User login
Life during COVID-19: A pandemic of silence
Our world has radically changed during the coronavirus disease 2019 (COVID-19) crisis, and this impact has quickly transformed many lives. Whether you’re on the front lines of the COVID-19 pandemic or waiting in eager anticipation to return to practice, there is no denying that a few months ago we could never have imagined the health care and humanitarian crisis that is now before us. While we are united in our longing for a better time, we couldn’t be further apart socially and emotionally … and I’m not just talking about 6 feet.
One thing that has been truly striking to me is the silence. While experts have suggested there is a “silent pandemic” of mental illness on the horizon,1 I’ve been struck by the actual silence that exists as we walk through our stores and neighborhoods. We’re not speaking to each other anymore; it’s almost as if we’re afraid to make eye contact with one another.
Humans are social creatures, and the isolation that many people are experiencing during this pandemic could have detrimental and lasting effects if we don’t take action. While I highly encourage and support efforts to employ social distancing and mitigate the spread of this illness, I’m increasingly concerned about another kind of truly silent pandemic brewing beneath the surface of the COVID-19 crisis. Even under the best conditions, many individuals with posttraumatic stress disorder, depression, anxiety, bipolar disorder, schizophrenia, and other psychiatric disorders may lack adequate social interaction and experience feelings of isolation. These individuals need connection—not silence.
What happens to people who already felt intense isolation before COVID-19 and may have had invaluable lifelines cut off during this time of social distancing? What about individuals with alcohol or substance use disorders, or families who are sheltered in place in unsafe or violent home conditions? How can they reach out in silence? How can we help?
Fostering human connection
To address this, we must actively work to engage our patients and communities. One simple way to help is to acknowledge the people you encounter. Yes, stay 6 feet apart, and wear appropriate personal protective equipment. However, it is still OK to smile and greet someone with a nod, a smile, or a “hello.” A genuine smile can still be seen in someone’s eyes. We need these types of human connection, perhaps now more than ever before. We need each other.
Most importantly, during this time, we need to be aware of individuals who are most at risk in this silent pandemic. We can offer our patients appointments via video conferencing. We can use texting, e-mail, social media, phone calls, and video conferencing to check in with our families, friends, and neighbors. We’re at war with a terrible foe, but let’s not let the human connection become collateral damage.
1. Galea S, Merchant RM, Lurie N, et al. The mental health consequences of COVID-19 and physical distancing: the need for prevention and early intervention [published online April 10, 2020]. JAMA Intern Med. 2020. doi: 10.1001/jamainternmed.2020.1562.
Our world has radically changed during the coronavirus disease 2019 (COVID-19) crisis, and this impact has quickly transformed many lives. Whether you’re on the front lines of the COVID-19 pandemic or waiting in eager anticipation to return to practice, there is no denying that a few months ago we could never have imagined the health care and humanitarian crisis that is now before us. While we are united in our longing for a better time, we couldn’t be further apart socially and emotionally … and I’m not just talking about 6 feet.
One thing that has been truly striking to me is the silence. While experts have suggested there is a “silent pandemic” of mental illness on the horizon,1 I’ve been struck by the actual silence that exists as we walk through our stores and neighborhoods. We’re not speaking to each other anymore; it’s almost as if we’re afraid to make eye contact with one another.
Humans are social creatures, and the isolation that many people are experiencing during this pandemic could have detrimental and lasting effects if we don’t take action. While I highly encourage and support efforts to employ social distancing and mitigate the spread of this illness, I’m increasingly concerned about another kind of truly silent pandemic brewing beneath the surface of the COVID-19 crisis. Even under the best conditions, many individuals with posttraumatic stress disorder, depression, anxiety, bipolar disorder, schizophrenia, and other psychiatric disorders may lack adequate social interaction and experience feelings of isolation. These individuals need connection—not silence.
What happens to people who already felt intense isolation before COVID-19 and may have had invaluable lifelines cut off during this time of social distancing? What about individuals with alcohol or substance use disorders, or families who are sheltered in place in unsafe or violent home conditions? How can they reach out in silence? How can we help?
Fostering human connection
To address this, we must actively work to engage our patients and communities. One simple way to help is to acknowledge the people you encounter. Yes, stay 6 feet apart, and wear appropriate personal protective equipment. However, it is still OK to smile and greet someone with a nod, a smile, or a “hello.” A genuine smile can still be seen in someone’s eyes. We need these types of human connection, perhaps now more than ever before. We need each other.
Most importantly, during this time, we need to be aware of individuals who are most at risk in this silent pandemic. We can offer our patients appointments via video conferencing. We can use texting, e-mail, social media, phone calls, and video conferencing to check in with our families, friends, and neighbors. We’re at war with a terrible foe, but let’s not let the human connection become collateral damage.
Our world has radically changed during the coronavirus disease 2019 (COVID-19) crisis, and this impact has quickly transformed many lives. Whether you’re on the front lines of the COVID-19 pandemic or waiting in eager anticipation to return to practice, there is no denying that a few months ago we could never have imagined the health care and humanitarian crisis that is now before us. While we are united in our longing for a better time, we couldn’t be further apart socially and emotionally … and I’m not just talking about 6 feet.
One thing that has been truly striking to me is the silence. While experts have suggested there is a “silent pandemic” of mental illness on the horizon,1 I’ve been struck by the actual silence that exists as we walk through our stores and neighborhoods. We’re not speaking to each other anymore; it’s almost as if we’re afraid to make eye contact with one another.
Humans are social creatures, and the isolation that many people are experiencing during this pandemic could have detrimental and lasting effects if we don’t take action. While I highly encourage and support efforts to employ social distancing and mitigate the spread of this illness, I’m increasingly concerned about another kind of truly silent pandemic brewing beneath the surface of the COVID-19 crisis. Even under the best conditions, many individuals with posttraumatic stress disorder, depression, anxiety, bipolar disorder, schizophrenia, and other psychiatric disorders may lack adequate social interaction and experience feelings of isolation. These individuals need connection—not silence.
What happens to people who already felt intense isolation before COVID-19 and may have had invaluable lifelines cut off during this time of social distancing? What about individuals with alcohol or substance use disorders, or families who are sheltered in place in unsafe or violent home conditions? How can they reach out in silence? How can we help?
Fostering human connection
To address this, we must actively work to engage our patients and communities. One simple way to help is to acknowledge the people you encounter. Yes, stay 6 feet apart, and wear appropriate personal protective equipment. However, it is still OK to smile and greet someone with a nod, a smile, or a “hello.” A genuine smile can still be seen in someone’s eyes. We need these types of human connection, perhaps now more than ever before. We need each other.
Most importantly, during this time, we need to be aware of individuals who are most at risk in this silent pandemic. We can offer our patients appointments via video conferencing. We can use texting, e-mail, social media, phone calls, and video conferencing to check in with our families, friends, and neighbors. We’re at war with a terrible foe, but let’s not let the human connection become collateral damage.
1. Galea S, Merchant RM, Lurie N, et al. The mental health consequences of COVID-19 and physical distancing: the need for prevention and early intervention [published online April 10, 2020]. JAMA Intern Med. 2020. doi: 10.1001/jamainternmed.2020.1562.
1. Galea S, Merchant RM, Lurie N, et al. The mental health consequences of COVID-19 and physical distancing: the need for prevention and early intervention [published online April 10, 2020]. JAMA Intern Med. 2020. doi: 10.1001/jamainternmed.2020.1562.
Neuropsychiatric manifestations of COVID-19
On March 11, 2020, the World Health Organization declared that coronavirus disease 2019 (COVID-19) was a pandemic.1 As of mid-May 2020, the illness had claimed more than 316,000 lives worldwide.2 The main symptoms of the respiratory illness caused by COVID-19 are fever, dry cough, and shortness of breath. However, disorders of consciousness also have been reported, especially in patients who succumb to the illness.3 In fact, approximately one-third of hospitalized COVID-19 patients experience neurologic symptoms.4 Although the most common of these symptoms are dizziness, headache, and loss of smell and taste, patients with more severe cases can experience acute cerebrovascular diseases and impaired consciousness.4 As such, psychiatrists assessing confusion should include COVID-19 in their differential diagnosis as a potential cause of altered mental status.
How COVID-19 might affect the CNS
Although primarily considered a respiratory illness, COVID-19 also may have neurotropic potential. The virus that causes COVID-19, severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2), is a beta-coronavirus. Two other highly pathogenic coronaviruses—SARS-CoV-1 and Middle East respiratory syndrome–related coronavirus (MERS-CoV)—are also beta-coronaviruses, and both have been reported to invade the CNS in some patients.5 These viruses are thought to invade cells via angiotensin-converting enzyme 2 (ACE2) receptors.6 These receptors are located on the epithelial cells of the respiratory and gastrointestinal (GI) tracts, but also are expressed in certain areas of the brain.7 Transmission to the brain could occur through various routes. However, the clinical symptom of loss of smell and taste hints to possible transmission of the virus from nasal cells to the olfactory bulb via trans-synaptic transmission in olfactory neurons.5,8,9
Immune injury via systemic inflammation is another proposed mechanism for nervous system damage.8,9 This has been described as “cytokine storm syndrome” and provides support to the role of immunotherapy in COVID-19 patients.10 Such inflammation has been long hypothesized as a contributor to psychiatric illnesses, especially neurocognitive disorders.11,12
Neuropsychiatric complications of COVID-19
Disorders of consciousness were identified early as a symptom of COVID-19.3 Subsequent studies and case reports have confirmed impaired consciousness as a possible symptom of COVID-19.4 The first case of encephalitis secondary to COVID-19 was reported by Chinese media on March 5, 2020 in Beijing, China.13 Subsequently, cases of encephalopathy secondary to COVID-19 have been reported in the United States. A 74-year-old man in Boca Raton, Florida who had recently returned from the Netherlands presented with altered mental status and was confirmed positive for COVID-19.14 A female airline worker in her late 50s who presented with altered mental status and tested positive for COVID-19 was found on imaging to have acute hemorrhagic necrotizing encephalopathy.15 There also have been cases of patients with confirmed COVID-19 who initially presented with complaints of seizures16 and Guillain-Barré syndrome.17 As such, neuropsychiatric complications of COVID-19 are being increasingly recognized and are important to consider during psychiatric assessments.
Consider COVID-19 when assessing altered mental status
Psychiatrists are often consulted to assess patients with impaired consciousness, mental status changes, or confusion. Acute changes to mentation raise concern for delirium. In fact, delirium should always be ruled out when assessing new psychiatric symptoms. The astute psychiatrist is aware of the myriad of medical contributors to delirium. However, because knowledge of COVID-19 is in its infancy, it can be easy to overlook this virus as a potential contributor to delirium. Even patients whose confusion seems to be more in line with a major neurocognitive disorder should be evaluated for COVID-19, because the sudden onset of cognitive impairment may be due to hypoxia, inflammatory damage, or cerebrovascular changes secondary to infection with the virus or its respiratory complications, such as acute respiratory distress syndrome (ARDS).18
The most obvious clues to the possible presence of COVID-19 in a patient who is confused would be fever, dry cough, and shortness of breath. Because ACE2 receptors are also located in the GI tract, nausea, vomiting, and diarrhea also are possible. However, patients who are confused may be poor historians, demonstrating behavioral symptoms that might make physical assessments challenging, or simply may be pre- or asymptomatic carriers of the virus. Hence, a thorough review of the patient’s history and collateral information is invaluable. A recent history of travel or contact with COVID-19–positive individuals should raise suspicion for viral infection. A patient who mentions a loss of taste or smell would also alert the psychiatrist to the possibility of COVID-19. A patient might not directly state this information, but may mention that he/she has been eating less or has not been disturbed by odors. Neuroimaging can be useful because patients with severe cases are at increased risk for acute cerebrovascular diseases.4 Also, ordering a chest CT may prove helpful because this testing is highly sensitive for COVID-19.19 If there is sufficient clinical evidence to suspect viral infection, testing for COVID-19 should be performed immediately.
It is important to be vigilant for the possibility of COVID-19 infection in patients who present with confusion. Because the virus is highly contagious, the threshold for COVID-19 testing should be low. Viral infection in patients can manifest in ways other than classic respiratory symptoms. Psychiatrists should be aware of COVID-19’s potential to invade the CNS and cause neuropsychiatric symptoms. When assessing confusion in any setting, the clinical and historical clues for COVID-19 should be kept in mind. This will allow patients with COVID-19 to be quickly diagnosed to initiate appropriate management and minimize progression of the illness. Additionally, this will allow for efficient quarantine of the patient to prevent the spread of the virus to others. As such, psychiatrists can play an important role in containing this virus and resolving the COVID-19 pandemic.
Continue to: Bottom Line
Bottom Line
Although primarily considered a respiratory illness, coronavirus disease 2019 (COVID-19) also may have the potential to invade the CNS and cause neuropsychiatric symptoms, such as impaired consciousness, encephalitis, or a loss of taste or smell. When assessing a patient who presents with confusion, be vigilant for the possibility of COVID-19.
Related Resources
- American Psychiatry Association. APA coronavirus resources. https://www.psychiatry.org/psychiatrists/covid-19-coronavirus#psych.
- Troyer EA, Kohn JN, Hong S. Are we facing a crashing wave of neuropsychiatric sequelae of COVID-19? Neuropsychiatric symptoms and potential immunologic mechanisms. Brain Behav Immun. 2020;S0889-1591(20)30489-X. doi: 10.1016/j.bbi.2020.04.027.
1. World Health Organization. Rolling updates on coronavirus disease (COVID-19). https://www.who.int/emergencies/diseases/novel-coronavirus-2019/events-as-they-happen. Updated May 1, 2020. Accessed May 4, 2020.
2. John Hopkins University. Coronavirus resource center. World map. https://coronavirus.jhu.edu/map.html. Accessed May 4, 2020.
3. Chen T, Wu D, Chen H, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. doi: 10.1136/bmj.m1091.
4. Mao L, Wang M, Chen S, et al. Neurologic manifestations of hospitalized patients with COVID-19 in Wuhan, China: a retrospective case series study [published online February 25, 2020]. JAMA Neurol. 2020;e201127. doi: 10.1101/2020.02.22.20026500.
5. Li YC, Bai WZ, Hashikawa T. The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients [published online February 27, 2020]. J Med Virol. 2020;92(6). doi: 10.1002/jmv.25728.
6. Baig AM, Khaleeq A, Ali E, et al. Evidence of the COVID-19 virus targeting the CNS: tissue distribution, host-virus interaction, and proposed neurotropic mechanisms. ACS Chem Neurosci. 2020;11(7):995-998.
7. Xia H, Lazartigues E. Angiotensin-converting enzyme 2: central regulator for cardiovascular function. Curr Hypertens Rep. 2010;12(3):170-175.
8. Steardo L, Steardo L Jr, Zorec R, et al. Neuroinfection may contribute to pathophysiology and clinical manifestations of COVID-19 [published online March 29, 2020]. Acta Physiol (Oxf). 2020;e13473. doi: 10.1111/apha.13473.
9. Wu Y, Xu X, Chen Z, et al. Nervous system involvement after infection with COVID-19 and other coronaviruses [published online March 30, 2020]. Brain Behav Immun. 2020;S0889-1591(20)30357-3. doi: 10.1016/j.bbi.2020.03.031.
10. Mehta P, McAuley DF, Brown M, et al; HLH Across Specialty Collaboration, UK. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033-1034.
11. McNeil JB, Hughes CG, Girard T, et al. Plasma biomarkers of inflammation, coagulation, and brain injury as predictors of delirium duration in older hospitalized patients. PLoS One. 2019;14(12):e0226412. doi: 10.1371/journal.pone.0226412.
12. Heneka MT, Carson MJ, Khoury JE, et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015;14(4):388‐405.
13. Beijing hospital confirms nervous system infections by novel coronavirus. XINHUANET. http://www.xinhuanet.com/english/2020-03/05/c_138846529.htm. Published May 3, 2020. Accessed May 4, 2020.
14. Filatov A, Sharma P, Hindi F, et al. Neurological complications of coronavirus disease (COVID-19): encephalopathy. Cureus. 2020;12(3):e7352. doi: 10.7759/cureus.7352.
15. Poyiadji N, Shahin G, Noujaim D, et al. COVID-19-associated acute hemorrhagic necrotizing encephalopathy: CT and MRI features [published online March 31, 2020]. Radiology. 2020;201187. doi: 10.1148/radiol.2020201187.
16. Karimi N, Razavi AS, Rouhani N. Frequent convulsive seizures in an adult patient with COVID-19: a case report. Iran Red Crescent Med J. 2020;22(3):e102828. doi: 10.5812/ircmj.102828.
17. Zhao H, Shen D, Zhou H, et al. Guillain-Barré syndrome associated with SARS-CoV-2 infection: causality or coincidence? Lancet Neurol. 2020;19(5):383-384.
18. Sasannejad C, Ely EW, Lahiri S. Long-term cognitive impairment after acute respiratory distress syndrome: a review of clinical impact and pathophysiological mechanisms. Crit Care. 2019;23(1):352.
19. Fang Y, Zhang H, Xie J, et al. Sensitivity of chest CT for COVID-19: comparison to RT-PCR [published online February 19, 2020]. Radiology. 2020;200432. doi: 10.1148/radiol.2020200432.
On March 11, 2020, the World Health Organization declared that coronavirus disease 2019 (COVID-19) was a pandemic.1 As of mid-May 2020, the illness had claimed more than 316,000 lives worldwide.2 The main symptoms of the respiratory illness caused by COVID-19 are fever, dry cough, and shortness of breath. However, disorders of consciousness also have been reported, especially in patients who succumb to the illness.3 In fact, approximately one-third of hospitalized COVID-19 patients experience neurologic symptoms.4 Although the most common of these symptoms are dizziness, headache, and loss of smell and taste, patients with more severe cases can experience acute cerebrovascular diseases and impaired consciousness.4 As such, psychiatrists assessing confusion should include COVID-19 in their differential diagnosis as a potential cause of altered mental status.
How COVID-19 might affect the CNS
Although primarily considered a respiratory illness, COVID-19 also may have neurotropic potential. The virus that causes COVID-19, severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2), is a beta-coronavirus. Two other highly pathogenic coronaviruses—SARS-CoV-1 and Middle East respiratory syndrome–related coronavirus (MERS-CoV)—are also beta-coronaviruses, and both have been reported to invade the CNS in some patients.5 These viruses are thought to invade cells via angiotensin-converting enzyme 2 (ACE2) receptors.6 These receptors are located on the epithelial cells of the respiratory and gastrointestinal (GI) tracts, but also are expressed in certain areas of the brain.7 Transmission to the brain could occur through various routes. However, the clinical symptom of loss of smell and taste hints to possible transmission of the virus from nasal cells to the olfactory bulb via trans-synaptic transmission in olfactory neurons.5,8,9
Immune injury via systemic inflammation is another proposed mechanism for nervous system damage.8,9 This has been described as “cytokine storm syndrome” and provides support to the role of immunotherapy in COVID-19 patients.10 Such inflammation has been long hypothesized as a contributor to psychiatric illnesses, especially neurocognitive disorders.11,12
Neuropsychiatric complications of COVID-19
Disorders of consciousness were identified early as a symptom of COVID-19.3 Subsequent studies and case reports have confirmed impaired consciousness as a possible symptom of COVID-19.4 The first case of encephalitis secondary to COVID-19 was reported by Chinese media on March 5, 2020 in Beijing, China.13 Subsequently, cases of encephalopathy secondary to COVID-19 have been reported in the United States. A 74-year-old man in Boca Raton, Florida who had recently returned from the Netherlands presented with altered mental status and was confirmed positive for COVID-19.14 A female airline worker in her late 50s who presented with altered mental status and tested positive for COVID-19 was found on imaging to have acute hemorrhagic necrotizing encephalopathy.15 There also have been cases of patients with confirmed COVID-19 who initially presented with complaints of seizures16 and Guillain-Barré syndrome.17 As such, neuropsychiatric complications of COVID-19 are being increasingly recognized and are important to consider during psychiatric assessments.
Consider COVID-19 when assessing altered mental status
Psychiatrists are often consulted to assess patients with impaired consciousness, mental status changes, or confusion. Acute changes to mentation raise concern for delirium. In fact, delirium should always be ruled out when assessing new psychiatric symptoms. The astute psychiatrist is aware of the myriad of medical contributors to delirium. However, because knowledge of COVID-19 is in its infancy, it can be easy to overlook this virus as a potential contributor to delirium. Even patients whose confusion seems to be more in line with a major neurocognitive disorder should be evaluated for COVID-19, because the sudden onset of cognitive impairment may be due to hypoxia, inflammatory damage, or cerebrovascular changes secondary to infection with the virus or its respiratory complications, such as acute respiratory distress syndrome (ARDS).18
The most obvious clues to the possible presence of COVID-19 in a patient who is confused would be fever, dry cough, and shortness of breath. Because ACE2 receptors are also located in the GI tract, nausea, vomiting, and diarrhea also are possible. However, patients who are confused may be poor historians, demonstrating behavioral symptoms that might make physical assessments challenging, or simply may be pre- or asymptomatic carriers of the virus. Hence, a thorough review of the patient’s history and collateral information is invaluable. A recent history of travel or contact with COVID-19–positive individuals should raise suspicion for viral infection. A patient who mentions a loss of taste or smell would also alert the psychiatrist to the possibility of COVID-19. A patient might not directly state this information, but may mention that he/she has been eating less or has not been disturbed by odors. Neuroimaging can be useful because patients with severe cases are at increased risk for acute cerebrovascular diseases.4 Also, ordering a chest CT may prove helpful because this testing is highly sensitive for COVID-19.19 If there is sufficient clinical evidence to suspect viral infection, testing for COVID-19 should be performed immediately.
It is important to be vigilant for the possibility of COVID-19 infection in patients who present with confusion. Because the virus is highly contagious, the threshold for COVID-19 testing should be low. Viral infection in patients can manifest in ways other than classic respiratory symptoms. Psychiatrists should be aware of COVID-19’s potential to invade the CNS and cause neuropsychiatric symptoms. When assessing confusion in any setting, the clinical and historical clues for COVID-19 should be kept in mind. This will allow patients with COVID-19 to be quickly diagnosed to initiate appropriate management and minimize progression of the illness. Additionally, this will allow for efficient quarantine of the patient to prevent the spread of the virus to others. As such, psychiatrists can play an important role in containing this virus and resolving the COVID-19 pandemic.
Continue to: Bottom Line
Bottom Line
Although primarily considered a respiratory illness, coronavirus disease 2019 (COVID-19) also may have the potential to invade the CNS and cause neuropsychiatric symptoms, such as impaired consciousness, encephalitis, or a loss of taste or smell. When assessing a patient who presents with confusion, be vigilant for the possibility of COVID-19.
Related Resources
- American Psychiatry Association. APA coronavirus resources. https://www.psychiatry.org/psychiatrists/covid-19-coronavirus#psych.
- Troyer EA, Kohn JN, Hong S. Are we facing a crashing wave of neuropsychiatric sequelae of COVID-19? Neuropsychiatric symptoms and potential immunologic mechanisms. Brain Behav Immun. 2020;S0889-1591(20)30489-X. doi: 10.1016/j.bbi.2020.04.027.
On March 11, 2020, the World Health Organization declared that coronavirus disease 2019 (COVID-19) was a pandemic.1 As of mid-May 2020, the illness had claimed more than 316,000 lives worldwide.2 The main symptoms of the respiratory illness caused by COVID-19 are fever, dry cough, and shortness of breath. However, disorders of consciousness also have been reported, especially in patients who succumb to the illness.3 In fact, approximately one-third of hospitalized COVID-19 patients experience neurologic symptoms.4 Although the most common of these symptoms are dizziness, headache, and loss of smell and taste, patients with more severe cases can experience acute cerebrovascular diseases and impaired consciousness.4 As such, psychiatrists assessing confusion should include COVID-19 in their differential diagnosis as a potential cause of altered mental status.
How COVID-19 might affect the CNS
Although primarily considered a respiratory illness, COVID-19 also may have neurotropic potential. The virus that causes COVID-19, severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2), is a beta-coronavirus. Two other highly pathogenic coronaviruses—SARS-CoV-1 and Middle East respiratory syndrome–related coronavirus (MERS-CoV)—are also beta-coronaviruses, and both have been reported to invade the CNS in some patients.5 These viruses are thought to invade cells via angiotensin-converting enzyme 2 (ACE2) receptors.6 These receptors are located on the epithelial cells of the respiratory and gastrointestinal (GI) tracts, but also are expressed in certain areas of the brain.7 Transmission to the brain could occur through various routes. However, the clinical symptom of loss of smell and taste hints to possible transmission of the virus from nasal cells to the olfactory bulb via trans-synaptic transmission in olfactory neurons.5,8,9
Immune injury via systemic inflammation is another proposed mechanism for nervous system damage.8,9 This has been described as “cytokine storm syndrome” and provides support to the role of immunotherapy in COVID-19 patients.10 Such inflammation has been long hypothesized as a contributor to psychiatric illnesses, especially neurocognitive disorders.11,12
Neuropsychiatric complications of COVID-19
Disorders of consciousness were identified early as a symptom of COVID-19.3 Subsequent studies and case reports have confirmed impaired consciousness as a possible symptom of COVID-19.4 The first case of encephalitis secondary to COVID-19 was reported by Chinese media on March 5, 2020 in Beijing, China.13 Subsequently, cases of encephalopathy secondary to COVID-19 have been reported in the United States. A 74-year-old man in Boca Raton, Florida who had recently returned from the Netherlands presented with altered mental status and was confirmed positive for COVID-19.14 A female airline worker in her late 50s who presented with altered mental status and tested positive for COVID-19 was found on imaging to have acute hemorrhagic necrotizing encephalopathy.15 There also have been cases of patients with confirmed COVID-19 who initially presented with complaints of seizures16 and Guillain-Barré syndrome.17 As such, neuropsychiatric complications of COVID-19 are being increasingly recognized and are important to consider during psychiatric assessments.
Consider COVID-19 when assessing altered mental status
Psychiatrists are often consulted to assess patients with impaired consciousness, mental status changes, or confusion. Acute changes to mentation raise concern for delirium. In fact, delirium should always be ruled out when assessing new psychiatric symptoms. The astute psychiatrist is aware of the myriad of medical contributors to delirium. However, because knowledge of COVID-19 is in its infancy, it can be easy to overlook this virus as a potential contributor to delirium. Even patients whose confusion seems to be more in line with a major neurocognitive disorder should be evaluated for COVID-19, because the sudden onset of cognitive impairment may be due to hypoxia, inflammatory damage, or cerebrovascular changes secondary to infection with the virus or its respiratory complications, such as acute respiratory distress syndrome (ARDS).18
The most obvious clues to the possible presence of COVID-19 in a patient who is confused would be fever, dry cough, and shortness of breath. Because ACE2 receptors are also located in the GI tract, nausea, vomiting, and diarrhea also are possible. However, patients who are confused may be poor historians, demonstrating behavioral symptoms that might make physical assessments challenging, or simply may be pre- or asymptomatic carriers of the virus. Hence, a thorough review of the patient’s history and collateral information is invaluable. A recent history of travel or contact with COVID-19–positive individuals should raise suspicion for viral infection. A patient who mentions a loss of taste or smell would also alert the psychiatrist to the possibility of COVID-19. A patient might not directly state this information, but may mention that he/she has been eating less or has not been disturbed by odors. Neuroimaging can be useful because patients with severe cases are at increased risk for acute cerebrovascular diseases.4 Also, ordering a chest CT may prove helpful because this testing is highly sensitive for COVID-19.19 If there is sufficient clinical evidence to suspect viral infection, testing for COVID-19 should be performed immediately.
It is important to be vigilant for the possibility of COVID-19 infection in patients who present with confusion. Because the virus is highly contagious, the threshold for COVID-19 testing should be low. Viral infection in patients can manifest in ways other than classic respiratory symptoms. Psychiatrists should be aware of COVID-19’s potential to invade the CNS and cause neuropsychiatric symptoms. When assessing confusion in any setting, the clinical and historical clues for COVID-19 should be kept in mind. This will allow patients with COVID-19 to be quickly diagnosed to initiate appropriate management and minimize progression of the illness. Additionally, this will allow for efficient quarantine of the patient to prevent the spread of the virus to others. As such, psychiatrists can play an important role in containing this virus and resolving the COVID-19 pandemic.
Continue to: Bottom Line
Bottom Line
Although primarily considered a respiratory illness, coronavirus disease 2019 (COVID-19) also may have the potential to invade the CNS and cause neuropsychiatric symptoms, such as impaired consciousness, encephalitis, or a loss of taste or smell. When assessing a patient who presents with confusion, be vigilant for the possibility of COVID-19.
Related Resources
- American Psychiatry Association. APA coronavirus resources. https://www.psychiatry.org/psychiatrists/covid-19-coronavirus#psych.
- Troyer EA, Kohn JN, Hong S. Are we facing a crashing wave of neuropsychiatric sequelae of COVID-19? Neuropsychiatric symptoms and potential immunologic mechanisms. Brain Behav Immun. 2020;S0889-1591(20)30489-X. doi: 10.1016/j.bbi.2020.04.027.
1. World Health Organization. Rolling updates on coronavirus disease (COVID-19). https://www.who.int/emergencies/diseases/novel-coronavirus-2019/events-as-they-happen. Updated May 1, 2020. Accessed May 4, 2020.
2. John Hopkins University. Coronavirus resource center. World map. https://coronavirus.jhu.edu/map.html. Accessed May 4, 2020.
3. Chen T, Wu D, Chen H, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. doi: 10.1136/bmj.m1091.
4. Mao L, Wang M, Chen S, et al. Neurologic manifestations of hospitalized patients with COVID-19 in Wuhan, China: a retrospective case series study [published online February 25, 2020]. JAMA Neurol. 2020;e201127. doi: 10.1101/2020.02.22.20026500.
5. Li YC, Bai WZ, Hashikawa T. The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients [published online February 27, 2020]. J Med Virol. 2020;92(6). doi: 10.1002/jmv.25728.
6. Baig AM, Khaleeq A, Ali E, et al. Evidence of the COVID-19 virus targeting the CNS: tissue distribution, host-virus interaction, and proposed neurotropic mechanisms. ACS Chem Neurosci. 2020;11(7):995-998.
7. Xia H, Lazartigues E. Angiotensin-converting enzyme 2: central regulator for cardiovascular function. Curr Hypertens Rep. 2010;12(3):170-175.
8. Steardo L, Steardo L Jr, Zorec R, et al. Neuroinfection may contribute to pathophysiology and clinical manifestations of COVID-19 [published online March 29, 2020]. Acta Physiol (Oxf). 2020;e13473. doi: 10.1111/apha.13473.
9. Wu Y, Xu X, Chen Z, et al. Nervous system involvement after infection with COVID-19 and other coronaviruses [published online March 30, 2020]. Brain Behav Immun. 2020;S0889-1591(20)30357-3. doi: 10.1016/j.bbi.2020.03.031.
10. Mehta P, McAuley DF, Brown M, et al; HLH Across Specialty Collaboration, UK. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033-1034.
11. McNeil JB, Hughes CG, Girard T, et al. Plasma biomarkers of inflammation, coagulation, and brain injury as predictors of delirium duration in older hospitalized patients. PLoS One. 2019;14(12):e0226412. doi: 10.1371/journal.pone.0226412.
12. Heneka MT, Carson MJ, Khoury JE, et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015;14(4):388‐405.
13. Beijing hospital confirms nervous system infections by novel coronavirus. XINHUANET. http://www.xinhuanet.com/english/2020-03/05/c_138846529.htm. Published May 3, 2020. Accessed May 4, 2020.
14. Filatov A, Sharma P, Hindi F, et al. Neurological complications of coronavirus disease (COVID-19): encephalopathy. Cureus. 2020;12(3):e7352. doi: 10.7759/cureus.7352.
15. Poyiadji N, Shahin G, Noujaim D, et al. COVID-19-associated acute hemorrhagic necrotizing encephalopathy: CT and MRI features [published online March 31, 2020]. Radiology. 2020;201187. doi: 10.1148/radiol.2020201187.
16. Karimi N, Razavi AS, Rouhani N. Frequent convulsive seizures in an adult patient with COVID-19: a case report. Iran Red Crescent Med J. 2020;22(3):e102828. doi: 10.5812/ircmj.102828.
17. Zhao H, Shen D, Zhou H, et al. Guillain-Barré syndrome associated with SARS-CoV-2 infection: causality or coincidence? Lancet Neurol. 2020;19(5):383-384.
18. Sasannejad C, Ely EW, Lahiri S. Long-term cognitive impairment after acute respiratory distress syndrome: a review of clinical impact and pathophysiological mechanisms. Crit Care. 2019;23(1):352.
19. Fang Y, Zhang H, Xie J, et al. Sensitivity of chest CT for COVID-19: comparison to RT-PCR [published online February 19, 2020]. Radiology. 2020;200432. doi: 10.1148/radiol.2020200432.
1. World Health Organization. Rolling updates on coronavirus disease (COVID-19). https://www.who.int/emergencies/diseases/novel-coronavirus-2019/events-as-they-happen. Updated May 1, 2020. Accessed May 4, 2020.
2. John Hopkins University. Coronavirus resource center. World map. https://coronavirus.jhu.edu/map.html. Accessed May 4, 2020.
3. Chen T, Wu D, Chen H, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. doi: 10.1136/bmj.m1091.
4. Mao L, Wang M, Chen S, et al. Neurologic manifestations of hospitalized patients with COVID-19 in Wuhan, China: a retrospective case series study [published online February 25, 2020]. JAMA Neurol. 2020;e201127. doi: 10.1101/2020.02.22.20026500.
5. Li YC, Bai WZ, Hashikawa T. The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients [published online February 27, 2020]. J Med Virol. 2020;92(6). doi: 10.1002/jmv.25728.
6. Baig AM, Khaleeq A, Ali E, et al. Evidence of the COVID-19 virus targeting the CNS: tissue distribution, host-virus interaction, and proposed neurotropic mechanisms. ACS Chem Neurosci. 2020;11(7):995-998.
7. Xia H, Lazartigues E. Angiotensin-converting enzyme 2: central regulator for cardiovascular function. Curr Hypertens Rep. 2010;12(3):170-175.
8. Steardo L, Steardo L Jr, Zorec R, et al. Neuroinfection may contribute to pathophysiology and clinical manifestations of COVID-19 [published online March 29, 2020]. Acta Physiol (Oxf). 2020;e13473. doi: 10.1111/apha.13473.
9. Wu Y, Xu X, Chen Z, et al. Nervous system involvement after infection with COVID-19 and other coronaviruses [published online March 30, 2020]. Brain Behav Immun. 2020;S0889-1591(20)30357-3. doi: 10.1016/j.bbi.2020.03.031.
10. Mehta P, McAuley DF, Brown M, et al; HLH Across Specialty Collaboration, UK. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033-1034.
11. McNeil JB, Hughes CG, Girard T, et al. Plasma biomarkers of inflammation, coagulation, and brain injury as predictors of delirium duration in older hospitalized patients. PLoS One. 2019;14(12):e0226412. doi: 10.1371/journal.pone.0226412.
12. Heneka MT, Carson MJ, Khoury JE, et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015;14(4):388‐405.
13. Beijing hospital confirms nervous system infections by novel coronavirus. XINHUANET. http://www.xinhuanet.com/english/2020-03/05/c_138846529.htm. Published May 3, 2020. Accessed May 4, 2020.
14. Filatov A, Sharma P, Hindi F, et al. Neurological complications of coronavirus disease (COVID-19): encephalopathy. Cureus. 2020;12(3):e7352. doi: 10.7759/cureus.7352.
15. Poyiadji N, Shahin G, Noujaim D, et al. COVID-19-associated acute hemorrhagic necrotizing encephalopathy: CT and MRI features [published online March 31, 2020]. Radiology. 2020;201187. doi: 10.1148/radiol.2020201187.
16. Karimi N, Razavi AS, Rouhani N. Frequent convulsive seizures in an adult patient with COVID-19: a case report. Iran Red Crescent Med J. 2020;22(3):e102828. doi: 10.5812/ircmj.102828.
17. Zhao H, Shen D, Zhou H, et al. Guillain-Barré syndrome associated with SARS-CoV-2 infection: causality or coincidence? Lancet Neurol. 2020;19(5):383-384.
18. Sasannejad C, Ely EW, Lahiri S. Long-term cognitive impairment after acute respiratory distress syndrome: a review of clinical impact and pathophysiological mechanisms. Crit Care. 2019;23(1):352.
19. Fang Y, Zhang H, Xie J, et al. Sensitivity of chest CT for COVID-19: comparison to RT-PCR [published online February 19, 2020]. Radiology. 2020;200432. doi: 10.1148/radiol.2020200432.
Newer anticoagulants linked to lower fracture risk in AFib
The direct oral anticoagulant (DOAC) drugs apixaban, dabigatran, and rivaroxaban are associated with a lower risk of osteoporotic fracture than is warfarin in patients with atrial fibrillation (AFib), according to a new retrospective analysis.
There was no difference in risk between individual DOAC medications.
The study drew from an EHR database of the Hong Kong Hospital Authority. It was led by Wallis C.Y. Lau, PhD, of the University of Hong Kong and appeared online May 19 in Annals of Internal Medicine.
Warfarin is suspected to contribute to osteoporotic fracturing in AFib patients, but previous studies returned mixed results. The more recently introduced DOACs were not tested for fracture risks, and it hasn’t been determined if individual DOACs have different risks. The question is even more important in AFib, in which patients are older and often have comorbidities that could predispose them to fractures.
The study included 23,515 patients with AFib who used anticoagulants. 3,241 used apixaban, 6,867 dabigatran, 3,866 rivaroxaban, and 9,541 used warfarin. The median follow-up was 423 days.
According to Cox proportional hazards model analyses, DOAC use was associated with fewer fractures than was warfarin (hazard ratio for apixaban vs. warfarin, 0.62; 95% confidence interval, 0.41-0.94; HR for dabigatran, 0.65; 95% CI, 0.49-0.86; HR for rivaroxaban, 0.52; 95% CI, 0.37-0.73). Subanalyses in men and women showed similar results (P for interaction >.05).
Head-to-head comparisons between individual DOACs yielded no statistically significant differences in osteoporotic fracture risk.
Although the findings couldn’t absolutely rule out a difference in osteoporotic fracture risk between different DOACs, the authors argue that any clinical significance would likely be small.
“Given the supportive evidence from experimental settings, findings from our study using clinical data, and the indirect evidence provided by the previous meta-analysis of randomized, controlled trials, there exists a compelling case for evaluating whether the risk for osteoporotic fractures should be considered at the point of prescribing an oral anticoagulant to minimize fracture risk,” the authors wrote.
The study is limited by the potential for residual confounding, the investigators noted.
The study was funded by the University of Hong Kong and University College London Strategic Partnership Fund.
SOURCE: Lau WCY et al. Ann Intern Med. 2020 May 19. doi: 10.7326/M19-3671.
The direct oral anticoagulant (DOAC) drugs apixaban, dabigatran, and rivaroxaban are associated with a lower risk of osteoporotic fracture than is warfarin in patients with atrial fibrillation (AFib), according to a new retrospective analysis.
There was no difference in risk between individual DOAC medications.
The study drew from an EHR database of the Hong Kong Hospital Authority. It was led by Wallis C.Y. Lau, PhD, of the University of Hong Kong and appeared online May 19 in Annals of Internal Medicine.
Warfarin is suspected to contribute to osteoporotic fracturing in AFib patients, but previous studies returned mixed results. The more recently introduced DOACs were not tested for fracture risks, and it hasn’t been determined if individual DOACs have different risks. The question is even more important in AFib, in which patients are older and often have comorbidities that could predispose them to fractures.
The study included 23,515 patients with AFib who used anticoagulants. 3,241 used apixaban, 6,867 dabigatran, 3,866 rivaroxaban, and 9,541 used warfarin. The median follow-up was 423 days.
According to Cox proportional hazards model analyses, DOAC use was associated with fewer fractures than was warfarin (hazard ratio for apixaban vs. warfarin, 0.62; 95% confidence interval, 0.41-0.94; HR for dabigatran, 0.65; 95% CI, 0.49-0.86; HR for rivaroxaban, 0.52; 95% CI, 0.37-0.73). Subanalyses in men and women showed similar results (P for interaction >.05).
Head-to-head comparisons between individual DOACs yielded no statistically significant differences in osteoporotic fracture risk.
Although the findings couldn’t absolutely rule out a difference in osteoporotic fracture risk between different DOACs, the authors argue that any clinical significance would likely be small.
“Given the supportive evidence from experimental settings, findings from our study using clinical data, and the indirect evidence provided by the previous meta-analysis of randomized, controlled trials, there exists a compelling case for evaluating whether the risk for osteoporotic fractures should be considered at the point of prescribing an oral anticoagulant to minimize fracture risk,” the authors wrote.
The study is limited by the potential for residual confounding, the investigators noted.
The study was funded by the University of Hong Kong and University College London Strategic Partnership Fund.
SOURCE: Lau WCY et al. Ann Intern Med. 2020 May 19. doi: 10.7326/M19-3671.
The direct oral anticoagulant (DOAC) drugs apixaban, dabigatran, and rivaroxaban are associated with a lower risk of osteoporotic fracture than is warfarin in patients with atrial fibrillation (AFib), according to a new retrospective analysis.
There was no difference in risk between individual DOAC medications.
The study drew from an EHR database of the Hong Kong Hospital Authority. It was led by Wallis C.Y. Lau, PhD, of the University of Hong Kong and appeared online May 19 in Annals of Internal Medicine.
Warfarin is suspected to contribute to osteoporotic fracturing in AFib patients, but previous studies returned mixed results. The more recently introduced DOACs were not tested for fracture risks, and it hasn’t been determined if individual DOACs have different risks. The question is even more important in AFib, in which patients are older and often have comorbidities that could predispose them to fractures.
The study included 23,515 patients with AFib who used anticoagulants. 3,241 used apixaban, 6,867 dabigatran, 3,866 rivaroxaban, and 9,541 used warfarin. The median follow-up was 423 days.
According to Cox proportional hazards model analyses, DOAC use was associated with fewer fractures than was warfarin (hazard ratio for apixaban vs. warfarin, 0.62; 95% confidence interval, 0.41-0.94; HR for dabigatran, 0.65; 95% CI, 0.49-0.86; HR for rivaroxaban, 0.52; 95% CI, 0.37-0.73). Subanalyses in men and women showed similar results (P for interaction >.05).
Head-to-head comparisons between individual DOACs yielded no statistically significant differences in osteoporotic fracture risk.
Although the findings couldn’t absolutely rule out a difference in osteoporotic fracture risk between different DOACs, the authors argue that any clinical significance would likely be small.
“Given the supportive evidence from experimental settings, findings from our study using clinical data, and the indirect evidence provided by the previous meta-analysis of randomized, controlled trials, there exists a compelling case for evaluating whether the risk for osteoporotic fractures should be considered at the point of prescribing an oral anticoagulant to minimize fracture risk,” the authors wrote.
The study is limited by the potential for residual confounding, the investigators noted.
The study was funded by the University of Hong Kong and University College London Strategic Partnership Fund.
SOURCE: Lau WCY et al. Ann Intern Med. 2020 May 19. doi: 10.7326/M19-3671.
FROM ANNALS OF INTERNAL MEDICINE
Patient-focused precautions, testing help blunt pandemic effects on heme-onc unit
Keeping hematologic oncology patients on their treatment regimens and caring for inpatients with hematologic malignancies remained “manageable” during the first 2 months of the COVID-19 pandemic at Levine Cancer Institute in Charlotte, N.C.
That level of manageability has partly been because a surge in cases so far hasn’t arrived at Levine or in most of the surrounding North Carolina and South Carolina communities it serves. As of May 15, 2020, the total number of confirmed and reported COVID-19 cases had reached about 19,000 in North Carolina, and just under 9,000 in South Carolina, out of a total population in the two states of close to 16 million. What’s happened instead at Levine Cancer Institute (LCI) has been a steady but low drumbeat of cases that, by mid-May 2020, totaled fewer than 10 patients with hematologic malignancies diagnosed with COVID-19.
“For a large system with multiple sites throughout North and South Carolina that saw 17,200 new patients in 2019 – including solid tumor, benign hematology, and malignant hematology patients – with 198,000 total patient visits, it is safe to say that we are off to a good start. However, we remain in the early throes of the pandemic and we will need to remain vigilant going forward,” said Peter Voorhees, MD, professor of medicine and director of Medical Operations and Outreach Services in LCI’s Department of Hematologic Oncology and Blood Disorders.
The limited effects to date of COVID-19 at LCI has been thanks to a regimen of great caution for preventing infections that’s been consistently conveyed to LCI patients from before the pandemic’s onset, liberal testing that started early, a proactive plan to defer and temporarily replace infusion care when medically appropriate, a novel staffing approach designed to minimize and contain potential staff outbreaks, and an early pivot to virtual patient contact when feasible.
COVID-19 has had limited penetration into the LCI case load because patients have, in general, “been very careful,” said Dr. Voorhees.
“My impression is that the incidence has been low partly because our patients, especially those with hematologic malignancies including those on active chemotherapy, were already getting warned to be cautious even before the coronavirus using distancing, masking, and meticulous hand hygiene,” he said in an interview that reviewed the steps LCI took starting in March to confront and manage the effects of the then-nascent pandemic. “Since we started screening asymptomatic patients in the inpatient and outpatient settings we have identified only one patient with COVID-19 infection, which supports the low rate of infection in our patient population thus far.”
Another key step was the launch of “robust” testing for the COVID-19 virus starting on March 9, using an in-house assay from LCI’s parent health system, Atrium Health, that delivered results within 24 hours. Testing became available at LCI “earlier than at many other health systems.” At first, testing was limited to patients or staff presenting with symptoms, but in the following weeks, it expanded to more patients, including those without symptoms who were scheduled for treatment at the apheresis center, cell donors and cell recipients, patients arriving for inpatient chemotherapy or cellular therapy, patients arriving from a skilled nursing facility or similar environments, and more recently, outpatient chemotherapy patients. “We’re now doing a lot of screening,” Dr. Voorhees said. “In general, screening has been well received because patients recognize that it’s for their own safety.”
Another piece of COVID-19 preparedness was a move toward technology as an alternative to face-to-face encounters between patients and staff. “We adopted virtual technology early.” When medically appropriate, they provided either video consultations with more tech-savvy patients or telephone-based virtual visits for patients who preferred a more familiar interface. As LCI starts the process of reentry for patients whose face-to-face encounters were deferred, virtual visits will remain an important facet of maintaining care while limiting exposure for appropriate patients and facilitating adequate space for social distancing in the clinics and infusion centers.
Atrium Health also launched a “virtual hospital” geared to intensified remote management of COVID-19 patients who aren’t sick enough for hospitalization. “People who test positive automatically enter the virtual hospital and have regular interactions with their team of providers,” with LCI providing additional support for their patients who get infected. Patients receive an equipment kit that lets them monitor and transmit their vital signs. The virtual hospital program also helps expedite personal needs like delivery of prescriptions and food. “It helps patients manage at home, and has been incredibly useful,” said Dr. Voorhees.
Perhaps the most challenging step LCI clinicians took to preclude a potential COVID-19 case surge was to review all patients receiving infusional therapy or planned cellular therapy and triage those who could potentially tolerate a temporary change to either an oral, at-home regimen or to a brief hold on their treatment. Some patients on maintenance, outpatient infusion-therapy regimens “expressed concern about coming to the clinic. We looked at the patients scheduled to come for infusions and decided which visits were essential and which were deferrable without disrupting care by briefly using a noninfusional approach,” said Dr. Voorhees. The number of patients who had their regimens modified or held was “relatively small,” and with the recent recognition that a surge of infections has not occurred, “we’re now rolling out cautious reentry of those patients back to their originally prescribed chemotherapy.”
In addition to concerns of exposure at infusion clinics, there are concerns about the heightened susceptibility of immunosuppressed hematologic oncology patients to COVID-19 and their risk for more severe infection. “Our view is that, if patients tested positive, continuing immunosuppressive treatment would likely be detrimental,” so when possible treatment is temporarily suspended and then resumed when the infection has cleared. “When patients test positive for a prolonged period, a decision to resume treatment must be in the best interests of the patient and weigh the benefits of resuming therapy against the risks of incurring a more severe infection by restarting potentially immunosuppressive therapy,” Dr. Voorhees said.
The enhanced risk that cancer patients face if they develop COVID-19 was documented in a recent review of 218 cancer patients hospitalized for COVID-19 during parts of March and April in a large New York health system. The results showed an overall mortality rate of 28%, including a 37% rate among 54 patients with hematologic malignancies and a 25% rate among 164 patients with solid tumors. The mortality rate “may not be quite as high as they reported because that depends on how many patients you test, but there is no question that patients with more comorbidities are at higher risk. Patients with active cancer on chemotherapy are a particularly vulnerable population, and many have expressed concerns about their vulnerability,” he observed.
For the few LCI patients who developed COVID-19 infection, the medical staff has had several therapeutic options they could match to each patient’s needs, with help from the Atrium Health infectious disease team. LCI and Atrium Health are participating in several COVID-19 clinical treatment trials, including an investigational convalescent plasma protocol spearheaded by the Mayo Clinic. They have also opened a randomized, phase 2 trial evaluating the safety and efficacy of selinexor (Xpovio), an oral drug that’s Food and Drug Administration approved for patients with multiple myeloma, for treatment of moderate or severe COVID-19 infection. Additional studies evaluating blockade of granulocyte-macrophage colony-stimulating factor, as well as inhaled antiviral therapy, have recently launched, and several additional studies are poised to open in the coming weeks.
The LCI and Atrium Health team also has a supply of the antiviral agent remdesivir as part of the FDA’s expanded access protocol and emergency use authorization. They also have a supply of and experience administering the interleukin-6 receptor inhibitor tocilizumab (Actemra), which showed some suggestion of efficacy in limited experience treating patients with severe or critical COVID-19 infections. Clinicians at LCI have not used the investigational and unproven agents hydroxychloroquine, chloroquine, and azithromycin to either prevent or treat COVID-19.
LCI also instituted measures to try to minimize the risk that staff members could become infected and transmit the virus while asymptomatic. Following conversations held early on with COVID-19–experienced health authorities in China and Italy, the patient-facing LCI staff split into two teams starting on March 23 that alternated responsibility for direct patient interactions every 2 weeks. When one of these teams was off from direct patient contact they continued to care for patients remotely through virtual technologies. The concept was that, if a staffer became infected while remaining asymptomatic during their contact with patients, their status would either become diagnosable or resolve during their 2 weeks away from seeing any patients. Perhaps in part because of this approach infections among staff members “have not been a big issue. We’ve had an incredibly low infection rate among the LCI staff,” Dr. Voorhees noted.
By mid-May, with the imminent threat of a sudden CODIV-19 surge moderated, heme-onc operations at LCI began to cautiously revert to more normal operations. “We’re continuing patient screening for signs and symptoms of COVID-19 infection, testing for asymptomatic infections, and requiring masking and social distancing in the clinics and hospitals, but we’re starting to slowly restore the number of patients at our clinics [virtual and face to face[ and infusion centers,” and the staff’s division into two teams ended. “The idea was to get past a surge and make sure our system was not overwhelmed. We anticipated a local surge in late April, but then it kept getting pushed back. Current projections are for the infection rate among LCI patients to remain low provided that community spread remains stable or, ideally, decreases.” The LCI infectious disease staff is closely monitoring infection rates for early recognition of an outbreak, with plans to follow any new cases with contact tracing. So far, the COVID-19 pandemic at LCI “has been very manageable,” Dr. Voorhees concluded.
“We’re now better positioned to deal with a case surge if it were to happen. We could resume the two-team approach, hospital-wide plans are now in place for a future surge, and we are now up and running with robust testing and inpatient and outpatient virtual technology. The first time, we were all learning on the fly.”
The LCI biostatistics team has been prospectively collecting the Institutes’s COVID-19 patient data, with plans to report their findings.
Dr. Voorhees has had financial relationships with Bristol-Myers Squibb/Celgene, Janssen, Novartis, and Oncopeptides, none of which are relevant to this article.
Keeping hematologic oncology patients on their treatment regimens and caring for inpatients with hematologic malignancies remained “manageable” during the first 2 months of the COVID-19 pandemic at Levine Cancer Institute in Charlotte, N.C.
That level of manageability has partly been because a surge in cases so far hasn’t arrived at Levine or in most of the surrounding North Carolina and South Carolina communities it serves. As of May 15, 2020, the total number of confirmed and reported COVID-19 cases had reached about 19,000 in North Carolina, and just under 9,000 in South Carolina, out of a total population in the two states of close to 16 million. What’s happened instead at Levine Cancer Institute (LCI) has been a steady but low drumbeat of cases that, by mid-May 2020, totaled fewer than 10 patients with hematologic malignancies diagnosed with COVID-19.
“For a large system with multiple sites throughout North and South Carolina that saw 17,200 new patients in 2019 – including solid tumor, benign hematology, and malignant hematology patients – with 198,000 total patient visits, it is safe to say that we are off to a good start. However, we remain in the early throes of the pandemic and we will need to remain vigilant going forward,” said Peter Voorhees, MD, professor of medicine and director of Medical Operations and Outreach Services in LCI’s Department of Hematologic Oncology and Blood Disorders.
The limited effects to date of COVID-19 at LCI has been thanks to a regimen of great caution for preventing infections that’s been consistently conveyed to LCI patients from before the pandemic’s onset, liberal testing that started early, a proactive plan to defer and temporarily replace infusion care when medically appropriate, a novel staffing approach designed to minimize and contain potential staff outbreaks, and an early pivot to virtual patient contact when feasible.
COVID-19 has had limited penetration into the LCI case load because patients have, in general, “been very careful,” said Dr. Voorhees.
“My impression is that the incidence has been low partly because our patients, especially those with hematologic malignancies including those on active chemotherapy, were already getting warned to be cautious even before the coronavirus using distancing, masking, and meticulous hand hygiene,” he said in an interview that reviewed the steps LCI took starting in March to confront and manage the effects of the then-nascent pandemic. “Since we started screening asymptomatic patients in the inpatient and outpatient settings we have identified only one patient with COVID-19 infection, which supports the low rate of infection in our patient population thus far.”
Another key step was the launch of “robust” testing for the COVID-19 virus starting on March 9, using an in-house assay from LCI’s parent health system, Atrium Health, that delivered results within 24 hours. Testing became available at LCI “earlier than at many other health systems.” At first, testing was limited to patients or staff presenting with symptoms, but in the following weeks, it expanded to more patients, including those without symptoms who were scheduled for treatment at the apheresis center, cell donors and cell recipients, patients arriving for inpatient chemotherapy or cellular therapy, patients arriving from a skilled nursing facility or similar environments, and more recently, outpatient chemotherapy patients. “We’re now doing a lot of screening,” Dr. Voorhees said. “In general, screening has been well received because patients recognize that it’s for their own safety.”
Another piece of COVID-19 preparedness was a move toward technology as an alternative to face-to-face encounters between patients and staff. “We adopted virtual technology early.” When medically appropriate, they provided either video consultations with more tech-savvy patients or telephone-based virtual visits for patients who preferred a more familiar interface. As LCI starts the process of reentry for patients whose face-to-face encounters were deferred, virtual visits will remain an important facet of maintaining care while limiting exposure for appropriate patients and facilitating adequate space for social distancing in the clinics and infusion centers.
Atrium Health also launched a “virtual hospital” geared to intensified remote management of COVID-19 patients who aren’t sick enough for hospitalization. “People who test positive automatically enter the virtual hospital and have regular interactions with their team of providers,” with LCI providing additional support for their patients who get infected. Patients receive an equipment kit that lets them monitor and transmit their vital signs. The virtual hospital program also helps expedite personal needs like delivery of prescriptions and food. “It helps patients manage at home, and has been incredibly useful,” said Dr. Voorhees.
Perhaps the most challenging step LCI clinicians took to preclude a potential COVID-19 case surge was to review all patients receiving infusional therapy or planned cellular therapy and triage those who could potentially tolerate a temporary change to either an oral, at-home regimen or to a brief hold on their treatment. Some patients on maintenance, outpatient infusion-therapy regimens “expressed concern about coming to the clinic. We looked at the patients scheduled to come for infusions and decided which visits were essential and which were deferrable without disrupting care by briefly using a noninfusional approach,” said Dr. Voorhees. The number of patients who had their regimens modified or held was “relatively small,” and with the recent recognition that a surge of infections has not occurred, “we’re now rolling out cautious reentry of those patients back to their originally prescribed chemotherapy.”
In addition to concerns of exposure at infusion clinics, there are concerns about the heightened susceptibility of immunosuppressed hematologic oncology patients to COVID-19 and their risk for more severe infection. “Our view is that, if patients tested positive, continuing immunosuppressive treatment would likely be detrimental,” so when possible treatment is temporarily suspended and then resumed when the infection has cleared. “When patients test positive for a prolonged period, a decision to resume treatment must be in the best interests of the patient and weigh the benefits of resuming therapy against the risks of incurring a more severe infection by restarting potentially immunosuppressive therapy,” Dr. Voorhees said.
The enhanced risk that cancer patients face if they develop COVID-19 was documented in a recent review of 218 cancer patients hospitalized for COVID-19 during parts of March and April in a large New York health system. The results showed an overall mortality rate of 28%, including a 37% rate among 54 patients with hematologic malignancies and a 25% rate among 164 patients with solid tumors. The mortality rate “may not be quite as high as they reported because that depends on how many patients you test, but there is no question that patients with more comorbidities are at higher risk. Patients with active cancer on chemotherapy are a particularly vulnerable population, and many have expressed concerns about their vulnerability,” he observed.
For the few LCI patients who developed COVID-19 infection, the medical staff has had several therapeutic options they could match to each patient’s needs, with help from the Atrium Health infectious disease team. LCI and Atrium Health are participating in several COVID-19 clinical treatment trials, including an investigational convalescent plasma protocol spearheaded by the Mayo Clinic. They have also opened a randomized, phase 2 trial evaluating the safety and efficacy of selinexor (Xpovio), an oral drug that’s Food and Drug Administration approved for patients with multiple myeloma, for treatment of moderate or severe COVID-19 infection. Additional studies evaluating blockade of granulocyte-macrophage colony-stimulating factor, as well as inhaled antiviral therapy, have recently launched, and several additional studies are poised to open in the coming weeks.
The LCI and Atrium Health team also has a supply of the antiviral agent remdesivir as part of the FDA’s expanded access protocol and emergency use authorization. They also have a supply of and experience administering the interleukin-6 receptor inhibitor tocilizumab (Actemra), which showed some suggestion of efficacy in limited experience treating patients with severe or critical COVID-19 infections. Clinicians at LCI have not used the investigational and unproven agents hydroxychloroquine, chloroquine, and azithromycin to either prevent or treat COVID-19.
LCI also instituted measures to try to minimize the risk that staff members could become infected and transmit the virus while asymptomatic. Following conversations held early on with COVID-19–experienced health authorities in China and Italy, the patient-facing LCI staff split into two teams starting on March 23 that alternated responsibility for direct patient interactions every 2 weeks. When one of these teams was off from direct patient contact they continued to care for patients remotely through virtual technologies. The concept was that, if a staffer became infected while remaining asymptomatic during their contact with patients, their status would either become diagnosable or resolve during their 2 weeks away from seeing any patients. Perhaps in part because of this approach infections among staff members “have not been a big issue. We’ve had an incredibly low infection rate among the LCI staff,” Dr. Voorhees noted.
By mid-May, with the imminent threat of a sudden CODIV-19 surge moderated, heme-onc operations at LCI began to cautiously revert to more normal operations. “We’re continuing patient screening for signs and symptoms of COVID-19 infection, testing for asymptomatic infections, and requiring masking and social distancing in the clinics and hospitals, but we’re starting to slowly restore the number of patients at our clinics [virtual and face to face[ and infusion centers,” and the staff’s division into two teams ended. “The idea was to get past a surge and make sure our system was not overwhelmed. We anticipated a local surge in late April, but then it kept getting pushed back. Current projections are for the infection rate among LCI patients to remain low provided that community spread remains stable or, ideally, decreases.” The LCI infectious disease staff is closely monitoring infection rates for early recognition of an outbreak, with plans to follow any new cases with contact tracing. So far, the COVID-19 pandemic at LCI “has been very manageable,” Dr. Voorhees concluded.
“We’re now better positioned to deal with a case surge if it were to happen. We could resume the two-team approach, hospital-wide plans are now in place for a future surge, and we are now up and running with robust testing and inpatient and outpatient virtual technology. The first time, we were all learning on the fly.”
The LCI biostatistics team has been prospectively collecting the Institutes’s COVID-19 patient data, with plans to report their findings.
Dr. Voorhees has had financial relationships with Bristol-Myers Squibb/Celgene, Janssen, Novartis, and Oncopeptides, none of which are relevant to this article.
Keeping hematologic oncology patients on their treatment regimens and caring for inpatients with hematologic malignancies remained “manageable” during the first 2 months of the COVID-19 pandemic at Levine Cancer Institute in Charlotte, N.C.
That level of manageability has partly been because a surge in cases so far hasn’t arrived at Levine or in most of the surrounding North Carolina and South Carolina communities it serves. As of May 15, 2020, the total number of confirmed and reported COVID-19 cases had reached about 19,000 in North Carolina, and just under 9,000 in South Carolina, out of a total population in the two states of close to 16 million. What’s happened instead at Levine Cancer Institute (LCI) has been a steady but low drumbeat of cases that, by mid-May 2020, totaled fewer than 10 patients with hematologic malignancies diagnosed with COVID-19.
“For a large system with multiple sites throughout North and South Carolina that saw 17,200 new patients in 2019 – including solid tumor, benign hematology, and malignant hematology patients – with 198,000 total patient visits, it is safe to say that we are off to a good start. However, we remain in the early throes of the pandemic and we will need to remain vigilant going forward,” said Peter Voorhees, MD, professor of medicine and director of Medical Operations and Outreach Services in LCI’s Department of Hematologic Oncology and Blood Disorders.
The limited effects to date of COVID-19 at LCI has been thanks to a regimen of great caution for preventing infections that’s been consistently conveyed to LCI patients from before the pandemic’s onset, liberal testing that started early, a proactive plan to defer and temporarily replace infusion care when medically appropriate, a novel staffing approach designed to minimize and contain potential staff outbreaks, and an early pivot to virtual patient contact when feasible.
COVID-19 has had limited penetration into the LCI case load because patients have, in general, “been very careful,” said Dr. Voorhees.
“My impression is that the incidence has been low partly because our patients, especially those with hematologic malignancies including those on active chemotherapy, were already getting warned to be cautious even before the coronavirus using distancing, masking, and meticulous hand hygiene,” he said in an interview that reviewed the steps LCI took starting in March to confront and manage the effects of the then-nascent pandemic. “Since we started screening asymptomatic patients in the inpatient and outpatient settings we have identified only one patient with COVID-19 infection, which supports the low rate of infection in our patient population thus far.”
Another key step was the launch of “robust” testing for the COVID-19 virus starting on March 9, using an in-house assay from LCI’s parent health system, Atrium Health, that delivered results within 24 hours. Testing became available at LCI “earlier than at many other health systems.” At first, testing was limited to patients or staff presenting with symptoms, but in the following weeks, it expanded to more patients, including those without symptoms who were scheduled for treatment at the apheresis center, cell donors and cell recipients, patients arriving for inpatient chemotherapy or cellular therapy, patients arriving from a skilled nursing facility or similar environments, and more recently, outpatient chemotherapy patients. “We’re now doing a lot of screening,” Dr. Voorhees said. “In general, screening has been well received because patients recognize that it’s for their own safety.”
Another piece of COVID-19 preparedness was a move toward technology as an alternative to face-to-face encounters between patients and staff. “We adopted virtual technology early.” When medically appropriate, they provided either video consultations with more tech-savvy patients or telephone-based virtual visits for patients who preferred a more familiar interface. As LCI starts the process of reentry for patients whose face-to-face encounters were deferred, virtual visits will remain an important facet of maintaining care while limiting exposure for appropriate patients and facilitating adequate space for social distancing in the clinics and infusion centers.
Atrium Health also launched a “virtual hospital” geared to intensified remote management of COVID-19 patients who aren’t sick enough for hospitalization. “People who test positive automatically enter the virtual hospital and have regular interactions with their team of providers,” with LCI providing additional support for their patients who get infected. Patients receive an equipment kit that lets them monitor and transmit their vital signs. The virtual hospital program also helps expedite personal needs like delivery of prescriptions and food. “It helps patients manage at home, and has been incredibly useful,” said Dr. Voorhees.
Perhaps the most challenging step LCI clinicians took to preclude a potential COVID-19 case surge was to review all patients receiving infusional therapy or planned cellular therapy and triage those who could potentially tolerate a temporary change to either an oral, at-home regimen or to a brief hold on their treatment. Some patients on maintenance, outpatient infusion-therapy regimens “expressed concern about coming to the clinic. We looked at the patients scheduled to come for infusions and decided which visits were essential and which were deferrable without disrupting care by briefly using a noninfusional approach,” said Dr. Voorhees. The number of patients who had their regimens modified or held was “relatively small,” and with the recent recognition that a surge of infections has not occurred, “we’re now rolling out cautious reentry of those patients back to their originally prescribed chemotherapy.”
In addition to concerns of exposure at infusion clinics, there are concerns about the heightened susceptibility of immunosuppressed hematologic oncology patients to COVID-19 and their risk for more severe infection. “Our view is that, if patients tested positive, continuing immunosuppressive treatment would likely be detrimental,” so when possible treatment is temporarily suspended and then resumed when the infection has cleared. “When patients test positive for a prolonged period, a decision to resume treatment must be in the best interests of the patient and weigh the benefits of resuming therapy against the risks of incurring a more severe infection by restarting potentially immunosuppressive therapy,” Dr. Voorhees said.
The enhanced risk that cancer patients face if they develop COVID-19 was documented in a recent review of 218 cancer patients hospitalized for COVID-19 during parts of March and April in a large New York health system. The results showed an overall mortality rate of 28%, including a 37% rate among 54 patients with hematologic malignancies and a 25% rate among 164 patients with solid tumors. The mortality rate “may not be quite as high as they reported because that depends on how many patients you test, but there is no question that patients with more comorbidities are at higher risk. Patients with active cancer on chemotherapy are a particularly vulnerable population, and many have expressed concerns about their vulnerability,” he observed.
For the few LCI patients who developed COVID-19 infection, the medical staff has had several therapeutic options they could match to each patient’s needs, with help from the Atrium Health infectious disease team. LCI and Atrium Health are participating in several COVID-19 clinical treatment trials, including an investigational convalescent plasma protocol spearheaded by the Mayo Clinic. They have also opened a randomized, phase 2 trial evaluating the safety and efficacy of selinexor (Xpovio), an oral drug that’s Food and Drug Administration approved for patients with multiple myeloma, for treatment of moderate or severe COVID-19 infection. Additional studies evaluating blockade of granulocyte-macrophage colony-stimulating factor, as well as inhaled antiviral therapy, have recently launched, and several additional studies are poised to open in the coming weeks.
The LCI and Atrium Health team also has a supply of the antiviral agent remdesivir as part of the FDA’s expanded access protocol and emergency use authorization. They also have a supply of and experience administering the interleukin-6 receptor inhibitor tocilizumab (Actemra), which showed some suggestion of efficacy in limited experience treating patients with severe or critical COVID-19 infections. Clinicians at LCI have not used the investigational and unproven agents hydroxychloroquine, chloroquine, and azithromycin to either prevent or treat COVID-19.
LCI also instituted measures to try to minimize the risk that staff members could become infected and transmit the virus while asymptomatic. Following conversations held early on with COVID-19–experienced health authorities in China and Italy, the patient-facing LCI staff split into two teams starting on March 23 that alternated responsibility for direct patient interactions every 2 weeks. When one of these teams was off from direct patient contact they continued to care for patients remotely through virtual technologies. The concept was that, if a staffer became infected while remaining asymptomatic during their contact with patients, their status would either become diagnosable or resolve during their 2 weeks away from seeing any patients. Perhaps in part because of this approach infections among staff members “have not been a big issue. We’ve had an incredibly low infection rate among the LCI staff,” Dr. Voorhees noted.
By mid-May, with the imminent threat of a sudden CODIV-19 surge moderated, heme-onc operations at LCI began to cautiously revert to more normal operations. “We’re continuing patient screening for signs and symptoms of COVID-19 infection, testing for asymptomatic infections, and requiring masking and social distancing in the clinics and hospitals, but we’re starting to slowly restore the number of patients at our clinics [virtual and face to face[ and infusion centers,” and the staff’s division into two teams ended. “The idea was to get past a surge and make sure our system was not overwhelmed. We anticipated a local surge in late April, but then it kept getting pushed back. Current projections are for the infection rate among LCI patients to remain low provided that community spread remains stable or, ideally, decreases.” The LCI infectious disease staff is closely monitoring infection rates for early recognition of an outbreak, with plans to follow any new cases with contact tracing. So far, the COVID-19 pandemic at LCI “has been very manageable,” Dr. Voorhees concluded.
“We’re now better positioned to deal with a case surge if it were to happen. We could resume the two-team approach, hospital-wide plans are now in place for a future surge, and we are now up and running with robust testing and inpatient and outpatient virtual technology. The first time, we were all learning on the fly.”
The LCI biostatistics team has been prospectively collecting the Institutes’s COVID-19 patient data, with plans to report their findings.
Dr. Voorhees has had financial relationships with Bristol-Myers Squibb/Celgene, Janssen, Novartis, and Oncopeptides, none of which are relevant to this article.
Psychiatrists’ pay increases, most happy with income, career
Psychiatrists continue to rank close to the bottom of the compensation ladder, but they made more this year than last year and they continue to enjoy their profession, findings from the newly released Medscape Psychiatrist Compensation Report 2020 show.
Psychiatrists’ average annual income this year rose to $268,000, up from $260,000 last year. Two-thirds of psychiatrists feel fairly compensated, similar to last year’s percentage.
Psychiatrists are below the middle earners of all physician specialties, ranking eighth from the bottom, just below neurologists ($280,000), but ahead of rheumatologists ($262,000) and internists ($251,000).
Orthopedists are the top earners ($511,000 annual pay), followed by plastic surgeons ($479,000), otolaryngologists ($455,000), and cardiologists ($438,000), according to the overall Medscape Physician Compensation Report 2020, which covers U.S. physicians as a whole. The survey included more than 17,000 physicians in more than 30 specialties.
COVID-19 impact
An important caveat is that data for this year’s report were collected prior to Feb. 10, 2020, and therefore reflect physician salary and income prior to the COVID-19 crisis, which has had a huge impact on physicians.
For example, since the start of the crisis, data show that physician practices have seen a 55% dip in revenue and a 60% dip in patient volume on average. Hospitals and physician groups nationwide have implemented layoffs, furloughs, and pay cuts.
In March, 43,000 health care workers were laid off; 9% of independent medical practices reported that they have closed their practices, at least temporarily.
There continues to be a gender pay gap in psychiatry, with male psychiatrists earning about 21% more than their female peers ($289,000 vs. $239,000). Among all specialists, men earn 31% more than women, similar to last year’s figure of 33%. There continues to be a 25% gender pay gap among primary care physicians.
Psychiatrists report that they are eligible for $26,000 in annual incentive bonuses. Such bonuses are highest among orthopedists ($96,000) and lowest among family medicine physicians ($24,000).
Close to one-third of psychiatrists (and physicians overall) who have incentive bonuses say the prospect of the bonus has encouraged them to work longer hours.
Two thirds of psychiatrists say they receive more than three quarters of their potential annual incentive bonus. On average, psychiatrists achieve 70% of their potential bonus, similar to physicians overall (67%).
However, COVID-19 may change that. Experts recently interviewed by Medscape Medical News noted that productivity benchmarks for physicians are likely to be lowered in light of plunging patient numbers from COVID-19, and bonuses are expected to take a hit.
Happy at work
On average, male psychiatrists spend 34.5 hours per week seeing patients, somewhat higher than female psychiatrists (31.5 hours); the average for all physicians is 37.9 hours per week.
Bureaucratic tasks continue to be a burden for physicians in all specialties. On average, psychiatrists spend 15.9 hours per week on paperwork and administration, about the same as physicians overall (15.6 hours).
Intensivists top the list regarding such tasks (19.1 hours), followed by internists (18.5 hours), infectious disease physicians (18.5 hours), and psychiatrists (18.3 hours). Anesthesiologists and ophthalmologists spend the least amount of time on paperwork/administration (10.0 and 9.8 hours per week, respectively).
What is most rewarding about being a psychiatrist? Making the world a better place (helping others) tops the list (28%), followed closely by relationships with and gratitude from patients (24%), being good at what they do/finding answers, diagnoses (20%), and making good money at a job they like (15%). A few cited teaching (6%) and pride in their profession (4%).
The most challenging part of practicing psychiatry is having so many rules and regulations (29%). Other challenges include dealing with difficult patients (18%), working with EHRs (13%), having to work long hours (11%), and trouble getting fair reimbursement (10%).
Despite the challenges,
Other key findings in the latest report regarding psychiatrists include the following:
- At 16%, psychiatrists rank toward the middle of physicians, potentially losing money on denied or resubmitted claims. Plastic surgery and emergency medicine have the highest percentage of claims denied or resubmitted (28% and 22%, respectively). One study found that, on average, 63% of denied claims are recoverable, but healthcare professionals spend about $118 per claim on appeals.
- Only 14% of psychiatrists say they use physician assistants to treat patients in their practices, while 46% use nurse practitioners; about half (51%) use neither for patient care. Of psychiatrists who work with physician assistants and nurse practitioners in their offices, 34% say these employees have helped boost profitability.
- 56% of psychiatrists say they will continue taking new and current Medicare/Medicaid patients; only 1% say they won’t take new Medicare patients and 22% are undecided.
- The large majority of psychiatrists rely on payers; 30% rely on fee-for-service arrangements and 14% on accountable care organizations for patient-based income.
- Only 12% of psychiatrists expect to participate in the merit-based incentive payment system and only 1% expect to participate in alternative payment models.
A version of this article originally appeared on Medscape.com.
Psychiatrists continue to rank close to the bottom of the compensation ladder, but they made more this year than last year and they continue to enjoy their profession, findings from the newly released Medscape Psychiatrist Compensation Report 2020 show.
Psychiatrists’ average annual income this year rose to $268,000, up from $260,000 last year. Two-thirds of psychiatrists feel fairly compensated, similar to last year’s percentage.
Psychiatrists are below the middle earners of all physician specialties, ranking eighth from the bottom, just below neurologists ($280,000), but ahead of rheumatologists ($262,000) and internists ($251,000).
Orthopedists are the top earners ($511,000 annual pay), followed by plastic surgeons ($479,000), otolaryngologists ($455,000), and cardiologists ($438,000), according to the overall Medscape Physician Compensation Report 2020, which covers U.S. physicians as a whole. The survey included more than 17,000 physicians in more than 30 specialties.
COVID-19 impact
An important caveat is that data for this year’s report were collected prior to Feb. 10, 2020, and therefore reflect physician salary and income prior to the COVID-19 crisis, which has had a huge impact on physicians.
For example, since the start of the crisis, data show that physician practices have seen a 55% dip in revenue and a 60% dip in patient volume on average. Hospitals and physician groups nationwide have implemented layoffs, furloughs, and pay cuts.
In March, 43,000 health care workers were laid off; 9% of independent medical practices reported that they have closed their practices, at least temporarily.
There continues to be a gender pay gap in psychiatry, with male psychiatrists earning about 21% more than their female peers ($289,000 vs. $239,000). Among all specialists, men earn 31% more than women, similar to last year’s figure of 33%. There continues to be a 25% gender pay gap among primary care physicians.
Psychiatrists report that they are eligible for $26,000 in annual incentive bonuses. Such bonuses are highest among orthopedists ($96,000) and lowest among family medicine physicians ($24,000).
Close to one-third of psychiatrists (and physicians overall) who have incentive bonuses say the prospect of the bonus has encouraged them to work longer hours.
Two thirds of psychiatrists say they receive more than three quarters of their potential annual incentive bonus. On average, psychiatrists achieve 70% of their potential bonus, similar to physicians overall (67%).
However, COVID-19 may change that. Experts recently interviewed by Medscape Medical News noted that productivity benchmarks for physicians are likely to be lowered in light of plunging patient numbers from COVID-19, and bonuses are expected to take a hit.
Happy at work
On average, male psychiatrists spend 34.5 hours per week seeing patients, somewhat higher than female psychiatrists (31.5 hours); the average for all physicians is 37.9 hours per week.
Bureaucratic tasks continue to be a burden for physicians in all specialties. On average, psychiatrists spend 15.9 hours per week on paperwork and administration, about the same as physicians overall (15.6 hours).
Intensivists top the list regarding such tasks (19.1 hours), followed by internists (18.5 hours), infectious disease physicians (18.5 hours), and psychiatrists (18.3 hours). Anesthesiologists and ophthalmologists spend the least amount of time on paperwork/administration (10.0 and 9.8 hours per week, respectively).
What is most rewarding about being a psychiatrist? Making the world a better place (helping others) tops the list (28%), followed closely by relationships with and gratitude from patients (24%), being good at what they do/finding answers, diagnoses (20%), and making good money at a job they like (15%). A few cited teaching (6%) and pride in their profession (4%).
The most challenging part of practicing psychiatry is having so many rules and regulations (29%). Other challenges include dealing with difficult patients (18%), working with EHRs (13%), having to work long hours (11%), and trouble getting fair reimbursement (10%).
Despite the challenges,
Other key findings in the latest report regarding psychiatrists include the following:
- At 16%, psychiatrists rank toward the middle of physicians, potentially losing money on denied or resubmitted claims. Plastic surgery and emergency medicine have the highest percentage of claims denied or resubmitted (28% and 22%, respectively). One study found that, on average, 63% of denied claims are recoverable, but healthcare professionals spend about $118 per claim on appeals.
- Only 14% of psychiatrists say they use physician assistants to treat patients in their practices, while 46% use nurse practitioners; about half (51%) use neither for patient care. Of psychiatrists who work with physician assistants and nurse practitioners in their offices, 34% say these employees have helped boost profitability.
- 56% of psychiatrists say they will continue taking new and current Medicare/Medicaid patients; only 1% say they won’t take new Medicare patients and 22% are undecided.
- The large majority of psychiatrists rely on payers; 30% rely on fee-for-service arrangements and 14% on accountable care organizations for patient-based income.
- Only 12% of psychiatrists expect to participate in the merit-based incentive payment system and only 1% expect to participate in alternative payment models.
A version of this article originally appeared on Medscape.com.
Psychiatrists continue to rank close to the bottom of the compensation ladder, but they made more this year than last year and they continue to enjoy their profession, findings from the newly released Medscape Psychiatrist Compensation Report 2020 show.
Psychiatrists’ average annual income this year rose to $268,000, up from $260,000 last year. Two-thirds of psychiatrists feel fairly compensated, similar to last year’s percentage.
Psychiatrists are below the middle earners of all physician specialties, ranking eighth from the bottom, just below neurologists ($280,000), but ahead of rheumatologists ($262,000) and internists ($251,000).
Orthopedists are the top earners ($511,000 annual pay), followed by plastic surgeons ($479,000), otolaryngologists ($455,000), and cardiologists ($438,000), according to the overall Medscape Physician Compensation Report 2020, which covers U.S. physicians as a whole. The survey included more than 17,000 physicians in more than 30 specialties.
COVID-19 impact
An important caveat is that data for this year’s report were collected prior to Feb. 10, 2020, and therefore reflect physician salary and income prior to the COVID-19 crisis, which has had a huge impact on physicians.
For example, since the start of the crisis, data show that physician practices have seen a 55% dip in revenue and a 60% dip in patient volume on average. Hospitals and physician groups nationwide have implemented layoffs, furloughs, and pay cuts.
In March, 43,000 health care workers were laid off; 9% of independent medical practices reported that they have closed their practices, at least temporarily.
There continues to be a gender pay gap in psychiatry, with male psychiatrists earning about 21% more than their female peers ($289,000 vs. $239,000). Among all specialists, men earn 31% more than women, similar to last year’s figure of 33%. There continues to be a 25% gender pay gap among primary care physicians.
Psychiatrists report that they are eligible for $26,000 in annual incentive bonuses. Such bonuses are highest among orthopedists ($96,000) and lowest among family medicine physicians ($24,000).
Close to one-third of psychiatrists (and physicians overall) who have incentive bonuses say the prospect of the bonus has encouraged them to work longer hours.
Two thirds of psychiatrists say they receive more than three quarters of their potential annual incentive bonus. On average, psychiatrists achieve 70% of their potential bonus, similar to physicians overall (67%).
However, COVID-19 may change that. Experts recently interviewed by Medscape Medical News noted that productivity benchmarks for physicians are likely to be lowered in light of plunging patient numbers from COVID-19, and bonuses are expected to take a hit.
Happy at work
On average, male psychiatrists spend 34.5 hours per week seeing patients, somewhat higher than female psychiatrists (31.5 hours); the average for all physicians is 37.9 hours per week.
Bureaucratic tasks continue to be a burden for physicians in all specialties. On average, psychiatrists spend 15.9 hours per week on paperwork and administration, about the same as physicians overall (15.6 hours).
Intensivists top the list regarding such tasks (19.1 hours), followed by internists (18.5 hours), infectious disease physicians (18.5 hours), and psychiatrists (18.3 hours). Anesthesiologists and ophthalmologists spend the least amount of time on paperwork/administration (10.0 and 9.8 hours per week, respectively).
What is most rewarding about being a psychiatrist? Making the world a better place (helping others) tops the list (28%), followed closely by relationships with and gratitude from patients (24%), being good at what they do/finding answers, diagnoses (20%), and making good money at a job they like (15%). A few cited teaching (6%) and pride in their profession (4%).
The most challenging part of practicing psychiatry is having so many rules and regulations (29%). Other challenges include dealing with difficult patients (18%), working with EHRs (13%), having to work long hours (11%), and trouble getting fair reimbursement (10%).
Despite the challenges,
Other key findings in the latest report regarding psychiatrists include the following:
- At 16%, psychiatrists rank toward the middle of physicians, potentially losing money on denied or resubmitted claims. Plastic surgery and emergency medicine have the highest percentage of claims denied or resubmitted (28% and 22%, respectively). One study found that, on average, 63% of denied claims are recoverable, but healthcare professionals spend about $118 per claim on appeals.
- Only 14% of psychiatrists say they use physician assistants to treat patients in their practices, while 46% use nurse practitioners; about half (51%) use neither for patient care. Of psychiatrists who work with physician assistants and nurse practitioners in their offices, 34% say these employees have helped boost profitability.
- 56% of psychiatrists say they will continue taking new and current Medicare/Medicaid patients; only 1% say they won’t take new Medicare patients and 22% are undecided.
- The large majority of psychiatrists rely on payers; 30% rely on fee-for-service arrangements and 14% on accountable care organizations for patient-based income.
- Only 12% of psychiatrists expect to participate in the merit-based incentive payment system and only 1% expect to participate in alternative payment models.
A version of this article originally appeared on Medscape.com.
Most patients with lichen sclerosus receive appropriate treatment
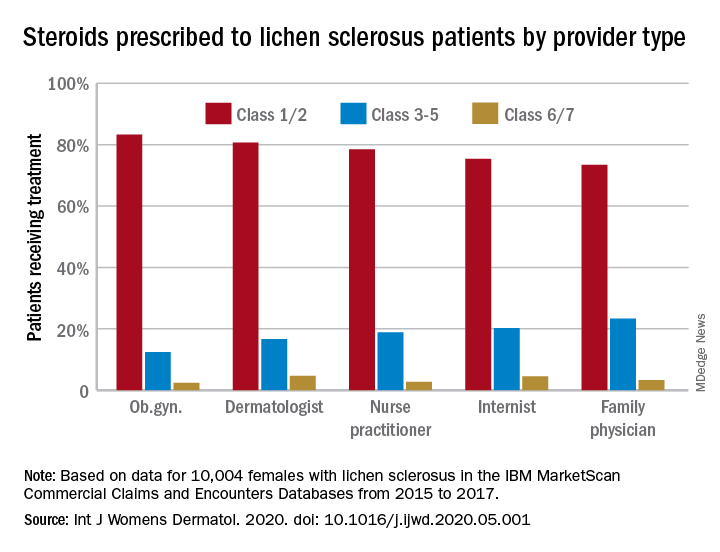
The claims-based prevalence of 0.05% found in the study is lower than previously reported, and only 16% of the diagnoses were in women aged 18-44 years, Laura E. Melnick, MD, and associates wrote after identifying 10,004 females aged 0-65 years with lichen sclerosus in the IBM MarketScan Commercial Claims and Encounters Databases from 2015 to 2017. The majority (79%) of those diagnosed were aged 45-65 years (average, 50.8 years).
In pediatric patients (up to age 17 years), the low prevalence (0.01%) “may be attributable to several factors including relative rarity, as well as variability in pediatric clinicians’ familiarity with [lichen sclerosus] and in patients’ clinical symptoms,” said Dr. Melnick and associates in the department of dermatology at New York University.
Just over half of all diagnoses (52.4%) were made by ob.gyns., with dermatologists next at 14.5%, followed by family physicians (6.5%), nurse practitioners (2.5%), and internists (0.4%), they reported in the International Journal of Women’s Dermatology.
Treatment for lichen sclerosus, in the form of high-potency topical corticosteroids, was mostly appropriate. Ob.gyns. prescribed class 1/2 steroids to 83% of their patients, tops among all clinicians. Dermatologists were just over 80%, and the other clinician categories were all over 70%, the investigators said.
“Understanding the current management of [lichen sclerosus] is important given that un- or undertreated disease can significantly impact patients’ quality of life, lead to increased lower urinary tract symptoms and irreversible architectural changes, and predispose women to squamous cell carcinoma,” they wrote.
SOURCE: Melnick LE et al. Int J Womens Dermatol. 2020. doi: 10.1016/j.ijwd.2020.05.001.

The claims-based prevalence of 0.05% found in the study is lower than previously reported, and only 16% of the diagnoses were in women aged 18-44 years, Laura E. Melnick, MD, and associates wrote after identifying 10,004 females aged 0-65 years with lichen sclerosus in the IBM MarketScan Commercial Claims and Encounters Databases from 2015 to 2017. The majority (79%) of those diagnosed were aged 45-65 years (average, 50.8 years).
In pediatric patients (up to age 17 years), the low prevalence (0.01%) “may be attributable to several factors including relative rarity, as well as variability in pediatric clinicians’ familiarity with [lichen sclerosus] and in patients’ clinical symptoms,” said Dr. Melnick and associates in the department of dermatology at New York University.
Just over half of all diagnoses (52.4%) were made by ob.gyns., with dermatologists next at 14.5%, followed by family physicians (6.5%), nurse practitioners (2.5%), and internists (0.4%), they reported in the International Journal of Women’s Dermatology.
Treatment for lichen sclerosus, in the form of high-potency topical corticosteroids, was mostly appropriate. Ob.gyns. prescribed class 1/2 steroids to 83% of their patients, tops among all clinicians. Dermatologists were just over 80%, and the other clinician categories were all over 70%, the investigators said.
“Understanding the current management of [lichen sclerosus] is important given that un- or undertreated disease can significantly impact patients’ quality of life, lead to increased lower urinary tract symptoms and irreversible architectural changes, and predispose women to squamous cell carcinoma,” they wrote.
SOURCE: Melnick LE et al. Int J Womens Dermatol. 2020. doi: 10.1016/j.ijwd.2020.05.001.

The claims-based prevalence of 0.05% found in the study is lower than previously reported, and only 16% of the diagnoses were in women aged 18-44 years, Laura E. Melnick, MD, and associates wrote after identifying 10,004 females aged 0-65 years with lichen sclerosus in the IBM MarketScan Commercial Claims and Encounters Databases from 2015 to 2017. The majority (79%) of those diagnosed were aged 45-65 years (average, 50.8 years).
In pediatric patients (up to age 17 years), the low prevalence (0.01%) “may be attributable to several factors including relative rarity, as well as variability in pediatric clinicians’ familiarity with [lichen sclerosus] and in patients’ clinical symptoms,” said Dr. Melnick and associates in the department of dermatology at New York University.
Just over half of all diagnoses (52.4%) were made by ob.gyns., with dermatologists next at 14.5%, followed by family physicians (6.5%), nurse practitioners (2.5%), and internists (0.4%), they reported in the International Journal of Women’s Dermatology.
Treatment for lichen sclerosus, in the form of high-potency topical corticosteroids, was mostly appropriate. Ob.gyns. prescribed class 1/2 steroids to 83% of their patients, tops among all clinicians. Dermatologists were just over 80%, and the other clinician categories were all over 70%, the investigators said.
“Understanding the current management of [lichen sclerosus] is important given that un- or undertreated disease can significantly impact patients’ quality of life, lead to increased lower urinary tract symptoms and irreversible architectural changes, and predispose women to squamous cell carcinoma,” they wrote.
SOURCE: Melnick LE et al. Int J Womens Dermatol. 2020. doi: 10.1016/j.ijwd.2020.05.001.
FROM THE INTERNATIONAL JOURNAL OF WOMEN’S DERMATOLOGY
Sarcopenic obesity: The wasting within
Case
The patient is a 65-year-old white female who recently was discovered to have a 2-cm spiculated lung mass in the right upper lobe. She is undergoing an evaluation at present but her main complaint today is that of profound weakness and fatigue. Her appetite and energy level are noticeably less; her family ascribes this to anxiety and depression. Her other medical problems include diabetes, hypertension, osteoporosis, and obesity. The patient believes that she’s lost about 20-25 pounds recently, though her family is skeptical, adding that “she’s been heavy all her life.” Her body mass index is 40. What additional interventions would you add to her workup?
Background
Sarcopenic obesity occurs as a natural consequence of aging. As a general rule, as many as half the women and a quarter of the men over age 80 years are affected. A total of about 18 million people are involved.
One thought as to etiology is that as one ages, proteolysis outdoes protein synthesis. Fat then replaces the body’s muscle, permeates the viscera, and becomes the prominent body form. Chronic lipodeposition leads to chronic inflammation which, in turn, augments protein catabolism. The elderly become less energetic and less active, and the muscle mass decreases further. A vicious cycle develops. Concurrently with obesity, patients suffer with the onset of dyslipidemia, osteoarthritis, osteoporosis (due to vitamin D deficiency), insulin resistance, and an overall increase in frailty.
Sarcopenic obesity also plays a prognostic role in the management of cancer patients where the presence of sarcopenia correlates with earlier death and decreased capacity for therapy. Patients seen as obese are less likely to receive the intensive care (particularly nutritional support) that patients seen as a higher risk receive. The cancer cachexia is less pronounced. The obesity seen externally masks the wasting within.
Diagnosis and treatment
Sarcopenic obesity suffers from an inexact definition. According to the World Health Organization, obesity is defined, officially, as a body mass index of greater than 30 kg/m2. Muscle mass is an important part of this entity, too, but the inclusion of muscle function in this definition brings, seemingly, a point of conjecture. Is muscle function necessary? By what scale do you measure it? This imprecision makes comparative research in the field somewhat more difficult.
As clinical acumen remains the major diagnostic approach to this disease, confirmatory testing for sarcopenic obesity comprises MRIs/CTs and dual energy x-ray absorptiometry (DXA) scans. Presently DXA is used to assess bone density in the diagnosis of osteoporosis. It also reveals the decreased lean appendicular (extremity) muscle mass which, along with the increased BMI, forms the basic diagnosis of sarcopenic obesity. DXA scans are favored over CTs for the assessment of appendicular lean muscle mass. DXA scans provide a relatively inexpensive method of estimating fat, muscle, and additionally, bone density. CTs are less favored because of their radiation exposure as well as their high cost. Assessing muscle strength, using handgrip dynomometry, is available though not widely advocated.
Of the myriad modalities tried in sarcopenic obesity, many have shortcomings. No particular diet format can be advocated. Hypocaloric diets, with or without protein supplementation, offer little advantage to a good physical exercise program. The administration of vitamin D, with calcium, can be of benefit to those sarcopenically obese patients suffering with osteoporosis. Other medications, as exemplified by testosterone, vitamin K, myostatin inhibitors, or mesenchymal stem cells, are either anecdotal or dubious in nature. More research is definitely needed.
The key component for the treatment of sarcopenic obesity is exercise, both aerobic and resistant. Physical exercise recruits muscle satellite cells into the muscle fibers strengthening their composition. Growth factors are also released that stimulate the production of muscle satellite cells. Muscle mass becomes augmented and fortified. Aerobic exercise counteracts the negative metabolic effects of lipids. Resistance training is felt to improve strength when in combination with aerobic exercise, compared with aerobic exercise alone. Research has shown that high-speed resistance training, over a 12-week period, had shown a greater improvement in muscle power and capacity when compared to low-speed training. It was also recommended that patients exercise only until fatigued, not until “failure,” as a stopping point. Programs must be customized to fit the individual.
Sarcopenic obesity is a form of deconditioning that occurs naturally with age but is compounded by cancer. Research into this disease is confounded by a lack of accepted definitions. Radiographic workup and lifestyle changes are the mainstay of medical management. The foremost diagnostic tool remains, as always, clinical suspicion.
Dr. Killeen is a physician in Tampa, Fla. He practices internal medicine, hematology, and oncology, and has worked in hospice and hospital medicine.
Recommended reading
Gruber ES et al. Sarcopenia and Sarcopenic Obesity are independent adverse prognostic factors in resectable pancreatic ductal adenocarcinoma. PLoS One. 2019;14(5): e02115915.10.1371/journal.pone.0215915 [PMID 31059520].
Lombardo M et al. Sarcopenic Obesity: Etiology and lifestyle therapy. Eur Rev Med Pharmacol Sci. 2019; 23: 7152-62.
Petroni M et al. Prevention and treatment of Sarcopenic Obesity in women. Nutrients. 2019; Jun 8.10.3390/nu1161302 [PMID 31181771].
Barcos VE, Arribas L. Sarcopenic Obesity: Hidden muscle wasting and its impact for survival and complications of cancer therapy. Ann Oncol. 2018;29(suppl. 2):ii1-ii9.
Zhang X et al. Association of Sarcopenic Obesity with the risk of all-cause mortality among adults over a broad range of different settings: An update meta-analysis. BMC Geriatr. 2018;19:183-97.
Key points
- • In sarcopenic obesity a patient’s muscle loss in mass can be clouded, overshadowed by the obese body habitus. The major diagnostic tool initially is clinical suspicion.
- • The diagnostic tests for sarcopenic obesity are DXA and CT scans.
- • The best treatment for sarcopenic obesity is a good exercise plan.
Quiz
1. What is the best treatment for sarcopenic obesity?
A. Testosterone
B. Vitamin K
C. Myostatin inhibitors
D. None of the above
Answer: D
There is no particular pharmaceutical treatment, to date, for sarcopenic obesity. Only an exercise program has proved to be of benefit. Those for whom fatigue might be problematic could benefit perhaps by doing “energy banking” or taking programmed naps/rest periods prior to exercise.
2. DXA scans are favored over CT scans because of which of the following?
A. Less cost
B. Capacity to diagnose osteoporosis
C. Less radiation exposure
D. All of the above
Answer: D
DXA scans offer all of the above advantages over CT scans. Also, patients with sarcopenic obesity found to be osteoporotic could be started on vitamin D and calcium supplementation.
3. Which of the following hamper the diagnosis and treatment of sarcopenic obesity?
A. The issue of muscle function
B. Difficulties in comparative research studies
C. Remembering that muscle wasting can occur without external evidence of cachexia
D. All of the above
Answer: D
Obtaining a precise definition of sarcopenic obesity and dealing with the issue of muscle strength and capacity make comparative studies difficult. The sarcopenic obese patient needs as much attention as the cachectic one as their wasting is from within.
4. In sarcopenic obesity and cancer the presence of sarcopenia is likely to lead to which of the following?
A. Earlier death
B. Decreased capacity for therapy
C. Less treatment focus compared to nonsarcopenic patients
D. All of the above
Answer: D
The presence of sarcopenia correlates to all of the above particularly as the obese patient is thought to require less intensive attention than others.
Case
The patient is a 65-year-old white female who recently was discovered to have a 2-cm spiculated lung mass in the right upper lobe. She is undergoing an evaluation at present but her main complaint today is that of profound weakness and fatigue. Her appetite and energy level are noticeably less; her family ascribes this to anxiety and depression. Her other medical problems include diabetes, hypertension, osteoporosis, and obesity. The patient believes that she’s lost about 20-25 pounds recently, though her family is skeptical, adding that “she’s been heavy all her life.” Her body mass index is 40. What additional interventions would you add to her workup?
Background
Sarcopenic obesity occurs as a natural consequence of aging. As a general rule, as many as half the women and a quarter of the men over age 80 years are affected. A total of about 18 million people are involved.
One thought as to etiology is that as one ages, proteolysis outdoes protein synthesis. Fat then replaces the body’s muscle, permeates the viscera, and becomes the prominent body form. Chronic lipodeposition leads to chronic inflammation which, in turn, augments protein catabolism. The elderly become less energetic and less active, and the muscle mass decreases further. A vicious cycle develops. Concurrently with obesity, patients suffer with the onset of dyslipidemia, osteoarthritis, osteoporosis (due to vitamin D deficiency), insulin resistance, and an overall increase in frailty.
Sarcopenic obesity also plays a prognostic role in the management of cancer patients where the presence of sarcopenia correlates with earlier death and decreased capacity for therapy. Patients seen as obese are less likely to receive the intensive care (particularly nutritional support) that patients seen as a higher risk receive. The cancer cachexia is less pronounced. The obesity seen externally masks the wasting within.
Diagnosis and treatment
Sarcopenic obesity suffers from an inexact definition. According to the World Health Organization, obesity is defined, officially, as a body mass index of greater than 30 kg/m2. Muscle mass is an important part of this entity, too, but the inclusion of muscle function in this definition brings, seemingly, a point of conjecture. Is muscle function necessary? By what scale do you measure it? This imprecision makes comparative research in the field somewhat more difficult.
As clinical acumen remains the major diagnostic approach to this disease, confirmatory testing for sarcopenic obesity comprises MRIs/CTs and dual energy x-ray absorptiometry (DXA) scans. Presently DXA is used to assess bone density in the diagnosis of osteoporosis. It also reveals the decreased lean appendicular (extremity) muscle mass which, along with the increased BMI, forms the basic diagnosis of sarcopenic obesity. DXA scans are favored over CTs for the assessment of appendicular lean muscle mass. DXA scans provide a relatively inexpensive method of estimating fat, muscle, and additionally, bone density. CTs are less favored because of their radiation exposure as well as their high cost. Assessing muscle strength, using handgrip dynomometry, is available though not widely advocated.
Of the myriad modalities tried in sarcopenic obesity, many have shortcomings. No particular diet format can be advocated. Hypocaloric diets, with or without protein supplementation, offer little advantage to a good physical exercise program. The administration of vitamin D, with calcium, can be of benefit to those sarcopenically obese patients suffering with osteoporosis. Other medications, as exemplified by testosterone, vitamin K, myostatin inhibitors, or mesenchymal stem cells, are either anecdotal or dubious in nature. More research is definitely needed.
The key component for the treatment of sarcopenic obesity is exercise, both aerobic and resistant. Physical exercise recruits muscle satellite cells into the muscle fibers strengthening their composition. Growth factors are also released that stimulate the production of muscle satellite cells. Muscle mass becomes augmented and fortified. Aerobic exercise counteracts the negative metabolic effects of lipids. Resistance training is felt to improve strength when in combination with aerobic exercise, compared with aerobic exercise alone. Research has shown that high-speed resistance training, over a 12-week period, had shown a greater improvement in muscle power and capacity when compared to low-speed training. It was also recommended that patients exercise only until fatigued, not until “failure,” as a stopping point. Programs must be customized to fit the individual.
Sarcopenic obesity is a form of deconditioning that occurs naturally with age but is compounded by cancer. Research into this disease is confounded by a lack of accepted definitions. Radiographic workup and lifestyle changes are the mainstay of medical management. The foremost diagnostic tool remains, as always, clinical suspicion.
Dr. Killeen is a physician in Tampa, Fla. He practices internal medicine, hematology, and oncology, and has worked in hospice and hospital medicine.
Recommended reading
Gruber ES et al. Sarcopenia and Sarcopenic Obesity are independent adverse prognostic factors in resectable pancreatic ductal adenocarcinoma. PLoS One. 2019;14(5): e02115915.10.1371/journal.pone.0215915 [PMID 31059520].
Lombardo M et al. Sarcopenic Obesity: Etiology and lifestyle therapy. Eur Rev Med Pharmacol Sci. 2019; 23: 7152-62.
Petroni M et al. Prevention and treatment of Sarcopenic Obesity in women. Nutrients. 2019; Jun 8.10.3390/nu1161302 [PMID 31181771].
Barcos VE, Arribas L. Sarcopenic Obesity: Hidden muscle wasting and its impact for survival and complications of cancer therapy. Ann Oncol. 2018;29(suppl. 2):ii1-ii9.
Zhang X et al. Association of Sarcopenic Obesity with the risk of all-cause mortality among adults over a broad range of different settings: An update meta-analysis. BMC Geriatr. 2018;19:183-97.
Key points
- • In sarcopenic obesity a patient’s muscle loss in mass can be clouded, overshadowed by the obese body habitus. The major diagnostic tool initially is clinical suspicion.
- • The diagnostic tests for sarcopenic obesity are DXA and CT scans.
- • The best treatment for sarcopenic obesity is a good exercise plan.
Quiz
1. What is the best treatment for sarcopenic obesity?
A. Testosterone
B. Vitamin K
C. Myostatin inhibitors
D. None of the above
Answer: D
There is no particular pharmaceutical treatment, to date, for sarcopenic obesity. Only an exercise program has proved to be of benefit. Those for whom fatigue might be problematic could benefit perhaps by doing “energy banking” or taking programmed naps/rest periods prior to exercise.
2. DXA scans are favored over CT scans because of which of the following?
A. Less cost
B. Capacity to diagnose osteoporosis
C. Less radiation exposure
D. All of the above
Answer: D
DXA scans offer all of the above advantages over CT scans. Also, patients with sarcopenic obesity found to be osteoporotic could be started on vitamin D and calcium supplementation.
3. Which of the following hamper the diagnosis and treatment of sarcopenic obesity?
A. The issue of muscle function
B. Difficulties in comparative research studies
C. Remembering that muscle wasting can occur without external evidence of cachexia
D. All of the above
Answer: D
Obtaining a precise definition of sarcopenic obesity and dealing with the issue of muscle strength and capacity make comparative studies difficult. The sarcopenic obese patient needs as much attention as the cachectic one as their wasting is from within.
4. In sarcopenic obesity and cancer the presence of sarcopenia is likely to lead to which of the following?
A. Earlier death
B. Decreased capacity for therapy
C. Less treatment focus compared to nonsarcopenic patients
D. All of the above
Answer: D
The presence of sarcopenia correlates to all of the above particularly as the obese patient is thought to require less intensive attention than others.
Case
The patient is a 65-year-old white female who recently was discovered to have a 2-cm spiculated lung mass in the right upper lobe. She is undergoing an evaluation at present but her main complaint today is that of profound weakness and fatigue. Her appetite and energy level are noticeably less; her family ascribes this to anxiety and depression. Her other medical problems include diabetes, hypertension, osteoporosis, and obesity. The patient believes that she’s lost about 20-25 pounds recently, though her family is skeptical, adding that “she’s been heavy all her life.” Her body mass index is 40. What additional interventions would you add to her workup?
Background
Sarcopenic obesity occurs as a natural consequence of aging. As a general rule, as many as half the women and a quarter of the men over age 80 years are affected. A total of about 18 million people are involved.
One thought as to etiology is that as one ages, proteolysis outdoes protein synthesis. Fat then replaces the body’s muscle, permeates the viscera, and becomes the prominent body form. Chronic lipodeposition leads to chronic inflammation which, in turn, augments protein catabolism. The elderly become less energetic and less active, and the muscle mass decreases further. A vicious cycle develops. Concurrently with obesity, patients suffer with the onset of dyslipidemia, osteoarthritis, osteoporosis (due to vitamin D deficiency), insulin resistance, and an overall increase in frailty.
Sarcopenic obesity also plays a prognostic role in the management of cancer patients where the presence of sarcopenia correlates with earlier death and decreased capacity for therapy. Patients seen as obese are less likely to receive the intensive care (particularly nutritional support) that patients seen as a higher risk receive. The cancer cachexia is less pronounced. The obesity seen externally masks the wasting within.
Diagnosis and treatment
Sarcopenic obesity suffers from an inexact definition. According to the World Health Organization, obesity is defined, officially, as a body mass index of greater than 30 kg/m2. Muscle mass is an important part of this entity, too, but the inclusion of muscle function in this definition brings, seemingly, a point of conjecture. Is muscle function necessary? By what scale do you measure it? This imprecision makes comparative research in the field somewhat more difficult.
As clinical acumen remains the major diagnostic approach to this disease, confirmatory testing for sarcopenic obesity comprises MRIs/CTs and dual energy x-ray absorptiometry (DXA) scans. Presently DXA is used to assess bone density in the diagnosis of osteoporosis. It also reveals the decreased lean appendicular (extremity) muscle mass which, along with the increased BMI, forms the basic diagnosis of sarcopenic obesity. DXA scans are favored over CTs for the assessment of appendicular lean muscle mass. DXA scans provide a relatively inexpensive method of estimating fat, muscle, and additionally, bone density. CTs are less favored because of their radiation exposure as well as their high cost. Assessing muscle strength, using handgrip dynomometry, is available though not widely advocated.
Of the myriad modalities tried in sarcopenic obesity, many have shortcomings. No particular diet format can be advocated. Hypocaloric diets, with or without protein supplementation, offer little advantage to a good physical exercise program. The administration of vitamin D, with calcium, can be of benefit to those sarcopenically obese patients suffering with osteoporosis. Other medications, as exemplified by testosterone, vitamin K, myostatin inhibitors, or mesenchymal stem cells, are either anecdotal or dubious in nature. More research is definitely needed.
The key component for the treatment of sarcopenic obesity is exercise, both aerobic and resistant. Physical exercise recruits muscle satellite cells into the muscle fibers strengthening their composition. Growth factors are also released that stimulate the production of muscle satellite cells. Muscle mass becomes augmented and fortified. Aerobic exercise counteracts the negative metabolic effects of lipids. Resistance training is felt to improve strength when in combination with aerobic exercise, compared with aerobic exercise alone. Research has shown that high-speed resistance training, over a 12-week period, had shown a greater improvement in muscle power and capacity when compared to low-speed training. It was also recommended that patients exercise only until fatigued, not until “failure,” as a stopping point. Programs must be customized to fit the individual.
Sarcopenic obesity is a form of deconditioning that occurs naturally with age but is compounded by cancer. Research into this disease is confounded by a lack of accepted definitions. Radiographic workup and lifestyle changes are the mainstay of medical management. The foremost diagnostic tool remains, as always, clinical suspicion.
Dr. Killeen is a physician in Tampa, Fla. He practices internal medicine, hematology, and oncology, and has worked in hospice and hospital medicine.
Recommended reading
Gruber ES et al. Sarcopenia and Sarcopenic Obesity are independent adverse prognostic factors in resectable pancreatic ductal adenocarcinoma. PLoS One. 2019;14(5): e02115915.10.1371/journal.pone.0215915 [PMID 31059520].
Lombardo M et al. Sarcopenic Obesity: Etiology and lifestyle therapy. Eur Rev Med Pharmacol Sci. 2019; 23: 7152-62.
Petroni M et al. Prevention and treatment of Sarcopenic Obesity in women. Nutrients. 2019; Jun 8.10.3390/nu1161302 [PMID 31181771].
Barcos VE, Arribas L. Sarcopenic Obesity: Hidden muscle wasting and its impact for survival and complications of cancer therapy. Ann Oncol. 2018;29(suppl. 2):ii1-ii9.
Zhang X et al. Association of Sarcopenic Obesity with the risk of all-cause mortality among adults over a broad range of different settings: An update meta-analysis. BMC Geriatr. 2018;19:183-97.
Key points
- • In sarcopenic obesity a patient’s muscle loss in mass can be clouded, overshadowed by the obese body habitus. The major diagnostic tool initially is clinical suspicion.
- • The diagnostic tests for sarcopenic obesity are DXA and CT scans.
- • The best treatment for sarcopenic obesity is a good exercise plan.
Quiz
1. What is the best treatment for sarcopenic obesity?
A. Testosterone
B. Vitamin K
C. Myostatin inhibitors
D. None of the above
Answer: D
There is no particular pharmaceutical treatment, to date, for sarcopenic obesity. Only an exercise program has proved to be of benefit. Those for whom fatigue might be problematic could benefit perhaps by doing “energy banking” or taking programmed naps/rest periods prior to exercise.
2. DXA scans are favored over CT scans because of which of the following?
A. Less cost
B. Capacity to diagnose osteoporosis
C. Less radiation exposure
D. All of the above
Answer: D
DXA scans offer all of the above advantages over CT scans. Also, patients with sarcopenic obesity found to be osteoporotic could be started on vitamin D and calcium supplementation.
3. Which of the following hamper the diagnosis and treatment of sarcopenic obesity?
A. The issue of muscle function
B. Difficulties in comparative research studies
C. Remembering that muscle wasting can occur without external evidence of cachexia
D. All of the above
Answer: D
Obtaining a precise definition of sarcopenic obesity and dealing with the issue of muscle strength and capacity make comparative studies difficult. The sarcopenic obese patient needs as much attention as the cachectic one as their wasting is from within.
4. In sarcopenic obesity and cancer the presence of sarcopenia is likely to lead to which of the following?
A. Earlier death
B. Decreased capacity for therapy
C. Less treatment focus compared to nonsarcopenic patients
D. All of the above
Answer: D
The presence of sarcopenia correlates to all of the above particularly as the obese patient is thought to require less intensive attention than others.
Low-dose erlotinib seems feasible for frail, elderly patients with NSCLC
, according to researchers.
They conducted a phase 2 trial to investigate whether one-third of the maximum tolerated dose of erlotinib could maintain sufficient plasma concentration of the drug while avoiding the adverse effects of higher doses. The results were published in JAMA Oncology.
Erlotinib and other epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs) have demonstrated efficacy in elderly patients with EGFR-positive NSCLC, according to study author Shingo Miyamoto, MD, of Japanese Red Cross Medical Center in Tokyo, and colleagues.
“With the increasing number of elderly patients with cancer, many of whom also have significant comorbidities, there is a considerable value in investigating whether EGFR-TKIs are effective for the frail population,” the authors wrote. They also noted that it is “difficult to identify the appropriate dose of molecular-targeted drugs.”
With this in mind, Dr. Miyamoto and colleagues conducted a single-arm, phase 2 trial of low-dose erlotinib in 80 chemotherapy-naive frail or elderly patients with EGFR-positive NSCLC. Frailty was defined by age and the Charlson Comorbidity Index. The patients’ median age was 80 years (range, 49-90 years).
Patients received erlotinib at 50 mg per day, which is one-third of the established maximum tolerated dose, for 4 weeks. Then, they were evaluated with radiologic imaging. Treatment continued until disease progression or unacceptable adverse events. Dosing was modified by treatment response or by adverse events.
Results
At last follow-up, 7 of the 80 patients were still receiving low-dose erlotinib. Reasons for discontinuation were disease progression (n = 60), patient request (n = 6), adverse events (n = 4), and death (n = 3).
The overall response rate was 60%, and the disease control rate was 90%. The researchers measured plasma erlotinib concentration in 48 patients and found it did not correlate with response.
The median progression-free survival was 9.3 months, and the median overall survival was 26.2 months.
Ten patients had erlotinib temporarily suspended because of adverse events. Five patients had their dose reduced to 25 mg because of adverse events, including oral mucositis, paronychia, erythema multiforme, diarrhea, and anorexia.
Two patients discontinued treatment because of adverse events. One patient had a cutaneous ulcer and bone infection. The other had oral mucositis.
Dr. Miyamoto and colleagues concluded that, “low-dose erlotinib was associated with efficacy and safety in frail patients with EGFR mutation–positive lung cancer. More research on the dosing strategy of target-based drugs is warranted, especially in frail patients in the real-world setting.”
Less is more
Sometimes, less can be more, said Mellar P. Davis, MD, an oncologist and section head of the palliative care department at Geisinger Medical System in Danville, Penn., who was not involved in this study.
“Why do patients benefit from small doses? It may be that there are fewer drug interruptions over time and patients are able to stay on schedule,” Dr. Davis said. “It may also be that erlotinib clearance is reduced in the elderly and comorbid patient. The reduced dose may, in fact, be the ‘therapeutic’ dose in this special population.”
Plasma levels were frequently in therapeutic ranges in this study, but patients who had subtherapeutic plasma levels also responded to therapy, Dr. Davis pointed out. The lower dose was shown to maintain sufficient concentrations of the treatment while reducing adverse effects.
However, Dr. Davis noted, this was not a randomized trial. “It is always a risk hedging bets on single-arm trials,” he said. “Randomized trials often prove phase 2 single-arm trials wrong.”
He added that quality-of-life measures are absent from the study. Erlotinib is a palliative drug with side effects, Dr. Davis noted.
“Control of cancer and cancer regression should improve symptoms and quality of life when balanced against treatment toxicity,” he said. “In this study, I would have thought that symptom improvement, performance score, and quality of life would have been the primary outcome or the co-primary outcome with disease control.”
Should a randomized, controlled trial of low-dose erlotinib be conducted in the frail/elderly population? “If one believes trials should be quantitatively based, the answer would be no,” Dr. Davis said. “Responses may be the same, and it would be expensive to prove that low-dose erlotinib is the same as standard doses when comparing survival.”
However, if one is interested in quality of life, particularly in this growing population, a trial that incorporated quality-of-life measures would make more sense, according to Dr. Davis. “For if one can achieve less toxicity and treat more patients and get the same duration of clinical benefit, then less will be more,” he concluded.
Dr. Davis reported having no conflicts of interest. Study authors disclosed relationships with Astellas Pharma, AstraZeneca, Bristol-Myers Squibb, and many other companies. Erlotinib is manufactured for OSI Pharmaceuticals, an affiliate of Astellas Pharma, and distributed by Genentech, a member of the Roche Group.
The study was supported by the Japan Agency for Medical Research and Development.
SOURCE: Miyamoto S et al. JAMA Oncol. 2020 May 14; e201250. doi: 10.1001/jamaoncol.2020.1250.
, according to researchers.
They conducted a phase 2 trial to investigate whether one-third of the maximum tolerated dose of erlotinib could maintain sufficient plasma concentration of the drug while avoiding the adverse effects of higher doses. The results were published in JAMA Oncology.
Erlotinib and other epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs) have demonstrated efficacy in elderly patients with EGFR-positive NSCLC, according to study author Shingo Miyamoto, MD, of Japanese Red Cross Medical Center in Tokyo, and colleagues.
“With the increasing number of elderly patients with cancer, many of whom also have significant comorbidities, there is a considerable value in investigating whether EGFR-TKIs are effective for the frail population,” the authors wrote. They also noted that it is “difficult to identify the appropriate dose of molecular-targeted drugs.”
With this in mind, Dr. Miyamoto and colleagues conducted a single-arm, phase 2 trial of low-dose erlotinib in 80 chemotherapy-naive frail or elderly patients with EGFR-positive NSCLC. Frailty was defined by age and the Charlson Comorbidity Index. The patients’ median age was 80 years (range, 49-90 years).
Patients received erlotinib at 50 mg per day, which is one-third of the established maximum tolerated dose, for 4 weeks. Then, they were evaluated with radiologic imaging. Treatment continued until disease progression or unacceptable adverse events. Dosing was modified by treatment response or by adverse events.
Results
At last follow-up, 7 of the 80 patients were still receiving low-dose erlotinib. Reasons for discontinuation were disease progression (n = 60), patient request (n = 6), adverse events (n = 4), and death (n = 3).
The overall response rate was 60%, and the disease control rate was 90%. The researchers measured plasma erlotinib concentration in 48 patients and found it did not correlate with response.
The median progression-free survival was 9.3 months, and the median overall survival was 26.2 months.
Ten patients had erlotinib temporarily suspended because of adverse events. Five patients had their dose reduced to 25 mg because of adverse events, including oral mucositis, paronychia, erythema multiforme, diarrhea, and anorexia.
Two patients discontinued treatment because of adverse events. One patient had a cutaneous ulcer and bone infection. The other had oral mucositis.
Dr. Miyamoto and colleagues concluded that, “low-dose erlotinib was associated with efficacy and safety in frail patients with EGFR mutation–positive lung cancer. More research on the dosing strategy of target-based drugs is warranted, especially in frail patients in the real-world setting.”
Less is more
Sometimes, less can be more, said Mellar P. Davis, MD, an oncologist and section head of the palliative care department at Geisinger Medical System in Danville, Penn., who was not involved in this study.
“Why do patients benefit from small doses? It may be that there are fewer drug interruptions over time and patients are able to stay on schedule,” Dr. Davis said. “It may also be that erlotinib clearance is reduced in the elderly and comorbid patient. The reduced dose may, in fact, be the ‘therapeutic’ dose in this special population.”
Plasma levels were frequently in therapeutic ranges in this study, but patients who had subtherapeutic plasma levels also responded to therapy, Dr. Davis pointed out. The lower dose was shown to maintain sufficient concentrations of the treatment while reducing adverse effects.
However, Dr. Davis noted, this was not a randomized trial. “It is always a risk hedging bets on single-arm trials,” he said. “Randomized trials often prove phase 2 single-arm trials wrong.”
He added that quality-of-life measures are absent from the study. Erlotinib is a palliative drug with side effects, Dr. Davis noted.
“Control of cancer and cancer regression should improve symptoms and quality of life when balanced against treatment toxicity,” he said. “In this study, I would have thought that symptom improvement, performance score, and quality of life would have been the primary outcome or the co-primary outcome with disease control.”
Should a randomized, controlled trial of low-dose erlotinib be conducted in the frail/elderly population? “If one believes trials should be quantitatively based, the answer would be no,” Dr. Davis said. “Responses may be the same, and it would be expensive to prove that low-dose erlotinib is the same as standard doses when comparing survival.”
However, if one is interested in quality of life, particularly in this growing population, a trial that incorporated quality-of-life measures would make more sense, according to Dr. Davis. “For if one can achieve less toxicity and treat more patients and get the same duration of clinical benefit, then less will be more,” he concluded.
Dr. Davis reported having no conflicts of interest. Study authors disclosed relationships with Astellas Pharma, AstraZeneca, Bristol-Myers Squibb, and many other companies. Erlotinib is manufactured for OSI Pharmaceuticals, an affiliate of Astellas Pharma, and distributed by Genentech, a member of the Roche Group.
The study was supported by the Japan Agency for Medical Research and Development.
SOURCE: Miyamoto S et al. JAMA Oncol. 2020 May 14; e201250. doi: 10.1001/jamaoncol.2020.1250.
, according to researchers.
They conducted a phase 2 trial to investigate whether one-third of the maximum tolerated dose of erlotinib could maintain sufficient plasma concentration of the drug while avoiding the adverse effects of higher doses. The results were published in JAMA Oncology.
Erlotinib and other epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs) have demonstrated efficacy in elderly patients with EGFR-positive NSCLC, according to study author Shingo Miyamoto, MD, of Japanese Red Cross Medical Center in Tokyo, and colleagues.
“With the increasing number of elderly patients with cancer, many of whom also have significant comorbidities, there is a considerable value in investigating whether EGFR-TKIs are effective for the frail population,” the authors wrote. They also noted that it is “difficult to identify the appropriate dose of molecular-targeted drugs.”
With this in mind, Dr. Miyamoto and colleagues conducted a single-arm, phase 2 trial of low-dose erlotinib in 80 chemotherapy-naive frail or elderly patients with EGFR-positive NSCLC. Frailty was defined by age and the Charlson Comorbidity Index. The patients’ median age was 80 years (range, 49-90 years).
Patients received erlotinib at 50 mg per day, which is one-third of the established maximum tolerated dose, for 4 weeks. Then, they were evaluated with radiologic imaging. Treatment continued until disease progression or unacceptable adverse events. Dosing was modified by treatment response or by adverse events.
Results
At last follow-up, 7 of the 80 patients were still receiving low-dose erlotinib. Reasons for discontinuation were disease progression (n = 60), patient request (n = 6), adverse events (n = 4), and death (n = 3).
The overall response rate was 60%, and the disease control rate was 90%. The researchers measured plasma erlotinib concentration in 48 patients and found it did not correlate with response.
The median progression-free survival was 9.3 months, and the median overall survival was 26.2 months.
Ten patients had erlotinib temporarily suspended because of adverse events. Five patients had their dose reduced to 25 mg because of adverse events, including oral mucositis, paronychia, erythema multiforme, diarrhea, and anorexia.
Two patients discontinued treatment because of adverse events. One patient had a cutaneous ulcer and bone infection. The other had oral mucositis.
Dr. Miyamoto and colleagues concluded that, “low-dose erlotinib was associated with efficacy and safety in frail patients with EGFR mutation–positive lung cancer. More research on the dosing strategy of target-based drugs is warranted, especially in frail patients in the real-world setting.”
Less is more
Sometimes, less can be more, said Mellar P. Davis, MD, an oncologist and section head of the palliative care department at Geisinger Medical System in Danville, Penn., who was not involved in this study.
“Why do patients benefit from small doses? It may be that there are fewer drug interruptions over time and patients are able to stay on schedule,” Dr. Davis said. “It may also be that erlotinib clearance is reduced in the elderly and comorbid patient. The reduced dose may, in fact, be the ‘therapeutic’ dose in this special population.”
Plasma levels were frequently in therapeutic ranges in this study, but patients who had subtherapeutic plasma levels also responded to therapy, Dr. Davis pointed out. The lower dose was shown to maintain sufficient concentrations of the treatment while reducing adverse effects.
However, Dr. Davis noted, this was not a randomized trial. “It is always a risk hedging bets on single-arm trials,” he said. “Randomized trials often prove phase 2 single-arm trials wrong.”
He added that quality-of-life measures are absent from the study. Erlotinib is a palliative drug with side effects, Dr. Davis noted.
“Control of cancer and cancer regression should improve symptoms and quality of life when balanced against treatment toxicity,” he said. “In this study, I would have thought that symptom improvement, performance score, and quality of life would have been the primary outcome or the co-primary outcome with disease control.”
Should a randomized, controlled trial of low-dose erlotinib be conducted in the frail/elderly population? “If one believes trials should be quantitatively based, the answer would be no,” Dr. Davis said. “Responses may be the same, and it would be expensive to prove that low-dose erlotinib is the same as standard doses when comparing survival.”
However, if one is interested in quality of life, particularly in this growing population, a trial that incorporated quality-of-life measures would make more sense, according to Dr. Davis. “For if one can achieve less toxicity and treat more patients and get the same duration of clinical benefit, then less will be more,” he concluded.
Dr. Davis reported having no conflicts of interest. Study authors disclosed relationships with Astellas Pharma, AstraZeneca, Bristol-Myers Squibb, and many other companies. Erlotinib is manufactured for OSI Pharmaceuticals, an affiliate of Astellas Pharma, and distributed by Genentech, a member of the Roche Group.
The study was supported by the Japan Agency for Medical Research and Development.
SOURCE: Miyamoto S et al. JAMA Oncol. 2020 May 14; e201250. doi: 10.1001/jamaoncol.2020.1250.
Painful Indurated Plaque on the Groin
The Diagnosis: Cutaneous Metastasis
Histopathology demonstrated ulceration of the epidermis with necrosis of the papillary dermis. There was a diffuse infiltration of pleomorphic and atypical epithelioid cells in the reticular dermis (Figure). Focally there was ductal and glandular differentiation. The stroma was sclerotic. At the deep aspect of the biopsy specimen, tumor cells intercalated between collagen bundles in linear strands. Atypical mitoses were common, and necrosis en masse was seen. An immunohistochemical panel also was performed. Tissue from the biopsy was strongly positive for CDX-2 and cytokeratin 20 and diffusely negative for cytokeratin 7, gross cystic disease fluid protein 15, and prostate-specific antigen. The other biopsy was sent for cultures and grew no organisms, which confirmed the diagnosis of cutaneous metastasis from the patient's primary colonic adenocarcinoma. Due to the poor prognosis and his overall poor health, our patient opted for palliative care.
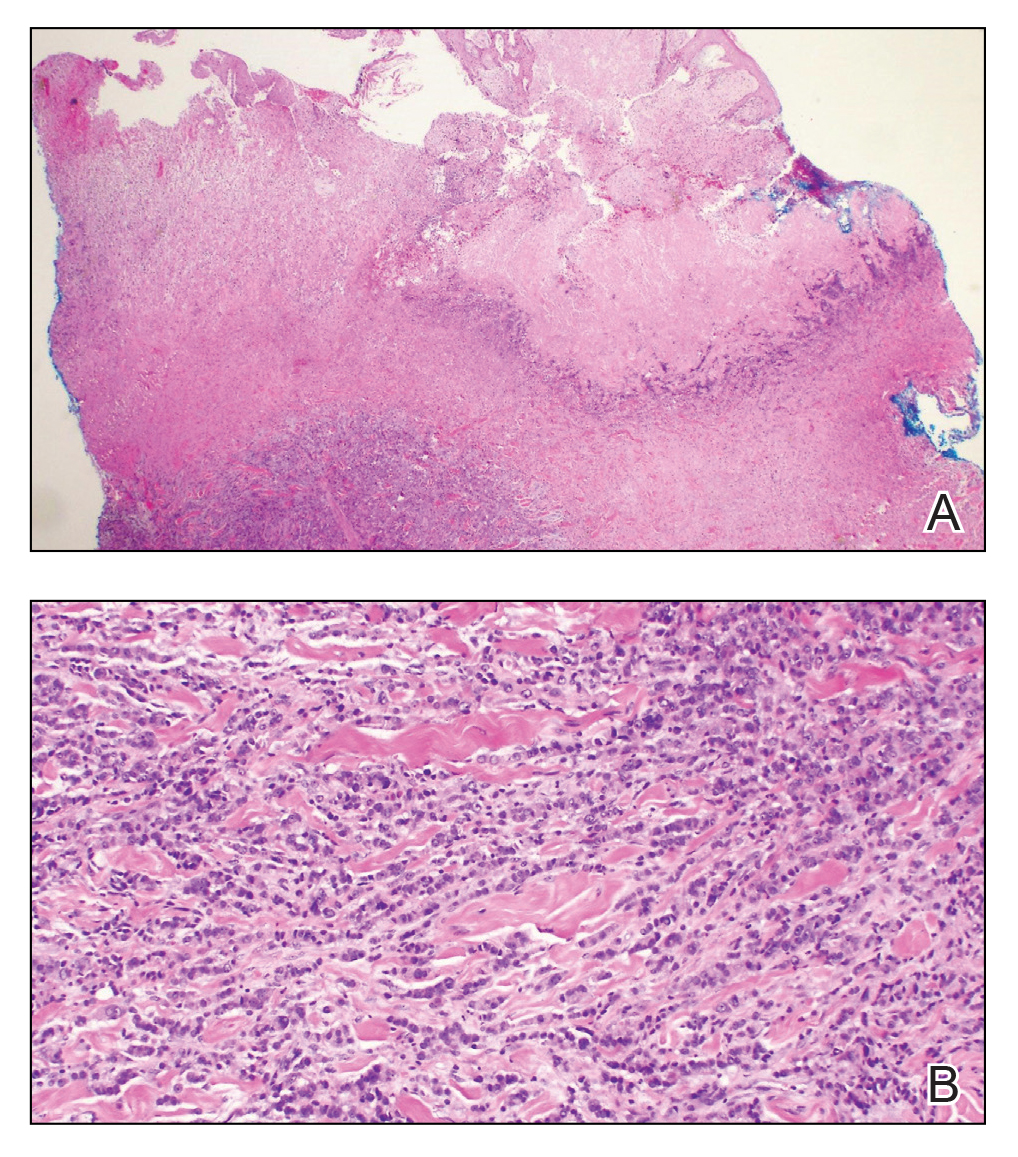
Based on large retrospective studies, the frequency of cutaneous metastasis for patients diagnosed with any malignancy is 0.7% to 9.0%.1-4 The third most common malignancy in both sexes is colorectal cancer, affecting approximately 5% of the US population.3 The frequency of cutaneous metastases from colorectal cancer is 0.81% to 3.9%.1,2,4,5 Generally, cutaneous metastases present within 2 to 3 years from diagnosis of primary malignancy.6,7 The most common sites for cutaneous metastases in a patient with colorectal cancer are the abdomen and pelvic region, often at surgical sites.1-4,6-9
The clinical presentation of cutaneous metastases varies greatly, and as a result, they commonly are misdiagnosed.6,7 Although treatment with many antibiotics and antifungals had failed in our patient, the examination still was concerning for a possible granulomatous infection vs malignancy. With the history of colon cancer, radiation treatment, and chemotherapy, the possible malignancy diagnoses included primary skin cancers, viral tumors, and cutaneous metastasis. The initial evaluations had focused on infectious causes and resulted in 6 weeks of misdiagnosis and inappropriate therapy. Despite cutaneous metastases being uncommon, there should be a high index of suspicion for lesions in patients who have a history of cancer, especially if the lesion does not respond to treatment.2,6,7
Physical examination in our patient showed a high tumor burden as well as evidence of carcinoma erysipeloides on the lower abdomen and thighs, in addition to carcinoma en cuirasse throughout the pubic region. Carcinoma erysipeloides was first described in 1893 in a patient with breast cancer: "The erythematous infiltration of the skin was very superficial, and was attended simply by redness with a slight degree of induration. Until touched by the finger the condition might easily have been taken for a slightly-marked form of erysipelas."10 The clinical findings are a result of lymphatic and vascular obstruction.3,9 The breast is the most common location to find carcinoma erysipeloides.3 It is an unusual occurrence to find it on the abdomen from colonic adenocarcinoma. The term cancer en cuirasse was coined in 1838 to describe the cutaneous manifestation of breast cancer that caused the skin to resemble the metal breastplate of a cuirasser.4 Similar to carcinoma erysipeloides, carcinoma en cuirasse most commonly is found as cutaneous metastasis from breast cancer, not from colonic adenocarcinoma.3
The histologic characteristics of cutaneous metastases in general are similar to the primary malignancy but can be more poorly differentiated.7 Generally, neoplastic cells are seen in the lymphatic and blood vessels, and a large portion of the tumor is confined to the deep dermis and in the subcutaneous fat.3,6 Histologic features of colonic adenocarcinoma metastases can demonstrate a well-differentiated, glandular architecture with mucin-secreting cells.3,8,9 There also is a histologic pattern of neoplastic cells arranging themselves between collagen bundles in linear strands; this finding more commonly is seen in adenocarcinoma of the breast but also was seen in our patient.3,9 With immunohistochemical staining, a truncated panel of cytokeratin 7, cytokeratin 20, and S-100 had a diagnostic accuracy of 100% for cutaneous metastases from colonic adenocarcinoma in one study. The pattern of all colonic adenocarcinomas was cytokeratin 20 positive and cytokeratin 7 and S-100 negative.6
Cutaneous metastases typically demonstrate widespread and rapidly progressive disease.3,9 Survival studies of cutaneous metastases showed that 48% to 66% of patients died within the first 6 months.3,6 Specifically, cutaneous metastases from colorectal cancers showed a median survival of 3 to 5 months.6,7 Currently there are no treatment guidelines for cutaneous metastases.
- Lookingbill DP, Spangler N, Helm K. Cutaneous metastases in patients with metastatic carcinoma: a retrospective study of 4020 patients. J Am Acad Dermatol. 1993;29(2 pt 1):228-236.
- Gul U, Kilic A, Gonul M, et al. Spectrum of cutaneous metastases in 1287 cases of internal malignancies: a study from Turkey. Acta Derm Venereol. 2007;87:160-162.
- Hussein MR. Skin metastasis: a pathologist's perspective. J Cutan Pathol. 2010;37:E1-E20.
- Schwartz RA. Cutaneous metastatic disease. J Am Acad Dermatol. 1995;33(2 pt 1):161-182; quiz 183-186.
- Hu S, Chen G, Wu C, et al. Rates of cutaneous metastases from different internal malignancies: experience from a Taiwanese medical center. J Am Acad Dermatol. 2009;60:379-387.
- Saeed S, Keehn C, Morgan M. Cutaneous metastasis: a clinical, pathological, and immunohistochemical appraisal. J Cutan Pathol. 2004;31:419-430.
- Sariya D, Ruth K, Adams-McDonnell R. Clinicopathologic correlation of cutaneous metastases: experience of a cancer center. Arch Dermatol. 2007;143:613-620.
- Brownstein M, Helwig E. Metastatic tumors of the skin. Cancer. 1972;29:1298-1307.
- McKee PH. Cutaneous metastases. J Cutan Pathol. 1985;12:239-250.
- Hutchinson J. Notes from congresses and continental hospitals: erythema-scirrhus of the skin in association with cancer of the breast. Arch Surg (London). 1893;4:220-222
The Diagnosis: Cutaneous Metastasis
Histopathology demonstrated ulceration of the epidermis with necrosis of the papillary dermis. There was a diffuse infiltration of pleomorphic and atypical epithelioid cells in the reticular dermis (Figure). Focally there was ductal and glandular differentiation. The stroma was sclerotic. At the deep aspect of the biopsy specimen, tumor cells intercalated between collagen bundles in linear strands. Atypical mitoses were common, and necrosis en masse was seen. An immunohistochemical panel also was performed. Tissue from the biopsy was strongly positive for CDX-2 and cytokeratin 20 and diffusely negative for cytokeratin 7, gross cystic disease fluid protein 15, and prostate-specific antigen. The other biopsy was sent for cultures and grew no organisms, which confirmed the diagnosis of cutaneous metastasis from the patient's primary colonic adenocarcinoma. Due to the poor prognosis and his overall poor health, our patient opted for palliative care.

Based on large retrospective studies, the frequency of cutaneous metastasis for patients diagnosed with any malignancy is 0.7% to 9.0%.1-4 The third most common malignancy in both sexes is colorectal cancer, affecting approximately 5% of the US population.3 The frequency of cutaneous metastases from colorectal cancer is 0.81% to 3.9%.1,2,4,5 Generally, cutaneous metastases present within 2 to 3 years from diagnosis of primary malignancy.6,7 The most common sites for cutaneous metastases in a patient with colorectal cancer are the abdomen and pelvic region, often at surgical sites.1-4,6-9
The clinical presentation of cutaneous metastases varies greatly, and as a result, they commonly are misdiagnosed.6,7 Although treatment with many antibiotics and antifungals had failed in our patient, the examination still was concerning for a possible granulomatous infection vs malignancy. With the history of colon cancer, radiation treatment, and chemotherapy, the possible malignancy diagnoses included primary skin cancers, viral tumors, and cutaneous metastasis. The initial evaluations had focused on infectious causes and resulted in 6 weeks of misdiagnosis and inappropriate therapy. Despite cutaneous metastases being uncommon, there should be a high index of suspicion for lesions in patients who have a history of cancer, especially if the lesion does not respond to treatment.2,6,7
Physical examination in our patient showed a high tumor burden as well as evidence of carcinoma erysipeloides on the lower abdomen and thighs, in addition to carcinoma en cuirasse throughout the pubic region. Carcinoma erysipeloides was first described in 1893 in a patient with breast cancer: "The erythematous infiltration of the skin was very superficial, and was attended simply by redness with a slight degree of induration. Until touched by the finger the condition might easily have been taken for a slightly-marked form of erysipelas."10 The clinical findings are a result of lymphatic and vascular obstruction.3,9 The breast is the most common location to find carcinoma erysipeloides.3 It is an unusual occurrence to find it on the abdomen from colonic adenocarcinoma. The term cancer en cuirasse was coined in 1838 to describe the cutaneous manifestation of breast cancer that caused the skin to resemble the metal breastplate of a cuirasser.4 Similar to carcinoma erysipeloides, carcinoma en cuirasse most commonly is found as cutaneous metastasis from breast cancer, not from colonic adenocarcinoma.3
The histologic characteristics of cutaneous metastases in general are similar to the primary malignancy but can be more poorly differentiated.7 Generally, neoplastic cells are seen in the lymphatic and blood vessels, and a large portion of the tumor is confined to the deep dermis and in the subcutaneous fat.3,6 Histologic features of colonic adenocarcinoma metastases can demonstrate a well-differentiated, glandular architecture with mucin-secreting cells.3,8,9 There also is a histologic pattern of neoplastic cells arranging themselves between collagen bundles in linear strands; this finding more commonly is seen in adenocarcinoma of the breast but also was seen in our patient.3,9 With immunohistochemical staining, a truncated panel of cytokeratin 7, cytokeratin 20, and S-100 had a diagnostic accuracy of 100% for cutaneous metastases from colonic adenocarcinoma in one study. The pattern of all colonic adenocarcinomas was cytokeratin 20 positive and cytokeratin 7 and S-100 negative.6
Cutaneous metastases typically demonstrate widespread and rapidly progressive disease.3,9 Survival studies of cutaneous metastases showed that 48% to 66% of patients died within the first 6 months.3,6 Specifically, cutaneous metastases from colorectal cancers showed a median survival of 3 to 5 months.6,7 Currently there are no treatment guidelines for cutaneous metastases.
The Diagnosis: Cutaneous Metastasis
Histopathology demonstrated ulceration of the epidermis with necrosis of the papillary dermis. There was a diffuse infiltration of pleomorphic and atypical epithelioid cells in the reticular dermis (Figure). Focally there was ductal and glandular differentiation. The stroma was sclerotic. At the deep aspect of the biopsy specimen, tumor cells intercalated between collagen bundles in linear strands. Atypical mitoses were common, and necrosis en masse was seen. An immunohistochemical panel also was performed. Tissue from the biopsy was strongly positive for CDX-2 and cytokeratin 20 and diffusely negative for cytokeratin 7, gross cystic disease fluid protein 15, and prostate-specific antigen. The other biopsy was sent for cultures and grew no organisms, which confirmed the diagnosis of cutaneous metastasis from the patient's primary colonic adenocarcinoma. Due to the poor prognosis and his overall poor health, our patient opted for palliative care.

Based on large retrospective studies, the frequency of cutaneous metastasis for patients diagnosed with any malignancy is 0.7% to 9.0%.1-4 The third most common malignancy in both sexes is colorectal cancer, affecting approximately 5% of the US population.3 The frequency of cutaneous metastases from colorectal cancer is 0.81% to 3.9%.1,2,4,5 Generally, cutaneous metastases present within 2 to 3 years from diagnosis of primary malignancy.6,7 The most common sites for cutaneous metastases in a patient with colorectal cancer are the abdomen and pelvic region, often at surgical sites.1-4,6-9
The clinical presentation of cutaneous metastases varies greatly, and as a result, they commonly are misdiagnosed.6,7 Although treatment with many antibiotics and antifungals had failed in our patient, the examination still was concerning for a possible granulomatous infection vs malignancy. With the history of colon cancer, radiation treatment, and chemotherapy, the possible malignancy diagnoses included primary skin cancers, viral tumors, and cutaneous metastasis. The initial evaluations had focused on infectious causes and resulted in 6 weeks of misdiagnosis and inappropriate therapy. Despite cutaneous metastases being uncommon, there should be a high index of suspicion for lesions in patients who have a history of cancer, especially if the lesion does not respond to treatment.2,6,7
Physical examination in our patient showed a high tumor burden as well as evidence of carcinoma erysipeloides on the lower abdomen and thighs, in addition to carcinoma en cuirasse throughout the pubic region. Carcinoma erysipeloides was first described in 1893 in a patient with breast cancer: "The erythematous infiltration of the skin was very superficial, and was attended simply by redness with a slight degree of induration. Until touched by the finger the condition might easily have been taken for a slightly-marked form of erysipelas."10 The clinical findings are a result of lymphatic and vascular obstruction.3,9 The breast is the most common location to find carcinoma erysipeloides.3 It is an unusual occurrence to find it on the abdomen from colonic adenocarcinoma. The term cancer en cuirasse was coined in 1838 to describe the cutaneous manifestation of breast cancer that caused the skin to resemble the metal breastplate of a cuirasser.4 Similar to carcinoma erysipeloides, carcinoma en cuirasse most commonly is found as cutaneous metastasis from breast cancer, not from colonic adenocarcinoma.3
The histologic characteristics of cutaneous metastases in general are similar to the primary malignancy but can be more poorly differentiated.7 Generally, neoplastic cells are seen in the lymphatic and blood vessels, and a large portion of the tumor is confined to the deep dermis and in the subcutaneous fat.3,6 Histologic features of colonic adenocarcinoma metastases can demonstrate a well-differentiated, glandular architecture with mucin-secreting cells.3,8,9 There also is a histologic pattern of neoplastic cells arranging themselves between collagen bundles in linear strands; this finding more commonly is seen in adenocarcinoma of the breast but also was seen in our patient.3,9 With immunohistochemical staining, a truncated panel of cytokeratin 7, cytokeratin 20, and S-100 had a diagnostic accuracy of 100% for cutaneous metastases from colonic adenocarcinoma in one study. The pattern of all colonic adenocarcinomas was cytokeratin 20 positive and cytokeratin 7 and S-100 negative.6
Cutaneous metastases typically demonstrate widespread and rapidly progressive disease.3,9 Survival studies of cutaneous metastases showed that 48% to 66% of patients died within the first 6 months.3,6 Specifically, cutaneous metastases from colorectal cancers showed a median survival of 3 to 5 months.6,7 Currently there are no treatment guidelines for cutaneous metastases.
- Lookingbill DP, Spangler N, Helm K. Cutaneous metastases in patients with metastatic carcinoma: a retrospective study of 4020 patients. J Am Acad Dermatol. 1993;29(2 pt 1):228-236.
- Gul U, Kilic A, Gonul M, et al. Spectrum of cutaneous metastases in 1287 cases of internal malignancies: a study from Turkey. Acta Derm Venereol. 2007;87:160-162.
- Hussein MR. Skin metastasis: a pathologist's perspective. J Cutan Pathol. 2010;37:E1-E20.
- Schwartz RA. Cutaneous metastatic disease. J Am Acad Dermatol. 1995;33(2 pt 1):161-182; quiz 183-186.
- Hu S, Chen G, Wu C, et al. Rates of cutaneous metastases from different internal malignancies: experience from a Taiwanese medical center. J Am Acad Dermatol. 2009;60:379-387.
- Saeed S, Keehn C, Morgan M. Cutaneous metastasis: a clinical, pathological, and immunohistochemical appraisal. J Cutan Pathol. 2004;31:419-430.
- Sariya D, Ruth K, Adams-McDonnell R. Clinicopathologic correlation of cutaneous metastases: experience of a cancer center. Arch Dermatol. 2007;143:613-620.
- Brownstein M, Helwig E. Metastatic tumors of the skin. Cancer. 1972;29:1298-1307.
- McKee PH. Cutaneous metastases. J Cutan Pathol. 1985;12:239-250.
- Hutchinson J. Notes from congresses and continental hospitals: erythema-scirrhus of the skin in association with cancer of the breast. Arch Surg (London). 1893;4:220-222
- Lookingbill DP, Spangler N, Helm K. Cutaneous metastases in patients with metastatic carcinoma: a retrospective study of 4020 patients. J Am Acad Dermatol. 1993;29(2 pt 1):228-236.
- Gul U, Kilic A, Gonul M, et al. Spectrum of cutaneous metastases in 1287 cases of internal malignancies: a study from Turkey. Acta Derm Venereol. 2007;87:160-162.
- Hussein MR. Skin metastasis: a pathologist's perspective. J Cutan Pathol. 2010;37:E1-E20.
- Schwartz RA. Cutaneous metastatic disease. J Am Acad Dermatol. 1995;33(2 pt 1):161-182; quiz 183-186.
- Hu S, Chen G, Wu C, et al. Rates of cutaneous metastases from different internal malignancies: experience from a Taiwanese medical center. J Am Acad Dermatol. 2009;60:379-387.
- Saeed S, Keehn C, Morgan M. Cutaneous metastasis: a clinical, pathological, and immunohistochemical appraisal. J Cutan Pathol. 2004;31:419-430.
- Sariya D, Ruth K, Adams-McDonnell R. Clinicopathologic correlation of cutaneous metastases: experience of a cancer center. Arch Dermatol. 2007;143:613-620.
- Brownstein M, Helwig E. Metastatic tumors of the skin. Cancer. 1972;29:1298-1307.
- McKee PH. Cutaneous metastases. J Cutan Pathol. 1985;12:239-250.
- Hutchinson J. Notes from congresses and continental hospitals: erythema-scirrhus of the skin in association with cancer of the breast. Arch Surg (London). 1893;4:220-222
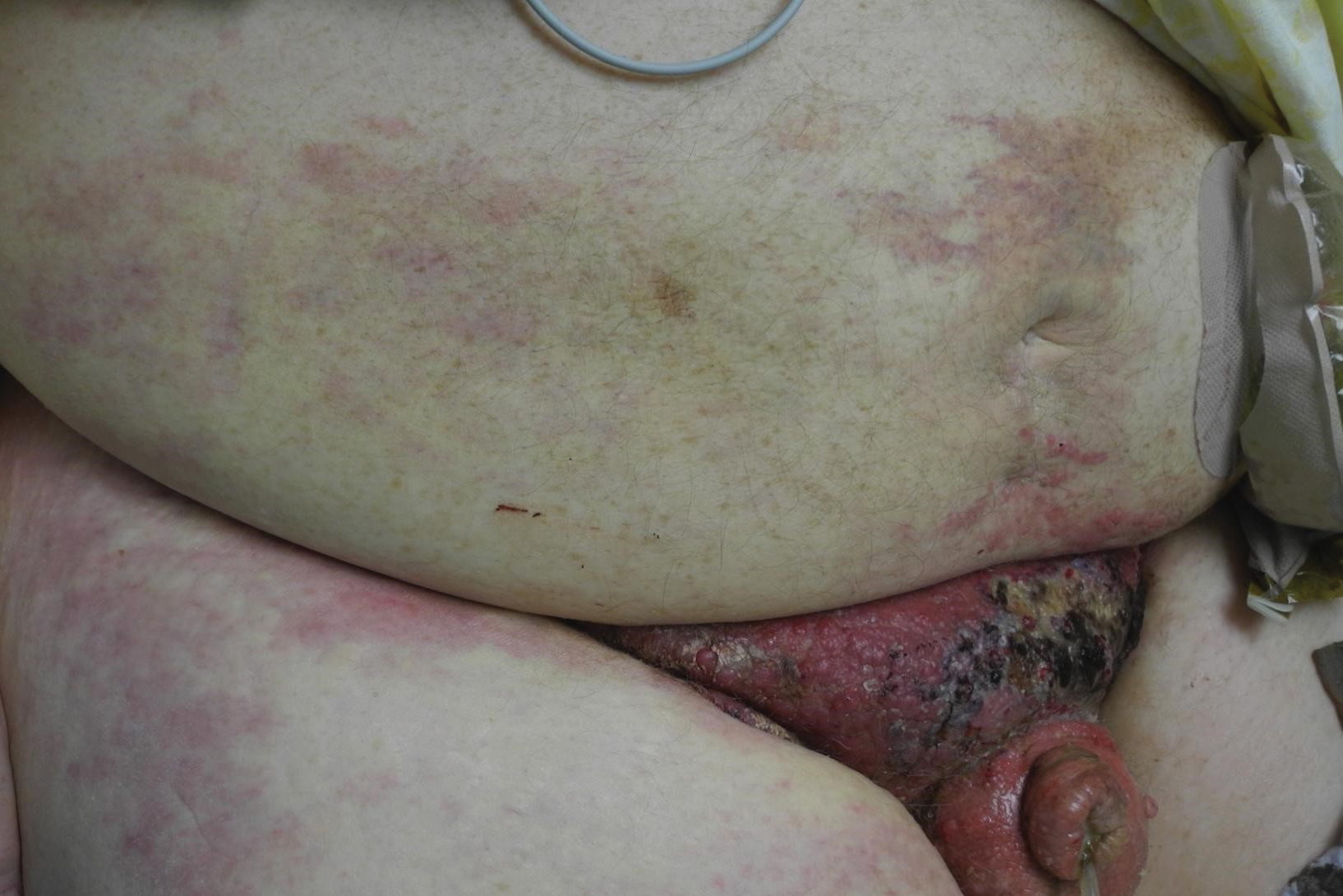
A 67-year-old man presented with a chronic lesion on the groin of 6 weeks' duration. The patient had a history of type 2 diabetes mellitus and colonic adenocarcinoma diagnosed 4 years prior that was treated with a colectomy, radiation therapy, and chemotherapy. Six weeks prior to the current presentation, the patient first sought treatment of swelling, redness, pain, and a bumpy texture on the groin. He was unsuccessfully managed by several physicians including at a long-term care facility where he was admitted and treated for presumed cellulitis. Attempted treatments included a topical antifungal, fluconazole, ciprofloxacin, metronidazole, cefepime, clindamycin, daptomycin, and vancomycin. The affected area continued to worsen along with the patient's overall health. He was transferred to the hospital for more advanced care and was evaluated by inpatient dermatology. Physical examination revealed firm, pink to red-brown, ulcerating papulonodules that coalesced into a large indurated plaque over the pubis, scrotum, penis, and inguinal folds (top). There also were red-violet, indurated plaques on the lower abdomen and bilateral proximal thighs (bottom). Punch biopsies were taken from the indurated area on the left side of the pubis--one for histopathologic evaluation and the other for bacterial, fungal, atypical mycobacterial, and Nocardia tissue cultures.
Implementation of a Patient Blood Management Program in a Large, Diverse Multi-Hospital System
From BJC HealthCare, St. Louis, MO.
Abstract
Background: There is limited literature relating to patient blood management (PBM) programs in large multi-hospital systems or addressing challenges of implementation across diverse systems comprised of community and academic hospitals.
Objective: To establish a PBM program to improve utilization of blood transfusion units at a multi-hospital system in the Midwest (BJC HealthCare).
Methods: High-impact strategies in establishing the PBM program included formation of Clinical Expert Councils (CECs) of providers, establishment of consensus utilization guidelines, and development of a robust reporting tool. CECs enabled collaboration and facilitated standardization across a complex system of academic, private practice, and tertiary facilities with a diverse community of medical providers. Consensus guidelines and the PBM reporting tool were key to creating meaningful reports to drive provider practice change.
Results: Over the 5 years following implementation of the PBM program, there has been a steady decrease in red blood cell (RBC) utilization. Noticeable changes have taken place at individual hospitals in the system, including reductions in transfusions falling outside guideline parameters from 300 per quarter to less than 8 per quarter at 1 of our community hospitals. No negative impact on patient care has been identified.
Conclusion: In response to current transfusion guidelines and the need for optimizing stewardship of blood product resources, this hospital system successfully implemented a robust PBM program that engaged academic and non-academic community providers and decreased utilization of blood transfusion resources in line with consensus guidelines.
Keywords: quality improvement; RBC transfusion; transfusion practices; provider practice change; utilization trends.
Evidence from clinical trials and published clinical guidelines support the adoption of a restrictive blood transfusion approach in hospitalized, stable patients as best practice.1-5 As such, the development and implementation of patient blood management (PBM) programs has become an increasingly important process improvement for reducing variability in transfusion practices and clinical outcomes.
As recently as 2013, BJC HealthCare, a multi-hospital system in the Midwest, had no standardized, system-wide blood management program, and transfusion practices varied widely across providers and between individual hospitals based on size, patient population, and resources. The system consisted of 13 hospitals, ranging from large tertiary to smaller community and academic hospitals. Although adults constituted the vast majority of the patient population, the hospital system also included a pediatric specialty hospital, St. Louis Children’s Hospital. In addition, some sites were staffed by private practice providers and others by university-based providers, including blood bank medical directors. Due to the diversity of settings and populations, efforts to align transfusion and other practices often faced multiple challenges. However, improving the management of blood transfusions was identified as a key resource stewardship priority in 2013, and implementation of a system-wide program began after extensive discussions and consensus approval by senior hospital system and medical leadership. The primary aim of the program was to optimize overall blood product resource stewardship. Specifically, we sought to control or reduce costs per patient-care episode using strategies that would not negatively impact patient care and could potentially even improve patient outcomes (eg, by avoiding unnecessary transfusions and their attendant risks).
There is a plethora of literature related to the implemention of PBM programs in individual hospitals,6-18 but few reports specifically relate to large multi-hospital health systems,19-21 or directly address the unique challenges of implementation across a diverse system of community and academic hospitals and providers.19 Here, we discuss our experience with establishing a PBM program in a large, diverse, multi-hospital health system, provide examples of innovative strategies, and address challenges faced and lessons learned. Future endeavors of the PBM program at BJC HealthCare are also described.
Setting
BJC HealthCare is one of the largest nonprofit health care organizations in the United States, delivering services to the greater St. Louis, southern Illinois, and mid-Missouri regions, and addressing the health care needs of urban, suburban, and rural communities. As of 2018, the system included 15 hospitals and multiple community health locations comprising more than 3400 staffed beds, 31,500 employees, and 4300 physicians with privileges. The system annually has more than 151,000 hospital admissions, 81,000 outpatient surgery visits, and 537,000 emergency department visits. In addition to inpatient and outpatient care, services include primary care, community health and wellness, workplace health, home health, community mental health, rehabilitation, long-term care, and hospice. As a nonprofit system, BJC is the largest provider of charity care, unreimbursed care, and community benefit in Missouri, highlighting the fact that resource stewardship is a critical issue across the entire system and the communities served.22
PBM Project
Preparation for large-scale change across several hospitals began with creating a framework for the initiative, which consisted of a “burning platform,” a guiding vision, and a coalition. The burning platform identifies the importance and urgency of a change and helps to establish commitment. Between 2012 and 2014, the American Association of Blood Banks (AABB) released new evidence-based guidelines and recommendations calling for more restrictive transfusion practices pertaining to red blood cells (RBCs; ie, a hemoglobin threshold of 7 to 8 g/dL) in both inpatient and outpatient care.2 In addition, use of single-unit transfusions was recognized as best practice by the AABB in the Choosing Wisely campaign.23 Historically, adult patients requiring transfusions were given 2 units in succession. The new recommendations provided a strong basis for changing transfusion practices at BJC. It was believed that aligning transfusion practices with the new guidelines was consistent with the mission and vision of the work: that these changes could lead to optimization of resources, cost control, reductions in unnecessary blood transfusions, and potentially improved care (eg, fewer transfusion-related complications). We used the national guidelines to initiate discussions and to identify clinical conditions and associated laboratory parameters for transfusion therapy.
Once this burning platform was established, a team comprised of physicians, blood bank experts, quality consultants, data analysts, and supply managers, referred to as the Outcomes Team, was formed to lead the change efforts across the system. Initial projects for the team included developing system-wide consensus-based transfusion guidelines, providing education to providers on the new evidence in transfusion practice, and sharing BJC-specific historical utilization data. The guiding principle for the group was that “blood is a valuable resource, but not without risk, and less is more.” In order to disseminate the vision of the initiative across the system, campaign signs with the slogans “7 is the new 10” (referring to the g/dL transfusion threshold) and “1 is the new 2” (referring to the new practice of the preferential transfusion of single units rather than 2 at a time) were displayed in system hospitals.
Last, a guiding coalition of system leaders was needed to help push the initiative forward and sustain the program once fully implemented. Thus, a multidisciplinary PBM Clinical Expert Counciel (CEC) was formed to assist with implementation and maintenance of the program.
Role of PBM Clinical Expert Council
The PBM CEC was designed to improve overall physician and expert engagement and provide a forum where stakeholders from across the system could participate to voice their expert opinion. CECs (which BJC formed in other clinical areas as well) are multidisciplinary teams consisting of clinical, administrative, and technical staff. The open, multidisciplinary structure of the councils allows for collaboration that promotes change across a complex multi-hospital system. Each hospital is represented by key physicians and technical leaders, opening opportunity for both horizontal and vertical partnership.
As part of the overall physician engagement strategy, the PBM CEC was launched across BJC in November 2013 as a decision-making body for gaining system consensus on matters relating to blood management. The initial goals for the PBM CEC were to share information and educate providers and others on the latest evidence, to subsequently debate and develop consensus for guidelines to be applied across BJC, and to identify and adopt gold standard practices to drive and sustain compliance across the system. More specifically, we wanted to focus on how to avoid unnecessary blood transfusions known to be associated with increased risk for adverse reactions, other morbidity, mortality, and longer length of stay. Council members met quarterly to address 6 key drivers: patient safety, informatics and data, quality improvement, efficiencies and workflows, education and competency, and communication and engagement. Members then voted to approve guidelines, policies, and procedures. The group continues to assist in updating and standardizing guidelines and providing input on improving the functionality of the PBM reporting tool.
Development of the PBM Reporting Tool
Providing and sharing data on blood utilization and practices with the CEC and hospital leaders was imperative to driving change. The Outcomes Team deliberated on how best to generate and provide such information, conducting comparisons between selected vendor-based tools and potential internal BJC solutions. After investigation, BJC leadership approved the development of an in-house PBM dashboard tool using Tableau Desktop (Tableau Software, Inc.). The tool consists of an executive page with 5 additional tabs for navigating to the appropriate information (Figure 1 and Figure 2); data within the tool are organized by facility, service, provider, ICD diagnosis, transfusion indication, and the Clinical Classifications Software category, as defined by the Agency for Healthcare Research and Quality.
The PBM reporting tool was launched on December 31, 2014. The next priority after the launch was to validate the tool’s blood utilization data and implement enhancements to make the tool more effective for users. A super-user group consisting of blood bank supervisors and managers was established. The goals of the user group were to preview any enhancements before presenting the tool to the larger CEC, test and validate data once new information was added, and share and prioritize future enhancements. User group meetings were held monthly to share best practices and discuss individual facilities’ blood utilization data. In addition, each facility’s representative(s) shared how they were driving changes in provider practice and discussed challenges specific to their facility. Enhancements suggested through the user group included: incorporation of additional lab values into the tool to correspond with other blood products (eg, fibrinogen, hematocrit, international normalized ratio, and platelet count), addition of the specific location where the blood product was administered, and standard naming conventions of locations to allow comparisons across facilities (eg, Emergency Department instead of ED, ER, or EU).
All hospital users were given access to a test version of the reporting tool where they could review enhancements, identify what worked well and what could be done better, and suggest corrections. As changes were made to the hospital lab systems, a sample of data was reviewed and validated with affected facilities to confirm the continued accuracy of the data. To ensure its practicality to users, the tool continues to be improved upon with input from council stakeholders and subject-matter experts.
Measurements
To monitor blood utilization across the health system, we tracked the total RBC units administered by hospital, service, and provider and also tracked pre- and post-transfusion hemoglobin values.
Results
Overall, the system has seen a steady decrease in RBC utilization over the 5 years since the PBM program was implemented (Table
In addition to system-wide improvement, noticeable changes have taken place at individual hospitals in the BJC system. For example, Boone Hospital Center in Columbia, Missouri, began critically reviewing all RBC transfusions starting in 2015 and, to raise awareness, communicating with any provider who transfused a patient outside of transfusion guidelines. Since then, Boone Hospital has seen a dramatic reduction in transfusions considered noncompliant (ie, falling outside guideline parameters), from 300 transfusions per quarter, down to less than 8 per quarter. St. Louis Children’s Hospital also began reviewing blood products utilized by providers that fell outside of the standardized guidelines. At this hospital, physician champions discuss any outliers with the providers involved and use multiple methods for disseminating information to providers, including grand rounds, faculty meetings, and new resident orientations.
Another success has been the partnership between Barnes Jewish St. Peters and Progress West Hospitals in providing PBM education. Their joint effort resulted in implementation of education modules in BJC’s internal learning system, and has provided PBM-related education to more than 367 nurses, blood bank staff, and physicians.
Challenges and Lessons Learned
Implementation of the PBM program was generally successful, but it was not without challenges. One of the biggest challenges was addressing the variation in care and practices across the hospital enterprise. Due to the varying sizes and service goals of individual hospitals, lack of standardization was a significant barrier to change. Gaining trust and buy-in was imperative to increasing compliance with new transfusion policies. The primary concern was finding a balance between respecting physician autonomy and emphasizing and aligning practices with new evidence in the literature. Thus, understanding and applying principles of thoughtful change management was imperative to advancing the framework of the PBM program. The CEC venue enabled collaboration among hospitals and staff and was ultimately used to facilitate the necessary standardization process. To gain the trust of hospital and medical staff, the Outcomes Team conducted several site visits, enabling face-to-face interaction with frontline staff and operational leaders. Moreover, the team’s emphasis on the use of the latest evidence-based guidelines in discussions with hospital and medical staff underscored the need for change.
Frank et al19 describes using an approach similar to our Outcomes Team at the Johns Hopkins Health System. A designated multidisciplinary quality improvement team, referred to as the “clinical community,” worked on implementing best practices for blood management across a system of 5 hospitals. The authors reported similar results, with an overall decrease in number of units transfused, as well as substantial cost savings.19 Our project, along with the project implemented by Frank et al, shows how a “consensus-community” approach, involving stakeholders and various experts across the system, can be be used to align practices among multiple hospitals.
Development of a robust PBM reporting tool was key to creating meaningful monthly reports and driving provider practice change. However, this did require several training sessions, site visits, and computer-based training. Members of the Outcomes Team engaged in one-on-one sessions with tool users as a way of addressing specific areas of concern raised by staff at individual blood banks, and also took part in system-wide initiatives. The team also attended blood bank staff meetings and hospital transfusion committee meetings to educate staff on the evidence and initiative, provide demos of the reporting tool, and allow for a more robust discussion of how the data could be used and shared with other departments. These sessions provided opportunities to identify and prioritize future enhancements, as well as opportunities for continued education and discussion at hospitals, which were critical to ongoing improvement of the reporting tool.
Conclusion and Future Directions
Blood products remain extremely valuable and scarce resources, and all health care professionals must work to prevent unnecessary transfusions and improve clinical outcomes by adhering to the latest evidence-based guidelines. In response to current transfusion guidelines and the need to optimize blood product resources, our system successfully implemented a robust PBM program that engaged both academic and non-academic providers and communities. Several elements of the program helped us overcome the challenges relating to standardization of transfusion practices: consensus-based development of guidelines using the latest scientific evidence; formation and utilization of the CEC venue to gain system-wide consensus around both guidelines and approaches to change; development of a trustworthy and accessible PBM reporting tool (as well as continuing education sessions to improve adoption and utilization of the tool); and ongoing multidisciplinary discussions and support of thoughtful change and sustaining activities. We have seen a system-wide decrease in the number of RBC units transfused (absolute and per case mix-adjusted patient day) since implementing the PBM program, and in the following years have noted a trending decrease in transfusion-related safety events. Although there was a slight increase in reported safety events from 2018 to 2019, this was likely due to the systematic implementation of a new electronic medical record system and improved reporting infrastructure.
Upcoming phases of our system-wide PBM program will include looking at opportunities to improve blood utilization in other specific clinical areas. For example, we have begun discussions with hematology and oncology experts across the system to expand their patient population data within the PBM reporting tool, and to identify areas of opportunity for provider practice change within their specialty. We are also reviewing cardiothoracic surgery transfusion data to identify opportunities for reducing blood utilization in specific clinical scenarios. In addition, we are working to incorporate our 2 newest hospital system members (Memorial Hospital East and Memorial Hospital Belleville) into the PBM program. In collaboration with perioperative leaders across the system, the surgical blood ordering process is being reviewed. The goal of this effort is to reduce blood products ordered in preparation for surgical procedures. We are also currently investigating whether an impact on safety events (ie, reduction in transfusion reactions) can yet be detected. Last, our health care system recently launched a system-wide electronic medical record, and we are eager to see how this will provide us with new methods to monitor and analyze blood administration and utilization data. We look forward to reporting on the expansion of our program and on any clinical outcome improvements gained through avoidance of unnecessary transfusions.
Acknowledgment: The authors thank the leadership within the Center for Clinical Excellence and Supply Chain at BJC HealthCare for their support of this manuscript, as well as all system participants who have contributed to these efforts, especially Mohammad Agha, MD, MHA, current physician leader of the PBM CEC, for his thoughtful edits of this manuscript.
Corresponding author: Audrey A. Gronemeyer, MPH, Center for Clinical Excellence, BJC HealthCare, 8300 Eager Road, Suite 400A, St. Louis, MO 63144; audrey.gronemeyer@bjc.org.
Financial disclosures: None.
1. Carson JL, Grossman BJ, Kleinman S, et al. Red blood cell transfusion: A clinical practice guideline from the AABB*. Ann Intern Med. 2012;157:49-58.
2. Goodnough LT, Levy JH, Murphy MF. Concepts of blood transfusion in adults. Lancet. 2013;381:1845-1854.
3. Hébert PC, Carson JL. Transfusion threshold of 7 g per deciliter—The new normal. N Engl J Med. 2014;371:1459-1461.
4. Gani F, Cerullo M, Ejaz A, et al. Implementation of a blood management program at a tertiary care hospital: Effect on transfusion practices and clinical outcomes among patients undergoing surgery. Ann Surg. 2019;269:1073-1079.
5. Podlasek SJ, Thakkar RN, Rotello LC, et al. Implementing a “why give 2 when 1 will do?” Choosing Wisely campaign. Transfusion. 2016;56:2164.
6. Boral LI, Bernard A, Hjorth T, et al. How do I implement a more restrictive transfusion trigger of hemoglobin level of 7 g/dL at my hospital? Transfusion. 2015;55:937-945.
7. Geissler RG, Kosters C, Franz D, et al. Utilization of blood components in trauma surgery: A single-center, retrospective analysis before and after the implementation of an educative PBM initiative. Transfuse Med Hemother. 2015;42:83-89.
8. Goel R, Cushing MM, Tobian AA. Pediatric patient blood management programs: Not just transfusing little adults. Transfus Med Rev. 2016;30:235-241.
9. Gupta PB, DeMario VM, Amin RM, et al. Patient blood management program improves blood use and clinical outcomes in orthopedic surgery. Anesthesiology. 2018;129;1082-1091.
10. Leahy MF, Roberts H, Mukhtar SA, et al. A pragmatic approach to embedding patient blood management in a tertiary hospital. Transfusion. 2014;54:1133-1145.
11. Leahy MF, Hofmann A, Towler S, et al. Improved outcomes and reduced costs associated with a health-system-wide patient blood management program: A retrospective observational study in four major adult tertiary-care hospitals. Transfusion. 2017;57:1347-1358.
12. Meybohm P, Herrmann E, Steinbicker AU, et al. Patient blood management is associated with a substantial reduction of red blood cell utilization and safe for patient’s outcome: A prospective, multicenter cohort study with a noninferiority design. Ann Surg. 2016;264:203-211.
13. Morgan PN, Coleman PL, Martinez-Garduno CM, et al. Implementation of a patient blood management program in an Australian private hospital orthopedic unit. J Blood Med. 2018;9;83-90.
14. Norgaard A, Stensballe J, de Lichtenberg TH, et al. Three-year follow-up of implementation of evidence-based transfusion practice in a tertiary hospital. Vox Sang. 2017;112:229-239.
15. Meuller MM, Van Remoortel H, Meybohm P, et al. Patient blood management: Recommendations from the 2018 Frankfurt Consensus Conference. JAMA. 2019;321:983-997.
16. Oliver JC, Griffin RL, Hannon T, Marques MB. The success of our patient blood management program depended on an institution-wide change in transfusion practices. Transfusion. 2014;54:2617-2624.
17. Thakkar RN, Lee KH, Ness PM, et al. Relative impact of a patient blood management program on utilization of all three major blood components. Transfusion. 2016;56:2212-2220.
18. Yang WW, Thakkar RN, Gehrie EA, et al. Single-unit transfusions and hemoglobin trigger: relative impact on red cell utilization. Transfusion. 2017;57:1163-1170.
19. Frank SM, Thakkar RN, Podlasek SJ, et al. Implementing a health system-wide patient blood management program with a clinical community approach. Anesthesiology. 2017;127;754-764.
20. Verdecchia NM, Wisniewski MK, Waters JH, et al. Changes in blood product utilization in a seven-hospital system after the implementation of a patient blood management program: A 9-year follow-up. Hematology. 2016;21:490-499.
21. Yazer MH, Waters JH. How do I implement a hospital-based blood management program? Transfusion. 2012;52:1640-1645.
22. BJC HealthCare. Facts and Figures.. BJC HealthCare website. www.bjc.org/About-Us/Facts-Figures. Accessed November 18, 2019.
23. Callum JL, Waters JH, Shaz BH, et al. The AABB recommendations for the Choosing Wisely campaign of the American Board of Internal Medicine. Transfusion. 2014;54:2344-2352.
From BJC HealthCare, St. Louis, MO.
Abstract
Background: There is limited literature relating to patient blood management (PBM) programs in large multi-hospital systems or addressing challenges of implementation across diverse systems comprised of community and academic hospitals.
Objective: To establish a PBM program to improve utilization of blood transfusion units at a multi-hospital system in the Midwest (BJC HealthCare).
Methods: High-impact strategies in establishing the PBM program included formation of Clinical Expert Councils (CECs) of providers, establishment of consensus utilization guidelines, and development of a robust reporting tool. CECs enabled collaboration and facilitated standardization across a complex system of academic, private practice, and tertiary facilities with a diverse community of medical providers. Consensus guidelines and the PBM reporting tool were key to creating meaningful reports to drive provider practice change.
Results: Over the 5 years following implementation of the PBM program, there has been a steady decrease in red blood cell (RBC) utilization. Noticeable changes have taken place at individual hospitals in the system, including reductions in transfusions falling outside guideline parameters from 300 per quarter to less than 8 per quarter at 1 of our community hospitals. No negative impact on patient care has been identified.
Conclusion: In response to current transfusion guidelines and the need for optimizing stewardship of blood product resources, this hospital system successfully implemented a robust PBM program that engaged academic and non-academic community providers and decreased utilization of blood transfusion resources in line with consensus guidelines.
Keywords: quality improvement; RBC transfusion; transfusion practices; provider practice change; utilization trends.
Evidence from clinical trials and published clinical guidelines support the adoption of a restrictive blood transfusion approach in hospitalized, stable patients as best practice.1-5 As such, the development and implementation of patient blood management (PBM) programs has become an increasingly important process improvement for reducing variability in transfusion practices and clinical outcomes.
As recently as 2013, BJC HealthCare, a multi-hospital system in the Midwest, had no standardized, system-wide blood management program, and transfusion practices varied widely across providers and between individual hospitals based on size, patient population, and resources. The system consisted of 13 hospitals, ranging from large tertiary to smaller community and academic hospitals. Although adults constituted the vast majority of the patient population, the hospital system also included a pediatric specialty hospital, St. Louis Children’s Hospital. In addition, some sites were staffed by private practice providers and others by university-based providers, including blood bank medical directors. Due to the diversity of settings and populations, efforts to align transfusion and other practices often faced multiple challenges. However, improving the management of blood transfusions was identified as a key resource stewardship priority in 2013, and implementation of a system-wide program began after extensive discussions and consensus approval by senior hospital system and medical leadership. The primary aim of the program was to optimize overall blood product resource stewardship. Specifically, we sought to control or reduce costs per patient-care episode using strategies that would not negatively impact patient care and could potentially even improve patient outcomes (eg, by avoiding unnecessary transfusions and their attendant risks).
There is a plethora of literature related to the implemention of PBM programs in individual hospitals,6-18 but few reports specifically relate to large multi-hospital health systems,19-21 or directly address the unique challenges of implementation across a diverse system of community and academic hospitals and providers.19 Here, we discuss our experience with establishing a PBM program in a large, diverse, multi-hospital health system, provide examples of innovative strategies, and address challenges faced and lessons learned. Future endeavors of the PBM program at BJC HealthCare are also described.
Setting
BJC HealthCare is one of the largest nonprofit health care organizations in the United States, delivering services to the greater St. Louis, southern Illinois, and mid-Missouri regions, and addressing the health care needs of urban, suburban, and rural communities. As of 2018, the system included 15 hospitals and multiple community health locations comprising more than 3400 staffed beds, 31,500 employees, and 4300 physicians with privileges. The system annually has more than 151,000 hospital admissions, 81,000 outpatient surgery visits, and 537,000 emergency department visits. In addition to inpatient and outpatient care, services include primary care, community health and wellness, workplace health, home health, community mental health, rehabilitation, long-term care, and hospice. As a nonprofit system, BJC is the largest provider of charity care, unreimbursed care, and community benefit in Missouri, highlighting the fact that resource stewardship is a critical issue across the entire system and the communities served.22
PBM Project
Preparation for large-scale change across several hospitals began with creating a framework for the initiative, which consisted of a “burning platform,” a guiding vision, and a coalition. The burning platform identifies the importance and urgency of a change and helps to establish commitment. Between 2012 and 2014, the American Association of Blood Banks (AABB) released new evidence-based guidelines and recommendations calling for more restrictive transfusion practices pertaining to red blood cells (RBCs; ie, a hemoglobin threshold of 7 to 8 g/dL) in both inpatient and outpatient care.2 In addition, use of single-unit transfusions was recognized as best practice by the AABB in the Choosing Wisely campaign.23 Historically, adult patients requiring transfusions were given 2 units in succession. The new recommendations provided a strong basis for changing transfusion practices at BJC. It was believed that aligning transfusion practices with the new guidelines was consistent with the mission and vision of the work: that these changes could lead to optimization of resources, cost control, reductions in unnecessary blood transfusions, and potentially improved care (eg, fewer transfusion-related complications). We used the national guidelines to initiate discussions and to identify clinical conditions and associated laboratory parameters for transfusion therapy.
Once this burning platform was established, a team comprised of physicians, blood bank experts, quality consultants, data analysts, and supply managers, referred to as the Outcomes Team, was formed to lead the change efforts across the system. Initial projects for the team included developing system-wide consensus-based transfusion guidelines, providing education to providers on the new evidence in transfusion practice, and sharing BJC-specific historical utilization data. The guiding principle for the group was that “blood is a valuable resource, but not without risk, and less is more.” In order to disseminate the vision of the initiative across the system, campaign signs with the slogans “7 is the new 10” (referring to the g/dL transfusion threshold) and “1 is the new 2” (referring to the new practice of the preferential transfusion of single units rather than 2 at a time) were displayed in system hospitals.
Last, a guiding coalition of system leaders was needed to help push the initiative forward and sustain the program once fully implemented. Thus, a multidisciplinary PBM Clinical Expert Counciel (CEC) was formed to assist with implementation and maintenance of the program.
Role of PBM Clinical Expert Council
The PBM CEC was designed to improve overall physician and expert engagement and provide a forum where stakeholders from across the system could participate to voice their expert opinion. CECs (which BJC formed in other clinical areas as well) are multidisciplinary teams consisting of clinical, administrative, and technical staff. The open, multidisciplinary structure of the councils allows for collaboration that promotes change across a complex multi-hospital system. Each hospital is represented by key physicians and technical leaders, opening opportunity for both horizontal and vertical partnership.
As part of the overall physician engagement strategy, the PBM CEC was launched across BJC in November 2013 as a decision-making body for gaining system consensus on matters relating to blood management. The initial goals for the PBM CEC were to share information and educate providers and others on the latest evidence, to subsequently debate and develop consensus for guidelines to be applied across BJC, and to identify and adopt gold standard practices to drive and sustain compliance across the system. More specifically, we wanted to focus on how to avoid unnecessary blood transfusions known to be associated with increased risk for adverse reactions, other morbidity, mortality, and longer length of stay. Council members met quarterly to address 6 key drivers: patient safety, informatics and data, quality improvement, efficiencies and workflows, education and competency, and communication and engagement. Members then voted to approve guidelines, policies, and procedures. The group continues to assist in updating and standardizing guidelines and providing input on improving the functionality of the PBM reporting tool.
Development of the PBM Reporting Tool
Providing and sharing data on blood utilization and practices with the CEC and hospital leaders was imperative to driving change. The Outcomes Team deliberated on how best to generate and provide such information, conducting comparisons between selected vendor-based tools and potential internal BJC solutions. After investigation, BJC leadership approved the development of an in-house PBM dashboard tool using Tableau Desktop (Tableau Software, Inc.). The tool consists of an executive page with 5 additional tabs for navigating to the appropriate information (Figure 1 and Figure 2); data within the tool are organized by facility, service, provider, ICD diagnosis, transfusion indication, and the Clinical Classifications Software category, as defined by the Agency for Healthcare Research and Quality.
The PBM reporting tool was launched on December 31, 2014. The next priority after the launch was to validate the tool’s blood utilization data and implement enhancements to make the tool more effective for users. A super-user group consisting of blood bank supervisors and managers was established. The goals of the user group were to preview any enhancements before presenting the tool to the larger CEC, test and validate data once new information was added, and share and prioritize future enhancements. User group meetings were held monthly to share best practices and discuss individual facilities’ blood utilization data. In addition, each facility’s representative(s) shared how they were driving changes in provider practice and discussed challenges specific to their facility. Enhancements suggested through the user group included: incorporation of additional lab values into the tool to correspond with other blood products (eg, fibrinogen, hematocrit, international normalized ratio, and platelet count), addition of the specific location where the blood product was administered, and standard naming conventions of locations to allow comparisons across facilities (eg, Emergency Department instead of ED, ER, or EU).
All hospital users were given access to a test version of the reporting tool where they could review enhancements, identify what worked well and what could be done better, and suggest corrections. As changes were made to the hospital lab systems, a sample of data was reviewed and validated with affected facilities to confirm the continued accuracy of the data. To ensure its practicality to users, the tool continues to be improved upon with input from council stakeholders and subject-matter experts.
Measurements
To monitor blood utilization across the health system, we tracked the total RBC units administered by hospital, service, and provider and also tracked pre- and post-transfusion hemoglobin values.
Results
Overall, the system has seen a steady decrease in RBC utilization over the 5 years since the PBM program was implemented (Table
In addition to system-wide improvement, noticeable changes have taken place at individual hospitals in the BJC system. For example, Boone Hospital Center in Columbia, Missouri, began critically reviewing all RBC transfusions starting in 2015 and, to raise awareness, communicating with any provider who transfused a patient outside of transfusion guidelines. Since then, Boone Hospital has seen a dramatic reduction in transfusions considered noncompliant (ie, falling outside guideline parameters), from 300 transfusions per quarter, down to less than 8 per quarter. St. Louis Children’s Hospital also began reviewing blood products utilized by providers that fell outside of the standardized guidelines. At this hospital, physician champions discuss any outliers with the providers involved and use multiple methods for disseminating information to providers, including grand rounds, faculty meetings, and new resident orientations.
Another success has been the partnership between Barnes Jewish St. Peters and Progress West Hospitals in providing PBM education. Their joint effort resulted in implementation of education modules in BJC’s internal learning system, and has provided PBM-related education to more than 367 nurses, blood bank staff, and physicians.
Challenges and Lessons Learned
Implementation of the PBM program was generally successful, but it was not without challenges. One of the biggest challenges was addressing the variation in care and practices across the hospital enterprise. Due to the varying sizes and service goals of individual hospitals, lack of standardization was a significant barrier to change. Gaining trust and buy-in was imperative to increasing compliance with new transfusion policies. The primary concern was finding a balance between respecting physician autonomy and emphasizing and aligning practices with new evidence in the literature. Thus, understanding and applying principles of thoughtful change management was imperative to advancing the framework of the PBM program. The CEC venue enabled collaboration among hospitals and staff and was ultimately used to facilitate the necessary standardization process. To gain the trust of hospital and medical staff, the Outcomes Team conducted several site visits, enabling face-to-face interaction with frontline staff and operational leaders. Moreover, the team’s emphasis on the use of the latest evidence-based guidelines in discussions with hospital and medical staff underscored the need for change.
Frank et al19 describes using an approach similar to our Outcomes Team at the Johns Hopkins Health System. A designated multidisciplinary quality improvement team, referred to as the “clinical community,” worked on implementing best practices for blood management across a system of 5 hospitals. The authors reported similar results, with an overall decrease in number of units transfused, as well as substantial cost savings.19 Our project, along with the project implemented by Frank et al, shows how a “consensus-community” approach, involving stakeholders and various experts across the system, can be be used to align practices among multiple hospitals.
Development of a robust PBM reporting tool was key to creating meaningful monthly reports and driving provider practice change. However, this did require several training sessions, site visits, and computer-based training. Members of the Outcomes Team engaged in one-on-one sessions with tool users as a way of addressing specific areas of concern raised by staff at individual blood banks, and also took part in system-wide initiatives. The team also attended blood bank staff meetings and hospital transfusion committee meetings to educate staff on the evidence and initiative, provide demos of the reporting tool, and allow for a more robust discussion of how the data could be used and shared with other departments. These sessions provided opportunities to identify and prioritize future enhancements, as well as opportunities for continued education and discussion at hospitals, which were critical to ongoing improvement of the reporting tool.
Conclusion and Future Directions
Blood products remain extremely valuable and scarce resources, and all health care professionals must work to prevent unnecessary transfusions and improve clinical outcomes by adhering to the latest evidence-based guidelines. In response to current transfusion guidelines and the need to optimize blood product resources, our system successfully implemented a robust PBM program that engaged both academic and non-academic providers and communities. Several elements of the program helped us overcome the challenges relating to standardization of transfusion practices: consensus-based development of guidelines using the latest scientific evidence; formation and utilization of the CEC venue to gain system-wide consensus around both guidelines and approaches to change; development of a trustworthy and accessible PBM reporting tool (as well as continuing education sessions to improve adoption and utilization of the tool); and ongoing multidisciplinary discussions and support of thoughtful change and sustaining activities. We have seen a system-wide decrease in the number of RBC units transfused (absolute and per case mix-adjusted patient day) since implementing the PBM program, and in the following years have noted a trending decrease in transfusion-related safety events. Although there was a slight increase in reported safety events from 2018 to 2019, this was likely due to the systematic implementation of a new electronic medical record system and improved reporting infrastructure.
Upcoming phases of our system-wide PBM program will include looking at opportunities to improve blood utilization in other specific clinical areas. For example, we have begun discussions with hematology and oncology experts across the system to expand their patient population data within the PBM reporting tool, and to identify areas of opportunity for provider practice change within their specialty. We are also reviewing cardiothoracic surgery transfusion data to identify opportunities for reducing blood utilization in specific clinical scenarios. In addition, we are working to incorporate our 2 newest hospital system members (Memorial Hospital East and Memorial Hospital Belleville) into the PBM program. In collaboration with perioperative leaders across the system, the surgical blood ordering process is being reviewed. The goal of this effort is to reduce blood products ordered in preparation for surgical procedures. We are also currently investigating whether an impact on safety events (ie, reduction in transfusion reactions) can yet be detected. Last, our health care system recently launched a system-wide electronic medical record, and we are eager to see how this will provide us with new methods to monitor and analyze blood administration and utilization data. We look forward to reporting on the expansion of our program and on any clinical outcome improvements gained through avoidance of unnecessary transfusions.
Acknowledgment: The authors thank the leadership within the Center for Clinical Excellence and Supply Chain at BJC HealthCare for their support of this manuscript, as well as all system participants who have contributed to these efforts, especially Mohammad Agha, MD, MHA, current physician leader of the PBM CEC, for his thoughtful edits of this manuscript.
Corresponding author: Audrey A. Gronemeyer, MPH, Center for Clinical Excellence, BJC HealthCare, 8300 Eager Road, Suite 400A, St. Louis, MO 63144; audrey.gronemeyer@bjc.org.
Financial disclosures: None.
From BJC HealthCare, St. Louis, MO.
Abstract
Background: There is limited literature relating to patient blood management (PBM) programs in large multi-hospital systems or addressing challenges of implementation across diverse systems comprised of community and academic hospitals.
Objective: To establish a PBM program to improve utilization of blood transfusion units at a multi-hospital system in the Midwest (BJC HealthCare).
Methods: High-impact strategies in establishing the PBM program included formation of Clinical Expert Councils (CECs) of providers, establishment of consensus utilization guidelines, and development of a robust reporting tool. CECs enabled collaboration and facilitated standardization across a complex system of academic, private practice, and tertiary facilities with a diverse community of medical providers. Consensus guidelines and the PBM reporting tool were key to creating meaningful reports to drive provider practice change.
Results: Over the 5 years following implementation of the PBM program, there has been a steady decrease in red blood cell (RBC) utilization. Noticeable changes have taken place at individual hospitals in the system, including reductions in transfusions falling outside guideline parameters from 300 per quarter to less than 8 per quarter at 1 of our community hospitals. No negative impact on patient care has been identified.
Conclusion: In response to current transfusion guidelines and the need for optimizing stewardship of blood product resources, this hospital system successfully implemented a robust PBM program that engaged academic and non-academic community providers and decreased utilization of blood transfusion resources in line with consensus guidelines.
Keywords: quality improvement; RBC transfusion; transfusion practices; provider practice change; utilization trends.
Evidence from clinical trials and published clinical guidelines support the adoption of a restrictive blood transfusion approach in hospitalized, stable patients as best practice.1-5 As such, the development and implementation of patient blood management (PBM) programs has become an increasingly important process improvement for reducing variability in transfusion practices and clinical outcomes.
As recently as 2013, BJC HealthCare, a multi-hospital system in the Midwest, had no standardized, system-wide blood management program, and transfusion practices varied widely across providers and between individual hospitals based on size, patient population, and resources. The system consisted of 13 hospitals, ranging from large tertiary to smaller community and academic hospitals. Although adults constituted the vast majority of the patient population, the hospital system also included a pediatric specialty hospital, St. Louis Children’s Hospital. In addition, some sites were staffed by private practice providers and others by university-based providers, including blood bank medical directors. Due to the diversity of settings and populations, efforts to align transfusion and other practices often faced multiple challenges. However, improving the management of blood transfusions was identified as a key resource stewardship priority in 2013, and implementation of a system-wide program began after extensive discussions and consensus approval by senior hospital system and medical leadership. The primary aim of the program was to optimize overall blood product resource stewardship. Specifically, we sought to control or reduce costs per patient-care episode using strategies that would not negatively impact patient care and could potentially even improve patient outcomes (eg, by avoiding unnecessary transfusions and their attendant risks).
There is a plethora of literature related to the implemention of PBM programs in individual hospitals,6-18 but few reports specifically relate to large multi-hospital health systems,19-21 or directly address the unique challenges of implementation across a diverse system of community and academic hospitals and providers.19 Here, we discuss our experience with establishing a PBM program in a large, diverse, multi-hospital health system, provide examples of innovative strategies, and address challenges faced and lessons learned. Future endeavors of the PBM program at BJC HealthCare are also described.
Setting
BJC HealthCare is one of the largest nonprofit health care organizations in the United States, delivering services to the greater St. Louis, southern Illinois, and mid-Missouri regions, and addressing the health care needs of urban, suburban, and rural communities. As of 2018, the system included 15 hospitals and multiple community health locations comprising more than 3400 staffed beds, 31,500 employees, and 4300 physicians with privileges. The system annually has more than 151,000 hospital admissions, 81,000 outpatient surgery visits, and 537,000 emergency department visits. In addition to inpatient and outpatient care, services include primary care, community health and wellness, workplace health, home health, community mental health, rehabilitation, long-term care, and hospice. As a nonprofit system, BJC is the largest provider of charity care, unreimbursed care, and community benefit in Missouri, highlighting the fact that resource stewardship is a critical issue across the entire system and the communities served.22
PBM Project
Preparation for large-scale change across several hospitals began with creating a framework for the initiative, which consisted of a “burning platform,” a guiding vision, and a coalition. The burning platform identifies the importance and urgency of a change and helps to establish commitment. Between 2012 and 2014, the American Association of Blood Banks (AABB) released new evidence-based guidelines and recommendations calling for more restrictive transfusion practices pertaining to red blood cells (RBCs; ie, a hemoglobin threshold of 7 to 8 g/dL) in both inpatient and outpatient care.2 In addition, use of single-unit transfusions was recognized as best practice by the AABB in the Choosing Wisely campaign.23 Historically, adult patients requiring transfusions were given 2 units in succession. The new recommendations provided a strong basis for changing transfusion practices at BJC. It was believed that aligning transfusion practices with the new guidelines was consistent with the mission and vision of the work: that these changes could lead to optimization of resources, cost control, reductions in unnecessary blood transfusions, and potentially improved care (eg, fewer transfusion-related complications). We used the national guidelines to initiate discussions and to identify clinical conditions and associated laboratory parameters for transfusion therapy.
Once this burning platform was established, a team comprised of physicians, blood bank experts, quality consultants, data analysts, and supply managers, referred to as the Outcomes Team, was formed to lead the change efforts across the system. Initial projects for the team included developing system-wide consensus-based transfusion guidelines, providing education to providers on the new evidence in transfusion practice, and sharing BJC-specific historical utilization data. The guiding principle for the group was that “blood is a valuable resource, but not without risk, and less is more.” In order to disseminate the vision of the initiative across the system, campaign signs with the slogans “7 is the new 10” (referring to the g/dL transfusion threshold) and “1 is the new 2” (referring to the new practice of the preferential transfusion of single units rather than 2 at a time) were displayed in system hospitals.
Last, a guiding coalition of system leaders was needed to help push the initiative forward and sustain the program once fully implemented. Thus, a multidisciplinary PBM Clinical Expert Counciel (CEC) was formed to assist with implementation and maintenance of the program.
Role of PBM Clinical Expert Council
The PBM CEC was designed to improve overall physician and expert engagement and provide a forum where stakeholders from across the system could participate to voice their expert opinion. CECs (which BJC formed in other clinical areas as well) are multidisciplinary teams consisting of clinical, administrative, and technical staff. The open, multidisciplinary structure of the councils allows for collaboration that promotes change across a complex multi-hospital system. Each hospital is represented by key physicians and technical leaders, opening opportunity for both horizontal and vertical partnership.
As part of the overall physician engagement strategy, the PBM CEC was launched across BJC in November 2013 as a decision-making body for gaining system consensus on matters relating to blood management. The initial goals for the PBM CEC were to share information and educate providers and others on the latest evidence, to subsequently debate and develop consensus for guidelines to be applied across BJC, and to identify and adopt gold standard practices to drive and sustain compliance across the system. More specifically, we wanted to focus on how to avoid unnecessary blood transfusions known to be associated with increased risk for adverse reactions, other morbidity, mortality, and longer length of stay. Council members met quarterly to address 6 key drivers: patient safety, informatics and data, quality improvement, efficiencies and workflows, education and competency, and communication and engagement. Members then voted to approve guidelines, policies, and procedures. The group continues to assist in updating and standardizing guidelines and providing input on improving the functionality of the PBM reporting tool.
Development of the PBM Reporting Tool
Providing and sharing data on blood utilization and practices with the CEC and hospital leaders was imperative to driving change. The Outcomes Team deliberated on how best to generate and provide such information, conducting comparisons between selected vendor-based tools and potential internal BJC solutions. After investigation, BJC leadership approved the development of an in-house PBM dashboard tool using Tableau Desktop (Tableau Software, Inc.). The tool consists of an executive page with 5 additional tabs for navigating to the appropriate information (Figure 1 and Figure 2); data within the tool are organized by facility, service, provider, ICD diagnosis, transfusion indication, and the Clinical Classifications Software category, as defined by the Agency for Healthcare Research and Quality.
The PBM reporting tool was launched on December 31, 2014. The next priority after the launch was to validate the tool’s blood utilization data and implement enhancements to make the tool more effective for users. A super-user group consisting of blood bank supervisors and managers was established. The goals of the user group were to preview any enhancements before presenting the tool to the larger CEC, test and validate data once new information was added, and share and prioritize future enhancements. User group meetings were held monthly to share best practices and discuss individual facilities’ blood utilization data. In addition, each facility’s representative(s) shared how they were driving changes in provider practice and discussed challenges specific to their facility. Enhancements suggested through the user group included: incorporation of additional lab values into the tool to correspond with other blood products (eg, fibrinogen, hematocrit, international normalized ratio, and platelet count), addition of the specific location where the blood product was administered, and standard naming conventions of locations to allow comparisons across facilities (eg, Emergency Department instead of ED, ER, or EU).
All hospital users were given access to a test version of the reporting tool where they could review enhancements, identify what worked well and what could be done better, and suggest corrections. As changes were made to the hospital lab systems, a sample of data was reviewed and validated with affected facilities to confirm the continued accuracy of the data. To ensure its practicality to users, the tool continues to be improved upon with input from council stakeholders and subject-matter experts.
Measurements
To monitor blood utilization across the health system, we tracked the total RBC units administered by hospital, service, and provider and also tracked pre- and post-transfusion hemoglobin values.
Results
Overall, the system has seen a steady decrease in RBC utilization over the 5 years since the PBM program was implemented (Table
In addition to system-wide improvement, noticeable changes have taken place at individual hospitals in the BJC system. For example, Boone Hospital Center in Columbia, Missouri, began critically reviewing all RBC transfusions starting in 2015 and, to raise awareness, communicating with any provider who transfused a patient outside of transfusion guidelines. Since then, Boone Hospital has seen a dramatic reduction in transfusions considered noncompliant (ie, falling outside guideline parameters), from 300 transfusions per quarter, down to less than 8 per quarter. St. Louis Children’s Hospital also began reviewing blood products utilized by providers that fell outside of the standardized guidelines. At this hospital, physician champions discuss any outliers with the providers involved and use multiple methods for disseminating information to providers, including grand rounds, faculty meetings, and new resident orientations.
Another success has been the partnership between Barnes Jewish St. Peters and Progress West Hospitals in providing PBM education. Their joint effort resulted in implementation of education modules in BJC’s internal learning system, and has provided PBM-related education to more than 367 nurses, blood bank staff, and physicians.
Challenges and Lessons Learned
Implementation of the PBM program was generally successful, but it was not without challenges. One of the biggest challenges was addressing the variation in care and practices across the hospital enterprise. Due to the varying sizes and service goals of individual hospitals, lack of standardization was a significant barrier to change. Gaining trust and buy-in was imperative to increasing compliance with new transfusion policies. The primary concern was finding a balance between respecting physician autonomy and emphasizing and aligning practices with new evidence in the literature. Thus, understanding and applying principles of thoughtful change management was imperative to advancing the framework of the PBM program. The CEC venue enabled collaboration among hospitals and staff and was ultimately used to facilitate the necessary standardization process. To gain the trust of hospital and medical staff, the Outcomes Team conducted several site visits, enabling face-to-face interaction with frontline staff and operational leaders. Moreover, the team’s emphasis on the use of the latest evidence-based guidelines in discussions with hospital and medical staff underscored the need for change.
Frank et al19 describes using an approach similar to our Outcomes Team at the Johns Hopkins Health System. A designated multidisciplinary quality improvement team, referred to as the “clinical community,” worked on implementing best practices for blood management across a system of 5 hospitals. The authors reported similar results, with an overall decrease in number of units transfused, as well as substantial cost savings.19 Our project, along with the project implemented by Frank et al, shows how a “consensus-community” approach, involving stakeholders and various experts across the system, can be be used to align practices among multiple hospitals.
Development of a robust PBM reporting tool was key to creating meaningful monthly reports and driving provider practice change. However, this did require several training sessions, site visits, and computer-based training. Members of the Outcomes Team engaged in one-on-one sessions with tool users as a way of addressing specific areas of concern raised by staff at individual blood banks, and also took part in system-wide initiatives. The team also attended blood bank staff meetings and hospital transfusion committee meetings to educate staff on the evidence and initiative, provide demos of the reporting tool, and allow for a more robust discussion of how the data could be used and shared with other departments. These sessions provided opportunities to identify and prioritize future enhancements, as well as opportunities for continued education and discussion at hospitals, which were critical to ongoing improvement of the reporting tool.
Conclusion and Future Directions
Blood products remain extremely valuable and scarce resources, and all health care professionals must work to prevent unnecessary transfusions and improve clinical outcomes by adhering to the latest evidence-based guidelines. In response to current transfusion guidelines and the need to optimize blood product resources, our system successfully implemented a robust PBM program that engaged both academic and non-academic providers and communities. Several elements of the program helped us overcome the challenges relating to standardization of transfusion practices: consensus-based development of guidelines using the latest scientific evidence; formation and utilization of the CEC venue to gain system-wide consensus around both guidelines and approaches to change; development of a trustworthy and accessible PBM reporting tool (as well as continuing education sessions to improve adoption and utilization of the tool); and ongoing multidisciplinary discussions and support of thoughtful change and sustaining activities. We have seen a system-wide decrease in the number of RBC units transfused (absolute and per case mix-adjusted patient day) since implementing the PBM program, and in the following years have noted a trending decrease in transfusion-related safety events. Although there was a slight increase in reported safety events from 2018 to 2019, this was likely due to the systematic implementation of a new electronic medical record system and improved reporting infrastructure.
Upcoming phases of our system-wide PBM program will include looking at opportunities to improve blood utilization in other specific clinical areas. For example, we have begun discussions with hematology and oncology experts across the system to expand their patient population data within the PBM reporting tool, and to identify areas of opportunity for provider practice change within their specialty. We are also reviewing cardiothoracic surgery transfusion data to identify opportunities for reducing blood utilization in specific clinical scenarios. In addition, we are working to incorporate our 2 newest hospital system members (Memorial Hospital East and Memorial Hospital Belleville) into the PBM program. In collaboration with perioperative leaders across the system, the surgical blood ordering process is being reviewed. The goal of this effort is to reduce blood products ordered in preparation for surgical procedures. We are also currently investigating whether an impact on safety events (ie, reduction in transfusion reactions) can yet be detected. Last, our health care system recently launched a system-wide electronic medical record, and we are eager to see how this will provide us with new methods to monitor and analyze blood administration and utilization data. We look forward to reporting on the expansion of our program and on any clinical outcome improvements gained through avoidance of unnecessary transfusions.
Acknowledgment: The authors thank the leadership within the Center for Clinical Excellence and Supply Chain at BJC HealthCare for their support of this manuscript, as well as all system participants who have contributed to these efforts, especially Mohammad Agha, MD, MHA, current physician leader of the PBM CEC, for his thoughtful edits of this manuscript.
Corresponding author: Audrey A. Gronemeyer, MPH, Center for Clinical Excellence, BJC HealthCare, 8300 Eager Road, Suite 400A, St. Louis, MO 63144; audrey.gronemeyer@bjc.org.
Financial disclosures: None.
1. Carson JL, Grossman BJ, Kleinman S, et al. Red blood cell transfusion: A clinical practice guideline from the AABB*. Ann Intern Med. 2012;157:49-58.
2. Goodnough LT, Levy JH, Murphy MF. Concepts of blood transfusion in adults. Lancet. 2013;381:1845-1854.
3. Hébert PC, Carson JL. Transfusion threshold of 7 g per deciliter—The new normal. N Engl J Med. 2014;371:1459-1461.
4. Gani F, Cerullo M, Ejaz A, et al. Implementation of a blood management program at a tertiary care hospital: Effect on transfusion practices and clinical outcomes among patients undergoing surgery. Ann Surg. 2019;269:1073-1079.
5. Podlasek SJ, Thakkar RN, Rotello LC, et al. Implementing a “why give 2 when 1 will do?” Choosing Wisely campaign. Transfusion. 2016;56:2164.
6. Boral LI, Bernard A, Hjorth T, et al. How do I implement a more restrictive transfusion trigger of hemoglobin level of 7 g/dL at my hospital? Transfusion. 2015;55:937-945.
7. Geissler RG, Kosters C, Franz D, et al. Utilization of blood components in trauma surgery: A single-center, retrospective analysis before and after the implementation of an educative PBM initiative. Transfuse Med Hemother. 2015;42:83-89.
8. Goel R, Cushing MM, Tobian AA. Pediatric patient blood management programs: Not just transfusing little adults. Transfus Med Rev. 2016;30:235-241.
9. Gupta PB, DeMario VM, Amin RM, et al. Patient blood management program improves blood use and clinical outcomes in orthopedic surgery. Anesthesiology. 2018;129;1082-1091.
10. Leahy MF, Roberts H, Mukhtar SA, et al. A pragmatic approach to embedding patient blood management in a tertiary hospital. Transfusion. 2014;54:1133-1145.
11. Leahy MF, Hofmann A, Towler S, et al. Improved outcomes and reduced costs associated with a health-system-wide patient blood management program: A retrospective observational study in four major adult tertiary-care hospitals. Transfusion. 2017;57:1347-1358.
12. Meybohm P, Herrmann E, Steinbicker AU, et al. Patient blood management is associated with a substantial reduction of red blood cell utilization and safe for patient’s outcome: A prospective, multicenter cohort study with a noninferiority design. Ann Surg. 2016;264:203-211.
13. Morgan PN, Coleman PL, Martinez-Garduno CM, et al. Implementation of a patient blood management program in an Australian private hospital orthopedic unit. J Blood Med. 2018;9;83-90.
14. Norgaard A, Stensballe J, de Lichtenberg TH, et al. Three-year follow-up of implementation of evidence-based transfusion practice in a tertiary hospital. Vox Sang. 2017;112:229-239.
15. Meuller MM, Van Remoortel H, Meybohm P, et al. Patient blood management: Recommendations from the 2018 Frankfurt Consensus Conference. JAMA. 2019;321:983-997.
16. Oliver JC, Griffin RL, Hannon T, Marques MB. The success of our patient blood management program depended on an institution-wide change in transfusion practices. Transfusion. 2014;54:2617-2624.
17. Thakkar RN, Lee KH, Ness PM, et al. Relative impact of a patient blood management program on utilization of all three major blood components. Transfusion. 2016;56:2212-2220.
18. Yang WW, Thakkar RN, Gehrie EA, et al. Single-unit transfusions and hemoglobin trigger: relative impact on red cell utilization. Transfusion. 2017;57:1163-1170.
19. Frank SM, Thakkar RN, Podlasek SJ, et al. Implementing a health system-wide patient blood management program with a clinical community approach. Anesthesiology. 2017;127;754-764.
20. Verdecchia NM, Wisniewski MK, Waters JH, et al. Changes in blood product utilization in a seven-hospital system after the implementation of a patient blood management program: A 9-year follow-up. Hematology. 2016;21:490-499.
21. Yazer MH, Waters JH. How do I implement a hospital-based blood management program? Transfusion. 2012;52:1640-1645.
22. BJC HealthCare. Facts and Figures.. BJC HealthCare website. www.bjc.org/About-Us/Facts-Figures. Accessed November 18, 2019.
23. Callum JL, Waters JH, Shaz BH, et al. The AABB recommendations for the Choosing Wisely campaign of the American Board of Internal Medicine. Transfusion. 2014;54:2344-2352.
1. Carson JL, Grossman BJ, Kleinman S, et al. Red blood cell transfusion: A clinical practice guideline from the AABB*. Ann Intern Med. 2012;157:49-58.
2. Goodnough LT, Levy JH, Murphy MF. Concepts of blood transfusion in adults. Lancet. 2013;381:1845-1854.
3. Hébert PC, Carson JL. Transfusion threshold of 7 g per deciliter—The new normal. N Engl J Med. 2014;371:1459-1461.
4. Gani F, Cerullo M, Ejaz A, et al. Implementation of a blood management program at a tertiary care hospital: Effect on transfusion practices and clinical outcomes among patients undergoing surgery. Ann Surg. 2019;269:1073-1079.
5. Podlasek SJ, Thakkar RN, Rotello LC, et al. Implementing a “why give 2 when 1 will do?” Choosing Wisely campaign. Transfusion. 2016;56:2164.
6. Boral LI, Bernard A, Hjorth T, et al. How do I implement a more restrictive transfusion trigger of hemoglobin level of 7 g/dL at my hospital? Transfusion. 2015;55:937-945.
7. Geissler RG, Kosters C, Franz D, et al. Utilization of blood components in trauma surgery: A single-center, retrospective analysis before and after the implementation of an educative PBM initiative. Transfuse Med Hemother. 2015;42:83-89.
8. Goel R, Cushing MM, Tobian AA. Pediatric patient blood management programs: Not just transfusing little adults. Transfus Med Rev. 2016;30:235-241.
9. Gupta PB, DeMario VM, Amin RM, et al. Patient blood management program improves blood use and clinical outcomes in orthopedic surgery. Anesthesiology. 2018;129;1082-1091.
10. Leahy MF, Roberts H, Mukhtar SA, et al. A pragmatic approach to embedding patient blood management in a tertiary hospital. Transfusion. 2014;54:1133-1145.
11. Leahy MF, Hofmann A, Towler S, et al. Improved outcomes and reduced costs associated with a health-system-wide patient blood management program: A retrospective observational study in four major adult tertiary-care hospitals. Transfusion. 2017;57:1347-1358.
12. Meybohm P, Herrmann E, Steinbicker AU, et al. Patient blood management is associated with a substantial reduction of red blood cell utilization and safe for patient’s outcome: A prospective, multicenter cohort study with a noninferiority design. Ann Surg. 2016;264:203-211.
13. Morgan PN, Coleman PL, Martinez-Garduno CM, et al. Implementation of a patient blood management program in an Australian private hospital orthopedic unit. J Blood Med. 2018;9;83-90.
14. Norgaard A, Stensballe J, de Lichtenberg TH, et al. Three-year follow-up of implementation of evidence-based transfusion practice in a tertiary hospital. Vox Sang. 2017;112:229-239.
15. Meuller MM, Van Remoortel H, Meybohm P, et al. Patient blood management: Recommendations from the 2018 Frankfurt Consensus Conference. JAMA. 2019;321:983-997.
16. Oliver JC, Griffin RL, Hannon T, Marques MB. The success of our patient blood management program depended on an institution-wide change in transfusion practices. Transfusion. 2014;54:2617-2624.
17. Thakkar RN, Lee KH, Ness PM, et al. Relative impact of a patient blood management program on utilization of all three major blood components. Transfusion. 2016;56:2212-2220.
18. Yang WW, Thakkar RN, Gehrie EA, et al. Single-unit transfusions and hemoglobin trigger: relative impact on red cell utilization. Transfusion. 2017;57:1163-1170.
19. Frank SM, Thakkar RN, Podlasek SJ, et al. Implementing a health system-wide patient blood management program with a clinical community approach. Anesthesiology. 2017;127;754-764.
20. Verdecchia NM, Wisniewski MK, Waters JH, et al. Changes in blood product utilization in a seven-hospital system after the implementation of a patient blood management program: A 9-year follow-up. Hematology. 2016;21:490-499.
21. Yazer MH, Waters JH. How do I implement a hospital-based blood management program? Transfusion. 2012;52:1640-1645.
22. BJC HealthCare. Facts and Figures.. BJC HealthCare website. www.bjc.org/About-Us/Facts-Figures. Accessed November 18, 2019.
23. Callum JL, Waters JH, Shaz BH, et al. The AABB recommendations for the Choosing Wisely campaign of the American Board of Internal Medicine. Transfusion. 2014;54:2344-2352.