User login
New COVID-19 strain has reached the U.S.
Deadline, citing a Centers for Disease Control and Prevention report, said 26 residents and 20 workers tested positive for COVID-19 at a skilled care nursing home. The facility has 83 residents and 116 employees.
On March 1, 28 specimens that had been subjected to whole genome sequencing were found to have “mutations aligning with the R.1 lineage,” Deadline said.
About 90% of the facility’s residents and 52% of the staff had received two COVID vaccine doses, the CDC said. Because of the high vaccination rate, the finding raises concerns about “reduced protective immunity” in relation to the R.1 variant, the CDC said.
However, the nursing home case appears to show that the vaccine keeps most people from getting extremely sick, the CDC said. The vaccine was 86.5% protective against symptomatic illness among residents and 87.1% protective for employees.
“Compared with unvaccinated persons, vaccinated persons had reduced risk for SARS-CoV-2 infection and symptomatic COVID-19,” the CDC said. The vaccination of nursing home residents and health care workers “is essential to reduce the risk for symptomatic COVID-19, as is continued focus on infection prevention and control practices,” the CDC said.
Since being reported in Kentucky, R.1 has been detected more than 10,000 times in the United States, Forbes reported, basing that number on entries in the GISAID SARS-CoV-2 database.
Overall, more than 42 million cases of COVID have been reported since the start of the pandemic.
Deadline reported that the R.1 strain was first detected in Japan in January among three members of one family. The family members had no history of traveling abroad, Deadline said, citing an National Institutes of Health report.
The CDC has not classified R.1 as a variant of concern yet but noted it has “several mutations of importance” and “demonstrates evidence of increasing virus transmissibility.”
A version of this article first appeared on WebMD.com.
Deadline, citing a Centers for Disease Control and Prevention report, said 26 residents and 20 workers tested positive for COVID-19 at a skilled care nursing home. The facility has 83 residents and 116 employees.
On March 1, 28 specimens that had been subjected to whole genome sequencing were found to have “mutations aligning with the R.1 lineage,” Deadline said.
About 90% of the facility’s residents and 52% of the staff had received two COVID vaccine doses, the CDC said. Because of the high vaccination rate, the finding raises concerns about “reduced protective immunity” in relation to the R.1 variant, the CDC said.
However, the nursing home case appears to show that the vaccine keeps most people from getting extremely sick, the CDC said. The vaccine was 86.5% protective against symptomatic illness among residents and 87.1% protective for employees.
“Compared with unvaccinated persons, vaccinated persons had reduced risk for SARS-CoV-2 infection and symptomatic COVID-19,” the CDC said. The vaccination of nursing home residents and health care workers “is essential to reduce the risk for symptomatic COVID-19, as is continued focus on infection prevention and control practices,” the CDC said.
Since being reported in Kentucky, R.1 has been detected more than 10,000 times in the United States, Forbes reported, basing that number on entries in the GISAID SARS-CoV-2 database.
Overall, more than 42 million cases of COVID have been reported since the start of the pandemic.
Deadline reported that the R.1 strain was first detected in Japan in January among three members of one family. The family members had no history of traveling abroad, Deadline said, citing an National Institutes of Health report.
The CDC has not classified R.1 as a variant of concern yet but noted it has “several mutations of importance” and “demonstrates evidence of increasing virus transmissibility.”
A version of this article first appeared on WebMD.com.
Deadline, citing a Centers for Disease Control and Prevention report, said 26 residents and 20 workers tested positive for COVID-19 at a skilled care nursing home. The facility has 83 residents and 116 employees.
On March 1, 28 specimens that had been subjected to whole genome sequencing were found to have “mutations aligning with the R.1 lineage,” Deadline said.
About 90% of the facility’s residents and 52% of the staff had received two COVID vaccine doses, the CDC said. Because of the high vaccination rate, the finding raises concerns about “reduced protective immunity” in relation to the R.1 variant, the CDC said.
However, the nursing home case appears to show that the vaccine keeps most people from getting extremely sick, the CDC said. The vaccine was 86.5% protective against symptomatic illness among residents and 87.1% protective for employees.
“Compared with unvaccinated persons, vaccinated persons had reduced risk for SARS-CoV-2 infection and symptomatic COVID-19,” the CDC said. The vaccination of nursing home residents and health care workers “is essential to reduce the risk for symptomatic COVID-19, as is continued focus on infection prevention and control practices,” the CDC said.
Since being reported in Kentucky, R.1 has been detected more than 10,000 times in the United States, Forbes reported, basing that number on entries in the GISAID SARS-CoV-2 database.
Overall, more than 42 million cases of COVID have been reported since the start of the pandemic.
Deadline reported that the R.1 strain was first detected in Japan in January among three members of one family. The family members had no history of traveling abroad, Deadline said, citing an National Institutes of Health report.
The CDC has not classified R.1 as a variant of concern yet but noted it has “several mutations of importance” and “demonstrates evidence of increasing virus transmissibility.”
A version of this article first appeared on WebMD.com.
Sexual assault in women tied to increased stroke, dementia risk
Traumatic experiences, especially sexual assault, may put women at greater risk for poor brain health.
In the Ms Brain study, middle-aged women with trauma exposure had a greater volume of white matter hyperintensities (WMHs) than those without trauma. In addition, the differences persisted even after adjusting for depressive or post-traumatic stress symptoms.
WMHs are “an important indicator of small vessel disease in the brain and have been linked to future stroke risk, dementia risk, and mortality,” lead investigator Rebecca Thurston, PhD, from the University of Pittsburgh, told this news organization.
“What I take from this is, really, that sexual assault has implications for women’s health, far beyond exclusively mental health outcomes, but also for their cardiovascular health, as we have shown in other work and for their stroke and dementia risk as we are seeing in the present work,” Dr. Thurston added.
The study was presented at the North American Menopause Society (NAMS) Annual Meeting in Washington, D.C., and has been accepted for publication in the journal Brain Imaging and Behavior.
Beyond the usual suspects
As part of the study, 145 women (mean age, 59 years) free of clinical cardiovascular disease, stroke, or dementia provided their medical history, including history of traumatic experiences, depression, and post-traumatic stress disorder and underwent magnetic resonance brain imaging for WMHs.
More than two-thirds (68%) of the women reported at least one trauma, most commonly sexual assault (23%).
In multivariate analysis, women with trauma exposure had greater WMH volume than women without trauma (P = .01), with sexual assault most strongly associated with greater WMH volume (P = .02).
The associations persisted after adjusting for depressive or post-traumatic stress symptoms.
“A history of sexual assault was particularly related to white matter hyperintensities in the parietal lobe, and these kinds of white matter hyperintensities have been linked to Alzheimer’s disease in a fairly pronounced way,” Dr. Thurston said.
“When we think about risk factors for stroke, dementia, we need to think beyond exclusively our usual suspects and also think about women [who experienced] psychological trauma and experienced sexual assault in particular. So ask about it and consider it part of your screening regimen,” she added.
‘Burgeoning’ literature
Commenting on the findings, Charles Nemeroff, MD, PhD, professor and chair, department of psychiatry and behavioral sciences, Dell Medical School, University of Texas at Austin, and director of its Institute for Early Life Adversity Research, said the research adds to the “burgeoning literature on the long term neurobiological consequences of trauma and more specifically, sexual abuse, on brain imaging measures.”
“Our group and others reported several years ago that patients with mood disorders, more specifically bipolar disorder and major depression, had higher rates of WMH than matched controls. Those older studies did not control for a history of early life adversity such as childhood maltreatment,” Dr. Nemeroff said.
“In addition to this finding of increased WMH in subjects exposed to trauma is a very large literature documenting other central nervous system (CNS) changes in this population, including cortical thinning in certain brain areas and clearly an emerging finding that different forms of childhood maltreatment are associated with quite distinct structural brain alterations in adulthood,” he noted.
The study was supported by grants from the National Institutes of Health. Dr. Thurston and Dr. Nemeroff have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Traumatic experiences, especially sexual assault, may put women at greater risk for poor brain health.
In the Ms Brain study, middle-aged women with trauma exposure had a greater volume of white matter hyperintensities (WMHs) than those without trauma. In addition, the differences persisted even after adjusting for depressive or post-traumatic stress symptoms.
WMHs are “an important indicator of small vessel disease in the brain and have been linked to future stroke risk, dementia risk, and mortality,” lead investigator Rebecca Thurston, PhD, from the University of Pittsburgh, told this news organization.
“What I take from this is, really, that sexual assault has implications for women’s health, far beyond exclusively mental health outcomes, but also for their cardiovascular health, as we have shown in other work and for their stroke and dementia risk as we are seeing in the present work,” Dr. Thurston added.
The study was presented at the North American Menopause Society (NAMS) Annual Meeting in Washington, D.C., and has been accepted for publication in the journal Brain Imaging and Behavior.
Beyond the usual suspects
As part of the study, 145 women (mean age, 59 years) free of clinical cardiovascular disease, stroke, or dementia provided their medical history, including history of traumatic experiences, depression, and post-traumatic stress disorder and underwent magnetic resonance brain imaging for WMHs.
More than two-thirds (68%) of the women reported at least one trauma, most commonly sexual assault (23%).
In multivariate analysis, women with trauma exposure had greater WMH volume than women without trauma (P = .01), with sexual assault most strongly associated with greater WMH volume (P = .02).
The associations persisted after adjusting for depressive or post-traumatic stress symptoms.
“A history of sexual assault was particularly related to white matter hyperintensities in the parietal lobe, and these kinds of white matter hyperintensities have been linked to Alzheimer’s disease in a fairly pronounced way,” Dr. Thurston said.
“When we think about risk factors for stroke, dementia, we need to think beyond exclusively our usual suspects and also think about women [who experienced] psychological trauma and experienced sexual assault in particular. So ask about it and consider it part of your screening regimen,” she added.
‘Burgeoning’ literature
Commenting on the findings, Charles Nemeroff, MD, PhD, professor and chair, department of psychiatry and behavioral sciences, Dell Medical School, University of Texas at Austin, and director of its Institute for Early Life Adversity Research, said the research adds to the “burgeoning literature on the long term neurobiological consequences of trauma and more specifically, sexual abuse, on brain imaging measures.”
“Our group and others reported several years ago that patients with mood disorders, more specifically bipolar disorder and major depression, had higher rates of WMH than matched controls. Those older studies did not control for a history of early life adversity such as childhood maltreatment,” Dr. Nemeroff said.
“In addition to this finding of increased WMH in subjects exposed to trauma is a very large literature documenting other central nervous system (CNS) changes in this population, including cortical thinning in certain brain areas and clearly an emerging finding that different forms of childhood maltreatment are associated with quite distinct structural brain alterations in adulthood,” he noted.
The study was supported by grants from the National Institutes of Health. Dr. Thurston and Dr. Nemeroff have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Traumatic experiences, especially sexual assault, may put women at greater risk for poor brain health.
In the Ms Brain study, middle-aged women with trauma exposure had a greater volume of white matter hyperintensities (WMHs) than those without trauma. In addition, the differences persisted even after adjusting for depressive or post-traumatic stress symptoms.
WMHs are “an important indicator of small vessel disease in the brain and have been linked to future stroke risk, dementia risk, and mortality,” lead investigator Rebecca Thurston, PhD, from the University of Pittsburgh, told this news organization.
“What I take from this is, really, that sexual assault has implications for women’s health, far beyond exclusively mental health outcomes, but also for their cardiovascular health, as we have shown in other work and for their stroke and dementia risk as we are seeing in the present work,” Dr. Thurston added.
The study was presented at the North American Menopause Society (NAMS) Annual Meeting in Washington, D.C., and has been accepted for publication in the journal Brain Imaging and Behavior.
Beyond the usual suspects
As part of the study, 145 women (mean age, 59 years) free of clinical cardiovascular disease, stroke, or dementia provided their medical history, including history of traumatic experiences, depression, and post-traumatic stress disorder and underwent magnetic resonance brain imaging for WMHs.
More than two-thirds (68%) of the women reported at least one trauma, most commonly sexual assault (23%).
In multivariate analysis, women with trauma exposure had greater WMH volume than women without trauma (P = .01), with sexual assault most strongly associated with greater WMH volume (P = .02).
The associations persisted after adjusting for depressive or post-traumatic stress symptoms.
“A history of sexual assault was particularly related to white matter hyperintensities in the parietal lobe, and these kinds of white matter hyperintensities have been linked to Alzheimer’s disease in a fairly pronounced way,” Dr. Thurston said.
“When we think about risk factors for stroke, dementia, we need to think beyond exclusively our usual suspects and also think about women [who experienced] psychological trauma and experienced sexual assault in particular. So ask about it and consider it part of your screening regimen,” she added.
‘Burgeoning’ literature
Commenting on the findings, Charles Nemeroff, MD, PhD, professor and chair, department of psychiatry and behavioral sciences, Dell Medical School, University of Texas at Austin, and director of its Institute for Early Life Adversity Research, said the research adds to the “burgeoning literature on the long term neurobiological consequences of trauma and more specifically, sexual abuse, on brain imaging measures.”
“Our group and others reported several years ago that patients with mood disorders, more specifically bipolar disorder and major depression, had higher rates of WMH than matched controls. Those older studies did not control for a history of early life adversity such as childhood maltreatment,” Dr. Nemeroff said.
“In addition to this finding of increased WMH in subjects exposed to trauma is a very large literature documenting other central nervous system (CNS) changes in this population, including cortical thinning in certain brain areas and clearly an emerging finding that different forms of childhood maltreatment are associated with quite distinct structural brain alterations in adulthood,” he noted.
The study was supported by grants from the National Institutes of Health. Dr. Thurston and Dr. Nemeroff have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
COVID-19 vaccines in pregnancy may protect baby, too
Women who receive COVID-19 vaccines during pregnancy pass antibodies to their babies, which could protect newborns from the disease, research has shown.
.
Researchers with New York University Langone Health conducted a study that included pregnant women who had received at least one dose of an mRNA COVID-19 vaccine (Pfizer/BioNTech or Moderna) by June 4.
All neonates had antibodies to the spike protein at high titers, the researchers found.
Unlike similar prior studies, the researchers also looked for antibodies to the nucleocapsid protein, which would have indicated the presence of antibodies from natural COVID-19 infection. They did not detect antibodies to the nucleocapsid protein, and the lack of these antibodies suggests that the antibodies to the spike protein resulted from vaccination and not from prior infection, the researchers said.
The participants had a median time from completion of the vaccine series to delivery of 13 weeks. The study was published online in the American Journal of Obstetrics & Gynecology MFM.
“The presence of these anti-spike antibodies in the cord blood should, at least in theory, offer these newborns some degree of protection,” said study investigator Ashley S. Roman, MD, director of the division of maternal-fetal medicine at NYU Langone Health. “While the primary rationale for vaccination during pregnancy is to keep moms healthy and keep moms out of the hospital, the outstanding question to us was whether there is any fetal or neonatal benefit conferred by receiving the vaccine during pregnancy.”
Questions remain about the degree and durability of protection for newborns from these antibodies. An ongoing study, MOMI-VAX, aims to systematically measure antibody levels in mothers who receive COVID-19 vaccines during pregnancy and in their babies over time.
The present study contributes welcome preliminary evidence suggesting a benefit to infants, said Emily Adhikari, MD, of the University of Texas Southwestern Medical Center in Dallas, who was not involved in the study.
Still, “the main concern and our priority as obstetricians is to vaccinate pregnant women to protect them from severe or critical illness,” she said.
Although most individuals infected with SARS-CoV-2 recover, a significant portion of pregnant women get seriously sick, Dr. Adhikari said. “With this recent Delta surge, we are seeing more pregnant patients who are sicker,” said Dr. Adhikari, who has published research from one hospital describing this trend.
When weighing whether patients should receive COVID-19 vaccines in pregnancy, the risks from infection have outweighed any risk from vaccination to such an extent that there is “not a comparison to make,” Dr. Adhikari said. “The risks of the infection are so much higher.
“For me, it is a matter of making sure that my patient understands that we have really good safety data on these vaccines and there is no reason to think that a pregnant person would be harmed by them. On the contrary, the benefit is to protect and maybe even save your life,” Dr. Adhikari said. “And now we have more evidence that the fetus may also benefit.”
The rationale for vaccinations during pregnancy can vary, Dr. Roman said. Flu shots in pregnancy mainly are intended to protect the mother, though they confer protection for newborns as well. With the whooping cough vaccine given in the third trimester, however, the primary aim is to protect the baby from whooping cough in the first months of life, Dr. Roman said.
“I think it is really important for pregnant women to understand that antibodies crossing the placenta is a good thing,” she added.
As patients who already have received COVID-19 vaccines become pregnant and may become eligible for a booster dose, Dr. Adhikari will offer it, she said, though she has confidence in the protection provided by the initial immune response.
Dr. Roman and Dr. Adhikari had no disclosures.
Women who receive COVID-19 vaccines during pregnancy pass antibodies to their babies, which could protect newborns from the disease, research has shown.
.
Researchers with New York University Langone Health conducted a study that included pregnant women who had received at least one dose of an mRNA COVID-19 vaccine (Pfizer/BioNTech or Moderna) by June 4.
All neonates had antibodies to the spike protein at high titers, the researchers found.
Unlike similar prior studies, the researchers also looked for antibodies to the nucleocapsid protein, which would have indicated the presence of antibodies from natural COVID-19 infection. They did not detect antibodies to the nucleocapsid protein, and the lack of these antibodies suggests that the antibodies to the spike protein resulted from vaccination and not from prior infection, the researchers said.
The participants had a median time from completion of the vaccine series to delivery of 13 weeks. The study was published online in the American Journal of Obstetrics & Gynecology MFM.
“The presence of these anti-spike antibodies in the cord blood should, at least in theory, offer these newborns some degree of protection,” said study investigator Ashley S. Roman, MD, director of the division of maternal-fetal medicine at NYU Langone Health. “While the primary rationale for vaccination during pregnancy is to keep moms healthy and keep moms out of the hospital, the outstanding question to us was whether there is any fetal or neonatal benefit conferred by receiving the vaccine during pregnancy.”
Questions remain about the degree and durability of protection for newborns from these antibodies. An ongoing study, MOMI-VAX, aims to systematically measure antibody levels in mothers who receive COVID-19 vaccines during pregnancy and in their babies over time.
The present study contributes welcome preliminary evidence suggesting a benefit to infants, said Emily Adhikari, MD, of the University of Texas Southwestern Medical Center in Dallas, who was not involved in the study.
Still, “the main concern and our priority as obstetricians is to vaccinate pregnant women to protect them from severe or critical illness,” she said.
Although most individuals infected with SARS-CoV-2 recover, a significant portion of pregnant women get seriously sick, Dr. Adhikari said. “With this recent Delta surge, we are seeing more pregnant patients who are sicker,” said Dr. Adhikari, who has published research from one hospital describing this trend.
When weighing whether patients should receive COVID-19 vaccines in pregnancy, the risks from infection have outweighed any risk from vaccination to such an extent that there is “not a comparison to make,” Dr. Adhikari said. “The risks of the infection are so much higher.
“For me, it is a matter of making sure that my patient understands that we have really good safety data on these vaccines and there is no reason to think that a pregnant person would be harmed by them. On the contrary, the benefit is to protect and maybe even save your life,” Dr. Adhikari said. “And now we have more evidence that the fetus may also benefit.”
The rationale for vaccinations during pregnancy can vary, Dr. Roman said. Flu shots in pregnancy mainly are intended to protect the mother, though they confer protection for newborns as well. With the whooping cough vaccine given in the third trimester, however, the primary aim is to protect the baby from whooping cough in the first months of life, Dr. Roman said.
“I think it is really important for pregnant women to understand that antibodies crossing the placenta is a good thing,” she added.
As patients who already have received COVID-19 vaccines become pregnant and may become eligible for a booster dose, Dr. Adhikari will offer it, she said, though she has confidence in the protection provided by the initial immune response.
Dr. Roman and Dr. Adhikari had no disclosures.
Women who receive COVID-19 vaccines during pregnancy pass antibodies to their babies, which could protect newborns from the disease, research has shown.
.
Researchers with New York University Langone Health conducted a study that included pregnant women who had received at least one dose of an mRNA COVID-19 vaccine (Pfizer/BioNTech or Moderna) by June 4.
All neonates had antibodies to the spike protein at high titers, the researchers found.
Unlike similar prior studies, the researchers also looked for antibodies to the nucleocapsid protein, which would have indicated the presence of antibodies from natural COVID-19 infection. They did not detect antibodies to the nucleocapsid protein, and the lack of these antibodies suggests that the antibodies to the spike protein resulted from vaccination and not from prior infection, the researchers said.
The participants had a median time from completion of the vaccine series to delivery of 13 weeks. The study was published online in the American Journal of Obstetrics & Gynecology MFM.
“The presence of these anti-spike antibodies in the cord blood should, at least in theory, offer these newborns some degree of protection,” said study investigator Ashley S. Roman, MD, director of the division of maternal-fetal medicine at NYU Langone Health. “While the primary rationale for vaccination during pregnancy is to keep moms healthy and keep moms out of the hospital, the outstanding question to us was whether there is any fetal or neonatal benefit conferred by receiving the vaccine during pregnancy.”
Questions remain about the degree and durability of protection for newborns from these antibodies. An ongoing study, MOMI-VAX, aims to systematically measure antibody levels in mothers who receive COVID-19 vaccines during pregnancy and in their babies over time.
The present study contributes welcome preliminary evidence suggesting a benefit to infants, said Emily Adhikari, MD, of the University of Texas Southwestern Medical Center in Dallas, who was not involved in the study.
Still, “the main concern and our priority as obstetricians is to vaccinate pregnant women to protect them from severe or critical illness,” she said.
Although most individuals infected with SARS-CoV-2 recover, a significant portion of pregnant women get seriously sick, Dr. Adhikari said. “With this recent Delta surge, we are seeing more pregnant patients who are sicker,” said Dr. Adhikari, who has published research from one hospital describing this trend.
When weighing whether patients should receive COVID-19 vaccines in pregnancy, the risks from infection have outweighed any risk from vaccination to such an extent that there is “not a comparison to make,” Dr. Adhikari said. “The risks of the infection are so much higher.
“For me, it is a matter of making sure that my patient understands that we have really good safety data on these vaccines and there is no reason to think that a pregnant person would be harmed by them. On the contrary, the benefit is to protect and maybe even save your life,” Dr. Adhikari said. “And now we have more evidence that the fetus may also benefit.”
The rationale for vaccinations during pregnancy can vary, Dr. Roman said. Flu shots in pregnancy mainly are intended to protect the mother, though they confer protection for newborns as well. With the whooping cough vaccine given in the third trimester, however, the primary aim is to protect the baby from whooping cough in the first months of life, Dr. Roman said.
“I think it is really important for pregnant women to understand that antibodies crossing the placenta is a good thing,” she added.
As patients who already have received COVID-19 vaccines become pregnant and may become eligible for a booster dose, Dr. Adhikari will offer it, she said, though she has confidence in the protection provided by the initial immune response.
Dr. Roman and Dr. Adhikari had no disclosures.
FROM AMERICAN JOURNAL OF OBSTETRICS & GYNECOLOGY MFM
Smart watch glucose monitoring on the horizon
Earlier this year, technology news sites reported that the Apple Watch Series 7 and the Samsung Galaxy Watch 4 were going to have integrated optical sensors for checking interstitial fluid glucose levels with no blood sampling needed. By the summer, new articles indicated that the glucose sensing watches would not be released this year for either Apple or Samsung.
For now, the newest technology available for monitoring glucose is continuous glucose monitoring (CGM), which involves a tiny sensor being inserted under the skin. The sensor tests glucose every few minutes, and a transmitter wirelessly sends the information to a monitor, which may be part of an insulin pump or a separate device. Some CGMs send information directly to a smartphone or tablet, according to the National Institutes of Health.
In 1999 the Food and Drug Administration approved the first CGM, which was only approved for downloading 3 days of data at a doctor’s office. Interestingly, the first real-time CGM device for patients to use on their own was a watch, the Glucowatch Biographer. Because of irritation and other issues, that watch never caught on. In 2006 and 2008, Dexcom and then Abbott released the first real-time CGMs that allowed patients to frequently check their own blood sugars.1,2
How CGM has advanced diabetes management
The advent of CGM has advanced the field of diabetes management in many ways.
It has allowed patients to get real time feedback on how their behavior affects their blood sugar. The use of CGM along with the ensuing behavioral changes actually leads to a decrease in hemoglobin A1c, along with a lower risk of hypoglycemia. CGM has also resulted in patients having a better understanding of several aspects of glucose control, including glucose variability and nocturnal hypoglycemia.
Affordable, readily accessible CGM monitors that allow patients to intermittently use CGM have become available over the last 3 years.
In the United States alone, 34.2 million people have diabetes – nearly 1 in every 10 people. Many do not do self-monitoring of blood glucose and most do not use CGM. The current alternative to CGM – self monitoring of blood glucose – is cumbersome, and, since it requires regular finger sticks, is painful. Also, there is significant cost to each test strip that is used to self-monitor, and most insurance limits the number of times a day a patient can check their blood sugar. CGM used to be reserved only for patients who use multiple doses of insulin daily, and only began being approved for use for patients on basal insulin alone in June 2021.3
Most primary care doctors are just beginning to learn how to interpret CGM data.
Smart watch glucose monitoring predictions
When smart watch glucose monitoring arrives, it will suddenly change the playing field for patients with diabetes and their doctors alike.
We expect it to bring down the price of CGM and make it readily available to any patient who owns a smart watch with that function.
For doctors, the new technology will result in them suddenly being asked to advise their patients on how to use the data generated by watch-based CGM.
Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, Philadelphia, and associate director of the family medicine residency program at Abington (Pa.) Hospital–Jefferson Health. They have no conflicts related to the content of this piece. Dr. Persampiere is a second-year resident in the family medicine residency program at Abington Jefferson Health. You can contact them at fpnews@mdedge.com.
References
1. Hirsh I. Introduction: History of Glucose Monitoring, in “Role of Continuous Glucose Monitoring in Diabetes Treatment.” American Diabetes Association. 2018.
2. Peters A. The Evidence Base for Continuous Glucose Monitoring, in “Role of Continuous Glucose Monitoring in Diabetes Treatment.” American Diabetes Association 2018.
3. “Medicare Loosening Restrictions for Continuous Glucose Monitor (CGM) Coverage,” Healthline. 2021 Jul 13.
Earlier this year, technology news sites reported that the Apple Watch Series 7 and the Samsung Galaxy Watch 4 were going to have integrated optical sensors for checking interstitial fluid glucose levels with no blood sampling needed. By the summer, new articles indicated that the glucose sensing watches would not be released this year for either Apple or Samsung.
For now, the newest technology available for monitoring glucose is continuous glucose monitoring (CGM), which involves a tiny sensor being inserted under the skin. The sensor tests glucose every few minutes, and a transmitter wirelessly sends the information to a monitor, which may be part of an insulin pump or a separate device. Some CGMs send information directly to a smartphone or tablet, according to the National Institutes of Health.
In 1999 the Food and Drug Administration approved the first CGM, which was only approved for downloading 3 days of data at a doctor’s office. Interestingly, the first real-time CGM device for patients to use on their own was a watch, the Glucowatch Biographer. Because of irritation and other issues, that watch never caught on. In 2006 and 2008, Dexcom and then Abbott released the first real-time CGMs that allowed patients to frequently check their own blood sugars.1,2
How CGM has advanced diabetes management
The advent of CGM has advanced the field of diabetes management in many ways.
It has allowed patients to get real time feedback on how their behavior affects their blood sugar. The use of CGM along with the ensuing behavioral changes actually leads to a decrease in hemoglobin A1c, along with a lower risk of hypoglycemia. CGM has also resulted in patients having a better understanding of several aspects of glucose control, including glucose variability and nocturnal hypoglycemia.
Affordable, readily accessible CGM monitors that allow patients to intermittently use CGM have become available over the last 3 years.
In the United States alone, 34.2 million people have diabetes – nearly 1 in every 10 people. Many do not do self-monitoring of blood glucose and most do not use CGM. The current alternative to CGM – self monitoring of blood glucose – is cumbersome, and, since it requires regular finger sticks, is painful. Also, there is significant cost to each test strip that is used to self-monitor, and most insurance limits the number of times a day a patient can check their blood sugar. CGM used to be reserved only for patients who use multiple doses of insulin daily, and only began being approved for use for patients on basal insulin alone in June 2021.3
Most primary care doctors are just beginning to learn how to interpret CGM data.
Smart watch glucose monitoring predictions
When smart watch glucose monitoring arrives, it will suddenly change the playing field for patients with diabetes and their doctors alike.
We expect it to bring down the price of CGM and make it readily available to any patient who owns a smart watch with that function.
For doctors, the new technology will result in them suddenly being asked to advise their patients on how to use the data generated by watch-based CGM.
Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, Philadelphia, and associate director of the family medicine residency program at Abington (Pa.) Hospital–Jefferson Health. They have no conflicts related to the content of this piece. Dr. Persampiere is a second-year resident in the family medicine residency program at Abington Jefferson Health. You can contact them at fpnews@mdedge.com.
References
1. Hirsh I. Introduction: History of Glucose Monitoring, in “Role of Continuous Glucose Monitoring in Diabetes Treatment.” American Diabetes Association. 2018.
2. Peters A. The Evidence Base for Continuous Glucose Monitoring, in “Role of Continuous Glucose Monitoring in Diabetes Treatment.” American Diabetes Association 2018.
3. “Medicare Loosening Restrictions for Continuous Glucose Monitor (CGM) Coverage,” Healthline. 2021 Jul 13.
Earlier this year, technology news sites reported that the Apple Watch Series 7 and the Samsung Galaxy Watch 4 were going to have integrated optical sensors for checking interstitial fluid glucose levels with no blood sampling needed. By the summer, new articles indicated that the glucose sensing watches would not be released this year for either Apple or Samsung.
For now, the newest technology available for monitoring glucose is continuous glucose monitoring (CGM), which involves a tiny sensor being inserted under the skin. The sensor tests glucose every few minutes, and a transmitter wirelessly sends the information to a monitor, which may be part of an insulin pump or a separate device. Some CGMs send information directly to a smartphone or tablet, according to the National Institutes of Health.
In 1999 the Food and Drug Administration approved the first CGM, which was only approved for downloading 3 days of data at a doctor’s office. Interestingly, the first real-time CGM device for patients to use on their own was a watch, the Glucowatch Biographer. Because of irritation and other issues, that watch never caught on. In 2006 and 2008, Dexcom and then Abbott released the first real-time CGMs that allowed patients to frequently check their own blood sugars.1,2
How CGM has advanced diabetes management
The advent of CGM has advanced the field of diabetes management in many ways.
It has allowed patients to get real time feedback on how their behavior affects their blood sugar. The use of CGM along with the ensuing behavioral changes actually leads to a decrease in hemoglobin A1c, along with a lower risk of hypoglycemia. CGM has also resulted in patients having a better understanding of several aspects of glucose control, including glucose variability and nocturnal hypoglycemia.
Affordable, readily accessible CGM monitors that allow patients to intermittently use CGM have become available over the last 3 years.
In the United States alone, 34.2 million people have diabetes – nearly 1 in every 10 people. Many do not do self-monitoring of blood glucose and most do not use CGM. The current alternative to CGM – self monitoring of blood glucose – is cumbersome, and, since it requires regular finger sticks, is painful. Also, there is significant cost to each test strip that is used to self-monitor, and most insurance limits the number of times a day a patient can check their blood sugar. CGM used to be reserved only for patients who use multiple doses of insulin daily, and only began being approved for use for patients on basal insulin alone in June 2021.3
Most primary care doctors are just beginning to learn how to interpret CGM data.
Smart watch glucose monitoring predictions
When smart watch glucose monitoring arrives, it will suddenly change the playing field for patients with diabetes and their doctors alike.
We expect it to bring down the price of CGM and make it readily available to any patient who owns a smart watch with that function.
For doctors, the new technology will result in them suddenly being asked to advise their patients on how to use the data generated by watch-based CGM.
Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, Philadelphia, and associate director of the family medicine residency program at Abington (Pa.) Hospital–Jefferson Health. They have no conflicts related to the content of this piece. Dr. Persampiere is a second-year resident in the family medicine residency program at Abington Jefferson Health. You can contact them at fpnews@mdedge.com.
References
1. Hirsh I. Introduction: History of Glucose Monitoring, in “Role of Continuous Glucose Monitoring in Diabetes Treatment.” American Diabetes Association. 2018.
2. Peters A. The Evidence Base for Continuous Glucose Monitoring, in “Role of Continuous Glucose Monitoring in Diabetes Treatment.” American Diabetes Association 2018.
3. “Medicare Loosening Restrictions for Continuous Glucose Monitor (CGM) Coverage,” Healthline. 2021 Jul 13.
Investigative botulinum toxin formulation shows prolonged effect
, according to results of a phase 3 clinical trial presented at the virtual International Congress of Parkinson’s Disease and Movement Disorders.
The ASPEN-1 trial evaluated 301 patients with moderate to severe cervical dystonia for up to 36 weeks and found that those receiving two doses of DaxibotulinumtoxinA, known as DAXI, versus placebo improved their scores on the Toronto Western Spasmodic Torticollis Rating Scale (TWSTRS), said Joseph Jankovic, MD, professor of neurology and director of the Parkinson’s Disease Center and Movement Disorders Clinic at Baylor College of Medicine in Houston.
“Botulinum neurotoxin is clearly the treatment of choice for cervical dystonia,” Dr. Jankovic said in an interview. “While the majority of patients obtain satisfactory benefit from BoNT injections, some experience adverse effects such as neck weakness and difficulty swallowing.” Another limitation of BoNT is that its effects wear off after about 3 months or less and patients have to be re-injected, he said.
“This is why I am quite encouraged by the results of the DAXI study that suggest that this formulation of BoNT (type A) may have a longer response and relatively few side effects,” he said.
Patients in the study were randomized 1:3:3 to placebo, DAXI 125U or DAXI 250U. The average TWSTRS score upon enrollment was 43.3. The placebo group had a mean ± standard error TWSTRS improvement of 4.3 ± 1.8 at 4 or 6 weeks, while the treatment groups had mean ± SE improvements of 12.7 ± 1.3 for 125U and 10.9 ± 1.2 for 250U (P = .0006 vs. placebo). They translate into improvements of 12%, 31%, and 27% for the placebo and low- and high-dose treatment groups, respectively.
“Even though paradoxically it seems the high-dose group did slightly less well than the low-dose group, there was no difference between the two groups,” Dr. Jankovic said in the presentation.
The median duration of benefit was 24 weeks in the low-dose group and 20.3 weeks in the high-dose group.
The treatment groups demonstrated similar benefit compared with placebo in TWSTRS subscales for disease severity, disability, and pain, Dr. Jankovic said. “The majority of the patients had little better, moderately better, or very much better from the botulinum toxin injection with respect to clinical global impression of change and patient global impression of change,” he said.
Likewise, both the Clinician Global Impression of Change (CGIC) and Patient Global Impression of Change (PGIC) demonstrated improvement versus placebo: 77.6% and 76.9% in the 125U and 250U doses versus 45.7% for the former; and 71.2% and 73.1% versus 41.3% for the latter.
Side effects “were remarkably minimal,” Dr. Jankovic said, “but I want to call attention to the low frequency of neck weakness or dysphagia in comparison with other studies of botulinum toxin in cervical dystonia.” The rates of dysphagia were 1.6% and 3.9% in the 125U and 250U treatment groups, respectively. Sixteen of the 255 patients in the treatment groups reported muscular weakness or musculoskeletal pain, and seven had dysphagia.
The rate of dysphagia after injection is noteworthy, said David Charles, MD, professor and vice chair of neurology at Vanderbilt University in Nashville, Tenn., who was not involved in the research. “The one thing we worry about most in people with cervical dystonia are swallowing and choking – dysphagia – and the numbers are very modest: 2 out of 127 in the 125U dose and 5 of 130 in the 250U dose,” he said. “That’s a very low rate of that adverse event.”
The duration of action for both doses is “rather remarkable,” Dr. Charles said. “With the other formulations, my patients are coming back every 12 weeks for treatment; the BoNT helps so much that [these] patients make their appointments every 3 months for as far out as they can,” he said. “This could potentially mean two or three trips a year as opposed to four trips a year.”
The trial was funded by Revance Therapeutics. Dr. Jankovic is an investigator for Revance, and three coauthors are employees of Revance. Dr. Charles is a consultant to the company.
, according to results of a phase 3 clinical trial presented at the virtual International Congress of Parkinson’s Disease and Movement Disorders.
The ASPEN-1 trial evaluated 301 patients with moderate to severe cervical dystonia for up to 36 weeks and found that those receiving two doses of DaxibotulinumtoxinA, known as DAXI, versus placebo improved their scores on the Toronto Western Spasmodic Torticollis Rating Scale (TWSTRS), said Joseph Jankovic, MD, professor of neurology and director of the Parkinson’s Disease Center and Movement Disorders Clinic at Baylor College of Medicine in Houston.
“Botulinum neurotoxin is clearly the treatment of choice for cervical dystonia,” Dr. Jankovic said in an interview. “While the majority of patients obtain satisfactory benefit from BoNT injections, some experience adverse effects such as neck weakness and difficulty swallowing.” Another limitation of BoNT is that its effects wear off after about 3 months or less and patients have to be re-injected, he said.
“This is why I am quite encouraged by the results of the DAXI study that suggest that this formulation of BoNT (type A) may have a longer response and relatively few side effects,” he said.
Patients in the study were randomized 1:3:3 to placebo, DAXI 125U or DAXI 250U. The average TWSTRS score upon enrollment was 43.3. The placebo group had a mean ± standard error TWSTRS improvement of 4.3 ± 1.8 at 4 or 6 weeks, while the treatment groups had mean ± SE improvements of 12.7 ± 1.3 for 125U and 10.9 ± 1.2 for 250U (P = .0006 vs. placebo). They translate into improvements of 12%, 31%, and 27% for the placebo and low- and high-dose treatment groups, respectively.
“Even though paradoxically it seems the high-dose group did slightly less well than the low-dose group, there was no difference between the two groups,” Dr. Jankovic said in the presentation.
The median duration of benefit was 24 weeks in the low-dose group and 20.3 weeks in the high-dose group.
The treatment groups demonstrated similar benefit compared with placebo in TWSTRS subscales for disease severity, disability, and pain, Dr. Jankovic said. “The majority of the patients had little better, moderately better, or very much better from the botulinum toxin injection with respect to clinical global impression of change and patient global impression of change,” he said.
Likewise, both the Clinician Global Impression of Change (CGIC) and Patient Global Impression of Change (PGIC) demonstrated improvement versus placebo: 77.6% and 76.9% in the 125U and 250U doses versus 45.7% for the former; and 71.2% and 73.1% versus 41.3% for the latter.
Side effects “were remarkably minimal,” Dr. Jankovic said, “but I want to call attention to the low frequency of neck weakness or dysphagia in comparison with other studies of botulinum toxin in cervical dystonia.” The rates of dysphagia were 1.6% and 3.9% in the 125U and 250U treatment groups, respectively. Sixteen of the 255 patients in the treatment groups reported muscular weakness or musculoskeletal pain, and seven had dysphagia.
The rate of dysphagia after injection is noteworthy, said David Charles, MD, professor and vice chair of neurology at Vanderbilt University in Nashville, Tenn., who was not involved in the research. “The one thing we worry about most in people with cervical dystonia are swallowing and choking – dysphagia – and the numbers are very modest: 2 out of 127 in the 125U dose and 5 of 130 in the 250U dose,” he said. “That’s a very low rate of that adverse event.”
The duration of action for both doses is “rather remarkable,” Dr. Charles said. “With the other formulations, my patients are coming back every 12 weeks for treatment; the BoNT helps so much that [these] patients make their appointments every 3 months for as far out as they can,” he said. “This could potentially mean two or three trips a year as opposed to four trips a year.”
The trial was funded by Revance Therapeutics. Dr. Jankovic is an investigator for Revance, and three coauthors are employees of Revance. Dr. Charles is a consultant to the company.
, according to results of a phase 3 clinical trial presented at the virtual International Congress of Parkinson’s Disease and Movement Disorders.
The ASPEN-1 trial evaluated 301 patients with moderate to severe cervical dystonia for up to 36 weeks and found that those receiving two doses of DaxibotulinumtoxinA, known as DAXI, versus placebo improved their scores on the Toronto Western Spasmodic Torticollis Rating Scale (TWSTRS), said Joseph Jankovic, MD, professor of neurology and director of the Parkinson’s Disease Center and Movement Disorders Clinic at Baylor College of Medicine in Houston.
“Botulinum neurotoxin is clearly the treatment of choice for cervical dystonia,” Dr. Jankovic said in an interview. “While the majority of patients obtain satisfactory benefit from BoNT injections, some experience adverse effects such as neck weakness and difficulty swallowing.” Another limitation of BoNT is that its effects wear off after about 3 months or less and patients have to be re-injected, he said.
“This is why I am quite encouraged by the results of the DAXI study that suggest that this formulation of BoNT (type A) may have a longer response and relatively few side effects,” he said.
Patients in the study were randomized 1:3:3 to placebo, DAXI 125U or DAXI 250U. The average TWSTRS score upon enrollment was 43.3. The placebo group had a mean ± standard error TWSTRS improvement of 4.3 ± 1.8 at 4 or 6 weeks, while the treatment groups had mean ± SE improvements of 12.7 ± 1.3 for 125U and 10.9 ± 1.2 for 250U (P = .0006 vs. placebo). They translate into improvements of 12%, 31%, and 27% for the placebo and low- and high-dose treatment groups, respectively.
“Even though paradoxically it seems the high-dose group did slightly less well than the low-dose group, there was no difference between the two groups,” Dr. Jankovic said in the presentation.
The median duration of benefit was 24 weeks in the low-dose group and 20.3 weeks in the high-dose group.
The treatment groups demonstrated similar benefit compared with placebo in TWSTRS subscales for disease severity, disability, and pain, Dr. Jankovic said. “The majority of the patients had little better, moderately better, or very much better from the botulinum toxin injection with respect to clinical global impression of change and patient global impression of change,” he said.
Likewise, both the Clinician Global Impression of Change (CGIC) and Patient Global Impression of Change (PGIC) demonstrated improvement versus placebo: 77.6% and 76.9% in the 125U and 250U doses versus 45.7% for the former; and 71.2% and 73.1% versus 41.3% for the latter.
Side effects “were remarkably minimal,” Dr. Jankovic said, “but I want to call attention to the low frequency of neck weakness or dysphagia in comparison with other studies of botulinum toxin in cervical dystonia.” The rates of dysphagia were 1.6% and 3.9% in the 125U and 250U treatment groups, respectively. Sixteen of the 255 patients in the treatment groups reported muscular weakness or musculoskeletal pain, and seven had dysphagia.
The rate of dysphagia after injection is noteworthy, said David Charles, MD, professor and vice chair of neurology at Vanderbilt University in Nashville, Tenn., who was not involved in the research. “The one thing we worry about most in people with cervical dystonia are swallowing and choking – dysphagia – and the numbers are very modest: 2 out of 127 in the 125U dose and 5 of 130 in the 250U dose,” he said. “That’s a very low rate of that adverse event.”
The duration of action for both doses is “rather remarkable,” Dr. Charles said. “With the other formulations, my patients are coming back every 12 weeks for treatment; the BoNT helps so much that [these] patients make their appointments every 3 months for as far out as they can,” he said. “This could potentially mean two or three trips a year as opposed to four trips a year.”
The trial was funded by Revance Therapeutics. Dr. Jankovic is an investigator for Revance, and three coauthors are employees of Revance. Dr. Charles is a consultant to the company.
FROM MDS VIRTUAL CONGRESS 2021
Efforts target underrepresented populations in Parkinson’s disease genetic studies
, attendees at the International Congress of Parkinson’s Disease and Movement Disorders were told.
“Through the years, as we’ve increased the number of individuals that we’ve included in our genetic studies, the number of risk factors that we’ve been able to identify has increased exponentially,” said Ignacio F. Mata, PhD, a neurogeneticist and principal investigator with the Genomic Medicine Institute of the Cleveland Clinic Lerner Research Institute. “This is all due to collaborations.”
Dr. Mata reviewed no fewer than seven initiatives that are gathering genetic data from people with Parkinson’s disease in Central and South America, India, China, Africa, Oceania, the Middle East, and Central Asia, along with efforts to target diverse populations in London and African Americans in the United States.
“One of the problems that we’ve had in the past is that most of the studies have been done just with individuals that are of European ancestry, so there’s a big gap of other populations that we haven’t been able to study,” Dr. Mata said. “And this is true for all of the current studies that are ongoing here in the United States.” That includes the Parkinson’s Progression Markers Initiative, he said, in which fewer than 6% of participants are non-European. Dr. Mata is also the lead in the Global Parkinson’s Genetics Program (GP2) for underrepresented populations.
Lack of diversity in genetic studies isn’t an issue in Parkinson’s studies alone, Dr. Mata said. “This is a generalized problem across all genetic studies,” he said, citing a 2016 analysis that found the proportion of participants in genome-wide studies was 96% European descent in 2009, shifting to 80% by 2016. “There’s still a big gap because most of the non-European populations came mostly from Asia,” Dr. Mata said, with Latinos and people of African descent representing less than 1% of the study populations.
In an interview, Dr. Mata noted there are a multitude of reasons for enrolling more diverse populations. “We’re going to be able to use genetics to create new treatments and do risk prediction – the so-called precision or personalized medicine,” he said. “We’re leaving a big chunk of the population behind if we don’t include those individuals.”
Scientific basis for diversity
There are a multitude of scientific reasons for doing so, too, said Dr. Mata. “In the whole genome we try to find gene variants that modify the risk for certain disease,” he said. “These regions can be quite large, so increasing the number of individuals that come from different genetic backgrounds can actually help us reduce the number of regions that need to be studied to find the causal variants.”
Andrew Singleton, PhD, director of the Center for Alzheimer’s and Related Dementias at the National Institute of Aging in Bethesda, Md., concurred that enrolling more diverse populations can speed up research for targeting genetic variants.
“We can use the differences in genetics to narrow down our search for variants, reduce the places where we’re looking for risk variants, and reduce the number of genes we’re looking at,” he said in an interview.
Roy Alcalay, MD, professor of neurology at Columbia University Irving Medical Center in New York, offered two more scientific reasons for more diverse study populations “in addition to being more ethically appropriate,” he said. “One is, you may identify new genes that you wouldn’t have identified otherwise; and also in the genes that already exist, you may recognize that some of the pathogenic variants may be more prevalent in populations that were unknown.”
One of the challenges in casting a wider net is that much of the research funding has been concentrated in the United States and Europe, Dr. Mata said. And even in the United States, with large, diverse populations, minority groups are underrepresented in these studies, he said, but a potential solution is emerging. “This is something that we’re learning now with COVID,” he said. “We can do a lot remotely. This should help bring some of those barriers down.”
Cultural barriers are also foreboding. “Individuals may not feel comfortable participating in research,” he said in the interview. “I see this especially in the Hispanic community; many don’t understand what they can do with genetic material, or they’re afraid it will be shared with police, and if they’re here in nonofficial immigration status, they’re afraid they could be deported. There are a lot of misconceptions about genetic research.”
An initiative of the Parkinson’s Foundation PD GENEration Study is to provide free genetic tests and give the patient a report on genetic counseling “to empower patients,” Dr. Mata said.
Solutions for targeting underrepresented groups are emerging, Dr. Singleton said. “Actually there’s a really elegant solution, which is that in the populations that we go into and work with, we make sure the ownership of those cohorts, the ownership of the science and the analysis belongs to those populations,” he said.
“Part of that is creating infrastructure on site,” Dr. Singleton added. “Another part is providing training and outreach so we can help to train a whole new generation of scientists and researchers who can work in those populations embedded within those populations. They’re really the champion of moving that research forward.”
Dr. Alcalay credited Dr. Mata for his work with cohorts in Central and South America and in reaching out to other countries to recruit more diverse populations for genetic Parkinson’s studies. “And it’s not just because it’s the politically correct thing to do, about inclusivity and diversity,” Dr. Alcalay said. “It’s because it’s really meaningful. In addition to being ethically more appropriate, it will advance the entire field.
“I also really think it’s a no-brainer,” Dr. Alcalay said. “It’s something that needs to happen.”
Dr. Mata receives grant funding from the National Institutes of Health. Dr. Singleton and Dr. Alcalay have no relevant disclosures.
, attendees at the International Congress of Parkinson’s Disease and Movement Disorders were told.
“Through the years, as we’ve increased the number of individuals that we’ve included in our genetic studies, the number of risk factors that we’ve been able to identify has increased exponentially,” said Ignacio F. Mata, PhD, a neurogeneticist and principal investigator with the Genomic Medicine Institute of the Cleveland Clinic Lerner Research Institute. “This is all due to collaborations.”
Dr. Mata reviewed no fewer than seven initiatives that are gathering genetic data from people with Parkinson’s disease in Central and South America, India, China, Africa, Oceania, the Middle East, and Central Asia, along with efforts to target diverse populations in London and African Americans in the United States.
“One of the problems that we’ve had in the past is that most of the studies have been done just with individuals that are of European ancestry, so there’s a big gap of other populations that we haven’t been able to study,” Dr. Mata said. “And this is true for all of the current studies that are ongoing here in the United States.” That includes the Parkinson’s Progression Markers Initiative, he said, in which fewer than 6% of participants are non-European. Dr. Mata is also the lead in the Global Parkinson’s Genetics Program (GP2) for underrepresented populations.
Lack of diversity in genetic studies isn’t an issue in Parkinson’s studies alone, Dr. Mata said. “This is a generalized problem across all genetic studies,” he said, citing a 2016 analysis that found the proportion of participants in genome-wide studies was 96% European descent in 2009, shifting to 80% by 2016. “There’s still a big gap because most of the non-European populations came mostly from Asia,” Dr. Mata said, with Latinos and people of African descent representing less than 1% of the study populations.
In an interview, Dr. Mata noted there are a multitude of reasons for enrolling more diverse populations. “We’re going to be able to use genetics to create new treatments and do risk prediction – the so-called precision or personalized medicine,” he said. “We’re leaving a big chunk of the population behind if we don’t include those individuals.”
Scientific basis for diversity
There are a multitude of scientific reasons for doing so, too, said Dr. Mata. “In the whole genome we try to find gene variants that modify the risk for certain disease,” he said. “These regions can be quite large, so increasing the number of individuals that come from different genetic backgrounds can actually help us reduce the number of regions that need to be studied to find the causal variants.”
Andrew Singleton, PhD, director of the Center for Alzheimer’s and Related Dementias at the National Institute of Aging in Bethesda, Md., concurred that enrolling more diverse populations can speed up research for targeting genetic variants.
“We can use the differences in genetics to narrow down our search for variants, reduce the places where we’re looking for risk variants, and reduce the number of genes we’re looking at,” he said in an interview.
Roy Alcalay, MD, professor of neurology at Columbia University Irving Medical Center in New York, offered two more scientific reasons for more diverse study populations “in addition to being more ethically appropriate,” he said. “One is, you may identify new genes that you wouldn’t have identified otherwise; and also in the genes that already exist, you may recognize that some of the pathogenic variants may be more prevalent in populations that were unknown.”
One of the challenges in casting a wider net is that much of the research funding has been concentrated in the United States and Europe, Dr. Mata said. And even in the United States, with large, diverse populations, minority groups are underrepresented in these studies, he said, but a potential solution is emerging. “This is something that we’re learning now with COVID,” he said. “We can do a lot remotely. This should help bring some of those barriers down.”
Cultural barriers are also foreboding. “Individuals may not feel comfortable participating in research,” he said in the interview. “I see this especially in the Hispanic community; many don’t understand what they can do with genetic material, or they’re afraid it will be shared with police, and if they’re here in nonofficial immigration status, they’re afraid they could be deported. There are a lot of misconceptions about genetic research.”
An initiative of the Parkinson’s Foundation PD GENEration Study is to provide free genetic tests and give the patient a report on genetic counseling “to empower patients,” Dr. Mata said.
Solutions for targeting underrepresented groups are emerging, Dr. Singleton said. “Actually there’s a really elegant solution, which is that in the populations that we go into and work with, we make sure the ownership of those cohorts, the ownership of the science and the analysis belongs to those populations,” he said.
“Part of that is creating infrastructure on site,” Dr. Singleton added. “Another part is providing training and outreach so we can help to train a whole new generation of scientists and researchers who can work in those populations embedded within those populations. They’re really the champion of moving that research forward.”
Dr. Alcalay credited Dr. Mata for his work with cohorts in Central and South America and in reaching out to other countries to recruit more diverse populations for genetic Parkinson’s studies. “And it’s not just because it’s the politically correct thing to do, about inclusivity and diversity,” Dr. Alcalay said. “It’s because it’s really meaningful. In addition to being ethically more appropriate, it will advance the entire field.
“I also really think it’s a no-brainer,” Dr. Alcalay said. “It’s something that needs to happen.”
Dr. Mata receives grant funding from the National Institutes of Health. Dr. Singleton and Dr. Alcalay have no relevant disclosures.
, attendees at the International Congress of Parkinson’s Disease and Movement Disorders were told.
“Through the years, as we’ve increased the number of individuals that we’ve included in our genetic studies, the number of risk factors that we’ve been able to identify has increased exponentially,” said Ignacio F. Mata, PhD, a neurogeneticist and principal investigator with the Genomic Medicine Institute of the Cleveland Clinic Lerner Research Institute. “This is all due to collaborations.”
Dr. Mata reviewed no fewer than seven initiatives that are gathering genetic data from people with Parkinson’s disease in Central and South America, India, China, Africa, Oceania, the Middle East, and Central Asia, along with efforts to target diverse populations in London and African Americans in the United States.
“One of the problems that we’ve had in the past is that most of the studies have been done just with individuals that are of European ancestry, so there’s a big gap of other populations that we haven’t been able to study,” Dr. Mata said. “And this is true for all of the current studies that are ongoing here in the United States.” That includes the Parkinson’s Progression Markers Initiative, he said, in which fewer than 6% of participants are non-European. Dr. Mata is also the lead in the Global Parkinson’s Genetics Program (GP2) for underrepresented populations.
Lack of diversity in genetic studies isn’t an issue in Parkinson’s studies alone, Dr. Mata said. “This is a generalized problem across all genetic studies,” he said, citing a 2016 analysis that found the proportion of participants in genome-wide studies was 96% European descent in 2009, shifting to 80% by 2016. “There’s still a big gap because most of the non-European populations came mostly from Asia,” Dr. Mata said, with Latinos and people of African descent representing less than 1% of the study populations.
In an interview, Dr. Mata noted there are a multitude of reasons for enrolling more diverse populations. “We’re going to be able to use genetics to create new treatments and do risk prediction – the so-called precision or personalized medicine,” he said. “We’re leaving a big chunk of the population behind if we don’t include those individuals.”
Scientific basis for diversity
There are a multitude of scientific reasons for doing so, too, said Dr. Mata. “In the whole genome we try to find gene variants that modify the risk for certain disease,” he said. “These regions can be quite large, so increasing the number of individuals that come from different genetic backgrounds can actually help us reduce the number of regions that need to be studied to find the causal variants.”
Andrew Singleton, PhD, director of the Center for Alzheimer’s and Related Dementias at the National Institute of Aging in Bethesda, Md., concurred that enrolling more diverse populations can speed up research for targeting genetic variants.
“We can use the differences in genetics to narrow down our search for variants, reduce the places where we’re looking for risk variants, and reduce the number of genes we’re looking at,” he said in an interview.
Roy Alcalay, MD, professor of neurology at Columbia University Irving Medical Center in New York, offered two more scientific reasons for more diverse study populations “in addition to being more ethically appropriate,” he said. “One is, you may identify new genes that you wouldn’t have identified otherwise; and also in the genes that already exist, you may recognize that some of the pathogenic variants may be more prevalent in populations that were unknown.”
One of the challenges in casting a wider net is that much of the research funding has been concentrated in the United States and Europe, Dr. Mata said. And even in the United States, with large, diverse populations, minority groups are underrepresented in these studies, he said, but a potential solution is emerging. “This is something that we’re learning now with COVID,” he said. “We can do a lot remotely. This should help bring some of those barriers down.”
Cultural barriers are also foreboding. “Individuals may not feel comfortable participating in research,” he said in the interview. “I see this especially in the Hispanic community; many don’t understand what they can do with genetic material, or they’re afraid it will be shared with police, and if they’re here in nonofficial immigration status, they’re afraid they could be deported. There are a lot of misconceptions about genetic research.”
An initiative of the Parkinson’s Foundation PD GENEration Study is to provide free genetic tests and give the patient a report on genetic counseling “to empower patients,” Dr. Mata said.
Solutions for targeting underrepresented groups are emerging, Dr. Singleton said. “Actually there’s a really elegant solution, which is that in the populations that we go into and work with, we make sure the ownership of those cohorts, the ownership of the science and the analysis belongs to those populations,” he said.
“Part of that is creating infrastructure on site,” Dr. Singleton added. “Another part is providing training and outreach so we can help to train a whole new generation of scientists and researchers who can work in those populations embedded within those populations. They’re really the champion of moving that research forward.”
Dr. Alcalay credited Dr. Mata for his work with cohorts in Central and South America and in reaching out to other countries to recruit more diverse populations for genetic Parkinson’s studies. “And it’s not just because it’s the politically correct thing to do, about inclusivity and diversity,” Dr. Alcalay said. “It’s because it’s really meaningful. In addition to being ethically more appropriate, it will advance the entire field.
“I also really think it’s a no-brainer,” Dr. Alcalay said. “It’s something that needs to happen.”
Dr. Mata receives grant funding from the National Institutes of Health. Dr. Singleton and Dr. Alcalay have no relevant disclosures.
FROM MDS VIRTUAL CONGRESS 2021
Time to hit pause on ‘pausing’ puberty in gender-dysphoric youth
Teens are identifying as transgender in record numbers. In 2017, 3-4 in 100 teens in the United States reported that they are or may be transgender. A more recent 2021 study suggests that the rate of transgender identification among America’s youth may be as high as 9 in 100. All of the major gender centers in the world have reported a several-thousand-percent increase in youth presenting with gender distress.
How do we reconcile these numbers with 2013 data reporting the prevalence of adult gender dysphoria to be a rare 2-14 in 100,000? Reflection is warranted because many U.S. medical societies support providing youth who have transgender identification (over 1 million children and adolescents, using the latest estimates) with access to powerful endocrine interventions.
GnRH analogues (colloquially known as “puberty blockers”) are now available at Tanner stage 2 of puberty – a threshold crossed by females as young as 8-9 years old. Cross-sex hormones and surgeries follow, and mastectomies are now available to children as young as 13. Genital-altering surgeries, as well as the removal of the ovaries, uterus, and testes, can be obtained as soon as a patient turns 18.
What’s driving this massive increase in trans-identified youth? What are the risks, benefits, and uncertainties associated with hormonal and surgical interventions? Do such interventions improve the long-term psychological health of gender-dysphoric youth? How many will regret the irreversible changes made to their bodies during what may have been a temporary phase in their development?
We don’t know the answers to these questions, but we need to figure them out before offering such interventions. Frontline clinicians – especially those working with youth – will not be able to remain on the sidelines of this issue for much longer. Each clinician considering writing a prescription for puberty blockers or cross-sex hormones, or generating a referral for surgery, will need to answer for themselves: Just because I can, does it mean I should?
What’s contributing to the rapid rise of gender-dysphoric youth?
The etiology of the rapid rise of transgender identifications in young people is vigorously debated. Proponents of hormonal and surgical interventions for youth argue that the several-thousand-percent increase in the numbers of youth seeking gender reassignment is a reflection of more social acceptance of transgender identities, allowing more young people to “come out.” But closer examination of this claim reveals several inconsistencies.
Because adolescent and young adult females now account for 6-8 in 10 of the presenting cases (previously, prepubertal males were more common), one would expect a commensurate increase in the rate of transgender identification in older females. This has not occurred. In addition, more than three-quarters of currently presenting cases have significant mental health problems or suffer from neurocognitive comorbidities such as autism spectrum disorder or attention-deficit/hyperactivity disorder – a much higher burden of mental health comorbidities than the historical cohort with gender dysphoria.
There is legitimate concern that these comorbid mental health conditions, as well as the influence of social groups and online immersion into transgender topics, may be playing a role in the rapidly growing rate of transgender identification among these particularly vulnerable youth.
The initial study positing the theory that social influence is playing a role in the increased incidence of “late” or adolescent-onset (vs. childhood-onset) transgender-identified youth was harshly attacked by proponents of medical transitioning of youth, despite the fact that the study utilized methods similar to those used in other areas of health research. The study underwent an unprecedented second peer review and emerged with largely unchanged conclusions.
Since the study’s publication, a number of mental health clinicians working directly with gender-distressed youth have corroborated a rapid onset of transgender identification among teens with previously gender-normative childhoods.
Pioneers in gender dysphoria treatment are changing course
Several European countries that were pioneers in pediatric medical transition are now reversing course toward far more caution after their own evidence evaluations failed to show that medically transitioning gender-distressed youth improves mental health outcomes. In Sweden, following Karolinska Hospital’s announcement that it will no longer transition people under 18 outside of strictly regulated clinical trials, a number of other pediatric gender clinics followed suit and made the same decision.
In the United Kingdom, Keira Bell – a young woman who was treated with “affirmative” hormonal and surgical interventions before detransitioning – brought a challenge against the national gender clinic. Her landmark case and the UK High Court’s original judgement against the clinic have highlighted the urgency to reassess treatment approaches for the increasingly varied presentations of gender dysphoria in young people. As this article went to press, the UK’s national gender clinic won its appeal against Keira Bell, meaning that doctors there will once again be able to decide whether their patients under 16 can properly consent to puberty blockers. Keira Bell said she is disappointed with this decision and will be seeking permission to appeal to the Supreme Court. She said the medical service had become “politicized,” and added: “A global conversation has begun and has been shaped by this case. It has shone a light into the dark corners of a medical scandal that is harming children and harmed me. There is more to be done.”
And the UK National Health Service (NHS) has already commissioned an independent systematic review of data, which concluded that the evidence of benefit of hormonal interventions in gender dysphoric youth is of very low certainty and must be carefully weighed against the risks. An independent taskforce has also been convened to reassess the country’s approach to treating gender dysphoric youth.
Finland has arguably undertaken the biggest change of all. An early adopter of pediatric medical transition, researchers there noticed that adolescents who had mental health struggles at baseline failed to improve after transition. The Finnish national Gender Identity Development services issued new treatment guidelines in 2020 stating that psychotherapy, rather than gender reassignment, should be the first line of treatment for gender-dysphoric youth.
Leaders of America’s medical societies have been slower to respond. Recently, the Society for Evidence-Based Gender Medicine applied to share information about youth gender transitions at the yearly meeting of the American Academy of Pediatrics (AAP). The application was denied without explanation, despite the fact that 80% of rank-and-file pediatricians who voted on AAP resolutions days earlier endorsed a resolution calling for a reassessment of the evidence and more caution regarding gender transitions of minors.
The AAP leadership apparently ignored the resounding support for this resolution, but the clear message from that vote is that frontline pediatricians do not agree with the “one size fits all” approach of automatically affirming gender-distressed youth as transgender and proceeding to gender reassignment.
What we know and don’t know
There is now growing evidence that the “gender-affirming” model, based on the unproven assumption that gender reassignment is the best way to help gender-distressed youth, is not living up to its promise. This should not be surprising. Despite more than 50 years of experience with mature adult gender transitions, there is a lack of convincing evidence that transitions improve the psychological functioning of those with gender dysphoria, and studies on regret have been plagued by high dropout rates that prevent meaningful conclusions for practitioners and patients alike. Pediatric transitions are a much more recent phenomenon, with little to no quality data to guide decision-making.
We are witnessing a growing number of vocal regretters who underwent gender reassignment as teens and young adults under “gender-affirming” care protocols in recent years. A review of stories on the subreddit r/detrans, which counts over 20,000 members (not all are detransitioners, as the forum is open to those fully detransitioned, partially detransitioned, desisted [those who identified as transgender for a period of time in their youth but no longer do], and questioning their transition) is flush with first-hand accounts of regret and should be mandatory reading for any clinician who is considering becoming a prescriber of gender-affirmative care.
Here is a brief outline of what we know – and more importantly, what we don’t know – about the practice of medically transitioning minors.
Most cases of early childhood-onset gender dysphoria self-resolve. Eleven out of 11 studies that followed the trajectory of gender-variant youth show that the most common outcome is natural resolution of gender dysphoria around or after puberty. Among those diagnosed as having gender identity disorder, 67% no longer met the diagnostic criteria as adults; among those subthreshold for diagnosis, 93% were not gender dysphoric as adults. Gender dysphoria in childhood is a far better predictor of future homosexuality than of future trans identity.
The future trajectory of people whose transgender identity emerged during or after puberty is entirely unknown. No one has studied future trajectories of patients whose transgender identity emerged for the first time after the onset of puberty – a previously rare but now increasingly common presentation. Growing numbers of young detransitioners and desisters are precisely from this demographic, suggesting that a transgender identity that emerges in adolescence may not be durable.
Social transition does not improve mental health outcomes. Recent studies show that while socially transitioned children can thrive in the short term, they do not fare any better than their non–socially transitioned dysphoric peers. It appears that peer relations, not the social transition status, predict mental health in gender-dysphoric children. We don’t yet know the long-term trajectories of socially transitioned minors, but emerging evidence suggests that they may be more likely to persist with gender-related distress rather than outgrow it, as previously observed. This in turn necessitates decades of invasive and risky medical interventions. In fact, the Dutch researchers who pioneered the protocol used to medically transition minors explicitly and strongly discouraged social transition of children and early adolescents.
Nearly 100% of children who begin puberty blockers will proceed to cross-sex hormones and surgeries. The two main studies that have evaluated the effects of puberty blockers on mental health found no improvements or improvements of marginal clinical significance. Both studies are also at critical risk of bias due to the absence of control groups.
Four additional studies looking at the mental health effects of puberty blockers were plagued by design limitations and also failed to show any convincing positive effects on psychological health. However, one effect of puberty blockers has been consistently replicated: At least four studies show that virtually all of the children who start puberty blockers proceed to cross-sex hormones. This suggests that rather than being a pause button, puberty blockers may serve as the “gas pedal” for gender transition.
Most of the long-term health risks are largely unknown. No long-term studies exist of patients who underwent medical transition as teens or young adults. Therefore, our ability to assess risks vs. benefits is limited. Puberty blockers have been demonstrated to significantly impair bone health, and it is not clear whether this will result in future osteoporosis. Cross-sex hormones are associated with roughly 3-5 times the risk for heart attacks and strokes, though long-term studies are of insufficient quality for accurate risk assessments. Other risks associated with these endocrine interventions will come to light as the practice continues to scale and as young people spend years and decades on these interventions. The risks to fertility are largely unknown, but it is almost certain that if puberty blockers are given at the early stages of puberty and followed by cross-sex hormones, sterility will result.
The medical pathway of “affirmative care” rests on a single Dutch study that is not applicable to the current populations of gender-dysphoric youth. Most of the youth presenting for care today would have been explicitly disqualified by the original Dutch protocol, as most have significant mental health comorbidities and post-puberty onset of trans identities. This fact has been recognized by the principal investigators of the Dutch protocol itself, who have recently begun to sound the alarm about the potential misapplication of their protocol and who suggest that psychotherapy – rather than gender reassignment – is more appropriate for many of the currently presenting cases.
On suicidality
The urgency to put gender-dysphoric youth through gender reassignment despite the dearth of evidence appears to stem from the notion that if we don’t intervene medically and in short order, these youth will commit suicide. However, studies using quality data reveal a markedly different reality.
While gender-dysphoric youth do have elevated rates of suicidality, it’s not uniquely high. In fact, it’s roughly similar to the rate of suicidality found in populations of youth referred for other mental health conditions. Quality long-term studies that explored whether transition leads to reduced suicidality have not been able to demonstrate a reduction.
Medicine has a pattern of enthusiastically embracing unproven medical interventions, only to find out years or decades later that the harms from those interventions outweigh the benefits. We owe it to our patients to be transparent about the limits of our knowledge and the fact that the “affirmative care” pathway is largely irreversible.
When the benefits of an intervention have not been shown to outweigh the risks, medical ethics dictate that such interventions should not occur outside of clinical trials. We must not conflate medical care for gender-dysphoric youth with experimental and risky interventions that are based on low-quality evidence. It’s time to hit pause on gender transitions for youth.
A brief history of the Dutch protocol
Before the mid-1990s, medical transition was primarily reserved for mature adults. However, noting the “never-disappearing masculine appearance” of many adult male transitioners, a team of Dutch researchers hypothesized that it might be appropriate to provide early intervention to a carefully selected group of adolescents before the irreversible physical changes of puberty occur.
To differentiate the majority of gender-dysphoric children who would outgrow their cross-sex identification by adulthood from the few who would probably not have resolution and would wish to transition later in life, the Dutch gender clinic designed a rigorous screening protocol, with multidisciplinary teams closely following prospective candidates for several years.
To qualify for early intervention, the adolescents had to have had persistent and severe cross-sex identification from early childhood (cases of adolescent-onset trans identity were disqualified); the distress had to worsen during puberty; and the adolescents had to be free from any other significant mental health conditions. For qualifying adolescents, puberty blockers were initiated no earlier than 12 years of age, cross-sex hormones at 16, and surgeries upon turning 18. Ongoing psychotherapy was provided through the entire assessment and intervention period.
The Dutch team published the final results of their research in 2014. The authors reported that at the average age of 21 (approximately 1.5 years post surgery), the young people were free from gender dysphoria and functioning well. Despite a postsurgical death from infection, several new diagnoses of metabolic illness, and multiple dropouts, the Western world enthusiastically embraced the early-intervention model. Concerningly, the only attempt to replicate the Dutch protocol outside of the Netherlands failed to show any psychological improvements, and to date, no long-term outcome data are available for the cohort of the 55 treated Dutch adolescents.
These progressively irreversible interventions form the basis of the “Dutch Protocol.” Currently, this protocol is being scaled in ways it was never designed for. For example, it strongly discouraged childhood social transition and did not transition adolescents with postpubertal onset of transgender identity or those with significant mental health comorbidities. Yet, treating such cases with the interventions outlined in the Dutch protocol is now common, and the age of eligibility for hormonal and surgical interventions has progressively lowered, with children as young as 8 now eligible to begin puberty blockers.
William Malone, MD, is an assistant professor of endocrinology practicing in Southern Idaho and an adviser to the Society for Evidence-Based Gender Medicine. A version of this article first appeared on Medscape.com.
Teens are identifying as transgender in record numbers. In 2017, 3-4 in 100 teens in the United States reported that they are or may be transgender. A more recent 2021 study suggests that the rate of transgender identification among America’s youth may be as high as 9 in 100. All of the major gender centers in the world have reported a several-thousand-percent increase in youth presenting with gender distress.
How do we reconcile these numbers with 2013 data reporting the prevalence of adult gender dysphoria to be a rare 2-14 in 100,000? Reflection is warranted because many U.S. medical societies support providing youth who have transgender identification (over 1 million children and adolescents, using the latest estimates) with access to powerful endocrine interventions.
GnRH analogues (colloquially known as “puberty blockers”) are now available at Tanner stage 2 of puberty – a threshold crossed by females as young as 8-9 years old. Cross-sex hormones and surgeries follow, and mastectomies are now available to children as young as 13. Genital-altering surgeries, as well as the removal of the ovaries, uterus, and testes, can be obtained as soon as a patient turns 18.
What’s driving this massive increase in trans-identified youth? What are the risks, benefits, and uncertainties associated with hormonal and surgical interventions? Do such interventions improve the long-term psychological health of gender-dysphoric youth? How many will regret the irreversible changes made to their bodies during what may have been a temporary phase in their development?
We don’t know the answers to these questions, but we need to figure them out before offering such interventions. Frontline clinicians – especially those working with youth – will not be able to remain on the sidelines of this issue for much longer. Each clinician considering writing a prescription for puberty blockers or cross-sex hormones, or generating a referral for surgery, will need to answer for themselves: Just because I can, does it mean I should?
What’s contributing to the rapid rise of gender-dysphoric youth?
The etiology of the rapid rise of transgender identifications in young people is vigorously debated. Proponents of hormonal and surgical interventions for youth argue that the several-thousand-percent increase in the numbers of youth seeking gender reassignment is a reflection of more social acceptance of transgender identities, allowing more young people to “come out.” But closer examination of this claim reveals several inconsistencies.
Because adolescent and young adult females now account for 6-8 in 10 of the presenting cases (previously, prepubertal males were more common), one would expect a commensurate increase in the rate of transgender identification in older females. This has not occurred. In addition, more than three-quarters of currently presenting cases have significant mental health problems or suffer from neurocognitive comorbidities such as autism spectrum disorder or attention-deficit/hyperactivity disorder – a much higher burden of mental health comorbidities than the historical cohort with gender dysphoria.
There is legitimate concern that these comorbid mental health conditions, as well as the influence of social groups and online immersion into transgender topics, may be playing a role in the rapidly growing rate of transgender identification among these particularly vulnerable youth.
The initial study positing the theory that social influence is playing a role in the increased incidence of “late” or adolescent-onset (vs. childhood-onset) transgender-identified youth was harshly attacked by proponents of medical transitioning of youth, despite the fact that the study utilized methods similar to those used in other areas of health research. The study underwent an unprecedented second peer review and emerged with largely unchanged conclusions.
Since the study’s publication, a number of mental health clinicians working directly with gender-distressed youth have corroborated a rapid onset of transgender identification among teens with previously gender-normative childhoods.
Pioneers in gender dysphoria treatment are changing course
Several European countries that were pioneers in pediatric medical transition are now reversing course toward far more caution after their own evidence evaluations failed to show that medically transitioning gender-distressed youth improves mental health outcomes. In Sweden, following Karolinska Hospital’s announcement that it will no longer transition people under 18 outside of strictly regulated clinical trials, a number of other pediatric gender clinics followed suit and made the same decision.
In the United Kingdom, Keira Bell – a young woman who was treated with “affirmative” hormonal and surgical interventions before detransitioning – brought a challenge against the national gender clinic. Her landmark case and the UK High Court’s original judgement against the clinic have highlighted the urgency to reassess treatment approaches for the increasingly varied presentations of gender dysphoria in young people. As this article went to press, the UK’s national gender clinic won its appeal against Keira Bell, meaning that doctors there will once again be able to decide whether their patients under 16 can properly consent to puberty blockers. Keira Bell said she is disappointed with this decision and will be seeking permission to appeal to the Supreme Court. She said the medical service had become “politicized,” and added: “A global conversation has begun and has been shaped by this case. It has shone a light into the dark corners of a medical scandal that is harming children and harmed me. There is more to be done.”
And the UK National Health Service (NHS) has already commissioned an independent systematic review of data, which concluded that the evidence of benefit of hormonal interventions in gender dysphoric youth is of very low certainty and must be carefully weighed against the risks. An independent taskforce has also been convened to reassess the country’s approach to treating gender dysphoric youth.
Finland has arguably undertaken the biggest change of all. An early adopter of pediatric medical transition, researchers there noticed that adolescents who had mental health struggles at baseline failed to improve after transition. The Finnish national Gender Identity Development services issued new treatment guidelines in 2020 stating that psychotherapy, rather than gender reassignment, should be the first line of treatment for gender-dysphoric youth.
Leaders of America’s medical societies have been slower to respond. Recently, the Society for Evidence-Based Gender Medicine applied to share information about youth gender transitions at the yearly meeting of the American Academy of Pediatrics (AAP). The application was denied without explanation, despite the fact that 80% of rank-and-file pediatricians who voted on AAP resolutions days earlier endorsed a resolution calling for a reassessment of the evidence and more caution regarding gender transitions of minors.
The AAP leadership apparently ignored the resounding support for this resolution, but the clear message from that vote is that frontline pediatricians do not agree with the “one size fits all” approach of automatically affirming gender-distressed youth as transgender and proceeding to gender reassignment.
What we know and don’t know
There is now growing evidence that the “gender-affirming” model, based on the unproven assumption that gender reassignment is the best way to help gender-distressed youth, is not living up to its promise. This should not be surprising. Despite more than 50 years of experience with mature adult gender transitions, there is a lack of convincing evidence that transitions improve the psychological functioning of those with gender dysphoria, and studies on regret have been plagued by high dropout rates that prevent meaningful conclusions for practitioners and patients alike. Pediatric transitions are a much more recent phenomenon, with little to no quality data to guide decision-making.
We are witnessing a growing number of vocal regretters who underwent gender reassignment as teens and young adults under “gender-affirming” care protocols in recent years. A review of stories on the subreddit r/detrans, which counts over 20,000 members (not all are detransitioners, as the forum is open to those fully detransitioned, partially detransitioned, desisted [those who identified as transgender for a period of time in their youth but no longer do], and questioning their transition) is flush with first-hand accounts of regret and should be mandatory reading for any clinician who is considering becoming a prescriber of gender-affirmative care.
Here is a brief outline of what we know – and more importantly, what we don’t know – about the practice of medically transitioning minors.
Most cases of early childhood-onset gender dysphoria self-resolve. Eleven out of 11 studies that followed the trajectory of gender-variant youth show that the most common outcome is natural resolution of gender dysphoria around or after puberty. Among those diagnosed as having gender identity disorder, 67% no longer met the diagnostic criteria as adults; among those subthreshold for diagnosis, 93% were not gender dysphoric as adults. Gender dysphoria in childhood is a far better predictor of future homosexuality than of future trans identity.
The future trajectory of people whose transgender identity emerged during or after puberty is entirely unknown. No one has studied future trajectories of patients whose transgender identity emerged for the first time after the onset of puberty – a previously rare but now increasingly common presentation. Growing numbers of young detransitioners and desisters are precisely from this demographic, suggesting that a transgender identity that emerges in adolescence may not be durable.
Social transition does not improve mental health outcomes. Recent studies show that while socially transitioned children can thrive in the short term, they do not fare any better than their non–socially transitioned dysphoric peers. It appears that peer relations, not the social transition status, predict mental health in gender-dysphoric children. We don’t yet know the long-term trajectories of socially transitioned minors, but emerging evidence suggests that they may be more likely to persist with gender-related distress rather than outgrow it, as previously observed. This in turn necessitates decades of invasive and risky medical interventions. In fact, the Dutch researchers who pioneered the protocol used to medically transition minors explicitly and strongly discouraged social transition of children and early adolescents.
Nearly 100% of children who begin puberty blockers will proceed to cross-sex hormones and surgeries. The two main studies that have evaluated the effects of puberty blockers on mental health found no improvements or improvements of marginal clinical significance. Both studies are also at critical risk of bias due to the absence of control groups.
Four additional studies looking at the mental health effects of puberty blockers were plagued by design limitations and also failed to show any convincing positive effects on psychological health. However, one effect of puberty blockers has been consistently replicated: At least four studies show that virtually all of the children who start puberty blockers proceed to cross-sex hormones. This suggests that rather than being a pause button, puberty blockers may serve as the “gas pedal” for gender transition.
Most of the long-term health risks are largely unknown. No long-term studies exist of patients who underwent medical transition as teens or young adults. Therefore, our ability to assess risks vs. benefits is limited. Puberty blockers have been demonstrated to significantly impair bone health, and it is not clear whether this will result in future osteoporosis. Cross-sex hormones are associated with roughly 3-5 times the risk for heart attacks and strokes, though long-term studies are of insufficient quality for accurate risk assessments. Other risks associated with these endocrine interventions will come to light as the practice continues to scale and as young people spend years and decades on these interventions. The risks to fertility are largely unknown, but it is almost certain that if puberty blockers are given at the early stages of puberty and followed by cross-sex hormones, sterility will result.
The medical pathway of “affirmative care” rests on a single Dutch study that is not applicable to the current populations of gender-dysphoric youth. Most of the youth presenting for care today would have been explicitly disqualified by the original Dutch protocol, as most have significant mental health comorbidities and post-puberty onset of trans identities. This fact has been recognized by the principal investigators of the Dutch protocol itself, who have recently begun to sound the alarm about the potential misapplication of their protocol and who suggest that psychotherapy – rather than gender reassignment – is more appropriate for many of the currently presenting cases.
On suicidality
The urgency to put gender-dysphoric youth through gender reassignment despite the dearth of evidence appears to stem from the notion that if we don’t intervene medically and in short order, these youth will commit suicide. However, studies using quality data reveal a markedly different reality.
While gender-dysphoric youth do have elevated rates of suicidality, it’s not uniquely high. In fact, it’s roughly similar to the rate of suicidality found in populations of youth referred for other mental health conditions. Quality long-term studies that explored whether transition leads to reduced suicidality have not been able to demonstrate a reduction.
Medicine has a pattern of enthusiastically embracing unproven medical interventions, only to find out years or decades later that the harms from those interventions outweigh the benefits. We owe it to our patients to be transparent about the limits of our knowledge and the fact that the “affirmative care” pathway is largely irreversible.
When the benefits of an intervention have not been shown to outweigh the risks, medical ethics dictate that such interventions should not occur outside of clinical trials. We must not conflate medical care for gender-dysphoric youth with experimental and risky interventions that are based on low-quality evidence. It’s time to hit pause on gender transitions for youth.
A brief history of the Dutch protocol
Before the mid-1990s, medical transition was primarily reserved for mature adults. However, noting the “never-disappearing masculine appearance” of many adult male transitioners, a team of Dutch researchers hypothesized that it might be appropriate to provide early intervention to a carefully selected group of adolescents before the irreversible physical changes of puberty occur.
To differentiate the majority of gender-dysphoric children who would outgrow their cross-sex identification by adulthood from the few who would probably not have resolution and would wish to transition later in life, the Dutch gender clinic designed a rigorous screening protocol, with multidisciplinary teams closely following prospective candidates for several years.
To qualify for early intervention, the adolescents had to have had persistent and severe cross-sex identification from early childhood (cases of adolescent-onset trans identity were disqualified); the distress had to worsen during puberty; and the adolescents had to be free from any other significant mental health conditions. For qualifying adolescents, puberty blockers were initiated no earlier than 12 years of age, cross-sex hormones at 16, and surgeries upon turning 18. Ongoing psychotherapy was provided through the entire assessment and intervention period.
The Dutch team published the final results of their research in 2014. The authors reported that at the average age of 21 (approximately 1.5 years post surgery), the young people were free from gender dysphoria and functioning well. Despite a postsurgical death from infection, several new diagnoses of metabolic illness, and multiple dropouts, the Western world enthusiastically embraced the early-intervention model. Concerningly, the only attempt to replicate the Dutch protocol outside of the Netherlands failed to show any psychological improvements, and to date, no long-term outcome data are available for the cohort of the 55 treated Dutch adolescents.
These progressively irreversible interventions form the basis of the “Dutch Protocol.” Currently, this protocol is being scaled in ways it was never designed for. For example, it strongly discouraged childhood social transition and did not transition adolescents with postpubertal onset of transgender identity or those with significant mental health comorbidities. Yet, treating such cases with the interventions outlined in the Dutch protocol is now common, and the age of eligibility for hormonal and surgical interventions has progressively lowered, with children as young as 8 now eligible to begin puberty blockers.
William Malone, MD, is an assistant professor of endocrinology practicing in Southern Idaho and an adviser to the Society for Evidence-Based Gender Medicine. A version of this article first appeared on Medscape.com.
Teens are identifying as transgender in record numbers. In 2017, 3-4 in 100 teens in the United States reported that they are or may be transgender. A more recent 2021 study suggests that the rate of transgender identification among America’s youth may be as high as 9 in 100. All of the major gender centers in the world have reported a several-thousand-percent increase in youth presenting with gender distress.
How do we reconcile these numbers with 2013 data reporting the prevalence of adult gender dysphoria to be a rare 2-14 in 100,000? Reflection is warranted because many U.S. medical societies support providing youth who have transgender identification (over 1 million children and adolescents, using the latest estimates) with access to powerful endocrine interventions.
GnRH analogues (colloquially known as “puberty blockers”) are now available at Tanner stage 2 of puberty – a threshold crossed by females as young as 8-9 years old. Cross-sex hormones and surgeries follow, and mastectomies are now available to children as young as 13. Genital-altering surgeries, as well as the removal of the ovaries, uterus, and testes, can be obtained as soon as a patient turns 18.
What’s driving this massive increase in trans-identified youth? What are the risks, benefits, and uncertainties associated with hormonal and surgical interventions? Do such interventions improve the long-term psychological health of gender-dysphoric youth? How many will regret the irreversible changes made to their bodies during what may have been a temporary phase in their development?
We don’t know the answers to these questions, but we need to figure them out before offering such interventions. Frontline clinicians – especially those working with youth – will not be able to remain on the sidelines of this issue for much longer. Each clinician considering writing a prescription for puberty blockers or cross-sex hormones, or generating a referral for surgery, will need to answer for themselves: Just because I can, does it mean I should?
What’s contributing to the rapid rise of gender-dysphoric youth?
The etiology of the rapid rise of transgender identifications in young people is vigorously debated. Proponents of hormonal and surgical interventions for youth argue that the several-thousand-percent increase in the numbers of youth seeking gender reassignment is a reflection of more social acceptance of transgender identities, allowing more young people to “come out.” But closer examination of this claim reveals several inconsistencies.
Because adolescent and young adult females now account for 6-8 in 10 of the presenting cases (previously, prepubertal males were more common), one would expect a commensurate increase in the rate of transgender identification in older females. This has not occurred. In addition, more than three-quarters of currently presenting cases have significant mental health problems or suffer from neurocognitive comorbidities such as autism spectrum disorder or attention-deficit/hyperactivity disorder – a much higher burden of mental health comorbidities than the historical cohort with gender dysphoria.
There is legitimate concern that these comorbid mental health conditions, as well as the influence of social groups and online immersion into transgender topics, may be playing a role in the rapidly growing rate of transgender identification among these particularly vulnerable youth.
The initial study positing the theory that social influence is playing a role in the increased incidence of “late” or adolescent-onset (vs. childhood-onset) transgender-identified youth was harshly attacked by proponents of medical transitioning of youth, despite the fact that the study utilized methods similar to those used in other areas of health research. The study underwent an unprecedented second peer review and emerged with largely unchanged conclusions.
Since the study’s publication, a number of mental health clinicians working directly with gender-distressed youth have corroborated a rapid onset of transgender identification among teens with previously gender-normative childhoods.
Pioneers in gender dysphoria treatment are changing course
Several European countries that were pioneers in pediatric medical transition are now reversing course toward far more caution after their own evidence evaluations failed to show that medically transitioning gender-distressed youth improves mental health outcomes. In Sweden, following Karolinska Hospital’s announcement that it will no longer transition people under 18 outside of strictly regulated clinical trials, a number of other pediatric gender clinics followed suit and made the same decision.
In the United Kingdom, Keira Bell – a young woman who was treated with “affirmative” hormonal and surgical interventions before detransitioning – brought a challenge against the national gender clinic. Her landmark case and the UK High Court’s original judgement against the clinic have highlighted the urgency to reassess treatment approaches for the increasingly varied presentations of gender dysphoria in young people. As this article went to press, the UK’s national gender clinic won its appeal against Keira Bell, meaning that doctors there will once again be able to decide whether their patients under 16 can properly consent to puberty blockers. Keira Bell said she is disappointed with this decision and will be seeking permission to appeal to the Supreme Court. She said the medical service had become “politicized,” and added: “A global conversation has begun and has been shaped by this case. It has shone a light into the dark corners of a medical scandal that is harming children and harmed me. There is more to be done.”
And the UK National Health Service (NHS) has already commissioned an independent systematic review of data, which concluded that the evidence of benefit of hormonal interventions in gender dysphoric youth is of very low certainty and must be carefully weighed against the risks. An independent taskforce has also been convened to reassess the country’s approach to treating gender dysphoric youth.
Finland has arguably undertaken the biggest change of all. An early adopter of pediatric medical transition, researchers there noticed that adolescents who had mental health struggles at baseline failed to improve after transition. The Finnish national Gender Identity Development services issued new treatment guidelines in 2020 stating that psychotherapy, rather than gender reassignment, should be the first line of treatment for gender-dysphoric youth.
Leaders of America’s medical societies have been slower to respond. Recently, the Society for Evidence-Based Gender Medicine applied to share information about youth gender transitions at the yearly meeting of the American Academy of Pediatrics (AAP). The application was denied without explanation, despite the fact that 80% of rank-and-file pediatricians who voted on AAP resolutions days earlier endorsed a resolution calling for a reassessment of the evidence and more caution regarding gender transitions of minors.
The AAP leadership apparently ignored the resounding support for this resolution, but the clear message from that vote is that frontline pediatricians do not agree with the “one size fits all” approach of automatically affirming gender-distressed youth as transgender and proceeding to gender reassignment.
What we know and don’t know
There is now growing evidence that the “gender-affirming” model, based on the unproven assumption that gender reassignment is the best way to help gender-distressed youth, is not living up to its promise. This should not be surprising. Despite more than 50 years of experience with mature adult gender transitions, there is a lack of convincing evidence that transitions improve the psychological functioning of those with gender dysphoria, and studies on regret have been plagued by high dropout rates that prevent meaningful conclusions for practitioners and patients alike. Pediatric transitions are a much more recent phenomenon, with little to no quality data to guide decision-making.
We are witnessing a growing number of vocal regretters who underwent gender reassignment as teens and young adults under “gender-affirming” care protocols in recent years. A review of stories on the subreddit r/detrans, which counts over 20,000 members (not all are detransitioners, as the forum is open to those fully detransitioned, partially detransitioned, desisted [those who identified as transgender for a period of time in their youth but no longer do], and questioning their transition) is flush with first-hand accounts of regret and should be mandatory reading for any clinician who is considering becoming a prescriber of gender-affirmative care.
Here is a brief outline of what we know – and more importantly, what we don’t know – about the practice of medically transitioning minors.
Most cases of early childhood-onset gender dysphoria self-resolve. Eleven out of 11 studies that followed the trajectory of gender-variant youth show that the most common outcome is natural resolution of gender dysphoria around or after puberty. Among those diagnosed as having gender identity disorder, 67% no longer met the diagnostic criteria as adults; among those subthreshold for diagnosis, 93% were not gender dysphoric as adults. Gender dysphoria in childhood is a far better predictor of future homosexuality than of future trans identity.
The future trajectory of people whose transgender identity emerged during or after puberty is entirely unknown. No one has studied future trajectories of patients whose transgender identity emerged for the first time after the onset of puberty – a previously rare but now increasingly common presentation. Growing numbers of young detransitioners and desisters are precisely from this demographic, suggesting that a transgender identity that emerges in adolescence may not be durable.
Social transition does not improve mental health outcomes. Recent studies show that while socially transitioned children can thrive in the short term, they do not fare any better than their non–socially transitioned dysphoric peers. It appears that peer relations, not the social transition status, predict mental health in gender-dysphoric children. We don’t yet know the long-term trajectories of socially transitioned minors, but emerging evidence suggests that they may be more likely to persist with gender-related distress rather than outgrow it, as previously observed. This in turn necessitates decades of invasive and risky medical interventions. In fact, the Dutch researchers who pioneered the protocol used to medically transition minors explicitly and strongly discouraged social transition of children and early adolescents.
Nearly 100% of children who begin puberty blockers will proceed to cross-sex hormones and surgeries. The two main studies that have evaluated the effects of puberty blockers on mental health found no improvements or improvements of marginal clinical significance. Both studies are also at critical risk of bias due to the absence of control groups.
Four additional studies looking at the mental health effects of puberty blockers were plagued by design limitations and also failed to show any convincing positive effects on psychological health. However, one effect of puberty blockers has been consistently replicated: At least four studies show that virtually all of the children who start puberty blockers proceed to cross-sex hormones. This suggests that rather than being a pause button, puberty blockers may serve as the “gas pedal” for gender transition.
Most of the long-term health risks are largely unknown. No long-term studies exist of patients who underwent medical transition as teens or young adults. Therefore, our ability to assess risks vs. benefits is limited. Puberty blockers have been demonstrated to significantly impair bone health, and it is not clear whether this will result in future osteoporosis. Cross-sex hormones are associated with roughly 3-5 times the risk for heart attacks and strokes, though long-term studies are of insufficient quality for accurate risk assessments. Other risks associated with these endocrine interventions will come to light as the practice continues to scale and as young people spend years and decades on these interventions. The risks to fertility are largely unknown, but it is almost certain that if puberty blockers are given at the early stages of puberty and followed by cross-sex hormones, sterility will result.
The medical pathway of “affirmative care” rests on a single Dutch study that is not applicable to the current populations of gender-dysphoric youth. Most of the youth presenting for care today would have been explicitly disqualified by the original Dutch protocol, as most have significant mental health comorbidities and post-puberty onset of trans identities. This fact has been recognized by the principal investigators of the Dutch protocol itself, who have recently begun to sound the alarm about the potential misapplication of their protocol and who suggest that psychotherapy – rather than gender reassignment – is more appropriate for many of the currently presenting cases.
On suicidality
The urgency to put gender-dysphoric youth through gender reassignment despite the dearth of evidence appears to stem from the notion that if we don’t intervene medically and in short order, these youth will commit suicide. However, studies using quality data reveal a markedly different reality.
While gender-dysphoric youth do have elevated rates of suicidality, it’s not uniquely high. In fact, it’s roughly similar to the rate of suicidality found in populations of youth referred for other mental health conditions. Quality long-term studies that explored whether transition leads to reduced suicidality have not been able to demonstrate a reduction.
Medicine has a pattern of enthusiastically embracing unproven medical interventions, only to find out years or decades later that the harms from those interventions outweigh the benefits. We owe it to our patients to be transparent about the limits of our knowledge and the fact that the “affirmative care” pathway is largely irreversible.
When the benefits of an intervention have not been shown to outweigh the risks, medical ethics dictate that such interventions should not occur outside of clinical trials. We must not conflate medical care for gender-dysphoric youth with experimental and risky interventions that are based on low-quality evidence. It’s time to hit pause on gender transitions for youth.
A brief history of the Dutch protocol
Before the mid-1990s, medical transition was primarily reserved for mature adults. However, noting the “never-disappearing masculine appearance” of many adult male transitioners, a team of Dutch researchers hypothesized that it might be appropriate to provide early intervention to a carefully selected group of adolescents before the irreversible physical changes of puberty occur.
To differentiate the majority of gender-dysphoric children who would outgrow their cross-sex identification by adulthood from the few who would probably not have resolution and would wish to transition later in life, the Dutch gender clinic designed a rigorous screening protocol, with multidisciplinary teams closely following prospective candidates for several years.
To qualify for early intervention, the adolescents had to have had persistent and severe cross-sex identification from early childhood (cases of adolescent-onset trans identity were disqualified); the distress had to worsen during puberty; and the adolescents had to be free from any other significant mental health conditions. For qualifying adolescents, puberty blockers were initiated no earlier than 12 years of age, cross-sex hormones at 16, and surgeries upon turning 18. Ongoing psychotherapy was provided through the entire assessment and intervention period.
The Dutch team published the final results of their research in 2014. The authors reported that at the average age of 21 (approximately 1.5 years post surgery), the young people were free from gender dysphoria and functioning well. Despite a postsurgical death from infection, several new diagnoses of metabolic illness, and multiple dropouts, the Western world enthusiastically embraced the early-intervention model. Concerningly, the only attempt to replicate the Dutch protocol outside of the Netherlands failed to show any psychological improvements, and to date, no long-term outcome data are available for the cohort of the 55 treated Dutch adolescents.
These progressively irreversible interventions form the basis of the “Dutch Protocol.” Currently, this protocol is being scaled in ways it was never designed for. For example, it strongly discouraged childhood social transition and did not transition adolescents with postpubertal onset of transgender identity or those with significant mental health comorbidities. Yet, treating such cases with the interventions outlined in the Dutch protocol is now common, and the age of eligibility for hormonal and surgical interventions has progressively lowered, with children as young as 8 now eligible to begin puberty blockers.
William Malone, MD, is an assistant professor of endocrinology practicing in Southern Idaho and an adviser to the Society for Evidence-Based Gender Medicine. A version of this article first appeared on Medscape.com.
Synthetic triglyceride shows potential in Huntington’s disease
, according to data presented at the International Congress of Parkinson’s Disease and Movement Disorders.
Reporting results of TRIHEP3 and an extension study, Fanny Mochel, MD, PhD, of Sorbonne University in Paris and the Paris Brain Institute, said in an interview that her group is the only one investigating triheptanoin to target caudate atrophy in Huntington’s disease. The Food and Drug Administration last year approved triheptanoin for the treatment of long-chain fatty acid oxidation disorders.
“The main findings are two observations: that patients were clinically stable based on their gradation of total motor score (TMS) on UHDRS (Unified Huntington’s Disease Rating Scale) after 1 year,” Dr. Mochel said in an interview. “The other is that we observed a reduction of the caudate atrophy progression that we usually see over 1 year by about 50%.”
TRIHEP3 randomized 100 patients with early-stage Huntington’s disease to triheptanoin 1g/kg daily and placebo. It followed on previous research in which the group used 31-phosphorus brain MR spectroscopy to demonstrate triheptanoin restored a normal brain energetic profile in patients with Huntington’s disease. TRIHEP3 was a 6-month randomized controlled trial at two centers, followed by a 6-month open-label phase. After that, 42 patients opted to participate in the 1-year extension study.
TRIHEP3 found no difference in caudate boundary shift integral (cBSI) at 6 months – the primary endpoint. But in the extension study, TMS tended to stabilize in patients treated for 1 year (0.6 ± 5.1), compared with those treated for 6 months (2.5 ± 4.5, P = .072).
Using a placebo control group from an external study of patients with Huntington’s disease with what Dr. Mochel described as “identical clinical characteristics,” she said the research confirmed TMS clinical stability in treated patients at 1 year (2.6 ± 4.6 vs. 0.6 ± 5.1, P = .057) and found significantly lower caudate atrophy (–3% vs. –6.7%, compared with baseline, P < .001).
Dr. Mochel also noted that Diffusion Tensor Imaging and Fixed-based analyses (FBA) showed fewer alterations in fiber metrics at 24 months in patients treated from baseline. FBA also showed improved fiber trophicity at 24 months in both groups.
‘The first good news’
Dr. Mochel noted that the Huntington’s disease community had been shaken in the spring by the failure of three trials of gene-targeting therapies for Huntington’s disease. Roche halted a phase 3 study of its antisense oligonucleotide (ASO) tominersen, and Wave Life Sciences scuttled two ASO programs in phase 1/2 trials.
“Triheptanoin is not going to cure Huntington’s disease; it’s a disease with many components, but it does work on the energy aspects and that seems to stabilize patients over the time of observation,” Dr. Mochel said. “That’s the first good news.”
She also noted that side effects were mainly gastrointestinal in nature, and they typically resolved with dietary management.
As a target in Huntington disease, the caudate nucleus is highly desirable, and caudate atrophy has been shown to occur even before the onset of motor symptoms, said N. Ahmad Aziz, MD, PhD, a neurologist and epidemiologist at the German Center for Neurodegenerative Diseases at the University of Bonn (Germany). “In this light, the findings of the trial conducted by Dr. Mochel and colleagues, which suggest that triheptanoin intake may slow down the rate of caudate atrophy in patients with early-stage Huntington’s disease, are highly promising,” Dr. Aziz said in an interview.
However, he noted that the improvement in caudate atrophy was only a secondary endpoint in the extension study. “Nevertheless, given triheptanoin’s biologically plausible mechanism of action – i.e., provision of substrates to the Krebs cycle and at least partial restoration of the well-documented defective mitochondrial function in Huntington’s disease – combined with its apparently relatively mild side-effect profile and good tolerability, I think that the preliminary findings of this trial are very promising and justify a larger phase 3 trial,” Dr. Aziz said.
Dr. Mochel said that the findings are prompting the investigators to consider just that.
Dr. Mochel has received consulting fees from and conducted investigator‐sponsored studies supported by Ultragenyx Pharmaceuticals. Dr. Aziz has no relevant financial relationships to disclose.
, according to data presented at the International Congress of Parkinson’s Disease and Movement Disorders.
Reporting results of TRIHEP3 and an extension study, Fanny Mochel, MD, PhD, of Sorbonne University in Paris and the Paris Brain Institute, said in an interview that her group is the only one investigating triheptanoin to target caudate atrophy in Huntington’s disease. The Food and Drug Administration last year approved triheptanoin for the treatment of long-chain fatty acid oxidation disorders.
“The main findings are two observations: that patients were clinically stable based on their gradation of total motor score (TMS) on UHDRS (Unified Huntington’s Disease Rating Scale) after 1 year,” Dr. Mochel said in an interview. “The other is that we observed a reduction of the caudate atrophy progression that we usually see over 1 year by about 50%.”
TRIHEP3 randomized 100 patients with early-stage Huntington’s disease to triheptanoin 1g/kg daily and placebo. It followed on previous research in which the group used 31-phosphorus brain MR spectroscopy to demonstrate triheptanoin restored a normal brain energetic profile in patients with Huntington’s disease. TRIHEP3 was a 6-month randomized controlled trial at two centers, followed by a 6-month open-label phase. After that, 42 patients opted to participate in the 1-year extension study.
TRIHEP3 found no difference in caudate boundary shift integral (cBSI) at 6 months – the primary endpoint. But in the extension study, TMS tended to stabilize in patients treated for 1 year (0.6 ± 5.1), compared with those treated for 6 months (2.5 ± 4.5, P = .072).
Using a placebo control group from an external study of patients with Huntington’s disease with what Dr. Mochel described as “identical clinical characteristics,” she said the research confirmed TMS clinical stability in treated patients at 1 year (2.6 ± 4.6 vs. 0.6 ± 5.1, P = .057) and found significantly lower caudate atrophy (–3% vs. –6.7%, compared with baseline, P < .001).
Dr. Mochel also noted that Diffusion Tensor Imaging and Fixed-based analyses (FBA) showed fewer alterations in fiber metrics at 24 months in patients treated from baseline. FBA also showed improved fiber trophicity at 24 months in both groups.
‘The first good news’
Dr. Mochel noted that the Huntington’s disease community had been shaken in the spring by the failure of three trials of gene-targeting therapies for Huntington’s disease. Roche halted a phase 3 study of its antisense oligonucleotide (ASO) tominersen, and Wave Life Sciences scuttled two ASO programs in phase 1/2 trials.
“Triheptanoin is not going to cure Huntington’s disease; it’s a disease with many components, but it does work on the energy aspects and that seems to stabilize patients over the time of observation,” Dr. Mochel said. “That’s the first good news.”
She also noted that side effects were mainly gastrointestinal in nature, and they typically resolved with dietary management.
As a target in Huntington disease, the caudate nucleus is highly desirable, and caudate atrophy has been shown to occur even before the onset of motor symptoms, said N. Ahmad Aziz, MD, PhD, a neurologist and epidemiologist at the German Center for Neurodegenerative Diseases at the University of Bonn (Germany). “In this light, the findings of the trial conducted by Dr. Mochel and colleagues, which suggest that triheptanoin intake may slow down the rate of caudate atrophy in patients with early-stage Huntington’s disease, are highly promising,” Dr. Aziz said in an interview.
However, he noted that the improvement in caudate atrophy was only a secondary endpoint in the extension study. “Nevertheless, given triheptanoin’s biologically plausible mechanism of action – i.e., provision of substrates to the Krebs cycle and at least partial restoration of the well-documented defective mitochondrial function in Huntington’s disease – combined with its apparently relatively mild side-effect profile and good tolerability, I think that the preliminary findings of this trial are very promising and justify a larger phase 3 trial,” Dr. Aziz said.
Dr. Mochel said that the findings are prompting the investigators to consider just that.
Dr. Mochel has received consulting fees from and conducted investigator‐sponsored studies supported by Ultragenyx Pharmaceuticals. Dr. Aziz has no relevant financial relationships to disclose.
, according to data presented at the International Congress of Parkinson’s Disease and Movement Disorders.
Reporting results of TRIHEP3 and an extension study, Fanny Mochel, MD, PhD, of Sorbonne University in Paris and the Paris Brain Institute, said in an interview that her group is the only one investigating triheptanoin to target caudate atrophy in Huntington’s disease. The Food and Drug Administration last year approved triheptanoin for the treatment of long-chain fatty acid oxidation disorders.
“The main findings are two observations: that patients were clinically stable based on their gradation of total motor score (TMS) on UHDRS (Unified Huntington’s Disease Rating Scale) after 1 year,” Dr. Mochel said in an interview. “The other is that we observed a reduction of the caudate atrophy progression that we usually see over 1 year by about 50%.”
TRIHEP3 randomized 100 patients with early-stage Huntington’s disease to triheptanoin 1g/kg daily and placebo. It followed on previous research in which the group used 31-phosphorus brain MR spectroscopy to demonstrate triheptanoin restored a normal brain energetic profile in patients with Huntington’s disease. TRIHEP3 was a 6-month randomized controlled trial at two centers, followed by a 6-month open-label phase. After that, 42 patients opted to participate in the 1-year extension study.
TRIHEP3 found no difference in caudate boundary shift integral (cBSI) at 6 months – the primary endpoint. But in the extension study, TMS tended to stabilize in patients treated for 1 year (0.6 ± 5.1), compared with those treated for 6 months (2.5 ± 4.5, P = .072).
Using a placebo control group from an external study of patients with Huntington’s disease with what Dr. Mochel described as “identical clinical characteristics,” she said the research confirmed TMS clinical stability in treated patients at 1 year (2.6 ± 4.6 vs. 0.6 ± 5.1, P = .057) and found significantly lower caudate atrophy (–3% vs. –6.7%, compared with baseline, P < .001).
Dr. Mochel also noted that Diffusion Tensor Imaging and Fixed-based analyses (FBA) showed fewer alterations in fiber metrics at 24 months in patients treated from baseline. FBA also showed improved fiber trophicity at 24 months in both groups.
‘The first good news’
Dr. Mochel noted that the Huntington’s disease community had been shaken in the spring by the failure of three trials of gene-targeting therapies for Huntington’s disease. Roche halted a phase 3 study of its antisense oligonucleotide (ASO) tominersen, and Wave Life Sciences scuttled two ASO programs in phase 1/2 trials.
“Triheptanoin is not going to cure Huntington’s disease; it’s a disease with many components, but it does work on the energy aspects and that seems to stabilize patients over the time of observation,” Dr. Mochel said. “That’s the first good news.”
She also noted that side effects were mainly gastrointestinal in nature, and they typically resolved with dietary management.
As a target in Huntington disease, the caudate nucleus is highly desirable, and caudate atrophy has been shown to occur even before the onset of motor symptoms, said N. Ahmad Aziz, MD, PhD, a neurologist and epidemiologist at the German Center for Neurodegenerative Diseases at the University of Bonn (Germany). “In this light, the findings of the trial conducted by Dr. Mochel and colleagues, which suggest that triheptanoin intake may slow down the rate of caudate atrophy in patients with early-stage Huntington’s disease, are highly promising,” Dr. Aziz said in an interview.
However, he noted that the improvement in caudate atrophy was only a secondary endpoint in the extension study. “Nevertheless, given triheptanoin’s biologically plausible mechanism of action – i.e., provision of substrates to the Krebs cycle and at least partial restoration of the well-documented defective mitochondrial function in Huntington’s disease – combined with its apparently relatively mild side-effect profile and good tolerability, I think that the preliminary findings of this trial are very promising and justify a larger phase 3 trial,” Dr. Aziz said.
Dr. Mochel said that the findings are prompting the investigators to consider just that.
Dr. Mochel has received consulting fees from and conducted investigator‐sponsored studies supported by Ultragenyx Pharmaceuticals. Dr. Aziz has no relevant financial relationships to disclose.
FROM MDS VIRTUAL CONGRESS 2021
Mean leadership
The differences between the mean and median of leadership data
Let me apologize for misleading all of you; this is not an article about malignant physician leaders; instead, it goes over the numbers and trends uncovered by the 2020 State of Hospital Medicine report (SoHM).1 The hospital medicine leader ends up doing many tasks like planning, growth, collaboration, finance, recruiting, scheduling, onboarding, coaching, and most near and dear to our hearts, putting out the fires and conflict resolution.
Ratio of leadership FTE to physician hospitalists FTE
If my pun has already put you off, you can avoid reading the rest of the piece and go to the 2020 SoHM to look at pages 52 (Table 3.7c), 121 (Table 4.7c), and 166 (Table 5.7c). It has a newly added table (3.7c), and it is phenomenal; it is the ratio of leadership FTE to physician hospitalists FTE. As an avid user of SoHM, I always ended up doing a makeshift calculation to “guesstimate” this number. Now that we have it calculated for us and the ultimate revelation lies in its narrow range across all groups. We might differ in the region, employment type, academics, teaching, or size, but this range is relatively narrow.
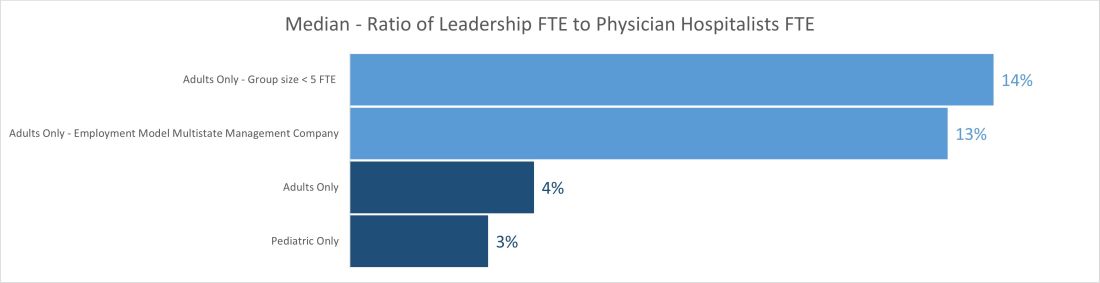
The median ratio of leadership FTE to total FTE lies between 2% and 5% in pediatric groups and between 3% and 6% for most adult groups. The only two outliers are on the adult side, with less than 5 FTE and multistate management companies. The higher median for the less than 5 FTE group size is understandable because of the small number of hospitalist FTEs that the leader’s time must be spread over. Even a small amount of dedicated leadership time will result in a high ratio of leader time to hospitalist clinical time if the group is very small. The multistate management company is probably a result of multiple layers of physician leadership (for example, regional medical directors) and travel-related time adjustments. Still, it raises the question of why the local leadership is not developed to decrease the leadership cost and better access.
Another helpful pattern is the decrease in standard deviation with the increase in group size. The hospital medicine leaders and CEOs of the hospital need to watch this number closely; any extremes on high or low side would be indicators for a deep dive in leadership structure and health.
Total number and total dedicated FTE for all physician leaders
Once we start seeing the differences between the mean and median of leadership data, we can see the median is relatively static while the mean has increased year after year and took a big jump in the 2020 SoHM. The chart below shows trends for the number of individuals in leadership positions (“Total No” and total FTEs allocated to leadership (“Total FTE”) over the last several surveys. The data is heavily skewed toward the right (positive); so, it makes sense to use the median in this case rather than mean. A few factors could explain the right skew of data.
- Large groups of 30 or more hospitalists are increasing, and so is their leadership need.
- There is more recognition of the need for dedicated leadership individuals and FTE.
- The leadership is getting less concentrated among just one or a few leaders.
- Outliers on the high side.
- Lower bounds of 0 or 0.1 FTE.
Highest-ranked leader dedicated FTE and premium compensation
Another pleasing trend is an increase in dedicated FTE for the highest-paid leader. Like any skill-set development, leadership requires the investment of deliberate practice, financial acumen, negotiation skills, and increased vulnerability. Time helps way more in developing these skill sets than money. SoHM trends show increase in dedicated FTE for the highest physician leader over the years and static premium compensation.
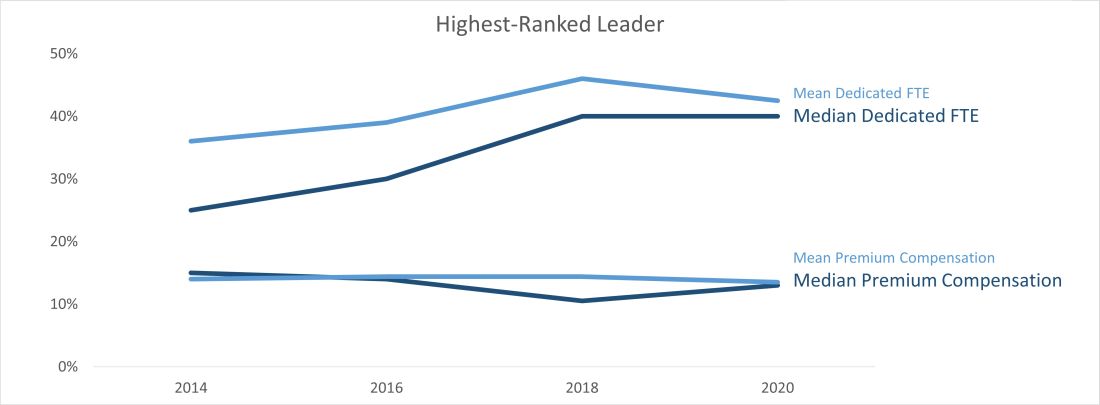
At last, we can say median leadership is always better than “mean” leadership in skewed data. Pun apart, every group needs leadership, and SoHM offers a nice window to the trends in leadership amongst many practice groups. It is a valuable resource for every group.
Dr. Chadha is chief of the division of hospital medicine at the University of Kentucky Healthcare, Lexington. He actively leads efforts of recruiting, practice analysis, and operation of the group. He is finishing his first tenure in the Practice Analysis Committee. He is often found spending a lot more than required time with spreadsheets and graphs.
Reference
1. 2020 State of Hospital Medicine. www.hospitalmedicine.org/practice-management/shms-state-of-hospital-medicine/
The differences between the mean and median of leadership data
The differences between the mean and median of leadership data
Let me apologize for misleading all of you; this is not an article about malignant physician leaders; instead, it goes over the numbers and trends uncovered by the 2020 State of Hospital Medicine report (SoHM).1 The hospital medicine leader ends up doing many tasks like planning, growth, collaboration, finance, recruiting, scheduling, onboarding, coaching, and most near and dear to our hearts, putting out the fires and conflict resolution.
Ratio of leadership FTE to physician hospitalists FTE
If my pun has already put you off, you can avoid reading the rest of the piece and go to the 2020 SoHM to look at pages 52 (Table 3.7c), 121 (Table 4.7c), and 166 (Table 5.7c). It has a newly added table (3.7c), and it is phenomenal; it is the ratio of leadership FTE to physician hospitalists FTE. As an avid user of SoHM, I always ended up doing a makeshift calculation to “guesstimate” this number. Now that we have it calculated for us and the ultimate revelation lies in its narrow range across all groups. We might differ in the region, employment type, academics, teaching, or size, but this range is relatively narrow.

The median ratio of leadership FTE to total FTE lies between 2% and 5% in pediatric groups and between 3% and 6% for most adult groups. The only two outliers are on the adult side, with less than 5 FTE and multistate management companies. The higher median for the less than 5 FTE group size is understandable because of the small number of hospitalist FTEs that the leader’s time must be spread over. Even a small amount of dedicated leadership time will result in a high ratio of leader time to hospitalist clinical time if the group is very small. The multistate management company is probably a result of multiple layers of physician leadership (for example, regional medical directors) and travel-related time adjustments. Still, it raises the question of why the local leadership is not developed to decrease the leadership cost and better access.
Another helpful pattern is the decrease in standard deviation with the increase in group size. The hospital medicine leaders and CEOs of the hospital need to watch this number closely; any extremes on high or low side would be indicators for a deep dive in leadership structure and health.
Total number and total dedicated FTE for all physician leaders
Once we start seeing the differences between the mean and median of leadership data, we can see the median is relatively static while the mean has increased year after year and took a big jump in the 2020 SoHM. The chart below shows trends for the number of individuals in leadership positions (“Total No” and total FTEs allocated to leadership (“Total FTE”) over the last several surveys. The data is heavily skewed toward the right (positive); so, it makes sense to use the median in this case rather than mean. A few factors could explain the right skew of data.
- Large groups of 30 or more hospitalists are increasing, and so is their leadership need.
- There is more recognition of the need for dedicated leadership individuals and FTE.
- The leadership is getting less concentrated among just one or a few leaders.
- Outliers on the high side.
- Lower bounds of 0 or 0.1 FTE.
Highest-ranked leader dedicated FTE and premium compensation
Another pleasing trend is an increase in dedicated FTE for the highest-paid leader. Like any skill-set development, leadership requires the investment of deliberate practice, financial acumen, negotiation skills, and increased vulnerability. Time helps way more in developing these skill sets than money. SoHM trends show increase in dedicated FTE for the highest physician leader over the years and static premium compensation.

At last, we can say median leadership is always better than “mean” leadership in skewed data. Pun apart, every group needs leadership, and SoHM offers a nice window to the trends in leadership amongst many practice groups. It is a valuable resource for every group.
Dr. Chadha is chief of the division of hospital medicine at the University of Kentucky Healthcare, Lexington. He actively leads efforts of recruiting, practice analysis, and operation of the group. He is finishing his first tenure in the Practice Analysis Committee. He is often found spending a lot more than required time with spreadsheets and graphs.
Reference
1. 2020 State of Hospital Medicine. www.hospitalmedicine.org/practice-management/shms-state-of-hospital-medicine/
Let me apologize for misleading all of you; this is not an article about malignant physician leaders; instead, it goes over the numbers and trends uncovered by the 2020 State of Hospital Medicine report (SoHM).1 The hospital medicine leader ends up doing many tasks like planning, growth, collaboration, finance, recruiting, scheduling, onboarding, coaching, and most near and dear to our hearts, putting out the fires and conflict resolution.
Ratio of leadership FTE to physician hospitalists FTE
If my pun has already put you off, you can avoid reading the rest of the piece and go to the 2020 SoHM to look at pages 52 (Table 3.7c), 121 (Table 4.7c), and 166 (Table 5.7c). It has a newly added table (3.7c), and it is phenomenal; it is the ratio of leadership FTE to physician hospitalists FTE. As an avid user of SoHM, I always ended up doing a makeshift calculation to “guesstimate” this number. Now that we have it calculated for us and the ultimate revelation lies in its narrow range across all groups. We might differ in the region, employment type, academics, teaching, or size, but this range is relatively narrow.

The median ratio of leadership FTE to total FTE lies between 2% and 5% in pediatric groups and between 3% and 6% for most adult groups. The only two outliers are on the adult side, with less than 5 FTE and multistate management companies. The higher median for the less than 5 FTE group size is understandable because of the small number of hospitalist FTEs that the leader’s time must be spread over. Even a small amount of dedicated leadership time will result in a high ratio of leader time to hospitalist clinical time if the group is very small. The multistate management company is probably a result of multiple layers of physician leadership (for example, regional medical directors) and travel-related time adjustments. Still, it raises the question of why the local leadership is not developed to decrease the leadership cost and better access.
Another helpful pattern is the decrease in standard deviation with the increase in group size. The hospital medicine leaders and CEOs of the hospital need to watch this number closely; any extremes on high or low side would be indicators for a deep dive in leadership structure and health.
Total number and total dedicated FTE for all physician leaders
Once we start seeing the differences between the mean and median of leadership data, we can see the median is relatively static while the mean has increased year after year and took a big jump in the 2020 SoHM. The chart below shows trends for the number of individuals in leadership positions (“Total No” and total FTEs allocated to leadership (“Total FTE”) over the last several surveys. The data is heavily skewed toward the right (positive); so, it makes sense to use the median in this case rather than mean. A few factors could explain the right skew of data.
- Large groups of 30 or more hospitalists are increasing, and so is their leadership need.
- There is more recognition of the need for dedicated leadership individuals and FTE.
- The leadership is getting less concentrated among just one or a few leaders.
- Outliers on the high side.
- Lower bounds of 0 or 0.1 FTE.
Highest-ranked leader dedicated FTE and premium compensation
Another pleasing trend is an increase in dedicated FTE for the highest-paid leader. Like any skill-set development, leadership requires the investment of deliberate practice, financial acumen, negotiation skills, and increased vulnerability. Time helps way more in developing these skill sets than money. SoHM trends show increase in dedicated FTE for the highest physician leader over the years and static premium compensation.

At last, we can say median leadership is always better than “mean” leadership in skewed data. Pun apart, every group needs leadership, and SoHM offers a nice window to the trends in leadership amongst many practice groups. It is a valuable resource for every group.
Dr. Chadha is chief of the division of hospital medicine at the University of Kentucky Healthcare, Lexington. He actively leads efforts of recruiting, practice analysis, and operation of the group. He is finishing his first tenure in the Practice Analysis Committee. He is often found spending a lot more than required time with spreadsheets and graphs.
Reference
1. 2020 State of Hospital Medicine. www.hospitalmedicine.org/practice-management/shms-state-of-hospital-medicine/
Pandemic restrictions ignite innovative pivot for psychiatry
As medical school faculty members – and our students – know well, the COVID-19 pandemic forced us to become creative and shift much of our curricula online. Many hospitals chose to limit medical student rotations because of safety concerns. Students fell victim to canceled psychiatry rotations and electives during the pandemic’s early days. Privacy issues, combined with stigma tied to mental illness, made this shift to virtual instruction particularly challenging. But as a field, we persevered! And, as we learned during our shift toward telemedicine, many of the changes we made in medical education are probably here to stay.
Our team at the New York Institute of Technology College of Osteopathic Medicine (NYITCOM) was able to implement a novel curriculum that allowed our students to learn psychiatry and maintain high-quality medical school education.
We developed an online course for third-year students’ rotation in psychiatry, with several modules that focused on a variety of psychiatric topics and disorders, including the basic classifications and categories of depression, anxiety, personality disorders, and psychotic disorders. There were also video encounters available showing actual patient encounters. On completion of the online module, a faculty session was held to discuss topics of concern/confusion to the students, areas of interest, and a variety of related topics, such as professionalism in psychiatry, essentials of the mental status exam, management of diverse populations, and COVID repercussions in psychiatry.
For fourth-year students, we developed a telemedicine psychiatry elective, which allowed the students to observe psychiatric evaluations, psychiatric medication review visits, and even follow-up psychotherapy sessions, with the school’s clinical psychologists. The new method was minimally invasive, and it was accepted by patients and welcomed by the students.
During a time when hospitals were limiting onsite student rotations and discouraging patient contact, medical students still needed to experience patient interactions. As the director of the school’s Center for Behavioral Health, I designed an additional program that allowed students to participate in observing patients who presented with psychiatric complaints and symptoms. It had to be confidential in nature, accessible, and safe.
I recalled my own training in a hospital setting, where students and residents were allowed to observe a patient being evaluated by an attending, through a one-way mirror. It was a method that was acceptable at the time in a hospital, but unfortunately, not in a private office setting. As such, students and residents experienced such an interaction in acute inpatient and/or outpatient clinics of a hospital. The experience was invaluable.
The concept was simple, yet very efficient. The clinicians in the Center for Behavioral Health were seeing all patients with psychiatric needs via a HIPAA-compliant telemedicine platform. Access was granted for students – with the patient’s consent – and they “entered the session” without being seen or heard. This presented little to no distraction to the patient, and the student was able to observe a range of clinical sessions.
The course also provided online supplemental modules, including essential psychiatric topics, psychopharmacology, and a psychotherapeutic module that discussed a myriad of therapeutic interventions. In addition, the student was supervised weekly by the course director, the psychopharmacologist, and the clinical psychologist. The course director provided daily wrap-up reviews as well.
Originally, this new approach was envisioned as a temporary solution for use during the pandemic. But it has become clear that this approach would be beneficial post pandemic as well. Most of the students who participated in the course were actually interested in pursuing psychiatry as their future specialty. It allowed them to observe a population of patients firsthand that they might encounter in private practice, as opposed to only hospital settings.
Being present in a session with a patient with psychiatric symptoms and diagnoses has always been a challenge. Many patients refuse to have another medical professional in the room because of the intimate details being discussed and their associated stigma. The patients’ inability to see or hear the student during the sessions allows them to ignore the students’ presence – or at least not be intimidated by it. This, therefore, allows the students access and affords them a unique and memorable educational experience.
The pandemic curtailed and altered medical students’ traditional exposure to patients, but we found innovative ways to redefine it. As difficult as COVID-19 has been for the health care community, we have been able to use the restrictions forced by the pandemic to identify innovative ways to improve the education of our medical students.
In addition to serving as director of the Center for Behavioral Health at NYITCOM in Old Westbury, N.Y., Dr. Jarkon is assistant professor in the department of family medicine. She has no disclosures.
As medical school faculty members – and our students – know well, the COVID-19 pandemic forced us to become creative and shift much of our curricula online. Many hospitals chose to limit medical student rotations because of safety concerns. Students fell victim to canceled psychiatry rotations and electives during the pandemic’s early days. Privacy issues, combined with stigma tied to mental illness, made this shift to virtual instruction particularly challenging. But as a field, we persevered! And, as we learned during our shift toward telemedicine, many of the changes we made in medical education are probably here to stay.
Our team at the New York Institute of Technology College of Osteopathic Medicine (NYITCOM) was able to implement a novel curriculum that allowed our students to learn psychiatry and maintain high-quality medical school education.
We developed an online course for third-year students’ rotation in psychiatry, with several modules that focused on a variety of psychiatric topics and disorders, including the basic classifications and categories of depression, anxiety, personality disorders, and psychotic disorders. There were also video encounters available showing actual patient encounters. On completion of the online module, a faculty session was held to discuss topics of concern/confusion to the students, areas of interest, and a variety of related topics, such as professionalism in psychiatry, essentials of the mental status exam, management of diverse populations, and COVID repercussions in psychiatry.
For fourth-year students, we developed a telemedicine psychiatry elective, which allowed the students to observe psychiatric evaluations, psychiatric medication review visits, and even follow-up psychotherapy sessions, with the school’s clinical psychologists. The new method was minimally invasive, and it was accepted by patients and welcomed by the students.
During a time when hospitals were limiting onsite student rotations and discouraging patient contact, medical students still needed to experience patient interactions. As the director of the school’s Center for Behavioral Health, I designed an additional program that allowed students to participate in observing patients who presented with psychiatric complaints and symptoms. It had to be confidential in nature, accessible, and safe.
I recalled my own training in a hospital setting, where students and residents were allowed to observe a patient being evaluated by an attending, through a one-way mirror. It was a method that was acceptable at the time in a hospital, but unfortunately, not in a private office setting. As such, students and residents experienced such an interaction in acute inpatient and/or outpatient clinics of a hospital. The experience was invaluable.
The concept was simple, yet very efficient. The clinicians in the Center for Behavioral Health were seeing all patients with psychiatric needs via a HIPAA-compliant telemedicine platform. Access was granted for students – with the patient’s consent – and they “entered the session” without being seen or heard. This presented little to no distraction to the patient, and the student was able to observe a range of clinical sessions.
The course also provided online supplemental modules, including essential psychiatric topics, psychopharmacology, and a psychotherapeutic module that discussed a myriad of therapeutic interventions. In addition, the student was supervised weekly by the course director, the psychopharmacologist, and the clinical psychologist. The course director provided daily wrap-up reviews as well.
Originally, this new approach was envisioned as a temporary solution for use during the pandemic. But it has become clear that this approach would be beneficial post pandemic as well. Most of the students who participated in the course were actually interested in pursuing psychiatry as their future specialty. It allowed them to observe a population of patients firsthand that they might encounter in private practice, as opposed to only hospital settings.
Being present in a session with a patient with psychiatric symptoms and diagnoses has always been a challenge. Many patients refuse to have another medical professional in the room because of the intimate details being discussed and their associated stigma. The patients’ inability to see or hear the student during the sessions allows them to ignore the students’ presence – or at least not be intimidated by it. This, therefore, allows the students access and affords them a unique and memorable educational experience.
The pandemic curtailed and altered medical students’ traditional exposure to patients, but we found innovative ways to redefine it. As difficult as COVID-19 has been for the health care community, we have been able to use the restrictions forced by the pandemic to identify innovative ways to improve the education of our medical students.
In addition to serving as director of the Center for Behavioral Health at NYITCOM in Old Westbury, N.Y., Dr. Jarkon is assistant professor in the department of family medicine. She has no disclosures.
As medical school faculty members – and our students – know well, the COVID-19 pandemic forced us to become creative and shift much of our curricula online. Many hospitals chose to limit medical student rotations because of safety concerns. Students fell victim to canceled psychiatry rotations and electives during the pandemic’s early days. Privacy issues, combined with stigma tied to mental illness, made this shift to virtual instruction particularly challenging. But as a field, we persevered! And, as we learned during our shift toward telemedicine, many of the changes we made in medical education are probably here to stay.
Our team at the New York Institute of Technology College of Osteopathic Medicine (NYITCOM) was able to implement a novel curriculum that allowed our students to learn psychiatry and maintain high-quality medical school education.
We developed an online course for third-year students’ rotation in psychiatry, with several modules that focused on a variety of psychiatric topics and disorders, including the basic classifications and categories of depression, anxiety, personality disorders, and psychotic disorders. There were also video encounters available showing actual patient encounters. On completion of the online module, a faculty session was held to discuss topics of concern/confusion to the students, areas of interest, and a variety of related topics, such as professionalism in psychiatry, essentials of the mental status exam, management of diverse populations, and COVID repercussions in psychiatry.
For fourth-year students, we developed a telemedicine psychiatry elective, which allowed the students to observe psychiatric evaluations, psychiatric medication review visits, and even follow-up psychotherapy sessions, with the school’s clinical psychologists. The new method was minimally invasive, and it was accepted by patients and welcomed by the students.
During a time when hospitals were limiting onsite student rotations and discouraging patient contact, medical students still needed to experience patient interactions. As the director of the school’s Center for Behavioral Health, I designed an additional program that allowed students to participate in observing patients who presented with psychiatric complaints and symptoms. It had to be confidential in nature, accessible, and safe.
I recalled my own training in a hospital setting, where students and residents were allowed to observe a patient being evaluated by an attending, through a one-way mirror. It was a method that was acceptable at the time in a hospital, but unfortunately, not in a private office setting. As such, students and residents experienced such an interaction in acute inpatient and/or outpatient clinics of a hospital. The experience was invaluable.
The concept was simple, yet very efficient. The clinicians in the Center for Behavioral Health were seeing all patients with psychiatric needs via a HIPAA-compliant telemedicine platform. Access was granted for students – with the patient’s consent – and they “entered the session” without being seen or heard. This presented little to no distraction to the patient, and the student was able to observe a range of clinical sessions.
The course also provided online supplemental modules, including essential psychiatric topics, psychopharmacology, and a psychotherapeutic module that discussed a myriad of therapeutic interventions. In addition, the student was supervised weekly by the course director, the psychopharmacologist, and the clinical psychologist. The course director provided daily wrap-up reviews as well.
Originally, this new approach was envisioned as a temporary solution for use during the pandemic. But it has become clear that this approach would be beneficial post pandemic as well. Most of the students who participated in the course were actually interested in pursuing psychiatry as their future specialty. It allowed them to observe a population of patients firsthand that they might encounter in private practice, as opposed to only hospital settings.
Being present in a session with a patient with psychiatric symptoms and diagnoses has always been a challenge. Many patients refuse to have another medical professional in the room because of the intimate details being discussed and their associated stigma. The patients’ inability to see or hear the student during the sessions allows them to ignore the students’ presence – or at least not be intimidated by it. This, therefore, allows the students access and affords them a unique and memorable educational experience.
The pandemic curtailed and altered medical students’ traditional exposure to patients, but we found innovative ways to redefine it. As difficult as COVID-19 has been for the health care community, we have been able to use the restrictions forced by the pandemic to identify innovative ways to improve the education of our medical students.
In addition to serving as director of the Center for Behavioral Health at NYITCOM in Old Westbury, N.Y., Dr. Jarkon is assistant professor in the department of family medicine. She has no disclosures.