User login
Tool-less but not clueless
There is apparently some debate about which of our ancestors was the first to use tools. It probably was Homo habilis, the “handy man.” But it could have been a relative of Lucy, of the Australopithecus afarensis tribe. Regardless of which pile of chipped rocks looks more tool-like to you, it is generally agreed that our ability to make and use tools is one of the key ingredients to our evolutionary success.
I have always enjoyed the feel of good quality knife when I am woodcarving, and the tool collection hanging on the wall over my work bench is one of my most prized possessions. But when I was practicing general pediatrics, I could never really warm up to the screening tools that were being touted as must-haves for detecting developmental delays.
It turns out I was not alone. A recent study published in Pediatrics found that the number of pediatricians who reported using developmental screening tools increased from 21% to 63% between 2002 and 2016. (Pediatrics. 2020 Apr. doi: 10.1542/peds.2019-0851). However, this means that, despite a significant increase in usage, more than a third of pediatricians still are not employing screening tools. Does this suggest that one out of every three pediatricians, including me and maybe you, is a knuckle-dragging pre–Homo sapiens practicing in blissful and clueless ignorance?
Mei Elansary MD, MPhil, and Michael Silverstein, MD, MPH, who wrote a companion commentary in the same journal, suggested that maybe those of us who have resisted the call to be tool users aren’t prehistoric ignoramuses (Pediatrics. 2020 Apr. doi: 10.1542/peds.2020-0164). They observed that, regardless of whether the pediatricians were using screening tools, more than 40% of the those surveyed did not refer patients for early intervention.
The commentators pointed out that the decision of when, whom, and how to screen must be viewed as part of a “complicated web of changing epidemiology, time and reimbursement constraints, and service availability.” They observe that pediatricians facing this landscape in upheaval “default to what they know best: clinical judgment.” Citing one study of the management of febrile infants, the authors point out that relying on guidelines doesn’t always result in improved clinical care.
My decision of when to refer a patient for early intervention was based on what I had observed over a series of visits and whether I thought that the early intervention resources available in my community would have a significant benefit for any particular child. Because I crafted my practice around a model that put a strong emphasis on continuity, my patients almost never saw another provider for a health maintenance visit and usually saw me for their sick visits, including ear rechecks.
I guess you could argue that there are situations in which seeing a variety of providers, each with a slightly different perspective, might benefit the patient. But when we are talking about a domain like development that is defined by change, or lack of change, over time, multiple observations by a single observer usually can be more valuable.
If I were practicing in a situation in which I didn’t have the luxury of continuity, I think I would be more likely to use a screening tool. Although I have found screening guidelines can be helpful as mnemonics in some situations, they aren’t equally applicable in all clinical settings.
While I may be asking for trouble by questioning anything even remotely related to the concept of early intervention, I must say that I wholeheartedly agree with Dr. Elansary and Dr. Silverstein when they wrote “the pediatrics community may have something to learn from the significant minority of pediatricians who do not practice formalized screening.”
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at pdnews@mdedge.com.
There is apparently some debate about which of our ancestors was the first to use tools. It probably was Homo habilis, the “handy man.” But it could have been a relative of Lucy, of the Australopithecus afarensis tribe. Regardless of which pile of chipped rocks looks more tool-like to you, it is generally agreed that our ability to make and use tools is one of the key ingredients to our evolutionary success.
I have always enjoyed the feel of good quality knife when I am woodcarving, and the tool collection hanging on the wall over my work bench is one of my most prized possessions. But when I was practicing general pediatrics, I could never really warm up to the screening tools that were being touted as must-haves for detecting developmental delays.
It turns out I was not alone. A recent study published in Pediatrics found that the number of pediatricians who reported using developmental screening tools increased from 21% to 63% between 2002 and 2016. (Pediatrics. 2020 Apr. doi: 10.1542/peds.2019-0851). However, this means that, despite a significant increase in usage, more than a third of pediatricians still are not employing screening tools. Does this suggest that one out of every three pediatricians, including me and maybe you, is a knuckle-dragging pre–Homo sapiens practicing in blissful and clueless ignorance?
Mei Elansary MD, MPhil, and Michael Silverstein, MD, MPH, who wrote a companion commentary in the same journal, suggested that maybe those of us who have resisted the call to be tool users aren’t prehistoric ignoramuses (Pediatrics. 2020 Apr. doi: 10.1542/peds.2020-0164). They observed that, regardless of whether the pediatricians were using screening tools, more than 40% of the those surveyed did not refer patients for early intervention.
The commentators pointed out that the decision of when, whom, and how to screen must be viewed as part of a “complicated web of changing epidemiology, time and reimbursement constraints, and service availability.” They observe that pediatricians facing this landscape in upheaval “default to what they know best: clinical judgment.” Citing one study of the management of febrile infants, the authors point out that relying on guidelines doesn’t always result in improved clinical care.
My decision of when to refer a patient for early intervention was based on what I had observed over a series of visits and whether I thought that the early intervention resources available in my community would have a significant benefit for any particular child. Because I crafted my practice around a model that put a strong emphasis on continuity, my patients almost never saw another provider for a health maintenance visit and usually saw me for their sick visits, including ear rechecks.
I guess you could argue that there are situations in which seeing a variety of providers, each with a slightly different perspective, might benefit the patient. But when we are talking about a domain like development that is defined by change, or lack of change, over time, multiple observations by a single observer usually can be more valuable.
If I were practicing in a situation in which I didn’t have the luxury of continuity, I think I would be more likely to use a screening tool. Although I have found screening guidelines can be helpful as mnemonics in some situations, they aren’t equally applicable in all clinical settings.
While I may be asking for trouble by questioning anything even remotely related to the concept of early intervention, I must say that I wholeheartedly agree with Dr. Elansary and Dr. Silverstein when they wrote “the pediatrics community may have something to learn from the significant minority of pediatricians who do not practice formalized screening.”
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at pdnews@mdedge.com.
There is apparently some debate about which of our ancestors was the first to use tools. It probably was Homo habilis, the “handy man.” But it could have been a relative of Lucy, of the Australopithecus afarensis tribe. Regardless of which pile of chipped rocks looks more tool-like to you, it is generally agreed that our ability to make and use tools is one of the key ingredients to our evolutionary success.
I have always enjoyed the feel of good quality knife when I am woodcarving, and the tool collection hanging on the wall over my work bench is one of my most prized possessions. But when I was practicing general pediatrics, I could never really warm up to the screening tools that were being touted as must-haves for detecting developmental delays.
It turns out I was not alone. A recent study published in Pediatrics found that the number of pediatricians who reported using developmental screening tools increased from 21% to 63% between 2002 and 2016. (Pediatrics. 2020 Apr. doi: 10.1542/peds.2019-0851). However, this means that, despite a significant increase in usage, more than a third of pediatricians still are not employing screening tools. Does this suggest that one out of every three pediatricians, including me and maybe you, is a knuckle-dragging pre–Homo sapiens practicing in blissful and clueless ignorance?
Mei Elansary MD, MPhil, and Michael Silverstein, MD, MPH, who wrote a companion commentary in the same journal, suggested that maybe those of us who have resisted the call to be tool users aren’t prehistoric ignoramuses (Pediatrics. 2020 Apr. doi: 10.1542/peds.2020-0164). They observed that, regardless of whether the pediatricians were using screening tools, more than 40% of the those surveyed did not refer patients for early intervention.
The commentators pointed out that the decision of when, whom, and how to screen must be viewed as part of a “complicated web of changing epidemiology, time and reimbursement constraints, and service availability.” They observe that pediatricians facing this landscape in upheaval “default to what they know best: clinical judgment.” Citing one study of the management of febrile infants, the authors point out that relying on guidelines doesn’t always result in improved clinical care.
My decision of when to refer a patient for early intervention was based on what I had observed over a series of visits and whether I thought that the early intervention resources available in my community would have a significant benefit for any particular child. Because I crafted my practice around a model that put a strong emphasis on continuity, my patients almost never saw another provider for a health maintenance visit and usually saw me for their sick visits, including ear rechecks.
I guess you could argue that there are situations in which seeing a variety of providers, each with a slightly different perspective, might benefit the patient. But when we are talking about a domain like development that is defined by change, or lack of change, over time, multiple observations by a single observer usually can be more valuable.
If I were practicing in a situation in which I didn’t have the luxury of continuity, I think I would be more likely to use a screening tool. Although I have found screening guidelines can be helpful as mnemonics in some situations, they aren’t equally applicable in all clinical settings.
While I may be asking for trouble by questioning anything even remotely related to the concept of early intervention, I must say that I wholeheartedly agree with Dr. Elansary and Dr. Silverstein when they wrote “the pediatrics community may have something to learn from the significant minority of pediatricians who do not practice formalized screening.”
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at pdnews@mdedge.com.
An unexplained exacerbation of depression, anxiety, and panic
CASE Depression, anxiety, and panic attacks
At the urging of his parents Mr. P, age 33, presents to the partial hospitalization program (PHP) for worsening depression and anxiety, daily panic attacks with accompanying diaphoresis and headache, and the possibility that he may have taken an overdose of zolpidem. Mr. P denies taking an intentional overdose of zolpidem, claiming instead that he was having a sleep-walking episode and did not realize how many pills he took.
In addition to daily panic attacks, Mr. P reports having trouble falling asleep, overwhelming sadness, and daily passive suicidal ideation without a plan or active intent.
Mr. P cannot identify a specific trigger to this most recent exacerbation of depressed/anxious mood, but instead describes it as slowly building over the past 6 to 8 months. Mr. P says the panic attacks occur without warning and states, “I feel like my heart is going to jump out of my chest; I get a terrible headache, and I sweat like crazy. Sometimes I just feel like I’m about to pass out or die.” Although these episodes had been present for approximately 2 years, they now occur almost daily.
HISTORY Inconsistent adherence
For the last year, Mr. P had been taking alprazolam, 0.5 mg twice daily, and paroxetine, 20 mg/d, and these medications provided moderate relief of his depressive/anxious symptoms. However, he stopped taking both medications approximately 3 or 4 weeks ago when he ran out. He also takes propranolol, 20 mg/d, sporadically, for hypertension. In the past, he had been prescribed carvedilol, clonidine, and lisinopril—all with varying degrees of relief of his hypertension. He denies a family history of hypertension or any other chronic or acute health problems. He reports that he has been sober from alcohol for 19 months but smokes 1 to 2 marijuana cigarettes a day.
EVALUATION Elevated blood pressure and pulse
Mr. P’s physical examination and medical review of systems are unremarkable, except for an elevated blood pressure (190/110 mm Hg) and pulse (92 beats per minute); he also has a headache. A repeat blood pressure test later in the day is 172/94 mm Hg, with a pulse of 100 beats per minute. His urine drug screen is positive only for delta-9-tetrahydrocannabinol (THC).
[polldaddy:10558304]
The author’s observations
A CBC with differential is helpful for ruling out infection and anemia as causes of anxiety and depression.1 In Mr. P’s case, there were no concerning symptoms that pointed to anemia or infection as likely causes of his anxiety, depression, or panic attacks. A TSH level also would be reasonable, because hyperthyroidism can present as anxiety, while hyperthyroidism or hypothyroidism each can present as depression.1 However, both Mr. P’s medical history and physical examination were not concerning for thyroid disease, making it unlikely that he had either of those conditions. A review of Mr. P’s medical records indicated that within the past 6 months, his primary care physician (PCP) had ordered a CBC and TSH test; the results of both were within normal limits.
Serum porphyrin tests can exclude porphyria as a contributor to Mr. P’s anxiety and depression. Porphyrias are a group of 8 inherited disorders that involve accumulation of heme precursors (porphyrins) in the CNS and subcutaneous tissue.2 Collectively, porphyrias affect approximately 1 in 200,000 people.2 Anxiety and depression are strongly associated with porphyria, but do not occur secondary to the illness; depression and anxiety appear to be intrinsic personality features in people with porphyria.3 Skin lesions and abdominal pain are the most common symptoms,3 and there is a higher incidence of hypertension in people with porphyria than in the general population.4 Mr. P does not report any heritable disorders, nor does he appear to have any CNS disturbance or unusual cutaneous lesions, which makes it unlikely that this disorder is related to his psychiatric symptoms.
Continue to: A serum metanephrines test measures...
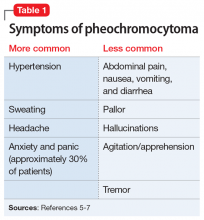
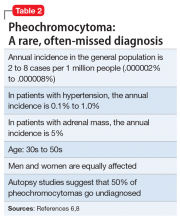
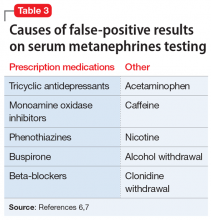
A serum metanephrines test measures the metabolites of epinephrine and norepinephrine. These catecholamines are produced in excess by an adrenal or extra-adrenal tumor seen in pheochromocytoma. The classic triad of symptoms of pheochromocytoma are hypertension, sweating, and headache; approximately 30% of patients report significant anxiety and panic (Table 15-7). This type of tumor is rare, with an annual incidence of only 2 to 8 cases per 1 million individuals. Among people with hypertension, the annual incidence is 0.1% to 1.0%, and for those with an adrenal mass, the annual incidence is 5% (Table 26,8). Autopsy studies suggest that up to 50% of pheochromocytomas are undiagnosed.8 Left untreated, pheochromocytoma can result in hypertensive crisis, arrhythmia, myocardial infarction, multisystem organ failure, and premature death.7Table 36,7 highlights some causes of false-positive serum on metanephrines testing.
EVALUATION Metanephrines testing
Mr. P has what appears to be treatment-resistant hypertension, accompanied by the classic symptoms observed in most patients with pheochromocytoma. Because Mr. P is participating in the PHP 6 days per week for 6 hours each day, visiting his PCP would be inconvenient, so the treatment team orders the serum metanephrines test. If a positive result is found, Mr. P will be referred to his PCP for further assessment and follow-up care with endocrinology.
TREATMENT Pharmacotherapy to target anxiety and panic
Next, the treatment team establishes a safety plan for Mr. P, and restarts paroxetine, 20 mg/d, to target his depressed and anxious mood. Alprazolam, 0.5 mg twice daily, is started to target anxious mood and panic symptoms, and to allow time for the anxiolytic properties of the paroxetine to become fully effective. The alprazolam will be tapered and stopped after 2 weeks. Mr. P is started on hydroxyzine, 1 to 2 25-mg tablets 2 to 3 times daily as needed for anxious mood and panic symptoms.
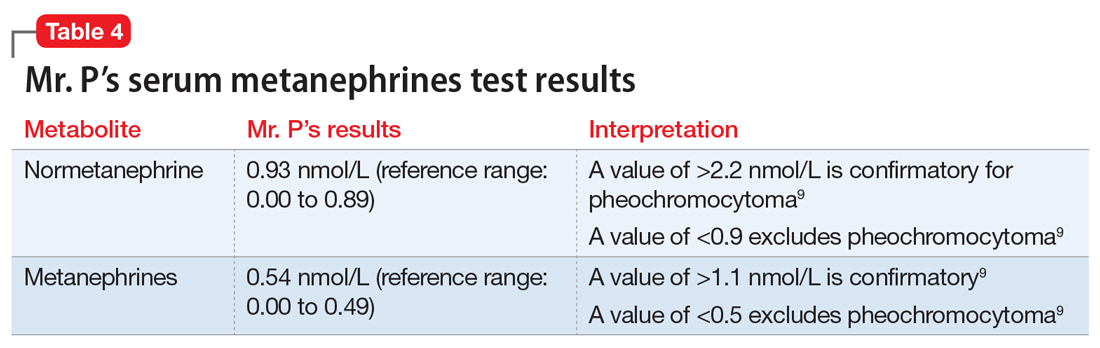
The serum metanephrines test results are equivocal, with a slight elevation of both epinephrine and norepinephrine that is too low to confirm a diagnosis of pheochromocytoma but too elevated to exclude it (Table 49). With Mr. P’s consent, the treatment team contacts his PCP and convey the results of this test. Mr. P schedules an appointment with his PCP for the following week for further assessment and confirmatory pheochromocytoma testing.
After 1 week, Mr. P remains anxious, with a slight reduction in panic attacks from multiple attacks each day to 3 or 4 attacks per week. The team considers adding an additional anxiolytic agent.
[polldaddy:10558305]
Continue to: The author's observations
The author’s observations
The triad of symptoms in pheochromocytoma results directly from the intermittent release of catecholamines into systemic circulation. Surges of epinephrine and norepinephrine lead to headaches, palpitations, diaphoresis, and (less commonly) gastrointestinal symptoms such as nausea, vomiting, and constipation. Persistent or episodic hypertension may be present, with 13% of patients maintaining a normal blood pressure.5-7 Patients with pheochromocytoma-related anxiety typically have substantial or complete resolution of anxiety and panic attacks after tumor resection.6,8,10
Because of their ability to raise catecholamine levels, several medications, including some psychotropics, can lead to false-positive results on serum and urine metanephrines testing. Tricyclic antidepressants and beta-blockers can cause false-positive results on plasma assays, while buspirone can cause false-positives on urinalysis assays.5 Trazodone, on the other hand, exhibits no catecholaminergic activity and its alpha-1 adrenergic antagonism may actually have some benefit in pheochromocytoma.11 Alpha-1 adrenergic antagonism with doxazosin, prazosin, or terazosin is the first-line of treatment in reducing pheochromocytoma-related hypertension.7 Treatment with a beta-blocker is safe only after alpha-adrenergic blockade occurs. While beta-blockers are useful for reducing the palpitations and anxiety observed in patients with pheochromocytoma, they must not be used alone due to the risk of hypertensive crisis resulting from unopposed alpha-adrenergic agonist activated vasoconstriction.5,7
TREATMENT CBT provides benefit
Mr. P decides against receiving an additional agent for anxiety and instead decides to wait for the outcome of the confirmatory pheochromocytoma testing. He continues to take alprazolam, and both his depressed mood and anxiety improve. His panic attacks continue to lessen, and he appears to benefit from cognitive-behavioral therapy provided during group therapy. Mr. P is advised by his PCP to taper and stop the alprazolam 3 to 5 days before his 24-hour urine metanephrines test because benzodiazepines can lead to false-positive results on a urinalysis assay.7
OUTCOME Remission of anxiety and depression
Mr. P has a repeat serum metanephrines test and a 24-hour urinalysis assay. Both are negative for pheochromocytoma. His PCP refers him to cardiology for management of treatment-resistant hypertension. He is discharged from the PHP and continues psychotherapy for depression and anxiety in an intensive outpatient program (IOP). Throughout his PHP and IOP treatments, he continues to take paroxetine and hydroxyzine. He achieves a successful remission of his anxiety and depression, with partial but significant remission of his panic attacks.
The author’s observations
Although Mr. P did not have pheochromocytoma, it is important to rule out this rare condition in patients who present with treatment-resistant hypertension and/or treatment-resistant anxiety.
Continue to: Bottom Line
Bottom Line
Pheochromocytoma is a tumor of the adrenal gland. The classic triad of symptoms of this rare condition is hypertension, sweating, and headache; approximately 30% of patients report significant anxiety and panic. Several medications, including tricyclic antidepressants, beta-blockers, and buspirone, can lead to false-positive results on the serum and urine metanephrines testing used to diagnose pheochromocytoma.
Related Resources
- National Organization for Rare Disorders. Rare Disease Database: pheochromocytoma. www.rarediseases.org/rare-diseases/pheochromocytoma/.
- Young WF Jr. Clinical presentation and diagnosis of pheochromocytoma. UpToDate. www.uptodate.com/contents/clinical-presentation-and-diagnosis-of-pheochromocytoma. Published January 2020.
Drug Brand Names
Alprazolam • Xanax
Amitriptyline • Elavil
Buspirone • Buspar
Carvedilol • Coreg
Clonidine • Catapres
Doxazosin • Cardura
Hydroxyzine • Vistaril
Lisinopril • Prinivil, Zestril
Paroxetine • Paxil
Prazosin • Minipress
Propranolol • Inderal
Terazosin • Hytrin
Trazodone • Desyrel
Zolpidem • Ambien
1. Morrison J. When psychological problems mask medical disorders: a guide for psychotherapists. 2nd ed. New York, NY: The Guilford Press; 2015.
2. American Porphyria Foundation. About porphyria. https://porphyriafoundation.org/patients/about-porphyria. Accessed May 13, 2020.
3. Millward L, Kelly P, King A, et al. Anxiety and depression in the acute porphyrias. J Inherit Metab Dis. 2005;28(6):1099-1107.
4. Bonkovsky H, Maddukuri VC, Yazici C, et al. Acute porphyrias in the USA: features of 108 subjects from porphyria consortium. Am J Med. 2014;127(12):1233-1241.
5. Tsirlin A, Oo Y, Sharma R, et al. Pheochromocytoma: a review. Maturitas. 2014;77(3):229-238.
6. Leung A, Zun L, Nordstrom K, et al. Psychiatric emergencies for physicians: clinical management and approach to distinguishing pheochromocytoma from psychiatric and thyrotoxic diseases in the emergency room. J Emerg Med. 2017;53(5):712-716.
7. Garg M, Kharb S, Brar KS, et al. Medical management of pheochromocytoma: role of the endocrinologist. Indian J Endocrinol and Metab. 2011;15(suppl 4):S329-S336. doi: 10.4103/2230-8210.86976.
8. Zardawi I. Phaeochromocytoma masquerading as anxiety and depression. Am J Case Rep. 2013;14:161-163.
9. ARUP Laboratories. Test directory. https://www.aruplab.com. Accessed February 11, 2020.
10. Sriram P, Raghavan V. Pheochromocytoma presenting as anxiety disorder: a case report. Asian J Psychiatr. 2017;29:83-84.
11. Stahl SM. Stahl’s essential psychopharmacology: neuroscientific basis and practical applications. Cambridge, UK: Cambridge University Press; 2013.
CASE Depression, anxiety, and panic attacks
At the urging of his parents Mr. P, age 33, presents to the partial hospitalization program (PHP) for worsening depression and anxiety, daily panic attacks with accompanying diaphoresis and headache, and the possibility that he may have taken an overdose of zolpidem. Mr. P denies taking an intentional overdose of zolpidem, claiming instead that he was having a sleep-walking episode and did not realize how many pills he took.
In addition to daily panic attacks, Mr. P reports having trouble falling asleep, overwhelming sadness, and daily passive suicidal ideation without a plan or active intent.
Mr. P cannot identify a specific trigger to this most recent exacerbation of depressed/anxious mood, but instead describes it as slowly building over the past 6 to 8 months. Mr. P says the panic attacks occur without warning and states, “I feel like my heart is going to jump out of my chest; I get a terrible headache, and I sweat like crazy. Sometimes I just feel like I’m about to pass out or die.” Although these episodes had been present for approximately 2 years, they now occur almost daily.
HISTORY Inconsistent adherence
For the last year, Mr. P had been taking alprazolam, 0.5 mg twice daily, and paroxetine, 20 mg/d, and these medications provided moderate relief of his depressive/anxious symptoms. However, he stopped taking both medications approximately 3 or 4 weeks ago when he ran out. He also takes propranolol, 20 mg/d, sporadically, for hypertension. In the past, he had been prescribed carvedilol, clonidine, and lisinopril—all with varying degrees of relief of his hypertension. He denies a family history of hypertension or any other chronic or acute health problems. He reports that he has been sober from alcohol for 19 months but smokes 1 to 2 marijuana cigarettes a day.
EVALUATION Elevated blood pressure and pulse
Mr. P’s physical examination and medical review of systems are unremarkable, except for an elevated blood pressure (190/110 mm Hg) and pulse (92 beats per minute); he also has a headache. A repeat blood pressure test later in the day is 172/94 mm Hg, with a pulse of 100 beats per minute. His urine drug screen is positive only for delta-9-tetrahydrocannabinol (THC).
[polldaddy:10558304]
The author’s observations
A CBC with differential is helpful for ruling out infection and anemia as causes of anxiety and depression.1 In Mr. P’s case, there were no concerning symptoms that pointed to anemia or infection as likely causes of his anxiety, depression, or panic attacks. A TSH level also would be reasonable, because hyperthyroidism can present as anxiety, while hyperthyroidism or hypothyroidism each can present as depression.1 However, both Mr. P’s medical history and physical examination were not concerning for thyroid disease, making it unlikely that he had either of those conditions. A review of Mr. P’s medical records indicated that within the past 6 months, his primary care physician (PCP) had ordered a CBC and TSH test; the results of both were within normal limits.
Serum porphyrin tests can exclude porphyria as a contributor to Mr. P’s anxiety and depression. Porphyrias are a group of 8 inherited disorders that involve accumulation of heme precursors (porphyrins) in the CNS and subcutaneous tissue.2 Collectively, porphyrias affect approximately 1 in 200,000 people.2 Anxiety and depression are strongly associated with porphyria, but do not occur secondary to the illness; depression and anxiety appear to be intrinsic personality features in people with porphyria.3 Skin lesions and abdominal pain are the most common symptoms,3 and there is a higher incidence of hypertension in people with porphyria than in the general population.4 Mr. P does not report any heritable disorders, nor does he appear to have any CNS disturbance or unusual cutaneous lesions, which makes it unlikely that this disorder is related to his psychiatric symptoms.
Continue to: A serum metanephrines test measures...
A serum metanephrines test measures the metabolites of epinephrine and norepinephrine. These catecholamines are produced in excess by an adrenal or extra-adrenal tumor seen in pheochromocytoma. The classic triad of symptoms of pheochromocytoma are hypertension, sweating, and headache; approximately 30% of patients report significant anxiety and panic (Table 15-7). This type of tumor is rare, with an annual incidence of only 2 to 8 cases per 1 million individuals. Among people with hypertension, the annual incidence is 0.1% to 1.0%, and for those with an adrenal mass, the annual incidence is 5% (Table 26,8). Autopsy studies suggest that up to 50% of pheochromocytomas are undiagnosed.8 Left untreated, pheochromocytoma can result in hypertensive crisis, arrhythmia, myocardial infarction, multisystem organ failure, and premature death.7Table 36,7 highlights some causes of false-positive serum on metanephrines testing.
EVALUATION Metanephrines testing
Mr. P has what appears to be treatment-resistant hypertension, accompanied by the classic symptoms observed in most patients with pheochromocytoma. Because Mr. P is participating in the PHP 6 days per week for 6 hours each day, visiting his PCP would be inconvenient, so the treatment team orders the serum metanephrines test. If a positive result is found, Mr. P will be referred to his PCP for further assessment and follow-up care with endocrinology.
TREATMENT Pharmacotherapy to target anxiety and panic
Next, the treatment team establishes a safety plan for Mr. P, and restarts paroxetine, 20 mg/d, to target his depressed and anxious mood. Alprazolam, 0.5 mg twice daily, is started to target anxious mood and panic symptoms, and to allow time for the anxiolytic properties of the paroxetine to become fully effective. The alprazolam will be tapered and stopped after 2 weeks. Mr. P is started on hydroxyzine, 1 to 2 25-mg tablets 2 to 3 times daily as needed for anxious mood and panic symptoms.
The serum metanephrines test results are equivocal, with a slight elevation of both epinephrine and norepinephrine that is too low to confirm a diagnosis of pheochromocytoma but too elevated to exclude it (Table 49). With Mr. P’s consent, the treatment team contacts his PCP and convey the results of this test. Mr. P schedules an appointment with his PCP for the following week for further assessment and confirmatory pheochromocytoma testing.
After 1 week, Mr. P remains anxious, with a slight reduction in panic attacks from multiple attacks each day to 3 or 4 attacks per week. The team considers adding an additional anxiolytic agent.
[polldaddy:10558305]
Continue to: The author's observations
The author’s observations
The triad of symptoms in pheochromocytoma results directly from the intermittent release of catecholamines into systemic circulation. Surges of epinephrine and norepinephrine lead to headaches, palpitations, diaphoresis, and (less commonly) gastrointestinal symptoms such as nausea, vomiting, and constipation. Persistent or episodic hypertension may be present, with 13% of patients maintaining a normal blood pressure.5-7 Patients with pheochromocytoma-related anxiety typically have substantial or complete resolution of anxiety and panic attacks after tumor resection.6,8,10
Because of their ability to raise catecholamine levels, several medications, including some psychotropics, can lead to false-positive results on serum and urine metanephrines testing. Tricyclic antidepressants and beta-blockers can cause false-positive results on plasma assays, while buspirone can cause false-positives on urinalysis assays.5 Trazodone, on the other hand, exhibits no catecholaminergic activity and its alpha-1 adrenergic antagonism may actually have some benefit in pheochromocytoma.11 Alpha-1 adrenergic antagonism with doxazosin, prazosin, or terazosin is the first-line of treatment in reducing pheochromocytoma-related hypertension.7 Treatment with a beta-blocker is safe only after alpha-adrenergic blockade occurs. While beta-blockers are useful for reducing the palpitations and anxiety observed in patients with pheochromocytoma, they must not be used alone due to the risk of hypertensive crisis resulting from unopposed alpha-adrenergic agonist activated vasoconstriction.5,7
TREATMENT CBT provides benefit
Mr. P decides against receiving an additional agent for anxiety and instead decides to wait for the outcome of the confirmatory pheochromocytoma testing. He continues to take alprazolam, and both his depressed mood and anxiety improve. His panic attacks continue to lessen, and he appears to benefit from cognitive-behavioral therapy provided during group therapy. Mr. P is advised by his PCP to taper and stop the alprazolam 3 to 5 days before his 24-hour urine metanephrines test because benzodiazepines can lead to false-positive results on a urinalysis assay.7
OUTCOME Remission of anxiety and depression
Mr. P has a repeat serum metanephrines test and a 24-hour urinalysis assay. Both are negative for pheochromocytoma. His PCP refers him to cardiology for management of treatment-resistant hypertension. He is discharged from the PHP and continues psychotherapy for depression and anxiety in an intensive outpatient program (IOP). Throughout his PHP and IOP treatments, he continues to take paroxetine and hydroxyzine. He achieves a successful remission of his anxiety and depression, with partial but significant remission of his panic attacks.
The author’s observations
Although Mr. P did not have pheochromocytoma, it is important to rule out this rare condition in patients who present with treatment-resistant hypertension and/or treatment-resistant anxiety.
Continue to: Bottom Line
Bottom Line
Pheochromocytoma is a tumor of the adrenal gland. The classic triad of symptoms of this rare condition is hypertension, sweating, and headache; approximately 30% of patients report significant anxiety and panic. Several medications, including tricyclic antidepressants, beta-blockers, and buspirone, can lead to false-positive results on the serum and urine metanephrines testing used to diagnose pheochromocytoma.
Related Resources
- National Organization for Rare Disorders. Rare Disease Database: pheochromocytoma. www.rarediseases.org/rare-diseases/pheochromocytoma/.
- Young WF Jr. Clinical presentation and diagnosis of pheochromocytoma. UpToDate. www.uptodate.com/contents/clinical-presentation-and-diagnosis-of-pheochromocytoma. Published January 2020.
Drug Brand Names
Alprazolam • Xanax
Amitriptyline • Elavil
Buspirone • Buspar
Carvedilol • Coreg
Clonidine • Catapres
Doxazosin • Cardura
Hydroxyzine • Vistaril
Lisinopril • Prinivil, Zestril
Paroxetine • Paxil
Prazosin • Minipress
Propranolol • Inderal
Terazosin • Hytrin
Trazodone • Desyrel
Zolpidem • Ambien
CASE Depression, anxiety, and panic attacks
At the urging of his parents Mr. P, age 33, presents to the partial hospitalization program (PHP) for worsening depression and anxiety, daily panic attacks with accompanying diaphoresis and headache, and the possibility that he may have taken an overdose of zolpidem. Mr. P denies taking an intentional overdose of zolpidem, claiming instead that he was having a sleep-walking episode and did not realize how many pills he took.
In addition to daily panic attacks, Mr. P reports having trouble falling asleep, overwhelming sadness, and daily passive suicidal ideation without a plan or active intent.
Mr. P cannot identify a specific trigger to this most recent exacerbation of depressed/anxious mood, but instead describes it as slowly building over the past 6 to 8 months. Mr. P says the panic attacks occur without warning and states, “I feel like my heart is going to jump out of my chest; I get a terrible headache, and I sweat like crazy. Sometimes I just feel like I’m about to pass out or die.” Although these episodes had been present for approximately 2 years, they now occur almost daily.
HISTORY Inconsistent adherence
For the last year, Mr. P had been taking alprazolam, 0.5 mg twice daily, and paroxetine, 20 mg/d, and these medications provided moderate relief of his depressive/anxious symptoms. However, he stopped taking both medications approximately 3 or 4 weeks ago when he ran out. He also takes propranolol, 20 mg/d, sporadically, for hypertension. In the past, he had been prescribed carvedilol, clonidine, and lisinopril—all with varying degrees of relief of his hypertension. He denies a family history of hypertension or any other chronic or acute health problems. He reports that he has been sober from alcohol for 19 months but smokes 1 to 2 marijuana cigarettes a day.
EVALUATION Elevated blood pressure and pulse
Mr. P’s physical examination and medical review of systems are unremarkable, except for an elevated blood pressure (190/110 mm Hg) and pulse (92 beats per minute); he also has a headache. A repeat blood pressure test later in the day is 172/94 mm Hg, with a pulse of 100 beats per minute. His urine drug screen is positive only for delta-9-tetrahydrocannabinol (THC).
[polldaddy:10558304]
The author’s observations
A CBC with differential is helpful for ruling out infection and anemia as causes of anxiety and depression.1 In Mr. P’s case, there were no concerning symptoms that pointed to anemia or infection as likely causes of his anxiety, depression, or panic attacks. A TSH level also would be reasonable, because hyperthyroidism can present as anxiety, while hyperthyroidism or hypothyroidism each can present as depression.1 However, both Mr. P’s medical history and physical examination were not concerning for thyroid disease, making it unlikely that he had either of those conditions. A review of Mr. P’s medical records indicated that within the past 6 months, his primary care physician (PCP) had ordered a CBC and TSH test; the results of both were within normal limits.
Serum porphyrin tests can exclude porphyria as a contributor to Mr. P’s anxiety and depression. Porphyrias are a group of 8 inherited disorders that involve accumulation of heme precursors (porphyrins) in the CNS and subcutaneous tissue.2 Collectively, porphyrias affect approximately 1 in 200,000 people.2 Anxiety and depression are strongly associated with porphyria, but do not occur secondary to the illness; depression and anxiety appear to be intrinsic personality features in people with porphyria.3 Skin lesions and abdominal pain are the most common symptoms,3 and there is a higher incidence of hypertension in people with porphyria than in the general population.4 Mr. P does not report any heritable disorders, nor does he appear to have any CNS disturbance or unusual cutaneous lesions, which makes it unlikely that this disorder is related to his psychiatric symptoms.
Continue to: A serum metanephrines test measures...
A serum metanephrines test measures the metabolites of epinephrine and norepinephrine. These catecholamines are produced in excess by an adrenal or extra-adrenal tumor seen in pheochromocytoma. The classic triad of symptoms of pheochromocytoma are hypertension, sweating, and headache; approximately 30% of patients report significant anxiety and panic (Table 15-7). This type of tumor is rare, with an annual incidence of only 2 to 8 cases per 1 million individuals. Among people with hypertension, the annual incidence is 0.1% to 1.0%, and for those with an adrenal mass, the annual incidence is 5% (Table 26,8). Autopsy studies suggest that up to 50% of pheochromocytomas are undiagnosed.8 Left untreated, pheochromocytoma can result in hypertensive crisis, arrhythmia, myocardial infarction, multisystem organ failure, and premature death.7Table 36,7 highlights some causes of false-positive serum on metanephrines testing.
EVALUATION Metanephrines testing
Mr. P has what appears to be treatment-resistant hypertension, accompanied by the classic symptoms observed in most patients with pheochromocytoma. Because Mr. P is participating in the PHP 6 days per week for 6 hours each day, visiting his PCP would be inconvenient, so the treatment team orders the serum metanephrines test. If a positive result is found, Mr. P will be referred to his PCP for further assessment and follow-up care with endocrinology.
TREATMENT Pharmacotherapy to target anxiety and panic
Next, the treatment team establishes a safety plan for Mr. P, and restarts paroxetine, 20 mg/d, to target his depressed and anxious mood. Alprazolam, 0.5 mg twice daily, is started to target anxious mood and panic symptoms, and to allow time for the anxiolytic properties of the paroxetine to become fully effective. The alprazolam will be tapered and stopped after 2 weeks. Mr. P is started on hydroxyzine, 1 to 2 25-mg tablets 2 to 3 times daily as needed for anxious mood and panic symptoms.
The serum metanephrines test results are equivocal, with a slight elevation of both epinephrine and norepinephrine that is too low to confirm a diagnosis of pheochromocytoma but too elevated to exclude it (Table 49). With Mr. P’s consent, the treatment team contacts his PCP and convey the results of this test. Mr. P schedules an appointment with his PCP for the following week for further assessment and confirmatory pheochromocytoma testing.
After 1 week, Mr. P remains anxious, with a slight reduction in panic attacks from multiple attacks each day to 3 or 4 attacks per week. The team considers adding an additional anxiolytic agent.
[polldaddy:10558305]
Continue to: The author's observations
The author’s observations
The triad of symptoms in pheochromocytoma results directly from the intermittent release of catecholamines into systemic circulation. Surges of epinephrine and norepinephrine lead to headaches, palpitations, diaphoresis, and (less commonly) gastrointestinal symptoms such as nausea, vomiting, and constipation. Persistent or episodic hypertension may be present, with 13% of patients maintaining a normal blood pressure.5-7 Patients with pheochromocytoma-related anxiety typically have substantial or complete resolution of anxiety and panic attacks after tumor resection.6,8,10
Because of their ability to raise catecholamine levels, several medications, including some psychotropics, can lead to false-positive results on serum and urine metanephrines testing. Tricyclic antidepressants and beta-blockers can cause false-positive results on plasma assays, while buspirone can cause false-positives on urinalysis assays.5 Trazodone, on the other hand, exhibits no catecholaminergic activity and its alpha-1 adrenergic antagonism may actually have some benefit in pheochromocytoma.11 Alpha-1 adrenergic antagonism with doxazosin, prazosin, or terazosin is the first-line of treatment in reducing pheochromocytoma-related hypertension.7 Treatment with a beta-blocker is safe only after alpha-adrenergic blockade occurs. While beta-blockers are useful for reducing the palpitations and anxiety observed in patients with pheochromocytoma, they must not be used alone due to the risk of hypertensive crisis resulting from unopposed alpha-adrenergic agonist activated vasoconstriction.5,7
TREATMENT CBT provides benefit
Mr. P decides against receiving an additional agent for anxiety and instead decides to wait for the outcome of the confirmatory pheochromocytoma testing. He continues to take alprazolam, and both his depressed mood and anxiety improve. His panic attacks continue to lessen, and he appears to benefit from cognitive-behavioral therapy provided during group therapy. Mr. P is advised by his PCP to taper and stop the alprazolam 3 to 5 days before his 24-hour urine metanephrines test because benzodiazepines can lead to false-positive results on a urinalysis assay.7
OUTCOME Remission of anxiety and depression
Mr. P has a repeat serum metanephrines test and a 24-hour urinalysis assay. Both are negative for pheochromocytoma. His PCP refers him to cardiology for management of treatment-resistant hypertension. He is discharged from the PHP and continues psychotherapy for depression and anxiety in an intensive outpatient program (IOP). Throughout his PHP and IOP treatments, he continues to take paroxetine and hydroxyzine. He achieves a successful remission of his anxiety and depression, with partial but significant remission of his panic attacks.
The author’s observations
Although Mr. P did not have pheochromocytoma, it is important to rule out this rare condition in patients who present with treatment-resistant hypertension and/or treatment-resistant anxiety.
Continue to: Bottom Line
Bottom Line
Pheochromocytoma is a tumor of the adrenal gland. The classic triad of symptoms of this rare condition is hypertension, sweating, and headache; approximately 30% of patients report significant anxiety and panic. Several medications, including tricyclic antidepressants, beta-blockers, and buspirone, can lead to false-positive results on the serum and urine metanephrines testing used to diagnose pheochromocytoma.
Related Resources
- National Organization for Rare Disorders. Rare Disease Database: pheochromocytoma. www.rarediseases.org/rare-diseases/pheochromocytoma/.
- Young WF Jr. Clinical presentation and diagnosis of pheochromocytoma. UpToDate. www.uptodate.com/contents/clinical-presentation-and-diagnosis-of-pheochromocytoma. Published January 2020.
Drug Brand Names
Alprazolam • Xanax
Amitriptyline • Elavil
Buspirone • Buspar
Carvedilol • Coreg
Clonidine • Catapres
Doxazosin • Cardura
Hydroxyzine • Vistaril
Lisinopril • Prinivil, Zestril
Paroxetine • Paxil
Prazosin • Minipress
Propranolol • Inderal
Terazosin • Hytrin
Trazodone • Desyrel
Zolpidem • Ambien
1. Morrison J. When psychological problems mask medical disorders: a guide for psychotherapists. 2nd ed. New York, NY: The Guilford Press; 2015.
2. American Porphyria Foundation. About porphyria. https://porphyriafoundation.org/patients/about-porphyria. Accessed May 13, 2020.
3. Millward L, Kelly P, King A, et al. Anxiety and depression in the acute porphyrias. J Inherit Metab Dis. 2005;28(6):1099-1107.
4. Bonkovsky H, Maddukuri VC, Yazici C, et al. Acute porphyrias in the USA: features of 108 subjects from porphyria consortium. Am J Med. 2014;127(12):1233-1241.
5. Tsirlin A, Oo Y, Sharma R, et al. Pheochromocytoma: a review. Maturitas. 2014;77(3):229-238.
6. Leung A, Zun L, Nordstrom K, et al. Psychiatric emergencies for physicians: clinical management and approach to distinguishing pheochromocytoma from psychiatric and thyrotoxic diseases in the emergency room. J Emerg Med. 2017;53(5):712-716.
7. Garg M, Kharb S, Brar KS, et al. Medical management of pheochromocytoma: role of the endocrinologist. Indian J Endocrinol and Metab. 2011;15(suppl 4):S329-S336. doi: 10.4103/2230-8210.86976.
8. Zardawi I. Phaeochromocytoma masquerading as anxiety and depression. Am J Case Rep. 2013;14:161-163.
9. ARUP Laboratories. Test directory. https://www.aruplab.com. Accessed February 11, 2020.
10. Sriram P, Raghavan V. Pheochromocytoma presenting as anxiety disorder: a case report. Asian J Psychiatr. 2017;29:83-84.
11. Stahl SM. Stahl’s essential psychopharmacology: neuroscientific basis and practical applications. Cambridge, UK: Cambridge University Press; 2013.
1. Morrison J. When psychological problems mask medical disorders: a guide for psychotherapists. 2nd ed. New York, NY: The Guilford Press; 2015.
2. American Porphyria Foundation. About porphyria. https://porphyriafoundation.org/patients/about-porphyria. Accessed May 13, 2020.
3. Millward L, Kelly P, King A, et al. Anxiety and depression in the acute porphyrias. J Inherit Metab Dis. 2005;28(6):1099-1107.
4. Bonkovsky H, Maddukuri VC, Yazici C, et al. Acute porphyrias in the USA: features of 108 subjects from porphyria consortium. Am J Med. 2014;127(12):1233-1241.
5. Tsirlin A, Oo Y, Sharma R, et al. Pheochromocytoma: a review. Maturitas. 2014;77(3):229-238.
6. Leung A, Zun L, Nordstrom K, et al. Psychiatric emergencies for physicians: clinical management and approach to distinguishing pheochromocytoma from psychiatric and thyrotoxic diseases in the emergency room. J Emerg Med. 2017;53(5):712-716.
7. Garg M, Kharb S, Brar KS, et al. Medical management of pheochromocytoma: role of the endocrinologist. Indian J Endocrinol and Metab. 2011;15(suppl 4):S329-S336. doi: 10.4103/2230-8210.86976.
8. Zardawi I. Phaeochromocytoma masquerading as anxiety and depression. Am J Case Rep. 2013;14:161-163.
9. ARUP Laboratories. Test directory. https://www.aruplab.com. Accessed February 11, 2020.
10. Sriram P, Raghavan V. Pheochromocytoma presenting as anxiety disorder: a case report. Asian J Psychiatr. 2017;29:83-84.
11. Stahl SM. Stahl’s essential psychopharmacology: neuroscientific basis and practical applications. Cambridge, UK: Cambridge University Press; 2013.
Cannabidiol for psychosis: A review of 4 studies
There has been increasing interest in the medicinal use of cannabidiol (CBD) for a wide variety of health conditions. CBD is one of more than 80 chemicals identified in the Cannabis sativa plant, otherwise known as marijuana or hemp. Delta-9-tetrahydrocannabinol (THC) is the psychoactive ingredient found in marijuana that produces a “high.” CBD, which is one of the most abundant cannabinoids in Cannabis sativa, does not produce any psychotomimetic effects.
The strongest scientific evidence supporting CBD for medicinal purposes is for its effectiveness in treating certain childhood epilepsy syndromes that typically do not respond to antiseizure medications. Currently, the only FDA-approved CBD product is a prescription oil cannabidiol (brand name: Epidiolex) for treating 2 types of epilepsy. Aside from Epidiolex, state laws governing the use of CBD vary. CBD is being studied as a treatment for a wide range of psychiatric conditions, including bipolar disorder, schizophrenia, dystonia, insomnia, and anxiety. Research supporting CBD’s benefits is limited, and the US National Library of Medicine’s MedlinePlus indicates there is “insufficient evidence to rate effectiveness” for these indications.1
Despite having been legalized for medicinal use in many states, CBD is classified as a Schedule I controlled substance by the US Drug Enforcement Agency. Because of this classification, little has been done to regulate and oversee the sale of products containing CBD. In a 2017 study of 84 CBD products sold by 31 companies online, Bonn-Miller et al2 found that nearly 70% percent of products were inaccurately labeled. In this study, blind testing found that only approximately 31% of products contained within 10% of the amount of CBD that was listed on the label. These researchers also found that some products contained components not listed on the label, including THC.2
The relationship between cannabis and psychosis or psychotic symptoms has been investigated for decades. Some recent studies that examined the effects of CBD on psychosis found that individuals who use CBD may experience fewer positive psychotic symptoms compared with placebo. This raises the question of whether CBD may have a role in the treatment of schizophrenia and other psychotic disorders. One of the first studies on this issue was conducted by Leweke et al,3 who compared oral CBD, up to 800 mg/d, with the antipsychotic amisulpride, up to 800 mg/d, in 39 patients with an acute exacerbation of psychotic symptoms. Amisulpride is used outside the United States to treat psychosis, but is FDA-approved only as an antiemetic. Patients were treated for 4 weeks. By Day 28, there was a significant reduction in positive symptoms as measured using the Positive and Negative Syndrome Scale (PANSS), with no significant difference in efficacy between the treatments. Similar findings emerged for negative, total, and general symptoms, with significant reductions by Day 28 in both treatment arms, and no significant between-treatment differences.
These findings were the first robust indication that CBD may have antipsychotic efficacy. However, of greater interest may be CBD’s markedly superior adverse effect profile. Predictably, amisulpride significantly increased extrapyramidal symptoms (EPS), weight gain, and prolactin levels from baseline to Day 28. However, no significant change was found in any of these adverse effects in the CBD group, and the between-treatment difference was significant (all P < .01).
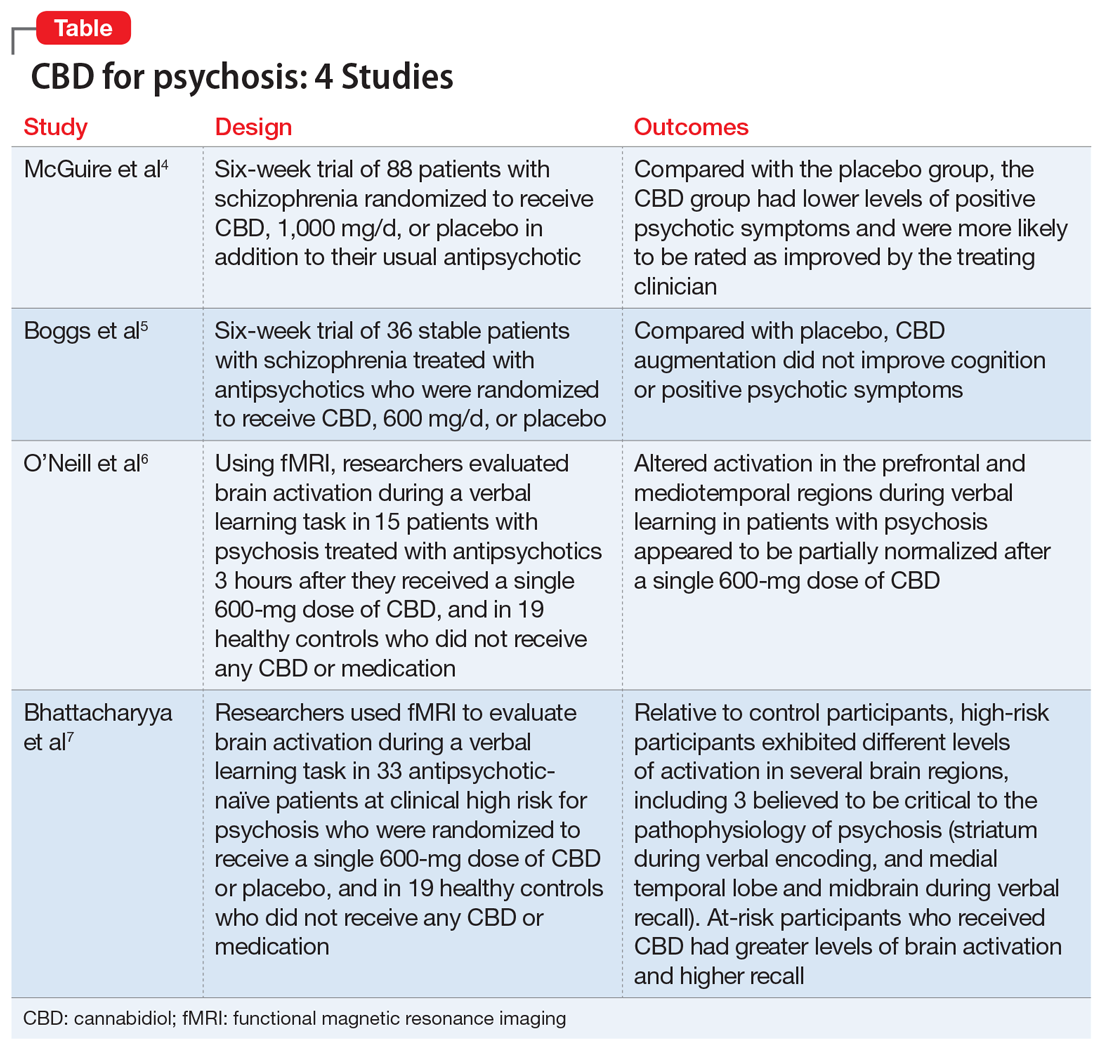
Here we review 4 recent studies that evaluated CBD as a treatment for schizophrenia. These studies are summarized in the Table.4-7
Continue to: McGuire P, et al...
1. McGuire P, Robson P, Cubala WJ, et al. Cannabidiol (CBD) as an adjunctive therapy in schizophrenia: a multicenter randomized controlled trial. Am J Psychiatry. 2018;175(3):225-231.
Antipsychotic medications act through blockade of central dopamine D2 receptors. For most patients, antipsychotics effectively treat positive psychotic symptoms, which are driven by elevated dopamine function. However, these medications have minimal effects on negative symptoms and cognitive impairment, features of schizophrenia that are not driven by elevated dopamine. Compounds exhibiting a mechanism of action unlike that of current antipsychotics may improve the treatment and outcomes of patients with schizophrenia. The mechanism of action of CBD is unclear, but it does not appear to involve the direct antagonism of dopamine receptors. Human and animal research study findings indicate that CBD has antipsychotic properties. McGuire et al4 assessed the safety and effectiveness of CBD as an adjunctive treatment of schizophrenia.
Study design
- In this double-blind parallel-group trial conducted at 15 hospitals in the United Kingdom, Romania, and Poland, 88 patients with schizophrenia received CBD (1,000 mg/d; N = 43) or placebo (N = 45) as adjunct to the antipsychotic medication they had been prescribed. Patients had previously demonstrated at least a partial response to antipsychotic treatment, and were taking stable doses of an antipsychotic for ≥4 weeks.
- Evaluations of symptoms, general functioning, cognitive performance, and EPS were completed at baseline and on Days 8, 22, and 43 (± 3 days). Current substance use was assessed using a semi-structured interview, and reassessed at the end of treatment.
- The key endpoints were the patients’ level of functioning, severity of symptoms, and cognitive performance. Participants were assessed before and after treatment using the PANSS, the Brief Assessment of Cognition in Schizophrenia (BACS), the Global Assessment of Functioning scale (GAF), and the improvement and severity scales of the Clinical Global Impressions Scale (CGI-I and CGI-S, respectively).
- The clinicians’ impression of illness severity and symptom improvement and patient- or caregiver-reported impressions of general functioning and sleep also were noted.
Outcomes
- After 6 weeks, compared with the placebo group, the CBD group had lower levels of positive psychotic symptoms and were more likely to be rated as improved and as not severely unwell by the treating clinician. Patients in the CBD group also showed greater improvements in cognitive performance and in overall functioning, although these were not statistically significant.
- Similar levels of negative psychotic symptoms, overall psychopathology, and general psychopathology were observed in the CBD and placebo groups. The CBD group had a higher proportion of treatment responders (≥20% improvement in PANSS total score) than did the placebo group; however, the total number of responders per group was small (12 and 6 patients, respectively). At baseline, most patients in both groups were classified as moderately, markedly, or severely ill (83.4% in the CBD group vs 79.6% in placebo group). By the end of treatment, this decreased to 54.8% in the CBD group and 63.6% in the placebo group. Clinicians rated 78.6% of patients in the CBD group as “improved” on the CGI-I, compared with 54.6% of patients in the placebo group.
Conclusion
- CBD treatment adjunctive to antipsychotics was associated with significant effects on positive psychotic symptoms and on CGI-I and illness severity. Improvements in cognitive performance and level of overall functioning were also seen, but were not statistically significant.
- Although the effect on positive symptoms was modest, improvement occurred in patients being treated with appropriate dosages of antipsychotics, which suggests CBD provided benefits over and above the effect of antipsychotic treatment. Moreover, the changes in CGI-I and CGI-S scores indicated that the improvement was evident to the treating psychiatrists, and may therefore be clinically meaningful.
Continue to: Boggs DL, et al...
2. Boggs DL, Surti T, Gupta A, et al. The effects of cannabidiol (CBD) on cognition and symptoms in outpatients with chronic schizophrenia a randomized placebo controlled trial. Psychopharmacology (Berl). 2018;235(7):1923-1932.
Schizophrenia is associated with cognitive deficits in learning, recall, attention, working memory, and executive function. The cognitive impairments associated with schizophrenia (CIAS) are independent of phase of illness and often persist after other symptoms have been effectively treated. These impairments are the strongest predictor of functional outcome, even more so than psychotic symptoms.
Antipsychotics have limited efficacy for CIAS, which highlights the need for CIAS treatments that target other nondopaminergic neurotransmitter systems. The endocannabinoid system, which has been implicated in schizophrenia and in cognition, is a potential target. Several cannabinoids impair memory and attention. The main psychoactive component of marijuana, THC, is a cannabinoid receptor type 1 (CB1R) partial agonist. Administration of THC produces significant deficits in verbal learning, attention, and working memory.
Researchers have hypothesized that CB1R blockade or modulation of cannabinoid levels may offer a novel target for treating CIAS. Boggs et al5 compared the cognitive, symptomatic, and adverse effects of CBD vs placebo.
Study design
- In this 6-week, randomized, placebo-controlled study conducted in Connecticut from September 2009 to May 2012, 36 stable patients with schizophrenia who were treated with antipsychotics were randomized to also receive oral CBD, 600 mg/d, or placebo.
- Cognition was assessed using the t score of the MATRICS Consensus Cognitive Battery (MCCB) composite and subscales at baseline and the end of study. An increase in MCCB t score indicates an improvement in cognitive ability. Psychotic symptoms were assessed using the PANSS at baseline, Week 2, Week 4, and Week 6.
Outcomes
- CBD augmentation did not improve MCCB performance or psychotic symptoms. There was no main effect of time or medication on MCCB composite score, but a significant drug × time effect was observed.
- Post-hoc analyses revealed that only patients who received placebo improved over time. The lack of a similar improvement with CBD might be related to the greater incidence of sedation among the CBD group (20%) vs the placebo group (5%). Both the MCCB composite score and reasoning and problem-solving domain scores were higher at baseline and endpoint for patients who received CBD, which suggests that the observed improvement in the placebo group could represent a regression to the mean.
- There was a significant decrease in PANSS scores over time, but there was no significant drug × time interaction.
Conclusion
- CBD augmentation was not associated with an improvement in MCCB score. This is consistent with data from other clinical trials4,8 that suggested that CBD (at a wide range of doses) does not have significant beneficial effects on cognition in patients with schizophrenia.
- Additionally, CBD did not improve psychotic symptoms. These results are in contrast to published case reports9,10 and 2 published clinical trials3,4 that found CBD (800 mg/d) was as efficacious as amisulpride in reducing positive psychotic symptoms, and a small but statistically significant improvement in PANSS positive scores with CBD (1,000 mg/d) compared with placebo. However, these results are similar to those of a separate study11 that evaluated the same 600-mg/d dose of CBD used by Boggs et al.5 At 600 mg/d, CBD produced very small improvements in PANSS total scores (~2.4) that were not statistically significant. A higher CBD dose may be needed to reduce psychotic symptoms in patients with schizophrenia.
Continue to: O’Neill A, et al...
3. O’Neill A, Wilson R, Blest-Hopley G, et al. Normalization of mediotemporal and prefrontal activity, and mediotemporal-striatal connectivity, may underlie antipsychotic effects of cannabidiol in psychosis. Psychol Med. 2020;1-11. doi: 10.1017/S0033291719003519.
In addition to their key roles in the psychopathology of psychosis, the mediotemporal and prefrontal cortices are involved in learning and memory, and the striatum plays a role in encoding contextual information associated with memories. Because deficits in verbal learning and memory are one of the most commonly reported impairments in patients with psychosis, O’Neill et al6 used functional MRI (fMRI) to examine brain activity during a verbal learning task in patients with psychosis after taking CBD or placebo.
Study design
- In a double-blind, randomized, placebo-controlled, crossover study, researchers investigated the effects of a single dose of CBD in 15 patients with psychosis who were treated with antipsychotics. Three hours after taking a 600-mg dose of CBD or placebo, these participants were scanned using fMRI while performing a verbal paired associate (VPA) learning task. Nineteen healthy controls underwent fMRI in identical conditions, but without any medication administration.
- The fMRI measured brain activation using the blood oxygen level–dependent (BOLD) hemodynamic responses of the brain. The fMRI signals were studied in the mediotemporal, prefrontal, and striatal regions.
- The VPA task presented word pairs visually, and the accuracy of responses were recorded online. The VPA task was comprised of 3 conditions: encoding, recall, and baseline.
- Results during each phase of the VPA task were compared.
Outcomes
- While completing the VPA task after taking placebo, compared with healthy controls, patients with psychosis demonstrated a different pattern of activity in the prefrontal and mediotemporal brain areas. Specifically, during verbal encoding, the placebo group showed altered activation in prefrontal regions. During verbal recall, the placebo group showed altered activation in prefrontal and mediotemporal regions, as well as increased mediotemporal-striatal functional connectivity.
- After participants received CBD, activation in these brain areas became more like the activation seen in controls. CBD attenuated dysfunction in these regions such that activation was intermediate between the placebo condition and the control group. CBD also attenuated functional connectivity between the hippocampus and striatum, and lead to reduced symptoms in patients with psychosis (as measured by PANSS total score).
Conclusion
- Altered activation in prefrontal and mediotemporal regions during verbal learning in patients with psychosis appeared to be partially normalized after a single 600-mg dose of CBD. Results also showed improvement in PANSS total score with CBD.
- These findings suggest that a single dose of CBD may partially attenuate the dysfunctional prefrontal and mediotemporal activation that is believed to underlie the dopamine dysfunction that leads to psychotic symptoms. These effects, along with a reduction in psychotic symptoms, suggest that normalization of altered prefrontal and mediotemporal function and mediotemporal-striatal connectivity may underlie the antipsychotic effects of CBD in established psychosis.
Continue to: Bhattacharyya S, et al...
4. Bhattacharyya S, Wilson R, Appiah-Kusi E, et al. Effect of cannabidiol on medial temporal, midbrain, and striatal dysfunction in people at clinical high risk of psychosis: a randomized clinical trial. JAMA Psychiatry. 2018;75(11):1107-1117.
Current preclinical models suggest that psychosis involves a disturbance of activity in the medial temporal lobe (MTL) that drives dopamine dysfunction in the striatum and midbrain. THC, which produces psychotomimetic effects, impacts the function of the striatum (verbal memoryand salience processing) andamygdala (emotional processing), and alters the functional connectivity of these regions. Compared with THC, CBD has broadly opposite neural and behavioral effects, including opposing effects on the activation of these regions. Bhattacharyya et al7 examined the neurocognitive mechanisms that underlie the therapeutic effects of CBD in psychosis and sought to understand whether CBD would attenuate functional abnormalities in the MTL, midbrain, and striatum.
Study design
- A randomized, double-blind, placebo-controlled trial examined 33 antipsychotic-naïve participants at clinical high risk (CHR) for psychosis and 19 healthy controls. The CHR group was randomized to CBD, 600 mg, or placebo.
- Three hours after taking CBD or placebo, CHR participants were studied using fMRI while performing a VPA learning task, which engages verbal learning and recall in the MTL, midbrain and striatum. Control participants did not receive any medication but underwent fMRI while performing the VPA task.
- The VPA task presented word pairs visually, and the accuracy of responses was recorded online. It was comprised of 3 conditions: encoding, recall, and baseline.
Outcomes
- Brain activation was analyzed in 15 participants in the CBD group, 16 in the placebo group, and 19 in the control group. Activation during encoding was observed in the striatum (specifically, the right caudate). Activation during recall was observed in the midbrain and the MTL (specifically, the parahippocampus).
- Brain activation levels in all 3 regions were lowest in the placebo group, intermediate in the CBD group, and greatest in the healthy control group. For all participants, the total recall score was directly correlated with the activation level in the left MTL (parahippocampus) during recall.
Conclusion
- Relative to controls, CHR participants exhibited different levels of activation in several regions, including the 3 areas thought to be critical to the pathophysiology of psychosis: the striatum during verbal encoding, and the MTL and midbrain during verbal recall.
- Compared with those who received placebo, CHR participants who received CBD before completing the VPA task demonstrated greater levels of brain activation and higher recall score.
- These findings suggest that CBD may partially normalize alterations in MTL, striatal, and midbrain function associated with CHR of psychosis. Because these regions are implicated in the pathophysiology of psychosis, the impact of CBD at these sites may contribute to the therapeutic effects of CBD that have been reported by some patients with psychosis.
Continue to: Conflicting data highlights...
Conflicting data highlights the need for longer, larger studies
Research findings on the use of CBD for psychotic symptoms in patients with schizophrenia have been conflicting. Some early research suggests that taking CBD 4 times daily for 4 weeks improves psychotic symptoms and might be as effective as the antipsychotic amisulpride. However, other early research suggests that taking CBD for 14 days is not beneficial. The conflicting results might be related to the CBD dose used and duration of treatment.
Davies and Bhattacharya12 recently reviewed evidence regarding the efficacy of CBD as a potential novel treatment for psychotic disorders.They concluded that CBD represents a promising potential novel treatment for patients with psychosis. It also appears that CBD may improve the disease trajectory of individuals with early psychosis and comorbid cannabis misuse.13 CBD use has also been associated with a decrease in symptoms of psychosis and changes in brain activity during verbal memory tasks in patients at high risk of psychosis.6 However, before CBD can become a viable treatment option for psychosis, the promising findings in these initial clinical studies must be replicated in large-scale trials with appropriate treatment duration.
1. US National Library of Medicine. MedlinePlus. Cannabidiol (CBD). https://medlineplus.gov/druginfo/natural/1439.html. Accessed May 14, 2020.
2. Bonn-Miller MO, Loflin MJE, Thomas BF, et al. Labeling accuracy of cannabidiol extracts sold online. JAMA. 2017;318(17):1708-1709.
3. Leweke FM, Piomelli D, Pahlisch F, et al. Cannabidiol enhances anandamide signaling and alleviates psychotic symptoms of schizophrenia. Transl Psychiatry. 2012;2(3):e94.
4. McGuire P, Robson P, Cubala WJ, et al. Cannabidiol (CBD) as an adjunctive therapy in schizophrenia: a multicenter randomized controlled trial. Am J Psychiatry. 2018;175(3):225-231.
5. Boggs DL, Surti T, Gupta A, et al. The effects of cannabidiol (CBD) on cognition and symptoms in outpatients with chronic schizophrenia a randomized placebo controlled trial. Psychopharmacology (Berl). 2018;235(7):1923-1932.
6. O’Neill A, Wilson R, Blest-Hopley G, et al. Normalization of mediotemporal and prefrontal activity, and mediotemporal-striatal connectivity, may underlie antipsychotic effects of cannabidiol in psychosis. Psychol Med. 2020;1-11. doi: 10.1017/S0033291719003519.
7. Bhattacharyya S, Wilson R, Appiah-Kusi E, et al. Effect of cannabidiol on medial temporal, midbrain, and striatal dysfunction in people at clinical high risk of psychosis: a randomized clinical trial. JAMA Psychiatry. 2018;75(11):1107-1117.
8. Hallak JE, Machado-de-Sousa JP, Crippa JAS, et al. Performance of schizophrenic patients in the Stroop color word test and electrodermal responsiveness after acute administration of cannabidiol (CBD). Rev Bras Psiquiatr. 2010;32(1):56-61.
9. Zuardi AW, Morais SL, Guimaraes FS, et al. Antipsychotic effect of cannabidiol. J Clin Psychiatry. 1995;56(10):485-486.
10. Zuardi AW, Hallak JE, Dursun SM, et al. Cannabidiol monotherapy for treatment-resistant schizophrenia. J Psychopharmacol. 2006;20(5):683-686.
11. Leweke FM, Hellmich M, Pahlisch F, et al. Modulation of the endocannabinoid system as a potential new target in the treatment of schizophrenia. Schizophr Res. 2014; 153(1):S47.
12. Davies C, Bhattacharyya S. Cannabidiol as a potential treatment for psychosis. Ther Adv Psychopharmacol. 2019;9. doi:10.1177/2045125319881916.
13. Hahn B. The potential of cannabidiol treatment for cannabis users with recent-onset psychosis. Schizophr Bull. 2018;44(1):46-53.
There has been increasing interest in the medicinal use of cannabidiol (CBD) for a wide variety of health conditions. CBD is one of more than 80 chemicals identified in the Cannabis sativa plant, otherwise known as marijuana or hemp. Delta-9-tetrahydrocannabinol (THC) is the psychoactive ingredient found in marijuana that produces a “high.” CBD, which is one of the most abundant cannabinoids in Cannabis sativa, does not produce any psychotomimetic effects.
The strongest scientific evidence supporting CBD for medicinal purposes is for its effectiveness in treating certain childhood epilepsy syndromes that typically do not respond to antiseizure medications. Currently, the only FDA-approved CBD product is a prescription oil cannabidiol (brand name: Epidiolex) for treating 2 types of epilepsy. Aside from Epidiolex, state laws governing the use of CBD vary. CBD is being studied as a treatment for a wide range of psychiatric conditions, including bipolar disorder, schizophrenia, dystonia, insomnia, and anxiety. Research supporting CBD’s benefits is limited, and the US National Library of Medicine’s MedlinePlus indicates there is “insufficient evidence to rate effectiveness” for these indications.1
Despite having been legalized for medicinal use in many states, CBD is classified as a Schedule I controlled substance by the US Drug Enforcement Agency. Because of this classification, little has been done to regulate and oversee the sale of products containing CBD. In a 2017 study of 84 CBD products sold by 31 companies online, Bonn-Miller et al2 found that nearly 70% percent of products were inaccurately labeled. In this study, blind testing found that only approximately 31% of products contained within 10% of the amount of CBD that was listed on the label. These researchers also found that some products contained components not listed on the label, including THC.2
The relationship between cannabis and psychosis or psychotic symptoms has been investigated for decades. Some recent studies that examined the effects of CBD on psychosis found that individuals who use CBD may experience fewer positive psychotic symptoms compared with placebo. This raises the question of whether CBD may have a role in the treatment of schizophrenia and other psychotic disorders. One of the first studies on this issue was conducted by Leweke et al,3 who compared oral CBD, up to 800 mg/d, with the antipsychotic amisulpride, up to 800 mg/d, in 39 patients with an acute exacerbation of psychotic symptoms. Amisulpride is used outside the United States to treat psychosis, but is FDA-approved only as an antiemetic. Patients were treated for 4 weeks. By Day 28, there was a significant reduction in positive symptoms as measured using the Positive and Negative Syndrome Scale (PANSS), with no significant difference in efficacy between the treatments. Similar findings emerged for negative, total, and general symptoms, with significant reductions by Day 28 in both treatment arms, and no significant between-treatment differences.
These findings were the first robust indication that CBD may have antipsychotic efficacy. However, of greater interest may be CBD’s markedly superior adverse effect profile. Predictably, amisulpride significantly increased extrapyramidal symptoms (EPS), weight gain, and prolactin levels from baseline to Day 28. However, no significant change was found in any of these adverse effects in the CBD group, and the between-treatment difference was significant (all P < .01).
Here we review 4 recent studies that evaluated CBD as a treatment for schizophrenia. These studies are summarized in the Table.4-7
Continue to: McGuire P, et al...
1. McGuire P, Robson P, Cubala WJ, et al. Cannabidiol (CBD) as an adjunctive therapy in schizophrenia: a multicenter randomized controlled trial. Am J Psychiatry. 2018;175(3):225-231.
Antipsychotic medications act through blockade of central dopamine D2 receptors. For most patients, antipsychotics effectively treat positive psychotic symptoms, which are driven by elevated dopamine function. However, these medications have minimal effects on negative symptoms and cognitive impairment, features of schizophrenia that are not driven by elevated dopamine. Compounds exhibiting a mechanism of action unlike that of current antipsychotics may improve the treatment and outcomes of patients with schizophrenia. The mechanism of action of CBD is unclear, but it does not appear to involve the direct antagonism of dopamine receptors. Human and animal research study findings indicate that CBD has antipsychotic properties. McGuire et al4 assessed the safety and effectiveness of CBD as an adjunctive treatment of schizophrenia.
Study design
- In this double-blind parallel-group trial conducted at 15 hospitals in the United Kingdom, Romania, and Poland, 88 patients with schizophrenia received CBD (1,000 mg/d; N = 43) or placebo (N = 45) as adjunct to the antipsychotic medication they had been prescribed. Patients had previously demonstrated at least a partial response to antipsychotic treatment, and were taking stable doses of an antipsychotic for ≥4 weeks.
- Evaluations of symptoms, general functioning, cognitive performance, and EPS were completed at baseline and on Days 8, 22, and 43 (± 3 days). Current substance use was assessed using a semi-structured interview, and reassessed at the end of treatment.
- The key endpoints were the patients’ level of functioning, severity of symptoms, and cognitive performance. Participants were assessed before and after treatment using the PANSS, the Brief Assessment of Cognition in Schizophrenia (BACS), the Global Assessment of Functioning scale (GAF), and the improvement and severity scales of the Clinical Global Impressions Scale (CGI-I and CGI-S, respectively).
- The clinicians’ impression of illness severity and symptom improvement and patient- or caregiver-reported impressions of general functioning and sleep also were noted.
Outcomes
- After 6 weeks, compared with the placebo group, the CBD group had lower levels of positive psychotic symptoms and were more likely to be rated as improved and as not severely unwell by the treating clinician. Patients in the CBD group also showed greater improvements in cognitive performance and in overall functioning, although these were not statistically significant.
- Similar levels of negative psychotic symptoms, overall psychopathology, and general psychopathology were observed in the CBD and placebo groups. The CBD group had a higher proportion of treatment responders (≥20% improvement in PANSS total score) than did the placebo group; however, the total number of responders per group was small (12 and 6 patients, respectively). At baseline, most patients in both groups were classified as moderately, markedly, or severely ill (83.4% in the CBD group vs 79.6% in placebo group). By the end of treatment, this decreased to 54.8% in the CBD group and 63.6% in the placebo group. Clinicians rated 78.6% of patients in the CBD group as “improved” on the CGI-I, compared with 54.6% of patients in the placebo group.
Conclusion
- CBD treatment adjunctive to antipsychotics was associated with significant effects on positive psychotic symptoms and on CGI-I and illness severity. Improvements in cognitive performance and level of overall functioning were also seen, but were not statistically significant.
- Although the effect on positive symptoms was modest, improvement occurred in patients being treated with appropriate dosages of antipsychotics, which suggests CBD provided benefits over and above the effect of antipsychotic treatment. Moreover, the changes in CGI-I and CGI-S scores indicated that the improvement was evident to the treating psychiatrists, and may therefore be clinically meaningful.
Continue to: Boggs DL, et al...
2. Boggs DL, Surti T, Gupta A, et al. The effects of cannabidiol (CBD) on cognition and symptoms in outpatients with chronic schizophrenia a randomized placebo controlled trial. Psychopharmacology (Berl). 2018;235(7):1923-1932.
Schizophrenia is associated with cognitive deficits in learning, recall, attention, working memory, and executive function. The cognitive impairments associated with schizophrenia (CIAS) are independent of phase of illness and often persist after other symptoms have been effectively treated. These impairments are the strongest predictor of functional outcome, even more so than psychotic symptoms.
Antipsychotics have limited efficacy for CIAS, which highlights the need for CIAS treatments that target other nondopaminergic neurotransmitter systems. The endocannabinoid system, which has been implicated in schizophrenia and in cognition, is a potential target. Several cannabinoids impair memory and attention. The main psychoactive component of marijuana, THC, is a cannabinoid receptor type 1 (CB1R) partial agonist. Administration of THC produces significant deficits in verbal learning, attention, and working memory.
Researchers have hypothesized that CB1R blockade or modulation of cannabinoid levels may offer a novel target for treating CIAS. Boggs et al5 compared the cognitive, symptomatic, and adverse effects of CBD vs placebo.
Study design
- In this 6-week, randomized, placebo-controlled study conducted in Connecticut from September 2009 to May 2012, 36 stable patients with schizophrenia who were treated with antipsychotics were randomized to also receive oral CBD, 600 mg/d, or placebo.
- Cognition was assessed using the t score of the MATRICS Consensus Cognitive Battery (MCCB) composite and subscales at baseline and the end of study. An increase in MCCB t score indicates an improvement in cognitive ability. Psychotic symptoms were assessed using the PANSS at baseline, Week 2, Week 4, and Week 6.
Outcomes
- CBD augmentation did not improve MCCB performance or psychotic symptoms. There was no main effect of time or medication on MCCB composite score, but a significant drug × time effect was observed.
- Post-hoc analyses revealed that only patients who received placebo improved over time. The lack of a similar improvement with CBD might be related to the greater incidence of sedation among the CBD group (20%) vs the placebo group (5%). Both the MCCB composite score and reasoning and problem-solving domain scores were higher at baseline and endpoint for patients who received CBD, which suggests that the observed improvement in the placebo group could represent a regression to the mean.
- There was a significant decrease in PANSS scores over time, but there was no significant drug × time interaction.
Conclusion
- CBD augmentation was not associated with an improvement in MCCB score. This is consistent with data from other clinical trials4,8 that suggested that CBD (at a wide range of doses) does not have significant beneficial effects on cognition in patients with schizophrenia.
- Additionally, CBD did not improve psychotic symptoms. These results are in contrast to published case reports9,10 and 2 published clinical trials3,4 that found CBD (800 mg/d) was as efficacious as amisulpride in reducing positive psychotic symptoms, and a small but statistically significant improvement in PANSS positive scores with CBD (1,000 mg/d) compared with placebo. However, these results are similar to those of a separate study11 that evaluated the same 600-mg/d dose of CBD used by Boggs et al.5 At 600 mg/d, CBD produced very small improvements in PANSS total scores (~2.4) that were not statistically significant. A higher CBD dose may be needed to reduce psychotic symptoms in patients with schizophrenia.
Continue to: O’Neill A, et al...
3. O’Neill A, Wilson R, Blest-Hopley G, et al. Normalization of mediotemporal and prefrontal activity, and mediotemporal-striatal connectivity, may underlie antipsychotic effects of cannabidiol in psychosis. Psychol Med. 2020;1-11. doi: 10.1017/S0033291719003519.
In addition to their key roles in the psychopathology of psychosis, the mediotemporal and prefrontal cortices are involved in learning and memory, and the striatum plays a role in encoding contextual information associated with memories. Because deficits in verbal learning and memory are one of the most commonly reported impairments in patients with psychosis, O’Neill et al6 used functional MRI (fMRI) to examine brain activity during a verbal learning task in patients with psychosis after taking CBD or placebo.
Study design
- In a double-blind, randomized, placebo-controlled, crossover study, researchers investigated the effects of a single dose of CBD in 15 patients with psychosis who were treated with antipsychotics. Three hours after taking a 600-mg dose of CBD or placebo, these participants were scanned using fMRI while performing a verbal paired associate (VPA) learning task. Nineteen healthy controls underwent fMRI in identical conditions, but without any medication administration.
- The fMRI measured brain activation using the blood oxygen level–dependent (BOLD) hemodynamic responses of the brain. The fMRI signals were studied in the mediotemporal, prefrontal, and striatal regions.
- The VPA task presented word pairs visually, and the accuracy of responses were recorded online. The VPA task was comprised of 3 conditions: encoding, recall, and baseline.
- Results during each phase of the VPA task were compared.
Outcomes
- While completing the VPA task after taking placebo, compared with healthy controls, patients with psychosis demonstrated a different pattern of activity in the prefrontal and mediotemporal brain areas. Specifically, during verbal encoding, the placebo group showed altered activation in prefrontal regions. During verbal recall, the placebo group showed altered activation in prefrontal and mediotemporal regions, as well as increased mediotemporal-striatal functional connectivity.
- After participants received CBD, activation in these brain areas became more like the activation seen in controls. CBD attenuated dysfunction in these regions such that activation was intermediate between the placebo condition and the control group. CBD also attenuated functional connectivity between the hippocampus and striatum, and lead to reduced symptoms in patients with psychosis (as measured by PANSS total score).
Conclusion
- Altered activation in prefrontal and mediotemporal regions during verbal learning in patients with psychosis appeared to be partially normalized after a single 600-mg dose of CBD. Results also showed improvement in PANSS total score with CBD.
- These findings suggest that a single dose of CBD may partially attenuate the dysfunctional prefrontal and mediotemporal activation that is believed to underlie the dopamine dysfunction that leads to psychotic symptoms. These effects, along with a reduction in psychotic symptoms, suggest that normalization of altered prefrontal and mediotemporal function and mediotemporal-striatal connectivity may underlie the antipsychotic effects of CBD in established psychosis.
Continue to: Bhattacharyya S, et al...
4. Bhattacharyya S, Wilson R, Appiah-Kusi E, et al. Effect of cannabidiol on medial temporal, midbrain, and striatal dysfunction in people at clinical high risk of psychosis: a randomized clinical trial. JAMA Psychiatry. 2018;75(11):1107-1117.
Current preclinical models suggest that psychosis involves a disturbance of activity in the medial temporal lobe (MTL) that drives dopamine dysfunction in the striatum and midbrain. THC, which produces psychotomimetic effects, impacts the function of the striatum (verbal memoryand salience processing) andamygdala (emotional processing), and alters the functional connectivity of these regions. Compared with THC, CBD has broadly opposite neural and behavioral effects, including opposing effects on the activation of these regions. Bhattacharyya et al7 examined the neurocognitive mechanisms that underlie the therapeutic effects of CBD in psychosis and sought to understand whether CBD would attenuate functional abnormalities in the MTL, midbrain, and striatum.
Study design
- A randomized, double-blind, placebo-controlled trial examined 33 antipsychotic-naïve participants at clinical high risk (CHR) for psychosis and 19 healthy controls. The CHR group was randomized to CBD, 600 mg, or placebo.
- Three hours after taking CBD or placebo, CHR participants were studied using fMRI while performing a VPA learning task, which engages verbal learning and recall in the MTL, midbrain and striatum. Control participants did not receive any medication but underwent fMRI while performing the VPA task.
- The VPA task presented word pairs visually, and the accuracy of responses was recorded online. It was comprised of 3 conditions: encoding, recall, and baseline.
Outcomes
- Brain activation was analyzed in 15 participants in the CBD group, 16 in the placebo group, and 19 in the control group. Activation during encoding was observed in the striatum (specifically, the right caudate). Activation during recall was observed in the midbrain and the MTL (specifically, the parahippocampus).
- Brain activation levels in all 3 regions were lowest in the placebo group, intermediate in the CBD group, and greatest in the healthy control group. For all participants, the total recall score was directly correlated with the activation level in the left MTL (parahippocampus) during recall.
Conclusion
- Relative to controls, CHR participants exhibited different levels of activation in several regions, including the 3 areas thought to be critical to the pathophysiology of psychosis: the striatum during verbal encoding, and the MTL and midbrain during verbal recall.
- Compared with those who received placebo, CHR participants who received CBD before completing the VPA task demonstrated greater levels of brain activation and higher recall score.
- These findings suggest that CBD may partially normalize alterations in MTL, striatal, and midbrain function associated with CHR of psychosis. Because these regions are implicated in the pathophysiology of psychosis, the impact of CBD at these sites may contribute to the therapeutic effects of CBD that have been reported by some patients with psychosis.
Continue to: Conflicting data highlights...
Conflicting data highlights the need for longer, larger studies
Research findings on the use of CBD for psychotic symptoms in patients with schizophrenia have been conflicting. Some early research suggests that taking CBD 4 times daily for 4 weeks improves psychotic symptoms and might be as effective as the antipsychotic amisulpride. However, other early research suggests that taking CBD for 14 days is not beneficial. The conflicting results might be related to the CBD dose used and duration of treatment.
Davies and Bhattacharya12 recently reviewed evidence regarding the efficacy of CBD as a potential novel treatment for psychotic disorders.They concluded that CBD represents a promising potential novel treatment for patients with psychosis. It also appears that CBD may improve the disease trajectory of individuals with early psychosis and comorbid cannabis misuse.13 CBD use has also been associated with a decrease in symptoms of psychosis and changes in brain activity during verbal memory tasks in patients at high risk of psychosis.6 However, before CBD can become a viable treatment option for psychosis, the promising findings in these initial clinical studies must be replicated in large-scale trials with appropriate treatment duration.
There has been increasing interest in the medicinal use of cannabidiol (CBD) for a wide variety of health conditions. CBD is one of more than 80 chemicals identified in the Cannabis sativa plant, otherwise known as marijuana or hemp. Delta-9-tetrahydrocannabinol (THC) is the psychoactive ingredient found in marijuana that produces a “high.” CBD, which is one of the most abundant cannabinoids in Cannabis sativa, does not produce any psychotomimetic effects.
The strongest scientific evidence supporting CBD for medicinal purposes is for its effectiveness in treating certain childhood epilepsy syndromes that typically do not respond to antiseizure medications. Currently, the only FDA-approved CBD product is a prescription oil cannabidiol (brand name: Epidiolex) for treating 2 types of epilepsy. Aside from Epidiolex, state laws governing the use of CBD vary. CBD is being studied as a treatment for a wide range of psychiatric conditions, including bipolar disorder, schizophrenia, dystonia, insomnia, and anxiety. Research supporting CBD’s benefits is limited, and the US National Library of Medicine’s MedlinePlus indicates there is “insufficient evidence to rate effectiveness” for these indications.1
Despite having been legalized for medicinal use in many states, CBD is classified as a Schedule I controlled substance by the US Drug Enforcement Agency. Because of this classification, little has been done to regulate and oversee the sale of products containing CBD. In a 2017 study of 84 CBD products sold by 31 companies online, Bonn-Miller et al2 found that nearly 70% percent of products were inaccurately labeled. In this study, blind testing found that only approximately 31% of products contained within 10% of the amount of CBD that was listed on the label. These researchers also found that some products contained components not listed on the label, including THC.2
The relationship between cannabis and psychosis or psychotic symptoms has been investigated for decades. Some recent studies that examined the effects of CBD on psychosis found that individuals who use CBD may experience fewer positive psychotic symptoms compared with placebo. This raises the question of whether CBD may have a role in the treatment of schizophrenia and other psychotic disorders. One of the first studies on this issue was conducted by Leweke et al,3 who compared oral CBD, up to 800 mg/d, with the antipsychotic amisulpride, up to 800 mg/d, in 39 patients with an acute exacerbation of psychotic symptoms. Amisulpride is used outside the United States to treat psychosis, but is FDA-approved only as an antiemetic. Patients were treated for 4 weeks. By Day 28, there was a significant reduction in positive symptoms as measured using the Positive and Negative Syndrome Scale (PANSS), with no significant difference in efficacy between the treatments. Similar findings emerged for negative, total, and general symptoms, with significant reductions by Day 28 in both treatment arms, and no significant between-treatment differences.
These findings were the first robust indication that CBD may have antipsychotic efficacy. However, of greater interest may be CBD’s markedly superior adverse effect profile. Predictably, amisulpride significantly increased extrapyramidal symptoms (EPS), weight gain, and prolactin levels from baseline to Day 28. However, no significant change was found in any of these adverse effects in the CBD group, and the between-treatment difference was significant (all P < .01).
Here we review 4 recent studies that evaluated CBD as a treatment for schizophrenia. These studies are summarized in the Table.4-7
Continue to: McGuire P, et al...
1. McGuire P, Robson P, Cubala WJ, et al. Cannabidiol (CBD) as an adjunctive therapy in schizophrenia: a multicenter randomized controlled trial. Am J Psychiatry. 2018;175(3):225-231.
Antipsychotic medications act through blockade of central dopamine D2 receptors. For most patients, antipsychotics effectively treat positive psychotic symptoms, which are driven by elevated dopamine function. However, these medications have minimal effects on negative symptoms and cognitive impairment, features of schizophrenia that are not driven by elevated dopamine. Compounds exhibiting a mechanism of action unlike that of current antipsychotics may improve the treatment and outcomes of patients with schizophrenia. The mechanism of action of CBD is unclear, but it does not appear to involve the direct antagonism of dopamine receptors. Human and animal research study findings indicate that CBD has antipsychotic properties. McGuire et al4 assessed the safety and effectiveness of CBD as an adjunctive treatment of schizophrenia.
Study design
- In this double-blind parallel-group trial conducted at 15 hospitals in the United Kingdom, Romania, and Poland, 88 patients with schizophrenia received CBD (1,000 mg/d; N = 43) or placebo (N = 45) as adjunct to the antipsychotic medication they had been prescribed. Patients had previously demonstrated at least a partial response to antipsychotic treatment, and were taking stable doses of an antipsychotic for ≥4 weeks.
- Evaluations of symptoms, general functioning, cognitive performance, and EPS were completed at baseline and on Days 8, 22, and 43 (± 3 days). Current substance use was assessed using a semi-structured interview, and reassessed at the end of treatment.
- The key endpoints were the patients’ level of functioning, severity of symptoms, and cognitive performance. Participants were assessed before and after treatment using the PANSS, the Brief Assessment of Cognition in Schizophrenia (BACS), the Global Assessment of Functioning scale (GAF), and the improvement and severity scales of the Clinical Global Impressions Scale (CGI-I and CGI-S, respectively).
- The clinicians’ impression of illness severity and symptom improvement and patient- or caregiver-reported impressions of general functioning and sleep also were noted.
Outcomes
- After 6 weeks, compared with the placebo group, the CBD group had lower levels of positive psychotic symptoms and were more likely to be rated as improved and as not severely unwell by the treating clinician. Patients in the CBD group also showed greater improvements in cognitive performance and in overall functioning, although these were not statistically significant.
- Similar levels of negative psychotic symptoms, overall psychopathology, and general psychopathology were observed in the CBD and placebo groups. The CBD group had a higher proportion of treatment responders (≥20% improvement in PANSS total score) than did the placebo group; however, the total number of responders per group was small (12 and 6 patients, respectively). At baseline, most patients in both groups were classified as moderately, markedly, or severely ill (83.4% in the CBD group vs 79.6% in placebo group). By the end of treatment, this decreased to 54.8% in the CBD group and 63.6% in the placebo group. Clinicians rated 78.6% of patients in the CBD group as “improved” on the CGI-I, compared with 54.6% of patients in the placebo group.
Conclusion
- CBD treatment adjunctive to antipsychotics was associated with significant effects on positive psychotic symptoms and on CGI-I and illness severity. Improvements in cognitive performance and level of overall functioning were also seen, but were not statistically significant.
- Although the effect on positive symptoms was modest, improvement occurred in patients being treated with appropriate dosages of antipsychotics, which suggests CBD provided benefits over and above the effect of antipsychotic treatment. Moreover, the changes in CGI-I and CGI-S scores indicated that the improvement was evident to the treating psychiatrists, and may therefore be clinically meaningful.
Continue to: Boggs DL, et al...
2. Boggs DL, Surti T, Gupta A, et al. The effects of cannabidiol (CBD) on cognition and symptoms in outpatients with chronic schizophrenia a randomized placebo controlled trial. Psychopharmacology (Berl). 2018;235(7):1923-1932.
Schizophrenia is associated with cognitive deficits in learning, recall, attention, working memory, and executive function. The cognitive impairments associated with schizophrenia (CIAS) are independent of phase of illness and often persist after other symptoms have been effectively treated. These impairments are the strongest predictor of functional outcome, even more so than psychotic symptoms.
Antipsychotics have limited efficacy for CIAS, which highlights the need for CIAS treatments that target other nondopaminergic neurotransmitter systems. The endocannabinoid system, which has been implicated in schizophrenia and in cognition, is a potential target. Several cannabinoids impair memory and attention. The main psychoactive component of marijuana, THC, is a cannabinoid receptor type 1 (CB1R) partial agonist. Administration of THC produces significant deficits in verbal learning, attention, and working memory.
Researchers have hypothesized that CB1R blockade or modulation of cannabinoid levels may offer a novel target for treating CIAS. Boggs et al5 compared the cognitive, symptomatic, and adverse effects of CBD vs placebo.
Study design
- In this 6-week, randomized, placebo-controlled study conducted in Connecticut from September 2009 to May 2012, 36 stable patients with schizophrenia who were treated with antipsychotics were randomized to also receive oral CBD, 600 mg/d, or placebo.
- Cognition was assessed using the t score of the MATRICS Consensus Cognitive Battery (MCCB) composite and subscales at baseline and the end of study. An increase in MCCB t score indicates an improvement in cognitive ability. Psychotic symptoms were assessed using the PANSS at baseline, Week 2, Week 4, and Week 6.
Outcomes
- CBD augmentation did not improve MCCB performance or psychotic symptoms. There was no main effect of time or medication on MCCB composite score, but a significant drug × time effect was observed.
- Post-hoc analyses revealed that only patients who received placebo improved over time. The lack of a similar improvement with CBD might be related to the greater incidence of sedation among the CBD group (20%) vs the placebo group (5%). Both the MCCB composite score and reasoning and problem-solving domain scores were higher at baseline and endpoint for patients who received CBD, which suggests that the observed improvement in the placebo group could represent a regression to the mean.
- There was a significant decrease in PANSS scores over time, but there was no significant drug × time interaction.
Conclusion
- CBD augmentation was not associated with an improvement in MCCB score. This is consistent with data from other clinical trials4,8 that suggested that CBD (at a wide range of doses) does not have significant beneficial effects on cognition in patients with schizophrenia.
- Additionally, CBD did not improve psychotic symptoms. These results are in contrast to published case reports9,10 and 2 published clinical trials3,4 that found CBD (800 mg/d) was as efficacious as amisulpride in reducing positive psychotic symptoms, and a small but statistically significant improvement in PANSS positive scores with CBD (1,000 mg/d) compared with placebo. However, these results are similar to those of a separate study11 that evaluated the same 600-mg/d dose of CBD used by Boggs et al.5 At 600 mg/d, CBD produced very small improvements in PANSS total scores (~2.4) that were not statistically significant. A higher CBD dose may be needed to reduce psychotic symptoms in patients with schizophrenia.
Continue to: O’Neill A, et al...
3. O’Neill A, Wilson R, Blest-Hopley G, et al. Normalization of mediotemporal and prefrontal activity, and mediotemporal-striatal connectivity, may underlie antipsychotic effects of cannabidiol in psychosis. Psychol Med. 2020;1-11. doi: 10.1017/S0033291719003519.
In addition to their key roles in the psychopathology of psychosis, the mediotemporal and prefrontal cortices are involved in learning and memory, and the striatum plays a role in encoding contextual information associated with memories. Because deficits in verbal learning and memory are one of the most commonly reported impairments in patients with psychosis, O’Neill et al6 used functional MRI (fMRI) to examine brain activity during a verbal learning task in patients with psychosis after taking CBD or placebo.
Study design
- In a double-blind, randomized, placebo-controlled, crossover study, researchers investigated the effects of a single dose of CBD in 15 patients with psychosis who were treated with antipsychotics. Three hours after taking a 600-mg dose of CBD or placebo, these participants were scanned using fMRI while performing a verbal paired associate (VPA) learning task. Nineteen healthy controls underwent fMRI in identical conditions, but without any medication administration.
- The fMRI measured brain activation using the blood oxygen level–dependent (BOLD) hemodynamic responses of the brain. The fMRI signals were studied in the mediotemporal, prefrontal, and striatal regions.
- The VPA task presented word pairs visually, and the accuracy of responses were recorded online. The VPA task was comprised of 3 conditions: encoding, recall, and baseline.
- Results during each phase of the VPA task were compared.
Outcomes
- While completing the VPA task after taking placebo, compared with healthy controls, patients with psychosis demonstrated a different pattern of activity in the prefrontal and mediotemporal brain areas. Specifically, during verbal encoding, the placebo group showed altered activation in prefrontal regions. During verbal recall, the placebo group showed altered activation in prefrontal and mediotemporal regions, as well as increased mediotemporal-striatal functional connectivity.
- After participants received CBD, activation in these brain areas became more like the activation seen in controls. CBD attenuated dysfunction in these regions such that activation was intermediate between the placebo condition and the control group. CBD also attenuated functional connectivity between the hippocampus and striatum, and lead to reduced symptoms in patients with psychosis (as measured by PANSS total score).
Conclusion
- Altered activation in prefrontal and mediotemporal regions during verbal learning in patients with psychosis appeared to be partially normalized after a single 600-mg dose of CBD. Results also showed improvement in PANSS total score with CBD.
- These findings suggest that a single dose of CBD may partially attenuate the dysfunctional prefrontal and mediotemporal activation that is believed to underlie the dopamine dysfunction that leads to psychotic symptoms. These effects, along with a reduction in psychotic symptoms, suggest that normalization of altered prefrontal and mediotemporal function and mediotemporal-striatal connectivity may underlie the antipsychotic effects of CBD in established psychosis.
Continue to: Bhattacharyya S, et al...
4. Bhattacharyya S, Wilson R, Appiah-Kusi E, et al. Effect of cannabidiol on medial temporal, midbrain, and striatal dysfunction in people at clinical high risk of psychosis: a randomized clinical trial. JAMA Psychiatry. 2018;75(11):1107-1117.
Current preclinical models suggest that psychosis involves a disturbance of activity in the medial temporal lobe (MTL) that drives dopamine dysfunction in the striatum and midbrain. THC, which produces psychotomimetic effects, impacts the function of the striatum (verbal memoryand salience processing) andamygdala (emotional processing), and alters the functional connectivity of these regions. Compared with THC, CBD has broadly opposite neural and behavioral effects, including opposing effects on the activation of these regions. Bhattacharyya et al7 examined the neurocognitive mechanisms that underlie the therapeutic effects of CBD in psychosis and sought to understand whether CBD would attenuate functional abnormalities in the MTL, midbrain, and striatum.
Study design
- A randomized, double-blind, placebo-controlled trial examined 33 antipsychotic-naïve participants at clinical high risk (CHR) for psychosis and 19 healthy controls. The CHR group was randomized to CBD, 600 mg, or placebo.
- Three hours after taking CBD or placebo, CHR participants were studied using fMRI while performing a VPA learning task, which engages verbal learning and recall in the MTL, midbrain and striatum. Control participants did not receive any medication but underwent fMRI while performing the VPA task.
- The VPA task presented word pairs visually, and the accuracy of responses was recorded online. It was comprised of 3 conditions: encoding, recall, and baseline.
Outcomes
- Brain activation was analyzed in 15 participants in the CBD group, 16 in the placebo group, and 19 in the control group. Activation during encoding was observed in the striatum (specifically, the right caudate). Activation during recall was observed in the midbrain and the MTL (specifically, the parahippocampus).
- Brain activation levels in all 3 regions were lowest in the placebo group, intermediate in the CBD group, and greatest in the healthy control group. For all participants, the total recall score was directly correlated with the activation level in the left MTL (parahippocampus) during recall.
Conclusion
- Relative to controls, CHR participants exhibited different levels of activation in several regions, including the 3 areas thought to be critical to the pathophysiology of psychosis: the striatum during verbal encoding, and the MTL and midbrain during verbal recall.
- Compared with those who received placebo, CHR participants who received CBD before completing the VPA task demonstrated greater levels of brain activation and higher recall score.
- These findings suggest that CBD may partially normalize alterations in MTL, striatal, and midbrain function associated with CHR of psychosis. Because these regions are implicated in the pathophysiology of psychosis, the impact of CBD at these sites may contribute to the therapeutic effects of CBD that have been reported by some patients with psychosis.
Continue to: Conflicting data highlights...
Conflicting data highlights the need for longer, larger studies
Research findings on the use of CBD for psychotic symptoms in patients with schizophrenia have been conflicting. Some early research suggests that taking CBD 4 times daily for 4 weeks improves psychotic symptoms and might be as effective as the antipsychotic amisulpride. However, other early research suggests that taking CBD for 14 days is not beneficial. The conflicting results might be related to the CBD dose used and duration of treatment.
Davies and Bhattacharya12 recently reviewed evidence regarding the efficacy of CBD as a potential novel treatment for psychotic disorders.They concluded that CBD represents a promising potential novel treatment for patients with psychosis. It also appears that CBD may improve the disease trajectory of individuals with early psychosis and comorbid cannabis misuse.13 CBD use has also been associated with a decrease in symptoms of psychosis and changes in brain activity during verbal memory tasks in patients at high risk of psychosis.6 However, before CBD can become a viable treatment option for psychosis, the promising findings in these initial clinical studies must be replicated in large-scale trials with appropriate treatment duration.
1. US National Library of Medicine. MedlinePlus. Cannabidiol (CBD). https://medlineplus.gov/druginfo/natural/1439.html. Accessed May 14, 2020.
2. Bonn-Miller MO, Loflin MJE, Thomas BF, et al. Labeling accuracy of cannabidiol extracts sold online. JAMA. 2017;318(17):1708-1709.
3. Leweke FM, Piomelli D, Pahlisch F, et al. Cannabidiol enhances anandamide signaling and alleviates psychotic symptoms of schizophrenia. Transl Psychiatry. 2012;2(3):e94.
4. McGuire P, Robson P, Cubala WJ, et al. Cannabidiol (CBD) as an adjunctive therapy in schizophrenia: a multicenter randomized controlled trial. Am J Psychiatry. 2018;175(3):225-231.
5. Boggs DL, Surti T, Gupta A, et al. The effects of cannabidiol (CBD) on cognition and symptoms in outpatients with chronic schizophrenia a randomized placebo controlled trial. Psychopharmacology (Berl). 2018;235(7):1923-1932.
6. O’Neill A, Wilson R, Blest-Hopley G, et al. Normalization of mediotemporal and prefrontal activity, and mediotemporal-striatal connectivity, may underlie antipsychotic effects of cannabidiol in psychosis. Psychol Med. 2020;1-11. doi: 10.1017/S0033291719003519.
7. Bhattacharyya S, Wilson R, Appiah-Kusi E, et al. Effect of cannabidiol on medial temporal, midbrain, and striatal dysfunction in people at clinical high risk of psychosis: a randomized clinical trial. JAMA Psychiatry. 2018;75(11):1107-1117.
8. Hallak JE, Machado-de-Sousa JP, Crippa JAS, et al. Performance of schizophrenic patients in the Stroop color word test and electrodermal responsiveness after acute administration of cannabidiol (CBD). Rev Bras Psiquiatr. 2010;32(1):56-61.
9. Zuardi AW, Morais SL, Guimaraes FS, et al. Antipsychotic effect of cannabidiol. J Clin Psychiatry. 1995;56(10):485-486.
10. Zuardi AW, Hallak JE, Dursun SM, et al. Cannabidiol monotherapy for treatment-resistant schizophrenia. J Psychopharmacol. 2006;20(5):683-686.
11. Leweke FM, Hellmich M, Pahlisch F, et al. Modulation of the endocannabinoid system as a potential new target in the treatment of schizophrenia. Schizophr Res. 2014; 153(1):S47.
12. Davies C, Bhattacharyya S. Cannabidiol as a potential treatment for psychosis. Ther Adv Psychopharmacol. 2019;9. doi:10.1177/2045125319881916.
13. Hahn B. The potential of cannabidiol treatment for cannabis users with recent-onset psychosis. Schizophr Bull. 2018;44(1):46-53.
1. US National Library of Medicine. MedlinePlus. Cannabidiol (CBD). https://medlineplus.gov/druginfo/natural/1439.html. Accessed May 14, 2020.
2. Bonn-Miller MO, Loflin MJE, Thomas BF, et al. Labeling accuracy of cannabidiol extracts sold online. JAMA. 2017;318(17):1708-1709.
3. Leweke FM, Piomelli D, Pahlisch F, et al. Cannabidiol enhances anandamide signaling and alleviates psychotic symptoms of schizophrenia. Transl Psychiatry. 2012;2(3):e94.
4. McGuire P, Robson P, Cubala WJ, et al. Cannabidiol (CBD) as an adjunctive therapy in schizophrenia: a multicenter randomized controlled trial. Am J Psychiatry. 2018;175(3):225-231.
5. Boggs DL, Surti T, Gupta A, et al. The effects of cannabidiol (CBD) on cognition and symptoms in outpatients with chronic schizophrenia a randomized placebo controlled trial. Psychopharmacology (Berl). 2018;235(7):1923-1932.
6. O’Neill A, Wilson R, Blest-Hopley G, et al. Normalization of mediotemporal and prefrontal activity, and mediotemporal-striatal connectivity, may underlie antipsychotic effects of cannabidiol in psychosis. Psychol Med. 2020;1-11. doi: 10.1017/S0033291719003519.
7. Bhattacharyya S, Wilson R, Appiah-Kusi E, et al. Effect of cannabidiol on medial temporal, midbrain, and striatal dysfunction in people at clinical high risk of psychosis: a randomized clinical trial. JAMA Psychiatry. 2018;75(11):1107-1117.
8. Hallak JE, Machado-de-Sousa JP, Crippa JAS, et al. Performance of schizophrenic patients in the Stroop color word test and electrodermal responsiveness after acute administration of cannabidiol (CBD). Rev Bras Psiquiatr. 2010;32(1):56-61.
9. Zuardi AW, Morais SL, Guimaraes FS, et al. Antipsychotic effect of cannabidiol. J Clin Psychiatry. 1995;56(10):485-486.
10. Zuardi AW, Hallak JE, Dursun SM, et al. Cannabidiol monotherapy for treatment-resistant schizophrenia. J Psychopharmacol. 2006;20(5):683-686.
11. Leweke FM, Hellmich M, Pahlisch F, et al. Modulation of the endocannabinoid system as a potential new target in the treatment of schizophrenia. Schizophr Res. 2014; 153(1):S47.
12. Davies C, Bhattacharyya S. Cannabidiol as a potential treatment for psychosis. Ther Adv Psychopharmacol. 2019;9. doi:10.1177/2045125319881916.
13. Hahn B. The potential of cannabidiol treatment for cannabis users with recent-onset psychosis. Schizophr Bull. 2018;44(1):46-53.
Stop calling it ‘behavioral health’: Psychiatry is much more
Psychiatry has been historically plagued by absurd misnomers. It started with the laughable “mental hygiene,” coined by William Sweetser, MD, in 1843, 1 year before the original 13 members of the Association of Medical Superintendents of American Institutions for the Insane established what in 1921 was renamed the American Psychiatric Association. Mental hygiene evokes an image of psychiatrists scrubbing the brains of mentally ill patients with soap and water! That term was neither medically nor scientifically appropriate, but it stuck for decades.
Enter “mental health.” In 1949, the National Institute of Mental Health was established. It is the 5th oldest of the 27 Institutes and Centers of the National Institutes of Health. Then, in 1963, Congress passed the Community Mental Health Act, which established Community Mental Health Centers around the country. It is perplexing that the term “health” was used instead of “illness,” when psychiatry is a medical specialty that treats mental disorders. Health is certainly the goal of all medical specialties, but cardiology was never called “heart health,” neurology was never called “brain health,” and pediatrics was never called “children’s health.” Like all its sister medical specialties, psychiatry treats disease and syndromes, but somehow, it has been transmogrified into “mental health.” Perhaps it was meant to be a euphemism to disguise and avert the unfortunate stigma associated with mental illness back during the institutionalization era.
The advent of ‘behavioral health’
Then suddenly, the term “behavioral health” was coined and began to be used as a substitute for psychiatry, further distorting psychiatry’s medical identity. Behavioral health is completely different from psychiatry. It refers to healthy behaviors that people should uphold throughout their lives to maintain their overall health and well-being, including eating a balanced diet, exercising regularly, avoiding tobacco and drugs of abuse, practicing safe sex, and establishing meaningful social relationships. So behavioral health promotes a healthy lifestyle, and that could very aptly apply to cardiology, pulmonology, nephrology, or hepatology, where good nutrition and avoiding weight gain, smoking, and sedentary living can reduce the risk for various medical diseases and early mortality. For dermatologists, behavioral health is avoiding sunburn, and for dentists, it is regular brushing and flossing.
Thus, behavioral health is a term that broadly promotes physical health and well-being, and should not be conflated with mental disorders. It is by no means synonymous with psychiatry, a medical discipline that addresses serious disorders of thought, emotions, affect, delusions, hallucinations, suicide, homicide, impulsivity, obsessions and compulsions, motivation, memory, attention, and judgment. Psychiatry is far more than behaviors that promote healthy living. Psychiatry contends with acute and chronic mental disorders, similar to other chronic medical conditions such as chronic heart, lung, gastrointestinal, or kidney diseases. Psychiatric disorders can emerge in individuals despite—and irrespective of—a healthy lifestyle promoted by behavioral health. Most psychiatric disorders have been shown to be highly genetic, and can be triggered by gene-environment interactions, even in the context of a healthful life that behavioral health advocates and fecundates.
I dislike conspiracy theories, but it is legitimate to inquire: Was there a “malicious intent” by insurance companies and managed-care entities when they abruptly replaced the medically accurate term “psychiatry” with the counterfactual “behavioral health”? Did they intend to portray psychiatry as somehow “different” from other medical specialties? Did this phraseological acrobatics facilitate and justify the carving out of psychiatric and addiction care, cursed with an anemic budget and absence of parity for persons with psychiatric brain disorders? Somehow, using behavioral health instead of psychiatry has the unfortunate connotation that patients with mental illness are “misbehaving” by not practicing healthy living, rather than being genuinely medically ill through no fault of their own. That’s a surreptitious de-medicalization of psychiatric brain disorders. It is very likely that the same companies that propagated behavioral health are the ones who came up with the demeaning term “providers,” which lumps physicians with nonphysicians, diluting the medical identify of psychiatrists, and implying a non-equivalence of psychiatric disorders with other medical conditions, which perpetuates stigma.
An erroneous epithet
We are psychiatric physicians, not “behavioral health advisors.” We are graduates of medical schools where we had clinical psychiatric experiences rotating with internal medicine, surgery, obstetrics and gynecology, and pediatrics. We did not have behavioral health rotations. And after graduating with an MD, we spent 4 additional years in psychiatric residency training, not behavioral health training, and we treated very sick patients in emergency departments and on inpatient units, not on behavioral health wards. We receive our board certification from the American Board of Psychiatry and Neurology, not from a behavioral health board. As psychiatrists, we are regularly consulted on the cases of medical and surgical patients who develop psychiatric disorders, which has absolutely nothing to do with behavioral health. Our psychiatric outpatient clinics require extensive medical knowledge and psychopharmacological skills, not behavioral health.
As part of our work as physicians and psychiatrists, we do counsel patients on adopting a healthy lifestyle because many of them have comorbid medical conditions such as diabetes, hypertension, dyslipidemia, chronic obstructive pulmonary disease, asthma, and kidney and gastrointestinal disorders. We practice collaborative care with primary care physicians so we can jointly manage patients’ physical and mental disorders, and help them optimize their lifestyles. Thus, behavioral health is a tiny component of what psychiatrists do, and it does not come close to defining our comprehensive medical care. Similarly, neurologists and cardiologists should not be labeled as behavior health specialties simply because they counsel their patients on how to lower the risk of strokes or heart attacks due to unhealthy lifestyles.
So, let’s call a spade a spade. Psychiatry is psychiatric medical care, not behavioral health. Let’s abandon this erroneous epithet and change the signs outside hospitals and clinics to “psychiatric medicine” facilities. I guarantee that orthopedists would not like it all if you call their specialty “bone health,” and may break your leg if you label their discipline “bone hygiene”… after washing it with soap and water, of course!
Psychiatry has been historically plagued by absurd misnomers. It started with the laughable “mental hygiene,” coined by William Sweetser, MD, in 1843, 1 year before the original 13 members of the Association of Medical Superintendents of American Institutions for the Insane established what in 1921 was renamed the American Psychiatric Association. Mental hygiene evokes an image of psychiatrists scrubbing the brains of mentally ill patients with soap and water! That term was neither medically nor scientifically appropriate, but it stuck for decades.
Enter “mental health.” In 1949, the National Institute of Mental Health was established. It is the 5th oldest of the 27 Institutes and Centers of the National Institutes of Health. Then, in 1963, Congress passed the Community Mental Health Act, which established Community Mental Health Centers around the country. It is perplexing that the term “health” was used instead of “illness,” when psychiatry is a medical specialty that treats mental disorders. Health is certainly the goal of all medical specialties, but cardiology was never called “heart health,” neurology was never called “brain health,” and pediatrics was never called “children’s health.” Like all its sister medical specialties, psychiatry treats disease and syndromes, but somehow, it has been transmogrified into “mental health.” Perhaps it was meant to be a euphemism to disguise and avert the unfortunate stigma associated with mental illness back during the institutionalization era.
The advent of ‘behavioral health’
Then suddenly, the term “behavioral health” was coined and began to be used as a substitute for psychiatry, further distorting psychiatry’s medical identity. Behavioral health is completely different from psychiatry. It refers to healthy behaviors that people should uphold throughout their lives to maintain their overall health and well-being, including eating a balanced diet, exercising regularly, avoiding tobacco and drugs of abuse, practicing safe sex, and establishing meaningful social relationships. So behavioral health promotes a healthy lifestyle, and that could very aptly apply to cardiology, pulmonology, nephrology, or hepatology, where good nutrition and avoiding weight gain, smoking, and sedentary living can reduce the risk for various medical diseases and early mortality. For dermatologists, behavioral health is avoiding sunburn, and for dentists, it is regular brushing and flossing.
Thus, behavioral health is a term that broadly promotes physical health and well-being, and should not be conflated with mental disorders. It is by no means synonymous with psychiatry, a medical discipline that addresses serious disorders of thought, emotions, affect, delusions, hallucinations, suicide, homicide, impulsivity, obsessions and compulsions, motivation, memory, attention, and judgment. Psychiatry is far more than behaviors that promote healthy living. Psychiatry contends with acute and chronic mental disorders, similar to other chronic medical conditions such as chronic heart, lung, gastrointestinal, or kidney diseases. Psychiatric disorders can emerge in individuals despite—and irrespective of—a healthy lifestyle promoted by behavioral health. Most psychiatric disorders have been shown to be highly genetic, and can be triggered by gene-environment interactions, even in the context of a healthful life that behavioral health advocates and fecundates.
I dislike conspiracy theories, but it is legitimate to inquire: Was there a “malicious intent” by insurance companies and managed-care entities when they abruptly replaced the medically accurate term “psychiatry” with the counterfactual “behavioral health”? Did they intend to portray psychiatry as somehow “different” from other medical specialties? Did this phraseological acrobatics facilitate and justify the carving out of psychiatric and addiction care, cursed with an anemic budget and absence of parity for persons with psychiatric brain disorders? Somehow, using behavioral health instead of psychiatry has the unfortunate connotation that patients with mental illness are “misbehaving” by not practicing healthy living, rather than being genuinely medically ill through no fault of their own. That’s a surreptitious de-medicalization of psychiatric brain disorders. It is very likely that the same companies that propagated behavioral health are the ones who came up with the demeaning term “providers,” which lumps physicians with nonphysicians, diluting the medical identify of psychiatrists, and implying a non-equivalence of psychiatric disorders with other medical conditions, which perpetuates stigma.
An erroneous epithet
We are psychiatric physicians, not “behavioral health advisors.” We are graduates of medical schools where we had clinical psychiatric experiences rotating with internal medicine, surgery, obstetrics and gynecology, and pediatrics. We did not have behavioral health rotations. And after graduating with an MD, we spent 4 additional years in psychiatric residency training, not behavioral health training, and we treated very sick patients in emergency departments and on inpatient units, not on behavioral health wards. We receive our board certification from the American Board of Psychiatry and Neurology, not from a behavioral health board. As psychiatrists, we are regularly consulted on the cases of medical and surgical patients who develop psychiatric disorders, which has absolutely nothing to do with behavioral health. Our psychiatric outpatient clinics require extensive medical knowledge and psychopharmacological skills, not behavioral health.
As part of our work as physicians and psychiatrists, we do counsel patients on adopting a healthy lifestyle because many of them have comorbid medical conditions such as diabetes, hypertension, dyslipidemia, chronic obstructive pulmonary disease, asthma, and kidney and gastrointestinal disorders. We practice collaborative care with primary care physicians so we can jointly manage patients’ physical and mental disorders, and help them optimize their lifestyles. Thus, behavioral health is a tiny component of what psychiatrists do, and it does not come close to defining our comprehensive medical care. Similarly, neurologists and cardiologists should not be labeled as behavior health specialties simply because they counsel their patients on how to lower the risk of strokes or heart attacks due to unhealthy lifestyles.
So, let’s call a spade a spade. Psychiatry is psychiatric medical care, not behavioral health. Let’s abandon this erroneous epithet and change the signs outside hospitals and clinics to “psychiatric medicine” facilities. I guarantee that orthopedists would not like it all if you call their specialty “bone health,” and may break your leg if you label their discipline “bone hygiene”… after washing it with soap and water, of course!
Psychiatry has been historically plagued by absurd misnomers. It started with the laughable “mental hygiene,” coined by William Sweetser, MD, in 1843, 1 year before the original 13 members of the Association of Medical Superintendents of American Institutions for the Insane established what in 1921 was renamed the American Psychiatric Association. Mental hygiene evokes an image of psychiatrists scrubbing the brains of mentally ill patients with soap and water! That term was neither medically nor scientifically appropriate, but it stuck for decades.
Enter “mental health.” In 1949, the National Institute of Mental Health was established. It is the 5th oldest of the 27 Institutes and Centers of the National Institutes of Health. Then, in 1963, Congress passed the Community Mental Health Act, which established Community Mental Health Centers around the country. It is perplexing that the term “health” was used instead of “illness,” when psychiatry is a medical specialty that treats mental disorders. Health is certainly the goal of all medical specialties, but cardiology was never called “heart health,” neurology was never called “brain health,” and pediatrics was never called “children’s health.” Like all its sister medical specialties, psychiatry treats disease and syndromes, but somehow, it has been transmogrified into “mental health.” Perhaps it was meant to be a euphemism to disguise and avert the unfortunate stigma associated with mental illness back during the institutionalization era.
The advent of ‘behavioral health’
Then suddenly, the term “behavioral health” was coined and began to be used as a substitute for psychiatry, further distorting psychiatry’s medical identity. Behavioral health is completely different from psychiatry. It refers to healthy behaviors that people should uphold throughout their lives to maintain their overall health and well-being, including eating a balanced diet, exercising regularly, avoiding tobacco and drugs of abuse, practicing safe sex, and establishing meaningful social relationships. So behavioral health promotes a healthy lifestyle, and that could very aptly apply to cardiology, pulmonology, nephrology, or hepatology, where good nutrition and avoiding weight gain, smoking, and sedentary living can reduce the risk for various medical diseases and early mortality. For dermatologists, behavioral health is avoiding sunburn, and for dentists, it is regular brushing and flossing.
Thus, behavioral health is a term that broadly promotes physical health and well-being, and should not be conflated with mental disorders. It is by no means synonymous with psychiatry, a medical discipline that addresses serious disorders of thought, emotions, affect, delusions, hallucinations, suicide, homicide, impulsivity, obsessions and compulsions, motivation, memory, attention, and judgment. Psychiatry is far more than behaviors that promote healthy living. Psychiatry contends with acute and chronic mental disorders, similar to other chronic medical conditions such as chronic heart, lung, gastrointestinal, or kidney diseases. Psychiatric disorders can emerge in individuals despite—and irrespective of—a healthy lifestyle promoted by behavioral health. Most psychiatric disorders have been shown to be highly genetic, and can be triggered by gene-environment interactions, even in the context of a healthful life that behavioral health advocates and fecundates.
I dislike conspiracy theories, but it is legitimate to inquire: Was there a “malicious intent” by insurance companies and managed-care entities when they abruptly replaced the medically accurate term “psychiatry” with the counterfactual “behavioral health”? Did they intend to portray psychiatry as somehow “different” from other medical specialties? Did this phraseological acrobatics facilitate and justify the carving out of psychiatric and addiction care, cursed with an anemic budget and absence of parity for persons with psychiatric brain disorders? Somehow, using behavioral health instead of psychiatry has the unfortunate connotation that patients with mental illness are “misbehaving” by not practicing healthy living, rather than being genuinely medically ill through no fault of their own. That’s a surreptitious de-medicalization of psychiatric brain disorders. It is very likely that the same companies that propagated behavioral health are the ones who came up with the demeaning term “providers,” which lumps physicians with nonphysicians, diluting the medical identify of psychiatrists, and implying a non-equivalence of psychiatric disorders with other medical conditions, which perpetuates stigma.
An erroneous epithet
We are psychiatric physicians, not “behavioral health advisors.” We are graduates of medical schools where we had clinical psychiatric experiences rotating with internal medicine, surgery, obstetrics and gynecology, and pediatrics. We did not have behavioral health rotations. And after graduating with an MD, we spent 4 additional years in psychiatric residency training, not behavioral health training, and we treated very sick patients in emergency departments and on inpatient units, not on behavioral health wards. We receive our board certification from the American Board of Psychiatry and Neurology, not from a behavioral health board. As psychiatrists, we are regularly consulted on the cases of medical and surgical patients who develop psychiatric disorders, which has absolutely nothing to do with behavioral health. Our psychiatric outpatient clinics require extensive medical knowledge and psychopharmacological skills, not behavioral health.
As part of our work as physicians and psychiatrists, we do counsel patients on adopting a healthy lifestyle because many of them have comorbid medical conditions such as diabetes, hypertension, dyslipidemia, chronic obstructive pulmonary disease, asthma, and kidney and gastrointestinal disorders. We practice collaborative care with primary care physicians so we can jointly manage patients’ physical and mental disorders, and help them optimize their lifestyles. Thus, behavioral health is a tiny component of what psychiatrists do, and it does not come close to defining our comprehensive medical care. Similarly, neurologists and cardiologists should not be labeled as behavior health specialties simply because they counsel their patients on how to lower the risk of strokes or heart attacks due to unhealthy lifestyles.
So, let’s call a spade a spade. Psychiatry is psychiatric medical care, not behavioral health. Let’s abandon this erroneous epithet and change the signs outside hospitals and clinics to “psychiatric medicine” facilities. I guarantee that orthopedists would not like it all if you call their specialty “bone health,” and may break your leg if you label their discipline “bone hygiene”… after washing it with soap and water, of course!
Remdesivir shortens COVID-19 time to recovery in published study
Much-anticipated results from the National Institute of Allergy and Infectious Diseases’ clinical trial of remdesivir in COVID-19 patients published in the New England Journal of Medicine suggest remdesivir shortens the disease course for hospitalized COVID-19 patients.
The agency reported initial promising results from the study earlier this month, which prompted the Food and Drug Administration to issue an emergency use authorization (EUA) for the drug, but the full data and results have not been widely available until now.
The findings also suggest remdesivir should be started, if possible, before patients have such severe pulmonary disease that they require mechanical ventilation, according to the study authors.
The published results are “completely consistent” with the NIAID’s earlier announcement, H. Clifford Lane, MD, deputy director for clinical research and special projects at the NIAID, said in an interview. “The benefit appeared to be the greatest for the patients who are hospitalized with severe disease who require supplemental oxygen.”
Given the limited supply of remdesivir, physicians have been eager to see the full data to ensure they use the drug most effectively, Daniel Kaul, MD, a professor of infectious diseases at the University of Michigan, Ann Arbor, said in an interview. Hospitals in states across the country, including New York, Michigan, and Washington, have received limited supplies of the drug in the last couple of weeks since the FDA’s authorization.
“I am losing my patience waiting for #remdesivir data. I was willing to give them a week to verify the numbers, triple proof the tables, cautiously frame conclusions. But it’s gone on too long. We are rationing with no rationale. We are floating on whisps [sic] of data, adrift,” Kate Stephenson, MD, an infectious diseases specialist at the Center for Virology and Vaccine Research at Harvard Medical School, Boston, wrote on Twitter May 18. After reading the paper, she tweeted Friday evening that she was “relieved to see convincing benefit – I was starting to worry!”
In the midst of a public health crisis, however, it is not unusual to make an announcement about trial results before the full dataset has been analyzed, said Dr. Lane. The NIAID followed a similar playbook for the PALM trial evaluating possible Ebola treatments in the Democratic Republic of Congo, with the independent monitoring board recommending the trial be terminated early in response to positive results from two of the four candidate drugs.
“When you have a result you think is of public health importance, you don’t wait for it to be published in a peer-reviewed journal,” said Dr. Lane, a coauthor of the study. The lag time from announcement to study publication was a result of the time it took to write up the paper for publication and go through peer review, Dr. Lane added. He also noted that the FDA had access to the data when the agency wrote its guidance for physicians administering the drug to patients under the EUA.
The authors opted not to publish the initial findings on a preprint server because they felt it was important to undergo peer review, said Dr. Lane. “The last thing you want for something this critical is for incomplete data to be out there, or you don’t have everything audited to the level that you want.”
Trial details
In the ACTT-1 randomized, placebo-controlled, double-blinded trial, researchers enrolled 1,063 patients from Feb. 21 to April 19, 2020, at 60 trial sites and 13 subsites worldwide (45 sites in the United States). The remdesivir group had 541 patients, and the placebo group had 522. A small number of patients (49 in the remdesivir group and 53 in the placebo group) discontinued treatment before day 10 because of an adverse event or withdrawn consent. When data collection for this preliminary analysis ended in late April, 301 patients had not recovered and had not completed their final follow-up visit.
Most of the patients had one (27%) or more (52.1%) preexisting conditions, including hypertension (49.6%), obesity (37%), and type 2 diabetes mellitus (29.7%). Mean patient age was 58.9 years, and the majority of patients were men (64.3%). The median number of days from symptom onset to randomization was 9, and 53.6% of the patients were white, 20.6% were black, 12.6% were Asian, 23.4% were Hispanic or Latino, and the ethnicity of 13.6% were not reported or reported as other.
Patients received one 200-mg loading dose on the first day of the trial, and then one 100-mg maintenance dose every day for days 2 through 10, or until discharge or death. Patients in the control group of the study received a matching placebo on the same schedule and volume. The clinical status of each patient was assessed every day, from day 1 through day 29 of his or her hospital stay, according to an eight-category ordinal scale.
Time to recovery was defined as the first day during the 28-day enrollment period that a patient’s clinical status met a 1 (not hospitalization, no activity limitations), 2 (not hospitalized, activity limitation, oxygen requirement or both), or 3 (hospitalized, not requiring supplemental oxygen or medical care if hospitalization was extended for infection-control reasons) on the eight-category scale. A score of 4 indicated a patient was hospitalized and needed ongoing medical care, but did not require supplemental oxygen; a score of 8 signified death.
The analysis found remdesivir patients had a median time to recovery of 11 days, compared with the median 15 days for patients on the placebo (rate ratio for recovery, 1.32; 95% confidence interval, 1.12-1.55; P < .001). Mortality was also lower in the remdesivir group (hazard ratio for death, 0.70; 95% CI, 0.47-1.04), but the result was not statistically significant. By 14 days, the Kaplan-Meier estimate of mortality was 7.1 % in the remdesivir group and 11.9% in the placebo group.
Patients receiving oxygen, but not yet requiring high-flow oxygen, mechanical ventilation, or extracorporeal membrane oxygenation, seemed to fare best from treatment with remdesivir (these patients had a baseline ordinal score of 5). That may be a result of the larger sample size of these patients, the researchers note in the study. The study authors were unable to estimate the recovery time for the most severely ill patients (category 7), possibly because the follow-up time was too short to fully evaluate this subgroup.
“There is clear and consistent evidence of clinically significant benefit for those hospitalized on oxygen but not yet requiring mechanical ventilation,” Dr. Kaul, who was not involved in the study, said after seeing the published results. “Surprisingly, early dosing as measured from time to onset of symptoms did not seem to make a difference.”
Dr. Kaul said there is still the possibility that remdesivir could benefit patients on mechanical ventilation, but “clinicians will have to determine if the evidence suggesting no benefit in those who are intubated is strong enough to justify using this currently scarce resource in that population versus limiting use to those requiring oxygen but not on mechanical ventilation.”
Site investigators estimated that just four serious adverse events (two in each group) in enrolled patients were related to remdesivir or placebo. No deaths were attributed to the treatments, although acute respiratory failure, hypotension, acute kidney injury, and viral pneumonia were slightly more common in patients receiving the placebo than those receiving remdesivir.
The researchers plan to publish a follow-up study in the coming weeks or months, after the full cohort has completed 28 days of follow-up, Dr. Lane said. In future studies, the agency will likely focus on comparing remdesivir with combinations of remdesivir with other treatments, like the anti-inflammatory baricitinib.
A version of this article originally appeared on Medscape.com.
Much-anticipated results from the National Institute of Allergy and Infectious Diseases’ clinical trial of remdesivir in COVID-19 patients published in the New England Journal of Medicine suggest remdesivir shortens the disease course for hospitalized COVID-19 patients.
The agency reported initial promising results from the study earlier this month, which prompted the Food and Drug Administration to issue an emergency use authorization (EUA) for the drug, but the full data and results have not been widely available until now.
The findings also suggest remdesivir should be started, if possible, before patients have such severe pulmonary disease that they require mechanical ventilation, according to the study authors.
The published results are “completely consistent” with the NIAID’s earlier announcement, H. Clifford Lane, MD, deputy director for clinical research and special projects at the NIAID, said in an interview. “The benefit appeared to be the greatest for the patients who are hospitalized with severe disease who require supplemental oxygen.”
Given the limited supply of remdesivir, physicians have been eager to see the full data to ensure they use the drug most effectively, Daniel Kaul, MD, a professor of infectious diseases at the University of Michigan, Ann Arbor, said in an interview. Hospitals in states across the country, including New York, Michigan, and Washington, have received limited supplies of the drug in the last couple of weeks since the FDA’s authorization.
“I am losing my patience waiting for #remdesivir data. I was willing to give them a week to verify the numbers, triple proof the tables, cautiously frame conclusions. But it’s gone on too long. We are rationing with no rationale. We are floating on whisps [sic] of data, adrift,” Kate Stephenson, MD, an infectious diseases specialist at the Center for Virology and Vaccine Research at Harvard Medical School, Boston, wrote on Twitter May 18. After reading the paper, she tweeted Friday evening that she was “relieved to see convincing benefit – I was starting to worry!”
In the midst of a public health crisis, however, it is not unusual to make an announcement about trial results before the full dataset has been analyzed, said Dr. Lane. The NIAID followed a similar playbook for the PALM trial evaluating possible Ebola treatments in the Democratic Republic of Congo, with the independent monitoring board recommending the trial be terminated early in response to positive results from two of the four candidate drugs.
“When you have a result you think is of public health importance, you don’t wait for it to be published in a peer-reviewed journal,” said Dr. Lane, a coauthor of the study. The lag time from announcement to study publication was a result of the time it took to write up the paper for publication and go through peer review, Dr. Lane added. He also noted that the FDA had access to the data when the agency wrote its guidance for physicians administering the drug to patients under the EUA.
The authors opted not to publish the initial findings on a preprint server because they felt it was important to undergo peer review, said Dr. Lane. “The last thing you want for something this critical is for incomplete data to be out there, or you don’t have everything audited to the level that you want.”
Trial details
In the ACTT-1 randomized, placebo-controlled, double-blinded trial, researchers enrolled 1,063 patients from Feb. 21 to April 19, 2020, at 60 trial sites and 13 subsites worldwide (45 sites in the United States). The remdesivir group had 541 patients, and the placebo group had 522. A small number of patients (49 in the remdesivir group and 53 in the placebo group) discontinued treatment before day 10 because of an adverse event or withdrawn consent. When data collection for this preliminary analysis ended in late April, 301 patients had not recovered and had not completed their final follow-up visit.
Most of the patients had one (27%) or more (52.1%) preexisting conditions, including hypertension (49.6%), obesity (37%), and type 2 diabetes mellitus (29.7%). Mean patient age was 58.9 years, and the majority of patients were men (64.3%). The median number of days from symptom onset to randomization was 9, and 53.6% of the patients were white, 20.6% were black, 12.6% were Asian, 23.4% were Hispanic or Latino, and the ethnicity of 13.6% were not reported or reported as other.
Patients received one 200-mg loading dose on the first day of the trial, and then one 100-mg maintenance dose every day for days 2 through 10, or until discharge or death. Patients in the control group of the study received a matching placebo on the same schedule and volume. The clinical status of each patient was assessed every day, from day 1 through day 29 of his or her hospital stay, according to an eight-category ordinal scale.
Time to recovery was defined as the first day during the 28-day enrollment period that a patient’s clinical status met a 1 (not hospitalization, no activity limitations), 2 (not hospitalized, activity limitation, oxygen requirement or both), or 3 (hospitalized, not requiring supplemental oxygen or medical care if hospitalization was extended for infection-control reasons) on the eight-category scale. A score of 4 indicated a patient was hospitalized and needed ongoing medical care, but did not require supplemental oxygen; a score of 8 signified death.
The analysis found remdesivir patients had a median time to recovery of 11 days, compared with the median 15 days for patients on the placebo (rate ratio for recovery, 1.32; 95% confidence interval, 1.12-1.55; P < .001). Mortality was also lower in the remdesivir group (hazard ratio for death, 0.70; 95% CI, 0.47-1.04), but the result was not statistically significant. By 14 days, the Kaplan-Meier estimate of mortality was 7.1 % in the remdesivir group and 11.9% in the placebo group.
Patients receiving oxygen, but not yet requiring high-flow oxygen, mechanical ventilation, or extracorporeal membrane oxygenation, seemed to fare best from treatment with remdesivir (these patients had a baseline ordinal score of 5). That may be a result of the larger sample size of these patients, the researchers note in the study. The study authors were unable to estimate the recovery time for the most severely ill patients (category 7), possibly because the follow-up time was too short to fully evaluate this subgroup.
“There is clear and consistent evidence of clinically significant benefit for those hospitalized on oxygen but not yet requiring mechanical ventilation,” Dr. Kaul, who was not involved in the study, said after seeing the published results. “Surprisingly, early dosing as measured from time to onset of symptoms did not seem to make a difference.”
Dr. Kaul said there is still the possibility that remdesivir could benefit patients on mechanical ventilation, but “clinicians will have to determine if the evidence suggesting no benefit in those who are intubated is strong enough to justify using this currently scarce resource in that population versus limiting use to those requiring oxygen but not on mechanical ventilation.”
Site investigators estimated that just four serious adverse events (two in each group) in enrolled patients were related to remdesivir or placebo. No deaths were attributed to the treatments, although acute respiratory failure, hypotension, acute kidney injury, and viral pneumonia were slightly more common in patients receiving the placebo than those receiving remdesivir.
The researchers plan to publish a follow-up study in the coming weeks or months, after the full cohort has completed 28 days of follow-up, Dr. Lane said. In future studies, the agency will likely focus on comparing remdesivir with combinations of remdesivir with other treatments, like the anti-inflammatory baricitinib.
A version of this article originally appeared on Medscape.com.
Much-anticipated results from the National Institute of Allergy and Infectious Diseases’ clinical trial of remdesivir in COVID-19 patients published in the New England Journal of Medicine suggest remdesivir shortens the disease course for hospitalized COVID-19 patients.
The agency reported initial promising results from the study earlier this month, which prompted the Food and Drug Administration to issue an emergency use authorization (EUA) for the drug, but the full data and results have not been widely available until now.
The findings also suggest remdesivir should be started, if possible, before patients have such severe pulmonary disease that they require mechanical ventilation, according to the study authors.
The published results are “completely consistent” with the NIAID’s earlier announcement, H. Clifford Lane, MD, deputy director for clinical research and special projects at the NIAID, said in an interview. “The benefit appeared to be the greatest for the patients who are hospitalized with severe disease who require supplemental oxygen.”
Given the limited supply of remdesivir, physicians have been eager to see the full data to ensure they use the drug most effectively, Daniel Kaul, MD, a professor of infectious diseases at the University of Michigan, Ann Arbor, said in an interview. Hospitals in states across the country, including New York, Michigan, and Washington, have received limited supplies of the drug in the last couple of weeks since the FDA’s authorization.
“I am losing my patience waiting for #remdesivir data. I was willing to give them a week to verify the numbers, triple proof the tables, cautiously frame conclusions. But it’s gone on too long. We are rationing with no rationale. We are floating on whisps [sic] of data, adrift,” Kate Stephenson, MD, an infectious diseases specialist at the Center for Virology and Vaccine Research at Harvard Medical School, Boston, wrote on Twitter May 18. After reading the paper, she tweeted Friday evening that she was “relieved to see convincing benefit – I was starting to worry!”
In the midst of a public health crisis, however, it is not unusual to make an announcement about trial results before the full dataset has been analyzed, said Dr. Lane. The NIAID followed a similar playbook for the PALM trial evaluating possible Ebola treatments in the Democratic Republic of Congo, with the independent monitoring board recommending the trial be terminated early in response to positive results from two of the four candidate drugs.
“When you have a result you think is of public health importance, you don’t wait for it to be published in a peer-reviewed journal,” said Dr. Lane, a coauthor of the study. The lag time from announcement to study publication was a result of the time it took to write up the paper for publication and go through peer review, Dr. Lane added. He also noted that the FDA had access to the data when the agency wrote its guidance for physicians administering the drug to patients under the EUA.
The authors opted not to publish the initial findings on a preprint server because they felt it was important to undergo peer review, said Dr. Lane. “The last thing you want for something this critical is for incomplete data to be out there, or you don’t have everything audited to the level that you want.”
Trial details
In the ACTT-1 randomized, placebo-controlled, double-blinded trial, researchers enrolled 1,063 patients from Feb. 21 to April 19, 2020, at 60 trial sites and 13 subsites worldwide (45 sites in the United States). The remdesivir group had 541 patients, and the placebo group had 522. A small number of patients (49 in the remdesivir group and 53 in the placebo group) discontinued treatment before day 10 because of an adverse event or withdrawn consent. When data collection for this preliminary analysis ended in late April, 301 patients had not recovered and had not completed their final follow-up visit.
Most of the patients had one (27%) or more (52.1%) preexisting conditions, including hypertension (49.6%), obesity (37%), and type 2 diabetes mellitus (29.7%). Mean patient age was 58.9 years, and the majority of patients were men (64.3%). The median number of days from symptom onset to randomization was 9, and 53.6% of the patients were white, 20.6% were black, 12.6% were Asian, 23.4% were Hispanic or Latino, and the ethnicity of 13.6% were not reported or reported as other.
Patients received one 200-mg loading dose on the first day of the trial, and then one 100-mg maintenance dose every day for days 2 through 10, or until discharge or death. Patients in the control group of the study received a matching placebo on the same schedule and volume. The clinical status of each patient was assessed every day, from day 1 through day 29 of his or her hospital stay, according to an eight-category ordinal scale.
Time to recovery was defined as the first day during the 28-day enrollment period that a patient’s clinical status met a 1 (not hospitalization, no activity limitations), 2 (not hospitalized, activity limitation, oxygen requirement or both), or 3 (hospitalized, not requiring supplemental oxygen or medical care if hospitalization was extended for infection-control reasons) on the eight-category scale. A score of 4 indicated a patient was hospitalized and needed ongoing medical care, but did not require supplemental oxygen; a score of 8 signified death.
The analysis found remdesivir patients had a median time to recovery of 11 days, compared with the median 15 days for patients on the placebo (rate ratio for recovery, 1.32; 95% confidence interval, 1.12-1.55; P < .001). Mortality was also lower in the remdesivir group (hazard ratio for death, 0.70; 95% CI, 0.47-1.04), but the result was not statistically significant. By 14 days, the Kaplan-Meier estimate of mortality was 7.1 % in the remdesivir group and 11.9% in the placebo group.
Patients receiving oxygen, but not yet requiring high-flow oxygen, mechanical ventilation, or extracorporeal membrane oxygenation, seemed to fare best from treatment with remdesivir (these patients had a baseline ordinal score of 5). That may be a result of the larger sample size of these patients, the researchers note in the study. The study authors were unable to estimate the recovery time for the most severely ill patients (category 7), possibly because the follow-up time was too short to fully evaluate this subgroup.
“There is clear and consistent evidence of clinically significant benefit for those hospitalized on oxygen but not yet requiring mechanical ventilation,” Dr. Kaul, who was not involved in the study, said after seeing the published results. “Surprisingly, early dosing as measured from time to onset of symptoms did not seem to make a difference.”
Dr. Kaul said there is still the possibility that remdesivir could benefit patients on mechanical ventilation, but “clinicians will have to determine if the evidence suggesting no benefit in those who are intubated is strong enough to justify using this currently scarce resource in that population versus limiting use to those requiring oxygen but not on mechanical ventilation.”
Site investigators estimated that just four serious adverse events (two in each group) in enrolled patients were related to remdesivir or placebo. No deaths were attributed to the treatments, although acute respiratory failure, hypotension, acute kidney injury, and viral pneumonia were slightly more common in patients receiving the placebo than those receiving remdesivir.
The researchers plan to publish a follow-up study in the coming weeks or months, after the full cohort has completed 28 days of follow-up, Dr. Lane said. In future studies, the agency will likely focus on comparing remdesivir with combinations of remdesivir with other treatments, like the anti-inflammatory baricitinib.
A version of this article originally appeared on Medscape.com.
The injustice of pre-authorization
I agree with Dr. Nasrallah’s clear description of the malign nature of the pre-authorization system, as described in his editorial “Pre-authorization is illegal, unethical, and adversely disrupts patient care” (From the Editor,
As an example of the latter, I was recently told by a pharmacist that I needed to call the insurer to justify why a patient was going from a prescription for #30 citalopram to #45 citalopram. The request had triggered a quantity limit. The pharmacist had explained to the insurer that more pills were required because the dosage was being lowered from 40 to 30 mg/d. Because there are no 30-mg tablets available, it made most sense for the patient to take one and a half 20-mg tablets, which totals 45 pills per month.
The insurer—probably a screener, not a pharmacist—would not accept that explanation and insisted that I call them myself. I bitterly resented how casually the insurer expected busy doctors to interrupt their clinical work to comply with arbitrary micromanagement of pill quantities! I’ve seldom seen such nonsense in more than 40 years of practice.
When doctors call these insurers, they are connected to a screener, but never a pharmacist. The screener asks a series of questions prompted by a computer. We give them verbal answers, but they don’t comprehend what they input into their system. The reasons we give to the screener may not even make it into the report that the screener passes on to the staff member who makes the decision. The doctor is not told what is in the report, or who is reviewing it. So much for transparency in this era that supposedly values it!
In any case, answering all the computer-prompted questions can take a long time. And time, as we know (but they do not), is not elastic.
Serious consequences may ensue if an insurer denies coverage for the doctor’s first choice. Many patients cannot afford to pay hundreds of extra dollars out of pocket. The insurer may ask the doctor to choose a different medication. Aside from the disrespect for the doctor’s decision implied by such a request, another problem is that the patient knows the new medication is his/her doctor’s second (or third) choice. Any positive placebo effect that may have existed before has now been lost. Most doctors would be glad to have a positive placebo effect augmenting the physiologic effects of the medication, especially when the patient is already feeling helpless or hopeless. These negative feelings would likely increase when the patient feels pressured into starting a medication that they know was their doctor’s second choice.
These are just a few reasons pre-authorization is a horrid system; Dr. Nasrallah covered many others in his editorial. The system, as currently structured, needs to be eliminated.
Arthur Mode, MD
Private psychiatric practice
Falls Church, Virginia
Disclosure: The author reports no financial relationships with any companies whose products are mentioned in this article, or with manufacturers of competing products.
Continue to: Hooray for...
Hooray for Dr. Nasrallah’s editorial about pre-authorization! I worry, however, that he missed some important considerations.
He writes, “The welfare of the patient is not on the insurance company’s radar screen, perhaps because it is crowded out by dollar signs.” But the welfare of the patient is exactly what is on their radar screens! If the patient dies, the insurance company profits, because it will not have to pay for treatment. This is like having a Red Sox employee manage the Yankees, except we are talking about human lives, not baseball games. Dr. Nasrallah asks (but does not answer), “How did for-profit insurance companies empower themselves to tyrannize clinical practice so that the treatment administered isn’t customized to the patient’s need but instead to fatten the profits of the insurance company?” The answer: Physicians let them. Many physicians are paid by insurers directly or through work for clinics or hospitals. He who pays the piper calls the tune. And because employers often select the insurer, patients have no say.
Honesty is most important. Pre-authorization is a dishonest term, because pre-authorization actually is pre-denial. The term pre-authorization should be replaced by “pre-denial.” It is also fraudulent when insurance companies call themselves health care companies, because they only provide insurance, not health care. Similarly, the term “evidence-based medicine” is typically only an excuse that insurers use to refuse to cover the cost of treatment. In another scenario of Dr. Nasrallah’s patient with treatment-resistant depression who responded to modafinil, what if the evidence for using this medication was based on the patient’s psychiatric history alone, without any evidence from a meta-analysis of randomized controlled trials? That would not be “evidence-based” in the dishonest world of insurance. Evidence to insurers does not include what is evident in the patient’s response to a given treatment.
What about amnesty, especially for physicians who work in the so-called pre-authorization denial business? Some even claim to be peers (ie, the “peer to peer reviews” they conduct) and insist they cannot be on speakerphone, so that their identity is kept secret from the patient. Not all of these “physicians” are incompetent. Not all of them have criminal minds or lack empathy. Some may have had exceptional circumstances leading them to such a profession, which Dr. Nasrallah correctly notes as felonious behavior. For these physicians, I think some kind of amnesty program would be appropriate, rather than prosecution.
John Jacobs, MD
Private psychiatric practice
Manchester, New Hampshire
Disclosure: The author reports no financial relationships with any companies whose products are mentioned in this article, or with manufacturers of competing products.
Continue to: I have just finished reading...
I have just finished reading Dr. Nasrallah’s editorial about pre-authorization. I agree with everything he said, but I do have a couple of comments:
1. Many of our colleagues do not accept insurance because their practices operate on a cash basis. This seems to obviate the problem of pre-authorization, and suggests that if we truly want to get rid of pre-authorization, we should get rid of insurance.
2. In practices that do not accept insurance, some patients may be filing their own insurance claims. Do you have any information on this approach? Are patients able to apply pressure to their insurance companies? Do patients get frustrated with their insurance companies and pay cash, rather than trying to negotiate with their insurance companies?
Katherine Hankins, MD
Private psychiatric practice
Omaha, Nebraska
Disclosure: The author reports no financial relationships with any companies whose products are mentioned in this article, or with manufacturers of competing products.
Continue to: Why not address...
Why not address the underlying (and actual) cause of the “pre-authorization” scam/scandal: the private health insurance industry.
Other countries in the western world have figured out how to provide guaranteed health care to their citizens without resorting to a costly insurance industry. This parasitic business suborns 10% to 20% of the health care bill while wasting our money on withholding health care deemed “not eligible” for patients who need it. Meanwhile, the executives who manage this insurance racket are paid enormous salaries not to deliver services.
Moreover, we reap a double loss to the health care system because hospitals must employ a building full of clerks to submit (and then, when rejected, re-submit) bills for reimbursement of hospital charges.
Franz Kafka would immediately grasp the despicable workings of this self-serving scheme.
David Link, MD
Associate Professor of Pediatrics
Harvard Medical School
Boston, Massachusetts
Disclosure: The author reports no financial relationships with any companies whose products are mentioned in this article, or with manufacturers of competing products.
Continue to: Dr. Nasrallah responds
Dr. Nasrallah responds
Thanks to all my colleagues who commented on (and unanimously agreed with) my editorial. It is clearly one of the most outrageous hurdles that all psychiatric practitioners face every day.
For the sake of our patients who deserve optimal medical care (laboratory tests, procedures, and medications), insurance companies must be tightly regulated to avoid second-guessing the treating clinicians, and readily cover what is prescribed. Some patients who can afford it resort to paying out of pocket for privacy reasons or for rapid access to psychiatric care, and may or may not file for insurance coverage, but they will certainly receive what their psychiatrist deems appropriate after a direct evaluation.
I hope the American Psychiatric Association and American Medical Association will continue to forcefully pursue legislation to eliminate pre-authorization and restore some sanity to the critical process of good clinical care.
Henry A. Nasrallah, MD
Professor of Psychiatry, Neurology, and Neuroscience
Medical Director: Neuropsychiatry
Director, Schizophrenia and Neuropsychiatry Programs
University of Cincinnati College of Medicine
Cincinnati, Ohio
Professor Emeritus, Saint Louis University
St. Louis, Missouri
I agree with Dr. Nasrallah’s clear description of the malign nature of the pre-authorization system, as described in his editorial “Pre-authorization is illegal, unethical, and adversely disrupts patient care” (From the Editor,
As an example of the latter, I was recently told by a pharmacist that I needed to call the insurer to justify why a patient was going from a prescription for #30 citalopram to #45 citalopram. The request had triggered a quantity limit. The pharmacist had explained to the insurer that more pills were required because the dosage was being lowered from 40 to 30 mg/d. Because there are no 30-mg tablets available, it made most sense for the patient to take one and a half 20-mg tablets, which totals 45 pills per month.
The insurer—probably a screener, not a pharmacist—would not accept that explanation and insisted that I call them myself. I bitterly resented how casually the insurer expected busy doctors to interrupt their clinical work to comply with arbitrary micromanagement of pill quantities! I’ve seldom seen such nonsense in more than 40 years of practice.
When doctors call these insurers, they are connected to a screener, but never a pharmacist. The screener asks a series of questions prompted by a computer. We give them verbal answers, but they don’t comprehend what they input into their system. The reasons we give to the screener may not even make it into the report that the screener passes on to the staff member who makes the decision. The doctor is not told what is in the report, or who is reviewing it. So much for transparency in this era that supposedly values it!
In any case, answering all the computer-prompted questions can take a long time. And time, as we know (but they do not), is not elastic.
Serious consequences may ensue if an insurer denies coverage for the doctor’s first choice. Many patients cannot afford to pay hundreds of extra dollars out of pocket. The insurer may ask the doctor to choose a different medication. Aside from the disrespect for the doctor’s decision implied by such a request, another problem is that the patient knows the new medication is his/her doctor’s second (or third) choice. Any positive placebo effect that may have existed before has now been lost. Most doctors would be glad to have a positive placebo effect augmenting the physiologic effects of the medication, especially when the patient is already feeling helpless or hopeless. These negative feelings would likely increase when the patient feels pressured into starting a medication that they know was their doctor’s second choice.
These are just a few reasons pre-authorization is a horrid system; Dr. Nasrallah covered many others in his editorial. The system, as currently structured, needs to be eliminated.
Arthur Mode, MD
Private psychiatric practice
Falls Church, Virginia
Disclosure: The author reports no financial relationships with any companies whose products are mentioned in this article, or with manufacturers of competing products.
Continue to: Hooray for...
Hooray for Dr. Nasrallah’s editorial about pre-authorization! I worry, however, that he missed some important considerations.
He writes, “The welfare of the patient is not on the insurance company’s radar screen, perhaps because it is crowded out by dollar signs.” But the welfare of the patient is exactly what is on their radar screens! If the patient dies, the insurance company profits, because it will not have to pay for treatment. This is like having a Red Sox employee manage the Yankees, except we are talking about human lives, not baseball games. Dr. Nasrallah asks (but does not answer), “How did for-profit insurance companies empower themselves to tyrannize clinical practice so that the treatment administered isn’t customized to the patient’s need but instead to fatten the profits of the insurance company?” The answer: Physicians let them. Many physicians are paid by insurers directly or through work for clinics or hospitals. He who pays the piper calls the tune. And because employers often select the insurer, patients have no say.
Honesty is most important. Pre-authorization is a dishonest term, because pre-authorization actually is pre-denial. The term pre-authorization should be replaced by “pre-denial.” It is also fraudulent when insurance companies call themselves health care companies, because they only provide insurance, not health care. Similarly, the term “evidence-based medicine” is typically only an excuse that insurers use to refuse to cover the cost of treatment. In another scenario of Dr. Nasrallah’s patient with treatment-resistant depression who responded to modafinil, what if the evidence for using this medication was based on the patient’s psychiatric history alone, without any evidence from a meta-analysis of randomized controlled trials? That would not be “evidence-based” in the dishonest world of insurance. Evidence to insurers does not include what is evident in the patient’s response to a given treatment.
What about amnesty, especially for physicians who work in the so-called pre-authorization denial business? Some even claim to be peers (ie, the “peer to peer reviews” they conduct) and insist they cannot be on speakerphone, so that their identity is kept secret from the patient. Not all of these “physicians” are incompetent. Not all of them have criminal minds or lack empathy. Some may have had exceptional circumstances leading them to such a profession, which Dr. Nasrallah correctly notes as felonious behavior. For these physicians, I think some kind of amnesty program would be appropriate, rather than prosecution.
John Jacobs, MD
Private psychiatric practice
Manchester, New Hampshire
Disclosure: The author reports no financial relationships with any companies whose products are mentioned in this article, or with manufacturers of competing products.
Continue to: I have just finished reading...
I have just finished reading Dr. Nasrallah’s editorial about pre-authorization. I agree with everything he said, but I do have a couple of comments:
1. Many of our colleagues do not accept insurance because their practices operate on a cash basis. This seems to obviate the problem of pre-authorization, and suggests that if we truly want to get rid of pre-authorization, we should get rid of insurance.
2. In practices that do not accept insurance, some patients may be filing their own insurance claims. Do you have any information on this approach? Are patients able to apply pressure to their insurance companies? Do patients get frustrated with their insurance companies and pay cash, rather than trying to negotiate with their insurance companies?
Katherine Hankins, MD
Private psychiatric practice
Omaha, Nebraska
Disclosure: The author reports no financial relationships with any companies whose products are mentioned in this article, or with manufacturers of competing products.
Continue to: Why not address...
Why not address the underlying (and actual) cause of the “pre-authorization” scam/scandal: the private health insurance industry.
Other countries in the western world have figured out how to provide guaranteed health care to their citizens without resorting to a costly insurance industry. This parasitic business suborns 10% to 20% of the health care bill while wasting our money on withholding health care deemed “not eligible” for patients who need it. Meanwhile, the executives who manage this insurance racket are paid enormous salaries not to deliver services.
Moreover, we reap a double loss to the health care system because hospitals must employ a building full of clerks to submit (and then, when rejected, re-submit) bills for reimbursement of hospital charges.
Franz Kafka would immediately grasp the despicable workings of this self-serving scheme.
David Link, MD
Associate Professor of Pediatrics
Harvard Medical School
Boston, Massachusetts
Disclosure: The author reports no financial relationships with any companies whose products are mentioned in this article, or with manufacturers of competing products.
Continue to: Dr. Nasrallah responds
Dr. Nasrallah responds
Thanks to all my colleagues who commented on (and unanimously agreed with) my editorial. It is clearly one of the most outrageous hurdles that all psychiatric practitioners face every day.
For the sake of our patients who deserve optimal medical care (laboratory tests, procedures, and medications), insurance companies must be tightly regulated to avoid second-guessing the treating clinicians, and readily cover what is prescribed. Some patients who can afford it resort to paying out of pocket for privacy reasons or for rapid access to psychiatric care, and may or may not file for insurance coverage, but they will certainly receive what their psychiatrist deems appropriate after a direct evaluation.
I hope the American Psychiatric Association and American Medical Association will continue to forcefully pursue legislation to eliminate pre-authorization and restore some sanity to the critical process of good clinical care.
Henry A. Nasrallah, MD
Professor of Psychiatry, Neurology, and Neuroscience
Medical Director: Neuropsychiatry
Director, Schizophrenia and Neuropsychiatry Programs
University of Cincinnati College of Medicine
Cincinnati, Ohio
Professor Emeritus, Saint Louis University
St. Louis, Missouri
I agree with Dr. Nasrallah’s clear description of the malign nature of the pre-authorization system, as described in his editorial “Pre-authorization is illegal, unethical, and adversely disrupts patient care” (From the Editor,
As an example of the latter, I was recently told by a pharmacist that I needed to call the insurer to justify why a patient was going from a prescription for #30 citalopram to #45 citalopram. The request had triggered a quantity limit. The pharmacist had explained to the insurer that more pills were required because the dosage was being lowered from 40 to 30 mg/d. Because there are no 30-mg tablets available, it made most sense for the patient to take one and a half 20-mg tablets, which totals 45 pills per month.
The insurer—probably a screener, not a pharmacist—would not accept that explanation and insisted that I call them myself. I bitterly resented how casually the insurer expected busy doctors to interrupt their clinical work to comply with arbitrary micromanagement of pill quantities! I’ve seldom seen such nonsense in more than 40 years of practice.
When doctors call these insurers, they are connected to a screener, but never a pharmacist. The screener asks a series of questions prompted by a computer. We give them verbal answers, but they don’t comprehend what they input into their system. The reasons we give to the screener may not even make it into the report that the screener passes on to the staff member who makes the decision. The doctor is not told what is in the report, or who is reviewing it. So much for transparency in this era that supposedly values it!
In any case, answering all the computer-prompted questions can take a long time. And time, as we know (but they do not), is not elastic.
Serious consequences may ensue if an insurer denies coverage for the doctor’s first choice. Many patients cannot afford to pay hundreds of extra dollars out of pocket. The insurer may ask the doctor to choose a different medication. Aside from the disrespect for the doctor’s decision implied by such a request, another problem is that the patient knows the new medication is his/her doctor’s second (or third) choice. Any positive placebo effect that may have existed before has now been lost. Most doctors would be glad to have a positive placebo effect augmenting the physiologic effects of the medication, especially when the patient is already feeling helpless or hopeless. These negative feelings would likely increase when the patient feels pressured into starting a medication that they know was their doctor’s second choice.
These are just a few reasons pre-authorization is a horrid system; Dr. Nasrallah covered many others in his editorial. The system, as currently structured, needs to be eliminated.
Arthur Mode, MD
Private psychiatric practice
Falls Church, Virginia
Disclosure: The author reports no financial relationships with any companies whose products are mentioned in this article, or with manufacturers of competing products.
Continue to: Hooray for...
Hooray for Dr. Nasrallah’s editorial about pre-authorization! I worry, however, that he missed some important considerations.
He writes, “The welfare of the patient is not on the insurance company’s radar screen, perhaps because it is crowded out by dollar signs.” But the welfare of the patient is exactly what is on their radar screens! If the patient dies, the insurance company profits, because it will not have to pay for treatment. This is like having a Red Sox employee manage the Yankees, except we are talking about human lives, not baseball games. Dr. Nasrallah asks (but does not answer), “How did for-profit insurance companies empower themselves to tyrannize clinical practice so that the treatment administered isn’t customized to the patient’s need but instead to fatten the profits of the insurance company?” The answer: Physicians let them. Many physicians are paid by insurers directly or through work for clinics or hospitals. He who pays the piper calls the tune. And because employers often select the insurer, patients have no say.
Honesty is most important. Pre-authorization is a dishonest term, because pre-authorization actually is pre-denial. The term pre-authorization should be replaced by “pre-denial.” It is also fraudulent when insurance companies call themselves health care companies, because they only provide insurance, not health care. Similarly, the term “evidence-based medicine” is typically only an excuse that insurers use to refuse to cover the cost of treatment. In another scenario of Dr. Nasrallah’s patient with treatment-resistant depression who responded to modafinil, what if the evidence for using this medication was based on the patient’s psychiatric history alone, without any evidence from a meta-analysis of randomized controlled trials? That would not be “evidence-based” in the dishonest world of insurance. Evidence to insurers does not include what is evident in the patient’s response to a given treatment.
What about amnesty, especially for physicians who work in the so-called pre-authorization denial business? Some even claim to be peers (ie, the “peer to peer reviews” they conduct) and insist they cannot be on speakerphone, so that their identity is kept secret from the patient. Not all of these “physicians” are incompetent. Not all of them have criminal minds or lack empathy. Some may have had exceptional circumstances leading them to such a profession, which Dr. Nasrallah correctly notes as felonious behavior. For these physicians, I think some kind of amnesty program would be appropriate, rather than prosecution.
John Jacobs, MD
Private psychiatric practice
Manchester, New Hampshire
Disclosure: The author reports no financial relationships with any companies whose products are mentioned in this article, or with manufacturers of competing products.
Continue to: I have just finished reading...
I have just finished reading Dr. Nasrallah’s editorial about pre-authorization. I agree with everything he said, but I do have a couple of comments:
1. Many of our colleagues do not accept insurance because their practices operate on a cash basis. This seems to obviate the problem of pre-authorization, and suggests that if we truly want to get rid of pre-authorization, we should get rid of insurance.
2. In practices that do not accept insurance, some patients may be filing their own insurance claims. Do you have any information on this approach? Are patients able to apply pressure to their insurance companies? Do patients get frustrated with their insurance companies and pay cash, rather than trying to negotiate with their insurance companies?
Katherine Hankins, MD
Private psychiatric practice
Omaha, Nebraska
Disclosure: The author reports no financial relationships with any companies whose products are mentioned in this article, or with manufacturers of competing products.
Continue to: Why not address...
Why not address the underlying (and actual) cause of the “pre-authorization” scam/scandal: the private health insurance industry.
Other countries in the western world have figured out how to provide guaranteed health care to their citizens without resorting to a costly insurance industry. This parasitic business suborns 10% to 20% of the health care bill while wasting our money on withholding health care deemed “not eligible” for patients who need it. Meanwhile, the executives who manage this insurance racket are paid enormous salaries not to deliver services.
Moreover, we reap a double loss to the health care system because hospitals must employ a building full of clerks to submit (and then, when rejected, re-submit) bills for reimbursement of hospital charges.
Franz Kafka would immediately grasp the despicable workings of this self-serving scheme.
David Link, MD
Associate Professor of Pediatrics
Harvard Medical School
Boston, Massachusetts
Disclosure: The author reports no financial relationships with any companies whose products are mentioned in this article, or with manufacturers of competing products.
Continue to: Dr. Nasrallah responds
Dr. Nasrallah responds
Thanks to all my colleagues who commented on (and unanimously agreed with) my editorial. It is clearly one of the most outrageous hurdles that all psychiatric practitioners face every day.
For the sake of our patients who deserve optimal medical care (laboratory tests, procedures, and medications), insurance companies must be tightly regulated to avoid second-guessing the treating clinicians, and readily cover what is prescribed. Some patients who can afford it resort to paying out of pocket for privacy reasons or for rapid access to psychiatric care, and may or may not file for insurance coverage, but they will certainly receive what their psychiatrist deems appropriate after a direct evaluation.
I hope the American Psychiatric Association and American Medical Association will continue to forcefully pursue legislation to eliminate pre-authorization and restore some sanity to the critical process of good clinical care.
Henry A. Nasrallah, MD
Professor of Psychiatry, Neurology, and Neuroscience
Medical Director: Neuropsychiatry
Director, Schizophrenia and Neuropsychiatry Programs
University of Cincinnati College of Medicine
Cincinnati, Ohio
Professor Emeritus, Saint Louis University
St. Louis, Missouri
Armchair epidemiology
Real epidemiologists are out knocking on doors, chasing down contacts, or hunched over their computers trying to make sense out of screens full of data and maps. A few are trying valiantly to talk some sense into our elected officials.
This leaves the rest of us with time on our hands to fabricate our own less-than-scientific explanations for the behavior of the SARS-CoV-2 virus. So I have decided to put on hold my current mental challenge of choosing which pasta shape to pair with the sauce I’ve prepared from an online recipe. Here is my educated guess based on what I can glean from media sources that may have been filtered through a variety politically biased lenses. Remember, I did go to medical school; however, when I was in college the DNA helix was still just theoretical.
From those halcyon days of mid-February when our attention was focused on the Diamond Princess quarantined in Yokohama Harbor, it didn’t take a board-certified epidemiologist to suspect that the virus was spreading through the ventilating system in the ship’s tight quarters. Subsequent outbreaks on U.S. and French military ships suggests a similar explanation.
While still not proven, it sounds like SARS-CoV-2 jumped to humans from bats. It should not surprise us that having evolved in a dense population of mammals it would thrive in other high-density populations such as New York and nursing homes. Because we have lacked a robust testing capability, it has been less obvious until recently that, while it is easily transmitted, the virus has infected many who are asymptomatic (“Antibody surveys suggesting vast undercount of coronavirus infections may be unreliable,” Gretchen Vogel, Science, April 21, 2020). Subsequent surveys seem to confirm this higher level carrier state; it suggests that the virus is far less deadly than was previously suggested. However, it seems to be a crafty little bug attacking just about any organ system it lands on.
I don’t think any of us are surprised that the elderly population with weakened immune systems, particularly those in congregate housing, has been much more vulnerable. However, many of the deaths among younger apparently healthy people have defied explanation. The anecdotal observations that physicians, particularly those who practice in-your-face medicine (e.g., ophthalmologists and otolaryngologists) may be more vulnerable raises the issue of viral load. It may be that, although it can be extremely contagious, the virus is not terribly dangerous for most people until the inoculum dose of the virus reaches a certain level. To my knowledge this dose is unknown.
A published survey of more than 300 outbreaks from 120 Chinese cities also may support my suspicion that viral load is of critical importance. The researchers found that all the “identified outbreaks of three or more cases occurred in an indoor environment, which confirms that sharing indoor space is a major SARS-CoV-2 infection risk” (Huan Qian et al. “Indoor transmission of SARS-CoV-2,” MedRxiv. 2020 Apr 7. doi: 10.1101/2020.04.04.20053058). Again, this data shouldn’t surprise us when we look back at what little we know about the outbreaks in the confined spaces on cruise ships and in nursing homes.
I’m not sure that we have any data that helps us determine whether wearing a mask in an outdoor space has any more than symbolic value when we are talking about this particular virus. We may read that the virus in a droplet can survive on the surface it lands on for 8 minutes, and we can see those slow motion videos of the impressive plume of snot spray released by a sneeze. It would seem obvious that even outside someone within 10 feet of the sneeze has a good chance of being infected. However, how much of a threat is the asymptomatic carrier who passes within three feet of you while you are out on lovely summer day stroll? This armchair epidemiologist suspects that, when we are talking about an outside space, the 6-foot guideline for small groups of a dozen or less is overly restrictive. But until we know, I’m staying put in my armchair ... outside on the porch overlooking Casco Bay.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” He has no disclosures. Email him at pdnews@mdedge.com.
Real epidemiologists are out knocking on doors, chasing down contacts, or hunched over their computers trying to make sense out of screens full of data and maps. A few are trying valiantly to talk some sense into our elected officials.
This leaves the rest of us with time on our hands to fabricate our own less-than-scientific explanations for the behavior of the SARS-CoV-2 virus. So I have decided to put on hold my current mental challenge of choosing which pasta shape to pair with the sauce I’ve prepared from an online recipe. Here is my educated guess based on what I can glean from media sources that may have been filtered through a variety politically biased lenses. Remember, I did go to medical school; however, when I was in college the DNA helix was still just theoretical.
From those halcyon days of mid-February when our attention was focused on the Diamond Princess quarantined in Yokohama Harbor, it didn’t take a board-certified epidemiologist to suspect that the virus was spreading through the ventilating system in the ship’s tight quarters. Subsequent outbreaks on U.S. and French military ships suggests a similar explanation.
While still not proven, it sounds like SARS-CoV-2 jumped to humans from bats. It should not surprise us that having evolved in a dense population of mammals it would thrive in other high-density populations such as New York and nursing homes. Because we have lacked a robust testing capability, it has been less obvious until recently that, while it is easily transmitted, the virus has infected many who are asymptomatic (“Antibody surveys suggesting vast undercount of coronavirus infections may be unreliable,” Gretchen Vogel, Science, April 21, 2020). Subsequent surveys seem to confirm this higher level carrier state; it suggests that the virus is far less deadly than was previously suggested. However, it seems to be a crafty little bug attacking just about any organ system it lands on.
I don’t think any of us are surprised that the elderly population with weakened immune systems, particularly those in congregate housing, has been much more vulnerable. However, many of the deaths among younger apparently healthy people have defied explanation. The anecdotal observations that physicians, particularly those who practice in-your-face medicine (e.g., ophthalmologists and otolaryngologists) may be more vulnerable raises the issue of viral load. It may be that, although it can be extremely contagious, the virus is not terribly dangerous for most people until the inoculum dose of the virus reaches a certain level. To my knowledge this dose is unknown.
A published survey of more than 300 outbreaks from 120 Chinese cities also may support my suspicion that viral load is of critical importance. The researchers found that all the “identified outbreaks of three or more cases occurred in an indoor environment, which confirms that sharing indoor space is a major SARS-CoV-2 infection risk” (Huan Qian et al. “Indoor transmission of SARS-CoV-2,” MedRxiv. 2020 Apr 7. doi: 10.1101/2020.04.04.20053058). Again, this data shouldn’t surprise us when we look back at what little we know about the outbreaks in the confined spaces on cruise ships and in nursing homes.
I’m not sure that we have any data that helps us determine whether wearing a mask in an outdoor space has any more than symbolic value when we are talking about this particular virus. We may read that the virus in a droplet can survive on the surface it lands on for 8 minutes, and we can see those slow motion videos of the impressive plume of snot spray released by a sneeze. It would seem obvious that even outside someone within 10 feet of the sneeze has a good chance of being infected. However, how much of a threat is the asymptomatic carrier who passes within three feet of you while you are out on lovely summer day stroll? This armchair epidemiologist suspects that, when we are talking about an outside space, the 6-foot guideline for small groups of a dozen or less is overly restrictive. But until we know, I’m staying put in my armchair ... outside on the porch overlooking Casco Bay.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” He has no disclosures. Email him at pdnews@mdedge.com.
Real epidemiologists are out knocking on doors, chasing down contacts, or hunched over their computers trying to make sense out of screens full of data and maps. A few are trying valiantly to talk some sense into our elected officials.
This leaves the rest of us with time on our hands to fabricate our own less-than-scientific explanations for the behavior of the SARS-CoV-2 virus. So I have decided to put on hold my current mental challenge of choosing which pasta shape to pair with the sauce I’ve prepared from an online recipe. Here is my educated guess based on what I can glean from media sources that may have been filtered through a variety politically biased lenses. Remember, I did go to medical school; however, when I was in college the DNA helix was still just theoretical.
From those halcyon days of mid-February when our attention was focused on the Diamond Princess quarantined in Yokohama Harbor, it didn’t take a board-certified epidemiologist to suspect that the virus was spreading through the ventilating system in the ship’s tight quarters. Subsequent outbreaks on U.S. and French military ships suggests a similar explanation.
While still not proven, it sounds like SARS-CoV-2 jumped to humans from bats. It should not surprise us that having evolved in a dense population of mammals it would thrive in other high-density populations such as New York and nursing homes. Because we have lacked a robust testing capability, it has been less obvious until recently that, while it is easily transmitted, the virus has infected many who are asymptomatic (“Antibody surveys suggesting vast undercount of coronavirus infections may be unreliable,” Gretchen Vogel, Science, April 21, 2020). Subsequent surveys seem to confirm this higher level carrier state; it suggests that the virus is far less deadly than was previously suggested. However, it seems to be a crafty little bug attacking just about any organ system it lands on.
I don’t think any of us are surprised that the elderly population with weakened immune systems, particularly those in congregate housing, has been much more vulnerable. However, many of the deaths among younger apparently healthy people have defied explanation. The anecdotal observations that physicians, particularly those who practice in-your-face medicine (e.g., ophthalmologists and otolaryngologists) may be more vulnerable raises the issue of viral load. It may be that, although it can be extremely contagious, the virus is not terribly dangerous for most people until the inoculum dose of the virus reaches a certain level. To my knowledge this dose is unknown.
A published survey of more than 300 outbreaks from 120 Chinese cities also may support my suspicion that viral load is of critical importance. The researchers found that all the “identified outbreaks of three or more cases occurred in an indoor environment, which confirms that sharing indoor space is a major SARS-CoV-2 infection risk” (Huan Qian et al. “Indoor transmission of SARS-CoV-2,” MedRxiv. 2020 Apr 7. doi: 10.1101/2020.04.04.20053058). Again, this data shouldn’t surprise us when we look back at what little we know about the outbreaks in the confined spaces on cruise ships and in nursing homes.
I’m not sure that we have any data that helps us determine whether wearing a mask in an outdoor space has any more than symbolic value when we are talking about this particular virus. We may read that the virus in a droplet can survive on the surface it lands on for 8 minutes, and we can see those slow motion videos of the impressive plume of snot spray released by a sneeze. It would seem obvious that even outside someone within 10 feet of the sneeze has a good chance of being infected. However, how much of a threat is the asymptomatic carrier who passes within three feet of you while you are out on lovely summer day stroll? This armchair epidemiologist suspects that, when we are talking about an outside space, the 6-foot guideline for small groups of a dozen or less is overly restrictive. But until we know, I’m staying put in my armchair ... outside on the porch overlooking Casco Bay.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” He has no disclosures. Email him at pdnews@mdedge.com.
Time series analysis of poison control data
The US Poison Control Centers’ National Poison Data System (NPDS) publishes annual reports describing exposures to various substances among the general population.1 Table 22B of each NPDS report shows the number of outcomes from exposures to different pharmacologic treatments in the United States, including psychotropic medications.2 In this Table, the relative morbidity (RM) of a medication is calculated as the ratio of serious outcomes (SO) to single exposures (SE), where SO = moderate + major + death. In this article, I use the NPDS data to demonstrate how time series analysis of the RM ratios for hypertension and psychiatric medications can help predict SO associated with these agents, which may help guide clinicians’ prescribing decisions.2,3
Time series analysis of hypertension medications
Due to the high prevalence of hypertension, it is not surprising that more suicide deaths occur each year from calcium channel blockers (CCB) than from lithium (37 vs 2, according to 2017 NPDS data).3 I used time series analysis to compare SO during 2006-2017 for 5 classes of hypertension medications: CCB, beta blockers (BB), angiotensin-converting enzyme inhibitors (ACEI), angiotensin receptor blockers (ARB), and diuretics (Figure 1).
Time series analysis of 2006-2017 data predicted the following number of deaths for 2018: CCB ≥33, BB ≥17, ACEI ≤2, ARB 0, and diuretics ≤1. The observed deaths in 2018 were 41, 23, 0, 0, and 1, respectively.2 The 2018 predicted RM were CCB 10.66%, BB 11.10%, ACEI 3.51%, ARB 2.04%, and diuretics 3.38%. The 2018 observed RM for these medications were 11.01%, 11.37%, 3.02%, 2.40%, and 2.88%, respectively.2
Because the NPDS data for hypertension medications was only provided by class, in order to detect differences within each class, I used the relative lethality (RL) equation: RL = 310x / LD50, where x is the maximum daily dose of a medication prescribed for 30 days, and LD50 is the rat oral lethal dose 50. The RL equation represents the ratio of a 30-day supply of medication to the human equivalent LD50 for a 60-kg person.4 The RL equation is useful for comparing the safety of various medications, and can help clinicians avoid prescribing a lethal amount of a given medication (Figure 2). For example, the equation shows that among CCB, felodipine is 466 times safer than verapamil and 101 times safer than diltiazem. Not surprisingly, 2006-2018 data shows many deaths via intentional verapamil or diltiazem overdose vs only 1 reference to felodipine. A regression model shows significant correlation and causality between RL and SO over time.5 Integrating all 3 mathematical models suggests that the higher RM of CCB and BB may be caused by the high RL of verapamil, diltiazem, nicardipine, propranolol, and labetalol.
These mathematical models can help physicians consider whether to switch the patient’s current medication to another class with a lower RM. For patients who need a BB or CCB, prescribing a medication with a lower RL within the same class may be another option. The data suggest that avoiding hypertension medications with RL >100% may significantly decrease morbidity and mortality.
Predicting serious outcomes of psychiatric medications
The 2018 NPDS data for psychiatric medications show similarly important results.2 For example, the lithium RM is predictable over time (Figure 3) and has been consistently the highest among psychiatric medications. Using 2006-2017 NPDS data,3 I predicted that the 2018 lithium RM would be 41.56%. The 2018 observed lithium RM was 41.45%.2 I created a linear regression model for each NPDS report from 2013 to 2018 to illustrate the correlation between RL and adjusted SO for 13 psychiatric medications.2,3,6,7 To account for different sample sizes among medications, the lithium SE for each respective year was used for all medications (adjusted SO = SE × RM). A time series analysis of these regression models shows that SO data points hover in the same y-axis region from year to year, with a corresponding RL on the x-axis: escitalopram 6.33%, citalopram 15.50%, mirtazapine 28.47%, paroxetine 37.35%, sertraline 46.72%, fluoxetine 54.87%, venlafaxine 99.64%, duloxetine 133.33%, trazodone 269.57%, bupropion 289.42%, amitriptyline 387.50%, doxepin 632.65%, and lithium 1062.86% (Figure 4). Every year, the scatter plot shape remains approximately the same, which suggests that both SO and RM can be predicted over time. Medications with RL >300% have SO ≈ 1500 (RM ≈ 40%), and those with RL <100% have SO ≈ 500 (RM ≈ 13%).
Time series analysis of NPDS data sheds light on hidden patterns. It may help clinicians discern patterns of potential SO associated with various hypertension and psychiatric medications. RL based on rat experimental data is highly correlated to RM based on human observational data, and the causality is self-evident. On a global scale, data-driven prescribing of medications with RL <100% could potentially help prevent millions of SO every year.
1. National Poison Data System Annual Reports. American Association of Poison Control Centers. https://www.aapcc.org/annual-reports. Updated November 2019. Accessed May 5, 2020.
2. Gummin DD, Mowry JB, Spyker DA, et al. 2018 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 36th Annual Report. Clin Toxicol (Phila). 2019;57(12):1220-1413.
3. Gummin DD, Mowry JB, Spyker DA, et al. 2017 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 35th Annual Report. Clin Toxicol (Phila). 2018;56(12):1213-1415.
4. Giurca D. Decreasing suicide risk with math. Current Psychiatry. 2018;17(2):57-59,A,B.
5. Giurca D. Data-driven prescribing. Current Psychiatry. 2018;17(10):e6-e8.
6. Mowry JB, Spyker DA, Brooks DE, et al. 2015 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 33rd Annual Report. Clin Toxicol (Phila). 2016;54(10):924-1109.
7. Gummin DD, Mowry JB, Spyker DA, et al. 2016 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 34th Annual Report. Clin Toxicol (Phila). 2017;55(10):1072-1252.
The US Poison Control Centers’ National Poison Data System (NPDS) publishes annual reports describing exposures to various substances among the general population.1 Table 22B of each NPDS report shows the number of outcomes from exposures to different pharmacologic treatments in the United States, including psychotropic medications.2 In this Table, the relative morbidity (RM) of a medication is calculated as the ratio of serious outcomes (SO) to single exposures (SE), where SO = moderate + major + death. In this article, I use the NPDS data to demonstrate how time series analysis of the RM ratios for hypertension and psychiatric medications can help predict SO associated with these agents, which may help guide clinicians’ prescribing decisions.2,3
Time series analysis of hypertension medications
Due to the high prevalence of hypertension, it is not surprising that more suicide deaths occur each year from calcium channel blockers (CCB) than from lithium (37 vs 2, according to 2017 NPDS data).3 I used time series analysis to compare SO during 2006-2017 for 5 classes of hypertension medications: CCB, beta blockers (BB), angiotensin-converting enzyme inhibitors (ACEI), angiotensin receptor blockers (ARB), and diuretics (Figure 1).
Time series analysis of 2006-2017 data predicted the following number of deaths for 2018: CCB ≥33, BB ≥17, ACEI ≤2, ARB 0, and diuretics ≤1. The observed deaths in 2018 were 41, 23, 0, 0, and 1, respectively.2 The 2018 predicted RM were CCB 10.66%, BB 11.10%, ACEI 3.51%, ARB 2.04%, and diuretics 3.38%. The 2018 observed RM for these medications were 11.01%, 11.37%, 3.02%, 2.40%, and 2.88%, respectively.2
Because the NPDS data for hypertension medications was only provided by class, in order to detect differences within each class, I used the relative lethality (RL) equation: RL = 310x / LD50, where x is the maximum daily dose of a medication prescribed for 30 days, and LD50 is the rat oral lethal dose 50. The RL equation represents the ratio of a 30-day supply of medication to the human equivalent LD50 for a 60-kg person.4 The RL equation is useful for comparing the safety of various medications, and can help clinicians avoid prescribing a lethal amount of a given medication (Figure 2). For example, the equation shows that among CCB, felodipine is 466 times safer than verapamil and 101 times safer than diltiazem. Not surprisingly, 2006-2018 data shows many deaths via intentional verapamil or diltiazem overdose vs only 1 reference to felodipine. A regression model shows significant correlation and causality between RL and SO over time.5 Integrating all 3 mathematical models suggests that the higher RM of CCB and BB may be caused by the high RL of verapamil, diltiazem, nicardipine, propranolol, and labetalol.
These mathematical models can help physicians consider whether to switch the patient’s current medication to another class with a lower RM. For patients who need a BB or CCB, prescribing a medication with a lower RL within the same class may be another option. The data suggest that avoiding hypertension medications with RL >100% may significantly decrease morbidity and mortality.
Predicting serious outcomes of psychiatric medications
The 2018 NPDS data for psychiatric medications show similarly important results.2 For example, the lithium RM is predictable over time (Figure 3) and has been consistently the highest among psychiatric medications. Using 2006-2017 NPDS data,3 I predicted that the 2018 lithium RM would be 41.56%. The 2018 observed lithium RM was 41.45%.2 I created a linear regression model for each NPDS report from 2013 to 2018 to illustrate the correlation between RL and adjusted SO for 13 psychiatric medications.2,3,6,7 To account for different sample sizes among medications, the lithium SE for each respective year was used for all medications (adjusted SO = SE × RM). A time series analysis of these regression models shows that SO data points hover in the same y-axis region from year to year, with a corresponding RL on the x-axis: escitalopram 6.33%, citalopram 15.50%, mirtazapine 28.47%, paroxetine 37.35%, sertraline 46.72%, fluoxetine 54.87%, venlafaxine 99.64%, duloxetine 133.33%, trazodone 269.57%, bupropion 289.42%, amitriptyline 387.50%, doxepin 632.65%, and lithium 1062.86% (Figure 4). Every year, the scatter plot shape remains approximately the same, which suggests that both SO and RM can be predicted over time. Medications with RL >300% have SO ≈ 1500 (RM ≈ 40%), and those with RL <100% have SO ≈ 500 (RM ≈ 13%).
Time series analysis of NPDS data sheds light on hidden patterns. It may help clinicians discern patterns of potential SO associated with various hypertension and psychiatric medications. RL based on rat experimental data is highly correlated to RM based on human observational data, and the causality is self-evident. On a global scale, data-driven prescribing of medications with RL <100% could potentially help prevent millions of SO every year.
The US Poison Control Centers’ National Poison Data System (NPDS) publishes annual reports describing exposures to various substances among the general population.1 Table 22B of each NPDS report shows the number of outcomes from exposures to different pharmacologic treatments in the United States, including psychotropic medications.2 In this Table, the relative morbidity (RM) of a medication is calculated as the ratio of serious outcomes (SO) to single exposures (SE), where SO = moderate + major + death. In this article, I use the NPDS data to demonstrate how time series analysis of the RM ratios for hypertension and psychiatric medications can help predict SO associated with these agents, which may help guide clinicians’ prescribing decisions.2,3
Time series analysis of hypertension medications
Due to the high prevalence of hypertension, it is not surprising that more suicide deaths occur each year from calcium channel blockers (CCB) than from lithium (37 vs 2, according to 2017 NPDS data).3 I used time series analysis to compare SO during 2006-2017 for 5 classes of hypertension medications: CCB, beta blockers (BB), angiotensin-converting enzyme inhibitors (ACEI), angiotensin receptor blockers (ARB), and diuretics (Figure 1).
Time series analysis of 2006-2017 data predicted the following number of deaths for 2018: CCB ≥33, BB ≥17, ACEI ≤2, ARB 0, and diuretics ≤1. The observed deaths in 2018 were 41, 23, 0, 0, and 1, respectively.2 The 2018 predicted RM were CCB 10.66%, BB 11.10%, ACEI 3.51%, ARB 2.04%, and diuretics 3.38%. The 2018 observed RM for these medications were 11.01%, 11.37%, 3.02%, 2.40%, and 2.88%, respectively.2
Because the NPDS data for hypertension medications was only provided by class, in order to detect differences within each class, I used the relative lethality (RL) equation: RL = 310x / LD50, where x is the maximum daily dose of a medication prescribed for 30 days, and LD50 is the rat oral lethal dose 50. The RL equation represents the ratio of a 30-day supply of medication to the human equivalent LD50 for a 60-kg person.4 The RL equation is useful for comparing the safety of various medications, and can help clinicians avoid prescribing a lethal amount of a given medication (Figure 2). For example, the equation shows that among CCB, felodipine is 466 times safer than verapamil and 101 times safer than diltiazem. Not surprisingly, 2006-2018 data shows many deaths via intentional verapamil or diltiazem overdose vs only 1 reference to felodipine. A regression model shows significant correlation and causality between RL and SO over time.5 Integrating all 3 mathematical models suggests that the higher RM of CCB and BB may be caused by the high RL of verapamil, diltiazem, nicardipine, propranolol, and labetalol.
These mathematical models can help physicians consider whether to switch the patient’s current medication to another class with a lower RM. For patients who need a BB or CCB, prescribing a medication with a lower RL within the same class may be another option. The data suggest that avoiding hypertension medications with RL >100% may significantly decrease morbidity and mortality.
Predicting serious outcomes of psychiatric medications
The 2018 NPDS data for psychiatric medications show similarly important results.2 For example, the lithium RM is predictable over time (Figure 3) and has been consistently the highest among psychiatric medications. Using 2006-2017 NPDS data,3 I predicted that the 2018 lithium RM would be 41.56%. The 2018 observed lithium RM was 41.45%.2 I created a linear regression model for each NPDS report from 2013 to 2018 to illustrate the correlation between RL and adjusted SO for 13 psychiatric medications.2,3,6,7 To account for different sample sizes among medications, the lithium SE for each respective year was used for all medications (adjusted SO = SE × RM). A time series analysis of these regression models shows that SO data points hover in the same y-axis region from year to year, with a corresponding RL on the x-axis: escitalopram 6.33%, citalopram 15.50%, mirtazapine 28.47%, paroxetine 37.35%, sertraline 46.72%, fluoxetine 54.87%, venlafaxine 99.64%, duloxetine 133.33%, trazodone 269.57%, bupropion 289.42%, amitriptyline 387.50%, doxepin 632.65%, and lithium 1062.86% (Figure 4). Every year, the scatter plot shape remains approximately the same, which suggests that both SO and RM can be predicted over time. Medications with RL >300% have SO ≈ 1500 (RM ≈ 40%), and those with RL <100% have SO ≈ 500 (RM ≈ 13%).
Time series analysis of NPDS data sheds light on hidden patterns. It may help clinicians discern patterns of potential SO associated with various hypertension and psychiatric medications. RL based on rat experimental data is highly correlated to RM based on human observational data, and the causality is self-evident. On a global scale, data-driven prescribing of medications with RL <100% could potentially help prevent millions of SO every year.
1. National Poison Data System Annual Reports. American Association of Poison Control Centers. https://www.aapcc.org/annual-reports. Updated November 2019. Accessed May 5, 2020.
2. Gummin DD, Mowry JB, Spyker DA, et al. 2018 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 36th Annual Report. Clin Toxicol (Phila). 2019;57(12):1220-1413.
3. Gummin DD, Mowry JB, Spyker DA, et al. 2017 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 35th Annual Report. Clin Toxicol (Phila). 2018;56(12):1213-1415.
4. Giurca D. Decreasing suicide risk with math. Current Psychiatry. 2018;17(2):57-59,A,B.
5. Giurca D. Data-driven prescribing. Current Psychiatry. 2018;17(10):e6-e8.
6. Mowry JB, Spyker DA, Brooks DE, et al. 2015 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 33rd Annual Report. Clin Toxicol (Phila). 2016;54(10):924-1109.
7. Gummin DD, Mowry JB, Spyker DA, et al. 2016 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 34th Annual Report. Clin Toxicol (Phila). 2017;55(10):1072-1252.
1. National Poison Data System Annual Reports. American Association of Poison Control Centers. https://www.aapcc.org/annual-reports. Updated November 2019. Accessed May 5, 2020.
2. Gummin DD, Mowry JB, Spyker DA, et al. 2018 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 36th Annual Report. Clin Toxicol (Phila). 2019;57(12):1220-1413.
3. Gummin DD, Mowry JB, Spyker DA, et al. 2017 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 35th Annual Report. Clin Toxicol (Phila). 2018;56(12):1213-1415.
4. Giurca D. Decreasing suicide risk with math. Current Psychiatry. 2018;17(2):57-59,A,B.
5. Giurca D. Data-driven prescribing. Current Psychiatry. 2018;17(10):e6-e8.
6. Mowry JB, Spyker DA, Brooks DE, et al. 2015 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 33rd Annual Report. Clin Toxicol (Phila). 2016;54(10):924-1109.
7. Gummin DD, Mowry JB, Spyker DA, et al. 2016 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 34th Annual Report. Clin Toxicol (Phila). 2017;55(10):1072-1252.
Telepsychiatry during COVID-19: Understanding the rules
In addition to affecting our personal lives, coronavirus disease 2019 (COVID-19) has altered the way we practice psychiatry. Telepsychiatry—the delivery of mental health services via remote communication—is being used to replace face-to-face outpatient encounters. Several rules and regulations governing the provision of care and prescribing have been temporarily modified or suspended to allow clinicians to more easily use telepsychiatry to care for their patients. Although these requirements are continually changing, here I review some of the telepsychiatry rules and regulations clinicians need to understand to minimize their risk for liability.
Changes in light of COVID-19
In March 2020, the Centers for Medicare & Medicaid Services (CMS) released guidance that allows Medicare beneficiaries to receive various services at home through telehealth without having to travel to a doctor’s office or hospital.1 Many commercial insurers also are allowing patients to receive telehealth services in their home. The US Department of Health & Human Services Office for Civil Rights, which enforces the Health Insurance Portability and Accountability Act (HIPAA), reported in March 2020 that it will not impose penalties for not complying with HIPAA requirements on clinicians who provide good-faith telepsychiatry during the COVID-19 crisis.2
Clinicians who want to use audio or video remote communication to provide any type of telehealth services (not just those related to COVID-19) should use “non-public facing” products.2 Non-public facing products (eg, Skype, WhatsApp video call, Zoom) allow only the intended parties to participate in the communication.3 Usually, these products employ end-to-end encryption, which allows only those engaging in communication to see and hear what is transmitted.3 To limit access and verify the participants, these products also support individual user accounts, login names, and passwords.3 In addition, these products usually allow participants and/or “the host” to exert some degree of control over particular features, such as choosing to record the communication, mute, or turn off the video or audio signal.3 When using these products, clinicians should enable all available encryption and privacy modes.2
“Public-facing” products (eg, Facebook Live, TikTok, Twitch) should not be used to provide telepsychiatry services because they are designed to be open to the public or allow for wide or indiscriminate access to the communication.2,3 Clinicians who desire additional privacy protections (and a more permanent solution) should choose a HIPAA-compliant telehealth vendor (eg, Doxy.me, VSee, Zoom for Healthcare) and obtain a Business Associate Agreement with the vendor to ensure data protection and security.2,4
Regardless of the product, obtain informed consent from your patients that authorizes the use of remote communication.4 Inform your patients of any potential privacy or security breaches, the need for interactions to be conducted in a location that provides privacy, and whether the specific technology used is HIPAA-compliant.4 Document that your patients understand these issues before using remote communication.4
How licensing requirements have changed
As of March 31, 2020, the CMS temporarily waived the requirement that out-of-state clinicians be licensed in the state where they are providing services to Medicare beneficiaries.5 The CMS waived this requirement for clinicians who meet the following 4 conditions5,6:
- must be enrolled in Medicare
- must possess a valid license to practice in the state that relates to his/her Medicare enrollment
- are furnishing services—whether in person or via telepsychiatry—in a state where the emergency is occurring to contribute to relief efforts in his/her professional capacity
- are not excluded from practicing in any state that is part of the nationally declared emergency area.
Note that individual state licensure requirements continue to apply unless waived by the state.6 Therefore, in order for clinicians to see Medicare patients via remote communication under the 4 conditions described above, the state also would have to waive its licensure requirements for the type of practice for which the clinicians are licensed in their own state.6 Regarding commercial payers, in general, clinicians providing telepsychiatry services need a license to practice in the state where the patient is located at the time services are provided.6 During the COVID-19 pandemic, many governors issued executive orders waiving licensure requirements, and many have accelerated granting temporary licenses to out-of-state clinicians who wish to provide telepsychiatry services to the residents of their state.4
Continue to: Prescribing via telepsychiatry
Prescribing via telepsychiatry
Effective March 31, 2020 and lasting for the duration of COVID-19 emergency declaration, the Drug Enforcement Agency (DEA) suspended the Ryan Haight Online Pharmacy Consumer Protection Act of 2008, which requires clinicians to conduct initial, in-person examinations of patients before they can prescribe controlled substances electronically.6,7 The DEA suspension allows clinicians to prescribe controlled substances after conducting an initial evaluation via remote communication. In addition, the DEA waived the requirement that a clinician needs to hold a DEA license in the state where the patient is located to be able to prescribe a controlled substance electronically.4,6 However, you still must comply with all other state laws and regulations for prescribing controlled substances.4
Staying informed
Although several telepsychiatry rules and regulations have been modified or suspended during the COVID-19 pandemic, the standard of care for services rendered via telepsychiatry remains the same as services provided via face-to-face encounters, including patient evaluation and assessment, treatment plans, medication, and documentation.4 Clinicians can keep up-to-date on how practicing telepsychiatry may evolve during these times by using the following resources from the American Psychiatric Association:
- Telepsychiatry Toolkit: www.psychiatry.org/psychiatrists/practice/telepsychiatry
- Practice Guidance for COVID-19: www.psychiatry.org/psychiatrists/covid-19-coronavirus/practice-guidance-for-covid-19.
1. Centers for Medicare and Medicaid Services. COVID-19: President Trump expands telehealth benefits for Medicare beneficiaries during COVID-19 outbreak. https://www.cms.gov/outreach-and-educationoutreachffsprovpartprogprovider-partnership-email-archive/2020-03-17. Published March 17, 2020. Accessed May 6, 2020.
2. US Department of Health & Human Services. Notification of enforcement discretion for telehealth remote communications during the COVID-19 nationwide public health emergency. https://www.hhs.gov/hipaa/for-professionals/special-topics/emergency-preparedness/notification-enforcement-discretion-telehealth/index.html. Updated March 30, 2020. Accessed May 6, 2020.
3. US Department of Health & Human Services. What is a “non-public facing” remote communication product? https://www.hhs.gov/hipaa/for-professionals/faq/3024/what-is-a-non-public-facing-remote-communication-product/index.html. Updated April 10, 2020. Accessed May 6, 2020.
4. Huben-Kearney A. Risk management amid a global pandemic. Psychiatric News. https://psychnews.psychiatryonline.org/doi/10.1176/appi.pn.2020.5a38. Published April 28, 2020. Accessed May 6, 2020.
5. Centers for Medicare & Medicaid Services. COVID-19 emergency declaration blanket waivers for health care providers. https://www.cms.gov/files/document/summary-covid-19-emergency-declaration-waivers.pdf. Published April 29, 2020. Accessed May 6, 2020.
6. American Psychiatric Association. Update on telehealth restrictions in response to COVID-19. https://www.psychiatry.org/psychiatrists/practice/telepsychiatry/blog/apa-resources-on-telepsychiatry-and-covid-19. Updated May 1, 2020. Accessed May 6, 2020.
7. US Drug Enforcement Agency. How to prescribe controlled substances to patients during the COVID-19 public health emergency. https://www.deadiversion.usdoj.gov/GDP/(DEA-DC-023)(DEA075)Decision_Tree_(Final)_33120_2007.pdf. Published March 31, 2020. Accessed on May 6, 2020.
In addition to affecting our personal lives, coronavirus disease 2019 (COVID-19) has altered the way we practice psychiatry. Telepsychiatry—the delivery of mental health services via remote communication—is being used to replace face-to-face outpatient encounters. Several rules and regulations governing the provision of care and prescribing have been temporarily modified or suspended to allow clinicians to more easily use telepsychiatry to care for their patients. Although these requirements are continually changing, here I review some of the telepsychiatry rules and regulations clinicians need to understand to minimize their risk for liability.
Changes in light of COVID-19
In March 2020, the Centers for Medicare & Medicaid Services (CMS) released guidance that allows Medicare beneficiaries to receive various services at home through telehealth without having to travel to a doctor’s office or hospital.1 Many commercial insurers also are allowing patients to receive telehealth services in their home. The US Department of Health & Human Services Office for Civil Rights, which enforces the Health Insurance Portability and Accountability Act (HIPAA), reported in March 2020 that it will not impose penalties for not complying with HIPAA requirements on clinicians who provide good-faith telepsychiatry during the COVID-19 crisis.2
Clinicians who want to use audio or video remote communication to provide any type of telehealth services (not just those related to COVID-19) should use “non-public facing” products.2 Non-public facing products (eg, Skype, WhatsApp video call, Zoom) allow only the intended parties to participate in the communication.3 Usually, these products employ end-to-end encryption, which allows only those engaging in communication to see and hear what is transmitted.3 To limit access and verify the participants, these products also support individual user accounts, login names, and passwords.3 In addition, these products usually allow participants and/or “the host” to exert some degree of control over particular features, such as choosing to record the communication, mute, or turn off the video or audio signal.3 When using these products, clinicians should enable all available encryption and privacy modes.2
“Public-facing” products (eg, Facebook Live, TikTok, Twitch) should not be used to provide telepsychiatry services because they are designed to be open to the public or allow for wide or indiscriminate access to the communication.2,3 Clinicians who desire additional privacy protections (and a more permanent solution) should choose a HIPAA-compliant telehealth vendor (eg, Doxy.me, VSee, Zoom for Healthcare) and obtain a Business Associate Agreement with the vendor to ensure data protection and security.2,4
Regardless of the product, obtain informed consent from your patients that authorizes the use of remote communication.4 Inform your patients of any potential privacy or security breaches, the need for interactions to be conducted in a location that provides privacy, and whether the specific technology used is HIPAA-compliant.4 Document that your patients understand these issues before using remote communication.4
How licensing requirements have changed
As of March 31, 2020, the CMS temporarily waived the requirement that out-of-state clinicians be licensed in the state where they are providing services to Medicare beneficiaries.5 The CMS waived this requirement for clinicians who meet the following 4 conditions5,6:
- must be enrolled in Medicare
- must possess a valid license to practice in the state that relates to his/her Medicare enrollment
- are furnishing services—whether in person or via telepsychiatry—in a state where the emergency is occurring to contribute to relief efforts in his/her professional capacity
- are not excluded from practicing in any state that is part of the nationally declared emergency area.
Note that individual state licensure requirements continue to apply unless waived by the state.6 Therefore, in order for clinicians to see Medicare patients via remote communication under the 4 conditions described above, the state also would have to waive its licensure requirements for the type of practice for which the clinicians are licensed in their own state.6 Regarding commercial payers, in general, clinicians providing telepsychiatry services need a license to practice in the state where the patient is located at the time services are provided.6 During the COVID-19 pandemic, many governors issued executive orders waiving licensure requirements, and many have accelerated granting temporary licenses to out-of-state clinicians who wish to provide telepsychiatry services to the residents of their state.4
Continue to: Prescribing via telepsychiatry
Prescribing via telepsychiatry
Effective March 31, 2020 and lasting for the duration of COVID-19 emergency declaration, the Drug Enforcement Agency (DEA) suspended the Ryan Haight Online Pharmacy Consumer Protection Act of 2008, which requires clinicians to conduct initial, in-person examinations of patients before they can prescribe controlled substances electronically.6,7 The DEA suspension allows clinicians to prescribe controlled substances after conducting an initial evaluation via remote communication. In addition, the DEA waived the requirement that a clinician needs to hold a DEA license in the state where the patient is located to be able to prescribe a controlled substance electronically.4,6 However, you still must comply with all other state laws and regulations for prescribing controlled substances.4
Staying informed
Although several telepsychiatry rules and regulations have been modified or suspended during the COVID-19 pandemic, the standard of care for services rendered via telepsychiatry remains the same as services provided via face-to-face encounters, including patient evaluation and assessment, treatment plans, medication, and documentation.4 Clinicians can keep up-to-date on how practicing telepsychiatry may evolve during these times by using the following resources from the American Psychiatric Association:
- Telepsychiatry Toolkit: www.psychiatry.org/psychiatrists/practice/telepsychiatry
- Practice Guidance for COVID-19: www.psychiatry.org/psychiatrists/covid-19-coronavirus/practice-guidance-for-covid-19.
In addition to affecting our personal lives, coronavirus disease 2019 (COVID-19) has altered the way we practice psychiatry. Telepsychiatry—the delivery of mental health services via remote communication—is being used to replace face-to-face outpatient encounters. Several rules and regulations governing the provision of care and prescribing have been temporarily modified or suspended to allow clinicians to more easily use telepsychiatry to care for their patients. Although these requirements are continually changing, here I review some of the telepsychiatry rules and regulations clinicians need to understand to minimize their risk for liability.
Changes in light of COVID-19
In March 2020, the Centers for Medicare & Medicaid Services (CMS) released guidance that allows Medicare beneficiaries to receive various services at home through telehealth without having to travel to a doctor’s office or hospital.1 Many commercial insurers also are allowing patients to receive telehealth services in their home. The US Department of Health & Human Services Office for Civil Rights, which enforces the Health Insurance Portability and Accountability Act (HIPAA), reported in March 2020 that it will not impose penalties for not complying with HIPAA requirements on clinicians who provide good-faith telepsychiatry during the COVID-19 crisis.2
Clinicians who want to use audio or video remote communication to provide any type of telehealth services (not just those related to COVID-19) should use “non-public facing” products.2 Non-public facing products (eg, Skype, WhatsApp video call, Zoom) allow only the intended parties to participate in the communication.3 Usually, these products employ end-to-end encryption, which allows only those engaging in communication to see and hear what is transmitted.3 To limit access and verify the participants, these products also support individual user accounts, login names, and passwords.3 In addition, these products usually allow participants and/or “the host” to exert some degree of control over particular features, such as choosing to record the communication, mute, or turn off the video or audio signal.3 When using these products, clinicians should enable all available encryption and privacy modes.2
“Public-facing” products (eg, Facebook Live, TikTok, Twitch) should not be used to provide telepsychiatry services because they are designed to be open to the public or allow for wide or indiscriminate access to the communication.2,3 Clinicians who desire additional privacy protections (and a more permanent solution) should choose a HIPAA-compliant telehealth vendor (eg, Doxy.me, VSee, Zoom for Healthcare) and obtain a Business Associate Agreement with the vendor to ensure data protection and security.2,4
Regardless of the product, obtain informed consent from your patients that authorizes the use of remote communication.4 Inform your patients of any potential privacy or security breaches, the need for interactions to be conducted in a location that provides privacy, and whether the specific technology used is HIPAA-compliant.4 Document that your patients understand these issues before using remote communication.4
How licensing requirements have changed
As of March 31, 2020, the CMS temporarily waived the requirement that out-of-state clinicians be licensed in the state where they are providing services to Medicare beneficiaries.5 The CMS waived this requirement for clinicians who meet the following 4 conditions5,6:
- must be enrolled in Medicare
- must possess a valid license to practice in the state that relates to his/her Medicare enrollment
- are furnishing services—whether in person or via telepsychiatry—in a state where the emergency is occurring to contribute to relief efforts in his/her professional capacity
- are not excluded from practicing in any state that is part of the nationally declared emergency area.
Note that individual state licensure requirements continue to apply unless waived by the state.6 Therefore, in order for clinicians to see Medicare patients via remote communication under the 4 conditions described above, the state also would have to waive its licensure requirements for the type of practice for which the clinicians are licensed in their own state.6 Regarding commercial payers, in general, clinicians providing telepsychiatry services need a license to practice in the state where the patient is located at the time services are provided.6 During the COVID-19 pandemic, many governors issued executive orders waiving licensure requirements, and many have accelerated granting temporary licenses to out-of-state clinicians who wish to provide telepsychiatry services to the residents of their state.4
Continue to: Prescribing via telepsychiatry
Prescribing via telepsychiatry
Effective March 31, 2020 and lasting for the duration of COVID-19 emergency declaration, the Drug Enforcement Agency (DEA) suspended the Ryan Haight Online Pharmacy Consumer Protection Act of 2008, which requires clinicians to conduct initial, in-person examinations of patients before they can prescribe controlled substances electronically.6,7 The DEA suspension allows clinicians to prescribe controlled substances after conducting an initial evaluation via remote communication. In addition, the DEA waived the requirement that a clinician needs to hold a DEA license in the state where the patient is located to be able to prescribe a controlled substance electronically.4,6 However, you still must comply with all other state laws and regulations for prescribing controlled substances.4
Staying informed
Although several telepsychiatry rules and regulations have been modified or suspended during the COVID-19 pandemic, the standard of care for services rendered via telepsychiatry remains the same as services provided via face-to-face encounters, including patient evaluation and assessment, treatment plans, medication, and documentation.4 Clinicians can keep up-to-date on how practicing telepsychiatry may evolve during these times by using the following resources from the American Psychiatric Association:
- Telepsychiatry Toolkit: www.psychiatry.org/psychiatrists/practice/telepsychiatry
- Practice Guidance for COVID-19: www.psychiatry.org/psychiatrists/covid-19-coronavirus/practice-guidance-for-covid-19.
1. Centers for Medicare and Medicaid Services. COVID-19: President Trump expands telehealth benefits for Medicare beneficiaries during COVID-19 outbreak. https://www.cms.gov/outreach-and-educationoutreachffsprovpartprogprovider-partnership-email-archive/2020-03-17. Published March 17, 2020. Accessed May 6, 2020.
2. US Department of Health & Human Services. Notification of enforcement discretion for telehealth remote communications during the COVID-19 nationwide public health emergency. https://www.hhs.gov/hipaa/for-professionals/special-topics/emergency-preparedness/notification-enforcement-discretion-telehealth/index.html. Updated March 30, 2020. Accessed May 6, 2020.
3. US Department of Health & Human Services. What is a “non-public facing” remote communication product? https://www.hhs.gov/hipaa/for-professionals/faq/3024/what-is-a-non-public-facing-remote-communication-product/index.html. Updated April 10, 2020. Accessed May 6, 2020.
4. Huben-Kearney A. Risk management amid a global pandemic. Psychiatric News. https://psychnews.psychiatryonline.org/doi/10.1176/appi.pn.2020.5a38. Published April 28, 2020. Accessed May 6, 2020.
5. Centers for Medicare & Medicaid Services. COVID-19 emergency declaration blanket waivers for health care providers. https://www.cms.gov/files/document/summary-covid-19-emergency-declaration-waivers.pdf. Published April 29, 2020. Accessed May 6, 2020.
6. American Psychiatric Association. Update on telehealth restrictions in response to COVID-19. https://www.psychiatry.org/psychiatrists/practice/telepsychiatry/blog/apa-resources-on-telepsychiatry-and-covid-19. Updated May 1, 2020. Accessed May 6, 2020.
7. US Drug Enforcement Agency. How to prescribe controlled substances to patients during the COVID-19 public health emergency. https://www.deadiversion.usdoj.gov/GDP/(DEA-DC-023)(DEA075)Decision_Tree_(Final)_33120_2007.pdf. Published March 31, 2020. Accessed on May 6, 2020.
1. Centers for Medicare and Medicaid Services. COVID-19: President Trump expands telehealth benefits for Medicare beneficiaries during COVID-19 outbreak. https://www.cms.gov/outreach-and-educationoutreachffsprovpartprogprovider-partnership-email-archive/2020-03-17. Published March 17, 2020. Accessed May 6, 2020.
2. US Department of Health & Human Services. Notification of enforcement discretion for telehealth remote communications during the COVID-19 nationwide public health emergency. https://www.hhs.gov/hipaa/for-professionals/special-topics/emergency-preparedness/notification-enforcement-discretion-telehealth/index.html. Updated March 30, 2020. Accessed May 6, 2020.
3. US Department of Health & Human Services. What is a “non-public facing” remote communication product? https://www.hhs.gov/hipaa/for-professionals/faq/3024/what-is-a-non-public-facing-remote-communication-product/index.html. Updated April 10, 2020. Accessed May 6, 2020.
4. Huben-Kearney A. Risk management amid a global pandemic. Psychiatric News. https://psychnews.psychiatryonline.org/doi/10.1176/appi.pn.2020.5a38. Published April 28, 2020. Accessed May 6, 2020.
5. Centers for Medicare & Medicaid Services. COVID-19 emergency declaration blanket waivers for health care providers. https://www.cms.gov/files/document/summary-covid-19-emergency-declaration-waivers.pdf. Published April 29, 2020. Accessed May 6, 2020.
6. American Psychiatric Association. Update on telehealth restrictions in response to COVID-19. https://www.psychiatry.org/psychiatrists/practice/telepsychiatry/blog/apa-resources-on-telepsychiatry-and-covid-19. Updated May 1, 2020. Accessed May 6, 2020.
7. US Drug Enforcement Agency. How to prescribe controlled substances to patients during the COVID-19 public health emergency. https://www.deadiversion.usdoj.gov/GDP/(DEA-DC-023)(DEA075)Decision_Tree_(Final)_33120_2007.pdf. Published March 31, 2020. Accessed on May 6, 2020.
Major GI bleeding risk calculated for primary prevention aspirin in elderly
in a new analysis from the large randomized ASPREE trial released as part of the annual Digestive Disease Week.
The analysis identified several independent risk factors for major GI bleeding – advanced age, hypertension, obesity, smoking, and chronic kidney disease – according to Suzanne E. Mahady, MBBS, PhD, a gastroenterologist and clinical epidemiologist at Monash University in Melbourne.
“To date, there [are] no comparable data assessing aspirin-related bleeding in older healthy people from a large randomized, controlled trial. Previous data [have] been observational, with variable definitions of significant bleeding, and retrospective. We derived a standard definition for bleeding, used physicians to adjudicate bleeding endpoints, and followed older people for 5 years,” she explained in an interview.
“Our data on bleeding [are] novel,” Dr. Mahady added. “It will help clinicians assess who is most at risk of bleeding with aspirin and target modifiable bleeding risk factors where possible.”
ASPREE was a double-blind trial including 19,114 apparently healthy Australian and American adults age 70 or older, or age 65-plus for blacks and Hispanics in the United States. Participants were randomized to 100 mg/day of enteric-coated aspirin or placebo. At a median 4.7 years of follow-up, there was no between-group difference in major adverse cardiovascular events, a lack of benefit accompanied by a 38% greater risk of major hemorrhage risk and a statistically significant 14% increase in all-cause mortality in the aspirin group. (N Engl J Med. 2018 Oct 18;379[16]:1509-18). The chief contributor to the excess mortality in the aspirin group was their 31% greater risk of cancer-related death (N Engl J Med. 2018 Oct 18;379[16]:1519-28).
The new analysis of severe GI bleeding documented an absolute 5-year risk of 0.2% for 70-year-olds and 0.4% in 80-year-olds on aspirin. In 80-year-olds with additional GI bleeding risk factors as identified in the study, the rate reached up to 5.5%. The risk of major upper GI bleeding events was 87% greater in the aspirin group, compared with placebo-treated controls, and the risk of serious lower GI bleeding was increased 36%.
ASPREE coinvestigator Andrew T. Chan, MD, said that the bleeding data should prove useful in future efforts to appropriately weight the risks and benefits of low-dose aspirin treatment.
“We need to better understand how to incorporate bleeding risk in clinical decision making about how to use aspirin among older adults because aspirin has many potential benefits, including prevention of colorectal cancer,” said Dr. Chan, a gastroenterologist and professor of medicine at Harvard Medical School and director for cancer epidemiology at Massachusetts General Hospital, both in Boston.
However, ASPREE has soured cardiologists on the decades-long practice of recommending aspirin for primary prevention of cardiovascular disease in older individuals. In response to the publication of primary outcomes in ASPREE, which was closely bracketed by publication of the largely negative results of the randomized ARRIVE and ASCEND trials in a collective 47,000-plus randomized patients, the American College of Cardiology/American Heart Association clipped aspirin’s role for primary prevention of atherosclerotic cardiovascular disease. The current recommendation is that low-dose aspirin should not be administered on a routine basis for primary cardiovascular prevention in people above age 70, nor in adults at any age at increased bleeding risk, although the practice “might be considered” for primary prevention in select higher atherosclerotic cardiovascular disease–risk 40- to 70-year-olds, provided they are not at increased bleeding risk (J Am Coll Cardiol. 2019 Sep. doi: 10.1016/j.jacc.2019.03.010).
Dr. Mahady reported having no financial conflicts of interest. Dr. Chan serves as a consultant to Bayer Pharma, Janssen, and Pfizer.
in a new analysis from the large randomized ASPREE trial released as part of the annual Digestive Disease Week.
The analysis identified several independent risk factors for major GI bleeding – advanced age, hypertension, obesity, smoking, and chronic kidney disease – according to Suzanne E. Mahady, MBBS, PhD, a gastroenterologist and clinical epidemiologist at Monash University in Melbourne.
“To date, there [are] no comparable data assessing aspirin-related bleeding in older healthy people from a large randomized, controlled trial. Previous data [have] been observational, with variable definitions of significant bleeding, and retrospective. We derived a standard definition for bleeding, used physicians to adjudicate bleeding endpoints, and followed older people for 5 years,” she explained in an interview.
“Our data on bleeding [are] novel,” Dr. Mahady added. “It will help clinicians assess who is most at risk of bleeding with aspirin and target modifiable bleeding risk factors where possible.”
ASPREE was a double-blind trial including 19,114 apparently healthy Australian and American adults age 70 or older, or age 65-plus for blacks and Hispanics in the United States. Participants were randomized to 100 mg/day of enteric-coated aspirin or placebo. At a median 4.7 years of follow-up, there was no between-group difference in major adverse cardiovascular events, a lack of benefit accompanied by a 38% greater risk of major hemorrhage risk and a statistically significant 14% increase in all-cause mortality in the aspirin group. (N Engl J Med. 2018 Oct 18;379[16]:1509-18). The chief contributor to the excess mortality in the aspirin group was their 31% greater risk of cancer-related death (N Engl J Med. 2018 Oct 18;379[16]:1519-28).
The new analysis of severe GI bleeding documented an absolute 5-year risk of 0.2% for 70-year-olds and 0.4% in 80-year-olds on aspirin. In 80-year-olds with additional GI bleeding risk factors as identified in the study, the rate reached up to 5.5%. The risk of major upper GI bleeding events was 87% greater in the aspirin group, compared with placebo-treated controls, and the risk of serious lower GI bleeding was increased 36%.
ASPREE coinvestigator Andrew T. Chan, MD, said that the bleeding data should prove useful in future efforts to appropriately weight the risks and benefits of low-dose aspirin treatment.
“We need to better understand how to incorporate bleeding risk in clinical decision making about how to use aspirin among older adults because aspirin has many potential benefits, including prevention of colorectal cancer,” said Dr. Chan, a gastroenterologist and professor of medicine at Harvard Medical School and director for cancer epidemiology at Massachusetts General Hospital, both in Boston.
However, ASPREE has soured cardiologists on the decades-long practice of recommending aspirin for primary prevention of cardiovascular disease in older individuals. In response to the publication of primary outcomes in ASPREE, which was closely bracketed by publication of the largely negative results of the randomized ARRIVE and ASCEND trials in a collective 47,000-plus randomized patients, the American College of Cardiology/American Heart Association clipped aspirin’s role for primary prevention of atherosclerotic cardiovascular disease. The current recommendation is that low-dose aspirin should not be administered on a routine basis for primary cardiovascular prevention in people above age 70, nor in adults at any age at increased bleeding risk, although the practice “might be considered” for primary prevention in select higher atherosclerotic cardiovascular disease–risk 40- to 70-year-olds, provided they are not at increased bleeding risk (J Am Coll Cardiol. 2019 Sep. doi: 10.1016/j.jacc.2019.03.010).
Dr. Mahady reported having no financial conflicts of interest. Dr. Chan serves as a consultant to Bayer Pharma, Janssen, and Pfizer.
in a new analysis from the large randomized ASPREE trial released as part of the annual Digestive Disease Week.
The analysis identified several independent risk factors for major GI bleeding – advanced age, hypertension, obesity, smoking, and chronic kidney disease – according to Suzanne E. Mahady, MBBS, PhD, a gastroenterologist and clinical epidemiologist at Monash University in Melbourne.
“To date, there [are] no comparable data assessing aspirin-related bleeding in older healthy people from a large randomized, controlled trial. Previous data [have] been observational, with variable definitions of significant bleeding, and retrospective. We derived a standard definition for bleeding, used physicians to adjudicate bleeding endpoints, and followed older people for 5 years,” she explained in an interview.
“Our data on bleeding [are] novel,” Dr. Mahady added. “It will help clinicians assess who is most at risk of bleeding with aspirin and target modifiable bleeding risk factors where possible.”
ASPREE was a double-blind trial including 19,114 apparently healthy Australian and American adults age 70 or older, or age 65-plus for blacks and Hispanics in the United States. Participants were randomized to 100 mg/day of enteric-coated aspirin or placebo. At a median 4.7 years of follow-up, there was no between-group difference in major adverse cardiovascular events, a lack of benefit accompanied by a 38% greater risk of major hemorrhage risk and a statistically significant 14% increase in all-cause mortality in the aspirin group. (N Engl J Med. 2018 Oct 18;379[16]:1509-18). The chief contributor to the excess mortality in the aspirin group was their 31% greater risk of cancer-related death (N Engl J Med. 2018 Oct 18;379[16]:1519-28).
The new analysis of severe GI bleeding documented an absolute 5-year risk of 0.2% for 70-year-olds and 0.4% in 80-year-olds on aspirin. In 80-year-olds with additional GI bleeding risk factors as identified in the study, the rate reached up to 5.5%. The risk of major upper GI bleeding events was 87% greater in the aspirin group, compared with placebo-treated controls, and the risk of serious lower GI bleeding was increased 36%.
ASPREE coinvestigator Andrew T. Chan, MD, said that the bleeding data should prove useful in future efforts to appropriately weight the risks and benefits of low-dose aspirin treatment.
“We need to better understand how to incorporate bleeding risk in clinical decision making about how to use aspirin among older adults because aspirin has many potential benefits, including prevention of colorectal cancer,” said Dr. Chan, a gastroenterologist and professor of medicine at Harvard Medical School and director for cancer epidemiology at Massachusetts General Hospital, both in Boston.
However, ASPREE has soured cardiologists on the decades-long practice of recommending aspirin for primary prevention of cardiovascular disease in older individuals. In response to the publication of primary outcomes in ASPREE, which was closely bracketed by publication of the largely negative results of the randomized ARRIVE and ASCEND trials in a collective 47,000-plus randomized patients, the American College of Cardiology/American Heart Association clipped aspirin’s role for primary prevention of atherosclerotic cardiovascular disease. The current recommendation is that low-dose aspirin should not be administered on a routine basis for primary cardiovascular prevention in people above age 70, nor in adults at any age at increased bleeding risk, although the practice “might be considered” for primary prevention in select higher atherosclerotic cardiovascular disease–risk 40- to 70-year-olds, provided they are not at increased bleeding risk (J Am Coll Cardiol. 2019 Sep. doi: 10.1016/j.jacc.2019.03.010).
Dr. Mahady reported having no financial conflicts of interest. Dr. Chan serves as a consultant to Bayer Pharma, Janssen, and Pfizer.
FROM DDW 2020