User login
At Last, a Nasal Epinephrine Spray
This summer, the US Food and Drug Administration (FDA) fast-tracked approval of the first-in-its-class nasal epinephrine (neffy). It’s a very welcome addition to our anaphylaxis treatment armamentarium. In healthy volunteers, neffy achieved similar serum epinephrine levels, rises in blood pressure, and pulse compared with IM epinephrine.
The Need for Neffy
It was just a few days ago that I saw a new patient with fire ant anaphylaxis. The last time he tried to use an injectable epinephrine pen, he made two mistakes. First, he placed the wrong end against his thigh, and when it did not inject, he depressed it with his thumb — in other words, he injected his thumb with epinephrine. Of course, that cannot happen with neffy.
I recall a few years ago, a child experienced anaphylaxis but the parent was hesitant to administer the EAI (epinephrine autoinjector). The parent drove to the emergency room but was delayed by traffic, and by the time they reached the ER, the patient had suffered a respiratory arrest and passed away.
Patients are not the only ones who are hesitant to administer epinephrine. Some clinicians do not treat anaphylaxis appropriately. As an allergist, I see patients after-the-fact for diagnosis and management. Patients often tell me of systemic allergic reactions treated with IV antihistamines/corticosteroids and even sometimes with nebulized beta agonists, but not epinephrine.
My opinion is that it’s not just needle phobia. As I mentioned, in my Medscape commentary “Injectable Epinephrine: An Epidemic of Misuse,” I believe it’s due to a misunderstanding of the guidelines and a sense that epinephrine is a potent medication to be used sparingly. Clinicians and patients must understand that epinephrine is a naturally occurring hormone and administration leads to serum levels seen under other natural circumstances (eg, stress — the fight-or-flight surge). The aforementioned article also includes a patient handout, “Don’t Fear Epinephrine,” which I encourage you to read and distribute.
The potential benefits of neffy are clear:
- It should overcome fear of injection ergo being more likely to be used, and used earlier, by both patient/family member and clinicians.
- It’s easier to carry than many larger devices (though not the AUVI-Q).
- It cannot be injected incorrectly.
- Expiration is 8 months longer than the EAI.
- There are no pharmacist substitutions (as there is no equivalent device).
Potential Problems With Neffy and Some Suggested Solutions
As promising and beneficial as it is, I wonder about a few training issues. In the office, patients can be trained with a (reusable) injectable epinephrine trainer but not with a nasal spray device trainer in the office (an important alternative is a small model of a nose in the office for patient education). A training device should also be included in the neffy prescription, as with the EAI.
Neffy and Patients With Nasal Polyps or Nasal Surgery
It’s more complicated than that neffy cannot be used with patients who have had nasal polyps or nasal surgery. It’s really about how much healthy nasal mucosa is required for absorption. Nasal surgery may be simple or complex. Nasal polyps may be obstructive or resolved with nasal steroid or biologic therapy. Nasal polyps affect 2% of the population, but 35% of pediatric food allergy (FA) patients develop allergic rhinitis (AR), and these AR symptoms present even when not triggered by FA. AR is present at baseline in patients with FA. How does this influence neffy absorption? For FA patients who have anaphylactic reactions with severe nasal reactions, neffy absorption could be further compromised, something that has not been studied.
Insurance Coverage
As we don’t yet know the comparative efficacy of neffy in anaphylactic episodes, it’s likely that patients, especially with more severe food sensitivities, will be prescribed both the nasal and IM devices. The question remains whether insurance will cover both.
In “mild cases,” I suspect that doctors might be more inclined to prescribe neffy.
Conclusion
Delay in epinephrine use is frequent despite the clear indication during anaphylactic episodes, which in turn increases risk for mortality. Neffy will probably save many lives.
Dr. Stadtmauer serves on the advisory board of Medscape. He is in private practice in New York City and is affiliated with the Mount Sinai School of Medicine.
A version of this article first appeared on Medscape.com.
This summer, the US Food and Drug Administration (FDA) fast-tracked approval of the first-in-its-class nasal epinephrine (neffy). It’s a very welcome addition to our anaphylaxis treatment armamentarium. In healthy volunteers, neffy achieved similar serum epinephrine levels, rises in blood pressure, and pulse compared with IM epinephrine.
The Need for Neffy
It was just a few days ago that I saw a new patient with fire ant anaphylaxis. The last time he tried to use an injectable epinephrine pen, he made two mistakes. First, he placed the wrong end against his thigh, and when it did not inject, he depressed it with his thumb — in other words, he injected his thumb with epinephrine. Of course, that cannot happen with neffy.
I recall a few years ago, a child experienced anaphylaxis but the parent was hesitant to administer the EAI (epinephrine autoinjector). The parent drove to the emergency room but was delayed by traffic, and by the time they reached the ER, the patient had suffered a respiratory arrest and passed away.
Patients are not the only ones who are hesitant to administer epinephrine. Some clinicians do not treat anaphylaxis appropriately. As an allergist, I see patients after-the-fact for diagnosis and management. Patients often tell me of systemic allergic reactions treated with IV antihistamines/corticosteroids and even sometimes with nebulized beta agonists, but not epinephrine.
My opinion is that it’s not just needle phobia. As I mentioned, in my Medscape commentary “Injectable Epinephrine: An Epidemic of Misuse,” I believe it’s due to a misunderstanding of the guidelines and a sense that epinephrine is a potent medication to be used sparingly. Clinicians and patients must understand that epinephrine is a naturally occurring hormone and administration leads to serum levels seen under other natural circumstances (eg, stress — the fight-or-flight surge). The aforementioned article also includes a patient handout, “Don’t Fear Epinephrine,” which I encourage you to read and distribute.
The potential benefits of neffy are clear:
- It should overcome fear of injection ergo being more likely to be used, and used earlier, by both patient/family member and clinicians.
- It’s easier to carry than many larger devices (though not the AUVI-Q).
- It cannot be injected incorrectly.
- Expiration is 8 months longer than the EAI.
- There are no pharmacist substitutions (as there is no equivalent device).
Potential Problems With Neffy and Some Suggested Solutions
As promising and beneficial as it is, I wonder about a few training issues. In the office, patients can be trained with a (reusable) injectable epinephrine trainer but not with a nasal spray device trainer in the office (an important alternative is a small model of a nose in the office for patient education). A training device should also be included in the neffy prescription, as with the EAI.
Neffy and Patients With Nasal Polyps or Nasal Surgery
It’s more complicated than that neffy cannot be used with patients who have had nasal polyps or nasal surgery. It’s really about how much healthy nasal mucosa is required for absorption. Nasal surgery may be simple or complex. Nasal polyps may be obstructive or resolved with nasal steroid or biologic therapy. Nasal polyps affect 2% of the population, but 35% of pediatric food allergy (FA) patients develop allergic rhinitis (AR), and these AR symptoms present even when not triggered by FA. AR is present at baseline in patients with FA. How does this influence neffy absorption? For FA patients who have anaphylactic reactions with severe nasal reactions, neffy absorption could be further compromised, something that has not been studied.
Insurance Coverage
As we don’t yet know the comparative efficacy of neffy in anaphylactic episodes, it’s likely that patients, especially with more severe food sensitivities, will be prescribed both the nasal and IM devices. The question remains whether insurance will cover both.
In “mild cases,” I suspect that doctors might be more inclined to prescribe neffy.
Conclusion
Delay in epinephrine use is frequent despite the clear indication during anaphylactic episodes, which in turn increases risk for mortality. Neffy will probably save many lives.
Dr. Stadtmauer serves on the advisory board of Medscape. He is in private practice in New York City and is affiliated with the Mount Sinai School of Medicine.
A version of this article first appeared on Medscape.com.
This summer, the US Food and Drug Administration (FDA) fast-tracked approval of the first-in-its-class nasal epinephrine (neffy). It’s a very welcome addition to our anaphylaxis treatment armamentarium. In healthy volunteers, neffy achieved similar serum epinephrine levels, rises in blood pressure, and pulse compared with IM epinephrine.
The Need for Neffy
It was just a few days ago that I saw a new patient with fire ant anaphylaxis. The last time he tried to use an injectable epinephrine pen, he made two mistakes. First, he placed the wrong end against his thigh, and when it did not inject, he depressed it with his thumb — in other words, he injected his thumb with epinephrine. Of course, that cannot happen with neffy.
I recall a few years ago, a child experienced anaphylaxis but the parent was hesitant to administer the EAI (epinephrine autoinjector). The parent drove to the emergency room but was delayed by traffic, and by the time they reached the ER, the patient had suffered a respiratory arrest and passed away.
Patients are not the only ones who are hesitant to administer epinephrine. Some clinicians do not treat anaphylaxis appropriately. As an allergist, I see patients after-the-fact for diagnosis and management. Patients often tell me of systemic allergic reactions treated with IV antihistamines/corticosteroids and even sometimes with nebulized beta agonists, but not epinephrine.
My opinion is that it’s not just needle phobia. As I mentioned, in my Medscape commentary “Injectable Epinephrine: An Epidemic of Misuse,” I believe it’s due to a misunderstanding of the guidelines and a sense that epinephrine is a potent medication to be used sparingly. Clinicians and patients must understand that epinephrine is a naturally occurring hormone and administration leads to serum levels seen under other natural circumstances (eg, stress — the fight-or-flight surge). The aforementioned article also includes a patient handout, “Don’t Fear Epinephrine,” which I encourage you to read and distribute.
The potential benefits of neffy are clear:
- It should overcome fear of injection ergo being more likely to be used, and used earlier, by both patient/family member and clinicians.
- It’s easier to carry than many larger devices (though not the AUVI-Q).
- It cannot be injected incorrectly.
- Expiration is 8 months longer than the EAI.
- There are no pharmacist substitutions (as there is no equivalent device).
Potential Problems With Neffy and Some Suggested Solutions
As promising and beneficial as it is, I wonder about a few training issues. In the office, patients can be trained with a (reusable) injectable epinephrine trainer but not with a nasal spray device trainer in the office (an important alternative is a small model of a nose in the office for patient education). A training device should also be included in the neffy prescription, as with the EAI.
Neffy and Patients With Nasal Polyps or Nasal Surgery
It’s more complicated than that neffy cannot be used with patients who have had nasal polyps or nasal surgery. It’s really about how much healthy nasal mucosa is required for absorption. Nasal surgery may be simple or complex. Nasal polyps may be obstructive or resolved with nasal steroid or biologic therapy. Nasal polyps affect 2% of the population, but 35% of pediatric food allergy (FA) patients develop allergic rhinitis (AR), and these AR symptoms present even when not triggered by FA. AR is present at baseline in patients with FA. How does this influence neffy absorption? For FA patients who have anaphylactic reactions with severe nasal reactions, neffy absorption could be further compromised, something that has not been studied.
Insurance Coverage
As we don’t yet know the comparative efficacy of neffy in anaphylactic episodes, it’s likely that patients, especially with more severe food sensitivities, will be prescribed both the nasal and IM devices. The question remains whether insurance will cover both.
In “mild cases,” I suspect that doctors might be more inclined to prescribe neffy.
Conclusion
Delay in epinephrine use is frequent despite the clear indication during anaphylactic episodes, which in turn increases risk for mortality. Neffy will probably save many lives.
Dr. Stadtmauer serves on the advisory board of Medscape. He is in private practice in New York City and is affiliated with the Mount Sinai School of Medicine.
A version of this article first appeared on Medscape.com.
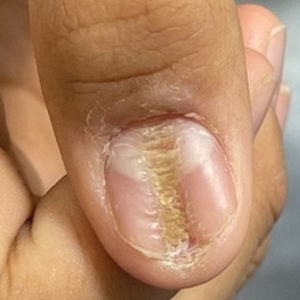
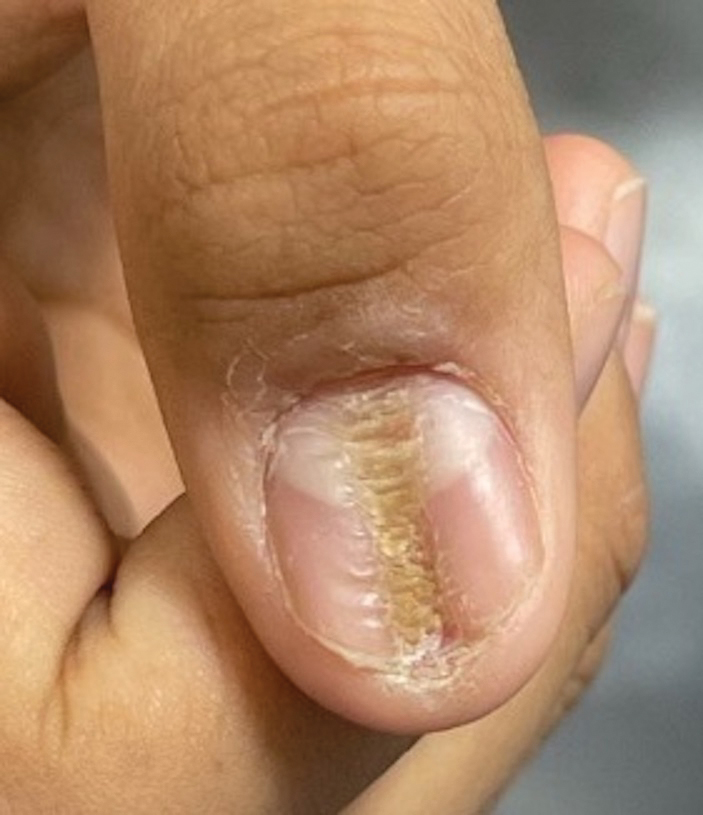
Longitudinal Depression on the Right Thumbnail
THE DIAGNOSIS: Habit-Tic Deformity
Habit-tic deformity is a cause of nail dystrophy that commonly arises in children and adults due to subconscious repetitive and self-injurious manipulation of the nail bed or cuticle, which ultimately damages the nail matrix.1,2 It can be considered a variant of onychotillomania.1
Characteristic features of habit-tic deformity include a longitudinal depression on the central nail plate with transverse ridges,1 which can be more prominent on the dominant hand.3 Patients typically note a long duration of nail deformity, often without insight into its etiology.2 Diagnosis relies on careful assessment of the clinical presentation and the patient’s history to rule out other differential diagnoses. Based on our patient’s clinical presentation and history, we excluded wart, squamous cell carcinoma, eczema, psoriasis, lichen planus, autoimmune connective tissue disease, onychomycosis, paronychia, pincer nail deformity, and Beau line as potential diagnoses. Biopsy also can be performed to exclude these diagnoses from the differential if the cause is unclear following clinical examination.
Treatment for habit-tic deformity involves identifying and addressing the underlying habit. Barrier methods such as bandages and cyanoacrylate adhesives that prevent further manipulation of the nail matrix are effective treatments for habit-tic deformity.2 A multidisciplinary approach with psychiatry may be optimal to identify underlying psychological comorbidities and break the habit through behavior interventions and medications.4 Nail dystrophy generally improves once the habit is disrupted; however, a younger age of onset may carry a worse prognosis.3 Patients should be counseled that the affected nail may never grow normally.
Our patient was advised to use fluocinonide ointment 0.05% to reduce inflammation of the proximal nail fold and to cover the thumbnail with a bandage to prevent picking. He also was counseled that the nail may show ongoing abnormal growth. Minimal improvement was noted after 6 months.
- Rieder EA, Tosti A. Onychotillomania: an underrecognized disorder. J Am Acad Dermatol. 2016;75:1245-1250.doi:10.1016/j.jaad.2016
- Ring DS. Inexpensive solution for habit-tic deformity. Arch Dermatol. 2010;146:1222-1223. doi:10.1001/archdermatol.2010.287
- Horne MI, Utzig JB, Rieder EA, et al. Alopecia areata and habit tic deformities. Skin Appendage Disord. 2018;4:323-325. doi:10.1159/000486540
- Sonthalia S, Sharma P, Kapoor J, et al. Habit tic deformity: need fora comprehensive approach. Skin Appendage Disord. 2019;5:117-118.doi:10.1159/000489320 .05.036
THE DIAGNOSIS: Habit-Tic Deformity
Habit-tic deformity is a cause of nail dystrophy that commonly arises in children and adults due to subconscious repetitive and self-injurious manipulation of the nail bed or cuticle, which ultimately damages the nail matrix.1,2 It can be considered a variant of onychotillomania.1
Characteristic features of habit-tic deformity include a longitudinal depression on the central nail plate with transverse ridges,1 which can be more prominent on the dominant hand.3 Patients typically note a long duration of nail deformity, often without insight into its etiology.2 Diagnosis relies on careful assessment of the clinical presentation and the patient’s history to rule out other differential diagnoses. Based on our patient’s clinical presentation and history, we excluded wart, squamous cell carcinoma, eczema, psoriasis, lichen planus, autoimmune connective tissue disease, onychomycosis, paronychia, pincer nail deformity, and Beau line as potential diagnoses. Biopsy also can be performed to exclude these diagnoses from the differential if the cause is unclear following clinical examination.
Treatment for habit-tic deformity involves identifying and addressing the underlying habit. Barrier methods such as bandages and cyanoacrylate adhesives that prevent further manipulation of the nail matrix are effective treatments for habit-tic deformity.2 A multidisciplinary approach with psychiatry may be optimal to identify underlying psychological comorbidities and break the habit through behavior interventions and medications.4 Nail dystrophy generally improves once the habit is disrupted; however, a younger age of onset may carry a worse prognosis.3 Patients should be counseled that the affected nail may never grow normally.
Our patient was advised to use fluocinonide ointment 0.05% to reduce inflammation of the proximal nail fold and to cover the thumbnail with a bandage to prevent picking. He also was counseled that the nail may show ongoing abnormal growth. Minimal improvement was noted after 6 months.
THE DIAGNOSIS: Habit-Tic Deformity
Habit-tic deformity is a cause of nail dystrophy that commonly arises in children and adults due to subconscious repetitive and self-injurious manipulation of the nail bed or cuticle, which ultimately damages the nail matrix.1,2 It can be considered a variant of onychotillomania.1
Characteristic features of habit-tic deformity include a longitudinal depression on the central nail plate with transverse ridges,1 which can be more prominent on the dominant hand.3 Patients typically note a long duration of nail deformity, often without insight into its etiology.2 Diagnosis relies on careful assessment of the clinical presentation and the patient’s history to rule out other differential diagnoses. Based on our patient’s clinical presentation and history, we excluded wart, squamous cell carcinoma, eczema, psoriasis, lichen planus, autoimmune connective tissue disease, onychomycosis, paronychia, pincer nail deformity, and Beau line as potential diagnoses. Biopsy also can be performed to exclude these diagnoses from the differential if the cause is unclear following clinical examination.
Treatment for habit-tic deformity involves identifying and addressing the underlying habit. Barrier methods such as bandages and cyanoacrylate adhesives that prevent further manipulation of the nail matrix are effective treatments for habit-tic deformity.2 A multidisciplinary approach with psychiatry may be optimal to identify underlying psychological comorbidities and break the habit through behavior interventions and medications.4 Nail dystrophy generally improves once the habit is disrupted; however, a younger age of onset may carry a worse prognosis.3 Patients should be counseled that the affected nail may never grow normally.
Our patient was advised to use fluocinonide ointment 0.05% to reduce inflammation of the proximal nail fold and to cover the thumbnail with a bandage to prevent picking. He also was counseled that the nail may show ongoing abnormal growth. Minimal improvement was noted after 6 months.
- Rieder EA, Tosti A. Onychotillomania: an underrecognized disorder. J Am Acad Dermatol. 2016;75:1245-1250.doi:10.1016/j.jaad.2016
- Ring DS. Inexpensive solution for habit-tic deformity. Arch Dermatol. 2010;146:1222-1223. doi:10.1001/archdermatol.2010.287
- Horne MI, Utzig JB, Rieder EA, et al. Alopecia areata and habit tic deformities. Skin Appendage Disord. 2018;4:323-325. doi:10.1159/000486540
- Sonthalia S, Sharma P, Kapoor J, et al. Habit tic deformity: need fora comprehensive approach. Skin Appendage Disord. 2019;5:117-118.doi:10.1159/000489320 .05.036
- Rieder EA, Tosti A. Onychotillomania: an underrecognized disorder. J Am Acad Dermatol. 2016;75:1245-1250.doi:10.1016/j.jaad.2016
- Ring DS. Inexpensive solution for habit-tic deformity. Arch Dermatol. 2010;146:1222-1223. doi:10.1001/archdermatol.2010.287
- Horne MI, Utzig JB, Rieder EA, et al. Alopecia areata and habit tic deformities. Skin Appendage Disord. 2018;4:323-325. doi:10.1159/000486540
- Sonthalia S, Sharma P, Kapoor J, et al. Habit tic deformity: need fora comprehensive approach. Skin Appendage Disord. 2019;5:117-118.doi:10.1159/000489320 .05.036
A healthy 13-year-old boy presented to the dermatology department with dystrophy of the right thumbnail of 3 to 4 years’ duration. A 5-mm-wide, depressed median longitudinal groove with a fir tree pattern was noted on the central nail plate. The patient noted that the groove had been gradually deepening. There was erythema, edema, and lichenification of the proximal nailfold without vascular changes, and the lunula was enlarged. No hyperkeratosis, subungual debris, erythematous nail folds, or inward curvature of the lateral aspects of the nail were noted. The patient denied any pruritus, pain, discomfort, or bleeding; he also denied any recent illness or trauma to the nail. None of the other nails were affected, and no other lesions or rashes were observed elsewhere on the body. The patient was unsure if he picked at the nail but acknowledged that he may have done so subconsciously. He had no history of eczema, psoriasis, or autoimmune connective tissue disorders.
On the Road to Care: Travel Nurses Still in Demand
Ashly Doran has worked at seven hospitals in four states since she graduated from nursing school in 2020. No, she isn’t job-hopping. Her travel nursing assignments have ranged from level 1 trauma center emergency rooms in big cities to small medical-surgical units in the suburbs. After each 13-week assignment, Doran packs up her belongings and her cats and moves to a new post.
“Travel nursing is so flexible,” she said. “I decide where I want to go and how much I want to make and start looking for travel contracts in that area.”
Nationwide nursing shortages have forced hospitals to hire travel nurses to fill staffing gaps. During the COVID-19 pandemic, the demand for travel nurses increased by 35%. While there is still a demand for nurses to fill short-term contracts, data show that demand has declined 42% between January and July 2022 and has continued the downward trend.
“What we’re seeing now is a shift…to a pre-pandemic market,” said Rachel Neill, RN, senior clinician advocate at Vivian Health. “Travel [nursing] is not going away — there will always be a need for hospital systems and facilities to fill gaps — but hospitals have shifted more into a traditional ... operational environment.”
Traveling a Different Path
For some registered nurses (RNs), short-term assignments offer opportunities to gain experience in different facilities or explore new locations before settling into permanent positions. Even experienced RNs embrace travel nursing for the flexible schedules and opportunities to take longer breaks between contracts.
Burnout and turnover among nurses are high, and flexible schedules, including controlling when to work, are essential to sustaining a clinical nursing career. In fact, 34% of nurses called travel nursing an “ideal option” for their lifestyle, with 14% viewing it as an option for career progression.
Travel nursing is especially appealing to Millennials and Generation Z, according to Brian Weirich, RN, chief nurse innovation officer at Bon Secours Mercy Health in Cincinnati, Ohio. In fact, the average age of a travel nurse is 35 compared with an average age of 52 for all RNs.
These are generations that are more focused on reducing school loan debt and gaining experience, not 401(k) and health insurance, he said in an interview. Pay is also a factor. The average pay for travel nurses was $2588 per month, compared with $1375 for permanent staff nurses.
During the pandemic, Weirich recalls groups of nurses resigning to take travel assignments together. The RNs picked desirable locations, accepted short-term assignments, and moved together, “making top dollar in locations they wanted to explore with their best friends.”
It’s been more than a decade since Kelly Spurlock traded a permanent nursing role in Lake Placid, Florida, for short-term nursing contracts in intensive care units in 20 states.
Spurlock works with a recruiter at Ingenovis Health to secure new contracts and considers travel assignments “working vacations.” In the process of exploring new places and meeting new people, Spurlock believes that travel nursing allows her to prioritize patient care.
“I can be at the bedside and be an advocate for my patient but also keep out of the spotlight for the political part of what we do,” she explained.
The Road Ahead
The appeal of travel nursing is taking new nursing assignments in different cities and earning higher salaries, but there are downsides, too. Travel nurses often receive fewer benefits than staff nurses and end up with less favorable assignments; their levels of dissatisfaction and burnout are also higher, and their sense of work-life balance is lower than staff nurses.
Most travel contracts last between 4 and 13 weeks. Hospitals often put policies and practices in place that limit the number of back-to-back contracts that traveling nurses can accept, which means that RNs can either convert to core staff or move on to new assignments once their contract term is up.
Weirich noted that some hospitals devote considerable effort to recruiting traveling nurses to full-time roles, adding, “There are active initiatives ... to make it such a good experience that they want to stay.”
On the flip side, contracts can be terminated without notice, leaving traveling nurses scrambling to find a new assignment and a new place to live on short notice.
“You’re there as long as the hospital needs you,” said Neill. “You could sign a 12- or 15-week contract, and their needs change a month in, and ... there are budget cuts, and they can’t pay salaries anymore, so they are laying off their nurses.”
Declining demand for travel nurses has made it harder to line up back-to-back contracts. Despite being available for work, Doran once waited 6 weeks to secure a new assignment and had to live off her savings.
Spurlock believes increased competition and declining wages — pay for travel nurses declined more than 9% from January 2023 to January 2024 — have made travel nursing less attractive.
“There has been such an influx of travel nurses ... because of COVID,” said Spurlock. “The rates have now come down [and] everybody’s fighting for jobs, and ... it’s very difficult to get a job that’s paying decent money.”
Despite the challenges, Spurlock continues learning new things from each assignment and hopes to work as a travel nurse until retirement. Doran has worked at hospitals in Washington, Oregon, California, and Wisconsin and would like to add Montana, Utah, and Nevada to the list. The goal: Continue accepting assignments in different cities and states until she finds the place where she wants to put down roots.
“Nursing is a great job, but it’s a hard job [and] it can take its toll at times,” Neill said. It’s important that nurses know their goals and values to be able to find a good fitting position. “And the beauty of it is that travel can be a great way to explore and add some flexibility.”
A version of this article first appeared on Medscape.com.
Ashly Doran has worked at seven hospitals in four states since she graduated from nursing school in 2020. No, she isn’t job-hopping. Her travel nursing assignments have ranged from level 1 trauma center emergency rooms in big cities to small medical-surgical units in the suburbs. After each 13-week assignment, Doran packs up her belongings and her cats and moves to a new post.
“Travel nursing is so flexible,” she said. “I decide where I want to go and how much I want to make and start looking for travel contracts in that area.”
Nationwide nursing shortages have forced hospitals to hire travel nurses to fill staffing gaps. During the COVID-19 pandemic, the demand for travel nurses increased by 35%. While there is still a demand for nurses to fill short-term contracts, data show that demand has declined 42% between January and July 2022 and has continued the downward trend.
“What we’re seeing now is a shift…to a pre-pandemic market,” said Rachel Neill, RN, senior clinician advocate at Vivian Health. “Travel [nursing] is not going away — there will always be a need for hospital systems and facilities to fill gaps — but hospitals have shifted more into a traditional ... operational environment.”
Traveling a Different Path
For some registered nurses (RNs), short-term assignments offer opportunities to gain experience in different facilities or explore new locations before settling into permanent positions. Even experienced RNs embrace travel nursing for the flexible schedules and opportunities to take longer breaks between contracts.
Burnout and turnover among nurses are high, and flexible schedules, including controlling when to work, are essential to sustaining a clinical nursing career. In fact, 34% of nurses called travel nursing an “ideal option” for their lifestyle, with 14% viewing it as an option for career progression.
Travel nursing is especially appealing to Millennials and Generation Z, according to Brian Weirich, RN, chief nurse innovation officer at Bon Secours Mercy Health in Cincinnati, Ohio. In fact, the average age of a travel nurse is 35 compared with an average age of 52 for all RNs.
These are generations that are more focused on reducing school loan debt and gaining experience, not 401(k) and health insurance, he said in an interview. Pay is also a factor. The average pay for travel nurses was $2588 per month, compared with $1375 for permanent staff nurses.
During the pandemic, Weirich recalls groups of nurses resigning to take travel assignments together. The RNs picked desirable locations, accepted short-term assignments, and moved together, “making top dollar in locations they wanted to explore with their best friends.”
It’s been more than a decade since Kelly Spurlock traded a permanent nursing role in Lake Placid, Florida, for short-term nursing contracts in intensive care units in 20 states.
Spurlock works with a recruiter at Ingenovis Health to secure new contracts and considers travel assignments “working vacations.” In the process of exploring new places and meeting new people, Spurlock believes that travel nursing allows her to prioritize patient care.
“I can be at the bedside and be an advocate for my patient but also keep out of the spotlight for the political part of what we do,” she explained.
The Road Ahead
The appeal of travel nursing is taking new nursing assignments in different cities and earning higher salaries, but there are downsides, too. Travel nurses often receive fewer benefits than staff nurses and end up with less favorable assignments; their levels of dissatisfaction and burnout are also higher, and their sense of work-life balance is lower than staff nurses.
Most travel contracts last between 4 and 13 weeks. Hospitals often put policies and practices in place that limit the number of back-to-back contracts that traveling nurses can accept, which means that RNs can either convert to core staff or move on to new assignments once their contract term is up.
Weirich noted that some hospitals devote considerable effort to recruiting traveling nurses to full-time roles, adding, “There are active initiatives ... to make it such a good experience that they want to stay.”
On the flip side, contracts can be terminated without notice, leaving traveling nurses scrambling to find a new assignment and a new place to live on short notice.
“You’re there as long as the hospital needs you,” said Neill. “You could sign a 12- or 15-week contract, and their needs change a month in, and ... there are budget cuts, and they can’t pay salaries anymore, so they are laying off their nurses.”
Declining demand for travel nurses has made it harder to line up back-to-back contracts. Despite being available for work, Doran once waited 6 weeks to secure a new assignment and had to live off her savings.
Spurlock believes increased competition and declining wages — pay for travel nurses declined more than 9% from January 2023 to January 2024 — have made travel nursing less attractive.
“There has been such an influx of travel nurses ... because of COVID,” said Spurlock. “The rates have now come down [and] everybody’s fighting for jobs, and ... it’s very difficult to get a job that’s paying decent money.”
Despite the challenges, Spurlock continues learning new things from each assignment and hopes to work as a travel nurse until retirement. Doran has worked at hospitals in Washington, Oregon, California, and Wisconsin and would like to add Montana, Utah, and Nevada to the list. The goal: Continue accepting assignments in different cities and states until she finds the place where she wants to put down roots.
“Nursing is a great job, but it’s a hard job [and] it can take its toll at times,” Neill said. It’s important that nurses know their goals and values to be able to find a good fitting position. “And the beauty of it is that travel can be a great way to explore and add some flexibility.”
A version of this article first appeared on Medscape.com.
Ashly Doran has worked at seven hospitals in four states since she graduated from nursing school in 2020. No, she isn’t job-hopping. Her travel nursing assignments have ranged from level 1 trauma center emergency rooms in big cities to small medical-surgical units in the suburbs. After each 13-week assignment, Doran packs up her belongings and her cats and moves to a new post.
“Travel nursing is so flexible,” she said. “I decide where I want to go and how much I want to make and start looking for travel contracts in that area.”
Nationwide nursing shortages have forced hospitals to hire travel nurses to fill staffing gaps. During the COVID-19 pandemic, the demand for travel nurses increased by 35%. While there is still a demand for nurses to fill short-term contracts, data show that demand has declined 42% between January and July 2022 and has continued the downward trend.
“What we’re seeing now is a shift…to a pre-pandemic market,” said Rachel Neill, RN, senior clinician advocate at Vivian Health. “Travel [nursing] is not going away — there will always be a need for hospital systems and facilities to fill gaps — but hospitals have shifted more into a traditional ... operational environment.”
Traveling a Different Path
For some registered nurses (RNs), short-term assignments offer opportunities to gain experience in different facilities or explore new locations before settling into permanent positions. Even experienced RNs embrace travel nursing for the flexible schedules and opportunities to take longer breaks between contracts.
Burnout and turnover among nurses are high, and flexible schedules, including controlling when to work, are essential to sustaining a clinical nursing career. In fact, 34% of nurses called travel nursing an “ideal option” for their lifestyle, with 14% viewing it as an option for career progression.
Travel nursing is especially appealing to Millennials and Generation Z, according to Brian Weirich, RN, chief nurse innovation officer at Bon Secours Mercy Health in Cincinnati, Ohio. In fact, the average age of a travel nurse is 35 compared with an average age of 52 for all RNs.
These are generations that are more focused on reducing school loan debt and gaining experience, not 401(k) and health insurance, he said in an interview. Pay is also a factor. The average pay for travel nurses was $2588 per month, compared with $1375 for permanent staff nurses.
During the pandemic, Weirich recalls groups of nurses resigning to take travel assignments together. The RNs picked desirable locations, accepted short-term assignments, and moved together, “making top dollar in locations they wanted to explore with their best friends.”
It’s been more than a decade since Kelly Spurlock traded a permanent nursing role in Lake Placid, Florida, for short-term nursing contracts in intensive care units in 20 states.
Spurlock works with a recruiter at Ingenovis Health to secure new contracts and considers travel assignments “working vacations.” In the process of exploring new places and meeting new people, Spurlock believes that travel nursing allows her to prioritize patient care.
“I can be at the bedside and be an advocate for my patient but also keep out of the spotlight for the political part of what we do,” she explained.
The Road Ahead
The appeal of travel nursing is taking new nursing assignments in different cities and earning higher salaries, but there are downsides, too. Travel nurses often receive fewer benefits than staff nurses and end up with less favorable assignments; their levels of dissatisfaction and burnout are also higher, and their sense of work-life balance is lower than staff nurses.
Most travel contracts last between 4 and 13 weeks. Hospitals often put policies and practices in place that limit the number of back-to-back contracts that traveling nurses can accept, which means that RNs can either convert to core staff or move on to new assignments once their contract term is up.
Weirich noted that some hospitals devote considerable effort to recruiting traveling nurses to full-time roles, adding, “There are active initiatives ... to make it such a good experience that they want to stay.”
On the flip side, contracts can be terminated without notice, leaving traveling nurses scrambling to find a new assignment and a new place to live on short notice.
“You’re there as long as the hospital needs you,” said Neill. “You could sign a 12- or 15-week contract, and their needs change a month in, and ... there are budget cuts, and they can’t pay salaries anymore, so they are laying off their nurses.”
Declining demand for travel nurses has made it harder to line up back-to-back contracts. Despite being available for work, Doran once waited 6 weeks to secure a new assignment and had to live off her savings.
Spurlock believes increased competition and declining wages — pay for travel nurses declined more than 9% from January 2023 to January 2024 — have made travel nursing less attractive.
“There has been such an influx of travel nurses ... because of COVID,” said Spurlock. “The rates have now come down [and] everybody’s fighting for jobs, and ... it’s very difficult to get a job that’s paying decent money.”
Despite the challenges, Spurlock continues learning new things from each assignment and hopes to work as a travel nurse until retirement. Doran has worked at hospitals in Washington, Oregon, California, and Wisconsin and would like to add Montana, Utah, and Nevada to the list. The goal: Continue accepting assignments in different cities and states until she finds the place where she wants to put down roots.
“Nursing is a great job, but it’s a hard job [and] it can take its toll at times,” Neill said. It’s important that nurses know their goals and values to be able to find a good fitting position. “And the beauty of it is that travel can be a great way to explore and add some flexibility.”
A version of this article first appeared on Medscape.com.
The Bad News Behind the Rise in Locum Tenens
I’ve worked locum tenens off and on since 1982. Flexible schedules allowed me to write several books, pursue a parallel career as a medical journalist, lead medical missions in the Philippines, and develop modest expertise as an underwater photographer.
But the recent rise in locum tenens practitioners signals trouble for medicine.
A Multibillion-Dollar Industry
Roughly 52,000 US doctors work locum tenens full or part time. In annual reports by CHG Healthcare, two thirds of healthcare facilities surveyed report using locums and more than half expect to maintain or increase their use in 2024.
Another measure of the industry’s growth is that membership of The National Association of Locum Tenens Organizations (NALTO), formed in 2001 to lead this fledgling industry, has doubled since 2019. Currently, NALTO has 148 member agencies.
Why Locums?
What used to be the preserve of older physicians transitioning to retirement is now becoming a career choice. According to the 2024 Survey of Locum Tenens Physicians and Advanced Practice Professionals by AMN Healthcare, 81% of respondents said they started taking locum tenens assignments immediately after finishing medical training or in mid-career. What entices doctors to move from place to place, repeatedly adapt to new facilities and electronic medical records, live in cheap hotels, and work without paid vacations, health insurance, or retirement benefits?
Supplemental income is one reason. But the elephant in the room is clearly burnout. Rates of burnout in practicing doctors and physicians-in-training have exceeded 50%. Burnout results in medical errors, malpractice suits, and increased healthcare costs.
A recent Doximity poll of 7590 physicians revealed that 63% would not want their children to pursue a medical career. And in a Medscape survey of 7000 physicians, a third of docs under 40 would not choose medicine again if they had a do-over. If a career in medicine brings high income and privileged status, why do so many physicians regret it and discourage their children from taking the same path?
Where Is Marcus Welby, MD?
Private practice is an endangered species that no one is trying to save. According to a 2022 AMA survey, 44% of physicians owned their practices compared with 76% of physicians in the 1980s. Even fewer younger physicians are choosing private practice. Among physicians under 45 years of age, only 32% owned their practices. Most physicians are now employees, not employers. They have lost control over their duties and work hours.
In 2022, barely 13% of physicians were in solo practice. The iconic Dr Marcus Welby of the 1970s TV series has transmuted from an idealized physician to an implausible figure. (My medical students have never heard of him.)
Hospitals and health systems have purchased many private medical groups. Private-equity companies own close to 1000 physician practices and staff up to 40% of emergency rooms. For these firms, profits are paramount.
Canary in a Coal Mine
Locum tenens offers physicians unprecedented flexibility where they work, when they work, and how much they work. It provides an escape from overwhelming and unsatisfying clinical practice. While some physicians have fled to nonclinical careers, locums physicians can practice medicine without the burdens of administration, hospital politics, and ever-increasing overhead.
The locum tenens paradox is that its successful growth indicates a deteriorating traditional healthcare model. Locum tenens is not the problem, but it’s also not the solution. At best, locums is a pair of crutches that helps the current system limp along.
Healthcare is increasingly controlled by those who prioritize profit, not patients. If physicians become nothing more than complicit cogs in a dysfunctional system, burnout will fester. The profession will fail to attract the best and the brightest, the doctor shortage will increase, and the quality of patient care will decline. Everyone will suffer.
It’s already happening.
Andrew Wilner is an associate professor of neurology at the University of Tennessee Health Science Center, Memphis. He reported conflicts of interest from Accordant Health Services.
A version of this article first appeared on Medscape.com.
I’ve worked locum tenens off and on since 1982. Flexible schedules allowed me to write several books, pursue a parallel career as a medical journalist, lead medical missions in the Philippines, and develop modest expertise as an underwater photographer.
But the recent rise in locum tenens practitioners signals trouble for medicine.
A Multibillion-Dollar Industry
Roughly 52,000 US doctors work locum tenens full or part time. In annual reports by CHG Healthcare, two thirds of healthcare facilities surveyed report using locums and more than half expect to maintain or increase their use in 2024.
Another measure of the industry’s growth is that membership of The National Association of Locum Tenens Organizations (NALTO), formed in 2001 to lead this fledgling industry, has doubled since 2019. Currently, NALTO has 148 member agencies.
Why Locums?
What used to be the preserve of older physicians transitioning to retirement is now becoming a career choice. According to the 2024 Survey of Locum Tenens Physicians and Advanced Practice Professionals by AMN Healthcare, 81% of respondents said they started taking locum tenens assignments immediately after finishing medical training or in mid-career. What entices doctors to move from place to place, repeatedly adapt to new facilities and electronic medical records, live in cheap hotels, and work without paid vacations, health insurance, or retirement benefits?
Supplemental income is one reason. But the elephant in the room is clearly burnout. Rates of burnout in practicing doctors and physicians-in-training have exceeded 50%. Burnout results in medical errors, malpractice suits, and increased healthcare costs.
A recent Doximity poll of 7590 physicians revealed that 63% would not want their children to pursue a medical career. And in a Medscape survey of 7000 physicians, a third of docs under 40 would not choose medicine again if they had a do-over. If a career in medicine brings high income and privileged status, why do so many physicians regret it and discourage their children from taking the same path?
Where Is Marcus Welby, MD?
Private practice is an endangered species that no one is trying to save. According to a 2022 AMA survey, 44% of physicians owned their practices compared with 76% of physicians in the 1980s. Even fewer younger physicians are choosing private practice. Among physicians under 45 years of age, only 32% owned their practices. Most physicians are now employees, not employers. They have lost control over their duties and work hours.
In 2022, barely 13% of physicians were in solo practice. The iconic Dr Marcus Welby of the 1970s TV series has transmuted from an idealized physician to an implausible figure. (My medical students have never heard of him.)
Hospitals and health systems have purchased many private medical groups. Private-equity companies own close to 1000 physician practices and staff up to 40% of emergency rooms. For these firms, profits are paramount.
Canary in a Coal Mine
Locum tenens offers physicians unprecedented flexibility where they work, when they work, and how much they work. It provides an escape from overwhelming and unsatisfying clinical practice. While some physicians have fled to nonclinical careers, locums physicians can practice medicine without the burdens of administration, hospital politics, and ever-increasing overhead.
The locum tenens paradox is that its successful growth indicates a deteriorating traditional healthcare model. Locum tenens is not the problem, but it’s also not the solution. At best, locums is a pair of crutches that helps the current system limp along.
Healthcare is increasingly controlled by those who prioritize profit, not patients. If physicians become nothing more than complicit cogs in a dysfunctional system, burnout will fester. The profession will fail to attract the best and the brightest, the doctor shortage will increase, and the quality of patient care will decline. Everyone will suffer.
It’s already happening.
Andrew Wilner is an associate professor of neurology at the University of Tennessee Health Science Center, Memphis. He reported conflicts of interest from Accordant Health Services.
A version of this article first appeared on Medscape.com.
I’ve worked locum tenens off and on since 1982. Flexible schedules allowed me to write several books, pursue a parallel career as a medical journalist, lead medical missions in the Philippines, and develop modest expertise as an underwater photographer.
But the recent rise in locum tenens practitioners signals trouble for medicine.
A Multibillion-Dollar Industry
Roughly 52,000 US doctors work locum tenens full or part time. In annual reports by CHG Healthcare, two thirds of healthcare facilities surveyed report using locums and more than half expect to maintain or increase their use in 2024.
Another measure of the industry’s growth is that membership of The National Association of Locum Tenens Organizations (NALTO), formed in 2001 to lead this fledgling industry, has doubled since 2019. Currently, NALTO has 148 member agencies.
Why Locums?
What used to be the preserve of older physicians transitioning to retirement is now becoming a career choice. According to the 2024 Survey of Locum Tenens Physicians and Advanced Practice Professionals by AMN Healthcare, 81% of respondents said they started taking locum tenens assignments immediately after finishing medical training or in mid-career. What entices doctors to move from place to place, repeatedly adapt to new facilities and electronic medical records, live in cheap hotels, and work without paid vacations, health insurance, or retirement benefits?
Supplemental income is one reason. But the elephant in the room is clearly burnout. Rates of burnout in practicing doctors and physicians-in-training have exceeded 50%. Burnout results in medical errors, malpractice suits, and increased healthcare costs.
A recent Doximity poll of 7590 physicians revealed that 63% would not want their children to pursue a medical career. And in a Medscape survey of 7000 physicians, a third of docs under 40 would not choose medicine again if they had a do-over. If a career in medicine brings high income and privileged status, why do so many physicians regret it and discourage their children from taking the same path?
Where Is Marcus Welby, MD?
Private practice is an endangered species that no one is trying to save. According to a 2022 AMA survey, 44% of physicians owned their practices compared with 76% of physicians in the 1980s. Even fewer younger physicians are choosing private practice. Among physicians under 45 years of age, only 32% owned their practices. Most physicians are now employees, not employers. They have lost control over their duties and work hours.
In 2022, barely 13% of physicians were in solo practice. The iconic Dr Marcus Welby of the 1970s TV series has transmuted from an idealized physician to an implausible figure. (My medical students have never heard of him.)
Hospitals and health systems have purchased many private medical groups. Private-equity companies own close to 1000 physician practices and staff up to 40% of emergency rooms. For these firms, profits are paramount.
Canary in a Coal Mine
Locum tenens offers physicians unprecedented flexibility where they work, when they work, and how much they work. It provides an escape from overwhelming and unsatisfying clinical practice. While some physicians have fled to nonclinical careers, locums physicians can practice medicine without the burdens of administration, hospital politics, and ever-increasing overhead.
The locum tenens paradox is that its successful growth indicates a deteriorating traditional healthcare model. Locum tenens is not the problem, but it’s also not the solution. At best, locums is a pair of crutches that helps the current system limp along.
Healthcare is increasingly controlled by those who prioritize profit, not patients. If physicians become nothing more than complicit cogs in a dysfunctional system, burnout will fester. The profession will fail to attract the best and the brightest, the doctor shortage will increase, and the quality of patient care will decline. Everyone will suffer.
It’s already happening.
Andrew Wilner is an associate professor of neurology at the University of Tennessee Health Science Center, Memphis. He reported conflicts of interest from Accordant Health Services.
A version of this article first appeared on Medscape.com.
Myasthenia Gravis Highlights From AANEM 2024
The latest data on myasthenia gravis (MG) research, reported at the American Association of Neuromuscular and Electrodiagnostic Medicine 2024 Annual Meeting, are presented by Dr Pushpa Narayanswami of Harvard Medical School in Boston, Massachusetts.
Dr Narayanswami begins with a safety, tolerability, and efficacy study for subcutaneous efgartigimod. Results showed that the mean change in MG activities of daily living (MG-ADL) was no different between the fixed-dose and cyclic regimens, demonstrating another dosage option for patients.
Next, Dr Narayanswami discusses two separate complement C5 inhibitor therapy trials. The first was a global registry study looking at ravulizumab. Patient cohorts consisted of those who started and remained on ravulizumab vs another that switched from initial eculizumab to ravulizumab. In both groups, MG-ADL was improved. The other study investigated zilucoplan in acetylcholine receptor autoantibody–positive generalized MG patient populations; similarly, researchers found favorable results.
She then details a study looking at the safety outcomes in pregnant patients treated with eculizumab. Because of limited disease-specific data in the registry, further investigation is recommended.
Finally, Dr Narayanaswami examines results for inebilizumab, a first-in-class anti-CD19 B cell–depleting agent. The drug demonstrated safety and beneficial efficacy compared with placebo in seropositive generalized MG patients.
--
Pushpa Narayanaswami, MD, Associate Professor, Department of Neurology, Harvard Medical School; Vice Chair of Clinical Operations, Department of Neurology, Beth Israel Deaconess Medical Center, Boston, Massachusetts
Pushpa Narayanaswami, MD, has disclosed the following relevant financial relationships:
Serve(d) as an advisor or consultant for: Alexion; Argenx; Janssen; Dianthus; UCB; GSK
Received research grant from: Alexion; UCB; Dianthus; Janssen
The latest data on myasthenia gravis (MG) research, reported at the American Association of Neuromuscular and Electrodiagnostic Medicine 2024 Annual Meeting, are presented by Dr Pushpa Narayanswami of Harvard Medical School in Boston, Massachusetts.
Dr Narayanswami begins with a safety, tolerability, and efficacy study for subcutaneous efgartigimod. Results showed that the mean change in MG activities of daily living (MG-ADL) was no different between the fixed-dose and cyclic regimens, demonstrating another dosage option for patients.
Next, Dr Narayanswami discusses two separate complement C5 inhibitor therapy trials. The first was a global registry study looking at ravulizumab. Patient cohorts consisted of those who started and remained on ravulizumab vs another that switched from initial eculizumab to ravulizumab. In both groups, MG-ADL was improved. The other study investigated zilucoplan in acetylcholine receptor autoantibody–positive generalized MG patient populations; similarly, researchers found favorable results.
She then details a study looking at the safety outcomes in pregnant patients treated with eculizumab. Because of limited disease-specific data in the registry, further investigation is recommended.
Finally, Dr Narayanaswami examines results for inebilizumab, a first-in-class anti-CD19 B cell–depleting agent. The drug demonstrated safety and beneficial efficacy compared with placebo in seropositive generalized MG patients.
--
Pushpa Narayanaswami, MD, Associate Professor, Department of Neurology, Harvard Medical School; Vice Chair of Clinical Operations, Department of Neurology, Beth Israel Deaconess Medical Center, Boston, Massachusetts
Pushpa Narayanaswami, MD, has disclosed the following relevant financial relationships:
Serve(d) as an advisor or consultant for: Alexion; Argenx; Janssen; Dianthus; UCB; GSK
Received research grant from: Alexion; UCB; Dianthus; Janssen
The latest data on myasthenia gravis (MG) research, reported at the American Association of Neuromuscular and Electrodiagnostic Medicine 2024 Annual Meeting, are presented by Dr Pushpa Narayanswami of Harvard Medical School in Boston, Massachusetts.
Dr Narayanswami begins with a safety, tolerability, and efficacy study for subcutaneous efgartigimod. Results showed that the mean change in MG activities of daily living (MG-ADL) was no different between the fixed-dose and cyclic regimens, demonstrating another dosage option for patients.
Next, Dr Narayanswami discusses two separate complement C5 inhibitor therapy trials. The first was a global registry study looking at ravulizumab. Patient cohorts consisted of those who started and remained on ravulizumab vs another that switched from initial eculizumab to ravulizumab. In both groups, MG-ADL was improved. The other study investigated zilucoplan in acetylcholine receptor autoantibody–positive generalized MG patient populations; similarly, researchers found favorable results.
She then details a study looking at the safety outcomes in pregnant patients treated with eculizumab. Because of limited disease-specific data in the registry, further investigation is recommended.
Finally, Dr Narayanaswami examines results for inebilizumab, a first-in-class anti-CD19 B cell–depleting agent. The drug demonstrated safety and beneficial efficacy compared with placebo in seropositive generalized MG patients.
--
Pushpa Narayanaswami, MD, Associate Professor, Department of Neurology, Harvard Medical School; Vice Chair of Clinical Operations, Department of Neurology, Beth Israel Deaconess Medical Center, Boston, Massachusetts
Pushpa Narayanaswami, MD, has disclosed the following relevant financial relationships:
Serve(d) as an advisor or consultant for: Alexion; Argenx; Janssen; Dianthus; UCB; GSK
Received research grant from: Alexion; UCB; Dianthus; Janssen

A History of Concussion Linked to Maternal Mental Illness
A history of concussion can have serious long-term mental health implications for women, even years after giving birth, according to a new study.
Researchers looked at all people who delivered babies in Ontario, Canada, and found that those with a predelivery history of concussion were 25% more likely to have a serious mental illness up to 14 years after giving birth than those with no history of concussion.
The findings indicate the need for early identification and screening of women with a history of concussion, as well as ongoing, long-term supports to prevent adverse psychiatric outcomes, wrote the authors.
“I played a lot of sports growing up, and I definitely would not have thought about how a concussion could affect childbearing or parenting,” author Samantha Krueger, RM, MSc, told this news organization. She completed the research as part of her studies at the University of Toronto, Ontario.
The data were published on November 4 in The Journal of Clinical Psychiatry.
Implications for Prevention
“Birthing people, and women in general, are an often-overlooked population in the scientific literature on traumatic brain injury, including concussion. There is a potential interplay between concussion history and the challenges of being a new parent (such as labor and birth, lack of sleep, and increased noise) that make this an important population to study,” said Krueger.
The researchers conducted a population-based cohort study of all women who gave birth in Ontario between 2007 and 2017. Follow-up continued until 2021. The primary outcome was severe maternal mental illness, which was defined as a psychiatric emergency department visit, psychiatric hospital admission, or self-harm or suicide in the 14 years after delivery.
The researchers identified 18,064 women with a predelivery history of concussion and 736,689 women without a history of concussion during the study period. Women with a predelivery history of concussion were more likely than those without such a history to live in a rural area and have a history of assault or mental illness.
Overall, 11.3% (n = 2033) of the women with a predelivery history of concussion developed severe maternal mental illness (14.7 per 1000 person-years), compared with 6.8% (n = 49,928) of the women without a predelivery history of concussion (7.9 per 1000 person-years).
The adjusted hazard ratio (aHR) was 1.25. The association was strongest in women who had a predelivery history of concussion but no history of mental illness (aHR, 1.33).
“We hope to increase awareness of the seriousness of having a concussion, even when it is considered a mild head injury,” Krueger said. “The results have important implications for concussion prevention measures for young people and for the provision of postpartum supports (such as mental health and other social supports like sleep relief) to mitigate the risk of serious mental illness outcomes in birthing people with a history of concussion.”
Healthcare providers, including maternity care providers, should be asking about concussion history and providing mental health screening and supports to clients and their families to detect mental illness before a serious outcome occurs, Krueger added.
“Maternity care providers can help birthing people and their families set up supports for after the baby is born and teach families about mental health symptoms to look out for. It’s also important that providers be certain that their care is trauma informed to avoid triggering a trauma response when providing care,” she said.
Area of Concern
“This research is novel and highlights an area of major concern,” Simon Sherry, PhD, professor of psychology and neuroscience at Dalhousie University in Halifax, Nova Scotia, Canada, told this news organization. Sherry did not participate in the study.
“Postpartum depression occurs in approximately 10%-25% of mothers, but it is likely that many more cases go undiagnosed. It is attributed to hormonal changes, genetic predisposition, and environmental factors, and while previous depression or mental illness is frequently considered a risk factor, traumatic brain injuries or concussions usually are not,” Sherry said.
“Mothers are already an at-risk population for mental illness, as illustrated by the high rates of postpartum depression, and so are people with a history of concussion or traumatic brain injury. What sets this study apart is that it shows the heightened risk for women with the combination of those two distinct risk factors. Identifying these risk factors is essential to providing preventive care. If care providers know a patient is at increased risk when starting a pregnancy, then they will likely catch warning signs earlier,” he said.
“Additionally, as the article suggests, maternal mental health often is not studied beyond the first postpartum year,” Sherry said.
“Mental health struggles during the first postpartum year have largely been normalized as part of the transition into parenthood, but mental health issues among parents later in life are less accepted. After birth, so much emphasis is moved from the parent to the child. Parents rightly prioritize their children, but our job as care providers is to ensure we are also prioritizing them. The prolonged period of this study helps illustrate how important the practice of prioritizing mothers’ mental health is,” he added.
The study was supported by ICES, which is funded by an annual grant from the Ontario Ministry of Health and the Ministry of Long-Term Care. The Canadian Institutes of Health Research also supported the study. Krueger is supported by a Canadian Institutes of Health Research Canada Graduate Scholarship Masters Award. Sherry reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A history of concussion can have serious long-term mental health implications for women, even years after giving birth, according to a new study.
Researchers looked at all people who delivered babies in Ontario, Canada, and found that those with a predelivery history of concussion were 25% more likely to have a serious mental illness up to 14 years after giving birth than those with no history of concussion.
The findings indicate the need for early identification and screening of women with a history of concussion, as well as ongoing, long-term supports to prevent adverse psychiatric outcomes, wrote the authors.
“I played a lot of sports growing up, and I definitely would not have thought about how a concussion could affect childbearing or parenting,” author Samantha Krueger, RM, MSc, told this news organization. She completed the research as part of her studies at the University of Toronto, Ontario.
The data were published on November 4 in The Journal of Clinical Psychiatry.
Implications for Prevention
“Birthing people, and women in general, are an often-overlooked population in the scientific literature on traumatic brain injury, including concussion. There is a potential interplay between concussion history and the challenges of being a new parent (such as labor and birth, lack of sleep, and increased noise) that make this an important population to study,” said Krueger.
The researchers conducted a population-based cohort study of all women who gave birth in Ontario between 2007 and 2017. Follow-up continued until 2021. The primary outcome was severe maternal mental illness, which was defined as a psychiatric emergency department visit, psychiatric hospital admission, or self-harm or suicide in the 14 years after delivery.
The researchers identified 18,064 women with a predelivery history of concussion and 736,689 women without a history of concussion during the study period. Women with a predelivery history of concussion were more likely than those without such a history to live in a rural area and have a history of assault or mental illness.
Overall, 11.3% (n = 2033) of the women with a predelivery history of concussion developed severe maternal mental illness (14.7 per 1000 person-years), compared with 6.8% (n = 49,928) of the women without a predelivery history of concussion (7.9 per 1000 person-years).
The adjusted hazard ratio (aHR) was 1.25. The association was strongest in women who had a predelivery history of concussion but no history of mental illness (aHR, 1.33).
“We hope to increase awareness of the seriousness of having a concussion, even when it is considered a mild head injury,” Krueger said. “The results have important implications for concussion prevention measures for young people and for the provision of postpartum supports (such as mental health and other social supports like sleep relief) to mitigate the risk of serious mental illness outcomes in birthing people with a history of concussion.”
Healthcare providers, including maternity care providers, should be asking about concussion history and providing mental health screening and supports to clients and their families to detect mental illness before a serious outcome occurs, Krueger added.
“Maternity care providers can help birthing people and their families set up supports for after the baby is born and teach families about mental health symptoms to look out for. It’s also important that providers be certain that their care is trauma informed to avoid triggering a trauma response when providing care,” she said.
Area of Concern
“This research is novel and highlights an area of major concern,” Simon Sherry, PhD, professor of psychology and neuroscience at Dalhousie University in Halifax, Nova Scotia, Canada, told this news organization. Sherry did not participate in the study.
“Postpartum depression occurs in approximately 10%-25% of mothers, but it is likely that many more cases go undiagnosed. It is attributed to hormonal changes, genetic predisposition, and environmental factors, and while previous depression or mental illness is frequently considered a risk factor, traumatic brain injuries or concussions usually are not,” Sherry said.
“Mothers are already an at-risk population for mental illness, as illustrated by the high rates of postpartum depression, and so are people with a history of concussion or traumatic brain injury. What sets this study apart is that it shows the heightened risk for women with the combination of those two distinct risk factors. Identifying these risk factors is essential to providing preventive care. If care providers know a patient is at increased risk when starting a pregnancy, then they will likely catch warning signs earlier,” he said.
“Additionally, as the article suggests, maternal mental health often is not studied beyond the first postpartum year,” Sherry said.
“Mental health struggles during the first postpartum year have largely been normalized as part of the transition into parenthood, but mental health issues among parents later in life are less accepted. After birth, so much emphasis is moved from the parent to the child. Parents rightly prioritize their children, but our job as care providers is to ensure we are also prioritizing them. The prolonged period of this study helps illustrate how important the practice of prioritizing mothers’ mental health is,” he added.
The study was supported by ICES, which is funded by an annual grant from the Ontario Ministry of Health and the Ministry of Long-Term Care. The Canadian Institutes of Health Research also supported the study. Krueger is supported by a Canadian Institutes of Health Research Canada Graduate Scholarship Masters Award. Sherry reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A history of concussion can have serious long-term mental health implications for women, even years after giving birth, according to a new study.
Researchers looked at all people who delivered babies in Ontario, Canada, and found that those with a predelivery history of concussion were 25% more likely to have a serious mental illness up to 14 years after giving birth than those with no history of concussion.
The findings indicate the need for early identification and screening of women with a history of concussion, as well as ongoing, long-term supports to prevent adverse psychiatric outcomes, wrote the authors.
“I played a lot of sports growing up, and I definitely would not have thought about how a concussion could affect childbearing or parenting,” author Samantha Krueger, RM, MSc, told this news organization. She completed the research as part of her studies at the University of Toronto, Ontario.
The data were published on November 4 in The Journal of Clinical Psychiatry.
Implications for Prevention
“Birthing people, and women in general, are an often-overlooked population in the scientific literature on traumatic brain injury, including concussion. There is a potential interplay between concussion history and the challenges of being a new parent (such as labor and birth, lack of sleep, and increased noise) that make this an important population to study,” said Krueger.
The researchers conducted a population-based cohort study of all women who gave birth in Ontario between 2007 and 2017. Follow-up continued until 2021. The primary outcome was severe maternal mental illness, which was defined as a psychiatric emergency department visit, psychiatric hospital admission, or self-harm or suicide in the 14 years after delivery.
The researchers identified 18,064 women with a predelivery history of concussion and 736,689 women without a history of concussion during the study period. Women with a predelivery history of concussion were more likely than those without such a history to live in a rural area and have a history of assault or mental illness.
Overall, 11.3% (n = 2033) of the women with a predelivery history of concussion developed severe maternal mental illness (14.7 per 1000 person-years), compared with 6.8% (n = 49,928) of the women without a predelivery history of concussion (7.9 per 1000 person-years).
The adjusted hazard ratio (aHR) was 1.25. The association was strongest in women who had a predelivery history of concussion but no history of mental illness (aHR, 1.33).
“We hope to increase awareness of the seriousness of having a concussion, even when it is considered a mild head injury,” Krueger said. “The results have important implications for concussion prevention measures for young people and for the provision of postpartum supports (such as mental health and other social supports like sleep relief) to mitigate the risk of serious mental illness outcomes in birthing people with a history of concussion.”
Healthcare providers, including maternity care providers, should be asking about concussion history and providing mental health screening and supports to clients and their families to detect mental illness before a serious outcome occurs, Krueger added.
“Maternity care providers can help birthing people and their families set up supports for after the baby is born and teach families about mental health symptoms to look out for. It’s also important that providers be certain that their care is trauma informed to avoid triggering a trauma response when providing care,” she said.
Area of Concern
“This research is novel and highlights an area of major concern,” Simon Sherry, PhD, professor of psychology and neuroscience at Dalhousie University in Halifax, Nova Scotia, Canada, told this news organization. Sherry did not participate in the study.
“Postpartum depression occurs in approximately 10%-25% of mothers, but it is likely that many more cases go undiagnosed. It is attributed to hormonal changes, genetic predisposition, and environmental factors, and while previous depression or mental illness is frequently considered a risk factor, traumatic brain injuries or concussions usually are not,” Sherry said.
“Mothers are already an at-risk population for mental illness, as illustrated by the high rates of postpartum depression, and so are people with a history of concussion or traumatic brain injury. What sets this study apart is that it shows the heightened risk for women with the combination of those two distinct risk factors. Identifying these risk factors is essential to providing preventive care. If care providers know a patient is at increased risk when starting a pregnancy, then they will likely catch warning signs earlier,” he said.
“Additionally, as the article suggests, maternal mental health often is not studied beyond the first postpartum year,” Sherry said.
“Mental health struggles during the first postpartum year have largely been normalized as part of the transition into parenthood, but mental health issues among parents later in life are less accepted. After birth, so much emphasis is moved from the parent to the child. Parents rightly prioritize their children, but our job as care providers is to ensure we are also prioritizing them. The prolonged period of this study helps illustrate how important the practice of prioritizing mothers’ mental health is,” he added.
The study was supported by ICES, which is funded by an annual grant from the Ontario Ministry of Health and the Ministry of Long-Term Care. The Canadian Institutes of Health Research also supported the study. Krueger is supported by a Canadian Institutes of Health Research Canada Graduate Scholarship Masters Award. Sherry reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF CLINICAL PSYCHIATRY
4 Simple Hacks to Get Paid for Lifestyle Medicine
This transcript has been edited for clarity.
As primary care doctors, lifestyle medicine is supposed to be a pillar of our practice. Per the evidence, lifestyle medicine can prevent up to 80% of chronic disease. It’s a real irony, then, that it’s the thing we’re least likely to be paid to do.
Thankfully, though, there are a few hacks to help you keep your patients healthy and yourself financially healthy at the same time.
No. 1: Be as accurate in your coding as possible. We all know working on things like sleep, exercise, and diet with patients takes time, so bill for it. With time-based billing, in particular, you can account for both the time spent in face-to-face encounters and the time spent afterward on documentation and care coordination. Make sure to capture that.
No. 2: Try group visits on for size. Group visit models are great for lifestyle medicine. They give you the flexibility to include longer conversations and deeper lessons on a range of subjects while still getting paid for what you do. Want to host a cooking class? Group visit. Want to bring in a personal trainer or hold a dance class or exercise dance class? Group visit. Meditation, yoga, or even a sleep hygiene class? Group visit.
While there are a few tricks to getting paid for group visits, they’re the same things, such as documenting time and the various parts of the visit, that are key to getting paid for regular visits. They have the bonus of fighting burnout and making your own practice more meaningful as well.
No. 3: Think about joining a value-based care arrangement. While only accounting for 10% of the market right now, value-based care (VBC) is growing rapidly, and it’s easy to see why. By trading quality for the hamster wheel of billing widgets, physicians are freed up to think more about how best to take care of patients, including incorporating more lifestyle medicine. Some VBC models even have their own electronic medical records, freeing you from outdated structures when it comes to documenting patient visits.
No. 4: direct primary care. Direct primary care cuts out the middlemen of payers, letting patients pay physician practices directly for their own care. Like VBC, it opens up possibilities for practicing better medicine, including lifestyle medicine. In addition, it’s often very affordable, with a family of four often paying around $80 a month for a membership for the entire family. It’s a win-win for the doctor and the patient.
Lifestyle medicine is a great way to improve both your patients’ and your own well-being. With a few flexes, it can improve your wallet’s well-being, too.
Tamaan K. Osbourne-Roberts, President/CEO, Happiness by the Numbers, Denver, Colorado, has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
As primary care doctors, lifestyle medicine is supposed to be a pillar of our practice. Per the evidence, lifestyle medicine can prevent up to 80% of chronic disease. It’s a real irony, then, that it’s the thing we’re least likely to be paid to do.
Thankfully, though, there are a few hacks to help you keep your patients healthy and yourself financially healthy at the same time.
No. 1: Be as accurate in your coding as possible. We all know working on things like sleep, exercise, and diet with patients takes time, so bill for it. With time-based billing, in particular, you can account for both the time spent in face-to-face encounters and the time spent afterward on documentation and care coordination. Make sure to capture that.
No. 2: Try group visits on for size. Group visit models are great for lifestyle medicine. They give you the flexibility to include longer conversations and deeper lessons on a range of subjects while still getting paid for what you do. Want to host a cooking class? Group visit. Want to bring in a personal trainer or hold a dance class or exercise dance class? Group visit. Meditation, yoga, or even a sleep hygiene class? Group visit.
While there are a few tricks to getting paid for group visits, they’re the same things, such as documenting time and the various parts of the visit, that are key to getting paid for regular visits. They have the bonus of fighting burnout and making your own practice more meaningful as well.
No. 3: Think about joining a value-based care arrangement. While only accounting for 10% of the market right now, value-based care (VBC) is growing rapidly, and it’s easy to see why. By trading quality for the hamster wheel of billing widgets, physicians are freed up to think more about how best to take care of patients, including incorporating more lifestyle medicine. Some VBC models even have their own electronic medical records, freeing you from outdated structures when it comes to documenting patient visits.
No. 4: direct primary care. Direct primary care cuts out the middlemen of payers, letting patients pay physician practices directly for their own care. Like VBC, it opens up possibilities for practicing better medicine, including lifestyle medicine. In addition, it’s often very affordable, with a family of four often paying around $80 a month for a membership for the entire family. It’s a win-win for the doctor and the patient.
Lifestyle medicine is a great way to improve both your patients’ and your own well-being. With a few flexes, it can improve your wallet’s well-being, too.
Tamaan K. Osbourne-Roberts, President/CEO, Happiness by the Numbers, Denver, Colorado, has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
As primary care doctors, lifestyle medicine is supposed to be a pillar of our practice. Per the evidence, lifestyle medicine can prevent up to 80% of chronic disease. It’s a real irony, then, that it’s the thing we’re least likely to be paid to do.
Thankfully, though, there are a few hacks to help you keep your patients healthy and yourself financially healthy at the same time.
No. 1: Be as accurate in your coding as possible. We all know working on things like sleep, exercise, and diet with patients takes time, so bill for it. With time-based billing, in particular, you can account for both the time spent in face-to-face encounters and the time spent afterward on documentation and care coordination. Make sure to capture that.
No. 2: Try group visits on for size. Group visit models are great for lifestyle medicine. They give you the flexibility to include longer conversations and deeper lessons on a range of subjects while still getting paid for what you do. Want to host a cooking class? Group visit. Want to bring in a personal trainer or hold a dance class or exercise dance class? Group visit. Meditation, yoga, or even a sleep hygiene class? Group visit.
While there are a few tricks to getting paid for group visits, they’re the same things, such as documenting time and the various parts of the visit, that are key to getting paid for regular visits. They have the bonus of fighting burnout and making your own practice more meaningful as well.
No. 3: Think about joining a value-based care arrangement. While only accounting for 10% of the market right now, value-based care (VBC) is growing rapidly, and it’s easy to see why. By trading quality for the hamster wheel of billing widgets, physicians are freed up to think more about how best to take care of patients, including incorporating more lifestyle medicine. Some VBC models even have their own electronic medical records, freeing you from outdated structures when it comes to documenting patient visits.
No. 4: direct primary care. Direct primary care cuts out the middlemen of payers, letting patients pay physician practices directly for their own care. Like VBC, it opens up possibilities for practicing better medicine, including lifestyle medicine. In addition, it’s often very affordable, with a family of four often paying around $80 a month for a membership for the entire family. It’s a win-win for the doctor and the patient.
Lifestyle medicine is a great way to improve both your patients’ and your own well-being. With a few flexes, it can improve your wallet’s well-being, too.
Tamaan K. Osbourne-Roberts, President/CEO, Happiness by the Numbers, Denver, Colorado, has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Treating Obesity May Reduce Pelvic Organ Prolapse Risk
TOPLINE:
People with central obesity (CO), characterized by excess fat around the abdomen, are at a greater risk for pelvic organ prolapse (POP), particularly those who are younger than 60 years or without a history of hysterectomy. Also, women who have overweight but do not have CO are at greater risk.
METHODOLOGY:
- Researchers conducted a prospective cohort study to estimate the association between CO and general obesity and the risk for POP in individuals using the UK Biobank.
- A total of 251,143 participants (median age, 57 years) without preexisting POP were included, of whom 60.9% were postmenopausal and 17.2% had undergone hysterectomy before enrollment.
- Participants were followed for a median duration of 13.8 years, and POP cases were identified using International Classification of Diseases, 10th Revision (ICD-10) codes.
- Waist circumference, height, and body weight were measured at enrollment for the calculation of waist/height ratio and body mass index (BMI); CO was defined as a waist/height ratio ≥ 0.5.
- The relative risk of POP for the various combinations of waist/height ratio and BMI was evaluated against the reference group (waist/height ratio < 0.5; BMI < 25) using Cox proportional hazards models.
TAKEAWAY:
- During the follow-up period, 9781 cases of POP were identified, of which 71.2% occurred in a single pelvic compartment.
- Around 21.7% of all POP cases were attributable to CO; 2% were attributable to being overweight without CO.
- The risk for POP was 48% higher in individuals with CO regardless of BMI (hazard ratio [HR], 1.48; 95% CI, 1.41-1.56) and 23% higher in those who had overweight without CO (HR, 1.23; 95% CI, 1.14-1.34).
- The association between POP and CO was further strengthened in individuals who were younger than 60 years and those without a history of hysterectomy.
IN PRACTICE:
“We found that waist/height ratio combined with BMI could help differentiate individuals with varying risks of prolapse more accurately. Among individuals within the same BMI category, waist/height ratio can vary, with those having a higher ratio generally facing a greater risk of POP, compared with those with a normal ratio. Therefore, they should not be grouped together based solely on a single measure of obesity. In addition, this combination can help identify more individuals at high risk for POP, compared with using either alone,” the study authors wrote.
SOURCE:
This study was led by Keyi Si, PhD, of Tongji University in Shanghai, China, and was published online in Obstetrics & Gynecology.
LIMITATIONS:
Differences in healthcare-seeking behavior could have biased the association between obesity and risk for POP, as individuals with obesity may have been less likely to notice or report symptoms of POP. The diagnosis of POP was according to ICD-10 codes rather than physical examination, which may have affected accuracy. Other limitations included missing data on delivery mode and history of constipation.
DISCLOSURES:
This study was supported by grants from the National Natural Science Foundation of China, the Science and Technology Commission of Shanghai Municipality, the Shanghai Hospital Development Center, and the Shanghai First Maternity and Infant Hospital. The authors reported no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
People with central obesity (CO), characterized by excess fat around the abdomen, are at a greater risk for pelvic organ prolapse (POP), particularly those who are younger than 60 years or without a history of hysterectomy. Also, women who have overweight but do not have CO are at greater risk.
METHODOLOGY:
- Researchers conducted a prospective cohort study to estimate the association between CO and general obesity and the risk for POP in individuals using the UK Biobank.
- A total of 251,143 participants (median age, 57 years) without preexisting POP were included, of whom 60.9% were postmenopausal and 17.2% had undergone hysterectomy before enrollment.
- Participants were followed for a median duration of 13.8 years, and POP cases were identified using International Classification of Diseases, 10th Revision (ICD-10) codes.
- Waist circumference, height, and body weight were measured at enrollment for the calculation of waist/height ratio and body mass index (BMI); CO was defined as a waist/height ratio ≥ 0.5.
- The relative risk of POP for the various combinations of waist/height ratio and BMI was evaluated against the reference group (waist/height ratio < 0.5; BMI < 25) using Cox proportional hazards models.
TAKEAWAY:
- During the follow-up period, 9781 cases of POP were identified, of which 71.2% occurred in a single pelvic compartment.
- Around 21.7% of all POP cases were attributable to CO; 2% were attributable to being overweight without CO.
- The risk for POP was 48% higher in individuals with CO regardless of BMI (hazard ratio [HR], 1.48; 95% CI, 1.41-1.56) and 23% higher in those who had overweight without CO (HR, 1.23; 95% CI, 1.14-1.34).
- The association between POP and CO was further strengthened in individuals who were younger than 60 years and those without a history of hysterectomy.
IN PRACTICE:
“We found that waist/height ratio combined with BMI could help differentiate individuals with varying risks of prolapse more accurately. Among individuals within the same BMI category, waist/height ratio can vary, with those having a higher ratio generally facing a greater risk of POP, compared with those with a normal ratio. Therefore, they should not be grouped together based solely on a single measure of obesity. In addition, this combination can help identify more individuals at high risk for POP, compared with using either alone,” the study authors wrote.
SOURCE:
This study was led by Keyi Si, PhD, of Tongji University in Shanghai, China, and was published online in Obstetrics & Gynecology.
LIMITATIONS:
Differences in healthcare-seeking behavior could have biased the association between obesity and risk for POP, as individuals with obesity may have been less likely to notice or report symptoms of POP. The diagnosis of POP was according to ICD-10 codes rather than physical examination, which may have affected accuracy. Other limitations included missing data on delivery mode and history of constipation.
DISCLOSURES:
This study was supported by grants from the National Natural Science Foundation of China, the Science and Technology Commission of Shanghai Municipality, the Shanghai Hospital Development Center, and the Shanghai First Maternity and Infant Hospital. The authors reported no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
People with central obesity (CO), characterized by excess fat around the abdomen, are at a greater risk for pelvic organ prolapse (POP), particularly those who are younger than 60 years or without a history of hysterectomy. Also, women who have overweight but do not have CO are at greater risk.
METHODOLOGY:
- Researchers conducted a prospective cohort study to estimate the association between CO and general obesity and the risk for POP in individuals using the UK Biobank.
- A total of 251,143 participants (median age, 57 years) without preexisting POP were included, of whom 60.9% were postmenopausal and 17.2% had undergone hysterectomy before enrollment.
- Participants were followed for a median duration of 13.8 years, and POP cases were identified using International Classification of Diseases, 10th Revision (ICD-10) codes.
- Waist circumference, height, and body weight were measured at enrollment for the calculation of waist/height ratio and body mass index (BMI); CO was defined as a waist/height ratio ≥ 0.5.
- The relative risk of POP for the various combinations of waist/height ratio and BMI was evaluated against the reference group (waist/height ratio < 0.5; BMI < 25) using Cox proportional hazards models.
TAKEAWAY:
- During the follow-up period, 9781 cases of POP were identified, of which 71.2% occurred in a single pelvic compartment.
- Around 21.7% of all POP cases were attributable to CO; 2% were attributable to being overweight without CO.
- The risk for POP was 48% higher in individuals with CO regardless of BMI (hazard ratio [HR], 1.48; 95% CI, 1.41-1.56) and 23% higher in those who had overweight without CO (HR, 1.23; 95% CI, 1.14-1.34).
- The association between POP and CO was further strengthened in individuals who were younger than 60 years and those without a history of hysterectomy.
IN PRACTICE:
“We found that waist/height ratio combined with BMI could help differentiate individuals with varying risks of prolapse more accurately. Among individuals within the same BMI category, waist/height ratio can vary, with those having a higher ratio generally facing a greater risk of POP, compared with those with a normal ratio. Therefore, they should not be grouped together based solely on a single measure of obesity. In addition, this combination can help identify more individuals at high risk for POP, compared with using either alone,” the study authors wrote.
SOURCE:
This study was led by Keyi Si, PhD, of Tongji University in Shanghai, China, and was published online in Obstetrics & Gynecology.
LIMITATIONS:
Differences in healthcare-seeking behavior could have biased the association between obesity and risk for POP, as individuals with obesity may have been less likely to notice or report symptoms of POP. The diagnosis of POP was according to ICD-10 codes rather than physical examination, which may have affected accuracy. Other limitations included missing data on delivery mode and history of constipation.
DISCLOSURES:
This study was supported by grants from the National Natural Science Foundation of China, the Science and Technology Commission of Shanghai Municipality, the Shanghai Hospital Development Center, and the Shanghai First Maternity and Infant Hospital. The authors reported no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Knowledge Gaps About Obesity Medicine Seen in Primary Care
SAN ANTONIO — Despite the prevalence of obesity in primary care, there appear to be major knowledge gaps among providers regarding obesity management, new research suggests.
Anonymous surveys of 96 primary care providers at a Boston, Massachusetts, safety-net hospital revealed that participants had limited understanding of criteria for prescribing antiobesity medications (AOM), and expressed discomfort in prescribing AOMs because of knowledge concerns, especially for non–glucagon-like peptide 1 (GLP-1) receptor agonists. One third reported that they didn’t prescribe AOMs, and rates of referral for bariatric surgery were also low.
The findings were presented at the Obesity Society’s annual Obesity Week meeting by Alejandro Campos, MD, a third-year resident in the section of internal medicine, Boston Medical Center, and the Department of Medicine, Boston University.
“I think it comes down to education. ... Not only training primary care physicians or residents about criteria and pathophysiology, but also stigma. Perceptions need to be addressed from the start of training in the healthcare field,” Campos told this news organization in an interview.
During his presentation, Campos noted this is the first such study in the setting of a safety-net hospital, which cares for lower-income people who experience disproportionate rates of obesity. But, “these findings are similar to ones observed from non–safety-net settings, which can indicate some potential transferability.”
Asked to comment, session moderator John D. Clark, MD, PhD, Chief Population Health Officer at Sharp Rees-Stealy Medical Group, San Diego, California, told this news organization that the findings didn’t surprise him. “I’d say that medical education around obesity has definitely improved, and training is improving but it’s not uniform. The treatment of obesity as a disease, especially with some of the newer medical treatments, is not standard of care and practiced widely.”
The study involved a standard-model Knowledge, Attitudes, and Practices questionnaire, distributed electronically for anonymous responses among both trained and in-training primary care providers. It contained a total of 43 items, 7 of them demographic, 11 on knowledge, 9 regarding attitudes, and 16 asking about practices.
The hospital is the largest safety-net hospital in New England, with a patient population that includes 58% enrolled in Medicaid, 32% Black/African American individuals, 24% identifying as Hispanic/Latino individuals, and 37% living below the poverty line.
The 96 responding providers (from a total 350 invited) all worked in either family medicine or internal medicine. The trained providers included both attending MDs and nurse practitioners, while those in-training were residents in one of those two specialties. Two thirds were women. The majority were aged 20-30 years (49.45%) or 31-40 years (27.47%).
Overall, 73.63% reported having received some type of obesity training. Just over half (52.08%) reported receiving that training during medical or nursing school, while 43.75% reported receiving it during residency.
When asked to choose from a list of conditions to pick which are considered weight-related comorbidities, between 80% and 90% choose type 2 diabetes, obstructive sleep apnea (OSA), hypertension, hyperlipidemia, nonalcoholic fatty liver disease, and coronary artery disease. Fewer, but still a majority, also listed osteoarthritis and gastroesophageal reflux disease. However, respondents were less likely to cite cancer, mood disorders, or chronic kidney disease as being related to obesity.
Asked to list benefits of a 10% body weight loss, most recognized reductions in OSA, glycemia, cardiovascular disease risk, osteoarthritis, and hepatic steatosis. But, only about half knew weight loss could also improve urinary incontinence.
Only 25% could correctly name both indications for AOMs. Just 27.1% knew that one was a body mass index (BMI) ≥ 27 with comorbidities, while 46.9% knew BMI ≥ 30 without comorbidities was an AOM indication. Only 9.4% were correct on both of those indications for bariatric surgery.
“Reassuringly,” Campos said, the majority either “disagreed” or “strongly disagreed” that “lack of will power” contributes to obesity. However, more than 20% agreed that “lack of exercise or physical activity” contributed.
Overall, 73% of the trained providers and 59% of those in training reported that they prescribe AOMs. Asked about their comfort level in prescribing specific types of AOMs, many more endorsed semaglutide and liraglutide than older medications such as bupropion/naltrexone and phentermine/topiramate.
Asked about factors that influence their comfort with prescribing AOMs, the top five factors selected, in order, were side-effect knowledge, insurance coverage, safety issues, and dosing knowledge. Fewer respondents endorsed “patient’s ideas, concerns, and expectations,” cost, or efficacy.
Referrals to nutrition services were endorsed more often than to obesity medicine specialists or bariatric surgery.
Asked about barriers to obesity treatment in their practices, “time constraints” was the most frequently endorsed, followed by “lack of training or knowledge,” “patient adherence and motivation,” and “limited resources.”
“What are the future directives? We feel we have the need to provide ongoing obesity management, education and assistance to primary care providers, including support for securing coverage for treatments,” Campos said.
He added that Boston Medical Center is now developing and implementing an embedded weight management program within primary care “to assist the front line of obesity care.”
Asked by this news organization whether he believes the rise of GLP-1 drugs will make a difference, Campos said “Definitely, I think with that momentum obesity medicine as a whole will gain more attention and hopefully more implementation in the curricula for medical and nursing schools, because in the end it requires a multidisciplinary approach.”
Campos and Clark had no disclosures.
A version of this article first appeared on Medscape.com.
SAN ANTONIO — Despite the prevalence of obesity in primary care, there appear to be major knowledge gaps among providers regarding obesity management, new research suggests.
Anonymous surveys of 96 primary care providers at a Boston, Massachusetts, safety-net hospital revealed that participants had limited understanding of criteria for prescribing antiobesity medications (AOM), and expressed discomfort in prescribing AOMs because of knowledge concerns, especially for non–glucagon-like peptide 1 (GLP-1) receptor agonists. One third reported that they didn’t prescribe AOMs, and rates of referral for bariatric surgery were also low.
The findings were presented at the Obesity Society’s annual Obesity Week meeting by Alejandro Campos, MD, a third-year resident in the section of internal medicine, Boston Medical Center, and the Department of Medicine, Boston University.
“I think it comes down to education. ... Not only training primary care physicians or residents about criteria and pathophysiology, but also stigma. Perceptions need to be addressed from the start of training in the healthcare field,” Campos told this news organization in an interview.
During his presentation, Campos noted this is the first such study in the setting of a safety-net hospital, which cares for lower-income people who experience disproportionate rates of obesity. But, “these findings are similar to ones observed from non–safety-net settings, which can indicate some potential transferability.”
Asked to comment, session moderator John D. Clark, MD, PhD, Chief Population Health Officer at Sharp Rees-Stealy Medical Group, San Diego, California, told this news organization that the findings didn’t surprise him. “I’d say that medical education around obesity has definitely improved, and training is improving but it’s not uniform. The treatment of obesity as a disease, especially with some of the newer medical treatments, is not standard of care and practiced widely.”
The study involved a standard-model Knowledge, Attitudes, and Practices questionnaire, distributed electronically for anonymous responses among both trained and in-training primary care providers. It contained a total of 43 items, 7 of them demographic, 11 on knowledge, 9 regarding attitudes, and 16 asking about practices.
The hospital is the largest safety-net hospital in New England, with a patient population that includes 58% enrolled in Medicaid, 32% Black/African American individuals, 24% identifying as Hispanic/Latino individuals, and 37% living below the poverty line.
The 96 responding providers (from a total 350 invited) all worked in either family medicine or internal medicine. The trained providers included both attending MDs and nurse practitioners, while those in-training were residents in one of those two specialties. Two thirds were women. The majority were aged 20-30 years (49.45%) or 31-40 years (27.47%).
Overall, 73.63% reported having received some type of obesity training. Just over half (52.08%) reported receiving that training during medical or nursing school, while 43.75% reported receiving it during residency.
When asked to choose from a list of conditions to pick which are considered weight-related comorbidities, between 80% and 90% choose type 2 diabetes, obstructive sleep apnea (OSA), hypertension, hyperlipidemia, nonalcoholic fatty liver disease, and coronary artery disease. Fewer, but still a majority, also listed osteoarthritis and gastroesophageal reflux disease. However, respondents were less likely to cite cancer, mood disorders, or chronic kidney disease as being related to obesity.
Asked to list benefits of a 10% body weight loss, most recognized reductions in OSA, glycemia, cardiovascular disease risk, osteoarthritis, and hepatic steatosis. But, only about half knew weight loss could also improve urinary incontinence.
Only 25% could correctly name both indications for AOMs. Just 27.1% knew that one was a body mass index (BMI) ≥ 27 with comorbidities, while 46.9% knew BMI ≥ 30 without comorbidities was an AOM indication. Only 9.4% were correct on both of those indications for bariatric surgery.
“Reassuringly,” Campos said, the majority either “disagreed” or “strongly disagreed” that “lack of will power” contributes to obesity. However, more than 20% agreed that “lack of exercise or physical activity” contributed.
Overall, 73% of the trained providers and 59% of those in training reported that they prescribe AOMs. Asked about their comfort level in prescribing specific types of AOMs, many more endorsed semaglutide and liraglutide than older medications such as bupropion/naltrexone and phentermine/topiramate.
Asked about factors that influence their comfort with prescribing AOMs, the top five factors selected, in order, were side-effect knowledge, insurance coverage, safety issues, and dosing knowledge. Fewer respondents endorsed “patient’s ideas, concerns, and expectations,” cost, or efficacy.
Referrals to nutrition services were endorsed more often than to obesity medicine specialists or bariatric surgery.
Asked about barriers to obesity treatment in their practices, “time constraints” was the most frequently endorsed, followed by “lack of training or knowledge,” “patient adherence and motivation,” and “limited resources.”
“What are the future directives? We feel we have the need to provide ongoing obesity management, education and assistance to primary care providers, including support for securing coverage for treatments,” Campos said.
He added that Boston Medical Center is now developing and implementing an embedded weight management program within primary care “to assist the front line of obesity care.”
Asked by this news organization whether he believes the rise of GLP-1 drugs will make a difference, Campos said “Definitely, I think with that momentum obesity medicine as a whole will gain more attention and hopefully more implementation in the curricula for medical and nursing schools, because in the end it requires a multidisciplinary approach.”
Campos and Clark had no disclosures.
A version of this article first appeared on Medscape.com.
SAN ANTONIO — Despite the prevalence of obesity in primary care, there appear to be major knowledge gaps among providers regarding obesity management, new research suggests.
Anonymous surveys of 96 primary care providers at a Boston, Massachusetts, safety-net hospital revealed that participants had limited understanding of criteria for prescribing antiobesity medications (AOM), and expressed discomfort in prescribing AOMs because of knowledge concerns, especially for non–glucagon-like peptide 1 (GLP-1) receptor agonists. One third reported that they didn’t prescribe AOMs, and rates of referral for bariatric surgery were also low.
The findings were presented at the Obesity Society’s annual Obesity Week meeting by Alejandro Campos, MD, a third-year resident in the section of internal medicine, Boston Medical Center, and the Department of Medicine, Boston University.
“I think it comes down to education. ... Not only training primary care physicians or residents about criteria and pathophysiology, but also stigma. Perceptions need to be addressed from the start of training in the healthcare field,” Campos told this news organization in an interview.
During his presentation, Campos noted this is the first such study in the setting of a safety-net hospital, which cares for lower-income people who experience disproportionate rates of obesity. But, “these findings are similar to ones observed from non–safety-net settings, which can indicate some potential transferability.”
Asked to comment, session moderator John D. Clark, MD, PhD, Chief Population Health Officer at Sharp Rees-Stealy Medical Group, San Diego, California, told this news organization that the findings didn’t surprise him. “I’d say that medical education around obesity has definitely improved, and training is improving but it’s not uniform. The treatment of obesity as a disease, especially with some of the newer medical treatments, is not standard of care and practiced widely.”
The study involved a standard-model Knowledge, Attitudes, and Practices questionnaire, distributed electronically for anonymous responses among both trained and in-training primary care providers. It contained a total of 43 items, 7 of them demographic, 11 on knowledge, 9 regarding attitudes, and 16 asking about practices.
The hospital is the largest safety-net hospital in New England, with a patient population that includes 58% enrolled in Medicaid, 32% Black/African American individuals, 24% identifying as Hispanic/Latino individuals, and 37% living below the poverty line.
The 96 responding providers (from a total 350 invited) all worked in either family medicine or internal medicine. The trained providers included both attending MDs and nurse practitioners, while those in-training were residents in one of those two specialties. Two thirds were women. The majority were aged 20-30 years (49.45%) or 31-40 years (27.47%).
Overall, 73.63% reported having received some type of obesity training. Just over half (52.08%) reported receiving that training during medical or nursing school, while 43.75% reported receiving it during residency.
When asked to choose from a list of conditions to pick which are considered weight-related comorbidities, between 80% and 90% choose type 2 diabetes, obstructive sleep apnea (OSA), hypertension, hyperlipidemia, nonalcoholic fatty liver disease, and coronary artery disease. Fewer, but still a majority, also listed osteoarthritis and gastroesophageal reflux disease. However, respondents were less likely to cite cancer, mood disorders, or chronic kidney disease as being related to obesity.
Asked to list benefits of a 10% body weight loss, most recognized reductions in OSA, glycemia, cardiovascular disease risk, osteoarthritis, and hepatic steatosis. But, only about half knew weight loss could also improve urinary incontinence.
Only 25% could correctly name both indications for AOMs. Just 27.1% knew that one was a body mass index (BMI) ≥ 27 with comorbidities, while 46.9% knew BMI ≥ 30 without comorbidities was an AOM indication. Only 9.4% were correct on both of those indications for bariatric surgery.
“Reassuringly,” Campos said, the majority either “disagreed” or “strongly disagreed” that “lack of will power” contributes to obesity. However, more than 20% agreed that “lack of exercise or physical activity” contributed.
Overall, 73% of the trained providers and 59% of those in training reported that they prescribe AOMs. Asked about their comfort level in prescribing specific types of AOMs, many more endorsed semaglutide and liraglutide than older medications such as bupropion/naltrexone and phentermine/topiramate.
Asked about factors that influence their comfort with prescribing AOMs, the top five factors selected, in order, were side-effect knowledge, insurance coverage, safety issues, and dosing knowledge. Fewer respondents endorsed “patient’s ideas, concerns, and expectations,” cost, or efficacy.
Referrals to nutrition services were endorsed more often than to obesity medicine specialists or bariatric surgery.
Asked about barriers to obesity treatment in their practices, “time constraints” was the most frequently endorsed, followed by “lack of training or knowledge,” “patient adherence and motivation,” and “limited resources.”
“What are the future directives? We feel we have the need to provide ongoing obesity management, education and assistance to primary care providers, including support for securing coverage for treatments,” Campos said.
He added that Boston Medical Center is now developing and implementing an embedded weight management program within primary care “to assist the front line of obesity care.”
Asked by this news organization whether he believes the rise of GLP-1 drugs will make a difference, Campos said “Definitely, I think with that momentum obesity medicine as a whole will gain more attention and hopefully more implementation in the curricula for medical and nursing schools, because in the end it requires a multidisciplinary approach.”
Campos and Clark had no disclosures.
A version of this article first appeared on Medscape.com.
FROM OBESITY WEEK 2024
AI-Assisted Colonoscopy Linked to Higher Rate of Benign Lesion Removal
PHILADELPHIA — according to a study presented at the annual meeting of the American College of Gastroenterology (ACG).
In particular, AIAC led to a statistically and clinically significant increase in the proportion of exams that detected lesions that after resection were all found to be benign, compared with unassisted colonoscopy.
“The potential implications include increased procedural risks, as well as costs, such as pathology costs and other healthcare expenditures, without any additional colorectal cancer prevention benefit,” said lead author Tessa Herman, MD, chief resident of internal medicine at the University of Minnesota, Minneapolis, and Minneapolis Veterans Affairs Health Care System.
In a previous implementation trial at the Minneapolis VA Medical Center, Herman and colleagues compared ADR between a group of patients undergoing AIAC and a historical cohort of patients who had non–AI-assisted colonoscopy.
In this subsequent study, the research team conducted an ad hoc analysis of data from the previous trial to determine the proportion of colonoscopies for screening, surveillance, and positive fecal immunochemical tests which detect lesions that after resection are all found to be benign. They excluded colonoscopies conducted for diagnostic indications or inflammatory bowel disease, as well as incomplete colonoscopies, and for those with inadequate bowel preparation.
Overall, they studied 441 non-AIAC colonoscopies (between November 2022 and April 2023) and 599 AIAC colonoscopies (between May 2023 and October 2023). The groups were balanced, and there were no significant differences in patient demographics, endoscopists, AI technology, procedure time, or average number of polyps detected.
In the non-AIAC cohort, 37 cases (8.4%) had polypectomies that revealed only benign lesions, as compared with 74 cases (12.4%) in the AIAC cohort. The most common resected lesions were benign colonic mucosa, lymphoid aggregates, and hyperplastic polyps.
Applied to the 15 million colonoscopies conducted in the United States per year, the findings indicate that full adoption of AIAC could result in about 600,000 more colonoscopies in which only benign, nonadenomatous lesions are removed, compared with traditional colonoscopy, Herman said.
More study of AIAC is needed, said Daniel Pambianco, MD, managing partner of GastroHealth-Charlottesville in Virginia and the 2023 ACG president. “This technology is in a fledging stage, and the more data we have, the more helpful it’ll be to know if we’re removing the right lesions at a better rate.”
“There’s a hope that assistance will improve detection, removal of polyps, and ultimately, colon cancer,” added Pambianco, who comoderated the session on colorectal cancer prevention.
Future longitudinal studies should monitor both ADR and benign lesion resection rates with AIAC, and modeling studies could determine the benefits and costs of the technology, Herman said. In addition, development of hybrid CADe and computer-aided diagnosis systems could mitigate concerns about excessive benign lesion resection with AI tools.
Clinicians already are able to find colon mucosa that are polypoid or lymphoid aggregates during colonoscopy without AI assistance, said the session’s comoderator, Sita Chokhavatia, MD, AGAF, a gastroenterologist with Valley Medical Group in Ridgewood, New Jersey.
“Instead, we need a tool that can help us to not remove these polyps that are not neoplastic,” she said. “With future developments, we may be able to take it to the next step where the algorithm tells us that it’s benign and not to touch it.”
The study was named an ACG Newsworthy Abstract. Herman, Pambianco, and Chokhavatia reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
PHILADELPHIA — according to a study presented at the annual meeting of the American College of Gastroenterology (ACG).
In particular, AIAC led to a statistically and clinically significant increase in the proportion of exams that detected lesions that after resection were all found to be benign, compared with unassisted colonoscopy.
“The potential implications include increased procedural risks, as well as costs, such as pathology costs and other healthcare expenditures, without any additional colorectal cancer prevention benefit,” said lead author Tessa Herman, MD, chief resident of internal medicine at the University of Minnesota, Minneapolis, and Minneapolis Veterans Affairs Health Care System.
In a previous implementation trial at the Minneapolis VA Medical Center, Herman and colleagues compared ADR between a group of patients undergoing AIAC and a historical cohort of patients who had non–AI-assisted colonoscopy.
In this subsequent study, the research team conducted an ad hoc analysis of data from the previous trial to determine the proportion of colonoscopies for screening, surveillance, and positive fecal immunochemical tests which detect lesions that after resection are all found to be benign. They excluded colonoscopies conducted for diagnostic indications or inflammatory bowel disease, as well as incomplete colonoscopies, and for those with inadequate bowel preparation.
Overall, they studied 441 non-AIAC colonoscopies (between November 2022 and April 2023) and 599 AIAC colonoscopies (between May 2023 and October 2023). The groups were balanced, and there were no significant differences in patient demographics, endoscopists, AI technology, procedure time, or average number of polyps detected.
In the non-AIAC cohort, 37 cases (8.4%) had polypectomies that revealed only benign lesions, as compared with 74 cases (12.4%) in the AIAC cohort. The most common resected lesions were benign colonic mucosa, lymphoid aggregates, and hyperplastic polyps.
Applied to the 15 million colonoscopies conducted in the United States per year, the findings indicate that full adoption of AIAC could result in about 600,000 more colonoscopies in which only benign, nonadenomatous lesions are removed, compared with traditional colonoscopy, Herman said.
More study of AIAC is needed, said Daniel Pambianco, MD, managing partner of GastroHealth-Charlottesville in Virginia and the 2023 ACG president. “This technology is in a fledging stage, and the more data we have, the more helpful it’ll be to know if we’re removing the right lesions at a better rate.”
“There’s a hope that assistance will improve detection, removal of polyps, and ultimately, colon cancer,” added Pambianco, who comoderated the session on colorectal cancer prevention.
Future longitudinal studies should monitor both ADR and benign lesion resection rates with AIAC, and modeling studies could determine the benefits and costs of the technology, Herman said. In addition, development of hybrid CADe and computer-aided diagnosis systems could mitigate concerns about excessive benign lesion resection with AI tools.
Clinicians already are able to find colon mucosa that are polypoid or lymphoid aggregates during colonoscopy without AI assistance, said the session’s comoderator, Sita Chokhavatia, MD, AGAF, a gastroenterologist with Valley Medical Group in Ridgewood, New Jersey.
“Instead, we need a tool that can help us to not remove these polyps that are not neoplastic,” she said. “With future developments, we may be able to take it to the next step where the algorithm tells us that it’s benign and not to touch it.”
The study was named an ACG Newsworthy Abstract. Herman, Pambianco, and Chokhavatia reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
PHILADELPHIA — according to a study presented at the annual meeting of the American College of Gastroenterology (ACG).
In particular, AIAC led to a statistically and clinically significant increase in the proportion of exams that detected lesions that after resection were all found to be benign, compared with unassisted colonoscopy.
“The potential implications include increased procedural risks, as well as costs, such as pathology costs and other healthcare expenditures, without any additional colorectal cancer prevention benefit,” said lead author Tessa Herman, MD, chief resident of internal medicine at the University of Minnesota, Minneapolis, and Minneapolis Veterans Affairs Health Care System.
In a previous implementation trial at the Minneapolis VA Medical Center, Herman and colleagues compared ADR between a group of patients undergoing AIAC and a historical cohort of patients who had non–AI-assisted colonoscopy.
In this subsequent study, the research team conducted an ad hoc analysis of data from the previous trial to determine the proportion of colonoscopies for screening, surveillance, and positive fecal immunochemical tests which detect lesions that after resection are all found to be benign. They excluded colonoscopies conducted for diagnostic indications or inflammatory bowel disease, as well as incomplete colonoscopies, and for those with inadequate bowel preparation.
Overall, they studied 441 non-AIAC colonoscopies (between November 2022 and April 2023) and 599 AIAC colonoscopies (between May 2023 and October 2023). The groups were balanced, and there were no significant differences in patient demographics, endoscopists, AI technology, procedure time, or average number of polyps detected.
In the non-AIAC cohort, 37 cases (8.4%) had polypectomies that revealed only benign lesions, as compared with 74 cases (12.4%) in the AIAC cohort. The most common resected lesions were benign colonic mucosa, lymphoid aggregates, and hyperplastic polyps.
Applied to the 15 million colonoscopies conducted in the United States per year, the findings indicate that full adoption of AIAC could result in about 600,000 more colonoscopies in which only benign, nonadenomatous lesions are removed, compared with traditional colonoscopy, Herman said.
More study of AIAC is needed, said Daniel Pambianco, MD, managing partner of GastroHealth-Charlottesville in Virginia and the 2023 ACG president. “This technology is in a fledging stage, and the more data we have, the more helpful it’ll be to know if we’re removing the right lesions at a better rate.”
“There’s a hope that assistance will improve detection, removal of polyps, and ultimately, colon cancer,” added Pambianco, who comoderated the session on colorectal cancer prevention.
Future longitudinal studies should monitor both ADR and benign lesion resection rates with AIAC, and modeling studies could determine the benefits and costs of the technology, Herman said. In addition, development of hybrid CADe and computer-aided diagnosis systems could mitigate concerns about excessive benign lesion resection with AI tools.
Clinicians already are able to find colon mucosa that are polypoid or lymphoid aggregates during colonoscopy without AI assistance, said the session’s comoderator, Sita Chokhavatia, MD, AGAF, a gastroenterologist with Valley Medical Group in Ridgewood, New Jersey.
“Instead, we need a tool that can help us to not remove these polyps that are not neoplastic,” she said. “With future developments, we may be able to take it to the next step where the algorithm tells us that it’s benign and not to touch it.”
The study was named an ACG Newsworthy Abstract. Herman, Pambianco, and Chokhavatia reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM ACG 2024