User login
Digital algorithm better predicts risk for postpartum hemorrhage
A digital algorithm using 24 patient characteristics identifies far more women who are likely to develop a postpartum hemorrhage than currently used tools to predict the risk for bleeding after delivery, according to a study published in the Journal of the American Medical Informatics Association.
About 1 in 10 of the roughly 700 pregnancy-related deaths in the United States are caused by postpartum hemorrhage, according to the U.S. Centers for Disease Control and Prevention. These deaths disproportionately occur among Black women, for whom studies show the risk of dying from a postpartum hemorrhage is fivefold greater than that of White women.
“Postpartum hemorrhage is a preventable medical emergency but remains the leading cause of maternal mortality globally,” the study’s senior author Li Li, MD, senior vice president of clinical informatics at Sema4, a company that uses artificial intelligence and machine learning to develop data-based clinical tools, told this news organization. “Early intervention is critical for reducing postpartum hemorrhage morbidity and mortality.”
Porous predictors
Existing tools for risk prediction are not particularly effective, Dr. Li said. For example, the American College of Obstetricians and Gynecologists’ (ACOG) Safe Motherhood Initiative offers checklists of clinical characteristics to classify women as low, medium, or high risk. However, 40% of the women classified as low risk based on this type of tool experience a hemorrhage.
ACOG also recommends quantifying blood loss during delivery or immediately after to identify women who are hemorrhaging, because imprecise estimates from clinicians may delay urgently needed care. Yet many hospitals have not implemented methods for measuring bleeding, said Dr. Li, who also is an assistant professor of genetics and genomic sciences at the Icahn School of Medicine at Mount Sinai, New York.
To develop a more precise way of identifying women at risk, Dr. Li and colleagues turned to artificial intelligence technology to create a “digital phenotype” based on approximately 72,000 births in the Mount Sinai Health System.
The digital tool retrospectively identified about 6,600 cases of postpartum hemorrhage, about 3.8 times the roughly 1,700 cases that would have been predicted based on methods that estimate blood loss. A blinded physician review of a subset of 45 patient charts – including 26 patients who experienced a hemorrhage, 11 who didn’t, and 6 with unclear outcomes – found that the digital approach was 89% percent accurate at identifying cases, whereas blood loss–based methods were accurate 67% of the time.
Several of the 24 characteristics included in the model appear in other risk predictors, including whether a woman has had a previous cesarean delivery or prior postdelivery bleeding and whether she has anemia or related blood disorders. Among the rest were risk factors that have been identified in the literature, including maternal blood pressure, time from admission to delivery, and average pulse during hospitalization. Five more features were new: red blood cell count and distribution width, mean corpuscular hemoglobin, absolute neutrophil count, and white blood cell count.
“These [new] values are easily obtainable from standard blood draws in the hospital but are not currently used in clinical practice to estimate postpartum hemorrhage risk,” Dr. Li said.
In a related retrospective study, Dr. Li and her colleagues used the new tool to classify women into high, low, or medium risk categories. They found that 28% of the women the algorithm classified as high risk experienced a postpartum hemorrhage compared with 15% to 19% of the women classified as high risk by standard predictive tools. They also identified potential “inflection points” where changes in vital signs may suggest a substantial increase in risk. For example, women whose median blood pressure during labor and delivery was above 132 mm Hg had an 11% average increase in their risk for bleeding.
By more precisely identifying women at risk, the new method “could be used to pre-emptively allocate resources that can ultimately reduce postpartum hemorrhage morbidity and mortality,” Dr. Li said. Sema4 is launching a prospective clinical trial to further assess the algorithm, she added.
Finding the continuum of risk
Holly Ende, MD, an obstetric anesthesiologist at Vanderbilt University Medical Center, Nashville, Tenn., said approaches that leverage electronic health records to identify women at risk for hemorrhage have many advantages over currently used tools.
“Machine learning models or statistical models are able to take into account many more risk factors and weigh each of those risk factors based on how much they contribute to risk,” Dr. Ende, who was not involved in the new studies, told this news organization. “We can stratify women more on a continuum.”
But digital approaches have potential downsides.
“Machine learning algorithms can be developed in such a way that perpetuates racial bias, and it’s important to be aware of potential biases in coded algorithms,” Dr. Li said. To help reduce such bias, they used a database that included a racially and ethnically diverse patient population, but she acknowledged that additional research is needed.
Dr. Ende, the coauthor of a commentary in Obstetrics & Gynecology on risk assessment for postpartum hemorrhage, said algorithm developers must be sensitive to pre-existing disparities in health care that may affect the data they use to build the software.
She pointed to uterine atony – a known risk factor for hemorrhage – as an example. In her own research, she and her colleagues identified women with atony by searching their medical records for medications used to treat the condition. But when they ran their model, Black women were less likely to develop uterine atony, which the team knew wasn’t true in the real world. They traced the problem to an existing disparity in obstetric care: Black women with uterine atony were less likely than women of other races to receive medications for the condition.
“People need to be cognizant as they are developing these types of prediction models and be extremely careful to avoid perpetuating any disparities in care,” Dr. Ende cautioned. On the other hand, if carefully developed, these tools might also help reduce disparities in health care by standardizing risk stratification and clinical practices, she said.
In addition to independent validation of data-based risk prediction tools, Dr. Ende said ensuring that clinicians are properly trained to use these tools is crucial.
“Implementation may be the biggest limitation,” she said.
Dr. Ende and Dr. Li have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A digital algorithm using 24 patient characteristics identifies far more women who are likely to develop a postpartum hemorrhage than currently used tools to predict the risk for bleeding after delivery, according to a study published in the Journal of the American Medical Informatics Association.
About 1 in 10 of the roughly 700 pregnancy-related deaths in the United States are caused by postpartum hemorrhage, according to the U.S. Centers for Disease Control and Prevention. These deaths disproportionately occur among Black women, for whom studies show the risk of dying from a postpartum hemorrhage is fivefold greater than that of White women.
“Postpartum hemorrhage is a preventable medical emergency but remains the leading cause of maternal mortality globally,” the study’s senior author Li Li, MD, senior vice president of clinical informatics at Sema4, a company that uses artificial intelligence and machine learning to develop data-based clinical tools, told this news organization. “Early intervention is critical for reducing postpartum hemorrhage morbidity and mortality.”
Porous predictors
Existing tools for risk prediction are not particularly effective, Dr. Li said. For example, the American College of Obstetricians and Gynecologists’ (ACOG) Safe Motherhood Initiative offers checklists of clinical characteristics to classify women as low, medium, or high risk. However, 40% of the women classified as low risk based on this type of tool experience a hemorrhage.
ACOG also recommends quantifying blood loss during delivery or immediately after to identify women who are hemorrhaging, because imprecise estimates from clinicians may delay urgently needed care. Yet many hospitals have not implemented methods for measuring bleeding, said Dr. Li, who also is an assistant professor of genetics and genomic sciences at the Icahn School of Medicine at Mount Sinai, New York.
To develop a more precise way of identifying women at risk, Dr. Li and colleagues turned to artificial intelligence technology to create a “digital phenotype” based on approximately 72,000 births in the Mount Sinai Health System.
The digital tool retrospectively identified about 6,600 cases of postpartum hemorrhage, about 3.8 times the roughly 1,700 cases that would have been predicted based on methods that estimate blood loss. A blinded physician review of a subset of 45 patient charts – including 26 patients who experienced a hemorrhage, 11 who didn’t, and 6 with unclear outcomes – found that the digital approach was 89% percent accurate at identifying cases, whereas blood loss–based methods were accurate 67% of the time.
Several of the 24 characteristics included in the model appear in other risk predictors, including whether a woman has had a previous cesarean delivery or prior postdelivery bleeding and whether she has anemia or related blood disorders. Among the rest were risk factors that have been identified in the literature, including maternal blood pressure, time from admission to delivery, and average pulse during hospitalization. Five more features were new: red blood cell count and distribution width, mean corpuscular hemoglobin, absolute neutrophil count, and white blood cell count.
“These [new] values are easily obtainable from standard blood draws in the hospital but are not currently used in clinical practice to estimate postpartum hemorrhage risk,” Dr. Li said.
In a related retrospective study, Dr. Li and her colleagues used the new tool to classify women into high, low, or medium risk categories. They found that 28% of the women the algorithm classified as high risk experienced a postpartum hemorrhage compared with 15% to 19% of the women classified as high risk by standard predictive tools. They also identified potential “inflection points” where changes in vital signs may suggest a substantial increase in risk. For example, women whose median blood pressure during labor and delivery was above 132 mm Hg had an 11% average increase in their risk for bleeding.
By more precisely identifying women at risk, the new method “could be used to pre-emptively allocate resources that can ultimately reduce postpartum hemorrhage morbidity and mortality,” Dr. Li said. Sema4 is launching a prospective clinical trial to further assess the algorithm, she added.
Finding the continuum of risk
Holly Ende, MD, an obstetric anesthesiologist at Vanderbilt University Medical Center, Nashville, Tenn., said approaches that leverage electronic health records to identify women at risk for hemorrhage have many advantages over currently used tools.
“Machine learning models or statistical models are able to take into account many more risk factors and weigh each of those risk factors based on how much they contribute to risk,” Dr. Ende, who was not involved in the new studies, told this news organization. “We can stratify women more on a continuum.”
But digital approaches have potential downsides.
“Machine learning algorithms can be developed in such a way that perpetuates racial bias, and it’s important to be aware of potential biases in coded algorithms,” Dr. Li said. To help reduce such bias, they used a database that included a racially and ethnically diverse patient population, but she acknowledged that additional research is needed.
Dr. Ende, the coauthor of a commentary in Obstetrics & Gynecology on risk assessment for postpartum hemorrhage, said algorithm developers must be sensitive to pre-existing disparities in health care that may affect the data they use to build the software.
She pointed to uterine atony – a known risk factor for hemorrhage – as an example. In her own research, she and her colleagues identified women with atony by searching their medical records for medications used to treat the condition. But when they ran their model, Black women were less likely to develop uterine atony, which the team knew wasn’t true in the real world. They traced the problem to an existing disparity in obstetric care: Black women with uterine atony were less likely than women of other races to receive medications for the condition.
“People need to be cognizant as they are developing these types of prediction models and be extremely careful to avoid perpetuating any disparities in care,” Dr. Ende cautioned. On the other hand, if carefully developed, these tools might also help reduce disparities in health care by standardizing risk stratification and clinical practices, she said.
In addition to independent validation of data-based risk prediction tools, Dr. Ende said ensuring that clinicians are properly trained to use these tools is crucial.
“Implementation may be the biggest limitation,” she said.
Dr. Ende and Dr. Li have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A digital algorithm using 24 patient characteristics identifies far more women who are likely to develop a postpartum hemorrhage than currently used tools to predict the risk for bleeding after delivery, according to a study published in the Journal of the American Medical Informatics Association.
About 1 in 10 of the roughly 700 pregnancy-related deaths in the United States are caused by postpartum hemorrhage, according to the U.S. Centers for Disease Control and Prevention. These deaths disproportionately occur among Black women, for whom studies show the risk of dying from a postpartum hemorrhage is fivefold greater than that of White women.
“Postpartum hemorrhage is a preventable medical emergency but remains the leading cause of maternal mortality globally,” the study’s senior author Li Li, MD, senior vice president of clinical informatics at Sema4, a company that uses artificial intelligence and machine learning to develop data-based clinical tools, told this news organization. “Early intervention is critical for reducing postpartum hemorrhage morbidity and mortality.”
Porous predictors
Existing tools for risk prediction are not particularly effective, Dr. Li said. For example, the American College of Obstetricians and Gynecologists’ (ACOG) Safe Motherhood Initiative offers checklists of clinical characteristics to classify women as low, medium, or high risk. However, 40% of the women classified as low risk based on this type of tool experience a hemorrhage.
ACOG also recommends quantifying blood loss during delivery or immediately after to identify women who are hemorrhaging, because imprecise estimates from clinicians may delay urgently needed care. Yet many hospitals have not implemented methods for measuring bleeding, said Dr. Li, who also is an assistant professor of genetics and genomic sciences at the Icahn School of Medicine at Mount Sinai, New York.
To develop a more precise way of identifying women at risk, Dr. Li and colleagues turned to artificial intelligence technology to create a “digital phenotype” based on approximately 72,000 births in the Mount Sinai Health System.
The digital tool retrospectively identified about 6,600 cases of postpartum hemorrhage, about 3.8 times the roughly 1,700 cases that would have been predicted based on methods that estimate blood loss. A blinded physician review of a subset of 45 patient charts – including 26 patients who experienced a hemorrhage, 11 who didn’t, and 6 with unclear outcomes – found that the digital approach was 89% percent accurate at identifying cases, whereas blood loss–based methods were accurate 67% of the time.
Several of the 24 characteristics included in the model appear in other risk predictors, including whether a woman has had a previous cesarean delivery or prior postdelivery bleeding and whether she has anemia or related blood disorders. Among the rest were risk factors that have been identified in the literature, including maternal blood pressure, time from admission to delivery, and average pulse during hospitalization. Five more features were new: red blood cell count and distribution width, mean corpuscular hemoglobin, absolute neutrophil count, and white blood cell count.
“These [new] values are easily obtainable from standard blood draws in the hospital but are not currently used in clinical practice to estimate postpartum hemorrhage risk,” Dr. Li said.
In a related retrospective study, Dr. Li and her colleagues used the new tool to classify women into high, low, or medium risk categories. They found that 28% of the women the algorithm classified as high risk experienced a postpartum hemorrhage compared with 15% to 19% of the women classified as high risk by standard predictive tools. They also identified potential “inflection points” where changes in vital signs may suggest a substantial increase in risk. For example, women whose median blood pressure during labor and delivery was above 132 mm Hg had an 11% average increase in their risk for bleeding.
By more precisely identifying women at risk, the new method “could be used to pre-emptively allocate resources that can ultimately reduce postpartum hemorrhage morbidity and mortality,” Dr. Li said. Sema4 is launching a prospective clinical trial to further assess the algorithm, she added.
Finding the continuum of risk
Holly Ende, MD, an obstetric anesthesiologist at Vanderbilt University Medical Center, Nashville, Tenn., said approaches that leverage electronic health records to identify women at risk for hemorrhage have many advantages over currently used tools.
“Machine learning models or statistical models are able to take into account many more risk factors and weigh each of those risk factors based on how much they contribute to risk,” Dr. Ende, who was not involved in the new studies, told this news organization. “We can stratify women more on a continuum.”
But digital approaches have potential downsides.
“Machine learning algorithms can be developed in such a way that perpetuates racial bias, and it’s important to be aware of potential biases in coded algorithms,” Dr. Li said. To help reduce such bias, they used a database that included a racially and ethnically diverse patient population, but she acknowledged that additional research is needed.
Dr. Ende, the coauthor of a commentary in Obstetrics & Gynecology on risk assessment for postpartum hemorrhage, said algorithm developers must be sensitive to pre-existing disparities in health care that may affect the data they use to build the software.
She pointed to uterine atony – a known risk factor for hemorrhage – as an example. In her own research, she and her colleagues identified women with atony by searching their medical records for medications used to treat the condition. But when they ran their model, Black women were less likely to develop uterine atony, which the team knew wasn’t true in the real world. They traced the problem to an existing disparity in obstetric care: Black women with uterine atony were less likely than women of other races to receive medications for the condition.
“People need to be cognizant as they are developing these types of prediction models and be extremely careful to avoid perpetuating any disparities in care,” Dr. Ende cautioned. On the other hand, if carefully developed, these tools might also help reduce disparities in health care by standardizing risk stratification and clinical practices, she said.
In addition to independent validation of data-based risk prediction tools, Dr. Ende said ensuring that clinicians are properly trained to use these tools is crucial.
“Implementation may be the biggest limitation,” she said.
Dr. Ende and Dr. Li have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
This doc still supports NP/PA-led care ... with caveats
Two years ago, I argued that independent care from nurse practitioners (NPs) and physician assistants (PAs) would not have ill effects on health outcomes. To the surprise of no one, NPs and PAs embraced the argument; physicians clobbered it.
My case had three pegs: One was that medicine isn’t rocket science and clinicians control a lot less than we think we do. The second peg was that technology levels the playing field of clinical care. High-sensitivity troponin assays, for instance, make missing MI a lot less likely. The third peg was empirical: Studies have found little difference in MD versus non–MD-led care. Looking back, I now see empiricism as the weakest part of the argument because the studies had so many limitations.
I update this viewpoint now because health care is increasingly delivered by NPs and PAs. And there are two concerning trends regarding NP education and experience. First is that nurses are turning to advanced practitioner training earlier in their careers – without gathering much bedside experience. And these training programs are increasingly likely to be online, with minimal hands-on clinical tutoring.
Education and experience pop in my head often. Not every day, but many days I think back to my lucky 7 years in Indiana learning under the supervision of master clinicians – at a time when trainees were allowed the leeway to make decisions ... and mistakes. Then, when I joined private practice, I continued to learn from experienced practitioners.
It would be foolish to argue that training and experience aren’t important.
But here’s the thing:
I will make three points: First, I will bolster two of my old arguments as to why we shouldn’t be worried about non-MD clinicians, then I will propose some ideas to increase confidence in NP and PA care.
Health care does not equal health
On the matter of how much clinicians affect outcomes, a recently published randomized controlled trial performed in India found that subsidizing insurance care led to increased utilization of hospital services but had no significant effect on health outcomes. This follows the RAND and Oregon Health Insurance studies in the United States, which largely reported similar results.
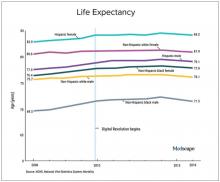
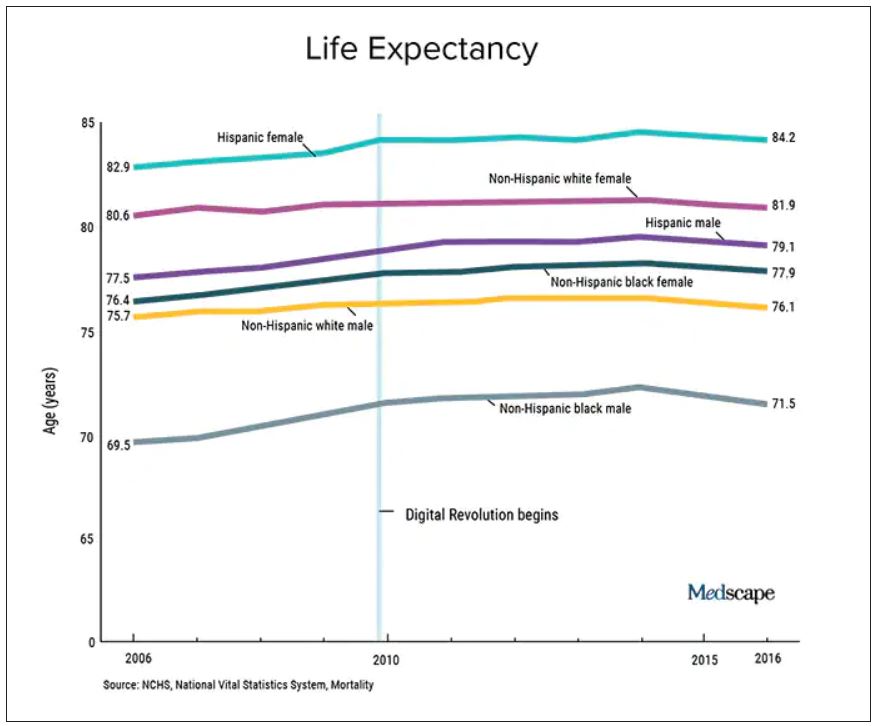
We should also not dismiss the fact that – despite the massive technology gains over the past half-century in digital health and artificial intelligence and increased use of quality measures, new drugs and procedures, and mega-medical centers – the average lifespan of Americans is flat to declining (in most ethnic and racial groups). Worse than no gains in longevity, perhaps, is that death from diseases like dementia and Parkinson’s disease are on the rise.
A neutral Martian would look down and wonder why all this health care hasn’t translated to longer and better lives. The causes of this paradox remain speculative, and are for another column, but the point remains that – on average – more health care is clearly not delivering more health. And if that is true, one may deduce that much of U.S. health care is marginal when it comes to affecting major outcomes.
It’s about the delta
Logos trumps pathos. Sure, my physician colleagues can tell scary anecdotes of bad outcomes caused by an inexperienced NP or PA. I would counter that by saying I have sat on our hospital’s peer review committee for 2 decades, including the era before NPs or PAs were practicing, and I have plenty of stories of physician errors. These include, of course, my own errors.
Logos: We must consider the difference between non–MD-led care and MD-led care.
My arguments from 2020 remain relevant today. Most medical problems are not engineering puzzles. Many, perhaps most, patients fall into an easy protocol – say, chest pain, dyspnea, or atrial fibrillation. With basic training, a motivated serious person quickly gains skill in recognizing and treating everyday problems.
And just 2 years on, technology further levels the playing field. Consider radiology in 2022 – it’s easy to take for granted the speed of the CT scan, the fidelity of the MRI, and the easy access to both in the U.S. hospital system. Less experienced clinicians have never had more tools to assist with diagnostics and therapeutics.
The expansion of team-based care has also mitigated the effects of inexperience. It took Americans longer than Canadians to figure out how helpful pharmacists could be. Pharmacists in my hospital now help us dose complicated medicines and protect us against prescribing errors.
Then there is the immediate access to online information. Gone are the days when you had to memorize long-QT syndromes. Book knowledge – that I spent years acquiring – now comes in seconds. The other day an NP corrected me. I asked, Are you sure? Boom, she took out her phone and showed me the evidence.
In sum, if it were even possible to measure the clinical competence of care from NP and PA versus physicians, there would be two bell-shaped curves with a tremendous amount of overlap. And that overlap would steadily increase as a given NP or PA gathered experience. (The NP in our electrophysiology division has more than 25 years’ experience in heart rhythm care, and it is common for colleagues to call her before one of us docs. Rightly so.)
Three basic proposals regarding NP and PA care
To ensure quality of care, I have three proposals.
It has always seemed strange to me that an NP or PA can flip from one field to another without a period of training. I can’t just change practice from electrophysiology to dermatology without doing a residency. But NPs and PAs can.
My first proposal would be that NPs and PAs spend a substantial period of training in a field before practice – a legit apprenticeship. The duration of this period is a matter of debate, but it ought to be standardized.
My second proposal is that, if physicians are required to pass certification exams, so should NPs. (PAs have an exam every 10 years.) The exam should be the same as (or very similar to) the physician exam, and it should be specific to their field of practice.
While I have argued (and still feel) that the American Board of Internal Medicine brand of certification is dubious, the fact remains that physicians must maintain proficiency in their field. Requiring NPs and PAs to do the same would help foster specialization. And while I can’t cite empirical evidence, specialization seems super-important. We have NPs at my hospital who have been in the same area for years, and they exude clinical competence.
Finally, I have come to believe that the best way for nearly any clinician to practice medicine is as part of a team. (The exception being primary care in rural areas where there are clinician shortages.)
On the matter of team care, I’ve practiced for a long time, but nearly every day I run situations by a colleague; often this person is an NP. The economist Friedrich Hayek proposed that dispersed knowledge always outpaces the wisdom of any individual. That notion pertains well to the increasing complexities and specialization of modern medical practice.
A person who commits to learning one area of medicine, enjoys helping people, asks often for help, and has the support of colleagues is set up to be a successful clinician – whether the letters after their name are APRN, PA, DO, or MD.
Dr. Mandrola practices cardiac electrophysiology in Louisville, Ky. He did not report any relevant financial disclosures. A version of this article first appeared on Medscape.com.
Two years ago, I argued that independent care from nurse practitioners (NPs) and physician assistants (PAs) would not have ill effects on health outcomes. To the surprise of no one, NPs and PAs embraced the argument; physicians clobbered it.
My case had three pegs: One was that medicine isn’t rocket science and clinicians control a lot less than we think we do. The second peg was that technology levels the playing field of clinical care. High-sensitivity troponin assays, for instance, make missing MI a lot less likely. The third peg was empirical: Studies have found little difference in MD versus non–MD-led care. Looking back, I now see empiricism as the weakest part of the argument because the studies had so many limitations.
I update this viewpoint now because health care is increasingly delivered by NPs and PAs. And there are two concerning trends regarding NP education and experience. First is that nurses are turning to advanced practitioner training earlier in their careers – without gathering much bedside experience. And these training programs are increasingly likely to be online, with minimal hands-on clinical tutoring.
Education and experience pop in my head often. Not every day, but many days I think back to my lucky 7 years in Indiana learning under the supervision of master clinicians – at a time when trainees were allowed the leeway to make decisions ... and mistakes. Then, when I joined private practice, I continued to learn from experienced practitioners.
It would be foolish to argue that training and experience aren’t important.
But here’s the thing:
I will make three points: First, I will bolster two of my old arguments as to why we shouldn’t be worried about non-MD clinicians, then I will propose some ideas to increase confidence in NP and PA care.
Health care does not equal health
On the matter of how much clinicians affect outcomes, a recently published randomized controlled trial performed in India found that subsidizing insurance care led to increased utilization of hospital services but had no significant effect on health outcomes. This follows the RAND and Oregon Health Insurance studies in the United States, which largely reported similar results.
We should also not dismiss the fact that – despite the massive technology gains over the past half-century in digital health and artificial intelligence and increased use of quality measures, new drugs and procedures, and mega-medical centers – the average lifespan of Americans is flat to declining (in most ethnic and racial groups). Worse than no gains in longevity, perhaps, is that death from diseases like dementia and Parkinson’s disease are on the rise.
A neutral Martian would look down and wonder why all this health care hasn’t translated to longer and better lives. The causes of this paradox remain speculative, and are for another column, but the point remains that – on average – more health care is clearly not delivering more health. And if that is true, one may deduce that much of U.S. health care is marginal when it comes to affecting major outcomes.
It’s about the delta
Logos trumps pathos. Sure, my physician colleagues can tell scary anecdotes of bad outcomes caused by an inexperienced NP or PA. I would counter that by saying I have sat on our hospital’s peer review committee for 2 decades, including the era before NPs or PAs were practicing, and I have plenty of stories of physician errors. These include, of course, my own errors.
Logos: We must consider the difference between non–MD-led care and MD-led care.
My arguments from 2020 remain relevant today. Most medical problems are not engineering puzzles. Many, perhaps most, patients fall into an easy protocol – say, chest pain, dyspnea, or atrial fibrillation. With basic training, a motivated serious person quickly gains skill in recognizing and treating everyday problems.
And just 2 years on, technology further levels the playing field. Consider radiology in 2022 – it’s easy to take for granted the speed of the CT scan, the fidelity of the MRI, and the easy access to both in the U.S. hospital system. Less experienced clinicians have never had more tools to assist with diagnostics and therapeutics.
The expansion of team-based care has also mitigated the effects of inexperience. It took Americans longer than Canadians to figure out how helpful pharmacists could be. Pharmacists in my hospital now help us dose complicated medicines and protect us against prescribing errors.
Then there is the immediate access to online information. Gone are the days when you had to memorize long-QT syndromes. Book knowledge – that I spent years acquiring – now comes in seconds. The other day an NP corrected me. I asked, Are you sure? Boom, she took out her phone and showed me the evidence.
In sum, if it were even possible to measure the clinical competence of care from NP and PA versus physicians, there would be two bell-shaped curves with a tremendous amount of overlap. And that overlap would steadily increase as a given NP or PA gathered experience. (The NP in our electrophysiology division has more than 25 years’ experience in heart rhythm care, and it is common for colleagues to call her before one of us docs. Rightly so.)
Three basic proposals regarding NP and PA care
To ensure quality of care, I have three proposals.
It has always seemed strange to me that an NP or PA can flip from one field to another without a period of training. I can’t just change practice from electrophysiology to dermatology without doing a residency. But NPs and PAs can.
My first proposal would be that NPs and PAs spend a substantial period of training in a field before practice – a legit apprenticeship. The duration of this period is a matter of debate, but it ought to be standardized.
My second proposal is that, if physicians are required to pass certification exams, so should NPs. (PAs have an exam every 10 years.) The exam should be the same as (or very similar to) the physician exam, and it should be specific to their field of practice.
While I have argued (and still feel) that the American Board of Internal Medicine brand of certification is dubious, the fact remains that physicians must maintain proficiency in their field. Requiring NPs and PAs to do the same would help foster specialization. And while I can’t cite empirical evidence, specialization seems super-important. We have NPs at my hospital who have been in the same area for years, and they exude clinical competence.
Finally, I have come to believe that the best way for nearly any clinician to practice medicine is as part of a team. (The exception being primary care in rural areas where there are clinician shortages.)
On the matter of team care, I’ve practiced for a long time, but nearly every day I run situations by a colleague; often this person is an NP. The economist Friedrich Hayek proposed that dispersed knowledge always outpaces the wisdom of any individual. That notion pertains well to the increasing complexities and specialization of modern medical practice.
A person who commits to learning one area of medicine, enjoys helping people, asks often for help, and has the support of colleagues is set up to be a successful clinician – whether the letters after their name are APRN, PA, DO, or MD.
Dr. Mandrola practices cardiac electrophysiology in Louisville, Ky. He did not report any relevant financial disclosures. A version of this article first appeared on Medscape.com.
Two years ago, I argued that independent care from nurse practitioners (NPs) and physician assistants (PAs) would not have ill effects on health outcomes. To the surprise of no one, NPs and PAs embraced the argument; physicians clobbered it.
My case had three pegs: One was that medicine isn’t rocket science and clinicians control a lot less than we think we do. The second peg was that technology levels the playing field of clinical care. High-sensitivity troponin assays, for instance, make missing MI a lot less likely. The third peg was empirical: Studies have found little difference in MD versus non–MD-led care. Looking back, I now see empiricism as the weakest part of the argument because the studies had so many limitations.
I update this viewpoint now because health care is increasingly delivered by NPs and PAs. And there are two concerning trends regarding NP education and experience. First is that nurses are turning to advanced practitioner training earlier in their careers – without gathering much bedside experience. And these training programs are increasingly likely to be online, with minimal hands-on clinical tutoring.
Education and experience pop in my head often. Not every day, but many days I think back to my lucky 7 years in Indiana learning under the supervision of master clinicians – at a time when trainees were allowed the leeway to make decisions ... and mistakes. Then, when I joined private practice, I continued to learn from experienced practitioners.
It would be foolish to argue that training and experience aren’t important.
But here’s the thing:
I will make three points: First, I will bolster two of my old arguments as to why we shouldn’t be worried about non-MD clinicians, then I will propose some ideas to increase confidence in NP and PA care.
Health care does not equal health
On the matter of how much clinicians affect outcomes, a recently published randomized controlled trial performed in India found that subsidizing insurance care led to increased utilization of hospital services but had no significant effect on health outcomes. This follows the RAND and Oregon Health Insurance studies in the United States, which largely reported similar results.
We should also not dismiss the fact that – despite the massive technology gains over the past half-century in digital health and artificial intelligence and increased use of quality measures, new drugs and procedures, and mega-medical centers – the average lifespan of Americans is flat to declining (in most ethnic and racial groups). Worse than no gains in longevity, perhaps, is that death from diseases like dementia and Parkinson’s disease are on the rise.
A neutral Martian would look down and wonder why all this health care hasn’t translated to longer and better lives. The causes of this paradox remain speculative, and are for another column, but the point remains that – on average – more health care is clearly not delivering more health. And if that is true, one may deduce that much of U.S. health care is marginal when it comes to affecting major outcomes.
It’s about the delta
Logos trumps pathos. Sure, my physician colleagues can tell scary anecdotes of bad outcomes caused by an inexperienced NP or PA. I would counter that by saying I have sat on our hospital’s peer review committee for 2 decades, including the era before NPs or PAs were practicing, and I have plenty of stories of physician errors. These include, of course, my own errors.
Logos: We must consider the difference between non–MD-led care and MD-led care.
My arguments from 2020 remain relevant today. Most medical problems are not engineering puzzles. Many, perhaps most, patients fall into an easy protocol – say, chest pain, dyspnea, or atrial fibrillation. With basic training, a motivated serious person quickly gains skill in recognizing and treating everyday problems.
And just 2 years on, technology further levels the playing field. Consider radiology in 2022 – it’s easy to take for granted the speed of the CT scan, the fidelity of the MRI, and the easy access to both in the U.S. hospital system. Less experienced clinicians have never had more tools to assist with diagnostics and therapeutics.
The expansion of team-based care has also mitigated the effects of inexperience. It took Americans longer than Canadians to figure out how helpful pharmacists could be. Pharmacists in my hospital now help us dose complicated medicines and protect us against prescribing errors.
Then there is the immediate access to online information. Gone are the days when you had to memorize long-QT syndromes. Book knowledge – that I spent years acquiring – now comes in seconds. The other day an NP corrected me. I asked, Are you sure? Boom, she took out her phone and showed me the evidence.
In sum, if it were even possible to measure the clinical competence of care from NP and PA versus physicians, there would be two bell-shaped curves with a tremendous amount of overlap. And that overlap would steadily increase as a given NP or PA gathered experience. (The NP in our electrophysiology division has more than 25 years’ experience in heart rhythm care, and it is common for colleagues to call her before one of us docs. Rightly so.)
Three basic proposals regarding NP and PA care
To ensure quality of care, I have three proposals.
It has always seemed strange to me that an NP or PA can flip from one field to another without a period of training. I can’t just change practice from electrophysiology to dermatology without doing a residency. But NPs and PAs can.
My first proposal would be that NPs and PAs spend a substantial period of training in a field before practice – a legit apprenticeship. The duration of this period is a matter of debate, but it ought to be standardized.
My second proposal is that, if physicians are required to pass certification exams, so should NPs. (PAs have an exam every 10 years.) The exam should be the same as (or very similar to) the physician exam, and it should be specific to their field of practice.
While I have argued (and still feel) that the American Board of Internal Medicine brand of certification is dubious, the fact remains that physicians must maintain proficiency in their field. Requiring NPs and PAs to do the same would help foster specialization. And while I can’t cite empirical evidence, specialization seems super-important. We have NPs at my hospital who have been in the same area for years, and they exude clinical competence.
Finally, I have come to believe that the best way for nearly any clinician to practice medicine is as part of a team. (The exception being primary care in rural areas where there are clinician shortages.)
On the matter of team care, I’ve practiced for a long time, but nearly every day I run situations by a colleague; often this person is an NP. The economist Friedrich Hayek proposed that dispersed knowledge always outpaces the wisdom of any individual. That notion pertains well to the increasing complexities and specialization of modern medical practice.
A person who commits to learning one area of medicine, enjoys helping people, asks often for help, and has the support of colleagues is set up to be a successful clinician – whether the letters after their name are APRN, PA, DO, or MD.
Dr. Mandrola practices cardiac electrophysiology in Louisville, Ky. He did not report any relevant financial disclosures. A version of this article first appeared on Medscape.com.
Seven ways doctors could get better payment from insurers
, say experts in physician-payer contracts.
Many doctors sign long-term agreements and then forget about them, says Marcia Brauchler, president and founder of Physicians’ Ally, Littleton, Colorado, a health care consulting company. “The average doctor is trying to run a practice on 2010 rates because they haven’t touched their insurance contracts for 10 years,” she says.
Payers also make a lot of money by adopting dozens of unilateral policy and procedure changes every year that they know physicians are too busy to read. They are counting on the fact that few doctors will understand what the policy changes are and that even fewer will contest them, says Greg Brodek, JD, chair of the health law practice group and head of the managed care litigation practice at Duane Morris, who represents doctors in disputes with payers.
These experts say doctors can push back on one-sided payer contracts and negotiate changes. Mr. Brodek says some practices have more leverage than others to influence payers – if they are larger, in a specialty that the payer needs in its network, or located in a remote area where the payer has limited options.
Here are seven key areas to pay attention to:
1. Long-term contracts. Most doctors sign multiyear “evergreen” contracts that renew automatically every year. This allows insurers to continue to pay doctors the same rate for years.
To avoid this, doctors should negotiate new rates when their agreements renew or, if they prefer, ask that a cost-of-living adjustment be included in the multiyear contract that applies to subsequent years, says Ms. Brauchler.
2. Fee schedules. Payers will “whitewash” what they’re paying you by saying it’s 100% of the payer fee schedule. When it comes to Medicare, they may be paying you a lot less, says Ms. Brauchler.
“My biggest takeaway is to compare the CPT codes of the payer’s fee schedule against what Medicare allows. For example, for CPT code 99213, a 15-minute established office visit, if Medicare pays you $100 and Aetna pays you $75.00, you’re getting 75% of Medicare,” says Ms. Brauchler. To avoid this, doctors should ask that the contract state that reimbursement be made according to Medicare’s medical policies rather than the payer’s.
3. Audits. Commercial payers will claim they have a contractual right to conduct pre- and post-payment audits of physicians’ claims that can result in reduced payments. The contract only states that if doctors correctly submit claims, they will get paid, not that they will have to go through extra steps, which is a breach of their agreement, says Mr. Brodek.
In his experience, 90% of payers back down when asked to provide the contractual basis to conduct these audits. “Or, they take the position that it’s not in the contract but that they have a policy.”
4. Contract amendments versus policies and procedures. This is a huge area that needs to be clarified in contracts and monitored by providers throughout their relationships with payers. Contracts have three elements: the parties, the services provided, and the payment. Changing any one of those terms requires an amendment and advance written notice that has to be delivered to the other party in a certain way, such as by overnight delivery, says Mr. Brodek.
In addition, both parties have to sign that they agree to an amendment. “But, that’s too cumbersome and complicated for payers who have decided to adopt policies instead. These are unilateral changes made with no advance notice given, since the payer typically posts the change on its website,” says Mr. Brodek.
5. Recoupment efforts. Payers will review claims after they’re paid and contact the doctor saying they found a mistake, such as inappropriate coding. They will claim that the doctor now owes them a large sum of money based on a percentage of claims reviewed. “They typically send the doctor a letter that ends with, ‘If you do not pay this amount within 30 days, we will offset the amount due against future payments that we would otherwise make to you,’” says Mr. Brodek.
He recommends that contracts include the doctors’ right to contest an audit so the “payer doesn’t have the unilateral right to disregard the initial coding that the doctor appropriately assigned to the claim and recoup the money anyway,” says Mr. Brodek.
6. Medical network rentals and products. Most contracts say that payers can rent out their medical networks to other health plans, such as HMOs, and that the clinicians agree to comply with all of their policies and procedures. The agreement may also cover the products of other plans.
“The problem is that physicians are not given information about the other plans, including their terms and conditions for getting paid,” says Mr. Brodek. If a problem with payment arises, they have no written agreement with that plan, which makes it harder to enforce.
“That’s why we recommend that doctors negotiate agreements that only cover the main payer. Most of the time, the payer is amenable to putting that language in the contract,” he says.
7. Payer products. In the past several years, a typical contract has included appendices that list the payer’s products, such as Medicare, workers compensation, auto insurance liability, or health care exchange products. Many clinicians don’t realize they can pick the plans they want to participate in by accepting or opting out, says Mr. Brodek.
“We advise clients to limit the contract to what you want covered and to make informed decisions, because some products have low fees set by the states, such as workers compensation and health care exchanges,” says Mr. Brodek.
A version of this article first appeared on Medscape.com.
, say experts in physician-payer contracts.
Many doctors sign long-term agreements and then forget about them, says Marcia Brauchler, president and founder of Physicians’ Ally, Littleton, Colorado, a health care consulting company. “The average doctor is trying to run a practice on 2010 rates because they haven’t touched their insurance contracts for 10 years,” she says.
Payers also make a lot of money by adopting dozens of unilateral policy and procedure changes every year that they know physicians are too busy to read. They are counting on the fact that few doctors will understand what the policy changes are and that even fewer will contest them, says Greg Brodek, JD, chair of the health law practice group and head of the managed care litigation practice at Duane Morris, who represents doctors in disputes with payers.
These experts say doctors can push back on one-sided payer contracts and negotiate changes. Mr. Brodek says some practices have more leverage than others to influence payers – if they are larger, in a specialty that the payer needs in its network, or located in a remote area where the payer has limited options.
Here are seven key areas to pay attention to:
1. Long-term contracts. Most doctors sign multiyear “evergreen” contracts that renew automatically every year. This allows insurers to continue to pay doctors the same rate for years.
To avoid this, doctors should negotiate new rates when their agreements renew or, if they prefer, ask that a cost-of-living adjustment be included in the multiyear contract that applies to subsequent years, says Ms. Brauchler.
2. Fee schedules. Payers will “whitewash” what they’re paying you by saying it’s 100% of the payer fee schedule. When it comes to Medicare, they may be paying you a lot less, says Ms. Brauchler.
“My biggest takeaway is to compare the CPT codes of the payer’s fee schedule against what Medicare allows. For example, for CPT code 99213, a 15-minute established office visit, if Medicare pays you $100 and Aetna pays you $75.00, you’re getting 75% of Medicare,” says Ms. Brauchler. To avoid this, doctors should ask that the contract state that reimbursement be made according to Medicare’s medical policies rather than the payer’s.
3. Audits. Commercial payers will claim they have a contractual right to conduct pre- and post-payment audits of physicians’ claims that can result in reduced payments. The contract only states that if doctors correctly submit claims, they will get paid, not that they will have to go through extra steps, which is a breach of their agreement, says Mr. Brodek.
In his experience, 90% of payers back down when asked to provide the contractual basis to conduct these audits. “Or, they take the position that it’s not in the contract but that they have a policy.”
4. Contract amendments versus policies and procedures. This is a huge area that needs to be clarified in contracts and monitored by providers throughout their relationships with payers. Contracts have three elements: the parties, the services provided, and the payment. Changing any one of those terms requires an amendment and advance written notice that has to be delivered to the other party in a certain way, such as by overnight delivery, says Mr. Brodek.
In addition, both parties have to sign that they agree to an amendment. “But, that’s too cumbersome and complicated for payers who have decided to adopt policies instead. These are unilateral changes made with no advance notice given, since the payer typically posts the change on its website,” says Mr. Brodek.
5. Recoupment efforts. Payers will review claims after they’re paid and contact the doctor saying they found a mistake, such as inappropriate coding. They will claim that the doctor now owes them a large sum of money based on a percentage of claims reviewed. “They typically send the doctor a letter that ends with, ‘If you do not pay this amount within 30 days, we will offset the amount due against future payments that we would otherwise make to you,’” says Mr. Brodek.
He recommends that contracts include the doctors’ right to contest an audit so the “payer doesn’t have the unilateral right to disregard the initial coding that the doctor appropriately assigned to the claim and recoup the money anyway,” says Mr. Brodek.
6. Medical network rentals and products. Most contracts say that payers can rent out their medical networks to other health plans, such as HMOs, and that the clinicians agree to comply with all of their policies and procedures. The agreement may also cover the products of other plans.
“The problem is that physicians are not given information about the other plans, including their terms and conditions for getting paid,” says Mr. Brodek. If a problem with payment arises, they have no written agreement with that plan, which makes it harder to enforce.
“That’s why we recommend that doctors negotiate agreements that only cover the main payer. Most of the time, the payer is amenable to putting that language in the contract,” he says.
7. Payer products. In the past several years, a typical contract has included appendices that list the payer’s products, such as Medicare, workers compensation, auto insurance liability, or health care exchange products. Many clinicians don’t realize they can pick the plans they want to participate in by accepting or opting out, says Mr. Brodek.
“We advise clients to limit the contract to what you want covered and to make informed decisions, because some products have low fees set by the states, such as workers compensation and health care exchanges,” says Mr. Brodek.
A version of this article first appeared on Medscape.com.
, say experts in physician-payer contracts.
Many doctors sign long-term agreements and then forget about them, says Marcia Brauchler, president and founder of Physicians’ Ally, Littleton, Colorado, a health care consulting company. “The average doctor is trying to run a practice on 2010 rates because they haven’t touched their insurance contracts for 10 years,” she says.
Payers also make a lot of money by adopting dozens of unilateral policy and procedure changes every year that they know physicians are too busy to read. They are counting on the fact that few doctors will understand what the policy changes are and that even fewer will contest them, says Greg Brodek, JD, chair of the health law practice group and head of the managed care litigation practice at Duane Morris, who represents doctors in disputes with payers.
These experts say doctors can push back on one-sided payer contracts and negotiate changes. Mr. Brodek says some practices have more leverage than others to influence payers – if they are larger, in a specialty that the payer needs in its network, or located in a remote area where the payer has limited options.
Here are seven key areas to pay attention to:
1. Long-term contracts. Most doctors sign multiyear “evergreen” contracts that renew automatically every year. This allows insurers to continue to pay doctors the same rate for years.
To avoid this, doctors should negotiate new rates when their agreements renew or, if they prefer, ask that a cost-of-living adjustment be included in the multiyear contract that applies to subsequent years, says Ms. Brauchler.
2. Fee schedules. Payers will “whitewash” what they’re paying you by saying it’s 100% of the payer fee schedule. When it comes to Medicare, they may be paying you a lot less, says Ms. Brauchler.
“My biggest takeaway is to compare the CPT codes of the payer’s fee schedule against what Medicare allows. For example, for CPT code 99213, a 15-minute established office visit, if Medicare pays you $100 and Aetna pays you $75.00, you’re getting 75% of Medicare,” says Ms. Brauchler. To avoid this, doctors should ask that the contract state that reimbursement be made according to Medicare’s medical policies rather than the payer’s.
3. Audits. Commercial payers will claim they have a contractual right to conduct pre- and post-payment audits of physicians’ claims that can result in reduced payments. The contract only states that if doctors correctly submit claims, they will get paid, not that they will have to go through extra steps, which is a breach of their agreement, says Mr. Brodek.
In his experience, 90% of payers back down when asked to provide the contractual basis to conduct these audits. “Or, they take the position that it’s not in the contract but that they have a policy.”
4. Contract amendments versus policies and procedures. This is a huge area that needs to be clarified in contracts and monitored by providers throughout their relationships with payers. Contracts have three elements: the parties, the services provided, and the payment. Changing any one of those terms requires an amendment and advance written notice that has to be delivered to the other party in a certain way, such as by overnight delivery, says Mr. Brodek.
In addition, both parties have to sign that they agree to an amendment. “But, that’s too cumbersome and complicated for payers who have decided to adopt policies instead. These are unilateral changes made with no advance notice given, since the payer typically posts the change on its website,” says Mr. Brodek.
5. Recoupment efforts. Payers will review claims after they’re paid and contact the doctor saying they found a mistake, such as inappropriate coding. They will claim that the doctor now owes them a large sum of money based on a percentage of claims reviewed. “They typically send the doctor a letter that ends with, ‘If you do not pay this amount within 30 days, we will offset the amount due against future payments that we would otherwise make to you,’” says Mr. Brodek.
He recommends that contracts include the doctors’ right to contest an audit so the “payer doesn’t have the unilateral right to disregard the initial coding that the doctor appropriately assigned to the claim and recoup the money anyway,” says Mr. Brodek.
6. Medical network rentals and products. Most contracts say that payers can rent out their medical networks to other health plans, such as HMOs, and that the clinicians agree to comply with all of their policies and procedures. The agreement may also cover the products of other plans.
“The problem is that physicians are not given information about the other plans, including their terms and conditions for getting paid,” says Mr. Brodek. If a problem with payment arises, they have no written agreement with that plan, which makes it harder to enforce.
“That’s why we recommend that doctors negotiate agreements that only cover the main payer. Most of the time, the payer is amenable to putting that language in the contract,” he says.
7. Payer products. In the past several years, a typical contract has included appendices that list the payer’s products, such as Medicare, workers compensation, auto insurance liability, or health care exchange products. Many clinicians don’t realize they can pick the plans they want to participate in by accepting or opting out, says Mr. Brodek.
“We advise clients to limit the contract to what you want covered and to make informed decisions, because some products have low fees set by the states, such as workers compensation and health care exchanges,” says Mr. Brodek.
A version of this article first appeared on Medscape.com.
Ways to make sure 2022 doesn’t stink for docs
Depending on the data you’re looking at, 40%-60% of physicians are burned out.
Research studies and the eye test reveal the painfully obvious: Colleagues are tired, winded, spent, and at times way past burned out. People aren’t asking me if they’re burned out. They know they’re burned out; heck, they can even recite the Maslach burnout inventory, forward and backward, in a mask, or while completing a COVID quarantine. A fair share of people know the key steps to prevent burnout and promote recovery.
What I’m starting to see more of is, “Why should I even bother to recover from this? Why pick myself up again just to get another occupational stress injury (burnout, demoralization, moral injury, etc.)?” In other words, it’s not just simply about negating burnout; it’s about supporting and facilitating the motivation to work.
We’ve been through so much with COVID that it might be challenging to remember when you saw a truly engaged work environment. No doubt, we have outstanding professionals across medicine who answer the bell every day. However, if you’ve been looking closely, many teams/units have lost a bit of the zip and pep. The synergy and trust aren’t as smooth, and at noon, everyone counts the hours to the end of the shift.
You may be thinking, Well, of course, they are; we’re still amid a pandemic, and people have been through hell. Your observation would be correct, except I’ve personally seen some teams weather the pandemic storm and still remain engaged (some even more involved).
The No. 1 consult result for the GW Resiliency and Well-Being Center, where I work, has been on lectures for burnout. The R&WC has given so many of these lectures that my dreams take the form of a PowerPoint presentation. Overall the talks have gone very well. We’ve added skills sections on practices of whole-person care. We’ve blitzed the daylights out of restorative sleep, yet I know we are still searching for the correct narrative.
Motivated staff, faculty, and students will genuinely take in the information and follow the recommendations; however, they still struggle to find that drive and zest for work. Yes, moving from burnout to neutral is reasonable but likely won’t move the needle of your professional or personal life. We need to have the emotional energy and the clear desire to utilize that energy for a meaningful purpose.
Talking about burnout in specific ways is straightforward and, in my opinion, much easier than talking about engagement. Part of the challenge when trying to discuss engagement is that people can feel invalidated or that you’re telling them to be stoic. Or worse yet, that the problem of burnout primarily lies with them. It’s essential to recognize the role of an organizational factor in burnout (approximately 80%, depending on the study); still, even if you address burnout, people may not be miserable, but it doesn’t mean they will stay at their current job (please cue intro music for the Great Resignation).
Engagement models have existed for some time and certainly have gained much more attention in health care settings over the past 2 decades. Engagement can be described as having three components: dedication, vigor, and absorption. When a person is filling all three of these components over time, presto – you get the much-sought-after state of the supremely engaged professional.
These models definitely give us excellent starting points to approach engagement from a pre-COVID era. In COVID and beyond, I’m not sure how these models will stand up in a hybrid work environment, where autonomy and flexibility could be more valued than ever. Personally, COVID revealed some things I was missing in my work pre-COVID:
- Time to think and process. This was one of the great things about being a consultation-liaison psychiatrist; it was literally feast or famine.
- Doing what I’m talented at and really enjoy.
- Time is short, and I want to be more present in the life of my family.
The list above isn’t exhaustive, but I’ve found them to be my own personal recipe for being engaged. Over the next series of articles, I’m going to focus on engagement and factors related to key resilience. These articles will be informed by a front-line view from my colleagues, and hopefully start to separate the myth from reality on the subject of health professional engagement and resilience.
Everyone be safe and well!
A version of this article first appeared on Medscape.com.
Depending on the data you’re looking at, 40%-60% of physicians are burned out.
Research studies and the eye test reveal the painfully obvious: Colleagues are tired, winded, spent, and at times way past burned out. People aren’t asking me if they’re burned out. They know they’re burned out; heck, they can even recite the Maslach burnout inventory, forward and backward, in a mask, or while completing a COVID quarantine. A fair share of people know the key steps to prevent burnout and promote recovery.
What I’m starting to see more of is, “Why should I even bother to recover from this? Why pick myself up again just to get another occupational stress injury (burnout, demoralization, moral injury, etc.)?” In other words, it’s not just simply about negating burnout; it’s about supporting and facilitating the motivation to work.
We’ve been through so much with COVID that it might be challenging to remember when you saw a truly engaged work environment. No doubt, we have outstanding professionals across medicine who answer the bell every day. However, if you’ve been looking closely, many teams/units have lost a bit of the zip and pep. The synergy and trust aren’t as smooth, and at noon, everyone counts the hours to the end of the shift.
You may be thinking, Well, of course, they are; we’re still amid a pandemic, and people have been through hell. Your observation would be correct, except I’ve personally seen some teams weather the pandemic storm and still remain engaged (some even more involved).
The No. 1 consult result for the GW Resiliency and Well-Being Center, where I work, has been on lectures for burnout. The R&WC has given so many of these lectures that my dreams take the form of a PowerPoint presentation. Overall the talks have gone very well. We’ve added skills sections on practices of whole-person care. We’ve blitzed the daylights out of restorative sleep, yet I know we are still searching for the correct narrative.
Motivated staff, faculty, and students will genuinely take in the information and follow the recommendations; however, they still struggle to find that drive and zest for work. Yes, moving from burnout to neutral is reasonable but likely won’t move the needle of your professional or personal life. We need to have the emotional energy and the clear desire to utilize that energy for a meaningful purpose.
Talking about burnout in specific ways is straightforward and, in my opinion, much easier than talking about engagement. Part of the challenge when trying to discuss engagement is that people can feel invalidated or that you’re telling them to be stoic. Or worse yet, that the problem of burnout primarily lies with them. It’s essential to recognize the role of an organizational factor in burnout (approximately 80%, depending on the study); still, even if you address burnout, people may not be miserable, but it doesn’t mean they will stay at their current job (please cue intro music for the Great Resignation).
Engagement models have existed for some time and certainly have gained much more attention in health care settings over the past 2 decades. Engagement can be described as having three components: dedication, vigor, and absorption. When a person is filling all three of these components over time, presto – you get the much-sought-after state of the supremely engaged professional.
These models definitely give us excellent starting points to approach engagement from a pre-COVID era. In COVID and beyond, I’m not sure how these models will stand up in a hybrid work environment, where autonomy and flexibility could be more valued than ever. Personally, COVID revealed some things I was missing in my work pre-COVID:
- Time to think and process. This was one of the great things about being a consultation-liaison psychiatrist; it was literally feast or famine.
- Doing what I’m talented at and really enjoy.
- Time is short, and I want to be more present in the life of my family.
The list above isn’t exhaustive, but I’ve found them to be my own personal recipe for being engaged. Over the next series of articles, I’m going to focus on engagement and factors related to key resilience. These articles will be informed by a front-line view from my colleagues, and hopefully start to separate the myth from reality on the subject of health professional engagement and resilience.
Everyone be safe and well!
A version of this article first appeared on Medscape.com.
Depending on the data you’re looking at, 40%-60% of physicians are burned out.
Research studies and the eye test reveal the painfully obvious: Colleagues are tired, winded, spent, and at times way past burned out. People aren’t asking me if they’re burned out. They know they’re burned out; heck, they can even recite the Maslach burnout inventory, forward and backward, in a mask, or while completing a COVID quarantine. A fair share of people know the key steps to prevent burnout and promote recovery.
What I’m starting to see more of is, “Why should I even bother to recover from this? Why pick myself up again just to get another occupational stress injury (burnout, demoralization, moral injury, etc.)?” In other words, it’s not just simply about negating burnout; it’s about supporting and facilitating the motivation to work.
We’ve been through so much with COVID that it might be challenging to remember when you saw a truly engaged work environment. No doubt, we have outstanding professionals across medicine who answer the bell every day. However, if you’ve been looking closely, many teams/units have lost a bit of the zip and pep. The synergy and trust aren’t as smooth, and at noon, everyone counts the hours to the end of the shift.
You may be thinking, Well, of course, they are; we’re still amid a pandemic, and people have been through hell. Your observation would be correct, except I’ve personally seen some teams weather the pandemic storm and still remain engaged (some even more involved).
The No. 1 consult result for the GW Resiliency and Well-Being Center, where I work, has been on lectures for burnout. The R&WC has given so many of these lectures that my dreams take the form of a PowerPoint presentation. Overall the talks have gone very well. We’ve added skills sections on practices of whole-person care. We’ve blitzed the daylights out of restorative sleep, yet I know we are still searching for the correct narrative.
Motivated staff, faculty, and students will genuinely take in the information and follow the recommendations; however, they still struggle to find that drive and zest for work. Yes, moving from burnout to neutral is reasonable but likely won’t move the needle of your professional or personal life. We need to have the emotional energy and the clear desire to utilize that energy for a meaningful purpose.
Talking about burnout in specific ways is straightforward and, in my opinion, much easier than talking about engagement. Part of the challenge when trying to discuss engagement is that people can feel invalidated or that you’re telling them to be stoic. Or worse yet, that the problem of burnout primarily lies with them. It’s essential to recognize the role of an organizational factor in burnout (approximately 80%, depending on the study); still, even if you address burnout, people may not be miserable, but it doesn’t mean they will stay at their current job (please cue intro music for the Great Resignation).
Engagement models have existed for some time and certainly have gained much more attention in health care settings over the past 2 decades. Engagement can be described as having three components: dedication, vigor, and absorption. When a person is filling all three of these components over time, presto – you get the much-sought-after state of the supremely engaged professional.
These models definitely give us excellent starting points to approach engagement from a pre-COVID era. In COVID and beyond, I’m not sure how these models will stand up in a hybrid work environment, where autonomy and flexibility could be more valued than ever. Personally, COVID revealed some things I was missing in my work pre-COVID:
- Time to think and process. This was one of the great things about being a consultation-liaison psychiatrist; it was literally feast or famine.
- Doing what I’m talented at and really enjoy.
- Time is short, and I want to be more present in the life of my family.
The list above isn’t exhaustive, but I’ve found them to be my own personal recipe for being engaged. Over the next series of articles, I’m going to focus on engagement and factors related to key resilience. These articles will be informed by a front-line view from my colleagues, and hopefully start to separate the myth from reality on the subject of health professional engagement and resilience.
Everyone be safe and well!
A version of this article first appeared on Medscape.com.
Does COVID-19 induce type 1 diabetes in kids? Jury still out
Two new studies from different parts of the world have identified an increase in the incidence of type 1 diabetes in children since the COVID-19 pandemic began, but the reasons still aren’t clear.
The findings from the two studies, in Germany and the United States, align closely, endocrinologist Jane J. Kim, MD, professor of pediatrics and principal investigator of the U.S. study, told this news organization. “I think that the general conclusion based on their data and our data is that there appears to be an increased rate of new type 1 diabetes diagnoses in children since the onset of the pandemic.”
Dr. Kim noted that because her group’s data pertain to just a single center, she is “heartened to see that the [German team’s] general conclusions are the same as ours.” Moreover, she pointed out that other studies examining this question came from Europe early in the pandemic, whereas “now both they [the German group] and we have had the opportunity to look at what’s happening over a longer period of time.”
But the reason for the association remains unclear. Some answers may be forthcoming from a database designed in mid-2020 specifically to examine the relationship between COVID-19 and new-onset diabetes. Called CoviDiab, the registry aims “to establish the extent and characteristics of new-onset, COVID-19–related diabetes and to investigate its pathogenesis, management, and outcomes,” according to the website.
The first new study, a multicenter German diabetes registry study, was published online Jan. 17 in Diabetes Care by Clemens Kamrath, MD, of Justus Liebig University, Giessen, Germany, and colleagues.
The other, from Rady Children’s Hospital of San Diego, was published online Jan. 24 in JAMA Pediatrics by Bethany L. Gottesman, MD, and colleagues, all with the University of California, San Diego.
Mechanisms likely to differ for type 1 versus type 2 diabetes
Neither the German nor the U.S. investigators were able to directly correlate current or prior SARS-CoV-2 infection in children with the subsequent development of type 1 diabetes.
Earlier this month, a study from the U.S. Centers for Disease Control and Prevention did examine that issue, but it also included youth with type 2 diabetes and did not separate out the two groups.
Dr. Kim said her institution has also seen an increase in type 2 diabetes among youth since the COVID-19 pandemic began but did not include that in their current article.
“When we started looking at our data, diabetes and COVID-19 in adults had been relatively well established. To see an increase in type 2 [diabetes] was not so surprising to our group. But we had the sense we were seeing more patients with type 1, and when we looked at our hospital that was very much the case. I think that was a surprise to people,” said Dr. Kim.
Although a direct effect of SARS-CoV-2 on pancreatic beta cells has been proposed, in both the German and San Diego datasets the diagnosis of type 1 diabetes was confirmed with autoantibodies that are typically present years prior to the onset of clinical symptoms.
The German group suggests possible other explanations for the link, including the lack of immune system exposure to other common pediatric infections during pandemic-necessitated social distancing – the so-called hygiene hypothesis – as well as the possible role of psychological stress, which several studies have linked to type 1 diabetes.
But as of now, Dr. Kim said, “Nobody really knows.”
Is the effect direct or indirect?
Using data from the multicenter German Diabetes Prospective Follow-up Registry, Dr. Kamrath and colleagues compared the incidence of type 1 diabetes in children and adolescents from Jan. 1, 2020 through June 30, 2021 with the incidence in 2011-2019.
During the pandemic period, a total of 5,162 youth were newly diagnosed with type 1 diabetes at 236 German centers. That incidence, 24.4 per 100,000 patient-years, was significantly higher than the 21.2 per 100,000 patient-years expected based on the prior decade, with an incidence rate ratio of 1.15 (P < .001). The increase was similar in both males and females.
There was a difference by age, however, as the phenomenon appeared to be limited to the preadolescent age groups. The incidence rate ratios (IRRs) for ages below 6 years and 6-11 years were 1.23 and 1.18 (both P < .001), respectively, compared to a nonsignificant IRR of 1.06 (P = .13) in those aged 12-17 years.
Compared with the expected monthly incidence, the observed incidence was significantly higher in June 2020 (IRR, 1.43; P = .003), July 2020 (IRR, 1.48; P < 0.001), March 2021 (IRR, 1.29; P = .028), and June 2021 (IRR, 1.39; P = .01).
Among the 3,851 patients for whom data on type 1 diabetes-associated autoantibodies were available, the adjusted rates of autoantibody negativity did not differ from 2018-2019 during the entire pandemic period or during the year 2020 or the first half of 2021.
“Therefore, the increase in the incidence of type 1 diabetes in children appears to be due to immune-mediated type 1 diabetes. However, because autoimmunity and progressive beta-cell destruction typically begin long before the clinical diagnosis of type 1 diabetes, we were surprised to see the incidence of type 1 diabetes followed the peak incidence of COVID-19 and also the pandemic containment measures by only approximately 3 months,” Dr. Kamrath and colleagues write.
Taken together, they say, the data suggest that “the impact on type 1 diabetes incidence is not due to infection with SARS-CoV-2 but rather a consequence of environmental changes resulting from the pandemic itself or pandemic containment measures.”
Similar findings at a U.S. children’s hospital
In the cross-sectional study in San Diego, Dr. Gottesman and colleagues looked at the electronic medical records (EMRs) at Rady Children’s Hospital for patients aged younger than 19 years with at least one positive type 1 diabetes antibody titer.
During March 19, 2020 to March 18, 2021, a total of 187 children were admitted for new-onset type 1 diabetes, compared with just 119 the previous year, a 57% increase.
From July 2020 through February 2021, the number of new type 1 diabetes diagnoses significantly exceeded the number expected based on a quarterly moving average of each of the preceding 5 years.
Only four of the 187 patients (2.1%) diagnosed during the pandemic period had a COVID-19 infection at the time of presentation. Antibody testing to assess prior infection wasn’t feasible, and now that children are receiving the vaccine – and therefore most will have antibodies – “we’ve lost our window of opportunity to look at that question,” Dr. Kim noted.
As has been previously shown, there was an increase in the percentage of patients presenting with diabetic ketoacidosis during the pandemic compared with the prior 5 years (49.7% vs. 40.7% requiring insulin infusion). However, there was no difference in mean age at presentation, body mass index, A1c, or percentage requiring admission to intensive care.
Because these data only go through March 2021, Dr. Kim noted, “We need to see what’s happening with these different variants. We’ll have a chance to look in a month or two to see the effects of Omicron on the rates of diabetes in the hospital.”
Will CoviDiab answer the question?
Data from CoviDiab will include diabetes type in adults and children, registry coprincipal investigator Francesco Rubino, MD, of King’s College London, told this news organization.
“We aimed at having as many as possible cases of new-onset diabetes for which we can have also a minimum set of clinical data including type of diabetes and A1c. By looking at this information we can infer whether a role of COVID-19 in triggering diabetes is clinically plausible – or not – and what type of diabetes is most frequently associated with COVID-19 as this also speaks about mechanisms of action.”
Dr. Rubino said that the CoviDiab team is approaching the data with the assumption that, at least in adults diagnosed with type 2 diabetes, the explanation might be that the person already had undiagnosed diabetes or that the hyperglycemia may be stress-induced and temporary.
“We’re looking at this question with a skeptical eye ... Is it just an association, or does the virus have a role in inducing diabetes from scratch, or can the virus advance pathophysiology in a way that it ends up in full-blown diabetes in predisposed individuals?”
While no single study will prove that SARS-CoV-2 causes diabetes, “combining observations from various studies and approaches we may get a higher degree of certainty,” Dr. Rubino said, noting that the CoviDiab team plans to publish data from the first 800 cases “soon.”
Dr. Kim has reported no relevant financial relationships. Dr. Rubino has reported receiving grants from Ethicon and Medtronic, personal fees from GI Dynamic, Keyron, Novo Nordisk, Ethicon, and Medtronic.
A version of this article first appeared on Medscape.com.
Two new studies from different parts of the world have identified an increase in the incidence of type 1 diabetes in children since the COVID-19 pandemic began, but the reasons still aren’t clear.
The findings from the two studies, in Germany and the United States, align closely, endocrinologist Jane J. Kim, MD, professor of pediatrics and principal investigator of the U.S. study, told this news organization. “I think that the general conclusion based on their data and our data is that there appears to be an increased rate of new type 1 diabetes diagnoses in children since the onset of the pandemic.”
Dr. Kim noted that because her group’s data pertain to just a single center, she is “heartened to see that the [German team’s] general conclusions are the same as ours.” Moreover, she pointed out that other studies examining this question came from Europe early in the pandemic, whereas “now both they [the German group] and we have had the opportunity to look at what’s happening over a longer period of time.”
But the reason for the association remains unclear. Some answers may be forthcoming from a database designed in mid-2020 specifically to examine the relationship between COVID-19 and new-onset diabetes. Called CoviDiab, the registry aims “to establish the extent and characteristics of new-onset, COVID-19–related diabetes and to investigate its pathogenesis, management, and outcomes,” according to the website.
The first new study, a multicenter German diabetes registry study, was published online Jan. 17 in Diabetes Care by Clemens Kamrath, MD, of Justus Liebig University, Giessen, Germany, and colleagues.
The other, from Rady Children’s Hospital of San Diego, was published online Jan. 24 in JAMA Pediatrics by Bethany L. Gottesman, MD, and colleagues, all with the University of California, San Diego.
Mechanisms likely to differ for type 1 versus type 2 diabetes
Neither the German nor the U.S. investigators were able to directly correlate current or prior SARS-CoV-2 infection in children with the subsequent development of type 1 diabetes.
Earlier this month, a study from the U.S. Centers for Disease Control and Prevention did examine that issue, but it also included youth with type 2 diabetes and did not separate out the two groups.
Dr. Kim said her institution has also seen an increase in type 2 diabetes among youth since the COVID-19 pandemic began but did not include that in their current article.
“When we started looking at our data, diabetes and COVID-19 in adults had been relatively well established. To see an increase in type 2 [diabetes] was not so surprising to our group. But we had the sense we were seeing more patients with type 1, and when we looked at our hospital that was very much the case. I think that was a surprise to people,” said Dr. Kim.
Although a direct effect of SARS-CoV-2 on pancreatic beta cells has been proposed, in both the German and San Diego datasets the diagnosis of type 1 diabetes was confirmed with autoantibodies that are typically present years prior to the onset of clinical symptoms.
The German group suggests possible other explanations for the link, including the lack of immune system exposure to other common pediatric infections during pandemic-necessitated social distancing – the so-called hygiene hypothesis – as well as the possible role of psychological stress, which several studies have linked to type 1 diabetes.
But as of now, Dr. Kim said, “Nobody really knows.”
Is the effect direct or indirect?
Using data from the multicenter German Diabetes Prospective Follow-up Registry, Dr. Kamrath and colleagues compared the incidence of type 1 diabetes in children and adolescents from Jan. 1, 2020 through June 30, 2021 with the incidence in 2011-2019.
During the pandemic period, a total of 5,162 youth were newly diagnosed with type 1 diabetes at 236 German centers. That incidence, 24.4 per 100,000 patient-years, was significantly higher than the 21.2 per 100,000 patient-years expected based on the prior decade, with an incidence rate ratio of 1.15 (P < .001). The increase was similar in both males and females.
There was a difference by age, however, as the phenomenon appeared to be limited to the preadolescent age groups. The incidence rate ratios (IRRs) for ages below 6 years and 6-11 years were 1.23 and 1.18 (both P < .001), respectively, compared to a nonsignificant IRR of 1.06 (P = .13) in those aged 12-17 years.
Compared with the expected monthly incidence, the observed incidence was significantly higher in June 2020 (IRR, 1.43; P = .003), July 2020 (IRR, 1.48; P < 0.001), March 2021 (IRR, 1.29; P = .028), and June 2021 (IRR, 1.39; P = .01).
Among the 3,851 patients for whom data on type 1 diabetes-associated autoantibodies were available, the adjusted rates of autoantibody negativity did not differ from 2018-2019 during the entire pandemic period or during the year 2020 or the first half of 2021.
“Therefore, the increase in the incidence of type 1 diabetes in children appears to be due to immune-mediated type 1 diabetes. However, because autoimmunity and progressive beta-cell destruction typically begin long before the clinical diagnosis of type 1 diabetes, we were surprised to see the incidence of type 1 diabetes followed the peak incidence of COVID-19 and also the pandemic containment measures by only approximately 3 months,” Dr. Kamrath and colleagues write.
Taken together, they say, the data suggest that “the impact on type 1 diabetes incidence is not due to infection with SARS-CoV-2 but rather a consequence of environmental changes resulting from the pandemic itself or pandemic containment measures.”
Similar findings at a U.S. children’s hospital
In the cross-sectional study in San Diego, Dr. Gottesman and colleagues looked at the electronic medical records (EMRs) at Rady Children’s Hospital for patients aged younger than 19 years with at least one positive type 1 diabetes antibody titer.
During March 19, 2020 to March 18, 2021, a total of 187 children were admitted for new-onset type 1 diabetes, compared with just 119 the previous year, a 57% increase.
From July 2020 through February 2021, the number of new type 1 diabetes diagnoses significantly exceeded the number expected based on a quarterly moving average of each of the preceding 5 years.
Only four of the 187 patients (2.1%) diagnosed during the pandemic period had a COVID-19 infection at the time of presentation. Antibody testing to assess prior infection wasn’t feasible, and now that children are receiving the vaccine – and therefore most will have antibodies – “we’ve lost our window of opportunity to look at that question,” Dr. Kim noted.
As has been previously shown, there was an increase in the percentage of patients presenting with diabetic ketoacidosis during the pandemic compared with the prior 5 years (49.7% vs. 40.7% requiring insulin infusion). However, there was no difference in mean age at presentation, body mass index, A1c, or percentage requiring admission to intensive care.
Because these data only go through March 2021, Dr. Kim noted, “We need to see what’s happening with these different variants. We’ll have a chance to look in a month or two to see the effects of Omicron on the rates of diabetes in the hospital.”
Will CoviDiab answer the question?
Data from CoviDiab will include diabetes type in adults and children, registry coprincipal investigator Francesco Rubino, MD, of King’s College London, told this news organization.
“We aimed at having as many as possible cases of new-onset diabetes for which we can have also a minimum set of clinical data including type of diabetes and A1c. By looking at this information we can infer whether a role of COVID-19 in triggering diabetes is clinically plausible – or not – and what type of diabetes is most frequently associated with COVID-19 as this also speaks about mechanisms of action.”
Dr. Rubino said that the CoviDiab team is approaching the data with the assumption that, at least in adults diagnosed with type 2 diabetes, the explanation might be that the person already had undiagnosed diabetes or that the hyperglycemia may be stress-induced and temporary.
“We’re looking at this question with a skeptical eye ... Is it just an association, or does the virus have a role in inducing diabetes from scratch, or can the virus advance pathophysiology in a way that it ends up in full-blown diabetes in predisposed individuals?”
While no single study will prove that SARS-CoV-2 causes diabetes, “combining observations from various studies and approaches we may get a higher degree of certainty,” Dr. Rubino said, noting that the CoviDiab team plans to publish data from the first 800 cases “soon.”
Dr. Kim has reported no relevant financial relationships. Dr. Rubino has reported receiving grants from Ethicon and Medtronic, personal fees from GI Dynamic, Keyron, Novo Nordisk, Ethicon, and Medtronic.
A version of this article first appeared on Medscape.com.
Two new studies from different parts of the world have identified an increase in the incidence of type 1 diabetes in children since the COVID-19 pandemic began, but the reasons still aren’t clear.
The findings from the two studies, in Germany and the United States, align closely, endocrinologist Jane J. Kim, MD, professor of pediatrics and principal investigator of the U.S. study, told this news organization. “I think that the general conclusion based on their data and our data is that there appears to be an increased rate of new type 1 diabetes diagnoses in children since the onset of the pandemic.”
Dr. Kim noted that because her group’s data pertain to just a single center, she is “heartened to see that the [German team’s] general conclusions are the same as ours.” Moreover, she pointed out that other studies examining this question came from Europe early in the pandemic, whereas “now both they [the German group] and we have had the opportunity to look at what’s happening over a longer period of time.”
But the reason for the association remains unclear. Some answers may be forthcoming from a database designed in mid-2020 specifically to examine the relationship between COVID-19 and new-onset diabetes. Called CoviDiab, the registry aims “to establish the extent and characteristics of new-onset, COVID-19–related diabetes and to investigate its pathogenesis, management, and outcomes,” according to the website.
The first new study, a multicenter German diabetes registry study, was published online Jan. 17 in Diabetes Care by Clemens Kamrath, MD, of Justus Liebig University, Giessen, Germany, and colleagues.
The other, from Rady Children’s Hospital of San Diego, was published online Jan. 24 in JAMA Pediatrics by Bethany L. Gottesman, MD, and colleagues, all with the University of California, San Diego.
Mechanisms likely to differ for type 1 versus type 2 diabetes
Neither the German nor the U.S. investigators were able to directly correlate current or prior SARS-CoV-2 infection in children with the subsequent development of type 1 diabetes.
Earlier this month, a study from the U.S. Centers for Disease Control and Prevention did examine that issue, but it also included youth with type 2 diabetes and did not separate out the two groups.
Dr. Kim said her institution has also seen an increase in type 2 diabetes among youth since the COVID-19 pandemic began but did not include that in their current article.
“When we started looking at our data, diabetes and COVID-19 in adults had been relatively well established. To see an increase in type 2 [diabetes] was not so surprising to our group. But we had the sense we were seeing more patients with type 1, and when we looked at our hospital that was very much the case. I think that was a surprise to people,” said Dr. Kim.
Although a direct effect of SARS-CoV-2 on pancreatic beta cells has been proposed, in both the German and San Diego datasets the diagnosis of type 1 diabetes was confirmed with autoantibodies that are typically present years prior to the onset of clinical symptoms.
The German group suggests possible other explanations for the link, including the lack of immune system exposure to other common pediatric infections during pandemic-necessitated social distancing – the so-called hygiene hypothesis – as well as the possible role of psychological stress, which several studies have linked to type 1 diabetes.
But as of now, Dr. Kim said, “Nobody really knows.”
Is the effect direct or indirect?
Using data from the multicenter German Diabetes Prospective Follow-up Registry, Dr. Kamrath and colleagues compared the incidence of type 1 diabetes in children and adolescents from Jan. 1, 2020 through June 30, 2021 with the incidence in 2011-2019.
During the pandemic period, a total of 5,162 youth were newly diagnosed with type 1 diabetes at 236 German centers. That incidence, 24.4 per 100,000 patient-years, was significantly higher than the 21.2 per 100,000 patient-years expected based on the prior decade, with an incidence rate ratio of 1.15 (P < .001). The increase was similar in both males and females.
There was a difference by age, however, as the phenomenon appeared to be limited to the preadolescent age groups. The incidence rate ratios (IRRs) for ages below 6 years and 6-11 years were 1.23 and 1.18 (both P < .001), respectively, compared to a nonsignificant IRR of 1.06 (P = .13) in those aged 12-17 years.
Compared with the expected monthly incidence, the observed incidence was significantly higher in June 2020 (IRR, 1.43; P = .003), July 2020 (IRR, 1.48; P < 0.001), March 2021 (IRR, 1.29; P = .028), and June 2021 (IRR, 1.39; P = .01).
Among the 3,851 patients for whom data on type 1 diabetes-associated autoantibodies were available, the adjusted rates of autoantibody negativity did not differ from 2018-2019 during the entire pandemic period or during the year 2020 or the first half of 2021.
“Therefore, the increase in the incidence of type 1 diabetes in children appears to be due to immune-mediated type 1 diabetes. However, because autoimmunity and progressive beta-cell destruction typically begin long before the clinical diagnosis of type 1 diabetes, we were surprised to see the incidence of type 1 diabetes followed the peak incidence of COVID-19 and also the pandemic containment measures by only approximately 3 months,” Dr. Kamrath and colleagues write.
Taken together, they say, the data suggest that “the impact on type 1 diabetes incidence is not due to infection with SARS-CoV-2 but rather a consequence of environmental changes resulting from the pandemic itself or pandemic containment measures.”
Similar findings at a U.S. children’s hospital
In the cross-sectional study in San Diego, Dr. Gottesman and colleagues looked at the electronic medical records (EMRs) at Rady Children’s Hospital for patients aged younger than 19 years with at least one positive type 1 diabetes antibody titer.
During March 19, 2020 to March 18, 2021, a total of 187 children were admitted for new-onset type 1 diabetes, compared with just 119 the previous year, a 57% increase.
From July 2020 through February 2021, the number of new type 1 diabetes diagnoses significantly exceeded the number expected based on a quarterly moving average of each of the preceding 5 years.
Only four of the 187 patients (2.1%) diagnosed during the pandemic period had a COVID-19 infection at the time of presentation. Antibody testing to assess prior infection wasn’t feasible, and now that children are receiving the vaccine – and therefore most will have antibodies – “we’ve lost our window of opportunity to look at that question,” Dr. Kim noted.
As has been previously shown, there was an increase in the percentage of patients presenting with diabetic ketoacidosis during the pandemic compared with the prior 5 years (49.7% vs. 40.7% requiring insulin infusion). However, there was no difference in mean age at presentation, body mass index, A1c, or percentage requiring admission to intensive care.
Because these data only go through March 2021, Dr. Kim noted, “We need to see what’s happening with these different variants. We’ll have a chance to look in a month or two to see the effects of Omicron on the rates of diabetes in the hospital.”
Will CoviDiab answer the question?
Data from CoviDiab will include diabetes type in adults and children, registry coprincipal investigator Francesco Rubino, MD, of King’s College London, told this news organization.
“We aimed at having as many as possible cases of new-onset diabetes for which we can have also a minimum set of clinical data including type of diabetes and A1c. By looking at this information we can infer whether a role of COVID-19 in triggering diabetes is clinically plausible – or not – and what type of diabetes is most frequently associated with COVID-19 as this also speaks about mechanisms of action.”
Dr. Rubino said that the CoviDiab team is approaching the data with the assumption that, at least in adults diagnosed with type 2 diabetes, the explanation might be that the person already had undiagnosed diabetes or that the hyperglycemia may be stress-induced and temporary.
“We’re looking at this question with a skeptical eye ... Is it just an association, or does the virus have a role in inducing diabetes from scratch, or can the virus advance pathophysiology in a way that it ends up in full-blown diabetes in predisposed individuals?”
While no single study will prove that SARS-CoV-2 causes diabetes, “combining observations from various studies and approaches we may get a higher degree of certainty,” Dr. Rubino said, noting that the CoviDiab team plans to publish data from the first 800 cases “soon.”
Dr. Kim has reported no relevant financial relationships. Dr. Rubino has reported receiving grants from Ethicon and Medtronic, personal fees from GI Dynamic, Keyron, Novo Nordisk, Ethicon, and Medtronic.
A version of this article first appeared on Medscape.com.
How to help adults meet dietary recommendations
Dietary guidelines provide scientifically sound and practical advice that, if followed by every person, would probably result in less obesity, type 2 diabetes, cardiovascular disease, cancer, and bone loss. But few US adults meet these recommendations, according to a recent report in the CDC’s Morbidity and Mortality Weekly Report (MMWR).1
Data from the 2019 Behavioral Risk Factor Surveillance system indicate that only 12.3% of US adults consumed the recommended amount of fruit and 10% the recommended amount of vegetables (more on that shortly). Women were more likely than men to meet the requirements for fruit (14.5% vs 10.1%) and vegetable (12.4% vs 7.6%) intake. The vegetable recommendation was more likely to be met by those in higher income households than those in the lowest income categories (12.2% vs 6.8%).1
Just what’s recommended? The most recent dietary guidelines from the Department of Agriculture suggest that adults should consume 1.5 to 2 cup-equivalents of fruits and 2 to 3 cup-equivalents of vegetables each day.2 What is a cup-equivalent? Examples include: 1 cup of a raw, or cooked, vegetable or fruit; 1 cup of fruit juice; 2 cups of leafy salad greens; or 1/2 cup of a dried fruit or vegetable. Additional recommendations are that added sugar constitute < 10% of calories per day, saturated fat < 10% of calories per day, and sodium < 2300 mg per day.
Simplify the message to this … There’s an easy message for clinicians to provide to patients: Consume 2 cups of fruit and 2 to 3 cups of vegetables per day; increase intake of whole grains, seafood, nuts, and seeds; choose fat-free and low-fat dairy products; and avoid sugary beverages and foods. But as we know, recommending that patients do something and actually having them do it are often 2 different things. So how can we tip the scales in a healthier direction?
Advise patients not to go it alone. The US Preventive Services Task Force recommends intensive behavioral interventions to alter eating habits. These interventions include individual or group counseling sessions over extended periods (eg, 6 hours of contact time over 6 to 18 months), including some 1-on-1 time with a specially trained professional, such as a primary care physician, nurse, registered dietitian, or nutritionist. The good news is that, for those with cardiovascular risk factors (dyslipidemia, elevated blood pressure, type 2 diabetes, and hypertension), this is a level “B” recommendation—meaning these interventions should be covered by commercial health insurance with no out-of-pocket cost to patients.3
1. Lee SH, Moore LV, Park S, et al. Adults meeting fruit and vegetable intake recommendations—United States, 2019. MMWR Morb Mortal Wkly Rep. 2022;71:1-9. Accessed January 18, 2022. www.cdc.gov/mmwr/volumes/71/wr/mm7101a1.htm
2. USDA. Dietary guidelines for Americans 2020-2025. Ninth ed. Accessed January 18, 2022. www.dietaryguidelines.gov/sites/default/files/2020-12/Dietary_Guidelines_for_Americans_2020-2025.pdf
3. USPSTF. Healthy diet and physical activity for cardiovascular disease prevention in adults with cardiovascular risk factors: behavioral counseling interventions. Final recommendation statement. Published November 24, 2020. Accessed January 18, 2022. www.uspreventiveservicestaskforce.org/uspstf/recommendation/healthy-diet-and-physical-activity-counseling-adults-with-high-risk-of-cvd
Dietary guidelines provide scientifically sound and practical advice that, if followed by every person, would probably result in less obesity, type 2 diabetes, cardiovascular disease, cancer, and bone loss. But few US adults meet these recommendations, according to a recent report in the CDC’s Morbidity and Mortality Weekly Report (MMWR).1
Data from the 2019 Behavioral Risk Factor Surveillance system indicate that only 12.3% of US adults consumed the recommended amount of fruit and 10% the recommended amount of vegetables (more on that shortly). Women were more likely than men to meet the requirements for fruit (14.5% vs 10.1%) and vegetable (12.4% vs 7.6%) intake. The vegetable recommendation was more likely to be met by those in higher income households than those in the lowest income categories (12.2% vs 6.8%).1
Just what’s recommended? The most recent dietary guidelines from the Department of Agriculture suggest that adults should consume 1.5 to 2 cup-equivalents of fruits and 2 to 3 cup-equivalents of vegetables each day.2 What is a cup-equivalent? Examples include: 1 cup of a raw, or cooked, vegetable or fruit; 1 cup of fruit juice; 2 cups of leafy salad greens; or 1/2 cup of a dried fruit or vegetable. Additional recommendations are that added sugar constitute < 10% of calories per day, saturated fat < 10% of calories per day, and sodium < 2300 mg per day.
Simplify the message to this … There’s an easy message for clinicians to provide to patients: Consume 2 cups of fruit and 2 to 3 cups of vegetables per day; increase intake of whole grains, seafood, nuts, and seeds; choose fat-free and low-fat dairy products; and avoid sugary beverages and foods. But as we know, recommending that patients do something and actually having them do it are often 2 different things. So how can we tip the scales in a healthier direction?
Advise patients not to go it alone. The US Preventive Services Task Force recommends intensive behavioral interventions to alter eating habits. These interventions include individual or group counseling sessions over extended periods (eg, 6 hours of contact time over 6 to 18 months), including some 1-on-1 time with a specially trained professional, such as a primary care physician, nurse, registered dietitian, or nutritionist. The good news is that, for those with cardiovascular risk factors (dyslipidemia, elevated blood pressure, type 2 diabetes, and hypertension), this is a level “B” recommendation—meaning these interventions should be covered by commercial health insurance with no out-of-pocket cost to patients.3
Dietary guidelines provide scientifically sound and practical advice that, if followed by every person, would probably result in less obesity, type 2 diabetes, cardiovascular disease, cancer, and bone loss. But few US adults meet these recommendations, according to a recent report in the CDC’s Morbidity and Mortality Weekly Report (MMWR).1
Data from the 2019 Behavioral Risk Factor Surveillance system indicate that only 12.3% of US adults consumed the recommended amount of fruit and 10% the recommended amount of vegetables (more on that shortly). Women were more likely than men to meet the requirements for fruit (14.5% vs 10.1%) and vegetable (12.4% vs 7.6%) intake. The vegetable recommendation was more likely to be met by those in higher income households than those in the lowest income categories (12.2% vs 6.8%).1
Just what’s recommended? The most recent dietary guidelines from the Department of Agriculture suggest that adults should consume 1.5 to 2 cup-equivalents of fruits and 2 to 3 cup-equivalents of vegetables each day.2 What is a cup-equivalent? Examples include: 1 cup of a raw, or cooked, vegetable or fruit; 1 cup of fruit juice; 2 cups of leafy salad greens; or 1/2 cup of a dried fruit or vegetable. Additional recommendations are that added sugar constitute < 10% of calories per day, saturated fat < 10% of calories per day, and sodium < 2300 mg per day.
Simplify the message to this … There’s an easy message for clinicians to provide to patients: Consume 2 cups of fruit and 2 to 3 cups of vegetables per day; increase intake of whole grains, seafood, nuts, and seeds; choose fat-free and low-fat dairy products; and avoid sugary beverages and foods. But as we know, recommending that patients do something and actually having them do it are often 2 different things. So how can we tip the scales in a healthier direction?
Advise patients not to go it alone. The US Preventive Services Task Force recommends intensive behavioral interventions to alter eating habits. These interventions include individual or group counseling sessions over extended periods (eg, 6 hours of contact time over 6 to 18 months), including some 1-on-1 time with a specially trained professional, such as a primary care physician, nurse, registered dietitian, or nutritionist. The good news is that, for those with cardiovascular risk factors (dyslipidemia, elevated blood pressure, type 2 diabetes, and hypertension), this is a level “B” recommendation—meaning these interventions should be covered by commercial health insurance with no out-of-pocket cost to patients.3
1. Lee SH, Moore LV, Park S, et al. Adults meeting fruit and vegetable intake recommendations—United States, 2019. MMWR Morb Mortal Wkly Rep. 2022;71:1-9. Accessed January 18, 2022. www.cdc.gov/mmwr/volumes/71/wr/mm7101a1.htm
2. USDA. Dietary guidelines for Americans 2020-2025. Ninth ed. Accessed January 18, 2022. www.dietaryguidelines.gov/sites/default/files/2020-12/Dietary_Guidelines_for_Americans_2020-2025.pdf
3. USPSTF. Healthy diet and physical activity for cardiovascular disease prevention in adults with cardiovascular risk factors: behavioral counseling interventions. Final recommendation statement. Published November 24, 2020. Accessed January 18, 2022. www.uspreventiveservicestaskforce.org/uspstf/recommendation/healthy-diet-and-physical-activity-counseling-adults-with-high-risk-of-cvd
1. Lee SH, Moore LV, Park S, et al. Adults meeting fruit and vegetable intake recommendations—United States, 2019. MMWR Morb Mortal Wkly Rep. 2022;71:1-9. Accessed January 18, 2022. www.cdc.gov/mmwr/volumes/71/wr/mm7101a1.htm
2. USDA. Dietary guidelines for Americans 2020-2025. Ninth ed. Accessed January 18, 2022. www.dietaryguidelines.gov/sites/default/files/2020-12/Dietary_Guidelines_for_Americans_2020-2025.pdf
3. USPSTF. Healthy diet and physical activity for cardiovascular disease prevention in adults with cardiovascular risk factors: behavioral counseling interventions. Final recommendation statement. Published November 24, 2020. Accessed January 18, 2022. www.uspreventiveservicestaskforce.org/uspstf/recommendation/healthy-diet-and-physical-activity-counseling-adults-with-high-risk-of-cvd
Pruritic Papules on the Trunk, Extremities, and Face
The Diagnosis: Gamasoidosis
An entomologist confirmed the specimen as an avian mite in either the Dermanyssus or Ornithonyssus genera (quiz image [bottom]). The patient was asked whether any bird had nested around her bedroom, and she affirmed that a woodpecker had nested outside her bedroom closet that spring. She subsequently discovered it had burrowed a hole into her closet wall. She and her husband removed the nest, and within 4 weeks, the eruption permanently cleared.
Gamasoidosis, or avian mite dermatitis, often is an overlooked, difficult-to-make diagnosis that is increasing in prevalence.1 Bird mites are ectoparasitic arthropods that are 0.3 to 1 mm in length. They have egg-shaped bodies with 4 pairs of legs; they are a translucent brown color before feeding and red after feeding.2 Although most avian mites cannot subsist off human blood, if the mites are without an avian host, such as after affected birds abandon their nests, the mites will bite humans.3 Studies have discovered the presence of mammalian erythrocytes in the digestive tracts of one species of bird mite, Dermanyssus gallinae, suggesting that at least one form of avian mite may feed off humans but typically cannot reproduce without an avian blood meal.4 Individuals with gamasoidosis often are exposed to avian mites by owning birds as pets, rearing chickens or messenger pigeons, or having bird’s nests around their bedrooms or air conditioning units.1
Most people who develop avian mite dermatitis are the only affected member of the family to develop pruritus and papules since the reaction requires both bites and hypersensitivity to them; however, there are cases of nuclear families all reacting to avian mite bites.2,4 As in this case, hypersensitivity to avian mite bites causes exquisitely pruritic 2- to 5-mm papules, vesicles, or urticarial lesions that may be diagnosed as papular urticaria or misdiagnosed as scabies. Although bird mites may carry bacteria such as Salmonella, Spirochaete, Rickettsia, and Pasteurella, they have not demonstrated an ability to pass these on to human vectors.5,6
Bird mites will spend most of their lives on avian hosts but can spread to humans through direct contact or through air.7 Mites can go through floors, walls, ceilings, or most commonly through ventilation or air conditioning units. Increasing urbanization, especially in warmer climates where avian mites thrive, has increased the prevalence of gamasoidosis.1
Avian mite dermatitis commonly can be mistaken for scabies, but the mites can be seen with the naked eye and cannot form burrows, unlike scabies.4,8 Avian mites usually are not found on human skin since they leave the host after feeding and move with surprising speed.8 Pediculosis corporis (body lice) results from an infestation of Pediculus humanus corporis. At 2- to 4-mm long, this louse is much larger than a bird mite. Body lice rarely are found on the skin but rather live and lay eggs on clothing, particularly along the seams. The body louse has an elongated body with 3 segments and short antennae. Pthirus pubis (pubic lice) measure 1.5 to 2.0 mm in adulthood and have wider, more crablike bodies compared to body or hair lice or avian mites. Lice, being insects, have 6 legs as opposed to mites, being arachnids, having 8 legs. Cheyletiella are 0.5-mm long, nonburrowing mites commonly found on cats, dogs, and rabbits. Cheyletiella blakei affects cats. They look somewhat similar to bird mites but have hooklike palps extending from their heads instead of antennae.
Antihistamines and topical corticosteroids may reduce discomfort from avian bites but are not curative.2,9 The most efficient way to treat gamasoidosis is to remove any affected birds or nearby bird’s nests, as the mites cannot survive more than a few weeks to months without feeding on an avian host.8 It also may be necessary to fumigate infested rooms.10
The diagnosis of avian mite dermatitis often is missed to the frustration of the patient and clinician alike. Becoming familiar with this bite reaction will help clinicians diagnose this dermatologic conundrum.
- Wambier CG, de Farias Wambier SP. Gamasoidosis illustrated— from the nest to dermoscopy. An Bras Dermatol. 2012;87:926-927. doi:10.1590/s0365-05962012000600021
- Collgros H, Iglesias-Sancho M, Aldunce MJ, et al. Dermanyssus gallinae (chicken mite): an underdiagnosed environmental infestation. Clin Exp Dermatol. 2013;38:374-377.
- Akdemir C, Gülcan E, Tanritanir P. Case report: Dermanyssus gallinae in a patient with pruritus and skin lesions. Turkiye Parazitol Derg. 2009;33:242-244.
- Williams RW. An infestation of a human habitation by Dermanyssus gallinae (de Geer, 1778)(Acarina: Dermanyssidae) in New York resulting in sanguisugent attacks upon the occupants. Am J Trop Med Hyg. 1958;7:627-629.
- Walker A. The Arthropods of Humans and Domestic Animals. A Guide to Preliminary Identification. Chapman and Hall; 1994.
- Vaiente MC, Chauve C, Zenner L. Experimental infection of Salmonella enteritidis by the poultry red mite, Dermanyssus gallinae. Vet Parasitol. 2007;146:329-336.
- Regan AM, Metersky ML, Craven DE. Nosocomial dermatitis and pruritus caused by pigeon mite infestation. Arch Intern Med. 1987;147:2185-2187.
- Orton DI, Warren LJ, Wilkinson JD. Avian mite dermatitis. Clin Exp Dermatol. 2000;25:129-131.
- Bassini-Silva R, de Castro Jacinavicius F, Akashi Hernandes F, et al. Dermatitis in humans caused by Ornithonyssus bursa (Berlese 1888) (Mesostigmata: Macronyssidae) and new records from Brazil. Rev Bras Parasitol Vet. 2019;28:134-139.
- Watson CR. Human infestation with bird mites in Wollongong. Commun Dis Intell Q Rep. 2003;27:259-261.
The Diagnosis: Gamasoidosis
An entomologist confirmed the specimen as an avian mite in either the Dermanyssus or Ornithonyssus genera (quiz image [bottom]). The patient was asked whether any bird had nested around her bedroom, and she affirmed that a woodpecker had nested outside her bedroom closet that spring. She subsequently discovered it had burrowed a hole into her closet wall. She and her husband removed the nest, and within 4 weeks, the eruption permanently cleared.
Gamasoidosis, or avian mite dermatitis, often is an overlooked, difficult-to-make diagnosis that is increasing in prevalence.1 Bird mites are ectoparasitic arthropods that are 0.3 to 1 mm in length. They have egg-shaped bodies with 4 pairs of legs; they are a translucent brown color before feeding and red after feeding.2 Although most avian mites cannot subsist off human blood, if the mites are without an avian host, such as after affected birds abandon their nests, the mites will bite humans.3 Studies have discovered the presence of mammalian erythrocytes in the digestive tracts of one species of bird mite, Dermanyssus gallinae, suggesting that at least one form of avian mite may feed off humans but typically cannot reproduce without an avian blood meal.4 Individuals with gamasoidosis often are exposed to avian mites by owning birds as pets, rearing chickens or messenger pigeons, or having bird’s nests around their bedrooms or air conditioning units.1
Most people who develop avian mite dermatitis are the only affected member of the family to develop pruritus and papules since the reaction requires both bites and hypersensitivity to them; however, there are cases of nuclear families all reacting to avian mite bites.2,4 As in this case, hypersensitivity to avian mite bites causes exquisitely pruritic 2- to 5-mm papules, vesicles, or urticarial lesions that may be diagnosed as papular urticaria or misdiagnosed as scabies. Although bird mites may carry bacteria such as Salmonella, Spirochaete, Rickettsia, and Pasteurella, they have not demonstrated an ability to pass these on to human vectors.5,6
Bird mites will spend most of their lives on avian hosts but can spread to humans through direct contact or through air.7 Mites can go through floors, walls, ceilings, or most commonly through ventilation or air conditioning units. Increasing urbanization, especially in warmer climates where avian mites thrive, has increased the prevalence of gamasoidosis.1
Avian mite dermatitis commonly can be mistaken for scabies, but the mites can be seen with the naked eye and cannot form burrows, unlike scabies.4,8 Avian mites usually are not found on human skin since they leave the host after feeding and move with surprising speed.8 Pediculosis corporis (body lice) results from an infestation of Pediculus humanus corporis. At 2- to 4-mm long, this louse is much larger than a bird mite. Body lice rarely are found on the skin but rather live and lay eggs on clothing, particularly along the seams. The body louse has an elongated body with 3 segments and short antennae. Pthirus pubis (pubic lice) measure 1.5 to 2.0 mm in adulthood and have wider, more crablike bodies compared to body or hair lice or avian mites. Lice, being insects, have 6 legs as opposed to mites, being arachnids, having 8 legs. Cheyletiella are 0.5-mm long, nonburrowing mites commonly found on cats, dogs, and rabbits. Cheyletiella blakei affects cats. They look somewhat similar to bird mites but have hooklike palps extending from their heads instead of antennae.
Antihistamines and topical corticosteroids may reduce discomfort from avian bites but are not curative.2,9 The most efficient way to treat gamasoidosis is to remove any affected birds or nearby bird’s nests, as the mites cannot survive more than a few weeks to months without feeding on an avian host.8 It also may be necessary to fumigate infested rooms.10
The diagnosis of avian mite dermatitis often is missed to the frustration of the patient and clinician alike. Becoming familiar with this bite reaction will help clinicians diagnose this dermatologic conundrum.
The Diagnosis: Gamasoidosis
An entomologist confirmed the specimen as an avian mite in either the Dermanyssus or Ornithonyssus genera (quiz image [bottom]). The patient was asked whether any bird had nested around her bedroom, and she affirmed that a woodpecker had nested outside her bedroom closet that spring. She subsequently discovered it had burrowed a hole into her closet wall. She and her husband removed the nest, and within 4 weeks, the eruption permanently cleared.
Gamasoidosis, or avian mite dermatitis, often is an overlooked, difficult-to-make diagnosis that is increasing in prevalence.1 Bird mites are ectoparasitic arthropods that are 0.3 to 1 mm in length. They have egg-shaped bodies with 4 pairs of legs; they are a translucent brown color before feeding and red after feeding.2 Although most avian mites cannot subsist off human blood, if the mites are without an avian host, such as after affected birds abandon their nests, the mites will bite humans.3 Studies have discovered the presence of mammalian erythrocytes in the digestive tracts of one species of bird mite, Dermanyssus gallinae, suggesting that at least one form of avian mite may feed off humans but typically cannot reproduce without an avian blood meal.4 Individuals with gamasoidosis often are exposed to avian mites by owning birds as pets, rearing chickens or messenger pigeons, or having bird’s nests around their bedrooms or air conditioning units.1
Most people who develop avian mite dermatitis are the only affected member of the family to develop pruritus and papules since the reaction requires both bites and hypersensitivity to them; however, there are cases of nuclear families all reacting to avian mite bites.2,4 As in this case, hypersensitivity to avian mite bites causes exquisitely pruritic 2- to 5-mm papules, vesicles, or urticarial lesions that may be diagnosed as papular urticaria or misdiagnosed as scabies. Although bird mites may carry bacteria such as Salmonella, Spirochaete, Rickettsia, and Pasteurella, they have not demonstrated an ability to pass these on to human vectors.5,6
Bird mites will spend most of their lives on avian hosts but can spread to humans through direct contact or through air.7 Mites can go through floors, walls, ceilings, or most commonly through ventilation or air conditioning units. Increasing urbanization, especially in warmer climates where avian mites thrive, has increased the prevalence of gamasoidosis.1
Avian mite dermatitis commonly can be mistaken for scabies, but the mites can be seen with the naked eye and cannot form burrows, unlike scabies.4,8 Avian mites usually are not found on human skin since they leave the host after feeding and move with surprising speed.8 Pediculosis corporis (body lice) results from an infestation of Pediculus humanus corporis. At 2- to 4-mm long, this louse is much larger than a bird mite. Body lice rarely are found on the skin but rather live and lay eggs on clothing, particularly along the seams. The body louse has an elongated body with 3 segments and short antennae. Pthirus pubis (pubic lice) measure 1.5 to 2.0 mm in adulthood and have wider, more crablike bodies compared to body or hair lice or avian mites. Lice, being insects, have 6 legs as opposed to mites, being arachnids, having 8 legs. Cheyletiella are 0.5-mm long, nonburrowing mites commonly found on cats, dogs, and rabbits. Cheyletiella blakei affects cats. They look somewhat similar to bird mites but have hooklike palps extending from their heads instead of antennae.
Antihistamines and topical corticosteroids may reduce discomfort from avian bites but are not curative.2,9 The most efficient way to treat gamasoidosis is to remove any affected birds or nearby bird’s nests, as the mites cannot survive more than a few weeks to months without feeding on an avian host.8 It also may be necessary to fumigate infested rooms.10
The diagnosis of avian mite dermatitis often is missed to the frustration of the patient and clinician alike. Becoming familiar with this bite reaction will help clinicians diagnose this dermatologic conundrum.
- Wambier CG, de Farias Wambier SP. Gamasoidosis illustrated— from the nest to dermoscopy. An Bras Dermatol. 2012;87:926-927. doi:10.1590/s0365-05962012000600021
- Collgros H, Iglesias-Sancho M, Aldunce MJ, et al. Dermanyssus gallinae (chicken mite): an underdiagnosed environmental infestation. Clin Exp Dermatol. 2013;38:374-377.
- Akdemir C, Gülcan E, Tanritanir P. Case report: Dermanyssus gallinae in a patient with pruritus and skin lesions. Turkiye Parazitol Derg. 2009;33:242-244.
- Williams RW. An infestation of a human habitation by Dermanyssus gallinae (de Geer, 1778)(Acarina: Dermanyssidae) in New York resulting in sanguisugent attacks upon the occupants. Am J Trop Med Hyg. 1958;7:627-629.
- Walker A. The Arthropods of Humans and Domestic Animals. A Guide to Preliminary Identification. Chapman and Hall; 1994.
- Vaiente MC, Chauve C, Zenner L. Experimental infection of Salmonella enteritidis by the poultry red mite, Dermanyssus gallinae. Vet Parasitol. 2007;146:329-336.
- Regan AM, Metersky ML, Craven DE. Nosocomial dermatitis and pruritus caused by pigeon mite infestation. Arch Intern Med. 1987;147:2185-2187.
- Orton DI, Warren LJ, Wilkinson JD. Avian mite dermatitis. Clin Exp Dermatol. 2000;25:129-131.
- Bassini-Silva R, de Castro Jacinavicius F, Akashi Hernandes F, et al. Dermatitis in humans caused by Ornithonyssus bursa (Berlese 1888) (Mesostigmata: Macronyssidae) and new records from Brazil. Rev Bras Parasitol Vet. 2019;28:134-139.
- Watson CR. Human infestation with bird mites in Wollongong. Commun Dis Intell Q Rep. 2003;27:259-261.
- Wambier CG, de Farias Wambier SP. Gamasoidosis illustrated— from the nest to dermoscopy. An Bras Dermatol. 2012;87:926-927. doi:10.1590/s0365-05962012000600021
- Collgros H, Iglesias-Sancho M, Aldunce MJ, et al. Dermanyssus gallinae (chicken mite): an underdiagnosed environmental infestation. Clin Exp Dermatol. 2013;38:374-377.
- Akdemir C, Gülcan E, Tanritanir P. Case report: Dermanyssus gallinae in a patient with pruritus and skin lesions. Turkiye Parazitol Derg. 2009;33:242-244.
- Williams RW. An infestation of a human habitation by Dermanyssus gallinae (de Geer, 1778)(Acarina: Dermanyssidae) in New York resulting in sanguisugent attacks upon the occupants. Am J Trop Med Hyg. 1958;7:627-629.
- Walker A. The Arthropods of Humans and Domestic Animals. A Guide to Preliminary Identification. Chapman and Hall; 1994.
- Vaiente MC, Chauve C, Zenner L. Experimental infection of Salmonella enteritidis by the poultry red mite, Dermanyssus gallinae. Vet Parasitol. 2007;146:329-336.
- Regan AM, Metersky ML, Craven DE. Nosocomial dermatitis and pruritus caused by pigeon mite infestation. Arch Intern Med. 1987;147:2185-2187.
- Orton DI, Warren LJ, Wilkinson JD. Avian mite dermatitis. Clin Exp Dermatol. 2000;25:129-131.
- Bassini-Silva R, de Castro Jacinavicius F, Akashi Hernandes F, et al. Dermatitis in humans caused by Ornithonyssus bursa (Berlese 1888) (Mesostigmata: Macronyssidae) and new records from Brazil. Rev Bras Parasitol Vet. 2019;28:134-139.
- Watson CR. Human infestation with bird mites in Wollongong. Commun Dis Intell Q Rep. 2003;27:259-261.
A 69-year-old woman presented in early summer in southeastern Michigan with several itchy bumps (top) of 4 to 5 weeks’ duration that erupted and remitted over the trunk, extremities, and face. She had taken no new medications. She had an asymptomatic cat and no exposure to anyone else who had been itching. Physical examination revealed approximately a dozen 2- to 5-mm edematous papules on the trunk, arms, shins, thighs, and left cheek, as well as one 3-mm vesicle on the forearm. No burrows could be identified on physical examination. Lesions treated with betamethasone dipropionate cream 0.05% improved, but new lesions continued to arise. An exterminator was contacted but found no signs of bedbugs or other infestations. Later, the patient reported seeing 3 tiny black dots crawl across the screen of her cell phone as she read in bed. She was able to capture them on tape and bring them to her appointment. The specimens were approximately 1 mm in length (bottom).
No amount of alcohol safe for the heart: WHF
The widely held notion that consuming small to moderate amounts of alcohol is good for cardiovascular health is not supported by the data, the World Heart Federation says in a new policy brief.
In fact, the evidence is clear that any level of drinking can contribute to loss of a healthy life, the organization says.
“Over the past several decades, the prevalence of cardiovascular disease has nearly doubled, and alcohol has played a major role in the incidence of much of it,” the WHF said in the brief.
“The portrayal of alcohol as necessary for a vibrant social life has diverted attention from the harms of alcohol use, as have the frequent and widely publicized claims that moderate drinking, such as a glass of red wine a day, can offer protection against cardiovascular disease,” Monika Arora, PhD, member of the WHF advocacy committee and coauthor of the brief, said in a news release.
“These claims are at best misinformed and at worst an attempt by the alcohol industry to mislead the public about the danger of their product,” Dr. Arora added.
The WHF conclusions follow a report in the Lancet based on the Global Burden of Diseases, Injuries, and Risk Factors Study (GBD), which found that there is no safe level of alcohol consumption.
In 2019, nearly 2.4 million deaths were attributed to alcohol, accounting for 4.3% of all deaths globally and 12.6% of deaths in men 15 to 49 years of age.
Even small amounts of alcohol have been shown to raise the risk for cardiovascular disease, including coronary disease, stroke, heart failure, hypertensive heart disease, cardiomyopathy, atrial fibrillation, and aneurysm, the WHF notes.
Studies that claim otherwise are largely based on purely observational research, which fails to account for relevant cofactors, the organization writes.
Based on their summary of the evidence to date, there is no reliable correlation between moderate alcohol consumption and a lower risk for cardiovascular disease.
Alcohol use is also a “major avoidable risk factor” for cancer, digestive diseases, intentional and unintentional injuries, and several infectious diseases, the WHF says.
Alcohol use also has significant economic and social costs, which include costs to individuals and health systems, productivity losses, as well as the increased risk for violence, homelessness, and criminal activity.
The WHF policy brief calls for “urgent and decisive action” to tackle the unprecedented rise in alcohol-related death and disability worldwide.
Recommended actions include boosting restrictions on alcohol availability; advancing and enforcing drinking and driving countermeasures; increasing access to screening, brief interventions, and treatment for alcohol use disorder; enforcing bans on alcohol advertising; establishing a uniform minimum legal drinking age; and mandating health warnings on alcohol products.
A version of this article first appeared on Medscape.com.
The widely held notion that consuming small to moderate amounts of alcohol is good for cardiovascular health is not supported by the data, the World Heart Federation says in a new policy brief.
In fact, the evidence is clear that any level of drinking can contribute to loss of a healthy life, the organization says.
“Over the past several decades, the prevalence of cardiovascular disease has nearly doubled, and alcohol has played a major role in the incidence of much of it,” the WHF said in the brief.
“The portrayal of alcohol as necessary for a vibrant social life has diverted attention from the harms of alcohol use, as have the frequent and widely publicized claims that moderate drinking, such as a glass of red wine a day, can offer protection against cardiovascular disease,” Monika Arora, PhD, member of the WHF advocacy committee and coauthor of the brief, said in a news release.
“These claims are at best misinformed and at worst an attempt by the alcohol industry to mislead the public about the danger of their product,” Dr. Arora added.
The WHF conclusions follow a report in the Lancet based on the Global Burden of Diseases, Injuries, and Risk Factors Study (GBD), which found that there is no safe level of alcohol consumption.
In 2019, nearly 2.4 million deaths were attributed to alcohol, accounting for 4.3% of all deaths globally and 12.6% of deaths in men 15 to 49 years of age.
Even small amounts of alcohol have been shown to raise the risk for cardiovascular disease, including coronary disease, stroke, heart failure, hypertensive heart disease, cardiomyopathy, atrial fibrillation, and aneurysm, the WHF notes.
Studies that claim otherwise are largely based on purely observational research, which fails to account for relevant cofactors, the organization writes.
Based on their summary of the evidence to date, there is no reliable correlation between moderate alcohol consumption and a lower risk for cardiovascular disease.
Alcohol use is also a “major avoidable risk factor” for cancer, digestive diseases, intentional and unintentional injuries, and several infectious diseases, the WHF says.
Alcohol use also has significant economic and social costs, which include costs to individuals and health systems, productivity losses, as well as the increased risk for violence, homelessness, and criminal activity.
The WHF policy brief calls for “urgent and decisive action” to tackle the unprecedented rise in alcohol-related death and disability worldwide.
Recommended actions include boosting restrictions on alcohol availability; advancing and enforcing drinking and driving countermeasures; increasing access to screening, brief interventions, and treatment for alcohol use disorder; enforcing bans on alcohol advertising; establishing a uniform minimum legal drinking age; and mandating health warnings on alcohol products.
A version of this article first appeared on Medscape.com.
The widely held notion that consuming small to moderate amounts of alcohol is good for cardiovascular health is not supported by the data, the World Heart Federation says in a new policy brief.
In fact, the evidence is clear that any level of drinking can contribute to loss of a healthy life, the organization says.
“Over the past several decades, the prevalence of cardiovascular disease has nearly doubled, and alcohol has played a major role in the incidence of much of it,” the WHF said in the brief.
“The portrayal of alcohol as necessary for a vibrant social life has diverted attention from the harms of alcohol use, as have the frequent and widely publicized claims that moderate drinking, such as a glass of red wine a day, can offer protection against cardiovascular disease,” Monika Arora, PhD, member of the WHF advocacy committee and coauthor of the brief, said in a news release.
“These claims are at best misinformed and at worst an attempt by the alcohol industry to mislead the public about the danger of their product,” Dr. Arora added.
The WHF conclusions follow a report in the Lancet based on the Global Burden of Diseases, Injuries, and Risk Factors Study (GBD), which found that there is no safe level of alcohol consumption.
In 2019, nearly 2.4 million deaths were attributed to alcohol, accounting for 4.3% of all deaths globally and 12.6% of deaths in men 15 to 49 years of age.
Even small amounts of alcohol have been shown to raise the risk for cardiovascular disease, including coronary disease, stroke, heart failure, hypertensive heart disease, cardiomyopathy, atrial fibrillation, and aneurysm, the WHF notes.
Studies that claim otherwise are largely based on purely observational research, which fails to account for relevant cofactors, the organization writes.
Based on their summary of the evidence to date, there is no reliable correlation between moderate alcohol consumption and a lower risk for cardiovascular disease.
Alcohol use is also a “major avoidable risk factor” for cancer, digestive diseases, intentional and unintentional injuries, and several infectious diseases, the WHF says.
Alcohol use also has significant economic and social costs, which include costs to individuals and health systems, productivity losses, as well as the increased risk for violence, homelessness, and criminal activity.
The WHF policy brief calls for “urgent and decisive action” to tackle the unprecedented rise in alcohol-related death and disability worldwide.
Recommended actions include boosting restrictions on alcohol availability; advancing and enforcing drinking and driving countermeasures; increasing access to screening, brief interventions, and treatment for alcohol use disorder; enforcing bans on alcohol advertising; establishing a uniform minimum legal drinking age; and mandating health warnings on alcohol products.
A version of this article first appeared on Medscape.com.
Gut bacteria linked with long COVID
While links have been found between the gut’s microbiome and COVID-19, as well as other diseases, this is the first published research to show a link specifically to COVID’s long-term effects, the investigators, based at the Chinese University of Hong Kong, wrote in Gut.
“To our knowledge, this is the first study to show that altered gut microbiome composition is strongly associated with persistent symptoms in patients with COVID-19 up to 6 months after clearance of SARS-CoV-2 virus,” said Siew Ng, MBBS, PhD, associate director at the university’s Center for Gut Microbiota Research.
At three hospitals, the researchers enrolled 106 patients with COVID-19 from February to August 2020 with stool samples at admission and at 1 month and 6 months after discharge, and compared them with people who did not have COVID, recruited in 2019. The severity of COVID in the enrolled patients was mostly mild to moderate.
At 3 months, 86 of the patients with COVID had post–acute COVID-19 syndrome (PACS) – defined as at least one persistent, otherwise unexplained symptom 4 weeks after clearance of the virus. And 81 patients had PACS at 6 months, most commonly fatigue, poor memory, hair loss, anxiety, and trouble sleeping.
Using stool samples for their analysis, the researchers found that, broadly, the diversity of the types of bacteria, and the abundance of these bacteria, were significantly lower at 6 months for those with PACS, compared with those without PACS and with controls (P < .05 and P < .0001, respectively). Among those with PACS, 28 bacteria species were diminished and 14 were enriched, both at baseline and follow-up. Those patients who had COVID but not PACS showed just 25 alterations of bacteria species at the time of hospital admission, and they all normalized by 6 months.
Having respiratory symptoms at 6 months was linked with higher levels of opportunistic pathogens such as Streptococcus anginosus and S. vestibularis. Neuropsychiatric symptoms and fatigue were associated with nosocomial pathogens that are linked to opportunistic infections, such as Clostridium innocuum and Actinomyces naeslundii (P < .05).
Bacteria known for producing butyrate, a beneficial fatty acid, were significantly depleted in those patients with hair loss. And certain of these bacteria, including Bifidobacterium pseudocatenulatum and Faecalibacterium prausnitzii, had the largest inverse correlations with PACS at 6 months (P < .05), the researchers found.
“Particular gut microbial profiles may indicate heightened susceptibility,” Dr. Ng said.
Although the findings were drawn from patients with earlier strains of the COVID-19 virus, the findings still apply to new variants, including Omicron, since these pose the same problem of persistent disruption of the immune system, Dr. Ng said.
Her group is conducting trials to look at how modulating the microbiome might prevent long COVID and boost antibodies after vaccination in high-risk people, she said.
“Gut microbiota influences the health of the host,” Dr. Ng said. “It provides crucial benefits in the form of immune system development, prevention of infections, nutrient acquisition, and brain and nervous system functionality. Considering the millions of people infected during the ongoing pandemic, our findings are a strong impetus for consideration of microbiota modulation to facilitate timely recovery and reduce the burden of post–acute COVID-19 syndrome.”
John Haran, MD, PhD, associate professor of microbiology and physiological systems and emergency medicine at the University of Massachusetts, Worcester, said the research adds to the evidence base on the gut microbiome’s links to COVID, but there was likely be no clinical impact yet. Still, he said the findings linking specific species to specific symptoms was particularly interesting.
“Very early on during hospitalization, [the researchers] saw these differences and correlated out with people who have longer symptoms, and especially different groups of people that have longer symptoms, too,” said Dr. Haran, who has done research on the topic. “It’s very different if you have different symptoms, for example, you keep coughing for months versus you have brain fog and fatigue, or other debilitating symptoms.”
Dr. Haran noted that the findings didn’t identify bacteria types especially linked to COVID, but rather species that have already been found to be associated with a “bad” microbiome. He also pointed out that the patients enrolled in the study were not vaccinated, because vaccines weren’t available at the time. Still, further study to see whether modulation of gut bacteria can be a therapy seems worthwhile.
“Microbiome modulation is pretty safe, and that’s really the next big step that needs to be taken in this,” he said.
For now, the findings don’t give the clinician much new ammunition for treatment.
“We’re not there yet,” he added. “It’s not as if clinicians are going to tell their COVID patients: ‘Go out and buy some kale.’ ”
Eugene Chang, MD, professor of medicine at the University of Chicago, who has studied the gut microbiome and gastrointestinal disease, said it’s “too preliminary” to say whether the findings could lead to a clinical impact. The measures used merely identify the microbes present, but not what they are doing.
“These measures are unlikely to perform well enough to be useful for risk assessment or predicting clinical outcomes,” he said. “That being said, advances in technology are being made where next generations of metrics could be developed and useful as stratifiers and predictors of risk.”
Seeing shifting patterns associated with certain symptoms, he said, is “notable because it suggests that the disturbances of the gut microbiota in PACS are significant.”
But he said it’s important to know whether these changes are a cause of PACS in some way or just an effect of it.
“If causative or contributory – this has to be proven – then ‘microbiota modulation’ would make sense and could be a priority for development,” he said. “If merely an effect, these metrics and better ones to come could be useful as predictors or measures of the patient’s general state of health.”
As seen in his group’s work and other work, he said, “the gut microbiota is highly sensitive to changes in their ecosystem, which is influenced by the health state of the patient.”
Dr. Ng, Dr. Haran, and Dr. Chang reported no relevant disclosures.
This article was updated Jan. 27, 2022.
While links have been found between the gut’s microbiome and COVID-19, as well as other diseases, this is the first published research to show a link specifically to COVID’s long-term effects, the investigators, based at the Chinese University of Hong Kong, wrote in Gut.
“To our knowledge, this is the first study to show that altered gut microbiome composition is strongly associated with persistent symptoms in patients with COVID-19 up to 6 months after clearance of SARS-CoV-2 virus,” said Siew Ng, MBBS, PhD, associate director at the university’s Center for Gut Microbiota Research.
At three hospitals, the researchers enrolled 106 patients with COVID-19 from February to August 2020 with stool samples at admission and at 1 month and 6 months after discharge, and compared them with people who did not have COVID, recruited in 2019. The severity of COVID in the enrolled patients was mostly mild to moderate.
At 3 months, 86 of the patients with COVID had post–acute COVID-19 syndrome (PACS) – defined as at least one persistent, otherwise unexplained symptom 4 weeks after clearance of the virus. And 81 patients had PACS at 6 months, most commonly fatigue, poor memory, hair loss, anxiety, and trouble sleeping.
Using stool samples for their analysis, the researchers found that, broadly, the diversity of the types of bacteria, and the abundance of these bacteria, were significantly lower at 6 months for those with PACS, compared with those without PACS and with controls (P < .05 and P < .0001, respectively). Among those with PACS, 28 bacteria species were diminished and 14 were enriched, both at baseline and follow-up. Those patients who had COVID but not PACS showed just 25 alterations of bacteria species at the time of hospital admission, and they all normalized by 6 months.
Having respiratory symptoms at 6 months was linked with higher levels of opportunistic pathogens such as Streptococcus anginosus and S. vestibularis. Neuropsychiatric symptoms and fatigue were associated with nosocomial pathogens that are linked to opportunistic infections, such as Clostridium innocuum and Actinomyces naeslundii (P < .05).
Bacteria known for producing butyrate, a beneficial fatty acid, were significantly depleted in those patients with hair loss. And certain of these bacteria, including Bifidobacterium pseudocatenulatum and Faecalibacterium prausnitzii, had the largest inverse correlations with PACS at 6 months (P < .05), the researchers found.
“Particular gut microbial profiles may indicate heightened susceptibility,” Dr. Ng said.
Although the findings were drawn from patients with earlier strains of the COVID-19 virus, the findings still apply to new variants, including Omicron, since these pose the same problem of persistent disruption of the immune system, Dr. Ng said.
Her group is conducting trials to look at how modulating the microbiome might prevent long COVID and boost antibodies after vaccination in high-risk people, she said.
“Gut microbiota influences the health of the host,” Dr. Ng said. “It provides crucial benefits in the form of immune system development, prevention of infections, nutrient acquisition, and brain and nervous system functionality. Considering the millions of people infected during the ongoing pandemic, our findings are a strong impetus for consideration of microbiota modulation to facilitate timely recovery and reduce the burden of post–acute COVID-19 syndrome.”
John Haran, MD, PhD, associate professor of microbiology and physiological systems and emergency medicine at the University of Massachusetts, Worcester, said the research adds to the evidence base on the gut microbiome’s links to COVID, but there was likely be no clinical impact yet. Still, he said the findings linking specific species to specific symptoms was particularly interesting.
“Very early on during hospitalization, [the researchers] saw these differences and correlated out with people who have longer symptoms, and especially different groups of people that have longer symptoms, too,” said Dr. Haran, who has done research on the topic. “It’s very different if you have different symptoms, for example, you keep coughing for months versus you have brain fog and fatigue, or other debilitating symptoms.”
Dr. Haran noted that the findings didn’t identify bacteria types especially linked to COVID, but rather species that have already been found to be associated with a “bad” microbiome. He also pointed out that the patients enrolled in the study were not vaccinated, because vaccines weren’t available at the time. Still, further study to see whether modulation of gut bacteria can be a therapy seems worthwhile.
“Microbiome modulation is pretty safe, and that’s really the next big step that needs to be taken in this,” he said.
For now, the findings don’t give the clinician much new ammunition for treatment.
“We’re not there yet,” he added. “It’s not as if clinicians are going to tell their COVID patients: ‘Go out and buy some kale.’ ”
Eugene Chang, MD, professor of medicine at the University of Chicago, who has studied the gut microbiome and gastrointestinal disease, said it’s “too preliminary” to say whether the findings could lead to a clinical impact. The measures used merely identify the microbes present, but not what they are doing.
“These measures are unlikely to perform well enough to be useful for risk assessment or predicting clinical outcomes,” he said. “That being said, advances in technology are being made where next generations of metrics could be developed and useful as stratifiers and predictors of risk.”
Seeing shifting patterns associated with certain symptoms, he said, is “notable because it suggests that the disturbances of the gut microbiota in PACS are significant.”
But he said it’s important to know whether these changes are a cause of PACS in some way or just an effect of it.
“If causative or contributory – this has to be proven – then ‘microbiota modulation’ would make sense and could be a priority for development,” he said. “If merely an effect, these metrics and better ones to come could be useful as predictors or measures of the patient’s general state of health.”
As seen in his group’s work and other work, he said, “the gut microbiota is highly sensitive to changes in their ecosystem, which is influenced by the health state of the patient.”
Dr. Ng, Dr. Haran, and Dr. Chang reported no relevant disclosures.
This article was updated Jan. 27, 2022.
While links have been found between the gut’s microbiome and COVID-19, as well as other diseases, this is the first published research to show a link specifically to COVID’s long-term effects, the investigators, based at the Chinese University of Hong Kong, wrote in Gut.
“To our knowledge, this is the first study to show that altered gut microbiome composition is strongly associated with persistent symptoms in patients with COVID-19 up to 6 months after clearance of SARS-CoV-2 virus,” said Siew Ng, MBBS, PhD, associate director at the university’s Center for Gut Microbiota Research.
At three hospitals, the researchers enrolled 106 patients with COVID-19 from February to August 2020 with stool samples at admission and at 1 month and 6 months after discharge, and compared them with people who did not have COVID, recruited in 2019. The severity of COVID in the enrolled patients was mostly mild to moderate.
At 3 months, 86 of the patients with COVID had post–acute COVID-19 syndrome (PACS) – defined as at least one persistent, otherwise unexplained symptom 4 weeks after clearance of the virus. And 81 patients had PACS at 6 months, most commonly fatigue, poor memory, hair loss, anxiety, and trouble sleeping.
Using stool samples for their analysis, the researchers found that, broadly, the diversity of the types of bacteria, and the abundance of these bacteria, were significantly lower at 6 months for those with PACS, compared with those without PACS and with controls (P < .05 and P < .0001, respectively). Among those with PACS, 28 bacteria species were diminished and 14 were enriched, both at baseline and follow-up. Those patients who had COVID but not PACS showed just 25 alterations of bacteria species at the time of hospital admission, and they all normalized by 6 months.
Having respiratory symptoms at 6 months was linked with higher levels of opportunistic pathogens such as Streptococcus anginosus and S. vestibularis. Neuropsychiatric symptoms and fatigue were associated with nosocomial pathogens that are linked to opportunistic infections, such as Clostridium innocuum and Actinomyces naeslundii (P < .05).
Bacteria known for producing butyrate, a beneficial fatty acid, were significantly depleted in those patients with hair loss. And certain of these bacteria, including Bifidobacterium pseudocatenulatum and Faecalibacterium prausnitzii, had the largest inverse correlations with PACS at 6 months (P < .05), the researchers found.
“Particular gut microbial profiles may indicate heightened susceptibility,” Dr. Ng said.
Although the findings were drawn from patients with earlier strains of the COVID-19 virus, the findings still apply to new variants, including Omicron, since these pose the same problem of persistent disruption of the immune system, Dr. Ng said.
Her group is conducting trials to look at how modulating the microbiome might prevent long COVID and boost antibodies after vaccination in high-risk people, she said.
“Gut microbiota influences the health of the host,” Dr. Ng said. “It provides crucial benefits in the form of immune system development, prevention of infections, nutrient acquisition, and brain and nervous system functionality. Considering the millions of people infected during the ongoing pandemic, our findings are a strong impetus for consideration of microbiota modulation to facilitate timely recovery and reduce the burden of post–acute COVID-19 syndrome.”
John Haran, MD, PhD, associate professor of microbiology and physiological systems and emergency medicine at the University of Massachusetts, Worcester, said the research adds to the evidence base on the gut microbiome’s links to COVID, but there was likely be no clinical impact yet. Still, he said the findings linking specific species to specific symptoms was particularly interesting.
“Very early on during hospitalization, [the researchers] saw these differences and correlated out with people who have longer symptoms, and especially different groups of people that have longer symptoms, too,” said Dr. Haran, who has done research on the topic. “It’s very different if you have different symptoms, for example, you keep coughing for months versus you have brain fog and fatigue, or other debilitating symptoms.”
Dr. Haran noted that the findings didn’t identify bacteria types especially linked to COVID, but rather species that have already been found to be associated with a “bad” microbiome. He also pointed out that the patients enrolled in the study were not vaccinated, because vaccines weren’t available at the time. Still, further study to see whether modulation of gut bacteria can be a therapy seems worthwhile.
“Microbiome modulation is pretty safe, and that’s really the next big step that needs to be taken in this,” he said.
For now, the findings don’t give the clinician much new ammunition for treatment.
“We’re not there yet,” he added. “It’s not as if clinicians are going to tell their COVID patients: ‘Go out and buy some kale.’ ”
Eugene Chang, MD, professor of medicine at the University of Chicago, who has studied the gut microbiome and gastrointestinal disease, said it’s “too preliminary” to say whether the findings could lead to a clinical impact. The measures used merely identify the microbes present, but not what they are doing.
“These measures are unlikely to perform well enough to be useful for risk assessment or predicting clinical outcomes,” he said. “That being said, advances in technology are being made where next generations of metrics could be developed and useful as stratifiers and predictors of risk.”
Seeing shifting patterns associated with certain symptoms, he said, is “notable because it suggests that the disturbances of the gut microbiota in PACS are significant.”
But he said it’s important to know whether these changes are a cause of PACS in some way or just an effect of it.
“If causative or contributory – this has to be proven – then ‘microbiota modulation’ would make sense and could be a priority for development,” he said. “If merely an effect, these metrics and better ones to come could be useful as predictors or measures of the patient’s general state of health.”
As seen in his group’s work and other work, he said, “the gut microbiota is highly sensitive to changes in their ecosystem, which is influenced by the health state of the patient.”
Dr. Ng, Dr. Haran, and Dr. Chang reported no relevant disclosures.
This article was updated Jan. 27, 2022.
FROM GUT
Expert Update on Acute Bacterial Skin and Skin Structure Infection Treatment Options in the Community Setting
The goal of this activity is to improve healthcare providers’ knowledge on the treatment of patients with acute bacterial skin and skin structure infections (ABSSSI) in the outpatient setting as well as how to best incorporate novel antimicrobials into patient care plans.
Click here to access this content now
The goal of this activity is to improve healthcare providers’ knowledge on the treatment of patients with acute bacterial skin and skin structure infections (ABSSSI) in the outpatient setting as well as how to best incorporate novel antimicrobials into patient care plans.
Click here to access this content now
The goal of this activity is to improve healthcare providers’ knowledge on the treatment of patients with acute bacterial skin and skin structure infections (ABSSSI) in the outpatient setting as well as how to best incorporate novel antimicrobials into patient care plans.
Click here to access this content now