User login
Pandemic effect: Telemedicine is now a ‘must-have’ service
If people try telemedicine, they’ll like telemedicine. And if they want to avoid a doctor’s office, as most people do these days, they’ll try telemedicine. That is the message coming from 1,000 people surveyed for DocASAP, a provider of online patient access and engagement systems.

Here are a couple of numbers: 92% of those who made a telemedicine visit said they were satisfied with the overall appointment experience, and 91% said that they are more likely to schedule a telemedicine visit instead of an in-person appointment. All of the survey respondents had visited a health care provider in the past year, and 40% already had made a telemedicine visit, DocASAP reported.
Puneet Maheshwari, DocASAP cofounder and CEO, said in a statement. “As providers continue to adopt innovative technology to power a more seamless, end-to-end digital consumer experience, I expect telehealth to become fully integrated into overall care management.”
For now, though, COVID-19 is an overriding concern and health care facilities are suspect. When respondents were asked to identify the types of public facilities where they felt safe, hospitals were named by 32%, doctors’ offices by 26%, and ED/urgent care by just 12%, the DocASAP report said. Even public transportation got 13%.
The safest place to be, according to 42% of the respondents? The grocery store.
Of those surveyed, 43% “indicated they will not feel safe entering any health care setting until at least the fall,” the company said. An even higher share of patients, 68%, canceled or postponed an in-person appointment during the pandemic.
“No longer are remote health services viewed as ‘nice to have’ – they are now a must-have care delivery option,” DocASAP said in their report.
Safety concerns involving COVID-19, named by 47% of the sample, were the leading factor that would influence patients’ decision to schedule a telemedicine visit. Insurance coverage was next at 43%, followed by “ease of accessing quality care” at 40%, the report said.
Among those who had made a telemedicine visit, scheduling the appointment was the most satisfying aspect of the experience, according to 54% of respondents, with day-of-appointment wait time next at 38% and quality of the video/audio technology tied with preappointment communication at almost 33%, the survey data show.
Conversely, scheduling the appointment also was declared the most frustrating aspect of the telemedicine experience, although the total in that category was a much lower 29%.
“The pandemic has thrust profound change on every aspect of life, particularly health care. … Innovations – like digital and telehealth solutions – designed to meet patient needs will likely become embedded into the health care delivery system,” DocASAP said.
The survey was commissioned by DocASAP and conducted by marketing research company OnePoll on June 29-30, 2020.
If people try telemedicine, they’ll like telemedicine. And if they want to avoid a doctor’s office, as most people do these days, they’ll try telemedicine. That is the message coming from 1,000 people surveyed for DocASAP, a provider of online patient access and engagement systems.

Here are a couple of numbers: 92% of those who made a telemedicine visit said they were satisfied with the overall appointment experience, and 91% said that they are more likely to schedule a telemedicine visit instead of an in-person appointment. All of the survey respondents had visited a health care provider in the past year, and 40% already had made a telemedicine visit, DocASAP reported.
Puneet Maheshwari, DocASAP cofounder and CEO, said in a statement. “As providers continue to adopt innovative technology to power a more seamless, end-to-end digital consumer experience, I expect telehealth to become fully integrated into overall care management.”
For now, though, COVID-19 is an overriding concern and health care facilities are suspect. When respondents were asked to identify the types of public facilities where they felt safe, hospitals were named by 32%, doctors’ offices by 26%, and ED/urgent care by just 12%, the DocASAP report said. Even public transportation got 13%.
The safest place to be, according to 42% of the respondents? The grocery store.
Of those surveyed, 43% “indicated they will not feel safe entering any health care setting until at least the fall,” the company said. An even higher share of patients, 68%, canceled or postponed an in-person appointment during the pandemic.
“No longer are remote health services viewed as ‘nice to have’ – they are now a must-have care delivery option,” DocASAP said in their report.
Safety concerns involving COVID-19, named by 47% of the sample, were the leading factor that would influence patients’ decision to schedule a telemedicine visit. Insurance coverage was next at 43%, followed by “ease of accessing quality care” at 40%, the report said.
Among those who had made a telemedicine visit, scheduling the appointment was the most satisfying aspect of the experience, according to 54% of respondents, with day-of-appointment wait time next at 38% and quality of the video/audio technology tied with preappointment communication at almost 33%, the survey data show.
Conversely, scheduling the appointment also was declared the most frustrating aspect of the telemedicine experience, although the total in that category was a much lower 29%.
“The pandemic has thrust profound change on every aspect of life, particularly health care. … Innovations – like digital and telehealth solutions – designed to meet patient needs will likely become embedded into the health care delivery system,” DocASAP said.
The survey was commissioned by DocASAP and conducted by marketing research company OnePoll on June 29-30, 2020.
If people try telemedicine, they’ll like telemedicine. And if they want to avoid a doctor’s office, as most people do these days, they’ll try telemedicine. That is the message coming from 1,000 people surveyed for DocASAP, a provider of online patient access and engagement systems.

Here are a couple of numbers: 92% of those who made a telemedicine visit said they were satisfied with the overall appointment experience, and 91% said that they are more likely to schedule a telemedicine visit instead of an in-person appointment. All of the survey respondents had visited a health care provider in the past year, and 40% already had made a telemedicine visit, DocASAP reported.
Puneet Maheshwari, DocASAP cofounder and CEO, said in a statement. “As providers continue to adopt innovative technology to power a more seamless, end-to-end digital consumer experience, I expect telehealth to become fully integrated into overall care management.”
For now, though, COVID-19 is an overriding concern and health care facilities are suspect. When respondents were asked to identify the types of public facilities where they felt safe, hospitals were named by 32%, doctors’ offices by 26%, and ED/urgent care by just 12%, the DocASAP report said. Even public transportation got 13%.
The safest place to be, according to 42% of the respondents? The grocery store.
Of those surveyed, 43% “indicated they will not feel safe entering any health care setting until at least the fall,” the company said. An even higher share of patients, 68%, canceled or postponed an in-person appointment during the pandemic.
“No longer are remote health services viewed as ‘nice to have’ – they are now a must-have care delivery option,” DocASAP said in their report.
Safety concerns involving COVID-19, named by 47% of the sample, were the leading factor that would influence patients’ decision to schedule a telemedicine visit. Insurance coverage was next at 43%, followed by “ease of accessing quality care” at 40%, the report said.
Among those who had made a telemedicine visit, scheduling the appointment was the most satisfying aspect of the experience, according to 54% of respondents, with day-of-appointment wait time next at 38% and quality of the video/audio technology tied with preappointment communication at almost 33%, the survey data show.
Conversely, scheduling the appointment also was declared the most frustrating aspect of the telemedicine experience, although the total in that category was a much lower 29%.
“The pandemic has thrust profound change on every aspect of life, particularly health care. … Innovations – like digital and telehealth solutions – designed to meet patient needs will likely become embedded into the health care delivery system,” DocASAP said.
The survey was commissioned by DocASAP and conducted by marketing research company OnePoll on June 29-30, 2020.
FDA clamps down on compliance for gluten-free products
To retain the label of “gluten free,” manufacturers of foods that are fermented and hydrolyzed, or that contain fermented or hydrolyzed ingredients, must make and keep detailed records of the manufacturing and production process, according to a final rule issued by the Food and Drug Administration.
In an announcement released on Aug. 12, the FDA stated that manufacturers must confirm that food products such as soy sauce, yogurt, sauerkraut, pickles, cheese, and green olives, as well as distilled foods such as vinegar, meet the definition of gluten free before the fermentation or hydrolysis process. In addition, the rule states that “the manufacturer has adequately evaluated the potential for cross-contact with gluten during the manufacturing process; and if necessary, measures are in place to prevent the introduction of gluten into the food during the manufacturing process,” according to the FDA.
Gluten breaks down during fermentation and hydrolysis, and the gluten-free status of products manufactured in this way can’t be confirmed after the process using currently available methods, according to the FDA.
The new rule is designed to ensure that products labeled as gluten-free meet the definition of gluten free, which remains unchanged from the FDA guidance in 2013.
“The FDA continues to work to protect people with celiac disease, which impacts at least 3 million Americans,” FDA Commissioner Stephen M. Hahn, MD, said in a statement.
“The agency has taken a number of steps on this front by first establishing a standardized definition of gluten free, and now by continuing to work to ensure manufacturers are keeping the products that are labeled with this claim gluten free,” he emphasized.
The final rule states that manufacturers will not need to keep such records if and when other analytical methods are developed, but in the meantime products that do not meet the definition will be deemed misbranded, according to the FDA.
To retain the label of “gluten free,” manufacturers of foods that are fermented and hydrolyzed, or that contain fermented or hydrolyzed ingredients, must make and keep detailed records of the manufacturing and production process, according to a final rule issued by the Food and Drug Administration.
In an announcement released on Aug. 12, the FDA stated that manufacturers must confirm that food products such as soy sauce, yogurt, sauerkraut, pickles, cheese, and green olives, as well as distilled foods such as vinegar, meet the definition of gluten free before the fermentation or hydrolysis process. In addition, the rule states that “the manufacturer has adequately evaluated the potential for cross-contact with gluten during the manufacturing process; and if necessary, measures are in place to prevent the introduction of gluten into the food during the manufacturing process,” according to the FDA.
Gluten breaks down during fermentation and hydrolysis, and the gluten-free status of products manufactured in this way can’t be confirmed after the process using currently available methods, according to the FDA.
The new rule is designed to ensure that products labeled as gluten-free meet the definition of gluten free, which remains unchanged from the FDA guidance in 2013.
“The FDA continues to work to protect people with celiac disease, which impacts at least 3 million Americans,” FDA Commissioner Stephen M. Hahn, MD, said in a statement.
“The agency has taken a number of steps on this front by first establishing a standardized definition of gluten free, and now by continuing to work to ensure manufacturers are keeping the products that are labeled with this claim gluten free,” he emphasized.
The final rule states that manufacturers will not need to keep such records if and when other analytical methods are developed, but in the meantime products that do not meet the definition will be deemed misbranded, according to the FDA.
To retain the label of “gluten free,” manufacturers of foods that are fermented and hydrolyzed, or that contain fermented or hydrolyzed ingredients, must make and keep detailed records of the manufacturing and production process, according to a final rule issued by the Food and Drug Administration.
In an announcement released on Aug. 12, the FDA stated that manufacturers must confirm that food products such as soy sauce, yogurt, sauerkraut, pickles, cheese, and green olives, as well as distilled foods such as vinegar, meet the definition of gluten free before the fermentation or hydrolysis process. In addition, the rule states that “the manufacturer has adequately evaluated the potential for cross-contact with gluten during the manufacturing process; and if necessary, measures are in place to prevent the introduction of gluten into the food during the manufacturing process,” according to the FDA.
Gluten breaks down during fermentation and hydrolysis, and the gluten-free status of products manufactured in this way can’t be confirmed after the process using currently available methods, according to the FDA.
The new rule is designed to ensure that products labeled as gluten-free meet the definition of gluten free, which remains unchanged from the FDA guidance in 2013.
“The FDA continues to work to protect people with celiac disease, which impacts at least 3 million Americans,” FDA Commissioner Stephen M. Hahn, MD, said in a statement.
“The agency has taken a number of steps on this front by first establishing a standardized definition of gluten free, and now by continuing to work to ensure manufacturers are keeping the products that are labeled with this claim gluten free,” he emphasized.
The final rule states that manufacturers will not need to keep such records if and when other analytical methods are developed, but in the meantime products that do not meet the definition will be deemed misbranded, according to the FDA.
Cancer treatments bring concerns for hospitalists
Advances in cancer treatment have brought a range of potential issues hospitalists are likely to see in admitted patients – many of which can escalate quickly into life-threatening emergencies if they’re not handled properly, an oncologist said in a presentation at HM20 Virtual, hosted by the Society of Hospital Medicine.
Checkpoint inhibitors and CAR T-cell therapy – revolutions in fighting cancer but potential instigators of serious side effects because of the way they set the immune system in motion – can have consequences throughout the body, said Megan Kruse, MD, an oncologist at the Cleveland Clinic.
Checkpoint inhibitors, which cause the body to essentially take its foot off the break of the immune system, in particular have diverse effects, Dr. Kruse said.
“Suffice it to say that any odd symptom in any organ system in a patient on immunotherapy, or with a history of immunotherapy, can be cause for concern,” she said. Most common are skin, gut, endocrine, lung, and musculoskeletal involvement. Cardiovascular, hematologic, renal, neurologic, and ophthalmological effects are less common, but when they happen, they’re often dramatic and need urgent management.
With these medications –which include anti–programmed death-1 agents pembrolizumab and nivolumab and anti–PD-ligand 1 agents atezolizumab and avelumab, among others – rash is often seen first, followed by diarrhea and colitis. Hypophysitis, which requires intervention, and liver toxicity, which usually tapers off on its own, often occur about 6-8 weeks into treatment. There are no rigid rules for the arrival of these symptoms, however, Dr. Kruse said.
“We must have a high index of suspicion. ... They really can occur at any point after a patient has had even one dose of an immunologic agent,” she said.
In more serious cases, steroids are typically the go-to treatment, she added, because they will quickly tamp down the immune activation brought on by the medications.
“When these drugs first came out, we were all very concerned about adding steroids,” she said. “In follow-up studies, it actually looks like we don’t attenuate the anticancer response very much by instituting steroids when clinically appropriate. And so you all should feel very comfortable adding steroids while waiting to talk to oncology.”
In these cases, the steroid taper is done very slowly, over weeks or even months.
With CAR T-cell therapy – in which patients receive T cells to target liquid tumors – cytokine release syndrome (CRS) can occur, often within 14 days after treatment. Dr. Kruse cautioned that it can present with symptoms similar to tumor lysis syndrome or sepsis.
“Patients are at a high risk of bacterial infection, so antibiotics are advised,” she said.
In these cases, fever is often a harbinger, often arriving at least a day before the rest of the symptoms of CRS.
Early treatment with the interleukin-6 inhibitor tocilizumab is recommended for these patients, she said. This agent has been shown to have a 69% response rate in severe CRS and has no known effect on the efficacy of the CAR T-cell treatment.
Dr. Kruse also touched on several other conditions that can rise to the level of emergencies in cancer treatment:
- In cases of neutropenic fever, patients should be treated as soon as possible with antibiotics, and some solid-tumor patients at lower risk can be treated as outpatients, she said. Those with hematologic cancer, however, will need inpatient care.
- For tumor lysis syndrome with renal failure, fluids should be started quickly. Rasburicase, a recombinant urate oxidase enzyme, can be considered in some cases, but requires caution.
- In cases of spinal cord compression, a full spine MRI should be completed because about a third of patients have multilevel involvement. Steroids should be started as soon as possible.
In a question-and-answer session, much of the discussion focused on when outpatient care for neutropenic fever was possible. Dr. Kruse said those who need to be admitted for neutropenic fever treatment tend to be those with hematologic malignancies because their treatment is so myelosuppressive.
“Their window of complications is longer,” she said. Solid tumor patients, on the other hand, will usually improve “fairly rapidly” in about 3-4 days.
Many session viewers expressed surprise at the possibility of outpatient neutropenic fever treatment. Dr. Kruse said that the Cleveland Clinic’s incorporation of this approach has included the input of neutropenic fever risk index scoring into their electronic medical record and a good deal of in-service training.
Asked about appropriate swabbing of patients for COVID-19 before chemotherapy, Dr. Kruse said that her center screens only patients who need to be hospitalized for the treatment – those with a high incidence of prolonged neutropenia.
“For our typical outpatients who are receiving chemotherapy,” she said, “we are not swabbing them.” But they have intense fever screening and distance measures in place.
Dr. Kruse reported advisory board involvement for Novartis Oncology and consulting for Puma Biotechnology.
Advances in cancer treatment have brought a range of potential issues hospitalists are likely to see in admitted patients – many of which can escalate quickly into life-threatening emergencies if they’re not handled properly, an oncologist said in a presentation at HM20 Virtual, hosted by the Society of Hospital Medicine.
Checkpoint inhibitors and CAR T-cell therapy – revolutions in fighting cancer but potential instigators of serious side effects because of the way they set the immune system in motion – can have consequences throughout the body, said Megan Kruse, MD, an oncologist at the Cleveland Clinic.
Checkpoint inhibitors, which cause the body to essentially take its foot off the break of the immune system, in particular have diverse effects, Dr. Kruse said.
“Suffice it to say that any odd symptom in any organ system in a patient on immunotherapy, or with a history of immunotherapy, can be cause for concern,” she said. Most common are skin, gut, endocrine, lung, and musculoskeletal involvement. Cardiovascular, hematologic, renal, neurologic, and ophthalmological effects are less common, but when they happen, they’re often dramatic and need urgent management.
With these medications –which include anti–programmed death-1 agents pembrolizumab and nivolumab and anti–PD-ligand 1 agents atezolizumab and avelumab, among others – rash is often seen first, followed by diarrhea and colitis. Hypophysitis, which requires intervention, and liver toxicity, which usually tapers off on its own, often occur about 6-8 weeks into treatment. There are no rigid rules for the arrival of these symptoms, however, Dr. Kruse said.
“We must have a high index of suspicion. ... They really can occur at any point after a patient has had even one dose of an immunologic agent,” she said.
In more serious cases, steroids are typically the go-to treatment, she added, because they will quickly tamp down the immune activation brought on by the medications.
“When these drugs first came out, we were all very concerned about adding steroids,” she said. “In follow-up studies, it actually looks like we don’t attenuate the anticancer response very much by instituting steroids when clinically appropriate. And so you all should feel very comfortable adding steroids while waiting to talk to oncology.”
In these cases, the steroid taper is done very slowly, over weeks or even months.
With CAR T-cell therapy – in which patients receive T cells to target liquid tumors – cytokine release syndrome (CRS) can occur, often within 14 days after treatment. Dr. Kruse cautioned that it can present with symptoms similar to tumor lysis syndrome or sepsis.
“Patients are at a high risk of bacterial infection, so antibiotics are advised,” she said.
In these cases, fever is often a harbinger, often arriving at least a day before the rest of the symptoms of CRS.
Early treatment with the interleukin-6 inhibitor tocilizumab is recommended for these patients, she said. This agent has been shown to have a 69% response rate in severe CRS and has no known effect on the efficacy of the CAR T-cell treatment.
Dr. Kruse also touched on several other conditions that can rise to the level of emergencies in cancer treatment:
- In cases of neutropenic fever, patients should be treated as soon as possible with antibiotics, and some solid-tumor patients at lower risk can be treated as outpatients, she said. Those with hematologic cancer, however, will need inpatient care.
- For tumor lysis syndrome with renal failure, fluids should be started quickly. Rasburicase, a recombinant urate oxidase enzyme, can be considered in some cases, but requires caution.
- In cases of spinal cord compression, a full spine MRI should be completed because about a third of patients have multilevel involvement. Steroids should be started as soon as possible.
In a question-and-answer session, much of the discussion focused on when outpatient care for neutropenic fever was possible. Dr. Kruse said those who need to be admitted for neutropenic fever treatment tend to be those with hematologic malignancies because their treatment is so myelosuppressive.
“Their window of complications is longer,” she said. Solid tumor patients, on the other hand, will usually improve “fairly rapidly” in about 3-4 days.
Many session viewers expressed surprise at the possibility of outpatient neutropenic fever treatment. Dr. Kruse said that the Cleveland Clinic’s incorporation of this approach has included the input of neutropenic fever risk index scoring into their electronic medical record and a good deal of in-service training.
Asked about appropriate swabbing of patients for COVID-19 before chemotherapy, Dr. Kruse said that her center screens only patients who need to be hospitalized for the treatment – those with a high incidence of prolonged neutropenia.
“For our typical outpatients who are receiving chemotherapy,” she said, “we are not swabbing them.” But they have intense fever screening and distance measures in place.
Dr. Kruse reported advisory board involvement for Novartis Oncology and consulting for Puma Biotechnology.
Advances in cancer treatment have brought a range of potential issues hospitalists are likely to see in admitted patients – many of which can escalate quickly into life-threatening emergencies if they’re not handled properly, an oncologist said in a presentation at HM20 Virtual, hosted by the Society of Hospital Medicine.
Checkpoint inhibitors and CAR T-cell therapy – revolutions in fighting cancer but potential instigators of serious side effects because of the way they set the immune system in motion – can have consequences throughout the body, said Megan Kruse, MD, an oncologist at the Cleveland Clinic.
Checkpoint inhibitors, which cause the body to essentially take its foot off the break of the immune system, in particular have diverse effects, Dr. Kruse said.
“Suffice it to say that any odd symptom in any organ system in a patient on immunotherapy, or with a history of immunotherapy, can be cause for concern,” she said. Most common are skin, gut, endocrine, lung, and musculoskeletal involvement. Cardiovascular, hematologic, renal, neurologic, and ophthalmological effects are less common, but when they happen, they’re often dramatic and need urgent management.
With these medications –which include anti–programmed death-1 agents pembrolizumab and nivolumab and anti–PD-ligand 1 agents atezolizumab and avelumab, among others – rash is often seen first, followed by diarrhea and colitis. Hypophysitis, which requires intervention, and liver toxicity, which usually tapers off on its own, often occur about 6-8 weeks into treatment. There are no rigid rules for the arrival of these symptoms, however, Dr. Kruse said.
“We must have a high index of suspicion. ... They really can occur at any point after a patient has had even one dose of an immunologic agent,” she said.
In more serious cases, steroids are typically the go-to treatment, she added, because they will quickly tamp down the immune activation brought on by the medications.
“When these drugs first came out, we were all very concerned about adding steroids,” she said. “In follow-up studies, it actually looks like we don’t attenuate the anticancer response very much by instituting steroids when clinically appropriate. And so you all should feel very comfortable adding steroids while waiting to talk to oncology.”
In these cases, the steroid taper is done very slowly, over weeks or even months.
With CAR T-cell therapy – in which patients receive T cells to target liquid tumors – cytokine release syndrome (CRS) can occur, often within 14 days after treatment. Dr. Kruse cautioned that it can present with symptoms similar to tumor lysis syndrome or sepsis.
“Patients are at a high risk of bacterial infection, so antibiotics are advised,” she said.
In these cases, fever is often a harbinger, often arriving at least a day before the rest of the symptoms of CRS.
Early treatment with the interleukin-6 inhibitor tocilizumab is recommended for these patients, she said. This agent has been shown to have a 69% response rate in severe CRS and has no known effect on the efficacy of the CAR T-cell treatment.
Dr. Kruse also touched on several other conditions that can rise to the level of emergencies in cancer treatment:
- In cases of neutropenic fever, patients should be treated as soon as possible with antibiotics, and some solid-tumor patients at lower risk can be treated as outpatients, she said. Those with hematologic cancer, however, will need inpatient care.
- For tumor lysis syndrome with renal failure, fluids should be started quickly. Rasburicase, a recombinant urate oxidase enzyme, can be considered in some cases, but requires caution.
- In cases of spinal cord compression, a full spine MRI should be completed because about a third of patients have multilevel involvement. Steroids should be started as soon as possible.
In a question-and-answer session, much of the discussion focused on when outpatient care for neutropenic fever was possible. Dr. Kruse said those who need to be admitted for neutropenic fever treatment tend to be those with hematologic malignancies because their treatment is so myelosuppressive.
“Their window of complications is longer,” she said. Solid tumor patients, on the other hand, will usually improve “fairly rapidly” in about 3-4 days.
Many session viewers expressed surprise at the possibility of outpatient neutropenic fever treatment. Dr. Kruse said that the Cleveland Clinic’s incorporation of this approach has included the input of neutropenic fever risk index scoring into their electronic medical record and a good deal of in-service training.
Asked about appropriate swabbing of patients for COVID-19 before chemotherapy, Dr. Kruse said that her center screens only patients who need to be hospitalized for the treatment – those with a high incidence of prolonged neutropenia.
“For our typical outpatients who are receiving chemotherapy,” she said, “we are not swabbing them.” But they have intense fever screening and distance measures in place.
Dr. Kruse reported advisory board involvement for Novartis Oncology and consulting for Puma Biotechnology.
FROM HM20 VIRTUAL
Welcome to week 2 of HM20 Virtual!
The Society of Hospital Medicine prides itself on bringing a broad range of experts together with the largest gathering of hospitalists at any conference – virtual or otherwise! Hospitalists, nurse practitioners, physician assistants, executives, pharmacists, educators, and practitioners of many hospital-based specialties make HM20 Virtual a unique educational experience.
We know that patients depend on you to have pertinent, updated, and timely information for their acute care needs. HM20 Virtual can provide the information you need to stay abreast in this complex and ever-changing year. From COVID-19 to common diagnosis, from racism/bias to blood glucose, from peds to pulmonary embolism, HM20 Virtual covers important topics for all acute care and hospital clinicians and professionals.
This year’s conference is something new. To meet the ever-changing challenges that the year 2020 has brought all of us, HM20 Virtual has addressed one of the limitations of an online conference: personal interactions. With Simulive sessions, you will have the opportunity to chat with fellow participants and interact with the expert faculty in real time! Of course, all Simulive sessions will be available on demand after the fact for those of you who need alternate times to watch.
Be sure to attend some (or all!) of this week’s Simulive sessions. There is something for everyone:
- On Tuesday, Aug. 18, Sam Brondfield, MD, will discuss oncologic work-ups, and James Kim, MD, will make antibiotics simple (where was Dr. Kim for my medical school training?).
- Wednesday, Aug. 19, circles back to another epidemic, the opioid crisis, presented by Theresa Vettese, MD. Dr. Alfred Burger updates us on Clinical Practice Guidelines, and Jeff Trost, MD, brings us up to speed on the effects of COVID-19 and the heart.
- Thursday, Aug. 20, wraps up week 2 of HM20 Virtual with Population Health by Adam Myers, MD, and Updates in Pneumonia by Joanna Bonsall, MD.
The personal interactions don’t have to stop there! HM20 Virtual also features Special Interest Forums. Check out the list and find out how to join by visiting the HM20 Virtual website.
We look forward to “seeing” you at HM20 Virtual. We always want your feedback; however, in this socially distanced, travel-limited world, your input is more important now than ever. Be sure to let us know how this new format works for your learning, networking, and professional needs.
On behalf of the SHM board of directors, the SHM staff, and myself, we hope you enjoy HM20 Virtual. Through this meeting’s rich selection of educational opportunities – and the innovative approaches in a world dominated by the coronavirus – SHM continues to further its mission to promote excellence in the practice of hospital medicine. SHM remains at the forefront of health care today, empowering hospitalists and transforming patient care.
Dr. Howell is CEO of the Society of Hospital Medicine.
The Society of Hospital Medicine prides itself on bringing a broad range of experts together with the largest gathering of hospitalists at any conference – virtual or otherwise! Hospitalists, nurse practitioners, physician assistants, executives, pharmacists, educators, and practitioners of many hospital-based specialties make HM20 Virtual a unique educational experience.
We know that patients depend on you to have pertinent, updated, and timely information for their acute care needs. HM20 Virtual can provide the information you need to stay abreast in this complex and ever-changing year. From COVID-19 to common diagnosis, from racism/bias to blood glucose, from peds to pulmonary embolism, HM20 Virtual covers important topics for all acute care and hospital clinicians and professionals.
This year’s conference is something new. To meet the ever-changing challenges that the year 2020 has brought all of us, HM20 Virtual has addressed one of the limitations of an online conference: personal interactions. With Simulive sessions, you will have the opportunity to chat with fellow participants and interact with the expert faculty in real time! Of course, all Simulive sessions will be available on demand after the fact for those of you who need alternate times to watch.
Be sure to attend some (or all!) of this week’s Simulive sessions. There is something for everyone:
- On Tuesday, Aug. 18, Sam Brondfield, MD, will discuss oncologic work-ups, and James Kim, MD, will make antibiotics simple (where was Dr. Kim for my medical school training?).
- Wednesday, Aug. 19, circles back to another epidemic, the opioid crisis, presented by Theresa Vettese, MD. Dr. Alfred Burger updates us on Clinical Practice Guidelines, and Jeff Trost, MD, brings us up to speed on the effects of COVID-19 and the heart.
- Thursday, Aug. 20, wraps up week 2 of HM20 Virtual with Population Health by Adam Myers, MD, and Updates in Pneumonia by Joanna Bonsall, MD.
The personal interactions don’t have to stop there! HM20 Virtual also features Special Interest Forums. Check out the list and find out how to join by visiting the HM20 Virtual website.
We look forward to “seeing” you at HM20 Virtual. We always want your feedback; however, in this socially distanced, travel-limited world, your input is more important now than ever. Be sure to let us know how this new format works for your learning, networking, and professional needs.
On behalf of the SHM board of directors, the SHM staff, and myself, we hope you enjoy HM20 Virtual. Through this meeting’s rich selection of educational opportunities – and the innovative approaches in a world dominated by the coronavirus – SHM continues to further its mission to promote excellence in the practice of hospital medicine. SHM remains at the forefront of health care today, empowering hospitalists and transforming patient care.
Dr. Howell is CEO of the Society of Hospital Medicine.
The Society of Hospital Medicine prides itself on bringing a broad range of experts together with the largest gathering of hospitalists at any conference – virtual or otherwise! Hospitalists, nurse practitioners, physician assistants, executives, pharmacists, educators, and practitioners of many hospital-based specialties make HM20 Virtual a unique educational experience.
We know that patients depend on you to have pertinent, updated, and timely information for their acute care needs. HM20 Virtual can provide the information you need to stay abreast in this complex and ever-changing year. From COVID-19 to common diagnosis, from racism/bias to blood glucose, from peds to pulmonary embolism, HM20 Virtual covers important topics for all acute care and hospital clinicians and professionals.
This year’s conference is something new. To meet the ever-changing challenges that the year 2020 has brought all of us, HM20 Virtual has addressed one of the limitations of an online conference: personal interactions. With Simulive sessions, you will have the opportunity to chat with fellow participants and interact with the expert faculty in real time! Of course, all Simulive sessions will be available on demand after the fact for those of you who need alternate times to watch.
Be sure to attend some (or all!) of this week’s Simulive sessions. There is something for everyone:
- On Tuesday, Aug. 18, Sam Brondfield, MD, will discuss oncologic work-ups, and James Kim, MD, will make antibiotics simple (where was Dr. Kim for my medical school training?).
- Wednesday, Aug. 19, circles back to another epidemic, the opioid crisis, presented by Theresa Vettese, MD. Dr. Alfred Burger updates us on Clinical Practice Guidelines, and Jeff Trost, MD, brings us up to speed on the effects of COVID-19 and the heart.
- Thursday, Aug. 20, wraps up week 2 of HM20 Virtual with Population Health by Adam Myers, MD, and Updates in Pneumonia by Joanna Bonsall, MD.
The personal interactions don’t have to stop there! HM20 Virtual also features Special Interest Forums. Check out the list and find out how to join by visiting the HM20 Virtual website.
We look forward to “seeing” you at HM20 Virtual. We always want your feedback; however, in this socially distanced, travel-limited world, your input is more important now than ever. Be sure to let us know how this new format works for your learning, networking, and professional needs.
On behalf of the SHM board of directors, the SHM staff, and myself, we hope you enjoy HM20 Virtual. Through this meeting’s rich selection of educational opportunities – and the innovative approaches in a world dominated by the coronavirus – SHM continues to further its mission to promote excellence in the practice of hospital medicine. SHM remains at the forefront of health care today, empowering hospitalists and transforming patient care.
Dr. Howell is CEO of the Society of Hospital Medicine.
COVID-19/heart connection: What hospitalists need to know
The heart-related manifestations of COVID-19 are a serious matter, but no one should make the mistake of thinking of COVID-19 as primarily a cardiac disease, according to Jeffrey C. Trost, MD, a cardiologist at Johns Hopkins University, Baltimore.
he said in offering a preview of his upcoming clinical update at HM20 Virtual, hosted by the Society of Hospital Medicine.
For this reason, in his clinical update talk, titled “COVID-19 and the Heart: What Every Hospitalist Should Know,” he’ll urge hospitalists to be conservative in ordering cardiac biomarker tests such troponin and natriuretic peptide levels. The focus should appropriately be on the subset of COVID-19 patients having the same symptoms suggestive of acute coronary syndrome, heart failure, or new-onset cardiomyopathy that would trigger laboratory testing in non–COVID-19 patients.
“Be more selective. Definitely do not routinely monitor troponin or [N-terminal of the prohormone brain natriuretic peptide] in patients just because they have COVID-19. A lot of patients with COVID-19 have these labs drawn, especially in the emergency department. We see a high signal-to-noise ratio: not infrequently the values are abnormal, and yet we don’t really know what that means,” said Dr. Trost, who is also director of the cardiac catheterization laboratory at Johns Hopkins Bayview Medical Center.
COVID-19 patients with preexisting heart disease are clearly at increased risk of severe forms of the infectious illness. In his talk, Dr. Trost will review the epidemiology of this association. He’ll also discuss the varied cardiac manifestations of COVID-19, consisting of myocarditis or other forms of new-onset cardiomyopathy, acute coronary syndrome, heart failure, and arrhythmias.
Many questions regarding COVID-19 and the heart remain unanswered for now, such as the mechanism and long-term implications of the phenomenon of ST-elevation acute coronary syndrome with chest pain in the presence of unobstructed coronary arteries, which Dr. Trost and others have encountered. Or the extent to which COVID-19–associated myocarditis is directly virus mediated as opposed to an autoimmune process.
“We’re relying completely on case reports at this point,” according to the cardiologist.
But one major issue has, thankfully, been put to rest on the basis of persuasive evidence which Dr. Trost plans to highlight: Millions of patients on ACE inhibitors or angiotensin receptor blockers can now rest assured that taking those medications doesn’t place them at increased risk of becoming infected with the novel coronavirus or, if infected, developing severe complications of COVID-19. Earlier in the pandemic that had been a legitimate theoretic concern based upon a plausible mechanism.
“I think we as physicians can now confidently say that we don’t need to stop these medicines in folks,” Dr. Trost said.
COVID-19 and the Heart: What Every Hospitalist Should Know
Live Q&A: Wednesday, Aug. 19, 3:30 p.m. to 4:30 p.m. ET
The heart-related manifestations of COVID-19 are a serious matter, but no one should make the mistake of thinking of COVID-19 as primarily a cardiac disease, according to Jeffrey C. Trost, MD, a cardiologist at Johns Hopkins University, Baltimore.
he said in offering a preview of his upcoming clinical update at HM20 Virtual, hosted by the Society of Hospital Medicine.
For this reason, in his clinical update talk, titled “COVID-19 and the Heart: What Every Hospitalist Should Know,” he’ll urge hospitalists to be conservative in ordering cardiac biomarker tests such troponin and natriuretic peptide levels. The focus should appropriately be on the subset of COVID-19 patients having the same symptoms suggestive of acute coronary syndrome, heart failure, or new-onset cardiomyopathy that would trigger laboratory testing in non–COVID-19 patients.
“Be more selective. Definitely do not routinely monitor troponin or [N-terminal of the prohormone brain natriuretic peptide] in patients just because they have COVID-19. A lot of patients with COVID-19 have these labs drawn, especially in the emergency department. We see a high signal-to-noise ratio: not infrequently the values are abnormal, and yet we don’t really know what that means,” said Dr. Trost, who is also director of the cardiac catheterization laboratory at Johns Hopkins Bayview Medical Center.
COVID-19 patients with preexisting heart disease are clearly at increased risk of severe forms of the infectious illness. In his talk, Dr. Trost will review the epidemiology of this association. He’ll also discuss the varied cardiac manifestations of COVID-19, consisting of myocarditis or other forms of new-onset cardiomyopathy, acute coronary syndrome, heart failure, and arrhythmias.
Many questions regarding COVID-19 and the heart remain unanswered for now, such as the mechanism and long-term implications of the phenomenon of ST-elevation acute coronary syndrome with chest pain in the presence of unobstructed coronary arteries, which Dr. Trost and others have encountered. Or the extent to which COVID-19–associated myocarditis is directly virus mediated as opposed to an autoimmune process.
“We’re relying completely on case reports at this point,” according to the cardiologist.
But one major issue has, thankfully, been put to rest on the basis of persuasive evidence which Dr. Trost plans to highlight: Millions of patients on ACE inhibitors or angiotensin receptor blockers can now rest assured that taking those medications doesn’t place them at increased risk of becoming infected with the novel coronavirus or, if infected, developing severe complications of COVID-19. Earlier in the pandemic that had been a legitimate theoretic concern based upon a plausible mechanism.
“I think we as physicians can now confidently say that we don’t need to stop these medicines in folks,” Dr. Trost said.
COVID-19 and the Heart: What Every Hospitalist Should Know
Live Q&A: Wednesday, Aug. 19, 3:30 p.m. to 4:30 p.m. ET
The heart-related manifestations of COVID-19 are a serious matter, but no one should make the mistake of thinking of COVID-19 as primarily a cardiac disease, according to Jeffrey C. Trost, MD, a cardiologist at Johns Hopkins University, Baltimore.
he said in offering a preview of his upcoming clinical update at HM20 Virtual, hosted by the Society of Hospital Medicine.
For this reason, in his clinical update talk, titled “COVID-19 and the Heart: What Every Hospitalist Should Know,” he’ll urge hospitalists to be conservative in ordering cardiac biomarker tests such troponin and natriuretic peptide levels. The focus should appropriately be on the subset of COVID-19 patients having the same symptoms suggestive of acute coronary syndrome, heart failure, or new-onset cardiomyopathy that would trigger laboratory testing in non–COVID-19 patients.
“Be more selective. Definitely do not routinely monitor troponin or [N-terminal of the prohormone brain natriuretic peptide] in patients just because they have COVID-19. A lot of patients with COVID-19 have these labs drawn, especially in the emergency department. We see a high signal-to-noise ratio: not infrequently the values are abnormal, and yet we don’t really know what that means,” said Dr. Trost, who is also director of the cardiac catheterization laboratory at Johns Hopkins Bayview Medical Center.
COVID-19 patients with preexisting heart disease are clearly at increased risk of severe forms of the infectious illness. In his talk, Dr. Trost will review the epidemiology of this association. He’ll also discuss the varied cardiac manifestations of COVID-19, consisting of myocarditis or other forms of new-onset cardiomyopathy, acute coronary syndrome, heart failure, and arrhythmias.
Many questions regarding COVID-19 and the heart remain unanswered for now, such as the mechanism and long-term implications of the phenomenon of ST-elevation acute coronary syndrome with chest pain in the presence of unobstructed coronary arteries, which Dr. Trost and others have encountered. Or the extent to which COVID-19–associated myocarditis is directly virus mediated as opposed to an autoimmune process.
“We’re relying completely on case reports at this point,” according to the cardiologist.
But one major issue has, thankfully, been put to rest on the basis of persuasive evidence which Dr. Trost plans to highlight: Millions of patients on ACE inhibitors or angiotensin receptor blockers can now rest assured that taking those medications doesn’t place them at increased risk of becoming infected with the novel coronavirus or, if infected, developing severe complications of COVID-19. Earlier in the pandemic that had been a legitimate theoretic concern based upon a plausible mechanism.
“I think we as physicians can now confidently say that we don’t need to stop these medicines in folks,” Dr. Trost said.
COVID-19 and the Heart: What Every Hospitalist Should Know
Live Q&A: Wednesday, Aug. 19, 3:30 p.m. to 4:30 p.m. ET
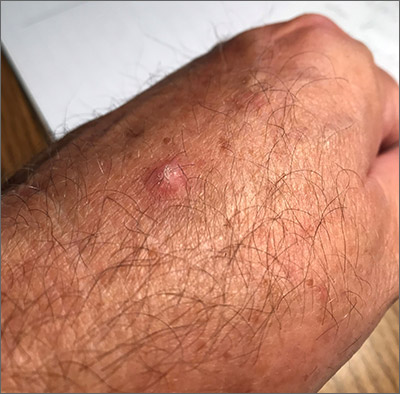
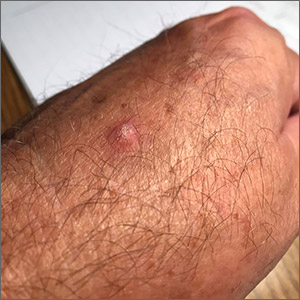
Scaly hand papule
This pink raised nodule underlying a scaly surface was suspicious for squamous cell carcinoma (SCC). Since this was a virtual visit, and the lesion required pathology due to the likelihood of cancer, the patient was brought into the clinic for additional evaluation. A broad-based deep shave biopsy was performed to remove the visible lesion. Pathology showed SCC in situ, with borders uninvolved.
Patients who have had AKs are extremely likely to develop additional AKs. A notable percentage of AKs will, over time, develop into SCC in situ and then invasive SCC if not treated. While cryosurgery of an SK should not result in SCC, it’s most likely that in this case, an AK adjacent to the SK progressed to the SCC in situ.
There are multiple treatments available for SCC in situ. Topical imiquimod has been shown to be somewhat effective in stimulating the immune system, thus leading to resolution of SCC in situ. But there is a significant risk of recurrence. Topical 5-FU can be utilized on a daily or twice daily basis for 2 weeks (or up to several months). The risk of recurrence ranges from 7% to 33%. Electrodesiccation and curettage is often used for SCC in situ, with recurrence rates of 2% to 19%. Cryosurgery for SCC in situ requires an aggressive freeze, with freeze times of up to 30 seconds. Photodynamic therapy also is an option; however, it requires multiple sessions and is more costly than other treatment options.
This patient’s borders were uninvolved on pathology, but it was possible that there was some residual SCC in situ due to the standard “bread loaf slicing” used for routine pathology. To treat possible residual SCC in situ at the wound site and surrounding tissue, the patient was given a prescription for topical 5-FU to apply twice daily for 6 weeks. The patient was instructed to return for follow-up in 6 months, or sooner, if any problems arose.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
Shimizu I, Cruz A, Chang KH, et al. Treatment of squamous cell carcinoma in situ: a review. Dermatol Surg. 2011;37:1394-1411.
This pink raised nodule underlying a scaly surface was suspicious for squamous cell carcinoma (SCC). Since this was a virtual visit, and the lesion required pathology due to the likelihood of cancer, the patient was brought into the clinic for additional evaluation. A broad-based deep shave biopsy was performed to remove the visible lesion. Pathology showed SCC in situ, with borders uninvolved.
Patients who have had AKs are extremely likely to develop additional AKs. A notable percentage of AKs will, over time, develop into SCC in situ and then invasive SCC if not treated. While cryosurgery of an SK should not result in SCC, it’s most likely that in this case, an AK adjacent to the SK progressed to the SCC in situ.
There are multiple treatments available for SCC in situ. Topical imiquimod has been shown to be somewhat effective in stimulating the immune system, thus leading to resolution of SCC in situ. But there is a significant risk of recurrence. Topical 5-FU can be utilized on a daily or twice daily basis for 2 weeks (or up to several months). The risk of recurrence ranges from 7% to 33%. Electrodesiccation and curettage is often used for SCC in situ, with recurrence rates of 2% to 19%. Cryosurgery for SCC in situ requires an aggressive freeze, with freeze times of up to 30 seconds. Photodynamic therapy also is an option; however, it requires multiple sessions and is more costly than other treatment options.
This patient’s borders were uninvolved on pathology, but it was possible that there was some residual SCC in situ due to the standard “bread loaf slicing” used for routine pathology. To treat possible residual SCC in situ at the wound site and surrounding tissue, the patient was given a prescription for topical 5-FU to apply twice daily for 6 weeks. The patient was instructed to return for follow-up in 6 months, or sooner, if any problems arose.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
This pink raised nodule underlying a scaly surface was suspicious for squamous cell carcinoma (SCC). Since this was a virtual visit, and the lesion required pathology due to the likelihood of cancer, the patient was brought into the clinic for additional evaluation. A broad-based deep shave biopsy was performed to remove the visible lesion. Pathology showed SCC in situ, with borders uninvolved.
Patients who have had AKs are extremely likely to develop additional AKs. A notable percentage of AKs will, over time, develop into SCC in situ and then invasive SCC if not treated. While cryosurgery of an SK should not result in SCC, it’s most likely that in this case, an AK adjacent to the SK progressed to the SCC in situ.
There are multiple treatments available for SCC in situ. Topical imiquimod has been shown to be somewhat effective in stimulating the immune system, thus leading to resolution of SCC in situ. But there is a significant risk of recurrence. Topical 5-FU can be utilized on a daily or twice daily basis for 2 weeks (or up to several months). The risk of recurrence ranges from 7% to 33%. Electrodesiccation and curettage is often used for SCC in situ, with recurrence rates of 2% to 19%. Cryosurgery for SCC in situ requires an aggressive freeze, with freeze times of up to 30 seconds. Photodynamic therapy also is an option; however, it requires multiple sessions and is more costly than other treatment options.
This patient’s borders were uninvolved on pathology, but it was possible that there was some residual SCC in situ due to the standard “bread loaf slicing” used for routine pathology. To treat possible residual SCC in situ at the wound site and surrounding tissue, the patient was given a prescription for topical 5-FU to apply twice daily for 6 weeks. The patient was instructed to return for follow-up in 6 months, or sooner, if any problems arose.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
Shimizu I, Cruz A, Chang KH, et al. Treatment of squamous cell carcinoma in situ: a review. Dermatol Surg. 2011;37:1394-1411.
Shimizu I, Cruz A, Chang KH, et al. Treatment of squamous cell carcinoma in situ: a review. Dermatol Surg. 2011;37:1394-1411.
Psoriasis in Patients of Color: Differences in Morphology, Clinical Presentation, and Treatment
Psoriasis is a chronic inflammatory skin disease that affects 2% to 3% of individuals worldwide.1 Despite extensive research, the majority of clinical data are in white patients with limited data in patients of color, yet a number of differences are known. The prevalence of psoriasis differs among racial and ethnic groups, with lower prevalence in racial minorities.2 A cross-sectional American study using data from 2009 through 2010 showed the prevalence for psoriasis was 3.6% in white patients, 1.9% in black patients, 1.6% in Hispanic patients, and 1.4% in other racial groups.3 Psoriasis presents differently in patients of color, both in morphology and severity. Cultural differences and stigma may contribute to the differences seen in severity but also to the psychological impact and treatment choices in patients of color compared to white patients.4 It has even been theorized that treatment efficacy could differ because of potential genetic differences.5 Psoriasis in patients of color is an emerging clinical issue that requires further attention so that dermatologists can learn about, diagnose, and treat them.
We report 3 cases of patients of color with psoriasis who presented to an urban and racially diverse dermatology clinic affiliated with Scarborough General Hospital in Toronto, Ontario, Canada. A retrospective chart review was performed on these high-yield representative cases to demonstrate differences in color and morphology, disease severity, and treatment in patients of various races seen at our clinic. After informed consent was obtained, photographs were taken of patient cutaneous findings to illustrate these differences. Discussion with these selected patients yielded supplementary qualitative data, highlighting individual perspectives of their disease.
Case Series
Patient 1
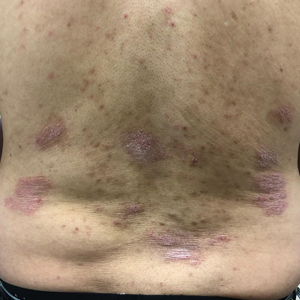
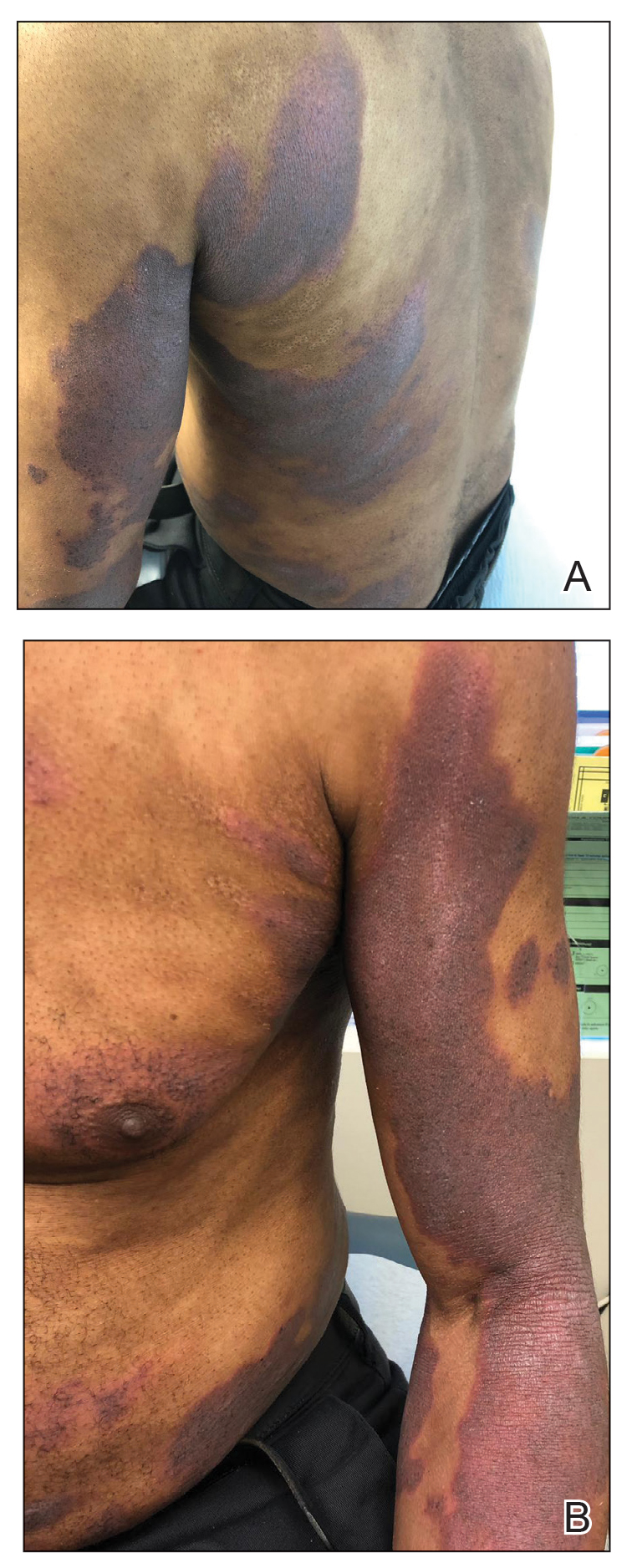
A 53-year-old black man from Grenada presented to our clinic with a history of psoriasis for a number of years that presented as violaceous plaques throughout large portions of the body (Figure 1). He previously had achieved inadequate results while using topical therapies, methotrexate, acitretin, apremilast, ustekinumab, ixekizumab, and guselkumab at adequate or even maximum doses. His disease affected 30% of the body surface area, with a psoriasis area and severity index score of 27 and a dermatology life quality index score of 23. The patient’s life was quite affected by psoriasis, with emphasis on choice of clothing worn and effect on body image. He also discussed the stigma psoriasis may have in black patients, stating that he has been told multiple times that “black people do not get psoriasis.”
Patient 2
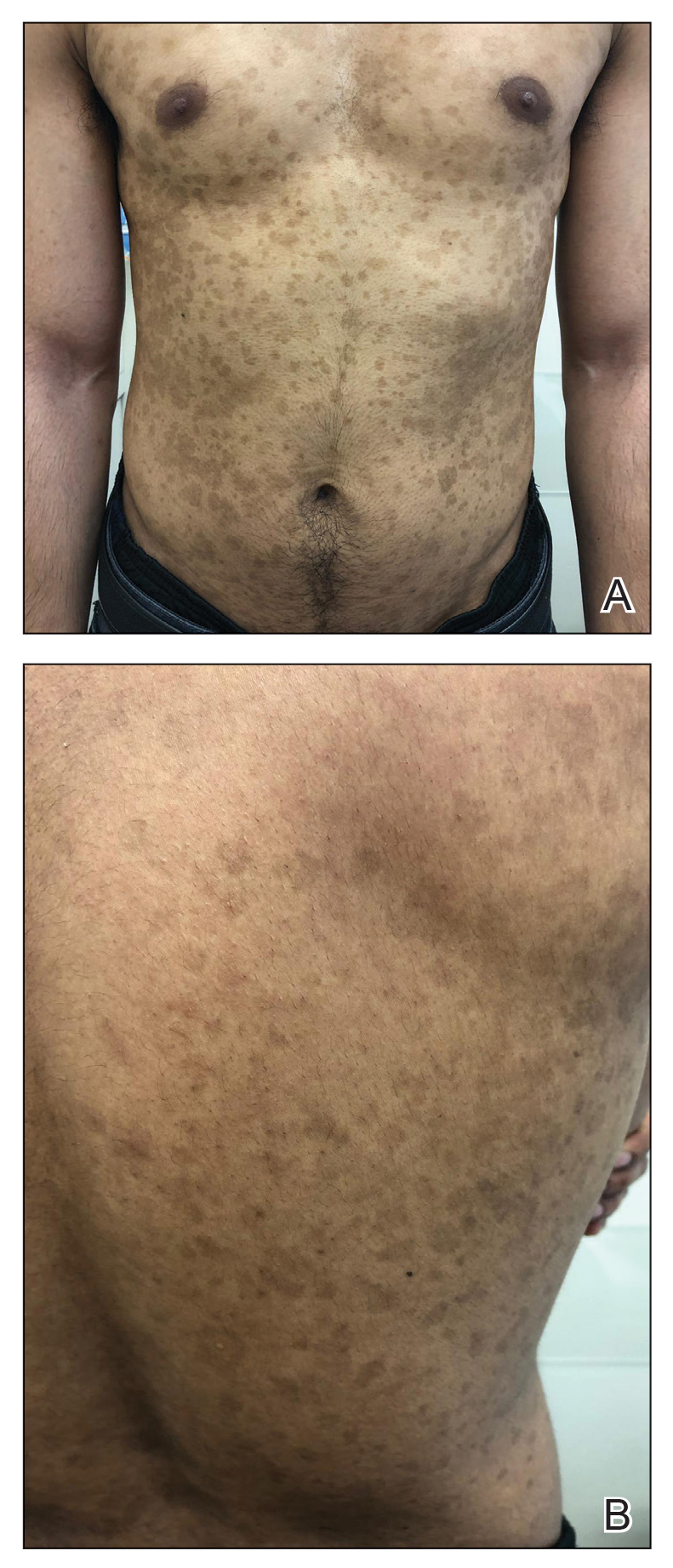
A 27-year-old man from India presented with guttate psoriasis (Figure 2). He was treated with methotrexate 2 years prior and currently is on maintenance therapy with topical treatments alone. His main concerns pertained to the persistent dyschromia that occurred secondary to the psoriatic lesions. Through discussion, the patient stated that he “would do anything to get rid of it.”
Patient 3
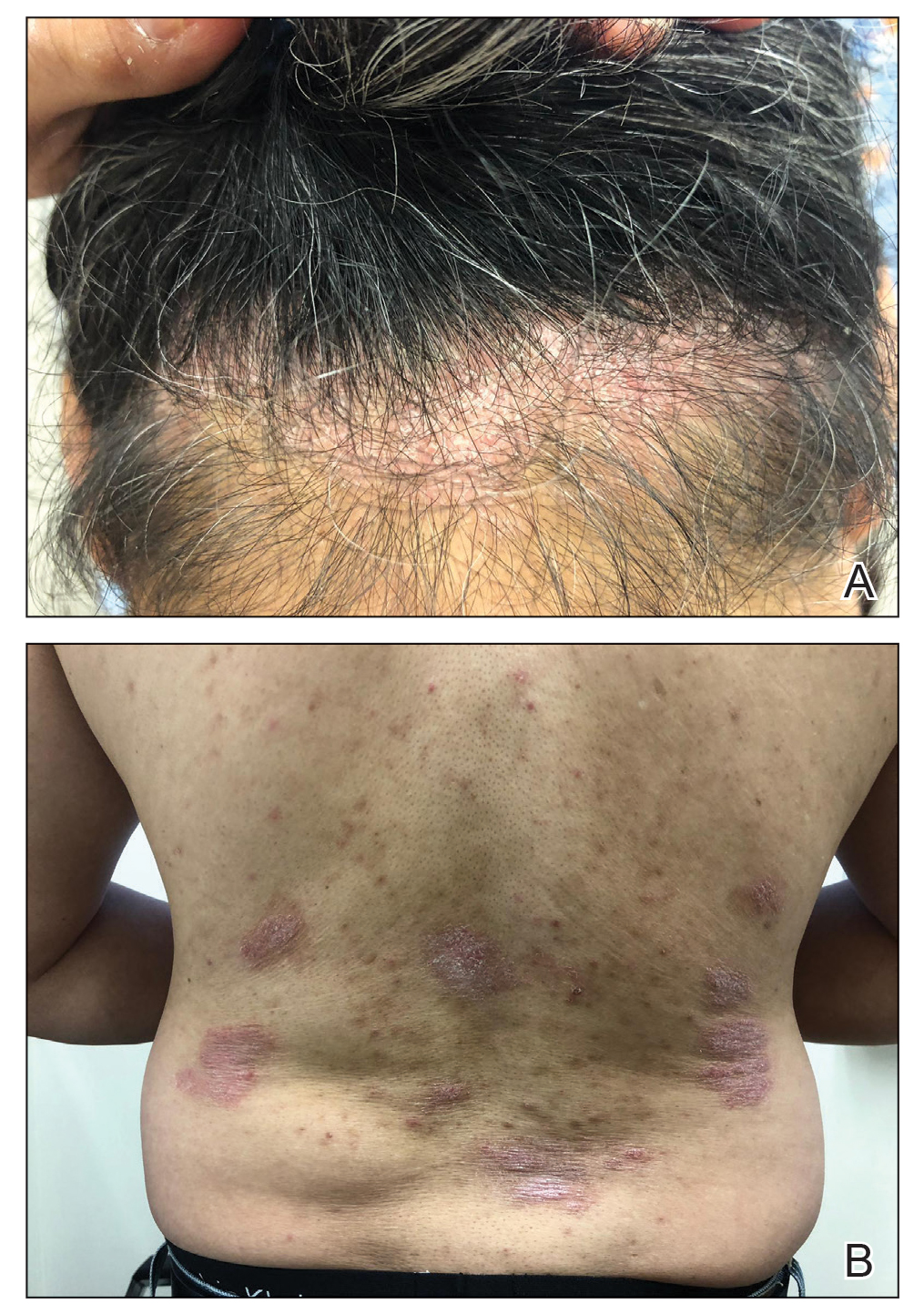
A 49-year-old man from the Philippines presented to our clinic with plaque psoriasis that predominantly affected the trunk and scalp (Figure 3). He had been treated with methotrexate and phototherapy with suboptimal efficacy and was planning for biologic therapy. Although he had active plaques on the trunk, the patient stated, “I am most bothered by my scalp,” particularly referring to the itch and scale and their effects on hair and hairstyling.
Comment
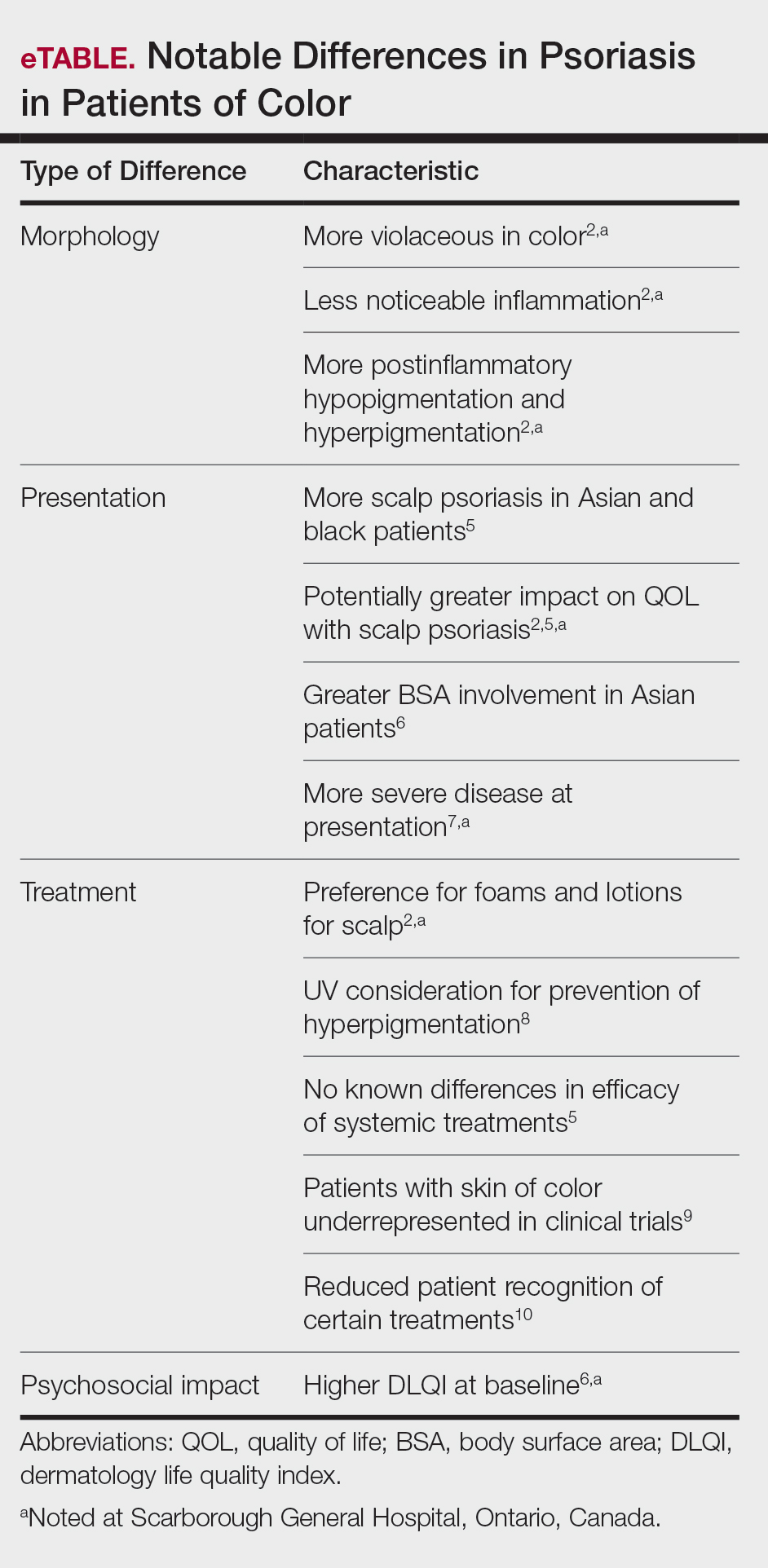
Clinical differences in patients of color with psoriasis affect the management of the disease. Special consideration should be given to variances in morphology, presentation, treatment, and psychosocial factors in the management of psoriasis for these patient populations, as summarized in the eTable.
Morphology
At our clinic, patients of color have been found to have differences in morphology, including lesions that are more violaceous in color, as seen in patient 1; less noticeable inflammation; and more postinflammatory hypopigmentation and hyperpigmentation changes, as seen in patient 2. These changes are supported by the literature and differ from typical psoriasis plaques, which are pink-red and have more overlying scale. The varied morphology also may affect the differential, and other mimickers may be considered, such as lichen planus, cutaneous lupus erythematosus, and sarcoidosis.2
Presentation
There are differences in presentation among patients of color, particularly in distribution, type of psoriasis, and severity. As seen in patient 3, Asian and black patients are more likely to present with scalp psoriasis.2,5 Hairstyling and hair care practices can differ considerably between racial groups. Given the differences in hairstyling, scalp psoriasis also may have a greater impact on patient quality of life (QOL).
Racial differences affect the type of psoriasis seen. Asian patients are more likely to present with pustular and erythrodermic psoriasis and less likely to present with inverse psoriasis compared to white patients. Hispanic patients are more likely to present with pustular psoriasis.11 Black patients have been reported to have lower frequencies of psoriatic arthritis compared to white patients.12 Recognition of these differences may help guide initial choice for therapeutics.
Notably, patients of color may present with much more severe psoriasis, particularly Asian and Hispanic patients.7 One retrospective study looking at patients with psoriasis treated with etanercept found that Asian patients were more likely to have greater baseline body surface area involvement.6 An American cross-sectional study reported higher psoriasis area and severity index scores in black patients compared to white patients,12 possibly because patients of color do not normalize the experience of having psoriasis and feel stigmatized, which can cause delays in seeking medical attention and worsen disease burden. For patient 1, the stigma of black patients having psoriasis affected his body image and may have led to a delay in seeking medical attention due to him not believing it was possible for people of his skin color to have psoriasis. Increased disease severity may contribute to treatment resistance or numerous trials of topicals or biologics before the disease improves. Patient education in the community as well as patient support groups are paramount, and increased awareness of psoriasis can help improve disease management.
Treatment
Topical therapies are the first-line treatment of psoriasis. Although there is no evidence showing differences in topical treatment efficacy, patient preference for different topical treatments may vary based on race. For example, patients with Afro-textured hair may prefer foams and lotions and would avoid shampoo therapies, as frequent hair washing may not be feasible with certain hairstyles and may cause hair breakage or dryness.2
UV therapy can be an effective treatment modality for patients with psoriasis. The strength of therapy tends to be dictated by the Fitzpatrick skin phototype rather than race. Darker-skinned individuals may have an increased risk for hyperpigmentation, so caution should be taken to prevent burning during therapy. Suberythemogenic dosing—70% of minimal erythema dose—of narrowband UVB treatments has shown the same efficacy as using minimal erythema dose in patients with darker skin types in addition to fair-skinned patients.8
Although we found poor efficacy of systemic treatments in patient 1, to our knowledge, studies examining the efficacy of systemic therapeutic options have not shown differences in patients of color.6,13 Studies show similar efficacy in treatments among races, particularly biologic therapies.5 However, patients with skin of color historically have been underrepresented in clinical trials,9 which may contribute to these patients, particularly black patients, being less familiar with biologics as a treatment option for psoriasis, as reported by Takeshita et al.10 Therefore, patient-centered discussions regarding treatment choices are important to ensure patients understand all options available to manage their disease.
Psychosocial Impact
Because of its chronic remitting course, psoriasis has a notable psychosocial impact on the lives of all patients, though the literature suggests there may be more of an impact on QOL in patients of color. Higher baseline dermatology life quality index scores have been reported in patients of color compared to white patients.6 Kerr et al12 reported significantly greater psoriasis area and severity index scores (P=.06) and greater psychological impact in black patients compared to white patients. Stress also was more likely to be reported as a trigger for psoriasis in patients of Hispanic background compared to white patients.14 Many patients report body image issues with large physical lesions; however, the difference may lie in personal and cultural views about psoriasis, as one of our patients stated, “black people do not get psoriasis.” In addition to the cosmetic challenges that patients face with active lesions, postinflammatory pigmentary changes can be equally as burdensome to patients, as one of our patients stated he “would do anything to get rid of it.” Increased rates of depression and anxiety in patients of color can worsen their outlook on the condition.15,16 The increased stigma and burden of psoriasis in patients of color calls for clinicians to counsel and address psoriasis in a holistic way and refer patients to psoriasis support groups when appropriate. Although the burden of psoriasis is clear, more studies can be carried out to investigate the impact on QOL in different ethnic populations.
Dermatology Education
Although differences have been found in patients of color with psoriasis, dissemination of this knowledge continues to be a challenge. In dermatology residency programs, the majority of teaching is provided with examples of skin diseases in white patients, which can complicate pattern recognition and diagnostic ability for trainees. Although dermatologists recognize that ethnic skin has unique dermatologic considerations, there is a persistent need for increasing skin of color education within dermatology residency programs.17,18 Implementing more educational programs on skin of color has been proposed, and these programs will continue to be in demand as our population increasingly diversifies.19
Conclusion
Psoriasis in patients of color carries unique challenges when compared to psoriasis in white patients. Differences in morphology and presentation can make the disease difficult to accurately diagnose. These differences in addition to cultural differences may contribute to a greater impact on QOL and psychological health. Although treatment preferences and recognition may differ, treatment efficacy has so far been similar, albeit with a low proportion of patients with skin of color included in clinical trials.
Further focus should now lie within knowledge translation of these differences, which would normalize the condition for patients, support them seeking medical attention sooner, and inform them of all treatment options possible. For clinicians, more attention on the differences would help make earlier diagnoses, personalize physician-patient conversations, and advocate for further education on this issue in residency training programs.
- National Psoriasis Foundation. Statistics. https://www.psoriasis.org/content/statistics. Accessed July 14, 2020.
- Alexis AF, Blackcloud P. Psoriasis in skin of color: epidemiology, genetics, clinical presentation, and treatment nuances. J Clin Aesthet Dermatol. 2014;7:16-24.
- Rachakonda TD, Schupp CW, Armstrong AW. Psoriasis prevalence among adults in the United States. J Am Acad Dermatol. 2014;70:512-516.
- Goff KL, Karimkhani C, Boyers LN, et al. The global burden of psoriatic skin disease. Br J Dermatol. 2015;172:1665-1668.
- Kaufman BP, Alexis AF. Psoriasis in skin of color: insights into the epidemiology, clinical presentation, genetics, quality-of-life impact, and treatment of psoriasis in non-white racial/ethnic groups. Am J Clin Dermatol. 2018;19:405-423.
- Shah SK, Arthur A, Yang YC, et al. A retrospective study to investigate racial and ethnic variations in the treatment of psoriasis with etanercept. J Drugs Dermatol. 2011;10:866-872.
- Abrouk M, Lee K, Brodsky M, et al. Ethnicity affects the presenting severity of psoriasis. J Am Acad Dermatol. 2017;77:180-182.
- Youssef RM, Mahgoub D, Mashaly HM, et al. Different narrowband UVB dosage regimens in dark skinned psoriatics: a preliminary study. Photodermatol Photoimmunol Photomed. 2008;24:256-259.
- Charrow A, Xia F Di, Joyce C, et al. Diversity in dermatology clinical trials: a systematic review. JAMA Dermatol. 2017;153:193-198.
- Takeshita J, Eriksen WT, Raziano VT, et al. Racial differences in perceptions of psoriasis therapies: implications for racial disparities in psoriasis treatment. J Invest Dermatol. 2019;139:1672-1679.
- Yan D, Afifi L, Jeon C, et al. A cross-sectional study of the distribution of psoriasis subtypes in different ethno-racial groups. Dermatol Online J. 2018;24. pii:13030/qt5z21q4k2.
- Kerr GS, Qaiyumi S, Richards J, et al. Psoriasis and psoriatic arthritis in African-American patients—the need to measure disease burden. Clin Rheumatol. 2015;34:1753-1759.
- Edson-Heredia E, Sterling KL, Alatorre CI, et al. Heterogeneity of response to biologic treatment: perspective for psoriasis. J Invest Dermatol. 2014;134:18-23.
- Yan D, Afifi L, Jeon C, et al. A cross-sectional study of psoriasis triggers among different ethno-racial groups. J Am Acad Dermatol. 2017;77:756-758.
- Bailey RK, Mokonogho J, Kumar A. Racial and ethnic differences in depression: current perspectives. Neuropsychiatr Dis Treat. 2019;15:603-609.
- Jackson C, Maibach H. Ethnic and socioeconomic disparities in dermatology. J Dermatolog Treat. 2016;27:290-291.
- Salam A, Dadzie OE. Dermatology training in the U.K.: does it reflect the changing demographics of our population? Br J Dermatol. 2013;169:1360-1362.
- Nijhawan RI, Jacob SE, Woolery-Lloyd H. Skin of color education in dermatology residency programs: does residency training reflect the changing demographics of the United States? J Am Acad Dermatol. 2008;59:615-618.
- Ogunyemi B, Miller-Monthrope Y. The state of ethnic dermatology in Canada. J Cutan Med Surg. 2017;21:464-466.
Psoriasis is a chronic inflammatory skin disease that affects 2% to 3% of individuals worldwide.1 Despite extensive research, the majority of clinical data are in white patients with limited data in patients of color, yet a number of differences are known. The prevalence of psoriasis differs among racial and ethnic groups, with lower prevalence in racial minorities.2 A cross-sectional American study using data from 2009 through 2010 showed the prevalence for psoriasis was 3.6% in white patients, 1.9% in black patients, 1.6% in Hispanic patients, and 1.4% in other racial groups.3 Psoriasis presents differently in patients of color, both in morphology and severity. Cultural differences and stigma may contribute to the differences seen in severity but also to the psychological impact and treatment choices in patients of color compared to white patients.4 It has even been theorized that treatment efficacy could differ because of potential genetic differences.5 Psoriasis in patients of color is an emerging clinical issue that requires further attention so that dermatologists can learn about, diagnose, and treat them.
We report 3 cases of patients of color with psoriasis who presented to an urban and racially diverse dermatology clinic affiliated with Scarborough General Hospital in Toronto, Ontario, Canada. A retrospective chart review was performed on these high-yield representative cases to demonstrate differences in color and morphology, disease severity, and treatment in patients of various races seen at our clinic. After informed consent was obtained, photographs were taken of patient cutaneous findings to illustrate these differences. Discussion with these selected patients yielded supplementary qualitative data, highlighting individual perspectives of their disease.
Case Series
Patient 1
A 53-year-old black man from Grenada presented to our clinic with a history of psoriasis for a number of years that presented as violaceous plaques throughout large portions of the body (Figure 1). He previously had achieved inadequate results while using topical therapies, methotrexate, acitretin, apremilast, ustekinumab, ixekizumab, and guselkumab at adequate or even maximum doses. His disease affected 30% of the body surface area, with a psoriasis area and severity index score of 27 and a dermatology life quality index score of 23. The patient’s life was quite affected by psoriasis, with emphasis on choice of clothing worn and effect on body image. He also discussed the stigma psoriasis may have in black patients, stating that he has been told multiple times that “black people do not get psoriasis.”

Patient 2
A 27-year-old man from India presented with guttate psoriasis (Figure 2). He was treated with methotrexate 2 years prior and currently is on maintenance therapy with topical treatments alone. His main concerns pertained to the persistent dyschromia that occurred secondary to the psoriatic lesions. Through discussion, the patient stated that he “would do anything to get rid of it.”

Patient 3
A 49-year-old man from the Philippines presented to our clinic with plaque psoriasis that predominantly affected the trunk and scalp (Figure 3). He had been treated with methotrexate and phototherapy with suboptimal efficacy and was planning for biologic therapy. Although he had active plaques on the trunk, the patient stated, “I am most bothered by my scalp,” particularly referring to the itch and scale and their effects on hair and hairstyling.

Comment
Clinical differences in patients of color with psoriasis affect the management of the disease. Special consideration should be given to variances in morphology, presentation, treatment, and psychosocial factors in the management of psoriasis for these patient populations, as summarized in the eTable.

Morphology
At our clinic, patients of color have been found to have differences in morphology, including lesions that are more violaceous in color, as seen in patient 1; less noticeable inflammation; and more postinflammatory hypopigmentation and hyperpigmentation changes, as seen in patient 2. These changes are supported by the literature and differ from typical psoriasis plaques, which are pink-red and have more overlying scale. The varied morphology also may affect the differential, and other mimickers may be considered, such as lichen planus, cutaneous lupus erythematosus, and sarcoidosis.2
Presentation
There are differences in presentation among patients of color, particularly in distribution, type of psoriasis, and severity. As seen in patient 3, Asian and black patients are more likely to present with scalp psoriasis.2,5 Hairstyling and hair care practices can differ considerably between racial groups. Given the differences in hairstyling, scalp psoriasis also may have a greater impact on patient quality of life (QOL).
Racial differences affect the type of psoriasis seen. Asian patients are more likely to present with pustular and erythrodermic psoriasis and less likely to present with inverse psoriasis compared to white patients. Hispanic patients are more likely to present with pustular psoriasis.11 Black patients have been reported to have lower frequencies of psoriatic arthritis compared to white patients.12 Recognition of these differences may help guide initial choice for therapeutics.
Notably, patients of color may present with much more severe psoriasis, particularly Asian and Hispanic patients.7 One retrospective study looking at patients with psoriasis treated with etanercept found that Asian patients were more likely to have greater baseline body surface area involvement.6 An American cross-sectional study reported higher psoriasis area and severity index scores in black patients compared to white patients,12 possibly because patients of color do not normalize the experience of having psoriasis and feel stigmatized, which can cause delays in seeking medical attention and worsen disease burden. For patient 1, the stigma of black patients having psoriasis affected his body image and may have led to a delay in seeking medical attention due to him not believing it was possible for people of his skin color to have psoriasis. Increased disease severity may contribute to treatment resistance or numerous trials of topicals or biologics before the disease improves. Patient education in the community as well as patient support groups are paramount, and increased awareness of psoriasis can help improve disease management.
Treatment
Topical therapies are the first-line treatment of psoriasis. Although there is no evidence showing differences in topical treatment efficacy, patient preference for different topical treatments may vary based on race. For example, patients with Afro-textured hair may prefer foams and lotions and would avoid shampoo therapies, as frequent hair washing may not be feasible with certain hairstyles and may cause hair breakage or dryness.2
UV therapy can be an effective treatment modality for patients with psoriasis. The strength of therapy tends to be dictated by the Fitzpatrick skin phototype rather than race. Darker-skinned individuals may have an increased risk for hyperpigmentation, so caution should be taken to prevent burning during therapy. Suberythemogenic dosing—70% of minimal erythema dose—of narrowband UVB treatments has shown the same efficacy as using minimal erythema dose in patients with darker skin types in addition to fair-skinned patients.8
Although we found poor efficacy of systemic treatments in patient 1, to our knowledge, studies examining the efficacy of systemic therapeutic options have not shown differences in patients of color.6,13 Studies show similar efficacy in treatments among races, particularly biologic therapies.5 However, patients with skin of color historically have been underrepresented in clinical trials,9 which may contribute to these patients, particularly black patients, being less familiar with biologics as a treatment option for psoriasis, as reported by Takeshita et al.10 Therefore, patient-centered discussions regarding treatment choices are important to ensure patients understand all options available to manage their disease.
Psychosocial Impact
Because of its chronic remitting course, psoriasis has a notable psychosocial impact on the lives of all patients, though the literature suggests there may be more of an impact on QOL in patients of color. Higher baseline dermatology life quality index scores have been reported in patients of color compared to white patients.6 Kerr et al12 reported significantly greater psoriasis area and severity index scores (P=.06) and greater psychological impact in black patients compared to white patients. Stress also was more likely to be reported as a trigger for psoriasis in patients of Hispanic background compared to white patients.14 Many patients report body image issues with large physical lesions; however, the difference may lie in personal and cultural views about psoriasis, as one of our patients stated, “black people do not get psoriasis.” In addition to the cosmetic challenges that patients face with active lesions, postinflammatory pigmentary changes can be equally as burdensome to patients, as one of our patients stated he “would do anything to get rid of it.” Increased rates of depression and anxiety in patients of color can worsen their outlook on the condition.15,16 The increased stigma and burden of psoriasis in patients of color calls for clinicians to counsel and address psoriasis in a holistic way and refer patients to psoriasis support groups when appropriate. Although the burden of psoriasis is clear, more studies can be carried out to investigate the impact on QOL in different ethnic populations.
Dermatology Education
Although differences have been found in patients of color with psoriasis, dissemination of this knowledge continues to be a challenge. In dermatology residency programs, the majority of teaching is provided with examples of skin diseases in white patients, which can complicate pattern recognition and diagnostic ability for trainees. Although dermatologists recognize that ethnic skin has unique dermatologic considerations, there is a persistent need for increasing skin of color education within dermatology residency programs.17,18 Implementing more educational programs on skin of color has been proposed, and these programs will continue to be in demand as our population increasingly diversifies.19
Conclusion
Psoriasis in patients of color carries unique challenges when compared to psoriasis in white patients. Differences in morphology and presentation can make the disease difficult to accurately diagnose. These differences in addition to cultural differences may contribute to a greater impact on QOL and psychological health. Although treatment preferences and recognition may differ, treatment efficacy has so far been similar, albeit with a low proportion of patients with skin of color included in clinical trials.
Further focus should now lie within knowledge translation of these differences, which would normalize the condition for patients, support them seeking medical attention sooner, and inform them of all treatment options possible. For clinicians, more attention on the differences would help make earlier diagnoses, personalize physician-patient conversations, and advocate for further education on this issue in residency training programs.
Psoriasis is a chronic inflammatory skin disease that affects 2% to 3% of individuals worldwide.1 Despite extensive research, the majority of clinical data are in white patients with limited data in patients of color, yet a number of differences are known. The prevalence of psoriasis differs among racial and ethnic groups, with lower prevalence in racial minorities.2 A cross-sectional American study using data from 2009 through 2010 showed the prevalence for psoriasis was 3.6% in white patients, 1.9% in black patients, 1.6% in Hispanic patients, and 1.4% in other racial groups.3 Psoriasis presents differently in patients of color, both in morphology and severity. Cultural differences and stigma may contribute to the differences seen in severity but also to the psychological impact and treatment choices in patients of color compared to white patients.4 It has even been theorized that treatment efficacy could differ because of potential genetic differences.5 Psoriasis in patients of color is an emerging clinical issue that requires further attention so that dermatologists can learn about, diagnose, and treat them.
We report 3 cases of patients of color with psoriasis who presented to an urban and racially diverse dermatology clinic affiliated with Scarborough General Hospital in Toronto, Ontario, Canada. A retrospective chart review was performed on these high-yield representative cases to demonstrate differences in color and morphology, disease severity, and treatment in patients of various races seen at our clinic. After informed consent was obtained, photographs were taken of patient cutaneous findings to illustrate these differences. Discussion with these selected patients yielded supplementary qualitative data, highlighting individual perspectives of their disease.
Case Series
Patient 1
A 53-year-old black man from Grenada presented to our clinic with a history of psoriasis for a number of years that presented as violaceous plaques throughout large portions of the body (Figure 1). He previously had achieved inadequate results while using topical therapies, methotrexate, acitretin, apremilast, ustekinumab, ixekizumab, and guselkumab at adequate or even maximum doses. His disease affected 30% of the body surface area, with a psoriasis area and severity index score of 27 and a dermatology life quality index score of 23. The patient’s life was quite affected by psoriasis, with emphasis on choice of clothing worn and effect on body image. He also discussed the stigma psoriasis may have in black patients, stating that he has been told multiple times that “black people do not get psoriasis.”

Patient 2
A 27-year-old man from India presented with guttate psoriasis (Figure 2). He was treated with methotrexate 2 years prior and currently is on maintenance therapy with topical treatments alone. His main concerns pertained to the persistent dyschromia that occurred secondary to the psoriatic lesions. Through discussion, the patient stated that he “would do anything to get rid of it.”

Patient 3
A 49-year-old man from the Philippines presented to our clinic with plaque psoriasis that predominantly affected the trunk and scalp (Figure 3). He had been treated with methotrexate and phototherapy with suboptimal efficacy and was planning for biologic therapy. Although he had active plaques on the trunk, the patient stated, “I am most bothered by my scalp,” particularly referring to the itch and scale and their effects on hair and hairstyling.

Comment
Clinical differences in patients of color with psoriasis affect the management of the disease. Special consideration should be given to variances in morphology, presentation, treatment, and psychosocial factors in the management of psoriasis for these patient populations, as summarized in the eTable.

Morphology
At our clinic, patients of color have been found to have differences in morphology, including lesions that are more violaceous in color, as seen in patient 1; less noticeable inflammation; and more postinflammatory hypopigmentation and hyperpigmentation changes, as seen in patient 2. These changes are supported by the literature and differ from typical psoriasis plaques, which are pink-red and have more overlying scale. The varied morphology also may affect the differential, and other mimickers may be considered, such as lichen planus, cutaneous lupus erythematosus, and sarcoidosis.2
Presentation
There are differences in presentation among patients of color, particularly in distribution, type of psoriasis, and severity. As seen in patient 3, Asian and black patients are more likely to present with scalp psoriasis.2,5 Hairstyling and hair care practices can differ considerably between racial groups. Given the differences in hairstyling, scalp psoriasis also may have a greater impact on patient quality of life (QOL).
Racial differences affect the type of psoriasis seen. Asian patients are more likely to present with pustular and erythrodermic psoriasis and less likely to present with inverse psoriasis compared to white patients. Hispanic patients are more likely to present with pustular psoriasis.11 Black patients have been reported to have lower frequencies of psoriatic arthritis compared to white patients.12 Recognition of these differences may help guide initial choice for therapeutics.
Notably, patients of color may present with much more severe psoriasis, particularly Asian and Hispanic patients.7 One retrospective study looking at patients with psoriasis treated with etanercept found that Asian patients were more likely to have greater baseline body surface area involvement.6 An American cross-sectional study reported higher psoriasis area and severity index scores in black patients compared to white patients,12 possibly because patients of color do not normalize the experience of having psoriasis and feel stigmatized, which can cause delays in seeking medical attention and worsen disease burden. For patient 1, the stigma of black patients having psoriasis affected his body image and may have led to a delay in seeking medical attention due to him not believing it was possible for people of his skin color to have psoriasis. Increased disease severity may contribute to treatment resistance or numerous trials of topicals or biologics before the disease improves. Patient education in the community as well as patient support groups are paramount, and increased awareness of psoriasis can help improve disease management.
Treatment
Topical therapies are the first-line treatment of psoriasis. Although there is no evidence showing differences in topical treatment efficacy, patient preference for different topical treatments may vary based on race. For example, patients with Afro-textured hair may prefer foams and lotions and would avoid shampoo therapies, as frequent hair washing may not be feasible with certain hairstyles and may cause hair breakage or dryness.2
UV therapy can be an effective treatment modality for patients with psoriasis. The strength of therapy tends to be dictated by the Fitzpatrick skin phototype rather than race. Darker-skinned individuals may have an increased risk for hyperpigmentation, so caution should be taken to prevent burning during therapy. Suberythemogenic dosing—70% of minimal erythema dose—of narrowband UVB treatments has shown the same efficacy as using minimal erythema dose in patients with darker skin types in addition to fair-skinned patients.8
Although we found poor efficacy of systemic treatments in patient 1, to our knowledge, studies examining the efficacy of systemic therapeutic options have not shown differences in patients of color.6,13 Studies show similar efficacy in treatments among races, particularly biologic therapies.5 However, patients with skin of color historically have been underrepresented in clinical trials,9 which may contribute to these patients, particularly black patients, being less familiar with biologics as a treatment option for psoriasis, as reported by Takeshita et al.10 Therefore, patient-centered discussions regarding treatment choices are important to ensure patients understand all options available to manage their disease.
Psychosocial Impact
Because of its chronic remitting course, psoriasis has a notable psychosocial impact on the lives of all patients, though the literature suggests there may be more of an impact on QOL in patients of color. Higher baseline dermatology life quality index scores have been reported in patients of color compared to white patients.6 Kerr et al12 reported significantly greater psoriasis area and severity index scores (P=.06) and greater psychological impact in black patients compared to white patients. Stress also was more likely to be reported as a trigger for psoriasis in patients of Hispanic background compared to white patients.14 Many patients report body image issues with large physical lesions; however, the difference may lie in personal and cultural views about psoriasis, as one of our patients stated, “black people do not get psoriasis.” In addition to the cosmetic challenges that patients face with active lesions, postinflammatory pigmentary changes can be equally as burdensome to patients, as one of our patients stated he “would do anything to get rid of it.” Increased rates of depression and anxiety in patients of color can worsen their outlook on the condition.15,16 The increased stigma and burden of psoriasis in patients of color calls for clinicians to counsel and address psoriasis in a holistic way and refer patients to psoriasis support groups when appropriate. Although the burden of psoriasis is clear, more studies can be carried out to investigate the impact on QOL in different ethnic populations.
Dermatology Education
Although differences have been found in patients of color with psoriasis, dissemination of this knowledge continues to be a challenge. In dermatology residency programs, the majority of teaching is provided with examples of skin diseases in white patients, which can complicate pattern recognition and diagnostic ability for trainees. Although dermatologists recognize that ethnic skin has unique dermatologic considerations, there is a persistent need for increasing skin of color education within dermatology residency programs.17,18 Implementing more educational programs on skin of color has been proposed, and these programs will continue to be in demand as our population increasingly diversifies.19
Conclusion
Psoriasis in patients of color carries unique challenges when compared to psoriasis in white patients. Differences in morphology and presentation can make the disease difficult to accurately diagnose. These differences in addition to cultural differences may contribute to a greater impact on QOL and psychological health. Although treatment preferences and recognition may differ, treatment efficacy has so far been similar, albeit with a low proportion of patients with skin of color included in clinical trials.
Further focus should now lie within knowledge translation of these differences, which would normalize the condition for patients, support them seeking medical attention sooner, and inform them of all treatment options possible. For clinicians, more attention on the differences would help make earlier diagnoses, personalize physician-patient conversations, and advocate for further education on this issue in residency training programs.
- National Psoriasis Foundation. Statistics. https://www.psoriasis.org/content/statistics. Accessed July 14, 2020.
- Alexis AF, Blackcloud P. Psoriasis in skin of color: epidemiology, genetics, clinical presentation, and treatment nuances. J Clin Aesthet Dermatol. 2014;7:16-24.
- Rachakonda TD, Schupp CW, Armstrong AW. Psoriasis prevalence among adults in the United States. J Am Acad Dermatol. 2014;70:512-516.
- Goff KL, Karimkhani C, Boyers LN, et al. The global burden of psoriatic skin disease. Br J Dermatol. 2015;172:1665-1668.
- Kaufman BP, Alexis AF. Psoriasis in skin of color: insights into the epidemiology, clinical presentation, genetics, quality-of-life impact, and treatment of psoriasis in non-white racial/ethnic groups. Am J Clin Dermatol. 2018;19:405-423.
- Shah SK, Arthur A, Yang YC, et al. A retrospective study to investigate racial and ethnic variations in the treatment of psoriasis with etanercept. J Drugs Dermatol. 2011;10:866-872.
- Abrouk M, Lee K, Brodsky M, et al. Ethnicity affects the presenting severity of psoriasis. J Am Acad Dermatol. 2017;77:180-182.
- Youssef RM, Mahgoub D, Mashaly HM, et al. Different narrowband UVB dosage regimens in dark skinned psoriatics: a preliminary study. Photodermatol Photoimmunol Photomed. 2008;24:256-259.
- Charrow A, Xia F Di, Joyce C, et al. Diversity in dermatology clinical trials: a systematic review. JAMA Dermatol. 2017;153:193-198.
- Takeshita J, Eriksen WT, Raziano VT, et al. Racial differences in perceptions of psoriasis therapies: implications for racial disparities in psoriasis treatment. J Invest Dermatol. 2019;139:1672-1679.
- Yan D, Afifi L, Jeon C, et al. A cross-sectional study of the distribution of psoriasis subtypes in different ethno-racial groups. Dermatol Online J. 2018;24. pii:13030/qt5z21q4k2.
- Kerr GS, Qaiyumi S, Richards J, et al. Psoriasis and psoriatic arthritis in African-American patients—the need to measure disease burden. Clin Rheumatol. 2015;34:1753-1759.
- Edson-Heredia E, Sterling KL, Alatorre CI, et al. Heterogeneity of response to biologic treatment: perspective for psoriasis. J Invest Dermatol. 2014;134:18-23.
- Yan D, Afifi L, Jeon C, et al. A cross-sectional study of psoriasis triggers among different ethno-racial groups. J Am Acad Dermatol. 2017;77:756-758.
- Bailey RK, Mokonogho J, Kumar A. Racial and ethnic differences in depression: current perspectives. Neuropsychiatr Dis Treat. 2019;15:603-609.
- Jackson C, Maibach H. Ethnic and socioeconomic disparities in dermatology. J Dermatolog Treat. 2016;27:290-291.
- Salam A, Dadzie OE. Dermatology training in the U.K.: does it reflect the changing demographics of our population? Br J Dermatol. 2013;169:1360-1362.
- Nijhawan RI, Jacob SE, Woolery-Lloyd H. Skin of color education in dermatology residency programs: does residency training reflect the changing demographics of the United States? J Am Acad Dermatol. 2008;59:615-618.
- Ogunyemi B, Miller-Monthrope Y. The state of ethnic dermatology in Canada. J Cutan Med Surg. 2017;21:464-466.
- National Psoriasis Foundation. Statistics. https://www.psoriasis.org/content/statistics. Accessed July 14, 2020.
- Alexis AF, Blackcloud P. Psoriasis in skin of color: epidemiology, genetics, clinical presentation, and treatment nuances. J Clin Aesthet Dermatol. 2014;7:16-24.
- Rachakonda TD, Schupp CW, Armstrong AW. Psoriasis prevalence among adults in the United States. J Am Acad Dermatol. 2014;70:512-516.
- Goff KL, Karimkhani C, Boyers LN, et al. The global burden of psoriatic skin disease. Br J Dermatol. 2015;172:1665-1668.
- Kaufman BP, Alexis AF. Psoriasis in skin of color: insights into the epidemiology, clinical presentation, genetics, quality-of-life impact, and treatment of psoriasis in non-white racial/ethnic groups. Am J Clin Dermatol. 2018;19:405-423.
- Shah SK, Arthur A, Yang YC, et al. A retrospective study to investigate racial and ethnic variations in the treatment of psoriasis with etanercept. J Drugs Dermatol. 2011;10:866-872.
- Abrouk M, Lee K, Brodsky M, et al. Ethnicity affects the presenting severity of psoriasis. J Am Acad Dermatol. 2017;77:180-182.
- Youssef RM, Mahgoub D, Mashaly HM, et al. Different narrowband UVB dosage regimens in dark skinned psoriatics: a preliminary study. Photodermatol Photoimmunol Photomed. 2008;24:256-259.
- Charrow A, Xia F Di, Joyce C, et al. Diversity in dermatology clinical trials: a systematic review. JAMA Dermatol. 2017;153:193-198.
- Takeshita J, Eriksen WT, Raziano VT, et al. Racial differences in perceptions of psoriasis therapies: implications for racial disparities in psoriasis treatment. J Invest Dermatol. 2019;139:1672-1679.
- Yan D, Afifi L, Jeon C, et al. A cross-sectional study of the distribution of psoriasis subtypes in different ethno-racial groups. Dermatol Online J. 2018;24. pii:13030/qt5z21q4k2.
- Kerr GS, Qaiyumi S, Richards J, et al. Psoriasis and psoriatic arthritis in African-American patients—the need to measure disease burden. Clin Rheumatol. 2015;34:1753-1759.
- Edson-Heredia E, Sterling KL, Alatorre CI, et al. Heterogeneity of response to biologic treatment: perspective for psoriasis. J Invest Dermatol. 2014;134:18-23.
- Yan D, Afifi L, Jeon C, et al. A cross-sectional study of psoriasis triggers among different ethno-racial groups. J Am Acad Dermatol. 2017;77:756-758.
- Bailey RK, Mokonogho J, Kumar A. Racial and ethnic differences in depression: current perspectives. Neuropsychiatr Dis Treat. 2019;15:603-609.
- Jackson C, Maibach H. Ethnic and socioeconomic disparities in dermatology. J Dermatolog Treat. 2016;27:290-291.
- Salam A, Dadzie OE. Dermatology training in the U.K.: does it reflect the changing demographics of our population? Br J Dermatol. 2013;169:1360-1362.
- Nijhawan RI, Jacob SE, Woolery-Lloyd H. Skin of color education in dermatology residency programs: does residency training reflect the changing demographics of the United States? J Am Acad Dermatol. 2008;59:615-618.
- Ogunyemi B, Miller-Monthrope Y. The state of ethnic dermatology in Canada. J Cutan Med Surg. 2017;21:464-466.
Practice Points
- There are key differences in psoriasis in patients with skin of color, including the morphology, clinical presentation, treatment, and psychosocial impact.
- Recognition and awareness of these differences may normalize the condition for patients, support them seeking medical attention sooner, and better inform them of all possible treatment options.
- Advocating further education on these differences in residency training and continuing medical education programs may help physicians make earlier diagnoses and personalize physician-patient conversations.
Tales of the Pandemic
After learning about coronavirus disease 2019 (COVID-19) on the news, we were all aware that it would eventually affect our lives and our dermatology practices. However, once the COVID-19 pandemic arrived in the United States, we were under a shelter-in-place order, schools were shut, and most businesses were closed within a few weeks.
As dermatologists, we were considered essential workers, and our offices could remain open. However, as the numbers of cases accelerated in New York City—the global epicenter of the pandemic—and we approached our peak, I closed down my practice, except for emergencies.
One of the first medical challenges dermatologists faced in the early days of the COVID-19 pandemic was the proper management of our psoriasis patients. The major concern was that patients on biologics and other immunomodulatory therapies might be at an increased risk for COVID-19 infection and increased morbidity if affected. I received a multitude of telephone calls from patients taking these therapies who expressed high levels of concern and anxiety and were looking for direction as to whether they should continue their medications.
Early on, several of our professional societies provided guidelines regarding the use of systemic immunosuppressive agents during the COVID-19 pandemic. On April 15, 2020, the American Academy of Dermatology (AAD) advised, “Dermatologists must delicately balance the risk of immunosuppression with the risk of disease flare requiring urgent intervention with patient-specific risks.”1 The AAD strongly recommended that patients should not stop their ongoing systemic immunosuppressive therapy without consulting their physicians. The AAD’s guidance provided specific recommendations for the following groups: (1) patients on systemic immunosuppressive agents who have not tested positive or exhibited signs/symptoms of COVID-19, (2) patients on systemic immunosuppressive agents who have tested positive for COVID-19 or exhibit signs/symptoms of COVID-19, (3) patients who have halted systemic immunosuppressive therapy after testing positive for COVID-19 (in whom it recommended physicians could reinitiate treatment), and (4) patients being considered for systemic immunosuppressive agents.1
The National Psoriasis Foundation (NPF) also recognized the need for additional guidelines for health care providers and patients on managing psoriatic disease during the COVID-19 pandemic. In June 2020, the NPF formed a COVID-19 Task Force, which released its own recommendations for adult and pediatric patients with psoriatic disease.2 Similar to the AAD, the NPF COVID-19 Task Force recommended that patients do not stop biologic or oral therapies for psoriasis during the current health crisis, stating the following: “While some uncertainties remain, initial data suggest that the benefit of continuing treatments for psoriatic diseases outweighs the hypothetical risks associated with immune modulating treatment of poor COVID-19–related outcomes for most patients.” Individuals in high-risk groups were advised to consult their health care providers regarding whether they should continue or alter therapy during the pandemic, and the clinical decision would be guided by the specific treatment regimen; the patient’s age, disease characteristics, and underlying medical conditions; or any particular concerns. Additionally, the task force emphasized that patients with psoriatic disease should continue to follow common sense measures to lower the risk of becoming infected with COVID-19, including practicing physical distancing, wearing face coverings in public settings, and washing their hands regularly.2
We remain in the midst of the COVID-19 pandemic with no true guidance as to the future course and impact of the infection. It is important to realize that our understanding of the coronavirus and its impact on our patients is constantly evolving. I encourage all providers to stay current with updates on clinical guidelines. In addition, we should pay attention to the myriad of clinical trials and registries now underway, as they may provide more insight into optimal clinical management in these challenging times.
Most importantly, stay safe!
- American Academy of Dermatology. Guidance on the use of medications during COVID-19 outbreak. https://assets.ctfassets.net/1ny4yoiyrqia/PicgNuD0IpYd9MSOwab47/5e6d85324e7b5aafed45dde0ac4ea21e/Guidance_on_medications_AHTF_approved_April_15.pdf. Updated April 15, 2020. Accessed July 27, 2020.
- National Psoriasis Foundation. NPF forms COVID-19 Task Force. https://www.psoriasis.org/advance/coronavirus. Updated July 7, 2020. Accessed July 27, 2020.
After learning about coronavirus disease 2019 (COVID-19) on the news, we were all aware that it would eventually affect our lives and our dermatology practices. However, once the COVID-19 pandemic arrived in the United States, we were under a shelter-in-place order, schools were shut, and most businesses were closed within a few weeks.
As dermatologists, we were considered essential workers, and our offices could remain open. However, as the numbers of cases accelerated in New York City—the global epicenter of the pandemic—and we approached our peak, I closed down my practice, except for emergencies.
One of the first medical challenges dermatologists faced in the early days of the COVID-19 pandemic was the proper management of our psoriasis patients. The major concern was that patients on biologics and other immunomodulatory therapies might be at an increased risk for COVID-19 infection and increased morbidity if affected. I received a multitude of telephone calls from patients taking these therapies who expressed high levels of concern and anxiety and were looking for direction as to whether they should continue their medications.
Early on, several of our professional societies provided guidelines regarding the use of systemic immunosuppressive agents during the COVID-19 pandemic. On April 15, 2020, the American Academy of Dermatology (AAD) advised, “Dermatologists must delicately balance the risk of immunosuppression with the risk of disease flare requiring urgent intervention with patient-specific risks.”1 The AAD strongly recommended that patients should not stop their ongoing systemic immunosuppressive therapy without consulting their physicians. The AAD’s guidance provided specific recommendations for the following groups: (1) patients on systemic immunosuppressive agents who have not tested positive or exhibited signs/symptoms of COVID-19, (2) patients on systemic immunosuppressive agents who have tested positive for COVID-19 or exhibit signs/symptoms of COVID-19, (3) patients who have halted systemic immunosuppressive therapy after testing positive for COVID-19 (in whom it recommended physicians could reinitiate treatment), and (4) patients being considered for systemic immunosuppressive agents.1
The National Psoriasis Foundation (NPF) also recognized the need for additional guidelines for health care providers and patients on managing psoriatic disease during the COVID-19 pandemic. In June 2020, the NPF formed a COVID-19 Task Force, which released its own recommendations for adult and pediatric patients with psoriatic disease.2 Similar to the AAD, the NPF COVID-19 Task Force recommended that patients do not stop biologic or oral therapies for psoriasis during the current health crisis, stating the following: “While some uncertainties remain, initial data suggest that the benefit of continuing treatments for psoriatic diseases outweighs the hypothetical risks associated with immune modulating treatment of poor COVID-19–related outcomes for most patients.” Individuals in high-risk groups were advised to consult their health care providers regarding whether they should continue or alter therapy during the pandemic, and the clinical decision would be guided by the specific treatment regimen; the patient’s age, disease characteristics, and underlying medical conditions; or any particular concerns. Additionally, the task force emphasized that patients with psoriatic disease should continue to follow common sense measures to lower the risk of becoming infected with COVID-19, including practicing physical distancing, wearing face coverings in public settings, and washing their hands regularly.2
We remain in the midst of the COVID-19 pandemic with no true guidance as to the future course and impact of the infection. It is important to realize that our understanding of the coronavirus and its impact on our patients is constantly evolving. I encourage all providers to stay current with updates on clinical guidelines. In addition, we should pay attention to the myriad of clinical trials and registries now underway, as they may provide more insight into optimal clinical management in these challenging times.
Most importantly, stay safe!
After learning about coronavirus disease 2019 (COVID-19) on the news, we were all aware that it would eventually affect our lives and our dermatology practices. However, once the COVID-19 pandemic arrived in the United States, we were under a shelter-in-place order, schools were shut, and most businesses were closed within a few weeks.
As dermatologists, we were considered essential workers, and our offices could remain open. However, as the numbers of cases accelerated in New York City—the global epicenter of the pandemic—and we approached our peak, I closed down my practice, except for emergencies.
One of the first medical challenges dermatologists faced in the early days of the COVID-19 pandemic was the proper management of our psoriasis patients. The major concern was that patients on biologics and other immunomodulatory therapies might be at an increased risk for COVID-19 infection and increased morbidity if affected. I received a multitude of telephone calls from patients taking these therapies who expressed high levels of concern and anxiety and were looking for direction as to whether they should continue their medications.
Early on, several of our professional societies provided guidelines regarding the use of systemic immunosuppressive agents during the COVID-19 pandemic. On April 15, 2020, the American Academy of Dermatology (AAD) advised, “Dermatologists must delicately balance the risk of immunosuppression with the risk of disease flare requiring urgent intervention with patient-specific risks.”1 The AAD strongly recommended that patients should not stop their ongoing systemic immunosuppressive therapy without consulting their physicians. The AAD’s guidance provided specific recommendations for the following groups: (1) patients on systemic immunosuppressive agents who have not tested positive or exhibited signs/symptoms of COVID-19, (2) patients on systemic immunosuppressive agents who have tested positive for COVID-19 or exhibit signs/symptoms of COVID-19, (3) patients who have halted systemic immunosuppressive therapy after testing positive for COVID-19 (in whom it recommended physicians could reinitiate treatment), and (4) patients being considered for systemic immunosuppressive agents.1
The National Psoriasis Foundation (NPF) also recognized the need for additional guidelines for health care providers and patients on managing psoriatic disease during the COVID-19 pandemic. In June 2020, the NPF formed a COVID-19 Task Force, which released its own recommendations for adult and pediatric patients with psoriatic disease.2 Similar to the AAD, the NPF COVID-19 Task Force recommended that patients do not stop biologic or oral therapies for psoriasis during the current health crisis, stating the following: “While some uncertainties remain, initial data suggest that the benefit of continuing treatments for psoriatic diseases outweighs the hypothetical risks associated with immune modulating treatment of poor COVID-19–related outcomes for most patients.” Individuals in high-risk groups were advised to consult their health care providers regarding whether they should continue or alter therapy during the pandemic, and the clinical decision would be guided by the specific treatment regimen; the patient’s age, disease characteristics, and underlying medical conditions; or any particular concerns. Additionally, the task force emphasized that patients with psoriatic disease should continue to follow common sense measures to lower the risk of becoming infected with COVID-19, including practicing physical distancing, wearing face coverings in public settings, and washing their hands regularly.2
We remain in the midst of the COVID-19 pandemic with no true guidance as to the future course and impact of the infection. It is important to realize that our understanding of the coronavirus and its impact on our patients is constantly evolving. I encourage all providers to stay current with updates on clinical guidelines. In addition, we should pay attention to the myriad of clinical trials and registries now underway, as they may provide more insight into optimal clinical management in these challenging times.
Most importantly, stay safe!
- American Academy of Dermatology. Guidance on the use of medications during COVID-19 outbreak. https://assets.ctfassets.net/1ny4yoiyrqia/PicgNuD0IpYd9MSOwab47/5e6d85324e7b5aafed45dde0ac4ea21e/Guidance_on_medications_AHTF_approved_April_15.pdf. Updated April 15, 2020. Accessed July 27, 2020.
- National Psoriasis Foundation. NPF forms COVID-19 Task Force. https://www.psoriasis.org/advance/coronavirus. Updated July 7, 2020. Accessed July 27, 2020.
- American Academy of Dermatology. Guidance on the use of medications during COVID-19 outbreak. https://assets.ctfassets.net/1ny4yoiyrqia/PicgNuD0IpYd9MSOwab47/5e6d85324e7b5aafed45dde0ac4ea21e/Guidance_on_medications_AHTF_approved_April_15.pdf. Updated April 15, 2020. Accessed July 27, 2020.
- National Psoriasis Foundation. NPF forms COVID-19 Task Force. https://www.psoriasis.org/advance/coronavirus. Updated July 7, 2020. Accessed July 27, 2020.
Management of Psoriasis With Biologics in Clinical Practice: An Update for 2020
The advent of biologic therapy over the last 2 decades has transformed the treatment of psoriasis; patients who either are not good candidates for or have an inadequate response to traditional treatments (topicals and/or phototherapy) now have numerous options for treatment.1 Patients burdened by extensive disease, recurrent flares, and stubborn treatment areas are ideal candidates for biologics. There are 11 biologics approved by the US Food and Drug Administration (FDA)(Table) for treating moderate to severe plaque psoriasis as supported by grade A evidence. The FDA has authorized 1 new biologic—risankizumab—since the joint guidelines from the American Academy of Dermatology and National Psoriasis Foundation were released for the treatment of psoriasis with biologics.2 This article aims to address updates on recent clinical trial findings (April 2019 to April 2020) regarding biologic therapy initiation and maintenance for adult patients. Prescribers should use this update as guidance for determining the appropriate biologic class based on patient characteristics and for approaching biologic-experienced patients with refractory psoriasis. This update also may serve as a reference for the recommended dosing regimens of the 11 approved biologics.
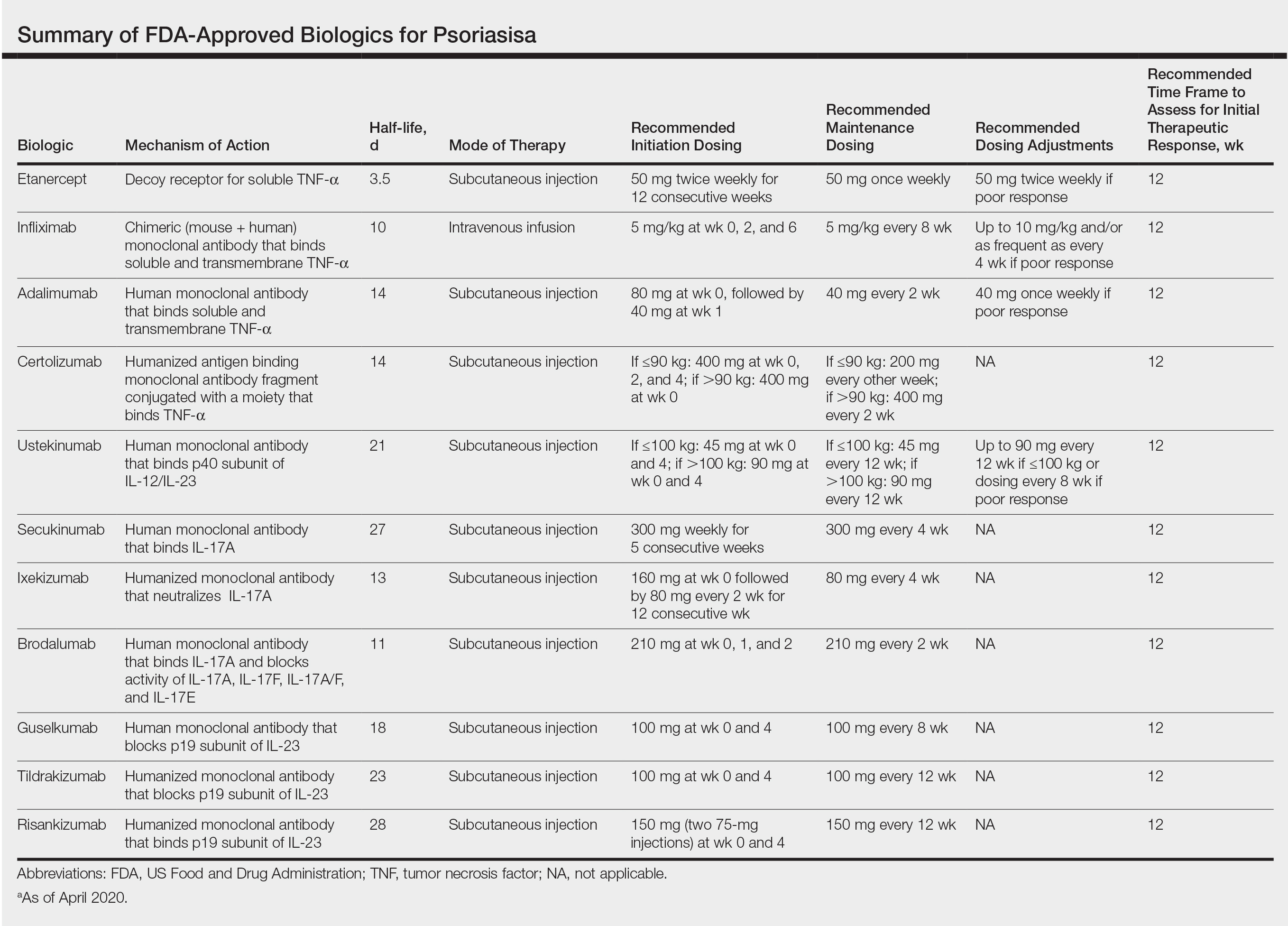
Using Risankizumab
Risankizumab is a new biologic that selectively targets the IL-23 pathway by binding the p19 subunit of IL-23. It was approved by the FDA in April 2019. Two recent studies have demonstrated the efficacy of risankizumab in disease management.3,4
IMMvent was a double-blind, 2-part, phase 3, randomized controlled trial (RCT) of participants 18 years and older (N=605) with moderate to severe psoriasis (with or without psoriatic arthritis) across 11 countries.3 Inclusion criteria consisted of psoriasis involving at least 10% of the body surface area (BSA), absolute psoriasis area and severity index (PASI) score of 12 or higher, and static physician global assessment (sPGA) score of 3 or higher. Prior biologic treatment did not preclude study entry (excluding risankizumab or adalimumab), and nearly 40% of participants previously had been on a different biologic. Notably, this trial allowed for inclusion of patients with prior malignancy (>5 years prior) and patients who tested positive for exposure to tuberculosis (TB) but were not shown to have active TB (provided appropriate treatment for latent TB was started). Study participants identified as white (81%), Asian (14%), black/African American (4%), or other ethnicity (1%). Part A involved administration of 150 mg risankizumab (n=301) at weeks 0 and 4 or 80 mg adalimumab (n=304) loading dose at week 0 followed by 40 mg at week 1 and 40 mg every other week thereafter until the end of week 15. At week 16 there was a significant difference in proportion of participants achieving 90% or more improvement (PASI-90) with risankizumab (72%) vs adalimumab (47%)(P<.0001) and achieving an sPGA score of 0 or 1 (clear or almost clear) with risankizumab (84%) vs adalimumab (60%)(P<.0001). In part B (weeks 16–44), adalimumab immediate responder (PASI ≥50 to PASI <90) participants were re-randomized to continue adalimumab 40 mg every other week (starting from week 17 and stopping at week 44) or switch to 150 mg risankizumab administered at weeks 16, 20, and 32. Patients taking risankizumab in part A continued the drug, administered at weeks 16 and 28. At week 44, there was a significant difference in percentage of participants achieving PASI-90 with risankizumab (66%) vs adalimumab (21%)(P<.0001).3
IMMhance was another double-blind phase 3 RCT with 2 parts that assessed the clinical efficacy of risankizumab compared to placebo in patients 18 years or older (N=507) across 9 countries with the same inclusion criteria for patients as IMMvent.4 Part A involved administration of 150 mg risankizumab (n=407) or placebo (n=100) at weeks 0 and 4 using a 4:1 random allocation ratio. At week 16, regardless of initial treatment, all participants received 150 mg risankizumab. Treatment results at week 16 showed a significant difference in percentage of participants achieving PASI-90 with risankizumab (73.2%) vs placebo (2.0%)(P<.001) and sPGA score of 0 or 1 with risankizumab (83.5%) vs placebo (7.0%)(P<.001). Furthermore, in part B (weeks 16–104), at week 28 participants on risankizumab with an sPGA score of 0 or 1 were randomized with a 1:2 allocation ratio to continue 150 mg risankizumab or switch to placebo to produce a treatment withdrawal effect. Part B results showed a significant difference in the proportion of participants achieving an sPGA score of 0 or 1 with risankizumab (87.4%) vs placebo (61.3%)(P<.001) at week 52 and at week 104 with risankizumab (81.1%) vs placebo (7.1%)(P<.001). Risankizumab was well tolerated, with the most common adverse events (AEs) being nasopharyngitis (23.4%), upper respiratory tract infection (15.4%), and headache (6.8%). Serious AEs included cancer (2.6%; 2.2 events per 100 patient-years), hepatic events (4.6%) including hepatic cirrhosis (0.2%), and serious infections (1.8%; 1.4 events per 100 patient-years).4
Overall, the strengths of risankizumab with regard to its clinical efficacy and utility in biologic-experienced patients were confirmed in these studies. The inclusion of patients with prior treated malignancy and positive TB tests also was more in line with what one might encounter with real-world practice and, as such, provided valuable data to help aid treatment decisions. These 2 studies provided valuable evidence for the therapeutic benefit and relatively mild safety profile of risankizumab in treatment of moderate to severe psoriasis for patients with and without prior biologic therapy.
Choosing a Biologic
Refractory psoriasis involves nonresponse (primary failure) or return of disease symptoms after initial improvement (secondary failure) with a biologic. Selecting a biologic for patients who have experienced prior biologic failure is difficult. It is still unknown whether it is more efficacious for patients to try a same-class drug or a biologic targeting a different inflammatory pathway or cytokine. Studies have shown mixed results regarding how to manage patients with biologic failure, with both approaches demonstrating positive outcomes.
One analysis of the Corrona Psoriasis Registry included 144 patients, the majority of whom (89.8%) were biologic experienced, who began secukinumab treatment and returned for a 6-month follow-up (5–9 months).5 Patients enrolled in the registry were 18 years or older, had been diagnosed with psoriasis by a dermatologist, and initiated or switched an FDA-approved systemic agent or biologic within the last 12 months. Of biologic-experienced participants, 37.7% had used 3 or more biologics. More than half of included participants were either male (55%) or obese (53.4%). Comorbidities included hypertension (43.2%), hyperlipidemia (33.9%), anxiety (20.3%), diabetes mellitus (15.3%), cardiovascular disease (14.4%), and depression (13.6%). After 6 months of treatment, there was significant improvement in the involvement of BSA (mean difference, −12.1), investigator global assessment score (−1.5), dermatology life quality index (DLQI)(−4.8), pain (−23.2), itch (−30.8), fatigue (−8.8), and work productivity (−9.2)(P<.01). Secukinumab therapy displayed notable reduction in symptom severity in this population with difficult-to-treat psoriasis. Its relative success in this cohort provides support for its use in treating patients who have failed other classes of biologics.5
Evidence supporting reduction of pruritus and pain with secukinumab also was notable. The CLEAR phase 3 RCT involved participants treated with 300 mg secukinumab every week for the first 4 weeks and then every 4 weeks thereafter for 48 weeks (n=312), up to 100 weeks (n=277).6 Participants had complete relief of pain (score 0), itching, and scaling at week 16 (69.4%, 49.7%, and 61.2%, respectively), week 52 (67.1%, 48.9%, and 53.3%, respectively), and week 104 (70.9%, 47.4%, and 54.8%, respectively). Reported AEs included candida infections (7.2%), malignant or unspecified tumors (1.5%), and neutropenia (<1%).6
Researchers investigated intraclass switching to brodalumab with prior failure of IL-17 inhibitors. An open-label study involved participants (n=39) with prior failure with secukinumab or ixekizumab therapy.7 Participants were administered 210 mg brodalumab with standard dosing at weeks 0, 1, and 2, and then every 2 weeks thereafter. At week 16, 69% of participants achieved PASI-75, 44% achieved PASI-90, 28% achieved PASI-100, and 62% achieved an sPGA score of 0 or 1. The authors attributed the relative success of brodalumab compared to prior anti–IL-17 agents to inhibition of the IL-17 receptor with brodalumab rather than the IL-17A ligand.7 Brodalumab may be a useful alternative biologic for patients with nonresponse to and secondary failure with biologics, including the IL-17A inhibitors.
Recent findings support effective skin clearance and improved symptom management with ixekizumab and ustekinumab. Of note, ixekizumab was reported to provide rapid improvement in skin lesions and quality of life to a greater extent than guselkumab.
The IXORA-R double-blinded RCT compared the clinical benefit of participants 18 years and older taking standard approved dosages of ixekizumab (n=520) or guselkumab (n=507).8 Patients were included if they had plaque psoriasis for at least 6 months before baseline, an sPGA score of at least 3, PASI score of 12 or higher, 10% or greater BSA, no prior IL-17 inhibitor failure, no use of IL-23 p19 inhibitors, and no use of any biologic within the specified period prior to baseline. At week 12, ixekizumab showed superior clinical improvement measured by the proportion of participants achieving complete skin clearance (ie, PASI-100)(41%) compared to guselkumab (25%)(P<.001). There were more participants taking ixekizumab who reported DLQI of 0 or 1 (no impact of disease on quality of life)(34%) compared to guselkumab (21%)(P<.001) as early on as week 4. The most common AE was upper respiratory tract infection (7%) in both groups. The risk of treatment-emergent AEs (56%), discontinuation because of AEs (2%), and serious AEs (3%) were comparable in both groups. The number of injection-site reactions was higher with ixekizumab (13%) vs guselkumab (3%). The authors concluded that ixekizumab offers the ability to provide rapid relief of symptoms, which is associated with improved DLQI.8
Response to ustekinumab therapy was assessed in a patient cohort enrolled in the Corrona Psoriasis Registry. This study involved 178 participants 18 years and older with psoriasis involvement of 3% or greater BSA who were treated with ustekinumab.9 By their 6-month follow-up visit, 55.6% of participants achieved adequate treatment response (BSA improving to <3% or 75% from enrollment). Increasing patient age was significantly associated with decreased likelihood of achieving a response (odds ratio, 0.981 [95% confidence interval, 0.962-0.999]; P=.049). Ustekinumab is a practical option for psoriasis treatment that seems to yield better results in younger patients.9 This evidence reveals that increased patient age is a characteristic that may contribute to poor treatment response and should be considered when choosing the best fit for biologic therapy.
Final Thoughts
Using evidence-based interventions to treat patients is the cornerstone of ethical and high-quality medical care. This guide sought to provide relevant updates in a variety of both comparator and pivotal trials, with the goal of summarizing clinically relevant information that may be extracted from these trials to guide patient care. It is not an exhaustive review but may be utilized as a reference tool to fine-tune selection criteria in choosing 1 of 11 biologics for the treatment of psoriasis.
- Pithadia DJ, Reynolds KA, Lee EB, et al. Translating the 2019 AAD-NPF Guidelines of Care for the Management of Psoriasis With Biologics to clinical practice. Cutis. 2019;104(suppl 2):12-16.
- Menter A, Strober BE, Kaplan DH, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics [published online February 13, 2019]. J Am Acad Dermatol. 2019;80:1029-1072.
- Reich K, Gooderham M, Thaçi D, et al. Risankizumab compared with adalimumab in patients with moderate-to-severe plaque psoriasis (IMMvent): a randomised, double-blind, active-comparator-controlled phase 3 trial. Lancet. 2019;394:576-586.
- Blauvelt A, Leonardi CL, Gooderham M, et al. Efficacy and safety of continuous risankizumab therapy vs treatment withdrawal in patients with moderate to severe plaque psoriasis: a phase 3 randomized clinical trial. JAMA Dermatol. 2020;156:649-658.
- Strober BE, Germino R, Guana A, et al. US real-world effectiveness of secukinumab for the treatment of psoriasis: 6-month analysis from the Corrona Psoriasis Registry. J Dermatolog Treat. 2020;31:333-341.
- Thaçi D, Puig L, Reich K, et al. Secukinumab demonstrates sustained efficacy in clearing skin and improving patient-reported outcomes in patients with moderate-to-severe psoriasis through 2 years of treatment: results from the CLEAR study. J Am Acad Dermatol. 2019;81:1405-1409.
- Kimmel G, Chima M, Kim HJ, et al. Brodalumab in the treatment of moderate to severe psoriasis in patients when previous anti-interleukin 17A therapies have failed. J Am Acad Dermatol. 2019;81:857-859.
- Blauvelt A, Papp K, Gottlieb A, et al. A head‐to‐head comparison of ixekizumab vs. guselkumab in patients with moderate‐to‐severe plaque psoriasis: 12‐week efficacy, safety and speed of response from a randomized, double‐blinded trial. Br J Dermatol. 2020;182:1348-1358.
- Van Voorhees AS, Mason MA, Harrold LR, et al. Characterization of insufficient responders to ustekinumab in patients with moderate-to-severe psoriasis in the US Corrona Psoriasis Registry [published online February 27, 2020]. J Dermatolog Treat. doi:10.1080/09546634.2020.1720586.
The advent of biologic therapy over the last 2 decades has transformed the treatment of psoriasis; patients who either are not good candidates for or have an inadequate response to traditional treatments (topicals and/or phototherapy) now have numerous options for treatment.1 Patients burdened by extensive disease, recurrent flares, and stubborn treatment areas are ideal candidates for biologics. There are 11 biologics approved by the US Food and Drug Administration (FDA)(Table) for treating moderate to severe plaque psoriasis as supported by grade A evidence. The FDA has authorized 1 new biologic—risankizumab—since the joint guidelines from the American Academy of Dermatology and National Psoriasis Foundation were released for the treatment of psoriasis with biologics.2 This article aims to address updates on recent clinical trial findings (April 2019 to April 2020) regarding biologic therapy initiation and maintenance for adult patients. Prescribers should use this update as guidance for determining the appropriate biologic class based on patient characteristics and for approaching biologic-experienced patients with refractory psoriasis. This update also may serve as a reference for the recommended dosing regimens of the 11 approved biologics.

Using Risankizumab
Risankizumab is a new biologic that selectively targets the IL-23 pathway by binding the p19 subunit of IL-23. It was approved by the FDA in April 2019. Two recent studies have demonstrated the efficacy of risankizumab in disease management.3,4
IMMvent was a double-blind, 2-part, phase 3, randomized controlled trial (RCT) of participants 18 years and older (N=605) with moderate to severe psoriasis (with or without psoriatic arthritis) across 11 countries.3 Inclusion criteria consisted of psoriasis involving at least 10% of the body surface area (BSA), absolute psoriasis area and severity index (PASI) score of 12 or higher, and static physician global assessment (sPGA) score of 3 or higher. Prior biologic treatment did not preclude study entry (excluding risankizumab or adalimumab), and nearly 40% of participants previously had been on a different biologic. Notably, this trial allowed for inclusion of patients with prior malignancy (>5 years prior) and patients who tested positive for exposure to tuberculosis (TB) but were not shown to have active TB (provided appropriate treatment for latent TB was started). Study participants identified as white (81%), Asian (14%), black/African American (4%), or other ethnicity (1%). Part A involved administration of 150 mg risankizumab (n=301) at weeks 0 and 4 or 80 mg adalimumab (n=304) loading dose at week 0 followed by 40 mg at week 1 and 40 mg every other week thereafter until the end of week 15. At week 16 there was a significant difference in proportion of participants achieving 90% or more improvement (PASI-90) with risankizumab (72%) vs adalimumab (47%)(P<.0001) and achieving an sPGA score of 0 or 1 (clear or almost clear) with risankizumab (84%) vs adalimumab (60%)(P<.0001). In part B (weeks 16–44), adalimumab immediate responder (PASI ≥50 to PASI <90) participants were re-randomized to continue adalimumab 40 mg every other week (starting from week 17 and stopping at week 44) or switch to 150 mg risankizumab administered at weeks 16, 20, and 32. Patients taking risankizumab in part A continued the drug, administered at weeks 16 and 28. At week 44, there was a significant difference in percentage of participants achieving PASI-90 with risankizumab (66%) vs adalimumab (21%)(P<.0001).3
IMMhance was another double-blind phase 3 RCT with 2 parts that assessed the clinical efficacy of risankizumab compared to placebo in patients 18 years or older (N=507) across 9 countries with the same inclusion criteria for patients as IMMvent.4 Part A involved administration of 150 mg risankizumab (n=407) or placebo (n=100) at weeks 0 and 4 using a 4:1 random allocation ratio. At week 16, regardless of initial treatment, all participants received 150 mg risankizumab. Treatment results at week 16 showed a significant difference in percentage of participants achieving PASI-90 with risankizumab (73.2%) vs placebo (2.0%)(P<.001) and sPGA score of 0 or 1 with risankizumab (83.5%) vs placebo (7.0%)(P<.001). Furthermore, in part B (weeks 16–104), at week 28 participants on risankizumab with an sPGA score of 0 or 1 were randomized with a 1:2 allocation ratio to continue 150 mg risankizumab or switch to placebo to produce a treatment withdrawal effect. Part B results showed a significant difference in the proportion of participants achieving an sPGA score of 0 or 1 with risankizumab (87.4%) vs placebo (61.3%)(P<.001) at week 52 and at week 104 with risankizumab (81.1%) vs placebo (7.1%)(P<.001). Risankizumab was well tolerated, with the most common adverse events (AEs) being nasopharyngitis (23.4%), upper respiratory tract infection (15.4%), and headache (6.8%). Serious AEs included cancer (2.6%; 2.2 events per 100 patient-years), hepatic events (4.6%) including hepatic cirrhosis (0.2%), and serious infections (1.8%; 1.4 events per 100 patient-years).4
Overall, the strengths of risankizumab with regard to its clinical efficacy and utility in biologic-experienced patients were confirmed in these studies. The inclusion of patients with prior treated malignancy and positive TB tests also was more in line with what one might encounter with real-world practice and, as such, provided valuable data to help aid treatment decisions. These 2 studies provided valuable evidence for the therapeutic benefit and relatively mild safety profile of risankizumab in treatment of moderate to severe psoriasis for patients with and without prior biologic therapy.
Choosing a Biologic
Refractory psoriasis involves nonresponse (primary failure) or return of disease symptoms after initial improvement (secondary failure) with a biologic. Selecting a biologic for patients who have experienced prior biologic failure is difficult. It is still unknown whether it is more efficacious for patients to try a same-class drug or a biologic targeting a different inflammatory pathway or cytokine. Studies have shown mixed results regarding how to manage patients with biologic failure, with both approaches demonstrating positive outcomes.
One analysis of the Corrona Psoriasis Registry included 144 patients, the majority of whom (89.8%) were biologic experienced, who began secukinumab treatment and returned for a 6-month follow-up (5–9 months).5 Patients enrolled in the registry were 18 years or older, had been diagnosed with psoriasis by a dermatologist, and initiated or switched an FDA-approved systemic agent or biologic within the last 12 months. Of biologic-experienced participants, 37.7% had used 3 or more biologics. More than half of included participants were either male (55%) or obese (53.4%). Comorbidities included hypertension (43.2%), hyperlipidemia (33.9%), anxiety (20.3%), diabetes mellitus (15.3%), cardiovascular disease (14.4%), and depression (13.6%). After 6 months of treatment, there was significant improvement in the involvement of BSA (mean difference, −12.1), investigator global assessment score (−1.5), dermatology life quality index (DLQI)(−4.8), pain (−23.2), itch (−30.8), fatigue (−8.8), and work productivity (−9.2)(P<.01). Secukinumab therapy displayed notable reduction in symptom severity in this population with difficult-to-treat psoriasis. Its relative success in this cohort provides support for its use in treating patients who have failed other classes of biologics.5
Evidence supporting reduction of pruritus and pain with secukinumab also was notable. The CLEAR phase 3 RCT involved participants treated with 300 mg secukinumab every week for the first 4 weeks and then every 4 weeks thereafter for 48 weeks (n=312), up to 100 weeks (n=277).6 Participants had complete relief of pain (score 0), itching, and scaling at week 16 (69.4%, 49.7%, and 61.2%, respectively), week 52 (67.1%, 48.9%, and 53.3%, respectively), and week 104 (70.9%, 47.4%, and 54.8%, respectively). Reported AEs included candida infections (7.2%), malignant or unspecified tumors (1.5%), and neutropenia (<1%).6
Researchers investigated intraclass switching to brodalumab with prior failure of IL-17 inhibitors. An open-label study involved participants (n=39) with prior failure with secukinumab or ixekizumab therapy.7 Participants were administered 210 mg brodalumab with standard dosing at weeks 0, 1, and 2, and then every 2 weeks thereafter. At week 16, 69% of participants achieved PASI-75, 44% achieved PASI-90, 28% achieved PASI-100, and 62% achieved an sPGA score of 0 or 1. The authors attributed the relative success of brodalumab compared to prior anti–IL-17 agents to inhibition of the IL-17 receptor with brodalumab rather than the IL-17A ligand.7 Brodalumab may be a useful alternative biologic for patients with nonresponse to and secondary failure with biologics, including the IL-17A inhibitors.
Recent findings support effective skin clearance and improved symptom management with ixekizumab and ustekinumab. Of note, ixekizumab was reported to provide rapid improvement in skin lesions and quality of life to a greater extent than guselkumab.
The IXORA-R double-blinded RCT compared the clinical benefit of participants 18 years and older taking standard approved dosages of ixekizumab (n=520) or guselkumab (n=507).8 Patients were included if they had plaque psoriasis for at least 6 months before baseline, an sPGA score of at least 3, PASI score of 12 or higher, 10% or greater BSA, no prior IL-17 inhibitor failure, no use of IL-23 p19 inhibitors, and no use of any biologic within the specified period prior to baseline. At week 12, ixekizumab showed superior clinical improvement measured by the proportion of participants achieving complete skin clearance (ie, PASI-100)(41%) compared to guselkumab (25%)(P<.001). There were more participants taking ixekizumab who reported DLQI of 0 or 1 (no impact of disease on quality of life)(34%) compared to guselkumab (21%)(P<.001) as early on as week 4. The most common AE was upper respiratory tract infection (7%) in both groups. The risk of treatment-emergent AEs (56%), discontinuation because of AEs (2%), and serious AEs (3%) were comparable in both groups. The number of injection-site reactions was higher with ixekizumab (13%) vs guselkumab (3%). The authors concluded that ixekizumab offers the ability to provide rapid relief of symptoms, which is associated with improved DLQI.8
Response to ustekinumab therapy was assessed in a patient cohort enrolled in the Corrona Psoriasis Registry. This study involved 178 participants 18 years and older with psoriasis involvement of 3% or greater BSA who were treated with ustekinumab.9 By their 6-month follow-up visit, 55.6% of participants achieved adequate treatment response (BSA improving to <3% or 75% from enrollment). Increasing patient age was significantly associated with decreased likelihood of achieving a response (odds ratio, 0.981 [95% confidence interval, 0.962-0.999]; P=.049). Ustekinumab is a practical option for psoriasis treatment that seems to yield better results in younger patients.9 This evidence reveals that increased patient age is a characteristic that may contribute to poor treatment response and should be considered when choosing the best fit for biologic therapy.
Final Thoughts
Using evidence-based interventions to treat patients is the cornerstone of ethical and high-quality medical care. This guide sought to provide relevant updates in a variety of both comparator and pivotal trials, with the goal of summarizing clinically relevant information that may be extracted from these trials to guide patient care. It is not an exhaustive review but may be utilized as a reference tool to fine-tune selection criteria in choosing 1 of 11 biologics for the treatment of psoriasis.
The advent of biologic therapy over the last 2 decades has transformed the treatment of psoriasis; patients who either are not good candidates for or have an inadequate response to traditional treatments (topicals and/or phototherapy) now have numerous options for treatment.1 Patients burdened by extensive disease, recurrent flares, and stubborn treatment areas are ideal candidates for biologics. There are 11 biologics approved by the US Food and Drug Administration (FDA)(Table) for treating moderate to severe plaque psoriasis as supported by grade A evidence. The FDA has authorized 1 new biologic—risankizumab—since the joint guidelines from the American Academy of Dermatology and National Psoriasis Foundation were released for the treatment of psoriasis with biologics.2 This article aims to address updates on recent clinical trial findings (April 2019 to April 2020) regarding biologic therapy initiation and maintenance for adult patients. Prescribers should use this update as guidance for determining the appropriate biologic class based on patient characteristics and for approaching biologic-experienced patients with refractory psoriasis. This update also may serve as a reference for the recommended dosing regimens of the 11 approved biologics.

Using Risankizumab
Risankizumab is a new biologic that selectively targets the IL-23 pathway by binding the p19 subunit of IL-23. It was approved by the FDA in April 2019. Two recent studies have demonstrated the efficacy of risankizumab in disease management.3,4
IMMvent was a double-blind, 2-part, phase 3, randomized controlled trial (RCT) of participants 18 years and older (N=605) with moderate to severe psoriasis (with or without psoriatic arthritis) across 11 countries.3 Inclusion criteria consisted of psoriasis involving at least 10% of the body surface area (BSA), absolute psoriasis area and severity index (PASI) score of 12 or higher, and static physician global assessment (sPGA) score of 3 or higher. Prior biologic treatment did not preclude study entry (excluding risankizumab or adalimumab), and nearly 40% of participants previously had been on a different biologic. Notably, this trial allowed for inclusion of patients with prior malignancy (>5 years prior) and patients who tested positive for exposure to tuberculosis (TB) but were not shown to have active TB (provided appropriate treatment for latent TB was started). Study participants identified as white (81%), Asian (14%), black/African American (4%), or other ethnicity (1%). Part A involved administration of 150 mg risankizumab (n=301) at weeks 0 and 4 or 80 mg adalimumab (n=304) loading dose at week 0 followed by 40 mg at week 1 and 40 mg every other week thereafter until the end of week 15. At week 16 there was a significant difference in proportion of participants achieving 90% or more improvement (PASI-90) with risankizumab (72%) vs adalimumab (47%)(P<.0001) and achieving an sPGA score of 0 or 1 (clear or almost clear) with risankizumab (84%) vs adalimumab (60%)(P<.0001). In part B (weeks 16–44), adalimumab immediate responder (PASI ≥50 to PASI <90) participants were re-randomized to continue adalimumab 40 mg every other week (starting from week 17 and stopping at week 44) or switch to 150 mg risankizumab administered at weeks 16, 20, and 32. Patients taking risankizumab in part A continued the drug, administered at weeks 16 and 28. At week 44, there was a significant difference in percentage of participants achieving PASI-90 with risankizumab (66%) vs adalimumab (21%)(P<.0001).3
IMMhance was another double-blind phase 3 RCT with 2 parts that assessed the clinical efficacy of risankizumab compared to placebo in patients 18 years or older (N=507) across 9 countries with the same inclusion criteria for patients as IMMvent.4 Part A involved administration of 150 mg risankizumab (n=407) or placebo (n=100) at weeks 0 and 4 using a 4:1 random allocation ratio. At week 16, regardless of initial treatment, all participants received 150 mg risankizumab. Treatment results at week 16 showed a significant difference in percentage of participants achieving PASI-90 with risankizumab (73.2%) vs placebo (2.0%)(P<.001) and sPGA score of 0 or 1 with risankizumab (83.5%) vs placebo (7.0%)(P<.001). Furthermore, in part B (weeks 16–104), at week 28 participants on risankizumab with an sPGA score of 0 or 1 were randomized with a 1:2 allocation ratio to continue 150 mg risankizumab or switch to placebo to produce a treatment withdrawal effect. Part B results showed a significant difference in the proportion of participants achieving an sPGA score of 0 or 1 with risankizumab (87.4%) vs placebo (61.3%)(P<.001) at week 52 and at week 104 with risankizumab (81.1%) vs placebo (7.1%)(P<.001). Risankizumab was well tolerated, with the most common adverse events (AEs) being nasopharyngitis (23.4%), upper respiratory tract infection (15.4%), and headache (6.8%). Serious AEs included cancer (2.6%; 2.2 events per 100 patient-years), hepatic events (4.6%) including hepatic cirrhosis (0.2%), and serious infections (1.8%; 1.4 events per 100 patient-years).4
Overall, the strengths of risankizumab with regard to its clinical efficacy and utility in biologic-experienced patients were confirmed in these studies. The inclusion of patients with prior treated malignancy and positive TB tests also was more in line with what one might encounter with real-world practice and, as such, provided valuable data to help aid treatment decisions. These 2 studies provided valuable evidence for the therapeutic benefit and relatively mild safety profile of risankizumab in treatment of moderate to severe psoriasis for patients with and without prior biologic therapy.
Choosing a Biologic
Refractory psoriasis involves nonresponse (primary failure) or return of disease symptoms after initial improvement (secondary failure) with a biologic. Selecting a biologic for patients who have experienced prior biologic failure is difficult. It is still unknown whether it is more efficacious for patients to try a same-class drug or a biologic targeting a different inflammatory pathway or cytokine. Studies have shown mixed results regarding how to manage patients with biologic failure, with both approaches demonstrating positive outcomes.
One analysis of the Corrona Psoriasis Registry included 144 patients, the majority of whom (89.8%) were biologic experienced, who began secukinumab treatment and returned for a 6-month follow-up (5–9 months).5 Patients enrolled in the registry were 18 years or older, had been diagnosed with psoriasis by a dermatologist, and initiated or switched an FDA-approved systemic agent or biologic within the last 12 months. Of biologic-experienced participants, 37.7% had used 3 or more biologics. More than half of included participants were either male (55%) or obese (53.4%). Comorbidities included hypertension (43.2%), hyperlipidemia (33.9%), anxiety (20.3%), diabetes mellitus (15.3%), cardiovascular disease (14.4%), and depression (13.6%). After 6 months of treatment, there was significant improvement in the involvement of BSA (mean difference, −12.1), investigator global assessment score (−1.5), dermatology life quality index (DLQI)(−4.8), pain (−23.2), itch (−30.8), fatigue (−8.8), and work productivity (−9.2)(P<.01). Secukinumab therapy displayed notable reduction in symptom severity in this population with difficult-to-treat psoriasis. Its relative success in this cohort provides support for its use in treating patients who have failed other classes of biologics.5
Evidence supporting reduction of pruritus and pain with secukinumab also was notable. The CLEAR phase 3 RCT involved participants treated with 300 mg secukinumab every week for the first 4 weeks and then every 4 weeks thereafter for 48 weeks (n=312), up to 100 weeks (n=277).6 Participants had complete relief of pain (score 0), itching, and scaling at week 16 (69.4%, 49.7%, and 61.2%, respectively), week 52 (67.1%, 48.9%, and 53.3%, respectively), and week 104 (70.9%, 47.4%, and 54.8%, respectively). Reported AEs included candida infections (7.2%), malignant or unspecified tumors (1.5%), and neutropenia (<1%).6
Researchers investigated intraclass switching to brodalumab with prior failure of IL-17 inhibitors. An open-label study involved participants (n=39) with prior failure with secukinumab or ixekizumab therapy.7 Participants were administered 210 mg brodalumab with standard dosing at weeks 0, 1, and 2, and then every 2 weeks thereafter. At week 16, 69% of participants achieved PASI-75, 44% achieved PASI-90, 28% achieved PASI-100, and 62% achieved an sPGA score of 0 or 1. The authors attributed the relative success of brodalumab compared to prior anti–IL-17 agents to inhibition of the IL-17 receptor with brodalumab rather than the IL-17A ligand.7 Brodalumab may be a useful alternative biologic for patients with nonresponse to and secondary failure with biologics, including the IL-17A inhibitors.
Recent findings support effective skin clearance and improved symptom management with ixekizumab and ustekinumab. Of note, ixekizumab was reported to provide rapid improvement in skin lesions and quality of life to a greater extent than guselkumab.
The IXORA-R double-blinded RCT compared the clinical benefit of participants 18 years and older taking standard approved dosages of ixekizumab (n=520) or guselkumab (n=507).8 Patients were included if they had plaque psoriasis for at least 6 months before baseline, an sPGA score of at least 3, PASI score of 12 or higher, 10% or greater BSA, no prior IL-17 inhibitor failure, no use of IL-23 p19 inhibitors, and no use of any biologic within the specified period prior to baseline. At week 12, ixekizumab showed superior clinical improvement measured by the proportion of participants achieving complete skin clearance (ie, PASI-100)(41%) compared to guselkumab (25%)(P<.001). There were more participants taking ixekizumab who reported DLQI of 0 or 1 (no impact of disease on quality of life)(34%) compared to guselkumab (21%)(P<.001) as early on as week 4. The most common AE was upper respiratory tract infection (7%) in both groups. The risk of treatment-emergent AEs (56%), discontinuation because of AEs (2%), and serious AEs (3%) were comparable in both groups. The number of injection-site reactions was higher with ixekizumab (13%) vs guselkumab (3%). The authors concluded that ixekizumab offers the ability to provide rapid relief of symptoms, which is associated with improved DLQI.8
Response to ustekinumab therapy was assessed in a patient cohort enrolled in the Corrona Psoriasis Registry. This study involved 178 participants 18 years and older with psoriasis involvement of 3% or greater BSA who were treated with ustekinumab.9 By their 6-month follow-up visit, 55.6% of participants achieved adequate treatment response (BSA improving to <3% or 75% from enrollment). Increasing patient age was significantly associated with decreased likelihood of achieving a response (odds ratio, 0.981 [95% confidence interval, 0.962-0.999]; P=.049). Ustekinumab is a practical option for psoriasis treatment that seems to yield better results in younger patients.9 This evidence reveals that increased patient age is a characteristic that may contribute to poor treatment response and should be considered when choosing the best fit for biologic therapy.
Final Thoughts
Using evidence-based interventions to treat patients is the cornerstone of ethical and high-quality medical care. This guide sought to provide relevant updates in a variety of both comparator and pivotal trials, with the goal of summarizing clinically relevant information that may be extracted from these trials to guide patient care. It is not an exhaustive review but may be utilized as a reference tool to fine-tune selection criteria in choosing 1 of 11 biologics for the treatment of psoriasis.
- Pithadia DJ, Reynolds KA, Lee EB, et al. Translating the 2019 AAD-NPF Guidelines of Care for the Management of Psoriasis With Biologics to clinical practice. Cutis. 2019;104(suppl 2):12-16.
- Menter A, Strober BE, Kaplan DH, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics [published online February 13, 2019]. J Am Acad Dermatol. 2019;80:1029-1072.
- Reich K, Gooderham M, Thaçi D, et al. Risankizumab compared with adalimumab in patients with moderate-to-severe plaque psoriasis (IMMvent): a randomised, double-blind, active-comparator-controlled phase 3 trial. Lancet. 2019;394:576-586.
- Blauvelt A, Leonardi CL, Gooderham M, et al. Efficacy and safety of continuous risankizumab therapy vs treatment withdrawal in patients with moderate to severe plaque psoriasis: a phase 3 randomized clinical trial. JAMA Dermatol. 2020;156:649-658.
- Strober BE, Germino R, Guana A, et al. US real-world effectiveness of secukinumab for the treatment of psoriasis: 6-month analysis from the Corrona Psoriasis Registry. J Dermatolog Treat. 2020;31:333-341.
- Thaçi D, Puig L, Reich K, et al. Secukinumab demonstrates sustained efficacy in clearing skin and improving patient-reported outcomes in patients with moderate-to-severe psoriasis through 2 years of treatment: results from the CLEAR study. J Am Acad Dermatol. 2019;81:1405-1409.
- Kimmel G, Chima M, Kim HJ, et al. Brodalumab in the treatment of moderate to severe psoriasis in patients when previous anti-interleukin 17A therapies have failed. J Am Acad Dermatol. 2019;81:857-859.
- Blauvelt A, Papp K, Gottlieb A, et al. A head‐to‐head comparison of ixekizumab vs. guselkumab in patients with moderate‐to‐severe plaque psoriasis: 12‐week efficacy, safety and speed of response from a randomized, double‐blinded trial. Br J Dermatol. 2020;182:1348-1358.
- Van Voorhees AS, Mason MA, Harrold LR, et al. Characterization of insufficient responders to ustekinumab in patients with moderate-to-severe psoriasis in the US Corrona Psoriasis Registry [published online February 27, 2020]. J Dermatolog Treat. doi:10.1080/09546634.2020.1720586.
- Pithadia DJ, Reynolds KA, Lee EB, et al. Translating the 2019 AAD-NPF Guidelines of Care for the Management of Psoriasis With Biologics to clinical practice. Cutis. 2019;104(suppl 2):12-16.
- Menter A, Strober BE, Kaplan DH, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics [published online February 13, 2019]. J Am Acad Dermatol. 2019;80:1029-1072.
- Reich K, Gooderham M, Thaçi D, et al. Risankizumab compared with adalimumab in patients with moderate-to-severe plaque psoriasis (IMMvent): a randomised, double-blind, active-comparator-controlled phase 3 trial. Lancet. 2019;394:576-586.
- Blauvelt A, Leonardi CL, Gooderham M, et al. Efficacy and safety of continuous risankizumab therapy vs treatment withdrawal in patients with moderate to severe plaque psoriasis: a phase 3 randomized clinical trial. JAMA Dermatol. 2020;156:649-658.
- Strober BE, Germino R, Guana A, et al. US real-world effectiveness of secukinumab for the treatment of psoriasis: 6-month analysis from the Corrona Psoriasis Registry. J Dermatolog Treat. 2020;31:333-341.
- Thaçi D, Puig L, Reich K, et al. Secukinumab demonstrates sustained efficacy in clearing skin and improving patient-reported outcomes in patients with moderate-to-severe psoriasis through 2 years of treatment: results from the CLEAR study. J Am Acad Dermatol. 2019;81:1405-1409.
- Kimmel G, Chima M, Kim HJ, et al. Brodalumab in the treatment of moderate to severe psoriasis in patients when previous anti-interleukin 17A therapies have failed. J Am Acad Dermatol. 2019;81:857-859.
- Blauvelt A, Papp K, Gottlieb A, et al. A head‐to‐head comparison of ixekizumab vs. guselkumab in patients with moderate‐to‐severe plaque psoriasis: 12‐week efficacy, safety and speed of response from a randomized, double‐blinded trial. Br J Dermatol. 2020;182:1348-1358.
- Van Voorhees AS, Mason MA, Harrold LR, et al. Characterization of insufficient responders to ustekinumab in patients with moderate-to-severe psoriasis in the US Corrona Psoriasis Registry [published online February 27, 2020]. J Dermatolog Treat. doi:10.1080/09546634.2020.1720586.
Practice Points
- Inform patients about current data guiding treatment from clinical trials of biologics.
- Explain to patients that finding the treatment that is the best fit for them may require trial and error, as everyone responds to treatments differently.
- Consult with patients about misconceptions and potential fears about biologics and what the protocol is for monitoring safety during treatment.
Treatment of Psoriasis in Pregnancy
Historically, there have been limited data available on the management of psoriasis in pregnancy. The most comprehensive discussion of treatment guidelines is from 2012.1 In the interim, many biologics have been approved for treating psoriasis, with slow accumulation of pregnancy safety data. The 2019 American Academy of Dermatology–National Psoriasis Foundation guidelines on biologics for psoriasis contain updated information but also highlight the paucity of pregnancy safety data.2 This gap is in part a consequence of the exclusion and disenrollment of pregnant women from clinical trials.3 Additionally, lack of detection through registries contributes; pregnancy capture in registries is low compared to the expected number of pregnancies estimated from US Census data.4 Despite these shortcomings, psoriasis patients who are already pregnant or are considering becoming pregnant frequently are encountered in practice and may need treatment. This article reviews the evidence on commonly used treatments for psoriasis in pregnancy.
Background
For many patients, psoriasis improves during pregnancy5,6 and becomes worse postpartum. In a prospective study, most patients reported improvement in pregnancy corresponding to a significant decrease in
In addition to the maternal disease state, the issue of pregnancy outcomes is paramount. In the inflammatory bowel disease and rheumatology literature, it is established that uncontrolled disease is associated with poorer pregnancy outcomes.8-10 Guidelines vary among societies on the use of biologics in pregnancy generally (eTable 11,2,9,11-24), but some societies recommend systemic agents to achieve disease control during pregnancy.9,25
Assessing the potential interplay between disease severity and outcomes in pregnant women with psoriasis is further complicated by the slowly growing body of literature demonstrating that women with psoriasis have more comorbidities26 and worse pregnancy outcomes.27,28 Pregnant psoriasis patients are more likely to smoke, have depression, and be overweight or obese prior to pregnancy and are less likely to take prenatal vitamins.26 They also have an increased risk for cesarean birth, gestational diabetes, gestational hypertension, and preeclampsia.28 In contrast to these prior studies, a systematic review revealed no risk for adverse outcomes in pregnant women with psoriasis.29
Assessment of Treatments for Psoriasis in Pregnancy
In light of these issues, treatment of psoriasis during pregnancy should be assessed from several vantage points. Of note, the US Food and Drug Administration changed its classification scheme in 2015 to a more narrative format called the Pregnancy and Lactation Labeling Rule.30 Prior classifications, however, provide a reasonable starting point for categorizing the safety of drugs (Table31). Importantly, time of exposure to systemic agents also matters; first-trimester exposure is more likely to affect embryogenesis, whereas second- and third-trimester exposures are more prone to affect other aspects of fetal growth. eTable 2 provides data on the use of oral and topical medications to treat psoriasis in pregnancy.1,8,22,32-45

Topical Agents
Topical steroids are largely understood to be reasonable treatment options, though consideration of potency, formulation, area of application, and use of occlusion is important.1,46 Risk for orofacial cleft has been noted with first-trimester topical steroid exposure, though a 2015 Cochrane review update determined that the relative risk of this association was not significantly elevated.32
The impact of topical calcipotriene and salicylic acid has not been studied in human pregnancies,1 but systemic absorption can occur for both. There is potential for vitamin D toxicity with calcipotriene46; consequently, use during pregnancy is not recommended.1,46 Some authors recommend against topical salicylic acid in pregnancy; others report that limited exposure is permissible.47 In fact, as salicylic acid commonly is found in over-the-counter acne products, many women of childbearing potential likely have quotidian exposure.
Preterm delivery and low birthweight have been reported with oral tacrolimus; however, risk with topical tacrolimus is thought to be low1 because the molecular size likely prohibits notable absorption.47 Evidence for the use of anthralin and coal tar also is scarce. First-trimester coal tar use should be avoided; subsequent use in pregnancy should be restricted given concern for adverse outcomes.1
Phototherapy
Broadband or narrowband UVB therapy is recommended as second-line therapy in pregnancy. No cases of fetal risk or premature delivery associated with UVB therapy were found in our search.1 Phototherapy can exacerbate melasma47 and decrease folate levels48; as such, some authors recommend folate supplementation in females of childbearing age who are being treated with phototherapy.49 Psoralen, used in psoralen plus UVA therapy, is mutagenic and therefore contraindicated in pregnancy.1
Oral Medications
Both methotrexate, which is a teratogen, abortifacient, and mutagen,1 and systemic retinoids, which are teratogens, are contraindicated in pregnancy.1,47 Acitretin labeling recommends avoiding pregnancy for 3 years posttreatment50 because alcohol intake prolongs the medication’s half-life.22
Apremilast use is not documented in pregnant psoriasis patients51; an ongoing registry of the Organization of Tetralogy Information Specialists has not reported publicly to date.52 Animal studies of apremilast have documented dose-related decreased birthweight and fetal loss.22
Safety data for systemic steroids, used infrequently in psoriasis, are not well established. First-trimester prednisone exposure has been associated with prematurity, low birthweight, and congenital abnormalities.38 A separate evaluation of 1047 children exposed to betamethasone in utero failed to demonstrate significant change in birthweight or head circumference. However, repeat antenatal corticosteroid exposure was associated with attention problems at 2 years of age.39
Data regarding cyclosporine use, derived primarily from organ transplant recipients, suggest elevated risk for prematurity and low birthweight.53,54 A meta-analysis demonstrated that organ transplant recipients taking cyclosporine had a nonsignificantly elevated odds ratio for congenital malformations, prematurity, and low birthweight.42 Cyclosporine use for psoriasis in pregnancy is not well described; in a study, rates of prematurity and low birthweight were both 21%.43 Limited data are available for Janus kinase inhibitors, none of which are approved for psoriasis, though clinical trials in psoriasis and psoriatic arthritis are underway (ClinicalTrials.gov identifiers NCT04246372, NCT03104374, NCT03104400).
Biologics and Small-Molecule Inhibitors
Limited data on biologics in pregnancy exist25 (eTable 3). Placental transport of IgG antibodies, including biologics, increases throughout pregnancy, especially in the third trimester.82 Infants of mothers treated with a biologic with potential for placental transfer are therefore considered by some authors to be immunosuppressed during the first months of life.2
Looking globally across biologics used for psoriasis, limited safety data are encouraging. In a review of PSOLAR (Psoriasis Longitudinal Assessment and Registry), 83 pregnancies with biologic exposure resulted in 59 live births (71%); 18 spontaneous abortions (22%); 6 induced abortions (7%); no congenital abnormalities; and 7 reports of neonatal problems, including respiratory issues, ABO blood group mismatch, hospitalization, and opioid withdrawal.83
Use of tumor necrosis factor (TNF) inhibitors in pregnancy has the most data25 and is considered a reasonable treatment option. Historically, there was concern about the risk for VACTERL syndrome (vertebral defects, anal atresia, cardiac defects, tracheoesophageal fistula, renal anomalies, limb abnormalities) with exposure to a TNF inhibitor,25,84-86 but further reports have alleviated these concerns. Active transplacental transport occurs for adalimumab, infliximab, and golimumab,87 but given structural differences, transport of certolizumab and etanercept is substantially less.88,89 In the CRIB study of placental transfer of certolizumab from mother to infant (N=14), pharmacokinetic data demonstrated no quantifiable certolizumab levels in 13 infants and minimal levels in 1 infant at birth.88 There are fewer data available on the use of other biologics in pregnancy, but for those in which active placental transport is relevant, similar concerns (ie, immunosuppression) might arise (eTable 3).
Concern over biologics largely involves risk for newborn immunosuppression. A case report detailed a Crohn disease patient treated with infliximab who gave birth to an infant who died of disseminated bacille Calmette-Guérin infection at 4.5 months after receiving the vaccine at 3 months.90 This case underscores the importance of delaying live vaccination in infants born to mothers who were treated with a biologic during pregnancy. Authors have provided various data on how long to avoid vaccination; some state as long as 1 year.91
In pregnant females with inflammatory bowel disease treated with a biologic, no correlation was observed among maternal, placental, and infant serum biologic levels and neonatal infection. However, an association between preterm birth and the level of the biologic in maternal and placental (but not infant) serum and preterm birth was observed.92
In another report from the same registry, combination therapy with a TNF inhibitor and another immunomodulator led to an increased risk for infection in infants at 12 months of age, compared to infants exposed to monotherapy89 or exposed to neither agent.93 A strategy to circumvent this potential problem is to avoid treatment with actively transported molecules in the third trimester.
Conclusion
Limited data exist to guide providers who are treating pregnant women with psoriasis. Our understanding of treatment of psoriasis in pregnancy is limited as a consequence of regulations surrounding clinical trials and inadequate detection of pregnancies in registries. Further efforts are necessary to better understand the relationship between psoriasis and pregnancy and how to manage pregnant women with psoriasis.
- Bae YS, Van Voorhees AS, Hsu S, et al. Review of treatment options for psoriasis in pregnant or lactating women: from the medical board of the National Psoriasis Foundation. J Am Acad Dermatol. 2012;67:459-477.
- Menter A, Strober BE, Kaplan DH, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol. 2019;80:1029-1072.
- Flood KS, Porter ML, Kimball AB. Use of biologics in pregnancy: limitations stemming from clinical trials and registry experience. J Eur Acad Dermatol Venereol. 2019;33:E276-E277.
- Horn EJ, Chambers CD, Menter A, et al. Pregnancy outcomes in psoriasis: why do we know so little? J Am Acad Dermatol. 2009;61:E5-E8.
- Raychaudhuri SP, Navare T, Gross J, et al. Clinical course of psoriasis during pregnancy. Int J Dermatol. 2003;42:518-520.
- Boyd AS, Morris LF, Phillips CM, et al. Psoriasis and pregnancy: hormone and immune system interaction. Int J Dermatol. 1996;35:169-172.
- Murase JE, Chan KK, Garite TJ, et al. Hormonal effect on psoriasis in pregnancy and post partum. Arch Dermatol. 2005;14:601-606.
- Götestam Skorpen C, Hoeltzenbein M, Tincani A, et al. The EULAR points to consider for use of antirheumatic drugs before pregnancy, and during pregnancy and lactation. Ann Rheum Dis. 2016;75:795-810.
- Nguyen GC, Seow CH, Maxwell C, et al. The Toronto consensus statements for the management of inflammatory bowel disease in pregnancy. Gastroenterology. 2016;150:734-757.
- Wise J. Rheumatic diseases should be actively treated in pregnancy, new guidelines say. BMJ. 2016;532:i312.
- Puig L, Carrascosa JM, Carretero G, et al. Spanish evidence-based guidelines on the treatment of psoriasis with biologic agents, 2013. part 1: on efficacy and choice of treatment. Actas Dermosifiliogr. 2013;104:694-709.
- Girolomoni G, Altomore G, Ayala F, et al. Differential management of mild-to-severe psoriasis with biologic drugs: an Italian Delphi consensus expert panel. J Dermatolog Treat. 2015;26:128-133.
- Yeung J, Gooderham MJ, Grewal P, et al. Management of plaque psoriasis with biologic therapies in women of child-bearing potential consensus paper. J Cutan Med Surg. 2020;24:3S-14S.
- Louthrenoo W, Kasitanon N, Kathamort W, et al. 2016 updated Thai Rheumatism Association Recommendations for the use of biologic and targeted synthetic disease-modifying anti-rheumatic drugs in patients with rheumatoid arthritis. Int J Rheum Dis. 2017;20:1166-1184.
- Flint J, Panchal S, Hurrell A, et al. BSR and BHPR guideline on prescribing drugs in pregnancy and breastfeeding—part I: standard and biologic disease modifying anti-rheumatic drugs and corticosteroids. Rheumatology (Oxford). 2016;55:1693-1697.
- van der Woude CJ, Ardizzone S, Bengtson MB, et al. The second European evidenced-based consensus on reproduction and pregnancy in inflammatory bowel disease. J Crohns Colitis. 2015;9:107-124.
- Orlando A, Armuzz A, Papi C, et al. The Italian Society of Gastroenterology (SIGE) and the Italian Group for the study of Inflammatory Bowel Disease (IG-IBD) Clinical Practice Guidelines: the use of tumor necrosis factor-alpha antagonist therapy in inflammatory bowel disease. Dig Liver Dis. 2011;43:1-20.
- Puchner A, Grochenig HP, Sautner J, et al. Immunosuppressives and biologics during pregnancy and lactation. Wien Klin Wochenschr. 2019;131:29-44.
- ACOG Committee opinion no. 776: immune modulating therapies in pregnancy and lactation. Obstet Gynecol. 2019;133:E287-E297.
- Lamb CA, Kennedy NA, Raine T, et al. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut. 2019;68:S1-S106.
- Goëb V, Ardizzone M, Arnard L, et al. Recommendations for using TNF-α antagonists and French Clinical Practice Guidelines endorsed by the French National Authority for Health. Joint Bone Spine. 2013;80:574-581.
- Rademaker M, Agnew K, Andrews M, et al. Psoriasis in those planning a family, pregnant or breast-feeding. the Australasian Psoriasis Collaboration. Australas J Dermatol. 2018;59:86-100.
- Mahadevan U, Robinson C, Bernasko N, et al. Inflammatory bowel disease in pregnancy clinical care pathway: a report from the American Gastroenterological Association IBD Parenthood Project Working Group. Gastroenterology. 2019;156:1508-1524.
- Mahadevan U, Cucchiara S, Hyam JS, et al. The London position statement of the World Congress of Gastroenterology on biological therapy for IBD with the European Crohn’s and Colitis Organisation: pregnancy and pediatrics. Am J Gastroenterol. 2011;106:214-223.
- Porter ML, Lockwood SJ, Kimball AB. Update on biologic safety for patients with psoriasis during pregnancy. Int J Womens Dermatol. 2017;3:21-25.
- Bandoli G, Johnson DL, Jones KL, et al. Potentially modifiable risk factors for adverse pregnancy outcomes in women with psoriasis. Br J Dermatol. 2010;163:334-339.
- Lima XT, Janakiraman V, Hughes MD, et al. The impact of psoriasis on pregnancy outcomes. J Invest Dermatol. 2012;132:85-91.
- Bröms G, Haerskjold A, Granath F, et al. Effect of maternal psoriasis on pregnancy and birth outcomes: a population-based cohort study from Denmark and Sweden. Acta Derm Venereol. 2018;98:728-734.
- Bobotsis R, Gulliver WP, Monaghan K, et al. Psoriasis and adverse pregnancy outcomes: a systematic review of observational studies. Br J Dermatol. 2016;175:464-472.
- Blattner CM, Danesh M, Safaee M, et al. Understanding the new FDA pregnancy and lactation labeling rules. Int J Womens Dermatol. 2016;2:5-7.
- Pernia S, DeMaagd G. The new pregnancy and lactation labeling rule. P T. 2016;4:713-715.
- Chi C-C, Wang S-H, Wojnarowska F, et al. Safety of topical corticosteroids in pregnancy. Cochrane Database Syst Rev. 2015:CD007346.
- Chi CC, Wang SH, Kirtschig G. Safety of topical corticosteroids in pregnancy. JAMA Dermatol. 2016;152:934-935.
- Dovonex (calcipotriene) Cream, 0.005% [package insert]. Dublin, Ireland: Leo Laboratories, Ltd; March 2015.
- Franssen ME, van der Wilt GJ, de Jong PC, et al. A retrospective study of the teratogenicity of dermatological coal tar products. Acta Derm Venereol. 1999;79:390-391.
- Garbis H, Elefant E, Bertolotti E, et al. Pregnancy outcome after periconceptional and first-trimester exposure to methoxsalen photochemotherapy. Arch Dermatol. 1995;131:492-493.
- Horizon Pharma USA. RAYOS (prednisone). https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/202020s000lbl.pdf https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/202020s000lbl.pdf.
- Park-Wyllie L, Mazzotta P, Pastuszak A, et al. Birth defects after maternal exposure to corticosteroids: prospective cohort study and meta-analysis of epidemiological studies. Teratology. 2000;62:385-392.
- Crowther CA, Doyle LW, Haslam RR, et al. Outcomes at 2 years of age after repeat doses of antenatal corticosteroids. N Engl J Med. 2007;357:1179-1189.
- Palmsten K, Rolland M, Herbert MF, et al. Patterns of prednisone use during pregnancy in women with rheumatoid arthritis: daily and cumulative dose. Pharmacoepidemiol Drug Saf. 2018;27:430-438.
- Groth K, Brännström M, Mölne J, et al. Cyclosporine A exposure during pregnancy in mice: effects on reproductive performance in mothers and offspring. Hum Reprod. 2010;25:697-704.
- Bar Oz B, Hackman R, Einarson T, et al. Pregnancy outcome after cyclosporine therapy during pregnancy: a meta-analysis. Transplantation. 2001;71:1051-1055.
- Paziana K, Del Monaco M, Cardonick E, et al. Ciclosporin use during pregnancy. Drug Saf. 2013;36:279-294.
- Lamarque V, Leleu MF, Monka C, et al. Analysis of 629 pregnancy outcomes in transplant recipients treated with Sandimmun. Transplant Proc. 1997;29:2480.
- Otezla (apremilast) tablets, for oral use [package insert]. Summit, NJ: Celgene Corporation; June 2017. http://www.accessdata.fda.gov/drugsatfda_docs/label/2017/205437s006lbl.pdf. Accessed July 8, 2020.
- Kurizky PS, de Castro Ferreira C, Nogueira LSC, et al. Treatment of psoriasis and psoriatic arthritis during pregnancy and breastfeeding. An Bras Dermatol. 2015;90:367-375.
- Murase JE, Heller MM, Butler DC. Safety of dermatologic medications in pregnancy and lactation. J Am Acad Dermatol. 2014;70:401.e1-401.e4.
- El-Saie LT, Rabie AR, Kamel MI, et al. Effect of narrowband ultraviolet B phototherapy on serum folic acid levels in patients with psoriasis. Lasers Med Sci. 2011;26:481-485.
- Murase JE, Koo JY, Berger TG. Narrowband ultraviolet B phototherapy influences serum folate levels in patients with vitiligo. J Am Acad Dermatol. 2010;62:710-711.
- Soriatane (acitretin) capsules [package insert]. Morrisville, NC: Stiefel Laboratories, Inc; April 2011. http://www.accessdata.fda.gov/drugsatfda_docs/label/2011/019821s018mg.pdf. Accessed July 8, 2020.
- Kaushik SB, Lebwohl MG. Psoriasis: which therapy for which patient: focus on special populations and chronic infections. J Am Acad Dermatol. 2019;80:43-53.
- Help us better understand the effects of Otezla in pregnancy. MotherToBaby website. https://mothertobaby.org/ongoing-study/otezla/. Accessed July 8, 2020.
- Bangsgaard N, Rørbye C, Skov L. Treating psoriasis during pregnancy: safety and efficacy of treatments. Am J Clin Dermatol. 2015;16:389-398.
- Tyler K. Dermatologic therapy in pregnancy. Clin Obstet Gynecol. 2015;58:112-118.
- Luu M, Benzenine E, Doret M, et al. Continuous anti–TNF-α use throughout pregnancy: possible complications for the mother but not for the fetus. a retrospective cohort on the French National Health Insurance Database (EVASION). Am J Gastroenterol. 2018;113:1669-1677.
- Bröms G, Granath F, Ekbom A, et al. Low risk of birth defects for infants whose mothers are treated with anti-tumor necrosis factor agents during pregnancy. Clin Gastroenterol Hepatol. 2016;14:234-241.
- Mirdamadi K, Salinas T, Vali R, et al. Meta-analysis of pregnancy outcomes after exposure to TNF-α inhibitors during pregnancy for the treatment of arthritic diseases. J Popul Ther Clin Pharmacol. 2018;25:E53-E56.
- Shihab Z, Yeomans ND, De Cruz P. Anti-tumour necrosis factor α therapies and inflammatory bowel disease pregnancy outcomes: a meta-analysis. J Crohns Colitis. 2016;10:979-988.
- Bröms G, Kieler H, Ekbom A, et al. Anti-TNF treatment during pregnancy and birth outcomes: a population-based study from Denmark, Finland, and Sweden. Pharmacoepidemiol Drug Saf. 2020;29:316-327.
- Diav-Citrin O, Otcheretianski-Volodarsky A, Shechtman S, et al. Pregnancy outcome following gestational exposure to TNF-alpha-inhibitors: a prospective, comparative, observational study. Reprod Toxicol. 2014;43:78-84.
- FDA determinations for pregnant and nursing women. National Psoriasis Foundation website. http://www.psoriasis.org/pregnancy/fda-determinations. Accessed July 8, 2020.
- Østensen M. Safety issues of biologics in pregnant patients with rheumatic diseases. Ann N Y Acad Sci. 2014;1317:32-38.
- Chambers CD, Johnson DL, Luo Y, et al. Pregnancy outcome in women treated with adalimumab for the treatment of rheumatoid arthritis: the OTIS Autoimmune Diseases in Pregnancy Project. Arthritis Rheum. 2012;64:2466.
- Clowse ME, Wolf DC, Forger F, et al. Pregnancy outcomes after exposure to certolizumab pegol: updated results from a pharmacovigilance safety database. Arthritis Rheumatol. 2018;70:1399-1407.
- Carman WJ, Accortt NA, Anthony MS, et al. Pregnancy and infant outcomes including major congenital malformations among women with chronic inflammatory arthritis or psoriasis, with and without etanercept use. Pharmacoepidemiol Drug Saf. 2017;26:1109-1118.
- Janssen. SIMPONI (golilumab). https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/125289s0064lbl.pdf.
- Yurkon K, Guo CY, Harrison D, et al. Pregnancy outcomes in women with dermatologic conditions exposed to infliximab. J Am Acad Dermatol. 2014;70:AB179.
- Watson N, Wu K, Farr P, et al. Ustekinumab exposure during conception and pregnancy in patients with chronic plaque psoriasis: a case series of 10 pregnancies. Br J Dermatol. 2019;180:195-196.
- Naureckas S, Slater J, Gearhart N, et al. Pregnancy outcomes in women with psoriasis and psoriatic arthritis exposed to ustekinumab. J Am Acad Dermatol. 2016;74:AB264.
- Haycraft K, DiRuggiero D, Rozzo SJ, et al. Outcomes of pregnancies from tildrakizumab phases I to III clinical development program. J Clin Aesthet Dermatol. 2019;12:S27-S28.
- Tremfya (guselkumab) injection, for subcutaneous use [package insert]. Horsham, PA: Janssen Biotech, Inc; July 2017. http://www.accessdata.fda.gov/drugsatfda_docs/label/2017/761061s000lbl.pdf. Accessed Juy 8, 2020.
- Skyrizi (risankizumab-rzaa) injection, for subcutaneous use [package insert]. Northi Chicago, IL; April 2019. http://www.accessdata.fda.gov/drugsatfda_docs/label/2019/761105s000lbl.pdf. Accessed July 8, 2020.
- Siliq (brodalumab) injection, for subcutaneous use [package insert]. Bridgewater, NJ: Valeant Pharmaceuticals North America LLC; February 2017. http://www.accessdata.fda.gov/drugsatfda_docs/label/2017/761032lbl.pdf. Accessed July 8, 2020.
- Feldman S, Pangallo B, Xu W, et al. Ixekizumab and pregnancy outcome. J Am Acad Dermatol. 2017;76:AB419.
- Clarke DO, Hilbish KG, Waters DG, et al. Assessment of ixekizumab, an interleukin-17A monoclonal antibody, for potential effects on reproduction and development, including immune system function, in cynomolgus monkeys. Reprod Toxicol. 2015;58:160-173.
- Warren RB, Reich K, Langley RG, et al. Secukinumab in pregnancy: outcomes in psoriasis, psoriatic arthritis and ankylosing spondylitis from the global safety database. Br J Dermatol. 2018;179:1205-1207.
- Nardin C, Colas M, Curie V, et al. Pregnancy after tubal sterilization in a woman treated with biologics for severe psoriasis. Dermatol Ther (Heidelb). 2018;8:323-326.
- Xeljanz (tofacitinib) tablets for oral administration [package insert]. New York, NY: Pfizer; November 2012. http://www.accessdata.fda.gov/drugsatfda_docs/label/2012/203214s000lbl.pdf. Accessed July 8, 2020.
- Pfizer. Xeljanz (tofacitinib). https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/203214s018lbl.pdf.
- Mahadevan U, Dubinsky M, Su C, et al. Outcomes of pregnancies with maternal/paternal exposure in the tofacitinib safety databases for ulcerative colitis. Inflamm Bowel Dis. 2018;24:2494-2500.
- Clowse ME, Feldman SR, Isaacs JD, et al. Pregnancy outcomes in the tofacitinib safety databases for rheumatoid arthritis and psoriasis. Drug Saf. 2016;39:755-762.
- Malek A, Sager R, Kuhn P, et al. Evolution of maternofetal transport of immunoglobulins during human pregnancy. Am J Reprod Immunol. 1996;36:248-255.
- Kimball AB, Crow JA, Ridley K, et al. Pregnancy outcomes in women with moderate to severe psoriasis: the PSOLAR experience. J Am Acad Dermatol. 2014;70(suppl 1):AB179.
- Carter JD, Valeriano J, Vasey FB. Tumor necrosis factor-alpha inhibition and VATER association: a causal relationship. J Rheumatol. 2006;33:1014-1017.
- Carter JD, Ladhani A, Ricca LR, et al. A safety assessment of tumor necrosis factor antagonists during pregnancy: a review of the Food and Drug Administration database. J Rheumatol. 2009;36:635-641.
- Koren G, Inoue M. Do tumor necrosis factor inhibitors cause malformations in humans? J Rheumatol. 2009;36:465-466.
- Johansen C, Jimenez-Solem E, Haerskjold A, et al. The use and safety of TNF inhibitors during pregnancy in women with psoriasis: a review. Int J Mol Sci. 2018;19:E1349.
- Mariette X, Forger F, Abraham B, et al. Lack of placental transfer of certolizumab pegol during pregnancy: results from CRIB, a prospective, postmarketing, pharmacokinetic study. Ann Rheum Dis. 2018;77:228-233.
- Mahadevan U, Wolf DC, Dubinsky M, et al. Placental transfer of anti-tumor necrosis factor agents in pregnant patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2013;11:286-292.
- Cheent K, Nolan J, Sharig S, et al. Case report: fatal case of disseminated BCG infection in an infant born to a mother taking infliximab for Crohn’s disease. J Crohns Colitis. 2010;4:603-605.
- Julsgaard M, Christensen LA, Gibson PR, et al. Concentrations of adalimumab and infliximab in mothers and newborns, and effects on infection. Gastroenterology. 2016;151:110-119.
- Mahadevan U, Martin C, Kane SV, et al. Do infant serum levels of biologic agents at birth correlate with risk of adverse outcomes? results from the PIANO registry. Gastroenterology. 2016;150:S91-S92.
- Mahadevan U, Martin CF, Sandler RS, et al. PIANO: a 1000 patient prospective registry of pregnancy outcomes in women with IBD exposed to immunomodulators and biologic therapy [AGA abstract 865]. Gastroenterology. 2012;142:S-149.
Historically, there have been limited data available on the management of psoriasis in pregnancy. The most comprehensive discussion of treatment guidelines is from 2012.1 In the interim, many biologics have been approved for treating psoriasis, with slow accumulation of pregnancy safety data. The 2019 American Academy of Dermatology–National Psoriasis Foundation guidelines on biologics for psoriasis contain updated information but also highlight the paucity of pregnancy safety data.2 This gap is in part a consequence of the exclusion and disenrollment of pregnant women from clinical trials.3 Additionally, lack of detection through registries contributes; pregnancy capture in registries is low compared to the expected number of pregnancies estimated from US Census data.4 Despite these shortcomings, psoriasis patients who are already pregnant or are considering becoming pregnant frequently are encountered in practice and may need treatment. This article reviews the evidence on commonly used treatments for psoriasis in pregnancy.
Background
For many patients, psoriasis improves during pregnancy5,6 and becomes worse postpartum. In a prospective study, most patients reported improvement in pregnancy corresponding to a significant decrease in
In addition to the maternal disease state, the issue of pregnancy outcomes is paramount. In the inflammatory bowel disease and rheumatology literature, it is established that uncontrolled disease is associated with poorer pregnancy outcomes.8-10 Guidelines vary among societies on the use of biologics in pregnancy generally (eTable 11,2,9,11-24), but some societies recommend systemic agents to achieve disease control during pregnancy.9,25
Assessing the potential interplay between disease severity and outcomes in pregnant women with psoriasis is further complicated by the slowly growing body of literature demonstrating that women with psoriasis have more comorbidities26 and worse pregnancy outcomes.27,28 Pregnant psoriasis patients are more likely to smoke, have depression, and be overweight or obese prior to pregnancy and are less likely to take prenatal vitamins.26 They also have an increased risk for cesarean birth, gestational diabetes, gestational hypertension, and preeclampsia.28 In contrast to these prior studies, a systematic review revealed no risk for adverse outcomes in pregnant women with psoriasis.29
Assessment of Treatments for Psoriasis in Pregnancy
In light of these issues, treatment of psoriasis during pregnancy should be assessed from several vantage points. Of note, the US Food and Drug Administration changed its classification scheme in 2015 to a more narrative format called the Pregnancy and Lactation Labeling Rule.30 Prior classifications, however, provide a reasonable starting point for categorizing the safety of drugs (Table31). Importantly, time of exposure to systemic agents also matters; first-trimester exposure is more likely to affect embryogenesis, whereas second- and third-trimester exposures are more prone to affect other aspects of fetal growth. eTable 2 provides data on the use of oral and topical medications to treat psoriasis in pregnancy.1,8,22,32-45

Topical Agents
Topical steroids are largely understood to be reasonable treatment options, though consideration of potency, formulation, area of application, and use of occlusion is important.1,46 Risk for orofacial cleft has been noted with first-trimester topical steroid exposure, though a 2015 Cochrane review update determined that the relative risk of this association was not significantly elevated.32
The impact of topical calcipotriene and salicylic acid has not been studied in human pregnancies,1 but systemic absorption can occur for both. There is potential for vitamin D toxicity with calcipotriene46; consequently, use during pregnancy is not recommended.1,46 Some authors recommend against topical salicylic acid in pregnancy; others report that limited exposure is permissible.47 In fact, as salicylic acid commonly is found in over-the-counter acne products, many women of childbearing potential likely have quotidian exposure.
Preterm delivery and low birthweight have been reported with oral tacrolimus; however, risk with topical tacrolimus is thought to be low1 because the molecular size likely prohibits notable absorption.47 Evidence for the use of anthralin and coal tar also is scarce. First-trimester coal tar use should be avoided; subsequent use in pregnancy should be restricted given concern for adverse outcomes.1
Phototherapy
Broadband or narrowband UVB therapy is recommended as second-line therapy in pregnancy. No cases of fetal risk or premature delivery associated with UVB therapy were found in our search.1 Phototherapy can exacerbate melasma47 and decrease folate levels48; as such, some authors recommend folate supplementation in females of childbearing age who are being treated with phototherapy.49 Psoralen, used in psoralen plus UVA therapy, is mutagenic and therefore contraindicated in pregnancy.1
Oral Medications
Both methotrexate, which is a teratogen, abortifacient, and mutagen,1 and systemic retinoids, which are teratogens, are contraindicated in pregnancy.1,47 Acitretin labeling recommends avoiding pregnancy for 3 years posttreatment50 because alcohol intake prolongs the medication’s half-life.22
Apremilast use is not documented in pregnant psoriasis patients51; an ongoing registry of the Organization of Tetralogy Information Specialists has not reported publicly to date.52 Animal studies of apremilast have documented dose-related decreased birthweight and fetal loss.22
Safety data for systemic steroids, used infrequently in psoriasis, are not well established. First-trimester prednisone exposure has been associated with prematurity, low birthweight, and congenital abnormalities.38 A separate evaluation of 1047 children exposed to betamethasone in utero failed to demonstrate significant change in birthweight or head circumference. However, repeat antenatal corticosteroid exposure was associated with attention problems at 2 years of age.39
Data regarding cyclosporine use, derived primarily from organ transplant recipients, suggest elevated risk for prematurity and low birthweight.53,54 A meta-analysis demonstrated that organ transplant recipients taking cyclosporine had a nonsignificantly elevated odds ratio for congenital malformations, prematurity, and low birthweight.42 Cyclosporine use for psoriasis in pregnancy is not well described; in a study, rates of prematurity and low birthweight were both 21%.43 Limited data are available for Janus kinase inhibitors, none of which are approved for psoriasis, though clinical trials in psoriasis and psoriatic arthritis are underway (ClinicalTrials.gov identifiers NCT04246372, NCT03104374, NCT03104400).
Biologics and Small-Molecule Inhibitors
Limited data on biologics in pregnancy exist25 (eTable 3). Placental transport of IgG antibodies, including biologics, increases throughout pregnancy, especially in the third trimester.82 Infants of mothers treated with a biologic with potential for placental transfer are therefore considered by some authors to be immunosuppressed during the first months of life.2
Looking globally across biologics used for psoriasis, limited safety data are encouraging. In a review of PSOLAR (Psoriasis Longitudinal Assessment and Registry), 83 pregnancies with biologic exposure resulted in 59 live births (71%); 18 spontaneous abortions (22%); 6 induced abortions (7%); no congenital abnormalities; and 7 reports of neonatal problems, including respiratory issues, ABO blood group mismatch, hospitalization, and opioid withdrawal.83
Use of tumor necrosis factor (TNF) inhibitors in pregnancy has the most data25 and is considered a reasonable treatment option. Historically, there was concern about the risk for VACTERL syndrome (vertebral defects, anal atresia, cardiac defects, tracheoesophageal fistula, renal anomalies, limb abnormalities) with exposure to a TNF inhibitor,25,84-86 but further reports have alleviated these concerns. Active transplacental transport occurs for adalimumab, infliximab, and golimumab,87 but given structural differences, transport of certolizumab and etanercept is substantially less.88,89 In the CRIB study of placental transfer of certolizumab from mother to infant (N=14), pharmacokinetic data demonstrated no quantifiable certolizumab levels in 13 infants and minimal levels in 1 infant at birth.88 There are fewer data available on the use of other biologics in pregnancy, but for those in which active placental transport is relevant, similar concerns (ie, immunosuppression) might arise (eTable 3).
Concern over biologics largely involves risk for newborn immunosuppression. A case report detailed a Crohn disease patient treated with infliximab who gave birth to an infant who died of disseminated bacille Calmette-Guérin infection at 4.5 months after receiving the vaccine at 3 months.90 This case underscores the importance of delaying live vaccination in infants born to mothers who were treated with a biologic during pregnancy. Authors have provided various data on how long to avoid vaccination; some state as long as 1 year.91
In pregnant females with inflammatory bowel disease treated with a biologic, no correlation was observed among maternal, placental, and infant serum biologic levels and neonatal infection. However, an association between preterm birth and the level of the biologic in maternal and placental (but not infant) serum and preterm birth was observed.92
In another report from the same registry, combination therapy with a TNF inhibitor and another immunomodulator led to an increased risk for infection in infants at 12 months of age, compared to infants exposed to monotherapy89 or exposed to neither agent.93 A strategy to circumvent this potential problem is to avoid treatment with actively transported molecules in the third trimester.
Conclusion
Limited data exist to guide providers who are treating pregnant women with psoriasis. Our understanding of treatment of psoriasis in pregnancy is limited as a consequence of regulations surrounding clinical trials and inadequate detection of pregnancies in registries. Further efforts are necessary to better understand the relationship between psoriasis and pregnancy and how to manage pregnant women with psoriasis.
Historically, there have been limited data available on the management of psoriasis in pregnancy. The most comprehensive discussion of treatment guidelines is from 2012.1 In the interim, many biologics have been approved for treating psoriasis, with slow accumulation of pregnancy safety data. The 2019 American Academy of Dermatology–National Psoriasis Foundation guidelines on biologics for psoriasis contain updated information but also highlight the paucity of pregnancy safety data.2 This gap is in part a consequence of the exclusion and disenrollment of pregnant women from clinical trials.3 Additionally, lack of detection through registries contributes; pregnancy capture in registries is low compared to the expected number of pregnancies estimated from US Census data.4 Despite these shortcomings, psoriasis patients who are already pregnant or are considering becoming pregnant frequently are encountered in practice and may need treatment. This article reviews the evidence on commonly used treatments for psoriasis in pregnancy.
Background
For many patients, psoriasis improves during pregnancy5,6 and becomes worse postpartum. In a prospective study, most patients reported improvement in pregnancy corresponding to a significant decrease in
In addition to the maternal disease state, the issue of pregnancy outcomes is paramount. In the inflammatory bowel disease and rheumatology literature, it is established that uncontrolled disease is associated with poorer pregnancy outcomes.8-10 Guidelines vary among societies on the use of biologics in pregnancy generally (eTable 11,2,9,11-24), but some societies recommend systemic agents to achieve disease control during pregnancy.9,25
Assessing the potential interplay between disease severity and outcomes in pregnant women with psoriasis is further complicated by the slowly growing body of literature demonstrating that women with psoriasis have more comorbidities26 and worse pregnancy outcomes.27,28 Pregnant psoriasis patients are more likely to smoke, have depression, and be overweight or obese prior to pregnancy and are less likely to take prenatal vitamins.26 They also have an increased risk for cesarean birth, gestational diabetes, gestational hypertension, and preeclampsia.28 In contrast to these prior studies, a systematic review revealed no risk for adverse outcomes in pregnant women with psoriasis.29
Assessment of Treatments for Psoriasis in Pregnancy
In light of these issues, treatment of psoriasis during pregnancy should be assessed from several vantage points. Of note, the US Food and Drug Administration changed its classification scheme in 2015 to a more narrative format called the Pregnancy and Lactation Labeling Rule.30 Prior classifications, however, provide a reasonable starting point for categorizing the safety of drugs (Table31). Importantly, time of exposure to systemic agents also matters; first-trimester exposure is more likely to affect embryogenesis, whereas second- and third-trimester exposures are more prone to affect other aspects of fetal growth. eTable 2 provides data on the use of oral and topical medications to treat psoriasis in pregnancy.1,8,22,32-45

Topical Agents
Topical steroids are largely understood to be reasonable treatment options, though consideration of potency, formulation, area of application, and use of occlusion is important.1,46 Risk for orofacial cleft has been noted with first-trimester topical steroid exposure, though a 2015 Cochrane review update determined that the relative risk of this association was not significantly elevated.32
The impact of topical calcipotriene and salicylic acid has not been studied in human pregnancies,1 but systemic absorption can occur for both. There is potential for vitamin D toxicity with calcipotriene46; consequently, use during pregnancy is not recommended.1,46 Some authors recommend against topical salicylic acid in pregnancy; others report that limited exposure is permissible.47 In fact, as salicylic acid commonly is found in over-the-counter acne products, many women of childbearing potential likely have quotidian exposure.
Preterm delivery and low birthweight have been reported with oral tacrolimus; however, risk with topical tacrolimus is thought to be low1 because the molecular size likely prohibits notable absorption.47 Evidence for the use of anthralin and coal tar also is scarce. First-trimester coal tar use should be avoided; subsequent use in pregnancy should be restricted given concern for adverse outcomes.1
Phototherapy
Broadband or narrowband UVB therapy is recommended as second-line therapy in pregnancy. No cases of fetal risk or premature delivery associated with UVB therapy were found in our search.1 Phototherapy can exacerbate melasma47 and decrease folate levels48; as such, some authors recommend folate supplementation in females of childbearing age who are being treated with phototherapy.49 Psoralen, used in psoralen plus UVA therapy, is mutagenic and therefore contraindicated in pregnancy.1
Oral Medications
Both methotrexate, which is a teratogen, abortifacient, and mutagen,1 and systemic retinoids, which are teratogens, are contraindicated in pregnancy.1,47 Acitretin labeling recommends avoiding pregnancy for 3 years posttreatment50 because alcohol intake prolongs the medication’s half-life.22
Apremilast use is not documented in pregnant psoriasis patients51; an ongoing registry of the Organization of Tetralogy Information Specialists has not reported publicly to date.52 Animal studies of apremilast have documented dose-related decreased birthweight and fetal loss.22
Safety data for systemic steroids, used infrequently in psoriasis, are not well established. First-trimester prednisone exposure has been associated with prematurity, low birthweight, and congenital abnormalities.38 A separate evaluation of 1047 children exposed to betamethasone in utero failed to demonstrate significant change in birthweight or head circumference. However, repeat antenatal corticosteroid exposure was associated with attention problems at 2 years of age.39
Data regarding cyclosporine use, derived primarily from organ transplant recipients, suggest elevated risk for prematurity and low birthweight.53,54 A meta-analysis demonstrated that organ transplant recipients taking cyclosporine had a nonsignificantly elevated odds ratio for congenital malformations, prematurity, and low birthweight.42 Cyclosporine use for psoriasis in pregnancy is not well described; in a study, rates of prematurity and low birthweight were both 21%.43 Limited data are available for Janus kinase inhibitors, none of which are approved for psoriasis, though clinical trials in psoriasis and psoriatic arthritis are underway (ClinicalTrials.gov identifiers NCT04246372, NCT03104374, NCT03104400).
Biologics and Small-Molecule Inhibitors
Limited data on biologics in pregnancy exist25 (eTable 3). Placental transport of IgG antibodies, including biologics, increases throughout pregnancy, especially in the third trimester.82 Infants of mothers treated with a biologic with potential for placental transfer are therefore considered by some authors to be immunosuppressed during the first months of life.2
Looking globally across biologics used for psoriasis, limited safety data are encouraging. In a review of PSOLAR (Psoriasis Longitudinal Assessment and Registry), 83 pregnancies with biologic exposure resulted in 59 live births (71%); 18 spontaneous abortions (22%); 6 induced abortions (7%); no congenital abnormalities; and 7 reports of neonatal problems, including respiratory issues, ABO blood group mismatch, hospitalization, and opioid withdrawal.83
Use of tumor necrosis factor (TNF) inhibitors in pregnancy has the most data25 and is considered a reasonable treatment option. Historically, there was concern about the risk for VACTERL syndrome (vertebral defects, anal atresia, cardiac defects, tracheoesophageal fistula, renal anomalies, limb abnormalities) with exposure to a TNF inhibitor,25,84-86 but further reports have alleviated these concerns. Active transplacental transport occurs for adalimumab, infliximab, and golimumab,87 but given structural differences, transport of certolizumab and etanercept is substantially less.88,89 In the CRIB study of placental transfer of certolizumab from mother to infant (N=14), pharmacokinetic data demonstrated no quantifiable certolizumab levels in 13 infants and minimal levels in 1 infant at birth.88 There are fewer data available on the use of other biologics in pregnancy, but for those in which active placental transport is relevant, similar concerns (ie, immunosuppression) might arise (eTable 3).
Concern over biologics largely involves risk for newborn immunosuppression. A case report detailed a Crohn disease patient treated with infliximab who gave birth to an infant who died of disseminated bacille Calmette-Guérin infection at 4.5 months after receiving the vaccine at 3 months.90 This case underscores the importance of delaying live vaccination in infants born to mothers who were treated with a biologic during pregnancy. Authors have provided various data on how long to avoid vaccination; some state as long as 1 year.91
In pregnant females with inflammatory bowel disease treated with a biologic, no correlation was observed among maternal, placental, and infant serum biologic levels and neonatal infection. However, an association between preterm birth and the level of the biologic in maternal and placental (but not infant) serum and preterm birth was observed.92
In another report from the same registry, combination therapy with a TNF inhibitor and another immunomodulator led to an increased risk for infection in infants at 12 months of age, compared to infants exposed to monotherapy89 or exposed to neither agent.93 A strategy to circumvent this potential problem is to avoid treatment with actively transported molecules in the third trimester.
Conclusion
Limited data exist to guide providers who are treating pregnant women with psoriasis. Our understanding of treatment of psoriasis in pregnancy is limited as a consequence of regulations surrounding clinical trials and inadequate detection of pregnancies in registries. Further efforts are necessary to better understand the relationship between psoriasis and pregnancy and how to manage pregnant women with psoriasis.
- Bae YS, Van Voorhees AS, Hsu S, et al. Review of treatment options for psoriasis in pregnant or lactating women: from the medical board of the National Psoriasis Foundation. J Am Acad Dermatol. 2012;67:459-477.
- Menter A, Strober BE, Kaplan DH, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol. 2019;80:1029-1072.
- Flood KS, Porter ML, Kimball AB. Use of biologics in pregnancy: limitations stemming from clinical trials and registry experience. J Eur Acad Dermatol Venereol. 2019;33:E276-E277.
- Horn EJ, Chambers CD, Menter A, et al. Pregnancy outcomes in psoriasis: why do we know so little? J Am Acad Dermatol. 2009;61:E5-E8.
- Raychaudhuri SP, Navare T, Gross J, et al. Clinical course of psoriasis during pregnancy. Int J Dermatol. 2003;42:518-520.
- Boyd AS, Morris LF, Phillips CM, et al. Psoriasis and pregnancy: hormone and immune system interaction. Int J Dermatol. 1996;35:169-172.
- Murase JE, Chan KK, Garite TJ, et al. Hormonal effect on psoriasis in pregnancy and post partum. Arch Dermatol. 2005;14:601-606.
- Götestam Skorpen C, Hoeltzenbein M, Tincani A, et al. The EULAR points to consider for use of antirheumatic drugs before pregnancy, and during pregnancy and lactation. Ann Rheum Dis. 2016;75:795-810.
- Nguyen GC, Seow CH, Maxwell C, et al. The Toronto consensus statements for the management of inflammatory bowel disease in pregnancy. Gastroenterology. 2016;150:734-757.
- Wise J. Rheumatic diseases should be actively treated in pregnancy, new guidelines say. BMJ. 2016;532:i312.
- Puig L, Carrascosa JM, Carretero G, et al. Spanish evidence-based guidelines on the treatment of psoriasis with biologic agents, 2013. part 1: on efficacy and choice of treatment. Actas Dermosifiliogr. 2013;104:694-709.
- Girolomoni G, Altomore G, Ayala F, et al. Differential management of mild-to-severe psoriasis with biologic drugs: an Italian Delphi consensus expert panel. J Dermatolog Treat. 2015;26:128-133.
- Yeung J, Gooderham MJ, Grewal P, et al. Management of plaque psoriasis with biologic therapies in women of child-bearing potential consensus paper. J Cutan Med Surg. 2020;24:3S-14S.
- Louthrenoo W, Kasitanon N, Kathamort W, et al. 2016 updated Thai Rheumatism Association Recommendations for the use of biologic and targeted synthetic disease-modifying anti-rheumatic drugs in patients with rheumatoid arthritis. Int J Rheum Dis. 2017;20:1166-1184.
- Flint J, Panchal S, Hurrell A, et al. BSR and BHPR guideline on prescribing drugs in pregnancy and breastfeeding—part I: standard and biologic disease modifying anti-rheumatic drugs and corticosteroids. Rheumatology (Oxford). 2016;55:1693-1697.
- van der Woude CJ, Ardizzone S, Bengtson MB, et al. The second European evidenced-based consensus on reproduction and pregnancy in inflammatory bowel disease. J Crohns Colitis. 2015;9:107-124.
- Orlando A, Armuzz A, Papi C, et al. The Italian Society of Gastroenterology (SIGE) and the Italian Group for the study of Inflammatory Bowel Disease (IG-IBD) Clinical Practice Guidelines: the use of tumor necrosis factor-alpha antagonist therapy in inflammatory bowel disease. Dig Liver Dis. 2011;43:1-20.
- Puchner A, Grochenig HP, Sautner J, et al. Immunosuppressives and biologics during pregnancy and lactation. Wien Klin Wochenschr. 2019;131:29-44.
- ACOG Committee opinion no. 776: immune modulating therapies in pregnancy and lactation. Obstet Gynecol. 2019;133:E287-E297.
- Lamb CA, Kennedy NA, Raine T, et al. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut. 2019;68:S1-S106.
- Goëb V, Ardizzone M, Arnard L, et al. Recommendations for using TNF-α antagonists and French Clinical Practice Guidelines endorsed by the French National Authority for Health. Joint Bone Spine. 2013;80:574-581.
- Rademaker M, Agnew K, Andrews M, et al. Psoriasis in those planning a family, pregnant or breast-feeding. the Australasian Psoriasis Collaboration. Australas J Dermatol. 2018;59:86-100.
- Mahadevan U, Robinson C, Bernasko N, et al. Inflammatory bowel disease in pregnancy clinical care pathway: a report from the American Gastroenterological Association IBD Parenthood Project Working Group. Gastroenterology. 2019;156:1508-1524.
- Mahadevan U, Cucchiara S, Hyam JS, et al. The London position statement of the World Congress of Gastroenterology on biological therapy for IBD with the European Crohn’s and Colitis Organisation: pregnancy and pediatrics. Am J Gastroenterol. 2011;106:214-223.
- Porter ML, Lockwood SJ, Kimball AB. Update on biologic safety for patients with psoriasis during pregnancy. Int J Womens Dermatol. 2017;3:21-25.
- Bandoli G, Johnson DL, Jones KL, et al. Potentially modifiable risk factors for adverse pregnancy outcomes in women with psoriasis. Br J Dermatol. 2010;163:334-339.
- Lima XT, Janakiraman V, Hughes MD, et al. The impact of psoriasis on pregnancy outcomes. J Invest Dermatol. 2012;132:85-91.
- Bröms G, Haerskjold A, Granath F, et al. Effect of maternal psoriasis on pregnancy and birth outcomes: a population-based cohort study from Denmark and Sweden. Acta Derm Venereol. 2018;98:728-734.
- Bobotsis R, Gulliver WP, Monaghan K, et al. Psoriasis and adverse pregnancy outcomes: a systematic review of observational studies. Br J Dermatol. 2016;175:464-472.
- Blattner CM, Danesh M, Safaee M, et al. Understanding the new FDA pregnancy and lactation labeling rules. Int J Womens Dermatol. 2016;2:5-7.
- Pernia S, DeMaagd G. The new pregnancy and lactation labeling rule. P T. 2016;4:713-715.
- Chi C-C, Wang S-H, Wojnarowska F, et al. Safety of topical corticosteroids in pregnancy. Cochrane Database Syst Rev. 2015:CD007346.
- Chi CC, Wang SH, Kirtschig G. Safety of topical corticosteroids in pregnancy. JAMA Dermatol. 2016;152:934-935.
- Dovonex (calcipotriene) Cream, 0.005% [package insert]. Dublin, Ireland: Leo Laboratories, Ltd; March 2015.
- Franssen ME, van der Wilt GJ, de Jong PC, et al. A retrospective study of the teratogenicity of dermatological coal tar products. Acta Derm Venereol. 1999;79:390-391.
- Garbis H, Elefant E, Bertolotti E, et al. Pregnancy outcome after periconceptional and first-trimester exposure to methoxsalen photochemotherapy. Arch Dermatol. 1995;131:492-493.
- Horizon Pharma USA. RAYOS (prednisone). https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/202020s000lbl.pdf https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/202020s000lbl.pdf.
- Park-Wyllie L, Mazzotta P, Pastuszak A, et al. Birth defects after maternal exposure to corticosteroids: prospective cohort study and meta-analysis of epidemiological studies. Teratology. 2000;62:385-392.
- Crowther CA, Doyle LW, Haslam RR, et al. Outcomes at 2 years of age after repeat doses of antenatal corticosteroids. N Engl J Med. 2007;357:1179-1189.
- Palmsten K, Rolland M, Herbert MF, et al. Patterns of prednisone use during pregnancy in women with rheumatoid arthritis: daily and cumulative dose. Pharmacoepidemiol Drug Saf. 2018;27:430-438.
- Groth K, Brännström M, Mölne J, et al. Cyclosporine A exposure during pregnancy in mice: effects on reproductive performance in mothers and offspring. Hum Reprod. 2010;25:697-704.
- Bar Oz B, Hackman R, Einarson T, et al. Pregnancy outcome after cyclosporine therapy during pregnancy: a meta-analysis. Transplantation. 2001;71:1051-1055.
- Paziana K, Del Monaco M, Cardonick E, et al. Ciclosporin use during pregnancy. Drug Saf. 2013;36:279-294.
- Lamarque V, Leleu MF, Monka C, et al. Analysis of 629 pregnancy outcomes in transplant recipients treated with Sandimmun. Transplant Proc. 1997;29:2480.
- Otezla (apremilast) tablets, for oral use [package insert]. Summit, NJ: Celgene Corporation; June 2017. http://www.accessdata.fda.gov/drugsatfda_docs/label/2017/205437s006lbl.pdf. Accessed July 8, 2020.
- Kurizky PS, de Castro Ferreira C, Nogueira LSC, et al. Treatment of psoriasis and psoriatic arthritis during pregnancy and breastfeeding. An Bras Dermatol. 2015;90:367-375.
- Murase JE, Heller MM, Butler DC. Safety of dermatologic medications in pregnancy and lactation. J Am Acad Dermatol. 2014;70:401.e1-401.e4.
- El-Saie LT, Rabie AR, Kamel MI, et al. Effect of narrowband ultraviolet B phototherapy on serum folic acid levels in patients with psoriasis. Lasers Med Sci. 2011;26:481-485.
- Murase JE, Koo JY, Berger TG. Narrowband ultraviolet B phototherapy influences serum folate levels in patients with vitiligo. J Am Acad Dermatol. 2010;62:710-711.
- Soriatane (acitretin) capsules [package insert]. Morrisville, NC: Stiefel Laboratories, Inc; April 2011. http://www.accessdata.fda.gov/drugsatfda_docs/label/2011/019821s018mg.pdf. Accessed July 8, 2020.
- Kaushik SB, Lebwohl MG. Psoriasis: which therapy for which patient: focus on special populations and chronic infections. J Am Acad Dermatol. 2019;80:43-53.
- Help us better understand the effects of Otezla in pregnancy. MotherToBaby website. https://mothertobaby.org/ongoing-study/otezla/. Accessed July 8, 2020.
- Bangsgaard N, Rørbye C, Skov L. Treating psoriasis during pregnancy: safety and efficacy of treatments. Am J Clin Dermatol. 2015;16:389-398.
- Tyler K. Dermatologic therapy in pregnancy. Clin Obstet Gynecol. 2015;58:112-118.
- Luu M, Benzenine E, Doret M, et al. Continuous anti–TNF-α use throughout pregnancy: possible complications for the mother but not for the fetus. a retrospective cohort on the French National Health Insurance Database (EVASION). Am J Gastroenterol. 2018;113:1669-1677.
- Bröms G, Granath F, Ekbom A, et al. Low risk of birth defects for infants whose mothers are treated with anti-tumor necrosis factor agents during pregnancy. Clin Gastroenterol Hepatol. 2016;14:234-241.
- Mirdamadi K, Salinas T, Vali R, et al. Meta-analysis of pregnancy outcomes after exposure to TNF-α inhibitors during pregnancy for the treatment of arthritic diseases. J Popul Ther Clin Pharmacol. 2018;25:E53-E56.
- Shihab Z, Yeomans ND, De Cruz P. Anti-tumour necrosis factor α therapies and inflammatory bowel disease pregnancy outcomes: a meta-analysis. J Crohns Colitis. 2016;10:979-988.
- Bröms G, Kieler H, Ekbom A, et al. Anti-TNF treatment during pregnancy and birth outcomes: a population-based study from Denmark, Finland, and Sweden. Pharmacoepidemiol Drug Saf. 2020;29:316-327.
- Diav-Citrin O, Otcheretianski-Volodarsky A, Shechtman S, et al. Pregnancy outcome following gestational exposure to TNF-alpha-inhibitors: a prospective, comparative, observational study. Reprod Toxicol. 2014;43:78-84.
- FDA determinations for pregnant and nursing women. National Psoriasis Foundation website. http://www.psoriasis.org/pregnancy/fda-determinations. Accessed July 8, 2020.
- Østensen M. Safety issues of biologics in pregnant patients with rheumatic diseases. Ann N Y Acad Sci. 2014;1317:32-38.
- Chambers CD, Johnson DL, Luo Y, et al. Pregnancy outcome in women treated with adalimumab for the treatment of rheumatoid arthritis: the OTIS Autoimmune Diseases in Pregnancy Project. Arthritis Rheum. 2012;64:2466.
- Clowse ME, Wolf DC, Forger F, et al. Pregnancy outcomes after exposure to certolizumab pegol: updated results from a pharmacovigilance safety database. Arthritis Rheumatol. 2018;70:1399-1407.
- Carman WJ, Accortt NA, Anthony MS, et al. Pregnancy and infant outcomes including major congenital malformations among women with chronic inflammatory arthritis or psoriasis, with and without etanercept use. Pharmacoepidemiol Drug Saf. 2017;26:1109-1118.
- Janssen. SIMPONI (golilumab). https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/125289s0064lbl.pdf.
- Yurkon K, Guo CY, Harrison D, et al. Pregnancy outcomes in women with dermatologic conditions exposed to infliximab. J Am Acad Dermatol. 2014;70:AB179.
- Watson N, Wu K, Farr P, et al. Ustekinumab exposure during conception and pregnancy in patients with chronic plaque psoriasis: a case series of 10 pregnancies. Br J Dermatol. 2019;180:195-196.
- Naureckas S, Slater J, Gearhart N, et al. Pregnancy outcomes in women with psoriasis and psoriatic arthritis exposed to ustekinumab. J Am Acad Dermatol. 2016;74:AB264.
- Haycraft K, DiRuggiero D, Rozzo SJ, et al. Outcomes of pregnancies from tildrakizumab phases I to III clinical development program. J Clin Aesthet Dermatol. 2019;12:S27-S28.
- Tremfya (guselkumab) injection, for subcutaneous use [package insert]. Horsham, PA: Janssen Biotech, Inc; July 2017. http://www.accessdata.fda.gov/drugsatfda_docs/label/2017/761061s000lbl.pdf. Accessed Juy 8, 2020.
- Skyrizi (risankizumab-rzaa) injection, for subcutaneous use [package insert]. Northi Chicago, IL; April 2019. http://www.accessdata.fda.gov/drugsatfda_docs/label/2019/761105s000lbl.pdf. Accessed July 8, 2020.
- Siliq (brodalumab) injection, for subcutaneous use [package insert]. Bridgewater, NJ: Valeant Pharmaceuticals North America LLC; February 2017. http://www.accessdata.fda.gov/drugsatfda_docs/label/2017/761032lbl.pdf. Accessed July 8, 2020.
- Feldman S, Pangallo B, Xu W, et al. Ixekizumab and pregnancy outcome. J Am Acad Dermatol. 2017;76:AB419.
- Clarke DO, Hilbish KG, Waters DG, et al. Assessment of ixekizumab, an interleukin-17A monoclonal antibody, for potential effects on reproduction and development, including immune system function, in cynomolgus monkeys. Reprod Toxicol. 2015;58:160-173.
- Warren RB, Reich K, Langley RG, et al. Secukinumab in pregnancy: outcomes in psoriasis, psoriatic arthritis and ankylosing spondylitis from the global safety database. Br J Dermatol. 2018;179:1205-1207.
- Nardin C, Colas M, Curie V, et al. Pregnancy after tubal sterilization in a woman treated with biologics for severe psoriasis. Dermatol Ther (Heidelb). 2018;8:323-326.
- Xeljanz (tofacitinib) tablets for oral administration [package insert]. New York, NY: Pfizer; November 2012. http://www.accessdata.fda.gov/drugsatfda_docs/label/2012/203214s000lbl.pdf. Accessed July 8, 2020.
- Pfizer. Xeljanz (tofacitinib). https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/203214s018lbl.pdf.
- Mahadevan U, Dubinsky M, Su C, et al. Outcomes of pregnancies with maternal/paternal exposure in the tofacitinib safety databases for ulcerative colitis. Inflamm Bowel Dis. 2018;24:2494-2500.
- Clowse ME, Feldman SR, Isaacs JD, et al. Pregnancy outcomes in the tofacitinib safety databases for rheumatoid arthritis and psoriasis. Drug Saf. 2016;39:755-762.
- Malek A, Sager R, Kuhn P, et al. Evolution of maternofetal transport of immunoglobulins during human pregnancy. Am J Reprod Immunol. 1996;36:248-255.
- Kimball AB, Crow JA, Ridley K, et al. Pregnancy outcomes in women with moderate to severe psoriasis: the PSOLAR experience. J Am Acad Dermatol. 2014;70(suppl 1):AB179.
- Carter JD, Valeriano J, Vasey FB. Tumor necrosis factor-alpha inhibition and VATER association: a causal relationship. J Rheumatol. 2006;33:1014-1017.
- Carter JD, Ladhani A, Ricca LR, et al. A safety assessment of tumor necrosis factor antagonists during pregnancy: a review of the Food and Drug Administration database. J Rheumatol. 2009;36:635-641.
- Koren G, Inoue M. Do tumor necrosis factor inhibitors cause malformations in humans? J Rheumatol. 2009;36:465-466.
- Johansen C, Jimenez-Solem E, Haerskjold A, et al. The use and safety of TNF inhibitors during pregnancy in women with psoriasis: a review. Int J Mol Sci. 2018;19:E1349.
- Mariette X, Forger F, Abraham B, et al. Lack of placental transfer of certolizumab pegol during pregnancy: results from CRIB, a prospective, postmarketing, pharmacokinetic study. Ann Rheum Dis. 2018;77:228-233.
- Mahadevan U, Wolf DC, Dubinsky M, et al. Placental transfer of anti-tumor necrosis factor agents in pregnant patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2013;11:286-292.
- Cheent K, Nolan J, Sharig S, et al. Case report: fatal case of disseminated BCG infection in an infant born to a mother taking infliximab for Crohn’s disease. J Crohns Colitis. 2010;4:603-605.
- Julsgaard M, Christensen LA, Gibson PR, et al. Concentrations of adalimumab and infliximab in mothers and newborns, and effects on infection. Gastroenterology. 2016;151:110-119.
- Mahadevan U, Martin C, Kane SV, et al. Do infant serum levels of biologic agents at birth correlate with risk of adverse outcomes? results from the PIANO registry. Gastroenterology. 2016;150:S91-S92.
- Mahadevan U, Martin CF, Sandler RS, et al. PIANO: a 1000 patient prospective registry of pregnancy outcomes in women with IBD exposed to immunomodulators and biologic therapy [AGA abstract 865]. Gastroenterology. 2012;142:S-149.
- Bae YS, Van Voorhees AS, Hsu S, et al. Review of treatment options for psoriasis in pregnant or lactating women: from the medical board of the National Psoriasis Foundation. J Am Acad Dermatol. 2012;67:459-477.
- Menter A, Strober BE, Kaplan DH, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol. 2019;80:1029-1072.
- Flood KS, Porter ML, Kimball AB. Use of biologics in pregnancy: limitations stemming from clinical trials and registry experience. J Eur Acad Dermatol Venereol. 2019;33:E276-E277.
- Horn EJ, Chambers CD, Menter A, et al. Pregnancy outcomes in psoriasis: why do we know so little? J Am Acad Dermatol. 2009;61:E5-E8.
- Raychaudhuri SP, Navare T, Gross J, et al. Clinical course of psoriasis during pregnancy. Int J Dermatol. 2003;42:518-520.
- Boyd AS, Morris LF, Phillips CM, et al. Psoriasis and pregnancy: hormone and immune system interaction. Int J Dermatol. 1996;35:169-172.
- Murase JE, Chan KK, Garite TJ, et al. Hormonal effect on psoriasis in pregnancy and post partum. Arch Dermatol. 2005;14:601-606.
- Götestam Skorpen C, Hoeltzenbein M, Tincani A, et al. The EULAR points to consider for use of antirheumatic drugs before pregnancy, and during pregnancy and lactation. Ann Rheum Dis. 2016;75:795-810.
- Nguyen GC, Seow CH, Maxwell C, et al. The Toronto consensus statements for the management of inflammatory bowel disease in pregnancy. Gastroenterology. 2016;150:734-757.
- Wise J. Rheumatic diseases should be actively treated in pregnancy, new guidelines say. BMJ. 2016;532:i312.
- Puig L, Carrascosa JM, Carretero G, et al. Spanish evidence-based guidelines on the treatment of psoriasis with biologic agents, 2013. part 1: on efficacy and choice of treatment. Actas Dermosifiliogr. 2013;104:694-709.
- Girolomoni G, Altomore G, Ayala F, et al. Differential management of mild-to-severe psoriasis with biologic drugs: an Italian Delphi consensus expert panel. J Dermatolog Treat. 2015;26:128-133.
- Yeung J, Gooderham MJ, Grewal P, et al. Management of plaque psoriasis with biologic therapies in women of child-bearing potential consensus paper. J Cutan Med Surg. 2020;24:3S-14S.
- Louthrenoo W, Kasitanon N, Kathamort W, et al. 2016 updated Thai Rheumatism Association Recommendations for the use of biologic and targeted synthetic disease-modifying anti-rheumatic drugs in patients with rheumatoid arthritis. Int J Rheum Dis. 2017;20:1166-1184.
- Flint J, Panchal S, Hurrell A, et al. BSR and BHPR guideline on prescribing drugs in pregnancy and breastfeeding—part I: standard and biologic disease modifying anti-rheumatic drugs and corticosteroids. Rheumatology (Oxford). 2016;55:1693-1697.
- van der Woude CJ, Ardizzone S, Bengtson MB, et al. The second European evidenced-based consensus on reproduction and pregnancy in inflammatory bowel disease. J Crohns Colitis. 2015;9:107-124.
- Orlando A, Armuzz A, Papi C, et al. The Italian Society of Gastroenterology (SIGE) and the Italian Group for the study of Inflammatory Bowel Disease (IG-IBD) Clinical Practice Guidelines: the use of tumor necrosis factor-alpha antagonist therapy in inflammatory bowel disease. Dig Liver Dis. 2011;43:1-20.
- Puchner A, Grochenig HP, Sautner J, et al. Immunosuppressives and biologics during pregnancy and lactation. Wien Klin Wochenschr. 2019;131:29-44.
- ACOG Committee opinion no. 776: immune modulating therapies in pregnancy and lactation. Obstet Gynecol. 2019;133:E287-E297.
- Lamb CA, Kennedy NA, Raine T, et al. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut. 2019;68:S1-S106.
- Goëb V, Ardizzone M, Arnard L, et al. Recommendations for using TNF-α antagonists and French Clinical Practice Guidelines endorsed by the French National Authority for Health. Joint Bone Spine. 2013;80:574-581.
- Rademaker M, Agnew K, Andrews M, et al. Psoriasis in those planning a family, pregnant or breast-feeding. the Australasian Psoriasis Collaboration. Australas J Dermatol. 2018;59:86-100.
- Mahadevan U, Robinson C, Bernasko N, et al. Inflammatory bowel disease in pregnancy clinical care pathway: a report from the American Gastroenterological Association IBD Parenthood Project Working Group. Gastroenterology. 2019;156:1508-1524.
- Mahadevan U, Cucchiara S, Hyam JS, et al. The London position statement of the World Congress of Gastroenterology on biological therapy for IBD with the European Crohn’s and Colitis Organisation: pregnancy and pediatrics. Am J Gastroenterol. 2011;106:214-223.
- Porter ML, Lockwood SJ, Kimball AB. Update on biologic safety for patients with psoriasis during pregnancy. Int J Womens Dermatol. 2017;3:21-25.
- Bandoli G, Johnson DL, Jones KL, et al. Potentially modifiable risk factors for adverse pregnancy outcomes in women with psoriasis. Br J Dermatol. 2010;163:334-339.
- Lima XT, Janakiraman V, Hughes MD, et al. The impact of psoriasis on pregnancy outcomes. J Invest Dermatol. 2012;132:85-91.
- Bröms G, Haerskjold A, Granath F, et al. Effect of maternal psoriasis on pregnancy and birth outcomes: a population-based cohort study from Denmark and Sweden. Acta Derm Venereol. 2018;98:728-734.
- Bobotsis R, Gulliver WP, Monaghan K, et al. Psoriasis and adverse pregnancy outcomes: a systematic review of observational studies. Br J Dermatol. 2016;175:464-472.
- Blattner CM, Danesh M, Safaee M, et al. Understanding the new FDA pregnancy and lactation labeling rules. Int J Womens Dermatol. 2016;2:5-7.
- Pernia S, DeMaagd G. The new pregnancy and lactation labeling rule. P T. 2016;4:713-715.
- Chi C-C, Wang S-H, Wojnarowska F, et al. Safety of topical corticosteroids in pregnancy. Cochrane Database Syst Rev. 2015:CD007346.
- Chi CC, Wang SH, Kirtschig G. Safety of topical corticosteroids in pregnancy. JAMA Dermatol. 2016;152:934-935.
- Dovonex (calcipotriene) Cream, 0.005% [package insert]. Dublin, Ireland: Leo Laboratories, Ltd; March 2015.
- Franssen ME, van der Wilt GJ, de Jong PC, et al. A retrospective study of the teratogenicity of dermatological coal tar products. Acta Derm Venereol. 1999;79:390-391.
- Garbis H, Elefant E, Bertolotti E, et al. Pregnancy outcome after periconceptional and first-trimester exposure to methoxsalen photochemotherapy. Arch Dermatol. 1995;131:492-493.
- Horizon Pharma USA. RAYOS (prednisone). https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/202020s000lbl.pdf https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/202020s000lbl.pdf.
- Park-Wyllie L, Mazzotta P, Pastuszak A, et al. Birth defects after maternal exposure to corticosteroids: prospective cohort study and meta-analysis of epidemiological studies. Teratology. 2000;62:385-392.
- Crowther CA, Doyle LW, Haslam RR, et al. Outcomes at 2 years of age after repeat doses of antenatal corticosteroids. N Engl J Med. 2007;357:1179-1189.
- Palmsten K, Rolland M, Herbert MF, et al. Patterns of prednisone use during pregnancy in women with rheumatoid arthritis: daily and cumulative dose. Pharmacoepidemiol Drug Saf. 2018;27:430-438.
- Groth K, Brännström M, Mölne J, et al. Cyclosporine A exposure during pregnancy in mice: effects on reproductive performance in mothers and offspring. Hum Reprod. 2010;25:697-704.
- Bar Oz B, Hackman R, Einarson T, et al. Pregnancy outcome after cyclosporine therapy during pregnancy: a meta-analysis. Transplantation. 2001;71:1051-1055.
- Paziana K, Del Monaco M, Cardonick E, et al. Ciclosporin use during pregnancy. Drug Saf. 2013;36:279-294.
- Lamarque V, Leleu MF, Monka C, et al. Analysis of 629 pregnancy outcomes in transplant recipients treated with Sandimmun. Transplant Proc. 1997;29:2480.
- Otezla (apremilast) tablets, for oral use [package insert]. Summit, NJ: Celgene Corporation; June 2017. http://www.accessdata.fda.gov/drugsatfda_docs/label/2017/205437s006lbl.pdf. Accessed July 8, 2020.
- Kurizky PS, de Castro Ferreira C, Nogueira LSC, et al. Treatment of psoriasis and psoriatic arthritis during pregnancy and breastfeeding. An Bras Dermatol. 2015;90:367-375.
- Murase JE, Heller MM, Butler DC. Safety of dermatologic medications in pregnancy and lactation. J Am Acad Dermatol. 2014;70:401.e1-401.e4.
- El-Saie LT, Rabie AR, Kamel MI, et al. Effect of narrowband ultraviolet B phototherapy on serum folic acid levels in patients with psoriasis. Lasers Med Sci. 2011;26:481-485.
- Murase JE, Koo JY, Berger TG. Narrowband ultraviolet B phototherapy influences serum folate levels in patients with vitiligo. J Am Acad Dermatol. 2010;62:710-711.
- Soriatane (acitretin) capsules [package insert]. Morrisville, NC: Stiefel Laboratories, Inc; April 2011. http://www.accessdata.fda.gov/drugsatfda_docs/label/2011/019821s018mg.pdf. Accessed July 8, 2020.
- Kaushik SB, Lebwohl MG. Psoriasis: which therapy for which patient: focus on special populations and chronic infections. J Am Acad Dermatol. 2019;80:43-53.
- Help us better understand the effects of Otezla in pregnancy. MotherToBaby website. https://mothertobaby.org/ongoing-study/otezla/. Accessed July 8, 2020.
- Bangsgaard N, Rørbye C, Skov L. Treating psoriasis during pregnancy: safety and efficacy of treatments. Am J Clin Dermatol. 2015;16:389-398.
- Tyler K. Dermatologic therapy in pregnancy. Clin Obstet Gynecol. 2015;58:112-118.
- Luu M, Benzenine E, Doret M, et al. Continuous anti–TNF-α use throughout pregnancy: possible complications for the mother but not for the fetus. a retrospective cohort on the French National Health Insurance Database (EVASION). Am J Gastroenterol. 2018;113:1669-1677.
- Bröms G, Granath F, Ekbom A, et al. Low risk of birth defects for infants whose mothers are treated with anti-tumor necrosis factor agents during pregnancy. Clin Gastroenterol Hepatol. 2016;14:234-241.
- Mirdamadi K, Salinas T, Vali R, et al. Meta-analysis of pregnancy outcomes after exposure to TNF-α inhibitors during pregnancy for the treatment of arthritic diseases. J Popul Ther Clin Pharmacol. 2018;25:E53-E56.
- Shihab Z, Yeomans ND, De Cruz P. Anti-tumour necrosis factor α therapies and inflammatory bowel disease pregnancy outcomes: a meta-analysis. J Crohns Colitis. 2016;10:979-988.
- Bröms G, Kieler H, Ekbom A, et al. Anti-TNF treatment during pregnancy and birth outcomes: a population-based study from Denmark, Finland, and Sweden. Pharmacoepidemiol Drug Saf. 2020;29:316-327.
- Diav-Citrin O, Otcheretianski-Volodarsky A, Shechtman S, et al. Pregnancy outcome following gestational exposure to TNF-alpha-inhibitors: a prospective, comparative, observational study. Reprod Toxicol. 2014;43:78-84.
- FDA determinations for pregnant and nursing women. National Psoriasis Foundation website. http://www.psoriasis.org/pregnancy/fda-determinations. Accessed July 8, 2020.
- Østensen M. Safety issues of biologics in pregnant patients with rheumatic diseases. Ann N Y Acad Sci. 2014;1317:32-38.
- Chambers CD, Johnson DL, Luo Y, et al. Pregnancy outcome in women treated with adalimumab for the treatment of rheumatoid arthritis: the OTIS Autoimmune Diseases in Pregnancy Project. Arthritis Rheum. 2012;64:2466.
- Clowse ME, Wolf DC, Forger F, et al. Pregnancy outcomes after exposure to certolizumab pegol: updated results from a pharmacovigilance safety database. Arthritis Rheumatol. 2018;70:1399-1407.
- Carman WJ, Accortt NA, Anthony MS, et al. Pregnancy and infant outcomes including major congenital malformations among women with chronic inflammatory arthritis or psoriasis, with and without etanercept use. Pharmacoepidemiol Drug Saf. 2017;26:1109-1118.
- Janssen. SIMPONI (golilumab). https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/125289s0064lbl.pdf.
- Yurkon K, Guo CY, Harrison D, et al. Pregnancy outcomes in women with dermatologic conditions exposed to infliximab. J Am Acad Dermatol. 2014;70:AB179.
- Watson N, Wu K, Farr P, et al. Ustekinumab exposure during conception and pregnancy in patients with chronic plaque psoriasis: a case series of 10 pregnancies. Br J Dermatol. 2019;180:195-196.
- Naureckas S, Slater J, Gearhart N, et al. Pregnancy outcomes in women with psoriasis and psoriatic arthritis exposed to ustekinumab. J Am Acad Dermatol. 2016;74:AB264.
- Haycraft K, DiRuggiero D, Rozzo SJ, et al. Outcomes of pregnancies from tildrakizumab phases I to III clinical development program. J Clin Aesthet Dermatol. 2019;12:S27-S28.
- Tremfya (guselkumab) injection, for subcutaneous use [package insert]. Horsham, PA: Janssen Biotech, Inc; July 2017. http://www.accessdata.fda.gov/drugsatfda_docs/label/2017/761061s000lbl.pdf. Accessed Juy 8, 2020.
- Skyrizi (risankizumab-rzaa) injection, for subcutaneous use [package insert]. Northi Chicago, IL; April 2019. http://www.accessdata.fda.gov/drugsatfda_docs/label/2019/761105s000lbl.pdf. Accessed July 8, 2020.
- Siliq (brodalumab) injection, for subcutaneous use [package insert]. Bridgewater, NJ: Valeant Pharmaceuticals North America LLC; February 2017. http://www.accessdata.fda.gov/drugsatfda_docs/label/2017/761032lbl.pdf. Accessed July 8, 2020.
- Feldman S, Pangallo B, Xu W, et al. Ixekizumab and pregnancy outcome. J Am Acad Dermatol. 2017;76:AB419.
- Clarke DO, Hilbish KG, Waters DG, et al. Assessment of ixekizumab, an interleukin-17A monoclonal antibody, for potential effects on reproduction and development, including immune system function, in cynomolgus monkeys. Reprod Toxicol. 2015;58:160-173.
- Warren RB, Reich K, Langley RG, et al. Secukinumab in pregnancy: outcomes in psoriasis, psoriatic arthritis and ankylosing spondylitis from the global safety database. Br J Dermatol. 2018;179:1205-1207.
- Nardin C, Colas M, Curie V, et al. Pregnancy after tubal sterilization in a woman treated with biologics for severe psoriasis. Dermatol Ther (Heidelb). 2018;8:323-326.
- Xeljanz (tofacitinib) tablets for oral administration [package insert]. New York, NY: Pfizer; November 2012. http://www.accessdata.fda.gov/drugsatfda_docs/label/2012/203214s000lbl.pdf. Accessed July 8, 2020.
- Pfizer. Xeljanz (tofacitinib). https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/203214s018lbl.pdf.
- Mahadevan U, Dubinsky M, Su C, et al. Outcomes of pregnancies with maternal/paternal exposure in the tofacitinib safety databases for ulcerative colitis. Inflamm Bowel Dis. 2018;24:2494-2500.
- Clowse ME, Feldman SR, Isaacs JD, et al. Pregnancy outcomes in the tofacitinib safety databases for rheumatoid arthritis and psoriasis. Drug Saf. 2016;39:755-762.
- Malek A, Sager R, Kuhn P, et al. Evolution of maternofetal transport of immunoglobulins during human pregnancy. Am J Reprod Immunol. 1996;36:248-255.
- Kimball AB, Crow JA, Ridley K, et al. Pregnancy outcomes in women with moderate to severe psoriasis: the PSOLAR experience. J Am Acad Dermatol. 2014;70(suppl 1):AB179.
- Carter JD, Valeriano J, Vasey FB. Tumor necrosis factor-alpha inhibition and VATER association: a causal relationship. J Rheumatol. 2006;33:1014-1017.
- Carter JD, Ladhani A, Ricca LR, et al. A safety assessment of tumor necrosis factor antagonists during pregnancy: a review of the Food and Drug Administration database. J Rheumatol. 2009;36:635-641.
- Koren G, Inoue M. Do tumor necrosis factor inhibitors cause malformations in humans? J Rheumatol. 2009;36:465-466.
- Johansen C, Jimenez-Solem E, Haerskjold A, et al. The use and safety of TNF inhibitors during pregnancy in women with psoriasis: a review. Int J Mol Sci. 2018;19:E1349.
- Mariette X, Forger F, Abraham B, et al. Lack of placental transfer of certolizumab pegol during pregnancy: results from CRIB, a prospective, postmarketing, pharmacokinetic study. Ann Rheum Dis. 2018;77:228-233.
- Mahadevan U, Wolf DC, Dubinsky M, et al. Placental transfer of anti-tumor necrosis factor agents in pregnant patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2013;11:286-292.
- Cheent K, Nolan J, Sharig S, et al. Case report: fatal case of disseminated BCG infection in an infant born to a mother taking infliximab for Crohn’s disease. J Crohns Colitis. 2010;4:603-605.
- Julsgaard M, Christensen LA, Gibson PR, et al. Concentrations of adalimumab and infliximab in mothers and newborns, and effects on infection. Gastroenterology. 2016;151:110-119.
- Mahadevan U, Martin C, Kane SV, et al. Do infant serum levels of biologic agents at birth correlate with risk of adverse outcomes? results from the PIANO registry. Gastroenterology. 2016;150:S91-S92.
- Mahadevan U, Martin CF, Sandler RS, et al. PIANO: a 1000 patient prospective registry of pregnancy outcomes in women with IBD exposed to immunomodulators and biologic therapy [AGA abstract 865]. Gastroenterology. 2012;142:S-149.
Practice Points
- Robust safety data often are lacking for the use of topical and systemic agents to treat psoriasis in pregnancy.
- Professional society guidelines on the use of systemic agents in pregnancy vary among dermatology, gastroenterology, and rheumatology organizations.