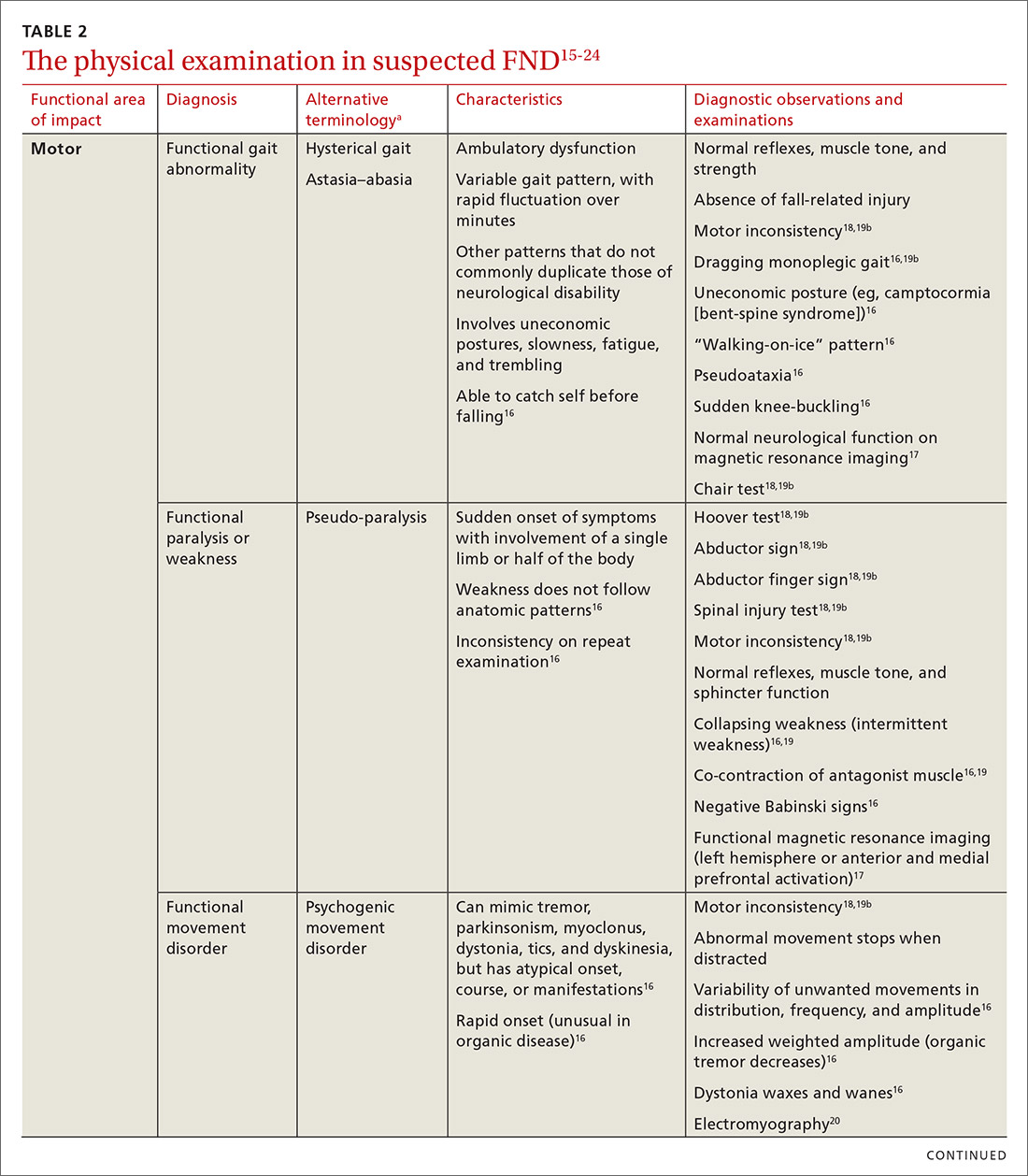
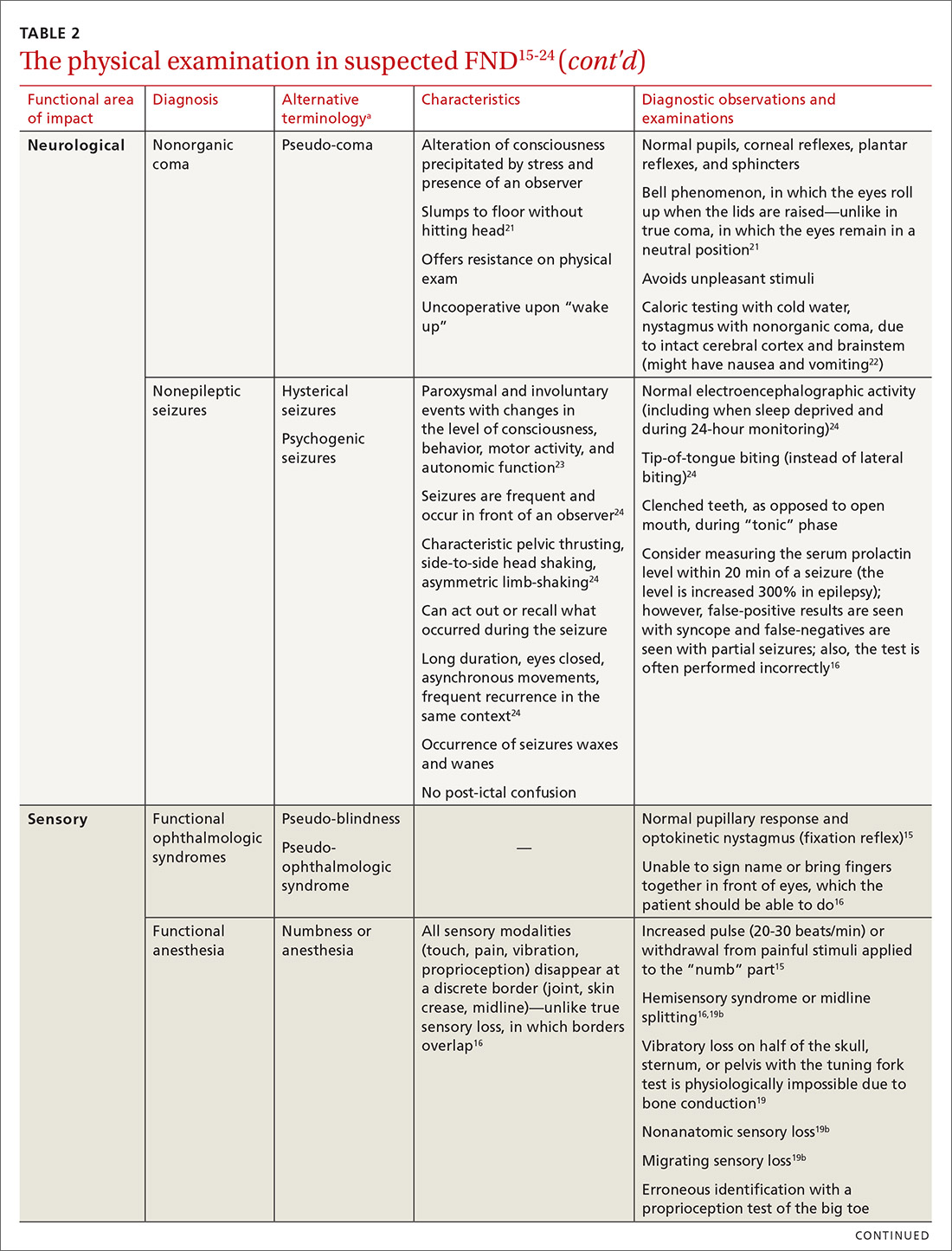
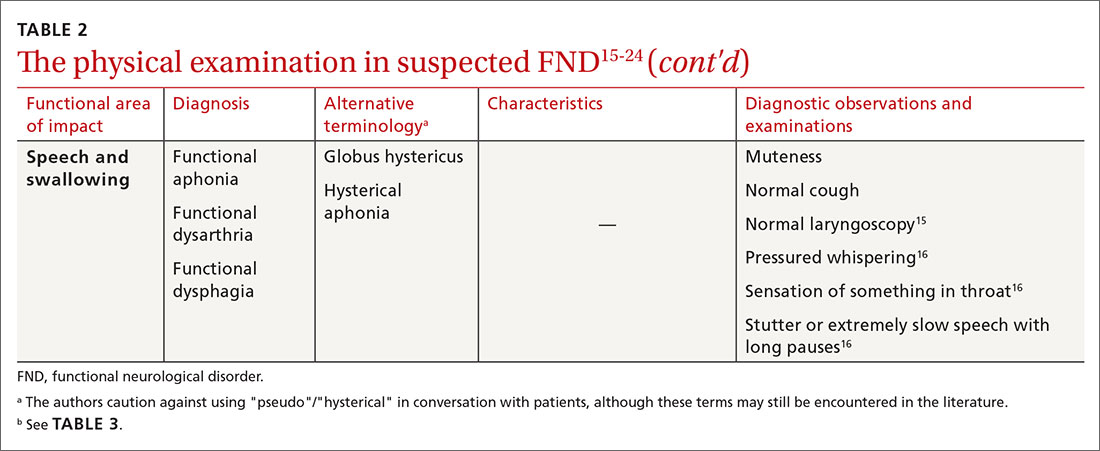
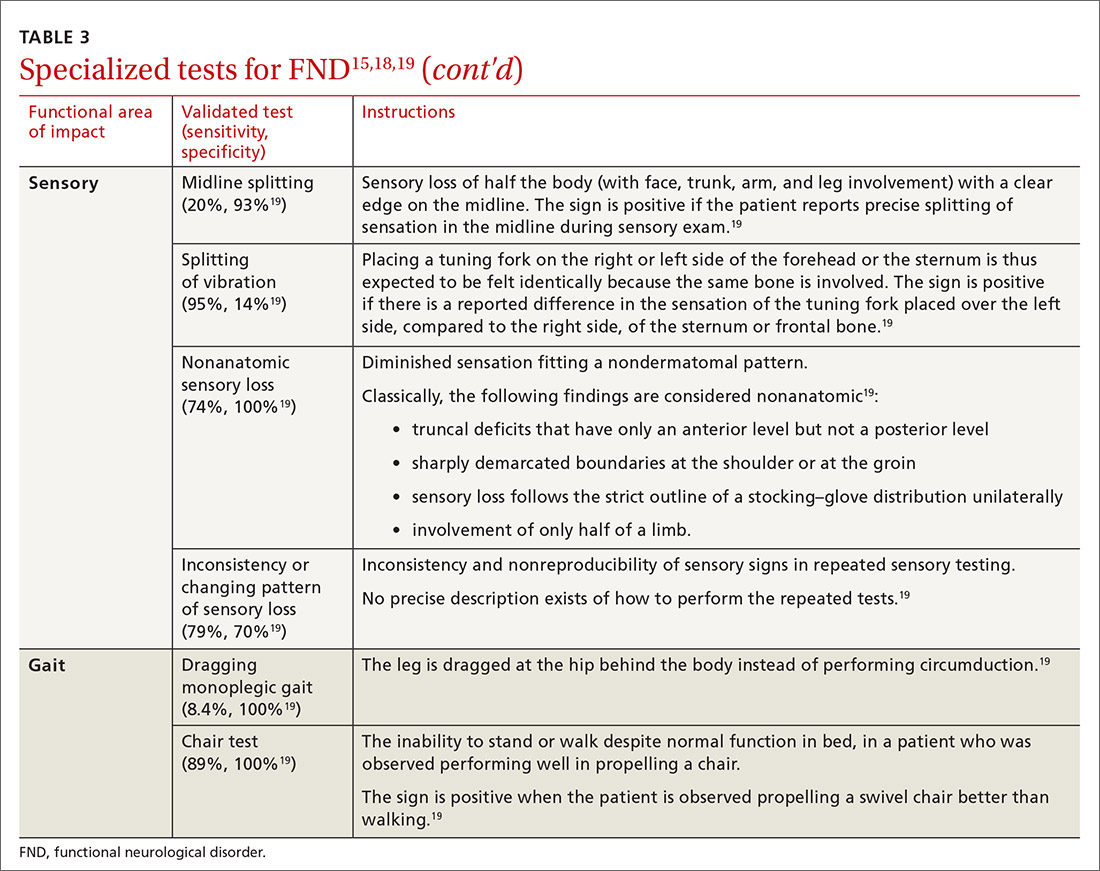
User login
Near-infrared imaging for tumors deep in tissue, such as GISTs
A new technique in which near-infrared hyperspectral imaging (NIR-HSI) is combined with machine learning could be useful for detecting cancers deep within tissue, suggests a research team from Japan.
An example of such a cancer is gastrointestinal stromal tumor (GIST). These tumors are often located in the submucosal layer and are covered by mucosal tissue, making diagnosis by conventional endoscopy difficult.
In a study to assess the new technique, the researchers examined 12 GIST lesions that were surgically resected. Seven of these lesions were totally covered by a mucosal layer (thickness, 0.4-2.5 mm); three lesions were partially covered.
Using NIR-HSI with machine learning, the researchers found that GIST lesions appeared green and that normal tissue appeared yellow. Calculation of classified pixels showed that the technique detected the GISTs with a specificity of 73%, a sensitivity of 91.3%, and an accuracy of 86.1%.
The findings were published in Nature’s Scientific Reports.
“There are many situations where we need to be able to detect cancers deep in the tissue that are not visible during surgery or diagnosis,” said lead author Toshihiro Takamatsu, PhD, assistant professor, Tokyo University of Science. “Near-infrared hyperspectral imaging has a strong potential to detect deep lesions,” he said.
In this study, the imaging was performed ex vivo.
“The data presented in the paper were not obtained endoscopically but utilized surgical specimens to demonstrate proof of concept. Additional data would be required to demonstrate its feasibility with endoscopy,” noted Margaret von Mehren, MD, chief, division of sarcoma medical oncology, Fox Chase Cancer Center, Philadelphia, who was approached for comment.
Dr. Takamatsu said his team is currently developing prototypes of laparoscopes and endoscopes for NIR-HSI.
“This technology is being developed to be added onto endoscopy, and as such I think may well be feasible,” Dr. von Mehren commented.
Currently diagnosed by endoscopy and biopsy
GISTs are found predominantly in the stomach (60%) and the small intestine (30%). Although some are detected after the occurrence of symptoms such as pain, gastrointestinal bleeding, and bowel obstruction, most cases are asymptomatic.
The authors note that endoscopic examination is the primary tool for detecting GISTs. Lesions usually first appear as submucosal tumors. Direct observation cannot differentially diagnose these tumors, and biopsies may have a low diagnostic yield, because the lesions are often deep and not easily accessible.
Endoscopic ultrasound-guided fine-needle aspiration can be used for taking samples for biopsy, but it can be technically demanding, and making a definitive diagnosis of GIST requires time-consuming immunohistochemical procedures, the authors write. A high-throughput, simple diagnostic technique for identifying GISTs located under the mucosa is needed. They report on the potential of NIR-HSI for diagnosing submucosal tumors that present deep within organs.
Study details
For the study, the team worked on GIST lesions that had been surgically resected from 12 patients. The median size of the tumors was 41 mm (range, 24-80 mm).
The researchers imaged each specimen with an NIR hyperspectral camera from the aspect of the mucosal surface.
The site of the GIST was defined by a pathologist who used the NIR image to prepare training data for normal regions and regions with GISTs. A machine learning algorithm–support vector machine was then used to predict normal and GIST regions.
The results were displayed using color-coded regions. The team says the results from this small study show that the technique has “great potential” in the diagnosis of GISTs as well as other tumors that are located deep within tissue.
“I think the potential benefit of such a diagnostic tool is with small lesions when trying to differentiate benign findings, such as leiomyomas,” Dr. von Mehren commented.
She pointed out that this study comes from Japan, where endoscopy is routinely used for surveillance of gastric cancer. Thus, many cases of GIST may be diagnosed before symptoms occur and when lesions are small. Such a practice is less common in other parts of the world, she told this news organization.
Overall, she sees this technique as having the most utility in cases involving small lesions, inasmuch as larger lesions are easily assessed through biopsy with endoscopic ultrasound, and pathologic assessment is not a lengthy procedure. “In addition, as we now appreciate that GISTs with different molecular drivers require different therapeutic approaches, I continue to see a role for tissue biopsies in the diagnostic workup of GISTs,” she said.
“Current approaches using endoscopic ultrasound have defined criteria for lesions more likely to be GISTs rather than benign lesions,” she added. “I would want to see a comparison of near-infrared hyperspectral imaging data compared to endoscopic ultrasound to see if this provides additional benefit to our current approaches.”
The study was partially funded by the National Cancer Center Research and Development Fund. The authors and Dr. von Mehren have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A new technique in which near-infrared hyperspectral imaging (NIR-HSI) is combined with machine learning could be useful for detecting cancers deep within tissue, suggests a research team from Japan.
An example of such a cancer is gastrointestinal stromal tumor (GIST). These tumors are often located in the submucosal layer and are covered by mucosal tissue, making diagnosis by conventional endoscopy difficult.
In a study to assess the new technique, the researchers examined 12 GIST lesions that were surgically resected. Seven of these lesions were totally covered by a mucosal layer (thickness, 0.4-2.5 mm); three lesions were partially covered.
Using NIR-HSI with machine learning, the researchers found that GIST lesions appeared green and that normal tissue appeared yellow. Calculation of classified pixels showed that the technique detected the GISTs with a specificity of 73%, a sensitivity of 91.3%, and an accuracy of 86.1%.
The findings were published in Nature’s Scientific Reports.
“There are many situations where we need to be able to detect cancers deep in the tissue that are not visible during surgery or diagnosis,” said lead author Toshihiro Takamatsu, PhD, assistant professor, Tokyo University of Science. “Near-infrared hyperspectral imaging has a strong potential to detect deep lesions,” he said.
In this study, the imaging was performed ex vivo.
“The data presented in the paper were not obtained endoscopically but utilized surgical specimens to demonstrate proof of concept. Additional data would be required to demonstrate its feasibility with endoscopy,” noted Margaret von Mehren, MD, chief, division of sarcoma medical oncology, Fox Chase Cancer Center, Philadelphia, who was approached for comment.
Dr. Takamatsu said his team is currently developing prototypes of laparoscopes and endoscopes for NIR-HSI.
“This technology is being developed to be added onto endoscopy, and as such I think may well be feasible,” Dr. von Mehren commented.
Currently diagnosed by endoscopy and biopsy
GISTs are found predominantly in the stomach (60%) and the small intestine (30%). Although some are detected after the occurrence of symptoms such as pain, gastrointestinal bleeding, and bowel obstruction, most cases are asymptomatic.
The authors note that endoscopic examination is the primary tool for detecting GISTs. Lesions usually first appear as submucosal tumors. Direct observation cannot differentially diagnose these tumors, and biopsies may have a low diagnostic yield, because the lesions are often deep and not easily accessible.
Endoscopic ultrasound-guided fine-needle aspiration can be used for taking samples for biopsy, but it can be technically demanding, and making a definitive diagnosis of GIST requires time-consuming immunohistochemical procedures, the authors write. A high-throughput, simple diagnostic technique for identifying GISTs located under the mucosa is needed. They report on the potential of NIR-HSI for diagnosing submucosal tumors that present deep within organs.
Study details
For the study, the team worked on GIST lesions that had been surgically resected from 12 patients. The median size of the tumors was 41 mm (range, 24-80 mm).
The researchers imaged each specimen with an NIR hyperspectral camera from the aspect of the mucosal surface.
The site of the GIST was defined by a pathologist who used the NIR image to prepare training data for normal regions and regions with GISTs. A machine learning algorithm–support vector machine was then used to predict normal and GIST regions.
The results were displayed using color-coded regions. The team says the results from this small study show that the technique has “great potential” in the diagnosis of GISTs as well as other tumors that are located deep within tissue.
“I think the potential benefit of such a diagnostic tool is with small lesions when trying to differentiate benign findings, such as leiomyomas,” Dr. von Mehren commented.
She pointed out that this study comes from Japan, where endoscopy is routinely used for surveillance of gastric cancer. Thus, many cases of GIST may be diagnosed before symptoms occur and when lesions are small. Such a practice is less common in other parts of the world, she told this news organization.
Overall, she sees this technique as having the most utility in cases involving small lesions, inasmuch as larger lesions are easily assessed through biopsy with endoscopic ultrasound, and pathologic assessment is not a lengthy procedure. “In addition, as we now appreciate that GISTs with different molecular drivers require different therapeutic approaches, I continue to see a role for tissue biopsies in the diagnostic workup of GISTs,” she said.
“Current approaches using endoscopic ultrasound have defined criteria for lesions more likely to be GISTs rather than benign lesions,” she added. “I would want to see a comparison of near-infrared hyperspectral imaging data compared to endoscopic ultrasound to see if this provides additional benefit to our current approaches.”
The study was partially funded by the National Cancer Center Research and Development Fund. The authors and Dr. von Mehren have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A new technique in which near-infrared hyperspectral imaging (NIR-HSI) is combined with machine learning could be useful for detecting cancers deep within tissue, suggests a research team from Japan.
An example of such a cancer is gastrointestinal stromal tumor (GIST). These tumors are often located in the submucosal layer and are covered by mucosal tissue, making diagnosis by conventional endoscopy difficult.
In a study to assess the new technique, the researchers examined 12 GIST lesions that were surgically resected. Seven of these lesions were totally covered by a mucosal layer (thickness, 0.4-2.5 mm); three lesions were partially covered.
Using NIR-HSI with machine learning, the researchers found that GIST lesions appeared green and that normal tissue appeared yellow. Calculation of classified pixels showed that the technique detected the GISTs with a specificity of 73%, a sensitivity of 91.3%, and an accuracy of 86.1%.
The findings were published in Nature’s Scientific Reports.
“There are many situations where we need to be able to detect cancers deep in the tissue that are not visible during surgery or diagnosis,” said lead author Toshihiro Takamatsu, PhD, assistant professor, Tokyo University of Science. “Near-infrared hyperspectral imaging has a strong potential to detect deep lesions,” he said.
In this study, the imaging was performed ex vivo.
“The data presented in the paper were not obtained endoscopically but utilized surgical specimens to demonstrate proof of concept. Additional data would be required to demonstrate its feasibility with endoscopy,” noted Margaret von Mehren, MD, chief, division of sarcoma medical oncology, Fox Chase Cancer Center, Philadelphia, who was approached for comment.
Dr. Takamatsu said his team is currently developing prototypes of laparoscopes and endoscopes for NIR-HSI.
“This technology is being developed to be added onto endoscopy, and as such I think may well be feasible,” Dr. von Mehren commented.
Currently diagnosed by endoscopy and biopsy
GISTs are found predominantly in the stomach (60%) and the small intestine (30%). Although some are detected after the occurrence of symptoms such as pain, gastrointestinal bleeding, and bowel obstruction, most cases are asymptomatic.
The authors note that endoscopic examination is the primary tool for detecting GISTs. Lesions usually first appear as submucosal tumors. Direct observation cannot differentially diagnose these tumors, and biopsies may have a low diagnostic yield, because the lesions are often deep and not easily accessible.
Endoscopic ultrasound-guided fine-needle aspiration can be used for taking samples for biopsy, but it can be technically demanding, and making a definitive diagnosis of GIST requires time-consuming immunohistochemical procedures, the authors write. A high-throughput, simple diagnostic technique for identifying GISTs located under the mucosa is needed. They report on the potential of NIR-HSI for diagnosing submucosal tumors that present deep within organs.
Study details
For the study, the team worked on GIST lesions that had been surgically resected from 12 patients. The median size of the tumors was 41 mm (range, 24-80 mm).
The researchers imaged each specimen with an NIR hyperspectral camera from the aspect of the mucosal surface.
The site of the GIST was defined by a pathologist who used the NIR image to prepare training data for normal regions and regions with GISTs. A machine learning algorithm–support vector machine was then used to predict normal and GIST regions.
The results were displayed using color-coded regions. The team says the results from this small study show that the technique has “great potential” in the diagnosis of GISTs as well as other tumors that are located deep within tissue.
“I think the potential benefit of such a diagnostic tool is with small lesions when trying to differentiate benign findings, such as leiomyomas,” Dr. von Mehren commented.
She pointed out that this study comes from Japan, where endoscopy is routinely used for surveillance of gastric cancer. Thus, many cases of GIST may be diagnosed before symptoms occur and when lesions are small. Such a practice is less common in other parts of the world, she told this news organization.
Overall, she sees this technique as having the most utility in cases involving small lesions, inasmuch as larger lesions are easily assessed through biopsy with endoscopic ultrasound, and pathologic assessment is not a lengthy procedure. “In addition, as we now appreciate that GISTs with different molecular drivers require different therapeutic approaches, I continue to see a role for tissue biopsies in the diagnostic workup of GISTs,” she said.
“Current approaches using endoscopic ultrasound have defined criteria for lesions more likely to be GISTs rather than benign lesions,” she added. “I would want to see a comparison of near-infrared hyperspectral imaging data compared to endoscopic ultrasound to see if this provides additional benefit to our current approaches.”
The study was partially funded by the National Cancer Center Research and Development Fund. The authors and Dr. von Mehren have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FDA supports robotic device as hysterectomy helper
Surgeons have a new tool for use in benign hysterectomies with the Food & Drug Administration’s authorization for marketing of the Hominis Surgical System, a robotic-assisted surgical device. The marketing authorization was granted to Memic Innovative Surgery.
The FDA reviewed the device through the De Novo classification review process, a regulatory pathway for low- to moderate-risk devices of a new type.
The robotically assisted surgical device (RASD) is designed to facilitate transvaginal hysterectomy procedures and salpingo-oophorectomy procedures in patients without cancer.
RASDs are not robots and require human control, but they allow a surgeon to use computer technology to control and move surgical instruments inserted through incisions or orifices. “RASD technology facilitates performing minimally invasive surgery and complex tasks in confined areas inside the body,” according to an FDA press release announcing the authorization.
“The FDA continues to support advancements in safe and effective medical devices that can improve patient experiences when undergoing surgical procedures,” Binita Ashar, MD, of the Office of Surgical and Infection Control Devices in the FDA’s Center for Devices and Radiological Health, said in the press release. The device represents another minimally invasive option for noncancerous conditions requiring gynecologic surgery.
The FDA also is establishing controls to ensure safety and effectiveness for RASDs, including labeling and performance testing requirements. “When met, the special controls, along with general controls, provide reasonable assurance of safety and effectiveness for devices of this type,” according to the press release.
The Hominis Surgical System involves the use of minimally invasive surgical instruments inserted through the vagina. A video camera is inserted laparoscopically through an abdominal incision; the camera allows the surgeon to visualize the instruments inside the patient.
“The FDA will require the manufacturer to develop and provide a comprehensive training program for surgeons and operating room staff to complete before operation of the device,” according to the press release.
The FDA reviewed data from a clinical study of 30 patients aged 37-79 years who underwent transvaginal total hysterectomy with salpingo-oophorectomy or salpingectomy for benign conditions.
Observed adverse events included minor blood loss, urinary tract infection and delayed healing of the closure made at the top of the vagina (vaginal cuff) that is done as part of a hysterectomy, according to the FDA. However, all 30 procedures were completed with no need for conversion to an open or other procedure.
Surgeons have a new tool for use in benign hysterectomies with the Food & Drug Administration’s authorization for marketing of the Hominis Surgical System, a robotic-assisted surgical device. The marketing authorization was granted to Memic Innovative Surgery.
The FDA reviewed the device through the De Novo classification review process, a regulatory pathway for low- to moderate-risk devices of a new type.
The robotically assisted surgical device (RASD) is designed to facilitate transvaginal hysterectomy procedures and salpingo-oophorectomy procedures in patients without cancer.
RASDs are not robots and require human control, but they allow a surgeon to use computer technology to control and move surgical instruments inserted through incisions or orifices. “RASD technology facilitates performing minimally invasive surgery and complex tasks in confined areas inside the body,” according to an FDA press release announcing the authorization.
“The FDA continues to support advancements in safe and effective medical devices that can improve patient experiences when undergoing surgical procedures,” Binita Ashar, MD, of the Office of Surgical and Infection Control Devices in the FDA’s Center for Devices and Radiological Health, said in the press release. The device represents another minimally invasive option for noncancerous conditions requiring gynecologic surgery.
The FDA also is establishing controls to ensure safety and effectiveness for RASDs, including labeling and performance testing requirements. “When met, the special controls, along with general controls, provide reasonable assurance of safety and effectiveness for devices of this type,” according to the press release.
The Hominis Surgical System involves the use of minimally invasive surgical instruments inserted through the vagina. A video camera is inserted laparoscopically through an abdominal incision; the camera allows the surgeon to visualize the instruments inside the patient.
“The FDA will require the manufacturer to develop and provide a comprehensive training program for surgeons and operating room staff to complete before operation of the device,” according to the press release.
The FDA reviewed data from a clinical study of 30 patients aged 37-79 years who underwent transvaginal total hysterectomy with salpingo-oophorectomy or salpingectomy for benign conditions.
Observed adverse events included minor blood loss, urinary tract infection and delayed healing of the closure made at the top of the vagina (vaginal cuff) that is done as part of a hysterectomy, according to the FDA. However, all 30 procedures were completed with no need for conversion to an open or other procedure.
Surgeons have a new tool for use in benign hysterectomies with the Food & Drug Administration’s authorization for marketing of the Hominis Surgical System, a robotic-assisted surgical device. The marketing authorization was granted to Memic Innovative Surgery.
The FDA reviewed the device through the De Novo classification review process, a regulatory pathway for low- to moderate-risk devices of a new type.
The robotically assisted surgical device (RASD) is designed to facilitate transvaginal hysterectomy procedures and salpingo-oophorectomy procedures in patients without cancer.
RASDs are not robots and require human control, but they allow a surgeon to use computer technology to control and move surgical instruments inserted through incisions or orifices. “RASD technology facilitates performing minimally invasive surgery and complex tasks in confined areas inside the body,” according to an FDA press release announcing the authorization.
“The FDA continues to support advancements in safe and effective medical devices that can improve patient experiences when undergoing surgical procedures,” Binita Ashar, MD, of the Office of Surgical and Infection Control Devices in the FDA’s Center for Devices and Radiological Health, said in the press release. The device represents another minimally invasive option for noncancerous conditions requiring gynecologic surgery.
The FDA also is establishing controls to ensure safety and effectiveness for RASDs, including labeling and performance testing requirements. “When met, the special controls, along with general controls, provide reasonable assurance of safety and effectiveness for devices of this type,” according to the press release.
The Hominis Surgical System involves the use of minimally invasive surgical instruments inserted through the vagina. A video camera is inserted laparoscopically through an abdominal incision; the camera allows the surgeon to visualize the instruments inside the patient.
“The FDA will require the manufacturer to develop and provide a comprehensive training program for surgeons and operating room staff to complete before operation of the device,” according to the press release.
The FDA reviewed data from a clinical study of 30 patients aged 37-79 years who underwent transvaginal total hysterectomy with salpingo-oophorectomy or salpingectomy for benign conditions.
Observed adverse events included minor blood loss, urinary tract infection and delayed healing of the closure made at the top of the vagina (vaginal cuff) that is done as part of a hysterectomy, according to the FDA. However, all 30 procedures were completed with no need for conversion to an open or other procedure.
Vagisil offered teens a vaginal ‘glow up.’ Docs cry foul
Late one night in early February, Jen Gunter, MD, was scrolling online when she discovered a new “feminine hygiene” product being marketed for teen girls. The new vanilla clementine scented wipes and cleansers with confetti-colored packaging and a cute name (OMV!) irked Dr. Gunter because they are designed for girls to use to “freshen” their vaginal area.
Dr. Gunter, a San Francisco-based gynecologist and author of “The Vagina Bible,” has built a reputation as a fierce advocate for women’s health and debunker of pseudoscience. She has called out jade eggs and “detox pearls” and various other items that promise to improve the vagina but that she and other doctors warn could actually be harmful. And, in her view, this product is no different.
She fired off a tweet that became the first volley in a vociferous social media countercampaign: “Hey @vagisil going to call you out here for this predatory line of products aimed at teen girls. Why do you think teen vulvas need special cleaning? To be prepped for men? Because they are dirty. Anxiously awaiting your answer as are all my followers.”
Vagisil responded on Instagram that “we want to clarify any confusion or the underlying belief that OMV! was developed because there is something wrong with teens or that vulvas/vaginas are inherently dirty. That is not the case. All-Day Fresh Wash is an all-over body wash, that is safe, gentle, and pH-balanced for sensitive vulvar area skin.”
Dr. Gunter’s Feb. 4 tweet attracted more than 8,300 likes, 1,300 retweets and hundreds of comments, but that was just the beginning. Dr. Gunter has continued to tweet about the OMV! product line – and has inspired dozens of other gynecologists to join in.
‘Your vagina is fine’
Dr. Gunter and other gynecologists have long delivered the message that water alone is sufficient to cleanse the vulvar area and that the vagina itself is self-cleaning. Research into the vaginal microbiome reveals the role of lactobacilli in preventing urogenital diseases. “Disturbances in your vagina microbiome are hard to undo,” says Jocelyn Fitzgerald, MD, a urogynecologist and pelvic reconstructive surgeon at Magee-Womens Hospital at the University of Pittsburgh Medical Center.
To underscore that message, Dr. Fitzgerald recently tweeted in support of Dr. Gunter’s Twitter thread: “Honestly, the @vagisil marketing campaign is a brilliant one because using their products while your vagina is perfectly fine will destroy your microbiome, give you real Bacterial Vaginosis, and prompt you to buy more Vagisil. DON’T FALL FOR IT GIRLS YOUR VAGINA IS FINE.”
In an emailed response to this news organization, a Vagisil spokesperson said, “We follow industry best practices for testing and OMV! products are rigorously assessed for safety and quality. In addition, we work with respected, independent clinical labs that follow strict testing protocols, using board-certified gynecologists and dermatologists to test our products before launch.”
However, beyond the potential for irritation or misuse, the gynecologists zeroed in on the underlying message that girls would feel more confident if they used the wipes and cleanser. For example, the company suggested that teens could use the wipes to get rid of “period funk.”
“There is no such thing as period funk!” gynecologist Danielle Jones, MD, exclaimed in a video on YouTube, where she has a channel called Mama Doctor Jones – with 700,000 subscribers. “All you need is ordinary hygiene. Period funk is not a thing! And if you feel like something is going on because there’s an odor that is abnormal, you need to talk to your doctor.”
Adult women often use wipes and special cleansers in the vaginal area. An online survey of 1,435 Canadian women, published in BMC Women’s Health in 2018, found 42% had used vaginal wipes, 12% had used vaginal washes or cleansers – and 4% had used them internally.
When it launched OMV! in July, Vagisil said it had engaged 2,500 teens and their mothers in creating the product, which it said was “designed to meet the cleansing and care needs of a new generation of young women.”
That extension of a product most commonly used by adult women to teenagers – who often feel self-conscious about their bodies – is exactly what bothers Dr. Gunter. “BTW I am sorry I am subjecting you all to my @vagisil outrage, but preying on teens and amplifying patriarchal shame of normal bodily functions to sell an irritating product is not acceptable. I’m not stopping until they take that OMV! product line down everywhere,” she said in a Feb. 8 tweet that attracted more than 7,900 likes.
No ‘glow up’ needed
Dr. Gunter’s tweets tapped into collective anger over the shaming of women’s bodies. The OMV! marketing suggested that teens could get a “glow up” with the products.
“Your vulva doesn’t need a ‘glow up.’ It’s fine like it is. And if it’s not, talk to your doctor,” Dr. Jones said in her Feb. 8 video, which has had almost 350,000 views, with 28,000 likes and only 149 dislikes.
“They’re very clearly pathologizing normal physiology,” Dr. Jones says. “They’re creating language that makes people feel as though their normal bodily functions have to be somehow fixed or changed.”
Dr. Gunter says she specifically wanted to prevent Vagisil from leveraging social media to influence teen girls. With her stream of tweets and support from colleagues around the country, she has sparked a prolonged online conversation.
“I am encouraged by the strong response on social media from both other enraged ob.gyns. and health care professionals as well the response from a lot of women and men,” Dr. Gunter said in an interview. “We have effectively blocked [Vagisil] from using social media.”
In its response to this news organization, Vagisil noted, “We are a brand run by women with daughters of our own.” While defending the products, Vagisil acknowledged the criticisms: “We are always listening to our consumers and our expert partners so that we continuously evolve. We appreciate the perspective that our language choice surrounding periods may perpetuate an old idea and have already begun to make changes to address this.”
Dr. Gunter says she plans to stay on topic. “Given the number of people outraged, I suspect if they venture out on social media again the reaction will be swift,” she said. “Hopefully we have made OMV! toxic for influencers as well.”
In fact, she’s ready to take on “the entire predatory feminine hygiene market. I’m sick of their false claims about balancing pH and not-so-subtle suggestions that vaginas and vulvas and menstruation stink. These products cause psychological harm as well as physical harm from their irritants,” she said.
A version of this article first appeared on Medscape.com.
Late one night in early February, Jen Gunter, MD, was scrolling online when she discovered a new “feminine hygiene” product being marketed for teen girls. The new vanilla clementine scented wipes and cleansers with confetti-colored packaging and a cute name (OMV!) irked Dr. Gunter because they are designed for girls to use to “freshen” their vaginal area.
Dr. Gunter, a San Francisco-based gynecologist and author of “The Vagina Bible,” has built a reputation as a fierce advocate for women’s health and debunker of pseudoscience. She has called out jade eggs and “detox pearls” and various other items that promise to improve the vagina but that she and other doctors warn could actually be harmful. And, in her view, this product is no different.
She fired off a tweet that became the first volley in a vociferous social media countercampaign: “Hey @vagisil going to call you out here for this predatory line of products aimed at teen girls. Why do you think teen vulvas need special cleaning? To be prepped for men? Because they are dirty. Anxiously awaiting your answer as are all my followers.”
Vagisil responded on Instagram that “we want to clarify any confusion or the underlying belief that OMV! was developed because there is something wrong with teens or that vulvas/vaginas are inherently dirty. That is not the case. All-Day Fresh Wash is an all-over body wash, that is safe, gentle, and pH-balanced for sensitive vulvar area skin.”
Dr. Gunter’s Feb. 4 tweet attracted more than 8,300 likes, 1,300 retweets and hundreds of comments, but that was just the beginning. Dr. Gunter has continued to tweet about the OMV! product line – and has inspired dozens of other gynecologists to join in.
‘Your vagina is fine’
Dr. Gunter and other gynecologists have long delivered the message that water alone is sufficient to cleanse the vulvar area and that the vagina itself is self-cleaning. Research into the vaginal microbiome reveals the role of lactobacilli in preventing urogenital diseases. “Disturbances in your vagina microbiome are hard to undo,” says Jocelyn Fitzgerald, MD, a urogynecologist and pelvic reconstructive surgeon at Magee-Womens Hospital at the University of Pittsburgh Medical Center.
To underscore that message, Dr. Fitzgerald recently tweeted in support of Dr. Gunter’s Twitter thread: “Honestly, the @vagisil marketing campaign is a brilliant one because using their products while your vagina is perfectly fine will destroy your microbiome, give you real Bacterial Vaginosis, and prompt you to buy more Vagisil. DON’T FALL FOR IT GIRLS YOUR VAGINA IS FINE.”
In an emailed response to this news organization, a Vagisil spokesperson said, “We follow industry best practices for testing and OMV! products are rigorously assessed for safety and quality. In addition, we work with respected, independent clinical labs that follow strict testing protocols, using board-certified gynecologists and dermatologists to test our products before launch.”
However, beyond the potential for irritation or misuse, the gynecologists zeroed in on the underlying message that girls would feel more confident if they used the wipes and cleanser. For example, the company suggested that teens could use the wipes to get rid of “period funk.”
“There is no such thing as period funk!” gynecologist Danielle Jones, MD, exclaimed in a video on YouTube, where she has a channel called Mama Doctor Jones – with 700,000 subscribers. “All you need is ordinary hygiene. Period funk is not a thing! And if you feel like something is going on because there’s an odor that is abnormal, you need to talk to your doctor.”
Adult women often use wipes and special cleansers in the vaginal area. An online survey of 1,435 Canadian women, published in BMC Women’s Health in 2018, found 42% had used vaginal wipes, 12% had used vaginal washes or cleansers – and 4% had used them internally.
When it launched OMV! in July, Vagisil said it had engaged 2,500 teens and their mothers in creating the product, which it said was “designed to meet the cleansing and care needs of a new generation of young women.”
That extension of a product most commonly used by adult women to teenagers – who often feel self-conscious about their bodies – is exactly what bothers Dr. Gunter. “BTW I am sorry I am subjecting you all to my @vagisil outrage, but preying on teens and amplifying patriarchal shame of normal bodily functions to sell an irritating product is not acceptable. I’m not stopping until they take that OMV! product line down everywhere,” she said in a Feb. 8 tweet that attracted more than 7,900 likes.
No ‘glow up’ needed
Dr. Gunter’s tweets tapped into collective anger over the shaming of women’s bodies. The OMV! marketing suggested that teens could get a “glow up” with the products.
“Your vulva doesn’t need a ‘glow up.’ It’s fine like it is. And if it’s not, talk to your doctor,” Dr. Jones said in her Feb. 8 video, which has had almost 350,000 views, with 28,000 likes and only 149 dislikes.
“They’re very clearly pathologizing normal physiology,” Dr. Jones says. “They’re creating language that makes people feel as though their normal bodily functions have to be somehow fixed or changed.”
Dr. Gunter says she specifically wanted to prevent Vagisil from leveraging social media to influence teen girls. With her stream of tweets and support from colleagues around the country, she has sparked a prolonged online conversation.
“I am encouraged by the strong response on social media from both other enraged ob.gyns. and health care professionals as well the response from a lot of women and men,” Dr. Gunter said in an interview. “We have effectively blocked [Vagisil] from using social media.”
In its response to this news organization, Vagisil noted, “We are a brand run by women with daughters of our own.” While defending the products, Vagisil acknowledged the criticisms: “We are always listening to our consumers and our expert partners so that we continuously evolve. We appreciate the perspective that our language choice surrounding periods may perpetuate an old idea and have already begun to make changes to address this.”
Dr. Gunter says she plans to stay on topic. “Given the number of people outraged, I suspect if they venture out on social media again the reaction will be swift,” she said. “Hopefully we have made OMV! toxic for influencers as well.”
In fact, she’s ready to take on “the entire predatory feminine hygiene market. I’m sick of their false claims about balancing pH and not-so-subtle suggestions that vaginas and vulvas and menstruation stink. These products cause psychological harm as well as physical harm from their irritants,” she said.
A version of this article first appeared on Medscape.com.
Late one night in early February, Jen Gunter, MD, was scrolling online when she discovered a new “feminine hygiene” product being marketed for teen girls. The new vanilla clementine scented wipes and cleansers with confetti-colored packaging and a cute name (OMV!) irked Dr. Gunter because they are designed for girls to use to “freshen” their vaginal area.
Dr. Gunter, a San Francisco-based gynecologist and author of “The Vagina Bible,” has built a reputation as a fierce advocate for women’s health and debunker of pseudoscience. She has called out jade eggs and “detox pearls” and various other items that promise to improve the vagina but that she and other doctors warn could actually be harmful. And, in her view, this product is no different.
She fired off a tweet that became the first volley in a vociferous social media countercampaign: “Hey @vagisil going to call you out here for this predatory line of products aimed at teen girls. Why do you think teen vulvas need special cleaning? To be prepped for men? Because they are dirty. Anxiously awaiting your answer as are all my followers.”
Vagisil responded on Instagram that “we want to clarify any confusion or the underlying belief that OMV! was developed because there is something wrong with teens or that vulvas/vaginas are inherently dirty. That is not the case. All-Day Fresh Wash is an all-over body wash, that is safe, gentle, and pH-balanced for sensitive vulvar area skin.”
Dr. Gunter’s Feb. 4 tweet attracted more than 8,300 likes, 1,300 retweets and hundreds of comments, but that was just the beginning. Dr. Gunter has continued to tweet about the OMV! product line – and has inspired dozens of other gynecologists to join in.
‘Your vagina is fine’
Dr. Gunter and other gynecologists have long delivered the message that water alone is sufficient to cleanse the vulvar area and that the vagina itself is self-cleaning. Research into the vaginal microbiome reveals the role of lactobacilli in preventing urogenital diseases. “Disturbances in your vagina microbiome are hard to undo,” says Jocelyn Fitzgerald, MD, a urogynecologist and pelvic reconstructive surgeon at Magee-Womens Hospital at the University of Pittsburgh Medical Center.
To underscore that message, Dr. Fitzgerald recently tweeted in support of Dr. Gunter’s Twitter thread: “Honestly, the @vagisil marketing campaign is a brilliant one because using their products while your vagina is perfectly fine will destroy your microbiome, give you real Bacterial Vaginosis, and prompt you to buy more Vagisil. DON’T FALL FOR IT GIRLS YOUR VAGINA IS FINE.”
In an emailed response to this news organization, a Vagisil spokesperson said, “We follow industry best practices for testing and OMV! products are rigorously assessed for safety and quality. In addition, we work with respected, independent clinical labs that follow strict testing protocols, using board-certified gynecologists and dermatologists to test our products before launch.”
However, beyond the potential for irritation or misuse, the gynecologists zeroed in on the underlying message that girls would feel more confident if they used the wipes and cleanser. For example, the company suggested that teens could use the wipes to get rid of “period funk.”
“There is no such thing as period funk!” gynecologist Danielle Jones, MD, exclaimed in a video on YouTube, where she has a channel called Mama Doctor Jones – with 700,000 subscribers. “All you need is ordinary hygiene. Period funk is not a thing! And if you feel like something is going on because there’s an odor that is abnormal, you need to talk to your doctor.”
Adult women often use wipes and special cleansers in the vaginal area. An online survey of 1,435 Canadian women, published in BMC Women’s Health in 2018, found 42% had used vaginal wipes, 12% had used vaginal washes or cleansers – and 4% had used them internally.
When it launched OMV! in July, Vagisil said it had engaged 2,500 teens and their mothers in creating the product, which it said was “designed to meet the cleansing and care needs of a new generation of young women.”
That extension of a product most commonly used by adult women to teenagers – who often feel self-conscious about their bodies – is exactly what bothers Dr. Gunter. “BTW I am sorry I am subjecting you all to my @vagisil outrage, but preying on teens and amplifying patriarchal shame of normal bodily functions to sell an irritating product is not acceptable. I’m not stopping until they take that OMV! product line down everywhere,” she said in a Feb. 8 tweet that attracted more than 7,900 likes.
No ‘glow up’ needed
Dr. Gunter’s tweets tapped into collective anger over the shaming of women’s bodies. The OMV! marketing suggested that teens could get a “glow up” with the products.
“Your vulva doesn’t need a ‘glow up.’ It’s fine like it is. And if it’s not, talk to your doctor,” Dr. Jones said in her Feb. 8 video, which has had almost 350,000 views, with 28,000 likes and only 149 dislikes.
“They’re very clearly pathologizing normal physiology,” Dr. Jones says. “They’re creating language that makes people feel as though their normal bodily functions have to be somehow fixed or changed.”
Dr. Gunter says she specifically wanted to prevent Vagisil from leveraging social media to influence teen girls. With her stream of tweets and support from colleagues around the country, she has sparked a prolonged online conversation.
“I am encouraged by the strong response on social media from both other enraged ob.gyns. and health care professionals as well the response from a lot of women and men,” Dr. Gunter said in an interview. “We have effectively blocked [Vagisil] from using social media.”
In its response to this news organization, Vagisil noted, “We are a brand run by women with daughters of our own.” While defending the products, Vagisil acknowledged the criticisms: “We are always listening to our consumers and our expert partners so that we continuously evolve. We appreciate the perspective that our language choice surrounding periods may perpetuate an old idea and have already begun to make changes to address this.”
Dr. Gunter says she plans to stay on topic. “Given the number of people outraged, I suspect if they venture out on social media again the reaction will be swift,” she said. “Hopefully we have made OMV! toxic for influencers as well.”
In fact, she’s ready to take on “the entire predatory feminine hygiene market. I’m sick of their false claims about balancing pH and not-so-subtle suggestions that vaginas and vulvas and menstruation stink. These products cause psychological harm as well as physical harm from their irritants,” she said.
A version of this article first appeared on Medscape.com.
New skin papules
A 49-year-old woman with a history of end-stage renal disease, uncontrolled type 2 diabetes, and congestive heart failure visited the hospital for an acute heart failure exacerbation secondary to missed dialysis appointments. On admission, her provider noted that she had tender, pruritic lesions on the extensor surface of her arms. She said they had appeared 2 to 3 months after she started dialysis. She had attempted to control the pain and pruritus with over-the-counter topical hydrocortisone and oral diphenhydramine but nothing provided relief. She was recommended for follow-up at the hospital for further examination and biopsy of one of her lesions.
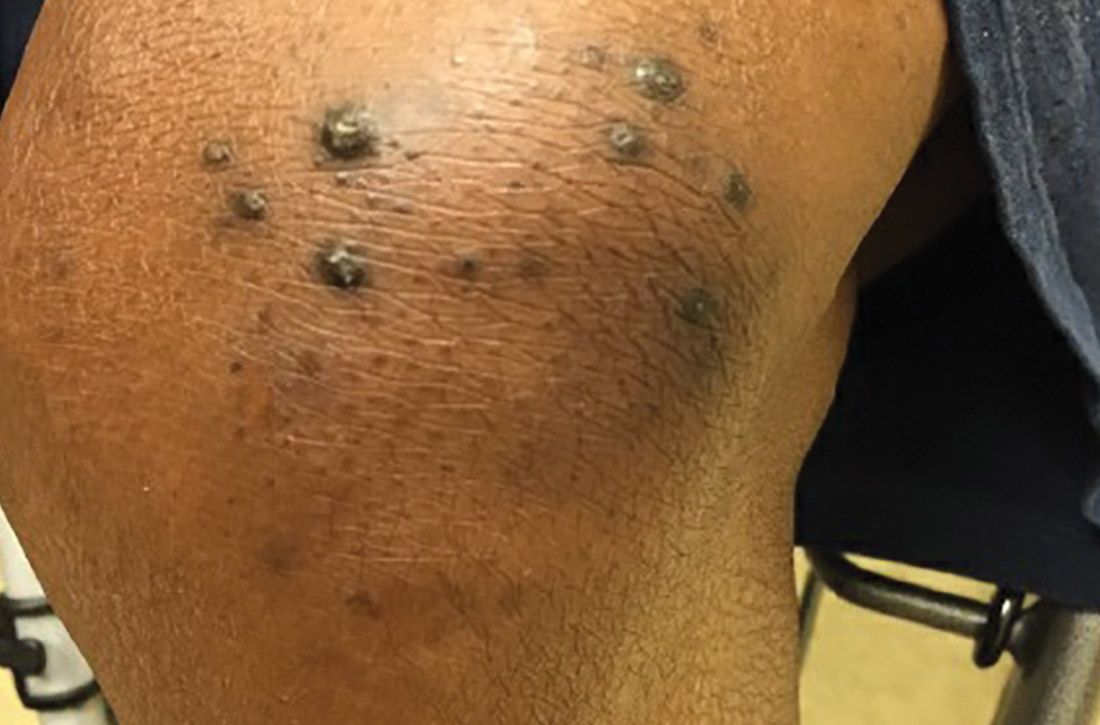
At this follow-up visit, the patient noted that the lesions had spread to her left knee. Multiple firm discrete papules and nodules, with central hyperkeratotic plugs, were noted along the extensor surfaces of her forearms, left extensor knee, and around her ankles (FIGURES 1A and 1B). Some of the lesions were tender. Examination of the rest of her skin was normal. A punch biopsy was obtained.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Kyrle disease
The patient’s end-stage renal disease and type 2 diabetes—along with findings from the physical examination—led us to suspect Kyrle disease. The punch biopsy, as well as the characteristic keratotic plugs (FIGURE 2) within epidermal invagination that was bordered by hyperkeratotic epidermis, confirmed the diagnosis.
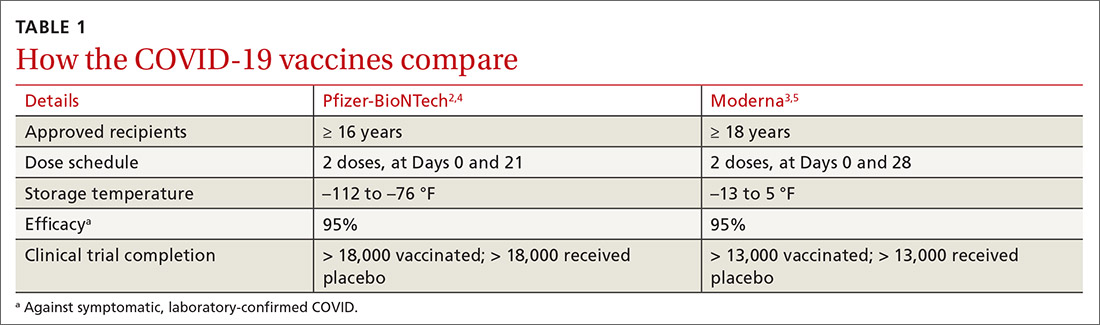
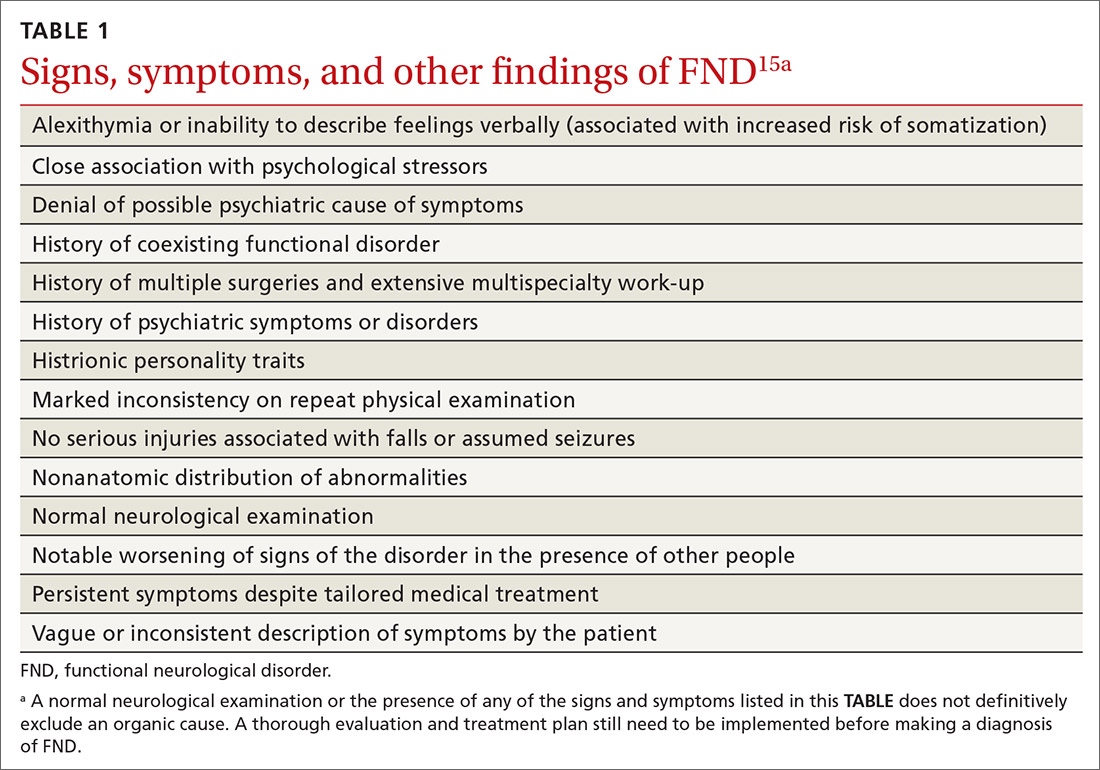
Kyrle disease (also known as hyperkeratosis follicularis et follicularis in cutem penetrans) is a rare skin condition. It is 1 of 4 skin conditions that are classified as perforating skin disorders; the other 3 are elastosis perforans serpiginosa, reactive perforating collagenosis, and perforating folliculitis (TABLE1,2).3 Perforating skin disorders share the common characteristic of transepidermal elimination of material from the upper dermis.4 These disorders are typically classified based on the nature of the eliminated material and the type of epidermal disruption.5
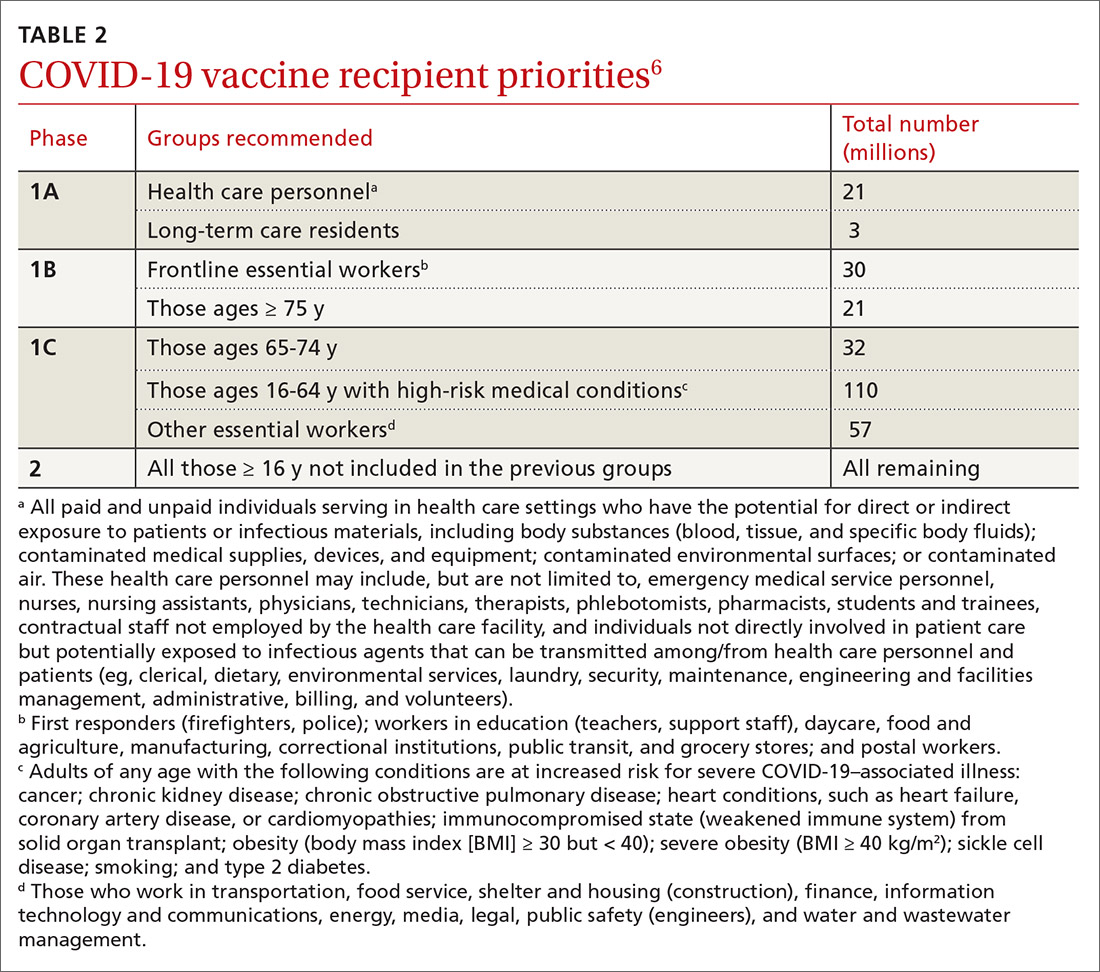
There are 2 forms of Kyrle disease: an inherited form often seen in childhood that is not associated with systemic disease and an acquired form that occurs in adulthood, most commonly among women ages 35 to 70 years who have systemic disease.3,4,6 The acquired form of Kyrle disease is associated with diabetes and renal failure, but there is a lack of data on its pathogenesis.7,8
Characteristic findings include discrete pruritic, dry papules and nodules with central keratotic plugs that are occasionally tender. These can manifest over the extensor surface of the extremities, trunk, face, and scalp.4,7,9 Lesions most commonly manifest on the extensor surfaces of the lower extremities.
Other conditions that feature pruritic lesions
In addition to the other perforating skin disorders described in the TABLE,1,2 the differential for Kyrle disease includes the following:
Prurigo nodularis (PN) is a skin disorder in which the manifestation of extremely pruritic nodules leads to vigorous scratching and secondary infections. These lesions typically have a grouped and symmetrically distributed appearance. They often appear on extensor surfaces of upper and lower extremities.10 PN has no known etiology, but like Kyrle disease, is associated with renal failure. Biopsy can help to distinguish PN from Kyrle disease.
Continue to: Hypertrophic lichen planus
Hypertrophic lichen planus is a pruritic skin disorder characterized by the “6 Ps”: planar, purple, polygonal, pruritic, papules, and plaques. These lesions can mimic the early stages of Kyrle disease.11 However, in the later stages of Kyrle disease, discrete papules with hyperkeratotic plugs develop, whereas large plaques will be seen with lichen planus.
Keratosis pilaris (KP) is an extremely common, yet benign, disorder in which hair follicles become keratinized.12 KP can feature rough papules that are often described as “goosebumps” or having a sandpaper–like appearance. These papules often affect the upper arms. KP usually manifests in adolescents or young adults and tends to improve with age.12 The lesions are typically smaller than those seen in Kyrle disease and are asymptomatic. In addition, KP is not associated with systemic disease.
Target symptoms and any underlying conditions
In patients who have an acquired form of the disease, symptoms may improve by
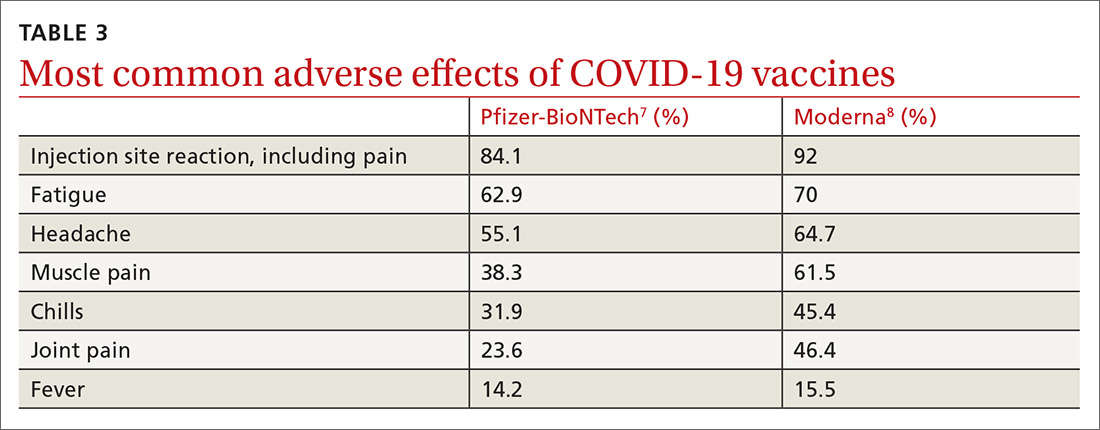
For patients whose Kyrle disease is inherited or whose underlying condition is not easily treated, there are a number of treatment options to consider. First-line treatment includes topical keratolytics (salicylic acid and urea), topical retinoids, and ultraviolet light therapy.5,7 Systemic retinoids, topical steroids, cryotherapy, electrosurgery, CO2 laser surgery, and surgical excision have also been used with some success.7,14 Oral histamines and emollients also may help to relieve the pruritus. Lesions often recur upon discontinuation of therapy.
Our patient was referred to Dermatology for ultraviolet light therapy. She was also treated with topical 12% ammonium lactate twice daily. Within a few months, she reported improvement of her symptoms.
1. Rapini R. Perforating disorders. Plastic Surgery Key. Published April 22, 2017. Accessed February 18, 2021. https://plasticsurgerykey.com/perforating-disorders/
2. Patterson JW. The perforating disorders. J Am Acad Dermatol. 1984;10:561-581
3. Azad K, Hajirnis K, Sawant S, et al. Kyrle’s disease. Indian Dermatol Online J. 2013;4:378-379.
4. Arora K, Hajirnis KA, Sawant S, et al. Perforating disorders of the skin. Indian J Pathol Microbiol. 2013;56:355-358.
5. Ataseven A, Ozturk P, Kucukosmanoglu I, et al. Kyrle’s disease. BMJ Case Rep. 2014;2014: bcr2013009905.
6. Cunningham SR, Walsh M, Matthews R. Kyrle’s disease. J Am Acad Dermatol. 1987;16(pt 1):117-123.
7. Nair PA, Jivani NB, Diwan NG. Kyrle’s disease in a patient of diabetes mellitus and chronic renal failure on dialysis. J Family Med Prim Care. 2015;4:284-286.
8. Hurwitz RM, Melton ME, Creech FT 3rd, et al. Perforating folliculitis in association with hemodialysis. Am J Dermatopathol. 1982;4:101-108.
9. Kolla PK, Desai M, Pathapati RM, et al. Cutaneous manifestations in patients with chronic kidney disease on maintenance hemodialysis. ISRN Dermatol. 2012;2012:679619.
10. Lee MR, Shumack S. Prurigo nodularis: a review. Australas J Dermatol. 2005;46:211-220.
11. Usatine RP, Tinitigan M. Diagnosis and treatment of lichen planus. Am Fam Physician. 2011;84:53-60.
12. Thomas M, Khopkar US. Keratosis pilaris revisited: is it more than just a follicular keratosis? Int J Trichology. 2012;4:255-258.
13. Chang P, Fernández V. Acquired perforating disease: report of nine cases. Int J Dermatol. 1993;32:874-876.
14. Wagner G, Sachse MM. Acquired reactive perforating dermatosis. J Dtsch Dermatol Ges. 2013;11:723-729.
A 49-year-old woman with a history of end-stage renal disease, uncontrolled type 2 diabetes, and congestive heart failure visited the hospital for an acute heart failure exacerbation secondary to missed dialysis appointments. On admission, her provider noted that she had tender, pruritic lesions on the extensor surface of her arms. She said they had appeared 2 to 3 months after she started dialysis. She had attempted to control the pain and pruritus with over-the-counter topical hydrocortisone and oral diphenhydramine but nothing provided relief. She was recommended for follow-up at the hospital for further examination and biopsy of one of her lesions.
At this follow-up visit, the patient noted that the lesions had spread to her left knee. Multiple firm discrete papules and nodules, with central hyperkeratotic plugs, were noted along the extensor surfaces of her forearms, left extensor knee, and around her ankles (FIGURES 1A and 1B). Some of the lesions were tender. Examination of the rest of her skin was normal. A punch biopsy was obtained.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Kyrle disease
The patient’s end-stage renal disease and type 2 diabetes—along with findings from the physical examination—led us to suspect Kyrle disease. The punch biopsy, as well as the characteristic keratotic plugs (FIGURE 2) within epidermal invagination that was bordered by hyperkeratotic epidermis, confirmed the diagnosis.
Kyrle disease (also known as hyperkeratosis follicularis et follicularis in cutem penetrans) is a rare skin condition. It is 1 of 4 skin conditions that are classified as perforating skin disorders; the other 3 are elastosis perforans serpiginosa, reactive perforating collagenosis, and perforating folliculitis (TABLE1,2).3 Perforating skin disorders share the common characteristic of transepidermal elimination of material from the upper dermis.4 These disorders are typically classified based on the nature of the eliminated material and the type of epidermal disruption.5
There are 2 forms of Kyrle disease: an inherited form often seen in childhood that is not associated with systemic disease and an acquired form that occurs in adulthood, most commonly among women ages 35 to 70 years who have systemic disease.3,4,6 The acquired form of Kyrle disease is associated with diabetes and renal failure, but there is a lack of data on its pathogenesis.7,8
Characteristic findings include discrete pruritic, dry papules and nodules with central keratotic plugs that are occasionally tender. These can manifest over the extensor surface of the extremities, trunk, face, and scalp.4,7,9 Lesions most commonly manifest on the extensor surfaces of the lower extremities.
Other conditions that feature pruritic lesions
In addition to the other perforating skin disorders described in the TABLE,1,2 the differential for Kyrle disease includes the following:
Prurigo nodularis (PN) is a skin disorder in which the manifestation of extremely pruritic nodules leads to vigorous scratching and secondary infections. These lesions typically have a grouped and symmetrically distributed appearance. They often appear on extensor surfaces of upper and lower extremities.10 PN has no known etiology, but like Kyrle disease, is associated with renal failure. Biopsy can help to distinguish PN from Kyrle disease.
Continue to: Hypertrophic lichen planus
Hypertrophic lichen planus is a pruritic skin disorder characterized by the “6 Ps”: planar, purple, polygonal, pruritic, papules, and plaques. These lesions can mimic the early stages of Kyrle disease.11 However, in the later stages of Kyrle disease, discrete papules with hyperkeratotic plugs develop, whereas large plaques will be seen with lichen planus.
Keratosis pilaris (KP) is an extremely common, yet benign, disorder in which hair follicles become keratinized.12 KP can feature rough papules that are often described as “goosebumps” or having a sandpaper–like appearance. These papules often affect the upper arms. KP usually manifests in adolescents or young adults and tends to improve with age.12 The lesions are typically smaller than those seen in Kyrle disease and are asymptomatic. In addition, KP is not associated with systemic disease.
Target symptoms and any underlying conditions
In patients who have an acquired form of the disease, symptoms may improve by
For patients whose Kyrle disease is inherited or whose underlying condition is not easily treated, there are a number of treatment options to consider. First-line treatment includes topical keratolytics (salicylic acid and urea), topical retinoids, and ultraviolet light therapy.5,7 Systemic retinoids, topical steroids, cryotherapy, electrosurgery, CO2 laser surgery, and surgical excision have also been used with some success.7,14 Oral histamines and emollients also may help to relieve the pruritus. Lesions often recur upon discontinuation of therapy.
Our patient was referred to Dermatology for ultraviolet light therapy. She was also treated with topical 12% ammonium lactate twice daily. Within a few months, she reported improvement of her symptoms.
A 49-year-old woman with a history of end-stage renal disease, uncontrolled type 2 diabetes, and congestive heart failure visited the hospital for an acute heart failure exacerbation secondary to missed dialysis appointments. On admission, her provider noted that she had tender, pruritic lesions on the extensor surface of her arms. She said they had appeared 2 to 3 months after she started dialysis. She had attempted to control the pain and pruritus with over-the-counter topical hydrocortisone and oral diphenhydramine but nothing provided relief. She was recommended for follow-up at the hospital for further examination and biopsy of one of her lesions.
At this follow-up visit, the patient noted that the lesions had spread to her left knee. Multiple firm discrete papules and nodules, with central hyperkeratotic plugs, were noted along the extensor surfaces of her forearms, left extensor knee, and around her ankles (FIGURES 1A and 1B). Some of the lesions were tender. Examination of the rest of her skin was normal. A punch biopsy was obtained.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Kyrle disease
The patient’s end-stage renal disease and type 2 diabetes—along with findings from the physical examination—led us to suspect Kyrle disease. The punch biopsy, as well as the characteristic keratotic plugs (FIGURE 2) within epidermal invagination that was bordered by hyperkeratotic epidermis, confirmed the diagnosis.
Kyrle disease (also known as hyperkeratosis follicularis et follicularis in cutem penetrans) is a rare skin condition. It is 1 of 4 skin conditions that are classified as perforating skin disorders; the other 3 are elastosis perforans serpiginosa, reactive perforating collagenosis, and perforating folliculitis (TABLE1,2).3 Perforating skin disorders share the common characteristic of transepidermal elimination of material from the upper dermis.4 These disorders are typically classified based on the nature of the eliminated material and the type of epidermal disruption.5
There are 2 forms of Kyrle disease: an inherited form often seen in childhood that is not associated with systemic disease and an acquired form that occurs in adulthood, most commonly among women ages 35 to 70 years who have systemic disease.3,4,6 The acquired form of Kyrle disease is associated with diabetes and renal failure, but there is a lack of data on its pathogenesis.7,8
Characteristic findings include discrete pruritic, dry papules and nodules with central keratotic plugs that are occasionally tender. These can manifest over the extensor surface of the extremities, trunk, face, and scalp.4,7,9 Lesions most commonly manifest on the extensor surfaces of the lower extremities.
Other conditions that feature pruritic lesions
In addition to the other perforating skin disorders described in the TABLE,1,2 the differential for Kyrle disease includes the following:
Prurigo nodularis (PN) is a skin disorder in which the manifestation of extremely pruritic nodules leads to vigorous scratching and secondary infections. These lesions typically have a grouped and symmetrically distributed appearance. They often appear on extensor surfaces of upper and lower extremities.10 PN has no known etiology, but like Kyrle disease, is associated with renal failure. Biopsy can help to distinguish PN from Kyrle disease.
Continue to: Hypertrophic lichen planus
Hypertrophic lichen planus is a pruritic skin disorder characterized by the “6 Ps”: planar, purple, polygonal, pruritic, papules, and plaques. These lesions can mimic the early stages of Kyrle disease.11 However, in the later stages of Kyrle disease, discrete papules with hyperkeratotic plugs develop, whereas large plaques will be seen with lichen planus.
Keratosis pilaris (KP) is an extremely common, yet benign, disorder in which hair follicles become keratinized.12 KP can feature rough papules that are often described as “goosebumps” or having a sandpaper–like appearance. These papules often affect the upper arms. KP usually manifests in adolescents or young adults and tends to improve with age.12 The lesions are typically smaller than those seen in Kyrle disease and are asymptomatic. In addition, KP is not associated with systemic disease.
Target symptoms and any underlying conditions
In patients who have an acquired form of the disease, symptoms may improve by
For patients whose Kyrle disease is inherited or whose underlying condition is not easily treated, there are a number of treatment options to consider. First-line treatment includes topical keratolytics (salicylic acid and urea), topical retinoids, and ultraviolet light therapy.5,7 Systemic retinoids, topical steroids, cryotherapy, electrosurgery, CO2 laser surgery, and surgical excision have also been used with some success.7,14 Oral histamines and emollients also may help to relieve the pruritus. Lesions often recur upon discontinuation of therapy.
Our patient was referred to Dermatology for ultraviolet light therapy. She was also treated with topical 12% ammonium lactate twice daily. Within a few months, she reported improvement of her symptoms.
1. Rapini R. Perforating disorders. Plastic Surgery Key. Published April 22, 2017. Accessed February 18, 2021. https://plasticsurgerykey.com/perforating-disorders/
2. Patterson JW. The perforating disorders. J Am Acad Dermatol. 1984;10:561-581
3. Azad K, Hajirnis K, Sawant S, et al. Kyrle’s disease. Indian Dermatol Online J. 2013;4:378-379.
4. Arora K, Hajirnis KA, Sawant S, et al. Perforating disorders of the skin. Indian J Pathol Microbiol. 2013;56:355-358.
5. Ataseven A, Ozturk P, Kucukosmanoglu I, et al. Kyrle’s disease. BMJ Case Rep. 2014;2014: bcr2013009905.
6. Cunningham SR, Walsh M, Matthews R. Kyrle’s disease. J Am Acad Dermatol. 1987;16(pt 1):117-123.
7. Nair PA, Jivani NB, Diwan NG. Kyrle’s disease in a patient of diabetes mellitus and chronic renal failure on dialysis. J Family Med Prim Care. 2015;4:284-286.
8. Hurwitz RM, Melton ME, Creech FT 3rd, et al. Perforating folliculitis in association with hemodialysis. Am J Dermatopathol. 1982;4:101-108.
9. Kolla PK, Desai M, Pathapati RM, et al. Cutaneous manifestations in patients with chronic kidney disease on maintenance hemodialysis. ISRN Dermatol. 2012;2012:679619.
10. Lee MR, Shumack S. Prurigo nodularis: a review. Australas J Dermatol. 2005;46:211-220.
11. Usatine RP, Tinitigan M. Diagnosis and treatment of lichen planus. Am Fam Physician. 2011;84:53-60.
12. Thomas M, Khopkar US. Keratosis pilaris revisited: is it more than just a follicular keratosis? Int J Trichology. 2012;4:255-258.
13. Chang P, Fernández V. Acquired perforating disease: report of nine cases. Int J Dermatol. 1993;32:874-876.
14. Wagner G, Sachse MM. Acquired reactive perforating dermatosis. J Dtsch Dermatol Ges. 2013;11:723-729.
1. Rapini R. Perforating disorders. Plastic Surgery Key. Published April 22, 2017. Accessed February 18, 2021. https://plasticsurgerykey.com/perforating-disorders/
2. Patterson JW. The perforating disorders. J Am Acad Dermatol. 1984;10:561-581
3. Azad K, Hajirnis K, Sawant S, et al. Kyrle’s disease. Indian Dermatol Online J. 2013;4:378-379.
4. Arora K, Hajirnis KA, Sawant S, et al. Perforating disorders of the skin. Indian J Pathol Microbiol. 2013;56:355-358.
5. Ataseven A, Ozturk P, Kucukosmanoglu I, et al. Kyrle’s disease. BMJ Case Rep. 2014;2014: bcr2013009905.
6. Cunningham SR, Walsh M, Matthews R. Kyrle’s disease. J Am Acad Dermatol. 1987;16(pt 1):117-123.
7. Nair PA, Jivani NB, Diwan NG. Kyrle’s disease in a patient of diabetes mellitus and chronic renal failure on dialysis. J Family Med Prim Care. 2015;4:284-286.
8. Hurwitz RM, Melton ME, Creech FT 3rd, et al. Perforating folliculitis in association with hemodialysis. Am J Dermatopathol. 1982;4:101-108.
9. Kolla PK, Desai M, Pathapati RM, et al. Cutaneous manifestations in patients with chronic kidney disease on maintenance hemodialysis. ISRN Dermatol. 2012;2012:679619.
10. Lee MR, Shumack S. Prurigo nodularis: a review. Australas J Dermatol. 2005;46:211-220.
11. Usatine RP, Tinitigan M. Diagnosis and treatment of lichen planus. Am Fam Physician. 2011;84:53-60.
12. Thomas M, Khopkar US. Keratosis pilaris revisited: is it more than just a follicular keratosis? Int J Trichology. 2012;4:255-258.
13. Chang P, Fernández V. Acquired perforating disease: report of nine cases. Int J Dermatol. 1993;32:874-876.
14. Wagner G, Sachse MM. Acquired reactive perforating dermatosis. J Dtsch Dermatol Ges. 2013;11:723-729.
‘Phenomenal’ results with CAR T cells in R/R multiple myeloma
Patients with multiple myeloma that has continued to progress despite many lines of therapy have shown deep and durable responses to a new chimeric antigen receptor (CAR) T-cell therapy, idecabtagene vicleucel (ide-cel, under development by Bristol-Myers Squibb and Bluebird Bio).
An expert not involved in the trial described the results as “phenomenal.”
Krina Patel, MD, an associate professor in the department of lymphoma/myeloma at the University of Texas MD Anderson Cancer Center, Houston, said that “the response rate of 73% in a patient population with a median of six lines of therapy, and with one-third of those patients achieving a deep response of complete response or better, is phenomenal.”
“We are very excited as a myeloma community for this study of idecabtagene vicleucel for relapsed/refractory patients,” Dr. Patel said.
The new data on ide-cell, from a trial in 128 patients, were published Feb. 25 in the New England Journal of Medicine.
Lead investigator of the study Nikhil Munshi, MD, of Dana-Farber Cancer Institute, Boston, said: “The results of this trial represent a true turning point in the treatment of this disease. In my 30 years of treating myeloma, I have not seen any other therapy as effective in this group of patients.”
Both experts highlighted the poor prognosis for this population of relapsed/refractory patients. Recent decades have seen a flurry of new agents for myeloma, and there are now three main classes of agents: immunomodulatory agents, proteasome inhibitors, and anti-CD38 antibodies. Nevertheless, in some patients, the disease continues to progress. For patients who have failed all three classes of drugs, the median progression-free survival is about 3-4 months, with a median overall survival of 8-9 months.
Product is awaiting approval
Ide-cel is currently awaiting FDA approval, with a decision date slated for March 27.
Several CAR T-cell products are already marketed for use in certain leukemias and lymphomas, and there is another for use in multiple myeloma, ciltacabtagene autoleucel (cilta-cel, under development by Janssen), that is awaiting approval in Europe.
Strong and sustained responses
The trial involved 128 patients treated with ide-cel infusions. At the time of data cutoff for this report (Jan. 14, 2020), 62 patients remained in the primary study. Of the 128 treated patients, the median age was 61 years and the median time since diagnosis was 6 years. About half (51%) had a high tumor burden (≥50% bone marrow plasma cells), 39% had extramedullary disease, 16% had stage III disease, and 35% had a high-risk cytogenetic abnormality, defined as del(17p), t(4;14), or t(14;16).
Patients in the cohort had received a median of six previous antimyeloma regimens (range, 3-16), and most of the patients (120, 94%) had undergone autologous hematopoietic stem cell transplants. In addition, the majority of patients (84%) had disease that was triple refractory (to an immunomodulatory agent, a proteasome inhibitor, and an anti-CD38 antibody), 60% had disease that was penta exposed (to bortezomib, carfilzomib, lenalidomide, pomalidomide, and daratumumab), and 26% had disease that was penta refractory.
At a median follow-up of 13.3 months, 94 of 128 patients (73%) showed a response to therapy (P < .001), with 42 (33%) showing a complete or stringent complete response, and 67 patients (52%) showing a “very good partial response or better.”
Overall median progression-free survival was 8.8 months at the 450×106 dose but more than double that (20.2 months) for patients who achieved a complete or stringent complete response. Estimated median overall survival was 19.4 months, with an overall survival of 78% at 12 months. The authors noted that overall survival data are not yet mature.
After experiencing disease progression, 28 patients were retreated with ide-cel, with 6 patients showing a second response. The durations of response ranged from 1.9 to 6.8 months.
All patients in the cohort experienced adverse events, primarily grade 3 or 4 events that occurred in 127 patients (99%). The most common events reported were hematologic toxicities, including neutropenia in 114 patients (89%), anemia in 77 (60%), and thrombocytopenia in 67 (52%), and were at least partially related to the lymphodepleting chemotherapy administered before ide-cel infusion, the authors note. Cytokine-release syndrome occurred in 107 patients (84%), primarily grade 1 or 2.
“Results of the KarMMa study support substantial antitumor activity for ide-cel across a target dose range of 150×106 to 450×106 CAR+ T cells,” the authors conclude. “The 450×106 dose appeared to be somewhat more effective than the other doses.”
New option?
“What this study further highlights is that higher cell dose tends to increase cell expansion, which correlates to improved response and duration of response,” said Dr. Patel.
Importantly, multiple vulnerable subgroups experienced impressive outcomes, such as those who are older or with high risk or extramedullary disease, she noted.
“My patients who have undergone this therapy, albeit on other clinical trials, all say that their quality of life during this time of remission is priceless,” Dr. Patel added. “The is the first therapy in the relapsed/refractory setting that allows patients to have a significant chemo-free period. We need to find more ways to do this for our patients.”
The study was supported by Bluebird Bio and Bristol-Myers Squibb. Dr. Patel has served on the advisory board for Janssen and Bristol-Myers Squibb. She also reports a speaking engagement with Oncopeptides. Dr. Munshi acts as a consultant for several pharmaceutical companies, and many coauthors also have relationships with industry, as listed in the original article.
A version of this article first appeared on Medscape.com.
Patients with multiple myeloma that has continued to progress despite many lines of therapy have shown deep and durable responses to a new chimeric antigen receptor (CAR) T-cell therapy, idecabtagene vicleucel (ide-cel, under development by Bristol-Myers Squibb and Bluebird Bio).
An expert not involved in the trial described the results as “phenomenal.”
Krina Patel, MD, an associate professor in the department of lymphoma/myeloma at the University of Texas MD Anderson Cancer Center, Houston, said that “the response rate of 73% in a patient population with a median of six lines of therapy, and with one-third of those patients achieving a deep response of complete response or better, is phenomenal.”
“We are very excited as a myeloma community for this study of idecabtagene vicleucel for relapsed/refractory patients,” Dr. Patel said.
The new data on ide-cell, from a trial in 128 patients, were published Feb. 25 in the New England Journal of Medicine.
Lead investigator of the study Nikhil Munshi, MD, of Dana-Farber Cancer Institute, Boston, said: “The results of this trial represent a true turning point in the treatment of this disease. In my 30 years of treating myeloma, I have not seen any other therapy as effective in this group of patients.”
Both experts highlighted the poor prognosis for this population of relapsed/refractory patients. Recent decades have seen a flurry of new agents for myeloma, and there are now three main classes of agents: immunomodulatory agents, proteasome inhibitors, and anti-CD38 antibodies. Nevertheless, in some patients, the disease continues to progress. For patients who have failed all three classes of drugs, the median progression-free survival is about 3-4 months, with a median overall survival of 8-9 months.
Product is awaiting approval
Ide-cel is currently awaiting FDA approval, with a decision date slated for March 27.
Several CAR T-cell products are already marketed for use in certain leukemias and lymphomas, and there is another for use in multiple myeloma, ciltacabtagene autoleucel (cilta-cel, under development by Janssen), that is awaiting approval in Europe.
Strong and sustained responses
The trial involved 128 patients treated with ide-cel infusions. At the time of data cutoff for this report (Jan. 14, 2020), 62 patients remained in the primary study. Of the 128 treated patients, the median age was 61 years and the median time since diagnosis was 6 years. About half (51%) had a high tumor burden (≥50% bone marrow plasma cells), 39% had extramedullary disease, 16% had stage III disease, and 35% had a high-risk cytogenetic abnormality, defined as del(17p), t(4;14), or t(14;16).
Patients in the cohort had received a median of six previous antimyeloma regimens (range, 3-16), and most of the patients (120, 94%) had undergone autologous hematopoietic stem cell transplants. In addition, the majority of patients (84%) had disease that was triple refractory (to an immunomodulatory agent, a proteasome inhibitor, and an anti-CD38 antibody), 60% had disease that was penta exposed (to bortezomib, carfilzomib, lenalidomide, pomalidomide, and daratumumab), and 26% had disease that was penta refractory.
At a median follow-up of 13.3 months, 94 of 128 patients (73%) showed a response to therapy (P < .001), with 42 (33%) showing a complete or stringent complete response, and 67 patients (52%) showing a “very good partial response or better.”
Overall median progression-free survival was 8.8 months at the 450×106 dose but more than double that (20.2 months) for patients who achieved a complete or stringent complete response. Estimated median overall survival was 19.4 months, with an overall survival of 78% at 12 months. The authors noted that overall survival data are not yet mature.
After experiencing disease progression, 28 patients were retreated with ide-cel, with 6 patients showing a second response. The durations of response ranged from 1.9 to 6.8 months.
All patients in the cohort experienced adverse events, primarily grade 3 or 4 events that occurred in 127 patients (99%). The most common events reported were hematologic toxicities, including neutropenia in 114 patients (89%), anemia in 77 (60%), and thrombocytopenia in 67 (52%), and were at least partially related to the lymphodepleting chemotherapy administered before ide-cel infusion, the authors note. Cytokine-release syndrome occurred in 107 patients (84%), primarily grade 1 or 2.
“Results of the KarMMa study support substantial antitumor activity for ide-cel across a target dose range of 150×106 to 450×106 CAR+ T cells,” the authors conclude. “The 450×106 dose appeared to be somewhat more effective than the other doses.”
New option?
“What this study further highlights is that higher cell dose tends to increase cell expansion, which correlates to improved response and duration of response,” said Dr. Patel.
Importantly, multiple vulnerable subgroups experienced impressive outcomes, such as those who are older or with high risk or extramedullary disease, she noted.
“My patients who have undergone this therapy, albeit on other clinical trials, all say that their quality of life during this time of remission is priceless,” Dr. Patel added. “The is the first therapy in the relapsed/refractory setting that allows patients to have a significant chemo-free period. We need to find more ways to do this for our patients.”
The study was supported by Bluebird Bio and Bristol-Myers Squibb. Dr. Patel has served on the advisory board for Janssen and Bristol-Myers Squibb. She also reports a speaking engagement with Oncopeptides. Dr. Munshi acts as a consultant for several pharmaceutical companies, and many coauthors also have relationships with industry, as listed in the original article.
A version of this article first appeared on Medscape.com.
Patients with multiple myeloma that has continued to progress despite many lines of therapy have shown deep and durable responses to a new chimeric antigen receptor (CAR) T-cell therapy, idecabtagene vicleucel (ide-cel, under development by Bristol-Myers Squibb and Bluebird Bio).
An expert not involved in the trial described the results as “phenomenal.”
Krina Patel, MD, an associate professor in the department of lymphoma/myeloma at the University of Texas MD Anderson Cancer Center, Houston, said that “the response rate of 73% in a patient population with a median of six lines of therapy, and with one-third of those patients achieving a deep response of complete response or better, is phenomenal.”
“We are very excited as a myeloma community for this study of idecabtagene vicleucel for relapsed/refractory patients,” Dr. Patel said.
The new data on ide-cell, from a trial in 128 patients, were published Feb. 25 in the New England Journal of Medicine.
Lead investigator of the study Nikhil Munshi, MD, of Dana-Farber Cancer Institute, Boston, said: “The results of this trial represent a true turning point in the treatment of this disease. In my 30 years of treating myeloma, I have not seen any other therapy as effective in this group of patients.”
Both experts highlighted the poor prognosis for this population of relapsed/refractory patients. Recent decades have seen a flurry of new agents for myeloma, and there are now three main classes of agents: immunomodulatory agents, proteasome inhibitors, and anti-CD38 antibodies. Nevertheless, in some patients, the disease continues to progress. For patients who have failed all three classes of drugs, the median progression-free survival is about 3-4 months, with a median overall survival of 8-9 months.
Product is awaiting approval
Ide-cel is currently awaiting FDA approval, with a decision date slated for March 27.
Several CAR T-cell products are already marketed for use in certain leukemias and lymphomas, and there is another for use in multiple myeloma, ciltacabtagene autoleucel (cilta-cel, under development by Janssen), that is awaiting approval in Europe.
Strong and sustained responses
The trial involved 128 patients treated with ide-cel infusions. At the time of data cutoff for this report (Jan. 14, 2020), 62 patients remained in the primary study. Of the 128 treated patients, the median age was 61 years and the median time since diagnosis was 6 years. About half (51%) had a high tumor burden (≥50% bone marrow plasma cells), 39% had extramedullary disease, 16% had stage III disease, and 35% had a high-risk cytogenetic abnormality, defined as del(17p), t(4;14), or t(14;16).
Patients in the cohort had received a median of six previous antimyeloma regimens (range, 3-16), and most of the patients (120, 94%) had undergone autologous hematopoietic stem cell transplants. In addition, the majority of patients (84%) had disease that was triple refractory (to an immunomodulatory agent, a proteasome inhibitor, and an anti-CD38 antibody), 60% had disease that was penta exposed (to bortezomib, carfilzomib, lenalidomide, pomalidomide, and daratumumab), and 26% had disease that was penta refractory.
At a median follow-up of 13.3 months, 94 of 128 patients (73%) showed a response to therapy (P < .001), with 42 (33%) showing a complete or stringent complete response, and 67 patients (52%) showing a “very good partial response or better.”
Overall median progression-free survival was 8.8 months at the 450×106 dose but more than double that (20.2 months) for patients who achieved a complete or stringent complete response. Estimated median overall survival was 19.4 months, with an overall survival of 78% at 12 months. The authors noted that overall survival data are not yet mature.
After experiencing disease progression, 28 patients were retreated with ide-cel, with 6 patients showing a second response. The durations of response ranged from 1.9 to 6.8 months.
All patients in the cohort experienced adverse events, primarily grade 3 or 4 events that occurred in 127 patients (99%). The most common events reported were hematologic toxicities, including neutropenia in 114 patients (89%), anemia in 77 (60%), and thrombocytopenia in 67 (52%), and were at least partially related to the lymphodepleting chemotherapy administered before ide-cel infusion, the authors note. Cytokine-release syndrome occurred in 107 patients (84%), primarily grade 1 or 2.
“Results of the KarMMa study support substantial antitumor activity for ide-cel across a target dose range of 150×106 to 450×106 CAR+ T cells,” the authors conclude. “The 450×106 dose appeared to be somewhat more effective than the other doses.”
New option?
“What this study further highlights is that higher cell dose tends to increase cell expansion, which correlates to improved response and duration of response,” said Dr. Patel.
Importantly, multiple vulnerable subgroups experienced impressive outcomes, such as those who are older or with high risk or extramedullary disease, she noted.
“My patients who have undergone this therapy, albeit on other clinical trials, all say that their quality of life during this time of remission is priceless,” Dr. Patel added. “The is the first therapy in the relapsed/refractory setting that allows patients to have a significant chemo-free period. We need to find more ways to do this for our patients.”
The study was supported by Bluebird Bio and Bristol-Myers Squibb. Dr. Patel has served on the advisory board for Janssen and Bristol-Myers Squibb. She also reports a speaking engagement with Oncopeptides. Dr. Munshi acts as a consultant for several pharmaceutical companies, and many coauthors also have relationships with industry, as listed in the original article.
A version of this article first appeared on Medscape.com.
Pembrolizumab SCLC indication withdrawn in U.S.
The move does not affect any of the drug’s other indications. The immunotherapy is used in the treatment of many different types of cancer.
The SCLC indication had been granted an accelerated approval by the Food and Drug Administration in 2019 based on tumor response rate and durability of response data from patient cohorts in two trials. However, the anti-PD-1 therapy failed to demonstrate statistically significant improved overall survival in a confirmatory trial, which is mandated after an accelerated approval.
The FDA is conducting “an industry-wide evaluation of indications based on accelerated approvals that have not yet met their postmarketing requirements,” said Merck.
In February of 2021, an indication for durvalumab (Imfinzi) was withdrawn by AstraZeneca in concert with the FDA after the drug failed to improve overall survival in unresectable metastatic bladder cancer in a confirmatory trial, as reported by Medscape Medical News.
“We will continue to rigorously evaluate the benefits of [pembrolizumab] in small cell lung cancer and other types of cancer, in pursuit of Merck’s mission to save and improve lives,” Roy Baynes, MD, chief medical officer, Merck Research Laboratories, said in the company statement
Dr. Baynes also championed the value of accelerated approvals.
“The accelerated pathways created by the FDA have been integral to the remarkable progress in oncology care over the past 5 years and have helped many cancer patients with advanced disease, including small cell lung cancer, access new treatments,” he said.
However, in the past, the FDA has been criticized for approving new cancer drugs based on surrogate markers such as response rates because, in many cases, subsequent studies often show that the drug fails to improve overall survival.
For example, a 2015 study found that 36 (67%) of 54 cancer drug approvals from 2008 to 2012 were made on the basis of surrogate markers – either tumor response rate or progression-free survival. Over a median follow-up period of 4.4 years, only 5 of those 36 drugs were shown in randomized studies to improve overall survival, as reported by Medscape Medical News.
The FDA says that it instituted the accelerated approval program to “allow for earlier approval of drugs that treat serious conditions, and that fill an unmet medical need based on a surrogate endpoint.” The program was started in 1992, in the midst of the HIV/AIDS epidemic.
In 2020, the nonprofit Friends of Cancer Research issued a white paper calling for reform in the accelerated approval process, which included a proposal to add risk assessment to surrogate endpoints that would factor in variables such as toxicity.
A version of this article first appeared on Medscape.com.
The move does not affect any of the drug’s other indications. The immunotherapy is used in the treatment of many different types of cancer.
The SCLC indication had been granted an accelerated approval by the Food and Drug Administration in 2019 based on tumor response rate and durability of response data from patient cohorts in two trials. However, the anti-PD-1 therapy failed to demonstrate statistically significant improved overall survival in a confirmatory trial, which is mandated after an accelerated approval.
The FDA is conducting “an industry-wide evaluation of indications based on accelerated approvals that have not yet met their postmarketing requirements,” said Merck.
In February of 2021, an indication for durvalumab (Imfinzi) was withdrawn by AstraZeneca in concert with the FDA after the drug failed to improve overall survival in unresectable metastatic bladder cancer in a confirmatory trial, as reported by Medscape Medical News.
“We will continue to rigorously evaluate the benefits of [pembrolizumab] in small cell lung cancer and other types of cancer, in pursuit of Merck’s mission to save and improve lives,” Roy Baynes, MD, chief medical officer, Merck Research Laboratories, said in the company statement
Dr. Baynes also championed the value of accelerated approvals.
“The accelerated pathways created by the FDA have been integral to the remarkable progress in oncology care over the past 5 years and have helped many cancer patients with advanced disease, including small cell lung cancer, access new treatments,” he said.
However, in the past, the FDA has been criticized for approving new cancer drugs based on surrogate markers such as response rates because, in many cases, subsequent studies often show that the drug fails to improve overall survival.
For example, a 2015 study found that 36 (67%) of 54 cancer drug approvals from 2008 to 2012 were made on the basis of surrogate markers – either tumor response rate or progression-free survival. Over a median follow-up period of 4.4 years, only 5 of those 36 drugs were shown in randomized studies to improve overall survival, as reported by Medscape Medical News.
The FDA says that it instituted the accelerated approval program to “allow for earlier approval of drugs that treat serious conditions, and that fill an unmet medical need based on a surrogate endpoint.” The program was started in 1992, in the midst of the HIV/AIDS epidemic.
In 2020, the nonprofit Friends of Cancer Research issued a white paper calling for reform in the accelerated approval process, which included a proposal to add risk assessment to surrogate endpoints that would factor in variables such as toxicity.
A version of this article first appeared on Medscape.com.
The move does not affect any of the drug’s other indications. The immunotherapy is used in the treatment of many different types of cancer.
The SCLC indication had been granted an accelerated approval by the Food and Drug Administration in 2019 based on tumor response rate and durability of response data from patient cohorts in two trials. However, the anti-PD-1 therapy failed to demonstrate statistically significant improved overall survival in a confirmatory trial, which is mandated after an accelerated approval.
The FDA is conducting “an industry-wide evaluation of indications based on accelerated approvals that have not yet met their postmarketing requirements,” said Merck.
In February of 2021, an indication for durvalumab (Imfinzi) was withdrawn by AstraZeneca in concert with the FDA after the drug failed to improve overall survival in unresectable metastatic bladder cancer in a confirmatory trial, as reported by Medscape Medical News.
“We will continue to rigorously evaluate the benefits of [pembrolizumab] in small cell lung cancer and other types of cancer, in pursuit of Merck’s mission to save and improve lives,” Roy Baynes, MD, chief medical officer, Merck Research Laboratories, said in the company statement
Dr. Baynes also championed the value of accelerated approvals.
“The accelerated pathways created by the FDA have been integral to the remarkable progress in oncology care over the past 5 years and have helped many cancer patients with advanced disease, including small cell lung cancer, access new treatments,” he said.
However, in the past, the FDA has been criticized for approving new cancer drugs based on surrogate markers such as response rates because, in many cases, subsequent studies often show that the drug fails to improve overall survival.
For example, a 2015 study found that 36 (67%) of 54 cancer drug approvals from 2008 to 2012 were made on the basis of surrogate markers – either tumor response rate or progression-free survival. Over a median follow-up period of 4.4 years, only 5 of those 36 drugs were shown in randomized studies to improve overall survival, as reported by Medscape Medical News.
The FDA says that it instituted the accelerated approval program to “allow for earlier approval of drugs that treat serious conditions, and that fill an unmet medical need based on a surrogate endpoint.” The program was started in 1992, in the midst of the HIV/AIDS epidemic.
In 2020, the nonprofit Friends of Cancer Research issued a white paper calling for reform in the accelerated approval process, which included a proposal to add risk assessment to surrogate endpoints that would factor in variables such as toxicity.
A version of this article first appeared on Medscape.com.
14-year-old girl • history of bullying • lack of social support • multiple linear scars on breasts • Dx?
THE CASE
A 14-year-old girl with no significant medical history presented to the office accompanied by her mother for a routine well-adolescent visit. She attended school online due to a history of severe bullying and, when interviewed alone, admitted to a lack of a social life as a result. On questioning, she denied tobacco, alcohol, or illicit drug use. Her gender identity was female. Her sexual orientation was toward both males and females, but she was not sexually active. She denied exposure to physical or emotional violence at home and said she did not feel depressed or think about suicide.
Physical examination revealed multiple erythematous linear scars surrounding the areola of both breasts. When questioned about these lesions, she admitted to cutting herself on the breasts during the past several months but again denied suicidal intent. She believed that her behavior was a normal coping mechanism.
The physical exam was otherwise normal. Lab results, including thyroid-stimulating hormone and complete blood count, were within normal limits.
THE DIAGNOSIS
The physical exam findings and the patient’s self report pointed to a diagnosis of nonsuicidal self-injurious (NSSI) behavior involving cutting.
DISCUSSION
The NSSI behavior displayed by this patient is a common biopsychosocial disorder observed in adolescents. Self-injury is defined as the deliberate injuring of body tissues without suicidal intent.1 Self-injurious behavior typically begins when patients are 13 to 16 years of age, and cutting is the most common form. Most acts occur on the arms, legs, wrists, and stomach.2 Studies have shown that the prevalence of this behavior is on the rise among adolescents, from about 7% in 2014 to between 14% and 24% in 2015.3
Risk for suicide. Although a feature of NSSI is the lack of suicidal intent, this type of high-risk behavior is associated with past, present, and future suicide attempts. It is important for physicians to identify NSSI in an adolescent, as it is linked to a 7-fold increased risk for a suicide attempt.3
Screening for NSSI. Less than one-fifth of adolescents who injure themselves come to the attention of health care providers.4 We propose that primary care physicians add NSSI to the list of risky behaviors—including drug abuse, sexual activity, and depression—for which they screen during well-child visits.
Continue to: Identifying risk factors
Identifying risk factors. The case patient experienced bullying and reported a nonheterosexual orientation, both of which have been demonstrated as strong risk factors for NSSI.5 Female gender has also been identified as a risk factor for NSSI.3
In adolescent psychiatric samples, prevalence rates of NSSI were found to be as high as 60% for 1 incident of NSSI and around 50% for repetitive NSSI.6 NSSI coincides with other psychiatric comorbidities, including eating disorders, mood disorders (depression), anxiety disorders, posttraumatic stress disorder, and borderline personality disorder.3 In a study of 93 subjects, each of whom was a self-reported abuse survivor with a history of self-injury, 96% were in therapy for diagnoses that included posttraumatic stress disorder (73%), dissociative disorder (40%), borderline personality disorder (37%), and multiple personality disorder (29%).7
The experience of adverse childhood events also increases risk for NSSI. This includes parental neglect, abuse, or deprivation.6 Insecure paternal attachment and parental neglect are significant predictors for women, while childhood separation is a primary predictor for men.8 Indirect childhood maltreatment, such as witnessing domestic violence or increased parental critique, is also associated with NSSI.8 NSSI is also more prevalent among young people who identify with a subculture such as gothic or emo.6
Why they do it and how to help
In multiple studies aimed at identifying reasons for self-injury, converging evidence suggests that nearly all patients act with the intent of alleviating negative affect.9 Patients self-harm to regulate distress, anxiety, and frustration that they perceive to be intolerable.9 They may self-harm to generate feeling when emotionally empty or to avert suicidal intent.9 For others, self-harm is a way to communicate their distress.
How to proceed. After a physician identifies NSSI, the patient should be assessed for suicidality and medical severity of the injury.3 Factors associated with higher likelihood of suicidality in patients with NSSI include multiple self-injurious methods and locations, early age of onset, longer history of NSSI, recent worsening of the injuries, simultaneous substance use, and the perception that the patient is addicted to self-injury.10
Continue to: It is also important...
It is also important to ask the patient whether she or he has told anyone about the behavior. Participation in NSSI communities may reinforce it.3
Treatment found to be effective for NSSI involves dialectical behavioral therapy, cognitive behavioral therapy, and mentalization-based therapy.11
Our patient was admitted to the hospital several weeks after her well visit because she expressed suicidal ideation. After being discharged, she was referred to outpatient Psychiatry with a treatment plan that included cognitive behavioral therapy.
THE TAKEAWAY
While our patient may have concealed her self-injurious experience because of stigma and concern about others’ reactions, there were several risk factors for NSSI in her history that prompted further investigation with a skin exam.
If a patient presents with 1 or more risk factors, an initial assessment for possible NSSI should be performed with detailed history-taking and a skin exam. Once NSSI is identified, the initial response and tone of questioning toward the patient need to convey a sense of genuine curiosity about the patient’s experience. From there, the physician can avail the patient to the proper treatment modalities.
NSSI patients can be resistant to sharing and participating in support groups. However, a referred counselor can follow up with a stepwise approach to slowly gain the trust of the individual, find the root cause, and get the patient to a point where she or he is ready to start the necessary treatment.
1. Klonsky ED, Glenn CR. Resisting urges to self-injure. Behav Cogn Psychother. 2008;36:211-220. doi: 10.1017/S1352465808004128
2. Whitlock J, Eckenrode J, Silverman D. Self-injurious behaviors in a college population. Pediatrics. 2006;117:1939-1948. doi: 10.1542/peds.2005-2543
3. Lewis SP, Heath NL. Non-suicidal self-injury among youth. J Pediatr. 2015;166:526-630. doi: 10.1016/j.jpeds.2014.11.062
4. Ystgaard M, Arensman E, Hawton K, et al. Deliberate self-harm in adolescents: comparison between those who receive help following self-harm and those who do not. J Adolesc. 2009;32: 875-891.
5. Lereya ST, Copeland WE, Costello EJ, et al. Adult mental health consequences of peer bullying and maltreatment in childhood: two cohorts in two countries. Lancet Psychiatry. 2015;2:524-531. doi: 10.1016/S2215-0366(15)00165-0
6. Brown RC, Plener PL. Non-suicidal self-injury in adolescence. Curr Psychiatry Rep. 2017;19:20. doi: 10.1007/s11920-017-0767-9
7. Briere J, Gil E. Self-mutilation in clinical and general population samples: prevalence, correlates, and functions. Am J Orthopsychiatry. 1998;68:609-620. doi:10.1037/h0080369
8. Gratz KL, Conrad SD, Roemer L. Risk factors for deliberate self-harm among college students. Am J Orthopsychiatry. 2002;1:128-140. doi: 10.1037//0002-9432.72.1.128
9. Klonsky ED. The functions of deliberate self-injury: a review of the evidence. Clin Psychol Rev. 2007;27:226-239.
10. Nock MK, Joiner Jr. TE, Gordon KH, et al. Non-suicidal self-injury among adolescents: diagnostic correlates and relation to suicide attempts. Psychiatry Res. 2006;144:65-72. doi: 10.1016/j.psychres.2006.05.010
11. Lewis SP, Baker TG. The possible risks of self-injury websites: a content analysis. Arch Suicide Res. 2011;15:390-396. doi: 10.1080/13811118.2011.616154
THE CASE
A 14-year-old girl with no significant medical history presented to the office accompanied by her mother for a routine well-adolescent visit. She attended school online due to a history of severe bullying and, when interviewed alone, admitted to a lack of a social life as a result. On questioning, she denied tobacco, alcohol, or illicit drug use. Her gender identity was female. Her sexual orientation was toward both males and females, but she was not sexually active. She denied exposure to physical or emotional violence at home and said she did not feel depressed or think about suicide.
Physical examination revealed multiple erythematous linear scars surrounding the areola of both breasts. When questioned about these lesions, she admitted to cutting herself on the breasts during the past several months but again denied suicidal intent. She believed that her behavior was a normal coping mechanism.
The physical exam was otherwise normal. Lab results, including thyroid-stimulating hormone and complete blood count, were within normal limits.
THE DIAGNOSIS
The physical exam findings and the patient’s self report pointed to a diagnosis of nonsuicidal self-injurious (NSSI) behavior involving cutting.
DISCUSSION
The NSSI behavior displayed by this patient is a common biopsychosocial disorder observed in adolescents. Self-injury is defined as the deliberate injuring of body tissues without suicidal intent.1 Self-injurious behavior typically begins when patients are 13 to 16 years of age, and cutting is the most common form. Most acts occur on the arms, legs, wrists, and stomach.2 Studies have shown that the prevalence of this behavior is on the rise among adolescents, from about 7% in 2014 to between 14% and 24% in 2015.3
Risk for suicide. Although a feature of NSSI is the lack of suicidal intent, this type of high-risk behavior is associated with past, present, and future suicide attempts. It is important for physicians to identify NSSI in an adolescent, as it is linked to a 7-fold increased risk for a suicide attempt.3
Screening for NSSI. Less than one-fifth of adolescents who injure themselves come to the attention of health care providers.4 We propose that primary care physicians add NSSI to the list of risky behaviors—including drug abuse, sexual activity, and depression—for which they screen during well-child visits.
Continue to: Identifying risk factors
Identifying risk factors. The case patient experienced bullying and reported a nonheterosexual orientation, both of which have been demonstrated as strong risk factors for NSSI.5 Female gender has also been identified as a risk factor for NSSI.3
In adolescent psychiatric samples, prevalence rates of NSSI were found to be as high as 60% for 1 incident of NSSI and around 50% for repetitive NSSI.6 NSSI coincides with other psychiatric comorbidities, including eating disorders, mood disorders (depression), anxiety disorders, posttraumatic stress disorder, and borderline personality disorder.3 In a study of 93 subjects, each of whom was a self-reported abuse survivor with a history of self-injury, 96% were in therapy for diagnoses that included posttraumatic stress disorder (73%), dissociative disorder (40%), borderline personality disorder (37%), and multiple personality disorder (29%).7
The experience of adverse childhood events also increases risk for NSSI. This includes parental neglect, abuse, or deprivation.6 Insecure paternal attachment and parental neglect are significant predictors for women, while childhood separation is a primary predictor for men.8 Indirect childhood maltreatment, such as witnessing domestic violence or increased parental critique, is also associated with NSSI.8 NSSI is also more prevalent among young people who identify with a subculture such as gothic or emo.6
Why they do it and how to help
In multiple studies aimed at identifying reasons for self-injury, converging evidence suggests that nearly all patients act with the intent of alleviating negative affect.9 Patients self-harm to regulate distress, anxiety, and frustration that they perceive to be intolerable.9 They may self-harm to generate feeling when emotionally empty or to avert suicidal intent.9 For others, self-harm is a way to communicate their distress.
How to proceed. After a physician identifies NSSI, the patient should be assessed for suicidality and medical severity of the injury.3 Factors associated with higher likelihood of suicidality in patients with NSSI include multiple self-injurious methods and locations, early age of onset, longer history of NSSI, recent worsening of the injuries, simultaneous substance use, and the perception that the patient is addicted to self-injury.10
Continue to: It is also important...
It is also important to ask the patient whether she or he has told anyone about the behavior. Participation in NSSI communities may reinforce it.3
Treatment found to be effective for NSSI involves dialectical behavioral therapy, cognitive behavioral therapy, and mentalization-based therapy.11
Our patient was admitted to the hospital several weeks after her well visit because she expressed suicidal ideation. After being discharged, she was referred to outpatient Psychiatry with a treatment plan that included cognitive behavioral therapy.
THE TAKEAWAY
While our patient may have concealed her self-injurious experience because of stigma and concern about others’ reactions, there were several risk factors for NSSI in her history that prompted further investigation with a skin exam.
If a patient presents with 1 or more risk factors, an initial assessment for possible NSSI should be performed with detailed history-taking and a skin exam. Once NSSI is identified, the initial response and tone of questioning toward the patient need to convey a sense of genuine curiosity about the patient’s experience. From there, the physician can avail the patient to the proper treatment modalities.
NSSI patients can be resistant to sharing and participating in support groups. However, a referred counselor can follow up with a stepwise approach to slowly gain the trust of the individual, find the root cause, and get the patient to a point where she or he is ready to start the necessary treatment.
THE CASE
A 14-year-old girl with no significant medical history presented to the office accompanied by her mother for a routine well-adolescent visit. She attended school online due to a history of severe bullying and, when interviewed alone, admitted to a lack of a social life as a result. On questioning, she denied tobacco, alcohol, or illicit drug use. Her gender identity was female. Her sexual orientation was toward both males and females, but she was not sexually active. She denied exposure to physical or emotional violence at home and said she did not feel depressed or think about suicide.
Physical examination revealed multiple erythematous linear scars surrounding the areola of both breasts. When questioned about these lesions, she admitted to cutting herself on the breasts during the past several months but again denied suicidal intent. She believed that her behavior was a normal coping mechanism.
The physical exam was otherwise normal. Lab results, including thyroid-stimulating hormone and complete blood count, were within normal limits.
THE DIAGNOSIS
The physical exam findings and the patient’s self report pointed to a diagnosis of nonsuicidal self-injurious (NSSI) behavior involving cutting.
DISCUSSION
The NSSI behavior displayed by this patient is a common biopsychosocial disorder observed in adolescents. Self-injury is defined as the deliberate injuring of body tissues without suicidal intent.1 Self-injurious behavior typically begins when patients are 13 to 16 years of age, and cutting is the most common form. Most acts occur on the arms, legs, wrists, and stomach.2 Studies have shown that the prevalence of this behavior is on the rise among adolescents, from about 7% in 2014 to between 14% and 24% in 2015.3
Risk for suicide. Although a feature of NSSI is the lack of suicidal intent, this type of high-risk behavior is associated with past, present, and future suicide attempts. It is important for physicians to identify NSSI in an adolescent, as it is linked to a 7-fold increased risk for a suicide attempt.3
Screening for NSSI. Less than one-fifth of adolescents who injure themselves come to the attention of health care providers.4 We propose that primary care physicians add NSSI to the list of risky behaviors—including drug abuse, sexual activity, and depression—for which they screen during well-child visits.
Continue to: Identifying risk factors
Identifying risk factors. The case patient experienced bullying and reported a nonheterosexual orientation, both of which have been demonstrated as strong risk factors for NSSI.5 Female gender has also been identified as a risk factor for NSSI.3
In adolescent psychiatric samples, prevalence rates of NSSI were found to be as high as 60% for 1 incident of NSSI and around 50% for repetitive NSSI.6 NSSI coincides with other psychiatric comorbidities, including eating disorders, mood disorders (depression), anxiety disorders, posttraumatic stress disorder, and borderline personality disorder.3 In a study of 93 subjects, each of whom was a self-reported abuse survivor with a history of self-injury, 96% were in therapy for diagnoses that included posttraumatic stress disorder (73%), dissociative disorder (40%), borderline personality disorder (37%), and multiple personality disorder (29%).7
The experience of adverse childhood events also increases risk for NSSI. This includes parental neglect, abuse, or deprivation.6 Insecure paternal attachment and parental neglect are significant predictors for women, while childhood separation is a primary predictor for men.8 Indirect childhood maltreatment, such as witnessing domestic violence or increased parental critique, is also associated with NSSI.8 NSSI is also more prevalent among young people who identify with a subculture such as gothic or emo.6
Why they do it and how to help
In multiple studies aimed at identifying reasons for self-injury, converging evidence suggests that nearly all patients act with the intent of alleviating negative affect.9 Patients self-harm to regulate distress, anxiety, and frustration that they perceive to be intolerable.9 They may self-harm to generate feeling when emotionally empty or to avert suicidal intent.9 For others, self-harm is a way to communicate their distress.
How to proceed. After a physician identifies NSSI, the patient should be assessed for suicidality and medical severity of the injury.3 Factors associated with higher likelihood of suicidality in patients with NSSI include multiple self-injurious methods and locations, early age of onset, longer history of NSSI, recent worsening of the injuries, simultaneous substance use, and the perception that the patient is addicted to self-injury.10
Continue to: It is also important...
It is also important to ask the patient whether she or he has told anyone about the behavior. Participation in NSSI communities may reinforce it.3
Treatment found to be effective for NSSI involves dialectical behavioral therapy, cognitive behavioral therapy, and mentalization-based therapy.11
Our patient was admitted to the hospital several weeks after her well visit because she expressed suicidal ideation. After being discharged, she was referred to outpatient Psychiatry with a treatment plan that included cognitive behavioral therapy.
THE TAKEAWAY
While our patient may have concealed her self-injurious experience because of stigma and concern about others’ reactions, there were several risk factors for NSSI in her history that prompted further investigation with a skin exam.
If a patient presents with 1 or more risk factors, an initial assessment for possible NSSI should be performed with detailed history-taking and a skin exam. Once NSSI is identified, the initial response and tone of questioning toward the patient need to convey a sense of genuine curiosity about the patient’s experience. From there, the physician can avail the patient to the proper treatment modalities.
NSSI patients can be resistant to sharing and participating in support groups. However, a referred counselor can follow up with a stepwise approach to slowly gain the trust of the individual, find the root cause, and get the patient to a point where she or he is ready to start the necessary treatment.
1. Klonsky ED, Glenn CR. Resisting urges to self-injure. Behav Cogn Psychother. 2008;36:211-220. doi: 10.1017/S1352465808004128
2. Whitlock J, Eckenrode J, Silverman D. Self-injurious behaviors in a college population. Pediatrics. 2006;117:1939-1948. doi: 10.1542/peds.2005-2543
3. Lewis SP, Heath NL. Non-suicidal self-injury among youth. J Pediatr. 2015;166:526-630. doi: 10.1016/j.jpeds.2014.11.062
4. Ystgaard M, Arensman E, Hawton K, et al. Deliberate self-harm in adolescents: comparison between those who receive help following self-harm and those who do not. J Adolesc. 2009;32: 875-891.
5. Lereya ST, Copeland WE, Costello EJ, et al. Adult mental health consequences of peer bullying and maltreatment in childhood: two cohorts in two countries. Lancet Psychiatry. 2015;2:524-531. doi: 10.1016/S2215-0366(15)00165-0
6. Brown RC, Plener PL. Non-suicidal self-injury in adolescence. Curr Psychiatry Rep. 2017;19:20. doi: 10.1007/s11920-017-0767-9
7. Briere J, Gil E. Self-mutilation in clinical and general population samples: prevalence, correlates, and functions. Am J Orthopsychiatry. 1998;68:609-620. doi:10.1037/h0080369
8. Gratz KL, Conrad SD, Roemer L. Risk factors for deliberate self-harm among college students. Am J Orthopsychiatry. 2002;1:128-140. doi: 10.1037//0002-9432.72.1.128
9. Klonsky ED. The functions of deliberate self-injury: a review of the evidence. Clin Psychol Rev. 2007;27:226-239.
10. Nock MK, Joiner Jr. TE, Gordon KH, et al. Non-suicidal self-injury among adolescents: diagnostic correlates and relation to suicide attempts. Psychiatry Res. 2006;144:65-72. doi: 10.1016/j.psychres.2006.05.010
11. Lewis SP, Baker TG. The possible risks of self-injury websites: a content analysis. Arch Suicide Res. 2011;15:390-396. doi: 10.1080/13811118.2011.616154
1. Klonsky ED, Glenn CR. Resisting urges to self-injure. Behav Cogn Psychother. 2008;36:211-220. doi: 10.1017/S1352465808004128
2. Whitlock J, Eckenrode J, Silverman D. Self-injurious behaviors in a college population. Pediatrics. 2006;117:1939-1948. doi: 10.1542/peds.2005-2543
3. Lewis SP, Heath NL. Non-suicidal self-injury among youth. J Pediatr. 2015;166:526-630. doi: 10.1016/j.jpeds.2014.11.062
4. Ystgaard M, Arensman E, Hawton K, et al. Deliberate self-harm in adolescents: comparison between those who receive help following self-harm and those who do not. J Adolesc. 2009;32: 875-891.
5. Lereya ST, Copeland WE, Costello EJ, et al. Adult mental health consequences of peer bullying and maltreatment in childhood: two cohorts in two countries. Lancet Psychiatry. 2015;2:524-531. doi: 10.1016/S2215-0366(15)00165-0
6. Brown RC, Plener PL. Non-suicidal self-injury in adolescence. Curr Psychiatry Rep. 2017;19:20. doi: 10.1007/s11920-017-0767-9
7. Briere J, Gil E. Self-mutilation in clinical and general population samples: prevalence, correlates, and functions. Am J Orthopsychiatry. 1998;68:609-620. doi:10.1037/h0080369
8. Gratz KL, Conrad SD, Roemer L. Risk factors for deliberate self-harm among college students. Am J Orthopsychiatry. 2002;1:128-140. doi: 10.1037//0002-9432.72.1.128
9. Klonsky ED. The functions of deliberate self-injury: a review of the evidence. Clin Psychol Rev. 2007;27:226-239.
10. Nock MK, Joiner Jr. TE, Gordon KH, et al. Non-suicidal self-injury among adolescents: diagnostic correlates and relation to suicide attempts. Psychiatry Res. 2006;144:65-72. doi: 10.1016/j.psychres.2006.05.010
11. Lewis SP, Baker TG. The possible risks of self-injury websites: a content analysis. Arch Suicide Res. 2011;15:390-396. doi: 10.1080/13811118.2011.616154
ACIP recommendations for COVID-19 vaccines—and more
The year 2020 was challenging for public health agencies and especially for the Centers for Disease Control and Prevention (CDC) and its Advisory Committee on Immunization Practices (ACIP). In a normal year, the ACIP meets in person 3 times for a total of 6 days of deliberations. In 2020, there were 10 meetings (all but 1 using Zoom) covering 14 days. Much of the time was dedicated to the COVID-19 pandemic, the vaccines being developed to prevent COVID-19, and the prioritization of those who should receive the vaccines first.
The ACIP also made recommendations for the use of influenza vaccines in the 2020-2021 season, approved the adult and pediatric immunization schedules for 2021, and approved the use of 2 new vaccines, one to protect against meningococcal meningitis and the other to prevent Ebola virus disease. The influenza recommendations were covered in the October 2020 Practice Alert,1 and the immunization schedules can be found on the CDC website at www.cdc.gov/vaccines/schedules/hcp/index.html.
COVID-19 vaccines
Two COVID-19 vaccines have been approved for use in the United States. The first was the Pfizer-BioNTech COVID-19 vaccine, approved by the Food and Drug Administration (FDA) on December 11 and recommended for use by the ACIP on December 12.2 The second vaccine, from Moderna, was approved by the FDA on December 18 and recommended by the ACIP on December 19.3 Both were approved by the FDA under an Emergency Use Authorization (EUA) and were approved by the ACIP for use while the EUA is in effect. Both vaccines must eventually undergo regular approval by the FDA and will be reconsidered by the ACIP regarding use in non–public health emergency conditions. A description of the EUA process and measures taken to assure efficacy and safety, before and after approval, were discussed in the September 2020 audiocast.
Both COVID-19 vaccines consist of nucleoside-modified mRNA encapsulated with lipid nanoparticles, which encode for a spike glycoprotein of SARS-CoV-2, the virus that causes COVID-19. Both vaccines require 2 doses (separated by 3 weeks for the Pfizer vaccine and 4 weeks for the Moderna vaccine) and are approved for use only in adults and older adolescents (ages ≥ 16 years for the Pfizer vaccine and ≥ 18 years for the Moderna vaccine) (TABLE 12-5).
In anticipation of vaccine shortages immediately after approval for use and a high demand for the vaccine, the ACIP developed a list of high-priority groups who should receive the vaccine in ranked order.6 States are encouraged, but not required, to follow this priority list (TABLE 26).
Caveats with usage. Both COVID-19 vaccines are very reactogenic, causing local and systemic adverse effects that patients should be warned about (TABLE 37,8). These reactions are usually mild to moderate and last 24 hours or less. Acetaminophen can alleviate these symptoms but should not be used to prevent them. In addition, both vaccines have stringent cold-storage requirements; once the vaccines are thawed, they must be used within a defined time-period.
Neither vaccine is listed as preferred. And they are not interchangeable; both recommended doses should be completed with the same vaccine. More details about the use of these vaccines were discussed in the January 2021 audiocast (www.mdedge.com/familymedicine/article/234239/coronavirus-updates/covid-19-vaccines-rollout-risks-and-reason-still) and can be located on the CDC website (www.cdc.gov/vaccines/covid-19/info-by-product/pfizer/reactogenicity.html; www.cdc.gov/vaccines/covid-19/info-by-product/moderna/reactogenicity.html).
Continue to: Much remains unknown...
Much remains unknown regarding the use of these COVID-19 vaccines:
- What is their duration of protection, and will booster doses be needed?
- Will they protect against asymptomatic infection and carrier states, and thereby prevent transmission?
- Can they be co-administered with other vaccines?
- Will they be efficacious and safe to use during pregnancy and breastfeeding?
These issues will need to be addressed before they are recommended for non–public health emergency use.
Quadrivalent meningococcal conjugate vaccine (MenACWY)
In June 2020, the ACIP added a third quadrivalent meningococcal conjugate vaccine to its recommended list of vaccines that are FDA-approved for meningococcal disease (TABLE 49). The new vaccine fills a void left by the meningococcal polysaccharide vaccine (MPSV4), which is no longer marketed in the United States. MPSV4 was previously the only meningococcal vaccine approved for individuals 55 years and older.
The new vaccine, MenACWY-TT (MenQuadfi), is approved for those ages 2 years and older, including those > 55 years. It is anticipated that MenQuadfi will, in the near future, be licensed and approved for individuals 6 months and older and will replace MenACWY-D (Menactra). (Both are manufactured by Sanofi Pasteur.)
Groups for whom a MenACWY vaccine is recommended are listed in TABLE 5.9 A full description of current, updated recommendations for the prevention of meningococcal disease is also available.9
Continue to: Ebola virus (EBOV) vaccine
Ebola virus (EBOV) vaccine
A vaccine to prevent Ebola virus disease (EVD) is available by special request in the United States. Recombinant vesicular stomatitis virus-based Ebola virus vaccine, abbreviated as rVSVΔG-ZEBOV-GP (brand name, ERVBO) is manufactured by Merck and received approval by the FDA on December 19, 2019, for use in those ages 18 years and older. It is a live, attenuated vaccine.
The ACIP has recommended pre-exposure vaccination with rVSVΔG-ZEBOV-GP for adults 18 years or older who are at risk of exposure to EBOV while responding to an outbreak of EVD; while working as health care personnel at a federally designated Ebola Treatment Center; or while working at biosafety-level 4 facilities.10 The vaccine is protective against just 1 of 4 EBOV species, Zaire ebolavirus, which has been the cause of most reported EVD outbreaks, including the 2 largest EVD outbreaks in history that occurred in West Africa and the Republic of Congo.
It is estimated that EBOV outbreaks have infected more than 31,000 people and resulted in more than 12,000 deaths worldwide.11 Only 11 people infected with EBOV have been treated in the United States, all related to the 2014-2016 large outbreaks in West Africa. Nine of these cases were imported and only 1 resulted in transmission, to 2 people.10 The mammalian species that are suspected as intermediate hosts for EBOV are not present in the United States, which prevents EBOV from becoming endemic here.
The rVSVΔG-ZEBOV-GP vaccine was tested in a large trial in Africa during the 2014 outbreak. Its effectiveness was 100% (95% confidence interval, 63.5%-100%). The most common adverse effects were injection site pain, swelling, and redness. Mild-to-moderate systemic symptoms can occur within the first 2 days following vaccination, and include headache (37%), fever (34%), muscle pain (33%), fatigue (19%), joint pain (18%), nausea (8%), arthritis (5%), rash (4%), and
Since the vaccine contains a live virus that causes stomatitis in animals, it is possible that the virus could be transmitted to humans and other animals through close contact. Accordingly, the CDC has published some precautions including, but not limited to, not donating blood and, for 6 weeks after vaccination, avoiding contact with those who are immunosuppressed.10 The vaccine is not commercially available in the United States and must be obtained from the CDC. Information on requesting the vaccine is available at www.cdc.gov/vhf/ebola/clinicians/vaccine/.
1. Campos-Outcalt D. Prospects and challenges for the upcoming influenza season. J Fam Pract 2020;69:406-411.
2. Oliver SE, Gargano JW, Marin M, et al. The Advisory Committee on Immunization Practices’ interim recommendation for use of Pfizer-BioNTech COVID-19 vaccine-United States, December 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1922-1924.
3. Oliver SE, Gargano JW, Marin M, et al. The Advisory Committee on Immunization Practices’ interim recommendation for use of Moderna COVID-19 vaccine-United States, December 2020. MMWR Morb Mortal Wkly Rep. 2021;69:1653-1656.
4. CDC. Pfizer-BioNTech COVID-19 vaccine. Accessed February 17, 2021. www.cdc.gov/vaccines/covid-19/info-by-product/pfizer/index.html
5. CDC. Moderna COVID-19 vaccine. Accessed February 17, 2021. www.cdc.gov/vaccines/covid-19/info-by-product/moderna/index.html#:~:text=How%20to%20Store%20the%20Moderna%20COVID%2D19%20Vaccine&text=Vaccine%20may%20be%20stored%20in,for%20this%20vaccine%20is%20tighter
6. Dooling K, Marin M, Wallace M, et al. The Advisory Committee on Immunization Practices’ updated interim recommendation for allocation of COVID-19 Vaccine—United States, December 2020. MMWR Morb Mortal Wkly Rep. 2021;69:1657-1660.
7. FDA. Fact sheet for healthcare providers administering vaccine. [Pfizer–BioNTech]. Accessed February 17, 2021. www.fda.gov/media/144413/download
8. FDA. Fact sheet for healthcare providers administering vaccine. [Moderna]. Accessed February 17, 2021. www.fda.gov/media/144637/download
9. Mbaeyi SA, Bozio CH, Duffy J, et al. Meningococcal vaccination: recommendations of the Advisory Committee on Immunization Practices, United States, 2020. MMWR Recomm Rep. 2020;69:1-41.
10. Choi MJ, Cossaboom CM, Whitesell AN, et al. Use of Ebola vaccine: Recommendations of the Advisory Committee on Immunization Practices—United States, 2020. MMWR Recomm Rep. 2021;70:1-12.
11. CDC. Ebola background. Accessed February 17, 2021. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2020-02/Ebola-02-Choi-508.pdf
The year 2020 was challenging for public health agencies and especially for the Centers for Disease Control and Prevention (CDC) and its Advisory Committee on Immunization Practices (ACIP). In a normal year, the ACIP meets in person 3 times for a total of 6 days of deliberations. In 2020, there were 10 meetings (all but 1 using Zoom) covering 14 days. Much of the time was dedicated to the COVID-19 pandemic, the vaccines being developed to prevent COVID-19, and the prioritization of those who should receive the vaccines first.
The ACIP also made recommendations for the use of influenza vaccines in the 2020-2021 season, approved the adult and pediatric immunization schedules for 2021, and approved the use of 2 new vaccines, one to protect against meningococcal meningitis and the other to prevent Ebola virus disease. The influenza recommendations were covered in the October 2020 Practice Alert,1 and the immunization schedules can be found on the CDC website at www.cdc.gov/vaccines/schedules/hcp/index.html.
COVID-19 vaccines
Two COVID-19 vaccines have been approved for use in the United States. The first was the Pfizer-BioNTech COVID-19 vaccine, approved by the Food and Drug Administration (FDA) on December 11 and recommended for use by the ACIP on December 12.2 The second vaccine, from Moderna, was approved by the FDA on December 18 and recommended by the ACIP on December 19.3 Both were approved by the FDA under an Emergency Use Authorization (EUA) and were approved by the ACIP for use while the EUA is in effect. Both vaccines must eventually undergo regular approval by the FDA and will be reconsidered by the ACIP regarding use in non–public health emergency conditions. A description of the EUA process and measures taken to assure efficacy and safety, before and after approval, were discussed in the September 2020 audiocast.
Both COVID-19 vaccines consist of nucleoside-modified mRNA encapsulated with lipid nanoparticles, which encode for a spike glycoprotein of SARS-CoV-2, the virus that causes COVID-19. Both vaccines require 2 doses (separated by 3 weeks for the Pfizer vaccine and 4 weeks for the Moderna vaccine) and are approved for use only in adults and older adolescents (ages ≥ 16 years for the Pfizer vaccine and ≥ 18 years for the Moderna vaccine) (TABLE 12-5).
In anticipation of vaccine shortages immediately after approval for use and a high demand for the vaccine, the ACIP developed a list of high-priority groups who should receive the vaccine in ranked order.6 States are encouraged, but not required, to follow this priority list (TABLE 26).
Caveats with usage. Both COVID-19 vaccines are very reactogenic, causing local and systemic adverse effects that patients should be warned about (TABLE 37,8). These reactions are usually mild to moderate and last 24 hours or less. Acetaminophen can alleviate these symptoms but should not be used to prevent them. In addition, both vaccines have stringent cold-storage requirements; once the vaccines are thawed, they must be used within a defined time-period.
Neither vaccine is listed as preferred. And they are not interchangeable; both recommended doses should be completed with the same vaccine. More details about the use of these vaccines were discussed in the January 2021 audiocast (www.mdedge.com/familymedicine/article/234239/coronavirus-updates/covid-19-vaccines-rollout-risks-and-reason-still) and can be located on the CDC website (www.cdc.gov/vaccines/covid-19/info-by-product/pfizer/reactogenicity.html; www.cdc.gov/vaccines/covid-19/info-by-product/moderna/reactogenicity.html).
Continue to: Much remains unknown...
Much remains unknown regarding the use of these COVID-19 vaccines:
- What is their duration of protection, and will booster doses be needed?
- Will they protect against asymptomatic infection and carrier states, and thereby prevent transmission?
- Can they be co-administered with other vaccines?
- Will they be efficacious and safe to use during pregnancy and breastfeeding?
These issues will need to be addressed before they are recommended for non–public health emergency use.
Quadrivalent meningococcal conjugate vaccine (MenACWY)
In June 2020, the ACIP added a third quadrivalent meningococcal conjugate vaccine to its recommended list of vaccines that are FDA-approved for meningococcal disease (TABLE 49). The new vaccine fills a void left by the meningococcal polysaccharide vaccine (MPSV4), which is no longer marketed in the United States. MPSV4 was previously the only meningococcal vaccine approved for individuals 55 years and older.
The new vaccine, MenACWY-TT (MenQuadfi), is approved for those ages 2 years and older, including those > 55 years. It is anticipated that MenQuadfi will, in the near future, be licensed and approved for individuals 6 months and older and will replace MenACWY-D (Menactra). (Both are manufactured by Sanofi Pasteur.)
Groups for whom a MenACWY vaccine is recommended are listed in TABLE 5.9 A full description of current, updated recommendations for the prevention of meningococcal disease is also available.9
Continue to: Ebola virus (EBOV) vaccine
Ebola virus (EBOV) vaccine
A vaccine to prevent Ebola virus disease (EVD) is available by special request in the United States. Recombinant vesicular stomatitis virus-based Ebola virus vaccine, abbreviated as rVSVΔG-ZEBOV-GP (brand name, ERVBO) is manufactured by Merck and received approval by the FDA on December 19, 2019, for use in those ages 18 years and older. It is a live, attenuated vaccine.
The ACIP has recommended pre-exposure vaccination with rVSVΔG-ZEBOV-GP for adults 18 years or older who are at risk of exposure to EBOV while responding to an outbreak of EVD; while working as health care personnel at a federally designated Ebola Treatment Center; or while working at biosafety-level 4 facilities.10 The vaccine is protective against just 1 of 4 EBOV species, Zaire ebolavirus, which has been the cause of most reported EVD outbreaks, including the 2 largest EVD outbreaks in history that occurred in West Africa and the Republic of Congo.
It is estimated that EBOV outbreaks have infected more than 31,000 people and resulted in more than 12,000 deaths worldwide.11 Only 11 people infected with EBOV have been treated in the United States, all related to the 2014-2016 large outbreaks in West Africa. Nine of these cases were imported and only 1 resulted in transmission, to 2 people.10 The mammalian species that are suspected as intermediate hosts for EBOV are not present in the United States, which prevents EBOV from becoming endemic here.
The rVSVΔG-ZEBOV-GP vaccine was tested in a large trial in Africa during the 2014 outbreak. Its effectiveness was 100% (95% confidence interval, 63.5%-100%). The most common adverse effects were injection site pain, swelling, and redness. Mild-to-moderate systemic symptoms can occur within the first 2 days following vaccination, and include headache (37%), fever (34%), muscle pain (33%), fatigue (19%), joint pain (18%), nausea (8%), arthritis (5%), rash (4%), and
Since the vaccine contains a live virus that causes stomatitis in animals, it is possible that the virus could be transmitted to humans and other animals through close contact. Accordingly, the CDC has published some precautions including, but not limited to, not donating blood and, for 6 weeks after vaccination, avoiding contact with those who are immunosuppressed.10 The vaccine is not commercially available in the United States and must be obtained from the CDC. Information on requesting the vaccine is available at www.cdc.gov/vhf/ebola/clinicians/vaccine/.
The year 2020 was challenging for public health agencies and especially for the Centers for Disease Control and Prevention (CDC) and its Advisory Committee on Immunization Practices (ACIP). In a normal year, the ACIP meets in person 3 times for a total of 6 days of deliberations. In 2020, there were 10 meetings (all but 1 using Zoom) covering 14 days. Much of the time was dedicated to the COVID-19 pandemic, the vaccines being developed to prevent COVID-19, and the prioritization of those who should receive the vaccines first.
The ACIP also made recommendations for the use of influenza vaccines in the 2020-2021 season, approved the adult and pediatric immunization schedules for 2021, and approved the use of 2 new vaccines, one to protect against meningococcal meningitis and the other to prevent Ebola virus disease. The influenza recommendations were covered in the October 2020 Practice Alert,1 and the immunization schedules can be found on the CDC website at www.cdc.gov/vaccines/schedules/hcp/index.html.
COVID-19 vaccines
Two COVID-19 vaccines have been approved for use in the United States. The first was the Pfizer-BioNTech COVID-19 vaccine, approved by the Food and Drug Administration (FDA) on December 11 and recommended for use by the ACIP on December 12.2 The second vaccine, from Moderna, was approved by the FDA on December 18 and recommended by the ACIP on December 19.3 Both were approved by the FDA under an Emergency Use Authorization (EUA) and were approved by the ACIP for use while the EUA is in effect. Both vaccines must eventually undergo regular approval by the FDA and will be reconsidered by the ACIP regarding use in non–public health emergency conditions. A description of the EUA process and measures taken to assure efficacy and safety, before and after approval, were discussed in the September 2020 audiocast.
Both COVID-19 vaccines consist of nucleoside-modified mRNA encapsulated with lipid nanoparticles, which encode for a spike glycoprotein of SARS-CoV-2, the virus that causes COVID-19. Both vaccines require 2 doses (separated by 3 weeks for the Pfizer vaccine and 4 weeks for the Moderna vaccine) and are approved for use only in adults and older adolescents (ages ≥ 16 years for the Pfizer vaccine and ≥ 18 years for the Moderna vaccine) (TABLE 12-5).
In anticipation of vaccine shortages immediately after approval for use and a high demand for the vaccine, the ACIP developed a list of high-priority groups who should receive the vaccine in ranked order.6 States are encouraged, but not required, to follow this priority list (TABLE 26).
Caveats with usage. Both COVID-19 vaccines are very reactogenic, causing local and systemic adverse effects that patients should be warned about (TABLE 37,8). These reactions are usually mild to moderate and last 24 hours or less. Acetaminophen can alleviate these symptoms but should not be used to prevent them. In addition, both vaccines have stringent cold-storage requirements; once the vaccines are thawed, they must be used within a defined time-period.
Neither vaccine is listed as preferred. And they are not interchangeable; both recommended doses should be completed with the same vaccine. More details about the use of these vaccines were discussed in the January 2021 audiocast (www.mdedge.com/familymedicine/article/234239/coronavirus-updates/covid-19-vaccines-rollout-risks-and-reason-still) and can be located on the CDC website (www.cdc.gov/vaccines/covid-19/info-by-product/pfizer/reactogenicity.html; www.cdc.gov/vaccines/covid-19/info-by-product/moderna/reactogenicity.html).
Continue to: Much remains unknown...
Much remains unknown regarding the use of these COVID-19 vaccines:
- What is their duration of protection, and will booster doses be needed?
- Will they protect against asymptomatic infection and carrier states, and thereby prevent transmission?
- Can they be co-administered with other vaccines?
- Will they be efficacious and safe to use during pregnancy and breastfeeding?
These issues will need to be addressed before they are recommended for non–public health emergency use.
Quadrivalent meningococcal conjugate vaccine (MenACWY)
In June 2020, the ACIP added a third quadrivalent meningococcal conjugate vaccine to its recommended list of vaccines that are FDA-approved for meningococcal disease (TABLE 49). The new vaccine fills a void left by the meningococcal polysaccharide vaccine (MPSV4), which is no longer marketed in the United States. MPSV4 was previously the only meningococcal vaccine approved for individuals 55 years and older.
The new vaccine, MenACWY-TT (MenQuadfi), is approved for those ages 2 years and older, including those > 55 years. It is anticipated that MenQuadfi will, in the near future, be licensed and approved for individuals 6 months and older and will replace MenACWY-D (Menactra). (Both are manufactured by Sanofi Pasteur.)
Groups for whom a MenACWY vaccine is recommended are listed in TABLE 5.9 A full description of current, updated recommendations for the prevention of meningococcal disease is also available.9
Continue to: Ebola virus (EBOV) vaccine
Ebola virus (EBOV) vaccine
A vaccine to prevent Ebola virus disease (EVD) is available by special request in the United States. Recombinant vesicular stomatitis virus-based Ebola virus vaccine, abbreviated as rVSVΔG-ZEBOV-GP (brand name, ERVBO) is manufactured by Merck and received approval by the FDA on December 19, 2019, for use in those ages 18 years and older. It is a live, attenuated vaccine.
The ACIP has recommended pre-exposure vaccination with rVSVΔG-ZEBOV-GP for adults 18 years or older who are at risk of exposure to EBOV while responding to an outbreak of EVD; while working as health care personnel at a federally designated Ebola Treatment Center; or while working at biosafety-level 4 facilities.10 The vaccine is protective against just 1 of 4 EBOV species, Zaire ebolavirus, which has been the cause of most reported EVD outbreaks, including the 2 largest EVD outbreaks in history that occurred in West Africa and the Republic of Congo.
It is estimated that EBOV outbreaks have infected more than 31,000 people and resulted in more than 12,000 deaths worldwide.11 Only 11 people infected with EBOV have been treated in the United States, all related to the 2014-2016 large outbreaks in West Africa. Nine of these cases were imported and only 1 resulted in transmission, to 2 people.10 The mammalian species that are suspected as intermediate hosts for EBOV are not present in the United States, which prevents EBOV from becoming endemic here.
The rVSVΔG-ZEBOV-GP vaccine was tested in a large trial in Africa during the 2014 outbreak. Its effectiveness was 100% (95% confidence interval, 63.5%-100%). The most common adverse effects were injection site pain, swelling, and redness. Mild-to-moderate systemic symptoms can occur within the first 2 days following vaccination, and include headache (37%), fever (34%), muscle pain (33%), fatigue (19%), joint pain (18%), nausea (8%), arthritis (5%), rash (4%), and
Since the vaccine contains a live virus that causes stomatitis in animals, it is possible that the virus could be transmitted to humans and other animals through close contact. Accordingly, the CDC has published some precautions including, but not limited to, not donating blood and, for 6 weeks after vaccination, avoiding contact with those who are immunosuppressed.10 The vaccine is not commercially available in the United States and must be obtained from the CDC. Information on requesting the vaccine is available at www.cdc.gov/vhf/ebola/clinicians/vaccine/.
1. Campos-Outcalt D. Prospects and challenges for the upcoming influenza season. J Fam Pract 2020;69:406-411.
2. Oliver SE, Gargano JW, Marin M, et al. The Advisory Committee on Immunization Practices’ interim recommendation for use of Pfizer-BioNTech COVID-19 vaccine-United States, December 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1922-1924.
3. Oliver SE, Gargano JW, Marin M, et al. The Advisory Committee on Immunization Practices’ interim recommendation for use of Moderna COVID-19 vaccine-United States, December 2020. MMWR Morb Mortal Wkly Rep. 2021;69:1653-1656.
4. CDC. Pfizer-BioNTech COVID-19 vaccine. Accessed February 17, 2021. www.cdc.gov/vaccines/covid-19/info-by-product/pfizer/index.html
5. CDC. Moderna COVID-19 vaccine. Accessed February 17, 2021. www.cdc.gov/vaccines/covid-19/info-by-product/moderna/index.html#:~:text=How%20to%20Store%20the%20Moderna%20COVID%2D19%20Vaccine&text=Vaccine%20may%20be%20stored%20in,for%20this%20vaccine%20is%20tighter
6. Dooling K, Marin M, Wallace M, et al. The Advisory Committee on Immunization Practices’ updated interim recommendation for allocation of COVID-19 Vaccine—United States, December 2020. MMWR Morb Mortal Wkly Rep. 2021;69:1657-1660.
7. FDA. Fact sheet for healthcare providers administering vaccine. [Pfizer–BioNTech]. Accessed February 17, 2021. www.fda.gov/media/144413/download
8. FDA. Fact sheet for healthcare providers administering vaccine. [Moderna]. Accessed February 17, 2021. www.fda.gov/media/144637/download
9. Mbaeyi SA, Bozio CH, Duffy J, et al. Meningococcal vaccination: recommendations of the Advisory Committee on Immunization Practices, United States, 2020. MMWR Recomm Rep. 2020;69:1-41.
10. Choi MJ, Cossaboom CM, Whitesell AN, et al. Use of Ebola vaccine: Recommendations of the Advisory Committee on Immunization Practices—United States, 2020. MMWR Recomm Rep. 2021;70:1-12.
11. CDC. Ebola background. Accessed February 17, 2021. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2020-02/Ebola-02-Choi-508.pdf
1. Campos-Outcalt D. Prospects and challenges for the upcoming influenza season. J Fam Pract 2020;69:406-411.
2. Oliver SE, Gargano JW, Marin M, et al. The Advisory Committee on Immunization Practices’ interim recommendation for use of Pfizer-BioNTech COVID-19 vaccine-United States, December 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1922-1924.
3. Oliver SE, Gargano JW, Marin M, et al. The Advisory Committee on Immunization Practices’ interim recommendation for use of Moderna COVID-19 vaccine-United States, December 2020. MMWR Morb Mortal Wkly Rep. 2021;69:1653-1656.
4. CDC. Pfizer-BioNTech COVID-19 vaccine. Accessed February 17, 2021. www.cdc.gov/vaccines/covid-19/info-by-product/pfizer/index.html
5. CDC. Moderna COVID-19 vaccine. Accessed February 17, 2021. www.cdc.gov/vaccines/covid-19/info-by-product/moderna/index.html#:~:text=How%20to%20Store%20the%20Moderna%20COVID%2D19%20Vaccine&text=Vaccine%20may%20be%20stored%20in,for%20this%20vaccine%20is%20tighter
6. Dooling K, Marin M, Wallace M, et al. The Advisory Committee on Immunization Practices’ updated interim recommendation for allocation of COVID-19 Vaccine—United States, December 2020. MMWR Morb Mortal Wkly Rep. 2021;69:1657-1660.
7. FDA. Fact sheet for healthcare providers administering vaccine. [Pfizer–BioNTech]. Accessed February 17, 2021. www.fda.gov/media/144413/download
8. FDA. Fact sheet for healthcare providers administering vaccine. [Moderna]. Accessed February 17, 2021. www.fda.gov/media/144637/download
9. Mbaeyi SA, Bozio CH, Duffy J, et al. Meningococcal vaccination: recommendations of the Advisory Committee on Immunization Practices, United States, 2020. MMWR Recomm Rep. 2020;69:1-41.
10. Choi MJ, Cossaboom CM, Whitesell AN, et al. Use of Ebola vaccine: Recommendations of the Advisory Committee on Immunization Practices—United States, 2020. MMWR Recomm Rep. 2021;70:1-12.
11. CDC. Ebola background. Accessed February 17, 2021. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2020-02/Ebola-02-Choi-508.pdf
AT PRESS TIME
The US Food and Drug Administration issued an Emergency Use Authorization for a third COVID-19 vaccine. The single-dose vaccine was developed by the Janssen Pharmaceutical Companies of Johnson & Johnson. For more information, go to www.mdedge.com/familymedicine
Conservative or surgical management for that shoulder dislocation?
The shoulder, or glenohumeral joint, is the most commonly dislocated large joint; dislocation occurs at a rate of 23.9 per 100,000 person/years.1,2 There are 2 types of dislocation: traumatic anterior dislocation, which accounts for roughly 90% of dislocations, and posterior dislocation (10%).3 Anterior dislocation typically occurs when the patient’s shoulder is forcefully abducted and externally rotated.
The diagnosis is made after review of the history and mechanism of injury and performance of a complete physical exam with imaging studies—the most critical component of diagnosis.4 Standard radiographs (anteroposterior, axillary, and scapular Y) can confirm the presence of a dislocation; once the diagnosis is confirmed, closed reduction of the joint should be performed.1 (Methods of reduction are beyond the scope of this article but have been recently reviewed.5)
Risk for recurrence drives management choices
Following an initial shoulder dislocation, the risk of recurrence is high.6,7 Rates vary based on age, pathology after dislocation, activity level, type of immobilization, and whether surgery was performed. Overall, age is the strongest predictor of recurrence: 72% of patients ages 12 to 22 years, 56% of those ages 23 to 29 years, and 27% of those older than 30 years experience recurrence.6 Patients who have recurrent dislocations are at risk for arthropathy, fear of instability, and worsening surgical outcomes.6
Reducing the risk of a recurrent shoulder dislocation has been the focus of intense study. Proponents of surgical stabilization argue that surgery—rather than a trial of conservative treatment—is best when you consider the high risk of recurrence in young athletes (the population primarily studied), the soft-tissue and bony damage caused by recurrent instability, and the predictable improvement in quality of life following surgery.
In a recent systematic review and meta-analysis, there was evidence that, for first-time traumatic shoulder dislocations, early surgery led to fewer repeat shoulder dislocations (number needed to treat [NNT] = 2-4.7). However, a significant number of patients primarily treated nonoperatively did not experience a repeat shoulder dislocation within 2 years.2
The conflicting results from randomized trials comparing operative intervention to conservative management have led surgeons and physicians in other specialties to take different approaches to the management of shoulder dislocation.2 In this review, we aim to summarize considerations for conservative vs surgical management and provide clinical guidance for primary care physicians.
When to try conservative management
Although the initial treatment after a traumatic anterior shoulder dislocation has been debated, a recent meta-analysis of randomized controlled trials showed that at least half of first-time dislocations are successfully treated with conservative management.2 Management can include immobilization for comfort and/or physical therapy. Age will play a role, as mentioned earlier; in general, patients older than 30 have a significant decrease in recurrence rate and are good candidates for conservative therapy.6 It should be noted that much of the research with regard to management of shoulder dislocations has been done in an athletic population.
Continue to: Immobilization may benefit some
Immobilization may benefit some
Recent evidence has determined that the duration of immobilization in internal rotation does not impact recurrent instability.8,9 In patients older than 30, the rate of repeat dislocation is lower, and early mobilization after 1 week is advocated to avoid joint stiffness and minimize the risk of adhesive capsulitis.10
Arm position during immobilization remains controversial.11 In a classic study by Itoi et al, immobilization for 3 weeks in internal rotation vs 10° of external rotation was associated with a recurrence rate of 42% vs 26%, respectively.12 In this study, immobilization in 10° of external rotation was especially beneficial for patients ages 30 years or younger.12
Cadaveric and magnetic resonance imaging (MRI) studies have shown external rotation may improve the odds of labral tear healing by positioning the damaged and intact parts of the glenoid labrum in closer proximity.13 While this is theoretically plausible, a recent Cochrane review found insufficient evidence to determine whether immobilization in external rotation has any benefits beyond those offered by internal rotation.14 A recent systematic review and meta-analysis found that immobilization in external rotation vs internal rotation after a first-time traumatic shoulder dislocation did not change outcomes.2 With that said, most would prefer to immobilize in the internal rotation position for ease.
More research is needed. A Cochrane review highlighted the need for continued research.14 Additionally, most of the available randomized controlled trials to date have consisted of young men, with the majority of dislocations related to sports activities. Women, nonathletes, and older patients have been understudied to date; extrapolating current research to those groups of patients may not be appropriate and should be a focus for future research.2
Physical therapy: The conservative standard of care
Rehabilitation after glenohumeral joint dislocation is the current standard of care in conservative management to reduce the risk for repeat dislocation.15 Depending on the specific characteristics of the instability pattern, the approach may be adapted to the patient. A recent review focused on the following 4 key points: (1) restoration of rotator cuff strength, focusing on the eccentric capacity of the external rotators, (2) normalization of rotational range of motion with particular focus on internal range of motion, (3) optimization of the flexibility and muscle performance of the scapular muscles, and (4) increasing the functional sport-specific load on the shoulder girdle.
Continue to: A common approach to the care of...
A common approach to the care of a patient after a glenohumeral joint dislocation is to place the patient’s shoulder in a sling for comfort, with permitted pain-free isometric exercise along with passive and assisted elevation up to 100°.16 This is followed by a nonaggressive rehabilitation protocol for 2 months until full recovery, which includes progressive range of motion, strength, proprioception, and return to functional activities.16
More aggressive return-to-play protocols with accelerated timelines and functional progression have been studied, including in a multicenter observational study that followed 45 contact intercollegiate athletes prospectively after in-season anterior glenohumeral instability. Thirty-three of 45 (73%) athletes returned to sport for either all or part of the season after a median 5 days lost from competition, with 12 athletes (27%) successfully completing the season without recurrence. Athletes with a subluxation event were 5.3 times more likely to return to sport during the same season, compared with those with dislocations.17
Dynamic bracing may also allow for a safe and quicker return to sport in athletes18 but recently was shown to not impact recurrent dislocation risk.19
Return to play should be based on subjective assessment as well as objective measurements of range of motion, strength, and dynamic function.15 Patients who continue to have significant weakness and pain at 2 to 3 weeks post injury despite physical therapy should be re-evaluated with an MRI for concomitant rotator cuff tears and need for surgical referral.20
When to consider surgical intervention
In a recent meta-analysis, recurrent dislocation and instability occurred at a rate of 52.9% following nonsurgical treatment.2 The decision to perform surgical intervention is typically made following failure of conservative management. Other considerations include age, gender, bone loss, and cartilage defect.21,22 Age younger than 30 years, participation in competition, contact sports, and male gender have been associated with an increased risk of recurrence.23-25 For this reason, obtaining an MRI at time of first dislocation can help facilitate surgical decisions if the patient is at high risk for surgical need.26
Continue to: An increasing number...
An increasing number of dislocations portends a poor outcome with nonoperative treatment. Kao et al demonstrated a second dislocation leads to another dislocation in 19.6% of cases, while 44.3% of those with a third dislocation event will sustain another dislocation.24 Surgery should be considered for patients with recurrent instability events to prevent persistent instability and decrease the amount of bone loss that can occur with repetitive dislocations.
What are the surgical options?
Several surgical options exist to remedy the unstable shoulder. Procedures can range from an arthroscopic repair to an open stabilization combined with structural bone graft to replace a bone defect caused by repetitive dislocations.
Arthroscopic techniques have become the mainstay of treatment and account for 71% of stabilization procedures performed.21 These techniques cause less pain in the early postoperative period and provide for a faster return to work.27 Arthroscopy has the additional advantage of allowing for complete visualization of the glenohumeral joint to identify and address concomitant pathology, such as intra-articular loose bodies or rotator cuff tears.
Open repair was the mainstay of treatment prior to development of arthroscopic techniques. Some surgeons still prefer this method—especially in high-risk groups—because of a lower risk of recurrent disloca-tion.28 Open techniques often involve detachment and repair of the upper subscapularis tendon and are more likely to produce long-term losses in external rotation range of motion.28
Which one is appropriate for your patient? The decision to pursue an open or arthroscopic procedure and to augment with bone graft depends on the amount of glenoid and humeral head bone loss, patient activity level, risk of recurrent dislocation, and surgeon preference.
Continue to: For the nonathletic population...
For the nonathletic population, the timing of injury is less critical and surgery is typically recommended after conservative treatment has failed. In an athletic population, the timing of injury is a necessary consideration. An injury midseason may be “rehabbed” in hopes of returning to play. Individuals with injuries occurring at the end of a season, who are unable to regain desired function, and/or with peri-articular fractures or associated full-thickness rotator cuff tears may benefit from sooner surgical intervention.21
Owens et al have described appropriate surgical indications and recommendations for an in-season athlete.21 In this particular algorithm, the authors suggest obtaining an MRI for decision making, but this is specific to in-season athletes wishing to return to play. In general, an MRI is not always indicated for patients who wish to receive conservative therapy but would be indicated for surgical considerations. The algorithm otherwise uses bone and soft-tissue injury, recurrent instability, and timing in the season to help determine management.21
Outcomes: Surgery has advantages …
Recurrence rates following surgical intervention are considerably lower than with conservative management, especially among young, active individuals. A recent systematic review by Donohue et al demonstrated recurrent instability rates following surgical intervention as low as 2.4%.29 One study comparing the outcome of arthroscopic repair vs conservative management showed that the risk of postoperative instability was reduced by 20% compared to other treatments.7 Furthermore, early surgical fixation can improve quality of life, produce better functional outcomes, decrease time away from activity, increase patient satisfaction, and slow the development of glenohumeral osteoarthritis produced from recurrent instability.2,7
Complications. Surgery does carry inherent risks of infection, anesthesia effects, surgical complications, and surgical failure. Recurrent instability is the most common complication following surgical shoulder stabilization. Rates of recurrent instability after surgical stabilization depend on patient age, activity level, and amount of bone loss: males younger than 18 years who participate in contact competitive sports and have significant bone loss are more likely to have recurrent dislocation after surgery.23 The type of surgical procedure selected may decrease this risk.
While the open procedures decrease risk of postoperative instability, these surgeries can pose a significant risk of complications. Major complications for specific open techniques have been reported in up to 30% of patients30 and are associated with lower levels of surgeon experience.31 While the healing of bones and ligaments is always a concern, 1 of the most feared complications following stabilization surgery is iatrogenic nerve injury. Because of the axillary nerve’s close proximity to the inferior glenoid, this nerve can be injured without meticulous care and can result in paralysis of the deltoid muscle. This injury poses a major impediment to normal shoulder function. Some procedures may cause nerve injuries in up to 10% of patients, although most injuries are transient.32
Continue to: Bottom line
Bottom line
Due to the void of evidence-based guidelines for conservative vs surgical management of primary shoulder dislocation, it would be prudent to have a risk-benefit discussion with patients regarding treatment options.
Patients older than 30 years and those with uncomplicated injuries are best suited for conservative management of primary shoulder dislocations. Immobilization is debated and may not change outcomes, but a progressive rehabilitative program after the initial acute injury is helpful. Risk factors for failing conservative management include recurrent dislocation, subsequent arthropathy, and additional concomitant bone or soft-tissue injuries.
Patients younger than 30 years who have complicated injuries with bone or cartilage loss, rotator cuff tears, or recurrent instability, and highly physically active individuals are best suited for surgical management. Shoulder arthroscopy has become the mainstay of surgical treatment for shoulder dislocations. Outcomes are favorable and dislocation recurrence is low after surgical repair. Surgery does carry its own inherent risks of infection, anesthesia effects, complications during surgery, and surgical failure leading to recurrent instability.
Cayce Onks, DO, MS, ATC, Penn State Hershey, Milton S. Hershey Medical Center, Penn State College of Medicine, Family and Community Medicine H154, 500 University Drive, PO Box 850, Hershey, PA 17033-0850; conks@pennstatehealth.psu.edu
1. Lin K, James E, Spitzer E, et al. Pediatric and adolescent anterior shoulder instability: clinical management of first time dislocators. Curr Opin Pediatr. 2018;30:49-56.
2. Kavaja L, Lähdeoja T, Malmivaara A, et al. Treatment after traumatic shoulder dislocation: a systematic review with a network meta-analysis. Br J Sports Med. 2018;52:1498-1506.
3. Brelin A, Dickens JF. Posterior shoulder instability. Sports Med Arthrosc Rev. 2017;25:136-143.
4. Galvin JW, Ernat JJ, Waterman BR, et al. The epidemiology and natural history of anterior shoulder dislocation. Curr Rev Musculoskelet Med. 2017;10:411-424.
5. Rozzi SL, Anderson JM, Doberstein ST, et al. National Athletic Trainers’ Association position statement: immediate management of appendicular joint dislocations. J Athl Train. 2018;53:1117-1128.
6. Hovelius L, Saeboe M. Arthropathy after primary anterior shoulder dislocation: 223 shoulders prospectively followed up for twenty-five years. J Shoulder Elbow Surg. 2009;18:339-347.
7. Polyzois I, Dattani R, Gupta R, et al. Traumatic first time shoulder dislocation: surgery vs non-operative treatment. Arch Bone Jt Surg. 2016;4:104-108.
8. Cox CL, Kuhn JE. Operative versus nonoperative treatment of acute shoulder dislocation in the athlete. Curr Sports Med Rep. 2008;7:263-268.
9. Kuhn JE. Treating the initial anterior shoulder dislocation—an evidence-based medicine approach. Sports Med Arthrosc Rev. 2006;14:192-198.
10. Smith TO. Immobilization following traumatic anterior glenohumeral joint dislocation: a literature review. Injury. 2006;37:228-237.
11. Liavaag S, Brox JI, Pripp AH, et al. Immobilization in external rotation after primary shoulder dislocation did not reduce the risk of recurrence: a randomized controlled trial. J Bone Joint Surg Am. 2011;93:897-904.
12. Itoi E, Hatakeyama Y, Sato T, et al. Immobilization in external rotation after shoulder dislocation reduces the risk of recurrence: a randomized controlled trial. J Bone Joint Surg Am. 2007;89:2124-2131.
13. Miller BS, Sonnabend DH, Hatrick C, et al. Should acute anterior dislocations of the shoulder be immobilized in external rotation? A cadaveric study. J Shoulder Elbow Surg. 2004;13:589-592.
14. Hanchard NCA, Goodchild LM, Kottam L. Conservative management following closed reduction of traumatic anterior dislocation of the shoulder. Cochrane Database Syst Rev. 2014;(4):CD004962.
15. Cools AM, Borms D, Castelein B, et al. Evidence-based rehabilitation of athletes with glenohumeral instability. Knee Surg Sports Traumatol Arthrosc. 2016;24:382-389.
16. Lafuente JLA, Marco SM, Pequerul JMG. Controversies in the management of the first time shoulder dislocation. Open Orthop J. 2017;11:1001-1010.
17. Dickens JF, Owens BD, Cameron KL, et al. Return to play and recurrent instability after in-season anterior shoulder instability: a prospective multicenter study. Am J Sports Med. 2014;42:2842-2850.
18. Conti M, Garofalo R, Castagna A, et al. Dynamic brace is a good option to treat first anterior shoulder dislocation in season. Musculoskelet Surg. 2017;101(suppl 2):169-173.
19. Shanley E, Thigpen C, Brooks J, et al. Return to sport as an outcome measure for shoulder instability. Am J Sports Med. 2019;47:1062-1067.
20. Gombera MM, Sekiya JK. Rotator cuff tear and glenohumeral instability. Clin Orthop Relat Res. 2014;472:2448-2456.
21. Owens BD, Dickens JF, Kilcoyne KG, et al. Management of mid-season traumatic anterior shoulder instability in athletes. J Am Acad Orthop Surg. 2012;20:518-526.
22. Ozturk BY, Maak TG, Fabricant P, et al. Return to sports after arthroscopic anterior stabilization in patients aged younger than 25 years. Arthroscopy. 2013;29:1922-1931.
23. Balg F, Boileau P. The instability severity index score. A simple preoperative score to select patients for arthroscopic or open shoulder stabilisation. J Bone Joint Surg Br. 2007;89:1470-1477.
24. Kao J-T, Chang C-L, Su W-R, et al. Incidence of recurrence after shoulder dislocation: a nationwide database study. J Shoulder Elbow Surg. 2018;27:1519-1525.
25. Porcillini G, Campi F, Pegreffi F, et al. Predisposing factors for recurrent shoulder dislocation after arthroscopic treatment. J Bone Joint Surg Am. 2009;91:2537-2542.
26. Magee T. 3T MRI of the shoulder: is MR arthrography necessary? AJR Am J Roentgenol. 2009;192:86-92.
27. Green MR, Christensen KP. Arthroscopic versus open Bankart procedures: a comparison of early morbidity and complications. Arthroscopy. 1993;9:371-374.
28. Khatri K, Arora H, Chaudhary S, et al. Meta-analysis of randomized controlled trials involving anterior shoulder instability. Open Orthop J. 2018;12:411-418.
29. Donohue MA, Owens BD, Dickens JF. Return to play following anterior shoulder dislocations and stabilization surgery. Clin Sports Med. 2016;35:545-561.
30. Griesser MJ, Harris JD, McCoy BW, et al. Complications and re-operations after Bristow-Latarjet shoulder stabilization: a systematic review. J Shoulder Elbow Surg. 2013;22:286-292.
31. Ekhtiari S, Horner NS, Bedi A, et al. The learning curve for the Latarjet procedure: a systematic review. Orthop J Sports Med. 2018;6:2325967118786930.
32. Shah AA, Butler RB, Romanowski J, et al. Short-term complications of the Latarjet procedure. J Bone Joint Surg Am. 2012;94:495-501.
The shoulder, or glenohumeral joint, is the most commonly dislocated large joint; dislocation occurs at a rate of 23.9 per 100,000 person/years.1,2 There are 2 types of dislocation: traumatic anterior dislocation, which accounts for roughly 90% of dislocations, and posterior dislocation (10%).3 Anterior dislocation typically occurs when the patient’s shoulder is forcefully abducted and externally rotated.
The diagnosis is made after review of the history and mechanism of injury and performance of a complete physical exam with imaging studies—the most critical component of diagnosis.4 Standard radiographs (anteroposterior, axillary, and scapular Y) can confirm the presence of a dislocation; once the diagnosis is confirmed, closed reduction of the joint should be performed.1 (Methods of reduction are beyond the scope of this article but have been recently reviewed.5)
Risk for recurrence drives management choices
Following an initial shoulder dislocation, the risk of recurrence is high.6,7 Rates vary based on age, pathology after dislocation, activity level, type of immobilization, and whether surgery was performed. Overall, age is the strongest predictor of recurrence: 72% of patients ages 12 to 22 years, 56% of those ages 23 to 29 years, and 27% of those older than 30 years experience recurrence.6 Patients who have recurrent dislocations are at risk for arthropathy, fear of instability, and worsening surgical outcomes.6
Reducing the risk of a recurrent shoulder dislocation has been the focus of intense study. Proponents of surgical stabilization argue that surgery—rather than a trial of conservative treatment—is best when you consider the high risk of recurrence in young athletes (the population primarily studied), the soft-tissue and bony damage caused by recurrent instability, and the predictable improvement in quality of life following surgery.
In a recent systematic review and meta-analysis, there was evidence that, for first-time traumatic shoulder dislocations, early surgery led to fewer repeat shoulder dislocations (number needed to treat [NNT] = 2-4.7). However, a significant number of patients primarily treated nonoperatively did not experience a repeat shoulder dislocation within 2 years.2
The conflicting results from randomized trials comparing operative intervention to conservative management have led surgeons and physicians in other specialties to take different approaches to the management of shoulder dislocation.2 In this review, we aim to summarize considerations for conservative vs surgical management and provide clinical guidance for primary care physicians.
When to try conservative management
Although the initial treatment after a traumatic anterior shoulder dislocation has been debated, a recent meta-analysis of randomized controlled trials showed that at least half of first-time dislocations are successfully treated with conservative management.2 Management can include immobilization for comfort and/or physical therapy. Age will play a role, as mentioned earlier; in general, patients older than 30 have a significant decrease in recurrence rate and are good candidates for conservative therapy.6 It should be noted that much of the research with regard to management of shoulder dislocations has been done in an athletic population.
Continue to: Immobilization may benefit some
Immobilization may benefit some
Recent evidence has determined that the duration of immobilization in internal rotation does not impact recurrent instability.8,9 In patients older than 30, the rate of repeat dislocation is lower, and early mobilization after 1 week is advocated to avoid joint stiffness and minimize the risk of adhesive capsulitis.10
Arm position during immobilization remains controversial.11 In a classic study by Itoi et al, immobilization for 3 weeks in internal rotation vs 10° of external rotation was associated with a recurrence rate of 42% vs 26%, respectively.12 In this study, immobilization in 10° of external rotation was especially beneficial for patients ages 30 years or younger.12
Cadaveric and magnetic resonance imaging (MRI) studies have shown external rotation may improve the odds of labral tear healing by positioning the damaged and intact parts of the glenoid labrum in closer proximity.13 While this is theoretically plausible, a recent Cochrane review found insufficient evidence to determine whether immobilization in external rotation has any benefits beyond those offered by internal rotation.14 A recent systematic review and meta-analysis found that immobilization in external rotation vs internal rotation after a first-time traumatic shoulder dislocation did not change outcomes.2 With that said, most would prefer to immobilize in the internal rotation position for ease.
More research is needed. A Cochrane review highlighted the need for continued research.14 Additionally, most of the available randomized controlled trials to date have consisted of young men, with the majority of dislocations related to sports activities. Women, nonathletes, and older patients have been understudied to date; extrapolating current research to those groups of patients may not be appropriate and should be a focus for future research.2
Physical therapy: The conservative standard of care
Rehabilitation after glenohumeral joint dislocation is the current standard of care in conservative management to reduce the risk for repeat dislocation.15 Depending on the specific characteristics of the instability pattern, the approach may be adapted to the patient. A recent review focused on the following 4 key points: (1) restoration of rotator cuff strength, focusing on the eccentric capacity of the external rotators, (2) normalization of rotational range of motion with particular focus on internal range of motion, (3) optimization of the flexibility and muscle performance of the scapular muscles, and (4) increasing the functional sport-specific load on the shoulder girdle.
Continue to: A common approach to the care of...
A common approach to the care of a patient after a glenohumeral joint dislocation is to place the patient’s shoulder in a sling for comfort, with permitted pain-free isometric exercise along with passive and assisted elevation up to 100°.16 This is followed by a nonaggressive rehabilitation protocol for 2 months until full recovery, which includes progressive range of motion, strength, proprioception, and return to functional activities.16
More aggressive return-to-play protocols with accelerated timelines and functional progression have been studied, including in a multicenter observational study that followed 45 contact intercollegiate athletes prospectively after in-season anterior glenohumeral instability. Thirty-three of 45 (73%) athletes returned to sport for either all or part of the season after a median 5 days lost from competition, with 12 athletes (27%) successfully completing the season without recurrence. Athletes with a subluxation event were 5.3 times more likely to return to sport during the same season, compared with those with dislocations.17
Dynamic bracing may also allow for a safe and quicker return to sport in athletes18 but recently was shown to not impact recurrent dislocation risk.19
Return to play should be based on subjective assessment as well as objective measurements of range of motion, strength, and dynamic function.15 Patients who continue to have significant weakness and pain at 2 to 3 weeks post injury despite physical therapy should be re-evaluated with an MRI for concomitant rotator cuff tears and need for surgical referral.20
When to consider surgical intervention
In a recent meta-analysis, recurrent dislocation and instability occurred at a rate of 52.9% following nonsurgical treatment.2 The decision to perform surgical intervention is typically made following failure of conservative management. Other considerations include age, gender, bone loss, and cartilage defect.21,22 Age younger than 30 years, participation in competition, contact sports, and male gender have been associated with an increased risk of recurrence.23-25 For this reason, obtaining an MRI at time of first dislocation can help facilitate surgical decisions if the patient is at high risk for surgical need.26
Continue to: An increasing number...
An increasing number of dislocations portends a poor outcome with nonoperative treatment. Kao et al demonstrated a second dislocation leads to another dislocation in 19.6% of cases, while 44.3% of those with a third dislocation event will sustain another dislocation.24 Surgery should be considered for patients with recurrent instability events to prevent persistent instability and decrease the amount of bone loss that can occur with repetitive dislocations.
What are the surgical options?
Several surgical options exist to remedy the unstable shoulder. Procedures can range from an arthroscopic repair to an open stabilization combined with structural bone graft to replace a bone defect caused by repetitive dislocations.
Arthroscopic techniques have become the mainstay of treatment and account for 71% of stabilization procedures performed.21 These techniques cause less pain in the early postoperative period and provide for a faster return to work.27 Arthroscopy has the additional advantage of allowing for complete visualization of the glenohumeral joint to identify and address concomitant pathology, such as intra-articular loose bodies or rotator cuff tears.
Open repair was the mainstay of treatment prior to development of arthroscopic techniques. Some surgeons still prefer this method—especially in high-risk groups—because of a lower risk of recurrent disloca-tion.28 Open techniques often involve detachment and repair of the upper subscapularis tendon and are more likely to produce long-term losses in external rotation range of motion.28
Which one is appropriate for your patient? The decision to pursue an open or arthroscopic procedure and to augment with bone graft depends on the amount of glenoid and humeral head bone loss, patient activity level, risk of recurrent dislocation, and surgeon preference.
Continue to: For the nonathletic population...
For the nonathletic population, the timing of injury is less critical and surgery is typically recommended after conservative treatment has failed. In an athletic population, the timing of injury is a necessary consideration. An injury midseason may be “rehabbed” in hopes of returning to play. Individuals with injuries occurring at the end of a season, who are unable to regain desired function, and/or with peri-articular fractures or associated full-thickness rotator cuff tears may benefit from sooner surgical intervention.21
Owens et al have described appropriate surgical indications and recommendations for an in-season athlete.21 In this particular algorithm, the authors suggest obtaining an MRI for decision making, but this is specific to in-season athletes wishing to return to play. In general, an MRI is not always indicated for patients who wish to receive conservative therapy but would be indicated for surgical considerations. The algorithm otherwise uses bone and soft-tissue injury, recurrent instability, and timing in the season to help determine management.21
Outcomes: Surgery has advantages …
Recurrence rates following surgical intervention are considerably lower than with conservative management, especially among young, active individuals. A recent systematic review by Donohue et al demonstrated recurrent instability rates following surgical intervention as low as 2.4%.29 One study comparing the outcome of arthroscopic repair vs conservative management showed that the risk of postoperative instability was reduced by 20% compared to other treatments.7 Furthermore, early surgical fixation can improve quality of life, produce better functional outcomes, decrease time away from activity, increase patient satisfaction, and slow the development of glenohumeral osteoarthritis produced from recurrent instability.2,7
Complications. Surgery does carry inherent risks of infection, anesthesia effects, surgical complications, and surgical failure. Recurrent instability is the most common complication following surgical shoulder stabilization. Rates of recurrent instability after surgical stabilization depend on patient age, activity level, and amount of bone loss: males younger than 18 years who participate in contact competitive sports and have significant bone loss are more likely to have recurrent dislocation after surgery.23 The type of surgical procedure selected may decrease this risk.
While the open procedures decrease risk of postoperative instability, these surgeries can pose a significant risk of complications. Major complications for specific open techniques have been reported in up to 30% of patients30 and are associated with lower levels of surgeon experience.31 While the healing of bones and ligaments is always a concern, 1 of the most feared complications following stabilization surgery is iatrogenic nerve injury. Because of the axillary nerve’s close proximity to the inferior glenoid, this nerve can be injured without meticulous care and can result in paralysis of the deltoid muscle. This injury poses a major impediment to normal shoulder function. Some procedures may cause nerve injuries in up to 10% of patients, although most injuries are transient.32
Continue to: Bottom line
Bottom line
Due to the void of evidence-based guidelines for conservative vs surgical management of primary shoulder dislocation, it would be prudent to have a risk-benefit discussion with patients regarding treatment options.
Patients older than 30 years and those with uncomplicated injuries are best suited for conservative management of primary shoulder dislocations. Immobilization is debated and may not change outcomes, but a progressive rehabilitative program after the initial acute injury is helpful. Risk factors for failing conservative management include recurrent dislocation, subsequent arthropathy, and additional concomitant bone or soft-tissue injuries.
Patients younger than 30 years who have complicated injuries with bone or cartilage loss, rotator cuff tears, or recurrent instability, and highly physically active individuals are best suited for surgical management. Shoulder arthroscopy has become the mainstay of surgical treatment for shoulder dislocations. Outcomes are favorable and dislocation recurrence is low after surgical repair. Surgery does carry its own inherent risks of infection, anesthesia effects, complications during surgery, and surgical failure leading to recurrent instability.
Cayce Onks, DO, MS, ATC, Penn State Hershey, Milton S. Hershey Medical Center, Penn State College of Medicine, Family and Community Medicine H154, 500 University Drive, PO Box 850, Hershey, PA 17033-0850; conks@pennstatehealth.psu.edu
The shoulder, or glenohumeral joint, is the most commonly dislocated large joint; dislocation occurs at a rate of 23.9 per 100,000 person/years.1,2 There are 2 types of dislocation: traumatic anterior dislocation, which accounts for roughly 90% of dislocations, and posterior dislocation (10%).3 Anterior dislocation typically occurs when the patient’s shoulder is forcefully abducted and externally rotated.
The diagnosis is made after review of the history and mechanism of injury and performance of a complete physical exam with imaging studies—the most critical component of diagnosis.4 Standard radiographs (anteroposterior, axillary, and scapular Y) can confirm the presence of a dislocation; once the diagnosis is confirmed, closed reduction of the joint should be performed.1 (Methods of reduction are beyond the scope of this article but have been recently reviewed.5)
Risk for recurrence drives management choices
Following an initial shoulder dislocation, the risk of recurrence is high.6,7 Rates vary based on age, pathology after dislocation, activity level, type of immobilization, and whether surgery was performed. Overall, age is the strongest predictor of recurrence: 72% of patients ages 12 to 22 years, 56% of those ages 23 to 29 years, and 27% of those older than 30 years experience recurrence.6 Patients who have recurrent dislocations are at risk for arthropathy, fear of instability, and worsening surgical outcomes.6
Reducing the risk of a recurrent shoulder dislocation has been the focus of intense study. Proponents of surgical stabilization argue that surgery—rather than a trial of conservative treatment—is best when you consider the high risk of recurrence in young athletes (the population primarily studied), the soft-tissue and bony damage caused by recurrent instability, and the predictable improvement in quality of life following surgery.
In a recent systematic review and meta-analysis, there was evidence that, for first-time traumatic shoulder dislocations, early surgery led to fewer repeat shoulder dislocations (number needed to treat [NNT] = 2-4.7). However, a significant number of patients primarily treated nonoperatively did not experience a repeat shoulder dislocation within 2 years.2
The conflicting results from randomized trials comparing operative intervention to conservative management have led surgeons and physicians in other specialties to take different approaches to the management of shoulder dislocation.2 In this review, we aim to summarize considerations for conservative vs surgical management and provide clinical guidance for primary care physicians.
When to try conservative management
Although the initial treatment after a traumatic anterior shoulder dislocation has been debated, a recent meta-analysis of randomized controlled trials showed that at least half of first-time dislocations are successfully treated with conservative management.2 Management can include immobilization for comfort and/or physical therapy. Age will play a role, as mentioned earlier; in general, patients older than 30 have a significant decrease in recurrence rate and are good candidates for conservative therapy.6 It should be noted that much of the research with regard to management of shoulder dislocations has been done in an athletic population.
Continue to: Immobilization may benefit some
Immobilization may benefit some
Recent evidence has determined that the duration of immobilization in internal rotation does not impact recurrent instability.8,9 In patients older than 30, the rate of repeat dislocation is lower, and early mobilization after 1 week is advocated to avoid joint stiffness and minimize the risk of adhesive capsulitis.10
Arm position during immobilization remains controversial.11 In a classic study by Itoi et al, immobilization for 3 weeks in internal rotation vs 10° of external rotation was associated with a recurrence rate of 42% vs 26%, respectively.12 In this study, immobilization in 10° of external rotation was especially beneficial for patients ages 30 years or younger.12
Cadaveric and magnetic resonance imaging (MRI) studies have shown external rotation may improve the odds of labral tear healing by positioning the damaged and intact parts of the glenoid labrum in closer proximity.13 While this is theoretically plausible, a recent Cochrane review found insufficient evidence to determine whether immobilization in external rotation has any benefits beyond those offered by internal rotation.14 A recent systematic review and meta-analysis found that immobilization in external rotation vs internal rotation after a first-time traumatic shoulder dislocation did not change outcomes.2 With that said, most would prefer to immobilize in the internal rotation position for ease.
More research is needed. A Cochrane review highlighted the need for continued research.14 Additionally, most of the available randomized controlled trials to date have consisted of young men, with the majority of dislocations related to sports activities. Women, nonathletes, and older patients have been understudied to date; extrapolating current research to those groups of patients may not be appropriate and should be a focus for future research.2
Physical therapy: The conservative standard of care
Rehabilitation after glenohumeral joint dislocation is the current standard of care in conservative management to reduce the risk for repeat dislocation.15 Depending on the specific characteristics of the instability pattern, the approach may be adapted to the patient. A recent review focused on the following 4 key points: (1) restoration of rotator cuff strength, focusing on the eccentric capacity of the external rotators, (2) normalization of rotational range of motion with particular focus on internal range of motion, (3) optimization of the flexibility and muscle performance of the scapular muscles, and (4) increasing the functional sport-specific load on the shoulder girdle.
Continue to: A common approach to the care of...
A common approach to the care of a patient after a glenohumeral joint dislocation is to place the patient’s shoulder in a sling for comfort, with permitted pain-free isometric exercise along with passive and assisted elevation up to 100°.16 This is followed by a nonaggressive rehabilitation protocol for 2 months until full recovery, which includes progressive range of motion, strength, proprioception, and return to functional activities.16
More aggressive return-to-play protocols with accelerated timelines and functional progression have been studied, including in a multicenter observational study that followed 45 contact intercollegiate athletes prospectively after in-season anterior glenohumeral instability. Thirty-three of 45 (73%) athletes returned to sport for either all or part of the season after a median 5 days lost from competition, with 12 athletes (27%) successfully completing the season without recurrence. Athletes with a subluxation event were 5.3 times more likely to return to sport during the same season, compared with those with dislocations.17
Dynamic bracing may also allow for a safe and quicker return to sport in athletes18 but recently was shown to not impact recurrent dislocation risk.19
Return to play should be based on subjective assessment as well as objective measurements of range of motion, strength, and dynamic function.15 Patients who continue to have significant weakness and pain at 2 to 3 weeks post injury despite physical therapy should be re-evaluated with an MRI for concomitant rotator cuff tears and need for surgical referral.20
When to consider surgical intervention
In a recent meta-analysis, recurrent dislocation and instability occurred at a rate of 52.9% following nonsurgical treatment.2 The decision to perform surgical intervention is typically made following failure of conservative management. Other considerations include age, gender, bone loss, and cartilage defect.21,22 Age younger than 30 years, participation in competition, contact sports, and male gender have been associated with an increased risk of recurrence.23-25 For this reason, obtaining an MRI at time of first dislocation can help facilitate surgical decisions if the patient is at high risk for surgical need.26
Continue to: An increasing number...
An increasing number of dislocations portends a poor outcome with nonoperative treatment. Kao et al demonstrated a second dislocation leads to another dislocation in 19.6% of cases, while 44.3% of those with a third dislocation event will sustain another dislocation.24 Surgery should be considered for patients with recurrent instability events to prevent persistent instability and decrease the amount of bone loss that can occur with repetitive dislocations.
What are the surgical options?
Several surgical options exist to remedy the unstable shoulder. Procedures can range from an arthroscopic repair to an open stabilization combined with structural bone graft to replace a bone defect caused by repetitive dislocations.
Arthroscopic techniques have become the mainstay of treatment and account for 71% of stabilization procedures performed.21 These techniques cause less pain in the early postoperative period and provide for a faster return to work.27 Arthroscopy has the additional advantage of allowing for complete visualization of the glenohumeral joint to identify and address concomitant pathology, such as intra-articular loose bodies or rotator cuff tears.
Open repair was the mainstay of treatment prior to development of arthroscopic techniques. Some surgeons still prefer this method—especially in high-risk groups—because of a lower risk of recurrent disloca-tion.28 Open techniques often involve detachment and repair of the upper subscapularis tendon and are more likely to produce long-term losses in external rotation range of motion.28
Which one is appropriate for your patient? The decision to pursue an open or arthroscopic procedure and to augment with bone graft depends on the amount of glenoid and humeral head bone loss, patient activity level, risk of recurrent dislocation, and surgeon preference.
Continue to: For the nonathletic population...
For the nonathletic population, the timing of injury is less critical and surgery is typically recommended after conservative treatment has failed. In an athletic population, the timing of injury is a necessary consideration. An injury midseason may be “rehabbed” in hopes of returning to play. Individuals with injuries occurring at the end of a season, who are unable to regain desired function, and/or with peri-articular fractures or associated full-thickness rotator cuff tears may benefit from sooner surgical intervention.21
Owens et al have described appropriate surgical indications and recommendations for an in-season athlete.21 In this particular algorithm, the authors suggest obtaining an MRI for decision making, but this is specific to in-season athletes wishing to return to play. In general, an MRI is not always indicated for patients who wish to receive conservative therapy but would be indicated for surgical considerations. The algorithm otherwise uses bone and soft-tissue injury, recurrent instability, and timing in the season to help determine management.21
Outcomes: Surgery has advantages …
Recurrence rates following surgical intervention are considerably lower than with conservative management, especially among young, active individuals. A recent systematic review by Donohue et al demonstrated recurrent instability rates following surgical intervention as low as 2.4%.29 One study comparing the outcome of arthroscopic repair vs conservative management showed that the risk of postoperative instability was reduced by 20% compared to other treatments.7 Furthermore, early surgical fixation can improve quality of life, produce better functional outcomes, decrease time away from activity, increase patient satisfaction, and slow the development of glenohumeral osteoarthritis produced from recurrent instability.2,7
Complications. Surgery does carry inherent risks of infection, anesthesia effects, surgical complications, and surgical failure. Recurrent instability is the most common complication following surgical shoulder stabilization. Rates of recurrent instability after surgical stabilization depend on patient age, activity level, and amount of bone loss: males younger than 18 years who participate in contact competitive sports and have significant bone loss are more likely to have recurrent dislocation after surgery.23 The type of surgical procedure selected may decrease this risk.
While the open procedures decrease risk of postoperative instability, these surgeries can pose a significant risk of complications. Major complications for specific open techniques have been reported in up to 30% of patients30 and are associated with lower levels of surgeon experience.31 While the healing of bones and ligaments is always a concern, 1 of the most feared complications following stabilization surgery is iatrogenic nerve injury. Because of the axillary nerve’s close proximity to the inferior glenoid, this nerve can be injured without meticulous care and can result in paralysis of the deltoid muscle. This injury poses a major impediment to normal shoulder function. Some procedures may cause nerve injuries in up to 10% of patients, although most injuries are transient.32
Continue to: Bottom line
Bottom line
Due to the void of evidence-based guidelines for conservative vs surgical management of primary shoulder dislocation, it would be prudent to have a risk-benefit discussion with patients regarding treatment options.
Patients older than 30 years and those with uncomplicated injuries are best suited for conservative management of primary shoulder dislocations. Immobilization is debated and may not change outcomes, but a progressive rehabilitative program after the initial acute injury is helpful. Risk factors for failing conservative management include recurrent dislocation, subsequent arthropathy, and additional concomitant bone or soft-tissue injuries.
Patients younger than 30 years who have complicated injuries with bone or cartilage loss, rotator cuff tears, or recurrent instability, and highly physically active individuals are best suited for surgical management. Shoulder arthroscopy has become the mainstay of surgical treatment for shoulder dislocations. Outcomes are favorable and dislocation recurrence is low after surgical repair. Surgery does carry its own inherent risks of infection, anesthesia effects, complications during surgery, and surgical failure leading to recurrent instability.
Cayce Onks, DO, MS, ATC, Penn State Hershey, Milton S. Hershey Medical Center, Penn State College of Medicine, Family and Community Medicine H154, 500 University Drive, PO Box 850, Hershey, PA 17033-0850; conks@pennstatehealth.psu.edu
1. Lin K, James E, Spitzer E, et al. Pediatric and adolescent anterior shoulder instability: clinical management of first time dislocators. Curr Opin Pediatr. 2018;30:49-56.
2. Kavaja L, Lähdeoja T, Malmivaara A, et al. Treatment after traumatic shoulder dislocation: a systematic review with a network meta-analysis. Br J Sports Med. 2018;52:1498-1506.
3. Brelin A, Dickens JF. Posterior shoulder instability. Sports Med Arthrosc Rev. 2017;25:136-143.
4. Galvin JW, Ernat JJ, Waterman BR, et al. The epidemiology and natural history of anterior shoulder dislocation. Curr Rev Musculoskelet Med. 2017;10:411-424.
5. Rozzi SL, Anderson JM, Doberstein ST, et al. National Athletic Trainers’ Association position statement: immediate management of appendicular joint dislocations. J Athl Train. 2018;53:1117-1128.
6. Hovelius L, Saeboe M. Arthropathy after primary anterior shoulder dislocation: 223 shoulders prospectively followed up for twenty-five years. J Shoulder Elbow Surg. 2009;18:339-347.
7. Polyzois I, Dattani R, Gupta R, et al. Traumatic first time shoulder dislocation: surgery vs non-operative treatment. Arch Bone Jt Surg. 2016;4:104-108.
8. Cox CL, Kuhn JE. Operative versus nonoperative treatment of acute shoulder dislocation in the athlete. Curr Sports Med Rep. 2008;7:263-268.
9. Kuhn JE. Treating the initial anterior shoulder dislocation—an evidence-based medicine approach. Sports Med Arthrosc Rev. 2006;14:192-198.
10. Smith TO. Immobilization following traumatic anterior glenohumeral joint dislocation: a literature review. Injury. 2006;37:228-237.
11. Liavaag S, Brox JI, Pripp AH, et al. Immobilization in external rotation after primary shoulder dislocation did not reduce the risk of recurrence: a randomized controlled trial. J Bone Joint Surg Am. 2011;93:897-904.
12. Itoi E, Hatakeyama Y, Sato T, et al. Immobilization in external rotation after shoulder dislocation reduces the risk of recurrence: a randomized controlled trial. J Bone Joint Surg Am. 2007;89:2124-2131.
13. Miller BS, Sonnabend DH, Hatrick C, et al. Should acute anterior dislocations of the shoulder be immobilized in external rotation? A cadaveric study. J Shoulder Elbow Surg. 2004;13:589-592.
14. Hanchard NCA, Goodchild LM, Kottam L. Conservative management following closed reduction of traumatic anterior dislocation of the shoulder. Cochrane Database Syst Rev. 2014;(4):CD004962.
15. Cools AM, Borms D, Castelein B, et al. Evidence-based rehabilitation of athletes with glenohumeral instability. Knee Surg Sports Traumatol Arthrosc. 2016;24:382-389.
16. Lafuente JLA, Marco SM, Pequerul JMG. Controversies in the management of the first time shoulder dislocation. Open Orthop J. 2017;11:1001-1010.
17. Dickens JF, Owens BD, Cameron KL, et al. Return to play and recurrent instability after in-season anterior shoulder instability: a prospective multicenter study. Am J Sports Med. 2014;42:2842-2850.
18. Conti M, Garofalo R, Castagna A, et al. Dynamic brace is a good option to treat first anterior shoulder dislocation in season. Musculoskelet Surg. 2017;101(suppl 2):169-173.
19. Shanley E, Thigpen C, Brooks J, et al. Return to sport as an outcome measure for shoulder instability. Am J Sports Med. 2019;47:1062-1067.
20. Gombera MM, Sekiya JK. Rotator cuff tear and glenohumeral instability. Clin Orthop Relat Res. 2014;472:2448-2456.
21. Owens BD, Dickens JF, Kilcoyne KG, et al. Management of mid-season traumatic anterior shoulder instability in athletes. J Am Acad Orthop Surg. 2012;20:518-526.
22. Ozturk BY, Maak TG, Fabricant P, et al. Return to sports after arthroscopic anterior stabilization in patients aged younger than 25 years. Arthroscopy. 2013;29:1922-1931.
23. Balg F, Boileau P. The instability severity index score. A simple preoperative score to select patients for arthroscopic or open shoulder stabilisation. J Bone Joint Surg Br. 2007;89:1470-1477.
24. Kao J-T, Chang C-L, Su W-R, et al. Incidence of recurrence after shoulder dislocation: a nationwide database study. J Shoulder Elbow Surg. 2018;27:1519-1525.
25. Porcillini G, Campi F, Pegreffi F, et al. Predisposing factors for recurrent shoulder dislocation after arthroscopic treatment. J Bone Joint Surg Am. 2009;91:2537-2542.
26. Magee T. 3T MRI of the shoulder: is MR arthrography necessary? AJR Am J Roentgenol. 2009;192:86-92.
27. Green MR, Christensen KP. Arthroscopic versus open Bankart procedures: a comparison of early morbidity and complications. Arthroscopy. 1993;9:371-374.
28. Khatri K, Arora H, Chaudhary S, et al. Meta-analysis of randomized controlled trials involving anterior shoulder instability. Open Orthop J. 2018;12:411-418.
29. Donohue MA, Owens BD, Dickens JF. Return to play following anterior shoulder dislocations and stabilization surgery. Clin Sports Med. 2016;35:545-561.
30. Griesser MJ, Harris JD, McCoy BW, et al. Complications and re-operations after Bristow-Latarjet shoulder stabilization: a systematic review. J Shoulder Elbow Surg. 2013;22:286-292.
31. Ekhtiari S, Horner NS, Bedi A, et al. The learning curve for the Latarjet procedure: a systematic review. Orthop J Sports Med. 2018;6:2325967118786930.
32. Shah AA, Butler RB, Romanowski J, et al. Short-term complications of the Latarjet procedure. J Bone Joint Surg Am. 2012;94:495-501.
1. Lin K, James E, Spitzer E, et al. Pediatric and adolescent anterior shoulder instability: clinical management of first time dislocators. Curr Opin Pediatr. 2018;30:49-56.
2. Kavaja L, Lähdeoja T, Malmivaara A, et al. Treatment after traumatic shoulder dislocation: a systematic review with a network meta-analysis. Br J Sports Med. 2018;52:1498-1506.
3. Brelin A, Dickens JF. Posterior shoulder instability. Sports Med Arthrosc Rev. 2017;25:136-143.
4. Galvin JW, Ernat JJ, Waterman BR, et al. The epidemiology and natural history of anterior shoulder dislocation. Curr Rev Musculoskelet Med. 2017;10:411-424.
5. Rozzi SL, Anderson JM, Doberstein ST, et al. National Athletic Trainers’ Association position statement: immediate management of appendicular joint dislocations. J Athl Train. 2018;53:1117-1128.
6. Hovelius L, Saeboe M. Arthropathy after primary anterior shoulder dislocation: 223 shoulders prospectively followed up for twenty-five years. J Shoulder Elbow Surg. 2009;18:339-347.
7. Polyzois I, Dattani R, Gupta R, et al. Traumatic first time shoulder dislocation: surgery vs non-operative treatment. Arch Bone Jt Surg. 2016;4:104-108.
8. Cox CL, Kuhn JE. Operative versus nonoperative treatment of acute shoulder dislocation in the athlete. Curr Sports Med Rep. 2008;7:263-268.
9. Kuhn JE. Treating the initial anterior shoulder dislocation—an evidence-based medicine approach. Sports Med Arthrosc Rev. 2006;14:192-198.
10. Smith TO. Immobilization following traumatic anterior glenohumeral joint dislocation: a literature review. Injury. 2006;37:228-237.
11. Liavaag S, Brox JI, Pripp AH, et al. Immobilization in external rotation after primary shoulder dislocation did not reduce the risk of recurrence: a randomized controlled trial. J Bone Joint Surg Am. 2011;93:897-904.
12. Itoi E, Hatakeyama Y, Sato T, et al. Immobilization in external rotation after shoulder dislocation reduces the risk of recurrence: a randomized controlled trial. J Bone Joint Surg Am. 2007;89:2124-2131.
13. Miller BS, Sonnabend DH, Hatrick C, et al. Should acute anterior dislocations of the shoulder be immobilized in external rotation? A cadaveric study. J Shoulder Elbow Surg. 2004;13:589-592.
14. Hanchard NCA, Goodchild LM, Kottam L. Conservative management following closed reduction of traumatic anterior dislocation of the shoulder. Cochrane Database Syst Rev. 2014;(4):CD004962.
15. Cools AM, Borms D, Castelein B, et al. Evidence-based rehabilitation of athletes with glenohumeral instability. Knee Surg Sports Traumatol Arthrosc. 2016;24:382-389.
16. Lafuente JLA, Marco SM, Pequerul JMG. Controversies in the management of the first time shoulder dislocation. Open Orthop J. 2017;11:1001-1010.
17. Dickens JF, Owens BD, Cameron KL, et al. Return to play and recurrent instability after in-season anterior shoulder instability: a prospective multicenter study. Am J Sports Med. 2014;42:2842-2850.
18. Conti M, Garofalo R, Castagna A, et al. Dynamic brace is a good option to treat first anterior shoulder dislocation in season. Musculoskelet Surg. 2017;101(suppl 2):169-173.
19. Shanley E, Thigpen C, Brooks J, et al. Return to sport as an outcome measure for shoulder instability. Am J Sports Med. 2019;47:1062-1067.
20. Gombera MM, Sekiya JK. Rotator cuff tear and glenohumeral instability. Clin Orthop Relat Res. 2014;472:2448-2456.
21. Owens BD, Dickens JF, Kilcoyne KG, et al. Management of mid-season traumatic anterior shoulder instability in athletes. J Am Acad Orthop Surg. 2012;20:518-526.
22. Ozturk BY, Maak TG, Fabricant P, et al. Return to sports after arthroscopic anterior stabilization in patients aged younger than 25 years. Arthroscopy. 2013;29:1922-1931.
23. Balg F, Boileau P. The instability severity index score. A simple preoperative score to select patients for arthroscopic or open shoulder stabilisation. J Bone Joint Surg Br. 2007;89:1470-1477.
24. Kao J-T, Chang C-L, Su W-R, et al. Incidence of recurrence after shoulder dislocation: a nationwide database study. J Shoulder Elbow Surg. 2018;27:1519-1525.
25. Porcillini G, Campi F, Pegreffi F, et al. Predisposing factors for recurrent shoulder dislocation after arthroscopic treatment. J Bone Joint Surg Am. 2009;91:2537-2542.
26. Magee T. 3T MRI of the shoulder: is MR arthrography necessary? AJR Am J Roentgenol. 2009;192:86-92.
27. Green MR, Christensen KP. Arthroscopic versus open Bankart procedures: a comparison of early morbidity and complications. Arthroscopy. 1993;9:371-374.
28. Khatri K, Arora H, Chaudhary S, et al. Meta-analysis of randomized controlled trials involving anterior shoulder instability. Open Orthop J. 2018;12:411-418.
29. Donohue MA, Owens BD, Dickens JF. Return to play following anterior shoulder dislocations and stabilization surgery. Clin Sports Med. 2016;35:545-561.
30. Griesser MJ, Harris JD, McCoy BW, et al. Complications and re-operations after Bristow-Latarjet shoulder stabilization: a systematic review. J Shoulder Elbow Surg. 2013;22:286-292.
31. Ekhtiari S, Horner NS, Bedi A, et al. The learning curve for the Latarjet procedure: a systematic review. Orthop J Sports Med. 2018;6:2325967118786930.
32. Shah AA, Butler RB, Romanowski J, et al. Short-term complications of the Latarjet procedure. J Bone Joint Surg Am. 2012;94:495-501.
PRACTICE RECOMMENDATIONS
› Start with conservative management of shoulder dislocation in patients older than 30 years and those with uncomplicated injuries. B
› Discourage strict immobilization; its utility is debated and it may not change outcomes. B
› Recommend a progressive rehabilitative program after the initial acute shoulder injury. B
› Consider surgical management for patients younger than 30 years who have complicated injuries with bone or cartilage loss, rotator cuff tears, or recurrent instability or for the highly physically active individual. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Functional neurological disorder: A practical guide to an elusive Dx
CASE
John D,* a 25-year-old patient with an otherwise unremarkable medical history, describes 2 months of daily headache, lower-extremity weakness, and unsteady gait that began fairly suddenly during his first deployment in the US Army. He explains that these symptoms affected his ability to perform his duties and necessitated an early return stateside for evaluation and treatment.
Mr. D denies precipitating trauma or unusual environmental exposures. He reports that, stateside now, symptoms continue to affect his ability to work and attend to personal and family responsibilities.
Asked about stressors, Mr. D notes the birth of his first child approximately 3 months ago, while he was deployed, and marital stressors. He denies suicidal or homicidal ideation.
* The patient’s name has been changed to protect his identity.
The challenge of identifying and managing FND
A functional neurological disorder (FND) is a constellation of psychological, physiological, and neurological symptoms, without an identifiable organic etiology, a conscious decision, or secondary gain for the patient,1 that adversely impacts functioning in 1 or more significant life domains.
Given the high throughput of patients in primary care practices, family physicians can expect to encounter suspected cases of FND in their practices. Regrettably, however, a lack of familiarity with the disorder and its related problems (eg, nonorganic paralysis, sensory loss, nonepileptic seizures, and abnormal movements) can add as much as $20,000 in excess direct and indirect costs of care for every such patient.1 In this article, we synthesize the recent literature on FND so that family physicians can expand their acumen in understanding, identifying, and evaluating patients whose presentation suggests FND.
An underrecognized entity
A precise estimate of the prevalence of FND is difficult to determine because the disorder is underrecognized and misdiagnosed and because it is often accompanied by the confounding of psychological and physiological comorbidities. A 2012 study estimated the annual incidence of FND to be 4 to 12 cases for every 100,000 people2; in primary care and outpatient neurology settings, prevalence is 6% to 22% of all patients.3,4 Stone and colleagues identified functional neurological symptoms as the second most common reason for outpatient neurology consultation,5 with 1 nonepileptic seizure patient seen for every 6 epileptic patients, and functional weakness presenting at the same rate as multiple sclerosis.6
Continue to: Demographics of patients with FND...
Demographics of patients with FND vary, depending on presenting neurological symptoms and disorder subtype. Existing data indicate a correlation between FND and younger age, female sex, physical disability,7 and a history of abuse or trauma.3,8 A challenge in concretely ascertaining the prevalence of FND is that conditions such as fibromyalgia, chronic pelvic pain, globus hystericus, and nonepileptic seizures can also be characterized as medically unexplained functional disorders, even within the network of neurology care.4
Misdiagnosis and bias are not uncommon
Ambiguity in classifying and evaluating FND can affect physicians’ perceptions, assessment, and care of patients with suggestive presenting symptoms. A major early challenge in diagnosing FND is the inconsistency of characterizing terminology (pseudoneurological, somatic, dissociative, conversion, psychogenic, hysterical, factitious, functional, medically unexplained9,10) and definitions in the literature. Neurological symptoms of unidentifiable organic cause can greatly diminish quality of life4; FND is a scientifically and clinically useful diagnosis for many combinations of nonrandomly co-occurring symptoms and clinical signs.
The pitfall of misdiagnosis. Remain cautious about making a diagnosis of FND by exclusion, which might yield an incorrect or false-negative finding because of an atypical presentation. It is important to avoid misdiagnosis by prematurely closing the differential diagnosis; instead, keep in mind that a medically unexplained diagnosis might be better explained by conducting a robust social and medical history and obtaining additional or collateral data, or both, along with appropriate consultation.4,9
Misdiagnosis can lead to a circuitous and costly work-up, with the potential to increase the patient’s distress. You can reduce this burden with early recognition of FND and centralized management of multidisciplinary care, which are more likely to lead to an accurate and timely diagnosis—paramount to empowering patients with access to the correct information and meaningful support needed to enhance treatment and self-care.9
Bias, haste, and dismissal are unproductive. Even with a clear definition of FND, it is not uncommon for a physician to rapidly assess a patient’s clinical signs, make a diagnosis of “unknown etiology,” or openly question the veracity of complaints. Furthermore, be aware of inadvertently characterizing FND using the prefix “pseudo” or the term “hysterical,” which can be psychologically discomforting for many patients, who legitimately experience inexplicable symptoms. Such pejoratives can lead to stigmatizing and misleading assessments and treatment paths4—courses of action that can cause early and, possibly, irreparable harm to the patient–physician relationship and increase the patient’s inclination to go “doctor-shopping,” with associated loss of continuity of care.
Why is it difficult to diagnose FND?
The latest (5th) edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) describes conversion, somatoform disorder, and FND synonymously.DSM-5 diagnostic criteria for conversion disorderare11:
- a specified type of symptom or deficit of altered voluntary motor or sensory function (eg, weakness, difficulty swallowing, slurred speech, seizures)
- clinical evidence of the incompatibility of the symptom or deficit and any recognized neurological or medical disorder
- incapability of better explaining the symptom or deficit as another medical or mental disorder.
- The symptom or deficit causes distress or impairment that (1) is clinically significant in occupational, social, or other important areas of function or (2) warrants medical evaluation.
The overarching feature of these criteria is the inconsistency of symptoms with recognized neurological, physiological, or psychiatric conditions. Although identification of psychological factors can help clarify and provide a treatment direction, such identification is not essential for making a diagnosis of FND. Malingering does not need to be refuted as part of establishing the diagnosis.12
Continue to: In contrast...
In contrast, the World Health Organization’s ICD-10 Classification of Mental and Behavioural Disorders groups diagnostic criteria for FND among the dissociative disorders13:
- Clinical features are specified for the individual dissociative disorder (motor, sensory, convulsions, mixed).
- Evidence is absent of a physical disorder that might explain symptoms.
- Evidence of psychological causation is present in clear temporal association with stressful events and problems or disturbed relationships, even if the patient denies such association.
Note the emphasis on psychological causation and exclusion of purposeful simulation of symptoms, as opposed to a primarily unconscious disconnection from the patient’s body or environment.
ICD-10 guidelines acknowledge the difficulty of finding definitive evidence of a psychological cause and recommend provisional diagnosis of FND if psychological factors are not readily apparent.14 Of note, many patients with FND are affected psychologically by their condition, with an impact on mood, behaviors, and interpersonal interactions, although not necessarily to a clinically diagnostic degree. Therefore, a psychiatric diagnosis alone is not a necessary precursor for the diagnosis of an FND.
CASE
History. Mr. D’s history is positive for light alcohol consumption (“2 or 3 cans of beer on weekends”) and chewing tobacco (he reports stopping 6 months earlier) and negative for substance abuse. The family history is positive for maternal hypertension and paternal suicide when the patient was 10 years old (no other known paternal history).
Physical findings. The review of systems is positive for intermittent palpitations, lower-extremity weakness causing unsteady gait, and generalized headache.
Vital signs are within normal limits, including blood pressure (120/82 mm Hg) and heart rate (110 beats/min). The patient is not in acute distress; he is awake, alert, and oriented × 3. No murmurs are heard; lungs are clear bilaterally to auscultation. There is no tenderness on abdominal palpation, and no hepatomegaly or splenomegaly; bowel sounds are normal. No significant bruising or lacerations are noted.
Neurology exam. Cranial nerves II-XII are intact. Pupils are equal and reactive to light. Reflexes are 2+ bilaterally. Muscle strength and tone are normal; no tremors are noted. Babinski signs are normal. A Romberg test is positive (swaying).
Continue to: Mr. D has an antalgic gait...
Mr. D has an antalgic gait with significant swaying (without falling); bent posture; and unsteadiness that requires a cane. However, he is able to get up and off the exam table without assistance, and to propel himself, by rolling a chair forward and backward, without difficulty.
Conducting a diagnostic examination
Taking the history. Certain clues can aid in the diagnosis of FND (TABLE 1).15 For example, the patient might have been seen in multiple specialty practices for a multitude of vague symptoms indicative of potentially related conditions (eg, chronic fatigue, allergies and sensitivities, fibromyalgia, and other chronic pain). The history might include repeated surgeries to investigate those symptoms (eg, laparoscopy, or hysterectomy at an early age). Taking time and care to explore all clinical clues, patient reports, and collateral data are therefore key to making an accurate diagnosis.
Note any discrepancies between the severity of reported symptoms and functional ability. A technique that can help elucidate a complex or ambiguous medical presentation is to ask the patient to list all their symptoms at the beginning of the interview. This has threefold benefit: You get a broad picture of the problem; the patient is unburdened of their concerns and experiences your validation; and a long list of symptoms can be an early clue to a diagnosis of FND.
Other helpful questions to determine the impact of symptoms on the patient’s well-being include inquiries about16:
- functional impairment
- onset and course of symptoms
- potential causal or correlating events
- dissociative episodes
- previous diagnoses and treatments
- the patient’s perceptions of, and emotional response to, their illness
- a history of abuse.
The physical examination to determine the presence of FND varies, depending on the functional area of impact (eg, motor, neurological, sensory, speech and swallowing). Pay particular attention to presenting signs and clues, and balance them with the patient’s report (or lack of report). Endeavor to demonstrate positive functional signs, such as a positive Hoover test, which relies on the principle of synergistic muscle contraction. You might see evidence of inconsistency, such as weakness or a change in gait, under observation, that seemingly resolves when the patient is getting on and off the exam table.16Table 215-24 describes areas affected by FND, characteristics of the disorder, and related diagnostic examinations.
Table 315,18,19 reviews validated special exams that can aid in making the diagnosis. Additional special tests are discussed in the literature.15-24 These tests can be helpful in narrowing the differential diagnosis but have not been validated and should be used with caution.
Some clinical signs associated with FND might be affected by other factors, including socioeconomic status, limited access to health care, low health literacy, poor communication skills, and physician bias. Keep these factors in mind during the visit, to avoid contributing further to health disparities among groups of patients affected by these problems.
Continue to: CASE
CASE
The work-up over the next month for Mr. D includes numerous studies, all yielding results that are negative or within normal limits: visual acuity; electrocardiography and an event monitor; laboratory testing (including a complete blood count, comprehensive metabolic panel, thyroid-stimulating hormone, creatine kinase, erythrocyte sedimentation rate, C-reactive protein, vitamin B12, folate, and vitamin D); magnetic resonance imaging of the brain and lumbar spine; lumbar puncture; and electromyography.
The score on the 9-item Patient Health Questionnaire for depression is 4 (severity: “none or minimal”); on the 7-item Generalized Anxiety Disorder scale, 0 (“no anxiety disorder”).
Referral. A neurology work-up of headache, lower extremity weakness, and unsteady gait to address several diagnostic possibilities, including migraine and multiple sclerosis, is within normal limits. A cardiology work-up of palpitations is negative for arrhythmias and other concerning findings.
Mr. D declines psychiatric and psychological evaluations.
Building a differential diagnosisis a formidable task
The differential diagnosis of FND is vast. It includes neurological, physiological, and psychiatric symptoms and disorders; somatization; and malingering (Table 4).6 Any disorder or condition in these areas that is in the differential diagnosis can be precipitated or exacerbated by stress; most, however, do not involve loss of physical function.12 In addition, the diagnosis of an FND does not necessarily exclude an organic disorder.
A patient’s presentation becomes complicated—and more difficult to treat—when functional symptoms and an unrelated underlying or early-stage neurological condition coexist. For example, a patient with epilepsy might also have dissociative seizures atop their organic disorder. Neurological disease is considered a risk factor for an overlying FND—just as the risk of depression or anxiety runs concurrently with other chronic diseases.14
Focus on clinical signs to narrow the differential. A thorough social and medical history and physical examination, as discussed earlier, help narrow the differential diagnosis of organic and medically unexplained disorders. Well-defined imaging or laboratory protocols do not exist to guide physicians to a definitive diagnosis, however.
Continue to: Psychiatric conditions
Psychiatric conditions can coexist with the diagnosis of FND, but might be unrelated. A systematic review of the literature showed that 17% to 42% of patients with FND had a concurrent anxiety disorder. Depression disorders were co-diagnosed in 19% to 71% of patients with FND; dissociative and personality disorders were noted, as well.25 However, coexisting psychiatric diagnosis might more likely be associated with distress from the presenting functional neurological symptoms, not linked to the FND diagnosis itself.12 This shift in understanding is reflected in the description of FND in the DSM-5.11
CASE
Mr. D reports debilitating headaches at return office visits. Trials of abortive triptans provide no relief; neither do control medications (beta-blockers, coenzyme Q10, magnesium, onabotulinumtoxinA [Botox], topiramate, and valproate). Lower-extremity weakness and unsteadiness are managed with supportive devices, including a cane, and physical therapy.
Importance of establishing a multidisciplinary approach
The complexity of FND lends itself to a multidisciplinary approach during evaluation and, eventually, for treatment. The assessment and diagnostic intervention that you provide, along with the contributions of consulted specialists (including neurology, physical and occupational therapy, psychiatry, psychology, and other mental health professionals) establishes a team-based approach that can increase the patient’s sense of support and reduce excessive testing and unnecessary medications, surgeries, and other treatments.26
Family physicians are in the ideal position to recognize the patient’s functional capacity and the quality of symptoms and to provide timely referral (eg, to Neurology and Psychiatry) for confirmation of the diagnosis and then treatment.
Evidence-based treatment options include:
- psychotherapy, with an emphasis on cognitive behavioral therapy
- physical therapy
- psychopharmacology
- promising combinations of physical and psychological treatment to improve long-term functionality.27
A promising diagnostic tool
The most significant update in the FND literature is on functional neuroimaging for assessing the disorder. Early findings suggest an intricate relationship between mind and body regarding the pathological distortion in FND. And, there is clear evidence that neuroimaging—specifically, functional magnetic resonance imaging—shows changes in brain activity that correspond to the patient’s symptom report. That said, imaging is not the recommended standard of care in the initial work-up of FND because of its cost and the fact that the diagnosis is principally a clinical undertaking.17,28
Call to action
Offer a generous ear. Begin the diagnostic pursuit by listening carefully and fully to the patient’s complaints, without arriving at a diagnosis with unwarranted bias or haste. This endeavor might require support from other clinical staff (eg, nurses, social workers, case managers) because the diagnostic process can be arduous and lengthy.
Continue to: Convey the diagnosis with sensitivity
Convey the diagnosis with sensitivity. Inquire about the patient’s perceptions and impairments to best personalize your diagnostic explanations. Delivery of the diagnosis might affect the patient’s acceptance and compliance with further testing and treatment of what is generally a persistent and treatment-resistant disorder; poor delivery of diagnostic information can impair the patient–physician relationship and increase the risk of disjointed care. Many patients find that improved patient–physician communication is therapeutic.29
Let the patient know that you’re taking her seriously. Validate patient concerns with a nonstigmatizing diagnostic label; discuss the diagnostic parameters and cause of symptoms in layman’s terms; and emphasize the potential for reversibility.30 Some patients are not satisfied with having a diagnosis of FND until they are reassured with normal results of testing and provided with referral; even then, some seek further reassurance.
Key tenets of managing care for patients who have been given a diagnosis of FND include:
- nonjudgmental, positive regard
- meaningful expression of empathy
- multidisciplinary coordination
- avoidance of unnecessary testing and harmful treatments
- descriptive and contextual explanations of the diagnosis.
Last, keep in mind that the course of treatment for FND is potentially prolonged and multilayered.
CASE
After many visits with his family physician and the neurology and cardiology specialists, as well as an extensive work-up, the physician approaches Mr. D with the possibility of a diagnosis of FND and proposes a multidisciplinary plan that includes:
- a course of physical and occupational therapy
- development of individualized cognitive behavioral tools
- weekly personal and marital counseling
- initiation of a selective serotonin reuptake inhibitor for anxiety
- monthly visits with his family physician.
Months after his return from deployment for evaluation and treatment, Mr. D is able to return to military duty. He reports that his quality of life has improved.
CORRESPONDENCE
Roselyn W. Clemente Fuentes, MD, FAAFP, Eglin Family Medicine Residency, 307 Boatner Road, Eglin AFB, FL 32547; roselynjan.w.fuentes.mil@mail.mil.
1. Konnopka A, Schaefert R, Heinrich S, et al. Economics of medically unexplained symptoms: a systematic review of the literature. Psychother Psychosom. 2012;81:265-275.
2. Carson AJ, Brown R, David AS, et al; on behalf of UK-FNS. Functional (conversion) neurological symptoms: research since the millennium. J Neurol Neurosurg Psychiatry. 2012;83:842-850.
3. Stone J, Carson A, Duncan R, et al. Who is referred to neurology clinics?—the diagnoses made in 3781 new patients. Clin Neurol Neurosurg. 2010;112:747-751.
4. Evens A, Vendetta L, Krebs K, et al. Medically unexplained neurologic symptoms: a primer for physicians who make the initial encounter. Am J Med. 2015;128:1059-1064.
5. Stone J, Reuber M, Carson A. Functional symptoms in neurology: mimics and chameleons. Pract Neurol. 2013;13:104-113.
6. Stone J, Warlow C, Sharpe M. The symptom of functional weakness: a controlled study of 107 patients. Brain. 2010;133:1537-1551.
7. Carson A, Stone J, Hibberd C, et al. Disability, distress and unemployment in neurology outpatients with symptoms ‘unexplained by organic disease’. J Neurol Neurosurg Psychiatry. 2011;82:810-813.
8. Fink P, Hansen MS, Oxhøj M-L. The prevalence of somatoform disorders among internal medical inpatients. J Psychosom Res. 2004;56:413-418.
9. Thomas LE. Are your patient’s medically unexplained symptoms really “all in her head”? Med Hypotheses. 2012;78:542-547.
10. Ding JM, Kanaan RAA. What should we say to patients with unexplained neurological symptoms? How explanation affects offence. J Psychosom Res. 2016;91:55-60.
11. Diagnostic and Statistical Manual of Mental Disorders (DSM-5). 5th ed. American Psychiatric Association; 2013.
12. Stone J, LaFrance WC Jr, Levenson JL, et al. Issues for DSM-5: Conversion disorder. Am J Psychiatry. 2010;167:626-627.
13. The ICD-10 Classification of Mental and Behavioural Disorders: Clinical Descriptions and Diagnostic Guidelines. World Health Organization; 1994. Accessed January 21, 2021. www.who.int/classifications/icd/en/bluebook.pdf
14. Stone J, Carson A, Duncan R, et al. Which neurological diseases are most likely to be associated with “symptoms unexplained by organic disease.” J Neurol. 2012;259:33-38.
15. Shaibani A, Sabbagh M. Pseudoneurologic syndromes: recognition and diagnosis. Am Fam Physician. 1998;57:2485-2494.
16. Stone J, Carson A, Sharpe M. Functional symptoms and signs in neurology: assessment and diagnosis. J Neurol Neurosurg Psychiatry. 2005;76(suppl 1):i2-i12.
17. Vuilleumier P. Brain circuits implicated in psychogenic paralysis in conversion disorders and hypnosis. Neurophysiol Clin. 2014;44:323-337.
18. McKee K, Glass S, Adams C, et al. The inpatient assessment and management of motor functional neurological disorders: an interdisciplinary perspective. Psychosomatics. 2018;59:358-368.
19. Daum C, Hubschmid M, Aybek S. The value of ‘positive’ clinical signs for weakness, sensory and gait disorders in conversion disorder: a systematic and narrative review. J Neurol Neurosurg Psychiatry. 2014;85:180-190.
20. Brown P, Thompson PD. Electrophysiological aids to the diagnosis of psychogenic jerks, spasms, and tremor. Mov Disord. 2001;16:595-599.
21. Ludwig L, McWhirter L, Williams S, et al. Functional coma. In: Hallett M, Stone J, Carson A, eds. Handbook of Clinical Neurology: Volume 139: Functional Neurologic Disorders. 1st ed. Academic Press; 2016:313.
22. Miller NR, Subramanian PS, Patel VR. Walsh and Hoyt’s Clinical Neuro-ophthalmology. 3rd ed. Wolters Kluwer; 2016:512-513.
23. Takazaki K, Stransky AD, Miller G. Psychogenic nonepileptic seizures: diagnosis, management, and bioethics. Pediatr Neurol. 2016;62:3-8.
24. Sahaya K, Dholakia SA, Sahota PK. Psychogenic non-epileptic seizures: a challenging entity. J Clin Neurosci. 2011;18:1602-1607.
25. Gelauff J, Stone J, Edwards M, et al. The prognosis of functional (psychogenic) motor symptoms: a systematic review. J Neurol Neurosurg Psychiatry. 2014;85:220-226.
26. Kranick SM, Gorrindo T, Hallett M. Psychogenic movement disorders and motor conversion: a roadmap for collaboration between neurology and psychiatry. Psychosomatics. 2011;52:109-116.
27. Edwards MJ, Bhatia KP. Functional (psychogenic) movement disorders: merging mind and brain. Lancet Neurol. 2012;11:250-260.
28. Burgmer M, Kugel H, Pfleiderer B, et al. The mirror neuron system under hypnosis—brain substrates of voluntary and involuntary motor activation in hypnotic paralysis. Cortex. 2013;49:437-445.
29. van Bokhoven MA, Koch H, van der Weijden T, et al. Influence of watchful waiting on satisfaction and anxiety among patients seeking care for unexplained complaints, Ann Fam Med. 2009;7:112-120.
30. Stone J, Carson A, Hallet M. Explanation as treatment for functional neurologic disorders. Handb Clin Neurol. 2016;139:543-553.
CASE
John D,* a 25-year-old patient with an otherwise unremarkable medical history, describes 2 months of daily headache, lower-extremity weakness, and unsteady gait that began fairly suddenly during his first deployment in the US Army. He explains that these symptoms affected his ability to perform his duties and necessitated an early return stateside for evaluation and treatment.
Mr. D denies precipitating trauma or unusual environmental exposures. He reports that, stateside now, symptoms continue to affect his ability to work and attend to personal and family responsibilities.
Asked about stressors, Mr. D notes the birth of his first child approximately 3 months ago, while he was deployed, and marital stressors. He denies suicidal or homicidal ideation.
* The patient’s name has been changed to protect his identity.
The challenge of identifying and managing FND
A functional neurological disorder (FND) is a constellation of psychological, physiological, and neurological symptoms, without an identifiable organic etiology, a conscious decision, or secondary gain for the patient,1 that adversely impacts functioning in 1 or more significant life domains.
Given the high throughput of patients in primary care practices, family physicians can expect to encounter suspected cases of FND in their practices. Regrettably, however, a lack of familiarity with the disorder and its related problems (eg, nonorganic paralysis, sensory loss, nonepileptic seizures, and abnormal movements) can add as much as $20,000 in excess direct and indirect costs of care for every such patient.1 In this article, we synthesize the recent literature on FND so that family physicians can expand their acumen in understanding, identifying, and evaluating patients whose presentation suggests FND.
An underrecognized entity
A precise estimate of the prevalence of FND is difficult to determine because the disorder is underrecognized and misdiagnosed and because it is often accompanied by the confounding of psychological and physiological comorbidities. A 2012 study estimated the annual incidence of FND to be 4 to 12 cases for every 100,000 people2; in primary care and outpatient neurology settings, prevalence is 6% to 22% of all patients.3,4 Stone and colleagues identified functional neurological symptoms as the second most common reason for outpatient neurology consultation,5 with 1 nonepileptic seizure patient seen for every 6 epileptic patients, and functional weakness presenting at the same rate as multiple sclerosis.6
Continue to: Demographics of patients with FND...
Demographics of patients with FND vary, depending on presenting neurological symptoms and disorder subtype. Existing data indicate a correlation between FND and younger age, female sex, physical disability,7 and a history of abuse or trauma.3,8 A challenge in concretely ascertaining the prevalence of FND is that conditions such as fibromyalgia, chronic pelvic pain, globus hystericus, and nonepileptic seizures can also be characterized as medically unexplained functional disorders, even within the network of neurology care.4
Misdiagnosis and bias are not uncommon
Ambiguity in classifying and evaluating FND can affect physicians’ perceptions, assessment, and care of patients with suggestive presenting symptoms. A major early challenge in diagnosing FND is the inconsistency of characterizing terminology (pseudoneurological, somatic, dissociative, conversion, psychogenic, hysterical, factitious, functional, medically unexplained9,10) and definitions in the literature. Neurological symptoms of unidentifiable organic cause can greatly diminish quality of life4; FND is a scientifically and clinically useful diagnosis for many combinations of nonrandomly co-occurring symptoms and clinical signs.
The pitfall of misdiagnosis. Remain cautious about making a diagnosis of FND by exclusion, which might yield an incorrect or false-negative finding because of an atypical presentation. It is important to avoid misdiagnosis by prematurely closing the differential diagnosis; instead, keep in mind that a medically unexplained diagnosis might be better explained by conducting a robust social and medical history and obtaining additional or collateral data, or both, along with appropriate consultation.4,9
Misdiagnosis can lead to a circuitous and costly work-up, with the potential to increase the patient’s distress. You can reduce this burden with early recognition of FND and centralized management of multidisciplinary care, which are more likely to lead to an accurate and timely diagnosis—paramount to empowering patients with access to the correct information and meaningful support needed to enhance treatment and self-care.9
Bias, haste, and dismissal are unproductive. Even with a clear definition of FND, it is not uncommon for a physician to rapidly assess a patient’s clinical signs, make a diagnosis of “unknown etiology,” or openly question the veracity of complaints. Furthermore, be aware of inadvertently characterizing FND using the prefix “pseudo” or the term “hysterical,” which can be psychologically discomforting for many patients, who legitimately experience inexplicable symptoms. Such pejoratives can lead to stigmatizing and misleading assessments and treatment paths4—courses of action that can cause early and, possibly, irreparable harm to the patient–physician relationship and increase the patient’s inclination to go “doctor-shopping,” with associated loss of continuity of care.
Why is it difficult to diagnose FND?
The latest (5th) edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) describes conversion, somatoform disorder, and FND synonymously.DSM-5 diagnostic criteria for conversion disorderare11:
- a specified type of symptom or deficit of altered voluntary motor or sensory function (eg, weakness, difficulty swallowing, slurred speech, seizures)
- clinical evidence of the incompatibility of the symptom or deficit and any recognized neurological or medical disorder
- incapability of better explaining the symptom or deficit as another medical or mental disorder.
- The symptom or deficit causes distress or impairment that (1) is clinically significant in occupational, social, or other important areas of function or (2) warrants medical evaluation.
The overarching feature of these criteria is the inconsistency of symptoms with recognized neurological, physiological, or psychiatric conditions. Although identification of psychological factors can help clarify and provide a treatment direction, such identification is not essential for making a diagnosis of FND. Malingering does not need to be refuted as part of establishing the diagnosis.12
Continue to: In contrast...
In contrast, the World Health Organization’s ICD-10 Classification of Mental and Behavioural Disorders groups diagnostic criteria for FND among the dissociative disorders13:
- Clinical features are specified for the individual dissociative disorder (motor, sensory, convulsions, mixed).
- Evidence is absent of a physical disorder that might explain symptoms.
- Evidence of psychological causation is present in clear temporal association with stressful events and problems or disturbed relationships, even if the patient denies such association.
Note the emphasis on psychological causation and exclusion of purposeful simulation of symptoms, as opposed to a primarily unconscious disconnection from the patient’s body or environment.
ICD-10 guidelines acknowledge the difficulty of finding definitive evidence of a psychological cause and recommend provisional diagnosis of FND if psychological factors are not readily apparent.14 Of note, many patients with FND are affected psychologically by their condition, with an impact on mood, behaviors, and interpersonal interactions, although not necessarily to a clinically diagnostic degree. Therefore, a psychiatric diagnosis alone is not a necessary precursor for the diagnosis of an FND.
CASE
History. Mr. D’s history is positive for light alcohol consumption (“2 or 3 cans of beer on weekends”) and chewing tobacco (he reports stopping 6 months earlier) and negative for substance abuse. The family history is positive for maternal hypertension and paternal suicide when the patient was 10 years old (no other known paternal history).
Physical findings. The review of systems is positive for intermittent palpitations, lower-extremity weakness causing unsteady gait, and generalized headache.
Vital signs are within normal limits, including blood pressure (120/82 mm Hg) and heart rate (110 beats/min). The patient is not in acute distress; he is awake, alert, and oriented × 3. No murmurs are heard; lungs are clear bilaterally to auscultation. There is no tenderness on abdominal palpation, and no hepatomegaly or splenomegaly; bowel sounds are normal. No significant bruising or lacerations are noted.
Neurology exam. Cranial nerves II-XII are intact. Pupils are equal and reactive to light. Reflexes are 2+ bilaterally. Muscle strength and tone are normal; no tremors are noted. Babinski signs are normal. A Romberg test is positive (swaying).
Continue to: Mr. D has an antalgic gait...
Mr. D has an antalgic gait with significant swaying (without falling); bent posture; and unsteadiness that requires a cane. However, he is able to get up and off the exam table without assistance, and to propel himself, by rolling a chair forward and backward, without difficulty.
Conducting a diagnostic examination
Taking the history. Certain clues can aid in the diagnosis of FND (TABLE 1).15 For example, the patient might have been seen in multiple specialty practices for a multitude of vague symptoms indicative of potentially related conditions (eg, chronic fatigue, allergies and sensitivities, fibromyalgia, and other chronic pain). The history might include repeated surgeries to investigate those symptoms (eg, laparoscopy, or hysterectomy at an early age). Taking time and care to explore all clinical clues, patient reports, and collateral data are therefore key to making an accurate diagnosis.
Note any discrepancies between the severity of reported symptoms and functional ability. A technique that can help elucidate a complex or ambiguous medical presentation is to ask the patient to list all their symptoms at the beginning of the interview. This has threefold benefit: You get a broad picture of the problem; the patient is unburdened of their concerns and experiences your validation; and a long list of symptoms can be an early clue to a diagnosis of FND.
Other helpful questions to determine the impact of symptoms on the patient’s well-being include inquiries about16:
- functional impairment
- onset and course of symptoms
- potential causal or correlating events
- dissociative episodes
- previous diagnoses and treatments
- the patient’s perceptions of, and emotional response to, their illness
- a history of abuse.
The physical examination to determine the presence of FND varies, depending on the functional area of impact (eg, motor, neurological, sensory, speech and swallowing). Pay particular attention to presenting signs and clues, and balance them with the patient’s report (or lack of report). Endeavor to demonstrate positive functional signs, such as a positive Hoover test, which relies on the principle of synergistic muscle contraction. You might see evidence of inconsistency, such as weakness or a change in gait, under observation, that seemingly resolves when the patient is getting on and off the exam table.16Table 215-24 describes areas affected by FND, characteristics of the disorder, and related diagnostic examinations.
Table 315,18,19 reviews validated special exams that can aid in making the diagnosis. Additional special tests are discussed in the literature.15-24 These tests can be helpful in narrowing the differential diagnosis but have not been validated and should be used with caution.
Some clinical signs associated with FND might be affected by other factors, including socioeconomic status, limited access to health care, low health literacy, poor communication skills, and physician bias. Keep these factors in mind during the visit, to avoid contributing further to health disparities among groups of patients affected by these problems.
Continue to: CASE
CASE
The work-up over the next month for Mr. D includes numerous studies, all yielding results that are negative or within normal limits: visual acuity; electrocardiography and an event monitor; laboratory testing (including a complete blood count, comprehensive metabolic panel, thyroid-stimulating hormone, creatine kinase, erythrocyte sedimentation rate, C-reactive protein, vitamin B12, folate, and vitamin D); magnetic resonance imaging of the brain and lumbar spine; lumbar puncture; and electromyography.
The score on the 9-item Patient Health Questionnaire for depression is 4 (severity: “none or minimal”); on the 7-item Generalized Anxiety Disorder scale, 0 (“no anxiety disorder”).
Referral. A neurology work-up of headache, lower extremity weakness, and unsteady gait to address several diagnostic possibilities, including migraine and multiple sclerosis, is within normal limits. A cardiology work-up of palpitations is negative for arrhythmias and other concerning findings.
Mr. D declines psychiatric and psychological evaluations.
Building a differential diagnosisis a formidable task
The differential diagnosis of FND is vast. It includes neurological, physiological, and psychiatric symptoms and disorders; somatization; and malingering (Table 4).6 Any disorder or condition in these areas that is in the differential diagnosis can be precipitated or exacerbated by stress; most, however, do not involve loss of physical function.12 In addition, the diagnosis of an FND does not necessarily exclude an organic disorder.
A patient’s presentation becomes complicated—and more difficult to treat—when functional symptoms and an unrelated underlying or early-stage neurological condition coexist. For example, a patient with epilepsy might also have dissociative seizures atop their organic disorder. Neurological disease is considered a risk factor for an overlying FND—just as the risk of depression or anxiety runs concurrently with other chronic diseases.14
Focus on clinical signs to narrow the differential. A thorough social and medical history and physical examination, as discussed earlier, help narrow the differential diagnosis of organic and medically unexplained disorders. Well-defined imaging or laboratory protocols do not exist to guide physicians to a definitive diagnosis, however.
Continue to: Psychiatric conditions
Psychiatric conditions can coexist with the diagnosis of FND, but might be unrelated. A systematic review of the literature showed that 17% to 42% of patients with FND had a concurrent anxiety disorder. Depression disorders were co-diagnosed in 19% to 71% of patients with FND; dissociative and personality disorders were noted, as well.25 However, coexisting psychiatric diagnosis might more likely be associated with distress from the presenting functional neurological symptoms, not linked to the FND diagnosis itself.12 This shift in understanding is reflected in the description of FND in the DSM-5.11
CASE
Mr. D reports debilitating headaches at return office visits. Trials of abortive triptans provide no relief; neither do control medications (beta-blockers, coenzyme Q10, magnesium, onabotulinumtoxinA [Botox], topiramate, and valproate). Lower-extremity weakness and unsteadiness are managed with supportive devices, including a cane, and physical therapy.
Importance of establishing a multidisciplinary approach
The complexity of FND lends itself to a multidisciplinary approach during evaluation and, eventually, for treatment. The assessment and diagnostic intervention that you provide, along with the contributions of consulted specialists (including neurology, physical and occupational therapy, psychiatry, psychology, and other mental health professionals) establishes a team-based approach that can increase the patient’s sense of support and reduce excessive testing and unnecessary medications, surgeries, and other treatments.26
Family physicians are in the ideal position to recognize the patient’s functional capacity and the quality of symptoms and to provide timely referral (eg, to Neurology and Psychiatry) for confirmation of the diagnosis and then treatment.
Evidence-based treatment options include:
- psychotherapy, with an emphasis on cognitive behavioral therapy
- physical therapy
- psychopharmacology
- promising combinations of physical and psychological treatment to improve long-term functionality.27
A promising diagnostic tool
The most significant update in the FND literature is on functional neuroimaging for assessing the disorder. Early findings suggest an intricate relationship between mind and body regarding the pathological distortion in FND. And, there is clear evidence that neuroimaging—specifically, functional magnetic resonance imaging—shows changes in brain activity that correspond to the patient’s symptom report. That said, imaging is not the recommended standard of care in the initial work-up of FND because of its cost and the fact that the diagnosis is principally a clinical undertaking.17,28
Call to action
Offer a generous ear. Begin the diagnostic pursuit by listening carefully and fully to the patient’s complaints, without arriving at a diagnosis with unwarranted bias or haste. This endeavor might require support from other clinical staff (eg, nurses, social workers, case managers) because the diagnostic process can be arduous and lengthy.
Continue to: Convey the diagnosis with sensitivity
Convey the diagnosis with sensitivity. Inquire about the patient’s perceptions and impairments to best personalize your diagnostic explanations. Delivery of the diagnosis might affect the patient’s acceptance and compliance with further testing and treatment of what is generally a persistent and treatment-resistant disorder; poor delivery of diagnostic information can impair the patient–physician relationship and increase the risk of disjointed care. Many patients find that improved patient–physician communication is therapeutic.29
Let the patient know that you’re taking her seriously. Validate patient concerns with a nonstigmatizing diagnostic label; discuss the diagnostic parameters and cause of symptoms in layman’s terms; and emphasize the potential for reversibility.30 Some patients are not satisfied with having a diagnosis of FND until they are reassured with normal results of testing and provided with referral; even then, some seek further reassurance.
Key tenets of managing care for patients who have been given a diagnosis of FND include:
- nonjudgmental, positive regard
- meaningful expression of empathy
- multidisciplinary coordination
- avoidance of unnecessary testing and harmful treatments
- descriptive and contextual explanations of the diagnosis.
Last, keep in mind that the course of treatment for FND is potentially prolonged and multilayered.
CASE
After many visits with his family physician and the neurology and cardiology specialists, as well as an extensive work-up, the physician approaches Mr. D with the possibility of a diagnosis of FND and proposes a multidisciplinary plan that includes:
- a course of physical and occupational therapy
- development of individualized cognitive behavioral tools
- weekly personal and marital counseling
- initiation of a selective serotonin reuptake inhibitor for anxiety
- monthly visits with his family physician.
Months after his return from deployment for evaluation and treatment, Mr. D is able to return to military duty. He reports that his quality of life has improved.
CORRESPONDENCE
Roselyn W. Clemente Fuentes, MD, FAAFP, Eglin Family Medicine Residency, 307 Boatner Road, Eglin AFB, FL 32547; roselynjan.w.fuentes.mil@mail.mil.
CASE
John D,* a 25-year-old patient with an otherwise unremarkable medical history, describes 2 months of daily headache, lower-extremity weakness, and unsteady gait that began fairly suddenly during his first deployment in the US Army. He explains that these symptoms affected his ability to perform his duties and necessitated an early return stateside for evaluation and treatment.
Mr. D denies precipitating trauma or unusual environmental exposures. He reports that, stateside now, symptoms continue to affect his ability to work and attend to personal and family responsibilities.
Asked about stressors, Mr. D notes the birth of his first child approximately 3 months ago, while he was deployed, and marital stressors. He denies suicidal or homicidal ideation.
* The patient’s name has been changed to protect his identity.
The challenge of identifying and managing FND
A functional neurological disorder (FND) is a constellation of psychological, physiological, and neurological symptoms, without an identifiable organic etiology, a conscious decision, or secondary gain for the patient,1 that adversely impacts functioning in 1 or more significant life domains.
Given the high throughput of patients in primary care practices, family physicians can expect to encounter suspected cases of FND in their practices. Regrettably, however, a lack of familiarity with the disorder and its related problems (eg, nonorganic paralysis, sensory loss, nonepileptic seizures, and abnormal movements) can add as much as $20,000 in excess direct and indirect costs of care for every such patient.1 In this article, we synthesize the recent literature on FND so that family physicians can expand their acumen in understanding, identifying, and evaluating patients whose presentation suggests FND.
An underrecognized entity
A precise estimate of the prevalence of FND is difficult to determine because the disorder is underrecognized and misdiagnosed and because it is often accompanied by the confounding of psychological and physiological comorbidities. A 2012 study estimated the annual incidence of FND to be 4 to 12 cases for every 100,000 people2; in primary care and outpatient neurology settings, prevalence is 6% to 22% of all patients.3,4 Stone and colleagues identified functional neurological symptoms as the second most common reason for outpatient neurology consultation,5 with 1 nonepileptic seizure patient seen for every 6 epileptic patients, and functional weakness presenting at the same rate as multiple sclerosis.6
Continue to: Demographics of patients with FND...
Demographics of patients with FND vary, depending on presenting neurological symptoms and disorder subtype. Existing data indicate a correlation between FND and younger age, female sex, physical disability,7 and a history of abuse or trauma.3,8 A challenge in concretely ascertaining the prevalence of FND is that conditions such as fibromyalgia, chronic pelvic pain, globus hystericus, and nonepileptic seizures can also be characterized as medically unexplained functional disorders, even within the network of neurology care.4
Misdiagnosis and bias are not uncommon
Ambiguity in classifying and evaluating FND can affect physicians’ perceptions, assessment, and care of patients with suggestive presenting symptoms. A major early challenge in diagnosing FND is the inconsistency of characterizing terminology (pseudoneurological, somatic, dissociative, conversion, psychogenic, hysterical, factitious, functional, medically unexplained9,10) and definitions in the literature. Neurological symptoms of unidentifiable organic cause can greatly diminish quality of life4; FND is a scientifically and clinically useful diagnosis for many combinations of nonrandomly co-occurring symptoms and clinical signs.
The pitfall of misdiagnosis. Remain cautious about making a diagnosis of FND by exclusion, which might yield an incorrect or false-negative finding because of an atypical presentation. It is important to avoid misdiagnosis by prematurely closing the differential diagnosis; instead, keep in mind that a medically unexplained diagnosis might be better explained by conducting a robust social and medical history and obtaining additional or collateral data, or both, along with appropriate consultation.4,9
Misdiagnosis can lead to a circuitous and costly work-up, with the potential to increase the patient’s distress. You can reduce this burden with early recognition of FND and centralized management of multidisciplinary care, which are more likely to lead to an accurate and timely diagnosis—paramount to empowering patients with access to the correct information and meaningful support needed to enhance treatment and self-care.9
Bias, haste, and dismissal are unproductive. Even with a clear definition of FND, it is not uncommon for a physician to rapidly assess a patient’s clinical signs, make a diagnosis of “unknown etiology,” or openly question the veracity of complaints. Furthermore, be aware of inadvertently characterizing FND using the prefix “pseudo” or the term “hysterical,” which can be psychologically discomforting for many patients, who legitimately experience inexplicable symptoms. Such pejoratives can lead to stigmatizing and misleading assessments and treatment paths4—courses of action that can cause early and, possibly, irreparable harm to the patient–physician relationship and increase the patient’s inclination to go “doctor-shopping,” with associated loss of continuity of care.
Why is it difficult to diagnose FND?
The latest (5th) edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) describes conversion, somatoform disorder, and FND synonymously.DSM-5 diagnostic criteria for conversion disorderare11:
- a specified type of symptom or deficit of altered voluntary motor or sensory function (eg, weakness, difficulty swallowing, slurred speech, seizures)
- clinical evidence of the incompatibility of the symptom or deficit and any recognized neurological or medical disorder
- incapability of better explaining the symptom or deficit as another medical or mental disorder.
- The symptom or deficit causes distress or impairment that (1) is clinically significant in occupational, social, or other important areas of function or (2) warrants medical evaluation.
The overarching feature of these criteria is the inconsistency of symptoms with recognized neurological, physiological, or psychiatric conditions. Although identification of psychological factors can help clarify and provide a treatment direction, such identification is not essential for making a diagnosis of FND. Malingering does not need to be refuted as part of establishing the diagnosis.12
Continue to: In contrast...
In contrast, the World Health Organization’s ICD-10 Classification of Mental and Behavioural Disorders groups diagnostic criteria for FND among the dissociative disorders13:
- Clinical features are specified for the individual dissociative disorder (motor, sensory, convulsions, mixed).
- Evidence is absent of a physical disorder that might explain symptoms.
- Evidence of psychological causation is present in clear temporal association with stressful events and problems or disturbed relationships, even if the patient denies such association.
Note the emphasis on psychological causation and exclusion of purposeful simulation of symptoms, as opposed to a primarily unconscious disconnection from the patient’s body or environment.
ICD-10 guidelines acknowledge the difficulty of finding definitive evidence of a psychological cause and recommend provisional diagnosis of FND if psychological factors are not readily apparent.14 Of note, many patients with FND are affected psychologically by their condition, with an impact on mood, behaviors, and interpersonal interactions, although not necessarily to a clinically diagnostic degree. Therefore, a psychiatric diagnosis alone is not a necessary precursor for the diagnosis of an FND.
CASE
History. Mr. D’s history is positive for light alcohol consumption (“2 or 3 cans of beer on weekends”) and chewing tobacco (he reports stopping 6 months earlier) and negative for substance abuse. The family history is positive for maternal hypertension and paternal suicide when the patient was 10 years old (no other known paternal history).
Physical findings. The review of systems is positive for intermittent palpitations, lower-extremity weakness causing unsteady gait, and generalized headache.
Vital signs are within normal limits, including blood pressure (120/82 mm Hg) and heart rate (110 beats/min). The patient is not in acute distress; he is awake, alert, and oriented × 3. No murmurs are heard; lungs are clear bilaterally to auscultation. There is no tenderness on abdominal palpation, and no hepatomegaly or splenomegaly; bowel sounds are normal. No significant bruising or lacerations are noted.
Neurology exam. Cranial nerves II-XII are intact. Pupils are equal and reactive to light. Reflexes are 2+ bilaterally. Muscle strength and tone are normal; no tremors are noted. Babinski signs are normal. A Romberg test is positive (swaying).
Continue to: Mr. D has an antalgic gait...
Mr. D has an antalgic gait with significant swaying (without falling); bent posture; and unsteadiness that requires a cane. However, he is able to get up and off the exam table without assistance, and to propel himself, by rolling a chair forward and backward, without difficulty.
Conducting a diagnostic examination
Taking the history. Certain clues can aid in the diagnosis of FND (TABLE 1).15 For example, the patient might have been seen in multiple specialty practices for a multitude of vague symptoms indicative of potentially related conditions (eg, chronic fatigue, allergies and sensitivities, fibromyalgia, and other chronic pain). The history might include repeated surgeries to investigate those symptoms (eg, laparoscopy, or hysterectomy at an early age). Taking time and care to explore all clinical clues, patient reports, and collateral data are therefore key to making an accurate diagnosis.
Note any discrepancies between the severity of reported symptoms and functional ability. A technique that can help elucidate a complex or ambiguous medical presentation is to ask the patient to list all their symptoms at the beginning of the interview. This has threefold benefit: You get a broad picture of the problem; the patient is unburdened of their concerns and experiences your validation; and a long list of symptoms can be an early clue to a diagnosis of FND.
Other helpful questions to determine the impact of symptoms on the patient’s well-being include inquiries about16:
- functional impairment
- onset and course of symptoms
- potential causal or correlating events
- dissociative episodes
- previous diagnoses and treatments
- the patient’s perceptions of, and emotional response to, their illness
- a history of abuse.
The physical examination to determine the presence of FND varies, depending on the functional area of impact (eg, motor, neurological, sensory, speech and swallowing). Pay particular attention to presenting signs and clues, and balance them with the patient’s report (or lack of report). Endeavor to demonstrate positive functional signs, such as a positive Hoover test, which relies on the principle of synergistic muscle contraction. You might see evidence of inconsistency, such as weakness or a change in gait, under observation, that seemingly resolves when the patient is getting on and off the exam table.16Table 215-24 describes areas affected by FND, characteristics of the disorder, and related diagnostic examinations.
Table 315,18,19 reviews validated special exams that can aid in making the diagnosis. Additional special tests are discussed in the literature.15-24 These tests can be helpful in narrowing the differential diagnosis but have not been validated and should be used with caution.
Some clinical signs associated with FND might be affected by other factors, including socioeconomic status, limited access to health care, low health literacy, poor communication skills, and physician bias. Keep these factors in mind during the visit, to avoid contributing further to health disparities among groups of patients affected by these problems.
Continue to: CASE
CASE
The work-up over the next month for Mr. D includes numerous studies, all yielding results that are negative or within normal limits: visual acuity; electrocardiography and an event monitor; laboratory testing (including a complete blood count, comprehensive metabolic panel, thyroid-stimulating hormone, creatine kinase, erythrocyte sedimentation rate, C-reactive protein, vitamin B12, folate, and vitamin D); magnetic resonance imaging of the brain and lumbar spine; lumbar puncture; and electromyography.
The score on the 9-item Patient Health Questionnaire for depression is 4 (severity: “none or minimal”); on the 7-item Generalized Anxiety Disorder scale, 0 (“no anxiety disorder”).
Referral. A neurology work-up of headache, lower extremity weakness, and unsteady gait to address several diagnostic possibilities, including migraine and multiple sclerosis, is within normal limits. A cardiology work-up of palpitations is negative for arrhythmias and other concerning findings.
Mr. D declines psychiatric and psychological evaluations.
Building a differential diagnosisis a formidable task
The differential diagnosis of FND is vast. It includes neurological, physiological, and psychiatric symptoms and disorders; somatization; and malingering (Table 4).6 Any disorder or condition in these areas that is in the differential diagnosis can be precipitated or exacerbated by stress; most, however, do not involve loss of physical function.12 In addition, the diagnosis of an FND does not necessarily exclude an organic disorder.
A patient’s presentation becomes complicated—and more difficult to treat—when functional symptoms and an unrelated underlying or early-stage neurological condition coexist. For example, a patient with epilepsy might also have dissociative seizures atop their organic disorder. Neurological disease is considered a risk factor for an overlying FND—just as the risk of depression or anxiety runs concurrently with other chronic diseases.14
Focus on clinical signs to narrow the differential. A thorough social and medical history and physical examination, as discussed earlier, help narrow the differential diagnosis of organic and medically unexplained disorders. Well-defined imaging or laboratory protocols do not exist to guide physicians to a definitive diagnosis, however.
Continue to: Psychiatric conditions
Psychiatric conditions can coexist with the diagnosis of FND, but might be unrelated. A systematic review of the literature showed that 17% to 42% of patients with FND had a concurrent anxiety disorder. Depression disorders were co-diagnosed in 19% to 71% of patients with FND; dissociative and personality disorders were noted, as well.25 However, coexisting psychiatric diagnosis might more likely be associated with distress from the presenting functional neurological symptoms, not linked to the FND diagnosis itself.12 This shift in understanding is reflected in the description of FND in the DSM-5.11
CASE
Mr. D reports debilitating headaches at return office visits. Trials of abortive triptans provide no relief; neither do control medications (beta-blockers, coenzyme Q10, magnesium, onabotulinumtoxinA [Botox], topiramate, and valproate). Lower-extremity weakness and unsteadiness are managed with supportive devices, including a cane, and physical therapy.
Importance of establishing a multidisciplinary approach
The complexity of FND lends itself to a multidisciplinary approach during evaluation and, eventually, for treatment. The assessment and diagnostic intervention that you provide, along with the contributions of consulted specialists (including neurology, physical and occupational therapy, psychiatry, psychology, and other mental health professionals) establishes a team-based approach that can increase the patient’s sense of support and reduce excessive testing and unnecessary medications, surgeries, and other treatments.26
Family physicians are in the ideal position to recognize the patient’s functional capacity and the quality of symptoms and to provide timely referral (eg, to Neurology and Psychiatry) for confirmation of the diagnosis and then treatment.
Evidence-based treatment options include:
- psychotherapy, with an emphasis on cognitive behavioral therapy
- physical therapy
- psychopharmacology
- promising combinations of physical and psychological treatment to improve long-term functionality.27
A promising diagnostic tool
The most significant update in the FND literature is on functional neuroimaging for assessing the disorder. Early findings suggest an intricate relationship between mind and body regarding the pathological distortion in FND. And, there is clear evidence that neuroimaging—specifically, functional magnetic resonance imaging—shows changes in brain activity that correspond to the patient’s symptom report. That said, imaging is not the recommended standard of care in the initial work-up of FND because of its cost and the fact that the diagnosis is principally a clinical undertaking.17,28
Call to action
Offer a generous ear. Begin the diagnostic pursuit by listening carefully and fully to the patient’s complaints, without arriving at a diagnosis with unwarranted bias or haste. This endeavor might require support from other clinical staff (eg, nurses, social workers, case managers) because the diagnostic process can be arduous and lengthy.
Continue to: Convey the diagnosis with sensitivity
Convey the diagnosis with sensitivity. Inquire about the patient’s perceptions and impairments to best personalize your diagnostic explanations. Delivery of the diagnosis might affect the patient’s acceptance and compliance with further testing and treatment of what is generally a persistent and treatment-resistant disorder; poor delivery of diagnostic information can impair the patient–physician relationship and increase the risk of disjointed care. Many patients find that improved patient–physician communication is therapeutic.29
Let the patient know that you’re taking her seriously. Validate patient concerns with a nonstigmatizing diagnostic label; discuss the diagnostic parameters and cause of symptoms in layman’s terms; and emphasize the potential for reversibility.30 Some patients are not satisfied with having a diagnosis of FND until they are reassured with normal results of testing and provided with referral; even then, some seek further reassurance.
Key tenets of managing care for patients who have been given a diagnosis of FND include:
- nonjudgmental, positive regard
- meaningful expression of empathy
- multidisciplinary coordination
- avoidance of unnecessary testing and harmful treatments
- descriptive and contextual explanations of the diagnosis.
Last, keep in mind that the course of treatment for FND is potentially prolonged and multilayered.
CASE
After many visits with his family physician and the neurology and cardiology specialists, as well as an extensive work-up, the physician approaches Mr. D with the possibility of a diagnosis of FND and proposes a multidisciplinary plan that includes:
- a course of physical and occupational therapy
- development of individualized cognitive behavioral tools
- weekly personal and marital counseling
- initiation of a selective serotonin reuptake inhibitor for anxiety
- monthly visits with his family physician.
Months after his return from deployment for evaluation and treatment, Mr. D is able to return to military duty. He reports that his quality of life has improved.
CORRESPONDENCE
Roselyn W. Clemente Fuentes, MD, FAAFP, Eglin Family Medicine Residency, 307 Boatner Road, Eglin AFB, FL 32547; roselynjan.w.fuentes.mil@mail.mil.
1. Konnopka A, Schaefert R, Heinrich S, et al. Economics of medically unexplained symptoms: a systematic review of the literature. Psychother Psychosom. 2012;81:265-275.
2. Carson AJ, Brown R, David AS, et al; on behalf of UK-FNS. Functional (conversion) neurological symptoms: research since the millennium. J Neurol Neurosurg Psychiatry. 2012;83:842-850.
3. Stone J, Carson A, Duncan R, et al. Who is referred to neurology clinics?—the diagnoses made in 3781 new patients. Clin Neurol Neurosurg. 2010;112:747-751.
4. Evens A, Vendetta L, Krebs K, et al. Medically unexplained neurologic symptoms: a primer for physicians who make the initial encounter. Am J Med. 2015;128:1059-1064.
5. Stone J, Reuber M, Carson A. Functional symptoms in neurology: mimics and chameleons. Pract Neurol. 2013;13:104-113.
6. Stone J, Warlow C, Sharpe M. The symptom of functional weakness: a controlled study of 107 patients. Brain. 2010;133:1537-1551.
7. Carson A, Stone J, Hibberd C, et al. Disability, distress and unemployment in neurology outpatients with symptoms ‘unexplained by organic disease’. J Neurol Neurosurg Psychiatry. 2011;82:810-813.
8. Fink P, Hansen MS, Oxhøj M-L. The prevalence of somatoform disorders among internal medical inpatients. J Psychosom Res. 2004;56:413-418.
9. Thomas LE. Are your patient’s medically unexplained symptoms really “all in her head”? Med Hypotheses. 2012;78:542-547.
10. Ding JM, Kanaan RAA. What should we say to patients with unexplained neurological symptoms? How explanation affects offence. J Psychosom Res. 2016;91:55-60.
11. Diagnostic and Statistical Manual of Mental Disorders (DSM-5). 5th ed. American Psychiatric Association; 2013.
12. Stone J, LaFrance WC Jr, Levenson JL, et al. Issues for DSM-5: Conversion disorder. Am J Psychiatry. 2010;167:626-627.
13. The ICD-10 Classification of Mental and Behavioural Disorders: Clinical Descriptions and Diagnostic Guidelines. World Health Organization; 1994. Accessed January 21, 2021. www.who.int/classifications/icd/en/bluebook.pdf
14. Stone J, Carson A, Duncan R, et al. Which neurological diseases are most likely to be associated with “symptoms unexplained by organic disease.” J Neurol. 2012;259:33-38.
15. Shaibani A, Sabbagh M. Pseudoneurologic syndromes: recognition and diagnosis. Am Fam Physician. 1998;57:2485-2494.
16. Stone J, Carson A, Sharpe M. Functional symptoms and signs in neurology: assessment and diagnosis. J Neurol Neurosurg Psychiatry. 2005;76(suppl 1):i2-i12.
17. Vuilleumier P. Brain circuits implicated in psychogenic paralysis in conversion disorders and hypnosis. Neurophysiol Clin. 2014;44:323-337.
18. McKee K, Glass S, Adams C, et al. The inpatient assessment and management of motor functional neurological disorders: an interdisciplinary perspective. Psychosomatics. 2018;59:358-368.
19. Daum C, Hubschmid M, Aybek S. The value of ‘positive’ clinical signs for weakness, sensory and gait disorders in conversion disorder: a systematic and narrative review. J Neurol Neurosurg Psychiatry. 2014;85:180-190.
20. Brown P, Thompson PD. Electrophysiological aids to the diagnosis of psychogenic jerks, spasms, and tremor. Mov Disord. 2001;16:595-599.
21. Ludwig L, McWhirter L, Williams S, et al. Functional coma. In: Hallett M, Stone J, Carson A, eds. Handbook of Clinical Neurology: Volume 139: Functional Neurologic Disorders. 1st ed. Academic Press; 2016:313.
22. Miller NR, Subramanian PS, Patel VR. Walsh and Hoyt’s Clinical Neuro-ophthalmology. 3rd ed. Wolters Kluwer; 2016:512-513.
23. Takazaki K, Stransky AD, Miller G. Psychogenic nonepileptic seizures: diagnosis, management, and bioethics. Pediatr Neurol. 2016;62:3-8.
24. Sahaya K, Dholakia SA, Sahota PK. Psychogenic non-epileptic seizures: a challenging entity. J Clin Neurosci. 2011;18:1602-1607.
25. Gelauff J, Stone J, Edwards M, et al. The prognosis of functional (psychogenic) motor symptoms: a systematic review. J Neurol Neurosurg Psychiatry. 2014;85:220-226.
26. Kranick SM, Gorrindo T, Hallett M. Psychogenic movement disorders and motor conversion: a roadmap for collaboration between neurology and psychiatry. Psychosomatics. 2011;52:109-116.
27. Edwards MJ, Bhatia KP. Functional (psychogenic) movement disorders: merging mind and brain. Lancet Neurol. 2012;11:250-260.
28. Burgmer M, Kugel H, Pfleiderer B, et al. The mirror neuron system under hypnosis—brain substrates of voluntary and involuntary motor activation in hypnotic paralysis. Cortex. 2013;49:437-445.
29. van Bokhoven MA, Koch H, van der Weijden T, et al. Influence of watchful waiting on satisfaction and anxiety among patients seeking care for unexplained complaints, Ann Fam Med. 2009;7:112-120.
30. Stone J, Carson A, Hallet M. Explanation as treatment for functional neurologic disorders. Handb Clin Neurol. 2016;139:543-553.
1. Konnopka A, Schaefert R, Heinrich S, et al. Economics of medically unexplained symptoms: a systematic review of the literature. Psychother Psychosom. 2012;81:265-275.
2. Carson AJ, Brown R, David AS, et al; on behalf of UK-FNS. Functional (conversion) neurological symptoms: research since the millennium. J Neurol Neurosurg Psychiatry. 2012;83:842-850.
3. Stone J, Carson A, Duncan R, et al. Who is referred to neurology clinics?—the diagnoses made in 3781 new patients. Clin Neurol Neurosurg. 2010;112:747-751.
4. Evens A, Vendetta L, Krebs K, et al. Medically unexplained neurologic symptoms: a primer for physicians who make the initial encounter. Am J Med. 2015;128:1059-1064.
5. Stone J, Reuber M, Carson A. Functional symptoms in neurology: mimics and chameleons. Pract Neurol. 2013;13:104-113.
6. Stone J, Warlow C, Sharpe M. The symptom of functional weakness: a controlled study of 107 patients. Brain. 2010;133:1537-1551.
7. Carson A, Stone J, Hibberd C, et al. Disability, distress and unemployment in neurology outpatients with symptoms ‘unexplained by organic disease’. J Neurol Neurosurg Psychiatry. 2011;82:810-813.
8. Fink P, Hansen MS, Oxhøj M-L. The prevalence of somatoform disorders among internal medical inpatients. J Psychosom Res. 2004;56:413-418.
9. Thomas LE. Are your patient’s medically unexplained symptoms really “all in her head”? Med Hypotheses. 2012;78:542-547.
10. Ding JM, Kanaan RAA. What should we say to patients with unexplained neurological symptoms? How explanation affects offence. J Psychosom Res. 2016;91:55-60.
11. Diagnostic and Statistical Manual of Mental Disorders (DSM-5). 5th ed. American Psychiatric Association; 2013.
12. Stone J, LaFrance WC Jr, Levenson JL, et al. Issues for DSM-5: Conversion disorder. Am J Psychiatry. 2010;167:626-627.
13. The ICD-10 Classification of Mental and Behavioural Disorders: Clinical Descriptions and Diagnostic Guidelines. World Health Organization; 1994. Accessed January 21, 2021. www.who.int/classifications/icd/en/bluebook.pdf
14. Stone J, Carson A, Duncan R, et al. Which neurological diseases are most likely to be associated with “symptoms unexplained by organic disease.” J Neurol. 2012;259:33-38.
15. Shaibani A, Sabbagh M. Pseudoneurologic syndromes: recognition and diagnosis. Am Fam Physician. 1998;57:2485-2494.
16. Stone J, Carson A, Sharpe M. Functional symptoms and signs in neurology: assessment and diagnosis. J Neurol Neurosurg Psychiatry. 2005;76(suppl 1):i2-i12.
17. Vuilleumier P. Brain circuits implicated in psychogenic paralysis in conversion disorders and hypnosis. Neurophysiol Clin. 2014;44:323-337.
18. McKee K, Glass S, Adams C, et al. The inpatient assessment and management of motor functional neurological disorders: an interdisciplinary perspective. Psychosomatics. 2018;59:358-368.
19. Daum C, Hubschmid M, Aybek S. The value of ‘positive’ clinical signs for weakness, sensory and gait disorders in conversion disorder: a systematic and narrative review. J Neurol Neurosurg Psychiatry. 2014;85:180-190.
20. Brown P, Thompson PD. Electrophysiological aids to the diagnosis of psychogenic jerks, spasms, and tremor. Mov Disord. 2001;16:595-599.
21. Ludwig L, McWhirter L, Williams S, et al. Functional coma. In: Hallett M, Stone J, Carson A, eds. Handbook of Clinical Neurology: Volume 139: Functional Neurologic Disorders. 1st ed. Academic Press; 2016:313.
22. Miller NR, Subramanian PS, Patel VR. Walsh and Hoyt’s Clinical Neuro-ophthalmology. 3rd ed. Wolters Kluwer; 2016:512-513.
23. Takazaki K, Stransky AD, Miller G. Psychogenic nonepileptic seizures: diagnosis, management, and bioethics. Pediatr Neurol. 2016;62:3-8.
24. Sahaya K, Dholakia SA, Sahota PK. Psychogenic non-epileptic seizures: a challenging entity. J Clin Neurosci. 2011;18:1602-1607.
25. Gelauff J, Stone J, Edwards M, et al. The prognosis of functional (psychogenic) motor symptoms: a systematic review. J Neurol Neurosurg Psychiatry. 2014;85:220-226.
26. Kranick SM, Gorrindo T, Hallett M. Psychogenic movement disorders and motor conversion: a roadmap for collaboration between neurology and psychiatry. Psychosomatics. 2011;52:109-116.
27. Edwards MJ, Bhatia KP. Functional (psychogenic) movement disorders: merging mind and brain. Lancet Neurol. 2012;11:250-260.
28. Burgmer M, Kugel H, Pfleiderer B, et al. The mirror neuron system under hypnosis—brain substrates of voluntary and involuntary motor activation in hypnotic paralysis. Cortex. 2013;49:437-445.
29. van Bokhoven MA, Koch H, van der Weijden T, et al. Influence of watchful waiting on satisfaction and anxiety among patients seeking care for unexplained complaints, Ann Fam Med. 2009;7:112-120.
30. Stone J, Carson A, Hallet M. Explanation as treatment for functional neurologic disorders. Handb Clin Neurol. 2016;139:543-553.
PRACTICE RECOMMENDATIONS
› Avoid using stigmatizing terminology (eg, adding the prefix “pseudo” or the adjective “hysterical”) to characterize a suspected functional neurological disorder (FND) or a medically unexplained disorder. C
› Refrain from ordering functional magnetic resonance imaging as part of the routine evaluation of suspected FND. C
› Validate the patient‘s concerns with an appropriate diagnostic label; use layman’s terms to discuss the diagnostic parameters of FND and the cause of symptoms; and emphasize treatment possibilities and plans. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series