User login
Do antidepressants increase the risk of brain bleeds?
Contrary to previous findings, results of a large observational study show. However, at least one expert urged caution in interpreting the finding.
“These findings are important, especially since depression is common after stroke and SSRIs are some of the first drugs considered for people,” Mithilesh Siddu, MD, of the University of Miami/Jackson Memorial Hospital, also in Miami, said in a statement.
However, Dr. Siddu said “more research is needed to confirm our findings and to also examine if SSRIs prescribed after a stroke may be linked to risk of a second stroke.”
The findings were released ahead of the study’s scheduled presentation at the annual meeting of the American Academy of Neurology.
Widely prescribed
SSRIs, the most widely prescribed antidepressant in the United States, have previously been linked to an increased risk of ICH, possibly as a result of impaired platelet function.
To investigate further, the researchers analyzed data from the Florida Stroke Registry (FSR). They identified 127,915 patients who suffered ICH from January 2010 to December 2019 and for whom information on antidepressant use was available.
They analyzed the proportion of cases presenting with ICH among antidepressant users and the rate of SSRI prescription among stroke patients discharged on antidepressant therapy.
The researchers found that 11% of those who had been prescribed antidepressants had an ICH, compared with 14% of those who had not.
Antidepressant users were more likely to be female; non-Hispanic White; have hypertension; have diabetes; and use oral anticoagulants, antiplatelets, and statins prior to hospital presentation for ICH.
In multivariable analyses adjusting for age, race, prior history of hypertension, diabetes and prior oral anticoagulant, antiplatelet and statin use, antidepressant users were just as likely to present with spontaneous ICH as nonantidepressant users (odds ratio, 0.92; 95% confidence interval, 0.85-1.01).
A total of 3.4% of all ICH patients and 9% of those in whom specific antidepressant information was available were discharged home on an antidepressant, most commonly an SSRI (74%).
The authors noted a key limitation of the study: Some details regarding the length, dosage, and type of antidepressants were not available.
Interpret with caution
In a comment, Shaheen Lakhan, MD, PhD, a neurologist in Newton, Mass., and executive director of the Global Neuroscience Initiative Foundation, urged caution in making any firm conclusions based on this study.
“We have two questions here: One, is SSRI use a risk factor for first-time intracerebral hemorrhage, and two, is SSRI use after an ICH a risk factor for additional hemorrhages,” said Dr. Lakhan, who was not involved with the study.
“This study incompletely addresses the first because it is known that SSRIs have a variety of potencies. For instance, paroxetine is a strong inhibitor of serotonin reuptake, whereas bupropion is weak. Hypothetically, the former has a greater risk of ICH. Because this study did not stratify by type of antidepressant, it is not possible to tease these out,” Dr. Lakhan said.
“The second question is completely unaddressed by this study and is the real concern in clinical practice, because the chance of rebleed is much higher than the risk of first-time ICH in the general population,” he added.
The study had no specific funding. Dr. Siddu and Dr. Lakhan disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Contrary to previous findings, results of a large observational study show. However, at least one expert urged caution in interpreting the finding.
“These findings are important, especially since depression is common after stroke and SSRIs are some of the first drugs considered for people,” Mithilesh Siddu, MD, of the University of Miami/Jackson Memorial Hospital, also in Miami, said in a statement.
However, Dr. Siddu said “more research is needed to confirm our findings and to also examine if SSRIs prescribed after a stroke may be linked to risk of a second stroke.”
The findings were released ahead of the study’s scheduled presentation at the annual meeting of the American Academy of Neurology.
Widely prescribed
SSRIs, the most widely prescribed antidepressant in the United States, have previously been linked to an increased risk of ICH, possibly as a result of impaired platelet function.
To investigate further, the researchers analyzed data from the Florida Stroke Registry (FSR). They identified 127,915 patients who suffered ICH from January 2010 to December 2019 and for whom information on antidepressant use was available.
They analyzed the proportion of cases presenting with ICH among antidepressant users and the rate of SSRI prescription among stroke patients discharged on antidepressant therapy.
The researchers found that 11% of those who had been prescribed antidepressants had an ICH, compared with 14% of those who had not.
Antidepressant users were more likely to be female; non-Hispanic White; have hypertension; have diabetes; and use oral anticoagulants, antiplatelets, and statins prior to hospital presentation for ICH.
In multivariable analyses adjusting for age, race, prior history of hypertension, diabetes and prior oral anticoagulant, antiplatelet and statin use, antidepressant users were just as likely to present with spontaneous ICH as nonantidepressant users (odds ratio, 0.92; 95% confidence interval, 0.85-1.01).
A total of 3.4% of all ICH patients and 9% of those in whom specific antidepressant information was available were discharged home on an antidepressant, most commonly an SSRI (74%).
The authors noted a key limitation of the study: Some details regarding the length, dosage, and type of antidepressants were not available.
Interpret with caution
In a comment, Shaheen Lakhan, MD, PhD, a neurologist in Newton, Mass., and executive director of the Global Neuroscience Initiative Foundation, urged caution in making any firm conclusions based on this study.
“We have two questions here: One, is SSRI use a risk factor for first-time intracerebral hemorrhage, and two, is SSRI use after an ICH a risk factor for additional hemorrhages,” said Dr. Lakhan, who was not involved with the study.
“This study incompletely addresses the first because it is known that SSRIs have a variety of potencies. For instance, paroxetine is a strong inhibitor of serotonin reuptake, whereas bupropion is weak. Hypothetically, the former has a greater risk of ICH. Because this study did not stratify by type of antidepressant, it is not possible to tease these out,” Dr. Lakhan said.
“The second question is completely unaddressed by this study and is the real concern in clinical practice, because the chance of rebleed is much higher than the risk of first-time ICH in the general population,” he added.
The study had no specific funding. Dr. Siddu and Dr. Lakhan disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Contrary to previous findings, results of a large observational study show. However, at least one expert urged caution in interpreting the finding.
“These findings are important, especially since depression is common after stroke and SSRIs are some of the first drugs considered for people,” Mithilesh Siddu, MD, of the University of Miami/Jackson Memorial Hospital, also in Miami, said in a statement.
However, Dr. Siddu said “more research is needed to confirm our findings and to also examine if SSRIs prescribed after a stroke may be linked to risk of a second stroke.”
The findings were released ahead of the study’s scheduled presentation at the annual meeting of the American Academy of Neurology.
Widely prescribed
SSRIs, the most widely prescribed antidepressant in the United States, have previously been linked to an increased risk of ICH, possibly as a result of impaired platelet function.
To investigate further, the researchers analyzed data from the Florida Stroke Registry (FSR). They identified 127,915 patients who suffered ICH from January 2010 to December 2019 and for whom information on antidepressant use was available.
They analyzed the proportion of cases presenting with ICH among antidepressant users and the rate of SSRI prescription among stroke patients discharged on antidepressant therapy.
The researchers found that 11% of those who had been prescribed antidepressants had an ICH, compared with 14% of those who had not.
Antidepressant users were more likely to be female; non-Hispanic White; have hypertension; have diabetes; and use oral anticoagulants, antiplatelets, and statins prior to hospital presentation for ICH.
In multivariable analyses adjusting for age, race, prior history of hypertension, diabetes and prior oral anticoagulant, antiplatelet and statin use, antidepressant users were just as likely to present with spontaneous ICH as nonantidepressant users (odds ratio, 0.92; 95% confidence interval, 0.85-1.01).
A total of 3.4% of all ICH patients and 9% of those in whom specific antidepressant information was available were discharged home on an antidepressant, most commonly an SSRI (74%).
The authors noted a key limitation of the study: Some details regarding the length, dosage, and type of antidepressants were not available.
Interpret with caution
In a comment, Shaheen Lakhan, MD, PhD, a neurologist in Newton, Mass., and executive director of the Global Neuroscience Initiative Foundation, urged caution in making any firm conclusions based on this study.
“We have two questions here: One, is SSRI use a risk factor for first-time intracerebral hemorrhage, and two, is SSRI use after an ICH a risk factor for additional hemorrhages,” said Dr. Lakhan, who was not involved with the study.
“This study incompletely addresses the first because it is known that SSRIs have a variety of potencies. For instance, paroxetine is a strong inhibitor of serotonin reuptake, whereas bupropion is weak. Hypothetically, the former has a greater risk of ICH. Because this study did not stratify by type of antidepressant, it is not possible to tease these out,” Dr. Lakhan said.
“The second question is completely unaddressed by this study and is the real concern in clinical practice, because the chance of rebleed is much higher than the risk of first-time ICH in the general population,” he added.
The study had no specific funding. Dr. Siddu and Dr. Lakhan disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM AAN 2021
Natalizumab postinfusion reactions rare; is monitoring necessary?
new studies show.
Collectively, the results suggest the need to rethink the drug’s mandatory 1-hour postinfusion observation period – particularly when unnecessarily spending time in medical settings is discouraged because of concerns regarding COVID-19, the researchers concluded.
Their findings “highlight a potential opportunity to improve and streamline the infusion and postinfusion monitoring process,” reported the authors of one of the studies. The findings were presented at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
Infusion reactions were rare
“In this systematic review of almost 10,000 natalizumab infusions, all infusion-related adverse events were mild, and no clinically relevant safety concerns were associated with natalizumab infusions,” they said.
The 1-hour postinfusion observation period for natalizumab, approved for the treatment of relapsing remitting MS (RRMS), is mandated by the Food and Drug Administration, as well as the European Medicines Agency, and applies to each dose, regardless of treatment duration, owing to concerns of infusion reactions. However, previous evidence has indicated that reactions are rare and are usually mild.
In addition to adding burden to the treatment regimen for patients and providers alike, any extended time in an environment where there is concern of heightened risk for SARS-CoV-2 exposure is a concern.
To evaluate the frequency, severity, and timing of infusion reactions, Yujie Wang, MD, of the department of neurology at the University of Washington, Seattle, and colleagues reviewed medical records of all patients who received natalizumab at the University of Washington MS Center’s infusion suite between July 2012 and September 2020.
Among 333 patients with RRMS, 9,682 infusions of natalizumab were provided over the study period, with a mean of 27 infusions per patient (range, 1-174). The mean age of the patients was 41 years, and 87 (26%) were male.
Overall, 33 infusion-related adverse events were reported in 26 patients, representing 0.34% of total infusions and 7.8% of patients.
In 77% of cases, the adverse event occurred during the infusion. In 92% of cases, the adverse event occurred within the first 6 months of treatment.
All of the events were described as mild. The most common were itching, gastrointestinal problems, headache, and flushing.
None of the reactions required emergency care or hospitalization. Symptoms were either self-managed or were managed easily with standard care. The treatment was continued in all cases.
“For physicians and providers who care for patients with MS and are comfortable with infusible therapies, it is no surprise that rates of clinically significant infusion reactions were low,” Dr. Wang said. “It is indeed consistent with prior studies that reactions generally occur during rather than post infusion.”
The authors underscored the array of potential benefits in making changes to the requirement. “Anticipated benefits may include reducing SARS-CoV2 exposure risks for patients and staff, reducing patients’ treatment burden, increasing efficiency, as well as improving access to care without neglecting patient safety.”
Additional studies show consistent findings
Several other recent studies have shown similar results. In a study published in Multiple Sclerosis in October 2020, researchers with the Amsterdam University Medical Center found that, among 14,174 natalizumab infusions provided to 225 patients with RRMS between 2006 and 2018, 276 infusion-related adverse events occurred (1.95%) among 60 patients.
There were 11 severe infusion-related adverse events in nine patients (4.0%). All documented severe reactions occurred during the infusion. Among 19 moderate adverse events, 17 occurred during the infusion.
The researchers noted that the majority of patients who experienced severe infusion reactions had detectable antibodies against natalizumab. Such antibodies are associated with a higher risk for infusion-related adverse events.
Patients who did not have any symptoms of a reaction during the infusion had no clinically relevant moderate or severe reactions.
“Thus, the need for postinfusion observation will depend on the patients’ clinical status during the infusion,” they wrote. “Consequently, our data suggest that patients who do not have an infusion-related adverse event while receiving natalizumab treatment do not need to stay in the hospital for an additional observation hour.”
Rapid infusion protocol
In another recent study published in Multiple Sclerosis and Related Disorders in January 2021, researchers in Australia reported on the use of a rapid infusion protocol of natalizumab and ocrelizumab. The protocol was implemented to reduce the amount of time patients are required to spend in clinical settings during the COVID-19 pandemic.
In their analysis of 269 rapid infusions of natalizumab and 100 rapid infusions of ocrelizumab, there were two infusion-related reactions in the natalizumab group and eight in the ocrelizumab group.
All the reactions were mild to moderate, and no discontinuations were required. None of the reactions occurred during the postinfusion observation period.
“In the setting of COVID-19 pandemic, rapid infusion protocols could potentially save hospital resources and limit patient exposure to a high-risk clinical setting while still maintaining ongoing treatment of multiple sclerosis,” the authors wrote.
Under the rapid infusion protocol, patients receive three standard doses for 1 hour followed by 30 minutes of observation. In addition, infusions are reduced to 30 minutes, explained lead author Louise Rath, of clinical neurosciences, Alfred Health, in Melbourne.
“For our cohort of patients, the side effects were minimal,” she said.
“Rapid infusions allowed patients to have option of hospital in-home or office, ensuring work was not at risk by infusion,” she added. “Our governance has been very supportive, and we will be keeping rapid infusion post COVID.”
The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
new studies show.
Collectively, the results suggest the need to rethink the drug’s mandatory 1-hour postinfusion observation period – particularly when unnecessarily spending time in medical settings is discouraged because of concerns regarding COVID-19, the researchers concluded.
Their findings “highlight a potential opportunity to improve and streamline the infusion and postinfusion monitoring process,” reported the authors of one of the studies. The findings were presented at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
Infusion reactions were rare
“In this systematic review of almost 10,000 natalizumab infusions, all infusion-related adverse events were mild, and no clinically relevant safety concerns were associated with natalizumab infusions,” they said.
The 1-hour postinfusion observation period for natalizumab, approved for the treatment of relapsing remitting MS (RRMS), is mandated by the Food and Drug Administration, as well as the European Medicines Agency, and applies to each dose, regardless of treatment duration, owing to concerns of infusion reactions. However, previous evidence has indicated that reactions are rare and are usually mild.
In addition to adding burden to the treatment regimen for patients and providers alike, any extended time in an environment where there is concern of heightened risk for SARS-CoV-2 exposure is a concern.
To evaluate the frequency, severity, and timing of infusion reactions, Yujie Wang, MD, of the department of neurology at the University of Washington, Seattle, and colleagues reviewed medical records of all patients who received natalizumab at the University of Washington MS Center’s infusion suite between July 2012 and September 2020.
Among 333 patients with RRMS, 9,682 infusions of natalizumab were provided over the study period, with a mean of 27 infusions per patient (range, 1-174). The mean age of the patients was 41 years, and 87 (26%) were male.
Overall, 33 infusion-related adverse events were reported in 26 patients, representing 0.34% of total infusions and 7.8% of patients.
In 77% of cases, the adverse event occurred during the infusion. In 92% of cases, the adverse event occurred within the first 6 months of treatment.
All of the events were described as mild. The most common were itching, gastrointestinal problems, headache, and flushing.
None of the reactions required emergency care or hospitalization. Symptoms were either self-managed or were managed easily with standard care. The treatment was continued in all cases.
“For physicians and providers who care for patients with MS and are comfortable with infusible therapies, it is no surprise that rates of clinically significant infusion reactions were low,” Dr. Wang said. “It is indeed consistent with prior studies that reactions generally occur during rather than post infusion.”
The authors underscored the array of potential benefits in making changes to the requirement. “Anticipated benefits may include reducing SARS-CoV2 exposure risks for patients and staff, reducing patients’ treatment burden, increasing efficiency, as well as improving access to care without neglecting patient safety.”
Additional studies show consistent findings
Several other recent studies have shown similar results. In a study published in Multiple Sclerosis in October 2020, researchers with the Amsterdam University Medical Center found that, among 14,174 natalizumab infusions provided to 225 patients with RRMS between 2006 and 2018, 276 infusion-related adverse events occurred (1.95%) among 60 patients.
There were 11 severe infusion-related adverse events in nine patients (4.0%). All documented severe reactions occurred during the infusion. Among 19 moderate adverse events, 17 occurred during the infusion.
The researchers noted that the majority of patients who experienced severe infusion reactions had detectable antibodies against natalizumab. Such antibodies are associated with a higher risk for infusion-related adverse events.
Patients who did not have any symptoms of a reaction during the infusion had no clinically relevant moderate or severe reactions.
“Thus, the need for postinfusion observation will depend on the patients’ clinical status during the infusion,” they wrote. “Consequently, our data suggest that patients who do not have an infusion-related adverse event while receiving natalizumab treatment do not need to stay in the hospital for an additional observation hour.”
Rapid infusion protocol
In another recent study published in Multiple Sclerosis and Related Disorders in January 2021, researchers in Australia reported on the use of a rapid infusion protocol of natalizumab and ocrelizumab. The protocol was implemented to reduce the amount of time patients are required to spend in clinical settings during the COVID-19 pandemic.
In their analysis of 269 rapid infusions of natalizumab and 100 rapid infusions of ocrelizumab, there were two infusion-related reactions in the natalizumab group and eight in the ocrelizumab group.
All the reactions were mild to moderate, and no discontinuations were required. None of the reactions occurred during the postinfusion observation period.
“In the setting of COVID-19 pandemic, rapid infusion protocols could potentially save hospital resources and limit patient exposure to a high-risk clinical setting while still maintaining ongoing treatment of multiple sclerosis,” the authors wrote.
Under the rapid infusion protocol, patients receive three standard doses for 1 hour followed by 30 minutes of observation. In addition, infusions are reduced to 30 minutes, explained lead author Louise Rath, of clinical neurosciences, Alfred Health, in Melbourne.
“For our cohort of patients, the side effects were minimal,” she said.
“Rapid infusions allowed patients to have option of hospital in-home or office, ensuring work was not at risk by infusion,” she added. “Our governance has been very supportive, and we will be keeping rapid infusion post COVID.”
The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
new studies show.
Collectively, the results suggest the need to rethink the drug’s mandatory 1-hour postinfusion observation period – particularly when unnecessarily spending time in medical settings is discouraged because of concerns regarding COVID-19, the researchers concluded.
Their findings “highlight a potential opportunity to improve and streamline the infusion and postinfusion monitoring process,” reported the authors of one of the studies. The findings were presented at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
Infusion reactions were rare
“In this systematic review of almost 10,000 natalizumab infusions, all infusion-related adverse events were mild, and no clinically relevant safety concerns were associated with natalizumab infusions,” they said.
The 1-hour postinfusion observation period for natalizumab, approved for the treatment of relapsing remitting MS (RRMS), is mandated by the Food and Drug Administration, as well as the European Medicines Agency, and applies to each dose, regardless of treatment duration, owing to concerns of infusion reactions. However, previous evidence has indicated that reactions are rare and are usually mild.
In addition to adding burden to the treatment regimen for patients and providers alike, any extended time in an environment where there is concern of heightened risk for SARS-CoV-2 exposure is a concern.
To evaluate the frequency, severity, and timing of infusion reactions, Yujie Wang, MD, of the department of neurology at the University of Washington, Seattle, and colleagues reviewed medical records of all patients who received natalizumab at the University of Washington MS Center’s infusion suite between July 2012 and September 2020.
Among 333 patients with RRMS, 9,682 infusions of natalizumab were provided over the study period, with a mean of 27 infusions per patient (range, 1-174). The mean age of the patients was 41 years, and 87 (26%) were male.
Overall, 33 infusion-related adverse events were reported in 26 patients, representing 0.34% of total infusions and 7.8% of patients.
In 77% of cases, the adverse event occurred during the infusion. In 92% of cases, the adverse event occurred within the first 6 months of treatment.
All of the events were described as mild. The most common were itching, gastrointestinal problems, headache, and flushing.
None of the reactions required emergency care or hospitalization. Symptoms were either self-managed or were managed easily with standard care. The treatment was continued in all cases.
“For physicians and providers who care for patients with MS and are comfortable with infusible therapies, it is no surprise that rates of clinically significant infusion reactions were low,” Dr. Wang said. “It is indeed consistent with prior studies that reactions generally occur during rather than post infusion.”
The authors underscored the array of potential benefits in making changes to the requirement. “Anticipated benefits may include reducing SARS-CoV2 exposure risks for patients and staff, reducing patients’ treatment burden, increasing efficiency, as well as improving access to care without neglecting patient safety.”
Additional studies show consistent findings
Several other recent studies have shown similar results. In a study published in Multiple Sclerosis in October 2020, researchers with the Amsterdam University Medical Center found that, among 14,174 natalizumab infusions provided to 225 patients with RRMS between 2006 and 2018, 276 infusion-related adverse events occurred (1.95%) among 60 patients.
There were 11 severe infusion-related adverse events in nine patients (4.0%). All documented severe reactions occurred during the infusion. Among 19 moderate adverse events, 17 occurred during the infusion.
The researchers noted that the majority of patients who experienced severe infusion reactions had detectable antibodies against natalizumab. Such antibodies are associated with a higher risk for infusion-related adverse events.
Patients who did not have any symptoms of a reaction during the infusion had no clinically relevant moderate or severe reactions.
“Thus, the need for postinfusion observation will depend on the patients’ clinical status during the infusion,” they wrote. “Consequently, our data suggest that patients who do not have an infusion-related adverse event while receiving natalizumab treatment do not need to stay in the hospital for an additional observation hour.”
Rapid infusion protocol
In another recent study published in Multiple Sclerosis and Related Disorders in January 2021, researchers in Australia reported on the use of a rapid infusion protocol of natalizumab and ocrelizumab. The protocol was implemented to reduce the amount of time patients are required to spend in clinical settings during the COVID-19 pandemic.
In their analysis of 269 rapid infusions of natalizumab and 100 rapid infusions of ocrelizumab, there were two infusion-related reactions in the natalizumab group and eight in the ocrelizumab group.
All the reactions were mild to moderate, and no discontinuations were required. None of the reactions occurred during the postinfusion observation period.
“In the setting of COVID-19 pandemic, rapid infusion protocols could potentially save hospital resources and limit patient exposure to a high-risk clinical setting while still maintaining ongoing treatment of multiple sclerosis,” the authors wrote.
Under the rapid infusion protocol, patients receive three standard doses for 1 hour followed by 30 minutes of observation. In addition, infusions are reduced to 30 minutes, explained lead author Louise Rath, of clinical neurosciences, Alfred Health, in Melbourne.
“For our cohort of patients, the side effects were minimal,” she said.
“Rapid infusions allowed patients to have option of hospital in-home or office, ensuring work was not at risk by infusion,” she added. “Our governance has been very supportive, and we will be keeping rapid infusion post COVID.”
The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ACTRIMS 2021
Docs become dog groomers and warehouse workers after COVID-19 work loss
One of the biggest conundrums of the COVID-19 pandemic has been the simultaneous panic-hiring of medical professionals in hot spots and significant downsizing of staff across the country. From huge hospital systems to private practices, the stoppage of breast reductions and knee replacements, not to mention the drops in motor vehicle accidents and bar fights, have quieted operating rooms and emergency departments and put doctors’ jobs on the chopping block. A widely cited survey suggests that 21% of doctors have had a work reduction due to COVID-19.
For many American doctors, this is their first extended period of unemployment. Unlike engineers or those with MBAs who might see their fortunes rise and fall with the whims of recessions and boom times, physicians are not exactly accustomed to being laid off. However, doctors were already smarting for years due to falling salaries and decreased autonomy, punctuated by endless clicks on electronic medical records software.
Stephanie Eschenbach Morgan, MD, a breast radiologist in North Carolina, trained for 10 years after college before earning a true physician’s salary.
“Being furloughed was awful. Initially, it was only going to be 2 weeks, and then it turned into 2 months with no pay,” she reflected.
Dr. Eschenbach Morgan and her surgeon husband, who lost a full quarter’s salary, had to ask for grace periods on their credit card and mortgage payments because they had paid a large tax bill right before the pandemic began. “We couldn’t get any stimulus help, so that added insult to injury,” she said.
With her time spent waiting in a holding pattern, Dr. Eschenbach Morgan homeschooled her two young children and started putting a home gym together. She went on a home organizing spree, started a garden, and, perhaps most impressively, caught up with 5 years of photo albums.
A bonus she noted: “I didn’t set an alarm for 2 months.”
Shella Farooki, MD, a radiologist in California, was also focused on homeschooling, itself a demanding job, and veered toward retirement. When one of her work contracts furloughed her (“at one point, I made $30K a month for [their business]”), she started saving money at home, teaching the kids, and applied for a Paycheck Protection Program loan. Her husband, a hospitalist, had had his shifts cut. Dr. Farooki tried a radiology artificial intelligence firm but backed out when she was asked to read 9,200 studies for them for $2,000 per month.
Now, she thinks about leaving medicine “every day.”
Some doctors are questioning whether they should be in medicine in the first place. Family medicine physician Jonathan Polak, MD, faced with his own pink slip, turned to pink T-shirts instead. His girlfriend manages an outlet of the teen fashion retailer Justice. Dr. Polak, who finished his residency just 2 years ago, didn’t hesitate to take a $10-an-hour gig as a stock doc, once even finding himself delivering a shelving unit from the shuttering store to a physician fleeing the city for rural New Hampshire to “escape.”
There’s no escape for him – yet. Saddled with “astronomical” student loans, he had considered grocery store work as well. Dr. Polak knows he can’t work part time or go into teaching long term, as he might like.
Even so, he’s doing everything he can to not be in patient care for the long haul – it’s just not what he thought it would be.
“The culture of medicine, bureaucracy, endless paperwork and charting, and threat of litigation sucks a lot of the joy out of it to the point that I don’t see myself doing it forever when imagining myself 5-10 years into it.”
Still, he recently took an 18-month hospital contract that will force him to move to Florida, but he’s also been turning himself into a veritable Renaissance man; composing music, training for an ultramarathon, studying the latest medical findings, roadtripping, and launching a podcast about dog grooming with a master groomer. “We found parallels between medicine and dog grooming,” he says, somewhat convincingly.
Also working the ruff life is Jen Tserng, MD, a former forensic pathologist who landed on news websites in recent years for becoming a professional dogwalker and housesitter without a permanent home. Dr. Tserng knows doctors were restless and unhappy before COVID-19, their thoughts wandering where the grass might be greener.
As her profile grew, she found her inbox gathering messages from disaffected medical minions: students with a fear of failing or staring down residency application season and employed doctors sick of the constant grind. As she recounted those de facto life coach conversations (“What do you really enjoy?” “Do you really like dogs?”) by phone from New York, she said matter-of-factly, “They don’t call because of COVID. They call because they hate their lives.”
Michelle Mudge-Riley, MD, a physician in Texas, has been seeing this shift for some time as well. She recently held a virtual version of her Physicians Helping Physicians conference, where doctors hear from their peers working successfully in fields like pharmaceuticals and real estate investing.
When COVID-19 hit, Dr. Mudge-Riley quickly pivoted to a virtual platform, where the MDs and DOs huddled in breakout rooms having honest chats about their fears and tentative hopes about their new careers.
“There has been increased interest in nonclinical exploration into full- and part-time careers, as well as side hustles, since COVID began,” she said. “Many physicians have had their hours or pay cut, and some have been laid off. Others are furloughed. Some just want out of an environment where they don’t feel safe.”
An ear, nose, and throat surgeon, Maansi Doshi, MD, from central California, didn’t feel safe – so she left. She had returned from India sick with a mystery virus right as the pandemic began (she said her COVID-19 tests were all negative) and was waiting to get well enough to go back to her private practice job. However, she said she clashed with Trump-supporting colleagues she feared might not be taking the pandemic seriously enough.
Finally getting over a relapse of her mystery virus, Dr. Doshi emailed her resignation in May. Her husband, family practice doctor Mark Mangiapane, MD, gave his job notice weeks later in solidarity because he worked in the same building. Together, they have embraced gardening, a Peloton splurge, and learning business skills to open private practices – solo primary care for him; ENT with a focus on her favorite surgery, rhinoplasty, for her.
Dr. Mangiapane had considered editing medical brochures and also tried to apply for a job as a county public health officer in rural California, but he received his own shock when he learned the county intended to open schools in the midst of the pandemic despite advisement to the contrary by the former health officer.
He retreated from job listings altogether after hearing his would-be peers were getting death threats – targeting their children.
Both doctors felt COVID-19 pushed them beyond their comfort zones. “If COVID hadn’t happened, I would be working. ... Be ‘owned.’ In a weird way, COVID made me more independent and take a risk with my career.”
Obstetrician Kwandaa Roberts, MD, certainly did; she took a budding interest in decorating dollhouses straight to Instagram and national news fame, and she is now a TV-show expert on “Sell This House.”
Like Dr. Doshi and Dr. Mangiapane, Dr. Polak wants to be more in control of his future – even if selling T-shirts at a mall means a certain loss of status along the way.
“Aside from my passion to learn and to have that connection with people, I went into medicine ... because of the job security I thought existed,” he said. “I would say that my getting furloughed has changed my view of the United States in a dramatic way. I do not feel as confident in the U.S. economy and general way of life as I did a year ago. And I am taking a number of steps to put myself in a more fluid, adaptable position in case another crisis like this occurs or if the current state of things worsens.”
A version of this article first appeared on Medscape.com.
One of the biggest conundrums of the COVID-19 pandemic has been the simultaneous panic-hiring of medical professionals in hot spots and significant downsizing of staff across the country. From huge hospital systems to private practices, the stoppage of breast reductions and knee replacements, not to mention the drops in motor vehicle accidents and bar fights, have quieted operating rooms and emergency departments and put doctors’ jobs on the chopping block. A widely cited survey suggests that 21% of doctors have had a work reduction due to COVID-19.
For many American doctors, this is their first extended period of unemployment. Unlike engineers or those with MBAs who might see their fortunes rise and fall with the whims of recessions and boom times, physicians are not exactly accustomed to being laid off. However, doctors were already smarting for years due to falling salaries and decreased autonomy, punctuated by endless clicks on electronic medical records software.
Stephanie Eschenbach Morgan, MD, a breast radiologist in North Carolina, trained for 10 years after college before earning a true physician’s salary.
“Being furloughed was awful. Initially, it was only going to be 2 weeks, and then it turned into 2 months with no pay,” she reflected.
Dr. Eschenbach Morgan and her surgeon husband, who lost a full quarter’s salary, had to ask for grace periods on their credit card and mortgage payments because they had paid a large tax bill right before the pandemic began. “We couldn’t get any stimulus help, so that added insult to injury,” she said.
With her time spent waiting in a holding pattern, Dr. Eschenbach Morgan homeschooled her two young children and started putting a home gym together. She went on a home organizing spree, started a garden, and, perhaps most impressively, caught up with 5 years of photo albums.
A bonus she noted: “I didn’t set an alarm for 2 months.”
Shella Farooki, MD, a radiologist in California, was also focused on homeschooling, itself a demanding job, and veered toward retirement. When one of her work contracts furloughed her (“at one point, I made $30K a month for [their business]”), she started saving money at home, teaching the kids, and applied for a Paycheck Protection Program loan. Her husband, a hospitalist, had had his shifts cut. Dr. Farooki tried a radiology artificial intelligence firm but backed out when she was asked to read 9,200 studies for them for $2,000 per month.
Now, she thinks about leaving medicine “every day.”
Some doctors are questioning whether they should be in medicine in the first place. Family medicine physician Jonathan Polak, MD, faced with his own pink slip, turned to pink T-shirts instead. His girlfriend manages an outlet of the teen fashion retailer Justice. Dr. Polak, who finished his residency just 2 years ago, didn’t hesitate to take a $10-an-hour gig as a stock doc, once even finding himself delivering a shelving unit from the shuttering store to a physician fleeing the city for rural New Hampshire to “escape.”
There’s no escape for him – yet. Saddled with “astronomical” student loans, he had considered grocery store work as well. Dr. Polak knows he can’t work part time or go into teaching long term, as he might like.
Even so, he’s doing everything he can to not be in patient care for the long haul – it’s just not what he thought it would be.
“The culture of medicine, bureaucracy, endless paperwork and charting, and threat of litigation sucks a lot of the joy out of it to the point that I don’t see myself doing it forever when imagining myself 5-10 years into it.”
Still, he recently took an 18-month hospital contract that will force him to move to Florida, but he’s also been turning himself into a veritable Renaissance man; composing music, training for an ultramarathon, studying the latest medical findings, roadtripping, and launching a podcast about dog grooming with a master groomer. “We found parallels between medicine and dog grooming,” he says, somewhat convincingly.
Also working the ruff life is Jen Tserng, MD, a former forensic pathologist who landed on news websites in recent years for becoming a professional dogwalker and housesitter without a permanent home. Dr. Tserng knows doctors were restless and unhappy before COVID-19, their thoughts wandering where the grass might be greener.
As her profile grew, she found her inbox gathering messages from disaffected medical minions: students with a fear of failing or staring down residency application season and employed doctors sick of the constant grind. As she recounted those de facto life coach conversations (“What do you really enjoy?” “Do you really like dogs?”) by phone from New York, she said matter-of-factly, “They don’t call because of COVID. They call because they hate their lives.”
Michelle Mudge-Riley, MD, a physician in Texas, has been seeing this shift for some time as well. She recently held a virtual version of her Physicians Helping Physicians conference, where doctors hear from their peers working successfully in fields like pharmaceuticals and real estate investing.
When COVID-19 hit, Dr. Mudge-Riley quickly pivoted to a virtual platform, where the MDs and DOs huddled in breakout rooms having honest chats about their fears and tentative hopes about their new careers.
“There has been increased interest in nonclinical exploration into full- and part-time careers, as well as side hustles, since COVID began,” she said. “Many physicians have had their hours or pay cut, and some have been laid off. Others are furloughed. Some just want out of an environment where they don’t feel safe.”
An ear, nose, and throat surgeon, Maansi Doshi, MD, from central California, didn’t feel safe – so she left. She had returned from India sick with a mystery virus right as the pandemic began (she said her COVID-19 tests were all negative) and was waiting to get well enough to go back to her private practice job. However, she said she clashed with Trump-supporting colleagues she feared might not be taking the pandemic seriously enough.
Finally getting over a relapse of her mystery virus, Dr. Doshi emailed her resignation in May. Her husband, family practice doctor Mark Mangiapane, MD, gave his job notice weeks later in solidarity because he worked in the same building. Together, they have embraced gardening, a Peloton splurge, and learning business skills to open private practices – solo primary care for him; ENT with a focus on her favorite surgery, rhinoplasty, for her.
Dr. Mangiapane had considered editing medical brochures and also tried to apply for a job as a county public health officer in rural California, but he received his own shock when he learned the county intended to open schools in the midst of the pandemic despite advisement to the contrary by the former health officer.
He retreated from job listings altogether after hearing his would-be peers were getting death threats – targeting their children.
Both doctors felt COVID-19 pushed them beyond their comfort zones. “If COVID hadn’t happened, I would be working. ... Be ‘owned.’ In a weird way, COVID made me more independent and take a risk with my career.”
Obstetrician Kwandaa Roberts, MD, certainly did; she took a budding interest in decorating dollhouses straight to Instagram and national news fame, and she is now a TV-show expert on “Sell This House.”
Like Dr. Doshi and Dr. Mangiapane, Dr. Polak wants to be more in control of his future – even if selling T-shirts at a mall means a certain loss of status along the way.
“Aside from my passion to learn and to have that connection with people, I went into medicine ... because of the job security I thought existed,” he said. “I would say that my getting furloughed has changed my view of the United States in a dramatic way. I do not feel as confident in the U.S. economy and general way of life as I did a year ago. And I am taking a number of steps to put myself in a more fluid, adaptable position in case another crisis like this occurs or if the current state of things worsens.”
A version of this article first appeared on Medscape.com.
One of the biggest conundrums of the COVID-19 pandemic has been the simultaneous panic-hiring of medical professionals in hot spots and significant downsizing of staff across the country. From huge hospital systems to private practices, the stoppage of breast reductions and knee replacements, not to mention the drops in motor vehicle accidents and bar fights, have quieted operating rooms and emergency departments and put doctors’ jobs on the chopping block. A widely cited survey suggests that 21% of doctors have had a work reduction due to COVID-19.
For many American doctors, this is their first extended period of unemployment. Unlike engineers or those with MBAs who might see their fortunes rise and fall with the whims of recessions and boom times, physicians are not exactly accustomed to being laid off. However, doctors were already smarting for years due to falling salaries and decreased autonomy, punctuated by endless clicks on electronic medical records software.
Stephanie Eschenbach Morgan, MD, a breast radiologist in North Carolina, trained for 10 years after college before earning a true physician’s salary.
“Being furloughed was awful. Initially, it was only going to be 2 weeks, and then it turned into 2 months with no pay,” she reflected.
Dr. Eschenbach Morgan and her surgeon husband, who lost a full quarter’s salary, had to ask for grace periods on their credit card and mortgage payments because they had paid a large tax bill right before the pandemic began. “We couldn’t get any stimulus help, so that added insult to injury,” she said.
With her time spent waiting in a holding pattern, Dr. Eschenbach Morgan homeschooled her two young children and started putting a home gym together. She went on a home organizing spree, started a garden, and, perhaps most impressively, caught up with 5 years of photo albums.
A bonus she noted: “I didn’t set an alarm for 2 months.”
Shella Farooki, MD, a radiologist in California, was also focused on homeschooling, itself a demanding job, and veered toward retirement. When one of her work contracts furloughed her (“at one point, I made $30K a month for [their business]”), she started saving money at home, teaching the kids, and applied for a Paycheck Protection Program loan. Her husband, a hospitalist, had had his shifts cut. Dr. Farooki tried a radiology artificial intelligence firm but backed out when she was asked to read 9,200 studies for them for $2,000 per month.
Now, she thinks about leaving medicine “every day.”
Some doctors are questioning whether they should be in medicine in the first place. Family medicine physician Jonathan Polak, MD, faced with his own pink slip, turned to pink T-shirts instead. His girlfriend manages an outlet of the teen fashion retailer Justice. Dr. Polak, who finished his residency just 2 years ago, didn’t hesitate to take a $10-an-hour gig as a stock doc, once even finding himself delivering a shelving unit from the shuttering store to a physician fleeing the city for rural New Hampshire to “escape.”
There’s no escape for him – yet. Saddled with “astronomical” student loans, he had considered grocery store work as well. Dr. Polak knows he can’t work part time or go into teaching long term, as he might like.
Even so, he’s doing everything he can to not be in patient care for the long haul – it’s just not what he thought it would be.
“The culture of medicine, bureaucracy, endless paperwork and charting, and threat of litigation sucks a lot of the joy out of it to the point that I don’t see myself doing it forever when imagining myself 5-10 years into it.”
Still, he recently took an 18-month hospital contract that will force him to move to Florida, but he’s also been turning himself into a veritable Renaissance man; composing music, training for an ultramarathon, studying the latest medical findings, roadtripping, and launching a podcast about dog grooming with a master groomer. “We found parallels between medicine and dog grooming,” he says, somewhat convincingly.
Also working the ruff life is Jen Tserng, MD, a former forensic pathologist who landed on news websites in recent years for becoming a professional dogwalker and housesitter without a permanent home. Dr. Tserng knows doctors were restless and unhappy before COVID-19, their thoughts wandering where the grass might be greener.
As her profile grew, she found her inbox gathering messages from disaffected medical minions: students with a fear of failing or staring down residency application season and employed doctors sick of the constant grind. As she recounted those de facto life coach conversations (“What do you really enjoy?” “Do you really like dogs?”) by phone from New York, she said matter-of-factly, “They don’t call because of COVID. They call because they hate their lives.”
Michelle Mudge-Riley, MD, a physician in Texas, has been seeing this shift for some time as well. She recently held a virtual version of her Physicians Helping Physicians conference, where doctors hear from their peers working successfully in fields like pharmaceuticals and real estate investing.
When COVID-19 hit, Dr. Mudge-Riley quickly pivoted to a virtual platform, where the MDs and DOs huddled in breakout rooms having honest chats about their fears and tentative hopes about their new careers.
“There has been increased interest in nonclinical exploration into full- and part-time careers, as well as side hustles, since COVID began,” she said. “Many physicians have had their hours or pay cut, and some have been laid off. Others are furloughed. Some just want out of an environment where they don’t feel safe.”
An ear, nose, and throat surgeon, Maansi Doshi, MD, from central California, didn’t feel safe – so she left. She had returned from India sick with a mystery virus right as the pandemic began (she said her COVID-19 tests were all negative) and was waiting to get well enough to go back to her private practice job. However, she said she clashed with Trump-supporting colleagues she feared might not be taking the pandemic seriously enough.
Finally getting over a relapse of her mystery virus, Dr. Doshi emailed her resignation in May. Her husband, family practice doctor Mark Mangiapane, MD, gave his job notice weeks later in solidarity because he worked in the same building. Together, they have embraced gardening, a Peloton splurge, and learning business skills to open private practices – solo primary care for him; ENT with a focus on her favorite surgery, rhinoplasty, for her.
Dr. Mangiapane had considered editing medical brochures and also tried to apply for a job as a county public health officer in rural California, but he received his own shock when he learned the county intended to open schools in the midst of the pandemic despite advisement to the contrary by the former health officer.
He retreated from job listings altogether after hearing his would-be peers were getting death threats – targeting their children.
Both doctors felt COVID-19 pushed them beyond their comfort zones. “If COVID hadn’t happened, I would be working. ... Be ‘owned.’ In a weird way, COVID made me more independent and take a risk with my career.”
Obstetrician Kwandaa Roberts, MD, certainly did; she took a budding interest in decorating dollhouses straight to Instagram and national news fame, and she is now a TV-show expert on “Sell This House.”
Like Dr. Doshi and Dr. Mangiapane, Dr. Polak wants to be more in control of his future – even if selling T-shirts at a mall means a certain loss of status along the way.
“Aside from my passion to learn and to have that connection with people, I went into medicine ... because of the job security I thought existed,” he said. “I would say that my getting furloughed has changed my view of the United States in a dramatic way. I do not feel as confident in the U.S. economy and general way of life as I did a year ago. And I am taking a number of steps to put myself in a more fluid, adaptable position in case another crisis like this occurs or if the current state of things worsens.”
A version of this article first appeared on Medscape.com.
Novel oral agent effective in teens with atopic dermatitis
Abrocitinib, an investigational drug proven to be a safe and effective treatment for moderate to severe atopic dermatitis (AD) in adults 18 years and older, is also safe and effective in patients aged 12-17 years, according to a randomized trial of the oral, once-daily Janus kinase (JAK) 1 selective inhibitor, used in combination with medicated topical therapy.
The results, from the phase 3 JADE TEEN study, were presented during an oral abstract session at the annual meeting of the American Academy of Allergy, Asthma, and Immunology, held virtually this year.
“We’re very excited about the introduction of oral JAKs into our armamentarium for atopic dermatitis,” lead author Lawrence Eichenfield, MD, professor of dermatology and pediatrics, University of California, San Diego, and chief of pediatric and adolescent dermatology, Rady Children’s Hospital, also in San Diego, said in an interview.
AD ranges in severity, and there is a great deal of moderate to severe AD that has a tremendous negative impact on the individual, Dr. Eichenfield said. “Traditionally we have treated it with intermittent topical corticosteroids, but this has left a significant percentage of patients without long-term disease control.”
JAK inhibitors are effective mediators of the inflammation response that occurs in moderate to severe AD. They inhibit the stimulation of the JAK pathway and allow anti-inflammatory effects and therefore have potential, especially in more severe disease, Dr. Eichenfield said.
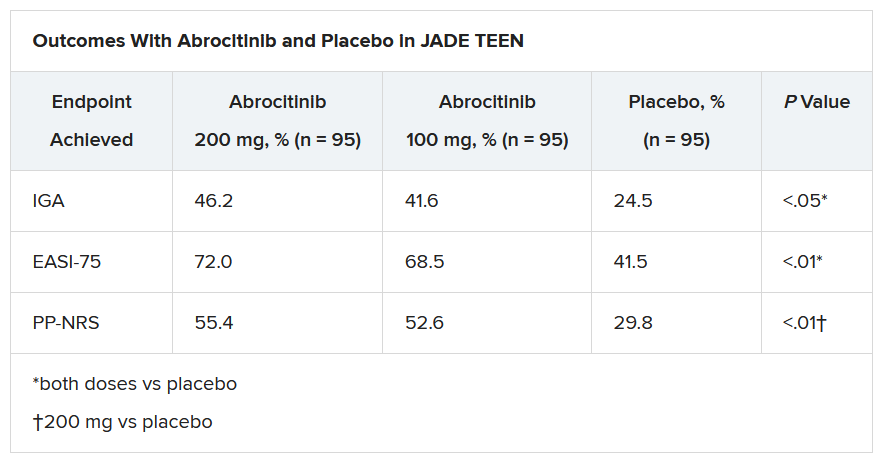
In the current study, which is a spin-off of the original study that looked at abrocitinib in adults, he and his team randomly assigned 285 teens (mean age, 14.9 years; 50.9% male; 56.1% White) with moderate to severe AD to receive one of the following treatments for 12 weeks: abrocitinib 200 mg plus topical therapy (95); abrocitinib 100 mg plus topical therapy (95); or placebo, which consisted of topical therapy alone (95).
The primary endpoints were an Investigator’s Global Assessment response of clear or almost clear (scores of 0 and 1, respectively), with an improvement of at least 2 points, and an improvement in Eczema Area and Severity Index score of at least 75% at week 12.
Secondary endpoints included an improvement in Peak Pruritus Numerical Rating Scale (PP-NRS) response of at least 4 points at week 12.
The teens who received abrocitinib along with medicated topical therapy showed significant improvement in the severity of their AD at the end of the 12-week period, compared with those in the placebo group.
“The percentage of patients achieving essentially no itch, as captured in the fact that more than half of those on the higher dose of abrocitinib made it to no itch, is a new data point and is important to note,” Dr. Eichenfield said. “A lot of the other medicines don’t really get a significant percentage of the population to an itch score of 0 to 1. This drug brought about a rapid and profound itch relief.”
He added: “The results from JADE TEEN extend the drug’s utility in this younger population and show that abrocitinib performs the same with regard to efficacy and safety in the teenagers. Having atopic dermatitis that does not respond to treatment is especially hard for adolescents, but now we know that abrocitinib will be safe and effective and so we now have something to offer these kids.”
“Abrocitinib achieved a good response in this study that was statistically significant, compared to standard treatment,” Jonathan A. Bernstein, MD, professor of medicine at the University of Cincinnati, commented in an interview.
“JAK inhibitors are very promising, and this study adds to that promise. They play an important role in atopic dermatitis, so obviously, teenagers with AD represent an important population,” said Dr. Bernstein, who was not part of the study. “These results are very encouraging, and I think that we will probably see some of these JAK inhibitors approved by the FDA, if not this year, probably next.”
The study was sponsored by Pfizer. Dr. Eichenfield serves as an investigator, speaker, and consultant for Pfizer; and as an investigator, speaker, consultant, and/or is on a data safety monitoring board for AbbVie, Almirall, Amgen, Arcutis, Asana, Dermavant, Dermira, Forte, Galderma, Ichnos/Glenmark, Incyte, LEO, Lilly, L’Oreal, Novartis, Regeneron, Sanofi-Genzyme, and Verrica. Dr. Bernstein disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Abrocitinib, an investigational drug proven to be a safe and effective treatment for moderate to severe atopic dermatitis (AD) in adults 18 years and older, is also safe and effective in patients aged 12-17 years, according to a randomized trial of the oral, once-daily Janus kinase (JAK) 1 selective inhibitor, used in combination with medicated topical therapy.
The results, from the phase 3 JADE TEEN study, were presented during an oral abstract session at the annual meeting of the American Academy of Allergy, Asthma, and Immunology, held virtually this year.
“We’re very excited about the introduction of oral JAKs into our armamentarium for atopic dermatitis,” lead author Lawrence Eichenfield, MD, professor of dermatology and pediatrics, University of California, San Diego, and chief of pediatric and adolescent dermatology, Rady Children’s Hospital, also in San Diego, said in an interview.
AD ranges in severity, and there is a great deal of moderate to severe AD that has a tremendous negative impact on the individual, Dr. Eichenfield said. “Traditionally we have treated it with intermittent topical corticosteroids, but this has left a significant percentage of patients without long-term disease control.”
JAK inhibitors are effective mediators of the inflammation response that occurs in moderate to severe AD. They inhibit the stimulation of the JAK pathway and allow anti-inflammatory effects and therefore have potential, especially in more severe disease, Dr. Eichenfield said.
In the current study, which is a spin-off of the original study that looked at abrocitinib in adults, he and his team randomly assigned 285 teens (mean age, 14.9 years; 50.9% male; 56.1% White) with moderate to severe AD to receive one of the following treatments for 12 weeks: abrocitinib 200 mg plus topical therapy (95); abrocitinib 100 mg plus topical therapy (95); or placebo, which consisted of topical therapy alone (95).
The primary endpoints were an Investigator’s Global Assessment response of clear or almost clear (scores of 0 and 1, respectively), with an improvement of at least 2 points, and an improvement in Eczema Area and Severity Index score of at least 75% at week 12.
Secondary endpoints included an improvement in Peak Pruritus Numerical Rating Scale (PP-NRS) response of at least 4 points at week 12.
The teens who received abrocitinib along with medicated topical therapy showed significant improvement in the severity of their AD at the end of the 12-week period, compared with those in the placebo group.
“The percentage of patients achieving essentially no itch, as captured in the fact that more than half of those on the higher dose of abrocitinib made it to no itch, is a new data point and is important to note,” Dr. Eichenfield said. “A lot of the other medicines don’t really get a significant percentage of the population to an itch score of 0 to 1. This drug brought about a rapid and profound itch relief.”
He added: “The results from JADE TEEN extend the drug’s utility in this younger population and show that abrocitinib performs the same with regard to efficacy and safety in the teenagers. Having atopic dermatitis that does not respond to treatment is especially hard for adolescents, but now we know that abrocitinib will be safe and effective and so we now have something to offer these kids.”
“Abrocitinib achieved a good response in this study that was statistically significant, compared to standard treatment,” Jonathan A. Bernstein, MD, professor of medicine at the University of Cincinnati, commented in an interview.
“JAK inhibitors are very promising, and this study adds to that promise. They play an important role in atopic dermatitis, so obviously, teenagers with AD represent an important population,” said Dr. Bernstein, who was not part of the study. “These results are very encouraging, and I think that we will probably see some of these JAK inhibitors approved by the FDA, if not this year, probably next.”
The study was sponsored by Pfizer. Dr. Eichenfield serves as an investigator, speaker, and consultant for Pfizer; and as an investigator, speaker, consultant, and/or is on a data safety monitoring board for AbbVie, Almirall, Amgen, Arcutis, Asana, Dermavant, Dermira, Forte, Galderma, Ichnos/Glenmark, Incyte, LEO, Lilly, L’Oreal, Novartis, Regeneron, Sanofi-Genzyme, and Verrica. Dr. Bernstein disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Abrocitinib, an investigational drug proven to be a safe and effective treatment for moderate to severe atopic dermatitis (AD) in adults 18 years and older, is also safe and effective in patients aged 12-17 years, according to a randomized trial of the oral, once-daily Janus kinase (JAK) 1 selective inhibitor, used in combination with medicated topical therapy.
The results, from the phase 3 JADE TEEN study, were presented during an oral abstract session at the annual meeting of the American Academy of Allergy, Asthma, and Immunology, held virtually this year.
“We’re very excited about the introduction of oral JAKs into our armamentarium for atopic dermatitis,” lead author Lawrence Eichenfield, MD, professor of dermatology and pediatrics, University of California, San Diego, and chief of pediatric and adolescent dermatology, Rady Children’s Hospital, also in San Diego, said in an interview.
AD ranges in severity, and there is a great deal of moderate to severe AD that has a tremendous negative impact on the individual, Dr. Eichenfield said. “Traditionally we have treated it with intermittent topical corticosteroids, but this has left a significant percentage of patients without long-term disease control.”
JAK inhibitors are effective mediators of the inflammation response that occurs in moderate to severe AD. They inhibit the stimulation of the JAK pathway and allow anti-inflammatory effects and therefore have potential, especially in more severe disease, Dr. Eichenfield said.
In the current study, which is a spin-off of the original study that looked at abrocitinib in adults, he and his team randomly assigned 285 teens (mean age, 14.9 years; 50.9% male; 56.1% White) with moderate to severe AD to receive one of the following treatments for 12 weeks: abrocitinib 200 mg plus topical therapy (95); abrocitinib 100 mg plus topical therapy (95); or placebo, which consisted of topical therapy alone (95).
The primary endpoints were an Investigator’s Global Assessment response of clear or almost clear (scores of 0 and 1, respectively), with an improvement of at least 2 points, and an improvement in Eczema Area and Severity Index score of at least 75% at week 12.
Secondary endpoints included an improvement in Peak Pruritus Numerical Rating Scale (PP-NRS) response of at least 4 points at week 12.
The teens who received abrocitinib along with medicated topical therapy showed significant improvement in the severity of their AD at the end of the 12-week period, compared with those in the placebo group.
“The percentage of patients achieving essentially no itch, as captured in the fact that more than half of those on the higher dose of abrocitinib made it to no itch, is a new data point and is important to note,” Dr. Eichenfield said. “A lot of the other medicines don’t really get a significant percentage of the population to an itch score of 0 to 1. This drug brought about a rapid and profound itch relief.”
He added: “The results from JADE TEEN extend the drug’s utility in this younger population and show that abrocitinib performs the same with regard to efficacy and safety in the teenagers. Having atopic dermatitis that does not respond to treatment is especially hard for adolescents, but now we know that abrocitinib will be safe and effective and so we now have something to offer these kids.”
“Abrocitinib achieved a good response in this study that was statistically significant, compared to standard treatment,” Jonathan A. Bernstein, MD, professor of medicine at the University of Cincinnati, commented in an interview.
“JAK inhibitors are very promising, and this study adds to that promise. They play an important role in atopic dermatitis, so obviously, teenagers with AD represent an important population,” said Dr. Bernstein, who was not part of the study. “These results are very encouraging, and I think that we will probably see some of these JAK inhibitors approved by the FDA, if not this year, probably next.”
The study was sponsored by Pfizer. Dr. Eichenfield serves as an investigator, speaker, and consultant for Pfizer; and as an investigator, speaker, consultant, and/or is on a data safety monitoring board for AbbVie, Almirall, Amgen, Arcutis, Asana, Dermavant, Dermira, Forte, Galderma, Ichnos/Glenmark, Incyte, LEO, Lilly, L’Oreal, Novartis, Regeneron, Sanofi-Genzyme, and Verrica. Dr. Bernstein disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
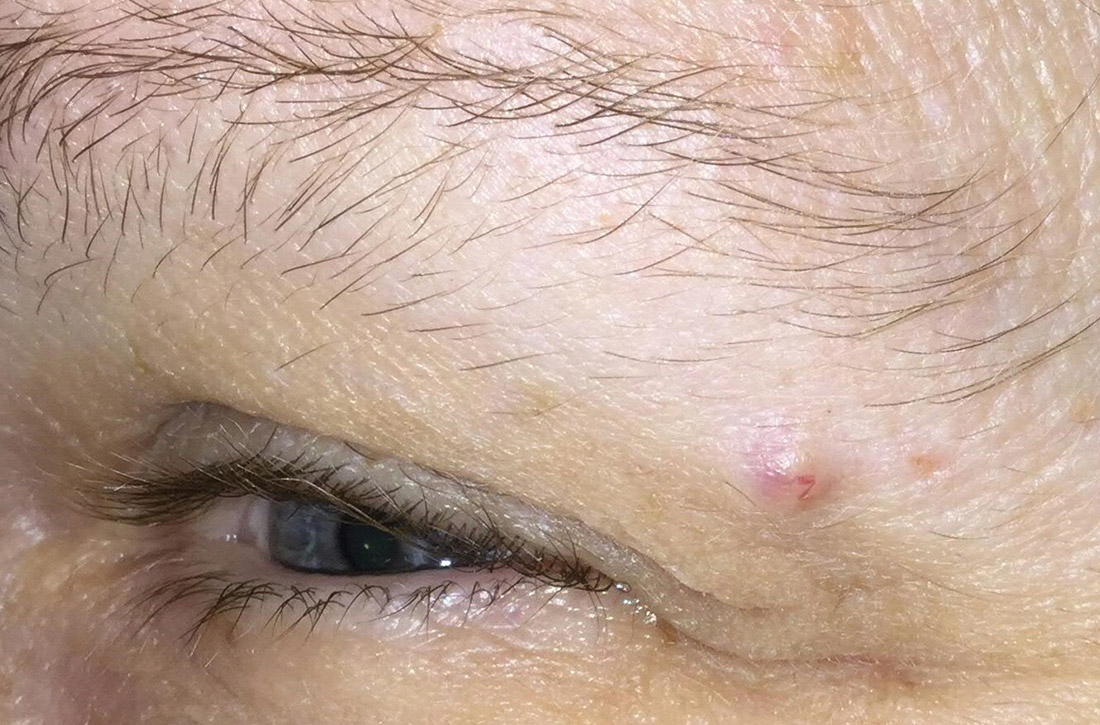
Slow-growing lesion on eyebrow
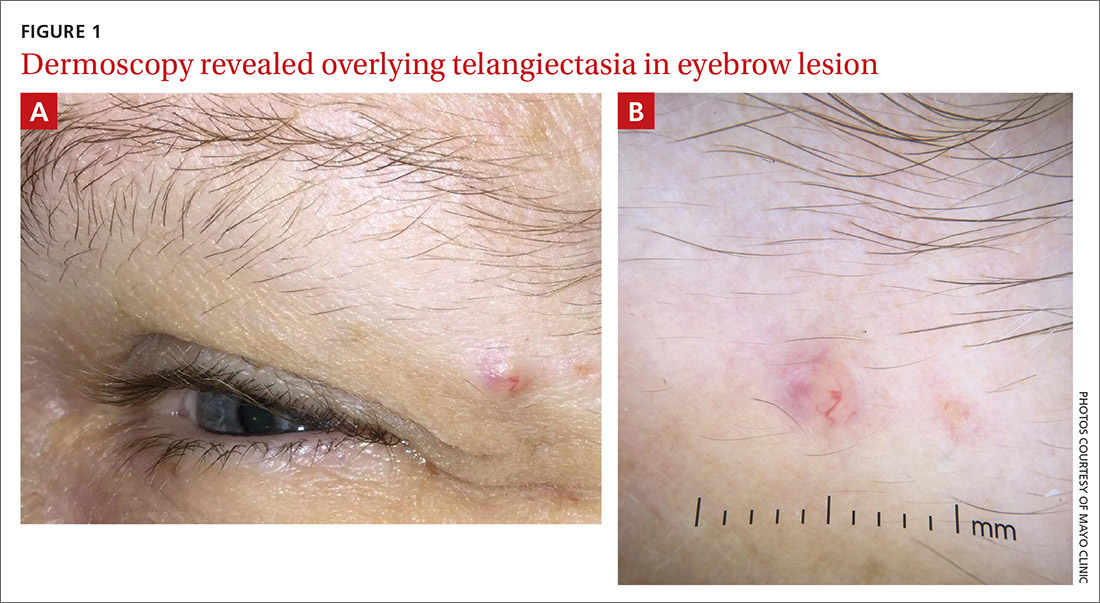
A 51-year-old woman presented to the family medicine clinic for evaluation of a slightly tender skin lesion on her left eyebrow. The lesion had been slowly growing for a year.
The patient’s family history included multiple family members with colon or breast cancer and other relatives with pancreatic and prostate cancer. A colonoscopy performed a year earlier on the patient was negative. The patient’s past medical history included hypertension, major depressive disorder, hyperlipidemia, and venous insufficiency. She also had a colon polyp history.
Physical examination of the eyebrow showed a 3-mm papule that was firm on palpation. Dermoscopy of the lesion revealed a yellow papule with
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Sebaceous carcinoma
A rapid teledermatology consultation helped us to determine that this was a sebaceous lesion, but its location and the overlying telangiectasia raised concerns for malignancy. After shared decision-making with the patient, she agreed to proceed with a biopsy. We first made an incision into the lesion, which was hard, demonstrating that it was not cystic. A shave biopsy was then completed. The dermatopathology findings showed clear-cell change consisting of bubbly or foamy cytoplasm, with scalloping of the nuclei, which is characteristic of a sebaceous origin. There were tumor cells that were enlarged with pleomorphism, multiple nucleoli, and scattered mitotic figures. These findings pointed to a diagnosis of sebaceous carcinoma.
Sebaceous carcinomas most commonly manifest on the eyelids. They can originate from the Meibomian glands as well as from pilosebaceous glands at other sites on the body.1 They are rare, accounting for only 1% to 5% of eyelid malignancies, and occur in approximately 2 per 1 million people.1 Tumors can invade locally and metastasize, particularly to surrounding lymph nodes. Periocular pathology may sometimes lead to misdiagnosis, which contributes to a mortality rate that has been reported as high as 20%.1 Suspicion for malignancy may arise due to ulceration, bleeding, pain, or rapid growth.
A lesson in considering the full differential
While sebaceous lesions on the eyelid and eyebrow are often benign, this case underscored the importance of considering the more worrisome elements in the differential. The differential diagnosis for lesions in the area of the eye include the following:
Sebaceous hyperplasia is a common condition (typically among older patients) in which sebaceous glands increase in size and number.2 The classic clinical feature is yellow or skin-colored papules. The lesions typically manifest on the face—particularly on the forehead. They are benign and often have a central umbilication.2
Sebaceous adenomas are benign tumors that may manifest as tan, skin-colored, pink, or yellow papules or nodules.2 The lesions are usually asymptomatic, small, and slow growing.2
Continue to: Basal and squamous cell carcinomas
Basal and squamous cell carcinomas. Basal cell carcinomas often feature translucent lesions on areas of the skin that are exposed to sunlight. These lesions often have slightly rolled border edges or overlying branching telangiectasia and may be nodular.3 Squamous cell carcinomas often feature scaled, reddened patches that may become tender and ulcerate.4
Hordeolums and chalazions. A hordeolum (or stye) is a painful, acute, localized swelling of the eyelid.5 These often develop externally at the lid margin from infection of the follicle. A chalazion is characterized by a persistent, nontender mass that results from small, noninfectious obstruction of the Meibomian glands with secondary granulomatous inflammation.5
Dermoscopy can (and did) help with the Dx
Dermoscopy can help confirm whether a lesion has a sebaceous origin because it would show yellow globules with “crown vessel” telangiectasias that classically do not cross midline.6 Unfortunately, the findings of yellow globules and dermal vessels do not adequately differentiate benign from malignant lesions.6 Carcinomas can manifest in an undifferentiated way early in their course.
Sebaceous carcinomas can be associated with the autosomal dominant Muir-Torre syndrome, a subset of the Lynch syndrome.7,8 Colorectal and genitourinary carcinomas are the most common internal malignancies seen in patients with Muir-Torre syndrome.9
Patients benefit from Mohs surgery
Treatment outcomes for sebaceous carcinoma appear to be improved by Mohs surgery. In a recent review of 1265 patients with early-stage sebaceous carcinomas, Su et al found that 234 patients who were treated with Mohs surgery had improved overall survival, compared with 1031 who were treated with surgical excision.10
Continue to: Our patient
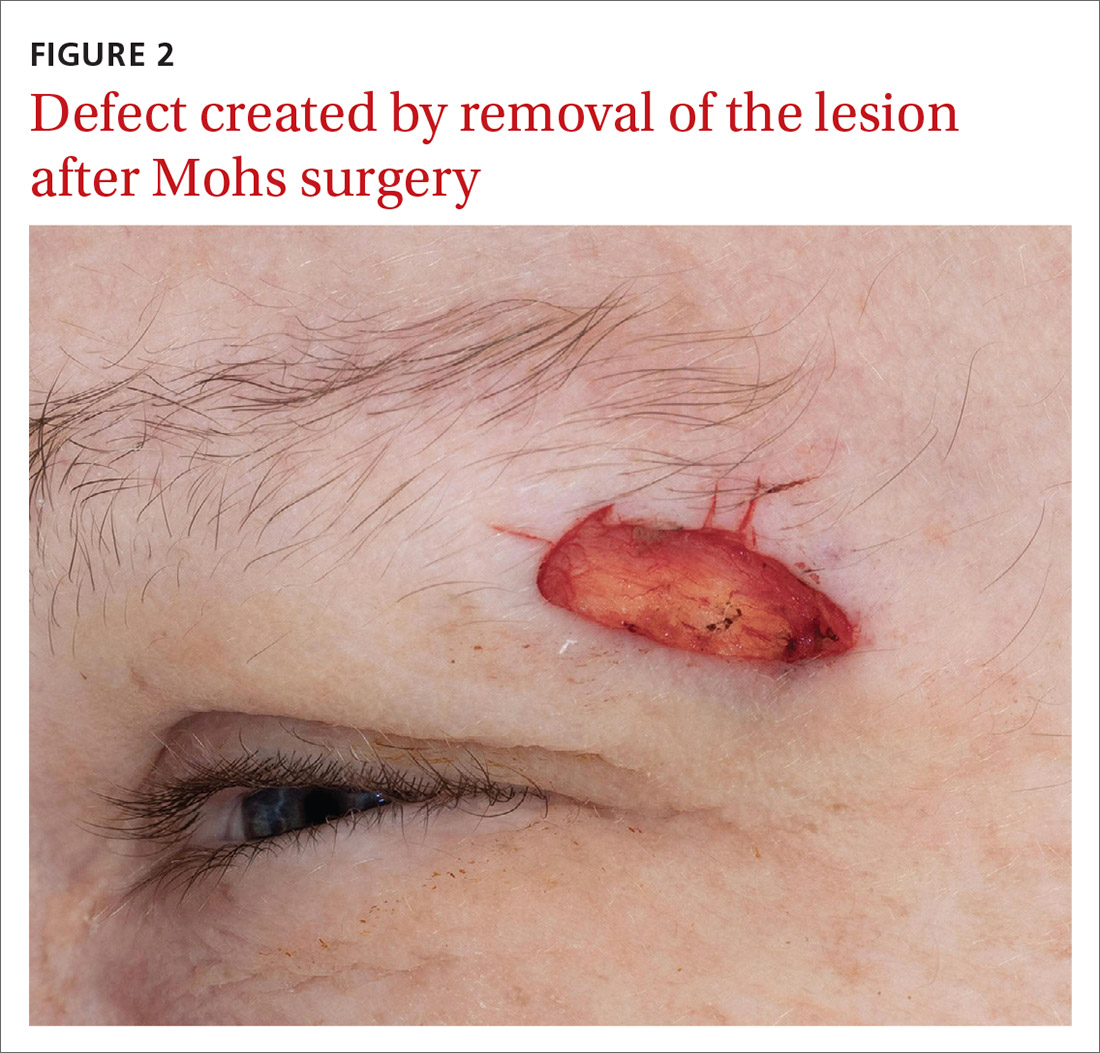
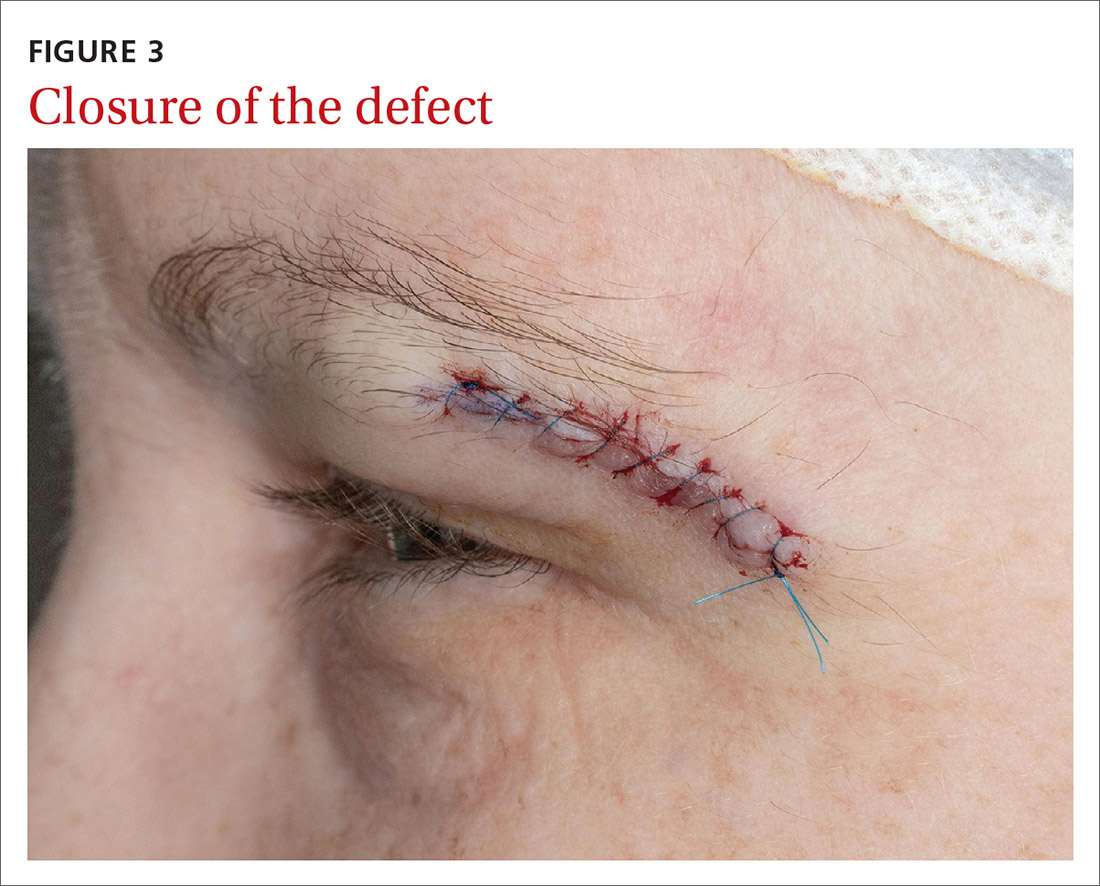
Our patient was referred to a Mohs surgeon who removed the lesion (FIGURES 2 and 3). Given the overall small tumor size, a sentinel lymph node biopsy was not necessary. Because of the patient’s family history, which was suggestive of a genetic predisposition to cancer, she requested a clinical genetics consultation for definitive testing. She went on to pursue genetic testing, which came back negative for Lynch syndrome genes.
The dermatologist recommended yearly skin examination for 5 years for the patient.
1. Kahana A, Pribila HT, Nelson CC, et al. Sebaceous cell carcinoma. In: Levin LA, Albert DM, eds. Ocular Disease: Mechanisms and Management. Saunders/Elsevier; 2010:396-407.
2. Iacobelli J, Harvey NT, Wood BA. Sebaceous lesions of the skin. Pathology. 2017;49:688-697.
3. Marzuka AG, Book SE. Basal cell carcinoma: pathogenesis, epidemiology, clinical features, diagnosis, histopathology, and management. Yale J Biol Med. 2015;88:167-179.
4. Smith H, Patel A. When to suspect a non-melanoma skin cancer. BMJ. 2020;368:m692.
5. Sun MT, Huang S, Huilgol SC, et al. Eyelid lesions in general practice. Aust J Gen Pract. 2019;48:509-514.
6. Kim NH, Zell DS, Kolm I, et al. The dermoscopic differential diagnosis of yellow lobularlike structures. Arch Dermatol. 2008;144:962.
7. EG, Bell AJY, Barlow KA. Multiple primary carcinomata of the colon, duodenum, and larynx associated with kerato-acanthomata of the face. Br J Surg. 1967;54:191-195.
8. Torre D. Multiple sebaceous tumors. Arch Dermatol. 1968;98:549-55.
9. Cohen PR, Kohn SR, Kurzrock R. Association of sebaceous gland tumors and internal malignancy: the Muir-Torre syndrome. Am J Med. 1991;90:606-613.
10. Su C, Nguyen KA, Bai HX, et al. Comparison of Mohs surgery and surgical excision in the treatment of localized sebaceous carcinoma. Dermatol Surg. 2019;45:1125-1135.
A 51-year-old woman presented to the family medicine clinic for evaluation of a slightly tender skin lesion on her left eyebrow. The lesion had been slowly growing for a year.
The patient’s family history included multiple family members with colon or breast cancer and other relatives with pancreatic and prostate cancer. A colonoscopy performed a year earlier on the patient was negative. The patient’s past medical history included hypertension, major depressive disorder, hyperlipidemia, and venous insufficiency. She also had a colon polyp history.
Physical examination of the eyebrow showed a 3-mm papule that was firm on palpation. Dermoscopy of the lesion revealed a yellow papule with
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Sebaceous carcinoma
A rapid teledermatology consultation helped us to determine that this was a sebaceous lesion, but its location and the overlying telangiectasia raised concerns for malignancy. After shared decision-making with the patient, she agreed to proceed with a biopsy. We first made an incision into the lesion, which was hard, demonstrating that it was not cystic. A shave biopsy was then completed. The dermatopathology findings showed clear-cell change consisting of bubbly or foamy cytoplasm, with scalloping of the nuclei, which is characteristic of a sebaceous origin. There were tumor cells that were enlarged with pleomorphism, multiple nucleoli, and scattered mitotic figures. These findings pointed to a diagnosis of sebaceous carcinoma.
Sebaceous carcinomas most commonly manifest on the eyelids. They can originate from the Meibomian glands as well as from pilosebaceous glands at other sites on the body.1 They are rare, accounting for only 1% to 5% of eyelid malignancies, and occur in approximately 2 per 1 million people.1 Tumors can invade locally and metastasize, particularly to surrounding lymph nodes. Periocular pathology may sometimes lead to misdiagnosis, which contributes to a mortality rate that has been reported as high as 20%.1 Suspicion for malignancy may arise due to ulceration, bleeding, pain, or rapid growth.
A lesson in considering the full differential
While sebaceous lesions on the eyelid and eyebrow are often benign, this case underscored the importance of considering the more worrisome elements in the differential. The differential diagnosis for lesions in the area of the eye include the following:
Sebaceous hyperplasia is a common condition (typically among older patients) in which sebaceous glands increase in size and number.2 The classic clinical feature is yellow or skin-colored papules. The lesions typically manifest on the face—particularly on the forehead. They are benign and often have a central umbilication.2
Sebaceous adenomas are benign tumors that may manifest as tan, skin-colored, pink, or yellow papules or nodules.2 The lesions are usually asymptomatic, small, and slow growing.2
Continue to: Basal and squamous cell carcinomas
Basal and squamous cell carcinomas. Basal cell carcinomas often feature translucent lesions on areas of the skin that are exposed to sunlight. These lesions often have slightly rolled border edges or overlying branching telangiectasia and may be nodular.3 Squamous cell carcinomas often feature scaled, reddened patches that may become tender and ulcerate.4
Hordeolums and chalazions. A hordeolum (or stye) is a painful, acute, localized swelling of the eyelid.5 These often develop externally at the lid margin from infection of the follicle. A chalazion is characterized by a persistent, nontender mass that results from small, noninfectious obstruction of the Meibomian glands with secondary granulomatous inflammation.5
Dermoscopy can (and did) help with the Dx
Dermoscopy can help confirm whether a lesion has a sebaceous origin because it would show yellow globules with “crown vessel” telangiectasias that classically do not cross midline.6 Unfortunately, the findings of yellow globules and dermal vessels do not adequately differentiate benign from malignant lesions.6 Carcinomas can manifest in an undifferentiated way early in their course.
Sebaceous carcinomas can be associated with the autosomal dominant Muir-Torre syndrome, a subset of the Lynch syndrome.7,8 Colorectal and genitourinary carcinomas are the most common internal malignancies seen in patients with Muir-Torre syndrome.9
Patients benefit from Mohs surgery
Treatment outcomes for sebaceous carcinoma appear to be improved by Mohs surgery. In a recent review of 1265 patients with early-stage sebaceous carcinomas, Su et al found that 234 patients who were treated with Mohs surgery had improved overall survival, compared with 1031 who were treated with surgical excision.10
Continue to: Our patient
Our patient was referred to a Mohs surgeon who removed the lesion (FIGURES 2 and 3). Given the overall small tumor size, a sentinel lymph node biopsy was not necessary. Because of the patient’s family history, which was suggestive of a genetic predisposition to cancer, she requested a clinical genetics consultation for definitive testing. She went on to pursue genetic testing, which came back negative for Lynch syndrome genes.
The dermatologist recommended yearly skin examination for 5 years for the patient.
A 51-year-old woman presented to the family medicine clinic for evaluation of a slightly tender skin lesion on her left eyebrow. The lesion had been slowly growing for a year.
The patient’s family history included multiple family members with colon or breast cancer and other relatives with pancreatic and prostate cancer. A colonoscopy performed a year earlier on the patient was negative. The patient’s past medical history included hypertension, major depressive disorder, hyperlipidemia, and venous insufficiency. She also had a colon polyp history.
Physical examination of the eyebrow showed a 3-mm papule that was firm on palpation. Dermoscopy of the lesion revealed a yellow papule with
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Sebaceous carcinoma
A rapid teledermatology consultation helped us to determine that this was a sebaceous lesion, but its location and the overlying telangiectasia raised concerns for malignancy. After shared decision-making with the patient, she agreed to proceed with a biopsy. We first made an incision into the lesion, which was hard, demonstrating that it was not cystic. A shave biopsy was then completed. The dermatopathology findings showed clear-cell change consisting of bubbly or foamy cytoplasm, with scalloping of the nuclei, which is characteristic of a sebaceous origin. There were tumor cells that were enlarged with pleomorphism, multiple nucleoli, and scattered mitotic figures. These findings pointed to a diagnosis of sebaceous carcinoma.
Sebaceous carcinomas most commonly manifest on the eyelids. They can originate from the Meibomian glands as well as from pilosebaceous glands at other sites on the body.1 They are rare, accounting for only 1% to 5% of eyelid malignancies, and occur in approximately 2 per 1 million people.1 Tumors can invade locally and metastasize, particularly to surrounding lymph nodes. Periocular pathology may sometimes lead to misdiagnosis, which contributes to a mortality rate that has been reported as high as 20%.1 Suspicion for malignancy may arise due to ulceration, bleeding, pain, or rapid growth.
A lesson in considering the full differential
While sebaceous lesions on the eyelid and eyebrow are often benign, this case underscored the importance of considering the more worrisome elements in the differential. The differential diagnosis for lesions in the area of the eye include the following:
Sebaceous hyperplasia is a common condition (typically among older patients) in which sebaceous glands increase in size and number.2 The classic clinical feature is yellow or skin-colored papules. The lesions typically manifest on the face—particularly on the forehead. They are benign and often have a central umbilication.2
Sebaceous adenomas are benign tumors that may manifest as tan, skin-colored, pink, or yellow papules or nodules.2 The lesions are usually asymptomatic, small, and slow growing.2
Continue to: Basal and squamous cell carcinomas
Basal and squamous cell carcinomas. Basal cell carcinomas often feature translucent lesions on areas of the skin that are exposed to sunlight. These lesions often have slightly rolled border edges or overlying branching telangiectasia and may be nodular.3 Squamous cell carcinomas often feature scaled, reddened patches that may become tender and ulcerate.4
Hordeolums and chalazions. A hordeolum (or stye) is a painful, acute, localized swelling of the eyelid.5 These often develop externally at the lid margin from infection of the follicle. A chalazion is characterized by a persistent, nontender mass that results from small, noninfectious obstruction of the Meibomian glands with secondary granulomatous inflammation.5
Dermoscopy can (and did) help with the Dx
Dermoscopy can help confirm whether a lesion has a sebaceous origin because it would show yellow globules with “crown vessel” telangiectasias that classically do not cross midline.6 Unfortunately, the findings of yellow globules and dermal vessels do not adequately differentiate benign from malignant lesions.6 Carcinomas can manifest in an undifferentiated way early in their course.
Sebaceous carcinomas can be associated with the autosomal dominant Muir-Torre syndrome, a subset of the Lynch syndrome.7,8 Colorectal and genitourinary carcinomas are the most common internal malignancies seen in patients with Muir-Torre syndrome.9
Patients benefit from Mohs surgery
Treatment outcomes for sebaceous carcinoma appear to be improved by Mohs surgery. In a recent review of 1265 patients with early-stage sebaceous carcinomas, Su et al found that 234 patients who were treated with Mohs surgery had improved overall survival, compared with 1031 who were treated with surgical excision.10
Continue to: Our patient
Our patient was referred to a Mohs surgeon who removed the lesion (FIGURES 2 and 3). Given the overall small tumor size, a sentinel lymph node biopsy was not necessary. Because of the patient’s family history, which was suggestive of a genetic predisposition to cancer, she requested a clinical genetics consultation for definitive testing. She went on to pursue genetic testing, which came back negative for Lynch syndrome genes.
The dermatologist recommended yearly skin examination for 5 years for the patient.
1. Kahana A, Pribila HT, Nelson CC, et al. Sebaceous cell carcinoma. In: Levin LA, Albert DM, eds. Ocular Disease: Mechanisms and Management. Saunders/Elsevier; 2010:396-407.
2. Iacobelli J, Harvey NT, Wood BA. Sebaceous lesions of the skin. Pathology. 2017;49:688-697.
3. Marzuka AG, Book SE. Basal cell carcinoma: pathogenesis, epidemiology, clinical features, diagnosis, histopathology, and management. Yale J Biol Med. 2015;88:167-179.
4. Smith H, Patel A. When to suspect a non-melanoma skin cancer. BMJ. 2020;368:m692.
5. Sun MT, Huang S, Huilgol SC, et al. Eyelid lesions in general practice. Aust J Gen Pract. 2019;48:509-514.
6. Kim NH, Zell DS, Kolm I, et al. The dermoscopic differential diagnosis of yellow lobularlike structures. Arch Dermatol. 2008;144:962.
7. EG, Bell AJY, Barlow KA. Multiple primary carcinomata of the colon, duodenum, and larynx associated with kerato-acanthomata of the face. Br J Surg. 1967;54:191-195.
8. Torre D. Multiple sebaceous tumors. Arch Dermatol. 1968;98:549-55.
9. Cohen PR, Kohn SR, Kurzrock R. Association of sebaceous gland tumors and internal malignancy: the Muir-Torre syndrome. Am J Med. 1991;90:606-613.
10. Su C, Nguyen KA, Bai HX, et al. Comparison of Mohs surgery and surgical excision in the treatment of localized sebaceous carcinoma. Dermatol Surg. 2019;45:1125-1135.
1. Kahana A, Pribila HT, Nelson CC, et al. Sebaceous cell carcinoma. In: Levin LA, Albert DM, eds. Ocular Disease: Mechanisms and Management. Saunders/Elsevier; 2010:396-407.
2. Iacobelli J, Harvey NT, Wood BA. Sebaceous lesions of the skin. Pathology. 2017;49:688-697.
3. Marzuka AG, Book SE. Basal cell carcinoma: pathogenesis, epidemiology, clinical features, diagnosis, histopathology, and management. Yale J Biol Med. 2015;88:167-179.
4. Smith H, Patel A. When to suspect a non-melanoma skin cancer. BMJ. 2020;368:m692.
5. Sun MT, Huang S, Huilgol SC, et al. Eyelid lesions in general practice. Aust J Gen Pract. 2019;48:509-514.
6. Kim NH, Zell DS, Kolm I, et al. The dermoscopic differential diagnosis of yellow lobularlike structures. Arch Dermatol. 2008;144:962.
7. EG, Bell AJY, Barlow KA. Multiple primary carcinomata of the colon, duodenum, and larynx associated with kerato-acanthomata of the face. Br J Surg. 1967;54:191-195.
8. Torre D. Multiple sebaceous tumors. Arch Dermatol. 1968;98:549-55.
9. Cohen PR, Kohn SR, Kurzrock R. Association of sebaceous gland tumors and internal malignancy: the Muir-Torre syndrome. Am J Med. 1991;90:606-613.
10. Su C, Nguyen KA, Bai HX, et al. Comparison of Mohs surgery and surgical excision in the treatment of localized sebaceous carcinoma. Dermatol Surg. 2019;45:1125-1135.
Is metformin effective for reducing weight in obese or overweight adolescents?
EVIDENCE SUMMARY
Metformin has modest effects on body weight
A large systematic review and meta-analysis (38 RCTs; n = 2199) published in 2020 evaluated metformin therapy in children and adolescents (including those with metabolic disease, growth problems, and psychological disorders in addition to obesity and overweight).1 Over an average of 6 months, metformin use modestly reduced BMI (weighted mean difference [WMD] = –1.07 kg/m2; 95% CI, –1.43 to –0.72 kg/m2) and body weight (WMD = –2.51 kg; 95% CI, –3.14 to –1.89 kg) for all participants.1
However, the authors also performed a meta-analysis of trials involving obese or overweight youth without other comorbidities. Participants in these trials ranged in age from 7 to 17 years (mean not supplied; most trials, 12-15 years), had a BMI greater than the 95th percentile for age, and took doses of metformin ranging from 1500 to 3000 mg (most trials, 1500-2000 mg/d for 24 weeks).1 In this analysis, metformin reduced body weight (8 trials; n = 616; WMD = –2.06 kg; 95% CI, –3.47 to –0.65 kg) and body fat mass (–1.9%; 95% CI, –3.25% to –0.56%). But it did not reduce BMI (12 trials; n = 826; WMD = –0.76 kg/m2; 95 % CI, –1.61 to 0.08 kg/m2) or improve lean body mass (2 trials; N = 98; WMD = –0.74 kg; 95% CI, –2.4 to 0.91 kg).1
The authors of this meta-analysis did not include an evaluation of the quality of the individual RCTs.
Metformin has benefits but also adverse effects
A 2016 Cochrane systematic review and meta-analysis assessed 8 trials (total n = 543) evaluating metformin vs placebo in adolescents prescribed exercise and lifestyle support.2 This meta-analysis included 4 trials (n = 294) with obese or overweight adolescents that were also included in the newer meta-analysis,1 as well as 4 trials involving obese adolescents with insulin resistance. The authors did not assess the effects of metformin on obese or overweight adolescents separately.
Over 6 months, metformin use reduced BMI (WMD = –1.35 kg/m2; 95% CI –2 to –0.69 kg/m2).2 Metformin commonly produced gastrointestinal symptoms: diarrhea, flatulence (rates not given), and nausea in 15% to 42% compared with 3% to 21% with placebo (no comparison statistic supplied), however rarely to the point of discontinuation (< 5%).2 Nine participants withdrew due to adverse effects: 5 in the metformin group and 4 in the placebo group. The authors rated the quality of the included trials as low to moderate.
An evidence report and systematic review (42 RCTs; total n = 6956) compared the efficacy of several approaches for weight loss in adolescents, including metformin (6 of the 8 RCTs included in the 2020 meta-analysis1) and lifestyle interventions.3 Interventions comprising exercise and diet counseling for > 26 hours over 6 to 12 months produced decreases in BMI (–0.86 kg/m2; 95% CI –1.44 to –0.29 kg/m2) but not weight (–2 kg; 95% CI –3.2 to 1.2 kg).3
Recommendations from others
The US Preventive Services Task Force states that metformin treatment in adolescents who are overweight or obese produces a small reduction in BMI when compared to placebo, but the clinical significance of this reduction is unclear.3
Editor’s takeaway
The idea of using medications for weight loss remains seductive, given how hard it can be for patients to achieve significant, lasting weight loss through lifestyle modification. Evidence suggests that metformin can help in this regard but not enough to recommend it. In addition, metformin therapy is associated with gastrointestinal adverse effects.
1. Sadeghi A, Mousavi SM, Mokhtari T, et al. Metformin therapy reduces obesity indices in children and adolescents: a systematic review and meta-analysis of randomized clinical trials. Child Obes. 2020;16:174-191.
2. Mead E, Atkinson G, Richter B, et al. Drug interventions for the treatment of obesity in children and adolescents. Cochrane Database Syst Rev. 2016;11:CD012436.
3. O’Connor EA, Evans CV, Burda BU, et al. Screening for obesity and intervention for weight management in children and adolescents: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2017;317:2427-2444.
EVIDENCE SUMMARY
Metformin has modest effects on body weight
A large systematic review and meta-analysis (38 RCTs; n = 2199) published in 2020 evaluated metformin therapy in children and adolescents (including those with metabolic disease, growth problems, and psychological disorders in addition to obesity and overweight).1 Over an average of 6 months, metformin use modestly reduced BMI (weighted mean difference [WMD] = –1.07 kg/m2; 95% CI, –1.43 to –0.72 kg/m2) and body weight (WMD = –2.51 kg; 95% CI, –3.14 to –1.89 kg) for all participants.1
However, the authors also performed a meta-analysis of trials involving obese or overweight youth without other comorbidities. Participants in these trials ranged in age from 7 to 17 years (mean not supplied; most trials, 12-15 years), had a BMI greater than the 95th percentile for age, and took doses of metformin ranging from 1500 to 3000 mg (most trials, 1500-2000 mg/d for 24 weeks).1 In this analysis, metformin reduced body weight (8 trials; n = 616; WMD = –2.06 kg; 95% CI, –3.47 to –0.65 kg) and body fat mass (–1.9%; 95% CI, –3.25% to –0.56%). But it did not reduce BMI (12 trials; n = 826; WMD = –0.76 kg/m2; 95 % CI, –1.61 to 0.08 kg/m2) or improve lean body mass (2 trials; N = 98; WMD = –0.74 kg; 95% CI, –2.4 to 0.91 kg).1
The authors of this meta-analysis did not include an evaluation of the quality of the individual RCTs.
Metformin has benefits but also adverse effects
A 2016 Cochrane systematic review and meta-analysis assessed 8 trials (total n = 543) evaluating metformin vs placebo in adolescents prescribed exercise and lifestyle support.2 This meta-analysis included 4 trials (n = 294) with obese or overweight adolescents that were also included in the newer meta-analysis,1 as well as 4 trials involving obese adolescents with insulin resistance. The authors did not assess the effects of metformin on obese or overweight adolescents separately.
Over 6 months, metformin use reduced BMI (WMD = –1.35 kg/m2; 95% CI –2 to –0.69 kg/m2).2 Metformin commonly produced gastrointestinal symptoms: diarrhea, flatulence (rates not given), and nausea in 15% to 42% compared with 3% to 21% with placebo (no comparison statistic supplied), however rarely to the point of discontinuation (< 5%).2 Nine participants withdrew due to adverse effects: 5 in the metformin group and 4 in the placebo group. The authors rated the quality of the included trials as low to moderate.
An evidence report and systematic review (42 RCTs; total n = 6956) compared the efficacy of several approaches for weight loss in adolescents, including metformin (6 of the 8 RCTs included in the 2020 meta-analysis1) and lifestyle interventions.3 Interventions comprising exercise and diet counseling for > 26 hours over 6 to 12 months produced decreases in BMI (–0.86 kg/m2; 95% CI –1.44 to –0.29 kg/m2) but not weight (–2 kg; 95% CI –3.2 to 1.2 kg).3
Recommendations from others
The US Preventive Services Task Force states that metformin treatment in adolescents who are overweight or obese produces a small reduction in BMI when compared to placebo, but the clinical significance of this reduction is unclear.3
Editor’s takeaway
The idea of using medications for weight loss remains seductive, given how hard it can be for patients to achieve significant, lasting weight loss through lifestyle modification. Evidence suggests that metformin can help in this regard but not enough to recommend it. In addition, metformin therapy is associated with gastrointestinal adverse effects.
EVIDENCE SUMMARY
Metformin has modest effects on body weight
A large systematic review and meta-analysis (38 RCTs; n = 2199) published in 2020 evaluated metformin therapy in children and adolescents (including those with metabolic disease, growth problems, and psychological disorders in addition to obesity and overweight).1 Over an average of 6 months, metformin use modestly reduced BMI (weighted mean difference [WMD] = –1.07 kg/m2; 95% CI, –1.43 to –0.72 kg/m2) and body weight (WMD = –2.51 kg; 95% CI, –3.14 to –1.89 kg) for all participants.1
However, the authors also performed a meta-analysis of trials involving obese or overweight youth without other comorbidities. Participants in these trials ranged in age from 7 to 17 years (mean not supplied; most trials, 12-15 years), had a BMI greater than the 95th percentile for age, and took doses of metformin ranging from 1500 to 3000 mg (most trials, 1500-2000 mg/d for 24 weeks).1 In this analysis, metformin reduced body weight (8 trials; n = 616; WMD = –2.06 kg; 95% CI, –3.47 to –0.65 kg) and body fat mass (–1.9%; 95% CI, –3.25% to –0.56%). But it did not reduce BMI (12 trials; n = 826; WMD = –0.76 kg/m2; 95 % CI, –1.61 to 0.08 kg/m2) or improve lean body mass (2 trials; N = 98; WMD = –0.74 kg; 95% CI, –2.4 to 0.91 kg).1
The authors of this meta-analysis did not include an evaluation of the quality of the individual RCTs.
Metformin has benefits but also adverse effects
A 2016 Cochrane systematic review and meta-analysis assessed 8 trials (total n = 543) evaluating metformin vs placebo in adolescents prescribed exercise and lifestyle support.2 This meta-analysis included 4 trials (n = 294) with obese or overweight adolescents that were also included in the newer meta-analysis,1 as well as 4 trials involving obese adolescents with insulin resistance. The authors did not assess the effects of metformin on obese or overweight adolescents separately.
Over 6 months, metformin use reduced BMI (WMD = –1.35 kg/m2; 95% CI –2 to –0.69 kg/m2).2 Metformin commonly produced gastrointestinal symptoms: diarrhea, flatulence (rates not given), and nausea in 15% to 42% compared with 3% to 21% with placebo (no comparison statistic supplied), however rarely to the point of discontinuation (< 5%).2 Nine participants withdrew due to adverse effects: 5 in the metformin group and 4 in the placebo group. The authors rated the quality of the included trials as low to moderate.
An evidence report and systematic review (42 RCTs; total n = 6956) compared the efficacy of several approaches for weight loss in adolescents, including metformin (6 of the 8 RCTs included in the 2020 meta-analysis1) and lifestyle interventions.3 Interventions comprising exercise and diet counseling for > 26 hours over 6 to 12 months produced decreases in BMI (–0.86 kg/m2; 95% CI –1.44 to –0.29 kg/m2) but not weight (–2 kg; 95% CI –3.2 to 1.2 kg).3
Recommendations from others
The US Preventive Services Task Force states that metformin treatment in adolescents who are overweight or obese produces a small reduction in BMI when compared to placebo, but the clinical significance of this reduction is unclear.3
Editor’s takeaway
The idea of using medications for weight loss remains seductive, given how hard it can be for patients to achieve significant, lasting weight loss through lifestyle modification. Evidence suggests that metformin can help in this regard but not enough to recommend it. In addition, metformin therapy is associated with gastrointestinal adverse effects.
1. Sadeghi A, Mousavi SM, Mokhtari T, et al. Metformin therapy reduces obesity indices in children and adolescents: a systematic review and meta-analysis of randomized clinical trials. Child Obes. 2020;16:174-191.
2. Mead E, Atkinson G, Richter B, et al. Drug interventions for the treatment of obesity in children and adolescents. Cochrane Database Syst Rev. 2016;11:CD012436.
3. O’Connor EA, Evans CV, Burda BU, et al. Screening for obesity and intervention for weight management in children and adolescents: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2017;317:2427-2444.
1. Sadeghi A, Mousavi SM, Mokhtari T, et al. Metformin therapy reduces obesity indices in children and adolescents: a systematic review and meta-analysis of randomized clinical trials. Child Obes. 2020;16:174-191.
2. Mead E, Atkinson G, Richter B, et al. Drug interventions for the treatment of obesity in children and adolescents. Cochrane Database Syst Rev. 2016;11:CD012436.
3. O’Connor EA, Evans CV, Burda BU, et al. Screening for obesity and intervention for weight management in children and adolescents: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2017;317:2427-2444.
EVIDENCE-BASED ANSWER:
Yes, to some degree—but it is of uncertain clinical significance. Over a period of 6 months, metformin modestly reduced weight (–2.1 kg) and body fat mass (–1.9%), but not body mass index (BMI) or lean body mass, in adolescents who were overweight or obese. This is comparable to lifestyle interventions (diet and exercise) supported with > 26 hours of counseling, which modestly improved BMI but not weight. (Strength of recommendation [SOR]: A, based on a large meta-analysis of randomized controlled trials [RCTs] of variable quality).
Which detoxification regimens are effective for alcohol withdrawal syndrome?
EVIDENCE SUMMARY
Benzodiazepines work—but how do they compare?
A 2010 Cochrane meta-analysis of 64 RCTs and controlled clinical trials (CCTs; N = 4309) evaluated the use of benzodiazepines for treatment of AWS in adults.1 This systematic review compared benzodiazepines
- vs placebo (10 studies)
- vs other drugs, including phenobarbital, carbamazepine, topiramate, lamotrigine, gabapentin, haloperidol, clonidine, hydroxyzine, propranolol, and baclofen (42 studies)
- to other benzodiazepines, including chlordiazepoxide, alprazolam, diazepam, and lorazepam (18 studies)
- in combination with other drugs vs other drugs alone (3 studies)
- administered on a fixed schedule vs symptom-triggered administration (3 studies).
Primary outcomes included efficacy (alcohol withdrawal seizures, alcohol withdrawal delirium, alcohol withdrawal symptoms, global improvement), safety (adverse events and severe, life-threatening adverse events), and acceptability (dropouts and dropouts due to adverse events).
Benzodiazepines performed better than placebo for seizures in 3 studies (N = 324), with a relative risk (RR) of 0.16 (95% confidence interval [CI], 0.04-0.69). Studies assessing the described outcomes between benzodiazepines and other drugs were often of small sample size and heterogeneous in interventions and outcomes, limiting the ability to draw clear conclusions regarding benzodiazepine superiority. Comparisons of different benzodiazepines with each other and comparisons of benzodiazepines combined with other drugs vs other drugs alone did not reach statistical significance. Data on harms of benzodiazepines were lacking.
Anticonvulsants are not better than placebo for AWS
Another 2010 Cochrane meta-analysis of 56 RCTs and CCTs (N = 4076) evaluated the use of anticonvulsants for AWS.2 This systematic review compared anticonvulsants
- vs placebo (17 studies)
- vs other drugs, such as bromocriptine, piracetam, gamma-hydroxybutyric acid, trifluoperazine, clonidine, and various benzodiazepines (32 studies)
- to other anticonvulsants (10 studies)
- in combination with other drugs vs other drugs alone (6 studies)
- in combination with other drugs vs different anticonvulsants (1 study).
Primary outcomes included reductions in alcohol withdrawal seizures, adverse events, and acceptability of medication as indicated by participant dropouts.
Anticonvulsants were not superior to placebo for any outcome. Three studies (N = 260) favored carbamazepine over benzodiazepine (oxazepam or lorazepam) for 1 secondary outcome: a reduction of Clinical Institute Withdrawal Assessment of Alcohol Scale (CIWA-Ar) score (maximum score of 7; mean difference [MD] = –1 [95% CI, –1.9 to –0.2]).
Continue to: Gabapentin is effective; less sedating than chlordiazepoxide
Gabapentin is effective; less sedating than chlordiazepoxide
A 2013 RCT of US veterans with AWS (N = 26; 25 men; average age, 53.5 years) compared gabapentin and chlordiazepoxide.3 Endpoints were ratings on the Epworth Sleepiness Scale (ESS; maximum score = 24), Penn Alcohol Craving Scale (PACS; maximum score, 30), and CIWA-Ar.
In the early treatment period (Days 1-4), ESS and PACS scores did not differ significantly between groups. At end of treatment (Days 5-7), ESS and PACS scores were lower in gabapentin-treated patients (ESS: MD = –3.7; 95% CI, –7.2 to –0.19; P = .04; PACS: MD = –6.05; 95% CI –12.82 to 0.72; P = .08). CIWA-Ar did not differ between treatment groups.
Recommendations from others
In January 2020, the American Society of Addiction Medicine (ASAM) published a clinical practice guideline for alcohol withdrawal management. Protocols for diagnosis, assessment, level of care determination, and management are delineated.4
Benzodiazepines are the first-line treatment for moderate-to-severe AWS, or when there is risk for severe AWS. In the ambulatory setting, when AWS is mild and there is no risk for worsening, AWS can be managed with supportive care or with either benzodiazepines, gabapentin, or carbamazepine as monotherapy. ASAM recommends long-acting benzodiazepines (eg, chlordiazepoxide or diazepam) over short-acting benzodiazepines (eg, alprazolam or lorazepam), except in the elderly and those with liver or lung disease.5
Editor’s takeaway
Dozens of small trials and meta-analyses confirm the benefits (sometimes marginal) of sedation to treat alcohol withdrawal. Given that the evidence fails to point to the superiority of 1 agent over another, it seems reasonable to make treatment decisions based on physician and perhaps patient preference. This review does not support a change in clinical practice.
1. Amato L, Minozzi S, Vecchi S, et al. Benzodiazepines for alcohol withdrawal. Cochrane Database Syst Rev. 2010;(3):CD005063.
2. Minozzi S, Amato L, Vecchi S, et al. Anticonvulsants for alcohol withdrawal. Cochrane Database Syst Rev. 2010;(3):CD005064.
3. Stock CJ, Carpenter L, Ying J, et al. Gabapentin versus chlordiazepoxide for outpatient alcohol detoxification treatment. Ann Pharmacother. 2013;47:961-969.
4. American Society of Addiction Medicine. The ASAM Clinical Practice Guideline on Alcohol Withdrawal Management 2020. Accessed March 2, 2021. www.asam.org/docs/default-source/quality-science/the_asam_clinical_practice_guideline_on_alcohol-1.pdf
5. Ries RK, Fiellin DA, Miller SC, et al. The ASAM Principles of Addiction Medicine. 4th ed. Lippincott Williams & Wilkins; 2014.
EVIDENCE SUMMARY
Benzodiazepines work—but how do they compare?
A 2010 Cochrane meta-analysis of 64 RCTs and controlled clinical trials (CCTs; N = 4309) evaluated the use of benzodiazepines for treatment of AWS in adults.1 This systematic review compared benzodiazepines
- vs placebo (10 studies)
- vs other drugs, including phenobarbital, carbamazepine, topiramate, lamotrigine, gabapentin, haloperidol, clonidine, hydroxyzine, propranolol, and baclofen (42 studies)
- to other benzodiazepines, including chlordiazepoxide, alprazolam, diazepam, and lorazepam (18 studies)
- in combination with other drugs vs other drugs alone (3 studies)
- administered on a fixed schedule vs symptom-triggered administration (3 studies).
Primary outcomes included efficacy (alcohol withdrawal seizures, alcohol withdrawal delirium, alcohol withdrawal symptoms, global improvement), safety (adverse events and severe, life-threatening adverse events), and acceptability (dropouts and dropouts due to adverse events).
Benzodiazepines performed better than placebo for seizures in 3 studies (N = 324), with a relative risk (RR) of 0.16 (95% confidence interval [CI], 0.04-0.69). Studies assessing the described outcomes between benzodiazepines and other drugs were often of small sample size and heterogeneous in interventions and outcomes, limiting the ability to draw clear conclusions regarding benzodiazepine superiority. Comparisons of different benzodiazepines with each other and comparisons of benzodiazepines combined with other drugs vs other drugs alone did not reach statistical significance. Data on harms of benzodiazepines were lacking.
Anticonvulsants are not better than placebo for AWS
Another 2010 Cochrane meta-analysis of 56 RCTs and CCTs (N = 4076) evaluated the use of anticonvulsants for AWS.2 This systematic review compared anticonvulsants
- vs placebo (17 studies)
- vs other drugs, such as bromocriptine, piracetam, gamma-hydroxybutyric acid, trifluoperazine, clonidine, and various benzodiazepines (32 studies)
- to other anticonvulsants (10 studies)
- in combination with other drugs vs other drugs alone (6 studies)
- in combination with other drugs vs different anticonvulsants (1 study).
Primary outcomes included reductions in alcohol withdrawal seizures, adverse events, and acceptability of medication as indicated by participant dropouts.
Anticonvulsants were not superior to placebo for any outcome. Three studies (N = 260) favored carbamazepine over benzodiazepine (oxazepam or lorazepam) for 1 secondary outcome: a reduction of Clinical Institute Withdrawal Assessment of Alcohol Scale (CIWA-Ar) score (maximum score of 7; mean difference [MD] = –1 [95% CI, –1.9 to –0.2]).
Continue to: Gabapentin is effective; less sedating than chlordiazepoxide
Gabapentin is effective; less sedating than chlordiazepoxide
A 2013 RCT of US veterans with AWS (N = 26; 25 men; average age, 53.5 years) compared gabapentin and chlordiazepoxide.3 Endpoints were ratings on the Epworth Sleepiness Scale (ESS; maximum score = 24), Penn Alcohol Craving Scale (PACS; maximum score, 30), and CIWA-Ar.
In the early treatment period (Days 1-4), ESS and PACS scores did not differ significantly between groups. At end of treatment (Days 5-7), ESS and PACS scores were lower in gabapentin-treated patients (ESS: MD = –3.7; 95% CI, –7.2 to –0.19; P = .04; PACS: MD = –6.05; 95% CI –12.82 to 0.72; P = .08). CIWA-Ar did not differ between treatment groups.
Recommendations from others
In January 2020, the American Society of Addiction Medicine (ASAM) published a clinical practice guideline for alcohol withdrawal management. Protocols for diagnosis, assessment, level of care determination, and management are delineated.4
Benzodiazepines are the first-line treatment for moderate-to-severe AWS, or when there is risk for severe AWS. In the ambulatory setting, when AWS is mild and there is no risk for worsening, AWS can be managed with supportive care or with either benzodiazepines, gabapentin, or carbamazepine as monotherapy. ASAM recommends long-acting benzodiazepines (eg, chlordiazepoxide or diazepam) over short-acting benzodiazepines (eg, alprazolam or lorazepam), except in the elderly and those with liver or lung disease.5
Editor’s takeaway
Dozens of small trials and meta-analyses confirm the benefits (sometimes marginal) of sedation to treat alcohol withdrawal. Given that the evidence fails to point to the superiority of 1 agent over another, it seems reasonable to make treatment decisions based on physician and perhaps patient preference. This review does not support a change in clinical practice.
EVIDENCE SUMMARY
Benzodiazepines work—but how do they compare?
A 2010 Cochrane meta-analysis of 64 RCTs and controlled clinical trials (CCTs; N = 4309) evaluated the use of benzodiazepines for treatment of AWS in adults.1 This systematic review compared benzodiazepines
- vs placebo (10 studies)
- vs other drugs, including phenobarbital, carbamazepine, topiramate, lamotrigine, gabapentin, haloperidol, clonidine, hydroxyzine, propranolol, and baclofen (42 studies)
- to other benzodiazepines, including chlordiazepoxide, alprazolam, diazepam, and lorazepam (18 studies)
- in combination with other drugs vs other drugs alone (3 studies)
- administered on a fixed schedule vs symptom-triggered administration (3 studies).
Primary outcomes included efficacy (alcohol withdrawal seizures, alcohol withdrawal delirium, alcohol withdrawal symptoms, global improvement), safety (adverse events and severe, life-threatening adverse events), and acceptability (dropouts and dropouts due to adverse events).
Benzodiazepines performed better than placebo for seizures in 3 studies (N = 324), with a relative risk (RR) of 0.16 (95% confidence interval [CI], 0.04-0.69). Studies assessing the described outcomes between benzodiazepines and other drugs were often of small sample size and heterogeneous in interventions and outcomes, limiting the ability to draw clear conclusions regarding benzodiazepine superiority. Comparisons of different benzodiazepines with each other and comparisons of benzodiazepines combined with other drugs vs other drugs alone did not reach statistical significance. Data on harms of benzodiazepines were lacking.
Anticonvulsants are not better than placebo for AWS
Another 2010 Cochrane meta-analysis of 56 RCTs and CCTs (N = 4076) evaluated the use of anticonvulsants for AWS.2 This systematic review compared anticonvulsants
- vs placebo (17 studies)
- vs other drugs, such as bromocriptine, piracetam, gamma-hydroxybutyric acid, trifluoperazine, clonidine, and various benzodiazepines (32 studies)
- to other anticonvulsants (10 studies)
- in combination with other drugs vs other drugs alone (6 studies)
- in combination with other drugs vs different anticonvulsants (1 study).
Primary outcomes included reductions in alcohol withdrawal seizures, adverse events, and acceptability of medication as indicated by participant dropouts.
Anticonvulsants were not superior to placebo for any outcome. Three studies (N = 260) favored carbamazepine over benzodiazepine (oxazepam or lorazepam) for 1 secondary outcome: a reduction of Clinical Institute Withdrawal Assessment of Alcohol Scale (CIWA-Ar) score (maximum score of 7; mean difference [MD] = –1 [95% CI, –1.9 to –0.2]).
Continue to: Gabapentin is effective; less sedating than chlordiazepoxide
Gabapentin is effective; less sedating than chlordiazepoxide
A 2013 RCT of US veterans with AWS (N = 26; 25 men; average age, 53.5 years) compared gabapentin and chlordiazepoxide.3 Endpoints were ratings on the Epworth Sleepiness Scale (ESS; maximum score = 24), Penn Alcohol Craving Scale (PACS; maximum score, 30), and CIWA-Ar.
In the early treatment period (Days 1-4), ESS and PACS scores did not differ significantly between groups. At end of treatment (Days 5-7), ESS and PACS scores were lower in gabapentin-treated patients (ESS: MD = –3.7; 95% CI, –7.2 to –0.19; P = .04; PACS: MD = –6.05; 95% CI –12.82 to 0.72; P = .08). CIWA-Ar did not differ between treatment groups.
Recommendations from others
In January 2020, the American Society of Addiction Medicine (ASAM) published a clinical practice guideline for alcohol withdrawal management. Protocols for diagnosis, assessment, level of care determination, and management are delineated.4
Benzodiazepines are the first-line treatment for moderate-to-severe AWS, or when there is risk for severe AWS. In the ambulatory setting, when AWS is mild and there is no risk for worsening, AWS can be managed with supportive care or with either benzodiazepines, gabapentin, or carbamazepine as monotherapy. ASAM recommends long-acting benzodiazepines (eg, chlordiazepoxide or diazepam) over short-acting benzodiazepines (eg, alprazolam or lorazepam), except in the elderly and those with liver or lung disease.5
Editor’s takeaway
Dozens of small trials and meta-analyses confirm the benefits (sometimes marginal) of sedation to treat alcohol withdrawal. Given that the evidence fails to point to the superiority of 1 agent over another, it seems reasonable to make treatment decisions based on physician and perhaps patient preference. This review does not support a change in clinical practice.
1. Amato L, Minozzi S, Vecchi S, et al. Benzodiazepines for alcohol withdrawal. Cochrane Database Syst Rev. 2010;(3):CD005063.
2. Minozzi S, Amato L, Vecchi S, et al. Anticonvulsants for alcohol withdrawal. Cochrane Database Syst Rev. 2010;(3):CD005064.
3. Stock CJ, Carpenter L, Ying J, et al. Gabapentin versus chlordiazepoxide for outpatient alcohol detoxification treatment. Ann Pharmacother. 2013;47:961-969.
4. American Society of Addiction Medicine. The ASAM Clinical Practice Guideline on Alcohol Withdrawal Management 2020. Accessed March 2, 2021. www.asam.org/docs/default-source/quality-science/the_asam_clinical_practice_guideline_on_alcohol-1.pdf
5. Ries RK, Fiellin DA, Miller SC, et al. The ASAM Principles of Addiction Medicine. 4th ed. Lippincott Williams & Wilkins; 2014.
1. Amato L, Minozzi S, Vecchi S, et al. Benzodiazepines for alcohol withdrawal. Cochrane Database Syst Rev. 2010;(3):CD005063.
2. Minozzi S, Amato L, Vecchi S, et al. Anticonvulsants for alcohol withdrawal. Cochrane Database Syst Rev. 2010;(3):CD005064.
3. Stock CJ, Carpenter L, Ying J, et al. Gabapentin versus chlordiazepoxide for outpatient alcohol detoxification treatment. Ann Pharmacother. 2013;47:961-969.
4. American Society of Addiction Medicine. The ASAM Clinical Practice Guideline on Alcohol Withdrawal Management 2020. Accessed March 2, 2021. www.asam.org/docs/default-source/quality-science/the_asam_clinical_practice_guideline_on_alcohol-1.pdf
5. Ries RK, Fiellin DA, Miller SC, et al. The ASAM Principles of Addiction Medicine. 4th ed. Lippincott Williams & Wilkins; 2014.
EVIDENCE-BASED ANSWER:
Benzodiazepines remain the first-line regimen for alcohol withdrawal syndrome (AWS) and are the only class more effective than placebo for reducing seizure (strength of recommendation [SOR]: B, based on 3 medium-quality randomized controlled trials [RCTs]). Anticonvulsants are no more effective than placebo at reducing seizures (SOR: B, based on 10 moderate-quality RCTs). Gabapentin reduces withdrawal symptoms and is less sedating than benzodiazepines (SOR: B, based on 1 medium-quality RCT). Carbamazepine also reduces withdrawal symptoms (SOR: B, based on 3 RCTs). Evidence of benzodiazepine superiority to other drugs with respect to safety is lacking (SOR: A, based on a meta-analysis).
The Physician Support Line: One psychiatrist strives to make a difference
Have you ever had a really good idea about how to improve the delivery of mental health services? An idea that would help people, but that would require passion, innovation, and hard work to implement, and one that immediately is beset with a list of reasons why it can not be implemented?
Mona Masood, DO, had an idea. The Pennsylvania psychiatrist was asked to help moderate a Facebook group started by one of her infectious disease colleagues last winter – a private Facebook group for physicians working with COVID-19 patients.
“The group was getting posts from frontline workers about how depressed and hopeless they were feeling.” Dr. Masood said. “People were posting about how they were having escape fantasies and how they regretted becoming physicians. It became clear that there was a need for more support.”
psychiatrist volunteers would take calls from physicians who needed someone to talk to – the psychiatrist would provide a sympathetic ear and have a list of resources, but this would be support, not treatment. There would be no prescriptions, no treatment relationship, no reporting to licensing boards or employers. The calls would be anonymous.
She posted her idea on the Facebook group, and the response was immediate. “There were a lot of emails – 200 psychiatrists responded saying: “Sign me up.” A Zoom meeting was set up, and the process was set in motion.
Dr. Masood used a Google document for weekly sign-ups so the volunteer psychiatrists could choose times. “We had to pay for an upgraded Google suite package for that many users. Getting this up and running was like the saying about building a plane as you fly it,” Dr. Masood said. “It forced so much so quickly because there was this acknowledgment that the need was there.”
Initially, the support line launched with a telehealth platform, but there was a problem. “Many doctors don’t want to be seen; they worry about being recognized.” Dr. Masood researched hotline phone services and was able to get one for a reduced fee. The volunteers have an App on their smartphones that enables them to log in at the start of their shifts and log out at the end. In addition to the logistics of coordinating the volunteers – now numbering over 700 – the group found a health care law firm that provided pro bono services to review the policies and procedures.
Now that the support line is running, Dr. Masood is able to set up the day’s volunteers for the support line connection in a few minutes each morning, but the beginning was not easy. Her private practice transitioned to telemedicine, and her two children were home with one in virtual school. “At first, it was like another full-time job.” She still remains available for trouble-shooting during the day. It’s a project she has taken on with passion.
The support line began as a response to watching colleagues struggle with COVID. Since it launched, there have been approximately 2,000 calls. Calls typically last for 20 to 90 minutes, and no one has called with a suicidal crisis. It is now open to doctors and medical students looking for support for any reason. “Physicians call with all kinds of issues. In the first 3 months, it was COVID, but then they called with other concerns – there were doctors who called with election anxiety, really anything that affects the general public also affects us.”
The group has also offered Saturday didactic sessions for volunteers and weekly debriefing sessions. Dr. Masood has been approached by Vibrant Emotional Health, the administrator of the National Suicide Prevention Lifeline, about resources to help with funding – until now, this endeavor has had no financing – and she is hopeful that their financial support will allow the support line to sustain itself and grow. Future directions include advocating for systemic change in how physician mental health and wellness issues are addressed.
The Physician Support Line was one psychiatrist’s vision for how to address a problem. Like so many things related to this pandemic, it happened quickly and with surprising efficiency. Implementing this service, however, was not easy – it required hard work, innovative thinking, and passion. Those looking for someone to listen can call 1-888-409-0141 and psychiatrists who wish to volunteer can sign up at physiciansupportline.com/volunteer-info.
Dr. Miller is coauthor of “Committed: The Battle Over Involuntary Psychiatric Care” (Johns Hopkins University Press, 2018). She has a private practice and is assistant professor of psychiatry and behavioral sciences at Johns Hopkins University, both in Baltimore.
Have you ever had a really good idea about how to improve the delivery of mental health services? An idea that would help people, but that would require passion, innovation, and hard work to implement, and one that immediately is beset with a list of reasons why it can not be implemented?
Mona Masood, DO, had an idea. The Pennsylvania psychiatrist was asked to help moderate a Facebook group started by one of her infectious disease colleagues last winter – a private Facebook group for physicians working with COVID-19 patients.
“The group was getting posts from frontline workers about how depressed and hopeless they were feeling.” Dr. Masood said. “People were posting about how they were having escape fantasies and how they regretted becoming physicians. It became clear that there was a need for more support.”
psychiatrist volunteers would take calls from physicians who needed someone to talk to – the psychiatrist would provide a sympathetic ear and have a list of resources, but this would be support, not treatment. There would be no prescriptions, no treatment relationship, no reporting to licensing boards or employers. The calls would be anonymous.
She posted her idea on the Facebook group, and the response was immediate. “There were a lot of emails – 200 psychiatrists responded saying: “Sign me up.” A Zoom meeting was set up, and the process was set in motion.
Dr. Masood used a Google document for weekly sign-ups so the volunteer psychiatrists could choose times. “We had to pay for an upgraded Google suite package for that many users. Getting this up and running was like the saying about building a plane as you fly it,” Dr. Masood said. “It forced so much so quickly because there was this acknowledgment that the need was there.”
Initially, the support line launched with a telehealth platform, but there was a problem. “Many doctors don’t want to be seen; they worry about being recognized.” Dr. Masood researched hotline phone services and was able to get one for a reduced fee. The volunteers have an App on their smartphones that enables them to log in at the start of their shifts and log out at the end. In addition to the logistics of coordinating the volunteers – now numbering over 700 – the group found a health care law firm that provided pro bono services to review the policies and procedures.
Now that the support line is running, Dr. Masood is able to set up the day’s volunteers for the support line connection in a few minutes each morning, but the beginning was not easy. Her private practice transitioned to telemedicine, and her two children were home with one in virtual school. “At first, it was like another full-time job.” She still remains available for trouble-shooting during the day. It’s a project she has taken on with passion.
The support line began as a response to watching colleagues struggle with COVID. Since it launched, there have been approximately 2,000 calls. Calls typically last for 20 to 90 minutes, and no one has called with a suicidal crisis. It is now open to doctors and medical students looking for support for any reason. “Physicians call with all kinds of issues. In the first 3 months, it was COVID, but then they called with other concerns – there were doctors who called with election anxiety, really anything that affects the general public also affects us.”
The group has also offered Saturday didactic sessions for volunteers and weekly debriefing sessions. Dr. Masood has been approached by Vibrant Emotional Health, the administrator of the National Suicide Prevention Lifeline, about resources to help with funding – until now, this endeavor has had no financing – and she is hopeful that their financial support will allow the support line to sustain itself and grow. Future directions include advocating for systemic change in how physician mental health and wellness issues are addressed.
The Physician Support Line was one psychiatrist’s vision for how to address a problem. Like so many things related to this pandemic, it happened quickly and with surprising efficiency. Implementing this service, however, was not easy – it required hard work, innovative thinking, and passion. Those looking for someone to listen can call 1-888-409-0141 and psychiatrists who wish to volunteer can sign up at physiciansupportline.com/volunteer-info.
Dr. Miller is coauthor of “Committed: The Battle Over Involuntary Psychiatric Care” (Johns Hopkins University Press, 2018). She has a private practice and is assistant professor of psychiatry and behavioral sciences at Johns Hopkins University, both in Baltimore.
Have you ever had a really good idea about how to improve the delivery of mental health services? An idea that would help people, but that would require passion, innovation, and hard work to implement, and one that immediately is beset with a list of reasons why it can not be implemented?
Mona Masood, DO, had an idea. The Pennsylvania psychiatrist was asked to help moderate a Facebook group started by one of her infectious disease colleagues last winter – a private Facebook group for physicians working with COVID-19 patients.
“The group was getting posts from frontline workers about how depressed and hopeless they were feeling.” Dr. Masood said. “People were posting about how they were having escape fantasies and how they regretted becoming physicians. It became clear that there was a need for more support.”
psychiatrist volunteers would take calls from physicians who needed someone to talk to – the psychiatrist would provide a sympathetic ear and have a list of resources, but this would be support, not treatment. There would be no prescriptions, no treatment relationship, no reporting to licensing boards or employers. The calls would be anonymous.
She posted her idea on the Facebook group, and the response was immediate. “There were a lot of emails – 200 psychiatrists responded saying: “Sign me up.” A Zoom meeting was set up, and the process was set in motion.
Dr. Masood used a Google document for weekly sign-ups so the volunteer psychiatrists could choose times. “We had to pay for an upgraded Google suite package for that many users. Getting this up and running was like the saying about building a plane as you fly it,” Dr. Masood said. “It forced so much so quickly because there was this acknowledgment that the need was there.”
Initially, the support line launched with a telehealth platform, but there was a problem. “Many doctors don’t want to be seen; they worry about being recognized.” Dr. Masood researched hotline phone services and was able to get one for a reduced fee. The volunteers have an App on their smartphones that enables them to log in at the start of their shifts and log out at the end. In addition to the logistics of coordinating the volunteers – now numbering over 700 – the group found a health care law firm that provided pro bono services to review the policies and procedures.
Now that the support line is running, Dr. Masood is able to set up the day’s volunteers for the support line connection in a few minutes each morning, but the beginning was not easy. Her private practice transitioned to telemedicine, and her two children were home with one in virtual school. “At first, it was like another full-time job.” She still remains available for trouble-shooting during the day. It’s a project she has taken on with passion.
The support line began as a response to watching colleagues struggle with COVID. Since it launched, there have been approximately 2,000 calls. Calls typically last for 20 to 90 minutes, and no one has called with a suicidal crisis. It is now open to doctors and medical students looking for support for any reason. “Physicians call with all kinds of issues. In the first 3 months, it was COVID, but then they called with other concerns – there were doctors who called with election anxiety, really anything that affects the general public also affects us.”
The group has also offered Saturday didactic sessions for volunteers and weekly debriefing sessions. Dr. Masood has been approached by Vibrant Emotional Health, the administrator of the National Suicide Prevention Lifeline, about resources to help with funding – until now, this endeavor has had no financing – and she is hopeful that their financial support will allow the support line to sustain itself and grow. Future directions include advocating for systemic change in how physician mental health and wellness issues are addressed.
The Physician Support Line was one psychiatrist’s vision for how to address a problem. Like so many things related to this pandemic, it happened quickly and with surprising efficiency. Implementing this service, however, was not easy – it required hard work, innovative thinking, and passion. Those looking for someone to listen can call 1-888-409-0141 and psychiatrists who wish to volunteer can sign up at physiciansupportline.com/volunteer-info.
Dr. Miller is coauthor of “Committed: The Battle Over Involuntary Psychiatric Care” (Johns Hopkins University Press, 2018). She has a private practice and is assistant professor of psychiatry and behavioral sciences at Johns Hopkins University, both in Baltimore.
We need to apply the evidence to nonphysician practice
The Journal of Family Practice rightfully places a high priority on evidence-based practice by including “strength of evidence” qualifiers to help physicians analyze scientific studies and emphasizing campaigns that encourage good stewardship of medical resources. The editorial “When patients don’t get the care they should” (J Fam Pract. 2020;69:427) struck on an often-neglected aspect of evidence-based practice: the increase in care provided by nonphysician practitioners.
Henry Silver, MD, created the first pediatric nurse practitioner (NP) training program at the University of Colorado in 1965. That same year, Eugene Stead, MD, created the first physician assistant (PA) program at Duke University. The goal of both professions was simple: to create physician extenders to reach medically needy patients in underserved areas. But over the past 20 years, NPs and PAs have increasingly sought—and legislatively gained—independent practice, the right to treat patients without physician supervision.
Here’s where evidence-based practice comes in. Despite claims by NP advocates that “50 years of evidence” shows safe and effective practice, the truth is that there is no scientific evidence that nonphysicians can practice safely and effectively without physician supervision. The best meta-analysis of nurse practitioner care, a Cochrane review, found only 18 studies of adequate quality to analyze.1 Of these, only 3 were performed in the United States, and every single study in the Cochrane review involved nurses working under physician supervision or following physician-created protocols. Yes, even supposedly independent NPs in Mary Mundinger’s famous 2000 study were practicing under a collaborating physician, as required by New York statute at the time. In addition, NPs in the study were assigned a physician mentor and received an additional 9 months of training with medical residents.
Regarding the emphasis for physicians to “choose wisely,” research raises concerns about an overuse of health care resources by nonphysician practitioners.Studies show that nonphysician practitioners order more labs2 and radiographic tests3 than physicians; prescribe more medications, including opioids,4 antipsychotics,4 and antibiotics5 than physicians; place lower-quality referrals than physicians6; and perform significantly more biopsies than physicians to diagnose malignant neoplasms in patients < 65 years.7
As the rate of nonphysician practitioners increases (significantly outpacing the growth of physicians), we must be cognizant of the rising risks to our patients in the absence of appropriate physician oversight.8 This issue is so concerning to me that I co-authored a book on the subject.8 I encourage all physicians to educate themselves on this topic and make practice decisions with the evidence in mind.
1. Laurant M, van der Biezen M, Wijers N, et al. Nurses as substitutes for doctors in primary care. Cochrane Database of Syst Rev. 2018;(7):CD001271. doi: 10.1002/14651858.CD001271.pub3
2. Flynn, BC. The effectiveness of nurse clinicians’ service delivery. AJPH. 1974;64:604-611.
3. Hughes DR, Jiang M, Duszak R. A comparison of diagnostic imaging ordering patterns between advanced practice clinicians and primary care physicians following office-based evaluation and management visits. JAMA Intern Med. 2015;175:101–107. doi:10.1001/jamainternmed.2014.6349
4. Muench, U, Perloff J, Thomas C, et al. Prescribing practices by nurse practitioners and primary care physicians: a descriptive analysis of Medicare beneficiaries. Journal of Nursing Regulation. 2017;8:21-30. doi: https://doi.org/10.1016/S2155-8256(17)30071-6
5. Sanchez GV, Hersh AL, Shapiro DJ, et al. Outpatient antibiotic prescribing among United States nurse practitioners and physician assistants. Open Forum Infect Dis. 2016;10:ofw168. doi: 10.1093/ofid/ofw168.
6. Lohr RH, West CP, Beliveau M, et al. Comparison of the quality of patient referrals from physicians, physician assistants, and nurse practitioners. Mayo Clin Proc. 2013;88:1266‐1271. doi:10.1016/j.mayocp.2013.08.013
7. Nault A, Zhang C, Kim KM, et al. Biopsy use in skin cancer diagnosis: comparing dermatology physicians and advanced practice professionals. JAMA Dermatol. 2015;151:899-901. doi:10.1001/jamadermatol.2015.0173
8. Al-Agba N, Bernard R. Patients at Risk: The Rise of the Nurse Practitioner and Physician Assistant in Healthcare. Universal Publishers; 2020.
The Journal of Family Practice rightfully places a high priority on evidence-based practice by including “strength of evidence” qualifiers to help physicians analyze scientific studies and emphasizing campaigns that encourage good stewardship of medical resources. The editorial “When patients don’t get the care they should” (J Fam Pract. 2020;69:427) struck on an often-neglected aspect of evidence-based practice: the increase in care provided by nonphysician practitioners.
Henry Silver, MD, created the first pediatric nurse practitioner (NP) training program at the University of Colorado in 1965. That same year, Eugene Stead, MD, created the first physician assistant (PA) program at Duke University. The goal of both professions was simple: to create physician extenders to reach medically needy patients in underserved areas. But over the past 20 years, NPs and PAs have increasingly sought—and legislatively gained—independent practice, the right to treat patients without physician supervision.
Here’s where evidence-based practice comes in. Despite claims by NP advocates that “50 years of evidence” shows safe and effective practice, the truth is that there is no scientific evidence that nonphysicians can practice safely and effectively without physician supervision. The best meta-analysis of nurse practitioner care, a Cochrane review, found only 18 studies of adequate quality to analyze.1 Of these, only 3 were performed in the United States, and every single study in the Cochrane review involved nurses working under physician supervision or following physician-created protocols. Yes, even supposedly independent NPs in Mary Mundinger’s famous 2000 study were practicing under a collaborating physician, as required by New York statute at the time. In addition, NPs in the study were assigned a physician mentor and received an additional 9 months of training with medical residents.
Regarding the emphasis for physicians to “choose wisely,” research raises concerns about an overuse of health care resources by nonphysician practitioners.Studies show that nonphysician practitioners order more labs2 and radiographic tests3 than physicians; prescribe more medications, including opioids,4 antipsychotics,4 and antibiotics5 than physicians; place lower-quality referrals than physicians6; and perform significantly more biopsies than physicians to diagnose malignant neoplasms in patients < 65 years.7
As the rate of nonphysician practitioners increases (significantly outpacing the growth of physicians), we must be cognizant of the rising risks to our patients in the absence of appropriate physician oversight.8 This issue is so concerning to me that I co-authored a book on the subject.8 I encourage all physicians to educate themselves on this topic and make practice decisions with the evidence in mind.
The Journal of Family Practice rightfully places a high priority on evidence-based practice by including “strength of evidence” qualifiers to help physicians analyze scientific studies and emphasizing campaigns that encourage good stewardship of medical resources. The editorial “When patients don’t get the care they should” (J Fam Pract. 2020;69:427) struck on an often-neglected aspect of evidence-based practice: the increase in care provided by nonphysician practitioners.
Henry Silver, MD, created the first pediatric nurse practitioner (NP) training program at the University of Colorado in 1965. That same year, Eugene Stead, MD, created the first physician assistant (PA) program at Duke University. The goal of both professions was simple: to create physician extenders to reach medically needy patients in underserved areas. But over the past 20 years, NPs and PAs have increasingly sought—and legislatively gained—independent practice, the right to treat patients without physician supervision.
Here’s where evidence-based practice comes in. Despite claims by NP advocates that “50 years of evidence” shows safe and effective practice, the truth is that there is no scientific evidence that nonphysicians can practice safely and effectively without physician supervision. The best meta-analysis of nurse practitioner care, a Cochrane review, found only 18 studies of adequate quality to analyze.1 Of these, only 3 were performed in the United States, and every single study in the Cochrane review involved nurses working under physician supervision or following physician-created protocols. Yes, even supposedly independent NPs in Mary Mundinger’s famous 2000 study were practicing under a collaborating physician, as required by New York statute at the time. In addition, NPs in the study were assigned a physician mentor and received an additional 9 months of training with medical residents.
Regarding the emphasis for physicians to “choose wisely,” research raises concerns about an overuse of health care resources by nonphysician practitioners.Studies show that nonphysician practitioners order more labs2 and radiographic tests3 than physicians; prescribe more medications, including opioids,4 antipsychotics,4 and antibiotics5 than physicians; place lower-quality referrals than physicians6; and perform significantly more biopsies than physicians to diagnose malignant neoplasms in patients < 65 years.7
As the rate of nonphysician practitioners increases (significantly outpacing the growth of physicians), we must be cognizant of the rising risks to our patients in the absence of appropriate physician oversight.8 This issue is so concerning to me that I co-authored a book on the subject.8 I encourage all physicians to educate themselves on this topic and make practice decisions with the evidence in mind.
1. Laurant M, van der Biezen M, Wijers N, et al. Nurses as substitutes for doctors in primary care. Cochrane Database of Syst Rev. 2018;(7):CD001271. doi: 10.1002/14651858.CD001271.pub3
2. Flynn, BC. The effectiveness of nurse clinicians’ service delivery. AJPH. 1974;64:604-611.
3. Hughes DR, Jiang M, Duszak R. A comparison of diagnostic imaging ordering patterns between advanced practice clinicians and primary care physicians following office-based evaluation and management visits. JAMA Intern Med. 2015;175:101–107. doi:10.1001/jamainternmed.2014.6349
4. Muench, U, Perloff J, Thomas C, et al. Prescribing practices by nurse practitioners and primary care physicians: a descriptive analysis of Medicare beneficiaries. Journal of Nursing Regulation. 2017;8:21-30. doi: https://doi.org/10.1016/S2155-8256(17)30071-6
5. Sanchez GV, Hersh AL, Shapiro DJ, et al. Outpatient antibiotic prescribing among United States nurse practitioners and physician assistants. Open Forum Infect Dis. 2016;10:ofw168. doi: 10.1093/ofid/ofw168.
6. Lohr RH, West CP, Beliveau M, et al. Comparison of the quality of patient referrals from physicians, physician assistants, and nurse practitioners. Mayo Clin Proc. 2013;88:1266‐1271. doi:10.1016/j.mayocp.2013.08.013
7. Nault A, Zhang C, Kim KM, et al. Biopsy use in skin cancer diagnosis: comparing dermatology physicians and advanced practice professionals. JAMA Dermatol. 2015;151:899-901. doi:10.1001/jamadermatol.2015.0173
8. Al-Agba N, Bernard R. Patients at Risk: The Rise of the Nurse Practitioner and Physician Assistant in Healthcare. Universal Publishers; 2020.
1. Laurant M, van der Biezen M, Wijers N, et al. Nurses as substitutes for doctors in primary care. Cochrane Database of Syst Rev. 2018;(7):CD001271. doi: 10.1002/14651858.CD001271.pub3
2. Flynn, BC. The effectiveness of nurse clinicians’ service delivery. AJPH. 1974;64:604-611.
3. Hughes DR, Jiang M, Duszak R. A comparison of diagnostic imaging ordering patterns between advanced practice clinicians and primary care physicians following office-based evaluation and management visits. JAMA Intern Med. 2015;175:101–107. doi:10.1001/jamainternmed.2014.6349
4. Muench, U, Perloff J, Thomas C, et al. Prescribing practices by nurse practitioners and primary care physicians: a descriptive analysis of Medicare beneficiaries. Journal of Nursing Regulation. 2017;8:21-30. doi: https://doi.org/10.1016/S2155-8256(17)30071-6
5. Sanchez GV, Hersh AL, Shapiro DJ, et al. Outpatient antibiotic prescribing among United States nurse practitioners and physician assistants. Open Forum Infect Dis. 2016;10:ofw168. doi: 10.1093/ofid/ofw168.
6. Lohr RH, West CP, Beliveau M, et al. Comparison of the quality of patient referrals from physicians, physician assistants, and nurse practitioners. Mayo Clin Proc. 2013;88:1266‐1271. doi:10.1016/j.mayocp.2013.08.013
7. Nault A, Zhang C, Kim KM, et al. Biopsy use in skin cancer diagnosis: comparing dermatology physicians and advanced practice professionals. JAMA Dermatol. 2015;151:899-901. doi:10.1001/jamadermatol.2015.0173
8. Al-Agba N, Bernard R. Patients at Risk: The Rise of the Nurse Practitioner and Physician Assistant in Healthcare. Universal Publishers; 2020.
Near-infrared imaging for tumors deep in tissue, such as GISTs
A new technique in which near-infrared hyperspectral imaging (NIR-HSI) is combined with machine learning could be useful for detecting cancers deep within tissue, suggests a research team from Japan.
An example of such a cancer is gastrointestinal stromal tumor (GIST). These tumors are often located in the submucosal layer and are covered by mucosal tissue, making diagnosis by conventional endoscopy difficult.
In a study to assess the new technique, the researchers examined 12 GIST lesions that were surgically resected. Seven of these lesions were totally covered by a mucosal layer (thickness, 0.4-2.5 mm); three lesions were partially covered.
Using NIR-HSI with machine learning, the researchers found that GIST lesions appeared green and that normal tissue appeared yellow. Calculation of classified pixels showed that the technique detected the GISTs with a specificity of 73%, a sensitivity of 91.3%, and an accuracy of 86.1%.
The findings were published in Nature’s Scientific Reports.
“There are many situations where we need to be able to detect cancers deep in the tissue that are not visible during surgery or diagnosis,” said lead author Toshihiro Takamatsu, PhD, assistant professor, Tokyo University of Science. “Near-infrared hyperspectral imaging has a strong potential to detect deep lesions,” he said.
In this study, the imaging was performed ex vivo.
“The data presented in the paper were not obtained endoscopically but utilized surgical specimens to demonstrate proof of concept. Additional data would be required to demonstrate its feasibility with endoscopy,” noted Margaret von Mehren, MD, chief, division of sarcoma medical oncology, Fox Chase Cancer Center, Philadelphia, who was approached for comment.
Dr. Takamatsu said his team is currently developing prototypes of laparoscopes and endoscopes for NIR-HSI.
“This technology is being developed to be added onto endoscopy, and as such I think may well be feasible,” Dr. von Mehren commented.
Currently diagnosed by endoscopy and biopsy
GISTs are found predominantly in the stomach (60%) and the small intestine (30%). Although some are detected after the occurrence of symptoms such as pain, gastrointestinal bleeding, and bowel obstruction, most cases are asymptomatic.
The authors note that endoscopic examination is the primary tool for detecting GISTs. Lesions usually first appear as submucosal tumors. Direct observation cannot differentially diagnose these tumors, and biopsies may have a low diagnostic yield, because the lesions are often deep and not easily accessible.
Endoscopic ultrasound-guided fine-needle aspiration can be used for taking samples for biopsy, but it can be technically demanding, and making a definitive diagnosis of GIST requires time-consuming immunohistochemical procedures, the authors write. A high-throughput, simple diagnostic technique for identifying GISTs located under the mucosa is needed. They report on the potential of NIR-HSI for diagnosing submucosal tumors that present deep within organs.
Study details
For the study, the team worked on GIST lesions that had been surgically resected from 12 patients. The median size of the tumors was 41 mm (range, 24-80 mm).
The researchers imaged each specimen with an NIR hyperspectral camera from the aspect of the mucosal surface.
The site of the GIST was defined by a pathologist who used the NIR image to prepare training data for normal regions and regions with GISTs. A machine learning algorithm–support vector machine was then used to predict normal and GIST regions.
The results were displayed using color-coded regions. The team says the results from this small study show that the technique has “great potential” in the diagnosis of GISTs as well as other tumors that are located deep within tissue.
“I think the potential benefit of such a diagnostic tool is with small lesions when trying to differentiate benign findings, such as leiomyomas,” Dr. von Mehren commented.
She pointed out that this study comes from Japan, where endoscopy is routinely used for surveillance of gastric cancer. Thus, many cases of GIST may be diagnosed before symptoms occur and when lesions are small. Such a practice is less common in other parts of the world, she told this news organization.
Overall, she sees this technique as having the most utility in cases involving small lesions, inasmuch as larger lesions are easily assessed through biopsy with endoscopic ultrasound, and pathologic assessment is not a lengthy procedure. “In addition, as we now appreciate that GISTs with different molecular drivers require different therapeutic approaches, I continue to see a role for tissue biopsies in the diagnostic workup of GISTs,” she said.
“Current approaches using endoscopic ultrasound have defined criteria for lesions more likely to be GISTs rather than benign lesions,” she added. “I would want to see a comparison of near-infrared hyperspectral imaging data compared to endoscopic ultrasound to see if this provides additional benefit to our current approaches.”
The study was partially funded by the National Cancer Center Research and Development Fund. The authors and Dr. von Mehren have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A new technique in which near-infrared hyperspectral imaging (NIR-HSI) is combined with machine learning could be useful for detecting cancers deep within tissue, suggests a research team from Japan.
An example of such a cancer is gastrointestinal stromal tumor (GIST). These tumors are often located in the submucosal layer and are covered by mucosal tissue, making diagnosis by conventional endoscopy difficult.
In a study to assess the new technique, the researchers examined 12 GIST lesions that were surgically resected. Seven of these lesions were totally covered by a mucosal layer (thickness, 0.4-2.5 mm); three lesions were partially covered.
Using NIR-HSI with machine learning, the researchers found that GIST lesions appeared green and that normal tissue appeared yellow. Calculation of classified pixels showed that the technique detected the GISTs with a specificity of 73%, a sensitivity of 91.3%, and an accuracy of 86.1%.
The findings were published in Nature’s Scientific Reports.
“There are many situations where we need to be able to detect cancers deep in the tissue that are not visible during surgery or diagnosis,” said lead author Toshihiro Takamatsu, PhD, assistant professor, Tokyo University of Science. “Near-infrared hyperspectral imaging has a strong potential to detect deep lesions,” he said.
In this study, the imaging was performed ex vivo.
“The data presented in the paper were not obtained endoscopically but utilized surgical specimens to demonstrate proof of concept. Additional data would be required to demonstrate its feasibility with endoscopy,” noted Margaret von Mehren, MD, chief, division of sarcoma medical oncology, Fox Chase Cancer Center, Philadelphia, who was approached for comment.
Dr. Takamatsu said his team is currently developing prototypes of laparoscopes and endoscopes for NIR-HSI.
“This technology is being developed to be added onto endoscopy, and as such I think may well be feasible,” Dr. von Mehren commented.
Currently diagnosed by endoscopy and biopsy
GISTs are found predominantly in the stomach (60%) and the small intestine (30%). Although some are detected after the occurrence of symptoms such as pain, gastrointestinal bleeding, and bowel obstruction, most cases are asymptomatic.
The authors note that endoscopic examination is the primary tool for detecting GISTs. Lesions usually first appear as submucosal tumors. Direct observation cannot differentially diagnose these tumors, and biopsies may have a low diagnostic yield, because the lesions are often deep and not easily accessible.
Endoscopic ultrasound-guided fine-needle aspiration can be used for taking samples for biopsy, but it can be technically demanding, and making a definitive diagnosis of GIST requires time-consuming immunohistochemical procedures, the authors write. A high-throughput, simple diagnostic technique for identifying GISTs located under the mucosa is needed. They report on the potential of NIR-HSI for diagnosing submucosal tumors that present deep within organs.
Study details
For the study, the team worked on GIST lesions that had been surgically resected from 12 patients. The median size of the tumors was 41 mm (range, 24-80 mm).
The researchers imaged each specimen with an NIR hyperspectral camera from the aspect of the mucosal surface.
The site of the GIST was defined by a pathologist who used the NIR image to prepare training data for normal regions and regions with GISTs. A machine learning algorithm–support vector machine was then used to predict normal and GIST regions.
The results were displayed using color-coded regions. The team says the results from this small study show that the technique has “great potential” in the diagnosis of GISTs as well as other tumors that are located deep within tissue.
“I think the potential benefit of such a diagnostic tool is with small lesions when trying to differentiate benign findings, such as leiomyomas,” Dr. von Mehren commented.
She pointed out that this study comes from Japan, where endoscopy is routinely used for surveillance of gastric cancer. Thus, many cases of GIST may be diagnosed before symptoms occur and when lesions are small. Such a practice is less common in other parts of the world, she told this news organization.
Overall, she sees this technique as having the most utility in cases involving small lesions, inasmuch as larger lesions are easily assessed through biopsy with endoscopic ultrasound, and pathologic assessment is not a lengthy procedure. “In addition, as we now appreciate that GISTs with different molecular drivers require different therapeutic approaches, I continue to see a role for tissue biopsies in the diagnostic workup of GISTs,” she said.
“Current approaches using endoscopic ultrasound have defined criteria for lesions more likely to be GISTs rather than benign lesions,” she added. “I would want to see a comparison of near-infrared hyperspectral imaging data compared to endoscopic ultrasound to see if this provides additional benefit to our current approaches.”
The study was partially funded by the National Cancer Center Research and Development Fund. The authors and Dr. von Mehren have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A new technique in which near-infrared hyperspectral imaging (NIR-HSI) is combined with machine learning could be useful for detecting cancers deep within tissue, suggests a research team from Japan.
An example of such a cancer is gastrointestinal stromal tumor (GIST). These tumors are often located in the submucosal layer and are covered by mucosal tissue, making diagnosis by conventional endoscopy difficult.
In a study to assess the new technique, the researchers examined 12 GIST lesions that were surgically resected. Seven of these lesions were totally covered by a mucosal layer (thickness, 0.4-2.5 mm); three lesions were partially covered.
Using NIR-HSI with machine learning, the researchers found that GIST lesions appeared green and that normal tissue appeared yellow. Calculation of classified pixels showed that the technique detected the GISTs with a specificity of 73%, a sensitivity of 91.3%, and an accuracy of 86.1%.
The findings were published in Nature’s Scientific Reports.
“There are many situations where we need to be able to detect cancers deep in the tissue that are not visible during surgery or diagnosis,” said lead author Toshihiro Takamatsu, PhD, assistant professor, Tokyo University of Science. “Near-infrared hyperspectral imaging has a strong potential to detect deep lesions,” he said.
In this study, the imaging was performed ex vivo.
“The data presented in the paper were not obtained endoscopically but utilized surgical specimens to demonstrate proof of concept. Additional data would be required to demonstrate its feasibility with endoscopy,” noted Margaret von Mehren, MD, chief, division of sarcoma medical oncology, Fox Chase Cancer Center, Philadelphia, who was approached for comment.
Dr. Takamatsu said his team is currently developing prototypes of laparoscopes and endoscopes for NIR-HSI.
“This technology is being developed to be added onto endoscopy, and as such I think may well be feasible,” Dr. von Mehren commented.
Currently diagnosed by endoscopy and biopsy
GISTs are found predominantly in the stomach (60%) and the small intestine (30%). Although some are detected after the occurrence of symptoms such as pain, gastrointestinal bleeding, and bowel obstruction, most cases are asymptomatic.
The authors note that endoscopic examination is the primary tool for detecting GISTs. Lesions usually first appear as submucosal tumors. Direct observation cannot differentially diagnose these tumors, and biopsies may have a low diagnostic yield, because the lesions are often deep and not easily accessible.
Endoscopic ultrasound-guided fine-needle aspiration can be used for taking samples for biopsy, but it can be technically demanding, and making a definitive diagnosis of GIST requires time-consuming immunohistochemical procedures, the authors write. A high-throughput, simple diagnostic technique for identifying GISTs located under the mucosa is needed. They report on the potential of NIR-HSI for diagnosing submucosal tumors that present deep within organs.
Study details
For the study, the team worked on GIST lesions that had been surgically resected from 12 patients. The median size of the tumors was 41 mm (range, 24-80 mm).
The researchers imaged each specimen with an NIR hyperspectral camera from the aspect of the mucosal surface.
The site of the GIST was defined by a pathologist who used the NIR image to prepare training data for normal regions and regions with GISTs. A machine learning algorithm–support vector machine was then used to predict normal and GIST regions.
The results were displayed using color-coded regions. The team says the results from this small study show that the technique has “great potential” in the diagnosis of GISTs as well as other tumors that are located deep within tissue.
“I think the potential benefit of such a diagnostic tool is with small lesions when trying to differentiate benign findings, such as leiomyomas,” Dr. von Mehren commented.
She pointed out that this study comes from Japan, where endoscopy is routinely used for surveillance of gastric cancer. Thus, many cases of GIST may be diagnosed before symptoms occur and when lesions are small. Such a practice is less common in other parts of the world, she told this news organization.
Overall, she sees this technique as having the most utility in cases involving small lesions, inasmuch as larger lesions are easily assessed through biopsy with endoscopic ultrasound, and pathologic assessment is not a lengthy procedure. “In addition, as we now appreciate that GISTs with different molecular drivers require different therapeutic approaches, I continue to see a role for tissue biopsies in the diagnostic workup of GISTs,” she said.
“Current approaches using endoscopic ultrasound have defined criteria for lesions more likely to be GISTs rather than benign lesions,” she added. “I would want to see a comparison of near-infrared hyperspectral imaging data compared to endoscopic ultrasound to see if this provides additional benefit to our current approaches.”
The study was partially funded by the National Cancer Center Research and Development Fund. The authors and Dr. von Mehren have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.