children and young adults and calls for more research, including studies following the short- and long-term cardiovascular effects.
It also reports that COVID-19 vaccines have been found to prevent severe COVID-19 disease and decrease the risk of developing MIS-C by 91% among children ages 12-18 years.
On returning to sports, it says data suggest it is safe for young people with mild or asymptomatic COVID-19 to resume exercise after recovery from symptoms. For those with more serious infections, it recommends additional tests, including cardiac enzyme levels, electrocardiogram, and echocardiogram, before returning to sports or strenuous physical exercise.
The scientific statement was published online on in Circulation.
“Two years into the pandemic and with vast amounts of research conducted in children with COVID-19, this statement summarizes what we know so far related to COVID-19 in children,” said chair of the statement writing group Pei-Ni Jone, MD, from the Children’s Hospital Colorado, Aurora.
Analysis of the latest research indicates children generally have mild symptoms from SARS-CoV-2 infection. In the U.S., as of Feb. 24, 2022, children under 18 years of age have accounted for 17.6% of total COVID-19 cases and about 0.1% of deaths from the virus, the report states.
In addition, young adults, ages 18-29 years, have accounted for 21.3% of cases and 0.8% of deaths from COVID-19.
Like adults, children with underlying medical conditions such as chronic lung disease or obesity and those who are immunocompromised are more likely to be hospitalized, to be admitted to an intensive care unit, and to die of COVID-19, the statement notes. There are conflicting reports on the risk of severe COVID-19 in children and young adults with congenital heart disease, with some reports suggesting a slightly increased risk of severe COVID-19.
In terms of cardiovascular complications of COVID-19 in children, arrhythmias have included ventricular tachycardia and atrial tachycardia, as well as first-degree atrioventricular block. Although arrhythmias generally self-resolve without the need for treatment, prophylactic antiarrhythmics have been administered in some cases, and death caused by recurrent ventricular tachycardia in an adolescent with hypertrophic cardiomyopathy has been described.
Elevations of troponin, electrocardiographic abnormalities, including ST-segment changes, and delayed gadolinium enhancement on cardiac magnetic resonance imaging have been seen in those with myocardial involvement. Although death is rare, both sudden cardiac death and death after intensive medical and supportive therapies have occurred in children with severe myocardial involvement.
In a large retrospective pediatric case series of SARS-CoV-2–associated deaths in individuals under 21 years of age, the median age at death was 17 years, 63% were male, 28% were Black, and 46% were Hispanic. Of those who died, 86% had a comorbid condition, with obesity (42%) and asthma (29%) being the most common.
But the report concludes that: “Although children with comorbidities are at increased risk for symptomatic SARS-CoV-2 infection, compared with healthy children, cardiovascular complications, severe illness, and death are uncommon.”
MIS-C: Rare but severe
The authors of the statement explain that children and some young adults may develop MIS-C, a relatively rare but severe inflammatory syndrome generally occurring 2-6 weeks after infection with SARS-CoV-2 that can affect the heart and multiple organ systems.
In the first year of the pandemic, more than 2,600 cases of MIS-C were reported to the Centers for Disease Control and Prevention, at an estimated rate of 1 case per 3,164 cases of SARS-CoV-2 infection in children, with MIS-C disproportionately affecting Hispanic and Black children.
As many as 50% of children with MIS-C have myocardial involvement, including decreased left ventricular function, coronary artery dilation or aneurysms, myocarditis, elevated troponin and BNP or NT-proBNP, or pericardial effusion. Acute-phase reactants, including C-reactive protein, D-dimer, ferritin, and fibrinogen, can be significantly elevated in MIS-C, neutrophil/lymphocyte ratio may be higher, and platelet counts lower than those with non–MIS-C febrile illnesses.
Fortunately, the outcome of MIS-C is generally very good, with resolution of inflammation and cardiovascular abnormalities within 1-4 weeks of diagnosis, the report says.
However, there have been reports of progression of coronary artery aneurysms after discharge, highlighting the potential for long-term complications. Death resulting from MIS-C is rare, with a mortality rate of 1.4%-1.9%.
Compared with children and young adults who died of acute SARS-CoV-2 infection, most of the fatalities from MIS-C were in previously healthy individuals without comorbidities.
The authors recommend structured follow-up of patients with MIS-C because of concern about progression of cardiac complications and an unclear long-term prognosis.
The statement notes that the first-line treatment for MIS-C is typically intravenous immunoglobulin (IVIG) and patients with poor ventricular function may need to have IVIG in divided doses to tolerate the fluid load.
Supportive treatment for heart failure and vasoplegic shock often requires aggressive management in an ICU for administration of inotropes and vasoactive medications. Antiplatelet therapy with low-dose aspirin is considered in patients with coronary artery involvement, and anticoagulation is added, depending on the degree of coronary artery dilation.
COVID-19 vaccination
The statement notes that vaccines can prevent patients from getting COVID-19 and decrease the risk of MIS-C by 91% among children 12-18 years of age.
On vaccine-associated myocarditis, it concludes the benefits of getting the vaccines outweigh the risks.
For example, for every 1 million doses of the mRNA COVID-19 vaccines in males ages 12-29 years (the highest risk group for vaccine-associated myocarditis), it is estimated that 11,000 COVID-19 cases, 560 hospitalizations, and six deaths would be prevented, whereas 39-47 cases of myocarditis would be expected.
But it adds that the CDC is continuing to follow myocarditis in children and young adults closely, particularly a possible connection to the mRNA COVID-19 vaccines.
The statement says that more research is needed to better understand the mechanisms and optimal treatment approaches for SARS-CoV-2 infection, vaccine-associated myocarditis, the long-term outcomes of both COVID-19 and MIS-C, and the impact of these various conditions on the heart in children and young adults. In addition, any new antiviral therapies need to be tested in clinical trials focused on children.
“Although much has been learned about how the virus impacts children’s and young adult’s hearts, how to best treat cardiovascular complications, and prevent severe illness, continued clinical research trials are needed to better understand the long-term cardiovascular impacts,” Dr. Jone said. “It is also important to address health disparities that have become more apparent during the pandemic. We must work to ensure all children receive equal access to vaccination and high-quality care.”
A version of this article first appeared on Medscape.com.
Cardiovascular complications are uncommon for children and young adults after COVID-19 disease or SARS-CoV-2 infection, according to a new scientific statement from the American Heart Association.
However, the infection can cause some children and young people to experience arrhythmias, myocarditis, pericarditis, or multisystem inflammatory syndrome (MIS-C), a new condition identified during the pandemic, it notes.
The statement details what has been learned about how to treat, manage, and prevent cardiovascular complications associated with COVID-19 in children and young adults and calls for more research, including studies following the short- and long-term cardiovascular effects.
It also reports that COVID-19 vaccines have been found to prevent severe COVID-19 disease and decrease the risk of developing MIS-C by 91% among children ages 12-18 years.
On returning to sports, it says data suggest it is safe for young people with mild or asymptomatic COVID-19 to resume exercise after recovery from symptoms. For those with more serious infections, it recommends additional tests, including cardiac enzyme levels, electrocardiogram, and echocardiogram, before returning to sports or strenuous physical exercise.
The scientific statement was published online on in Circulation.
“Two years into the pandemic and with vast amounts of research conducted in children with COVID-19, this statement summarizes what we know so far related to COVID-19 in children,” said chair of the statement writing group Pei-Ni Jone, MD, from the Children’s Hospital Colorado, Aurora.
Analysis of the latest research indicates children generally have mild symptoms from SARS-CoV-2 infection. In the U.S., as of Feb. 24, 2022, children under 18 years of age have accounted for 17.6% of total COVID-19 cases and about 0.1% of deaths from the virus, the report states.
In addition, young adults, ages 18-29 years, have accounted for 21.3% of cases and 0.8% of deaths from COVID-19.
Like adults, children with underlying medical conditions such as chronic lung disease or obesity and those who are immunocompromised are more likely to be hospitalized, to be admitted to an intensive care unit, and to die of COVID-19, the statement notes. There are conflicting reports on the risk of severe COVID-19 in children and young adults with congenital heart disease, with some reports suggesting a slightly increased risk of severe COVID-19.
In terms of cardiovascular complications of COVID-19 in children, arrhythmias have included ventricular tachycardia and atrial tachycardia, as well as first-degree atrioventricular block. Although arrhythmias generally self-resolve without the need for treatment, prophylactic antiarrhythmics have been administered in some cases, and death caused by recurrent ventricular tachycardia in an adolescent with hypertrophic cardiomyopathy has been described.
Elevations of troponin, electrocardiographic abnormalities, including ST-segment changes, and delayed gadolinium enhancement on cardiac magnetic resonance imaging have been seen in those with myocardial involvement. Although death is rare, both sudden cardiac death and death after intensive medical and supportive therapies have occurred in children with severe myocardial involvement.
In a large retrospective pediatric case series of SARS-CoV-2–associated deaths in individuals under 21 years of age, the median age at death was 17 years, 63% were male, 28% were Black, and 46% were Hispanic. Of those who died, 86% had a comorbid condition, with obesity (42%) and asthma (29%) being the most common.
But the report concludes that: “Although children with comorbidities are at increased risk for symptomatic SARS-CoV-2 infection, compared with healthy children, cardiovascular complications, severe illness, and death are uncommon.”
MIS-C: Rare but severe
The authors of the statement explain that children and some young adults may develop MIS-C, a relatively rare but severe inflammatory syndrome generally occurring 2-6 weeks after infection with SARS-CoV-2 that can affect the heart and multiple organ systems.
In the first year of the pandemic, more than 2,600 cases of MIS-C were reported to the Centers for Disease Control and Prevention, at an estimated rate of 1 case per 3,164 cases of SARS-CoV-2 infection in children, with MIS-C disproportionately affecting Hispanic and Black children.
As many as 50% of children with MIS-C have myocardial involvement, including decreased left ventricular function, coronary artery dilation or aneurysms, myocarditis, elevated troponin and BNP or NT-proBNP, or pericardial effusion. Acute-phase reactants, including C-reactive protein, D-dimer, ferritin, and fibrinogen, can be significantly elevated in MIS-C, neutrophil/lymphocyte ratio may be higher, and platelet counts lower than those with non–MIS-C febrile illnesses.
Fortunately, the outcome of MIS-C is generally very good, with resolution of inflammation and cardiovascular abnormalities within 1-4 weeks of diagnosis, the report says.
However, there have been reports of progression of coronary artery aneurysms after discharge, highlighting the potential for long-term complications. Death resulting from MIS-C is rare, with a mortality rate of 1.4%-1.9%.
Compared with children and young adults who died of acute SARS-CoV-2 infection, most of the fatalities from MIS-C were in previously healthy individuals without comorbidities.
The authors recommend structured follow-up of patients with MIS-C because of concern about progression of cardiac complications and an unclear long-term prognosis.
The statement notes that the first-line treatment for MIS-C is typically intravenous immunoglobulin (IVIG) and patients with poor ventricular function may need to have IVIG in divided doses to tolerate the fluid load.
Supportive treatment for heart failure and vasoplegic shock often requires aggressive management in an ICU for administration of inotropes and vasoactive medications. Antiplatelet therapy with low-dose aspirin is considered in patients with coronary artery involvement, and anticoagulation is added, depending on the degree of coronary artery dilation.
COVID-19 vaccination
The statement notes that vaccines can prevent patients from getting COVID-19 and decrease the risk of MIS-C by 91% among children 12-18 years of age.
On vaccine-associated myocarditis, it concludes the benefits of getting the vaccines outweigh the risks.
For example, for every 1 million doses of the mRNA COVID-19 vaccines in males ages 12-29 years (the highest risk group for vaccine-associated myocarditis), it is estimated that 11,000 COVID-19 cases, 560 hospitalizations, and six deaths would be prevented, whereas 39-47 cases of myocarditis would be expected.
But it adds that the CDC is continuing to follow myocarditis in children and young adults closely, particularly a possible connection to the mRNA COVID-19 vaccines.
The statement says that more research is needed to better understand the mechanisms and optimal treatment approaches for SARS-CoV-2 infection, vaccine-associated myocarditis, the long-term outcomes of both COVID-19 and MIS-C, and the impact of these various conditions on the heart in children and young adults. In addition, any new antiviral therapies need to be tested in clinical trials focused on children.
“Although much has been learned about how the virus impacts children’s and young adult’s hearts, how to best treat cardiovascular complications, and prevent severe illness, continued clinical research trials are needed to better understand the long-term cardiovascular impacts,” Dr. Jone said. “It is also important to address health disparities that have become more apparent during the pandemic. We must work to ensure all children receive equal access to vaccination and high-quality care.”
A version of this article first appeared on Medscape.com.
Cardiovascular complications are uncommon for children and young adults after COVID-19 disease or SARS-CoV-2 infection, according to a new scientific statement from the American Heart Association.
However, the infection can cause some children and young people to experience arrhythmias, myocarditis, pericarditis, or multisystem inflammatory syndrome (MIS-C), a new condition identified during the pandemic, it notes.
The statement details what has been learned about how to treat, manage, and prevent cardiovascular complications associated with COVID-19 in children and young adults and calls for more research, including studies following the short- and long-term cardiovascular effects.
It also reports that COVID-19 vaccines have been found to prevent severe COVID-19 disease and decrease the risk of developing MIS-C by 91% among children ages 12-18 years.
On returning to sports, it says data suggest it is safe for young people with mild or asymptomatic COVID-19 to resume exercise after recovery from symptoms. For those with more serious infections, it recommends additional tests, including cardiac enzyme levels, electrocardiogram, and echocardiogram, before returning to sports or strenuous physical exercise.
The scientific statement was published online on in Circulation.
“Two years into the pandemic and with vast amounts of research conducted in children with COVID-19, this statement summarizes what we know so far related to COVID-19 in children,” said chair of the statement writing group Pei-Ni Jone, MD, from the Children’s Hospital Colorado, Aurora.
Analysis of the latest research indicates children generally have mild symptoms from SARS-CoV-2 infection. In the U.S., as of Feb. 24, 2022, children under 18 years of age have accounted for 17.6% of total COVID-19 cases and about 0.1% of deaths from the virus, the report states.
In addition, young adults, ages 18-29 years, have accounted for 21.3% of cases and 0.8% of deaths from COVID-19.
Like adults, children with underlying medical conditions such as chronic lung disease or obesity and those who are immunocompromised are more likely to be hospitalized, to be admitted to an intensive care unit, and to die of COVID-19, the statement notes. There are conflicting reports on the risk of severe COVID-19 in children and young adults with congenital heart disease, with some reports suggesting a slightly increased risk of severe COVID-19.
In terms of cardiovascular complications of COVID-19 in children, arrhythmias have included ventricular tachycardia and atrial tachycardia, as well as first-degree atrioventricular block. Although arrhythmias generally self-resolve without the need for treatment, prophylactic antiarrhythmics have been administered in some cases, and death caused by recurrent ventricular tachycardia in an adolescent with hypertrophic cardiomyopathy has been described.
Elevations of troponin, electrocardiographic abnormalities, including ST-segment changes, and delayed gadolinium enhancement on cardiac magnetic resonance imaging have been seen in those with myocardial involvement. Although death is rare, both sudden cardiac death and death after intensive medical and supportive therapies have occurred in children with severe myocardial involvement.
In a large retrospective pediatric case series of SARS-CoV-2–associated deaths in individuals under 21 years of age, the median age at death was 17 years, 63% were male, 28% were Black, and 46% were Hispanic. Of those who died, 86% had a comorbid condition, with obesity (42%) and asthma (29%) being the most common.
But the report concludes that: “Although children with comorbidities are at increased risk for symptomatic SARS-CoV-2 infection, compared with healthy children, cardiovascular complications, severe illness, and death are uncommon.”
MIS-C: Rare but severe
The authors of the statement explain that children and some young adults may develop MIS-C, a relatively rare but severe inflammatory syndrome generally occurring 2-6 weeks after infection with SARS-CoV-2 that can affect the heart and multiple organ systems.
In the first year of the pandemic, more than 2,600 cases of MIS-C were reported to the Centers for Disease Control and Prevention, at an estimated rate of 1 case per 3,164 cases of SARS-CoV-2 infection in children, with MIS-C disproportionately affecting Hispanic and Black children.
As many as 50% of children with MIS-C have myocardial involvement, including decreased left ventricular function, coronary artery dilation or aneurysms, myocarditis, elevated troponin and BNP or NT-proBNP, or pericardial effusion. Acute-phase reactants, including C-reactive protein, D-dimer, ferritin, and fibrinogen, can be significantly elevated in MIS-C, neutrophil/lymphocyte ratio may be higher, and platelet counts lower than those with non–MIS-C febrile illnesses.
Fortunately, the outcome of MIS-C is generally very good, with resolution of inflammation and cardiovascular abnormalities within 1-4 weeks of diagnosis, the report says.
However, there have been reports of progression of coronary artery aneurysms after discharge, highlighting the potential for long-term complications. Death resulting from MIS-C is rare, with a mortality rate of 1.4%-1.9%.
Compared with children and young adults who died of acute SARS-CoV-2 infection, most of the fatalities from MIS-C were in previously healthy individuals without comorbidities.
The authors recommend structured follow-up of patients with MIS-C because of concern about progression of cardiac complications and an unclear long-term prognosis.
The statement notes that the first-line treatment for MIS-C is typically intravenous immunoglobulin (IVIG) and patients with poor ventricular function may need to have IVIG in divided doses to tolerate the fluid load.
Supportive treatment for heart failure and vasoplegic shock often requires aggressive management in an ICU for administration of inotropes and vasoactive medications. Antiplatelet therapy with low-dose aspirin is considered in patients with coronary artery involvement, and anticoagulation is added, depending on the degree of coronary artery dilation.
COVID-19 vaccination
The statement notes that vaccines can prevent patients from getting COVID-19 and decrease the risk of MIS-C by 91% among children 12-18 years of age.
On vaccine-associated myocarditis, it concludes the benefits of getting the vaccines outweigh the risks.
For example, for every 1 million doses of the mRNA COVID-19 vaccines in males ages 12-29 years (the highest risk group for vaccine-associated myocarditis), it is estimated that 11,000 COVID-19 cases, 560 hospitalizations, and six deaths would be prevented, whereas 39-47 cases of myocarditis would be expected.
But it adds that the CDC is continuing to follow myocarditis in children and young adults closely, particularly a possible connection to the mRNA COVID-19 vaccines.
The statement says that more research is needed to better understand the mechanisms and optimal treatment approaches for SARS-CoV-2 infection, vaccine-associated myocarditis, the long-term outcomes of both COVID-19 and MIS-C, and the impact of these various conditions on the heart in children and young adults. In addition, any new antiviral therapies need to be tested in clinical trials focused on children.
“Although much has been learned about how the virus impacts children’s and young adult’s hearts, how to best treat cardiovascular complications, and prevent severe illness, continued clinical research trials are needed to better understand the long-term cardiovascular impacts,” Dr. Jone said. “It is also important to address health disparities that have become more apparent during the pandemic. We must work to ensure all children receive equal access to vaccination and high-quality care.”
A version of this article first appeared on Medscape.com.
FROM CIRCULATION
Central centrifugal cicatricial alopecia
THE PRESENTATION
A Early central centrifugal cicatricial alopecia with a small central patch of hair loss in a 45-year-old Black woman.
B Late central centrifugal cicatricial alopecia with a large central patch of hair loss in a 43-year-old Black woman.
Scarring alopecia is a collection of hair loss disorders including chronic cutaneous lupus erythematosus (discoid lupus), lichen planopilaris, dissecting cellulitis, acne keloidalis, and central centrifugal cicatricial alopecia.1 CCCA (formerly hot comb alopecia or follicular degeneration syndrome) is a progressive, scarring, inflammatory alopecia and represents the most common form of scarring alopecia in women of African descent. It results in permanent destruction of hair follicles.
Epidemiology
CCCA predominantly affects women of African descent but also may affect men. The prevalence of CCCA in those of African descent has varied in the literature. Khumalo2 reported a prevalence of 1.2% for women younger than 50 years and 6.7% in women older than 50 years. CCCA has been reported in other ethnic groups, such as those of Asian descent.3
Historically, hair care practices that are more common in those of African descent, such as high-tension hairstyles as well as heat and chemical hair relaxers, were implicated in the development of CCCA. However, the causes of CCCA are most likely multifactorial, including family history, genetic mutations, and hair care practices.4-7PADI3 mutations likely predispose some women to CCCA. Mutations in PADI3, which encodes peptidyl arginine deiminase 3 (an enzyme that modifies proteins crucial for the formation of hair shafts), were found in some patients with CCCA.8 Moreover, other genetic defects also likely play a role.7
Key clinical features
Early recognition is key for patients with CCCA.
- CCCA begins in the central scalp (crown area, vertex) and spreads centrifugally.
- Scalp symptoms such as tenderness, pain, a tingling or crawling sensation, and itching may occur.9 Some patients may not have any symptoms at all, and hair loss may progress painlessly.
- Central hair breakage—forme fruste CCCA—may be a presenting sign of CCCA.9
- Loss of follicular ostia and mottled hypopigmented and hyperpigmented macules are common findings.6
- CCCA can be diagnosed clinically and by histopathology.
Worth noting
Patients may experience hair loss and scalp symptoms for years before seeking medical evaluation. In some cultures, hair breakage or itching on the top of the scalp may be viewed as a normal occurrence in life.
It is important to set patient expectations that CCCA is a scarring alopecia, and the initial goal often is to maintain the patient's existing hair. However, hair and areas responding to treatment should still be treated. Without any intervention, the resulting scarring from CCCA may permanently scar follicles on the entire scalp.
Continue to: Due to the inflammatory...
Due to the inflammatory nature of CCCA, potent topical corticosteroids (eg, clobetasol propionate), intralesional corticosteroids (eg, triamcinolone acetonide), and oral antiinflammatory agents (eg, doxycycline) are utilized in the treatment of CCCA. Minoxidil is another treatment option. Adjuvant therapies such as topical metformin also have been tried.10 Importantly, treatment of CCCA may halt further permanent destruction of hair follicles, but scalp symptoms may reappear periodically and require re-treatment with anti-inflammatory agents.
Health care highlight
Thorough scalp examination and awareness of clinical features of CCCA may prompt earlier diagnosis and prevent future severe permanent alopecia. Clinicians should encourage patients with suggestive signs or symptoms of CCCA to seek care from a dermatologist.
1. Sperling LC. Scarring alopecia and the dermatopathologist. J Cutan Pathol. 2001;28:333-342. doi:10.1034/ j.1600-0560.2001.280701.x
2. Khumalo NP. Prevalence of central centrifugal cicatricial alopecia. Arch Dermatol. 2011;147:1453-1454. doi:10.1001/ archderm.147.12.1453
3. Su HJ, Cheng AY, Liu CH, et al. Primary scarring alopecia: a retrospective study of 89 patients in Taiwan [published online January 16, 2018]. J Dermatol. 2018;45:450-455. doi:10.1111/ 1346-8138.14217
4. Sperling LC, Cowper SE. The histopathology of primary cicatricial alopecia. Semin Cutan Med Surg. 2006;25:41-50
5. Dlova NC, Forder M. Central centrifugal cicatricial alopecia: possible familial aetiology in two African families from South Africa. Int J Dermatol. 2012;51(supp 1):17-20, 20-23.
6. Ogunleye TA, Quinn CR, McMichael A. Alopecia. In: Taylor SC, Kelly AP, Lim HW, et al, eds. Dermatology for Skin of Color. McGraw Hill; 2016:253-264.
7. Uitto J. Genetic susceptibility to alopecia [published online February 13, 2019]. N Engl J Med. 2019;380:873-876. doi:10.1056/ NEJMe1900042
8. Malki L, Sarig O, Romano MT, et al. Variant PADI3 in central centrifugal cicatricial alopecia. N Engl J Med. 2019;380:833-841.
9. Callender VD, Wright DR, Davis EC, et al. Hair breakage as a presenting sign of early or occult central centrifugal cicatricial alopecia: clinicopathologic findings in 9 patients. Arch Dermatol. 2012;148:1047-1052.
10. Araoye EF, Thomas JAL, Aguh CU. Hair regrowth in 2 patients with recalcitrant central centrifugal cicatricial alopecia after use of topical metformin. JAAD Case Rep. 2020;6:106-108. doi:10.1016/ j.jdcr.2019.12.008.
THE PRESENTATION
A Early central centrifugal cicatricial alopecia with a small central patch of hair loss in a 45-year-old Black woman.
B Late central centrifugal cicatricial alopecia with a large central patch of hair loss in a 43-year-old Black woman.
Scarring alopecia is a collection of hair loss disorders including chronic cutaneous lupus erythematosus (discoid lupus), lichen planopilaris, dissecting cellulitis, acne keloidalis, and central centrifugal cicatricial alopecia.1 CCCA (formerly hot comb alopecia or follicular degeneration syndrome) is a progressive, scarring, inflammatory alopecia and represents the most common form of scarring alopecia in women of African descent. It results in permanent destruction of hair follicles.
Epidemiology
CCCA predominantly affects women of African descent but also may affect men. The prevalence of CCCA in those of African descent has varied in the literature. Khumalo2 reported a prevalence of 1.2% for women younger than 50 years and 6.7% in women older than 50 years. CCCA has been reported in other ethnic groups, such as those of Asian descent.3
Historically, hair care practices that are more common in those of African descent, such as high-tension hairstyles as well as heat and chemical hair relaxers, were implicated in the development of CCCA. However, the causes of CCCA are most likely multifactorial, including family history, genetic mutations, and hair care practices.4-7PADI3 mutations likely predispose some women to CCCA. Mutations in PADI3, which encodes peptidyl arginine deiminase 3 (an enzyme that modifies proteins crucial for the formation of hair shafts), were found in some patients with CCCA.8 Moreover, other genetic defects also likely play a role.7
Key clinical features
Early recognition is key for patients with CCCA.
- CCCA begins in the central scalp (crown area, vertex) and spreads centrifugally.
- Scalp symptoms such as tenderness, pain, a tingling or crawling sensation, and itching may occur.9 Some patients may not have any symptoms at all, and hair loss may progress painlessly.
- Central hair breakage—forme fruste CCCA—may be a presenting sign of CCCA.9
- Loss of follicular ostia and mottled hypopigmented and hyperpigmented macules are common findings.6
- CCCA can be diagnosed clinically and by histopathology.
Worth noting
Patients may experience hair loss and scalp symptoms for years before seeking medical evaluation. In some cultures, hair breakage or itching on the top of the scalp may be viewed as a normal occurrence in life.
It is important to set patient expectations that CCCA is a scarring alopecia, and the initial goal often is to maintain the patient's existing hair. However, hair and areas responding to treatment should still be treated. Without any intervention, the resulting scarring from CCCA may permanently scar follicles on the entire scalp.
Continue to: Due to the inflammatory...
Due to the inflammatory nature of CCCA, potent topical corticosteroids (eg, clobetasol propionate), intralesional corticosteroids (eg, triamcinolone acetonide), and oral antiinflammatory agents (eg, doxycycline) are utilized in the treatment of CCCA. Minoxidil is another treatment option. Adjuvant therapies such as topical metformin also have been tried.10 Importantly, treatment of CCCA may halt further permanent destruction of hair follicles, but scalp symptoms may reappear periodically and require re-treatment with anti-inflammatory agents.
Health care highlight
Thorough scalp examination and awareness of clinical features of CCCA may prompt earlier diagnosis and prevent future severe permanent alopecia. Clinicians should encourage patients with suggestive signs or symptoms of CCCA to seek care from a dermatologist.
THE PRESENTATION
A Early central centrifugal cicatricial alopecia with a small central patch of hair loss in a 45-year-old Black woman.
B Late central centrifugal cicatricial alopecia with a large central patch of hair loss in a 43-year-old Black woman.
Scarring alopecia is a collection of hair loss disorders including chronic cutaneous lupus erythematosus (discoid lupus), lichen planopilaris, dissecting cellulitis, acne keloidalis, and central centrifugal cicatricial alopecia.1 CCCA (formerly hot comb alopecia or follicular degeneration syndrome) is a progressive, scarring, inflammatory alopecia and represents the most common form of scarring alopecia in women of African descent. It results in permanent destruction of hair follicles.
Epidemiology
CCCA predominantly affects women of African descent but also may affect men. The prevalence of CCCA in those of African descent has varied in the literature. Khumalo2 reported a prevalence of 1.2% for women younger than 50 years and 6.7% in women older than 50 years. CCCA has been reported in other ethnic groups, such as those of Asian descent.3
Historically, hair care practices that are more common in those of African descent, such as high-tension hairstyles as well as heat and chemical hair relaxers, were implicated in the development of CCCA. However, the causes of CCCA are most likely multifactorial, including family history, genetic mutations, and hair care practices.4-7PADI3 mutations likely predispose some women to CCCA. Mutations in PADI3, which encodes peptidyl arginine deiminase 3 (an enzyme that modifies proteins crucial for the formation of hair shafts), were found in some patients with CCCA.8 Moreover, other genetic defects also likely play a role.7
Key clinical features
Early recognition is key for patients with CCCA.
- CCCA begins in the central scalp (crown area, vertex) and spreads centrifugally.
- Scalp symptoms such as tenderness, pain, a tingling or crawling sensation, and itching may occur.9 Some patients may not have any symptoms at all, and hair loss may progress painlessly.
- Central hair breakage—forme fruste CCCA—may be a presenting sign of CCCA.9
- Loss of follicular ostia and mottled hypopigmented and hyperpigmented macules are common findings.6
- CCCA can be diagnosed clinically and by histopathology.
Worth noting
Patients may experience hair loss and scalp symptoms for years before seeking medical evaluation. In some cultures, hair breakage or itching on the top of the scalp may be viewed as a normal occurrence in life.
It is important to set patient expectations that CCCA is a scarring alopecia, and the initial goal often is to maintain the patient's existing hair. However, hair and areas responding to treatment should still be treated. Without any intervention, the resulting scarring from CCCA may permanently scar follicles on the entire scalp.
Continue to: Due to the inflammatory...
Due to the inflammatory nature of CCCA, potent topical corticosteroids (eg, clobetasol propionate), intralesional corticosteroids (eg, triamcinolone acetonide), and oral antiinflammatory agents (eg, doxycycline) are utilized in the treatment of CCCA. Minoxidil is another treatment option. Adjuvant therapies such as topical metformin also have been tried.10 Importantly, treatment of CCCA may halt further permanent destruction of hair follicles, but scalp symptoms may reappear periodically and require re-treatment with anti-inflammatory agents.
Health care highlight
Thorough scalp examination and awareness of clinical features of CCCA may prompt earlier diagnosis and prevent future severe permanent alopecia. Clinicians should encourage patients with suggestive signs or symptoms of CCCA to seek care from a dermatologist.
1. Sperling LC. Scarring alopecia and the dermatopathologist. J Cutan Pathol. 2001;28:333-342. doi:10.1034/ j.1600-0560.2001.280701.x
2. Khumalo NP. Prevalence of central centrifugal cicatricial alopecia. Arch Dermatol. 2011;147:1453-1454. doi:10.1001/ archderm.147.12.1453
3. Su HJ, Cheng AY, Liu CH, et al. Primary scarring alopecia: a retrospective study of 89 patients in Taiwan [published online January 16, 2018]. J Dermatol. 2018;45:450-455. doi:10.1111/ 1346-8138.14217
4. Sperling LC, Cowper SE. The histopathology of primary cicatricial alopecia. Semin Cutan Med Surg. 2006;25:41-50
5. Dlova NC, Forder M. Central centrifugal cicatricial alopecia: possible familial aetiology in two African families from South Africa. Int J Dermatol. 2012;51(supp 1):17-20, 20-23.
6. Ogunleye TA, Quinn CR, McMichael A. Alopecia. In: Taylor SC, Kelly AP, Lim HW, et al, eds. Dermatology for Skin of Color. McGraw Hill; 2016:253-264.
7. Uitto J. Genetic susceptibility to alopecia [published online February 13, 2019]. N Engl J Med. 2019;380:873-876. doi:10.1056/ NEJMe1900042
8. Malki L, Sarig O, Romano MT, et al. Variant PADI3 in central centrifugal cicatricial alopecia. N Engl J Med. 2019;380:833-841.
9. Callender VD, Wright DR, Davis EC, et al. Hair breakage as a presenting sign of early or occult central centrifugal cicatricial alopecia: clinicopathologic findings in 9 patients. Arch Dermatol. 2012;148:1047-1052.
10. Araoye EF, Thomas JAL, Aguh CU. Hair regrowth in 2 patients with recalcitrant central centrifugal cicatricial alopecia after use of topical metformin. JAAD Case Rep. 2020;6:106-108. doi:10.1016/ j.jdcr.2019.12.008.
1. Sperling LC. Scarring alopecia and the dermatopathologist. J Cutan Pathol. 2001;28:333-342. doi:10.1034/ j.1600-0560.2001.280701.x
2. Khumalo NP. Prevalence of central centrifugal cicatricial alopecia. Arch Dermatol. 2011;147:1453-1454. doi:10.1001/ archderm.147.12.1453
3. Su HJ, Cheng AY, Liu CH, et al. Primary scarring alopecia: a retrospective study of 89 patients in Taiwan [published online January 16, 2018]. J Dermatol. 2018;45:450-455. doi:10.1111/ 1346-8138.14217
4. Sperling LC, Cowper SE. The histopathology of primary cicatricial alopecia. Semin Cutan Med Surg. 2006;25:41-50
5. Dlova NC, Forder M. Central centrifugal cicatricial alopecia: possible familial aetiology in two African families from South Africa. Int J Dermatol. 2012;51(supp 1):17-20, 20-23.
6. Ogunleye TA, Quinn CR, McMichael A. Alopecia. In: Taylor SC, Kelly AP, Lim HW, et al, eds. Dermatology for Skin of Color. McGraw Hill; 2016:253-264.
7. Uitto J. Genetic susceptibility to alopecia [published online February 13, 2019]. N Engl J Med. 2019;380:873-876. doi:10.1056/ NEJMe1900042
8. Malki L, Sarig O, Romano MT, et al. Variant PADI3 in central centrifugal cicatricial alopecia. N Engl J Med. 2019;380:833-841.
9. Callender VD, Wright DR, Davis EC, et al. Hair breakage as a presenting sign of early or occult central centrifugal cicatricial alopecia: clinicopathologic findings in 9 patients. Arch Dermatol. 2012;148:1047-1052.
10. Araoye EF, Thomas JAL, Aguh CU. Hair regrowth in 2 patients with recalcitrant central centrifugal cicatricial alopecia after use of topical metformin. JAAD Case Rep. 2020;6:106-108. doi:10.1016/ j.jdcr.2019.12.008.