User login
NAFLD may predict arrhythmia recurrence post-AFib ablation
Increasingly recognized as an independent risk factor for new-onset atrial fibrillation (AFib), new research suggests for the first time that nonalcoholic fatty liver disease (NAFLD) also confers a higher risk for arrhythmia recurrence after AFib ablation.
Over 29 months of postablation follow-up, 56% of patients with NAFLD suffered bouts of arrhythmia, compared with 31% of patients without NAFLD, matched on the basis of age, sex, body mass index (BMI), ejection fraction within 5%, and AFib type (P < .0001).
The presence of NAFLD was an independent predictor of arrhythmia recurrence in multivariable analyses adjusted for several confounders, including hemoglobin A1c, BMI, and AFib type (hazard ratio, 3.0; 95% confidence interval, 1.94-4.68).
The association is concerning given that one in four adults in the United States has NAFLD, and up to 6.1 million Americans are estimated to have Afib. Previous studies, such as ARREST-AF and LEGACY, however, have demonstrated the benefits of aggressive preablation cardiometabolic risk factor modification on long-term AFib ablation success.
Indeed, none of the NAFLD patients in the present study who lost at least 10% of their body weight had recurrent arrhythmia, compared with 31% who lost less than 10%, and 91% who gained weight prior to ablation (P < .0001).
All 22 patients whose A1c increased during the 12 months prior to ablation had recurrent arrhythmia, compared with 36% of patients whose A1c improved (P < .0001).
“I don’t think the findings of the study were particularly surprising, given what we know. It’s just further reinforcement of the essential role of risk-factor modification,” lead author Eoin Donnellan, MD, Cleveland Clinic, said in an interview.
The results were published Augus 12 in JACC Clinical Electrophysiology.
For the study, the researchers examined data from 267 consecutive patients with a mean BMI of 32.7 kg/m2 who underwent radiofrequency ablation (98%) or cryoablation (2%) at the Cleveland Clinic between January 2013 and December 2017.
All patients were followed for at least 12 months after ablation and had scheduled clinic visits at 3, 6, and 12 months after pulmonary vein isolation, and annually thereafter.
NAFLD was diagnosed in 89 patients prior to ablation on the basis of CT imaging and abdominal ultrasound or MRI. On the basis of NAFLD-Fibrosis Score (NAFLD-FS), 13 patients had a low probability of liver fibrosis (F0-F2), 54 had an indeterminate probability, and 22 a high probability of fibrosis (F3-F4).
Compared with patients with no or early fibrosis (F0-F2), patients with advanced liver fibrosis (F3-F4) had almost a threefold increase in AFib recurrence (82% vs. 31%; P = .003).
“Cardiologists should make an effort to risk-stratify NAFLD patients either by NAFLD-FS or [an] alternative option, such as transient elastography or MR elastography, given these observations, rather than viewing it as either present or absence [sic] and involve expert multidisciplinary team care early in the clinical course of NAFLD patients with evidence of advanced fibrosis,” Dr. Donnellan and colleagues wrote.
Coauthor Thomas G. Cotter, MD, department of gastroenterology and hepatology, University of Chicago, said in an interview that cardiologists could use just the NAFLD-FS as part of an algorithm for an AFib.
“Because if it shows low risk, then it’s very, very likely the patient will be fine,” he said. “To use more advanced noninvasive testing, there are subtleties in the interpretation that would require referral to a liver doctor or a gastroenterologist and the cost of referring might bulk up the costs. But the NAFLD-FS is freely available and is a validated tool.”
Although it hasn’t specifically been validated in patients with AFib, the NAFLD-FS has been shown to correlate with the development of coronary artery disease (CAD) and was recommended for clinical use in U.S. multisociety guidelines for NAFLD.
The score is calculated using six readily available clinical variables (age, BMI, hyperglycemia or diabetes, AST/ALT, platelets, and albumin). It does not include family history or alcohol consumption, which should be carefully detailed given the large overlap between NAFLD and alcohol-related liver disease, Dr. Cotter observed.
Of note, the study excluded patients with alcohol consumption of more than 30 g/day in men and more than 20 g/day in women, chronic viral hepatitis, Wilson’s disease, and hereditary hemochromatosis.
Finally, CT imaging revealed that epicardial fat volume (EFV) was greater in patients with NAFLD than in those without NAFLD (248 vs. 223 mL; P = .01).
Although increased amounts of epicardial fat have been associated with CAD, there was no significant difference in EFV between patients who did and did not develop recurrent arrhythmia (238 vs. 229 mL; P = .5). Nor was EFV associated with arrhythmia recurrence on Cox proportional hazards analysis (HR, 1.001; P = .17).
“We hypothesized that the increased risk of arrhythmia recurrence may be mediated in part by an increased epicardial fat volume,” Dr. Donnellan said. “The existing literature exploring the link between epicardial fat volume and A[Fib] burden and recurrence is conflicting. But in both this study and our bariatric surgery study, epicardial fat volume was not a significant predictor of arrhythmia recurrence on multivariable analysis.”
It’s likely that the increased recurrence risk is caused by several mechanisms, including NAFLD’s deleterious impact on cardiac structure and function, the bidirectional relationship between NAFLD and sleep apnea, and transcription of proinflammatory cytokines and low-grade systemic inflammation, he suggested.
“Patients with NAFLD represent a particularly high-risk population for arrhythmia recurrence. NAFLD is a reversible disease, and a multidisciplinary approach incorporating dietary and lifestyle interventions should by instituted prior to ablation,” Dr. Donnellan and colleagues concluded.
They noted that serial abdominal imaging to assess for preablation changes in NAFLD was limited in patients and that only 56% of control subjects underwent dedicated abdominal imaging to rule out hepatic steatosis. Also, the heterogeneity of imaging modalities used to diagnose NAFLD may have influenced the results and the study’s single-center, retrospective design limits their generalizability.
The authors reported having no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Increasingly recognized as an independent risk factor for new-onset atrial fibrillation (AFib), new research suggests for the first time that nonalcoholic fatty liver disease (NAFLD) also confers a higher risk for arrhythmia recurrence after AFib ablation.
Over 29 months of postablation follow-up, 56% of patients with NAFLD suffered bouts of arrhythmia, compared with 31% of patients without NAFLD, matched on the basis of age, sex, body mass index (BMI), ejection fraction within 5%, and AFib type (P < .0001).
The presence of NAFLD was an independent predictor of arrhythmia recurrence in multivariable analyses adjusted for several confounders, including hemoglobin A1c, BMI, and AFib type (hazard ratio, 3.0; 95% confidence interval, 1.94-4.68).
The association is concerning given that one in four adults in the United States has NAFLD, and up to 6.1 million Americans are estimated to have Afib. Previous studies, such as ARREST-AF and LEGACY, however, have demonstrated the benefits of aggressive preablation cardiometabolic risk factor modification on long-term AFib ablation success.
Indeed, none of the NAFLD patients in the present study who lost at least 10% of their body weight had recurrent arrhythmia, compared with 31% who lost less than 10%, and 91% who gained weight prior to ablation (P < .0001).
All 22 patients whose A1c increased during the 12 months prior to ablation had recurrent arrhythmia, compared with 36% of patients whose A1c improved (P < .0001).
“I don’t think the findings of the study were particularly surprising, given what we know. It’s just further reinforcement of the essential role of risk-factor modification,” lead author Eoin Donnellan, MD, Cleveland Clinic, said in an interview.
The results were published Augus 12 in JACC Clinical Electrophysiology.
For the study, the researchers examined data from 267 consecutive patients with a mean BMI of 32.7 kg/m2 who underwent radiofrequency ablation (98%) or cryoablation (2%) at the Cleveland Clinic between January 2013 and December 2017.
All patients were followed for at least 12 months after ablation and had scheduled clinic visits at 3, 6, and 12 months after pulmonary vein isolation, and annually thereafter.
NAFLD was diagnosed in 89 patients prior to ablation on the basis of CT imaging and abdominal ultrasound or MRI. On the basis of NAFLD-Fibrosis Score (NAFLD-FS), 13 patients had a low probability of liver fibrosis (F0-F2), 54 had an indeterminate probability, and 22 a high probability of fibrosis (F3-F4).
Compared with patients with no or early fibrosis (F0-F2), patients with advanced liver fibrosis (F3-F4) had almost a threefold increase in AFib recurrence (82% vs. 31%; P = .003).
“Cardiologists should make an effort to risk-stratify NAFLD patients either by NAFLD-FS or [an] alternative option, such as transient elastography or MR elastography, given these observations, rather than viewing it as either present or absence [sic] and involve expert multidisciplinary team care early in the clinical course of NAFLD patients with evidence of advanced fibrosis,” Dr. Donnellan and colleagues wrote.
Coauthor Thomas G. Cotter, MD, department of gastroenterology and hepatology, University of Chicago, said in an interview that cardiologists could use just the NAFLD-FS as part of an algorithm for an AFib.
“Because if it shows low risk, then it’s very, very likely the patient will be fine,” he said. “To use more advanced noninvasive testing, there are subtleties in the interpretation that would require referral to a liver doctor or a gastroenterologist and the cost of referring might bulk up the costs. But the NAFLD-FS is freely available and is a validated tool.”
Although it hasn’t specifically been validated in patients with AFib, the NAFLD-FS has been shown to correlate with the development of coronary artery disease (CAD) and was recommended for clinical use in U.S. multisociety guidelines for NAFLD.
The score is calculated using six readily available clinical variables (age, BMI, hyperglycemia or diabetes, AST/ALT, platelets, and albumin). It does not include family history or alcohol consumption, which should be carefully detailed given the large overlap between NAFLD and alcohol-related liver disease, Dr. Cotter observed.
Of note, the study excluded patients with alcohol consumption of more than 30 g/day in men and more than 20 g/day in women, chronic viral hepatitis, Wilson’s disease, and hereditary hemochromatosis.
Finally, CT imaging revealed that epicardial fat volume (EFV) was greater in patients with NAFLD than in those without NAFLD (248 vs. 223 mL; P = .01).
Although increased amounts of epicardial fat have been associated with CAD, there was no significant difference in EFV between patients who did and did not develop recurrent arrhythmia (238 vs. 229 mL; P = .5). Nor was EFV associated with arrhythmia recurrence on Cox proportional hazards analysis (HR, 1.001; P = .17).
“We hypothesized that the increased risk of arrhythmia recurrence may be mediated in part by an increased epicardial fat volume,” Dr. Donnellan said. “The existing literature exploring the link between epicardial fat volume and A[Fib] burden and recurrence is conflicting. But in both this study and our bariatric surgery study, epicardial fat volume was not a significant predictor of arrhythmia recurrence on multivariable analysis.”
It’s likely that the increased recurrence risk is caused by several mechanisms, including NAFLD’s deleterious impact on cardiac structure and function, the bidirectional relationship between NAFLD and sleep apnea, and transcription of proinflammatory cytokines and low-grade systemic inflammation, he suggested.
“Patients with NAFLD represent a particularly high-risk population for arrhythmia recurrence. NAFLD is a reversible disease, and a multidisciplinary approach incorporating dietary and lifestyle interventions should by instituted prior to ablation,” Dr. Donnellan and colleagues concluded.
They noted that serial abdominal imaging to assess for preablation changes in NAFLD was limited in patients and that only 56% of control subjects underwent dedicated abdominal imaging to rule out hepatic steatosis. Also, the heterogeneity of imaging modalities used to diagnose NAFLD may have influenced the results and the study’s single-center, retrospective design limits their generalizability.
The authors reported having no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Increasingly recognized as an independent risk factor for new-onset atrial fibrillation (AFib), new research suggests for the first time that nonalcoholic fatty liver disease (NAFLD) also confers a higher risk for arrhythmia recurrence after AFib ablation.
Over 29 months of postablation follow-up, 56% of patients with NAFLD suffered bouts of arrhythmia, compared with 31% of patients without NAFLD, matched on the basis of age, sex, body mass index (BMI), ejection fraction within 5%, and AFib type (P < .0001).
The presence of NAFLD was an independent predictor of arrhythmia recurrence in multivariable analyses adjusted for several confounders, including hemoglobin A1c, BMI, and AFib type (hazard ratio, 3.0; 95% confidence interval, 1.94-4.68).
The association is concerning given that one in four adults in the United States has NAFLD, and up to 6.1 million Americans are estimated to have Afib. Previous studies, such as ARREST-AF and LEGACY, however, have demonstrated the benefits of aggressive preablation cardiometabolic risk factor modification on long-term AFib ablation success.
Indeed, none of the NAFLD patients in the present study who lost at least 10% of their body weight had recurrent arrhythmia, compared with 31% who lost less than 10%, and 91% who gained weight prior to ablation (P < .0001).
All 22 patients whose A1c increased during the 12 months prior to ablation had recurrent arrhythmia, compared with 36% of patients whose A1c improved (P < .0001).
“I don’t think the findings of the study were particularly surprising, given what we know. It’s just further reinforcement of the essential role of risk-factor modification,” lead author Eoin Donnellan, MD, Cleveland Clinic, said in an interview.
The results were published Augus 12 in JACC Clinical Electrophysiology.
For the study, the researchers examined data from 267 consecutive patients with a mean BMI of 32.7 kg/m2 who underwent radiofrequency ablation (98%) or cryoablation (2%) at the Cleveland Clinic between January 2013 and December 2017.
All patients were followed for at least 12 months after ablation and had scheduled clinic visits at 3, 6, and 12 months after pulmonary vein isolation, and annually thereafter.
NAFLD was diagnosed in 89 patients prior to ablation on the basis of CT imaging and abdominal ultrasound or MRI. On the basis of NAFLD-Fibrosis Score (NAFLD-FS), 13 patients had a low probability of liver fibrosis (F0-F2), 54 had an indeterminate probability, and 22 a high probability of fibrosis (F3-F4).
Compared with patients with no or early fibrosis (F0-F2), patients with advanced liver fibrosis (F3-F4) had almost a threefold increase in AFib recurrence (82% vs. 31%; P = .003).
“Cardiologists should make an effort to risk-stratify NAFLD patients either by NAFLD-FS or [an] alternative option, such as transient elastography or MR elastography, given these observations, rather than viewing it as either present or absence [sic] and involve expert multidisciplinary team care early in the clinical course of NAFLD patients with evidence of advanced fibrosis,” Dr. Donnellan and colleagues wrote.
Coauthor Thomas G. Cotter, MD, department of gastroenterology and hepatology, University of Chicago, said in an interview that cardiologists could use just the NAFLD-FS as part of an algorithm for an AFib.
“Because if it shows low risk, then it’s very, very likely the patient will be fine,” he said. “To use more advanced noninvasive testing, there are subtleties in the interpretation that would require referral to a liver doctor or a gastroenterologist and the cost of referring might bulk up the costs. But the NAFLD-FS is freely available and is a validated tool.”
Although it hasn’t specifically been validated in patients with AFib, the NAFLD-FS has been shown to correlate with the development of coronary artery disease (CAD) and was recommended for clinical use in U.S. multisociety guidelines for NAFLD.
The score is calculated using six readily available clinical variables (age, BMI, hyperglycemia or diabetes, AST/ALT, platelets, and albumin). It does not include family history or alcohol consumption, which should be carefully detailed given the large overlap between NAFLD and alcohol-related liver disease, Dr. Cotter observed.
Of note, the study excluded patients with alcohol consumption of more than 30 g/day in men and more than 20 g/day in women, chronic viral hepatitis, Wilson’s disease, and hereditary hemochromatosis.
Finally, CT imaging revealed that epicardial fat volume (EFV) was greater in patients with NAFLD than in those without NAFLD (248 vs. 223 mL; P = .01).
Although increased amounts of epicardial fat have been associated with CAD, there was no significant difference in EFV between patients who did and did not develop recurrent arrhythmia (238 vs. 229 mL; P = .5). Nor was EFV associated with arrhythmia recurrence on Cox proportional hazards analysis (HR, 1.001; P = .17).
“We hypothesized that the increased risk of arrhythmia recurrence may be mediated in part by an increased epicardial fat volume,” Dr. Donnellan said. “The existing literature exploring the link between epicardial fat volume and A[Fib] burden and recurrence is conflicting. But in both this study and our bariatric surgery study, epicardial fat volume was not a significant predictor of arrhythmia recurrence on multivariable analysis.”
It’s likely that the increased recurrence risk is caused by several mechanisms, including NAFLD’s deleterious impact on cardiac structure and function, the bidirectional relationship between NAFLD and sleep apnea, and transcription of proinflammatory cytokines and low-grade systemic inflammation, he suggested.
“Patients with NAFLD represent a particularly high-risk population for arrhythmia recurrence. NAFLD is a reversible disease, and a multidisciplinary approach incorporating dietary and lifestyle interventions should by instituted prior to ablation,” Dr. Donnellan and colleagues concluded.
They noted that serial abdominal imaging to assess for preablation changes in NAFLD was limited in patients and that only 56% of control subjects underwent dedicated abdominal imaging to rule out hepatic steatosis. Also, the heterogeneity of imaging modalities used to diagnose NAFLD may have influenced the results and the study’s single-center, retrospective design limits their generalizability.
The authors reported having no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Examining Biomarkers and the Pulmonologist’s Role in Lung Cancer Treatment
In this two-part supplement to CHEST Physician, Brett Bade, MD, (Yale School of Medicine) and Gerard A. Silvestri, MD, MS, FCCP (Medical University of South Carolina) discuss the role that pulmonologists play in lung cancer treatment as well as the clinical relevance of biomarkers.
The content in this supplement is based on proceedings from the Lung Cancer Summit hosted by the American College of Chest Physicians last July. The summit successfully fostered key relationships between clinicians, educators, scientists, advocacy groups, payers, and government entities. Attendees identified opportunities to align efforts toward moving forward a research agenda, supporting the faster-paced adoption of key innovations in care, and improving the accessibility of those innovations to all patient populations. These papers highlight the key clinical elements of the summit. Dr. Silvestri served as Chair of the Summit Steering Committee.
The supplement includes:
Part 1: Biomarker Use for Pulmonologists: Pulmonary Nodule Management
- Factors that influence the evaluation and management of indeterminate solid nodules
- Summary of key guidelines
- Emergence of biomarkers in clinical trials
Part 2: Biomarker Use in Metastatic Non-Small Cell Lung Cancer: What the Pulmonologist Needs to Know
- Expansion of systemic lung cancer treatments in recent years
- Clinical trials demonstrating improved patient outcomes
- Biomarkers being used in targeted therapies
In this two-part supplement to CHEST Physician, Brett Bade, MD, (Yale School of Medicine) and Gerard A. Silvestri, MD, MS, FCCP (Medical University of South Carolina) discuss the role that pulmonologists play in lung cancer treatment as well as the clinical relevance of biomarkers.
The content in this supplement is based on proceedings from the Lung Cancer Summit hosted by the American College of Chest Physicians last July. The summit successfully fostered key relationships between clinicians, educators, scientists, advocacy groups, payers, and government entities. Attendees identified opportunities to align efforts toward moving forward a research agenda, supporting the faster-paced adoption of key innovations in care, and improving the accessibility of those innovations to all patient populations. These papers highlight the key clinical elements of the summit. Dr. Silvestri served as Chair of the Summit Steering Committee.
The supplement includes:
Part 1: Biomarker Use for Pulmonologists: Pulmonary Nodule Management
- Factors that influence the evaluation and management of indeterminate solid nodules
- Summary of key guidelines
- Emergence of biomarkers in clinical trials
Part 2: Biomarker Use in Metastatic Non-Small Cell Lung Cancer: What the Pulmonologist Needs to Know
- Expansion of systemic lung cancer treatments in recent years
- Clinical trials demonstrating improved patient outcomes
- Biomarkers being used in targeted therapies
In this two-part supplement to CHEST Physician, Brett Bade, MD, (Yale School of Medicine) and Gerard A. Silvestri, MD, MS, FCCP (Medical University of South Carolina) discuss the role that pulmonologists play in lung cancer treatment as well as the clinical relevance of biomarkers.
The content in this supplement is based on proceedings from the Lung Cancer Summit hosted by the American College of Chest Physicians last July. The summit successfully fostered key relationships between clinicians, educators, scientists, advocacy groups, payers, and government entities. Attendees identified opportunities to align efforts toward moving forward a research agenda, supporting the faster-paced adoption of key innovations in care, and improving the accessibility of those innovations to all patient populations. These papers highlight the key clinical elements of the summit. Dr. Silvestri served as Chair of the Summit Steering Committee.
The supplement includes:
Part 1: Biomarker Use for Pulmonologists: Pulmonary Nodule Management
- Factors that influence the evaluation and management of indeterminate solid nodules
- Summary of key guidelines
- Emergence of biomarkers in clinical trials
Part 2: Biomarker Use in Metastatic Non-Small Cell Lung Cancer: What the Pulmonologist Needs to Know
- Expansion of systemic lung cancer treatments in recent years
- Clinical trials demonstrating improved patient outcomes
- Biomarkers being used in targeted therapies
Deaths, despair tied to drug dependence are accelerating amid COVID-19
Patients with OUDs need assistance now more than ever.
The Centers for Disease Control and Prevention reported recently that opioid overdose deaths will increase to a new U.S. record, and more are expected as pandemic-related overdose deaths are yet to be counted.1
Specifically, according to the CDC, 70,980 people died from fatal overdoses in 2019,2 which is record high. Experts such as Bruce A. Goldberger, PhD, fear that the 2020 numbers could rise even higher, exacerbated by the coronavirus pandemic.
Deaths from drug overdoses remain higher than the peak yearly death totals ever recorded for car accidents, guns, or AIDS. Overdose deaths have accelerated further – pushing down overall life expectancy in the United States.3 Headlines purporting to identify good news in drug death figures don’t always get below top-level data. Deaths and despair tied to drug dependence are indeed accelerating. I am concerned about these alarmingly dangerous trends.
Synthetic opioids such as fentanyl accounted for about 3,000 deaths in 2013. By 2019, they accounted for more than 37,137.4 In addition, 16,539 deaths involved stimulants such as methamphetamine, and 16,196 deaths involved cocaine, the most recent CDC reporting shows. Opioids continue to play a role in U.S. “deaths of despair,” or rising fatalities from drugs, suicides, and alcohol among Americans without employment, hope of job opportunities, or college degrees.5 As the American Medical Association has warned,6 more people are dying from overdoses amid the COVID-19 pandemic. Clinicians need to be aware of trends so that we can help our patients navigate these challenges.
Fentanyl presents dangers
Experts had predicted that the pandemic, by limiting access to treatment, rescue, or overdose services, and increasing time at home and in the neighborhood, would result in more tragedy. In addition, the shift from prescription opioids to heroin and now to fentanyl has made deaths more common.
Fentanyls – synthetic opioids – are involved in more than half of overdose deaths, and in many of the cocaine and methamphetamine-related deaths, which also are on the rise. Fentanyl is about 100 times more potent than morphine and 50 times more potent than heroin. Breathing can stop after use of just 2 mg of fentanyl, which is about as much as trace amounts of table salt. Fentanyl has replaced heroin in many cities as the pandemic changed the relative ease of importing raw drugs such as heroin.
Another important trend is that fentanyl production and distribution throughout the United States have expanded. The ease of manufacture in unregulated sectors of the Chinese and Mexican economies is difficult for U.S. authorities to curb or eliminate. The Internet promotes novel strategies for synthesizing the substance, spreading its production across many labs; suppliers use the U.S. Postal Service for distribution, and e-commerce helps to get the drug from manufacturers to U.S. consumers for fentanyl transactions.
A recent RAND report observes that, for only $10 through the postal service, suppliers can ship a 1-kg parcel from China to the United States, and private shipments cost about $100.7 And with large volumes of legal trade between the two countries making rigorous scrutiny of products difficult, especially given the light weight of fentanyl, suppliers find it relatively easy to hide illicit substances in licit shipments. Opioid users have made the switch to fentanyl, and have seen fentanyl added to cocaine and methamphetamine they buy on the streets.
OUD and buprenorphine
Fentanyl is one part of the overdose crisis. Opioid use disorder (OUD) is the other. Both need to be addressed if we are to make any progress in this epidemic of death and dependency.
The OUD crisis continues amid the pandemic – and isn’t going away.8 Slips, relapses, and overdoses are all too common. Medication-assisted treatment (MAT) and OUD treatment programs are essential parts of our response to overdose initiatives. After naloxone rescue, the best anti-overdose response is to get the OUD patient into treatment with MATs. Patients with OUD have continuously high risks of overdose. The best outcomes appear to be related to treatment duration of greater than 2 years. But it is common to see patients with OUDs who have been in treatment multiple times, taking MATs, dropping out, overdosing, and dying. Some have been described as treatment resistant.9 It is clear that treatment can work, but also that even evidence-based treatments often fail.10
A recent study compared OUD patients who continued treatment for 6-9 months to those patients who had continued MAT treatment for 15-18 months. The longer the treatment, the fewer emergencies, prescriptions, or hospitalizations.11
But this study reminds us that all OUD patients, whether they are currently buprenorphine treated or not, experience overdoses and emergency department interventions. Short and longer treatment groups have a similar nonfatal overdose rate, about 6%, and went to the emergency department at a high rate, above 40%. Discontinuation of buprenorphine treatment is a major risk factor in opioid relapse, emergency department visits, and overdose. Cures are not common. Whether an OUD patient is being treated or has been treated in the past, carrying naloxone (brand name Narcan), makes sense and can save lives.
Methadone still considered most effective
Methadone is a synthetic opioid first studied as a treatment for OUD at Rockefeller University in New York City in the 1960s. Methadone may be the most effective treatment for OUD in promoting treatment retention for years, decreasing intravenous drug use, and decreasing deaths.12 It has been studied and safely used in treatment programs for decades. Methadone is typically administered in a clinic, daily, and with observation. In addition, methadone patients periodically take urine drug tests, which can distinguish methadone from substances of abuse. They also receive counseling. But methadone can be prescribed and administered only in methadone clinics in the United States. It is available for prescription in primary care clinics in Great Britain, Canada, and Australia.13 Numerous experts have suggested passing new legislation aimed at changing how methadone can be prescribed. Allowing primary care to administer methadone, just like buprenorphine, can improve access and benefit OUD patients.12
Availability of Narcan is critical
A comprehensive treatment model for OUDs includes prescribing naloxone, encouraging those patients with an OUD and their loved ones to have naloxone with them, and providing MATs and appropriate therapies, such as counseling.
As described by Allison L. Pitt and colleagues at Stanford (Calif.) University,14 the United States might be on track to have up to 500,000 deaths tied to opioid overdoses that might occur over the next 5 years. They modeled the effect on overdose of a long list of interventions, but only a few had an impact. At the top of the list was naloxone availability. We need to focus on saving lives by increasing naloxone availability, improving initiation, and expanding access to MAT, and increasing psychosocial treatment to improve outcomes, increase life-years and quality-adjusted life-years, and reduce opioid-related deaths. When Ms. Pitt and colleagues looked at what would make the most impact in reducing OUD deaths, it was naloxone. Pain patients on higher doses of opioids, nonprescription opioid users, OUD patients should be given naloxone prescriptions. While many can give a Heimlich to a choking person or CPR, few have naloxone to rescue a person who has overdosed on opioids. If an overdose is suspected, it should be administered by anyone who has it, as soon as possible. Then, the person who is intervening should call 911.
What we can do today
At this moment, clinicians can follow the Surgeon General’s advice,15 and prescribe naloxone.
We should give naloxone to OUD patients and their families, to pain patients at dosages of greater than or equal to 50 MME. Our top priorities should be patients with comorbid pain syndromes, those being treated with benzodiazepines and sleeping medications, and patients with alcohol use disorders. This is also an important intervention for those who binge drink, and have sleep apnea, and heart and respiratory diseases.
Naloxone is available without a prescription in at least 43 states. Naloxone is available in harm reduction programs and in hospitals, and is carried by emergency medical staff, law enforcement, and EMTs. It also is available on the streets, though it does not appear to have a dollar value like opioids or even buprenorphine. Also, the availability of naloxone in pharmacies has made it easier for family members and caregivers of pain patients or those with OUD to have it to administer in an emergency.
An excellent place for MDs to start is to do more to encourage all patients with OUD to carry naloxone, for their loved ones to carry naloxone, and for their homes to have naloxone nearby in the bedroom or bathroom. It is not logical to expect a person with an OUD to rescue themselves. Current and past OUD patients, as well as their loved ones, are at high risk – and should have naloxone nearby at all times.
Naloxone reverses an opioid overdose, but it should be thought about like cardioversion or CPR rather than a treatment for an underlying disease. Increasing access to buprenorphine, buprenorphine + naloxone, and naltrexone treatment for OUDs is an important organizing principle. Initiation of MAT treatment in the emergency setting or most anywhere and any place a patient with an OUD can begin treatment is necessary. Treatment with buprenorphine or methadone reduces opioid overdose and opioid-related acute care use.16
Reducing racial disparities in OUD treatment is necessary, because buprenorphine treatment is concentrated among White patients who either use private insurance or are self-pay.17 Reducing barriers to methadone program licenses, expanding sites for distribution,18 prescribing methadone in an office setting might help. Clinicians can do a better job of explaining the risks associated with opioid prescriptions, including diversion and overdose, and the benefits of OUD treatment. So, To reduce opioid overdoses, we must increase physician competencies in addiction medicine.
Dr. Gold is professor of psychiatry (adjunct) at Washington University, St. Louis. He is the 17th Distinguished Alumni Professor at the University of Florida, Gainesville. For more than 40 years, Dr. Gold has worked on developing models for understanding the effects of opioid, tobacco, cocaine, and other drugs, as well as food, on the brain and behavior. He disclosed financial ties with ADAPT Pharma and Magstim Ltd.
References
1. Kamp J. Overdose deaths rise, may reach record level, federal data show. Wall Street Journal. 2020 Jul 15.
2. 12 month–ending provisional number of drug overdose drugs. Centers for Disease Control and Prevention. 2020 Jul 5.
3. Katz J et al. In shadow of pandemic, U.S. drug overdose deaths resurge to record. New York Times. 2020 Jul 15.
4. Gold MS. The fentanyl crisis is only getting worse. Addiction Policy Forum. Updated 2020 Mar 12.
5. Gold MS. Mo Med. 2020-Mar-Apr;117(2):99-101.
6. Reports of increases in opioid-related overdoses and other concerns during the COVID-19 pandemic. American Medical Association. Issue brief. Updated 2020 Jul 20.
7. Pardo B et al. The future of fentanyl and other synthetic opioids. RAND report.
8. Gold MS. New challenges in the opioid epidemic. Addiction Policy Forum. 2020 Jun 4.
9. Patterson Silver Wolf DA and Gold MS. J Neurol Sci. 2020;411:116718.
10. Oesterle TS et al. Mayo Clin Proc. 2019;94(10):2072-86.
11. Connery HS and Weiss RD. Am J Psychiatry. 2020;177(2):104-6.
12. Kleber HD. JAMA. 2008;300(19):2303-5.
13. Samet JH et al. N Engl J Med. 2018;379(1):7-8.
14. Pitt AL et al. Am J Public Health. 2018;108(10):1394-1400.
15. U.S. Surgeon General’s Advisory on Naloxone and Opioid Overdose. hhs.gov.
16. Wakeman SE et al. JAMA Netw Open. 2020;3(2):e1920622.
17. Lagisetty PA et al. JAMA Psychiatry. 2019;76(9):979-81.
18. Kleinman RA. JAMA Psychiatry. 2020 Jul 15. doi: 10.1001/jamapsychiatry.2020.1624.
Patients with OUDs need assistance now more than ever.
Patients with OUDs need assistance now more than ever.
The Centers for Disease Control and Prevention reported recently that opioid overdose deaths will increase to a new U.S. record, and more are expected as pandemic-related overdose deaths are yet to be counted.1
Specifically, according to the CDC, 70,980 people died from fatal overdoses in 2019,2 which is record high. Experts such as Bruce A. Goldberger, PhD, fear that the 2020 numbers could rise even higher, exacerbated by the coronavirus pandemic.
Deaths from drug overdoses remain higher than the peak yearly death totals ever recorded for car accidents, guns, or AIDS. Overdose deaths have accelerated further – pushing down overall life expectancy in the United States.3 Headlines purporting to identify good news in drug death figures don’t always get below top-level data. Deaths and despair tied to drug dependence are indeed accelerating. I am concerned about these alarmingly dangerous trends.
Synthetic opioids such as fentanyl accounted for about 3,000 deaths in 2013. By 2019, they accounted for more than 37,137.4 In addition, 16,539 deaths involved stimulants such as methamphetamine, and 16,196 deaths involved cocaine, the most recent CDC reporting shows. Opioids continue to play a role in U.S. “deaths of despair,” or rising fatalities from drugs, suicides, and alcohol among Americans without employment, hope of job opportunities, or college degrees.5 As the American Medical Association has warned,6 more people are dying from overdoses amid the COVID-19 pandemic. Clinicians need to be aware of trends so that we can help our patients navigate these challenges.
Fentanyl presents dangers
Experts had predicted that the pandemic, by limiting access to treatment, rescue, or overdose services, and increasing time at home and in the neighborhood, would result in more tragedy. In addition, the shift from prescription opioids to heroin and now to fentanyl has made deaths more common.
Fentanyls – synthetic opioids – are involved in more than half of overdose deaths, and in many of the cocaine and methamphetamine-related deaths, which also are on the rise. Fentanyl is about 100 times more potent than morphine and 50 times more potent than heroin. Breathing can stop after use of just 2 mg of fentanyl, which is about as much as trace amounts of table salt. Fentanyl has replaced heroin in many cities as the pandemic changed the relative ease of importing raw drugs such as heroin.
Another important trend is that fentanyl production and distribution throughout the United States have expanded. The ease of manufacture in unregulated sectors of the Chinese and Mexican economies is difficult for U.S. authorities to curb or eliminate. The Internet promotes novel strategies for synthesizing the substance, spreading its production across many labs; suppliers use the U.S. Postal Service for distribution, and e-commerce helps to get the drug from manufacturers to U.S. consumers for fentanyl transactions.
A recent RAND report observes that, for only $10 through the postal service, suppliers can ship a 1-kg parcel from China to the United States, and private shipments cost about $100.7 And with large volumes of legal trade between the two countries making rigorous scrutiny of products difficult, especially given the light weight of fentanyl, suppliers find it relatively easy to hide illicit substances in licit shipments. Opioid users have made the switch to fentanyl, and have seen fentanyl added to cocaine and methamphetamine they buy on the streets.
OUD and buprenorphine
Fentanyl is one part of the overdose crisis. Opioid use disorder (OUD) is the other. Both need to be addressed if we are to make any progress in this epidemic of death and dependency.
The OUD crisis continues amid the pandemic – and isn’t going away.8 Slips, relapses, and overdoses are all too common. Medication-assisted treatment (MAT) and OUD treatment programs are essential parts of our response to overdose initiatives. After naloxone rescue, the best anti-overdose response is to get the OUD patient into treatment with MATs. Patients with OUD have continuously high risks of overdose. The best outcomes appear to be related to treatment duration of greater than 2 years. But it is common to see patients with OUDs who have been in treatment multiple times, taking MATs, dropping out, overdosing, and dying. Some have been described as treatment resistant.9 It is clear that treatment can work, but also that even evidence-based treatments often fail.10
A recent study compared OUD patients who continued treatment for 6-9 months to those patients who had continued MAT treatment for 15-18 months. The longer the treatment, the fewer emergencies, prescriptions, or hospitalizations.11
But this study reminds us that all OUD patients, whether they are currently buprenorphine treated or not, experience overdoses and emergency department interventions. Short and longer treatment groups have a similar nonfatal overdose rate, about 6%, and went to the emergency department at a high rate, above 40%. Discontinuation of buprenorphine treatment is a major risk factor in opioid relapse, emergency department visits, and overdose. Cures are not common. Whether an OUD patient is being treated or has been treated in the past, carrying naloxone (brand name Narcan), makes sense and can save lives.
Methadone still considered most effective
Methadone is a synthetic opioid first studied as a treatment for OUD at Rockefeller University in New York City in the 1960s. Methadone may be the most effective treatment for OUD in promoting treatment retention for years, decreasing intravenous drug use, and decreasing deaths.12 It has been studied and safely used in treatment programs for decades. Methadone is typically administered in a clinic, daily, and with observation. In addition, methadone patients periodically take urine drug tests, which can distinguish methadone from substances of abuse. They also receive counseling. But methadone can be prescribed and administered only in methadone clinics in the United States. It is available for prescription in primary care clinics in Great Britain, Canada, and Australia.13 Numerous experts have suggested passing new legislation aimed at changing how methadone can be prescribed. Allowing primary care to administer methadone, just like buprenorphine, can improve access and benefit OUD patients.12
Availability of Narcan is critical
A comprehensive treatment model for OUDs includes prescribing naloxone, encouraging those patients with an OUD and their loved ones to have naloxone with them, and providing MATs and appropriate therapies, such as counseling.
As described by Allison L. Pitt and colleagues at Stanford (Calif.) University,14 the United States might be on track to have up to 500,000 deaths tied to opioid overdoses that might occur over the next 5 years. They modeled the effect on overdose of a long list of interventions, but only a few had an impact. At the top of the list was naloxone availability. We need to focus on saving lives by increasing naloxone availability, improving initiation, and expanding access to MAT, and increasing psychosocial treatment to improve outcomes, increase life-years and quality-adjusted life-years, and reduce opioid-related deaths. When Ms. Pitt and colleagues looked at what would make the most impact in reducing OUD deaths, it was naloxone. Pain patients on higher doses of opioids, nonprescription opioid users, OUD patients should be given naloxone prescriptions. While many can give a Heimlich to a choking person or CPR, few have naloxone to rescue a person who has overdosed on opioids. If an overdose is suspected, it should be administered by anyone who has it, as soon as possible. Then, the person who is intervening should call 911.
What we can do today
At this moment, clinicians can follow the Surgeon General’s advice,15 and prescribe naloxone.
We should give naloxone to OUD patients and their families, to pain patients at dosages of greater than or equal to 50 MME. Our top priorities should be patients with comorbid pain syndromes, those being treated with benzodiazepines and sleeping medications, and patients with alcohol use disorders. This is also an important intervention for those who binge drink, and have sleep apnea, and heart and respiratory diseases.
Naloxone is available without a prescription in at least 43 states. Naloxone is available in harm reduction programs and in hospitals, and is carried by emergency medical staff, law enforcement, and EMTs. It also is available on the streets, though it does not appear to have a dollar value like opioids or even buprenorphine. Also, the availability of naloxone in pharmacies has made it easier for family members and caregivers of pain patients or those with OUD to have it to administer in an emergency.
An excellent place for MDs to start is to do more to encourage all patients with OUD to carry naloxone, for their loved ones to carry naloxone, and for their homes to have naloxone nearby in the bedroom or bathroom. It is not logical to expect a person with an OUD to rescue themselves. Current and past OUD patients, as well as their loved ones, are at high risk – and should have naloxone nearby at all times.
Naloxone reverses an opioid overdose, but it should be thought about like cardioversion or CPR rather than a treatment for an underlying disease. Increasing access to buprenorphine, buprenorphine + naloxone, and naltrexone treatment for OUDs is an important organizing principle. Initiation of MAT treatment in the emergency setting or most anywhere and any place a patient with an OUD can begin treatment is necessary. Treatment with buprenorphine or methadone reduces opioid overdose and opioid-related acute care use.16
Reducing racial disparities in OUD treatment is necessary, because buprenorphine treatment is concentrated among White patients who either use private insurance or are self-pay.17 Reducing barriers to methadone program licenses, expanding sites for distribution,18 prescribing methadone in an office setting might help. Clinicians can do a better job of explaining the risks associated with opioid prescriptions, including diversion and overdose, and the benefits of OUD treatment. So, To reduce opioid overdoses, we must increase physician competencies in addiction medicine.
Dr. Gold is professor of psychiatry (adjunct) at Washington University, St. Louis. He is the 17th Distinguished Alumni Professor at the University of Florida, Gainesville. For more than 40 years, Dr. Gold has worked on developing models for understanding the effects of opioid, tobacco, cocaine, and other drugs, as well as food, on the brain and behavior. He disclosed financial ties with ADAPT Pharma and Magstim Ltd.
References
1. Kamp J. Overdose deaths rise, may reach record level, federal data show. Wall Street Journal. 2020 Jul 15.
2. 12 month–ending provisional number of drug overdose drugs. Centers for Disease Control and Prevention. 2020 Jul 5.
3. Katz J et al. In shadow of pandemic, U.S. drug overdose deaths resurge to record. New York Times. 2020 Jul 15.
4. Gold MS. The fentanyl crisis is only getting worse. Addiction Policy Forum. Updated 2020 Mar 12.
5. Gold MS. Mo Med. 2020-Mar-Apr;117(2):99-101.
6. Reports of increases in opioid-related overdoses and other concerns during the COVID-19 pandemic. American Medical Association. Issue brief. Updated 2020 Jul 20.
7. Pardo B et al. The future of fentanyl and other synthetic opioids. RAND report.
8. Gold MS. New challenges in the opioid epidemic. Addiction Policy Forum. 2020 Jun 4.
9. Patterson Silver Wolf DA and Gold MS. J Neurol Sci. 2020;411:116718.
10. Oesterle TS et al. Mayo Clin Proc. 2019;94(10):2072-86.
11. Connery HS and Weiss RD. Am J Psychiatry. 2020;177(2):104-6.
12. Kleber HD. JAMA. 2008;300(19):2303-5.
13. Samet JH et al. N Engl J Med. 2018;379(1):7-8.
14. Pitt AL et al. Am J Public Health. 2018;108(10):1394-1400.
15. U.S. Surgeon General’s Advisory on Naloxone and Opioid Overdose. hhs.gov.
16. Wakeman SE et al. JAMA Netw Open. 2020;3(2):e1920622.
17. Lagisetty PA et al. JAMA Psychiatry. 2019;76(9):979-81.
18. Kleinman RA. JAMA Psychiatry. 2020 Jul 15. doi: 10.1001/jamapsychiatry.2020.1624.
The Centers for Disease Control and Prevention reported recently that opioid overdose deaths will increase to a new U.S. record, and more are expected as pandemic-related overdose deaths are yet to be counted.1
Specifically, according to the CDC, 70,980 people died from fatal overdoses in 2019,2 which is record high. Experts such as Bruce A. Goldberger, PhD, fear that the 2020 numbers could rise even higher, exacerbated by the coronavirus pandemic.
Deaths from drug overdoses remain higher than the peak yearly death totals ever recorded for car accidents, guns, or AIDS. Overdose deaths have accelerated further – pushing down overall life expectancy in the United States.3 Headlines purporting to identify good news in drug death figures don’t always get below top-level data. Deaths and despair tied to drug dependence are indeed accelerating. I am concerned about these alarmingly dangerous trends.
Synthetic opioids such as fentanyl accounted for about 3,000 deaths in 2013. By 2019, they accounted for more than 37,137.4 In addition, 16,539 deaths involved stimulants such as methamphetamine, and 16,196 deaths involved cocaine, the most recent CDC reporting shows. Opioids continue to play a role in U.S. “deaths of despair,” or rising fatalities from drugs, suicides, and alcohol among Americans without employment, hope of job opportunities, or college degrees.5 As the American Medical Association has warned,6 more people are dying from overdoses amid the COVID-19 pandemic. Clinicians need to be aware of trends so that we can help our patients navigate these challenges.
Fentanyl presents dangers
Experts had predicted that the pandemic, by limiting access to treatment, rescue, or overdose services, and increasing time at home and in the neighborhood, would result in more tragedy. In addition, the shift from prescription opioids to heroin and now to fentanyl has made deaths more common.
Fentanyls – synthetic opioids – are involved in more than half of overdose deaths, and in many of the cocaine and methamphetamine-related deaths, which also are on the rise. Fentanyl is about 100 times more potent than morphine and 50 times more potent than heroin. Breathing can stop after use of just 2 mg of fentanyl, which is about as much as trace amounts of table salt. Fentanyl has replaced heroin in many cities as the pandemic changed the relative ease of importing raw drugs such as heroin.
Another important trend is that fentanyl production and distribution throughout the United States have expanded. The ease of manufacture in unregulated sectors of the Chinese and Mexican economies is difficult for U.S. authorities to curb or eliminate. The Internet promotes novel strategies for synthesizing the substance, spreading its production across many labs; suppliers use the U.S. Postal Service for distribution, and e-commerce helps to get the drug from manufacturers to U.S. consumers for fentanyl transactions.
A recent RAND report observes that, for only $10 through the postal service, suppliers can ship a 1-kg parcel from China to the United States, and private shipments cost about $100.7 And with large volumes of legal trade between the two countries making rigorous scrutiny of products difficult, especially given the light weight of fentanyl, suppliers find it relatively easy to hide illicit substances in licit shipments. Opioid users have made the switch to fentanyl, and have seen fentanyl added to cocaine and methamphetamine they buy on the streets.
OUD and buprenorphine
Fentanyl is one part of the overdose crisis. Opioid use disorder (OUD) is the other. Both need to be addressed if we are to make any progress in this epidemic of death and dependency.
The OUD crisis continues amid the pandemic – and isn’t going away.8 Slips, relapses, and overdoses are all too common. Medication-assisted treatment (MAT) and OUD treatment programs are essential parts of our response to overdose initiatives. After naloxone rescue, the best anti-overdose response is to get the OUD patient into treatment with MATs. Patients with OUD have continuously high risks of overdose. The best outcomes appear to be related to treatment duration of greater than 2 years. But it is common to see patients with OUDs who have been in treatment multiple times, taking MATs, dropping out, overdosing, and dying. Some have been described as treatment resistant.9 It is clear that treatment can work, but also that even evidence-based treatments often fail.10
A recent study compared OUD patients who continued treatment for 6-9 months to those patients who had continued MAT treatment for 15-18 months. The longer the treatment, the fewer emergencies, prescriptions, or hospitalizations.11
But this study reminds us that all OUD patients, whether they are currently buprenorphine treated or not, experience overdoses and emergency department interventions. Short and longer treatment groups have a similar nonfatal overdose rate, about 6%, and went to the emergency department at a high rate, above 40%. Discontinuation of buprenorphine treatment is a major risk factor in opioid relapse, emergency department visits, and overdose. Cures are not common. Whether an OUD patient is being treated or has been treated in the past, carrying naloxone (brand name Narcan), makes sense and can save lives.
Methadone still considered most effective
Methadone is a synthetic opioid first studied as a treatment for OUD at Rockefeller University in New York City in the 1960s. Methadone may be the most effective treatment for OUD in promoting treatment retention for years, decreasing intravenous drug use, and decreasing deaths.12 It has been studied and safely used in treatment programs for decades. Methadone is typically administered in a clinic, daily, and with observation. In addition, methadone patients periodically take urine drug tests, which can distinguish methadone from substances of abuse. They also receive counseling. But methadone can be prescribed and administered only in methadone clinics in the United States. It is available for prescription in primary care clinics in Great Britain, Canada, and Australia.13 Numerous experts have suggested passing new legislation aimed at changing how methadone can be prescribed. Allowing primary care to administer methadone, just like buprenorphine, can improve access and benefit OUD patients.12
Availability of Narcan is critical
A comprehensive treatment model for OUDs includes prescribing naloxone, encouraging those patients with an OUD and their loved ones to have naloxone with them, and providing MATs and appropriate therapies, such as counseling.
As described by Allison L. Pitt and colleagues at Stanford (Calif.) University,14 the United States might be on track to have up to 500,000 deaths tied to opioid overdoses that might occur over the next 5 years. They modeled the effect on overdose of a long list of interventions, but only a few had an impact. At the top of the list was naloxone availability. We need to focus on saving lives by increasing naloxone availability, improving initiation, and expanding access to MAT, and increasing psychosocial treatment to improve outcomes, increase life-years and quality-adjusted life-years, and reduce opioid-related deaths. When Ms. Pitt and colleagues looked at what would make the most impact in reducing OUD deaths, it was naloxone. Pain patients on higher doses of opioids, nonprescription opioid users, OUD patients should be given naloxone prescriptions. While many can give a Heimlich to a choking person or CPR, few have naloxone to rescue a person who has overdosed on opioids. If an overdose is suspected, it should be administered by anyone who has it, as soon as possible. Then, the person who is intervening should call 911.
What we can do today
At this moment, clinicians can follow the Surgeon General’s advice,15 and prescribe naloxone.
We should give naloxone to OUD patients and their families, to pain patients at dosages of greater than or equal to 50 MME. Our top priorities should be patients with comorbid pain syndromes, those being treated with benzodiazepines and sleeping medications, and patients with alcohol use disorders. This is also an important intervention for those who binge drink, and have sleep apnea, and heart and respiratory diseases.
Naloxone is available without a prescription in at least 43 states. Naloxone is available in harm reduction programs and in hospitals, and is carried by emergency medical staff, law enforcement, and EMTs. It also is available on the streets, though it does not appear to have a dollar value like opioids or even buprenorphine. Also, the availability of naloxone in pharmacies has made it easier for family members and caregivers of pain patients or those with OUD to have it to administer in an emergency.
An excellent place for MDs to start is to do more to encourage all patients with OUD to carry naloxone, for their loved ones to carry naloxone, and for their homes to have naloxone nearby in the bedroom or bathroom. It is not logical to expect a person with an OUD to rescue themselves. Current and past OUD patients, as well as their loved ones, are at high risk – and should have naloxone nearby at all times.
Naloxone reverses an opioid overdose, but it should be thought about like cardioversion or CPR rather than a treatment for an underlying disease. Increasing access to buprenorphine, buprenorphine + naloxone, and naltrexone treatment for OUDs is an important organizing principle. Initiation of MAT treatment in the emergency setting or most anywhere and any place a patient with an OUD can begin treatment is necessary. Treatment with buprenorphine or methadone reduces opioid overdose and opioid-related acute care use.16
Reducing racial disparities in OUD treatment is necessary, because buprenorphine treatment is concentrated among White patients who either use private insurance or are self-pay.17 Reducing barriers to methadone program licenses, expanding sites for distribution,18 prescribing methadone in an office setting might help. Clinicians can do a better job of explaining the risks associated with opioid prescriptions, including diversion and overdose, and the benefits of OUD treatment. So, To reduce opioid overdoses, we must increase physician competencies in addiction medicine.
Dr. Gold is professor of psychiatry (adjunct) at Washington University, St. Louis. He is the 17th Distinguished Alumni Professor at the University of Florida, Gainesville. For more than 40 years, Dr. Gold has worked on developing models for understanding the effects of opioid, tobacco, cocaine, and other drugs, as well as food, on the brain and behavior. He disclosed financial ties with ADAPT Pharma and Magstim Ltd.
References
1. Kamp J. Overdose deaths rise, may reach record level, federal data show. Wall Street Journal. 2020 Jul 15.
2. 12 month–ending provisional number of drug overdose drugs. Centers for Disease Control and Prevention. 2020 Jul 5.
3. Katz J et al. In shadow of pandemic, U.S. drug overdose deaths resurge to record. New York Times. 2020 Jul 15.
4. Gold MS. The fentanyl crisis is only getting worse. Addiction Policy Forum. Updated 2020 Mar 12.
5. Gold MS. Mo Med. 2020-Mar-Apr;117(2):99-101.
6. Reports of increases in opioid-related overdoses and other concerns during the COVID-19 pandemic. American Medical Association. Issue brief. Updated 2020 Jul 20.
7. Pardo B et al. The future of fentanyl and other synthetic opioids. RAND report.
8. Gold MS. New challenges in the opioid epidemic. Addiction Policy Forum. 2020 Jun 4.
9. Patterson Silver Wolf DA and Gold MS. J Neurol Sci. 2020;411:116718.
10. Oesterle TS et al. Mayo Clin Proc. 2019;94(10):2072-86.
11. Connery HS and Weiss RD. Am J Psychiatry. 2020;177(2):104-6.
12. Kleber HD. JAMA. 2008;300(19):2303-5.
13. Samet JH et al. N Engl J Med. 2018;379(1):7-8.
14. Pitt AL et al. Am J Public Health. 2018;108(10):1394-1400.
15. U.S. Surgeon General’s Advisory on Naloxone and Opioid Overdose. hhs.gov.
16. Wakeman SE et al. JAMA Netw Open. 2020;3(2):e1920622.
17. Lagisetty PA et al. JAMA Psychiatry. 2019;76(9):979-81.
18. Kleinman RA. JAMA Psychiatry. 2020 Jul 15. doi: 10.1001/jamapsychiatry.2020.1624.
Polygenic risk score may predict VTE in adolescents, but not adults, with ALL
Although patients with acute lymphoblastic leukemia (ALL) are at known risk of venous thromboembolism (VTE), there was no overall genetic correlation found to be associated with that susceptibility in the overall population. However, a significant genetic predisposition to VTE was found in adolescent ALL patients, according to a report published in Thrombosis Research.
The researchers assessed the prospectively registered VTE events and collected germline DNA in patients aged 1-45.9 years in the Nordic Society of Pediatric Hematology and Oncology (NOPHO) ALL2008 study, which took place from 2008 to 2016. The researchers performed polygenic risk score (PRS) analysis on VTE development in the NOPHO cohort, according to Kirsten Brunsvig Jarvis, MD, of Oslo University Hospital, and colleagues.
The researchers used summary statistics from two large genomewide association studies on VTE in adults (the International Network of Venous Thromboembolism Clinical Research Networks [INVENT] consortium and the UK Biobank).
Of 1,252 patients with ALL in the genetic cohort, 89 developed VTE (2.5-year cumulative incidence, 7.2%; 95% confidence interval,5.7-8.6) at a median 12.7 weeks from diagnosis.
Overall, an analysis of single-nucleotide polymorphisms (SNPs) from INVENT and UK Biobank studies did not reveal evidence of polygenic correlation with VTE in patients with ALL, the researchers reported. However, when separating adolescents aged 10.0-17.9 years (n = 231) from adults aged 18 years or older (n = 127), they saw polygenic overlap between the INVENT study and thromboembolism development in the adolescent population.
The best-fit polygenic risk score, including 16,144 SNPs, was associated with VTE in adolescents with ALL at a hazard ratio of 1.76 (95% CI, 1.23-2.52; P = .02).
Adolescent vs. adult risk
The researchers expressed surprise that they did not find evidence of genetic overlap in adults. But they stated that, in general, VTE occurs more frequently in adults as part of natural aging, while children and adolescents are physiologically protected. This might explain why genetics might play a stronger role in the high-risk situation of cancer and chemotherapy in adolescents who do not have as many additional exogenic risk factors as adults.
“The usefulness of genetic studies on [V]TE in the general adult population is limited when it comes to understanding the etiology of [V]TE in patients with ALL. However, we found evidence of polygenic overlap in subgroup analysis of adolescents aged 10.0-17.9 years with ALL, and we believe the genetics of [V]TE in this group should be further explored in future risk prediction models for identification of those who might benefit from thromboprophylaxis,” the researchers concluded.
The study was supported by research grant from the South-Eastern Norway Regional Health Authority. The authors reported that they had no conflicts of interest.
SOURCE: Jarvis KB et al. Thromb Res. 2020 Aug 11.doi: 10.1016/j.thromres.2020.08.015.
Although patients with acute lymphoblastic leukemia (ALL) are at known risk of venous thromboembolism (VTE), there was no overall genetic correlation found to be associated with that susceptibility in the overall population. However, a significant genetic predisposition to VTE was found in adolescent ALL patients, according to a report published in Thrombosis Research.
The researchers assessed the prospectively registered VTE events and collected germline DNA in patients aged 1-45.9 years in the Nordic Society of Pediatric Hematology and Oncology (NOPHO) ALL2008 study, which took place from 2008 to 2016. The researchers performed polygenic risk score (PRS) analysis on VTE development in the NOPHO cohort, according to Kirsten Brunsvig Jarvis, MD, of Oslo University Hospital, and colleagues.
The researchers used summary statistics from two large genomewide association studies on VTE in adults (the International Network of Venous Thromboembolism Clinical Research Networks [INVENT] consortium and the UK Biobank).
Of 1,252 patients with ALL in the genetic cohort, 89 developed VTE (2.5-year cumulative incidence, 7.2%; 95% confidence interval,5.7-8.6) at a median 12.7 weeks from diagnosis.
Overall, an analysis of single-nucleotide polymorphisms (SNPs) from INVENT and UK Biobank studies did not reveal evidence of polygenic correlation with VTE in patients with ALL, the researchers reported. However, when separating adolescents aged 10.0-17.9 years (n = 231) from adults aged 18 years or older (n = 127), they saw polygenic overlap between the INVENT study and thromboembolism development in the adolescent population.
The best-fit polygenic risk score, including 16,144 SNPs, was associated with VTE in adolescents with ALL at a hazard ratio of 1.76 (95% CI, 1.23-2.52; P = .02).
Adolescent vs. adult risk
The researchers expressed surprise that they did not find evidence of genetic overlap in adults. But they stated that, in general, VTE occurs more frequently in adults as part of natural aging, while children and adolescents are physiologically protected. This might explain why genetics might play a stronger role in the high-risk situation of cancer and chemotherapy in adolescents who do not have as many additional exogenic risk factors as adults.
“The usefulness of genetic studies on [V]TE in the general adult population is limited when it comes to understanding the etiology of [V]TE in patients with ALL. However, we found evidence of polygenic overlap in subgroup analysis of adolescents aged 10.0-17.9 years with ALL, and we believe the genetics of [V]TE in this group should be further explored in future risk prediction models for identification of those who might benefit from thromboprophylaxis,” the researchers concluded.
The study was supported by research grant from the South-Eastern Norway Regional Health Authority. The authors reported that they had no conflicts of interest.
SOURCE: Jarvis KB et al. Thromb Res. 2020 Aug 11.doi: 10.1016/j.thromres.2020.08.015.
Although patients with acute lymphoblastic leukemia (ALL) are at known risk of venous thromboembolism (VTE), there was no overall genetic correlation found to be associated with that susceptibility in the overall population. However, a significant genetic predisposition to VTE was found in adolescent ALL patients, according to a report published in Thrombosis Research.
The researchers assessed the prospectively registered VTE events and collected germline DNA in patients aged 1-45.9 years in the Nordic Society of Pediatric Hematology and Oncology (NOPHO) ALL2008 study, which took place from 2008 to 2016. The researchers performed polygenic risk score (PRS) analysis on VTE development in the NOPHO cohort, according to Kirsten Brunsvig Jarvis, MD, of Oslo University Hospital, and colleagues.
The researchers used summary statistics from two large genomewide association studies on VTE in adults (the International Network of Venous Thromboembolism Clinical Research Networks [INVENT] consortium and the UK Biobank).
Of 1,252 patients with ALL in the genetic cohort, 89 developed VTE (2.5-year cumulative incidence, 7.2%; 95% confidence interval,5.7-8.6) at a median 12.7 weeks from diagnosis.
Overall, an analysis of single-nucleotide polymorphisms (SNPs) from INVENT and UK Biobank studies did not reveal evidence of polygenic correlation with VTE in patients with ALL, the researchers reported. However, when separating adolescents aged 10.0-17.9 years (n = 231) from adults aged 18 years or older (n = 127), they saw polygenic overlap between the INVENT study and thromboembolism development in the adolescent population.
The best-fit polygenic risk score, including 16,144 SNPs, was associated with VTE in adolescents with ALL at a hazard ratio of 1.76 (95% CI, 1.23-2.52; P = .02).
Adolescent vs. adult risk
The researchers expressed surprise that they did not find evidence of genetic overlap in adults. But they stated that, in general, VTE occurs more frequently in adults as part of natural aging, while children and adolescents are physiologically protected. This might explain why genetics might play a stronger role in the high-risk situation of cancer and chemotherapy in adolescents who do not have as many additional exogenic risk factors as adults.
“The usefulness of genetic studies on [V]TE in the general adult population is limited when it comes to understanding the etiology of [V]TE in patients with ALL. However, we found evidence of polygenic overlap in subgroup analysis of adolescents aged 10.0-17.9 years with ALL, and we believe the genetics of [V]TE in this group should be further explored in future risk prediction models for identification of those who might benefit from thromboprophylaxis,” the researchers concluded.
The study was supported by research grant from the South-Eastern Norway Regional Health Authority. The authors reported that they had no conflicts of interest.
SOURCE: Jarvis KB et al. Thromb Res. 2020 Aug 11.doi: 10.1016/j.thromres.2020.08.015.
FROM THROMBOSIS RESEARCH
Swallowable ‘sponge on string’ to diagnose esophageal cancer
An experimental cell-collection device that can be administered without anesthesia in a primary care practice was shown to be better at detecting Barrett esophagus than the standard of care in a community-based clinical trial.
Use of this patient-swallowed device, called Cytosponge-TFF3, could allow clinicians to diagnose esophageal conditions such as dysplasia or cancer at an earlier and potentially curable stage, said the investigators. However, it would also increase the likelihood of unnecessary endoscopies, owing to false-positive results.
“In this multicenter, pragmatic, randomized controlled trial we found that an invitation to have a Cytosponge-TFF3 test led to increased diagnosis of Barrett’s esophagus when compared with usual care by general practitioners,” write Rebecca C. Fitzgerald, MD, from the Hutchison/MRC Research Center in Cambridge, England, and colleagues.
The study was published online on Aug. 1 in The Lancet.
“This is a very important study, a landmark study,” said Stephen J. Meltzer, MD, professor of medicine and oncology at Johns Hopkins University, Baltimore, who was approached for comment.
“What it shows is that if you opt to have this procedure, you’re much more likely to have your Barrett’s diagnosed than if you don’t opt to have it,” he said.
He congratulated Dr. Fitzgerald and colleagues for successful completion of a large, primary practice–based clinical utility study.
“Those studies are very difficult to do. This is looking at the actual impact of an intervention, which is the sponge,” he said in an interview.
Soaking up cells
Dr. Meltzer was senior author of a case-control study published in 2019 in Clinical Cancer Research that described use of a similar device. As previously reported, that device, called EsophaCap, uses a “methylation on bead” technique to collect DNA on a swallowed sponge. The DNA is then extracted from the sponge and analyzed with a methylation biomarker panel.
Like the EsophaCap device, the Cytosponge-TFF3 device consists of a compressed, gelatin-coated collection sponge attached to a thread. The patient swallows the device. After the gelatin dissolves and the sponge expands, it is gently withdrawn through the esophagus, picking up cells as it passes through.
The collected cells are then analyzed with an in vitro test for biomarker trefoil factor 3 (TFF3), a sign of intestinal metaplasia that is a histopathologic hallmark of Barrett esophagus, the authors explained.
Cytosponge-TFF3 study
The study by Dr. Fitzgerald and colleagues was conducted in patients taking medications for gastroesophageal reflux. The patients were undergoing treatment at 109 general practice clinics in England.
Eligible patients included adults aged 50 years and older who had been taking acid-suppressing medication for gastroesophageal reflux for more than 6 months and had not undergone endoscopy within the previous 5 years.
The study was randomized at both the clinic level (cluster randomization) and the individual patient level. Patients were assigned to either standard management of gastroesophageal reflux, with endoscopies performed only if recommended by the practitioner, or to the intervention group, where individuals received usual care and were offered the Cytosponge-TFF3 procedure. Those patients whose samples yielded TFF3-positive cells subsequently underwent endoscopy.
Among 6,834 patients assigned to the intervention group, 2,679 (39%) expressed willingness to undergo the Cytosponge-TFF3 procedure. Of this group, 1,750 patients met all of the eligibility criteria on telephone screening and underwent the procedure.
The large majority of patients (95%) who agreed to undergo the procedure were able to swallow the capsule and the attached thread.
Patients in the intervention group who declined the Cytosponge-TFF3 and all patients assigned to the usual-care arm underwent endoscopy only at the recommendation of their primary practitioner.
During a mean follow-up of 12 months, 140 of the 6,834 patients in the intervention group (2%) were diagnosed with Barrett esophagus, compared with 13 of 6,388 patients in the usual-care group (0.2%). The absolute difference per 1000 person-years, the trial’s primary endpoint, was 18.3. The rate ratio adjusted for cluster randomization was 10.6 (P < .001).
A total of four patients in the intervention group were diagnosed with dysplastic Barrett esophagus, and five were diagnosed with stage I esophagogastric cancer. No patients in the usual-care group were diagnosed with either condition.
Of the 1,654 patients in the intervention group who opted for the Cytosponge device and swallowed it successfully, 221 underwent endoscopy after testing positive for TFF3. Of these patients, 131 (59%) were diagnosed with either Barrett esophagus or cancer.
The most common adverse event with the Cytosponge procedure was sore throat, reported by 4% of those who opted for it. In one patient, the thread became detach from the Cytopsonge, necessitating endoscopy to remove the device.
Promising, but refinements needed
In an editorial accompanying the study, Yuri Hanada, MD, and Kenneth K. Wang, MD, from the department of gastroenterology at the Mayo Clinic in Rochester, Minn., said that the Cytosponge-TFF3 procedure “is a promising nonendoscopic screening tool and will represent a component in the screening for Barrett’s esophagus and esophagogastric cancer.”
They noted, however, that it is unlikely to be the sole screening tool for Barrett esophagus and that its use in primary practice may be problematic during the COVID-19 pandemic, because of the release of aerosolized particles as the sponge is withdrawn from the esophagus.
“It might also be necessary to enrich disease prevalence in the screened population by limiting this population to males and people with other risk factors, in order to make this test more cost-effective than previously shown,” they wrote.
Acceptance rate low?
Dr. Meltzer noted that, despite being less invasive than endoscopy, only 39% of the group who could try it agreed to do so.
“It was kind of surprising, because in my experience, when I offer it to my patients, the acceptance is much higher, but that’s not in a controlled clinical trial situation, so I don’t really know what the true percentage is,” he said.
He pointed out that the patients he sees in his clinic are more likely to be symptomatic and highly motivated to accept a test, in contrast to the general patient population in the study.
He also noted that the endoscopy-confirmed prevalence rate of Barrett esophagus or cancer in 221 patients in the intervention group was 59%, suggesting that 41% underwent an unnecessary endoscopy after the Cytosponge screening.
Dr. Fitzgerald and colleagues acknowledged the potential for overdiagnosis with screening. They noted a debate as to whether 1 cm or short segments of Barrett esophagus are a cause for clinical concern.
They also note that the TFF3 test (used in the CytoSponge device) is sensitive and detects some short segments of Barrett esophagus and that, “since this was a pragmatic trial that relied on a coded diagnosis of Barrett’s esophagus, we also identified patients in the usual care group who had short segments of Barrett’s esophagus (1 cm or less in length) and were diagnosed as having the condition, reflecting the variable practice in U.K. hospitals.
“We expect that these patients can be reassured and probably do not require surveillance,” they continued. “This expectation is consistent with the clinical guidelines, which suggest that patients with over 1 cm of salmon-colored epithelium containing intestinal metaplasia should be monitored.”
The study was funded by Cancer Research UK, the U.K. National Institute for Health Research, the U.K. National Health Service, Medtronic, and the Medical Research Council. Dr. Fitzgerald is named on patents related to the Cytosponge-TFF3 test. Dr. Meltzer has cofounded a company, Capsulomics, to commercialize the methylation biomarker panel used in EsophaCap studies. Dr. Wang has received research funding from eNose for research on a device used in a screening study of Barrett esophagus.
Help your patients better understand the risks, testing and treatment options for Barrett’s esophagus by sharing education from the AGA GI Patient Center at http://ow.ly/p9hU30r4oya.
This article first appeared on Medscape.com.
An experimental cell-collection device that can be administered without anesthesia in a primary care practice was shown to be better at detecting Barrett esophagus than the standard of care in a community-based clinical trial.
Use of this patient-swallowed device, called Cytosponge-TFF3, could allow clinicians to diagnose esophageal conditions such as dysplasia or cancer at an earlier and potentially curable stage, said the investigators. However, it would also increase the likelihood of unnecessary endoscopies, owing to false-positive results.
“In this multicenter, pragmatic, randomized controlled trial we found that an invitation to have a Cytosponge-TFF3 test led to increased diagnosis of Barrett’s esophagus when compared with usual care by general practitioners,” write Rebecca C. Fitzgerald, MD, from the Hutchison/MRC Research Center in Cambridge, England, and colleagues.
The study was published online on Aug. 1 in The Lancet.
“This is a very important study, a landmark study,” said Stephen J. Meltzer, MD, professor of medicine and oncology at Johns Hopkins University, Baltimore, who was approached for comment.
“What it shows is that if you opt to have this procedure, you’re much more likely to have your Barrett’s diagnosed than if you don’t opt to have it,” he said.
He congratulated Dr. Fitzgerald and colleagues for successful completion of a large, primary practice–based clinical utility study.
“Those studies are very difficult to do. This is looking at the actual impact of an intervention, which is the sponge,” he said in an interview.
Soaking up cells
Dr. Meltzer was senior author of a case-control study published in 2019 in Clinical Cancer Research that described use of a similar device. As previously reported, that device, called EsophaCap, uses a “methylation on bead” technique to collect DNA on a swallowed sponge. The DNA is then extracted from the sponge and analyzed with a methylation biomarker panel.
Like the EsophaCap device, the Cytosponge-TFF3 device consists of a compressed, gelatin-coated collection sponge attached to a thread. The patient swallows the device. After the gelatin dissolves and the sponge expands, it is gently withdrawn through the esophagus, picking up cells as it passes through.
The collected cells are then analyzed with an in vitro test for biomarker trefoil factor 3 (TFF3), a sign of intestinal metaplasia that is a histopathologic hallmark of Barrett esophagus, the authors explained.
Cytosponge-TFF3 study
The study by Dr. Fitzgerald and colleagues was conducted in patients taking medications for gastroesophageal reflux. The patients were undergoing treatment at 109 general practice clinics in England.
Eligible patients included adults aged 50 years and older who had been taking acid-suppressing medication for gastroesophageal reflux for more than 6 months and had not undergone endoscopy within the previous 5 years.
The study was randomized at both the clinic level (cluster randomization) and the individual patient level. Patients were assigned to either standard management of gastroesophageal reflux, with endoscopies performed only if recommended by the practitioner, or to the intervention group, where individuals received usual care and were offered the Cytosponge-TFF3 procedure. Those patients whose samples yielded TFF3-positive cells subsequently underwent endoscopy.
Among 6,834 patients assigned to the intervention group, 2,679 (39%) expressed willingness to undergo the Cytosponge-TFF3 procedure. Of this group, 1,750 patients met all of the eligibility criteria on telephone screening and underwent the procedure.
The large majority of patients (95%) who agreed to undergo the procedure were able to swallow the capsule and the attached thread.
Patients in the intervention group who declined the Cytosponge-TFF3 and all patients assigned to the usual-care arm underwent endoscopy only at the recommendation of their primary practitioner.
During a mean follow-up of 12 months, 140 of the 6,834 patients in the intervention group (2%) were diagnosed with Barrett esophagus, compared with 13 of 6,388 patients in the usual-care group (0.2%). The absolute difference per 1000 person-years, the trial’s primary endpoint, was 18.3. The rate ratio adjusted for cluster randomization was 10.6 (P < .001).
A total of four patients in the intervention group were diagnosed with dysplastic Barrett esophagus, and five were diagnosed with stage I esophagogastric cancer. No patients in the usual-care group were diagnosed with either condition.
Of the 1,654 patients in the intervention group who opted for the Cytosponge device and swallowed it successfully, 221 underwent endoscopy after testing positive for TFF3. Of these patients, 131 (59%) were diagnosed with either Barrett esophagus or cancer.
The most common adverse event with the Cytosponge procedure was sore throat, reported by 4% of those who opted for it. In one patient, the thread became detach from the Cytopsonge, necessitating endoscopy to remove the device.
Promising, but refinements needed
In an editorial accompanying the study, Yuri Hanada, MD, and Kenneth K. Wang, MD, from the department of gastroenterology at the Mayo Clinic in Rochester, Minn., said that the Cytosponge-TFF3 procedure “is a promising nonendoscopic screening tool and will represent a component in the screening for Barrett’s esophagus and esophagogastric cancer.”
They noted, however, that it is unlikely to be the sole screening tool for Barrett esophagus and that its use in primary practice may be problematic during the COVID-19 pandemic, because of the release of aerosolized particles as the sponge is withdrawn from the esophagus.
“It might also be necessary to enrich disease prevalence in the screened population by limiting this population to males and people with other risk factors, in order to make this test more cost-effective than previously shown,” they wrote.
Acceptance rate low?
Dr. Meltzer noted that, despite being less invasive than endoscopy, only 39% of the group who could try it agreed to do so.
“It was kind of surprising, because in my experience, when I offer it to my patients, the acceptance is much higher, but that’s not in a controlled clinical trial situation, so I don’t really know what the true percentage is,” he said.
He pointed out that the patients he sees in his clinic are more likely to be symptomatic and highly motivated to accept a test, in contrast to the general patient population in the study.
He also noted that the endoscopy-confirmed prevalence rate of Barrett esophagus or cancer in 221 patients in the intervention group was 59%, suggesting that 41% underwent an unnecessary endoscopy after the Cytosponge screening.
Dr. Fitzgerald and colleagues acknowledged the potential for overdiagnosis with screening. They noted a debate as to whether 1 cm or short segments of Barrett esophagus are a cause for clinical concern.
They also note that the TFF3 test (used in the CytoSponge device) is sensitive and detects some short segments of Barrett esophagus and that, “since this was a pragmatic trial that relied on a coded diagnosis of Barrett’s esophagus, we also identified patients in the usual care group who had short segments of Barrett’s esophagus (1 cm or less in length) and were diagnosed as having the condition, reflecting the variable practice in U.K. hospitals.
“We expect that these patients can be reassured and probably do not require surveillance,” they continued. “This expectation is consistent with the clinical guidelines, which suggest that patients with over 1 cm of salmon-colored epithelium containing intestinal metaplasia should be monitored.”
The study was funded by Cancer Research UK, the U.K. National Institute for Health Research, the U.K. National Health Service, Medtronic, and the Medical Research Council. Dr. Fitzgerald is named on patents related to the Cytosponge-TFF3 test. Dr. Meltzer has cofounded a company, Capsulomics, to commercialize the methylation biomarker panel used in EsophaCap studies. Dr. Wang has received research funding from eNose for research on a device used in a screening study of Barrett esophagus.
Help your patients better understand the risks, testing and treatment options for Barrett’s esophagus by sharing education from the AGA GI Patient Center at http://ow.ly/p9hU30r4oya.
This article first appeared on Medscape.com.
An experimental cell-collection device that can be administered without anesthesia in a primary care practice was shown to be better at detecting Barrett esophagus than the standard of care in a community-based clinical trial.
Use of this patient-swallowed device, called Cytosponge-TFF3, could allow clinicians to diagnose esophageal conditions such as dysplasia or cancer at an earlier and potentially curable stage, said the investigators. However, it would also increase the likelihood of unnecessary endoscopies, owing to false-positive results.
“In this multicenter, pragmatic, randomized controlled trial we found that an invitation to have a Cytosponge-TFF3 test led to increased diagnosis of Barrett’s esophagus when compared with usual care by general practitioners,” write Rebecca C. Fitzgerald, MD, from the Hutchison/MRC Research Center in Cambridge, England, and colleagues.
The study was published online on Aug. 1 in The Lancet.
“This is a very important study, a landmark study,” said Stephen J. Meltzer, MD, professor of medicine and oncology at Johns Hopkins University, Baltimore, who was approached for comment.
“What it shows is that if you opt to have this procedure, you’re much more likely to have your Barrett’s diagnosed than if you don’t opt to have it,” he said.
He congratulated Dr. Fitzgerald and colleagues for successful completion of a large, primary practice–based clinical utility study.
“Those studies are very difficult to do. This is looking at the actual impact of an intervention, which is the sponge,” he said in an interview.
Soaking up cells
Dr. Meltzer was senior author of a case-control study published in 2019 in Clinical Cancer Research that described use of a similar device. As previously reported, that device, called EsophaCap, uses a “methylation on bead” technique to collect DNA on a swallowed sponge. The DNA is then extracted from the sponge and analyzed with a methylation biomarker panel.
Like the EsophaCap device, the Cytosponge-TFF3 device consists of a compressed, gelatin-coated collection sponge attached to a thread. The patient swallows the device. After the gelatin dissolves and the sponge expands, it is gently withdrawn through the esophagus, picking up cells as it passes through.
The collected cells are then analyzed with an in vitro test for biomarker trefoil factor 3 (TFF3), a sign of intestinal metaplasia that is a histopathologic hallmark of Barrett esophagus, the authors explained.
Cytosponge-TFF3 study
The study by Dr. Fitzgerald and colleagues was conducted in patients taking medications for gastroesophageal reflux. The patients were undergoing treatment at 109 general practice clinics in England.
Eligible patients included adults aged 50 years and older who had been taking acid-suppressing medication for gastroesophageal reflux for more than 6 months and had not undergone endoscopy within the previous 5 years.
The study was randomized at both the clinic level (cluster randomization) and the individual patient level. Patients were assigned to either standard management of gastroesophageal reflux, with endoscopies performed only if recommended by the practitioner, or to the intervention group, where individuals received usual care and were offered the Cytosponge-TFF3 procedure. Those patients whose samples yielded TFF3-positive cells subsequently underwent endoscopy.
Among 6,834 patients assigned to the intervention group, 2,679 (39%) expressed willingness to undergo the Cytosponge-TFF3 procedure. Of this group, 1,750 patients met all of the eligibility criteria on telephone screening and underwent the procedure.
The large majority of patients (95%) who agreed to undergo the procedure were able to swallow the capsule and the attached thread.
Patients in the intervention group who declined the Cytosponge-TFF3 and all patients assigned to the usual-care arm underwent endoscopy only at the recommendation of their primary practitioner.
During a mean follow-up of 12 months, 140 of the 6,834 patients in the intervention group (2%) were diagnosed with Barrett esophagus, compared with 13 of 6,388 patients in the usual-care group (0.2%). The absolute difference per 1000 person-years, the trial’s primary endpoint, was 18.3. The rate ratio adjusted for cluster randomization was 10.6 (P < .001).
A total of four patients in the intervention group were diagnosed with dysplastic Barrett esophagus, and five were diagnosed with stage I esophagogastric cancer. No patients in the usual-care group were diagnosed with either condition.
Of the 1,654 patients in the intervention group who opted for the Cytosponge device and swallowed it successfully, 221 underwent endoscopy after testing positive for TFF3. Of these patients, 131 (59%) were diagnosed with either Barrett esophagus or cancer.
The most common adverse event with the Cytosponge procedure was sore throat, reported by 4% of those who opted for it. In one patient, the thread became detach from the Cytopsonge, necessitating endoscopy to remove the device.
Promising, but refinements needed
In an editorial accompanying the study, Yuri Hanada, MD, and Kenneth K. Wang, MD, from the department of gastroenterology at the Mayo Clinic in Rochester, Minn., said that the Cytosponge-TFF3 procedure “is a promising nonendoscopic screening tool and will represent a component in the screening for Barrett’s esophagus and esophagogastric cancer.”
They noted, however, that it is unlikely to be the sole screening tool for Barrett esophagus and that its use in primary practice may be problematic during the COVID-19 pandemic, because of the release of aerosolized particles as the sponge is withdrawn from the esophagus.
“It might also be necessary to enrich disease prevalence in the screened population by limiting this population to males and people with other risk factors, in order to make this test more cost-effective than previously shown,” they wrote.
Acceptance rate low?
Dr. Meltzer noted that, despite being less invasive than endoscopy, only 39% of the group who could try it agreed to do so.
“It was kind of surprising, because in my experience, when I offer it to my patients, the acceptance is much higher, but that’s not in a controlled clinical trial situation, so I don’t really know what the true percentage is,” he said.
He pointed out that the patients he sees in his clinic are more likely to be symptomatic and highly motivated to accept a test, in contrast to the general patient population in the study.
He also noted that the endoscopy-confirmed prevalence rate of Barrett esophagus or cancer in 221 patients in the intervention group was 59%, suggesting that 41% underwent an unnecessary endoscopy after the Cytosponge screening.
Dr. Fitzgerald and colleagues acknowledged the potential for overdiagnosis with screening. They noted a debate as to whether 1 cm or short segments of Barrett esophagus are a cause for clinical concern.
They also note that the TFF3 test (used in the CytoSponge device) is sensitive and detects some short segments of Barrett esophagus and that, “since this was a pragmatic trial that relied on a coded diagnosis of Barrett’s esophagus, we also identified patients in the usual care group who had short segments of Barrett’s esophagus (1 cm or less in length) and were diagnosed as having the condition, reflecting the variable practice in U.K. hospitals.
“We expect that these patients can be reassured and probably do not require surveillance,” they continued. “This expectation is consistent with the clinical guidelines, which suggest that patients with over 1 cm of salmon-colored epithelium containing intestinal metaplasia should be monitored.”
The study was funded by Cancer Research UK, the U.K. National Institute for Health Research, the U.K. National Health Service, Medtronic, and the Medical Research Council. Dr. Fitzgerald is named on patents related to the Cytosponge-TFF3 test. Dr. Meltzer has cofounded a company, Capsulomics, to commercialize the methylation biomarker panel used in EsophaCap studies. Dr. Wang has received research funding from eNose for research on a device used in a screening study of Barrett esophagus.
Help your patients better understand the risks, testing and treatment options for Barrett’s esophagus by sharing education from the AGA GI Patient Center at http://ow.ly/p9hU30r4oya.
This article first appeared on Medscape.com.
Impaired senses, especially smell, linked to dementia
new research suggests. The study, which included almost 1,800 participants, adds to emerging evidence that even mild levels of multisensory impairment are associated with accelerated cognitive aging, the researchers noted.
Clinicians should be aware of this link between sensory impairment and dementia risk, said lead author Willa Brenowitz, PhD, assistant professor, department of psychiatry and behavioral sciences, University of California, San Francisco. “Many of these impairments are treatable, or at least physicians can monitor them; and this can improve quality of life, even if it doesn’t improve dementia risk.”
The findings were published online July 12 in Alzheimer’s and Dementia.
Additive effects
Previous research has focused on the link between dementia and individual senses, but this new work is unique in that it focuses on the additive effects of multiple impairments in sensory function, said Dr. Brenowitz. The study included 1,794 dementia-free participants in their 70s from the Health, Aging and Body Composition study, a prospective cohort study of healthy Black and White men and women.
Researchers tested participants’ hearing using a pure tone average without hearing aids and vision using contrast sensitivity with glasses permitted. They also measured vibrations in the big toe to assess touch and had participants identify distinctive odors such as paint thinner, roses, lemons, and onions to assess smell.
A score of 0-3 was assigned based on sample quartiles for each of the four sensory functions. Individuals with the best quartile were assigned a score of 0 and those with the worst were assigned a score of 3.
The investigators added scores across all senses to create a summary score of multisensory function (0-12) and classified the participants into tertiles of good, medium, and poor. Individuals with a score of 0 would have good function in all senses, whereas those with 12 would have poor function in all senses. Those with medium scores could have a mix of impairments.
Participants with good multisensory function were more likely to be healthier than those with poor function. They were also significantly more likely to have completed high school (85.0% vs. 72.1%), were significantly less likely to have diabetes (16.9% vs. 27.9%), and were marginally less likely to have cardiovascular disease, high blood pressure, and history of stroke.
Investigators measured cognition using the Modified Mini-Mental State (3MS) examination, a test of global cognitive function, and the Digit Symbol Substitution Test (DSST), a measure of cognitive processing speed. Cognitive testing was carried out at the beginning of the study and repeated every other year.
Dementia was defined as the use of dementia medication, being hospitalized with dementia as a primary or secondary diagnosis, or having a 3MS score 1.5 standard deviations lower than the race-stratified Health ABC study baseline mean.
Over an average follow-up of 6.3 years, 18% of participants developed dementia.
Dose-response increase
Results showed that, with worsening multisensory function score, the risk for dementia increased in a dose-response manner. In models adjusted for demographics and health conditions, participants with a poor multisensory function score were more than twice as likely to develop dementia than those with a good score (hazard ratio, 2.05; 95% confidence interval, 1.50-2.81; P < .001). Those with a middle multisensory function score were 1.45 times more likely to develop dementia (HR, 1.45; 95% CI, 1.09-1.91; P < .001).
Even a 1-point worse multisensory function score was associated with a 14% higher risk for dementia (95% CI, 8%-21%), while a 4-point worse score was associated with 71% higher risk for dementia (95% CI, 38%-211%).
Smell was the sensory function most strongly associated with dementia risk. Participants whose sense of smell declined by 10% had a 19% higher risk for dementia versus a 1%-3% higher risk for declines in vision, hearing, and touch.
It is not clear why smell was a stronger determinant of dementia risk. However, loss of this sense is often considered to be a marker for Alzheimer’s disease “because it is closely linked with brain regions that are affected” in that disease, said Dr. Brenowitz.
However, that does not necessarily mean smell is more important than vision or hearing, she added. “Even if hearing and vision have a smaller contribution to dementia, they have a stronger potential for intervention.” The findings suggest “some additive or cumulative” effects for loss of the different senses. “There’s an association above and beyond those which can be attributed to individual sensory domains,” she said.
Frailty link
After including mobility, which is a potential mediator, estimates for the multisensory function score were slightly lower. “Walking speed is pretty strongly associated with dementia risk,” Dr. Brenowitz noted. Physical frailty might help explain the link between sensory impairment and dementia risk. “It’s not clear if that’s because people with dementia are declining or because people with frailty are especially vulnerable to dementia,” she said.
The researchers also assessed the role of social support, another potential mechanism by which sensory decline, especially in hearing and vision, could influence dementia risk. Although the study did not find substantial differences in social support measures, the investigators noted that questions assessing social support were limited in scope.
Interactions between multisensory function score and race, APOE e4 allele status, and sex were not significant.
Worsening multisensory function was also linked to faster annual rates of cognitive decline as measured by both the 3MS and DSST. Each 1-point worse score was associated with faster decline (P < .05), even after adjustment for demographics and health conditions.
Possible mechanisms
A number of possible mechanisms may explain the link between poor sensory function and dementia. It could be that neurodegeneration underlying dementia affects the senses, or vision and/or hearing loss leads to social isolation and poor mental health, which in turn could affect dementia risk, the researchers wrote. It also is possible that cardiovascular disease or diabetes affect both dementia risk and sensory impairment.
Dr. Brenowitz noted that, because cognitive tests rely on a certain degree of vision and hearing, impairment of these senses may complicate such tests. Still to be determined is whether correcting sensory impairments, such as wearing corrective lenses or hearing aids, affects dementia risk.
Meanwhile, it might be a good idea to more regularly check sensory function, especially vision and hearing, the researchers suggested. These functions affect various aspects of health and can be assessed rather easily. However, because smell is so strongly associated with dementia risk, Dr. Brenowitz said she would like to see it also become “part of a screening tool.”
A possible study limitation cited was that the researchers checked sensory function only once. “Most likely, some of these would change over time, but at least it captured sensory function at one point,” Dr. Brenowitz said.
“Sheds further light”
Commenting on the study, Jo V. Rushworth, PhD, associate professor and national teaching fellow, De Montfort University Leicester (England), said it “sheds further light on the emerging links” between multisensory impairment and cognitive decline leading to dementia. “The authors show that people with even mild loss of function in various senses are more likely to develop cognitive impairment.”
Dr. Rushworth was not involved with the study but has done research in the area.
The current results suggest that measuring patients’ hearing, vision, sense of smell, and touch might “flag at-risk groups” who could be targeted for dementia prevention strategies, Dr. Rushworth noted. Such tests are noninvasive and potentially less distressing than other methods of diagnosing dementia. “Importantly, the relatively low cost and simplicity of sensory tests offer the potential for more frequent testing and the use of these methods in areas of the world where medical facilities and resources are limited.”
This new study raises the question of whether the observed sensory impairments are a cause or an effect of dementia, Dr. Rushworth noted. “As the authors suggest, decreased sensory function can lead to a decrease in social engagement, mobility, and other factors which would usually contribute to counteracting cognitive decline.”
The study raises other questions, too, said Dr. Rushworth. She noted that the participants who experienced more severe sensory impairments were, on average, 2 years older than those with the least impairments. “To what degree were the observed sensory deficits linked to normal aging rather than dementia?”
As well, Dr. Rushworth pointed out that the molecular mechanisms that “kick-start” dementia are believed to occur in midlife – so possibly at an age younger than the study participants. “Do younger people of a ‘predementia’ age range display multisensory impairments?”
Because study participants could wear glasses during vision tests but were not allowed to wear hearing aids for the hearing tests, further standardization of sensory impairment is required, Dr. Rushworth said.
“Future studies will be essential in determining the value of clinical measurement of multisensory impairment as a possible dementia indicator and prevention strategy,” she concluded.
The study was funded by the National Institute on Aging, the National Institute of Nursing Research, and the Alzheimer’s Association. Dr. Brenowitz and Dr. Rushworth have reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
new research suggests. The study, which included almost 1,800 participants, adds to emerging evidence that even mild levels of multisensory impairment are associated with accelerated cognitive aging, the researchers noted.
Clinicians should be aware of this link between sensory impairment and dementia risk, said lead author Willa Brenowitz, PhD, assistant professor, department of psychiatry and behavioral sciences, University of California, San Francisco. “Many of these impairments are treatable, or at least physicians can monitor them; and this can improve quality of life, even if it doesn’t improve dementia risk.”
The findings were published online July 12 in Alzheimer’s and Dementia.
Additive effects
Previous research has focused on the link between dementia and individual senses, but this new work is unique in that it focuses on the additive effects of multiple impairments in sensory function, said Dr. Brenowitz. The study included 1,794 dementia-free participants in their 70s from the Health, Aging and Body Composition study, a prospective cohort study of healthy Black and White men and women.
Researchers tested participants’ hearing using a pure tone average without hearing aids and vision using contrast sensitivity with glasses permitted. They also measured vibrations in the big toe to assess touch and had participants identify distinctive odors such as paint thinner, roses, lemons, and onions to assess smell.
A score of 0-3 was assigned based on sample quartiles for each of the four sensory functions. Individuals with the best quartile were assigned a score of 0 and those with the worst were assigned a score of 3.
The investigators added scores across all senses to create a summary score of multisensory function (0-12) and classified the participants into tertiles of good, medium, and poor. Individuals with a score of 0 would have good function in all senses, whereas those with 12 would have poor function in all senses. Those with medium scores could have a mix of impairments.
Participants with good multisensory function were more likely to be healthier than those with poor function. They were also significantly more likely to have completed high school (85.0% vs. 72.1%), were significantly less likely to have diabetes (16.9% vs. 27.9%), and were marginally less likely to have cardiovascular disease, high blood pressure, and history of stroke.
Investigators measured cognition using the Modified Mini-Mental State (3MS) examination, a test of global cognitive function, and the Digit Symbol Substitution Test (DSST), a measure of cognitive processing speed. Cognitive testing was carried out at the beginning of the study and repeated every other year.
Dementia was defined as the use of dementia medication, being hospitalized with dementia as a primary or secondary diagnosis, or having a 3MS score 1.5 standard deviations lower than the race-stratified Health ABC study baseline mean.
Over an average follow-up of 6.3 years, 18% of participants developed dementia.
Dose-response increase
Results showed that, with worsening multisensory function score, the risk for dementia increased in a dose-response manner. In models adjusted for demographics and health conditions, participants with a poor multisensory function score were more than twice as likely to develop dementia than those with a good score (hazard ratio, 2.05; 95% confidence interval, 1.50-2.81; P < .001). Those with a middle multisensory function score were 1.45 times more likely to develop dementia (HR, 1.45; 95% CI, 1.09-1.91; P < .001).
Even a 1-point worse multisensory function score was associated with a 14% higher risk for dementia (95% CI, 8%-21%), while a 4-point worse score was associated with 71% higher risk for dementia (95% CI, 38%-211%).
Smell was the sensory function most strongly associated with dementia risk. Participants whose sense of smell declined by 10% had a 19% higher risk for dementia versus a 1%-3% higher risk for declines in vision, hearing, and touch.
It is not clear why smell was a stronger determinant of dementia risk. However, loss of this sense is often considered to be a marker for Alzheimer’s disease “because it is closely linked with brain regions that are affected” in that disease, said Dr. Brenowitz.
However, that does not necessarily mean smell is more important than vision or hearing, she added. “Even if hearing and vision have a smaller contribution to dementia, they have a stronger potential for intervention.” The findings suggest “some additive or cumulative” effects for loss of the different senses. “There’s an association above and beyond those which can be attributed to individual sensory domains,” she said.
Frailty link
After including mobility, which is a potential mediator, estimates for the multisensory function score were slightly lower. “Walking speed is pretty strongly associated with dementia risk,” Dr. Brenowitz noted. Physical frailty might help explain the link between sensory impairment and dementia risk. “It’s not clear if that’s because people with dementia are declining or because people with frailty are especially vulnerable to dementia,” she said.
The researchers also assessed the role of social support, another potential mechanism by which sensory decline, especially in hearing and vision, could influence dementia risk. Although the study did not find substantial differences in social support measures, the investigators noted that questions assessing social support were limited in scope.
Interactions between multisensory function score and race, APOE e4 allele status, and sex were not significant.
Worsening multisensory function was also linked to faster annual rates of cognitive decline as measured by both the 3MS and DSST. Each 1-point worse score was associated with faster decline (P < .05), even after adjustment for demographics and health conditions.
Possible mechanisms
A number of possible mechanisms may explain the link between poor sensory function and dementia. It could be that neurodegeneration underlying dementia affects the senses, or vision and/or hearing loss leads to social isolation and poor mental health, which in turn could affect dementia risk, the researchers wrote. It also is possible that cardiovascular disease or diabetes affect both dementia risk and sensory impairment.
Dr. Brenowitz noted that, because cognitive tests rely on a certain degree of vision and hearing, impairment of these senses may complicate such tests. Still to be determined is whether correcting sensory impairments, such as wearing corrective lenses or hearing aids, affects dementia risk.
Meanwhile, it might be a good idea to more regularly check sensory function, especially vision and hearing, the researchers suggested. These functions affect various aspects of health and can be assessed rather easily. However, because smell is so strongly associated with dementia risk, Dr. Brenowitz said she would like to see it also become “part of a screening tool.”
A possible study limitation cited was that the researchers checked sensory function only once. “Most likely, some of these would change over time, but at least it captured sensory function at one point,” Dr. Brenowitz said.
“Sheds further light”
Commenting on the study, Jo V. Rushworth, PhD, associate professor and national teaching fellow, De Montfort University Leicester (England), said it “sheds further light on the emerging links” between multisensory impairment and cognitive decline leading to dementia. “The authors show that people with even mild loss of function in various senses are more likely to develop cognitive impairment.”
Dr. Rushworth was not involved with the study but has done research in the area.
The current results suggest that measuring patients’ hearing, vision, sense of smell, and touch might “flag at-risk groups” who could be targeted for dementia prevention strategies, Dr. Rushworth noted. Such tests are noninvasive and potentially less distressing than other methods of diagnosing dementia. “Importantly, the relatively low cost and simplicity of sensory tests offer the potential for more frequent testing and the use of these methods in areas of the world where medical facilities and resources are limited.”
This new study raises the question of whether the observed sensory impairments are a cause or an effect of dementia, Dr. Rushworth noted. “As the authors suggest, decreased sensory function can lead to a decrease in social engagement, mobility, and other factors which would usually contribute to counteracting cognitive decline.”
The study raises other questions, too, said Dr. Rushworth. She noted that the participants who experienced more severe sensory impairments were, on average, 2 years older than those with the least impairments. “To what degree were the observed sensory deficits linked to normal aging rather than dementia?”
As well, Dr. Rushworth pointed out that the molecular mechanisms that “kick-start” dementia are believed to occur in midlife – so possibly at an age younger than the study participants. “Do younger people of a ‘predementia’ age range display multisensory impairments?”
Because study participants could wear glasses during vision tests but were not allowed to wear hearing aids for the hearing tests, further standardization of sensory impairment is required, Dr. Rushworth said.
“Future studies will be essential in determining the value of clinical measurement of multisensory impairment as a possible dementia indicator and prevention strategy,” she concluded.
The study was funded by the National Institute on Aging, the National Institute of Nursing Research, and the Alzheimer’s Association. Dr. Brenowitz and Dr. Rushworth have reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
new research suggests. The study, which included almost 1,800 participants, adds to emerging evidence that even mild levels of multisensory impairment are associated with accelerated cognitive aging, the researchers noted.
Clinicians should be aware of this link between sensory impairment and dementia risk, said lead author Willa Brenowitz, PhD, assistant professor, department of psychiatry and behavioral sciences, University of California, San Francisco. “Many of these impairments are treatable, or at least physicians can monitor them; and this can improve quality of life, even if it doesn’t improve dementia risk.”
The findings were published online July 12 in Alzheimer’s and Dementia.
Additive effects
Previous research has focused on the link between dementia and individual senses, but this new work is unique in that it focuses on the additive effects of multiple impairments in sensory function, said Dr. Brenowitz. The study included 1,794 dementia-free participants in their 70s from the Health, Aging and Body Composition study, a prospective cohort study of healthy Black and White men and women.
Researchers tested participants’ hearing using a pure tone average without hearing aids and vision using contrast sensitivity with glasses permitted. They also measured vibrations in the big toe to assess touch and had participants identify distinctive odors such as paint thinner, roses, lemons, and onions to assess smell.
A score of 0-3 was assigned based on sample quartiles for each of the four sensory functions. Individuals with the best quartile were assigned a score of 0 and those with the worst were assigned a score of 3.
The investigators added scores across all senses to create a summary score of multisensory function (0-12) and classified the participants into tertiles of good, medium, and poor. Individuals with a score of 0 would have good function in all senses, whereas those with 12 would have poor function in all senses. Those with medium scores could have a mix of impairments.
Participants with good multisensory function were more likely to be healthier than those with poor function. They were also significantly more likely to have completed high school (85.0% vs. 72.1%), were significantly less likely to have diabetes (16.9% vs. 27.9%), and were marginally less likely to have cardiovascular disease, high blood pressure, and history of stroke.
Investigators measured cognition using the Modified Mini-Mental State (3MS) examination, a test of global cognitive function, and the Digit Symbol Substitution Test (DSST), a measure of cognitive processing speed. Cognitive testing was carried out at the beginning of the study and repeated every other year.
Dementia was defined as the use of dementia medication, being hospitalized with dementia as a primary or secondary diagnosis, or having a 3MS score 1.5 standard deviations lower than the race-stratified Health ABC study baseline mean.
Over an average follow-up of 6.3 years, 18% of participants developed dementia.
Dose-response increase
Results showed that, with worsening multisensory function score, the risk for dementia increased in a dose-response manner. In models adjusted for demographics and health conditions, participants with a poor multisensory function score were more than twice as likely to develop dementia than those with a good score (hazard ratio, 2.05; 95% confidence interval, 1.50-2.81; P < .001). Those with a middle multisensory function score were 1.45 times more likely to develop dementia (HR, 1.45; 95% CI, 1.09-1.91; P < .001).
Even a 1-point worse multisensory function score was associated with a 14% higher risk for dementia (95% CI, 8%-21%), while a 4-point worse score was associated with 71% higher risk for dementia (95% CI, 38%-211%).
Smell was the sensory function most strongly associated with dementia risk. Participants whose sense of smell declined by 10% had a 19% higher risk for dementia versus a 1%-3% higher risk for declines in vision, hearing, and touch.
It is not clear why smell was a stronger determinant of dementia risk. However, loss of this sense is often considered to be a marker for Alzheimer’s disease “because it is closely linked with brain regions that are affected” in that disease, said Dr. Brenowitz.
However, that does not necessarily mean smell is more important than vision or hearing, she added. “Even if hearing and vision have a smaller contribution to dementia, they have a stronger potential for intervention.” The findings suggest “some additive or cumulative” effects for loss of the different senses. “There’s an association above and beyond those which can be attributed to individual sensory domains,” she said.
Frailty link
After including mobility, which is a potential mediator, estimates for the multisensory function score were slightly lower. “Walking speed is pretty strongly associated with dementia risk,” Dr. Brenowitz noted. Physical frailty might help explain the link between sensory impairment and dementia risk. “It’s not clear if that’s because people with dementia are declining or because people with frailty are especially vulnerable to dementia,” she said.
The researchers also assessed the role of social support, another potential mechanism by which sensory decline, especially in hearing and vision, could influence dementia risk. Although the study did not find substantial differences in social support measures, the investigators noted that questions assessing social support were limited in scope.
Interactions between multisensory function score and race, APOE e4 allele status, and sex were not significant.
Worsening multisensory function was also linked to faster annual rates of cognitive decline as measured by both the 3MS and DSST. Each 1-point worse score was associated with faster decline (P < .05), even after adjustment for demographics and health conditions.
Possible mechanisms
A number of possible mechanisms may explain the link between poor sensory function and dementia. It could be that neurodegeneration underlying dementia affects the senses, or vision and/or hearing loss leads to social isolation and poor mental health, which in turn could affect dementia risk, the researchers wrote. It also is possible that cardiovascular disease or diabetes affect both dementia risk and sensory impairment.
Dr. Brenowitz noted that, because cognitive tests rely on a certain degree of vision and hearing, impairment of these senses may complicate such tests. Still to be determined is whether correcting sensory impairments, such as wearing corrective lenses or hearing aids, affects dementia risk.
Meanwhile, it might be a good idea to more regularly check sensory function, especially vision and hearing, the researchers suggested. These functions affect various aspects of health and can be assessed rather easily. However, because smell is so strongly associated with dementia risk, Dr. Brenowitz said she would like to see it also become “part of a screening tool.”
A possible study limitation cited was that the researchers checked sensory function only once. “Most likely, some of these would change over time, but at least it captured sensory function at one point,” Dr. Brenowitz said.
“Sheds further light”
Commenting on the study, Jo V. Rushworth, PhD, associate professor and national teaching fellow, De Montfort University Leicester (England), said it “sheds further light on the emerging links” between multisensory impairment and cognitive decline leading to dementia. “The authors show that people with even mild loss of function in various senses are more likely to develop cognitive impairment.”
Dr. Rushworth was not involved with the study but has done research in the area.
The current results suggest that measuring patients’ hearing, vision, sense of smell, and touch might “flag at-risk groups” who could be targeted for dementia prevention strategies, Dr. Rushworth noted. Such tests are noninvasive and potentially less distressing than other methods of diagnosing dementia. “Importantly, the relatively low cost and simplicity of sensory tests offer the potential for more frequent testing and the use of these methods in areas of the world where medical facilities and resources are limited.”
This new study raises the question of whether the observed sensory impairments are a cause or an effect of dementia, Dr. Rushworth noted. “As the authors suggest, decreased sensory function can lead to a decrease in social engagement, mobility, and other factors which would usually contribute to counteracting cognitive decline.”
The study raises other questions, too, said Dr. Rushworth. She noted that the participants who experienced more severe sensory impairments were, on average, 2 years older than those with the least impairments. “To what degree were the observed sensory deficits linked to normal aging rather than dementia?”
As well, Dr. Rushworth pointed out that the molecular mechanisms that “kick-start” dementia are believed to occur in midlife – so possibly at an age younger than the study participants. “Do younger people of a ‘predementia’ age range display multisensory impairments?”
Because study participants could wear glasses during vision tests but were not allowed to wear hearing aids for the hearing tests, further standardization of sensory impairment is required, Dr. Rushworth said.
“Future studies will be essential in determining the value of clinical measurement of multisensory impairment as a possible dementia indicator and prevention strategy,” she concluded.
The study was funded by the National Institute on Aging, the National Institute of Nursing Research, and the Alzheimer’s Association. Dr. Brenowitz and Dr. Rushworth have reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Risk stratification key in acute pulmonary embolism
All intermediate-risk pulmonary embolism is not the same, Victor F. Tapson, MD, declared at HM20 Virtual, hosted by the Society of Hospital Medicine.
This additional classification is worthwhile because it has important treatment implications.
Patients with intermediate- to low-risk PE, along with those who have truly low-risk PE, require anticoagulation only. In contrast, patients with intermediate- to high-risk PE are at increased risk of decompensation. They have a much higher in-hospital mortality than those with intermediate- to low-risk PE. So hospitalists may want to consult their hospitals’ PE response team (PERT), if there is one, or whoever on staff is involved in helping make decisions about the appropriateness of more aggressive interventions, such as catheter-directed thrombolysis or catheter-directed clot extraction, said Dr. Tapson, director of the venous thromboembolism and pulmonary vascular disease research program at Cedars-Sinai Medical Center in Los Angeles.
“We don’t have evidence of any real proven mortality difference yet in the intermediate-high risk PE group by being more aggressive. I think if the right patients were studied we could see a mortality difference. But one thing I’ve noted is that by being more aggressive – in a cautious manner, in selected patients – we clearly shorten the hospital stay by doing catheter-directed therapy in some of these folks. It saves money,” he observed.
Once the diagnosis of PE is confirmed, the first priority is to get anticoagulation started in all patients with an acceptable bleeding risk, since there is convincing evidence that anticoagulation reduces mortality in PE. The 2019 European Society of Cardiology guidelines recommend a direct-acting oral anticoagulant over warfarin on the basis of persuasive evidence of lower risk of major bleeding coupled with equal or better effectiveness in preventing recurrent PE.
Dr. Tapson said it’s worthwhile for hospitalists to take a close look at these European guidelines (Eur Respir J. 2019 Oct 9. doi: 10.1183/13993003.01647-2019).
“I think our Europeans friends did a really nice job with those guidelines. They’re great guidelines, better than many of the others out there. I think they’re very, very usable,” he said. “I took part in the ACCP [American College of Chest Physicians] guidelines for years. I think they’re very rigorous in terms of the evidence base, but because they’re so rigorous there’s just tons of 2C recommendations, which are basically suggestions. The ESC guidelines are more robust.”
Risk stratification
Once anticoagulation is on board, the next task is risk stratification to determine the need for more aggressive therapy. A high-risk PE is best defined hemodynamically as one causing a systolic blood pressure below 90 mm Hg for at least 15 minutes. The term “high risk” is increasingly replacing “massive” PE, because the size of the clot doesn’t necessarily correlate with its hemodynamic impact.
An intermediate-risk PE is marked by a simplified Pulmonary Embolism Severity Index (sPESI) score of 1 or more, right ventricular dysfunction on echocardiography or CT angiography, or an elevated cardiac troponin level.
The sPESI is a validated, user-friendly tool that grants 1 point each for age over 80, background cardiopulmonary disease, a systolic blood pressure below 100 mm Hg, cancer, a heart rate of 110 bpm or more, and an oxygen saturation level below 90%.
“All you really need to know about a patient’s sPESI score is: Is it more than zero?” he explained.
Indeed, patients with an sPESI score of 0 have a 30-day mortality of 1%. With a score of 1 or more, however, that risk jumps to 10.9%.
No scoring system is 100% accurate, though, and Dr. Tapson emphasized that clinician gestalt plays an important role in PE risk stratification. In terms of clinical indicators of risk, he pays special attention to heart rate.
“I think if I had to pick the one thing that drives my decision the most about whether someone needs more aggressive therapy than anticoagulation, it’s probably heart rate,” he said. “If the heart rate is 70, the patient is probably very stable. Of course, that might not hold up in a patient with conduction problems or who is on a beta blocker, but in general if I see someone who looks good, has a relatively small PE, and a low heart rate, it makes me feel much better. If the heart rate is 130 or 120, I’m much more concerned.”
Both the European guidelines and the PERT Consortium guidelines on the diagnosis, treatment, and follow-up of acute PE (Clin Appl Thromb Hemost. 2019 Jun 17. doi: 10.1177/1076029619853037), which Dr. Tapson coauthored, recommend substratifying intermediate-risk PE into intermediate to low or intermediate to high risk. It’s a straightforward matter: If a patient has either right ventricular dysfunction on imaging or an elevated cardiac troponin, that’s an intermediate- to low-risk PE warranting anticoagulation only. On the other hand, if both right ventricular dysfunction and an elevated troponin are present, the patient has an intermediate- to high-risk PE. Since this distinction translates to a difference in outcome, a consultation with PERT or an experienced PE interventionalist is in order for the intermediate- to high-risk PE, he said.
Dr. Tapson reported receiving research funding from Bayer, Bristol-Myers Squibb, Janssen, BiO2, EKOS/BTG, and Daiichi. He is also a consultant to Janssen and BiO2, and on speakers’ bureaus for EKOS/BTG and Janssen.
All intermediate-risk pulmonary embolism is not the same, Victor F. Tapson, MD, declared at HM20 Virtual, hosted by the Society of Hospital Medicine.
This additional classification is worthwhile because it has important treatment implications.
Patients with intermediate- to low-risk PE, along with those who have truly low-risk PE, require anticoagulation only. In contrast, patients with intermediate- to high-risk PE are at increased risk of decompensation. They have a much higher in-hospital mortality than those with intermediate- to low-risk PE. So hospitalists may want to consult their hospitals’ PE response team (PERT), if there is one, or whoever on staff is involved in helping make decisions about the appropriateness of more aggressive interventions, such as catheter-directed thrombolysis or catheter-directed clot extraction, said Dr. Tapson, director of the venous thromboembolism and pulmonary vascular disease research program at Cedars-Sinai Medical Center in Los Angeles.
“We don’t have evidence of any real proven mortality difference yet in the intermediate-high risk PE group by being more aggressive. I think if the right patients were studied we could see a mortality difference. But one thing I’ve noted is that by being more aggressive – in a cautious manner, in selected patients – we clearly shorten the hospital stay by doing catheter-directed therapy in some of these folks. It saves money,” he observed.
Once the diagnosis of PE is confirmed, the first priority is to get anticoagulation started in all patients with an acceptable bleeding risk, since there is convincing evidence that anticoagulation reduces mortality in PE. The 2019 European Society of Cardiology guidelines recommend a direct-acting oral anticoagulant over warfarin on the basis of persuasive evidence of lower risk of major bleeding coupled with equal or better effectiveness in preventing recurrent PE.
Dr. Tapson said it’s worthwhile for hospitalists to take a close look at these European guidelines (Eur Respir J. 2019 Oct 9. doi: 10.1183/13993003.01647-2019).
“I think our Europeans friends did a really nice job with those guidelines. They’re great guidelines, better than many of the others out there. I think they’re very, very usable,” he said. “I took part in the ACCP [American College of Chest Physicians] guidelines for years. I think they’re very rigorous in terms of the evidence base, but because they’re so rigorous there’s just tons of 2C recommendations, which are basically suggestions. The ESC guidelines are more robust.”
Risk stratification
Once anticoagulation is on board, the next task is risk stratification to determine the need for more aggressive therapy. A high-risk PE is best defined hemodynamically as one causing a systolic blood pressure below 90 mm Hg for at least 15 minutes. The term “high risk” is increasingly replacing “massive” PE, because the size of the clot doesn’t necessarily correlate with its hemodynamic impact.
An intermediate-risk PE is marked by a simplified Pulmonary Embolism Severity Index (sPESI) score of 1 or more, right ventricular dysfunction on echocardiography or CT angiography, or an elevated cardiac troponin level.
The sPESI is a validated, user-friendly tool that grants 1 point each for age over 80, background cardiopulmonary disease, a systolic blood pressure below 100 mm Hg, cancer, a heart rate of 110 bpm or more, and an oxygen saturation level below 90%.
“All you really need to know about a patient’s sPESI score is: Is it more than zero?” he explained.
Indeed, patients with an sPESI score of 0 have a 30-day mortality of 1%. With a score of 1 or more, however, that risk jumps to 10.9%.
No scoring system is 100% accurate, though, and Dr. Tapson emphasized that clinician gestalt plays an important role in PE risk stratification. In terms of clinical indicators of risk, he pays special attention to heart rate.
“I think if I had to pick the one thing that drives my decision the most about whether someone needs more aggressive therapy than anticoagulation, it’s probably heart rate,” he said. “If the heart rate is 70, the patient is probably very stable. Of course, that might not hold up in a patient with conduction problems or who is on a beta blocker, but in general if I see someone who looks good, has a relatively small PE, and a low heart rate, it makes me feel much better. If the heart rate is 130 or 120, I’m much more concerned.”
Both the European guidelines and the PERT Consortium guidelines on the diagnosis, treatment, and follow-up of acute PE (Clin Appl Thromb Hemost. 2019 Jun 17. doi: 10.1177/1076029619853037), which Dr. Tapson coauthored, recommend substratifying intermediate-risk PE into intermediate to low or intermediate to high risk. It’s a straightforward matter: If a patient has either right ventricular dysfunction on imaging or an elevated cardiac troponin, that’s an intermediate- to low-risk PE warranting anticoagulation only. On the other hand, if both right ventricular dysfunction and an elevated troponin are present, the patient has an intermediate- to high-risk PE. Since this distinction translates to a difference in outcome, a consultation with PERT or an experienced PE interventionalist is in order for the intermediate- to high-risk PE, he said.
Dr. Tapson reported receiving research funding from Bayer, Bristol-Myers Squibb, Janssen, BiO2, EKOS/BTG, and Daiichi. He is also a consultant to Janssen and BiO2, and on speakers’ bureaus for EKOS/BTG and Janssen.
All intermediate-risk pulmonary embolism is not the same, Victor F. Tapson, MD, declared at HM20 Virtual, hosted by the Society of Hospital Medicine.
This additional classification is worthwhile because it has important treatment implications.
Patients with intermediate- to low-risk PE, along with those who have truly low-risk PE, require anticoagulation only. In contrast, patients with intermediate- to high-risk PE are at increased risk of decompensation. They have a much higher in-hospital mortality than those with intermediate- to low-risk PE. So hospitalists may want to consult their hospitals’ PE response team (PERT), if there is one, or whoever on staff is involved in helping make decisions about the appropriateness of more aggressive interventions, such as catheter-directed thrombolysis or catheter-directed clot extraction, said Dr. Tapson, director of the venous thromboembolism and pulmonary vascular disease research program at Cedars-Sinai Medical Center in Los Angeles.
“We don’t have evidence of any real proven mortality difference yet in the intermediate-high risk PE group by being more aggressive. I think if the right patients were studied we could see a mortality difference. But one thing I’ve noted is that by being more aggressive – in a cautious manner, in selected patients – we clearly shorten the hospital stay by doing catheter-directed therapy in some of these folks. It saves money,” he observed.
Once the diagnosis of PE is confirmed, the first priority is to get anticoagulation started in all patients with an acceptable bleeding risk, since there is convincing evidence that anticoagulation reduces mortality in PE. The 2019 European Society of Cardiology guidelines recommend a direct-acting oral anticoagulant over warfarin on the basis of persuasive evidence of lower risk of major bleeding coupled with equal or better effectiveness in preventing recurrent PE.
Dr. Tapson said it’s worthwhile for hospitalists to take a close look at these European guidelines (Eur Respir J. 2019 Oct 9. doi: 10.1183/13993003.01647-2019).
“I think our Europeans friends did a really nice job with those guidelines. They’re great guidelines, better than many of the others out there. I think they’re very, very usable,” he said. “I took part in the ACCP [American College of Chest Physicians] guidelines for years. I think they’re very rigorous in terms of the evidence base, but because they’re so rigorous there’s just tons of 2C recommendations, which are basically suggestions. The ESC guidelines are more robust.”
Risk stratification
Once anticoagulation is on board, the next task is risk stratification to determine the need for more aggressive therapy. A high-risk PE is best defined hemodynamically as one causing a systolic blood pressure below 90 mm Hg for at least 15 minutes. The term “high risk” is increasingly replacing “massive” PE, because the size of the clot doesn’t necessarily correlate with its hemodynamic impact.
An intermediate-risk PE is marked by a simplified Pulmonary Embolism Severity Index (sPESI) score of 1 or more, right ventricular dysfunction on echocardiography or CT angiography, or an elevated cardiac troponin level.
The sPESI is a validated, user-friendly tool that grants 1 point each for age over 80, background cardiopulmonary disease, a systolic blood pressure below 100 mm Hg, cancer, a heart rate of 110 bpm or more, and an oxygen saturation level below 90%.
“All you really need to know about a patient’s sPESI score is: Is it more than zero?” he explained.
Indeed, patients with an sPESI score of 0 have a 30-day mortality of 1%. With a score of 1 or more, however, that risk jumps to 10.9%.
No scoring system is 100% accurate, though, and Dr. Tapson emphasized that clinician gestalt plays an important role in PE risk stratification. In terms of clinical indicators of risk, he pays special attention to heart rate.
“I think if I had to pick the one thing that drives my decision the most about whether someone needs more aggressive therapy than anticoagulation, it’s probably heart rate,” he said. “If the heart rate is 70, the patient is probably very stable. Of course, that might not hold up in a patient with conduction problems or who is on a beta blocker, but in general if I see someone who looks good, has a relatively small PE, and a low heart rate, it makes me feel much better. If the heart rate is 130 or 120, I’m much more concerned.”
Both the European guidelines and the PERT Consortium guidelines on the diagnosis, treatment, and follow-up of acute PE (Clin Appl Thromb Hemost. 2019 Jun 17. doi: 10.1177/1076029619853037), which Dr. Tapson coauthored, recommend substratifying intermediate-risk PE into intermediate to low or intermediate to high risk. It’s a straightforward matter: If a patient has either right ventricular dysfunction on imaging or an elevated cardiac troponin, that’s an intermediate- to low-risk PE warranting anticoagulation only. On the other hand, if both right ventricular dysfunction and an elevated troponin are present, the patient has an intermediate- to high-risk PE. Since this distinction translates to a difference in outcome, a consultation with PERT or an experienced PE interventionalist is in order for the intermediate- to high-risk PE, he said.
Dr. Tapson reported receiving research funding from Bayer, Bristol-Myers Squibb, Janssen, BiO2, EKOS/BTG, and Daiichi. He is also a consultant to Janssen and BiO2, and on speakers’ bureaus for EKOS/BTG and Janssen.
FROM HM20 VIRTUAL
Nonhealing Ulcerative Hand Wound
The Diagnosis: Neutrophilic Dermatosis of the Dorsal Hands
Microscopic specimen analysis demonstrated epidermal ulceration, a diffuse dermal neutrophilic infiltrate, and papillary edema (Figure) consistent with neutrophilic dermatosis of the dorsal hands (NDDH). Special stains and cultures were negative for bacterial and fungal organisms. The patient was treated with high-dose oral prednisone 80 mg daily for 1 week (tapered over the course of 7 weeks) and dapsone gel 5% twice daily with rapid wound resolution. An extensive review of systems, age-appropriate malignancy screening, and laboratory evaluation did not demonstrate underlying systemic illness, infection, or malignancy.
Neutrophilic dermatosis of the dorsal hands commonly arises alongside traumatic injury and presents as a nonhealing hand wound.1 It is considered a localized variant of acute febrile neutrophilic dermatosis (Sweet syndrome), a systemic inflammatory condition characterized by fever, malaise, neutrophilia, and elevated inflammatory markers.1,2 Cutaneous lesions are variable and may include pustular nodules; tender, purulent, violaceous plaques with ulceration and crusting; or hemorrhagic bullae resembling coagulopathy or an infectious etiology.1,3 Leukocytoclastic vasculitis may present with bullous or ulcerative lesions and also histologically resembles NDDH.4 Although ulceration typically is not common in Sweet syndrome, the ulcerated lesions with elevated, edematous, and violaceous borders in our patient were characteristic of NDDH.
Neutrophilic dermatosis of the dorsal hands, similar to Sweet syndrome, may arise along with malignancy, infection (eg, respiratory, gastrointestinal, hepatitis C virus), systemic illnesses (eg, inflammatory bowel disease, colitis, rheumatoid arthritis, Raynaud phenomenon), or environmental exposure (eg, fertilizer) or with the use of certain medications (eg, thalidomide, minocycline).1-3,5 Both solid tumors (eg, breast and lung carcinomas) as well as hematologic disturbances (eg, leukemia, myelodysplasia, lymphoma) have been associated with NDDH.1-3 Although NDDH appears to be idiopathic, all patients should undergo an extensive review of systems, laboratory evaluation, and age-appropriate malignancy screening.
Given the rarity of NDDH, necrotic lesion appearance, and potential for secondary infection, patients often are misdiagnosed with infectious etiologies, including necrotizing fasciitis.1,3,6,7 Lesions of blastomycosislike pyoderma also may be pustular or ulcerative with elevated borders resembling NDDH.8 The pathogenesis of this rare condition remains uncertain. Although systemic antibiotics are a commonly utilized treatment modality, their efficacy may be primarily related to their anti-inflammatory properties.8
Mycobacterium marinum is an aquatic nontuberculous mycobacterium that causes ulcerated, nodular, or pustular cutaneous granulomas that may resemble the lesions of NDDH.9 Similar to NDDH, lesions develop in areas of minor skin trauma, often on the upper extremities. At-risk individuals include those in frequent contact with aquatic environments, lending to the term fish tank granuloma. Diagnosis is made through culture, tissue biopsy, or the presence of acid-fast bacilli. Antibiotics such as doxycycline, surgical debridement, or cryotherapy are effective treatments.9
Unlike infectious etiologies of similarly appearing lesions, primary lesions of NDDH are aseptic. Treatment with antibiotics is ineffective, and surgical intervention can result in devastating expansion of existing wounds as well as development of new lesions at surgical margins due to the pathergy effect and Koebner phenomenon.3,6 The initiation of systemic corticosteroids and/or dapsone results in prompt resolution of NDDH.1 In recalcitrant cases or when steroids are contraindicated, other medications may be used including dapsone, colchicine, potassium iodide, indomethacin, or biologics.2
Atypical pyoderma gangrenosum is a bullous variant of pyoderma gangrenosum that is clinically and histologically indistinguishable from NDDH.2,10 Atypical pyoderma gangrenosum frequently presents on the upper extremities, exhibits a pathergy response to trauma, is associated with similar systemic diseases, and is treated identically to NDDH. There is some degree of uncertainty about the classification and pathophysiology of atypical pyoderma gangrenosum, NDDH, and Sweet syndrome. The compelling similarities may indicate that these cutaneous disorders represent a spectrum of the same disease.2,10
Consideration of NDDH in the differential of nonhealing hand wounds is paramount to prevent progression and iatrogenic morbidity associated with delayed and missed diagnosis. Early recognition of NDDH may allow for earlier diagnosis of frequently associated systemic illnesses and malignancies.
- DiCaudo DJ, Connolly SM. Neutrophilic dermatosis (pustular vasculitis) of the dorsal hands: a report of 7 cases and review of the literature. Arch Dermatol. 2002;138:361-365.
- Walling HW, Snipes CJ, Gerami P, et al. The relationship between neutrophilic dermatosis of the dorsal hands and Sweet syndrome: report of 9 cases and comparison to atypical pyoderma gangrenosum. Arch Dermatol. 2006;142:57-63.
- Cheng AMY, Cheng HS, Smith BJ, et al. Neutrophilic dermatosis of the hands: a review of 17 cases. J Hand Surg Am. 2018;43:185.E1-185.E5.
- Russell JP, Gibson LE. Primary cutaneous small vessel vasculitis: approach to diagnosis and treatment. Int J Dermatol. 2006;45:3-13.
- Kaur S, Gupta D, Garg B, et al. Neutrophilic dermatosis of the dorsal hands. Indian Dermatol Online J. 2015;6:42-45.
- Cooke-Norris RH, Youse JS, Gibson LE. Neutrophilic dermatosis of the hands: an underrecognized hematological condition that may result in unnecessary surgery. Am J Hematol. 2009;84:60-61.
- Kroshinsky D, Alloo A, Rothschild B, et al. Necrotizing Sweet syndrome: a new variant of neutrophilic dermatosis mimicking necrotizing fasciitis. J Am Acad Dermatol. 2012;67:945-954.
- Hongal AA, Gejje S. Blastomycosis-like pyoderma--a rare case report. J Clin Diagn Res. 2016;10:WD03-WD04.
- Petrini B. Mycobacterium marinum: ubiquitous agent of waterborne granulomatous skin infections. Eur J Clin Microbiol Infect Dis. 2006;25:609-613.
- Ahronowitz I, Harp J, Shinkai K. Etiology and management of pyoderma gangrenosum: a comprehensive review. Am J Clin Dermatol. 2012;13:191-211.
The Diagnosis: Neutrophilic Dermatosis of the Dorsal Hands
Microscopic specimen analysis demonstrated epidermal ulceration, a diffuse dermal neutrophilic infiltrate, and papillary edema (Figure) consistent with neutrophilic dermatosis of the dorsal hands (NDDH). Special stains and cultures were negative for bacterial and fungal organisms. The patient was treated with high-dose oral prednisone 80 mg daily for 1 week (tapered over the course of 7 weeks) and dapsone gel 5% twice daily with rapid wound resolution. An extensive review of systems, age-appropriate malignancy screening, and laboratory evaluation did not demonstrate underlying systemic illness, infection, or malignancy.

Neutrophilic dermatosis of the dorsal hands commonly arises alongside traumatic injury and presents as a nonhealing hand wound.1 It is considered a localized variant of acute febrile neutrophilic dermatosis (Sweet syndrome), a systemic inflammatory condition characterized by fever, malaise, neutrophilia, and elevated inflammatory markers.1,2 Cutaneous lesions are variable and may include pustular nodules; tender, purulent, violaceous plaques with ulceration and crusting; or hemorrhagic bullae resembling coagulopathy or an infectious etiology.1,3 Leukocytoclastic vasculitis may present with bullous or ulcerative lesions and also histologically resembles NDDH.4 Although ulceration typically is not common in Sweet syndrome, the ulcerated lesions with elevated, edematous, and violaceous borders in our patient were characteristic of NDDH.
Neutrophilic dermatosis of the dorsal hands, similar to Sweet syndrome, may arise along with malignancy, infection (eg, respiratory, gastrointestinal, hepatitis C virus), systemic illnesses (eg, inflammatory bowel disease, colitis, rheumatoid arthritis, Raynaud phenomenon), or environmental exposure (eg, fertilizer) or with the use of certain medications (eg, thalidomide, minocycline).1-3,5 Both solid tumors (eg, breast and lung carcinomas) as well as hematologic disturbances (eg, leukemia, myelodysplasia, lymphoma) have been associated with NDDH.1-3 Although NDDH appears to be idiopathic, all patients should undergo an extensive review of systems, laboratory evaluation, and age-appropriate malignancy screening.
Given the rarity of NDDH, necrotic lesion appearance, and potential for secondary infection, patients often are misdiagnosed with infectious etiologies, including necrotizing fasciitis.1,3,6,7 Lesions of blastomycosislike pyoderma also may be pustular or ulcerative with elevated borders resembling NDDH.8 The pathogenesis of this rare condition remains uncertain. Although systemic antibiotics are a commonly utilized treatment modality, their efficacy may be primarily related to their anti-inflammatory properties.8
Mycobacterium marinum is an aquatic nontuberculous mycobacterium that causes ulcerated, nodular, or pustular cutaneous granulomas that may resemble the lesions of NDDH.9 Similar to NDDH, lesions develop in areas of minor skin trauma, often on the upper extremities. At-risk individuals include those in frequent contact with aquatic environments, lending to the term fish tank granuloma. Diagnosis is made through culture, tissue biopsy, or the presence of acid-fast bacilli. Antibiotics such as doxycycline, surgical debridement, or cryotherapy are effective treatments.9
Unlike infectious etiologies of similarly appearing lesions, primary lesions of NDDH are aseptic. Treatment with antibiotics is ineffective, and surgical intervention can result in devastating expansion of existing wounds as well as development of new lesions at surgical margins due to the pathergy effect and Koebner phenomenon.3,6 The initiation of systemic corticosteroids and/or dapsone results in prompt resolution of NDDH.1 In recalcitrant cases or when steroids are contraindicated, other medications may be used including dapsone, colchicine, potassium iodide, indomethacin, or biologics.2
Atypical pyoderma gangrenosum is a bullous variant of pyoderma gangrenosum that is clinically and histologically indistinguishable from NDDH.2,10 Atypical pyoderma gangrenosum frequently presents on the upper extremities, exhibits a pathergy response to trauma, is associated with similar systemic diseases, and is treated identically to NDDH. There is some degree of uncertainty about the classification and pathophysiology of atypical pyoderma gangrenosum, NDDH, and Sweet syndrome. The compelling similarities may indicate that these cutaneous disorders represent a spectrum of the same disease.2,10
Consideration of NDDH in the differential of nonhealing hand wounds is paramount to prevent progression and iatrogenic morbidity associated with delayed and missed diagnosis. Early recognition of NDDH may allow for earlier diagnosis of frequently associated systemic illnesses and malignancies.
The Diagnosis: Neutrophilic Dermatosis of the Dorsal Hands
Microscopic specimen analysis demonstrated epidermal ulceration, a diffuse dermal neutrophilic infiltrate, and papillary edema (Figure) consistent with neutrophilic dermatosis of the dorsal hands (NDDH). Special stains and cultures were negative for bacterial and fungal organisms. The patient was treated with high-dose oral prednisone 80 mg daily for 1 week (tapered over the course of 7 weeks) and dapsone gel 5% twice daily with rapid wound resolution. An extensive review of systems, age-appropriate malignancy screening, and laboratory evaluation did not demonstrate underlying systemic illness, infection, or malignancy.

Neutrophilic dermatosis of the dorsal hands commonly arises alongside traumatic injury and presents as a nonhealing hand wound.1 It is considered a localized variant of acute febrile neutrophilic dermatosis (Sweet syndrome), a systemic inflammatory condition characterized by fever, malaise, neutrophilia, and elevated inflammatory markers.1,2 Cutaneous lesions are variable and may include pustular nodules; tender, purulent, violaceous plaques with ulceration and crusting; or hemorrhagic bullae resembling coagulopathy or an infectious etiology.1,3 Leukocytoclastic vasculitis may present with bullous or ulcerative lesions and also histologically resembles NDDH.4 Although ulceration typically is not common in Sweet syndrome, the ulcerated lesions with elevated, edematous, and violaceous borders in our patient were characteristic of NDDH.
Neutrophilic dermatosis of the dorsal hands, similar to Sweet syndrome, may arise along with malignancy, infection (eg, respiratory, gastrointestinal, hepatitis C virus), systemic illnesses (eg, inflammatory bowel disease, colitis, rheumatoid arthritis, Raynaud phenomenon), or environmental exposure (eg, fertilizer) or with the use of certain medications (eg, thalidomide, minocycline).1-3,5 Both solid tumors (eg, breast and lung carcinomas) as well as hematologic disturbances (eg, leukemia, myelodysplasia, lymphoma) have been associated with NDDH.1-3 Although NDDH appears to be idiopathic, all patients should undergo an extensive review of systems, laboratory evaluation, and age-appropriate malignancy screening.
Given the rarity of NDDH, necrotic lesion appearance, and potential for secondary infection, patients often are misdiagnosed with infectious etiologies, including necrotizing fasciitis.1,3,6,7 Lesions of blastomycosislike pyoderma also may be pustular or ulcerative with elevated borders resembling NDDH.8 The pathogenesis of this rare condition remains uncertain. Although systemic antibiotics are a commonly utilized treatment modality, their efficacy may be primarily related to their anti-inflammatory properties.8
Mycobacterium marinum is an aquatic nontuberculous mycobacterium that causes ulcerated, nodular, or pustular cutaneous granulomas that may resemble the lesions of NDDH.9 Similar to NDDH, lesions develop in areas of minor skin trauma, often on the upper extremities. At-risk individuals include those in frequent contact with aquatic environments, lending to the term fish tank granuloma. Diagnosis is made through culture, tissue biopsy, or the presence of acid-fast bacilli. Antibiotics such as doxycycline, surgical debridement, or cryotherapy are effective treatments.9
Unlike infectious etiologies of similarly appearing lesions, primary lesions of NDDH are aseptic. Treatment with antibiotics is ineffective, and surgical intervention can result in devastating expansion of existing wounds as well as development of new lesions at surgical margins due to the pathergy effect and Koebner phenomenon.3,6 The initiation of systemic corticosteroids and/or dapsone results in prompt resolution of NDDH.1 In recalcitrant cases or when steroids are contraindicated, other medications may be used including dapsone, colchicine, potassium iodide, indomethacin, or biologics.2
Atypical pyoderma gangrenosum is a bullous variant of pyoderma gangrenosum that is clinically and histologically indistinguishable from NDDH.2,10 Atypical pyoderma gangrenosum frequently presents on the upper extremities, exhibits a pathergy response to trauma, is associated with similar systemic diseases, and is treated identically to NDDH. There is some degree of uncertainty about the classification and pathophysiology of atypical pyoderma gangrenosum, NDDH, and Sweet syndrome. The compelling similarities may indicate that these cutaneous disorders represent a spectrum of the same disease.2,10
Consideration of NDDH in the differential of nonhealing hand wounds is paramount to prevent progression and iatrogenic morbidity associated with delayed and missed diagnosis. Early recognition of NDDH may allow for earlier diagnosis of frequently associated systemic illnesses and malignancies.
- DiCaudo DJ, Connolly SM. Neutrophilic dermatosis (pustular vasculitis) of the dorsal hands: a report of 7 cases and review of the literature. Arch Dermatol. 2002;138:361-365.
- Walling HW, Snipes CJ, Gerami P, et al. The relationship between neutrophilic dermatosis of the dorsal hands and Sweet syndrome: report of 9 cases and comparison to atypical pyoderma gangrenosum. Arch Dermatol. 2006;142:57-63.
- Cheng AMY, Cheng HS, Smith BJ, et al. Neutrophilic dermatosis of the hands: a review of 17 cases. J Hand Surg Am. 2018;43:185.E1-185.E5.
- Russell JP, Gibson LE. Primary cutaneous small vessel vasculitis: approach to diagnosis and treatment. Int J Dermatol. 2006;45:3-13.
- Kaur S, Gupta D, Garg B, et al. Neutrophilic dermatosis of the dorsal hands. Indian Dermatol Online J. 2015;6:42-45.
- Cooke-Norris RH, Youse JS, Gibson LE. Neutrophilic dermatosis of the hands: an underrecognized hematological condition that may result in unnecessary surgery. Am J Hematol. 2009;84:60-61.
- Kroshinsky D, Alloo A, Rothschild B, et al. Necrotizing Sweet syndrome: a new variant of neutrophilic dermatosis mimicking necrotizing fasciitis. J Am Acad Dermatol. 2012;67:945-954.
- Hongal AA, Gejje S. Blastomycosis-like pyoderma--a rare case report. J Clin Diagn Res. 2016;10:WD03-WD04.
- Petrini B. Mycobacterium marinum: ubiquitous agent of waterborne granulomatous skin infections. Eur J Clin Microbiol Infect Dis. 2006;25:609-613.
- Ahronowitz I, Harp J, Shinkai K. Etiology and management of pyoderma gangrenosum: a comprehensive review. Am J Clin Dermatol. 2012;13:191-211.
- DiCaudo DJ, Connolly SM. Neutrophilic dermatosis (pustular vasculitis) of the dorsal hands: a report of 7 cases and review of the literature. Arch Dermatol. 2002;138:361-365.
- Walling HW, Snipes CJ, Gerami P, et al. The relationship between neutrophilic dermatosis of the dorsal hands and Sweet syndrome: report of 9 cases and comparison to atypical pyoderma gangrenosum. Arch Dermatol. 2006;142:57-63.
- Cheng AMY, Cheng HS, Smith BJ, et al. Neutrophilic dermatosis of the hands: a review of 17 cases. J Hand Surg Am. 2018;43:185.E1-185.E5.
- Russell JP, Gibson LE. Primary cutaneous small vessel vasculitis: approach to diagnosis and treatment. Int J Dermatol. 2006;45:3-13.
- Kaur S, Gupta D, Garg B, et al. Neutrophilic dermatosis of the dorsal hands. Indian Dermatol Online J. 2015;6:42-45.
- Cooke-Norris RH, Youse JS, Gibson LE. Neutrophilic dermatosis of the hands: an underrecognized hematological condition that may result in unnecessary surgery. Am J Hematol. 2009;84:60-61.
- Kroshinsky D, Alloo A, Rothschild B, et al. Necrotizing Sweet syndrome: a new variant of neutrophilic dermatosis mimicking necrotizing fasciitis. J Am Acad Dermatol. 2012;67:945-954.
- Hongal AA, Gejje S. Blastomycosis-like pyoderma--a rare case report. J Clin Diagn Res. 2016;10:WD03-WD04.
- Petrini B. Mycobacterium marinum: ubiquitous agent of waterborne granulomatous skin infections. Eur J Clin Microbiol Infect Dis. 2006;25:609-613.
- Ahronowitz I, Harp J, Shinkai K. Etiology and management of pyoderma gangrenosum: a comprehensive review. Am J Clin Dermatol. 2012;13:191-211.
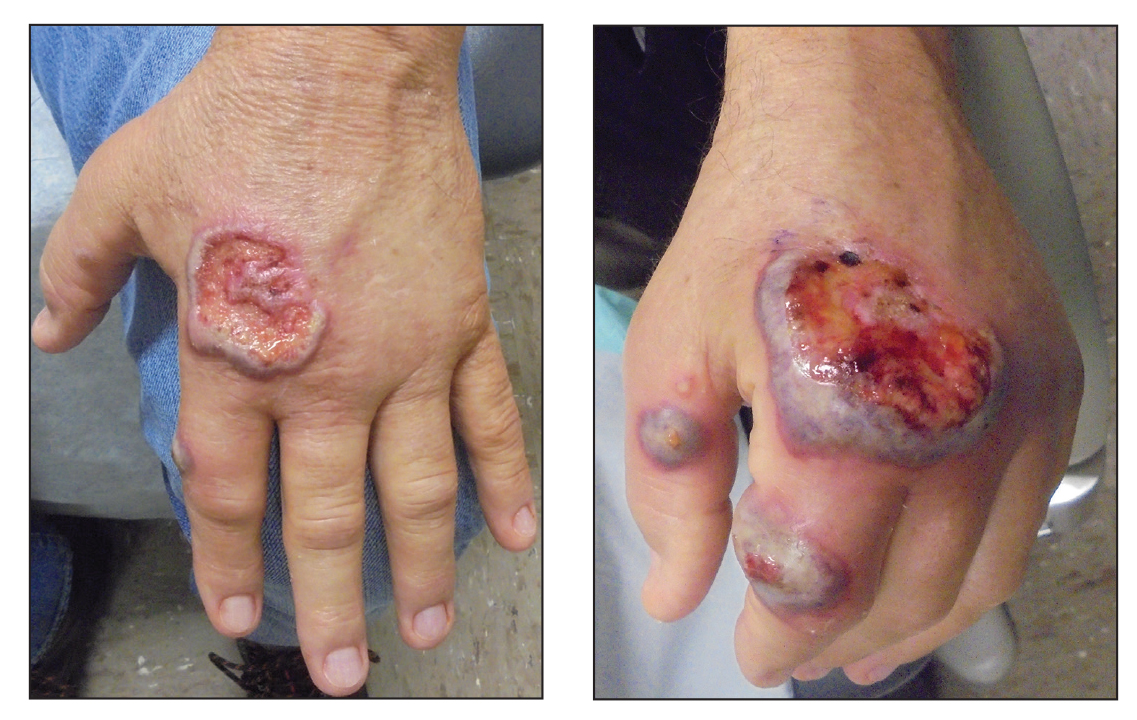
A 63-year-old man presented with an expanding wound on the dorsal aspect of the left hand after striking it on a wall. He sustained a small laceration that progressively became more edematous and developed a violaceous border. He presented to the emergency department the following day and was prescribed bacitracin with no improvement in the lesion. He returned to the emergency department after the symptoms worsened and was subsequently prescribed a 10-day course of oral trimethoprim-sulfamethoxazole (1600/320 mg) twice daily. Physical examination at a follow-up visit 11 days after the initial injury revealed an expanding, 4.3×5.0-cm, ulcerated wound with surrounding erythema and serosanguineous drainage (left). He was started on a 10-day course of amoxicillin–clavulanic acid (1750/250 mg) twice daily and underwent debridement the same day. On postoperative day 2 (13 days following the onset of symptoms), the wound had not improved, and 2 new 1-cm bullae on the left first and second fingers had progressed (right). Erythrocyte sedimentation rate (33 mm/h [reference range, 0–10 mm/h]) and C-reactive protein (3.701 mg/dL [reference range, 0–0.747 mg/dL]) were elevated; however, other laboratory studies, including a complete blood cell count, were within reference range. He remained afebrile, and a review of systems was normal. Punch biopsy specimens were obtained.
Action and awareness are needed to increase immunization rates
August was National Immunization Awareness Month. ... just in time to address the precipitous drop in immunization delivered during the early months of the pandemic.
In May, the Centers for Disease Control and Prevention reported substantial reductions in vaccine doses ordered through the Vaccines for Children program after the declaration of national emergency because of COVID-19 on March 13. Approximately 2.5 million fewer doses of routine, noninfluenza vaccines were administered between Jan. 6 and April 2020, compared with a similar period last year (MMWR Morb Mortal Wkly Rep. 2020 May 15;69[19]:591-3). Declines in immunization rates were echoed by states and municipalities across the United States. Last month, the health system in which I work reported 40,000 children behind on at least one vaccine.
We all know that, when immunization rates drop, outbreaks of vaccine-preventable diseases follow. In order and that is going to take more than a single month.
Identify patients who’ve missed vaccinations
Simply being open and ready to vaccinate is not enough. The Centers for Disease Control and Prevention urges providers to identify patients who have missed vaccines, and call them to schedule in-person visits. Proactively let parents know about strategies implemented in your office to ensure a safe environment.
Pediatricians are accustomed to an influx of patients in the summer, as parents make sure their children have all of the vaccines required for school attendance. As noted in a Washington Post article from Aug. 4, 2020, schools have traditionally served as a backstop for immunization rates. But as many school districts opt to take education online this fall, the implications for vaccine requirements are unclear. District of Columbia public schools continue to require immunization for virtual school attendance, but it is not clear how easily this can be enforced. To read about how other school districts have chosen to address – or not address – immunization requirements for school, visit the the Immunization Action Coalition’s Repository of Resources for Maintaining Immunization during the COVID-19 Pandemic. The repository links to international, national, and state-level policies and guidance and advocacy materials, including talking points, webinars, press releases, media articles from around the United States and social media posts, as well as telehealth resources.
Get some inspiration to talk about vaccination
Need a little inspiration for talking to parents about vaccines? Check out the CDC’s #HowIRecommend video series. These are short videos, most under a minute in length, that explain the importance of vaccination, how to effectively address questions from parents about vaccine safety, and how clinicians routinely recommend same day vaccination to their patients. These videos are part of the CDC’s National Immunization Awareness Month (NIAM) toolkit for communication with health care professionals. A companion toolkit for communicating with parents and patients contains sample social media messages with graphics, along with educational resources to share with parents.
The “Comprehensive Vaccine Education Program – From Training to Practice,” a free online program offered by the Pediatric Infectious Diseases Society, takes a deeper dive into strategies to combat vaccine misinformation and address vaccine hesitancy. Available modules cover vaccine fundamentals, vaccine safety, clinical manifestations of vaccine-preventable diseases, and communication skills that lead to more effective conversations with patients and parents. The curriculum also includes the newest edition of The Vaccine Handbook app, a comprehensive source of practical information for vaccine providers.
Educate young children about vaccines
Don’t leave young children out of the conversation. Vax-Force is a children’s book that explores how vaccination works inside the human body. Dr. Vaxson the pediatrician explains how trusted doctors and scientists made Vicky the Vaccine. Her mission is to tell Willy the White Blood Cell and his Antibuddies how to find and fight bad-guy germs like measles, tetanus, and polio. The book was written by Kelsey Rowe, MD, while she was a medical student at Saint Louis University School of Medicine. Dr. Rowe, now a pediatric resident, notes, “In a world where anti-vaccination rhetoric threatens the health of our global community, this book’s mission is to teach children and adults alike that getting vaccinations is a safe, effective, and even exciting thing to do.” The book is available for purchase at https://www.vax-force.com/, and a small part of every sale is donated to Unicef USA.
Consider vaccination advocacy in your communities
Vaccinate Your Family, a national, nonprofit organization dedicated to protecting people of all ages from vaccine-preventable diseases, suggests that health care providers need to take an active role in raising immunization rates, not just in their own practices, but in their communities. One way to do this is to submit an opinion piece or letter to the editor to a local newspaper describing why it’s important for parents to make sure their child’s immunizations are current. Those who have never written an opinion-editorial should look at the guidance developed by Voices for Vaccines.
How are we doing?
Early data suggest a rebound in immunization rates in May and June, but that is unlikely to close the gap created by disruptions in health care delivery earlier in the year. Collectively, we need to set ambitious goals. Are we just trying to reach prepandemic immunization levels? In Kentucky, where I practice, only 71% of kids aged 19-45 months had received all doses of seven routinely recommended vaccines (≥4 DTaP doses, ≥3 polio doses, ≥1 MMR dose, Hib full series, ≥3 HepB doses, ≥1 varicella dose, and ≥4 PCV doses) based on 2017 National Immunization Survey data. The Healthy People 2020 target goal is 80%. Only 55% of Kentucky girls aged 13-17 years received at least one dose of HPV vaccine, and rates in boys were even lower. Flu vaccine coverage in children 6 months to 17 years also was 55%. The status quo sets the bar too low. To see how your state is doing, check out the interactive map developed by the American Academy of Pediatrics.
Are we attempting to avoid disaster or can we seize the opportunity to protect more children than ever from vaccine-preventable diseases? The latter would really be something to celebrate.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She said she had no relevant financial disclosures. Email her at pdnews@mdedge.com.
August was National Immunization Awareness Month. ... just in time to address the precipitous drop in immunization delivered during the early months of the pandemic.
In May, the Centers for Disease Control and Prevention reported substantial reductions in vaccine doses ordered through the Vaccines for Children program after the declaration of national emergency because of COVID-19 on March 13. Approximately 2.5 million fewer doses of routine, noninfluenza vaccines were administered between Jan. 6 and April 2020, compared with a similar period last year (MMWR Morb Mortal Wkly Rep. 2020 May 15;69[19]:591-3). Declines in immunization rates were echoed by states and municipalities across the United States. Last month, the health system in which I work reported 40,000 children behind on at least one vaccine.
We all know that, when immunization rates drop, outbreaks of vaccine-preventable diseases follow. In order and that is going to take more than a single month.
Identify patients who’ve missed vaccinations
Simply being open and ready to vaccinate is not enough. The Centers for Disease Control and Prevention urges providers to identify patients who have missed vaccines, and call them to schedule in-person visits. Proactively let parents know about strategies implemented in your office to ensure a safe environment.
Pediatricians are accustomed to an influx of patients in the summer, as parents make sure their children have all of the vaccines required for school attendance. As noted in a Washington Post article from Aug. 4, 2020, schools have traditionally served as a backstop for immunization rates. But as many school districts opt to take education online this fall, the implications for vaccine requirements are unclear. District of Columbia public schools continue to require immunization for virtual school attendance, but it is not clear how easily this can be enforced. To read about how other school districts have chosen to address – or not address – immunization requirements for school, visit the the Immunization Action Coalition’s Repository of Resources for Maintaining Immunization during the COVID-19 Pandemic. The repository links to international, national, and state-level policies and guidance and advocacy materials, including talking points, webinars, press releases, media articles from around the United States and social media posts, as well as telehealth resources.
Get some inspiration to talk about vaccination
Need a little inspiration for talking to parents about vaccines? Check out the CDC’s #HowIRecommend video series. These are short videos, most under a minute in length, that explain the importance of vaccination, how to effectively address questions from parents about vaccine safety, and how clinicians routinely recommend same day vaccination to their patients. These videos are part of the CDC’s National Immunization Awareness Month (NIAM) toolkit for communication with health care professionals. A companion toolkit for communicating with parents and patients contains sample social media messages with graphics, along with educational resources to share with parents.
The “Comprehensive Vaccine Education Program – From Training to Practice,” a free online program offered by the Pediatric Infectious Diseases Society, takes a deeper dive into strategies to combat vaccine misinformation and address vaccine hesitancy. Available modules cover vaccine fundamentals, vaccine safety, clinical manifestations of vaccine-preventable diseases, and communication skills that lead to more effective conversations with patients and parents. The curriculum also includes the newest edition of The Vaccine Handbook app, a comprehensive source of practical information for vaccine providers.
Educate young children about vaccines
Don’t leave young children out of the conversation. Vax-Force is a children’s book that explores how vaccination works inside the human body. Dr. Vaxson the pediatrician explains how trusted doctors and scientists made Vicky the Vaccine. Her mission is to tell Willy the White Blood Cell and his Antibuddies how to find and fight bad-guy germs like measles, tetanus, and polio. The book was written by Kelsey Rowe, MD, while she was a medical student at Saint Louis University School of Medicine. Dr. Rowe, now a pediatric resident, notes, “In a world where anti-vaccination rhetoric threatens the health of our global community, this book’s mission is to teach children and adults alike that getting vaccinations is a safe, effective, and even exciting thing to do.” The book is available for purchase at https://www.vax-force.com/, and a small part of every sale is donated to Unicef USA.
Consider vaccination advocacy in your communities
Vaccinate Your Family, a national, nonprofit organization dedicated to protecting people of all ages from vaccine-preventable diseases, suggests that health care providers need to take an active role in raising immunization rates, not just in their own practices, but in their communities. One way to do this is to submit an opinion piece or letter to the editor to a local newspaper describing why it’s important for parents to make sure their child’s immunizations are current. Those who have never written an opinion-editorial should look at the guidance developed by Voices for Vaccines.
How are we doing?
Early data suggest a rebound in immunization rates in May and June, but that is unlikely to close the gap created by disruptions in health care delivery earlier in the year. Collectively, we need to set ambitious goals. Are we just trying to reach prepandemic immunization levels? In Kentucky, where I practice, only 71% of kids aged 19-45 months had received all doses of seven routinely recommended vaccines (≥4 DTaP doses, ≥3 polio doses, ≥1 MMR dose, Hib full series, ≥3 HepB doses, ≥1 varicella dose, and ≥4 PCV doses) based on 2017 National Immunization Survey data. The Healthy People 2020 target goal is 80%. Only 55% of Kentucky girls aged 13-17 years received at least one dose of HPV vaccine, and rates in boys were even lower. Flu vaccine coverage in children 6 months to 17 years also was 55%. The status quo sets the bar too low. To see how your state is doing, check out the interactive map developed by the American Academy of Pediatrics.
Are we attempting to avoid disaster or can we seize the opportunity to protect more children than ever from vaccine-preventable diseases? The latter would really be something to celebrate.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She said she had no relevant financial disclosures. Email her at pdnews@mdedge.com.
August was National Immunization Awareness Month. ... just in time to address the precipitous drop in immunization delivered during the early months of the pandemic.
In May, the Centers for Disease Control and Prevention reported substantial reductions in vaccine doses ordered through the Vaccines for Children program after the declaration of national emergency because of COVID-19 on March 13. Approximately 2.5 million fewer doses of routine, noninfluenza vaccines were administered between Jan. 6 and April 2020, compared with a similar period last year (MMWR Morb Mortal Wkly Rep. 2020 May 15;69[19]:591-3). Declines in immunization rates were echoed by states and municipalities across the United States. Last month, the health system in which I work reported 40,000 children behind on at least one vaccine.
We all know that, when immunization rates drop, outbreaks of vaccine-preventable diseases follow. In order and that is going to take more than a single month.
Identify patients who’ve missed vaccinations
Simply being open and ready to vaccinate is not enough. The Centers for Disease Control and Prevention urges providers to identify patients who have missed vaccines, and call them to schedule in-person visits. Proactively let parents know about strategies implemented in your office to ensure a safe environment.
Pediatricians are accustomed to an influx of patients in the summer, as parents make sure their children have all of the vaccines required for school attendance. As noted in a Washington Post article from Aug. 4, 2020, schools have traditionally served as a backstop for immunization rates. But as many school districts opt to take education online this fall, the implications for vaccine requirements are unclear. District of Columbia public schools continue to require immunization for virtual school attendance, but it is not clear how easily this can be enforced. To read about how other school districts have chosen to address – or not address – immunization requirements for school, visit the the Immunization Action Coalition’s Repository of Resources for Maintaining Immunization during the COVID-19 Pandemic. The repository links to international, national, and state-level policies and guidance and advocacy materials, including talking points, webinars, press releases, media articles from around the United States and social media posts, as well as telehealth resources.
Get some inspiration to talk about vaccination
Need a little inspiration for talking to parents about vaccines? Check out the CDC’s #HowIRecommend video series. These are short videos, most under a minute in length, that explain the importance of vaccination, how to effectively address questions from parents about vaccine safety, and how clinicians routinely recommend same day vaccination to their patients. These videos are part of the CDC’s National Immunization Awareness Month (NIAM) toolkit for communication with health care professionals. A companion toolkit for communicating with parents and patients contains sample social media messages with graphics, along with educational resources to share with parents.
The “Comprehensive Vaccine Education Program – From Training to Practice,” a free online program offered by the Pediatric Infectious Diseases Society, takes a deeper dive into strategies to combat vaccine misinformation and address vaccine hesitancy. Available modules cover vaccine fundamentals, vaccine safety, clinical manifestations of vaccine-preventable diseases, and communication skills that lead to more effective conversations with patients and parents. The curriculum also includes the newest edition of The Vaccine Handbook app, a comprehensive source of practical information for vaccine providers.
Educate young children about vaccines
Don’t leave young children out of the conversation. Vax-Force is a children’s book that explores how vaccination works inside the human body. Dr. Vaxson the pediatrician explains how trusted doctors and scientists made Vicky the Vaccine. Her mission is to tell Willy the White Blood Cell and his Antibuddies how to find and fight bad-guy germs like measles, tetanus, and polio. The book was written by Kelsey Rowe, MD, while she was a medical student at Saint Louis University School of Medicine. Dr. Rowe, now a pediatric resident, notes, “In a world where anti-vaccination rhetoric threatens the health of our global community, this book’s mission is to teach children and adults alike that getting vaccinations is a safe, effective, and even exciting thing to do.” The book is available for purchase at https://www.vax-force.com/, and a small part of every sale is donated to Unicef USA.
Consider vaccination advocacy in your communities
Vaccinate Your Family, a national, nonprofit organization dedicated to protecting people of all ages from vaccine-preventable diseases, suggests that health care providers need to take an active role in raising immunization rates, not just in their own practices, but in their communities. One way to do this is to submit an opinion piece or letter to the editor to a local newspaper describing why it’s important for parents to make sure their child’s immunizations are current. Those who have never written an opinion-editorial should look at the guidance developed by Voices for Vaccines.
How are we doing?
Early data suggest a rebound in immunization rates in May and June, but that is unlikely to close the gap created by disruptions in health care delivery earlier in the year. Collectively, we need to set ambitious goals. Are we just trying to reach prepandemic immunization levels? In Kentucky, where I practice, only 71% of kids aged 19-45 months had received all doses of seven routinely recommended vaccines (≥4 DTaP doses, ≥3 polio doses, ≥1 MMR dose, Hib full series, ≥3 HepB doses, ≥1 varicella dose, and ≥4 PCV doses) based on 2017 National Immunization Survey data. The Healthy People 2020 target goal is 80%. Only 55% of Kentucky girls aged 13-17 years received at least one dose of HPV vaccine, and rates in boys were even lower. Flu vaccine coverage in children 6 months to 17 years also was 55%. The status quo sets the bar too low. To see how your state is doing, check out the interactive map developed by the American Academy of Pediatrics.
Are we attempting to avoid disaster or can we seize the opportunity to protect more children than ever from vaccine-preventable diseases? The latter would really be something to celebrate.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She said she had no relevant financial disclosures. Email her at pdnews@mdedge.com.
FDA approves viltolarsen (Viltepso) for Duchenne muscular dystrophy
The FDA approved golodirsen (Vyondys 53, Sarepta Therapeutics) for this indication last year.
“The FDA is committed to fostering drug development for serious neurological disorders like Duchenne muscular dystrophy,” Billy Dunn, MD, director, Office of Neuroscience of the FDA’s Center for Drug Evaluation and Research, said in a statement.
The approval of viltolarsen provides “an important treatment option for Duchenne muscular dystrophy patients with this confirmed mutation,” Dr. Dunn said.
Viltolarsen is an antisense oligonucleotide that promotes production of functional dystrophin by masking exon 53 in the dystrophin gene. It was evaluated in two studies involving 32 male patients.
In one study of 16 patients, the increase in dystrophin production was established in eight patients receiving viltolarsen at the recommended dose. In this study, dystrophin levels increased, on average, from 0.6% of normal at baseline to 5.9% of normal at week 25.
The increase in dystrophin production is “reasonably likely to predict clinical benefit,” but a “clinical benefit of the drug has not been established,” the FDA said.
In making the decision, the FDA considered the potential risks associated with the drug, the life-threatening and debilitating nature of the disease, and the lack of available therapies.
Viltolarsen was approved under the FDA’s accelerated approval pathway, which provides for the approval of drugs that treat serious or life-threatening diseases and generally offer a meaningful advantage over existing treatments.
As part of the accelerated approval, the FDA requires the company to do a clinical trial to confirm the drug’s clinical benefit. If the trial fails to verify clinical benefit, the FDA may start proceedings to withdraw approval of the drug, the agency said.
The most common side effects with viltolarsen are upper respiratory tract infection, injection-site reaction, cough, and fever.
Kidney toxicity was not observed in the clinical studies, but the clinical experience with the drug is limited, and kidney toxicity, including potentially fatal glomerulonephritis, has been observed with some antisense oligonucleotides.
“Kidney function should be monitored in patients taking Viltepso,” the FDA advises.
A version of this article originally appeared on Medscape.com.
The FDA approved golodirsen (Vyondys 53, Sarepta Therapeutics) for this indication last year.
“The FDA is committed to fostering drug development for serious neurological disorders like Duchenne muscular dystrophy,” Billy Dunn, MD, director, Office of Neuroscience of the FDA’s Center for Drug Evaluation and Research, said in a statement.
The approval of viltolarsen provides “an important treatment option for Duchenne muscular dystrophy patients with this confirmed mutation,” Dr. Dunn said.
Viltolarsen is an antisense oligonucleotide that promotes production of functional dystrophin by masking exon 53 in the dystrophin gene. It was evaluated in two studies involving 32 male patients.
In one study of 16 patients, the increase in dystrophin production was established in eight patients receiving viltolarsen at the recommended dose. In this study, dystrophin levels increased, on average, from 0.6% of normal at baseline to 5.9% of normal at week 25.
The increase in dystrophin production is “reasonably likely to predict clinical benefit,” but a “clinical benefit of the drug has not been established,” the FDA said.
In making the decision, the FDA considered the potential risks associated with the drug, the life-threatening and debilitating nature of the disease, and the lack of available therapies.
Viltolarsen was approved under the FDA’s accelerated approval pathway, which provides for the approval of drugs that treat serious or life-threatening diseases and generally offer a meaningful advantage over existing treatments.
As part of the accelerated approval, the FDA requires the company to do a clinical trial to confirm the drug’s clinical benefit. If the trial fails to verify clinical benefit, the FDA may start proceedings to withdraw approval of the drug, the agency said.
The most common side effects with viltolarsen are upper respiratory tract infection, injection-site reaction, cough, and fever.
Kidney toxicity was not observed in the clinical studies, but the clinical experience with the drug is limited, and kidney toxicity, including potentially fatal glomerulonephritis, has been observed with some antisense oligonucleotides.
“Kidney function should be monitored in patients taking Viltepso,” the FDA advises.
A version of this article originally appeared on Medscape.com.
The FDA approved golodirsen (Vyondys 53, Sarepta Therapeutics) for this indication last year.
“The FDA is committed to fostering drug development for serious neurological disorders like Duchenne muscular dystrophy,” Billy Dunn, MD, director, Office of Neuroscience of the FDA’s Center for Drug Evaluation and Research, said in a statement.
The approval of viltolarsen provides “an important treatment option for Duchenne muscular dystrophy patients with this confirmed mutation,” Dr. Dunn said.
Viltolarsen is an antisense oligonucleotide that promotes production of functional dystrophin by masking exon 53 in the dystrophin gene. It was evaluated in two studies involving 32 male patients.
In one study of 16 patients, the increase in dystrophin production was established in eight patients receiving viltolarsen at the recommended dose. In this study, dystrophin levels increased, on average, from 0.6% of normal at baseline to 5.9% of normal at week 25.
The increase in dystrophin production is “reasonably likely to predict clinical benefit,” but a “clinical benefit of the drug has not been established,” the FDA said.
In making the decision, the FDA considered the potential risks associated with the drug, the life-threatening and debilitating nature of the disease, and the lack of available therapies.
Viltolarsen was approved under the FDA’s accelerated approval pathway, which provides for the approval of drugs that treat serious or life-threatening diseases and generally offer a meaningful advantage over existing treatments.
As part of the accelerated approval, the FDA requires the company to do a clinical trial to confirm the drug’s clinical benefit. If the trial fails to verify clinical benefit, the FDA may start proceedings to withdraw approval of the drug, the agency said.
The most common side effects with viltolarsen are upper respiratory tract infection, injection-site reaction, cough, and fever.
Kidney toxicity was not observed in the clinical studies, but the clinical experience with the drug is limited, and kidney toxicity, including potentially fatal glomerulonephritis, has been observed with some antisense oligonucleotides.
“Kidney function should be monitored in patients taking Viltepso,” the FDA advises.
A version of this article originally appeared on Medscape.com.