User login
Progressive Axillary Hyperpigmentation
The Diagnosis: Dowling-Degos Disease
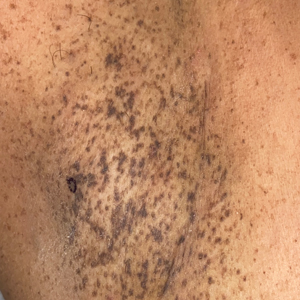
Histopathology demonstrated elongation of the epidermal rete ridges with increased basal pigmentation, suprapapillary epithelial thinning, dermal melanophages, and a mild lymphocytic infiltrate (Figure). Given the clinical and histologic findings, a diagnosis of Dowling-Degos disease (DDD) was made. The patient was counseled on the increased risk for her children developing DDD. Treatment with the erbium:YAG (Er:YAG) laser subsequently was initiated.
Dowling-Degos disease (also known as reticulate pigmented anomaly of the flexures) is an uncommon autosomal-dominant condition characterized by reticular hyperpigmentation involving the flexural and intertriginous sites. Classic DDD commonly is caused by lossof-function mutations in the keratin 5 gene, KRT51; however, DDD also may result from loss-of-function mutations in the protein O-fucosyltransferase 1, POFUT1, and protein O-glucosyltransferase 1, POGLUT1, genes.2
Rare cases of DDD associated with hidradenitis suppurativa are caused by mutations in the presenilin enhancer protein 2 gene, PSENEN.3
Of note, a missense mutation in KRT5 is implicated in epidermolysis bullosa simplex with mottled pigmentation. Onset of DDD typically occurs during the third to fourth decades of life. Reticulated hyperpigmented macules initially occur in the axillae and groin and progressively increase over time to involve the neck, inframammary folds, trunk, and flexural surfaces of the arms and thighs. Patients additionally may present with pitted perioral scars, comedolike lesions on the back and neck, epidermoid cysts, and hidradenitis suppurativa. Keratoacanthoma and squamous cell carcinoma rarely have been reported in association with classic DDD.4,5
Dowling-Degos disease usually is asymptomatic, though pruritus seldom may occur in the affected flexural areas. Histologically, the epidermal rete ridges are elongated in a filiform or antlerlike pattern with increased pigmentation of the basal layer and thinning of the suprapapillary epithelium. Dermal melanosis and a mild perivascular lymphohistiocytic infiltrate also are present with no increase in the number of melanocytes.6,7 Galli-Galli disease is a variant of DDD that shares similar clinical and histologic features of DDD but is distinguished from DDD by suprabasilar nondyskeratotic acantholysis on histology.8
Regarding other differential diagnoses for our patient, acanthosis nigricans may be distinguished clinically by the presence of velvety and/or verrucous plaques, commonly in the neck folds and axillae. Histologically, acanthosis nigricans is distinct from DDD and involves hyperkeratosis, acanthosis, and epidermal papillomatosis. Our patient had no history of diabetes mellitus or insulin resistance. Granular parakeratosis presents with hyperpigmented hyperkeratotic papules and plaques classically confined to the axillary region; however, the involvement of other intertriginous areas may occur. Histologically, granular parakeratosis demonstrates compact parakeratosis with small bluish keratohyalin granules within the stratum corneum. Confluent and reticulated papillomatosis presents with red-brown keratotic papules that initially appear in the intermammary region and spread laterally forming a reticulated pattern. Histology is similar to acanthosis nigricans and demonstrates hyperkeratosis, acanthosis, and papillomatosis. Inverse psoriasis presents with symmetric and sharply demarcated, erythematous, nonscaly plaques in the intertriginous areas. The plaques of inverse psoriasis may be pruritic and/or sore and occasionally may become macerated. Inverse psoriasis shares similar histologic findings compared to classic plaque psoriasis but may have less confluent parakeratosis.
Treatment of DDD essentially is reserved for cosmetic reasons. Topical hydroquinone, tretinoin, and corticosteroids have been used with limited to no success.5,9 Beneficial results after treatment with the Er:YAG laser have been reported.10
- Betz RC, Planko L, Eigelshoven S, et al. Loss-of-function mutations in the keratin 5 gene lead to Dowling-Degos disease. Am J Hum Genet. 2006;78:510-519.
- Basmanav FB, Oprisoreanu AM, Pasternack SM, et al. Mutations in POGLUT1, encoding protein O-glucosyltransferase 1, cause autosomaldominant Dowling-Degos disease. Am J Hum Genet. 2014;94:135-143.
- Pavlovsky M, Sarig O, Eskin-Schwartz M, et al. A phenotype combining hidradenitis suppurativa with Dowling-Degos disease caused by a founder mutation in PSENEN. Br J Dermatol. 2018;178:502-508.
- Ujihara M, Kamakura T, Ikeda M, et al. Dowling-Degos disease associated with squamous cell carcinomas on the dappled pigmentation. Br J Dermatol. 2002;147:568-571.
- Weber LA, Kantor GR, Bergfeld WF. Reticulate pigmented anomaly of the flexures (Dowling-Degos disease): a case report associated with hidradenitis suppurativa and squamous cell carcinoma. Cutis. 1990;45:446-450.
- Jones EW, Grice K. Reticulate pigmented anomaly of the flexures. Dowing Degos disease, a new genodermatosis. Arch Dermatol. 1978;114:1150-1157.
- Kim YC, Davis MD, Schanbacher CF, et al. Dowling-Degos disease (reticulate pigmented anomaly of the flexures): a clinical and histopathologic study of 6 cases. J Am Acad Dermatol. 1999; 40:462-467.
- Reisenauer AK, Wordingham SV, York J, et al. Heterozygous frameshift mutation in keratin 5 in a family with Galli-Galli disease. Br J Dermatol. 2014;170:1362-1365.
- Oppolzer G, Schwarz T, Duschet P, et al. Dowling-Degos disease: unsuccessful therapeutic trial with retinoids [in German]. Hautarzt. 1987;38:615-618.
- Wenzel G, Petrow W, Tappe K, et al. Treatment of Dowling-Degos disease with Er:YAG-laser: results after 2.5 years. Dermatol Surg. 2003;29:1161-1162.
The Diagnosis: Dowling-Degos Disease
Histopathology demonstrated elongation of the epidermal rete ridges with increased basal pigmentation, suprapapillary epithelial thinning, dermal melanophages, and a mild lymphocytic infiltrate (Figure). Given the clinical and histologic findings, a diagnosis of Dowling-Degos disease (DDD) was made. The patient was counseled on the increased risk for her children developing DDD. Treatment with the erbium:YAG (Er:YAG) laser subsequently was initiated.
Dowling-Degos disease (also known as reticulate pigmented anomaly of the flexures) is an uncommon autosomal-dominant condition characterized by reticular hyperpigmentation involving the flexural and intertriginous sites. Classic DDD commonly is caused by lossof-function mutations in the keratin 5 gene, KRT51; however, DDD also may result from loss-of-function mutations in the protein O-fucosyltransferase 1, POFUT1, and protein O-glucosyltransferase 1, POGLUT1, genes.2
Rare cases of DDD associated with hidradenitis suppurativa are caused by mutations in the presenilin enhancer protein 2 gene, PSENEN.3
Of note, a missense mutation in KRT5 is implicated in epidermolysis bullosa simplex with mottled pigmentation. Onset of DDD typically occurs during the third to fourth decades of life. Reticulated hyperpigmented macules initially occur in the axillae and groin and progressively increase over time to involve the neck, inframammary folds, trunk, and flexural surfaces of the arms and thighs. Patients additionally may present with pitted perioral scars, comedolike lesions on the back and neck, epidermoid cysts, and hidradenitis suppurativa. Keratoacanthoma and squamous cell carcinoma rarely have been reported in association with classic DDD.4,5
Dowling-Degos disease usually is asymptomatic, though pruritus seldom may occur in the affected flexural areas. Histologically, the epidermal rete ridges are elongated in a filiform or antlerlike pattern with increased pigmentation of the basal layer and thinning of the suprapapillary epithelium. Dermal melanosis and a mild perivascular lymphohistiocytic infiltrate also are present with no increase in the number of melanocytes.6,7 Galli-Galli disease is a variant of DDD that shares similar clinical and histologic features of DDD but is distinguished from DDD by suprabasilar nondyskeratotic acantholysis on histology.8
Regarding other differential diagnoses for our patient, acanthosis nigricans may be distinguished clinically by the presence of velvety and/or verrucous plaques, commonly in the neck folds and axillae. Histologically, acanthosis nigricans is distinct from DDD and involves hyperkeratosis, acanthosis, and epidermal papillomatosis. Our patient had no history of diabetes mellitus or insulin resistance. Granular parakeratosis presents with hyperpigmented hyperkeratotic papules and plaques classically confined to the axillary region; however, the involvement of other intertriginous areas may occur. Histologically, granular parakeratosis demonstrates compact parakeratosis with small bluish keratohyalin granules within the stratum corneum. Confluent and reticulated papillomatosis presents with red-brown keratotic papules that initially appear in the intermammary region and spread laterally forming a reticulated pattern. Histology is similar to acanthosis nigricans and demonstrates hyperkeratosis, acanthosis, and papillomatosis. Inverse psoriasis presents with symmetric and sharply demarcated, erythematous, nonscaly plaques in the intertriginous areas. The plaques of inverse psoriasis may be pruritic and/or sore and occasionally may become macerated. Inverse psoriasis shares similar histologic findings compared to classic plaque psoriasis but may have less confluent parakeratosis.
Treatment of DDD essentially is reserved for cosmetic reasons. Topical hydroquinone, tretinoin, and corticosteroids have been used with limited to no success.5,9 Beneficial results after treatment with the Er:YAG laser have been reported.10
The Diagnosis: Dowling-Degos Disease
Histopathology demonstrated elongation of the epidermal rete ridges with increased basal pigmentation, suprapapillary epithelial thinning, dermal melanophages, and a mild lymphocytic infiltrate (Figure). Given the clinical and histologic findings, a diagnosis of Dowling-Degos disease (DDD) was made. The patient was counseled on the increased risk for her children developing DDD. Treatment with the erbium:YAG (Er:YAG) laser subsequently was initiated.
Dowling-Degos disease (also known as reticulate pigmented anomaly of the flexures) is an uncommon autosomal-dominant condition characterized by reticular hyperpigmentation involving the flexural and intertriginous sites. Classic DDD commonly is caused by lossof-function mutations in the keratin 5 gene, KRT51; however, DDD also may result from loss-of-function mutations in the protein O-fucosyltransferase 1, POFUT1, and protein O-glucosyltransferase 1, POGLUT1, genes.2
Rare cases of DDD associated with hidradenitis suppurativa are caused by mutations in the presenilin enhancer protein 2 gene, PSENEN.3
Of note, a missense mutation in KRT5 is implicated in epidermolysis bullosa simplex with mottled pigmentation. Onset of DDD typically occurs during the third to fourth decades of life. Reticulated hyperpigmented macules initially occur in the axillae and groin and progressively increase over time to involve the neck, inframammary folds, trunk, and flexural surfaces of the arms and thighs. Patients additionally may present with pitted perioral scars, comedolike lesions on the back and neck, epidermoid cysts, and hidradenitis suppurativa. Keratoacanthoma and squamous cell carcinoma rarely have been reported in association with classic DDD.4,5
Dowling-Degos disease usually is asymptomatic, though pruritus seldom may occur in the affected flexural areas. Histologically, the epidermal rete ridges are elongated in a filiform or antlerlike pattern with increased pigmentation of the basal layer and thinning of the suprapapillary epithelium. Dermal melanosis and a mild perivascular lymphohistiocytic infiltrate also are present with no increase in the number of melanocytes.6,7 Galli-Galli disease is a variant of DDD that shares similar clinical and histologic features of DDD but is distinguished from DDD by suprabasilar nondyskeratotic acantholysis on histology.8
Regarding other differential diagnoses for our patient, acanthosis nigricans may be distinguished clinically by the presence of velvety and/or verrucous plaques, commonly in the neck folds and axillae. Histologically, acanthosis nigricans is distinct from DDD and involves hyperkeratosis, acanthosis, and epidermal papillomatosis. Our patient had no history of diabetes mellitus or insulin resistance. Granular parakeratosis presents with hyperpigmented hyperkeratotic papules and plaques classically confined to the axillary region; however, the involvement of other intertriginous areas may occur. Histologically, granular parakeratosis demonstrates compact parakeratosis with small bluish keratohyalin granules within the stratum corneum. Confluent and reticulated papillomatosis presents with red-brown keratotic papules that initially appear in the intermammary region and spread laterally forming a reticulated pattern. Histology is similar to acanthosis nigricans and demonstrates hyperkeratosis, acanthosis, and papillomatosis. Inverse psoriasis presents with symmetric and sharply demarcated, erythematous, nonscaly plaques in the intertriginous areas. The plaques of inverse psoriasis may be pruritic and/or sore and occasionally may become macerated. Inverse psoriasis shares similar histologic findings compared to classic plaque psoriasis but may have less confluent parakeratosis.
Treatment of DDD essentially is reserved for cosmetic reasons. Topical hydroquinone, tretinoin, and corticosteroids have been used with limited to no success.5,9 Beneficial results after treatment with the Er:YAG laser have been reported.10
- Betz RC, Planko L, Eigelshoven S, et al. Loss-of-function mutations in the keratin 5 gene lead to Dowling-Degos disease. Am J Hum Genet. 2006;78:510-519.
- Basmanav FB, Oprisoreanu AM, Pasternack SM, et al. Mutations in POGLUT1, encoding protein O-glucosyltransferase 1, cause autosomaldominant Dowling-Degos disease. Am J Hum Genet. 2014;94:135-143.
- Pavlovsky M, Sarig O, Eskin-Schwartz M, et al. A phenotype combining hidradenitis suppurativa with Dowling-Degos disease caused by a founder mutation in PSENEN. Br J Dermatol. 2018;178:502-508.
- Ujihara M, Kamakura T, Ikeda M, et al. Dowling-Degos disease associated with squamous cell carcinomas on the dappled pigmentation. Br J Dermatol. 2002;147:568-571.
- Weber LA, Kantor GR, Bergfeld WF. Reticulate pigmented anomaly of the flexures (Dowling-Degos disease): a case report associated with hidradenitis suppurativa and squamous cell carcinoma. Cutis. 1990;45:446-450.
- Jones EW, Grice K. Reticulate pigmented anomaly of the flexures. Dowing Degos disease, a new genodermatosis. Arch Dermatol. 1978;114:1150-1157.
- Kim YC, Davis MD, Schanbacher CF, et al. Dowling-Degos disease (reticulate pigmented anomaly of the flexures): a clinical and histopathologic study of 6 cases. J Am Acad Dermatol. 1999; 40:462-467.
- Reisenauer AK, Wordingham SV, York J, et al. Heterozygous frameshift mutation in keratin 5 in a family with Galli-Galli disease. Br J Dermatol. 2014;170:1362-1365.
- Oppolzer G, Schwarz T, Duschet P, et al. Dowling-Degos disease: unsuccessful therapeutic trial with retinoids [in German]. Hautarzt. 1987;38:615-618.
- Wenzel G, Petrow W, Tappe K, et al. Treatment of Dowling-Degos disease with Er:YAG-laser: results after 2.5 years. Dermatol Surg. 2003;29:1161-1162.
- Betz RC, Planko L, Eigelshoven S, et al. Loss-of-function mutations in the keratin 5 gene lead to Dowling-Degos disease. Am J Hum Genet. 2006;78:510-519.
- Basmanav FB, Oprisoreanu AM, Pasternack SM, et al. Mutations in POGLUT1, encoding protein O-glucosyltransferase 1, cause autosomaldominant Dowling-Degos disease. Am J Hum Genet. 2014;94:135-143.
- Pavlovsky M, Sarig O, Eskin-Schwartz M, et al. A phenotype combining hidradenitis suppurativa with Dowling-Degos disease caused by a founder mutation in PSENEN. Br J Dermatol. 2018;178:502-508.
- Ujihara M, Kamakura T, Ikeda M, et al. Dowling-Degos disease associated with squamous cell carcinomas on the dappled pigmentation. Br J Dermatol. 2002;147:568-571.
- Weber LA, Kantor GR, Bergfeld WF. Reticulate pigmented anomaly of the flexures (Dowling-Degos disease): a case report associated with hidradenitis suppurativa and squamous cell carcinoma. Cutis. 1990;45:446-450.
- Jones EW, Grice K. Reticulate pigmented anomaly of the flexures. Dowing Degos disease, a new genodermatosis. Arch Dermatol. 1978;114:1150-1157.
- Kim YC, Davis MD, Schanbacher CF, et al. Dowling-Degos disease (reticulate pigmented anomaly of the flexures): a clinical and histopathologic study of 6 cases. J Am Acad Dermatol. 1999; 40:462-467.
- Reisenauer AK, Wordingham SV, York J, et al. Heterozygous frameshift mutation in keratin 5 in a family with Galli-Galli disease. Br J Dermatol. 2014;170:1362-1365.
- Oppolzer G, Schwarz T, Duschet P, et al. Dowling-Degos disease: unsuccessful therapeutic trial with retinoids [in German]. Hautarzt. 1987;38:615-618.
- Wenzel G, Petrow W, Tappe K, et al. Treatment of Dowling-Degos disease with Er:YAG-laser: results after 2.5 years. Dermatol Surg. 2003;29:1161-1162.
A 50-year-old Hispanic woman presented with asymptomatic, progressive, brown hyperpigmentation involving the axillae, neck, upper back, and inframammary areas of 5 years’ duration. She had no other notable medical history; family history was unremarkable. She had been treated with topical hydroquinone and tretinoin by an outside physician without improvement. Physical examination revealed reticulated hyperpigmented macules and patches involving the inverse regions of the neck, axillae, and inframammary regions. Additionally, acneform pitted scars involving the perioral region were seen. A 4.0-mm punch biopsy of the right axilla was performed.
FDA rejects teplizumab for type 1 diabetes delay
The U.S. , despite narrow endorsement in a 10-7 vote in favor of approval by one of its advisory panels in May.
According to the company, the FDA did not cite any clinical deficiencies related to the efficacy and safety data packages submitted as part of the biologics license application for teplizumab.
Rather, the sticking point appears to be a study in healthy volunteers that had been raised as an issue with Provention Bio in April.
That study was designed to compare the planned commercial product with the product originally manufactured for clinical trials, but the former was not pharmacologically comparable to the latter, the FDA said in its complete response letter, issued on July 2.
The company expects, later this quarter, to obtain data from a substudy in patients receiving 12 days of therapy in the ongoing PROTECT trial of newly diagnosed patients with type 1 diabetes, which it hopes will help alleviate the FDA’s concerns.
“Upon review of the results from this substudy, the company will determine whether to submit these data to the FDA for its review ... to support pharmacokinetic comparability or otherwise justify why pharmacokinetic comparability is not necessary,” it said in its statement.
The FDA’s complete response letter had also mentioned additional issues related to product quality that Provention believes it has or will be able to address in the short term.
Teplizumab delays type 1 diabetes onset by years
Phase 2 data showing that a 14-day teplizumab infusion delayed the onset of type 1 diabetes by 2 years in high-risk relatives of people with the condition were called “game-changing” when presented at the American Diabetes Association 2019 Scientific Sessions and simultaneously published in the New England Journal of Medicine. These were the data considered by the FDA advisory panel in May.
In response to the FDA decision, the type 1 diabetes research and advocacy organization JDRF said: “It is unfortunate that the FDA has not approved teplizumab at this time and instead has requested additional information from the sponsor. We look forward to Provention Bio addressing the issues outlined in the Complete Response Letter and working with the FDA to bring this option to market safely.”
Teplizumab is one of several potential disease-modifying therapies being studied for type 1 diabetes administered either soon after diagnosis or to asymptomatic individuals with high-risk autoantibodies.
“Disease-modifying therapies such as teplizumab will help address the unmet needs of people with type 1 diabetes and those at risk for developing the disease. In the meantime, our organization will continue to support the research of other disease-modifying therapies that put us on the critical pathway to preventing and ultimately curing type 1 diabetes,” JDRF said in a statement.
A version of this article first appeared on Medscape.com.
The U.S. , despite narrow endorsement in a 10-7 vote in favor of approval by one of its advisory panels in May.
According to the company, the FDA did not cite any clinical deficiencies related to the efficacy and safety data packages submitted as part of the biologics license application for teplizumab.
Rather, the sticking point appears to be a study in healthy volunteers that had been raised as an issue with Provention Bio in April.
That study was designed to compare the planned commercial product with the product originally manufactured for clinical trials, but the former was not pharmacologically comparable to the latter, the FDA said in its complete response letter, issued on July 2.
The company expects, later this quarter, to obtain data from a substudy in patients receiving 12 days of therapy in the ongoing PROTECT trial of newly diagnosed patients with type 1 diabetes, which it hopes will help alleviate the FDA’s concerns.
“Upon review of the results from this substudy, the company will determine whether to submit these data to the FDA for its review ... to support pharmacokinetic comparability or otherwise justify why pharmacokinetic comparability is not necessary,” it said in its statement.
The FDA’s complete response letter had also mentioned additional issues related to product quality that Provention believes it has or will be able to address in the short term.
Teplizumab delays type 1 diabetes onset by years
Phase 2 data showing that a 14-day teplizumab infusion delayed the onset of type 1 diabetes by 2 years in high-risk relatives of people with the condition were called “game-changing” when presented at the American Diabetes Association 2019 Scientific Sessions and simultaneously published in the New England Journal of Medicine. These were the data considered by the FDA advisory panel in May.
In response to the FDA decision, the type 1 diabetes research and advocacy organization JDRF said: “It is unfortunate that the FDA has not approved teplizumab at this time and instead has requested additional information from the sponsor. We look forward to Provention Bio addressing the issues outlined in the Complete Response Letter and working with the FDA to bring this option to market safely.”
Teplizumab is one of several potential disease-modifying therapies being studied for type 1 diabetes administered either soon after diagnosis or to asymptomatic individuals with high-risk autoantibodies.
“Disease-modifying therapies such as teplizumab will help address the unmet needs of people with type 1 diabetes and those at risk for developing the disease. In the meantime, our organization will continue to support the research of other disease-modifying therapies that put us on the critical pathway to preventing and ultimately curing type 1 diabetes,” JDRF said in a statement.
A version of this article first appeared on Medscape.com.
The U.S. , despite narrow endorsement in a 10-7 vote in favor of approval by one of its advisory panels in May.
According to the company, the FDA did not cite any clinical deficiencies related to the efficacy and safety data packages submitted as part of the biologics license application for teplizumab.
Rather, the sticking point appears to be a study in healthy volunteers that had been raised as an issue with Provention Bio in April.
That study was designed to compare the planned commercial product with the product originally manufactured for clinical trials, but the former was not pharmacologically comparable to the latter, the FDA said in its complete response letter, issued on July 2.
The company expects, later this quarter, to obtain data from a substudy in patients receiving 12 days of therapy in the ongoing PROTECT trial of newly diagnosed patients with type 1 diabetes, which it hopes will help alleviate the FDA’s concerns.
“Upon review of the results from this substudy, the company will determine whether to submit these data to the FDA for its review ... to support pharmacokinetic comparability or otherwise justify why pharmacokinetic comparability is not necessary,” it said in its statement.
The FDA’s complete response letter had also mentioned additional issues related to product quality that Provention believes it has or will be able to address in the short term.
Teplizumab delays type 1 diabetes onset by years
Phase 2 data showing that a 14-day teplizumab infusion delayed the onset of type 1 diabetes by 2 years in high-risk relatives of people with the condition were called “game-changing” when presented at the American Diabetes Association 2019 Scientific Sessions and simultaneously published in the New England Journal of Medicine. These were the data considered by the FDA advisory panel in May.
In response to the FDA decision, the type 1 diabetes research and advocacy organization JDRF said: “It is unfortunate that the FDA has not approved teplizumab at this time and instead has requested additional information from the sponsor. We look forward to Provention Bio addressing the issues outlined in the Complete Response Letter and working with the FDA to bring this option to market safely.”
Teplizumab is one of several potential disease-modifying therapies being studied for type 1 diabetes administered either soon after diagnosis or to asymptomatic individuals with high-risk autoantibodies.
“Disease-modifying therapies such as teplizumab will help address the unmet needs of people with type 1 diabetes and those at risk for developing the disease. In the meantime, our organization will continue to support the research of other disease-modifying therapies that put us on the critical pathway to preventing and ultimately curing type 1 diabetes,” JDRF said in a statement.
A version of this article first appeared on Medscape.com.
Therapeutic Approaches in Advanced Breast Cancer
More than 280,000 women in the United States will be diagnosed with invasive breast cancer this year. For those with metastatic breast cancer with distant spread, the 5-year survival rate is approximately 28%. Whether advanced disease is discovered at initial diagnosis or in relapsed disease, it is imperative to understand the molecular characteristics of the metastatic tumor.
Dr Susan Domchek, from the University of Pennsylvania, discusses the importance of retesting for estrogen receptor, progesterone receptor, and HER2/neu on a metastatic tumor focus in order to identify potential discordance between the primary cancer and metastatic disease.
Additionally, Dr Domchek discusses the importance of molecular testing for targetable mutations, including P13K and germline BRCA1/2, for which approved therapies have shown survival benefit.
The list of targetable mutations in breast cancer continues to expand. In the tumor-agnostic studies, pembrolizumab has shown survival benefit in tumors that have mismatch repair deficiency and microsatellite instability, and TRK inhibitors have shown efficacy in tumors positive for NTRK fusions. Numerous clinical trials are available looking at additional molecular-based therapies.
--
Susan M. Domchek, MD, Basser Professor, Department of Oncology; Executive Director, Basser Center for BRCA, Abramson Cancer Center, University of Pennsylvania, Philadelphia.
Susan M. Domchek, MD, has disclosed the following relevant financial relationships: Received income in an amount equal to or greater than $250 from: AstraZeneca; Clovis; Bristol Myers Squibb.
More than 280,000 women in the United States will be diagnosed with invasive breast cancer this year. For those with metastatic breast cancer with distant spread, the 5-year survival rate is approximately 28%. Whether advanced disease is discovered at initial diagnosis or in relapsed disease, it is imperative to understand the molecular characteristics of the metastatic tumor.
Dr Susan Domchek, from the University of Pennsylvania, discusses the importance of retesting for estrogen receptor, progesterone receptor, and HER2/neu on a metastatic tumor focus in order to identify potential discordance between the primary cancer and metastatic disease.
Additionally, Dr Domchek discusses the importance of molecular testing for targetable mutations, including P13K and germline BRCA1/2, for which approved therapies have shown survival benefit.
The list of targetable mutations in breast cancer continues to expand. In the tumor-agnostic studies, pembrolizumab has shown survival benefit in tumors that have mismatch repair deficiency and microsatellite instability, and TRK inhibitors have shown efficacy in tumors positive for NTRK fusions. Numerous clinical trials are available looking at additional molecular-based therapies.
--
Susan M. Domchek, MD, Basser Professor, Department of Oncology; Executive Director, Basser Center for BRCA, Abramson Cancer Center, University of Pennsylvania, Philadelphia.
Susan M. Domchek, MD, has disclosed the following relevant financial relationships: Received income in an amount equal to or greater than $250 from: AstraZeneca; Clovis; Bristol Myers Squibb.
More than 280,000 women in the United States will be diagnosed with invasive breast cancer this year. For those with metastatic breast cancer with distant spread, the 5-year survival rate is approximately 28%. Whether advanced disease is discovered at initial diagnosis or in relapsed disease, it is imperative to understand the molecular characteristics of the metastatic tumor.
Dr Susan Domchek, from the University of Pennsylvania, discusses the importance of retesting for estrogen receptor, progesterone receptor, and HER2/neu on a metastatic tumor focus in order to identify potential discordance between the primary cancer and metastatic disease.
Additionally, Dr Domchek discusses the importance of molecular testing for targetable mutations, including P13K and germline BRCA1/2, for which approved therapies have shown survival benefit.
The list of targetable mutations in breast cancer continues to expand. In the tumor-agnostic studies, pembrolizumab has shown survival benefit in tumors that have mismatch repair deficiency and microsatellite instability, and TRK inhibitors have shown efficacy in tumors positive for NTRK fusions. Numerous clinical trials are available looking at additional molecular-based therapies.
--
Susan M. Domchek, MD, Basser Professor, Department of Oncology; Executive Director, Basser Center for BRCA, Abramson Cancer Center, University of Pennsylvania, Philadelphia.
Susan M. Domchek, MD, has disclosed the following relevant financial relationships: Received income in an amount equal to or greater than $250 from: AstraZeneca; Clovis; Bristol Myers Squibb.

Musical instruments can throw skin out of tune
Violin and viola players can pay a price for the music they create: Many suffer from skin irritation and inflammation where the instruments touch their necks and upper bodies.
“These skin conditions are disfiguring, and they also carry so much psychological burden. Not only are these patients under constant pressure to perform at their maximum at all times, it really is troublesome when there is a barrier between you and performing art that you absolutely love,” lead author Henry Lim, an osteopathic medical student at the University of North Texas Health Science Center at Fort Worth, said in an interview.
The results of the literature review were presented in a poster at the Inaugural Symposium for Inflammatory Skin Disease.
Mr. Lim, who has a special interest in skin, said his own musical experience inspired the research. “Throughout my experience as a violinist, I faced many dermatologic issues because of my violin, and it affected my performance,” he said. “As time went on, I recognized that many other stringed instrumentalists were dealing with similar issues but chose to live with it because it came with the territory.”
One physician told Mr. Lim that he needed to quit in order to permanently treat his skin problems. He didn’t accept this answer and instead launched the literature review with colleagues Marshall Hall, MPH, also an osteopathic medical student with an interest in dermatology, and Sajid Surve, DO, codirector of the UNT Texas Center for Performing Arts Health.
Mr. Lim and colleagues evaluated 23 articles, which included case studies and literature reviews, about dermatitis in violinists, violists, cellists, bassists, guitarists and harpists. “Stringed instrumentalists are the highest at-risk population compared to performers who play other types of instruments,” Mr. Lim said.
The poster he presented at the meeting largely focuses on fiddler’s neck, which he defined as “simply dermatitis related to friction and allergic irritation from playing violin or viola.” Many people, he noted, are allergic to nickel, and the bracket that secures the violin’s chin rest “most often contains nickel. Even a very small concentration of nickel can cause massive reactions, and we found that the C string of a viola – the thickest, lowest-sounding string – contains a nickel concentration of up to 37%.”
Gold-coated strings are an alternative option, he said, but they’re more expensive.
Stringed instrumentalists may also be allergic to rosin applied to “bow hairs,” which is the hair – typically from horses – that is used to string bows, also described in the poster. “We found that there is an overall common allergy to the main ingredient called colophony,” Mr. Lim said. The legendary violin maker Antonio Stradivari “was rumored to have used colophony and another irritating ingredient called propolis in the wood varnish of his instruments. Because he was such a great influence on the art of violin crafting, his technique is still used in the modern era, which may be another contributing factor to the allergic reactions seen in stringed instrumentalists.”
(In the poster, the authors refer to one of the articles in the review, which described a violin maker allergic to colophony and propolis, who was treated with cetirizine, mild corticosteroids, and avoidance.)
What should dermatologists know about skin conditions in these musicians? Mr. Hall, one of the coauthors of the report, suggested they invite the patients to play their instruments during a visit. “The musicians may not understand that they are doing certain things with their movements, but looking from a clinical lens, we are able to see how their biomechanics and posture [are] contributing to their dermatitis,” he said.
Dr. Surve, the other coauthor, also suggested speaking to the patient’s teacher, coach, or mentor. “Keeping that person in the loop regarding what you are seeing and recommending will go a long way towards helping your patient,” he said. “If the teacher doesn’t understand or agree with what you’re trying to accomplish, they may try to undermine your plan of care. But if they are on board, they become a valuable tool for facilitating and reinforcing it.”
As for treatments, avoidance of the instruments is the most effective, but is simply not feasible for many musicians. “Certain interventions like creating a barrier between the musician and the instrument can reduce the risk of contact dermatitis without compromising the quality [of playing] as much,” Mr. Hall said. The poster reported that a handkerchief was used for this purpose in one case attributed to nickel sulfate in a 16-year-old .
Purchasing more expensive instrument materials to prevent reactions is another option, he said, and players can also purchase stands. But musicians may be resistant to any treatment that changes how the instruments sound or forces them to adjust the way they do things, he cautioned.
No funding for the study or author disclosures were reported.
Violin and viola players can pay a price for the music they create: Many suffer from skin irritation and inflammation where the instruments touch their necks and upper bodies.
“These skin conditions are disfiguring, and they also carry so much psychological burden. Not only are these patients under constant pressure to perform at their maximum at all times, it really is troublesome when there is a barrier between you and performing art that you absolutely love,” lead author Henry Lim, an osteopathic medical student at the University of North Texas Health Science Center at Fort Worth, said in an interview.
The results of the literature review were presented in a poster at the Inaugural Symposium for Inflammatory Skin Disease.
Mr. Lim, who has a special interest in skin, said his own musical experience inspired the research. “Throughout my experience as a violinist, I faced many dermatologic issues because of my violin, and it affected my performance,” he said. “As time went on, I recognized that many other stringed instrumentalists were dealing with similar issues but chose to live with it because it came with the territory.”
One physician told Mr. Lim that he needed to quit in order to permanently treat his skin problems. He didn’t accept this answer and instead launched the literature review with colleagues Marshall Hall, MPH, also an osteopathic medical student with an interest in dermatology, and Sajid Surve, DO, codirector of the UNT Texas Center for Performing Arts Health.
Mr. Lim and colleagues evaluated 23 articles, which included case studies and literature reviews, about dermatitis in violinists, violists, cellists, bassists, guitarists and harpists. “Stringed instrumentalists are the highest at-risk population compared to performers who play other types of instruments,” Mr. Lim said.
The poster he presented at the meeting largely focuses on fiddler’s neck, which he defined as “simply dermatitis related to friction and allergic irritation from playing violin or viola.” Many people, he noted, are allergic to nickel, and the bracket that secures the violin’s chin rest “most often contains nickel. Even a very small concentration of nickel can cause massive reactions, and we found that the C string of a viola – the thickest, lowest-sounding string – contains a nickel concentration of up to 37%.”
Gold-coated strings are an alternative option, he said, but they’re more expensive.
Stringed instrumentalists may also be allergic to rosin applied to “bow hairs,” which is the hair – typically from horses – that is used to string bows, also described in the poster. “We found that there is an overall common allergy to the main ingredient called colophony,” Mr. Lim said. The legendary violin maker Antonio Stradivari “was rumored to have used colophony and another irritating ingredient called propolis in the wood varnish of his instruments. Because he was such a great influence on the art of violin crafting, his technique is still used in the modern era, which may be another contributing factor to the allergic reactions seen in stringed instrumentalists.”
(In the poster, the authors refer to one of the articles in the review, which described a violin maker allergic to colophony and propolis, who was treated with cetirizine, mild corticosteroids, and avoidance.)
What should dermatologists know about skin conditions in these musicians? Mr. Hall, one of the coauthors of the report, suggested they invite the patients to play their instruments during a visit. “The musicians may not understand that they are doing certain things with their movements, but looking from a clinical lens, we are able to see how their biomechanics and posture [are] contributing to their dermatitis,” he said.
Dr. Surve, the other coauthor, also suggested speaking to the patient’s teacher, coach, or mentor. “Keeping that person in the loop regarding what you are seeing and recommending will go a long way towards helping your patient,” he said. “If the teacher doesn’t understand or agree with what you’re trying to accomplish, they may try to undermine your plan of care. But if they are on board, they become a valuable tool for facilitating and reinforcing it.”
As for treatments, avoidance of the instruments is the most effective, but is simply not feasible for many musicians. “Certain interventions like creating a barrier between the musician and the instrument can reduce the risk of contact dermatitis without compromising the quality [of playing] as much,” Mr. Hall said. The poster reported that a handkerchief was used for this purpose in one case attributed to nickel sulfate in a 16-year-old .
Purchasing more expensive instrument materials to prevent reactions is another option, he said, and players can also purchase stands. But musicians may be resistant to any treatment that changes how the instruments sound or forces them to adjust the way they do things, he cautioned.
No funding for the study or author disclosures were reported.
Violin and viola players can pay a price for the music they create: Many suffer from skin irritation and inflammation where the instruments touch their necks and upper bodies.
“These skin conditions are disfiguring, and they also carry so much psychological burden. Not only are these patients under constant pressure to perform at their maximum at all times, it really is troublesome when there is a barrier between you and performing art that you absolutely love,” lead author Henry Lim, an osteopathic medical student at the University of North Texas Health Science Center at Fort Worth, said in an interview.
The results of the literature review were presented in a poster at the Inaugural Symposium for Inflammatory Skin Disease.
Mr. Lim, who has a special interest in skin, said his own musical experience inspired the research. “Throughout my experience as a violinist, I faced many dermatologic issues because of my violin, and it affected my performance,” he said. “As time went on, I recognized that many other stringed instrumentalists were dealing with similar issues but chose to live with it because it came with the territory.”
One physician told Mr. Lim that he needed to quit in order to permanently treat his skin problems. He didn’t accept this answer and instead launched the literature review with colleagues Marshall Hall, MPH, also an osteopathic medical student with an interest in dermatology, and Sajid Surve, DO, codirector of the UNT Texas Center for Performing Arts Health.
Mr. Lim and colleagues evaluated 23 articles, which included case studies and literature reviews, about dermatitis in violinists, violists, cellists, bassists, guitarists and harpists. “Stringed instrumentalists are the highest at-risk population compared to performers who play other types of instruments,” Mr. Lim said.
The poster he presented at the meeting largely focuses on fiddler’s neck, which he defined as “simply dermatitis related to friction and allergic irritation from playing violin or viola.” Many people, he noted, are allergic to nickel, and the bracket that secures the violin’s chin rest “most often contains nickel. Even a very small concentration of nickel can cause massive reactions, and we found that the C string of a viola – the thickest, lowest-sounding string – contains a nickel concentration of up to 37%.”
Gold-coated strings are an alternative option, he said, but they’re more expensive.
Stringed instrumentalists may also be allergic to rosin applied to “bow hairs,” which is the hair – typically from horses – that is used to string bows, also described in the poster. “We found that there is an overall common allergy to the main ingredient called colophony,” Mr. Lim said. The legendary violin maker Antonio Stradivari “was rumored to have used colophony and another irritating ingredient called propolis in the wood varnish of his instruments. Because he was such a great influence on the art of violin crafting, his technique is still used in the modern era, which may be another contributing factor to the allergic reactions seen in stringed instrumentalists.”
(In the poster, the authors refer to one of the articles in the review, which described a violin maker allergic to colophony and propolis, who was treated with cetirizine, mild corticosteroids, and avoidance.)
What should dermatologists know about skin conditions in these musicians? Mr. Hall, one of the coauthors of the report, suggested they invite the patients to play their instruments during a visit. “The musicians may not understand that they are doing certain things with their movements, but looking from a clinical lens, we are able to see how their biomechanics and posture [are] contributing to their dermatitis,” he said.
Dr. Surve, the other coauthor, also suggested speaking to the patient’s teacher, coach, or mentor. “Keeping that person in the loop regarding what you are seeing and recommending will go a long way towards helping your patient,” he said. “If the teacher doesn’t understand or agree with what you’re trying to accomplish, they may try to undermine your plan of care. But if they are on board, they become a valuable tool for facilitating and reinforcing it.”
As for treatments, avoidance of the instruments is the most effective, but is simply not feasible for many musicians. “Certain interventions like creating a barrier between the musician and the instrument can reduce the risk of contact dermatitis without compromising the quality [of playing] as much,” Mr. Hall said. The poster reported that a handkerchief was used for this purpose in one case attributed to nickel sulfate in a 16-year-old .
Purchasing more expensive instrument materials to prevent reactions is another option, he said, and players can also purchase stands. But musicians may be resistant to any treatment that changes how the instruments sound or forces them to adjust the way they do things, he cautioned.
No funding for the study or author disclosures were reported.
FROM SISD 2021
3 cases of hormone therapy optimized to match the patient problem
There are dozens of medications containing combinations of estrogen and progestin. I am often confused by the bewildering proliferation of generic brand names used to describe the same estrogen-progestin (E-P) regimen. For example, the combination medication containing ethinyl estradiol 20 µg plus norethindrone acetate (NEA) 1 mg is available under at least 5 different names: Lo Estrin 1/20 (Warner Chilcot), Junel 1/20 (Teva Pharmaceuticals), Microgestin Fe 1/20 (Mayne Pharma), Gildess 1/20 (Qualitest Pharmaceuticals), and Larin 1/20 (Novast Laboratories). To reduce the confusion, it is often useful to select a single preferred estrogen and progestin and use the dose combinations that are available to treat a wide range of gynecology problems (TABLE). In this editorial I focus on using various dose combinations of ethinyl estradiol and NEA to treat 3 common gynecologic problems.

CASE 1 Polycystic ovary syndrome
A 19-year-old woman reports 4 spontaneous menses in the past year and bothersome facial hair and acne. Her total testosterone concentration is at the upper limit of normal (0.46 ng/mL) and her sex hormone binding globulin (SHBG) concentration is at the lower limit of normal (35 nM). For treatment of the patient’s menstrual disorder, what is an optimal E-P combination?
Prioritize the use of an estrogen-dominant medication
Based on the Rotterdam criteria this woman has polycystic ovary syndrome (PCOS).1 In women with PCOS, luteinizing hormone (LH) secretion is increased, stimulating excessive ovarian production of testosterone.2 In addition, many women with PCOS have decreased hepatic secretion of SHBG, a binding protein that prevents testosterone from entering cells, resulting in excessive bioavailable testosterone.3 The Endocrine Society recommends that women with PCOS who have menstrual dysfunction or hirsutism be treated initially with a combination E-P hormone medication.1 Combination E-P medications suppress pituitary secretion of LH, thereby reducing ovarian production of testosterone, and ethinyl estradiol increases hepatic secretion of SHBG, reducing bioavailable testosterone. These two goals are best accomplished with an oral E-P hormone medication containing ethinyl estradiol doses of 20 µg to 30 µg per pill. An E-P hormone medication containing pills with an ethinyl estradiol dose ≤ 10 µg-daily may stimulate less hepatic production of SHBG than a pill with an ethinyl estradiol dose of 20 µg or 30 µg daily.4,5 In addition, E-P pills containing levonorgestrel suppress SHBG hormone secretion compared with E-P pills with other progestins.6 Therefore, levonorgestrel-containing E-P pills should not be prioritized for use in women with PCOS because the estrogen-induced increase in SHBG will be blunted by levonorgestrel.
CASE 2 Moderate to severe pelvic pain caused by endometriosis
A 25-year-old woman (G0) with severe dysmenorrhea had a laparoscopy showing endometriosis lesions in the cul-de-sac and a peritoneal window near the left uterosacral ligament. Biopsy showed endometriosis. Postoperatively, the patient was treated with an E-P pill containing 30 µg ethinyl estradiol and 0.15 mg desogestrel per pill using a continuous-dosing protocol. During the year following the laparoscopy, her pelvic pain symptoms gradually increased until they became severe, preventing her from performing daily activities on multiple days per month. She was prescribed elagolix but her insurance did not approve the treatment. What alternative treatment would you prescribe?
Continue to: Use progestin-dominant pills to treat pelvic pain...
Use progestin-dominant pills to treat pelvic pain
Cellular activity in endometriosis lesions is stimulated by estradiol and inhibited by a high concentration of androgenic progestins or androgens. This simplified endocrine paradigm explains the effectiveness of hormonal treatments that suppress ovarian estradiol production, including leuprolide, elagolix, medroxyprogesterone acetate, and NEA. For the woman in the above case, I would advocate for elagolix treatment but, following the insurance denial of the prescription, an alternative treatment for moderate or severe pelvic pain caused by endometriosis would be a progestin-dominant hormone medication (for example, NEA 5 mg daily). Norethindrone acetate 5 mg daily may be associated with bothersome adverse effects including weight gain (16% of patients; mean weight gain, 3.1 kg), acne (10%), mood lability (9%), hot flashes (8%), depression (6%), scalp hair loss (4%), headache (4%), nausea (3%), and deepening of the voice (1%).7
I sometimes see women with moderate to severe pelvic pain caused by endometriosis being treated with norethindrone 0.35 mg daily. This dose of norethindrone is suboptimal for pain treatment because it does not reliably suppress ovarian production of estradiol. In addition, the cells in endometriosis lesions are often resistant to the effects of progesterone, requiring higher dosages to produce secretory or decidual changes. In most situations, I recommend against the use of norethindrone 0.35 mg daily for the treatment of pelvic pain caused by endometriosis.
Patients commonly ask if NEA 5 mg daily has contraceptive efficacy. Although it is not approved at this dosage by the US Food and Drug Administration as a contraceptive,8 norethindrone 0.35 mg daily is approved as a progestin-only contraceptive.9 Norethindrone acetate is rapidly and completely deacetylated to norethindrone and the disposition of oral NEA is indistinguishable from that of norethindrone (which is the FDA-approved dosage mentioned above). Since norethindrone 0.35 mg daily is approved as a contraceptive, it is highly likely that NEA 5 mg daily has contraceptive efficacy, especially if there is good adherence with the daily medication.
CASE 3 Perimenopausal AUB
A 45-year-old woman reports varying menstrual cycle lengths from 24 to 60 days with very heavy menses in some cycles. Pelvic ultrasonography shows no abnormality. Endometrial biopsy shows a proliferative endometrium. Her serum progesterone level, obtained 1 week before the onset of menses, is < 3 ng/mL. She has no past history of heavy menses, easy bruising, excessive bleeding with procedures, or a family history of bleeding problems. She also reports occasional hot flashes that wake her from sleep.
Use an estrogen step-down regimen to manage postmenopause transition
This patient is likely in the perimenopause transition, and the abnormal uterine bleeding (AUB) is caused, in part, by oligo- or anovulation. Perimenopausal women with AUB may have cycles characterized by above normal ovarian estradiol production and below normal progesterone production, or frank anovulation.10 Elevated ovarian estrogen and low progesterone production sets the stage for heavy bleeding in the perimenopause, regardless of the presence of uterine pathology such as fibroids.
For perimenopausal women, one option for treatment of AUB due to anovulation is to prescribe an estrogen step-down regimen. For the 45-year-old woman in this case, initiating treatment with an E-P pill containing ethinyl estradiol 10 µg and NEA 1 mg will likely control the AUB and her occasional hot flash.11 As the woman ages, the ethinyl estradiol dose can be decreased to pills containing 5 µg and then 2.5 µg, covering the transition into postmenopause. Once the woman is in the postmenopause, treatment with transdermal estradiol and oral micronized progesterone is an option to treat menopausal vasomotor symptoms.
Optimize estrogen and progestin treatment for your patients
Many gynecologic problems are effectively treated by estrogen and/or progestin steroids. The dose of estrogen and progestin should be tailored to the specific problem. For PCOS, the estrogen dose selected should be sufficient to safely stimulate hepatic SHBG production. For endometriosis, if a GnRH antagonist is not available to the patient, a high-dose progestin, such as NEA 5 mg, may be an effective treatment. During the perimenopause transition in a woman with AUB, a treatment plan using a sequential E-P step-down program might control symptoms and help smoothly glide the patient into the postmenopause. ●
- Legro RS, Arslanian SA, Ehrmann DA, et al. Diagnosis and treatment of polycystic ovary syndrome: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2013;98:4565-4592. doi: 10.1210/jc.2013-2350.
- Rosenfield RL, Ehrmann DA. The pathogenesis of polycystic ovary syndrome (PCOS): the hypothesis of PCOS as functional ovarian hyperandrogenism revisited. Endocr Rev. 2016;37:467-520. doi: 10.1210/er.2015-1104.
- Zhu JL, Chen Z, Feng WJ, et al. Sex hormone-binding globulin and polycystic ovary syndrome. Clin Chim Acta. 2019;499:142-148. doi: 10.1016/j.cca.2019.09.010.
- Oner G, Muderris II. A prospective randomized trial comparing low-dose ethinyl estradiol and drospirenone 24/4 combined oral contraceptive vs. ethinyl estradiol and drospirenone 21/7 combined oral contraceptive in the treatment of hirsutism. Contraception. 2011;84:508-511. doi: 10.1016/j.contraception.2011.03.002.
- Boyd RA, Zegarac EA, Posvar EL, et al. Minimal androgenic activity of a new oral contraceptive containing norethindrone acetate and graduated doses of ethinyl estradiol. Contraception. 2001;63:71-76. doi: 10.1016/s0010-7824(01)00179-2.
- Thorneycroft IH, Stanczyk FZ, Bradshaw KD, et al. Effect of low-dose oral contraceptives on androgenic markers and acne. Contraception. 1999;60:255-262. doi: 10.1016/s0010-7824(99)00093-1.
- Kaser DJ, Missmer SA, Berry KF, et al. Use of norethindrone acetate alone for postoperative suppression of endometriosis symptoms. J Pediatr Adolesc Gynecol. 2012;25:105-108. doi: 10.1016/j.jpag.2011.09.013.
- Aygestin [package insert]. Pomona, NY: Duramed Pharmaceuticals; 2007.
- Camila [package insert]. Greenville, NC; Mayne Pharma; 2018.
- Santoro N, Brown JR, Adel T, et al. Characterization of reproductive hormonal dynamics in the perimenopause. J Clin Endocrinol Metab. 1996;81:1495-1501. doi: 10.1210/jcem.81.4.8636357.
- Speroff L, Symons J, Kempfert N, et al; FemHrt Study Investigators. The effect of varying low-dose combinations of norethindrone acetate and ethinyl estradiol (Femhrt) on the frequency and intensity of vasomotor symptoms. Menopause. 2000;7:383-390. doi: 10.1097/00042192-200011000-00003.
There are dozens of medications containing combinations of estrogen and progestin. I am often confused by the bewildering proliferation of generic brand names used to describe the same estrogen-progestin (E-P) regimen. For example, the combination medication containing ethinyl estradiol 20 µg plus norethindrone acetate (NEA) 1 mg is available under at least 5 different names: Lo Estrin 1/20 (Warner Chilcot), Junel 1/20 (Teva Pharmaceuticals), Microgestin Fe 1/20 (Mayne Pharma), Gildess 1/20 (Qualitest Pharmaceuticals), and Larin 1/20 (Novast Laboratories). To reduce the confusion, it is often useful to select a single preferred estrogen and progestin and use the dose combinations that are available to treat a wide range of gynecology problems (TABLE). In this editorial I focus on using various dose combinations of ethinyl estradiol and NEA to treat 3 common gynecologic problems.

CASE 1 Polycystic ovary syndrome
A 19-year-old woman reports 4 spontaneous menses in the past year and bothersome facial hair and acne. Her total testosterone concentration is at the upper limit of normal (0.46 ng/mL) and her sex hormone binding globulin (SHBG) concentration is at the lower limit of normal (35 nM). For treatment of the patient’s menstrual disorder, what is an optimal E-P combination?
Prioritize the use of an estrogen-dominant medication
Based on the Rotterdam criteria this woman has polycystic ovary syndrome (PCOS).1 In women with PCOS, luteinizing hormone (LH) secretion is increased, stimulating excessive ovarian production of testosterone.2 In addition, many women with PCOS have decreased hepatic secretion of SHBG, a binding protein that prevents testosterone from entering cells, resulting in excessive bioavailable testosterone.3 The Endocrine Society recommends that women with PCOS who have menstrual dysfunction or hirsutism be treated initially with a combination E-P hormone medication.1 Combination E-P medications suppress pituitary secretion of LH, thereby reducing ovarian production of testosterone, and ethinyl estradiol increases hepatic secretion of SHBG, reducing bioavailable testosterone. These two goals are best accomplished with an oral E-P hormone medication containing ethinyl estradiol doses of 20 µg to 30 µg per pill. An E-P hormone medication containing pills with an ethinyl estradiol dose ≤ 10 µg-daily may stimulate less hepatic production of SHBG than a pill with an ethinyl estradiol dose of 20 µg or 30 µg daily.4,5 In addition, E-P pills containing levonorgestrel suppress SHBG hormone secretion compared with E-P pills with other progestins.6 Therefore, levonorgestrel-containing E-P pills should not be prioritized for use in women with PCOS because the estrogen-induced increase in SHBG will be blunted by levonorgestrel.
CASE 2 Moderate to severe pelvic pain caused by endometriosis
A 25-year-old woman (G0) with severe dysmenorrhea had a laparoscopy showing endometriosis lesions in the cul-de-sac and a peritoneal window near the left uterosacral ligament. Biopsy showed endometriosis. Postoperatively, the patient was treated with an E-P pill containing 30 µg ethinyl estradiol and 0.15 mg desogestrel per pill using a continuous-dosing protocol. During the year following the laparoscopy, her pelvic pain symptoms gradually increased until they became severe, preventing her from performing daily activities on multiple days per month. She was prescribed elagolix but her insurance did not approve the treatment. What alternative treatment would you prescribe?
Continue to: Use progestin-dominant pills to treat pelvic pain...
Use progestin-dominant pills to treat pelvic pain
Cellular activity in endometriosis lesions is stimulated by estradiol and inhibited by a high concentration of androgenic progestins or androgens. This simplified endocrine paradigm explains the effectiveness of hormonal treatments that suppress ovarian estradiol production, including leuprolide, elagolix, medroxyprogesterone acetate, and NEA. For the woman in the above case, I would advocate for elagolix treatment but, following the insurance denial of the prescription, an alternative treatment for moderate or severe pelvic pain caused by endometriosis would be a progestin-dominant hormone medication (for example, NEA 5 mg daily). Norethindrone acetate 5 mg daily may be associated with bothersome adverse effects including weight gain (16% of patients; mean weight gain, 3.1 kg), acne (10%), mood lability (9%), hot flashes (8%), depression (6%), scalp hair loss (4%), headache (4%), nausea (3%), and deepening of the voice (1%).7
I sometimes see women with moderate to severe pelvic pain caused by endometriosis being treated with norethindrone 0.35 mg daily. This dose of norethindrone is suboptimal for pain treatment because it does not reliably suppress ovarian production of estradiol. In addition, the cells in endometriosis lesions are often resistant to the effects of progesterone, requiring higher dosages to produce secretory or decidual changes. In most situations, I recommend against the use of norethindrone 0.35 mg daily for the treatment of pelvic pain caused by endometriosis.
Patients commonly ask if NEA 5 mg daily has contraceptive efficacy. Although it is not approved at this dosage by the US Food and Drug Administration as a contraceptive,8 norethindrone 0.35 mg daily is approved as a progestin-only contraceptive.9 Norethindrone acetate is rapidly and completely deacetylated to norethindrone and the disposition of oral NEA is indistinguishable from that of norethindrone (which is the FDA-approved dosage mentioned above). Since norethindrone 0.35 mg daily is approved as a contraceptive, it is highly likely that NEA 5 mg daily has contraceptive efficacy, especially if there is good adherence with the daily medication.
CASE 3 Perimenopausal AUB
A 45-year-old woman reports varying menstrual cycle lengths from 24 to 60 days with very heavy menses in some cycles. Pelvic ultrasonography shows no abnormality. Endometrial biopsy shows a proliferative endometrium. Her serum progesterone level, obtained 1 week before the onset of menses, is < 3 ng/mL. She has no past history of heavy menses, easy bruising, excessive bleeding with procedures, or a family history of bleeding problems. She also reports occasional hot flashes that wake her from sleep.
Use an estrogen step-down regimen to manage postmenopause transition
This patient is likely in the perimenopause transition, and the abnormal uterine bleeding (AUB) is caused, in part, by oligo- or anovulation. Perimenopausal women with AUB may have cycles characterized by above normal ovarian estradiol production and below normal progesterone production, or frank anovulation.10 Elevated ovarian estrogen and low progesterone production sets the stage for heavy bleeding in the perimenopause, regardless of the presence of uterine pathology such as fibroids.
For perimenopausal women, one option for treatment of AUB due to anovulation is to prescribe an estrogen step-down regimen. For the 45-year-old woman in this case, initiating treatment with an E-P pill containing ethinyl estradiol 10 µg and NEA 1 mg will likely control the AUB and her occasional hot flash.11 As the woman ages, the ethinyl estradiol dose can be decreased to pills containing 5 µg and then 2.5 µg, covering the transition into postmenopause. Once the woman is in the postmenopause, treatment with transdermal estradiol and oral micronized progesterone is an option to treat menopausal vasomotor symptoms.
Optimize estrogen and progestin treatment for your patients
Many gynecologic problems are effectively treated by estrogen and/or progestin steroids. The dose of estrogen and progestin should be tailored to the specific problem. For PCOS, the estrogen dose selected should be sufficient to safely stimulate hepatic SHBG production. For endometriosis, if a GnRH antagonist is not available to the patient, a high-dose progestin, such as NEA 5 mg, may be an effective treatment. During the perimenopause transition in a woman with AUB, a treatment plan using a sequential E-P step-down program might control symptoms and help smoothly glide the patient into the postmenopause. ●
There are dozens of medications containing combinations of estrogen and progestin. I am often confused by the bewildering proliferation of generic brand names used to describe the same estrogen-progestin (E-P) regimen. For example, the combination medication containing ethinyl estradiol 20 µg plus norethindrone acetate (NEA) 1 mg is available under at least 5 different names: Lo Estrin 1/20 (Warner Chilcot), Junel 1/20 (Teva Pharmaceuticals), Microgestin Fe 1/20 (Mayne Pharma), Gildess 1/20 (Qualitest Pharmaceuticals), and Larin 1/20 (Novast Laboratories). To reduce the confusion, it is often useful to select a single preferred estrogen and progestin and use the dose combinations that are available to treat a wide range of gynecology problems (TABLE). In this editorial I focus on using various dose combinations of ethinyl estradiol and NEA to treat 3 common gynecologic problems.

CASE 1 Polycystic ovary syndrome
A 19-year-old woman reports 4 spontaneous menses in the past year and bothersome facial hair and acne. Her total testosterone concentration is at the upper limit of normal (0.46 ng/mL) and her sex hormone binding globulin (SHBG) concentration is at the lower limit of normal (35 nM). For treatment of the patient’s menstrual disorder, what is an optimal E-P combination?
Prioritize the use of an estrogen-dominant medication
Based on the Rotterdam criteria this woman has polycystic ovary syndrome (PCOS).1 In women with PCOS, luteinizing hormone (LH) secretion is increased, stimulating excessive ovarian production of testosterone.2 In addition, many women with PCOS have decreased hepatic secretion of SHBG, a binding protein that prevents testosterone from entering cells, resulting in excessive bioavailable testosterone.3 The Endocrine Society recommends that women with PCOS who have menstrual dysfunction or hirsutism be treated initially with a combination E-P hormone medication.1 Combination E-P medications suppress pituitary secretion of LH, thereby reducing ovarian production of testosterone, and ethinyl estradiol increases hepatic secretion of SHBG, reducing bioavailable testosterone. These two goals are best accomplished with an oral E-P hormone medication containing ethinyl estradiol doses of 20 µg to 30 µg per pill. An E-P hormone medication containing pills with an ethinyl estradiol dose ≤ 10 µg-daily may stimulate less hepatic production of SHBG than a pill with an ethinyl estradiol dose of 20 µg or 30 µg daily.4,5 In addition, E-P pills containing levonorgestrel suppress SHBG hormone secretion compared with E-P pills with other progestins.6 Therefore, levonorgestrel-containing E-P pills should not be prioritized for use in women with PCOS because the estrogen-induced increase in SHBG will be blunted by levonorgestrel.
CASE 2 Moderate to severe pelvic pain caused by endometriosis
A 25-year-old woman (G0) with severe dysmenorrhea had a laparoscopy showing endometriosis lesions in the cul-de-sac and a peritoneal window near the left uterosacral ligament. Biopsy showed endometriosis. Postoperatively, the patient was treated with an E-P pill containing 30 µg ethinyl estradiol and 0.15 mg desogestrel per pill using a continuous-dosing protocol. During the year following the laparoscopy, her pelvic pain symptoms gradually increased until they became severe, preventing her from performing daily activities on multiple days per month. She was prescribed elagolix but her insurance did not approve the treatment. What alternative treatment would you prescribe?
Continue to: Use progestin-dominant pills to treat pelvic pain...
Use progestin-dominant pills to treat pelvic pain
Cellular activity in endometriosis lesions is stimulated by estradiol and inhibited by a high concentration of androgenic progestins or androgens. This simplified endocrine paradigm explains the effectiveness of hormonal treatments that suppress ovarian estradiol production, including leuprolide, elagolix, medroxyprogesterone acetate, and NEA. For the woman in the above case, I would advocate for elagolix treatment but, following the insurance denial of the prescription, an alternative treatment for moderate or severe pelvic pain caused by endometriosis would be a progestin-dominant hormone medication (for example, NEA 5 mg daily). Norethindrone acetate 5 mg daily may be associated with bothersome adverse effects including weight gain (16% of patients; mean weight gain, 3.1 kg), acne (10%), mood lability (9%), hot flashes (8%), depression (6%), scalp hair loss (4%), headache (4%), nausea (3%), and deepening of the voice (1%).7
I sometimes see women with moderate to severe pelvic pain caused by endometriosis being treated with norethindrone 0.35 mg daily. This dose of norethindrone is suboptimal for pain treatment because it does not reliably suppress ovarian production of estradiol. In addition, the cells in endometriosis lesions are often resistant to the effects of progesterone, requiring higher dosages to produce secretory or decidual changes. In most situations, I recommend against the use of norethindrone 0.35 mg daily for the treatment of pelvic pain caused by endometriosis.
Patients commonly ask if NEA 5 mg daily has contraceptive efficacy. Although it is not approved at this dosage by the US Food and Drug Administration as a contraceptive,8 norethindrone 0.35 mg daily is approved as a progestin-only contraceptive.9 Norethindrone acetate is rapidly and completely deacetylated to norethindrone and the disposition of oral NEA is indistinguishable from that of norethindrone (which is the FDA-approved dosage mentioned above). Since norethindrone 0.35 mg daily is approved as a contraceptive, it is highly likely that NEA 5 mg daily has contraceptive efficacy, especially if there is good adherence with the daily medication.
CASE 3 Perimenopausal AUB
A 45-year-old woman reports varying menstrual cycle lengths from 24 to 60 days with very heavy menses in some cycles. Pelvic ultrasonography shows no abnormality. Endometrial biopsy shows a proliferative endometrium. Her serum progesterone level, obtained 1 week before the onset of menses, is < 3 ng/mL. She has no past history of heavy menses, easy bruising, excessive bleeding with procedures, or a family history of bleeding problems. She also reports occasional hot flashes that wake her from sleep.
Use an estrogen step-down regimen to manage postmenopause transition
This patient is likely in the perimenopause transition, and the abnormal uterine bleeding (AUB) is caused, in part, by oligo- or anovulation. Perimenopausal women with AUB may have cycles characterized by above normal ovarian estradiol production and below normal progesterone production, or frank anovulation.10 Elevated ovarian estrogen and low progesterone production sets the stage for heavy bleeding in the perimenopause, regardless of the presence of uterine pathology such as fibroids.
For perimenopausal women, one option for treatment of AUB due to anovulation is to prescribe an estrogen step-down regimen. For the 45-year-old woman in this case, initiating treatment with an E-P pill containing ethinyl estradiol 10 µg and NEA 1 mg will likely control the AUB and her occasional hot flash.11 As the woman ages, the ethinyl estradiol dose can be decreased to pills containing 5 µg and then 2.5 µg, covering the transition into postmenopause. Once the woman is in the postmenopause, treatment with transdermal estradiol and oral micronized progesterone is an option to treat menopausal vasomotor symptoms.
Optimize estrogen and progestin treatment for your patients
Many gynecologic problems are effectively treated by estrogen and/or progestin steroids. The dose of estrogen and progestin should be tailored to the specific problem. For PCOS, the estrogen dose selected should be sufficient to safely stimulate hepatic SHBG production. For endometriosis, if a GnRH antagonist is not available to the patient, a high-dose progestin, such as NEA 5 mg, may be an effective treatment. During the perimenopause transition in a woman with AUB, a treatment plan using a sequential E-P step-down program might control symptoms and help smoothly glide the patient into the postmenopause. ●
- Legro RS, Arslanian SA, Ehrmann DA, et al. Diagnosis and treatment of polycystic ovary syndrome: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2013;98:4565-4592. doi: 10.1210/jc.2013-2350.
- Rosenfield RL, Ehrmann DA. The pathogenesis of polycystic ovary syndrome (PCOS): the hypothesis of PCOS as functional ovarian hyperandrogenism revisited. Endocr Rev. 2016;37:467-520. doi: 10.1210/er.2015-1104.
- Zhu JL, Chen Z, Feng WJ, et al. Sex hormone-binding globulin and polycystic ovary syndrome. Clin Chim Acta. 2019;499:142-148. doi: 10.1016/j.cca.2019.09.010.
- Oner G, Muderris II. A prospective randomized trial comparing low-dose ethinyl estradiol and drospirenone 24/4 combined oral contraceptive vs. ethinyl estradiol and drospirenone 21/7 combined oral contraceptive in the treatment of hirsutism. Contraception. 2011;84:508-511. doi: 10.1016/j.contraception.2011.03.002.
- Boyd RA, Zegarac EA, Posvar EL, et al. Minimal androgenic activity of a new oral contraceptive containing norethindrone acetate and graduated doses of ethinyl estradiol. Contraception. 2001;63:71-76. doi: 10.1016/s0010-7824(01)00179-2.
- Thorneycroft IH, Stanczyk FZ, Bradshaw KD, et al. Effect of low-dose oral contraceptives on androgenic markers and acne. Contraception. 1999;60:255-262. doi: 10.1016/s0010-7824(99)00093-1.
- Kaser DJ, Missmer SA, Berry KF, et al. Use of norethindrone acetate alone for postoperative suppression of endometriosis symptoms. J Pediatr Adolesc Gynecol. 2012;25:105-108. doi: 10.1016/j.jpag.2011.09.013.
- Aygestin [package insert]. Pomona, NY: Duramed Pharmaceuticals; 2007.
- Camila [package insert]. Greenville, NC; Mayne Pharma; 2018.
- Santoro N, Brown JR, Adel T, et al. Characterization of reproductive hormonal dynamics in the perimenopause. J Clin Endocrinol Metab. 1996;81:1495-1501. doi: 10.1210/jcem.81.4.8636357.
- Speroff L, Symons J, Kempfert N, et al; FemHrt Study Investigators. The effect of varying low-dose combinations of norethindrone acetate and ethinyl estradiol (Femhrt) on the frequency and intensity of vasomotor symptoms. Menopause. 2000;7:383-390. doi: 10.1097/00042192-200011000-00003.
- Legro RS, Arslanian SA, Ehrmann DA, et al. Diagnosis and treatment of polycystic ovary syndrome: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2013;98:4565-4592. doi: 10.1210/jc.2013-2350.
- Rosenfield RL, Ehrmann DA. The pathogenesis of polycystic ovary syndrome (PCOS): the hypothesis of PCOS as functional ovarian hyperandrogenism revisited. Endocr Rev. 2016;37:467-520. doi: 10.1210/er.2015-1104.
- Zhu JL, Chen Z, Feng WJ, et al. Sex hormone-binding globulin and polycystic ovary syndrome. Clin Chim Acta. 2019;499:142-148. doi: 10.1016/j.cca.2019.09.010.
- Oner G, Muderris II. A prospective randomized trial comparing low-dose ethinyl estradiol and drospirenone 24/4 combined oral contraceptive vs. ethinyl estradiol and drospirenone 21/7 combined oral contraceptive in the treatment of hirsutism. Contraception. 2011;84:508-511. doi: 10.1016/j.contraception.2011.03.002.
- Boyd RA, Zegarac EA, Posvar EL, et al. Minimal androgenic activity of a new oral contraceptive containing norethindrone acetate and graduated doses of ethinyl estradiol. Contraception. 2001;63:71-76. doi: 10.1016/s0010-7824(01)00179-2.
- Thorneycroft IH, Stanczyk FZ, Bradshaw KD, et al. Effect of low-dose oral contraceptives on androgenic markers and acne. Contraception. 1999;60:255-262. doi: 10.1016/s0010-7824(99)00093-1.
- Kaser DJ, Missmer SA, Berry KF, et al. Use of norethindrone acetate alone for postoperative suppression of endometriosis symptoms. J Pediatr Adolesc Gynecol. 2012;25:105-108. doi: 10.1016/j.jpag.2011.09.013.
- Aygestin [package insert]. Pomona, NY: Duramed Pharmaceuticals; 2007.
- Camila [package insert]. Greenville, NC; Mayne Pharma; 2018.
- Santoro N, Brown JR, Adel T, et al. Characterization of reproductive hormonal dynamics in the perimenopause. J Clin Endocrinol Metab. 1996;81:1495-1501. doi: 10.1210/jcem.81.4.8636357.
- Speroff L, Symons J, Kempfert N, et al; FemHrt Study Investigators. The effect of varying low-dose combinations of norethindrone acetate and ethinyl estradiol (Femhrt) on the frequency and intensity of vasomotor symptoms. Menopause. 2000;7:383-390. doi: 10.1097/00042192-200011000-00003.
Focus on cancer risk
Hereditary cancer risk assessment is the key to identifying patients and families who are at increased risk for developing cancer. The knowledge generated by cancer risk assessment impacts clinical decisions that obstetricians and gynecologists and their patients make every day. Previvors—patients predisposed to developing cancer, because of their family history or a pathogenic gene variant, who have not had cancer—benefit from counseling, heightened surveillance, and medical and surgical options.
For the last 25 years, this field has been growing dramatically, and although the scientific advances are present, only 15.3% of patients with a personal history of breast or ovarian cancer who meet hereditary cancer testing criteria have been tested.1 As many as 1 in 4 women who present for a gynecologic examination may have a personal history or a family history that qualifies them for genetic testing.2
Cancer risk app considerations
The ability to leverage mobile device applications can provide clinicians and patients with a useful screening tool to identify women who are at increased cancer risk. Only a handful of apps are available today and most are geared to patients. Such apps explore the different testing modalities, including genetic testing, as well as treatment options. When evaluating the best app for patients, using the ACOG-recommended rubric shown on page 35, the qualities to keep in mind and that should score 4 out of 4 include design, authority, usefulness, and accuracy.
A few apps provide reminders for appointments, such as mammograms, magnetic resonance imaging, or breast self-exams, and allow patients to track treatment plans. To date, no app addresses prevention and treatment opportunities that are specific to patients who have a hereditary predisposition. At least one app lists hereditary cancer testing guidelines. Many more apps are geared toward individuals with cancer rather than toward previvors.
As ObGyns, we have an opportunity to educate and identify women and, subsequently, better counsel women identified as at increased risk for developing cancer. We can utilize medical apps to efficiently incorporate this screening into clinical practice. ●
- Childers P, Childers KK, Maggard-Gibbons M, et al. National estimates of genetic testing in women with a history of breast or ovarian cancer. J Clin Oncol. 2017;35:3800-3806.
- DeFrancesco M, Waldman RN, Pearlstone MM, et al. Hereditary cancer risk assessment and genetic testing in a community practice setting. Obstet Gynecol. 2018;132:1121-1129.
Hereditary cancer risk assessment is the key to identifying patients and families who are at increased risk for developing cancer. The knowledge generated by cancer risk assessment impacts clinical decisions that obstetricians and gynecologists and their patients make every day. Previvors—patients predisposed to developing cancer, because of their family history or a pathogenic gene variant, who have not had cancer—benefit from counseling, heightened surveillance, and medical and surgical options.
For the last 25 years, this field has been growing dramatically, and although the scientific advances are present, only 15.3% of patients with a personal history of breast or ovarian cancer who meet hereditary cancer testing criteria have been tested.1 As many as 1 in 4 women who present for a gynecologic examination may have a personal history or a family history that qualifies them for genetic testing.2
Cancer risk app considerations
The ability to leverage mobile device applications can provide clinicians and patients with a useful screening tool to identify women who are at increased cancer risk. Only a handful of apps are available today and most are geared to patients. Such apps explore the different testing modalities, including genetic testing, as well as treatment options. When evaluating the best app for patients, using the ACOG-recommended rubric shown on page 35, the qualities to keep in mind and that should score 4 out of 4 include design, authority, usefulness, and accuracy.
A few apps provide reminders for appointments, such as mammograms, magnetic resonance imaging, or breast self-exams, and allow patients to track treatment plans. To date, no app addresses prevention and treatment opportunities that are specific to patients who have a hereditary predisposition. At least one app lists hereditary cancer testing guidelines. Many more apps are geared toward individuals with cancer rather than toward previvors.
As ObGyns, we have an opportunity to educate and identify women and, subsequently, better counsel women identified as at increased risk for developing cancer. We can utilize medical apps to efficiently incorporate this screening into clinical practice. ●
Hereditary cancer risk assessment is the key to identifying patients and families who are at increased risk for developing cancer. The knowledge generated by cancer risk assessment impacts clinical decisions that obstetricians and gynecologists and their patients make every day. Previvors—patients predisposed to developing cancer, because of their family history or a pathogenic gene variant, who have not had cancer—benefit from counseling, heightened surveillance, and medical and surgical options.
For the last 25 years, this field has been growing dramatically, and although the scientific advances are present, only 15.3% of patients with a personal history of breast or ovarian cancer who meet hereditary cancer testing criteria have been tested.1 As many as 1 in 4 women who present for a gynecologic examination may have a personal history or a family history that qualifies them for genetic testing.2
Cancer risk app considerations
The ability to leverage mobile device applications can provide clinicians and patients with a useful screening tool to identify women who are at increased cancer risk. Only a handful of apps are available today and most are geared to patients. Such apps explore the different testing modalities, including genetic testing, as well as treatment options. When evaluating the best app for patients, using the ACOG-recommended rubric shown on page 35, the qualities to keep in mind and that should score 4 out of 4 include design, authority, usefulness, and accuracy.
A few apps provide reminders for appointments, such as mammograms, magnetic resonance imaging, or breast self-exams, and allow patients to track treatment plans. To date, no app addresses prevention and treatment opportunities that are specific to patients who have a hereditary predisposition. At least one app lists hereditary cancer testing guidelines. Many more apps are geared toward individuals with cancer rather than toward previvors.
As ObGyns, we have an opportunity to educate and identify women and, subsequently, better counsel women identified as at increased risk for developing cancer. We can utilize medical apps to efficiently incorporate this screening into clinical practice. ●
- Childers P, Childers KK, Maggard-Gibbons M, et al. National estimates of genetic testing in women with a history of breast or ovarian cancer. J Clin Oncol. 2017;35:3800-3806.
- DeFrancesco M, Waldman RN, Pearlstone MM, et al. Hereditary cancer risk assessment and genetic testing in a community practice setting. Obstet Gynecol. 2018;132:1121-1129.
- Childers P, Childers KK, Maggard-Gibbons M, et al. National estimates of genetic testing in women with a history of breast or ovarian cancer. J Clin Oncol. 2017;35:3800-3806.
- DeFrancesco M, Waldman RN, Pearlstone MM, et al. Hereditary cancer risk assessment and genetic testing in a community practice setting. Obstet Gynecol. 2018;132:1121-1129.
Mobile apps in ObGyn practice: Tools for enhancing women’s preventive health care
Evolutionary changes in ObGyn
Preventive medicine guidelines have evolved to reflect enhanced cervical cancer screening tests, longer-acting contraceptive options, and better data on the lack of utility of the annual pelvic exam that has changed the focus of the annual visit for both physicians and patients.1 These changes allow us to pivot and leverage the trust we build with our patients to make meaningful impacts in preventing chronic disease, improving prepregnancy health, reducing maternal mortality and morbidity, and improving the quality and longevity of our patients’ lives. New guidelines, coupled with the knowledge of the leading causes of morbidity for women, provide the chance to incorporate areas of screening and intervention that, while we are capable of addressing, we traditionally have not done so for various reasons.
The ACOG Presidential Task Force identified 5 areas of preventive health that significantly influence the long-term morbidity of women: obesity, cardiovascular disease, preconception counseling, diabetes, and cancer risk. ObGyns are uniquely positioned to identify and initiate the conversation and subsequently manage, treat, and address these critical health areas. To make this daunting task more manageable, the Task Force not only published webinars to address the clinical knowledge pertaining to these areas of health but also specifically looked at how to use technology to aid obstetrician-gynecologists in addressing them with patients.
Making use of technology in clinical practice
Technology is emerging as an influential player in health care. Major corporations, such as Amazon, Google, Apple, and Facebook, are making headlines in health care as they consider strategies (moves) to revolutionize technology and, in turn, patient visits like we have never seen before. Examples include incorporating artificial intelligence in a patient’s care and allowing better access for primary care.
The changes that we will see over the next 10 years, influenced by industry, will be more than those seen in our lifetime. To prepare for these changes, we need to incorporate technology into our daily practice. This encompasses much more than just the electronic medical record. Consequently, the Task Force intentionally looked at mobile medical apps to aid physicians in addressing the 5 specific areas of preventive health identified.
While a small step compared with what is to come, apps are a great resource to leverage in making this transition. However, with hundreds of thousands of medical apps available in app stores and the constant updates and iterations of each, it would be impossible to recommend any single app. There is much value in having a framework to use to efficiently measure the benefit of an app that you or your patient comes across in clinical practice. The objective of this series was to provide clinicians with an effective tool to evaluate a medical app that could be used, for example, when addressing obesity or optimizing prepregnancy health.
Continue to: The recommended rubric for evaluating apps...
The recommended rubric for evaluating apps
To evaluate mobile drug information apps, the Task Force members recommend a user-friendly, convenient rubric developed by the American Society of Health-System Pharmacists (ASHP) (see page 35). The rubric can help obstetrician-gynecologists evaluate and compare the value of various medical apps that specifically address obesity, diabetes mellitus, cardiovascular disease, improving maternal morbidity with enhanced preconception counseling, and cancer risk assessment.
The authors of this Task Force series have attempted to highlight the key features of an app as it pertains to a particular area of focus. It is important to keep in mind the primary user and the goal when choosing or recommending an app for practice or for patient use. The ASHP’s rubric is a tool meant to aid clinicians in evaluating medical apps, but it is ultimately the user’s decision to determine if the deficiencies of an app should deter its use. Although all the criteria are relevant and important, as medical experts it is incumbent on us to pay careful attention to the accuracy, authority, objectivity, timeliness, and security of any app we consider incorporating into clinical practice.
While integrating the use of medical apps into clinical practice will be novel for some, for others, junior Fellows in particular, it has become part of their practice and education. Dr. Eva Hoffmann, Chief Resident in the NYU Langone Health System, offers this perspective: “As medical trainees we use mobile apps to enhance our patient interaction and guide high-quality, continuous care. In today’s modern technological world, apps help keep us up to date with the ever-changing guidelines in pregnancy and routine gynecologic care as well as communicate directly and discreetly with a patient whenever the need arises. The most significant apps provide guidance on abnormal Pap results, indicated deliveries prior to 39 weeks, and the ability to respond to obstetrical emergencies. They also allow for quick society-endorsed references in seconds. Apps have changed the way that we practice by providing evidence-based medicine literally at our fingertips—in a shareable and communicable way—making the practice of medicine even more efficient and effective.”
Opportunity to reaffirm expertise
Dr. Chalas’ initiative was meant to shed light on the opportunity obstetrician-gynecologists have to reassert themselves as women’s health experts, to consider redefining their practice by incorporating new preventive guidelines, and to leverage medical apps for achieving better health outcomes for women across their lifetime. We hope that by opening a dialogue about how ubiquitous medical apps are (for both physicians and patients) in today’s health arena, how many apps are inaccurate and/or misused, and how a simple rubric can be used to assess an app’s value, you are inspired and feel more comfortable to incorporate medical apps into your practice.
Health care will continually undergo advancements, and as a specialty we must evolve to address women’s needs. Obstetrician-gynecologists are well suited to contribute significantly to the well-being of women and mothers. We can leverage technology-based apps to help us redefine our roles and priorities at the patient’s annual visit. We can reaffirm ourselves as the leading women’s health care physicians.
An additional resource
To enhance your understanding of apps and how to evaluate them, Dr. Katherine Chen’s App Review series in
In appreciation
The members of this Task Force want to thank the Editorial Board and staff of
- Women’s Preventive Services Initiative website. Recommendations for well-woman care: a well-woman chart. https:// www.womenspreventivehealth.org/wellwomanchart/. Accessed June 11, 2021.
- Chyjek K, Farag S, Chen KT. Rating pregnancy wheel applications using the APPLICATIONS scoring system. Obstet Gynecol. 2015;125:1478-1483.
Evolutionary changes in ObGyn
Preventive medicine guidelines have evolved to reflect enhanced cervical cancer screening tests, longer-acting contraceptive options, and better data on the lack of utility of the annual pelvic exam that has changed the focus of the annual visit for both physicians and patients.1 These changes allow us to pivot and leverage the trust we build with our patients to make meaningful impacts in preventing chronic disease, improving prepregnancy health, reducing maternal mortality and morbidity, and improving the quality and longevity of our patients’ lives. New guidelines, coupled with the knowledge of the leading causes of morbidity for women, provide the chance to incorporate areas of screening and intervention that, while we are capable of addressing, we traditionally have not done so for various reasons.
The ACOG Presidential Task Force identified 5 areas of preventive health that significantly influence the long-term morbidity of women: obesity, cardiovascular disease, preconception counseling, diabetes, and cancer risk. ObGyns are uniquely positioned to identify and initiate the conversation and subsequently manage, treat, and address these critical health areas. To make this daunting task more manageable, the Task Force not only published webinars to address the clinical knowledge pertaining to these areas of health but also specifically looked at how to use technology to aid obstetrician-gynecologists in addressing them with patients.
Making use of technology in clinical practice
Technology is emerging as an influential player in health care. Major corporations, such as Amazon, Google, Apple, and Facebook, are making headlines in health care as they consider strategies (moves) to revolutionize technology and, in turn, patient visits like we have never seen before. Examples include incorporating artificial intelligence in a patient’s care and allowing better access for primary care.
The changes that we will see over the next 10 years, influenced by industry, will be more than those seen in our lifetime. To prepare for these changes, we need to incorporate technology into our daily practice. This encompasses much more than just the electronic medical record. Consequently, the Task Force intentionally looked at mobile medical apps to aid physicians in addressing the 5 specific areas of preventive health identified.
While a small step compared with what is to come, apps are a great resource to leverage in making this transition. However, with hundreds of thousands of medical apps available in app stores and the constant updates and iterations of each, it would be impossible to recommend any single app. There is much value in having a framework to use to efficiently measure the benefit of an app that you or your patient comes across in clinical practice. The objective of this series was to provide clinicians with an effective tool to evaluate a medical app that could be used, for example, when addressing obesity or optimizing prepregnancy health.
Continue to: The recommended rubric for evaluating apps...
The recommended rubric for evaluating apps
To evaluate mobile drug information apps, the Task Force members recommend a user-friendly, convenient rubric developed by the American Society of Health-System Pharmacists (ASHP) (see page 35). The rubric can help obstetrician-gynecologists evaluate and compare the value of various medical apps that specifically address obesity, diabetes mellitus, cardiovascular disease, improving maternal morbidity with enhanced preconception counseling, and cancer risk assessment.
The authors of this Task Force series have attempted to highlight the key features of an app as it pertains to a particular area of focus. It is important to keep in mind the primary user and the goal when choosing or recommending an app for practice or for patient use. The ASHP’s rubric is a tool meant to aid clinicians in evaluating medical apps, but it is ultimately the user’s decision to determine if the deficiencies of an app should deter its use. Although all the criteria are relevant and important, as medical experts it is incumbent on us to pay careful attention to the accuracy, authority, objectivity, timeliness, and security of any app we consider incorporating into clinical practice.
While integrating the use of medical apps into clinical practice will be novel for some, for others, junior Fellows in particular, it has become part of their practice and education. Dr. Eva Hoffmann, Chief Resident in the NYU Langone Health System, offers this perspective: “As medical trainees we use mobile apps to enhance our patient interaction and guide high-quality, continuous care. In today’s modern technological world, apps help keep us up to date with the ever-changing guidelines in pregnancy and routine gynecologic care as well as communicate directly and discreetly with a patient whenever the need arises. The most significant apps provide guidance on abnormal Pap results, indicated deliveries prior to 39 weeks, and the ability to respond to obstetrical emergencies. They also allow for quick society-endorsed references in seconds. Apps have changed the way that we practice by providing evidence-based medicine literally at our fingertips—in a shareable and communicable way—making the practice of medicine even more efficient and effective.”
Opportunity to reaffirm expertise
Dr. Chalas’ initiative was meant to shed light on the opportunity obstetrician-gynecologists have to reassert themselves as women’s health experts, to consider redefining their practice by incorporating new preventive guidelines, and to leverage medical apps for achieving better health outcomes for women across their lifetime. We hope that by opening a dialogue about how ubiquitous medical apps are (for both physicians and patients) in today’s health arena, how many apps are inaccurate and/or misused, and how a simple rubric can be used to assess an app’s value, you are inspired and feel more comfortable to incorporate medical apps into your practice.
Health care will continually undergo advancements, and as a specialty we must evolve to address women’s needs. Obstetrician-gynecologists are well suited to contribute significantly to the well-being of women and mothers. We can leverage technology-based apps to help us redefine our roles and priorities at the patient’s annual visit. We can reaffirm ourselves as the leading women’s health care physicians.
An additional resource
To enhance your understanding of apps and how to evaluate them, Dr. Katherine Chen’s App Review series in
In appreciation
The members of this Task Force want to thank the Editorial Board and staff of
Evolutionary changes in ObGyn
Preventive medicine guidelines have evolved to reflect enhanced cervical cancer screening tests, longer-acting contraceptive options, and better data on the lack of utility of the annual pelvic exam that has changed the focus of the annual visit for both physicians and patients.1 These changes allow us to pivot and leverage the trust we build with our patients to make meaningful impacts in preventing chronic disease, improving prepregnancy health, reducing maternal mortality and morbidity, and improving the quality and longevity of our patients’ lives. New guidelines, coupled with the knowledge of the leading causes of morbidity for women, provide the chance to incorporate areas of screening and intervention that, while we are capable of addressing, we traditionally have not done so for various reasons.
The ACOG Presidential Task Force identified 5 areas of preventive health that significantly influence the long-term morbidity of women: obesity, cardiovascular disease, preconception counseling, diabetes, and cancer risk. ObGyns are uniquely positioned to identify and initiate the conversation and subsequently manage, treat, and address these critical health areas. To make this daunting task more manageable, the Task Force not only published webinars to address the clinical knowledge pertaining to these areas of health but also specifically looked at how to use technology to aid obstetrician-gynecologists in addressing them with patients.
Making use of technology in clinical practice
Technology is emerging as an influential player in health care. Major corporations, such as Amazon, Google, Apple, and Facebook, are making headlines in health care as they consider strategies (moves) to revolutionize technology and, in turn, patient visits like we have never seen before. Examples include incorporating artificial intelligence in a patient’s care and allowing better access for primary care.
The changes that we will see over the next 10 years, influenced by industry, will be more than those seen in our lifetime. To prepare for these changes, we need to incorporate technology into our daily practice. This encompasses much more than just the electronic medical record. Consequently, the Task Force intentionally looked at mobile medical apps to aid physicians in addressing the 5 specific areas of preventive health identified.
While a small step compared with what is to come, apps are a great resource to leverage in making this transition. However, with hundreds of thousands of medical apps available in app stores and the constant updates and iterations of each, it would be impossible to recommend any single app. There is much value in having a framework to use to efficiently measure the benefit of an app that you or your patient comes across in clinical practice. The objective of this series was to provide clinicians with an effective tool to evaluate a medical app that could be used, for example, when addressing obesity or optimizing prepregnancy health.
Continue to: The recommended rubric for evaluating apps...
The recommended rubric for evaluating apps
To evaluate mobile drug information apps, the Task Force members recommend a user-friendly, convenient rubric developed by the American Society of Health-System Pharmacists (ASHP) (see page 35). The rubric can help obstetrician-gynecologists evaluate and compare the value of various medical apps that specifically address obesity, diabetes mellitus, cardiovascular disease, improving maternal morbidity with enhanced preconception counseling, and cancer risk assessment.
The authors of this Task Force series have attempted to highlight the key features of an app as it pertains to a particular area of focus. It is important to keep in mind the primary user and the goal when choosing or recommending an app for practice or for patient use. The ASHP’s rubric is a tool meant to aid clinicians in evaluating medical apps, but it is ultimately the user’s decision to determine if the deficiencies of an app should deter its use. Although all the criteria are relevant and important, as medical experts it is incumbent on us to pay careful attention to the accuracy, authority, objectivity, timeliness, and security of any app we consider incorporating into clinical practice.
While integrating the use of medical apps into clinical practice will be novel for some, for others, junior Fellows in particular, it has become part of their practice and education. Dr. Eva Hoffmann, Chief Resident in the NYU Langone Health System, offers this perspective: “As medical trainees we use mobile apps to enhance our patient interaction and guide high-quality, continuous care. In today’s modern technological world, apps help keep us up to date with the ever-changing guidelines in pregnancy and routine gynecologic care as well as communicate directly and discreetly with a patient whenever the need arises. The most significant apps provide guidance on abnormal Pap results, indicated deliveries prior to 39 weeks, and the ability to respond to obstetrical emergencies. They also allow for quick society-endorsed references in seconds. Apps have changed the way that we practice by providing evidence-based medicine literally at our fingertips—in a shareable and communicable way—making the practice of medicine even more efficient and effective.”
Opportunity to reaffirm expertise
Dr. Chalas’ initiative was meant to shed light on the opportunity obstetrician-gynecologists have to reassert themselves as women’s health experts, to consider redefining their practice by incorporating new preventive guidelines, and to leverage medical apps for achieving better health outcomes for women across their lifetime. We hope that by opening a dialogue about how ubiquitous medical apps are (for both physicians and patients) in today’s health arena, how many apps are inaccurate and/or misused, and how a simple rubric can be used to assess an app’s value, you are inspired and feel more comfortable to incorporate medical apps into your practice.
Health care will continually undergo advancements, and as a specialty we must evolve to address women’s needs. Obstetrician-gynecologists are well suited to contribute significantly to the well-being of women and mothers. We can leverage technology-based apps to help us redefine our roles and priorities at the patient’s annual visit. We can reaffirm ourselves as the leading women’s health care physicians.
An additional resource
To enhance your understanding of apps and how to evaluate them, Dr. Katherine Chen’s App Review series in
In appreciation
The members of this Task Force want to thank the Editorial Board and staff of
- Women’s Preventive Services Initiative website. Recommendations for well-woman care: a well-woman chart. https:// www.womenspreventivehealth.org/wellwomanchart/. Accessed June 11, 2021.
- Chyjek K, Farag S, Chen KT. Rating pregnancy wheel applications using the APPLICATIONS scoring system. Obstet Gynecol. 2015;125:1478-1483.
- Women’s Preventive Services Initiative website. Recommendations for well-woman care: a well-woman chart. https:// www.womenspreventivehealth.org/wellwomanchart/. Accessed June 11, 2021.
- Chyjek K, Farag S, Chen KT. Rating pregnancy wheel applications using the APPLICATIONS scoring system. Obstet Gynecol. 2015;125:1478-1483.
Texas doctor accused of vaccine theft faces grand jury
Hasan Gokal, MD, was fired from his job and charged with theft by a public servant. A judge dismissed the theft charge in January 2021, saying there was no probable cause, but prosecutors took the accusation to the Harris County Grand Jury, which on June 30 decided no prosecution was warranted, the Associated Press reported.
“I came as a practicing ER doctor into public health and as an ER doctor, I err on the side of life and that’s how I chose to make my decision,” Dr. Gokal told the Associated Press. “It was the right thing to do and it meant saving more lives.”
Dr. Gokal, 48, was supervising a COVID-19 vaccination clinic Dec. 29, 2020, in Humble, Tex., when the clinic closed for the day with an open vial containing nine doses of Moderna vaccine, the New York Times reported.
Since the vaccine would expire in 6 hours, Dr. Gokal scrambled to find people with medical conditions who needed vaccinating, he said. He gave the last dose to his wife, who has a lung condition, pulmonary sarcoidosis.
Dr. Gokal said he contacted his supervisor before acting and provided documentation the next day. He was fired for breaking protocol and then charged with theft.
“He abused his position to place his friends and family in line in front of people who had gone through the lawful process to be there,” Harris County District Attorney Kim Ogg said in a January statement. “What he did was illegal and he’ll be held accountable under the law.”
The AP reported that on June 30 the DA’s office issued a statement saying: “We respect the decision of the grand jury in this and every case. Evidence, not public opinion, is the guiding principle of our work.”
The AP said numerous doctors voiced support for Dr. Gokal and that the Texas Medical Board dismissed an investigation against him.
Dr. Gokal told the AP he’d still like to work in public health. Since being fired by the health department, he’s worked part time in the emergency departments at two Houston hospitals.
A version of this article first appeared on WebMD.com.
Hasan Gokal, MD, was fired from his job and charged with theft by a public servant. A judge dismissed the theft charge in January 2021, saying there was no probable cause, but prosecutors took the accusation to the Harris County Grand Jury, which on June 30 decided no prosecution was warranted, the Associated Press reported.
“I came as a practicing ER doctor into public health and as an ER doctor, I err on the side of life and that’s how I chose to make my decision,” Dr. Gokal told the Associated Press. “It was the right thing to do and it meant saving more lives.”
Dr. Gokal, 48, was supervising a COVID-19 vaccination clinic Dec. 29, 2020, in Humble, Tex., when the clinic closed for the day with an open vial containing nine doses of Moderna vaccine, the New York Times reported.
Since the vaccine would expire in 6 hours, Dr. Gokal scrambled to find people with medical conditions who needed vaccinating, he said. He gave the last dose to his wife, who has a lung condition, pulmonary sarcoidosis.
Dr. Gokal said he contacted his supervisor before acting and provided documentation the next day. He was fired for breaking protocol and then charged with theft.
“He abused his position to place his friends and family in line in front of people who had gone through the lawful process to be there,” Harris County District Attorney Kim Ogg said in a January statement. “What he did was illegal and he’ll be held accountable under the law.”
The AP reported that on June 30 the DA’s office issued a statement saying: “We respect the decision of the grand jury in this and every case. Evidence, not public opinion, is the guiding principle of our work.”
The AP said numerous doctors voiced support for Dr. Gokal and that the Texas Medical Board dismissed an investigation against him.
Dr. Gokal told the AP he’d still like to work in public health. Since being fired by the health department, he’s worked part time in the emergency departments at two Houston hospitals.
A version of this article first appeared on WebMD.com.
Hasan Gokal, MD, was fired from his job and charged with theft by a public servant. A judge dismissed the theft charge in January 2021, saying there was no probable cause, but prosecutors took the accusation to the Harris County Grand Jury, which on June 30 decided no prosecution was warranted, the Associated Press reported.
“I came as a practicing ER doctor into public health and as an ER doctor, I err on the side of life and that’s how I chose to make my decision,” Dr. Gokal told the Associated Press. “It was the right thing to do and it meant saving more lives.”
Dr. Gokal, 48, was supervising a COVID-19 vaccination clinic Dec. 29, 2020, in Humble, Tex., when the clinic closed for the day with an open vial containing nine doses of Moderna vaccine, the New York Times reported.
Since the vaccine would expire in 6 hours, Dr. Gokal scrambled to find people with medical conditions who needed vaccinating, he said. He gave the last dose to his wife, who has a lung condition, pulmonary sarcoidosis.
Dr. Gokal said he contacted his supervisor before acting and provided documentation the next day. He was fired for breaking protocol and then charged with theft.
“He abused his position to place his friends and family in line in front of people who had gone through the lawful process to be there,” Harris County District Attorney Kim Ogg said in a January statement. “What he did was illegal and he’ll be held accountable under the law.”
The AP reported that on June 30 the DA’s office issued a statement saying: “We respect the decision of the grand jury in this and every case. Evidence, not public opinion, is the guiding principle of our work.”
The AP said numerous doctors voiced support for Dr. Gokal and that the Texas Medical Board dismissed an investigation against him.
Dr. Gokal told the AP he’d still like to work in public health. Since being fired by the health department, he’s worked part time in the emergency departments at two Houston hospitals.
A version of this article first appeared on WebMD.com.
Researchers follow development of axial SpA in first-degree relatives of patients
Healthy first-degree relatives of individuals with HLA-B27–positive axial spondyloarthritis who also were HLA-B27 positive were at increased risk for developing the disease themselves within 1 year, based on data from an ongoing prospective cohort study that involved 202 first-degree relatives.
Axial spondyloarthritis (axSpA) generally arises between ages 18 and 40 years, but diagnosis can be delayed, in part because of the lack of biomarkers and nonspecific symptoms, wrote Henriëtte M.Y. de Jong, MD, PhD, of the University of Amsterdam, and colleagues.
Individuals who carry the HLA-B27 gene are predisposed to axSpA, and their first-degree relatives (FDRs) are at increased risk as well, the researchers said. Therefore, “studying [FDRs] could help to identify clinical signs, imaging abnormalities, and biomarkers that are predictive of development of axSpA,” they said.
In a study published in Arthritis Care & Research, the investigators reviewed data from patients in the Pre-SpA cohort, a 5-year prospective study of healthy-seeming FDRs of patients with HLA-B27–positive axSpA. The researchers previously reported that up to one-third of 51 FDRs had clinical features associated with SpA at baseline, despite the lack of a diagnosis.
The current study included an additional 151 FDRs who had answered yearly questions about back pain and undergone a yearly physical exam and plain radiographs and MRI imaging at baseline.
Overall, 65% reported back pain at baseline and 19% met criteria for inflammatory back pain, with a median visual analog score (VAS) for back pain of 22. No active arthritis was noted, but 5 FDRs reported a past arthritis diagnosis, 48 reported arthralgia, and 16 had at least one tender joint on physical exam. Eight FDRs had past diagnoses of enthesitis, and one had a history of dactylitis.
In assessing disease activity, the researchers found an elevated C-reactive protein (CRP) level in 24 FDRs and 11 had an elevated erythrocyte sedimentation rate (ESR).
On MRI of the sacroiliac joint at baseline, 10% of the FDRs had SPARCC (Spondyloarthritis Research Consortium of Canada) scores of 2 or higher, 4% had scores of 5 or higher, and 4% had deep lesions.
A total of 123 FDRs had complete data at a 1-year follow-up visit.
“All features were equally distributed between HLA-B27–positive and –negative FDRs,” the researchers noted. However, at the end of the 1-year follow-up period, seven (6%) of the FDRs were clinically diagnosed with axSpA, and six of them were HLA-B27 positive. Disease activity measures had increased at 1 year in all seven patients with newly diagnosed axSpA.
The study findings were limited by several factors, including the possible channeling of FDRs with current complaints of back pain into the study and the inability to confirm details of family and medical history, the researchers noted. However, the VAS back pain scores reported by the FDRs suggest that this pain was not a fixture in daily life, they wrote.
The results confirm the prevalent subclinical signs of SpA in healthy FDRs of patients with axSpA who were positive and negative for HLA-B27, but also confirm that clinical progression occurred primarily in the HLA-B27–positive patients in conjunction with inflammatory back pain, the researchers said.
“Further follow-up of the Pre-SpA cohort will give more robust insight into the characteristics of FDRs that progress towards clinical SpA, thereby hopefully enabling the characterization of high-risk FDRs,” they concluded.
The Pre-SpA cohort is supported by the Dutch Arthritis Society. Lead author Dr. de Jong had no financial conflicts to disclose. One coauthor is employed by UCB, and several others disclosed relationships with AbbVie, Eli Lilly, Janssen, Novartis, Pfizer, and UCB.
Healthy first-degree relatives of individuals with HLA-B27–positive axial spondyloarthritis who also were HLA-B27 positive were at increased risk for developing the disease themselves within 1 year, based on data from an ongoing prospective cohort study that involved 202 first-degree relatives.
Axial spondyloarthritis (axSpA) generally arises between ages 18 and 40 years, but diagnosis can be delayed, in part because of the lack of biomarkers and nonspecific symptoms, wrote Henriëtte M.Y. de Jong, MD, PhD, of the University of Amsterdam, and colleagues.
Individuals who carry the HLA-B27 gene are predisposed to axSpA, and their first-degree relatives (FDRs) are at increased risk as well, the researchers said. Therefore, “studying [FDRs] could help to identify clinical signs, imaging abnormalities, and biomarkers that are predictive of development of axSpA,” they said.
In a study published in Arthritis Care & Research, the investigators reviewed data from patients in the Pre-SpA cohort, a 5-year prospective study of healthy-seeming FDRs of patients with HLA-B27–positive axSpA. The researchers previously reported that up to one-third of 51 FDRs had clinical features associated with SpA at baseline, despite the lack of a diagnosis.
The current study included an additional 151 FDRs who had answered yearly questions about back pain and undergone a yearly physical exam and plain radiographs and MRI imaging at baseline.
Overall, 65% reported back pain at baseline and 19% met criteria for inflammatory back pain, with a median visual analog score (VAS) for back pain of 22. No active arthritis was noted, but 5 FDRs reported a past arthritis diagnosis, 48 reported arthralgia, and 16 had at least one tender joint on physical exam. Eight FDRs had past diagnoses of enthesitis, and one had a history of dactylitis.
In assessing disease activity, the researchers found an elevated C-reactive protein (CRP) level in 24 FDRs and 11 had an elevated erythrocyte sedimentation rate (ESR).
On MRI of the sacroiliac joint at baseline, 10% of the FDRs had SPARCC (Spondyloarthritis Research Consortium of Canada) scores of 2 or higher, 4% had scores of 5 or higher, and 4% had deep lesions.
A total of 123 FDRs had complete data at a 1-year follow-up visit.
“All features were equally distributed between HLA-B27–positive and –negative FDRs,” the researchers noted. However, at the end of the 1-year follow-up period, seven (6%) of the FDRs were clinically diagnosed with axSpA, and six of them were HLA-B27 positive. Disease activity measures had increased at 1 year in all seven patients with newly diagnosed axSpA.
The study findings were limited by several factors, including the possible channeling of FDRs with current complaints of back pain into the study and the inability to confirm details of family and medical history, the researchers noted. However, the VAS back pain scores reported by the FDRs suggest that this pain was not a fixture in daily life, they wrote.
The results confirm the prevalent subclinical signs of SpA in healthy FDRs of patients with axSpA who were positive and negative for HLA-B27, but also confirm that clinical progression occurred primarily in the HLA-B27–positive patients in conjunction with inflammatory back pain, the researchers said.
“Further follow-up of the Pre-SpA cohort will give more robust insight into the characteristics of FDRs that progress towards clinical SpA, thereby hopefully enabling the characterization of high-risk FDRs,” they concluded.
The Pre-SpA cohort is supported by the Dutch Arthritis Society. Lead author Dr. de Jong had no financial conflicts to disclose. One coauthor is employed by UCB, and several others disclosed relationships with AbbVie, Eli Lilly, Janssen, Novartis, Pfizer, and UCB.
Healthy first-degree relatives of individuals with HLA-B27–positive axial spondyloarthritis who also were HLA-B27 positive were at increased risk for developing the disease themselves within 1 year, based on data from an ongoing prospective cohort study that involved 202 first-degree relatives.
Axial spondyloarthritis (axSpA) generally arises between ages 18 and 40 years, but diagnosis can be delayed, in part because of the lack of biomarkers and nonspecific symptoms, wrote Henriëtte M.Y. de Jong, MD, PhD, of the University of Amsterdam, and colleagues.
Individuals who carry the HLA-B27 gene are predisposed to axSpA, and their first-degree relatives (FDRs) are at increased risk as well, the researchers said. Therefore, “studying [FDRs] could help to identify clinical signs, imaging abnormalities, and biomarkers that are predictive of development of axSpA,” they said.
In a study published in Arthritis Care & Research, the investigators reviewed data from patients in the Pre-SpA cohort, a 5-year prospective study of healthy-seeming FDRs of patients with HLA-B27–positive axSpA. The researchers previously reported that up to one-third of 51 FDRs had clinical features associated with SpA at baseline, despite the lack of a diagnosis.
The current study included an additional 151 FDRs who had answered yearly questions about back pain and undergone a yearly physical exam and plain radiographs and MRI imaging at baseline.
Overall, 65% reported back pain at baseline and 19% met criteria for inflammatory back pain, with a median visual analog score (VAS) for back pain of 22. No active arthritis was noted, but 5 FDRs reported a past arthritis diagnosis, 48 reported arthralgia, and 16 had at least one tender joint on physical exam. Eight FDRs had past diagnoses of enthesitis, and one had a history of dactylitis.
In assessing disease activity, the researchers found an elevated C-reactive protein (CRP) level in 24 FDRs and 11 had an elevated erythrocyte sedimentation rate (ESR).
On MRI of the sacroiliac joint at baseline, 10% of the FDRs had SPARCC (Spondyloarthritis Research Consortium of Canada) scores of 2 or higher, 4% had scores of 5 or higher, and 4% had deep lesions.
A total of 123 FDRs had complete data at a 1-year follow-up visit.
“All features were equally distributed between HLA-B27–positive and –negative FDRs,” the researchers noted. However, at the end of the 1-year follow-up period, seven (6%) of the FDRs were clinically diagnosed with axSpA, and six of them were HLA-B27 positive. Disease activity measures had increased at 1 year in all seven patients with newly diagnosed axSpA.
The study findings were limited by several factors, including the possible channeling of FDRs with current complaints of back pain into the study and the inability to confirm details of family and medical history, the researchers noted. However, the VAS back pain scores reported by the FDRs suggest that this pain was not a fixture in daily life, they wrote.
The results confirm the prevalent subclinical signs of SpA in healthy FDRs of patients with axSpA who were positive and negative for HLA-B27, but also confirm that clinical progression occurred primarily in the HLA-B27–positive patients in conjunction with inflammatory back pain, the researchers said.
“Further follow-up of the Pre-SpA cohort will give more robust insight into the characteristics of FDRs that progress towards clinical SpA, thereby hopefully enabling the characterization of high-risk FDRs,” they concluded.
The Pre-SpA cohort is supported by the Dutch Arthritis Society. Lead author Dr. de Jong had no financial conflicts to disclose. One coauthor is employed by UCB, and several others disclosed relationships with AbbVie, Eli Lilly, Janssen, Novartis, Pfizer, and UCB.
FROM ARTHRITIS CARE & RESEARCH
Bleeding events tied to higher mortality in patients with factor V inhibition
Coagulation factor V (FV) inhibitor is a rare disease with a mortality rate of nearly 15%. Increased mortality was significantly associated with the incidence of major bleeding, according to a review of PubMed case reports published in Thrombosis Update.
FV autoantibodies are most often detected in patients in the postoperative state, in those who have received a blood transfusion, in patients treated with antibiotics, and in those with immune diseases, according to the online report by Hideo Wada, MD, PhD, of the Mie Prefectural General Medical Center, Yokkaichi, Japan, and colleagues. These patients who acquired immune FV inhibitor (AIFVD) vary widely in symptoms from asymptomatic to mild or severe hemorrhagic manifestations, with some reports of thrombotic complications, the authors added.
Their review assessed the PubMed literature from Jan. 1, 1968, to July 31, 2020, and found 212 case reports on acquired FV deficiency. Of these, 150 cases with confirmed FV inhibitor positivity were included. The 150 reported cases of FV inhibitor were primarily from the United States (n = 48) and Japan (n = 43). The median patient age was 68.0 years, and the female to male ratio of patients was 0.47, according to the authors. The largest associated percentage of underlying conditions were postoperative state (25.3%), idiopathic (18.7%), infection (12.7%) and malignant neoplasms and autoimmune disease, at 7.3% each.
Major bleeds
A total of 73 cases were positive for major bleeding (48.7%) and 30 cases were negative (20.0%), while the rest were undetermined (31.3%). The FV activity was ≤ 28% in all patients with FV inhibitor.
The overall mortality rate was 14.6%, with half of the nonsurvivors dying of major bleeding. The mortality rate was more than twofold higher in the group with major bleeding (23.3% mortality) compared to the group without major bleeding (10.0%), yielding an odds ratio of 2.73 of death because of a major bleed. The most frequent types of fatal bleeding were intracranial bleeding and gastrointestinal bleeding. Of the 20 deaths reported in 135 patients with data, the causes of death were major bleeding (12 patients), infection (6 patients) and thrombosis (2 patients). Remission was observed in three of the nonsurvivors, indicating that even after remission, patients with FV inhibitor might still be susceptible to infection or thrombosis, according to the authors.
“[Major bleeding] should be treated aggressively; however, the best treatment is not clear and even patients in remission should be followed closely due to the risk of death from infection or thrombosis,” the authors concluded.
They reported having no conflicts of interest.
Coagulation factor V (FV) inhibitor is a rare disease with a mortality rate of nearly 15%. Increased mortality was significantly associated with the incidence of major bleeding, according to a review of PubMed case reports published in Thrombosis Update.
FV autoantibodies are most often detected in patients in the postoperative state, in those who have received a blood transfusion, in patients treated with antibiotics, and in those with immune diseases, according to the online report by Hideo Wada, MD, PhD, of the Mie Prefectural General Medical Center, Yokkaichi, Japan, and colleagues. These patients who acquired immune FV inhibitor (AIFVD) vary widely in symptoms from asymptomatic to mild or severe hemorrhagic manifestations, with some reports of thrombotic complications, the authors added.
Their review assessed the PubMed literature from Jan. 1, 1968, to July 31, 2020, and found 212 case reports on acquired FV deficiency. Of these, 150 cases with confirmed FV inhibitor positivity were included. The 150 reported cases of FV inhibitor were primarily from the United States (n = 48) and Japan (n = 43). The median patient age was 68.0 years, and the female to male ratio of patients was 0.47, according to the authors. The largest associated percentage of underlying conditions were postoperative state (25.3%), idiopathic (18.7%), infection (12.7%) and malignant neoplasms and autoimmune disease, at 7.3% each.
Major bleeds
A total of 73 cases were positive for major bleeding (48.7%) and 30 cases were negative (20.0%), while the rest were undetermined (31.3%). The FV activity was ≤ 28% in all patients with FV inhibitor.
The overall mortality rate was 14.6%, with half of the nonsurvivors dying of major bleeding. The mortality rate was more than twofold higher in the group with major bleeding (23.3% mortality) compared to the group without major bleeding (10.0%), yielding an odds ratio of 2.73 of death because of a major bleed. The most frequent types of fatal bleeding were intracranial bleeding and gastrointestinal bleeding. Of the 20 deaths reported in 135 patients with data, the causes of death were major bleeding (12 patients), infection (6 patients) and thrombosis (2 patients). Remission was observed in three of the nonsurvivors, indicating that even after remission, patients with FV inhibitor might still be susceptible to infection or thrombosis, according to the authors.
“[Major bleeding] should be treated aggressively; however, the best treatment is not clear and even patients in remission should be followed closely due to the risk of death from infection or thrombosis,” the authors concluded.
They reported having no conflicts of interest.
Coagulation factor V (FV) inhibitor is a rare disease with a mortality rate of nearly 15%. Increased mortality was significantly associated with the incidence of major bleeding, according to a review of PubMed case reports published in Thrombosis Update.
FV autoantibodies are most often detected in patients in the postoperative state, in those who have received a blood transfusion, in patients treated with antibiotics, and in those with immune diseases, according to the online report by Hideo Wada, MD, PhD, of the Mie Prefectural General Medical Center, Yokkaichi, Japan, and colleagues. These patients who acquired immune FV inhibitor (AIFVD) vary widely in symptoms from asymptomatic to mild or severe hemorrhagic manifestations, with some reports of thrombotic complications, the authors added.
Their review assessed the PubMed literature from Jan. 1, 1968, to July 31, 2020, and found 212 case reports on acquired FV deficiency. Of these, 150 cases with confirmed FV inhibitor positivity were included. The 150 reported cases of FV inhibitor were primarily from the United States (n = 48) and Japan (n = 43). The median patient age was 68.0 years, and the female to male ratio of patients was 0.47, according to the authors. The largest associated percentage of underlying conditions were postoperative state (25.3%), idiopathic (18.7%), infection (12.7%) and malignant neoplasms and autoimmune disease, at 7.3% each.
Major bleeds
A total of 73 cases were positive for major bleeding (48.7%) and 30 cases were negative (20.0%), while the rest were undetermined (31.3%). The FV activity was ≤ 28% in all patients with FV inhibitor.
The overall mortality rate was 14.6%, with half of the nonsurvivors dying of major bleeding. The mortality rate was more than twofold higher in the group with major bleeding (23.3% mortality) compared to the group without major bleeding (10.0%), yielding an odds ratio of 2.73 of death because of a major bleed. The most frequent types of fatal bleeding were intracranial bleeding and gastrointestinal bleeding. Of the 20 deaths reported in 135 patients with data, the causes of death were major bleeding (12 patients), infection (6 patients) and thrombosis (2 patients). Remission was observed in three of the nonsurvivors, indicating that even after remission, patients with FV inhibitor might still be susceptible to infection or thrombosis, according to the authors.
“[Major bleeding] should be treated aggressively; however, the best treatment is not clear and even patients in remission should be followed closely due to the risk of death from infection or thrombosis,” the authors concluded.
They reported having no conflicts of interest.
FROM THROMBOSIS UPDATE