User login
Pediatric data on novel axillary hyperhidrosis treatment reported
PARIS – Two compelling reasons exist to take excessive sweating in children and adolescents more seriously, Lawrence J. Green, MD, asserted at the annual congress of the European Academy of Dermatology and Venereology.
One is that this is a surprisingly common and embarrassing medical condition that can have a profound adverse developmental impact in young people at a time when they are engaged in forming their self-image.
The other reason to get serious about addressing primary axillary hyperhidrosis in pediatric patients is the recent approval of glycopyrronium tosylate as a topical therapy, Dr. Green, a dermatologist at George Washington University, Washington. The treatment, glycopyrronium pads (Qbrexza), was approved by the Food and Drug Administration for the topical treatment of primary axillary hyperhidrosis in patients aged 9 years and older in June 2018, and will be available in October 2018.
He presented new data from a 44-week, open-label extension of two pivotal 4-week, phase 3, randomized, double-blind, placebo-controlled trials known as ATMOS-1 and ATMOS-2. The new post hoc analysis from the extension study, known as the ARIDO study, provides reassurance that the product remains both safe and durably effective with longterm use.
Dr. Green’s analysis focused on the 44 pediatric participants aged 9-16 years. That’s because even though primary axillary hyperhidrosis affects people of all ages, with an estimated 4.8% prevalence in the U.S. population – 5.3 million people – it is more common in children and adolescents than adults. And it hits them particularly hard.
“Hyperhidrosis is largely underdiagnosed and undertreated, particularly among pediatric patients,” he said. “The impact on quality of life is comparable to or greater than acne, psoriasis, or eczema.”
The glycopyrronium pad is self-applied as a once-daily wipe. Glycopyrronium is an anticholinergic agent, which blocks sweat production by inhibiting the receptors that activate sweat glands.
Dr. Green noted several key findings from the 44-week ARIDO analysis, presented for the first time at the EADV congress.
The median absolute decrease in sweat production in pediatric patients at 44 weeks as measured gravimetrically was 50.3 mg per 5 minutes from a baseline of 150 mg per 5 minutes, comparable with the mean 75 mg reduction from a baseline of 175 mg in the 507-patient older cohort. However, Dr. Green advised not to make too much of this endpoint, as sweat production is notoriously difficult to measure accurately. In addition, an individual’s sweat rate can vary widely depending upon a multitude of factors, including ambient temperature and even what a patient is thinking about. The FDA recognizes this and therefore elevated several validated patient-reported outcomes to the status of coprimary endpoints in the clinical trials.
A positive result on one such patient-reported outcome, the Hyperhidrosis Severity Scale, was achieved in 57% of pediatric patients and 64% of adults at week 44 of open-label therapy. This required at least a 2-grade improvement from baseline, when roughly 60% of youths had a score of 3 and the remainder scored 4 on the 1-4 point scale.
From a mean baseline score of 9.2 on the Children’s Dermatology Life Quality Index, the pediatric group averaged a mean 6.2-point improvement at week 44, while adults experienced a mean 8.7-point improvement on the Dermatology Life Quality Index from a baseline of 11.25.
There was no diminution in treatment efficacy through 44 weeks, Dr. Green noted. Treatment-emergent adverse events consisted largely of transient mild to moderate anticholinergic effects, which seldom led to study discontinuation.
Dilated pupils and blurred vision were more common in children than adults (7.9% and 10.5% vs. 5.1% and 6.4%, respectively). “Why that is I can only speculate. Kids do tend to touch their eyes more often than adults. Pretty much everything else was the same. The adverse events can be worked around by educating people to use the pads appropriately. We saw the anticholinergic side effects more often in the first 4 weeks of the double-blind trials than in the longterm extension because once patients learned how to use the pad and not touch themselves afterwards, the adverse events came down,” he said.
The studies were sponsored by Dermira. Dr. Green has received research funding from and been a consultant to the company.
PARIS – Two compelling reasons exist to take excessive sweating in children and adolescents more seriously, Lawrence J. Green, MD, asserted at the annual congress of the European Academy of Dermatology and Venereology.
One is that this is a surprisingly common and embarrassing medical condition that can have a profound adverse developmental impact in young people at a time when they are engaged in forming their self-image.
The other reason to get serious about addressing primary axillary hyperhidrosis in pediatric patients is the recent approval of glycopyrronium tosylate as a topical therapy, Dr. Green, a dermatologist at George Washington University, Washington. The treatment, glycopyrronium pads (Qbrexza), was approved by the Food and Drug Administration for the topical treatment of primary axillary hyperhidrosis in patients aged 9 years and older in June 2018, and will be available in October 2018.
He presented new data from a 44-week, open-label extension of two pivotal 4-week, phase 3, randomized, double-blind, placebo-controlled trials known as ATMOS-1 and ATMOS-2. The new post hoc analysis from the extension study, known as the ARIDO study, provides reassurance that the product remains both safe and durably effective with longterm use.
Dr. Green’s analysis focused on the 44 pediatric participants aged 9-16 years. That’s because even though primary axillary hyperhidrosis affects people of all ages, with an estimated 4.8% prevalence in the U.S. population – 5.3 million people – it is more common in children and adolescents than adults. And it hits them particularly hard.
“Hyperhidrosis is largely underdiagnosed and undertreated, particularly among pediatric patients,” he said. “The impact on quality of life is comparable to or greater than acne, psoriasis, or eczema.”
The glycopyrronium pad is self-applied as a once-daily wipe. Glycopyrronium is an anticholinergic agent, which blocks sweat production by inhibiting the receptors that activate sweat glands.
Dr. Green noted several key findings from the 44-week ARIDO analysis, presented for the first time at the EADV congress.
The median absolute decrease in sweat production in pediatric patients at 44 weeks as measured gravimetrically was 50.3 mg per 5 minutes from a baseline of 150 mg per 5 minutes, comparable with the mean 75 mg reduction from a baseline of 175 mg in the 507-patient older cohort. However, Dr. Green advised not to make too much of this endpoint, as sweat production is notoriously difficult to measure accurately. In addition, an individual’s sweat rate can vary widely depending upon a multitude of factors, including ambient temperature and even what a patient is thinking about. The FDA recognizes this and therefore elevated several validated patient-reported outcomes to the status of coprimary endpoints in the clinical trials.
A positive result on one such patient-reported outcome, the Hyperhidrosis Severity Scale, was achieved in 57% of pediatric patients and 64% of adults at week 44 of open-label therapy. This required at least a 2-grade improvement from baseline, when roughly 60% of youths had a score of 3 and the remainder scored 4 on the 1-4 point scale.
From a mean baseline score of 9.2 on the Children’s Dermatology Life Quality Index, the pediatric group averaged a mean 6.2-point improvement at week 44, while adults experienced a mean 8.7-point improvement on the Dermatology Life Quality Index from a baseline of 11.25.
There was no diminution in treatment efficacy through 44 weeks, Dr. Green noted. Treatment-emergent adverse events consisted largely of transient mild to moderate anticholinergic effects, which seldom led to study discontinuation.
Dilated pupils and blurred vision were more common in children than adults (7.9% and 10.5% vs. 5.1% and 6.4%, respectively). “Why that is I can only speculate. Kids do tend to touch their eyes more often than adults. Pretty much everything else was the same. The adverse events can be worked around by educating people to use the pads appropriately. We saw the anticholinergic side effects more often in the first 4 weeks of the double-blind trials than in the longterm extension because once patients learned how to use the pad and not touch themselves afterwards, the adverse events came down,” he said.
The studies were sponsored by Dermira. Dr. Green has received research funding from and been a consultant to the company.
PARIS – Two compelling reasons exist to take excessive sweating in children and adolescents more seriously, Lawrence J. Green, MD, asserted at the annual congress of the European Academy of Dermatology and Venereology.
One is that this is a surprisingly common and embarrassing medical condition that can have a profound adverse developmental impact in young people at a time when they are engaged in forming their self-image.
The other reason to get serious about addressing primary axillary hyperhidrosis in pediatric patients is the recent approval of glycopyrronium tosylate as a topical therapy, Dr. Green, a dermatologist at George Washington University, Washington. The treatment, glycopyrronium pads (Qbrexza), was approved by the Food and Drug Administration for the topical treatment of primary axillary hyperhidrosis in patients aged 9 years and older in June 2018, and will be available in October 2018.
He presented new data from a 44-week, open-label extension of two pivotal 4-week, phase 3, randomized, double-blind, placebo-controlled trials known as ATMOS-1 and ATMOS-2. The new post hoc analysis from the extension study, known as the ARIDO study, provides reassurance that the product remains both safe and durably effective with longterm use.
Dr. Green’s analysis focused on the 44 pediatric participants aged 9-16 years. That’s because even though primary axillary hyperhidrosis affects people of all ages, with an estimated 4.8% prevalence in the U.S. population – 5.3 million people – it is more common in children and adolescents than adults. And it hits them particularly hard.
“Hyperhidrosis is largely underdiagnosed and undertreated, particularly among pediatric patients,” he said. “The impact on quality of life is comparable to or greater than acne, psoriasis, or eczema.”
The glycopyrronium pad is self-applied as a once-daily wipe. Glycopyrronium is an anticholinergic agent, which blocks sweat production by inhibiting the receptors that activate sweat glands.
Dr. Green noted several key findings from the 44-week ARIDO analysis, presented for the first time at the EADV congress.
The median absolute decrease in sweat production in pediatric patients at 44 weeks as measured gravimetrically was 50.3 mg per 5 minutes from a baseline of 150 mg per 5 minutes, comparable with the mean 75 mg reduction from a baseline of 175 mg in the 507-patient older cohort. However, Dr. Green advised not to make too much of this endpoint, as sweat production is notoriously difficult to measure accurately. In addition, an individual’s sweat rate can vary widely depending upon a multitude of factors, including ambient temperature and even what a patient is thinking about. The FDA recognizes this and therefore elevated several validated patient-reported outcomes to the status of coprimary endpoints in the clinical trials.
A positive result on one such patient-reported outcome, the Hyperhidrosis Severity Scale, was achieved in 57% of pediatric patients and 64% of adults at week 44 of open-label therapy. This required at least a 2-grade improvement from baseline, when roughly 60% of youths had a score of 3 and the remainder scored 4 on the 1-4 point scale.
From a mean baseline score of 9.2 on the Children’s Dermatology Life Quality Index, the pediatric group averaged a mean 6.2-point improvement at week 44, while adults experienced a mean 8.7-point improvement on the Dermatology Life Quality Index from a baseline of 11.25.
There was no diminution in treatment efficacy through 44 weeks, Dr. Green noted. Treatment-emergent adverse events consisted largely of transient mild to moderate anticholinergic effects, which seldom led to study discontinuation.
Dilated pupils and blurred vision were more common in children than adults (7.9% and 10.5% vs. 5.1% and 6.4%, respectively). “Why that is I can only speculate. Kids do tend to touch their eyes more often than adults. Pretty much everything else was the same. The adverse events can be worked around by educating people to use the pads appropriately. We saw the anticholinergic side effects more often in the first 4 weeks of the double-blind trials than in the longterm extension because once patients learned how to use the pad and not touch themselves afterwards, the adverse events came down,” he said.
The studies were sponsored by Dermira. Dr. Green has received research funding from and been a consultant to the company.
REPORTING FROM THE EADV CONGRESS
Key clinical point: Glycopyrronium tosylate pads address a common and undertreated medical condition in children: primary axillary hyperhidrosis.
Major finding: Mean scores on the Children’s Dermatology Life Quality Index improved by an average of 6.2 points from a baseline of 9.2 in children aged 9-16 years with primary axillary hyperhidrosis treated with once-daily glycopyrronium tosylate pads during an open-label, 44-week study.
Study details: This was a post hoc analysis of 44 patients aged 9-16 years and 507 patients aged 17 years and older who participated in a 44-week, open-label extension study of once-daily glycopyrronium tosylate pads for treatment of primary axillary hyperhidrosis.
Disclosures: The study was sponsored by Dermira. The presenter has received research funding from and been a consultant to the company.
Congenital syphilis rates continue skyrocketing alongside other STDs
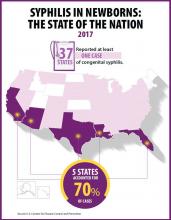
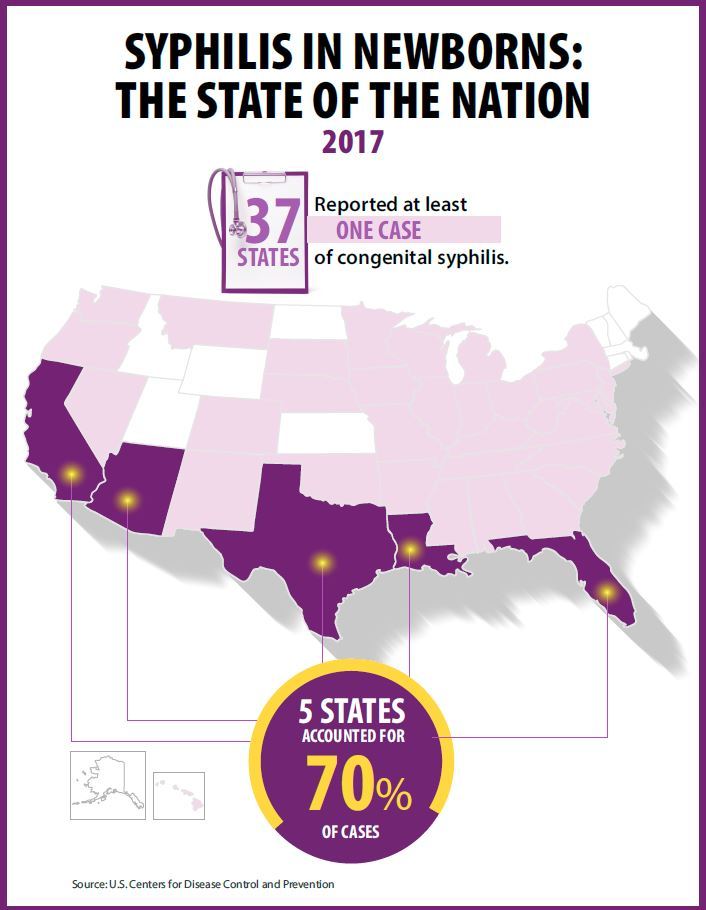
Rapidly increasing cases of newborn syphilis have reached their highest prevalence in 2 decades, according to a new report by the Centers for Disease Control and Prevention on sexually transmitted disease surveillance in 2017.
Newborn syphilis incidence has more than doubled, from 362 cases in 2013 to 918 cases in 2017, resulting in 64 syphilitic stillbirths and 13 infant deaths that year, according to data published in Sexually Transmitted Disease Surveillance 2017.
At least one case was reported in 37 states last year, and the greatest burden of cases occurred in California, Arizona, Texas, Louisiana, and Florida, together accounting for 70% of all 2017 cases.
“The resurgence of syphilis, and particularly congenital syphilis, is not an arbitrary event, but rather a symptom of a deteriorating public health infrastructure and lack of access to health care,” wrote Gail Bolan, MD, director of the Division of STD Prevention at the CDC’s National Center for HIV/AIDS, Viral Hepatitis, STD and TB Prevention. “It is exposing hidden, fragile populations in need that are not getting the health care and preventive services they deserve.”
Dr. Bolan recommends modernizing surveillance to capture more of the cases in populations without ready access to diagnosis and treatment and in those choosing not to access care.
“It is imperative that federal, state, and local programs employ strategies that maximize long-term population impact by reducing STD incidence and promoting sexual, reproductive, maternal, and infant health,” she wrote. “Further, it will be important for us to measure and monitor the adverse health consequences of STDs, such as ocular and neurosyphilis, pelvic inflammatory disease, ectopic pregnancy, infertility, HIV, congenital syphilis, and neonatal herpes.”
Multiple sources contributed data to the report: state and local STD programs’ notifiable disease reporting, private and federal national surveys, and specific projects that collect STD prevalence data, including the National Job Training Program, the STD Surveillance Network and the Gonococcal Isolate Surveillance Project.
The four nationally notifiable STDs are chlamydia, gonorrhea, syphilis, and chancroid.
The rise in newborn syphilis cases, currently at 23.3 cases per 100,000 live births, mirrors the increased U.S. prevalence of both primary and secondary syphilis in 2017, with 9.5 cases per 100,000 people. Syphilis has increased every year since 2000-2001, when prevalence was at a record low.
Chlamydia and gonorrhea rates climb too
The report also noted increases in the prevalence of other STDs. Chlamydia, the most common STD, increased 6.9% as compared to 2016, with 528.8 cases per 100,000 people. This increase occurred in all U.S. regions and independently of sex, race, or ethnicity, though rates were highest in teens and young adults. Nearly two-thirds of chlamydia cases in 2017 occurred in people ages 15-24 years old.
Reported rates were higher in women than in men, likely due to women’s increased likelihood of undergoing screening, the report suggested. Better surveillance may also partly explain the climb in men’s cases.
“Increases in rates among men may reflect an increased number of men, including gay, bisexual and other men who have sex with men (collectively referred to as MSM) being tested and diagnosed with a chlamydial infection due to increased availability of urine testing and extragenital screening,” according to the report.
The CDC received reports of more than a half million gonorrhea infections in 2017 (555,608 cases), an increase of 18.6% since the previous year, including a 19.3% increase among men and a 17.8% increase among women.
“The magnitude of the increase among men suggests either increased transmission, increased case ascertainment (e.g., through increased extra-genital screening among MSM), or both,” the authors wrote. “The concurrent increase in cases reported among women suggests parallel increases in heterosexual transmission, increased screening among women, or both.”
Overall, gonorrhea cases have skyrocketed 75.2% since their historic low in 2009, compounding the problem of antibiotic resistance that has limited CDC-recommended treatment to just ceftriaxone and azithromycin.
The report was supported by the Centers for Disease Control and Prevention. The authors did not report having any disclosures.
SOURCE: Centers for Disease Control and Prevention. Sexually Transmitted Disease Surveillance 2017; https://www.cdc.gov/std/stats
Rapidly increasing cases of newborn syphilis have reached their highest prevalence in 2 decades, according to a new report by the Centers for Disease Control and Prevention on sexually transmitted disease surveillance in 2017.
Newborn syphilis incidence has more than doubled, from 362 cases in 2013 to 918 cases in 2017, resulting in 64 syphilitic stillbirths and 13 infant deaths that year, according to data published in Sexually Transmitted Disease Surveillance 2017.
At least one case was reported in 37 states last year, and the greatest burden of cases occurred in California, Arizona, Texas, Louisiana, and Florida, together accounting for 70% of all 2017 cases.
“The resurgence of syphilis, and particularly congenital syphilis, is not an arbitrary event, but rather a symptom of a deteriorating public health infrastructure and lack of access to health care,” wrote Gail Bolan, MD, director of the Division of STD Prevention at the CDC’s National Center for HIV/AIDS, Viral Hepatitis, STD and TB Prevention. “It is exposing hidden, fragile populations in need that are not getting the health care and preventive services they deserve.”
Dr. Bolan recommends modernizing surveillance to capture more of the cases in populations without ready access to diagnosis and treatment and in those choosing not to access care.
“It is imperative that federal, state, and local programs employ strategies that maximize long-term population impact by reducing STD incidence and promoting sexual, reproductive, maternal, and infant health,” she wrote. “Further, it will be important for us to measure and monitor the adverse health consequences of STDs, such as ocular and neurosyphilis, pelvic inflammatory disease, ectopic pregnancy, infertility, HIV, congenital syphilis, and neonatal herpes.”
Multiple sources contributed data to the report: state and local STD programs’ notifiable disease reporting, private and federal national surveys, and specific projects that collect STD prevalence data, including the National Job Training Program, the STD Surveillance Network and the Gonococcal Isolate Surveillance Project.
The four nationally notifiable STDs are chlamydia, gonorrhea, syphilis, and chancroid.
The rise in newborn syphilis cases, currently at 23.3 cases per 100,000 live births, mirrors the increased U.S. prevalence of both primary and secondary syphilis in 2017, with 9.5 cases per 100,000 people. Syphilis has increased every year since 2000-2001, when prevalence was at a record low.
Chlamydia and gonorrhea rates climb too
The report also noted increases in the prevalence of other STDs. Chlamydia, the most common STD, increased 6.9% as compared to 2016, with 528.8 cases per 100,000 people. This increase occurred in all U.S. regions and independently of sex, race, or ethnicity, though rates were highest in teens and young adults. Nearly two-thirds of chlamydia cases in 2017 occurred in people ages 15-24 years old.
Reported rates were higher in women than in men, likely due to women’s increased likelihood of undergoing screening, the report suggested. Better surveillance may also partly explain the climb in men’s cases.
“Increases in rates among men may reflect an increased number of men, including gay, bisexual and other men who have sex with men (collectively referred to as MSM) being tested and diagnosed with a chlamydial infection due to increased availability of urine testing and extragenital screening,” according to the report.
The CDC received reports of more than a half million gonorrhea infections in 2017 (555,608 cases), an increase of 18.6% since the previous year, including a 19.3% increase among men and a 17.8% increase among women.
“The magnitude of the increase among men suggests either increased transmission, increased case ascertainment (e.g., through increased extra-genital screening among MSM), or both,” the authors wrote. “The concurrent increase in cases reported among women suggests parallel increases in heterosexual transmission, increased screening among women, or both.”
Overall, gonorrhea cases have skyrocketed 75.2% since their historic low in 2009, compounding the problem of antibiotic resistance that has limited CDC-recommended treatment to just ceftriaxone and azithromycin.
The report was supported by the Centers for Disease Control and Prevention. The authors did not report having any disclosures.
SOURCE: Centers for Disease Control and Prevention. Sexually Transmitted Disease Surveillance 2017; https://www.cdc.gov/std/stats
Rapidly increasing cases of newborn syphilis have reached their highest prevalence in 2 decades, according to a new report by the Centers for Disease Control and Prevention on sexually transmitted disease surveillance in 2017.
Newborn syphilis incidence has more than doubled, from 362 cases in 2013 to 918 cases in 2017, resulting in 64 syphilitic stillbirths and 13 infant deaths that year, according to data published in Sexually Transmitted Disease Surveillance 2017.
At least one case was reported in 37 states last year, and the greatest burden of cases occurred in California, Arizona, Texas, Louisiana, and Florida, together accounting for 70% of all 2017 cases.
“The resurgence of syphilis, and particularly congenital syphilis, is not an arbitrary event, but rather a symptom of a deteriorating public health infrastructure and lack of access to health care,” wrote Gail Bolan, MD, director of the Division of STD Prevention at the CDC’s National Center for HIV/AIDS, Viral Hepatitis, STD and TB Prevention. “It is exposing hidden, fragile populations in need that are not getting the health care and preventive services they deserve.”
Dr. Bolan recommends modernizing surveillance to capture more of the cases in populations without ready access to diagnosis and treatment and in those choosing not to access care.
“It is imperative that federal, state, and local programs employ strategies that maximize long-term population impact by reducing STD incidence and promoting sexual, reproductive, maternal, and infant health,” she wrote. “Further, it will be important for us to measure and monitor the adverse health consequences of STDs, such as ocular and neurosyphilis, pelvic inflammatory disease, ectopic pregnancy, infertility, HIV, congenital syphilis, and neonatal herpes.”
Multiple sources contributed data to the report: state and local STD programs’ notifiable disease reporting, private and federal national surveys, and specific projects that collect STD prevalence data, including the National Job Training Program, the STD Surveillance Network and the Gonococcal Isolate Surveillance Project.
The four nationally notifiable STDs are chlamydia, gonorrhea, syphilis, and chancroid.
The rise in newborn syphilis cases, currently at 23.3 cases per 100,000 live births, mirrors the increased U.S. prevalence of both primary and secondary syphilis in 2017, with 9.5 cases per 100,000 people. Syphilis has increased every year since 2000-2001, when prevalence was at a record low.
Chlamydia and gonorrhea rates climb too
The report also noted increases in the prevalence of other STDs. Chlamydia, the most common STD, increased 6.9% as compared to 2016, with 528.8 cases per 100,000 people. This increase occurred in all U.S. regions and independently of sex, race, or ethnicity, though rates were highest in teens and young adults. Nearly two-thirds of chlamydia cases in 2017 occurred in people ages 15-24 years old.
Reported rates were higher in women than in men, likely due to women’s increased likelihood of undergoing screening, the report suggested. Better surveillance may also partly explain the climb in men’s cases.
“Increases in rates among men may reflect an increased number of men, including gay, bisexual and other men who have sex with men (collectively referred to as MSM) being tested and diagnosed with a chlamydial infection due to increased availability of urine testing and extragenital screening,” according to the report.
The CDC received reports of more than a half million gonorrhea infections in 2017 (555,608 cases), an increase of 18.6% since the previous year, including a 19.3% increase among men and a 17.8% increase among women.
“The magnitude of the increase among men suggests either increased transmission, increased case ascertainment (e.g., through increased extra-genital screening among MSM), or both,” the authors wrote. “The concurrent increase in cases reported among women suggests parallel increases in heterosexual transmission, increased screening among women, or both.”
Overall, gonorrhea cases have skyrocketed 75.2% since their historic low in 2009, compounding the problem of antibiotic resistance that has limited CDC-recommended treatment to just ceftriaxone and azithromycin.
The report was supported by the Centers for Disease Control and Prevention. The authors did not report having any disclosures.
SOURCE: Centers for Disease Control and Prevention. Sexually Transmitted Disease Surveillance 2017; https://www.cdc.gov/std/stats
Key clinical point: Newborn syphilis cases have more than doubled in 5 years along with substantial increases in chlamydia, gonorrhea, and syphilis.
Major finding: 918 cases of newborn syphilis were reported in 37 states in 2017.
Study details: The findings are based on data from public health notifiable disease reports and multiple federal and private surveillance projects.
Disclosures: The report was supported by the Centers for Disease Control and Prevention. The authors did not report having any disclosures.
Source: Centers for Disease Control and Prevention. Sexually Transmitted Disease Surveillance 2017.
How to manage school failure
The start of the school year brings excitement and some expected anxiety, around seeing friends and undertaking new challenges. While setbacks, small failures, and disappointments are an essential part of a child’s mastery of new challenges, academic and otherwise, occasionally a child will experience school failure in many areas. When this happens, the school usually will engage parents to help understand and address what might be interfering with the child’s performance at school. Parents may turn to their trusted pediatricians for guidance in sorting out school failure, as the list of possible causes is very long. By asking the right questions and knowing your patient, you can efficiently investigate this problem so that your patient may quickly get back on track, both academically and in overall development.
Are their academic problems a striking change from prior years? If your patients previously had managed coursework with ease, then there is a new problem interfering with their performance, unless they are young enough that earlier years were not as challenging. Possibly a previous school was not as demanding or new academic expectations such as writing an essay or a dramatic increase in reading expectations have exposed a learning disability or attentional issue that is interfering with performance. This can be sorted out by asking more specific questions about their function. Do they struggle more with reading, essay writing, or math? Do they struggle with sustained attention on assignments or handing in completed work? Your patients can help answer these questions, as can as parents and teachers. Neuropsychological testing can elucidate specific learning disabilities or indicate marked problems with attention, working memory, or processing speed that may be improved with cognitive coaching, in-class strategies, and even medications. With older patients, a new problem is less likely to be the first presentation of an underlying learning or attentional issue and will need further investigation.
Do your patients still enjoy school or are they resisting attending? Students who are avoiding school may be struggling with anxiety. This may be a consequence of their academic struggles, as they try to avoid the shame, embarrassment, or discomfort of their failure to understand material, keep up, or perform. Alternately, the anxiety may have come first, leading to an inability to manage the challenges of school and then failure academically. Similarly, a mood disorder such as depression can create problems with attention, energy, interest, and motivation that make it difficult to attend and participate in school.
Ask about any family history of school problems and psychiatric disorders as these issues often run in families. Ask if there is anxiety around academic or social performance or more generalized anxiety. Are they experiencing trouble with sleep, energy, appetite? Have they withdrawn from other interests? Are they more tearful or irritable in all settings? When these symptoms are universal (i.e., occurring across settings and affecting school), there is likely an underlying psychiatric disorder driving them, and they require a full psychiatric assessment. It is worth noting that often children or adolescents with mood or anxiety disorders will experience somatic symptoms such as stomach aches or headaches alongside the loss of energy and motivation. They may come to the pediatrician first, and it is important to investigate the likely psychiatric illnesses (anxiety in prepubertal children and anxiety or depression in adolescents) as well as the more esoteric medical problems that could be causing such universal impairment in a child or teenager. Stigma still exists around psychiatric illness and it is powerful when a pediatrician can tell a family that these illnesses are common in young people (affecting nearly 20% of children by the age of 18) and very treatable.
Drug and alcohol abuse may be associated with another psychiatric illness or can be independent problems that interfere with the healthy development and school performance of young people, including middle school students. Find out if your patients are drinking alcohol, using marijuana, vaping, utilizing prescription medications that are not their own, or using other illegal drugs. Substance use that has led to problems at school is by definition a problem (in addition to being illegal) and will not improve without treatment. These young people need a full psychiatric evaluation, and they and their parents require specialized treatment and support to address the substance abuse problem.
Of course, school failure may represent other sources of stress. It is critical to find out from your patients if they feel safe at school. Are they being bullied or threatened? Do they have a safe way to get to and from school? Has something else occurred at the school that has left them feeling vigilant and unable to concentrate on classwork? While bullying or living in a neighborhood plagued by violence may not be easy problems to fix, it is critical to find out about them so the adults – parents, teachers, and others – can provide the students with support while directly addressing the safety issue. Do not fail to find out if the fear is at home. Children who are managing physical or sexual abuse may be too stressed to complete homework or even attend school. A caring, curious pediatrician will be a lifeline to a safer future for these children.
Similarly, it is important to find out if your patient is managing less dramatic stresses at home. Perhaps a parent has been seriously ill, working two jobs, or managing a problem with drugs or alcohol, and your patient is caring for that person, or for siblings, instead of keeping up with schoolwork. Perhaps there has been a stressful loss or transition, such as the death of a grandparent or pet, the loss of a job, or a big move, or family discord/violence that has made it difficult for your patient to focus on homework or interfered with parental supervision or homework help. Perhaps your patient has gotten a job to help the family financially and has no time for homework. Bringing such a challenge out into the open and rallying support for your patient and the family in these circumstances is often enough to foster adaptation to these stresses and a return to healthy function in school.
Finally, it is possible that school failure is a function of milder imbalances in a young person’s life. Some children may respond to the expanded independence of adolescence by making poor choices. When do they go to bed at night? Are they staying up late playing video games or surfing the web? Not all insomnia represents illness. Find out how much independence your patients have and how they are managing their time and responsibilities. Help them to think about how to protect time for both responsibilities and relaxation. You also may help the parents of these young people think about how to set expectations and basic rules while stepping back appropriately to allow for expanding independence in ways that will help their children to flourish.
Once defined, Setting reasonable expectations, curriculum adjustments, any needed psychiatric treatment, building on a child’s strengths, and paying attention to self-esteem are the hallmarks of effective interventions.
Dr. Swick is an attending psychiatrist in the division of child psychiatry at Massachusetts General Hospital, Boston, and director of the Parenting at a Challenging Time (PACT) Program at the Vernon Cancer Center at Newton Wellesley Hospital, also in Boston. Dr. Jellinek is professor emeritus of psychiatry and pediatrics, Harvard Medical School, Boston. Email them at pdnews@mdedge.com.
The start of the school year brings excitement and some expected anxiety, around seeing friends and undertaking new challenges. While setbacks, small failures, and disappointments are an essential part of a child’s mastery of new challenges, academic and otherwise, occasionally a child will experience school failure in many areas. When this happens, the school usually will engage parents to help understand and address what might be interfering with the child’s performance at school. Parents may turn to their trusted pediatricians for guidance in sorting out school failure, as the list of possible causes is very long. By asking the right questions and knowing your patient, you can efficiently investigate this problem so that your patient may quickly get back on track, both academically and in overall development.
Are their academic problems a striking change from prior years? If your patients previously had managed coursework with ease, then there is a new problem interfering with their performance, unless they are young enough that earlier years were not as challenging. Possibly a previous school was not as demanding or new academic expectations such as writing an essay or a dramatic increase in reading expectations have exposed a learning disability or attentional issue that is interfering with performance. This can be sorted out by asking more specific questions about their function. Do they struggle more with reading, essay writing, or math? Do they struggle with sustained attention on assignments or handing in completed work? Your patients can help answer these questions, as can as parents and teachers. Neuropsychological testing can elucidate specific learning disabilities or indicate marked problems with attention, working memory, or processing speed that may be improved with cognitive coaching, in-class strategies, and even medications. With older patients, a new problem is less likely to be the first presentation of an underlying learning or attentional issue and will need further investigation.
Do your patients still enjoy school or are they resisting attending? Students who are avoiding school may be struggling with anxiety. This may be a consequence of their academic struggles, as they try to avoid the shame, embarrassment, or discomfort of their failure to understand material, keep up, or perform. Alternately, the anxiety may have come first, leading to an inability to manage the challenges of school and then failure academically. Similarly, a mood disorder such as depression can create problems with attention, energy, interest, and motivation that make it difficult to attend and participate in school.
Ask about any family history of school problems and psychiatric disorders as these issues often run in families. Ask if there is anxiety around academic or social performance or more generalized anxiety. Are they experiencing trouble with sleep, energy, appetite? Have they withdrawn from other interests? Are they more tearful or irritable in all settings? When these symptoms are universal (i.e., occurring across settings and affecting school), there is likely an underlying psychiatric disorder driving them, and they require a full psychiatric assessment. It is worth noting that often children or adolescents with mood or anxiety disorders will experience somatic symptoms such as stomach aches or headaches alongside the loss of energy and motivation. They may come to the pediatrician first, and it is important to investigate the likely psychiatric illnesses (anxiety in prepubertal children and anxiety or depression in adolescents) as well as the more esoteric medical problems that could be causing such universal impairment in a child or teenager. Stigma still exists around psychiatric illness and it is powerful when a pediatrician can tell a family that these illnesses are common in young people (affecting nearly 20% of children by the age of 18) and very treatable.
Drug and alcohol abuse may be associated with another psychiatric illness or can be independent problems that interfere with the healthy development and school performance of young people, including middle school students. Find out if your patients are drinking alcohol, using marijuana, vaping, utilizing prescription medications that are not their own, or using other illegal drugs. Substance use that has led to problems at school is by definition a problem (in addition to being illegal) and will not improve without treatment. These young people need a full psychiatric evaluation, and they and their parents require specialized treatment and support to address the substance abuse problem.
Of course, school failure may represent other sources of stress. It is critical to find out from your patients if they feel safe at school. Are they being bullied or threatened? Do they have a safe way to get to and from school? Has something else occurred at the school that has left them feeling vigilant and unable to concentrate on classwork? While bullying or living in a neighborhood plagued by violence may not be easy problems to fix, it is critical to find out about them so the adults – parents, teachers, and others – can provide the students with support while directly addressing the safety issue. Do not fail to find out if the fear is at home. Children who are managing physical or sexual abuse may be too stressed to complete homework or even attend school. A caring, curious pediatrician will be a lifeline to a safer future for these children.
Similarly, it is important to find out if your patient is managing less dramatic stresses at home. Perhaps a parent has been seriously ill, working two jobs, or managing a problem with drugs or alcohol, and your patient is caring for that person, or for siblings, instead of keeping up with schoolwork. Perhaps there has been a stressful loss or transition, such as the death of a grandparent or pet, the loss of a job, or a big move, or family discord/violence that has made it difficult for your patient to focus on homework or interfered with parental supervision or homework help. Perhaps your patient has gotten a job to help the family financially and has no time for homework. Bringing such a challenge out into the open and rallying support for your patient and the family in these circumstances is often enough to foster adaptation to these stresses and a return to healthy function in school.
Finally, it is possible that school failure is a function of milder imbalances in a young person’s life. Some children may respond to the expanded independence of adolescence by making poor choices. When do they go to bed at night? Are they staying up late playing video games or surfing the web? Not all insomnia represents illness. Find out how much independence your patients have and how they are managing their time and responsibilities. Help them to think about how to protect time for both responsibilities and relaxation. You also may help the parents of these young people think about how to set expectations and basic rules while stepping back appropriately to allow for expanding independence in ways that will help their children to flourish.
Once defined, Setting reasonable expectations, curriculum adjustments, any needed psychiatric treatment, building on a child’s strengths, and paying attention to self-esteem are the hallmarks of effective interventions.
Dr. Swick is an attending psychiatrist in the division of child psychiatry at Massachusetts General Hospital, Boston, and director of the Parenting at a Challenging Time (PACT) Program at the Vernon Cancer Center at Newton Wellesley Hospital, also in Boston. Dr. Jellinek is professor emeritus of psychiatry and pediatrics, Harvard Medical School, Boston. Email them at pdnews@mdedge.com.
The start of the school year brings excitement and some expected anxiety, around seeing friends and undertaking new challenges. While setbacks, small failures, and disappointments are an essential part of a child’s mastery of new challenges, academic and otherwise, occasionally a child will experience school failure in many areas. When this happens, the school usually will engage parents to help understand and address what might be interfering with the child’s performance at school. Parents may turn to their trusted pediatricians for guidance in sorting out school failure, as the list of possible causes is very long. By asking the right questions and knowing your patient, you can efficiently investigate this problem so that your patient may quickly get back on track, both academically and in overall development.
Are their academic problems a striking change from prior years? If your patients previously had managed coursework with ease, then there is a new problem interfering with their performance, unless they are young enough that earlier years were not as challenging. Possibly a previous school was not as demanding or new academic expectations such as writing an essay or a dramatic increase in reading expectations have exposed a learning disability or attentional issue that is interfering with performance. This can be sorted out by asking more specific questions about their function. Do they struggle more with reading, essay writing, or math? Do they struggle with sustained attention on assignments or handing in completed work? Your patients can help answer these questions, as can as parents and teachers. Neuropsychological testing can elucidate specific learning disabilities or indicate marked problems with attention, working memory, or processing speed that may be improved with cognitive coaching, in-class strategies, and even medications. With older patients, a new problem is less likely to be the first presentation of an underlying learning or attentional issue and will need further investigation.
Do your patients still enjoy school or are they resisting attending? Students who are avoiding school may be struggling with anxiety. This may be a consequence of their academic struggles, as they try to avoid the shame, embarrassment, or discomfort of their failure to understand material, keep up, or perform. Alternately, the anxiety may have come first, leading to an inability to manage the challenges of school and then failure academically. Similarly, a mood disorder such as depression can create problems with attention, energy, interest, and motivation that make it difficult to attend and participate in school.
Ask about any family history of school problems and psychiatric disorders as these issues often run in families. Ask if there is anxiety around academic or social performance or more generalized anxiety. Are they experiencing trouble with sleep, energy, appetite? Have they withdrawn from other interests? Are they more tearful or irritable in all settings? When these symptoms are universal (i.e., occurring across settings and affecting school), there is likely an underlying psychiatric disorder driving them, and they require a full psychiatric assessment. It is worth noting that often children or adolescents with mood or anxiety disorders will experience somatic symptoms such as stomach aches or headaches alongside the loss of energy and motivation. They may come to the pediatrician first, and it is important to investigate the likely psychiatric illnesses (anxiety in prepubertal children and anxiety or depression in adolescents) as well as the more esoteric medical problems that could be causing such universal impairment in a child or teenager. Stigma still exists around psychiatric illness and it is powerful when a pediatrician can tell a family that these illnesses are common in young people (affecting nearly 20% of children by the age of 18) and very treatable.
Drug and alcohol abuse may be associated with another psychiatric illness or can be independent problems that interfere with the healthy development and school performance of young people, including middle school students. Find out if your patients are drinking alcohol, using marijuana, vaping, utilizing prescription medications that are not their own, or using other illegal drugs. Substance use that has led to problems at school is by definition a problem (in addition to being illegal) and will not improve without treatment. These young people need a full psychiatric evaluation, and they and their parents require specialized treatment and support to address the substance abuse problem.
Of course, school failure may represent other sources of stress. It is critical to find out from your patients if they feel safe at school. Are they being bullied or threatened? Do they have a safe way to get to and from school? Has something else occurred at the school that has left them feeling vigilant and unable to concentrate on classwork? While bullying or living in a neighborhood plagued by violence may not be easy problems to fix, it is critical to find out about them so the adults – parents, teachers, and others – can provide the students with support while directly addressing the safety issue. Do not fail to find out if the fear is at home. Children who are managing physical or sexual abuse may be too stressed to complete homework or even attend school. A caring, curious pediatrician will be a lifeline to a safer future for these children.
Similarly, it is important to find out if your patient is managing less dramatic stresses at home. Perhaps a parent has been seriously ill, working two jobs, or managing a problem with drugs or alcohol, and your patient is caring for that person, or for siblings, instead of keeping up with schoolwork. Perhaps there has been a stressful loss or transition, such as the death of a grandparent or pet, the loss of a job, or a big move, or family discord/violence that has made it difficult for your patient to focus on homework or interfered with parental supervision or homework help. Perhaps your patient has gotten a job to help the family financially and has no time for homework. Bringing such a challenge out into the open and rallying support for your patient and the family in these circumstances is often enough to foster adaptation to these stresses and a return to healthy function in school.
Finally, it is possible that school failure is a function of milder imbalances in a young person’s life. Some children may respond to the expanded independence of adolescence by making poor choices. When do they go to bed at night? Are they staying up late playing video games or surfing the web? Not all insomnia represents illness. Find out how much independence your patients have and how they are managing their time and responsibilities. Help them to think about how to protect time for both responsibilities and relaxation. You also may help the parents of these young people think about how to set expectations and basic rules while stepping back appropriately to allow for expanding independence in ways that will help their children to flourish.
Once defined, Setting reasonable expectations, curriculum adjustments, any needed psychiatric treatment, building on a child’s strengths, and paying attention to self-esteem are the hallmarks of effective interventions.
Dr. Swick is an attending psychiatrist in the division of child psychiatry at Massachusetts General Hospital, Boston, and director of the Parenting at a Challenging Time (PACT) Program at the Vernon Cancer Center at Newton Wellesley Hospital, also in Boston. Dr. Jellinek is professor emeritus of psychiatry and pediatrics, Harvard Medical School, Boston. Email them at pdnews@mdedge.com.
Five mistakes to avoid when starting a locum tenens position
Beware inefficient placement systems
For the last 8 years I have worked as a locum tenens hospitalist. I began on this path when it was the least popular option upon graduation from residency.
I did countless hours of research trying to find accurate information about locum tenens companies, but never found anything written by physicians, only by the companies themselves. So, I stepped into this field blindfolded and learned the hard way. Since than, I have worked with over 16 locum tenens companies, 14 hospitals, and eight electronic medical record systems.
Through these experiences I’ve realized that, unfortunately, some locum tenens companies do not act with the professionalism and efficiency that both physicians and hospital systems would expect. This can lead to more stress than an actual employed position for physicians, and poor coverage with enormous costs for hospitals.
I decided to take matters into my own hands because I wanted to make the locum tenens system easier to navigate. I believe that the system can play a role in decreasing physician burnout, and I deeply understand the need that hospitals have to serve their patients with a shortage of doctors. As locum tenens physicians, we serve a need and shouldn’t have to deal with inefficient placement systems.
Here are five mistakes to avoid for physicians that are first entering into the locum tenens world:
1. Beware choosing a “factory mill” locum tenens company. Bigger companies have higher overhead, which usually means that they take more of a margin from physicians. Generally speaking, larger locum tenens companies pay their recruiters a lower percentage commission, so each recruiter has more physicians. This can lead to mistakes which can cause stress for both physicians and hospitals.
2. Beware long travel. Hourly rates that are $5-$10 dollars more per hour in remote locations are attractive. However, the amount of travel needed to get to these locations may not be worth it. When negotiating a rate, make sure not to lose sight of the amount of time it will take to travel to the hospital or outpatient location.
3. Beware short-term placements. There are a lot of hospitals that just need one or two weeks covered. Even if it’s at a much higher rate, the amount of paper work and credentialing hassle may not be worth the amount of time you work there. The greater number of cumulative hospitals worked, the longer credentialing will take in future locum tenens placements.
4. Beware using multiple travel services. Stick to one airline, one rental car company, and one hotel chain. This way when you are not working, you may be able to use the points earned during your work days for future vacations.
5. Beware companies that are not organized. If you find that a locum tenens company is asking you to do all of the paperwork to get credentialed, move on. This can be a red flag and may mean they lack credentialing staff. You should never have to fill out your own paperwork; rather you should be the one that simply reviews and corrects it.
Dr. Arora works as a liaison between hospitals, physicians, and locum tenens companies and is a member of The Hospitalist’s editorial advisory board. She negotiates rates and expectations with multiple locum tenens companies on behalf of physicians in all fields of practice and does not own or endorse a locum tenens company. You can contact her at www.doctorsliaison.com.
Beware inefficient placement systems
Beware inefficient placement systems
For the last 8 years I have worked as a locum tenens hospitalist. I began on this path when it was the least popular option upon graduation from residency.
I did countless hours of research trying to find accurate information about locum tenens companies, but never found anything written by physicians, only by the companies themselves. So, I stepped into this field blindfolded and learned the hard way. Since than, I have worked with over 16 locum tenens companies, 14 hospitals, and eight electronic medical record systems.
Through these experiences I’ve realized that, unfortunately, some locum tenens companies do not act with the professionalism and efficiency that both physicians and hospital systems would expect. This can lead to more stress than an actual employed position for physicians, and poor coverage with enormous costs for hospitals.
I decided to take matters into my own hands because I wanted to make the locum tenens system easier to navigate. I believe that the system can play a role in decreasing physician burnout, and I deeply understand the need that hospitals have to serve their patients with a shortage of doctors. As locum tenens physicians, we serve a need and shouldn’t have to deal with inefficient placement systems.
Here are five mistakes to avoid for physicians that are first entering into the locum tenens world:
1. Beware choosing a “factory mill” locum tenens company. Bigger companies have higher overhead, which usually means that they take more of a margin from physicians. Generally speaking, larger locum tenens companies pay their recruiters a lower percentage commission, so each recruiter has more physicians. This can lead to mistakes which can cause stress for both physicians and hospitals.
2. Beware long travel. Hourly rates that are $5-$10 dollars more per hour in remote locations are attractive. However, the amount of travel needed to get to these locations may not be worth it. When negotiating a rate, make sure not to lose sight of the amount of time it will take to travel to the hospital or outpatient location.
3. Beware short-term placements. There are a lot of hospitals that just need one or two weeks covered. Even if it’s at a much higher rate, the amount of paper work and credentialing hassle may not be worth the amount of time you work there. The greater number of cumulative hospitals worked, the longer credentialing will take in future locum tenens placements.
4. Beware using multiple travel services. Stick to one airline, one rental car company, and one hotel chain. This way when you are not working, you may be able to use the points earned during your work days for future vacations.
5. Beware companies that are not organized. If you find that a locum tenens company is asking you to do all of the paperwork to get credentialed, move on. This can be a red flag and may mean they lack credentialing staff. You should never have to fill out your own paperwork; rather you should be the one that simply reviews and corrects it.
Dr. Arora works as a liaison between hospitals, physicians, and locum tenens companies and is a member of The Hospitalist’s editorial advisory board. She negotiates rates and expectations with multiple locum tenens companies on behalf of physicians in all fields of practice and does not own or endorse a locum tenens company. You can contact her at www.doctorsliaison.com.
For the last 8 years I have worked as a locum tenens hospitalist. I began on this path when it was the least popular option upon graduation from residency.
I did countless hours of research trying to find accurate information about locum tenens companies, but never found anything written by physicians, only by the companies themselves. So, I stepped into this field blindfolded and learned the hard way. Since than, I have worked with over 16 locum tenens companies, 14 hospitals, and eight electronic medical record systems.
Through these experiences I’ve realized that, unfortunately, some locum tenens companies do not act with the professionalism and efficiency that both physicians and hospital systems would expect. This can lead to more stress than an actual employed position for physicians, and poor coverage with enormous costs for hospitals.
I decided to take matters into my own hands because I wanted to make the locum tenens system easier to navigate. I believe that the system can play a role in decreasing physician burnout, and I deeply understand the need that hospitals have to serve their patients with a shortage of doctors. As locum tenens physicians, we serve a need and shouldn’t have to deal with inefficient placement systems.
Here are five mistakes to avoid for physicians that are first entering into the locum tenens world:
1. Beware choosing a “factory mill” locum tenens company. Bigger companies have higher overhead, which usually means that they take more of a margin from physicians. Generally speaking, larger locum tenens companies pay their recruiters a lower percentage commission, so each recruiter has more physicians. This can lead to mistakes which can cause stress for both physicians and hospitals.
2. Beware long travel. Hourly rates that are $5-$10 dollars more per hour in remote locations are attractive. However, the amount of travel needed to get to these locations may not be worth it. When negotiating a rate, make sure not to lose sight of the amount of time it will take to travel to the hospital or outpatient location.
3. Beware short-term placements. There are a lot of hospitals that just need one or two weeks covered. Even if it’s at a much higher rate, the amount of paper work and credentialing hassle may not be worth the amount of time you work there. The greater number of cumulative hospitals worked, the longer credentialing will take in future locum tenens placements.
4. Beware using multiple travel services. Stick to one airline, one rental car company, and one hotel chain. This way when you are not working, you may be able to use the points earned during your work days for future vacations.
5. Beware companies that are not organized. If you find that a locum tenens company is asking you to do all of the paperwork to get credentialed, move on. This can be a red flag and may mean they lack credentialing staff. You should never have to fill out your own paperwork; rather you should be the one that simply reviews and corrects it.
Dr. Arora works as a liaison between hospitals, physicians, and locum tenens companies and is a member of The Hospitalist’s editorial advisory board. She negotiates rates and expectations with multiple locum tenens companies on behalf of physicians in all fields of practice and does not own or endorse a locum tenens company. You can contact her at www.doctorsliaison.com.
Complex Ankle and Hindfoot Arthrodesis Using Circular External Fixation
ABSTRACT
Surgical reconstruction of the ankle and hindfoot in patients with diabetes, Charcot neuroarthropathy, osteomyelitis, deformity, and/or bone loss can be challenging and often results in amputation. In these patients, conventional internal fixation with plates, screws, and intramedullary nails is often not feasible because of ongoing infection or poor bone stock and soft tissue quality. The Ilizarov method of ankle and hindfoot arthrodesis is a well-established technique for limb reconstruction that uses circular external fixation to achieve solid bony fusion, optimal leg length, and eradication of infection in cases of complex pathology. This article discusses indications, contraindications, pearls, and pitfalls of performing ankle and hindfoot arthrodesis using the Ilizarov technique.
Continue to: Patients with complex ankle and hindfoot deformity...
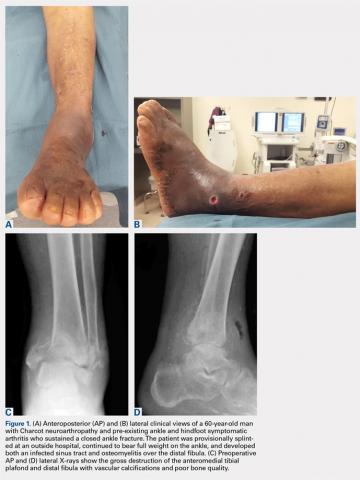
Patients with complex ankle and hindfoot deformity present a unique challenge to both nonoperative management and surgical reconstruction. Nonoperative management focuses on wound care, bracing, and immobilization using ankle-foot orthoses, total contact casts, and Charcot restraint orthotic walker boots for external stabilization. Fusion using the Ilizarov technique with circular fixation is a salvage limb-preservation procedure that has shown good results in select patient populations.1-5 Indications include post-traumatic, degenerative, and rheumatoid arthritis, osteomyelitis, tumors, neuromuscular conditions, and salvage of failed ankle and hindfoot procedures.6-9 Relative contraindications include wet gangrene, severe limb ischemia, and soft tissue compromise requiring urgent amputation. In addition, circular frames are not recommended in patients who are unable to comply with postoperative restrictions, and pin and wire care for the duration of frame placement because of personal, psychological, or socioeconomic reasons.
The Ilizarov technique of ring fixation provides dynamic, modular, and rigid fixation in multiple planes to control shear, bending, and rotational forces, and allows for early weight-bearing and postoperative adjustments as needed.10,11 Percutaneously placed half-pins and wires allow for solid fixation in the setting of both poor bone and soft tissue quality, and fusion can be achieved in the presence of active infection in a 1-stage procedure. The goal of ankle and hindfoot fusion using the Ilizarov technique is to achieve an infection-free, stable, plantigrade foot with neutral ankle alignment to allow for patient ambulation and return to activities of daily living.
Nonunion rates with circular fixation are reported to be as high as 16% to 54%, due to medical comorbidities, such as smoking, peripheral vascular disease, and Charcot neuroarthropathy.1Charcot, in particular, is a risk factor for nonunion as patients lack protective sensation, and have a higher rate of wound dehiscence, noncompliance with weight-bearing precautions, pin site infections, and frame breakage. In these patients, tibiotalocalcaneal (TTC) arthrodesis is preferred over the isolated ankle, or subtalar fusion to both provide a stable platform for ambulation and reduce the incidence of adjacent joint breakdown. Common complications of the Ilizarov technique include pin site infections, wire breakage, talar necrosis, and tibial stress fractures after frame removal.1,2,6,11-13 Circular frames are typically maintained for 3 to 8 months, until solid fusion is achieved radiographically. Frames are removed in the operating room with the concurrent examination of the fusion sites under anesthesia followed by a period of protected weight-bearing in a cast or tall controlled ankle motion (CAM) boot.
This article reviews several technical details, tips, and tricks that can help improve the intraoperative and postoperative outcomes of combined ankle and hindfoot arthrodesis using the Ilizarov technique with circular external fixation.
Continue to: SURGICAL TECHNIQUE...
SURGICAL TECHNIQUE
SETUP AND APPROACH
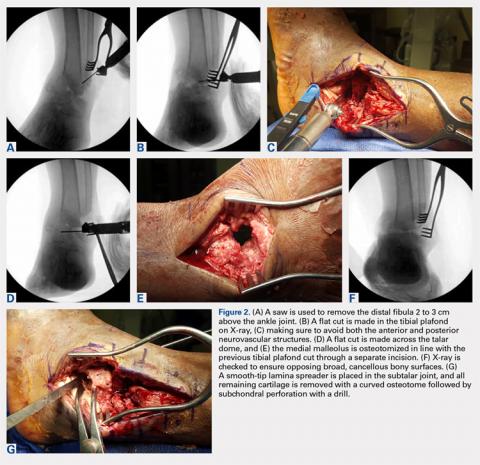
Patients are positioned supine with padding under the operative extremity to achieve neutral leg rotation (Figures 1A-1D). A thigh tourniquet is placed with the foot positioned at the end of the bed and on top of the radiolucent padding to avoid interference of the contralateral leg during lateral X-rays. After sterile prepping and draping, the extremity is exsanguinated above the level of an active infection, and the tourniquet inflated.
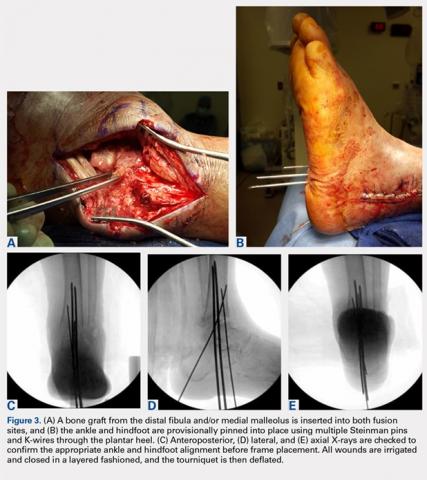
For isolated ankle arthrodesis, an anterior or lateral approach can be used, while for TTC arthrodesis, a lateral approach is required to access both the ankle and subtalar joints. A 10-cm longitudinal incision is made along the distal fibula, curving slightly and anteriorly along the distal extent of the incision. Dissection is continued down to bone using full thickness flaps, and the distal fibula is removed 2 to 3 cm above the ankle joint using a saw and osteotome (Figures 2A-2G). The distal fibula can be used subsequently as bone grafts depending on the quality of bone. The peroneal tendons are retracted posteriorly, and dissection is then continued to the posterior facet of the subtalar joint.
JOINT PREPARATION AND ALIGNMENT
Both the anterior and posterior neurovascular bundles are protected along the distal tibia with Hohmann retractors while a saw is used to create flat cuts across the tibial plafond and talus to allow apposition of flat, broad cancellous bony surfaces. Flat cuts followed by later joint compression will often shorten the limb by 2 to 3 cm. This leg length discrepancy can later be accommodated using a shoe lift, as needed. All retained hardware and/or infected and necrotic tissues in the ankle and hindfoot are removed using a rongeur and a pituitary rongeur.
The medial malleolus is osteotomized vertically using a direct medial incision and approach with full thickness flaps, and in line with the previous tibial plafond, is both cut and removed. The medial malleolus can also be used for bone grafts in fusion sites. A smooth-tip lamina spreader is placed in the subtalar joint for distraction and a curved osteotome, curettes, and a small rongeur are used to remove all remaining cartilage from the subtalar joint. Flat cuts in the subtalar joint can remove excessive bone, particularly from the inferior aspect of the talus. The subchondral bone is perforated using a 2.5- to 3.0-mm drill bit and a curved osteotome.
A bone graft from the distal fibula and medial malleolus, with or without the addition of allograft adjuvants, is placed evenly across the ankle and subtalar joints (Figures 3A-3E). At this point, the ankle and subtalar joints can be manipulated in multiple planes to achieve neutral coronal, sagittal, and axial alignment. With both the ankle and hindfoot held in a neutral position, multiple Steinman pins and K-wires in different orientations are inserted through the plantar aspect of the heel to hold the ankle and subtalar joints in place temporarily. Wires are cut short to prevent interference with subsequent foot olive wire placement through the frame.
Continue to: X-rays should be carefully checked...
X-rays should be carefully checked to ensure proper alignment. Wounds are gently irrigated, and vancomycin powder (2 g) can be placed within wounds for local antibiotic delivery. Lateral tissues are sharply debulked to allow for decreased tension on the incision, and small ulcers can be excised in their entirety. Wounds are closed in a layered fashion using 0-polydioxanone (PDS, Ethicon) suture for deep tissue, 2-0 PDS for subcutaneous tissue, and 2-0 nylon for skin closure. The tourniquet is deflated for the remainder of the case to reduce limb ischemia during frame placement.
CIRCULAR FRAME CONCEPTS AND PLACEMENT
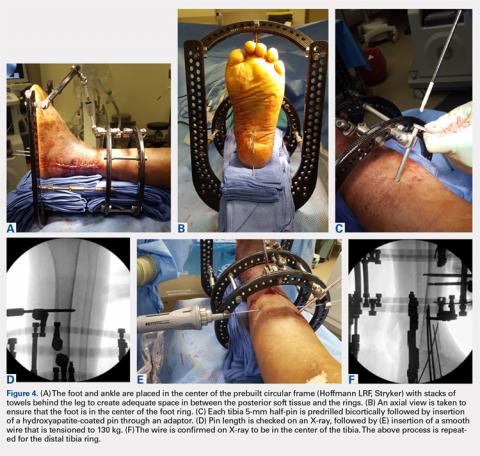
The majority of circular frames for both ankle and hindfoot fusion have multiple ring sizes available in aluminum and radiolucent carbon fiber reinforced polymer (Hoffmann LRF, Stryker). Rings are available in full, open, segment, and both short- and long-foot options. Frames can be sterilized in a prebuilt 3 to 4 ring construct with 4 static or dynamic (telescopic) struts (100-277 mm). The most commonly used tibia and foot ring sizes are 155 cm, 180 cm, and 210 cm. Ring size should be able to accommodate posterior soft tissue swelling and avoid circumferential soft tissue abrasion against the rings. Anterior foot arches are used for increased construct stability and can be locked to the distal tibia ring for weight-bearing support. Wire and half-pin bolts, adaptors, and nuts are used to join each ring of the frame to the patient’s bone.
For TTC arthrodesis, 2 rings are typically used in the tibia, and 1 ring is used in the foot. For isolated ankle arthrodesis, an additional ring can be added with olive wires in the talus to permit compression only across the ankle joint. Multiple points of fixation are used in each ring in different planes to achieve both maximal stability and rotational control. If a single wire or half-pin becomes infected and requires removal, there are still multiple other points of fixation in the ring to maintain stability. Fixation within each ring should be off axis compared with the adjacent ring to both avoid stress risers and increase construct rigidity.
The prebuilt frame is checked on the back table to ensure proper orientation and component alignment. The frame is then placed over both the foot and ankle, and multiple stacks of towels are placed behind the heel, ankle, and calf to center the foot and ankle in the frame (Figures 4A-4F). At least 4 to 6 cm of space is needed in between the posterior soft tissues and each ring to accommodate postoperative swelling. On the lateral view, the foot ring should be in the mid-portion of the calcaneus. If there is a concern, particularly in Charcot patients, regarding early weight-bearing noncompliance, the foot ring can be placed flush with the plantar aspect of the foot, and olive wires can be inserted using longer adaptors. The frame should be checked from multiple viewpoints to ensure that both the foot and ankle are centered and in neutral rotation.
Continue to: TIBIA RING FIXATION...
TIBIA RING FIXATION
Tibia rings can be fixed using 2 to 3 half-pins (4-6 mm) alone or 2 half-pins in combination with a smooth wire. A small incision is made over the area of planned half-pin insertion, and the periosteum is cleared away using a hemostat. An adaptor sleeve is used, and the bone is drilled bicortically, followed by insertion of the half-pin. Hydroxyapatite-coated pins are used to improve the strength of the bone-pin interface and reduce the incidence of pin tract infections. Pins are inserted along both the anterior and medial aspects of the tibia, avoiding the thick lateral musculature. Care is taken to protect the medial neurovascular structures during pin placement following established Ilizarov safe zones.
After each pin is placed in the bone, the pin is secured to the adaptor that is then tightened to the ring. This process is repeated for both the proximal and distal tibia rings. Pins should be placed above and below each ring to avoid creating stress risers. During smooth wire placement, each wire is pushed by hand through the soft tissues and then drilled into the bone while the exposed segment is held with a damp sponge to reduce the incidence of thermal bone necrosis. Once the wire is drilled bicortically, a mallet is used to tap the wire through the remaining soft tissues to avoid wrapping them up in the wire. Each wire should be parallel to the ring to get an even line of compression.
Each wire is secured on 1 end and then tensioned to 130 kg using a hand tensioner. An additional tool can be placed in the wire adaptor to prevent the wire from bending during tensioning. If the wire is passing above or below the ring, longer wire adaptors should be used to build to the wire. The wire should never be bent toward the ring as this can increase the likelihood of improper pin tensioning and breakage. Wire placement should be avoided posteriorly as this can make it difficult to secure and/or tension wires, and also increases the risk of damage to posterior structures.
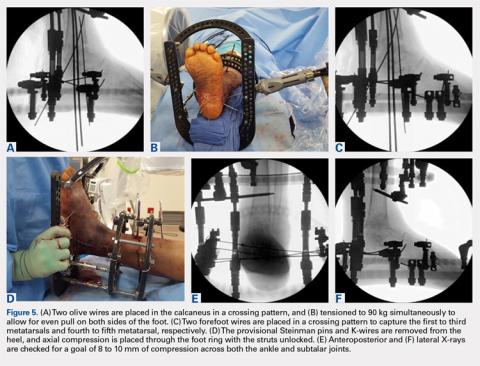
Ring fixation in the distal tibia near the plafond may require 1 half-pin and 2 wires to avoid damage to the tibialis anterior and posterior tibial tendons. In this case, smooth wires should be placed in a crossing pattern and tensioned simultaneously to avoid pulling the ankle away from the center of the frame. Wires should be bent and curved over each ring and then cut to facilitate subsequent removal.
FOOT RING FIXATION
In the foot, olive wires are used to increase fixation against bone. For each olive wire, a small incision is made to accommodate the diameter of the olive through the soft tissue. Similar to the distal tibia, 2 olive wires should be placed above and below the foot ring in a crossing pattern through the calcaneus (Figures 5A-5F). The axial view of the frame should be checked to ensure proper wire orientation. When using olive wires, it is essential to tension both at the same time to 90 kg, as the foot can be pulled medially or laterally in the frame if 1 wire is tensioned before the other.
Forefoot olive wires should also be placed in a crossing pattern, with 1 wire fixed through the first, second, and third metatarsals, and 1 wire through the fourth and fifth metatarsals. Additional forefoot olive wires can be placed if compression is needed across the midfoot or Chopart joints for fusion. Multiple X-rays should be checked to ensure that the calcaneus and forefoot olive wires are firmly fixed both in and against bone.
Continue to: JOINT COMPRESSION AND FINAL FRAME ADJUSTMENTS...
JOINT COMPRESSION AND FINAL FRAME ADJUSTMENTS
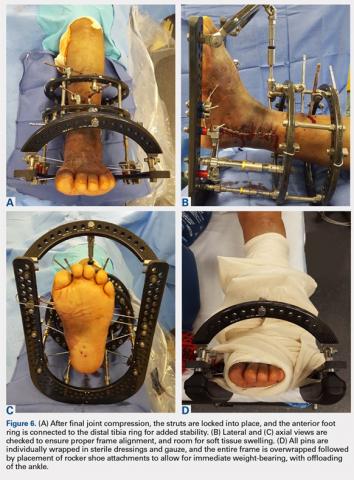
Once all rings are secured to the bone with half-pins and wires, the previously placed Steinman pins, and K-wires through the heel are removed. Both ankle and subtalar joint alignments are rechecked, and then axial compression is placed through the foot ring with the knee extended and the struts unlocked. Static or telescopic struts are used to achieve 8 to 10 mm of bony compression. X-rays are taken before and after to analyze final joint compression and alignment. Struts should be sequentially tightened (1/2 turn of a static strut) 1 at a time as final tightening of 1 strut alone can bind and interfere with both the compression and tightening of the remaining struts.
Once final compression is achieved, the struts are locked, and the front foot arch is closed anteriorly and connected to the distal tibia ring for increased stability (Figures 6A-6D). Each pin and wire is covered in a sterile dressing followed by gauze to allow for soft tissue padding. The entire frame is then overwrapped in bias stockinette rolls or ace wraps.
Walking attachments can be added immediately to the frame that allows for early weight-bearing. Rocker shoe attachments with a 15° anterior and posterior slope and rubber soles can help offload the ankle and subtalar joints, decrease pressure on heel strike, and reduce ankle motion during ambulation (Hoffmann LRF, Stryker).
POSTOPERATIVE PROTOCOL
Depending on individual characteristics, patients can be immediately weight-bearing in the circular frame. Patients with Charcot neuroarthropathy are recommended to remain non-weight-bearing for the first 2 months to reduce the likelihood of pin, wire, and frame breakage along with nonunion. Pin and wire site care and maintenance are initiated the day after surgery and continue on a daily basis for the duration of frame placement. Sutures are removed 4 to 5 weeks after surgery to ensure adequate wound healing. Serial X-rays are taken monthly to analyze fusion sites.
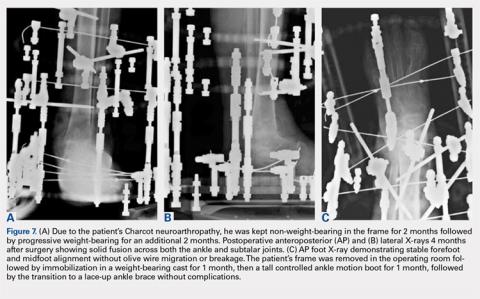
If pins or wires become infected, patients are placed on oral antibiotics, and both pins and wires can be removed or exchanged in the operating room. Once fusion is achieved in 3 to 8 months (Figures 7A-7C), the frame is removed in the operating room, and fusion sites are examined under dynamic fluoroscopy. If fusion is confirmed, patients are made weight-bearing as tolerated in a short-leg cast or tall CAM boot for 6 to 8 weeks, and then transitioned to an ankle brace in an accommodative shoe.
Continue to: DISCUSSION...
DISCUSSION
A key aspect of recovery after ankle and hindfoot fusion using the Ilizarov technique is balancing pin care, soft tissue swelling, and weight-bearing status. The average time patients will spend in the frame is approximately 25 to 28 weeks, but can range from 12 to 84 weeks.1,2Given the considerable variability in both soft tissue healing and bony union, patients should be extensively counseled before surgery to set expectations correctly and ensure that they have the necessary help and support to care for the frame during the treatment period. Patients should be followed closely during the first 6 weeks to ensure that pins and wires do not become infected or break, as both of these issues require immediate intervention.
In a review of 11 patients who underwent tibiocalcaneal arthrodesis using an Ilizarov external fixator for infected talar nonunions or extrusions, Rochman and colleagues8 reported an 81% rate of successful fusion with a final mean American Orthopaedic Foot and Ankle Society score of 65 (out of a maximum 86). Similar results were reported by Saltzman9 in a series of 8 patients with diffuse ankle osteomyelitis treated with resection of all infected tissue and hybrid-frame compression arthrodesis. All patients received 6 weeks of intravenous antibiotics, and frames were removed at 3 months, and walking casts were applied for 1 to 2 additional months. Ankle sepsis was eradicated in all patients, and 7/8 (87.5%) ankles successfully fused at an average of 13.5 weeks (range, 10-16 weeks). One limb required below-knee amputation at 5 weeks due to non-reconstructible vascular insufficiency. At an average of 3.4-year follow-up, none of the 7 fused ankles required further surgery.
Fragomen and colleagues1 retrospectively reviewed 101 patients who underwent complex ankle fusion using the Ilizarov technique and found that 76/91 (83.5%) patients achieved fusion at an average of 25 weeks (range, 10-65 weeks). Smoking was associated with a 54% rate of nonunion and 15/19 (79%) patients with Charcot neuroarthropathy achieved ankle fusion, but had a subsequent subtalar joint failure, thus highlighting the need for TTC arthrodesis in Charcot patients. Salem and colleagues2 reviewed 21 Ilizarov ankle fusions and reported that all patients achieved fusion at an average of 28 weeks (range, 12-84 weeks). Complications occurred in 11 patients, including 2 nonunions that healed after revision frame application and 4 pin tract infections.
CONCLUSION
Overall, the Ilizarov technique using circular external fixation is a powerful tool that can be used to treat a variety of disorders including complex foot and ankle deformity and infection. While case series generally show favorable outcomes, patients must be informed that this technique is a salvage procedure for limb preservation that requires meticulous operative technique, diligent postoperative care, and tight control of medical comorbidities, such as blood sugar levels in individuals with diabetes to achieve a successful outcome.
1. Fragomen AT, Borst E, Schachter L, Lyman S, Rozbruch SR. Complex ankle arthrodesis using the Ilizarov method yields high rate of fusion. Clin Orthop Relat Res. 2012;470(10):2864-2873. doi:10.1007/s11999-012-2470-9.
2. Salem KH, Kinzl L, Schmelz A. Ankle arthrodesis using Ilizarov ring fixators: a review of 22 cases. Foot Ankle Int. 2006;27(10):764-770. doi:10.1177/107110070602701002.
3. Cierny G 3rd, Cook WG, Mader JT. Ankle arthrodesis in the presence of ongoing sepsis. Indications, methods, and results. Orthop Clin North Am. 1989;20(4):709-721.
4. Dalla Paola L, Brocco E, Ceccacci T, et al. Limb salvage in Charcot foot and ankle osteomyelitis: combined use single stage/double stage of arthrodesis and external fixation. Foot Ankle Int. 2009;30(11):1065-1070. doi:10.3113/FAI.2009.1065.
5. Eylon S, Porat S, Bor N, Leibner ED. Outcome of Ilizarov ankle arthrodesis. Foot Ankle Int. 2007;28(8):873-879. doi:10.3113/FAI.2007.0873.
6. Kalish S, Fleming J, Weinstein R. External fixators for elective rearfoot and ankle arthrodesis. Techniques and indications. Clin Podiatr Med Surg. 2003;20(1):65-96, vi.
7. Kollig E, Esenwein SA, Muhr G, Kutscha-Lissberg F. Fusion of the septic ankle: experience with 15 cases using hybrid external fixation. J Trauma. 2003;55(4):685-691. doi:10.1097/01.TA.0000051933.83342.E4.
8. Rochman R, Jackson Hutson J, Alade O. Tibiocalcaneal arthrodesis using the Ilizarov technique in the presence of bone loss and infection of the talus. Foot Ankle Int. 2008;29(10):1001-1008. doi:10.3113/FAI.2008.1001.
9. Saltzman CL. Salvage of diffuse ankle osteomyelitis by single-stage resection and circumferential frame compression arthrodesis. Iowa Orthop J. 2005;2547-52.
10. Fragomen AT, Rozbruch SR. The mechanics of external fixation. HSS J. 2007;3(1):13-29. doi:10.1007/s11420-006-9025-0.
11. Hawkins BJ, Langerman RJ, Anger DM, Calhoun JH. The Ilizarov technique in ankle fusion. Clin Orthop Relat Res. 1994;(303):217-225.
12. Jones CP, Youngblood CS, Waldrop N, Davis WH, Pinzur MS. Tibial Stress Fracture Secondary to Half-Pins in Circular Ring External Fixation for Charcot Foot. Foot Ankle Int. 2014;35(6):572-577. doi:10.1177/1071100714531229.
13. Kazmers NH, Fragomen AT, Rozbruch SR. Prevention of pin site infection in external fixation: a review of the literature. Strategies Trauma Limb Reconstr. 2016;11(2):75-85. doi:10.1007/s11751-016-0256-4.
ABSTRACT
Surgical reconstruction of the ankle and hindfoot in patients with diabetes, Charcot neuroarthropathy, osteomyelitis, deformity, and/or bone loss can be challenging and often results in amputation. In these patients, conventional internal fixation with plates, screws, and intramedullary nails is often not feasible because of ongoing infection or poor bone stock and soft tissue quality. The Ilizarov method of ankle and hindfoot arthrodesis is a well-established technique for limb reconstruction that uses circular external fixation to achieve solid bony fusion, optimal leg length, and eradication of infection in cases of complex pathology. This article discusses indications, contraindications, pearls, and pitfalls of performing ankle and hindfoot arthrodesis using the Ilizarov technique.
Continue to: Patients with complex ankle and hindfoot deformity...
Patients with complex ankle and hindfoot deformity present a unique challenge to both nonoperative management and surgical reconstruction. Nonoperative management focuses on wound care, bracing, and immobilization using ankle-foot orthoses, total contact casts, and Charcot restraint orthotic walker boots for external stabilization. Fusion using the Ilizarov technique with circular fixation is a salvage limb-preservation procedure that has shown good results in select patient populations.1-5 Indications include post-traumatic, degenerative, and rheumatoid arthritis, osteomyelitis, tumors, neuromuscular conditions, and salvage of failed ankle and hindfoot procedures.6-9 Relative contraindications include wet gangrene, severe limb ischemia, and soft tissue compromise requiring urgent amputation. In addition, circular frames are not recommended in patients who are unable to comply with postoperative restrictions, and pin and wire care for the duration of frame placement because of personal, psychological, or socioeconomic reasons.
The Ilizarov technique of ring fixation provides dynamic, modular, and rigid fixation in multiple planes to control shear, bending, and rotational forces, and allows for early weight-bearing and postoperative adjustments as needed.10,11 Percutaneously placed half-pins and wires allow for solid fixation in the setting of both poor bone and soft tissue quality, and fusion can be achieved in the presence of active infection in a 1-stage procedure. The goal of ankle and hindfoot fusion using the Ilizarov technique is to achieve an infection-free, stable, plantigrade foot with neutral ankle alignment to allow for patient ambulation and return to activities of daily living.
Nonunion rates with circular fixation are reported to be as high as 16% to 54%, due to medical comorbidities, such as smoking, peripheral vascular disease, and Charcot neuroarthropathy.1Charcot, in particular, is a risk factor for nonunion as patients lack protective sensation, and have a higher rate of wound dehiscence, noncompliance with weight-bearing precautions, pin site infections, and frame breakage. In these patients, tibiotalocalcaneal (TTC) arthrodesis is preferred over the isolated ankle, or subtalar fusion to both provide a stable platform for ambulation and reduce the incidence of adjacent joint breakdown. Common complications of the Ilizarov technique include pin site infections, wire breakage, talar necrosis, and tibial stress fractures after frame removal.1,2,6,11-13 Circular frames are typically maintained for 3 to 8 months, until solid fusion is achieved radiographically. Frames are removed in the operating room with the concurrent examination of the fusion sites under anesthesia followed by a period of protected weight-bearing in a cast or tall controlled ankle motion (CAM) boot.
This article reviews several technical details, tips, and tricks that can help improve the intraoperative and postoperative outcomes of combined ankle and hindfoot arthrodesis using the Ilizarov technique with circular external fixation.
Continue to: SURGICAL TECHNIQUE...
SURGICAL TECHNIQUE
SETUP AND APPROACH
Patients are positioned supine with padding under the operative extremity to achieve neutral leg rotation (Figures 1A-1D). A thigh tourniquet is placed with the foot positioned at the end of the bed and on top of the radiolucent padding to avoid interference of the contralateral leg during lateral X-rays. After sterile prepping and draping, the extremity is exsanguinated above the level of an active infection, and the tourniquet inflated.
For isolated ankle arthrodesis, an anterior or lateral approach can be used, while for TTC arthrodesis, a lateral approach is required to access both the ankle and subtalar joints. A 10-cm longitudinal incision is made along the distal fibula, curving slightly and anteriorly along the distal extent of the incision. Dissection is continued down to bone using full thickness flaps, and the distal fibula is removed 2 to 3 cm above the ankle joint using a saw and osteotome (Figures 2A-2G). The distal fibula can be used subsequently as bone grafts depending on the quality of bone. The peroneal tendons are retracted posteriorly, and dissection is then continued to the posterior facet of the subtalar joint.
JOINT PREPARATION AND ALIGNMENT
Both the anterior and posterior neurovascular bundles are protected along the distal tibia with Hohmann retractors while a saw is used to create flat cuts across the tibial plafond and talus to allow apposition of flat, broad cancellous bony surfaces. Flat cuts followed by later joint compression will often shorten the limb by 2 to 3 cm. This leg length discrepancy can later be accommodated using a shoe lift, as needed. All retained hardware and/or infected and necrotic tissues in the ankle and hindfoot are removed using a rongeur and a pituitary rongeur.
The medial malleolus is osteotomized vertically using a direct medial incision and approach with full thickness flaps, and in line with the previous tibial plafond, is both cut and removed. The medial malleolus can also be used for bone grafts in fusion sites. A smooth-tip lamina spreader is placed in the subtalar joint for distraction and a curved osteotome, curettes, and a small rongeur are used to remove all remaining cartilage from the subtalar joint. Flat cuts in the subtalar joint can remove excessive bone, particularly from the inferior aspect of the talus. The subchondral bone is perforated using a 2.5- to 3.0-mm drill bit and a curved osteotome.
A bone graft from the distal fibula and medial malleolus, with or without the addition of allograft adjuvants, is placed evenly across the ankle and subtalar joints (Figures 3A-3E). At this point, the ankle and subtalar joints can be manipulated in multiple planes to achieve neutral coronal, sagittal, and axial alignment. With both the ankle and hindfoot held in a neutral position, multiple Steinman pins and K-wires in different orientations are inserted through the plantar aspect of the heel to hold the ankle and subtalar joints in place temporarily. Wires are cut short to prevent interference with subsequent foot olive wire placement through the frame.
Continue to: X-rays should be carefully checked...
X-rays should be carefully checked to ensure proper alignment. Wounds are gently irrigated, and vancomycin powder (2 g) can be placed within wounds for local antibiotic delivery. Lateral tissues are sharply debulked to allow for decreased tension on the incision, and small ulcers can be excised in their entirety. Wounds are closed in a layered fashion using 0-polydioxanone (PDS, Ethicon) suture for deep tissue, 2-0 PDS for subcutaneous tissue, and 2-0 nylon for skin closure. The tourniquet is deflated for the remainder of the case to reduce limb ischemia during frame placement.
CIRCULAR FRAME CONCEPTS AND PLACEMENT
The majority of circular frames for both ankle and hindfoot fusion have multiple ring sizes available in aluminum and radiolucent carbon fiber reinforced polymer (Hoffmann LRF, Stryker). Rings are available in full, open, segment, and both short- and long-foot options. Frames can be sterilized in a prebuilt 3 to 4 ring construct with 4 static or dynamic (telescopic) struts (100-277 mm). The most commonly used tibia and foot ring sizes are 155 cm, 180 cm, and 210 cm. Ring size should be able to accommodate posterior soft tissue swelling and avoid circumferential soft tissue abrasion against the rings. Anterior foot arches are used for increased construct stability and can be locked to the distal tibia ring for weight-bearing support. Wire and half-pin bolts, adaptors, and nuts are used to join each ring of the frame to the patient’s bone.
For TTC arthrodesis, 2 rings are typically used in the tibia, and 1 ring is used in the foot. For isolated ankle arthrodesis, an additional ring can be added with olive wires in the talus to permit compression only across the ankle joint. Multiple points of fixation are used in each ring in different planes to achieve both maximal stability and rotational control. If a single wire or half-pin becomes infected and requires removal, there are still multiple other points of fixation in the ring to maintain stability. Fixation within each ring should be off axis compared with the adjacent ring to both avoid stress risers and increase construct rigidity.
The prebuilt frame is checked on the back table to ensure proper orientation and component alignment. The frame is then placed over both the foot and ankle, and multiple stacks of towels are placed behind the heel, ankle, and calf to center the foot and ankle in the frame (Figures 4A-4F). At least 4 to 6 cm of space is needed in between the posterior soft tissues and each ring to accommodate postoperative swelling. On the lateral view, the foot ring should be in the mid-portion of the calcaneus. If there is a concern, particularly in Charcot patients, regarding early weight-bearing noncompliance, the foot ring can be placed flush with the plantar aspect of the foot, and olive wires can be inserted using longer adaptors. The frame should be checked from multiple viewpoints to ensure that both the foot and ankle are centered and in neutral rotation.
Continue to: TIBIA RING FIXATION...
TIBIA RING FIXATION
Tibia rings can be fixed using 2 to 3 half-pins (4-6 mm) alone or 2 half-pins in combination with a smooth wire. A small incision is made over the area of planned half-pin insertion, and the periosteum is cleared away using a hemostat. An adaptor sleeve is used, and the bone is drilled bicortically, followed by insertion of the half-pin. Hydroxyapatite-coated pins are used to improve the strength of the bone-pin interface and reduce the incidence of pin tract infections. Pins are inserted along both the anterior and medial aspects of the tibia, avoiding the thick lateral musculature. Care is taken to protect the medial neurovascular structures during pin placement following established Ilizarov safe zones.
After each pin is placed in the bone, the pin is secured to the adaptor that is then tightened to the ring. This process is repeated for both the proximal and distal tibia rings. Pins should be placed above and below each ring to avoid creating stress risers. During smooth wire placement, each wire is pushed by hand through the soft tissues and then drilled into the bone while the exposed segment is held with a damp sponge to reduce the incidence of thermal bone necrosis. Once the wire is drilled bicortically, a mallet is used to tap the wire through the remaining soft tissues to avoid wrapping them up in the wire. Each wire should be parallel to the ring to get an even line of compression.
Each wire is secured on 1 end and then tensioned to 130 kg using a hand tensioner. An additional tool can be placed in the wire adaptor to prevent the wire from bending during tensioning. If the wire is passing above or below the ring, longer wire adaptors should be used to build to the wire. The wire should never be bent toward the ring as this can increase the likelihood of improper pin tensioning and breakage. Wire placement should be avoided posteriorly as this can make it difficult to secure and/or tension wires, and also increases the risk of damage to posterior structures.
Ring fixation in the distal tibia near the plafond may require 1 half-pin and 2 wires to avoid damage to the tibialis anterior and posterior tibial tendons. In this case, smooth wires should be placed in a crossing pattern and tensioned simultaneously to avoid pulling the ankle away from the center of the frame. Wires should be bent and curved over each ring and then cut to facilitate subsequent removal.
FOOT RING FIXATION
In the foot, olive wires are used to increase fixation against bone. For each olive wire, a small incision is made to accommodate the diameter of the olive through the soft tissue. Similar to the distal tibia, 2 olive wires should be placed above and below the foot ring in a crossing pattern through the calcaneus (Figures 5A-5F). The axial view of the frame should be checked to ensure proper wire orientation. When using olive wires, it is essential to tension both at the same time to 90 kg, as the foot can be pulled medially or laterally in the frame if 1 wire is tensioned before the other.
Forefoot olive wires should also be placed in a crossing pattern, with 1 wire fixed through the first, second, and third metatarsals, and 1 wire through the fourth and fifth metatarsals. Additional forefoot olive wires can be placed if compression is needed across the midfoot or Chopart joints for fusion. Multiple X-rays should be checked to ensure that the calcaneus and forefoot olive wires are firmly fixed both in and against bone.
Continue to: JOINT COMPRESSION AND FINAL FRAME ADJUSTMENTS...
JOINT COMPRESSION AND FINAL FRAME ADJUSTMENTS
Once all rings are secured to the bone with half-pins and wires, the previously placed Steinman pins, and K-wires through the heel are removed. Both ankle and subtalar joint alignments are rechecked, and then axial compression is placed through the foot ring with the knee extended and the struts unlocked. Static or telescopic struts are used to achieve 8 to 10 mm of bony compression. X-rays are taken before and after to analyze final joint compression and alignment. Struts should be sequentially tightened (1/2 turn of a static strut) 1 at a time as final tightening of 1 strut alone can bind and interfere with both the compression and tightening of the remaining struts.
Once final compression is achieved, the struts are locked, and the front foot arch is closed anteriorly and connected to the distal tibia ring for increased stability (Figures 6A-6D). Each pin and wire is covered in a sterile dressing followed by gauze to allow for soft tissue padding. The entire frame is then overwrapped in bias stockinette rolls or ace wraps.
Walking attachments can be added immediately to the frame that allows for early weight-bearing. Rocker shoe attachments with a 15° anterior and posterior slope and rubber soles can help offload the ankle and subtalar joints, decrease pressure on heel strike, and reduce ankle motion during ambulation (Hoffmann LRF, Stryker).
POSTOPERATIVE PROTOCOL
Depending on individual characteristics, patients can be immediately weight-bearing in the circular frame. Patients with Charcot neuroarthropathy are recommended to remain non-weight-bearing for the first 2 months to reduce the likelihood of pin, wire, and frame breakage along with nonunion. Pin and wire site care and maintenance are initiated the day after surgery and continue on a daily basis for the duration of frame placement. Sutures are removed 4 to 5 weeks after surgery to ensure adequate wound healing. Serial X-rays are taken monthly to analyze fusion sites.
If pins or wires become infected, patients are placed on oral antibiotics, and both pins and wires can be removed or exchanged in the operating room. Once fusion is achieved in 3 to 8 months (Figures 7A-7C), the frame is removed in the operating room, and fusion sites are examined under dynamic fluoroscopy. If fusion is confirmed, patients are made weight-bearing as tolerated in a short-leg cast or tall CAM boot for 6 to 8 weeks, and then transitioned to an ankle brace in an accommodative shoe.
Continue to: DISCUSSION...
DISCUSSION
A key aspect of recovery after ankle and hindfoot fusion using the Ilizarov technique is balancing pin care, soft tissue swelling, and weight-bearing status. The average time patients will spend in the frame is approximately 25 to 28 weeks, but can range from 12 to 84 weeks.1,2Given the considerable variability in both soft tissue healing and bony union, patients should be extensively counseled before surgery to set expectations correctly and ensure that they have the necessary help and support to care for the frame during the treatment period. Patients should be followed closely during the first 6 weeks to ensure that pins and wires do not become infected or break, as both of these issues require immediate intervention.
In a review of 11 patients who underwent tibiocalcaneal arthrodesis using an Ilizarov external fixator for infected talar nonunions or extrusions, Rochman and colleagues8 reported an 81% rate of successful fusion with a final mean American Orthopaedic Foot and Ankle Society score of 65 (out of a maximum 86). Similar results were reported by Saltzman9 in a series of 8 patients with diffuse ankle osteomyelitis treated with resection of all infected tissue and hybrid-frame compression arthrodesis. All patients received 6 weeks of intravenous antibiotics, and frames were removed at 3 months, and walking casts were applied for 1 to 2 additional months. Ankle sepsis was eradicated in all patients, and 7/8 (87.5%) ankles successfully fused at an average of 13.5 weeks (range, 10-16 weeks). One limb required below-knee amputation at 5 weeks due to non-reconstructible vascular insufficiency. At an average of 3.4-year follow-up, none of the 7 fused ankles required further surgery.
Fragomen and colleagues1 retrospectively reviewed 101 patients who underwent complex ankle fusion using the Ilizarov technique and found that 76/91 (83.5%) patients achieved fusion at an average of 25 weeks (range, 10-65 weeks). Smoking was associated with a 54% rate of nonunion and 15/19 (79%) patients with Charcot neuroarthropathy achieved ankle fusion, but had a subsequent subtalar joint failure, thus highlighting the need for TTC arthrodesis in Charcot patients. Salem and colleagues2 reviewed 21 Ilizarov ankle fusions and reported that all patients achieved fusion at an average of 28 weeks (range, 12-84 weeks). Complications occurred in 11 patients, including 2 nonunions that healed after revision frame application and 4 pin tract infections.
CONCLUSION
Overall, the Ilizarov technique using circular external fixation is a powerful tool that can be used to treat a variety of disorders including complex foot and ankle deformity and infection. While case series generally show favorable outcomes, patients must be informed that this technique is a salvage procedure for limb preservation that requires meticulous operative technique, diligent postoperative care, and tight control of medical comorbidities, such as blood sugar levels in individuals with diabetes to achieve a successful outcome.
ABSTRACT
Surgical reconstruction of the ankle and hindfoot in patients with diabetes, Charcot neuroarthropathy, osteomyelitis, deformity, and/or bone loss can be challenging and often results in amputation. In these patients, conventional internal fixation with plates, screws, and intramedullary nails is often not feasible because of ongoing infection or poor bone stock and soft tissue quality. The Ilizarov method of ankle and hindfoot arthrodesis is a well-established technique for limb reconstruction that uses circular external fixation to achieve solid bony fusion, optimal leg length, and eradication of infection in cases of complex pathology. This article discusses indications, contraindications, pearls, and pitfalls of performing ankle and hindfoot arthrodesis using the Ilizarov technique.
Continue to: Patients with complex ankle and hindfoot deformity...
Patients with complex ankle and hindfoot deformity present a unique challenge to both nonoperative management and surgical reconstruction. Nonoperative management focuses on wound care, bracing, and immobilization using ankle-foot orthoses, total contact casts, and Charcot restraint orthotic walker boots for external stabilization. Fusion using the Ilizarov technique with circular fixation is a salvage limb-preservation procedure that has shown good results in select patient populations.1-5 Indications include post-traumatic, degenerative, and rheumatoid arthritis, osteomyelitis, tumors, neuromuscular conditions, and salvage of failed ankle and hindfoot procedures.6-9 Relative contraindications include wet gangrene, severe limb ischemia, and soft tissue compromise requiring urgent amputation. In addition, circular frames are not recommended in patients who are unable to comply with postoperative restrictions, and pin and wire care for the duration of frame placement because of personal, psychological, or socioeconomic reasons.
The Ilizarov technique of ring fixation provides dynamic, modular, and rigid fixation in multiple planes to control shear, bending, and rotational forces, and allows for early weight-bearing and postoperative adjustments as needed.10,11 Percutaneously placed half-pins and wires allow for solid fixation in the setting of both poor bone and soft tissue quality, and fusion can be achieved in the presence of active infection in a 1-stage procedure. The goal of ankle and hindfoot fusion using the Ilizarov technique is to achieve an infection-free, stable, plantigrade foot with neutral ankle alignment to allow for patient ambulation and return to activities of daily living.
Nonunion rates with circular fixation are reported to be as high as 16% to 54%, due to medical comorbidities, such as smoking, peripheral vascular disease, and Charcot neuroarthropathy.1Charcot, in particular, is a risk factor for nonunion as patients lack protective sensation, and have a higher rate of wound dehiscence, noncompliance with weight-bearing precautions, pin site infections, and frame breakage. In these patients, tibiotalocalcaneal (TTC) arthrodesis is preferred over the isolated ankle, or subtalar fusion to both provide a stable platform for ambulation and reduce the incidence of adjacent joint breakdown. Common complications of the Ilizarov technique include pin site infections, wire breakage, talar necrosis, and tibial stress fractures after frame removal.1,2,6,11-13 Circular frames are typically maintained for 3 to 8 months, until solid fusion is achieved radiographically. Frames are removed in the operating room with the concurrent examination of the fusion sites under anesthesia followed by a period of protected weight-bearing in a cast or tall controlled ankle motion (CAM) boot.
This article reviews several technical details, tips, and tricks that can help improve the intraoperative and postoperative outcomes of combined ankle and hindfoot arthrodesis using the Ilizarov technique with circular external fixation.
Continue to: SURGICAL TECHNIQUE...
SURGICAL TECHNIQUE
SETUP AND APPROACH
Patients are positioned supine with padding under the operative extremity to achieve neutral leg rotation (Figures 1A-1D). A thigh tourniquet is placed with the foot positioned at the end of the bed and on top of the radiolucent padding to avoid interference of the contralateral leg during lateral X-rays. After sterile prepping and draping, the extremity is exsanguinated above the level of an active infection, and the tourniquet inflated.
For isolated ankle arthrodesis, an anterior or lateral approach can be used, while for TTC arthrodesis, a lateral approach is required to access both the ankle and subtalar joints. A 10-cm longitudinal incision is made along the distal fibula, curving slightly and anteriorly along the distal extent of the incision. Dissection is continued down to bone using full thickness flaps, and the distal fibula is removed 2 to 3 cm above the ankle joint using a saw and osteotome (Figures 2A-2G). The distal fibula can be used subsequently as bone grafts depending on the quality of bone. The peroneal tendons are retracted posteriorly, and dissection is then continued to the posterior facet of the subtalar joint.
JOINT PREPARATION AND ALIGNMENT
Both the anterior and posterior neurovascular bundles are protected along the distal tibia with Hohmann retractors while a saw is used to create flat cuts across the tibial plafond and talus to allow apposition of flat, broad cancellous bony surfaces. Flat cuts followed by later joint compression will often shorten the limb by 2 to 3 cm. This leg length discrepancy can later be accommodated using a shoe lift, as needed. All retained hardware and/or infected and necrotic tissues in the ankle and hindfoot are removed using a rongeur and a pituitary rongeur.
The medial malleolus is osteotomized vertically using a direct medial incision and approach with full thickness flaps, and in line with the previous tibial plafond, is both cut and removed. The medial malleolus can also be used for bone grafts in fusion sites. A smooth-tip lamina spreader is placed in the subtalar joint for distraction and a curved osteotome, curettes, and a small rongeur are used to remove all remaining cartilage from the subtalar joint. Flat cuts in the subtalar joint can remove excessive bone, particularly from the inferior aspect of the talus. The subchondral bone is perforated using a 2.5- to 3.0-mm drill bit and a curved osteotome.
A bone graft from the distal fibula and medial malleolus, with or without the addition of allograft adjuvants, is placed evenly across the ankle and subtalar joints (Figures 3A-3E). At this point, the ankle and subtalar joints can be manipulated in multiple planes to achieve neutral coronal, sagittal, and axial alignment. With both the ankle and hindfoot held in a neutral position, multiple Steinman pins and K-wires in different orientations are inserted through the plantar aspect of the heel to hold the ankle and subtalar joints in place temporarily. Wires are cut short to prevent interference with subsequent foot olive wire placement through the frame.
Continue to: X-rays should be carefully checked...
X-rays should be carefully checked to ensure proper alignment. Wounds are gently irrigated, and vancomycin powder (2 g) can be placed within wounds for local antibiotic delivery. Lateral tissues are sharply debulked to allow for decreased tension on the incision, and small ulcers can be excised in their entirety. Wounds are closed in a layered fashion using 0-polydioxanone (PDS, Ethicon) suture for deep tissue, 2-0 PDS for subcutaneous tissue, and 2-0 nylon for skin closure. The tourniquet is deflated for the remainder of the case to reduce limb ischemia during frame placement.
CIRCULAR FRAME CONCEPTS AND PLACEMENT
The majority of circular frames for both ankle and hindfoot fusion have multiple ring sizes available in aluminum and radiolucent carbon fiber reinforced polymer (Hoffmann LRF, Stryker). Rings are available in full, open, segment, and both short- and long-foot options. Frames can be sterilized in a prebuilt 3 to 4 ring construct with 4 static or dynamic (telescopic) struts (100-277 mm). The most commonly used tibia and foot ring sizes are 155 cm, 180 cm, and 210 cm. Ring size should be able to accommodate posterior soft tissue swelling and avoid circumferential soft tissue abrasion against the rings. Anterior foot arches are used for increased construct stability and can be locked to the distal tibia ring for weight-bearing support. Wire and half-pin bolts, adaptors, and nuts are used to join each ring of the frame to the patient’s bone.
For TTC arthrodesis, 2 rings are typically used in the tibia, and 1 ring is used in the foot. For isolated ankle arthrodesis, an additional ring can be added with olive wires in the talus to permit compression only across the ankle joint. Multiple points of fixation are used in each ring in different planes to achieve both maximal stability and rotational control. If a single wire or half-pin becomes infected and requires removal, there are still multiple other points of fixation in the ring to maintain stability. Fixation within each ring should be off axis compared with the adjacent ring to both avoid stress risers and increase construct rigidity.
The prebuilt frame is checked on the back table to ensure proper orientation and component alignment. The frame is then placed over both the foot and ankle, and multiple stacks of towels are placed behind the heel, ankle, and calf to center the foot and ankle in the frame (Figures 4A-4F). At least 4 to 6 cm of space is needed in between the posterior soft tissues and each ring to accommodate postoperative swelling. On the lateral view, the foot ring should be in the mid-portion of the calcaneus. If there is a concern, particularly in Charcot patients, regarding early weight-bearing noncompliance, the foot ring can be placed flush with the plantar aspect of the foot, and olive wires can be inserted using longer adaptors. The frame should be checked from multiple viewpoints to ensure that both the foot and ankle are centered and in neutral rotation.
Continue to: TIBIA RING FIXATION...
TIBIA RING FIXATION
Tibia rings can be fixed using 2 to 3 half-pins (4-6 mm) alone or 2 half-pins in combination with a smooth wire. A small incision is made over the area of planned half-pin insertion, and the periosteum is cleared away using a hemostat. An adaptor sleeve is used, and the bone is drilled bicortically, followed by insertion of the half-pin. Hydroxyapatite-coated pins are used to improve the strength of the bone-pin interface and reduce the incidence of pin tract infections. Pins are inserted along both the anterior and medial aspects of the tibia, avoiding the thick lateral musculature. Care is taken to protect the medial neurovascular structures during pin placement following established Ilizarov safe zones.
After each pin is placed in the bone, the pin is secured to the adaptor that is then tightened to the ring. This process is repeated for both the proximal and distal tibia rings. Pins should be placed above and below each ring to avoid creating stress risers. During smooth wire placement, each wire is pushed by hand through the soft tissues and then drilled into the bone while the exposed segment is held with a damp sponge to reduce the incidence of thermal bone necrosis. Once the wire is drilled bicortically, a mallet is used to tap the wire through the remaining soft tissues to avoid wrapping them up in the wire. Each wire should be parallel to the ring to get an even line of compression.
Each wire is secured on 1 end and then tensioned to 130 kg using a hand tensioner. An additional tool can be placed in the wire adaptor to prevent the wire from bending during tensioning. If the wire is passing above or below the ring, longer wire adaptors should be used to build to the wire. The wire should never be bent toward the ring as this can increase the likelihood of improper pin tensioning and breakage. Wire placement should be avoided posteriorly as this can make it difficult to secure and/or tension wires, and also increases the risk of damage to posterior structures.
Ring fixation in the distal tibia near the plafond may require 1 half-pin and 2 wires to avoid damage to the tibialis anterior and posterior tibial tendons. In this case, smooth wires should be placed in a crossing pattern and tensioned simultaneously to avoid pulling the ankle away from the center of the frame. Wires should be bent and curved over each ring and then cut to facilitate subsequent removal.
FOOT RING FIXATION
In the foot, olive wires are used to increase fixation against bone. For each olive wire, a small incision is made to accommodate the diameter of the olive through the soft tissue. Similar to the distal tibia, 2 olive wires should be placed above and below the foot ring in a crossing pattern through the calcaneus (Figures 5A-5F). The axial view of the frame should be checked to ensure proper wire orientation. When using olive wires, it is essential to tension both at the same time to 90 kg, as the foot can be pulled medially or laterally in the frame if 1 wire is tensioned before the other.
Forefoot olive wires should also be placed in a crossing pattern, with 1 wire fixed through the first, second, and third metatarsals, and 1 wire through the fourth and fifth metatarsals. Additional forefoot olive wires can be placed if compression is needed across the midfoot or Chopart joints for fusion. Multiple X-rays should be checked to ensure that the calcaneus and forefoot olive wires are firmly fixed both in and against bone.
Continue to: JOINT COMPRESSION AND FINAL FRAME ADJUSTMENTS...
JOINT COMPRESSION AND FINAL FRAME ADJUSTMENTS
Once all rings are secured to the bone with half-pins and wires, the previously placed Steinman pins, and K-wires through the heel are removed. Both ankle and subtalar joint alignments are rechecked, and then axial compression is placed through the foot ring with the knee extended and the struts unlocked. Static or telescopic struts are used to achieve 8 to 10 mm of bony compression. X-rays are taken before and after to analyze final joint compression and alignment. Struts should be sequentially tightened (1/2 turn of a static strut) 1 at a time as final tightening of 1 strut alone can bind and interfere with both the compression and tightening of the remaining struts.
Once final compression is achieved, the struts are locked, and the front foot arch is closed anteriorly and connected to the distal tibia ring for increased stability (Figures 6A-6D). Each pin and wire is covered in a sterile dressing followed by gauze to allow for soft tissue padding. The entire frame is then overwrapped in bias stockinette rolls or ace wraps.
Walking attachments can be added immediately to the frame that allows for early weight-bearing. Rocker shoe attachments with a 15° anterior and posterior slope and rubber soles can help offload the ankle and subtalar joints, decrease pressure on heel strike, and reduce ankle motion during ambulation (Hoffmann LRF, Stryker).
POSTOPERATIVE PROTOCOL
Depending on individual characteristics, patients can be immediately weight-bearing in the circular frame. Patients with Charcot neuroarthropathy are recommended to remain non-weight-bearing for the first 2 months to reduce the likelihood of pin, wire, and frame breakage along with nonunion. Pin and wire site care and maintenance are initiated the day after surgery and continue on a daily basis for the duration of frame placement. Sutures are removed 4 to 5 weeks after surgery to ensure adequate wound healing. Serial X-rays are taken monthly to analyze fusion sites.
If pins or wires become infected, patients are placed on oral antibiotics, and both pins and wires can be removed or exchanged in the operating room. Once fusion is achieved in 3 to 8 months (Figures 7A-7C), the frame is removed in the operating room, and fusion sites are examined under dynamic fluoroscopy. If fusion is confirmed, patients are made weight-bearing as tolerated in a short-leg cast or tall CAM boot for 6 to 8 weeks, and then transitioned to an ankle brace in an accommodative shoe.
Continue to: DISCUSSION...
DISCUSSION
A key aspect of recovery after ankle and hindfoot fusion using the Ilizarov technique is balancing pin care, soft tissue swelling, and weight-bearing status. The average time patients will spend in the frame is approximately 25 to 28 weeks, but can range from 12 to 84 weeks.1,2Given the considerable variability in both soft tissue healing and bony union, patients should be extensively counseled before surgery to set expectations correctly and ensure that they have the necessary help and support to care for the frame during the treatment period. Patients should be followed closely during the first 6 weeks to ensure that pins and wires do not become infected or break, as both of these issues require immediate intervention.
In a review of 11 patients who underwent tibiocalcaneal arthrodesis using an Ilizarov external fixator for infected talar nonunions or extrusions, Rochman and colleagues8 reported an 81% rate of successful fusion with a final mean American Orthopaedic Foot and Ankle Society score of 65 (out of a maximum 86). Similar results were reported by Saltzman9 in a series of 8 patients with diffuse ankle osteomyelitis treated with resection of all infected tissue and hybrid-frame compression arthrodesis. All patients received 6 weeks of intravenous antibiotics, and frames were removed at 3 months, and walking casts were applied for 1 to 2 additional months. Ankle sepsis was eradicated in all patients, and 7/8 (87.5%) ankles successfully fused at an average of 13.5 weeks (range, 10-16 weeks). One limb required below-knee amputation at 5 weeks due to non-reconstructible vascular insufficiency. At an average of 3.4-year follow-up, none of the 7 fused ankles required further surgery.
Fragomen and colleagues1 retrospectively reviewed 101 patients who underwent complex ankle fusion using the Ilizarov technique and found that 76/91 (83.5%) patients achieved fusion at an average of 25 weeks (range, 10-65 weeks). Smoking was associated with a 54% rate of nonunion and 15/19 (79%) patients with Charcot neuroarthropathy achieved ankle fusion, but had a subsequent subtalar joint failure, thus highlighting the need for TTC arthrodesis in Charcot patients. Salem and colleagues2 reviewed 21 Ilizarov ankle fusions and reported that all patients achieved fusion at an average of 28 weeks (range, 12-84 weeks). Complications occurred in 11 patients, including 2 nonunions that healed after revision frame application and 4 pin tract infections.
CONCLUSION
Overall, the Ilizarov technique using circular external fixation is a powerful tool that can be used to treat a variety of disorders including complex foot and ankle deformity and infection. While case series generally show favorable outcomes, patients must be informed that this technique is a salvage procedure for limb preservation that requires meticulous operative technique, diligent postoperative care, and tight control of medical comorbidities, such as blood sugar levels in individuals with diabetes to achieve a successful outcome.
1. Fragomen AT, Borst E, Schachter L, Lyman S, Rozbruch SR. Complex ankle arthrodesis using the Ilizarov method yields high rate of fusion. Clin Orthop Relat Res. 2012;470(10):2864-2873. doi:10.1007/s11999-012-2470-9.
2. Salem KH, Kinzl L, Schmelz A. Ankle arthrodesis using Ilizarov ring fixators: a review of 22 cases. Foot Ankle Int. 2006;27(10):764-770. doi:10.1177/107110070602701002.
3. Cierny G 3rd, Cook WG, Mader JT. Ankle arthrodesis in the presence of ongoing sepsis. Indications, methods, and results. Orthop Clin North Am. 1989;20(4):709-721.
4. Dalla Paola L, Brocco E, Ceccacci T, et al. Limb salvage in Charcot foot and ankle osteomyelitis: combined use single stage/double stage of arthrodesis and external fixation. Foot Ankle Int. 2009;30(11):1065-1070. doi:10.3113/FAI.2009.1065.
5. Eylon S, Porat S, Bor N, Leibner ED. Outcome of Ilizarov ankle arthrodesis. Foot Ankle Int. 2007;28(8):873-879. doi:10.3113/FAI.2007.0873.
6. Kalish S, Fleming J, Weinstein R. External fixators for elective rearfoot and ankle arthrodesis. Techniques and indications. Clin Podiatr Med Surg. 2003;20(1):65-96, vi.
7. Kollig E, Esenwein SA, Muhr G, Kutscha-Lissberg F. Fusion of the septic ankle: experience with 15 cases using hybrid external fixation. J Trauma. 2003;55(4):685-691. doi:10.1097/01.TA.0000051933.83342.E4.
8. Rochman R, Jackson Hutson J, Alade O. Tibiocalcaneal arthrodesis using the Ilizarov technique in the presence of bone loss and infection of the talus. Foot Ankle Int. 2008;29(10):1001-1008. doi:10.3113/FAI.2008.1001.
9. Saltzman CL. Salvage of diffuse ankle osteomyelitis by single-stage resection and circumferential frame compression arthrodesis. Iowa Orthop J. 2005;2547-52.
10. Fragomen AT, Rozbruch SR. The mechanics of external fixation. HSS J. 2007;3(1):13-29. doi:10.1007/s11420-006-9025-0.
11. Hawkins BJ, Langerman RJ, Anger DM, Calhoun JH. The Ilizarov technique in ankle fusion. Clin Orthop Relat Res. 1994;(303):217-225.
12. Jones CP, Youngblood CS, Waldrop N, Davis WH, Pinzur MS. Tibial Stress Fracture Secondary to Half-Pins in Circular Ring External Fixation for Charcot Foot. Foot Ankle Int. 2014;35(6):572-577. doi:10.1177/1071100714531229.
13. Kazmers NH, Fragomen AT, Rozbruch SR. Prevention of pin site infection in external fixation: a review of the literature. Strategies Trauma Limb Reconstr. 2016;11(2):75-85. doi:10.1007/s11751-016-0256-4.
1. Fragomen AT, Borst E, Schachter L, Lyman S, Rozbruch SR. Complex ankle arthrodesis using the Ilizarov method yields high rate of fusion. Clin Orthop Relat Res. 2012;470(10):2864-2873. doi:10.1007/s11999-012-2470-9.
2. Salem KH, Kinzl L, Schmelz A. Ankle arthrodesis using Ilizarov ring fixators: a review of 22 cases. Foot Ankle Int. 2006;27(10):764-770. doi:10.1177/107110070602701002.
3. Cierny G 3rd, Cook WG, Mader JT. Ankle arthrodesis in the presence of ongoing sepsis. Indications, methods, and results. Orthop Clin North Am. 1989;20(4):709-721.
4. Dalla Paola L, Brocco E, Ceccacci T, et al. Limb salvage in Charcot foot and ankle osteomyelitis: combined use single stage/double stage of arthrodesis and external fixation. Foot Ankle Int. 2009;30(11):1065-1070. doi:10.3113/FAI.2009.1065.
5. Eylon S, Porat S, Bor N, Leibner ED. Outcome of Ilizarov ankle arthrodesis. Foot Ankle Int. 2007;28(8):873-879. doi:10.3113/FAI.2007.0873.
6. Kalish S, Fleming J, Weinstein R. External fixators for elective rearfoot and ankle arthrodesis. Techniques and indications. Clin Podiatr Med Surg. 2003;20(1):65-96, vi.
7. Kollig E, Esenwein SA, Muhr G, Kutscha-Lissberg F. Fusion of the septic ankle: experience with 15 cases using hybrid external fixation. J Trauma. 2003;55(4):685-691. doi:10.1097/01.TA.0000051933.83342.E4.
8. Rochman R, Jackson Hutson J, Alade O. Tibiocalcaneal arthrodesis using the Ilizarov technique in the presence of bone loss and infection of the talus. Foot Ankle Int. 2008;29(10):1001-1008. doi:10.3113/FAI.2008.1001.
9. Saltzman CL. Salvage of diffuse ankle osteomyelitis by single-stage resection and circumferential frame compression arthrodesis. Iowa Orthop J. 2005;2547-52.
10. Fragomen AT, Rozbruch SR. The mechanics of external fixation. HSS J. 2007;3(1):13-29. doi:10.1007/s11420-006-9025-0.
11. Hawkins BJ, Langerman RJ, Anger DM, Calhoun JH. The Ilizarov technique in ankle fusion. Clin Orthop Relat Res. 1994;(303):217-225.
12. Jones CP, Youngblood CS, Waldrop N, Davis WH, Pinzur MS. Tibial Stress Fracture Secondary to Half-Pins in Circular Ring External Fixation for Charcot Foot. Foot Ankle Int. 2014;35(6):572-577. doi:10.1177/1071100714531229.
13. Kazmers NH, Fragomen AT, Rozbruch SR. Prevention of pin site infection in external fixation: a review of the literature. Strategies Trauma Limb Reconstr. 2016;11(2):75-85. doi:10.1007/s11751-016-0256-4.
TAKE-HOME POINTS
- Ankle and hindfoot fusion using circular external fixation is a useful surgical technique in patients with diabetes, Charcot, osteomyelitis, deformity, and/or bone and soft tissue compromise in order to obtain solid bony fusion, stable limb alignment, and eradication of infection in cases of complex pathology.
- Deformity correction with osteotomies and meticulous joint preparation is required in order to obtain broad, cancellous bony surfaces for fusion with neutral alignment. Autograft from the distal fibula and/or medial malleolus can be combined with bone allograft to assist with joint fusion.
- The ankle and hindfoot are provisionally pinned into neutral coronal and sagittal alignment through the plantar surface of the foot using large K-wires prior to placement of the lower leg in the center of a circular 3-ring compression frame. Typically, 2 to 3 points of fixation are used per ring with a combination of half-pins and smooth wires.
- Ring attachments are built up or down to the level of the half-pins and wires in order to prevent pins and wires from bending, breaking, or causing iatrogenic deformity during tensioning. Crossing olive wires are used in the midfoot and calcaneus with dual tensioning devices to ensure an even pull on both sides of the foot.
- Dynamic or static compression struts are used to obtain 8 to 10 mm of compression across the ankle and hindfoot, followed by addition of an anterior foot ring to increase construct rigidity. Daily pin care is started 3 to 4 days after surgery and patients are kept non-weight-bearing for approximately 2 months in the frame with a total frame period of 3 to 8 months depending on bony healing on X-ray.
Neoadjuvant combo yields high pathologic response in NSCLC
TORONTO – Neoadjuvant combined chemotherapy and immunotherapy (CT-IO) yielded high pathologic response rates for locally advanced, potentially resectable non-small cell lung cancer in the phase 2 multicenter study Nadim study.
All 30 adults with stage IIIA N2 non-small cell lung cancer (NSCLC) who enrolled in the ongoing single-arm, open-label study and have undergone surgery to date, had resectable tumors following neoadjuvant treatment, Mariano Provencio, MD, of Hospital Puerta de Hierro, Madrid, Spain, reported at the World Conference on Lung Cancer.
The complete and partial response rates following the treatment were 10% and 60%, respectively, and the remaining patients had stable disease, Dr. Provencio said, noting that an additional 10 enrolled patients have completed treatment and are awaiting resection, and another 3 are still completing treatment.
The overall major pathologic response rate was 80%, including 75% with complete remission, he said at the conference, which was sponsored by the International Association for the Study of Lung Cancer.
“With a median follow-up of 4 months, no patients suffered any recurrence,” he said. “We have been unable to identify any significant factor related to the response, but we still do not have information about biomarkers.”
The study participants had a median age of 64 years, most (73%) were men, and all were current or former smokers. All received the planned neoadjuvant doses, which included 3 cycles of intravenous nivolumab at a dose of 360 mg every 3 weeks, plus 200 mg/m2 of paclitaxel and carboplatin at AUC 6 every 3 weeks.
“The most important toxicity was hematological toxicity related to chemotherapy; there were very few episodes of non-hematological toxicity,” he said.
Tumors were then assessed, and surgery–lobectomy in 90% of cases–was performed in the 3rd or 4th week after day 21 of the third neoadjuvant treatment cycle.
“There were no intraoperative complications, and 7 patients experienced some postoperative complications, but there was no postoperative mortality,” he said.
Adjuvant treatment included 240 mg of intravenous nivolumab every 2 weeks for 4 months and then 480 mg every 4 weeks for 8 months after resection.
The primary end-point of the study is 24-month progression-free survival.
CT-IO has a high response rate and longer survival in unselected patients with metastatic NSCLC, but there were previously no data about this combination in the neoadjuvant setting, Dr. Provencio said.
The findings of this ongoing study are limited by their preliminary nature and the related lack of information about overall survival and biomarkers, Dr. Provencio said.
“But the higher complete remission and major pathological response rates make this a promising future treatment in stage 3 [NSCLC,] he said.
Indeed, the Nadim study thus far has shown “an amazing result that needs to be verified ... [there is] no prospective proof yet in this context that major pathologic response is parlayed with improved [progression-free survival] or [overall survival],” said discussant Corey Langer, MD, professor of medicine and director of thoracic oncology at the University of Pennsylvania, Philadelphia. “We need to track the late sequelae and [central nervous system] recurrences.”
Dr. Provencio reported relationships with AstraZeneca, Bristol-Meyers Squibb, Fabre, Merck, Pierre, Roche, and Bristol-Meyers Squibb. Dr. Langer reported relationships with numerous companies and organizations.
SOURCE: Provencio, Mario et al., WCLC 2018 Abstract OA01.05.
TORONTO – Neoadjuvant combined chemotherapy and immunotherapy (CT-IO) yielded high pathologic response rates for locally advanced, potentially resectable non-small cell lung cancer in the phase 2 multicenter study Nadim study.
All 30 adults with stage IIIA N2 non-small cell lung cancer (NSCLC) who enrolled in the ongoing single-arm, open-label study and have undergone surgery to date, had resectable tumors following neoadjuvant treatment, Mariano Provencio, MD, of Hospital Puerta de Hierro, Madrid, Spain, reported at the World Conference on Lung Cancer.
The complete and partial response rates following the treatment were 10% and 60%, respectively, and the remaining patients had stable disease, Dr. Provencio said, noting that an additional 10 enrolled patients have completed treatment and are awaiting resection, and another 3 are still completing treatment.
The overall major pathologic response rate was 80%, including 75% with complete remission, he said at the conference, which was sponsored by the International Association for the Study of Lung Cancer.
“With a median follow-up of 4 months, no patients suffered any recurrence,” he said. “We have been unable to identify any significant factor related to the response, but we still do not have information about biomarkers.”
The study participants had a median age of 64 years, most (73%) were men, and all were current or former smokers. All received the planned neoadjuvant doses, which included 3 cycles of intravenous nivolumab at a dose of 360 mg every 3 weeks, plus 200 mg/m2 of paclitaxel and carboplatin at AUC 6 every 3 weeks.
“The most important toxicity was hematological toxicity related to chemotherapy; there were very few episodes of non-hematological toxicity,” he said.
Tumors were then assessed, and surgery–lobectomy in 90% of cases–was performed in the 3rd or 4th week after day 21 of the third neoadjuvant treatment cycle.
“There were no intraoperative complications, and 7 patients experienced some postoperative complications, but there was no postoperative mortality,” he said.
Adjuvant treatment included 240 mg of intravenous nivolumab every 2 weeks for 4 months and then 480 mg every 4 weeks for 8 months after resection.
The primary end-point of the study is 24-month progression-free survival.
CT-IO has a high response rate and longer survival in unselected patients with metastatic NSCLC, but there were previously no data about this combination in the neoadjuvant setting, Dr. Provencio said.
The findings of this ongoing study are limited by their preliminary nature and the related lack of information about overall survival and biomarkers, Dr. Provencio said.
“But the higher complete remission and major pathological response rates make this a promising future treatment in stage 3 [NSCLC,] he said.
Indeed, the Nadim study thus far has shown “an amazing result that needs to be verified ... [there is] no prospective proof yet in this context that major pathologic response is parlayed with improved [progression-free survival] or [overall survival],” said discussant Corey Langer, MD, professor of medicine and director of thoracic oncology at the University of Pennsylvania, Philadelphia. “We need to track the late sequelae and [central nervous system] recurrences.”
Dr. Provencio reported relationships with AstraZeneca, Bristol-Meyers Squibb, Fabre, Merck, Pierre, Roche, and Bristol-Meyers Squibb. Dr. Langer reported relationships with numerous companies and organizations.
SOURCE: Provencio, Mario et al., WCLC 2018 Abstract OA01.05.
TORONTO – Neoadjuvant combined chemotherapy and immunotherapy (CT-IO) yielded high pathologic response rates for locally advanced, potentially resectable non-small cell lung cancer in the phase 2 multicenter study Nadim study.
All 30 adults with stage IIIA N2 non-small cell lung cancer (NSCLC) who enrolled in the ongoing single-arm, open-label study and have undergone surgery to date, had resectable tumors following neoadjuvant treatment, Mariano Provencio, MD, of Hospital Puerta de Hierro, Madrid, Spain, reported at the World Conference on Lung Cancer.
The complete and partial response rates following the treatment were 10% and 60%, respectively, and the remaining patients had stable disease, Dr. Provencio said, noting that an additional 10 enrolled patients have completed treatment and are awaiting resection, and another 3 are still completing treatment.
The overall major pathologic response rate was 80%, including 75% with complete remission, he said at the conference, which was sponsored by the International Association for the Study of Lung Cancer.
“With a median follow-up of 4 months, no patients suffered any recurrence,” he said. “We have been unable to identify any significant factor related to the response, but we still do not have information about biomarkers.”
The study participants had a median age of 64 years, most (73%) were men, and all were current or former smokers. All received the planned neoadjuvant doses, which included 3 cycles of intravenous nivolumab at a dose of 360 mg every 3 weeks, plus 200 mg/m2 of paclitaxel and carboplatin at AUC 6 every 3 weeks.
“The most important toxicity was hematological toxicity related to chemotherapy; there were very few episodes of non-hematological toxicity,” he said.
Tumors were then assessed, and surgery–lobectomy in 90% of cases–was performed in the 3rd or 4th week after day 21 of the third neoadjuvant treatment cycle.
“There were no intraoperative complications, and 7 patients experienced some postoperative complications, but there was no postoperative mortality,” he said.
Adjuvant treatment included 240 mg of intravenous nivolumab every 2 weeks for 4 months and then 480 mg every 4 weeks for 8 months after resection.
The primary end-point of the study is 24-month progression-free survival.
CT-IO has a high response rate and longer survival in unselected patients with metastatic NSCLC, but there were previously no data about this combination in the neoadjuvant setting, Dr. Provencio said.
The findings of this ongoing study are limited by their preliminary nature and the related lack of information about overall survival and biomarkers, Dr. Provencio said.
“But the higher complete remission and major pathological response rates make this a promising future treatment in stage 3 [NSCLC,] he said.
Indeed, the Nadim study thus far has shown “an amazing result that needs to be verified ... [there is] no prospective proof yet in this context that major pathologic response is parlayed with improved [progression-free survival] or [overall survival],” said discussant Corey Langer, MD, professor of medicine and director of thoracic oncology at the University of Pennsylvania, Philadelphia. “We need to track the late sequelae and [central nervous system] recurrences.”
Dr. Provencio reported relationships with AstraZeneca, Bristol-Meyers Squibb, Fabre, Merck, Pierre, Roche, and Bristol-Meyers Squibb. Dr. Langer reported relationships with numerous companies and organizations.
SOURCE: Provencio, Mario et al., WCLC 2018 Abstract OA01.05.
REPORTING FROM WCLC 2018
Key clinical point: Neoadjuvant CT-IO yielded high pathologic response rates for stage IIIA N2 NSCLC.
Major finding: The major pathologic response rate was 80%, including 10% with a complete response.
Study details: A phase 2, single-arm, open-label study of 30 patients.
Disclosures: Dr. Provencio reported relationships with AstraZeneca, Bristol-Meyers Squibb, Fabre, Merck, Pierre, Roche, and Bristol-Meyers Squibb. Dr. Langer reported relationships with numerous companies and organizations.
Source: Provencio, Mario et al., WCLC 2018 Abstract OA01.05.
Transition to noninjected drug use lowers risk of HCV infection
A protective effect was seen with regard to hepatitis C virus (HCV) transmission among individuals who “reversed transitioned” to noninjected drug use and in persons who used noninjected drugs in addition to injecting, according to an interview study of 846 drug users.
Data were collected between January 2007 and December 2017 as part of a long-running study of persons entering Mount Sinai Beth Israel drug detoxification and methadone maintenance programs, reported Don C. Des Jarlais, MD, of the Icahn School of Medicine at Mount Sinai, New York, and his colleagues.
Among the 846 individuals studied, men comprised 79%; 41% were white, 15% African American, and 40% Hispanic, with a mean age of 35 years. They were all currently persons who injected drugs (PWID), according to the report published in Drug and Alcohol Dependence.
A total of 97 persons (11%) “reverse transitioned” back to noninjected drug use, according to the researchers; this reverse transitioning was strongly associated with lower HCV positivity, compared with those who continued injecting (30% vs. 47%, respectively; P less than .005).
There was a substantial difference in the HCV prevalence between PWID who reported noninjected use of heroin (43%) versus PWID who did not report noninjected use of heroin (53%) (P = .005).
Distributive needle sharing was common in HCV seropositives who were injecting cocaine in the 6 months prior to the interview: 28% of the HCV seropositives injecting cocaine reported distributive sharing versus 14% of the HCV seropositives not injecting cocaine (P = .001).
“Harm reduction for PWID has been largely defined in terms of ‘safer injection,’ using sterile injection equipment, proper preparation of the injecting site, etc. We would like to suggest that harm reduction for PWID be broadened to include reverse transitions back to exclusive noninjecting use and frequent substitution of noninjecting for injecting use,” the investigators suggested.
The study was funded by the U.S. National Institute on Drug Abuse. The authors reported that they had no conflicts of interest.
SOURCE: Des Jarlais DC et al. Drug Alcohol Depend. 2018 Sep 12. doi: 10.1016/j.drugalcdep.2018.07.034.
A protective effect was seen with regard to hepatitis C virus (HCV) transmission among individuals who “reversed transitioned” to noninjected drug use and in persons who used noninjected drugs in addition to injecting, according to an interview study of 846 drug users.
Data were collected between January 2007 and December 2017 as part of a long-running study of persons entering Mount Sinai Beth Israel drug detoxification and methadone maintenance programs, reported Don C. Des Jarlais, MD, of the Icahn School of Medicine at Mount Sinai, New York, and his colleagues.
Among the 846 individuals studied, men comprised 79%; 41% were white, 15% African American, and 40% Hispanic, with a mean age of 35 years. They were all currently persons who injected drugs (PWID), according to the report published in Drug and Alcohol Dependence.
A total of 97 persons (11%) “reverse transitioned” back to noninjected drug use, according to the researchers; this reverse transitioning was strongly associated with lower HCV positivity, compared with those who continued injecting (30% vs. 47%, respectively; P less than .005).
There was a substantial difference in the HCV prevalence between PWID who reported noninjected use of heroin (43%) versus PWID who did not report noninjected use of heroin (53%) (P = .005).
Distributive needle sharing was common in HCV seropositives who were injecting cocaine in the 6 months prior to the interview: 28% of the HCV seropositives injecting cocaine reported distributive sharing versus 14% of the HCV seropositives not injecting cocaine (P = .001).
“Harm reduction for PWID has been largely defined in terms of ‘safer injection,’ using sterile injection equipment, proper preparation of the injecting site, etc. We would like to suggest that harm reduction for PWID be broadened to include reverse transitions back to exclusive noninjecting use and frequent substitution of noninjecting for injecting use,” the investigators suggested.
The study was funded by the U.S. National Institute on Drug Abuse. The authors reported that they had no conflicts of interest.
SOURCE: Des Jarlais DC et al. Drug Alcohol Depend. 2018 Sep 12. doi: 10.1016/j.drugalcdep.2018.07.034.
A protective effect was seen with regard to hepatitis C virus (HCV) transmission among individuals who “reversed transitioned” to noninjected drug use and in persons who used noninjected drugs in addition to injecting, according to an interview study of 846 drug users.
Data were collected between January 2007 and December 2017 as part of a long-running study of persons entering Mount Sinai Beth Israel drug detoxification and methadone maintenance programs, reported Don C. Des Jarlais, MD, of the Icahn School of Medicine at Mount Sinai, New York, and his colleagues.
Among the 846 individuals studied, men comprised 79%; 41% were white, 15% African American, and 40% Hispanic, with a mean age of 35 years. They were all currently persons who injected drugs (PWID), according to the report published in Drug and Alcohol Dependence.
A total of 97 persons (11%) “reverse transitioned” back to noninjected drug use, according to the researchers; this reverse transitioning was strongly associated with lower HCV positivity, compared with those who continued injecting (30% vs. 47%, respectively; P less than .005).
There was a substantial difference in the HCV prevalence between PWID who reported noninjected use of heroin (43%) versus PWID who did not report noninjected use of heroin (53%) (P = .005).
Distributive needle sharing was common in HCV seropositives who were injecting cocaine in the 6 months prior to the interview: 28% of the HCV seropositives injecting cocaine reported distributive sharing versus 14% of the HCV seropositives not injecting cocaine (P = .001).
“Harm reduction for PWID has been largely defined in terms of ‘safer injection,’ using sterile injection equipment, proper preparation of the injecting site, etc. We would like to suggest that harm reduction for PWID be broadened to include reverse transitions back to exclusive noninjecting use and frequent substitution of noninjecting for injecting use,” the investigators suggested.
The study was funded by the U.S. National Institute on Drug Abuse. The authors reported that they had no conflicts of interest.
SOURCE: Des Jarlais DC et al. Drug Alcohol Depend. 2018 Sep 12. doi: 10.1016/j.drugalcdep.2018.07.034.
FROM DRUG AND ALCOHOL DEPENDENCE
Key clinical point: Encouraging reverse transitioning to noninjected drug use could help persons who injected drugs lower their risk of hepatitis C virus infection.
Major finding: Reverse transitioning was strongly associated with lower hepatitis C virus positivity, compared with continued drug injection (P less than .005).
Study details: An interview study of 846 drug users.
Disclosures: The study was funded by the U.S. National Institute on Drug Abuse. The authors reported that they had no conflicts of interest.
Source: Des Jarlais DC et al. Drug Alcohol Depend. 2018 Sep 12. doi: 10.1016/j.drugalcdep.2018.07.034.
5 things to know about Trump’s new ‘public charge’ immigration proposal
A proposed rule from the White House would make it harder for legal immigrants to get green cards if they have received certain kinds of public assistance – including Medicaid, food stamps, and housing subsidies. Green cards allow them to live and work permanently in the United States.
“Those seeking to immigrate to the United States must show they can support themselves financially,” Homeland Security Secretary Kirstjen Nielsen said in a statement.
The proposal, announced Sept. 22, marks a new frontier in the administration’s long-term effort to curb immigration, both legal and illegal. It already has spurred intense criticism from Democrats, anti-poverty activists, health care organizations, and immigrants’ rights advocates, who call its restrictions unprecedented.
“We are operating in an overall climate of tremendous fear and anxiety as a result of the administration’s overall approach to immigration enforcement and immigration policy,” said Mark Greenberg, a senior fellow at the Migration Policy Institute, which studies migration and refugee policies at local, national, and international levels. He is also a former Obama administration official.
But what effect would this proposal have?
It’s a complicated question, touching upon vast government programs, with billions of dollars at stake. While the implications aren’t all immediately clear, Kaiser Health News breaks down some of the key elements.
1. First thing first: What is the White House proposing?
The Trump administration wants to redefine a status known as “public charge” – a category used to determine whether someone seeking permanent resident status is “likely to become primarily dependent on the government for subsistence.”
In the past, people have been at risk of being defined a “public charge” if they took cash welfare – known as Temporary Assistance for Needy Families, or Supplemental Security Income – or federal help paying for long-term care. (Immigrants must be in the country legally for 5 years before being eligible for TANF or SSI.)
And that “public charge” designation could undermine their applications for permanent residence.
The new rule would expand the list to include some health insurance, food, and housing programs. Specifically, it would penalize green-card applicants for using Medicaid, a federal-state health plan for low-income people. (Penalties would not apply for using Medicaid in certain emergencies or for some Medicaid services provided through schools and disability programs.)
Using food stamps, Section 8 rental assistance, and federal housing vouchers also would count against applicants. Enrollment in a Medicare Part D program subsidy to help low-income people buy prescription drugs would work against them, too.
The proposal “is definitely a dramatic change from how public charge works today,” said Kelly Whitener, an associate professor at Georgetown University’s Center for Children and Families who specializes in pediatric health benefits and managed-care systems.
A leaked version of the rule from March suggested officials then were also considering penalizing those who receive subsidies to buy health insurance on the Affordable Care Act marketplaces. But that idea was not in the proposal. The marketplace subsidies are aimed at people at a generally higher income bracket than the beneficiaries targeted in the Trump plan, Whitener noted.
“They’re really homing in on low-income immigrants,” she added.
Nielsen said the proposed rule is “intended to promote immigrant self-sufficiency and protect finite resources.”
2. Is this as unprecedented as critics say?
Yes.
Public charge is an old idea. In the 1990s, lawmakers expanded it to consider explicitly whether people had received cash-based welfare.
But including programs like Medicaid and food stamps, which are much wider in scope, is a significant change. It would more likely hit working people – the majority of people on Medicaid are themselves employed, and almost 80% live in families with at least one working member, according to data compiled by the Kaiser Family Foundation. (Kaiser Health News is an editorially independent program of the foundation.)
Children who are American citizens but whose parents are immigrants could be more likely to suffer repercussions, said some experts. When parents opt out of public assistance for fear of their own legal status, their kids are less likely to be enrolled in programs such as the Children’s Health Insurance Program, or CHIP, for which they would qualify.
To be clear, receiving public aid wouldn’t necessarily stop people from getting a green card. But it would tilt the odds against them.
“Another piece is the enormous discretion the administration will have under its proposal in making judgments about who gets admitted to the country and who gets a green card,” said the Migration Policy Institute’s Greenberg.
3. When will the policy shift take effect?
This is an early step in the complex federal rule-making process. And a lot could still change.
Once the proposed rule appears in the Federal Register, a 60-day countdown starts, during which anyone can weigh in with comments.
A final rule likely wouldn’t take effect until 2019.
And DHS is still seeking input on some details. For instance, it hasn’t decided whether CHIP would be counted as one of the “public charge” eligible programs.
In the interim, people who had received public benefits before the rule took effect would not be penalized for doing so.
4. Already, though, the proposal is having effects
DHS estimates that 2.5% of eligible immigrants would drop out of public benefits programs because of this change – which would tally about $1.5 billion worth of federal money per year. But others expect a much larger impact.
“The chilling effects will be vastly greater than the individuals directly affected,” Greenberg said. “There’s considerable reason to believe that [the White House estimate] may be a significant understatement.”
In the proposed rule, DHS notes that the changes could result in “worse health outcomes,” “increased use of emergency rooms,” “increased prevalence of communicable diseases,” “increased rates of poverty,” and other concerns.
Given the complexity of these programs and the proposed rule – and the high stakes at play – low-income immigrants would be much more likely to avoid public benefits altogether, immigration experts said. Millions of immigrants are likely to be affected directly or indirectly, according to the Center for Law and Social Policy, a D.C.-based nonprofit organization.
That could have stark health implications.
Take free vaccines, for which children are often eligible and which would not be subject to the public charge rule. Families afraid of jeopardizing a green card could still be more likely to opt out of that service, Whitener said.
Already, she added, there are reports of people declining federal assistance – even though nothing has yet happened.
“The fear factor cannot be underestimated,” she said.
5. Will people sue?
Legal action is likely.
Officials such as California Attorney General Xavier Becerra, who has frequently clashed with the White House, are weighing challenges to the rule.
“The Trump Administration’s proposal punishes hard-working immigrant families – even targeting children who are citizens – for utilizing programs that provide basic nutrition and healthcare. This is an assault on our families and our communities,” Becerra said in a statement.
But these actions depend on the final shape of the regulation, which could change through the rule-making process.
“They are likely to receive a very large number of sharply critical comments, and there is no way to know what changes they might make as a result,” Greenberg said.
Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente. KHN’s coverage of children’s health care issues is supported in part by the Heising-Simons Foundation.
A proposed rule from the White House would make it harder for legal immigrants to get green cards if they have received certain kinds of public assistance – including Medicaid, food stamps, and housing subsidies. Green cards allow them to live and work permanently in the United States.
“Those seeking to immigrate to the United States must show they can support themselves financially,” Homeland Security Secretary Kirstjen Nielsen said in a statement.
The proposal, announced Sept. 22, marks a new frontier in the administration’s long-term effort to curb immigration, both legal and illegal. It already has spurred intense criticism from Democrats, anti-poverty activists, health care organizations, and immigrants’ rights advocates, who call its restrictions unprecedented.
“We are operating in an overall climate of tremendous fear and anxiety as a result of the administration’s overall approach to immigration enforcement and immigration policy,” said Mark Greenberg, a senior fellow at the Migration Policy Institute, which studies migration and refugee policies at local, national, and international levels. He is also a former Obama administration official.
But what effect would this proposal have?
It’s a complicated question, touching upon vast government programs, with billions of dollars at stake. While the implications aren’t all immediately clear, Kaiser Health News breaks down some of the key elements.
1. First thing first: What is the White House proposing?
The Trump administration wants to redefine a status known as “public charge” – a category used to determine whether someone seeking permanent resident status is “likely to become primarily dependent on the government for subsistence.”
In the past, people have been at risk of being defined a “public charge” if they took cash welfare – known as Temporary Assistance for Needy Families, or Supplemental Security Income – or federal help paying for long-term care. (Immigrants must be in the country legally for 5 years before being eligible for TANF or SSI.)
And that “public charge” designation could undermine their applications for permanent residence.
The new rule would expand the list to include some health insurance, food, and housing programs. Specifically, it would penalize green-card applicants for using Medicaid, a federal-state health plan for low-income people. (Penalties would not apply for using Medicaid in certain emergencies or for some Medicaid services provided through schools and disability programs.)
Using food stamps, Section 8 rental assistance, and federal housing vouchers also would count against applicants. Enrollment in a Medicare Part D program subsidy to help low-income people buy prescription drugs would work against them, too.
The proposal “is definitely a dramatic change from how public charge works today,” said Kelly Whitener, an associate professor at Georgetown University’s Center for Children and Families who specializes in pediatric health benefits and managed-care systems.
A leaked version of the rule from March suggested officials then were also considering penalizing those who receive subsidies to buy health insurance on the Affordable Care Act marketplaces. But that idea was not in the proposal. The marketplace subsidies are aimed at people at a generally higher income bracket than the beneficiaries targeted in the Trump plan, Whitener noted.
“They’re really homing in on low-income immigrants,” she added.
Nielsen said the proposed rule is “intended to promote immigrant self-sufficiency and protect finite resources.”
2. Is this as unprecedented as critics say?
Yes.
Public charge is an old idea. In the 1990s, lawmakers expanded it to consider explicitly whether people had received cash-based welfare.
But including programs like Medicaid and food stamps, which are much wider in scope, is a significant change. It would more likely hit working people – the majority of people on Medicaid are themselves employed, and almost 80% live in families with at least one working member, according to data compiled by the Kaiser Family Foundation. (Kaiser Health News is an editorially independent program of the foundation.)
Children who are American citizens but whose parents are immigrants could be more likely to suffer repercussions, said some experts. When parents opt out of public assistance for fear of their own legal status, their kids are less likely to be enrolled in programs such as the Children’s Health Insurance Program, or CHIP, for which they would qualify.
To be clear, receiving public aid wouldn’t necessarily stop people from getting a green card. But it would tilt the odds against them.
“Another piece is the enormous discretion the administration will have under its proposal in making judgments about who gets admitted to the country and who gets a green card,” said the Migration Policy Institute’s Greenberg.
3. When will the policy shift take effect?
This is an early step in the complex federal rule-making process. And a lot could still change.
Once the proposed rule appears in the Federal Register, a 60-day countdown starts, during which anyone can weigh in with comments.
A final rule likely wouldn’t take effect until 2019.
And DHS is still seeking input on some details. For instance, it hasn’t decided whether CHIP would be counted as one of the “public charge” eligible programs.
In the interim, people who had received public benefits before the rule took effect would not be penalized for doing so.
4. Already, though, the proposal is having effects
DHS estimates that 2.5% of eligible immigrants would drop out of public benefits programs because of this change – which would tally about $1.5 billion worth of federal money per year. But others expect a much larger impact.
“The chilling effects will be vastly greater than the individuals directly affected,” Greenberg said. “There’s considerable reason to believe that [the White House estimate] may be a significant understatement.”
In the proposed rule, DHS notes that the changes could result in “worse health outcomes,” “increased use of emergency rooms,” “increased prevalence of communicable diseases,” “increased rates of poverty,” and other concerns.
Given the complexity of these programs and the proposed rule – and the high stakes at play – low-income immigrants would be much more likely to avoid public benefits altogether, immigration experts said. Millions of immigrants are likely to be affected directly or indirectly, according to the Center for Law and Social Policy, a D.C.-based nonprofit organization.
That could have stark health implications.
Take free vaccines, for which children are often eligible and which would not be subject to the public charge rule. Families afraid of jeopardizing a green card could still be more likely to opt out of that service, Whitener said.
Already, she added, there are reports of people declining federal assistance – even though nothing has yet happened.
“The fear factor cannot be underestimated,” she said.
5. Will people sue?
Legal action is likely.
Officials such as California Attorney General Xavier Becerra, who has frequently clashed with the White House, are weighing challenges to the rule.
“The Trump Administration’s proposal punishes hard-working immigrant families – even targeting children who are citizens – for utilizing programs that provide basic nutrition and healthcare. This is an assault on our families and our communities,” Becerra said in a statement.
But these actions depend on the final shape of the regulation, which could change through the rule-making process.
“They are likely to receive a very large number of sharply critical comments, and there is no way to know what changes they might make as a result,” Greenberg said.
Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente. KHN’s coverage of children’s health care issues is supported in part by the Heising-Simons Foundation.
A proposed rule from the White House would make it harder for legal immigrants to get green cards if they have received certain kinds of public assistance – including Medicaid, food stamps, and housing subsidies. Green cards allow them to live and work permanently in the United States.
“Those seeking to immigrate to the United States must show they can support themselves financially,” Homeland Security Secretary Kirstjen Nielsen said in a statement.
The proposal, announced Sept. 22, marks a new frontier in the administration’s long-term effort to curb immigration, both legal and illegal. It already has spurred intense criticism from Democrats, anti-poverty activists, health care organizations, and immigrants’ rights advocates, who call its restrictions unprecedented.
“We are operating in an overall climate of tremendous fear and anxiety as a result of the administration’s overall approach to immigration enforcement and immigration policy,” said Mark Greenberg, a senior fellow at the Migration Policy Institute, which studies migration and refugee policies at local, national, and international levels. He is also a former Obama administration official.
But what effect would this proposal have?
It’s a complicated question, touching upon vast government programs, with billions of dollars at stake. While the implications aren’t all immediately clear, Kaiser Health News breaks down some of the key elements.
1. First thing first: What is the White House proposing?
The Trump administration wants to redefine a status known as “public charge” – a category used to determine whether someone seeking permanent resident status is “likely to become primarily dependent on the government for subsistence.”
In the past, people have been at risk of being defined a “public charge” if they took cash welfare – known as Temporary Assistance for Needy Families, or Supplemental Security Income – or federal help paying for long-term care. (Immigrants must be in the country legally for 5 years before being eligible for TANF or SSI.)
And that “public charge” designation could undermine their applications for permanent residence.
The new rule would expand the list to include some health insurance, food, and housing programs. Specifically, it would penalize green-card applicants for using Medicaid, a federal-state health plan for low-income people. (Penalties would not apply for using Medicaid in certain emergencies or for some Medicaid services provided through schools and disability programs.)
Using food stamps, Section 8 rental assistance, and federal housing vouchers also would count against applicants. Enrollment in a Medicare Part D program subsidy to help low-income people buy prescription drugs would work against them, too.
The proposal “is definitely a dramatic change from how public charge works today,” said Kelly Whitener, an associate professor at Georgetown University’s Center for Children and Families who specializes in pediatric health benefits and managed-care systems.
A leaked version of the rule from March suggested officials then were also considering penalizing those who receive subsidies to buy health insurance on the Affordable Care Act marketplaces. But that idea was not in the proposal. The marketplace subsidies are aimed at people at a generally higher income bracket than the beneficiaries targeted in the Trump plan, Whitener noted.
“They’re really homing in on low-income immigrants,” she added.
Nielsen said the proposed rule is “intended to promote immigrant self-sufficiency and protect finite resources.”
2. Is this as unprecedented as critics say?
Yes.
Public charge is an old idea. In the 1990s, lawmakers expanded it to consider explicitly whether people had received cash-based welfare.
But including programs like Medicaid and food stamps, which are much wider in scope, is a significant change. It would more likely hit working people – the majority of people on Medicaid are themselves employed, and almost 80% live in families with at least one working member, according to data compiled by the Kaiser Family Foundation. (Kaiser Health News is an editorially independent program of the foundation.)
Children who are American citizens but whose parents are immigrants could be more likely to suffer repercussions, said some experts. When parents opt out of public assistance for fear of their own legal status, their kids are less likely to be enrolled in programs such as the Children’s Health Insurance Program, or CHIP, for which they would qualify.
To be clear, receiving public aid wouldn’t necessarily stop people from getting a green card. But it would tilt the odds against them.
“Another piece is the enormous discretion the administration will have under its proposal in making judgments about who gets admitted to the country and who gets a green card,” said the Migration Policy Institute’s Greenberg.
3. When will the policy shift take effect?
This is an early step in the complex federal rule-making process. And a lot could still change.
Once the proposed rule appears in the Federal Register, a 60-day countdown starts, during which anyone can weigh in with comments.
A final rule likely wouldn’t take effect until 2019.
And DHS is still seeking input on some details. For instance, it hasn’t decided whether CHIP would be counted as one of the “public charge” eligible programs.
In the interim, people who had received public benefits before the rule took effect would not be penalized for doing so.
4. Already, though, the proposal is having effects
DHS estimates that 2.5% of eligible immigrants would drop out of public benefits programs because of this change – which would tally about $1.5 billion worth of federal money per year. But others expect a much larger impact.
“The chilling effects will be vastly greater than the individuals directly affected,” Greenberg said. “There’s considerable reason to believe that [the White House estimate] may be a significant understatement.”
In the proposed rule, DHS notes that the changes could result in “worse health outcomes,” “increased use of emergency rooms,” “increased prevalence of communicable diseases,” “increased rates of poverty,” and other concerns.
Given the complexity of these programs and the proposed rule – and the high stakes at play – low-income immigrants would be much more likely to avoid public benefits altogether, immigration experts said. Millions of immigrants are likely to be affected directly or indirectly, according to the Center for Law and Social Policy, a D.C.-based nonprofit organization.
That could have stark health implications.
Take free vaccines, for which children are often eligible and which would not be subject to the public charge rule. Families afraid of jeopardizing a green card could still be more likely to opt out of that service, Whitener said.
Already, she added, there are reports of people declining federal assistance – even though nothing has yet happened.
“The fear factor cannot be underestimated,” she said.
5. Will people sue?
Legal action is likely.
Officials such as California Attorney General Xavier Becerra, who has frequently clashed with the White House, are weighing challenges to the rule.
“The Trump Administration’s proposal punishes hard-working immigrant families – even targeting children who are citizens – for utilizing programs that provide basic nutrition and healthcare. This is an assault on our families and our communities,” Becerra said in a statement.
But these actions depend on the final shape of the regulation, which could change through the rule-making process.
“They are likely to receive a very large number of sharply critical comments, and there is no way to know what changes they might make as a result,” Greenberg said.
Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente. KHN’s coverage of children’s health care issues is supported in part by the Heising-Simons Foundation.
Mutation in patients with chronic HCV raises risk of liver cancer
An adenine mutation in the MUTYH base repair excision gene increased the risk of developing hepatocellular carcinoma (HCC) in patients with chronic hepatitis C virus (HCV). The mutation was discovered in an analysis of 19 tagging single-nucleotide polymorphism (SNP) variants examined for multiple base repair excision genes (MUTYH, OGG1, and MTH1), according to the results of a retrospective analysis of patient genotypes reported in Free Radical Biology and Medicine (2018;129:88-96).
In addition, the researchers examined MUTYH-null mice to assess the involvement of oxidative stress and DNA repair enzymes in hepatocarcinogenesis.
One significant SNP was found and confirmed from 93 Japanese patients with chronic HCV (38 with HCC and 55 controls), according to Akira Sakurada, MD, of Sapporo (Japan) Medical University, and his colleagues.
Patients diagnosed as having chronic HCV between 2007 and 2015 were enrolled. All patients were 45 years of age or older with detectable anti-HCV antibodies and HCV-RNA, as well as histopathological evidence of chronic hepatitis on liver biopsy. An exploratory cohort of 40 patients was first used before the 93 patient confirmatory analysis.
Genomic DNA was extracted from peripheral mononuclear cells of all of the patients studied.
Dr. Sakurada and his colleagues found significant associations for the single intron SNP (rs3219487) in the MUTYH gene. Human MutY homolog (MUTYH) protein is responsible for recognition and removal of an inappropriately inserted adenine (A).
The risk of developing HCC in patients with A/A or G/A genotypes was higher than in those with the G/G genotype (odds ratio = 9.27, 95% confidence interval = 2.39-32.1, P = .0005). In addition, they found that MUTYH mRNA levels were significantly lower in G/A or A/A genotyped subjects as compared with the wild type (G/G) (P = .0157 and P = .0108, respectively).
In their mouse model, the investigators found that liver tumors developed in MUTYH-null mice. As a physiological confirmation of their results, the researchers investigated a MUTYH-null mouse model. They found that decreased MUTYH activity in the null mice resulted in the development of HCC in these animals after 12 months of a high iron diet, but no tumors were observed when a dietary antioxidant (N-Acetyl-L-cysteine) was also provided.
“HCC may develop even in patients with an SVR [sustained virologic response]. This may be a problem especially for the numerous elderly patients. Our study suggests that SNP genotyping may predict the risk of developing HCC in such patients. We propose that in the setting of appropriate screening intervals, stratification of patients for whom antioxidant treatment would be appropriate could be achieved in this manner,” the researchers concluded.
The study was supported by grants from the Japan Society for Scientific Research. The authors reported they had nothing to disclose.
SOURCE: Sakurada A et al. Free Radic Biol Med. 2018;129:88-96.
An adenine mutation in the MUTYH base repair excision gene increased the risk of developing hepatocellular carcinoma (HCC) in patients with chronic hepatitis C virus (HCV). The mutation was discovered in an analysis of 19 tagging single-nucleotide polymorphism (SNP) variants examined for multiple base repair excision genes (MUTYH, OGG1, and MTH1), according to the results of a retrospective analysis of patient genotypes reported in Free Radical Biology and Medicine (2018;129:88-96).
In addition, the researchers examined MUTYH-null mice to assess the involvement of oxidative stress and DNA repair enzymes in hepatocarcinogenesis.
One significant SNP was found and confirmed from 93 Japanese patients with chronic HCV (38 with HCC and 55 controls), according to Akira Sakurada, MD, of Sapporo (Japan) Medical University, and his colleagues.
Patients diagnosed as having chronic HCV between 2007 and 2015 were enrolled. All patients were 45 years of age or older with detectable anti-HCV antibodies and HCV-RNA, as well as histopathological evidence of chronic hepatitis on liver biopsy. An exploratory cohort of 40 patients was first used before the 93 patient confirmatory analysis.
Genomic DNA was extracted from peripheral mononuclear cells of all of the patients studied.
Dr. Sakurada and his colleagues found significant associations for the single intron SNP (rs3219487) in the MUTYH gene. Human MutY homolog (MUTYH) protein is responsible for recognition and removal of an inappropriately inserted adenine (A).
The risk of developing HCC in patients with A/A or G/A genotypes was higher than in those with the G/G genotype (odds ratio = 9.27, 95% confidence interval = 2.39-32.1, P = .0005). In addition, they found that MUTYH mRNA levels were significantly lower in G/A or A/A genotyped subjects as compared with the wild type (G/G) (P = .0157 and P = .0108, respectively).
In their mouse model, the investigators found that liver tumors developed in MUTYH-null mice. As a physiological confirmation of their results, the researchers investigated a MUTYH-null mouse model. They found that decreased MUTYH activity in the null mice resulted in the development of HCC in these animals after 12 months of a high iron diet, but no tumors were observed when a dietary antioxidant (N-Acetyl-L-cysteine) was also provided.
“HCC may develop even in patients with an SVR [sustained virologic response]. This may be a problem especially for the numerous elderly patients. Our study suggests that SNP genotyping may predict the risk of developing HCC in such patients. We propose that in the setting of appropriate screening intervals, stratification of patients for whom antioxidant treatment would be appropriate could be achieved in this manner,” the researchers concluded.
The study was supported by grants from the Japan Society for Scientific Research. The authors reported they had nothing to disclose.
SOURCE: Sakurada A et al. Free Radic Biol Med. 2018;129:88-96.
An adenine mutation in the MUTYH base repair excision gene increased the risk of developing hepatocellular carcinoma (HCC) in patients with chronic hepatitis C virus (HCV). The mutation was discovered in an analysis of 19 tagging single-nucleotide polymorphism (SNP) variants examined for multiple base repair excision genes (MUTYH, OGG1, and MTH1), according to the results of a retrospective analysis of patient genotypes reported in Free Radical Biology and Medicine (2018;129:88-96).
In addition, the researchers examined MUTYH-null mice to assess the involvement of oxidative stress and DNA repair enzymes in hepatocarcinogenesis.
One significant SNP was found and confirmed from 93 Japanese patients with chronic HCV (38 with HCC and 55 controls), according to Akira Sakurada, MD, of Sapporo (Japan) Medical University, and his colleagues.
Patients diagnosed as having chronic HCV between 2007 and 2015 were enrolled. All patients were 45 years of age or older with detectable anti-HCV antibodies and HCV-RNA, as well as histopathological evidence of chronic hepatitis on liver biopsy. An exploratory cohort of 40 patients was first used before the 93 patient confirmatory analysis.
Genomic DNA was extracted from peripheral mononuclear cells of all of the patients studied.
Dr. Sakurada and his colleagues found significant associations for the single intron SNP (rs3219487) in the MUTYH gene. Human MutY homolog (MUTYH) protein is responsible for recognition and removal of an inappropriately inserted adenine (A).
The risk of developing HCC in patients with A/A or G/A genotypes was higher than in those with the G/G genotype (odds ratio = 9.27, 95% confidence interval = 2.39-32.1, P = .0005). In addition, they found that MUTYH mRNA levels were significantly lower in G/A or A/A genotyped subjects as compared with the wild type (G/G) (P = .0157 and P = .0108, respectively).
In their mouse model, the investigators found that liver tumors developed in MUTYH-null mice. As a physiological confirmation of their results, the researchers investigated a MUTYH-null mouse model. They found that decreased MUTYH activity in the null mice resulted in the development of HCC in these animals after 12 months of a high iron diet, but no tumors were observed when a dietary antioxidant (N-Acetyl-L-cysteine) was also provided.
“HCC may develop even in patients with an SVR [sustained virologic response]. This may be a problem especially for the numerous elderly patients. Our study suggests that SNP genotyping may predict the risk of developing HCC in such patients. We propose that in the setting of appropriate screening intervals, stratification of patients for whom antioxidant treatment would be appropriate could be achieved in this manner,” the researchers concluded.
The study was supported by grants from the Japan Society for Scientific Research. The authors reported they had nothing to disclose.
SOURCE: Sakurada A et al. Free Radic Biol Med. 2018;129:88-96.
FROM FREE RADICAL BIOLOGY AND MEDICINE
Key clinical point: An adenine mutation in the MUTYH gene increased the risk of developing liver cancer in patients with chronic HCV.
Major finding: Hepatocellular carcinoma risk with the mutation was significantly higher than in patients with the standard genotype (OR = 9.27, P = .0005).
Study details: Nineteen SNPs in base excision repair genes were examined in 93 patients with chronic hepatitis C.
Disclosures: The study was supported by grants from the Japan Society for Scientific Research. The authors reported they had nothing to disclose.
Source: Sakurada A et al. Free Radic Biol Med. 2018;129:88-96.
FDA approves duvelisib for CLL/SLL and FL
The U.S. Food and Drug Administration (FDA) has approved duvelisib (Copiktra™), a dual PI3K delta/gamma inhibitor, for two indications.
Duvelisib has full FDA approval to treat adults with relapsed or refractory chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) who have received at least two prior therapies.
Duvelisib also has accelerated approval to treat adults with relapsed or refractory follicular lymphoma (FL) who have received at least two prior systemic therapies.
Accelerated approval is based on a surrogate or intermediate endpoint—in this case, overall response rate—that is reasonably likely to predict clinical benefit. Continued approval of duvelisib in FL may be contingent upon results of confirmatory trials verifying that the drug provides a clinical benefit.
Duvelisib will be available in the U.S. immediately, according to Verastem Inc., the company marketing the drug.
Verastem said it will help patients access duvelisib through the Verastem Cares™ program, which is designed to provide information and assistance to patients who are prescribed duvelisib.
The prescribing information for duvelisib includes a boxed warning detailing four fatal and/or serious toxicities associated with the drug—infections, diarrhea or colitis, cutaneous reactions, and pneumonitis.
Verastem said it is implementing an informational risk evaluation and mitigation strategy to provide appropriate dosing and safety information for duvelisib.
The recommended dose of duvelisib is 25 mg orally twice daily, taken continuously in 28-day treatment cycles.
The FDA assessed the new drug application for duvelisib under priority review. The FDA also granted duvelisib fast track designation in CLL and FL as well as orphan drug designation for CLL/SLL and FL.
The FDA’s approval of duvelisib is supported by data from the phase 3 DUO trial and the phase 2 DYNAMO trial. Updated results from both studies are available in the prescribing information for duvelisib.
DUO trial
DUO included 319 patients with CLL (n=312) or SLL (n=7) who had received at least one prior therapy. They were randomized to receive either duvelisib (25 mg orally twice daily) or ofatumumab (initial infusion of 300 mg followed by 7 weekly infusions and 4 monthly infusions of 2000 mg).
The following efficacy results were observed in patients who had received at least two prior therapies, which includes 95 patients in the duvelisib arm and 101 in the ofatumumab arm.
The overall response rate was 78% in the duvelisib arm and 39% in the ofatumumab arm. All responses in both arms were partial responses.
The median progression-free survival was 16.4 months with duvelisib and 9.1 months with ofatumumab (hazard ratio=0.40).
The safety results include all patients treated with duvelisib or ofatumumab.
Twelve percent of patients in the duvelisib arm had fatal adverse events (AEs) within 30 days of the last dose. The same was true of 4% of patients treated with ofatumumab.
Serious AEs occurred in 73% of patients treated with duvelisib. The most common were infection (38%) and diarrhea/colitis (23%).
Thirty-six percent of patients discontinued duvelisib. Most discontinuations were due to diarrhea/ colitis, infection, and rash. Twenty-nine percent of patients in the duvelisib arm required dose reductions, most often due to diarrhea/colitis and rash.
DYNAMO trial
DYNAMO enrolled patients with indolent non-Hodgkin lymphoma whose disease was refractory to both rituximab and chemotherapy or radioimmunotherapy.
There were 83 patients with FL. They had a median of 3 prior anticancer regimens (range, 1-10).
The patients received duvelisib at 25 mg orally twice daily until disease progression or unacceptable toxicity.
The overall response rate was 42%. One patient achieved a complete response, and 34 had a partial response.
Forty-three percent of responders maintained their response at 6 months, and 17% maintained their response at 12 months.
Serious AEs occurred in 58% of FL patients. The most common were diarrhea/colitis, pneumonia, renal insufficiency, rash, and sepsis.
AEs occurring in at least 20% of FL patients included diarrhea/colitis, nausea, fatigue, musculoskeletal pain, rash, neutropenia, cough, anemia, pyrexia, headache, mucositis, abdominal pain, vomiting, transaminase elevation, and thrombocytopenia.
Twenty-nine percent of FL patients discontinued duvelisib, and 23% had dose reductions. Most discontinuations were due to diarrhea/colitis and rash, and most dose reductions were due to transaminase elevation, diarrhea/colitis, lipase increase, and infection.
The U.S. Food and Drug Administration (FDA) has approved duvelisib (Copiktra™), a dual PI3K delta/gamma inhibitor, for two indications.
Duvelisib has full FDA approval to treat adults with relapsed or refractory chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) who have received at least two prior therapies.
Duvelisib also has accelerated approval to treat adults with relapsed or refractory follicular lymphoma (FL) who have received at least two prior systemic therapies.
Accelerated approval is based on a surrogate or intermediate endpoint—in this case, overall response rate—that is reasonably likely to predict clinical benefit. Continued approval of duvelisib in FL may be contingent upon results of confirmatory trials verifying that the drug provides a clinical benefit.
Duvelisib will be available in the U.S. immediately, according to Verastem Inc., the company marketing the drug.
Verastem said it will help patients access duvelisib through the Verastem Cares™ program, which is designed to provide information and assistance to patients who are prescribed duvelisib.
The prescribing information for duvelisib includes a boxed warning detailing four fatal and/or serious toxicities associated with the drug—infections, diarrhea or colitis, cutaneous reactions, and pneumonitis.
Verastem said it is implementing an informational risk evaluation and mitigation strategy to provide appropriate dosing and safety information for duvelisib.
The recommended dose of duvelisib is 25 mg orally twice daily, taken continuously in 28-day treatment cycles.
The FDA assessed the new drug application for duvelisib under priority review. The FDA also granted duvelisib fast track designation in CLL and FL as well as orphan drug designation for CLL/SLL and FL.
The FDA’s approval of duvelisib is supported by data from the phase 3 DUO trial and the phase 2 DYNAMO trial. Updated results from both studies are available in the prescribing information for duvelisib.
DUO trial
DUO included 319 patients with CLL (n=312) or SLL (n=7) who had received at least one prior therapy. They were randomized to receive either duvelisib (25 mg orally twice daily) or ofatumumab (initial infusion of 300 mg followed by 7 weekly infusions and 4 monthly infusions of 2000 mg).
The following efficacy results were observed in patients who had received at least two prior therapies, which includes 95 patients in the duvelisib arm and 101 in the ofatumumab arm.
The overall response rate was 78% in the duvelisib arm and 39% in the ofatumumab arm. All responses in both arms were partial responses.
The median progression-free survival was 16.4 months with duvelisib and 9.1 months with ofatumumab (hazard ratio=0.40).
The safety results include all patients treated with duvelisib or ofatumumab.
Twelve percent of patients in the duvelisib arm had fatal adverse events (AEs) within 30 days of the last dose. The same was true of 4% of patients treated with ofatumumab.
Serious AEs occurred in 73% of patients treated with duvelisib. The most common were infection (38%) and diarrhea/colitis (23%).
Thirty-six percent of patients discontinued duvelisib. Most discontinuations were due to diarrhea/ colitis, infection, and rash. Twenty-nine percent of patients in the duvelisib arm required dose reductions, most often due to diarrhea/colitis and rash.
DYNAMO trial
DYNAMO enrolled patients with indolent non-Hodgkin lymphoma whose disease was refractory to both rituximab and chemotherapy or radioimmunotherapy.
There were 83 patients with FL. They had a median of 3 prior anticancer regimens (range, 1-10).
The patients received duvelisib at 25 mg orally twice daily until disease progression or unacceptable toxicity.
The overall response rate was 42%. One patient achieved a complete response, and 34 had a partial response.
Forty-three percent of responders maintained their response at 6 months, and 17% maintained their response at 12 months.
Serious AEs occurred in 58% of FL patients. The most common were diarrhea/colitis, pneumonia, renal insufficiency, rash, and sepsis.
AEs occurring in at least 20% of FL patients included diarrhea/colitis, nausea, fatigue, musculoskeletal pain, rash, neutropenia, cough, anemia, pyrexia, headache, mucositis, abdominal pain, vomiting, transaminase elevation, and thrombocytopenia.
Twenty-nine percent of FL patients discontinued duvelisib, and 23% had dose reductions. Most discontinuations were due to diarrhea/colitis and rash, and most dose reductions were due to transaminase elevation, diarrhea/colitis, lipase increase, and infection.
The U.S. Food and Drug Administration (FDA) has approved duvelisib (Copiktra™), a dual PI3K delta/gamma inhibitor, for two indications.
Duvelisib has full FDA approval to treat adults with relapsed or refractory chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) who have received at least two prior therapies.
Duvelisib also has accelerated approval to treat adults with relapsed or refractory follicular lymphoma (FL) who have received at least two prior systemic therapies.
Accelerated approval is based on a surrogate or intermediate endpoint—in this case, overall response rate—that is reasonably likely to predict clinical benefit. Continued approval of duvelisib in FL may be contingent upon results of confirmatory trials verifying that the drug provides a clinical benefit.
Duvelisib will be available in the U.S. immediately, according to Verastem Inc., the company marketing the drug.
Verastem said it will help patients access duvelisib through the Verastem Cares™ program, which is designed to provide information and assistance to patients who are prescribed duvelisib.
The prescribing information for duvelisib includes a boxed warning detailing four fatal and/or serious toxicities associated with the drug—infections, diarrhea or colitis, cutaneous reactions, and pneumonitis.
Verastem said it is implementing an informational risk evaluation and mitigation strategy to provide appropriate dosing and safety information for duvelisib.
The recommended dose of duvelisib is 25 mg orally twice daily, taken continuously in 28-day treatment cycles.
The FDA assessed the new drug application for duvelisib under priority review. The FDA also granted duvelisib fast track designation in CLL and FL as well as orphan drug designation for CLL/SLL and FL.
The FDA’s approval of duvelisib is supported by data from the phase 3 DUO trial and the phase 2 DYNAMO trial. Updated results from both studies are available in the prescribing information for duvelisib.
DUO trial
DUO included 319 patients with CLL (n=312) or SLL (n=7) who had received at least one prior therapy. They were randomized to receive either duvelisib (25 mg orally twice daily) or ofatumumab (initial infusion of 300 mg followed by 7 weekly infusions and 4 monthly infusions of 2000 mg).
The following efficacy results were observed in patients who had received at least two prior therapies, which includes 95 patients in the duvelisib arm and 101 in the ofatumumab arm.
The overall response rate was 78% in the duvelisib arm and 39% in the ofatumumab arm. All responses in both arms were partial responses.
The median progression-free survival was 16.4 months with duvelisib and 9.1 months with ofatumumab (hazard ratio=0.40).
The safety results include all patients treated with duvelisib or ofatumumab.
Twelve percent of patients in the duvelisib arm had fatal adverse events (AEs) within 30 days of the last dose. The same was true of 4% of patients treated with ofatumumab.
Serious AEs occurred in 73% of patients treated with duvelisib. The most common were infection (38%) and diarrhea/colitis (23%).
Thirty-six percent of patients discontinued duvelisib. Most discontinuations were due to diarrhea/ colitis, infection, and rash. Twenty-nine percent of patients in the duvelisib arm required dose reductions, most often due to diarrhea/colitis and rash.
DYNAMO trial
DYNAMO enrolled patients with indolent non-Hodgkin lymphoma whose disease was refractory to both rituximab and chemotherapy or radioimmunotherapy.
There were 83 patients with FL. They had a median of 3 prior anticancer regimens (range, 1-10).
The patients received duvelisib at 25 mg orally twice daily until disease progression or unacceptable toxicity.
The overall response rate was 42%. One patient achieved a complete response, and 34 had a partial response.
Forty-three percent of responders maintained their response at 6 months, and 17% maintained their response at 12 months.
Serious AEs occurred in 58% of FL patients. The most common were diarrhea/colitis, pneumonia, renal insufficiency, rash, and sepsis.
AEs occurring in at least 20% of FL patients included diarrhea/colitis, nausea, fatigue, musculoskeletal pain, rash, neutropenia, cough, anemia, pyrexia, headache, mucositis, abdominal pain, vomiting, transaminase elevation, and thrombocytopenia.
Twenty-nine percent of FL patients discontinued duvelisib, and 23% had dose reductions. Most discontinuations were due to diarrhea/colitis and rash, and most dose reductions were due to transaminase elevation, diarrhea/colitis, lipase increase, and infection.