User login
C. difficile vaccine: Pfizer’s phase 3 CLOVER trial shows mixed results
, a phase 3 study involving 17,500 adults aged 50 and older that evaluated their candidate vaccine (PF-06425090) against Clostridioides difficile (C. diff) for the prevention of C. diff. infection (CDI).
The bad news is that the trial didn’t meet its efficacy endpoint – the prevention of primary CDI. According to a Pfizer press release, “Vaccine efficacy under the primary endpoint was 31% (96.4%, confidence interval -38.7, 66.6) following the third dose and 28.6% (96.4%, CI -28.4, 61.0) following the second dose. For all CDI cases recorded at 14 days post dose 3, vaccine efficacy was 49%, 47%, and 31% up to 12 months, 24 months, and at final analysis, respectively.”
This news organization requested an interview with a Pfizer spokesperson, but the company declined to comment further.
The good news is that the vaccine did meet its secondary endpoint. There were no cases of CDI requiring medical attention among vaccine recipients; by comparison, there were 11 cases among those who received placebo.
The Centers for Disease Control and Prevention classifies C. diff with other antimicrobial resistance “threat” organisms, as the two often go hand in hand. Their 2019 report noted that in 2017, 223,900 people in the United States required hospitalization for CDI, and at least 12,800 died. C. diff is the most common cause of health care-associated infection and increasingly occurs outside of acute care hospitals. Age older than 65 is a risk factor for disease. And at least 20% of patients experience recurrence.
The trial enrolled people older than 50 who were at higher risk of CDI because of having received antibiotics within the previous 12 weeks or because they were likely to have contact with health care systems. They received three doses of an investigational vaccine containing detoxified toxins A and B. These are the principal virulence factors produced by C. diff. Doses were given at 0, 1, and 6 months.
This news organization asked C. diff specialist David Aronoff, MD, chair of the department of medicine at Indiana University, for comment. Dr. Aronoff was not involved in the Pfizer clinical trials. He told this news organization via email, “Given the very low number of cases, I am impressed, from the limited data that have been made available, that the vaccine appears to have efficacy of around 50% for reducing CDI and, importantly, might reduce the severity of disease significantly, possibly preventing hospitalizations or worse clinical outcomes. It is unclear if the vaccine reduces the risk of recurrent CDI, but that would be a strong finding if true. I think we need to see these data after being subject to peer review, to better define its potential role in preventing CDI on a larger scale.”
Asked about the numbers needed to treat and cost-effectiveness of treatment, Dr. Aronoff added, “It is not clear how many people would need to receive the vaccine to prevent one hospitalization from CDI, or one death, or one case. Because the study groups had fewer episodes of CDI than anticipated, it watered down the power of this investigation to provide definitive answers regarding its true efficacy.”
Dr. Aronoff concluded, “All things considered, I am a cup half-full type of person on these topline results, since there are indications of reducing disease incidence and severity. We can build on these results.”
Dr. Aronoff had a basic science C. diff research grant from Pfizer in 2018-2019 that was not related to vaccines or therapeutics.
A version of this article first appeared on Medscape.com.
, a phase 3 study involving 17,500 adults aged 50 and older that evaluated their candidate vaccine (PF-06425090) against Clostridioides difficile (C. diff) for the prevention of C. diff. infection (CDI).
The bad news is that the trial didn’t meet its efficacy endpoint – the prevention of primary CDI. According to a Pfizer press release, “Vaccine efficacy under the primary endpoint was 31% (96.4%, confidence interval -38.7, 66.6) following the third dose and 28.6% (96.4%, CI -28.4, 61.0) following the second dose. For all CDI cases recorded at 14 days post dose 3, vaccine efficacy was 49%, 47%, and 31% up to 12 months, 24 months, and at final analysis, respectively.”
This news organization requested an interview with a Pfizer spokesperson, but the company declined to comment further.
The good news is that the vaccine did meet its secondary endpoint. There were no cases of CDI requiring medical attention among vaccine recipients; by comparison, there were 11 cases among those who received placebo.
The Centers for Disease Control and Prevention classifies C. diff with other antimicrobial resistance “threat” organisms, as the two often go hand in hand. Their 2019 report noted that in 2017, 223,900 people in the United States required hospitalization for CDI, and at least 12,800 died. C. diff is the most common cause of health care-associated infection and increasingly occurs outside of acute care hospitals. Age older than 65 is a risk factor for disease. And at least 20% of patients experience recurrence.
The trial enrolled people older than 50 who were at higher risk of CDI because of having received antibiotics within the previous 12 weeks or because they were likely to have contact with health care systems. They received three doses of an investigational vaccine containing detoxified toxins A and B. These are the principal virulence factors produced by C. diff. Doses were given at 0, 1, and 6 months.
This news organization asked C. diff specialist David Aronoff, MD, chair of the department of medicine at Indiana University, for comment. Dr. Aronoff was not involved in the Pfizer clinical trials. He told this news organization via email, “Given the very low number of cases, I am impressed, from the limited data that have been made available, that the vaccine appears to have efficacy of around 50% for reducing CDI and, importantly, might reduce the severity of disease significantly, possibly preventing hospitalizations or worse clinical outcomes. It is unclear if the vaccine reduces the risk of recurrent CDI, but that would be a strong finding if true. I think we need to see these data after being subject to peer review, to better define its potential role in preventing CDI on a larger scale.”
Asked about the numbers needed to treat and cost-effectiveness of treatment, Dr. Aronoff added, “It is not clear how many people would need to receive the vaccine to prevent one hospitalization from CDI, or one death, or one case. Because the study groups had fewer episodes of CDI than anticipated, it watered down the power of this investigation to provide definitive answers regarding its true efficacy.”
Dr. Aronoff concluded, “All things considered, I am a cup half-full type of person on these topline results, since there are indications of reducing disease incidence and severity. We can build on these results.”
Dr. Aronoff had a basic science C. diff research grant from Pfizer in 2018-2019 that was not related to vaccines or therapeutics.
A version of this article first appeared on Medscape.com.
, a phase 3 study involving 17,500 adults aged 50 and older that evaluated their candidate vaccine (PF-06425090) against Clostridioides difficile (C. diff) for the prevention of C. diff. infection (CDI).
The bad news is that the trial didn’t meet its efficacy endpoint – the prevention of primary CDI. According to a Pfizer press release, “Vaccine efficacy under the primary endpoint was 31% (96.4%, confidence interval -38.7, 66.6) following the third dose and 28.6% (96.4%, CI -28.4, 61.0) following the second dose. For all CDI cases recorded at 14 days post dose 3, vaccine efficacy was 49%, 47%, and 31% up to 12 months, 24 months, and at final analysis, respectively.”
This news organization requested an interview with a Pfizer spokesperson, but the company declined to comment further.
The good news is that the vaccine did meet its secondary endpoint. There were no cases of CDI requiring medical attention among vaccine recipients; by comparison, there were 11 cases among those who received placebo.
The Centers for Disease Control and Prevention classifies C. diff with other antimicrobial resistance “threat” organisms, as the two often go hand in hand. Their 2019 report noted that in 2017, 223,900 people in the United States required hospitalization for CDI, and at least 12,800 died. C. diff is the most common cause of health care-associated infection and increasingly occurs outside of acute care hospitals. Age older than 65 is a risk factor for disease. And at least 20% of patients experience recurrence.
The trial enrolled people older than 50 who were at higher risk of CDI because of having received antibiotics within the previous 12 weeks or because they were likely to have contact with health care systems. They received three doses of an investigational vaccine containing detoxified toxins A and B. These are the principal virulence factors produced by C. diff. Doses were given at 0, 1, and 6 months.
This news organization asked C. diff specialist David Aronoff, MD, chair of the department of medicine at Indiana University, for comment. Dr. Aronoff was not involved in the Pfizer clinical trials. He told this news organization via email, “Given the very low number of cases, I am impressed, from the limited data that have been made available, that the vaccine appears to have efficacy of around 50% for reducing CDI and, importantly, might reduce the severity of disease significantly, possibly preventing hospitalizations or worse clinical outcomes. It is unclear if the vaccine reduces the risk of recurrent CDI, but that would be a strong finding if true. I think we need to see these data after being subject to peer review, to better define its potential role in preventing CDI on a larger scale.”
Asked about the numbers needed to treat and cost-effectiveness of treatment, Dr. Aronoff added, “It is not clear how many people would need to receive the vaccine to prevent one hospitalization from CDI, or one death, or one case. Because the study groups had fewer episodes of CDI than anticipated, it watered down the power of this investigation to provide definitive answers regarding its true efficacy.”
Dr. Aronoff concluded, “All things considered, I am a cup half-full type of person on these topline results, since there are indications of reducing disease incidence and severity. We can build on these results.”
Dr. Aronoff had a basic science C. diff research grant from Pfizer in 2018-2019 that was not related to vaccines or therapeutics.
A version of this article first appeared on Medscape.com.
New guidelines on MRI use in patients with MS explained
MS affects approximately one million people in the United States. As family physicians, these guidelines are important to know, because we are often the ones who make the initial diagnosis of MS. Similarly, if we order the wrong imaging study, we can miss making an accurate diagnosis.
The new guidelines (MAGNIMS), which were sponsored by the Consortium of Multiple Sclerosis Centres, were published in August. The documents offers detailed guidance on the use of standardized MRI protocols as well as the use of IV gadolinium contrast agents, including in children and pregnant patients.
It is advised to use 3-D techniques (as opposed to two-dimensional) and it is noted that this is becoming more clinically available. Sagittal 3-D T2-weighted fluid-attenuated inversion recovery (FLAIR) is the core sequence considered for MS diagnosis and monitoring because of its high sensitivity. High-quality 2-D pulse sequences can be used alternatively when 3-D FLAIR is not available.
When 3 T scanners are not available, 1.5 T scanners are sufficient. However, 3 T scanners do have a higher detection rate for MS lesions. In evaluating the imaging, T2 lesion counts, gadolinium lesion counts, and interval changes should be reported.
The use of GBCAs (gadolinium-based contrast agents) is needed to diagnose MS and rule out other diseases. The time between injection of contrast should ideally be 10 minutes but no less than 5. Optic nerve MRI is recommended only in patients with atypical symptoms, such as new visual symptoms. Spinal cord MRI is also not routinely advised unless it is needed for prognosis.
When the initial MRI does not meet the full criteria of MS, brain MRI should be repeated every 6-12 months in suspected cases. The same modality should be used each time. After treatment is started, it is recommended to perform MRI without GBCAs for 3 months and annual follow ups. The use of GBCAs-free MRIs for routine follow up is a new recommendation compared to previous ones. However, if the use of GBCAs would change the management, then they should be utilized for monitoring.
The same imaging standards are recommended in pediatric patients. Spinal cord MRI should be utilized in kids with spinal cord symptoms or inconclusive brain MRI. Similar scan frequency is recommended as in adults. MRI is not contraindicated during pregnancy but should be decided on an individual basis. Standard protocols should be used as well as a magnetic field strength of 1.5 T. GBCAs should not be used during pregnancy. There are no limitations in the postpartum period.
The complete set of guidelines is quite extensive and adds to the previous guidelines published in 2017. They were first published in The Lancet Neurology.
While most of these patients will be referred to neurologists, as the primary care physician it is our responsibility to know all aspects of our patients’ diseases and treatments. While we may not be actively treating MS in these patients, we need to know their medications, how they interact with others, and how their disease is progressing
Additionally, we may be the ones asked to order MRIs for monitoring. It is imperative that we know the guidelines for how to do this.
Dr. Girgis practices family medicine in South River, N.J., and is a clinical assistant professor of family medicine at Robert Wood Johnson Medical School, New Brunswick, N.J. You can contact her at fpnews@mdedge.com.
MS affects approximately one million people in the United States. As family physicians, these guidelines are important to know, because we are often the ones who make the initial diagnosis of MS. Similarly, if we order the wrong imaging study, we can miss making an accurate diagnosis.
The new guidelines (MAGNIMS), which were sponsored by the Consortium of Multiple Sclerosis Centres, were published in August. The documents offers detailed guidance on the use of standardized MRI protocols as well as the use of IV gadolinium contrast agents, including in children and pregnant patients.
It is advised to use 3-D techniques (as opposed to two-dimensional) and it is noted that this is becoming more clinically available. Sagittal 3-D T2-weighted fluid-attenuated inversion recovery (FLAIR) is the core sequence considered for MS diagnosis and monitoring because of its high sensitivity. High-quality 2-D pulse sequences can be used alternatively when 3-D FLAIR is not available.
When 3 T scanners are not available, 1.5 T scanners are sufficient. However, 3 T scanners do have a higher detection rate for MS lesions. In evaluating the imaging, T2 lesion counts, gadolinium lesion counts, and interval changes should be reported.
The use of GBCAs (gadolinium-based contrast agents) is needed to diagnose MS and rule out other diseases. The time between injection of contrast should ideally be 10 minutes but no less than 5. Optic nerve MRI is recommended only in patients with atypical symptoms, such as new visual symptoms. Spinal cord MRI is also not routinely advised unless it is needed for prognosis.
When the initial MRI does not meet the full criteria of MS, brain MRI should be repeated every 6-12 months in suspected cases. The same modality should be used each time. After treatment is started, it is recommended to perform MRI without GBCAs for 3 months and annual follow ups. The use of GBCAs-free MRIs for routine follow up is a new recommendation compared to previous ones. However, if the use of GBCAs would change the management, then they should be utilized for monitoring.
The same imaging standards are recommended in pediatric patients. Spinal cord MRI should be utilized in kids with spinal cord symptoms or inconclusive brain MRI. Similar scan frequency is recommended as in adults. MRI is not contraindicated during pregnancy but should be decided on an individual basis. Standard protocols should be used as well as a magnetic field strength of 1.5 T. GBCAs should not be used during pregnancy. There are no limitations in the postpartum period.
The complete set of guidelines is quite extensive and adds to the previous guidelines published in 2017. They were first published in The Lancet Neurology.
While most of these patients will be referred to neurologists, as the primary care physician it is our responsibility to know all aspects of our patients’ diseases and treatments. While we may not be actively treating MS in these patients, we need to know their medications, how they interact with others, and how their disease is progressing
Additionally, we may be the ones asked to order MRIs for monitoring. It is imperative that we know the guidelines for how to do this.
Dr. Girgis practices family medicine in South River, N.J., and is a clinical assistant professor of family medicine at Robert Wood Johnson Medical School, New Brunswick, N.J. You can contact her at fpnews@mdedge.com.
MS affects approximately one million people in the United States. As family physicians, these guidelines are important to know, because we are often the ones who make the initial diagnosis of MS. Similarly, if we order the wrong imaging study, we can miss making an accurate diagnosis.
The new guidelines (MAGNIMS), which were sponsored by the Consortium of Multiple Sclerosis Centres, were published in August. The documents offers detailed guidance on the use of standardized MRI protocols as well as the use of IV gadolinium contrast agents, including in children and pregnant patients.
It is advised to use 3-D techniques (as opposed to two-dimensional) and it is noted that this is becoming more clinically available. Sagittal 3-D T2-weighted fluid-attenuated inversion recovery (FLAIR) is the core sequence considered for MS diagnosis and monitoring because of its high sensitivity. High-quality 2-D pulse sequences can be used alternatively when 3-D FLAIR is not available.
When 3 T scanners are not available, 1.5 T scanners are sufficient. However, 3 T scanners do have a higher detection rate for MS lesions. In evaluating the imaging, T2 lesion counts, gadolinium lesion counts, and interval changes should be reported.
The use of GBCAs (gadolinium-based contrast agents) is needed to diagnose MS and rule out other diseases. The time between injection of contrast should ideally be 10 minutes but no less than 5. Optic nerve MRI is recommended only in patients with atypical symptoms, such as new visual symptoms. Spinal cord MRI is also not routinely advised unless it is needed for prognosis.
When the initial MRI does not meet the full criteria of MS, brain MRI should be repeated every 6-12 months in suspected cases. The same modality should be used each time. After treatment is started, it is recommended to perform MRI without GBCAs for 3 months and annual follow ups. The use of GBCAs-free MRIs for routine follow up is a new recommendation compared to previous ones. However, if the use of GBCAs would change the management, then they should be utilized for monitoring.
The same imaging standards are recommended in pediatric patients. Spinal cord MRI should be utilized in kids with spinal cord symptoms or inconclusive brain MRI. Similar scan frequency is recommended as in adults. MRI is not contraindicated during pregnancy but should be decided on an individual basis. Standard protocols should be used as well as a magnetic field strength of 1.5 T. GBCAs should not be used during pregnancy. There are no limitations in the postpartum period.
The complete set of guidelines is quite extensive and adds to the previous guidelines published in 2017. They were first published in The Lancet Neurology.
While most of these patients will be referred to neurologists, as the primary care physician it is our responsibility to know all aspects of our patients’ diseases and treatments. While we may not be actively treating MS in these patients, we need to know their medications, how they interact with others, and how their disease is progressing
Additionally, we may be the ones asked to order MRIs for monitoring. It is imperative that we know the guidelines for how to do this.
Dr. Girgis practices family medicine in South River, N.J., and is a clinical assistant professor of family medicine at Robert Wood Johnson Medical School, New Brunswick, N.J. You can contact her at fpnews@mdedge.com.
New carcinogens added to toxicology list
From environmental tobacco smoke to ultraviolet (UV) radiation, diesel exhaust particulates, lead, and now, chronic infection with Helicobacter pylori (H pylori) —the Report on Carcinogens has regularly updated the list of substances known or “reasonably anticipated” to cause cancer.
The 15th report, which is prepared by the National Toxicology Program (NTP) for the Department of Health and Human Services, has 8 new entries, bringing the number of human carcinogens (eg, metals, pesticides, and drugs) on the list to 256. (The first report, released in 1980, listed 26.)
In 1971, then-President Nixon declared “war on cancer” (the second leading cause of death in the United States) and signed the National Cancer Act. In 1978, Congress ordered the Report on Carcinogens, to educate the public and health professionals on potential environmental carcinogenic hazards.
Perhaps disheartening to know that even with 256 entries, the list probably understates the number of carcinogens humans and other creatures are exposed to. But things can change with time. Each list goes through a rigorous round of reviews. Sometimes substances are “delisted” after, for instance, litigation or new research. Saccharin, for example, was removed from the ninth edition. It was listed as “reasonably anticipated” in 1981, based on “sufficient evidence of carcinogenicity in experimental animals.” It was removed, however, after extensive review of decades of saccharin use determined that the data were not sufficient to meet current criteria. Further research had revealed, also, that the observed bladder tumors in rats arose from a mechanism not relevant to humans.
Other entries, such as the controversial listing of the cancer drug tamoxifen, walk a fine line between risk and benefit. Tamoxifen, first listed in the ninth report (and still in the 15th report), was included because studies revealed that it could increase the risk of uterine cancer in women. But there also was conclusive evidence that it may prevent or delay breast cancer in women who are at high risk.
Ultimately, the report’s authors make it clear that it is for informative value and guidance, not necessarily a dictate. As one report put it: “Personal decisions concerning voluntary exposures to carcinogenic agents need to be based on additional information that is beyond the scope” of the report.
“As the identification of carcinogens is a key step in cancer prevention,” said Rick Woychik, PhD, director of the National Institute of Environmental Health Sciences and NTP, “publication of the report represents an important government activity towards improving public health.”
A version of this article first appeared on Medscape.com.
From environmental tobacco smoke to ultraviolet (UV) radiation, diesel exhaust particulates, lead, and now, chronic infection with Helicobacter pylori (H pylori) —the Report on Carcinogens has regularly updated the list of substances known or “reasonably anticipated” to cause cancer.
The 15th report, which is prepared by the National Toxicology Program (NTP) for the Department of Health and Human Services, has 8 new entries, bringing the number of human carcinogens (eg, metals, pesticides, and drugs) on the list to 256. (The first report, released in 1980, listed 26.)
In 1971, then-President Nixon declared “war on cancer” (the second leading cause of death in the United States) and signed the National Cancer Act. In 1978, Congress ordered the Report on Carcinogens, to educate the public and health professionals on potential environmental carcinogenic hazards.
Perhaps disheartening to know that even with 256 entries, the list probably understates the number of carcinogens humans and other creatures are exposed to. But things can change with time. Each list goes through a rigorous round of reviews. Sometimes substances are “delisted” after, for instance, litigation or new research. Saccharin, for example, was removed from the ninth edition. It was listed as “reasonably anticipated” in 1981, based on “sufficient evidence of carcinogenicity in experimental animals.” It was removed, however, after extensive review of decades of saccharin use determined that the data were not sufficient to meet current criteria. Further research had revealed, also, that the observed bladder tumors in rats arose from a mechanism not relevant to humans.
Other entries, such as the controversial listing of the cancer drug tamoxifen, walk a fine line between risk and benefit. Tamoxifen, first listed in the ninth report (and still in the 15th report), was included because studies revealed that it could increase the risk of uterine cancer in women. But there also was conclusive evidence that it may prevent or delay breast cancer in women who are at high risk.
Ultimately, the report’s authors make it clear that it is for informative value and guidance, not necessarily a dictate. As one report put it: “Personal decisions concerning voluntary exposures to carcinogenic agents need to be based on additional information that is beyond the scope” of the report.
“As the identification of carcinogens is a key step in cancer prevention,” said Rick Woychik, PhD, director of the National Institute of Environmental Health Sciences and NTP, “publication of the report represents an important government activity towards improving public health.”
A version of this article first appeared on Medscape.com.
From environmental tobacco smoke to ultraviolet (UV) radiation, diesel exhaust particulates, lead, and now, chronic infection with Helicobacter pylori (H pylori) —the Report on Carcinogens has regularly updated the list of substances known or “reasonably anticipated” to cause cancer.
The 15th report, which is prepared by the National Toxicology Program (NTP) for the Department of Health and Human Services, has 8 new entries, bringing the number of human carcinogens (eg, metals, pesticides, and drugs) on the list to 256. (The first report, released in 1980, listed 26.)
In 1971, then-President Nixon declared “war on cancer” (the second leading cause of death in the United States) and signed the National Cancer Act. In 1978, Congress ordered the Report on Carcinogens, to educate the public and health professionals on potential environmental carcinogenic hazards.
Perhaps disheartening to know that even with 256 entries, the list probably understates the number of carcinogens humans and other creatures are exposed to. But things can change with time. Each list goes through a rigorous round of reviews. Sometimes substances are “delisted” after, for instance, litigation or new research. Saccharin, for example, was removed from the ninth edition. It was listed as “reasonably anticipated” in 1981, based on “sufficient evidence of carcinogenicity in experimental animals.” It was removed, however, after extensive review of decades of saccharin use determined that the data were not sufficient to meet current criteria. Further research had revealed, also, that the observed bladder tumors in rats arose from a mechanism not relevant to humans.
Other entries, such as the controversial listing of the cancer drug tamoxifen, walk a fine line between risk and benefit. Tamoxifen, first listed in the ninth report (and still in the 15th report), was included because studies revealed that it could increase the risk of uterine cancer in women. But there also was conclusive evidence that it may prevent or delay breast cancer in women who are at high risk.
Ultimately, the report’s authors make it clear that it is for informative value and guidance, not necessarily a dictate. As one report put it: “Personal decisions concerning voluntary exposures to carcinogenic agents need to be based on additional information that is beyond the scope” of the report.
“As the identification of carcinogens is a key step in cancer prevention,” said Rick Woychik, PhD, director of the National Institute of Environmental Health Sciences and NTP, “publication of the report represents an important government activity towards improving public health.”
A version of this article first appeared on Medscape.com.
If you’ve never had COVID, should you relax or worry?
If you’re among those people in the United States who never had COVID-19, how should you think about your risk?
According to the Centers for Disease Control and Prevention (CDC), more than half of people in the United States are in the never-got-COVID category.
The CDC estimated that by the end of January, 43.4% in the United States had developed antibodies to SARS-CoV-2 triggered by infection, not by vaccination — suggesting nearly 60% of people have never been infected.
Now mask mandates are lifting, and daily case and death numbers are plunging. According to the New York Times tracker, new cases are down 51% for the past 2 weeks, and deaths have fallen 30% in that period.
So as those who have so far escaped the virus venture further out into reopened environments, should they worry more or less about risk than their previously infected counterparts?
No “suit of armor”
William Schaffner, MD, an infectious disease expert at Vanderbilt University School of Medicine in Nashville, Tenn., said in an interview that science has not been able to determine why some people have been able to be stay COVID-free when the virus was raging and exposure was ubiquitous.
He said it’s important to remember that though some people think they have never had COVID, they may have been asymptomatic or attributed mild symptoms to something else.
“People may have conceivably — but we can’t define them yet — different capacities to ward off viruses or bacteria,” Dr. Schaffner said.
Could it be that some people have a better immune system or genetic component or environmental reason that they are less susceptible to infectious disease? “We can’t define that in 2022 medicine, but it could be,” he said.
More is known about why people with the same COVID exposure may have different levels of illness severity.
“They’re more likely to get seriously ill if they have a list of predisposing conditions — if they’re older, if they’re frail, if they have underlying illness or are obese. All of those things clearly impair the body’s response to virus,” Dr. Schaffner said.
He warns those who have never been infected, though, not to assume they have “a suit of armor.”
All should continue to follow guidance on getting vaccinated, and those vaccinated should continue to get boosted, Dr. Schaffner said.
“Clearly, the data show that if you are vaccinated and boosted, you’re protected much more securely against severe disease,” he said.
If the never-COVIDs develop a respiratory infection, they should still get tested for COVID, Dr. Schaffner said.
He said while both vaccines and previous natural infection offer protection, the duration of that protection is not yet known.
“We have to stay tuned,” Dr. Schaffner said. “There may be a recommendation in the future to get a booster annually or something like that. We need to be open to those down the road.”
Amesh Adalja, MD, senior scholar at the Johns Hopkins Center for Health Security in Baltimore, says it’s unclear why some people have been able to avoid COVID.
“The explanation is likely multifactorial and involves behaviors as well as possible idiosyncrasies with their immune systems that are genetically based,” he said. “It also may be the case that inapparent infections occurred and went undiagnosed.”
Dr. Adalja agrees, though, that this isn’t the time to get overconfident with risk-taking where COVID is concerned.
“People who have not knowingly been infected with COVID should be vaccinated, and after that, be assured that they are protected against serious disease from this virus,” he said.
Genetic protection?
A new study in Nature Genetics explains a potential genetic relationship. Study authors found evidence that levels of expression of angiotensin-converting enzyme 2 (ACE2) – which helps regulate blood pressure, wound healing, and inflammation, but has also been shown to serve as an entry point into cells for some coronaviruses like SARS-CoV-2 — influence COVID-19 risk.
Manuel A. Ferreira, PhD, an executive director for analytic genetics at Regeneron Pharmaceuticals, said in an interview that ACE2 receptors — what he calls the “gateways” for SARS-CoV-2 to enter the body — are different in people who have inherited a particular allele.
The researchers have found that that allele is associated with lower risk of SARS-CoV-2 infection.
“It’s quite substantial -- a 40% risk reduction if you carry the allele that reduces ACE2 expression,” he said. They were not able to discern from this study, however, whether that could predict severity of disease.
The team also looked at a series of six genetic variants elsewhere in the genome and developed a risk score to see if it was possible to predict who might be more susceptible to severe COVID.
Dr. Ferreira said the score only slightly improved predictive abilities beyond factors such as age, sex, weight, and comorbidities. Further information will help hone the ability to predict the likelihood of developing severe disease on the basis of genetics, Ferreira said.
“As we identify more genetic risk factors for COVID — variants like the ACE2 variant that will affect your risk of having COVID — the more informative the risk score will be,” he said.
Several authors of the Nature Genetics article are current and/or former employees of AncestryDNA and may hold equity in AncestryDNA. Several are Regeneron employees and/or hold stock in the company. Dr. Ferreira is an employee of Regeneron and holds stock in the company. Dr. Schaffner and Dr. Adalja report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
If you’re among those people in the United States who never had COVID-19, how should you think about your risk?
According to the Centers for Disease Control and Prevention (CDC), more than half of people in the United States are in the never-got-COVID category.
The CDC estimated that by the end of January, 43.4% in the United States had developed antibodies to SARS-CoV-2 triggered by infection, not by vaccination — suggesting nearly 60% of people have never been infected.
Now mask mandates are lifting, and daily case and death numbers are plunging. According to the New York Times tracker, new cases are down 51% for the past 2 weeks, and deaths have fallen 30% in that period.
So as those who have so far escaped the virus venture further out into reopened environments, should they worry more or less about risk than their previously infected counterparts?
No “suit of armor”
William Schaffner, MD, an infectious disease expert at Vanderbilt University School of Medicine in Nashville, Tenn., said in an interview that science has not been able to determine why some people have been able to be stay COVID-free when the virus was raging and exposure was ubiquitous.
He said it’s important to remember that though some people think they have never had COVID, they may have been asymptomatic or attributed mild symptoms to something else.
“People may have conceivably — but we can’t define them yet — different capacities to ward off viruses or bacteria,” Dr. Schaffner said.
Could it be that some people have a better immune system or genetic component or environmental reason that they are less susceptible to infectious disease? “We can’t define that in 2022 medicine, but it could be,” he said.
More is known about why people with the same COVID exposure may have different levels of illness severity.
“They’re more likely to get seriously ill if they have a list of predisposing conditions — if they’re older, if they’re frail, if they have underlying illness or are obese. All of those things clearly impair the body’s response to virus,” Dr. Schaffner said.
He warns those who have never been infected, though, not to assume they have “a suit of armor.”
All should continue to follow guidance on getting vaccinated, and those vaccinated should continue to get boosted, Dr. Schaffner said.
“Clearly, the data show that if you are vaccinated and boosted, you’re protected much more securely against severe disease,” he said.
If the never-COVIDs develop a respiratory infection, they should still get tested for COVID, Dr. Schaffner said.
He said while both vaccines and previous natural infection offer protection, the duration of that protection is not yet known.
“We have to stay tuned,” Dr. Schaffner said. “There may be a recommendation in the future to get a booster annually or something like that. We need to be open to those down the road.”
Amesh Adalja, MD, senior scholar at the Johns Hopkins Center for Health Security in Baltimore, says it’s unclear why some people have been able to avoid COVID.
“The explanation is likely multifactorial and involves behaviors as well as possible idiosyncrasies with their immune systems that are genetically based,” he said. “It also may be the case that inapparent infections occurred and went undiagnosed.”
Dr. Adalja agrees, though, that this isn’t the time to get overconfident with risk-taking where COVID is concerned.
“People who have not knowingly been infected with COVID should be vaccinated, and after that, be assured that they are protected against serious disease from this virus,” he said.
Genetic protection?
A new study in Nature Genetics explains a potential genetic relationship. Study authors found evidence that levels of expression of angiotensin-converting enzyme 2 (ACE2) – which helps regulate blood pressure, wound healing, and inflammation, but has also been shown to serve as an entry point into cells for some coronaviruses like SARS-CoV-2 — influence COVID-19 risk.
Manuel A. Ferreira, PhD, an executive director for analytic genetics at Regeneron Pharmaceuticals, said in an interview that ACE2 receptors — what he calls the “gateways” for SARS-CoV-2 to enter the body — are different in people who have inherited a particular allele.
The researchers have found that that allele is associated with lower risk of SARS-CoV-2 infection.
“It’s quite substantial -- a 40% risk reduction if you carry the allele that reduces ACE2 expression,” he said. They were not able to discern from this study, however, whether that could predict severity of disease.
The team also looked at a series of six genetic variants elsewhere in the genome and developed a risk score to see if it was possible to predict who might be more susceptible to severe COVID.
Dr. Ferreira said the score only slightly improved predictive abilities beyond factors such as age, sex, weight, and comorbidities. Further information will help hone the ability to predict the likelihood of developing severe disease on the basis of genetics, Ferreira said.
“As we identify more genetic risk factors for COVID — variants like the ACE2 variant that will affect your risk of having COVID — the more informative the risk score will be,” he said.
Several authors of the Nature Genetics article are current and/or former employees of AncestryDNA and may hold equity in AncestryDNA. Several are Regeneron employees and/or hold stock in the company. Dr. Ferreira is an employee of Regeneron and holds stock in the company. Dr. Schaffner and Dr. Adalja report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
If you’re among those people in the United States who never had COVID-19, how should you think about your risk?
According to the Centers for Disease Control and Prevention (CDC), more than half of people in the United States are in the never-got-COVID category.
The CDC estimated that by the end of January, 43.4% in the United States had developed antibodies to SARS-CoV-2 triggered by infection, not by vaccination — suggesting nearly 60% of people have never been infected.
Now mask mandates are lifting, and daily case and death numbers are plunging. According to the New York Times tracker, new cases are down 51% for the past 2 weeks, and deaths have fallen 30% in that period.
So as those who have so far escaped the virus venture further out into reopened environments, should they worry more or less about risk than their previously infected counterparts?
No “suit of armor”
William Schaffner, MD, an infectious disease expert at Vanderbilt University School of Medicine in Nashville, Tenn., said in an interview that science has not been able to determine why some people have been able to be stay COVID-free when the virus was raging and exposure was ubiquitous.
He said it’s important to remember that though some people think they have never had COVID, they may have been asymptomatic or attributed mild symptoms to something else.
“People may have conceivably — but we can’t define them yet — different capacities to ward off viruses or bacteria,” Dr. Schaffner said.
Could it be that some people have a better immune system or genetic component or environmental reason that they are less susceptible to infectious disease? “We can’t define that in 2022 medicine, but it could be,” he said.
More is known about why people with the same COVID exposure may have different levels of illness severity.
“They’re more likely to get seriously ill if they have a list of predisposing conditions — if they’re older, if they’re frail, if they have underlying illness or are obese. All of those things clearly impair the body’s response to virus,” Dr. Schaffner said.
He warns those who have never been infected, though, not to assume they have “a suit of armor.”
All should continue to follow guidance on getting vaccinated, and those vaccinated should continue to get boosted, Dr. Schaffner said.
“Clearly, the data show that if you are vaccinated and boosted, you’re protected much more securely against severe disease,” he said.
If the never-COVIDs develop a respiratory infection, they should still get tested for COVID, Dr. Schaffner said.
He said while both vaccines and previous natural infection offer protection, the duration of that protection is not yet known.
“We have to stay tuned,” Dr. Schaffner said. “There may be a recommendation in the future to get a booster annually or something like that. We need to be open to those down the road.”
Amesh Adalja, MD, senior scholar at the Johns Hopkins Center for Health Security in Baltimore, says it’s unclear why some people have been able to avoid COVID.
“The explanation is likely multifactorial and involves behaviors as well as possible idiosyncrasies with their immune systems that are genetically based,” he said. “It also may be the case that inapparent infections occurred and went undiagnosed.”
Dr. Adalja agrees, though, that this isn’t the time to get overconfident with risk-taking where COVID is concerned.
“People who have not knowingly been infected with COVID should be vaccinated, and after that, be assured that they are protected against serious disease from this virus,” he said.
Genetic protection?
A new study in Nature Genetics explains a potential genetic relationship. Study authors found evidence that levels of expression of angiotensin-converting enzyme 2 (ACE2) – which helps regulate blood pressure, wound healing, and inflammation, but has also been shown to serve as an entry point into cells for some coronaviruses like SARS-CoV-2 — influence COVID-19 risk.
Manuel A. Ferreira, PhD, an executive director for analytic genetics at Regeneron Pharmaceuticals, said in an interview that ACE2 receptors — what he calls the “gateways” for SARS-CoV-2 to enter the body — are different in people who have inherited a particular allele.
The researchers have found that that allele is associated with lower risk of SARS-CoV-2 infection.
“It’s quite substantial -- a 40% risk reduction if you carry the allele that reduces ACE2 expression,” he said. They were not able to discern from this study, however, whether that could predict severity of disease.
The team also looked at a series of six genetic variants elsewhere in the genome and developed a risk score to see if it was possible to predict who might be more susceptible to severe COVID.
Dr. Ferreira said the score only slightly improved predictive abilities beyond factors such as age, sex, weight, and comorbidities. Further information will help hone the ability to predict the likelihood of developing severe disease on the basis of genetics, Ferreira said.
“As we identify more genetic risk factors for COVID — variants like the ACE2 variant that will affect your risk of having COVID — the more informative the risk score will be,” he said.
Several authors of the Nature Genetics article are current and/or former employees of AncestryDNA and may hold equity in AncestryDNA. Several are Regeneron employees and/or hold stock in the company. Dr. Ferreira is an employee of Regeneron and holds stock in the company. Dr. Schaffner and Dr. Adalja report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Height an ‘overlooked risk factor’ for colorectal cancer?
A new meta-analysis provides more evidence that
“There are well-known modifiable dietary associations for colorectal cancer, such as processed red meats and smoking, but guidelines currently are fixated on family history, and height is clinically neglected when it comes to risk screening,” study investigator Gerard Mullin, MD, with Johns Hopkins University, Baltimore, said in a news release. This large study “builds on evidence that taller height is an overlooked risk factor and should be considered when evaluating and recommending patients for colorectal cancer screenings.”
The study was published online March 1 in Cancer Epidemiology, Biomarkers & Prevention.
The evidence: Height and cancer risk
Height has been actively studied as a potential nonmodifiable risk factor for a range of cancers, including CRC.
In one large prospective study of postmenopausal women, researchers found a modest but statistically significant positive association between height and risk for any cancer and for melanoma, multiple myeloma, and cancers of the thyroid, ovary, colorectum, and endometrium.
A separate study found that tall men, especially those who are long-legged, may be at increased risk for prostate cancer, including high-grade tumors, relative to men of more modest stature.
However, the study authors point out, past studies have also produced mixed results, used inconsistent measures of height, and failed to include the risk of adenomas.
In the current meta-analysis, the investigators included 47 international, observational studies involving 280,644 adults with CRC and 14,139 cases of colorectal adenoma.
Because the definition of tallness differs around the world, the researchers compared the highest versus the lowest height percentile of various study groups. The findings were adjusted for demographic, socioeconomic, behavioral, and other known risk factors for CRC.
Overall, the investigators found that the tallest individuals within the highest percentile of height had a 24% higher risk of developing CRC compared to the shortest individuals within the lowest percentile (hazard ratio [HR], 1.24; P < .001).
In addition, they found that every 10-cm increase (about 4 inches) in height was associated with a 14% increased risk of developing CRC (HR, 1.14; P < .001) and a 6% increased likelihood of adenomas (odds ratio [OR], 1.06; P = .03).
In the United States, the average height for men is 5 feet, 9 inches, and for women it is 5 feet, 4 inches, which means men who are 6 feet, 1 inch and women who are 5 feet, 8 inches or taller have a 14% increased risk of CRC and a 6% increased risk of adenomas, the researchers explained.
According to co–first author Elinor Zhou, MD, also with Johns Hopkins University, a potential explanation for this link “is that adult height correlates with body organ size. More active proliferation in organs of taller people could increase the possibility of mutations leading to malignant transformation.”
The study authors said more research is needed to identify particular subgroups of tall people at risk for CRC.
“For instance, tall athletes and individuals with inherited tallness, such as those with Marfan syndrome, could be screened earlier and the impact of height further explored,” Dr. Zhou said.
Plus, Dr. Zhou added, more studies are needed to “definitively say at what height you would need earlier colorectal cancer screening.”
The current study was supported by grants from Bloomberg Philanthropies, intramural funds, and the Johns Hopkins Cancer Center Support Grant. The authors declared no conflicts of interest.
A version of this article first appeared on Medscape.com.
A new meta-analysis provides more evidence that
“There are well-known modifiable dietary associations for colorectal cancer, such as processed red meats and smoking, but guidelines currently are fixated on family history, and height is clinically neglected when it comes to risk screening,” study investigator Gerard Mullin, MD, with Johns Hopkins University, Baltimore, said in a news release. This large study “builds on evidence that taller height is an overlooked risk factor and should be considered when evaluating and recommending patients for colorectal cancer screenings.”
The study was published online March 1 in Cancer Epidemiology, Biomarkers & Prevention.
The evidence: Height and cancer risk
Height has been actively studied as a potential nonmodifiable risk factor for a range of cancers, including CRC.
In one large prospective study of postmenopausal women, researchers found a modest but statistically significant positive association between height and risk for any cancer and for melanoma, multiple myeloma, and cancers of the thyroid, ovary, colorectum, and endometrium.
A separate study found that tall men, especially those who are long-legged, may be at increased risk for prostate cancer, including high-grade tumors, relative to men of more modest stature.
However, the study authors point out, past studies have also produced mixed results, used inconsistent measures of height, and failed to include the risk of adenomas.
In the current meta-analysis, the investigators included 47 international, observational studies involving 280,644 adults with CRC and 14,139 cases of colorectal adenoma.
Because the definition of tallness differs around the world, the researchers compared the highest versus the lowest height percentile of various study groups. The findings were adjusted for demographic, socioeconomic, behavioral, and other known risk factors for CRC.
Overall, the investigators found that the tallest individuals within the highest percentile of height had a 24% higher risk of developing CRC compared to the shortest individuals within the lowest percentile (hazard ratio [HR], 1.24; P < .001).
In addition, they found that every 10-cm increase (about 4 inches) in height was associated with a 14% increased risk of developing CRC (HR, 1.14; P < .001) and a 6% increased likelihood of adenomas (odds ratio [OR], 1.06; P = .03).
In the United States, the average height for men is 5 feet, 9 inches, and for women it is 5 feet, 4 inches, which means men who are 6 feet, 1 inch and women who are 5 feet, 8 inches or taller have a 14% increased risk of CRC and a 6% increased risk of adenomas, the researchers explained.
According to co–first author Elinor Zhou, MD, also with Johns Hopkins University, a potential explanation for this link “is that adult height correlates with body organ size. More active proliferation in organs of taller people could increase the possibility of mutations leading to malignant transformation.”
The study authors said more research is needed to identify particular subgroups of tall people at risk for CRC.
“For instance, tall athletes and individuals with inherited tallness, such as those with Marfan syndrome, could be screened earlier and the impact of height further explored,” Dr. Zhou said.
Plus, Dr. Zhou added, more studies are needed to “definitively say at what height you would need earlier colorectal cancer screening.”
The current study was supported by grants from Bloomberg Philanthropies, intramural funds, and the Johns Hopkins Cancer Center Support Grant. The authors declared no conflicts of interest.
A version of this article first appeared on Medscape.com.
A new meta-analysis provides more evidence that
“There are well-known modifiable dietary associations for colorectal cancer, such as processed red meats and smoking, but guidelines currently are fixated on family history, and height is clinically neglected when it comes to risk screening,” study investigator Gerard Mullin, MD, with Johns Hopkins University, Baltimore, said in a news release. This large study “builds on evidence that taller height is an overlooked risk factor and should be considered when evaluating and recommending patients for colorectal cancer screenings.”
The study was published online March 1 in Cancer Epidemiology, Biomarkers & Prevention.
The evidence: Height and cancer risk
Height has been actively studied as a potential nonmodifiable risk factor for a range of cancers, including CRC.
In one large prospective study of postmenopausal women, researchers found a modest but statistically significant positive association between height and risk for any cancer and for melanoma, multiple myeloma, and cancers of the thyroid, ovary, colorectum, and endometrium.
A separate study found that tall men, especially those who are long-legged, may be at increased risk for prostate cancer, including high-grade tumors, relative to men of more modest stature.
However, the study authors point out, past studies have also produced mixed results, used inconsistent measures of height, and failed to include the risk of adenomas.
In the current meta-analysis, the investigators included 47 international, observational studies involving 280,644 adults with CRC and 14,139 cases of colorectal adenoma.
Because the definition of tallness differs around the world, the researchers compared the highest versus the lowest height percentile of various study groups. The findings were adjusted for demographic, socioeconomic, behavioral, and other known risk factors for CRC.
Overall, the investigators found that the tallest individuals within the highest percentile of height had a 24% higher risk of developing CRC compared to the shortest individuals within the lowest percentile (hazard ratio [HR], 1.24; P < .001).
In addition, they found that every 10-cm increase (about 4 inches) in height was associated with a 14% increased risk of developing CRC (HR, 1.14; P < .001) and a 6% increased likelihood of adenomas (odds ratio [OR], 1.06; P = .03).
In the United States, the average height for men is 5 feet, 9 inches, and for women it is 5 feet, 4 inches, which means men who are 6 feet, 1 inch and women who are 5 feet, 8 inches or taller have a 14% increased risk of CRC and a 6% increased risk of adenomas, the researchers explained.
According to co–first author Elinor Zhou, MD, also with Johns Hopkins University, a potential explanation for this link “is that adult height correlates with body organ size. More active proliferation in organs of taller people could increase the possibility of mutations leading to malignant transformation.”
The study authors said more research is needed to identify particular subgroups of tall people at risk for CRC.
“For instance, tall athletes and individuals with inherited tallness, such as those with Marfan syndrome, could be screened earlier and the impact of height further explored,” Dr. Zhou said.
Plus, Dr. Zhou added, more studies are needed to “definitively say at what height you would need earlier colorectal cancer screening.”
The current study was supported by grants from Bloomberg Philanthropies, intramural funds, and the Johns Hopkins Cancer Center Support Grant. The authors declared no conflicts of interest.
A version of this article first appeared on Medscape.com.
FROM CANCER EPIDEMIOLOGY, BIOMARKERS & PREVENTION
Wake Forest Cancer Center director fired, advisory board resigns
and withdrew their endorsement for renewal of the center’s National Cancer Institute comprehensive cancer center support grant.
The move was prompted by the abrupt firing of center director Boris Pasche, MD, PhD, on February 10, one day after NCI renewed a multimillion dollar grant.
The Cancer Letter broke the story and published the resignation letter from the EAB. It was signed by board chair Gerold Bepler, MD, PhD, CEO and director of the Karmanos Cancer Institute, Detroit, on behalf of the board.
The mass resignation of an EAB, a panel of outside experts that help shepherd cancer centers through the NCI grant process, is “highly unusual,” according to The Cancer Letter. It also raises concerns about the “immediate future” of Wake Forest’s cancer center, the publication added.
Numerous people involved with the situation did not respond or declined to comment when this news organization requested additional information and updates, including questions about the reason for Dr. Pasche’s termination; whether or not withdrawal of the endorsement puts Wake’s NCI designation in jeopardy; and if the EAB is being reconstituted.
A written statement from Wake Forest simply said that “the situation involving Dr. Pasche is an administrative decision. Various administrative changes occur regularly in organizations across the country. Dr. Pasche remains employed by Atrium Health Wake Forest Baptist. We are very grateful to Dr. Pasche for his years of service and many contributions to the mission and vision of our NCI-designated Comprehensive Cancer Center in Winston-Salem.”
Wake’s cancer center is in the process of combining with the Atrium Health Levine Cancer Center, which is not NCI-designated, following Atrium Health system’s recent acquisition of the Wake Forest Baptist Medical Center.
The NCI renewal notice, dated February 9, states that Dr. Pasche “and his leadership team have built a robust, transdisciplinary center that includes 140 scientists.”
Dr. Pasche was fired a day later.
The EAB resignation letter states that during Wake Forest’s recent NCI review process, “leadership gave their glowing endorsement of Dr. Pasche...This endorsement included unequivocal statements of support for Dr. Pasche’s oversight of the combined Atrium-Wake Forest cancer program.”
“What followed was his rapid dismissal after the...notice of award was issued, following a period during which the approach to integration was apparently being revisited,” Dr. Bepler said on behalf of the board.
“It is with sadness and dismay that we witnessed the change in approach by the institutional leadership towards” the merger, he wrote.
The Cancer Letter quotes an unnamed board member as saying, “EABs for cancer centers can only provide value to the center when there is openness and transparency in the process. In the absence of such, I believe the members felt that there was no further utility in providing guidance to the organization.”
The resignation letter was sent to the interim director of Wake’s cancer center, radiation oncologist William Blackstock, Jr, MD, and also copied to Atrium-Wake and NCI leadership.
The resignation letter endorsed Dr. Blackstock’s qualifications to run the center, and noted that as the board is reconstituted, “some of us would be honored to discuss participation...if there is unequivocal evidence from the health system’s senior management for support of a single, academically driven, comprehensive, and integrated cancer center.”
A version of this article first appeared on Medscape.com.
and withdrew their endorsement for renewal of the center’s National Cancer Institute comprehensive cancer center support grant.
The move was prompted by the abrupt firing of center director Boris Pasche, MD, PhD, on February 10, one day after NCI renewed a multimillion dollar grant.
The Cancer Letter broke the story and published the resignation letter from the EAB. It was signed by board chair Gerold Bepler, MD, PhD, CEO and director of the Karmanos Cancer Institute, Detroit, on behalf of the board.
The mass resignation of an EAB, a panel of outside experts that help shepherd cancer centers through the NCI grant process, is “highly unusual,” according to The Cancer Letter. It also raises concerns about the “immediate future” of Wake Forest’s cancer center, the publication added.
Numerous people involved with the situation did not respond or declined to comment when this news organization requested additional information and updates, including questions about the reason for Dr. Pasche’s termination; whether or not withdrawal of the endorsement puts Wake’s NCI designation in jeopardy; and if the EAB is being reconstituted.
A written statement from Wake Forest simply said that “the situation involving Dr. Pasche is an administrative decision. Various administrative changes occur regularly in organizations across the country. Dr. Pasche remains employed by Atrium Health Wake Forest Baptist. We are very grateful to Dr. Pasche for his years of service and many contributions to the mission and vision of our NCI-designated Comprehensive Cancer Center in Winston-Salem.”
Wake’s cancer center is in the process of combining with the Atrium Health Levine Cancer Center, which is not NCI-designated, following Atrium Health system’s recent acquisition of the Wake Forest Baptist Medical Center.
The NCI renewal notice, dated February 9, states that Dr. Pasche “and his leadership team have built a robust, transdisciplinary center that includes 140 scientists.”
Dr. Pasche was fired a day later.
The EAB resignation letter states that during Wake Forest’s recent NCI review process, “leadership gave their glowing endorsement of Dr. Pasche...This endorsement included unequivocal statements of support for Dr. Pasche’s oversight of the combined Atrium-Wake Forest cancer program.”
“What followed was his rapid dismissal after the...notice of award was issued, following a period during which the approach to integration was apparently being revisited,” Dr. Bepler said on behalf of the board.
“It is with sadness and dismay that we witnessed the change in approach by the institutional leadership towards” the merger, he wrote.
The Cancer Letter quotes an unnamed board member as saying, “EABs for cancer centers can only provide value to the center when there is openness and transparency in the process. In the absence of such, I believe the members felt that there was no further utility in providing guidance to the organization.”
The resignation letter was sent to the interim director of Wake’s cancer center, radiation oncologist William Blackstock, Jr, MD, and also copied to Atrium-Wake and NCI leadership.
The resignation letter endorsed Dr. Blackstock’s qualifications to run the center, and noted that as the board is reconstituted, “some of us would be honored to discuss participation...if there is unequivocal evidence from the health system’s senior management for support of a single, academically driven, comprehensive, and integrated cancer center.”
A version of this article first appeared on Medscape.com.
and withdrew their endorsement for renewal of the center’s National Cancer Institute comprehensive cancer center support grant.
The move was prompted by the abrupt firing of center director Boris Pasche, MD, PhD, on February 10, one day after NCI renewed a multimillion dollar grant.
The Cancer Letter broke the story and published the resignation letter from the EAB. It was signed by board chair Gerold Bepler, MD, PhD, CEO and director of the Karmanos Cancer Institute, Detroit, on behalf of the board.
The mass resignation of an EAB, a panel of outside experts that help shepherd cancer centers through the NCI grant process, is “highly unusual,” according to The Cancer Letter. It also raises concerns about the “immediate future” of Wake Forest’s cancer center, the publication added.
Numerous people involved with the situation did not respond or declined to comment when this news organization requested additional information and updates, including questions about the reason for Dr. Pasche’s termination; whether or not withdrawal of the endorsement puts Wake’s NCI designation in jeopardy; and if the EAB is being reconstituted.
A written statement from Wake Forest simply said that “the situation involving Dr. Pasche is an administrative decision. Various administrative changes occur regularly in organizations across the country. Dr. Pasche remains employed by Atrium Health Wake Forest Baptist. We are very grateful to Dr. Pasche for his years of service and many contributions to the mission and vision of our NCI-designated Comprehensive Cancer Center in Winston-Salem.”
Wake’s cancer center is in the process of combining with the Atrium Health Levine Cancer Center, which is not NCI-designated, following Atrium Health system’s recent acquisition of the Wake Forest Baptist Medical Center.
The NCI renewal notice, dated February 9, states that Dr. Pasche “and his leadership team have built a robust, transdisciplinary center that includes 140 scientists.”
Dr. Pasche was fired a day later.
The EAB resignation letter states that during Wake Forest’s recent NCI review process, “leadership gave their glowing endorsement of Dr. Pasche...This endorsement included unequivocal statements of support for Dr. Pasche’s oversight of the combined Atrium-Wake Forest cancer program.”
“What followed was his rapid dismissal after the...notice of award was issued, following a period during which the approach to integration was apparently being revisited,” Dr. Bepler said on behalf of the board.
“It is with sadness and dismay that we witnessed the change in approach by the institutional leadership towards” the merger, he wrote.
The Cancer Letter quotes an unnamed board member as saying, “EABs for cancer centers can only provide value to the center when there is openness and transparency in the process. In the absence of such, I believe the members felt that there was no further utility in providing guidance to the organization.”
The resignation letter was sent to the interim director of Wake’s cancer center, radiation oncologist William Blackstock, Jr, MD, and also copied to Atrium-Wake and NCI leadership.
The resignation letter endorsed Dr. Blackstock’s qualifications to run the center, and noted that as the board is reconstituted, “some of us would be honored to discuss participation...if there is unequivocal evidence from the health system’s senior management for support of a single, academically driven, comprehensive, and integrated cancer center.”
A version of this article first appeared on Medscape.com.
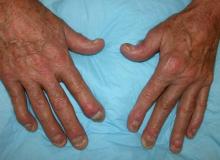
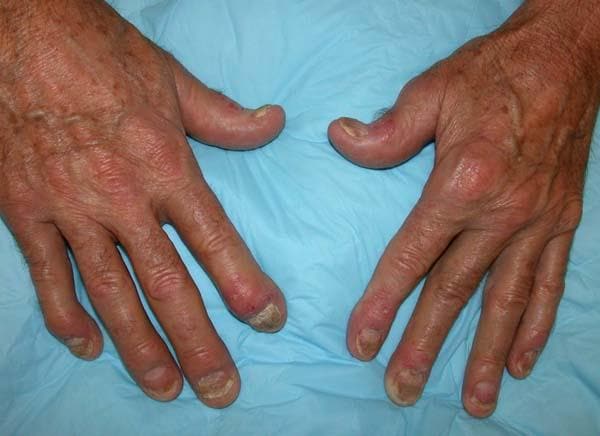
Painful swelling of fingers and toe
Although psoriatic arthritis is not the only disease associated with dactylitis — other culprits are sarcoidosis, septic arthritis, tuberculosis, and gout — dactylitis is one of the characteristic symptoms of psoriatic arthritis. Dactylitis is seen in as many as 35% of patients with psoriatic disease. Dactylitis clinically presents — as in this patient — with sausage-like swelling of the digits. It is included in the Classification Criteria for Psoriatic Arthritis (CASPAR) as one of the hallmarks of psoriatic arthritis.
Dactylitis has been thought to be a result of the concomitant swelling and inflammation of the flexor tendon sheaths of the metacarpophalangeal, metatarsophalangeal, or interphalangeal joints. Flexor tenosynovitis can be detected by examination with MRI and ultrasound. Dactylitis is associated with radiologically evident erosive damage to the joints.
Patients with psoriatic arthritis are typically seronegative for rheumatoid factor and antinuclear antibody; antinuclear antibody titers in persons with psoriatic arthritis do not differ from those of age- and sex-matched controls. C-reactive protein may be elevated but is often normal. Lack of C-reactive protein elevation, however, does not mean that systemic inflammation is absent, but rather indicates that different markers are needed that allow better quantification of systemic inflammation in psoriasis and psoriatic arthritis.
Herbert S. Diamond, MD, Professor of Medicine (retired), Temple University School of Medicine, University of Pittsburgh; Chairman, Department of Medicine Emeritus, Western Pennsylvania Hospital, Pittsburgh, PA.
Herbert S. Diamond, MD, has disclosed no relevant financial relationships.
Although psoriatic arthritis is not the only disease associated with dactylitis — other culprits are sarcoidosis, septic arthritis, tuberculosis, and gout — dactylitis is one of the characteristic symptoms of psoriatic arthritis. Dactylitis is seen in as many as 35% of patients with psoriatic disease. Dactylitis clinically presents — as in this patient — with sausage-like swelling of the digits. It is included in the Classification Criteria for Psoriatic Arthritis (CASPAR) as one of the hallmarks of psoriatic arthritis.
Dactylitis has been thought to be a result of the concomitant swelling and inflammation of the flexor tendon sheaths of the metacarpophalangeal, metatarsophalangeal, or interphalangeal joints. Flexor tenosynovitis can be detected by examination with MRI and ultrasound. Dactylitis is associated with radiologically evident erosive damage to the joints.
Patients with psoriatic arthritis are typically seronegative for rheumatoid factor and antinuclear antibody; antinuclear antibody titers in persons with psoriatic arthritis do not differ from those of age- and sex-matched controls. C-reactive protein may be elevated but is often normal. Lack of C-reactive protein elevation, however, does not mean that systemic inflammation is absent, but rather indicates that different markers are needed that allow better quantification of systemic inflammation in psoriasis and psoriatic arthritis.
Herbert S. Diamond, MD, Professor of Medicine (retired), Temple University School of Medicine, University of Pittsburgh; Chairman, Department of Medicine Emeritus, Western Pennsylvania Hospital, Pittsburgh, PA.
Herbert S. Diamond, MD, has disclosed no relevant financial relationships.
Although psoriatic arthritis is not the only disease associated with dactylitis — other culprits are sarcoidosis, septic arthritis, tuberculosis, and gout — dactylitis is one of the characteristic symptoms of psoriatic arthritis. Dactylitis is seen in as many as 35% of patients with psoriatic disease. Dactylitis clinically presents — as in this patient — with sausage-like swelling of the digits. It is included in the Classification Criteria for Psoriatic Arthritis (CASPAR) as one of the hallmarks of psoriatic arthritis.
Dactylitis has been thought to be a result of the concomitant swelling and inflammation of the flexor tendon sheaths of the metacarpophalangeal, metatarsophalangeal, or interphalangeal joints. Flexor tenosynovitis can be detected by examination with MRI and ultrasound. Dactylitis is associated with radiologically evident erosive damage to the joints.
Patients with psoriatic arthritis are typically seronegative for rheumatoid factor and antinuclear antibody; antinuclear antibody titers in persons with psoriatic arthritis do not differ from those of age- and sex-matched controls. C-reactive protein may be elevated but is often normal. Lack of C-reactive protein elevation, however, does not mean that systemic inflammation is absent, but rather indicates that different markers are needed that allow better quantification of systemic inflammation in psoriasis and psoriatic arthritis.
Herbert S. Diamond, MD, Professor of Medicine (retired), Temple University School of Medicine, University of Pittsburgh; Chairman, Department of Medicine Emeritus, Western Pennsylvania Hospital, Pittsburgh, PA.
Herbert S. Diamond, MD, has disclosed no relevant financial relationships.
A 35-year-old man presents with painful swelling of his right index and ring fingers as well as the fourth toe on his right foot, which has persisted for 5 days. He cannot perform his daily activities owing to severe pain in the affected fingers and toes. His medical history is unremarkable. His paternal uncle had psoriasis, which was successfully treated with adalimumab.
Physical assessment reveals tender, fusiform, swollen soft tissues in the affected fingertips, the fourth toe, and swollen palms. Nails are pitted. Hand radiography reveals mild edema of the soft tissue of the index and ring fingers but no significant joint abnormalities. Enthesitis is not present. Laboratory tests reveal a negative human leukocyte antigen B27 (HLA-B27) test, negative rheumatoid factor, negative antinuclear antibody, and normal C-reactive protein.
Dactylitis was diagnosed on the basis of clinical symptoms, radiographic results, and laboratory findings.
ESC suspension of Russia, Belarus societies sparks controversy
, provoking a heated discussion on whether medical organizations should become involved in politics.
“In the light of the continued aggression against Ukraine by the leaderships of the Russian Federation and Belarus, the European Society of Cardiology (ESC) has temporarily suspended the memberships of the Russian Society of Cardiology and the Belarussian Society of Cardiologists in the ESC,” the ESC statement reads.
“Individuals based in the Russian Federation or in Belarus are excluded from active participation in any ESC event or activity,” it states.
“The ESC very much regrets the effect this may have on individual Russian and Belarussian cardiologists and scientists, but the message to Russian and Belarussian leadership must be distinct and unequivocal,” it adds.
This action from the ESC has provoked a storm of heated discussions on the issue.
In a Twitter thread on the subject, Italian cardiologist Giuseppe Galati, MD, writes: “An astonishing decision by ESC that’s excluding all the Russian and Belarussian scientists from ESC congresses and activities. Treating doctors and scientists as [if] they are Putin and are responsible for the war.”
Dr. Galati adds: “A strong message that brings us to 70 years ago. ESC is promoting exclusion and not inclusion and diversity.”
Another commentator on the thread says: “It is a very unfortunate decision. Science, medicine should not be involved in politics. We are colleagues gathering together during congresses to exchange information for the sake of our patients. Politics should not overshadow this.”
And another added: “I think most cardiologists from Russia will not be able to participate in the events anyway, since international payments will soon be impossible from Russia. But it is wrong to limit the rights of doctors because of their nationality.”
But others support the ESC’s stance. Polish cardiologist Blazej Michalski, MD, says: “I think it is [a] good decision. Russians if they do not actively support dictatorship of Putin, the silence is also an agreement.” He adds: “I am proud of ESC. They did what they were supposed to do.”
A Twitter poll started by Ali Elzieny, MD, a cardiologist from Boston, titled “Do you agree that ESC suspend membership of Russian Society of Cardiology?” as of March 8 had 1,300 votes, with 61% of respondents disagreeing with the ESC decision and 39% in favor.
Medical societies respond
Several other medical societies have issued communications appearing to disagree with the action by the ESC.
The American College of Cardiology issued a statement saying medicine should be above politics.
“The American College of Cardiology believes that patients come first, and now, more than ever, there is a need to rally around our members across the globe to ensure that they have the support and resources they need to care for their patients,” said ACC President Dipti Itchhaporia, MD.
“Medicine is above politics and ACC will not exclude any of our colleagues who are working toward a shared mission of improving heart health. The College has a long history of working across borders to improve heart health and remains committed to that now and in the future. The ACC continues to express its support and concern for our members in the Ukraine and the patients they are working to treat on the frontlines,” the ACC statement added.
The Tele-Cardiology Working Group of the International Society for Telemedicine & eHealth (ISFTEH) also issued a statement disagreeing with the action from the ESC.
“In light of recent events, the cardiology working group of the ISFTEH will not restrict access to its events to cardiologists with regards to their nationality, religious beliefs or other characteristics that may seem discriminatory. We believe medical information should be widely available for all, especially for those doctors that find themselves in difficulty,” it said.
The European Academy of Neurology (EAN) said: “EAN is looking at ways to give practical support to Ukrainian neurologists and healthcare professionals there. EAN is not considering suspension of any individual member based on country of residence or nationality or any National Society member.”
But one oncology professional group has also cut ties with Russia.
The international cancer specialist network, OncoAlert, issued a statement saying it has severed all cooperation with doctors in Russia as part of the Western sanctions.
“The OncoAlert Network is non-political, but we cannot stand idle and not take a stand against this aggression towards our Ukrainian friends & colleagues,” OncoAlert said, adding that it will be pulling out of all collaborations and congresses in Russia. That statement was also greeted with a barrage of criticism on Twitter, mainly from Russian users.
A version of this article first appeared on Medscape.com.
, provoking a heated discussion on whether medical organizations should become involved in politics.
“In the light of the continued aggression against Ukraine by the leaderships of the Russian Federation and Belarus, the European Society of Cardiology (ESC) has temporarily suspended the memberships of the Russian Society of Cardiology and the Belarussian Society of Cardiologists in the ESC,” the ESC statement reads.
“Individuals based in the Russian Federation or in Belarus are excluded from active participation in any ESC event or activity,” it states.
“The ESC very much regrets the effect this may have on individual Russian and Belarussian cardiologists and scientists, but the message to Russian and Belarussian leadership must be distinct and unequivocal,” it adds.
This action from the ESC has provoked a storm of heated discussions on the issue.
In a Twitter thread on the subject, Italian cardiologist Giuseppe Galati, MD, writes: “An astonishing decision by ESC that’s excluding all the Russian and Belarussian scientists from ESC congresses and activities. Treating doctors and scientists as [if] they are Putin and are responsible for the war.”
Dr. Galati adds: “A strong message that brings us to 70 years ago. ESC is promoting exclusion and not inclusion and diversity.”
Another commentator on the thread says: “It is a very unfortunate decision. Science, medicine should not be involved in politics. We are colleagues gathering together during congresses to exchange information for the sake of our patients. Politics should not overshadow this.”
And another added: “I think most cardiologists from Russia will not be able to participate in the events anyway, since international payments will soon be impossible from Russia. But it is wrong to limit the rights of doctors because of their nationality.”
But others support the ESC’s stance. Polish cardiologist Blazej Michalski, MD, says: “I think it is [a] good decision. Russians if they do not actively support dictatorship of Putin, the silence is also an agreement.” He adds: “I am proud of ESC. They did what they were supposed to do.”
A Twitter poll started by Ali Elzieny, MD, a cardiologist from Boston, titled “Do you agree that ESC suspend membership of Russian Society of Cardiology?” as of March 8 had 1,300 votes, with 61% of respondents disagreeing with the ESC decision and 39% in favor.
Medical societies respond
Several other medical societies have issued communications appearing to disagree with the action by the ESC.
The American College of Cardiology issued a statement saying medicine should be above politics.
“The American College of Cardiology believes that patients come first, and now, more than ever, there is a need to rally around our members across the globe to ensure that they have the support and resources they need to care for their patients,” said ACC President Dipti Itchhaporia, MD.
“Medicine is above politics and ACC will not exclude any of our colleagues who are working toward a shared mission of improving heart health. The College has a long history of working across borders to improve heart health and remains committed to that now and in the future. The ACC continues to express its support and concern for our members in the Ukraine and the patients they are working to treat on the frontlines,” the ACC statement added.
The Tele-Cardiology Working Group of the International Society for Telemedicine & eHealth (ISFTEH) also issued a statement disagreeing with the action from the ESC.
“In light of recent events, the cardiology working group of the ISFTEH will not restrict access to its events to cardiologists with regards to their nationality, religious beliefs or other characteristics that may seem discriminatory. We believe medical information should be widely available for all, especially for those doctors that find themselves in difficulty,” it said.
The European Academy of Neurology (EAN) said: “EAN is looking at ways to give practical support to Ukrainian neurologists and healthcare professionals there. EAN is not considering suspension of any individual member based on country of residence or nationality or any National Society member.”
But one oncology professional group has also cut ties with Russia.
The international cancer specialist network, OncoAlert, issued a statement saying it has severed all cooperation with doctors in Russia as part of the Western sanctions.
“The OncoAlert Network is non-political, but we cannot stand idle and not take a stand against this aggression towards our Ukrainian friends & colleagues,” OncoAlert said, adding that it will be pulling out of all collaborations and congresses in Russia. That statement was also greeted with a barrage of criticism on Twitter, mainly from Russian users.
A version of this article first appeared on Medscape.com.
, provoking a heated discussion on whether medical organizations should become involved in politics.
“In the light of the continued aggression against Ukraine by the leaderships of the Russian Federation and Belarus, the European Society of Cardiology (ESC) has temporarily suspended the memberships of the Russian Society of Cardiology and the Belarussian Society of Cardiologists in the ESC,” the ESC statement reads.
“Individuals based in the Russian Federation or in Belarus are excluded from active participation in any ESC event or activity,” it states.
“The ESC very much regrets the effect this may have on individual Russian and Belarussian cardiologists and scientists, but the message to Russian and Belarussian leadership must be distinct and unequivocal,” it adds.
This action from the ESC has provoked a storm of heated discussions on the issue.
In a Twitter thread on the subject, Italian cardiologist Giuseppe Galati, MD, writes: “An astonishing decision by ESC that’s excluding all the Russian and Belarussian scientists from ESC congresses and activities. Treating doctors and scientists as [if] they are Putin and are responsible for the war.”
Dr. Galati adds: “A strong message that brings us to 70 years ago. ESC is promoting exclusion and not inclusion and diversity.”
Another commentator on the thread says: “It is a very unfortunate decision. Science, medicine should not be involved in politics. We are colleagues gathering together during congresses to exchange information for the sake of our patients. Politics should not overshadow this.”
And another added: “I think most cardiologists from Russia will not be able to participate in the events anyway, since international payments will soon be impossible from Russia. But it is wrong to limit the rights of doctors because of their nationality.”
But others support the ESC’s stance. Polish cardiologist Blazej Michalski, MD, says: “I think it is [a] good decision. Russians if they do not actively support dictatorship of Putin, the silence is also an agreement.” He adds: “I am proud of ESC. They did what they were supposed to do.”
A Twitter poll started by Ali Elzieny, MD, a cardiologist from Boston, titled “Do you agree that ESC suspend membership of Russian Society of Cardiology?” as of March 8 had 1,300 votes, with 61% of respondents disagreeing with the ESC decision and 39% in favor.
Medical societies respond
Several other medical societies have issued communications appearing to disagree with the action by the ESC.
The American College of Cardiology issued a statement saying medicine should be above politics.
“The American College of Cardiology believes that patients come first, and now, more than ever, there is a need to rally around our members across the globe to ensure that they have the support and resources they need to care for their patients,” said ACC President Dipti Itchhaporia, MD.
“Medicine is above politics and ACC will not exclude any of our colleagues who are working toward a shared mission of improving heart health. The College has a long history of working across borders to improve heart health and remains committed to that now and in the future. The ACC continues to express its support and concern for our members in the Ukraine and the patients they are working to treat on the frontlines,” the ACC statement added.
The Tele-Cardiology Working Group of the International Society for Telemedicine & eHealth (ISFTEH) also issued a statement disagreeing with the action from the ESC.
“In light of recent events, the cardiology working group of the ISFTEH will not restrict access to its events to cardiologists with regards to their nationality, religious beliefs or other characteristics that may seem discriminatory. We believe medical information should be widely available for all, especially for those doctors that find themselves in difficulty,” it said.
The European Academy of Neurology (EAN) said: “EAN is looking at ways to give practical support to Ukrainian neurologists and healthcare professionals there. EAN is not considering suspension of any individual member based on country of residence or nationality or any National Society member.”
But one oncology professional group has also cut ties with Russia.
The international cancer specialist network, OncoAlert, issued a statement saying it has severed all cooperation with doctors in Russia as part of the Western sanctions.
“The OncoAlert Network is non-political, but we cannot stand idle and not take a stand against this aggression towards our Ukrainian friends & colleagues,” OncoAlert said, adding that it will be pulling out of all collaborations and congresses in Russia. That statement was also greeted with a barrage of criticism on Twitter, mainly from Russian users.
A version of this article first appeared on Medscape.com.
Intermittent joint aches
Fundamental changes in the initial pharmacologic approach to psoriatic arthritis were made in the 2018 American College Rheumatology/National Psoriasis (ACR/NPF) guidelines for the treatment of psoriatic arthritis. Previously, methotrexate had been widely used as the first-line agent. The 2018 guidelines recommend a tumor necrosis factor (TNF) inhibitor over methotrexate and other oral small molecules (leflunomide, cyclosporine, and apremilast).
Herein is a broad summary of the guidelines:
· Treat with TNF inhibitor over oral small molecule; may consider oral small molecule with mild psoriatic arthritis and psoriasis, patient preference, and/or contraindication to TNF inhibitor
· Treat with TNF inhibitor over interleukin (IL)-17 inhibitor; may consider IL-17 inhibitor with severe psoriasis or contraindication to TNF inhibitor
· Treat with TNF inhibitor over interleukin (IL)-12/23 inhibitor; may consider IL-12/23 inhibitor with severe psoriasis or contraindication to TNF inhibitor
· Treat with oral small molecule over IL-17 inhibitor; may consider IL-17 inhibitor with severe psoriasis and/or psoriatic arthritis
· Treat with oral small molecule over IL-12/23 inhibitor; may consider IL-12/23 inhibitor with severe psoriasis or psoriatic arthritis, or concomitant inflammatory bowel disease
· Treat with methotrexate over nonsteroidal anti-inflammatory drugs; may consider nonsteroidals for mild psoriatic arthritis and psoriasis
· Treat with IL-17 inhibitor over IL-12/23 inhibitor; may consider IL-12/23 inhibitor in a patient with concomitant inflammatory bowel disease
Note that these recommendations are based on conditional evidence (ie, low to very low quality). In fact, in the entire guideline document, only 6% of the recommendations are strong, whereas 96% are conditional. This emphasizes the importance of evaluating each patient individually and engaging in a discussion to choose optimal therapy.
Another set of guidelines, from the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA), was last updated in 2015. Since then, many of the agents above have been introduced. Updated GRAPPA guidelines are expected to be released later this year.
Herbert S. Diamond, MD, Professor of Medicine (retired), Temple University School of Medicine, University of Pittsburgh; Chairman, Department of Medicine Emeritus, Western Pennsylvania Hospital, Pittsburgh, PA.
Herbert S. Diamond, MD, has disclosed no relevant financial relationships.
Fundamental changes in the initial pharmacologic approach to psoriatic arthritis were made in the 2018 American College Rheumatology/National Psoriasis (ACR/NPF) guidelines for the treatment of psoriatic arthritis. Previously, methotrexate had been widely used as the first-line agent. The 2018 guidelines recommend a tumor necrosis factor (TNF) inhibitor over methotrexate and other oral small molecules (leflunomide, cyclosporine, and apremilast).
Herein is a broad summary of the guidelines:
· Treat with TNF inhibitor over oral small molecule; may consider oral small molecule with mild psoriatic arthritis and psoriasis, patient preference, and/or contraindication to TNF inhibitor
· Treat with TNF inhibitor over interleukin (IL)-17 inhibitor; may consider IL-17 inhibitor with severe psoriasis or contraindication to TNF inhibitor
· Treat with TNF inhibitor over interleukin (IL)-12/23 inhibitor; may consider IL-12/23 inhibitor with severe psoriasis or contraindication to TNF inhibitor
· Treat with oral small molecule over IL-17 inhibitor; may consider IL-17 inhibitor with severe psoriasis and/or psoriatic arthritis
· Treat with oral small molecule over IL-12/23 inhibitor; may consider IL-12/23 inhibitor with severe psoriasis or psoriatic arthritis, or concomitant inflammatory bowel disease
· Treat with methotrexate over nonsteroidal anti-inflammatory drugs; may consider nonsteroidals for mild psoriatic arthritis and psoriasis
· Treat with IL-17 inhibitor over IL-12/23 inhibitor; may consider IL-12/23 inhibitor in a patient with concomitant inflammatory bowel disease
Note that these recommendations are based on conditional evidence (ie, low to very low quality). In fact, in the entire guideline document, only 6% of the recommendations are strong, whereas 96% are conditional. This emphasizes the importance of evaluating each patient individually and engaging in a discussion to choose optimal therapy.
Another set of guidelines, from the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA), was last updated in 2015. Since then, many of the agents above have been introduced. Updated GRAPPA guidelines are expected to be released later this year.
Herbert S. Diamond, MD, Professor of Medicine (retired), Temple University School of Medicine, University of Pittsburgh; Chairman, Department of Medicine Emeritus, Western Pennsylvania Hospital, Pittsburgh, PA.
Herbert S. Diamond, MD, has disclosed no relevant financial relationships.
Fundamental changes in the initial pharmacologic approach to psoriatic arthritis were made in the 2018 American College Rheumatology/National Psoriasis (ACR/NPF) guidelines for the treatment of psoriatic arthritis. Previously, methotrexate had been widely used as the first-line agent. The 2018 guidelines recommend a tumor necrosis factor (TNF) inhibitor over methotrexate and other oral small molecules (leflunomide, cyclosporine, and apremilast).
Herein is a broad summary of the guidelines:
· Treat with TNF inhibitor over oral small molecule; may consider oral small molecule with mild psoriatic arthritis and psoriasis, patient preference, and/or contraindication to TNF inhibitor
· Treat with TNF inhibitor over interleukin (IL)-17 inhibitor; may consider IL-17 inhibitor with severe psoriasis or contraindication to TNF inhibitor
· Treat with TNF inhibitor over interleukin (IL)-12/23 inhibitor; may consider IL-12/23 inhibitor with severe psoriasis or contraindication to TNF inhibitor
· Treat with oral small molecule over IL-17 inhibitor; may consider IL-17 inhibitor with severe psoriasis and/or psoriatic arthritis
· Treat with oral small molecule over IL-12/23 inhibitor; may consider IL-12/23 inhibitor with severe psoriasis or psoriatic arthritis, or concomitant inflammatory bowel disease
· Treat with methotrexate over nonsteroidal anti-inflammatory drugs; may consider nonsteroidals for mild psoriatic arthritis and psoriasis
· Treat with IL-17 inhibitor over IL-12/23 inhibitor; may consider IL-12/23 inhibitor in a patient with concomitant inflammatory bowel disease
Note that these recommendations are based on conditional evidence (ie, low to very low quality). In fact, in the entire guideline document, only 6% of the recommendations are strong, whereas 96% are conditional. This emphasizes the importance of evaluating each patient individually and engaging in a discussion to choose optimal therapy.
Another set of guidelines, from the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA), was last updated in 2015. Since then, many of the agents above have been introduced. Updated GRAPPA guidelines are expected to be released later this year.
Herbert S. Diamond, MD, Professor of Medicine (retired), Temple University School of Medicine, University of Pittsburgh; Chairman, Department of Medicine Emeritus, Western Pennsylvania Hospital, Pittsburgh, PA.
Herbert S. Diamond, MD, has disclosed no relevant financial relationships.
A 56-year-old man presents because of intermittent joint aches and difficulty picking up his grandchild and cleaning his home. He has a 6-year history of scalp psoriasis that he has controlled with a salicylic acid shampoo. On physical examination, he has tenderness over both elbows and in his metacarpophalangeal and proximal interphalangeal (PIP) joints on both hands. Swollen joints are noted in the proximal and distal joints of the right hand. His fingernails show uniform pitting.
Neurologic examination shows no sensory deficits or hyperesthesia. Motor examination is unremarkable, and chest and abdominal findings are unremarkable. Blood pressure is 138/90 mm Hg. Radiographic imaging shows asymmetric erosive changes with very small areas of bony proliferation in the PIP joints.There is asymmetric narrowing of the joint space in the interphalangeal joints. Laboratory findings reveal an erythrocyte sedimentation rate of 35 mm/h, negative rheumatoid factor, negative antinuclear antibody, and C-reactive protein of 7 mg/dL.
These findings are consistent with a diagnosis of psoriatic arthritis. This patient has severe psoriatic arthritis based on radiographic evidence of erosive disease.
Home blood pressure testing better than at clinics: Study
Everyone’s been there. Which one’s right?
The answer: Perhaps neither. Individual measures of blood pressure are not as accurate as taking multiple readings over a day and averaging them.
Blood pressure varies throughout the day – by about 30 points for systolic pressure, or the pressure when the heart beats – and one or two measurements in a doctor’s office may not accurately reflect the average figure, said Beverly B. Green, MD, a senior investigator at Kaiser Permanente Washington Health Research Institute in Seattle.
Average blood pressure reading is the only measurement on which a doctor can accurately diagnose and treat high blood pressure, she said. A new study by Dr. Green and other researchers at Kaiser Permanente showed that giving patients the chance to monitor their blood pressure at home could help get more reliable measurements.
Nearly one in four adults in the United States with high blood pressure are unaware they have the condition and are not getting treatment to control it. Without treatment, the condition can cause heart attacks, strokes, kidney damage, and other potentially life-threatening health problems.
Current guidelines for diagnosing high blood pressure recommend that patients whose pressure is high in the clinic get tested again to confirm the results. While the guidelines recommend home monitoring before diagnosing high blood pressure, research shows that doctors continue to measure blood pressure in their clinics for the second reading.
In their study, Dr. Green and colleagues found that home readings were more accurate than measurements taken in clinics or at pharmacy kiosks.
“Home blood pressure monitoring was a better option, because it was more accurate” than clinic blood pressure readings, Green said. A companion study found that patients preferred taking their blood pressure at home.
For their study, Dr. Green’s group used Kaiser’s electronic health record system to identify people at high risk for high blood pressure based on a recent clinic visit. They then randomly assigned the participants to get their follow-up blood pressure readings in the clinic, at home, or at kiosks in clinics or pharmacies.
Each participant also received a 24-hour ambulatory blood pressure monitor (ABPM). These devices, which people must wear continuously for 24 hours, have cuffs that inflate every 20-0 minutes during the day and every 30-60 minutes at night. Although ABPMs are the preferred test for accurately diagnosing high blood pressure, they aren’t available for widespread use.
The Kaiser researchers found that people’s systolic BP readings at clinics were generally lower than their ABPM measurements, leading to undiagnosed high BP in more than 50% of cases. Kiosk readings were much higher than the ABPM measurements and tended to overdiagnose high BP.
The value of home monitoring
Branden Villavaso, a 48-year-old attorney in New Orleans who was diagnosed with high BP at age 32, attributes his condition to genetics. He says an at-home monitor plus the occasional use of an ABPM finally provided his doctor with an accurate assessment of his condition.
Thanks to this aggressive approach, over the past 3 years, Mr. Villavaso’s diastolic reading has dropped from a previous range of between 90 and 100 to a healthier but not quite ideal value of about 80. Meanwhile, his systolic pressure has dropped to about 120, well below the goal of 130.
Mr. Villavaso said his doctor has relied on the averages of the BP readings to tailor his medication, and he also credited his wife, Chloe, a clinical nurse specialist, for monitoring his progress.
While previous studies have found similar benefits for measuring BP at home, Dr. Green said the latest study may offer the most powerful evidence to date because of the large number of people who took part, the involvement of primary care clinics, and the use of real-world health care professionals to take measurements instead of people who usually do health research. She said this study is the first to compare kiosk and ABPM results.
“The study indicates that assisting patients with getting access to valid blood pressure readings so they can measure their blood pressure at home will give a better picture of the true burden of [high BP],” said Keith C. Ferdinand, MD, a cardiologist at Tulane University, New Orleans.
He recommended patients select a home monitoring device from www.validatebp.org, a noncommercial website that lists home BP systems that have proven to be accurate.
“We know that [high blood pressure] is the most common and powerful cause of heart disease and death,” Dr. Ferdinand said. “Patients are pleased to participate in shared decision-making and actively assist in the control of a potentially deadly disease.”
A version of this article first appeared on WebMD.com.
Everyone’s been there. Which one’s right?
The answer: Perhaps neither. Individual measures of blood pressure are not as accurate as taking multiple readings over a day and averaging them.
Blood pressure varies throughout the day – by about 30 points for systolic pressure, or the pressure when the heart beats – and one or two measurements in a doctor’s office may not accurately reflect the average figure, said Beverly B. Green, MD, a senior investigator at Kaiser Permanente Washington Health Research Institute in Seattle.
Average blood pressure reading is the only measurement on which a doctor can accurately diagnose and treat high blood pressure, she said. A new study by Dr. Green and other researchers at Kaiser Permanente showed that giving patients the chance to monitor their blood pressure at home could help get more reliable measurements.
Nearly one in four adults in the United States with high blood pressure are unaware they have the condition and are not getting treatment to control it. Without treatment, the condition can cause heart attacks, strokes, kidney damage, and other potentially life-threatening health problems.
Current guidelines for diagnosing high blood pressure recommend that patients whose pressure is high in the clinic get tested again to confirm the results. While the guidelines recommend home monitoring before diagnosing high blood pressure, research shows that doctors continue to measure blood pressure in their clinics for the second reading.
In their study, Dr. Green and colleagues found that home readings were more accurate than measurements taken in clinics or at pharmacy kiosks.
“Home blood pressure monitoring was a better option, because it was more accurate” than clinic blood pressure readings, Green said. A companion study found that patients preferred taking their blood pressure at home.
For their study, Dr. Green’s group used Kaiser’s electronic health record system to identify people at high risk for high blood pressure based on a recent clinic visit. They then randomly assigned the participants to get their follow-up blood pressure readings in the clinic, at home, or at kiosks in clinics or pharmacies.
Each participant also received a 24-hour ambulatory blood pressure monitor (ABPM). These devices, which people must wear continuously for 24 hours, have cuffs that inflate every 20-0 minutes during the day and every 30-60 minutes at night. Although ABPMs are the preferred test for accurately diagnosing high blood pressure, they aren’t available for widespread use.
The Kaiser researchers found that people’s systolic BP readings at clinics were generally lower than their ABPM measurements, leading to undiagnosed high BP in more than 50% of cases. Kiosk readings were much higher than the ABPM measurements and tended to overdiagnose high BP.
The value of home monitoring
Branden Villavaso, a 48-year-old attorney in New Orleans who was diagnosed with high BP at age 32, attributes his condition to genetics. He says an at-home monitor plus the occasional use of an ABPM finally provided his doctor with an accurate assessment of his condition.
Thanks to this aggressive approach, over the past 3 years, Mr. Villavaso’s diastolic reading has dropped from a previous range of between 90 and 100 to a healthier but not quite ideal value of about 80. Meanwhile, his systolic pressure has dropped to about 120, well below the goal of 130.
Mr. Villavaso said his doctor has relied on the averages of the BP readings to tailor his medication, and he also credited his wife, Chloe, a clinical nurse specialist, for monitoring his progress.
While previous studies have found similar benefits for measuring BP at home, Dr. Green said the latest study may offer the most powerful evidence to date because of the large number of people who took part, the involvement of primary care clinics, and the use of real-world health care professionals to take measurements instead of people who usually do health research. She said this study is the first to compare kiosk and ABPM results.
“The study indicates that assisting patients with getting access to valid blood pressure readings so they can measure their blood pressure at home will give a better picture of the true burden of [high BP],” said Keith C. Ferdinand, MD, a cardiologist at Tulane University, New Orleans.
He recommended patients select a home monitoring device from www.validatebp.org, a noncommercial website that lists home BP systems that have proven to be accurate.
“We know that [high blood pressure] is the most common and powerful cause of heart disease and death,” Dr. Ferdinand said. “Patients are pleased to participate in shared decision-making and actively assist in the control of a potentially deadly disease.”
A version of this article first appeared on WebMD.com.
Everyone’s been there. Which one’s right?
The answer: Perhaps neither. Individual measures of blood pressure are not as accurate as taking multiple readings over a day and averaging them.
Blood pressure varies throughout the day – by about 30 points for systolic pressure, or the pressure when the heart beats – and one or two measurements in a doctor’s office may not accurately reflect the average figure, said Beverly B. Green, MD, a senior investigator at Kaiser Permanente Washington Health Research Institute in Seattle.
Average blood pressure reading is the only measurement on which a doctor can accurately diagnose and treat high blood pressure, she said. A new study by Dr. Green and other researchers at Kaiser Permanente showed that giving patients the chance to monitor their blood pressure at home could help get more reliable measurements.
Nearly one in four adults in the United States with high blood pressure are unaware they have the condition and are not getting treatment to control it. Without treatment, the condition can cause heart attacks, strokes, kidney damage, and other potentially life-threatening health problems.
Current guidelines for diagnosing high blood pressure recommend that patients whose pressure is high in the clinic get tested again to confirm the results. While the guidelines recommend home monitoring before diagnosing high blood pressure, research shows that doctors continue to measure blood pressure in their clinics for the second reading.
In their study, Dr. Green and colleagues found that home readings were more accurate than measurements taken in clinics or at pharmacy kiosks.
“Home blood pressure monitoring was a better option, because it was more accurate” than clinic blood pressure readings, Green said. A companion study found that patients preferred taking their blood pressure at home.
For their study, Dr. Green’s group used Kaiser’s electronic health record system to identify people at high risk for high blood pressure based on a recent clinic visit. They then randomly assigned the participants to get their follow-up blood pressure readings in the clinic, at home, or at kiosks in clinics or pharmacies.
Each participant also received a 24-hour ambulatory blood pressure monitor (ABPM). These devices, which people must wear continuously for 24 hours, have cuffs that inflate every 20-0 minutes during the day and every 30-60 minutes at night. Although ABPMs are the preferred test for accurately diagnosing high blood pressure, they aren’t available for widespread use.
The Kaiser researchers found that people’s systolic BP readings at clinics were generally lower than their ABPM measurements, leading to undiagnosed high BP in more than 50% of cases. Kiosk readings were much higher than the ABPM measurements and tended to overdiagnose high BP.
The value of home monitoring
Branden Villavaso, a 48-year-old attorney in New Orleans who was diagnosed with high BP at age 32, attributes his condition to genetics. He says an at-home monitor plus the occasional use of an ABPM finally provided his doctor with an accurate assessment of his condition.
Thanks to this aggressive approach, over the past 3 years, Mr. Villavaso’s diastolic reading has dropped from a previous range of between 90 and 100 to a healthier but not quite ideal value of about 80. Meanwhile, his systolic pressure has dropped to about 120, well below the goal of 130.
Mr. Villavaso said his doctor has relied on the averages of the BP readings to tailor his medication, and he also credited his wife, Chloe, a clinical nurse specialist, for monitoring his progress.
While previous studies have found similar benefits for measuring BP at home, Dr. Green said the latest study may offer the most powerful evidence to date because of the large number of people who took part, the involvement of primary care clinics, and the use of real-world health care professionals to take measurements instead of people who usually do health research. She said this study is the first to compare kiosk and ABPM results.
“The study indicates that assisting patients with getting access to valid blood pressure readings so they can measure their blood pressure at home will give a better picture of the true burden of [high BP],” said Keith C. Ferdinand, MD, a cardiologist at Tulane University, New Orleans.
He recommended patients select a home monitoring device from www.validatebp.org, a noncommercial website that lists home BP systems that have proven to be accurate.
“We know that [high blood pressure] is the most common and powerful cause of heart disease and death,” Dr. Ferdinand said. “Patients are pleased to participate in shared decision-making and actively assist in the control of a potentially deadly disease.”
A version of this article first appeared on WebMD.com.
FROM THE JOURNAL OF GENERAL INTERNAL MEDICINE