User login
Psychiatry trainees drive COVID-19 palliative care in New York
As SARS-CoV-2 cases surged in New York this past spring, one hospital system met the growing demand for palliative care in COVID-19 patients in acute care and emergency settings by training and redeploying psychiatry trainees, producing 100 consultations during a crisis period. Developers of this program wrote about their experience in the Journal of Pain and Symptom Management.
Research shows that psychiatrists can play an important, complementary role in palliative care, but not many models have explored this in practice. Over a 45-day period in March and April, New York Presbyterian/Columbia University Irving Medical Center saw an influx of 7,600 COVID-19 patients. Many were critically ill, and palliative care needs skyrocketed. Initial efforts to install a palliative care team at the emergency department and a proactive consultation model in the step-down units failed to meet demand for consults.
COVID-19 patients present unique challenges. Their clinical trajectory is less clear than those with cancer or other illnesses, Daniel Shalev, MD, a fellow in hospice and palliative medicine at Columbia University/New York State Psychiatric Institute, New York, and the study’s first author, said in an interview. “Ethical and systems issues around distribution of scarce resources may inflect patients’ and physicians’ responses,” Dr. Shalev said. “And families may not be able to be at the bedside with patients.”
To rapidly expand the palliative care workforce and meet patient needs, Dr. Shalev and colleagues recruited 16 psychiatry trainees from NYP, Columbia University Irving Medical Center, and Weill Cornell Medicine to work at NYP/Columbia University Irving Medical Center’s section of adult palliative medicine. Senior general psychiatry residents, child and adolescent psychiatry fellows, addiction psychiatry fellows, and postresidency T32 research fellows became part of a psychiatry-palliative care liaison team, offering psychosocial support and care goal strategies to patients and families.
Already well-versed in serious illness communication and psychosocial aspects of medical illness, the residents and fellows received additional training and education about SARS-CoV-2 and goals-of-care conversations. Child and adolescent psychiatry fellows participated in a communication workshop about the virus at Weill Cornell Medicine.
Working closely with the medical center’s palliative care service, the liaison team did consults around the clock at the ED under the supervision of a consultation-liaison (C-L) psychiatrist specializing in primary palliative care skills. The team managed 16 cases a day during the peak of New York’s COVID-19 outbreak, operating on a rotating schedule of one to three shifts weekly. Some shifts took place remotely to reduce exposure to the virus.
“We were fortunate that New York Presbyterian was early and aggressive in ensuring all clinical staff had personal protective equipment” in the treatment of COVID-19 patients, Dr. Shalev said.
The C-L psychiatry coordinator served as a traffic controller of sorts, overseeing daily staffing changes, maintaining a psychiatry–palliative care liaison team–shared patient list, and ensuring follow-up and continuity on patient care. The rotating schedule freed up time for trainees to meet other research and outpatient obligations.
The liaison team held a meeting each morning and accompanied the adult palliative care service on its daily virtual rounds to help streamline case management and care coordination among the various palliative care channels. Modifications in personnel took place as cases started to recede. Overall, the team participated in 100 consultations.
The findings show that there is significant overlap in psychiatry and palliative care skill sets, Dr. Shalev said. “Furthermore, many patients benefiting from palliative care services have mental health needs. But there are gaps between psychiatry and palliative care, including a lack of collaboration and cross-training. Our model showed how easily our disciplines can work together to improve the care available to all patients,” he added.
Some things could have gone more smoothly. Working under the duress of a pandemic, project leaders didn’t have enough time to train and supervise the team about advanced symptom management. Psychiatry staff members also weren’t as comfortable with nonpsychiatric symptom management as serious illness communication and psychiatric symptom management. Dr. Shalev expects these growth areas to improve over time.
The model could easily translate to other facilities, he believes. As of this writing, the liaison team was transitioning to a longer-term assignment involving patients on mechanical ventilation and their families.
The program increased access to care during a time of limited resources,and successfully combined psychiatric and palliative services – two specialties that, at times, can have conflicting recommendations, noted Maria I. Lapid, MD, a professor of psychiatry at the Mayo Clinic in Rochester, Minn., and a faculty member of the Mayo Clinic Center for Palliative Medicine, who was not part of the study. As urgent training for psychiatric trainees proved useful in the current crisis, long-term psychiatric programs will need to explore and consider how to integrate palliative care training into the psychiatric curriculum.
“Not only is this relevant in the current pandemic, but this will continue to be relevant in the context of the rapidly aging population” in the United States, said Dr. Lapid.
Dr. Shalev and colleagues declared no conflicts of interest in their study. Their research received no funds or grants from public, commercial, or nonprofit agencies.
SOURCE: Shalev D et al. J Pain Symptom Manage. 2020 Jun 13. doi.org/10.1016/j.jpainsymman.2020.06.009.
As SARS-CoV-2 cases surged in New York this past spring, one hospital system met the growing demand for palliative care in COVID-19 patients in acute care and emergency settings by training and redeploying psychiatry trainees, producing 100 consultations during a crisis period. Developers of this program wrote about their experience in the Journal of Pain and Symptom Management.
Research shows that psychiatrists can play an important, complementary role in palliative care, but not many models have explored this in practice. Over a 45-day period in March and April, New York Presbyterian/Columbia University Irving Medical Center saw an influx of 7,600 COVID-19 patients. Many were critically ill, and palliative care needs skyrocketed. Initial efforts to install a palliative care team at the emergency department and a proactive consultation model in the step-down units failed to meet demand for consults.
COVID-19 patients present unique challenges. Their clinical trajectory is less clear than those with cancer or other illnesses, Daniel Shalev, MD, a fellow in hospice and palliative medicine at Columbia University/New York State Psychiatric Institute, New York, and the study’s first author, said in an interview. “Ethical and systems issues around distribution of scarce resources may inflect patients’ and physicians’ responses,” Dr. Shalev said. “And families may not be able to be at the bedside with patients.”
To rapidly expand the palliative care workforce and meet patient needs, Dr. Shalev and colleagues recruited 16 psychiatry trainees from NYP, Columbia University Irving Medical Center, and Weill Cornell Medicine to work at NYP/Columbia University Irving Medical Center’s section of adult palliative medicine. Senior general psychiatry residents, child and adolescent psychiatry fellows, addiction psychiatry fellows, and postresidency T32 research fellows became part of a psychiatry-palliative care liaison team, offering psychosocial support and care goal strategies to patients and families.
Already well-versed in serious illness communication and psychosocial aspects of medical illness, the residents and fellows received additional training and education about SARS-CoV-2 and goals-of-care conversations. Child and adolescent psychiatry fellows participated in a communication workshop about the virus at Weill Cornell Medicine.
Working closely with the medical center’s palliative care service, the liaison team did consults around the clock at the ED under the supervision of a consultation-liaison (C-L) psychiatrist specializing in primary palliative care skills. The team managed 16 cases a day during the peak of New York’s COVID-19 outbreak, operating on a rotating schedule of one to three shifts weekly. Some shifts took place remotely to reduce exposure to the virus.
“We were fortunate that New York Presbyterian was early and aggressive in ensuring all clinical staff had personal protective equipment” in the treatment of COVID-19 patients, Dr. Shalev said.
The C-L psychiatry coordinator served as a traffic controller of sorts, overseeing daily staffing changes, maintaining a psychiatry–palliative care liaison team–shared patient list, and ensuring follow-up and continuity on patient care. The rotating schedule freed up time for trainees to meet other research and outpatient obligations.
The liaison team held a meeting each morning and accompanied the adult palliative care service on its daily virtual rounds to help streamline case management and care coordination among the various palliative care channels. Modifications in personnel took place as cases started to recede. Overall, the team participated in 100 consultations.
The findings show that there is significant overlap in psychiatry and palliative care skill sets, Dr. Shalev said. “Furthermore, many patients benefiting from palliative care services have mental health needs. But there are gaps between psychiatry and palliative care, including a lack of collaboration and cross-training. Our model showed how easily our disciplines can work together to improve the care available to all patients,” he added.
Some things could have gone more smoothly. Working under the duress of a pandemic, project leaders didn’t have enough time to train and supervise the team about advanced symptom management. Psychiatry staff members also weren’t as comfortable with nonpsychiatric symptom management as serious illness communication and psychiatric symptom management. Dr. Shalev expects these growth areas to improve over time.
The model could easily translate to other facilities, he believes. As of this writing, the liaison team was transitioning to a longer-term assignment involving patients on mechanical ventilation and their families.
The program increased access to care during a time of limited resources,and successfully combined psychiatric and palliative services – two specialties that, at times, can have conflicting recommendations, noted Maria I. Lapid, MD, a professor of psychiatry at the Mayo Clinic in Rochester, Minn., and a faculty member of the Mayo Clinic Center for Palliative Medicine, who was not part of the study. As urgent training for psychiatric trainees proved useful in the current crisis, long-term psychiatric programs will need to explore and consider how to integrate palliative care training into the psychiatric curriculum.
“Not only is this relevant in the current pandemic, but this will continue to be relevant in the context of the rapidly aging population” in the United States, said Dr. Lapid.
Dr. Shalev and colleagues declared no conflicts of interest in their study. Their research received no funds or grants from public, commercial, or nonprofit agencies.
SOURCE: Shalev D et al. J Pain Symptom Manage. 2020 Jun 13. doi.org/10.1016/j.jpainsymman.2020.06.009.
As SARS-CoV-2 cases surged in New York this past spring, one hospital system met the growing demand for palliative care in COVID-19 patients in acute care and emergency settings by training and redeploying psychiatry trainees, producing 100 consultations during a crisis period. Developers of this program wrote about their experience in the Journal of Pain and Symptom Management.
Research shows that psychiatrists can play an important, complementary role in palliative care, but not many models have explored this in practice. Over a 45-day period in March and April, New York Presbyterian/Columbia University Irving Medical Center saw an influx of 7,600 COVID-19 patients. Many were critically ill, and palliative care needs skyrocketed. Initial efforts to install a palliative care team at the emergency department and a proactive consultation model in the step-down units failed to meet demand for consults.
COVID-19 patients present unique challenges. Their clinical trajectory is less clear than those with cancer or other illnesses, Daniel Shalev, MD, a fellow in hospice and palliative medicine at Columbia University/New York State Psychiatric Institute, New York, and the study’s first author, said in an interview. “Ethical and systems issues around distribution of scarce resources may inflect patients’ and physicians’ responses,” Dr. Shalev said. “And families may not be able to be at the bedside with patients.”
To rapidly expand the palliative care workforce and meet patient needs, Dr. Shalev and colleagues recruited 16 psychiatry trainees from NYP, Columbia University Irving Medical Center, and Weill Cornell Medicine to work at NYP/Columbia University Irving Medical Center’s section of adult palliative medicine. Senior general psychiatry residents, child and adolescent psychiatry fellows, addiction psychiatry fellows, and postresidency T32 research fellows became part of a psychiatry-palliative care liaison team, offering psychosocial support and care goal strategies to patients and families.
Already well-versed in serious illness communication and psychosocial aspects of medical illness, the residents and fellows received additional training and education about SARS-CoV-2 and goals-of-care conversations. Child and adolescent psychiatry fellows participated in a communication workshop about the virus at Weill Cornell Medicine.
Working closely with the medical center’s palliative care service, the liaison team did consults around the clock at the ED under the supervision of a consultation-liaison (C-L) psychiatrist specializing in primary palliative care skills. The team managed 16 cases a day during the peak of New York’s COVID-19 outbreak, operating on a rotating schedule of one to three shifts weekly. Some shifts took place remotely to reduce exposure to the virus.
“We were fortunate that New York Presbyterian was early and aggressive in ensuring all clinical staff had personal protective equipment” in the treatment of COVID-19 patients, Dr. Shalev said.
The C-L psychiatry coordinator served as a traffic controller of sorts, overseeing daily staffing changes, maintaining a psychiatry–palliative care liaison team–shared patient list, and ensuring follow-up and continuity on patient care. The rotating schedule freed up time for trainees to meet other research and outpatient obligations.
The liaison team held a meeting each morning and accompanied the adult palliative care service on its daily virtual rounds to help streamline case management and care coordination among the various palliative care channels. Modifications in personnel took place as cases started to recede. Overall, the team participated in 100 consultations.
The findings show that there is significant overlap in psychiatry and palliative care skill sets, Dr. Shalev said. “Furthermore, many patients benefiting from palliative care services have mental health needs. But there are gaps between psychiatry and palliative care, including a lack of collaboration and cross-training. Our model showed how easily our disciplines can work together to improve the care available to all patients,” he added.
Some things could have gone more smoothly. Working under the duress of a pandemic, project leaders didn’t have enough time to train and supervise the team about advanced symptom management. Psychiatry staff members also weren’t as comfortable with nonpsychiatric symptom management as serious illness communication and psychiatric symptom management. Dr. Shalev expects these growth areas to improve over time.
The model could easily translate to other facilities, he believes. As of this writing, the liaison team was transitioning to a longer-term assignment involving patients on mechanical ventilation and their families.
The program increased access to care during a time of limited resources,and successfully combined psychiatric and palliative services – two specialties that, at times, can have conflicting recommendations, noted Maria I. Lapid, MD, a professor of psychiatry at the Mayo Clinic in Rochester, Minn., and a faculty member of the Mayo Clinic Center for Palliative Medicine, who was not part of the study. As urgent training for psychiatric trainees proved useful in the current crisis, long-term psychiatric programs will need to explore and consider how to integrate palliative care training into the psychiatric curriculum.
“Not only is this relevant in the current pandemic, but this will continue to be relevant in the context of the rapidly aging population” in the United States, said Dr. Lapid.
Dr. Shalev and colleagues declared no conflicts of interest in their study. Their research received no funds or grants from public, commercial, or nonprofit agencies.
SOURCE: Shalev D et al. J Pain Symptom Manage. 2020 Jun 13. doi.org/10.1016/j.jpainsymman.2020.06.009.
Hyperpigmentation of the legs
A 90-year-old man was admitted from the Emergency Department (ED) to our inpatient service for difficulty urinating and hematuria. In the ED, a complete blood count (CBC) with differential and a urinalysis were performed. CBC showed a mild normocytic anemia, consistent with the patient’s known chronic kidney disease. The urinalysis revealed moderate blood, trace ketones, proteinuria, small leukocyte esterases, positive nitrites, and more than 182 red blood cells—findings suspicious for a urinary tract infection. Computed tomography of the abdomen and pelvis was notable for a soft-tissue mass in the bladder.
He had a history of coronary artery disease (treated with stent placement), atrial fibrillation, congestive heart failure, hypothyroidism, gastroesophageal reflux disease, gastrointestinal bleeding, chronic obstructive pulmonary disease, a 60-pack-per-year history of tobacco dependence, chronic kidney disease, prostate cancer, benign prostatic hypertrophy, peripheral vascular disease, and gout. Medications included digoxin, metoprolol, torsemide, aspirin, levothyroxine, fluticasone, albuterol, omeprazole, diclofenac, escitalopram, and minocycline.
About 5 years earlier, doctors had discovered a popliteal thrombosis that required emergent thrombectomy of the infragenicular popliteal artery, thromboembolectomy of the right posterior tibial artery, graft angioplasty of the right posterior tibial artery, and right anterior fasciotomy for compartment syndrome.
Ten months later, an abscess formed at the incision site. His physician irrigated the popliteal wound and prescribed intravenous (IV) vancomycin. However, the patient developed an allergy and IV daptomycin was initiated and followed by chronic antibiotic suppression with oral minocycline 100 mg bid for about 3.5 years. Skin discoloration appeared within a year of starting the minocycline.
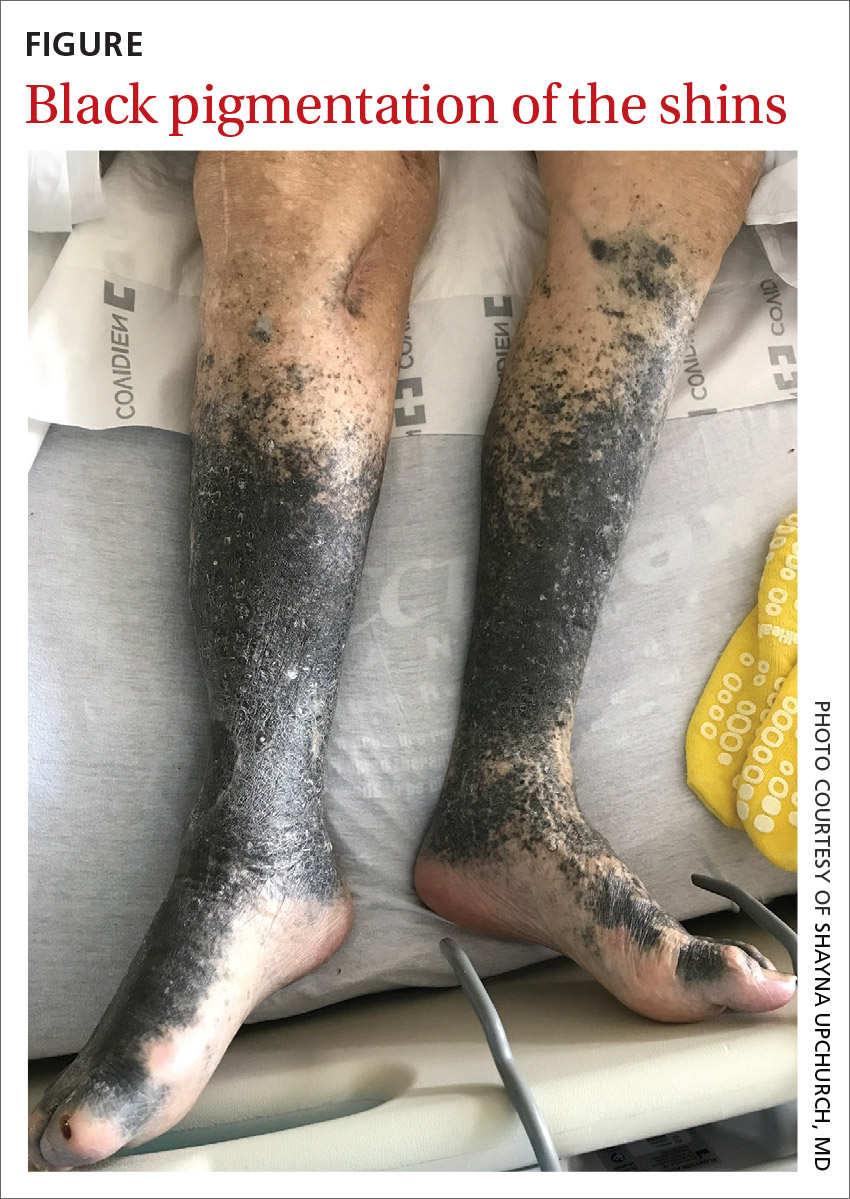
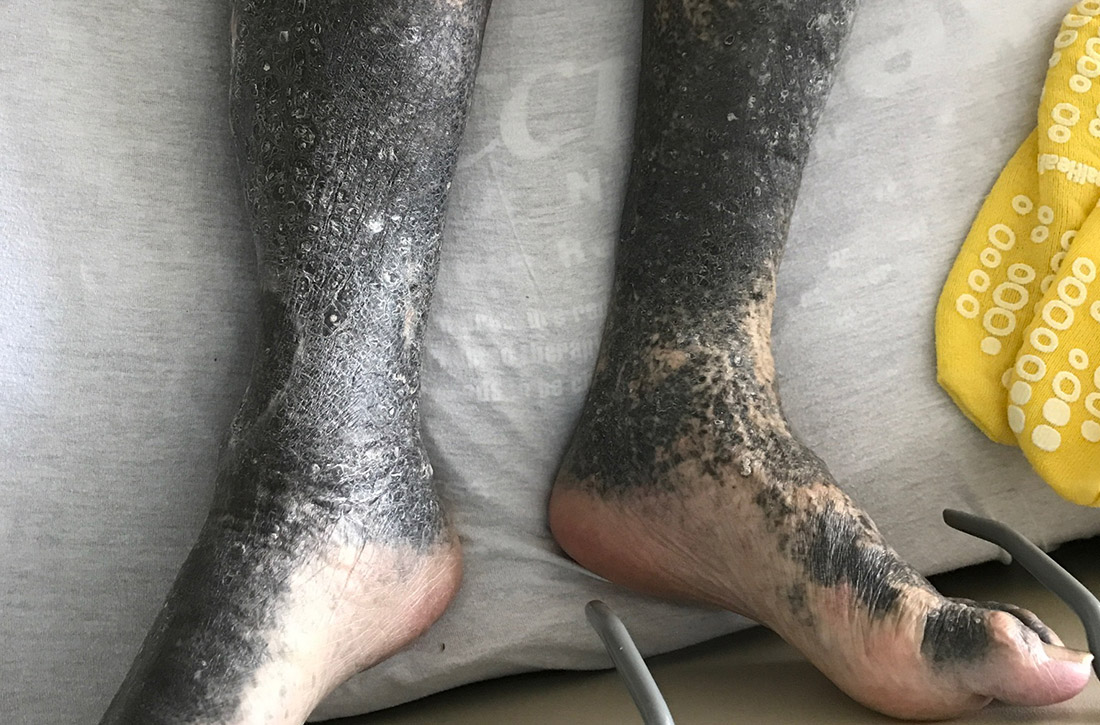
During his hospitalization on our service, we noted black pigmentation of both legs (FIGURE). He had intact strength and sensation in his legs, 1+ pitting edema, no pain upon palpation, and 2+ distal pulses. The patient was well appearing and in no acute distress.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Minocycline-induced hyperpigmentation
The patient’s clinical presentation of chronic blue-black hyperpigmentation on the anterior shins of both legs after a prolonged antibiotic course led us to conclude that this was an adverse effect of minocycline. Commonly, doctors use minocycline to treat acne, rosacea, and rheumatoid arthritis. In this case, it was used to provide chronic antimicrobial suppression.
Not an uncommon reaction for a patient like ours. One small study conducted in an orthopedic patient population found that 54% of patients receiving long-term minocycline suppression developed hyperpigmentation after a mean follow-up of nearly 5 years.1 The hyperpigmentation is solely cosmetic and without known clinical complications, but it can be distressing for patients.
There are 3 types of minocycline-induced hyperpigmentation:
- Type I is a circumscribed blue-black pigmentation that manifests in skin that previously was inflamed or scarred, such as facial acne scars.2 Histopathologic findings include black pigment granules in macrophages and throughout the dermis that stain with Perls Prussian blue iron.3
- Type II (which our patient had) is circumscribed blue-black pigmentation that appears in previously normal skin of the forearms or lower legs—especially the shins.3 On histopathology, black pigment granules are found in the dermis with macrophages that stain with Perls Prussian blue iron and Fontana-Masson.3
- Type III is a diffuse muddy brown hyperpigmentation in previously normal, sun-exposed skin.2 Histopathologic findings include increased melanin in basal keratinocytes and dermal melanophages that stain with Fontana-Masson.3
Types II and III may be related to cumulative dosing, whereas type I can occur at any point during treatment.2
Differential includes pigmentation disorders
The differential diagnosis includes Addison disease, argyria, hemochromatosis, and polycythemia vera, which all can cause diffuse blue-gray patches.4 Brown-violet pigmentation on sun-exposed areas, redness, and itching are more typical of Riehl melanosis.4
Continue to: Diltiazem
Diltiazem can produce slate-gray to blue-gray reticulated hyperpigmentation.5 Other drugs that can induce slate-gray macules or patches include amiodarone, chlorpromazine, imipramine, and desipramine.5
Treatment is simple, resolution takes time
The treatment for this condition is cessation of minocycline use. Pigmentation fades slowly and may persist for years. There has been successful treatment of type I and III minocycline-induced hyperpigmentation with the alexandrite 755 nm Q-switched laser combined with fractional photothermolysis.3,6 Unfortunately, insurance coverage is limited because these treatments are cosmetic in nature.
Given that hyperpigmentation is a known adverse effect of minocycline use, it’s important to counsel patients about the possibility prior to initiating treatment. It’s also important to monitor for signs of changing pigmentation to prevent psychological distress.
In this case, a biopsy was deemed unnecessary, as the antibiotic was the most likely cause of the pigmentation. The patient’s outpatient dermatologist recommended changing therapy if a medically appropriate alternative was available. Doxycycline would have been a reasonable alternative; however, the patient died shortly after his presentation to our hospital due to his multiple comorbidities.
CORRESPONDENCE
Bich-May Nguyen, MD, MPH, 14023 Southwest Freeway, Sugar Land, TX 77478; Bich-May.Nguyen@memorialhermann.org
1. Hanada Y, Berbari EF, Steckelberg JM. Minocycline-induced cutaneous hyperpigmentation in an orthopedic patient population. Open Forum Infect Dis. 2016;3:ofv107.
2. Mouton RW, Jordaan HF, Schneider JW. A new type of minocycline-induced cutaneous hyperpigmentation. Clin Exp Dermatol. 2004;29:8-14.
3. D’Agostino ML, Risser J, Robinson-Bostom L. Imipramine-induced hyperpigmentation: a case report and review of the literature. J Cutan Pathol. 2009;36:799-803.
4. Nisar MS, Iyer K, Brodell RT, et al. Minocycline-induced hyperpigmentation: comparison of 3 Q-switched lasers to reverse its effects. Clin Cosmet Investig Dermatol. 2013;6:159-162.
5. Scherschun L, Lee MW, Lim HW. Diltiazem-associated photodistributed hyperpigmentation. Arch Dermatol. 2001;137:179-182.
6. Vangipuram RK, DeLozier WL, Geddes E, et al. Complete resolution of minocycline pigmentation following a single treatment with non-ablative 1550-nm fractional resurfacing in combination with the 755-nm Q-switched alexandrite laser. Lasers Surg Med. 2016;48:234-237.
A 90-year-old man was admitted from the Emergency Department (ED) to our inpatient service for difficulty urinating and hematuria. In the ED, a complete blood count (CBC) with differential and a urinalysis were performed. CBC showed a mild normocytic anemia, consistent with the patient’s known chronic kidney disease. The urinalysis revealed moderate blood, trace ketones, proteinuria, small leukocyte esterases, positive nitrites, and more than 182 red blood cells—findings suspicious for a urinary tract infection. Computed tomography of the abdomen and pelvis was notable for a soft-tissue mass in the bladder.
He had a history of coronary artery disease (treated with stent placement), atrial fibrillation, congestive heart failure, hypothyroidism, gastroesophageal reflux disease, gastrointestinal bleeding, chronic obstructive pulmonary disease, a 60-pack-per-year history of tobacco dependence, chronic kidney disease, prostate cancer, benign prostatic hypertrophy, peripheral vascular disease, and gout. Medications included digoxin, metoprolol, torsemide, aspirin, levothyroxine, fluticasone, albuterol, omeprazole, diclofenac, escitalopram, and minocycline.
About 5 years earlier, doctors had discovered a popliteal thrombosis that required emergent thrombectomy of the infragenicular popliteal artery, thromboembolectomy of the right posterior tibial artery, graft angioplasty of the right posterior tibial artery, and right anterior fasciotomy for compartment syndrome.
Ten months later, an abscess formed at the incision site. His physician irrigated the popliteal wound and prescribed intravenous (IV) vancomycin. However, the patient developed an allergy and IV daptomycin was initiated and followed by chronic antibiotic suppression with oral minocycline 100 mg bid for about 3.5 years. Skin discoloration appeared within a year of starting the minocycline.
During his hospitalization on our service, we noted black pigmentation of both legs (FIGURE). He had intact strength and sensation in his legs, 1+ pitting edema, no pain upon palpation, and 2+ distal pulses. The patient was well appearing and in no acute distress.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Minocycline-induced hyperpigmentation
The patient’s clinical presentation of chronic blue-black hyperpigmentation on the anterior shins of both legs after a prolonged antibiotic course led us to conclude that this was an adverse effect of minocycline. Commonly, doctors use minocycline to treat acne, rosacea, and rheumatoid arthritis. In this case, it was used to provide chronic antimicrobial suppression.
Not an uncommon reaction for a patient like ours. One small study conducted in an orthopedic patient population found that 54% of patients receiving long-term minocycline suppression developed hyperpigmentation after a mean follow-up of nearly 5 years.1 The hyperpigmentation is solely cosmetic and without known clinical complications, but it can be distressing for patients.
There are 3 types of minocycline-induced hyperpigmentation:
- Type I is a circumscribed blue-black pigmentation that manifests in skin that previously was inflamed or scarred, such as facial acne scars.2 Histopathologic findings include black pigment granules in macrophages and throughout the dermis that stain with Perls Prussian blue iron.3
- Type II (which our patient had) is circumscribed blue-black pigmentation that appears in previously normal skin of the forearms or lower legs—especially the shins.3 On histopathology, black pigment granules are found in the dermis with macrophages that stain with Perls Prussian blue iron and Fontana-Masson.3
- Type III is a diffuse muddy brown hyperpigmentation in previously normal, sun-exposed skin.2 Histopathologic findings include increased melanin in basal keratinocytes and dermal melanophages that stain with Fontana-Masson.3
Types II and III may be related to cumulative dosing, whereas type I can occur at any point during treatment.2
Differential includes pigmentation disorders
The differential diagnosis includes Addison disease, argyria, hemochromatosis, and polycythemia vera, which all can cause diffuse blue-gray patches.4 Brown-violet pigmentation on sun-exposed areas, redness, and itching are more typical of Riehl melanosis.4
Continue to: Diltiazem
Diltiazem can produce slate-gray to blue-gray reticulated hyperpigmentation.5 Other drugs that can induce slate-gray macules or patches include amiodarone, chlorpromazine, imipramine, and desipramine.5
Treatment is simple, resolution takes time
The treatment for this condition is cessation of minocycline use. Pigmentation fades slowly and may persist for years. There has been successful treatment of type I and III minocycline-induced hyperpigmentation with the alexandrite 755 nm Q-switched laser combined with fractional photothermolysis.3,6 Unfortunately, insurance coverage is limited because these treatments are cosmetic in nature.
Given that hyperpigmentation is a known adverse effect of minocycline use, it’s important to counsel patients about the possibility prior to initiating treatment. It’s also important to monitor for signs of changing pigmentation to prevent psychological distress.
In this case, a biopsy was deemed unnecessary, as the antibiotic was the most likely cause of the pigmentation. The patient’s outpatient dermatologist recommended changing therapy if a medically appropriate alternative was available. Doxycycline would have been a reasonable alternative; however, the patient died shortly after his presentation to our hospital due to his multiple comorbidities.
CORRESPONDENCE
Bich-May Nguyen, MD, MPH, 14023 Southwest Freeway, Sugar Land, TX 77478; Bich-May.Nguyen@memorialhermann.org
A 90-year-old man was admitted from the Emergency Department (ED) to our inpatient service for difficulty urinating and hematuria. In the ED, a complete blood count (CBC) with differential and a urinalysis were performed. CBC showed a mild normocytic anemia, consistent with the patient’s known chronic kidney disease. The urinalysis revealed moderate blood, trace ketones, proteinuria, small leukocyte esterases, positive nitrites, and more than 182 red blood cells—findings suspicious for a urinary tract infection. Computed tomography of the abdomen and pelvis was notable for a soft-tissue mass in the bladder.
He had a history of coronary artery disease (treated with stent placement), atrial fibrillation, congestive heart failure, hypothyroidism, gastroesophageal reflux disease, gastrointestinal bleeding, chronic obstructive pulmonary disease, a 60-pack-per-year history of tobacco dependence, chronic kidney disease, prostate cancer, benign prostatic hypertrophy, peripheral vascular disease, and gout. Medications included digoxin, metoprolol, torsemide, aspirin, levothyroxine, fluticasone, albuterol, omeprazole, diclofenac, escitalopram, and minocycline.
About 5 years earlier, doctors had discovered a popliteal thrombosis that required emergent thrombectomy of the infragenicular popliteal artery, thromboembolectomy of the right posterior tibial artery, graft angioplasty of the right posterior tibial artery, and right anterior fasciotomy for compartment syndrome.
Ten months later, an abscess formed at the incision site. His physician irrigated the popliteal wound and prescribed intravenous (IV) vancomycin. However, the patient developed an allergy and IV daptomycin was initiated and followed by chronic antibiotic suppression with oral minocycline 100 mg bid for about 3.5 years. Skin discoloration appeared within a year of starting the minocycline.
During his hospitalization on our service, we noted black pigmentation of both legs (FIGURE). He had intact strength and sensation in his legs, 1+ pitting edema, no pain upon palpation, and 2+ distal pulses. The patient was well appearing and in no acute distress.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Minocycline-induced hyperpigmentation
The patient’s clinical presentation of chronic blue-black hyperpigmentation on the anterior shins of both legs after a prolonged antibiotic course led us to conclude that this was an adverse effect of minocycline. Commonly, doctors use minocycline to treat acne, rosacea, and rheumatoid arthritis. In this case, it was used to provide chronic antimicrobial suppression.
Not an uncommon reaction for a patient like ours. One small study conducted in an orthopedic patient population found that 54% of patients receiving long-term minocycline suppression developed hyperpigmentation after a mean follow-up of nearly 5 years.1 The hyperpigmentation is solely cosmetic and without known clinical complications, but it can be distressing for patients.
There are 3 types of minocycline-induced hyperpigmentation:
- Type I is a circumscribed blue-black pigmentation that manifests in skin that previously was inflamed or scarred, such as facial acne scars.2 Histopathologic findings include black pigment granules in macrophages and throughout the dermis that stain with Perls Prussian blue iron.3
- Type II (which our patient had) is circumscribed blue-black pigmentation that appears in previously normal skin of the forearms or lower legs—especially the shins.3 On histopathology, black pigment granules are found in the dermis with macrophages that stain with Perls Prussian blue iron and Fontana-Masson.3
- Type III is a diffuse muddy brown hyperpigmentation in previously normal, sun-exposed skin.2 Histopathologic findings include increased melanin in basal keratinocytes and dermal melanophages that stain with Fontana-Masson.3
Types II and III may be related to cumulative dosing, whereas type I can occur at any point during treatment.2
Differential includes pigmentation disorders
The differential diagnosis includes Addison disease, argyria, hemochromatosis, and polycythemia vera, which all can cause diffuse blue-gray patches.4 Brown-violet pigmentation on sun-exposed areas, redness, and itching are more typical of Riehl melanosis.4
Continue to: Diltiazem
Diltiazem can produce slate-gray to blue-gray reticulated hyperpigmentation.5 Other drugs that can induce slate-gray macules or patches include amiodarone, chlorpromazine, imipramine, and desipramine.5
Treatment is simple, resolution takes time
The treatment for this condition is cessation of minocycline use. Pigmentation fades slowly and may persist for years. There has been successful treatment of type I and III minocycline-induced hyperpigmentation with the alexandrite 755 nm Q-switched laser combined with fractional photothermolysis.3,6 Unfortunately, insurance coverage is limited because these treatments are cosmetic in nature.
Given that hyperpigmentation is a known adverse effect of minocycline use, it’s important to counsel patients about the possibility prior to initiating treatment. It’s also important to monitor for signs of changing pigmentation to prevent psychological distress.
In this case, a biopsy was deemed unnecessary, as the antibiotic was the most likely cause of the pigmentation. The patient’s outpatient dermatologist recommended changing therapy if a medically appropriate alternative was available. Doxycycline would have been a reasonable alternative; however, the patient died shortly after his presentation to our hospital due to his multiple comorbidities.
CORRESPONDENCE
Bich-May Nguyen, MD, MPH, 14023 Southwest Freeway, Sugar Land, TX 77478; Bich-May.Nguyen@memorialhermann.org
1. Hanada Y, Berbari EF, Steckelberg JM. Minocycline-induced cutaneous hyperpigmentation in an orthopedic patient population. Open Forum Infect Dis. 2016;3:ofv107.
2. Mouton RW, Jordaan HF, Schneider JW. A new type of minocycline-induced cutaneous hyperpigmentation. Clin Exp Dermatol. 2004;29:8-14.
3. D’Agostino ML, Risser J, Robinson-Bostom L. Imipramine-induced hyperpigmentation: a case report and review of the literature. J Cutan Pathol. 2009;36:799-803.
4. Nisar MS, Iyer K, Brodell RT, et al. Minocycline-induced hyperpigmentation: comparison of 3 Q-switched lasers to reverse its effects. Clin Cosmet Investig Dermatol. 2013;6:159-162.
5. Scherschun L, Lee MW, Lim HW. Diltiazem-associated photodistributed hyperpigmentation. Arch Dermatol. 2001;137:179-182.
6. Vangipuram RK, DeLozier WL, Geddes E, et al. Complete resolution of minocycline pigmentation following a single treatment with non-ablative 1550-nm fractional resurfacing in combination with the 755-nm Q-switched alexandrite laser. Lasers Surg Med. 2016;48:234-237.
1. Hanada Y, Berbari EF, Steckelberg JM. Minocycline-induced cutaneous hyperpigmentation in an orthopedic patient population. Open Forum Infect Dis. 2016;3:ofv107.
2. Mouton RW, Jordaan HF, Schneider JW. A new type of minocycline-induced cutaneous hyperpigmentation. Clin Exp Dermatol. 2004;29:8-14.
3. D’Agostino ML, Risser J, Robinson-Bostom L. Imipramine-induced hyperpigmentation: a case report and review of the literature. J Cutan Pathol. 2009;36:799-803.
4. Nisar MS, Iyer K, Brodell RT, et al. Minocycline-induced hyperpigmentation: comparison of 3 Q-switched lasers to reverse its effects. Clin Cosmet Investig Dermatol. 2013;6:159-162.
5. Scherschun L, Lee MW, Lim HW. Diltiazem-associated photodistributed hyperpigmentation. Arch Dermatol. 2001;137:179-182.
6. Vangipuram RK, DeLozier WL, Geddes E, et al. Complete resolution of minocycline pigmentation following a single treatment with non-ablative 1550-nm fractional resurfacing in combination with the 755-nm Q-switched alexandrite laser. Lasers Surg Med. 2016;48:234-237.
CHEST 2020: Premier education from the convenience of your home
After careful consideration, CHEST has decided to cancel the live, in-person CHEST Annual Meeting in Chicago, Illinois, this October and replace it with a 100% virtual event. The COVID-19 pandemic has provided the opportunity to look at different approaches for delivering education, and over the past several months, CHEST has done just that.
Due to the pandemic, we moved the CHEST Congress 2020, originally scheduled to take place in Bologna, Italy, to June 2021. On June 30, in partnership with the Italian Delegation, the CHEST Virtual Congress event took place with over 3,200 people registered, spanning over 100 countries. This event featured a robust program that included an international COVID panel, additional educational sessions, over 300 recorded poster presentations, and live, interactive games that kept attendees engaged throughout the day. There was also a surprise welcome message delivered by Dr. Anthony Fauci, the Director of the National Institute of Allergy and Infectious Diseases. We are excited to use the success of this virtual event as an opportunity to expand our knowledge and expertise, and deliver a fun, memorable CHEST 2020.
This October, CHEST will bring you the premier virtual education event in pulmonary, critical care, and sleep medicine, all from the comfort and safety of your home or institution. This year’s virtual Annual Meeting will include live, interactive education, including panel and case-based discussions, virtual networking opportunities, CHEST GAMES, and the space for you to connect, learn, and recharge with your peers…virtually.
Top faculty from across the field will bring you the latest in clinical developments related to the diagnosis, treatment, and management of pulmonary diseases, critical care complications, and sleep disorders. Nonclinical topics, like cultural diversity and burnout, that feature more prominently than ever in day-to-day practice, will be given equal weight. Sessions like, Being Me: Understanding ‘Otherness’ and Issues of Diversity, will rely on audience interaction to address scenarios involving bias and racism faced by the panel of presenters and members of the audience.
Crucial and quickly evolving information on COVID-19 will be front and center, including complications with COVID-19 recovery, COVID-19 management in complex situations, and additional discussions on updated drug trials, treatment plans, and practice management changes. We will focus on other challenges the pandemic has highlighted, helping educators with sessions such as APCCMPD: Education Lessons During a Pandemic and sharing key reminders to all on the fundamentals of pandemic preparation with When the Theoretical Becomes Real: Lessons from a Pandemic.
It is more important than ever to stay up to date on developments in health and medicine, but CHEST is putting equal weight on ensuring the experience of CHEST 2020 is a respite from the mental and physical exhaustion our community is experiencing during these unprecedented times. As ever, we will ensure you meet your educational needs. But together, we will also focus on supporting you in building resilience and giving you the tools to continue to find joy in medicine, even amidst the chaos of a pandemic. Thank you for your continued trust in CHEST, and we look forward to “seeing” you at CHEST 2020 October 18-21!
After careful consideration, CHEST has decided to cancel the live, in-person CHEST Annual Meeting in Chicago, Illinois, this October and replace it with a 100% virtual event. The COVID-19 pandemic has provided the opportunity to look at different approaches for delivering education, and over the past several months, CHEST has done just that.
Due to the pandemic, we moved the CHEST Congress 2020, originally scheduled to take place in Bologna, Italy, to June 2021. On June 30, in partnership with the Italian Delegation, the CHEST Virtual Congress event took place with over 3,200 people registered, spanning over 100 countries. This event featured a robust program that included an international COVID panel, additional educational sessions, over 300 recorded poster presentations, and live, interactive games that kept attendees engaged throughout the day. There was also a surprise welcome message delivered by Dr. Anthony Fauci, the Director of the National Institute of Allergy and Infectious Diseases. We are excited to use the success of this virtual event as an opportunity to expand our knowledge and expertise, and deliver a fun, memorable CHEST 2020.
This October, CHEST will bring you the premier virtual education event in pulmonary, critical care, and sleep medicine, all from the comfort and safety of your home or institution. This year’s virtual Annual Meeting will include live, interactive education, including panel and case-based discussions, virtual networking opportunities, CHEST GAMES, and the space for you to connect, learn, and recharge with your peers…virtually.
Top faculty from across the field will bring you the latest in clinical developments related to the diagnosis, treatment, and management of pulmonary diseases, critical care complications, and sleep disorders. Nonclinical topics, like cultural diversity and burnout, that feature more prominently than ever in day-to-day practice, will be given equal weight. Sessions like, Being Me: Understanding ‘Otherness’ and Issues of Diversity, will rely on audience interaction to address scenarios involving bias and racism faced by the panel of presenters and members of the audience.
Crucial and quickly evolving information on COVID-19 will be front and center, including complications with COVID-19 recovery, COVID-19 management in complex situations, and additional discussions on updated drug trials, treatment plans, and practice management changes. We will focus on other challenges the pandemic has highlighted, helping educators with sessions such as APCCMPD: Education Lessons During a Pandemic and sharing key reminders to all on the fundamentals of pandemic preparation with When the Theoretical Becomes Real: Lessons from a Pandemic.
It is more important than ever to stay up to date on developments in health and medicine, but CHEST is putting equal weight on ensuring the experience of CHEST 2020 is a respite from the mental and physical exhaustion our community is experiencing during these unprecedented times. As ever, we will ensure you meet your educational needs. But together, we will also focus on supporting you in building resilience and giving you the tools to continue to find joy in medicine, even amidst the chaos of a pandemic. Thank you for your continued trust in CHEST, and we look forward to “seeing” you at CHEST 2020 October 18-21!
After careful consideration, CHEST has decided to cancel the live, in-person CHEST Annual Meeting in Chicago, Illinois, this October and replace it with a 100% virtual event. The COVID-19 pandemic has provided the opportunity to look at different approaches for delivering education, and over the past several months, CHEST has done just that.
Due to the pandemic, we moved the CHEST Congress 2020, originally scheduled to take place in Bologna, Italy, to June 2021. On June 30, in partnership with the Italian Delegation, the CHEST Virtual Congress event took place with over 3,200 people registered, spanning over 100 countries. This event featured a robust program that included an international COVID panel, additional educational sessions, over 300 recorded poster presentations, and live, interactive games that kept attendees engaged throughout the day. There was also a surprise welcome message delivered by Dr. Anthony Fauci, the Director of the National Institute of Allergy and Infectious Diseases. We are excited to use the success of this virtual event as an opportunity to expand our knowledge and expertise, and deliver a fun, memorable CHEST 2020.
This October, CHEST will bring you the premier virtual education event in pulmonary, critical care, and sleep medicine, all from the comfort and safety of your home or institution. This year’s virtual Annual Meeting will include live, interactive education, including panel and case-based discussions, virtual networking opportunities, CHEST GAMES, and the space for you to connect, learn, and recharge with your peers…virtually.
Top faculty from across the field will bring you the latest in clinical developments related to the diagnosis, treatment, and management of pulmonary diseases, critical care complications, and sleep disorders. Nonclinical topics, like cultural diversity and burnout, that feature more prominently than ever in day-to-day practice, will be given equal weight. Sessions like, Being Me: Understanding ‘Otherness’ and Issues of Diversity, will rely on audience interaction to address scenarios involving bias and racism faced by the panel of presenters and members of the audience.
Crucial and quickly evolving information on COVID-19 will be front and center, including complications with COVID-19 recovery, COVID-19 management in complex situations, and additional discussions on updated drug trials, treatment plans, and practice management changes. We will focus on other challenges the pandemic has highlighted, helping educators with sessions such as APCCMPD: Education Lessons During a Pandemic and sharing key reminders to all on the fundamentals of pandemic preparation with When the Theoretical Becomes Real: Lessons from a Pandemic.
It is more important than ever to stay up to date on developments in health and medicine, but CHEST is putting equal weight on ensuring the experience of CHEST 2020 is a respite from the mental and physical exhaustion our community is experiencing during these unprecedented times. As ever, we will ensure you meet your educational needs. But together, we will also focus on supporting you in building resilience and giving you the tools to continue to find joy in medicine, even amidst the chaos of a pandemic. Thank you for your continued trust in CHEST, and we look forward to “seeing” you at CHEST 2020 October 18-21!
USPSTF expands options for cervical cancer screening
ILLUSTRATIVE CASE
A 35-year-old healthy woman without a history of high-grade precancerous cervical lesions, immunodeficiency, or exposure to diethylstilbestrol presents to your office for her routine health visit. During your conversation with her, she shares, “I read on the Internet that I only need to be tested for human papillomavirus, but I’m wondering how I’ll be checked for cervical cancer.” She asks for your opinion about cervical cancer screening methods.
The National Cancer Institute predicts that there will be 13,800 new cases of cervical cancer this year, with an estimated 4290 deaths.3 This type of cancer is primarily caused by high-risk human papillomavirus (hrHPV) infections. Fortunately, high-grade precancerous cervical lesions and cervical cancer can be detected with routine Papanicolaou (Pap) smears, which have led to a substantial decrease in the number of deaths from cervical cancer in the United States—from 2.8 per 100,000 women in 2000 to 2.3 deaths per 100,000 women in 2015.3 In addition to hrHPV infection, risk factors for cervical cancer include low socioeconomic status, cigarette smoking, marrying before 18 years of age, young age at first coitus, multiple sexual partners, multiple sexual partners of a partner, and multiple childbirths.4
Cervical cancer is associated with numerous negative outcomes, including a decrease in quality of life, decreased libido, poor mental health, infertility, negative body image, and death.5 This is particularly true among women of lower socioeconomic status or whose language differs from that of their primary health care provider.1,5
Given the enormous impact cervical cancer screening has made on the detection and mortality rate of this devastating disease,4,5 it is crucial to identify the types of screening tests and screening intervals that lead to the greatest benefit and least harm for all patient populations. The US Preventive Services Task Force (USPSTF) previously addressed this issue in 2012, concluding that cytology alone every 3 years for women ages 21 to 65 years and cytology alone every 3 years or co-testing with cytology and hrHPV every 5 years in women ages 30 to 65 years was of substantial benefit (strength of recommendation [SOR]: A).6
STUDY SUMMARY
Another option for some women: hrHPV testing alone every 5 years
In this 2018 systematic review and modeling study by the USPSTF, randomized controlled trials (RCTs) and cohort studies that compared cytology to hrHPV testing alone or co-testing (cytology with hrHPV) were used to determine the optimal frequency of, and age group for, cervical cancer screening that would yield the least harm and the most benefit from each of these screening methods.7-9
Similar to the previous recommendation, the USPSTF found that screening women < 21 years or > 65 years if previously adequately screened (defined as 3 consecutive negative screenings or 2 negative screenings within the past 10 years with the most recent being within the past 5 years) led to more harm than benefit. They therefore concluded that women in these age groups should not be screened routinely (SOR: D). The USPSTF also recommends against cervical cancer screening in women who have had a hysterectomy with removal of the cervix and who do not have a history of a high-grade precancerous lesion or cervical cancer (SOR: D).
However, for women ages 21 to 65 years, the USPSTF found that screening substantially reduces cervical cancer incidence and mortality, and that for women ages 21 to 29 years, screening every 3 years with cytology alone offers the best balance of benefits and harms (SOR: A). For women ages 30 to 65 years, the USPSTF recommends screening every 3 years with cytology alone or every 5 years with either primary hrHPV testing or co-testing (hrHPV with cytology) (SOR: A). The recommendations apply to all asymptomatic women with a cervix; exceptions include those with a history of a high-grade precancerous cervical lesion or cancer, in utero exposure to diethylstilbestrol, or a compromised immune system.
Continue to: The change
The change in this current set of recommendations by the USPSTF is the inclusion of screening with hrHPV alone every 5 years as an additional cervical cancer screening option for women ages 30 to 65 years. The decision to include this option was based largely on a decision analysis model commissioned by the USPSTF and reviewed along with clinical trials and cohort studies. The modeling studies found that both primary hrHPV testing alone and co-testing every 5 years prevented a similar number of cervical cancer cases and required a similar number of colposcopies.
Finally, the USPSTF emphasized that screening alone is not sufficient for the prevention of cervical cancer and that efforts should be made to create equitable access to follow-up of abnormal results and the provision of appropriate treatment.1,2
WHAT’S NEW
When it comes to cervical cancer screening, 3 solid options now exist
The previous USPSTF recommendation concluded that women ages 30 to 65 years should be screened with either cytology alone every 3 years or co-testing (cytology and hrHPV) every 5 years. This systematic review and modeling study concluded that any one of the stated screening methods would be adequately sensitive for detecting precancerous high-grade cervical lesions or cervical cancer: cytology every 3 years, primary hrHPV every 5 years, or co-testing every 5 years.7-9
CAVEATS
No studies comparing hrHPVto co-testing and no meta-analysis
No studies were found that directly compared primary hrHPV testing with co-testing.1 A meta-analysis could not be performed due to the methodological differences in RCTs and cohort studies reviewed. The new recommendation is unique in its reliance on modeling to simulate a direct comparison of these 2 screening methods.
CHALLENGES TO IMPLEMENTATION
Getting the word out and increasing comfort levels
The principal challenge to implementation lies in practitioners’ knowledge of this new recommendation and a possible low comfort level with ordering hrHPV testing alone. Patients will need to be engaged in shared decision-making to understand and make use of the 3 options.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Curry SJ, Krist AH, Owens DK, et al. Screening for cervical cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2018;320:674-686.
2. Melnikow J, Henderson JT, Burda BU, et al. Screening for cervical cancer with high-risk human papillomavirus testing: a systematic evidence review for the US Preventive Services Task Force. Evidence Synthesis No. 158. Rockville, MD: Agency for Healthcare Research and Quality; 2018.
3. National Cancer Institute. Cancer Stat Facts. Cervix uteri. https://seer.cancer.gov/statfacts/. Accessed July 1, 2020.
4. Momenimovahed Z, Salehiniya H. Incidence, mortality and risk factors of cervical cancer in the world. Biomed Res Ther. 2017;4:1795-1811.
5. Ashing-Giwa KT, Kagawa-Singer M, Padilla GV, et al. The impact of cervical cancer and dysplasia: a qualitative, multiethnic study. Psychooncology. 2004;13:709-728.
6. Moyer VA; US Preventive Services Task Force. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2012; 156:880-891.
7. Ronco G, Giorgi-Rossi P, Carozzi F, et al; New Technologies for Cervical Cancer Screening (NTCC) Working Group. Efficacy of human papillomavirus testing for the detection of invasive cervical cancers and cervical intraepithelial neoplasia: a randomized controlled trial. Lancet Oncol. 2010;11:249-257.
8. Ronco G, Giorgi-Rossi P, Carozzi F, et al; New Technologies for Cervical Cancer Screening Working Group. Results at recruitment from a randomized controlled trial comparing human papillomavirus testing alone with conventional cytology as the primary cervical cancer screening test. J Natl Cancer Inst. 2008;100:492-501.
9. Ogilvie GS, van Niekerk DJ, Krajden M, et al. A randomized controlled trial of human papillomavirus (HPV) testing for cervical cancer screening: trial design and preliminary results (HPV FOCAL Trial). BMC Cancer. 2010;10:111.
ILLUSTRATIVE CASE
A 35-year-old healthy woman without a history of high-grade precancerous cervical lesions, immunodeficiency, or exposure to diethylstilbestrol presents to your office for her routine health visit. During your conversation with her, she shares, “I read on the Internet that I only need to be tested for human papillomavirus, but I’m wondering how I’ll be checked for cervical cancer.” She asks for your opinion about cervical cancer screening methods.
The National Cancer Institute predicts that there will be 13,800 new cases of cervical cancer this year, with an estimated 4290 deaths.3 This type of cancer is primarily caused by high-risk human papillomavirus (hrHPV) infections. Fortunately, high-grade precancerous cervical lesions and cervical cancer can be detected with routine Papanicolaou (Pap) smears, which have led to a substantial decrease in the number of deaths from cervical cancer in the United States—from 2.8 per 100,000 women in 2000 to 2.3 deaths per 100,000 women in 2015.3 In addition to hrHPV infection, risk factors for cervical cancer include low socioeconomic status, cigarette smoking, marrying before 18 years of age, young age at first coitus, multiple sexual partners, multiple sexual partners of a partner, and multiple childbirths.4
Cervical cancer is associated with numerous negative outcomes, including a decrease in quality of life, decreased libido, poor mental health, infertility, negative body image, and death.5 This is particularly true among women of lower socioeconomic status or whose language differs from that of their primary health care provider.1,5
Given the enormous impact cervical cancer screening has made on the detection and mortality rate of this devastating disease,4,5 it is crucial to identify the types of screening tests and screening intervals that lead to the greatest benefit and least harm for all patient populations. The US Preventive Services Task Force (USPSTF) previously addressed this issue in 2012, concluding that cytology alone every 3 years for women ages 21 to 65 years and cytology alone every 3 years or co-testing with cytology and hrHPV every 5 years in women ages 30 to 65 years was of substantial benefit (strength of recommendation [SOR]: A).6
STUDY SUMMARY
Another option for some women: hrHPV testing alone every 5 years
In this 2018 systematic review and modeling study by the USPSTF, randomized controlled trials (RCTs) and cohort studies that compared cytology to hrHPV testing alone or co-testing (cytology with hrHPV) were used to determine the optimal frequency of, and age group for, cervical cancer screening that would yield the least harm and the most benefit from each of these screening methods.7-9
Similar to the previous recommendation, the USPSTF found that screening women < 21 years or > 65 years if previously adequately screened (defined as 3 consecutive negative screenings or 2 negative screenings within the past 10 years with the most recent being within the past 5 years) led to more harm than benefit. They therefore concluded that women in these age groups should not be screened routinely (SOR: D). The USPSTF also recommends against cervical cancer screening in women who have had a hysterectomy with removal of the cervix and who do not have a history of a high-grade precancerous lesion or cervical cancer (SOR: D).
However, for women ages 21 to 65 years, the USPSTF found that screening substantially reduces cervical cancer incidence and mortality, and that for women ages 21 to 29 years, screening every 3 years with cytology alone offers the best balance of benefits and harms (SOR: A). For women ages 30 to 65 years, the USPSTF recommends screening every 3 years with cytology alone or every 5 years with either primary hrHPV testing or co-testing (hrHPV with cytology) (SOR: A). The recommendations apply to all asymptomatic women with a cervix; exceptions include those with a history of a high-grade precancerous cervical lesion or cancer, in utero exposure to diethylstilbestrol, or a compromised immune system.
Continue to: The change
The change in this current set of recommendations by the USPSTF is the inclusion of screening with hrHPV alone every 5 years as an additional cervical cancer screening option for women ages 30 to 65 years. The decision to include this option was based largely on a decision analysis model commissioned by the USPSTF and reviewed along with clinical trials and cohort studies. The modeling studies found that both primary hrHPV testing alone and co-testing every 5 years prevented a similar number of cervical cancer cases and required a similar number of colposcopies.
Finally, the USPSTF emphasized that screening alone is not sufficient for the prevention of cervical cancer and that efforts should be made to create equitable access to follow-up of abnormal results and the provision of appropriate treatment.1,2
WHAT’S NEW
When it comes to cervical cancer screening, 3 solid options now exist
The previous USPSTF recommendation concluded that women ages 30 to 65 years should be screened with either cytology alone every 3 years or co-testing (cytology and hrHPV) every 5 years. This systematic review and modeling study concluded that any one of the stated screening methods would be adequately sensitive for detecting precancerous high-grade cervical lesions or cervical cancer: cytology every 3 years, primary hrHPV every 5 years, or co-testing every 5 years.7-9
CAVEATS
No studies comparing hrHPVto co-testing and no meta-analysis
No studies were found that directly compared primary hrHPV testing with co-testing.1 A meta-analysis could not be performed due to the methodological differences in RCTs and cohort studies reviewed. The new recommendation is unique in its reliance on modeling to simulate a direct comparison of these 2 screening methods.
CHALLENGES TO IMPLEMENTATION
Getting the word out and increasing comfort levels
The principal challenge to implementation lies in practitioners’ knowledge of this new recommendation and a possible low comfort level with ordering hrHPV testing alone. Patients will need to be engaged in shared decision-making to understand and make use of the 3 options.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
ILLUSTRATIVE CASE
A 35-year-old healthy woman without a history of high-grade precancerous cervical lesions, immunodeficiency, or exposure to diethylstilbestrol presents to your office for her routine health visit. During your conversation with her, she shares, “I read on the Internet that I only need to be tested for human papillomavirus, but I’m wondering how I’ll be checked for cervical cancer.” She asks for your opinion about cervical cancer screening methods.
The National Cancer Institute predicts that there will be 13,800 new cases of cervical cancer this year, with an estimated 4290 deaths.3 This type of cancer is primarily caused by high-risk human papillomavirus (hrHPV) infections. Fortunately, high-grade precancerous cervical lesions and cervical cancer can be detected with routine Papanicolaou (Pap) smears, which have led to a substantial decrease in the number of deaths from cervical cancer in the United States—from 2.8 per 100,000 women in 2000 to 2.3 deaths per 100,000 women in 2015.3 In addition to hrHPV infection, risk factors for cervical cancer include low socioeconomic status, cigarette smoking, marrying before 18 years of age, young age at first coitus, multiple sexual partners, multiple sexual partners of a partner, and multiple childbirths.4
Cervical cancer is associated with numerous negative outcomes, including a decrease in quality of life, decreased libido, poor mental health, infertility, negative body image, and death.5 This is particularly true among women of lower socioeconomic status or whose language differs from that of their primary health care provider.1,5
Given the enormous impact cervical cancer screening has made on the detection and mortality rate of this devastating disease,4,5 it is crucial to identify the types of screening tests and screening intervals that lead to the greatest benefit and least harm for all patient populations. The US Preventive Services Task Force (USPSTF) previously addressed this issue in 2012, concluding that cytology alone every 3 years for women ages 21 to 65 years and cytology alone every 3 years or co-testing with cytology and hrHPV every 5 years in women ages 30 to 65 years was of substantial benefit (strength of recommendation [SOR]: A).6
STUDY SUMMARY
Another option for some women: hrHPV testing alone every 5 years
In this 2018 systematic review and modeling study by the USPSTF, randomized controlled trials (RCTs) and cohort studies that compared cytology to hrHPV testing alone or co-testing (cytology with hrHPV) were used to determine the optimal frequency of, and age group for, cervical cancer screening that would yield the least harm and the most benefit from each of these screening methods.7-9
Similar to the previous recommendation, the USPSTF found that screening women < 21 years or > 65 years if previously adequately screened (defined as 3 consecutive negative screenings or 2 negative screenings within the past 10 years with the most recent being within the past 5 years) led to more harm than benefit. They therefore concluded that women in these age groups should not be screened routinely (SOR: D). The USPSTF also recommends against cervical cancer screening in women who have had a hysterectomy with removal of the cervix and who do not have a history of a high-grade precancerous lesion or cervical cancer (SOR: D).
However, for women ages 21 to 65 years, the USPSTF found that screening substantially reduces cervical cancer incidence and mortality, and that for women ages 21 to 29 years, screening every 3 years with cytology alone offers the best balance of benefits and harms (SOR: A). For women ages 30 to 65 years, the USPSTF recommends screening every 3 years with cytology alone or every 5 years with either primary hrHPV testing or co-testing (hrHPV with cytology) (SOR: A). The recommendations apply to all asymptomatic women with a cervix; exceptions include those with a history of a high-grade precancerous cervical lesion or cancer, in utero exposure to diethylstilbestrol, or a compromised immune system.
Continue to: The change
The change in this current set of recommendations by the USPSTF is the inclusion of screening with hrHPV alone every 5 years as an additional cervical cancer screening option for women ages 30 to 65 years. The decision to include this option was based largely on a decision analysis model commissioned by the USPSTF and reviewed along with clinical trials and cohort studies. The modeling studies found that both primary hrHPV testing alone and co-testing every 5 years prevented a similar number of cervical cancer cases and required a similar number of colposcopies.
Finally, the USPSTF emphasized that screening alone is not sufficient for the prevention of cervical cancer and that efforts should be made to create equitable access to follow-up of abnormal results and the provision of appropriate treatment.1,2
WHAT’S NEW
When it comes to cervical cancer screening, 3 solid options now exist
The previous USPSTF recommendation concluded that women ages 30 to 65 years should be screened with either cytology alone every 3 years or co-testing (cytology and hrHPV) every 5 years. This systematic review and modeling study concluded that any one of the stated screening methods would be adequately sensitive for detecting precancerous high-grade cervical lesions or cervical cancer: cytology every 3 years, primary hrHPV every 5 years, or co-testing every 5 years.7-9
CAVEATS
No studies comparing hrHPVto co-testing and no meta-analysis
No studies were found that directly compared primary hrHPV testing with co-testing.1 A meta-analysis could not be performed due to the methodological differences in RCTs and cohort studies reviewed. The new recommendation is unique in its reliance on modeling to simulate a direct comparison of these 2 screening methods.
CHALLENGES TO IMPLEMENTATION
Getting the word out and increasing comfort levels
The principal challenge to implementation lies in practitioners’ knowledge of this new recommendation and a possible low comfort level with ordering hrHPV testing alone. Patients will need to be engaged in shared decision-making to understand and make use of the 3 options.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Curry SJ, Krist AH, Owens DK, et al. Screening for cervical cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2018;320:674-686.
2. Melnikow J, Henderson JT, Burda BU, et al. Screening for cervical cancer with high-risk human papillomavirus testing: a systematic evidence review for the US Preventive Services Task Force. Evidence Synthesis No. 158. Rockville, MD: Agency for Healthcare Research and Quality; 2018.
3. National Cancer Institute. Cancer Stat Facts. Cervix uteri. https://seer.cancer.gov/statfacts/. Accessed July 1, 2020.
4. Momenimovahed Z, Salehiniya H. Incidence, mortality and risk factors of cervical cancer in the world. Biomed Res Ther. 2017;4:1795-1811.
5. Ashing-Giwa KT, Kagawa-Singer M, Padilla GV, et al. The impact of cervical cancer and dysplasia: a qualitative, multiethnic study. Psychooncology. 2004;13:709-728.
6. Moyer VA; US Preventive Services Task Force. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2012; 156:880-891.
7. Ronco G, Giorgi-Rossi P, Carozzi F, et al; New Technologies for Cervical Cancer Screening (NTCC) Working Group. Efficacy of human papillomavirus testing for the detection of invasive cervical cancers and cervical intraepithelial neoplasia: a randomized controlled trial. Lancet Oncol. 2010;11:249-257.
8. Ronco G, Giorgi-Rossi P, Carozzi F, et al; New Technologies for Cervical Cancer Screening Working Group. Results at recruitment from a randomized controlled trial comparing human papillomavirus testing alone with conventional cytology as the primary cervical cancer screening test. J Natl Cancer Inst. 2008;100:492-501.
9. Ogilvie GS, van Niekerk DJ, Krajden M, et al. A randomized controlled trial of human papillomavirus (HPV) testing for cervical cancer screening: trial design and preliminary results (HPV FOCAL Trial). BMC Cancer. 2010;10:111.
1. Curry SJ, Krist AH, Owens DK, et al. Screening for cervical cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2018;320:674-686.
2. Melnikow J, Henderson JT, Burda BU, et al. Screening for cervical cancer with high-risk human papillomavirus testing: a systematic evidence review for the US Preventive Services Task Force. Evidence Synthesis No. 158. Rockville, MD: Agency for Healthcare Research and Quality; 2018.
3. National Cancer Institute. Cancer Stat Facts. Cervix uteri. https://seer.cancer.gov/statfacts/. Accessed July 1, 2020.
4. Momenimovahed Z, Salehiniya H. Incidence, mortality and risk factors of cervical cancer in the world. Biomed Res Ther. 2017;4:1795-1811.
5. Ashing-Giwa KT, Kagawa-Singer M, Padilla GV, et al. The impact of cervical cancer and dysplasia: a qualitative, multiethnic study. Psychooncology. 2004;13:709-728.
6. Moyer VA; US Preventive Services Task Force. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2012; 156:880-891.
7. Ronco G, Giorgi-Rossi P, Carozzi F, et al; New Technologies for Cervical Cancer Screening (NTCC) Working Group. Efficacy of human papillomavirus testing for the detection of invasive cervical cancers and cervical intraepithelial neoplasia: a randomized controlled trial. Lancet Oncol. 2010;11:249-257.
8. Ronco G, Giorgi-Rossi P, Carozzi F, et al; New Technologies for Cervical Cancer Screening Working Group. Results at recruitment from a randomized controlled trial comparing human papillomavirus testing alone with conventional cytology as the primary cervical cancer screening test. J Natl Cancer Inst. 2008;100:492-501.
9. Ogilvie GS, van Niekerk DJ, Krajden M, et al. A randomized controlled trial of human papillomavirus (HPV) testing for cervical cancer screening: trial design and preliminary results (HPV FOCAL Trial). BMC Cancer. 2010;10:111.
PRACTICE CHANGER
Offer women ages 30 to 65 years the option of being screened for cervical cancer using a high-risk human papillomavirus assay every 5 years.1,2
STRENGTH OF RECOMMENDATION
A: Based on a US Preventive Services Task Force recommendation statement.
Curry SJ, Krist AH, Owens DK, et al. Screening for cervical cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2018;320:674-686.
Nine states have no board-certified pediatric dermatologist, analysis reveals
In fact, nine states do not have a single pediatric dermatologist.
The findings come from a cross-sectional analysis of national data presented by Sepideh Ashrafzadeh at the virtual annual meeting of the Society for Pediatric Dermatology.
“Nearly 82% of pediatricians report that their patients have difficulty accessing pediatric dermatologists [and] over 25% of pediatric dermatologists have a wait time of greater than 10 weeks for new patient appointments,” Ms. Ashrafzadeh, a student at Harvard Medical School, Boston, and associates wrote in their poster abstract. “While the shortage of pediatric dermatologists is well documented, little is known about the distribution of pediatric dermatologists across the U.S., which in turn affects families’ travel time and access to pediatric dermatologists. Defining the specific regions with greatest need for pediatric dermatology can help shape recruitment efforts and initiatives to increase access to pediatric dermatologists in areas with the greatest need.”
For the current study, the researchers drew from the SPD Directory in March 2020 to identify all U.S. board-certified pediatric dermatologists. They used the 2020 American Board of Pediatrics Directory and the 2020 Centers for Medicaid & Medicare Physician Compare Database to identify pediatric generalists, which were defined as pediatricians and family medicine physicians. They used the 2018 American Community Survey, published by the U.S. Census Bureau, to obtain the number of children ages 0-17 years in each county and state.
Next, Ms. Ashrafzadeh and colleagues tabulated the number of children, pediatric dermatologists, and pediatric generalists in each county and state, and calculated ratios of pediatric dermatologists and generalists to number of children. The Gini index, a standardized scale where 0 signifies equal distribution and 1 signifies complete maldistribution, was calculated for pediatric dermatologists and generalists relative to the population of children at the state level.
Of the 317 pediatric dermatologists included in the analysis, 243 (77%) were female, 194 (61%) worked in an academic center, and 311 (98%) worked in a metropolitan county. A pediatric dermatologist was present in 41 of 50 states (82%) and in 142 of 3,228 counties (4%). There was not a single pediatric dermatologist in 73 out of 158 counties (46%) with over 100,000 children, 19 out of 66 counties (29%) with over 200,000 children, and 4 out of 13 counties (31%) with over 500,000 children. Nine states had no pediatric dermatologists: Delaware, Idaho, Maine, Mississippi, Montana, Nevada, North Dakota, South Dakota, and Wyoming. States with the greatest density of pediatric dermatologists (range, 10.1-15.2 pediatric dermatologists per 1,000,000 children) were Wisconsin, Massachusetts, Rhode Island, and New Hampshire. The Gini index for the distribution of pediatric dermatologists relative to the population of children was 0.488, compared with 0.132 for that of pediatric generalists.
“To address the unmet pediatric dermatology need, educators and policymakers can create initiatives to recruit pediatric dermatologists and expand access to telehealth pediatric dermatology services in these high priority states and counties,” the researchers wrote in their abstract. “Future studies need to be done quantifying travel distances to pediatric dermatologists across the US as travel distances can further identify areas that are in great need of pediatric dermatologists.”
They acknowledged certain limitations of the study, including the fact that they may have missed board-certified pediatric dermatologists who are not listed in the SPD Directory. Ms. Ashrafzadeh and colleagues reported having no financial disclosures.
In fact, nine states do not have a single pediatric dermatologist.
The findings come from a cross-sectional analysis of national data presented by Sepideh Ashrafzadeh at the virtual annual meeting of the Society for Pediatric Dermatology.
“Nearly 82% of pediatricians report that their patients have difficulty accessing pediatric dermatologists [and] over 25% of pediatric dermatologists have a wait time of greater than 10 weeks for new patient appointments,” Ms. Ashrafzadeh, a student at Harvard Medical School, Boston, and associates wrote in their poster abstract. “While the shortage of pediatric dermatologists is well documented, little is known about the distribution of pediatric dermatologists across the U.S., which in turn affects families’ travel time and access to pediatric dermatologists. Defining the specific regions with greatest need for pediatric dermatology can help shape recruitment efforts and initiatives to increase access to pediatric dermatologists in areas with the greatest need.”
For the current study, the researchers drew from the SPD Directory in March 2020 to identify all U.S. board-certified pediatric dermatologists. They used the 2020 American Board of Pediatrics Directory and the 2020 Centers for Medicaid & Medicare Physician Compare Database to identify pediatric generalists, which were defined as pediatricians and family medicine physicians. They used the 2018 American Community Survey, published by the U.S. Census Bureau, to obtain the number of children ages 0-17 years in each county and state.
Next, Ms. Ashrafzadeh and colleagues tabulated the number of children, pediatric dermatologists, and pediatric generalists in each county and state, and calculated ratios of pediatric dermatologists and generalists to number of children. The Gini index, a standardized scale where 0 signifies equal distribution and 1 signifies complete maldistribution, was calculated for pediatric dermatologists and generalists relative to the population of children at the state level.
Of the 317 pediatric dermatologists included in the analysis, 243 (77%) were female, 194 (61%) worked in an academic center, and 311 (98%) worked in a metropolitan county. A pediatric dermatologist was present in 41 of 50 states (82%) and in 142 of 3,228 counties (4%). There was not a single pediatric dermatologist in 73 out of 158 counties (46%) with over 100,000 children, 19 out of 66 counties (29%) with over 200,000 children, and 4 out of 13 counties (31%) with over 500,000 children. Nine states had no pediatric dermatologists: Delaware, Idaho, Maine, Mississippi, Montana, Nevada, North Dakota, South Dakota, and Wyoming. States with the greatest density of pediatric dermatologists (range, 10.1-15.2 pediatric dermatologists per 1,000,000 children) were Wisconsin, Massachusetts, Rhode Island, and New Hampshire. The Gini index for the distribution of pediatric dermatologists relative to the population of children was 0.488, compared with 0.132 for that of pediatric generalists.
“To address the unmet pediatric dermatology need, educators and policymakers can create initiatives to recruit pediatric dermatologists and expand access to telehealth pediatric dermatology services in these high priority states and counties,” the researchers wrote in their abstract. “Future studies need to be done quantifying travel distances to pediatric dermatologists across the US as travel distances can further identify areas that are in great need of pediatric dermatologists.”
They acknowledged certain limitations of the study, including the fact that they may have missed board-certified pediatric dermatologists who are not listed in the SPD Directory. Ms. Ashrafzadeh and colleagues reported having no financial disclosures.
In fact, nine states do not have a single pediatric dermatologist.
The findings come from a cross-sectional analysis of national data presented by Sepideh Ashrafzadeh at the virtual annual meeting of the Society for Pediatric Dermatology.
“Nearly 82% of pediatricians report that their patients have difficulty accessing pediatric dermatologists [and] over 25% of pediatric dermatologists have a wait time of greater than 10 weeks for new patient appointments,” Ms. Ashrafzadeh, a student at Harvard Medical School, Boston, and associates wrote in their poster abstract. “While the shortage of pediatric dermatologists is well documented, little is known about the distribution of pediatric dermatologists across the U.S., which in turn affects families’ travel time and access to pediatric dermatologists. Defining the specific regions with greatest need for pediatric dermatology can help shape recruitment efforts and initiatives to increase access to pediatric dermatologists in areas with the greatest need.”
For the current study, the researchers drew from the SPD Directory in March 2020 to identify all U.S. board-certified pediatric dermatologists. They used the 2020 American Board of Pediatrics Directory and the 2020 Centers for Medicaid & Medicare Physician Compare Database to identify pediatric generalists, which were defined as pediatricians and family medicine physicians. They used the 2018 American Community Survey, published by the U.S. Census Bureau, to obtain the number of children ages 0-17 years in each county and state.
Next, Ms. Ashrafzadeh and colleagues tabulated the number of children, pediatric dermatologists, and pediatric generalists in each county and state, and calculated ratios of pediatric dermatologists and generalists to number of children. The Gini index, a standardized scale where 0 signifies equal distribution and 1 signifies complete maldistribution, was calculated for pediatric dermatologists and generalists relative to the population of children at the state level.
Of the 317 pediatric dermatologists included in the analysis, 243 (77%) were female, 194 (61%) worked in an academic center, and 311 (98%) worked in a metropolitan county. A pediatric dermatologist was present in 41 of 50 states (82%) and in 142 of 3,228 counties (4%). There was not a single pediatric dermatologist in 73 out of 158 counties (46%) with over 100,000 children, 19 out of 66 counties (29%) with over 200,000 children, and 4 out of 13 counties (31%) with over 500,000 children. Nine states had no pediatric dermatologists: Delaware, Idaho, Maine, Mississippi, Montana, Nevada, North Dakota, South Dakota, and Wyoming. States with the greatest density of pediatric dermatologists (range, 10.1-15.2 pediatric dermatologists per 1,000,000 children) were Wisconsin, Massachusetts, Rhode Island, and New Hampshire. The Gini index for the distribution of pediatric dermatologists relative to the population of children was 0.488, compared with 0.132 for that of pediatric generalists.
“To address the unmet pediatric dermatology need, educators and policymakers can create initiatives to recruit pediatric dermatologists and expand access to telehealth pediatric dermatology services in these high priority states and counties,” the researchers wrote in their abstract. “Future studies need to be done quantifying travel distances to pediatric dermatologists across the US as travel distances can further identify areas that are in great need of pediatric dermatologists.”
They acknowledged certain limitations of the study, including the fact that they may have missed board-certified pediatric dermatologists who are not listed in the SPD Directory. Ms. Ashrafzadeh and colleagues reported having no financial disclosures.
FROM SPD 2020
Quitting smoking after MI has huge benefits in young adults
Young adult smokers who stop smoking in the first year after an initial myocardial infarction are far less likely to die over the next 10 years than their peers who continue to smoke. Yet nearly two-thirds keep smoking after the event, according to new data from the Partners YOUNG-MI Registry.
“Smoking is one of the most common risk factors for developing an MI at a young age. ... This reinforces the need to have more young individuals avoid, or quit, the use of tobacco,” Ron Blankstein, MD, Brigham and Women’s Hospital and Harvard Medical School, Boston, said in an interview.
Yet, the finding that 62% of young adults continue to smoke 1 year after MI points to an “enormous need for better smoking cessation efforts following a heart attack,” he said.
“Powerful” message for clinicians
“This study joins an incredibly powerful body of evidence that says if you quit smoking, you’re going to live longer,” said Michael Fiore, MD, MPH, MBA, director of the University of Wisconsin Center for Tobacco Research and Intervention, Madison, who wasn’t involved in the study.
“As physicians, there is nothing we can do that will have a greater impact for our patients than quitting smoking. The study is a powerful call for clinicians to intervene with their patients that smoke – both if you have an MI or if you don’t,” Dr. Fiore told this news organization.
The study involved 2,072 individuals 50 years or younger (median age, 45 years; 81% male) who were hospitalized for an initial MI at two large academic medical centers in Boston. Of these, 33.9% were never-smokers, 13.6% were former smokers, and 52.5% were smokers at the time of their MI.
During a median follow-up of 10.2 years, those who quit smoking had a significantly lower rate of death from any cause (unadjusted hazard ratio, 0.35; 95% confidence interval, 0.19-0.63; P < .001) and a cardiovascular cause (HR, 0.29; 95% CI, 0.11-0.79; P = .02), relative to those who continued to smoke.
The results remained statistically significant in a propensity-matched analysis for both all-cause (HR, 0.30; 95% CI, 0.16-0.56; P < .001) and CV mortality (HR, 0.19; 95% CI, 0.06-0.56; P = .003).
“Although patients who quit smoking were similar to those who continued to smoke with respect to their baseline characteristics, smoking cessation was associated with an approximate 70%-80% reduction in all-cause and CV mortality,” the authors note in their article, published online July 8 in JAMA Network Open.
They say it’s also noteworthy that long-term death rates of never-smokers and former smokers who quit before the MI were nearly identical.
‘A failure of our health care system’
The bottom line, said Dr. Blankstein, is that it is “never too late to quit, and those who experience an MI should do so right away. Our health care system must help promote such efforts, as there is immense room for improvement.”
Dr. Fiore said: “When I see an article like this, it just reminds me that, if you’re really thinking about staying healthy, there is nothing better you can do to improve the quality and longevity of your life than quitting smoking.”
The observation that many patients continue to smoke after MI is a “failure of our health care system, and it’s an individual failure in that these individuals are not able to overcome their powerful nicotine dependence. It’s an unfortunate occurrence that’s resulting in unnecessary deaths,” said Dr. Fiore.
There is no “magic bullet” to overcome nicotine addiction, but there are approved treatments that can “substantially boost quit rates,” he noted.
The two most effective smoking-cessation treatments are varenicline (Chantix) and combination nicotine replacement therapy, a patch combined ideally with nicotine mini lozenges, particularly when combined with some brief counseling, said Fiore.
He encourages cardiologists to get their patients to commit to quitting and then link them to resources such as 1-800-QUIT-NOW or SmokeFree.gov.
Funding for the study was provided by grants from the National Heart, Lung, and Blood Institute. Dr. Blankstein reported receiving research support from Amgen and Astellas. Dr. Fiore had no relevant disclosures.
A version of this article originally appeared on Medscape.com.
Young adult smokers who stop smoking in the first year after an initial myocardial infarction are far less likely to die over the next 10 years than their peers who continue to smoke. Yet nearly two-thirds keep smoking after the event, according to new data from the Partners YOUNG-MI Registry.
“Smoking is one of the most common risk factors for developing an MI at a young age. ... This reinforces the need to have more young individuals avoid, or quit, the use of tobacco,” Ron Blankstein, MD, Brigham and Women’s Hospital and Harvard Medical School, Boston, said in an interview.
Yet, the finding that 62% of young adults continue to smoke 1 year after MI points to an “enormous need for better smoking cessation efforts following a heart attack,” he said.
“Powerful” message for clinicians
“This study joins an incredibly powerful body of evidence that says if you quit smoking, you’re going to live longer,” said Michael Fiore, MD, MPH, MBA, director of the University of Wisconsin Center for Tobacco Research and Intervention, Madison, who wasn’t involved in the study.
“As physicians, there is nothing we can do that will have a greater impact for our patients than quitting smoking. The study is a powerful call for clinicians to intervene with their patients that smoke – both if you have an MI or if you don’t,” Dr. Fiore told this news organization.
The study involved 2,072 individuals 50 years or younger (median age, 45 years; 81% male) who were hospitalized for an initial MI at two large academic medical centers in Boston. Of these, 33.9% were never-smokers, 13.6% were former smokers, and 52.5% were smokers at the time of their MI.
During a median follow-up of 10.2 years, those who quit smoking had a significantly lower rate of death from any cause (unadjusted hazard ratio, 0.35; 95% confidence interval, 0.19-0.63; P < .001) and a cardiovascular cause (HR, 0.29; 95% CI, 0.11-0.79; P = .02), relative to those who continued to smoke.
The results remained statistically significant in a propensity-matched analysis for both all-cause (HR, 0.30; 95% CI, 0.16-0.56; P < .001) and CV mortality (HR, 0.19; 95% CI, 0.06-0.56; P = .003).
“Although patients who quit smoking were similar to those who continued to smoke with respect to their baseline characteristics, smoking cessation was associated with an approximate 70%-80% reduction in all-cause and CV mortality,” the authors note in their article, published online July 8 in JAMA Network Open.
They say it’s also noteworthy that long-term death rates of never-smokers and former smokers who quit before the MI were nearly identical.
‘A failure of our health care system’
The bottom line, said Dr. Blankstein, is that it is “never too late to quit, and those who experience an MI should do so right away. Our health care system must help promote such efforts, as there is immense room for improvement.”
Dr. Fiore said: “When I see an article like this, it just reminds me that, if you’re really thinking about staying healthy, there is nothing better you can do to improve the quality and longevity of your life than quitting smoking.”
The observation that many patients continue to smoke after MI is a “failure of our health care system, and it’s an individual failure in that these individuals are not able to overcome their powerful nicotine dependence. It’s an unfortunate occurrence that’s resulting in unnecessary deaths,” said Dr. Fiore.
There is no “magic bullet” to overcome nicotine addiction, but there are approved treatments that can “substantially boost quit rates,” he noted.
The two most effective smoking-cessation treatments are varenicline (Chantix) and combination nicotine replacement therapy, a patch combined ideally with nicotine mini lozenges, particularly when combined with some brief counseling, said Fiore.
He encourages cardiologists to get their patients to commit to quitting and then link them to resources such as 1-800-QUIT-NOW or SmokeFree.gov.
Funding for the study was provided by grants from the National Heart, Lung, and Blood Institute. Dr. Blankstein reported receiving research support from Amgen and Astellas. Dr. Fiore had no relevant disclosures.
A version of this article originally appeared on Medscape.com.
Young adult smokers who stop smoking in the first year after an initial myocardial infarction are far less likely to die over the next 10 years than their peers who continue to smoke. Yet nearly two-thirds keep smoking after the event, according to new data from the Partners YOUNG-MI Registry.
“Smoking is one of the most common risk factors for developing an MI at a young age. ... This reinforces the need to have more young individuals avoid, or quit, the use of tobacco,” Ron Blankstein, MD, Brigham and Women’s Hospital and Harvard Medical School, Boston, said in an interview.
Yet, the finding that 62% of young adults continue to smoke 1 year after MI points to an “enormous need for better smoking cessation efforts following a heart attack,” he said.
“Powerful” message for clinicians
“This study joins an incredibly powerful body of evidence that says if you quit smoking, you’re going to live longer,” said Michael Fiore, MD, MPH, MBA, director of the University of Wisconsin Center for Tobacco Research and Intervention, Madison, who wasn’t involved in the study.
“As physicians, there is nothing we can do that will have a greater impact for our patients than quitting smoking. The study is a powerful call for clinicians to intervene with their patients that smoke – both if you have an MI or if you don’t,” Dr. Fiore told this news organization.
The study involved 2,072 individuals 50 years or younger (median age, 45 years; 81% male) who were hospitalized for an initial MI at two large academic medical centers in Boston. Of these, 33.9% were never-smokers, 13.6% were former smokers, and 52.5% were smokers at the time of their MI.
During a median follow-up of 10.2 years, those who quit smoking had a significantly lower rate of death from any cause (unadjusted hazard ratio, 0.35; 95% confidence interval, 0.19-0.63; P < .001) and a cardiovascular cause (HR, 0.29; 95% CI, 0.11-0.79; P = .02), relative to those who continued to smoke.
The results remained statistically significant in a propensity-matched analysis for both all-cause (HR, 0.30; 95% CI, 0.16-0.56; P < .001) and CV mortality (HR, 0.19; 95% CI, 0.06-0.56; P = .003).
“Although patients who quit smoking were similar to those who continued to smoke with respect to their baseline characteristics, smoking cessation was associated with an approximate 70%-80% reduction in all-cause and CV mortality,” the authors note in their article, published online July 8 in JAMA Network Open.
They say it’s also noteworthy that long-term death rates of never-smokers and former smokers who quit before the MI were nearly identical.
‘A failure of our health care system’
The bottom line, said Dr. Blankstein, is that it is “never too late to quit, and those who experience an MI should do so right away. Our health care system must help promote such efforts, as there is immense room for improvement.”
Dr. Fiore said: “When I see an article like this, it just reminds me that, if you’re really thinking about staying healthy, there is nothing better you can do to improve the quality and longevity of your life than quitting smoking.”
The observation that many patients continue to smoke after MI is a “failure of our health care system, and it’s an individual failure in that these individuals are not able to overcome their powerful nicotine dependence. It’s an unfortunate occurrence that’s resulting in unnecessary deaths,” said Dr. Fiore.
There is no “magic bullet” to overcome nicotine addiction, but there are approved treatments that can “substantially boost quit rates,” he noted.
The two most effective smoking-cessation treatments are varenicline (Chantix) and combination nicotine replacement therapy, a patch combined ideally with nicotine mini lozenges, particularly when combined with some brief counseling, said Fiore.
He encourages cardiologists to get their patients to commit to quitting and then link them to resources such as 1-800-QUIT-NOW or SmokeFree.gov.
Funding for the study was provided by grants from the National Heart, Lung, and Blood Institute. Dr. Blankstein reported receiving research support from Amgen and Astellas. Dr. Fiore had no relevant disclosures.
A version of this article originally appeared on Medscape.com.
Our CHEST year
Greetings. I hope that you are well and are enjoying the summer as best you can during these challenging times. Since the “CHEST year” has drawn to a close recently, I would like to offer my reflections, which were recently shared with the Board of Regents, as well as a glimpse of what is ahead for CHEST. There is just so much great work I want to share.
This past year has posed a number of challenges. COVID-19 has caused us to interact differently on both a social and a business level. CHEST Headquarters has been closed, and we have not had a live-learning course for more than 4 months. But our work has not faltered. We have been extremely productive during this period and have once again demonstrated our resiliency and innovative spirit; in our vernacular, we “Crushed It.”
While COVID-19 has presented us with a number of obstacles, it has presented us with a number of opportunities, and we have taken advantage of them. During this pandemic, CHEST has truly demonstrated its ability to provide a connection at a critical time, giving this phrase new meaning and urgency. We have created a new resource center for clinicians, developed patient education and awareness campaigns to support the public through this crisis, launched a webinar series, developed scientific guidance statements, and more. At the same time, we have invested in our technology and educational infrastructure to grow our capabilities and position CHEST for long-term success.
Prior to COVID-19, we spent a significant amount of time among the CHEST staff, Presidents, and Boards drafting and reviewing a concise strategy statement for CHEST to provide focus and clarity to its efforts and derive and tie together future strategies specific to learning, technology, and more. From this statement, we derived four key areas requiring our continued and explicit focus to achieve this goal:
• People: Ensure we attract, retain, and incentivize the right people (staff, leaders, and volunteers).
• Products: Foster an environment of innovation and product development resulting in overall revenue growth, as well as revenue from new products and services.
• Education: Ensure that CHEST education products and services are robust, differentiated, and scalable..
• Growth: Meet or exceed revenue and margin targets.
As long as the mission and strategy of the organization does not deviate, these goals should not change. However, how we go about executing on achieving these goals each year will depend on the context of our environment and be shaped by the specific initiatives planned affecting our People, Products, Education, and building toward Growth. This consistency is important to sustain a vibrant, aligned, and productive organization.
Beyond this groundwork, I also would like to list a series of things that, together, CHEST accomplished over the last year.
- Reviewed existing contracts and, where appropriate, renegotiated major contracts to ensure terms more favorable for CHEST.
- Hired and on-boarded a Chief Learning Officer to place greater emphasis on expanding CHEST educational programs. Analyzed current educational products and have begun repositioning our educational efforts to better serve our learners.
- Refined the one CHEST concept, realigned responsibilities throughout the organization in general, and the CHEST Foundation, in particular, to enhance resource readiness and productivity. Clarified relationship with industry by continuing to implement our Industry Partnership Guidelines and streamline efforts with our partners.
- Continued rollout and execution of our international event strategy. Successfully developed and held a program for CHEST Congress 2020 Italy with our CHEST Italian Delegation, in a virtual format, due to COVID, while enabling us to build momentum for a rescheduled meeting in 2021. We had over 2,000 virtual registrants from over 100 countries, and there was a thank you given to all attendees by Dr. Anthony Fauci, Director of the National Institute of Allergy and Infectious Diseases, to start off the program – what a success!
- Accelerated our digital transformation with an educational focus on virtual Board Review, CHEST 2020 Annual Meeting, online simulation.
- Forecasting strong financial outlook and improving financial reporting for FY19-20. Successful 2019 annual meeting:
o Total attendance 8,593—the largest attendance to date.
o Simulation Session Registration 979
o Exhibiting companies 160 SOLD OUT
o CHEST Annual Meeting 2019 delivered largest number of APPs and fellows attending in the last 5 years.
- Reintroduced CHEST into the advocacy and health policy arena through the successful acquisition of NAMDRC.
CHEST’s operating financial performance is solid, and well thought out efforts have kept CHEST on a growth trajectory over the last 7 years. During this same period (since 2011/12), our staff headcount has grown from 85 to a projected 121 this year; the new expertise and capabilities we have brought on board, combined with our highly talented and committed staff team, have contributed to this tremendous growth.
Our future is bright. These 2 two years have been very exciting for me both professionally and personally. I am grateful for the opportunity to work with all of you and serve as your CEO/EVP because together, we truly are making a significant difference in moving CHEST forward and crushing lung disease.
I know you are as proud of CHEST’s efforts this year as I am.
Thank you.
Greetings. I hope that you are well and are enjoying the summer as best you can during these challenging times. Since the “CHEST year” has drawn to a close recently, I would like to offer my reflections, which were recently shared with the Board of Regents, as well as a glimpse of what is ahead for CHEST. There is just so much great work I want to share.
This past year has posed a number of challenges. COVID-19 has caused us to interact differently on both a social and a business level. CHEST Headquarters has been closed, and we have not had a live-learning course for more than 4 months. But our work has not faltered. We have been extremely productive during this period and have once again demonstrated our resiliency and innovative spirit; in our vernacular, we “Crushed It.”
While COVID-19 has presented us with a number of obstacles, it has presented us with a number of opportunities, and we have taken advantage of them. During this pandemic, CHEST has truly demonstrated its ability to provide a connection at a critical time, giving this phrase new meaning and urgency. We have created a new resource center for clinicians, developed patient education and awareness campaigns to support the public through this crisis, launched a webinar series, developed scientific guidance statements, and more. At the same time, we have invested in our technology and educational infrastructure to grow our capabilities and position CHEST for long-term success.
Prior to COVID-19, we spent a significant amount of time among the CHEST staff, Presidents, and Boards drafting and reviewing a concise strategy statement for CHEST to provide focus and clarity to its efforts and derive and tie together future strategies specific to learning, technology, and more. From this statement, we derived four key areas requiring our continued and explicit focus to achieve this goal:
• People: Ensure we attract, retain, and incentivize the right people (staff, leaders, and volunteers).
• Products: Foster an environment of innovation and product development resulting in overall revenue growth, as well as revenue from new products and services.
• Education: Ensure that CHEST education products and services are robust, differentiated, and scalable..
• Growth: Meet or exceed revenue and margin targets.
As long as the mission and strategy of the organization does not deviate, these goals should not change. However, how we go about executing on achieving these goals each year will depend on the context of our environment and be shaped by the specific initiatives planned affecting our People, Products, Education, and building toward Growth. This consistency is important to sustain a vibrant, aligned, and productive organization.
Beyond this groundwork, I also would like to list a series of things that, together, CHEST accomplished over the last year.
- Reviewed existing contracts and, where appropriate, renegotiated major contracts to ensure terms more favorable for CHEST.
- Hired and on-boarded a Chief Learning Officer to place greater emphasis on expanding CHEST educational programs. Analyzed current educational products and have begun repositioning our educational efforts to better serve our learners.
- Refined the one CHEST concept, realigned responsibilities throughout the organization in general, and the CHEST Foundation, in particular, to enhance resource readiness and productivity. Clarified relationship with industry by continuing to implement our Industry Partnership Guidelines and streamline efforts with our partners.
- Continued rollout and execution of our international event strategy. Successfully developed and held a program for CHEST Congress 2020 Italy with our CHEST Italian Delegation, in a virtual format, due to COVID, while enabling us to build momentum for a rescheduled meeting in 2021. We had over 2,000 virtual registrants from over 100 countries, and there was a thank you given to all attendees by Dr. Anthony Fauci, Director of the National Institute of Allergy and Infectious Diseases, to start off the program – what a success!
- Accelerated our digital transformation with an educational focus on virtual Board Review, CHEST 2020 Annual Meeting, online simulation.
- Forecasting strong financial outlook and improving financial reporting for FY19-20. Successful 2019 annual meeting:
o Total attendance 8,593—the largest attendance to date.
o Simulation Session Registration 979
o Exhibiting companies 160 SOLD OUT
o CHEST Annual Meeting 2019 delivered largest number of APPs and fellows attending in the last 5 years.
- Reintroduced CHEST into the advocacy and health policy arena through the successful acquisition of NAMDRC.
CHEST’s operating financial performance is solid, and well thought out efforts have kept CHEST on a growth trajectory over the last 7 years. During this same period (since 2011/12), our staff headcount has grown from 85 to a projected 121 this year; the new expertise and capabilities we have brought on board, combined with our highly talented and committed staff team, have contributed to this tremendous growth.
Our future is bright. These 2 two years have been very exciting for me both professionally and personally. I am grateful for the opportunity to work with all of you and serve as your CEO/EVP because together, we truly are making a significant difference in moving CHEST forward and crushing lung disease.
I know you are as proud of CHEST’s efforts this year as I am.
Thank you.
Greetings. I hope that you are well and are enjoying the summer as best you can during these challenging times. Since the “CHEST year” has drawn to a close recently, I would like to offer my reflections, which were recently shared with the Board of Regents, as well as a glimpse of what is ahead for CHEST. There is just so much great work I want to share.
This past year has posed a number of challenges. COVID-19 has caused us to interact differently on both a social and a business level. CHEST Headquarters has been closed, and we have not had a live-learning course for more than 4 months. But our work has not faltered. We have been extremely productive during this period and have once again demonstrated our resiliency and innovative spirit; in our vernacular, we “Crushed It.”
While COVID-19 has presented us with a number of obstacles, it has presented us with a number of opportunities, and we have taken advantage of them. During this pandemic, CHEST has truly demonstrated its ability to provide a connection at a critical time, giving this phrase new meaning and urgency. We have created a new resource center for clinicians, developed patient education and awareness campaigns to support the public through this crisis, launched a webinar series, developed scientific guidance statements, and more. At the same time, we have invested in our technology and educational infrastructure to grow our capabilities and position CHEST for long-term success.
Prior to COVID-19, we spent a significant amount of time among the CHEST staff, Presidents, and Boards drafting and reviewing a concise strategy statement for CHEST to provide focus and clarity to its efforts and derive and tie together future strategies specific to learning, technology, and more. From this statement, we derived four key areas requiring our continued and explicit focus to achieve this goal:
• People: Ensure we attract, retain, and incentivize the right people (staff, leaders, and volunteers).
• Products: Foster an environment of innovation and product development resulting in overall revenue growth, as well as revenue from new products and services.
• Education: Ensure that CHEST education products and services are robust, differentiated, and scalable..
• Growth: Meet or exceed revenue and margin targets.
As long as the mission and strategy of the organization does not deviate, these goals should not change. However, how we go about executing on achieving these goals each year will depend on the context of our environment and be shaped by the specific initiatives planned affecting our People, Products, Education, and building toward Growth. This consistency is important to sustain a vibrant, aligned, and productive organization.
Beyond this groundwork, I also would like to list a series of things that, together, CHEST accomplished over the last year.
- Reviewed existing contracts and, where appropriate, renegotiated major contracts to ensure terms more favorable for CHEST.
- Hired and on-boarded a Chief Learning Officer to place greater emphasis on expanding CHEST educational programs. Analyzed current educational products and have begun repositioning our educational efforts to better serve our learners.
- Refined the one CHEST concept, realigned responsibilities throughout the organization in general, and the CHEST Foundation, in particular, to enhance resource readiness and productivity. Clarified relationship with industry by continuing to implement our Industry Partnership Guidelines and streamline efforts with our partners.
- Continued rollout and execution of our international event strategy. Successfully developed and held a program for CHEST Congress 2020 Italy with our CHEST Italian Delegation, in a virtual format, due to COVID, while enabling us to build momentum for a rescheduled meeting in 2021. We had over 2,000 virtual registrants from over 100 countries, and there was a thank you given to all attendees by Dr. Anthony Fauci, Director of the National Institute of Allergy and Infectious Diseases, to start off the program – what a success!
- Accelerated our digital transformation with an educational focus on virtual Board Review, CHEST 2020 Annual Meeting, online simulation.
- Forecasting strong financial outlook and improving financial reporting for FY19-20. Successful 2019 annual meeting:
o Total attendance 8,593—the largest attendance to date.
o Simulation Session Registration 979
o Exhibiting companies 160 SOLD OUT
o CHEST Annual Meeting 2019 delivered largest number of APPs and fellows attending in the last 5 years.
- Reintroduced CHEST into the advocacy and health policy arena through the successful acquisition of NAMDRC.
CHEST’s operating financial performance is solid, and well thought out efforts have kept CHEST on a growth trajectory over the last 7 years. During this same period (since 2011/12), our staff headcount has grown from 85 to a projected 121 this year; the new expertise and capabilities we have brought on board, combined with our highly talented and committed staff team, have contributed to this tremendous growth.
Our future is bright. These 2 two years have been very exciting for me both professionally and personally. I am grateful for the opportunity to work with all of you and serve as your CEO/EVP because together, we truly are making a significant difference in moving CHEST forward and crushing lung disease.
I know you are as proud of CHEST’s efforts this year as I am.
Thank you.
President’s report
Dear Colleagues,
We are now near 6 months into living with COVID-19. In Texas, we are experiencing the surge that much of the Northeast saw in March and April. The COVID-19 Task Force led by Dr. Steve Simpson (CHEST President-Elect) and with representation from the Critical Care, Chest Infections, and Disaster Response and Global Health NetWorks continues to meet regularly to keep our members updated on the latest research and rapidly changing clinical management of COVID-19 illness and the sequelae. COVID-19 has put our medical profession and our subspecialty under considerable stress, and CHEST has launched a new longitudinal Wellness Center led by Dr. Alex Niven, from Mayo Clinic, Rochester. These new resources will feature a wellness webinar series focused on mental health and wellness for clinicians during COVID-19 and beyond. CHEST received overwhelming positive feedback from members and attendees to the Women & Pulmonary Virtual Happy Hour that focused on sharing stories and building community. Many leaders have suggested other such topics and efforts that may be useful to the CHEST community. The CHEST Wellness Center will launch on July 15.
In addition to COVID-19 activities, our nation and the world have compelled a new powerful look at race relations, disparities, and diversity. I represented CHEST at a “White Coats for Black Lives” event in San Antonio. Following our nation’s call for racial equality, CHEST released a Statement of Equity that received overwhelmingly positive feedback and response from members via email and on social media. This statement clearly resonated with the CHEST community. We are asking our leadership and members to consider ways in which CHEST might continue to raise awareness and continue with efforts related to diversity and equity. CHEST also hosted an excellent webinar moderated by Dr. Demondes Haynes and Dr. Nneka Sederstrom in late June that offered a direct and meaningful dialogue on issues facing clinicians and patients of color, and the responsibility of those in leadership positions. CHEST leadership stand firm that racism and inequality are public health issues and are working to define how we further our efforts in this arena.
On June 17, CHEST held a 1-day Virtual CHEST Congress in conjunction with our the CHEST Italian Delegation, as COVID-19 prevented us from safely holding the live Congress in Bologna. We had 3,250 registered attendees. I was so impressed at what a virtual platform can deliver, complete with great educational sessions, including much on COVID-19, as well as capturing the CHEST experience with games, bocce, jeopardy etc! This gave CHEST an opportunity to explore further virtual-based education to reach our wider global audience. CHEST will still be holding an in-person Congress in Bologna, June 24-26, 2021.
CHEST will host three entirely virtual Board Review Courses this August in the areas of Pulmonary, Critical Care, and Pediatric Pulmonary Medicine. These courses will include a combination of pre-recorded lectures and live, interactive sessions. Audience response systems and SEEK questions will still be utilized. There’s still time to register, so don’t miss it! With time being a major commodity at present, all attendees will receive year-long access to all material!
I know you have been wondering about CHEST 2020, and as you have heard by now, CHEST 2020 in Chicago will be a virtual meeting. I am sure that this announcement came as no big surprise, but is certainly disappointing. As you can imagine this was a difficult decision, but one that was necessary based upon our new reality. It was compounded by limitations on the convention center venue under the Illinois reopening plan, and the fact that a large number of our faculty, as well as our attendees, are under a travel ban for the remainder of 2020 that will not allow them to travel to Chicago. The abstract and case report deadline closed June 1, and despite these circumstances, we saw our highest number of submissions to date! Late abstracts were due on July 17. We will be presenting standalone and complementary online offerings to ensure seamless delivery of critical education in formats that cater easily to our newly formed habits.
Thanks to our dedicated Scientific Program Committee Chair, Dr. Victor Test, and staff, we had already begun preparing for virtual CHEST Annual Meeting 2020. Here’s what you can expect:
• A memorable experience
• A highly interactive education program that includes audience Q&A, discussion threads, and audience response systems
• Opportunities for one-on-one discussions, networking, and access to faculty
• Industry-sponsored programs and a virtual exhibit hall
• Access to hundreds of narrated poster presentations, case reports, and research abstracts
• Competitive educational gaming where attendees can participate, win, or watch
• Dedicated COVID-19 update sessions
• CME and MOC credits
If you have already registered for CHEST 2020, you will have the option to transfer your registration to this new model. Our main focus is delivering the virtual program with the highest level of service that you have come to expect from CHEST and respect for our member’s time and current situation. I know Dr. Victor Test and the program committee will deliver a superb educational experience in a virtual meeting setting. Thank you for your support and understanding as we continue to evolve our events to meet the needs of our members while adapting to the best delivery methods.
Since so many fellows were unable to hold their live graduation events, and celebrations, we decided to send them off with a virtual event! On June 30 we held a Joint CHEST/ATS Respiratory Community Graduation Ceremony–for graduating fellows, and to welcome new fellows to our profession. The ceremony consisted of a combination of live and recorded messages from key leaders from both organizations. In addition, there was a keynote address from Dr. Rana Awdish, a critical care physician at Henry Ford Hospital in Detroit, who authored the bestselling book “In Shock: My Journey from Death to Recovery and the Redemptive Power of Hope.” I encourage you to watch the video on the Early Career Professionals page on our Chestnet.org website.
The National Association for Medical Direction of Respiratory Care (NAMDRC) merger with CHEST was finalized at the end of May. Look for more advocacy-related actions coming from CHEST. The newly formed Health Policy and Advocacy Committee is helping to set CHEST’s advocacy agendas in the legislative and regulatory arenas, engaging with policymakers and educating CHEST members on governmental affairs relevant to CHEST’s mission. Did you see the inaugural CHEST published, on-line issue of Washington Watchline, a newsletter that aims to keep CHEST members informed about governmental activities that affect physicians who provide clinical care in respiratory, critical care, and sleep medicine? Follow Washington Watchline to learn more about CHEST’s advocacy around regulatory, legislative, and payment issues that relate to the delivery of health care in support of CHEST’s mission. One of the features was Telemedicine, which many of us are now using and is likely to be a part of many of our practices going forward.
With new COVID-19 surges throughout many parts of the United States, CHEST has continued our volunteer matching program for areas of need, including to the Navaho Nations, where CHEST matched 20 volunteers and has had more than a half-dozen inquiries from our members. In addition, in conjunction with the Foundation, CHEST has partnered with American Mask Rally and started a campaign to distribute masks to frontline essential workers in underserved communities. CHEST received a generous donation from AstraZeneca and Glaxo Smith Kline to help in the global fight against COVID-19 to provide current and accurate information and education to frontline clinicians to allow them to provide the best patient outcomes. CHEST also partnered with the American Thoracic Society to launch a joint PSA/ media campaign entitled For My Lung Health Campaign, to provide credible resources for underserved Black and Latino communities, as these communities are disproportionally affected by COVID-19. At the time of this writing, over a million people have seen the related video, featuring tips for taking control of one’s health in these difficult and uncertain times.
So, in closing, thank you all for what you do in these challenging times. 2020 will certainly be a year to remember! Stay safe and stay well!
Stephanie
Dear Colleagues,
We are now near 6 months into living with COVID-19. In Texas, we are experiencing the surge that much of the Northeast saw in March and April. The COVID-19 Task Force led by Dr. Steve Simpson (CHEST President-Elect) and with representation from the Critical Care, Chest Infections, and Disaster Response and Global Health NetWorks continues to meet regularly to keep our members updated on the latest research and rapidly changing clinical management of COVID-19 illness and the sequelae. COVID-19 has put our medical profession and our subspecialty under considerable stress, and CHEST has launched a new longitudinal Wellness Center led by Dr. Alex Niven, from Mayo Clinic, Rochester. These new resources will feature a wellness webinar series focused on mental health and wellness for clinicians during COVID-19 and beyond. CHEST received overwhelming positive feedback from members and attendees to the Women & Pulmonary Virtual Happy Hour that focused on sharing stories and building community. Many leaders have suggested other such topics and efforts that may be useful to the CHEST community. The CHEST Wellness Center will launch on July 15.
In addition to COVID-19 activities, our nation and the world have compelled a new powerful look at race relations, disparities, and diversity. I represented CHEST at a “White Coats for Black Lives” event in San Antonio. Following our nation’s call for racial equality, CHEST released a Statement of Equity that received overwhelmingly positive feedback and response from members via email and on social media. This statement clearly resonated with the CHEST community. We are asking our leadership and members to consider ways in which CHEST might continue to raise awareness and continue with efforts related to diversity and equity. CHEST also hosted an excellent webinar moderated by Dr. Demondes Haynes and Dr. Nneka Sederstrom in late June that offered a direct and meaningful dialogue on issues facing clinicians and patients of color, and the responsibility of those in leadership positions. CHEST leadership stand firm that racism and inequality are public health issues and are working to define how we further our efforts in this arena.
On June 17, CHEST held a 1-day Virtual CHEST Congress in conjunction with our the CHEST Italian Delegation, as COVID-19 prevented us from safely holding the live Congress in Bologna. We had 3,250 registered attendees. I was so impressed at what a virtual platform can deliver, complete with great educational sessions, including much on COVID-19, as well as capturing the CHEST experience with games, bocce, jeopardy etc! This gave CHEST an opportunity to explore further virtual-based education to reach our wider global audience. CHEST will still be holding an in-person Congress in Bologna, June 24-26, 2021.
CHEST will host three entirely virtual Board Review Courses this August in the areas of Pulmonary, Critical Care, and Pediatric Pulmonary Medicine. These courses will include a combination of pre-recorded lectures and live, interactive sessions. Audience response systems and SEEK questions will still be utilized. There’s still time to register, so don’t miss it! With time being a major commodity at present, all attendees will receive year-long access to all material!
I know you have been wondering about CHEST 2020, and as you have heard by now, CHEST 2020 in Chicago will be a virtual meeting. I am sure that this announcement came as no big surprise, but is certainly disappointing. As you can imagine this was a difficult decision, but one that was necessary based upon our new reality. It was compounded by limitations on the convention center venue under the Illinois reopening plan, and the fact that a large number of our faculty, as well as our attendees, are under a travel ban for the remainder of 2020 that will not allow them to travel to Chicago. The abstract and case report deadline closed June 1, and despite these circumstances, we saw our highest number of submissions to date! Late abstracts were due on July 17. We will be presenting standalone and complementary online offerings to ensure seamless delivery of critical education in formats that cater easily to our newly formed habits.
Thanks to our dedicated Scientific Program Committee Chair, Dr. Victor Test, and staff, we had already begun preparing for virtual CHEST Annual Meeting 2020. Here’s what you can expect:
• A memorable experience
• A highly interactive education program that includes audience Q&A, discussion threads, and audience response systems
• Opportunities for one-on-one discussions, networking, and access to faculty
• Industry-sponsored programs and a virtual exhibit hall
• Access to hundreds of narrated poster presentations, case reports, and research abstracts
• Competitive educational gaming where attendees can participate, win, or watch
• Dedicated COVID-19 update sessions
• CME and MOC credits
If you have already registered for CHEST 2020, you will have the option to transfer your registration to this new model. Our main focus is delivering the virtual program with the highest level of service that you have come to expect from CHEST and respect for our member’s time and current situation. I know Dr. Victor Test and the program committee will deliver a superb educational experience in a virtual meeting setting. Thank you for your support and understanding as we continue to evolve our events to meet the needs of our members while adapting to the best delivery methods.
Since so many fellows were unable to hold their live graduation events, and celebrations, we decided to send them off with a virtual event! On June 30 we held a Joint CHEST/ATS Respiratory Community Graduation Ceremony–for graduating fellows, and to welcome new fellows to our profession. The ceremony consisted of a combination of live and recorded messages from key leaders from both organizations. In addition, there was a keynote address from Dr. Rana Awdish, a critical care physician at Henry Ford Hospital in Detroit, who authored the bestselling book “In Shock: My Journey from Death to Recovery and the Redemptive Power of Hope.” I encourage you to watch the video on the Early Career Professionals page on our Chestnet.org website.
The National Association for Medical Direction of Respiratory Care (NAMDRC) merger with CHEST was finalized at the end of May. Look for more advocacy-related actions coming from CHEST. The newly formed Health Policy and Advocacy Committee is helping to set CHEST’s advocacy agendas in the legislative and regulatory arenas, engaging with policymakers and educating CHEST members on governmental affairs relevant to CHEST’s mission. Did you see the inaugural CHEST published, on-line issue of Washington Watchline, a newsletter that aims to keep CHEST members informed about governmental activities that affect physicians who provide clinical care in respiratory, critical care, and sleep medicine? Follow Washington Watchline to learn more about CHEST’s advocacy around regulatory, legislative, and payment issues that relate to the delivery of health care in support of CHEST’s mission. One of the features was Telemedicine, which many of us are now using and is likely to be a part of many of our practices going forward.
With new COVID-19 surges throughout many parts of the United States, CHEST has continued our volunteer matching program for areas of need, including to the Navaho Nations, where CHEST matched 20 volunteers and has had more than a half-dozen inquiries from our members. In addition, in conjunction with the Foundation, CHEST has partnered with American Mask Rally and started a campaign to distribute masks to frontline essential workers in underserved communities. CHEST received a generous donation from AstraZeneca and Glaxo Smith Kline to help in the global fight against COVID-19 to provide current and accurate information and education to frontline clinicians to allow them to provide the best patient outcomes. CHEST also partnered with the American Thoracic Society to launch a joint PSA/ media campaign entitled For My Lung Health Campaign, to provide credible resources for underserved Black and Latino communities, as these communities are disproportionally affected by COVID-19. At the time of this writing, over a million people have seen the related video, featuring tips for taking control of one’s health in these difficult and uncertain times.
So, in closing, thank you all for what you do in these challenging times. 2020 will certainly be a year to remember! Stay safe and stay well!
Stephanie
Dear Colleagues,
We are now near 6 months into living with COVID-19. In Texas, we are experiencing the surge that much of the Northeast saw in March and April. The COVID-19 Task Force led by Dr. Steve Simpson (CHEST President-Elect) and with representation from the Critical Care, Chest Infections, and Disaster Response and Global Health NetWorks continues to meet regularly to keep our members updated on the latest research and rapidly changing clinical management of COVID-19 illness and the sequelae. COVID-19 has put our medical profession and our subspecialty under considerable stress, and CHEST has launched a new longitudinal Wellness Center led by Dr. Alex Niven, from Mayo Clinic, Rochester. These new resources will feature a wellness webinar series focused on mental health and wellness for clinicians during COVID-19 and beyond. CHEST received overwhelming positive feedback from members and attendees to the Women & Pulmonary Virtual Happy Hour that focused on sharing stories and building community. Many leaders have suggested other such topics and efforts that may be useful to the CHEST community. The CHEST Wellness Center will launch on July 15.
In addition to COVID-19 activities, our nation and the world have compelled a new powerful look at race relations, disparities, and diversity. I represented CHEST at a “White Coats for Black Lives” event in San Antonio. Following our nation’s call for racial equality, CHEST released a Statement of Equity that received overwhelmingly positive feedback and response from members via email and on social media. This statement clearly resonated with the CHEST community. We are asking our leadership and members to consider ways in which CHEST might continue to raise awareness and continue with efforts related to diversity and equity. CHEST also hosted an excellent webinar moderated by Dr. Demondes Haynes and Dr. Nneka Sederstrom in late June that offered a direct and meaningful dialogue on issues facing clinicians and patients of color, and the responsibility of those in leadership positions. CHEST leadership stand firm that racism and inequality are public health issues and are working to define how we further our efforts in this arena.
On June 17, CHEST held a 1-day Virtual CHEST Congress in conjunction with our the CHEST Italian Delegation, as COVID-19 prevented us from safely holding the live Congress in Bologna. We had 3,250 registered attendees. I was so impressed at what a virtual platform can deliver, complete with great educational sessions, including much on COVID-19, as well as capturing the CHEST experience with games, bocce, jeopardy etc! This gave CHEST an opportunity to explore further virtual-based education to reach our wider global audience. CHEST will still be holding an in-person Congress in Bologna, June 24-26, 2021.
CHEST will host three entirely virtual Board Review Courses this August in the areas of Pulmonary, Critical Care, and Pediatric Pulmonary Medicine. These courses will include a combination of pre-recorded lectures and live, interactive sessions. Audience response systems and SEEK questions will still be utilized. There’s still time to register, so don’t miss it! With time being a major commodity at present, all attendees will receive year-long access to all material!
I know you have been wondering about CHEST 2020, and as you have heard by now, CHEST 2020 in Chicago will be a virtual meeting. I am sure that this announcement came as no big surprise, but is certainly disappointing. As you can imagine this was a difficult decision, but one that was necessary based upon our new reality. It was compounded by limitations on the convention center venue under the Illinois reopening plan, and the fact that a large number of our faculty, as well as our attendees, are under a travel ban for the remainder of 2020 that will not allow them to travel to Chicago. The abstract and case report deadline closed June 1, and despite these circumstances, we saw our highest number of submissions to date! Late abstracts were due on July 17. We will be presenting standalone and complementary online offerings to ensure seamless delivery of critical education in formats that cater easily to our newly formed habits.
Thanks to our dedicated Scientific Program Committee Chair, Dr. Victor Test, and staff, we had already begun preparing for virtual CHEST Annual Meeting 2020. Here’s what you can expect:
• A memorable experience
• A highly interactive education program that includes audience Q&A, discussion threads, and audience response systems
• Opportunities for one-on-one discussions, networking, and access to faculty
• Industry-sponsored programs and a virtual exhibit hall
• Access to hundreds of narrated poster presentations, case reports, and research abstracts
• Competitive educational gaming where attendees can participate, win, or watch
• Dedicated COVID-19 update sessions
• CME and MOC credits
If you have already registered for CHEST 2020, you will have the option to transfer your registration to this new model. Our main focus is delivering the virtual program with the highest level of service that you have come to expect from CHEST and respect for our member’s time and current situation. I know Dr. Victor Test and the program committee will deliver a superb educational experience in a virtual meeting setting. Thank you for your support and understanding as we continue to evolve our events to meet the needs of our members while adapting to the best delivery methods.
Since so many fellows were unable to hold their live graduation events, and celebrations, we decided to send them off with a virtual event! On June 30 we held a Joint CHEST/ATS Respiratory Community Graduation Ceremony–for graduating fellows, and to welcome new fellows to our profession. The ceremony consisted of a combination of live and recorded messages from key leaders from both organizations. In addition, there was a keynote address from Dr. Rana Awdish, a critical care physician at Henry Ford Hospital in Detroit, who authored the bestselling book “In Shock: My Journey from Death to Recovery and the Redemptive Power of Hope.” I encourage you to watch the video on the Early Career Professionals page on our Chestnet.org website.
The National Association for Medical Direction of Respiratory Care (NAMDRC) merger with CHEST was finalized at the end of May. Look for more advocacy-related actions coming from CHEST. The newly formed Health Policy and Advocacy Committee is helping to set CHEST’s advocacy agendas in the legislative and regulatory arenas, engaging with policymakers and educating CHEST members on governmental affairs relevant to CHEST’s mission. Did you see the inaugural CHEST published, on-line issue of Washington Watchline, a newsletter that aims to keep CHEST members informed about governmental activities that affect physicians who provide clinical care in respiratory, critical care, and sleep medicine? Follow Washington Watchline to learn more about CHEST’s advocacy around regulatory, legislative, and payment issues that relate to the delivery of health care in support of CHEST’s mission. One of the features was Telemedicine, which many of us are now using and is likely to be a part of many of our practices going forward.
With new COVID-19 surges throughout many parts of the United States, CHEST has continued our volunteer matching program for areas of need, including to the Navaho Nations, where CHEST matched 20 volunteers and has had more than a half-dozen inquiries from our members. In addition, in conjunction with the Foundation, CHEST has partnered with American Mask Rally and started a campaign to distribute masks to frontline essential workers in underserved communities. CHEST received a generous donation from AstraZeneca and Glaxo Smith Kline to help in the global fight against COVID-19 to provide current and accurate information and education to frontline clinicians to allow them to provide the best patient outcomes. CHEST also partnered with the American Thoracic Society to launch a joint PSA/ media campaign entitled For My Lung Health Campaign, to provide credible resources for underserved Black and Latino communities, as these communities are disproportionally affected by COVID-19. At the time of this writing, over a million people have seen the related video, featuring tips for taking control of one’s health in these difficult and uncertain times.
So, in closing, thank you all for what you do in these challenging times. 2020 will certainly be a year to remember! Stay safe and stay well!
Stephanie
Socioeconomic status key factor in CPAP adherence in older adults
The benefits of continuous positive airway pressure therapy for patients with obstructive sleep apnea are well documented, but it only works if patients can adhere to the therapy.
A large national study of older Medicare patients with obstructive sleep apnea (OSA) has identified lower socioeconomic status and comorbidities as independent risk factors for nonadherence to continuous positive airway pressure (CPAP) therapy.
“[The] present results represent the largest study to date of rates and predictors of CPAP adherence among older adults in the United States. In our national sample of Medicare beneficiaries, adherence rates were generally lower than previously reported in smaller, clinic-based studies,” Emerson M. Wickwire, PhD, of the Sleep Disorders Center and division of pulmonary and critical care medicine at the University of Maryland, Baltimore, and colleagues wrote in Sleep.
Dr. Wickwire and colleagues estimated CPAP machine adherence using a 5% sample of Medicare claims data, identifying 3,229 Medicare beneficiaries with OSA who began CPAP therapy between 2009 and 2011. Individuals in the sample were aged at least 65 years with a new diagnosis of OSA, 88.1% of beneficiaries were white, and 52.3% were male.
The researchers applied objective adherence criteria set by the Centers for Medicare & Medicaid Services, which defines CPAP adherence as a patient using CPAP for at least 4 hours on 70% of nights, or CPAP use for 21 of 30 consecutive days within 90 days after beginning therapy.
Using CPAP machine charges as a measure of who adhered to therapy, they found 1,420 of 3,229 individuals (44%) achieved adherence under these criteria, which included making 13 monthly payments during their CPAP machine’s “rent-to-own” period. Partial adherence was found in 997 individuals (30.9%) who made between 4-12 payments on their CPAP machine, while 812 individuals (25.2%) made 4 payments or fewer on their CPAP machines, which the researchers classified as nonadherence. Nonadherers tended to be slightly younger (mean, 72.5 years vs. 79.2 years; P < .001) and had a higher number of comorbidities (35.2% vs. 30.4%; P = .002), compared with individuals with high adherence. Anxiety (odds ratio, 1.34; 95% confidence interval, 1.12-1.61), anemia (OR, 1.16; 95% CI, 1.02-1.32), fibromyalgia (OR, 1.19; 95% CI, 1.03-1.38), traumatic brain injury (OR, 1.58; 95% CI, 1.21-2.07), and Medicaid eligibility (OR, 1.48; 95% CI, 1.24-1.75) were all independently associated with lower CPAP adherence. Medicaid eligibility was considered an indicator of lower socioeconomic status.
Krishna M. Sundar, MD, FCCP, director at the Sleep-Wake Center in the University of Utah pulmonary division in Salt Lake City and CHEST Physician editorial board member, said in an interview that studies have shown early signs of adherence within the first few weeks are an important indicator of overall adherence to CPAP therapy. However, the use of CPAP machine payments in the study by Dr. Wickwire and colleagues was a novel way to track adherence.
Some of the issues with nonadherence may be related to challenges in using the technology, but it is the clinician’s role to communicate with patients about the effectiveness of CPAP and identifying reasons for nonadherence while also attempting to tease out the subtle socioeconomic factors related to nonadherence, Dr. Sundar noted. “We need to alter our practice to make sure that we communicate with these patients and better understand what are the social factors in getting the CPAP or utilizing CPAP, and also following these patients more closely, especially in the first month of starting CPAP therapy.
“Just because somebody has severe sleep apnea and other comorbid conditions does not mean that they’re going to wear the CPAP,” he said. “So, the fact that socioeconomic factors play an equal if not more important role in terms of predicting CPAP adherence. That is an important takeaway.”
Octavian C. Ioachimescu, MD, FCCP, of Emory University, Atlanta, and the Atlanta Veteran Affairs Administration and CHEST Physician editorial board member, said in an interview that the study raises a major question of what is next. “What can we offer to these patients, and what is the real-world compliance to that ‘next-best’ modality?” Dr. Ioachimescu said. “What are the outcomes of these individuals in the point-of-care environment, or ‘real world?’ ”
The analysis by the authors adds the perspective of a “real-world depiction of clinical care for patients with OSA,” Dr. Ioachimescu said. “One major lesson of such an analysis is that the health care goal setting that is referential to initial, randomized, well-controlled studies on highly selected patient populations need to be reassessed periodically from the point of view of actual results in the clinics.”
Clinicians may need to borrow ideas from other therapeutic fields to help improve patient adherence, he said. “[W]e may be able to develop and implement in the future peer involvement, behavioral and cognitive approaches, motivational enhancement interventions, as well as elements of acceptance and commitment techniques, all in the larger context of more integrated and in the same time individualized approaches to therapy.”
The investigators concluded that, “relative to Medicare-only beneficiaries, those eligible for both Medicare and Medicaid were significantly less likely to adhere to CPAP. Future research should seek to develop a deeper understanding of the mechanisms through which [socioeconomic status] and other social determinants impact patient experience throughout the OSA diagnostic and treatment process, including receiving, acclimating, and adhering to CPAP therapy.”
Dr. Sundar concurred with this assessment and said more research is needed on factors impacting adherence such as poverty, homelessness, and home support systems. “It’s not just coordinating with the patient. Clearly, more work is needed in understanding the social aspects of CPAP adherence.”
This study was funded in part by an investigator-initiated grant provided by ResMed to Dr. Wickmire’s institution, the University of Maryland, Baltimore. Dr. Wickmire reported being a scientific consultant to DayZz, Eisai, Merck, and Purdue and holds shares in WellTap. Dr. Oldstone is a ResMed employee and shareholder. Dr. Sundar reported being a cofounder of Hypnoscure, which creates software for population management of sleep apnea, and an investigator in trials where ResMed and Respironics devices were used. Dr. Ioachimescu reported no relevant financial disclosures.
SOURCE: Wickwire EM et al. Sleep. 2020 Jun 23. doi: 10.1093/sleep/zsaa122.
The benefits of continuous positive airway pressure therapy for patients with obstructive sleep apnea are well documented, but it only works if patients can adhere to the therapy.
A large national study of older Medicare patients with obstructive sleep apnea (OSA) has identified lower socioeconomic status and comorbidities as independent risk factors for nonadherence to continuous positive airway pressure (CPAP) therapy.
“[The] present results represent the largest study to date of rates and predictors of CPAP adherence among older adults in the United States. In our national sample of Medicare beneficiaries, adherence rates were generally lower than previously reported in smaller, clinic-based studies,” Emerson M. Wickwire, PhD, of the Sleep Disorders Center and division of pulmonary and critical care medicine at the University of Maryland, Baltimore, and colleagues wrote in Sleep.
Dr. Wickwire and colleagues estimated CPAP machine adherence using a 5% sample of Medicare claims data, identifying 3,229 Medicare beneficiaries with OSA who began CPAP therapy between 2009 and 2011. Individuals in the sample were aged at least 65 years with a new diagnosis of OSA, 88.1% of beneficiaries were white, and 52.3% were male.
The researchers applied objective adherence criteria set by the Centers for Medicare & Medicaid Services, which defines CPAP adherence as a patient using CPAP for at least 4 hours on 70% of nights, or CPAP use for 21 of 30 consecutive days within 90 days after beginning therapy.
Using CPAP machine charges as a measure of who adhered to therapy, they found 1,420 of 3,229 individuals (44%) achieved adherence under these criteria, which included making 13 monthly payments during their CPAP machine’s “rent-to-own” period. Partial adherence was found in 997 individuals (30.9%) who made between 4-12 payments on their CPAP machine, while 812 individuals (25.2%) made 4 payments or fewer on their CPAP machines, which the researchers classified as nonadherence. Nonadherers tended to be slightly younger (mean, 72.5 years vs. 79.2 years; P < .001) and had a higher number of comorbidities (35.2% vs. 30.4%; P = .002), compared with individuals with high adherence. Anxiety (odds ratio, 1.34; 95% confidence interval, 1.12-1.61), anemia (OR, 1.16; 95% CI, 1.02-1.32), fibromyalgia (OR, 1.19; 95% CI, 1.03-1.38), traumatic brain injury (OR, 1.58; 95% CI, 1.21-2.07), and Medicaid eligibility (OR, 1.48; 95% CI, 1.24-1.75) were all independently associated with lower CPAP adherence. Medicaid eligibility was considered an indicator of lower socioeconomic status.
Krishna M. Sundar, MD, FCCP, director at the Sleep-Wake Center in the University of Utah pulmonary division in Salt Lake City and CHEST Physician editorial board member, said in an interview that studies have shown early signs of adherence within the first few weeks are an important indicator of overall adherence to CPAP therapy. However, the use of CPAP machine payments in the study by Dr. Wickwire and colleagues was a novel way to track adherence.
Some of the issues with nonadherence may be related to challenges in using the technology, but it is the clinician’s role to communicate with patients about the effectiveness of CPAP and identifying reasons for nonadherence while also attempting to tease out the subtle socioeconomic factors related to nonadherence, Dr. Sundar noted. “We need to alter our practice to make sure that we communicate with these patients and better understand what are the social factors in getting the CPAP or utilizing CPAP, and also following these patients more closely, especially in the first month of starting CPAP therapy.
“Just because somebody has severe sleep apnea and other comorbid conditions does not mean that they’re going to wear the CPAP,” he said. “So, the fact that socioeconomic factors play an equal if not more important role in terms of predicting CPAP adherence. That is an important takeaway.”
Octavian C. Ioachimescu, MD, FCCP, of Emory University, Atlanta, and the Atlanta Veteran Affairs Administration and CHEST Physician editorial board member, said in an interview that the study raises a major question of what is next. “What can we offer to these patients, and what is the real-world compliance to that ‘next-best’ modality?” Dr. Ioachimescu said. “What are the outcomes of these individuals in the point-of-care environment, or ‘real world?’ ”
The analysis by the authors adds the perspective of a “real-world depiction of clinical care for patients with OSA,” Dr. Ioachimescu said. “One major lesson of such an analysis is that the health care goal setting that is referential to initial, randomized, well-controlled studies on highly selected patient populations need to be reassessed periodically from the point of view of actual results in the clinics.”
Clinicians may need to borrow ideas from other therapeutic fields to help improve patient adherence, he said. “[W]e may be able to develop and implement in the future peer involvement, behavioral and cognitive approaches, motivational enhancement interventions, as well as elements of acceptance and commitment techniques, all in the larger context of more integrated and in the same time individualized approaches to therapy.”
The investigators concluded that, “relative to Medicare-only beneficiaries, those eligible for both Medicare and Medicaid were significantly less likely to adhere to CPAP. Future research should seek to develop a deeper understanding of the mechanisms through which [socioeconomic status] and other social determinants impact patient experience throughout the OSA diagnostic and treatment process, including receiving, acclimating, and adhering to CPAP therapy.”
Dr. Sundar concurred with this assessment and said more research is needed on factors impacting adherence such as poverty, homelessness, and home support systems. “It’s not just coordinating with the patient. Clearly, more work is needed in understanding the social aspects of CPAP adherence.”
This study was funded in part by an investigator-initiated grant provided by ResMed to Dr. Wickmire’s institution, the University of Maryland, Baltimore. Dr. Wickmire reported being a scientific consultant to DayZz, Eisai, Merck, and Purdue and holds shares in WellTap. Dr. Oldstone is a ResMed employee and shareholder. Dr. Sundar reported being a cofounder of Hypnoscure, which creates software for population management of sleep apnea, and an investigator in trials where ResMed and Respironics devices were used. Dr. Ioachimescu reported no relevant financial disclosures.
SOURCE: Wickwire EM et al. Sleep. 2020 Jun 23. doi: 10.1093/sleep/zsaa122.
The benefits of continuous positive airway pressure therapy for patients with obstructive sleep apnea are well documented, but it only works if patients can adhere to the therapy.
A large national study of older Medicare patients with obstructive sleep apnea (OSA) has identified lower socioeconomic status and comorbidities as independent risk factors for nonadherence to continuous positive airway pressure (CPAP) therapy.
“[The] present results represent the largest study to date of rates and predictors of CPAP adherence among older adults in the United States. In our national sample of Medicare beneficiaries, adherence rates were generally lower than previously reported in smaller, clinic-based studies,” Emerson M. Wickwire, PhD, of the Sleep Disorders Center and division of pulmonary and critical care medicine at the University of Maryland, Baltimore, and colleagues wrote in Sleep.
Dr. Wickwire and colleagues estimated CPAP machine adherence using a 5% sample of Medicare claims data, identifying 3,229 Medicare beneficiaries with OSA who began CPAP therapy between 2009 and 2011. Individuals in the sample were aged at least 65 years with a new diagnosis of OSA, 88.1% of beneficiaries were white, and 52.3% were male.
The researchers applied objective adherence criteria set by the Centers for Medicare & Medicaid Services, which defines CPAP adherence as a patient using CPAP for at least 4 hours on 70% of nights, or CPAP use for 21 of 30 consecutive days within 90 days after beginning therapy.
Using CPAP machine charges as a measure of who adhered to therapy, they found 1,420 of 3,229 individuals (44%) achieved adherence under these criteria, which included making 13 monthly payments during their CPAP machine’s “rent-to-own” period. Partial adherence was found in 997 individuals (30.9%) who made between 4-12 payments on their CPAP machine, while 812 individuals (25.2%) made 4 payments or fewer on their CPAP machines, which the researchers classified as nonadherence. Nonadherers tended to be slightly younger (mean, 72.5 years vs. 79.2 years; P < .001) and had a higher number of comorbidities (35.2% vs. 30.4%; P = .002), compared with individuals with high adherence. Anxiety (odds ratio, 1.34; 95% confidence interval, 1.12-1.61), anemia (OR, 1.16; 95% CI, 1.02-1.32), fibromyalgia (OR, 1.19; 95% CI, 1.03-1.38), traumatic brain injury (OR, 1.58; 95% CI, 1.21-2.07), and Medicaid eligibility (OR, 1.48; 95% CI, 1.24-1.75) were all independently associated with lower CPAP adherence. Medicaid eligibility was considered an indicator of lower socioeconomic status.
Krishna M. Sundar, MD, FCCP, director at the Sleep-Wake Center in the University of Utah pulmonary division in Salt Lake City and CHEST Physician editorial board member, said in an interview that studies have shown early signs of adherence within the first few weeks are an important indicator of overall adherence to CPAP therapy. However, the use of CPAP machine payments in the study by Dr. Wickwire and colleagues was a novel way to track adherence.
Some of the issues with nonadherence may be related to challenges in using the technology, but it is the clinician’s role to communicate with patients about the effectiveness of CPAP and identifying reasons for nonadherence while also attempting to tease out the subtle socioeconomic factors related to nonadherence, Dr. Sundar noted. “We need to alter our practice to make sure that we communicate with these patients and better understand what are the social factors in getting the CPAP or utilizing CPAP, and also following these patients more closely, especially in the first month of starting CPAP therapy.
“Just because somebody has severe sleep apnea and other comorbid conditions does not mean that they’re going to wear the CPAP,” he said. “So, the fact that socioeconomic factors play an equal if not more important role in terms of predicting CPAP adherence. That is an important takeaway.”
Octavian C. Ioachimescu, MD, FCCP, of Emory University, Atlanta, and the Atlanta Veteran Affairs Administration and CHEST Physician editorial board member, said in an interview that the study raises a major question of what is next. “What can we offer to these patients, and what is the real-world compliance to that ‘next-best’ modality?” Dr. Ioachimescu said. “What are the outcomes of these individuals in the point-of-care environment, or ‘real world?’ ”
The analysis by the authors adds the perspective of a “real-world depiction of clinical care for patients with OSA,” Dr. Ioachimescu said. “One major lesson of such an analysis is that the health care goal setting that is referential to initial, randomized, well-controlled studies on highly selected patient populations need to be reassessed periodically from the point of view of actual results in the clinics.”
Clinicians may need to borrow ideas from other therapeutic fields to help improve patient adherence, he said. “[W]e may be able to develop and implement in the future peer involvement, behavioral and cognitive approaches, motivational enhancement interventions, as well as elements of acceptance and commitment techniques, all in the larger context of more integrated and in the same time individualized approaches to therapy.”
The investigators concluded that, “relative to Medicare-only beneficiaries, those eligible for both Medicare and Medicaid were significantly less likely to adhere to CPAP. Future research should seek to develop a deeper understanding of the mechanisms through which [socioeconomic status] and other social determinants impact patient experience throughout the OSA diagnostic and treatment process, including receiving, acclimating, and adhering to CPAP therapy.”
Dr. Sundar concurred with this assessment and said more research is needed on factors impacting adherence such as poverty, homelessness, and home support systems. “It’s not just coordinating with the patient. Clearly, more work is needed in understanding the social aspects of CPAP adherence.”
This study was funded in part by an investigator-initiated grant provided by ResMed to Dr. Wickmire’s institution, the University of Maryland, Baltimore. Dr. Wickmire reported being a scientific consultant to DayZz, Eisai, Merck, and Purdue and holds shares in WellTap. Dr. Oldstone is a ResMed employee and shareholder. Dr. Sundar reported being a cofounder of Hypnoscure, which creates software for population management of sleep apnea, and an investigator in trials where ResMed and Respironics devices were used. Dr. Ioachimescu reported no relevant financial disclosures.
SOURCE: Wickwire EM et al. Sleep. 2020 Jun 23. doi: 10.1093/sleep/zsaa122.
FROM SLEEP
A guide to managing disorders of the ear pinna and canal
Which antibiotics are most useful for infection following ear piercing? When is it safe to attempt removal of a foreign body from the ear canal, and which cerumenolytic agent may be best for ear wax? This review covers common ailments of the outer ear, which are often readily diagnosed given a patient’s history and thorough physical examination. We also address more complicated matters such as deciding when to refer for treatment of suspected malignant otitis externa, and which lab markers to follow when managing it yourself.
A (very) brief review of ear anatomy
Understanding the unique embryology and intricate anatomy of the external ear informs our understanding of predictable infections, growths, and malformations.
The external ear is composed of the external auditory canal and auricle. The external auditory canal has a lateral (external) cartilaginous portion and a medial (internal) bony portion. The auricular structure is complex and formed by the helix, antihelix (crura; scaphoid fossa), tragus, antitragus, conchae, and lobule. The auricle is composed of elastic cartilage covered by skin. The lobule is composed of skin, adipose tissue, and connective tissue.
Embryologically, the auricle, auditory canal, and middle ear form from ectoderm of the first 2 branchial arches during early gestation. The auricle forms from the fusion of soft-tissue swellings (hillocks). Three hillocks arise from the first branchial arch and 3 from the second branchial arch during the fifth and sixth weeks of gestation. Tissues from the second branchial arch comprise the lobule, antihelix, and caudal helix. The cartilage of the tragus forms from the first branchial arch. The ear canal forms from an epithelial invagination of the first branchial arch that also occurs during the fifth week of gestation.1
Infections
Perichondritis
Inflammation or infection of the connective tissue layer surrounding the auricular cartilage (perichondrium) results in perichondritis. Further extension of infection can lead to an auricular abscess. Both of these conditions can have serious consequences.
What you’ll see. The most common risk factor for perichondritis is the popular practice of cosmetic transcartilaginous piercing.2 Piercing of the helix, scapha, or anti-helix (often referred to as “high” ear piercing) causes localized trauma that can strip the adjacent perichondrium, decrease blood supply, create cartilaginous microfractures, and lead to devascularization. Rates of infection as high as 35% have been reported with high-ear piercing.3
The most common microbes associated with perichondritis and pinna abscess formation are Pseudomonas and Staphylococcus species.2 P
Continue to: How to treat
How to treat. The cornerstone of treatment is early detection and antimicrobial coverage with antipseudomonal antibiotics. Ciprofloxacin is the oral antibiotic of choice because of its ability to penetrate the tissue.4 Other options include clindamycin and third- or fourth-generation cephalosporins. If the wound becomes abscessed, perform (or refer for) early surgical incision and drainage.5 A failure to promptly recognize perichondritis or to mistakenly prescribe non-antipseudomonal antibiotics contributes to increased rates of hospitalization.2 Cosmetic deformity is the most common complication of perichondritis. This may require reconstructive surgery.
Otitis externa
Acute otitis externa (AOE; “swimmer’s ear”) is cellulitis of the skin and subdermis of the external ear canal. It is most prevalent in warm, moist climates and almost always associated with acute bacterial infection, most commonly P aeruginosa or S aureus.6 There is also an increased association with poor water quality (containing higher bacterial loads). Anything breaching the integrity of the ear canal can potentially predispose to the development of AOE. This includes trauma from cleaning, cerumen removal, scratching due to allergic conditions, and placement of hearing-aid devices.6
What you’ll see. Suspect AOE when signs or symptoms of ear canal inflammation have appeared rapidly (generally within 2 days) over the past 3 weeks.7 Findings include otalgia, itching, fullness, tragal tenderness, ear canal edema, erythema with or without otorrhea, lymphadenitis, or cellulitis of the pinna or adjacent skin.7 AOE must be distinguished from other causes of otalgia and otorrhea, including dermatitis and viral infection.
How to treat. Topical therapy is recommended for the initial treatment of uncomplicated AOE, usually given over 7 days. Multiple topical preparations are available, such as ciprofloxacin 0.2%/hydrocortisone 1.0%; neomycin/polymyxin B/hydrocortisone; ofloxacin 0.3%; or acetic acid 2.0%.7 Avoid these agents, though, if you suspect tympanic membrane rupture. Quinolone drops are the only topical antimicrobials approved for middle ear use.7
Systemic antibiotics are not recommended for the initial treatment of AOE. Topical agents deliver a much higher concentration of medication than can be achieved systemically. Consider systemic antibiotics if there is extension outside the ear canal, a concern for necrotizing otitis externa (more on this in a bit), or the patient is immunodeficient.8
Continue to: Patient (or parent) education...
Patient (or parent) education is important to ensure proper medication administration. The patient should lie down with the affected ear facing up. After the canal is filled with drops, the patient should remain in this position for 3 to 5 minutes. Gently massaging the tragus can augment delivery. Patients should keep the ear canal as dry as possible and avoid inserting objects (eg, hearing aids, ear buds, cotton-tipped applicators) into the canal for the duration of treatment. The delivery of topical antibiotics can be enhanced by wick placement. Prescribe analgesics (typically nonsteroidal anti-inflammatory agents) based on severity of pain.7
Have patients abstain from water sports for 7 to 10 days. Showering is acceptable with minimal ear exposure to water; bathing is preferred when possible. If there is no clinical improvement in 48 to 72 hours, ask patients to return for re-evaluation.8 Prevention is essential for patients with a history of recurrent otitis externa. Acetic acid solutions create an acidic environment within the canal to help prevent recurrent AOE. Ear plugs and petroleum jelly–soaked cotton plugs prior to water exposure may also help prevent recurrent AOE.
Malignant otitis externa
Malignant, or necrotizing, otitis externa is an aggressive disease form of otitis externa that is most common in individuals with diabetes or other immunodeficiency disorders.9 Most cases are due to infection with P aeruginosa.10 Prior to the availability of effective antibiotics, mortality rates in patients with necrotizing otitis externa were as high as 50%.11
What you’ll see. Patients typically present with severe ear pain, otorrhea, conductive hearing loss, and a feeling of fullness in the external ear canal. Physical examination reveals purulent otorrhea and a swollen, tender ear canal. Exposed bone may be visible, most often on the floor of the canal. The tympanic membrane and middle ear are seldom involved on initial presentation.
The infection often originates at the junction of the bony and cartilaginous portion of the external canal, spreading through the fissures of Santorini to the skull base. If not aggressively treated, the infection spreads medially to the tympanomastoid suture causing intracranial complications—usually a facial nerve neuropathy.
Continue to: Given these clinical findings...
Given these clinical findings, promptly order laboratory studies and imaging to confirm the diagnosis. The erythrocyte sedimentation rate and C-reactive protein level are typically elevated, and either can be used as a marker to follow treatment. Computed tomography (CT) helps to determine the location and extent of disease and is recommended as the initial diagnostic imaging modality for patients with suspected malignant otitis externa.12
Magnetic resonance imaging helps define soft-tissue changes, dural enhancement, and involvement of medullary bone, making this the preferred modality to monitor therapeutic response.12 Technetium bone scanning can also be used for the initial diagnosis (particularly if CT findings are normal and clinical suspicion is high) and for follow-up with treatment.
How to treat. Management involves a team approach with otolaryngology, radiology, neurology, endocrinology, and infectious disease specialists. Long term (6-8 weeks) antipseudomonal antibiotic treatment is typical.
Let culture results guide the choice of antibiotic. Fluoroquinolone therapy, usually ciprofloxacin, is used most often.12 Surgical intervention may be required for local debridement and drainage of abscesses. Close follow-up is necessary due to reports of recurrence up to 1 year after treatment. If left untreated, necrotizing otitis externa can lead to osteomyelitis, meningitis, septic thrombosis, cerebral abscess, and death.11
Cerumen impaction
The relatively small diameter of the external auditory canal increases the risk for impaction of cerumen and foreign bodies. Cerumen impaction, in particular, is a common primary care complaint. Cerumen forms when glandular secretions from the outer two-thirds of the ear canal mix with exfoliated skin. It functions as a lubricant for the ear canal and as a barrier against infection, water accumulation, and foreign bodies.13
Continue to: What you'll see
What you’ll see. You may encounter cerumen impaction in an asymptomatic patient when it prevents visualization of the external auditory canal or tympanic membrane, or when a patient complains of conductive hearing loss, tinnitus, dizziness, ear pain, itching, and cough.13 It is found in 1 in 10 children and 1 in 20 adults.13 There is a higher incidence in patients who are elderly, are cognitively impaired, or wear hearing devices or ear plugs.13,14 Asymptomatic cerumen impaction should not be treated. A recent clinical guideline provides a useful “do and don’t” list for patient education (TABLE).13
How to treat. In asymptomatic patients, the presence of cerumen on examination is not an indication for removal. Based on current guidelines,13 impacted cerumen can safely be removed from the ear canal of symptomatic patients in several ways:
- Manual removal with cerumen loop/spoon or alligator forceps. This method decreases the risk for infection because it limits moisture exposure. However, it should be performed by a health care provider trained in its use because of the risk for trauma to the ear canal and tympanic membrane.
- Irrigation of the ear using tap water or a 50-50 solution of hydrogen peroxide and water. Irrigation can be achieved with a syringe or jet irrigator using a modified tip. This method also has a risk for trauma to the ear canal and tympanic membrane and should only be performed by appropriately trained health care professionals.
- Use of cerumenolytic agents to soften and thin earwax and promote natural extrusion. Several types of cerumenolytic drops (water-based and oil-based) are available and appear to be equally effective. Water-based solutions contain hydrogen peroxide, docusate sodium, acetic acid, and sodium bicarbonate. Oil-based drops may contain peanut, almond, or olive oils. A thorough allergic history should be performed to avoid using products in patients with nut allergies. In head-to-head laboratory comparisons, distilled water appears to be the best cerumenolytic.15
Foreign bodies
Foreign bodies in the external auditory canal (typically beads, cotton tips, and insects) are more common in children than adults.16
What you’ll see. Most foreign bodies are lodged in the bony part of the external auditory canal, and many patients try to remove the object before seeking medical care. Removal requires adequate visualization and skill.17 Although patients may be asymptomatic, most complain of pain, fullness, decreased hearing, or otorrhea.
How to treat. Directly visible objects can often be removed without referral. Suction, irrigation, forceps, probes, and fine hooks have been used. Insect removal can be facilitated by first flooding the canal with xylocaine, alcohol, or mineral oil. Acetone may be used to dissolve foreign bodies containing Styrofoam or to loosen glues. If the object is a button battery, avoid irrigation to prevent liquefaction tissue necrosis.
Continue to: Complications of foreign body removal...
Complications of foreign body removal include pain, otitis externa, otitis media, and trauma to the ear or tympanic membrane. The likelihood of successful removal of the object decreases and the risk for complications increases with each subsequent attempt.17 Consult an otolaryngologist if sedation or anesthesia is required, the foreign body is tightly wedged, there is trauma to the ear canal or tympanic membrane, the foreign body has a sharp edge (eg, glass or wire), or removal attempts have been unsuccessful.
Trauma
Sports injuries, motor vehicle accidents, bites, falls, and burns are the primary causes of trauma to the external ear.18
What you’ll see. Blunt auricular trauma predisposes to infection, necrosis, and scar contracture. One of the most common sequelae is cauliflower ear. Trauma is particularly common with contact sports such as boxing, wrestling, or mixed martial arts. The skin of the auricle attaches directly to the perichondrium. Following blunt or shearing trauma to the auricle, hematomas form within the space between the perichondrium and cartilage of the anterior ear.19
How to treat. Small hematomas can be managed by aspiration, while larger ones generally require open drainage.20 Newer treatments involving pressure dressings and the use of fibrin glue have been proposed.20 Recommend that athletes participating in contact sports wear appropriate protective headgear to prevent auricular hematoma and cauliflower ear.
Neoplasm
Roughly 5% of all skin cancers involve the ear, most frequently the pinna due to chronic sun exposure.21 The most frequently occurring malignancy of the external ear is basal cell carcinoma (BCC), which is responsible for 80% of all nonmelanoma skin cancers.22
Continue to: What you'll see
What you’ll see. BCC of the ear usually involves the preauricular area and the helix. The risk for BCC is related to exposure to ultraviolet radiation. BCC of the ear is more common in men and can be particularly aggressive, highlighting the importance of prevention and prompt recognition. BCC typically presents as a fleshy papule that is often translucent or “pearly’” and has overlying telangiectasia and a “rolled” border. Central ulceration can occur as well.
How to treat. Usual treatment of BCC is surgical excision. Prevention is critical and centers on sun avoidance or the use of appropriate sunscreens.
In addition to BCC, exposure of the external ear to sunlight and ultraviolet radiation predisposes patients to the development of squamous cell carcinoma (SCC) and melanoma. SCC has a variety of presentations including papules, plaques, and nodules. SCC has a higher metastatic potential than does BCC.
Keloid
Keloids are an abnormal healing response to soft-tissue injury: benign fibrocartilaginous growths that extend beyond the original wound.
What you’ll see. Keloids are more common in dark-skinned individuals and tend to result from burns, surgical incisions, infection, trauma, tattooing, injections, piercings, and arthropod bites. In some cases, they arise spontaneously. Keloids are more common in areas of increased skin tension (chest, shoulders, back), but may occur on the ears—most commonly after piercing or trauma. Keloids present clinically as slow-growing rubbery or firm nodules. The diagnosis is typically based on clinical appearance but can be confirmed by histopathology.
Continue to: How to treat
How to treat. Treatments vary and include observation, excision, intralesional injections, cryotherapy, enzyme therapy, silicone gel application, and irradiation.23 Recurrence is common; no therapy has been proven to be universally superior or preferred.
Congenital malformations
Atresia
Disruption of embryologic development (failed invagination of the external auditory canal) can lead to a stenotic or absent ear canal (aural atresia). Aural atresia is also often associated with fusion of the incus and malleus. This condition occurs predominantly in males. Unilateral atresia is more common than bilateral atresia, and the right ear is more often involved than the left.24
Microtia
Microtia is the incomplete development of the pinna leading to a small or deformed pinna. Microtia can be unilateral or bilateral. As with atresia, microtia more commonly affects males and, if unilateral, the right side is more often affected than the left. Microtia can occur in isolation but is often associated with genetic syndromes such as Treacher Collins syndrome and craniofacial microsomia (Goldenhar syndrome). When microtia is identified (typically at birth or early infancy), audiologic testing and a thorough physical examination for evidence of associated defects should be performed. Consult with an audiologist, clinical geneticist, or pediatric otolaryngologist.
Pre-auricular pits
Pre-auricular pits (sinuses) are tiny indentations anterior to the helix and superior to the tragus. While pre-auricular pits are more common on the right side, they are bilateral in 25% to 50% of cases.25 Pre-auricular pits occur in up to 1% of white children, 5% of black children, and 10% of Asian children.25 Children with this condition should undergo formal audiologic testing as their risk for hearing loss is higher compared with the general population.26
The branchio-oto-renal syndrome (associated with pre-auricular pits and hearing loss) also features structural defects of the ear, renal anomalies and/or nasolacrimal duct stenosis or fistulas. If this syndrome is suspected, renal ultrasound imaging is warranted. Other indications for renal ultrasound in patients with a pre-auricular pit are any dysmorphic feature, a family history of deafness, an auricular malformation, or a maternal history of gestational diabetes.27 Pre-auricular pits do not require surgery unless they drain chronically or become recurrently infected. Complete surgical excision is the treatment of choice in these cases.
CORRESPONDENCE
Mark Stephens, MD, 1850 Park Avenue, State College, PA 16801; mstephens3@pennstatehealth.psu.edu
1. Cox TC, Camci ED, Vora S, et al. The genetics of auricular development and malformation: new findings in model systems driving future directions for microtia research. Eur J Med Genet. 2014;57:394-401.
2. Sosin M, Weissler JM, Pulcrano M, et al. Transcartilaginous ear piercing and infectious complications: a systematic review and critical analysis of outcomes. Laryngoscope. 2015;125:1827-1834.
3. Stirn A. Body piercing: medical consequences and psychological motivations. Lancet. 2003;361:1205-1215.
4. Liu ZW, Chokkalingam P. Piercing associated perichondritis of the pinna: are we treating it correctly? J Larygol Oncol. 2013;127:505-508.
5. Mitchell S, Ditta K, Minhas S, et al. Pinna abscesses: can we manage them better? A case series and review of the literature. Eur Arch Otorhinolaryngol. 2015;272:3163-3167.
6. Stone KE. Otitis externa. Pediatr Rev. 2007;28:77-78.
7. Rosenfeld RM, Schwartz SR, Cannon CR, et al. Clinical practice guideline: acute otitis externa. Otolaryngol Head Neck Surg. 2014;150(1 suppl):S1-S24.
8. Prentice P. American Academy of Otolaryngology: Head and Neck Surgery Foundation clinical practice guideline on acute otitis externa. Arch Dis Child Educ Pract Ed. 2015;100:197.
9. Unadkat S, Kanzara T, Watters G. Necrotising otitis externa in the immunocompetent patient. J Laryngol Otol. 2018;132:71-74.
10. Carfrae MJ, Kesser BW. Malignant otitis externa. Otolarngol Clin N Am. 2008;41:537-549.
11. Chandler JR, Malignant otitis externa. Laryngoscope. 1968;78:1257-1294.
12. Hollis S, Evans K. Management of malignant (necrotising) otitis externa. J Laryngol Otol. 2011;125:1212-1217.
13. Schwartz SR, Magit AE, Rosenfeld RM, et al. Clinical practice guideline (update): earwax (cerumen impaction). Otolaryngol Head Neck Surg. 2017;156:S1-S29.
14. Guest JF, Greener MJ, Robinson AC, et al. Impacted cerumen: composition, production, epidemiology and management. QJM. 2004;97:477-488.
15. Saxby C, Williams R, Hickey S. Finding the most effective cerumenolytic. J Laryngol Otol. 2013;127:1067-1070.
16. Awad AH, ElTaher M. ENT foreign bodies: an experience. Int Arch Otorhinolaryngol. 2018;22:146-151.
17. Heim SW, Maughan KL. Foreign bodies in the ear, nose, and throat. Am Fam Physician. 2007;76:1185-1189.
18. Sharma K, Goswami SC, Baruah DK. Auricular trauma and its management. Indian J Otolaryngol Head Neck Surg. 2006;58:232-234.
19. Haik J, Givol O, Kornhaber R, et al. Cauliflower ear–a minimally invasive treatment in a wrestling athlete: a case report. Int Med Case Rep J. 2018;11:5-7.
20. Ebrahimi A, Kazemi A, Rasouli HR, et al. Reconstructive surgery of auricular defects: an overview. Trauma Mon. 2015;20:e28202.
21. Warner E, Weston C, Barclay-Klingle N, et al. The swollen pinna. BMJ. 2017; 359; j5073.
22. Rubin AI, Chen EH, Ratner D. Basal cell carcinoma. N Engl J Med. 2005;353:2262-2269.
23. Ranjan SK, Ahmed A, Harsh V, et al. Giant bilateral keloids of the ear lobule: case report and brief review of the literature. J Family Med Prim Care. 2017;6:677-679.
24. Roland PS, Marple BF. Disorders of the external auditory canal. J Am Acad Audiol. 1997;8:367-378.
25. Scheinfeld NS, Silverberg NB, Weinberg JM, et al. The preauricular sinus: a review of its clinical presentation, treatment, and associations. Pediatr Dermatol. 2004;21:191-196.
26. Roth DA, Hildesheimer M, Bardestein S, et al. Preauricular skin tags and ear pits are associated with permanent hearing impairment in newborns. Pediatrics. 2008;122:e884-890.
27. Tan T, Constantinides H, Mitchell TE. The preauricular sinus: a review of its aetiology, clinical presentation and management. Int J Ped Otorhinolaryngol. 2005;69:1469-1474.
Which antibiotics are most useful for infection following ear piercing? When is it safe to attempt removal of a foreign body from the ear canal, and which cerumenolytic agent may be best for ear wax? This review covers common ailments of the outer ear, which are often readily diagnosed given a patient’s history and thorough physical examination. We also address more complicated matters such as deciding when to refer for treatment of suspected malignant otitis externa, and which lab markers to follow when managing it yourself.
A (very) brief review of ear anatomy
Understanding the unique embryology and intricate anatomy of the external ear informs our understanding of predictable infections, growths, and malformations.
The external ear is composed of the external auditory canal and auricle. The external auditory canal has a lateral (external) cartilaginous portion and a medial (internal) bony portion. The auricular structure is complex and formed by the helix, antihelix (crura; scaphoid fossa), tragus, antitragus, conchae, and lobule. The auricle is composed of elastic cartilage covered by skin. The lobule is composed of skin, adipose tissue, and connective tissue.
Embryologically, the auricle, auditory canal, and middle ear form from ectoderm of the first 2 branchial arches during early gestation. The auricle forms from the fusion of soft-tissue swellings (hillocks). Three hillocks arise from the first branchial arch and 3 from the second branchial arch during the fifth and sixth weeks of gestation. Tissues from the second branchial arch comprise the lobule, antihelix, and caudal helix. The cartilage of the tragus forms from the first branchial arch. The ear canal forms from an epithelial invagination of the first branchial arch that also occurs during the fifth week of gestation.1
Infections
Perichondritis
Inflammation or infection of the connective tissue layer surrounding the auricular cartilage (perichondrium) results in perichondritis. Further extension of infection can lead to an auricular abscess. Both of these conditions can have serious consequences.
What you’ll see. The most common risk factor for perichondritis is the popular practice of cosmetic transcartilaginous piercing.2 Piercing of the helix, scapha, or anti-helix (often referred to as “high” ear piercing) causes localized trauma that can strip the adjacent perichondrium, decrease blood supply, create cartilaginous microfractures, and lead to devascularization. Rates of infection as high as 35% have been reported with high-ear piercing.3
The most common microbes associated with perichondritis and pinna abscess formation are Pseudomonas and Staphylococcus species.2 P
Continue to: How to treat
How to treat. The cornerstone of treatment is early detection and antimicrobial coverage with antipseudomonal antibiotics. Ciprofloxacin is the oral antibiotic of choice because of its ability to penetrate the tissue.4 Other options include clindamycin and third- or fourth-generation cephalosporins. If the wound becomes abscessed, perform (or refer for) early surgical incision and drainage.5 A failure to promptly recognize perichondritis or to mistakenly prescribe non-antipseudomonal antibiotics contributes to increased rates of hospitalization.2 Cosmetic deformity is the most common complication of perichondritis. This may require reconstructive surgery.
Otitis externa
Acute otitis externa (AOE; “swimmer’s ear”) is cellulitis of the skin and subdermis of the external ear canal. It is most prevalent in warm, moist climates and almost always associated with acute bacterial infection, most commonly P aeruginosa or S aureus.6 There is also an increased association with poor water quality (containing higher bacterial loads). Anything breaching the integrity of the ear canal can potentially predispose to the development of AOE. This includes trauma from cleaning, cerumen removal, scratching due to allergic conditions, and placement of hearing-aid devices.6
What you’ll see. Suspect AOE when signs or symptoms of ear canal inflammation have appeared rapidly (generally within 2 days) over the past 3 weeks.7 Findings include otalgia, itching, fullness, tragal tenderness, ear canal edema, erythema with or without otorrhea, lymphadenitis, or cellulitis of the pinna or adjacent skin.7 AOE must be distinguished from other causes of otalgia and otorrhea, including dermatitis and viral infection.
How to treat. Topical therapy is recommended for the initial treatment of uncomplicated AOE, usually given over 7 days. Multiple topical preparations are available, such as ciprofloxacin 0.2%/hydrocortisone 1.0%; neomycin/polymyxin B/hydrocortisone; ofloxacin 0.3%; or acetic acid 2.0%.7 Avoid these agents, though, if you suspect tympanic membrane rupture. Quinolone drops are the only topical antimicrobials approved for middle ear use.7
Systemic antibiotics are not recommended for the initial treatment of AOE. Topical agents deliver a much higher concentration of medication than can be achieved systemically. Consider systemic antibiotics if there is extension outside the ear canal, a concern for necrotizing otitis externa (more on this in a bit), or the patient is immunodeficient.8
Continue to: Patient (or parent) education...
Patient (or parent) education is important to ensure proper medication administration. The patient should lie down with the affected ear facing up. After the canal is filled with drops, the patient should remain in this position for 3 to 5 minutes. Gently massaging the tragus can augment delivery. Patients should keep the ear canal as dry as possible and avoid inserting objects (eg, hearing aids, ear buds, cotton-tipped applicators) into the canal for the duration of treatment. The delivery of topical antibiotics can be enhanced by wick placement. Prescribe analgesics (typically nonsteroidal anti-inflammatory agents) based on severity of pain.7
Have patients abstain from water sports for 7 to 10 days. Showering is acceptable with minimal ear exposure to water; bathing is preferred when possible. If there is no clinical improvement in 48 to 72 hours, ask patients to return for re-evaluation.8 Prevention is essential for patients with a history of recurrent otitis externa. Acetic acid solutions create an acidic environment within the canal to help prevent recurrent AOE. Ear plugs and petroleum jelly–soaked cotton plugs prior to water exposure may also help prevent recurrent AOE.
Malignant otitis externa
Malignant, or necrotizing, otitis externa is an aggressive disease form of otitis externa that is most common in individuals with diabetes or other immunodeficiency disorders.9 Most cases are due to infection with P aeruginosa.10 Prior to the availability of effective antibiotics, mortality rates in patients with necrotizing otitis externa were as high as 50%.11
What you’ll see. Patients typically present with severe ear pain, otorrhea, conductive hearing loss, and a feeling of fullness in the external ear canal. Physical examination reveals purulent otorrhea and a swollen, tender ear canal. Exposed bone may be visible, most often on the floor of the canal. The tympanic membrane and middle ear are seldom involved on initial presentation.
The infection often originates at the junction of the bony and cartilaginous portion of the external canal, spreading through the fissures of Santorini to the skull base. If not aggressively treated, the infection spreads medially to the tympanomastoid suture causing intracranial complications—usually a facial nerve neuropathy.
Continue to: Given these clinical findings...
Given these clinical findings, promptly order laboratory studies and imaging to confirm the diagnosis. The erythrocyte sedimentation rate and C-reactive protein level are typically elevated, and either can be used as a marker to follow treatment. Computed tomography (CT) helps to determine the location and extent of disease and is recommended as the initial diagnostic imaging modality for patients with suspected malignant otitis externa.12
Magnetic resonance imaging helps define soft-tissue changes, dural enhancement, and involvement of medullary bone, making this the preferred modality to monitor therapeutic response.12 Technetium bone scanning can also be used for the initial diagnosis (particularly if CT findings are normal and clinical suspicion is high) and for follow-up with treatment.
How to treat. Management involves a team approach with otolaryngology, radiology, neurology, endocrinology, and infectious disease specialists. Long term (6-8 weeks) antipseudomonal antibiotic treatment is typical.
Let culture results guide the choice of antibiotic. Fluoroquinolone therapy, usually ciprofloxacin, is used most often.12 Surgical intervention may be required for local debridement and drainage of abscesses. Close follow-up is necessary due to reports of recurrence up to 1 year after treatment. If left untreated, necrotizing otitis externa can lead to osteomyelitis, meningitis, septic thrombosis, cerebral abscess, and death.11
Cerumen impaction
The relatively small diameter of the external auditory canal increases the risk for impaction of cerumen and foreign bodies. Cerumen impaction, in particular, is a common primary care complaint. Cerumen forms when glandular secretions from the outer two-thirds of the ear canal mix with exfoliated skin. It functions as a lubricant for the ear canal and as a barrier against infection, water accumulation, and foreign bodies.13
Continue to: What you'll see
What you’ll see. You may encounter cerumen impaction in an asymptomatic patient when it prevents visualization of the external auditory canal or tympanic membrane, or when a patient complains of conductive hearing loss, tinnitus, dizziness, ear pain, itching, and cough.13 It is found in 1 in 10 children and 1 in 20 adults.13 There is a higher incidence in patients who are elderly, are cognitively impaired, or wear hearing devices or ear plugs.13,14 Asymptomatic cerumen impaction should not be treated. A recent clinical guideline provides a useful “do and don’t” list for patient education (TABLE).13
How to treat. In asymptomatic patients, the presence of cerumen on examination is not an indication for removal. Based on current guidelines,13 impacted cerumen can safely be removed from the ear canal of symptomatic patients in several ways:
- Manual removal with cerumen loop/spoon or alligator forceps. This method decreases the risk for infection because it limits moisture exposure. However, it should be performed by a health care provider trained in its use because of the risk for trauma to the ear canal and tympanic membrane.
- Irrigation of the ear using tap water or a 50-50 solution of hydrogen peroxide and water. Irrigation can be achieved with a syringe or jet irrigator using a modified tip. This method also has a risk for trauma to the ear canal and tympanic membrane and should only be performed by appropriately trained health care professionals.
- Use of cerumenolytic agents to soften and thin earwax and promote natural extrusion. Several types of cerumenolytic drops (water-based and oil-based) are available and appear to be equally effective. Water-based solutions contain hydrogen peroxide, docusate sodium, acetic acid, and sodium bicarbonate. Oil-based drops may contain peanut, almond, or olive oils. A thorough allergic history should be performed to avoid using products in patients with nut allergies. In head-to-head laboratory comparisons, distilled water appears to be the best cerumenolytic.15
Foreign bodies
Foreign bodies in the external auditory canal (typically beads, cotton tips, and insects) are more common in children than adults.16
What you’ll see. Most foreign bodies are lodged in the bony part of the external auditory canal, and many patients try to remove the object before seeking medical care. Removal requires adequate visualization and skill.17 Although patients may be asymptomatic, most complain of pain, fullness, decreased hearing, or otorrhea.
How to treat. Directly visible objects can often be removed without referral. Suction, irrigation, forceps, probes, and fine hooks have been used. Insect removal can be facilitated by first flooding the canal with xylocaine, alcohol, or mineral oil. Acetone may be used to dissolve foreign bodies containing Styrofoam or to loosen glues. If the object is a button battery, avoid irrigation to prevent liquefaction tissue necrosis.
Continue to: Complications of foreign body removal...
Complications of foreign body removal include pain, otitis externa, otitis media, and trauma to the ear or tympanic membrane. The likelihood of successful removal of the object decreases and the risk for complications increases with each subsequent attempt.17 Consult an otolaryngologist if sedation or anesthesia is required, the foreign body is tightly wedged, there is trauma to the ear canal or tympanic membrane, the foreign body has a sharp edge (eg, glass or wire), or removal attempts have been unsuccessful.
Trauma
Sports injuries, motor vehicle accidents, bites, falls, and burns are the primary causes of trauma to the external ear.18
What you’ll see. Blunt auricular trauma predisposes to infection, necrosis, and scar contracture. One of the most common sequelae is cauliflower ear. Trauma is particularly common with contact sports such as boxing, wrestling, or mixed martial arts. The skin of the auricle attaches directly to the perichondrium. Following blunt or shearing trauma to the auricle, hematomas form within the space between the perichondrium and cartilage of the anterior ear.19
How to treat. Small hematomas can be managed by aspiration, while larger ones generally require open drainage.20 Newer treatments involving pressure dressings and the use of fibrin glue have been proposed.20 Recommend that athletes participating in contact sports wear appropriate protective headgear to prevent auricular hematoma and cauliflower ear.
Neoplasm
Roughly 5% of all skin cancers involve the ear, most frequently the pinna due to chronic sun exposure.21 The most frequently occurring malignancy of the external ear is basal cell carcinoma (BCC), which is responsible for 80% of all nonmelanoma skin cancers.22
Continue to: What you'll see
What you’ll see. BCC of the ear usually involves the preauricular area and the helix. The risk for BCC is related to exposure to ultraviolet radiation. BCC of the ear is more common in men and can be particularly aggressive, highlighting the importance of prevention and prompt recognition. BCC typically presents as a fleshy papule that is often translucent or “pearly’” and has overlying telangiectasia and a “rolled” border. Central ulceration can occur as well.
How to treat. Usual treatment of BCC is surgical excision. Prevention is critical and centers on sun avoidance or the use of appropriate sunscreens.
In addition to BCC, exposure of the external ear to sunlight and ultraviolet radiation predisposes patients to the development of squamous cell carcinoma (SCC) and melanoma. SCC has a variety of presentations including papules, plaques, and nodules. SCC has a higher metastatic potential than does BCC.
Keloid
Keloids are an abnormal healing response to soft-tissue injury: benign fibrocartilaginous growths that extend beyond the original wound.
What you’ll see. Keloids are more common in dark-skinned individuals and tend to result from burns, surgical incisions, infection, trauma, tattooing, injections, piercings, and arthropod bites. In some cases, they arise spontaneously. Keloids are more common in areas of increased skin tension (chest, shoulders, back), but may occur on the ears—most commonly after piercing or trauma. Keloids present clinically as slow-growing rubbery or firm nodules. The diagnosis is typically based on clinical appearance but can be confirmed by histopathology.
Continue to: How to treat
How to treat. Treatments vary and include observation, excision, intralesional injections, cryotherapy, enzyme therapy, silicone gel application, and irradiation.23 Recurrence is common; no therapy has been proven to be universally superior or preferred.
Congenital malformations
Atresia
Disruption of embryologic development (failed invagination of the external auditory canal) can lead to a stenotic or absent ear canal (aural atresia). Aural atresia is also often associated with fusion of the incus and malleus. This condition occurs predominantly in males. Unilateral atresia is more common than bilateral atresia, and the right ear is more often involved than the left.24
Microtia
Microtia is the incomplete development of the pinna leading to a small or deformed pinna. Microtia can be unilateral or bilateral. As with atresia, microtia more commonly affects males and, if unilateral, the right side is more often affected than the left. Microtia can occur in isolation but is often associated with genetic syndromes such as Treacher Collins syndrome and craniofacial microsomia (Goldenhar syndrome). When microtia is identified (typically at birth or early infancy), audiologic testing and a thorough physical examination for evidence of associated defects should be performed. Consult with an audiologist, clinical geneticist, or pediatric otolaryngologist.
Pre-auricular pits
Pre-auricular pits (sinuses) are tiny indentations anterior to the helix and superior to the tragus. While pre-auricular pits are more common on the right side, they are bilateral in 25% to 50% of cases.25 Pre-auricular pits occur in up to 1% of white children, 5% of black children, and 10% of Asian children.25 Children with this condition should undergo formal audiologic testing as their risk for hearing loss is higher compared with the general population.26
The branchio-oto-renal syndrome (associated with pre-auricular pits and hearing loss) also features structural defects of the ear, renal anomalies and/or nasolacrimal duct stenosis or fistulas. If this syndrome is suspected, renal ultrasound imaging is warranted. Other indications for renal ultrasound in patients with a pre-auricular pit are any dysmorphic feature, a family history of deafness, an auricular malformation, or a maternal history of gestational diabetes.27 Pre-auricular pits do not require surgery unless they drain chronically or become recurrently infected. Complete surgical excision is the treatment of choice in these cases.
CORRESPONDENCE
Mark Stephens, MD, 1850 Park Avenue, State College, PA 16801; mstephens3@pennstatehealth.psu.edu
Which antibiotics are most useful for infection following ear piercing? When is it safe to attempt removal of a foreign body from the ear canal, and which cerumenolytic agent may be best for ear wax? This review covers common ailments of the outer ear, which are often readily diagnosed given a patient’s history and thorough physical examination. We also address more complicated matters such as deciding when to refer for treatment of suspected malignant otitis externa, and which lab markers to follow when managing it yourself.
A (very) brief review of ear anatomy
Understanding the unique embryology and intricate anatomy of the external ear informs our understanding of predictable infections, growths, and malformations.
The external ear is composed of the external auditory canal and auricle. The external auditory canal has a lateral (external) cartilaginous portion and a medial (internal) bony portion. The auricular structure is complex and formed by the helix, antihelix (crura; scaphoid fossa), tragus, antitragus, conchae, and lobule. The auricle is composed of elastic cartilage covered by skin. The lobule is composed of skin, adipose tissue, and connective tissue.
Embryologically, the auricle, auditory canal, and middle ear form from ectoderm of the first 2 branchial arches during early gestation. The auricle forms from the fusion of soft-tissue swellings (hillocks). Three hillocks arise from the first branchial arch and 3 from the second branchial arch during the fifth and sixth weeks of gestation. Tissues from the second branchial arch comprise the lobule, antihelix, and caudal helix. The cartilage of the tragus forms from the first branchial arch. The ear canal forms from an epithelial invagination of the first branchial arch that also occurs during the fifth week of gestation.1
Infections
Perichondritis
Inflammation or infection of the connective tissue layer surrounding the auricular cartilage (perichondrium) results in perichondritis. Further extension of infection can lead to an auricular abscess. Both of these conditions can have serious consequences.
What you’ll see. The most common risk factor for perichondritis is the popular practice of cosmetic transcartilaginous piercing.2 Piercing of the helix, scapha, or anti-helix (often referred to as “high” ear piercing) causes localized trauma that can strip the adjacent perichondrium, decrease blood supply, create cartilaginous microfractures, and lead to devascularization. Rates of infection as high as 35% have been reported with high-ear piercing.3
The most common microbes associated with perichondritis and pinna abscess formation are Pseudomonas and Staphylococcus species.2 P
Continue to: How to treat
How to treat. The cornerstone of treatment is early detection and antimicrobial coverage with antipseudomonal antibiotics. Ciprofloxacin is the oral antibiotic of choice because of its ability to penetrate the tissue.4 Other options include clindamycin and third- or fourth-generation cephalosporins. If the wound becomes abscessed, perform (or refer for) early surgical incision and drainage.5 A failure to promptly recognize perichondritis or to mistakenly prescribe non-antipseudomonal antibiotics contributes to increased rates of hospitalization.2 Cosmetic deformity is the most common complication of perichondritis. This may require reconstructive surgery.
Otitis externa
Acute otitis externa (AOE; “swimmer’s ear”) is cellulitis of the skin and subdermis of the external ear canal. It is most prevalent in warm, moist climates and almost always associated with acute bacterial infection, most commonly P aeruginosa or S aureus.6 There is also an increased association with poor water quality (containing higher bacterial loads). Anything breaching the integrity of the ear canal can potentially predispose to the development of AOE. This includes trauma from cleaning, cerumen removal, scratching due to allergic conditions, and placement of hearing-aid devices.6
What you’ll see. Suspect AOE when signs or symptoms of ear canal inflammation have appeared rapidly (generally within 2 days) over the past 3 weeks.7 Findings include otalgia, itching, fullness, tragal tenderness, ear canal edema, erythema with or without otorrhea, lymphadenitis, or cellulitis of the pinna or adjacent skin.7 AOE must be distinguished from other causes of otalgia and otorrhea, including dermatitis and viral infection.
How to treat. Topical therapy is recommended for the initial treatment of uncomplicated AOE, usually given over 7 days. Multiple topical preparations are available, such as ciprofloxacin 0.2%/hydrocortisone 1.0%; neomycin/polymyxin B/hydrocortisone; ofloxacin 0.3%; or acetic acid 2.0%.7 Avoid these agents, though, if you suspect tympanic membrane rupture. Quinolone drops are the only topical antimicrobials approved for middle ear use.7
Systemic antibiotics are not recommended for the initial treatment of AOE. Topical agents deliver a much higher concentration of medication than can be achieved systemically. Consider systemic antibiotics if there is extension outside the ear canal, a concern for necrotizing otitis externa (more on this in a bit), or the patient is immunodeficient.8
Continue to: Patient (or parent) education...
Patient (or parent) education is important to ensure proper medication administration. The patient should lie down with the affected ear facing up. After the canal is filled with drops, the patient should remain in this position for 3 to 5 minutes. Gently massaging the tragus can augment delivery. Patients should keep the ear canal as dry as possible and avoid inserting objects (eg, hearing aids, ear buds, cotton-tipped applicators) into the canal for the duration of treatment. The delivery of topical antibiotics can be enhanced by wick placement. Prescribe analgesics (typically nonsteroidal anti-inflammatory agents) based on severity of pain.7
Have patients abstain from water sports for 7 to 10 days. Showering is acceptable with minimal ear exposure to water; bathing is preferred when possible. If there is no clinical improvement in 48 to 72 hours, ask patients to return for re-evaluation.8 Prevention is essential for patients with a history of recurrent otitis externa. Acetic acid solutions create an acidic environment within the canal to help prevent recurrent AOE. Ear plugs and petroleum jelly–soaked cotton plugs prior to water exposure may also help prevent recurrent AOE.
Malignant otitis externa
Malignant, or necrotizing, otitis externa is an aggressive disease form of otitis externa that is most common in individuals with diabetes or other immunodeficiency disorders.9 Most cases are due to infection with P aeruginosa.10 Prior to the availability of effective antibiotics, mortality rates in patients with necrotizing otitis externa were as high as 50%.11
What you’ll see. Patients typically present with severe ear pain, otorrhea, conductive hearing loss, and a feeling of fullness in the external ear canal. Physical examination reveals purulent otorrhea and a swollen, tender ear canal. Exposed bone may be visible, most often on the floor of the canal. The tympanic membrane and middle ear are seldom involved on initial presentation.
The infection often originates at the junction of the bony and cartilaginous portion of the external canal, spreading through the fissures of Santorini to the skull base. If not aggressively treated, the infection spreads medially to the tympanomastoid suture causing intracranial complications—usually a facial nerve neuropathy.
Continue to: Given these clinical findings...
Given these clinical findings, promptly order laboratory studies and imaging to confirm the diagnosis. The erythrocyte sedimentation rate and C-reactive protein level are typically elevated, and either can be used as a marker to follow treatment. Computed tomography (CT) helps to determine the location and extent of disease and is recommended as the initial diagnostic imaging modality for patients with suspected malignant otitis externa.12
Magnetic resonance imaging helps define soft-tissue changes, dural enhancement, and involvement of medullary bone, making this the preferred modality to monitor therapeutic response.12 Technetium bone scanning can also be used for the initial diagnosis (particularly if CT findings are normal and clinical suspicion is high) and for follow-up with treatment.
How to treat. Management involves a team approach with otolaryngology, radiology, neurology, endocrinology, and infectious disease specialists. Long term (6-8 weeks) antipseudomonal antibiotic treatment is typical.
Let culture results guide the choice of antibiotic. Fluoroquinolone therapy, usually ciprofloxacin, is used most often.12 Surgical intervention may be required for local debridement and drainage of abscesses. Close follow-up is necessary due to reports of recurrence up to 1 year after treatment. If left untreated, necrotizing otitis externa can lead to osteomyelitis, meningitis, septic thrombosis, cerebral abscess, and death.11
Cerumen impaction
The relatively small diameter of the external auditory canal increases the risk for impaction of cerumen and foreign bodies. Cerumen impaction, in particular, is a common primary care complaint. Cerumen forms when glandular secretions from the outer two-thirds of the ear canal mix with exfoliated skin. It functions as a lubricant for the ear canal and as a barrier against infection, water accumulation, and foreign bodies.13
Continue to: What you'll see
What you’ll see. You may encounter cerumen impaction in an asymptomatic patient when it prevents visualization of the external auditory canal or tympanic membrane, or when a patient complains of conductive hearing loss, tinnitus, dizziness, ear pain, itching, and cough.13 It is found in 1 in 10 children and 1 in 20 adults.13 There is a higher incidence in patients who are elderly, are cognitively impaired, or wear hearing devices or ear plugs.13,14 Asymptomatic cerumen impaction should not be treated. A recent clinical guideline provides a useful “do and don’t” list for patient education (TABLE).13
How to treat. In asymptomatic patients, the presence of cerumen on examination is not an indication for removal. Based on current guidelines,13 impacted cerumen can safely be removed from the ear canal of symptomatic patients in several ways:
- Manual removal with cerumen loop/spoon or alligator forceps. This method decreases the risk for infection because it limits moisture exposure. However, it should be performed by a health care provider trained in its use because of the risk for trauma to the ear canal and tympanic membrane.
- Irrigation of the ear using tap water or a 50-50 solution of hydrogen peroxide and water. Irrigation can be achieved with a syringe or jet irrigator using a modified tip. This method also has a risk for trauma to the ear canal and tympanic membrane and should only be performed by appropriately trained health care professionals.
- Use of cerumenolytic agents to soften and thin earwax and promote natural extrusion. Several types of cerumenolytic drops (water-based and oil-based) are available and appear to be equally effective. Water-based solutions contain hydrogen peroxide, docusate sodium, acetic acid, and sodium bicarbonate. Oil-based drops may contain peanut, almond, or olive oils. A thorough allergic history should be performed to avoid using products in patients with nut allergies. In head-to-head laboratory comparisons, distilled water appears to be the best cerumenolytic.15
Foreign bodies
Foreign bodies in the external auditory canal (typically beads, cotton tips, and insects) are more common in children than adults.16
What you’ll see. Most foreign bodies are lodged in the bony part of the external auditory canal, and many patients try to remove the object before seeking medical care. Removal requires adequate visualization and skill.17 Although patients may be asymptomatic, most complain of pain, fullness, decreased hearing, or otorrhea.
How to treat. Directly visible objects can often be removed without referral. Suction, irrigation, forceps, probes, and fine hooks have been used. Insect removal can be facilitated by first flooding the canal with xylocaine, alcohol, or mineral oil. Acetone may be used to dissolve foreign bodies containing Styrofoam or to loosen glues. If the object is a button battery, avoid irrigation to prevent liquefaction tissue necrosis.
Continue to: Complications of foreign body removal...
Complications of foreign body removal include pain, otitis externa, otitis media, and trauma to the ear or tympanic membrane. The likelihood of successful removal of the object decreases and the risk for complications increases with each subsequent attempt.17 Consult an otolaryngologist if sedation or anesthesia is required, the foreign body is tightly wedged, there is trauma to the ear canal or tympanic membrane, the foreign body has a sharp edge (eg, glass or wire), or removal attempts have been unsuccessful.
Trauma
Sports injuries, motor vehicle accidents, bites, falls, and burns are the primary causes of trauma to the external ear.18
What you’ll see. Blunt auricular trauma predisposes to infection, necrosis, and scar contracture. One of the most common sequelae is cauliflower ear. Trauma is particularly common with contact sports such as boxing, wrestling, or mixed martial arts. The skin of the auricle attaches directly to the perichondrium. Following blunt or shearing trauma to the auricle, hematomas form within the space between the perichondrium and cartilage of the anterior ear.19
How to treat. Small hematomas can be managed by aspiration, while larger ones generally require open drainage.20 Newer treatments involving pressure dressings and the use of fibrin glue have been proposed.20 Recommend that athletes participating in contact sports wear appropriate protective headgear to prevent auricular hematoma and cauliflower ear.
Neoplasm
Roughly 5% of all skin cancers involve the ear, most frequently the pinna due to chronic sun exposure.21 The most frequently occurring malignancy of the external ear is basal cell carcinoma (BCC), which is responsible for 80% of all nonmelanoma skin cancers.22
Continue to: What you'll see
What you’ll see. BCC of the ear usually involves the preauricular area and the helix. The risk for BCC is related to exposure to ultraviolet radiation. BCC of the ear is more common in men and can be particularly aggressive, highlighting the importance of prevention and prompt recognition. BCC typically presents as a fleshy papule that is often translucent or “pearly’” and has overlying telangiectasia and a “rolled” border. Central ulceration can occur as well.
How to treat. Usual treatment of BCC is surgical excision. Prevention is critical and centers on sun avoidance or the use of appropriate sunscreens.
In addition to BCC, exposure of the external ear to sunlight and ultraviolet radiation predisposes patients to the development of squamous cell carcinoma (SCC) and melanoma. SCC has a variety of presentations including papules, plaques, and nodules. SCC has a higher metastatic potential than does BCC.
Keloid
Keloids are an abnormal healing response to soft-tissue injury: benign fibrocartilaginous growths that extend beyond the original wound.
What you’ll see. Keloids are more common in dark-skinned individuals and tend to result from burns, surgical incisions, infection, trauma, tattooing, injections, piercings, and arthropod bites. In some cases, they arise spontaneously. Keloids are more common in areas of increased skin tension (chest, shoulders, back), but may occur on the ears—most commonly after piercing or trauma. Keloids present clinically as slow-growing rubbery or firm nodules. The diagnosis is typically based on clinical appearance but can be confirmed by histopathology.
Continue to: How to treat
How to treat. Treatments vary and include observation, excision, intralesional injections, cryotherapy, enzyme therapy, silicone gel application, and irradiation.23 Recurrence is common; no therapy has been proven to be universally superior or preferred.
Congenital malformations
Atresia
Disruption of embryologic development (failed invagination of the external auditory canal) can lead to a stenotic or absent ear canal (aural atresia). Aural atresia is also often associated with fusion of the incus and malleus. This condition occurs predominantly in males. Unilateral atresia is more common than bilateral atresia, and the right ear is more often involved than the left.24
Microtia
Microtia is the incomplete development of the pinna leading to a small or deformed pinna. Microtia can be unilateral or bilateral. As with atresia, microtia more commonly affects males and, if unilateral, the right side is more often affected than the left. Microtia can occur in isolation but is often associated with genetic syndromes such as Treacher Collins syndrome and craniofacial microsomia (Goldenhar syndrome). When microtia is identified (typically at birth or early infancy), audiologic testing and a thorough physical examination for evidence of associated defects should be performed. Consult with an audiologist, clinical geneticist, or pediatric otolaryngologist.
Pre-auricular pits
Pre-auricular pits (sinuses) are tiny indentations anterior to the helix and superior to the tragus. While pre-auricular pits are more common on the right side, they are bilateral in 25% to 50% of cases.25 Pre-auricular pits occur in up to 1% of white children, 5% of black children, and 10% of Asian children.25 Children with this condition should undergo formal audiologic testing as their risk for hearing loss is higher compared with the general population.26
The branchio-oto-renal syndrome (associated with pre-auricular pits and hearing loss) also features structural defects of the ear, renal anomalies and/or nasolacrimal duct stenosis or fistulas. If this syndrome is suspected, renal ultrasound imaging is warranted. Other indications for renal ultrasound in patients with a pre-auricular pit are any dysmorphic feature, a family history of deafness, an auricular malformation, or a maternal history of gestational diabetes.27 Pre-auricular pits do not require surgery unless they drain chronically or become recurrently infected. Complete surgical excision is the treatment of choice in these cases.
CORRESPONDENCE
Mark Stephens, MD, 1850 Park Avenue, State College, PA 16801; mstephens3@pennstatehealth.psu.edu
1. Cox TC, Camci ED, Vora S, et al. The genetics of auricular development and malformation: new findings in model systems driving future directions for microtia research. Eur J Med Genet. 2014;57:394-401.
2. Sosin M, Weissler JM, Pulcrano M, et al. Transcartilaginous ear piercing and infectious complications: a systematic review and critical analysis of outcomes. Laryngoscope. 2015;125:1827-1834.
3. Stirn A. Body piercing: medical consequences and psychological motivations. Lancet. 2003;361:1205-1215.
4. Liu ZW, Chokkalingam P. Piercing associated perichondritis of the pinna: are we treating it correctly? J Larygol Oncol. 2013;127:505-508.
5. Mitchell S, Ditta K, Minhas S, et al. Pinna abscesses: can we manage them better? A case series and review of the literature. Eur Arch Otorhinolaryngol. 2015;272:3163-3167.
6. Stone KE. Otitis externa. Pediatr Rev. 2007;28:77-78.
7. Rosenfeld RM, Schwartz SR, Cannon CR, et al. Clinical practice guideline: acute otitis externa. Otolaryngol Head Neck Surg. 2014;150(1 suppl):S1-S24.
8. Prentice P. American Academy of Otolaryngology: Head and Neck Surgery Foundation clinical practice guideline on acute otitis externa. Arch Dis Child Educ Pract Ed. 2015;100:197.
9. Unadkat S, Kanzara T, Watters G. Necrotising otitis externa in the immunocompetent patient. J Laryngol Otol. 2018;132:71-74.
10. Carfrae MJ, Kesser BW. Malignant otitis externa. Otolarngol Clin N Am. 2008;41:537-549.
11. Chandler JR, Malignant otitis externa. Laryngoscope. 1968;78:1257-1294.
12. Hollis S, Evans K. Management of malignant (necrotising) otitis externa. J Laryngol Otol. 2011;125:1212-1217.
13. Schwartz SR, Magit AE, Rosenfeld RM, et al. Clinical practice guideline (update): earwax (cerumen impaction). Otolaryngol Head Neck Surg. 2017;156:S1-S29.
14. Guest JF, Greener MJ, Robinson AC, et al. Impacted cerumen: composition, production, epidemiology and management. QJM. 2004;97:477-488.
15. Saxby C, Williams R, Hickey S. Finding the most effective cerumenolytic. J Laryngol Otol. 2013;127:1067-1070.
16. Awad AH, ElTaher M. ENT foreign bodies: an experience. Int Arch Otorhinolaryngol. 2018;22:146-151.
17. Heim SW, Maughan KL. Foreign bodies in the ear, nose, and throat. Am Fam Physician. 2007;76:1185-1189.
18. Sharma K, Goswami SC, Baruah DK. Auricular trauma and its management. Indian J Otolaryngol Head Neck Surg. 2006;58:232-234.
19. Haik J, Givol O, Kornhaber R, et al. Cauliflower ear–a minimally invasive treatment in a wrestling athlete: a case report. Int Med Case Rep J. 2018;11:5-7.
20. Ebrahimi A, Kazemi A, Rasouli HR, et al. Reconstructive surgery of auricular defects: an overview. Trauma Mon. 2015;20:e28202.
21. Warner E, Weston C, Barclay-Klingle N, et al. The swollen pinna. BMJ. 2017; 359; j5073.
22. Rubin AI, Chen EH, Ratner D. Basal cell carcinoma. N Engl J Med. 2005;353:2262-2269.
23. Ranjan SK, Ahmed A, Harsh V, et al. Giant bilateral keloids of the ear lobule: case report and brief review of the literature. J Family Med Prim Care. 2017;6:677-679.
24. Roland PS, Marple BF. Disorders of the external auditory canal. J Am Acad Audiol. 1997;8:367-378.
25. Scheinfeld NS, Silverberg NB, Weinberg JM, et al. The preauricular sinus: a review of its clinical presentation, treatment, and associations. Pediatr Dermatol. 2004;21:191-196.
26. Roth DA, Hildesheimer M, Bardestein S, et al. Preauricular skin tags and ear pits are associated with permanent hearing impairment in newborns. Pediatrics. 2008;122:e884-890.
27. Tan T, Constantinides H, Mitchell TE. The preauricular sinus: a review of its aetiology, clinical presentation and management. Int J Ped Otorhinolaryngol. 2005;69:1469-1474.
1. Cox TC, Camci ED, Vora S, et al. The genetics of auricular development and malformation: new findings in model systems driving future directions for microtia research. Eur J Med Genet. 2014;57:394-401.
2. Sosin M, Weissler JM, Pulcrano M, et al. Transcartilaginous ear piercing and infectious complications: a systematic review and critical analysis of outcomes. Laryngoscope. 2015;125:1827-1834.
3. Stirn A. Body piercing: medical consequences and psychological motivations. Lancet. 2003;361:1205-1215.
4. Liu ZW, Chokkalingam P. Piercing associated perichondritis of the pinna: are we treating it correctly? J Larygol Oncol. 2013;127:505-508.
5. Mitchell S, Ditta K, Minhas S, et al. Pinna abscesses: can we manage them better? A case series and review of the literature. Eur Arch Otorhinolaryngol. 2015;272:3163-3167.
6. Stone KE. Otitis externa. Pediatr Rev. 2007;28:77-78.
7. Rosenfeld RM, Schwartz SR, Cannon CR, et al. Clinical practice guideline: acute otitis externa. Otolaryngol Head Neck Surg. 2014;150(1 suppl):S1-S24.
8. Prentice P. American Academy of Otolaryngology: Head and Neck Surgery Foundation clinical practice guideline on acute otitis externa. Arch Dis Child Educ Pract Ed. 2015;100:197.
9. Unadkat S, Kanzara T, Watters G. Necrotising otitis externa in the immunocompetent patient. J Laryngol Otol. 2018;132:71-74.
10. Carfrae MJ, Kesser BW. Malignant otitis externa. Otolarngol Clin N Am. 2008;41:537-549.
11. Chandler JR, Malignant otitis externa. Laryngoscope. 1968;78:1257-1294.
12. Hollis S, Evans K. Management of malignant (necrotising) otitis externa. J Laryngol Otol. 2011;125:1212-1217.
13. Schwartz SR, Magit AE, Rosenfeld RM, et al. Clinical practice guideline (update): earwax (cerumen impaction). Otolaryngol Head Neck Surg. 2017;156:S1-S29.
14. Guest JF, Greener MJ, Robinson AC, et al. Impacted cerumen: composition, production, epidemiology and management. QJM. 2004;97:477-488.
15. Saxby C, Williams R, Hickey S. Finding the most effective cerumenolytic. J Laryngol Otol. 2013;127:1067-1070.
16. Awad AH, ElTaher M. ENT foreign bodies: an experience. Int Arch Otorhinolaryngol. 2018;22:146-151.
17. Heim SW, Maughan KL. Foreign bodies in the ear, nose, and throat. Am Fam Physician. 2007;76:1185-1189.
18. Sharma K, Goswami SC, Baruah DK. Auricular trauma and its management. Indian J Otolaryngol Head Neck Surg. 2006;58:232-234.
19. Haik J, Givol O, Kornhaber R, et al. Cauliflower ear–a minimally invasive treatment in a wrestling athlete: a case report. Int Med Case Rep J. 2018;11:5-7.
20. Ebrahimi A, Kazemi A, Rasouli HR, et al. Reconstructive surgery of auricular defects: an overview. Trauma Mon. 2015;20:e28202.
21. Warner E, Weston C, Barclay-Klingle N, et al. The swollen pinna. BMJ. 2017; 359; j5073.
22. Rubin AI, Chen EH, Ratner D. Basal cell carcinoma. N Engl J Med. 2005;353:2262-2269.
23. Ranjan SK, Ahmed A, Harsh V, et al. Giant bilateral keloids of the ear lobule: case report and brief review of the literature. J Family Med Prim Care. 2017;6:677-679.
24. Roland PS, Marple BF. Disorders of the external auditory canal. J Am Acad Audiol. 1997;8:367-378.
25. Scheinfeld NS, Silverberg NB, Weinberg JM, et al. The preauricular sinus: a review of its clinical presentation, treatment, and associations. Pediatr Dermatol. 2004;21:191-196.
26. Roth DA, Hildesheimer M, Bardestein S, et al. Preauricular skin tags and ear pits are associated with permanent hearing impairment in newborns. Pediatrics. 2008;122:e884-890.
27. Tan T, Constantinides H, Mitchell TE. The preauricular sinus: a review of its aetiology, clinical presentation and management. Int J Ped Otorhinolaryngol. 2005;69:1469-1474.
PRACTICE RECOMMENDATIONS
› Prescribe topical antibiotics for uncomplicated otitis externa, reserving systemic agents for infection extending outside the ear canal, necrotizing otitis externa, or patients who are immunodeficient. C
› Avoid clearing cerumen if a patient is asymptomatic and advise patients/parents on Do’s and Don’ts for ear wax accumulation. C
› Consider flooding the ear canal with xylocaine, alcohol, or mineral oil before attempting insect removal. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series