User login
NHLBI commits to a sickle cell cure
“We have new exigency and intensity of effort to enable curative strategies for sickle cell disease to move forward,” said W. Keith Hoots, MD, the director of the division of blood diseases at NHLBI.
The key word in the cure effort is partnership – whether it’s among federal agencies, with public and private organizations, or with patients and families.
“Developmental strategies are built on partnerships to enhance care and accelerate cure for sickle cell disease in the U.S. and worldwide,” Dr. Hoots said at the 12th annual symposium of the Foundation for Sickle Cell Disease Research in Washington.
The reach also extends internationally. Supporting research in sub-Saharan Africa has promised to accelerate the clinical trial process by bringing advanced research capabilities to a region with a very high per capita rate of SCD. While in the United States, infrastructure is being built for a future research network, with the goal of developing a secure database of shared elements that harmonize and unite existing data.
Future cohort studies, enhanced newborn screening, and higher uptake of hydroxyurea will all be supported as part of this effort, Dr. Hoots said.
In the United States, patients can participate in a meaningful way as citizen-scientists, as new technology makes it possible to crowdsource high-quality data collection securely.
And including both community organizations and primary care providers in the “circle of partners” means not only that advances are brought out to patients expeditiously but also that the voices of patients and families have a clear channel back to researchers and policy makers through formal patient engagement and lay participation at all levels, Dr. Hoots said.
“The number of presently interested partners may surprise you,” Dr. Hoots said.
This multifaceted approach allows for “multiple shots on goal, with the acceptance that there could potentially be some failures,” Dr. Hoots said. Keeping all players better connected, though, should allow efforts to be redirected when needed, with a particular focus on accelerating work toward genetic therapies for SCD.
Perhaps the flagship effort is the Cure Sickle Cell Disease Initiative, a new partnership focused on accelerating cure-focused SCD research by filling in gaps left in the network of other funding strategies.
NHLBI named Edward J. Benz Jr., MD, the president and CEO emeritus of Boston’s Dana-Farber Cancer Institute, as the executive director and the Emmes Corporation, a contract research organization with expertise in clinical trials, as the coordinating center.
Traveling the last mile
New strategies also need to focus on how to boost uptake of such currently available best practices in SCD treatment as hydroxyurea use. To that end, Dr. Hoots said, NHLBI is drawing on implementation science, a discipline that, in a medical setting, can help solve such “last-mile” problems as bringing best practices in SCD treatment to patients.
In clinical practice, this might look like solving transportation issues for family members so that appointments aren’t missed and hydroxyurea prescriptions are filled. For researchers, implementation science can help with thorny details of participant recruitment and retention.
Established in 2016, the Sickle Cell Disease Implementation Consortium comprises nine U.S. research centers and NHLBI, which are each seeking to recruit at least 300 participants with SCD, aged 15-45 years, to study effective identification of barriers to care, and the best means to overcome them.
However, Dr. Hoots said, NHLBI also will continue funding SCD research through the traditional investigator-initiated application process, in conjunction with “a suite of specialized programs that can support translational and clinical research in SCD.”
Some of the features rolling out within the Cure SCD Initiative are included in direct response to stakeholder feedback about pressing needs and top priorities. For example, an economic case needed to be made in order for insurance companies, public and private alike, to reimburse for genetic SCD treatments. This requires an understanding of the lifetime cost burden of SCD, as well as determining what the long-term follow-up of costs of gene therapy will be.
Patients, family members and those providing primary care for SCD patients all agreed that clinical trials should have endpoints that reflect meaningful outcomes for patients and should be designed with the input of both patients and providers.
When queried, sickle cell disease researchers expressed a need to identify common data elements in SCD research, and wished for a secure yet accessible national data warehouse for data from gene and cell therapy trials.
At present, there are three clinical trials of curative stem cell approaches for SCD registered with the Blood and Marrow Transplant Clinical Trials Network and several more early phase clinical trials underway, Dr. Hoots said. A primary focus is the use of autologous cells for genomic editing, gene therapy, and erythroid-specific vectors.
Genetic research
As an example of the new collaboration, research centers and biotechnology companies sent their cell and genetic therapy experts to an NIH-sponsored gathering in March 2017. By pooling expertise in this way, the group was able to “identify some unprecedented opportunities, as well as some necessary barriers to overcome,” he said. These players continue to collaborate in the ongoing clinical trials of novel – and potentially curative – SCD therapies.
The TOPMed (Trans-Omics for Precision Medicine) program is a key mechanism to support SCD-related genetic research. For example, Dr. Hoots said, TOPMed is being used in support of whole-genome sequencing in a longitudinal cohort of patients with SCD who receive transfusion care at four large centers in Brazil.
These renewed efforts, set against the backdrop of paradigm-shifting genetic therapies, represent new promise for a generation of individuals with SCD, Dr. Hoots said. “It takes all of us to address the SCD challenge.”
ASH initiatives
NHLBI isn’t alone in making SCD a priority. The American Society of Hematology also is putting a spotlight on the condition.
The ASH multifaceted sickle cell disease (SCD) initiative addresses the disease burden both within the United States and globally, said LaTasha Lee, PhD, senior manager of sickle cell disease policy and programs for ASH.
Speaking at the 12th annual symposium of the Foundation for Sickle Cell Disease Research, Dr. Lee said that four prongs make up the initiative: disease research, attention to global issues, a renewed focus on access to care in the United States, and work to develop ASH’s new SCD guidelines.
New guidelines on the management of acute and chronic complications of SCD are in the works, with an anticipated 2019 date for publication of five separate guidelines. Topics covered in the guidelines will include pain, cerebrovascular disease, cardiopulmonary and kidney disease, transfusion support, and stem cell transplantation.
“We have new exigency and intensity of effort to enable curative strategies for sickle cell disease to move forward,” said W. Keith Hoots, MD, the director of the division of blood diseases at NHLBI.
The key word in the cure effort is partnership – whether it’s among federal agencies, with public and private organizations, or with patients and families.
“Developmental strategies are built on partnerships to enhance care and accelerate cure for sickle cell disease in the U.S. and worldwide,” Dr. Hoots said at the 12th annual symposium of the Foundation for Sickle Cell Disease Research in Washington.
The reach also extends internationally. Supporting research in sub-Saharan Africa has promised to accelerate the clinical trial process by bringing advanced research capabilities to a region with a very high per capita rate of SCD. While in the United States, infrastructure is being built for a future research network, with the goal of developing a secure database of shared elements that harmonize and unite existing data.
Future cohort studies, enhanced newborn screening, and higher uptake of hydroxyurea will all be supported as part of this effort, Dr. Hoots said.
In the United States, patients can participate in a meaningful way as citizen-scientists, as new technology makes it possible to crowdsource high-quality data collection securely.
And including both community organizations and primary care providers in the “circle of partners” means not only that advances are brought out to patients expeditiously but also that the voices of patients and families have a clear channel back to researchers and policy makers through formal patient engagement and lay participation at all levels, Dr. Hoots said.
“The number of presently interested partners may surprise you,” Dr. Hoots said.
This multifaceted approach allows for “multiple shots on goal, with the acceptance that there could potentially be some failures,” Dr. Hoots said. Keeping all players better connected, though, should allow efforts to be redirected when needed, with a particular focus on accelerating work toward genetic therapies for SCD.
Perhaps the flagship effort is the Cure Sickle Cell Disease Initiative, a new partnership focused on accelerating cure-focused SCD research by filling in gaps left in the network of other funding strategies.
NHLBI named Edward J. Benz Jr., MD, the president and CEO emeritus of Boston’s Dana-Farber Cancer Institute, as the executive director and the Emmes Corporation, a contract research organization with expertise in clinical trials, as the coordinating center.
Traveling the last mile
New strategies also need to focus on how to boost uptake of such currently available best practices in SCD treatment as hydroxyurea use. To that end, Dr. Hoots said, NHLBI is drawing on implementation science, a discipline that, in a medical setting, can help solve such “last-mile” problems as bringing best practices in SCD treatment to patients.
In clinical practice, this might look like solving transportation issues for family members so that appointments aren’t missed and hydroxyurea prescriptions are filled. For researchers, implementation science can help with thorny details of participant recruitment and retention.
Established in 2016, the Sickle Cell Disease Implementation Consortium comprises nine U.S. research centers and NHLBI, which are each seeking to recruit at least 300 participants with SCD, aged 15-45 years, to study effective identification of barriers to care, and the best means to overcome them.
However, Dr. Hoots said, NHLBI also will continue funding SCD research through the traditional investigator-initiated application process, in conjunction with “a suite of specialized programs that can support translational and clinical research in SCD.”
Some of the features rolling out within the Cure SCD Initiative are included in direct response to stakeholder feedback about pressing needs and top priorities. For example, an economic case needed to be made in order for insurance companies, public and private alike, to reimburse for genetic SCD treatments. This requires an understanding of the lifetime cost burden of SCD, as well as determining what the long-term follow-up of costs of gene therapy will be.
Patients, family members and those providing primary care for SCD patients all agreed that clinical trials should have endpoints that reflect meaningful outcomes for patients and should be designed with the input of both patients and providers.
When queried, sickle cell disease researchers expressed a need to identify common data elements in SCD research, and wished for a secure yet accessible national data warehouse for data from gene and cell therapy trials.
At present, there are three clinical trials of curative stem cell approaches for SCD registered with the Blood and Marrow Transplant Clinical Trials Network and several more early phase clinical trials underway, Dr. Hoots said. A primary focus is the use of autologous cells for genomic editing, gene therapy, and erythroid-specific vectors.
Genetic research
As an example of the new collaboration, research centers and biotechnology companies sent their cell and genetic therapy experts to an NIH-sponsored gathering in March 2017. By pooling expertise in this way, the group was able to “identify some unprecedented opportunities, as well as some necessary barriers to overcome,” he said. These players continue to collaborate in the ongoing clinical trials of novel – and potentially curative – SCD therapies.
The TOPMed (Trans-Omics for Precision Medicine) program is a key mechanism to support SCD-related genetic research. For example, Dr. Hoots said, TOPMed is being used in support of whole-genome sequencing in a longitudinal cohort of patients with SCD who receive transfusion care at four large centers in Brazil.
These renewed efforts, set against the backdrop of paradigm-shifting genetic therapies, represent new promise for a generation of individuals with SCD, Dr. Hoots said. “It takes all of us to address the SCD challenge.”
ASH initiatives
NHLBI isn’t alone in making SCD a priority. The American Society of Hematology also is putting a spotlight on the condition.
The ASH multifaceted sickle cell disease (SCD) initiative addresses the disease burden both within the United States and globally, said LaTasha Lee, PhD, senior manager of sickle cell disease policy and programs for ASH.
Speaking at the 12th annual symposium of the Foundation for Sickle Cell Disease Research, Dr. Lee said that four prongs make up the initiative: disease research, attention to global issues, a renewed focus on access to care in the United States, and work to develop ASH’s new SCD guidelines.
New guidelines on the management of acute and chronic complications of SCD are in the works, with an anticipated 2019 date for publication of five separate guidelines. Topics covered in the guidelines will include pain, cerebrovascular disease, cardiopulmonary and kidney disease, transfusion support, and stem cell transplantation.
“We have new exigency and intensity of effort to enable curative strategies for sickle cell disease to move forward,” said W. Keith Hoots, MD, the director of the division of blood diseases at NHLBI.
The key word in the cure effort is partnership – whether it’s among federal agencies, with public and private organizations, or with patients and families.
“Developmental strategies are built on partnerships to enhance care and accelerate cure for sickle cell disease in the U.S. and worldwide,” Dr. Hoots said at the 12th annual symposium of the Foundation for Sickle Cell Disease Research in Washington.
The reach also extends internationally. Supporting research in sub-Saharan Africa has promised to accelerate the clinical trial process by bringing advanced research capabilities to a region with a very high per capita rate of SCD. While in the United States, infrastructure is being built for a future research network, with the goal of developing a secure database of shared elements that harmonize and unite existing data.
Future cohort studies, enhanced newborn screening, and higher uptake of hydroxyurea will all be supported as part of this effort, Dr. Hoots said.
In the United States, patients can participate in a meaningful way as citizen-scientists, as new technology makes it possible to crowdsource high-quality data collection securely.
And including both community organizations and primary care providers in the “circle of partners” means not only that advances are brought out to patients expeditiously but also that the voices of patients and families have a clear channel back to researchers and policy makers through formal patient engagement and lay participation at all levels, Dr. Hoots said.
“The number of presently interested partners may surprise you,” Dr. Hoots said.
This multifaceted approach allows for “multiple shots on goal, with the acceptance that there could potentially be some failures,” Dr. Hoots said. Keeping all players better connected, though, should allow efforts to be redirected when needed, with a particular focus on accelerating work toward genetic therapies for SCD.
Perhaps the flagship effort is the Cure Sickle Cell Disease Initiative, a new partnership focused on accelerating cure-focused SCD research by filling in gaps left in the network of other funding strategies.
NHLBI named Edward J. Benz Jr., MD, the president and CEO emeritus of Boston’s Dana-Farber Cancer Institute, as the executive director and the Emmes Corporation, a contract research organization with expertise in clinical trials, as the coordinating center.
Traveling the last mile
New strategies also need to focus on how to boost uptake of such currently available best practices in SCD treatment as hydroxyurea use. To that end, Dr. Hoots said, NHLBI is drawing on implementation science, a discipline that, in a medical setting, can help solve such “last-mile” problems as bringing best practices in SCD treatment to patients.
In clinical practice, this might look like solving transportation issues for family members so that appointments aren’t missed and hydroxyurea prescriptions are filled. For researchers, implementation science can help with thorny details of participant recruitment and retention.
Established in 2016, the Sickle Cell Disease Implementation Consortium comprises nine U.S. research centers and NHLBI, which are each seeking to recruit at least 300 participants with SCD, aged 15-45 years, to study effective identification of barriers to care, and the best means to overcome them.
However, Dr. Hoots said, NHLBI also will continue funding SCD research through the traditional investigator-initiated application process, in conjunction with “a suite of specialized programs that can support translational and clinical research in SCD.”
Some of the features rolling out within the Cure SCD Initiative are included in direct response to stakeholder feedback about pressing needs and top priorities. For example, an economic case needed to be made in order for insurance companies, public and private alike, to reimburse for genetic SCD treatments. This requires an understanding of the lifetime cost burden of SCD, as well as determining what the long-term follow-up of costs of gene therapy will be.
Patients, family members and those providing primary care for SCD patients all agreed that clinical trials should have endpoints that reflect meaningful outcomes for patients and should be designed with the input of both patients and providers.
When queried, sickle cell disease researchers expressed a need to identify common data elements in SCD research, and wished for a secure yet accessible national data warehouse for data from gene and cell therapy trials.
At present, there are three clinical trials of curative stem cell approaches for SCD registered with the Blood and Marrow Transplant Clinical Trials Network and several more early phase clinical trials underway, Dr. Hoots said. A primary focus is the use of autologous cells for genomic editing, gene therapy, and erythroid-specific vectors.
Genetic research
As an example of the new collaboration, research centers and biotechnology companies sent their cell and genetic therapy experts to an NIH-sponsored gathering in March 2017. By pooling expertise in this way, the group was able to “identify some unprecedented opportunities, as well as some necessary barriers to overcome,” he said. These players continue to collaborate in the ongoing clinical trials of novel – and potentially curative – SCD therapies.
The TOPMed (Trans-Omics for Precision Medicine) program is a key mechanism to support SCD-related genetic research. For example, Dr. Hoots said, TOPMed is being used in support of whole-genome sequencing in a longitudinal cohort of patients with SCD who receive transfusion care at four large centers in Brazil.
These renewed efforts, set against the backdrop of paradigm-shifting genetic therapies, represent new promise for a generation of individuals with SCD, Dr. Hoots said. “It takes all of us to address the SCD challenge.”
ASH initiatives
NHLBI isn’t alone in making SCD a priority. The American Society of Hematology also is putting a spotlight on the condition.
The ASH multifaceted sickle cell disease (SCD) initiative addresses the disease burden both within the United States and globally, said LaTasha Lee, PhD, senior manager of sickle cell disease policy and programs for ASH.
Speaking at the 12th annual symposium of the Foundation for Sickle Cell Disease Research, Dr. Lee said that four prongs make up the initiative: disease research, attention to global issues, a renewed focus on access to care in the United States, and work to develop ASH’s new SCD guidelines.
New guidelines on the management of acute and chronic complications of SCD are in the works, with an anticipated 2019 date for publication of five separate guidelines. Topics covered in the guidelines will include pain, cerebrovascular disease, cardiopulmonary and kidney disease, transfusion support, and stem cell transplantation.
Taking a global perspective on infectious disease
The Infectious Diseases Society of America, which has its annual meeting as part of IDWeek, has a particular emphasis on global health. In fact, “IDSA prioritizes promoting sufficient U.S. investment in global infectious diseases responses and research, and in using the voices of physician scientists to inform multilateral and national global health policies. IDSA is a trusted leader in shaping and advancing international and national policies and investments in global HIV, TB, health security, and antimicrobial resistance,” according to the global health policy page of the organization’s website.
So it is no surprise that the global face of infectious disease is a prominent focus of IDWeek 2018 as exemplified by the Global ID track.
Although many of the overall sessions have a global ID component, with research from other countries prominent in IDWeek 2018 presentations across all topics, there are particular sessions that focus on more prominent global issues and diseases. For example, tuberculosis and HIV in a global context are strongly represented in many sessions.
But of unique interest also are emerging diseases and those that are, for now, primarily geographically localized. For example, the Global Health and Travel Medicine session on Thursday features presentations on a wide range of such diseases, including babseiosis, brucellosis, Burkholderia pseudomallei, Chagas disease, and chikungunya virus.
Similarly, the session titled Adventures with Globally Acquired Infections on Friday covers diseases that are comparatively rare in the United States, but endemic in many areas of the world, including Leishmania major infection, Lassa fever, and malaria.
Friday’s late-breaking oral abstracts feature Emerging Infections, including details on recent outbreaks of Andes virus, Enterovirus A71, and Shigella that have spread into the United States.
And uniquely, Global ID and Transplant ID merge in a special session titled When Transplant Tours the World featuring disease risk in transplantation from a global perspective, including the growing issue of “transplant tourism.”
The Infectious Diseases Society of America, which has its annual meeting as part of IDWeek, has a particular emphasis on global health. In fact, “IDSA prioritizes promoting sufficient U.S. investment in global infectious diseases responses and research, and in using the voices of physician scientists to inform multilateral and national global health policies. IDSA is a trusted leader in shaping and advancing international and national policies and investments in global HIV, TB, health security, and antimicrobial resistance,” according to the global health policy page of the organization’s website.
So it is no surprise that the global face of infectious disease is a prominent focus of IDWeek 2018 as exemplified by the Global ID track.
Although many of the overall sessions have a global ID component, with research from other countries prominent in IDWeek 2018 presentations across all topics, there are particular sessions that focus on more prominent global issues and diseases. For example, tuberculosis and HIV in a global context are strongly represented in many sessions.
But of unique interest also are emerging diseases and those that are, for now, primarily geographically localized. For example, the Global Health and Travel Medicine session on Thursday features presentations on a wide range of such diseases, including babseiosis, brucellosis, Burkholderia pseudomallei, Chagas disease, and chikungunya virus.
Similarly, the session titled Adventures with Globally Acquired Infections on Friday covers diseases that are comparatively rare in the United States, but endemic in many areas of the world, including Leishmania major infection, Lassa fever, and malaria.
Friday’s late-breaking oral abstracts feature Emerging Infections, including details on recent outbreaks of Andes virus, Enterovirus A71, and Shigella that have spread into the United States.
And uniquely, Global ID and Transplant ID merge in a special session titled When Transplant Tours the World featuring disease risk in transplantation from a global perspective, including the growing issue of “transplant tourism.”
The Infectious Diseases Society of America, which has its annual meeting as part of IDWeek, has a particular emphasis on global health. In fact, “IDSA prioritizes promoting sufficient U.S. investment in global infectious diseases responses and research, and in using the voices of physician scientists to inform multilateral and national global health policies. IDSA is a trusted leader in shaping and advancing international and national policies and investments in global HIV, TB, health security, and antimicrobial resistance,” according to the global health policy page of the organization’s website.
So it is no surprise that the global face of infectious disease is a prominent focus of IDWeek 2018 as exemplified by the Global ID track.
Although many of the overall sessions have a global ID component, with research from other countries prominent in IDWeek 2018 presentations across all topics, there are particular sessions that focus on more prominent global issues and diseases. For example, tuberculosis and HIV in a global context are strongly represented in many sessions.
But of unique interest also are emerging diseases and those that are, for now, primarily geographically localized. For example, the Global Health and Travel Medicine session on Thursday features presentations on a wide range of such diseases, including babseiosis, brucellosis, Burkholderia pseudomallei, Chagas disease, and chikungunya virus.
Similarly, the session titled Adventures with Globally Acquired Infections on Friday covers diseases that are comparatively rare in the United States, but endemic in many areas of the world, including Leishmania major infection, Lassa fever, and malaria.
Friday’s late-breaking oral abstracts feature Emerging Infections, including details on recent outbreaks of Andes virus, Enterovirus A71, and Shigella that have spread into the United States.
And uniquely, Global ID and Transplant ID merge in a special session titled When Transplant Tours the World featuring disease risk in transplantation from a global perspective, including the growing issue of “transplant tourism.”
Getting the most out of IDWeek 2018
IDWeek, whose theme is Advancing Science, Improving Care, is the combined annual meeting of the Infectious Diseases Society of America (IDSA), the HIV Medicine Association (HIVMA), the Society for Healthcare Epidemiology of America (SHEA), and the Pediatric Infectious Diseases Society (PIDS). As always,
The organizers have provided an interactive planning tool for attendees to create their own schedule. Features of the personal planner include:
Browse/Search: Gives several options for searching or browsing the program, including searching to quickly find a specific author and/or topic of interest.
My Schedule: Displays the sessions or abstracts/posters that the user has chosen to attend concisely.
My Bookmarks: Allows the user to create a hyperlinked list of bookmarks for future reference, including sessions not attended.
Anyone who’s interested can see the entire program online here and can browse by date and by the program tracks that comprise the following: Adult ID, Epidemiology & Infection Control, Global ID, HIV-STD-TB, Investigative ID, Pediatric ID, Trainee, and Transplant ID.
According to the organizers, “with so many common issues and challenges cutting across our four disciplines, IDWeek provides an opportunity to learn from each other’s knowledge, experience and expertise, for the improvement of patient care and public health.”
IDWeek 2018 is being held at the Moscone Center in downtown San Francisco, with the official program beginning on Wed., Oct. 3, at 1:30 p.m., and ending on Sun., Oct. 7, at 10:45 a.m.
Valuable premeeting workshops will be offered on Tuesday, including sessions on antimicrobial stewardship and research training program and grant-writing strategies, and on Wednesday morning, when there will be workshops, including those on TB, hepatitis C, and how to use the Grading of Recommendation Assessment, Development and Evaluation framework to develop guidelines.
And while you are in San Francisco for the meeting, be sure to check out the various sights and entertainment opportunities available there. Many websites highlight the fun and interesting things to do while in the city and can help you plan your stay, including the comprehensive SanFranciscoTravel page.
IDWeek, whose theme is Advancing Science, Improving Care, is the combined annual meeting of the Infectious Diseases Society of America (IDSA), the HIV Medicine Association (HIVMA), the Society for Healthcare Epidemiology of America (SHEA), and the Pediatric Infectious Diseases Society (PIDS). As always,
The organizers have provided an interactive planning tool for attendees to create their own schedule. Features of the personal planner include:
Browse/Search: Gives several options for searching or browsing the program, including searching to quickly find a specific author and/or topic of interest.
My Schedule: Displays the sessions or abstracts/posters that the user has chosen to attend concisely.
My Bookmarks: Allows the user to create a hyperlinked list of bookmarks for future reference, including sessions not attended.
Anyone who’s interested can see the entire program online here and can browse by date and by the program tracks that comprise the following: Adult ID, Epidemiology & Infection Control, Global ID, HIV-STD-TB, Investigative ID, Pediatric ID, Trainee, and Transplant ID.
According to the organizers, “with so many common issues and challenges cutting across our four disciplines, IDWeek provides an opportunity to learn from each other’s knowledge, experience and expertise, for the improvement of patient care and public health.”
IDWeek 2018 is being held at the Moscone Center in downtown San Francisco, with the official program beginning on Wed., Oct. 3, at 1:30 p.m., and ending on Sun., Oct. 7, at 10:45 a.m.
Valuable premeeting workshops will be offered on Tuesday, including sessions on antimicrobial stewardship and research training program and grant-writing strategies, and on Wednesday morning, when there will be workshops, including those on TB, hepatitis C, and how to use the Grading of Recommendation Assessment, Development and Evaluation framework to develop guidelines.
And while you are in San Francisco for the meeting, be sure to check out the various sights and entertainment opportunities available there. Many websites highlight the fun and interesting things to do while in the city and can help you plan your stay, including the comprehensive SanFranciscoTravel page.
IDWeek, whose theme is Advancing Science, Improving Care, is the combined annual meeting of the Infectious Diseases Society of America (IDSA), the HIV Medicine Association (HIVMA), the Society for Healthcare Epidemiology of America (SHEA), and the Pediatric Infectious Diseases Society (PIDS). As always,
The organizers have provided an interactive planning tool for attendees to create their own schedule. Features of the personal planner include:
Browse/Search: Gives several options for searching or browsing the program, including searching to quickly find a specific author and/or topic of interest.
My Schedule: Displays the sessions or abstracts/posters that the user has chosen to attend concisely.
My Bookmarks: Allows the user to create a hyperlinked list of bookmarks for future reference, including sessions not attended.
Anyone who’s interested can see the entire program online here and can browse by date and by the program tracks that comprise the following: Adult ID, Epidemiology & Infection Control, Global ID, HIV-STD-TB, Investigative ID, Pediatric ID, Trainee, and Transplant ID.
According to the organizers, “with so many common issues and challenges cutting across our four disciplines, IDWeek provides an opportunity to learn from each other’s knowledge, experience and expertise, for the improvement of patient care and public health.”
IDWeek 2018 is being held at the Moscone Center in downtown San Francisco, with the official program beginning on Wed., Oct. 3, at 1:30 p.m., and ending on Sun., Oct. 7, at 10:45 a.m.
Valuable premeeting workshops will be offered on Tuesday, including sessions on antimicrobial stewardship and research training program and grant-writing strategies, and on Wednesday morning, when there will be workshops, including those on TB, hepatitis C, and how to use the Grading of Recommendation Assessment, Development and Evaluation framework to develop guidelines.
And while you are in San Francisco for the meeting, be sure to check out the various sights and entertainment opportunities available there. Many websites highlight the fun and interesting things to do while in the city and can help you plan your stay, including the comprehensive SanFranciscoTravel page.
FDA lifts partial hold on tazemetostat trials
The U.S. Food and Drug Administration (FDA) has lifted the partial clinical hold on trials of tazemetostat, an EZH2 inhibitor being developed to treat solid tumors and lymphomas.
The FDA had placed the hold in April, and this halted U.S.-based enrollment of new patients in tazemetostat clinical trials.
Now, Epizyme, Inc., the company developing tazemetostat, is in the process of reopening enrollment in all company-sponsored trials in the U.S.
The FDA had placed the partial hold on tazemetostat trials after an adverse event was observed in a pediatric patient on a phase 1 study.
The patient, who had advanced poorly differentiated chordoma, developed secondary T-cell lymphoblastic lymphoma (T-LBL) while taking tazemetostat. The patient has since discontinued tazemetostat and responded to treatment for T-LBL.
“This remains the only case of T-LBL we’ve seen in more than 750 patients treated with tazemetostat,” said Robert Bazemore, president and chief executive officer of Epizyme.
Due to this adverse event and the partial clinical hold, Epizyme began to assess the risk of T-LBL and other secondary malignancies potentially associated with tazemetostat.
The company also assessed the overall risks and benefits of tazemetostat treatment, conducting a review of the published literature and an examination of efficacy and safety data across all of its tazemetostat trials.
Epizyme concluded that the benefits of tazemetostat outweigh the risks, and the risk of T-LBL appears confined to pediatric patients who received higher doses of the drug. The phase 1 pediatric study in which the patient developed T-LBL included higher doses of tazemetostat than those used in the phase 2 adult studies.
Epizyme convened a panel of external scientific and medical experts who reviewed and validated the company’s findings.
“The team at Epizyme has worked diligently in collaboration with external experts and FDA over the past several months, culminating in decisions . . . to lift the partial clinical hold and allow re-opening of enrollment in our clinical trials,” Bazemore said.
He noted that the company is not making any substantial changes to trial designs or the patient populations involved in tazemetostat trials.
However, Epizyme is modifying dosing in the pediatric studies, improving patient monitoring, and making changes to exclusion criteria to reduce the potential risk of T-LBL and other secondary malignancies.
Bazemore said the lifting of the partial clinical hold allows Epizyme to turn its full attention to key priorities, including plans to submit a new drug application for tazemetostat in epithelioid sarcoma.
The company also plans to begin preparing for a potential new drug application for tazemetostat in follicular lymphoma.
Tazemetostat is currently under investigation as monotherapy in phase 2 trials of follicular lymphoma and solid tumor malignancies. The drug is also being studied as part of combination therapy for non-small cell lung cancer and diffuse large B-cell lymphoma (DLBCL).
In August, Epizyme announced its decision to stop developing tazemetostat for use as monotherapy or in combination with prednisolone for patients with DLBCL. However, tazemetostat is still under investigation as a potential treatment for DLBCL as part of other combination regimens.
Now that Epizyme has resolved the U.S. hold on tazemetostat trials, the company is working to resolve partial clinical holds placed in France and Germany to resume trial enrollment in those countries.
The U.S. Food and Drug Administration (FDA) has lifted the partial clinical hold on trials of tazemetostat, an EZH2 inhibitor being developed to treat solid tumors and lymphomas.
The FDA had placed the hold in April, and this halted U.S.-based enrollment of new patients in tazemetostat clinical trials.
Now, Epizyme, Inc., the company developing tazemetostat, is in the process of reopening enrollment in all company-sponsored trials in the U.S.
The FDA had placed the partial hold on tazemetostat trials after an adverse event was observed in a pediatric patient on a phase 1 study.
The patient, who had advanced poorly differentiated chordoma, developed secondary T-cell lymphoblastic lymphoma (T-LBL) while taking tazemetostat. The patient has since discontinued tazemetostat and responded to treatment for T-LBL.
“This remains the only case of T-LBL we’ve seen in more than 750 patients treated with tazemetostat,” said Robert Bazemore, president and chief executive officer of Epizyme.
Due to this adverse event and the partial clinical hold, Epizyme began to assess the risk of T-LBL and other secondary malignancies potentially associated with tazemetostat.
The company also assessed the overall risks and benefits of tazemetostat treatment, conducting a review of the published literature and an examination of efficacy and safety data across all of its tazemetostat trials.
Epizyme concluded that the benefits of tazemetostat outweigh the risks, and the risk of T-LBL appears confined to pediatric patients who received higher doses of the drug. The phase 1 pediatric study in which the patient developed T-LBL included higher doses of tazemetostat than those used in the phase 2 adult studies.
Epizyme convened a panel of external scientific and medical experts who reviewed and validated the company’s findings.
“The team at Epizyme has worked diligently in collaboration with external experts and FDA over the past several months, culminating in decisions . . . to lift the partial clinical hold and allow re-opening of enrollment in our clinical trials,” Bazemore said.
He noted that the company is not making any substantial changes to trial designs or the patient populations involved in tazemetostat trials.
However, Epizyme is modifying dosing in the pediatric studies, improving patient monitoring, and making changes to exclusion criteria to reduce the potential risk of T-LBL and other secondary malignancies.
Bazemore said the lifting of the partial clinical hold allows Epizyme to turn its full attention to key priorities, including plans to submit a new drug application for tazemetostat in epithelioid sarcoma.
The company also plans to begin preparing for a potential new drug application for tazemetostat in follicular lymphoma.
Tazemetostat is currently under investigation as monotherapy in phase 2 trials of follicular lymphoma and solid tumor malignancies. The drug is also being studied as part of combination therapy for non-small cell lung cancer and diffuse large B-cell lymphoma (DLBCL).
In August, Epizyme announced its decision to stop developing tazemetostat for use as monotherapy or in combination with prednisolone for patients with DLBCL. However, tazemetostat is still under investigation as a potential treatment for DLBCL as part of other combination regimens.
Now that Epizyme has resolved the U.S. hold on tazemetostat trials, the company is working to resolve partial clinical holds placed in France and Germany to resume trial enrollment in those countries.
The U.S. Food and Drug Administration (FDA) has lifted the partial clinical hold on trials of tazemetostat, an EZH2 inhibitor being developed to treat solid tumors and lymphomas.
The FDA had placed the hold in April, and this halted U.S.-based enrollment of new patients in tazemetostat clinical trials.
Now, Epizyme, Inc., the company developing tazemetostat, is in the process of reopening enrollment in all company-sponsored trials in the U.S.
The FDA had placed the partial hold on tazemetostat trials after an adverse event was observed in a pediatric patient on a phase 1 study.
The patient, who had advanced poorly differentiated chordoma, developed secondary T-cell lymphoblastic lymphoma (T-LBL) while taking tazemetostat. The patient has since discontinued tazemetostat and responded to treatment for T-LBL.
“This remains the only case of T-LBL we’ve seen in more than 750 patients treated with tazemetostat,” said Robert Bazemore, president and chief executive officer of Epizyme.
Due to this adverse event and the partial clinical hold, Epizyme began to assess the risk of T-LBL and other secondary malignancies potentially associated with tazemetostat.
The company also assessed the overall risks and benefits of tazemetostat treatment, conducting a review of the published literature and an examination of efficacy and safety data across all of its tazemetostat trials.
Epizyme concluded that the benefits of tazemetostat outweigh the risks, and the risk of T-LBL appears confined to pediatric patients who received higher doses of the drug. The phase 1 pediatric study in which the patient developed T-LBL included higher doses of tazemetostat than those used in the phase 2 adult studies.
Epizyme convened a panel of external scientific and medical experts who reviewed and validated the company’s findings.
“The team at Epizyme has worked diligently in collaboration with external experts and FDA over the past several months, culminating in decisions . . . to lift the partial clinical hold and allow re-opening of enrollment in our clinical trials,” Bazemore said.
He noted that the company is not making any substantial changes to trial designs or the patient populations involved in tazemetostat trials.
However, Epizyme is modifying dosing in the pediatric studies, improving patient monitoring, and making changes to exclusion criteria to reduce the potential risk of T-LBL and other secondary malignancies.
Bazemore said the lifting of the partial clinical hold allows Epizyme to turn its full attention to key priorities, including plans to submit a new drug application for tazemetostat in epithelioid sarcoma.
The company also plans to begin preparing for a potential new drug application for tazemetostat in follicular lymphoma.
Tazemetostat is currently under investigation as monotherapy in phase 2 trials of follicular lymphoma and solid tumor malignancies. The drug is also being studied as part of combination therapy for non-small cell lung cancer and diffuse large B-cell lymphoma (DLBCL).
In August, Epizyme announced its decision to stop developing tazemetostat for use as monotherapy or in combination with prednisolone for patients with DLBCL. However, tazemetostat is still under investigation as a potential treatment for DLBCL as part of other combination regimens.
Now that Epizyme has resolved the U.S. hold on tazemetostat trials, the company is working to resolve partial clinical holds placed in France and Germany to resume trial enrollment in those countries.
Blinatumomab approved to treat ALL in Japan
The Japanese Ministry of Health, Labour and Welfare has approved blinatumomab (Blincyto®) for the treatment of relapsed or refractory B-cell acute lymphoblastic leukemia (B-ALL).
Blinatumomab is the first and only bispecific T-cell engager immunotherapy construct approved globally.
The drug’s approval in Japan is based on data from the phase 3 TOWER study and the phase 1b/2 Horai study.
The TOWER trial (NCT02013167) enrolled 405 patients with Ph-negative, relapsed/refractory B-ALL, 376 of whom ultimately received treatment.
The patients received blinatumomab (n=267) or investigator’s choice of four protocol-defined standard of care (SOC) chemotherapy regimens (n=109):
- FLAG (fludarabine, high-dose cytarabine arabinoside, and granulocyte-colony stimulating factor), with or without an anthracycline (n=49, 45%)
- A high-dose cytarabine arabinoside-based regimen (n=19, 17%)
- A high-dose methotrexate-based regimen (n=22, 20%)
- A clofarabine-based regimen (n=19, 17%).
Blinatumomab demonstrated an improvement in median overall survival over SOC. The median overall survival was 7.7 months with blinatumomab and 4.0 months with SOC (hazard ratio for death=0.71; P=0.012).
Grade 3 or higher adverse events (AEs) of interest, according to the researchers, were:
- Infection (34% with blinatumomab and 52% with chemotherapy)
- Neutropenia (38% and 58%, respectively)
- Elevated liver enzymes (13% and 15%, respectively)
- Neurologic events (9% and 8%, respectively)
- Cytokine release syndrome (5% and 0%, respectively)
- Infusion reactions (3% and 1%, respectively)
- Lymphopenia (2% and 4%, respectively).
Fatal AEs occurred in 19% of patients in the blinatumomab arm and 17% of those in the SOC arm.
These results were published in The New England Journal of Medicine last year.
Horai
For this single-arm trial (NCT02412306), researchers evaluated blinatumomab in 35 Japanese adult and pediatric patients with relapsed or refractory B-ALL. An extension of this study is ongoing.
Efficacy data from Horai are not available.
According to Amgen, the major AEs occurring in adults on this trial were cytokine release syndrome (46.2%), pyrexia (46.2%), decrease in white blood cell count (38.5%), and decrease in platelet count (34.6%).
Major AEs in pediatric patients were elevated liver enzymes (66.7%), pyrexia (66.7%), cytokine release syndrome (55.6%), and abdominal pain (44.4%).
The Japanese Ministry of Health, Labour and Welfare has approved blinatumomab (Blincyto®) for the treatment of relapsed or refractory B-cell acute lymphoblastic leukemia (B-ALL).
Blinatumomab is the first and only bispecific T-cell engager immunotherapy construct approved globally.
The drug’s approval in Japan is based on data from the phase 3 TOWER study and the phase 1b/2 Horai study.
The TOWER trial (NCT02013167) enrolled 405 patients with Ph-negative, relapsed/refractory B-ALL, 376 of whom ultimately received treatment.
The patients received blinatumomab (n=267) or investigator’s choice of four protocol-defined standard of care (SOC) chemotherapy regimens (n=109):
- FLAG (fludarabine, high-dose cytarabine arabinoside, and granulocyte-colony stimulating factor), with or without an anthracycline (n=49, 45%)
- A high-dose cytarabine arabinoside-based regimen (n=19, 17%)
- A high-dose methotrexate-based regimen (n=22, 20%)
- A clofarabine-based regimen (n=19, 17%).
Blinatumomab demonstrated an improvement in median overall survival over SOC. The median overall survival was 7.7 months with blinatumomab and 4.0 months with SOC (hazard ratio for death=0.71; P=0.012).
Grade 3 or higher adverse events (AEs) of interest, according to the researchers, were:
- Infection (34% with blinatumomab and 52% with chemotherapy)
- Neutropenia (38% and 58%, respectively)
- Elevated liver enzymes (13% and 15%, respectively)
- Neurologic events (9% and 8%, respectively)
- Cytokine release syndrome (5% and 0%, respectively)
- Infusion reactions (3% and 1%, respectively)
- Lymphopenia (2% and 4%, respectively).
Fatal AEs occurred in 19% of patients in the blinatumomab arm and 17% of those in the SOC arm.
These results were published in The New England Journal of Medicine last year.
Horai
For this single-arm trial (NCT02412306), researchers evaluated blinatumomab in 35 Japanese adult and pediatric patients with relapsed or refractory B-ALL. An extension of this study is ongoing.
Efficacy data from Horai are not available.
According to Amgen, the major AEs occurring in adults on this trial were cytokine release syndrome (46.2%), pyrexia (46.2%), decrease in white blood cell count (38.5%), and decrease in platelet count (34.6%).
Major AEs in pediatric patients were elevated liver enzymes (66.7%), pyrexia (66.7%), cytokine release syndrome (55.6%), and abdominal pain (44.4%).
The Japanese Ministry of Health, Labour and Welfare has approved blinatumomab (Blincyto®) for the treatment of relapsed or refractory B-cell acute lymphoblastic leukemia (B-ALL).
Blinatumomab is the first and only bispecific T-cell engager immunotherapy construct approved globally.
The drug’s approval in Japan is based on data from the phase 3 TOWER study and the phase 1b/2 Horai study.
The TOWER trial (NCT02013167) enrolled 405 patients with Ph-negative, relapsed/refractory B-ALL, 376 of whom ultimately received treatment.
The patients received blinatumomab (n=267) or investigator’s choice of four protocol-defined standard of care (SOC) chemotherapy regimens (n=109):
- FLAG (fludarabine, high-dose cytarabine arabinoside, and granulocyte-colony stimulating factor), with or without an anthracycline (n=49, 45%)
- A high-dose cytarabine arabinoside-based regimen (n=19, 17%)
- A high-dose methotrexate-based regimen (n=22, 20%)
- A clofarabine-based regimen (n=19, 17%).
Blinatumomab demonstrated an improvement in median overall survival over SOC. The median overall survival was 7.7 months with blinatumomab and 4.0 months with SOC (hazard ratio for death=0.71; P=0.012).
Grade 3 or higher adverse events (AEs) of interest, according to the researchers, were:
- Infection (34% with blinatumomab and 52% with chemotherapy)
- Neutropenia (38% and 58%, respectively)
- Elevated liver enzymes (13% and 15%, respectively)
- Neurologic events (9% and 8%, respectively)
- Cytokine release syndrome (5% and 0%, respectively)
- Infusion reactions (3% and 1%, respectively)
- Lymphopenia (2% and 4%, respectively).
Fatal AEs occurred in 19% of patients in the blinatumomab arm and 17% of those in the SOC arm.
These results were published in The New England Journal of Medicine last year.
Horai
For this single-arm trial (NCT02412306), researchers evaluated blinatumomab in 35 Japanese adult and pediatric patients with relapsed or refractory B-ALL. An extension of this study is ongoing.
Efficacy data from Horai are not available.
According to Amgen, the major AEs occurring in adults on this trial were cytokine release syndrome (46.2%), pyrexia (46.2%), decrease in white blood cell count (38.5%), and decrease in platelet count (34.6%).
Major AEs in pediatric patients were elevated liver enzymes (66.7%), pyrexia (66.7%), cytokine release syndrome (55.6%), and abdominal pain (44.4%).
Managing procedural pain in a patient taking naltrexone
Mr. M, age 55, presents to his primary care physician (PCP) with hematochezia. Mr. M states that for the past week, he has noticed blood upon wiping after a bowel movement and is worried that he might have cancer.
Mr. M has a 10-year history of opioid use disorder as diagnosed by his psychiatrist. He is presently maintained on long-acting injectable naltrexone, 380 mg IM every 4 weeks, and has not used opioids for the past 1.5 years. Mr. M is also taking simvastatin, 40 mg, for dyslipidemia, lisinopril, 5 mg, for hypertension, and cetirizine, 5 mg as needed, for seasonal allergies.
A standard workup including a physical examination and laboratory tests are performed. Mr. M’s PCP would like for him to undergo a colonoscopy to investigate the etiology of the bleeding. In consultation with both the PCP and psychiatrist, the gastroenterologist determines that the colonoscopy can be performed within 48 hours with no changes to Mr. M’s medication regimen. The gastroenterologist utilizes a nonopioid, ketorolac, 30 mg IV, for pain management during the procedure. Diverticula were identified in the lower gastrointestinal tract and are treated endoscopically. Mr. M is successfully withdrawn from sedation with no adverse events or pain and continues to be in opioid remission.
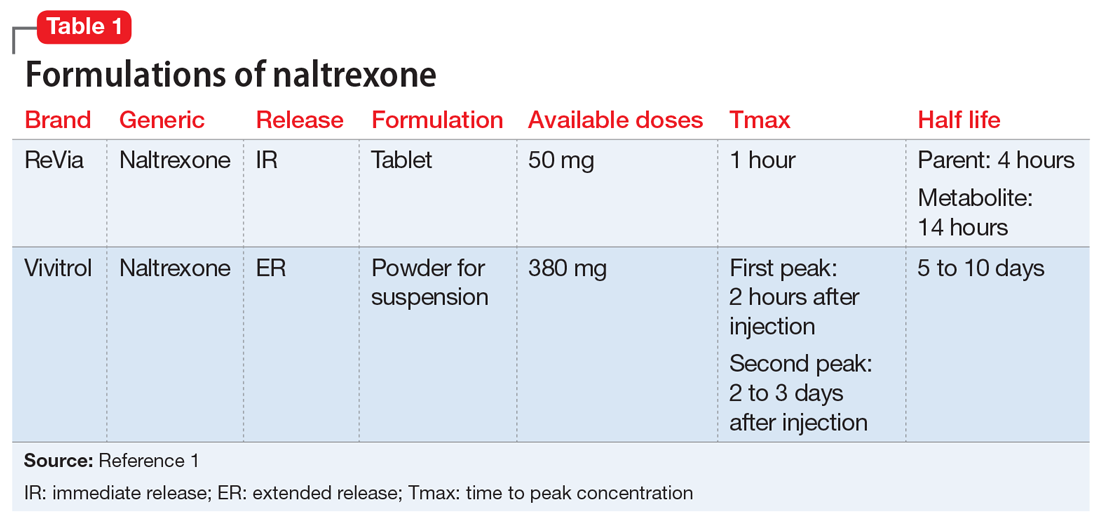
Naltrexone competitively antagonizes opioid receptors with the highest affinity for the µ-opioid receptor. It is approved for treatment of alcohol and opioid dependence following opioid detoxification.1 Its competitive inhibition at the µ-opioid receptor results in the inhibition of exogenous opioid effects. The medication is available as an orally administered tablet as well as a long-acting injection administered intramuscularly (Table 11). The long-acting injection can be useful in patients who have difficulty with adherence, because good adherence to naltrexone is required to maximize efficacy.
Due to its ability to block opioid analgesic effects, naltrexone presents a unique challenge for patients taking it who need to undergo procedures that require pain control. Pharmacologic regimens used during procedures often contain a sedative agent, such as propofol, and an opioid for analgesia. Alternative strategies are needed for patients taking naltrexone who require an opioid analgesic agent for procedures such as colonoscopies.
One strategy could be to withhold naltrexone before the procedure to ensure that the medication will not compete with the opioid agent to relieve pain. This strategy depends on the urgency of the procedure, the formulation of naltrexone being used, and patient-specific factors that may increase the risk for adverse events. For a non-urgent, elective procedure, it may be acceptable to hold oral naltrexone approximately 72 hours before the procedure. However, this is likely not a favorable approach for patients who may be at high risk for relapse or for patients who are receiving the long-acting formulation. Additionally, the use of an opioid agent intra- or post-operatively for pain may increase the risk of relapse. The use of opioids for such procedures may also be more difficult in a patient with a history of opioid abuse or dependence because he or she may have developed tolerance to opioids. Conversely, if a patient has been treated with naltrexone for an extended period, a lack of tolerance may increase the risk of respiratory depression with opioid administration due to upregulation of the opioid receptor.2
Continue to: Nonopioid analgesic agents
Nonopioid analgesic agents
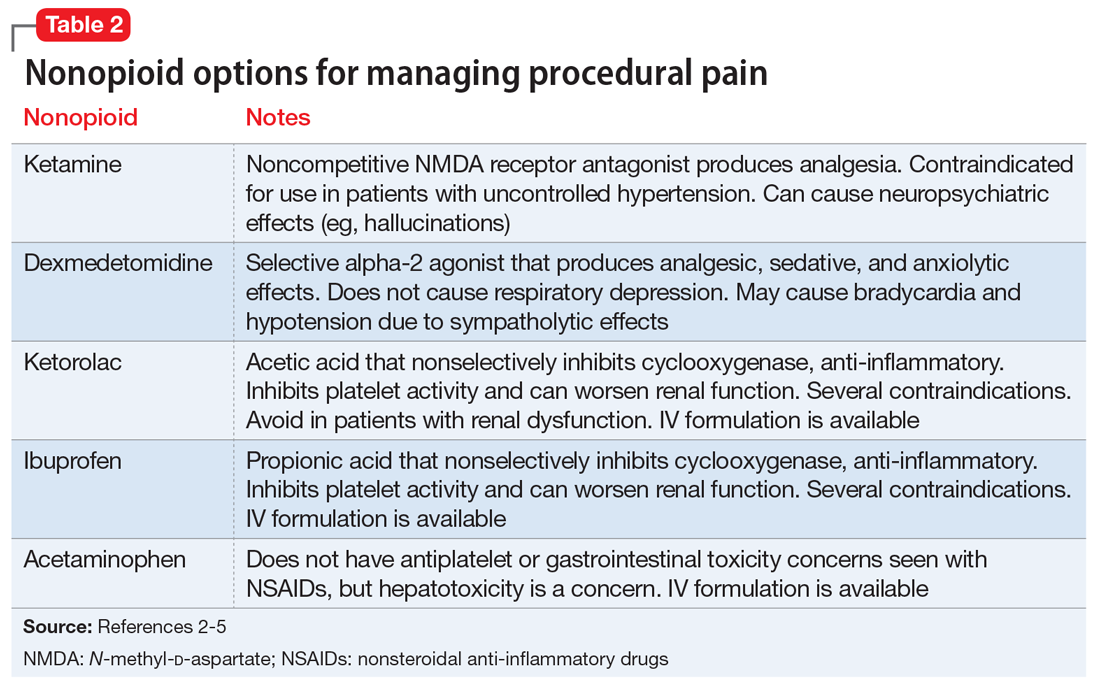
For a patient receiving naltrexone who needs to undergo a procedure, a multidisciplinary consultation between the patient’s psychiatrist and other clinicians is key for providing a regimen that is safe and effective. A nonopioid analgesic agent may be considered to avoid the problematic interactions possible in these patients (Table 23-5). Nonopioid regimens can be utilized alone or in combination, and may include the following3-5:
Ketamine is a non-competitive antagonist at the N-methyl-
Dexmedetomidine is an alpha-2 agonist that can provide sedative and analgesic effects. It can cause procedural hypotension and bradycardia, so caution is advised in patients with cardiac disease and hepatic and/or renal insufficiencies.
Nonsteroidal anti-inflammatory drugs (NSAIDs), such as ibuprofen or ketorolac, inhibit cyclooxygenase enzymes and can be considered in analgesic regimens. However, for most surgical procedures, the increased risk of bleeding due to platelet inhibition is a concern.
Continue to: Acetaminophen
Acetaminophen. Although its full mechanism of action has not been discovered, acetaminophen may also act on the cyclooxygenase pathway to produce analgesia. Compared with the oral formulation, IV acetaminophen is more expensive but may offer certain advantages, including faster plasma peak levels and lower production of acetaminophen’s toxic metabolite, N-acetyl-p-benzoquinone imine. Nonetheless, hepatotoxicity and overdose remain a concern.
The use of nonopioid analgesics during elective procedures that require pain control will allow continued use of an opioid antagonist such as naltrexone, while minimizing the risk for withdrawal or relapse. Their use must be evaluated on a case-by-case basis to ensure maximum safety and efficacy for each patient from both a medical and psychiatric standpoint. Overall, with the proper expertise and consultation, nonopioid pain regimens represent a reasonable alternative to opiates for patients who take naltrexone.
Related Resources
- American Society of Anesthesiologists. Standards guidelines and related resources. https://www.asahq.org/quality-and-practice-management/standards-guidelines-and-related-resources-search.
- American Society of Addiction Medicine. Clinical resources. https://www.asam.org/resources/guidelines-and-consensus-documents.
Drug Brand Names
Acetaminophen • Tylenol
Cetirizine • Zyrtec
Dexmedetomidine • Precedex
Ibuprofen • Caldolor (IV), Motrin (oral)
Ketamine • Ketalar
Ketorolac • Toradol
Lisinopril • Prinivil, Zestril
Naltrexone • ReVia, Vivitrol
Propofol • Diprivan
Simvastatin • Juvisync, Simcor
1. Vivitrol [package insert]. Waltham, MA: Alkermes, Inc.; 2015.
2. Yoburn BC, Duttaroy A, Shah S, et al. Opioid antagonist-induced receptor upregulation: effects of concurrent agonist administration. Brain Res Bull. 1994;33(2):237-240.
3. Vadivelu N, Chang D, Lumermann L, et al. Management of patients on abuse-deterrent opioids in the ambulatory surgery setting. Curr Pain Headache Rep. 2017;21(2):10.
4. Koh W, Nguyen KP, Jahr JS. Intravenous non-opioid analgesia for peri- and postoperative pain management: a scientific review of intravenous acetaminophen and ibuprofen. Korean J Anesthesiol. 2015;68(1):3-12.
5. Kaye AD, Cornett EM, Helander E, et al. An update on nonopioids: intravenous or oral analgesics for perioperative pain management. Anesthesiol Clin. 2017;35(2):e55-e71.
Mr. M, age 55, presents to his primary care physician (PCP) with hematochezia. Mr. M states that for the past week, he has noticed blood upon wiping after a bowel movement and is worried that he might have cancer.
Mr. M has a 10-year history of opioid use disorder as diagnosed by his psychiatrist. He is presently maintained on long-acting injectable naltrexone, 380 mg IM every 4 weeks, and has not used opioids for the past 1.5 years. Mr. M is also taking simvastatin, 40 mg, for dyslipidemia, lisinopril, 5 mg, for hypertension, and cetirizine, 5 mg as needed, for seasonal allergies.
A standard workup including a physical examination and laboratory tests are performed. Mr. M’s PCP would like for him to undergo a colonoscopy to investigate the etiology of the bleeding. In consultation with both the PCP and psychiatrist, the gastroenterologist determines that the colonoscopy can be performed within 48 hours with no changes to Mr. M’s medication regimen. The gastroenterologist utilizes a nonopioid, ketorolac, 30 mg IV, for pain management during the procedure. Diverticula were identified in the lower gastrointestinal tract and are treated endoscopically. Mr. M is successfully withdrawn from sedation with no adverse events or pain and continues to be in opioid remission.
Naltrexone competitively antagonizes opioid receptors with the highest affinity for the µ-opioid receptor. It is approved for treatment of alcohol and opioid dependence following opioid detoxification.1 Its competitive inhibition at the µ-opioid receptor results in the inhibition of exogenous opioid effects. The medication is available as an orally administered tablet as well as a long-acting injection administered intramuscularly (Table 11). The long-acting injection can be useful in patients who have difficulty with adherence, because good adherence to naltrexone is required to maximize efficacy.
Due to its ability to block opioid analgesic effects, naltrexone presents a unique challenge for patients taking it who need to undergo procedures that require pain control. Pharmacologic regimens used during procedures often contain a sedative agent, such as propofol, and an opioid for analgesia. Alternative strategies are needed for patients taking naltrexone who require an opioid analgesic agent for procedures such as colonoscopies.
One strategy could be to withhold naltrexone before the procedure to ensure that the medication will not compete with the opioid agent to relieve pain. This strategy depends on the urgency of the procedure, the formulation of naltrexone being used, and patient-specific factors that may increase the risk for adverse events. For a non-urgent, elective procedure, it may be acceptable to hold oral naltrexone approximately 72 hours before the procedure. However, this is likely not a favorable approach for patients who may be at high risk for relapse or for patients who are receiving the long-acting formulation. Additionally, the use of an opioid agent intra- or post-operatively for pain may increase the risk of relapse. The use of opioids for such procedures may also be more difficult in a patient with a history of opioid abuse or dependence because he or she may have developed tolerance to opioids. Conversely, if a patient has been treated with naltrexone for an extended period, a lack of tolerance may increase the risk of respiratory depression with opioid administration due to upregulation of the opioid receptor.2
Continue to: Nonopioid analgesic agents
Nonopioid analgesic agents
For a patient receiving naltrexone who needs to undergo a procedure, a multidisciplinary consultation between the patient’s psychiatrist and other clinicians is key for providing a regimen that is safe and effective. A nonopioid analgesic agent may be considered to avoid the problematic interactions possible in these patients (Table 23-5). Nonopioid regimens can be utilized alone or in combination, and may include the following3-5:
Ketamine is a non-competitive antagonist at the N-methyl-
Dexmedetomidine is an alpha-2 agonist that can provide sedative and analgesic effects. It can cause procedural hypotension and bradycardia, so caution is advised in patients with cardiac disease and hepatic and/or renal insufficiencies.
Nonsteroidal anti-inflammatory drugs (NSAIDs), such as ibuprofen or ketorolac, inhibit cyclooxygenase enzymes and can be considered in analgesic regimens. However, for most surgical procedures, the increased risk of bleeding due to platelet inhibition is a concern.
Continue to: Acetaminophen
Acetaminophen. Although its full mechanism of action has not been discovered, acetaminophen may also act on the cyclooxygenase pathway to produce analgesia. Compared with the oral formulation, IV acetaminophen is more expensive but may offer certain advantages, including faster plasma peak levels and lower production of acetaminophen’s toxic metabolite, N-acetyl-p-benzoquinone imine. Nonetheless, hepatotoxicity and overdose remain a concern.
The use of nonopioid analgesics during elective procedures that require pain control will allow continued use of an opioid antagonist such as naltrexone, while minimizing the risk for withdrawal or relapse. Their use must be evaluated on a case-by-case basis to ensure maximum safety and efficacy for each patient from both a medical and psychiatric standpoint. Overall, with the proper expertise and consultation, nonopioid pain regimens represent a reasonable alternative to opiates for patients who take naltrexone.
Related Resources
- American Society of Anesthesiologists. Standards guidelines and related resources. https://www.asahq.org/quality-and-practice-management/standards-guidelines-and-related-resources-search.
- American Society of Addiction Medicine. Clinical resources. https://www.asam.org/resources/guidelines-and-consensus-documents.
Drug Brand Names
Acetaminophen • Tylenol
Cetirizine • Zyrtec
Dexmedetomidine • Precedex
Ibuprofen • Caldolor (IV), Motrin (oral)
Ketamine • Ketalar
Ketorolac • Toradol
Lisinopril • Prinivil, Zestril
Naltrexone • ReVia, Vivitrol
Propofol • Diprivan
Simvastatin • Juvisync, Simcor
Mr. M, age 55, presents to his primary care physician (PCP) with hematochezia. Mr. M states that for the past week, he has noticed blood upon wiping after a bowel movement and is worried that he might have cancer.
Mr. M has a 10-year history of opioid use disorder as diagnosed by his psychiatrist. He is presently maintained on long-acting injectable naltrexone, 380 mg IM every 4 weeks, and has not used opioids for the past 1.5 years. Mr. M is also taking simvastatin, 40 mg, for dyslipidemia, lisinopril, 5 mg, for hypertension, and cetirizine, 5 mg as needed, for seasonal allergies.
A standard workup including a physical examination and laboratory tests are performed. Mr. M’s PCP would like for him to undergo a colonoscopy to investigate the etiology of the bleeding. In consultation with both the PCP and psychiatrist, the gastroenterologist determines that the colonoscopy can be performed within 48 hours with no changes to Mr. M’s medication regimen. The gastroenterologist utilizes a nonopioid, ketorolac, 30 mg IV, for pain management during the procedure. Diverticula were identified in the lower gastrointestinal tract and are treated endoscopically. Mr. M is successfully withdrawn from sedation with no adverse events or pain and continues to be in opioid remission.
Naltrexone competitively antagonizes opioid receptors with the highest affinity for the µ-opioid receptor. It is approved for treatment of alcohol and opioid dependence following opioid detoxification.1 Its competitive inhibition at the µ-opioid receptor results in the inhibition of exogenous opioid effects. The medication is available as an orally administered tablet as well as a long-acting injection administered intramuscularly (Table 11). The long-acting injection can be useful in patients who have difficulty with adherence, because good adherence to naltrexone is required to maximize efficacy.
Due to its ability to block opioid analgesic effects, naltrexone presents a unique challenge for patients taking it who need to undergo procedures that require pain control. Pharmacologic regimens used during procedures often contain a sedative agent, such as propofol, and an opioid for analgesia. Alternative strategies are needed for patients taking naltrexone who require an opioid analgesic agent for procedures such as colonoscopies.
One strategy could be to withhold naltrexone before the procedure to ensure that the medication will not compete with the opioid agent to relieve pain. This strategy depends on the urgency of the procedure, the formulation of naltrexone being used, and patient-specific factors that may increase the risk for adverse events. For a non-urgent, elective procedure, it may be acceptable to hold oral naltrexone approximately 72 hours before the procedure. However, this is likely not a favorable approach for patients who may be at high risk for relapse or for patients who are receiving the long-acting formulation. Additionally, the use of an opioid agent intra- or post-operatively for pain may increase the risk of relapse. The use of opioids for such procedures may also be more difficult in a patient with a history of opioid abuse or dependence because he or she may have developed tolerance to opioids. Conversely, if a patient has been treated with naltrexone for an extended period, a lack of tolerance may increase the risk of respiratory depression with opioid administration due to upregulation of the opioid receptor.2
Continue to: Nonopioid analgesic agents
Nonopioid analgesic agents
For a patient receiving naltrexone who needs to undergo a procedure, a multidisciplinary consultation between the patient’s psychiatrist and other clinicians is key for providing a regimen that is safe and effective. A nonopioid analgesic agent may be considered to avoid the problematic interactions possible in these patients (Table 23-5). Nonopioid regimens can be utilized alone or in combination, and may include the following3-5:
Ketamine is a non-competitive antagonist at the N-methyl-
Dexmedetomidine is an alpha-2 agonist that can provide sedative and analgesic effects. It can cause procedural hypotension and bradycardia, so caution is advised in patients with cardiac disease and hepatic and/or renal insufficiencies.
Nonsteroidal anti-inflammatory drugs (NSAIDs), such as ibuprofen or ketorolac, inhibit cyclooxygenase enzymes and can be considered in analgesic regimens. However, for most surgical procedures, the increased risk of bleeding due to platelet inhibition is a concern.
Continue to: Acetaminophen
Acetaminophen. Although its full mechanism of action has not been discovered, acetaminophen may also act on the cyclooxygenase pathway to produce analgesia. Compared with the oral formulation, IV acetaminophen is more expensive but may offer certain advantages, including faster plasma peak levels and lower production of acetaminophen’s toxic metabolite, N-acetyl-p-benzoquinone imine. Nonetheless, hepatotoxicity and overdose remain a concern.
The use of nonopioid analgesics during elective procedures that require pain control will allow continued use of an opioid antagonist such as naltrexone, while minimizing the risk for withdrawal or relapse. Their use must be evaluated on a case-by-case basis to ensure maximum safety and efficacy for each patient from both a medical and psychiatric standpoint. Overall, with the proper expertise and consultation, nonopioid pain regimens represent a reasonable alternative to opiates for patients who take naltrexone.
Related Resources
- American Society of Anesthesiologists. Standards guidelines and related resources. https://www.asahq.org/quality-and-practice-management/standards-guidelines-and-related-resources-search.
- American Society of Addiction Medicine. Clinical resources. https://www.asam.org/resources/guidelines-and-consensus-documents.
Drug Brand Names
Acetaminophen • Tylenol
Cetirizine • Zyrtec
Dexmedetomidine • Precedex
Ibuprofen • Caldolor (IV), Motrin (oral)
Ketamine • Ketalar
Ketorolac • Toradol
Lisinopril • Prinivil, Zestril
Naltrexone • ReVia, Vivitrol
Propofol • Diprivan
Simvastatin • Juvisync, Simcor
1. Vivitrol [package insert]. Waltham, MA: Alkermes, Inc.; 2015.
2. Yoburn BC, Duttaroy A, Shah S, et al. Opioid antagonist-induced receptor upregulation: effects of concurrent agonist administration. Brain Res Bull. 1994;33(2):237-240.
3. Vadivelu N, Chang D, Lumermann L, et al. Management of patients on abuse-deterrent opioids in the ambulatory surgery setting. Curr Pain Headache Rep. 2017;21(2):10.
4. Koh W, Nguyen KP, Jahr JS. Intravenous non-opioid analgesia for peri- and postoperative pain management: a scientific review of intravenous acetaminophen and ibuprofen. Korean J Anesthesiol. 2015;68(1):3-12.
5. Kaye AD, Cornett EM, Helander E, et al. An update on nonopioids: intravenous or oral analgesics for perioperative pain management. Anesthesiol Clin. 2017;35(2):e55-e71.
1. Vivitrol [package insert]. Waltham, MA: Alkermes, Inc.; 2015.
2. Yoburn BC, Duttaroy A, Shah S, et al. Opioid antagonist-induced receptor upregulation: effects of concurrent agonist administration. Brain Res Bull. 1994;33(2):237-240.
3. Vadivelu N, Chang D, Lumermann L, et al. Management of patients on abuse-deterrent opioids in the ambulatory surgery setting. Curr Pain Headache Rep. 2017;21(2):10.
4. Koh W, Nguyen KP, Jahr JS. Intravenous non-opioid analgesia for peri- and postoperative pain management: a scientific review of intravenous acetaminophen and ibuprofen. Korean J Anesthesiol. 2015;68(1):3-12.
5. Kaye AD, Cornett EM, Helander E, et al. An update on nonopioids: intravenous or oral analgesics for perioperative pain management. Anesthesiol Clin. 2017;35(2):e55-e71.
Atrial fib guidelines may fall short on oral anticoagulation
Anticoagulation thresholds based on CHA2DS2-VASc risk score varied from population to population, researchers reported in the Annals of Internal Medicine.
After accounting for differing rates of stroke in published studies, the benefit of warfarin anticoagulation varied nearly fourfold, said Sachin J. Shah, MD, of the University of California San Francisco and his associates. They called for guidelines that “better reflect the uncertainty in current thresholds of stroke risk score for recommending anticoagulation.”
Oral anticoagulation markedly reduces risk of ischemic stroke in patients with atrial fibrillation but increases the risk of major bleeding, including intracranial hemorrhage, which often is fatal. Therefore, when deciding whether to recommend oral anticoagulation, physicians must estimate clinical net benefit by quantifying the difference between reduction in stroke risk and increase in major bleeding risk, weighted by the severity of each outcome.
Guidelines on nonvalvular atrial fibrillation from the European Society of Cardiology and joint guidelines from the American Heart Association, American College of Cardiology, and Heart Rhythm Society (AHA/ACC/HRS) recommend oral anticoagulation when CHA2DS2-VASc (congestive heart failure, hypertension, age, diabetes, stroke, and vascular disease) risk score is 2 or greater. These guidelines implicitly assume that a particular CHA2DS2-VASc score denotes the same amount of risk across populations, even though a recent meta-analysis found otherwise, as the researchers noted.
To further test this assumption, they applied an existing Markov model to data from more than 33,000 members of the ATRIA-CVRN cohort. All patients had nonvalvular atrial fibrillation, were members of Kaiser Permanente Northern California, and were diagnosed during 1996-1997. About 81% had a CHA2DS2-VASc score of at least 2. For each patient, the researchers produced four estimates of the net clinical benefit of oral anticoagulation based on ischemic stroke rates from ATRIA, the Swedish AF cohort study, the SPORTIF study, and the Danish National Patient Registry.
Optimal anticoagulation thresholds were a CHA2DS2-VASc score of 3 or more using stroke rates from ATRIA, 2 or more based on Swedish AF rates, 1 or more based on SPORTIF rates, and 0 or more using rates from the Danish National Patient Registry. Oral anticoagulation thresholds were lower but still varied widely after accounting for the lower rates of intracranial hemorrhage associated with non–vitamin K antagonist therapy.
Therefore, current guidelines based on CHA2DS2-VASc score may need revising “in favor of more accurate, individualized assessments of risk for both ischemic stroke and major bleeding,” the investigators wrote. “Until such time, guidelines should better reflect the uncertainty of the current approach in which a patient’s CHA2DS2-VASc score is used as the primary basis for recommending oral anticoagulation.”
The study had no primary funding source. Dr. Shah reported having no conflicts of interest. Three coinvestigators disclosed research support from relevant pharmaceutical or device companies.
SOURCE: Shah SJ et al. Ann Intern Med. 2018 Sep 25. doi: 10.7326/M17-2762
Based on this study, the CHA2DS2-VASc score threshold for anticoagulation might not be a “one-size-fits all approach but rather a starting point for a more tailored assessment,” wrote Jennifer M. Wright, MD, and Craig T. January, MD, PhD, in an editorial accompanying the report.
The CHA2DS2-VASc algorithm uses fixed whole integers and therefore might lack the sensitivity and flexibility needed to accurately reflect the effects of its components, the experts wrote. “For example, female sex now seems to be a risk modifier, and its intensity depends on other risk factors.”
However, CHA2DS2-VASc remains the main way to assess net clinical benefit of oral anticoagulation for patients with anticoagulation, they conceded. “When it comes to the conversation about the risks and benefits of anticoagulation for our patients with atrial fibrillation, we must remember that each patient is an individual and has his or her own ‘score.’ ”
The editorialists are with the University of Wisconsin in Madison. They reported having no relevant conflicts of interest. These comments are based on their editorial (Ann Intern Med. 2018 Sep 25. doi: 10.7326/M18-2355).
Based on this study, the CHA2DS2-VASc score threshold for anticoagulation might not be a “one-size-fits all approach but rather a starting point for a more tailored assessment,” wrote Jennifer M. Wright, MD, and Craig T. January, MD, PhD, in an editorial accompanying the report.
The CHA2DS2-VASc algorithm uses fixed whole integers and therefore might lack the sensitivity and flexibility needed to accurately reflect the effects of its components, the experts wrote. “For example, female sex now seems to be a risk modifier, and its intensity depends on other risk factors.”
However, CHA2DS2-VASc remains the main way to assess net clinical benefit of oral anticoagulation for patients with anticoagulation, they conceded. “When it comes to the conversation about the risks and benefits of anticoagulation for our patients with atrial fibrillation, we must remember that each patient is an individual and has his or her own ‘score.’ ”
The editorialists are with the University of Wisconsin in Madison. They reported having no relevant conflicts of interest. These comments are based on their editorial (Ann Intern Med. 2018 Sep 25. doi: 10.7326/M18-2355).
Based on this study, the CHA2DS2-VASc score threshold for anticoagulation might not be a “one-size-fits all approach but rather a starting point for a more tailored assessment,” wrote Jennifer M. Wright, MD, and Craig T. January, MD, PhD, in an editorial accompanying the report.
The CHA2DS2-VASc algorithm uses fixed whole integers and therefore might lack the sensitivity and flexibility needed to accurately reflect the effects of its components, the experts wrote. “For example, female sex now seems to be a risk modifier, and its intensity depends on other risk factors.”
However, CHA2DS2-VASc remains the main way to assess net clinical benefit of oral anticoagulation for patients with anticoagulation, they conceded. “When it comes to the conversation about the risks and benefits of anticoagulation for our patients with atrial fibrillation, we must remember that each patient is an individual and has his or her own ‘score.’ ”
The editorialists are with the University of Wisconsin in Madison. They reported having no relevant conflicts of interest. These comments are based on their editorial (Ann Intern Med. 2018 Sep 25. doi: 10.7326/M18-2355).
Anticoagulation thresholds based on CHA2DS2-VASc risk score varied from population to population, researchers reported in the Annals of Internal Medicine.
After accounting for differing rates of stroke in published studies, the benefit of warfarin anticoagulation varied nearly fourfold, said Sachin J. Shah, MD, of the University of California San Francisco and his associates. They called for guidelines that “better reflect the uncertainty in current thresholds of stroke risk score for recommending anticoagulation.”
Oral anticoagulation markedly reduces risk of ischemic stroke in patients with atrial fibrillation but increases the risk of major bleeding, including intracranial hemorrhage, which often is fatal. Therefore, when deciding whether to recommend oral anticoagulation, physicians must estimate clinical net benefit by quantifying the difference between reduction in stroke risk and increase in major bleeding risk, weighted by the severity of each outcome.
Guidelines on nonvalvular atrial fibrillation from the European Society of Cardiology and joint guidelines from the American Heart Association, American College of Cardiology, and Heart Rhythm Society (AHA/ACC/HRS) recommend oral anticoagulation when CHA2DS2-VASc (congestive heart failure, hypertension, age, diabetes, stroke, and vascular disease) risk score is 2 or greater. These guidelines implicitly assume that a particular CHA2DS2-VASc score denotes the same amount of risk across populations, even though a recent meta-analysis found otherwise, as the researchers noted.
To further test this assumption, they applied an existing Markov model to data from more than 33,000 members of the ATRIA-CVRN cohort. All patients had nonvalvular atrial fibrillation, were members of Kaiser Permanente Northern California, and were diagnosed during 1996-1997. About 81% had a CHA2DS2-VASc score of at least 2. For each patient, the researchers produced four estimates of the net clinical benefit of oral anticoagulation based on ischemic stroke rates from ATRIA, the Swedish AF cohort study, the SPORTIF study, and the Danish National Patient Registry.
Optimal anticoagulation thresholds were a CHA2DS2-VASc score of 3 or more using stroke rates from ATRIA, 2 or more based on Swedish AF rates, 1 or more based on SPORTIF rates, and 0 or more using rates from the Danish National Patient Registry. Oral anticoagulation thresholds were lower but still varied widely after accounting for the lower rates of intracranial hemorrhage associated with non–vitamin K antagonist therapy.
Therefore, current guidelines based on CHA2DS2-VASc score may need revising “in favor of more accurate, individualized assessments of risk for both ischemic stroke and major bleeding,” the investigators wrote. “Until such time, guidelines should better reflect the uncertainty of the current approach in which a patient’s CHA2DS2-VASc score is used as the primary basis for recommending oral anticoagulation.”
The study had no primary funding source. Dr. Shah reported having no conflicts of interest. Three coinvestigators disclosed research support from relevant pharmaceutical or device companies.
SOURCE: Shah SJ et al. Ann Intern Med. 2018 Sep 25. doi: 10.7326/M17-2762
Anticoagulation thresholds based on CHA2DS2-VASc risk score varied from population to population, researchers reported in the Annals of Internal Medicine.
After accounting for differing rates of stroke in published studies, the benefit of warfarin anticoagulation varied nearly fourfold, said Sachin J. Shah, MD, of the University of California San Francisco and his associates. They called for guidelines that “better reflect the uncertainty in current thresholds of stroke risk score for recommending anticoagulation.”
Oral anticoagulation markedly reduces risk of ischemic stroke in patients with atrial fibrillation but increases the risk of major bleeding, including intracranial hemorrhage, which often is fatal. Therefore, when deciding whether to recommend oral anticoagulation, physicians must estimate clinical net benefit by quantifying the difference between reduction in stroke risk and increase in major bleeding risk, weighted by the severity of each outcome.
Guidelines on nonvalvular atrial fibrillation from the European Society of Cardiology and joint guidelines from the American Heart Association, American College of Cardiology, and Heart Rhythm Society (AHA/ACC/HRS) recommend oral anticoagulation when CHA2DS2-VASc (congestive heart failure, hypertension, age, diabetes, stroke, and vascular disease) risk score is 2 or greater. These guidelines implicitly assume that a particular CHA2DS2-VASc score denotes the same amount of risk across populations, even though a recent meta-analysis found otherwise, as the researchers noted.
To further test this assumption, they applied an existing Markov model to data from more than 33,000 members of the ATRIA-CVRN cohort. All patients had nonvalvular atrial fibrillation, were members of Kaiser Permanente Northern California, and were diagnosed during 1996-1997. About 81% had a CHA2DS2-VASc score of at least 2. For each patient, the researchers produced four estimates of the net clinical benefit of oral anticoagulation based on ischemic stroke rates from ATRIA, the Swedish AF cohort study, the SPORTIF study, and the Danish National Patient Registry.
Optimal anticoagulation thresholds were a CHA2DS2-VASc score of 3 or more using stroke rates from ATRIA, 2 or more based on Swedish AF rates, 1 or more based on SPORTIF rates, and 0 or more using rates from the Danish National Patient Registry. Oral anticoagulation thresholds were lower but still varied widely after accounting for the lower rates of intracranial hemorrhage associated with non–vitamin K antagonist therapy.
Therefore, current guidelines based on CHA2DS2-VASc score may need revising “in favor of more accurate, individualized assessments of risk for both ischemic stroke and major bleeding,” the investigators wrote. “Until such time, guidelines should better reflect the uncertainty of the current approach in which a patient’s CHA2DS2-VASc score is used as the primary basis for recommending oral anticoagulation.”
The study had no primary funding source. Dr. Shah reported having no conflicts of interest. Three coinvestigators disclosed research support from relevant pharmaceutical or device companies.
SOURCE: Shah SJ et al. Ann Intern Med. 2018 Sep 25. doi: 10.7326/M17-2762
FROM THE ANNALS OF INTERNAL MEDICINE
Key clinical point:
Major finding: After accounting for differing rates of stroke in published studies, the benefit of warfarin anticoagulation varied nearly fourfold. Anticoagulation thresholds were lower but still varied widely in a model of non–vitamin K antagonist therapy.
Study details: Markov state-transition model of 33,434 patients with nonvalvular atrial fibrillation.
Disclosures: The study had no primary funding source. Dr. Shah reported having no conflicts of interest. Three coinvestigators disclosed research support from relevant pharmaceutical or device companies.
Source: Shah SJ et al. Ann Intern Med. 2018 Sep 25. doi: 10.7326/M17-2762.
Nonablative laser adds benefits to low-dose isotretinoin as treatment for moderate to severe acne
A combination of low-dose isotretinoin and nonablative fractional laser (NAFL) was a safe and effective treatment for moderate to severe acne and also improved acne scars in a small Chinese study, investigators reported.
In the randomized, split-face, controlled study of 18 adult Asian patients with moderate to severe acne, low-dose isotretinoin alone effectively controlled papule and pustule acne lesions, whereas NAFL had the additional effect of reducing the number of comedones and improving boxcar atrophic scars, reported Weihui Zeng, MD, and associates from the department of dermatology at the Second Affiliated Hospital of Xi’an Jiaotong University, Shanxi, China.
The authors noted that many patients seen at their clinic cannot tolerate a 20 mg/day dose of isotretinoin because of severe mucocutaneous side effects and that treatment with nonablative lasers, which – in contrast to ablative lasers – use infrared radiation to penetrate the skin deeply and thereby selectively heat dermal tissue while sparing the epidermis, could be a treatment option for these patients.
Therefore, they set out to investigate a treatment plan combining 1,550-nm NAFL with low-dose isotretinoin (10 mg/day) in the 18 patients (mean age, 24 years; skin types II-IV) attending their outpatient dermatology clinic. Three laser treatments were administered at monthly intervals to one side of the face, with the other side of the face serving as a control. Each patient was on low-dose isotretinoin for 30-45 days before laser treatment. A revised Leeds acne-grading system was used.
At follow-up after the third treatment – 3 months after the first laser treatment – both sides of the face showed significant recovery in all participants, but there was greater improvement on the laser-treated side. The mean Leeds acne-grading scores decreased from 10.6 at baseline to 5.8 on the control side of the face, and from 10.4 at baseline to 3.5 on the laser-treated side. The changes in scores differed significantly between sides (P less than .05), and the number of comedones decreased more on the laser-treated side of the face than it did the control side.
Significant improvements were also seen with superficial scars (P less than .05) and deep boxcar atrophic scars (P less than .01) on the laser-treated sides of patients’ faces, compared with the control sides, but significant improvements were not seen with the number of papules and nodules or with icepick or rolling scars.
Patients reported discomfort after NAFL treatment, including pain (100%), sensation of heat (100%), erythema (94.5%), and edema (88.9%), which resolved spontaneously within 24 hours.
Most of the patients (n = 12; 66.7%) were satisfied after the last treatment, two (11.1%) were “very satisfied,” and four (22.2%) were neutral; none were dissatisfied.
“Low-dose isotretinoin effectively controlled papule and pustule acne lesions, whereas use of 1,550-nm Er:glass NAFL may significantly reduce the number of comedones and improve boxcar atrophic scars,” the authors wrote.
They authors reported no significant interest with commercial supporters.
SOURCE: Xia J et al. Dermatol Surg. 2018 Sep;44(9):1201-8.
A combination of low-dose isotretinoin and nonablative fractional laser (NAFL) was a safe and effective treatment for moderate to severe acne and also improved acne scars in a small Chinese study, investigators reported.
In the randomized, split-face, controlled study of 18 adult Asian patients with moderate to severe acne, low-dose isotretinoin alone effectively controlled papule and pustule acne lesions, whereas NAFL had the additional effect of reducing the number of comedones and improving boxcar atrophic scars, reported Weihui Zeng, MD, and associates from the department of dermatology at the Second Affiliated Hospital of Xi’an Jiaotong University, Shanxi, China.
The authors noted that many patients seen at their clinic cannot tolerate a 20 mg/day dose of isotretinoin because of severe mucocutaneous side effects and that treatment with nonablative lasers, which – in contrast to ablative lasers – use infrared radiation to penetrate the skin deeply and thereby selectively heat dermal tissue while sparing the epidermis, could be a treatment option for these patients.
Therefore, they set out to investigate a treatment plan combining 1,550-nm NAFL with low-dose isotretinoin (10 mg/day) in the 18 patients (mean age, 24 years; skin types II-IV) attending their outpatient dermatology clinic. Three laser treatments were administered at monthly intervals to one side of the face, with the other side of the face serving as a control. Each patient was on low-dose isotretinoin for 30-45 days before laser treatment. A revised Leeds acne-grading system was used.
At follow-up after the third treatment – 3 months after the first laser treatment – both sides of the face showed significant recovery in all participants, but there was greater improvement on the laser-treated side. The mean Leeds acne-grading scores decreased from 10.6 at baseline to 5.8 on the control side of the face, and from 10.4 at baseline to 3.5 on the laser-treated side. The changes in scores differed significantly between sides (P less than .05), and the number of comedones decreased more on the laser-treated side of the face than it did the control side.
Significant improvements were also seen with superficial scars (P less than .05) and deep boxcar atrophic scars (P less than .01) on the laser-treated sides of patients’ faces, compared with the control sides, but significant improvements were not seen with the number of papules and nodules or with icepick or rolling scars.
Patients reported discomfort after NAFL treatment, including pain (100%), sensation of heat (100%), erythema (94.5%), and edema (88.9%), which resolved spontaneously within 24 hours.
Most of the patients (n = 12; 66.7%) were satisfied after the last treatment, two (11.1%) were “very satisfied,” and four (22.2%) were neutral; none were dissatisfied.
“Low-dose isotretinoin effectively controlled papule and pustule acne lesions, whereas use of 1,550-nm Er:glass NAFL may significantly reduce the number of comedones and improve boxcar atrophic scars,” the authors wrote.
They authors reported no significant interest with commercial supporters.
SOURCE: Xia J et al. Dermatol Surg. 2018 Sep;44(9):1201-8.
A combination of low-dose isotretinoin and nonablative fractional laser (NAFL) was a safe and effective treatment for moderate to severe acne and also improved acne scars in a small Chinese study, investigators reported.
In the randomized, split-face, controlled study of 18 adult Asian patients with moderate to severe acne, low-dose isotretinoin alone effectively controlled papule and pustule acne lesions, whereas NAFL had the additional effect of reducing the number of comedones and improving boxcar atrophic scars, reported Weihui Zeng, MD, and associates from the department of dermatology at the Second Affiliated Hospital of Xi’an Jiaotong University, Shanxi, China.
The authors noted that many patients seen at their clinic cannot tolerate a 20 mg/day dose of isotretinoin because of severe mucocutaneous side effects and that treatment with nonablative lasers, which – in contrast to ablative lasers – use infrared radiation to penetrate the skin deeply and thereby selectively heat dermal tissue while sparing the epidermis, could be a treatment option for these patients.
Therefore, they set out to investigate a treatment plan combining 1,550-nm NAFL with low-dose isotretinoin (10 mg/day) in the 18 patients (mean age, 24 years; skin types II-IV) attending their outpatient dermatology clinic. Three laser treatments were administered at monthly intervals to one side of the face, with the other side of the face serving as a control. Each patient was on low-dose isotretinoin for 30-45 days before laser treatment. A revised Leeds acne-grading system was used.
At follow-up after the third treatment – 3 months after the first laser treatment – both sides of the face showed significant recovery in all participants, but there was greater improvement on the laser-treated side. The mean Leeds acne-grading scores decreased from 10.6 at baseline to 5.8 on the control side of the face, and from 10.4 at baseline to 3.5 on the laser-treated side. The changes in scores differed significantly between sides (P less than .05), and the number of comedones decreased more on the laser-treated side of the face than it did the control side.
Significant improvements were also seen with superficial scars (P less than .05) and deep boxcar atrophic scars (P less than .01) on the laser-treated sides of patients’ faces, compared with the control sides, but significant improvements were not seen with the number of papules and nodules or with icepick or rolling scars.
Patients reported discomfort after NAFL treatment, including pain (100%), sensation of heat (100%), erythema (94.5%), and edema (88.9%), which resolved spontaneously within 24 hours.
Most of the patients (n = 12; 66.7%) were satisfied after the last treatment, two (11.1%) were “very satisfied,” and four (22.2%) were neutral; none were dissatisfied.
“Low-dose isotretinoin effectively controlled papule and pustule acne lesions, whereas use of 1,550-nm Er:glass NAFL may significantly reduce the number of comedones and improve boxcar atrophic scars,” the authors wrote.
They authors reported no significant interest with commercial supporters.
SOURCE: Xia J et al. Dermatol Surg. 2018 Sep;44(9):1201-8.
FROM DERMATOLOGIC SURGERY
Key clinical point: Adding nonablative fractional laser (NAFL) treatment to low-dose isotretinoin may reduce comedones and improve boxcar atrophic scarring in people with moderate to severe acne .
Major finding: Low-dose isotretinoin effectively controlled papule and pustule acne lesions, whereas nonablative laser also reduced the number of comedones and improved boxcar atrophic scars.
Study details: A prospective randomized, controlled, split-face study of 18 Asian adult patients with moderate to severe acne vulgaris, treated with low-dose isotretinoin, as well as NAFL to one side of the face.
Disclosures: The authors reported no significant interests with commercial supporters.
Source: Xia J et al. Dermatol Surg. 2018 Sep;44(9):1201-8.
What is the Diagnosis - September 2018
At the visit, the girl’s skin scrapings were analyzed under the microscope with potassium hydroxide (KOH) and no fungal elements were seen. A culture from one of the lesions was positive for methicillin-sensitive Staphylococcus aureus.
She was diagnosed with bullous impetigo (BI).
Impetigo is the most common superficial skin infection and can present as a nonbullous (most common) and bullous (least common) form.1 Nonbullous impetigo is usually caused the Staphylococcus aureus or Streptococcus pyogenes and tends to occur at sites of prior trauma like insect bites, scratches, atopic dermatitis, or varicella. On the other hand, bullous impetigo is caused by the local production of exfoliative toxins (ETA or ETB) by phage group II of Staphylococcus aureus. The exfoliative toxin binds to desmoglin-1, one of the desmosomal proteins of the skin, causing acantholysis at the level of the granular layer and blister formation. Different from nonbullous impetigo, bullous impetigo tends to occur in normal, undamaged skin. Lesions are more common in neonates and young infants but children also can be affected.
The characteristic lesions in bullous impetigo are small blisters that enlarge to 1-cm to 5-cm bullae that easily rupture, leaving an erythematous plaque with a collarette of scale or “double ring scale,” with minimal crust and mild erythema. They commonly occur on the face, trunk, buttocks, and intertriginous areas. The lesions heal within 4-6 weeks, leaving no scarring. Associated systemic symptoms are rare but some patients can present with weakness, fever, and diarrhea. The toxin can disseminate and cause staphylococcal scalded skin syndrome in neonates or older patients with renal failure or immunodeficiency.
The transmission of Staphylococcus aureus can occur from colonized or infected family members, children in contact sports, as well as contact with animals such as dogs, cattle, and poultry.2 Transmission from a pet rabbit also has been reported. In our patient, transmission from her pet hamster could have occurred as the areas on the body where there were lesions were areas where she was holding and cuddling her new pet.
The differential diagnosis of the type of lesions our patient presented with includes tinea corporis, and bullous tinea, which also can be transmitted by animals such as kittens. A KOH analysis ruled out this diagnosis. Tinea skin lesions tend to be more scaly than bullous impetigo lesions, which are more inflamed and crusted. Bullous arthropod reactions should be considered in the differential diagnosis as well. Bullous bite reaction lesions present with tense bullae, as they are subepidermal in nature and are pruritic. Subacute cutaneous lupus lesions present as annular scaly plaques with an erythematous border and central clearing usually in sun exposed areas similar to the distribution of our patient. Severe contact dermatitis reactions also can blister and form similar lesions as seen in our patient but with the difference that our patient didn’t complain of pruritus, which is a characteristic feature of allergic contact dermatitis. In neonates or young infants with bullous lesions other conditions such as herpes simplex infection, epidermolysis bullosa, bullous pemphigoid, linear IgA bullous dermatosis, bullous mastocytosis, and bullous erythema multiforme should be considered in the differential diagnosis.
First line treatment for impetigo consists of the use of topical application of mupirocin (Bactroban) 2% ointment, retapamulin (Altabax) 1% ointment, or fusidic acid 2% cream. A Cochrane review compared systemic versus topical treatment for impetigo concluding that topical treatment with either mupirocin or retapamulin is equally if not more effective than oral antibiotics.3 Ozenoxacin (Xepi), a new nonfluorinated topical quinolone has recently been Food and Drug Administration approved for the treatment of localized impetigo in patients 2 months of age and older.4 When there is treatment failure with topical antibiotics, widespread disease, or systemic symptoms, oral antimicrobials should be consider, such as beta-lactamase–resistant penicillin, first-generation cephalosporins, or clindamycin. The use of bleach baths and general hygiene measures for 4 months can reduce the risks of recurrence in 20% of the patients as noted by a study by Kaplan et al.5
Our patient was treated with oral cephalexin for 7 days as well as topical mupirocin with fast resolution of the lesions. Sadly, the parents gave her hamster pet away.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. Email her at pdnews@mdedge.com
References
1. Am Fam Physician. 2014 Aug 15;90(4):229-35.
2. Zentralbl Bakteriol Mikrobiol Hyg A. 1987 Jun;265(1-2):218-26.
3. Cochrane Database Syst Rev. 2012 Jan 18;1:CD003261.
4. Ann Pharmacother. 2018 Jun 1:1060028018786510.
5. Clin Infect Dis. 2014 Mar;58(5):679-82.
At the visit, the girl’s skin scrapings were analyzed under the microscope with potassium hydroxide (KOH) and no fungal elements were seen. A culture from one of the lesions was positive for methicillin-sensitive Staphylococcus aureus.
She was diagnosed with bullous impetigo (BI).
Impetigo is the most common superficial skin infection and can present as a nonbullous (most common) and bullous (least common) form.1 Nonbullous impetigo is usually caused the Staphylococcus aureus or Streptococcus pyogenes and tends to occur at sites of prior trauma like insect bites, scratches, atopic dermatitis, or varicella. On the other hand, bullous impetigo is caused by the local production of exfoliative toxins (ETA or ETB) by phage group II of Staphylococcus aureus. The exfoliative toxin binds to desmoglin-1, one of the desmosomal proteins of the skin, causing acantholysis at the level of the granular layer and blister formation. Different from nonbullous impetigo, bullous impetigo tends to occur in normal, undamaged skin. Lesions are more common in neonates and young infants but children also can be affected.
The characteristic lesions in bullous impetigo are small blisters that enlarge to 1-cm to 5-cm bullae that easily rupture, leaving an erythematous plaque with a collarette of scale or “double ring scale,” with minimal crust and mild erythema. They commonly occur on the face, trunk, buttocks, and intertriginous areas. The lesions heal within 4-6 weeks, leaving no scarring. Associated systemic symptoms are rare but some patients can present with weakness, fever, and diarrhea. The toxin can disseminate and cause staphylococcal scalded skin syndrome in neonates or older patients with renal failure or immunodeficiency.
The transmission of Staphylococcus aureus can occur from colonized or infected family members, children in contact sports, as well as contact with animals such as dogs, cattle, and poultry.2 Transmission from a pet rabbit also has been reported. In our patient, transmission from her pet hamster could have occurred as the areas on the body where there were lesions were areas where she was holding and cuddling her new pet.
The differential diagnosis of the type of lesions our patient presented with includes tinea corporis, and bullous tinea, which also can be transmitted by animals such as kittens. A KOH analysis ruled out this diagnosis. Tinea skin lesions tend to be more scaly than bullous impetigo lesions, which are more inflamed and crusted. Bullous arthropod reactions should be considered in the differential diagnosis as well. Bullous bite reaction lesions present with tense bullae, as they are subepidermal in nature and are pruritic. Subacute cutaneous lupus lesions present as annular scaly plaques with an erythematous border and central clearing usually in sun exposed areas similar to the distribution of our patient. Severe contact dermatitis reactions also can blister and form similar lesions as seen in our patient but with the difference that our patient didn’t complain of pruritus, which is a characteristic feature of allergic contact dermatitis. In neonates or young infants with bullous lesions other conditions such as herpes simplex infection, epidermolysis bullosa, bullous pemphigoid, linear IgA bullous dermatosis, bullous mastocytosis, and bullous erythema multiforme should be considered in the differential diagnosis.
First line treatment for impetigo consists of the use of topical application of mupirocin (Bactroban) 2% ointment, retapamulin (Altabax) 1% ointment, or fusidic acid 2% cream. A Cochrane review compared systemic versus topical treatment for impetigo concluding that topical treatment with either mupirocin or retapamulin is equally if not more effective than oral antibiotics.3 Ozenoxacin (Xepi), a new nonfluorinated topical quinolone has recently been Food and Drug Administration approved for the treatment of localized impetigo in patients 2 months of age and older.4 When there is treatment failure with topical antibiotics, widespread disease, or systemic symptoms, oral antimicrobials should be consider, such as beta-lactamase–resistant penicillin, first-generation cephalosporins, or clindamycin. The use of bleach baths and general hygiene measures for 4 months can reduce the risks of recurrence in 20% of the patients as noted by a study by Kaplan et al.5
Our patient was treated with oral cephalexin for 7 days as well as topical mupirocin with fast resolution of the lesions. Sadly, the parents gave her hamster pet away.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. Email her at pdnews@mdedge.com
References
1. Am Fam Physician. 2014 Aug 15;90(4):229-35.
2. Zentralbl Bakteriol Mikrobiol Hyg A. 1987 Jun;265(1-2):218-26.
3. Cochrane Database Syst Rev. 2012 Jan 18;1:CD003261.
4. Ann Pharmacother. 2018 Jun 1:1060028018786510.
5. Clin Infect Dis. 2014 Mar;58(5):679-82.
At the visit, the girl’s skin scrapings were analyzed under the microscope with potassium hydroxide (KOH) and no fungal elements were seen. A culture from one of the lesions was positive for methicillin-sensitive Staphylococcus aureus.
She was diagnosed with bullous impetigo (BI).
Impetigo is the most common superficial skin infection and can present as a nonbullous (most common) and bullous (least common) form.1 Nonbullous impetigo is usually caused the Staphylococcus aureus or Streptococcus pyogenes and tends to occur at sites of prior trauma like insect bites, scratches, atopic dermatitis, or varicella. On the other hand, bullous impetigo is caused by the local production of exfoliative toxins (ETA or ETB) by phage group II of Staphylococcus aureus. The exfoliative toxin binds to desmoglin-1, one of the desmosomal proteins of the skin, causing acantholysis at the level of the granular layer and blister formation. Different from nonbullous impetigo, bullous impetigo tends to occur in normal, undamaged skin. Lesions are more common in neonates and young infants but children also can be affected.
The characteristic lesions in bullous impetigo are small blisters that enlarge to 1-cm to 5-cm bullae that easily rupture, leaving an erythematous plaque with a collarette of scale or “double ring scale,” with minimal crust and mild erythema. They commonly occur on the face, trunk, buttocks, and intertriginous areas. The lesions heal within 4-6 weeks, leaving no scarring. Associated systemic symptoms are rare but some patients can present with weakness, fever, and diarrhea. The toxin can disseminate and cause staphylococcal scalded skin syndrome in neonates or older patients with renal failure or immunodeficiency.
The transmission of Staphylococcus aureus can occur from colonized or infected family members, children in contact sports, as well as contact with animals such as dogs, cattle, and poultry.2 Transmission from a pet rabbit also has been reported. In our patient, transmission from her pet hamster could have occurred as the areas on the body where there were lesions were areas where she was holding and cuddling her new pet.
The differential diagnosis of the type of lesions our patient presented with includes tinea corporis, and bullous tinea, which also can be transmitted by animals such as kittens. A KOH analysis ruled out this diagnosis. Tinea skin lesions tend to be more scaly than bullous impetigo lesions, which are more inflamed and crusted. Bullous arthropod reactions should be considered in the differential diagnosis as well. Bullous bite reaction lesions present with tense bullae, as they are subepidermal in nature and are pruritic. Subacute cutaneous lupus lesions present as annular scaly plaques with an erythematous border and central clearing usually in sun exposed areas similar to the distribution of our patient. Severe contact dermatitis reactions also can blister and form similar lesions as seen in our patient but with the difference that our patient didn’t complain of pruritus, which is a characteristic feature of allergic contact dermatitis. In neonates or young infants with bullous lesions other conditions such as herpes simplex infection, epidermolysis bullosa, bullous pemphigoid, linear IgA bullous dermatosis, bullous mastocytosis, and bullous erythema multiforme should be considered in the differential diagnosis.
First line treatment for impetigo consists of the use of topical application of mupirocin (Bactroban) 2% ointment, retapamulin (Altabax) 1% ointment, or fusidic acid 2% cream. A Cochrane review compared systemic versus topical treatment for impetigo concluding that topical treatment with either mupirocin or retapamulin is equally if not more effective than oral antibiotics.3 Ozenoxacin (Xepi), a new nonfluorinated topical quinolone has recently been Food and Drug Administration approved for the treatment of localized impetigo in patients 2 months of age and older.4 When there is treatment failure with topical antibiotics, widespread disease, or systemic symptoms, oral antimicrobials should be consider, such as beta-lactamase–resistant penicillin, first-generation cephalosporins, or clindamycin. The use of bleach baths and general hygiene measures for 4 months can reduce the risks of recurrence in 20% of the patients as noted by a study by Kaplan et al.5
Our patient was treated with oral cephalexin for 7 days as well as topical mupirocin with fast resolution of the lesions. Sadly, the parents gave her hamster pet away.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. Email her at pdnews@mdedge.com
References
1. Am Fam Physician. 2014 Aug 15;90(4):229-35.
2. Zentralbl Bakteriol Mikrobiol Hyg A. 1987 Jun;265(1-2):218-26.
3. Cochrane Database Syst Rev. 2012 Jan 18;1:CD003261.
4. Ann Pharmacother. 2018 Jun 1:1060028018786510.
5. Clin Infect Dis. 2014 Mar;58(5):679-82.
On physical exam, the girl is in no acute distress. Her vital signs are stable, and she has no fever.
On skin examination, she has several erythematous, crusted scaly plaques with double ring of scale on the nose, ears, neck, upper chest, and few on the abdomen. On her left abdomen, there is a small blister. Her seborrheic dermatitis is well controlled with mild erythema behind her ears and minimal scale on her scalp.
Teva Announces FDA Approval of Ajovy (fremanezumab-vfrm)
Teva Pharmaceutical Industries Ltd. announced that the FDA approved Ajovy (fremanezumab-vfrm) injection for the preventive treatment of migraine in adults. Ajovy, a humanized monoclonal antibody that binds to the calcitonin gene-related peptide (CGRP) ligand and blocks its binding to the receptor, is the first and only anti-CGRP treatment for the prevention of migraine with quarterly (675 mg) and monthly (225 mg) dosing options.
“Migraine is a disabling neurological disease that affects more than 36 million people in the United States,” said Stephen Silberstein
Ajovy was evaluated in two Phase III, placebo-controlled clinical trials that enrolled patients with disabling migraine and was studied as both a stand-alone preventive treatment and in combination with oral preventive treatments. In these trials, patients experienced a reduction in monthly migraine days during a 12-week period. The most common adverse reactions (≥ 5% and greater than placebo) were injection site reactions.
Teva Pharmaceutical Industries Ltd. announced that the FDA approved Ajovy (fremanezumab-vfrm) injection for the preventive treatment of migraine in adults. Ajovy, a humanized monoclonal antibody that binds to the calcitonin gene-related peptide (CGRP) ligand and blocks its binding to the receptor, is the first and only anti-CGRP treatment for the prevention of migraine with quarterly (675 mg) and monthly (225 mg) dosing options.
“Migraine is a disabling neurological disease that affects more than 36 million people in the United States,” said Stephen Silberstein
Ajovy was evaluated in two Phase III, placebo-controlled clinical trials that enrolled patients with disabling migraine and was studied as both a stand-alone preventive treatment and in combination with oral preventive treatments. In these trials, patients experienced a reduction in monthly migraine days during a 12-week period. The most common adverse reactions (≥ 5% and greater than placebo) were injection site reactions.
Teva Pharmaceutical Industries Ltd. announced that the FDA approved Ajovy (fremanezumab-vfrm) injection for the preventive treatment of migraine in adults. Ajovy, a humanized monoclonal antibody that binds to the calcitonin gene-related peptide (CGRP) ligand and blocks its binding to the receptor, is the first and only anti-CGRP treatment for the prevention of migraine with quarterly (675 mg) and monthly (225 mg) dosing options.
“Migraine is a disabling neurological disease that affects more than 36 million people in the United States,” said Stephen Silberstein
Ajovy was evaluated in two Phase III, placebo-controlled clinical trials that enrolled patients with disabling migraine and was studied as both a stand-alone preventive treatment and in combination with oral preventive treatments. In these trials, patients experienced a reduction in monthly migraine days during a 12-week period. The most common adverse reactions (≥ 5% and greater than placebo) were injection site reactions.