User login
Scalp Wound Closures in Mohs Micrographic Surgery: A Survey of Staples vs Sutures
Limited data exist comparing staples and sutures for scalp closures during Mohs micrographic surgery (MMS). As a result, the closure method for these scalp wounds is based on surgeon preference without established consensus. The purpose of this study was to survey practicing Mohs surgeons on their scalp wound closure preferences as well as the clinical and economic variables that impact their decisions. Understanding practice habits can guide future trial design, with a goal of creating established criterion for MMS scalp wound closures.
Methods
An anonymous survey was distributed from April 2019 to June 2019 to fellowship-trained Mohs surgeons using an electronic mailing list from the American College of Mohs Surgery (ACMS). The 10-question survey was approved by the University of Kansas institutional review board and the executive committee of the ACMS. Surgeons were asked about their preferred method for scalp wound closure as well as clinical and economic variables that impacted those preferences. Respondents indicated their frequency of using deep sutures, epidermal sutures, and wound undermining on a sliding scale of 0% to 100%. Comparisons were made between practice habits, preferences, and surgeon demographics using t tests. Statistical significance was determined as P<.05.
Results
Sixty-eight ACMS fellowship-trained Mohs surgeons completed the survey. The average age of respondents was 45 years; 69.1% (n=47) of respondents were male, and 76.5% (n=52) practiced in a private setting (Table 1). Regardless of epidermal closure type, deep suture placement was used in an average (standard deviation [SD]) of 88.8% (19.5%) of cases overall, which did not statistically differ between years of Mohs experience or practice setting (Table 2). Wound undermining was performed in an average (SD) of 83.0% (24.3%) of cases overall and was more prevalent in private vs academic settings (87.6% [17.8%] vs 65.7% [35.0%]; P<.01). Epidermal sutures were used in an average (SD) of 27.1% (33.5%) of scalp wound cases overall. Surgeons with less experience (≤5 years) used them more frequently (average [SD], 42.7% [36.2%] of cases) than surgeons with more experience (≥16 years; average [SD], 18.8% [32.6%] of cases; P=.037). There was no significant difference between epidermal suture placement rates and practice setting (average [SD], 18.1% [28.1%] of cases for academic providers vs 30.0% [34.8%] of cases with private providers; P=.210).

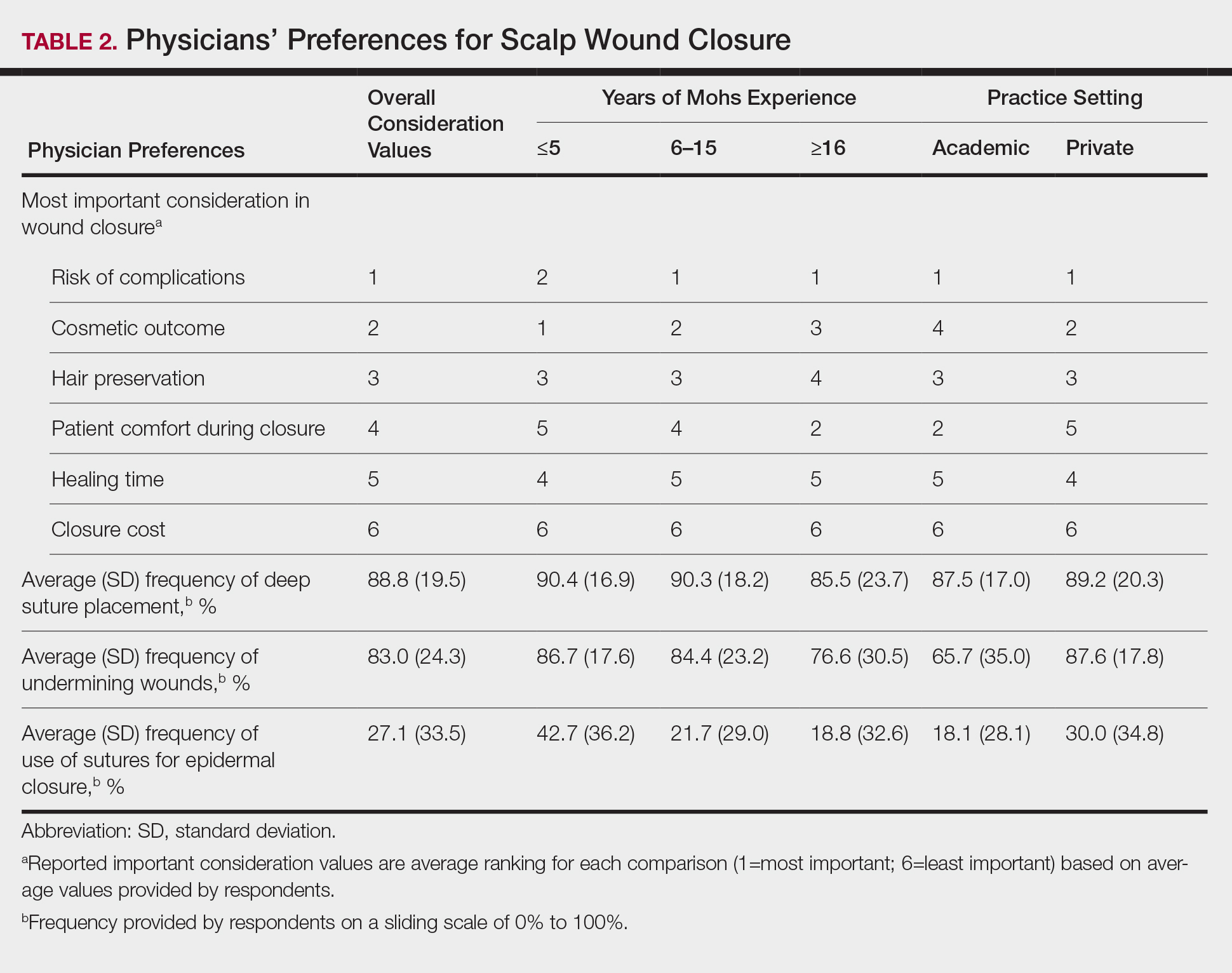
Clinical and economic factors that were most important during wound closure were ranked (beginning with most important) as the following: risk of complications, cosmetic outcome, hair preservation, patient comfort during closure, healing time, and closure cost. In all demographic cases, risk of complications was ranked 1 or 2 (1=most important; 6=least important) overall; cost was the least important factor overall (Table 2).
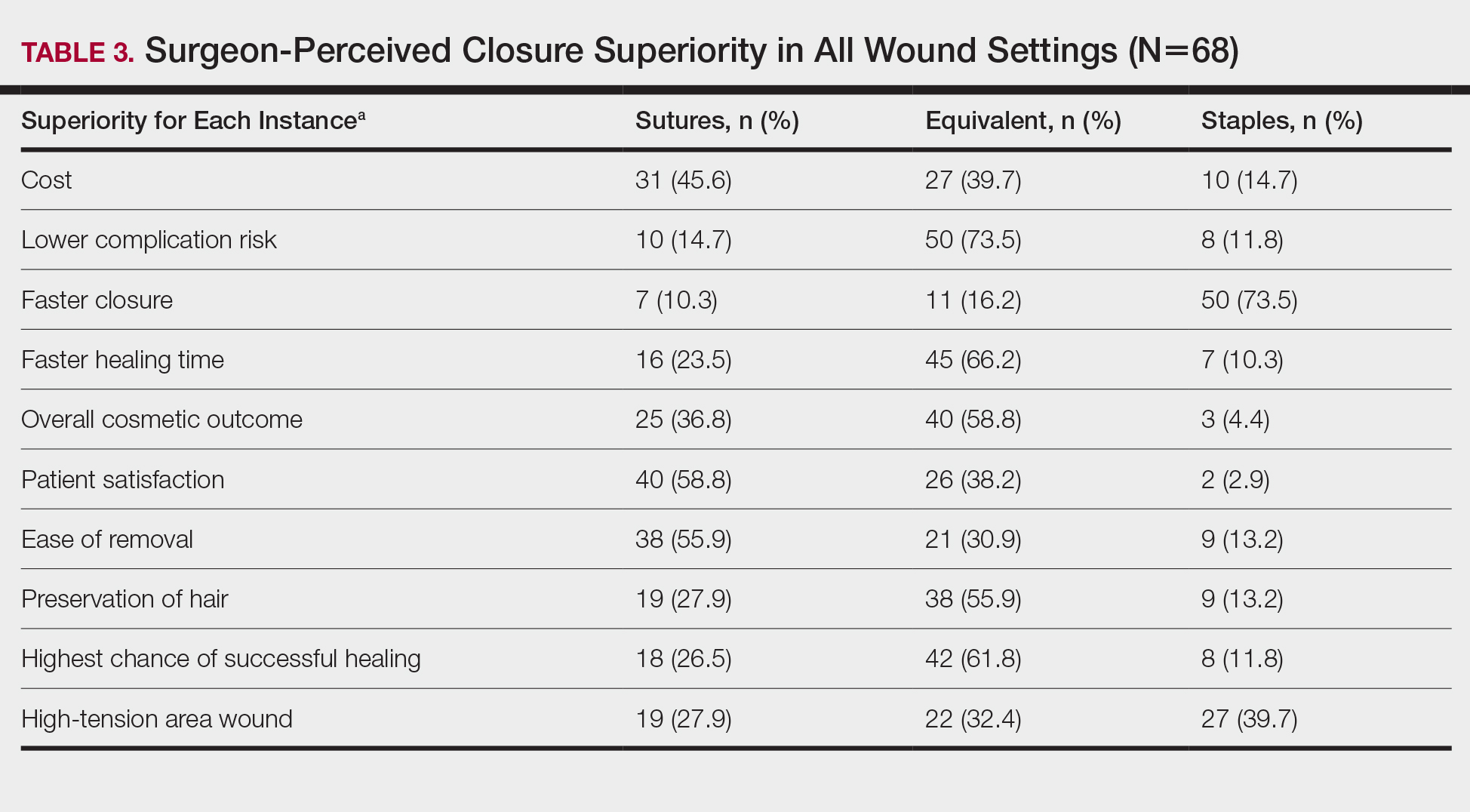
Surgeons perceived staples to be superior for speed of closure and for closing wounds in high-tension areas, whereas sutures were perceived as superior when considering cost of closure and ease of removal (Table 3). Successful healing rate, healing time, hair preservation, overall cosmetic outcome, and lower risk of complications were viewed as equivalent when comparing staples and sutures.
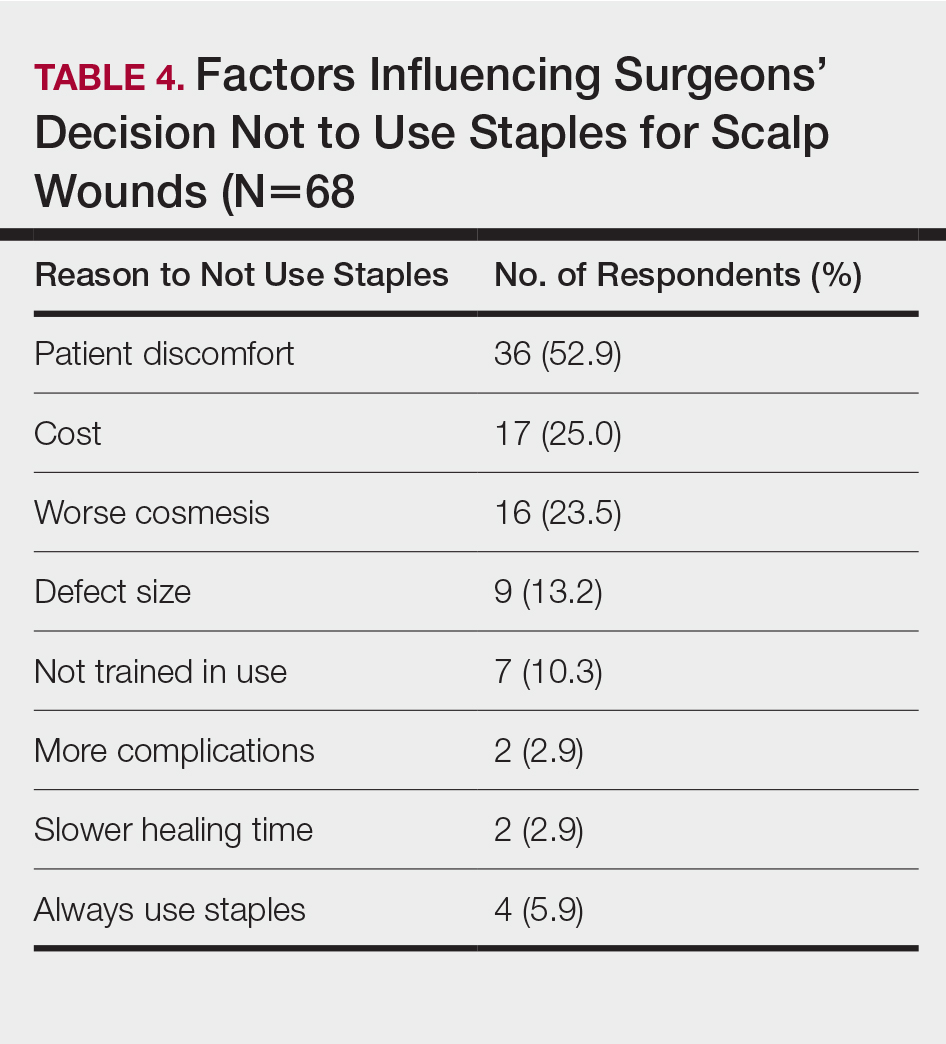
In cases in which surgeons did not use staples for closure, the most important factors for opting to not use them were patient discomfort (52.9% [n=36]), cost (25.0% [n=17]), and worse overall cosmetic outcome (23.5% [n=16])(Table 4). The most frequent locations outside of scalp wounds that physicians considered the use of staples for closure were the back (19.1% [n=13]), thigh (10.3% [n=7]), and shoulder (8.8% [n=6]).
Comment
Epidermal closure with sutures was reportedly used in an average of only 27.1% of scalp wound cases, with clinical factors such as cosmetic outcome, risk of complications, and closure time seen as either equivalent or inferior to staples. Our data suggest that surgeon closure perceptions generally are in agreement with established head and neck literature within different medical specialties that favor staple closures, particularly in high-tension areas.1 Interestingly, the most common reasons given for not using staples included patient discomfort, cost, and worse cosmetic outcomes, which are unsubstantiated with head and neck comparative studies.2-4
Although cost was the least important variable for determining closure type in our surveyed cohort, it is likely that the overall cost of closure is frequently underestimated. A higher material cost is noted with staples; however, the largest determinant of overall cost remains the surgeon’s time, which is reduced by factors of 10 or more when closing with staples.2,3 This difference—coupled with the unchanged cosmetic outcome and complication rates—makes staples more advantageous for high-tension scalp wounds.4 Moreover, the stapling technique is more reproducible than suturing, which requires more surgical skill and experience.
Limitations of this study include a lack of directly comparable data for staple and suture scalp wound closures. In addition, the small cohort of respondents in this preliminary study can serve to guide future studies.
Conclusion
Scalp wounds during MMS were most frequently closed using staples vs sutures, with the perception that these methods are equivalent in complication risk, cosmetic outcome, and overall patient satisfaction. These results agree with comparative literature for head and neck surgery and assist with establishing an epidemiologic baseline for future studies comparing their use during MMS.
- Ritchie AJ, Rocke LG. Staples versus sutures in the closure of scalp wounds: a prospective, double-blind, randomized trial. Injury. 1989;20:217-218.
- Batra J, Bekal RK, Byadgi S, et al. Comparison of skin staples and standard sutures for closing incisions after head and neck cancer surgery: a double-blind, randomized and prospective study. J Maxillofac Oral Surg. 2016;15:243-250.
- Kanegaye JT, Vance CW, Chan L, et al. Comparison of skin stapling devices and standard sutures for pediatric scalp lacerations: a randomized study of cost and time benefits. J Pediatr. 1997;130:808-813.
- Khan ANGA, Dayan PS, Miller S, et al. Cosmetic outcome of scalp wound closure with staples in the pediatric emergency department: a prospective, randomized trial. Pediatr Emerg Care. 2002;18:171-173.
Limited data exist comparing staples and sutures for scalp closures during Mohs micrographic surgery (MMS). As a result, the closure method for these scalp wounds is based on surgeon preference without established consensus. The purpose of this study was to survey practicing Mohs surgeons on their scalp wound closure preferences as well as the clinical and economic variables that impact their decisions. Understanding practice habits can guide future trial design, with a goal of creating established criterion for MMS scalp wound closures.
Methods
An anonymous survey was distributed from April 2019 to June 2019 to fellowship-trained Mohs surgeons using an electronic mailing list from the American College of Mohs Surgery (ACMS). The 10-question survey was approved by the University of Kansas institutional review board and the executive committee of the ACMS. Surgeons were asked about their preferred method for scalp wound closure as well as clinical and economic variables that impacted those preferences. Respondents indicated their frequency of using deep sutures, epidermal sutures, and wound undermining on a sliding scale of 0% to 100%. Comparisons were made between practice habits, preferences, and surgeon demographics using t tests. Statistical significance was determined as P<.05.
Results
Sixty-eight ACMS fellowship-trained Mohs surgeons completed the survey. The average age of respondents was 45 years; 69.1% (n=47) of respondents were male, and 76.5% (n=52) practiced in a private setting (Table 1). Regardless of epidermal closure type, deep suture placement was used in an average (standard deviation [SD]) of 88.8% (19.5%) of cases overall, which did not statistically differ between years of Mohs experience or practice setting (Table 2). Wound undermining was performed in an average (SD) of 83.0% (24.3%) of cases overall and was more prevalent in private vs academic settings (87.6% [17.8%] vs 65.7% [35.0%]; P<.01). Epidermal sutures were used in an average (SD) of 27.1% (33.5%) of scalp wound cases overall. Surgeons with less experience (≤5 years) used them more frequently (average [SD], 42.7% [36.2%] of cases) than surgeons with more experience (≥16 years; average [SD], 18.8% [32.6%] of cases; P=.037). There was no significant difference between epidermal suture placement rates and practice setting (average [SD], 18.1% [28.1%] of cases for academic providers vs 30.0% [34.8%] of cases with private providers; P=.210).


Clinical and economic factors that were most important during wound closure were ranked (beginning with most important) as the following: risk of complications, cosmetic outcome, hair preservation, patient comfort during closure, healing time, and closure cost. In all demographic cases, risk of complications was ranked 1 or 2 (1=most important; 6=least important) overall; cost was the least important factor overall (Table 2).
Surgeons perceived staples to be superior for speed of closure and for closing wounds in high-tension areas, whereas sutures were perceived as superior when considering cost of closure and ease of removal (Table 3). Successful healing rate, healing time, hair preservation, overall cosmetic outcome, and lower risk of complications were viewed as equivalent when comparing staples and sutures.

In cases in which surgeons did not use staples for closure, the most important factors for opting to not use them were patient discomfort (52.9% [n=36]), cost (25.0% [n=17]), and worse overall cosmetic outcome (23.5% [n=16])(Table 4). The most frequent locations outside of scalp wounds that physicians considered the use of staples for closure were the back (19.1% [n=13]), thigh (10.3% [n=7]), and shoulder (8.8% [n=6]).

Comment
Epidermal closure with sutures was reportedly used in an average of only 27.1% of scalp wound cases, with clinical factors such as cosmetic outcome, risk of complications, and closure time seen as either equivalent or inferior to staples. Our data suggest that surgeon closure perceptions generally are in agreement with established head and neck literature within different medical specialties that favor staple closures, particularly in high-tension areas.1 Interestingly, the most common reasons given for not using staples included patient discomfort, cost, and worse cosmetic outcomes, which are unsubstantiated with head and neck comparative studies.2-4
Although cost was the least important variable for determining closure type in our surveyed cohort, it is likely that the overall cost of closure is frequently underestimated. A higher material cost is noted with staples; however, the largest determinant of overall cost remains the surgeon’s time, which is reduced by factors of 10 or more when closing with staples.2,3 This difference—coupled with the unchanged cosmetic outcome and complication rates—makes staples more advantageous for high-tension scalp wounds.4 Moreover, the stapling technique is more reproducible than suturing, which requires more surgical skill and experience.
Limitations of this study include a lack of directly comparable data for staple and suture scalp wound closures. In addition, the small cohort of respondents in this preliminary study can serve to guide future studies.
Conclusion
Scalp wounds during MMS were most frequently closed using staples vs sutures, with the perception that these methods are equivalent in complication risk, cosmetic outcome, and overall patient satisfaction. These results agree with comparative literature for head and neck surgery and assist with establishing an epidemiologic baseline for future studies comparing their use during MMS.
Limited data exist comparing staples and sutures for scalp closures during Mohs micrographic surgery (MMS). As a result, the closure method for these scalp wounds is based on surgeon preference without established consensus. The purpose of this study was to survey practicing Mohs surgeons on their scalp wound closure preferences as well as the clinical and economic variables that impact their decisions. Understanding practice habits can guide future trial design, with a goal of creating established criterion for MMS scalp wound closures.
Methods
An anonymous survey was distributed from April 2019 to June 2019 to fellowship-trained Mohs surgeons using an electronic mailing list from the American College of Mohs Surgery (ACMS). The 10-question survey was approved by the University of Kansas institutional review board and the executive committee of the ACMS. Surgeons were asked about their preferred method for scalp wound closure as well as clinical and economic variables that impacted those preferences. Respondents indicated their frequency of using deep sutures, epidermal sutures, and wound undermining on a sliding scale of 0% to 100%. Comparisons were made between practice habits, preferences, and surgeon demographics using t tests. Statistical significance was determined as P<.05.
Results
Sixty-eight ACMS fellowship-trained Mohs surgeons completed the survey. The average age of respondents was 45 years; 69.1% (n=47) of respondents were male, and 76.5% (n=52) practiced in a private setting (Table 1). Regardless of epidermal closure type, deep suture placement was used in an average (standard deviation [SD]) of 88.8% (19.5%) of cases overall, which did not statistically differ between years of Mohs experience or practice setting (Table 2). Wound undermining was performed in an average (SD) of 83.0% (24.3%) of cases overall and was more prevalent in private vs academic settings (87.6% [17.8%] vs 65.7% [35.0%]; P<.01). Epidermal sutures were used in an average (SD) of 27.1% (33.5%) of scalp wound cases overall. Surgeons with less experience (≤5 years) used them more frequently (average [SD], 42.7% [36.2%] of cases) than surgeons with more experience (≥16 years; average [SD], 18.8% [32.6%] of cases; P=.037). There was no significant difference between epidermal suture placement rates and practice setting (average [SD], 18.1% [28.1%] of cases for academic providers vs 30.0% [34.8%] of cases with private providers; P=.210).


Clinical and economic factors that were most important during wound closure were ranked (beginning with most important) as the following: risk of complications, cosmetic outcome, hair preservation, patient comfort during closure, healing time, and closure cost. In all demographic cases, risk of complications was ranked 1 or 2 (1=most important; 6=least important) overall; cost was the least important factor overall (Table 2).
Surgeons perceived staples to be superior for speed of closure and for closing wounds in high-tension areas, whereas sutures were perceived as superior when considering cost of closure and ease of removal (Table 3). Successful healing rate, healing time, hair preservation, overall cosmetic outcome, and lower risk of complications were viewed as equivalent when comparing staples and sutures.

In cases in which surgeons did not use staples for closure, the most important factors for opting to not use them were patient discomfort (52.9% [n=36]), cost (25.0% [n=17]), and worse overall cosmetic outcome (23.5% [n=16])(Table 4). The most frequent locations outside of scalp wounds that physicians considered the use of staples for closure were the back (19.1% [n=13]), thigh (10.3% [n=7]), and shoulder (8.8% [n=6]).

Comment
Epidermal closure with sutures was reportedly used in an average of only 27.1% of scalp wound cases, with clinical factors such as cosmetic outcome, risk of complications, and closure time seen as either equivalent or inferior to staples. Our data suggest that surgeon closure perceptions generally are in agreement with established head and neck literature within different medical specialties that favor staple closures, particularly in high-tension areas.1 Interestingly, the most common reasons given for not using staples included patient discomfort, cost, and worse cosmetic outcomes, which are unsubstantiated with head and neck comparative studies.2-4
Although cost was the least important variable for determining closure type in our surveyed cohort, it is likely that the overall cost of closure is frequently underestimated. A higher material cost is noted with staples; however, the largest determinant of overall cost remains the surgeon’s time, which is reduced by factors of 10 or more when closing with staples.2,3 This difference—coupled with the unchanged cosmetic outcome and complication rates—makes staples more advantageous for high-tension scalp wounds.4 Moreover, the stapling technique is more reproducible than suturing, which requires more surgical skill and experience.
Limitations of this study include a lack of directly comparable data for staple and suture scalp wound closures. In addition, the small cohort of respondents in this preliminary study can serve to guide future studies.
Conclusion
Scalp wounds during MMS were most frequently closed using staples vs sutures, with the perception that these methods are equivalent in complication risk, cosmetic outcome, and overall patient satisfaction. These results agree with comparative literature for head and neck surgery and assist with establishing an epidemiologic baseline for future studies comparing their use during MMS.
- Ritchie AJ, Rocke LG. Staples versus sutures in the closure of scalp wounds: a prospective, double-blind, randomized trial. Injury. 1989;20:217-218.
- Batra J, Bekal RK, Byadgi S, et al. Comparison of skin staples and standard sutures for closing incisions after head and neck cancer surgery: a double-blind, randomized and prospective study. J Maxillofac Oral Surg. 2016;15:243-250.
- Kanegaye JT, Vance CW, Chan L, et al. Comparison of skin stapling devices and standard sutures for pediatric scalp lacerations: a randomized study of cost and time benefits. J Pediatr. 1997;130:808-813.
- Khan ANGA, Dayan PS, Miller S, et al. Cosmetic outcome of scalp wound closure with staples in the pediatric emergency department: a prospective, randomized trial. Pediatr Emerg Care. 2002;18:171-173.
- Ritchie AJ, Rocke LG. Staples versus sutures in the closure of scalp wounds: a prospective, double-blind, randomized trial. Injury. 1989;20:217-218.
- Batra J, Bekal RK, Byadgi S, et al. Comparison of skin staples and standard sutures for closing incisions after head and neck cancer surgery: a double-blind, randomized and prospective study. J Maxillofac Oral Surg. 2016;15:243-250.
- Kanegaye JT, Vance CW, Chan L, et al. Comparison of skin stapling devices and standard sutures for pediatric scalp lacerations: a randomized study of cost and time benefits. J Pediatr. 1997;130:808-813.
- Khan ANGA, Dayan PS, Miller S, et al. Cosmetic outcome of scalp wound closure with staples in the pediatric emergency department: a prospective, randomized trial. Pediatr Emerg Care. 2002;18:171-173.
Practice Points
- Scalp wounds present a unique challenge for closure during Mohs micrographic surgery due to the scalp's tendency to bleed, limited elasticity, and hair-bearing nature.
- Among fellowship-trained Mohs surgeons, scalp wounds were closed with staples more often than with epidermal sutures.
- Staples and sutures for scalp wounds were perceived to be equivalent in risk of complications, cosmetic outcome, and overall patient satisfaction.
- Compared to epidermal sutures, staples were perceived as advantageous in high-tension areas and for speed of closure.
Product News August 2020
FDA Approves Wynzora Cream for Plaque Psoriasis
MC2 Therapeutics announces US Food and Drug Administration (FDA) approval of Wynzora Cream (calcipotriene 0.005% and betamethasone dipropionate 0.064%) for once-daily treatment of plaque psoriasis in adults.
Wynzora Cream is based on PAD Technology, which enables stability of calcipotriene and betamethasone dipropionate in an aqueous formulation. Key features of PAD Technology formulations are high penetration of active ingredients to the target tissue, improved solubility and stability of active ingredients, high tolerability, and excellent treatment convenience. In the phase 3 trials conducted at multiple sites in the United States and the European Union, Wynzora Cream has demonstrated a combination of clinical efficacy, a favorable safety profile, and high convenience, offering overall better patient satisfaction in the topical treatment of plaque psoriasis in the real-world setting.
WynzoraCream is applied to affected areas once daily for up to 8 weeks and not more than 100 g per week. Patients should stop treatment when the plaque psoriasis is under control, unless a health care provider gives other instructions.
MC2 Therapeutics also has submitted a Marketing Authorization Application in the European Union for Wynzora Cream (50 µg/g calcipotriol and 0.5 mg/g betamethasone [as dipropionate]) for the treatment of plaque psoriasis. For more information, visit www.mc2therapeutics.com.
If you would like your product included in Product News, please email a press release to the Editorial Office at cutis@mdedge.com.
FDA Approves Wynzora Cream for Plaque Psoriasis
MC2 Therapeutics announces US Food and Drug Administration (FDA) approval of Wynzora Cream (calcipotriene 0.005% and betamethasone dipropionate 0.064%) for once-daily treatment of plaque psoriasis in adults.
Wynzora Cream is based on PAD Technology, which enables stability of calcipotriene and betamethasone dipropionate in an aqueous formulation. Key features of PAD Technology formulations are high penetration of active ingredients to the target tissue, improved solubility and stability of active ingredients, high tolerability, and excellent treatment convenience. In the phase 3 trials conducted at multiple sites in the United States and the European Union, Wynzora Cream has demonstrated a combination of clinical efficacy, a favorable safety profile, and high convenience, offering overall better patient satisfaction in the topical treatment of plaque psoriasis in the real-world setting.
WynzoraCream is applied to affected areas once daily for up to 8 weeks and not more than 100 g per week. Patients should stop treatment when the plaque psoriasis is under control, unless a health care provider gives other instructions.
MC2 Therapeutics also has submitted a Marketing Authorization Application in the European Union for Wynzora Cream (50 µg/g calcipotriol and 0.5 mg/g betamethasone [as dipropionate]) for the treatment of plaque psoriasis. For more information, visit www.mc2therapeutics.com.
If you would like your product included in Product News, please email a press release to the Editorial Office at cutis@mdedge.com.
FDA Approves Wynzora Cream for Plaque Psoriasis
MC2 Therapeutics announces US Food and Drug Administration (FDA) approval of Wynzora Cream (calcipotriene 0.005% and betamethasone dipropionate 0.064%) for once-daily treatment of plaque psoriasis in adults.
Wynzora Cream is based on PAD Technology, which enables stability of calcipotriene and betamethasone dipropionate in an aqueous formulation. Key features of PAD Technology formulations are high penetration of active ingredients to the target tissue, improved solubility and stability of active ingredients, high tolerability, and excellent treatment convenience. In the phase 3 trials conducted at multiple sites in the United States and the European Union, Wynzora Cream has demonstrated a combination of clinical efficacy, a favorable safety profile, and high convenience, offering overall better patient satisfaction in the topical treatment of plaque psoriasis in the real-world setting.
WynzoraCream is applied to affected areas once daily for up to 8 weeks and not more than 100 g per week. Patients should stop treatment when the plaque psoriasis is under control, unless a health care provider gives other instructions.
MC2 Therapeutics also has submitted a Marketing Authorization Application in the European Union for Wynzora Cream (50 µg/g calcipotriol and 0.5 mg/g betamethasone [as dipropionate]) for the treatment of plaque psoriasis. For more information, visit www.mc2therapeutics.com.
If you would like your product included in Product News, please email a press release to the Editorial Office at cutis@mdedge.com.
Assessment of Nail Content in the American Academy of Dermatology Patient Education Website
To the Editor:
Patients with skin, hair, or nail concerns often utilize online resources to self-diagnose or learn more about physician-diagnosed conditions. The American Academy of Dermatology (AAD) website offers the public access to informational pages categorized by disease or treatment (https://www.aad.org/public). We sought to evaluate the nail content by searching the Patients and Public section of the AAD website to qualitatively and quantitatively describe mentions of nail conditions. Psoriasis, psoriatic arthritis, atopic dermatitis, and ringworm content also were analyzed and compared to nail content. The analysis was performed on September 7, 2019.
Of the 73 topics listed in the Diseases and Treatments section of the site, 17 (23%) specifically mentioned nail symptoms or pathology (Table). Three additional topics—atopic dermatitis, cellulitis, and neurodermatitis—recommended keeping nails short to prevent injury from scratching. There was 1 mention of obtaining fungal cultures, 2 of nail scraping microscopy, 2 of nail clippings, and 2 of nail-related cancers. There were no mentions of nail biopsies. The total number of unique clinical images across all sections was 300, with 12 of nails. The video library contained 84 videos, of which 6 focused on nail health.
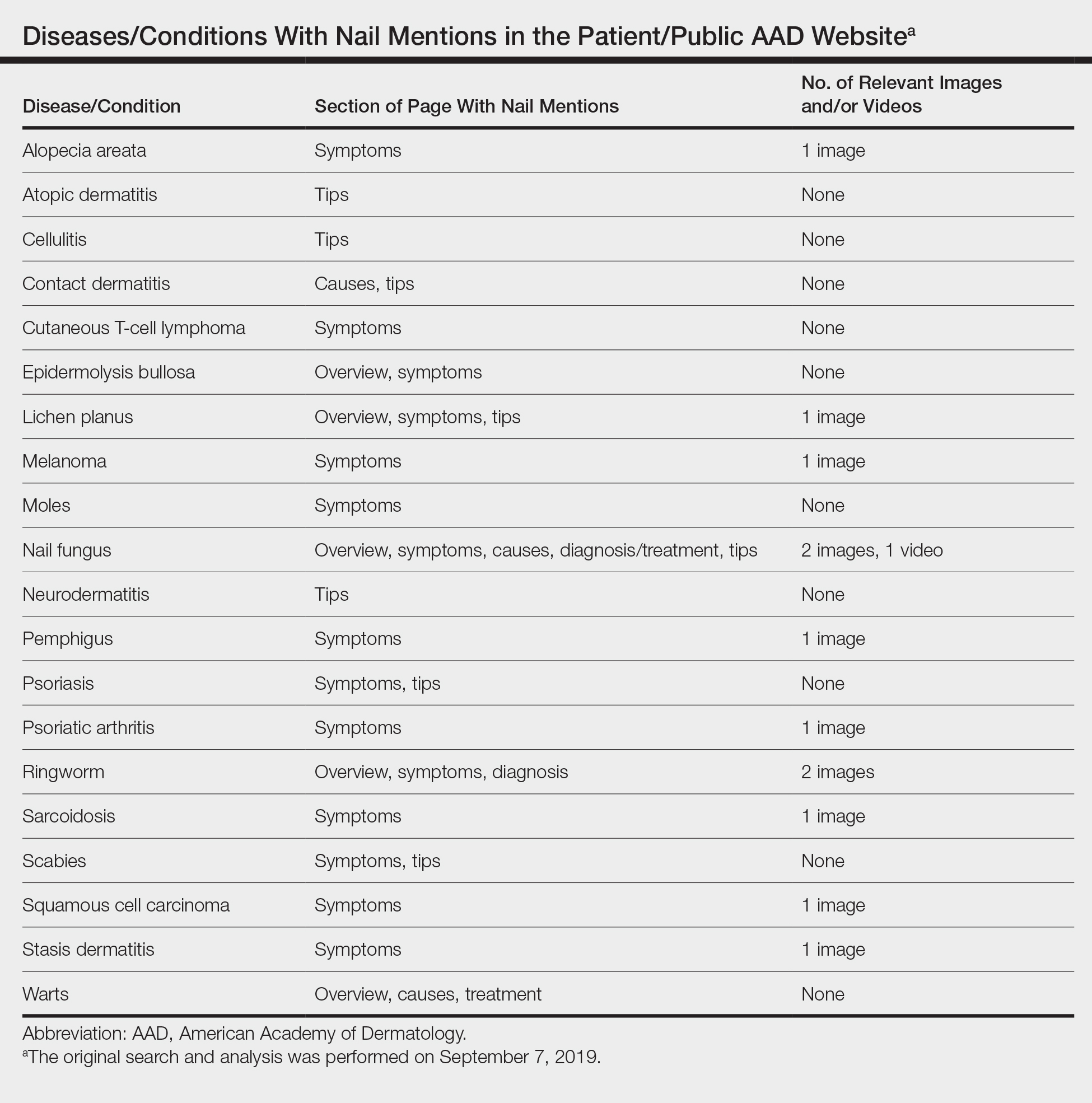
Our study demonstrated that nail content is underrepresented in the public education section of the AAD website. If patients are unable to find nail disease material on the AAD website, they may seek alternative sources that are unreliable. Prior studies have shown that patient Internet resources for subungual melanoma and onychomycosis often are inadequate in quality and readability.1,2
Representative photographs and key information on common nail diseases could be added to improve patient education. The atopic dermatitis section should include text on related nail changes with accompanying images. We also recommend including paronychia information and images as either a separate topic or in the cellulitis section. The contact dermatitis section mentions nail cosmetics as causative factors, but an image of roller-coaster onycholysis may be more helpful.3 Although the alopecia areata section mentions nail changes, this information should be added to the general hair loss section of the site, as many patients may initially seek out the latter category. Herpes simplex may affect nails, and an image showing these changes would be instructive. In addition, pyogenic granulomas and paronychia occur with isotretinoin use.4
Many of the included images were not representative of common clinical findings. The nail lichen planus image showed pitting instead of more typical findings of nail plate atrophy and pterygium. The nail melanoma image showed thickened yellow toenails and the fifth toenail with a thin gray-brown band instead of an isolated wide black band. The nail fungus section included images of superficial onychomycosis and severe onychodystrophy instead of showing more common changes such as distal onycholysis with subungual hyperkeratosis, which is typical of the most common subtype, distal lateral subungual onychomycosis.5 Onychomycosis was referenced again in the ringworm section with 1 image repeated from the nail fungus section and another image that appeared to be a subungual hematoma.
The AAD website offers important patient education resources; however, nail content is underrepresented on this platform. Dermatologists are experts on nail disease, and increased efforts are needed to educate the public about frequently encountered nail signs and symptoms that could signify a serious underlying condition.
After our original search and analysis, new nail topics, images, and videos have been added; therefore, there has been a positive trend toward new nail content being added to site, which will greatly benefit patients.
- Kang R, Lipner S. Assessment of internet sources on subungual melanoma [published online August 30, 2018]. Melanoma Res. doi:10.1097/CMR.0000000000000508.
- Kang R, Lipner S. Evaluation of onychomycosis information on the internet. J Drugs Dermatol. 2019;18:484-487.
- Rieder EA, Tosti A. Cosmetically induced disorders of the nail with update on contemporary nail manicures. J Clin Aesthet Dermatol. 2016;9:39-44.
- Arias-Santiago S, Husein-ElAhmed H, Aneiros-Cachaza J, et al. Uncommon side effects of isotretinoin therapy: paronychia and pyogenic granuloma. J Am Acad Dermatol. 2011;64:AB37.
- Lipner SR, Scher RK. Onychomycosis: clinical overview and diagnosis. J Am Acad Dermatol. 2019;80:835-851.
To the Editor:
Patients with skin, hair, or nail concerns often utilize online resources to self-diagnose or learn more about physician-diagnosed conditions. The American Academy of Dermatology (AAD) website offers the public access to informational pages categorized by disease or treatment (https://www.aad.org/public). We sought to evaluate the nail content by searching the Patients and Public section of the AAD website to qualitatively and quantitatively describe mentions of nail conditions. Psoriasis, psoriatic arthritis, atopic dermatitis, and ringworm content also were analyzed and compared to nail content. The analysis was performed on September 7, 2019.
Of the 73 topics listed in the Diseases and Treatments section of the site, 17 (23%) specifically mentioned nail symptoms or pathology (Table). Three additional topics—atopic dermatitis, cellulitis, and neurodermatitis—recommended keeping nails short to prevent injury from scratching. There was 1 mention of obtaining fungal cultures, 2 of nail scraping microscopy, 2 of nail clippings, and 2 of nail-related cancers. There were no mentions of nail biopsies. The total number of unique clinical images across all sections was 300, with 12 of nails. The video library contained 84 videos, of which 6 focused on nail health.

Our study demonstrated that nail content is underrepresented in the public education section of the AAD website. If patients are unable to find nail disease material on the AAD website, they may seek alternative sources that are unreliable. Prior studies have shown that patient Internet resources for subungual melanoma and onychomycosis often are inadequate in quality and readability.1,2
Representative photographs and key information on common nail diseases could be added to improve patient education. The atopic dermatitis section should include text on related nail changes with accompanying images. We also recommend including paronychia information and images as either a separate topic or in the cellulitis section. The contact dermatitis section mentions nail cosmetics as causative factors, but an image of roller-coaster onycholysis may be more helpful.3 Although the alopecia areata section mentions nail changes, this information should be added to the general hair loss section of the site, as many patients may initially seek out the latter category. Herpes simplex may affect nails, and an image showing these changes would be instructive. In addition, pyogenic granulomas and paronychia occur with isotretinoin use.4
Many of the included images were not representative of common clinical findings. The nail lichen planus image showed pitting instead of more typical findings of nail plate atrophy and pterygium. The nail melanoma image showed thickened yellow toenails and the fifth toenail with a thin gray-brown band instead of an isolated wide black band. The nail fungus section included images of superficial onychomycosis and severe onychodystrophy instead of showing more common changes such as distal onycholysis with subungual hyperkeratosis, which is typical of the most common subtype, distal lateral subungual onychomycosis.5 Onychomycosis was referenced again in the ringworm section with 1 image repeated from the nail fungus section and another image that appeared to be a subungual hematoma.
The AAD website offers important patient education resources; however, nail content is underrepresented on this platform. Dermatologists are experts on nail disease, and increased efforts are needed to educate the public about frequently encountered nail signs and symptoms that could signify a serious underlying condition.
After our original search and analysis, new nail topics, images, and videos have been added; therefore, there has been a positive trend toward new nail content being added to site, which will greatly benefit patients.
To the Editor:
Patients with skin, hair, or nail concerns often utilize online resources to self-diagnose or learn more about physician-diagnosed conditions. The American Academy of Dermatology (AAD) website offers the public access to informational pages categorized by disease or treatment (https://www.aad.org/public). We sought to evaluate the nail content by searching the Patients and Public section of the AAD website to qualitatively and quantitatively describe mentions of nail conditions. Psoriasis, psoriatic arthritis, atopic dermatitis, and ringworm content also were analyzed and compared to nail content. The analysis was performed on September 7, 2019.
Of the 73 topics listed in the Diseases and Treatments section of the site, 17 (23%) specifically mentioned nail symptoms or pathology (Table). Three additional topics—atopic dermatitis, cellulitis, and neurodermatitis—recommended keeping nails short to prevent injury from scratching. There was 1 mention of obtaining fungal cultures, 2 of nail scraping microscopy, 2 of nail clippings, and 2 of nail-related cancers. There were no mentions of nail biopsies. The total number of unique clinical images across all sections was 300, with 12 of nails. The video library contained 84 videos, of which 6 focused on nail health.

Our study demonstrated that nail content is underrepresented in the public education section of the AAD website. If patients are unable to find nail disease material on the AAD website, they may seek alternative sources that are unreliable. Prior studies have shown that patient Internet resources for subungual melanoma and onychomycosis often are inadequate in quality and readability.1,2
Representative photographs and key information on common nail diseases could be added to improve patient education. The atopic dermatitis section should include text on related nail changes with accompanying images. We also recommend including paronychia information and images as either a separate topic or in the cellulitis section. The contact dermatitis section mentions nail cosmetics as causative factors, but an image of roller-coaster onycholysis may be more helpful.3 Although the alopecia areata section mentions nail changes, this information should be added to the general hair loss section of the site, as many patients may initially seek out the latter category. Herpes simplex may affect nails, and an image showing these changes would be instructive. In addition, pyogenic granulomas and paronychia occur with isotretinoin use.4
Many of the included images were not representative of common clinical findings. The nail lichen planus image showed pitting instead of more typical findings of nail plate atrophy and pterygium. The nail melanoma image showed thickened yellow toenails and the fifth toenail with a thin gray-brown band instead of an isolated wide black band. The nail fungus section included images of superficial onychomycosis and severe onychodystrophy instead of showing more common changes such as distal onycholysis with subungual hyperkeratosis, which is typical of the most common subtype, distal lateral subungual onychomycosis.5 Onychomycosis was referenced again in the ringworm section with 1 image repeated from the nail fungus section and another image that appeared to be a subungual hematoma.
The AAD website offers important patient education resources; however, nail content is underrepresented on this platform. Dermatologists are experts on nail disease, and increased efforts are needed to educate the public about frequently encountered nail signs and symptoms that could signify a serious underlying condition.
After our original search and analysis, new nail topics, images, and videos have been added; therefore, there has been a positive trend toward new nail content being added to site, which will greatly benefit patients.
- Kang R, Lipner S. Assessment of internet sources on subungual melanoma [published online August 30, 2018]. Melanoma Res. doi:10.1097/CMR.0000000000000508.
- Kang R, Lipner S. Evaluation of onychomycosis information on the internet. J Drugs Dermatol. 2019;18:484-487.
- Rieder EA, Tosti A. Cosmetically induced disorders of the nail with update on contemporary nail manicures. J Clin Aesthet Dermatol. 2016;9:39-44.
- Arias-Santiago S, Husein-ElAhmed H, Aneiros-Cachaza J, et al. Uncommon side effects of isotretinoin therapy: paronychia and pyogenic granuloma. J Am Acad Dermatol. 2011;64:AB37.
- Lipner SR, Scher RK. Onychomycosis: clinical overview and diagnosis. J Am Acad Dermatol. 2019;80:835-851.
- Kang R, Lipner S. Assessment of internet sources on subungual melanoma [published online August 30, 2018]. Melanoma Res. doi:10.1097/CMR.0000000000000508.
- Kang R, Lipner S. Evaluation of onychomycosis information on the internet. J Drugs Dermatol. 2019;18:484-487.
- Rieder EA, Tosti A. Cosmetically induced disorders of the nail with update on contemporary nail manicures. J Clin Aesthet Dermatol. 2016;9:39-44.
- Arias-Santiago S, Husein-ElAhmed H, Aneiros-Cachaza J, et al. Uncommon side effects of isotretinoin therapy: paronychia and pyogenic granuloma. J Am Acad Dermatol. 2011;64:AB37.
- Lipner SR, Scher RK. Onychomycosis: clinical overview and diagnosis. J Am Acad Dermatol. 2019;80:835-851.
Practice Points
- Patients often utilize online resources to research skin, hair, and nail conditions.
- Nail signs and symptoms may represent a serious underlying condition, and nail content is underrepresented on the American Academy of Dermatology (AAD) Patients and Public section of the website.
- There is a need for more information on nail conditions on the AAD website, offering patients a more comprehensive online dermatology resource. Subsequently, there has been a positive trend toward new nail content being added to the site.
Laser Safety: The Need for Protocols
The use of lasers in dermatology has evolved and expanded since their first cutaneous use in 1963.1 As the fundamental understanding of the interaction of laser energy with biological tissues increased, the need for laser safety became apparent. Since then, lasers of varying wavelengths have been developed, each with its specific chromophore target and specific safety need. Protocols, such as a checklist, that have been shown to reduce adverse events in surgery and in the intensive care unit can be borrowed to decrease risk from laser injury and optimize laser safety in dermatology.2 The safety of the patient, the laser operator, and the other health care providers involved in the delivery of laser therapy led to the first US Food and Drug Administration (FDA) guidelines for laser use in 1984.3
There are 4 regulatory organizations for laser safety in the United States: the American National Standards Institute (ANSI), the Occupational Health and Safety Administration (OSHA), the FDA’s Center for Devices and Radiological Health, and The Joint Commission.
Laser Principles
The basic principles of lasers include transmission, absorption, scatter, and reflection, all occurring when laser light is applied to biological tissues. The effects of the laser are a function of the target tissue (the chromophore) and the wavelength of light being used.4 In the skin, there are 3 main endogenous chromophores: water, hemoglobin, and melanin. Some experts consider collagen to be a fourth and separate entity as a chromophore. Tattoos are considered exogenous chromophores.3 The basic principles of lasers are important to understand and keep in mind when discussing laser safety, as they are the mechanisms through which unintended consequences can occur.
Laser Safety
Ocular Hazards
Ocular hazards are a notable concern in laser surgery. The eye is uniquely susceptible to laser light, and eye injuries represent a majority of reported injuries, which can occur through direct beam, mirror reflection by surgical instruments, and beam reflection off the skin (4%–7% of light that hits the skin is reflected because of the refractive index between air and the stratum corneum).3 The different wavelengths of lasers affect different parts of the eye. The 3 parts of the eye affected most are the retina, cornea, and lens. Not only is the lens primarily at risk for acute (lenticular burns) and chronic (cataracts) injury from the laser, but secondarily the lens also can concentrate a laser beam onto the retina by a factor of 100,000 (Table 1).3
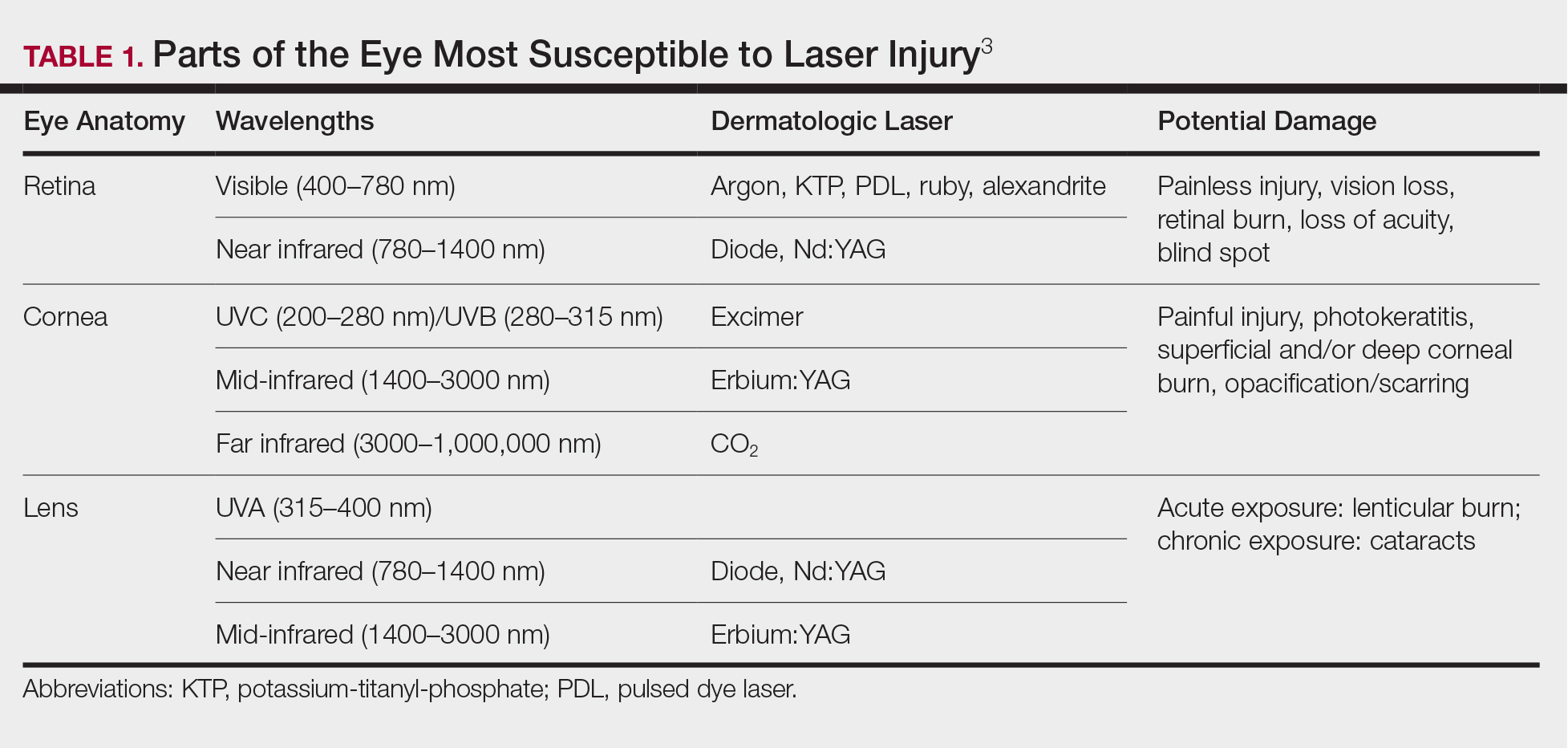
The use of ocular protective equipment, sometimes referred to as personal protective eyewear (PPE), is essential and is mandated by ANSI and OSHA for all class 3 and class 4 lasers. The eyewear must be labeled with the wavelength and the degree of optical protection—termed the optical density (OD) or filter factor—of each lens and should match the laser being used. Laser manufacturers, as required by ANSI, must provide the wavelength and OD of their lasers, and both can be found on each laser as well as in ANSI Z136.1.3
Vendors supplying PPE generally provide the material, usually glass or polycarbonate; color; visible light transmission, which is the actual amount of light that reaches one’s eye through the lens; filter specifications, which contain the OD at certain wavelengths; and the types of lasers for which each specific PPE is used. It is important to match the laser to the correct PPE. The use of multiple types of lasers in the same office or laser treatment area can present challenges regarding eye safety. Matching the PPE to the laser in use is critical, and therefore all steps to prevent error for patients and personnel should be employed. One recommendation is to place each laser in a separate room with the appropriate PPE hung outside on the door of that room.
When the treatment area is in the periocular region, protection of the patient’s cornea is essential. Leaded eye shields with nonreflective surfaces have been shown to offer the best protection.5 Prior to placement, anesthetic eye drops and lubrication are important for patient comfort and protection from corneal injury.
Laser-Generated Airborne Contaminants
Other hazards associated with laser use not directly related to the beam are laser-generated airborne contaminants (LGACs), including chemicals, viruses, bacteria, aerosolized blood products, and nanoparticles (<1 µm) known as ultrafine particles (UFPs). According to ANSI, electrosurgical devices and lasers generate the same smoke. The plume (surgical smoke) is known to contain as many as 60 chemicals, including but not limited to carbon monoxide, acrylonitrite, hydrocyanide, benzene, toluene, naphthalene, and formaldehyde. Several are known carcinogens, and others are environmental toxins.6,7
Smoke management is an important consideration for dermatologists and their patients and generally includes respiratory protection via masks and ventilation techniques. However, the practice is not universal, and oversight agencies such as OSHA and the National Institute for Occupational Safety and Health (NIOSH) provide guidelines only; they do not enforce. As such, smoke management is voluntary and not widely practiced. In a 2014 survey of 997 dermatologic surgeons who were asked if smoke management is used in their practice, 77% of respondents indicated no smoke management was used.6
The Surgical Plume: Composition
A 2014 study from the University of California, San Diego Department of Dermatology analyzed surgical smoke.6 The researchers placed the smoke collection probe 16 to 18 inches above the electrocautery site, which approximates the location of the surgeon’s head during the procedure. Assessing smoke composition, they found high levels of carcinogens and irritants. Two compounds found in their assay—1,3-butadiene and benzene—also are found in secondhand cigarette smoke. However, the concentrations in the plume were 17-fold higher for 1,3-butadiene and 10-fold higher for benzene than those found in secondhand cigarette smoke. The risk from chronic, long-term exposure to these airborne contaminants is notable, as benzene (a known carcinogen as determined by the US Department of Health and Human Services) is known to cause leukemia. For example, a busy Mohs surgeon can reach the equivalent of as many as 50 hours of continuous smoke exposure over the course of a year.6
The Surgical Plume: Particle Concentration
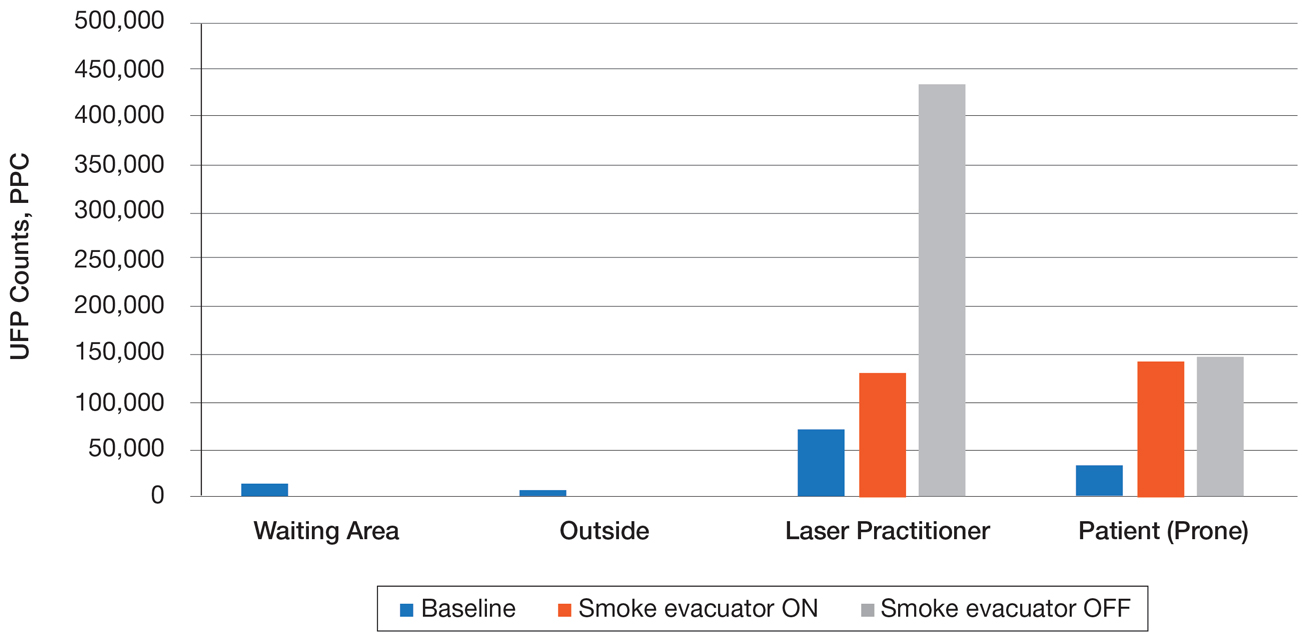
Ultrafine particles can bypass conventional filtering systems (surgical masks and N95 respirators) because of their extremely small size, which allows them to pass further into the lungs and all the way to the alveolar spaces. Geographic regions with high UFPs have been shown to have higher overall mortality rates, as well as higher rates of reactive airway disease, cardiovascular disease, and lung cancer. A 2016 study by Chuang et al7 published in JAMA Dermatology looked at the UFPs in the surgical plume from laser hair removal (LHR) procedures. The plume of LHR has a distinct odor and easily discernible particulates. The investigators measured the UFPs at the level of the laser practitioner and the patient’s face during LHR with a smoke evacuator turned on and again with it turned off for 30 seconds, and then compared them to UFPs measured in the treatment room, the waiting room, and outside the building. There were substantial increases in UFPs from the LHR procedure, especially for the laser practitioner, when the smoke evacuator was off. The ambient baseline particle count, as measured in the clinic waiting area, began at 15,300 particles per cubic centimeter (PPC), and once the LHR procedure began (smoke evacuator on), there was a greater than 8-fold PPC increase above baseline (15,300 PPC to 129,376 PPC) in UFPs measured for the laser practitioner. Importantly, during LHR when the smoke evacuator was turned off for 30 seconds, there was a more than 28-fold increase (15,300 PPC to 435,888 PPC) over baseline to the practitioner (Figure).7
The Surgical Plume: Viruses, Bacteria, and Aerosolized Blood Products
Viruses and bacteria are thought to be transmissible via the plume, and proviral human immunodeficiency virus DNA has been found in the plume as well as evacuator equipment used to reduce plume exposure.8 A study from 1988 found that CO2 laser users treating verrucae had human papillomavirus in the laser plume.9 A comparison study of CO2 laser users treating verrucae had an increased incidence of nasopharyngeal human papillomavirus infection when compared to a control group, and the plume also contained aerosolized blood.10 The American National Standards Institute, OSHA, and NIOSH all agree that LGAC control from lasers is necessary through respiratory protection and ventilation, but none of these organizations provides specific equipment recommendations. The American Society for Laser Medicine and Surgery has published a position statement on laser plume.11
The Surgical Plume: Smoke Management
Many virus particles and UFPs are less than 0.1 µm in size. It is important to note that neither surgical masks nor high-filtration masks, such as the N95 respirator, filter particles smaller than 0.1 µm. The first line of defense in smoke management is the local exhaust ventilation (LEV) system, which includes wall suction and/or a smoke evacuator. The smoke evacuator is considered the more important of the two. General filtration, such as wall suction, is a low-flow system and is really used for liquids. It can be used as a supplement to the smoke evacuator to control small amounts of plume if fitted with an in-line filter. There are 2 types of LEV filters: ultralow particulate air filters filter particles larger than 0.1
Of utmost importance when using a smoke evacuator system is suction tip placement. Placing the suction tip 1 cm from the tissue damage site has been shown to be 98.6% effective at removing laser plume. If moved to 2 cm, effectiveness decreases to less than 50%.11 Proper management recommendations based on current evidence suggest that use of a smoke evacuator and an approved fit-tested N95 respirator might provide maximum protection.6 In addition to plume exposure, tissue splatter can occur, especially during ablative (CO2) and tattoo laser therapy, which should prompt consideration of a face shield.11 There are several vendors and models available online, and a simple Internet search for surgical tissue splatter face shields will provide multiple options.
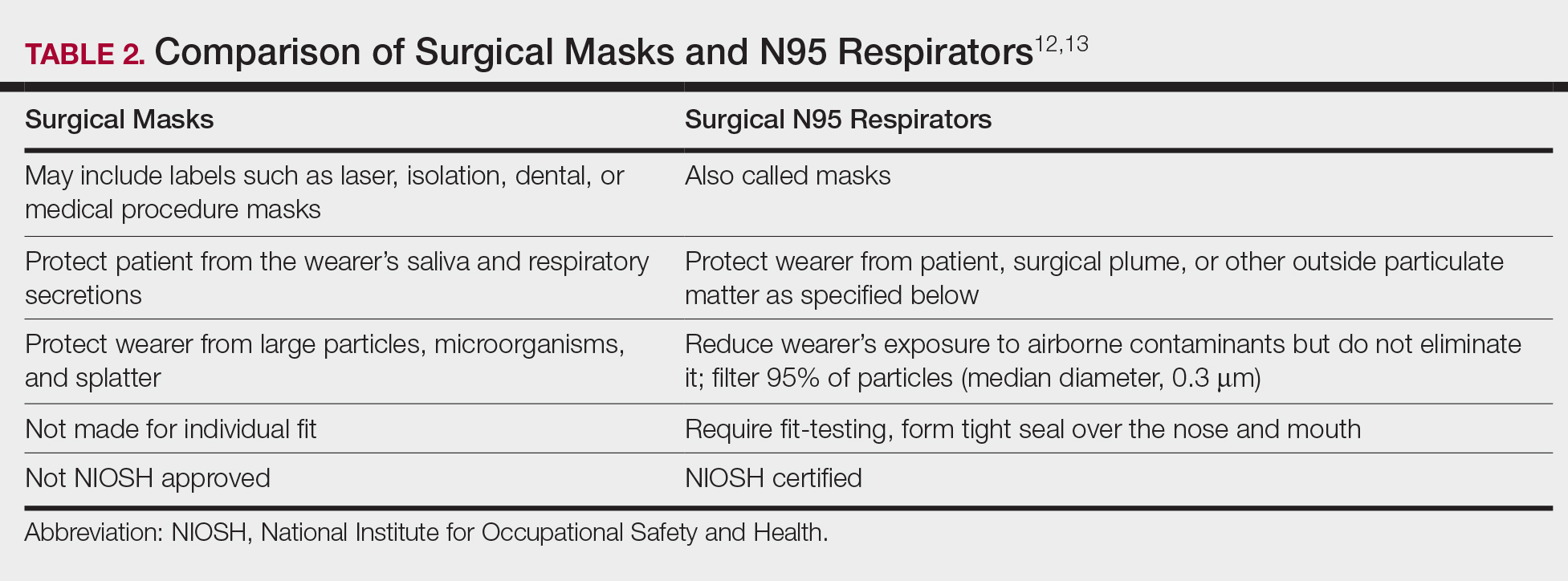
The standard surgical mask is not NIOSH approved and only effectively (99%) filters particles larger than 5 µm (vs 25% efficacy for 0.3-µm particles). Its main purpose is to protect the patient from the wearer.12
High-filtration masks, which capture particles as small as 0.1 µm, should be used instead. The surgical N95 respirator is a NIOSH-certified respirator and is recommended for use in cases when smoke management is necessary. The FDA does not test or certify these masks; it only clears them after reviewing manufacturer test data. Technically, to be called a surgical mask, it must be cleared by the FDA.12 The 95 of N95 indicates filter efficiency ratings of 95% when testing the filter efficiency using particles of approximately 0.3 µm in diameter (Table 2).13 Because 77% of surgical smoke particles are smaller than 1.1 µm, surgical masks and N95 respirators are never sufficient as stand-alone protection.14 An LEV system is much more important for safe surgical smoke management. However, recommendations call for the use of a smoke evacuator and a high-filtration mask together to obtain the most protection available.14
Fire Hazards
Fire hazards constitute another area of concern for the laser user and are seen with class 4 lasers. There usually are 2 types of fire hazards: electrical fires inside the laser (often faulty wiring) and flash fires (laser beam contacts flammable material). Flammable materials (eg, hair, hair products, makeup, fabrics, plastic, alcohol, chlorhexidine, aluminum chloride, elastic strap on safety goggles, gauze, drapes) should be identified and removed prior to laser use. CO2 and erbium:YAG lasers tend to pose the worst risk for flash fires.15
Precautions for fire control in the laser room should include fire extinguishers and/or fire extinguisher blankets, a water basin, and fire-resistant drapes available as needed. Flammable material such as gauze should be kept wet, or a nonflammable version should be used.3
Additional Safety Considerations
Whenever lasers are being used, it is important to cover any windows in the laser treatment area (LTA) to prevent the laser beam from passing through the glass window. Laser-blocking window covers are a requirement and are available from several vendors. Covers that block every laser class are available and come as a shade or a flat cover that is attached with Velcro or magnets. They also come with “Laser in Use” warning signs for additional safety. Access to the LTA when the laser is in use should be controlled and appropriate warning signs placed on the door to prevent inadvertent entry without proper PPE. Locking the door to the LTA while using the laser is an additional safety measure and can be included on a checklist.
For the dermatologist, the skin is a primary focus, and similar to the eye, can be at risk for injury. The most common type of injury resembles a sunburn, such as those seen in the UVB range, that appears as redness and sometimes blistering,15 which is an important consideration, and attention should be given to all those in the laser room.
Checklists
Checklists are ubiquitous throughout many occupations and many medical specialties. Their usefulness in preventing adverse events is well established. Any patient-provider encounter in which a series of sequential actions is required is a perfect situation for a checklist. In dermatologic laser surgery where the eye is uniquely susceptible to injury, a laser safety checklist is essential. Additionally, there are issues with LGACs and fire that are important to consider. Having protocols (ie, a checklist) in place that address these safety issues has been shown to reduce adverse outcomes.2 There are a number of templates available from various sources that can be customized to the laser treatment area. We provide a modifiable example (Table 3).
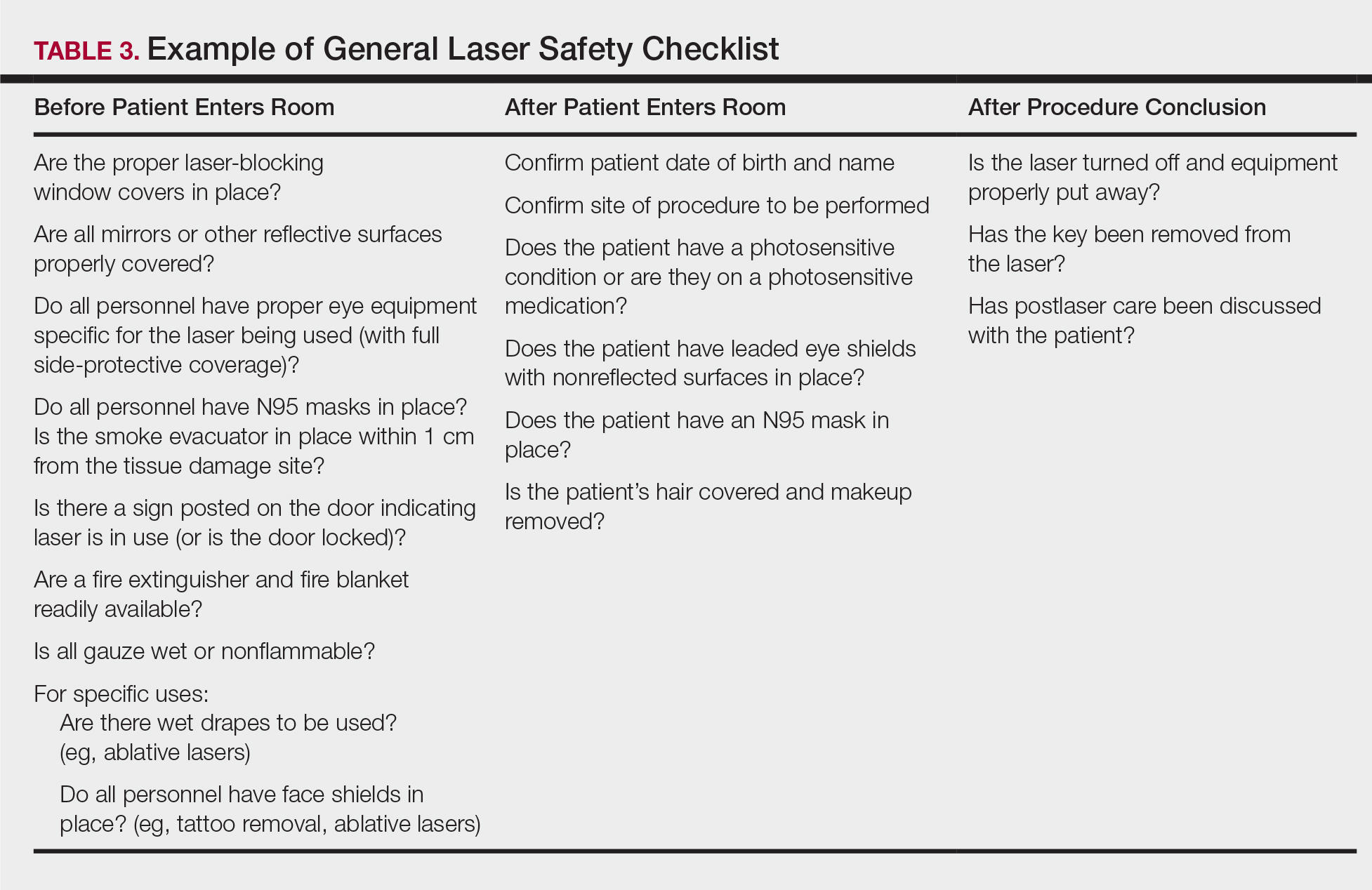
Conclusion
Laser usage in dermatologic surgery has increased. According to surveys from the American Society for Dermatologic Surgery, in 2012 there were approximately 2 million laser/light/energy-based procedures performed. By 2017, there were 3.27 million, up from 2.79 million in 2016, representing an approximate 1-year increase of 17%.16 Lasers have allowed interventions for skin, vascular, and aesthetic conditions that were once untreatable. As their use increases in number and broadens in scope, there also has been an increase in litigation alleging malpractice for misuse of the laser.17 Adverse events, which include photochemical or thermal injuries to the skin, pigmentation issues, scarring, plume-related issues, and fires, do occur. One solution to reduce the chance of an adverse outcome is to implement a checklist. Research using checklists has shown that adverse events are reduced when checklists are created and implemented properly. Improving checklist compliance also improves patient outcomes.17 The American National Standards Institute, in their ANSI Z136 series, and the World Health Organization provide checklist templates. We include our checklist for use in laser surgery (Table 3). Understanding that each laser treatment area is unique, the templates can serve as a starting point and can then be customized to suit the needs of each dermatologist.
- Goldman L, Blaney DJ, Kindel DJ, et al. Effect of the laser beam on the skin. J Invest Dermatol. 1963;40:121-122.
- Daggett C, Daggett A. The surgical check list revisited. Int J Surg Res Pract. 2017;4:051.
- Pritzker RN, Rohrer TE. Laser safety: standards and guidelines. In: Nouri K, ed. Handbook of Lasers in Dermatology. London, England: Springer; 2014:11-28.
- Husain Z, Alster TS. The role of lasers and intense pulsed light technology in dermatology. Clin Cosmet Investig Dermatol. 2016;9:29-40.
- Ries WR, Clymer MA, Reinisch L. Laser safety features of eye shields. Lasers Surg Med. 1996;18:309-315.
- Oganesyan G, Eimputh S, Kim SS, et al. Surgical smoke detection in dermatologic surgery. Dermatol Surg. 2014;40:1373-1377.
- Chuang GS, Farinelli W, Christiani DC, et al. Gaseous and particulate content of laser hair removal plume. JAMA Dermatol. 2016;152:1320-1326.
- Baggish MS, Poiesz BJ, Joret D, et al. Presence of human immunodeficiency DNA in laser smoke. Lasers Surg Med. 1991;11:197-203.
- Garden JM, O’Banion MK, Shelnitz LS, et al. Papilloma virus in the vapor of carbon dioxide laser-treated verrucae. JAMA. 1988;259:1199-1202.
- Gloster HM Jr, Roenigk RK. Risk of acquiring human papillomavirus from the plume produced by the carbon dioxide laser in the treatment of warts. J Am Acad Dermatol. 1995;32:436-441.
- American Society for Laser Medicine and Surgery. ASLMS laser and energy device plume position statement. http://www.aslms.org/for-professionals/professional-resources/safety-and-complications/aslms-laser-and-energy-device-plume-position-statement. Accessed October 4, 2019.
- A comparison of surgical masks, surgical N95 respirators, and industrial N95 respirators. OH&S website. https://ohsonline.com/Articles/2014/05/01/Comparison-Respiratory.aspx?Page=3. Published May 1, 2014. Accessed October 4, 2019.
- 3M Infection Prevention N95 particulate respirators, 1860/1860s and 1870. Frequently Asked Questions. http://multimedia.3m.com/mws/media/323208O/n95-particulate-respirators-1860-1860s-1870-faqs.pdf. Accessed October 4, 2019.
- Lewin JM, Brauer JA, Ostad A. Surgical smoke and the dermatologist. J Am Acad Dermatol. 2011;65:636-641.
- Arefiev K, Warycha M, Whiting D, et al. Flammability of topical preparations and surgical dressings in cutaneous and laser surgery: a controlled simulation study. J Am Acad Dermatol. 2012;67:700-705.
- ASDS survey on dermatologic procedures. American Society for Dermatologic Surgery website. https://www.asds.net/Medical-Professionals/Practice-Resources/ASDS-Survey-on-Dermatologic-Procedures. Accessed October 4, 2019.
- Jalian HR, Jalian CA, Avram MM. Common causes of injury and legal action in laser surgery. JAMA Dermatol. 2013;149:188-193.
The use of lasers in dermatology has evolved and expanded since their first cutaneous use in 1963.1 As the fundamental understanding of the interaction of laser energy with biological tissues increased, the need for laser safety became apparent. Since then, lasers of varying wavelengths have been developed, each with its specific chromophore target and specific safety need. Protocols, such as a checklist, that have been shown to reduce adverse events in surgery and in the intensive care unit can be borrowed to decrease risk from laser injury and optimize laser safety in dermatology.2 The safety of the patient, the laser operator, and the other health care providers involved in the delivery of laser therapy led to the first US Food and Drug Administration (FDA) guidelines for laser use in 1984.3
There are 4 regulatory organizations for laser safety in the United States: the American National Standards Institute (ANSI), the Occupational Health and Safety Administration (OSHA), the FDA’s Center for Devices and Radiological Health, and The Joint Commission.
Laser Principles
The basic principles of lasers include transmission, absorption, scatter, and reflection, all occurring when laser light is applied to biological tissues. The effects of the laser are a function of the target tissue (the chromophore) and the wavelength of light being used.4 In the skin, there are 3 main endogenous chromophores: water, hemoglobin, and melanin. Some experts consider collagen to be a fourth and separate entity as a chromophore. Tattoos are considered exogenous chromophores.3 The basic principles of lasers are important to understand and keep in mind when discussing laser safety, as they are the mechanisms through which unintended consequences can occur.
Laser Safety
Ocular Hazards
Ocular hazards are a notable concern in laser surgery. The eye is uniquely susceptible to laser light, and eye injuries represent a majority of reported injuries, which can occur through direct beam, mirror reflection by surgical instruments, and beam reflection off the skin (4%–7% of light that hits the skin is reflected because of the refractive index between air and the stratum corneum).3 The different wavelengths of lasers affect different parts of the eye. The 3 parts of the eye affected most are the retina, cornea, and lens. Not only is the lens primarily at risk for acute (lenticular burns) and chronic (cataracts) injury from the laser, but secondarily the lens also can concentrate a laser beam onto the retina by a factor of 100,000 (Table 1).3

The use of ocular protective equipment, sometimes referred to as personal protective eyewear (PPE), is essential and is mandated by ANSI and OSHA for all class 3 and class 4 lasers. The eyewear must be labeled with the wavelength and the degree of optical protection—termed the optical density (OD) or filter factor—of each lens and should match the laser being used. Laser manufacturers, as required by ANSI, must provide the wavelength and OD of their lasers, and both can be found on each laser as well as in ANSI Z136.1.3
Vendors supplying PPE generally provide the material, usually glass or polycarbonate; color; visible light transmission, which is the actual amount of light that reaches one’s eye through the lens; filter specifications, which contain the OD at certain wavelengths; and the types of lasers for which each specific PPE is used. It is important to match the laser to the correct PPE. The use of multiple types of lasers in the same office or laser treatment area can present challenges regarding eye safety. Matching the PPE to the laser in use is critical, and therefore all steps to prevent error for patients and personnel should be employed. One recommendation is to place each laser in a separate room with the appropriate PPE hung outside on the door of that room.
When the treatment area is in the periocular region, protection of the patient’s cornea is essential. Leaded eye shields with nonreflective surfaces have been shown to offer the best protection.5 Prior to placement, anesthetic eye drops and lubrication are important for patient comfort and protection from corneal injury.
Laser-Generated Airborne Contaminants
Other hazards associated with laser use not directly related to the beam are laser-generated airborne contaminants (LGACs), including chemicals, viruses, bacteria, aerosolized blood products, and nanoparticles (<1 µm) known as ultrafine particles (UFPs). According to ANSI, electrosurgical devices and lasers generate the same smoke. The plume (surgical smoke) is known to contain as many as 60 chemicals, including but not limited to carbon monoxide, acrylonitrite, hydrocyanide, benzene, toluene, naphthalene, and formaldehyde. Several are known carcinogens, and others are environmental toxins.6,7
Smoke management is an important consideration for dermatologists and their patients and generally includes respiratory protection via masks and ventilation techniques. However, the practice is not universal, and oversight agencies such as OSHA and the National Institute for Occupational Safety and Health (NIOSH) provide guidelines only; they do not enforce. As such, smoke management is voluntary and not widely practiced. In a 2014 survey of 997 dermatologic surgeons who were asked if smoke management is used in their practice, 77% of respondents indicated no smoke management was used.6
The Surgical Plume: Composition
A 2014 study from the University of California, San Diego Department of Dermatology analyzed surgical smoke.6 The researchers placed the smoke collection probe 16 to 18 inches above the electrocautery site, which approximates the location of the surgeon’s head during the procedure. Assessing smoke composition, they found high levels of carcinogens and irritants. Two compounds found in their assay—1,3-butadiene and benzene—also are found in secondhand cigarette smoke. However, the concentrations in the plume were 17-fold higher for 1,3-butadiene and 10-fold higher for benzene than those found in secondhand cigarette smoke. The risk from chronic, long-term exposure to these airborne contaminants is notable, as benzene (a known carcinogen as determined by the US Department of Health and Human Services) is known to cause leukemia. For example, a busy Mohs surgeon can reach the equivalent of as many as 50 hours of continuous smoke exposure over the course of a year.6
The Surgical Plume: Particle Concentration
Ultrafine particles can bypass conventional filtering systems (surgical masks and N95 respirators) because of their extremely small size, which allows them to pass further into the lungs and all the way to the alveolar spaces. Geographic regions with high UFPs have been shown to have higher overall mortality rates, as well as higher rates of reactive airway disease, cardiovascular disease, and lung cancer. A 2016 study by Chuang et al7 published in JAMA Dermatology looked at the UFPs in the surgical plume from laser hair removal (LHR) procedures. The plume of LHR has a distinct odor and easily discernible particulates. The investigators measured the UFPs at the level of the laser practitioner and the patient’s face during LHR with a smoke evacuator turned on and again with it turned off for 30 seconds, and then compared them to UFPs measured in the treatment room, the waiting room, and outside the building. There were substantial increases in UFPs from the LHR procedure, especially for the laser practitioner, when the smoke evacuator was off. The ambient baseline particle count, as measured in the clinic waiting area, began at 15,300 particles per cubic centimeter (PPC), and once the LHR procedure began (smoke evacuator on), there was a greater than 8-fold PPC increase above baseline (15,300 PPC to 129,376 PPC) in UFPs measured for the laser practitioner. Importantly, during LHR when the smoke evacuator was turned off for 30 seconds, there was a more than 28-fold increase (15,300 PPC to 435,888 PPC) over baseline to the practitioner (Figure).7

The Surgical Plume: Viruses, Bacteria, and Aerosolized Blood Products
Viruses and bacteria are thought to be transmissible via the plume, and proviral human immunodeficiency virus DNA has been found in the plume as well as evacuator equipment used to reduce plume exposure.8 A study from 1988 found that CO2 laser users treating verrucae had human papillomavirus in the laser plume.9 A comparison study of CO2 laser users treating verrucae had an increased incidence of nasopharyngeal human papillomavirus infection when compared to a control group, and the plume also contained aerosolized blood.10 The American National Standards Institute, OSHA, and NIOSH all agree that LGAC control from lasers is necessary through respiratory protection and ventilation, but none of these organizations provides specific equipment recommendations. The American Society for Laser Medicine and Surgery has published a position statement on laser plume.11
The Surgical Plume: Smoke Management
Many virus particles and UFPs are less than 0.1 µm in size. It is important to note that neither surgical masks nor high-filtration masks, such as the N95 respirator, filter particles smaller than 0.1 µm. The first line of defense in smoke management is the local exhaust ventilation (LEV) system, which includes wall suction and/or a smoke evacuator. The smoke evacuator is considered the more important of the two. General filtration, such as wall suction, is a low-flow system and is really used for liquids. It can be used as a supplement to the smoke evacuator to control small amounts of plume if fitted with an in-line filter. There are 2 types of LEV filters: ultralow particulate air filters filter particles larger than 0.1
Of utmost importance when using a smoke evacuator system is suction tip placement. Placing the suction tip 1 cm from the tissue damage site has been shown to be 98.6% effective at removing laser plume. If moved to 2 cm, effectiveness decreases to less than 50%.11 Proper management recommendations based on current evidence suggest that use of a smoke evacuator and an approved fit-tested N95 respirator might provide maximum protection.6 In addition to plume exposure, tissue splatter can occur, especially during ablative (CO2) and tattoo laser therapy, which should prompt consideration of a face shield.11 There are several vendors and models available online, and a simple Internet search for surgical tissue splatter face shields will provide multiple options.
The standard surgical mask is not NIOSH approved and only effectively (99%) filters particles larger than 5 µm (vs 25% efficacy for 0.3-µm particles). Its main purpose is to protect the patient from the wearer.12
High-filtration masks, which capture particles as small as 0.1 µm, should be used instead. The surgical N95 respirator is a NIOSH-certified respirator and is recommended for use in cases when smoke management is necessary. The FDA does not test or certify these masks; it only clears them after reviewing manufacturer test data. Technically, to be called a surgical mask, it must be cleared by the FDA.12 The 95 of N95 indicates filter efficiency ratings of 95% when testing the filter efficiency using particles of approximately 0.3 µm in diameter (Table 2).13 Because 77% of surgical smoke particles are smaller than 1.1 µm, surgical masks and N95 respirators are never sufficient as stand-alone protection.14 An LEV system is much more important for safe surgical smoke management. However, recommendations call for the use of a smoke evacuator and a high-filtration mask together to obtain the most protection available.14

Fire Hazards
Fire hazards constitute another area of concern for the laser user and are seen with class 4 lasers. There usually are 2 types of fire hazards: electrical fires inside the laser (often faulty wiring) and flash fires (laser beam contacts flammable material). Flammable materials (eg, hair, hair products, makeup, fabrics, plastic, alcohol, chlorhexidine, aluminum chloride, elastic strap on safety goggles, gauze, drapes) should be identified and removed prior to laser use. CO2 and erbium:YAG lasers tend to pose the worst risk for flash fires.15
Precautions for fire control in the laser room should include fire extinguishers and/or fire extinguisher blankets, a water basin, and fire-resistant drapes available as needed. Flammable material such as gauze should be kept wet, or a nonflammable version should be used.3
Additional Safety Considerations
Whenever lasers are being used, it is important to cover any windows in the laser treatment area (LTA) to prevent the laser beam from passing through the glass window. Laser-blocking window covers are a requirement and are available from several vendors. Covers that block every laser class are available and come as a shade or a flat cover that is attached with Velcro or magnets. They also come with “Laser in Use” warning signs for additional safety. Access to the LTA when the laser is in use should be controlled and appropriate warning signs placed on the door to prevent inadvertent entry without proper PPE. Locking the door to the LTA while using the laser is an additional safety measure and can be included on a checklist.
For the dermatologist, the skin is a primary focus, and similar to the eye, can be at risk for injury. The most common type of injury resembles a sunburn, such as those seen in the UVB range, that appears as redness and sometimes blistering,15 which is an important consideration, and attention should be given to all those in the laser room.
Checklists
Checklists are ubiquitous throughout many occupations and many medical specialties. Their usefulness in preventing adverse events is well established. Any patient-provider encounter in which a series of sequential actions is required is a perfect situation for a checklist. In dermatologic laser surgery where the eye is uniquely susceptible to injury, a laser safety checklist is essential. Additionally, there are issues with LGACs and fire that are important to consider. Having protocols (ie, a checklist) in place that address these safety issues has been shown to reduce adverse outcomes.2 There are a number of templates available from various sources that can be customized to the laser treatment area. We provide a modifiable example (Table 3).

Conclusion
Laser usage in dermatologic surgery has increased. According to surveys from the American Society for Dermatologic Surgery, in 2012 there were approximately 2 million laser/light/energy-based procedures performed. By 2017, there were 3.27 million, up from 2.79 million in 2016, representing an approximate 1-year increase of 17%.16 Lasers have allowed interventions for skin, vascular, and aesthetic conditions that were once untreatable. As their use increases in number and broadens in scope, there also has been an increase in litigation alleging malpractice for misuse of the laser.17 Adverse events, which include photochemical or thermal injuries to the skin, pigmentation issues, scarring, plume-related issues, and fires, do occur. One solution to reduce the chance of an adverse outcome is to implement a checklist. Research using checklists has shown that adverse events are reduced when checklists are created and implemented properly. Improving checklist compliance also improves patient outcomes.17 The American National Standards Institute, in their ANSI Z136 series, and the World Health Organization provide checklist templates. We include our checklist for use in laser surgery (Table 3). Understanding that each laser treatment area is unique, the templates can serve as a starting point and can then be customized to suit the needs of each dermatologist.
The use of lasers in dermatology has evolved and expanded since their first cutaneous use in 1963.1 As the fundamental understanding of the interaction of laser energy with biological tissues increased, the need for laser safety became apparent. Since then, lasers of varying wavelengths have been developed, each with its specific chromophore target and specific safety need. Protocols, such as a checklist, that have been shown to reduce adverse events in surgery and in the intensive care unit can be borrowed to decrease risk from laser injury and optimize laser safety in dermatology.2 The safety of the patient, the laser operator, and the other health care providers involved in the delivery of laser therapy led to the first US Food and Drug Administration (FDA) guidelines for laser use in 1984.3
There are 4 regulatory organizations for laser safety in the United States: the American National Standards Institute (ANSI), the Occupational Health and Safety Administration (OSHA), the FDA’s Center for Devices and Radiological Health, and The Joint Commission.
Laser Principles
The basic principles of lasers include transmission, absorption, scatter, and reflection, all occurring when laser light is applied to biological tissues. The effects of the laser are a function of the target tissue (the chromophore) and the wavelength of light being used.4 In the skin, there are 3 main endogenous chromophores: water, hemoglobin, and melanin. Some experts consider collagen to be a fourth and separate entity as a chromophore. Tattoos are considered exogenous chromophores.3 The basic principles of lasers are important to understand and keep in mind when discussing laser safety, as they are the mechanisms through which unintended consequences can occur.
Laser Safety
Ocular Hazards
Ocular hazards are a notable concern in laser surgery. The eye is uniquely susceptible to laser light, and eye injuries represent a majority of reported injuries, which can occur through direct beam, mirror reflection by surgical instruments, and beam reflection off the skin (4%–7% of light that hits the skin is reflected because of the refractive index between air and the stratum corneum).3 The different wavelengths of lasers affect different parts of the eye. The 3 parts of the eye affected most are the retina, cornea, and lens. Not only is the lens primarily at risk for acute (lenticular burns) and chronic (cataracts) injury from the laser, but secondarily the lens also can concentrate a laser beam onto the retina by a factor of 100,000 (Table 1).3

The use of ocular protective equipment, sometimes referred to as personal protective eyewear (PPE), is essential and is mandated by ANSI and OSHA for all class 3 and class 4 lasers. The eyewear must be labeled with the wavelength and the degree of optical protection—termed the optical density (OD) or filter factor—of each lens and should match the laser being used. Laser manufacturers, as required by ANSI, must provide the wavelength and OD of their lasers, and both can be found on each laser as well as in ANSI Z136.1.3
Vendors supplying PPE generally provide the material, usually glass or polycarbonate; color; visible light transmission, which is the actual amount of light that reaches one’s eye through the lens; filter specifications, which contain the OD at certain wavelengths; and the types of lasers for which each specific PPE is used. It is important to match the laser to the correct PPE. The use of multiple types of lasers in the same office or laser treatment area can present challenges regarding eye safety. Matching the PPE to the laser in use is critical, and therefore all steps to prevent error for patients and personnel should be employed. One recommendation is to place each laser in a separate room with the appropriate PPE hung outside on the door of that room.
When the treatment area is in the periocular region, protection of the patient’s cornea is essential. Leaded eye shields with nonreflective surfaces have been shown to offer the best protection.5 Prior to placement, anesthetic eye drops and lubrication are important for patient comfort and protection from corneal injury.
Laser-Generated Airborne Contaminants
Other hazards associated with laser use not directly related to the beam are laser-generated airborne contaminants (LGACs), including chemicals, viruses, bacteria, aerosolized blood products, and nanoparticles (<1 µm) known as ultrafine particles (UFPs). According to ANSI, electrosurgical devices and lasers generate the same smoke. The plume (surgical smoke) is known to contain as many as 60 chemicals, including but not limited to carbon monoxide, acrylonitrite, hydrocyanide, benzene, toluene, naphthalene, and formaldehyde. Several are known carcinogens, and others are environmental toxins.6,7
Smoke management is an important consideration for dermatologists and their patients and generally includes respiratory protection via masks and ventilation techniques. However, the practice is not universal, and oversight agencies such as OSHA and the National Institute for Occupational Safety and Health (NIOSH) provide guidelines only; they do not enforce. As such, smoke management is voluntary and not widely practiced. In a 2014 survey of 997 dermatologic surgeons who were asked if smoke management is used in their practice, 77% of respondents indicated no smoke management was used.6
The Surgical Plume: Composition
A 2014 study from the University of California, San Diego Department of Dermatology analyzed surgical smoke.6 The researchers placed the smoke collection probe 16 to 18 inches above the electrocautery site, which approximates the location of the surgeon’s head during the procedure. Assessing smoke composition, they found high levels of carcinogens and irritants. Two compounds found in their assay—1,3-butadiene and benzene—also are found in secondhand cigarette smoke. However, the concentrations in the plume were 17-fold higher for 1,3-butadiene and 10-fold higher for benzene than those found in secondhand cigarette smoke. The risk from chronic, long-term exposure to these airborne contaminants is notable, as benzene (a known carcinogen as determined by the US Department of Health and Human Services) is known to cause leukemia. For example, a busy Mohs surgeon can reach the equivalent of as many as 50 hours of continuous smoke exposure over the course of a year.6
The Surgical Plume: Particle Concentration
Ultrafine particles can bypass conventional filtering systems (surgical masks and N95 respirators) because of their extremely small size, which allows them to pass further into the lungs and all the way to the alveolar spaces. Geographic regions with high UFPs have been shown to have higher overall mortality rates, as well as higher rates of reactive airway disease, cardiovascular disease, and lung cancer. A 2016 study by Chuang et al7 published in JAMA Dermatology looked at the UFPs in the surgical plume from laser hair removal (LHR) procedures. The plume of LHR has a distinct odor and easily discernible particulates. The investigators measured the UFPs at the level of the laser practitioner and the patient’s face during LHR with a smoke evacuator turned on and again with it turned off for 30 seconds, and then compared them to UFPs measured in the treatment room, the waiting room, and outside the building. There were substantial increases in UFPs from the LHR procedure, especially for the laser practitioner, when the smoke evacuator was off. The ambient baseline particle count, as measured in the clinic waiting area, began at 15,300 particles per cubic centimeter (PPC), and once the LHR procedure began (smoke evacuator on), there was a greater than 8-fold PPC increase above baseline (15,300 PPC to 129,376 PPC) in UFPs measured for the laser practitioner. Importantly, during LHR when the smoke evacuator was turned off for 30 seconds, there was a more than 28-fold increase (15,300 PPC to 435,888 PPC) over baseline to the practitioner (Figure).7

The Surgical Plume: Viruses, Bacteria, and Aerosolized Blood Products
Viruses and bacteria are thought to be transmissible via the plume, and proviral human immunodeficiency virus DNA has been found in the plume as well as evacuator equipment used to reduce plume exposure.8 A study from 1988 found that CO2 laser users treating verrucae had human papillomavirus in the laser plume.9 A comparison study of CO2 laser users treating verrucae had an increased incidence of nasopharyngeal human papillomavirus infection when compared to a control group, and the plume also contained aerosolized blood.10 The American National Standards Institute, OSHA, and NIOSH all agree that LGAC control from lasers is necessary through respiratory protection and ventilation, but none of these organizations provides specific equipment recommendations. The American Society for Laser Medicine and Surgery has published a position statement on laser plume.11
The Surgical Plume: Smoke Management
Many virus particles and UFPs are less than 0.1 µm in size. It is important to note that neither surgical masks nor high-filtration masks, such as the N95 respirator, filter particles smaller than 0.1 µm. The first line of defense in smoke management is the local exhaust ventilation (LEV) system, which includes wall suction and/or a smoke evacuator. The smoke evacuator is considered the more important of the two. General filtration, such as wall suction, is a low-flow system and is really used for liquids. It can be used as a supplement to the smoke evacuator to control small amounts of plume if fitted with an in-line filter. There are 2 types of LEV filters: ultralow particulate air filters filter particles larger than 0.1
Of utmost importance when using a smoke evacuator system is suction tip placement. Placing the suction tip 1 cm from the tissue damage site has been shown to be 98.6% effective at removing laser plume. If moved to 2 cm, effectiveness decreases to less than 50%.11 Proper management recommendations based on current evidence suggest that use of a smoke evacuator and an approved fit-tested N95 respirator might provide maximum protection.6 In addition to plume exposure, tissue splatter can occur, especially during ablative (CO2) and tattoo laser therapy, which should prompt consideration of a face shield.11 There are several vendors and models available online, and a simple Internet search for surgical tissue splatter face shields will provide multiple options.
The standard surgical mask is not NIOSH approved and only effectively (99%) filters particles larger than 5 µm (vs 25% efficacy for 0.3-µm particles). Its main purpose is to protect the patient from the wearer.12
High-filtration masks, which capture particles as small as 0.1 µm, should be used instead. The surgical N95 respirator is a NIOSH-certified respirator and is recommended for use in cases when smoke management is necessary. The FDA does not test or certify these masks; it only clears them after reviewing manufacturer test data. Technically, to be called a surgical mask, it must be cleared by the FDA.12 The 95 of N95 indicates filter efficiency ratings of 95% when testing the filter efficiency using particles of approximately 0.3 µm in diameter (Table 2).13 Because 77% of surgical smoke particles are smaller than 1.1 µm, surgical masks and N95 respirators are never sufficient as stand-alone protection.14 An LEV system is much more important for safe surgical smoke management. However, recommendations call for the use of a smoke evacuator and a high-filtration mask together to obtain the most protection available.14

Fire Hazards
Fire hazards constitute another area of concern for the laser user and are seen with class 4 lasers. There usually are 2 types of fire hazards: electrical fires inside the laser (often faulty wiring) and flash fires (laser beam contacts flammable material). Flammable materials (eg, hair, hair products, makeup, fabrics, plastic, alcohol, chlorhexidine, aluminum chloride, elastic strap on safety goggles, gauze, drapes) should be identified and removed prior to laser use. CO2 and erbium:YAG lasers tend to pose the worst risk for flash fires.15
Precautions for fire control in the laser room should include fire extinguishers and/or fire extinguisher blankets, a water basin, and fire-resistant drapes available as needed. Flammable material such as gauze should be kept wet, or a nonflammable version should be used.3
Additional Safety Considerations
Whenever lasers are being used, it is important to cover any windows in the laser treatment area (LTA) to prevent the laser beam from passing through the glass window. Laser-blocking window covers are a requirement and are available from several vendors. Covers that block every laser class are available and come as a shade or a flat cover that is attached with Velcro or magnets. They also come with “Laser in Use” warning signs for additional safety. Access to the LTA when the laser is in use should be controlled and appropriate warning signs placed on the door to prevent inadvertent entry without proper PPE. Locking the door to the LTA while using the laser is an additional safety measure and can be included on a checklist.
For the dermatologist, the skin is a primary focus, and similar to the eye, can be at risk for injury. The most common type of injury resembles a sunburn, such as those seen in the UVB range, that appears as redness and sometimes blistering,15 which is an important consideration, and attention should be given to all those in the laser room.
Checklists
Checklists are ubiquitous throughout many occupations and many medical specialties. Their usefulness in preventing adverse events is well established. Any patient-provider encounter in which a series of sequential actions is required is a perfect situation for a checklist. In dermatologic laser surgery where the eye is uniquely susceptible to injury, a laser safety checklist is essential. Additionally, there are issues with LGACs and fire that are important to consider. Having protocols (ie, a checklist) in place that address these safety issues has been shown to reduce adverse outcomes.2 There are a number of templates available from various sources that can be customized to the laser treatment area. We provide a modifiable example (Table 3).

Conclusion
Laser usage in dermatologic surgery has increased. According to surveys from the American Society for Dermatologic Surgery, in 2012 there were approximately 2 million laser/light/energy-based procedures performed. By 2017, there were 3.27 million, up from 2.79 million in 2016, representing an approximate 1-year increase of 17%.16 Lasers have allowed interventions for skin, vascular, and aesthetic conditions that were once untreatable. As their use increases in number and broadens in scope, there also has been an increase in litigation alleging malpractice for misuse of the laser.17 Adverse events, which include photochemical or thermal injuries to the skin, pigmentation issues, scarring, plume-related issues, and fires, do occur. One solution to reduce the chance of an adverse outcome is to implement a checklist. Research using checklists has shown that adverse events are reduced when checklists are created and implemented properly. Improving checklist compliance also improves patient outcomes.17 The American National Standards Institute, in their ANSI Z136 series, and the World Health Organization provide checklist templates. We include our checklist for use in laser surgery (Table 3). Understanding that each laser treatment area is unique, the templates can serve as a starting point and can then be customized to suit the needs of each dermatologist.
- Goldman L, Blaney DJ, Kindel DJ, et al. Effect of the laser beam on the skin. J Invest Dermatol. 1963;40:121-122.
- Daggett C, Daggett A. The surgical check list revisited. Int J Surg Res Pract. 2017;4:051.
- Pritzker RN, Rohrer TE. Laser safety: standards and guidelines. In: Nouri K, ed. Handbook of Lasers in Dermatology. London, England: Springer; 2014:11-28.
- Husain Z, Alster TS. The role of lasers and intense pulsed light technology in dermatology. Clin Cosmet Investig Dermatol. 2016;9:29-40.
- Ries WR, Clymer MA, Reinisch L. Laser safety features of eye shields. Lasers Surg Med. 1996;18:309-315.
- Oganesyan G, Eimputh S, Kim SS, et al. Surgical smoke detection in dermatologic surgery. Dermatol Surg. 2014;40:1373-1377.
- Chuang GS, Farinelli W, Christiani DC, et al. Gaseous and particulate content of laser hair removal plume. JAMA Dermatol. 2016;152:1320-1326.
- Baggish MS, Poiesz BJ, Joret D, et al. Presence of human immunodeficiency DNA in laser smoke. Lasers Surg Med. 1991;11:197-203.
- Garden JM, O’Banion MK, Shelnitz LS, et al. Papilloma virus in the vapor of carbon dioxide laser-treated verrucae. JAMA. 1988;259:1199-1202.
- Gloster HM Jr, Roenigk RK. Risk of acquiring human papillomavirus from the plume produced by the carbon dioxide laser in the treatment of warts. J Am Acad Dermatol. 1995;32:436-441.
- American Society for Laser Medicine and Surgery. ASLMS laser and energy device plume position statement. http://www.aslms.org/for-professionals/professional-resources/safety-and-complications/aslms-laser-and-energy-device-plume-position-statement. Accessed October 4, 2019.
- A comparison of surgical masks, surgical N95 respirators, and industrial N95 respirators. OH&S website. https://ohsonline.com/Articles/2014/05/01/Comparison-Respiratory.aspx?Page=3. Published May 1, 2014. Accessed October 4, 2019.
- 3M Infection Prevention N95 particulate respirators, 1860/1860s and 1870. Frequently Asked Questions. http://multimedia.3m.com/mws/media/323208O/n95-particulate-respirators-1860-1860s-1870-faqs.pdf. Accessed October 4, 2019.
- Lewin JM, Brauer JA, Ostad A. Surgical smoke and the dermatologist. J Am Acad Dermatol. 2011;65:636-641.
- Arefiev K, Warycha M, Whiting D, et al. Flammability of topical preparations and surgical dressings in cutaneous and laser surgery: a controlled simulation study. J Am Acad Dermatol. 2012;67:700-705.
- ASDS survey on dermatologic procedures. American Society for Dermatologic Surgery website. https://www.asds.net/Medical-Professionals/Practice-Resources/ASDS-Survey-on-Dermatologic-Procedures. Accessed October 4, 2019.
- Jalian HR, Jalian CA, Avram MM. Common causes of injury and legal action in laser surgery. JAMA Dermatol. 2013;149:188-193.
- Goldman L, Blaney DJ, Kindel DJ, et al. Effect of the laser beam on the skin. J Invest Dermatol. 1963;40:121-122.
- Daggett C, Daggett A. The surgical check list revisited. Int J Surg Res Pract. 2017;4:051.
- Pritzker RN, Rohrer TE. Laser safety: standards and guidelines. In: Nouri K, ed. Handbook of Lasers in Dermatology. London, England: Springer; 2014:11-28.
- Husain Z, Alster TS. The role of lasers and intense pulsed light technology in dermatology. Clin Cosmet Investig Dermatol. 2016;9:29-40.
- Ries WR, Clymer MA, Reinisch L. Laser safety features of eye shields. Lasers Surg Med. 1996;18:309-315.
- Oganesyan G, Eimputh S, Kim SS, et al. Surgical smoke detection in dermatologic surgery. Dermatol Surg. 2014;40:1373-1377.
- Chuang GS, Farinelli W, Christiani DC, et al. Gaseous and particulate content of laser hair removal plume. JAMA Dermatol. 2016;152:1320-1326.
- Baggish MS, Poiesz BJ, Joret D, et al. Presence of human immunodeficiency DNA in laser smoke. Lasers Surg Med. 1991;11:197-203.
- Garden JM, O’Banion MK, Shelnitz LS, et al. Papilloma virus in the vapor of carbon dioxide laser-treated verrucae. JAMA. 1988;259:1199-1202.
- Gloster HM Jr, Roenigk RK. Risk of acquiring human papillomavirus from the plume produced by the carbon dioxide laser in the treatment of warts. J Am Acad Dermatol. 1995;32:436-441.
- American Society for Laser Medicine and Surgery. ASLMS laser and energy device plume position statement. http://www.aslms.org/for-professionals/professional-resources/safety-and-complications/aslms-laser-and-energy-device-plume-position-statement. Accessed October 4, 2019.
- A comparison of surgical masks, surgical N95 respirators, and industrial N95 respirators. OH&S website. https://ohsonline.com/Articles/2014/05/01/Comparison-Respiratory.aspx?Page=3. Published May 1, 2014. Accessed October 4, 2019.
- 3M Infection Prevention N95 particulate respirators, 1860/1860s and 1870. Frequently Asked Questions. http://multimedia.3m.com/mws/media/323208O/n95-particulate-respirators-1860-1860s-1870-faqs.pdf. Accessed October 4, 2019.
- Lewin JM, Brauer JA, Ostad A. Surgical smoke and the dermatologist. J Am Acad Dermatol. 2011;65:636-641.
- Arefiev K, Warycha M, Whiting D, et al. Flammability of topical preparations and surgical dressings in cutaneous and laser surgery: a controlled simulation study. J Am Acad Dermatol. 2012;67:700-705.
- ASDS survey on dermatologic procedures. American Society for Dermatologic Surgery website. https://www.asds.net/Medical-Professionals/Practice-Resources/ASDS-Survey-on-Dermatologic-Procedures. Accessed October 4, 2019.
- Jalian HR, Jalian CA, Avram MM. Common causes of injury and legal action in laser surgery. JAMA Dermatol. 2013;149:188-193.
Practice Points
- Laser therapy has evolved and expanded since its first cutaneous use in 1963.
- The 4 regulatory agencies for laser safety in the United States establish standards and guidelines, but implementation is voluntary.
- Ocular hazards, laser-generated airborne contaminants, fires, and unintended laser beam injuries constitute the main safety concerns.
- Safety protocols with a laser checklist can reduce adverse outcomes.
Translating the 2019 AAD-NPF Guidelines of Care for the Management of Psoriasis With Phototherapy
Psoriasis is a systemic immune-mediated disorder characterized by erythematous, scaly, well-demarcated plaques on the skin that affects approximately 3% of the world’s population.1 Although topical therapies often are the first-line treatment of mild to moderate psoriasis, approximately 1 in 6 individuals has moderate to severe disease that requires systemic treatment such as biologics or phototherapy.2 In patients with localized disease that is refractory to treatment or who have moderate to severe psoriasis requiring systemic treatment, phototherapy should be considered as a potential low-risk treatment option.
In July 2019, the American Academy of Dermatology (AAD) and National Psoriasis Foundation (NPF) released an updated set of guidelines for the use of phototherapy in treating adult patients with psoriasis.3 Since the prior guidelines were released in 2010, there have been numerous studies affirming the efficacy of phototherapy, with several large meta-analyses helping to refine clinical recommendations.4,5 Each treatment was ranked using Strength of Recommendation Taxonomy, with a score of A, B, or C based on the strength of the evidence supporting the given modality. With the ever-increasing number of treatment options for patients with psoriasis, these guidelines inform dermatologists of the recommendations for the initiation, maintenance, and optimization of phototherapy in the treatment of psoriasis.
The AAD-NPF recommendations discuss the mechanism of action, efficacy, safety, and frequency of adverse events of 10 commonly used phototherapy/photochemotherapy modalities. They also address dosing regimens, the potential to combine phototherapy with other therapies, and the efficacy of treatment modalities for different types of psoriasis.3 The purpose of this discussion is to present these guidelines in a condensed form for prescribers of phototherapy and to review the most clinically significant considerations during each step of treatment. Of note, we only highlight the treatment of adult patients and do not discuss information relevant to pediatric patients with psoriasis.
Choosing a Phototherapy Modality
Phototherapy may be considered for patients with psoriasis that affects more than 3% body surface area or for localized disease refractory to conventional treatments. UV light is believed to provide relief from psoriasis via multiple mechanisms, such as through favorable alterations in cytokine profiles, initiation of apoptosis, and local immunosupression.6 There is no single first-line phototherapeutic modality recommended for all patients with psoriasis. Rather, the decision to implement a particular modality should be individualized to the patient, considering factors such as percentage of body surface area affected by disease, quality-of-life assessment, comorbidities, lifestyle, and cost of treatment.
Of the 10 phototherapy modalities reviewed in these guidelines, 4 were ranked by the AAD and NPF as having grade A evidence for efficacy in the treatment of moderate to severe plaque psoriasis. Treatments with a grade A level of recommendation included narrowband UVB (NB-UVB), broadband UVB (BB-UVB), targeted phototherapy (excimer laser and excimer lamp), and
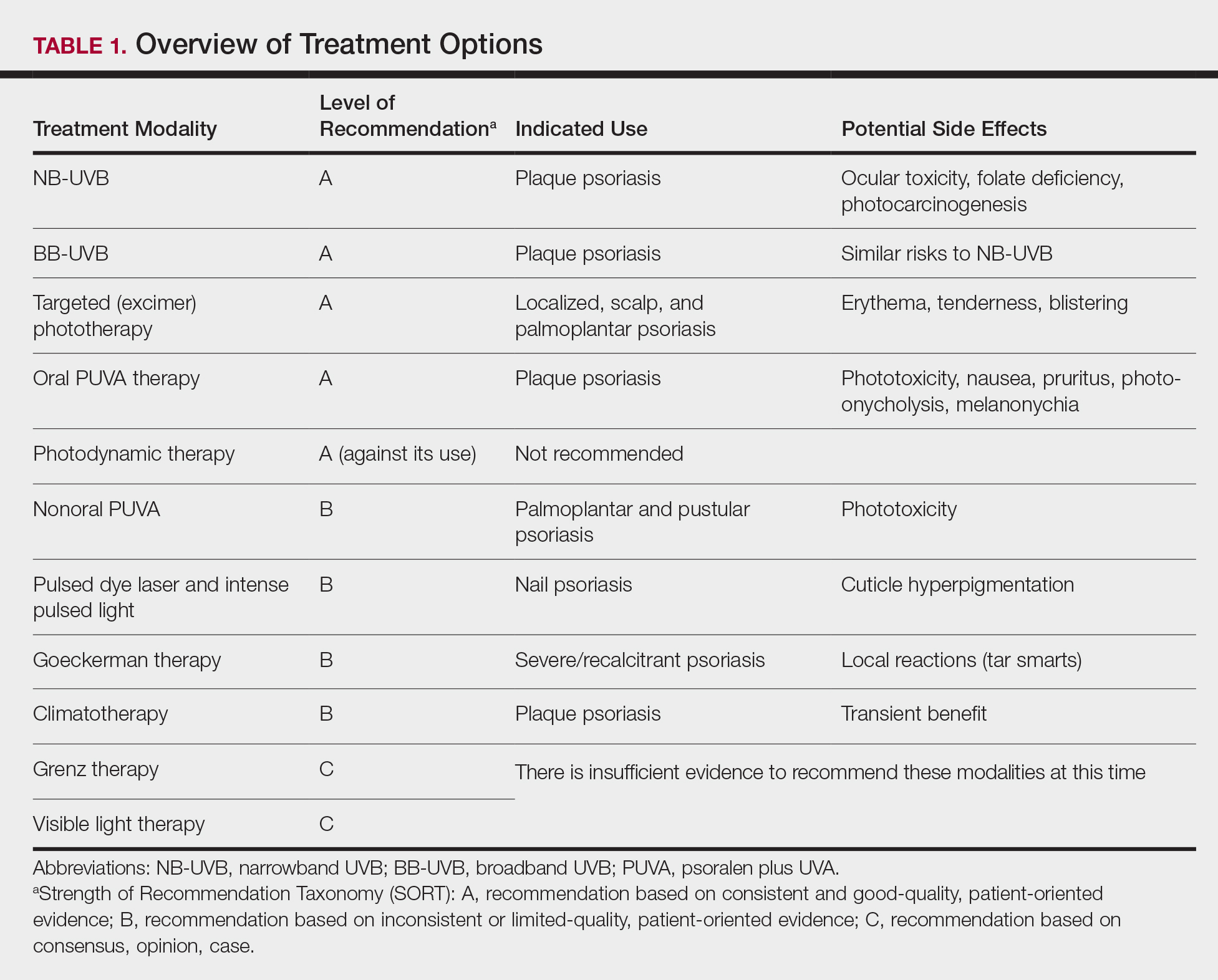
Studies have shown that the ideal wavelength needed to produce a therapeutic effect (ie, clearance of psoriatic plaques) is 304 to 313 nm. Wavelengths of 290 to 300 nm were found to be less therapeutic and more harmful, as they contributed to the development of sunburns.7 Broadband UVB phototherapy, with wavelengths ranging from 270 to 390 nm, exposes patients to a greater spectrum of radiation, thus making it more likely to cause sunburn and any theoretical form of sun-related damage, such as dysplasia and cancer. Compared with NB-UVB phototherapy, BB-UVB phototherapy is associated with a greater degree of sun damage–related side effects. Narrowband UVB, with a wavelength range of 311 to 313 nm, carries a grade A level of recommendation and should be considered as first-line monotherapy in patients with generalized plaque psoriasis, given its efficacy and promising safety profile. Multiple studies have shown that NB-UVB phototherapy is superior to BB-UVB phototherapy in the treatment of moderate to severe psoriasis in adults.8,9 In facilities where access to NB-UVB is limited, BB-UVB monotherapy is recommended as the treatment of generalized plaque psoriasis.
Psoralen plus UVA, which may be used topically (ie, bathwater PUVA) or taken orally, refers to treatment with photosensitizing psoralens. Psoralens are agents that intercalate with DNA and enhance the efficacy of phototherapy.10 Topical PUVA, with a grade B level of recommendation, is an effective treatment option for patients with localized disease and has been shown to be particularly efficacious in the treatment of palmoplantar pustular psoriasis. Oral PUVA is an effective option for psoriasis with a grade A recommendation, while bathwater PUVA has a grade B level of recommendation for moderate to severe plaque psoriasis. Oral PUVA is associated with greater systemic side effects (both acute and subacute) compared with NB-UVB and also is associated with photocarcinogenesis, particularly squamous cell carcinoma in white patients.11 Other side effects from PUVA include pigmented macules in sun-protected areas (known as PUVA lentigines), which may make evaluation of skin lesions challenging. Because of the increased risk for cancer with oral PUVA, NB-UVB is preferable as a first-line treatment vs PUVA, especially in patients with a history of skin cancer.12,13
Goeckerman therapy, which involves the synergistic combination of UVB and crude coal tar, is an older treatment that has shown efficacy in the treatment of severe or recalcitrant psoriasis (grade B level of recommendation). One prior case-control study comparing the efficacy of Goeckerman therapy with newer treatments, such as biologic therapies, steroids, and oral immunosuppressants, found a similar reduction in symptoms among both treatment groups, with longer disease-free periods in patients who received Goeckerman therapy than those who received newer therapies (22.3 years vs 4.6 months).14 However, Goeckerman therapy is utilized less frequently than more modern therapies because of the time required for treatment and declining insurance reimbursements for it. Climatotherapy, another older established therapy, involves the temporary or permanent relocation of patients to an environment that is favorable for disease control (grade B level of recommendation). Locations such as the Dead Sea and Canary Islands have been studied and shown to provide both subjective and objective improvement in patients’ psoriasis disease course. Patients had notable improvement in both their psoriasis area and severity index score and quality of life after a 3- to 4-week relocation to these areas.15,16 Access to climatotherapy and the transient nature of disease relief are apparent limitations of this treatment modality.
Grenz ray is a type of phototherapy that uses longer-wavelength ionizing radiation, which has low penetrance into surrounding tissues and a 95% absorption rate within the first 3 mm of the skin depth. This treatment has been used less frequently since the development of newer alternatives but should still be considered as a second line to UV therapy, especially in cases of recalcitrant disease and palmoplantar psoriasis, and when access to other treatment options is limited. Grenz ray has a grade C level of recommendation due to the paucity of evidence that supports its efficacy. Thus, it is not recommended as first-line therapy for the treatment of moderate to severe psoriasis. Visible light therapy is another treatment option that uses light in the visible wavelength spectrum but predominantly utilizes blue and red light. Psoriatic lesions are sensitive to light therapy because of the elevated levels of naturally occurring photosensitizing agents, called protoporphyrins, in these lesions.17 Several small studies have shown improvement in psoriatic lesions treated with visible light therapy, with blue light showing greater efficacy in lesional clearance than red light.18,19
Pulsed dye laser is a phototherapy modality that has shown efficacy in the treatment of nail psoriasis (grade B level of recommendation). One study comparing the effects of tazarotene cream 0.1% with pulsed dye laser and tazarotene cream 0.1% alone showed that patients receiving combination therapy had a greater decrease in
Initiation of Phototherapy
Prior to initiating phototherapy, it is important to assess the patient for any personal or family history of skin cancer, as phototherapy carries an increased risk for cutaneous malignancy, especially in patients with a history of skin cancer.22,23 All patients also should be evaluated for their Fitzpatrick skin type, and the minimal erythema dose should be defined for those initiating UVB treatment. These classifications can be useful for the initial determination of treatment dose and the prevention of treatment-related adverse events (TRAEs). A careful drug history also should be taken before the initiation of phototherapy to avoid photosensitizing reactions. Thiazide diuretics and tetracyclines are 2 such examples of medications commonly associated with photosensitizing reactions.24
Fitzpatrick skin type and/or minimal erythema dose testing also are essential in determining the appropriate initial NB-UVB dose required for treatment initiation (Table 2). Patient response to the initial NB-UVB trial will determine the optimal dosage for subsequent maintenance treatments.
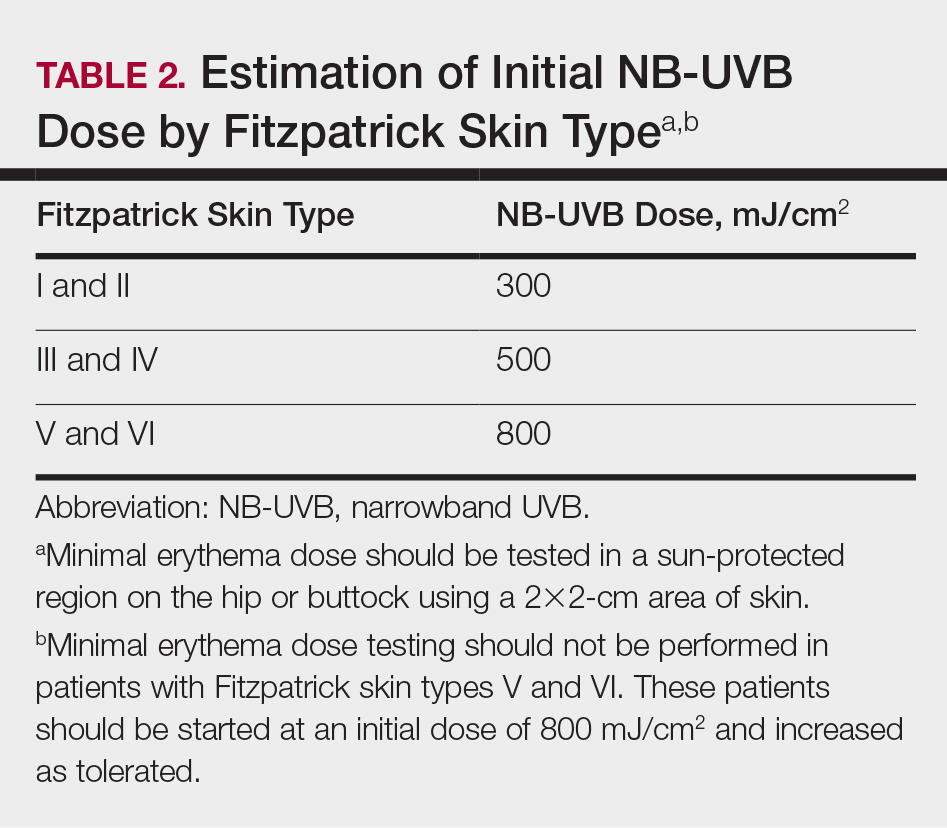
For patients unable or unwilling to commit to office-based or institution-based treatments, home NB-UVB is another therapeutic option. One study comparing patients with moderate to severe psoriasis who received home NB-UVB vs in-office treatment showed comparable psoriasis area and severity index scores and quality-of-life indices and no difference in the frequency of TRAE indices. It is important to note that patients who received home treatment had a significantly lower treatment burden (P≤.001) and greater treatment satisfaction than those receiving treatment in an office-based setting (P=.001).25
Assessment and Optimization of Phototherapy
After an appropriate starting dosage has been established, patients should be evaluated at each subsequent visit for the degree of treatment response. Excessive erythema (lasting more than 48 hours) or adverse effects, such as itching, stinging, or burning, are indications that the patient should have their dose adjusted back to the last dose without such adverse responses. Because tolerance to treatment develops over time, patients who miss a scheduled dose of NB-UVB phototherapy require their dose to be temporarily lowered. Targeted dosage of UVB phototherapy at a frequency of 2 to 3 times weekly is preferred over treatment 1 to 2 times weekly; however, consideration should be given toward patient preference.26 Dosing may be increased at a rate of 5% to 10% after each treatment, as tolerated, if there is no clearance of skin lesions with the original treatment dose. Patient skin type also is helpful in dictating the maximum phototherapy dose for each patient (Table 3).
Once a patient’s psoriatic lesions have cleared, the patient has the option to taper or indefinitely continue maintenance therapy. The established protocol for patients who choose to taper therapy is treatment twice weekly for 4 weeks, followed by once-weekly treatment for the second month. The maintenance dosage is held constant during the taper. For patients who prefer indefinite maintenance therapy, treatment is administered every 1 to 2 weeks, with a maintenance dosage that is approximately 25% lower than the original maintenance dosage.
Treatment Considerations
Efforts should be made to ensure that the long-term sequalae of phototherapy are minimized (Table 1). Development of cataracts is a recognized consequence of prolonged UVB exposure; therefore, eye protection is recommended during all UVB treatment sessions to reduce the risk for ocular toxicity. Although pregnancy is not a contraindication to phototherapy, except for PUVA, there is a dose-dependent degradation of folate with NB-UVB treatment, so folate supplementation (0.8 mg) is recommended during NB-UVB treatment to prevent development of neural tube defects in fetuses of patients who are pregnant or who may become pregnant.27
Although phototherapy carries the theoretical risk for photocarcinogenesis, multiple studies have shown no increased risk for malignancy with either NB-UVB or BB-UVB phototherapy.22,23 Regardless, patients who develop new-onset skin cancer while receiving any phototherapeutic treatment should discuss the potential risks and benefits of continued treatment with their physician. Providers should take extra caution prior to initiating treatment, especially in patients with a history of cutaneous malignancy. Because oral PUVA is a systemic therapy, it is associated with a greater risk for photocarcinogenesis than any other modality, particularly in fair-skinned individuals. Patients younger than 10 years; pregnant or nursing patients; and those with a history of lupus, xeroderma pigmentosum, or melanoma should not receive PUVA therapy because of their increased risk for photocarcinogenesis and TRAEs.
The decision to switch patients between different phototherapy modalities during treatment should be individualized to each patient based on factors such as disease severity, quality of life, and treatment burden. Other factors to consider include dosing frequency, treatment cost, and logistical factors, such as proximity to a treatment facility. Physicians should have a detailed discussion about the risks and benefits of continuing therapy for patients who develop new-onset skin cancer during phototherapy.
Final Thoughts
Phototherapy is an effective and safe treatment for patients with psoriasis who have localized and systemic disease. There are several treatment modalities that can be tailored to patient needs and preferences, such as home NB-UVB for patients who are unable or unwilling to undergo office-based treatments. Phototherapy has few absolute contraindications; however, relative contraindications to phototherapy exist. Patients with a history of skin cancer, photosensitivity disorders, and autoimmune diseases (eg, lupus) carry greater risks for adverse events, such as sun-related damage, cancer, and dysplasia. Because these conditions may preclude patients from pursuing phototherapy as a safe and effective approach to treating moderate to severe psoriasis, these patients should be considered for other therapies, such as biologic medications, which may carry a more favorable risk-benefit ratio given that individual’s background.
- Michalek IM, Loring B, John SM. A systematic review of worldwide epidemiology of psoriasis. J Eur Acad Dermatol Venereol. 2017;31:205-212.
- Yeung H, Takeshita J, Mehta NN, et al. Psoriasis severity and the prevalence of major medical comorbidity: a population-based study. JAMA Dermatol. 2013;149:1173-1179.
- Elmets CA, Lim HW, Stoff B, et al. Joint American Academy of Dermatology-National Psoriasis Foundation guidelines of care for the management and treatment of psoriasis with phototherapy. J Am Acad Dermatol. 2019;81:775-804.
- Archier E, Devaux S, Castela E, et al. Efficacy of psoralen UV-A therapy vs. narrowband UV-B therapy in chronic plaque psoriasis: a systematic literature review. J Eur Acad Dermatol Venereol. 2012;26(suppl 3):11-21.
- Chen X, Yang M, Cheng Y, et al. Narrow-band ultraviolet B phototherapy versus broad-band ultraviolet B or psoralen-ultraviolet A photochemotherapy for psoriasis. Cochrane Database Syst Rev. 2013;10:CD009481.
- Wong T, Hsu L, Liao W. Phototherapy in psoriasis: a review of mechanisms of action. J Cutan Med Surg. 2013;17:6-12.
- Parrish JA, Jaenicke KF. Action spectrum for phototherapy of psoriasis. J Invest Dermatol. 1981;76:359-362.
- Almutawa F, Alnomair N, Wang Y, et al. Systematic review of UV-based therapy for psoriasis. Am J Clin Dermatol. 2013;14:87-109.
- El-Mofty M, Mostafa WZ, Bosseila M, et al. A large scale analytical study on efficacy of different photo(chemo)therapeutic modalities in the treatment of psoriasis, vitiligo and mycosis fungoides. Dermatol Ther. 2010;23:428-434.
- Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 5. guidelines of care for the treatment of psoriasis with phototherapy and photochemotherapy. J Am Acad Dermatol. 2010;62:114-135.
- Murase JE, Lee EE, Koo J. Effect of ethnicity on the risk of developing nonmelanoma skin cancer following long-term PUVA therapy. Int J Dermatol. 2005;44:1016-1021.
- Bruynzeel I, Bergman W, Hartevelt HM, et al. 'High single-dose' European PUVA regimen also causes an excess of non-melanoma skin cancer. Br J Dermatol. 1991;124:49-55.
- Lindelöf B, Sigurgeirsson B, Tegner E, et al. PUVA and cancer risk: the Swedish follow-up study. Br J Dermatol. 1999;141:108-112.
- Chern E, Yau D, Ho JC, et al. Positive effect of modified Goeckerman regimen on quality of life and psychosocial distress in moderate and severe psoriasis. Acta Derm Venereol. 2011;91:447-451.
- Harari M, Czarnowicki T, Fluss R, et al. Patients with early-onset psoriasis achieve better results following Dead Sea climatotherapy. J Eur Acad Dermatol Venereol. 2012;26:554-559.
- Wahl AK, Langeland E, Larsen MH, et al. Positive changes in self-management and disease severity following climate therapy in people with psoriasis. Acta Dermatol Venereol. 2015;95:317-321.
- Bissonnette R, Zeng H, McLean DI, et al. Psoriatic plaques exhibit red autofluorescence that is due to protoporphyrin IX. J Invest Dermatol. 1998;111:586-591.
- Kleinpenning MM, Otero ME, van Erp PE, et al. Efficacy of blue light vs. red light in the treatment of psoriasis: a double-blind, randomized comparative study. J Eur Acad Dermatol Venereol. 2012;26:219-225.
- Weinstabl A, Hoff-Lesch S, Merk HF, et al. Prospective randomized study on the efficacy of blue light in the treatment of psoriasis vulgaris. Dermatology. 2011;223:251-259.
- Huang YC, Chou CL, Chiang YY. Efficacy of pulsed dye laser plus topical tazarotene versus topical tazarotene alone in psoriatic nail disease: a single-blind, intrapatient left-to-right controlled study. Lasers Surg Med. 2013;45:102-107.
- Tawfik AA. Novel treatment of nail psoriasis using the intense pulsed light: a one-year follow-up study. Dermatol Surg. 2014;40:763-768.
- Archier E, Devaux S, Castela E, et al. Carcinogenic risks of psoralen UV-A therapy and narrowband UV-B therapy in chronic plaque psoriasis: a systematic literature review. J Eur Acad Dermatol Venereol. 2012;26(suppl 3):22-31.
- Osmancevic A, Gillstedt M, Wennberg AM, et al. The risk of skin cancer in psoriasis patients treated with UVB therapy. Acta Dermatol Venereol. 2014;94:425-430.
- Dawe RS, Ibbotson SH. Drug-induced photosensitivity. Dermatol Clin. 2014;32:363-368.
- Koek MB, Buskens E, van Weelden H, et al. Home versus outpatient ultraviolet B phototherapy for mild to severe psoriasis: pragmatic multicentre randomised controlled non-inferiority trial (PLUTO study). BMJ. 2009;338:B1542.
- Almutawa F, Thalib L, Hekman D, et al. Efficacy of localized phototherapy and photodynamic therapy for psoriasis: a systematic review and meta-analysis. Photodermatol Photoimmunol Photomed. 2015;31:5-14.
- Zhang M, Goyert G, Lim HW. Folate and phototherapy: what should we inform our patients? J Am Acad Dermatol. 2017;77:958-964.
Psoriasis is a systemic immune-mediated disorder characterized by erythematous, scaly, well-demarcated plaques on the skin that affects approximately 3% of the world’s population.1 Although topical therapies often are the first-line treatment of mild to moderate psoriasis, approximately 1 in 6 individuals has moderate to severe disease that requires systemic treatment such as biologics or phototherapy.2 In patients with localized disease that is refractory to treatment or who have moderate to severe psoriasis requiring systemic treatment, phototherapy should be considered as a potential low-risk treatment option.
In July 2019, the American Academy of Dermatology (AAD) and National Psoriasis Foundation (NPF) released an updated set of guidelines for the use of phototherapy in treating adult patients with psoriasis.3 Since the prior guidelines were released in 2010, there have been numerous studies affirming the efficacy of phototherapy, with several large meta-analyses helping to refine clinical recommendations.4,5 Each treatment was ranked using Strength of Recommendation Taxonomy, with a score of A, B, or C based on the strength of the evidence supporting the given modality. With the ever-increasing number of treatment options for patients with psoriasis, these guidelines inform dermatologists of the recommendations for the initiation, maintenance, and optimization of phototherapy in the treatment of psoriasis.
The AAD-NPF recommendations discuss the mechanism of action, efficacy, safety, and frequency of adverse events of 10 commonly used phototherapy/photochemotherapy modalities. They also address dosing regimens, the potential to combine phototherapy with other therapies, and the efficacy of treatment modalities for different types of psoriasis.3 The purpose of this discussion is to present these guidelines in a condensed form for prescribers of phototherapy and to review the most clinically significant considerations during each step of treatment. Of note, we only highlight the treatment of adult patients and do not discuss information relevant to pediatric patients with psoriasis.
Choosing a Phototherapy Modality
Phototherapy may be considered for patients with psoriasis that affects more than 3% body surface area or for localized disease refractory to conventional treatments. UV light is believed to provide relief from psoriasis via multiple mechanisms, such as through favorable alterations in cytokine profiles, initiation of apoptosis, and local immunosupression.6 There is no single first-line phototherapeutic modality recommended for all patients with psoriasis. Rather, the decision to implement a particular modality should be individualized to the patient, considering factors such as percentage of body surface area affected by disease, quality-of-life assessment, comorbidities, lifestyle, and cost of treatment.
Of the 10 phototherapy modalities reviewed in these guidelines, 4 were ranked by the AAD and NPF as having grade A evidence for efficacy in the treatment of moderate to severe plaque psoriasis. Treatments with a grade A level of recommendation included narrowband UVB (NB-UVB), broadband UVB (BB-UVB), targeted phototherapy (excimer laser and excimer lamp), and

Studies have shown that the ideal wavelength needed to produce a therapeutic effect (ie, clearance of psoriatic plaques) is 304 to 313 nm. Wavelengths of 290 to 300 nm were found to be less therapeutic and more harmful, as they contributed to the development of sunburns.7 Broadband UVB phototherapy, with wavelengths ranging from 270 to 390 nm, exposes patients to a greater spectrum of radiation, thus making it more likely to cause sunburn and any theoretical form of sun-related damage, such as dysplasia and cancer. Compared with NB-UVB phototherapy, BB-UVB phototherapy is associated with a greater degree of sun damage–related side effects. Narrowband UVB, with a wavelength range of 311 to 313 nm, carries a grade A level of recommendation and should be considered as first-line monotherapy in patients with generalized plaque psoriasis, given its efficacy and promising safety profile. Multiple studies have shown that NB-UVB phototherapy is superior to BB-UVB phototherapy in the treatment of moderate to severe psoriasis in adults.8,9 In facilities where access to NB-UVB is limited, BB-UVB monotherapy is recommended as the treatment of generalized plaque psoriasis.
Psoralen plus UVA, which may be used topically (ie, bathwater PUVA) or taken orally, refers to treatment with photosensitizing psoralens. Psoralens are agents that intercalate with DNA and enhance the efficacy of phototherapy.10 Topical PUVA, with a grade B level of recommendation, is an effective treatment option for patients with localized disease and has been shown to be particularly efficacious in the treatment of palmoplantar pustular psoriasis. Oral PUVA is an effective option for psoriasis with a grade A recommendation, while bathwater PUVA has a grade B level of recommendation for moderate to severe plaque psoriasis. Oral PUVA is associated with greater systemic side effects (both acute and subacute) compared with NB-UVB and also is associated with photocarcinogenesis, particularly squamous cell carcinoma in white patients.11 Other side effects from PUVA include pigmented macules in sun-protected areas (known as PUVA lentigines), which may make evaluation of skin lesions challenging. Because of the increased risk for cancer with oral PUVA, NB-UVB is preferable as a first-line treatment vs PUVA, especially in patients with a history of skin cancer.12,13
Goeckerman therapy, which involves the synergistic combination of UVB and crude coal tar, is an older treatment that has shown efficacy in the treatment of severe or recalcitrant psoriasis (grade B level of recommendation). One prior case-control study comparing the efficacy of Goeckerman therapy with newer treatments, such as biologic therapies, steroids, and oral immunosuppressants, found a similar reduction in symptoms among both treatment groups, with longer disease-free periods in patients who received Goeckerman therapy than those who received newer therapies (22.3 years vs 4.6 months).14 However, Goeckerman therapy is utilized less frequently than more modern therapies because of the time required for treatment and declining insurance reimbursements for it. Climatotherapy, another older established therapy, involves the temporary or permanent relocation of patients to an environment that is favorable for disease control (grade B level of recommendation). Locations such as the Dead Sea and Canary Islands have been studied and shown to provide both subjective and objective improvement in patients’ psoriasis disease course. Patients had notable improvement in both their psoriasis area and severity index score and quality of life after a 3- to 4-week relocation to these areas.15,16 Access to climatotherapy and the transient nature of disease relief are apparent limitations of this treatment modality.
Grenz ray is a type of phototherapy that uses longer-wavelength ionizing radiation, which has low penetrance into surrounding tissues and a 95% absorption rate within the first 3 mm of the skin depth. This treatment has been used less frequently since the development of newer alternatives but should still be considered as a second line to UV therapy, especially in cases of recalcitrant disease and palmoplantar psoriasis, and when access to other treatment options is limited. Grenz ray has a grade C level of recommendation due to the paucity of evidence that supports its efficacy. Thus, it is not recommended as first-line therapy for the treatment of moderate to severe psoriasis. Visible light therapy is another treatment option that uses light in the visible wavelength spectrum but predominantly utilizes blue and red light. Psoriatic lesions are sensitive to light therapy because of the elevated levels of naturally occurring photosensitizing agents, called protoporphyrins, in these lesions.17 Several small studies have shown improvement in psoriatic lesions treated with visible light therapy, with blue light showing greater efficacy in lesional clearance than red light.18,19
Pulsed dye laser is a phototherapy modality that has shown efficacy in the treatment of nail psoriasis (grade B level of recommendation). One study comparing the effects of tazarotene cream 0.1% with pulsed dye laser and tazarotene cream 0.1% alone showed that patients receiving combination therapy had a greater decrease in
Initiation of Phototherapy
Prior to initiating phototherapy, it is important to assess the patient for any personal or family history of skin cancer, as phototherapy carries an increased risk for cutaneous malignancy, especially in patients with a history of skin cancer.22,23 All patients also should be evaluated for their Fitzpatrick skin type, and the minimal erythema dose should be defined for those initiating UVB treatment. These classifications can be useful for the initial determination of treatment dose and the prevention of treatment-related adverse events (TRAEs). A careful drug history also should be taken before the initiation of phototherapy to avoid photosensitizing reactions. Thiazide diuretics and tetracyclines are 2 such examples of medications commonly associated with photosensitizing reactions.24
Fitzpatrick skin type and/or minimal erythema dose testing also are essential in determining the appropriate initial NB-UVB dose required for treatment initiation (Table 2). Patient response to the initial NB-UVB trial will determine the optimal dosage for subsequent maintenance treatments.

For patients unable or unwilling to commit to office-based or institution-based treatments, home NB-UVB is another therapeutic option. One study comparing patients with moderate to severe psoriasis who received home NB-UVB vs in-office treatment showed comparable psoriasis area and severity index scores and quality-of-life indices and no difference in the frequency of TRAE indices. It is important to note that patients who received home treatment had a significantly lower treatment burden (P≤.001) and greater treatment satisfaction than those receiving treatment in an office-based setting (P=.001).25
Assessment and Optimization of Phototherapy
After an appropriate starting dosage has been established, patients should be evaluated at each subsequent visit for the degree of treatment response. Excessive erythema (lasting more than 48 hours) or adverse effects, such as itching, stinging, or burning, are indications that the patient should have their dose adjusted back to the last dose without such adverse responses. Because tolerance to treatment develops over time, patients who miss a scheduled dose of NB-UVB phototherapy require their dose to be temporarily lowered. Targeted dosage of UVB phototherapy at a frequency of 2 to 3 times weekly is preferred over treatment 1 to 2 times weekly; however, consideration should be given toward patient preference.26 Dosing may be increased at a rate of 5% to 10% after each treatment, as tolerated, if there is no clearance of skin lesions with the original treatment dose. Patient skin type also is helpful in dictating the maximum phototherapy dose for each patient (Table 3).

Once a patient’s psoriatic lesions have cleared, the patient has the option to taper or indefinitely continue maintenance therapy. The established protocol for patients who choose to taper therapy is treatment twice weekly for 4 weeks, followed by once-weekly treatment for the second month. The maintenance dosage is held constant during the taper. For patients who prefer indefinite maintenance therapy, treatment is administered every 1 to 2 weeks, with a maintenance dosage that is approximately 25% lower than the original maintenance dosage.
Treatment Considerations
Efforts should be made to ensure that the long-term sequalae of phototherapy are minimized (Table 1). Development of cataracts is a recognized consequence of prolonged UVB exposure; therefore, eye protection is recommended during all UVB treatment sessions to reduce the risk for ocular toxicity. Although pregnancy is not a contraindication to phototherapy, except for PUVA, there is a dose-dependent degradation of folate with NB-UVB treatment, so folate supplementation (0.8 mg) is recommended during NB-UVB treatment to prevent development of neural tube defects in fetuses of patients who are pregnant or who may become pregnant.27
Although phototherapy carries the theoretical risk for photocarcinogenesis, multiple studies have shown no increased risk for malignancy with either NB-UVB or BB-UVB phototherapy.22,23 Regardless, patients who develop new-onset skin cancer while receiving any phototherapeutic treatment should discuss the potential risks and benefits of continued treatment with their physician. Providers should take extra caution prior to initiating treatment, especially in patients with a history of cutaneous malignancy. Because oral PUVA is a systemic therapy, it is associated with a greater risk for photocarcinogenesis than any other modality, particularly in fair-skinned individuals. Patients younger than 10 years; pregnant or nursing patients; and those with a history of lupus, xeroderma pigmentosum, or melanoma should not receive PUVA therapy because of their increased risk for photocarcinogenesis and TRAEs.
The decision to switch patients between different phototherapy modalities during treatment should be individualized to each patient based on factors such as disease severity, quality of life, and treatment burden. Other factors to consider include dosing frequency, treatment cost, and logistical factors, such as proximity to a treatment facility. Physicians should have a detailed discussion about the risks and benefits of continuing therapy for patients who develop new-onset skin cancer during phototherapy.
Final Thoughts
Phototherapy is an effective and safe treatment for patients with psoriasis who have localized and systemic disease. There are several treatment modalities that can be tailored to patient needs and preferences, such as home NB-UVB for patients who are unable or unwilling to undergo office-based treatments. Phototherapy has few absolute contraindications; however, relative contraindications to phototherapy exist. Patients with a history of skin cancer, photosensitivity disorders, and autoimmune diseases (eg, lupus) carry greater risks for adverse events, such as sun-related damage, cancer, and dysplasia. Because these conditions may preclude patients from pursuing phototherapy as a safe and effective approach to treating moderate to severe psoriasis, these patients should be considered for other therapies, such as biologic medications, which may carry a more favorable risk-benefit ratio given that individual’s background.
Psoriasis is a systemic immune-mediated disorder characterized by erythematous, scaly, well-demarcated plaques on the skin that affects approximately 3% of the world’s population.1 Although topical therapies often are the first-line treatment of mild to moderate psoriasis, approximately 1 in 6 individuals has moderate to severe disease that requires systemic treatment such as biologics or phototherapy.2 In patients with localized disease that is refractory to treatment or who have moderate to severe psoriasis requiring systemic treatment, phototherapy should be considered as a potential low-risk treatment option.
In July 2019, the American Academy of Dermatology (AAD) and National Psoriasis Foundation (NPF) released an updated set of guidelines for the use of phototherapy in treating adult patients with psoriasis.3 Since the prior guidelines were released in 2010, there have been numerous studies affirming the efficacy of phototherapy, with several large meta-analyses helping to refine clinical recommendations.4,5 Each treatment was ranked using Strength of Recommendation Taxonomy, with a score of A, B, or C based on the strength of the evidence supporting the given modality. With the ever-increasing number of treatment options for patients with psoriasis, these guidelines inform dermatologists of the recommendations for the initiation, maintenance, and optimization of phototherapy in the treatment of psoriasis.
The AAD-NPF recommendations discuss the mechanism of action, efficacy, safety, and frequency of adverse events of 10 commonly used phototherapy/photochemotherapy modalities. They also address dosing regimens, the potential to combine phototherapy with other therapies, and the efficacy of treatment modalities for different types of psoriasis.3 The purpose of this discussion is to present these guidelines in a condensed form for prescribers of phototherapy and to review the most clinically significant considerations during each step of treatment. Of note, we only highlight the treatment of adult patients and do not discuss information relevant to pediatric patients with psoriasis.
Choosing a Phototherapy Modality
Phototherapy may be considered for patients with psoriasis that affects more than 3% body surface area or for localized disease refractory to conventional treatments. UV light is believed to provide relief from psoriasis via multiple mechanisms, such as through favorable alterations in cytokine profiles, initiation of apoptosis, and local immunosupression.6 There is no single first-line phototherapeutic modality recommended for all patients with psoriasis. Rather, the decision to implement a particular modality should be individualized to the patient, considering factors such as percentage of body surface area affected by disease, quality-of-life assessment, comorbidities, lifestyle, and cost of treatment.
Of the 10 phototherapy modalities reviewed in these guidelines, 4 were ranked by the AAD and NPF as having grade A evidence for efficacy in the treatment of moderate to severe plaque psoriasis. Treatments with a grade A level of recommendation included narrowband UVB (NB-UVB), broadband UVB (BB-UVB), targeted phototherapy (excimer laser and excimer lamp), and

Studies have shown that the ideal wavelength needed to produce a therapeutic effect (ie, clearance of psoriatic plaques) is 304 to 313 nm. Wavelengths of 290 to 300 nm were found to be less therapeutic and more harmful, as they contributed to the development of sunburns.7 Broadband UVB phototherapy, with wavelengths ranging from 270 to 390 nm, exposes patients to a greater spectrum of radiation, thus making it more likely to cause sunburn and any theoretical form of sun-related damage, such as dysplasia and cancer. Compared with NB-UVB phototherapy, BB-UVB phototherapy is associated with a greater degree of sun damage–related side effects. Narrowband UVB, with a wavelength range of 311 to 313 nm, carries a grade A level of recommendation and should be considered as first-line monotherapy in patients with generalized plaque psoriasis, given its efficacy and promising safety profile. Multiple studies have shown that NB-UVB phototherapy is superior to BB-UVB phototherapy in the treatment of moderate to severe psoriasis in adults.8,9 In facilities where access to NB-UVB is limited, BB-UVB monotherapy is recommended as the treatment of generalized plaque psoriasis.
Psoralen plus UVA, which may be used topically (ie, bathwater PUVA) or taken orally, refers to treatment with photosensitizing psoralens. Psoralens are agents that intercalate with DNA and enhance the efficacy of phototherapy.10 Topical PUVA, with a grade B level of recommendation, is an effective treatment option for patients with localized disease and has been shown to be particularly efficacious in the treatment of palmoplantar pustular psoriasis. Oral PUVA is an effective option for psoriasis with a grade A recommendation, while bathwater PUVA has a grade B level of recommendation for moderate to severe plaque psoriasis. Oral PUVA is associated with greater systemic side effects (both acute and subacute) compared with NB-UVB and also is associated with photocarcinogenesis, particularly squamous cell carcinoma in white patients.11 Other side effects from PUVA include pigmented macules in sun-protected areas (known as PUVA lentigines), which may make evaluation of skin lesions challenging. Because of the increased risk for cancer with oral PUVA, NB-UVB is preferable as a first-line treatment vs PUVA, especially in patients with a history of skin cancer.12,13
Goeckerman therapy, which involves the synergistic combination of UVB and crude coal tar, is an older treatment that has shown efficacy in the treatment of severe or recalcitrant psoriasis (grade B level of recommendation). One prior case-control study comparing the efficacy of Goeckerman therapy with newer treatments, such as biologic therapies, steroids, and oral immunosuppressants, found a similar reduction in symptoms among both treatment groups, with longer disease-free periods in patients who received Goeckerman therapy than those who received newer therapies (22.3 years vs 4.6 months).14 However, Goeckerman therapy is utilized less frequently than more modern therapies because of the time required for treatment and declining insurance reimbursements for it. Climatotherapy, another older established therapy, involves the temporary or permanent relocation of patients to an environment that is favorable for disease control (grade B level of recommendation). Locations such as the Dead Sea and Canary Islands have been studied and shown to provide both subjective and objective improvement in patients’ psoriasis disease course. Patients had notable improvement in both their psoriasis area and severity index score and quality of life after a 3- to 4-week relocation to these areas.15,16 Access to climatotherapy and the transient nature of disease relief are apparent limitations of this treatment modality.
Grenz ray is a type of phototherapy that uses longer-wavelength ionizing radiation, which has low penetrance into surrounding tissues and a 95% absorption rate within the first 3 mm of the skin depth. This treatment has been used less frequently since the development of newer alternatives but should still be considered as a second line to UV therapy, especially in cases of recalcitrant disease and palmoplantar psoriasis, and when access to other treatment options is limited. Grenz ray has a grade C level of recommendation due to the paucity of evidence that supports its efficacy. Thus, it is not recommended as first-line therapy for the treatment of moderate to severe psoriasis. Visible light therapy is another treatment option that uses light in the visible wavelength spectrum but predominantly utilizes blue and red light. Psoriatic lesions are sensitive to light therapy because of the elevated levels of naturally occurring photosensitizing agents, called protoporphyrins, in these lesions.17 Several small studies have shown improvement in psoriatic lesions treated with visible light therapy, with blue light showing greater efficacy in lesional clearance than red light.18,19
Pulsed dye laser is a phototherapy modality that has shown efficacy in the treatment of nail psoriasis (grade B level of recommendation). One study comparing the effects of tazarotene cream 0.1% with pulsed dye laser and tazarotene cream 0.1% alone showed that patients receiving combination therapy had a greater decrease in
Initiation of Phototherapy
Prior to initiating phototherapy, it is important to assess the patient for any personal or family history of skin cancer, as phototherapy carries an increased risk for cutaneous malignancy, especially in patients with a history of skin cancer.22,23 All patients also should be evaluated for their Fitzpatrick skin type, and the minimal erythema dose should be defined for those initiating UVB treatment. These classifications can be useful for the initial determination of treatment dose and the prevention of treatment-related adverse events (TRAEs). A careful drug history also should be taken before the initiation of phototherapy to avoid photosensitizing reactions. Thiazide diuretics and tetracyclines are 2 such examples of medications commonly associated with photosensitizing reactions.24
Fitzpatrick skin type and/or minimal erythema dose testing also are essential in determining the appropriate initial NB-UVB dose required for treatment initiation (Table 2). Patient response to the initial NB-UVB trial will determine the optimal dosage for subsequent maintenance treatments.

For patients unable or unwilling to commit to office-based or institution-based treatments, home NB-UVB is another therapeutic option. One study comparing patients with moderate to severe psoriasis who received home NB-UVB vs in-office treatment showed comparable psoriasis area and severity index scores and quality-of-life indices and no difference in the frequency of TRAE indices. It is important to note that patients who received home treatment had a significantly lower treatment burden (P≤.001) and greater treatment satisfaction than those receiving treatment in an office-based setting (P=.001).25
Assessment and Optimization of Phototherapy
After an appropriate starting dosage has been established, patients should be evaluated at each subsequent visit for the degree of treatment response. Excessive erythema (lasting more than 48 hours) or adverse effects, such as itching, stinging, or burning, are indications that the patient should have their dose adjusted back to the last dose without such adverse responses. Because tolerance to treatment develops over time, patients who miss a scheduled dose of NB-UVB phototherapy require their dose to be temporarily lowered. Targeted dosage of UVB phototherapy at a frequency of 2 to 3 times weekly is preferred over treatment 1 to 2 times weekly; however, consideration should be given toward patient preference.26 Dosing may be increased at a rate of 5% to 10% after each treatment, as tolerated, if there is no clearance of skin lesions with the original treatment dose. Patient skin type also is helpful in dictating the maximum phototherapy dose for each patient (Table 3).

Once a patient’s psoriatic lesions have cleared, the patient has the option to taper or indefinitely continue maintenance therapy. The established protocol for patients who choose to taper therapy is treatment twice weekly for 4 weeks, followed by once-weekly treatment for the second month. The maintenance dosage is held constant during the taper. For patients who prefer indefinite maintenance therapy, treatment is administered every 1 to 2 weeks, with a maintenance dosage that is approximately 25% lower than the original maintenance dosage.
Treatment Considerations
Efforts should be made to ensure that the long-term sequalae of phototherapy are minimized (Table 1). Development of cataracts is a recognized consequence of prolonged UVB exposure; therefore, eye protection is recommended during all UVB treatment sessions to reduce the risk for ocular toxicity. Although pregnancy is not a contraindication to phototherapy, except for PUVA, there is a dose-dependent degradation of folate with NB-UVB treatment, so folate supplementation (0.8 mg) is recommended during NB-UVB treatment to prevent development of neural tube defects in fetuses of patients who are pregnant or who may become pregnant.27
Although phototherapy carries the theoretical risk for photocarcinogenesis, multiple studies have shown no increased risk for malignancy with either NB-UVB or BB-UVB phototherapy.22,23 Regardless, patients who develop new-onset skin cancer while receiving any phototherapeutic treatment should discuss the potential risks and benefits of continued treatment with their physician. Providers should take extra caution prior to initiating treatment, especially in patients with a history of cutaneous malignancy. Because oral PUVA is a systemic therapy, it is associated with a greater risk for photocarcinogenesis than any other modality, particularly in fair-skinned individuals. Patients younger than 10 years; pregnant or nursing patients; and those with a history of lupus, xeroderma pigmentosum, or melanoma should not receive PUVA therapy because of their increased risk for photocarcinogenesis and TRAEs.
The decision to switch patients between different phototherapy modalities during treatment should be individualized to each patient based on factors such as disease severity, quality of life, and treatment burden. Other factors to consider include dosing frequency, treatment cost, and logistical factors, such as proximity to a treatment facility. Physicians should have a detailed discussion about the risks and benefits of continuing therapy for patients who develop new-onset skin cancer during phototherapy.
Final Thoughts
Phototherapy is an effective and safe treatment for patients with psoriasis who have localized and systemic disease. There are several treatment modalities that can be tailored to patient needs and preferences, such as home NB-UVB for patients who are unable or unwilling to undergo office-based treatments. Phototherapy has few absolute contraindications; however, relative contraindications to phototherapy exist. Patients with a history of skin cancer, photosensitivity disorders, and autoimmune diseases (eg, lupus) carry greater risks for adverse events, such as sun-related damage, cancer, and dysplasia. Because these conditions may preclude patients from pursuing phototherapy as a safe and effective approach to treating moderate to severe psoriasis, these patients should be considered for other therapies, such as biologic medications, which may carry a more favorable risk-benefit ratio given that individual’s background.
- Michalek IM, Loring B, John SM. A systematic review of worldwide epidemiology of psoriasis. J Eur Acad Dermatol Venereol. 2017;31:205-212.
- Yeung H, Takeshita J, Mehta NN, et al. Psoriasis severity and the prevalence of major medical comorbidity: a population-based study. JAMA Dermatol. 2013;149:1173-1179.
- Elmets CA, Lim HW, Stoff B, et al. Joint American Academy of Dermatology-National Psoriasis Foundation guidelines of care for the management and treatment of psoriasis with phototherapy. J Am Acad Dermatol. 2019;81:775-804.
- Archier E, Devaux S, Castela E, et al. Efficacy of psoralen UV-A therapy vs. narrowband UV-B therapy in chronic plaque psoriasis: a systematic literature review. J Eur Acad Dermatol Venereol. 2012;26(suppl 3):11-21.
- Chen X, Yang M, Cheng Y, et al. Narrow-band ultraviolet B phototherapy versus broad-band ultraviolet B or psoralen-ultraviolet A photochemotherapy for psoriasis. Cochrane Database Syst Rev. 2013;10:CD009481.
- Wong T, Hsu L, Liao W. Phototherapy in psoriasis: a review of mechanisms of action. J Cutan Med Surg. 2013;17:6-12.
- Parrish JA, Jaenicke KF. Action spectrum for phototherapy of psoriasis. J Invest Dermatol. 1981;76:359-362.
- Almutawa F, Alnomair N, Wang Y, et al. Systematic review of UV-based therapy for psoriasis. Am J Clin Dermatol. 2013;14:87-109.
- El-Mofty M, Mostafa WZ, Bosseila M, et al. A large scale analytical study on efficacy of different photo(chemo)therapeutic modalities in the treatment of psoriasis, vitiligo and mycosis fungoides. Dermatol Ther. 2010;23:428-434.
- Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 5. guidelines of care for the treatment of psoriasis with phototherapy and photochemotherapy. J Am Acad Dermatol. 2010;62:114-135.
- Murase JE, Lee EE, Koo J. Effect of ethnicity on the risk of developing nonmelanoma skin cancer following long-term PUVA therapy. Int J Dermatol. 2005;44:1016-1021.
- Bruynzeel I, Bergman W, Hartevelt HM, et al. 'High single-dose' European PUVA regimen also causes an excess of non-melanoma skin cancer. Br J Dermatol. 1991;124:49-55.
- Lindelöf B, Sigurgeirsson B, Tegner E, et al. PUVA and cancer risk: the Swedish follow-up study. Br J Dermatol. 1999;141:108-112.
- Chern E, Yau D, Ho JC, et al. Positive effect of modified Goeckerman regimen on quality of life and psychosocial distress in moderate and severe psoriasis. Acta Derm Venereol. 2011;91:447-451.
- Harari M, Czarnowicki T, Fluss R, et al. Patients with early-onset psoriasis achieve better results following Dead Sea climatotherapy. J Eur Acad Dermatol Venereol. 2012;26:554-559.
- Wahl AK, Langeland E, Larsen MH, et al. Positive changes in self-management and disease severity following climate therapy in people with psoriasis. Acta Dermatol Venereol. 2015;95:317-321.
- Bissonnette R, Zeng H, McLean DI, et al. Psoriatic plaques exhibit red autofluorescence that is due to protoporphyrin IX. J Invest Dermatol. 1998;111:586-591.
- Kleinpenning MM, Otero ME, van Erp PE, et al. Efficacy of blue light vs. red light in the treatment of psoriasis: a double-blind, randomized comparative study. J Eur Acad Dermatol Venereol. 2012;26:219-225.
- Weinstabl A, Hoff-Lesch S, Merk HF, et al. Prospective randomized study on the efficacy of blue light in the treatment of psoriasis vulgaris. Dermatology. 2011;223:251-259.
- Huang YC, Chou CL, Chiang YY. Efficacy of pulsed dye laser plus topical tazarotene versus topical tazarotene alone in psoriatic nail disease: a single-blind, intrapatient left-to-right controlled study. Lasers Surg Med. 2013;45:102-107.
- Tawfik AA. Novel treatment of nail psoriasis using the intense pulsed light: a one-year follow-up study. Dermatol Surg. 2014;40:763-768.
- Archier E, Devaux S, Castela E, et al. Carcinogenic risks of psoralen UV-A therapy and narrowband UV-B therapy in chronic plaque psoriasis: a systematic literature review. J Eur Acad Dermatol Venereol. 2012;26(suppl 3):22-31.
- Osmancevic A, Gillstedt M, Wennberg AM, et al. The risk of skin cancer in psoriasis patients treated with UVB therapy. Acta Dermatol Venereol. 2014;94:425-430.
- Dawe RS, Ibbotson SH. Drug-induced photosensitivity. Dermatol Clin. 2014;32:363-368.
- Koek MB, Buskens E, van Weelden H, et al. Home versus outpatient ultraviolet B phototherapy for mild to severe psoriasis: pragmatic multicentre randomised controlled non-inferiority trial (PLUTO study). BMJ. 2009;338:B1542.
- Almutawa F, Thalib L, Hekman D, et al. Efficacy of localized phototherapy and photodynamic therapy for psoriasis: a systematic review and meta-analysis. Photodermatol Photoimmunol Photomed. 2015;31:5-14.
- Zhang M, Goyert G, Lim HW. Folate and phototherapy: what should we inform our patients? J Am Acad Dermatol. 2017;77:958-964.
- Michalek IM, Loring B, John SM. A systematic review of worldwide epidemiology of psoriasis. J Eur Acad Dermatol Venereol. 2017;31:205-212.
- Yeung H, Takeshita J, Mehta NN, et al. Psoriasis severity and the prevalence of major medical comorbidity: a population-based study. JAMA Dermatol. 2013;149:1173-1179.
- Elmets CA, Lim HW, Stoff B, et al. Joint American Academy of Dermatology-National Psoriasis Foundation guidelines of care for the management and treatment of psoriasis with phototherapy. J Am Acad Dermatol. 2019;81:775-804.
- Archier E, Devaux S, Castela E, et al. Efficacy of psoralen UV-A therapy vs. narrowband UV-B therapy in chronic plaque psoriasis: a systematic literature review. J Eur Acad Dermatol Venereol. 2012;26(suppl 3):11-21.
- Chen X, Yang M, Cheng Y, et al. Narrow-band ultraviolet B phototherapy versus broad-band ultraviolet B or psoralen-ultraviolet A photochemotherapy for psoriasis. Cochrane Database Syst Rev. 2013;10:CD009481.
- Wong T, Hsu L, Liao W. Phototherapy in psoriasis: a review of mechanisms of action. J Cutan Med Surg. 2013;17:6-12.
- Parrish JA, Jaenicke KF. Action spectrum for phototherapy of psoriasis. J Invest Dermatol. 1981;76:359-362.
- Almutawa F, Alnomair N, Wang Y, et al. Systematic review of UV-based therapy for psoriasis. Am J Clin Dermatol. 2013;14:87-109.
- El-Mofty M, Mostafa WZ, Bosseila M, et al. A large scale analytical study on efficacy of different photo(chemo)therapeutic modalities in the treatment of psoriasis, vitiligo and mycosis fungoides. Dermatol Ther. 2010;23:428-434.
- Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 5. guidelines of care for the treatment of psoriasis with phototherapy and photochemotherapy. J Am Acad Dermatol. 2010;62:114-135.
- Murase JE, Lee EE, Koo J. Effect of ethnicity on the risk of developing nonmelanoma skin cancer following long-term PUVA therapy. Int J Dermatol. 2005;44:1016-1021.
- Bruynzeel I, Bergman W, Hartevelt HM, et al. 'High single-dose' European PUVA regimen also causes an excess of non-melanoma skin cancer. Br J Dermatol. 1991;124:49-55.
- Lindelöf B, Sigurgeirsson B, Tegner E, et al. PUVA and cancer risk: the Swedish follow-up study. Br J Dermatol. 1999;141:108-112.
- Chern E, Yau D, Ho JC, et al. Positive effect of modified Goeckerman regimen on quality of life and psychosocial distress in moderate and severe psoriasis. Acta Derm Venereol. 2011;91:447-451.
- Harari M, Czarnowicki T, Fluss R, et al. Patients with early-onset psoriasis achieve better results following Dead Sea climatotherapy. J Eur Acad Dermatol Venereol. 2012;26:554-559.
- Wahl AK, Langeland E, Larsen MH, et al. Positive changes in self-management and disease severity following climate therapy in people with psoriasis. Acta Dermatol Venereol. 2015;95:317-321.
- Bissonnette R, Zeng H, McLean DI, et al. Psoriatic plaques exhibit red autofluorescence that is due to protoporphyrin IX. J Invest Dermatol. 1998;111:586-591.
- Kleinpenning MM, Otero ME, van Erp PE, et al. Efficacy of blue light vs. red light in the treatment of psoriasis: a double-blind, randomized comparative study. J Eur Acad Dermatol Venereol. 2012;26:219-225.
- Weinstabl A, Hoff-Lesch S, Merk HF, et al. Prospective randomized study on the efficacy of blue light in the treatment of psoriasis vulgaris. Dermatology. 2011;223:251-259.
- Huang YC, Chou CL, Chiang YY. Efficacy of pulsed dye laser plus topical tazarotene versus topical tazarotene alone in psoriatic nail disease: a single-blind, intrapatient left-to-right controlled study. Lasers Surg Med. 2013;45:102-107.
- Tawfik AA. Novel treatment of nail psoriasis using the intense pulsed light: a one-year follow-up study. Dermatol Surg. 2014;40:763-768.
- Archier E, Devaux S, Castela E, et al. Carcinogenic risks of psoralen UV-A therapy and narrowband UV-B therapy in chronic plaque psoriasis: a systematic literature review. J Eur Acad Dermatol Venereol. 2012;26(suppl 3):22-31.
- Osmancevic A, Gillstedt M, Wennberg AM, et al. The risk of skin cancer in psoriasis patients treated with UVB therapy. Acta Dermatol Venereol. 2014;94:425-430.
- Dawe RS, Ibbotson SH. Drug-induced photosensitivity. Dermatol Clin. 2014;32:363-368.
- Koek MB, Buskens E, van Weelden H, et al. Home versus outpatient ultraviolet B phototherapy for mild to severe psoriasis: pragmatic multicentre randomised controlled non-inferiority trial (PLUTO study). BMJ. 2009;338:B1542.
- Almutawa F, Thalib L, Hekman D, et al. Efficacy of localized phototherapy and photodynamic therapy for psoriasis: a systematic review and meta-analysis. Photodermatol Photoimmunol Photomed. 2015;31:5-14.
- Zhang M, Goyert G, Lim HW. Folate and phototherapy: what should we inform our patients? J Am Acad Dermatol. 2017;77:958-964.
Practice Points
- Phototherapy should be considered as an effective and low-risk treatment of psoriasis.
- Narrowband UVB, broadband UVB, targeted phototherapy (excimer laser and excimer lamp), and oral psoralen plus UVA have all received a grade A level of recommendation for the treatment of psoriasis in adults.
Z-plasty for Correction of Standing Cutaneous Deformity
Practice Gap
Cutaneous head and neck reconstruction following Mohs micrographic surgery frequently presents the surgical dilemma of dog-ear formation during wound closure, often necessitating excision of additional tissue to correct the standing cone, which could pose the risk for an undesirable tension vector as well as encroachment upon additional cosmetic units or sensitive anatomic structures such as a free margin. A classic Z-plasty is a transposition flap (by definition, translocation of tissue laterally about a pivot point) that corrects a dog-ear deformity without skin excision by recruiting tissue from the axis of the standing cone and redistributing it along another.
The Technique
A classic Z-plasty is designed with 3 equal limb lengths (<1 cm each) at 60° angles, abutting the pedicle of the rotation or advancement flap. The limbs can extend away from the pedicle of the flap to minimize vascular compromise. In our patient, the theoretical standing cone was located at the lateral aspect of an O to L advancement flap (Figure 1). The 2 identical triangular flaps were elevated (Figure 2A), transposed around the pivot point (Figure 2B), and inset (Figure 3). The standing cone was corrected by redistribution of tissue without excision of additional tissue, resulting in a softer and thinner scar 2 weeks (Figure 4A) and 4 months (Figure 4B) postoperatively.
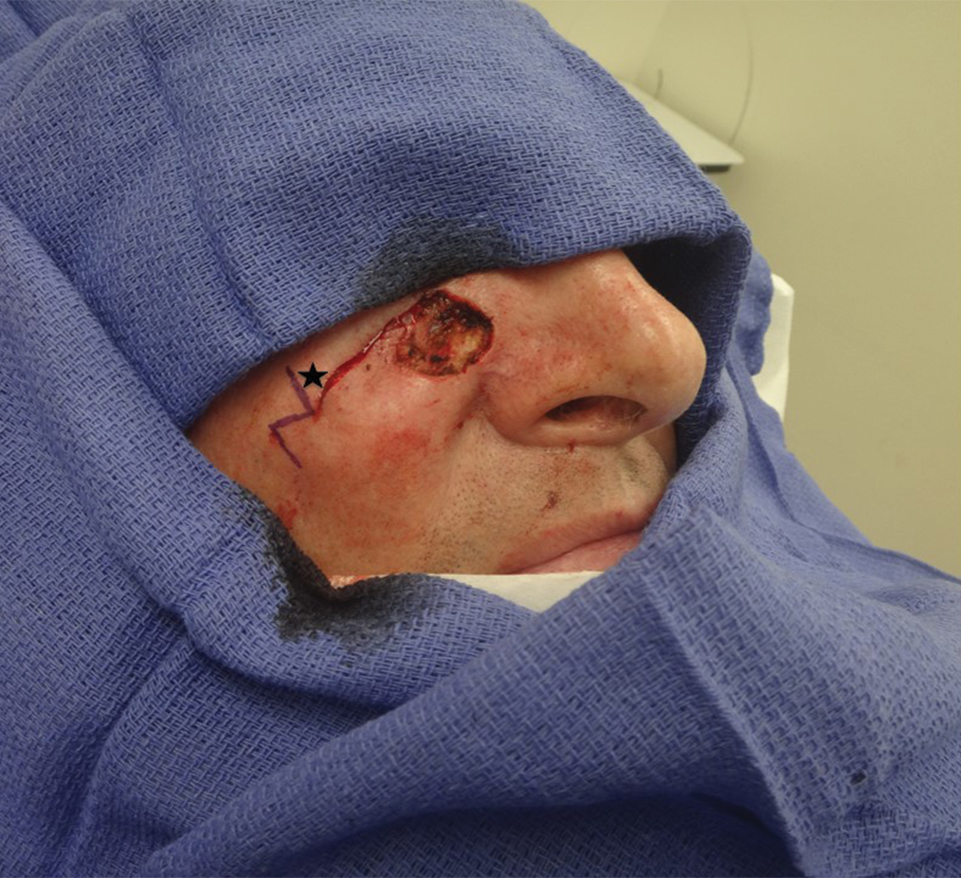

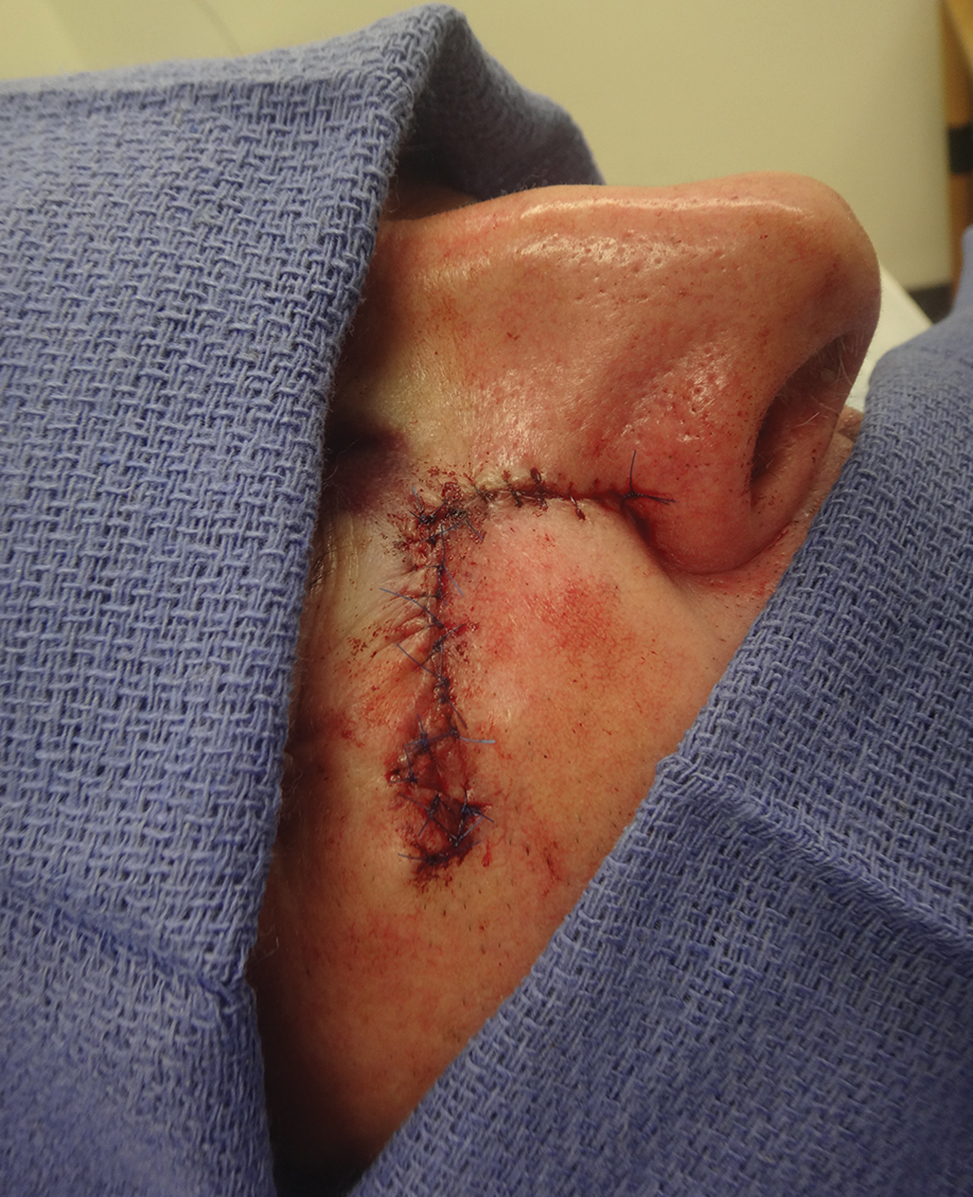

Practice Implications
This technique can be used to correct cones following primary wound repairs or flaps. The primary advantage of this technique for dog-ear correction is tissue sparing. Disadvantages include more complex surgical planning and longer scar length compared to excisional corrective techniques. Additionally, Z-plasty requires more time to execute compared to simpler techniques.1,2
- Frodel JL, Pawar SS, Wang TD. Z-Plasty. In: Baker SR, ed. Local Flaps in Facial Reconstruction. 3rd ed. Elsevier; 2014:317-338.
- Hundeshagen G, Zapata-Sirvent R, Goverman J, et al. Tissue rearrangements: the power of the Z-plasty. Clin Plast Surg. 2017;44:805-812.
Practice Gap
Cutaneous head and neck reconstruction following Mohs micrographic surgery frequently presents the surgical dilemma of dog-ear formation during wound closure, often necessitating excision of additional tissue to correct the standing cone, which could pose the risk for an undesirable tension vector as well as encroachment upon additional cosmetic units or sensitive anatomic structures such as a free margin. A classic Z-plasty is a transposition flap (by definition, translocation of tissue laterally about a pivot point) that corrects a dog-ear deformity without skin excision by recruiting tissue from the axis of the standing cone and redistributing it along another.
The Technique
A classic Z-plasty is designed with 3 equal limb lengths (<1 cm each) at 60° angles, abutting the pedicle of the rotation or advancement flap. The limbs can extend away from the pedicle of the flap to minimize vascular compromise. In our patient, the theoretical standing cone was located at the lateral aspect of an O to L advancement flap (Figure 1). The 2 identical triangular flaps were elevated (Figure 2A), transposed around the pivot point (Figure 2B), and inset (Figure 3). The standing cone was corrected by redistribution of tissue without excision of additional tissue, resulting in a softer and thinner scar 2 weeks (Figure 4A) and 4 months (Figure 4B) postoperatively.




Practice Implications
This technique can be used to correct cones following primary wound repairs or flaps. The primary advantage of this technique for dog-ear correction is tissue sparing. Disadvantages include more complex surgical planning and longer scar length compared to excisional corrective techniques. Additionally, Z-plasty requires more time to execute compared to simpler techniques.1,2
Practice Gap
Cutaneous head and neck reconstruction following Mohs micrographic surgery frequently presents the surgical dilemma of dog-ear formation during wound closure, often necessitating excision of additional tissue to correct the standing cone, which could pose the risk for an undesirable tension vector as well as encroachment upon additional cosmetic units or sensitive anatomic structures such as a free margin. A classic Z-plasty is a transposition flap (by definition, translocation of tissue laterally about a pivot point) that corrects a dog-ear deformity without skin excision by recruiting tissue from the axis of the standing cone and redistributing it along another.
The Technique
A classic Z-plasty is designed with 3 equal limb lengths (<1 cm each) at 60° angles, abutting the pedicle of the rotation or advancement flap. The limbs can extend away from the pedicle of the flap to minimize vascular compromise. In our patient, the theoretical standing cone was located at the lateral aspect of an O to L advancement flap (Figure 1). The 2 identical triangular flaps were elevated (Figure 2A), transposed around the pivot point (Figure 2B), and inset (Figure 3). The standing cone was corrected by redistribution of tissue without excision of additional tissue, resulting in a softer and thinner scar 2 weeks (Figure 4A) and 4 months (Figure 4B) postoperatively.




Practice Implications
This technique can be used to correct cones following primary wound repairs or flaps. The primary advantage of this technique for dog-ear correction is tissue sparing. Disadvantages include more complex surgical planning and longer scar length compared to excisional corrective techniques. Additionally, Z-plasty requires more time to execute compared to simpler techniques.1,2
- Frodel JL, Pawar SS, Wang TD. Z-Plasty. In: Baker SR, ed. Local Flaps in Facial Reconstruction. 3rd ed. Elsevier; 2014:317-338.
- Hundeshagen G, Zapata-Sirvent R, Goverman J, et al. Tissue rearrangements: the power of the Z-plasty. Clin Plast Surg. 2017;44:805-812.
- Frodel JL, Pawar SS, Wang TD. Z-Plasty. In: Baker SR, ed. Local Flaps in Facial Reconstruction. 3rd ed. Elsevier; 2014:317-338.
- Hundeshagen G, Zapata-Sirvent R, Goverman J, et al. Tissue rearrangements: the power of the Z-plasty. Clin Plast Surg. 2017;44:805-812.
APPlying Knowledge: Evidence for and Regulation of Mobile Apps for Dermatologists
Since the first mobile application (app) was developed in the 1990s, apps have become increasingly integrated into medical practice and training. More than 5.5 million apps were downloadable in 2019,1 of which more than 300,000 were health related.2 In the United States, more than 80% of physicians reported using smartphones for professional purposes in 2016.3 As the complexity of apps and their purpose of use has evolved, regulatory bodies have not adapted adequately to monitor apps that have broad-reaching consequences in medicine.
We review the primary literature on PubMed behind health-related apps that impact dermatologists as well as the government regulation of these apps, with a focus on the 3 most prevalent dermatology-related apps used by dermatology residents in the United States: VisualDx, UpToDate, and Mohs Surgery Appropriate Use Criteria. This prevalence is according to a survey emailed to all dermatology residents in the United States by the American Academy of Dermatology (AAD) in 2019 (unpublished data).
VisualDx
VisualDx, which aims to improve diagnostic accuracy and patient safety, contains peer-reviewed data and more than 32,000 images of dermatologic conditions. The editorial board includes more than 50 physicians. It provides opportunities for continuing medical education credit, is used in more than 2300 medical settings, and costs $399.99 annually for a subscription with partial features. Prior to the launch of the app in 2010, some health science professionals noted that the website version lacked references to primary sources.4 The same issue carried over to the app, which has evolved to offer artificial intelligence (AI) analysis of photographed skin lesions. However, there are no peer-reviewed publications showing positive impact of the app on diagnostic skills among dermatology residents or on patient outcomes.
UpToDate
UpToDate is a web-based database created in the early 1990s. A corresponding app was created around 2010. Both internal and independent research has demonstrated improved outcomes, and the app is advertised as the only clinical decision support resource associated with improved outcomes, as shown in more than 80 publications.5 UpToDate covers more than 11,800 medical topics and contains more than 35,000 graphics. It cites primary sources and uses a published system for grading recommendation strength and evidence quality. The data are processed and produced by a team of more than 7100 physicians as authors, editors, and reviewers. The platform grants continuing medical education credit and is used by more than 1.9 million clinicians in more than 190 countries. A 1-year subscription for an individual US-based physician costs $559. An observational study assessed UpToDate articles for potential conflicts of interest between authors and their recommendations. Of the 6 articles that met inclusion criteria of discussing management of medical conditions that have controversial or mostly brand-name treatment options, all had conflicts of interest, such as naming drugs from companies with which the authors and/or editors had financial relationships.6
Mohs Surgery Appropriate Use Criteria
The Mohs Surgery Appropriate Use Criteria app is a free clinical decision-making tool based on a consensus statement published in 2012 by the AAD, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and American Society for Mohs Surgery.7 It helps guide management of more than 200 dermatologic scenarios. Critique has been made that the criteria are partly based on expert opinion and data largely from the United States and has not been revised to incorporate newer data.8 There are no publications regarding the app itself.
Regulation of Health-Related Apps
Health-related apps that are designed for utilization by health care providers can be a valuable tool. However, given their prevalence, cost, and potential impact on patient lives, these apps should be well regulated and researched. The general paucity of peer-reviewed literature demonstrating the utility, safety, quality, and accuracy of health-related apps commonly used by providers is a reflection of insufficient mobile health regulation in the United States.
There are 3 primary government agencies responsible for regulating mobile medical apps: the US Food and Drug Administration (FDA), Federal Trade Commission, and Office for Civil Rights.9 The FDA does not regulate all medical devices. Apps intended for use in the diagnosis, cure, mitigation, prevention, or treatment of a disease or condition are considered to be medical devices.10 The FDA regulates those apps only if they are judged to pose more than minimal risk. Apps that are designed only to provide easy access to information related to health conditions or treatment are considered to be minimal risk but can develop into a different risk level such as by offering AI.11 Although the FDA does update its approach to medical devices, including apps and AI- and machine learning–based software, the rate and direction of update has not kept pace with the rapid evolution of apps.12 In 2019, the FDA began piloting a precertification program that grants long-term approval to organizations that develop apps instead of reviewing each app product individually.13 This decrease in premarket oversight is intended to expedite innovation with the hopeful upside of improving patient outcomes but is inconsistent, with the FDA still reviewing other types of medical devices individually.
For apps that are already in use, the Federal Trade Commission only gets involved in response to deceptive or unfair acts or practices relating to privacy, data security, and false or misleading claims about safety or performance. It may be more beneficial for consumers if those apps had a more stringent initial approval process. The Office for Civil Rights enforces the Health Insurance Portability and Accountability Act when relevant to apps.
Nongovernment agencies also are involved in app regulation. The FDA believes sharing more regulatory responsibility with private industry would promote efficiency.14 Google does not allow apps that contain false or misleading health claims,15 and Apple may scrutinize medical apps that could provide inaccurate data or be used for diagnosing or treating patients.16 Xcertia, a nonprofit organization founded by the American Medical Association and others, develops standards for the security, privacy, content, and operability of health-related apps, but those standards have not been adopted by other parties. Ultimately, nongovernment agencies are not responsible for public health and do not boast the government’s ability to enforce rules or ensure public safety.
Final Thoughts
The AAD survey of US dermatology residents found that the top consideration when choosing apps was up-to-date and accurate information; however, the 3 most prevalent apps among those same respondents did not need government approval and are not required to contain up-to-date data or to improve clinical outcomes, similar to most other health-related apps. This discrepancy is concerning considering the increasing utilization of apps for physician education and health care delivery and the increasing complexity of those apps. In light of these results, the potential decrease in federal premarket regulation suggested by the FDA’s precertification program seems inappropriate. It is important for the government to take responsibility for regulating health-related apps and to find a balance between too much regulation delaying innovation and too little regulation hurting physician training and patient care. It also is important for providers to be aware of the evidence and oversight behind the technologies they use for professional purposes.
- Clement J. Number of apps available in leading app stores as of 1st quarter 2020. Statista website. https://www.statista.com/statistics/276623/number-of-apps-available-in-leading-app-stores/. Published May 4, 2020. Accessed July 23, 2020.
- mHealth App Economics 2017/2018. Current Status and Future Trends in Mobile Health. Berlin, Germany: Research 2 Guidance; 2018.
- Healthcare Client Services. Professional usage of smartphones by doctors. Kantar website. https://www.kantarmedia.com/us/thinking-and-resources/blog/professional-usage-of-smartphones-by-doctors-2016. Published November 16, 2016. Accessed July 23, 2020.
- Skhal KJ, Koffel J. VisualDx. J Med Libr Assoc. 2007;95:470-471.
- UpToDate is the only clinical decision support resource associated with improved outcomes. UpToDate website. https://www.uptodate.com/home/research. Accessed July 29, 2020.
- Connolly SM, Baker DR, Coldiron BM, et al. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. J Am Acad Dermatol. 2012;67:531-550.
- Amber KT, Dhiman G, Goodman KW. Conflict of interest in online point-of-care clinical support websites. J Med Ethics. 2014;40:578-580.
- Croley JA, Joseph AK, Wagner RF Jr. Discrepancies in the Mohs micrographic surgery appropriate use criteria. J Am Acad Dermatol. 2020;82:E55.
- Mobile health apps interactive tool. Federal Trade Commission website. https://www.ftc.gov/tips-advice/business-center/guidance/mobile-health-apps-interactive-tool. Published April 2016. Accessed May 23, 2020.
- Federal Food, Drug, and Cosmetic Act, 21 USC §321 (2018).
- US Food and Drug Administration. Examples of software functions for which the FDA will exercise enforcement discretion. https://www.fda.gov/medical-devices/device-software-functions-including-mobile-medical-applications/examples-software-functions-which-fda-will-exercise-enforcement-discretion. Updated September 26, 2019. Accessed July 29, 2020.
- US Food and Drug Administration. Proposed regulatory framework for modifications to artificial intelligence/machine learning (AI/ML)‐based software as a medical device (SaMD). https://www.fda.gov/downloads/MedicalDevices/DigitalHealth/SoftwareasaMedicalDevice/UCM635052.pdf. Accessed July 23, 2020.
- US Food and Drug Administration. Digital health software precertification (pre-cert) program. https://www.fda.gov/medical-devices/digital-health/digital-health-software-precertification-pre-cert-program. Updated July 18, 2019. Accessed July 23, 2020.
- Gottlieb S. Fostering medical innovation: a plan for digital health devices. US Food and Drug Administration website. https://www.fda.gov/news-events/fda-voices/fostering-medical-innovation-plan-digital-health-devices. Published June 15, 2017. Accessed July 23, 2020.
- Restricted content: unapproved substances. Google Play website. https://play.google.com/about/restricted-content/unapproved-substances. Accessed July 23, 2020.
- App store review guidelines. Apple Developer website. https://developer.apple.com/app-store/review/guidelines. Updated March 4, 2020. Accessed July 23, 2020.
Since the first mobile application (app) was developed in the 1990s, apps have become increasingly integrated into medical practice and training. More than 5.5 million apps were downloadable in 2019,1 of which more than 300,000 were health related.2 In the United States, more than 80% of physicians reported using smartphones for professional purposes in 2016.3 As the complexity of apps and their purpose of use has evolved, regulatory bodies have not adapted adequately to monitor apps that have broad-reaching consequences in medicine.
We review the primary literature on PubMed behind health-related apps that impact dermatologists as well as the government regulation of these apps, with a focus on the 3 most prevalent dermatology-related apps used by dermatology residents in the United States: VisualDx, UpToDate, and Mohs Surgery Appropriate Use Criteria. This prevalence is according to a survey emailed to all dermatology residents in the United States by the American Academy of Dermatology (AAD) in 2019 (unpublished data).
VisualDx
VisualDx, which aims to improve diagnostic accuracy and patient safety, contains peer-reviewed data and more than 32,000 images of dermatologic conditions. The editorial board includes more than 50 physicians. It provides opportunities for continuing medical education credit, is used in more than 2300 medical settings, and costs $399.99 annually for a subscription with partial features. Prior to the launch of the app in 2010, some health science professionals noted that the website version lacked references to primary sources.4 The same issue carried over to the app, which has evolved to offer artificial intelligence (AI) analysis of photographed skin lesions. However, there are no peer-reviewed publications showing positive impact of the app on diagnostic skills among dermatology residents or on patient outcomes.
UpToDate
UpToDate is a web-based database created in the early 1990s. A corresponding app was created around 2010. Both internal and independent research has demonstrated improved outcomes, and the app is advertised as the only clinical decision support resource associated with improved outcomes, as shown in more than 80 publications.5 UpToDate covers more than 11,800 medical topics and contains more than 35,000 graphics. It cites primary sources and uses a published system for grading recommendation strength and evidence quality. The data are processed and produced by a team of more than 7100 physicians as authors, editors, and reviewers. The platform grants continuing medical education credit and is used by more than 1.9 million clinicians in more than 190 countries. A 1-year subscription for an individual US-based physician costs $559. An observational study assessed UpToDate articles for potential conflicts of interest between authors and their recommendations. Of the 6 articles that met inclusion criteria of discussing management of medical conditions that have controversial or mostly brand-name treatment options, all had conflicts of interest, such as naming drugs from companies with which the authors and/or editors had financial relationships.6
Mohs Surgery Appropriate Use Criteria
The Mohs Surgery Appropriate Use Criteria app is a free clinical decision-making tool based on a consensus statement published in 2012 by the AAD, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and American Society for Mohs Surgery.7 It helps guide management of more than 200 dermatologic scenarios. Critique has been made that the criteria are partly based on expert opinion and data largely from the United States and has not been revised to incorporate newer data.8 There are no publications regarding the app itself.
Regulation of Health-Related Apps
Health-related apps that are designed for utilization by health care providers can be a valuable tool. However, given their prevalence, cost, and potential impact on patient lives, these apps should be well regulated and researched. The general paucity of peer-reviewed literature demonstrating the utility, safety, quality, and accuracy of health-related apps commonly used by providers is a reflection of insufficient mobile health regulation in the United States.
There are 3 primary government agencies responsible for regulating mobile medical apps: the US Food and Drug Administration (FDA), Federal Trade Commission, and Office for Civil Rights.9 The FDA does not regulate all medical devices. Apps intended for use in the diagnosis, cure, mitigation, prevention, or treatment of a disease or condition are considered to be medical devices.10 The FDA regulates those apps only if they are judged to pose more than minimal risk. Apps that are designed only to provide easy access to information related to health conditions or treatment are considered to be minimal risk but can develop into a different risk level such as by offering AI.11 Although the FDA does update its approach to medical devices, including apps and AI- and machine learning–based software, the rate and direction of update has not kept pace with the rapid evolution of apps.12 In 2019, the FDA began piloting a precertification program that grants long-term approval to organizations that develop apps instead of reviewing each app product individually.13 This decrease in premarket oversight is intended to expedite innovation with the hopeful upside of improving patient outcomes but is inconsistent, with the FDA still reviewing other types of medical devices individually.
For apps that are already in use, the Federal Trade Commission only gets involved in response to deceptive or unfair acts or practices relating to privacy, data security, and false or misleading claims about safety or performance. It may be more beneficial for consumers if those apps had a more stringent initial approval process. The Office for Civil Rights enforces the Health Insurance Portability and Accountability Act when relevant to apps.
Nongovernment agencies also are involved in app regulation. The FDA believes sharing more regulatory responsibility with private industry would promote efficiency.14 Google does not allow apps that contain false or misleading health claims,15 and Apple may scrutinize medical apps that could provide inaccurate data or be used for diagnosing or treating patients.16 Xcertia, a nonprofit organization founded by the American Medical Association and others, develops standards for the security, privacy, content, and operability of health-related apps, but those standards have not been adopted by other parties. Ultimately, nongovernment agencies are not responsible for public health and do not boast the government’s ability to enforce rules or ensure public safety.
Final Thoughts
The AAD survey of US dermatology residents found that the top consideration when choosing apps was up-to-date and accurate information; however, the 3 most prevalent apps among those same respondents did not need government approval and are not required to contain up-to-date data or to improve clinical outcomes, similar to most other health-related apps. This discrepancy is concerning considering the increasing utilization of apps for physician education and health care delivery and the increasing complexity of those apps. In light of these results, the potential decrease in federal premarket regulation suggested by the FDA’s precertification program seems inappropriate. It is important for the government to take responsibility for regulating health-related apps and to find a balance between too much regulation delaying innovation and too little regulation hurting physician training and patient care. It also is important for providers to be aware of the evidence and oversight behind the technologies they use for professional purposes.
Since the first mobile application (app) was developed in the 1990s, apps have become increasingly integrated into medical practice and training. More than 5.5 million apps were downloadable in 2019,1 of which more than 300,000 were health related.2 In the United States, more than 80% of physicians reported using smartphones for professional purposes in 2016.3 As the complexity of apps and their purpose of use has evolved, regulatory bodies have not adapted adequately to monitor apps that have broad-reaching consequences in medicine.
We review the primary literature on PubMed behind health-related apps that impact dermatologists as well as the government regulation of these apps, with a focus on the 3 most prevalent dermatology-related apps used by dermatology residents in the United States: VisualDx, UpToDate, and Mohs Surgery Appropriate Use Criteria. This prevalence is according to a survey emailed to all dermatology residents in the United States by the American Academy of Dermatology (AAD) in 2019 (unpublished data).
VisualDx
VisualDx, which aims to improve diagnostic accuracy and patient safety, contains peer-reviewed data and more than 32,000 images of dermatologic conditions. The editorial board includes more than 50 physicians. It provides opportunities for continuing medical education credit, is used in more than 2300 medical settings, and costs $399.99 annually for a subscription with partial features. Prior to the launch of the app in 2010, some health science professionals noted that the website version lacked references to primary sources.4 The same issue carried over to the app, which has evolved to offer artificial intelligence (AI) analysis of photographed skin lesions. However, there are no peer-reviewed publications showing positive impact of the app on diagnostic skills among dermatology residents or on patient outcomes.
UpToDate
UpToDate is a web-based database created in the early 1990s. A corresponding app was created around 2010. Both internal and independent research has demonstrated improved outcomes, and the app is advertised as the only clinical decision support resource associated with improved outcomes, as shown in more than 80 publications.5 UpToDate covers more than 11,800 medical topics and contains more than 35,000 graphics. It cites primary sources and uses a published system for grading recommendation strength and evidence quality. The data are processed and produced by a team of more than 7100 physicians as authors, editors, and reviewers. The platform grants continuing medical education credit and is used by more than 1.9 million clinicians in more than 190 countries. A 1-year subscription for an individual US-based physician costs $559. An observational study assessed UpToDate articles for potential conflicts of interest between authors and their recommendations. Of the 6 articles that met inclusion criteria of discussing management of medical conditions that have controversial or mostly brand-name treatment options, all had conflicts of interest, such as naming drugs from companies with which the authors and/or editors had financial relationships.6
Mohs Surgery Appropriate Use Criteria
The Mohs Surgery Appropriate Use Criteria app is a free clinical decision-making tool based on a consensus statement published in 2012 by the AAD, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and American Society for Mohs Surgery.7 It helps guide management of more than 200 dermatologic scenarios. Critique has been made that the criteria are partly based on expert opinion and data largely from the United States and has not been revised to incorporate newer data.8 There are no publications regarding the app itself.
Regulation of Health-Related Apps
Health-related apps that are designed for utilization by health care providers can be a valuable tool. However, given their prevalence, cost, and potential impact on patient lives, these apps should be well regulated and researched. The general paucity of peer-reviewed literature demonstrating the utility, safety, quality, and accuracy of health-related apps commonly used by providers is a reflection of insufficient mobile health regulation in the United States.
There are 3 primary government agencies responsible for regulating mobile medical apps: the US Food and Drug Administration (FDA), Federal Trade Commission, and Office for Civil Rights.9 The FDA does not regulate all medical devices. Apps intended for use in the diagnosis, cure, mitigation, prevention, or treatment of a disease or condition are considered to be medical devices.10 The FDA regulates those apps only if they are judged to pose more than minimal risk. Apps that are designed only to provide easy access to information related to health conditions or treatment are considered to be minimal risk but can develop into a different risk level such as by offering AI.11 Although the FDA does update its approach to medical devices, including apps and AI- and machine learning–based software, the rate and direction of update has not kept pace with the rapid evolution of apps.12 In 2019, the FDA began piloting a precertification program that grants long-term approval to organizations that develop apps instead of reviewing each app product individually.13 This decrease in premarket oversight is intended to expedite innovation with the hopeful upside of improving patient outcomes but is inconsistent, with the FDA still reviewing other types of medical devices individually.
For apps that are already in use, the Federal Trade Commission only gets involved in response to deceptive or unfair acts or practices relating to privacy, data security, and false or misleading claims about safety or performance. It may be more beneficial for consumers if those apps had a more stringent initial approval process. The Office for Civil Rights enforces the Health Insurance Portability and Accountability Act when relevant to apps.
Nongovernment agencies also are involved in app regulation. The FDA believes sharing more regulatory responsibility with private industry would promote efficiency.14 Google does not allow apps that contain false or misleading health claims,15 and Apple may scrutinize medical apps that could provide inaccurate data or be used for diagnosing or treating patients.16 Xcertia, a nonprofit organization founded by the American Medical Association and others, develops standards for the security, privacy, content, and operability of health-related apps, but those standards have not been adopted by other parties. Ultimately, nongovernment agencies are not responsible for public health and do not boast the government’s ability to enforce rules or ensure public safety.
Final Thoughts
The AAD survey of US dermatology residents found that the top consideration when choosing apps was up-to-date and accurate information; however, the 3 most prevalent apps among those same respondents did not need government approval and are not required to contain up-to-date data or to improve clinical outcomes, similar to most other health-related apps. This discrepancy is concerning considering the increasing utilization of apps for physician education and health care delivery and the increasing complexity of those apps. In light of these results, the potential decrease in federal premarket regulation suggested by the FDA’s precertification program seems inappropriate. It is important for the government to take responsibility for regulating health-related apps and to find a balance between too much regulation delaying innovation and too little regulation hurting physician training and patient care. It also is important for providers to be aware of the evidence and oversight behind the technologies they use for professional purposes.
- Clement J. Number of apps available in leading app stores as of 1st quarter 2020. Statista website. https://www.statista.com/statistics/276623/number-of-apps-available-in-leading-app-stores/. Published May 4, 2020. Accessed July 23, 2020.
- mHealth App Economics 2017/2018. Current Status and Future Trends in Mobile Health. Berlin, Germany: Research 2 Guidance; 2018.
- Healthcare Client Services. Professional usage of smartphones by doctors. Kantar website. https://www.kantarmedia.com/us/thinking-and-resources/blog/professional-usage-of-smartphones-by-doctors-2016. Published November 16, 2016. Accessed July 23, 2020.
- Skhal KJ, Koffel J. VisualDx. J Med Libr Assoc. 2007;95:470-471.
- UpToDate is the only clinical decision support resource associated with improved outcomes. UpToDate website. https://www.uptodate.com/home/research. Accessed July 29, 2020.
- Connolly SM, Baker DR, Coldiron BM, et al. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. J Am Acad Dermatol. 2012;67:531-550.
- Amber KT, Dhiman G, Goodman KW. Conflict of interest in online point-of-care clinical support websites. J Med Ethics. 2014;40:578-580.
- Croley JA, Joseph AK, Wagner RF Jr. Discrepancies in the Mohs micrographic surgery appropriate use criteria. J Am Acad Dermatol. 2020;82:E55.
- Mobile health apps interactive tool. Federal Trade Commission website. https://www.ftc.gov/tips-advice/business-center/guidance/mobile-health-apps-interactive-tool. Published April 2016. Accessed May 23, 2020.
- Federal Food, Drug, and Cosmetic Act, 21 USC §321 (2018).
- US Food and Drug Administration. Examples of software functions for which the FDA will exercise enforcement discretion. https://www.fda.gov/medical-devices/device-software-functions-including-mobile-medical-applications/examples-software-functions-which-fda-will-exercise-enforcement-discretion. Updated September 26, 2019. Accessed July 29, 2020.
- US Food and Drug Administration. Proposed regulatory framework for modifications to artificial intelligence/machine learning (AI/ML)‐based software as a medical device (SaMD). https://www.fda.gov/downloads/MedicalDevices/DigitalHealth/SoftwareasaMedicalDevice/UCM635052.pdf. Accessed July 23, 2020.
- US Food and Drug Administration. Digital health software precertification (pre-cert) program. https://www.fda.gov/medical-devices/digital-health/digital-health-software-precertification-pre-cert-program. Updated July 18, 2019. Accessed July 23, 2020.
- Gottlieb S. Fostering medical innovation: a plan for digital health devices. US Food and Drug Administration website. https://www.fda.gov/news-events/fda-voices/fostering-medical-innovation-plan-digital-health-devices. Published June 15, 2017. Accessed July 23, 2020.
- Restricted content: unapproved substances. Google Play website. https://play.google.com/about/restricted-content/unapproved-substances. Accessed July 23, 2020.
- App store review guidelines. Apple Developer website. https://developer.apple.com/app-store/review/guidelines. Updated March 4, 2020. Accessed July 23, 2020.
- Clement J. Number of apps available in leading app stores as of 1st quarter 2020. Statista website. https://www.statista.com/statistics/276623/number-of-apps-available-in-leading-app-stores/. Published May 4, 2020. Accessed July 23, 2020.
- mHealth App Economics 2017/2018. Current Status and Future Trends in Mobile Health. Berlin, Germany: Research 2 Guidance; 2018.
- Healthcare Client Services. Professional usage of smartphones by doctors. Kantar website. https://www.kantarmedia.com/us/thinking-and-resources/blog/professional-usage-of-smartphones-by-doctors-2016. Published November 16, 2016. Accessed July 23, 2020.
- Skhal KJ, Koffel J. VisualDx. J Med Libr Assoc. 2007;95:470-471.
- UpToDate is the only clinical decision support resource associated with improved outcomes. UpToDate website. https://www.uptodate.com/home/research. Accessed July 29, 2020.
- Connolly SM, Baker DR, Coldiron BM, et al. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. J Am Acad Dermatol. 2012;67:531-550.
- Amber KT, Dhiman G, Goodman KW. Conflict of interest in online point-of-care clinical support websites. J Med Ethics. 2014;40:578-580.
- Croley JA, Joseph AK, Wagner RF Jr. Discrepancies in the Mohs micrographic surgery appropriate use criteria. J Am Acad Dermatol. 2020;82:E55.
- Mobile health apps interactive tool. Federal Trade Commission website. https://www.ftc.gov/tips-advice/business-center/guidance/mobile-health-apps-interactive-tool. Published April 2016. Accessed May 23, 2020.
- Federal Food, Drug, and Cosmetic Act, 21 USC §321 (2018).
- US Food and Drug Administration. Examples of software functions for which the FDA will exercise enforcement discretion. https://www.fda.gov/medical-devices/device-software-functions-including-mobile-medical-applications/examples-software-functions-which-fda-will-exercise-enforcement-discretion. Updated September 26, 2019. Accessed July 29, 2020.
- US Food and Drug Administration. Proposed regulatory framework for modifications to artificial intelligence/machine learning (AI/ML)‐based software as a medical device (SaMD). https://www.fda.gov/downloads/MedicalDevices/DigitalHealth/SoftwareasaMedicalDevice/UCM635052.pdf. Accessed July 23, 2020.
- US Food and Drug Administration. Digital health software precertification (pre-cert) program. https://www.fda.gov/medical-devices/digital-health/digital-health-software-precertification-pre-cert-program. Updated July 18, 2019. Accessed July 23, 2020.
- Gottlieb S. Fostering medical innovation: a plan for digital health devices. US Food and Drug Administration website. https://www.fda.gov/news-events/fda-voices/fostering-medical-innovation-plan-digital-health-devices. Published June 15, 2017. Accessed July 23, 2020.
- Restricted content: unapproved substances. Google Play website. https://play.google.com/about/restricted-content/unapproved-substances. Accessed July 23, 2020.
- App store review guidelines. Apple Developer website. https://developer.apple.com/app-store/review/guidelines. Updated March 4, 2020. Accessed July 23, 2020.
Practice Points
- Physicians who are selecting an app for self-education or patient care should take into consideration the strength of the evidence supporting the app as well as the rigor of any approval process the app had to undergo.
- Only a minority of health-related apps are regulated by the government. This regulation has not kept up with the evolution of app software and may become more indirect.
Utilization of Telehealth Services During the COVID-19 Pandemic
In 2017, lawmakers and insurers in the state of Texas approved the use of telehealth services in times of crisis.1 During the coronavirus disease 2019 (COVID-19) pandemic, our clinic has used telemedicine to provide remote care to dermatology patients. We posit that the quick introduction and implementation of telemedicine during this time of need will change the way we practice dermatology in the future.
At the University of Texas Medical Branch in Galveston, Texas, we primarily have used 2 forms of telemedicine during the COVID-19 pandemic: live face-to-face video communication (our institution primarily uses FaceTime), and a combination of telephone calls with store-and-forward images. All dermatology services at our institution were converted to telemedicine visits, and in-person office visits were only done if deemed necessary after triage by telemedicine in April and May 2020. This strategy removed the necessity for patients to leave their homes for their appointments, which not only saved them travel costs and time but also reduced the potential spread of COVID-19. Since this time, the clinic has reopened for in-person visits; however, patients still have the option to schedule a telehealth appointment if they prefer. Many patients still select the telehealth option for the above reasons.
Although routine skin checks were not always possible by video and/or store-and-forward images, telemedicine worked very well for follow-up visits, especially isotretinoin follow-ups. During the COVID-19 outbreak, iPLEDGE (https://www.ipledgeprogram.com/iPledgeUI/home.u) rapidly adapted to the use of telemedicine and even began to allow home pregnancy tests to be entered into the iPLEDGE system by health care providers. Isotretinoin follow-ups are especially useful for patients who do not require laboratory monitoring at the visit. Patients are easily evaluated, screened for side effects, and continued on their treatment if no concerns are found during the telemedicine visit. Patients who require laboratory monitoring are still able to schedule tests at our clinics or at free-standing laboratories near their homes without having an in-office dermatology appointment. At-home pregnancy tests are still being utilized as an option for patients electing for telehealth follow-ups. This strategy is both health conscious by protecting the patient from exposure to COVID-19 at a testing center and cost-effective, especially for our uninsured patients, while still meeting the safety check for iPLEDGE.
Additionally, we utilized store-and-forward telemedicine for hospital consultations. If the patient’s condition can easily be diagnosed by viewing unedited clinical images remotely, the clinician can further decrease the risk of COVID-19 spread and exposure by providing the consultation and treatment recommendations by telephone. In cases in which a diagnosis could not be made by reviewing clinical photographs remotely, an in-person visit would be done. We continue to use this strategy for our confirmed COVID-positive hospital consultations to help protect our faculty and residents and decrease the use of personal protective equipment. We propose this model could be instituted for patients admitted to hospitals without access to dermatology consultations. Store-and-forward photographs of worrisome lesions and rashes also can be used to triage visits. For example, a patient with a new-onset keratoacanthoma and a history of nonmelanoma skin cancer contacted our clinic during the pandemic and sent store-and-forward images for review. The patient was triaged by a telemedicine visit and was then brought into the clinic for biopsy based on his clinical photographs and history. Patients also have requested prescriptions for bimatoprost and tretinoin via telehealth, a service that many medical spas and online telehealth companies provide already but was not offered at our practice until now.
Telemedicine also has potentially helped decrease the number of patients going to urgent care clinics for dermatology-related issues. Additionally, we have utilized one provider per day to be the “on-call” dermatologist who would be doing telemedicine appointments for patients with new-onset conditions. This strategy not only minimized possible patient exposure to COVID-19 but also helped preserve resources at urgent care clinics and emergency departments, which currently are inundated with patients. Since we have reopened for in-person visits, we have been unable to sustain an on-call dermatologist for telemedicine but may re-employ this strategy in the future.
The unique experience of practicing medicine during a pandemic has and will affect the way we practice moving forward. The way telemedicine has been quickly and easily implemented by the health care community during the COVID-19 pandemic has taught our dermatologists the value of this method of health care delivery. We will likely continue to use telemedicine after the pandemic has been contained. Telemedicine has the potential to expand access to care to rural and underserved areas, hospitals without on-call dermatologists, and homebound patients. We also may be better able to provide isotretinoin to our patients who have deferred treatment due to difficulty with transportation to the monthly visits. Store-and-forward images could help patients referred to dermatology avoid long wait times for obvious skin cancers that would benefit from early treatment. Telemedicine visits also could potentially improve attendance for patients who forget about their appointment by calling them after they miss their scheduled appointment time and complete a telehealth encounter on the same day instead, which could help recover costs of no-show appointments for clinics.
It is still unclear how private insurance companies will adapt to the new use of telemedicine, but we hope they follow the lead of Medicare, which released a statement on March 6, 2020, supporting the implementation of telehealth services.2 Although Medicare has made adjustments to allow for equal reimbursement for telehealth appointments, private insurance companies still vary greatly. Many practices are struggling and some remained open despite shelter-in-place orders, but we propose telemedicine may be a safer alternative for patients and providers during the current health crisis that would keep billable services in place. It is still uncertain whether the laws enacted to make telemedicine accessible during this time will hold after COVID-19 is contained, but we are hopeful that living through the pandemic will bring some positive benefit to our practice and the patients we serve.
- Texas laws and regulations relating to telemedicine. Texas Medical Association website. https://www.texmed.org/Template.aspx?id=47554. Updated March 19, 2020. Accessed July 14, 2020.
- Centers for Medicare & Medicaid Services. President Trump expands telehealth benefits for Medicare beneficiaries during COVID 19 outbreak. https://www.cms.gov/newsroom/press-releases/president-trump-expands-telehealth-benefits-medicare-beneficiaries-during-covid-19-outbreak. Published March 17, 2020. Accessed July 14, 2020.
In 2017, lawmakers and insurers in the state of Texas approved the use of telehealth services in times of crisis.1 During the coronavirus disease 2019 (COVID-19) pandemic, our clinic has used telemedicine to provide remote care to dermatology patients. We posit that the quick introduction and implementation of telemedicine during this time of need will change the way we practice dermatology in the future.
At the University of Texas Medical Branch in Galveston, Texas, we primarily have used 2 forms of telemedicine during the COVID-19 pandemic: live face-to-face video communication (our institution primarily uses FaceTime), and a combination of telephone calls with store-and-forward images. All dermatology services at our institution were converted to telemedicine visits, and in-person office visits were only done if deemed necessary after triage by telemedicine in April and May 2020. This strategy removed the necessity for patients to leave their homes for their appointments, which not only saved them travel costs and time but also reduced the potential spread of COVID-19. Since this time, the clinic has reopened for in-person visits; however, patients still have the option to schedule a telehealth appointment if they prefer. Many patients still select the telehealth option for the above reasons.
Although routine skin checks were not always possible by video and/or store-and-forward images, telemedicine worked very well for follow-up visits, especially isotretinoin follow-ups. During the COVID-19 outbreak, iPLEDGE (https://www.ipledgeprogram.com/iPledgeUI/home.u) rapidly adapted to the use of telemedicine and even began to allow home pregnancy tests to be entered into the iPLEDGE system by health care providers. Isotretinoin follow-ups are especially useful for patients who do not require laboratory monitoring at the visit. Patients are easily evaluated, screened for side effects, and continued on their treatment if no concerns are found during the telemedicine visit. Patients who require laboratory monitoring are still able to schedule tests at our clinics or at free-standing laboratories near their homes without having an in-office dermatology appointment. At-home pregnancy tests are still being utilized as an option for patients electing for telehealth follow-ups. This strategy is both health conscious by protecting the patient from exposure to COVID-19 at a testing center and cost-effective, especially for our uninsured patients, while still meeting the safety check for iPLEDGE.
Additionally, we utilized store-and-forward telemedicine for hospital consultations. If the patient’s condition can easily be diagnosed by viewing unedited clinical images remotely, the clinician can further decrease the risk of COVID-19 spread and exposure by providing the consultation and treatment recommendations by telephone. In cases in which a diagnosis could not be made by reviewing clinical photographs remotely, an in-person visit would be done. We continue to use this strategy for our confirmed COVID-positive hospital consultations to help protect our faculty and residents and decrease the use of personal protective equipment. We propose this model could be instituted for patients admitted to hospitals without access to dermatology consultations. Store-and-forward photographs of worrisome lesions and rashes also can be used to triage visits. For example, a patient with a new-onset keratoacanthoma and a history of nonmelanoma skin cancer contacted our clinic during the pandemic and sent store-and-forward images for review. The patient was triaged by a telemedicine visit and was then brought into the clinic for biopsy based on his clinical photographs and history. Patients also have requested prescriptions for bimatoprost and tretinoin via telehealth, a service that many medical spas and online telehealth companies provide already but was not offered at our practice until now.
Telemedicine also has potentially helped decrease the number of patients going to urgent care clinics for dermatology-related issues. Additionally, we have utilized one provider per day to be the “on-call” dermatologist who would be doing telemedicine appointments for patients with new-onset conditions. This strategy not only minimized possible patient exposure to COVID-19 but also helped preserve resources at urgent care clinics and emergency departments, which currently are inundated with patients. Since we have reopened for in-person visits, we have been unable to sustain an on-call dermatologist for telemedicine but may re-employ this strategy in the future.
The unique experience of practicing medicine during a pandemic has and will affect the way we practice moving forward. The way telemedicine has been quickly and easily implemented by the health care community during the COVID-19 pandemic has taught our dermatologists the value of this method of health care delivery. We will likely continue to use telemedicine after the pandemic has been contained. Telemedicine has the potential to expand access to care to rural and underserved areas, hospitals without on-call dermatologists, and homebound patients. We also may be better able to provide isotretinoin to our patients who have deferred treatment due to difficulty with transportation to the monthly visits. Store-and-forward images could help patients referred to dermatology avoid long wait times for obvious skin cancers that would benefit from early treatment. Telemedicine visits also could potentially improve attendance for patients who forget about their appointment by calling them after they miss their scheduled appointment time and complete a telehealth encounter on the same day instead, which could help recover costs of no-show appointments for clinics.
It is still unclear how private insurance companies will adapt to the new use of telemedicine, but we hope they follow the lead of Medicare, which released a statement on March 6, 2020, supporting the implementation of telehealth services.2 Although Medicare has made adjustments to allow for equal reimbursement for telehealth appointments, private insurance companies still vary greatly. Many practices are struggling and some remained open despite shelter-in-place orders, but we propose telemedicine may be a safer alternative for patients and providers during the current health crisis that would keep billable services in place. It is still uncertain whether the laws enacted to make telemedicine accessible during this time will hold after COVID-19 is contained, but we are hopeful that living through the pandemic will bring some positive benefit to our practice and the patients we serve.
In 2017, lawmakers and insurers in the state of Texas approved the use of telehealth services in times of crisis.1 During the coronavirus disease 2019 (COVID-19) pandemic, our clinic has used telemedicine to provide remote care to dermatology patients. We posit that the quick introduction and implementation of telemedicine during this time of need will change the way we practice dermatology in the future.
At the University of Texas Medical Branch in Galveston, Texas, we primarily have used 2 forms of telemedicine during the COVID-19 pandemic: live face-to-face video communication (our institution primarily uses FaceTime), and a combination of telephone calls with store-and-forward images. All dermatology services at our institution were converted to telemedicine visits, and in-person office visits were only done if deemed necessary after triage by telemedicine in April and May 2020. This strategy removed the necessity for patients to leave their homes for their appointments, which not only saved them travel costs and time but also reduced the potential spread of COVID-19. Since this time, the clinic has reopened for in-person visits; however, patients still have the option to schedule a telehealth appointment if they prefer. Many patients still select the telehealth option for the above reasons.
Although routine skin checks were not always possible by video and/or store-and-forward images, telemedicine worked very well for follow-up visits, especially isotretinoin follow-ups. During the COVID-19 outbreak, iPLEDGE (https://www.ipledgeprogram.com/iPledgeUI/home.u) rapidly adapted to the use of telemedicine and even began to allow home pregnancy tests to be entered into the iPLEDGE system by health care providers. Isotretinoin follow-ups are especially useful for patients who do not require laboratory monitoring at the visit. Patients are easily evaluated, screened for side effects, and continued on their treatment if no concerns are found during the telemedicine visit. Patients who require laboratory monitoring are still able to schedule tests at our clinics or at free-standing laboratories near their homes without having an in-office dermatology appointment. At-home pregnancy tests are still being utilized as an option for patients electing for telehealth follow-ups. This strategy is both health conscious by protecting the patient from exposure to COVID-19 at a testing center and cost-effective, especially for our uninsured patients, while still meeting the safety check for iPLEDGE.
Additionally, we utilized store-and-forward telemedicine for hospital consultations. If the patient’s condition can easily be diagnosed by viewing unedited clinical images remotely, the clinician can further decrease the risk of COVID-19 spread and exposure by providing the consultation and treatment recommendations by telephone. In cases in which a diagnosis could not be made by reviewing clinical photographs remotely, an in-person visit would be done. We continue to use this strategy for our confirmed COVID-positive hospital consultations to help protect our faculty and residents and decrease the use of personal protective equipment. We propose this model could be instituted for patients admitted to hospitals without access to dermatology consultations. Store-and-forward photographs of worrisome lesions and rashes also can be used to triage visits. For example, a patient with a new-onset keratoacanthoma and a history of nonmelanoma skin cancer contacted our clinic during the pandemic and sent store-and-forward images for review. The patient was triaged by a telemedicine visit and was then brought into the clinic for biopsy based on his clinical photographs and history. Patients also have requested prescriptions for bimatoprost and tretinoin via telehealth, a service that many medical spas and online telehealth companies provide already but was not offered at our practice until now.
Telemedicine also has potentially helped decrease the number of patients going to urgent care clinics for dermatology-related issues. Additionally, we have utilized one provider per day to be the “on-call” dermatologist who would be doing telemedicine appointments for patients with new-onset conditions. This strategy not only minimized possible patient exposure to COVID-19 but also helped preserve resources at urgent care clinics and emergency departments, which currently are inundated with patients. Since we have reopened for in-person visits, we have been unable to sustain an on-call dermatologist for telemedicine but may re-employ this strategy in the future.
The unique experience of practicing medicine during a pandemic has and will affect the way we practice moving forward. The way telemedicine has been quickly and easily implemented by the health care community during the COVID-19 pandemic has taught our dermatologists the value of this method of health care delivery. We will likely continue to use telemedicine after the pandemic has been contained. Telemedicine has the potential to expand access to care to rural and underserved areas, hospitals without on-call dermatologists, and homebound patients. We also may be better able to provide isotretinoin to our patients who have deferred treatment due to difficulty with transportation to the monthly visits. Store-and-forward images could help patients referred to dermatology avoid long wait times for obvious skin cancers that would benefit from early treatment. Telemedicine visits also could potentially improve attendance for patients who forget about their appointment by calling them after they miss their scheduled appointment time and complete a telehealth encounter on the same day instead, which could help recover costs of no-show appointments for clinics.
It is still unclear how private insurance companies will adapt to the new use of telemedicine, but we hope they follow the lead of Medicare, which released a statement on March 6, 2020, supporting the implementation of telehealth services.2 Although Medicare has made adjustments to allow for equal reimbursement for telehealth appointments, private insurance companies still vary greatly. Many practices are struggling and some remained open despite shelter-in-place orders, but we propose telemedicine may be a safer alternative for patients and providers during the current health crisis that would keep billable services in place. It is still uncertain whether the laws enacted to make telemedicine accessible during this time will hold after COVID-19 is contained, but we are hopeful that living through the pandemic will bring some positive benefit to our practice and the patients we serve.
- Texas laws and regulations relating to telemedicine. Texas Medical Association website. https://www.texmed.org/Template.aspx?id=47554. Updated March 19, 2020. Accessed July 14, 2020.
- Centers for Medicare & Medicaid Services. President Trump expands telehealth benefits for Medicare beneficiaries during COVID 19 outbreak. https://www.cms.gov/newsroom/press-releases/president-trump-expands-telehealth-benefits-medicare-beneficiaries-during-covid-19-outbreak. Published March 17, 2020. Accessed July 14, 2020.
- Texas laws and regulations relating to telemedicine. Texas Medical Association website. https://www.texmed.org/Template.aspx?id=47554. Updated March 19, 2020. Accessed July 14, 2020.
- Centers for Medicare & Medicaid Services. President Trump expands telehealth benefits for Medicare beneficiaries during COVID 19 outbreak. https://www.cms.gov/newsroom/press-releases/president-trump-expands-telehealth-benefits-medicare-beneficiaries-during-covid-19-outbreak. Published March 17, 2020. Accessed July 14, 2020.
Practice Points
- Telehealth can increase access to dermatologic care for both inpatient hospital consultations and outpatient clinic visits, especially in areas lacking dermatologists.
- With the current iPLEDGE accommodations for coronavirus disease 19, we have been able to treat patients who live 3 hours away and cannot travel for monthly isotretinoin visits.
- Telehealth allows our providers to better triage benign vs potentially malignant conditions to schedule patients in a more appropriate time frame.
Management of Acute Opioid Toxicity in the Outpatient Setting
Dermatologists’ offices are not immune from potentially fatal medical events. As a result, it is imperative that dermatologists are well versed in how to manage emergency situations in an outpatient setting. We discuss signs, symptoms, and management of opioid toxicity with an instructive case from our outpatient, hospital-based dermatology clinic.
A 55-year-old woman presented for Mohs micrographic surgery for a large recurrent basal cell carcinoma on the right medial cheek. After informed consent was obtained and the procedure was discussed with the patient, she took one 0.5-mg tablet of clonazepam for perioperative anxiety, which was part of her standard home medication regimen and preoperative administration of clonazepam had been discussed with the treating physician prior to her appointment. During tissue processing, the patient waited alone in the procedure room, with nursing checks every 10 to 15 minutes. Roughly 30 minutes after the initial stage was taken and clear margins were confirmed, the patient was found to be somnolent and unresponsive to voice, light, or touch. Physical examination revealed pupillary constriction, labored breathing, and absent blink reflex. Subsequent examination of the arms, which initially were covered by sleeves, revealed track marks. She was only aroused by a deep sternal rub, which caused her to moan and open her eyes. Her vital signs remained stable, with oxygen saturation greater than 90% and respiratory rate greater than 12 breaths per minute, and a registered nurse remained at her bedside to monitor her clinical status and vitals. Because this event took place in a hospital setting and the patient adequately maintained her airway, respiratory rate, and oxygenation status, the decision was made to closely observe the patient in our clinic. Without additional intervention, the patient gradually regained full awareness, orientation, and mental capacity over the course of 90 minutes. She was ambulatory and conversant at the completion of the procedure, and she declined additional screening for drug abuse or transfer to an acute care facility. She elected for discharge and was accompanied by a family member to drive her home. Later, a search of the state’s prescription monitoring service revealed she had multiple prescriptions from numerous providers for benzodiazepines and opioids. We suspect that her intoxication was the result of ingestion or injection of an opioid medication when she left to visit the restroom unaccompanied, which occurred on at least one known occasion while awaiting tissue processing.
Patients may experience several side effects when using opioid analgesics, most commonly nausea and constipation. When opioids are used long-term, patients are at increased risk for developing fractures, as opioids may decrease bone mineral density by impairing the production of exogenous sex steroid hormones.1 Respiratory depression also can occur, especially when combined with alcohol and other medications such as benzodiazepines. Lastly, opioid dependence can develop in 1 week of regular use.1,2
If opioid overdose is suspected in the office setting, early intervention is critical. Rapid serum glucose should be obtained if a glucometer is available, as hypoglycemia can be confused with opioid toxicity and is easily correctable. If serum glucose is normal, the provider should notify emergency services. In a hospital setting, a rapid response or code can be initiated. In the office setting, dial 911. If not already in place, noninvasive continuous monitoring of the patient’s pulse, oxygen saturation, and blood pressure is needed.1
The provider’s primary concern should be ensuring the patient is adequately ventilated and oxygenated. If the patient’s respiratory rate is greater than 12 breaths per minute and oxygen saturation is greater than 90% on room air, as was the case with our patient, observe and reassess the patient frequently. If the oxygen saturation drops to less than 90% but the patient is breathing spontaneously, administer supplemental oxygen followed by naloxone. If the patient is breathing fewer than 12 breaths per minute, the airway can be maintained with the head tilt–chin lift technique while ventilating using a bag valve mask with supplemental oxygen, followed by administration of naloxone.1
Naloxone is a short-acting opioid antagonist used to treat potentially fatal respiratory depression associated with opioid overdose. It is available in intramuscular (IM), intravenous (IV), and intranasal forms. Intramuscular and IV administration are preferred due to a more rapid onset compared to intranasal. The dosage is 0.04 to 2 mg for IM or IV formulations and 4 mg for the intranasal formulation.1,3 The anterolateral thigh is the preferred IM injection site. Lower initial doses for the IM and IV forms generally are advisable because of the possibility of naloxone precipitating opioid withdrawal in opioid-dependent patients. Naloxone may be administered every 2 to 3 minutes until emergency personnel arrive. Repeat dosing of naloxone should be given until ventilation is greater than 12 breaths per minute while ensuring oxygen saturation is greater than 90%. If there is an inadequate response after 5 to 10 mg of naloxone administration, reconsider the diagnosis. If there is no response after naloxone administration, continue to provide respiratory support with the bag valve mask and supplemental oxygen. After the administration of naloxone, the patient should be transported to the nearest emergency department regardless of the clinical appearance, as naloxone’s half-life may be shorter than the ingested opioid, requiring further observation in a monitored setting.1,3
We recommend that dermatologists consider keeping naloxone in their offices. The medication is easily administered and has a relatively long shelf-life of 1 to 2 years, with a 10-mL vial of 0.4 mg/mL solution costing less than $200 in most cases.3 Increasing cases of opioid abuse could lead to more clinical scenarios similar to what we experienced. Proper identification and management of opioid overdose is within the purview of the dermatologist and can be lifesaving.
- Stolbach A, Hoffman RS. Acute opioid intoxication in adults. UpToDate website. https://www.uptodate.com/contents/acute-opioid-intoxication-in-adults?search=acute%20opioid%20intoxication%20in%20adults&source=search_result&selectedTitle=1~150&usage_type=default&display_rank=1. Updated October 1, 2019. Accessed July 23, 2020.
- Glass JS, Hardy CL, Meeks NM, et al. Acute pain management in dermatology: risk assessment and treatment. J Am Acad Dermatol. 2015;73:543-560.
- Pruyn S, Frey J, Baker B, et al. Quality assessment of expired naloxone products from first-responders’ supplies. Prehosp Emerg Care. 2018;23:647-653.
Dermatologists’ offices are not immune from potentially fatal medical events. As a result, it is imperative that dermatologists are well versed in how to manage emergency situations in an outpatient setting. We discuss signs, symptoms, and management of opioid toxicity with an instructive case from our outpatient, hospital-based dermatology clinic.
A 55-year-old woman presented for Mohs micrographic surgery for a large recurrent basal cell carcinoma on the right medial cheek. After informed consent was obtained and the procedure was discussed with the patient, she took one 0.5-mg tablet of clonazepam for perioperative anxiety, which was part of her standard home medication regimen and preoperative administration of clonazepam had been discussed with the treating physician prior to her appointment. During tissue processing, the patient waited alone in the procedure room, with nursing checks every 10 to 15 minutes. Roughly 30 minutes after the initial stage was taken and clear margins were confirmed, the patient was found to be somnolent and unresponsive to voice, light, or touch. Physical examination revealed pupillary constriction, labored breathing, and absent blink reflex. Subsequent examination of the arms, which initially were covered by sleeves, revealed track marks. She was only aroused by a deep sternal rub, which caused her to moan and open her eyes. Her vital signs remained stable, with oxygen saturation greater than 90% and respiratory rate greater than 12 breaths per minute, and a registered nurse remained at her bedside to monitor her clinical status and vitals. Because this event took place in a hospital setting and the patient adequately maintained her airway, respiratory rate, and oxygenation status, the decision was made to closely observe the patient in our clinic. Without additional intervention, the patient gradually regained full awareness, orientation, and mental capacity over the course of 90 minutes. She was ambulatory and conversant at the completion of the procedure, and she declined additional screening for drug abuse or transfer to an acute care facility. She elected for discharge and was accompanied by a family member to drive her home. Later, a search of the state’s prescription monitoring service revealed she had multiple prescriptions from numerous providers for benzodiazepines and opioids. We suspect that her intoxication was the result of ingestion or injection of an opioid medication when she left to visit the restroom unaccompanied, which occurred on at least one known occasion while awaiting tissue processing.
Patients may experience several side effects when using opioid analgesics, most commonly nausea and constipation. When opioids are used long-term, patients are at increased risk for developing fractures, as opioids may decrease bone mineral density by impairing the production of exogenous sex steroid hormones.1 Respiratory depression also can occur, especially when combined with alcohol and other medications such as benzodiazepines. Lastly, opioid dependence can develop in 1 week of regular use.1,2
If opioid overdose is suspected in the office setting, early intervention is critical. Rapid serum glucose should be obtained if a glucometer is available, as hypoglycemia can be confused with opioid toxicity and is easily correctable. If serum glucose is normal, the provider should notify emergency services. In a hospital setting, a rapid response or code can be initiated. In the office setting, dial 911. If not already in place, noninvasive continuous monitoring of the patient’s pulse, oxygen saturation, and blood pressure is needed.1
The provider’s primary concern should be ensuring the patient is adequately ventilated and oxygenated. If the patient’s respiratory rate is greater than 12 breaths per minute and oxygen saturation is greater than 90% on room air, as was the case with our patient, observe and reassess the patient frequently. If the oxygen saturation drops to less than 90% but the patient is breathing spontaneously, administer supplemental oxygen followed by naloxone. If the patient is breathing fewer than 12 breaths per minute, the airway can be maintained with the head tilt–chin lift technique while ventilating using a bag valve mask with supplemental oxygen, followed by administration of naloxone.1
Naloxone is a short-acting opioid antagonist used to treat potentially fatal respiratory depression associated with opioid overdose. It is available in intramuscular (IM), intravenous (IV), and intranasal forms. Intramuscular and IV administration are preferred due to a more rapid onset compared to intranasal. The dosage is 0.04 to 2 mg for IM or IV formulations and 4 mg for the intranasal formulation.1,3 The anterolateral thigh is the preferred IM injection site. Lower initial doses for the IM and IV forms generally are advisable because of the possibility of naloxone precipitating opioid withdrawal in opioid-dependent patients. Naloxone may be administered every 2 to 3 minutes until emergency personnel arrive. Repeat dosing of naloxone should be given until ventilation is greater than 12 breaths per minute while ensuring oxygen saturation is greater than 90%. If there is an inadequate response after 5 to 10 mg of naloxone administration, reconsider the diagnosis. If there is no response after naloxone administration, continue to provide respiratory support with the bag valve mask and supplemental oxygen. After the administration of naloxone, the patient should be transported to the nearest emergency department regardless of the clinical appearance, as naloxone’s half-life may be shorter than the ingested opioid, requiring further observation in a monitored setting.1,3
We recommend that dermatologists consider keeping naloxone in their offices. The medication is easily administered and has a relatively long shelf-life of 1 to 2 years, with a 10-mL vial of 0.4 mg/mL solution costing less than $200 in most cases.3 Increasing cases of opioid abuse could lead to more clinical scenarios similar to what we experienced. Proper identification and management of opioid overdose is within the purview of the dermatologist and can be lifesaving.
Dermatologists’ offices are not immune from potentially fatal medical events. As a result, it is imperative that dermatologists are well versed in how to manage emergency situations in an outpatient setting. We discuss signs, symptoms, and management of opioid toxicity with an instructive case from our outpatient, hospital-based dermatology clinic.
A 55-year-old woman presented for Mohs micrographic surgery for a large recurrent basal cell carcinoma on the right medial cheek. After informed consent was obtained and the procedure was discussed with the patient, she took one 0.5-mg tablet of clonazepam for perioperative anxiety, which was part of her standard home medication regimen and preoperative administration of clonazepam had been discussed with the treating physician prior to her appointment. During tissue processing, the patient waited alone in the procedure room, with nursing checks every 10 to 15 minutes. Roughly 30 minutes after the initial stage was taken and clear margins were confirmed, the patient was found to be somnolent and unresponsive to voice, light, or touch. Physical examination revealed pupillary constriction, labored breathing, and absent blink reflex. Subsequent examination of the arms, which initially were covered by sleeves, revealed track marks. She was only aroused by a deep sternal rub, which caused her to moan and open her eyes. Her vital signs remained stable, with oxygen saturation greater than 90% and respiratory rate greater than 12 breaths per minute, and a registered nurse remained at her bedside to monitor her clinical status and vitals. Because this event took place in a hospital setting and the patient adequately maintained her airway, respiratory rate, and oxygenation status, the decision was made to closely observe the patient in our clinic. Without additional intervention, the patient gradually regained full awareness, orientation, and mental capacity over the course of 90 minutes. She was ambulatory and conversant at the completion of the procedure, and she declined additional screening for drug abuse or transfer to an acute care facility. She elected for discharge and was accompanied by a family member to drive her home. Later, a search of the state’s prescription monitoring service revealed she had multiple prescriptions from numerous providers for benzodiazepines and opioids. We suspect that her intoxication was the result of ingestion or injection of an opioid medication when she left to visit the restroom unaccompanied, which occurred on at least one known occasion while awaiting tissue processing.
Patients may experience several side effects when using opioid analgesics, most commonly nausea and constipation. When opioids are used long-term, patients are at increased risk for developing fractures, as opioids may decrease bone mineral density by impairing the production of exogenous sex steroid hormones.1 Respiratory depression also can occur, especially when combined with alcohol and other medications such as benzodiazepines. Lastly, opioid dependence can develop in 1 week of regular use.1,2
If opioid overdose is suspected in the office setting, early intervention is critical. Rapid serum glucose should be obtained if a glucometer is available, as hypoglycemia can be confused with opioid toxicity and is easily correctable. If serum glucose is normal, the provider should notify emergency services. In a hospital setting, a rapid response or code can be initiated. In the office setting, dial 911. If not already in place, noninvasive continuous monitoring of the patient’s pulse, oxygen saturation, and blood pressure is needed.1
The provider’s primary concern should be ensuring the patient is adequately ventilated and oxygenated. If the patient’s respiratory rate is greater than 12 breaths per minute and oxygen saturation is greater than 90% on room air, as was the case with our patient, observe and reassess the patient frequently. If the oxygen saturation drops to less than 90% but the patient is breathing spontaneously, administer supplemental oxygen followed by naloxone. If the patient is breathing fewer than 12 breaths per minute, the airway can be maintained with the head tilt–chin lift technique while ventilating using a bag valve mask with supplemental oxygen, followed by administration of naloxone.1
Naloxone is a short-acting opioid antagonist used to treat potentially fatal respiratory depression associated with opioid overdose. It is available in intramuscular (IM), intravenous (IV), and intranasal forms. Intramuscular and IV administration are preferred due to a more rapid onset compared to intranasal. The dosage is 0.04 to 2 mg for IM or IV formulations and 4 mg for the intranasal formulation.1,3 The anterolateral thigh is the preferred IM injection site. Lower initial doses for the IM and IV forms generally are advisable because of the possibility of naloxone precipitating opioid withdrawal in opioid-dependent patients. Naloxone may be administered every 2 to 3 minutes until emergency personnel arrive. Repeat dosing of naloxone should be given until ventilation is greater than 12 breaths per minute while ensuring oxygen saturation is greater than 90%. If there is an inadequate response after 5 to 10 mg of naloxone administration, reconsider the diagnosis. If there is no response after naloxone administration, continue to provide respiratory support with the bag valve mask and supplemental oxygen. After the administration of naloxone, the patient should be transported to the nearest emergency department regardless of the clinical appearance, as naloxone’s half-life may be shorter than the ingested opioid, requiring further observation in a monitored setting.1,3
We recommend that dermatologists consider keeping naloxone in their offices. The medication is easily administered and has a relatively long shelf-life of 1 to 2 years, with a 10-mL vial of 0.4 mg/mL solution costing less than $200 in most cases.3 Increasing cases of opioid abuse could lead to more clinical scenarios similar to what we experienced. Proper identification and management of opioid overdose is within the purview of the dermatologist and can be lifesaving.
- Stolbach A, Hoffman RS. Acute opioid intoxication in adults. UpToDate website. https://www.uptodate.com/contents/acute-opioid-intoxication-in-adults?search=acute%20opioid%20intoxication%20in%20adults&source=search_result&selectedTitle=1~150&usage_type=default&display_rank=1. Updated October 1, 2019. Accessed July 23, 2020.
- Glass JS, Hardy CL, Meeks NM, et al. Acute pain management in dermatology: risk assessment and treatment. J Am Acad Dermatol. 2015;73:543-560.
- Pruyn S, Frey J, Baker B, et al. Quality assessment of expired naloxone products from first-responders’ supplies. Prehosp Emerg Care. 2018;23:647-653.
- Stolbach A, Hoffman RS. Acute opioid intoxication in adults. UpToDate website. https://www.uptodate.com/contents/acute-opioid-intoxication-in-adults?search=acute%20opioid%20intoxication%20in%20adults&source=search_result&selectedTitle=1~150&usage_type=default&display_rank=1. Updated October 1, 2019. Accessed July 23, 2020.
- Glass JS, Hardy CL, Meeks NM, et al. Acute pain management in dermatology: risk assessment and treatment. J Am Acad Dermatol. 2015;73:543-560.
- Pruyn S, Frey J, Baker B, et al. Quality assessment of expired naloxone products from first-responders’ supplies. Prehosp Emerg Care. 2018;23:647-653.
Practice Points
- Opioid overdose continues to be a major public health concern. Dermatologists may encounter opioid toxicity in their practice, and prompt recognition and treatment are crucial.
- Naloxone is a quick-acting, easy-to-use, and relatively inexpensive medication that can easily be stored and administered in dermatologists’ offices
Tattoo Hypersensitivity Reactions: Inky Business
Sometimes regrettable yet increasingly common, tattoos are an ancient art form used in modern times as a mark of artistic and cultural expression. Allergic contact dermatitis (ACD) to tattoo ink is rare, but the popularity of tattoos makes ACD an increasingly recognized occurrence. In a retrospective study of 38,543 patch-tested patients, only 29 (0.08%) had tattoo-related ACD, with the majority of patients being female and young adults. The most common contact allergy was to paraphenylenediamine (PPD), which occurred in 22 (76%) patients.1 In this article, we will walk you through the rainbow of tattoo ACD, covering hypersensitivity reactions to both temporary and permanent tattoo inks.
Temporary Tattoo Inks
Henna is the most common temporary tattoo ink. Derived from the plant Lawsonia inermis, henna is an orange dye that has been used in many parts of the world, particularly in Islamic and Hindu cultures, to dye skin, hair, and fabrics. Application of henna tattoos is common for weddings and other celebrations, and brides may wear elaborate henna patterns. To create these tattoos, henna powder is mixed with water and sometimes essential oils and is then applied to the skin for several hours. After application, the henna pigment lawsone (2-hydroxy-1,4-naphthoquinone) interacts with keratin and leaves a red-orange stain on the skin2; longer application time leads to a deeper color. Most traditional cutaneous henna designs fade in 2 to 6 weeks, but some last longer. Red henna generally is considered safe with low incidence of contact allergy. What is referred to as black henna usually is red henna mixed with PPD, a black dye, which is added to deepen the color. Paraphenylenediamine is highly sensitizing; patients can become sensitized to the PPD in the tattoo itself.2 One study confirmed the presence of PPD in black henna tattoos, with chemical analysis of common preparations revealing concentrations ranging from less than 1% to 30%.2 Patients who undergo patch testing for tattoo reactions often are strongly positive to PPD and have concomitant reactions to azo dyes, black rubber, and anesthetics. Other aromatic amines including aminophenols have been identified in black henna tattoo ink, and these chemicals also may contribute to ACD.3 Less common sources of contact allergy from temporary black henna tattoos include resorcinol,4 para-tertiary butylphenol formaldehyde resin,5 and fragrance.6
Clinically, ACD to PPD in temporary tattoos presents 1 to 3 days after application if the patient is already sensitized or 4 to 14 days if the patient is sensitized by the tattoo ink.2 Most patients notice erythema, edema, vesicles, papules, and/or bullae, but other less common reactions including generalized dermatitis, systemic symptoms, urticaria, and pustules have been described.2 Postinflammatory hypopigmentation or hyperpigmentation also can occur.
Because of the sensitizing nature of black henna tattoos, consumers are turning to natural temporary tattoos. Jagua temporary tattoos, with pigment derived from the sap of fruit from the Genipa americana tree, have been associated with ACD.7 This black dye is applied and washed off in a similar fashion to henna tattoos. Importantly, a recent analysis of jagua dye identified no PPD. In one case, a patient who developed ACD to a jagua tattoo was patch tested to components of the dye and had a positive reaction to genipin, a component of the fruit extract.7 Thus, jagua tattoos often are marketed as safe but are an emerging source of contact dermatitis to temporary tattoos.
Permanent Tattoo Inks
Permanent tattoos are created by injecting small amounts of ink into the dermis. As the name suggests, these tattoos are permanent. Tattoos are common; nearly one-third of Americans have at least 1 tattoo.1 Historically, tattoos were created using black pigment composed of amorphous carbon or black iron oxides.8,9 Metallic pigments (eg, mercury, chromium, cobalt, cadmium) were once used to add color to tattoos, but these metals are now only rarely used; in fact, a 2019 study of tattoo ink components identified 44 distinct pigments in 1416 permanent inks, with an average of 3 pigments per ink.8 Of the 44 pigments, 10 had metallic components including iron, barium, zinc, copper, molybdenum, and titanium. The remaining 34 pigments contained carbon, azo, diketopyrrolopyrrole, quinacridone, anthraquinone, dioxazine (purple), or quinophthalone (yellow) dyes. The authors noted that nearly one-quarter of the tattoo pigments identified in their study had been reported as contact allergens.8
Typically, reactions to permanent tattoo inks manifest as an eczematous dermatitis occurring weeks to years after tattoo application.9,10 The dermatitis usually is locally confined to the tattoo and may be limited to particular colors; occasionally, a new tattoo reaction may trigger concurrent inflammation in older tattoos. Many tattoo reactions occur as a response to red pigment but also have occurred with other tattoo ink components.9 Many researchers have speculated as to whether the reaction is related to the ink component itself or from the photochemical breakdown of the ink by exposure to UV radiation and/or laser therapy.9
Red Pigment
Red ink is the most common color reported to cause tattoo hypersensitivity reactions. Historically, red tattoo pigments include mercuric sulfide (vermilion, cinnabar), scarlet lake, cadmium red, carmine, and cochineal,11 but today’s tattoo inks primarily are composed of other pigments, such as quinacridone and azo dyes.12 Several cases of red tattoo ink hypersensitivity reactions exist in the literature, many without completion of patch tests or without positive patch tests to relevant red pigments.11-15
Black Pigment
In general, reactions to permanent black tattoo ink are rare; however, a few case reports exist. Black pigment can be created with India ink (carbon), logwood (chrome), iron oxide, and titanium.16,17 Shellac can be used as a binding agent in tattoo ink; there is at least one report of a reaction to black tattoo ink with a positive patch test to shellac and the original black ink.18
Metals
When utilized in tattoos, metals can create a variety of colors; several have been reported to cause ACD. There has been at least one reported case of a tattoo hypersensitivity reaction to a gold tattoo, with positive patch testing for gold sodium thiosulfate.19 Green tattoo inks also have been confirmed to contain metal. One case of nickel allergy from a green tattoo has been reported, with a positive patch test for nickel sulfate and tissue confirmation of the presence of nickel with micro X-ray fluorescence and laser ablation inductively coupled plasma mass spectrometry.20 Another case series described 3 patients with pruritus and chronic dermatitis associated with green tattoos who had positive patch tests to potassium dichromate, and the green tattoo pigment flared during patch testing. Chromium oxide was presumed to be present in the green tattoo pigment, and potassium dichromate avoidance in products and food improved both the pruritus and dermatitis.21
Azo Pigments
Azo pigments frequently are used in modern tattoos due to their vibrant colors. One case of hypersensitivity to azo pigment involved an eczematous ulcerated plaque overlying yellow, red, and green ink in a recently applied tattoo. Patch testing with the inks originally used in the tattoo was negative. The authors noted that the 3 problematic ink colors all contained pigment yellow 65—an azo pigment—and attributed the reaction to this dye.22 In another azo reaction, a patient had erythema and pruritus overlying a tattoo applied 1 month prior. Patch testing was positive for aminoazobenzene, an azo pigment that was present in the orange ink of the tattoo.23
Management of Tattoo Hypersensitivity Reactions
Hypersensitivity reactions to temporary tattoos are just that—temporary. Topical steroids and time generally will allow these reactions to resolve. In the setting of vigorous reactions, patients may develop postinflammatory hypopigmentation or hyperpigmentation that may last for months. Unfortunately, bullous tattoo reactions can lead to scarring and keloid formation, requiring more aggressive therapy.
Management of reactions to permanent tattoos is more challenging. High-potency topical steroids under occlusion or intralesional corticosteroid injections may aid in treating pruritus or discomfort. For severe reactions, oral corticosteroids may be required. Patients also may consider laser tattoo removal; however, providers should be aware that there have been rare reports of systemic urticarial reactions from this procedure.24,25 Obviously limited by location and size, excision also may be offered.
Patch Testing for Tattoo Ink Contact Allergy
When patients present for evaluation and management of tattoo ACD, it is important to also consider other causes, including granulomatous tattoo reaction, pseudolymphoma, and lichenoid tattoo reaction. A biopsy can be helpful if the diagnosis is in question.
Patch testing for contact allergy to temporary tattoo inks should include PPD, fragrance, aminophenols, resorcinol, para-tertiary butylphenol formaldehyde, and essential oils. Jagua currently is not available for commercial purchase but also should be considered if the patient has the original product or in research settings. If the individual tattoo ingredients can be identified, they also should be tested. In this scenario, recall reactions may occur; testing with the tattoo paste should be avoided if the prior reaction was severe. Importantly, patients with a PPD allergy should be counseled to avoid hair dyes that contain PPD. Many patients who are sensitized to PPD have strong reactions on patch testing and are at risk for severe reactions if PPD or PPD-related compounds are encountered in hair dye.
Patch testing for ACD to permanent tattoos is complex. In most cases, patch testing is of limited utility because many of the chemicals that have been reported to cause ACD are not commercially available. Additionally, a 2014 study of 90 patients with chronic tattoo reactions found that the majority had negative patch testing to the European baseline series (66%), disperse dyes (87%), and tattoo inks (87%–92%). The investigators theorized that the allergens causing tattoo reactions are formed by haptenization of “parent” chemicals in the dermis, meaning application of chemicals present in the original tattoo ink may not identify the relevant allergen.26 If patch testing is performed, it is most ideal if individual pigment ingredients can be identified. Allergens to be considered for testing include azo dyes, aromatic amines, iron oxide, barium, zinc, copper, molybdenum, titanium, gold sodium thiosulfate, nickel sulfate, carbon, shellac, diketopyrrolopyrrole, quinacridone, anthraquinone, dioxazine (purple), quinophthalone (yellow) dyes, mercuric sulfide (vermilion, cinnabar), scarlet lake, cadmium red, carmine, and cochineal, many of which are not commercially available for purchase.
Final Interpretation
As tattoos become increasingly trendy, tattoo ACD should be recognized by the astute dermatologist. The most common allergen associated with tattoo ACD is PPD, but other potential allergens include azo dyes and newer pigments. Unlike tattoos of the past, today’s inks are unlikely to contain toxic metals. Diagnosing ACD caused by permanent tattoo inks requires a high degree of suspicion, as patch testing may be of limited utility.
- Warshaw EM, Schlarbaum JP, Taylor JS, et al. Allergic reactions to tattoos: retrospective analysis of North American Contact Dermatitis Group data, 2001-2016. J Am Acad Dermatol. 2020;82:E61-E62.
- de Groot AC. Side-effects of henna and semi-permanent ‘black henna’ tattoos: a full review. Contact Dermatitis. 2013;69:1-25.
- Romita P, Foti C, Mascia P, et al. Eyebrow allergic contact dermatitis caused by m-aminophenol and toluene-2,5-diamine secondary to a temporary black henna tattoo. Contact Dermatitis. 2018;79:51-52.
- Ormerod E, Hughes TM, Stone N. Allergic contact dermatitis caused by resorcinol following a temporary black henna tattoo. Contact Dermatitis. 2017;77:187-188.
- Rodrigo-Nicolás B, de la Cuadra J, Sierra C, et al. Contact dermatitis from a temporary tattoo in a boy with contact allergy to p-tert butyl phenol formaldehyde resin. Dermatitis. 2014;25:37-38.
- Temesvári E, Podányi B, Pónyai G, et al. Fragrance sensitization caused by temporary henna tattoo. Contact Dermatitis. 2002;47:240.
- Bircher AJ, Scherer Hofmeier K, Schlegel U, et al. Genipin in temporary jagua tattoos—black dye causing severe allergic dermatitis. Dermatitis. 2019;30:375-376.
- Liszewski W, Warshaw EM. Pigments in American tattoo inks and their propensity to elicit allergic contact dermatitis. J Am Acad Dermatol. 2019;81:379-385.
- Serup J, Hutton Carlsen K, Dommershausen N, et al. Identification of pigments related to allergic tattoo reactions in 104 human skin biopsies. Contact Dermatitis. 2020;82:73-82.
- Bjerre RD, Ulrich NH, Linneberg A, et al. Adverse reactions to tattoos in the general population of Denmark. J Am Acad Dermatol. 2018;79:770-772.
- Bhardwaj SS, Brodell RT, Taylor JS. Red tattoo reactions. Contact Dermatitis. 2003;48:236-237.
- Gaudron S, Ferrier-Le Bouëdec MC, Franck F, et al. Azo pigments and quinacridones induce delayed hypersensitivity in red tattoos. Contact Dermatitis. 2015;72:97-105.
- de Winter RW, van der Bent SAS, van Esch M, et al. Allergic reaction to red cosmetic lip tattoo treated with hydroxychloroquine. Dermatitis. 2019;30:82-83.
- Greve B, Chytry R, Raulin C. Contact dermatitis from red tattoo pigment (quinacridone) with secondary spread. Contact Dermatitis. 2003;49:265-266.
- Ruiz-Villaverde R, Fernandez-Crehuet P, Aguayo-Carreras P, et al. Inflammatory reactions to red tattoo inks: three cases highlighting an emerging problem. Sultan Qaboos Univ Med J. 2018;18:E215-E218.
- Gallo R, Parodi A, Cozzani E, et al. Allergic reaction to India ink in a black tattoo. Contact Dermatitis. 1998;38:346-347.
- de Cuyper C, Lodewick E, Schreiver I, et al. Are metals involved in tattoo-related hypersensitivity reactions? a case report. Contact Dermatitis. 2017;77:397-405.
- González-Villanueva I, Hispán Ocete P, Silvestre Salvador JF. Allergic contact dermatitis caused by a black tattoo ink in a patient allergic to shellac. Contact Dermatitis. 2016;75:247-248.
- Tammaro A, Tuchinda P, Persechino S, et al. Contact allergic dermatitis to gold in a tattoo: a case report. Int J Immunopathol Pharmacol. 2011;24:1111-1113.
- van der Bent SAS, Berg T, Karst U, et al. Allergic reaction to a green tattoo with nickel as a possible allergen. Contact Dermatitis. 2019;81:64-66.
- Jacob SE, Castanedo-Tardan MP, Blyumin ML. Inflammation in green (chromium) tattoos during patch testing. Dermatitis. 2008;19:E33-E34.
- González-Villanueva I, Álvarez-Chinchilla P, Silvestre JF. Allergic reaction to 3 tattoo inks containing pigment yellow 65. Contact Dermatitis. 2018;79:107-108.
- Tammaro A, De Marco G, D’Arino A, et al. Aminoazobenzene in tattoo: another case of allergic contact dermatitis. Int J Dermatol. 2017;56:E79-E81.
- Willardson HB, Kobayashi TT, Arnold JG, et al. Diffuse urticarial reaction associated with titanium dioxide following laser tattoo removal treatments. Photomed Laser Surg. 2017;35:176‐180.
- England RW, Vogel P, Hagan L. Immediate cutaneous hypersensitivity after treatment of tattoo with Nd:YAG laser: a case report and review of the literature. Ann Allergy Asthma Immunol. 2002;89:215‐217.
- Serup J, Carlsen KH. Patch test study of 90 patients with tattoo reactions: negative outcome of allergy patch test to baseline batteries and culprit inks suggests allergen(s) are generated in the skin through haptenization. Contact Dermatitis. 2014;71:255-263.
Sometimes regrettable yet increasingly common, tattoos are an ancient art form used in modern times as a mark of artistic and cultural expression. Allergic contact dermatitis (ACD) to tattoo ink is rare, but the popularity of tattoos makes ACD an increasingly recognized occurrence. In a retrospective study of 38,543 patch-tested patients, only 29 (0.08%) had tattoo-related ACD, with the majority of patients being female and young adults. The most common contact allergy was to paraphenylenediamine (PPD), which occurred in 22 (76%) patients.1 In this article, we will walk you through the rainbow of tattoo ACD, covering hypersensitivity reactions to both temporary and permanent tattoo inks.
Temporary Tattoo Inks
Henna is the most common temporary tattoo ink. Derived from the plant Lawsonia inermis, henna is an orange dye that has been used in many parts of the world, particularly in Islamic and Hindu cultures, to dye skin, hair, and fabrics. Application of henna tattoos is common for weddings and other celebrations, and brides may wear elaborate henna patterns. To create these tattoos, henna powder is mixed with water and sometimes essential oils and is then applied to the skin for several hours. After application, the henna pigment lawsone (2-hydroxy-1,4-naphthoquinone) interacts with keratin and leaves a red-orange stain on the skin2; longer application time leads to a deeper color. Most traditional cutaneous henna designs fade in 2 to 6 weeks, but some last longer. Red henna generally is considered safe with low incidence of contact allergy. What is referred to as black henna usually is red henna mixed with PPD, a black dye, which is added to deepen the color. Paraphenylenediamine is highly sensitizing; patients can become sensitized to the PPD in the tattoo itself.2 One study confirmed the presence of PPD in black henna tattoos, with chemical analysis of common preparations revealing concentrations ranging from less than 1% to 30%.2 Patients who undergo patch testing for tattoo reactions often are strongly positive to PPD and have concomitant reactions to azo dyes, black rubber, and anesthetics. Other aromatic amines including aminophenols have been identified in black henna tattoo ink, and these chemicals also may contribute to ACD.3 Less common sources of contact allergy from temporary black henna tattoos include resorcinol,4 para-tertiary butylphenol formaldehyde resin,5 and fragrance.6
Clinically, ACD to PPD in temporary tattoos presents 1 to 3 days after application if the patient is already sensitized or 4 to 14 days if the patient is sensitized by the tattoo ink.2 Most patients notice erythema, edema, vesicles, papules, and/or bullae, but other less common reactions including generalized dermatitis, systemic symptoms, urticaria, and pustules have been described.2 Postinflammatory hypopigmentation or hyperpigmentation also can occur.
Because of the sensitizing nature of black henna tattoos, consumers are turning to natural temporary tattoos. Jagua temporary tattoos, with pigment derived from the sap of fruit from the Genipa americana tree, have been associated with ACD.7 This black dye is applied and washed off in a similar fashion to henna tattoos. Importantly, a recent analysis of jagua dye identified no PPD. In one case, a patient who developed ACD to a jagua tattoo was patch tested to components of the dye and had a positive reaction to genipin, a component of the fruit extract.7 Thus, jagua tattoos often are marketed as safe but are an emerging source of contact dermatitis to temporary tattoos.
Permanent Tattoo Inks
Permanent tattoos are created by injecting small amounts of ink into the dermis. As the name suggests, these tattoos are permanent. Tattoos are common; nearly one-third of Americans have at least 1 tattoo.1 Historically, tattoos were created using black pigment composed of amorphous carbon or black iron oxides.8,9 Metallic pigments (eg, mercury, chromium, cobalt, cadmium) were once used to add color to tattoos, but these metals are now only rarely used; in fact, a 2019 study of tattoo ink components identified 44 distinct pigments in 1416 permanent inks, with an average of 3 pigments per ink.8 Of the 44 pigments, 10 had metallic components including iron, barium, zinc, copper, molybdenum, and titanium. The remaining 34 pigments contained carbon, azo, diketopyrrolopyrrole, quinacridone, anthraquinone, dioxazine (purple), or quinophthalone (yellow) dyes. The authors noted that nearly one-quarter of the tattoo pigments identified in their study had been reported as contact allergens.8
Typically, reactions to permanent tattoo inks manifest as an eczematous dermatitis occurring weeks to years after tattoo application.9,10 The dermatitis usually is locally confined to the tattoo and may be limited to particular colors; occasionally, a new tattoo reaction may trigger concurrent inflammation in older tattoos. Many tattoo reactions occur as a response to red pigment but also have occurred with other tattoo ink components.9 Many researchers have speculated as to whether the reaction is related to the ink component itself or from the photochemical breakdown of the ink by exposure to UV radiation and/or laser therapy.9
Red Pigment
Red ink is the most common color reported to cause tattoo hypersensitivity reactions. Historically, red tattoo pigments include mercuric sulfide (vermilion, cinnabar), scarlet lake, cadmium red, carmine, and cochineal,11 but today’s tattoo inks primarily are composed of other pigments, such as quinacridone and azo dyes.12 Several cases of red tattoo ink hypersensitivity reactions exist in the literature, many without completion of patch tests or without positive patch tests to relevant red pigments.11-15
Black Pigment
In general, reactions to permanent black tattoo ink are rare; however, a few case reports exist. Black pigment can be created with India ink (carbon), logwood (chrome), iron oxide, and titanium.16,17 Shellac can be used as a binding agent in tattoo ink; there is at least one report of a reaction to black tattoo ink with a positive patch test to shellac and the original black ink.18
Metals
When utilized in tattoos, metals can create a variety of colors; several have been reported to cause ACD. There has been at least one reported case of a tattoo hypersensitivity reaction to a gold tattoo, with positive patch testing for gold sodium thiosulfate.19 Green tattoo inks also have been confirmed to contain metal. One case of nickel allergy from a green tattoo has been reported, with a positive patch test for nickel sulfate and tissue confirmation of the presence of nickel with micro X-ray fluorescence and laser ablation inductively coupled plasma mass spectrometry.20 Another case series described 3 patients with pruritus and chronic dermatitis associated with green tattoos who had positive patch tests to potassium dichromate, and the green tattoo pigment flared during patch testing. Chromium oxide was presumed to be present in the green tattoo pigment, and potassium dichromate avoidance in products and food improved both the pruritus and dermatitis.21
Azo Pigments
Azo pigments frequently are used in modern tattoos due to their vibrant colors. One case of hypersensitivity to azo pigment involved an eczematous ulcerated plaque overlying yellow, red, and green ink in a recently applied tattoo. Patch testing with the inks originally used in the tattoo was negative. The authors noted that the 3 problematic ink colors all contained pigment yellow 65—an azo pigment—and attributed the reaction to this dye.22 In another azo reaction, a patient had erythema and pruritus overlying a tattoo applied 1 month prior. Patch testing was positive for aminoazobenzene, an azo pigment that was present in the orange ink of the tattoo.23
Management of Tattoo Hypersensitivity Reactions
Hypersensitivity reactions to temporary tattoos are just that—temporary. Topical steroids and time generally will allow these reactions to resolve. In the setting of vigorous reactions, patients may develop postinflammatory hypopigmentation or hyperpigmentation that may last for months. Unfortunately, bullous tattoo reactions can lead to scarring and keloid formation, requiring more aggressive therapy.
Management of reactions to permanent tattoos is more challenging. High-potency topical steroids under occlusion or intralesional corticosteroid injections may aid in treating pruritus or discomfort. For severe reactions, oral corticosteroids may be required. Patients also may consider laser tattoo removal; however, providers should be aware that there have been rare reports of systemic urticarial reactions from this procedure.24,25 Obviously limited by location and size, excision also may be offered.
Patch Testing for Tattoo Ink Contact Allergy
When patients present for evaluation and management of tattoo ACD, it is important to also consider other causes, including granulomatous tattoo reaction, pseudolymphoma, and lichenoid tattoo reaction. A biopsy can be helpful if the diagnosis is in question.
Patch testing for contact allergy to temporary tattoo inks should include PPD, fragrance, aminophenols, resorcinol, para-tertiary butylphenol formaldehyde, and essential oils. Jagua currently is not available for commercial purchase but also should be considered if the patient has the original product or in research settings. If the individual tattoo ingredients can be identified, they also should be tested. In this scenario, recall reactions may occur; testing with the tattoo paste should be avoided if the prior reaction was severe. Importantly, patients with a PPD allergy should be counseled to avoid hair dyes that contain PPD. Many patients who are sensitized to PPD have strong reactions on patch testing and are at risk for severe reactions if PPD or PPD-related compounds are encountered in hair dye.
Patch testing for ACD to permanent tattoos is complex. In most cases, patch testing is of limited utility because many of the chemicals that have been reported to cause ACD are not commercially available. Additionally, a 2014 study of 90 patients with chronic tattoo reactions found that the majority had negative patch testing to the European baseline series (66%), disperse dyes (87%), and tattoo inks (87%–92%). The investigators theorized that the allergens causing tattoo reactions are formed by haptenization of “parent” chemicals in the dermis, meaning application of chemicals present in the original tattoo ink may not identify the relevant allergen.26 If patch testing is performed, it is most ideal if individual pigment ingredients can be identified. Allergens to be considered for testing include azo dyes, aromatic amines, iron oxide, barium, zinc, copper, molybdenum, titanium, gold sodium thiosulfate, nickel sulfate, carbon, shellac, diketopyrrolopyrrole, quinacridone, anthraquinone, dioxazine (purple), quinophthalone (yellow) dyes, mercuric sulfide (vermilion, cinnabar), scarlet lake, cadmium red, carmine, and cochineal, many of which are not commercially available for purchase.
Final Interpretation
As tattoos become increasingly trendy, tattoo ACD should be recognized by the astute dermatologist. The most common allergen associated with tattoo ACD is PPD, but other potential allergens include azo dyes and newer pigments. Unlike tattoos of the past, today’s inks are unlikely to contain toxic metals. Diagnosing ACD caused by permanent tattoo inks requires a high degree of suspicion, as patch testing may be of limited utility.
Sometimes regrettable yet increasingly common, tattoos are an ancient art form used in modern times as a mark of artistic and cultural expression. Allergic contact dermatitis (ACD) to tattoo ink is rare, but the popularity of tattoos makes ACD an increasingly recognized occurrence. In a retrospective study of 38,543 patch-tested patients, only 29 (0.08%) had tattoo-related ACD, with the majority of patients being female and young adults. The most common contact allergy was to paraphenylenediamine (PPD), which occurred in 22 (76%) patients.1 In this article, we will walk you through the rainbow of tattoo ACD, covering hypersensitivity reactions to both temporary and permanent tattoo inks.
Temporary Tattoo Inks
Henna is the most common temporary tattoo ink. Derived from the plant Lawsonia inermis, henna is an orange dye that has been used in many parts of the world, particularly in Islamic and Hindu cultures, to dye skin, hair, and fabrics. Application of henna tattoos is common for weddings and other celebrations, and brides may wear elaborate henna patterns. To create these tattoos, henna powder is mixed with water and sometimes essential oils and is then applied to the skin for several hours. After application, the henna pigment lawsone (2-hydroxy-1,4-naphthoquinone) interacts with keratin and leaves a red-orange stain on the skin2; longer application time leads to a deeper color. Most traditional cutaneous henna designs fade in 2 to 6 weeks, but some last longer. Red henna generally is considered safe with low incidence of contact allergy. What is referred to as black henna usually is red henna mixed with PPD, a black dye, which is added to deepen the color. Paraphenylenediamine is highly sensitizing; patients can become sensitized to the PPD in the tattoo itself.2 One study confirmed the presence of PPD in black henna tattoos, with chemical analysis of common preparations revealing concentrations ranging from less than 1% to 30%.2 Patients who undergo patch testing for tattoo reactions often are strongly positive to PPD and have concomitant reactions to azo dyes, black rubber, and anesthetics. Other aromatic amines including aminophenols have been identified in black henna tattoo ink, and these chemicals also may contribute to ACD.3 Less common sources of contact allergy from temporary black henna tattoos include resorcinol,4 para-tertiary butylphenol formaldehyde resin,5 and fragrance.6
Clinically, ACD to PPD in temporary tattoos presents 1 to 3 days after application if the patient is already sensitized or 4 to 14 days if the patient is sensitized by the tattoo ink.2 Most patients notice erythema, edema, vesicles, papules, and/or bullae, but other less common reactions including generalized dermatitis, systemic symptoms, urticaria, and pustules have been described.2 Postinflammatory hypopigmentation or hyperpigmentation also can occur.
Because of the sensitizing nature of black henna tattoos, consumers are turning to natural temporary tattoos. Jagua temporary tattoos, with pigment derived from the sap of fruit from the Genipa americana tree, have been associated with ACD.7 This black dye is applied and washed off in a similar fashion to henna tattoos. Importantly, a recent analysis of jagua dye identified no PPD. In one case, a patient who developed ACD to a jagua tattoo was patch tested to components of the dye and had a positive reaction to genipin, a component of the fruit extract.7 Thus, jagua tattoos often are marketed as safe but are an emerging source of contact dermatitis to temporary tattoos.
Permanent Tattoo Inks
Permanent tattoos are created by injecting small amounts of ink into the dermis. As the name suggests, these tattoos are permanent. Tattoos are common; nearly one-third of Americans have at least 1 tattoo.1 Historically, tattoos were created using black pigment composed of amorphous carbon or black iron oxides.8,9 Metallic pigments (eg, mercury, chromium, cobalt, cadmium) were once used to add color to tattoos, but these metals are now only rarely used; in fact, a 2019 study of tattoo ink components identified 44 distinct pigments in 1416 permanent inks, with an average of 3 pigments per ink.8 Of the 44 pigments, 10 had metallic components including iron, barium, zinc, copper, molybdenum, and titanium. The remaining 34 pigments contained carbon, azo, diketopyrrolopyrrole, quinacridone, anthraquinone, dioxazine (purple), or quinophthalone (yellow) dyes. The authors noted that nearly one-quarter of the tattoo pigments identified in their study had been reported as contact allergens.8
Typically, reactions to permanent tattoo inks manifest as an eczematous dermatitis occurring weeks to years after tattoo application.9,10 The dermatitis usually is locally confined to the tattoo and may be limited to particular colors; occasionally, a new tattoo reaction may trigger concurrent inflammation in older tattoos. Many tattoo reactions occur as a response to red pigment but also have occurred with other tattoo ink components.9 Many researchers have speculated as to whether the reaction is related to the ink component itself or from the photochemical breakdown of the ink by exposure to UV radiation and/or laser therapy.9
Red Pigment
Red ink is the most common color reported to cause tattoo hypersensitivity reactions. Historically, red tattoo pigments include mercuric sulfide (vermilion, cinnabar), scarlet lake, cadmium red, carmine, and cochineal,11 but today’s tattoo inks primarily are composed of other pigments, such as quinacridone and azo dyes.12 Several cases of red tattoo ink hypersensitivity reactions exist in the literature, many without completion of patch tests or without positive patch tests to relevant red pigments.11-15
Black Pigment
In general, reactions to permanent black tattoo ink are rare; however, a few case reports exist. Black pigment can be created with India ink (carbon), logwood (chrome), iron oxide, and titanium.16,17 Shellac can be used as a binding agent in tattoo ink; there is at least one report of a reaction to black tattoo ink with a positive patch test to shellac and the original black ink.18
Metals
When utilized in tattoos, metals can create a variety of colors; several have been reported to cause ACD. There has been at least one reported case of a tattoo hypersensitivity reaction to a gold tattoo, with positive patch testing for gold sodium thiosulfate.19 Green tattoo inks also have been confirmed to contain metal. One case of nickel allergy from a green tattoo has been reported, with a positive patch test for nickel sulfate and tissue confirmation of the presence of nickel with micro X-ray fluorescence and laser ablation inductively coupled plasma mass spectrometry.20 Another case series described 3 patients with pruritus and chronic dermatitis associated with green tattoos who had positive patch tests to potassium dichromate, and the green tattoo pigment flared during patch testing. Chromium oxide was presumed to be present in the green tattoo pigment, and potassium dichromate avoidance in products and food improved both the pruritus and dermatitis.21
Azo Pigments
Azo pigments frequently are used in modern tattoos due to their vibrant colors. One case of hypersensitivity to azo pigment involved an eczematous ulcerated plaque overlying yellow, red, and green ink in a recently applied tattoo. Patch testing with the inks originally used in the tattoo was negative. The authors noted that the 3 problematic ink colors all contained pigment yellow 65—an azo pigment—and attributed the reaction to this dye.22 In another azo reaction, a patient had erythema and pruritus overlying a tattoo applied 1 month prior. Patch testing was positive for aminoazobenzene, an azo pigment that was present in the orange ink of the tattoo.23
Management of Tattoo Hypersensitivity Reactions
Hypersensitivity reactions to temporary tattoos are just that—temporary. Topical steroids and time generally will allow these reactions to resolve. In the setting of vigorous reactions, patients may develop postinflammatory hypopigmentation or hyperpigmentation that may last for months. Unfortunately, bullous tattoo reactions can lead to scarring and keloid formation, requiring more aggressive therapy.
Management of reactions to permanent tattoos is more challenging. High-potency topical steroids under occlusion or intralesional corticosteroid injections may aid in treating pruritus or discomfort. For severe reactions, oral corticosteroids may be required. Patients also may consider laser tattoo removal; however, providers should be aware that there have been rare reports of systemic urticarial reactions from this procedure.24,25 Obviously limited by location and size, excision also may be offered.
Patch Testing for Tattoo Ink Contact Allergy
When patients present for evaluation and management of tattoo ACD, it is important to also consider other causes, including granulomatous tattoo reaction, pseudolymphoma, and lichenoid tattoo reaction. A biopsy can be helpful if the diagnosis is in question.
Patch testing for contact allergy to temporary tattoo inks should include PPD, fragrance, aminophenols, resorcinol, para-tertiary butylphenol formaldehyde, and essential oils. Jagua currently is not available for commercial purchase but also should be considered if the patient has the original product or in research settings. If the individual tattoo ingredients can be identified, they also should be tested. In this scenario, recall reactions may occur; testing with the tattoo paste should be avoided if the prior reaction was severe. Importantly, patients with a PPD allergy should be counseled to avoid hair dyes that contain PPD. Many patients who are sensitized to PPD have strong reactions on patch testing and are at risk for severe reactions if PPD or PPD-related compounds are encountered in hair dye.
Patch testing for ACD to permanent tattoos is complex. In most cases, patch testing is of limited utility because many of the chemicals that have been reported to cause ACD are not commercially available. Additionally, a 2014 study of 90 patients with chronic tattoo reactions found that the majority had negative patch testing to the European baseline series (66%), disperse dyes (87%), and tattoo inks (87%–92%). The investigators theorized that the allergens causing tattoo reactions are formed by haptenization of “parent” chemicals in the dermis, meaning application of chemicals present in the original tattoo ink may not identify the relevant allergen.26 If patch testing is performed, it is most ideal if individual pigment ingredients can be identified. Allergens to be considered for testing include azo dyes, aromatic amines, iron oxide, barium, zinc, copper, molybdenum, titanium, gold sodium thiosulfate, nickel sulfate, carbon, shellac, diketopyrrolopyrrole, quinacridone, anthraquinone, dioxazine (purple), quinophthalone (yellow) dyes, mercuric sulfide (vermilion, cinnabar), scarlet lake, cadmium red, carmine, and cochineal, many of which are not commercially available for purchase.
Final Interpretation
As tattoos become increasingly trendy, tattoo ACD should be recognized by the astute dermatologist. The most common allergen associated with tattoo ACD is PPD, but other potential allergens include azo dyes and newer pigments. Unlike tattoos of the past, today’s inks are unlikely to contain toxic metals. Diagnosing ACD caused by permanent tattoo inks requires a high degree of suspicion, as patch testing may be of limited utility.
- Warshaw EM, Schlarbaum JP, Taylor JS, et al. Allergic reactions to tattoos: retrospective analysis of North American Contact Dermatitis Group data, 2001-2016. J Am Acad Dermatol. 2020;82:E61-E62.
- de Groot AC. Side-effects of henna and semi-permanent ‘black henna’ tattoos: a full review. Contact Dermatitis. 2013;69:1-25.
- Romita P, Foti C, Mascia P, et al. Eyebrow allergic contact dermatitis caused by m-aminophenol and toluene-2,5-diamine secondary to a temporary black henna tattoo. Contact Dermatitis. 2018;79:51-52.
- Ormerod E, Hughes TM, Stone N. Allergic contact dermatitis caused by resorcinol following a temporary black henna tattoo. Contact Dermatitis. 2017;77:187-188.
- Rodrigo-Nicolás B, de la Cuadra J, Sierra C, et al. Contact dermatitis from a temporary tattoo in a boy with contact allergy to p-tert butyl phenol formaldehyde resin. Dermatitis. 2014;25:37-38.
- Temesvári E, Podányi B, Pónyai G, et al. Fragrance sensitization caused by temporary henna tattoo. Contact Dermatitis. 2002;47:240.
- Bircher AJ, Scherer Hofmeier K, Schlegel U, et al. Genipin in temporary jagua tattoos—black dye causing severe allergic dermatitis. Dermatitis. 2019;30:375-376.
- Liszewski W, Warshaw EM. Pigments in American tattoo inks and their propensity to elicit allergic contact dermatitis. J Am Acad Dermatol. 2019;81:379-385.
- Serup J, Hutton Carlsen K, Dommershausen N, et al. Identification of pigments related to allergic tattoo reactions in 104 human skin biopsies. Contact Dermatitis. 2020;82:73-82.
- Bjerre RD, Ulrich NH, Linneberg A, et al. Adverse reactions to tattoos in the general population of Denmark. J Am Acad Dermatol. 2018;79:770-772.
- Bhardwaj SS, Brodell RT, Taylor JS. Red tattoo reactions. Contact Dermatitis. 2003;48:236-237.
- Gaudron S, Ferrier-Le Bouëdec MC, Franck F, et al. Azo pigments and quinacridones induce delayed hypersensitivity in red tattoos. Contact Dermatitis. 2015;72:97-105.
- de Winter RW, van der Bent SAS, van Esch M, et al. Allergic reaction to red cosmetic lip tattoo treated with hydroxychloroquine. Dermatitis. 2019;30:82-83.
- Greve B, Chytry R, Raulin C. Contact dermatitis from red tattoo pigment (quinacridone) with secondary spread. Contact Dermatitis. 2003;49:265-266.
- Ruiz-Villaverde R, Fernandez-Crehuet P, Aguayo-Carreras P, et al. Inflammatory reactions to red tattoo inks: three cases highlighting an emerging problem. Sultan Qaboos Univ Med J. 2018;18:E215-E218.
- Gallo R, Parodi A, Cozzani E, et al. Allergic reaction to India ink in a black tattoo. Contact Dermatitis. 1998;38:346-347.
- de Cuyper C, Lodewick E, Schreiver I, et al. Are metals involved in tattoo-related hypersensitivity reactions? a case report. Contact Dermatitis. 2017;77:397-405.
- González-Villanueva I, Hispán Ocete P, Silvestre Salvador JF. Allergic contact dermatitis caused by a black tattoo ink in a patient allergic to shellac. Contact Dermatitis. 2016;75:247-248.
- Tammaro A, Tuchinda P, Persechino S, et al. Contact allergic dermatitis to gold in a tattoo: a case report. Int J Immunopathol Pharmacol. 2011;24:1111-1113.
- van der Bent SAS, Berg T, Karst U, et al. Allergic reaction to a green tattoo with nickel as a possible allergen. Contact Dermatitis. 2019;81:64-66.
- Jacob SE, Castanedo-Tardan MP, Blyumin ML. Inflammation in green (chromium) tattoos during patch testing. Dermatitis. 2008;19:E33-E34.
- González-Villanueva I, Álvarez-Chinchilla P, Silvestre JF. Allergic reaction to 3 tattoo inks containing pigment yellow 65. Contact Dermatitis. 2018;79:107-108.
- Tammaro A, De Marco G, D’Arino A, et al. Aminoazobenzene in tattoo: another case of allergic contact dermatitis. Int J Dermatol. 2017;56:E79-E81.
- Willardson HB, Kobayashi TT, Arnold JG, et al. Diffuse urticarial reaction associated with titanium dioxide following laser tattoo removal treatments. Photomed Laser Surg. 2017;35:176‐180.
- England RW, Vogel P, Hagan L. Immediate cutaneous hypersensitivity after treatment of tattoo with Nd:YAG laser: a case report and review of the literature. Ann Allergy Asthma Immunol. 2002;89:215‐217.
- Serup J, Carlsen KH. Patch test study of 90 patients with tattoo reactions: negative outcome of allergy patch test to baseline batteries and culprit inks suggests allergen(s) are generated in the skin through haptenization. Contact Dermatitis. 2014;71:255-263.
- Warshaw EM, Schlarbaum JP, Taylor JS, et al. Allergic reactions to tattoos: retrospective analysis of North American Contact Dermatitis Group data, 2001-2016. J Am Acad Dermatol. 2020;82:E61-E62.
- de Groot AC. Side-effects of henna and semi-permanent ‘black henna’ tattoos: a full review. Contact Dermatitis. 2013;69:1-25.
- Romita P, Foti C, Mascia P, et al. Eyebrow allergic contact dermatitis caused by m-aminophenol and toluene-2,5-diamine secondary to a temporary black henna tattoo. Contact Dermatitis. 2018;79:51-52.
- Ormerod E, Hughes TM, Stone N. Allergic contact dermatitis caused by resorcinol following a temporary black henna tattoo. Contact Dermatitis. 2017;77:187-188.
- Rodrigo-Nicolás B, de la Cuadra J, Sierra C, et al. Contact dermatitis from a temporary tattoo in a boy with contact allergy to p-tert butyl phenol formaldehyde resin. Dermatitis. 2014;25:37-38.
- Temesvári E, Podányi B, Pónyai G, et al. Fragrance sensitization caused by temporary henna tattoo. Contact Dermatitis. 2002;47:240.
- Bircher AJ, Scherer Hofmeier K, Schlegel U, et al. Genipin in temporary jagua tattoos—black dye causing severe allergic dermatitis. Dermatitis. 2019;30:375-376.
- Liszewski W, Warshaw EM. Pigments in American tattoo inks and their propensity to elicit allergic contact dermatitis. J Am Acad Dermatol. 2019;81:379-385.
- Serup J, Hutton Carlsen K, Dommershausen N, et al. Identification of pigments related to allergic tattoo reactions in 104 human skin biopsies. Contact Dermatitis. 2020;82:73-82.
- Bjerre RD, Ulrich NH, Linneberg A, et al. Adverse reactions to tattoos in the general population of Denmark. J Am Acad Dermatol. 2018;79:770-772.
- Bhardwaj SS, Brodell RT, Taylor JS. Red tattoo reactions. Contact Dermatitis. 2003;48:236-237.
- Gaudron S, Ferrier-Le Bouëdec MC, Franck F, et al. Azo pigments and quinacridones induce delayed hypersensitivity in red tattoos. Contact Dermatitis. 2015;72:97-105.
- de Winter RW, van der Bent SAS, van Esch M, et al. Allergic reaction to red cosmetic lip tattoo treated with hydroxychloroquine. Dermatitis. 2019;30:82-83.
- Greve B, Chytry R, Raulin C. Contact dermatitis from red tattoo pigment (quinacridone) with secondary spread. Contact Dermatitis. 2003;49:265-266.
- Ruiz-Villaverde R, Fernandez-Crehuet P, Aguayo-Carreras P, et al. Inflammatory reactions to red tattoo inks: three cases highlighting an emerging problem. Sultan Qaboos Univ Med J. 2018;18:E215-E218.
- Gallo R, Parodi A, Cozzani E, et al. Allergic reaction to India ink in a black tattoo. Contact Dermatitis. 1998;38:346-347.
- de Cuyper C, Lodewick E, Schreiver I, et al. Are metals involved in tattoo-related hypersensitivity reactions? a case report. Contact Dermatitis. 2017;77:397-405.
- González-Villanueva I, Hispán Ocete P, Silvestre Salvador JF. Allergic contact dermatitis caused by a black tattoo ink in a patient allergic to shellac. Contact Dermatitis. 2016;75:247-248.
- Tammaro A, Tuchinda P, Persechino S, et al. Contact allergic dermatitis to gold in a tattoo: a case report. Int J Immunopathol Pharmacol. 2011;24:1111-1113.
- van der Bent SAS, Berg T, Karst U, et al. Allergic reaction to a green tattoo with nickel as a possible allergen. Contact Dermatitis. 2019;81:64-66.
- Jacob SE, Castanedo-Tardan MP, Blyumin ML. Inflammation in green (chromium) tattoos during patch testing. Dermatitis. 2008;19:E33-E34.
- González-Villanueva I, Álvarez-Chinchilla P, Silvestre JF. Allergic reaction to 3 tattoo inks containing pigment yellow 65. Contact Dermatitis. 2018;79:107-108.
- Tammaro A, De Marco G, D’Arino A, et al. Aminoazobenzene in tattoo: another case of allergic contact dermatitis. Int J Dermatol. 2017;56:E79-E81.
- Willardson HB, Kobayashi TT, Arnold JG, et al. Diffuse urticarial reaction associated with titanium dioxide following laser tattoo removal treatments. Photomed Laser Surg. 2017;35:176‐180.
- England RW, Vogel P, Hagan L. Immediate cutaneous hypersensitivity after treatment of tattoo with Nd:YAG laser: a case report and review of the literature. Ann Allergy Asthma Immunol. 2002;89:215‐217.
- Serup J, Carlsen KH. Patch test study of 90 patients with tattoo reactions: negative outcome of allergy patch test to baseline batteries and culprit inks suggests allergen(s) are generated in the skin through haptenization. Contact Dermatitis. 2014;71:255-263.
Practice Points
- Temporary tattoo pigments include red henna, black henna, and jagua.
- Black henna tattoos contain paraphenylenediamine, the most common allergen in temporary tattoos.
- Modern permanent tattoo ink components include metals, carbon, azo, diketopyrrolopyrrole, quinacridone, anthraquinone, dioxazine (purple), or quinophthalone (yellow) dyes.
- Patch testing for tattoo contact allergy is complex and challenging.