User login
Real-World Experience With Automated Insulin Pump Technology in Veterans With Type 1 Diabetes
Insulin pump technology has been available since the 1970s. Innovation in insulin pumps has had significant impact on the management of diabetes mellitus (DM). In recent years, automated insulin pump technology (AIP) has proven to be a safe and effective way to treat DM. It has been studied mostly in highly organized randomized controlled trials (RCTs) in younger populations with type 1 DM (T1DM).1-3
One of the challenges in DM care has always been the wide variations in daily plasma glucose concentration that often cause major swings of hyperglycemia and hypoglycemia. Extreme variations in blood glucose have also been linked to adverse outcomes, including poor micro- and macrovascular outcomes.4,5 AIP technology is a hybrid closed-loop system that attempts to solve this problem by adjusting insulin delivery in response to real-time glucose information from a continuous glucose monitor (CGM). Glucose measurements are sent to the insulin pump in real time, which uses a specialized algorithm to determine whether insulin delivery should be up-titrated, down-titrated, or suspended.6
Several studies have shown that AIP technology reduces glucose variability and increases the percentage of time within the optimal glucose range.1-3,7 Its safety is especially indicated for patients with long-standing DM who often have hypoglycemia unawareness and recurrent episodes of hypoglycemia.7 Safety is the major advantage of the hybrid closed-loop system as long duration of DM makes patients particularly prone to emergency department (ED) visits and hospitalizations for severe hypoglycemia.8 Recurrent hypoglycemia also is associated with increased cardiovascular mortality in epidemiologic studies.9
Safety was the primary endpoint in the pivotal trial in a multicenter clinical study where 124 participants (mean age, 37.8 years; DM duration, 21.7 years; hemoglobin A1c [HbA1c], 7.4%) were monitored for 3 months while using a hybrid closed-loop pump, similar to the one used in our study.10 Remarkably, there were no device-related episodes of severe hypoglycemia or ketoacidosis. There was even a small but significant difference in HbA1c (7.4% at baseline, 6.9% at 3 months) and of the time in target range measured by CGM from 66.7% at baseline to 72.2% at 3 months). However, the mean age of the population studied was young (mean age, 37.8 years). It is unclear how these results would translate for a population of older patients with T1DM. Moreover, use of AIP systems have not been systematically tested outside of carefully controlled studies, as it would be in middle-aged veterans followed in outpatient US Department of Veterans Affairs (VA) clinics. Such an approach in the context of optimal glucose monitoring combined with use of structured DM education can significantly reduce impaired awareness of hypoglycemia in patients with T1DM of long duration.11
This is the first study to assess the feasibility of AIP technology in a real-world population of older veterans with T1DM in terms of safety and acceptability, because AIP has just recently become available for patient care in the Veterans Health Administration (VHA). This group of patients is of particular interest because they have been largely overlooked in earlier studies. They represent an older population with long-standing DM where hypoglycemia unawareness is often recurrent and incapacitating. In addition, long-standing DM makes optimal glycemic control mandatory to prevent microvascular complications.
Methods
In this retrospective review study,, we examined available data in patients with T1DM at the Malcom Randall VA Medical Center diabetes clinic in Gainesville, Florida, between March and December of 2018 who agreed to use AIP. In this clinic, the AIP system was offered to T1DM patients when the 4-year warranty of a previous insulin pump expired, they had frequent hypoglycemic events, or they were on multiple daily injections and were proficient with carbohydrate counting and adjusting insulin doses and willing to use an insulin pump. Veterans were trained on AIP use by a certified diabetes educator and pump trainer in sessions that lasted 2 to 4 hours depending on previous experience with AIP. Institutional review board approval was obtained at the University of Florida.
Demographic and clinical data before and after the initiation of AIP were collected, including standard insulin pump/CGM information for the Medtronic 670G and Guardian 3 Sensor AIPs. Several variables were evaluated, including age, gender, year of DM diagnosis, time of initiation of AIP, HbA1c, download data (percentage sensor wear, time in automated mode and manual mode, time in/above/below range, bolus information, insulin use, average sensor blood glucose, average meter blood glucose, pump settings), weight, body mass index (BMI), glucose meter information, history of hypoglycemia unawareness.
The primary outcome for this study was safety as assessed by percentage of time below target range on glucose sensor (time below target range is defined as < 70 mg/dL). We also addressed the secondary endpoint of efficacy as the percentage of time in-range defined as blood glucose per glucose sensor of 70 mg/dL to 180 mg/dL (efficacy), percentage of glucose sensor wear, and HbA1c.
Statistics
Comparisons of changes in continuous variables between groups were performed by an analysis of covariance (ANCOVA), adjusting for baseline levels. Fisher exact test (χ2) and unpaired t test were used to compare group differences at baseline for categorical and continuous variables, respectively, while Wilcoxon rank sum test was used for nonnormally distributed values. Changes in continuous measures within the same group were tested by paired t test or Wilcoxon matched-pairs signed rank test when applicable. Analyses were performed using Stata 11.0.
Results
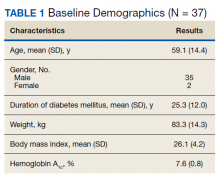
Thirty-seven veterans with T1DM using AIPs in 2018 were evaluated at baseline and at follow up visits (Tables 1 and 2). Time frame for follow-up was approximately 3 months, although there was some variation. Of note, the mean weight and BMI corresponded to mostly lean individuals, consistent with the diagnosis of T1DM.
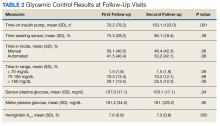
Time below target range hypoglycemia (sensor glucose < 70 mg/dL) remained low at each follow-up visit (both 1.5%). Percentage of time in automated mode increased from first to second follow-up visit after initiation of AIP (41% vs 53%, P = .06). Percentage of sensor wear numerically increased from first to second follow-up visit (75% vs 85%, P = .39), same as time in range, defined as sensor glucose 70 to 180 mg/dL, from first to second follow-up visit (70% vs 73%, P = .09). Time above range, defined as sensor glucose > 180 mg/dL, demonstrated a strong trend toward decreasing between follow-up appointments (29% to 25%; P = .09). HbA1c decreased from 7.6% to 7.3% (P = .005).
About half of the patients (18 of 37) reported hypoglycemia unawareness before the initiation of the 670G AIP. On follow-up visit 61% (11 of 18) reported significant improvement in awareness. Of the remaining 18 patients who reported normal awareness before automated mode, 17% (3 of 18) described a new onset unawareness.
Discussion
This study evaluated the safety of adopting a new DM technology in the real world of an outpatient VA clinic. To the best of our knowledge, this is the first study evaluating the use of AIP specifically in a population of middle-aged veterans with longstanding T1DM. After a mean 7 months of follow-up, participants accepted AIP use as evidenced by increased sensor wear over time and experienced improvements in DM measures that indicate successful use (ie, time in automated mode, which represents reduced glycemic variability). These results show success of an AIP approach in a demographically older group of patients.
AIP has been shown to have positive effects on glycemic control such as time in target glucose range (goal ≥ 70%). In our relatively small pilot study, there was trend for an improvement in the time in range from the first to second clinical follow-up visit, suggesting true patient involvement with the use of the device. Studies involving overall younger cohorts have proved that AIP technology is safe and efficacious for outpatient management of T1DM.7,10,12,13 However, they were all conducted under the safety of a research setting, and trials enrolled a younger population believed to adapt with more ease to this new technology. Tauschmann and colleagues performed a multicenter, parallel randomized controlled trial that compared hybrid closed-loop AIP therapy with sensor-augmented pump therapy in patients with suboptimal T1DM control.12 Results showed that the hybrid closed-loop system increased the time that the glucose concentration was within the target range (70-180 mg/dL) from 54% in the sensor-augmented pump group to 65% on the closed-loop system (P < .001). A small but significant improvement in HBA1c (from 8.0 -7.4%) and low rates of hypoglycemia (2.6% of time below 70 mg/dL) were also noted.12
A similar benefit was observed in a 2019 landmark study by Brown and colleagues of 168 patients with T1DM at 7 university medical centers who were treated for 6 months with either a closed-loop system (closed-loop group) or a sensor-augmented pump (control group) in a parallel-group, unblinded, randomized trial study.13 Mean (SD) time in the target range increased in the closed-loop group from 61% (17) at baseline to 71% (12) during the 6 months. HbA1c decreased from 7.4 to 7.1% and time ≤ 70 mg/dL was just 1.6%. However, only 13% of patients were aged ≥ 40 years in the study by Tauschmann and colleagues, and mean age was 33 years in the Brown and colleagues study.12,13 In contrast, the mean (SD) age in our study was 59 (14) years. Our pilot study also showed comparable, or somewhat better results, as mean time in target range was 72%, HbA1c was 7.3%, and time ≤ 70 mg/dL was just 1.5%.
In the only other single-center study in adults with T1DM (mean age 45 years), Faulds and colleagues evaluated changes in glycemic control and adherence in patient using the same hybrid closed-loop system.14 Treatment resulted in a decrease in HbA1c compared with baseline similar to our study, most notably for patients who had higher baseline HbA1c. However, over its short duration (6 to 12 weeks), there was decreased time in automated mode in study patients, likely due to treatment burden. Our study in older patients showed a similar reduction in HbA1c from baseline up to the 7-month visit but with increased sensor wear and time in automated mode.
There are many possible reasons for improved time in target range in our older population. Contrary to common belief that older age may be a barrier to adopting complex technology, it is likely that older age and longer duration of DM motivates adherence to a therapy that reduces glucose swings, offers a greater sense of safety and control, and improves quality of life. This is underscored by improvements over time in sensor wear and time in automated mode, measures of adherence, and successful AIP management. In support of a motivation factor to adopt insulin pump therapy in patients with long-standing T1DM, Faulds and colleagues found that older age and higher baseline HbA1c were associated with less time spent in hypoglycemia.14
The close supervision of patients by a certified diabetes educator and pump trainer may have helped improve glycemic control. Veterans received initial training, weekly follow-ups for 4 to 5 visits, and then bimonthly visits. There was also good access to the DM care team through a secure VA messaging system. This allowed for prompt troubleshooting and gave veterans the support they needed for the successful technology adoption.
The use of real-time CGM led to improvements in hypoglycemia unawareness. The nature of automated insulin delivery not only allows the patient to use a immediate CGM, but automatically lowers the delivery of insulin, further minimizing the risk of hypoglycemia.15 This combined approach explains the improvement in self-reported hypoglycemia unawareness in our cohort which decreased by 61%. As in our study, very recently Pratley and colleagues reported in a 6-month follow-up study that the greatest benefit of CGM was not the -0.3% improvement of glycemic control (similar in magnitude to our study) but the 47% decrease in the primary outcome of CGM-measured time in hypoglycemia.16
Hybrid closed-loop insulin delivery improves glucose control while reducing the risk of hypoglycemia. There is consensus that this approach is cost-effective and saves resources in the management of these complex patients, so prone to severe microvascular complications and hypoglycemia.17,18 A recent analysis by Pease and colleagues concluded that the hybrid closed-loop system was safer and more cost-effective when compared with the current standard of care, comprising insulin injections and capillary glucose testing.19 This held true even after several sensitivity analyses were performed, including baseline glycemic control, treatment effects, technology costs, age, and time horizon. This is relevant to the VHA, which at all times must consider the most cost-effective approach. Therefore, while there is no such debate about the cost-effectiveness of AIP technology for younger adults with T1DM, this study closes the knowledge gap for middle-aged veterans.7,10,12,13 The current study demonstrates that even for older patients with long-standing T1DM, when proper access to supplies and support services are made available, treatment is associated with considerable success.
Finally, AIP is well suited for telehealth applications. Data can be uploaded remotely and sent to VA health care providers, which can facilitate care without the need to travel. Distance is often a barrier for access and optimal care of veterans. The current COVID-19 pandemic is another barrier to access that may persist in the near future and adds value to AIP management.
There were a few challenges with use of AIP. Although transition to AIP was smooth for most patients already on insulin pump therapy, several noted requests for calibration in the middle of the night in automated mode, which affected sleep. Also, AIP technology requires some computer literacy to navigate the menu and address sensor calibrations, which can be a challenge for some. Based on our results, we would recommend AIP in veterans who are appropriately trained in carbohydrate counting, understand the principles of insulin therapy, and are able to navigate a computer screen menu. Most T1DM patients already using insulin pump meet those recommendations, thus, they are good candidates.
Limitations
There are some limitations to our study. The small sample size and single-center nature prevent generalization. Also, the veteran population cannot be extrapolated to other populations. For instance, the majority of the patients in this study were male.
Conclusions
We report that an AIP approach for patients with long-standing T1DM is well accepted and engages patients into monitoring their blood sugars and achieving better glycemic control. This was achieved with minimal hypoglycemia in a population where often hypoglycemia unawareness makes DM care a challenge. Future studies within the VHA are needed to fully assess the long-term benefits and cost-effectiveness of this technology in veterans.
1. Saunders A, Messer LH, Forlenza GP. MiniMed 670G hybrid closed loop artificial pancreas system for the treatment of type 1 diabetes mellitus: overview of its safety and efficacy. Expert Rev Med Devices. 2019;16(10):845-853. doi:10.1080/17434440.2019.1670639
2. Beato-Víbora PI, Quirós-López C, Lázaro-Martín L, et al. Impact of sensor-augmented pump therapy with predictive low-glucose suspend function on glycemic control and patient satisfaction in adults and children with type 1 diabetes. Diabetes Technol Ther. 2018;20(11):738-743. doi:10.1089/dia.2018.0199
3. De Ridder F, den Brinker M, De Block C. The road from intermittently scanned continuous glucose monitoring to hybrid closed-loop systems. Part B: results from randomized controlled trials. Ther Adv Endocrinol Metab. 2019;10:2042018819871903. Published 2019 Aug 30. doi:10.1177/2042018819871903
4. Monnier L, Colette C, Wojtusciszyn A, et al. Toward defining the threshold between low and high glucose variability in dabetes. Diabetes Care. 2017;40(7):832-838. doi:10.2337/dc16-1769
5. Monnier L, Colette C, Owens DR. The application of simple metrics in the assessment of glycaemic variability. Diabetes Metab. 2018;44(4):313-319. doi:10.1016/j.diabet.2018.02.008
6. Thabit H, Hovorka R. Coming of age: the artificial pancreas for type 1 diabetes. Diabetologia. 2016;59(9):1795-1805. doi:10.1007/s00125-016-4022-4
7. Anderson SM, Buckingham BA, Breton MD, et al. Hybrid closed-loop control is safe and effective for people with type 1 diabetes who are at moderate to high risk for hypoglycemia. Diabetes Technol Ther. 2019;21(6):356-363. doi:10.1089/dia.2019.0018
8. Liu J, Wang R, Ganz ML, Paprocki Y, Schneider D, Weatherall J. The burden of severe hypoglycemia in type 1 diabetes. Curr Med Res Opin. 2018;34(1):171-177. doi:10.1080/03007995.2017.1391079
9. Rawshani A, Sattar N, Franzén S, et al. Excess mortality and cardiovascular disease in young adults with type 1 diabetes in relation to age at onset: a nationwide, register-based cohort study. Lancet. 2018;392(10146):477-486. doi:10.1016/S0140-6736(18)31506-X
10. Bergenstal RM, Garg S, Weinzimer SA, et al. Safety of a hybrid closed-loop insulin delivery system in patients with type 1 diabetes. JAMA. 2016;316(13):1407-1408. doi:10.1001/jama.2016.11708
11. Little SA, Speight J, Leelarathna L, et al. Sustained reduction in severe hypoglycemia in adults with type 1 diabetes complicated by impaired awareness of hypoglycemia: two-year follow-up in the HypoCOMPaSS randomized clinical trial. Diabetes Care. 2018;41(8):1600-1607. doi:10.2337/dc17-2682
12. Tauschmann M, Thabit H, Bally L, et al. Closed-loop insulin delivery in suboptimally controlled type 1 diabetes: a multicentre, 12-week randomised trial [published correction appears in Lancet. 2018 Oct 13;392(10155):1310]. Lancet. 2018;392(10155):1321-1329. doi:10.1016/S0140-6736(18)31947-0
13. Brown SA, Kovatchev BP, Raghinaru D, et al. Six-month randomized, multicenter trial of closed-loop control in type 1 diabetes. N Engl J Med. 2019;381(18):1707-1717. doi:10.1056/NEJMoa1907863
14. Faulds ER, Zappe J, Dungan KM. Real-world implications of hybrid close loop (HCL) insulin delivery system. Endocr Pract. 2019;25(5):477-484. doi:10.4158/EP-2018-0515
15. Rickels MR, Peleckis AJ, Dalton-Bakes C, et al. Continuous glucose monitoring for hypoglycemia avoidance and glucose counterregulation in long-standing type 1 diabetes. J Clin Endocrinol Metab. 2018;103(1):105-114. doi:10.1210/jc.2017-01516
16. Pratley RE, Kanapka LG, Rickels MR, et al. Effect of continuous glucose monitoring on hypoglycemia in older adults with type 1 diabetes: a randomized clinical trial. JAMA. 2020;323(23):2397-2406. doi:10.1001/jama.2020.6928
17. Bekiari E, Kitsios K, Thabit H, et al. Artificial pancreas treatment for outpatients with type 1 diabetes: systematic review and meta-analysis. BMJ. 2018;361:k1310. Published 2018 Apr 18. doi:10.1136/bmj.k1310
18. American Diabetes Association. Addendum. 7. Diabetes technology: standards of medical care in diabetes-2020. Diabetes Care. 2020;43(suppl 1):S77-S88. Diabetes Care. 2020;43(8):1981. doi:10.2337/dc20-ad08c
19. Pease A, Zomer E, Liew D, et al. Cost-effectiveness analysis of a hybrid closed-loop system versus multiple daily injections and capillary glucose testing for adults with type 1 dabetes. Diabetes Technol Ther. 2020;22(11):812-821. doi:10.1089/dia.2020.0064
Insulin pump technology has been available since the 1970s. Innovation in insulin pumps has had significant impact on the management of diabetes mellitus (DM). In recent years, automated insulin pump technology (AIP) has proven to be a safe and effective way to treat DM. It has been studied mostly in highly organized randomized controlled trials (RCTs) in younger populations with type 1 DM (T1DM).1-3
One of the challenges in DM care has always been the wide variations in daily plasma glucose concentration that often cause major swings of hyperglycemia and hypoglycemia. Extreme variations in blood glucose have also been linked to adverse outcomes, including poor micro- and macrovascular outcomes.4,5 AIP technology is a hybrid closed-loop system that attempts to solve this problem by adjusting insulin delivery in response to real-time glucose information from a continuous glucose monitor (CGM). Glucose measurements are sent to the insulin pump in real time, which uses a specialized algorithm to determine whether insulin delivery should be up-titrated, down-titrated, or suspended.6
Several studies have shown that AIP technology reduces glucose variability and increases the percentage of time within the optimal glucose range.1-3,7 Its safety is especially indicated for patients with long-standing DM who often have hypoglycemia unawareness and recurrent episodes of hypoglycemia.7 Safety is the major advantage of the hybrid closed-loop system as long duration of DM makes patients particularly prone to emergency department (ED) visits and hospitalizations for severe hypoglycemia.8 Recurrent hypoglycemia also is associated with increased cardiovascular mortality in epidemiologic studies.9
Safety was the primary endpoint in the pivotal trial in a multicenter clinical study where 124 participants (mean age, 37.8 years; DM duration, 21.7 years; hemoglobin A1c [HbA1c], 7.4%) were monitored for 3 months while using a hybrid closed-loop pump, similar to the one used in our study.10 Remarkably, there were no device-related episodes of severe hypoglycemia or ketoacidosis. There was even a small but significant difference in HbA1c (7.4% at baseline, 6.9% at 3 months) and of the time in target range measured by CGM from 66.7% at baseline to 72.2% at 3 months). However, the mean age of the population studied was young (mean age, 37.8 years). It is unclear how these results would translate for a population of older patients with T1DM. Moreover, use of AIP systems have not been systematically tested outside of carefully controlled studies, as it would be in middle-aged veterans followed in outpatient US Department of Veterans Affairs (VA) clinics. Such an approach in the context of optimal glucose monitoring combined with use of structured DM education can significantly reduce impaired awareness of hypoglycemia in patients with T1DM of long duration.11
This is the first study to assess the feasibility of AIP technology in a real-world population of older veterans with T1DM in terms of safety and acceptability, because AIP has just recently become available for patient care in the Veterans Health Administration (VHA). This group of patients is of particular interest because they have been largely overlooked in earlier studies. They represent an older population with long-standing DM where hypoglycemia unawareness is often recurrent and incapacitating. In addition, long-standing DM makes optimal glycemic control mandatory to prevent microvascular complications.
Methods
In this retrospective review study,, we examined available data in patients with T1DM at the Malcom Randall VA Medical Center diabetes clinic in Gainesville, Florida, between March and December of 2018 who agreed to use AIP. In this clinic, the AIP system was offered to T1DM patients when the 4-year warranty of a previous insulin pump expired, they had frequent hypoglycemic events, or they were on multiple daily injections and were proficient with carbohydrate counting and adjusting insulin doses and willing to use an insulin pump. Veterans were trained on AIP use by a certified diabetes educator and pump trainer in sessions that lasted 2 to 4 hours depending on previous experience with AIP. Institutional review board approval was obtained at the University of Florida.
Demographic and clinical data before and after the initiation of AIP were collected, including standard insulin pump/CGM information for the Medtronic 670G and Guardian 3 Sensor AIPs. Several variables were evaluated, including age, gender, year of DM diagnosis, time of initiation of AIP, HbA1c, download data (percentage sensor wear, time in automated mode and manual mode, time in/above/below range, bolus information, insulin use, average sensor blood glucose, average meter blood glucose, pump settings), weight, body mass index (BMI), glucose meter information, history of hypoglycemia unawareness.
The primary outcome for this study was safety as assessed by percentage of time below target range on glucose sensor (time below target range is defined as < 70 mg/dL). We also addressed the secondary endpoint of efficacy as the percentage of time in-range defined as blood glucose per glucose sensor of 70 mg/dL to 180 mg/dL (efficacy), percentage of glucose sensor wear, and HbA1c.
Statistics
Comparisons of changes in continuous variables between groups were performed by an analysis of covariance (ANCOVA), adjusting for baseline levels. Fisher exact test (χ2) and unpaired t test were used to compare group differences at baseline for categorical and continuous variables, respectively, while Wilcoxon rank sum test was used for nonnormally distributed values. Changes in continuous measures within the same group were tested by paired t test or Wilcoxon matched-pairs signed rank test when applicable. Analyses were performed using Stata 11.0.
Results
Thirty-seven veterans with T1DM using AIPs in 2018 were evaluated at baseline and at follow up visits (Tables 1 and 2). Time frame for follow-up was approximately 3 months, although there was some variation. Of note, the mean weight and BMI corresponded to mostly lean individuals, consistent with the diagnosis of T1DM.
Time below target range hypoglycemia (sensor glucose < 70 mg/dL) remained low at each follow-up visit (both 1.5%). Percentage of time in automated mode increased from first to second follow-up visit after initiation of AIP (41% vs 53%, P = .06). Percentage of sensor wear numerically increased from first to second follow-up visit (75% vs 85%, P = .39), same as time in range, defined as sensor glucose 70 to 180 mg/dL, from first to second follow-up visit (70% vs 73%, P = .09). Time above range, defined as sensor glucose > 180 mg/dL, demonstrated a strong trend toward decreasing between follow-up appointments (29% to 25%; P = .09). HbA1c decreased from 7.6% to 7.3% (P = .005).
About half of the patients (18 of 37) reported hypoglycemia unawareness before the initiation of the 670G AIP. On follow-up visit 61% (11 of 18) reported significant improvement in awareness. Of the remaining 18 patients who reported normal awareness before automated mode, 17% (3 of 18) described a new onset unawareness.
Discussion
This study evaluated the safety of adopting a new DM technology in the real world of an outpatient VA clinic. To the best of our knowledge, this is the first study evaluating the use of AIP specifically in a population of middle-aged veterans with longstanding T1DM. After a mean 7 months of follow-up, participants accepted AIP use as evidenced by increased sensor wear over time and experienced improvements in DM measures that indicate successful use (ie, time in automated mode, which represents reduced glycemic variability). These results show success of an AIP approach in a demographically older group of patients.
AIP has been shown to have positive effects on glycemic control such as time in target glucose range (goal ≥ 70%). In our relatively small pilot study, there was trend for an improvement in the time in range from the first to second clinical follow-up visit, suggesting true patient involvement with the use of the device. Studies involving overall younger cohorts have proved that AIP technology is safe and efficacious for outpatient management of T1DM.7,10,12,13 However, they were all conducted under the safety of a research setting, and trials enrolled a younger population believed to adapt with more ease to this new technology. Tauschmann and colleagues performed a multicenter, parallel randomized controlled trial that compared hybrid closed-loop AIP therapy with sensor-augmented pump therapy in patients with suboptimal T1DM control.12 Results showed that the hybrid closed-loop system increased the time that the glucose concentration was within the target range (70-180 mg/dL) from 54% in the sensor-augmented pump group to 65% on the closed-loop system (P < .001). A small but significant improvement in HBA1c (from 8.0 -7.4%) and low rates of hypoglycemia (2.6% of time below 70 mg/dL) were also noted.12
A similar benefit was observed in a 2019 landmark study by Brown and colleagues of 168 patients with T1DM at 7 university medical centers who were treated for 6 months with either a closed-loop system (closed-loop group) or a sensor-augmented pump (control group) in a parallel-group, unblinded, randomized trial study.13 Mean (SD) time in the target range increased in the closed-loop group from 61% (17) at baseline to 71% (12) during the 6 months. HbA1c decreased from 7.4 to 7.1% and time ≤ 70 mg/dL was just 1.6%. However, only 13% of patients were aged ≥ 40 years in the study by Tauschmann and colleagues, and mean age was 33 years in the Brown and colleagues study.12,13 In contrast, the mean (SD) age in our study was 59 (14) years. Our pilot study also showed comparable, or somewhat better results, as mean time in target range was 72%, HbA1c was 7.3%, and time ≤ 70 mg/dL was just 1.5%.
In the only other single-center study in adults with T1DM (mean age 45 years), Faulds and colleagues evaluated changes in glycemic control and adherence in patient using the same hybrid closed-loop system.14 Treatment resulted in a decrease in HbA1c compared with baseline similar to our study, most notably for patients who had higher baseline HbA1c. However, over its short duration (6 to 12 weeks), there was decreased time in automated mode in study patients, likely due to treatment burden. Our study in older patients showed a similar reduction in HbA1c from baseline up to the 7-month visit but with increased sensor wear and time in automated mode.
There are many possible reasons for improved time in target range in our older population. Contrary to common belief that older age may be a barrier to adopting complex technology, it is likely that older age and longer duration of DM motivates adherence to a therapy that reduces glucose swings, offers a greater sense of safety and control, and improves quality of life. This is underscored by improvements over time in sensor wear and time in automated mode, measures of adherence, and successful AIP management. In support of a motivation factor to adopt insulin pump therapy in patients with long-standing T1DM, Faulds and colleagues found that older age and higher baseline HbA1c were associated with less time spent in hypoglycemia.14
The close supervision of patients by a certified diabetes educator and pump trainer may have helped improve glycemic control. Veterans received initial training, weekly follow-ups for 4 to 5 visits, and then bimonthly visits. There was also good access to the DM care team through a secure VA messaging system. This allowed for prompt troubleshooting and gave veterans the support they needed for the successful technology adoption.
The use of real-time CGM led to improvements in hypoglycemia unawareness. The nature of automated insulin delivery not only allows the patient to use a immediate CGM, but automatically lowers the delivery of insulin, further minimizing the risk of hypoglycemia.15 This combined approach explains the improvement in self-reported hypoglycemia unawareness in our cohort which decreased by 61%. As in our study, very recently Pratley and colleagues reported in a 6-month follow-up study that the greatest benefit of CGM was not the -0.3% improvement of glycemic control (similar in magnitude to our study) but the 47% decrease in the primary outcome of CGM-measured time in hypoglycemia.16
Hybrid closed-loop insulin delivery improves glucose control while reducing the risk of hypoglycemia. There is consensus that this approach is cost-effective and saves resources in the management of these complex patients, so prone to severe microvascular complications and hypoglycemia.17,18 A recent analysis by Pease and colleagues concluded that the hybrid closed-loop system was safer and more cost-effective when compared with the current standard of care, comprising insulin injections and capillary glucose testing.19 This held true even after several sensitivity analyses were performed, including baseline glycemic control, treatment effects, technology costs, age, and time horizon. This is relevant to the VHA, which at all times must consider the most cost-effective approach. Therefore, while there is no such debate about the cost-effectiveness of AIP technology for younger adults with T1DM, this study closes the knowledge gap for middle-aged veterans.7,10,12,13 The current study demonstrates that even for older patients with long-standing T1DM, when proper access to supplies and support services are made available, treatment is associated with considerable success.
Finally, AIP is well suited for telehealth applications. Data can be uploaded remotely and sent to VA health care providers, which can facilitate care without the need to travel. Distance is often a barrier for access and optimal care of veterans. The current COVID-19 pandemic is another barrier to access that may persist in the near future and adds value to AIP management.
There were a few challenges with use of AIP. Although transition to AIP was smooth for most patients already on insulin pump therapy, several noted requests for calibration in the middle of the night in automated mode, which affected sleep. Also, AIP technology requires some computer literacy to navigate the menu and address sensor calibrations, which can be a challenge for some. Based on our results, we would recommend AIP in veterans who are appropriately trained in carbohydrate counting, understand the principles of insulin therapy, and are able to navigate a computer screen menu. Most T1DM patients already using insulin pump meet those recommendations, thus, they are good candidates.
Limitations
There are some limitations to our study. The small sample size and single-center nature prevent generalization. Also, the veteran population cannot be extrapolated to other populations. For instance, the majority of the patients in this study were male.
Conclusions
We report that an AIP approach for patients with long-standing T1DM is well accepted and engages patients into monitoring their blood sugars and achieving better glycemic control. This was achieved with minimal hypoglycemia in a population where often hypoglycemia unawareness makes DM care a challenge. Future studies within the VHA are needed to fully assess the long-term benefits and cost-effectiveness of this technology in veterans.
Insulin pump technology has been available since the 1970s. Innovation in insulin pumps has had significant impact on the management of diabetes mellitus (DM). In recent years, automated insulin pump technology (AIP) has proven to be a safe and effective way to treat DM. It has been studied mostly in highly organized randomized controlled trials (RCTs) in younger populations with type 1 DM (T1DM).1-3
One of the challenges in DM care has always been the wide variations in daily plasma glucose concentration that often cause major swings of hyperglycemia and hypoglycemia. Extreme variations in blood glucose have also been linked to adverse outcomes, including poor micro- and macrovascular outcomes.4,5 AIP technology is a hybrid closed-loop system that attempts to solve this problem by adjusting insulin delivery in response to real-time glucose information from a continuous glucose monitor (CGM). Glucose measurements are sent to the insulin pump in real time, which uses a specialized algorithm to determine whether insulin delivery should be up-titrated, down-titrated, or suspended.6
Several studies have shown that AIP technology reduces glucose variability and increases the percentage of time within the optimal glucose range.1-3,7 Its safety is especially indicated for patients with long-standing DM who often have hypoglycemia unawareness and recurrent episodes of hypoglycemia.7 Safety is the major advantage of the hybrid closed-loop system as long duration of DM makes patients particularly prone to emergency department (ED) visits and hospitalizations for severe hypoglycemia.8 Recurrent hypoglycemia also is associated with increased cardiovascular mortality in epidemiologic studies.9
Safety was the primary endpoint in the pivotal trial in a multicenter clinical study where 124 participants (mean age, 37.8 years; DM duration, 21.7 years; hemoglobin A1c [HbA1c], 7.4%) were monitored for 3 months while using a hybrid closed-loop pump, similar to the one used in our study.10 Remarkably, there were no device-related episodes of severe hypoglycemia or ketoacidosis. There was even a small but significant difference in HbA1c (7.4% at baseline, 6.9% at 3 months) and of the time in target range measured by CGM from 66.7% at baseline to 72.2% at 3 months). However, the mean age of the population studied was young (mean age, 37.8 years). It is unclear how these results would translate for a population of older patients with T1DM. Moreover, use of AIP systems have not been systematically tested outside of carefully controlled studies, as it would be in middle-aged veterans followed in outpatient US Department of Veterans Affairs (VA) clinics. Such an approach in the context of optimal glucose monitoring combined with use of structured DM education can significantly reduce impaired awareness of hypoglycemia in patients with T1DM of long duration.11
This is the first study to assess the feasibility of AIP technology in a real-world population of older veterans with T1DM in terms of safety and acceptability, because AIP has just recently become available for patient care in the Veterans Health Administration (VHA). This group of patients is of particular interest because they have been largely overlooked in earlier studies. They represent an older population with long-standing DM where hypoglycemia unawareness is often recurrent and incapacitating. In addition, long-standing DM makes optimal glycemic control mandatory to prevent microvascular complications.
Methods
In this retrospective review study,, we examined available data in patients with T1DM at the Malcom Randall VA Medical Center diabetes clinic in Gainesville, Florida, between March and December of 2018 who agreed to use AIP. In this clinic, the AIP system was offered to T1DM patients when the 4-year warranty of a previous insulin pump expired, they had frequent hypoglycemic events, or they were on multiple daily injections and were proficient with carbohydrate counting and adjusting insulin doses and willing to use an insulin pump. Veterans were trained on AIP use by a certified diabetes educator and pump trainer in sessions that lasted 2 to 4 hours depending on previous experience with AIP. Institutional review board approval was obtained at the University of Florida.
Demographic and clinical data before and after the initiation of AIP were collected, including standard insulin pump/CGM information for the Medtronic 670G and Guardian 3 Sensor AIPs. Several variables were evaluated, including age, gender, year of DM diagnosis, time of initiation of AIP, HbA1c, download data (percentage sensor wear, time in automated mode and manual mode, time in/above/below range, bolus information, insulin use, average sensor blood glucose, average meter blood glucose, pump settings), weight, body mass index (BMI), glucose meter information, history of hypoglycemia unawareness.
The primary outcome for this study was safety as assessed by percentage of time below target range on glucose sensor (time below target range is defined as < 70 mg/dL). We also addressed the secondary endpoint of efficacy as the percentage of time in-range defined as blood glucose per glucose sensor of 70 mg/dL to 180 mg/dL (efficacy), percentage of glucose sensor wear, and HbA1c.
Statistics
Comparisons of changes in continuous variables between groups were performed by an analysis of covariance (ANCOVA), adjusting for baseline levels. Fisher exact test (χ2) and unpaired t test were used to compare group differences at baseline for categorical and continuous variables, respectively, while Wilcoxon rank sum test was used for nonnormally distributed values. Changes in continuous measures within the same group were tested by paired t test or Wilcoxon matched-pairs signed rank test when applicable. Analyses were performed using Stata 11.0.
Results
Thirty-seven veterans with T1DM using AIPs in 2018 were evaluated at baseline and at follow up visits (Tables 1 and 2). Time frame for follow-up was approximately 3 months, although there was some variation. Of note, the mean weight and BMI corresponded to mostly lean individuals, consistent with the diagnosis of T1DM.
Time below target range hypoglycemia (sensor glucose < 70 mg/dL) remained low at each follow-up visit (both 1.5%). Percentage of time in automated mode increased from first to second follow-up visit after initiation of AIP (41% vs 53%, P = .06). Percentage of sensor wear numerically increased from first to second follow-up visit (75% vs 85%, P = .39), same as time in range, defined as sensor glucose 70 to 180 mg/dL, from first to second follow-up visit (70% vs 73%, P = .09). Time above range, defined as sensor glucose > 180 mg/dL, demonstrated a strong trend toward decreasing between follow-up appointments (29% to 25%; P = .09). HbA1c decreased from 7.6% to 7.3% (P = .005).
About half of the patients (18 of 37) reported hypoglycemia unawareness before the initiation of the 670G AIP. On follow-up visit 61% (11 of 18) reported significant improvement in awareness. Of the remaining 18 patients who reported normal awareness before automated mode, 17% (3 of 18) described a new onset unawareness.
Discussion
This study evaluated the safety of adopting a new DM technology in the real world of an outpatient VA clinic. To the best of our knowledge, this is the first study evaluating the use of AIP specifically in a population of middle-aged veterans with longstanding T1DM. After a mean 7 months of follow-up, participants accepted AIP use as evidenced by increased sensor wear over time and experienced improvements in DM measures that indicate successful use (ie, time in automated mode, which represents reduced glycemic variability). These results show success of an AIP approach in a demographically older group of patients.
AIP has been shown to have positive effects on glycemic control such as time in target glucose range (goal ≥ 70%). In our relatively small pilot study, there was trend for an improvement in the time in range from the first to second clinical follow-up visit, suggesting true patient involvement with the use of the device. Studies involving overall younger cohorts have proved that AIP technology is safe and efficacious for outpatient management of T1DM.7,10,12,13 However, they were all conducted under the safety of a research setting, and trials enrolled a younger population believed to adapt with more ease to this new technology. Tauschmann and colleagues performed a multicenter, parallel randomized controlled trial that compared hybrid closed-loop AIP therapy with sensor-augmented pump therapy in patients with suboptimal T1DM control.12 Results showed that the hybrid closed-loop system increased the time that the glucose concentration was within the target range (70-180 mg/dL) from 54% in the sensor-augmented pump group to 65% on the closed-loop system (P < .001). A small but significant improvement in HBA1c (from 8.0 -7.4%) and low rates of hypoglycemia (2.6% of time below 70 mg/dL) were also noted.12
A similar benefit was observed in a 2019 landmark study by Brown and colleagues of 168 patients with T1DM at 7 university medical centers who were treated for 6 months with either a closed-loop system (closed-loop group) or a sensor-augmented pump (control group) in a parallel-group, unblinded, randomized trial study.13 Mean (SD) time in the target range increased in the closed-loop group from 61% (17) at baseline to 71% (12) during the 6 months. HbA1c decreased from 7.4 to 7.1% and time ≤ 70 mg/dL was just 1.6%. However, only 13% of patients were aged ≥ 40 years in the study by Tauschmann and colleagues, and mean age was 33 years in the Brown and colleagues study.12,13 In contrast, the mean (SD) age in our study was 59 (14) years. Our pilot study also showed comparable, or somewhat better results, as mean time in target range was 72%, HbA1c was 7.3%, and time ≤ 70 mg/dL was just 1.5%.
In the only other single-center study in adults with T1DM (mean age 45 years), Faulds and colleagues evaluated changes in glycemic control and adherence in patient using the same hybrid closed-loop system.14 Treatment resulted in a decrease in HbA1c compared with baseline similar to our study, most notably for patients who had higher baseline HbA1c. However, over its short duration (6 to 12 weeks), there was decreased time in automated mode in study patients, likely due to treatment burden. Our study in older patients showed a similar reduction in HbA1c from baseline up to the 7-month visit but with increased sensor wear and time in automated mode.
There are many possible reasons for improved time in target range in our older population. Contrary to common belief that older age may be a barrier to adopting complex technology, it is likely that older age and longer duration of DM motivates adherence to a therapy that reduces glucose swings, offers a greater sense of safety and control, and improves quality of life. This is underscored by improvements over time in sensor wear and time in automated mode, measures of adherence, and successful AIP management. In support of a motivation factor to adopt insulin pump therapy in patients with long-standing T1DM, Faulds and colleagues found that older age and higher baseline HbA1c were associated with less time spent in hypoglycemia.14
The close supervision of patients by a certified diabetes educator and pump trainer may have helped improve glycemic control. Veterans received initial training, weekly follow-ups for 4 to 5 visits, and then bimonthly visits. There was also good access to the DM care team through a secure VA messaging system. This allowed for prompt troubleshooting and gave veterans the support they needed for the successful technology adoption.
The use of real-time CGM led to improvements in hypoglycemia unawareness. The nature of automated insulin delivery not only allows the patient to use a immediate CGM, but automatically lowers the delivery of insulin, further minimizing the risk of hypoglycemia.15 This combined approach explains the improvement in self-reported hypoglycemia unawareness in our cohort which decreased by 61%. As in our study, very recently Pratley and colleagues reported in a 6-month follow-up study that the greatest benefit of CGM was not the -0.3% improvement of glycemic control (similar in magnitude to our study) but the 47% decrease in the primary outcome of CGM-measured time in hypoglycemia.16
Hybrid closed-loop insulin delivery improves glucose control while reducing the risk of hypoglycemia. There is consensus that this approach is cost-effective and saves resources in the management of these complex patients, so prone to severe microvascular complications and hypoglycemia.17,18 A recent analysis by Pease and colleagues concluded that the hybrid closed-loop system was safer and more cost-effective when compared with the current standard of care, comprising insulin injections and capillary glucose testing.19 This held true even after several sensitivity analyses were performed, including baseline glycemic control, treatment effects, technology costs, age, and time horizon. This is relevant to the VHA, which at all times must consider the most cost-effective approach. Therefore, while there is no such debate about the cost-effectiveness of AIP technology for younger adults with T1DM, this study closes the knowledge gap for middle-aged veterans.7,10,12,13 The current study demonstrates that even for older patients with long-standing T1DM, when proper access to supplies and support services are made available, treatment is associated with considerable success.
Finally, AIP is well suited for telehealth applications. Data can be uploaded remotely and sent to VA health care providers, which can facilitate care without the need to travel. Distance is often a barrier for access and optimal care of veterans. The current COVID-19 pandemic is another barrier to access that may persist in the near future and adds value to AIP management.
There were a few challenges with use of AIP. Although transition to AIP was smooth for most patients already on insulin pump therapy, several noted requests for calibration in the middle of the night in automated mode, which affected sleep. Also, AIP technology requires some computer literacy to navigate the menu and address sensor calibrations, which can be a challenge for some. Based on our results, we would recommend AIP in veterans who are appropriately trained in carbohydrate counting, understand the principles of insulin therapy, and are able to navigate a computer screen menu. Most T1DM patients already using insulin pump meet those recommendations, thus, they are good candidates.
Limitations
There are some limitations to our study. The small sample size and single-center nature prevent generalization. Also, the veteran population cannot be extrapolated to other populations. For instance, the majority of the patients in this study were male.
Conclusions
We report that an AIP approach for patients with long-standing T1DM is well accepted and engages patients into monitoring their blood sugars and achieving better glycemic control. This was achieved with minimal hypoglycemia in a population where often hypoglycemia unawareness makes DM care a challenge. Future studies within the VHA are needed to fully assess the long-term benefits and cost-effectiveness of this technology in veterans.
1. Saunders A, Messer LH, Forlenza GP. MiniMed 670G hybrid closed loop artificial pancreas system for the treatment of type 1 diabetes mellitus: overview of its safety and efficacy. Expert Rev Med Devices. 2019;16(10):845-853. doi:10.1080/17434440.2019.1670639
2. Beato-Víbora PI, Quirós-López C, Lázaro-Martín L, et al. Impact of sensor-augmented pump therapy with predictive low-glucose suspend function on glycemic control and patient satisfaction in adults and children with type 1 diabetes. Diabetes Technol Ther. 2018;20(11):738-743. doi:10.1089/dia.2018.0199
3. De Ridder F, den Brinker M, De Block C. The road from intermittently scanned continuous glucose monitoring to hybrid closed-loop systems. Part B: results from randomized controlled trials. Ther Adv Endocrinol Metab. 2019;10:2042018819871903. Published 2019 Aug 30. doi:10.1177/2042018819871903
4. Monnier L, Colette C, Wojtusciszyn A, et al. Toward defining the threshold between low and high glucose variability in dabetes. Diabetes Care. 2017;40(7):832-838. doi:10.2337/dc16-1769
5. Monnier L, Colette C, Owens DR. The application of simple metrics in the assessment of glycaemic variability. Diabetes Metab. 2018;44(4):313-319. doi:10.1016/j.diabet.2018.02.008
6. Thabit H, Hovorka R. Coming of age: the artificial pancreas for type 1 diabetes. Diabetologia. 2016;59(9):1795-1805. doi:10.1007/s00125-016-4022-4
7. Anderson SM, Buckingham BA, Breton MD, et al. Hybrid closed-loop control is safe and effective for people with type 1 diabetes who are at moderate to high risk for hypoglycemia. Diabetes Technol Ther. 2019;21(6):356-363. doi:10.1089/dia.2019.0018
8. Liu J, Wang R, Ganz ML, Paprocki Y, Schneider D, Weatherall J. The burden of severe hypoglycemia in type 1 diabetes. Curr Med Res Opin. 2018;34(1):171-177. doi:10.1080/03007995.2017.1391079
9. Rawshani A, Sattar N, Franzén S, et al. Excess mortality and cardiovascular disease in young adults with type 1 diabetes in relation to age at onset: a nationwide, register-based cohort study. Lancet. 2018;392(10146):477-486. doi:10.1016/S0140-6736(18)31506-X
10. Bergenstal RM, Garg S, Weinzimer SA, et al. Safety of a hybrid closed-loop insulin delivery system in patients with type 1 diabetes. JAMA. 2016;316(13):1407-1408. doi:10.1001/jama.2016.11708
11. Little SA, Speight J, Leelarathna L, et al. Sustained reduction in severe hypoglycemia in adults with type 1 diabetes complicated by impaired awareness of hypoglycemia: two-year follow-up in the HypoCOMPaSS randomized clinical trial. Diabetes Care. 2018;41(8):1600-1607. doi:10.2337/dc17-2682
12. Tauschmann M, Thabit H, Bally L, et al. Closed-loop insulin delivery in suboptimally controlled type 1 diabetes: a multicentre, 12-week randomised trial [published correction appears in Lancet. 2018 Oct 13;392(10155):1310]. Lancet. 2018;392(10155):1321-1329. doi:10.1016/S0140-6736(18)31947-0
13. Brown SA, Kovatchev BP, Raghinaru D, et al. Six-month randomized, multicenter trial of closed-loop control in type 1 diabetes. N Engl J Med. 2019;381(18):1707-1717. doi:10.1056/NEJMoa1907863
14. Faulds ER, Zappe J, Dungan KM. Real-world implications of hybrid close loop (HCL) insulin delivery system. Endocr Pract. 2019;25(5):477-484. doi:10.4158/EP-2018-0515
15. Rickels MR, Peleckis AJ, Dalton-Bakes C, et al. Continuous glucose monitoring for hypoglycemia avoidance and glucose counterregulation in long-standing type 1 diabetes. J Clin Endocrinol Metab. 2018;103(1):105-114. doi:10.1210/jc.2017-01516
16. Pratley RE, Kanapka LG, Rickels MR, et al. Effect of continuous glucose monitoring on hypoglycemia in older adults with type 1 diabetes: a randomized clinical trial. JAMA. 2020;323(23):2397-2406. doi:10.1001/jama.2020.6928
17. Bekiari E, Kitsios K, Thabit H, et al. Artificial pancreas treatment for outpatients with type 1 diabetes: systematic review and meta-analysis. BMJ. 2018;361:k1310. Published 2018 Apr 18. doi:10.1136/bmj.k1310
18. American Diabetes Association. Addendum. 7. Diabetes technology: standards of medical care in diabetes-2020. Diabetes Care. 2020;43(suppl 1):S77-S88. Diabetes Care. 2020;43(8):1981. doi:10.2337/dc20-ad08c
19. Pease A, Zomer E, Liew D, et al. Cost-effectiveness analysis of a hybrid closed-loop system versus multiple daily injections and capillary glucose testing for adults with type 1 dabetes. Diabetes Technol Ther. 2020;22(11):812-821. doi:10.1089/dia.2020.0064
1. Saunders A, Messer LH, Forlenza GP. MiniMed 670G hybrid closed loop artificial pancreas system for the treatment of type 1 diabetes mellitus: overview of its safety and efficacy. Expert Rev Med Devices. 2019;16(10):845-853. doi:10.1080/17434440.2019.1670639
2. Beato-Víbora PI, Quirós-López C, Lázaro-Martín L, et al. Impact of sensor-augmented pump therapy with predictive low-glucose suspend function on glycemic control and patient satisfaction in adults and children with type 1 diabetes. Diabetes Technol Ther. 2018;20(11):738-743. doi:10.1089/dia.2018.0199
3. De Ridder F, den Brinker M, De Block C. The road from intermittently scanned continuous glucose monitoring to hybrid closed-loop systems. Part B: results from randomized controlled trials. Ther Adv Endocrinol Metab. 2019;10:2042018819871903. Published 2019 Aug 30. doi:10.1177/2042018819871903
4. Monnier L, Colette C, Wojtusciszyn A, et al. Toward defining the threshold between low and high glucose variability in dabetes. Diabetes Care. 2017;40(7):832-838. doi:10.2337/dc16-1769
5. Monnier L, Colette C, Owens DR. The application of simple metrics in the assessment of glycaemic variability. Diabetes Metab. 2018;44(4):313-319. doi:10.1016/j.diabet.2018.02.008
6. Thabit H, Hovorka R. Coming of age: the artificial pancreas for type 1 diabetes. Diabetologia. 2016;59(9):1795-1805. doi:10.1007/s00125-016-4022-4
7. Anderson SM, Buckingham BA, Breton MD, et al. Hybrid closed-loop control is safe and effective for people with type 1 diabetes who are at moderate to high risk for hypoglycemia. Diabetes Technol Ther. 2019;21(6):356-363. doi:10.1089/dia.2019.0018
8. Liu J, Wang R, Ganz ML, Paprocki Y, Schneider D, Weatherall J. The burden of severe hypoglycemia in type 1 diabetes. Curr Med Res Opin. 2018;34(1):171-177. doi:10.1080/03007995.2017.1391079
9. Rawshani A, Sattar N, Franzén S, et al. Excess mortality and cardiovascular disease in young adults with type 1 diabetes in relation to age at onset: a nationwide, register-based cohort study. Lancet. 2018;392(10146):477-486. doi:10.1016/S0140-6736(18)31506-X
10. Bergenstal RM, Garg S, Weinzimer SA, et al. Safety of a hybrid closed-loop insulin delivery system in patients with type 1 diabetes. JAMA. 2016;316(13):1407-1408. doi:10.1001/jama.2016.11708
11. Little SA, Speight J, Leelarathna L, et al. Sustained reduction in severe hypoglycemia in adults with type 1 diabetes complicated by impaired awareness of hypoglycemia: two-year follow-up in the HypoCOMPaSS randomized clinical trial. Diabetes Care. 2018;41(8):1600-1607. doi:10.2337/dc17-2682
12. Tauschmann M, Thabit H, Bally L, et al. Closed-loop insulin delivery in suboptimally controlled type 1 diabetes: a multicentre, 12-week randomised trial [published correction appears in Lancet. 2018 Oct 13;392(10155):1310]. Lancet. 2018;392(10155):1321-1329. doi:10.1016/S0140-6736(18)31947-0
13. Brown SA, Kovatchev BP, Raghinaru D, et al. Six-month randomized, multicenter trial of closed-loop control in type 1 diabetes. N Engl J Med. 2019;381(18):1707-1717. doi:10.1056/NEJMoa1907863
14. Faulds ER, Zappe J, Dungan KM. Real-world implications of hybrid close loop (HCL) insulin delivery system. Endocr Pract. 2019;25(5):477-484. doi:10.4158/EP-2018-0515
15. Rickels MR, Peleckis AJ, Dalton-Bakes C, et al. Continuous glucose monitoring for hypoglycemia avoidance and glucose counterregulation in long-standing type 1 diabetes. J Clin Endocrinol Metab. 2018;103(1):105-114. doi:10.1210/jc.2017-01516
16. Pratley RE, Kanapka LG, Rickels MR, et al. Effect of continuous glucose monitoring on hypoglycemia in older adults with type 1 diabetes: a randomized clinical trial. JAMA. 2020;323(23):2397-2406. doi:10.1001/jama.2020.6928
17. Bekiari E, Kitsios K, Thabit H, et al. Artificial pancreas treatment for outpatients with type 1 diabetes: systematic review and meta-analysis. BMJ. 2018;361:k1310. Published 2018 Apr 18. doi:10.1136/bmj.k1310
18. American Diabetes Association. Addendum. 7. Diabetes technology: standards of medical care in diabetes-2020. Diabetes Care. 2020;43(suppl 1):S77-S88. Diabetes Care. 2020;43(8):1981. doi:10.2337/dc20-ad08c
19. Pease A, Zomer E, Liew D, et al. Cost-effectiveness analysis of a hybrid closed-loop system versus multiple daily injections and capillary glucose testing for adults with type 1 dabetes. Diabetes Technol Ther. 2020;22(11):812-821. doi:10.1089/dia.2020.0064
Nearly half of female surgeons surveyed lost a pregnancy
– according to an article published online July 28 in JAMA Surgery.
The authors, led by Erika L. Rangel, MD, division of general and gastrointestinal surgery, department of surgery, Brigham and Women’s Hospital, Boston, found that after the losses, the women took little or no time off.
Of 692 surgeons surveyed, 347 female surgeons had experienced a pregnancy loss. Of those, 244 had had a miscarriage at less than 10 weeks’ gestation, 92 had had a miscarriage between 10 and 20 weeks’ gestation, and 11 had had a stillbirth (loss at 20 weeks or later).
Most took no time off after miscarriage
After a miscarriage, 225 of 336 women (75%) took no time off work, and after a stillbirth, 5 of 11 (45%) took off 1 week or less, the authors found.
The study addressed an issue that people have talked about anecdotally or on social media, Dr. Rangel told this news organization.
“This was finally an opportunity to do a study of enough magnitude to show that there is a very quantifiable difference in complication rate, use of IVF [in vitro fertilization], and the age at which we have children. These are not just anecdotal stories,” she said.
For the study, a self-administered questionnaire was distributed electronically. Answers were collected from November 2020 to January 2021 through multiple U.S. surgical societies and social media among attending and resident surgeons with children. The control group for the study comprised 158 male surgeons who answered questions regarding their partners’ pregnancies.
Female surgeons had fewer children compared with male surgeons and their female partners (mean [SD],1.8 [0.8], versus 2.3 [1.1]; P < .001) and were more likely to delay having children because of surgical training (450 of 692 [65.0%] versus 69 of 158 [43.7%]; P < .001).
In addition, Dr. Rangel and colleagues found that 57% of female surgeons worked more than 60 hours a week during pregnancy and that 37% took more than six overnight calls.
The data show that female surgeons who operated 12 or more hours per week during the last trimester of pregnancy were at higher risk compared with those who operated fewer hours (odds ratio, 1.57; 95% confidence interval, 1.08-2.26).
“Pregnant surgeons should not be operating more than 12 hours a week when they are in the third trimester,” Dr. Rangel said.
“That is a modifiable risk factor,” she told this news organization. “It’s a very brief period of support – a couple of months of support for a woman who may do 25-30 more years of serving the public with surgical skills.”
She said that training programs should be organized so as to have colleagues cover operating room (OR) shifts to reduce the operating hours for pregnant colleagues. In addition, advanced practice health care professionals should be paid to take up the paperwork and perform non-OR care to reduce the stigma associated with pregnant trainees overburdening other surgical trainees.
‘It’s too big an ask’
Obstetrician-gynecologist Maryam Siddiqui, MD, said in an interview that she was particularly struck by the number of female surgeons who experience involuntary childlessness.
“That’s a big ask for people who want childbearing to be a part of the fulfillment of their life. It’s too big,” said Dr. Siddiqui, a gynecologic surgeon at UChicago Medicine.
She said the amount of detail in the article and the large number of participants were persuasive factors that can support establishing a more humane system than one in which one person at a time has to ask for change.
Pointing to the finding that three-fourths of the women in the study who had had miscarriages didn’t take time off, she said, “That’s not really humane. But they’re afraid to ask or they don’t want to reveal they’re trying [to get pregnant]. Why should you be afraid of building your family?”
The authors also found other adverse outcomes. Female surgeons were more likely to have musculoskeletal disorders compared with female nonsurgeon partners (36.9% versus 18.4%; P < .001), and they were more likely to undergo nonelective cesarean delivery (25.5% versus 15.3%; P = .01) and to experience postpartum depression (11.1% versus 5.7%; P = .04).
Dr. Siddiqui said the conditions that surgeons encounter on their return to work after childbirth are “a perfect storm” for postpartum depression among women who are not accustomed to being reliant on others.
Women often feel coerced into returning to work before they are physically or emotionally ready, then toggle back and forth from night shift to day shift, losing sleep, she said. “We can do better.”
One of the solutions, she said, is to provide better work coverage for the surgeon while she is pregnant and when she returns to work. That includes properly compensating the person covering for the surgeon by giving that person extra pay or additional time off.
“You have to value both people,” she said. “If both people are valued, there’s still collegiality.”
She acknowledged that that kind of compensation may be more readily available at large academic centers.
At UChicago, she said, they are creative with scheduling in training. For women at the height of pregnancy, rotations are less intensive, and trauma rotations are avoided.
Dr. Siddiqui said one of the most important aspects of the article is the authors’ list of two dozen ways, both big and small, to improve conditions.
Adopting such changes will become increasingly important for hiring and retaining female surgeons. “You want to work someplace where you’re respected as a whole person,” she said.
Sarah Blair, MD, a surgical oncologist at University of California, San Diego, stated that the number of miscarriages in particular provides disturbing proof of a problem women in surgery frequently discuss.
For nearly a decade, she led a women-in-surgery committee at UCSD in which they discussed such issues regarding pregnancy and medicine.
She said she hopes these data can help push for change in flexibility in residency so that women can graduate on time and have the families they want.
“There’s a movement away from time-based training to competency-based training, so maybe that will help women,” she said.
‘We have to figure this out’
“We will have to figure this out, because more than half of the people in medical school are women, and there are a lot more women in surgery than when I trained more than 20 years ago. It’s not a problem that’s going away,” she said.
One sign of improvement happened recently, Dr. Rangel said.
As previously reported, according to the American Board of Medical Specialties, as of July 1, 2021, residents and fellows are allowed a minimum 6 weeks away for medical leave or caregiving once during training, without having to use vacation time or sick leave and without having to extend their training.
“That’s huge,” she said. “But we still have a long way to go, because the residency programs still don’t have to have policy that abides that. It merely says you can take 6 weeks off and take your boards. It doesn’t say that the residency program has to allow you to take 6 weeks off.”
The authors noted that the United States and Papua New Guinea are the only countries in the world without federally mandated paid parental leave.
“Most U.S. female surgeons rely on their employer for this benefit, but only half of top-ranked medical schools offer paid leave, and 33%-65% of U.S. surgical training programs lack clear maternity leave policies,” she said.
Funding for the study was provided by the department of surgery at Brigham and Women’s Hospital. The study authors, Dr. Blair, and Dr. Siddiqui have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
– according to an article published online July 28 in JAMA Surgery.
The authors, led by Erika L. Rangel, MD, division of general and gastrointestinal surgery, department of surgery, Brigham and Women’s Hospital, Boston, found that after the losses, the women took little or no time off.
Of 692 surgeons surveyed, 347 female surgeons had experienced a pregnancy loss. Of those, 244 had had a miscarriage at less than 10 weeks’ gestation, 92 had had a miscarriage between 10 and 20 weeks’ gestation, and 11 had had a stillbirth (loss at 20 weeks or later).
Most took no time off after miscarriage
After a miscarriage, 225 of 336 women (75%) took no time off work, and after a stillbirth, 5 of 11 (45%) took off 1 week or less, the authors found.
The study addressed an issue that people have talked about anecdotally or on social media, Dr. Rangel told this news organization.
“This was finally an opportunity to do a study of enough magnitude to show that there is a very quantifiable difference in complication rate, use of IVF [in vitro fertilization], and the age at which we have children. These are not just anecdotal stories,” she said.
For the study, a self-administered questionnaire was distributed electronically. Answers were collected from November 2020 to January 2021 through multiple U.S. surgical societies and social media among attending and resident surgeons with children. The control group for the study comprised 158 male surgeons who answered questions regarding their partners’ pregnancies.
Female surgeons had fewer children compared with male surgeons and their female partners (mean [SD],1.8 [0.8], versus 2.3 [1.1]; P < .001) and were more likely to delay having children because of surgical training (450 of 692 [65.0%] versus 69 of 158 [43.7%]; P < .001).
In addition, Dr. Rangel and colleagues found that 57% of female surgeons worked more than 60 hours a week during pregnancy and that 37% took more than six overnight calls.
The data show that female surgeons who operated 12 or more hours per week during the last trimester of pregnancy were at higher risk compared with those who operated fewer hours (odds ratio, 1.57; 95% confidence interval, 1.08-2.26).
“Pregnant surgeons should not be operating more than 12 hours a week when they are in the third trimester,” Dr. Rangel said.
“That is a modifiable risk factor,” she told this news organization. “It’s a very brief period of support – a couple of months of support for a woman who may do 25-30 more years of serving the public with surgical skills.”
She said that training programs should be organized so as to have colleagues cover operating room (OR) shifts to reduce the operating hours for pregnant colleagues. In addition, advanced practice health care professionals should be paid to take up the paperwork and perform non-OR care to reduce the stigma associated with pregnant trainees overburdening other surgical trainees.
‘It’s too big an ask’
Obstetrician-gynecologist Maryam Siddiqui, MD, said in an interview that she was particularly struck by the number of female surgeons who experience involuntary childlessness.
“That’s a big ask for people who want childbearing to be a part of the fulfillment of their life. It’s too big,” said Dr. Siddiqui, a gynecologic surgeon at UChicago Medicine.
She said the amount of detail in the article and the large number of participants were persuasive factors that can support establishing a more humane system than one in which one person at a time has to ask for change.
Pointing to the finding that three-fourths of the women in the study who had had miscarriages didn’t take time off, she said, “That’s not really humane. But they’re afraid to ask or they don’t want to reveal they’re trying [to get pregnant]. Why should you be afraid of building your family?”
The authors also found other adverse outcomes. Female surgeons were more likely to have musculoskeletal disorders compared with female nonsurgeon partners (36.9% versus 18.4%; P < .001), and they were more likely to undergo nonelective cesarean delivery (25.5% versus 15.3%; P = .01) and to experience postpartum depression (11.1% versus 5.7%; P = .04).
Dr. Siddiqui said the conditions that surgeons encounter on their return to work after childbirth are “a perfect storm” for postpartum depression among women who are not accustomed to being reliant on others.
Women often feel coerced into returning to work before they are physically or emotionally ready, then toggle back and forth from night shift to day shift, losing sleep, she said. “We can do better.”
One of the solutions, she said, is to provide better work coverage for the surgeon while she is pregnant and when she returns to work. That includes properly compensating the person covering for the surgeon by giving that person extra pay or additional time off.
“You have to value both people,” she said. “If both people are valued, there’s still collegiality.”
She acknowledged that that kind of compensation may be more readily available at large academic centers.
At UChicago, she said, they are creative with scheduling in training. For women at the height of pregnancy, rotations are less intensive, and trauma rotations are avoided.
Dr. Siddiqui said one of the most important aspects of the article is the authors’ list of two dozen ways, both big and small, to improve conditions.
Adopting such changes will become increasingly important for hiring and retaining female surgeons. “You want to work someplace where you’re respected as a whole person,” she said.
Sarah Blair, MD, a surgical oncologist at University of California, San Diego, stated that the number of miscarriages in particular provides disturbing proof of a problem women in surgery frequently discuss.
For nearly a decade, she led a women-in-surgery committee at UCSD in which they discussed such issues regarding pregnancy and medicine.
She said she hopes these data can help push for change in flexibility in residency so that women can graduate on time and have the families they want.
“There’s a movement away from time-based training to competency-based training, so maybe that will help women,” she said.
‘We have to figure this out’
“We will have to figure this out, because more than half of the people in medical school are women, and there are a lot more women in surgery than when I trained more than 20 years ago. It’s not a problem that’s going away,” she said.
One sign of improvement happened recently, Dr. Rangel said.
As previously reported, according to the American Board of Medical Specialties, as of July 1, 2021, residents and fellows are allowed a minimum 6 weeks away for medical leave or caregiving once during training, without having to use vacation time or sick leave and without having to extend their training.
“That’s huge,” she said. “But we still have a long way to go, because the residency programs still don’t have to have policy that abides that. It merely says you can take 6 weeks off and take your boards. It doesn’t say that the residency program has to allow you to take 6 weeks off.”
The authors noted that the United States and Papua New Guinea are the only countries in the world without federally mandated paid parental leave.
“Most U.S. female surgeons rely on their employer for this benefit, but only half of top-ranked medical schools offer paid leave, and 33%-65% of U.S. surgical training programs lack clear maternity leave policies,” she said.
Funding for the study was provided by the department of surgery at Brigham and Women’s Hospital. The study authors, Dr. Blair, and Dr. Siddiqui have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
– according to an article published online July 28 in JAMA Surgery.
The authors, led by Erika L. Rangel, MD, division of general and gastrointestinal surgery, department of surgery, Brigham and Women’s Hospital, Boston, found that after the losses, the women took little or no time off.
Of 692 surgeons surveyed, 347 female surgeons had experienced a pregnancy loss. Of those, 244 had had a miscarriage at less than 10 weeks’ gestation, 92 had had a miscarriage between 10 and 20 weeks’ gestation, and 11 had had a stillbirth (loss at 20 weeks or later).
Most took no time off after miscarriage
After a miscarriage, 225 of 336 women (75%) took no time off work, and after a stillbirth, 5 of 11 (45%) took off 1 week or less, the authors found.
The study addressed an issue that people have talked about anecdotally or on social media, Dr. Rangel told this news organization.
“This was finally an opportunity to do a study of enough magnitude to show that there is a very quantifiable difference in complication rate, use of IVF [in vitro fertilization], and the age at which we have children. These are not just anecdotal stories,” she said.
For the study, a self-administered questionnaire was distributed electronically. Answers were collected from November 2020 to January 2021 through multiple U.S. surgical societies and social media among attending and resident surgeons with children. The control group for the study comprised 158 male surgeons who answered questions regarding their partners’ pregnancies.
Female surgeons had fewer children compared with male surgeons and their female partners (mean [SD],1.8 [0.8], versus 2.3 [1.1]; P < .001) and were more likely to delay having children because of surgical training (450 of 692 [65.0%] versus 69 of 158 [43.7%]; P < .001).
In addition, Dr. Rangel and colleagues found that 57% of female surgeons worked more than 60 hours a week during pregnancy and that 37% took more than six overnight calls.
The data show that female surgeons who operated 12 or more hours per week during the last trimester of pregnancy were at higher risk compared with those who operated fewer hours (odds ratio, 1.57; 95% confidence interval, 1.08-2.26).
“Pregnant surgeons should not be operating more than 12 hours a week when they are in the third trimester,” Dr. Rangel said.
“That is a modifiable risk factor,” she told this news organization. “It’s a very brief period of support – a couple of months of support for a woman who may do 25-30 more years of serving the public with surgical skills.”
She said that training programs should be organized so as to have colleagues cover operating room (OR) shifts to reduce the operating hours for pregnant colleagues. In addition, advanced practice health care professionals should be paid to take up the paperwork and perform non-OR care to reduce the stigma associated with pregnant trainees overburdening other surgical trainees.
‘It’s too big an ask’
Obstetrician-gynecologist Maryam Siddiqui, MD, said in an interview that she was particularly struck by the number of female surgeons who experience involuntary childlessness.
“That’s a big ask for people who want childbearing to be a part of the fulfillment of their life. It’s too big,” said Dr. Siddiqui, a gynecologic surgeon at UChicago Medicine.
She said the amount of detail in the article and the large number of participants were persuasive factors that can support establishing a more humane system than one in which one person at a time has to ask for change.
Pointing to the finding that three-fourths of the women in the study who had had miscarriages didn’t take time off, she said, “That’s not really humane. But they’re afraid to ask or they don’t want to reveal they’re trying [to get pregnant]. Why should you be afraid of building your family?”
The authors also found other adverse outcomes. Female surgeons were more likely to have musculoskeletal disorders compared with female nonsurgeon partners (36.9% versus 18.4%; P < .001), and they were more likely to undergo nonelective cesarean delivery (25.5% versus 15.3%; P = .01) and to experience postpartum depression (11.1% versus 5.7%; P = .04).
Dr. Siddiqui said the conditions that surgeons encounter on their return to work after childbirth are “a perfect storm” for postpartum depression among women who are not accustomed to being reliant on others.
Women often feel coerced into returning to work before they are physically or emotionally ready, then toggle back and forth from night shift to day shift, losing sleep, she said. “We can do better.”
One of the solutions, she said, is to provide better work coverage for the surgeon while she is pregnant and when she returns to work. That includes properly compensating the person covering for the surgeon by giving that person extra pay or additional time off.
“You have to value both people,” she said. “If both people are valued, there’s still collegiality.”
She acknowledged that that kind of compensation may be more readily available at large academic centers.
At UChicago, she said, they are creative with scheduling in training. For women at the height of pregnancy, rotations are less intensive, and trauma rotations are avoided.
Dr. Siddiqui said one of the most important aspects of the article is the authors’ list of two dozen ways, both big and small, to improve conditions.
Adopting such changes will become increasingly important for hiring and retaining female surgeons. “You want to work someplace where you’re respected as a whole person,” she said.
Sarah Blair, MD, a surgical oncologist at University of California, San Diego, stated that the number of miscarriages in particular provides disturbing proof of a problem women in surgery frequently discuss.
For nearly a decade, she led a women-in-surgery committee at UCSD in which they discussed such issues regarding pregnancy and medicine.
She said she hopes these data can help push for change in flexibility in residency so that women can graduate on time and have the families they want.
“There’s a movement away from time-based training to competency-based training, so maybe that will help women,” she said.
‘We have to figure this out’
“We will have to figure this out, because more than half of the people in medical school are women, and there are a lot more women in surgery than when I trained more than 20 years ago. It’s not a problem that’s going away,” she said.
One sign of improvement happened recently, Dr. Rangel said.
As previously reported, according to the American Board of Medical Specialties, as of July 1, 2021, residents and fellows are allowed a minimum 6 weeks away for medical leave or caregiving once during training, without having to use vacation time or sick leave and without having to extend their training.
“That’s huge,” she said. “But we still have a long way to go, because the residency programs still don’t have to have policy that abides that. It merely says you can take 6 weeks off and take your boards. It doesn’t say that the residency program has to allow you to take 6 weeks off.”
The authors noted that the United States and Papua New Guinea are the only countries in the world without federally mandated paid parental leave.
“Most U.S. female surgeons rely on their employer for this benefit, but only half of top-ranked medical schools offer paid leave, and 33%-65% of U.S. surgical training programs lack clear maternity leave policies,” she said.
Funding for the study was provided by the department of surgery at Brigham and Women’s Hospital. The study authors, Dr. Blair, and Dr. Siddiqui have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
COVID-19, hearings on Jan. 6 attack reignite interest in PTSD
After Sept. 11, 2001, and the subsequent long war in Iraq and Afghanistan, both mental health providers and the general public focused on posttraumatic stress disorder (PTSD). However, after almost 20 years of war and the COVID-19 epidemic, attention waned away from military service members and PTSD.
COVID-19–related PTSD and the hearings on the Jan. 6 attack on the Capitol have reignited interest in PTSD diagnosis and treatment. Testimony from police officers at the House select committee hearing about their experiences during the assault and PTSD was harrowing. One of the police officers had also served in Iraq, perhaps leading to “layered PTSD” – symptoms from war abroad and at home.
Thus, I thought a brief review of updates about diagnosis and treatment would be useful. Note: These are my opinions based on my extensive experience and do not represent the official opinion of my employer (MedStar Health).
PTSD was first classified as a disorder in 1980, based mainly on the experiences of military service members in Vietnam, as well as sexual assault victims and disaster survivors. Readers may look elsewhere for a fuller history of the disorder.
However, in brief, we have evolved from strict reliance on a variety of symptoms in the DSM (Diagnostic and Statistical Manual of Mental Disorders) to a more global determination of the experience of trauma and related symptoms of distress. We still rely for diagnosis on trauma-related anxiety and depression symptoms, such as nightmare, flashbacks, numbness, and disassociation.
Treatment has evolved. Patients may benefit from treatment even if they do not meet all the PTSD criteria. As many of my colleagues who treat patients have said, “if it smells like PTSD, treat it like PTSD.”
What is the most effective treatment? The literature declares that evidence-based treatments include two selective serotonin reuptake inhibitors (Zoloft and Paxil) and several psychotherapies. The psychotherapies include cognitive-behavioral therapies, exposure therapy, and EMDR (eye movement desensitization reprocessing).
The problem is that many patients cannot tolerate these therapies. SSRIs do have side effects, the most distressing being sexual dysfunction. Many service members do not enter the psychotherapies, or they drop out of trials, because they cannot tolerate the reimagining of their trauma.
I now counsel patients about the “three buckets” of treatment. The first bucket is medication, which as a psychiatrist is what I focus on. The second bucket is psychotherapy as discussed above. The third bucket is “everything else.”
“Everything else” includes a variety of methods the patients can use to reduce symptoms of anxiety, depression, and PTSD symptoms: exercising; deep breathing through the nose; doing yoga; doing meditation; playing or working with animals; gardening; and engaging in other activities that “self sooth.” I also recommend always doing “small acts of kindness” for others. I myself contribute to food banks and bring cookies or watermelons to the staff at my hospital.
Why is this approach useful? A menu of options gives control back to the patient. It provides activities that can reduce anxiety. Thinking about caring for others helps patients get out of their own “swamp of distress.”
We do live in very difficult times. We’re coping with COVID-19 Delta variant, attacks on the Capitol, and gun violence. I have not yet mentioned climate change, which is extremely frightening to many of us. So all providers need to be aware of all the strategies at our disposal to treat anxiety, depression, and PTSD.
Dr. Ritchie is chair of psychiatry at Medstar Washington (D.C.) Hospital Center. She has no conflicts of interest.
After Sept. 11, 2001, and the subsequent long war in Iraq and Afghanistan, both mental health providers and the general public focused on posttraumatic stress disorder (PTSD). However, after almost 20 years of war and the COVID-19 epidemic, attention waned away from military service members and PTSD.
COVID-19–related PTSD and the hearings on the Jan. 6 attack on the Capitol have reignited interest in PTSD diagnosis and treatment. Testimony from police officers at the House select committee hearing about their experiences during the assault and PTSD was harrowing. One of the police officers had also served in Iraq, perhaps leading to “layered PTSD” – symptoms from war abroad and at home.
Thus, I thought a brief review of updates about diagnosis and treatment would be useful. Note: These are my opinions based on my extensive experience and do not represent the official opinion of my employer (MedStar Health).
PTSD was first classified as a disorder in 1980, based mainly on the experiences of military service members in Vietnam, as well as sexual assault victims and disaster survivors. Readers may look elsewhere for a fuller history of the disorder.
However, in brief, we have evolved from strict reliance on a variety of symptoms in the DSM (Diagnostic and Statistical Manual of Mental Disorders) to a more global determination of the experience of trauma and related symptoms of distress. We still rely for diagnosis on trauma-related anxiety and depression symptoms, such as nightmare, flashbacks, numbness, and disassociation.
Treatment has evolved. Patients may benefit from treatment even if they do not meet all the PTSD criteria. As many of my colleagues who treat patients have said, “if it smells like PTSD, treat it like PTSD.”
What is the most effective treatment? The literature declares that evidence-based treatments include two selective serotonin reuptake inhibitors (Zoloft and Paxil) and several psychotherapies. The psychotherapies include cognitive-behavioral therapies, exposure therapy, and EMDR (eye movement desensitization reprocessing).
The problem is that many patients cannot tolerate these therapies. SSRIs do have side effects, the most distressing being sexual dysfunction. Many service members do not enter the psychotherapies, or they drop out of trials, because they cannot tolerate the reimagining of their trauma.
I now counsel patients about the “three buckets” of treatment. The first bucket is medication, which as a psychiatrist is what I focus on. The second bucket is psychotherapy as discussed above. The third bucket is “everything else.”
“Everything else” includes a variety of methods the patients can use to reduce symptoms of anxiety, depression, and PTSD symptoms: exercising; deep breathing through the nose; doing yoga; doing meditation; playing or working with animals; gardening; and engaging in other activities that “self sooth.” I also recommend always doing “small acts of kindness” for others. I myself contribute to food banks and bring cookies or watermelons to the staff at my hospital.
Why is this approach useful? A menu of options gives control back to the patient. It provides activities that can reduce anxiety. Thinking about caring for others helps patients get out of their own “swamp of distress.”
We do live in very difficult times. We’re coping with COVID-19 Delta variant, attacks on the Capitol, and gun violence. I have not yet mentioned climate change, which is extremely frightening to many of us. So all providers need to be aware of all the strategies at our disposal to treat anxiety, depression, and PTSD.
Dr. Ritchie is chair of psychiatry at Medstar Washington (D.C.) Hospital Center. She has no conflicts of interest.
After Sept. 11, 2001, and the subsequent long war in Iraq and Afghanistan, both mental health providers and the general public focused on posttraumatic stress disorder (PTSD). However, after almost 20 years of war and the COVID-19 epidemic, attention waned away from military service members and PTSD.
COVID-19–related PTSD and the hearings on the Jan. 6 attack on the Capitol have reignited interest in PTSD diagnosis and treatment. Testimony from police officers at the House select committee hearing about their experiences during the assault and PTSD was harrowing. One of the police officers had also served in Iraq, perhaps leading to “layered PTSD” – symptoms from war abroad and at home.
Thus, I thought a brief review of updates about diagnosis and treatment would be useful. Note: These are my opinions based on my extensive experience and do not represent the official opinion of my employer (MedStar Health).
PTSD was first classified as a disorder in 1980, based mainly on the experiences of military service members in Vietnam, as well as sexual assault victims and disaster survivors. Readers may look elsewhere for a fuller history of the disorder.
However, in brief, we have evolved from strict reliance on a variety of symptoms in the DSM (Diagnostic and Statistical Manual of Mental Disorders) to a more global determination of the experience of trauma and related symptoms of distress. We still rely for diagnosis on trauma-related anxiety and depression symptoms, such as nightmare, flashbacks, numbness, and disassociation.
Treatment has evolved. Patients may benefit from treatment even if they do not meet all the PTSD criteria. As many of my colleagues who treat patients have said, “if it smells like PTSD, treat it like PTSD.”
What is the most effective treatment? The literature declares that evidence-based treatments include two selective serotonin reuptake inhibitors (Zoloft and Paxil) and several psychotherapies. The psychotherapies include cognitive-behavioral therapies, exposure therapy, and EMDR (eye movement desensitization reprocessing).
The problem is that many patients cannot tolerate these therapies. SSRIs do have side effects, the most distressing being sexual dysfunction. Many service members do not enter the psychotherapies, or they drop out of trials, because they cannot tolerate the reimagining of their trauma.
I now counsel patients about the “three buckets” of treatment. The first bucket is medication, which as a psychiatrist is what I focus on. The second bucket is psychotherapy as discussed above. The third bucket is “everything else.”
“Everything else” includes a variety of methods the patients can use to reduce symptoms of anxiety, depression, and PTSD symptoms: exercising; deep breathing through the nose; doing yoga; doing meditation; playing or working with animals; gardening; and engaging in other activities that “self sooth.” I also recommend always doing “small acts of kindness” for others. I myself contribute to food banks and bring cookies or watermelons to the staff at my hospital.
Why is this approach useful? A menu of options gives control back to the patient. It provides activities that can reduce anxiety. Thinking about caring for others helps patients get out of their own “swamp of distress.”
We do live in very difficult times. We’re coping with COVID-19 Delta variant, attacks on the Capitol, and gun violence. I have not yet mentioned climate change, which is extremely frightening to many of us. So all providers need to be aware of all the strategies at our disposal to treat anxiety, depression, and PTSD.
Dr. Ritchie is chair of psychiatry at Medstar Washington (D.C.) Hospital Center. She has no conflicts of interest.
New investigational helmet device shrinks glioblastoma
This is the first time that the wearable Oncomagnetic device was tried with a patient.
The patient had end-stage recurrent glioblastoma and had undergone all standard therapy options. He wore the device for 5 weeks but died from an unrelated injury, so the treatment period was cut short.
A brain scan showed a 31% reduction of contrast-enhanced tumor volume, and an autopsy of his brain confirmed the rapid response to the treatment.
The case study was published online on July 22, 2021, in Frontiers in Oncology.
“I believe that there is a great potential with this device,” said study author David S. Baskin, MD, director of the Kenneth R. Peak Center for Brain and Pituitary Tumor Treatment in the department of neurosurgery at Houston Methodist Hospital. “This is a very exciting time.”
The team is now treating several patients with glioblastoma under compassionate use.
In an independent comment, Adilia Hormigo, MD, PhD, director of the neuro-oncology program at the Tisch Cancer Institute, Mount Sinai Health System, New York, noted that a clinical trial is needed to evaluate the device. “But this is an interesting idea, and we have to be open-minded in treating this fatal disease.”
Oscillating magnetic fields
The Oncomagnetic device consists of three oncoscillators that are attached to the outside of a helmet and are connected to a microprocessor-based electronic controller powered by a rechargeable battery.
It consists of a series of rotating magnets that produce oscillating magnetic fields that cover the entire brain, including the upper part of the brain stem. The device induces rapid apoptosis of glioblastoma cells, Dr. Baskin explained. Its mechanism of action involves disruption of the electron transport in the mitochondrial respiratory chain, causing an elevation of reactive oxygen species and caspase-dependent cancer cell death.
Dr. Baskin emphasized that the new Oncomagnetic device is very different from the Optune device (Novocare), which is already approved by the Food and Drug Administration and has been shown to increase survival among patients with glioblastoma. Optune uses tumor-treating fields (TTFs), which are electromagnetic waves that are delivered via an electric field generator through four transducer arrays that are placed on a shaved scalp. Preclinical studies indicated that the TTFs disrupt cell division by disrupting several steps in the mitotic process that are crucial for cell division.
Both of these devices “are using a type of external maneuver” rather than invasive intracranial approaches, said Dr. Hormingo. The experimental Oncomagnetic device may have an advantage in that it needs to be worn by the patient for fewer hours, she commented. A better understanding of the physics and underlying mechanism is needed, however. Clinical trials are an essential next step.
Most common brain cancer in adults
Glioblastoma is the most common malignant tumor of the brain in adults. Outcomes continue to be dismal. In more than 40 years, median survival has only modestly improved.
“We haven’t gotten very far with glioblastoma despite millions of dollars in research,” Dr. Baskin said. “With treatment, survival is about 15 months, and those are not very good months.”
Out of the box
Standard treatments for glioblastoma include surgery, radiotherapy, and chemotherapy, and many patients cannot tolerate some of these, Dr. Baskin noted. Hence, there is a great need for a different therapeutic approach that yields better outcomes with lower toxicity.
“We didn’t want to develop another chemotherapeutic agent that would help you live another 2 months,” he said in an interview. “We were trying to think out of the box.
“If you want to do something that will really make a difference in an aggressive tumor like glioblastoma, you have to attack something so basic that the tumor can’t evade it,” he said. “For example, with temozolomide, if it is unmethylated, the tumor can repair the DNA damage from the chemotherapy. Even if you’re sensitive to begin with, over time, the tumor will eventually become resistant.”
The new device stems from work by Dr. Baskin and colleagues on mitochondria, which he describes as the powerhouse of the cell. “Mitochondrial DNA can’t repair itself, so if you damage the mitochondria, you will damage the cell, and theoretically, it cannot repair itself,” he said.
In preclinical models, the oscillating magnetic fields generated by the new device were shown to kill patient-derived glioblastoma cells in cell culture without having cytotoxic effects on cortical neurons and normal human astrocytes. Animal studies also showed that it was effective and nontoxic, explained Dr. Baskin.
However, getting the device to human clinical trials has been slow going. “We wanted to start an early-phase trial for an investigational device, but the FDA is overwhelmed with COVID-related applications,” he said. “That has taken priority, and we understand that. So we were able to evaluate it on a patient through compassionate use via the [Food and Drug Administration]–approved Expanded Access Program.”
Exciting possibilities
The patient was a 53-year-old man who had undergone radiotherapy and chemotherapy, and the tumor was progressing. Imaging revealed the presence of leptomeningeal disease, which is associated with a poor outcome and a median survival of 3.5-3.9 months.
The patient was fitted with the helmet device and wore it under supervision for the first 3 days of treatment, during which time the strength of the oscillating magnetic fields was escalated. After this initial supervised phase, the treatment continued at home without supervision, using the same regimen as on the third day.
Treatment was first administered for 2 hours while under supervision and was then gradually increased to a maximum of 6 hours per day. The patient was evaluated clinically on days 7, 16, 30, and 44 after initiation of treatment. No serious adverse events were reported during treatment. The patient’s wife reported subjective improvement in speech and cognitive function.
Dr. Baskin noted that the patient had been experiencing falls for the past year and a half before treatment was initiated. “And then he tripped and fell and sustained a head injury that he subsequently died from,” he said.
Autopsy results confirmed the rapid response to treatment, and tumor shrinkage appeared to correlate with the treatment dose.
“Our results in the laboratory and with this patient open a new world of noninvasive and nontoxic therapy for brain cancer, with many exciting possibilities for the future,” Dr. Baskin commented.
He said his team has experimented with this approach with other tumor types in the laboratory, including triple-negative breast cancer and lung cancer. “We’ve only tried it in a culture so far, but it seems to melt the cancer cells,” he said.
The work was supported by a grant from the Translational Research Initiative of the Houston Methodist Research Institute and several foundations. Dr. Baskin and two coauthors are listed as inventors on a U.S. patent application filed by Houston Methodist Hospital for the device used in this report.
A version of this article first appeared on Medscape.com.
This is the first time that the wearable Oncomagnetic device was tried with a patient.
The patient had end-stage recurrent glioblastoma and had undergone all standard therapy options. He wore the device for 5 weeks but died from an unrelated injury, so the treatment period was cut short.
A brain scan showed a 31% reduction of contrast-enhanced tumor volume, and an autopsy of his brain confirmed the rapid response to the treatment.
The case study was published online on July 22, 2021, in Frontiers in Oncology.
“I believe that there is a great potential with this device,” said study author David S. Baskin, MD, director of the Kenneth R. Peak Center for Brain and Pituitary Tumor Treatment in the department of neurosurgery at Houston Methodist Hospital. “This is a very exciting time.”
The team is now treating several patients with glioblastoma under compassionate use.
In an independent comment, Adilia Hormigo, MD, PhD, director of the neuro-oncology program at the Tisch Cancer Institute, Mount Sinai Health System, New York, noted that a clinical trial is needed to evaluate the device. “But this is an interesting idea, and we have to be open-minded in treating this fatal disease.”
Oscillating magnetic fields
The Oncomagnetic device consists of three oncoscillators that are attached to the outside of a helmet and are connected to a microprocessor-based electronic controller powered by a rechargeable battery.
It consists of a series of rotating magnets that produce oscillating magnetic fields that cover the entire brain, including the upper part of the brain stem. The device induces rapid apoptosis of glioblastoma cells, Dr. Baskin explained. Its mechanism of action involves disruption of the electron transport in the mitochondrial respiratory chain, causing an elevation of reactive oxygen species and caspase-dependent cancer cell death.
Dr. Baskin emphasized that the new Oncomagnetic device is very different from the Optune device (Novocare), which is already approved by the Food and Drug Administration and has been shown to increase survival among patients with glioblastoma. Optune uses tumor-treating fields (TTFs), which are electromagnetic waves that are delivered via an electric field generator through four transducer arrays that are placed on a shaved scalp. Preclinical studies indicated that the TTFs disrupt cell division by disrupting several steps in the mitotic process that are crucial for cell division.
Both of these devices “are using a type of external maneuver” rather than invasive intracranial approaches, said Dr. Hormingo. The experimental Oncomagnetic device may have an advantage in that it needs to be worn by the patient for fewer hours, she commented. A better understanding of the physics and underlying mechanism is needed, however. Clinical trials are an essential next step.
Most common brain cancer in adults
Glioblastoma is the most common malignant tumor of the brain in adults. Outcomes continue to be dismal. In more than 40 years, median survival has only modestly improved.
“We haven’t gotten very far with glioblastoma despite millions of dollars in research,” Dr. Baskin said. “With treatment, survival is about 15 months, and those are not very good months.”
Out of the box
Standard treatments for glioblastoma include surgery, radiotherapy, and chemotherapy, and many patients cannot tolerate some of these, Dr. Baskin noted. Hence, there is a great need for a different therapeutic approach that yields better outcomes with lower toxicity.
“We didn’t want to develop another chemotherapeutic agent that would help you live another 2 months,” he said in an interview. “We were trying to think out of the box.
“If you want to do something that will really make a difference in an aggressive tumor like glioblastoma, you have to attack something so basic that the tumor can’t evade it,” he said. “For example, with temozolomide, if it is unmethylated, the tumor can repair the DNA damage from the chemotherapy. Even if you’re sensitive to begin with, over time, the tumor will eventually become resistant.”
The new device stems from work by Dr. Baskin and colleagues on mitochondria, which he describes as the powerhouse of the cell. “Mitochondrial DNA can’t repair itself, so if you damage the mitochondria, you will damage the cell, and theoretically, it cannot repair itself,” he said.
In preclinical models, the oscillating magnetic fields generated by the new device were shown to kill patient-derived glioblastoma cells in cell culture without having cytotoxic effects on cortical neurons and normal human astrocytes. Animal studies also showed that it was effective and nontoxic, explained Dr. Baskin.
However, getting the device to human clinical trials has been slow going. “We wanted to start an early-phase trial for an investigational device, but the FDA is overwhelmed with COVID-related applications,” he said. “That has taken priority, and we understand that. So we were able to evaluate it on a patient through compassionate use via the [Food and Drug Administration]–approved Expanded Access Program.”
Exciting possibilities
The patient was a 53-year-old man who had undergone radiotherapy and chemotherapy, and the tumor was progressing. Imaging revealed the presence of leptomeningeal disease, which is associated with a poor outcome and a median survival of 3.5-3.9 months.
The patient was fitted with the helmet device and wore it under supervision for the first 3 days of treatment, during which time the strength of the oscillating magnetic fields was escalated. After this initial supervised phase, the treatment continued at home without supervision, using the same regimen as on the third day.
Treatment was first administered for 2 hours while under supervision and was then gradually increased to a maximum of 6 hours per day. The patient was evaluated clinically on days 7, 16, 30, and 44 after initiation of treatment. No serious adverse events were reported during treatment. The patient’s wife reported subjective improvement in speech and cognitive function.
Dr. Baskin noted that the patient had been experiencing falls for the past year and a half before treatment was initiated. “And then he tripped and fell and sustained a head injury that he subsequently died from,” he said.
Autopsy results confirmed the rapid response to treatment, and tumor shrinkage appeared to correlate with the treatment dose.
“Our results in the laboratory and with this patient open a new world of noninvasive and nontoxic therapy for brain cancer, with many exciting possibilities for the future,” Dr. Baskin commented.
He said his team has experimented with this approach with other tumor types in the laboratory, including triple-negative breast cancer and lung cancer. “We’ve only tried it in a culture so far, but it seems to melt the cancer cells,” he said.
The work was supported by a grant from the Translational Research Initiative of the Houston Methodist Research Institute and several foundations. Dr. Baskin and two coauthors are listed as inventors on a U.S. patent application filed by Houston Methodist Hospital for the device used in this report.
A version of this article first appeared on Medscape.com.
This is the first time that the wearable Oncomagnetic device was tried with a patient.
The patient had end-stage recurrent glioblastoma and had undergone all standard therapy options. He wore the device for 5 weeks but died from an unrelated injury, so the treatment period was cut short.
A brain scan showed a 31% reduction of contrast-enhanced tumor volume, and an autopsy of his brain confirmed the rapid response to the treatment.
The case study was published online on July 22, 2021, in Frontiers in Oncology.
“I believe that there is a great potential with this device,” said study author David S. Baskin, MD, director of the Kenneth R. Peak Center for Brain and Pituitary Tumor Treatment in the department of neurosurgery at Houston Methodist Hospital. “This is a very exciting time.”
The team is now treating several patients with glioblastoma under compassionate use.
In an independent comment, Adilia Hormigo, MD, PhD, director of the neuro-oncology program at the Tisch Cancer Institute, Mount Sinai Health System, New York, noted that a clinical trial is needed to evaluate the device. “But this is an interesting idea, and we have to be open-minded in treating this fatal disease.”
Oscillating magnetic fields
The Oncomagnetic device consists of three oncoscillators that are attached to the outside of a helmet and are connected to a microprocessor-based electronic controller powered by a rechargeable battery.
It consists of a series of rotating magnets that produce oscillating magnetic fields that cover the entire brain, including the upper part of the brain stem. The device induces rapid apoptosis of glioblastoma cells, Dr. Baskin explained. Its mechanism of action involves disruption of the electron transport in the mitochondrial respiratory chain, causing an elevation of reactive oxygen species and caspase-dependent cancer cell death.
Dr. Baskin emphasized that the new Oncomagnetic device is very different from the Optune device (Novocare), which is already approved by the Food and Drug Administration and has been shown to increase survival among patients with glioblastoma. Optune uses tumor-treating fields (TTFs), which are electromagnetic waves that are delivered via an electric field generator through four transducer arrays that are placed on a shaved scalp. Preclinical studies indicated that the TTFs disrupt cell division by disrupting several steps in the mitotic process that are crucial for cell division.
Both of these devices “are using a type of external maneuver” rather than invasive intracranial approaches, said Dr. Hormingo. The experimental Oncomagnetic device may have an advantage in that it needs to be worn by the patient for fewer hours, she commented. A better understanding of the physics and underlying mechanism is needed, however. Clinical trials are an essential next step.
Most common brain cancer in adults
Glioblastoma is the most common malignant tumor of the brain in adults. Outcomes continue to be dismal. In more than 40 years, median survival has only modestly improved.
“We haven’t gotten very far with glioblastoma despite millions of dollars in research,” Dr. Baskin said. “With treatment, survival is about 15 months, and those are not very good months.”
Out of the box
Standard treatments for glioblastoma include surgery, radiotherapy, and chemotherapy, and many patients cannot tolerate some of these, Dr. Baskin noted. Hence, there is a great need for a different therapeutic approach that yields better outcomes with lower toxicity.
“We didn’t want to develop another chemotherapeutic agent that would help you live another 2 months,” he said in an interview. “We were trying to think out of the box.
“If you want to do something that will really make a difference in an aggressive tumor like glioblastoma, you have to attack something so basic that the tumor can’t evade it,” he said. “For example, with temozolomide, if it is unmethylated, the tumor can repair the DNA damage from the chemotherapy. Even if you’re sensitive to begin with, over time, the tumor will eventually become resistant.”
The new device stems from work by Dr. Baskin and colleagues on mitochondria, which he describes as the powerhouse of the cell. “Mitochondrial DNA can’t repair itself, so if you damage the mitochondria, you will damage the cell, and theoretically, it cannot repair itself,” he said.
In preclinical models, the oscillating magnetic fields generated by the new device were shown to kill patient-derived glioblastoma cells in cell culture without having cytotoxic effects on cortical neurons and normal human astrocytes. Animal studies also showed that it was effective and nontoxic, explained Dr. Baskin.
However, getting the device to human clinical trials has been slow going. “We wanted to start an early-phase trial for an investigational device, but the FDA is overwhelmed with COVID-related applications,” he said. “That has taken priority, and we understand that. So we were able to evaluate it on a patient through compassionate use via the [Food and Drug Administration]–approved Expanded Access Program.”
Exciting possibilities
The patient was a 53-year-old man who had undergone radiotherapy and chemotherapy, and the tumor was progressing. Imaging revealed the presence of leptomeningeal disease, which is associated with a poor outcome and a median survival of 3.5-3.9 months.
The patient was fitted with the helmet device and wore it under supervision for the first 3 days of treatment, during which time the strength of the oscillating magnetic fields was escalated. After this initial supervised phase, the treatment continued at home without supervision, using the same regimen as on the third day.
Treatment was first administered for 2 hours while under supervision and was then gradually increased to a maximum of 6 hours per day. The patient was evaluated clinically on days 7, 16, 30, and 44 after initiation of treatment. No serious adverse events were reported during treatment. The patient’s wife reported subjective improvement in speech and cognitive function.
Dr. Baskin noted that the patient had been experiencing falls for the past year and a half before treatment was initiated. “And then he tripped and fell and sustained a head injury that he subsequently died from,” he said.
Autopsy results confirmed the rapid response to treatment, and tumor shrinkage appeared to correlate with the treatment dose.
“Our results in the laboratory and with this patient open a new world of noninvasive and nontoxic therapy for brain cancer, with many exciting possibilities for the future,” Dr. Baskin commented.
He said his team has experimented with this approach with other tumor types in the laboratory, including triple-negative breast cancer and lung cancer. “We’ve only tried it in a culture so far, but it seems to melt the cancer cells,” he said.
The work was supported by a grant from the Translational Research Initiative of the Houston Methodist Research Institute and several foundations. Dr. Baskin and two coauthors are listed as inventors on a U.S. patent application filed by Houston Methodist Hospital for the device used in this report.
A version of this article first appeared on Medscape.com.
FDA’s fast-track approval process exposed as lax, in need of reform
an in-depth investigation published in The BMJ has determined.
“Despite the pathway’s good intentions to accelerate ‘the availability of drugs that treat serious diseases,’ experts are concerned that it is now being exploited – to the detriment of patients, who may be prescribed a drug that offers little benefit and possible harm, and to taxpayers,” writes Elisabeth Mahase, clinical reporter at The BMJ, who carried out the analysis.
The FDA’s accelerated approval pathway is intended to provide earlier access to drugs for serious diseases when there is lingering uncertainty at the time of approval regarding the drug’s ultimate clinical benefit.
Required studies rarely completed
As part of this fast-track pathway, drug manufacturers must conduct postapproval, phase 4 confirmatory trials to verify the anticipated clinical benefit. If these trials indicate no benefit, FDA approval can be withdrawn.
However, the analysis of FDA data shows once they are approved drugs are rarely taken off the market.
The BMJ investigation that analyzed data up to the end of 2020 shows that 112 of the 253 (44%) medications granted accelerated approval have not been confirmed to be effective.
In addition, 24 (21%) of these questionable drugs have been on the market for more than 5 years and some have been on the market for more than 20 years – often with a hefty price tag.
Furthermore, only 16 drugs approved through the accelerated approval process have ever been withdrawn, and most were shown to be ineffective, but in some cases the confirmatory trials were never done, Ms. Mahase reports.
For example, the COX-2 inhibitor celecoxib (Celebrex), which was granted accelerated approval in 1999 for the treatment of familial adenomatous polyposis, was on the market for 12 years before the FDA finally asked Pfizer to voluntarily withdraw it for this indication because efficacy trials were never completed.
As part of The BMJ’s investigation, Ms. Mahase asked manufacturers of the 24 drugs that have remained on the market for more than 5 years whether they had conducted the required phase 4 confirmatory trials. Six of the drugs had been withdrawn, approved, or postponed.
Of the remaining 18 drugs, the manufacturers provided the relevant trial information for only six. Only four drugmakers had started to recruit patients; two said they were still in discussion with the FDA over the final trial design.
“These products routinely have side effects, but the benefit information is a lot less certain. That’s what we’re concerned about – that we may have drugs on the market that don’t have any benefits, but certainly predictably have harms associated with them,” Huseyin Naci, PhD, MHS, with the London School of Economics, comments in the report.
Call for reform
As reported by this news organization, a 2015 report by the General Accountability Office (GAO) concluded that the FDA does not do an effective job of tracking the clinical efficacy or the safety of drugs with expedited approval after they hit the market.
In April of this year, the Institute for Clinical and Economic Review (ICER) cited a lack of “credible threats” to withdraw approval if companies don’t do confirmatory trials – meaning drugmakers have little incentive to do the trials.
“There are some instances where the companies really do seem to be taking advantage of the accelerated approval pathway and are using it in a way that makes it harder to get at the truth about whether these products really are safe and effective,” Rachel Sachs, JD, MPH, Washington University, St. Louis, said in The BMJ article.
In addition, the authors of a recent viewpoint article in JAMA Internal Medicine assert the recent approval of the controversial anti-amyloid drug aducanumab (Aduhelm, Biogen) shows that the accelerated approval pathway needs to be reformed.
Despite the concerns, Ms. Mahase said all experts who spoke to The BMJ believe the accelerated approval pathway is still useful and can be beneficial to patients, although some changes are needed.
One effective reform might be to have confirmatory trials designed, and even started, as part of accelerated approval.
“One important piece of the puzzle is for the FDA itself to be tougher on these companies, to hold them to the bargain that they have agreed to, and to take action when the company has not met their obligations,” Ms. Sachs told the journal.
An FDA spokesperson told the BMJ that the agency is “committed to working with sponsors to ensure that confirmatory studies are completed in a timely manner.”
“We expect sponsors to commit all resources needed to move trials forward as effectively as possible, with the aim of completing trials as soon as is feasible, while assuring the quality of the data and the robustness of the results,” the agency said.
A version of this article first appeared on Medscape.com.
an in-depth investigation published in The BMJ has determined.
“Despite the pathway’s good intentions to accelerate ‘the availability of drugs that treat serious diseases,’ experts are concerned that it is now being exploited – to the detriment of patients, who may be prescribed a drug that offers little benefit and possible harm, and to taxpayers,” writes Elisabeth Mahase, clinical reporter at The BMJ, who carried out the analysis.
The FDA’s accelerated approval pathway is intended to provide earlier access to drugs for serious diseases when there is lingering uncertainty at the time of approval regarding the drug’s ultimate clinical benefit.
Required studies rarely completed
As part of this fast-track pathway, drug manufacturers must conduct postapproval, phase 4 confirmatory trials to verify the anticipated clinical benefit. If these trials indicate no benefit, FDA approval can be withdrawn.
However, the analysis of FDA data shows once they are approved drugs are rarely taken off the market.
The BMJ investigation that analyzed data up to the end of 2020 shows that 112 of the 253 (44%) medications granted accelerated approval have not been confirmed to be effective.
In addition, 24 (21%) of these questionable drugs have been on the market for more than 5 years and some have been on the market for more than 20 years – often with a hefty price tag.
Furthermore, only 16 drugs approved through the accelerated approval process have ever been withdrawn, and most were shown to be ineffective, but in some cases the confirmatory trials were never done, Ms. Mahase reports.
For example, the COX-2 inhibitor celecoxib (Celebrex), which was granted accelerated approval in 1999 for the treatment of familial adenomatous polyposis, was on the market for 12 years before the FDA finally asked Pfizer to voluntarily withdraw it for this indication because efficacy trials were never completed.
As part of The BMJ’s investigation, Ms. Mahase asked manufacturers of the 24 drugs that have remained on the market for more than 5 years whether they had conducted the required phase 4 confirmatory trials. Six of the drugs had been withdrawn, approved, or postponed.
Of the remaining 18 drugs, the manufacturers provided the relevant trial information for only six. Only four drugmakers had started to recruit patients; two said they were still in discussion with the FDA over the final trial design.
“These products routinely have side effects, but the benefit information is a lot less certain. That’s what we’re concerned about – that we may have drugs on the market that don’t have any benefits, but certainly predictably have harms associated with them,” Huseyin Naci, PhD, MHS, with the London School of Economics, comments in the report.
Call for reform
As reported by this news organization, a 2015 report by the General Accountability Office (GAO) concluded that the FDA does not do an effective job of tracking the clinical efficacy or the safety of drugs with expedited approval after they hit the market.
In April of this year, the Institute for Clinical and Economic Review (ICER) cited a lack of “credible threats” to withdraw approval if companies don’t do confirmatory trials – meaning drugmakers have little incentive to do the trials.
“There are some instances where the companies really do seem to be taking advantage of the accelerated approval pathway and are using it in a way that makes it harder to get at the truth about whether these products really are safe and effective,” Rachel Sachs, JD, MPH, Washington University, St. Louis, said in The BMJ article.
In addition, the authors of a recent viewpoint article in JAMA Internal Medicine assert the recent approval of the controversial anti-amyloid drug aducanumab (Aduhelm, Biogen) shows that the accelerated approval pathway needs to be reformed.
Despite the concerns, Ms. Mahase said all experts who spoke to The BMJ believe the accelerated approval pathway is still useful and can be beneficial to patients, although some changes are needed.
One effective reform might be to have confirmatory trials designed, and even started, as part of accelerated approval.
“One important piece of the puzzle is for the FDA itself to be tougher on these companies, to hold them to the bargain that they have agreed to, and to take action when the company has not met their obligations,” Ms. Sachs told the journal.
An FDA spokesperson told the BMJ that the agency is “committed to working with sponsors to ensure that confirmatory studies are completed in a timely manner.”
“We expect sponsors to commit all resources needed to move trials forward as effectively as possible, with the aim of completing trials as soon as is feasible, while assuring the quality of the data and the robustness of the results,” the agency said.
A version of this article first appeared on Medscape.com.
an in-depth investigation published in The BMJ has determined.
“Despite the pathway’s good intentions to accelerate ‘the availability of drugs that treat serious diseases,’ experts are concerned that it is now being exploited – to the detriment of patients, who may be prescribed a drug that offers little benefit and possible harm, and to taxpayers,” writes Elisabeth Mahase, clinical reporter at The BMJ, who carried out the analysis.
The FDA’s accelerated approval pathway is intended to provide earlier access to drugs for serious diseases when there is lingering uncertainty at the time of approval regarding the drug’s ultimate clinical benefit.
Required studies rarely completed
As part of this fast-track pathway, drug manufacturers must conduct postapproval, phase 4 confirmatory trials to verify the anticipated clinical benefit. If these trials indicate no benefit, FDA approval can be withdrawn.
However, the analysis of FDA data shows once they are approved drugs are rarely taken off the market.
The BMJ investigation that analyzed data up to the end of 2020 shows that 112 of the 253 (44%) medications granted accelerated approval have not been confirmed to be effective.
In addition, 24 (21%) of these questionable drugs have been on the market for more than 5 years and some have been on the market for more than 20 years – often with a hefty price tag.
Furthermore, only 16 drugs approved through the accelerated approval process have ever been withdrawn, and most were shown to be ineffective, but in some cases the confirmatory trials were never done, Ms. Mahase reports.
For example, the COX-2 inhibitor celecoxib (Celebrex), which was granted accelerated approval in 1999 for the treatment of familial adenomatous polyposis, was on the market for 12 years before the FDA finally asked Pfizer to voluntarily withdraw it for this indication because efficacy trials were never completed.
As part of The BMJ’s investigation, Ms. Mahase asked manufacturers of the 24 drugs that have remained on the market for more than 5 years whether they had conducted the required phase 4 confirmatory trials. Six of the drugs had been withdrawn, approved, or postponed.
Of the remaining 18 drugs, the manufacturers provided the relevant trial information for only six. Only four drugmakers had started to recruit patients; two said they were still in discussion with the FDA over the final trial design.
“These products routinely have side effects, but the benefit information is a lot less certain. That’s what we’re concerned about – that we may have drugs on the market that don’t have any benefits, but certainly predictably have harms associated with them,” Huseyin Naci, PhD, MHS, with the London School of Economics, comments in the report.
Call for reform
As reported by this news organization, a 2015 report by the General Accountability Office (GAO) concluded that the FDA does not do an effective job of tracking the clinical efficacy or the safety of drugs with expedited approval after they hit the market.
In April of this year, the Institute for Clinical and Economic Review (ICER) cited a lack of “credible threats” to withdraw approval if companies don’t do confirmatory trials – meaning drugmakers have little incentive to do the trials.
“There are some instances where the companies really do seem to be taking advantage of the accelerated approval pathway and are using it in a way that makes it harder to get at the truth about whether these products really are safe and effective,” Rachel Sachs, JD, MPH, Washington University, St. Louis, said in The BMJ article.
In addition, the authors of a recent viewpoint article in JAMA Internal Medicine assert the recent approval of the controversial anti-amyloid drug aducanumab (Aduhelm, Biogen) shows that the accelerated approval pathway needs to be reformed.
Despite the concerns, Ms. Mahase said all experts who spoke to The BMJ believe the accelerated approval pathway is still useful and can be beneficial to patients, although some changes are needed.
One effective reform might be to have confirmatory trials designed, and even started, as part of accelerated approval.
“One important piece of the puzzle is for the FDA itself to be tougher on these companies, to hold them to the bargain that they have agreed to, and to take action when the company has not met their obligations,” Ms. Sachs told the journal.
An FDA spokesperson told the BMJ that the agency is “committed to working with sponsors to ensure that confirmatory studies are completed in a timely manner.”
“We expect sponsors to commit all resources needed to move trials forward as effectively as possible, with the aim of completing trials as soon as is feasible, while assuring the quality of the data and the robustness of the results,” the agency said.
A version of this article first appeared on Medscape.com.
Early transition to oral beta-lactams for low-risk S. aureus bacteremia may be acceptable
Background: There is consensus that LR-SAB can be safely treated with 14 days of antibiotic therapy, but the use of and/or proportion of duration of oral antibiotics is not clear. There is evidence that oral therapy has fewer treatment complications, compared with IV treatments. Objective of this study was to assess the safety of early oral switch (EOS) prior to 14 days for LR-SAB.
Study design: Retrospective cohort study.
Setting: Single institution tertiary care hospital in Wellington, New Zealand.
Synopsis: Study population included adults with health care–associated SAB deemed low risk (no positive blood cultures >72 hours after initial positive culture, no evidence of deep infection as determined by an infectious disease consultant, no nonremovable prosthetics). The primary outcome was occurrence of SAB-related complication (recurrence of SAB, deep-seated infection, readmission, attributable mortality) within 90 days.
Of the initial 469 episodes of SAB, 100 met inclusion, and 84 of those patients had EOS. Line infection was the source in a majority of patients (79% and 88% in EOS and IV, respectively). Only 5% of patients had MRSA. Overall, 86% of EOS patients were treated with an oral beta-lactam, within the EOS group, median duration of IV and oral antibiotics was 5 and 10 days, respectively. SAB recurrence within 90 days occurred in three (4%) and one (6%) patients in EOS vs. IV groups, respectively (P = .64). No deaths within 90 days were deemed attributable to SAB. Limitations include small size, single center, and observational, retrospective framework.
Bottom line: The study suggests that EOS with oral beta-lactams in selected patients with LR-SAB may be adequate; however, the study is too small to provide robust high-level evidence. Instead, the authors hope the data will lead to larger, more powerful prospective studies to examine if a simpler, cheaper, and in some ways safer treatment course is possible.
Citation: Bupha-Intr O et al. Efficacy of early oral switch with beta-lactams for low-risk Staphylococcus aureus bacteremia. Antimicrob Agents Chemother. 2020 Feb 3;AAC.02345-19. doi: 10.1128/AAC.02345-19.
Dr. Sneed is assistant professor of medicine, section of hospital medicine, at the University of Virginia School of Medicine, Charlottesville.
Background: There is consensus that LR-SAB can be safely treated with 14 days of antibiotic therapy, but the use of and/or proportion of duration of oral antibiotics is not clear. There is evidence that oral therapy has fewer treatment complications, compared with IV treatments. Objective of this study was to assess the safety of early oral switch (EOS) prior to 14 days for LR-SAB.
Study design: Retrospective cohort study.
Setting: Single institution tertiary care hospital in Wellington, New Zealand.
Synopsis: Study population included adults with health care–associated SAB deemed low risk (no positive blood cultures >72 hours after initial positive culture, no evidence of deep infection as determined by an infectious disease consultant, no nonremovable prosthetics). The primary outcome was occurrence of SAB-related complication (recurrence of SAB, deep-seated infection, readmission, attributable mortality) within 90 days.
Of the initial 469 episodes of SAB, 100 met inclusion, and 84 of those patients had EOS. Line infection was the source in a majority of patients (79% and 88% in EOS and IV, respectively). Only 5% of patients had MRSA. Overall, 86% of EOS patients were treated with an oral beta-lactam, within the EOS group, median duration of IV and oral antibiotics was 5 and 10 days, respectively. SAB recurrence within 90 days occurred in three (4%) and one (6%) patients in EOS vs. IV groups, respectively (P = .64). No deaths within 90 days were deemed attributable to SAB. Limitations include small size, single center, and observational, retrospective framework.
Bottom line: The study suggests that EOS with oral beta-lactams in selected patients with LR-SAB may be adequate; however, the study is too small to provide robust high-level evidence. Instead, the authors hope the data will lead to larger, more powerful prospective studies to examine if a simpler, cheaper, and in some ways safer treatment course is possible.
Citation: Bupha-Intr O et al. Efficacy of early oral switch with beta-lactams for low-risk Staphylococcus aureus bacteremia. Antimicrob Agents Chemother. 2020 Feb 3;AAC.02345-19. doi: 10.1128/AAC.02345-19.
Dr. Sneed is assistant professor of medicine, section of hospital medicine, at the University of Virginia School of Medicine, Charlottesville.
Background: There is consensus that LR-SAB can be safely treated with 14 days of antibiotic therapy, but the use of and/or proportion of duration of oral antibiotics is not clear. There is evidence that oral therapy has fewer treatment complications, compared with IV treatments. Objective of this study was to assess the safety of early oral switch (EOS) prior to 14 days for LR-SAB.
Study design: Retrospective cohort study.
Setting: Single institution tertiary care hospital in Wellington, New Zealand.
Synopsis: Study population included adults with health care–associated SAB deemed low risk (no positive blood cultures >72 hours after initial positive culture, no evidence of deep infection as determined by an infectious disease consultant, no nonremovable prosthetics). The primary outcome was occurrence of SAB-related complication (recurrence of SAB, deep-seated infection, readmission, attributable mortality) within 90 days.
Of the initial 469 episodes of SAB, 100 met inclusion, and 84 of those patients had EOS. Line infection was the source in a majority of patients (79% and 88% in EOS and IV, respectively). Only 5% of patients had MRSA. Overall, 86% of EOS patients were treated with an oral beta-lactam, within the EOS group, median duration of IV and oral antibiotics was 5 and 10 days, respectively. SAB recurrence within 90 days occurred in three (4%) and one (6%) patients in EOS vs. IV groups, respectively (P = .64). No deaths within 90 days were deemed attributable to SAB. Limitations include small size, single center, and observational, retrospective framework.
Bottom line: The study suggests that EOS with oral beta-lactams in selected patients with LR-SAB may be adequate; however, the study is too small to provide robust high-level evidence. Instead, the authors hope the data will lead to larger, more powerful prospective studies to examine if a simpler, cheaper, and in some ways safer treatment course is possible.
Citation: Bupha-Intr O et al. Efficacy of early oral switch with beta-lactams for low-risk Staphylococcus aureus bacteremia. Antimicrob Agents Chemother. 2020 Feb 3;AAC.02345-19. doi: 10.1128/AAC.02345-19.
Dr. Sneed is assistant professor of medicine, section of hospital medicine, at the University of Virginia School of Medicine, Charlottesville.
Inflammatory diet is linked to dementia
according to a new analysis of longitudinal data from the Framingham Heart Study Offspring Cohort.
The lack of an association with Alzheimer’s disease was a surprise because amyloid-beta prompts microglia and astrocytes to release markers of systemic inflammation, according to Debora Melo van Lent, PhD, who is a postdoctoral fellow at the University of Texas Health San Antonio – Glenn Biggs Institute for Alzheimer’s and Neurodegenerative Diseases. “We expected to see a relationship between higher DII scores and an increased risk for incident Alzheimer’s disease,” said Dr. Melo van Lent, who presented the findings at the 2021 Alzheimer’s Association International Conference.
Dr. Melo van Lent added that the most likely explanation is that the study was underpowered to produce a positive association, and the team is conducting further study in a larger population.
A modifiable risk factor
The study is the first to look at all-cause dementia and Alzheimer’s disease dementia and their association with DII, Dr. Melo van Lent said.
“As diet is a modifiable risk factor, we can actually do something about it. If we take a closer look at five components of the DII which are most anti-inflammatory, these components are present in green leafy vegetables, vegetables, fruit, soy, whole grains, and green and black tea. Most of these components are included in the Mediterranean diet. When we look at the three most proinflammatory components, they are present in high caloric products; such as butter or margarine, pastries and sweets, fried snacks, and red or processed meat. These components are present in ‘Western diets,’ which are discouraged,” said Dr. Melo van Lent.
The researchers analyzed data from 1,486 participants who were free of dementia, stroke, or other neurologic diseases at baseline. They analyzed DII scores both in a continuous range and divided into quartiles, using the first quartile as a reference.
The mean age of participants was 69 years, and 53% were women. During follow-up, 11.3% developed AD dementia, and 14.8% developed non-AD dementia.
In the continuous model, DII was associated with increased risk of all-cause dementia after adjusting for age, sex, APOE E4 status, body mass index, smoking, physical activity index score, total energy intake, lipid-lowering medications, and total cholesterol to HDL cholesterol ratio (hazard ratio, 1.18; P =.001). In the quartile analysis, after adjustments, compared with quartile 1, there was an increased risk of all-cause dementia for those in quartile 3 (HR, 1.69; P =.020) and quartile 4 (HR, 1.84; P =.013).
In the continuous analysis, after adjustments, there was an association between DII score and Alzheimer’s dementia (HR, 1.15; P =.020). In the quartile analysis, no associations were significant, though there was a trend of quartile 4 versus quartile 1 (HR, 1.65; P =.077).
The researchers found no significant interactions between higher DII scores and sex, the APOE E4 allele, or physical activity with respect to all-cause dementia or Alzheimer’s dementia.
Intertwined variables
The results were interesting, but cause and effect relationships can be difficult to tease out from such a study, according to Claire Sexton, DPhil, director of scientific programs and outreach at the Alzheimer’s Association, who was asked to comment on the study. Dr. Sexton noted that individuals who eat well are more likely to have energy to exercise, which could in turn help them to sleep better, and all of those factors could be involved in reducing dementia risk. “They’re all kind of intertwined. So in this study, they were taking into account physical activity, but they can’t take into account every single variable. It’s important for them to be followed up by randomized control trials.”
Dr. Sexton also referenced the U.S. Pointer study being conducted by the Alzheimer’s Association, which is examining various interventions related to diet, physical activity, and cognitive stimulation. “Whether intervening and improving people’s health behaviors then goes on to reduce their risk for dementia is a key question. We still need more results from studies to be reporting out before we get definitive answers,” she said.
The study was funded by the ASPEN Rhoads Research Foundation. Dr. Melo van Lent and Dr. Sexton have no relevant financial disclosures.
according to a new analysis of longitudinal data from the Framingham Heart Study Offspring Cohort.
The lack of an association with Alzheimer’s disease was a surprise because amyloid-beta prompts microglia and astrocytes to release markers of systemic inflammation, according to Debora Melo van Lent, PhD, who is a postdoctoral fellow at the University of Texas Health San Antonio – Glenn Biggs Institute for Alzheimer’s and Neurodegenerative Diseases. “We expected to see a relationship between higher DII scores and an increased risk for incident Alzheimer’s disease,” said Dr. Melo van Lent, who presented the findings at the 2021 Alzheimer’s Association International Conference.
Dr. Melo van Lent added that the most likely explanation is that the study was underpowered to produce a positive association, and the team is conducting further study in a larger population.
A modifiable risk factor
The study is the first to look at all-cause dementia and Alzheimer’s disease dementia and their association with DII, Dr. Melo van Lent said.
“As diet is a modifiable risk factor, we can actually do something about it. If we take a closer look at five components of the DII which are most anti-inflammatory, these components are present in green leafy vegetables, vegetables, fruit, soy, whole grains, and green and black tea. Most of these components are included in the Mediterranean diet. When we look at the three most proinflammatory components, they are present in high caloric products; such as butter or margarine, pastries and sweets, fried snacks, and red or processed meat. These components are present in ‘Western diets,’ which are discouraged,” said Dr. Melo van Lent.
The researchers analyzed data from 1,486 participants who were free of dementia, stroke, or other neurologic diseases at baseline. They analyzed DII scores both in a continuous range and divided into quartiles, using the first quartile as a reference.
The mean age of participants was 69 years, and 53% were women. During follow-up, 11.3% developed AD dementia, and 14.8% developed non-AD dementia.
In the continuous model, DII was associated with increased risk of all-cause dementia after adjusting for age, sex, APOE E4 status, body mass index, smoking, physical activity index score, total energy intake, lipid-lowering medications, and total cholesterol to HDL cholesterol ratio (hazard ratio, 1.18; P =.001). In the quartile analysis, after adjustments, compared with quartile 1, there was an increased risk of all-cause dementia for those in quartile 3 (HR, 1.69; P =.020) and quartile 4 (HR, 1.84; P =.013).
In the continuous analysis, after adjustments, there was an association between DII score and Alzheimer’s dementia (HR, 1.15; P =.020). In the quartile analysis, no associations were significant, though there was a trend of quartile 4 versus quartile 1 (HR, 1.65; P =.077).
The researchers found no significant interactions between higher DII scores and sex, the APOE E4 allele, or physical activity with respect to all-cause dementia or Alzheimer’s dementia.
Intertwined variables
The results were interesting, but cause and effect relationships can be difficult to tease out from such a study, according to Claire Sexton, DPhil, director of scientific programs and outreach at the Alzheimer’s Association, who was asked to comment on the study. Dr. Sexton noted that individuals who eat well are more likely to have energy to exercise, which could in turn help them to sleep better, and all of those factors could be involved in reducing dementia risk. “They’re all kind of intertwined. So in this study, they were taking into account physical activity, but they can’t take into account every single variable. It’s important for them to be followed up by randomized control trials.”
Dr. Sexton also referenced the U.S. Pointer study being conducted by the Alzheimer’s Association, which is examining various interventions related to diet, physical activity, and cognitive stimulation. “Whether intervening and improving people’s health behaviors then goes on to reduce their risk for dementia is a key question. We still need more results from studies to be reporting out before we get definitive answers,” she said.
The study was funded by the ASPEN Rhoads Research Foundation. Dr. Melo van Lent and Dr. Sexton have no relevant financial disclosures.
according to a new analysis of longitudinal data from the Framingham Heart Study Offspring Cohort.
The lack of an association with Alzheimer’s disease was a surprise because amyloid-beta prompts microglia and astrocytes to release markers of systemic inflammation, according to Debora Melo van Lent, PhD, who is a postdoctoral fellow at the University of Texas Health San Antonio – Glenn Biggs Institute for Alzheimer’s and Neurodegenerative Diseases. “We expected to see a relationship between higher DII scores and an increased risk for incident Alzheimer’s disease,” said Dr. Melo van Lent, who presented the findings at the 2021 Alzheimer’s Association International Conference.
Dr. Melo van Lent added that the most likely explanation is that the study was underpowered to produce a positive association, and the team is conducting further study in a larger population.
A modifiable risk factor
The study is the first to look at all-cause dementia and Alzheimer’s disease dementia and their association with DII, Dr. Melo van Lent said.
“As diet is a modifiable risk factor, we can actually do something about it. If we take a closer look at five components of the DII which are most anti-inflammatory, these components are present in green leafy vegetables, vegetables, fruit, soy, whole grains, and green and black tea. Most of these components are included in the Mediterranean diet. When we look at the three most proinflammatory components, they are present in high caloric products; such as butter or margarine, pastries and sweets, fried snacks, and red or processed meat. These components are present in ‘Western diets,’ which are discouraged,” said Dr. Melo van Lent.
The researchers analyzed data from 1,486 participants who were free of dementia, stroke, or other neurologic diseases at baseline. They analyzed DII scores both in a continuous range and divided into quartiles, using the first quartile as a reference.
The mean age of participants was 69 years, and 53% were women. During follow-up, 11.3% developed AD dementia, and 14.8% developed non-AD dementia.
In the continuous model, DII was associated with increased risk of all-cause dementia after adjusting for age, sex, APOE E4 status, body mass index, smoking, physical activity index score, total energy intake, lipid-lowering medications, and total cholesterol to HDL cholesterol ratio (hazard ratio, 1.18; P =.001). In the quartile analysis, after adjustments, compared with quartile 1, there was an increased risk of all-cause dementia for those in quartile 3 (HR, 1.69; P =.020) and quartile 4 (HR, 1.84; P =.013).
In the continuous analysis, after adjustments, there was an association between DII score and Alzheimer’s dementia (HR, 1.15; P =.020). In the quartile analysis, no associations were significant, though there was a trend of quartile 4 versus quartile 1 (HR, 1.65; P =.077).
The researchers found no significant interactions between higher DII scores and sex, the APOE E4 allele, or physical activity with respect to all-cause dementia or Alzheimer’s dementia.
Intertwined variables
The results were interesting, but cause and effect relationships can be difficult to tease out from such a study, according to Claire Sexton, DPhil, director of scientific programs and outreach at the Alzheimer’s Association, who was asked to comment on the study. Dr. Sexton noted that individuals who eat well are more likely to have energy to exercise, which could in turn help them to sleep better, and all of those factors could be involved in reducing dementia risk. “They’re all kind of intertwined. So in this study, they were taking into account physical activity, but they can’t take into account every single variable. It’s important for them to be followed up by randomized control trials.”
Dr. Sexton also referenced the U.S. Pointer study being conducted by the Alzheimer’s Association, which is examining various interventions related to diet, physical activity, and cognitive stimulation. “Whether intervening and improving people’s health behaviors then goes on to reduce their risk for dementia is a key question. We still need more results from studies to be reporting out before we get definitive answers,” she said.
The study was funded by the ASPEN Rhoads Research Foundation. Dr. Melo van Lent and Dr. Sexton have no relevant financial disclosures.
FROM AAIC 2021
‘A few mutations away’: The threat of a vaccine-proof variant
The Centers for Disease Control and Prevention Director Rochelle Walensky, MD, MPH, made a dire prediction during a media briefing this week that, if we weren’t already living within the reality of the COVID-19 pandemic, would sound more like a pitch for a movie about a dystopian future.
“For the amount of virus circulating in this country right now largely among unvaccinated people, the largest concern that we in public health and science are worried about is that the virus … [becomes] a very transmissible virus that has the potential to evade our vaccines in terms of how it protects us from severe disease and death,” Dr. Walensky told reporters on July 27.
A new, more elusive variant could be “just a few mutations away,” she said.
“That’s a very prescient comment,” Lewis Nelson, MD, professor and clinical chair of emergency medicine and chief of the division of medical toxicology at Rutgers New Jersey Medical School in Newark, told this news organization.
“We’ve gone through a few mutations already that have been named, and each one of them gets a little more transmissible,” he said. “That’s normal, natural selection and what you would expect to happen as viruses mutate from one strain to another.”
“What we’ve mostly seen this virus do is evolve to become more infectious,” said Stuart Ray, MD, when also asked to comment. “That is the remarkable feature of Delta – that it is so infectious.”
He said that the SARS-CoV-2 has evolved largely as expected, at least so far. “The potential for this virus to mutate has been something that has been a concern from early on.”
“The viral evolution is a bit like a ticking clock. The more we allow infections to occur, the more likely changes will occur. When we have lots of people infected, we give more chances to the virus to diversify and then adapt to selective pressures,” said Dr. Ray, vice-chair of medicine for data integrity and analytics and professor in the division of infectious diseases at Johns Hopkins School of Medicine in Baltimore.
Dr. Nelson said.
If this occurs, he added, “we will have an ineffective vaccine, essentially. And we’ll be back to where we were last March with a brand-new disease.”
Technology to the rescue?
The flexibility of mRNA vaccines is one potential solution. These vaccines could be more easily and quickly adapted to respond to a new, more vaccine-elusive variant.
“That’s absolutely reassuring,” Dr. Nelson said. For example, if a mutation changes the spike protein and vaccines no longer recognize it, a manufacturer could identify the new protein and incorporate that in a new mRNA vaccine.
“The problem is that some people are not taking the current vaccine,” he added. “I’m not sure what is going to make them take the next vaccine.”
Nothing appears certain
When asked how likely a new strain of SARS-CoV-2 could emerge that gets around vaccine protection, Dr. Nelson said, “I think [what] we’ve learned so far there is no way to predict anything” about this pandemic.
“The best way to prevent the virus from mutating is to prevent hosts, people, from getting sick with it,” he said. “That’s why it’s so important people should get immunized and wear masks.”
Both Dr. Nelson and Dr. Ray pointed out that it is in the best interest of the virus to evolve to be more transmissible and spread to more people. In contrast, a virus that causes people to get so sick that they isolate or die, thus halting transmission, works against viruses surviving evolutionarily.
Some viruses also mutate to become milder over time, but that has not been the case with SARS-CoV-2, Dr. Ray said.
Mutations not the only concern
Viruses have another mechanism that produces new strains, and it works even more quickly than mutations. Recombination, as it’s known, can occur when a person is infected with two different strains of the same virus. If the two versions enter the same cell, the viruses can swap genetic material and produce a third, altogether different strain.
Recombination has already been seen with influenza strains, where H and N genetic segments are swapped to yield H1N1, H1N2, and H3N2 versions of the flu, for example.
“In the early days of SARS-CoV-2 there was so little diversity that recombination did not matter,” Dr. Ray said. However, there are now distinct lineages of the virus circulating globally. If two of these lineages swap segments “this would make a very new viral sequence in one step without having to mutate to gain those differences.”
“The more diverse the strains that are circulating, the bigger a possibility this is,” Dr. Ray said.
Protected, for now
Dr. Walensky’s sober warning came at the same time the CDC released new guidance calling for the wearing of masks indoors in schools and in any location in the country where COVID-19 cases surpass 50 people per 100,000, also known as substantial or high transmission areas.
On a positive note, Dr. Walensky said: “Right now, fortunately, we are not there. The vaccines operate really well in protecting us from severe disease and death.”
A version of this article first appeared on Medscape.com.
The Centers for Disease Control and Prevention Director Rochelle Walensky, MD, MPH, made a dire prediction during a media briefing this week that, if we weren’t already living within the reality of the COVID-19 pandemic, would sound more like a pitch for a movie about a dystopian future.
“For the amount of virus circulating in this country right now largely among unvaccinated people, the largest concern that we in public health and science are worried about is that the virus … [becomes] a very transmissible virus that has the potential to evade our vaccines in terms of how it protects us from severe disease and death,” Dr. Walensky told reporters on July 27.
A new, more elusive variant could be “just a few mutations away,” she said.
“That’s a very prescient comment,” Lewis Nelson, MD, professor and clinical chair of emergency medicine and chief of the division of medical toxicology at Rutgers New Jersey Medical School in Newark, told this news organization.
“We’ve gone through a few mutations already that have been named, and each one of them gets a little more transmissible,” he said. “That’s normal, natural selection and what you would expect to happen as viruses mutate from one strain to another.”
“What we’ve mostly seen this virus do is evolve to become more infectious,” said Stuart Ray, MD, when also asked to comment. “That is the remarkable feature of Delta – that it is so infectious.”
He said that the SARS-CoV-2 has evolved largely as expected, at least so far. “The potential for this virus to mutate has been something that has been a concern from early on.”
“The viral evolution is a bit like a ticking clock. The more we allow infections to occur, the more likely changes will occur. When we have lots of people infected, we give more chances to the virus to diversify and then adapt to selective pressures,” said Dr. Ray, vice-chair of medicine for data integrity and analytics and professor in the division of infectious diseases at Johns Hopkins School of Medicine in Baltimore.
Dr. Nelson said.
If this occurs, he added, “we will have an ineffective vaccine, essentially. And we’ll be back to where we were last March with a brand-new disease.”
Technology to the rescue?
The flexibility of mRNA vaccines is one potential solution. These vaccines could be more easily and quickly adapted to respond to a new, more vaccine-elusive variant.
“That’s absolutely reassuring,” Dr. Nelson said. For example, if a mutation changes the spike protein and vaccines no longer recognize it, a manufacturer could identify the new protein and incorporate that in a new mRNA vaccine.
“The problem is that some people are not taking the current vaccine,” he added. “I’m not sure what is going to make them take the next vaccine.”
Nothing appears certain
When asked how likely a new strain of SARS-CoV-2 could emerge that gets around vaccine protection, Dr. Nelson said, “I think [what] we’ve learned so far there is no way to predict anything” about this pandemic.
“The best way to prevent the virus from mutating is to prevent hosts, people, from getting sick with it,” he said. “That’s why it’s so important people should get immunized and wear masks.”
Both Dr. Nelson and Dr. Ray pointed out that it is in the best interest of the virus to evolve to be more transmissible and spread to more people. In contrast, a virus that causes people to get so sick that they isolate or die, thus halting transmission, works against viruses surviving evolutionarily.
Some viruses also mutate to become milder over time, but that has not been the case with SARS-CoV-2, Dr. Ray said.
Mutations not the only concern
Viruses have another mechanism that produces new strains, and it works even more quickly than mutations. Recombination, as it’s known, can occur when a person is infected with two different strains of the same virus. If the two versions enter the same cell, the viruses can swap genetic material and produce a third, altogether different strain.
Recombination has already been seen with influenza strains, where H and N genetic segments are swapped to yield H1N1, H1N2, and H3N2 versions of the flu, for example.
“In the early days of SARS-CoV-2 there was so little diversity that recombination did not matter,” Dr. Ray said. However, there are now distinct lineages of the virus circulating globally. If two of these lineages swap segments “this would make a very new viral sequence in one step without having to mutate to gain those differences.”
“The more diverse the strains that are circulating, the bigger a possibility this is,” Dr. Ray said.
Protected, for now
Dr. Walensky’s sober warning came at the same time the CDC released new guidance calling for the wearing of masks indoors in schools and in any location in the country where COVID-19 cases surpass 50 people per 100,000, also known as substantial or high transmission areas.
On a positive note, Dr. Walensky said: “Right now, fortunately, we are not there. The vaccines operate really well in protecting us from severe disease and death.”
A version of this article first appeared on Medscape.com.
The Centers for Disease Control and Prevention Director Rochelle Walensky, MD, MPH, made a dire prediction during a media briefing this week that, if we weren’t already living within the reality of the COVID-19 pandemic, would sound more like a pitch for a movie about a dystopian future.
“For the amount of virus circulating in this country right now largely among unvaccinated people, the largest concern that we in public health and science are worried about is that the virus … [becomes] a very transmissible virus that has the potential to evade our vaccines in terms of how it protects us from severe disease and death,” Dr. Walensky told reporters on July 27.
A new, more elusive variant could be “just a few mutations away,” she said.
“That’s a very prescient comment,” Lewis Nelson, MD, professor and clinical chair of emergency medicine and chief of the division of medical toxicology at Rutgers New Jersey Medical School in Newark, told this news organization.
“We’ve gone through a few mutations already that have been named, and each one of them gets a little more transmissible,” he said. “That’s normal, natural selection and what you would expect to happen as viruses mutate from one strain to another.”
“What we’ve mostly seen this virus do is evolve to become more infectious,” said Stuart Ray, MD, when also asked to comment. “That is the remarkable feature of Delta – that it is so infectious.”
He said that the SARS-CoV-2 has evolved largely as expected, at least so far. “The potential for this virus to mutate has been something that has been a concern from early on.”
“The viral evolution is a bit like a ticking clock. The more we allow infections to occur, the more likely changes will occur. When we have lots of people infected, we give more chances to the virus to diversify and then adapt to selective pressures,” said Dr. Ray, vice-chair of medicine for data integrity and analytics and professor in the division of infectious diseases at Johns Hopkins School of Medicine in Baltimore.
Dr. Nelson said.
If this occurs, he added, “we will have an ineffective vaccine, essentially. And we’ll be back to where we were last March with a brand-new disease.”
Technology to the rescue?
The flexibility of mRNA vaccines is one potential solution. These vaccines could be more easily and quickly adapted to respond to a new, more vaccine-elusive variant.
“That’s absolutely reassuring,” Dr. Nelson said. For example, if a mutation changes the spike protein and vaccines no longer recognize it, a manufacturer could identify the new protein and incorporate that in a new mRNA vaccine.
“The problem is that some people are not taking the current vaccine,” he added. “I’m not sure what is going to make them take the next vaccine.”
Nothing appears certain
When asked how likely a new strain of SARS-CoV-2 could emerge that gets around vaccine protection, Dr. Nelson said, “I think [what] we’ve learned so far there is no way to predict anything” about this pandemic.
“The best way to prevent the virus from mutating is to prevent hosts, people, from getting sick with it,” he said. “That’s why it’s so important people should get immunized and wear masks.”
Both Dr. Nelson and Dr. Ray pointed out that it is in the best interest of the virus to evolve to be more transmissible and spread to more people. In contrast, a virus that causes people to get so sick that they isolate or die, thus halting transmission, works against viruses surviving evolutionarily.
Some viruses also mutate to become milder over time, but that has not been the case with SARS-CoV-2, Dr. Ray said.
Mutations not the only concern
Viruses have another mechanism that produces new strains, and it works even more quickly than mutations. Recombination, as it’s known, can occur when a person is infected with two different strains of the same virus. If the two versions enter the same cell, the viruses can swap genetic material and produce a third, altogether different strain.
Recombination has already been seen with influenza strains, where H and N genetic segments are swapped to yield H1N1, H1N2, and H3N2 versions of the flu, for example.
“In the early days of SARS-CoV-2 there was so little diversity that recombination did not matter,” Dr. Ray said. However, there are now distinct lineages of the virus circulating globally. If two of these lineages swap segments “this would make a very new viral sequence in one step without having to mutate to gain those differences.”
“The more diverse the strains that are circulating, the bigger a possibility this is,” Dr. Ray said.
Protected, for now
Dr. Walensky’s sober warning came at the same time the CDC released new guidance calling for the wearing of masks indoors in schools and in any location in the country where COVID-19 cases surpass 50 people per 100,000, also known as substantial or high transmission areas.
On a positive note, Dr. Walensky said: “Right now, fortunately, we are not there. The vaccines operate really well in protecting us from severe disease and death.”
A version of this article first appeared on Medscape.com.
Prevalence of dementia before age 65 much higher than expected
Results of a large meta-analysis show that currently 3.9 million individuals are living with young-onset dementia. Among these patients, symptoms of the disease start before age 65.
Recent global young-onset dementia estimates have ranged from 42.3 to 54.0 per 100,000 population, the researchers noted. However, the new study, which included 74 global studies with 2.7 million participants, shows that the global age-standardized prevalence of young-onset dementia is 119.00 per 100,000 among individuals aged 30-64 years; there was little difference in prevalence between men and women. On the basis of the latest population estimates, these new prevalence data imply that there are approximately 175,000 persons with young-onset dementia in the United States.
Although the new global estimate of young-onset dementia is higher than previously thought, “it is still probably an underestimation owing to lack of high-quality data. This should raise awareness for policy makers and health care professionals to organize more and better care for this subgroup of individuals with dementia,” wrote the investigators, with first author Stevie Hendriks, MSc, Maastricht (the Netherlands) University, and the Young-Onset Dementia Epidemiology Study Group.
The study was published online July 19, 2021, in JAMA Neurology.
‘Essential’ data
Young-onset dementia is exceedingly rare in those aged 30-63 years (1.1 per 100,000) but is more prevalent at age 60-64 years (77.4 per 100,000). “Our findings fit the general observation that prevalence of dementia increases exponentially from 60 years of age onward,” they wrote.
The prevalence of young-onset dementia was similar in men and women, lower in the United States than in Europe, highest in upper- to middle-income countries, and highest for Alzheimer’s disease, followed by vascular dementia and frontotemporal dementia.
Monitoring the prevalence of young-onset dementia is “essential” to adequately plan and organize health services, the investigators noted.
To ensure more accurate prevalence estimates in the future, “efforts should be made to conduct more cohort studies and to standardize procedures and reporting of prevalence studies. In addition, more data are needed from low-income countries as well as studies that include younger age ranges,” they said.
New insights
In an accompanying editorial, David S. Knopman, MD, department of neurology, Mayo Clinic, Rochester, Minn., noted that the study provides new insights into an “underappreciated problem.”.
Young-onset dementia is a “particularly disheartening diagnosis because it affects individuals in their prime years, in the midst of their careers, and while raising families,” Dr. Knopman wrote.
“Most dementia care is geared for older patients, and as a consequence, services are rarely available to address the needs of someone diagnosed with dementia in their 50s who has dependent children at home and a spouse who must continue working. Understanding the prevalence and incidence of young-onset dementia is a first step in addressing this challenge,” Dr. Knopman wrote.
He noted that the authors of this analysis have “done a service to the dementia community by collecting and analyzing the dozens of individual studies of young-onset dementia.
“The product, a rationally derived estimate of dementia prevalence across the population aged 30-64 years, provides a basis for initiating more efforts to improve methods for timely diagnosis and to address the unique needs of patients with young-onset dementia,” Dr. Knopman concluded.
A version of this article first appeared on Medscape.com.
Results of a large meta-analysis show that currently 3.9 million individuals are living with young-onset dementia. Among these patients, symptoms of the disease start before age 65.
Recent global young-onset dementia estimates have ranged from 42.3 to 54.0 per 100,000 population, the researchers noted. However, the new study, which included 74 global studies with 2.7 million participants, shows that the global age-standardized prevalence of young-onset dementia is 119.00 per 100,000 among individuals aged 30-64 years; there was little difference in prevalence between men and women. On the basis of the latest population estimates, these new prevalence data imply that there are approximately 175,000 persons with young-onset dementia in the United States.
Although the new global estimate of young-onset dementia is higher than previously thought, “it is still probably an underestimation owing to lack of high-quality data. This should raise awareness for policy makers and health care professionals to organize more and better care for this subgroup of individuals with dementia,” wrote the investigators, with first author Stevie Hendriks, MSc, Maastricht (the Netherlands) University, and the Young-Onset Dementia Epidemiology Study Group.
The study was published online July 19, 2021, in JAMA Neurology.
‘Essential’ data
Young-onset dementia is exceedingly rare in those aged 30-63 years (1.1 per 100,000) but is more prevalent at age 60-64 years (77.4 per 100,000). “Our findings fit the general observation that prevalence of dementia increases exponentially from 60 years of age onward,” they wrote.
The prevalence of young-onset dementia was similar in men and women, lower in the United States than in Europe, highest in upper- to middle-income countries, and highest for Alzheimer’s disease, followed by vascular dementia and frontotemporal dementia.
Monitoring the prevalence of young-onset dementia is “essential” to adequately plan and organize health services, the investigators noted.
To ensure more accurate prevalence estimates in the future, “efforts should be made to conduct more cohort studies and to standardize procedures and reporting of prevalence studies. In addition, more data are needed from low-income countries as well as studies that include younger age ranges,” they said.
New insights
In an accompanying editorial, David S. Knopman, MD, department of neurology, Mayo Clinic, Rochester, Minn., noted that the study provides new insights into an “underappreciated problem.”.
Young-onset dementia is a “particularly disheartening diagnosis because it affects individuals in their prime years, in the midst of their careers, and while raising families,” Dr. Knopman wrote.
“Most dementia care is geared for older patients, and as a consequence, services are rarely available to address the needs of someone diagnosed with dementia in their 50s who has dependent children at home and a spouse who must continue working. Understanding the prevalence and incidence of young-onset dementia is a first step in addressing this challenge,” Dr. Knopman wrote.
He noted that the authors of this analysis have “done a service to the dementia community by collecting and analyzing the dozens of individual studies of young-onset dementia.
“The product, a rationally derived estimate of dementia prevalence across the population aged 30-64 years, provides a basis for initiating more efforts to improve methods for timely diagnosis and to address the unique needs of patients with young-onset dementia,” Dr. Knopman concluded.
A version of this article first appeared on Medscape.com.
Results of a large meta-analysis show that currently 3.9 million individuals are living with young-onset dementia. Among these patients, symptoms of the disease start before age 65.
Recent global young-onset dementia estimates have ranged from 42.3 to 54.0 per 100,000 population, the researchers noted. However, the new study, which included 74 global studies with 2.7 million participants, shows that the global age-standardized prevalence of young-onset dementia is 119.00 per 100,000 among individuals aged 30-64 years; there was little difference in prevalence between men and women. On the basis of the latest population estimates, these new prevalence data imply that there are approximately 175,000 persons with young-onset dementia in the United States.
Although the new global estimate of young-onset dementia is higher than previously thought, “it is still probably an underestimation owing to lack of high-quality data. This should raise awareness for policy makers and health care professionals to organize more and better care for this subgroup of individuals with dementia,” wrote the investigators, with first author Stevie Hendriks, MSc, Maastricht (the Netherlands) University, and the Young-Onset Dementia Epidemiology Study Group.
The study was published online July 19, 2021, in JAMA Neurology.
‘Essential’ data
Young-onset dementia is exceedingly rare in those aged 30-63 years (1.1 per 100,000) but is more prevalent at age 60-64 years (77.4 per 100,000). “Our findings fit the general observation that prevalence of dementia increases exponentially from 60 years of age onward,” they wrote.
The prevalence of young-onset dementia was similar in men and women, lower in the United States than in Europe, highest in upper- to middle-income countries, and highest for Alzheimer’s disease, followed by vascular dementia and frontotemporal dementia.
Monitoring the prevalence of young-onset dementia is “essential” to adequately plan and organize health services, the investigators noted.
To ensure more accurate prevalence estimates in the future, “efforts should be made to conduct more cohort studies and to standardize procedures and reporting of prevalence studies. In addition, more data are needed from low-income countries as well as studies that include younger age ranges,” they said.
New insights
In an accompanying editorial, David S. Knopman, MD, department of neurology, Mayo Clinic, Rochester, Minn., noted that the study provides new insights into an “underappreciated problem.”.
Young-onset dementia is a “particularly disheartening diagnosis because it affects individuals in their prime years, in the midst of their careers, and while raising families,” Dr. Knopman wrote.
“Most dementia care is geared for older patients, and as a consequence, services are rarely available to address the needs of someone diagnosed with dementia in their 50s who has dependent children at home and a spouse who must continue working. Understanding the prevalence and incidence of young-onset dementia is a first step in addressing this challenge,” Dr. Knopman wrote.
He noted that the authors of this analysis have “done a service to the dementia community by collecting and analyzing the dozens of individual studies of young-onset dementia.
“The product, a rationally derived estimate of dementia prevalence across the population aged 30-64 years, provides a basis for initiating more efforts to improve methods for timely diagnosis and to address the unique needs of patients with young-onset dementia,” Dr. Knopman concluded.
A version of this article first appeared on Medscape.com.
FROM JAMA NEUROLOGY
‘Shocking’ early complications from teen-onset type 2 diabetes
Newly published data show alarmingly high rates and severity of early diabetes-specific complications in individuals who develop type 2 diabetes at a young age. This suggests intervention should be early and aggressive among these youngsters, said the researchers.
The results for the 500 young adult participants in the Treatment Options for Type 2 Diabetes in Adolescents and Youth 2 (TODAY 2) study were published online July 29 in the New England Journal of Medicine by the TODAY study group.
At follow-up – after originally participating in the TODAY trial when they were young teenagers – they had a mean age of 26.4 years.
At this time, more than two thirds had hypertension and half had dyslipidemia.
Overall, 60% had at least one diabetic microvascular complication (retinal disease, neuropathy, or diabetic kidney disease), and more than a quarter had two or more such complications.
“These data illustrate the serious personal and public health consequences of youth-onset type 2 diabetes in the transition to adulthood,” the researchers noted.
Don’t tread lightly just because they are young
“The fact that these youth are accumulating complications at a rapid rate and are broadly affected early in adulthood certainly suggests that aggressive therapy is needed, both for glycemic control and treatment of risk factors like hypertension and dyslipidemia,” study coauthor Philip S. Zeitler, MD, PhD, said in an interview.
“In the absence of studies specifically addressing this, we need to take a more aggressive approach than people might be inclined to, given that the age at diagnosis is young, around 14 years,” he added.
“Contrary to the inclination to be ‘gentle’ in treating them because they are kids, these data suggest that we can’t let these initial years go by without strong intervention, and we need to be prepared for polypharmacy.”
Unfortunately, as Dr. Zeitler and coauthors explained, youth-onset type 2 diabetes is characterized by a suboptimal response to currently approved diabetes medications.
New pediatric indications in the United States for drugs used to treat type 2 diabetes in adults, including the recent Food and Drug Administration approval of extended-release exenatide for children as young as 10 years of age, “helps, but only marginally,” said Dr. Zeitler, of the Clinical & Translational Research Center, Children’s Hospital Colorado, Aurora.
“In some cases, it will help get them covered by carriers, which is always good. But this is still a very limited set of medications. It doesn’t include more recently approved more potent glucagon-like peptide-1 (GLP-1) agonists, like semaglutide, and doesn’t include the sodium-glucose cotransporter 2 (SGLT2) inhibitors. Pediatricians are used to using medications off label and that is necessary here while we await further approvals,” he said.
And he noted that most individuals with youth-onset type 2 diabetes in the United States are covered by public insurance or are uninsured, depending on which state they live in. While the two major Medicaid programs in Colorado allow full access to adult formularies, that’s not the case everywhere. Moreover, patients often face further access barriers in states without expanded Medicaid.
Follow-up shows all metrics worsening over time
In TODAY 2, patients participated in an observational follow-up in their usual care settings in 2011-2020. At the start, they were receiving metformin with or without insulin for diabetes, but whether this continued and whether they were treated for other risk factors was down to individual circumstances.
Participants’ median A1c increased over time, and the percentage with A1c < 6% (< 48 mmol/mol) declined from 75% at the time of TODAY entry to just 19% at the 15-year end of follow-up.
The proportion with an A1c ≤ 10% (≤ 86 mmol/mol) rose from 0% at baseline to 34% at 15 years.
At that time, nearly 50% were taking both metformin and insulin, while more than a quarter were taking no medications.
The prevalence of hypertension increased from 19.2% at baseline to 67.5% at 15 years, while dyslipidemia rose from 20.8% to 51.6%.
Kidney disease prevalence increased from 8.0% at baseline to 54.8% at 15 years. Nerve disease rose from 1.0% to 32.4%. Retinal disease jumped from 13.7% with milder nonproliferative retinopathy in 2010-2011 to 51.0% with any eye disease in 2017-2018, including 8.8% with moderate to severe retinal changes and 3.5% with macular edema.
Overall, at the time of the last visit, 39.9% had no diabetes complications, 31.8% had one, 21.3% had two, and 7.1% had three complications.
Serious cardiovascular events in mid-20s
There were 17 adjudicated serious cardiovascular events, including four myocardial infarctions, six heart failure events, three diagnoses of coronary artery disease, and four strokes.
Six participants died, one each from myocardial infarction, kidney failure, and drug overdose, and three from sepsis.
Dr. Zeitler called the macrovascular events “shocking,” noting that although the numbers are small, for people in their mid-20s “they should be zero ... While we don’t yet know if the rates are the same or faster than in adults, even if they are the same, these kids are only in their late 20s, as opposed to adults experiencing these problems in their 50s, 60s, and 70s.
“The fact that these complications are occurring when these individuals should be in the prime of their life for both family and work has huge implications,” he stressed.
Findings have multiple causes
The reasons for the findings are both biologic and socioeconomic, Dr. Zeitler said.
“We know already that many kids with type 2 have rapid [deterioration of] beta-cell [function], which is probably very biologic. It stands to reason that an individual who can get diabetes as an adolescent probably has more fragile beta cells in some way,” he noted.
“But we also know that many other things contribute: stress, social determinants, access to quality care and medications, access to healthy foods and physical activity, availability of family supervision given the realities of families’ economic status and jobs, etc.”
It’s also known that youth with type 2 diabetes have much more severe insulin resistance than that of adults with the condition, and that “once the kids left ... the [TODAY] study, risk factor treatment in the community was less than ideal, and a lot of kids who met criteria for treatment of their blood pressure or lipids were not being treated. This is likely at least partly sociologic and partly the general pediatric hesitancy to use medications.”
He said the TODAY team will soon have some new data to show that “glycemia during the early years makes a difference, again supporting intensive intervention early on.”
The TODAY study was supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases. Dr. Zeitler had no further disclosures.
A version of this article first appeared on Medscape.com.
Newly published data show alarmingly high rates and severity of early diabetes-specific complications in individuals who develop type 2 diabetes at a young age. This suggests intervention should be early and aggressive among these youngsters, said the researchers.
The results for the 500 young adult participants in the Treatment Options for Type 2 Diabetes in Adolescents and Youth 2 (TODAY 2) study were published online July 29 in the New England Journal of Medicine by the TODAY study group.
At follow-up – after originally participating in the TODAY trial when they were young teenagers – they had a mean age of 26.4 years.
At this time, more than two thirds had hypertension and half had dyslipidemia.
Overall, 60% had at least one diabetic microvascular complication (retinal disease, neuropathy, or diabetic kidney disease), and more than a quarter had two or more such complications.
“These data illustrate the serious personal and public health consequences of youth-onset type 2 diabetes in the transition to adulthood,” the researchers noted.
Don’t tread lightly just because they are young
“The fact that these youth are accumulating complications at a rapid rate and are broadly affected early in adulthood certainly suggests that aggressive therapy is needed, both for glycemic control and treatment of risk factors like hypertension and dyslipidemia,” study coauthor Philip S. Zeitler, MD, PhD, said in an interview.
“In the absence of studies specifically addressing this, we need to take a more aggressive approach than people might be inclined to, given that the age at diagnosis is young, around 14 years,” he added.
“Contrary to the inclination to be ‘gentle’ in treating them because they are kids, these data suggest that we can’t let these initial years go by without strong intervention, and we need to be prepared for polypharmacy.”
Unfortunately, as Dr. Zeitler and coauthors explained, youth-onset type 2 diabetes is characterized by a suboptimal response to currently approved diabetes medications.
New pediatric indications in the United States for drugs used to treat type 2 diabetes in adults, including the recent Food and Drug Administration approval of extended-release exenatide for children as young as 10 years of age, “helps, but only marginally,” said Dr. Zeitler, of the Clinical & Translational Research Center, Children’s Hospital Colorado, Aurora.
“In some cases, it will help get them covered by carriers, which is always good. But this is still a very limited set of medications. It doesn’t include more recently approved more potent glucagon-like peptide-1 (GLP-1) agonists, like semaglutide, and doesn’t include the sodium-glucose cotransporter 2 (SGLT2) inhibitors. Pediatricians are used to using medications off label and that is necessary here while we await further approvals,” he said.
And he noted that most individuals with youth-onset type 2 diabetes in the United States are covered by public insurance or are uninsured, depending on which state they live in. While the two major Medicaid programs in Colorado allow full access to adult formularies, that’s not the case everywhere. Moreover, patients often face further access barriers in states without expanded Medicaid.
Follow-up shows all metrics worsening over time
In TODAY 2, patients participated in an observational follow-up in their usual care settings in 2011-2020. At the start, they were receiving metformin with or without insulin for diabetes, but whether this continued and whether they were treated for other risk factors was down to individual circumstances.
Participants’ median A1c increased over time, and the percentage with A1c < 6% (< 48 mmol/mol) declined from 75% at the time of TODAY entry to just 19% at the 15-year end of follow-up.
The proportion with an A1c ≤ 10% (≤ 86 mmol/mol) rose from 0% at baseline to 34% at 15 years.
At that time, nearly 50% were taking both metformin and insulin, while more than a quarter were taking no medications.
The prevalence of hypertension increased from 19.2% at baseline to 67.5% at 15 years, while dyslipidemia rose from 20.8% to 51.6%.
Kidney disease prevalence increased from 8.0% at baseline to 54.8% at 15 years. Nerve disease rose from 1.0% to 32.4%. Retinal disease jumped from 13.7% with milder nonproliferative retinopathy in 2010-2011 to 51.0% with any eye disease in 2017-2018, including 8.8% with moderate to severe retinal changes and 3.5% with macular edema.
Overall, at the time of the last visit, 39.9% had no diabetes complications, 31.8% had one, 21.3% had two, and 7.1% had three complications.
Serious cardiovascular events in mid-20s
There were 17 adjudicated serious cardiovascular events, including four myocardial infarctions, six heart failure events, three diagnoses of coronary artery disease, and four strokes.
Six participants died, one each from myocardial infarction, kidney failure, and drug overdose, and three from sepsis.
Dr. Zeitler called the macrovascular events “shocking,” noting that although the numbers are small, for people in their mid-20s “they should be zero ... While we don’t yet know if the rates are the same or faster than in adults, even if they are the same, these kids are only in their late 20s, as opposed to adults experiencing these problems in their 50s, 60s, and 70s.
“The fact that these complications are occurring when these individuals should be in the prime of their life for both family and work has huge implications,” he stressed.
Findings have multiple causes
The reasons for the findings are both biologic and socioeconomic, Dr. Zeitler said.
“We know already that many kids with type 2 have rapid [deterioration of] beta-cell [function], which is probably very biologic. It stands to reason that an individual who can get diabetes as an adolescent probably has more fragile beta cells in some way,” he noted.
“But we also know that many other things contribute: stress, social determinants, access to quality care and medications, access to healthy foods and physical activity, availability of family supervision given the realities of families’ economic status and jobs, etc.”
It’s also known that youth with type 2 diabetes have much more severe insulin resistance than that of adults with the condition, and that “once the kids left ... the [TODAY] study, risk factor treatment in the community was less than ideal, and a lot of kids who met criteria for treatment of their blood pressure or lipids were not being treated. This is likely at least partly sociologic and partly the general pediatric hesitancy to use medications.”
He said the TODAY team will soon have some new data to show that “glycemia during the early years makes a difference, again supporting intensive intervention early on.”
The TODAY study was supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases. Dr. Zeitler had no further disclosures.
A version of this article first appeared on Medscape.com.
Newly published data show alarmingly high rates and severity of early diabetes-specific complications in individuals who develop type 2 diabetes at a young age. This suggests intervention should be early and aggressive among these youngsters, said the researchers.
The results for the 500 young adult participants in the Treatment Options for Type 2 Diabetes in Adolescents and Youth 2 (TODAY 2) study were published online July 29 in the New England Journal of Medicine by the TODAY study group.
At follow-up – after originally participating in the TODAY trial when they were young teenagers – they had a mean age of 26.4 years.
At this time, more than two thirds had hypertension and half had dyslipidemia.
Overall, 60% had at least one diabetic microvascular complication (retinal disease, neuropathy, or diabetic kidney disease), and more than a quarter had two or more such complications.
“These data illustrate the serious personal and public health consequences of youth-onset type 2 diabetes in the transition to adulthood,” the researchers noted.
Don’t tread lightly just because they are young
“The fact that these youth are accumulating complications at a rapid rate and are broadly affected early in adulthood certainly suggests that aggressive therapy is needed, both for glycemic control and treatment of risk factors like hypertension and dyslipidemia,” study coauthor Philip S. Zeitler, MD, PhD, said in an interview.
“In the absence of studies specifically addressing this, we need to take a more aggressive approach than people might be inclined to, given that the age at diagnosis is young, around 14 years,” he added.
“Contrary to the inclination to be ‘gentle’ in treating them because they are kids, these data suggest that we can’t let these initial years go by without strong intervention, and we need to be prepared for polypharmacy.”
Unfortunately, as Dr. Zeitler and coauthors explained, youth-onset type 2 diabetes is characterized by a suboptimal response to currently approved diabetes medications.
New pediatric indications in the United States for drugs used to treat type 2 diabetes in adults, including the recent Food and Drug Administration approval of extended-release exenatide for children as young as 10 years of age, “helps, but only marginally,” said Dr. Zeitler, of the Clinical & Translational Research Center, Children’s Hospital Colorado, Aurora.
“In some cases, it will help get them covered by carriers, which is always good. But this is still a very limited set of medications. It doesn’t include more recently approved more potent glucagon-like peptide-1 (GLP-1) agonists, like semaglutide, and doesn’t include the sodium-glucose cotransporter 2 (SGLT2) inhibitors. Pediatricians are used to using medications off label and that is necessary here while we await further approvals,” he said.
And he noted that most individuals with youth-onset type 2 diabetes in the United States are covered by public insurance or are uninsured, depending on which state they live in. While the two major Medicaid programs in Colorado allow full access to adult formularies, that’s not the case everywhere. Moreover, patients often face further access barriers in states without expanded Medicaid.
Follow-up shows all metrics worsening over time
In TODAY 2, patients participated in an observational follow-up in their usual care settings in 2011-2020. At the start, they were receiving metformin with or without insulin for diabetes, but whether this continued and whether they were treated for other risk factors was down to individual circumstances.
Participants’ median A1c increased over time, and the percentage with A1c < 6% (< 48 mmol/mol) declined from 75% at the time of TODAY entry to just 19% at the 15-year end of follow-up.
The proportion with an A1c ≤ 10% (≤ 86 mmol/mol) rose from 0% at baseline to 34% at 15 years.
At that time, nearly 50% were taking both metformin and insulin, while more than a quarter were taking no medications.
The prevalence of hypertension increased from 19.2% at baseline to 67.5% at 15 years, while dyslipidemia rose from 20.8% to 51.6%.
Kidney disease prevalence increased from 8.0% at baseline to 54.8% at 15 years. Nerve disease rose from 1.0% to 32.4%. Retinal disease jumped from 13.7% with milder nonproliferative retinopathy in 2010-2011 to 51.0% with any eye disease in 2017-2018, including 8.8% with moderate to severe retinal changes and 3.5% with macular edema.
Overall, at the time of the last visit, 39.9% had no diabetes complications, 31.8% had one, 21.3% had two, and 7.1% had three complications.
Serious cardiovascular events in mid-20s
There were 17 adjudicated serious cardiovascular events, including four myocardial infarctions, six heart failure events, three diagnoses of coronary artery disease, and four strokes.
Six participants died, one each from myocardial infarction, kidney failure, and drug overdose, and three from sepsis.
Dr. Zeitler called the macrovascular events “shocking,” noting that although the numbers are small, for people in their mid-20s “they should be zero ... While we don’t yet know if the rates are the same or faster than in adults, even if they are the same, these kids are only in their late 20s, as opposed to adults experiencing these problems in their 50s, 60s, and 70s.
“The fact that these complications are occurring when these individuals should be in the prime of their life for both family and work has huge implications,” he stressed.
Findings have multiple causes
The reasons for the findings are both biologic and socioeconomic, Dr. Zeitler said.
“We know already that many kids with type 2 have rapid [deterioration of] beta-cell [function], which is probably very biologic. It stands to reason that an individual who can get diabetes as an adolescent probably has more fragile beta cells in some way,” he noted.
“But we also know that many other things contribute: stress, social determinants, access to quality care and medications, access to healthy foods and physical activity, availability of family supervision given the realities of families’ economic status and jobs, etc.”
It’s also known that youth with type 2 diabetes have much more severe insulin resistance than that of adults with the condition, and that “once the kids left ... the [TODAY] study, risk factor treatment in the community was less than ideal, and a lot of kids who met criteria for treatment of their blood pressure or lipids were not being treated. This is likely at least partly sociologic and partly the general pediatric hesitancy to use medications.”
He said the TODAY team will soon have some new data to show that “glycemia during the early years makes a difference, again supporting intensive intervention early on.”
The TODAY study was supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases. Dr. Zeitler had no further disclosures.
A version of this article first appeared on Medscape.com.