User login
Dual antiplatelet Tx for stroke prevention: Worth the risk?
The incidence of ischemic stroke in the United States is estimated to be more than 795,000 events each year.1 After an initial stroke, the rate of recurrence is 5% to 20% within the first year, with the greatest prevalence in the first 90 days following an event.2-5 Although dual antiplatelet therapy, often with aspirin and a P2Y12 inhibitor such as clopidogrel, reduces the risk for recurrent cardiovascular events, cerebrovascular events, and death following acute coronary syndromes and percutaneous intervention, the role of combination antiplatelet therapy for secondary prevention of ischemic stroke continues to be debated.6 Reconciling currently available data can be challenging, as many studies vary considerably in both the time to antiplatelet initiation and duration of therapy.
For many years, aspirin alone was the drug of choice for secondary prevention of noncardioembolic ischemic stroke.7 Efficacy is similar at dosages anywhere between 50 and 1500 mg/d; higher doses incur a greater risk for gastrointestinal hemorrhage.7 Current secondary prevention guidelines recommend a dosage of aspirin somewhere between 50 and 325 mg/d.7
Alternative agents have also been evaluated for secondary stroke prevention, but only clopidogrel is currently considered an acceptable alternative for monotherapy based on a subgroup analysis of the CAPRIE (Clopidogrel versus Aspirin in Patients at Risk of Ischaemic Events) trial.7,8 Other alternatives, including cilostazol, ticlopidine, and ticagrelor, are limited by a lack of data, adverse drug reactions, or unproven efficacy and are not recommended in current guidelines.7,9 The ongoing THALES (Acute Stroke or Transient Ischaemic Attack Treated with Ticagrelor and Aspirin for Prevention of Stroke and Death) trial, assessing combination ticagrelor and aspirin, may identify an additional option for antiplatelet therapy following acute stroke.10
The current guidelines from the American Heart Association/American Stroke Association (AHA/ASA) support the combination of aspirin and extended-release dipyridamole (ASA-ERDP) as a long-term alternative to aspirin monotherapy.7,11 Additionally, the combination of clopidogrel and aspirin (CLO-ASA) is now recommended for limited duration in the early management of ischemic stroke.11
This review will explore the role of dual antiplatelet therapy for secondary prevention of noncardioembolic ischemic stroke or transient ischemic attack (TIA), with particular focus on acute use of CLO-ASA.
Clopidogrel and aspirin: When to initiate, when to stop
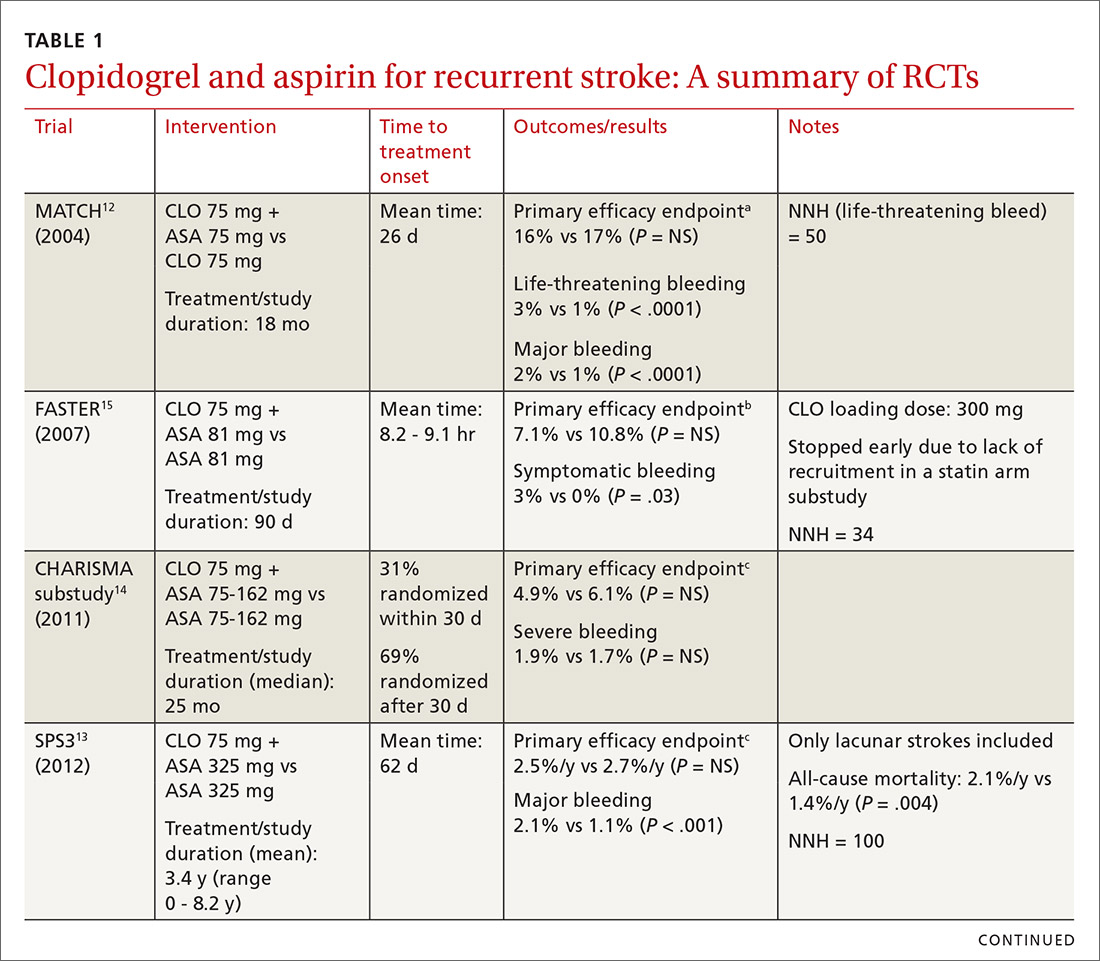
The combined use of clopidogrel and aspirin has been well-studied for secondary prevention of ischemic stroke and TIA. However, interpreting and applying the results of these trials can be challenging given key differences in both time to treatment initiation and the duration of combination therapy. Highlights of the major randomized controlled trials (RCTs) evaluating the safety and efficacy of CLO-ASA are detailed in TABLE 1.4,5,12-15
Initial trials evaluating CLO-ASA for secondary stroke prevention, including the MATCH (Management of ATherothrombosis with Clopidogrel in High-risk patients),12 SPS3 (Secondary Prevention of Small Subcortical Strokes),13 and CHARISMA (Clopidogrel for High Atherothrombotic Risk and Ischaemic Stabilization, Management and Avoidance)14 trials assessed the long-term benefits of combination therapy, with most patients initiating treatment a month or more following an initial stroke and continuing therapy for at least 18 months.12-14 Results from these trials indicate that long-term use (> 18 months) of CLO-ASA does not reduce recurrent events but increases rates of clinically significant bleeding.12-14
Continue to: A look at Tx timing
A look at Tx timing. Since these initial attempts failed to show a long-term benefit with CLO-ASA, subsequent trials attempted to establish an appropriate balance between the optimal time to initiate CLO-ASA and the optimal duration of therapy. The FASTER (Fast Assessment of Stroke and Transient ischaemic attack to prevent Early Recurrence) trial was a small pilot study of 392 patients randomized to CLO-ASA or aspirin within 24 hours of stroke or TIA onset and continued for only 3 months.15 While this trial did not find a significant reduction in ischemic or hemorrhagic stroke with combination therapy, there was a large numerical difference in event rates between the 2 groups (7.1% CLO-ASA vs 10.8% aspirin).15 An underpowered sample size (due to difficulty recruiting participants) is likely responsible for the lack of statistical significance.15 Despite the trial’s failure to show a benefit with acute use of CLO-ASA, it suggested a possible benefit that led to further investigation in the CHANCE (Clopidogrel in High-risk patients with Acute Non-disabling Cerebrovascular Events)5 and POINT (Platelet-Oriented Inhibition in New TIA and Minor Ischemic Stroke) 4 trials.
The CHANCE trial conducted in China included more than 5000 patients with acute minor ischemic stroke (National Institutes of Health Stroke Scale [NIHSS] score ≤ 3) or high-risk TIA (ABCD2 [a scale that assesses the risk of stroke on the basis of age, blood pressure, clinical features, duration of TIA, and presence or absence of diabetes] score ≥ 4).5 Similar to FASTER, patients were randomized within 24 hours of symptom onset to CLO-ASA or aspirin. However, CHANCE utilized combination therapy for only 21 days, after which the patients were continued on clopidogrel monotherapy for up to 90 days; the aspirin monotherapy group continued aspirin for 90 days.
After 90 days, patients initially using combination therapy had significantly lower rates of ischemic or hemorrhagic stroke vs those assigned to aspirin monotherapy. This result was driven heavily by the reduction in ischemic stroke (7.9% CLO-ASA vs 11.4% aspirin; P < .001). Additionally, there was no significant difference in moderate or severe bleeding events between the 2 groups.5 Efficacy and safety results were similar among a subgroup of patients who were randomized to treatment within 12 hours rather than 24 hours from symptom onset.16 The CHANCE trial was the first major study to demonstrate a clinical benefit of CLO-ASA to prevent recurrent stroke. Accordingly, the 2018 AHA/ASA guidelines included a new recommendation regarding secondary prevention for the use of CLO-ASA initiated within 24 hours and continued for 21 days following a minor stroke or TIA.11
A drawback of the CHANCE trial was its narrow patient population of only Chinese patients, which may limit applicability in clinical practice. There are known genetic variations in cytochrome P450 2C19 (CYP2C19) that may affect clopidogrel metabolism. CYP2C19 is responsible for the conversion of clopidogrel into its activated form in vivo. Carriers of a CYP2C19 loss-of-function allele may have reduced clopidogrel activation and subsequent reduced antiplatelet activity. Such loss-of-function alleles are more common in Asian populations vs non-Asian populations.17
A substudy of CHANCE found that CLO-ASA’s efficacy benefit was preserved in noncarrier patients; however, patients with the CYP2C19 loss-of-function allele did not benefit from combination therapy.18 Interestingly, these genetic differences did not affect bleeding outcomes. Given that approximately 60% of patients in the CHANCE substudy were loss-of-function allele carriers and that the overall study results still showed benefit with combination therapy, application of CHANCE’s findings to broader populations may not be a concern after all.18
Continue to: In efforts to gain insight...
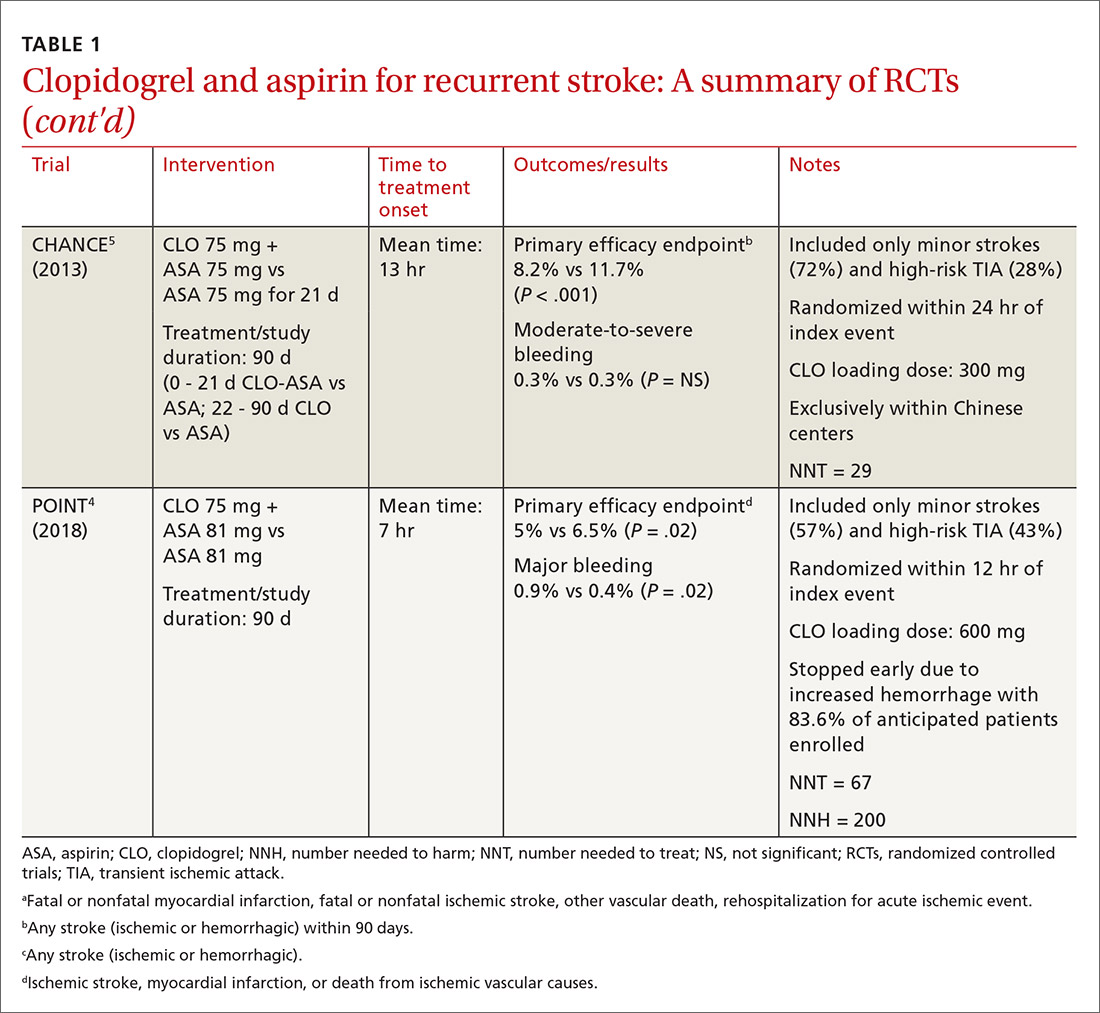
In efforts to gain insight on CLO-ASA’s use in a more diverse patient population, the POINT trial included almost 5000 patients, with 82% from the United States, who were randomized within 12 hours of symptom onset to CLO-ASA or aspirin monotherapy for 90 days.4 Similar to the CHANCE study, the POINT study included patients with mild ischemic strokes (NIHSS ≤ 3) or high-risk TIA (ABCD2 ≥ 4). Combination therapy significantly reduced the primary endpoint of ischemic stroke, myocardial infarction (MI), or death from an ischemic event. Contrary to CHANCE, there was a significant increase in major bleeding in those assigned to combination therapy, which resulted in the trial being stopped early.4
A closer look at safety differences. CHANCE and POINT were the first major trials to show a benefit of CLO-ASA for secondary prevention of stroke, yet their differences in safety outcomes, specifically major hemorrhage, argued for a deeper reconciliation of their results.4,5 While both trials initiated secondary prevention within 24 hours of symptom onset, the difference in duration of combination therapy (21 days in CHANCE vs 90 days in POINT) likely impacted the rates of hemorrhage. When results from POINT were stratified by time period, particularly within the first 30 days of therapy (similar to the 21-day treatment duration of CHANCE), combination therapy significantly reduced the primary endpoint of ischemic stroke, MI, or death from an ischemic event (3.9% CLO-ASA vs 5.8% aspirin; P = .02) without an increased risk for major hemorrhage. Between 30 and 90 days, this efficacy benefit disappeared. However, bleeding rates between groups continued to separate throughout the 90-day course. In this light, the 30-day outcomes of POINT are largely similar to CHANCE and support the short-term use of CLO-ASA for secondary prevention without an associated increase in major bleeding.4,5
Antiplatelet dosing in POINT and CHANCE may also play a role in the contrasting safety results between the trials.4,5 While both studies utilized clopidogrel loading doses, POINT used 600 mg while CHANCE used 300 mg. Clopidogrel maintenance dosing was the same at 75 mg/d. In CHANCE, aspirin dosing was protocolized to 75 mg/d; however, in POINT, 31% of patients used > 100 mg/d aspirin.4,5 It is possible that the higher doses of both aspirin and clopidogrel in the POINT trial contributed to the difference in the occurrence of major hemorrhage between the treatment groups in these trials.
The takeaway. Based on currently available data, patients who are best suited to benefit from CLO-ASA are those who have had minor noncardioembolic ischemic strokes or high-risk TIAs.4,5,11 Clopidogrel should be given as a 300-mg loading dose followed by 75 mg/d given concomitantly with aspirin at a dose no higher than 100 mg/d. CLO-ASA therapy should be initiated within 24 hours of symptom onset and be continued for no longer than 1 month, after which chronic preventive therapy with either aspirin or clopidogrel monotherapy should be started.4,5,11
Dipyridamole and aspirin: A controversial option
Since the approval of the combination product ASA-ERDP, there has been considerable controversy about using this combination over other therapies, such as aspirin or clopidogrel, for recurrent ischemic stroke prevention. Much of this controversy arises from limitations in the trial designs.
Continue to: The first trial to show benefit...
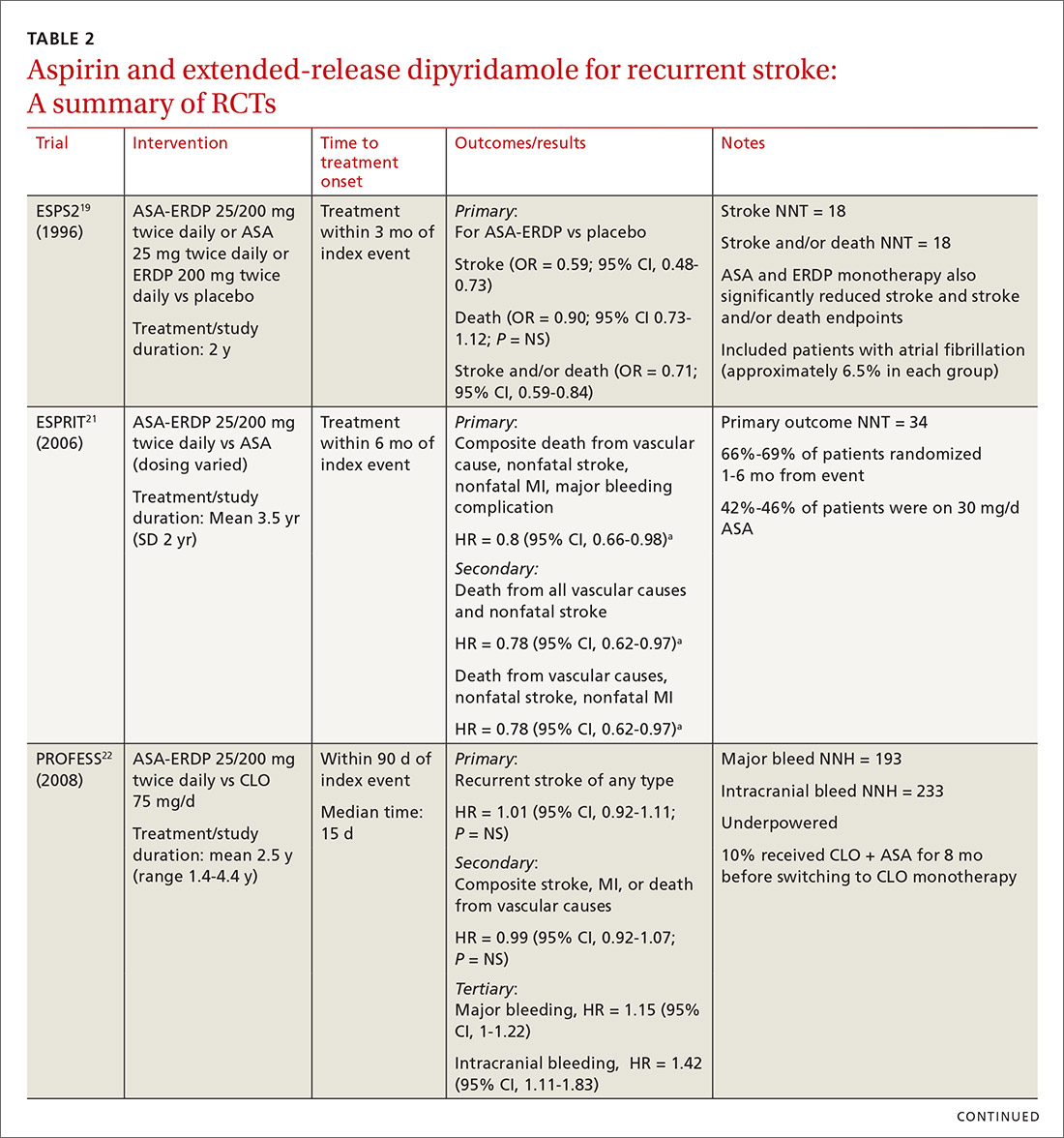
The first trial to show benefit with ASA-ERDP was ESPS2 (European Stroke Prevention Study 2), which demonstrated superiority of the combination over placebo in reducing recurrent stroke when treatment was added within 3 months of an index stroke.19 A few studies have evaluated ASA-ERDP compared to aspirin monotherapy; however, most of these studies were small and did not show any difference in outcomes.20 Only ESPRIT (European/Australasian Stroke Prevention in Reversible Ischaemia Trial)21 carried significant weight in a 2013 meta-analysis, which showed a significant reduction in recurrent events with the combination product compared to aspirin monotherapy.20
Both the ESPS2 and ESPRIT trials had significant limitations.19,21 Patients in both studies had vascular comorbidities including atherosclerotic cardiovascular disease (ASCVD); however, pharmacotherapies designated to treat these diseases were not mentioned in the demographic data, nor were these medications taken into consideration to limit potential bias.19,21 Retrospectively, a significant proportion of aspirin doses utilized as a control in ESPRIT were inferior to the guideline-recommended dosing with 42% to 46% of patients receiving 30 mg/d.21 Despite these controversies, ASA-ERDP is still considered an alternative to aspirin monotherapy in the guidelines.7
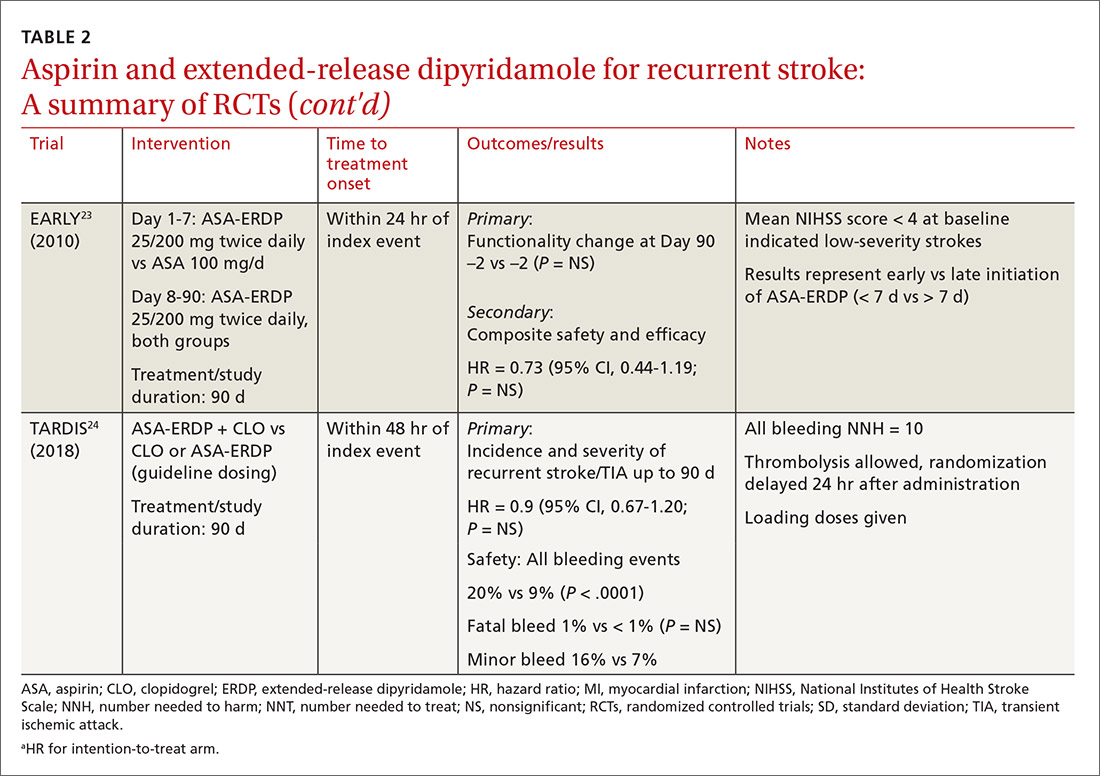
The timing of ASA-ERDP initiation appears to be inversely related to the efficacy of the combination over therapeutic alternatives. Studies in which the therapy was initiated 3 to 6 months from the index stroke indicated favorable outcomes for the combination when compared to ASA or ERDP monotherapy.19,21 Studies utilizing early initiation (ie, within 24 or 48 hours of the index event) or even within 3 weeks showed no difference in outcomes; however, this may be due in part to the use of clopidogrel or other combination antiplatelet therapy as active comparators.22-24
Early initiation of ASA-ERDP also demonstrated a higher risk of major and intracranial bleeding compared to clopidogrel.22 Additionally, use of triple therapy with ASA-ERDP plus clopidogrel increased bleeding events without improving efficacy.24 More recent studies of ASA-ERDP are focusing on earlier initiation of therapy; it is unknown whether the benefits of late initiation will be confirmed in future studies. Highlights of the major RCTs evaluating the safety and efficacy of ASA-ERDP are detailed in TABLE 219,21-24.
The takeaway. Methodological issues and potential confounding factors in many of the key trials for ASA-ERDP make it challenging to fully discern the role that ASA-ERDP may play in the secondary prevention of stroke. Further evidence utilizing appropriate controls, timing, and assessment of confounders is needed. Additionally, ASA-ERDP is plagued by tolerability issues such as headache, nausea, and vomiting, leading to higher rates of discontinuation than its comparators in clinical trials. Accordingly, the maintenance use of ASA-ERDP for secondary stroke prevention may be considered less preferred than other recommended alternatives such as aspirin or clopidogrel monotherapies.
CORRESPONDENCE
Robert S. Helmer, PharmD, BCPS, Department of Pharmacy Practice, Auburn University Harrison School of Pharmacy, 650 Clinic Drive, Suite 2100, Mobile, AL 36688; Rsh0011@auburn.edu.
1. CDC. Stroke Facts. Last updated January 31, 2020. www.cdc.gov/stroke/facts.htm. Accessed June 29, 2020.
2. Amarenco P, Lavallee PC, Labreuche J, et al. One-year risk of stroke after transient ischemic attack or minor stroke. N Engl J Med. 2016;374:1533-1542.
3. Amarenco P, Lavallee PC, Monteiro Tavares L, et al. Five-year risk of stroke after TIA or minor ischemic stroke. N Engl J Med. 2018;378:2182-2190.
4. Johnston SC, Easton JD, Farrant M, et al. Clopidogrel and aspirin in acute ischemic stroke and high-risk TIA. N Engl J Med. 2018;379:215-225.
5. Wang Y, Wang Y, Zhao X, et al. Clopidogrel with aspirin in acute minor stroke or transient ischemic attack. N Engl J Med. 2013;369:11-19.
6. Bowry AD, Brookhart MA, Choudhry NK. Meta-analysis of the efficacy and safety of clopidogrel plus aspirin as compared to antiplatelet monotherapy for the prevention of vascular events. Am J Cardiol. 2008;101:960-966.
7. Kernan WN, Ovbiagele B, Black HR, et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45:2160-2236.
8. Gent M, Beaumont D, Blanchard J, et al. A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). Lancet. 1996;348:1329-1339.
9. Lansberg MG, O’Donnell MJ, Khatri P, et al. Antithrombotic and thrombolytic therapy for ischemic stroke: Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e601S-e636S.
10. Johnston SC, Amarenco P, Denison H, et al. The acute stroke or transient ischemic attack treated with ticagrelor and aspirin for prevention of stroke and death (THALES) trial: rationale and design. Int J Stroke. 2019;14:745‐751.
11. Powers WJ, Rabinstein AA, Ackerson T, et al. 2018 Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2018;49:e46-e110.
12. Diener HC, Bogousslavsky J, Brass LM, et al. Aspirin and clopidogrel compared with clopidogrel alone after recent ischaemic stroke or transient ischaemic attack in high-risk patients (MATCH): randomised, double-blind, placebo-controlled trial. Lancet. 2004;364:331-337.
13. Benavente OR, Hart RG, McClure LA, et al. Effects of clopidogrel added to aspirin in patients with recent lacunar stroke. N Engl J Med. 2012;367:817-825.
14. Hankey GJ, Johnston SC, Easton JD, et al. Effect of clopidogrel plus ASA vs. ASA early after TIA and ischaemic stroke: a substudy of the CHARISMA trial. Int J Stroke. 2011;6:3-9.
15. Kennedy J, Hill MD, Ryckborst KJ, et al. Fast assessment of stroke and transient ischaemic attack to prevent early recurrence (FASTER): a randomised controlled pilot trial. Lancet Neurol. 2007;6:961-969.
16. Li Z, Wang Y, Zhao X, et al. Treatment effect of clopidogrel plus aspirin within 12 hours of acute minor stroke or transient ischemic attack. J Am Heart Assoc. 2016;5:e003038.
17. Scott SA, Sangkuhl K, Stein CM, et al. Clinical pharmacogenetics implementation consortium guidelines for CYP2C19 genotype and clopidogrel therapy: 2013 update. Clin Pharmacol Ther. 2013;94:317-323.
18. Wang Y, Zhao X, Lin J, et al. Association between CYP2C19 loss-of-function allele status and efficacy of clopidogrel for risk reduction among patients with minor stroke or transient ischemic attack. JAMA. 2016;316:70-78.
19. Diener HC, Cunha L, Forbes C, et al. European Stroke Prevention Study 2. Dipyridamole and acetylsalicylic acid in the secondary prevention of stroke. J Neurol Sci. 1996;143:1-13.
20. Li X, Zhou G, Zhou X, et al. The efficacy and safety of aspirin plus dipyridamole versus aspirin in secondary prevention following TIA or stroke: a meta-analysis of randomized controlled trials. J Neurol Sci. 2013;332:92-96.
21. Halkes PH, van Gijn J, Kapelle IJ, et al. Aspirin plus dipyridamole versus aspirin alone after cerebral ischaemia of arterial origin (ESPRIT): randomised controlled trial. Lancet. 2006;367:1665-1673.
22. Sacco RL, Diener HC, Yusuf S, et al. Aspirin and extended-release dipyridamole versus clopidogrel for recurrent stroke. N Engl J Med. 2008;359:1238-1251.
23. Dengler R, Diener HC, Schwartz A, et al. Early treatment with aspirin plus extended-release dipyridamole for transient ischaemic attack or ischaemic stroke within 24 h of symptom onset (EARLY trial): a randomised, open-label, blinded-endpoint trial. Lancet Neurol. 2010;9:159-166.
24. Bath PM, Woodhouse LJ, Appleton JP, et al. Antiplatelet therapy with aspirin, clopidogrel, and dipyridamole versus clopidogrel alone or aspirin and dipyridamole in patients with acute cerebral ischaemia (TARDIS): a randomised, open-label, phase 3 superiority trial. Lancet. 2018;391:850-859.
The incidence of ischemic stroke in the United States is estimated to be more than 795,000 events each year.1 After an initial stroke, the rate of recurrence is 5% to 20% within the first year, with the greatest prevalence in the first 90 days following an event.2-5 Although dual antiplatelet therapy, often with aspirin and a P2Y12 inhibitor such as clopidogrel, reduces the risk for recurrent cardiovascular events, cerebrovascular events, and death following acute coronary syndromes and percutaneous intervention, the role of combination antiplatelet therapy for secondary prevention of ischemic stroke continues to be debated.6 Reconciling currently available data can be challenging, as many studies vary considerably in both the time to antiplatelet initiation and duration of therapy.
For many years, aspirin alone was the drug of choice for secondary prevention of noncardioembolic ischemic stroke.7 Efficacy is similar at dosages anywhere between 50 and 1500 mg/d; higher doses incur a greater risk for gastrointestinal hemorrhage.7 Current secondary prevention guidelines recommend a dosage of aspirin somewhere between 50 and 325 mg/d.7
Alternative agents have also been evaluated for secondary stroke prevention, but only clopidogrel is currently considered an acceptable alternative for monotherapy based on a subgroup analysis of the CAPRIE (Clopidogrel versus Aspirin in Patients at Risk of Ischaemic Events) trial.7,8 Other alternatives, including cilostazol, ticlopidine, and ticagrelor, are limited by a lack of data, adverse drug reactions, or unproven efficacy and are not recommended in current guidelines.7,9 The ongoing THALES (Acute Stroke or Transient Ischaemic Attack Treated with Ticagrelor and Aspirin for Prevention of Stroke and Death) trial, assessing combination ticagrelor and aspirin, may identify an additional option for antiplatelet therapy following acute stroke.10
The current guidelines from the American Heart Association/American Stroke Association (AHA/ASA) support the combination of aspirin and extended-release dipyridamole (ASA-ERDP) as a long-term alternative to aspirin monotherapy.7,11 Additionally, the combination of clopidogrel and aspirin (CLO-ASA) is now recommended for limited duration in the early management of ischemic stroke.11
This review will explore the role of dual antiplatelet therapy for secondary prevention of noncardioembolic ischemic stroke or transient ischemic attack (TIA), with particular focus on acute use of CLO-ASA.
Clopidogrel and aspirin: When to initiate, when to stop
The combined use of clopidogrel and aspirin has been well-studied for secondary prevention of ischemic stroke and TIA. However, interpreting and applying the results of these trials can be challenging given key differences in both time to treatment initiation and the duration of combination therapy. Highlights of the major randomized controlled trials (RCTs) evaluating the safety and efficacy of CLO-ASA are detailed in TABLE 1.4,5,12-15
Initial trials evaluating CLO-ASA for secondary stroke prevention, including the MATCH (Management of ATherothrombosis with Clopidogrel in High-risk patients),12 SPS3 (Secondary Prevention of Small Subcortical Strokes),13 and CHARISMA (Clopidogrel for High Atherothrombotic Risk and Ischaemic Stabilization, Management and Avoidance)14 trials assessed the long-term benefits of combination therapy, with most patients initiating treatment a month or more following an initial stroke and continuing therapy for at least 18 months.12-14 Results from these trials indicate that long-term use (> 18 months) of CLO-ASA does not reduce recurrent events but increases rates of clinically significant bleeding.12-14
Continue to: A look at Tx timing
A look at Tx timing. Since these initial attempts failed to show a long-term benefit with CLO-ASA, subsequent trials attempted to establish an appropriate balance between the optimal time to initiate CLO-ASA and the optimal duration of therapy. The FASTER (Fast Assessment of Stroke and Transient ischaemic attack to prevent Early Recurrence) trial was a small pilot study of 392 patients randomized to CLO-ASA or aspirin within 24 hours of stroke or TIA onset and continued for only 3 months.15 While this trial did not find a significant reduction in ischemic or hemorrhagic stroke with combination therapy, there was a large numerical difference in event rates between the 2 groups (7.1% CLO-ASA vs 10.8% aspirin).15 An underpowered sample size (due to difficulty recruiting participants) is likely responsible for the lack of statistical significance.15 Despite the trial’s failure to show a benefit with acute use of CLO-ASA, it suggested a possible benefit that led to further investigation in the CHANCE (Clopidogrel in High-risk patients with Acute Non-disabling Cerebrovascular Events)5 and POINT (Platelet-Oriented Inhibition in New TIA and Minor Ischemic Stroke) 4 trials.
The CHANCE trial conducted in China included more than 5000 patients with acute minor ischemic stroke (National Institutes of Health Stroke Scale [NIHSS] score ≤ 3) or high-risk TIA (ABCD2 [a scale that assesses the risk of stroke on the basis of age, blood pressure, clinical features, duration of TIA, and presence or absence of diabetes] score ≥ 4).5 Similar to FASTER, patients were randomized within 24 hours of symptom onset to CLO-ASA or aspirin. However, CHANCE utilized combination therapy for only 21 days, after which the patients were continued on clopidogrel monotherapy for up to 90 days; the aspirin monotherapy group continued aspirin for 90 days.
After 90 days, patients initially using combination therapy had significantly lower rates of ischemic or hemorrhagic stroke vs those assigned to aspirin monotherapy. This result was driven heavily by the reduction in ischemic stroke (7.9% CLO-ASA vs 11.4% aspirin; P < .001). Additionally, there was no significant difference in moderate or severe bleeding events between the 2 groups.5 Efficacy and safety results were similar among a subgroup of patients who were randomized to treatment within 12 hours rather than 24 hours from symptom onset.16 The CHANCE trial was the first major study to demonstrate a clinical benefit of CLO-ASA to prevent recurrent stroke. Accordingly, the 2018 AHA/ASA guidelines included a new recommendation regarding secondary prevention for the use of CLO-ASA initiated within 24 hours and continued for 21 days following a minor stroke or TIA.11
A drawback of the CHANCE trial was its narrow patient population of only Chinese patients, which may limit applicability in clinical practice. There are known genetic variations in cytochrome P450 2C19 (CYP2C19) that may affect clopidogrel metabolism. CYP2C19 is responsible for the conversion of clopidogrel into its activated form in vivo. Carriers of a CYP2C19 loss-of-function allele may have reduced clopidogrel activation and subsequent reduced antiplatelet activity. Such loss-of-function alleles are more common in Asian populations vs non-Asian populations.17
A substudy of CHANCE found that CLO-ASA’s efficacy benefit was preserved in noncarrier patients; however, patients with the CYP2C19 loss-of-function allele did not benefit from combination therapy.18 Interestingly, these genetic differences did not affect bleeding outcomes. Given that approximately 60% of patients in the CHANCE substudy were loss-of-function allele carriers and that the overall study results still showed benefit with combination therapy, application of CHANCE’s findings to broader populations may not be a concern after all.18
Continue to: In efforts to gain insight...
In efforts to gain insight on CLO-ASA’s use in a more diverse patient population, the POINT trial included almost 5000 patients, with 82% from the United States, who were randomized within 12 hours of symptom onset to CLO-ASA or aspirin monotherapy for 90 days.4 Similar to the CHANCE study, the POINT study included patients with mild ischemic strokes (NIHSS ≤ 3) or high-risk TIA (ABCD2 ≥ 4). Combination therapy significantly reduced the primary endpoint of ischemic stroke, myocardial infarction (MI), or death from an ischemic event. Contrary to CHANCE, there was a significant increase in major bleeding in those assigned to combination therapy, which resulted in the trial being stopped early.4
A closer look at safety differences. CHANCE and POINT were the first major trials to show a benefit of CLO-ASA for secondary prevention of stroke, yet their differences in safety outcomes, specifically major hemorrhage, argued for a deeper reconciliation of their results.4,5 While both trials initiated secondary prevention within 24 hours of symptom onset, the difference in duration of combination therapy (21 days in CHANCE vs 90 days in POINT) likely impacted the rates of hemorrhage. When results from POINT were stratified by time period, particularly within the first 30 days of therapy (similar to the 21-day treatment duration of CHANCE), combination therapy significantly reduced the primary endpoint of ischemic stroke, MI, or death from an ischemic event (3.9% CLO-ASA vs 5.8% aspirin; P = .02) without an increased risk for major hemorrhage. Between 30 and 90 days, this efficacy benefit disappeared. However, bleeding rates between groups continued to separate throughout the 90-day course. In this light, the 30-day outcomes of POINT are largely similar to CHANCE and support the short-term use of CLO-ASA for secondary prevention without an associated increase in major bleeding.4,5
Antiplatelet dosing in POINT and CHANCE may also play a role in the contrasting safety results between the trials.4,5 While both studies utilized clopidogrel loading doses, POINT used 600 mg while CHANCE used 300 mg. Clopidogrel maintenance dosing was the same at 75 mg/d. In CHANCE, aspirin dosing was protocolized to 75 mg/d; however, in POINT, 31% of patients used > 100 mg/d aspirin.4,5 It is possible that the higher doses of both aspirin and clopidogrel in the POINT trial contributed to the difference in the occurrence of major hemorrhage between the treatment groups in these trials.
The takeaway. Based on currently available data, patients who are best suited to benefit from CLO-ASA are those who have had minor noncardioembolic ischemic strokes or high-risk TIAs.4,5,11 Clopidogrel should be given as a 300-mg loading dose followed by 75 mg/d given concomitantly with aspirin at a dose no higher than 100 mg/d. CLO-ASA therapy should be initiated within 24 hours of symptom onset and be continued for no longer than 1 month, after which chronic preventive therapy with either aspirin or clopidogrel monotherapy should be started.4,5,11
Dipyridamole and aspirin: A controversial option
Since the approval of the combination product ASA-ERDP, there has been considerable controversy about using this combination over other therapies, such as aspirin or clopidogrel, for recurrent ischemic stroke prevention. Much of this controversy arises from limitations in the trial designs.
Continue to: The first trial to show benefit...
The first trial to show benefit with ASA-ERDP was ESPS2 (European Stroke Prevention Study 2), which demonstrated superiority of the combination over placebo in reducing recurrent stroke when treatment was added within 3 months of an index stroke.19 A few studies have evaluated ASA-ERDP compared to aspirin monotherapy; however, most of these studies were small and did not show any difference in outcomes.20 Only ESPRIT (European/Australasian Stroke Prevention in Reversible Ischaemia Trial)21 carried significant weight in a 2013 meta-analysis, which showed a significant reduction in recurrent events with the combination product compared to aspirin monotherapy.20
Both the ESPS2 and ESPRIT trials had significant limitations.19,21 Patients in both studies had vascular comorbidities including atherosclerotic cardiovascular disease (ASCVD); however, pharmacotherapies designated to treat these diseases were not mentioned in the demographic data, nor were these medications taken into consideration to limit potential bias.19,21 Retrospectively, a significant proportion of aspirin doses utilized as a control in ESPRIT were inferior to the guideline-recommended dosing with 42% to 46% of patients receiving 30 mg/d.21 Despite these controversies, ASA-ERDP is still considered an alternative to aspirin monotherapy in the guidelines.7
The timing of ASA-ERDP initiation appears to be inversely related to the efficacy of the combination over therapeutic alternatives. Studies in which the therapy was initiated 3 to 6 months from the index stroke indicated favorable outcomes for the combination when compared to ASA or ERDP monotherapy.19,21 Studies utilizing early initiation (ie, within 24 or 48 hours of the index event) or even within 3 weeks showed no difference in outcomes; however, this may be due in part to the use of clopidogrel or other combination antiplatelet therapy as active comparators.22-24
Early initiation of ASA-ERDP also demonstrated a higher risk of major and intracranial bleeding compared to clopidogrel.22 Additionally, use of triple therapy with ASA-ERDP plus clopidogrel increased bleeding events without improving efficacy.24 More recent studies of ASA-ERDP are focusing on earlier initiation of therapy; it is unknown whether the benefits of late initiation will be confirmed in future studies. Highlights of the major RCTs evaluating the safety and efficacy of ASA-ERDP are detailed in TABLE 219,21-24.
The takeaway. Methodological issues and potential confounding factors in many of the key trials for ASA-ERDP make it challenging to fully discern the role that ASA-ERDP may play in the secondary prevention of stroke. Further evidence utilizing appropriate controls, timing, and assessment of confounders is needed. Additionally, ASA-ERDP is plagued by tolerability issues such as headache, nausea, and vomiting, leading to higher rates of discontinuation than its comparators in clinical trials. Accordingly, the maintenance use of ASA-ERDP for secondary stroke prevention may be considered less preferred than other recommended alternatives such as aspirin or clopidogrel monotherapies.
CORRESPONDENCE
Robert S. Helmer, PharmD, BCPS, Department of Pharmacy Practice, Auburn University Harrison School of Pharmacy, 650 Clinic Drive, Suite 2100, Mobile, AL 36688; Rsh0011@auburn.edu.
The incidence of ischemic stroke in the United States is estimated to be more than 795,000 events each year.1 After an initial stroke, the rate of recurrence is 5% to 20% within the first year, with the greatest prevalence in the first 90 days following an event.2-5 Although dual antiplatelet therapy, often with aspirin and a P2Y12 inhibitor such as clopidogrel, reduces the risk for recurrent cardiovascular events, cerebrovascular events, and death following acute coronary syndromes and percutaneous intervention, the role of combination antiplatelet therapy for secondary prevention of ischemic stroke continues to be debated.6 Reconciling currently available data can be challenging, as many studies vary considerably in both the time to antiplatelet initiation and duration of therapy.
For many years, aspirin alone was the drug of choice for secondary prevention of noncardioembolic ischemic stroke.7 Efficacy is similar at dosages anywhere between 50 and 1500 mg/d; higher doses incur a greater risk for gastrointestinal hemorrhage.7 Current secondary prevention guidelines recommend a dosage of aspirin somewhere between 50 and 325 mg/d.7
Alternative agents have also been evaluated for secondary stroke prevention, but only clopidogrel is currently considered an acceptable alternative for monotherapy based on a subgroup analysis of the CAPRIE (Clopidogrel versus Aspirin in Patients at Risk of Ischaemic Events) trial.7,8 Other alternatives, including cilostazol, ticlopidine, and ticagrelor, are limited by a lack of data, adverse drug reactions, or unproven efficacy and are not recommended in current guidelines.7,9 The ongoing THALES (Acute Stroke or Transient Ischaemic Attack Treated with Ticagrelor and Aspirin for Prevention of Stroke and Death) trial, assessing combination ticagrelor and aspirin, may identify an additional option for antiplatelet therapy following acute stroke.10
The current guidelines from the American Heart Association/American Stroke Association (AHA/ASA) support the combination of aspirin and extended-release dipyridamole (ASA-ERDP) as a long-term alternative to aspirin monotherapy.7,11 Additionally, the combination of clopidogrel and aspirin (CLO-ASA) is now recommended for limited duration in the early management of ischemic stroke.11
This review will explore the role of dual antiplatelet therapy for secondary prevention of noncardioembolic ischemic stroke or transient ischemic attack (TIA), with particular focus on acute use of CLO-ASA.
Clopidogrel and aspirin: When to initiate, when to stop
The combined use of clopidogrel and aspirin has been well-studied for secondary prevention of ischemic stroke and TIA. However, interpreting and applying the results of these trials can be challenging given key differences in both time to treatment initiation and the duration of combination therapy. Highlights of the major randomized controlled trials (RCTs) evaluating the safety and efficacy of CLO-ASA are detailed in TABLE 1.4,5,12-15
Initial trials evaluating CLO-ASA for secondary stroke prevention, including the MATCH (Management of ATherothrombosis with Clopidogrel in High-risk patients),12 SPS3 (Secondary Prevention of Small Subcortical Strokes),13 and CHARISMA (Clopidogrel for High Atherothrombotic Risk and Ischaemic Stabilization, Management and Avoidance)14 trials assessed the long-term benefits of combination therapy, with most patients initiating treatment a month or more following an initial stroke and continuing therapy for at least 18 months.12-14 Results from these trials indicate that long-term use (> 18 months) of CLO-ASA does not reduce recurrent events but increases rates of clinically significant bleeding.12-14
Continue to: A look at Tx timing
A look at Tx timing. Since these initial attempts failed to show a long-term benefit with CLO-ASA, subsequent trials attempted to establish an appropriate balance between the optimal time to initiate CLO-ASA and the optimal duration of therapy. The FASTER (Fast Assessment of Stroke and Transient ischaemic attack to prevent Early Recurrence) trial was a small pilot study of 392 patients randomized to CLO-ASA or aspirin within 24 hours of stroke or TIA onset and continued for only 3 months.15 While this trial did not find a significant reduction in ischemic or hemorrhagic stroke with combination therapy, there was a large numerical difference in event rates between the 2 groups (7.1% CLO-ASA vs 10.8% aspirin).15 An underpowered sample size (due to difficulty recruiting participants) is likely responsible for the lack of statistical significance.15 Despite the trial’s failure to show a benefit with acute use of CLO-ASA, it suggested a possible benefit that led to further investigation in the CHANCE (Clopidogrel in High-risk patients with Acute Non-disabling Cerebrovascular Events)5 and POINT (Platelet-Oriented Inhibition in New TIA and Minor Ischemic Stroke) 4 trials.
The CHANCE trial conducted in China included more than 5000 patients with acute minor ischemic stroke (National Institutes of Health Stroke Scale [NIHSS] score ≤ 3) or high-risk TIA (ABCD2 [a scale that assesses the risk of stroke on the basis of age, blood pressure, clinical features, duration of TIA, and presence or absence of diabetes] score ≥ 4).5 Similar to FASTER, patients were randomized within 24 hours of symptom onset to CLO-ASA or aspirin. However, CHANCE utilized combination therapy for only 21 days, after which the patients were continued on clopidogrel monotherapy for up to 90 days; the aspirin monotherapy group continued aspirin for 90 days.
After 90 days, patients initially using combination therapy had significantly lower rates of ischemic or hemorrhagic stroke vs those assigned to aspirin monotherapy. This result was driven heavily by the reduction in ischemic stroke (7.9% CLO-ASA vs 11.4% aspirin; P < .001). Additionally, there was no significant difference in moderate or severe bleeding events between the 2 groups.5 Efficacy and safety results were similar among a subgroup of patients who were randomized to treatment within 12 hours rather than 24 hours from symptom onset.16 The CHANCE trial was the first major study to demonstrate a clinical benefit of CLO-ASA to prevent recurrent stroke. Accordingly, the 2018 AHA/ASA guidelines included a new recommendation regarding secondary prevention for the use of CLO-ASA initiated within 24 hours and continued for 21 days following a minor stroke or TIA.11
A drawback of the CHANCE trial was its narrow patient population of only Chinese patients, which may limit applicability in clinical practice. There are known genetic variations in cytochrome P450 2C19 (CYP2C19) that may affect clopidogrel metabolism. CYP2C19 is responsible for the conversion of clopidogrel into its activated form in vivo. Carriers of a CYP2C19 loss-of-function allele may have reduced clopidogrel activation and subsequent reduced antiplatelet activity. Such loss-of-function alleles are more common in Asian populations vs non-Asian populations.17
A substudy of CHANCE found that CLO-ASA’s efficacy benefit was preserved in noncarrier patients; however, patients with the CYP2C19 loss-of-function allele did not benefit from combination therapy.18 Interestingly, these genetic differences did not affect bleeding outcomes. Given that approximately 60% of patients in the CHANCE substudy were loss-of-function allele carriers and that the overall study results still showed benefit with combination therapy, application of CHANCE’s findings to broader populations may not be a concern after all.18
Continue to: In efforts to gain insight...
In efforts to gain insight on CLO-ASA’s use in a more diverse patient population, the POINT trial included almost 5000 patients, with 82% from the United States, who were randomized within 12 hours of symptom onset to CLO-ASA or aspirin monotherapy for 90 days.4 Similar to the CHANCE study, the POINT study included patients with mild ischemic strokes (NIHSS ≤ 3) or high-risk TIA (ABCD2 ≥ 4). Combination therapy significantly reduced the primary endpoint of ischemic stroke, myocardial infarction (MI), or death from an ischemic event. Contrary to CHANCE, there was a significant increase in major bleeding in those assigned to combination therapy, which resulted in the trial being stopped early.4
A closer look at safety differences. CHANCE and POINT were the first major trials to show a benefit of CLO-ASA for secondary prevention of stroke, yet their differences in safety outcomes, specifically major hemorrhage, argued for a deeper reconciliation of their results.4,5 While both trials initiated secondary prevention within 24 hours of symptom onset, the difference in duration of combination therapy (21 days in CHANCE vs 90 days in POINT) likely impacted the rates of hemorrhage. When results from POINT were stratified by time period, particularly within the first 30 days of therapy (similar to the 21-day treatment duration of CHANCE), combination therapy significantly reduced the primary endpoint of ischemic stroke, MI, or death from an ischemic event (3.9% CLO-ASA vs 5.8% aspirin; P = .02) without an increased risk for major hemorrhage. Between 30 and 90 days, this efficacy benefit disappeared. However, bleeding rates between groups continued to separate throughout the 90-day course. In this light, the 30-day outcomes of POINT are largely similar to CHANCE and support the short-term use of CLO-ASA for secondary prevention without an associated increase in major bleeding.4,5
Antiplatelet dosing in POINT and CHANCE may also play a role in the contrasting safety results between the trials.4,5 While both studies utilized clopidogrel loading doses, POINT used 600 mg while CHANCE used 300 mg. Clopidogrel maintenance dosing was the same at 75 mg/d. In CHANCE, aspirin dosing was protocolized to 75 mg/d; however, in POINT, 31% of patients used > 100 mg/d aspirin.4,5 It is possible that the higher doses of both aspirin and clopidogrel in the POINT trial contributed to the difference in the occurrence of major hemorrhage between the treatment groups in these trials.
The takeaway. Based on currently available data, patients who are best suited to benefit from CLO-ASA are those who have had minor noncardioembolic ischemic strokes or high-risk TIAs.4,5,11 Clopidogrel should be given as a 300-mg loading dose followed by 75 mg/d given concomitantly with aspirin at a dose no higher than 100 mg/d. CLO-ASA therapy should be initiated within 24 hours of symptom onset and be continued for no longer than 1 month, after which chronic preventive therapy with either aspirin or clopidogrel monotherapy should be started.4,5,11
Dipyridamole and aspirin: A controversial option
Since the approval of the combination product ASA-ERDP, there has been considerable controversy about using this combination over other therapies, such as aspirin or clopidogrel, for recurrent ischemic stroke prevention. Much of this controversy arises from limitations in the trial designs.
Continue to: The first trial to show benefit...
The first trial to show benefit with ASA-ERDP was ESPS2 (European Stroke Prevention Study 2), which demonstrated superiority of the combination over placebo in reducing recurrent stroke when treatment was added within 3 months of an index stroke.19 A few studies have evaluated ASA-ERDP compared to aspirin monotherapy; however, most of these studies were small and did not show any difference in outcomes.20 Only ESPRIT (European/Australasian Stroke Prevention in Reversible Ischaemia Trial)21 carried significant weight in a 2013 meta-analysis, which showed a significant reduction in recurrent events with the combination product compared to aspirin monotherapy.20
Both the ESPS2 and ESPRIT trials had significant limitations.19,21 Patients in both studies had vascular comorbidities including atherosclerotic cardiovascular disease (ASCVD); however, pharmacotherapies designated to treat these diseases were not mentioned in the demographic data, nor were these medications taken into consideration to limit potential bias.19,21 Retrospectively, a significant proportion of aspirin doses utilized as a control in ESPRIT were inferior to the guideline-recommended dosing with 42% to 46% of patients receiving 30 mg/d.21 Despite these controversies, ASA-ERDP is still considered an alternative to aspirin monotherapy in the guidelines.7
The timing of ASA-ERDP initiation appears to be inversely related to the efficacy of the combination over therapeutic alternatives. Studies in which the therapy was initiated 3 to 6 months from the index stroke indicated favorable outcomes for the combination when compared to ASA or ERDP monotherapy.19,21 Studies utilizing early initiation (ie, within 24 or 48 hours of the index event) or even within 3 weeks showed no difference in outcomes; however, this may be due in part to the use of clopidogrel or other combination antiplatelet therapy as active comparators.22-24
Early initiation of ASA-ERDP also demonstrated a higher risk of major and intracranial bleeding compared to clopidogrel.22 Additionally, use of triple therapy with ASA-ERDP plus clopidogrel increased bleeding events without improving efficacy.24 More recent studies of ASA-ERDP are focusing on earlier initiation of therapy; it is unknown whether the benefits of late initiation will be confirmed in future studies. Highlights of the major RCTs evaluating the safety and efficacy of ASA-ERDP are detailed in TABLE 219,21-24.
The takeaway. Methodological issues and potential confounding factors in many of the key trials for ASA-ERDP make it challenging to fully discern the role that ASA-ERDP may play in the secondary prevention of stroke. Further evidence utilizing appropriate controls, timing, and assessment of confounders is needed. Additionally, ASA-ERDP is plagued by tolerability issues such as headache, nausea, and vomiting, leading to higher rates of discontinuation than its comparators in clinical trials. Accordingly, the maintenance use of ASA-ERDP for secondary stroke prevention may be considered less preferred than other recommended alternatives such as aspirin or clopidogrel monotherapies.
CORRESPONDENCE
Robert S. Helmer, PharmD, BCPS, Department of Pharmacy Practice, Auburn University Harrison School of Pharmacy, 650 Clinic Drive, Suite 2100, Mobile, AL 36688; Rsh0011@auburn.edu.
1. CDC. Stroke Facts. Last updated January 31, 2020. www.cdc.gov/stroke/facts.htm. Accessed June 29, 2020.
2. Amarenco P, Lavallee PC, Labreuche J, et al. One-year risk of stroke after transient ischemic attack or minor stroke. N Engl J Med. 2016;374:1533-1542.
3. Amarenco P, Lavallee PC, Monteiro Tavares L, et al. Five-year risk of stroke after TIA or minor ischemic stroke. N Engl J Med. 2018;378:2182-2190.
4. Johnston SC, Easton JD, Farrant M, et al. Clopidogrel and aspirin in acute ischemic stroke and high-risk TIA. N Engl J Med. 2018;379:215-225.
5. Wang Y, Wang Y, Zhao X, et al. Clopidogrel with aspirin in acute minor stroke or transient ischemic attack. N Engl J Med. 2013;369:11-19.
6. Bowry AD, Brookhart MA, Choudhry NK. Meta-analysis of the efficacy and safety of clopidogrel plus aspirin as compared to antiplatelet monotherapy for the prevention of vascular events. Am J Cardiol. 2008;101:960-966.
7. Kernan WN, Ovbiagele B, Black HR, et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45:2160-2236.
8. Gent M, Beaumont D, Blanchard J, et al. A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). Lancet. 1996;348:1329-1339.
9. Lansberg MG, O’Donnell MJ, Khatri P, et al. Antithrombotic and thrombolytic therapy for ischemic stroke: Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e601S-e636S.
10. Johnston SC, Amarenco P, Denison H, et al. The acute stroke or transient ischemic attack treated with ticagrelor and aspirin for prevention of stroke and death (THALES) trial: rationale and design. Int J Stroke. 2019;14:745‐751.
11. Powers WJ, Rabinstein AA, Ackerson T, et al. 2018 Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2018;49:e46-e110.
12. Diener HC, Bogousslavsky J, Brass LM, et al. Aspirin and clopidogrel compared with clopidogrel alone after recent ischaemic stroke or transient ischaemic attack in high-risk patients (MATCH): randomised, double-blind, placebo-controlled trial. Lancet. 2004;364:331-337.
13. Benavente OR, Hart RG, McClure LA, et al. Effects of clopidogrel added to aspirin in patients with recent lacunar stroke. N Engl J Med. 2012;367:817-825.
14. Hankey GJ, Johnston SC, Easton JD, et al. Effect of clopidogrel plus ASA vs. ASA early after TIA and ischaemic stroke: a substudy of the CHARISMA trial. Int J Stroke. 2011;6:3-9.
15. Kennedy J, Hill MD, Ryckborst KJ, et al. Fast assessment of stroke and transient ischaemic attack to prevent early recurrence (FASTER): a randomised controlled pilot trial. Lancet Neurol. 2007;6:961-969.
16. Li Z, Wang Y, Zhao X, et al. Treatment effect of clopidogrel plus aspirin within 12 hours of acute minor stroke or transient ischemic attack. J Am Heart Assoc. 2016;5:e003038.
17. Scott SA, Sangkuhl K, Stein CM, et al. Clinical pharmacogenetics implementation consortium guidelines for CYP2C19 genotype and clopidogrel therapy: 2013 update. Clin Pharmacol Ther. 2013;94:317-323.
18. Wang Y, Zhao X, Lin J, et al. Association between CYP2C19 loss-of-function allele status and efficacy of clopidogrel for risk reduction among patients with minor stroke or transient ischemic attack. JAMA. 2016;316:70-78.
19. Diener HC, Cunha L, Forbes C, et al. European Stroke Prevention Study 2. Dipyridamole and acetylsalicylic acid in the secondary prevention of stroke. J Neurol Sci. 1996;143:1-13.
20. Li X, Zhou G, Zhou X, et al. The efficacy and safety of aspirin plus dipyridamole versus aspirin in secondary prevention following TIA or stroke: a meta-analysis of randomized controlled trials. J Neurol Sci. 2013;332:92-96.
21. Halkes PH, van Gijn J, Kapelle IJ, et al. Aspirin plus dipyridamole versus aspirin alone after cerebral ischaemia of arterial origin (ESPRIT): randomised controlled trial. Lancet. 2006;367:1665-1673.
22. Sacco RL, Diener HC, Yusuf S, et al. Aspirin and extended-release dipyridamole versus clopidogrel for recurrent stroke. N Engl J Med. 2008;359:1238-1251.
23. Dengler R, Diener HC, Schwartz A, et al. Early treatment with aspirin plus extended-release dipyridamole for transient ischaemic attack or ischaemic stroke within 24 h of symptom onset (EARLY trial): a randomised, open-label, blinded-endpoint trial. Lancet Neurol. 2010;9:159-166.
24. Bath PM, Woodhouse LJ, Appleton JP, et al. Antiplatelet therapy with aspirin, clopidogrel, and dipyridamole versus clopidogrel alone or aspirin and dipyridamole in patients with acute cerebral ischaemia (TARDIS): a randomised, open-label, phase 3 superiority trial. Lancet. 2018;391:850-859.
1. CDC. Stroke Facts. Last updated January 31, 2020. www.cdc.gov/stroke/facts.htm. Accessed June 29, 2020.
2. Amarenco P, Lavallee PC, Labreuche J, et al. One-year risk of stroke after transient ischemic attack or minor stroke. N Engl J Med. 2016;374:1533-1542.
3. Amarenco P, Lavallee PC, Monteiro Tavares L, et al. Five-year risk of stroke after TIA or minor ischemic stroke. N Engl J Med. 2018;378:2182-2190.
4. Johnston SC, Easton JD, Farrant M, et al. Clopidogrel and aspirin in acute ischemic stroke and high-risk TIA. N Engl J Med. 2018;379:215-225.
5. Wang Y, Wang Y, Zhao X, et al. Clopidogrel with aspirin in acute minor stroke or transient ischemic attack. N Engl J Med. 2013;369:11-19.
6. Bowry AD, Brookhart MA, Choudhry NK. Meta-analysis of the efficacy and safety of clopidogrel plus aspirin as compared to antiplatelet monotherapy for the prevention of vascular events. Am J Cardiol. 2008;101:960-966.
7. Kernan WN, Ovbiagele B, Black HR, et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45:2160-2236.
8. Gent M, Beaumont D, Blanchard J, et al. A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). Lancet. 1996;348:1329-1339.
9. Lansberg MG, O’Donnell MJ, Khatri P, et al. Antithrombotic and thrombolytic therapy for ischemic stroke: Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e601S-e636S.
10. Johnston SC, Amarenco P, Denison H, et al. The acute stroke or transient ischemic attack treated with ticagrelor and aspirin for prevention of stroke and death (THALES) trial: rationale and design. Int J Stroke. 2019;14:745‐751.
11. Powers WJ, Rabinstein AA, Ackerson T, et al. 2018 Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2018;49:e46-e110.
12. Diener HC, Bogousslavsky J, Brass LM, et al. Aspirin and clopidogrel compared with clopidogrel alone after recent ischaemic stroke or transient ischaemic attack in high-risk patients (MATCH): randomised, double-blind, placebo-controlled trial. Lancet. 2004;364:331-337.
13. Benavente OR, Hart RG, McClure LA, et al. Effects of clopidogrel added to aspirin in patients with recent lacunar stroke. N Engl J Med. 2012;367:817-825.
14. Hankey GJ, Johnston SC, Easton JD, et al. Effect of clopidogrel plus ASA vs. ASA early after TIA and ischaemic stroke: a substudy of the CHARISMA trial. Int J Stroke. 2011;6:3-9.
15. Kennedy J, Hill MD, Ryckborst KJ, et al. Fast assessment of stroke and transient ischaemic attack to prevent early recurrence (FASTER): a randomised controlled pilot trial. Lancet Neurol. 2007;6:961-969.
16. Li Z, Wang Y, Zhao X, et al. Treatment effect of clopidogrel plus aspirin within 12 hours of acute minor stroke or transient ischemic attack. J Am Heart Assoc. 2016;5:e003038.
17. Scott SA, Sangkuhl K, Stein CM, et al. Clinical pharmacogenetics implementation consortium guidelines for CYP2C19 genotype and clopidogrel therapy: 2013 update. Clin Pharmacol Ther. 2013;94:317-323.
18. Wang Y, Zhao X, Lin J, et al. Association between CYP2C19 loss-of-function allele status and efficacy of clopidogrel for risk reduction among patients with minor stroke or transient ischemic attack. JAMA. 2016;316:70-78.
19. Diener HC, Cunha L, Forbes C, et al. European Stroke Prevention Study 2. Dipyridamole and acetylsalicylic acid in the secondary prevention of stroke. J Neurol Sci. 1996;143:1-13.
20. Li X, Zhou G, Zhou X, et al. The efficacy and safety of aspirin plus dipyridamole versus aspirin in secondary prevention following TIA or stroke: a meta-analysis of randomized controlled trials. J Neurol Sci. 2013;332:92-96.
21. Halkes PH, van Gijn J, Kapelle IJ, et al. Aspirin plus dipyridamole versus aspirin alone after cerebral ischaemia of arterial origin (ESPRIT): randomised controlled trial. Lancet. 2006;367:1665-1673.
22. Sacco RL, Diener HC, Yusuf S, et al. Aspirin and extended-release dipyridamole versus clopidogrel for recurrent stroke. N Engl J Med. 2008;359:1238-1251.
23. Dengler R, Diener HC, Schwartz A, et al. Early treatment with aspirin plus extended-release dipyridamole for transient ischaemic attack or ischaemic stroke within 24 h of symptom onset (EARLY trial): a randomised, open-label, blinded-endpoint trial. Lancet Neurol. 2010;9:159-166.
24. Bath PM, Woodhouse LJ, Appleton JP, et al. Antiplatelet therapy with aspirin, clopidogrel, and dipyridamole versus clopidogrel alone or aspirin and dipyridamole in patients with acute cerebral ischaemia (TARDIS): a randomised, open-label, phase 3 superiority trial. Lancet. 2018;391:850-859.
PRACTICE RECOMMENDATIONS
› Initiate combined clopidogrel plus aspirin within 24 hours of a minor stroke or TIA and continue for no longer than 1 month; then switch patients to aspirin or clopidogrel monotherapy. A
› Do not use combined clopidogrel plus aspirin for long-term secondary stroke prevention. A
› Limit use of aspirin plus extended-release dipyridamole as a first choice for secondary stroke prevention because of limitations in efficacy and poor tolerability. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Part 4: Monitoring for CKD in Diabetes Patients
Previously, we discussed assessment and treatment for dyslipidemia in patients with diabetes. Now we’ll explore how to monitor for kidney disease in this population.
CASE CONTINUED
Mr. W’s basic metabolic panel includes an estimated glomerular filtration rate (eGFR) of 55 ml/min/1.73 m2 (reference range, > 60 ml/min/1.73 m2). In the absence of any other markers of kidney disease, you obtain a spot urinary albumin-to-creatinine ratio (UACR). The UACR results show a ratio of 64 mg/g, confirming stage 3 chronic kidney disease (CKD).
Monitoring for Chronic Kidney Disease
CKD is characterized by persistent albuminuria, low eGFR, and manifestations of kidney damage, and it increases cardiovascular risk.2 According to the ADA, clinicians should obtain a UACR and eGFR at least annually in patients who have had type 1 diabetes for at least 5 years and in all patients with type 2 diabetes.2 Monitoring is needed twice a year for those who begin to show signs of albuminuria or a reduced eGFR. This helps define the presence or stage of CKD and allows for further treatment planning.
Notably, patients with an eGFR < 30 ml/min/1.73m2, an unclear cause of kidney disease, or signs of rapidly progressive disease (eg, decline in GFR category plus ≥ 25% decline in eGFR from baseline) should be seen by nephrology for further evaluation and treatment recommendations.2,36
Diabetes medications for kidney health. Sodium-glucose cotransporter-2 (SGLT2) inhibitors and glucagon-like peptide 1 (GLP-1) receptor agonists may be good candidates to promote kidney health in patients such as Mr. W. Recent trials show that SGLT2 inhibitors reduce the risk for progressive diabetic kidney disease, and the ADA recommends these medications for patients with CKD.2,16,36 GLP-1 receptor agonists also may be associated with a lower rate of development and progression of diabetic kidney disease, but this effect appears to be less robust.7,15,16 ADA guidelines recommend SGLT2 inhibitors for patients whose eGFR is adequate.37
ADA and AACE guidelines offer specific treatment recommendations on the use of SGLT2 inhibitors and GLP-1 receptor agonists in the management of diabetes.10,37 Note that neither SGLT2 inhibitors nor GLP-1 agonists are strictly under the purview of endocrinologists. Rather, multiple guidelines state that they can be utilized safely by a variety of practitioners.6,38,39
In the concluding part of this series, we will explore how to screen for peripheral neuropathy and diabetic retinopathy—identification of which can improve the patient’s quality of life.
1. Centers for Disease Control and Prevention. Diabetes incidence and prevalence. Diabetes Report Card 2017. www.cdc.gov/diabetes/library/reports/reportcard/incidence-2017.html. Published 2018. Accessed June 18, 2020.
2. Standards of Medical Care in Diabetes—2020 Abridged for Primary Care Providers. American Diabetes Association Clinical Diabetes. 2020;38(1):10-38.
3. Chen Y, Sloan FA, Yashkin AP. Adherence to diabetes guidelines for screening, physical activity and medication and onset of complications and death. J Diabetes Complications. 2015;29(8):1228-1233.
4. Mehta S, Mocarski M, Wisniewski T, et al. Primary care physicians’ utilization of type 2 diabetes screening guidelines and referrals to behavioral interventions: a survey-linked retrospective study. BMJ Open Diabetes Res Care. 2017;5(1):e000406.
5. Center for Disease Control and Prevention. Preventive care practices. Diabetes Report Card 2017. www.cdc.gov/diabetes/library/reports/reportcard/preventive-care.html. Published 2018. Accessed June 18, 2020.
6. Arnold SV, de Lemos JA, Rosenson RS, et al; GOULD Investigators. Use of guideline-recommended risk reduction strategies among patients with diabetes and atherosclerotic cardiovascular disease. Circulation. 2019;140(7):618-620.
7. Garber AJ, Handelsman Y, Grunberger G, et al. Consensus Statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm—2020 executive summary. Endocr Pract Endocr Pract. 2020;26(1):107-139.
8. American Diabetes Association. Comprehensive medical evaluation and assessment of comorbidities: standards of medical care in diabetes—2020. Diabetes Care. 2020;43(suppl 1):S37-S47.
9. Beck J, Greenwood DA, Blanton L, et al; 2017 Standards Revision Task Force. 2017 National Standards for diabetes self-management education and support. Diabetes Educ. 2017;43(5): 449-464.
10. Chrvala CA, Sherr D, Lipman RD. Diabetes self-management education for adults with type 2 diabetes mellitus: a systematic review of the effect on glycemic control. Patient Educ Couns. 2016;99(6):926-943.
11. Association of Diabetes Care & Education Specialists. Find a diabetes education program in your area. www.diabeteseducator.org/living-with-diabetes/find-an-education-program. Accessed June 15, 2020.
12. Estruch R, Ros E, Salas-Salvadó J, et al; PREDIMED Study Investigators. Primary prevention of cardiovascular disease with a Mediterranean diet supplemented with extra-virgin olive oil or nuts. NEJM. 2018;378(25):e34.
13. Centers for Disease Control and Prevention. Tips for better sleep. Sleep and sleep disorders. www.cdc.gov/sleep/about_sleep/sleep_hygiene.html. Reviewed July 15, 2016. Accessed June 18, 2020.
14. Doumit J, Prasad B. Sleep Apnea in Type 2 Diabetes. Diabetes Spectrum. 2016; 29(1): 14-19.
15. Marso SP, Daniels GH, Brown-Frandsen K, et al; LEADER Steering Committee on behalf of the LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311-322.
16. Perkovic V, Jardine MJ, Neal B, et al; CREDENCE Trial Investigators. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380(24):2295-2306.
17. Trends in Blood pressure control and treatment among type 2 diabetes with comorbid hypertension in the United States: 1988-2004. J Hypertens. 2009;27(9):1908-1916.
18. Emdin CA, Rahimi K, Neal B, et al. Blood pressure lowering in type 2 diabetes: a systematic review and meta-analysis. JAMA. 2015;313(6):603-615.
19. Vouri SM, Shaw RF, Waterbury NV, et al. Prevalence of achievement of A1c, blood pressure, and cholesterol (ABC) goal in veterans with diabetes. J Manag Care Pharm. 2011;17(4):304-312.
20. Kudo N, Yokokawa H, Fukuda H, et al. Achievement of target blood pressure levels among Japanese workers with hypertension and healthy lifestyle characteristics associated with therapeutic failure. Plos One. 2015;10(7):e0133641.
21. Carey RM, Whelton PK; 2017 ACC/AHA Hypertension Guideline Writing Committee. Prevention, detection, evaluation, and management of high blood pressure in adults: synopsis of the 2017 American College of Cardiology/American Heart Association Hypertension guideline. Ann Intern Med. 2018;168(5):351-358.
22. Deedwania PC. Blood pressure control in diabetes mellitus. Circulation. 2011;123:2776–2778.
23. Catalá-López F, Saint-Gerons DM, González-Bermejo D, et al. Cardiovascular and renal outcomes of renin-angiotensin system blockade in adult patients with diabetes mellitus: a systematic review with network meta-analyses. PLoS Med. 2016;13(3):e1001971.
24. Furberg CD, Wright JT Jr, Davis BR, et al; ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA. 2002;288(23):2981-2997.
25. Sleight P. The HOPE Study (Heart Outcomes Prevention Evaluation). J Renin-Angiotensin-Aldosterone Syst. 2000;1(1):18-20.
26. Tatti P, Pahor M, Byington RP, et al. Outcome results of the Fosinopril Versus Amlodipine Cardiovascular Events Randomized Trial (FACET) in patients with hypertension and NIDDM. Diabetes Care. 1998;21(4):597-603.
27. Schrier RW, Estacio RO, Jeffers B. Appropriate Blood Pressure Control in NIDDM (ABCD) Trial. Diabetologia. 1996;39(12):1646-1654.
28. Hansson L, Zanchetti A, Carruthers SG, et al; HOT Study Group. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) Randomised Trial. Lancet. 1998;351(9118):1755-1762.
29. Baigent C, Blackwell L, Emberson J, et al; Cholesterol Treatment Trialists’ (CTT) Collaboration. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376(9753):1670-1681.
30. Fu AZ, Zhang Q, Davies MJ, et al. Underutilization of statins in patients with type 2 diabetes in US clinical practice: a retrospective cohort study. Curr Med Res Opin. 2011;27(5):1035-1040.
31. Cannon CP, Blazing MA, Giugliano RP, et al; IMPROVE-IT Investigators. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015; 372:2387-2397
32. Sabatine MS, Giugliano RP, Keech AC, et al; the FOURIER Steering Committee and Investigators. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713-1722.
33. Schwartz GG, Steg PG, Szarek M, et al; ODYSSEY OUTCOMES Committees and Investigators. Alirocumab and Cardiovascular Outcomes after Acute Coronary Syndrome | NEJM. N Engl J Med. 2018;379:2097-2107.
34. Icosapent ethyl [package insert]. Bridgewater, NJ: Amarin Pharma, Inc.; 2019.
35. Bhatt DL, Steg PG, Miller M, et al; REDUCE-IT Investigators. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med. 2019;380:11-22
36. Bolton WK. Renal Physicians Association Clinical practice guideline: appropriate patient preparation for renal replacement therapy: guideline number 3. J Am Soc Nephrol. 2003;14(5):1406-1410.
37. American Diabetes Association. Pharmacologic Approaches to glycemic treatment: standards of medical care in diabetes—2020. Diabetes Care. 2020;43(suppl 1):S98-S110.
38. Qaseem A, Barry MJ, Humphrey LL, Forciea MA; Clinical Guidelines Committee of the American College of Physicians. Oral pharmacologic treatment of type 2 diabetes mellitus: a clinical practice guideline update from the American College of Physicians. Ann Intern Med. 2017;166(4):279-290.
39. Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Update Work Group. KDIGO 2017 Clinical Practice Guideline Update for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease–mineral and bone disorder (CKD-MBD). Kidney Int Suppl (2011). 2017;7(1):1-59.
40. Pop-Busui R, Boulton AJM, Feldman EL, et al. Diabetic neuropathy: a position statement by the American Diabetes Association. Diabetes Care. 2017;40(1):136-154.
41. Gupta V, Bansal R, Gupta A, Bhansali A. The sensitivity and specificity of nonmydriatic digital stereoscopic retinal imaging in detecting diabetic retinopathy. Indian J Ophthalmol. 2014;62(8):851-856.
42. Pérez MA, Bruce BB, Newman NJ, Biousse V. The use of retinal photography in non-ophthalmic settings and its potential for neurology. The Neurologist. 2012;18(6):350-355.
Previously, we discussed assessment and treatment for dyslipidemia in patients with diabetes. Now we’ll explore how to monitor for kidney disease in this population.
CASE CONTINUED
Mr. W’s basic metabolic panel includes an estimated glomerular filtration rate (eGFR) of 55 ml/min/1.73 m2 (reference range, > 60 ml/min/1.73 m2). In the absence of any other markers of kidney disease, you obtain a spot urinary albumin-to-creatinine ratio (UACR). The UACR results show a ratio of 64 mg/g, confirming stage 3 chronic kidney disease (CKD).
Monitoring for Chronic Kidney Disease
CKD is characterized by persistent albuminuria, low eGFR, and manifestations of kidney damage, and it increases cardiovascular risk.2 According to the ADA, clinicians should obtain a UACR and eGFR at least annually in patients who have had type 1 diabetes for at least 5 years and in all patients with type 2 diabetes.2 Monitoring is needed twice a year for those who begin to show signs of albuminuria or a reduced eGFR. This helps define the presence or stage of CKD and allows for further treatment planning.
Notably, patients with an eGFR < 30 ml/min/1.73m2, an unclear cause of kidney disease, or signs of rapidly progressive disease (eg, decline in GFR category plus ≥ 25% decline in eGFR from baseline) should be seen by nephrology for further evaluation and treatment recommendations.2,36
Diabetes medications for kidney health. Sodium-glucose cotransporter-2 (SGLT2) inhibitors and glucagon-like peptide 1 (GLP-1) receptor agonists may be good candidates to promote kidney health in patients such as Mr. W. Recent trials show that SGLT2 inhibitors reduce the risk for progressive diabetic kidney disease, and the ADA recommends these medications for patients with CKD.2,16,36 GLP-1 receptor agonists also may be associated with a lower rate of development and progression of diabetic kidney disease, but this effect appears to be less robust.7,15,16 ADA guidelines recommend SGLT2 inhibitors for patients whose eGFR is adequate.37
ADA and AACE guidelines offer specific treatment recommendations on the use of SGLT2 inhibitors and GLP-1 receptor agonists in the management of diabetes.10,37 Note that neither SGLT2 inhibitors nor GLP-1 agonists are strictly under the purview of endocrinologists. Rather, multiple guidelines state that they can be utilized safely by a variety of practitioners.6,38,39
In the concluding part of this series, we will explore how to screen for peripheral neuropathy and diabetic retinopathy—identification of which can improve the patient’s quality of life.
Previously, we discussed assessment and treatment for dyslipidemia in patients with diabetes. Now we’ll explore how to monitor for kidney disease in this population.
CASE CONTINUED
Mr. W’s basic metabolic panel includes an estimated glomerular filtration rate (eGFR) of 55 ml/min/1.73 m2 (reference range, > 60 ml/min/1.73 m2). In the absence of any other markers of kidney disease, you obtain a spot urinary albumin-to-creatinine ratio (UACR). The UACR results show a ratio of 64 mg/g, confirming stage 3 chronic kidney disease (CKD).
Monitoring for Chronic Kidney Disease
CKD is characterized by persistent albuminuria, low eGFR, and manifestations of kidney damage, and it increases cardiovascular risk.2 According to the ADA, clinicians should obtain a UACR and eGFR at least annually in patients who have had type 1 diabetes for at least 5 years and in all patients with type 2 diabetes.2 Monitoring is needed twice a year for those who begin to show signs of albuminuria or a reduced eGFR. This helps define the presence or stage of CKD and allows for further treatment planning.
Notably, patients with an eGFR < 30 ml/min/1.73m2, an unclear cause of kidney disease, or signs of rapidly progressive disease (eg, decline in GFR category plus ≥ 25% decline in eGFR from baseline) should be seen by nephrology for further evaluation and treatment recommendations.2,36
Diabetes medications for kidney health. Sodium-glucose cotransporter-2 (SGLT2) inhibitors and glucagon-like peptide 1 (GLP-1) receptor agonists may be good candidates to promote kidney health in patients such as Mr. W. Recent trials show that SGLT2 inhibitors reduce the risk for progressive diabetic kidney disease, and the ADA recommends these medications for patients with CKD.2,16,36 GLP-1 receptor agonists also may be associated with a lower rate of development and progression of diabetic kidney disease, but this effect appears to be less robust.7,15,16 ADA guidelines recommend SGLT2 inhibitors for patients whose eGFR is adequate.37
ADA and AACE guidelines offer specific treatment recommendations on the use of SGLT2 inhibitors and GLP-1 receptor agonists in the management of diabetes.10,37 Note that neither SGLT2 inhibitors nor GLP-1 agonists are strictly under the purview of endocrinologists. Rather, multiple guidelines state that they can be utilized safely by a variety of practitioners.6,38,39
In the concluding part of this series, we will explore how to screen for peripheral neuropathy and diabetic retinopathy—identification of which can improve the patient’s quality of life.
1. Centers for Disease Control and Prevention. Diabetes incidence and prevalence. Diabetes Report Card 2017. www.cdc.gov/diabetes/library/reports/reportcard/incidence-2017.html. Published 2018. Accessed June 18, 2020.
2. Standards of Medical Care in Diabetes—2020 Abridged for Primary Care Providers. American Diabetes Association Clinical Diabetes. 2020;38(1):10-38.
3. Chen Y, Sloan FA, Yashkin AP. Adherence to diabetes guidelines for screening, physical activity and medication and onset of complications and death. J Diabetes Complications. 2015;29(8):1228-1233.
4. Mehta S, Mocarski M, Wisniewski T, et al. Primary care physicians’ utilization of type 2 diabetes screening guidelines and referrals to behavioral interventions: a survey-linked retrospective study. BMJ Open Diabetes Res Care. 2017;5(1):e000406.
5. Center for Disease Control and Prevention. Preventive care practices. Diabetes Report Card 2017. www.cdc.gov/diabetes/library/reports/reportcard/preventive-care.html. Published 2018. Accessed June 18, 2020.
6. Arnold SV, de Lemos JA, Rosenson RS, et al; GOULD Investigators. Use of guideline-recommended risk reduction strategies among patients with diabetes and atherosclerotic cardiovascular disease. Circulation. 2019;140(7):618-620.
7. Garber AJ, Handelsman Y, Grunberger G, et al. Consensus Statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm—2020 executive summary. Endocr Pract Endocr Pract. 2020;26(1):107-139.
8. American Diabetes Association. Comprehensive medical evaluation and assessment of comorbidities: standards of medical care in diabetes—2020. Diabetes Care. 2020;43(suppl 1):S37-S47.
9. Beck J, Greenwood DA, Blanton L, et al; 2017 Standards Revision Task Force. 2017 National Standards for diabetes self-management education and support. Diabetes Educ. 2017;43(5): 449-464.
10. Chrvala CA, Sherr D, Lipman RD. Diabetes self-management education for adults with type 2 diabetes mellitus: a systematic review of the effect on glycemic control. Patient Educ Couns. 2016;99(6):926-943.
11. Association of Diabetes Care & Education Specialists. Find a diabetes education program in your area. www.diabeteseducator.org/living-with-diabetes/find-an-education-program. Accessed June 15, 2020.
12. Estruch R, Ros E, Salas-Salvadó J, et al; PREDIMED Study Investigators. Primary prevention of cardiovascular disease with a Mediterranean diet supplemented with extra-virgin olive oil or nuts. NEJM. 2018;378(25):e34.
13. Centers for Disease Control and Prevention. Tips for better sleep. Sleep and sleep disorders. www.cdc.gov/sleep/about_sleep/sleep_hygiene.html. Reviewed July 15, 2016. Accessed June 18, 2020.
14. Doumit J, Prasad B. Sleep Apnea in Type 2 Diabetes. Diabetes Spectrum. 2016; 29(1): 14-19.
15. Marso SP, Daniels GH, Brown-Frandsen K, et al; LEADER Steering Committee on behalf of the LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311-322.
16. Perkovic V, Jardine MJ, Neal B, et al; CREDENCE Trial Investigators. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380(24):2295-2306.
17. Trends in Blood pressure control and treatment among type 2 diabetes with comorbid hypertension in the United States: 1988-2004. J Hypertens. 2009;27(9):1908-1916.
18. Emdin CA, Rahimi K, Neal B, et al. Blood pressure lowering in type 2 diabetes: a systematic review and meta-analysis. JAMA. 2015;313(6):603-615.
19. Vouri SM, Shaw RF, Waterbury NV, et al. Prevalence of achievement of A1c, blood pressure, and cholesterol (ABC) goal in veterans with diabetes. J Manag Care Pharm. 2011;17(4):304-312.
20. Kudo N, Yokokawa H, Fukuda H, et al. Achievement of target blood pressure levels among Japanese workers with hypertension and healthy lifestyle characteristics associated with therapeutic failure. Plos One. 2015;10(7):e0133641.
21. Carey RM, Whelton PK; 2017 ACC/AHA Hypertension Guideline Writing Committee. Prevention, detection, evaluation, and management of high blood pressure in adults: synopsis of the 2017 American College of Cardiology/American Heart Association Hypertension guideline. Ann Intern Med. 2018;168(5):351-358.
22. Deedwania PC. Blood pressure control in diabetes mellitus. Circulation. 2011;123:2776–2778.
23. Catalá-López F, Saint-Gerons DM, González-Bermejo D, et al. Cardiovascular and renal outcomes of renin-angiotensin system blockade in adult patients with diabetes mellitus: a systematic review with network meta-analyses. PLoS Med. 2016;13(3):e1001971.
24. Furberg CD, Wright JT Jr, Davis BR, et al; ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA. 2002;288(23):2981-2997.
25. Sleight P. The HOPE Study (Heart Outcomes Prevention Evaluation). J Renin-Angiotensin-Aldosterone Syst. 2000;1(1):18-20.
26. Tatti P, Pahor M, Byington RP, et al. Outcome results of the Fosinopril Versus Amlodipine Cardiovascular Events Randomized Trial (FACET) in patients with hypertension and NIDDM. Diabetes Care. 1998;21(4):597-603.
27. Schrier RW, Estacio RO, Jeffers B. Appropriate Blood Pressure Control in NIDDM (ABCD) Trial. Diabetologia. 1996;39(12):1646-1654.
28. Hansson L, Zanchetti A, Carruthers SG, et al; HOT Study Group. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) Randomised Trial. Lancet. 1998;351(9118):1755-1762.
29. Baigent C, Blackwell L, Emberson J, et al; Cholesterol Treatment Trialists’ (CTT) Collaboration. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376(9753):1670-1681.
30. Fu AZ, Zhang Q, Davies MJ, et al. Underutilization of statins in patients with type 2 diabetes in US clinical practice: a retrospective cohort study. Curr Med Res Opin. 2011;27(5):1035-1040.
31. Cannon CP, Blazing MA, Giugliano RP, et al; IMPROVE-IT Investigators. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015; 372:2387-2397
32. Sabatine MS, Giugliano RP, Keech AC, et al; the FOURIER Steering Committee and Investigators. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713-1722.
33. Schwartz GG, Steg PG, Szarek M, et al; ODYSSEY OUTCOMES Committees and Investigators. Alirocumab and Cardiovascular Outcomes after Acute Coronary Syndrome | NEJM. N Engl J Med. 2018;379:2097-2107.
34. Icosapent ethyl [package insert]. Bridgewater, NJ: Amarin Pharma, Inc.; 2019.
35. Bhatt DL, Steg PG, Miller M, et al; REDUCE-IT Investigators. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med. 2019;380:11-22
36. Bolton WK. Renal Physicians Association Clinical practice guideline: appropriate patient preparation for renal replacement therapy: guideline number 3. J Am Soc Nephrol. 2003;14(5):1406-1410.
37. American Diabetes Association. Pharmacologic Approaches to glycemic treatment: standards of medical care in diabetes—2020. Diabetes Care. 2020;43(suppl 1):S98-S110.
38. Qaseem A, Barry MJ, Humphrey LL, Forciea MA; Clinical Guidelines Committee of the American College of Physicians. Oral pharmacologic treatment of type 2 diabetes mellitus: a clinical practice guideline update from the American College of Physicians. Ann Intern Med. 2017;166(4):279-290.
39. Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Update Work Group. KDIGO 2017 Clinical Practice Guideline Update for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease–mineral and bone disorder (CKD-MBD). Kidney Int Suppl (2011). 2017;7(1):1-59.
40. Pop-Busui R, Boulton AJM, Feldman EL, et al. Diabetic neuropathy: a position statement by the American Diabetes Association. Diabetes Care. 2017;40(1):136-154.
41. Gupta V, Bansal R, Gupta A, Bhansali A. The sensitivity and specificity of nonmydriatic digital stereoscopic retinal imaging in detecting diabetic retinopathy. Indian J Ophthalmol. 2014;62(8):851-856.
42. Pérez MA, Bruce BB, Newman NJ, Biousse V. The use of retinal photography in non-ophthalmic settings and its potential for neurology. The Neurologist. 2012;18(6):350-355.
1. Centers for Disease Control and Prevention. Diabetes incidence and prevalence. Diabetes Report Card 2017. www.cdc.gov/diabetes/library/reports/reportcard/incidence-2017.html. Published 2018. Accessed June 18, 2020.
2. Standards of Medical Care in Diabetes—2020 Abridged for Primary Care Providers. American Diabetes Association Clinical Diabetes. 2020;38(1):10-38.
3. Chen Y, Sloan FA, Yashkin AP. Adherence to diabetes guidelines for screening, physical activity and medication and onset of complications and death. J Diabetes Complications. 2015;29(8):1228-1233.
4. Mehta S, Mocarski M, Wisniewski T, et al. Primary care physicians’ utilization of type 2 diabetes screening guidelines and referrals to behavioral interventions: a survey-linked retrospective study. BMJ Open Diabetes Res Care. 2017;5(1):e000406.
5. Center for Disease Control and Prevention. Preventive care practices. Diabetes Report Card 2017. www.cdc.gov/diabetes/library/reports/reportcard/preventive-care.html. Published 2018. Accessed June 18, 2020.
6. Arnold SV, de Lemos JA, Rosenson RS, et al; GOULD Investigators. Use of guideline-recommended risk reduction strategies among patients with diabetes and atherosclerotic cardiovascular disease. Circulation. 2019;140(7):618-620.
7. Garber AJ, Handelsman Y, Grunberger G, et al. Consensus Statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm—2020 executive summary. Endocr Pract Endocr Pract. 2020;26(1):107-139.
8. American Diabetes Association. Comprehensive medical evaluation and assessment of comorbidities: standards of medical care in diabetes—2020. Diabetes Care. 2020;43(suppl 1):S37-S47.
9. Beck J, Greenwood DA, Blanton L, et al; 2017 Standards Revision Task Force. 2017 National Standards for diabetes self-management education and support. Diabetes Educ. 2017;43(5): 449-464.
10. Chrvala CA, Sherr D, Lipman RD. Diabetes self-management education for adults with type 2 diabetes mellitus: a systematic review of the effect on glycemic control. Patient Educ Couns. 2016;99(6):926-943.
11. Association of Diabetes Care & Education Specialists. Find a diabetes education program in your area. www.diabeteseducator.org/living-with-diabetes/find-an-education-program. Accessed June 15, 2020.
12. Estruch R, Ros E, Salas-Salvadó J, et al; PREDIMED Study Investigators. Primary prevention of cardiovascular disease with a Mediterranean diet supplemented with extra-virgin olive oil or nuts. NEJM. 2018;378(25):e34.
13. Centers for Disease Control and Prevention. Tips for better sleep. Sleep and sleep disorders. www.cdc.gov/sleep/about_sleep/sleep_hygiene.html. Reviewed July 15, 2016. Accessed June 18, 2020.
14. Doumit J, Prasad B. Sleep Apnea in Type 2 Diabetes. Diabetes Spectrum. 2016; 29(1): 14-19.
15. Marso SP, Daniels GH, Brown-Frandsen K, et al; LEADER Steering Committee on behalf of the LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311-322.
16. Perkovic V, Jardine MJ, Neal B, et al; CREDENCE Trial Investigators. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380(24):2295-2306.
17. Trends in Blood pressure control and treatment among type 2 diabetes with comorbid hypertension in the United States: 1988-2004. J Hypertens. 2009;27(9):1908-1916.
18. Emdin CA, Rahimi K, Neal B, et al. Blood pressure lowering in type 2 diabetes: a systematic review and meta-analysis. JAMA. 2015;313(6):603-615.
19. Vouri SM, Shaw RF, Waterbury NV, et al. Prevalence of achievement of A1c, blood pressure, and cholesterol (ABC) goal in veterans with diabetes. J Manag Care Pharm. 2011;17(4):304-312.
20. Kudo N, Yokokawa H, Fukuda H, et al. Achievement of target blood pressure levels among Japanese workers with hypertension and healthy lifestyle characteristics associated with therapeutic failure. Plos One. 2015;10(7):e0133641.
21. Carey RM, Whelton PK; 2017 ACC/AHA Hypertension Guideline Writing Committee. Prevention, detection, evaluation, and management of high blood pressure in adults: synopsis of the 2017 American College of Cardiology/American Heart Association Hypertension guideline. Ann Intern Med. 2018;168(5):351-358.
22. Deedwania PC. Blood pressure control in diabetes mellitus. Circulation. 2011;123:2776–2778.
23. Catalá-López F, Saint-Gerons DM, González-Bermejo D, et al. Cardiovascular and renal outcomes of renin-angiotensin system blockade in adult patients with diabetes mellitus: a systematic review with network meta-analyses. PLoS Med. 2016;13(3):e1001971.
24. Furberg CD, Wright JT Jr, Davis BR, et al; ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA. 2002;288(23):2981-2997.
25. Sleight P. The HOPE Study (Heart Outcomes Prevention Evaluation). J Renin-Angiotensin-Aldosterone Syst. 2000;1(1):18-20.
26. Tatti P, Pahor M, Byington RP, et al. Outcome results of the Fosinopril Versus Amlodipine Cardiovascular Events Randomized Trial (FACET) in patients with hypertension and NIDDM. Diabetes Care. 1998;21(4):597-603.
27. Schrier RW, Estacio RO, Jeffers B. Appropriate Blood Pressure Control in NIDDM (ABCD) Trial. Diabetologia. 1996;39(12):1646-1654.
28. Hansson L, Zanchetti A, Carruthers SG, et al; HOT Study Group. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) Randomised Trial. Lancet. 1998;351(9118):1755-1762.
29. Baigent C, Blackwell L, Emberson J, et al; Cholesterol Treatment Trialists’ (CTT) Collaboration. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376(9753):1670-1681.
30. Fu AZ, Zhang Q, Davies MJ, et al. Underutilization of statins in patients with type 2 diabetes in US clinical practice: a retrospective cohort study. Curr Med Res Opin. 2011;27(5):1035-1040.
31. Cannon CP, Blazing MA, Giugliano RP, et al; IMPROVE-IT Investigators. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015; 372:2387-2397
32. Sabatine MS, Giugliano RP, Keech AC, et al; the FOURIER Steering Committee and Investigators. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713-1722.
33. Schwartz GG, Steg PG, Szarek M, et al; ODYSSEY OUTCOMES Committees and Investigators. Alirocumab and Cardiovascular Outcomes after Acute Coronary Syndrome | NEJM. N Engl J Med. 2018;379:2097-2107.
34. Icosapent ethyl [package insert]. Bridgewater, NJ: Amarin Pharma, Inc.; 2019.
35. Bhatt DL, Steg PG, Miller M, et al; REDUCE-IT Investigators. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med. 2019;380:11-22
36. Bolton WK. Renal Physicians Association Clinical practice guideline: appropriate patient preparation for renal replacement therapy: guideline number 3. J Am Soc Nephrol. 2003;14(5):1406-1410.
37. American Diabetes Association. Pharmacologic Approaches to glycemic treatment: standards of medical care in diabetes—2020. Diabetes Care. 2020;43(suppl 1):S98-S110.
38. Qaseem A, Barry MJ, Humphrey LL, Forciea MA; Clinical Guidelines Committee of the American College of Physicians. Oral pharmacologic treatment of type 2 diabetes mellitus: a clinical practice guideline update from the American College of Physicians. Ann Intern Med. 2017;166(4):279-290.
39. Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Update Work Group. KDIGO 2017 Clinical Practice Guideline Update for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease–mineral and bone disorder (CKD-MBD). Kidney Int Suppl (2011). 2017;7(1):1-59.
40. Pop-Busui R, Boulton AJM, Feldman EL, et al. Diabetic neuropathy: a position statement by the American Diabetes Association. Diabetes Care. 2017;40(1):136-154.
41. Gupta V, Bansal R, Gupta A, Bhansali A. The sensitivity and specificity of nonmydriatic digital stereoscopic retinal imaging in detecting diabetic retinopathy. Indian J Ophthalmol. 2014;62(8):851-856.
42. Pérez MA, Bruce BB, Newman NJ, Biousse V. The use of retinal photography in non-ophthalmic settings and its potential for neurology. The Neurologist. 2012;18(6):350-355.
Confronting the epidemic of racism in ObGyn practice
CASE Black woman in stable labor expresses fear
A 29-year-old Black woman (G1) at 39 0/7 weeks’ gestation presents to your labor and delivery unit reporting leaking fluid and contractions. She is found to have ruptured membranes and reassuring fetal testing. Her cervix is 4 cm dilated, and you recommend admission for expectant management of labor. She is otherwise healthy and has no significant medical history.
As you are finishing admitting this patient, you ask if she has any remaining questions. She asks quietly, “Am I going to die today?”
You provide reassurance of her stable clinical picture, then pause and ask the patient about her fears. She looks at you and says, “They didn’t believe Serena Williams, so why would they believe me?”
Your patient is referencing Serena Williams’ harrowing and public postpartum course, complicated by a pulmonary embolism and several reoperations.1 While many of us in the medical field may read this account as a story of challenges with an ultimate triumph, many expectant Black mothers hold Serena’s experience as a cautionary tale about deep-rooted inequities in our health care system that lead to potentially dangerous outcomes.
Disparities in care
They are right to be concerned. In the United States, Black mothers are 4 times more likely to die during or after pregnancy, mostly from preventable causes,2 and nearly 50% more likely to have a preterm delivery.3 These disparities extend beyond the delivery room to all aspects of ObGyn care. Black women are 2 to 3 times more likely to die from cervical cancer, and they are more likely to be diagnosed at a later stage, thus rendering treatment less effective.4 Black patients also have a higher burden of obesity, diabetes, and cardiac disease, and when they present to the hospital, receive evidence-based treatment at lower rates compared with White patients.5
Mourning the deaths of Ahmaud Arbery, Breonna Taylor, and George Floyd, amongst the many other Black lives taken unjustly in the United States, has highlighted egregious practices against people of color embedded within the systems meant to protect and serve our communities. We as ObGyn physicians must take professional onus to recognize a devastating but humbling truth—systemic racism has long pervaded our health care practices and systems, and now more than ever, we must do more to stand by and for our patients.
As ObGyns, we help support patients through some of the happiest, most vulnerable, and potentially most dire moments of their lives. We help patients through the birth of their children, reproductive struggles, gynecologic concerns, and cancer diagnoses. Many of us chose this field for the privilege of caring for patients at these critical moments in their lives, but we have often neglected the racism present in our practices, our hospital settings, and the medical system itself. We often fail to acknowledge our own implicit bias and the role that we play in contributing to acts and experiences of racism that our patients and our colleagues face on a daily basis.
Racism in our origins
The history of obstetrics and gynecology shows us a long record of physicians perpetrating injustices that target marginalized communities of color. Dr. James Sims, often given the title of “father of modern gynecology,” performed numerous experiments on unanesthetized Black female slaves to develop procedures for fistulae repair and other surgical techniques.6 Throughout the twentieth century, dating as recent as 1979, state laws written in the name of public safety forcibly sterilized women of color to control an “undesirable population.”7 When a patient of color declines a method of long-acting reversible contraception, birth control pills, or tubal ligation, do you take the time to reflect on the potential context of the patient’s decision?
It is critical to recognize the legacy that these acts have on our patients today, leading to a higher burden of disease and an understandable distrust of the medical system. The uncovering of the unethical practices of the National Institutions of Health‒funded Tuskegee syphilis study, in which hundreds of Black men with latent syphilis were passively monitored despite the knowledge of a proven treatment, has attributed to a measurable decrease in life expectancy among Black males.8 Even as we face the COVID-19 pandemic, the undercurrent of racism continues to do harm. Black patients are 5 times more likely to be hospitalized with COVID-19 than their White counterparts. This disparity, in part, is a product of a higher burden of comorbidities and the privilege associated with shelter-in-place policies, which disproportionately strain communities of color.9
We as a medical community need to do better for our patients. No matter how difficult to confront, each of us must acknowledge our own biases and our duty to combat persistent and perpetual racism in our medical system. We need to commit to amplifying the voices of our Black patients and colleagues. It is not enough to celebrate diversity for performance sake—it is time to recognize that diversity saves lives.
We have a responsibility to rectify these traditions of injustice and work toward a safer, more equitable, healthy future for our patients and their families. While this pledge may seem daunting, changes at individual and systems levels can make a difference for all patients that come through our doors. In addition, to honor our oath to “do no harm,” we must act; Black lives matter, and we are charged as medical providers to help our patients thrive, especially those from historically oppressed communities and who continue to suffer inexcusable injustices in health care and beyond.
Take action
Here is a collection of ways to institute an antiracist environment and more equitable care for your patients.
Self-reflect and educate
- Learn about the role racism plays in ObGyn and modern medicine. One place to start: read “Medical Bondage: Race, Gender and the Origins of American Gynecology” by Deidre Cooper Owens. Also check out articles and key readings curated by the Black Mamas Matter Alliance.
- Introduce and sustain antiracism training for all staff in your clinic or hospital system. To start, consider taking these free and quick implicit bias tests at a staff or department meeting.
- Familiarize yourself and your colleagues with facets of reproductive justice—the human right to have children, to not have children, and to nurture children in a safe and healthy environment—and incorporate these values in your practice. Request trainings in reproductive justice from community groups like Sister Song.
- Sign up for updates for state and national bills addressing health inequity and access to reproductive health services. Show your support by calling your congress-people, testifying, or donating to a cause that promotes these bills. You can stay up to date on national issues with government affairs newsletters from the American College of Obstetricians and Gynecologists. Sign up here.
- Continue the conversation and re-evaluate your personal and institution’s efforts to combat racism and social and reproductive injustices.
Provide access to high-quality reproductive health care
- Ask your patients what barriers they faced to come to your clinic and receive the care they needed. Consider incorporating the following screening tools regarding social determinants of health: PRAPARE screening tool, AAFP screening tool.
- Promote access to insurance and support programs, including nutrition, exercise and wellness, and safe home and school environments. Look up resources available to your patients by their zip codes using AAFP’s Neighborhood Navigator.
- Help patients access their medications at affordable prices in their neighborhoods by using free apps. Use the GoodRx app to identify discounts for prescriptions at various pharmacies, and search the Bedsider app to find out how your patients can get their birth control for free and delivered to their homes.
- Expand access to language services for patients who do not speak English as their first language. If working in a resource-limited setting, use the Google Translate app. Print out these free handouts for birth control fact sheets in different languages.
- Establish standardized protocols for common treatment paradigms to reduce the influence of bias in clinical scenarios. For example, institute a protocol for managing postoperative pain to ensure equal access to treatment.
- Institute the AIM (Alliance for Innovation on Maternal Health) patient safety bundle on the Reduction of Peripartum Racial/Ethnic Disparities. Learn more about AIM’s maternal safety and quality improvement initiative to reduce maternal morbidity and mortality here.
Support a diverse workforce
- Designate and/or hire a Diversity and Inclusion Officer at your institution to ensure that hiring practices actively achieve a diverse workforce and that employees feel supported in the work environment. Consider coalition-building between hospitals, like the UPHS-CHOP Alliance of Minority Physicians.
- Recruit diverse applicants by advertising positions to groups that focus on the advancement of underrepresented minorities in medicine. Engage with your local chapter of the National Medical Association and American Medical Women’s Association.
- Have a system in place for anonymous reporting of incidents involving bias or discrimination against staff, and develop a protocol to ensure action is taken in case of such incidents.
- Institute a recurring conference or Grand Rounds across disciplines to discuss the impacts of bias and discrimination on patients and providers at your institution. View examples of these conferences here.
- Ensure invited speakers and other educational opportunities are comprised of diverse representation.
- Create a work environment with safe spaces for the discussion of racism, discrimination, and bias.
- Haskell R. Serena Williams on motherhood, marriage, and making her comeback. January 10, 2018. https://www.vogue.com/article/serena-williams-vogue-cover-interview-february-2018. Accessed July 1, 2020.
- Louis JM, Menard MK, Gee RE. Racial and ethnic disparities in maternal morbidity and mortality. Obstet Gynecol. 2015;125:690-694.
- Sigurdson K, Mitchell B, Liu J, et al. Racial/ethnic disparities in neonatal intensive care: a systematic review. Pediatrics. 2019;144:e20183114.
- Garner EI. Cervical cancer: disparities in screening, treatment, and survival. Cancer Epidemiol Biomarkers Prev. 2003;12:242s-247s.
- Arora S, Stouffer GA, Kucharska‐Newton A, et al. Fifteen‐year trends in management and outcomes of non–ST‐segment–elevation myocardial infarction among black and white patients: the ARIC community surveillance study, 2000–2014. J Am Heart Assoc. 2018;7:e010203.
- Zellars R. Black subjectivity and the origins of American gynecology. May 31, 2018. https://www.aaihs.org/black-subjectivity-and-the-origins-of-american-gynecology/. Accessed June 28, 2020.
- Ko K. Unwanted sterilization and eugenics programs in the United States. January 29, 2016. https://www.pbs.org/independentlens/blog/unwanted-sterilization-and-eugenics-programs-in-the-united-states/. Accessed June 28, 2020.
- Alsan M, Wanamaker M. Tuskegee and the health of black men. Q J Econ. 2018;133:407-455.
- Hooper MW, Nápoles AM, Pérez-Stable EJ. COVID-19 and racial/ethnic disparities. JAMA. 2020 May 11. doi: 10.1001/jama.2020.8598.
CASE Black woman in stable labor expresses fear
A 29-year-old Black woman (G1) at 39 0/7 weeks’ gestation presents to your labor and delivery unit reporting leaking fluid and contractions. She is found to have ruptured membranes and reassuring fetal testing. Her cervix is 4 cm dilated, and you recommend admission for expectant management of labor. She is otherwise healthy and has no significant medical history.
As you are finishing admitting this patient, you ask if she has any remaining questions. She asks quietly, “Am I going to die today?”
You provide reassurance of her stable clinical picture, then pause and ask the patient about her fears. She looks at you and says, “They didn’t believe Serena Williams, so why would they believe me?”
Your patient is referencing Serena Williams’ harrowing and public postpartum course, complicated by a pulmonary embolism and several reoperations.1 While many of us in the medical field may read this account as a story of challenges with an ultimate triumph, many expectant Black mothers hold Serena’s experience as a cautionary tale about deep-rooted inequities in our health care system that lead to potentially dangerous outcomes.
Disparities in care
They are right to be concerned. In the United States, Black mothers are 4 times more likely to die during or after pregnancy, mostly from preventable causes,2 and nearly 50% more likely to have a preterm delivery.3 These disparities extend beyond the delivery room to all aspects of ObGyn care. Black women are 2 to 3 times more likely to die from cervical cancer, and they are more likely to be diagnosed at a later stage, thus rendering treatment less effective.4 Black patients also have a higher burden of obesity, diabetes, and cardiac disease, and when they present to the hospital, receive evidence-based treatment at lower rates compared with White patients.5
Mourning the deaths of Ahmaud Arbery, Breonna Taylor, and George Floyd, amongst the many other Black lives taken unjustly in the United States, has highlighted egregious practices against people of color embedded within the systems meant to protect and serve our communities. We as ObGyn physicians must take professional onus to recognize a devastating but humbling truth—systemic racism has long pervaded our health care practices and systems, and now more than ever, we must do more to stand by and for our patients.
As ObGyns, we help support patients through some of the happiest, most vulnerable, and potentially most dire moments of their lives. We help patients through the birth of their children, reproductive struggles, gynecologic concerns, and cancer diagnoses. Many of us chose this field for the privilege of caring for patients at these critical moments in their lives, but we have often neglected the racism present in our practices, our hospital settings, and the medical system itself. We often fail to acknowledge our own implicit bias and the role that we play in contributing to acts and experiences of racism that our patients and our colleagues face on a daily basis.
Racism in our origins
The history of obstetrics and gynecology shows us a long record of physicians perpetrating injustices that target marginalized communities of color. Dr. James Sims, often given the title of “father of modern gynecology,” performed numerous experiments on unanesthetized Black female slaves to develop procedures for fistulae repair and other surgical techniques.6 Throughout the twentieth century, dating as recent as 1979, state laws written in the name of public safety forcibly sterilized women of color to control an “undesirable population.”7 When a patient of color declines a method of long-acting reversible contraception, birth control pills, or tubal ligation, do you take the time to reflect on the potential context of the patient’s decision?
It is critical to recognize the legacy that these acts have on our patients today, leading to a higher burden of disease and an understandable distrust of the medical system. The uncovering of the unethical practices of the National Institutions of Health‒funded Tuskegee syphilis study, in which hundreds of Black men with latent syphilis were passively monitored despite the knowledge of a proven treatment, has attributed to a measurable decrease in life expectancy among Black males.8 Even as we face the COVID-19 pandemic, the undercurrent of racism continues to do harm. Black patients are 5 times more likely to be hospitalized with COVID-19 than their White counterparts. This disparity, in part, is a product of a higher burden of comorbidities and the privilege associated with shelter-in-place policies, which disproportionately strain communities of color.9
We as a medical community need to do better for our patients. No matter how difficult to confront, each of us must acknowledge our own biases and our duty to combat persistent and perpetual racism in our medical system. We need to commit to amplifying the voices of our Black patients and colleagues. It is not enough to celebrate diversity for performance sake—it is time to recognize that diversity saves lives.
We have a responsibility to rectify these traditions of injustice and work toward a safer, more equitable, healthy future for our patients and their families. While this pledge may seem daunting, changes at individual and systems levels can make a difference for all patients that come through our doors. In addition, to honor our oath to “do no harm,” we must act; Black lives matter, and we are charged as medical providers to help our patients thrive, especially those from historically oppressed communities and who continue to suffer inexcusable injustices in health care and beyond.
Take action
Here is a collection of ways to institute an antiracist environment and more equitable care for your patients.
Self-reflect and educate
- Learn about the role racism plays in ObGyn and modern medicine. One place to start: read “Medical Bondage: Race, Gender and the Origins of American Gynecology” by Deidre Cooper Owens. Also check out articles and key readings curated by the Black Mamas Matter Alliance.
- Introduce and sustain antiracism training for all staff in your clinic or hospital system. To start, consider taking these free and quick implicit bias tests at a staff or department meeting.
- Familiarize yourself and your colleagues with facets of reproductive justice—the human right to have children, to not have children, and to nurture children in a safe and healthy environment—and incorporate these values in your practice. Request trainings in reproductive justice from community groups like Sister Song.
- Sign up for updates for state and national bills addressing health inequity and access to reproductive health services. Show your support by calling your congress-people, testifying, or donating to a cause that promotes these bills. You can stay up to date on national issues with government affairs newsletters from the American College of Obstetricians and Gynecologists. Sign up here.
- Continue the conversation and re-evaluate your personal and institution’s efforts to combat racism and social and reproductive injustices.
Provide access to high-quality reproductive health care
- Ask your patients what barriers they faced to come to your clinic and receive the care they needed. Consider incorporating the following screening tools regarding social determinants of health: PRAPARE screening tool, AAFP screening tool.
- Promote access to insurance and support programs, including nutrition, exercise and wellness, and safe home and school environments. Look up resources available to your patients by their zip codes using AAFP’s Neighborhood Navigator.
- Help patients access their medications at affordable prices in their neighborhoods by using free apps. Use the GoodRx app to identify discounts for prescriptions at various pharmacies, and search the Bedsider app to find out how your patients can get their birth control for free and delivered to their homes.
- Expand access to language services for patients who do not speak English as their first language. If working in a resource-limited setting, use the Google Translate app. Print out these free handouts for birth control fact sheets in different languages.
- Establish standardized protocols for common treatment paradigms to reduce the influence of bias in clinical scenarios. For example, institute a protocol for managing postoperative pain to ensure equal access to treatment.
- Institute the AIM (Alliance for Innovation on Maternal Health) patient safety bundle on the Reduction of Peripartum Racial/Ethnic Disparities. Learn more about AIM’s maternal safety and quality improvement initiative to reduce maternal morbidity and mortality here.
Support a diverse workforce
- Designate and/or hire a Diversity and Inclusion Officer at your institution to ensure that hiring practices actively achieve a diverse workforce and that employees feel supported in the work environment. Consider coalition-building between hospitals, like the UPHS-CHOP Alliance of Minority Physicians.
- Recruit diverse applicants by advertising positions to groups that focus on the advancement of underrepresented minorities in medicine. Engage with your local chapter of the National Medical Association and American Medical Women’s Association.
- Have a system in place for anonymous reporting of incidents involving bias or discrimination against staff, and develop a protocol to ensure action is taken in case of such incidents.
- Institute a recurring conference or Grand Rounds across disciplines to discuss the impacts of bias and discrimination on patients and providers at your institution. View examples of these conferences here.
- Ensure invited speakers and other educational opportunities are comprised of diverse representation.
- Create a work environment with safe spaces for the discussion of racism, discrimination, and bias.
CASE Black woman in stable labor expresses fear
A 29-year-old Black woman (G1) at 39 0/7 weeks’ gestation presents to your labor and delivery unit reporting leaking fluid and contractions. She is found to have ruptured membranes and reassuring fetal testing. Her cervix is 4 cm dilated, and you recommend admission for expectant management of labor. She is otherwise healthy and has no significant medical history.
As you are finishing admitting this patient, you ask if she has any remaining questions. She asks quietly, “Am I going to die today?”
You provide reassurance of her stable clinical picture, then pause and ask the patient about her fears. She looks at you and says, “They didn’t believe Serena Williams, so why would they believe me?”
Your patient is referencing Serena Williams’ harrowing and public postpartum course, complicated by a pulmonary embolism and several reoperations.1 While many of us in the medical field may read this account as a story of challenges with an ultimate triumph, many expectant Black mothers hold Serena’s experience as a cautionary tale about deep-rooted inequities in our health care system that lead to potentially dangerous outcomes.
Disparities in care
They are right to be concerned. In the United States, Black mothers are 4 times more likely to die during or after pregnancy, mostly from preventable causes,2 and nearly 50% more likely to have a preterm delivery.3 These disparities extend beyond the delivery room to all aspects of ObGyn care. Black women are 2 to 3 times more likely to die from cervical cancer, and they are more likely to be diagnosed at a later stage, thus rendering treatment less effective.4 Black patients also have a higher burden of obesity, diabetes, and cardiac disease, and when they present to the hospital, receive evidence-based treatment at lower rates compared with White patients.5
Mourning the deaths of Ahmaud Arbery, Breonna Taylor, and George Floyd, amongst the many other Black lives taken unjustly in the United States, has highlighted egregious practices against people of color embedded within the systems meant to protect and serve our communities. We as ObGyn physicians must take professional onus to recognize a devastating but humbling truth—systemic racism has long pervaded our health care practices and systems, and now more than ever, we must do more to stand by and for our patients.
As ObGyns, we help support patients through some of the happiest, most vulnerable, and potentially most dire moments of their lives. We help patients through the birth of their children, reproductive struggles, gynecologic concerns, and cancer diagnoses. Many of us chose this field for the privilege of caring for patients at these critical moments in their lives, but we have often neglected the racism present in our practices, our hospital settings, and the medical system itself. We often fail to acknowledge our own implicit bias and the role that we play in contributing to acts and experiences of racism that our patients and our colleagues face on a daily basis.
Racism in our origins
The history of obstetrics and gynecology shows us a long record of physicians perpetrating injustices that target marginalized communities of color. Dr. James Sims, often given the title of “father of modern gynecology,” performed numerous experiments on unanesthetized Black female slaves to develop procedures for fistulae repair and other surgical techniques.6 Throughout the twentieth century, dating as recent as 1979, state laws written in the name of public safety forcibly sterilized women of color to control an “undesirable population.”7 When a patient of color declines a method of long-acting reversible contraception, birth control pills, or tubal ligation, do you take the time to reflect on the potential context of the patient’s decision?
It is critical to recognize the legacy that these acts have on our patients today, leading to a higher burden of disease and an understandable distrust of the medical system. The uncovering of the unethical practices of the National Institutions of Health‒funded Tuskegee syphilis study, in which hundreds of Black men with latent syphilis were passively monitored despite the knowledge of a proven treatment, has attributed to a measurable decrease in life expectancy among Black males.8 Even as we face the COVID-19 pandemic, the undercurrent of racism continues to do harm. Black patients are 5 times more likely to be hospitalized with COVID-19 than their White counterparts. This disparity, in part, is a product of a higher burden of comorbidities and the privilege associated with shelter-in-place policies, which disproportionately strain communities of color.9
We as a medical community need to do better for our patients. No matter how difficult to confront, each of us must acknowledge our own biases and our duty to combat persistent and perpetual racism in our medical system. We need to commit to amplifying the voices of our Black patients and colleagues. It is not enough to celebrate diversity for performance sake—it is time to recognize that diversity saves lives.
We have a responsibility to rectify these traditions of injustice and work toward a safer, more equitable, healthy future for our patients and their families. While this pledge may seem daunting, changes at individual and systems levels can make a difference for all patients that come through our doors. In addition, to honor our oath to “do no harm,” we must act; Black lives matter, and we are charged as medical providers to help our patients thrive, especially those from historically oppressed communities and who continue to suffer inexcusable injustices in health care and beyond.
Take action
Here is a collection of ways to institute an antiracist environment and more equitable care for your patients.
Self-reflect and educate
- Learn about the role racism plays in ObGyn and modern medicine. One place to start: read “Medical Bondage: Race, Gender and the Origins of American Gynecology” by Deidre Cooper Owens. Also check out articles and key readings curated by the Black Mamas Matter Alliance.
- Introduce and sustain antiracism training for all staff in your clinic or hospital system. To start, consider taking these free and quick implicit bias tests at a staff or department meeting.
- Familiarize yourself and your colleagues with facets of reproductive justice—the human right to have children, to not have children, and to nurture children in a safe and healthy environment—and incorporate these values in your practice. Request trainings in reproductive justice from community groups like Sister Song.
- Sign up for updates for state and national bills addressing health inequity and access to reproductive health services. Show your support by calling your congress-people, testifying, or donating to a cause that promotes these bills. You can stay up to date on national issues with government affairs newsletters from the American College of Obstetricians and Gynecologists. Sign up here.
- Continue the conversation and re-evaluate your personal and institution’s efforts to combat racism and social and reproductive injustices.
Provide access to high-quality reproductive health care
- Ask your patients what barriers they faced to come to your clinic and receive the care they needed. Consider incorporating the following screening tools regarding social determinants of health: PRAPARE screening tool, AAFP screening tool.
- Promote access to insurance and support programs, including nutrition, exercise and wellness, and safe home and school environments. Look up resources available to your patients by their zip codes using AAFP’s Neighborhood Navigator.
- Help patients access their medications at affordable prices in their neighborhoods by using free apps. Use the GoodRx app to identify discounts for prescriptions at various pharmacies, and search the Bedsider app to find out how your patients can get their birth control for free and delivered to their homes.
- Expand access to language services for patients who do not speak English as their first language. If working in a resource-limited setting, use the Google Translate app. Print out these free handouts for birth control fact sheets in different languages.
- Establish standardized protocols for common treatment paradigms to reduce the influence of bias in clinical scenarios. For example, institute a protocol for managing postoperative pain to ensure equal access to treatment.
- Institute the AIM (Alliance for Innovation on Maternal Health) patient safety bundle on the Reduction of Peripartum Racial/Ethnic Disparities. Learn more about AIM’s maternal safety and quality improvement initiative to reduce maternal morbidity and mortality here.
Support a diverse workforce
- Designate and/or hire a Diversity and Inclusion Officer at your institution to ensure that hiring practices actively achieve a diverse workforce and that employees feel supported in the work environment. Consider coalition-building between hospitals, like the UPHS-CHOP Alliance of Minority Physicians.
- Recruit diverse applicants by advertising positions to groups that focus on the advancement of underrepresented minorities in medicine. Engage with your local chapter of the National Medical Association and American Medical Women’s Association.
- Have a system in place for anonymous reporting of incidents involving bias or discrimination against staff, and develop a protocol to ensure action is taken in case of such incidents.
- Institute a recurring conference or Grand Rounds across disciplines to discuss the impacts of bias and discrimination on patients and providers at your institution. View examples of these conferences here.
- Ensure invited speakers and other educational opportunities are comprised of diverse representation.
- Create a work environment with safe spaces for the discussion of racism, discrimination, and bias.
- Haskell R. Serena Williams on motherhood, marriage, and making her comeback. January 10, 2018. https://www.vogue.com/article/serena-williams-vogue-cover-interview-february-2018. Accessed July 1, 2020.
- Louis JM, Menard MK, Gee RE. Racial and ethnic disparities in maternal morbidity and mortality. Obstet Gynecol. 2015;125:690-694.
- Sigurdson K, Mitchell B, Liu J, et al. Racial/ethnic disparities in neonatal intensive care: a systematic review. Pediatrics. 2019;144:e20183114.
- Garner EI. Cervical cancer: disparities in screening, treatment, and survival. Cancer Epidemiol Biomarkers Prev. 2003;12:242s-247s.
- Arora S, Stouffer GA, Kucharska‐Newton A, et al. Fifteen‐year trends in management and outcomes of non–ST‐segment–elevation myocardial infarction among black and white patients: the ARIC community surveillance study, 2000–2014. J Am Heart Assoc. 2018;7:e010203.
- Zellars R. Black subjectivity and the origins of American gynecology. May 31, 2018. https://www.aaihs.org/black-subjectivity-and-the-origins-of-american-gynecology/. Accessed June 28, 2020.
- Ko K. Unwanted sterilization and eugenics programs in the United States. January 29, 2016. https://www.pbs.org/independentlens/blog/unwanted-sterilization-and-eugenics-programs-in-the-united-states/. Accessed June 28, 2020.
- Alsan M, Wanamaker M. Tuskegee and the health of black men. Q J Econ. 2018;133:407-455.
- Hooper MW, Nápoles AM, Pérez-Stable EJ. COVID-19 and racial/ethnic disparities. JAMA. 2020 May 11. doi: 10.1001/jama.2020.8598.
- Haskell R. Serena Williams on motherhood, marriage, and making her comeback. January 10, 2018. https://www.vogue.com/article/serena-williams-vogue-cover-interview-february-2018. Accessed July 1, 2020.
- Louis JM, Menard MK, Gee RE. Racial and ethnic disparities in maternal morbidity and mortality. Obstet Gynecol. 2015;125:690-694.
- Sigurdson K, Mitchell B, Liu J, et al. Racial/ethnic disparities in neonatal intensive care: a systematic review. Pediatrics. 2019;144:e20183114.
- Garner EI. Cervical cancer: disparities in screening, treatment, and survival. Cancer Epidemiol Biomarkers Prev. 2003;12:242s-247s.
- Arora S, Stouffer GA, Kucharska‐Newton A, et al. Fifteen‐year trends in management and outcomes of non–ST‐segment–elevation myocardial infarction among black and white patients: the ARIC community surveillance study, 2000–2014. J Am Heart Assoc. 2018;7:e010203.
- Zellars R. Black subjectivity and the origins of American gynecology. May 31, 2018. https://www.aaihs.org/black-subjectivity-and-the-origins-of-american-gynecology/. Accessed June 28, 2020.
- Ko K. Unwanted sterilization and eugenics programs in the United States. January 29, 2016. https://www.pbs.org/independentlens/blog/unwanted-sterilization-and-eugenics-programs-in-the-united-states/. Accessed June 28, 2020.
- Alsan M, Wanamaker M. Tuskegee and the health of black men. Q J Econ. 2018;133:407-455.
- Hooper MW, Nápoles AM, Pérez-Stable EJ. COVID-19 and racial/ethnic disparities. JAMA. 2020 May 11. doi: 10.1001/jama.2020.8598.
Used together, troponin and coronary calcium improve CV risk assessment
If either high sensitivity cardiac troponin (hs-cTnT) or coronary artery calcium (CAC) are elevated, the 10-year risk of atherosclerotic cardiovascular disease (ASCVD) climbs substantially, which suggests these biomarkers yield more prognostic information when they are used together, according to a cohort study with a median 15 years of follow-up.
Among those with a double negative result, meaning hs-cTnT was less than the limit of detection (<3 ng/L) and the CAC score was zero, only 2.8% developed ASCVD within 10 years, but the rates climbed to 4.6% if hs-cTnT was detectable and to 9.8% if the CAC score exceeded zero even when the other biomarker was negative.
“The increased risk for ASCVD among those with discordant results indicate that their prognostic information is complementary, favoring their conjoined use for risk prediction,” reported a multicenter team of investigators led by Allan S. Jaffe, MD, professor of laboratory medicine and pathology, Mayo Clinic, Rochester, Minn.
The study was performed with data from 6,749 participants in the Multi-Ethnic Study of Atherosclerosis (MESA), which is a longitudinal, community-based study funded by the National Heart, Lung, and Blood Institute. Over the course of long-term follow-up in a patient population that was about half female, 39% non-Hispanic white, 28% Black, 22% Hispanic American, and 12% Asian, ASCVD events were evaluated in relation to both biomarkers measured at baseline.
At baseline, both biomarkers were negative in 22%, both positive in 40%, and discordant in 38%.
After a median follow-up of 15 years, when 1,002 ASCVD events had occurred, the crude rate of ASCVD was 2.8 per 1,000 person-years in the double-negative group. When compared with this, the adjusted hazard ratio for ASCVD among those with double positive biomarkers was 3.5 (P < .00001). Increased risk was also highly significant if just hs-cTnT was positive (HR, 1.59; P = .003) or if just CAC was positive (HR, 2.74; P < .00001).
The added value of using both biomarkers to identify individuals at very low risk of ASCVD makes sense, according to the authors of an accompanying editorial. Written by a team led by John W. McEvoy, MB, BCh, National University of Ireland, Galway, the editorial explained why the information is complementary.
“CAC indicates subclinical atherosclerosis, whereas hs-cTnT indicates myocardial ischemia or damage, not just from coronary stenosis but also due to other conditions like hypertensive heart and left ventricular hypertrophy,” the authors stated.
Although they maintained that adding N-terminal pro-brain natriuretic peptide, which could be drawn from the same blood sample as hs-cTnT, might prove to be an even better but still simple strategy to identify low-risk patients, they praised the concept of combining biomarkers.
“If one’s wish is to identify truly low-risk individuals, then it appears that it takes two negative ASCVD biomarkers to make that wish come true,” the authors of the editorial concluded.
Relative to alternative methods of ASCVD risk assessment, measurement of these biomarkers might be useful for sparing patients from interventions, such as lipid lowering with statin therapy, being considered on the basis of conventional risk factors alone.
Dr. Jaffe said in an interview that he considers the two-biomarker assessment to be a useful tool in the low-risk population that he studied, but he does not consider this strategy as a substitute for other methods, such as those outline in the 2019 ACC/AHA guidelines that address the entire spectrum of risk, although work is planned to see if this approach can be extended to this broader group.*
“The data we have presented now is a good start and suggests that these two objective measures can identify those who are at very low risk and avoid adding individuals who may not be at as low risk if only one of the two tests is used,” Dr. Jaffe explained.
“Given there are now techniques to measure coronary calcium from any chest CT study, and that high sensitivity cardiac troponin is a relatively inexpensive test, putting them together should really help risk stratify patients,” he added.
When asked whether this approach will eventually replace conventional methods of ASCVD risk assessment, such as those proposed in the 2019 American College of Cardiology/American Heart Association guidelines for the primary prevention of cardiovascular disease (Circulation. 2019;140:e596-e646), he said maybe.
“The answer is that we will probe that question in our ongoing studies using continuous data in an attempt to evaluate how to use this approach to risk stratify larger numbers of individuals,” Dr. Jaffe replied.
The senior investigator, Dr. Jaffe, has consulting relationships with many pharmaceutical companies. The editorial authors had no relevant disclosures.
SOURCE: Sandoval Y et al. J Am Coll Cardiol. 2020;76:357-370.
*Correction, 7/27/20: An earlier version of this article mischaracterized Dr. Jaffe's statement.
If either high sensitivity cardiac troponin (hs-cTnT) or coronary artery calcium (CAC) are elevated, the 10-year risk of atherosclerotic cardiovascular disease (ASCVD) climbs substantially, which suggests these biomarkers yield more prognostic information when they are used together, according to a cohort study with a median 15 years of follow-up.
Among those with a double negative result, meaning hs-cTnT was less than the limit of detection (<3 ng/L) and the CAC score was zero, only 2.8% developed ASCVD within 10 years, but the rates climbed to 4.6% if hs-cTnT was detectable and to 9.8% if the CAC score exceeded zero even when the other biomarker was negative.
“The increased risk for ASCVD among those with discordant results indicate that their prognostic information is complementary, favoring their conjoined use for risk prediction,” reported a multicenter team of investigators led by Allan S. Jaffe, MD, professor of laboratory medicine and pathology, Mayo Clinic, Rochester, Minn.
The study was performed with data from 6,749 participants in the Multi-Ethnic Study of Atherosclerosis (MESA), which is a longitudinal, community-based study funded by the National Heart, Lung, and Blood Institute. Over the course of long-term follow-up in a patient population that was about half female, 39% non-Hispanic white, 28% Black, 22% Hispanic American, and 12% Asian, ASCVD events were evaluated in relation to both biomarkers measured at baseline.
At baseline, both biomarkers were negative in 22%, both positive in 40%, and discordant in 38%.
After a median follow-up of 15 years, when 1,002 ASCVD events had occurred, the crude rate of ASCVD was 2.8 per 1,000 person-years in the double-negative group. When compared with this, the adjusted hazard ratio for ASCVD among those with double positive biomarkers was 3.5 (P < .00001). Increased risk was also highly significant if just hs-cTnT was positive (HR, 1.59; P = .003) or if just CAC was positive (HR, 2.74; P < .00001).
The added value of using both biomarkers to identify individuals at very low risk of ASCVD makes sense, according to the authors of an accompanying editorial. Written by a team led by John W. McEvoy, MB, BCh, National University of Ireland, Galway, the editorial explained why the information is complementary.
“CAC indicates subclinical atherosclerosis, whereas hs-cTnT indicates myocardial ischemia or damage, not just from coronary stenosis but also due to other conditions like hypertensive heart and left ventricular hypertrophy,” the authors stated.
Although they maintained that adding N-terminal pro-brain natriuretic peptide, which could be drawn from the same blood sample as hs-cTnT, might prove to be an even better but still simple strategy to identify low-risk patients, they praised the concept of combining biomarkers.
“If one’s wish is to identify truly low-risk individuals, then it appears that it takes two negative ASCVD biomarkers to make that wish come true,” the authors of the editorial concluded.
Relative to alternative methods of ASCVD risk assessment, measurement of these biomarkers might be useful for sparing patients from interventions, such as lipid lowering with statin therapy, being considered on the basis of conventional risk factors alone.
Dr. Jaffe said in an interview that he considers the two-biomarker assessment to be a useful tool in the low-risk population that he studied, but he does not consider this strategy as a substitute for other methods, such as those outline in the 2019 ACC/AHA guidelines that address the entire spectrum of risk, although work is planned to see if this approach can be extended to this broader group.*
“The data we have presented now is a good start and suggests that these two objective measures can identify those who are at very low risk and avoid adding individuals who may not be at as low risk if only one of the two tests is used,” Dr. Jaffe explained.
“Given there are now techniques to measure coronary calcium from any chest CT study, and that high sensitivity cardiac troponin is a relatively inexpensive test, putting them together should really help risk stratify patients,” he added.
When asked whether this approach will eventually replace conventional methods of ASCVD risk assessment, such as those proposed in the 2019 American College of Cardiology/American Heart Association guidelines for the primary prevention of cardiovascular disease (Circulation. 2019;140:e596-e646), he said maybe.
“The answer is that we will probe that question in our ongoing studies using continuous data in an attempt to evaluate how to use this approach to risk stratify larger numbers of individuals,” Dr. Jaffe replied.
The senior investigator, Dr. Jaffe, has consulting relationships with many pharmaceutical companies. The editorial authors had no relevant disclosures.
SOURCE: Sandoval Y et al. J Am Coll Cardiol. 2020;76:357-370.
*Correction, 7/27/20: An earlier version of this article mischaracterized Dr. Jaffe's statement.
If either high sensitivity cardiac troponin (hs-cTnT) or coronary artery calcium (CAC) are elevated, the 10-year risk of atherosclerotic cardiovascular disease (ASCVD) climbs substantially, which suggests these biomarkers yield more prognostic information when they are used together, according to a cohort study with a median 15 years of follow-up.
Among those with a double negative result, meaning hs-cTnT was less than the limit of detection (<3 ng/L) and the CAC score was zero, only 2.8% developed ASCVD within 10 years, but the rates climbed to 4.6% if hs-cTnT was detectable and to 9.8% if the CAC score exceeded zero even when the other biomarker was negative.
“The increased risk for ASCVD among those with discordant results indicate that their prognostic information is complementary, favoring their conjoined use for risk prediction,” reported a multicenter team of investigators led by Allan S. Jaffe, MD, professor of laboratory medicine and pathology, Mayo Clinic, Rochester, Minn.
The study was performed with data from 6,749 participants in the Multi-Ethnic Study of Atherosclerosis (MESA), which is a longitudinal, community-based study funded by the National Heart, Lung, and Blood Institute. Over the course of long-term follow-up in a patient population that was about half female, 39% non-Hispanic white, 28% Black, 22% Hispanic American, and 12% Asian, ASCVD events were evaluated in relation to both biomarkers measured at baseline.
At baseline, both biomarkers were negative in 22%, both positive in 40%, and discordant in 38%.
After a median follow-up of 15 years, when 1,002 ASCVD events had occurred, the crude rate of ASCVD was 2.8 per 1,000 person-years in the double-negative group. When compared with this, the adjusted hazard ratio for ASCVD among those with double positive biomarkers was 3.5 (P < .00001). Increased risk was also highly significant if just hs-cTnT was positive (HR, 1.59; P = .003) or if just CAC was positive (HR, 2.74; P < .00001).
The added value of using both biomarkers to identify individuals at very low risk of ASCVD makes sense, according to the authors of an accompanying editorial. Written by a team led by John W. McEvoy, MB, BCh, National University of Ireland, Galway, the editorial explained why the information is complementary.
“CAC indicates subclinical atherosclerosis, whereas hs-cTnT indicates myocardial ischemia or damage, not just from coronary stenosis but also due to other conditions like hypertensive heart and left ventricular hypertrophy,” the authors stated.
Although they maintained that adding N-terminal pro-brain natriuretic peptide, which could be drawn from the same blood sample as hs-cTnT, might prove to be an even better but still simple strategy to identify low-risk patients, they praised the concept of combining biomarkers.
“If one’s wish is to identify truly low-risk individuals, then it appears that it takes two negative ASCVD biomarkers to make that wish come true,” the authors of the editorial concluded.
Relative to alternative methods of ASCVD risk assessment, measurement of these biomarkers might be useful for sparing patients from interventions, such as lipid lowering with statin therapy, being considered on the basis of conventional risk factors alone.
Dr. Jaffe said in an interview that he considers the two-biomarker assessment to be a useful tool in the low-risk population that he studied, but he does not consider this strategy as a substitute for other methods, such as those outline in the 2019 ACC/AHA guidelines that address the entire spectrum of risk, although work is planned to see if this approach can be extended to this broader group.*
“The data we have presented now is a good start and suggests that these two objective measures can identify those who are at very low risk and avoid adding individuals who may not be at as low risk if only one of the two tests is used,” Dr. Jaffe explained.
“Given there are now techniques to measure coronary calcium from any chest CT study, and that high sensitivity cardiac troponin is a relatively inexpensive test, putting them together should really help risk stratify patients,” he added.
When asked whether this approach will eventually replace conventional methods of ASCVD risk assessment, such as those proposed in the 2019 American College of Cardiology/American Heart Association guidelines for the primary prevention of cardiovascular disease (Circulation. 2019;140:e596-e646), he said maybe.
“The answer is that we will probe that question in our ongoing studies using continuous data in an attempt to evaluate how to use this approach to risk stratify larger numbers of individuals,” Dr. Jaffe replied.
The senior investigator, Dr. Jaffe, has consulting relationships with many pharmaceutical companies. The editorial authors had no relevant disclosures.
SOURCE: Sandoval Y et al. J Am Coll Cardiol. 2020;76:357-370.
*Correction, 7/27/20: An earlier version of this article mischaracterized Dr. Jaffe's statement.
FROM JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
Hot-off-the-press insights on heart failure
Hospitalists frequently encounter patients with heart failure – a complex, clinical syndrome, which has high prevalence, mortality, hospitalization rates, and health care costs.
The HM20 Virtual session “Updates in Heart Failure” will provide literature updates for all types of heart failure patient scenarios – patients with acute and chronic heart failure, those who are hospitalized with heart failure, and patients with heart failure with reduced ejection fraction (HFrEF) and heart failure with preserved ejection fraction (HFpEF). The popular session with questions and answers will be held on Aug. 25.
Presenter Dustin Smith, MD, SFHM, associate professor of medicine in the department of medicine at Emory University, Atlanta, and section chief for education in medical specialty at the Atlanta Veterans Affairs Medical Center, will discuss recent trends, diagnostics, therapeutics, and prognostics for heart failure. He’ll also provide a summary of recent changes to clinical practice guidelines.
“The significance of staying knowledgeable and updated regarding this common admission diagnosis cannot be overstated,” Dr. Smith said. Attendees of this clinical update should learn important practices from new evidence in literature, including an unearthed risk grade predictor of acute heart failure mortality, a diagnostic tool for HFpEF in euvolemic patients with unexplained dyspnea, an examination of the potassium “repletion reflex” in patients hospitalized with heart failure, dietary patterns associated with incident heart failure, and therapies efficacious for HFrEF and/or HFpEF.
“The goal of this session is for attendees to incorporate this new information into their clinical practice so they can optimally manage patients with heart failure,” Dr. Smith said.
The session is specifically curated to impact the clinical practice of hospitalists who provide care for patients with heart failure in the acute care setting and beyond. Key impact areas of clinical practice that will be tackled include:
- Augmenting one’s clinical acumen to diagnose HFpEF.
- Calculating mortality risk for patients with acute heart failure.
- Recognizing other predictors of risk for patients hospitalized with heart failure.
- Recommending dietary, medication, and interventional therapies to prevent future heart failure morbidity and mortality.
Dr. Smith will conclude each literature review with a summary of take-home learning points carefully selected to either change, modify, or confirm the current practice and teaching for providers who care for heart failure patients.
Although Dr. Smith has presented the “Updates in Heart Failure” session in various educational arenas in the past, this is a new update. He has gained vast experience and expertise in this area from conducting extensive and in-depth literature reviews on managing heart failure while preparing for presentations on this topic.
In addition, Dr. Smith has contributed to original research manuscripts, book chapters, and board review–style exam questions in cardiology – including heart failure – and evidence-based medicine topics as an author and editor. He has also sought out additional training and completed faculty development programs targeted at improving his knowledge and skill set to teach evidence-based clinical practice.
Dr. Smith had no relevant financial conflicts to disclose.
Updates in Heart Failure
Live Q&A – Tuesday, Aug. 25 1:00 p.m. to 2:00 p.m.
Hospitalists frequently encounter patients with heart failure – a complex, clinical syndrome, which has high prevalence, mortality, hospitalization rates, and health care costs.
The HM20 Virtual session “Updates in Heart Failure” will provide literature updates for all types of heart failure patient scenarios – patients with acute and chronic heart failure, those who are hospitalized with heart failure, and patients with heart failure with reduced ejection fraction (HFrEF) and heart failure with preserved ejection fraction (HFpEF). The popular session with questions and answers will be held on Aug. 25.
Presenter Dustin Smith, MD, SFHM, associate professor of medicine in the department of medicine at Emory University, Atlanta, and section chief for education in medical specialty at the Atlanta Veterans Affairs Medical Center, will discuss recent trends, diagnostics, therapeutics, and prognostics for heart failure. He’ll also provide a summary of recent changes to clinical practice guidelines.
“The significance of staying knowledgeable and updated regarding this common admission diagnosis cannot be overstated,” Dr. Smith said. Attendees of this clinical update should learn important practices from new evidence in literature, including an unearthed risk grade predictor of acute heart failure mortality, a diagnostic tool for HFpEF in euvolemic patients with unexplained dyspnea, an examination of the potassium “repletion reflex” in patients hospitalized with heart failure, dietary patterns associated with incident heart failure, and therapies efficacious for HFrEF and/or HFpEF.
“The goal of this session is for attendees to incorporate this new information into their clinical practice so they can optimally manage patients with heart failure,” Dr. Smith said.
The session is specifically curated to impact the clinical practice of hospitalists who provide care for patients with heart failure in the acute care setting and beyond. Key impact areas of clinical practice that will be tackled include:
- Augmenting one’s clinical acumen to diagnose HFpEF.
- Calculating mortality risk for patients with acute heart failure.
- Recognizing other predictors of risk for patients hospitalized with heart failure.
- Recommending dietary, medication, and interventional therapies to prevent future heart failure morbidity and mortality.
Dr. Smith will conclude each literature review with a summary of take-home learning points carefully selected to either change, modify, or confirm the current practice and teaching for providers who care for heart failure patients.
Although Dr. Smith has presented the “Updates in Heart Failure” session in various educational arenas in the past, this is a new update. He has gained vast experience and expertise in this area from conducting extensive and in-depth literature reviews on managing heart failure while preparing for presentations on this topic.
In addition, Dr. Smith has contributed to original research manuscripts, book chapters, and board review–style exam questions in cardiology – including heart failure – and evidence-based medicine topics as an author and editor. He has also sought out additional training and completed faculty development programs targeted at improving his knowledge and skill set to teach evidence-based clinical practice.
Dr. Smith had no relevant financial conflicts to disclose.
Updates in Heart Failure
Live Q&A – Tuesday, Aug. 25 1:00 p.m. to 2:00 p.m.
Hospitalists frequently encounter patients with heart failure – a complex, clinical syndrome, which has high prevalence, mortality, hospitalization rates, and health care costs.
The HM20 Virtual session “Updates in Heart Failure” will provide literature updates for all types of heart failure patient scenarios – patients with acute and chronic heart failure, those who are hospitalized with heart failure, and patients with heart failure with reduced ejection fraction (HFrEF) and heart failure with preserved ejection fraction (HFpEF). The popular session with questions and answers will be held on Aug. 25.
Presenter Dustin Smith, MD, SFHM, associate professor of medicine in the department of medicine at Emory University, Atlanta, and section chief for education in medical specialty at the Atlanta Veterans Affairs Medical Center, will discuss recent trends, diagnostics, therapeutics, and prognostics for heart failure. He’ll also provide a summary of recent changes to clinical practice guidelines.
“The significance of staying knowledgeable and updated regarding this common admission diagnosis cannot be overstated,” Dr. Smith said. Attendees of this clinical update should learn important practices from new evidence in literature, including an unearthed risk grade predictor of acute heart failure mortality, a diagnostic tool for HFpEF in euvolemic patients with unexplained dyspnea, an examination of the potassium “repletion reflex” in patients hospitalized with heart failure, dietary patterns associated with incident heart failure, and therapies efficacious for HFrEF and/or HFpEF.
“The goal of this session is for attendees to incorporate this new information into their clinical practice so they can optimally manage patients with heart failure,” Dr. Smith said.
The session is specifically curated to impact the clinical practice of hospitalists who provide care for patients with heart failure in the acute care setting and beyond. Key impact areas of clinical practice that will be tackled include:
- Augmenting one’s clinical acumen to diagnose HFpEF.
- Calculating mortality risk for patients with acute heart failure.
- Recognizing other predictors of risk for patients hospitalized with heart failure.
- Recommending dietary, medication, and interventional therapies to prevent future heart failure morbidity and mortality.
Dr. Smith will conclude each literature review with a summary of take-home learning points carefully selected to either change, modify, or confirm the current practice and teaching for providers who care for heart failure patients.
Although Dr. Smith has presented the “Updates in Heart Failure” session in various educational arenas in the past, this is a new update. He has gained vast experience and expertise in this area from conducting extensive and in-depth literature reviews on managing heart failure while preparing for presentations on this topic.
In addition, Dr. Smith has contributed to original research manuscripts, book chapters, and board review–style exam questions in cardiology – including heart failure – and evidence-based medicine topics as an author and editor. He has also sought out additional training and completed faculty development programs targeted at improving his knowledge and skill set to teach evidence-based clinical practice.
Dr. Smith had no relevant financial conflicts to disclose.
Updates in Heart Failure
Live Q&A – Tuesday, Aug. 25 1:00 p.m. to 2:00 p.m.
No link between topical steroids and fracture risk found in children with atopic dermatitis
suggest.
“Little has been published about the risk of fracture in children with atopic dermatitis on topical corticosteroids specifically,” one of the study authors, Reese L. Imhof, said in an interview following the virtual annual meeting of the Society for Pediatric Dermatology. “There are concerns, particularly among parents, regarding potential bone side effects through possible corticosteroid percutaneous absorption. Fears related to topical corticosteroid use likely stem from the fact that prolonged systemic corticosteroid use is associated with an increased risk of bone fractures.”
In an effort to determine the fracture risk in children who were diagnosed with atopic dermatitis (AD) prior to age 4 years and received topical corticosteroid treatment, Mr. Imhof, from Mayo Medical School, Rochester, Minn., and his associates used the Rochester Epidemiology Project records-linkage system to identify patients in Olmstead County, Minn., who received their first AD diagnosis prior to age 4 years between Jan. 1, 2004, through Dec. 31, 2017. Those who received topical corticosteroids listed in National Drug File-Reference Terminology class 8952 (anti-inflammatory, topical) or 8954 (anti-infective/anti-inflammatory combinations, topical) between Jan. 1, 2004, and Dec. 31, 2018 were included in the analysis and were followed to identify new bone fractures, excluding pathological fractures in neoplastic disease and skull or facial bone fractures.
The researchers conducted two analyses of the data. For the primary statistical analysis, they evaluated topical corticosteroid exposure as a binary time-dependent covariate in a Cox proportional hazard model using age as the time scale, with patients entering the risk set at the age of the first clinic visit rather than the age of their first AD diagnosis. Next, the researchers performed a landmark analysis as a sensitivity analysis. For this, each patient’s fourth birthday was defined as the starting point, since all included patients were diagnosed with AD prior to age 4 years.
Of the 7,505 patients first identified with AD, 3,542 were included in the primary analysis and 2,499 were included in the landmark analysis. In the primary analysis, 2,384 patients (67%) received a topical prescription for a topical corticosteroid prior to age 4 years, and an additional 190 (5%) received their first prescription after age 4 years. The researchers observed that 451 patients (13%) had a fracture after AD diagnosis at a median age of 7.4 years. The median age at last follow-up for the remaining 3,091 patients was 6.6 years. Evaluated as a time-dependent covariate, the use of a topical corticosteroid was associated with a nonsignificant 17% increased risk of fracture (hazard ratio, 1.17; P = .16).
In the landmark analysis, 1,722 patients (69%) were prescribed a topical corticosteroid prior to age 4 years. Of these patients, 333 (13%) had their first fracture after AD diagnosis, at a median age of 8.7 years. The median age at last follow-up for the remaining patients was 9.3 years. The researchers observed that, starting at 4 years of age, there was no association between topical corticosteroid use and risk of fracture (HR, 1.00; P = 1.00).
“Our findings suggest that topical corticosteroids do not significantly increase fracture risk in this pediatric population with atopic dermatitis,” Mr. Imhof said. “Dermatologists can use the results of this study to reassure parents of infants and young children, as most patients in our study received their first topical corticosteroid prescription prior to age 4.”
He acknowledged certain limitations of the study, such as its retrospective design and study population, which was predominantly white and resided in the upper Midwest. “Also, our study examined prescription data with the assumption made that topical corticosteroids were used as prescribed,” he said. “An additional limitation is that we evaluated ever versus never exposure to topical corticosteroids rather than cumulative duration of use and/or potency.”
Mr. Imhof and his colleagues reported having no financial disclosures.
suggest.
“Little has been published about the risk of fracture in children with atopic dermatitis on topical corticosteroids specifically,” one of the study authors, Reese L. Imhof, said in an interview following the virtual annual meeting of the Society for Pediatric Dermatology. “There are concerns, particularly among parents, regarding potential bone side effects through possible corticosteroid percutaneous absorption. Fears related to topical corticosteroid use likely stem from the fact that prolonged systemic corticosteroid use is associated with an increased risk of bone fractures.”
In an effort to determine the fracture risk in children who were diagnosed with atopic dermatitis (AD) prior to age 4 years and received topical corticosteroid treatment, Mr. Imhof, from Mayo Medical School, Rochester, Minn., and his associates used the Rochester Epidemiology Project records-linkage system to identify patients in Olmstead County, Minn., who received their first AD diagnosis prior to age 4 years between Jan. 1, 2004, through Dec. 31, 2017. Those who received topical corticosteroids listed in National Drug File-Reference Terminology class 8952 (anti-inflammatory, topical) or 8954 (anti-infective/anti-inflammatory combinations, topical) between Jan. 1, 2004, and Dec. 31, 2018 were included in the analysis and were followed to identify new bone fractures, excluding pathological fractures in neoplastic disease and skull or facial bone fractures.
The researchers conducted two analyses of the data. For the primary statistical analysis, they evaluated topical corticosteroid exposure as a binary time-dependent covariate in a Cox proportional hazard model using age as the time scale, with patients entering the risk set at the age of the first clinic visit rather than the age of their first AD diagnosis. Next, the researchers performed a landmark analysis as a sensitivity analysis. For this, each patient’s fourth birthday was defined as the starting point, since all included patients were diagnosed with AD prior to age 4 years.
Of the 7,505 patients first identified with AD, 3,542 were included in the primary analysis and 2,499 were included in the landmark analysis. In the primary analysis, 2,384 patients (67%) received a topical prescription for a topical corticosteroid prior to age 4 years, and an additional 190 (5%) received their first prescription after age 4 years. The researchers observed that 451 patients (13%) had a fracture after AD diagnosis at a median age of 7.4 years. The median age at last follow-up for the remaining 3,091 patients was 6.6 years. Evaluated as a time-dependent covariate, the use of a topical corticosteroid was associated with a nonsignificant 17% increased risk of fracture (hazard ratio, 1.17; P = .16).
In the landmark analysis, 1,722 patients (69%) were prescribed a topical corticosteroid prior to age 4 years. Of these patients, 333 (13%) had their first fracture after AD diagnosis, at a median age of 8.7 years. The median age at last follow-up for the remaining patients was 9.3 years. The researchers observed that, starting at 4 years of age, there was no association between topical corticosteroid use and risk of fracture (HR, 1.00; P = 1.00).
“Our findings suggest that topical corticosteroids do not significantly increase fracture risk in this pediatric population with atopic dermatitis,” Mr. Imhof said. “Dermatologists can use the results of this study to reassure parents of infants and young children, as most patients in our study received their first topical corticosteroid prescription prior to age 4.”
He acknowledged certain limitations of the study, such as its retrospective design and study population, which was predominantly white and resided in the upper Midwest. “Also, our study examined prescription data with the assumption made that topical corticosteroids were used as prescribed,” he said. “An additional limitation is that we evaluated ever versus never exposure to topical corticosteroids rather than cumulative duration of use and/or potency.”
Mr. Imhof and his colleagues reported having no financial disclosures.
suggest.
“Little has been published about the risk of fracture in children with atopic dermatitis on topical corticosteroids specifically,” one of the study authors, Reese L. Imhof, said in an interview following the virtual annual meeting of the Society for Pediatric Dermatology. “There are concerns, particularly among parents, regarding potential bone side effects through possible corticosteroid percutaneous absorption. Fears related to topical corticosteroid use likely stem from the fact that prolonged systemic corticosteroid use is associated with an increased risk of bone fractures.”
In an effort to determine the fracture risk in children who were diagnosed with atopic dermatitis (AD) prior to age 4 years and received topical corticosteroid treatment, Mr. Imhof, from Mayo Medical School, Rochester, Minn., and his associates used the Rochester Epidemiology Project records-linkage system to identify patients in Olmstead County, Minn., who received their first AD diagnosis prior to age 4 years between Jan. 1, 2004, through Dec. 31, 2017. Those who received topical corticosteroids listed in National Drug File-Reference Terminology class 8952 (anti-inflammatory, topical) or 8954 (anti-infective/anti-inflammatory combinations, topical) between Jan. 1, 2004, and Dec. 31, 2018 were included in the analysis and were followed to identify new bone fractures, excluding pathological fractures in neoplastic disease and skull or facial bone fractures.
The researchers conducted two analyses of the data. For the primary statistical analysis, they evaluated topical corticosteroid exposure as a binary time-dependent covariate in a Cox proportional hazard model using age as the time scale, with patients entering the risk set at the age of the first clinic visit rather than the age of their first AD diagnosis. Next, the researchers performed a landmark analysis as a sensitivity analysis. For this, each patient’s fourth birthday was defined as the starting point, since all included patients were diagnosed with AD prior to age 4 years.
Of the 7,505 patients first identified with AD, 3,542 were included in the primary analysis and 2,499 were included in the landmark analysis. In the primary analysis, 2,384 patients (67%) received a topical prescription for a topical corticosteroid prior to age 4 years, and an additional 190 (5%) received their first prescription after age 4 years. The researchers observed that 451 patients (13%) had a fracture after AD diagnosis at a median age of 7.4 years. The median age at last follow-up for the remaining 3,091 patients was 6.6 years. Evaluated as a time-dependent covariate, the use of a topical corticosteroid was associated with a nonsignificant 17% increased risk of fracture (hazard ratio, 1.17; P = .16).
In the landmark analysis, 1,722 patients (69%) were prescribed a topical corticosteroid prior to age 4 years. Of these patients, 333 (13%) had their first fracture after AD diagnosis, at a median age of 8.7 years. The median age at last follow-up for the remaining patients was 9.3 years. The researchers observed that, starting at 4 years of age, there was no association between topical corticosteroid use and risk of fracture (HR, 1.00; P = 1.00).
“Our findings suggest that topical corticosteroids do not significantly increase fracture risk in this pediatric population with atopic dermatitis,” Mr. Imhof said. “Dermatologists can use the results of this study to reassure parents of infants and young children, as most patients in our study received their first topical corticosteroid prescription prior to age 4.”
He acknowledged certain limitations of the study, such as its retrospective design and study population, which was predominantly white and resided in the upper Midwest. “Also, our study examined prescription data with the assumption made that topical corticosteroids were used as prescribed,” he said. “An additional limitation is that we evaluated ever versus never exposure to topical corticosteroids rather than cumulative duration of use and/or potency.”
Mr. Imhof and his colleagues reported having no financial disclosures.
FROM SPD 2020
How can hospitalists address health disparities for LGBTQ+ patients?
It is well established that lesbian, gay, bisexual, transgender, and queer (LGBTQ) patients suffer worse health outcomes, relative to patients who are heterosexual and cisgender – that is, those whose sense of personal identity and gender corresponds with their birth sex. The reasons for these disparities are multifactorial but include discrimination and limited provider knowledge about LGBTQ-specific health concerns.
These disparities – and what hospitalists can do to try to ameliorate them on the job – will be explored in a session at HM20 Virtual, “When the Answers Aren’t Straight Forward: Lesbian, Gay, Bisexual, Transgender, and Queer (LGBTQ) Health Updates for the Hospitalist.”
, according to Tyler Anstett, DO, copresenter and assistant professor in the division of hospital medicine at the University of Colorado. He and copresenter Keshav Khanijow, MD, an assistant professor in the division of hospital medicine, Northwestern University, Chicago, will share results from the Q-HEALTH (Quantifying Hospitalist Education and Awareness of LGBTQ Topics in Health) national survey of SHM members about their knowledge and attitudes regarding LGBTQ health. This survey, sponsored by SHM’s Education Committee, identified knowledge and comfort gaps in caring for LGBTQ+ patients. Most respondents say they are interested in receiving more didactic training on this topic, building on an introductory session on LGBTQ+ health presented at last year’s SHM Annual Conference. They also named the Annual Conference as one of their top venues for receiving such training.
The session at HM20 Virtual will cover the health disparities identified in LGBTQ+ populations, with case examples that highlight those disparities, Dr. Anstett said. “We will review results from Q-HEALTH, the SHM-wide survey on provider attitudes, knowledge, and comfort in caring for LGBTQ+ patients. Finally, the session will cover basic LGBTQ+ terminology and, through clinical scenarios, provide attendees with some basic skills for improving their practice for LGBTQ+ patients.”
With over 11 million Americans who identify as lesbian, gay, bisexual, transgender, and/or queer, hospitalists will certainly encounter patients of diverse sexual orientations and gender identities, Dr. Anstett said. Hospitalists should serve as allies for their patients, including for those who are LGBTQ+. Through this session, attendees can reflect on individual practice and learn how to educate others on LGBTQ+ health basics.
“We hope the cases we present will provide attendees with an introduction to the health issues the LGBTQ+ community faces with greater prevalence, and what hospitalists can be thinking about when they approach these issues,” Dr. Khanijow added.
Dr. Anstett and Dr. Khanijow had no relevant financial conflicts to disclose.
When the Answers Aren’t Straight Forward: Lesbian, Gay, Bisexual, Transgender, and Queer (LGBTQ) Health Updates for the Hospitalist
It is well established that lesbian, gay, bisexual, transgender, and queer (LGBTQ) patients suffer worse health outcomes, relative to patients who are heterosexual and cisgender – that is, those whose sense of personal identity and gender corresponds with their birth sex. The reasons for these disparities are multifactorial but include discrimination and limited provider knowledge about LGBTQ-specific health concerns.
These disparities – and what hospitalists can do to try to ameliorate them on the job – will be explored in a session at HM20 Virtual, “When the Answers Aren’t Straight Forward: Lesbian, Gay, Bisexual, Transgender, and Queer (LGBTQ) Health Updates for the Hospitalist.”
, according to Tyler Anstett, DO, copresenter and assistant professor in the division of hospital medicine at the University of Colorado. He and copresenter Keshav Khanijow, MD, an assistant professor in the division of hospital medicine, Northwestern University, Chicago, will share results from the Q-HEALTH (Quantifying Hospitalist Education and Awareness of LGBTQ Topics in Health) national survey of SHM members about their knowledge and attitudes regarding LGBTQ health. This survey, sponsored by SHM’s Education Committee, identified knowledge and comfort gaps in caring for LGBTQ+ patients. Most respondents say they are interested in receiving more didactic training on this topic, building on an introductory session on LGBTQ+ health presented at last year’s SHM Annual Conference. They also named the Annual Conference as one of their top venues for receiving such training.
The session at HM20 Virtual will cover the health disparities identified in LGBTQ+ populations, with case examples that highlight those disparities, Dr. Anstett said. “We will review results from Q-HEALTH, the SHM-wide survey on provider attitudes, knowledge, and comfort in caring for LGBTQ+ patients. Finally, the session will cover basic LGBTQ+ terminology and, through clinical scenarios, provide attendees with some basic skills for improving their practice for LGBTQ+ patients.”
With over 11 million Americans who identify as lesbian, gay, bisexual, transgender, and/or queer, hospitalists will certainly encounter patients of diverse sexual orientations and gender identities, Dr. Anstett said. Hospitalists should serve as allies for their patients, including for those who are LGBTQ+. Through this session, attendees can reflect on individual practice and learn how to educate others on LGBTQ+ health basics.
“We hope the cases we present will provide attendees with an introduction to the health issues the LGBTQ+ community faces with greater prevalence, and what hospitalists can be thinking about when they approach these issues,” Dr. Khanijow added.
Dr. Anstett and Dr. Khanijow had no relevant financial conflicts to disclose.
When the Answers Aren’t Straight Forward: Lesbian, Gay, Bisexual, Transgender, and Queer (LGBTQ) Health Updates for the Hospitalist
It is well established that lesbian, gay, bisexual, transgender, and queer (LGBTQ) patients suffer worse health outcomes, relative to patients who are heterosexual and cisgender – that is, those whose sense of personal identity and gender corresponds with their birth sex. The reasons for these disparities are multifactorial but include discrimination and limited provider knowledge about LGBTQ-specific health concerns.
These disparities – and what hospitalists can do to try to ameliorate them on the job – will be explored in a session at HM20 Virtual, “When the Answers Aren’t Straight Forward: Lesbian, Gay, Bisexual, Transgender, and Queer (LGBTQ) Health Updates for the Hospitalist.”
, according to Tyler Anstett, DO, copresenter and assistant professor in the division of hospital medicine at the University of Colorado. He and copresenter Keshav Khanijow, MD, an assistant professor in the division of hospital medicine, Northwestern University, Chicago, will share results from the Q-HEALTH (Quantifying Hospitalist Education and Awareness of LGBTQ Topics in Health) national survey of SHM members about their knowledge and attitudes regarding LGBTQ health. This survey, sponsored by SHM’s Education Committee, identified knowledge and comfort gaps in caring for LGBTQ+ patients. Most respondents say they are interested in receiving more didactic training on this topic, building on an introductory session on LGBTQ+ health presented at last year’s SHM Annual Conference. They also named the Annual Conference as one of their top venues for receiving such training.
The session at HM20 Virtual will cover the health disparities identified in LGBTQ+ populations, with case examples that highlight those disparities, Dr. Anstett said. “We will review results from Q-HEALTH, the SHM-wide survey on provider attitudes, knowledge, and comfort in caring for LGBTQ+ patients. Finally, the session will cover basic LGBTQ+ terminology and, through clinical scenarios, provide attendees with some basic skills for improving their practice for LGBTQ+ patients.”
With over 11 million Americans who identify as lesbian, gay, bisexual, transgender, and/or queer, hospitalists will certainly encounter patients of diverse sexual orientations and gender identities, Dr. Anstett said. Hospitalists should serve as allies for their patients, including for those who are LGBTQ+. Through this session, attendees can reflect on individual practice and learn how to educate others on LGBTQ+ health basics.
“We hope the cases we present will provide attendees with an introduction to the health issues the LGBTQ+ community faces with greater prevalence, and what hospitalists can be thinking about when they approach these issues,” Dr. Khanijow added.
Dr. Anstett and Dr. Khanijow had no relevant financial conflicts to disclose.
When the Answers Aren’t Straight Forward: Lesbian, Gay, Bisexual, Transgender, and Queer (LGBTQ) Health Updates for the Hospitalist
Early recognition of oncologic emergencies deemed ‘crucial’
During an oncologic emergency, making a clinical decision during the early diagnostic period is one of the most critical things a hospitalist can do when caring for patients with cancer. Hospitalists may not always be well versed in the symptoms of oncologic emergencies, though, particularly with newer treatments like immunotherapy and targeted therapies. They also may be tempted to contact colleagues in oncology when they may be qualified to handle these emergencies on their own.
At the end of her question-and-answer session, “Getting to Know Oncology Emergencies: Recognition and Management” to be presented on Aug. 12 at HM20 Virtual, the virtual annual meeting of the Society of Hospital Medicine, Megan Kruse, MD, hopes hospitalists will be able to recognize the signs and symptoms of “classic” oncologic emergencies they are likely to see in routine practice, as well as side effects of newer therapies they may not have encountered. Attendees will know how to manage these situations and understand when they need to involve a cancer specialist.
“Early recognition of these emergencies is crucial, and there are simple initial interventions that can make a big difference in patient outcomes,” said Dr. Kruse, an oncologist at the Cleveland Clinic.
In her presentation, Dr. Kruse will review oncologic emergencies that can occur in patients with acute leukemia such as acute blast crisis, as well as spinal cord compression and neutropenic fever. These complications are common in patients with cancer: Many cancers, such as multiple myeloma, lung cancer, and breast cancer, can cause spinal metastases that lead to spinal cord compression, while studies have shown neutropenic fever can occur in up to 80% of patients who undergo chemotherapy.
The presentation also will outline how hospitalists can manage specific side effects of immunotherapy and targeted therapies during an emergency situation. Dr. Kruse noted the session also will focus on when to start steroids for immune-related adverse event concerns and when to think about adding alternate immunosuppression. Complications of these therapies can differ from those of traditional chemotherapy, and not all hospitalists may be expecting them. Side effects from cancer therapy also can present months after treatment, further complicating the nature of oncologic emergencies in a hospital setting.
Recognizing the signs of such emergencies can be crucial for patients, especially if clinical decisions are made before a hospitalist can reach an oncologist for consult. Some decisions can be made by hospitalists themselves, while others may require specialty knowledge from an oncologist, Dr. Kruse noted. Regardless, it is important to consider cancer treatment history in a patient’s differential diagnosis.
Dr. Kruse has given presentations on oncologic emergencies at SHM annual conferences in the past, but notes this year’s virtual presentation will include more cases and examples of complications to improve recognition of these conditions. in patients with oncologic emergencies.
“I hope that attendees will leave with a better idea of what symptoms should be, warning signs of impending oncologic emergencies/complications, and what measures can be taken to treat these conditions prior to oncology service involvement,” Dr. Kruse said.
Dr. Kruse reported advisory board involvement for Novartis Oncology and consulting for Puma Biotechnology.
Getting to Know Oncology Emergencies: Recognition and Management
Live Q&A: Wednesday, Aug. 12, 1:00 p.m. to 2:00 p.m.
During an oncologic emergency, making a clinical decision during the early diagnostic period is one of the most critical things a hospitalist can do when caring for patients with cancer. Hospitalists may not always be well versed in the symptoms of oncologic emergencies, though, particularly with newer treatments like immunotherapy and targeted therapies. They also may be tempted to contact colleagues in oncology when they may be qualified to handle these emergencies on their own.
At the end of her question-and-answer session, “Getting to Know Oncology Emergencies: Recognition and Management” to be presented on Aug. 12 at HM20 Virtual, the virtual annual meeting of the Society of Hospital Medicine, Megan Kruse, MD, hopes hospitalists will be able to recognize the signs and symptoms of “classic” oncologic emergencies they are likely to see in routine practice, as well as side effects of newer therapies they may not have encountered. Attendees will know how to manage these situations and understand when they need to involve a cancer specialist.
“Early recognition of these emergencies is crucial, and there are simple initial interventions that can make a big difference in patient outcomes,” said Dr. Kruse, an oncologist at the Cleveland Clinic.
In her presentation, Dr. Kruse will review oncologic emergencies that can occur in patients with acute leukemia such as acute blast crisis, as well as spinal cord compression and neutropenic fever. These complications are common in patients with cancer: Many cancers, such as multiple myeloma, lung cancer, and breast cancer, can cause spinal metastases that lead to spinal cord compression, while studies have shown neutropenic fever can occur in up to 80% of patients who undergo chemotherapy.
The presentation also will outline how hospitalists can manage specific side effects of immunotherapy and targeted therapies during an emergency situation. Dr. Kruse noted the session also will focus on when to start steroids for immune-related adverse event concerns and when to think about adding alternate immunosuppression. Complications of these therapies can differ from those of traditional chemotherapy, and not all hospitalists may be expecting them. Side effects from cancer therapy also can present months after treatment, further complicating the nature of oncologic emergencies in a hospital setting.
Recognizing the signs of such emergencies can be crucial for patients, especially if clinical decisions are made before a hospitalist can reach an oncologist for consult. Some decisions can be made by hospitalists themselves, while others may require specialty knowledge from an oncologist, Dr. Kruse noted. Regardless, it is important to consider cancer treatment history in a patient’s differential diagnosis.
Dr. Kruse has given presentations on oncologic emergencies at SHM annual conferences in the past, but notes this year’s virtual presentation will include more cases and examples of complications to improve recognition of these conditions. in patients with oncologic emergencies.
“I hope that attendees will leave with a better idea of what symptoms should be, warning signs of impending oncologic emergencies/complications, and what measures can be taken to treat these conditions prior to oncology service involvement,” Dr. Kruse said.
Dr. Kruse reported advisory board involvement for Novartis Oncology and consulting for Puma Biotechnology.
Getting to Know Oncology Emergencies: Recognition and Management
Live Q&A: Wednesday, Aug. 12, 1:00 p.m. to 2:00 p.m.
During an oncologic emergency, making a clinical decision during the early diagnostic period is one of the most critical things a hospitalist can do when caring for patients with cancer. Hospitalists may not always be well versed in the symptoms of oncologic emergencies, though, particularly with newer treatments like immunotherapy and targeted therapies. They also may be tempted to contact colleagues in oncology when they may be qualified to handle these emergencies on their own.
At the end of her question-and-answer session, “Getting to Know Oncology Emergencies: Recognition and Management” to be presented on Aug. 12 at HM20 Virtual, the virtual annual meeting of the Society of Hospital Medicine, Megan Kruse, MD, hopes hospitalists will be able to recognize the signs and symptoms of “classic” oncologic emergencies they are likely to see in routine practice, as well as side effects of newer therapies they may not have encountered. Attendees will know how to manage these situations and understand when they need to involve a cancer specialist.
“Early recognition of these emergencies is crucial, and there are simple initial interventions that can make a big difference in patient outcomes,” said Dr. Kruse, an oncologist at the Cleveland Clinic.
In her presentation, Dr. Kruse will review oncologic emergencies that can occur in patients with acute leukemia such as acute blast crisis, as well as spinal cord compression and neutropenic fever. These complications are common in patients with cancer: Many cancers, such as multiple myeloma, lung cancer, and breast cancer, can cause spinal metastases that lead to spinal cord compression, while studies have shown neutropenic fever can occur in up to 80% of patients who undergo chemotherapy.
The presentation also will outline how hospitalists can manage specific side effects of immunotherapy and targeted therapies during an emergency situation. Dr. Kruse noted the session also will focus on when to start steroids for immune-related adverse event concerns and when to think about adding alternate immunosuppression. Complications of these therapies can differ from those of traditional chemotherapy, and not all hospitalists may be expecting them. Side effects from cancer therapy also can present months after treatment, further complicating the nature of oncologic emergencies in a hospital setting.
Recognizing the signs of such emergencies can be crucial for patients, especially if clinical decisions are made before a hospitalist can reach an oncologist for consult. Some decisions can be made by hospitalists themselves, while others may require specialty knowledge from an oncologist, Dr. Kruse noted. Regardless, it is important to consider cancer treatment history in a patient’s differential diagnosis.
Dr. Kruse has given presentations on oncologic emergencies at SHM annual conferences in the past, but notes this year’s virtual presentation will include more cases and examples of complications to improve recognition of these conditions. in patients with oncologic emergencies.
“I hope that attendees will leave with a better idea of what symptoms should be, warning signs of impending oncologic emergencies/complications, and what measures can be taken to treat these conditions prior to oncology service involvement,” Dr. Kruse said.
Dr. Kruse reported advisory board involvement for Novartis Oncology and consulting for Puma Biotechnology.
Getting to Know Oncology Emergencies: Recognition and Management
Live Q&A: Wednesday, Aug. 12, 1:00 p.m. to 2:00 p.m.
Trio of antibodies may enable earlier diagnosis of axSpA
Three autoantibodies to newly discovered axial spondyloarthritis peptides may improve early diagnosis of the disease, according to a cross-sectional cohort study reported in Arthritis & Rheumatology.
The Assessment in SpondyloArthritis International Society (ASAS) classification criteria were not intended for diagnosis and do not differentiate well between patients with early axial spondyloarthritis (axSpA) and patients with nonspecific chronic low back pain, note the investigators, who conducted their research under senior investigator Veerle Somers, PhD, professor of molecular biology at Hasselt (Belgium) University and vice dean of the School of Life Sciences at Transnationale Universiteit Limburg, also in Hasselt.
“Therefore, for many patients, axSpA diagnosis may be challenging and is often delayed by several years after the occurrence of first clinical symptoms, posing a problem for early treatment initiation,” they wrote.
The investigators used plasma samples from patients with early disease and an axSpA complementary DNA phage display library developed with synovial tissue to screen for IgG antibodies that displayed significantly higher reactivity to plasma pools from the early axSpA patients than healthy controls.
They then assessed presence of the antibodies with enzyme-linked immunosorbent assays in a mixed cohort (76 patients with early axSpA having mean disease duration of 2.8 years, 75 control patients with nonspecific chronic low back pain, 60 patients with RA, and 94 healthy controls) and in an axSpA-only cohort (174 patients, 79 of whom had early disease with mean disease duration of 1.4 years).
Screening identified antibodies to nine novel peptides – eight peptides showing partial homology to human proteins and one novel axSpA autoantigen, double homeobox protein 4 (DUX4) – that were more commonly present in patients with early axSpA than in healthy controls, Dr. Somers and coinvestigators reported.
Subsequent analyses focused on the three antibodies having the highest positive likelihood ratios for differentiating axSpA from chronic low back pain.
Some 14.2% of the combined group of all patients with early axSpA had at least one antibody in this panel, compared with just 5.3% of the patients with chronic low back pain (P = .0484), corresponding to 95% specificity.
Prevalence did not differ significantly from that in patients with RA (10.0%; P = .5025) or healthy controls (8.4%; P = .2292).
The positive likelihood ratio for confirming early axSpA using the three antibodies was 2.7, on par with the historical ratio of 2.5 seen for C-reactive protein (CRP), the currently used laboratory marker, the investigators noted.
Among the patients with chronic low back pain, the posttest probability for axSpA increased from 79% with presence of inflammatory back pain and positive test results for HLA-B27 and CRP to 91% with addition of testing for the three antibodies.
The researchers proposed that, “in combination with other laboratory markers such as HLA-B27 and CRP, antibodies against our [three peptides] ... could provide a novel tool for the diagnosis of a subset of axSpA patients,” but the three-peptide panel needs to be studied more in larger cohorts of early axSpA patients and controls with low back pain.
Findings in context
“The authors did a number of steps laudably,” James T. Rosenbaum, MD, chair of the division of arthritis and rheumatic diseases and the Edward E. Rosenbaum Professor of Inflammation Research at Oregon Health & Science University, Portland, commented in an interview. Specifically, they used a variety of appropriate controls, had discovery and validation sets, achieved a fairly good sample size, and applied the phage library technique.
“Despite this technological tour de force and the need for a sensitive and specific blood test to diagnose nonradiographic axSpA, this study is preliminary,” he cautioned. “For example, the authors found antibodies to DUX4 in 8% of axSpA patients versus 3% of healthy controls, 4% of patients with chronic low back pain, and 7% with RA. It took a combination of antigens to enhance the diagnostic accuracy of the ASAS criteria to diagnose axSpA. For each antigen that was studied, more than 80% of the axSpA patients had no detectable antibodies.”
Importantly, rheumatic diseases are often immune mediated without being autoimmune, calling into question the role of the antibodies, according to Dr. Rosenbaum.
“Even if further studies validate these observations, additional research needs to be done to support the concept that these antibodies cause disease as opposed to being mere epiphenomena as is suggested by the low prevalence,” he concluded. “Current hypotheses as to the cause of ankylosing spondylitis now point to the microbiome and autoinflammatory rather than autoimmune pathways, but the jury is still out.”
Dr. Somers and three coauthors disclosed having a patent pending on the markers. The study was funded by a personal grant from the Agency for Innovation by Science and Technology Flanders. Dr. Rosenbaum disclosed that he consults for AbbVie, Gilead, Novartis, Pfizer, Roche, and UCB.
SOURCE: Quaden D et al. Arthritis Rheumatol. 2020 Jul 8. doi: 10.1002/art.41427.
Three autoantibodies to newly discovered axial spondyloarthritis peptides may improve early diagnosis of the disease, according to a cross-sectional cohort study reported in Arthritis & Rheumatology.
The Assessment in SpondyloArthritis International Society (ASAS) classification criteria were not intended for diagnosis and do not differentiate well between patients with early axial spondyloarthritis (axSpA) and patients with nonspecific chronic low back pain, note the investigators, who conducted their research under senior investigator Veerle Somers, PhD, professor of molecular biology at Hasselt (Belgium) University and vice dean of the School of Life Sciences at Transnationale Universiteit Limburg, also in Hasselt.
“Therefore, for many patients, axSpA diagnosis may be challenging and is often delayed by several years after the occurrence of first clinical symptoms, posing a problem for early treatment initiation,” they wrote.
The investigators used plasma samples from patients with early disease and an axSpA complementary DNA phage display library developed with synovial tissue to screen for IgG antibodies that displayed significantly higher reactivity to plasma pools from the early axSpA patients than healthy controls.
They then assessed presence of the antibodies with enzyme-linked immunosorbent assays in a mixed cohort (76 patients with early axSpA having mean disease duration of 2.8 years, 75 control patients with nonspecific chronic low back pain, 60 patients with RA, and 94 healthy controls) and in an axSpA-only cohort (174 patients, 79 of whom had early disease with mean disease duration of 1.4 years).
Screening identified antibodies to nine novel peptides – eight peptides showing partial homology to human proteins and one novel axSpA autoantigen, double homeobox protein 4 (DUX4) – that were more commonly present in patients with early axSpA than in healthy controls, Dr. Somers and coinvestigators reported.
Subsequent analyses focused on the three antibodies having the highest positive likelihood ratios for differentiating axSpA from chronic low back pain.
Some 14.2% of the combined group of all patients with early axSpA had at least one antibody in this panel, compared with just 5.3% of the patients with chronic low back pain (P = .0484), corresponding to 95% specificity.
Prevalence did not differ significantly from that in patients with RA (10.0%; P = .5025) or healthy controls (8.4%; P = .2292).
The positive likelihood ratio for confirming early axSpA using the three antibodies was 2.7, on par with the historical ratio of 2.5 seen for C-reactive protein (CRP), the currently used laboratory marker, the investigators noted.
Among the patients with chronic low back pain, the posttest probability for axSpA increased from 79% with presence of inflammatory back pain and positive test results for HLA-B27 and CRP to 91% with addition of testing for the three antibodies.
The researchers proposed that, “in combination with other laboratory markers such as HLA-B27 and CRP, antibodies against our [three peptides] ... could provide a novel tool for the diagnosis of a subset of axSpA patients,” but the three-peptide panel needs to be studied more in larger cohorts of early axSpA patients and controls with low back pain.
Findings in context
“The authors did a number of steps laudably,” James T. Rosenbaum, MD, chair of the division of arthritis and rheumatic diseases and the Edward E. Rosenbaum Professor of Inflammation Research at Oregon Health & Science University, Portland, commented in an interview. Specifically, they used a variety of appropriate controls, had discovery and validation sets, achieved a fairly good sample size, and applied the phage library technique.
“Despite this technological tour de force and the need for a sensitive and specific blood test to diagnose nonradiographic axSpA, this study is preliminary,” he cautioned. “For example, the authors found antibodies to DUX4 in 8% of axSpA patients versus 3% of healthy controls, 4% of patients with chronic low back pain, and 7% with RA. It took a combination of antigens to enhance the diagnostic accuracy of the ASAS criteria to diagnose axSpA. For each antigen that was studied, more than 80% of the axSpA patients had no detectable antibodies.”
Importantly, rheumatic diseases are often immune mediated without being autoimmune, calling into question the role of the antibodies, according to Dr. Rosenbaum.
“Even if further studies validate these observations, additional research needs to be done to support the concept that these antibodies cause disease as opposed to being mere epiphenomena as is suggested by the low prevalence,” he concluded. “Current hypotheses as to the cause of ankylosing spondylitis now point to the microbiome and autoinflammatory rather than autoimmune pathways, but the jury is still out.”
Dr. Somers and three coauthors disclosed having a patent pending on the markers. The study was funded by a personal grant from the Agency for Innovation by Science and Technology Flanders. Dr. Rosenbaum disclosed that he consults for AbbVie, Gilead, Novartis, Pfizer, Roche, and UCB.
SOURCE: Quaden D et al. Arthritis Rheumatol. 2020 Jul 8. doi: 10.1002/art.41427.
Three autoantibodies to newly discovered axial spondyloarthritis peptides may improve early diagnosis of the disease, according to a cross-sectional cohort study reported in Arthritis & Rheumatology.
The Assessment in SpondyloArthritis International Society (ASAS) classification criteria were not intended for diagnosis and do not differentiate well between patients with early axial spondyloarthritis (axSpA) and patients with nonspecific chronic low back pain, note the investigators, who conducted their research under senior investigator Veerle Somers, PhD, professor of molecular biology at Hasselt (Belgium) University and vice dean of the School of Life Sciences at Transnationale Universiteit Limburg, also in Hasselt.
“Therefore, for many patients, axSpA diagnosis may be challenging and is often delayed by several years after the occurrence of first clinical symptoms, posing a problem for early treatment initiation,” they wrote.
The investigators used plasma samples from patients with early disease and an axSpA complementary DNA phage display library developed with synovial tissue to screen for IgG antibodies that displayed significantly higher reactivity to plasma pools from the early axSpA patients than healthy controls.
They then assessed presence of the antibodies with enzyme-linked immunosorbent assays in a mixed cohort (76 patients with early axSpA having mean disease duration of 2.8 years, 75 control patients with nonspecific chronic low back pain, 60 patients with RA, and 94 healthy controls) and in an axSpA-only cohort (174 patients, 79 of whom had early disease with mean disease duration of 1.4 years).
Screening identified antibodies to nine novel peptides – eight peptides showing partial homology to human proteins and one novel axSpA autoantigen, double homeobox protein 4 (DUX4) – that were more commonly present in patients with early axSpA than in healthy controls, Dr. Somers and coinvestigators reported.
Subsequent analyses focused on the three antibodies having the highest positive likelihood ratios for differentiating axSpA from chronic low back pain.
Some 14.2% of the combined group of all patients with early axSpA had at least one antibody in this panel, compared with just 5.3% of the patients with chronic low back pain (P = .0484), corresponding to 95% specificity.
Prevalence did not differ significantly from that in patients with RA (10.0%; P = .5025) or healthy controls (8.4%; P = .2292).
The positive likelihood ratio for confirming early axSpA using the three antibodies was 2.7, on par with the historical ratio of 2.5 seen for C-reactive protein (CRP), the currently used laboratory marker, the investigators noted.
Among the patients with chronic low back pain, the posttest probability for axSpA increased from 79% with presence of inflammatory back pain and positive test results for HLA-B27 and CRP to 91% with addition of testing for the three antibodies.
The researchers proposed that, “in combination with other laboratory markers such as HLA-B27 and CRP, antibodies against our [three peptides] ... could provide a novel tool for the diagnosis of a subset of axSpA patients,” but the three-peptide panel needs to be studied more in larger cohorts of early axSpA patients and controls with low back pain.
Findings in context
“The authors did a number of steps laudably,” James T. Rosenbaum, MD, chair of the division of arthritis and rheumatic diseases and the Edward E. Rosenbaum Professor of Inflammation Research at Oregon Health & Science University, Portland, commented in an interview. Specifically, they used a variety of appropriate controls, had discovery and validation sets, achieved a fairly good sample size, and applied the phage library technique.
“Despite this technological tour de force and the need for a sensitive and specific blood test to diagnose nonradiographic axSpA, this study is preliminary,” he cautioned. “For example, the authors found antibodies to DUX4 in 8% of axSpA patients versus 3% of healthy controls, 4% of patients with chronic low back pain, and 7% with RA. It took a combination of antigens to enhance the diagnostic accuracy of the ASAS criteria to diagnose axSpA. For each antigen that was studied, more than 80% of the axSpA patients had no detectable antibodies.”
Importantly, rheumatic diseases are often immune mediated without being autoimmune, calling into question the role of the antibodies, according to Dr. Rosenbaum.
“Even if further studies validate these observations, additional research needs to be done to support the concept that these antibodies cause disease as opposed to being mere epiphenomena as is suggested by the low prevalence,” he concluded. “Current hypotheses as to the cause of ankylosing spondylitis now point to the microbiome and autoinflammatory rather than autoimmune pathways, but the jury is still out.”
Dr. Somers and three coauthors disclosed having a patent pending on the markers. The study was funded by a personal grant from the Agency for Innovation by Science and Technology Flanders. Dr. Rosenbaum disclosed that he consults for AbbVie, Gilead, Novartis, Pfizer, Roche, and UCB.
SOURCE: Quaden D et al. Arthritis Rheumatol. 2020 Jul 8. doi: 10.1002/art.41427.
FROM ARTHRITIS & RHEUMATOLOGY
Simplifying the antibiotic selection process
Hospitalists are constantly battling infection.
James Soo Kim, MD, a hospitalist and assistant professor at Emory Healthcare in Atlanta, a presenter of the session “Antibiotics Made Ridiculously Simple” during HM20 Virtual, said that while he has given this talk at previous Society of Hospital Medicine Annual Conferences, the presentation has undergone significant changes over the years as the landscape of infectious disease treatment has shifted.
He hopes attendees of HM20 Virtual will appreciate the changes and encourages those who have attended his presentation in previous years to come see what is new, but admitted newcomers may think the presentation’s title is a bit of a misnomer.
“Despite the title of the talk, there really isn’t any way to make antibiotics ridiculously simple,” he said.
Dr. Kim, who is also an editorial board member for The Hospitalist, said the origin of “Antibiotics Made Ridiculously Simple” took place during his residency, where he had an interest in infectious disease. This interest carried over to his time in fellowship at the Keck School of Medicine of the University of Southern California – and was enough to become board certified in infectious disease by the American Board of Internal Medicine. Infectious disease continues to interest him now as an attending, he said, and since he joined Emory Healthcare in 2012, he has given a version of this presentation every year.
HM20 Virtual attendees will come away from the presentation with an idea of how to choose an antibiotic regimen, Dr. Kim said, including how to select an antibiotic when you’re worried about Pseudomonas, methicillin-resistant Staphylococcus aureus, and vancomycin-resistant Enterococcus or other likely organisms. “There are a variety of drugs out there that have activity against our ‘usual suspects,’ ” he said.
Attendees will also learn to select antibiotic options that have empiric coverage during a shortage of piperacillin/tazobactam (Zosyn), vancomycin, or your preferred drug of choice for treating common infections. He will also review the latest drugs that have been released over the past few years so attendees can add them to their armamentarium.
“I won’t necessarily expect attendees to use everything I talk about, but if you have a patient on service that infectious disease started Vabomere on, you’ll at least have a general idea of what they were worried about,” Dr. Kim said.
One practice pearl he hopes attendees take away from his presentation: Allergies to beta-lactam antibiotics like penicillin (PCN) derivatives are not as common as most providers and patients believe, and not giving these antibiotics to patients can actually decrease the chance that the patient gets appropriate therapy while also increasing the cost of care.
“I hope that my talk changes practice by making people aware of how infrequent true clinically significant PCN cross-reactions are so that patients can get more cost-effective and medically effective therapy,” he said.Dr. Kim reports no relevant financial disclosures.
Antibiotics Made Ridiculously Simple Live Q&A: Tuesday, August 18, 3:30-4:30 p.m.
Hospitalists are constantly battling infection.
James Soo Kim, MD, a hospitalist and assistant professor at Emory Healthcare in Atlanta, a presenter of the session “Antibiotics Made Ridiculously Simple” during HM20 Virtual, said that while he has given this talk at previous Society of Hospital Medicine Annual Conferences, the presentation has undergone significant changes over the years as the landscape of infectious disease treatment has shifted.
He hopes attendees of HM20 Virtual will appreciate the changes and encourages those who have attended his presentation in previous years to come see what is new, but admitted newcomers may think the presentation’s title is a bit of a misnomer.
“Despite the title of the talk, there really isn’t any way to make antibiotics ridiculously simple,” he said.
Dr. Kim, who is also an editorial board member for The Hospitalist, said the origin of “Antibiotics Made Ridiculously Simple” took place during his residency, where he had an interest in infectious disease. This interest carried over to his time in fellowship at the Keck School of Medicine of the University of Southern California – and was enough to become board certified in infectious disease by the American Board of Internal Medicine. Infectious disease continues to interest him now as an attending, he said, and since he joined Emory Healthcare in 2012, he has given a version of this presentation every year.
HM20 Virtual attendees will come away from the presentation with an idea of how to choose an antibiotic regimen, Dr. Kim said, including how to select an antibiotic when you’re worried about Pseudomonas, methicillin-resistant Staphylococcus aureus, and vancomycin-resistant Enterococcus or other likely organisms. “There are a variety of drugs out there that have activity against our ‘usual suspects,’ ” he said.
Attendees will also learn to select antibiotic options that have empiric coverage during a shortage of piperacillin/tazobactam (Zosyn), vancomycin, or your preferred drug of choice for treating common infections. He will also review the latest drugs that have been released over the past few years so attendees can add them to their armamentarium.
“I won’t necessarily expect attendees to use everything I talk about, but if you have a patient on service that infectious disease started Vabomere on, you’ll at least have a general idea of what they were worried about,” Dr. Kim said.
One practice pearl he hopes attendees take away from his presentation: Allergies to beta-lactam antibiotics like penicillin (PCN) derivatives are not as common as most providers and patients believe, and not giving these antibiotics to patients can actually decrease the chance that the patient gets appropriate therapy while also increasing the cost of care.
“I hope that my talk changes practice by making people aware of how infrequent true clinically significant PCN cross-reactions are so that patients can get more cost-effective and medically effective therapy,” he said.Dr. Kim reports no relevant financial disclosures.
Antibiotics Made Ridiculously Simple Live Q&A: Tuesday, August 18, 3:30-4:30 p.m.
Hospitalists are constantly battling infection.
James Soo Kim, MD, a hospitalist and assistant professor at Emory Healthcare in Atlanta, a presenter of the session “Antibiotics Made Ridiculously Simple” during HM20 Virtual, said that while he has given this talk at previous Society of Hospital Medicine Annual Conferences, the presentation has undergone significant changes over the years as the landscape of infectious disease treatment has shifted.
He hopes attendees of HM20 Virtual will appreciate the changes and encourages those who have attended his presentation in previous years to come see what is new, but admitted newcomers may think the presentation’s title is a bit of a misnomer.
“Despite the title of the talk, there really isn’t any way to make antibiotics ridiculously simple,” he said.
Dr. Kim, who is also an editorial board member for The Hospitalist, said the origin of “Antibiotics Made Ridiculously Simple” took place during his residency, where he had an interest in infectious disease. This interest carried over to his time in fellowship at the Keck School of Medicine of the University of Southern California – and was enough to become board certified in infectious disease by the American Board of Internal Medicine. Infectious disease continues to interest him now as an attending, he said, and since he joined Emory Healthcare in 2012, he has given a version of this presentation every year.
HM20 Virtual attendees will come away from the presentation with an idea of how to choose an antibiotic regimen, Dr. Kim said, including how to select an antibiotic when you’re worried about Pseudomonas, methicillin-resistant Staphylococcus aureus, and vancomycin-resistant Enterococcus or other likely organisms. “There are a variety of drugs out there that have activity against our ‘usual suspects,’ ” he said.
Attendees will also learn to select antibiotic options that have empiric coverage during a shortage of piperacillin/tazobactam (Zosyn), vancomycin, or your preferred drug of choice for treating common infections. He will also review the latest drugs that have been released over the past few years so attendees can add them to their armamentarium.
“I won’t necessarily expect attendees to use everything I talk about, but if you have a patient on service that infectious disease started Vabomere on, you’ll at least have a general idea of what they were worried about,” Dr. Kim said.
One practice pearl he hopes attendees take away from his presentation: Allergies to beta-lactam antibiotics like penicillin (PCN) derivatives are not as common as most providers and patients believe, and not giving these antibiotics to patients can actually decrease the chance that the patient gets appropriate therapy while also increasing the cost of care.
“I hope that my talk changes practice by making people aware of how infrequent true clinically significant PCN cross-reactions are so that patients can get more cost-effective and medically effective therapy,” he said.Dr. Kim reports no relevant financial disclosures.
Antibiotics Made Ridiculously Simple Live Q&A: Tuesday, August 18, 3:30-4:30 p.m.