User login
Doctor who claimed masks hurt health loses license
Steven Arthur LaTulippe’s advice to patients about face masking amounted to “gross negligence” in the practice of medicine and was grounds for discipline, the medical board said in a report.
Mr. LaTulippe, who had a family practice in Dallas, was fined $10,000, Insider reported. The board also said he’d overprescribed opioids for some patients.
The medical board report said Mr. LaTulippe and his wife, who ran the clinic with him, didn’t wear face masks while treating patients from March to December 2020.
Mr. LaTulippe told elderly and pediatric patients that mask wearing could hurt their health by exacerbating COPD and asthma and could contribute to heart attacks and other medical problems, the report said.
“Licensee asserts masks are likely to harm patients by increasing the body’s carbon dioxide content through rebreathing of gas trapped behind a mask,” the report said.
The report noted that “the amount of carbon dioxide rebreathed within a mask is trivial and would easily be expelled by an increase in minute ventilation so small it would not be noticed.”
The report said Mr. LaTulippe told patients they didn’t have to wear a mask in the clinic unless they were “acutely ill,” “coughing,” or “congested,” even though the Centers for Disease Control and Prevention and the Oregon governor had recommended masks be worn to prevent the spread of the virus.
Before coming into the office, patients weren’t asked if they’d had recent contact with anybody who was infected or showed COVID symptoms, the report said.
The medical board first suspended his license in September. He said he would not change his conduct concerning face masks.
“Licensee has confirmed that he will refuse to abide by the state’s COVID-19 protocols in the future as well, affirming that in a choice between losing his medical license versus wearing a mask in his clinic and requiring his patients and staff to wear a mask in his clinic, he will, ‘choose to sacrifice my medical license with no hesitation’ ” the medical board’s report said.
Mr. LaTulippe told the medical board that he was “a strong asset to the public in educating them on the real facts about this pandemic” and that “at least 98% of my patients were so extremely thankful that I did not wear a mask or demand wearing a mask in my clinic.”
The medical board found Mr. LaTulippe engaged in 8 instances of unprofessional or dishonorable conduct, 22 instances of negligence in the practice of medicine, and 5 instances of gross negligence in the practice of medicine.
A version of this article first appeared on WebMD.com.
Steven Arthur LaTulippe’s advice to patients about face masking amounted to “gross negligence” in the practice of medicine and was grounds for discipline, the medical board said in a report.
Mr. LaTulippe, who had a family practice in Dallas, was fined $10,000, Insider reported. The board also said he’d overprescribed opioids for some patients.
The medical board report said Mr. LaTulippe and his wife, who ran the clinic with him, didn’t wear face masks while treating patients from March to December 2020.
Mr. LaTulippe told elderly and pediatric patients that mask wearing could hurt their health by exacerbating COPD and asthma and could contribute to heart attacks and other medical problems, the report said.
“Licensee asserts masks are likely to harm patients by increasing the body’s carbon dioxide content through rebreathing of gas trapped behind a mask,” the report said.
The report noted that “the amount of carbon dioxide rebreathed within a mask is trivial and would easily be expelled by an increase in minute ventilation so small it would not be noticed.”
The report said Mr. LaTulippe told patients they didn’t have to wear a mask in the clinic unless they were “acutely ill,” “coughing,” or “congested,” even though the Centers for Disease Control and Prevention and the Oregon governor had recommended masks be worn to prevent the spread of the virus.
Before coming into the office, patients weren’t asked if they’d had recent contact with anybody who was infected or showed COVID symptoms, the report said.
The medical board first suspended his license in September. He said he would not change his conduct concerning face masks.
“Licensee has confirmed that he will refuse to abide by the state’s COVID-19 protocols in the future as well, affirming that in a choice between losing his medical license versus wearing a mask in his clinic and requiring his patients and staff to wear a mask in his clinic, he will, ‘choose to sacrifice my medical license with no hesitation’ ” the medical board’s report said.
Mr. LaTulippe told the medical board that he was “a strong asset to the public in educating them on the real facts about this pandemic” and that “at least 98% of my patients were so extremely thankful that I did not wear a mask or demand wearing a mask in my clinic.”
The medical board found Mr. LaTulippe engaged in 8 instances of unprofessional or dishonorable conduct, 22 instances of negligence in the practice of medicine, and 5 instances of gross negligence in the practice of medicine.
A version of this article first appeared on WebMD.com.
Steven Arthur LaTulippe’s advice to patients about face masking amounted to “gross negligence” in the practice of medicine and was grounds for discipline, the medical board said in a report.
Mr. LaTulippe, who had a family practice in Dallas, was fined $10,000, Insider reported. The board also said he’d overprescribed opioids for some patients.
The medical board report said Mr. LaTulippe and his wife, who ran the clinic with him, didn’t wear face masks while treating patients from March to December 2020.
Mr. LaTulippe told elderly and pediatric patients that mask wearing could hurt their health by exacerbating COPD and asthma and could contribute to heart attacks and other medical problems, the report said.
“Licensee asserts masks are likely to harm patients by increasing the body’s carbon dioxide content through rebreathing of gas trapped behind a mask,” the report said.
The report noted that “the amount of carbon dioxide rebreathed within a mask is trivial and would easily be expelled by an increase in minute ventilation so small it would not be noticed.”
The report said Mr. LaTulippe told patients they didn’t have to wear a mask in the clinic unless they were “acutely ill,” “coughing,” or “congested,” even though the Centers for Disease Control and Prevention and the Oregon governor had recommended masks be worn to prevent the spread of the virus.
Before coming into the office, patients weren’t asked if they’d had recent contact with anybody who was infected or showed COVID symptoms, the report said.
The medical board first suspended his license in September. He said he would not change his conduct concerning face masks.
“Licensee has confirmed that he will refuse to abide by the state’s COVID-19 protocols in the future as well, affirming that in a choice between losing his medical license versus wearing a mask in his clinic and requiring his patients and staff to wear a mask in his clinic, he will, ‘choose to sacrifice my medical license with no hesitation’ ” the medical board’s report said.
Mr. LaTulippe told the medical board that he was “a strong asset to the public in educating them on the real facts about this pandemic” and that “at least 98% of my patients were so extremely thankful that I did not wear a mask or demand wearing a mask in my clinic.”
The medical board found Mr. LaTulippe engaged in 8 instances of unprofessional or dishonorable conduct, 22 instances of negligence in the practice of medicine, and 5 instances of gross negligence in the practice of medicine.
A version of this article first appeared on WebMD.com.
AVAHO 2021 Meeting Posters and Abstracts
To view the abstracts and poster from this year's AVAHO 2021 meeting Click Here or on the cover image.
To view the abstracts and poster from this year's AVAHO 2021 meeting Click Here or on the cover image.
To view the abstracts and poster from this year's AVAHO 2021 meeting Click Here or on the cover image.
Pandemic affected home life of nearly 70% of female physicians with children
The survey, conducted by the Robert Graham Center and the American Board of Family Medicine from May to June 2020, examined the professional and personal experiences of being a mother and a primary care physician during the pandemic.
“The pandemic was hard for everyone, but for women who had children in the home, and it didn’t really matter what age, it seemed like the emotional impact was much harder,” study author Yalda Jabbarpour, MD, said in an interview.
The results of the survey of 89 female physicians who worked in the primary care specialty were published in the Journal of Mother Studies.
Dr. Jabbapour and her colleagues found that 67% of female physicians with children said the pandemic had a great “impact” on their home life compared with 25% of those without children. Furthermore, 41% of physician moms said COVID-19 greatly affected their work life, as opposed to 17% of their counterparts without children.
“Women are going into medicine at much higher rates. In primary care, it’s becoming close to the majority,” said Dr. Jabbarpour, a family physician and medical director of the Robert Graham Center for Policy Studies. “That has important workforce implications. If we’re not supporting our female physicians and they are greater than 50% of the physician workforce and they’re burning out, who’s going to have a doctor anymore?”
Child care challenges
Researchers found that the emotional toll female physicians experienced early on in the pandemic was indicative of the challenges they were facing. Some of those challenges included managing anxiety, increased stress from both work and home, and social isolation from friends and family.
Another challenge physician mothers had to deal with was fulfilling child care and homeschooling needs, as many women didn’t know what to do with their children and didn’t have external support from their employers.
Child care options vanished for many people during the pandemic, Emily Kaye, MD, MPH, who was not involved in the study, said in an interview.
“I think it was incredibly challenging for everyone and uniquely challenging for women who were young mothers, specifically with respect to child care” said Dr. Kaye, assistant professor in the department of oncology at St. Jude Children’s Research Hospital. “Many women were expected to just continue plugging on in the absence of any reasonable or safe form of child care.”
Some of the changes physician-mothers said they were required to make at home or in their personal lives included physical changes related to their family safety, such as decontaminating themselves in their garages before heading home after a shift. Some also reported that they had to find new ways to maintain emotional and mental health because of social isolation from family and friends.
The survey results, which were taken early on in the pandemic, highlight the need for health policies that support physician mothers and families, as women shoulder the burden of parenting and domestic responsibilities in heterosexual relationships, the researchers said.
“I’m hoping that people pay attention and start to implement more family friendly policies within their workplaces,” Dr. Jabbarpour said. “But during a pandemic, it was essential for [female health care workers] to go in, and they had nowhere to put their kids. [Therefore], the choice became leaving young children alone at home, putting them into daycare facilities that did remain open without knowing if they were [safe], or quitting their jobs. None of those choices are good.”
Community support as a potential solution
Dr. Kaye said she believes that there should be a “long overdue investment” in community support, affordable and accessible child care, flexible spending, paid family leave, and other forms of caregiving support.
“In order to keep women physicians in the workforce, we need to have a significant increase in investment in the social safety net in this country,” Dr. Kaye said.
Researchers said more studies should evaluate the role the COVID-19 pandemic had on the primary care workforce in the U.S., “with a specific emphasis on how the pandemic impacted mothers, and should more intentionally consider the further intersections of race and ethnicity in the experiences of physician-mothers.”
“I think people are burning out and then there’s all this anti-science, anti-health sentiment out there, which makes it harder,” Dr. Jabbarpour said. “If we did repeat this study now, I think things would be even more dire in the voices of the women that we heard.”
Dr. Jabbarpour and Dr. Kaye reported no disclosures.
The survey, conducted by the Robert Graham Center and the American Board of Family Medicine from May to June 2020, examined the professional and personal experiences of being a mother and a primary care physician during the pandemic.
“The pandemic was hard for everyone, but for women who had children in the home, and it didn’t really matter what age, it seemed like the emotional impact was much harder,” study author Yalda Jabbarpour, MD, said in an interview.
The results of the survey of 89 female physicians who worked in the primary care specialty were published in the Journal of Mother Studies.
Dr. Jabbapour and her colleagues found that 67% of female physicians with children said the pandemic had a great “impact” on their home life compared with 25% of those without children. Furthermore, 41% of physician moms said COVID-19 greatly affected their work life, as opposed to 17% of their counterparts without children.
“Women are going into medicine at much higher rates. In primary care, it’s becoming close to the majority,” said Dr. Jabbarpour, a family physician and medical director of the Robert Graham Center for Policy Studies. “That has important workforce implications. If we’re not supporting our female physicians and they are greater than 50% of the physician workforce and they’re burning out, who’s going to have a doctor anymore?”
Child care challenges
Researchers found that the emotional toll female physicians experienced early on in the pandemic was indicative of the challenges they were facing. Some of those challenges included managing anxiety, increased stress from both work and home, and social isolation from friends and family.
Another challenge physician mothers had to deal with was fulfilling child care and homeschooling needs, as many women didn’t know what to do with their children and didn’t have external support from their employers.
Child care options vanished for many people during the pandemic, Emily Kaye, MD, MPH, who was not involved in the study, said in an interview.
“I think it was incredibly challenging for everyone and uniquely challenging for women who were young mothers, specifically with respect to child care” said Dr. Kaye, assistant professor in the department of oncology at St. Jude Children’s Research Hospital. “Many women were expected to just continue plugging on in the absence of any reasonable or safe form of child care.”
Some of the changes physician-mothers said they were required to make at home or in their personal lives included physical changes related to their family safety, such as decontaminating themselves in their garages before heading home after a shift. Some also reported that they had to find new ways to maintain emotional and mental health because of social isolation from family and friends.
The survey results, which were taken early on in the pandemic, highlight the need for health policies that support physician mothers and families, as women shoulder the burden of parenting and domestic responsibilities in heterosexual relationships, the researchers said.
“I’m hoping that people pay attention and start to implement more family friendly policies within their workplaces,” Dr. Jabbarpour said. “But during a pandemic, it was essential for [female health care workers] to go in, and they had nowhere to put their kids. [Therefore], the choice became leaving young children alone at home, putting them into daycare facilities that did remain open without knowing if they were [safe], or quitting their jobs. None of those choices are good.”
Community support as a potential solution
Dr. Kaye said she believes that there should be a “long overdue investment” in community support, affordable and accessible child care, flexible spending, paid family leave, and other forms of caregiving support.
“In order to keep women physicians in the workforce, we need to have a significant increase in investment in the social safety net in this country,” Dr. Kaye said.
Researchers said more studies should evaluate the role the COVID-19 pandemic had on the primary care workforce in the U.S., “with a specific emphasis on how the pandemic impacted mothers, and should more intentionally consider the further intersections of race and ethnicity in the experiences of physician-mothers.”
“I think people are burning out and then there’s all this anti-science, anti-health sentiment out there, which makes it harder,” Dr. Jabbarpour said. “If we did repeat this study now, I think things would be even more dire in the voices of the women that we heard.”
Dr. Jabbarpour and Dr. Kaye reported no disclosures.
The survey, conducted by the Robert Graham Center and the American Board of Family Medicine from May to June 2020, examined the professional and personal experiences of being a mother and a primary care physician during the pandemic.
“The pandemic was hard for everyone, but for women who had children in the home, and it didn’t really matter what age, it seemed like the emotional impact was much harder,” study author Yalda Jabbarpour, MD, said in an interview.
The results of the survey of 89 female physicians who worked in the primary care specialty were published in the Journal of Mother Studies.
Dr. Jabbapour and her colleagues found that 67% of female physicians with children said the pandemic had a great “impact” on their home life compared with 25% of those without children. Furthermore, 41% of physician moms said COVID-19 greatly affected their work life, as opposed to 17% of their counterparts without children.
“Women are going into medicine at much higher rates. In primary care, it’s becoming close to the majority,” said Dr. Jabbarpour, a family physician and medical director of the Robert Graham Center for Policy Studies. “That has important workforce implications. If we’re not supporting our female physicians and they are greater than 50% of the physician workforce and they’re burning out, who’s going to have a doctor anymore?”
Child care challenges
Researchers found that the emotional toll female physicians experienced early on in the pandemic was indicative of the challenges they were facing. Some of those challenges included managing anxiety, increased stress from both work and home, and social isolation from friends and family.
Another challenge physician mothers had to deal with was fulfilling child care and homeschooling needs, as many women didn’t know what to do with their children and didn’t have external support from their employers.
Child care options vanished for many people during the pandemic, Emily Kaye, MD, MPH, who was not involved in the study, said in an interview.
“I think it was incredibly challenging for everyone and uniquely challenging for women who were young mothers, specifically with respect to child care” said Dr. Kaye, assistant professor in the department of oncology at St. Jude Children’s Research Hospital. “Many women were expected to just continue plugging on in the absence of any reasonable or safe form of child care.”
Some of the changes physician-mothers said they were required to make at home or in their personal lives included physical changes related to their family safety, such as decontaminating themselves in their garages before heading home after a shift. Some also reported that they had to find new ways to maintain emotional and mental health because of social isolation from family and friends.
The survey results, which were taken early on in the pandemic, highlight the need for health policies that support physician mothers and families, as women shoulder the burden of parenting and domestic responsibilities in heterosexual relationships, the researchers said.
“I’m hoping that people pay attention and start to implement more family friendly policies within their workplaces,” Dr. Jabbarpour said. “But during a pandemic, it was essential for [female health care workers] to go in, and they had nowhere to put their kids. [Therefore], the choice became leaving young children alone at home, putting them into daycare facilities that did remain open without knowing if they were [safe], or quitting their jobs. None of those choices are good.”
Community support as a potential solution
Dr. Kaye said she believes that there should be a “long overdue investment” in community support, affordable and accessible child care, flexible spending, paid family leave, and other forms of caregiving support.
“In order to keep women physicians in the workforce, we need to have a significant increase in investment in the social safety net in this country,” Dr. Kaye said.
Researchers said more studies should evaluate the role the COVID-19 pandemic had on the primary care workforce in the U.S., “with a specific emphasis on how the pandemic impacted mothers, and should more intentionally consider the further intersections of race and ethnicity in the experiences of physician-mothers.”
“I think people are burning out and then there’s all this anti-science, anti-health sentiment out there, which makes it harder,” Dr. Jabbarpour said. “If we did repeat this study now, I think things would be even more dire in the voices of the women that we heard.”
Dr. Jabbarpour and Dr. Kaye reported no disclosures.
FROM JOURNAL OF MOTHER STUDIES
Neoadjuvant immunotherapy for resectable head and neck cancer holds therapeutic benefit
The findings, published Sept. 2 in JAMA Otolaryngology Head & Neck Surgery, are noteworthy because the need for effective treatments in head and neck cancers is critical. It is the sixth most common malignancy in the world largely because of tobacco use and alcohol consumption and long-term outcomes are generally poor.
Currently, the standard care for patients with locoregionally advanced head and neck squamous cell carcinoma (HNSCC) includes the surgical removal of the tumor followed by chemotherapy and radiotherapy. Despite new treatments, including pembrolizumab and nivolumab for platinum-refractory advanced head and neck squamous cell carcinoma (HNSCC), there are high rates of recurrence.
“The emerging approach of neoadjuvant immunotherapy for solid cancers has set the ground for the integration of programmed cell death 1 (PD-1)/PD-1 ligand 1 (PD-L1) inhibitors into the neoadjuvant setting of head and neck squamous cell carcinoma treatment,” wrote the authors of the review which was led by Nidal Muhanna, MD, PhD, Tel Aviv Sourasky Medical Center, Tel Aviv University. “The results [of this analysis] demonstrated favorable outcomes and acceptable tolerance of the administration of neoadjuvant PD-1\PD-L1 inhibitors.”
The analysis included 10 studies
The review included 10 cohort studies and randomized clinical trials of 344 patients who were undergoing treatment for HNSCC between 2015 and 2021. In eight studies, patients received neoadjuvant immunotherapy, and in two studies, patients received combined immunotherapy with neoadjuvant chemotherapy and/or radiotherapy.
The overall pathological response rate was 9.7% (95% confidence interval, 3.1%-18.9%) for primary tumors treated with neoadjuvant immunotherapy or, 2.9% (95% CI, 0%-9.5%) for the pathological complete response rate (with a range of 0%-16.7%).
Treatment with neoadjuvant immunotherapy was ultimately found to be statistically significant (odds ratio, 0.07; 95% CI, 0.03-0.18). Plus, favorable associations for treatment with neoadjuvant immunotherapy were found with nodal pathological complete response. In two studies cited in the analysis, combination treatment with neoadjuvant immunotherapy and chemotherapy and/or radiation before surgery had an overall pathological complete response rate of 53%.
The major pathologic response in which less than 10% of the tumor remained after treatment varied greatly between 2.9% and 31.0% in five studies. The mean major pathologic response in these cases was 9.7%, which suggested a statistically significant association. A major pathologic response for lymph nodes was described in three studies as statistically significant for neoadjuvant immunotherapy.
In terms of adverse events, 8.4% (95% CI, 0.2%-23.2%) of patients were treated for autoimmune colitis, duodenal hemorrhage, mucositis, nausea, vomiting, and syncope.
Combination treatment with neoadjuvant immunotherapy with chemotherapy or radiotherapy continues to be evaluated in clinical trials (NCT03721757, NCT03635164, and NCT03618134). “Whether these combinations have synergistic effects or provide any therapeutic benefit, compared with single-agent therapy is still under investigation. It has been hypothesized that chemotherapy preceding the administration of neoadjuvant immunotherapy may increase antigen presentation by dendritic cells and enhance immune activation against the tumor, which can potentially increase therapeutic efficacy,” the authors wrote.
Other ongoing clinical trials will report survival data in the upcoming years. One of these trials is the IMSTAR-HN, a phase 3 clinical trial assessing neoadjuvant nivolumab with and without ipilimumab as first-line treatment with curative intent for HNSCC. It will report disease-free survival, overall survival, and progression-free survival outcomes.
The authors reported no disclosures.
The findings, published Sept. 2 in JAMA Otolaryngology Head & Neck Surgery, are noteworthy because the need for effective treatments in head and neck cancers is critical. It is the sixth most common malignancy in the world largely because of tobacco use and alcohol consumption and long-term outcomes are generally poor.
Currently, the standard care for patients with locoregionally advanced head and neck squamous cell carcinoma (HNSCC) includes the surgical removal of the tumor followed by chemotherapy and radiotherapy. Despite new treatments, including pembrolizumab and nivolumab for platinum-refractory advanced head and neck squamous cell carcinoma (HNSCC), there are high rates of recurrence.
“The emerging approach of neoadjuvant immunotherapy for solid cancers has set the ground for the integration of programmed cell death 1 (PD-1)/PD-1 ligand 1 (PD-L1) inhibitors into the neoadjuvant setting of head and neck squamous cell carcinoma treatment,” wrote the authors of the review which was led by Nidal Muhanna, MD, PhD, Tel Aviv Sourasky Medical Center, Tel Aviv University. “The results [of this analysis] demonstrated favorable outcomes and acceptable tolerance of the administration of neoadjuvant PD-1\PD-L1 inhibitors.”
The analysis included 10 studies
The review included 10 cohort studies and randomized clinical trials of 344 patients who were undergoing treatment for HNSCC between 2015 and 2021. In eight studies, patients received neoadjuvant immunotherapy, and in two studies, patients received combined immunotherapy with neoadjuvant chemotherapy and/or radiotherapy.
The overall pathological response rate was 9.7% (95% confidence interval, 3.1%-18.9%) for primary tumors treated with neoadjuvant immunotherapy or, 2.9% (95% CI, 0%-9.5%) for the pathological complete response rate (with a range of 0%-16.7%).
Treatment with neoadjuvant immunotherapy was ultimately found to be statistically significant (odds ratio, 0.07; 95% CI, 0.03-0.18). Plus, favorable associations for treatment with neoadjuvant immunotherapy were found with nodal pathological complete response. In two studies cited in the analysis, combination treatment with neoadjuvant immunotherapy and chemotherapy and/or radiation before surgery had an overall pathological complete response rate of 53%.
The major pathologic response in which less than 10% of the tumor remained after treatment varied greatly between 2.9% and 31.0% in five studies. The mean major pathologic response in these cases was 9.7%, which suggested a statistically significant association. A major pathologic response for lymph nodes was described in three studies as statistically significant for neoadjuvant immunotherapy.
In terms of adverse events, 8.4% (95% CI, 0.2%-23.2%) of patients were treated for autoimmune colitis, duodenal hemorrhage, mucositis, nausea, vomiting, and syncope.
Combination treatment with neoadjuvant immunotherapy with chemotherapy or radiotherapy continues to be evaluated in clinical trials (NCT03721757, NCT03635164, and NCT03618134). “Whether these combinations have synergistic effects or provide any therapeutic benefit, compared with single-agent therapy is still under investigation. It has been hypothesized that chemotherapy preceding the administration of neoadjuvant immunotherapy may increase antigen presentation by dendritic cells and enhance immune activation against the tumor, which can potentially increase therapeutic efficacy,” the authors wrote.
Other ongoing clinical trials will report survival data in the upcoming years. One of these trials is the IMSTAR-HN, a phase 3 clinical trial assessing neoadjuvant nivolumab with and without ipilimumab as first-line treatment with curative intent for HNSCC. It will report disease-free survival, overall survival, and progression-free survival outcomes.
The authors reported no disclosures.
The findings, published Sept. 2 in JAMA Otolaryngology Head & Neck Surgery, are noteworthy because the need for effective treatments in head and neck cancers is critical. It is the sixth most common malignancy in the world largely because of tobacco use and alcohol consumption and long-term outcomes are generally poor.
Currently, the standard care for patients with locoregionally advanced head and neck squamous cell carcinoma (HNSCC) includes the surgical removal of the tumor followed by chemotherapy and radiotherapy. Despite new treatments, including pembrolizumab and nivolumab for platinum-refractory advanced head and neck squamous cell carcinoma (HNSCC), there are high rates of recurrence.
“The emerging approach of neoadjuvant immunotherapy for solid cancers has set the ground for the integration of programmed cell death 1 (PD-1)/PD-1 ligand 1 (PD-L1) inhibitors into the neoadjuvant setting of head and neck squamous cell carcinoma treatment,” wrote the authors of the review which was led by Nidal Muhanna, MD, PhD, Tel Aviv Sourasky Medical Center, Tel Aviv University. “The results [of this analysis] demonstrated favorable outcomes and acceptable tolerance of the administration of neoadjuvant PD-1\PD-L1 inhibitors.”
The analysis included 10 studies
The review included 10 cohort studies and randomized clinical trials of 344 patients who were undergoing treatment for HNSCC between 2015 and 2021. In eight studies, patients received neoadjuvant immunotherapy, and in two studies, patients received combined immunotherapy with neoadjuvant chemotherapy and/or radiotherapy.
The overall pathological response rate was 9.7% (95% confidence interval, 3.1%-18.9%) for primary tumors treated with neoadjuvant immunotherapy or, 2.9% (95% CI, 0%-9.5%) for the pathological complete response rate (with a range of 0%-16.7%).
Treatment with neoadjuvant immunotherapy was ultimately found to be statistically significant (odds ratio, 0.07; 95% CI, 0.03-0.18). Plus, favorable associations for treatment with neoadjuvant immunotherapy were found with nodal pathological complete response. In two studies cited in the analysis, combination treatment with neoadjuvant immunotherapy and chemotherapy and/or radiation before surgery had an overall pathological complete response rate of 53%.
The major pathologic response in which less than 10% of the tumor remained after treatment varied greatly between 2.9% and 31.0% in five studies. The mean major pathologic response in these cases was 9.7%, which suggested a statistically significant association. A major pathologic response for lymph nodes was described in three studies as statistically significant for neoadjuvant immunotherapy.
In terms of adverse events, 8.4% (95% CI, 0.2%-23.2%) of patients were treated for autoimmune colitis, duodenal hemorrhage, mucositis, nausea, vomiting, and syncope.
Combination treatment with neoadjuvant immunotherapy with chemotherapy or radiotherapy continues to be evaluated in clinical trials (NCT03721757, NCT03635164, and NCT03618134). “Whether these combinations have synergistic effects or provide any therapeutic benefit, compared with single-agent therapy is still under investigation. It has been hypothesized that chemotherapy preceding the administration of neoadjuvant immunotherapy may increase antigen presentation by dendritic cells and enhance immune activation against the tumor, which can potentially increase therapeutic efficacy,” the authors wrote.
Other ongoing clinical trials will report survival data in the upcoming years. One of these trials is the IMSTAR-HN, a phase 3 clinical trial assessing neoadjuvant nivolumab with and without ipilimumab as first-line treatment with curative intent for HNSCC. It will report disease-free survival, overall survival, and progression-free survival outcomes.
The authors reported no disclosures.
FROM THE JOURNALS
Noise in medicine
A 26-year-old woman who reports a history of acyclovir-resistant herpes complains of a recurring, stinging rash around her mouth. Topical tacrolimus made it worse, she said. On exam, she has somewhat grouped pustules on her cutaneous lip. I mentioned her to colleagues, saying: “I’ve a patient with acyclovir-resistant herpes who isn’t improving on high-dose Valtrex.” They proffered a few alternative diagnoses and treatment recommendations. I tried several to no avail.
(it is after all only one condition). Nobel Prize–winning economist Daniel Kahneman, PhD, with two other authors, has written a brilliant book about this cognitive unreliability called “Noise: A Flaw in Human Judgment” (New York: Hachette Book Group, 2021).
Both bias and noise create trouble for us. Although biases get more attention, noise is both more prevalent and insidious. In a 2016 article, Dr. Kahneman and coauthors use a bathroom scale as an analogy to explain the difference. “We would say that the scale is biased if its readings are generally either too high or too low. A scale that consistently underestimates true weight by exactly 4 pounds is seriously biased but free of noise. A scale that gives two different readings when you step on it twice is noisy.” In the case presented, “measurements” by me and my colleagues were returning different “readings.” There is one true diagnosis and best treatment, yet because of noise, we waste time and resources by not getting it right the first time.
There is also evidence of bias in this case. For example, there’s probably some confirmation bias: The patient said she has a history of antiviral-resistant herpes; therefore, her rash might appear to be herpes. Also there might be salience bias: it’s easy to see how prominent pustules might be herpes simplex virus. Noise is an issue in many misdiagnoses, but trickier to see. In most instances, we don’t have the opportunity to get multiple assessments of the same case. When examined though, interrater reliability in medicine is often found to be shockingly low, an indication of how much noise there is in our clinical judgments. This leads to waste, frustration – and can even be dangerous when we’re trying to diagnose cancers such as melanoma, lung, or breast cancer.
Dr. Kahneman and colleagues have excellent recommendations on how to reduce noise, such as tips for good decision hygiene (e.g., using differential diagnoses) and using algorithms (e.g., calculating Apgar or LACE scores). I also liked their strategy of aggregating expert opinions. Fascinatingly, averaging multiple independent assessments is mathematically guaranteed to reduce noise. (God, I love economists). This is true of measurements and opinions: If you use 100 judgments for a case, you reduce noise by 90% (the noise is divided by the square root of the number of judgments averaged). So 20 colleagues’ opinions would reduce noise by almost 80%. However, those 20 opinions must be independent to avoid spurious agreement. (Again, math for the win.)
I showed photos of my patient to a few other dermatologists. They independently returned the same result: perioral dermatitis. This was the correct diagnosis and reminded me why grand rounds and tumor boards are such a great help. Multiple, independent assessments are more likely to get it right than just one opinion because we are canceling out the noise. But remember, grand rounds has to be old-school style – no looking at your coresident answers before giving yours!
Our patient cleared after restarting her topical tacrolimus and a bit of doxycycline. Credit the wisdom of the crowd. Reassuringly though, Dr. Kahneman also shows that expertise does matter in minimizing error. So that fellowship you did was still a great idea.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. He reports having no conflicts of interest. Write to him at dermnews@mdedge.com.
A 26-year-old woman who reports a history of acyclovir-resistant herpes complains of a recurring, stinging rash around her mouth. Topical tacrolimus made it worse, she said. On exam, she has somewhat grouped pustules on her cutaneous lip. I mentioned her to colleagues, saying: “I’ve a patient with acyclovir-resistant herpes who isn’t improving on high-dose Valtrex.” They proffered a few alternative diagnoses and treatment recommendations. I tried several to no avail.
(it is after all only one condition). Nobel Prize–winning economist Daniel Kahneman, PhD, with two other authors, has written a brilliant book about this cognitive unreliability called “Noise: A Flaw in Human Judgment” (New York: Hachette Book Group, 2021).
Both bias and noise create trouble for us. Although biases get more attention, noise is both more prevalent and insidious. In a 2016 article, Dr. Kahneman and coauthors use a bathroom scale as an analogy to explain the difference. “We would say that the scale is biased if its readings are generally either too high or too low. A scale that consistently underestimates true weight by exactly 4 pounds is seriously biased but free of noise. A scale that gives two different readings when you step on it twice is noisy.” In the case presented, “measurements” by me and my colleagues were returning different “readings.” There is one true diagnosis and best treatment, yet because of noise, we waste time and resources by not getting it right the first time.
There is also evidence of bias in this case. For example, there’s probably some confirmation bias: The patient said she has a history of antiviral-resistant herpes; therefore, her rash might appear to be herpes. Also there might be salience bias: it’s easy to see how prominent pustules might be herpes simplex virus. Noise is an issue in many misdiagnoses, but trickier to see. In most instances, we don’t have the opportunity to get multiple assessments of the same case. When examined though, interrater reliability in medicine is often found to be shockingly low, an indication of how much noise there is in our clinical judgments. This leads to waste, frustration – and can even be dangerous when we’re trying to diagnose cancers such as melanoma, lung, or breast cancer.
Dr. Kahneman and colleagues have excellent recommendations on how to reduce noise, such as tips for good decision hygiene (e.g., using differential diagnoses) and using algorithms (e.g., calculating Apgar or LACE scores). I also liked their strategy of aggregating expert opinions. Fascinatingly, averaging multiple independent assessments is mathematically guaranteed to reduce noise. (God, I love economists). This is true of measurements and opinions: If you use 100 judgments for a case, you reduce noise by 90% (the noise is divided by the square root of the number of judgments averaged). So 20 colleagues’ opinions would reduce noise by almost 80%. However, those 20 opinions must be independent to avoid spurious agreement. (Again, math for the win.)
I showed photos of my patient to a few other dermatologists. They independently returned the same result: perioral dermatitis. This was the correct diagnosis and reminded me why grand rounds and tumor boards are such a great help. Multiple, independent assessments are more likely to get it right than just one opinion because we are canceling out the noise. But remember, grand rounds has to be old-school style – no looking at your coresident answers before giving yours!
Our patient cleared after restarting her topical tacrolimus and a bit of doxycycline. Credit the wisdom of the crowd. Reassuringly though, Dr. Kahneman also shows that expertise does matter in minimizing error. So that fellowship you did was still a great idea.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. He reports having no conflicts of interest. Write to him at dermnews@mdedge.com.
A 26-year-old woman who reports a history of acyclovir-resistant herpes complains of a recurring, stinging rash around her mouth. Topical tacrolimus made it worse, she said. On exam, she has somewhat grouped pustules on her cutaneous lip. I mentioned her to colleagues, saying: “I’ve a patient with acyclovir-resistant herpes who isn’t improving on high-dose Valtrex.” They proffered a few alternative diagnoses and treatment recommendations. I tried several to no avail.
(it is after all only one condition). Nobel Prize–winning economist Daniel Kahneman, PhD, with two other authors, has written a brilliant book about this cognitive unreliability called “Noise: A Flaw in Human Judgment” (New York: Hachette Book Group, 2021).
Both bias and noise create trouble for us. Although biases get more attention, noise is both more prevalent and insidious. In a 2016 article, Dr. Kahneman and coauthors use a bathroom scale as an analogy to explain the difference. “We would say that the scale is biased if its readings are generally either too high or too low. A scale that consistently underestimates true weight by exactly 4 pounds is seriously biased but free of noise. A scale that gives two different readings when you step on it twice is noisy.” In the case presented, “measurements” by me and my colleagues were returning different “readings.” There is one true diagnosis and best treatment, yet because of noise, we waste time and resources by not getting it right the first time.
There is also evidence of bias in this case. For example, there’s probably some confirmation bias: The patient said she has a history of antiviral-resistant herpes; therefore, her rash might appear to be herpes. Also there might be salience bias: it’s easy to see how prominent pustules might be herpes simplex virus. Noise is an issue in many misdiagnoses, but trickier to see. In most instances, we don’t have the opportunity to get multiple assessments of the same case. When examined though, interrater reliability in medicine is often found to be shockingly low, an indication of how much noise there is in our clinical judgments. This leads to waste, frustration – and can even be dangerous when we’re trying to diagnose cancers such as melanoma, lung, or breast cancer.
Dr. Kahneman and colleagues have excellent recommendations on how to reduce noise, such as tips for good decision hygiene (e.g., using differential diagnoses) and using algorithms (e.g., calculating Apgar or LACE scores). I also liked their strategy of aggregating expert opinions. Fascinatingly, averaging multiple independent assessments is mathematically guaranteed to reduce noise. (God, I love economists). This is true of measurements and opinions: If you use 100 judgments for a case, you reduce noise by 90% (the noise is divided by the square root of the number of judgments averaged). So 20 colleagues’ opinions would reduce noise by almost 80%. However, those 20 opinions must be independent to avoid spurious agreement. (Again, math for the win.)
I showed photos of my patient to a few other dermatologists. They independently returned the same result: perioral dermatitis. This was the correct diagnosis and reminded me why grand rounds and tumor boards are such a great help. Multiple, independent assessments are more likely to get it right than just one opinion because we are canceling out the noise. But remember, grand rounds has to be old-school style – no looking at your coresident answers before giving yours!
Our patient cleared after restarting her topical tacrolimus and a bit of doxycycline. Credit the wisdom of the crowd. Reassuringly though, Dr. Kahneman also shows that expertise does matter in minimizing error. So that fellowship you did was still a great idea.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. He reports having no conflicts of interest. Write to him at dermnews@mdedge.com.
Real-world data generate debate on definition of flare in axial spondyloarthritis
How best to define axial spondyloarthritis (axSpA) flares in practice remains the subject of some debate as evidenced by the discussion that followed an abstract presentation at the 12th International Congress on Spondyloarthritides.
It’s an important topic, said Maxime Breban, MD, PhD, of Ambroise Paré Hospital in Paris, as flares can adversely affect patient outcomes. The absence of flares may also a useful measure of how well a patient is responding to treatment in clinical trials and whether a treatment can be tapered.
“There have been many ways to define flares in the past and there is no consensus,” he observed.
Although the Assessment of Spondyloarthritis International Society (ASAS) devised 12 preliminary definitions of flare in 2016, “these were not that good when we moved to patients,” Dr. Breban suggested.
The ASAS definitions were based on patient vignettes, he explained, and used a combination of variables from the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI), a visual analog scale (VAS) of pain, and the Ankylosing Spondylitis Disease Activity Score based on C-reactive protein (ASDAS-CRP).
The study that Dr. Breban presented looked at the performance of the ASAS preliminary definitions of axSpA flares in a real-life patient population, as well as prospectively determining how variations in BASDAI and VAS pain were associated with patient-perceived flares of disease.
A total of 99 patients took part in the study, recruited through a secure e-health platform called SPONDY+. Once a week, patients completed the BASDAI questionnaire and the pain VAS, and stated whether their disease had flared in the past week.
Receiver operating characteristic (ROC) curves were calculated to see how well the BASDAI and pain VAS identified patients who were experiencing a flare or had a recently resolved flare of axSpA.
Dr. Breban reported that variation in the BASDAI “appears a suitable variable to monitor the occurrence and resolution of patient-reported flare in axial spondylarthritis.”
In predicting a flare, the area under the curve (AUC) was significantly higher for the change in BASDAI than for the change in pain VAS, at a respective 0.81 and 0.77 (P = .01). However, both variables were similarly accurate in predicting the resolution of a flare, with respective AUCs of 0.78 and 0.80 (P = .3).
A 0.22-point increase in BASDAI was reported to be the best balance between sensitivity (70%) and specificity (79%) for a flare. However, this is “outside of what is possible within a test–retest situation,” Désirée van der Heijde, MD, PhD, of Leiden (the Netherlands) University Medical Center, said during discussion.
Dr. van der Heijde told Dr. Breban: “I understand that that comes out of your data, that that’s the best combination for sensitivity and specificity, but the next step is to decide if that makes sense.”
The ROC curves that Dr. Breban presented showed the range of sensitivities and specificities that could be achieved. If the specificity was increased to be 90% or higher, the specificity fell to 55%, with the change in BASDAI being an increase of 0.8 points. Conversely, bringing the sensitivity above 90% meant the specificity dropped to 39% and the change in BASDAI was a decrease of 0.1 point.
“So that means you can choose whatever you want as a cutoff,” Dr. Breban said. It depends on what you are aiming to do. “If you want to identify a flare, you can increase sensitivity, or specificity, according to what your purpose is,” he suggested.
“The next step, of course, is what to choose as a flare. Then it depends on how you want to use a flare if you want to use a flare to change the treatment,” agreed Dr. van der Heijde. “That was why, in the ASAS group, it was decided to have a high specificity so that you are not changing treatment all the time.”
In the data that Dr. Breban presented, the ASAS preliminary definitions were highly specific but lacked sensitivity. None of the ASAS definitions yielded sensitivity values higher than 37%, whereas specificity was higher than 95% for all of them.
The study’s design did not allow researchers to test the ASDAS-CRP as a definition of flare in its real-world patient sample. Thus, it is looking only at the patient’s perspective on flare, and there is a “huge discrepancy” between patient and physician-reported disease activity, Dr. van der Heijde noted. “So, I think before using your data to really choose the flare definition, I think we need to take it all into account.”
Maxime Dougados, MD, PhD, of Cochin Hospital in Paris, who has been “deeply involved in the elaboration of the definition of flare” added his thoughts: “Flare means for me, not a status, but a change,” he observed.
But if the aim of treating people with axSpA is to achieve a good or acceptable state of health, he questioned whether work should be continued to define the concept of a flare.
The definition of a flare was conceived for use in clinical trials mainly, Dr. van der Heijde noted. It helped to assess how changes in treatment might affect the outcomes of patients. In clinical practice, especially now with treat-to-target gaining more and more traction in axSpA, she agreed that perhaps the goal should be to focus more on the health status of patients.
Dr. Breban acknowledged that the SPONDY+ platform has been developed by bepatient with support from Merck Sharp & Dohme. No other disclosures were made.
How best to define axial spondyloarthritis (axSpA) flares in practice remains the subject of some debate as evidenced by the discussion that followed an abstract presentation at the 12th International Congress on Spondyloarthritides.
It’s an important topic, said Maxime Breban, MD, PhD, of Ambroise Paré Hospital in Paris, as flares can adversely affect patient outcomes. The absence of flares may also a useful measure of how well a patient is responding to treatment in clinical trials and whether a treatment can be tapered.
“There have been many ways to define flares in the past and there is no consensus,” he observed.
Although the Assessment of Spondyloarthritis International Society (ASAS) devised 12 preliminary definitions of flare in 2016, “these were not that good when we moved to patients,” Dr. Breban suggested.
The ASAS definitions were based on patient vignettes, he explained, and used a combination of variables from the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI), a visual analog scale (VAS) of pain, and the Ankylosing Spondylitis Disease Activity Score based on C-reactive protein (ASDAS-CRP).
The study that Dr. Breban presented looked at the performance of the ASAS preliminary definitions of axSpA flares in a real-life patient population, as well as prospectively determining how variations in BASDAI and VAS pain were associated with patient-perceived flares of disease.
A total of 99 patients took part in the study, recruited through a secure e-health platform called SPONDY+. Once a week, patients completed the BASDAI questionnaire and the pain VAS, and stated whether their disease had flared in the past week.
Receiver operating characteristic (ROC) curves were calculated to see how well the BASDAI and pain VAS identified patients who were experiencing a flare or had a recently resolved flare of axSpA.
Dr. Breban reported that variation in the BASDAI “appears a suitable variable to monitor the occurrence and resolution of patient-reported flare in axial spondylarthritis.”
In predicting a flare, the area under the curve (AUC) was significantly higher for the change in BASDAI than for the change in pain VAS, at a respective 0.81 and 0.77 (P = .01). However, both variables were similarly accurate in predicting the resolution of a flare, with respective AUCs of 0.78 and 0.80 (P = .3).
A 0.22-point increase in BASDAI was reported to be the best balance between sensitivity (70%) and specificity (79%) for a flare. However, this is “outside of what is possible within a test–retest situation,” Désirée van der Heijde, MD, PhD, of Leiden (the Netherlands) University Medical Center, said during discussion.
Dr. van der Heijde told Dr. Breban: “I understand that that comes out of your data, that that’s the best combination for sensitivity and specificity, but the next step is to decide if that makes sense.”
The ROC curves that Dr. Breban presented showed the range of sensitivities and specificities that could be achieved. If the specificity was increased to be 90% or higher, the specificity fell to 55%, with the change in BASDAI being an increase of 0.8 points. Conversely, bringing the sensitivity above 90% meant the specificity dropped to 39% and the change in BASDAI was a decrease of 0.1 point.
“So that means you can choose whatever you want as a cutoff,” Dr. Breban said. It depends on what you are aiming to do. “If you want to identify a flare, you can increase sensitivity, or specificity, according to what your purpose is,” he suggested.
“The next step, of course, is what to choose as a flare. Then it depends on how you want to use a flare if you want to use a flare to change the treatment,” agreed Dr. van der Heijde. “That was why, in the ASAS group, it was decided to have a high specificity so that you are not changing treatment all the time.”
In the data that Dr. Breban presented, the ASAS preliminary definitions were highly specific but lacked sensitivity. None of the ASAS definitions yielded sensitivity values higher than 37%, whereas specificity was higher than 95% for all of them.
The study’s design did not allow researchers to test the ASDAS-CRP as a definition of flare in its real-world patient sample. Thus, it is looking only at the patient’s perspective on flare, and there is a “huge discrepancy” between patient and physician-reported disease activity, Dr. van der Heijde noted. “So, I think before using your data to really choose the flare definition, I think we need to take it all into account.”
Maxime Dougados, MD, PhD, of Cochin Hospital in Paris, who has been “deeply involved in the elaboration of the definition of flare” added his thoughts: “Flare means for me, not a status, but a change,” he observed.
But if the aim of treating people with axSpA is to achieve a good or acceptable state of health, he questioned whether work should be continued to define the concept of a flare.
The definition of a flare was conceived for use in clinical trials mainly, Dr. van der Heijde noted. It helped to assess how changes in treatment might affect the outcomes of patients. In clinical practice, especially now with treat-to-target gaining more and more traction in axSpA, she agreed that perhaps the goal should be to focus more on the health status of patients.
Dr. Breban acknowledged that the SPONDY+ platform has been developed by bepatient with support from Merck Sharp & Dohme. No other disclosures were made.
How best to define axial spondyloarthritis (axSpA) flares in practice remains the subject of some debate as evidenced by the discussion that followed an abstract presentation at the 12th International Congress on Spondyloarthritides.
It’s an important topic, said Maxime Breban, MD, PhD, of Ambroise Paré Hospital in Paris, as flares can adversely affect patient outcomes. The absence of flares may also a useful measure of how well a patient is responding to treatment in clinical trials and whether a treatment can be tapered.
“There have been many ways to define flares in the past and there is no consensus,” he observed.
Although the Assessment of Spondyloarthritis International Society (ASAS) devised 12 preliminary definitions of flare in 2016, “these were not that good when we moved to patients,” Dr. Breban suggested.
The ASAS definitions were based on patient vignettes, he explained, and used a combination of variables from the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI), a visual analog scale (VAS) of pain, and the Ankylosing Spondylitis Disease Activity Score based on C-reactive protein (ASDAS-CRP).
The study that Dr. Breban presented looked at the performance of the ASAS preliminary definitions of axSpA flares in a real-life patient population, as well as prospectively determining how variations in BASDAI and VAS pain were associated with patient-perceived flares of disease.
A total of 99 patients took part in the study, recruited through a secure e-health platform called SPONDY+. Once a week, patients completed the BASDAI questionnaire and the pain VAS, and stated whether their disease had flared in the past week.
Receiver operating characteristic (ROC) curves were calculated to see how well the BASDAI and pain VAS identified patients who were experiencing a flare or had a recently resolved flare of axSpA.
Dr. Breban reported that variation in the BASDAI “appears a suitable variable to monitor the occurrence and resolution of patient-reported flare in axial spondylarthritis.”
In predicting a flare, the area under the curve (AUC) was significantly higher for the change in BASDAI than for the change in pain VAS, at a respective 0.81 and 0.77 (P = .01). However, both variables were similarly accurate in predicting the resolution of a flare, with respective AUCs of 0.78 and 0.80 (P = .3).
A 0.22-point increase in BASDAI was reported to be the best balance between sensitivity (70%) and specificity (79%) for a flare. However, this is “outside of what is possible within a test–retest situation,” Désirée van der Heijde, MD, PhD, of Leiden (the Netherlands) University Medical Center, said during discussion.
Dr. van der Heijde told Dr. Breban: “I understand that that comes out of your data, that that’s the best combination for sensitivity and specificity, but the next step is to decide if that makes sense.”
The ROC curves that Dr. Breban presented showed the range of sensitivities and specificities that could be achieved. If the specificity was increased to be 90% or higher, the specificity fell to 55%, with the change in BASDAI being an increase of 0.8 points. Conversely, bringing the sensitivity above 90% meant the specificity dropped to 39% and the change in BASDAI was a decrease of 0.1 point.
“So that means you can choose whatever you want as a cutoff,” Dr. Breban said. It depends on what you are aiming to do. “If you want to identify a flare, you can increase sensitivity, or specificity, according to what your purpose is,” he suggested.
“The next step, of course, is what to choose as a flare. Then it depends on how you want to use a flare if you want to use a flare to change the treatment,” agreed Dr. van der Heijde. “That was why, in the ASAS group, it was decided to have a high specificity so that you are not changing treatment all the time.”
In the data that Dr. Breban presented, the ASAS preliminary definitions were highly specific but lacked sensitivity. None of the ASAS definitions yielded sensitivity values higher than 37%, whereas specificity was higher than 95% for all of them.
The study’s design did not allow researchers to test the ASDAS-CRP as a definition of flare in its real-world patient sample. Thus, it is looking only at the patient’s perspective on flare, and there is a “huge discrepancy” between patient and physician-reported disease activity, Dr. van der Heijde noted. “So, I think before using your data to really choose the flare definition, I think we need to take it all into account.”
Maxime Dougados, MD, PhD, of Cochin Hospital in Paris, who has been “deeply involved in the elaboration of the definition of flare” added his thoughts: “Flare means for me, not a status, but a change,” he observed.
But if the aim of treating people with axSpA is to achieve a good or acceptable state of health, he questioned whether work should be continued to define the concept of a flare.
The definition of a flare was conceived for use in clinical trials mainly, Dr. van der Heijde noted. It helped to assess how changes in treatment might affect the outcomes of patients. In clinical practice, especially now with treat-to-target gaining more and more traction in axSpA, she agreed that perhaps the goal should be to focus more on the health status of patients.
Dr. Breban acknowledged that the SPONDY+ platform has been developed by bepatient with support from Merck Sharp & Dohme. No other disclosures were made.
FROM THE 2021 SPA CONGRESS
Flagellate Shiitake Mushroom Reaction With Histologic Features of Acute Generalized Exanthematous Pustulosis
To the Editor:
A 59-year-old man presented with a severely pruritic rash on the legs, arms, abdomen, groin, and buttocks of 3 days’ duration. He reported subjective fever and chills. Prior to the appearance of the rash, the patient and his family had eaten shiitake mushrooms daily for 3 days. He denied any new medications in the last several months or any recent upper respiratory or gastrointestinal tract illnesses. His medical history included type 2 diabetes mellitus and diabetes-induced end-stage renal disease requiring home peritoneal dialysis. His long-term medications for diabetes mellitus, hypertension, benign prostatic hyperplasia, hyperlipidemia, and insomnia included amlodipine, atorvastatin, finasteride, gabapentin, insulin glargine, linagliptin, metoprolol, and mirtazapine.
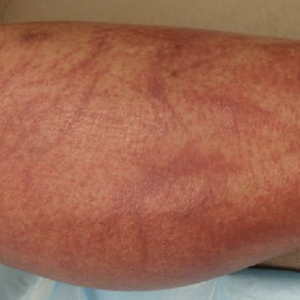
Physical examination revealed an afebrile man with medium brown skin tone and diffuse, bright red, erythematous patches on the lower legs, axillae, medial forearms, lateral trunk, lower abdomen, and groin. There were distinct flagellate, linear, red patches on the lower legs (Figure 1). In addition, small clusters of 1- to 2-mm superficial pustules were present on the right upper medial thigh and left forearm with micropapules grouped in the skin folds.
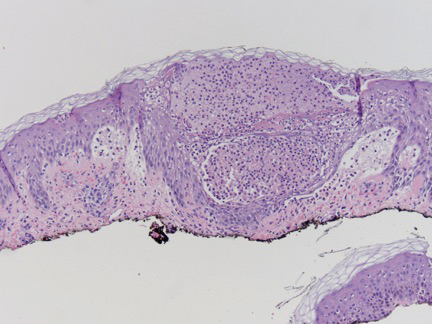
A shave biopsy specimen from a pustule on the right upper medial thigh revealed spongiotic dermatitis with neutrophilic subcorneal pustule formation and frequent eosinophils (Figure 2). The dermis contained scattered mixed inflammatory cells including neutrophils, eosinophils, lymphocytes, and histiocytes (Figure 3). These histologic findings were consistent with acute generalized exanthematous pustulosis (AGEP). No biopsy was performed on the flagellate patches due to its clinically distinct presentation and well-established association with shiitake mushroom ingestion.
The patient was treated with triamcinolone ointment and systemic corticosteroids to reduce pruritus and quickly clear the lesions due to his comorbidities. He recovered completely within 1 week and had no evidence of postinflammatory hyperpigmentation from the flagellate dermatitis.
Flagellate dermatitis is an intensely pruritic dermatitis characterized by 1-mm, disseminated, erythematous papules in a linear grouped arrangement secondary to koebnerization due to the patient scratching. It was first described in 1977 by Nakamura.1 Although it rarely is seen outside of China and Japan, there are well-established associations of flagellate dermatitis with bleomycin and shiitake mushroom (Lentinula edodes) ingestion. One key clinical difference between the two causes is that postinflammatory hyperpigmentation changes usually are seen with bleomycin-induced flagellate dermatitis and typically are not present with shiitake mushroom–induced flagellate dermatitis.2 Following ingestion of shiitake mushrooms, the median time of onset of presentation typically is 24 hours but ranges from 12 hours to 5 days. Most patients completely recover by 3 weeks, with or without treatment.3 Although the pathogenesis of shiitake mushroom–induced flagellate dermatitis is not clear, the most common theory is a toxic reaction to lentinan, a polysaccharide isolated from shiitake mushrooms. However, type I and IV allergic hypersensitivities also have been supported by the time of onset, clearance, severe pruritus, benefit from steroids and antihistamines, and lack of grouped outbreaks in people exposed to shared meals containing shiitake mushrooms.3,4 Furthermore, there is a case of patch test–confirmed allergic contact dermatitis to shiitake mushrooms, demonstrating a 1+ reaction at 96 hours to the cap of a shiitake mushroom but a negative pin-prick test at 20 minutes, suggesting type IV hypersensitivity.5 An additional case revealed a positive skin-prick test with formation of a 4-mm wheal and subsequent pruritic papules and vesicles appearing 48 to 72 hours later at the prick site.6 Subsequent cases have been reported in association with consumption of raw shiitake mushrooms, but cases have been reported after consumption of fully cooked mushrooms, which does not support a toxin-mediated theory, as cooking the mushroom before consumption likely would denature or change the structure of the suspected toxin.2
Acute generalized exanthematous pustulosis is a rare eruption that occurs due to ingestion of a causative agent, usually an antibiotic, and is characterized by the presence of fever and disseminated, erythematous, pinpoint, sterile pustules on the skin and mucous membranes. It affects 1 to 5 persons per million per year, with more than 90% of cases attributed to drug ingestion.7 Spontaneous resolution can be expected within 15 days of its onset; however, there is a mortality rate of up to 5% that occurs most often in those with severe comorbidities or in older patients, for whom systemic corticosteroid therapy may be justified.7,8 A multinational case-control study conducted to evaluate the risk of AGEP associated with certain drugs revealed macrolides (namely pristinamycin); β-lactam antibiotics including penicillin, aminopenicillin, and cephalosporin; quinolones; hydroxychloroquine; anti-infective sulfonamides; terbinafine; and diltiazem as the most strongly associated culprits.9 Our patient’s flagellate dermatitis was unique in that it also showed histologic features of AGEP. The pathogenesis of drug-induced AGEP has been partially elucidated and involves activation of drug-specific CD4+ and CD8+ T cells that migrate to the skin and participate in apoptotic signaling of keratinocytes and recruitment of neutrophils and eosinophils, which form subcorneal sterile pustules.7 In a study of severe cutaneous adverse drug reactions, 50% (7/14) of patients with AGEP had positive patch tests to the causative agent.10 This T cell–dependent response explains why the condition responds to systemic corticosteroids. Additionally, our case report of shiitake mushroom–induced flagellate dermatitis with histologic features of AGEP suggests that the pathogenesis of flagellate dermatitis may be a T cell–mediated type IV hypersensitivity reaction. The time of onset, lack of grouped outbreaks in those sharing shiitake mushroom–containing meals, severe pruritus, lack of cases demonstrating an anaphylactic or wheal and flare response, benefit of steroids, and a case with histologic features of AGEP all lend support to this theory.
We report a case of shiitake mushroom–induced flagellate dermatitis with histologic features of AGEP. The time course, histologic features of AGEP, absence of new medications, and resolution with discontinuation of shiitake mushrooms lends support of the hypothesis that the pathogenesis of shiitake mushroom–induced flagellate dermatitis is similar to AGEP’s type IV hypersensitivity reaction. To further elucidate its pathogenesis, skin prick testing and patch testing with shiitake mushrooms in patients exhibiting shiitake mushroom–induced flagellate dermatitis may prove to be beneficial.
- Nakamura T. Toxicoderma caused by shiitake (Lentinus edodes)[in Japanese]. Jpn J Clin Dermatol. 1977;31:65-68.
- Chu EY, Anand D, Dawn A, et al. Shiitake dermatitis: a report of 3 cases and review of the literature. Cutis. 2013;91:287-290.
- Boels D, Landreau A, Bruneau C, et al. Shiitake dermatitis recorded by French Poison Control Centers—new case series with clinical observations. Clin Toxicol (Phila). 2014;52:625-628.
- Nakamura T. Shiitake (Lentinus edodes) dermatitis. Contact Dermatitis. 1992;27:65-70.
- Curnow P, Tam M. Contact dermatitis to shiitake mushroom. Australas J Dermatol. 2003;44:155-157.
- Lippert U, Martin V, Schwertfeger C, et al. Shiitake dermatitis. Br J Dermatol. 2003;148:178-179.
- Fernando SL. Acute generalised exanthematous pustulosis. Australas J Dermatol. 2012;53:87-92.
- Sidoroff A, Halevy S, Bavinck JN, et al. Acute generalized exanthematous pustulosis (AGEP)—a clinical reaction pattern. J Cutan Pathol. 2001;28:113-119.
- Sidoroff A, Dunant A, Viboud C, et al. Risk factors for acute generalized exanthematous pustulosis (AGEP)—results of a multinational case-control study (EuroSCAR). Br J Dermatol. 2007;157:989-996.
- Wolkenstein P, Chosidow O, Flechet ML, et al. Patch testing in severe cutaneous adverse drug reactions, including Stevens-Johnson syndrome and toxic epidermal necrolysis. Contact Dermatitis. 1996;35:234-236.
To the Editor:
A 59-year-old man presented with a severely pruritic rash on the legs, arms, abdomen, groin, and buttocks of 3 days’ duration. He reported subjective fever and chills. Prior to the appearance of the rash, the patient and his family had eaten shiitake mushrooms daily for 3 days. He denied any new medications in the last several months or any recent upper respiratory or gastrointestinal tract illnesses. His medical history included type 2 diabetes mellitus and diabetes-induced end-stage renal disease requiring home peritoneal dialysis. His long-term medications for diabetes mellitus, hypertension, benign prostatic hyperplasia, hyperlipidemia, and insomnia included amlodipine, atorvastatin, finasteride, gabapentin, insulin glargine, linagliptin, metoprolol, and mirtazapine.
Physical examination revealed an afebrile man with medium brown skin tone and diffuse, bright red, erythematous patches on the lower legs, axillae, medial forearms, lateral trunk, lower abdomen, and groin. There were distinct flagellate, linear, red patches on the lower legs (Figure 1). In addition, small clusters of 1- to 2-mm superficial pustules were present on the right upper medial thigh and left forearm with micropapules grouped in the skin folds.
A shave biopsy specimen from a pustule on the right upper medial thigh revealed spongiotic dermatitis with neutrophilic subcorneal pustule formation and frequent eosinophils (Figure 2). The dermis contained scattered mixed inflammatory cells including neutrophils, eosinophils, lymphocytes, and histiocytes (Figure 3). These histologic findings were consistent with acute generalized exanthematous pustulosis (AGEP). No biopsy was performed on the flagellate patches due to its clinically distinct presentation and well-established association with shiitake mushroom ingestion.
The patient was treated with triamcinolone ointment and systemic corticosteroids to reduce pruritus and quickly clear the lesions due to his comorbidities. He recovered completely within 1 week and had no evidence of postinflammatory hyperpigmentation from the flagellate dermatitis.
Flagellate dermatitis is an intensely pruritic dermatitis characterized by 1-mm, disseminated, erythematous papules in a linear grouped arrangement secondary to koebnerization due to the patient scratching. It was first described in 1977 by Nakamura.1 Although it rarely is seen outside of China and Japan, there are well-established associations of flagellate dermatitis with bleomycin and shiitake mushroom (Lentinula edodes) ingestion. One key clinical difference between the two causes is that postinflammatory hyperpigmentation changes usually are seen with bleomycin-induced flagellate dermatitis and typically are not present with shiitake mushroom–induced flagellate dermatitis.2 Following ingestion of shiitake mushrooms, the median time of onset of presentation typically is 24 hours but ranges from 12 hours to 5 days. Most patients completely recover by 3 weeks, with or without treatment.3 Although the pathogenesis of shiitake mushroom–induced flagellate dermatitis is not clear, the most common theory is a toxic reaction to lentinan, a polysaccharide isolated from shiitake mushrooms. However, type I and IV allergic hypersensitivities also have been supported by the time of onset, clearance, severe pruritus, benefit from steroids and antihistamines, and lack of grouped outbreaks in people exposed to shared meals containing shiitake mushrooms.3,4 Furthermore, there is a case of patch test–confirmed allergic contact dermatitis to shiitake mushrooms, demonstrating a 1+ reaction at 96 hours to the cap of a shiitake mushroom but a negative pin-prick test at 20 minutes, suggesting type IV hypersensitivity.5 An additional case revealed a positive skin-prick test with formation of a 4-mm wheal and subsequent pruritic papules and vesicles appearing 48 to 72 hours later at the prick site.6 Subsequent cases have been reported in association with consumption of raw shiitake mushrooms, but cases have been reported after consumption of fully cooked mushrooms, which does not support a toxin-mediated theory, as cooking the mushroom before consumption likely would denature or change the structure of the suspected toxin.2
Acute generalized exanthematous pustulosis is a rare eruption that occurs due to ingestion of a causative agent, usually an antibiotic, and is characterized by the presence of fever and disseminated, erythematous, pinpoint, sterile pustules on the skin and mucous membranes. It affects 1 to 5 persons per million per year, with more than 90% of cases attributed to drug ingestion.7 Spontaneous resolution can be expected within 15 days of its onset; however, there is a mortality rate of up to 5% that occurs most often in those with severe comorbidities or in older patients, for whom systemic corticosteroid therapy may be justified.7,8 A multinational case-control study conducted to evaluate the risk of AGEP associated with certain drugs revealed macrolides (namely pristinamycin); β-lactam antibiotics including penicillin, aminopenicillin, and cephalosporin; quinolones; hydroxychloroquine; anti-infective sulfonamides; terbinafine; and diltiazem as the most strongly associated culprits.9 Our patient’s flagellate dermatitis was unique in that it also showed histologic features of AGEP. The pathogenesis of drug-induced AGEP has been partially elucidated and involves activation of drug-specific CD4+ and CD8+ T cells that migrate to the skin and participate in apoptotic signaling of keratinocytes and recruitment of neutrophils and eosinophils, which form subcorneal sterile pustules.7 In a study of severe cutaneous adverse drug reactions, 50% (7/14) of patients with AGEP had positive patch tests to the causative agent.10 This T cell–dependent response explains why the condition responds to systemic corticosteroids. Additionally, our case report of shiitake mushroom–induced flagellate dermatitis with histologic features of AGEP suggests that the pathogenesis of flagellate dermatitis may be a T cell–mediated type IV hypersensitivity reaction. The time of onset, lack of grouped outbreaks in those sharing shiitake mushroom–containing meals, severe pruritus, lack of cases demonstrating an anaphylactic or wheal and flare response, benefit of steroids, and a case with histologic features of AGEP all lend support to this theory.
We report a case of shiitake mushroom–induced flagellate dermatitis with histologic features of AGEP. The time course, histologic features of AGEP, absence of new medications, and resolution with discontinuation of shiitake mushrooms lends support of the hypothesis that the pathogenesis of shiitake mushroom–induced flagellate dermatitis is similar to AGEP’s type IV hypersensitivity reaction. To further elucidate its pathogenesis, skin prick testing and patch testing with shiitake mushrooms in patients exhibiting shiitake mushroom–induced flagellate dermatitis may prove to be beneficial.
To the Editor:
A 59-year-old man presented with a severely pruritic rash on the legs, arms, abdomen, groin, and buttocks of 3 days’ duration. He reported subjective fever and chills. Prior to the appearance of the rash, the patient and his family had eaten shiitake mushrooms daily for 3 days. He denied any new medications in the last several months or any recent upper respiratory or gastrointestinal tract illnesses. His medical history included type 2 diabetes mellitus and diabetes-induced end-stage renal disease requiring home peritoneal dialysis. His long-term medications for diabetes mellitus, hypertension, benign prostatic hyperplasia, hyperlipidemia, and insomnia included amlodipine, atorvastatin, finasteride, gabapentin, insulin glargine, linagliptin, metoprolol, and mirtazapine.
Physical examination revealed an afebrile man with medium brown skin tone and diffuse, bright red, erythematous patches on the lower legs, axillae, medial forearms, lateral trunk, lower abdomen, and groin. There were distinct flagellate, linear, red patches on the lower legs (Figure 1). In addition, small clusters of 1- to 2-mm superficial pustules were present on the right upper medial thigh and left forearm with micropapules grouped in the skin folds.
A shave biopsy specimen from a pustule on the right upper medial thigh revealed spongiotic dermatitis with neutrophilic subcorneal pustule formation and frequent eosinophils (Figure 2). The dermis contained scattered mixed inflammatory cells including neutrophils, eosinophils, lymphocytes, and histiocytes (Figure 3). These histologic findings were consistent with acute generalized exanthematous pustulosis (AGEP). No biopsy was performed on the flagellate patches due to its clinically distinct presentation and well-established association with shiitake mushroom ingestion.
The patient was treated with triamcinolone ointment and systemic corticosteroids to reduce pruritus and quickly clear the lesions due to his comorbidities. He recovered completely within 1 week and had no evidence of postinflammatory hyperpigmentation from the flagellate dermatitis.
Flagellate dermatitis is an intensely pruritic dermatitis characterized by 1-mm, disseminated, erythematous papules in a linear grouped arrangement secondary to koebnerization due to the patient scratching. It was first described in 1977 by Nakamura.1 Although it rarely is seen outside of China and Japan, there are well-established associations of flagellate dermatitis with bleomycin and shiitake mushroom (Lentinula edodes) ingestion. One key clinical difference between the two causes is that postinflammatory hyperpigmentation changes usually are seen with bleomycin-induced flagellate dermatitis and typically are not present with shiitake mushroom–induced flagellate dermatitis.2 Following ingestion of shiitake mushrooms, the median time of onset of presentation typically is 24 hours but ranges from 12 hours to 5 days. Most patients completely recover by 3 weeks, with or without treatment.3 Although the pathogenesis of shiitake mushroom–induced flagellate dermatitis is not clear, the most common theory is a toxic reaction to lentinan, a polysaccharide isolated from shiitake mushrooms. However, type I and IV allergic hypersensitivities also have been supported by the time of onset, clearance, severe pruritus, benefit from steroids and antihistamines, and lack of grouped outbreaks in people exposed to shared meals containing shiitake mushrooms.3,4 Furthermore, there is a case of patch test–confirmed allergic contact dermatitis to shiitake mushrooms, demonstrating a 1+ reaction at 96 hours to the cap of a shiitake mushroom but a negative pin-prick test at 20 minutes, suggesting type IV hypersensitivity.5 An additional case revealed a positive skin-prick test with formation of a 4-mm wheal and subsequent pruritic papules and vesicles appearing 48 to 72 hours later at the prick site.6 Subsequent cases have been reported in association with consumption of raw shiitake mushrooms, but cases have been reported after consumption of fully cooked mushrooms, which does not support a toxin-mediated theory, as cooking the mushroom before consumption likely would denature or change the structure of the suspected toxin.2
Acute generalized exanthematous pustulosis is a rare eruption that occurs due to ingestion of a causative agent, usually an antibiotic, and is characterized by the presence of fever and disseminated, erythematous, pinpoint, sterile pustules on the skin and mucous membranes. It affects 1 to 5 persons per million per year, with more than 90% of cases attributed to drug ingestion.7 Spontaneous resolution can be expected within 15 days of its onset; however, there is a mortality rate of up to 5% that occurs most often in those with severe comorbidities or in older patients, for whom systemic corticosteroid therapy may be justified.7,8 A multinational case-control study conducted to evaluate the risk of AGEP associated with certain drugs revealed macrolides (namely pristinamycin); β-lactam antibiotics including penicillin, aminopenicillin, and cephalosporin; quinolones; hydroxychloroquine; anti-infective sulfonamides; terbinafine; and diltiazem as the most strongly associated culprits.9 Our patient’s flagellate dermatitis was unique in that it also showed histologic features of AGEP. The pathogenesis of drug-induced AGEP has been partially elucidated and involves activation of drug-specific CD4+ and CD8+ T cells that migrate to the skin and participate in apoptotic signaling of keratinocytes and recruitment of neutrophils and eosinophils, which form subcorneal sterile pustules.7 In a study of severe cutaneous adverse drug reactions, 50% (7/14) of patients with AGEP had positive patch tests to the causative agent.10 This T cell–dependent response explains why the condition responds to systemic corticosteroids. Additionally, our case report of shiitake mushroom–induced flagellate dermatitis with histologic features of AGEP suggests that the pathogenesis of flagellate dermatitis may be a T cell–mediated type IV hypersensitivity reaction. The time of onset, lack of grouped outbreaks in those sharing shiitake mushroom–containing meals, severe pruritus, lack of cases demonstrating an anaphylactic or wheal and flare response, benefit of steroids, and a case with histologic features of AGEP all lend support to this theory.
We report a case of shiitake mushroom–induced flagellate dermatitis with histologic features of AGEP. The time course, histologic features of AGEP, absence of new medications, and resolution with discontinuation of shiitake mushrooms lends support of the hypothesis that the pathogenesis of shiitake mushroom–induced flagellate dermatitis is similar to AGEP’s type IV hypersensitivity reaction. To further elucidate its pathogenesis, skin prick testing and patch testing with shiitake mushrooms in patients exhibiting shiitake mushroom–induced flagellate dermatitis may prove to be beneficial.
- Nakamura T. Toxicoderma caused by shiitake (Lentinus edodes)[in Japanese]. Jpn J Clin Dermatol. 1977;31:65-68.
- Chu EY, Anand D, Dawn A, et al. Shiitake dermatitis: a report of 3 cases and review of the literature. Cutis. 2013;91:287-290.
- Boels D, Landreau A, Bruneau C, et al. Shiitake dermatitis recorded by French Poison Control Centers—new case series with clinical observations. Clin Toxicol (Phila). 2014;52:625-628.
- Nakamura T. Shiitake (Lentinus edodes) dermatitis. Contact Dermatitis. 1992;27:65-70.
- Curnow P, Tam M. Contact dermatitis to shiitake mushroom. Australas J Dermatol. 2003;44:155-157.
- Lippert U, Martin V, Schwertfeger C, et al. Shiitake dermatitis. Br J Dermatol. 2003;148:178-179.
- Fernando SL. Acute generalised exanthematous pustulosis. Australas J Dermatol. 2012;53:87-92.
- Sidoroff A, Halevy S, Bavinck JN, et al. Acute generalized exanthematous pustulosis (AGEP)—a clinical reaction pattern. J Cutan Pathol. 2001;28:113-119.
- Sidoroff A, Dunant A, Viboud C, et al. Risk factors for acute generalized exanthematous pustulosis (AGEP)—results of a multinational case-control study (EuroSCAR). Br J Dermatol. 2007;157:989-996.
- Wolkenstein P, Chosidow O, Flechet ML, et al. Patch testing in severe cutaneous adverse drug reactions, including Stevens-Johnson syndrome and toxic epidermal necrolysis. Contact Dermatitis. 1996;35:234-236.
- Nakamura T. Toxicoderma caused by shiitake (Lentinus edodes)[in Japanese]. Jpn J Clin Dermatol. 1977;31:65-68.
- Chu EY, Anand D, Dawn A, et al. Shiitake dermatitis: a report of 3 cases and review of the literature. Cutis. 2013;91:287-290.
- Boels D, Landreau A, Bruneau C, et al. Shiitake dermatitis recorded by French Poison Control Centers—new case series with clinical observations. Clin Toxicol (Phila). 2014;52:625-628.
- Nakamura T. Shiitake (Lentinus edodes) dermatitis. Contact Dermatitis. 1992;27:65-70.
- Curnow P, Tam M. Contact dermatitis to shiitake mushroom. Australas J Dermatol. 2003;44:155-157.
- Lippert U, Martin V, Schwertfeger C, et al. Shiitake dermatitis. Br J Dermatol. 2003;148:178-179.
- Fernando SL. Acute generalised exanthematous pustulosis. Australas J Dermatol. 2012;53:87-92.
- Sidoroff A, Halevy S, Bavinck JN, et al. Acute generalized exanthematous pustulosis (AGEP)—a clinical reaction pattern. J Cutan Pathol. 2001;28:113-119.
- Sidoroff A, Dunant A, Viboud C, et al. Risk factors for acute generalized exanthematous pustulosis (AGEP)—results of a multinational case-control study (EuroSCAR). Br J Dermatol. 2007;157:989-996.
- Wolkenstein P, Chosidow O, Flechet ML, et al. Patch testing in severe cutaneous adverse drug reactions, including Stevens-Johnson syndrome and toxic epidermal necrolysis. Contact Dermatitis. 1996;35:234-236.
Practice Points
- Ingestion of shiitake mushrooms and bleomycin is associated with flagellate dermatitis.
- Acute generalized exanthematous pustulosis (AGEP) is a rare condition associated with certain drug ingestion.
When children and teens with cancer get COVID-19
Although most children and adolescents with cancer have mild illness from COVID-19 infection, some do experience severe disease and a small percentage even die, according to a recent analysis.
The findings, published online in Lancet Oncology, represent the first global registry data spanning different income groups to report COVID-19 outcomes in pediatric oncology patients.
“We wanted to create a global pool of evidence to answer the question: Do we see severe [COVID-19] infection [in children with cancer]?” corresponding author Sheena Mukkada, MD, St. Jude Children’s Research Hospital, Memphis, said in an interview.
In a cohort of 1,319 pediatric patients followed for 30 days, Dr. Mukkada and colleagues reported that 80% of these patients had asymptomatic to moderate disease from COVID-19, while 1 in 5 experienced severe or critical illness and almost 4% died – four times the mortality rate observed in published cohorts of general pediatric patients.
The results highlight that “children and adolescents with cancer generally recover without incident from COVID-19, but can have a severe course of infection,” the authors concluded.
And knowing that some children can get very sick, investigators wanted “to identify who these patients are so that we can prioritize and protect that group,” she added.
Echoing that sentiment, Kathy Pritchard-Jones, MD, president of the International Society of Paediatric Oncology and coauthor on the study, noted in a press release that, “by working together to create this global registry, we have enabled hospitals around the world to rapidly share and learn how COVID-19 is affecting children with cancer.”
Dr. Pritchard-Jones commented that overall these results provide reassurance that “many children can continue their cancer treatment safely, but they also highlight important clinical features that may predict a more severe clinical course and the need for greater vigilance for some patients.”
Inside the Global Registry data
The Global Registry of COVID-19 in Childhood Cancer, created jointly by St. Jude Children’s Research Hospital and SIOP, included data from 131 institutions in 45 countries. Children recruited into the registry between April 2020 and February 2021 ranged in age from infancy to 18 years old.
Most patients remained asymptomatic (35%) or experienced mild to moderate illness (45%), though 20% did develop severe or critical illness.
The investigators highlighted several factors associated with a greater risk of developing more severe illness from COVID-19, which included cancer type, intensity of therapy, age, absolute lymphocyte count, and presence of comorbidities or COVID-19 symptoms.
Notably, more than 80% of either severe or critical infections occurred in patients with hematologic malignancies – with 56% of cases in patients with acute lymphoblastic lymphoma or acute lymphoblastic leukemia – followed by extracranial solid tumors (15.8%), and central nervous system tumors (2.7%).
In patients with acute lymphoblastic leukemia or acute lymphoblastic lymphoma, severe or critical disease was most common in those receiving induction therapy (30%), relapse or refractory therapy (30%), and those in the maintenance or continuation phase of therapy (19%).
Older age was associated with a higher likelihood of having severe disease – with the lowest risk in infants (9.7%) and the highest in the 15- to 18-year-old cohort (27.3%).
Patients with lymphopenia who had an absolute lymphocyte count of 300 cells per mm3 or less and an absolute neutrophil count of 500 cells per mm3 or more also had an elevated risk of severe illness from COVID-19.
Regarding whether the presence of lymphopenia or neutropenia should change the treatment approach, Dr. Mukkada noted that, when possible, these patients should receive antiviral treatment, such as remdesivir, if the center has antivirals, or be prioritized for hospital admission.
Modifying cancer treatment might be recommended if patients are highly lymphopenic or have very low neutrophil counts, but a more effective strategy is simply to ensure that age-eligible children and adolescents with cancer or who have had a hematopoietic stem-cell transplantation have been fully vaccinated against COVID-19. For children who are not yet age-eligible, everyone around them should be vaccinated.
Pediatric patients in low- and middle-income countries were also more likely to have severe or critical outcomes from COVID-19 (41.7%), compared with patients in other income groups (23.9%).
The impact of COVID-19 “has been felt in every corner of the world, but particularly in low- and middle-income countries, compared to high-income countries,” senior author Carlos Rodriguez-Galindo, MD, global director at St. Jude, said in a statement.
In terms of the intersection of cancer treatment and COVID diagnosis, almost 83% of pediatric patients were receiving treatment for their cancer. Chemotherapy was withheld in about 45% of these patients and some modification to the treatment regimen occurred in almost 56% of participants on active therapy.
“Treatment modifications were least common in patients from upper-middle–income countries, compared with other income groups,” the authors wrote.
Although an interesting observation, Dr. Mukkada noted that the registry data could not explain why treatment modifications occurred less frequently in upper-middle income countries as opposed to high-income and lower-income countries.
U.K. Monitoring Project
Not all studies, however, have found that COVID-19 infection is significantly more severe in children with cancer. In a 2020 report from the U.K. Paediatric Coronavirus Cancer Monitoring Project, researchers evaluated all children in the United Kingdom under the age of 16 diagnosed with COVID and cancer.
“[Given that] we had complete coverage of every center in the U.K. that cares for children with cancer, we are confident that we picked up at least all the severe or critical cases,” lead author Gerard Millen, MD, honorary clinical research fellow, University of Birmingham (England), said in an interview.
Between March 2020 and July 2020, Dr. Millen and colleagues identified 54 positive cases of COVID-19, 15 (28%) of which were asymptomatic, 34 (63%) mild, and 4 (7.4%) severe or critical – more in line with the incidence of severe illness reported in the general pediatric population.
“Thankfully, we had no children with cancer in the U.K. who died from COVID-19,” Dr. Millen noted. “Overall, in the U.K., we have taken the approach that the majority of children with cancer in this country are at very low risk from COVID-19 and that we do not have good evidence to modify their treatment.”
Dr. Millen pointed out that the data in the U.K. study were “remarkably similar” to those from the high-income countries in the global St. Jude/SIOP cohort, where 7.4% of patients in that cohort had severe or critical disease, compared with 7.4% of patients from their own U.K. cohort.
“I think many of the key differences between the two cohorts reflect the fact that access to treatment in many low- to middle-income countries is more challenging with many factors contributing to overall poorer outcomes for both cancer and noncancer metrics,” Dr. Millen said.
Both the U.K. and registry studies were performed prior to vaccinations becoming available to older children, and before the emergence of certain variants, including the Delta variant, which is responsible for the most recent surge of COVID-19 infections around the world.
Data on COVID-19 vaccination in children with cancer are limited but promising so far.
As for whether the Delta variant might affect outcomes for children with cancer and COVID-19, Dr. Mukkada could only speculate, but she noted that “what we are hearing anecdotally about the [Delta] disease being more severe, even in patients who don’t have cancer, is leading us to say that we can’t close the registry yet. We are still actively enrolling children.”
The study was funded by the American Lebanese Syrian Associated Charities and the National Cancer Institute. The study authors and Dr. Millen disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Although most children and adolescents with cancer have mild illness from COVID-19 infection, some do experience severe disease and a small percentage even die, according to a recent analysis.
The findings, published online in Lancet Oncology, represent the first global registry data spanning different income groups to report COVID-19 outcomes in pediatric oncology patients.
“We wanted to create a global pool of evidence to answer the question: Do we see severe [COVID-19] infection [in children with cancer]?” corresponding author Sheena Mukkada, MD, St. Jude Children’s Research Hospital, Memphis, said in an interview.
In a cohort of 1,319 pediatric patients followed for 30 days, Dr. Mukkada and colleagues reported that 80% of these patients had asymptomatic to moderate disease from COVID-19, while 1 in 5 experienced severe or critical illness and almost 4% died – four times the mortality rate observed in published cohorts of general pediatric patients.
The results highlight that “children and adolescents with cancer generally recover without incident from COVID-19, but can have a severe course of infection,” the authors concluded.
And knowing that some children can get very sick, investigators wanted “to identify who these patients are so that we can prioritize and protect that group,” she added.
Echoing that sentiment, Kathy Pritchard-Jones, MD, president of the International Society of Paediatric Oncology and coauthor on the study, noted in a press release that, “by working together to create this global registry, we have enabled hospitals around the world to rapidly share and learn how COVID-19 is affecting children with cancer.”
Dr. Pritchard-Jones commented that overall these results provide reassurance that “many children can continue their cancer treatment safely, but they also highlight important clinical features that may predict a more severe clinical course and the need for greater vigilance for some patients.”
Inside the Global Registry data
The Global Registry of COVID-19 in Childhood Cancer, created jointly by St. Jude Children’s Research Hospital and SIOP, included data from 131 institutions in 45 countries. Children recruited into the registry between April 2020 and February 2021 ranged in age from infancy to 18 years old.
Most patients remained asymptomatic (35%) or experienced mild to moderate illness (45%), though 20% did develop severe or critical illness.
The investigators highlighted several factors associated with a greater risk of developing more severe illness from COVID-19, which included cancer type, intensity of therapy, age, absolute lymphocyte count, and presence of comorbidities or COVID-19 symptoms.
Notably, more than 80% of either severe or critical infections occurred in patients with hematologic malignancies – with 56% of cases in patients with acute lymphoblastic lymphoma or acute lymphoblastic leukemia – followed by extracranial solid tumors (15.8%), and central nervous system tumors (2.7%).
In patients with acute lymphoblastic leukemia or acute lymphoblastic lymphoma, severe or critical disease was most common in those receiving induction therapy (30%), relapse or refractory therapy (30%), and those in the maintenance or continuation phase of therapy (19%).
Older age was associated with a higher likelihood of having severe disease – with the lowest risk in infants (9.7%) and the highest in the 15- to 18-year-old cohort (27.3%).
Patients with lymphopenia who had an absolute lymphocyte count of 300 cells per mm3 or less and an absolute neutrophil count of 500 cells per mm3 or more also had an elevated risk of severe illness from COVID-19.
Regarding whether the presence of lymphopenia or neutropenia should change the treatment approach, Dr. Mukkada noted that, when possible, these patients should receive antiviral treatment, such as remdesivir, if the center has antivirals, or be prioritized for hospital admission.
Modifying cancer treatment might be recommended if patients are highly lymphopenic or have very low neutrophil counts, but a more effective strategy is simply to ensure that age-eligible children and adolescents with cancer or who have had a hematopoietic stem-cell transplantation have been fully vaccinated against COVID-19. For children who are not yet age-eligible, everyone around them should be vaccinated.
Pediatric patients in low- and middle-income countries were also more likely to have severe or critical outcomes from COVID-19 (41.7%), compared with patients in other income groups (23.9%).
The impact of COVID-19 “has been felt in every corner of the world, but particularly in low- and middle-income countries, compared to high-income countries,” senior author Carlos Rodriguez-Galindo, MD, global director at St. Jude, said in a statement.
In terms of the intersection of cancer treatment and COVID diagnosis, almost 83% of pediatric patients were receiving treatment for their cancer. Chemotherapy was withheld in about 45% of these patients and some modification to the treatment regimen occurred in almost 56% of participants on active therapy.
“Treatment modifications were least common in patients from upper-middle–income countries, compared with other income groups,” the authors wrote.
Although an interesting observation, Dr. Mukkada noted that the registry data could not explain why treatment modifications occurred less frequently in upper-middle income countries as opposed to high-income and lower-income countries.
U.K. Monitoring Project
Not all studies, however, have found that COVID-19 infection is significantly more severe in children with cancer. In a 2020 report from the U.K. Paediatric Coronavirus Cancer Monitoring Project, researchers evaluated all children in the United Kingdom under the age of 16 diagnosed with COVID and cancer.
“[Given that] we had complete coverage of every center in the U.K. that cares for children with cancer, we are confident that we picked up at least all the severe or critical cases,” lead author Gerard Millen, MD, honorary clinical research fellow, University of Birmingham (England), said in an interview.
Between March 2020 and July 2020, Dr. Millen and colleagues identified 54 positive cases of COVID-19, 15 (28%) of which were asymptomatic, 34 (63%) mild, and 4 (7.4%) severe or critical – more in line with the incidence of severe illness reported in the general pediatric population.
“Thankfully, we had no children with cancer in the U.K. who died from COVID-19,” Dr. Millen noted. “Overall, in the U.K., we have taken the approach that the majority of children with cancer in this country are at very low risk from COVID-19 and that we do not have good evidence to modify their treatment.”
Dr. Millen pointed out that the data in the U.K. study were “remarkably similar” to those from the high-income countries in the global St. Jude/SIOP cohort, where 7.4% of patients in that cohort had severe or critical disease, compared with 7.4% of patients from their own U.K. cohort.
“I think many of the key differences between the two cohorts reflect the fact that access to treatment in many low- to middle-income countries is more challenging with many factors contributing to overall poorer outcomes for both cancer and noncancer metrics,” Dr. Millen said.
Both the U.K. and registry studies were performed prior to vaccinations becoming available to older children, and before the emergence of certain variants, including the Delta variant, which is responsible for the most recent surge of COVID-19 infections around the world.
Data on COVID-19 vaccination in children with cancer are limited but promising so far.
As for whether the Delta variant might affect outcomes for children with cancer and COVID-19, Dr. Mukkada could only speculate, but she noted that “what we are hearing anecdotally about the [Delta] disease being more severe, even in patients who don’t have cancer, is leading us to say that we can’t close the registry yet. We are still actively enrolling children.”
The study was funded by the American Lebanese Syrian Associated Charities and the National Cancer Institute. The study authors and Dr. Millen disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Although most children and adolescents with cancer have mild illness from COVID-19 infection, some do experience severe disease and a small percentage even die, according to a recent analysis.
The findings, published online in Lancet Oncology, represent the first global registry data spanning different income groups to report COVID-19 outcomes in pediatric oncology patients.
“We wanted to create a global pool of evidence to answer the question: Do we see severe [COVID-19] infection [in children with cancer]?” corresponding author Sheena Mukkada, MD, St. Jude Children’s Research Hospital, Memphis, said in an interview.
In a cohort of 1,319 pediatric patients followed for 30 days, Dr. Mukkada and colleagues reported that 80% of these patients had asymptomatic to moderate disease from COVID-19, while 1 in 5 experienced severe or critical illness and almost 4% died – four times the mortality rate observed in published cohorts of general pediatric patients.
The results highlight that “children and adolescents with cancer generally recover without incident from COVID-19, but can have a severe course of infection,” the authors concluded.
And knowing that some children can get very sick, investigators wanted “to identify who these patients are so that we can prioritize and protect that group,” she added.
Echoing that sentiment, Kathy Pritchard-Jones, MD, president of the International Society of Paediatric Oncology and coauthor on the study, noted in a press release that, “by working together to create this global registry, we have enabled hospitals around the world to rapidly share and learn how COVID-19 is affecting children with cancer.”
Dr. Pritchard-Jones commented that overall these results provide reassurance that “many children can continue their cancer treatment safely, but they also highlight important clinical features that may predict a more severe clinical course and the need for greater vigilance for some patients.”
Inside the Global Registry data
The Global Registry of COVID-19 in Childhood Cancer, created jointly by St. Jude Children’s Research Hospital and SIOP, included data from 131 institutions in 45 countries. Children recruited into the registry between April 2020 and February 2021 ranged in age from infancy to 18 years old.
Most patients remained asymptomatic (35%) or experienced mild to moderate illness (45%), though 20% did develop severe or critical illness.
The investigators highlighted several factors associated with a greater risk of developing more severe illness from COVID-19, which included cancer type, intensity of therapy, age, absolute lymphocyte count, and presence of comorbidities or COVID-19 symptoms.
Notably, more than 80% of either severe or critical infections occurred in patients with hematologic malignancies – with 56% of cases in patients with acute lymphoblastic lymphoma or acute lymphoblastic leukemia – followed by extracranial solid tumors (15.8%), and central nervous system tumors (2.7%).
In patients with acute lymphoblastic leukemia or acute lymphoblastic lymphoma, severe or critical disease was most common in those receiving induction therapy (30%), relapse or refractory therapy (30%), and those in the maintenance or continuation phase of therapy (19%).
Older age was associated with a higher likelihood of having severe disease – with the lowest risk in infants (9.7%) and the highest in the 15- to 18-year-old cohort (27.3%).
Patients with lymphopenia who had an absolute lymphocyte count of 300 cells per mm3 or less and an absolute neutrophil count of 500 cells per mm3 or more also had an elevated risk of severe illness from COVID-19.
Regarding whether the presence of lymphopenia or neutropenia should change the treatment approach, Dr. Mukkada noted that, when possible, these patients should receive antiviral treatment, such as remdesivir, if the center has antivirals, or be prioritized for hospital admission.
Modifying cancer treatment might be recommended if patients are highly lymphopenic or have very low neutrophil counts, but a more effective strategy is simply to ensure that age-eligible children and adolescents with cancer or who have had a hematopoietic stem-cell transplantation have been fully vaccinated against COVID-19. For children who are not yet age-eligible, everyone around them should be vaccinated.
Pediatric patients in low- and middle-income countries were also more likely to have severe or critical outcomes from COVID-19 (41.7%), compared with patients in other income groups (23.9%).
The impact of COVID-19 “has been felt in every corner of the world, but particularly in low- and middle-income countries, compared to high-income countries,” senior author Carlos Rodriguez-Galindo, MD, global director at St. Jude, said in a statement.
In terms of the intersection of cancer treatment and COVID diagnosis, almost 83% of pediatric patients were receiving treatment for their cancer. Chemotherapy was withheld in about 45% of these patients and some modification to the treatment regimen occurred in almost 56% of participants on active therapy.
“Treatment modifications were least common in patients from upper-middle–income countries, compared with other income groups,” the authors wrote.
Although an interesting observation, Dr. Mukkada noted that the registry data could not explain why treatment modifications occurred less frequently in upper-middle income countries as opposed to high-income and lower-income countries.
U.K. Monitoring Project
Not all studies, however, have found that COVID-19 infection is significantly more severe in children with cancer. In a 2020 report from the U.K. Paediatric Coronavirus Cancer Monitoring Project, researchers evaluated all children in the United Kingdom under the age of 16 diagnosed with COVID and cancer.
“[Given that] we had complete coverage of every center in the U.K. that cares for children with cancer, we are confident that we picked up at least all the severe or critical cases,” lead author Gerard Millen, MD, honorary clinical research fellow, University of Birmingham (England), said in an interview.
Between March 2020 and July 2020, Dr. Millen and colleagues identified 54 positive cases of COVID-19, 15 (28%) of which were asymptomatic, 34 (63%) mild, and 4 (7.4%) severe or critical – more in line with the incidence of severe illness reported in the general pediatric population.
“Thankfully, we had no children with cancer in the U.K. who died from COVID-19,” Dr. Millen noted. “Overall, in the U.K., we have taken the approach that the majority of children with cancer in this country are at very low risk from COVID-19 and that we do not have good evidence to modify their treatment.”
Dr. Millen pointed out that the data in the U.K. study were “remarkably similar” to those from the high-income countries in the global St. Jude/SIOP cohort, where 7.4% of patients in that cohort had severe or critical disease, compared with 7.4% of patients from their own U.K. cohort.
“I think many of the key differences between the two cohorts reflect the fact that access to treatment in many low- to middle-income countries is more challenging with many factors contributing to overall poorer outcomes for both cancer and noncancer metrics,” Dr. Millen said.
Both the U.K. and registry studies were performed prior to vaccinations becoming available to older children, and before the emergence of certain variants, including the Delta variant, which is responsible for the most recent surge of COVID-19 infections around the world.
Data on COVID-19 vaccination in children with cancer are limited but promising so far.
As for whether the Delta variant might affect outcomes for children with cancer and COVID-19, Dr. Mukkada could only speculate, but she noted that “what we are hearing anecdotally about the [Delta] disease being more severe, even in patients who don’t have cancer, is leading us to say that we can’t close the registry yet. We are still actively enrolling children.”
The study was funded by the American Lebanese Syrian Associated Charities and the National Cancer Institute. The study authors and Dr. Millen disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.