User login
Twelve-month overall survival benefit with ribociclib for metastatic breast cancer
“Based on these results, ribociclib and letrozole should be considered the preferred treatment option,” said lead investigator Gabriel N. Hortobagyi, MD, a breast cancer medical oncologist at the University of Texas MD Anderson Cancer Center, Houston.
He presented the definitive overall survival results from MONALESSA-2 which randomized 668 patients equally and in the first line to either ribociclib or placebo on a background of standard dose letrozole.
At a median follow up of 6.6 years, median overall survival with ribociclib was 63.9 months versus 51.4 months in the placebo arm, a 24% reduction in the relative risk of death (P = .004).
It was the first report of a median overall survival (OS) exceeding 5 years in a phase 3 trial for advanced breast cancer. The estimated 6-year OS rate was 44.2% for ribociclib versus 32.0% with placebo.
“These are really impressive results” and support the use of CDK 4/6 inhibitors in the front-line setting,” said study discussant Gonzalo Gomez Abuin, MD, a medical oncologist at Hospital Alemán in Bueno Aires.
Ribociclib and other CDK 4/6 inhibitors have shown consistent progression-free survival benefit for metastatic disease, but ribociclib is the first of the major phase 3 trials with definitive overall survival results. They have “been long awaited,” Dr. Abuin said.
The overall survival benefit in MONALESSA-2 began to emerge at around 20 months and continued to increase over time.
Women had no prior CDK4/6 inhibitor treatment, chemotherapy, or endocrine therapy for metastatic disease. “They represented a pure first-line population,” Dr. Hortobagyi said.
Among other benefits, the time to first chemotherapy was a median of 50.6 months with ribociclib versus 38.9 months with placebo, so patients “had an extra year of delay before chemotherapy was utilized,” he said.
In general, Dr. Abuin said, we “see a consistent benefit with CDK 4/6 inhibitors in metastatic breast cancer across different settings.”
However, “it’s a little intriguing” that in a subgroup analysis of non–de novo disease, the overall survival benefit with ribociclib had a hazard ratio of 0.91, whereas the progression-free survival benefit was robust and statistically significant in an earlier report.
“This has been an important question, but I would caution all of us not to make too much out of the forest plot,” Dr. Hortobagyi said.
“There are a number of hypotheses one could come up with that could explain why the de novo and non–de novo populations faired differently in overall survival as opposed to progression-free survival, but there is also the simple possibility that this is a statistical fluke,” he said.
“We are in the process of analyzing this particular observation. In the meantime, I think we should just take the overall survival results of the entire population as the lead answer, and not follow the subgroup analysis until further information is available,” Dr. Hortobagyi said.
No new ribociclib safety signals were observed in the trial. The most common adverse events were neutropenia and liver function abnormalities, but they were “largely asymptomatic laboratory findings and completely reversible,” he said.
Twice as many patients treated with ribociclib developed prolonged QT intervals, but again, “no clinical consequences of this EKG finding were detected,” Dr. Hortobagyi said.
Less than 1% of patients in the ribociclib arm developed interstitial lung disease. The majority of safety events occurred in the first 12 months of treatment.
The work was funded by Novartis, maker of both ribociclib and letrozole. Dr. Hortobagyi reported receiving an institutional grant from the company and personal fees related to the trial. Other investigators disclosed ties to Novartis. Dr. Abuin reported relationships with many companies, including Novartis.
This article was updated 9/24/21.
“Based on these results, ribociclib and letrozole should be considered the preferred treatment option,” said lead investigator Gabriel N. Hortobagyi, MD, a breast cancer medical oncologist at the University of Texas MD Anderson Cancer Center, Houston.
He presented the definitive overall survival results from MONALESSA-2 which randomized 668 patients equally and in the first line to either ribociclib or placebo on a background of standard dose letrozole.
At a median follow up of 6.6 years, median overall survival with ribociclib was 63.9 months versus 51.4 months in the placebo arm, a 24% reduction in the relative risk of death (P = .004).
It was the first report of a median overall survival (OS) exceeding 5 years in a phase 3 trial for advanced breast cancer. The estimated 6-year OS rate was 44.2% for ribociclib versus 32.0% with placebo.
“These are really impressive results” and support the use of CDK 4/6 inhibitors in the front-line setting,” said study discussant Gonzalo Gomez Abuin, MD, a medical oncologist at Hospital Alemán in Bueno Aires.
Ribociclib and other CDK 4/6 inhibitors have shown consistent progression-free survival benefit for metastatic disease, but ribociclib is the first of the major phase 3 trials with definitive overall survival results. They have “been long awaited,” Dr. Abuin said.
The overall survival benefit in MONALESSA-2 began to emerge at around 20 months and continued to increase over time.
Women had no prior CDK4/6 inhibitor treatment, chemotherapy, or endocrine therapy for metastatic disease. “They represented a pure first-line population,” Dr. Hortobagyi said.
Among other benefits, the time to first chemotherapy was a median of 50.6 months with ribociclib versus 38.9 months with placebo, so patients “had an extra year of delay before chemotherapy was utilized,” he said.
In general, Dr. Abuin said, we “see a consistent benefit with CDK 4/6 inhibitors in metastatic breast cancer across different settings.”
However, “it’s a little intriguing” that in a subgroup analysis of non–de novo disease, the overall survival benefit with ribociclib had a hazard ratio of 0.91, whereas the progression-free survival benefit was robust and statistically significant in an earlier report.
“This has been an important question, but I would caution all of us not to make too much out of the forest plot,” Dr. Hortobagyi said.
“There are a number of hypotheses one could come up with that could explain why the de novo and non–de novo populations faired differently in overall survival as opposed to progression-free survival, but there is also the simple possibility that this is a statistical fluke,” he said.
“We are in the process of analyzing this particular observation. In the meantime, I think we should just take the overall survival results of the entire population as the lead answer, and not follow the subgroup analysis until further information is available,” Dr. Hortobagyi said.
No new ribociclib safety signals were observed in the trial. The most common adverse events were neutropenia and liver function abnormalities, but they were “largely asymptomatic laboratory findings and completely reversible,” he said.
Twice as many patients treated with ribociclib developed prolonged QT intervals, but again, “no clinical consequences of this EKG finding were detected,” Dr. Hortobagyi said.
Less than 1% of patients in the ribociclib arm developed interstitial lung disease. The majority of safety events occurred in the first 12 months of treatment.
The work was funded by Novartis, maker of both ribociclib and letrozole. Dr. Hortobagyi reported receiving an institutional grant from the company and personal fees related to the trial. Other investigators disclosed ties to Novartis. Dr. Abuin reported relationships with many companies, including Novartis.
This article was updated 9/24/21.
“Based on these results, ribociclib and letrozole should be considered the preferred treatment option,” said lead investigator Gabriel N. Hortobagyi, MD, a breast cancer medical oncologist at the University of Texas MD Anderson Cancer Center, Houston.
He presented the definitive overall survival results from MONALESSA-2 which randomized 668 patients equally and in the first line to either ribociclib or placebo on a background of standard dose letrozole.
At a median follow up of 6.6 years, median overall survival with ribociclib was 63.9 months versus 51.4 months in the placebo arm, a 24% reduction in the relative risk of death (P = .004).
It was the first report of a median overall survival (OS) exceeding 5 years in a phase 3 trial for advanced breast cancer. The estimated 6-year OS rate was 44.2% for ribociclib versus 32.0% with placebo.
“These are really impressive results” and support the use of CDK 4/6 inhibitors in the front-line setting,” said study discussant Gonzalo Gomez Abuin, MD, a medical oncologist at Hospital Alemán in Bueno Aires.
Ribociclib and other CDK 4/6 inhibitors have shown consistent progression-free survival benefit for metastatic disease, but ribociclib is the first of the major phase 3 trials with definitive overall survival results. They have “been long awaited,” Dr. Abuin said.
The overall survival benefit in MONALESSA-2 began to emerge at around 20 months and continued to increase over time.
Women had no prior CDK4/6 inhibitor treatment, chemotherapy, or endocrine therapy for metastatic disease. “They represented a pure first-line population,” Dr. Hortobagyi said.
Among other benefits, the time to first chemotherapy was a median of 50.6 months with ribociclib versus 38.9 months with placebo, so patients “had an extra year of delay before chemotherapy was utilized,” he said.
In general, Dr. Abuin said, we “see a consistent benefit with CDK 4/6 inhibitors in metastatic breast cancer across different settings.”
However, “it’s a little intriguing” that in a subgroup analysis of non–de novo disease, the overall survival benefit with ribociclib had a hazard ratio of 0.91, whereas the progression-free survival benefit was robust and statistically significant in an earlier report.
“This has been an important question, but I would caution all of us not to make too much out of the forest plot,” Dr. Hortobagyi said.
“There are a number of hypotheses one could come up with that could explain why the de novo and non–de novo populations faired differently in overall survival as opposed to progression-free survival, but there is also the simple possibility that this is a statistical fluke,” he said.
“We are in the process of analyzing this particular observation. In the meantime, I think we should just take the overall survival results of the entire population as the lead answer, and not follow the subgroup analysis until further information is available,” Dr. Hortobagyi said.
No new ribociclib safety signals were observed in the trial. The most common adverse events were neutropenia and liver function abnormalities, but they were “largely asymptomatic laboratory findings and completely reversible,” he said.
Twice as many patients treated with ribociclib developed prolonged QT intervals, but again, “no clinical consequences of this EKG finding were detected,” Dr. Hortobagyi said.
Less than 1% of patients in the ribociclib arm developed interstitial lung disease. The majority of safety events occurred in the first 12 months of treatment.
The work was funded by Novartis, maker of both ribociclib and letrozole. Dr. Hortobagyi reported receiving an institutional grant from the company and personal fees related to the trial. Other investigators disclosed ties to Novartis. Dr. Abuin reported relationships with many companies, including Novartis.
This article was updated 9/24/21.
FROM ESMO 2021
Nurses ‘at the breaking point,’ consider quitting due to COVID issues: Survey
As hospitals have been flooded with critically ill patients, nurses have been overwhelmed.
“What we’re hearing from our nurses is really shocking,” Amanda Bettencourt, PhD, APRN, CCRN-K, president-elect of the American Association of Critical-Care Nurses (AACN), said in an interview. “They’re saying they’re at the breaking point.”
Between Aug. 26 and Aug. 30, the AACN surveyed more than 6,000 critical care nurses, zeroing in on four key questions regarding the pandemic and its impact on nursing. The results were alarming – not only with regard to individual nurses but also for the nursing profession and the future of health care. A full 66% of those surveyed said their experiences during the pandemic have caused them to consider leaving nursing. The respondents’ take on their colleagues was even more concerning. Ninety-two percent agreed with the following two statements: “I believe the pandemic has depleted nurses at my hospital. Their careers will be shorter than they intended.”
“This puts the entire health care system at risk,” says Dr. Bettencourt, assistant professor in the department of family and community health at the University of Pennsylvania School of Nursing, Philadelphia. Intensive care unit (ICU) nurses are highly trained and are skilled in caring for critically ill patients with complex medical needs. “It’s not easy to replace a critical care nurse when one leaves,” she said.
And when nurses leave, patients suffer, said Beth Wathen, MSN, RN, CCRN-K, president of the ACCN and frontline nurse at Children’s Hospital Colorado, in Aurora. “Hospitals can have all the beds and all the rooms and all the equipment they want, but without nurses and others at the front lines to provide that essential care, none of it really matters, whether we’re talking about caring for COVID patients or caring for patients with other health ailments.”
Heartbreak of the unvaccinated
The problem is not just overwork because of the flood of COVID-19 patients. The emotional strain is enormous as well. “What’s demoralizing for us is not that patients are sick and that it’s physically exhausting to take care of sick patients. We’re used to that,” said Dr. Bettencourt.
But few nurses have experienced the sheer magnitude of patients caused by this pandemic. “The past 18 months have been grueling,” says Ms. Wathen. “The burden on frontline caregivers and our nurses at the front line has been immense.”
The situation is made worse by how unnecessary much of the suffering is at this point. Seventy-six percent of the survey’s respondents agreed with the following statement: “People who hold out on getting vaccinated undermine nurses’ physical and mental well-being.” That comment doesn’t convey the nature or extent of the effect on caregivers’ well-being. “That 9 out of 10 of the people we’re seeing in ICU right now are unvaccinated just adds to the sense of heartbreak and frustration,” says Ms. Wathen. “These deaths don’t have to be happening right now. And that’s hard to bear witness to.”
The politicization of public health has also taken a toll. “That’s been the hard part of this entire pandemic,” says Ms. Wathen. “This really isn’t at all about politics. This is about your health; this is about my health. This is about our collective health as a community and as a country.”
Like the rest of the world, nurses are also concerned about their own loved ones. The survey statement, “I fear taking care of patients with COVID puts my family’s health at risk,” garnered 67% agreement. Ms. Wathen points out that nurses take the appropriate precautions but still worry about taking infection home to their families. “This disease is a tricky one,” she says. She points out that until this pandemic is over, in addition to being vaccinated, nurses and the public still need to be vigilant about wearing masks, social distancing, and taking other precautions to ensure the safety of us all. “Our individual decisions don’t just affect ourselves. They affect our family, the people in our circle, and the people in our community,” she said.
Avoiding a professional exodus
It’s too early yet to have reliable national data on how many nurses have already left their jobs because of COVID-19, but it is clear that there are too few nurses of all kinds. The American Nurses Association sent a letter to the U.S. Secretary of Health and Human Services urging the agency to declare the nursing shortage a crisis and to take immediate steps to find solutions.
The nursing shortage predates the pandemic, and COVID-19 has brought a simmering problem to the boil. Nurses are calling on the public and the health care system for help. From inside the industry, the needs are pretty much what they were before the pandemic. Dr. Bettencourt and Ms. Wathen point to the need for supportive leadership, healthy work environments, sufficient staffing to meet patients’ needs, and a voice in decisions, such as decisions about staffing, that affect nurses and their patients. Nurses want to be heard and appreciated. “It’s not that these are new things,” said Dr. Bettencourt. “We just need them even more now because we’re stressed even more than we were before.”
Critical care nurses have a different request of the public. They’re asking – pleading, actually – with the public to get vaccinated, wear masks in public, practice social distancing, and bring this pandemic to an end.
“COVID kills, and it’s a really difficult, tragic, and lonely death,” said Ms. Wathen. “We’ve witnessed hundreds of thousands of those deaths. But now we have a way to stop it. If many more people get vaccinated, we can stop this pandemic. And hopefully that will stop this current trend of nurses leaving.”
A version of this article first appeared on Medscape.com.
As hospitals have been flooded with critically ill patients, nurses have been overwhelmed.
“What we’re hearing from our nurses is really shocking,” Amanda Bettencourt, PhD, APRN, CCRN-K, president-elect of the American Association of Critical-Care Nurses (AACN), said in an interview. “They’re saying they’re at the breaking point.”
Between Aug. 26 and Aug. 30, the AACN surveyed more than 6,000 critical care nurses, zeroing in on four key questions regarding the pandemic and its impact on nursing. The results were alarming – not only with regard to individual nurses but also for the nursing profession and the future of health care. A full 66% of those surveyed said their experiences during the pandemic have caused them to consider leaving nursing. The respondents’ take on their colleagues was even more concerning. Ninety-two percent agreed with the following two statements: “I believe the pandemic has depleted nurses at my hospital. Their careers will be shorter than they intended.”
“This puts the entire health care system at risk,” says Dr. Bettencourt, assistant professor in the department of family and community health at the University of Pennsylvania School of Nursing, Philadelphia. Intensive care unit (ICU) nurses are highly trained and are skilled in caring for critically ill patients with complex medical needs. “It’s not easy to replace a critical care nurse when one leaves,” she said.
And when nurses leave, patients suffer, said Beth Wathen, MSN, RN, CCRN-K, president of the ACCN and frontline nurse at Children’s Hospital Colorado, in Aurora. “Hospitals can have all the beds and all the rooms and all the equipment they want, but without nurses and others at the front lines to provide that essential care, none of it really matters, whether we’re talking about caring for COVID patients or caring for patients with other health ailments.”
Heartbreak of the unvaccinated
The problem is not just overwork because of the flood of COVID-19 patients. The emotional strain is enormous as well. “What’s demoralizing for us is not that patients are sick and that it’s physically exhausting to take care of sick patients. We’re used to that,” said Dr. Bettencourt.
But few nurses have experienced the sheer magnitude of patients caused by this pandemic. “The past 18 months have been grueling,” says Ms. Wathen. “The burden on frontline caregivers and our nurses at the front line has been immense.”
The situation is made worse by how unnecessary much of the suffering is at this point. Seventy-six percent of the survey’s respondents agreed with the following statement: “People who hold out on getting vaccinated undermine nurses’ physical and mental well-being.” That comment doesn’t convey the nature or extent of the effect on caregivers’ well-being. “That 9 out of 10 of the people we’re seeing in ICU right now are unvaccinated just adds to the sense of heartbreak and frustration,” says Ms. Wathen. “These deaths don’t have to be happening right now. And that’s hard to bear witness to.”
The politicization of public health has also taken a toll. “That’s been the hard part of this entire pandemic,” says Ms. Wathen. “This really isn’t at all about politics. This is about your health; this is about my health. This is about our collective health as a community and as a country.”
Like the rest of the world, nurses are also concerned about their own loved ones. The survey statement, “I fear taking care of patients with COVID puts my family’s health at risk,” garnered 67% agreement. Ms. Wathen points out that nurses take the appropriate precautions but still worry about taking infection home to their families. “This disease is a tricky one,” she says. She points out that until this pandemic is over, in addition to being vaccinated, nurses and the public still need to be vigilant about wearing masks, social distancing, and taking other precautions to ensure the safety of us all. “Our individual decisions don’t just affect ourselves. They affect our family, the people in our circle, and the people in our community,” she said.
Avoiding a professional exodus
It’s too early yet to have reliable national data on how many nurses have already left their jobs because of COVID-19, but it is clear that there are too few nurses of all kinds. The American Nurses Association sent a letter to the U.S. Secretary of Health and Human Services urging the agency to declare the nursing shortage a crisis and to take immediate steps to find solutions.
The nursing shortage predates the pandemic, and COVID-19 has brought a simmering problem to the boil. Nurses are calling on the public and the health care system for help. From inside the industry, the needs are pretty much what they were before the pandemic. Dr. Bettencourt and Ms. Wathen point to the need for supportive leadership, healthy work environments, sufficient staffing to meet patients’ needs, and a voice in decisions, such as decisions about staffing, that affect nurses and their patients. Nurses want to be heard and appreciated. “It’s not that these are new things,” said Dr. Bettencourt. “We just need them even more now because we’re stressed even more than we were before.”
Critical care nurses have a different request of the public. They’re asking – pleading, actually – with the public to get vaccinated, wear masks in public, practice social distancing, and bring this pandemic to an end.
“COVID kills, and it’s a really difficult, tragic, and lonely death,” said Ms. Wathen. “We’ve witnessed hundreds of thousands of those deaths. But now we have a way to stop it. If many more people get vaccinated, we can stop this pandemic. And hopefully that will stop this current trend of nurses leaving.”
A version of this article first appeared on Medscape.com.
As hospitals have been flooded with critically ill patients, nurses have been overwhelmed.
“What we’re hearing from our nurses is really shocking,” Amanda Bettencourt, PhD, APRN, CCRN-K, president-elect of the American Association of Critical-Care Nurses (AACN), said in an interview. “They’re saying they’re at the breaking point.”
Between Aug. 26 and Aug. 30, the AACN surveyed more than 6,000 critical care nurses, zeroing in on four key questions regarding the pandemic and its impact on nursing. The results were alarming – not only with regard to individual nurses but also for the nursing profession and the future of health care. A full 66% of those surveyed said their experiences during the pandemic have caused them to consider leaving nursing. The respondents’ take on their colleagues was even more concerning. Ninety-two percent agreed with the following two statements: “I believe the pandemic has depleted nurses at my hospital. Their careers will be shorter than they intended.”
“This puts the entire health care system at risk,” says Dr. Bettencourt, assistant professor in the department of family and community health at the University of Pennsylvania School of Nursing, Philadelphia. Intensive care unit (ICU) nurses are highly trained and are skilled in caring for critically ill patients with complex medical needs. “It’s not easy to replace a critical care nurse when one leaves,” she said.
And when nurses leave, patients suffer, said Beth Wathen, MSN, RN, CCRN-K, president of the ACCN and frontline nurse at Children’s Hospital Colorado, in Aurora. “Hospitals can have all the beds and all the rooms and all the equipment they want, but without nurses and others at the front lines to provide that essential care, none of it really matters, whether we’re talking about caring for COVID patients or caring for patients with other health ailments.”
Heartbreak of the unvaccinated
The problem is not just overwork because of the flood of COVID-19 patients. The emotional strain is enormous as well. “What’s demoralizing for us is not that patients are sick and that it’s physically exhausting to take care of sick patients. We’re used to that,” said Dr. Bettencourt.
But few nurses have experienced the sheer magnitude of patients caused by this pandemic. “The past 18 months have been grueling,” says Ms. Wathen. “The burden on frontline caregivers and our nurses at the front line has been immense.”
The situation is made worse by how unnecessary much of the suffering is at this point. Seventy-six percent of the survey’s respondents agreed with the following statement: “People who hold out on getting vaccinated undermine nurses’ physical and mental well-being.” That comment doesn’t convey the nature or extent of the effect on caregivers’ well-being. “That 9 out of 10 of the people we’re seeing in ICU right now are unvaccinated just adds to the sense of heartbreak and frustration,” says Ms. Wathen. “These deaths don’t have to be happening right now. And that’s hard to bear witness to.”
The politicization of public health has also taken a toll. “That’s been the hard part of this entire pandemic,” says Ms. Wathen. “This really isn’t at all about politics. This is about your health; this is about my health. This is about our collective health as a community and as a country.”
Like the rest of the world, nurses are also concerned about their own loved ones. The survey statement, “I fear taking care of patients with COVID puts my family’s health at risk,” garnered 67% agreement. Ms. Wathen points out that nurses take the appropriate precautions but still worry about taking infection home to their families. “This disease is a tricky one,” she says. She points out that until this pandemic is over, in addition to being vaccinated, nurses and the public still need to be vigilant about wearing masks, social distancing, and taking other precautions to ensure the safety of us all. “Our individual decisions don’t just affect ourselves. They affect our family, the people in our circle, and the people in our community,” she said.
Avoiding a professional exodus
It’s too early yet to have reliable national data on how many nurses have already left their jobs because of COVID-19, but it is clear that there are too few nurses of all kinds. The American Nurses Association sent a letter to the U.S. Secretary of Health and Human Services urging the agency to declare the nursing shortage a crisis and to take immediate steps to find solutions.
The nursing shortage predates the pandemic, and COVID-19 has brought a simmering problem to the boil. Nurses are calling on the public and the health care system for help. From inside the industry, the needs are pretty much what they were before the pandemic. Dr. Bettencourt and Ms. Wathen point to the need for supportive leadership, healthy work environments, sufficient staffing to meet patients’ needs, and a voice in decisions, such as decisions about staffing, that affect nurses and their patients. Nurses want to be heard and appreciated. “It’s not that these are new things,” said Dr. Bettencourt. “We just need them even more now because we’re stressed even more than we were before.”
Critical care nurses have a different request of the public. They’re asking – pleading, actually – with the public to get vaccinated, wear masks in public, practice social distancing, and bring this pandemic to an end.
“COVID kills, and it’s a really difficult, tragic, and lonely death,” said Ms. Wathen. “We’ve witnessed hundreds of thousands of those deaths. But now we have a way to stop it. If many more people get vaccinated, we can stop this pandemic. And hopefully that will stop this current trend of nurses leaving.”
A version of this article first appeared on Medscape.com.
Texas doctor admits to violating abortion ban
A Texas doctor revealed in a Washington Post op-ed Sept. 18 that he violated the state ban on abortions performed beyond 6 weeks -- a move he knows could come with legal consequences.
San Antonio doctor Alan Braid, MD, said the new statewide restrictions reminded him of darker days during his 1972 obstetrics and gynecology residency, when he saw three teenagers die from illegal abortions.
“For me, it is 1972 all over again,” he wrote. “And that is why, on the morning of Sept. 6, I provided an abortion to a woman who, though still in her first trimester, was beyond the state’s new limit. I acted because I had a duty of care to this patient, as I do for all patients, and because she has a fundamental right to receive this care.”
“I fully understood that there could be legal consequences -- but I wanted to make sure that Texas didn’t get away with its bid to prevent this blatantly unconstitutional law from being tested,” he continued.
According to The Washington Post, Dr. Braid’s wish may come true. Two lawsuits against were filed Sept. 20. In one, a prisoner in Arkansas said he filed the suit in part because he could receive $10,000 if successful, according to the Post. The second was filed by a man in Chicago who wants the law struck down.
Dr. Braid’s op-ed is the first public admission to violating a Texas state law that took effect Sept. 1 banning abortion once a fetal heartbeat is detected. The controversial policy gives private citizens the right to bring civil litigation -- resulting in at least $10,000 in damages -- against providers and anyone else involved in the process.
Since the law went into effect, most patients seeking abortions are too far along to qualify, Dr. Braid wrote.
“I tell them that we can offer services only if we cannot see the presence of cardiac activity on an ultrasound, which usually occurs at about six weeks, before most people know they are pregnant. The tension is unbearable as they lie there, waiting to hear their fate,” he wrote.
“I understand that by providing an abortion beyond the new legal limit, I am taking a personal risk, but it’s something I believe in strongly,” he continued. “Represented by the Center for Reproductive Rights, my clinics are among the plaintiffs in an ongoing federal lawsuit to stop S.B. 8.”
A version of this article first appeared on WebMD.com .
A Texas doctor revealed in a Washington Post op-ed Sept. 18 that he violated the state ban on abortions performed beyond 6 weeks -- a move he knows could come with legal consequences.
San Antonio doctor Alan Braid, MD, said the new statewide restrictions reminded him of darker days during his 1972 obstetrics and gynecology residency, when he saw three teenagers die from illegal abortions.
“For me, it is 1972 all over again,” he wrote. “And that is why, on the morning of Sept. 6, I provided an abortion to a woman who, though still in her first trimester, was beyond the state’s new limit. I acted because I had a duty of care to this patient, as I do for all patients, and because she has a fundamental right to receive this care.”
“I fully understood that there could be legal consequences -- but I wanted to make sure that Texas didn’t get away with its bid to prevent this blatantly unconstitutional law from being tested,” he continued.
According to The Washington Post, Dr. Braid’s wish may come true. Two lawsuits against were filed Sept. 20. In one, a prisoner in Arkansas said he filed the suit in part because he could receive $10,000 if successful, according to the Post. The second was filed by a man in Chicago who wants the law struck down.
Dr. Braid’s op-ed is the first public admission to violating a Texas state law that took effect Sept. 1 banning abortion once a fetal heartbeat is detected. The controversial policy gives private citizens the right to bring civil litigation -- resulting in at least $10,000 in damages -- against providers and anyone else involved in the process.
Since the law went into effect, most patients seeking abortions are too far along to qualify, Dr. Braid wrote.
“I tell them that we can offer services only if we cannot see the presence of cardiac activity on an ultrasound, which usually occurs at about six weeks, before most people know they are pregnant. The tension is unbearable as they lie there, waiting to hear their fate,” he wrote.
“I understand that by providing an abortion beyond the new legal limit, I am taking a personal risk, but it’s something I believe in strongly,” he continued. “Represented by the Center for Reproductive Rights, my clinics are among the plaintiffs in an ongoing federal lawsuit to stop S.B. 8.”
A version of this article first appeared on WebMD.com .
A Texas doctor revealed in a Washington Post op-ed Sept. 18 that he violated the state ban on abortions performed beyond 6 weeks -- a move he knows could come with legal consequences.
San Antonio doctor Alan Braid, MD, said the new statewide restrictions reminded him of darker days during his 1972 obstetrics and gynecology residency, when he saw three teenagers die from illegal abortions.
“For me, it is 1972 all over again,” he wrote. “And that is why, on the morning of Sept. 6, I provided an abortion to a woman who, though still in her first trimester, was beyond the state’s new limit. I acted because I had a duty of care to this patient, as I do for all patients, and because she has a fundamental right to receive this care.”
“I fully understood that there could be legal consequences -- but I wanted to make sure that Texas didn’t get away with its bid to prevent this blatantly unconstitutional law from being tested,” he continued.
According to The Washington Post, Dr. Braid’s wish may come true. Two lawsuits against were filed Sept. 20. In one, a prisoner in Arkansas said he filed the suit in part because he could receive $10,000 if successful, according to the Post. The second was filed by a man in Chicago who wants the law struck down.
Dr. Braid’s op-ed is the first public admission to violating a Texas state law that took effect Sept. 1 banning abortion once a fetal heartbeat is detected. The controversial policy gives private citizens the right to bring civil litigation -- resulting in at least $10,000 in damages -- against providers and anyone else involved in the process.
Since the law went into effect, most patients seeking abortions are too far along to qualify, Dr. Braid wrote.
“I tell them that we can offer services only if we cannot see the presence of cardiac activity on an ultrasound, which usually occurs at about six weeks, before most people know they are pregnant. The tension is unbearable as they lie there, waiting to hear their fate,” he wrote.
“I understand that by providing an abortion beyond the new legal limit, I am taking a personal risk, but it’s something I believe in strongly,” he continued. “Represented by the Center for Reproductive Rights, my clinics are among the plaintiffs in an ongoing federal lawsuit to stop S.B. 8.”
A version of this article first appeared on WebMD.com .
Embedding diversity, equity, inclusion, and justice in hospital medicine
A road map for success
The language of equality in America’s founding was never truly embraced, resulting in a painful legacy of slavery, racial injustice, and gender inequality inherited by all generations. However, for as long as America has fallen short of this unfulfilled promise, individuals have dedicated their lives to the tireless work of correcting injustice. Although the process has been painstakingly slow, our nation has incrementally inched toward the promised vision of equality, and these efforts continue today. With increased attention to social justice movements such as #MeToo and Black Lives Matter, our collective social consciousness may be finally waking up to the systemic injustices embedded into our fundamental institutions.
Medicine is not immune to these injustices. Persistent underrepresentation of women and minorities remains in medical school faculty and the broader physician workforce, and the same inequities exist in hospital medicine.1-6 The report by the Association of American Medical Colleges (AAMC) on diversity in medicine highlights the impact widespread implicit and explicit bias has on creating exclusionary environments, exemplified by research demonstrating lower promotion rates in non-White faculty.7-8 The report calls us, as physicians, to a broader mission: “Focusing solely on increasing compositional diversity along the academic continuum is insufficient. To effectively enact institutional change at academic medical centers ... leaders must focus their efforts on developing inclusive, equity-minded environments.”7
We have a clear moral imperative to correct these shortcomings for our profession and our patients. It is incumbent on our institutions and hospital medicine groups (HMGs) to embark on the necessary process of systemic institutional change to address inequality and justice within our field.
A road map for DEI and justice in hospital medicine
The policies and biases allowing these inequities to persist have existed for decades, and superficial efforts will not bring sufficient change. Our institutions require new building blocks from which the foundation of a wholly inclusive and equal system of practice can be constructed. Encouragingly, some institutions and HMGs have taken steps to modernize their practices. We offer examples and suggestions of concrete practices to begin this journey, organizing these efforts into three broad categories:
1. Recruitment and retention
2. Scholarship, mentorship, and sponsorship
3. Community engagement and partnership.
Recruitment and retention
Improving equity and inclusion begins with recruitment. Search and hiring committees should be assembled intentionally, with gender balance, and ideally with diversity or equity experts invited to join. All members should receive unconscious bias training. For example, the University of Colorado utilizes a toolkit to ensure appropriate steps are followed in the recruitment process, including predetermined candidate selection criteria that are ranked in advance.
Job descriptions should be reviewed by a diversity expert, ensuring unbiased and ungendered language within written text. Advertisements should be wide-reaching, and the committee should consider asking applicants for a diversity statement. Interviews should include a variety of interviewers and interview types (e.g., 1:1, group, etc.). Letters of recommendation deserve special scrutiny; letters for women and minorities may be at risk of being shorter and less record focused, and may be subject to less professional respect, such as use of first names over honorifics or titles.
Once candidates are hired, institutions and HMGs should prioritize developing strategies to improve retention of a diverse workforce. This includes special attention to workplace culture, and thoughtfully striving for cultural intelligence within the group. Some examples may include developing affinity groups, such as underrepresented in medicine (UIM), women in medicine (WIM), or LGBTQ+ groups. Affinity groups provide a safe space for members and allies to support and uplift each other. Institutional and HMG leaders must educate themselves and their members on the importance of language (see table), and the more insidious forms of bias and discrimination that adversely affect workplace culture. Microinsults and microinvalidations, for example, can hurt and result in failure to recruit or turnover. 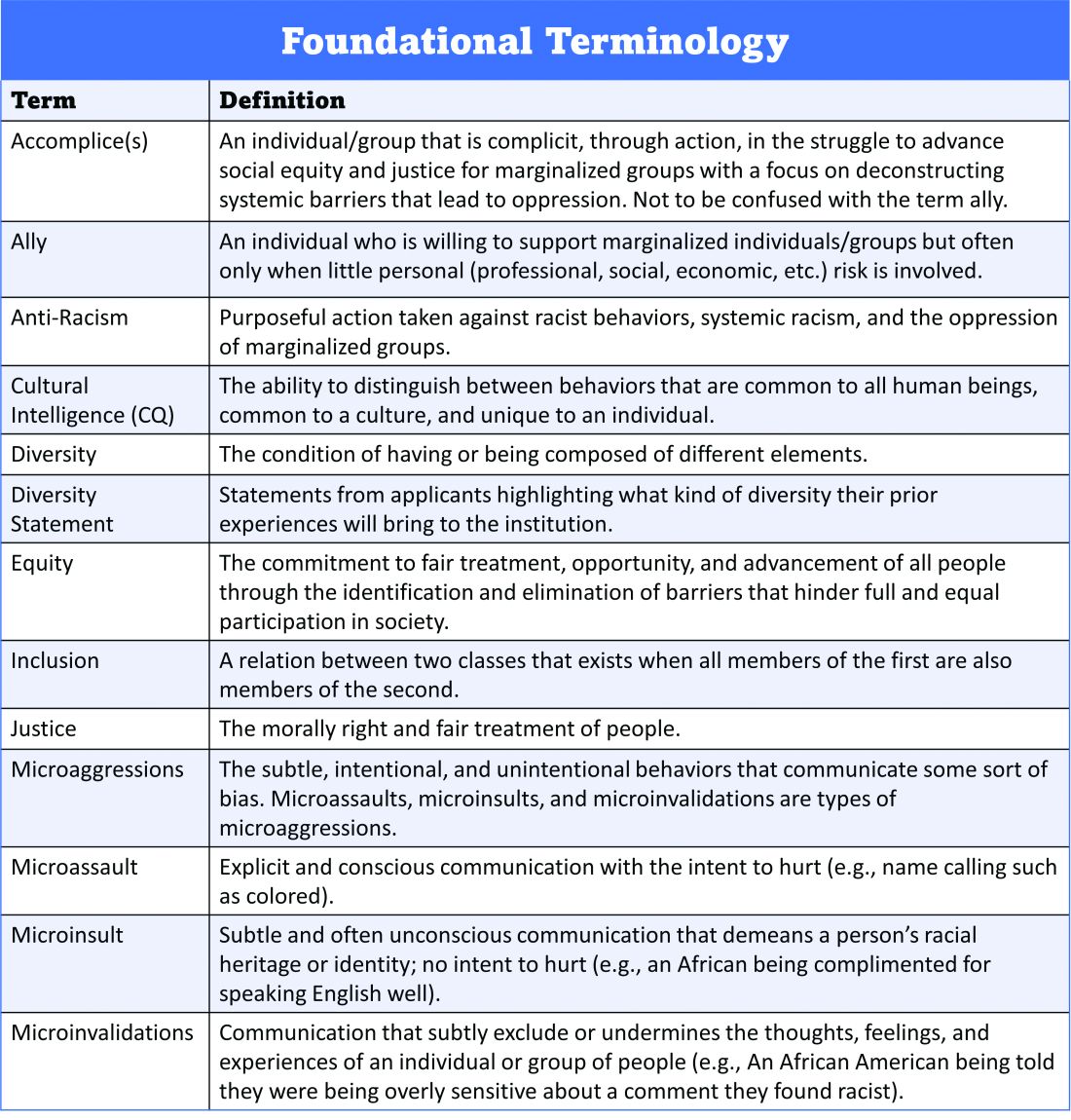
Conducting exit interviews when any hospitalist leaves is important to learn how to improve, but holding ‘stay’ interviews is mission critical. Stay interviews are an opportunity for HMG leaders to proactively understand why hospitalists stay, and what can be done to create more inclusive and equitable environments to retain them. This process creates psychological safety that brings challenges to the fore to be addressed, and spotlights best practices to be maintained and scaled.
Scholarship, mentorship, and sponsorship
Women and minorities are known to be over-mentored and under-sponsored. Sponsorship is defined by Ayyala et al. as “active support by someone appropriately placed in the organization who has significant influence on decision making processes or structures and who is advocating for the career advancement of an individual and recommends them for leadership roles, awards, or high-profile speaking opportunities.”9 While the goal of mentorship is professional development, sponsorship emphasizes professional advancement. Deliberate steps to both mentor and then sponsor diverse hospitalists and future hospitalists (including trainees) are important to ensure equity.
More inclusive HMGs can be bolstered by prioritizing peer education on the professional imperative that we have a diverse workforce and equitable, just workplaces. Academic institutions may use existing structures such as grand rounds to provide education on these crucial topics, and all HMGs can host journal clubs and professional development sessions on leadership competencies that foster inclusion and equity. Sessions coordinated by women and minorities are also a form of justice, by helping overcome barriers to career advancement. Diverse faculty presenting in educational venues will result in content that is relevant to more audience members and will exemplify that leaders and experts are of all races, ethnicities, genders, ages, and abilities.
Groups should prioritize mentoring trainees and early-career hospitalists on scholarly projects that examine equity in opportunities of care, which signals that this science is valued as much as basic research. When used to demonstrate areas needing improvement, these projects can drive meaningful change. Even projects as straightforward as studying diversity in conference presenters, disparities in adherence to guidelines, or QI projects on how race is portrayed in the medical record can be powerful tools in advancing equity.
A key part of mentoring is training hospitalists and future hospitalists in how to be an upstander, as in how to intervene when a peer or patient is affected by bias, harassment, or discrimination. Receiving such training can prepare hospitalists for these nearly inevitable experiences and receiving training during usual work hours communicates that this is a valuable and necessary professional competency.
Community engagement and partnership
Institutions and HMGs should deliberately work to promote community engagement and partnership within their groups. Beyond promoting health equity, community engagement also fosters inclusivity by allowing community members to share their ideas and give recommendations to the institutions that serve them.
There is a growing body of literature that demonstrates how disadvantages by individual and neighborhood-level socioeconomic status (SES) contribute to disparities in specific disease conditions.10-11 Strategies to narrow the gap in SES disadvantages may help reduce race-related health disparities. Institutions that engage the community and develop programs to promote health equity can do so through bidirectional exchange of knowledge and mutual benefit.
An institution-specific example is Medicine for the Greater Good at Johns Hopkins. The founders of this program wrote, “health is not synonymous with medicine. To truly care for our patients and their communities, health care professionals must understand how to deliver equitable health care that meets the needs of the diverse populations we care for. The mission of Medicine for the Greater Good is to promote health and wellness beyond the confines of the hospital through an interactive and engaging partnership with the community ...” Community engagement also provides an opportunity for growing the cultural intelligence of institutions and HMGs.
Tools for advancing comprehensive change – Repurposing PDSA cycles
Whether institutions and HMGs are at the beginning of their journey or further along in the work of reducing disparities, having a systematic approach for implementing and refining policies and procedures can cultivate more inclusive and equitable environments. Thankfully, hospitalists are already equipped with the fundamental tools needed to advance change across their institutions – QI processes in the form of Plan-Do-Study-Act (PDSA) cycles.
They allow a continuous cycle of successful incremental change based on direct evidence and experience. Any efforts to deconstruct systematic bias within our organizations must also be a continual process. Our female colleagues and colleagues of color need our institutions to engage unceasingly to bring about the equality they deserve. To that end, PDSA cycles are an apt tool to utilize in this work as they can naturally function in a never-ending process of improvement.
With PDSA as a model, we envision a cycle with steps that are intentionally purposed to fit the needs of equitable institutional change: Target-Engage-Assess-Modify. As highlighted (see graphic), these modifications ensure that stakeholders (i.e., those that unequal practices and policies affect the most) are engaged early and remain involved throughout the cycle. 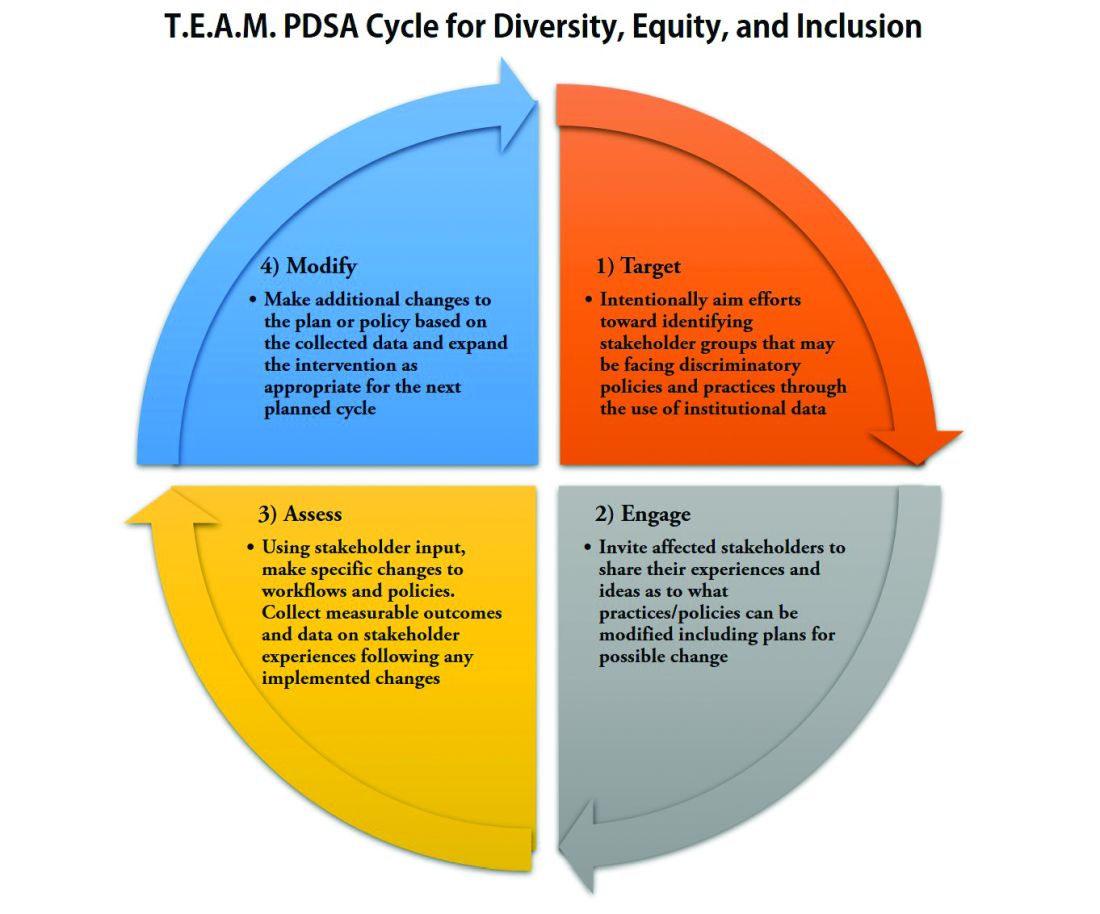
As hospitalists, we have significant work ahead to ensure that we develop and maintain a diverse, equitable and inclusive workforce. This work to bring change will not be easy and will require a considerable investment of time and resources. However, with the strategies and tools that we have outlined, our institutions and HMGs can start the change needed in our profession for our patients and the workforce. In doing so, we can all be accomplices in the fight to achieve racial and gender equity, and social justice.
Dr. Delapenha and Dr. Kisuule are based in the department of internal medicine, division of hospital medicine, at the Johns Hopkins University, Baltimore. Dr. Martin is based in the department of medicine, section of hospital medicine at the University of Chicago. Dr. Barrett is a hospitalist in the department of internal medicine, University of New Mexico, Albuquerque.
References
1. Diversity in Medicine: Facts and Figures 2019: Figure 19. Percentage of physicians by sex, 2018. AAMC website.
2. Diversity in Medicine: Facts and Figures 2019. Figure 16. Percentage of full-time U.S. medical school faculty by sex and race/ethnicity, 2018. AAMC website.
3. Diversity in Medicine: Facts and Figures 2019. Figure 15. Percentage of full-time U.S. medical school faculty by race/ethnicity, 2018. AAMC website.
4. Diversity in Medicine: Facts and Figures 2019. Figure 6. Percentage of acceptees to U.S. medical schools by race/ethnicity (alone), academic year 2018-2019. AAMC website.
5. Diversity in Medicine: Facts and Figures 2019 Figure 18. Percentage of all active physicians by race/ethnicity, 2018. AAMC website.
6. Herzke C et al. Gender issues in academic hospital medicine: A national survey of hospitalist leaders. J Gen Intern Med. 2020;35(6):1641-6.
7. Diversity in Medicine: Facts and Figures 2019. Fostering diversity and inclusion. AAMC website.
8. Diversity in Medicine: Facts and Figures 2019. Executive summary. AAMC website.
9. Ayyala MS et al. Mentorship is not enough: Exploring sponsorship and its role in career advancement in academic medicine. Acad Med. 2019;94(1):94-100.
10. Ejike OC et al. Contribution of individual and neighborhood factors to racial disparities in respiratory outcomes. Am J Respir Crit Care Med. 2021 Apr 15;203(8):987-97.
11. Galiatsatos P et al. The effect of community socioeconomic status on sepsis-attributable mortality. J Crit Care. 2018 Aug;46:129-33.
A road map for success
A road map for success
The language of equality in America’s founding was never truly embraced, resulting in a painful legacy of slavery, racial injustice, and gender inequality inherited by all generations. However, for as long as America has fallen short of this unfulfilled promise, individuals have dedicated their lives to the tireless work of correcting injustice. Although the process has been painstakingly slow, our nation has incrementally inched toward the promised vision of equality, and these efforts continue today. With increased attention to social justice movements such as #MeToo and Black Lives Matter, our collective social consciousness may be finally waking up to the systemic injustices embedded into our fundamental institutions.
Medicine is not immune to these injustices. Persistent underrepresentation of women and minorities remains in medical school faculty and the broader physician workforce, and the same inequities exist in hospital medicine.1-6 The report by the Association of American Medical Colleges (AAMC) on diversity in medicine highlights the impact widespread implicit and explicit bias has on creating exclusionary environments, exemplified by research demonstrating lower promotion rates in non-White faculty.7-8 The report calls us, as physicians, to a broader mission: “Focusing solely on increasing compositional diversity along the academic continuum is insufficient. To effectively enact institutional change at academic medical centers ... leaders must focus their efforts on developing inclusive, equity-minded environments.”7
We have a clear moral imperative to correct these shortcomings for our profession and our patients. It is incumbent on our institutions and hospital medicine groups (HMGs) to embark on the necessary process of systemic institutional change to address inequality and justice within our field.
A road map for DEI and justice in hospital medicine
The policies and biases allowing these inequities to persist have existed for decades, and superficial efforts will not bring sufficient change. Our institutions require new building blocks from which the foundation of a wholly inclusive and equal system of practice can be constructed. Encouragingly, some institutions and HMGs have taken steps to modernize their practices. We offer examples and suggestions of concrete practices to begin this journey, organizing these efforts into three broad categories:
1. Recruitment and retention
2. Scholarship, mentorship, and sponsorship
3. Community engagement and partnership.
Recruitment and retention
Improving equity and inclusion begins with recruitment. Search and hiring committees should be assembled intentionally, with gender balance, and ideally with diversity or equity experts invited to join. All members should receive unconscious bias training. For example, the University of Colorado utilizes a toolkit to ensure appropriate steps are followed in the recruitment process, including predetermined candidate selection criteria that are ranked in advance.
Job descriptions should be reviewed by a diversity expert, ensuring unbiased and ungendered language within written text. Advertisements should be wide-reaching, and the committee should consider asking applicants for a diversity statement. Interviews should include a variety of interviewers and interview types (e.g., 1:1, group, etc.). Letters of recommendation deserve special scrutiny; letters for women and minorities may be at risk of being shorter and less record focused, and may be subject to less professional respect, such as use of first names over honorifics or titles.
Once candidates are hired, institutions and HMGs should prioritize developing strategies to improve retention of a diverse workforce. This includes special attention to workplace culture, and thoughtfully striving for cultural intelligence within the group. Some examples may include developing affinity groups, such as underrepresented in medicine (UIM), women in medicine (WIM), or LGBTQ+ groups. Affinity groups provide a safe space for members and allies to support and uplift each other. Institutional and HMG leaders must educate themselves and their members on the importance of language (see table), and the more insidious forms of bias and discrimination that adversely affect workplace culture. Microinsults and microinvalidations, for example, can hurt and result in failure to recruit or turnover. 
Conducting exit interviews when any hospitalist leaves is important to learn how to improve, but holding ‘stay’ interviews is mission critical. Stay interviews are an opportunity for HMG leaders to proactively understand why hospitalists stay, and what can be done to create more inclusive and equitable environments to retain them. This process creates psychological safety that brings challenges to the fore to be addressed, and spotlights best practices to be maintained and scaled.
Scholarship, mentorship, and sponsorship
Women and minorities are known to be over-mentored and under-sponsored. Sponsorship is defined by Ayyala et al. as “active support by someone appropriately placed in the organization who has significant influence on decision making processes or structures and who is advocating for the career advancement of an individual and recommends them for leadership roles, awards, or high-profile speaking opportunities.”9 While the goal of mentorship is professional development, sponsorship emphasizes professional advancement. Deliberate steps to both mentor and then sponsor diverse hospitalists and future hospitalists (including trainees) are important to ensure equity.
More inclusive HMGs can be bolstered by prioritizing peer education on the professional imperative that we have a diverse workforce and equitable, just workplaces. Academic institutions may use existing structures such as grand rounds to provide education on these crucial topics, and all HMGs can host journal clubs and professional development sessions on leadership competencies that foster inclusion and equity. Sessions coordinated by women and minorities are also a form of justice, by helping overcome barriers to career advancement. Diverse faculty presenting in educational venues will result in content that is relevant to more audience members and will exemplify that leaders and experts are of all races, ethnicities, genders, ages, and abilities.
Groups should prioritize mentoring trainees and early-career hospitalists on scholarly projects that examine equity in opportunities of care, which signals that this science is valued as much as basic research. When used to demonstrate areas needing improvement, these projects can drive meaningful change. Even projects as straightforward as studying diversity in conference presenters, disparities in adherence to guidelines, or QI projects on how race is portrayed in the medical record can be powerful tools in advancing equity.
A key part of mentoring is training hospitalists and future hospitalists in how to be an upstander, as in how to intervene when a peer or patient is affected by bias, harassment, or discrimination. Receiving such training can prepare hospitalists for these nearly inevitable experiences and receiving training during usual work hours communicates that this is a valuable and necessary professional competency.
Community engagement and partnership
Institutions and HMGs should deliberately work to promote community engagement and partnership within their groups. Beyond promoting health equity, community engagement also fosters inclusivity by allowing community members to share their ideas and give recommendations to the institutions that serve them.
There is a growing body of literature that demonstrates how disadvantages by individual and neighborhood-level socioeconomic status (SES) contribute to disparities in specific disease conditions.10-11 Strategies to narrow the gap in SES disadvantages may help reduce race-related health disparities. Institutions that engage the community and develop programs to promote health equity can do so through bidirectional exchange of knowledge and mutual benefit.
An institution-specific example is Medicine for the Greater Good at Johns Hopkins. The founders of this program wrote, “health is not synonymous with medicine. To truly care for our patients and their communities, health care professionals must understand how to deliver equitable health care that meets the needs of the diverse populations we care for. The mission of Medicine for the Greater Good is to promote health and wellness beyond the confines of the hospital through an interactive and engaging partnership with the community ...” Community engagement also provides an opportunity for growing the cultural intelligence of institutions and HMGs.
Tools for advancing comprehensive change – Repurposing PDSA cycles
Whether institutions and HMGs are at the beginning of their journey or further along in the work of reducing disparities, having a systematic approach for implementing and refining policies and procedures can cultivate more inclusive and equitable environments. Thankfully, hospitalists are already equipped with the fundamental tools needed to advance change across their institutions – QI processes in the form of Plan-Do-Study-Act (PDSA) cycles.
They allow a continuous cycle of successful incremental change based on direct evidence and experience. Any efforts to deconstruct systematic bias within our organizations must also be a continual process. Our female colleagues and colleagues of color need our institutions to engage unceasingly to bring about the equality they deserve. To that end, PDSA cycles are an apt tool to utilize in this work as they can naturally function in a never-ending process of improvement.
With PDSA as a model, we envision a cycle with steps that are intentionally purposed to fit the needs of equitable institutional change: Target-Engage-Assess-Modify. As highlighted (see graphic), these modifications ensure that stakeholders (i.e., those that unequal practices and policies affect the most) are engaged early and remain involved throughout the cycle. 
As hospitalists, we have significant work ahead to ensure that we develop and maintain a diverse, equitable and inclusive workforce. This work to bring change will not be easy and will require a considerable investment of time and resources. However, with the strategies and tools that we have outlined, our institutions and HMGs can start the change needed in our profession for our patients and the workforce. In doing so, we can all be accomplices in the fight to achieve racial and gender equity, and social justice.
Dr. Delapenha and Dr. Kisuule are based in the department of internal medicine, division of hospital medicine, at the Johns Hopkins University, Baltimore. Dr. Martin is based in the department of medicine, section of hospital medicine at the University of Chicago. Dr. Barrett is a hospitalist in the department of internal medicine, University of New Mexico, Albuquerque.
References
1. Diversity in Medicine: Facts and Figures 2019: Figure 19. Percentage of physicians by sex, 2018. AAMC website.
2. Diversity in Medicine: Facts and Figures 2019. Figure 16. Percentage of full-time U.S. medical school faculty by sex and race/ethnicity, 2018. AAMC website.
3. Diversity in Medicine: Facts and Figures 2019. Figure 15. Percentage of full-time U.S. medical school faculty by race/ethnicity, 2018. AAMC website.
4. Diversity in Medicine: Facts and Figures 2019. Figure 6. Percentage of acceptees to U.S. medical schools by race/ethnicity (alone), academic year 2018-2019. AAMC website.
5. Diversity in Medicine: Facts and Figures 2019 Figure 18. Percentage of all active physicians by race/ethnicity, 2018. AAMC website.
6. Herzke C et al. Gender issues in academic hospital medicine: A national survey of hospitalist leaders. J Gen Intern Med. 2020;35(6):1641-6.
7. Diversity in Medicine: Facts and Figures 2019. Fostering diversity and inclusion. AAMC website.
8. Diversity in Medicine: Facts and Figures 2019. Executive summary. AAMC website.
9. Ayyala MS et al. Mentorship is not enough: Exploring sponsorship and its role in career advancement in academic medicine. Acad Med. 2019;94(1):94-100.
10. Ejike OC et al. Contribution of individual and neighborhood factors to racial disparities in respiratory outcomes. Am J Respir Crit Care Med. 2021 Apr 15;203(8):987-97.
11. Galiatsatos P et al. The effect of community socioeconomic status on sepsis-attributable mortality. J Crit Care. 2018 Aug;46:129-33.
The language of equality in America’s founding was never truly embraced, resulting in a painful legacy of slavery, racial injustice, and gender inequality inherited by all generations. However, for as long as America has fallen short of this unfulfilled promise, individuals have dedicated their lives to the tireless work of correcting injustice. Although the process has been painstakingly slow, our nation has incrementally inched toward the promised vision of equality, and these efforts continue today. With increased attention to social justice movements such as #MeToo and Black Lives Matter, our collective social consciousness may be finally waking up to the systemic injustices embedded into our fundamental institutions.
Medicine is not immune to these injustices. Persistent underrepresentation of women and minorities remains in medical school faculty and the broader physician workforce, and the same inequities exist in hospital medicine.1-6 The report by the Association of American Medical Colleges (AAMC) on diversity in medicine highlights the impact widespread implicit and explicit bias has on creating exclusionary environments, exemplified by research demonstrating lower promotion rates in non-White faculty.7-8 The report calls us, as physicians, to a broader mission: “Focusing solely on increasing compositional diversity along the academic continuum is insufficient. To effectively enact institutional change at academic medical centers ... leaders must focus their efforts on developing inclusive, equity-minded environments.”7
We have a clear moral imperative to correct these shortcomings for our profession and our patients. It is incumbent on our institutions and hospital medicine groups (HMGs) to embark on the necessary process of systemic institutional change to address inequality and justice within our field.
A road map for DEI and justice in hospital medicine
The policies and biases allowing these inequities to persist have existed for decades, and superficial efforts will not bring sufficient change. Our institutions require new building blocks from which the foundation of a wholly inclusive and equal system of practice can be constructed. Encouragingly, some institutions and HMGs have taken steps to modernize their practices. We offer examples and suggestions of concrete practices to begin this journey, organizing these efforts into three broad categories:
1. Recruitment and retention
2. Scholarship, mentorship, and sponsorship
3. Community engagement and partnership.
Recruitment and retention
Improving equity and inclusion begins with recruitment. Search and hiring committees should be assembled intentionally, with gender balance, and ideally with diversity or equity experts invited to join. All members should receive unconscious bias training. For example, the University of Colorado utilizes a toolkit to ensure appropriate steps are followed in the recruitment process, including predetermined candidate selection criteria that are ranked in advance.
Job descriptions should be reviewed by a diversity expert, ensuring unbiased and ungendered language within written text. Advertisements should be wide-reaching, and the committee should consider asking applicants for a diversity statement. Interviews should include a variety of interviewers and interview types (e.g., 1:1, group, etc.). Letters of recommendation deserve special scrutiny; letters for women and minorities may be at risk of being shorter and less record focused, and may be subject to less professional respect, such as use of first names over honorifics or titles.
Once candidates are hired, institutions and HMGs should prioritize developing strategies to improve retention of a diverse workforce. This includes special attention to workplace culture, and thoughtfully striving for cultural intelligence within the group. Some examples may include developing affinity groups, such as underrepresented in medicine (UIM), women in medicine (WIM), or LGBTQ+ groups. Affinity groups provide a safe space for members and allies to support and uplift each other. Institutional and HMG leaders must educate themselves and their members on the importance of language (see table), and the more insidious forms of bias and discrimination that adversely affect workplace culture. Microinsults and microinvalidations, for example, can hurt and result in failure to recruit or turnover. 
Conducting exit interviews when any hospitalist leaves is important to learn how to improve, but holding ‘stay’ interviews is mission critical. Stay interviews are an opportunity for HMG leaders to proactively understand why hospitalists stay, and what can be done to create more inclusive and equitable environments to retain them. This process creates psychological safety that brings challenges to the fore to be addressed, and spotlights best practices to be maintained and scaled.
Scholarship, mentorship, and sponsorship
Women and minorities are known to be over-mentored and under-sponsored. Sponsorship is defined by Ayyala et al. as “active support by someone appropriately placed in the organization who has significant influence on decision making processes or structures and who is advocating for the career advancement of an individual and recommends them for leadership roles, awards, or high-profile speaking opportunities.”9 While the goal of mentorship is professional development, sponsorship emphasizes professional advancement. Deliberate steps to both mentor and then sponsor diverse hospitalists and future hospitalists (including trainees) are important to ensure equity.
More inclusive HMGs can be bolstered by prioritizing peer education on the professional imperative that we have a diverse workforce and equitable, just workplaces. Academic institutions may use existing structures such as grand rounds to provide education on these crucial topics, and all HMGs can host journal clubs and professional development sessions on leadership competencies that foster inclusion and equity. Sessions coordinated by women and minorities are also a form of justice, by helping overcome barriers to career advancement. Diverse faculty presenting in educational venues will result in content that is relevant to more audience members and will exemplify that leaders and experts are of all races, ethnicities, genders, ages, and abilities.
Groups should prioritize mentoring trainees and early-career hospitalists on scholarly projects that examine equity in opportunities of care, which signals that this science is valued as much as basic research. When used to demonstrate areas needing improvement, these projects can drive meaningful change. Even projects as straightforward as studying diversity in conference presenters, disparities in adherence to guidelines, or QI projects on how race is portrayed in the medical record can be powerful tools in advancing equity.
A key part of mentoring is training hospitalists and future hospitalists in how to be an upstander, as in how to intervene when a peer or patient is affected by bias, harassment, or discrimination. Receiving such training can prepare hospitalists for these nearly inevitable experiences and receiving training during usual work hours communicates that this is a valuable and necessary professional competency.
Community engagement and partnership
Institutions and HMGs should deliberately work to promote community engagement and partnership within their groups. Beyond promoting health equity, community engagement also fosters inclusivity by allowing community members to share their ideas and give recommendations to the institutions that serve them.
There is a growing body of literature that demonstrates how disadvantages by individual and neighborhood-level socioeconomic status (SES) contribute to disparities in specific disease conditions.10-11 Strategies to narrow the gap in SES disadvantages may help reduce race-related health disparities. Institutions that engage the community and develop programs to promote health equity can do so through bidirectional exchange of knowledge and mutual benefit.
An institution-specific example is Medicine for the Greater Good at Johns Hopkins. The founders of this program wrote, “health is not synonymous with medicine. To truly care for our patients and their communities, health care professionals must understand how to deliver equitable health care that meets the needs of the diverse populations we care for. The mission of Medicine for the Greater Good is to promote health and wellness beyond the confines of the hospital through an interactive and engaging partnership with the community ...” Community engagement also provides an opportunity for growing the cultural intelligence of institutions and HMGs.
Tools for advancing comprehensive change – Repurposing PDSA cycles
Whether institutions and HMGs are at the beginning of their journey or further along in the work of reducing disparities, having a systematic approach for implementing and refining policies and procedures can cultivate more inclusive and equitable environments. Thankfully, hospitalists are already equipped with the fundamental tools needed to advance change across their institutions – QI processes in the form of Plan-Do-Study-Act (PDSA) cycles.
They allow a continuous cycle of successful incremental change based on direct evidence and experience. Any efforts to deconstruct systematic bias within our organizations must also be a continual process. Our female colleagues and colleagues of color need our institutions to engage unceasingly to bring about the equality they deserve. To that end, PDSA cycles are an apt tool to utilize in this work as they can naturally function in a never-ending process of improvement.
With PDSA as a model, we envision a cycle with steps that are intentionally purposed to fit the needs of equitable institutional change: Target-Engage-Assess-Modify. As highlighted (see graphic), these modifications ensure that stakeholders (i.e., those that unequal practices and policies affect the most) are engaged early and remain involved throughout the cycle. 
As hospitalists, we have significant work ahead to ensure that we develop and maintain a diverse, equitable and inclusive workforce. This work to bring change will not be easy and will require a considerable investment of time and resources. However, with the strategies and tools that we have outlined, our institutions and HMGs can start the change needed in our profession for our patients and the workforce. In doing so, we can all be accomplices in the fight to achieve racial and gender equity, and social justice.
Dr. Delapenha and Dr. Kisuule are based in the department of internal medicine, division of hospital medicine, at the Johns Hopkins University, Baltimore. Dr. Martin is based in the department of medicine, section of hospital medicine at the University of Chicago. Dr. Barrett is a hospitalist in the department of internal medicine, University of New Mexico, Albuquerque.
References
1. Diversity in Medicine: Facts and Figures 2019: Figure 19. Percentage of physicians by sex, 2018. AAMC website.
2. Diversity in Medicine: Facts and Figures 2019. Figure 16. Percentage of full-time U.S. medical school faculty by sex and race/ethnicity, 2018. AAMC website.
3. Diversity in Medicine: Facts and Figures 2019. Figure 15. Percentage of full-time U.S. medical school faculty by race/ethnicity, 2018. AAMC website.
4. Diversity in Medicine: Facts and Figures 2019. Figure 6. Percentage of acceptees to U.S. medical schools by race/ethnicity (alone), academic year 2018-2019. AAMC website.
5. Diversity in Medicine: Facts and Figures 2019 Figure 18. Percentage of all active physicians by race/ethnicity, 2018. AAMC website.
6. Herzke C et al. Gender issues in academic hospital medicine: A national survey of hospitalist leaders. J Gen Intern Med. 2020;35(6):1641-6.
7. Diversity in Medicine: Facts and Figures 2019. Fostering diversity and inclusion. AAMC website.
8. Diversity in Medicine: Facts and Figures 2019. Executive summary. AAMC website.
9. Ayyala MS et al. Mentorship is not enough: Exploring sponsorship and its role in career advancement in academic medicine. Acad Med. 2019;94(1):94-100.
10. Ejike OC et al. Contribution of individual and neighborhood factors to racial disparities in respiratory outcomes. Am J Respir Crit Care Med. 2021 Apr 15;203(8):987-97.
11. Galiatsatos P et al. The effect of community socioeconomic status on sepsis-attributable mortality. J Crit Care. 2018 Aug;46:129-33.
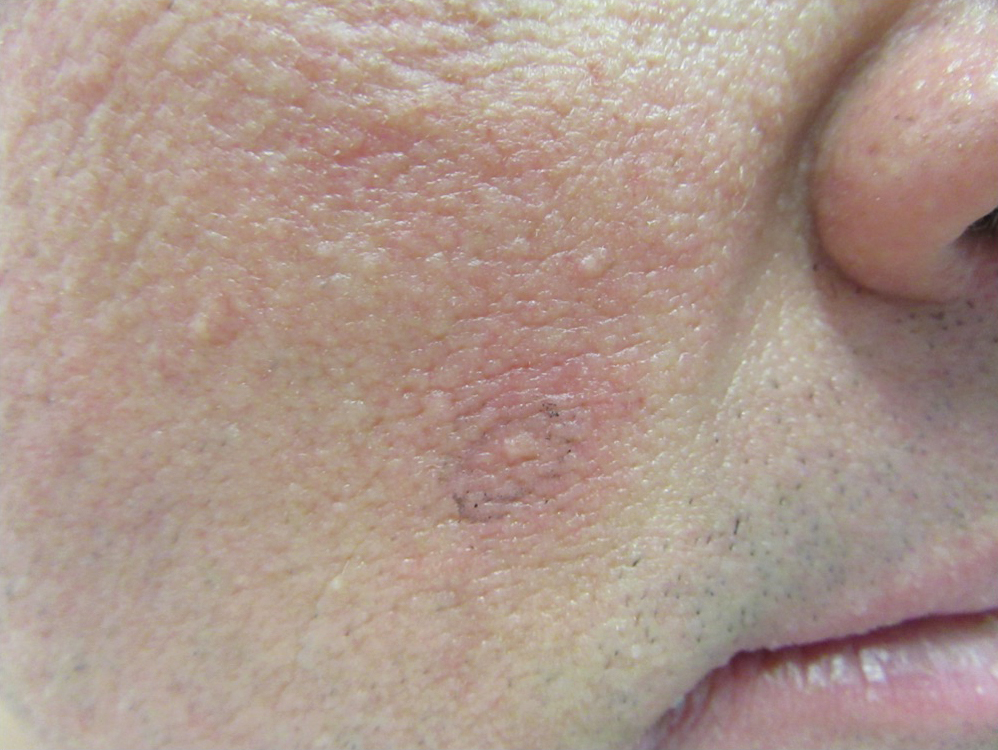
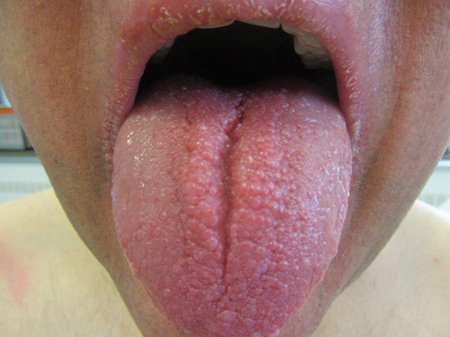
6-year-old with loud wheezing, difficulty breathing
The patient is probably presenting with a moderate asthma exacerbation. To confirm the diagnosis, spirometry assessments should be performed to establish the presence of baseline airway obstruction and determine its severity. Measurements should be obtained before and after inhalation of a short-acting bronchodilator. In addition, chest radiography can help to exclude other diagnoses, and pulse oximetry can exclude hypoxemia.
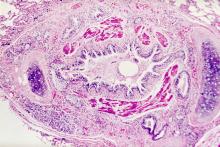
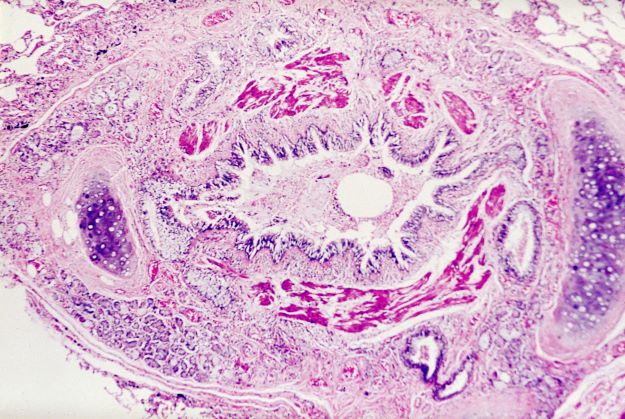
Asthma is a heterogeneous inflammatory disease and the most common chronic condition among children. Histologically, it is marked by vascular congestion, increased vascular permeability, increased tissue volume, and the presence of an exudate. Because the asthmatic lung undergoes a cycle of injury, repair, and regeneration, inflammation creates histologic changes and functional abnormalities in the airway mucosal epithelium. It is hypothesized that these changes play a major role in the pathophysiology of asthma.
Eosinophilic infiltration is a hallmark of this inflammatory activity. Histologic evaluations of the large airways may also reveal narrowing of lumina, bronchial and bronchiolar epithelial denudation, and mucus plugs. Other relevant findings can include epithelial desquamation and hyperplasia, goblet cell metaplasia, subbasement membrane thickening, smooth muscle hypertrophy or hyperplasia, and submucosal gland hypertrophy. In patients with severe asthma, the basement membrane may be significantly thickened. In terms of the small airways, pathologic study from the lungs of living patients is rarely undertaken, and therefore the histopathologic findings of asthma at the level of the distal airways and alveolated lung parenchyma remains largely unknown.
Asthma is treated using a stepwise approach. As directed for pediatric patients younger than 5 years, the patient in this case may be provided with an as-needed inhaled short-acting beta2-agonist (SABA) and should be followed up every 2-6 weeks while gaining control of her symptoms.
Nathan L. Boyer, MD, Assistant Professor, Department of Medicine, Uniformed Services University, Bethesda, Maryland; Chief of Critical Care, Landstuhl Regional Medical Center, Landstuhl, Germany.
Nathan L. Boyer, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
The patient is probably presenting with a moderate asthma exacerbation. To confirm the diagnosis, spirometry assessments should be performed to establish the presence of baseline airway obstruction and determine its severity. Measurements should be obtained before and after inhalation of a short-acting bronchodilator. In addition, chest radiography can help to exclude other diagnoses, and pulse oximetry can exclude hypoxemia.
Asthma is a heterogeneous inflammatory disease and the most common chronic condition among children. Histologically, it is marked by vascular congestion, increased vascular permeability, increased tissue volume, and the presence of an exudate. Because the asthmatic lung undergoes a cycle of injury, repair, and regeneration, inflammation creates histologic changes and functional abnormalities in the airway mucosal epithelium. It is hypothesized that these changes play a major role in the pathophysiology of asthma.
Eosinophilic infiltration is a hallmark of this inflammatory activity. Histologic evaluations of the large airways may also reveal narrowing of lumina, bronchial and bronchiolar epithelial denudation, and mucus plugs. Other relevant findings can include epithelial desquamation and hyperplasia, goblet cell metaplasia, subbasement membrane thickening, smooth muscle hypertrophy or hyperplasia, and submucosal gland hypertrophy. In patients with severe asthma, the basement membrane may be significantly thickened. In terms of the small airways, pathologic study from the lungs of living patients is rarely undertaken, and therefore the histopathologic findings of asthma at the level of the distal airways and alveolated lung parenchyma remains largely unknown.
Asthma is treated using a stepwise approach. As directed for pediatric patients younger than 5 years, the patient in this case may be provided with an as-needed inhaled short-acting beta2-agonist (SABA) and should be followed up every 2-6 weeks while gaining control of her symptoms.
Nathan L. Boyer, MD, Assistant Professor, Department of Medicine, Uniformed Services University, Bethesda, Maryland; Chief of Critical Care, Landstuhl Regional Medical Center, Landstuhl, Germany.
Nathan L. Boyer, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
The patient is probably presenting with a moderate asthma exacerbation. To confirm the diagnosis, spirometry assessments should be performed to establish the presence of baseline airway obstruction and determine its severity. Measurements should be obtained before and after inhalation of a short-acting bronchodilator. In addition, chest radiography can help to exclude other diagnoses, and pulse oximetry can exclude hypoxemia.
Asthma is a heterogeneous inflammatory disease and the most common chronic condition among children. Histologically, it is marked by vascular congestion, increased vascular permeability, increased tissue volume, and the presence of an exudate. Because the asthmatic lung undergoes a cycle of injury, repair, and regeneration, inflammation creates histologic changes and functional abnormalities in the airway mucosal epithelium. It is hypothesized that these changes play a major role in the pathophysiology of asthma.
Eosinophilic infiltration is a hallmark of this inflammatory activity. Histologic evaluations of the large airways may also reveal narrowing of lumina, bronchial and bronchiolar epithelial denudation, and mucus plugs. Other relevant findings can include epithelial desquamation and hyperplasia, goblet cell metaplasia, subbasement membrane thickening, smooth muscle hypertrophy or hyperplasia, and submucosal gland hypertrophy. In patients with severe asthma, the basement membrane may be significantly thickened. In terms of the small airways, pathologic study from the lungs of living patients is rarely undertaken, and therefore the histopathologic findings of asthma at the level of the distal airways and alveolated lung parenchyma remains largely unknown.
Asthma is treated using a stepwise approach. As directed for pediatric patients younger than 5 years, the patient in this case may be provided with an as-needed inhaled short-acting beta2-agonist (SABA) and should be followed up every 2-6 weeks while gaining control of her symptoms.
Nathan L. Boyer, MD, Assistant Professor, Department of Medicine, Uniformed Services University, Bethesda, Maryland; Chief of Critical Care, Landstuhl Regional Medical Center, Landstuhl, Germany.
Nathan L. Boyer, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
A 6-year-old girl presents with loud wheezing, difficulty breathing, and nasal flaring. She becomes particularly breathless while talking. The only abnormalities on chest CT are reduced airway luminal area and mucus plugs. Heart rate is 110 beats/min. Oxyhemoglobin saturation with room air is 94%. Skin examination is nonrevealing. The patient's mother cannot identify any factors that may have precipitated the episode.
Refined heart rate cutoffs may improve prognostic value of acute PE scoring systems
In patients with acute pulmonary embolism, using cutoff values other than 110 beats per minute might improve the prognostic value of heart rate at admission, a recent observational study suggests.
For identifying low-risk patients, a cutoff of 80 bpm increased the sensitivity of the simplified Pulmonary Embolism Severity Index (sPESI) from about 94% to nearly 99% among nonhypotensive patients with acute symptomatic pulmonary embolism (PE), according to results of the large, registry-based study.
Similarly, using a 140-bpm cutoff increased the specificity of the Bova score for identifying intermediate-high–risk patients from about 93% to 98% in the study, which was recently published in the journal CHEST.
“Although standard dichotomization of HR [i.e., HR less than 110 vs. greater than 110 bpm] may be useful for guideline recommendations, our results will allow for more accuracy regarding clinical decision-making,” wrote lead author Ana Jaureguízar, MD, of the University of Alcalá in Madrid, on behalf of the RIETE (Registro Informatizado de la Enfermedad TromboEmbólica) investigators.
Intuitive findings inform future research
These observational findings are intuitive and do at least have the potential to inform the design of future randomized clinical trials, according to Albert J. Polito, MD, chief of the division of pulmonary medicine and medical director for the lung center at Mercy Medical Center in Baltimore.
“In medicine, there is a spectrum of risk,” Dr. Polito said in an interview. “While we love our cutoffs, which in this case has traditionally always been that 110 beats per minute for heart rate, it makes sense that there would be some range of risks of bad outcomes.”
Building on the observations of the present study, subsequent prospective randomized studies could potentially aim to determine, for example, when thrombolytic therapy should be considered in nonhypotensive patients with acute PE and higher heart rates.
“It would not be easy to design, but it’s a straightforward question to ask whether patients with the highest heart rates are the ones who potentially might benefit the most from thrombolytic therapy,” Dr. Polito said.
Value of alternative HR cutoffs
Heart rate is a simple and easily available vital sign that is clearly linked to prognosis in patients with pulmonary embolism, authors of the RIETE registry study say in their report. Accordingly, a heart rate threshold of 110 bpm has made its way into scoring systems that seek to identify low-risk patients, such as the sPESI, and those focused on identifying higher-risk patients, such as the Bova score.
However, it has not been clear whether alternative HR cutoffs would improve upon the 110-bpm threshold, they added. At the low-risk end, more accurate scoring systems could optimize the selection of patients for home treatment, while at the intermediate-high–risk end, they could better select patients for close monitoring or advanced PE treatments.
Better granularity on heart rate risks?
To better define the prognostic value of different heart rate thresholds, investigators analyzed data from RIETE, a large, ongoing, multinational prospective registry including patients with objectively confirmed acute venous thromboembolism.
For 44,331 consecutive nonhypotensive symptomatic PEs, the overall rate of 30-day all-cause mortality was 5.1%, and the 30-day PE-related mortality was 1.9%, the authors report.
Significantly poorer outcomes were seen in patients with higher heart rates as compared to patients in the 80-99 bpm range, they also found. As compared to that reference range, odds ratios for 30-day all-cause death ranged from 1.5 for heart rates of 100-109, up to 2.4 for those with heart rates of 140 bpm or greater.
Likewise, patients with higher heart rates had a 1.7- to 2.4-fold greater risk of 30-day PE-related death as compared to the 80- to 99-bpm reference range, while patients with lower heart rates had lesser risk, the data published in CHEST show.
Toward refinement of prognostic scoring
Next, investigators sought to refine the prognostic scoring systems for low-risk PE (sPESI) and intermediate-high–risk PE (Bova).
For sPESI, they found that dropping the cutoff value from 110 to 100 bpm increased the sensitivity of the score from 93.4% to 95.3%. Going down even further to 80 bpm increased sensitivity to 98.8%, according to the report.
By going down from 110 to 80 bpm, the proportion of patients defined as low-risk dropped from 35% to 12%, according to the investigators.
For the Bova score, increasing the cutoff value from 110 to 120 bpm likewise increased specificity from 93.2% to 95%, while going up even further to 140 bpm increased specificity to 98.0%, the report shows.
In sensitivity analyses, the findings were not impacted by excluding younger patients, those who received reperfusion therapies, or those with atrial fibrillation, according to the study findings.
Potential implications for clinical practice
Taken together, these findings could serve as a resource to inform discussions regarding PE management that include whether home therapy or use of thrombolytic therapy is appropriate, investigators said in their report.
“For instance, among low-risk sPESI patients, those with borderline tachycardia [i.e., a heart rate between 100-109 bpm] might benefit from initial hospital observation for trending,” they wrote.
Dr. Jaureguízar reported no disclosures. One coinvestigator reported funding support from the Institute of Health Carlos III (ISCIII) and the European Development Regional Fund (ERDF). One coinvestigator reported consulting in litigation involving two models of inferior vena cava filters.
Dr. Polito reported no disclosures.
In patients with acute pulmonary embolism, using cutoff values other than 110 beats per minute might improve the prognostic value of heart rate at admission, a recent observational study suggests.
For identifying low-risk patients, a cutoff of 80 bpm increased the sensitivity of the simplified Pulmonary Embolism Severity Index (sPESI) from about 94% to nearly 99% among nonhypotensive patients with acute symptomatic pulmonary embolism (PE), according to results of the large, registry-based study.
Similarly, using a 140-bpm cutoff increased the specificity of the Bova score for identifying intermediate-high–risk patients from about 93% to 98% in the study, which was recently published in the journal CHEST.
“Although standard dichotomization of HR [i.e., HR less than 110 vs. greater than 110 bpm] may be useful for guideline recommendations, our results will allow for more accuracy regarding clinical decision-making,” wrote lead author Ana Jaureguízar, MD, of the University of Alcalá in Madrid, on behalf of the RIETE (Registro Informatizado de la Enfermedad TromboEmbólica) investigators.
Intuitive findings inform future research
These observational findings are intuitive and do at least have the potential to inform the design of future randomized clinical trials, according to Albert J. Polito, MD, chief of the division of pulmonary medicine and medical director for the lung center at Mercy Medical Center in Baltimore.
“In medicine, there is a spectrum of risk,” Dr. Polito said in an interview. “While we love our cutoffs, which in this case has traditionally always been that 110 beats per minute for heart rate, it makes sense that there would be some range of risks of bad outcomes.”
Building on the observations of the present study, subsequent prospective randomized studies could potentially aim to determine, for example, when thrombolytic therapy should be considered in nonhypotensive patients with acute PE and higher heart rates.
“It would not be easy to design, but it’s a straightforward question to ask whether patients with the highest heart rates are the ones who potentially might benefit the most from thrombolytic therapy,” Dr. Polito said.
Value of alternative HR cutoffs
Heart rate is a simple and easily available vital sign that is clearly linked to prognosis in patients with pulmonary embolism, authors of the RIETE registry study say in their report. Accordingly, a heart rate threshold of 110 bpm has made its way into scoring systems that seek to identify low-risk patients, such as the sPESI, and those focused on identifying higher-risk patients, such as the Bova score.
However, it has not been clear whether alternative HR cutoffs would improve upon the 110-bpm threshold, they added. At the low-risk end, more accurate scoring systems could optimize the selection of patients for home treatment, while at the intermediate-high–risk end, they could better select patients for close monitoring or advanced PE treatments.
Better granularity on heart rate risks?
To better define the prognostic value of different heart rate thresholds, investigators analyzed data from RIETE, a large, ongoing, multinational prospective registry including patients with objectively confirmed acute venous thromboembolism.
For 44,331 consecutive nonhypotensive symptomatic PEs, the overall rate of 30-day all-cause mortality was 5.1%, and the 30-day PE-related mortality was 1.9%, the authors report.
Significantly poorer outcomes were seen in patients with higher heart rates as compared to patients in the 80-99 bpm range, they also found. As compared to that reference range, odds ratios for 30-day all-cause death ranged from 1.5 for heart rates of 100-109, up to 2.4 for those with heart rates of 140 bpm or greater.
Likewise, patients with higher heart rates had a 1.7- to 2.4-fold greater risk of 30-day PE-related death as compared to the 80- to 99-bpm reference range, while patients with lower heart rates had lesser risk, the data published in CHEST show.
Toward refinement of prognostic scoring
Next, investigators sought to refine the prognostic scoring systems for low-risk PE (sPESI) and intermediate-high–risk PE (Bova).
For sPESI, they found that dropping the cutoff value from 110 to 100 bpm increased the sensitivity of the score from 93.4% to 95.3%. Going down even further to 80 bpm increased sensitivity to 98.8%, according to the report.
By going down from 110 to 80 bpm, the proportion of patients defined as low-risk dropped from 35% to 12%, according to the investigators.
For the Bova score, increasing the cutoff value from 110 to 120 bpm likewise increased specificity from 93.2% to 95%, while going up even further to 140 bpm increased specificity to 98.0%, the report shows.
In sensitivity analyses, the findings were not impacted by excluding younger patients, those who received reperfusion therapies, or those with atrial fibrillation, according to the study findings.
Potential implications for clinical practice
Taken together, these findings could serve as a resource to inform discussions regarding PE management that include whether home therapy or use of thrombolytic therapy is appropriate, investigators said in their report.
“For instance, among low-risk sPESI patients, those with borderline tachycardia [i.e., a heart rate between 100-109 bpm] might benefit from initial hospital observation for trending,” they wrote.
Dr. Jaureguízar reported no disclosures. One coinvestigator reported funding support from the Institute of Health Carlos III (ISCIII) and the European Development Regional Fund (ERDF). One coinvestigator reported consulting in litigation involving two models of inferior vena cava filters.
Dr. Polito reported no disclosures.
In patients with acute pulmonary embolism, using cutoff values other than 110 beats per minute might improve the prognostic value of heart rate at admission, a recent observational study suggests.
For identifying low-risk patients, a cutoff of 80 bpm increased the sensitivity of the simplified Pulmonary Embolism Severity Index (sPESI) from about 94% to nearly 99% among nonhypotensive patients with acute symptomatic pulmonary embolism (PE), according to results of the large, registry-based study.
Similarly, using a 140-bpm cutoff increased the specificity of the Bova score for identifying intermediate-high–risk patients from about 93% to 98% in the study, which was recently published in the journal CHEST.
“Although standard dichotomization of HR [i.e., HR less than 110 vs. greater than 110 bpm] may be useful for guideline recommendations, our results will allow for more accuracy regarding clinical decision-making,” wrote lead author Ana Jaureguízar, MD, of the University of Alcalá in Madrid, on behalf of the RIETE (Registro Informatizado de la Enfermedad TromboEmbólica) investigators.
Intuitive findings inform future research
These observational findings are intuitive and do at least have the potential to inform the design of future randomized clinical trials, according to Albert J. Polito, MD, chief of the division of pulmonary medicine and medical director for the lung center at Mercy Medical Center in Baltimore.
“In medicine, there is a spectrum of risk,” Dr. Polito said in an interview. “While we love our cutoffs, which in this case has traditionally always been that 110 beats per minute for heart rate, it makes sense that there would be some range of risks of bad outcomes.”
Building on the observations of the present study, subsequent prospective randomized studies could potentially aim to determine, for example, when thrombolytic therapy should be considered in nonhypotensive patients with acute PE and higher heart rates.
“It would not be easy to design, but it’s a straightforward question to ask whether patients with the highest heart rates are the ones who potentially might benefit the most from thrombolytic therapy,” Dr. Polito said.
Value of alternative HR cutoffs
Heart rate is a simple and easily available vital sign that is clearly linked to prognosis in patients with pulmonary embolism, authors of the RIETE registry study say in their report. Accordingly, a heart rate threshold of 110 bpm has made its way into scoring systems that seek to identify low-risk patients, such as the sPESI, and those focused on identifying higher-risk patients, such as the Bova score.
However, it has not been clear whether alternative HR cutoffs would improve upon the 110-bpm threshold, they added. At the low-risk end, more accurate scoring systems could optimize the selection of patients for home treatment, while at the intermediate-high–risk end, they could better select patients for close monitoring or advanced PE treatments.
Better granularity on heart rate risks?
To better define the prognostic value of different heart rate thresholds, investigators analyzed data from RIETE, a large, ongoing, multinational prospective registry including patients with objectively confirmed acute venous thromboembolism.
For 44,331 consecutive nonhypotensive symptomatic PEs, the overall rate of 30-day all-cause mortality was 5.1%, and the 30-day PE-related mortality was 1.9%, the authors report.
Significantly poorer outcomes were seen in patients with higher heart rates as compared to patients in the 80-99 bpm range, they also found. As compared to that reference range, odds ratios for 30-day all-cause death ranged from 1.5 for heart rates of 100-109, up to 2.4 for those with heart rates of 140 bpm or greater.
Likewise, patients with higher heart rates had a 1.7- to 2.4-fold greater risk of 30-day PE-related death as compared to the 80- to 99-bpm reference range, while patients with lower heart rates had lesser risk, the data published in CHEST show.
Toward refinement of prognostic scoring
Next, investigators sought to refine the prognostic scoring systems for low-risk PE (sPESI) and intermediate-high–risk PE (Bova).
For sPESI, they found that dropping the cutoff value from 110 to 100 bpm increased the sensitivity of the score from 93.4% to 95.3%. Going down even further to 80 bpm increased sensitivity to 98.8%, according to the report.
By going down from 110 to 80 bpm, the proportion of patients defined as low-risk dropped from 35% to 12%, according to the investigators.
For the Bova score, increasing the cutoff value from 110 to 120 bpm likewise increased specificity from 93.2% to 95%, while going up even further to 140 bpm increased specificity to 98.0%, the report shows.
In sensitivity analyses, the findings were not impacted by excluding younger patients, those who received reperfusion therapies, or those with atrial fibrillation, according to the study findings.
Potential implications for clinical practice
Taken together, these findings could serve as a resource to inform discussions regarding PE management that include whether home therapy or use of thrombolytic therapy is appropriate, investigators said in their report.
“For instance, among low-risk sPESI patients, those with borderline tachycardia [i.e., a heart rate between 100-109 bpm] might benefit from initial hospital observation for trending,” they wrote.
Dr. Jaureguízar reported no disclosures. One coinvestigator reported funding support from the Institute of Health Carlos III (ISCIII) and the European Development Regional Fund (ERDF). One coinvestigator reported consulting in litigation involving two models of inferior vena cava filters.
Dr. Polito reported no disclosures.
FROM CHEST
How do alcohol, obesity impact cirrhosis?
Alcohol intake and obesity are independent risk factors for morbidity among patients with cirrhosis, but the two factors do not appear to combine for a stronger effect (supra-additive), according to conclusions from a new analysis of participants in the UK Biobank study published in Hepatology.
The researchers analyzed data from the records of 489,285 individuals in the UK Biobank from May 2006 to July 2010. Researchers defined morbidity as first-time hospitalization for cirrhosis and calculated the cumulative incidence at 10 years among included individuals. The researchers defined obesity as body mass index of at least 30 kg/m2 and healthy BMI as 20-25. Safe drinking was defined as having fewer than 22 units per week for males or fewer than 15 units for females, harmful drinking was defined as more than 50 units per week for males or more than 35 for females, and hazardous drinking was defined as 22-49 units per week for males and 15-35 for females. The researchers assumed 2 units in a pint of beer or cider, 1.5 units in a glass of wine and “other” drinks, and 1 unit per measure of spirits.
The mean age was 57.0 years, and 45.4% were male. Overall, 24.3% of subjects were obese, 76.5% had safe levels of alcohol consumption, 19.7% had hazardous alcohol consumption, and 3.8% were classified as harmful drinkers.
Overall, harmful drinking was associated with 5.0 times the 10-year cumulative incidence of cirrhosis morbidity among harmful versus safe drinkers (1.51% vs. 0.30%). However, among those with a healthy BMI, harmful was associated with an 8.6-fold increase of cirrhosis morbidity, compared with safe drinkers (1.38% vs. 0.16%). On the other hand, obese patients with harmful drinking habits had a 3.6-fold increase over obese safe drinkers (1.99% vs. 0.56%).
When looked at according to BMI, 10-year cumulative incidence was 3.1 times higher in patients who with obesity versus those who with healthy BMI (0.65% vs. 0.21%). This varied strongly with drinking: Safe drinkers who with obesity had 3.7 times the incidence, compared with safe drinkers with healthy BMI (0.56% vs. 0.15%), and harmful drinkers who were obese had a 1.4-fold increased incidence, compared with harmful drinkers of a healthy weight (1.99% vs. 1.38%).
“In contrast to some previous studies, we found little evidence that [obesity and drinking] interacted supra-additively to modulate the risk of cirrhosis morbidity,” the authors wrote. “On the contrary, through a relative risk lens, the association between alcohol intake and cirrhosis morbidity was actually weaker for individuals with obesity than for individuals with a healthy BMI (indicating a sub-additive relationship).”
Fine-Gray regression modelling seemed to confirm that the relationship was sub-additive. After controlling for various factors, researchers found that harmful drinkers had a 6.84-fold increased risk at a healthy BMI, while the risk was only 3.14 times higher in obese patients (P interaction = 3.53 x 10–6).
The findings contradict previous studies, which suggested that high BMI and harmful drinking combined may produce much higher risk than either factor alone, possibly because obesity might “prime” the liver to be vulnerable to the effects of alcohol.
The authors suggest that the differences in findings may be caused by methodological limitations of the earlier studies, such as reliance on self-reported BMI data; small sample sizes and a relatively small number of liver events among those with obesity and harmful alcohol consumption; and the failure to use a competing risk perspective. The latter is relevant because alcohol and obesity are risk factors for other potentially fatal health conditions.
But the current study is not without its own limitations, according to Nancy Reau, MD, who is a professor of medicine and chair of hepatology at Rush University Medical Center in Chicago, who was asked to comment on the findings. Dr. Reau pointed out that the authors found the highest frequency of complications was observed in people with harmful alcohol intake whose BMI was under 20. That group may be composed of subjects with sarcopenia and end-stage liver disease from alcohol use. “Until you can separate these from the truly healthy BMI but [with harmful alcohol use], you can’t interpret this arm,” said Dr. Reau.
Beyond that, the researchers found increased risks of harm among individuals regardless of BMI, but the risks were highest among those with BMI over 30. Dr. Reau posited that the frequency might have been significantly greater at BMI higher than 35 and 40, but the researchers didn’t report results among these subcategories.
“In no way does this suggest that we need to ignore alcohol use in our patients with NAFLD [nonalcoholic fatty liver disease] or [nonalcoholic steatohepatitis],” said Dr. Reau.
In fact, she pointed to a figure in the paper that showed the highest increase in frequency among those with harmful alcohol use and obesity. “It’s clear that both conditions are much more serious than just obesity alone. It is incredibly important to council our NAFLD patients on appropriate alcohol use, [since] problematic drinking increases their risk. Problematic drinking remains a serious problem and increased awareness and linking to addiction services is important,” she said.
The authors reported no conflicts of interest. Dr. Reau has no relevant financial disclosures.
Alcohol intake and obesity are independent risk factors for morbidity among patients with cirrhosis, but the two factors do not appear to combine for a stronger effect (supra-additive), according to conclusions from a new analysis of participants in the UK Biobank study published in Hepatology.
The researchers analyzed data from the records of 489,285 individuals in the UK Biobank from May 2006 to July 2010. Researchers defined morbidity as first-time hospitalization for cirrhosis and calculated the cumulative incidence at 10 years among included individuals. The researchers defined obesity as body mass index of at least 30 kg/m2 and healthy BMI as 20-25. Safe drinking was defined as having fewer than 22 units per week for males or fewer than 15 units for females, harmful drinking was defined as more than 50 units per week for males or more than 35 for females, and hazardous drinking was defined as 22-49 units per week for males and 15-35 for females. The researchers assumed 2 units in a pint of beer or cider, 1.5 units in a glass of wine and “other” drinks, and 1 unit per measure of spirits.
The mean age was 57.0 years, and 45.4% were male. Overall, 24.3% of subjects were obese, 76.5% had safe levels of alcohol consumption, 19.7% had hazardous alcohol consumption, and 3.8% were classified as harmful drinkers.
Overall, harmful drinking was associated with 5.0 times the 10-year cumulative incidence of cirrhosis morbidity among harmful versus safe drinkers (1.51% vs. 0.30%). However, among those with a healthy BMI, harmful was associated with an 8.6-fold increase of cirrhosis morbidity, compared with safe drinkers (1.38% vs. 0.16%). On the other hand, obese patients with harmful drinking habits had a 3.6-fold increase over obese safe drinkers (1.99% vs. 0.56%).
When looked at according to BMI, 10-year cumulative incidence was 3.1 times higher in patients who with obesity versus those who with healthy BMI (0.65% vs. 0.21%). This varied strongly with drinking: Safe drinkers who with obesity had 3.7 times the incidence, compared with safe drinkers with healthy BMI (0.56% vs. 0.15%), and harmful drinkers who were obese had a 1.4-fold increased incidence, compared with harmful drinkers of a healthy weight (1.99% vs. 1.38%).
“In contrast to some previous studies, we found little evidence that [obesity and drinking] interacted supra-additively to modulate the risk of cirrhosis morbidity,” the authors wrote. “On the contrary, through a relative risk lens, the association between alcohol intake and cirrhosis morbidity was actually weaker for individuals with obesity than for individuals with a healthy BMI (indicating a sub-additive relationship).”
Fine-Gray regression modelling seemed to confirm that the relationship was sub-additive. After controlling for various factors, researchers found that harmful drinkers had a 6.84-fold increased risk at a healthy BMI, while the risk was only 3.14 times higher in obese patients (P interaction = 3.53 x 10–6).
The findings contradict previous studies, which suggested that high BMI and harmful drinking combined may produce much higher risk than either factor alone, possibly because obesity might “prime” the liver to be vulnerable to the effects of alcohol.
The authors suggest that the differences in findings may be caused by methodological limitations of the earlier studies, such as reliance on self-reported BMI data; small sample sizes and a relatively small number of liver events among those with obesity and harmful alcohol consumption; and the failure to use a competing risk perspective. The latter is relevant because alcohol and obesity are risk factors for other potentially fatal health conditions.
But the current study is not without its own limitations, according to Nancy Reau, MD, who is a professor of medicine and chair of hepatology at Rush University Medical Center in Chicago, who was asked to comment on the findings. Dr. Reau pointed out that the authors found the highest frequency of complications was observed in people with harmful alcohol intake whose BMI was under 20. That group may be composed of subjects with sarcopenia and end-stage liver disease from alcohol use. “Until you can separate these from the truly healthy BMI but [with harmful alcohol use], you can’t interpret this arm,” said Dr. Reau.
Beyond that, the researchers found increased risks of harm among individuals regardless of BMI, but the risks were highest among those with BMI over 30. Dr. Reau posited that the frequency might have been significantly greater at BMI higher than 35 and 40, but the researchers didn’t report results among these subcategories.
“In no way does this suggest that we need to ignore alcohol use in our patients with NAFLD [nonalcoholic fatty liver disease] or [nonalcoholic steatohepatitis],” said Dr. Reau.
In fact, she pointed to a figure in the paper that showed the highest increase in frequency among those with harmful alcohol use and obesity. “It’s clear that both conditions are much more serious than just obesity alone. It is incredibly important to council our NAFLD patients on appropriate alcohol use, [since] problematic drinking increases their risk. Problematic drinking remains a serious problem and increased awareness and linking to addiction services is important,” she said.
The authors reported no conflicts of interest. Dr. Reau has no relevant financial disclosures.
Alcohol intake and obesity are independent risk factors for morbidity among patients with cirrhosis, but the two factors do not appear to combine for a stronger effect (supra-additive), according to conclusions from a new analysis of participants in the UK Biobank study published in Hepatology.
The researchers analyzed data from the records of 489,285 individuals in the UK Biobank from May 2006 to July 2010. Researchers defined morbidity as first-time hospitalization for cirrhosis and calculated the cumulative incidence at 10 years among included individuals. The researchers defined obesity as body mass index of at least 30 kg/m2 and healthy BMI as 20-25. Safe drinking was defined as having fewer than 22 units per week for males or fewer than 15 units for females, harmful drinking was defined as more than 50 units per week for males or more than 35 for females, and hazardous drinking was defined as 22-49 units per week for males and 15-35 for females. The researchers assumed 2 units in a pint of beer or cider, 1.5 units in a glass of wine and “other” drinks, and 1 unit per measure of spirits.
The mean age was 57.0 years, and 45.4% were male. Overall, 24.3% of subjects were obese, 76.5% had safe levels of alcohol consumption, 19.7% had hazardous alcohol consumption, and 3.8% were classified as harmful drinkers.
Overall, harmful drinking was associated with 5.0 times the 10-year cumulative incidence of cirrhosis morbidity among harmful versus safe drinkers (1.51% vs. 0.30%). However, among those with a healthy BMI, harmful was associated with an 8.6-fold increase of cirrhosis morbidity, compared with safe drinkers (1.38% vs. 0.16%). On the other hand, obese patients with harmful drinking habits had a 3.6-fold increase over obese safe drinkers (1.99% vs. 0.56%).
When looked at according to BMI, 10-year cumulative incidence was 3.1 times higher in patients who with obesity versus those who with healthy BMI (0.65% vs. 0.21%). This varied strongly with drinking: Safe drinkers who with obesity had 3.7 times the incidence, compared with safe drinkers with healthy BMI (0.56% vs. 0.15%), and harmful drinkers who were obese had a 1.4-fold increased incidence, compared with harmful drinkers of a healthy weight (1.99% vs. 1.38%).
“In contrast to some previous studies, we found little evidence that [obesity and drinking] interacted supra-additively to modulate the risk of cirrhosis morbidity,” the authors wrote. “On the contrary, through a relative risk lens, the association between alcohol intake and cirrhosis morbidity was actually weaker for individuals with obesity than for individuals with a healthy BMI (indicating a sub-additive relationship).”
Fine-Gray regression modelling seemed to confirm that the relationship was sub-additive. After controlling for various factors, researchers found that harmful drinkers had a 6.84-fold increased risk at a healthy BMI, while the risk was only 3.14 times higher in obese patients (P interaction = 3.53 x 10–6).
The findings contradict previous studies, which suggested that high BMI and harmful drinking combined may produce much higher risk than either factor alone, possibly because obesity might “prime” the liver to be vulnerable to the effects of alcohol.
The authors suggest that the differences in findings may be caused by methodological limitations of the earlier studies, such as reliance on self-reported BMI data; small sample sizes and a relatively small number of liver events among those with obesity and harmful alcohol consumption; and the failure to use a competing risk perspective. The latter is relevant because alcohol and obesity are risk factors for other potentially fatal health conditions.
But the current study is not without its own limitations, according to Nancy Reau, MD, who is a professor of medicine and chair of hepatology at Rush University Medical Center in Chicago, who was asked to comment on the findings. Dr. Reau pointed out that the authors found the highest frequency of complications was observed in people with harmful alcohol intake whose BMI was under 20. That group may be composed of subjects with sarcopenia and end-stage liver disease from alcohol use. “Until you can separate these from the truly healthy BMI but [with harmful alcohol use], you can’t interpret this arm,” said Dr. Reau.
Beyond that, the researchers found increased risks of harm among individuals regardless of BMI, but the risks were highest among those with BMI over 30. Dr. Reau posited that the frequency might have been significantly greater at BMI higher than 35 and 40, but the researchers didn’t report results among these subcategories.
“In no way does this suggest that we need to ignore alcohol use in our patients with NAFLD [nonalcoholic fatty liver disease] or [nonalcoholic steatohepatitis],” said Dr. Reau.
In fact, she pointed to a figure in the paper that showed the highest increase in frequency among those with harmful alcohol use and obesity. “It’s clear that both conditions are much more serious than just obesity alone. It is incredibly important to council our NAFLD patients on appropriate alcohol use, [since] problematic drinking increases their risk. Problematic drinking remains a serious problem and increased awareness and linking to addiction services is important,” she said.
The authors reported no conflicts of interest. Dr. Reau has no relevant financial disclosures.
FROM HEPATOLOGY
Call for a move or boycott of big Texas cancer meeting
Docs won’t attend in person
Held annually in December, SABCS is the world’s largest breast cancer meeting and welcomes thousands of attendees every year.
The law banning abortions after 6 weeks, at which time a woman may not even realize that she is pregnant, has been described as the most restrictive in the United States. It also enables private citizens to bring civil lawsuits against people who assist a pregnant person seeking an abortion in violation of the ban.
If the meeting remains in Texas this year, then UCSF’s Laura Esserman, MD, director of the Carol Franc Buck Breast Cancer Center, has said that she and other university faculty and professionals from elsewhere will attend only online — a form of boycotting the in-person event.
Dr. Esserman and UCSF colleague Mary Helen Barcellos-Hoff, PhD, professor of radiation oncology, have emailed conference leaders encouraging them to move the meeting, according to a press statement issued by UCSF.
SABCS organizers told this news organization via email that they “are having serious discussions about this matter” and will update the public via their website and social media channels.
“It’s a terrible law, and it is absolutely not protective of women. I think that if Texas officials understood that when they pass laws inhospitable to women, we will not hold a major conference about women’s health in their state,” said Dr. Esserman.
Dr. Esserman received support for the idea to move the meeting earlier this month from peers on Twitter.
“In light of the Texas law that prohibits abortion past 6 weeks of pregnancy AND promotes vigilantism, directly harming women and their caregivers, the 2021 SABCS breast cancer meeting should be moved out of Texas to a place that supports Women’s rights and public health #sabcs,” tweeted Dr. Esserman on September 5.
The post generated more than 3,300 likes and retweets and 40-plus comments from readers, many of whom were healthcare professionals.
Supporters of the proposed move included Michael Feldman, MD, PhD, pathologist, University of Pennsylvania in Philadelphia; Sarah Sammons, MD, breast medical oncologist, Duke Cancer Center, Durham, N.C.; Anjali Thawani, MD, breast surgeon, Evanston, Ill.; Debora Barton, MD, Carisma Therapeutics, Philadelphia; Jane Hui, MD, surgical oncologist, University of Minnesota, Minneapolis; Erica Leith Mitchell, MD, surgeon, University of Tennessee, Memphis; and Rebecca Shatsky, MD, breast medical oncologist, University of California, San Diego.
Support for boycotting in-person attendance is growing nationally, Dr. Esserman said in the press statement, which also highlighted an “additional concern” about Texas laws prohibiting mandated masking and asking for vaccine status. Conference organizers said local ordinances will be used to require masking.
Notably, a Twitter search using #SABCS21, the meeting’s hashtag, indicates that COVID 19 — and not the restrictive abortion law — is the primary worry of would-be meeting attendees as of the last week or so.
Some said the combination of the two issues was influential in their decision not to attend in person.
Kelly Shanahan, MD, a former ob/gyn living with metastatic breast cancer in South Lake Tahoe, Calif., tweeted: “It’s a hybrid model this year. I was originally going in person but will not now because of their COVID behaviors and now this [the abortion law]. I will tune in virtually but I will not spend the $1500 on the hotel room, the hundreds of $$ on food and drink and Xmas presents. #SayNoToTX #SABCS21.”
Dr. Shanahan also replied to Dr. Esserman’s move-the-meeting-out-of-Texas tweet: “100% agree.”
UCSF’s Dr. Barcellos-Hoff believes moving the meeting would be a high-profile happening. “I think moving a meeting of that size would have an impact, largely because of the visibility of scientists who oppose the law.”
First organized in 1977, SABCS is jointly sponsored by the Cancer Therapy & Research Center at The University of Texas Health Science Center at San Antonio, the American Association for Cancer Research, and Baylor College of Medicine.
A version of this article first appeared on Medscape.com.
Docs won’t attend in person
Docs won’t attend in person
Held annually in December, SABCS is the world’s largest breast cancer meeting and welcomes thousands of attendees every year.
The law banning abortions after 6 weeks, at which time a woman may not even realize that she is pregnant, has been described as the most restrictive in the United States. It also enables private citizens to bring civil lawsuits against people who assist a pregnant person seeking an abortion in violation of the ban.
If the meeting remains in Texas this year, then UCSF’s Laura Esserman, MD, director of the Carol Franc Buck Breast Cancer Center, has said that she and other university faculty and professionals from elsewhere will attend only online — a form of boycotting the in-person event.
Dr. Esserman and UCSF colleague Mary Helen Barcellos-Hoff, PhD, professor of radiation oncology, have emailed conference leaders encouraging them to move the meeting, according to a press statement issued by UCSF.
SABCS organizers told this news organization via email that they “are having serious discussions about this matter” and will update the public via their website and social media channels.
“It’s a terrible law, and it is absolutely not protective of women. I think that if Texas officials understood that when they pass laws inhospitable to women, we will not hold a major conference about women’s health in their state,” said Dr. Esserman.
Dr. Esserman received support for the idea to move the meeting earlier this month from peers on Twitter.
“In light of the Texas law that prohibits abortion past 6 weeks of pregnancy AND promotes vigilantism, directly harming women and their caregivers, the 2021 SABCS breast cancer meeting should be moved out of Texas to a place that supports Women’s rights and public health #sabcs,” tweeted Dr. Esserman on September 5.
The post generated more than 3,300 likes and retweets and 40-plus comments from readers, many of whom were healthcare professionals.
Supporters of the proposed move included Michael Feldman, MD, PhD, pathologist, University of Pennsylvania in Philadelphia; Sarah Sammons, MD, breast medical oncologist, Duke Cancer Center, Durham, N.C.; Anjali Thawani, MD, breast surgeon, Evanston, Ill.; Debora Barton, MD, Carisma Therapeutics, Philadelphia; Jane Hui, MD, surgical oncologist, University of Minnesota, Minneapolis; Erica Leith Mitchell, MD, surgeon, University of Tennessee, Memphis; and Rebecca Shatsky, MD, breast medical oncologist, University of California, San Diego.
Support for boycotting in-person attendance is growing nationally, Dr. Esserman said in the press statement, which also highlighted an “additional concern” about Texas laws prohibiting mandated masking and asking for vaccine status. Conference organizers said local ordinances will be used to require masking.
Notably, a Twitter search using #SABCS21, the meeting’s hashtag, indicates that COVID 19 — and not the restrictive abortion law — is the primary worry of would-be meeting attendees as of the last week or so.
Some said the combination of the two issues was influential in their decision not to attend in person.
Kelly Shanahan, MD, a former ob/gyn living with metastatic breast cancer in South Lake Tahoe, Calif., tweeted: “It’s a hybrid model this year. I was originally going in person but will not now because of their COVID behaviors and now this [the abortion law]. I will tune in virtually but I will not spend the $1500 on the hotel room, the hundreds of $$ on food and drink and Xmas presents. #SayNoToTX #SABCS21.”
Dr. Shanahan also replied to Dr. Esserman’s move-the-meeting-out-of-Texas tweet: “100% agree.”
UCSF’s Dr. Barcellos-Hoff believes moving the meeting would be a high-profile happening. “I think moving a meeting of that size would have an impact, largely because of the visibility of scientists who oppose the law.”
First organized in 1977, SABCS is jointly sponsored by the Cancer Therapy & Research Center at The University of Texas Health Science Center at San Antonio, the American Association for Cancer Research, and Baylor College of Medicine.
A version of this article first appeared on Medscape.com.
Held annually in December, SABCS is the world’s largest breast cancer meeting and welcomes thousands of attendees every year.
The law banning abortions after 6 weeks, at which time a woman may not even realize that she is pregnant, has been described as the most restrictive in the United States. It also enables private citizens to bring civil lawsuits against people who assist a pregnant person seeking an abortion in violation of the ban.
If the meeting remains in Texas this year, then UCSF’s Laura Esserman, MD, director of the Carol Franc Buck Breast Cancer Center, has said that she and other university faculty and professionals from elsewhere will attend only online — a form of boycotting the in-person event.
Dr. Esserman and UCSF colleague Mary Helen Barcellos-Hoff, PhD, professor of radiation oncology, have emailed conference leaders encouraging them to move the meeting, according to a press statement issued by UCSF.
SABCS organizers told this news organization via email that they “are having serious discussions about this matter” and will update the public via their website and social media channels.
“It’s a terrible law, and it is absolutely not protective of women. I think that if Texas officials understood that when they pass laws inhospitable to women, we will not hold a major conference about women’s health in their state,” said Dr. Esserman.
Dr. Esserman received support for the idea to move the meeting earlier this month from peers on Twitter.
“In light of the Texas law that prohibits abortion past 6 weeks of pregnancy AND promotes vigilantism, directly harming women and their caregivers, the 2021 SABCS breast cancer meeting should be moved out of Texas to a place that supports Women’s rights and public health #sabcs,” tweeted Dr. Esserman on September 5.
The post generated more than 3,300 likes and retweets and 40-plus comments from readers, many of whom were healthcare professionals.
Supporters of the proposed move included Michael Feldman, MD, PhD, pathologist, University of Pennsylvania in Philadelphia; Sarah Sammons, MD, breast medical oncologist, Duke Cancer Center, Durham, N.C.; Anjali Thawani, MD, breast surgeon, Evanston, Ill.; Debora Barton, MD, Carisma Therapeutics, Philadelphia; Jane Hui, MD, surgical oncologist, University of Minnesota, Minneapolis; Erica Leith Mitchell, MD, surgeon, University of Tennessee, Memphis; and Rebecca Shatsky, MD, breast medical oncologist, University of California, San Diego.
Support for boycotting in-person attendance is growing nationally, Dr. Esserman said in the press statement, which also highlighted an “additional concern” about Texas laws prohibiting mandated masking and asking for vaccine status. Conference organizers said local ordinances will be used to require masking.
Notably, a Twitter search using #SABCS21, the meeting’s hashtag, indicates that COVID 19 — and not the restrictive abortion law — is the primary worry of would-be meeting attendees as of the last week or so.
Some said the combination of the two issues was influential in their decision not to attend in person.
Kelly Shanahan, MD, a former ob/gyn living with metastatic breast cancer in South Lake Tahoe, Calif., tweeted: “It’s a hybrid model this year. I was originally going in person but will not now because of their COVID behaviors and now this [the abortion law]. I will tune in virtually but I will not spend the $1500 on the hotel room, the hundreds of $$ on food and drink and Xmas presents. #SayNoToTX #SABCS21.”
Dr. Shanahan also replied to Dr. Esserman’s move-the-meeting-out-of-Texas tweet: “100% agree.”
UCSF’s Dr. Barcellos-Hoff believes moving the meeting would be a high-profile happening. “I think moving a meeting of that size would have an impact, largely because of the visibility of scientists who oppose the law.”
First organized in 1977, SABCS is jointly sponsored by the Cancer Therapy & Research Center at The University of Texas Health Science Center at San Antonio, the American Association for Cancer Research, and Baylor College of Medicine.
A version of this article first appeared on Medscape.com.
Moderate alcohol intake may curb subsequent diabetes after gestational diabetes
Among women with a history of gestational diabetes, alcohol intake of half a drink to one drink daily was associated with a 55% lower risk for subsequent type 2 diabetes, based on data from approximately 4,700 women in the Nurses’ Health Study II cohort.
However, the findings must be considered in the context of other risks and benefits of alcohol consumption before making statements or clinical recommendations, wrote Stefanie N. Hinkle, PhD, of the National Institutes of Health, Bethesda, Md., and colleagues.
Women with a history of gestational diabetes remain at increased risk for developing type 2 diabetes, so modifiable diet and lifestyle factors deserve further study, the researchers noted. Previous research has shown an association between light to moderate alcohol consumption and reduced risk of type 2 diabetes among women in the general population, but data on a similar risk reduction for women with a history of gestational diabetes are lacking, they added.
In a study published in JAMA Network Open, the researchers reviewed data from 4,740 women enrolled in the Nurses’ Health Study II who reported a history of gestational diabetes. These women were followed from Jan. 1, 1991, to Dec. 31, 2017, as part of the Diabetes & Women’s Health Study; dietary intake, including alcohol intake, was assessed every 4 years via validated food frequency questionnaires.
The average age at baseline was 38 years, and the median follow-up time was 24 years, yielding a total of 78,328 person-years of follow-up. Alcohol consumption was divided into four categories: none; 0.1 g/day to 4.9 g/day; 5.0 to 14.9 g/day, and 15.0 g/day or higher.
A total of 897 incident cases of type 2 diabetes were reported during the study period. After adjustment for multiple dietary and lifestyle variables, including diet and physical activity, only alcohol consumption of 5.0-14.9 g/day (approximately half a drink to one drink) was associated with a significantly decreased risk for incident type 2 diabetes (hazard ratio, 0.45) compared with women who reported no alcohol consumption.
On further adjustment for body mass index, women who reported alcohol consumption in the 5.0-14.9 g/day range had a 41% lower risk for developing incident type 2 diabetes (HR, 0.59); alcohol consumption in the other ranges remained unassociated with type 2 diabetes risk, although the researchers noted that these estimates were attenuated.
The median daily intake for women who consumed alcohol was 2.3 g/day, approximately one drink per week. Beer was the most frequently consumed type of alcohol.
When the researchers analyzed the data by alcohol type, notably, “only beer consumption of 1 or more servings a week was associated with a lower risk for type 2 diabetes,” although previous studies have suggested a stronger association in diabetes risk reduction with wine consumption vs. beer, the researchers noted.
The study findings were the potential for confounding factors not included in the adjustment, potential underreporting of alcohol intake, and potential screening bias toward women who were more health conscious, the researchers noted. Other limitations were lack of generalizability given that most of the study participants were white women, and a lack of data on binge drinking and whether alcohol was consumed with meals, they added. The study strengths included the prospective design, large size, long-term follow-up, and use of validated questionnaires, they said.
The researchers cautioned that the results should not be interpreted without considering other health outcomes. “Consistent with the 2020 Dietary Guidelines for Americans, which recommend that adults who do not consume alcohol do not initiate drinking, it may not be prudent for those with a history of gestational diabetes who do not consume alcohol to initiate drinking alcohol solely to reduce their risk for type 2 diabetes,” they emphasized.
Risk/benefit ratio for alcohol includes many factors
“There is a relative paucity of data regarding women’s long-term health as it may relate to pregnancy and pregnancy outcomes,” Angela Bianco, MD, of Mount Sinai Hospital, New York, said in an interview.
Dr. Bianco said she was surprised by some of the study findings.
“Generally speaking, I consider alcohol to be of little to no nutritional value, and to have a high sugar content/glycemic index,” she said. “However, a reduced incidence of adult-onset diabetes has been observed among moderate drinkers in other large prospective studies as well,” she noted. “In contrast, some studies have shown an increased risk of diabetes among a proportion of subjects in the top alcohol consumption category, while other studies have found no association. Possible inconsistencies may be due to differences in drinking patterns and the types of beverages consumed,” Dr. Bianco explained.
A key point for clinicians to keep in mind is that “the study may be flawed based on the different criteria used to make a diagnosis of history of gestational diabetes, the fact that they excluded patients that did not return the questionnaires, and the fact that respondents may not have answered correctly due to recall bias” or other reasons, Dr. Bianco said. “Additionally, those who responded obviously had access to health care, which in and of itself is a confounder,” she noted.
Another key point is that “the effect of alcohol being consumed with or without a meal was not examined,” said Dr. Bianco. “Alcohol concentration is reduced if consumed with meals. Alcohol can lead to hypoglycemia (from reduced gluconeogenesis) during fasting states, but after meals (postprandial states) it can result in lower glucose disposal and higher blood glucose levels,” she said. “The available literature suggests that alcohol may improve insulin sensitivity and reduce resistance, but there is likely a U-shaped association between alcohol consumption and the risk of diabetes,” Dr. Bianco noted. “There is likely a delicate balance between benefits and risks of alcohol intake. The inherent benefit/risk ratio must take into account with other potential comorbidities including BMI, activity level, stress, and preexisting conditions,” she said.
“Additional long-term studies engaging patients with diverse ethnic and socioeconomic backgrounds with detailed information regarding the role of nutrition, alcohol intake, tobacco and drug use, environmental exposures, and medical comorbidities need to be performed,” Dr. Bianco concluded.
The study was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, the National Institute of General Medical Sciences, and the National Institute of Diabetes and Digestive and Kidney Diseases; the Nurses’ Health Study II was supported by the National Institutes of Health. Lead author Dr. Hinkle and coauthor Cuilin Zhang, MD, are employees of the U.S. federal government. The researchers and Dr. Bianco had no financial conflicts to disclose.
Among women with a history of gestational diabetes, alcohol intake of half a drink to one drink daily was associated with a 55% lower risk for subsequent type 2 diabetes, based on data from approximately 4,700 women in the Nurses’ Health Study II cohort.
However, the findings must be considered in the context of other risks and benefits of alcohol consumption before making statements or clinical recommendations, wrote Stefanie N. Hinkle, PhD, of the National Institutes of Health, Bethesda, Md., and colleagues.
Women with a history of gestational diabetes remain at increased risk for developing type 2 diabetes, so modifiable diet and lifestyle factors deserve further study, the researchers noted. Previous research has shown an association between light to moderate alcohol consumption and reduced risk of type 2 diabetes among women in the general population, but data on a similar risk reduction for women with a history of gestational diabetes are lacking, they added.
In a study published in JAMA Network Open, the researchers reviewed data from 4,740 women enrolled in the Nurses’ Health Study II who reported a history of gestational diabetes. These women were followed from Jan. 1, 1991, to Dec. 31, 2017, as part of the Diabetes & Women’s Health Study; dietary intake, including alcohol intake, was assessed every 4 years via validated food frequency questionnaires.
The average age at baseline was 38 years, and the median follow-up time was 24 years, yielding a total of 78,328 person-years of follow-up. Alcohol consumption was divided into four categories: none; 0.1 g/day to 4.9 g/day; 5.0 to 14.9 g/day, and 15.0 g/day or higher.
A total of 897 incident cases of type 2 diabetes were reported during the study period. After adjustment for multiple dietary and lifestyle variables, including diet and physical activity, only alcohol consumption of 5.0-14.9 g/day (approximately half a drink to one drink) was associated with a significantly decreased risk for incident type 2 diabetes (hazard ratio, 0.45) compared with women who reported no alcohol consumption.
On further adjustment for body mass index, women who reported alcohol consumption in the 5.0-14.9 g/day range had a 41% lower risk for developing incident type 2 diabetes (HR, 0.59); alcohol consumption in the other ranges remained unassociated with type 2 diabetes risk, although the researchers noted that these estimates were attenuated.
The median daily intake for women who consumed alcohol was 2.3 g/day, approximately one drink per week. Beer was the most frequently consumed type of alcohol.
When the researchers analyzed the data by alcohol type, notably, “only beer consumption of 1 or more servings a week was associated with a lower risk for type 2 diabetes,” although previous studies have suggested a stronger association in diabetes risk reduction with wine consumption vs. beer, the researchers noted.
The study findings were the potential for confounding factors not included in the adjustment, potential underreporting of alcohol intake, and potential screening bias toward women who were more health conscious, the researchers noted. Other limitations were lack of generalizability given that most of the study participants were white women, and a lack of data on binge drinking and whether alcohol was consumed with meals, they added. The study strengths included the prospective design, large size, long-term follow-up, and use of validated questionnaires, they said.
The researchers cautioned that the results should not be interpreted without considering other health outcomes. “Consistent with the 2020 Dietary Guidelines for Americans, which recommend that adults who do not consume alcohol do not initiate drinking, it may not be prudent for those with a history of gestational diabetes who do not consume alcohol to initiate drinking alcohol solely to reduce their risk for type 2 diabetes,” they emphasized.
Risk/benefit ratio for alcohol includes many factors
“There is a relative paucity of data regarding women’s long-term health as it may relate to pregnancy and pregnancy outcomes,” Angela Bianco, MD, of Mount Sinai Hospital, New York, said in an interview.
Dr. Bianco said she was surprised by some of the study findings.
“Generally speaking, I consider alcohol to be of little to no nutritional value, and to have a high sugar content/glycemic index,” she said. “However, a reduced incidence of adult-onset diabetes has been observed among moderate drinkers in other large prospective studies as well,” she noted. “In contrast, some studies have shown an increased risk of diabetes among a proportion of subjects in the top alcohol consumption category, while other studies have found no association. Possible inconsistencies may be due to differences in drinking patterns and the types of beverages consumed,” Dr. Bianco explained.
A key point for clinicians to keep in mind is that “the study may be flawed based on the different criteria used to make a diagnosis of history of gestational diabetes, the fact that they excluded patients that did not return the questionnaires, and the fact that respondents may not have answered correctly due to recall bias” or other reasons, Dr. Bianco said. “Additionally, those who responded obviously had access to health care, which in and of itself is a confounder,” she noted.
Another key point is that “the effect of alcohol being consumed with or without a meal was not examined,” said Dr. Bianco. “Alcohol concentration is reduced if consumed with meals. Alcohol can lead to hypoglycemia (from reduced gluconeogenesis) during fasting states, but after meals (postprandial states) it can result in lower glucose disposal and higher blood glucose levels,” she said. “The available literature suggests that alcohol may improve insulin sensitivity and reduce resistance, but there is likely a U-shaped association between alcohol consumption and the risk of diabetes,” Dr. Bianco noted. “There is likely a delicate balance between benefits and risks of alcohol intake. The inherent benefit/risk ratio must take into account with other potential comorbidities including BMI, activity level, stress, and preexisting conditions,” she said.
“Additional long-term studies engaging patients with diverse ethnic and socioeconomic backgrounds with detailed information regarding the role of nutrition, alcohol intake, tobacco and drug use, environmental exposures, and medical comorbidities need to be performed,” Dr. Bianco concluded.
The study was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, the National Institute of General Medical Sciences, and the National Institute of Diabetes and Digestive and Kidney Diseases; the Nurses’ Health Study II was supported by the National Institutes of Health. Lead author Dr. Hinkle and coauthor Cuilin Zhang, MD, are employees of the U.S. federal government. The researchers and Dr. Bianco had no financial conflicts to disclose.
Among women with a history of gestational diabetes, alcohol intake of half a drink to one drink daily was associated with a 55% lower risk for subsequent type 2 diabetes, based on data from approximately 4,700 women in the Nurses’ Health Study II cohort.
However, the findings must be considered in the context of other risks and benefits of alcohol consumption before making statements or clinical recommendations, wrote Stefanie N. Hinkle, PhD, of the National Institutes of Health, Bethesda, Md., and colleagues.
Women with a history of gestational diabetes remain at increased risk for developing type 2 diabetes, so modifiable diet and lifestyle factors deserve further study, the researchers noted. Previous research has shown an association between light to moderate alcohol consumption and reduced risk of type 2 diabetes among women in the general population, but data on a similar risk reduction for women with a history of gestational diabetes are lacking, they added.
In a study published in JAMA Network Open, the researchers reviewed data from 4,740 women enrolled in the Nurses’ Health Study II who reported a history of gestational diabetes. These women were followed from Jan. 1, 1991, to Dec. 31, 2017, as part of the Diabetes & Women’s Health Study; dietary intake, including alcohol intake, was assessed every 4 years via validated food frequency questionnaires.
The average age at baseline was 38 years, and the median follow-up time was 24 years, yielding a total of 78,328 person-years of follow-up. Alcohol consumption was divided into four categories: none; 0.1 g/day to 4.9 g/day; 5.0 to 14.9 g/day, and 15.0 g/day or higher.
A total of 897 incident cases of type 2 diabetes were reported during the study period. After adjustment for multiple dietary and lifestyle variables, including diet and physical activity, only alcohol consumption of 5.0-14.9 g/day (approximately half a drink to one drink) was associated with a significantly decreased risk for incident type 2 diabetes (hazard ratio, 0.45) compared with women who reported no alcohol consumption.
On further adjustment for body mass index, women who reported alcohol consumption in the 5.0-14.9 g/day range had a 41% lower risk for developing incident type 2 diabetes (HR, 0.59); alcohol consumption in the other ranges remained unassociated with type 2 diabetes risk, although the researchers noted that these estimates were attenuated.
The median daily intake for women who consumed alcohol was 2.3 g/day, approximately one drink per week. Beer was the most frequently consumed type of alcohol.
When the researchers analyzed the data by alcohol type, notably, “only beer consumption of 1 or more servings a week was associated with a lower risk for type 2 diabetes,” although previous studies have suggested a stronger association in diabetes risk reduction with wine consumption vs. beer, the researchers noted.
The study findings were the potential for confounding factors not included in the adjustment, potential underreporting of alcohol intake, and potential screening bias toward women who were more health conscious, the researchers noted. Other limitations were lack of generalizability given that most of the study participants were white women, and a lack of data on binge drinking and whether alcohol was consumed with meals, they added. The study strengths included the prospective design, large size, long-term follow-up, and use of validated questionnaires, they said.
The researchers cautioned that the results should not be interpreted without considering other health outcomes. “Consistent with the 2020 Dietary Guidelines for Americans, which recommend that adults who do not consume alcohol do not initiate drinking, it may not be prudent for those with a history of gestational diabetes who do not consume alcohol to initiate drinking alcohol solely to reduce their risk for type 2 diabetes,” they emphasized.
Risk/benefit ratio for alcohol includes many factors
“There is a relative paucity of data regarding women’s long-term health as it may relate to pregnancy and pregnancy outcomes,” Angela Bianco, MD, of Mount Sinai Hospital, New York, said in an interview.
Dr. Bianco said she was surprised by some of the study findings.
“Generally speaking, I consider alcohol to be of little to no nutritional value, and to have a high sugar content/glycemic index,” she said. “However, a reduced incidence of adult-onset diabetes has been observed among moderate drinkers in other large prospective studies as well,” she noted. “In contrast, some studies have shown an increased risk of diabetes among a proportion of subjects in the top alcohol consumption category, while other studies have found no association. Possible inconsistencies may be due to differences in drinking patterns and the types of beverages consumed,” Dr. Bianco explained.
A key point for clinicians to keep in mind is that “the study may be flawed based on the different criteria used to make a diagnosis of history of gestational diabetes, the fact that they excluded patients that did not return the questionnaires, and the fact that respondents may not have answered correctly due to recall bias” or other reasons, Dr. Bianco said. “Additionally, those who responded obviously had access to health care, which in and of itself is a confounder,” she noted.
Another key point is that “the effect of alcohol being consumed with or without a meal was not examined,” said Dr. Bianco. “Alcohol concentration is reduced if consumed with meals. Alcohol can lead to hypoglycemia (from reduced gluconeogenesis) during fasting states, but after meals (postprandial states) it can result in lower glucose disposal and higher blood glucose levels,” she said. “The available literature suggests that alcohol may improve insulin sensitivity and reduce resistance, but there is likely a U-shaped association between alcohol consumption and the risk of diabetes,” Dr. Bianco noted. “There is likely a delicate balance between benefits and risks of alcohol intake. The inherent benefit/risk ratio must take into account with other potential comorbidities including BMI, activity level, stress, and preexisting conditions,” she said.
“Additional long-term studies engaging patients with diverse ethnic and socioeconomic backgrounds with detailed information regarding the role of nutrition, alcohol intake, tobacco and drug use, environmental exposures, and medical comorbidities need to be performed,” Dr. Bianco concluded.
The study was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, the National Institute of General Medical Sciences, and the National Institute of Diabetes and Digestive and Kidney Diseases; the Nurses’ Health Study II was supported by the National Institutes of Health. Lead author Dr. Hinkle and coauthor Cuilin Zhang, MD, are employees of the U.S. federal government. The researchers and Dr. Bianco had no financial conflicts to disclose.
FROM JAMA NETWORK OPEN
Overlapping Phenotypic Features of PTEN Hamartoma Tumor Syndrome and Birt-Hogg-Dubé Syndrome
To the Editor:
PTEN hamartoma tumor syndrome (PHTS) encompasses a spectrum of disorders that most commonly are caused by autosomal-dominant germline mutations in the phosphatase and tensin homolog, PTEN, tumor suppressor gene on chromosome 10q23. We describe a patient who presented with clinical features of PHTS and Birt-Hogg-Dubé syndrome (BHDS). Because the genetic mutations associated with both PHTS and BHDS result in altered mammalian target of rapamycin (mTOR) signaling, patients may have overlapping phenotypic features.
A 51-year-old man with a history of multiple carcinomas presented for evaluation of flesh-colored papules on the cheeks, nose, tongue, and hands, in addition to numerous skin tags on the neck, axillae, and lower abdomen bilaterally. His medical history was notable for several nasal and gastrointestinal tract polyps, chromophobe renal cell carcinoma, cutaneous lipomas, atypical carcinoid syndrome of the right lung, and a multinodular thyroid. His family history was notable for small cell lung cancer in his father, breast cancer and pancreatic cancer in his maternal aunt, esophageal cancer in his maternal grandfather, and celiac disease in his daughter.
Clinical examination revealed flesh-colored, dome-shaped papules measuring 1 to 2 mm in diameter on the nose and cheeks (Figure 1). He had hyperkeratotic papules on the dorsal fingers, consistent with acral keratoses. Additionally, multiple flesh-colored papules with a cobblestonelike appearance were noted on the oral mucosa (Figure 2). Other findings included pedunculated papules on the neck, axillae, and lower abdomen bilaterally, consistent with fibroepithelial polyps, as well as hyperpigmented velvety plaques on the axillae, characteristic of acanthosis nigricans (Figure 3). A shave biopsy of a papule on the right cheek revealed a proliferation of plump stellate fibroblasts, small blood vessels, and thick collagen bundles, characteristic of a fibrous papule (Figure 4).
Differential diagnoses for our patient included BHDS and Cowden syndrome (CS). Due to the combination of extensive family history of multiorgan cancers as well as the clinical findings, he was referred to a geneticist for further evaluation. Genetic analysis was positive for a heterozygous mutation variant of uncertain significance in the PTEN gene.
The PHTS disorders include CS, Bannayan-Riley-Ruvalcaba syndrome, Lhermitte-Duclos disease, Proteus syndrome, and Proteus-like syndrome (Table).1-9 Our patient’s clinical findings were indicative of CS, a rare genodermatosis characterized by multiple hamartomas and neoplasms of ectodermal, mesodermal, and endodermal origin.1 Most CS patients develop trichilemmomas of the central face, mucocutaneous papillomatous papules, and acral and plantar keratoses by the third decade of life.1 Importantly, CS patients have an increased risk for breast, thyroid, renal, endometrial, and colorectal cancers, as well as melanoma, with estimated lifetime risks of 85%, 35%, 33%, 28%, 9%, and 6%, respectively.2,10
Regarding the pathophysiology of PHTS disorders, PTEN encodes a phosphatase that inhibits phosphoinositide 3-kinase/Akt and mTOR signaling pathways, thereby controlling cell proliferation, cell-cycle progression, and apoptosis.2,3 Loss of PTEN function, as seen in CS patients, results in an increased risk for cancer.2 Other genetic diseases, including juvenile polyposis syndrome, Proteus syndrome, tuberous sclerosis, and Peutz-Jeghers syndrome, have phenotypic similarities to PHTS.3 Specifically, loss-of-function mutations of TSC1 and TSC2, tumor suppressor genes associated with tuberous sclerosis, similarly result in dysregulation of mTOR signaling.
Our patient also had some clinical features characteristic of BHDS, such as flesh-colored facial papules, acrochordonlike lesions, and chromophobe renal cell carcinoma.11 Birt-Hogg-Dubé syndrome most often is caused by an autosomal-dominant germline mutation in FLCN, a tumor suppressor gene.11 Interestingly, FLCN interacts with AMP-activated protein kinase to help regulate mTOR signaling, which may explain phenotypic similarities seen in CS and BHDS.12
Because the PHTS disorders and BHDS result in similar functional consequences on the mTOR signaling pathway, patients can present with overlapping clinical features that may be diagnostically challenging. Management includes patient education regarding cancer risk, surveillance for early detection of malignancy, and genetic counseling for family members.2 It is important for clinicians to appreciate phenotypic similarities between PHTS and other disorders affecting mTOR signaling to prevent delays in diagnosis.
- Nosé V. Genodermatosis affecting the skin and mucosa of the head and neck: clinicopathologic, genetic, and molecular aspect—PTEN-hamartoma tumor syndrome/Cowden syndrome. Head Neck Pathol. 2016;10:131-138.
- Porto A, Roider E, Ruzicka T. Cowden syndrome: report of a case and brief review of literature. An Bras Dermatol. 2013;88(6 suppl 1):S52-S55.
- Leslie N, Longy M. Inherited PTEN mutations and the prediction of phenotype. Semin Cell Dev Biol. 2016;52:30-38.
- The National Comprehensive Cancer Network (NCCN) clinical practice guidelines in oncology. genetic/familial high-risk assessment: breast and ovarian (version 1.2017). Published September 19, 2016. Accessed August 11, 2021. https://www.nccn.org/professionals/physician_gls/pdf/genetics_screening.pdf
- Laury AR, Bongiovanni M, Tille J, et al. Thyroid pathology in PTEN-hamartoma tumor syndrome: characteristic findings of a distinct entity. Thyroid. 2011;21:135-144.
- Eng C. PTEN hamartoma tumor syndrome. In: Pagon RA, Adam MP, Ardinger HH, et al, eds. GeneReviews. University of Washington; 2001.
- Golden N, Tjokorda MGB, Sri M, et al. Management of unusual dysplastic gangliocytoma of the cerebellum (Lhermitte-Duclos disease) in a developing country: case report and review of the literature. Asian J Neurosurg. 2016;11:170.
- Biesecker LG, Happle R, Mulliken JB, et al. Proteus syndrome: diagnostic criteria, differential diagnosis, and patient evaluation. Am J Med Genet. 1999;84:389-395.
- Busa T, Milh M, Degardin N, et al. Clinical presentation of PTEN mutations in childhood in the absence of family history of Cowden syndrome. Eur J Paediatr Neurol. 2015;19:188-192.
- Tan MH, Mester JL, Ngeow J, et al. Lifetime cancer risks in individuals with germline PTEN mutations. Clin Cancer Res. 2012;18:400-407.
- Ponti G, Pellacani G, Seidenari S, et al. Cancer-associated genodermatoses: skin neoplasms as clues to hereditary tumor syndromes. Crit Rev Oncol Hematol. 2013;85:239-256.
- Baba M, Hong S, Sharma N, et al. Folliculin encoded by the BHD gene interacts with a binding protein, FNIP1, and AMPK, and is involved in AMPK and mTOR signaling. Proc Natl Acad Sci U S A. 2006;103:15552-15557.
To the Editor:
PTEN hamartoma tumor syndrome (PHTS) encompasses a spectrum of disorders that most commonly are caused by autosomal-dominant germline mutations in the phosphatase and tensin homolog, PTEN, tumor suppressor gene on chromosome 10q23. We describe a patient who presented with clinical features of PHTS and Birt-Hogg-Dubé syndrome (BHDS). Because the genetic mutations associated with both PHTS and BHDS result in altered mammalian target of rapamycin (mTOR) signaling, patients may have overlapping phenotypic features.
A 51-year-old man with a history of multiple carcinomas presented for evaluation of flesh-colored papules on the cheeks, nose, tongue, and hands, in addition to numerous skin tags on the neck, axillae, and lower abdomen bilaterally. His medical history was notable for several nasal and gastrointestinal tract polyps, chromophobe renal cell carcinoma, cutaneous lipomas, atypical carcinoid syndrome of the right lung, and a multinodular thyroid. His family history was notable for small cell lung cancer in his father, breast cancer and pancreatic cancer in his maternal aunt, esophageal cancer in his maternal grandfather, and celiac disease in his daughter.
Clinical examination revealed flesh-colored, dome-shaped papules measuring 1 to 2 mm in diameter on the nose and cheeks (Figure 1). He had hyperkeratotic papules on the dorsal fingers, consistent with acral keratoses. Additionally, multiple flesh-colored papules with a cobblestonelike appearance were noted on the oral mucosa (Figure 2). Other findings included pedunculated papules on the neck, axillae, and lower abdomen bilaterally, consistent with fibroepithelial polyps, as well as hyperpigmented velvety plaques on the axillae, characteristic of acanthosis nigricans (Figure 3). A shave biopsy of a papule on the right cheek revealed a proliferation of plump stellate fibroblasts, small blood vessels, and thick collagen bundles, characteristic of a fibrous papule (Figure 4).
Differential diagnoses for our patient included BHDS and Cowden syndrome (CS). Due to the combination of extensive family history of multiorgan cancers as well as the clinical findings, he was referred to a geneticist for further evaluation. Genetic analysis was positive for a heterozygous mutation variant of uncertain significance in the PTEN gene.
The PHTS disorders include CS, Bannayan-Riley-Ruvalcaba syndrome, Lhermitte-Duclos disease, Proteus syndrome, and Proteus-like syndrome (Table).1-9 Our patient’s clinical findings were indicative of CS, a rare genodermatosis characterized by multiple hamartomas and neoplasms of ectodermal, mesodermal, and endodermal origin.1 Most CS patients develop trichilemmomas of the central face, mucocutaneous papillomatous papules, and acral and plantar keratoses by the third decade of life.1 Importantly, CS patients have an increased risk for breast, thyroid, renal, endometrial, and colorectal cancers, as well as melanoma, with estimated lifetime risks of 85%, 35%, 33%, 28%, 9%, and 6%, respectively.2,10
Regarding the pathophysiology of PHTS disorders, PTEN encodes a phosphatase that inhibits phosphoinositide 3-kinase/Akt and mTOR signaling pathways, thereby controlling cell proliferation, cell-cycle progression, and apoptosis.2,3 Loss of PTEN function, as seen in CS patients, results in an increased risk for cancer.2 Other genetic diseases, including juvenile polyposis syndrome, Proteus syndrome, tuberous sclerosis, and Peutz-Jeghers syndrome, have phenotypic similarities to PHTS.3 Specifically, loss-of-function mutations of TSC1 and TSC2, tumor suppressor genes associated with tuberous sclerosis, similarly result in dysregulation of mTOR signaling.
Our patient also had some clinical features characteristic of BHDS, such as flesh-colored facial papules, acrochordonlike lesions, and chromophobe renal cell carcinoma.11 Birt-Hogg-Dubé syndrome most often is caused by an autosomal-dominant germline mutation in FLCN, a tumor suppressor gene.11 Interestingly, FLCN interacts with AMP-activated protein kinase to help regulate mTOR signaling, which may explain phenotypic similarities seen in CS and BHDS.12
Because the PHTS disorders and BHDS result in similar functional consequences on the mTOR signaling pathway, patients can present with overlapping clinical features that may be diagnostically challenging. Management includes patient education regarding cancer risk, surveillance for early detection of malignancy, and genetic counseling for family members.2 It is important for clinicians to appreciate phenotypic similarities between PHTS and other disorders affecting mTOR signaling to prevent delays in diagnosis.
To the Editor:
PTEN hamartoma tumor syndrome (PHTS) encompasses a spectrum of disorders that most commonly are caused by autosomal-dominant germline mutations in the phosphatase and tensin homolog, PTEN, tumor suppressor gene on chromosome 10q23. We describe a patient who presented with clinical features of PHTS and Birt-Hogg-Dubé syndrome (BHDS). Because the genetic mutations associated with both PHTS and BHDS result in altered mammalian target of rapamycin (mTOR) signaling, patients may have overlapping phenotypic features.
A 51-year-old man with a history of multiple carcinomas presented for evaluation of flesh-colored papules on the cheeks, nose, tongue, and hands, in addition to numerous skin tags on the neck, axillae, and lower abdomen bilaterally. His medical history was notable for several nasal and gastrointestinal tract polyps, chromophobe renal cell carcinoma, cutaneous lipomas, atypical carcinoid syndrome of the right lung, and a multinodular thyroid. His family history was notable for small cell lung cancer in his father, breast cancer and pancreatic cancer in his maternal aunt, esophageal cancer in his maternal grandfather, and celiac disease in his daughter.
Clinical examination revealed flesh-colored, dome-shaped papules measuring 1 to 2 mm in diameter on the nose and cheeks (Figure 1). He had hyperkeratotic papules on the dorsal fingers, consistent with acral keratoses. Additionally, multiple flesh-colored papules with a cobblestonelike appearance were noted on the oral mucosa (Figure 2). Other findings included pedunculated papules on the neck, axillae, and lower abdomen bilaterally, consistent with fibroepithelial polyps, as well as hyperpigmented velvety plaques on the axillae, characteristic of acanthosis nigricans (Figure 3). A shave biopsy of a papule on the right cheek revealed a proliferation of plump stellate fibroblasts, small blood vessels, and thick collagen bundles, characteristic of a fibrous papule (Figure 4).
Differential diagnoses for our patient included BHDS and Cowden syndrome (CS). Due to the combination of extensive family history of multiorgan cancers as well as the clinical findings, he was referred to a geneticist for further evaluation. Genetic analysis was positive for a heterozygous mutation variant of uncertain significance in the PTEN gene.
The PHTS disorders include CS, Bannayan-Riley-Ruvalcaba syndrome, Lhermitte-Duclos disease, Proteus syndrome, and Proteus-like syndrome (Table).1-9 Our patient’s clinical findings were indicative of CS, a rare genodermatosis characterized by multiple hamartomas and neoplasms of ectodermal, mesodermal, and endodermal origin.1 Most CS patients develop trichilemmomas of the central face, mucocutaneous papillomatous papules, and acral and plantar keratoses by the third decade of life.1 Importantly, CS patients have an increased risk for breast, thyroid, renal, endometrial, and colorectal cancers, as well as melanoma, with estimated lifetime risks of 85%, 35%, 33%, 28%, 9%, and 6%, respectively.2,10
Regarding the pathophysiology of PHTS disorders, PTEN encodes a phosphatase that inhibits phosphoinositide 3-kinase/Akt and mTOR signaling pathways, thereby controlling cell proliferation, cell-cycle progression, and apoptosis.2,3 Loss of PTEN function, as seen in CS patients, results in an increased risk for cancer.2 Other genetic diseases, including juvenile polyposis syndrome, Proteus syndrome, tuberous sclerosis, and Peutz-Jeghers syndrome, have phenotypic similarities to PHTS.3 Specifically, loss-of-function mutations of TSC1 and TSC2, tumor suppressor genes associated with tuberous sclerosis, similarly result in dysregulation of mTOR signaling.
Our patient also had some clinical features characteristic of BHDS, such as flesh-colored facial papules, acrochordonlike lesions, and chromophobe renal cell carcinoma.11 Birt-Hogg-Dubé syndrome most often is caused by an autosomal-dominant germline mutation in FLCN, a tumor suppressor gene.11 Interestingly, FLCN interacts with AMP-activated protein kinase to help regulate mTOR signaling, which may explain phenotypic similarities seen in CS and BHDS.12
Because the PHTS disorders and BHDS result in similar functional consequences on the mTOR signaling pathway, patients can present with overlapping clinical features that may be diagnostically challenging. Management includes patient education regarding cancer risk, surveillance for early detection of malignancy, and genetic counseling for family members.2 It is important for clinicians to appreciate phenotypic similarities between PHTS and other disorders affecting mTOR signaling to prevent delays in diagnosis.
- Nosé V. Genodermatosis affecting the skin and mucosa of the head and neck: clinicopathologic, genetic, and molecular aspect—PTEN-hamartoma tumor syndrome/Cowden syndrome. Head Neck Pathol. 2016;10:131-138.
- Porto A, Roider E, Ruzicka T. Cowden syndrome: report of a case and brief review of literature. An Bras Dermatol. 2013;88(6 suppl 1):S52-S55.
- Leslie N, Longy M. Inherited PTEN mutations and the prediction of phenotype. Semin Cell Dev Biol. 2016;52:30-38.
- The National Comprehensive Cancer Network (NCCN) clinical practice guidelines in oncology. genetic/familial high-risk assessment: breast and ovarian (version 1.2017). Published September 19, 2016. Accessed August 11, 2021. https://www.nccn.org/professionals/physician_gls/pdf/genetics_screening.pdf
- Laury AR, Bongiovanni M, Tille J, et al. Thyroid pathology in PTEN-hamartoma tumor syndrome: characteristic findings of a distinct entity. Thyroid. 2011;21:135-144.
- Eng C. PTEN hamartoma tumor syndrome. In: Pagon RA, Adam MP, Ardinger HH, et al, eds. GeneReviews. University of Washington; 2001.
- Golden N, Tjokorda MGB, Sri M, et al. Management of unusual dysplastic gangliocytoma of the cerebellum (Lhermitte-Duclos disease) in a developing country: case report and review of the literature. Asian J Neurosurg. 2016;11:170.
- Biesecker LG, Happle R, Mulliken JB, et al. Proteus syndrome: diagnostic criteria, differential diagnosis, and patient evaluation. Am J Med Genet. 1999;84:389-395.
- Busa T, Milh M, Degardin N, et al. Clinical presentation of PTEN mutations in childhood in the absence of family history of Cowden syndrome. Eur J Paediatr Neurol. 2015;19:188-192.
- Tan MH, Mester JL, Ngeow J, et al. Lifetime cancer risks in individuals with germline PTEN mutations. Clin Cancer Res. 2012;18:400-407.
- Ponti G, Pellacani G, Seidenari S, et al. Cancer-associated genodermatoses: skin neoplasms as clues to hereditary tumor syndromes. Crit Rev Oncol Hematol. 2013;85:239-256.
- Baba M, Hong S, Sharma N, et al. Folliculin encoded by the BHD gene interacts with a binding protein, FNIP1, and AMPK, and is involved in AMPK and mTOR signaling. Proc Natl Acad Sci U S A. 2006;103:15552-15557.
- Nosé V. Genodermatosis affecting the skin and mucosa of the head and neck: clinicopathologic, genetic, and molecular aspect—PTEN-hamartoma tumor syndrome/Cowden syndrome. Head Neck Pathol. 2016;10:131-138.
- Porto A, Roider E, Ruzicka T. Cowden syndrome: report of a case and brief review of literature. An Bras Dermatol. 2013;88(6 suppl 1):S52-S55.
- Leslie N, Longy M. Inherited PTEN mutations and the prediction of phenotype. Semin Cell Dev Biol. 2016;52:30-38.
- The National Comprehensive Cancer Network (NCCN) clinical practice guidelines in oncology. genetic/familial high-risk assessment: breast and ovarian (version 1.2017). Published September 19, 2016. Accessed August 11, 2021. https://www.nccn.org/professionals/physician_gls/pdf/genetics_screening.pdf
- Laury AR, Bongiovanni M, Tille J, et al. Thyroid pathology in PTEN-hamartoma tumor syndrome: characteristic findings of a distinct entity. Thyroid. 2011;21:135-144.
- Eng C. PTEN hamartoma tumor syndrome. In: Pagon RA, Adam MP, Ardinger HH, et al, eds. GeneReviews. University of Washington; 2001.
- Golden N, Tjokorda MGB, Sri M, et al. Management of unusual dysplastic gangliocytoma of the cerebellum (Lhermitte-Duclos disease) in a developing country: case report and review of the literature. Asian J Neurosurg. 2016;11:170.
- Biesecker LG, Happle R, Mulliken JB, et al. Proteus syndrome: diagnostic criteria, differential diagnosis, and patient evaluation. Am J Med Genet. 1999;84:389-395.
- Busa T, Milh M, Degardin N, et al. Clinical presentation of PTEN mutations in childhood in the absence of family history of Cowden syndrome. Eur J Paediatr Neurol. 2015;19:188-192.
- Tan MH, Mester JL, Ngeow J, et al. Lifetime cancer risks in individuals with germline PTEN mutations. Clin Cancer Res. 2012;18:400-407.
- Ponti G, Pellacani G, Seidenari S, et al. Cancer-associated genodermatoses: skin neoplasms as clues to hereditary tumor syndromes. Crit Rev Oncol Hematol. 2013;85:239-256.
- Baba M, Hong S, Sharma N, et al. Folliculin encoded by the BHD gene interacts with a binding protein, FNIP1, and AMPK, and is involved in AMPK and mTOR signaling. Proc Natl Acad Sci U S A. 2006;103:15552-15557.
PRACTICE POINTS
- PTEN hamartoma tumor syndrome (PHTS) represents a spectrum of disorders caused by autosomal-dominant germline mutations in PTEN.
- Our patient presented with phenotypic features of PHTS and Birt-Hogg-Dubé syndrome. Given that both syndromes cause alterations in mammalian target of rapamycin signaling, overlapping phenotypic features may be seen.
- Recognizing overlapping phenotypic features of these syndromes will allow for timely diagnosis and surveillance for malignancy.