User login
Lenalidomide yields responses in a rare cutaneous lymphoma
The oral immunomodulatory drug lenalidomide is active and may provide prolonged responses in certain patients with a rare and aggressive subtype of primary cutaneous lymphoma, according to results of a phase 2 study.
In the study, which comprised 19 patients with primary cutaneous diffuse large B-cell lymphoma, leg type (PCDLBCL, LT), 5 patients (26.3%) had a response at 6 months, and there were still 3 patients in response at 12 months. The findings were reported in the Journal of Investigative Dermatology.
In an exploratory analysis, reducing the dose of lenalidomide was associated with prolonged response and improved survival, noted lead author Marie Beylot-Barry, MD, of the dermatology department, Hôpital Saint-André, CHU Bordeaux, France, and her colleagues.
“Lenalidomide at reduced doses may allow prolonged responses in few patients, and represents a therapeutic option in relapsing/refractory PCDLBCL, LT,” the researchers wrote.
Found mostly on the lower limbs of elderly patients, PCDLBCL, LT exhibits aggressive behavior and is associated with a high rate of skin recurrences. First-line therapy for the cutaneous lymphoma is typically rituximab and chemotherapy, regardless of clinical stage or patient age, the researchers wrote, though primary resistance or recurrence after treatment occurs in about half of patients. “In such relapsing or refractory cases, no treatment has demonstrated a sustained benefit thus far,” they noted.
Lenalidomide has already demonstrated efficacy in relapsed/refractory diffuse large B-cell lymphoma (DLBCL) and it induces inhibition of cell signaling, engaging NF-kappaB signaling. PCDLBCL, LT is marked by genetic alterations leading to the NF-kappaB pathway, which represents a therapeutic target.
Dr. Beylot-Barry and her colleagues initiated a multicenter, single-arm, phase 2 trial of 19 patients refractory/relapsing PCDLBCL, LT. Median progression-free survival in the trial was 4.9 months. The 6-month overall response rate – the primary endpoint of the trial – was 26.3%, which was not significantly superior to a prespecified 20% minimal response rate, according to the researchers.
“However, it was a stringent goal, and other secondary evaluations have to be considered in this context, such as a 6-month disease control rate at 42%,” they wrote.
Reduced doses were associated with improved outcomes, they added. Comparing the nine patients who had lenalidomide dose reductions to those who did not, there was a higher likelihood of 6- to 11-month overall response rate (44.4% vs. 10.0%; P = .11) and lower risk of disease progression or death (hazard ratio, 0.54; 95% confidence interval, 0.19-1.59; P = .27).
Grade 3 adverse events were primarily hematologic, and two deaths occurred (pulmonary embolism and sepsis).
Taken together, the encouraging results at reduced doses, the advanced age of the patients, and the high rate of adverse events suggests a role for lenalidomide as a part of combination treatment for PCDLBCL, LT in future trials, the researchers concluded.
The study was supported by grants from the French Ministry of Health and Celgene. The researchers reported having no financial disclosures.
SOURCE: Beylot-Barry M et al. J Invest Dermatol. 2018 Mar 26. doi: 10.1016/j.jid.2018.03.1516.
The oral immunomodulatory drug lenalidomide is active and may provide prolonged responses in certain patients with a rare and aggressive subtype of primary cutaneous lymphoma, according to results of a phase 2 study.
In the study, which comprised 19 patients with primary cutaneous diffuse large B-cell lymphoma, leg type (PCDLBCL, LT), 5 patients (26.3%) had a response at 6 months, and there were still 3 patients in response at 12 months. The findings were reported in the Journal of Investigative Dermatology.
In an exploratory analysis, reducing the dose of lenalidomide was associated with prolonged response and improved survival, noted lead author Marie Beylot-Barry, MD, of the dermatology department, Hôpital Saint-André, CHU Bordeaux, France, and her colleagues.
“Lenalidomide at reduced doses may allow prolonged responses in few patients, and represents a therapeutic option in relapsing/refractory PCDLBCL, LT,” the researchers wrote.
Found mostly on the lower limbs of elderly patients, PCDLBCL, LT exhibits aggressive behavior and is associated with a high rate of skin recurrences. First-line therapy for the cutaneous lymphoma is typically rituximab and chemotherapy, regardless of clinical stage or patient age, the researchers wrote, though primary resistance or recurrence after treatment occurs in about half of patients. “In such relapsing or refractory cases, no treatment has demonstrated a sustained benefit thus far,” they noted.
Lenalidomide has already demonstrated efficacy in relapsed/refractory diffuse large B-cell lymphoma (DLBCL) and it induces inhibition of cell signaling, engaging NF-kappaB signaling. PCDLBCL, LT is marked by genetic alterations leading to the NF-kappaB pathway, which represents a therapeutic target.
Dr. Beylot-Barry and her colleagues initiated a multicenter, single-arm, phase 2 trial of 19 patients refractory/relapsing PCDLBCL, LT. Median progression-free survival in the trial was 4.9 months. The 6-month overall response rate – the primary endpoint of the trial – was 26.3%, which was not significantly superior to a prespecified 20% minimal response rate, according to the researchers.
“However, it was a stringent goal, and other secondary evaluations have to be considered in this context, such as a 6-month disease control rate at 42%,” they wrote.
Reduced doses were associated with improved outcomes, they added. Comparing the nine patients who had lenalidomide dose reductions to those who did not, there was a higher likelihood of 6- to 11-month overall response rate (44.4% vs. 10.0%; P = .11) and lower risk of disease progression or death (hazard ratio, 0.54; 95% confidence interval, 0.19-1.59; P = .27).
Grade 3 adverse events were primarily hematologic, and two deaths occurred (pulmonary embolism and sepsis).
Taken together, the encouraging results at reduced doses, the advanced age of the patients, and the high rate of adverse events suggests a role for lenalidomide as a part of combination treatment for PCDLBCL, LT in future trials, the researchers concluded.
The study was supported by grants from the French Ministry of Health and Celgene. The researchers reported having no financial disclosures.
SOURCE: Beylot-Barry M et al. J Invest Dermatol. 2018 Mar 26. doi: 10.1016/j.jid.2018.03.1516.
The oral immunomodulatory drug lenalidomide is active and may provide prolonged responses in certain patients with a rare and aggressive subtype of primary cutaneous lymphoma, according to results of a phase 2 study.
In the study, which comprised 19 patients with primary cutaneous diffuse large B-cell lymphoma, leg type (PCDLBCL, LT), 5 patients (26.3%) had a response at 6 months, and there were still 3 patients in response at 12 months. The findings were reported in the Journal of Investigative Dermatology.
In an exploratory analysis, reducing the dose of lenalidomide was associated with prolonged response and improved survival, noted lead author Marie Beylot-Barry, MD, of the dermatology department, Hôpital Saint-André, CHU Bordeaux, France, and her colleagues.
“Lenalidomide at reduced doses may allow prolonged responses in few patients, and represents a therapeutic option in relapsing/refractory PCDLBCL, LT,” the researchers wrote.
Found mostly on the lower limbs of elderly patients, PCDLBCL, LT exhibits aggressive behavior and is associated with a high rate of skin recurrences. First-line therapy for the cutaneous lymphoma is typically rituximab and chemotherapy, regardless of clinical stage or patient age, the researchers wrote, though primary resistance or recurrence after treatment occurs in about half of patients. “In such relapsing or refractory cases, no treatment has demonstrated a sustained benefit thus far,” they noted.
Lenalidomide has already demonstrated efficacy in relapsed/refractory diffuse large B-cell lymphoma (DLBCL) and it induces inhibition of cell signaling, engaging NF-kappaB signaling. PCDLBCL, LT is marked by genetic alterations leading to the NF-kappaB pathway, which represents a therapeutic target.
Dr. Beylot-Barry and her colleagues initiated a multicenter, single-arm, phase 2 trial of 19 patients refractory/relapsing PCDLBCL, LT. Median progression-free survival in the trial was 4.9 months. The 6-month overall response rate – the primary endpoint of the trial – was 26.3%, which was not significantly superior to a prespecified 20% minimal response rate, according to the researchers.
“However, it was a stringent goal, and other secondary evaluations have to be considered in this context, such as a 6-month disease control rate at 42%,” they wrote.
Reduced doses were associated with improved outcomes, they added. Comparing the nine patients who had lenalidomide dose reductions to those who did not, there was a higher likelihood of 6- to 11-month overall response rate (44.4% vs. 10.0%; P = .11) and lower risk of disease progression or death (hazard ratio, 0.54; 95% confidence interval, 0.19-1.59; P = .27).
Grade 3 adverse events were primarily hematologic, and two deaths occurred (pulmonary embolism and sepsis).
Taken together, the encouraging results at reduced doses, the advanced age of the patients, and the high rate of adverse events suggests a role for lenalidomide as a part of combination treatment for PCDLBCL, LT in future trials, the researchers concluded.
The study was supported by grants from the French Ministry of Health and Celgene. The researchers reported having no financial disclosures.
SOURCE: Beylot-Barry M et al. J Invest Dermatol. 2018 Mar 26. doi: 10.1016/j.jid.2018.03.1516.
FROM THE JOURNAL OF INVESTIGATIVE DERMATOLOGY
Key clinical point:
Major finding: Five of 19 patients (26.3%) had a response at 6 months, and there were still 3 patients in response at 12 months.
Study details: A multicenter, single-arm, phase 2 trial of 19 patients refractory/relapsing PCDLBCL, LT.
Disclosures: The study was supported by grants from the French Ministry of Health and Celgene. The researchers reported having no financial disclosures.
Source: Beylot-Barry M et al. J Invest Dermatol. 2018 Mar 26. doi: 10.1016/j.jid.2018.03.1516.
Pediatric Dermatology Consult - March 2018
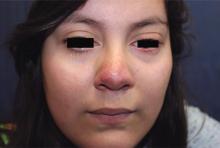
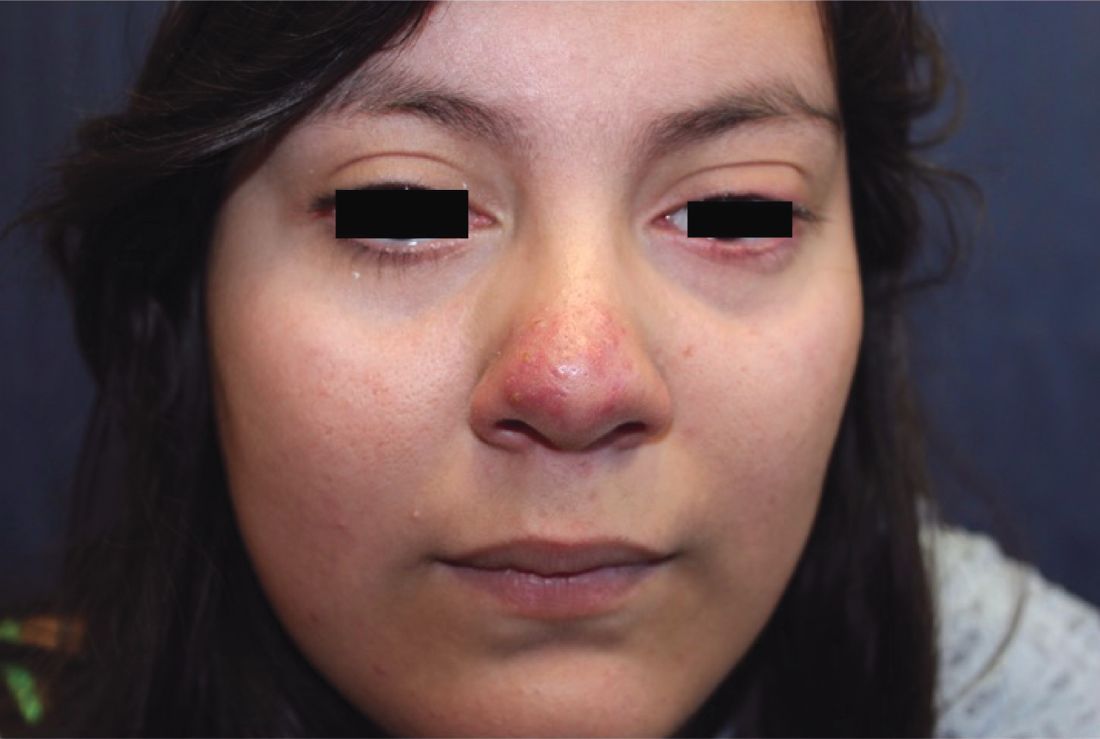
Rosacea is a chronic inflammatory skin disorder characterized by flushing, telangiectasia, erythema, papules, and pustules, most classically of the central face. Fair-skinned individuals and women are more commonly affected than are men, with age of onset typically around 30 years and older.1 In children and adolescents, rosacea is rare, especially among prepubertal children, so other papulopustular disorders should be considered when a rosacealike picture is present.2 Recurrent chalazia are seen with ocular rosacea and may be a clue to the diagnosis of acne rosacea. An individual’s subtype of rosacea also may transform with time to a different or an additional subtype.
Papulopustular rosacea (subtype II) is characterized by the presence of erythematous, dome-shaped papules distributed in crops in the central facial region. Cheeks, nasolabial folds, and the chin are most commonly affected. Pustules may or may not be present, but comedones are notably absent in an exclusively rosacea disease process. If comedones are present, a diagnosis of acne vulgaris should be considered instead of, or in addition to, rosacea. Pediatric patients with rosacea frequently present with papules and/or pustules, following the development of flushing.2
Ocular rosacea (subtype IV) may range in severity from mild blepharitis to severe keratitis and corneal vascularization. Patients may complain of a foreign body sensation. On external exam, lid margin telangiectasias, blepharitis, conjunctivitis, conjunctival injection, and recurrent chalazia may be frequently seen.3 Ocular rosacea may present without any signs of cutaneous disease; it may be the only form of rosacea (15% of patients in one study of 20 patients had only ocular rosacea)4 or may herald the development of cutaneous involvement. In fact, in children, ocular rosacea is frequently the first sign of disease. (A total of 55% of patients in the same study had both ocular and cutaneous rosacea, with ocular symptoms manifesting before the cutaneous disease). Thus an index of suspicion for rosacea should be maintained when a child presents with ocular findings.2
Other dermatitides resembling rosacea include steroid rosacea, perioral dermatitis, and idiopathic facial aseptic granuloma. Steroid rosacea, also known as iatrosacea, describes an eruption of erythema, papules, and telangiectasias that is clinically indistinguishable from rosacea.6 It results from chronic use of topical steroids, generally high potency, or abrupt withdrawal of steroids. Steroid rosacea should be treated by discontinuation of the steroid via tapered withdrawal.7 Perioral dermatitis, also known as periorificial dermatitis, may also appear rosacea-form. It usually is located around the perioral and perinasal areas, but may extend to the periocular area.8 Idiopathic facial septic granuloma describes erythematous to violaceous nodules of the cheeks and eyelid in children, with chalazia frequently present; it is thought to be associated with rosacea.9
Although the exact pathophysiology of rosacea is unknown, it is clear that the dysregulation of the innate immune system plays a key role in the pathogenesis of rosacea. Studies have found that patients with rosacea have increased expression of cathelicidin, and its activating serine protease, kallikrein.5 Interestingly, UV light, a known trigger of rosacea, induces expression of cathelicidin and its inflammatory cascade.5 Neurovascular signaling is also aberrantly upregulated; vanilloid and ankyrin receptors have been shown to be active in rosacea, and are activated by rosacea-exacerbating stimuli, such as heat, inflammation, and spices. Higher levels of Demodex folliculorum and Staphylococcus epidermis also have been consistently found on the skin of rosacea patients, compared with healthy subjects, though it is unclear what role these pathogens play in the development of rosacea.
Treatment of rosacea is very important given its profound impact on quality of life; one study found that the odds ratio for depression in individuals with rosacea is 4.81.10 Patient education is essential, and patients should be encouraged to identify specific triggers so they can minimize exposure when feasible. Common triggers include hot and cold temperature, sunlight, wind, spicy foods, alcohol, exercise, emotional stress, and certain medications such as niacin. Topical steroids frequently are exacerbating, so patients should be encouraged to avoid them and use moisturizers often, given their skin’s increased transepidermal water loss and susceptibility to irritation. In addition, sunscreens are essential to reduce inflammation from reactive oxygen species, which aggravate rosacea.11 For pharmaceutical therapeutics, topical sodium sulfacetamide, metronidazole, and azelaic acid have been shown to be effective in rosacea. For persistent erythema, topical alpha-adrenergic receptor agonists including brimonidine tartrate and oxymetazoline have been shown to reduce erythema by vasoconstricting blood vessels, although some products are associated with a rebound erythema on treatment discontinuation. For moderate to severe rosacea, low-dose oral doxycycline at anti-inflammatory doses (less than 50 mg daily) is the mainstay of therapy. Other oral antibiotics and topical permethrin have been used, and topical ivermectin 1% cream has been approved for inflammatory rosacea.11 Oral beta-blockers also have been successfully used in some patients to reduce erythema and flushing, as well as isotretinoin for refractory, severe rosacea with improvement.
Allison Han is a medical student at the University of California, San Diego. Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. There are no conflicts of interest or financial disclosures for Ms. Han or Dr. Eichenfield.
References
1. J Am Acad Dermatol. 2018 Jan;78(1):148-55.
2. Cutis. 2016 Jul;98(1):49-53.
3. J Eur Acad Dermatol Venereol. 2017 Oct;31(10):1732-8.
4. J Fr Ophtalmol. 2011 Dec;34(10):703-10.
5. J Am Acad Dermatol. 2015 May;72(5):749-58.
6. Indian J Dermatol. 2011 Jan;56(1):30-2.
7. Cutis, 2004. 74(2):99-103.
8. Pediatr Dermatol. 1992 Mar;9(1):22-6.
9. Pediatr Dermatol. 2015 Jul-Aug;32(4):e136-9.
10. Br J Dermatol. 2005 Dec;153(6):1176-81.
11. J Am Acad Dermatol. 2015 May;72(5):761-70.
Rosacea is a chronic inflammatory skin disorder characterized by flushing, telangiectasia, erythema, papules, and pustules, most classically of the central face. Fair-skinned individuals and women are more commonly affected than are men, with age of onset typically around 30 years and older.1 In children and adolescents, rosacea is rare, especially among prepubertal children, so other papulopustular disorders should be considered when a rosacealike picture is present.2 Recurrent chalazia are seen with ocular rosacea and may be a clue to the diagnosis of acne rosacea. An individual’s subtype of rosacea also may transform with time to a different or an additional subtype.
Papulopustular rosacea (subtype II) is characterized by the presence of erythematous, dome-shaped papules distributed in crops in the central facial region. Cheeks, nasolabial folds, and the chin are most commonly affected. Pustules may or may not be present, but comedones are notably absent in an exclusively rosacea disease process. If comedones are present, a diagnosis of acne vulgaris should be considered instead of, or in addition to, rosacea. Pediatric patients with rosacea frequently present with papules and/or pustules, following the development of flushing.2
Ocular rosacea (subtype IV) may range in severity from mild blepharitis to severe keratitis and corneal vascularization. Patients may complain of a foreign body sensation. On external exam, lid margin telangiectasias, blepharitis, conjunctivitis, conjunctival injection, and recurrent chalazia may be frequently seen.3 Ocular rosacea may present without any signs of cutaneous disease; it may be the only form of rosacea (15% of patients in one study of 20 patients had only ocular rosacea)4 or may herald the development of cutaneous involvement. In fact, in children, ocular rosacea is frequently the first sign of disease. (A total of 55% of patients in the same study had both ocular and cutaneous rosacea, with ocular symptoms manifesting before the cutaneous disease). Thus an index of suspicion for rosacea should be maintained when a child presents with ocular findings.2
Other dermatitides resembling rosacea include steroid rosacea, perioral dermatitis, and idiopathic facial aseptic granuloma. Steroid rosacea, also known as iatrosacea, describes an eruption of erythema, papules, and telangiectasias that is clinically indistinguishable from rosacea.6 It results from chronic use of topical steroids, generally high potency, or abrupt withdrawal of steroids. Steroid rosacea should be treated by discontinuation of the steroid via tapered withdrawal.7 Perioral dermatitis, also known as periorificial dermatitis, may also appear rosacea-form. It usually is located around the perioral and perinasal areas, but may extend to the periocular area.8 Idiopathic facial septic granuloma describes erythematous to violaceous nodules of the cheeks and eyelid in children, with chalazia frequently present; it is thought to be associated with rosacea.9
Although the exact pathophysiology of rosacea is unknown, it is clear that the dysregulation of the innate immune system plays a key role in the pathogenesis of rosacea. Studies have found that patients with rosacea have increased expression of cathelicidin, and its activating serine protease, kallikrein.5 Interestingly, UV light, a known trigger of rosacea, induces expression of cathelicidin and its inflammatory cascade.5 Neurovascular signaling is also aberrantly upregulated; vanilloid and ankyrin receptors have been shown to be active in rosacea, and are activated by rosacea-exacerbating stimuli, such as heat, inflammation, and spices. Higher levels of Demodex folliculorum and Staphylococcus epidermis also have been consistently found on the skin of rosacea patients, compared with healthy subjects, though it is unclear what role these pathogens play in the development of rosacea.
Treatment of rosacea is very important given its profound impact on quality of life; one study found that the odds ratio for depression in individuals with rosacea is 4.81.10 Patient education is essential, and patients should be encouraged to identify specific triggers so they can minimize exposure when feasible. Common triggers include hot and cold temperature, sunlight, wind, spicy foods, alcohol, exercise, emotional stress, and certain medications such as niacin. Topical steroids frequently are exacerbating, so patients should be encouraged to avoid them and use moisturizers often, given their skin’s increased transepidermal water loss and susceptibility to irritation. In addition, sunscreens are essential to reduce inflammation from reactive oxygen species, which aggravate rosacea.11 For pharmaceutical therapeutics, topical sodium sulfacetamide, metronidazole, and azelaic acid have been shown to be effective in rosacea. For persistent erythema, topical alpha-adrenergic receptor agonists including brimonidine tartrate and oxymetazoline have been shown to reduce erythema by vasoconstricting blood vessels, although some products are associated with a rebound erythema on treatment discontinuation. For moderate to severe rosacea, low-dose oral doxycycline at anti-inflammatory doses (less than 50 mg daily) is the mainstay of therapy. Other oral antibiotics and topical permethrin have been used, and topical ivermectin 1% cream has been approved for inflammatory rosacea.11 Oral beta-blockers also have been successfully used in some patients to reduce erythema and flushing, as well as isotretinoin for refractory, severe rosacea with improvement.
Allison Han is a medical student at the University of California, San Diego. Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. There are no conflicts of interest or financial disclosures for Ms. Han or Dr. Eichenfield.
References
1. J Am Acad Dermatol. 2018 Jan;78(1):148-55.
2. Cutis. 2016 Jul;98(1):49-53.
3. J Eur Acad Dermatol Venereol. 2017 Oct;31(10):1732-8.
4. J Fr Ophtalmol. 2011 Dec;34(10):703-10.
5. J Am Acad Dermatol. 2015 May;72(5):749-58.
6. Indian J Dermatol. 2011 Jan;56(1):30-2.
7. Cutis, 2004. 74(2):99-103.
8. Pediatr Dermatol. 1992 Mar;9(1):22-6.
9. Pediatr Dermatol. 2015 Jul-Aug;32(4):e136-9.
10. Br J Dermatol. 2005 Dec;153(6):1176-81.
11. J Am Acad Dermatol. 2015 May;72(5):761-70.
Rosacea is a chronic inflammatory skin disorder characterized by flushing, telangiectasia, erythema, papules, and pustules, most classically of the central face. Fair-skinned individuals and women are more commonly affected than are men, with age of onset typically around 30 years and older.1 In children and adolescents, rosacea is rare, especially among prepubertal children, so other papulopustular disorders should be considered when a rosacealike picture is present.2 Recurrent chalazia are seen with ocular rosacea and may be a clue to the diagnosis of acne rosacea. An individual’s subtype of rosacea also may transform with time to a different or an additional subtype.
Papulopustular rosacea (subtype II) is characterized by the presence of erythematous, dome-shaped papules distributed in crops in the central facial region. Cheeks, nasolabial folds, and the chin are most commonly affected. Pustules may or may not be present, but comedones are notably absent in an exclusively rosacea disease process. If comedones are present, a diagnosis of acne vulgaris should be considered instead of, or in addition to, rosacea. Pediatric patients with rosacea frequently present with papules and/or pustules, following the development of flushing.2
Ocular rosacea (subtype IV) may range in severity from mild blepharitis to severe keratitis and corneal vascularization. Patients may complain of a foreign body sensation. On external exam, lid margin telangiectasias, blepharitis, conjunctivitis, conjunctival injection, and recurrent chalazia may be frequently seen.3 Ocular rosacea may present without any signs of cutaneous disease; it may be the only form of rosacea (15% of patients in one study of 20 patients had only ocular rosacea)4 or may herald the development of cutaneous involvement. In fact, in children, ocular rosacea is frequently the first sign of disease. (A total of 55% of patients in the same study had both ocular and cutaneous rosacea, with ocular symptoms manifesting before the cutaneous disease). Thus an index of suspicion for rosacea should be maintained when a child presents with ocular findings.2
Other dermatitides resembling rosacea include steroid rosacea, perioral dermatitis, and idiopathic facial aseptic granuloma. Steroid rosacea, also known as iatrosacea, describes an eruption of erythema, papules, and telangiectasias that is clinically indistinguishable from rosacea.6 It results from chronic use of topical steroids, generally high potency, or abrupt withdrawal of steroids. Steroid rosacea should be treated by discontinuation of the steroid via tapered withdrawal.7 Perioral dermatitis, also known as periorificial dermatitis, may also appear rosacea-form. It usually is located around the perioral and perinasal areas, but may extend to the periocular area.8 Idiopathic facial septic granuloma describes erythematous to violaceous nodules of the cheeks and eyelid in children, with chalazia frequently present; it is thought to be associated with rosacea.9
Although the exact pathophysiology of rosacea is unknown, it is clear that the dysregulation of the innate immune system plays a key role in the pathogenesis of rosacea. Studies have found that patients with rosacea have increased expression of cathelicidin, and its activating serine protease, kallikrein.5 Interestingly, UV light, a known trigger of rosacea, induces expression of cathelicidin and its inflammatory cascade.5 Neurovascular signaling is also aberrantly upregulated; vanilloid and ankyrin receptors have been shown to be active in rosacea, and are activated by rosacea-exacerbating stimuli, such as heat, inflammation, and spices. Higher levels of Demodex folliculorum and Staphylococcus epidermis also have been consistently found on the skin of rosacea patients, compared with healthy subjects, though it is unclear what role these pathogens play in the development of rosacea.
Treatment of rosacea is very important given its profound impact on quality of life; one study found that the odds ratio for depression in individuals with rosacea is 4.81.10 Patient education is essential, and patients should be encouraged to identify specific triggers so they can minimize exposure when feasible. Common triggers include hot and cold temperature, sunlight, wind, spicy foods, alcohol, exercise, emotional stress, and certain medications such as niacin. Topical steroids frequently are exacerbating, so patients should be encouraged to avoid them and use moisturizers often, given their skin’s increased transepidermal water loss and susceptibility to irritation. In addition, sunscreens are essential to reduce inflammation from reactive oxygen species, which aggravate rosacea.11 For pharmaceutical therapeutics, topical sodium sulfacetamide, metronidazole, and azelaic acid have been shown to be effective in rosacea. For persistent erythema, topical alpha-adrenergic receptor agonists including brimonidine tartrate and oxymetazoline have been shown to reduce erythema by vasoconstricting blood vessels, although some products are associated with a rebound erythema on treatment discontinuation. For moderate to severe rosacea, low-dose oral doxycycline at anti-inflammatory doses (less than 50 mg daily) is the mainstay of therapy. Other oral antibiotics and topical permethrin have been used, and topical ivermectin 1% cream has been approved for inflammatory rosacea.11 Oral beta-blockers also have been successfully used in some patients to reduce erythema and flushing, as well as isotretinoin for refractory, severe rosacea with improvement.
Allison Han is a medical student at the University of California, San Diego. Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. There are no conflicts of interest or financial disclosures for Ms. Han or Dr. Eichenfield.
References
1. J Am Acad Dermatol. 2018 Jan;78(1):148-55.
2. Cutis. 2016 Jul;98(1):49-53.
3. J Eur Acad Dermatol Venereol. 2017 Oct;31(10):1732-8.
4. J Fr Ophtalmol. 2011 Dec;34(10):703-10.
5. J Am Acad Dermatol. 2015 May;72(5):749-58.
6. Indian J Dermatol. 2011 Jan;56(1):30-2.
7. Cutis, 2004. 74(2):99-103.
8. Pediatr Dermatol. 1992 Mar;9(1):22-6.
9. Pediatr Dermatol. 2015 Jul-Aug;32(4):e136-9.
10. Br J Dermatol. 2005 Dec;153(6):1176-81.
11. J Am Acad Dermatol. 2015 May;72(5):761-70.
A 16-year-old girl presented with a 6-month history of an erythematous eruption of small papules and pustules around the cheeks and nose. She states the erythema had started first, with periods of feeling flushed that became worse with sun exposure. She saw her primary care physician who prescribed topical steroids. After using the steroids, the rash became worse, and she developed papules and pustules.
A creative diversion
Do you have a creative diversion – a hobby for lack of a better word? One frequently hears of physicians who have creative skills not directly related to their professional careers. Furniture-building surgeons, fly-tying orthopedists, pediatrician poets, painting dermatologists ... I have even heard unsubstantiated claims that the traits that encourage individuals to become physicians make it more likely that they will have creative skills. Another one of those left brain/right brain things that probably doesn’t hold water.
If you do have a hobby or have the seed of a creative impulse you think could blossom into a hobby, I bet you wish that you could have an unlimited amount of time to invest in that activity. I am going to argue that this is another example of a situation in which you should be careful what you wish for.
When I was 9 or 10 years old, I bought a small carving of a sandpiper in a gift shop on Cape Cod. I still have it with its chipped bill and yellowed paper label on its driftwood base. That little bird triggered my interest in carving, and with gaps sometimes measured in decades I have been a self-taught bird carver. Some are attempts at realism with burned in feathers that takes weeks to complete. Others are free form painted whimsically, and are created in a few hours. They aren’t for sale, but to keep my inventory in check I distribute them as birthday and hostess gifts.
Ten years ago, after decades of visiting art galleries and grumbling to my wife, “I could do that,” I decided to try my hand at two-dimensional landscape painting. It was a fun challenge, and after a year or 2, I was ready to see what other people thought of my work. The first show that I entered stipulated that all of the entries be for sale. With no intention of parting with my work, I priced mine several orders of magnitude above what I thought they were worth.
One sold, and with that began a 7-year period during which pretty much anything I painted with a maritime theme sold for hundreds of dollars. It was a nice ego trip, but it took me down a dark path in which I began to choose my subjects and style based on what I knew would sell. Creating was no longer something I did for a change of pace. I was now retired, but painting had become my job. I felt burdened by the obligation to paint enough to cover the walls of the restaurant that graciously hung my work.
Luckily, the epiphany that I had sacrificed my creative diversion, which began with that little sandpiper, coincided with the restaurant’s decision to redecorate and the loss of much of my hanging space. I was now free to paint subjects I was interested in, and return to the comfort of carving when I felt the need to create.
If you already have a creative diversion, remember that a large part of its appeal is that it plays counterpoint to your job. Even if you are retired, a hobby provides a change of pace from which we can all benefit.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at pdnews@mdedge.com.
Do you have a creative diversion – a hobby for lack of a better word? One frequently hears of physicians who have creative skills not directly related to their professional careers. Furniture-building surgeons, fly-tying orthopedists, pediatrician poets, painting dermatologists ... I have even heard unsubstantiated claims that the traits that encourage individuals to become physicians make it more likely that they will have creative skills. Another one of those left brain/right brain things that probably doesn’t hold water.
If you do have a hobby or have the seed of a creative impulse you think could blossom into a hobby, I bet you wish that you could have an unlimited amount of time to invest in that activity. I am going to argue that this is another example of a situation in which you should be careful what you wish for.
When I was 9 or 10 years old, I bought a small carving of a sandpiper in a gift shop on Cape Cod. I still have it with its chipped bill and yellowed paper label on its driftwood base. That little bird triggered my interest in carving, and with gaps sometimes measured in decades I have been a self-taught bird carver. Some are attempts at realism with burned in feathers that takes weeks to complete. Others are free form painted whimsically, and are created in a few hours. They aren’t for sale, but to keep my inventory in check I distribute them as birthday and hostess gifts.
Ten years ago, after decades of visiting art galleries and grumbling to my wife, “I could do that,” I decided to try my hand at two-dimensional landscape painting. It was a fun challenge, and after a year or 2, I was ready to see what other people thought of my work. The first show that I entered stipulated that all of the entries be for sale. With no intention of parting with my work, I priced mine several orders of magnitude above what I thought they were worth.
One sold, and with that began a 7-year period during which pretty much anything I painted with a maritime theme sold for hundreds of dollars. It was a nice ego trip, but it took me down a dark path in which I began to choose my subjects and style based on what I knew would sell. Creating was no longer something I did for a change of pace. I was now retired, but painting had become my job. I felt burdened by the obligation to paint enough to cover the walls of the restaurant that graciously hung my work.
Luckily, the epiphany that I had sacrificed my creative diversion, which began with that little sandpiper, coincided with the restaurant’s decision to redecorate and the loss of much of my hanging space. I was now free to paint subjects I was interested in, and return to the comfort of carving when I felt the need to create.
If you already have a creative diversion, remember that a large part of its appeal is that it plays counterpoint to your job. Even if you are retired, a hobby provides a change of pace from which we can all benefit.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at pdnews@mdedge.com.
Do you have a creative diversion – a hobby for lack of a better word? One frequently hears of physicians who have creative skills not directly related to their professional careers. Furniture-building surgeons, fly-tying orthopedists, pediatrician poets, painting dermatologists ... I have even heard unsubstantiated claims that the traits that encourage individuals to become physicians make it more likely that they will have creative skills. Another one of those left brain/right brain things that probably doesn’t hold water.
If you do have a hobby or have the seed of a creative impulse you think could blossom into a hobby, I bet you wish that you could have an unlimited amount of time to invest in that activity. I am going to argue that this is another example of a situation in which you should be careful what you wish for.
When I was 9 or 10 years old, I bought a small carving of a sandpiper in a gift shop on Cape Cod. I still have it with its chipped bill and yellowed paper label on its driftwood base. That little bird triggered my interest in carving, and with gaps sometimes measured in decades I have been a self-taught bird carver. Some are attempts at realism with burned in feathers that takes weeks to complete. Others are free form painted whimsically, and are created in a few hours. They aren’t for sale, but to keep my inventory in check I distribute them as birthday and hostess gifts.
Ten years ago, after decades of visiting art galleries and grumbling to my wife, “I could do that,” I decided to try my hand at two-dimensional landscape painting. It was a fun challenge, and after a year or 2, I was ready to see what other people thought of my work. The first show that I entered stipulated that all of the entries be for sale. With no intention of parting with my work, I priced mine several orders of magnitude above what I thought they were worth.
One sold, and with that began a 7-year period during which pretty much anything I painted with a maritime theme sold for hundreds of dollars. It was a nice ego trip, but it took me down a dark path in which I began to choose my subjects and style based on what I knew would sell. Creating was no longer something I did for a change of pace. I was now retired, but painting had become my job. I felt burdened by the obligation to paint enough to cover the walls of the restaurant that graciously hung my work.
Luckily, the epiphany that I had sacrificed my creative diversion, which began with that little sandpiper, coincided with the restaurant’s decision to redecorate and the loss of much of my hanging space. I was now free to paint subjects I was interested in, and return to the comfort of carving when I felt the need to create.
If you already have a creative diversion, remember that a large part of its appeal is that it plays counterpoint to your job. Even if you are retired, a hobby provides a change of pace from which we can all benefit.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at pdnews@mdedge.com.
Ibrutinib preserves immune memory while fighting cGVHD
SALT LAKE CITY – Ibrutinib’s effectiveness in inhibiting chronic graft versus host disease (cGVHD) may hinge in part on inhibition of pre–germinal center B cells and follicular helper cells, according to a new analysis of clinical trial data.
The analysis also showed that ibrutinib preserved immune memory and type 1 T-helper cells.
Bita Sahaf, PhD, presented results of a “comprehensive and high dimensional proteomic approach” to data from 42 patients who were enrolled in a phase 1/2 clinical trial of ibrutinib for cGVHD (NCT02195869).
In that study, 80% of patients who had two or more organs affected by cGVHD responded in at least two organs; overall, two-thirds of patients had a complete or partial response with ibrutinib. The highest response rates were seen in disease affecting the skin, mouth, and gastrointestinal tract.
The new analysis used blood samples from trial participants collected before and during ibrutinib therapy to look for soluble plasma factors known to be related to inflammation, fibrosis, and cGVHD.
“A heat map of cytokines, chemokines, and factors associated with fibrosis shows a significant decrease following ibrutinib treatment,” Dr. Sahaf said during a top abstracts session at the combined annual meetings of the Center for International Blood & Marrow Transplant Research and the American Society for Blood and Marrow Transplantation.
In addition, inflammatory gene expression was reduced with ibrutinib use, with reductions in the chemokines nuclear factor kappa-B-1, CXCL10, CCL7, and CCL3 dropping by 2.6-fold, 2.3-fold, 25-fold, and 1.8-fold, respectively, after 3 months of ibrutinib therapy, Dr. Sahaf and her colleagues reported.
The investigators used several different techniques to tease apart the mechanisms behind ibrutinib’s effectiveness. Immunophenotyping was accomplished with cytometry by time of flight (CyTOF), a technique that uses transition element isotopes to tag antibodies, which are then analyzed on a cell-by-cell basis by a time-of-flight mass spectrometer.
Ibrutinib inhibits CD19+CD38+CD27+IgD+ pre–germinal center B cells as well as pathogenic CD4+ T follicular helper cells, both implicated in cGVHD, the investigators found. However, Th1 T cells were preserved in a patient-by-patient analysis.
The CyTOF technique also allowed a phosphorylation analysis showing ibrutinib’s blocking effect on Bruton’s tyrosine kinase (BTK) as well as IL-2 inducible T-cell kinase (ITK), with subsequent effects on the signaling molecule PLCgamma2. In individual patients, this inhibition was confirmed when BTK-activated B-cell populations were eliminated after ibrutinib therapy, Dr. Sahaf said.
Ibrutinib also decreased phosphorylation of ITK, with subsequent depletion of CD4+, CD185+, and BCL6+ follicular helper T cells, and of other T cell populations still to be characterized. However, neither CD4+Tbet+Th1 cells nor CD4+CD25+CD127dim Treg cells saw depletion.
Importantly, “CD8+ cytotoxic T cells persist,” said Dr. Sahaf. Phosphorylation of ITK, she said, “appears heterogeneous across most T-cell populations.
“These data support the clinical efficacy of ibrutinib in cGVHD and highlight ibrutinib’s multifactorial mechanism of action in this disease,” Dr. Sahaf, of Stanford (Calif.) University, and her collaborators wrote in the abstract accompanying the presentation.
In August 2017, ibrutinib became the first treatment approved by the Food and Drug Administration for cGVHD. It is indicated for adults who have failed at least one other therapy.
“These correlative studies suggest that ibrutinib impacts a number of the immunologic mechanisms underlying the development of chronic graft versus host disease,” Dr. Sahaf said. Taken together, her team’s work has shown a reduction in expression of inflammatory genes and cytokines, and a decrease in plasma levels of chemotactic, inflammatory, and fibrotic cytokines that all have been implicated in cGVHD pathogenesis. The selective inhibition of pre–germinal center B cells and the trend toward reduced follicular helper T cells also plays a role in ibrutinib’s effectiveness, she said.
Ibrutinib’s efficacy in damping down inflammatory pathways that lead to cGVHD does not come at the expense of other immune function, however. Immune memory and Th 1 cells were not affected by ibrutinib administration in the study population, Dr. Sahaf said. Comparing 33 ibrutinib-receiving patients who received intravenous immune globulin with three patients who did not, the investigators saw no differences in relative antibody concentrations for tetanus or Epstein-Barr virus between the two groups.
“Protective antibodies against tetanus and Epstein-Barr virus persist following ibrutinib therapy,” Dr. Sahaf said.
Next up is the iNTEGRATE trial (NCT02959944), a phase 3 study that will test ibrutinib plus prednisone as first-line therapy for cGVHD, Dr. Sahaf said. The research team will continue its extensive proteomics work in this study as well, she said.
Dr. Sahaf reported research funding from Pharmacyclics LLC, an AbbVie company, which markets ibrutinib. She also reported having patent, royalty, or intellectual property arrangements with Stanford University.
SOURCE: Sahaf, B et al. BMT Tandem Meetings, Abstract 2.
SALT LAKE CITY – Ibrutinib’s effectiveness in inhibiting chronic graft versus host disease (cGVHD) may hinge in part on inhibition of pre–germinal center B cells and follicular helper cells, according to a new analysis of clinical trial data.
The analysis also showed that ibrutinib preserved immune memory and type 1 T-helper cells.
Bita Sahaf, PhD, presented results of a “comprehensive and high dimensional proteomic approach” to data from 42 patients who were enrolled in a phase 1/2 clinical trial of ibrutinib for cGVHD (NCT02195869).
In that study, 80% of patients who had two or more organs affected by cGVHD responded in at least two organs; overall, two-thirds of patients had a complete or partial response with ibrutinib. The highest response rates were seen in disease affecting the skin, mouth, and gastrointestinal tract.
The new analysis used blood samples from trial participants collected before and during ibrutinib therapy to look for soluble plasma factors known to be related to inflammation, fibrosis, and cGVHD.
“A heat map of cytokines, chemokines, and factors associated with fibrosis shows a significant decrease following ibrutinib treatment,” Dr. Sahaf said during a top abstracts session at the combined annual meetings of the Center for International Blood & Marrow Transplant Research and the American Society for Blood and Marrow Transplantation.
In addition, inflammatory gene expression was reduced with ibrutinib use, with reductions in the chemokines nuclear factor kappa-B-1, CXCL10, CCL7, and CCL3 dropping by 2.6-fold, 2.3-fold, 25-fold, and 1.8-fold, respectively, after 3 months of ibrutinib therapy, Dr. Sahaf and her colleagues reported.
The investigators used several different techniques to tease apart the mechanisms behind ibrutinib’s effectiveness. Immunophenotyping was accomplished with cytometry by time of flight (CyTOF), a technique that uses transition element isotopes to tag antibodies, which are then analyzed on a cell-by-cell basis by a time-of-flight mass spectrometer.
Ibrutinib inhibits CD19+CD38+CD27+IgD+ pre–germinal center B cells as well as pathogenic CD4+ T follicular helper cells, both implicated in cGVHD, the investigators found. However, Th1 T cells were preserved in a patient-by-patient analysis.
The CyTOF technique also allowed a phosphorylation analysis showing ibrutinib’s blocking effect on Bruton’s tyrosine kinase (BTK) as well as IL-2 inducible T-cell kinase (ITK), with subsequent effects on the signaling molecule PLCgamma2. In individual patients, this inhibition was confirmed when BTK-activated B-cell populations were eliminated after ibrutinib therapy, Dr. Sahaf said.
Ibrutinib also decreased phosphorylation of ITK, with subsequent depletion of CD4+, CD185+, and BCL6+ follicular helper T cells, and of other T cell populations still to be characterized. However, neither CD4+Tbet+Th1 cells nor CD4+CD25+CD127dim Treg cells saw depletion.
Importantly, “CD8+ cytotoxic T cells persist,” said Dr. Sahaf. Phosphorylation of ITK, she said, “appears heterogeneous across most T-cell populations.
“These data support the clinical efficacy of ibrutinib in cGVHD and highlight ibrutinib’s multifactorial mechanism of action in this disease,” Dr. Sahaf, of Stanford (Calif.) University, and her collaborators wrote in the abstract accompanying the presentation.
In August 2017, ibrutinib became the first treatment approved by the Food and Drug Administration for cGVHD. It is indicated for adults who have failed at least one other therapy.
“These correlative studies suggest that ibrutinib impacts a number of the immunologic mechanisms underlying the development of chronic graft versus host disease,” Dr. Sahaf said. Taken together, her team’s work has shown a reduction in expression of inflammatory genes and cytokines, and a decrease in plasma levels of chemotactic, inflammatory, and fibrotic cytokines that all have been implicated in cGVHD pathogenesis. The selective inhibition of pre–germinal center B cells and the trend toward reduced follicular helper T cells also plays a role in ibrutinib’s effectiveness, she said.
Ibrutinib’s efficacy in damping down inflammatory pathways that lead to cGVHD does not come at the expense of other immune function, however. Immune memory and Th 1 cells were not affected by ibrutinib administration in the study population, Dr. Sahaf said. Comparing 33 ibrutinib-receiving patients who received intravenous immune globulin with three patients who did not, the investigators saw no differences in relative antibody concentrations for tetanus or Epstein-Barr virus between the two groups.
“Protective antibodies against tetanus and Epstein-Barr virus persist following ibrutinib therapy,” Dr. Sahaf said.
Next up is the iNTEGRATE trial (NCT02959944), a phase 3 study that will test ibrutinib plus prednisone as first-line therapy for cGVHD, Dr. Sahaf said. The research team will continue its extensive proteomics work in this study as well, she said.
Dr. Sahaf reported research funding from Pharmacyclics LLC, an AbbVie company, which markets ibrutinib. She also reported having patent, royalty, or intellectual property arrangements with Stanford University.
SOURCE: Sahaf, B et al. BMT Tandem Meetings, Abstract 2.
SALT LAKE CITY – Ibrutinib’s effectiveness in inhibiting chronic graft versus host disease (cGVHD) may hinge in part on inhibition of pre–germinal center B cells and follicular helper cells, according to a new analysis of clinical trial data.
The analysis also showed that ibrutinib preserved immune memory and type 1 T-helper cells.
Bita Sahaf, PhD, presented results of a “comprehensive and high dimensional proteomic approach” to data from 42 patients who were enrolled in a phase 1/2 clinical trial of ibrutinib for cGVHD (NCT02195869).
In that study, 80% of patients who had two or more organs affected by cGVHD responded in at least two organs; overall, two-thirds of patients had a complete or partial response with ibrutinib. The highest response rates were seen in disease affecting the skin, mouth, and gastrointestinal tract.
The new analysis used blood samples from trial participants collected before and during ibrutinib therapy to look for soluble plasma factors known to be related to inflammation, fibrosis, and cGVHD.
“A heat map of cytokines, chemokines, and factors associated with fibrosis shows a significant decrease following ibrutinib treatment,” Dr. Sahaf said during a top abstracts session at the combined annual meetings of the Center for International Blood & Marrow Transplant Research and the American Society for Blood and Marrow Transplantation.
In addition, inflammatory gene expression was reduced with ibrutinib use, with reductions in the chemokines nuclear factor kappa-B-1, CXCL10, CCL7, and CCL3 dropping by 2.6-fold, 2.3-fold, 25-fold, and 1.8-fold, respectively, after 3 months of ibrutinib therapy, Dr. Sahaf and her colleagues reported.
The investigators used several different techniques to tease apart the mechanisms behind ibrutinib’s effectiveness. Immunophenotyping was accomplished with cytometry by time of flight (CyTOF), a technique that uses transition element isotopes to tag antibodies, which are then analyzed on a cell-by-cell basis by a time-of-flight mass spectrometer.
Ibrutinib inhibits CD19+CD38+CD27+IgD+ pre–germinal center B cells as well as pathogenic CD4+ T follicular helper cells, both implicated in cGVHD, the investigators found. However, Th1 T cells were preserved in a patient-by-patient analysis.
The CyTOF technique also allowed a phosphorylation analysis showing ibrutinib’s blocking effect on Bruton’s tyrosine kinase (BTK) as well as IL-2 inducible T-cell kinase (ITK), with subsequent effects on the signaling molecule PLCgamma2. In individual patients, this inhibition was confirmed when BTK-activated B-cell populations were eliminated after ibrutinib therapy, Dr. Sahaf said.
Ibrutinib also decreased phosphorylation of ITK, with subsequent depletion of CD4+, CD185+, and BCL6+ follicular helper T cells, and of other T cell populations still to be characterized. However, neither CD4+Tbet+Th1 cells nor CD4+CD25+CD127dim Treg cells saw depletion.
Importantly, “CD8+ cytotoxic T cells persist,” said Dr. Sahaf. Phosphorylation of ITK, she said, “appears heterogeneous across most T-cell populations.
“These data support the clinical efficacy of ibrutinib in cGVHD and highlight ibrutinib’s multifactorial mechanism of action in this disease,” Dr. Sahaf, of Stanford (Calif.) University, and her collaborators wrote in the abstract accompanying the presentation.
In August 2017, ibrutinib became the first treatment approved by the Food and Drug Administration for cGVHD. It is indicated for adults who have failed at least one other therapy.
“These correlative studies suggest that ibrutinib impacts a number of the immunologic mechanisms underlying the development of chronic graft versus host disease,” Dr. Sahaf said. Taken together, her team’s work has shown a reduction in expression of inflammatory genes and cytokines, and a decrease in plasma levels of chemotactic, inflammatory, and fibrotic cytokines that all have been implicated in cGVHD pathogenesis. The selective inhibition of pre–germinal center B cells and the trend toward reduced follicular helper T cells also plays a role in ibrutinib’s effectiveness, she said.
Ibrutinib’s efficacy in damping down inflammatory pathways that lead to cGVHD does not come at the expense of other immune function, however. Immune memory and Th 1 cells were not affected by ibrutinib administration in the study population, Dr. Sahaf said. Comparing 33 ibrutinib-receiving patients who received intravenous immune globulin with three patients who did not, the investigators saw no differences in relative antibody concentrations for tetanus or Epstein-Barr virus between the two groups.
“Protective antibodies against tetanus and Epstein-Barr virus persist following ibrutinib therapy,” Dr. Sahaf said.
Next up is the iNTEGRATE trial (NCT02959944), a phase 3 study that will test ibrutinib plus prednisone as first-line therapy for cGVHD, Dr. Sahaf said. The research team will continue its extensive proteomics work in this study as well, she said.
Dr. Sahaf reported research funding from Pharmacyclics LLC, an AbbVie company, which markets ibrutinib. She also reported having patent, royalty, or intellectual property arrangements with Stanford University.
SOURCE: Sahaf, B et al. BMT Tandem Meetings, Abstract 2.
REPORTING FROM THE 2018 BMT TANDEM MEETINGS
Key clinical point:
Major finding: Inflammatory gene expression dropped between 1.8-fold and 25-fold for individual chemokines after ibrutinib treatment.
Study details: Comprehensive proteomics analysis of data from a phase 1/2 clinical trial of ibrutinib as second-line therapy for cGVHD.
Disclosures: The clinical trial was sponsored by Pharmacyclics LLC, an Abbvie company. Dr. Sahaf reported having patent, royalty, or intellectual property arrangements with Stanford University.
Source: Sahaf B et al. 2018 BMT Tandem Meetings. Abstract 2.
21-gene assay predicts survival in male and female breast cancer
A study of the molecular and genomic features of breast cancer in men, compared with those in women, highlights the prognostic value of a 21-gene breast recurrence score in both sexes, investigators say.
Men and women with estrogen receptor (ER)–positive breast cancer who had recurrence scores (RS) of 0 to 30 on the 21-gene assay (Oncotype DX) had excellent breast cancer–specific survival rates, which suggests that such patients could be spared from more aggressive treatments, such as chemotherapy, according to Suleiman Alfred Massarweh, MD, of Stanford (Calif.) University and his colleagues.
“Future adjuvant trials in ER-positive breast cancer may need to focus on targeting endocrine resistance in those patients with RS greater than 31 and may need to consider the weight of competing mortality risk when investigating the value of any additional treatment beyond endocrine therapy,” they wrote in the Journal of Clinical Oncology.
In 2016, an estimated 2,600 men were diagnosed with breast cancer in the United States.
“Approximately 95% of breast cancers diagnosed in men express the estrogen receptor and progesterone receptor (PR), which is a higher percentage than in women and suggests a key role for ER in the biology of breast cancer in men,” the investigators noted.
Although treatment of men with breast cancer has traditionally been extrapolated from treatment of women with breast cancer, genomic studies have suggested some key differences, the investigators noted, citing a study of the genomic landscape of male breast cancers presented at the 2014 San Antonio Breast Cancer Symposium.
In that study, investigators from the Memorial Sloan Kettering Cancer Center in New York and other institutions found that all male breast cancers in their sample of 64 patients were ER+ and human epidermal growth factor receptor 2 (HER2)–negative, predominantly of the luminal B subtype, and that the genetic alterations seen in male breast cancers frequently target DNA-repair fibroblast growth factor pathways. However, the pathways that are known to drive luminal cancers when mutated in women are seen less often among men, said Salvatore Piscuoglio, PhD, then a research fellow at MSKCC.
The current study helps to confirm and expand on the findings from that study, commented Steven J. Isakoff, MD, PhD, of the Massachusetts General Hospital Cancer Center in Boston, who was not involved in either study.
“I think it’s helpful to see in a larger dataset what the spectrum of oncotypes [Oncotype DX] looks like in men. In general, as the study described, we have a real lack of large-scale data in men and certainly no prospective data with oncotypes,” he said in an interview.
To get a better idea of the molecular characteristics of breast cancer in men and how they relate to breast cancer–specific mortality, Dr. Massarweh and his associates looked at deidentified 21-gene assay data from the Genomic Health Laboratory database on 3,806 men and 571,115 women with breast cancer with either no nodal involvement, micrometastases only, or one to three involved lymph nodes.
They also looked at survival data from the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) population of patients with breast cancer diagnosed during 2004-2012, which included data on 332 men and 55,842 women with ER-positive and/or PR-positive invasive breast cancer.
Among the entire 21-gene assay sample, they found that men were significantly older than women at the time of diagnosis, at a mean age of 64.2 vs. 59.1 years (P less than .001).
Both men and women had infiltrating ductal carcinoma as the most common histology; the prevalence was slightly higher among men at 87.6% versus 81.3% for women.
The average recurrence score in men was 16.8 versus 17.0 in women, a difference that was not statistically significant. A majority of both men and women had RS scores below 18 (65.8% and 58.2%, respectively), although significantly more men than women had RS scores of 31 or higher (12.4% vs. 7.4%; P less than .001).
“This relative predominance of high RS results in men was encountered across age groups but was most prominent in men younger than 40 years of age,” the investigators wrote.
At the other end of the scale, RS lower than 11, especially RS 0, were seen more frequently in men than in women, except among those younger than 40 years.
Looking at individual gene expression profiles, the authors found that mean gene expression was higher in men for genes associated with ER, proliferation, and invasion. ER expression was lowest and PR expression was highest in women younger than 50 years, but ER expression increased progressively with age.
Among men, those younger than 50 years had slightly lower ER and PR expression than did older men.
In the analysis of SEER survival data, they found that 5-year breast cancer severity score (BCSS) was 99% for men with RS below 18, 95.7% for those with RS between18 and 30, and 81% for those with RS of 31 or higher. Among women, 5-year BCSS was 99.5% for those with RS under 18, 98.6% for those with RS between 18 and 30, and 94.9% for those with RS of 31 or higher.
Five-year overall survival estimates were 92.6% for men with RS below 18, 86% for those with RS between 18 and 30, and 69% for those with RS of 31 or higher. Respective 5-year OS rates for women were 95%, 94.2%, and 89.9%.
“The 21-gene RS provided clear prognostic information in our cohort, with a significantly different 5-year BCSS determined by RS in both men and women,” the investigators wrote.
They noted that patients with low and intermediate RS have excellent prognoses regardless of nodal status, which suggests that these patients have more indolent disease and better outcomes than do patients with higher RS.
The more frequent use of adjuvant chemotherapy in the RS 31 and higher group indicates that “the prognostic utility of RS results is evident despite adjuvant chemotherapy use,” they wrote.
Dr. Isakoff pointed out, however, that the population in the study is from a registry of patients eligible for the 21-gene assay, which can only be used for patients with ER-positive and HER2-negative tumors.
“In other words, this is not a random sample. This is a sample of patients for whom the treating physician was on the fence about chemotherapy and in some way thought that getting an oncotype might be helpful,” he said.
He added that although the study findings “don’t change anything we have been doing, they provide reassurance that oncotype is a reasonable test to consider in patients with male breast cancer for whom we’re considering including or avoiding chemotherapy,” he said.
A funding source for the study was not reported. Dr. Massarweh disclosed stock or ownership in Radius Health, consulting for Novartis, and institutional research funding from multiple companies. Three coauthors are employees and stockholders of Genomic Health, maker of the Oncotype DX assay used in the study. Dr. Isakoff reported no conflicts of interest related to the study
SOURCE: Massarweh SA et al. 2018 Mar 27. doi: 10.1200/JCO.2017.76.8861.
A study of the molecular and genomic features of breast cancer in men, compared with those in women, highlights the prognostic value of a 21-gene breast recurrence score in both sexes, investigators say.
Men and women with estrogen receptor (ER)–positive breast cancer who had recurrence scores (RS) of 0 to 30 on the 21-gene assay (Oncotype DX) had excellent breast cancer–specific survival rates, which suggests that such patients could be spared from more aggressive treatments, such as chemotherapy, according to Suleiman Alfred Massarweh, MD, of Stanford (Calif.) University and his colleagues.
“Future adjuvant trials in ER-positive breast cancer may need to focus on targeting endocrine resistance in those patients with RS greater than 31 and may need to consider the weight of competing mortality risk when investigating the value of any additional treatment beyond endocrine therapy,” they wrote in the Journal of Clinical Oncology.
In 2016, an estimated 2,600 men were diagnosed with breast cancer in the United States.
“Approximately 95% of breast cancers diagnosed in men express the estrogen receptor and progesterone receptor (PR), which is a higher percentage than in women and suggests a key role for ER in the biology of breast cancer in men,” the investigators noted.
Although treatment of men with breast cancer has traditionally been extrapolated from treatment of women with breast cancer, genomic studies have suggested some key differences, the investigators noted, citing a study of the genomic landscape of male breast cancers presented at the 2014 San Antonio Breast Cancer Symposium.
In that study, investigators from the Memorial Sloan Kettering Cancer Center in New York and other institutions found that all male breast cancers in their sample of 64 patients were ER+ and human epidermal growth factor receptor 2 (HER2)–negative, predominantly of the luminal B subtype, and that the genetic alterations seen in male breast cancers frequently target DNA-repair fibroblast growth factor pathways. However, the pathways that are known to drive luminal cancers when mutated in women are seen less often among men, said Salvatore Piscuoglio, PhD, then a research fellow at MSKCC.
The current study helps to confirm and expand on the findings from that study, commented Steven J. Isakoff, MD, PhD, of the Massachusetts General Hospital Cancer Center in Boston, who was not involved in either study.
“I think it’s helpful to see in a larger dataset what the spectrum of oncotypes [Oncotype DX] looks like in men. In general, as the study described, we have a real lack of large-scale data in men and certainly no prospective data with oncotypes,” he said in an interview.
To get a better idea of the molecular characteristics of breast cancer in men and how they relate to breast cancer–specific mortality, Dr. Massarweh and his associates looked at deidentified 21-gene assay data from the Genomic Health Laboratory database on 3,806 men and 571,115 women with breast cancer with either no nodal involvement, micrometastases only, or one to three involved lymph nodes.
They also looked at survival data from the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) population of patients with breast cancer diagnosed during 2004-2012, which included data on 332 men and 55,842 women with ER-positive and/or PR-positive invasive breast cancer.
Among the entire 21-gene assay sample, they found that men were significantly older than women at the time of diagnosis, at a mean age of 64.2 vs. 59.1 years (P less than .001).
Both men and women had infiltrating ductal carcinoma as the most common histology; the prevalence was slightly higher among men at 87.6% versus 81.3% for women.
The average recurrence score in men was 16.8 versus 17.0 in women, a difference that was not statistically significant. A majority of both men and women had RS scores below 18 (65.8% and 58.2%, respectively), although significantly more men than women had RS scores of 31 or higher (12.4% vs. 7.4%; P less than .001).
“This relative predominance of high RS results in men was encountered across age groups but was most prominent in men younger than 40 years of age,” the investigators wrote.
At the other end of the scale, RS lower than 11, especially RS 0, were seen more frequently in men than in women, except among those younger than 40 years.
Looking at individual gene expression profiles, the authors found that mean gene expression was higher in men for genes associated with ER, proliferation, and invasion. ER expression was lowest and PR expression was highest in women younger than 50 years, but ER expression increased progressively with age.
Among men, those younger than 50 years had slightly lower ER and PR expression than did older men.
In the analysis of SEER survival data, they found that 5-year breast cancer severity score (BCSS) was 99% for men with RS below 18, 95.7% for those with RS between18 and 30, and 81% for those with RS of 31 or higher. Among women, 5-year BCSS was 99.5% for those with RS under 18, 98.6% for those with RS between 18 and 30, and 94.9% for those with RS of 31 or higher.
Five-year overall survival estimates were 92.6% for men with RS below 18, 86% for those with RS between 18 and 30, and 69% for those with RS of 31 or higher. Respective 5-year OS rates for women were 95%, 94.2%, and 89.9%.
“The 21-gene RS provided clear prognostic information in our cohort, with a significantly different 5-year BCSS determined by RS in both men and women,” the investigators wrote.
They noted that patients with low and intermediate RS have excellent prognoses regardless of nodal status, which suggests that these patients have more indolent disease and better outcomes than do patients with higher RS.
The more frequent use of adjuvant chemotherapy in the RS 31 and higher group indicates that “the prognostic utility of RS results is evident despite adjuvant chemotherapy use,” they wrote.
Dr. Isakoff pointed out, however, that the population in the study is from a registry of patients eligible for the 21-gene assay, which can only be used for patients with ER-positive and HER2-negative tumors.
“In other words, this is not a random sample. This is a sample of patients for whom the treating physician was on the fence about chemotherapy and in some way thought that getting an oncotype might be helpful,” he said.
He added that although the study findings “don’t change anything we have been doing, they provide reassurance that oncotype is a reasonable test to consider in patients with male breast cancer for whom we’re considering including or avoiding chemotherapy,” he said.
A funding source for the study was not reported. Dr. Massarweh disclosed stock or ownership in Radius Health, consulting for Novartis, and institutional research funding from multiple companies. Three coauthors are employees and stockholders of Genomic Health, maker of the Oncotype DX assay used in the study. Dr. Isakoff reported no conflicts of interest related to the study
SOURCE: Massarweh SA et al. 2018 Mar 27. doi: 10.1200/JCO.2017.76.8861.
A study of the molecular and genomic features of breast cancer in men, compared with those in women, highlights the prognostic value of a 21-gene breast recurrence score in both sexes, investigators say.
Men and women with estrogen receptor (ER)–positive breast cancer who had recurrence scores (RS) of 0 to 30 on the 21-gene assay (Oncotype DX) had excellent breast cancer–specific survival rates, which suggests that such patients could be spared from more aggressive treatments, such as chemotherapy, according to Suleiman Alfred Massarweh, MD, of Stanford (Calif.) University and his colleagues.
“Future adjuvant trials in ER-positive breast cancer may need to focus on targeting endocrine resistance in those patients with RS greater than 31 and may need to consider the weight of competing mortality risk when investigating the value of any additional treatment beyond endocrine therapy,” they wrote in the Journal of Clinical Oncology.
In 2016, an estimated 2,600 men were diagnosed with breast cancer in the United States.
“Approximately 95% of breast cancers diagnosed in men express the estrogen receptor and progesterone receptor (PR), which is a higher percentage than in women and suggests a key role for ER in the biology of breast cancer in men,” the investigators noted.
Although treatment of men with breast cancer has traditionally been extrapolated from treatment of women with breast cancer, genomic studies have suggested some key differences, the investigators noted, citing a study of the genomic landscape of male breast cancers presented at the 2014 San Antonio Breast Cancer Symposium.
In that study, investigators from the Memorial Sloan Kettering Cancer Center in New York and other institutions found that all male breast cancers in their sample of 64 patients were ER+ and human epidermal growth factor receptor 2 (HER2)–negative, predominantly of the luminal B subtype, and that the genetic alterations seen in male breast cancers frequently target DNA-repair fibroblast growth factor pathways. However, the pathways that are known to drive luminal cancers when mutated in women are seen less often among men, said Salvatore Piscuoglio, PhD, then a research fellow at MSKCC.
The current study helps to confirm and expand on the findings from that study, commented Steven J. Isakoff, MD, PhD, of the Massachusetts General Hospital Cancer Center in Boston, who was not involved in either study.
“I think it’s helpful to see in a larger dataset what the spectrum of oncotypes [Oncotype DX] looks like in men. In general, as the study described, we have a real lack of large-scale data in men and certainly no prospective data with oncotypes,” he said in an interview.
To get a better idea of the molecular characteristics of breast cancer in men and how they relate to breast cancer–specific mortality, Dr. Massarweh and his associates looked at deidentified 21-gene assay data from the Genomic Health Laboratory database on 3,806 men and 571,115 women with breast cancer with either no nodal involvement, micrometastases only, or one to three involved lymph nodes.
They also looked at survival data from the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) population of patients with breast cancer diagnosed during 2004-2012, which included data on 332 men and 55,842 women with ER-positive and/or PR-positive invasive breast cancer.
Among the entire 21-gene assay sample, they found that men were significantly older than women at the time of diagnosis, at a mean age of 64.2 vs. 59.1 years (P less than .001).
Both men and women had infiltrating ductal carcinoma as the most common histology; the prevalence was slightly higher among men at 87.6% versus 81.3% for women.
The average recurrence score in men was 16.8 versus 17.0 in women, a difference that was not statistically significant. A majority of both men and women had RS scores below 18 (65.8% and 58.2%, respectively), although significantly more men than women had RS scores of 31 or higher (12.4% vs. 7.4%; P less than .001).
“This relative predominance of high RS results in men was encountered across age groups but was most prominent in men younger than 40 years of age,” the investigators wrote.
At the other end of the scale, RS lower than 11, especially RS 0, were seen more frequently in men than in women, except among those younger than 40 years.
Looking at individual gene expression profiles, the authors found that mean gene expression was higher in men for genes associated with ER, proliferation, and invasion. ER expression was lowest and PR expression was highest in women younger than 50 years, but ER expression increased progressively with age.
Among men, those younger than 50 years had slightly lower ER and PR expression than did older men.
In the analysis of SEER survival data, they found that 5-year breast cancer severity score (BCSS) was 99% for men with RS below 18, 95.7% for those with RS between18 and 30, and 81% for those with RS of 31 or higher. Among women, 5-year BCSS was 99.5% for those with RS under 18, 98.6% for those with RS between 18 and 30, and 94.9% for those with RS of 31 or higher.
Five-year overall survival estimates were 92.6% for men with RS below 18, 86% for those with RS between 18 and 30, and 69% for those with RS of 31 or higher. Respective 5-year OS rates for women were 95%, 94.2%, and 89.9%.
“The 21-gene RS provided clear prognostic information in our cohort, with a significantly different 5-year BCSS determined by RS in both men and women,” the investigators wrote.
They noted that patients with low and intermediate RS have excellent prognoses regardless of nodal status, which suggests that these patients have more indolent disease and better outcomes than do patients with higher RS.
The more frequent use of adjuvant chemotherapy in the RS 31 and higher group indicates that “the prognostic utility of RS results is evident despite adjuvant chemotherapy use,” they wrote.
Dr. Isakoff pointed out, however, that the population in the study is from a registry of patients eligible for the 21-gene assay, which can only be used for patients with ER-positive and HER2-negative tumors.
“In other words, this is not a random sample. This is a sample of patients for whom the treating physician was on the fence about chemotherapy and in some way thought that getting an oncotype might be helpful,” he said.
He added that although the study findings “don’t change anything we have been doing, they provide reassurance that oncotype is a reasonable test to consider in patients with male breast cancer for whom we’re considering including or avoiding chemotherapy,” he said.
A funding source for the study was not reported. Dr. Massarweh disclosed stock or ownership in Radius Health, consulting for Novartis, and institutional research funding from multiple companies. Three coauthors are employees and stockholders of Genomic Health, maker of the Oncotype DX assay used in the study. Dr. Isakoff reported no conflicts of interest related to the study
SOURCE: Massarweh SA et al. 2018 Mar 27. doi: 10.1200/JCO.2017.76.8861.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: A 21-gene assay provides useful information about survival odds for men and women with breast cancer.
Major finding: A recurrence score of 31 or greater was associated with worse survival, particularly in men.
Study details: Retrospective review of genomic and surveillance data on 3,806 men and 571,115 women with breast cancer.
Disclosures: A funding source for the study was not reported. Dr. Massarweh disclosed stock or ownership in Radius Health, consulting for Novartis, and institutional research funding from multiple companies. Three coauthors are employees and stockholders of Genomic Health, maker of the Oncotype DX assay used in the study. Dr. Isakoff reported no conflicts of interest related to the study.
Source: Massarweh SA et al. 2018 Mar 27. doi: 10.1200/JCO.2017.76.8861.
FDA recalls kratom products for salmonella contamination
The Food and Drug Administration on April 3 recalled all products containing kratom manufactured by Triangle Pharmanaturals LLC, after a number of supplements tested positive for salmonella.
The FDA advises consumers to get rid of products including Raw Form Organics Maeng Da Kratom Emerald Green, Raw Form Organics Maeng Da Kratom Ivory White, and Raw Form Organics Maeng Da Kratom Ruby Red.
Evidence of the contamination was found after two samples were collected from a retail store in Oregon by the Oregon Public Health Division and tested positive for salmonella.
The recall was ordered after Triangle Pharmanaturals did not comply with a March 30 formal request from the FDA to voluntarily recall their products.
“This action is based on the imminent health risk posed by the contamination of this product with salmonella, and the refusal of this company to voluntarily act to protect its customers and issue a recall, despite our repeated requests and actions,” said FDA Commissioner Scott Gottlieb, M.D., said in a statement. “The action today is based on the risks posed by the contamination of this particular product with a potentially dangerous pathogen.”
At press time, Triangle Pharmanaturals did not respond to a request for comment.
This is the most recent in a list recalls of kratom products as part of an ongoing investigation of a salmonella outbreak by the FDA; however Triangle Pharmanaturals’ noncompliance is unique to the agency, according to an FDA representative.
“This is the first time the agency has issued a mandatory recall order to protect Americans from contaminated food products,” Michael Felberbaum, an FDA press officer, said in an interview. “This is the third time the FDA has invoked its mandatory recall authority, but the first time the agency ordered a mandatory recall because a company has opted not to voluntarily recall after the FDA’s notification of an opportunity to initiate a voluntary recall.”
Earlier in March, the CDC reported 87 people in 35 states infected with either Salmonella Javiana, Salmonela Okatie, or Salmonella Thompson, which have been associated with the outbreak.
While salmonella was identified in Triangle Pharmanaturals’ products, the strains identified are not currently linked to the outbreak.
Kratom, a plant that commonly grows in South East Asian countries like Thailand, Malaysia, Indonesia, and Papua New Guinea, has recently been used to produce food supplements and marketed as an alternative to addictive pain medication like opioids, as well as used to help treat opioid withdrawal symptoms.
Use of the food supplement has fired debate among physicians, patients, and public officials as all sides continue to determine its efficacy and how, or whether, it should be given a drug classification.
The Food and Drug Administration on April 3 recalled all products containing kratom manufactured by Triangle Pharmanaturals LLC, after a number of supplements tested positive for salmonella.
The FDA advises consumers to get rid of products including Raw Form Organics Maeng Da Kratom Emerald Green, Raw Form Organics Maeng Da Kratom Ivory White, and Raw Form Organics Maeng Da Kratom Ruby Red.
Evidence of the contamination was found after two samples were collected from a retail store in Oregon by the Oregon Public Health Division and tested positive for salmonella.
The recall was ordered after Triangle Pharmanaturals did not comply with a March 30 formal request from the FDA to voluntarily recall their products.
“This action is based on the imminent health risk posed by the contamination of this product with salmonella, and the refusal of this company to voluntarily act to protect its customers and issue a recall, despite our repeated requests and actions,” said FDA Commissioner Scott Gottlieb, M.D., said in a statement. “The action today is based on the risks posed by the contamination of this particular product with a potentially dangerous pathogen.”
At press time, Triangle Pharmanaturals did not respond to a request for comment.
This is the most recent in a list recalls of kratom products as part of an ongoing investigation of a salmonella outbreak by the FDA; however Triangle Pharmanaturals’ noncompliance is unique to the agency, according to an FDA representative.
“This is the first time the agency has issued a mandatory recall order to protect Americans from contaminated food products,” Michael Felberbaum, an FDA press officer, said in an interview. “This is the third time the FDA has invoked its mandatory recall authority, but the first time the agency ordered a mandatory recall because a company has opted not to voluntarily recall after the FDA’s notification of an opportunity to initiate a voluntary recall.”
Earlier in March, the CDC reported 87 people in 35 states infected with either Salmonella Javiana, Salmonela Okatie, or Salmonella Thompson, which have been associated with the outbreak.
While salmonella was identified in Triangle Pharmanaturals’ products, the strains identified are not currently linked to the outbreak.
Kratom, a plant that commonly grows in South East Asian countries like Thailand, Malaysia, Indonesia, and Papua New Guinea, has recently been used to produce food supplements and marketed as an alternative to addictive pain medication like opioids, as well as used to help treat opioid withdrawal symptoms.
Use of the food supplement has fired debate among physicians, patients, and public officials as all sides continue to determine its efficacy and how, or whether, it should be given a drug classification.
The Food and Drug Administration on April 3 recalled all products containing kratom manufactured by Triangle Pharmanaturals LLC, after a number of supplements tested positive for salmonella.
The FDA advises consumers to get rid of products including Raw Form Organics Maeng Da Kratom Emerald Green, Raw Form Organics Maeng Da Kratom Ivory White, and Raw Form Organics Maeng Da Kratom Ruby Red.
Evidence of the contamination was found after two samples were collected from a retail store in Oregon by the Oregon Public Health Division and tested positive for salmonella.
The recall was ordered after Triangle Pharmanaturals did not comply with a March 30 formal request from the FDA to voluntarily recall their products.
“This action is based on the imminent health risk posed by the contamination of this product with salmonella, and the refusal of this company to voluntarily act to protect its customers and issue a recall, despite our repeated requests and actions,” said FDA Commissioner Scott Gottlieb, M.D., said in a statement. “The action today is based on the risks posed by the contamination of this particular product with a potentially dangerous pathogen.”
At press time, Triangle Pharmanaturals did not respond to a request for comment.
This is the most recent in a list recalls of kratom products as part of an ongoing investigation of a salmonella outbreak by the FDA; however Triangle Pharmanaturals’ noncompliance is unique to the agency, according to an FDA representative.
“This is the first time the agency has issued a mandatory recall order to protect Americans from contaminated food products,” Michael Felberbaum, an FDA press officer, said in an interview. “This is the third time the FDA has invoked its mandatory recall authority, but the first time the agency ordered a mandatory recall because a company has opted not to voluntarily recall after the FDA’s notification of an opportunity to initiate a voluntary recall.”
Earlier in March, the CDC reported 87 people in 35 states infected with either Salmonella Javiana, Salmonela Okatie, or Salmonella Thompson, which have been associated with the outbreak.
While salmonella was identified in Triangle Pharmanaturals’ products, the strains identified are not currently linked to the outbreak.
Kratom, a plant that commonly grows in South East Asian countries like Thailand, Malaysia, Indonesia, and Papua New Guinea, has recently been used to produce food supplements and marketed as an alternative to addictive pain medication like opioids, as well as used to help treat opioid withdrawal symptoms.
Use of the food supplement has fired debate among physicians, patients, and public officials as all sides continue to determine its efficacy and how, or whether, it should be given a drug classification.
Developing a Pediatric Epilepsy Self-Management Protocol
Pediatric patients with epilepsy would benefit from a comprehensive self-management system that includes several modifiable targets such as adherence, self-sufficiency, attitudes about the disorder, and family variables, according to a review published in Epilepsia.
- To reach that conclusion, investigators studied English-based literature from 1985 to 2014, concentrating on countries with a very high human development index.
- They identified 25 studies related to self-management of epilepsy and found that individual and family-focused factors were most often analyzed for their ability to predict successful self-management.
- Psychosocial care needs and self-efficiency were identified as key factors linked to pediatric epilepsy self-management.
- Researchers identified adherence, self-efficacy for seizure management, attitudes toward epilepsy, and family variables as skills to concentrate on when developing a self-management model.
Smith G, Modi AC, Johnson EK, et al. Measurement in pediatric epilepsy self-management: a critical review. Epilepsia. 2018;59(3):509-522.
Pediatric patients with epilepsy would benefit from a comprehensive self-management system that includes several modifiable targets such as adherence, self-sufficiency, attitudes about the disorder, and family variables, according to a review published in Epilepsia.
- To reach that conclusion, investigators studied English-based literature from 1985 to 2014, concentrating on countries with a very high human development index.
- They identified 25 studies related to self-management of epilepsy and found that individual and family-focused factors were most often analyzed for their ability to predict successful self-management.
- Psychosocial care needs and self-efficiency were identified as key factors linked to pediatric epilepsy self-management.
- Researchers identified adherence, self-efficacy for seizure management, attitudes toward epilepsy, and family variables as skills to concentrate on when developing a self-management model.
Smith G, Modi AC, Johnson EK, et al. Measurement in pediatric epilepsy self-management: a critical review. Epilepsia. 2018;59(3):509-522.
Pediatric patients with epilepsy would benefit from a comprehensive self-management system that includes several modifiable targets such as adherence, self-sufficiency, attitudes about the disorder, and family variables, according to a review published in Epilepsia.
- To reach that conclusion, investigators studied English-based literature from 1985 to 2014, concentrating on countries with a very high human development index.
- They identified 25 studies related to self-management of epilepsy and found that individual and family-focused factors were most often analyzed for their ability to predict successful self-management.
- Psychosocial care needs and self-efficiency were identified as key factors linked to pediatric epilepsy self-management.
- Researchers identified adherence, self-efficacy for seizure management, attitudes toward epilepsy, and family variables as skills to concentrate on when developing a self-management model.
Smith G, Modi AC, Johnson EK, et al. Measurement in pediatric epilepsy self-management: a critical review. Epilepsia. 2018;59(3):509-522.
Linking EEG Oscillations to Epileptic Spasms
The occurrence rate of high-frequency oscillations is higher in children with drug-resistant multilobar epilepsy who experience epileptic spasms, when compared with those who do not, according to a study published in Epilepsia.
- Researchers found that the number of electrodes with high-rate fast ripple (FR) amplitude and the modulation index of coupling between slow and fast oscillations in all electrodes were significantly higher in children with epileptic spasms.
- The investigation involved 24 pediatric patients with drug-resistant multilobar onset epilepsy who had intracranial video EEGs done before multilobar resection.
- The modulation index was highest in 5 frequency bands among both epileptic spasm and nonepileptic spasm prone children.
- Researchers concluded that the greater number of high-rate FR electrodes provided evidence that there was more widespread epileptogenicity in the spasming patients, compared to those without spasms.
- Children with epileptic spasms who were free of seizures after surgery had strong coupling between slow oscillations and FRs.
Iimura Y, Jones K, Takada L, et al. Strong coupling between slow oscillations and wide fast ripples in children with epileptic spasms: Investigation of modulation index and occurrence rate. Epilepsia. 2018;59(3):544-554.
The occurrence rate of high-frequency oscillations is higher in children with drug-resistant multilobar epilepsy who experience epileptic spasms, when compared with those who do not, according to a study published in Epilepsia.
- Researchers found that the number of electrodes with high-rate fast ripple (FR) amplitude and the modulation index of coupling between slow and fast oscillations in all electrodes were significantly higher in children with epileptic spasms.
- The investigation involved 24 pediatric patients with drug-resistant multilobar onset epilepsy who had intracranial video EEGs done before multilobar resection.
- The modulation index was highest in 5 frequency bands among both epileptic spasm and nonepileptic spasm prone children.
- Researchers concluded that the greater number of high-rate FR electrodes provided evidence that there was more widespread epileptogenicity in the spasming patients, compared to those without spasms.
- Children with epileptic spasms who were free of seizures after surgery had strong coupling between slow oscillations and FRs.
Iimura Y, Jones K, Takada L, et al. Strong coupling between slow oscillations and wide fast ripples in children with epileptic spasms: Investigation of modulation index and occurrence rate. Epilepsia. 2018;59(3):544-554.
The occurrence rate of high-frequency oscillations is higher in children with drug-resistant multilobar epilepsy who experience epileptic spasms, when compared with those who do not, according to a study published in Epilepsia.
- Researchers found that the number of electrodes with high-rate fast ripple (FR) amplitude and the modulation index of coupling between slow and fast oscillations in all electrodes were significantly higher in children with epileptic spasms.
- The investigation involved 24 pediatric patients with drug-resistant multilobar onset epilepsy who had intracranial video EEGs done before multilobar resection.
- The modulation index was highest in 5 frequency bands among both epileptic spasm and nonepileptic spasm prone children.
- Researchers concluded that the greater number of high-rate FR electrodes provided evidence that there was more widespread epileptogenicity in the spasming patients, compared to those without spasms.
- Children with epileptic spasms who were free of seizures after surgery had strong coupling between slow oscillations and FRs.
Iimura Y, Jones K, Takada L, et al. Strong coupling between slow oscillations and wide fast ripples in children with epileptic spasms: Investigation of modulation index and occurrence rate. Epilepsia. 2018;59(3):544-554.
Angelman Syndrome Accompanied by Nonepileptic Myoclonus
Angelman syndrome, a neurogenetic disorder brought on by loss of the Ube3a gene, is often accompanied by nonepileptic myoclonus according to a study of 200 patients reported by the Massachusetts General Hospital and the Lurie Center for Autism.
- Myoclonus seizures were reported in 14% of patients with Angelman syndrome, with the first episode beginning before 8 years of age.
- The seizures were usually brief, unless the patient was experiencing myoclonic status, and EEGs showed interictal generalized spike and wave activity.
- 40% of patients older than 10 years had nonepileptic myoclonus.
- Nonepileptic myoclonus typically started during puberty or later.
- The nonepileptic myoclonus lasted from seconds to hours and always began in patients’ hands and, in some cases, spread to face and all extremities.
Pollack SF, Grocott OR, Parkin KA, et al. Myoclonus in Angelman syndrome [Published online ahead of print March 17, 2018]. Epilepsy Behav. https://doi.org/10.1016/j.yebeh.2018.02.006.
Angelman syndrome, a neurogenetic disorder brought on by loss of the Ube3a gene, is often accompanied by nonepileptic myoclonus according to a study of 200 patients reported by the Massachusetts General Hospital and the Lurie Center for Autism.
- Myoclonus seizures were reported in 14% of patients with Angelman syndrome, with the first episode beginning before 8 years of age.
- The seizures were usually brief, unless the patient was experiencing myoclonic status, and EEGs showed interictal generalized spike and wave activity.
- 40% of patients older than 10 years had nonepileptic myoclonus.
- Nonepileptic myoclonus typically started during puberty or later.
- The nonepileptic myoclonus lasted from seconds to hours and always began in patients’ hands and, in some cases, spread to face and all extremities.
Pollack SF, Grocott OR, Parkin KA, et al. Myoclonus in Angelman syndrome [Published online ahead of print March 17, 2018]. Epilepsy Behav. https://doi.org/10.1016/j.yebeh.2018.02.006.
Angelman syndrome, a neurogenetic disorder brought on by loss of the Ube3a gene, is often accompanied by nonepileptic myoclonus according to a study of 200 patients reported by the Massachusetts General Hospital and the Lurie Center for Autism.
- Myoclonus seizures were reported in 14% of patients with Angelman syndrome, with the first episode beginning before 8 years of age.
- The seizures were usually brief, unless the patient was experiencing myoclonic status, and EEGs showed interictal generalized spike and wave activity.
- 40% of patients older than 10 years had nonepileptic myoclonus.
- Nonepileptic myoclonus typically started during puberty or later.
- The nonepileptic myoclonus lasted from seconds to hours and always began in patients’ hands and, in some cases, spread to face and all extremities.
Pollack SF, Grocott OR, Parkin KA, et al. Myoclonus in Angelman syndrome [Published online ahead of print March 17, 2018]. Epilepsy Behav. https://doi.org/10.1016/j.yebeh.2018.02.006.
Evaluating fever in the first 90 days of life
Fever in the youngest of infants creates a challenge for the pediatric clinician. Fever is a common presentation for serious bacterial infection (SBI) although most fevers are due to viral infection. However, the clinical presentation does not necessarily differ, and the risk for a poor outcome in this age group is substantial.
In the early stages of my pediatric career, most febrile infants less than 90 days of age were evaluated for sepsis, admitted, and treated with antibiotics pending culture results. Group B streptococcal sepsis or Escherichia coli sepsis were common in the first month of life, and Haemophilus influenza type B or Streptococcus pneumoniae in the second and third months of life. The approach to fever in the first 90 days has changed following both the introduction of haemophilus and pneumococcal conjugate vaccines, the experience with risk stratification criteria for identifying infants at low risk for SBI, and the recognition of urinary tract infection (UTI) as a common source of infection in this age group as well as development of criteria for diagnosis.
A further nuance was subsequently added with the introduction of rapid diagnostics for viral infection. Byington et al. found that the majority of febrile infants less than 90 days of age had viral infection with enterovirus, respiratory syncytial virus (RSV), influenza or rotavirus.1 Using the Rochester risk stratification and the presence or absence of viral infection, she demonstrated that the risk of SBI was reduced in both high- and low-risk infants in the presence of viral infection; in low risk infants with viral infection, SBI was identified in 1.8%, compared with 3.1% in those without viral infection, and in high-risk infants. 5.5% has SBI when viral infection was found, compared to 16.7% in the absence of viral infection. She also proposed risk features to identify those infected with herpes simplex virus; age less than 42 days, vesicular rash, elevated alanine transaminase (ALT) and aspartate aminotransferase (AST), CSF pleocytosis, and seizure or twitching.
Greenhow et al. reported on the experience with “serious” bacterial infection in infants less than 90 days of age receiving care at Northern California Kaiser Permanente during the period 2005-2011.2 As pictured, the majority of children have UTI, and smaller numbers have bacteremia or meningitis. A small group of children with UTI have urosepsis as well; those with urosepsis can be differentiated from those with only UTI by age (less than 21 days), clinical exam (ill appearing), and elevated C reactive protein (greater than 20 mg/L) or elevated procalcitonin (greater than 0.5 ng/mL).3 Further evaluation of procalcitonin by other groups appears to validate its role in identifying children at low risk of SBI (procalcitonin less than 0.3 ng/mL).4
Currently, studies of febrile infants less than 90 days of age demonstrate that E. coli dominates in bacteremia, UTI, and meningitis, with Group B streptococcus as the next most frequent pathogen identified.2 Increasingly ampicillin resistance has been reported among E. coli isolates from both early- and late-onset disease as well as rare isolates that are resistant to third generation cephalosporins or gentamicin. Surveillance to identify changes in antimicrobial susceptibility will need to be ongoing to ensure that current approaches for initial therapy in high-risk infants aligns with current susceptibility patterns.
Dr. Pelton is chief of the section of pediatric infectious diseases and coordinator of the maternal-child HIV program at Boston Medical Center. He said he had no relevant financial disclosures. Email him at pdnews@mdedge.com.
References
1. Pediatrics. 2004 Jun;113(6):1662-6.
2. Pediatr Infect Dis J. 2014 Jun;33(6):595-9.
3. Pediatr Infect Dis J. 2015 Jan;34(1):17-21.
4. JAMA Pediatr. 2016;170(1):17-18.
5. “AAP Proposes Update to Evaluating, Managing Febrile Infants Guideline,” The Hospitalist, 2016.
Fever in the youngest of infants creates a challenge for the pediatric clinician. Fever is a common presentation for serious bacterial infection (SBI) although most fevers are due to viral infection. However, the clinical presentation does not necessarily differ, and the risk for a poor outcome in this age group is substantial.
In the early stages of my pediatric career, most febrile infants less than 90 days of age were evaluated for sepsis, admitted, and treated with antibiotics pending culture results. Group B streptococcal sepsis or Escherichia coli sepsis were common in the first month of life, and Haemophilus influenza type B or Streptococcus pneumoniae in the second and third months of life. The approach to fever in the first 90 days has changed following both the introduction of haemophilus and pneumococcal conjugate vaccines, the experience with risk stratification criteria for identifying infants at low risk for SBI, and the recognition of urinary tract infection (UTI) as a common source of infection in this age group as well as development of criteria for diagnosis.
A further nuance was subsequently added with the introduction of rapid diagnostics for viral infection. Byington et al. found that the majority of febrile infants less than 90 days of age had viral infection with enterovirus, respiratory syncytial virus (RSV), influenza or rotavirus.1 Using the Rochester risk stratification and the presence or absence of viral infection, she demonstrated that the risk of SBI was reduced in both high- and low-risk infants in the presence of viral infection; in low risk infants with viral infection, SBI was identified in 1.8%, compared with 3.1% in those without viral infection, and in high-risk infants. 5.5% has SBI when viral infection was found, compared to 16.7% in the absence of viral infection. She also proposed risk features to identify those infected with herpes simplex virus; age less than 42 days, vesicular rash, elevated alanine transaminase (ALT) and aspartate aminotransferase (AST), CSF pleocytosis, and seizure or twitching.
Greenhow et al. reported on the experience with “serious” bacterial infection in infants less than 90 days of age receiving care at Northern California Kaiser Permanente during the period 2005-2011.2 As pictured, the majority of children have UTI, and smaller numbers have bacteremia or meningitis. A small group of children with UTI have urosepsis as well; those with urosepsis can be differentiated from those with only UTI by age (less than 21 days), clinical exam (ill appearing), and elevated C reactive protein (greater than 20 mg/L) or elevated procalcitonin (greater than 0.5 ng/mL).3 Further evaluation of procalcitonin by other groups appears to validate its role in identifying children at low risk of SBI (procalcitonin less than 0.3 ng/mL).4
Currently, studies of febrile infants less than 90 days of age demonstrate that E. coli dominates in bacteremia, UTI, and meningitis, with Group B streptococcus as the next most frequent pathogen identified.2 Increasingly ampicillin resistance has been reported among E. coli isolates from both early- and late-onset disease as well as rare isolates that are resistant to third generation cephalosporins or gentamicin. Surveillance to identify changes in antimicrobial susceptibility will need to be ongoing to ensure that current approaches for initial therapy in high-risk infants aligns with current susceptibility patterns.
Dr. Pelton is chief of the section of pediatric infectious diseases and coordinator of the maternal-child HIV program at Boston Medical Center. He said he had no relevant financial disclosures. Email him at pdnews@mdedge.com.
References
1. Pediatrics. 2004 Jun;113(6):1662-6.
2. Pediatr Infect Dis J. 2014 Jun;33(6):595-9.
3. Pediatr Infect Dis J. 2015 Jan;34(1):17-21.
4. JAMA Pediatr. 2016;170(1):17-18.
5. “AAP Proposes Update to Evaluating, Managing Febrile Infants Guideline,” The Hospitalist, 2016.
Fever in the youngest of infants creates a challenge for the pediatric clinician. Fever is a common presentation for serious bacterial infection (SBI) although most fevers are due to viral infection. However, the clinical presentation does not necessarily differ, and the risk for a poor outcome in this age group is substantial.
In the early stages of my pediatric career, most febrile infants less than 90 days of age were evaluated for sepsis, admitted, and treated with antibiotics pending culture results. Group B streptococcal sepsis or Escherichia coli sepsis were common in the first month of life, and Haemophilus influenza type B or Streptococcus pneumoniae in the second and third months of life. The approach to fever in the first 90 days has changed following both the introduction of haemophilus and pneumococcal conjugate vaccines, the experience with risk stratification criteria for identifying infants at low risk for SBI, and the recognition of urinary tract infection (UTI) as a common source of infection in this age group as well as development of criteria for diagnosis.
A further nuance was subsequently added with the introduction of rapid diagnostics for viral infection. Byington et al. found that the majority of febrile infants less than 90 days of age had viral infection with enterovirus, respiratory syncytial virus (RSV), influenza or rotavirus.1 Using the Rochester risk stratification and the presence or absence of viral infection, she demonstrated that the risk of SBI was reduced in both high- and low-risk infants in the presence of viral infection; in low risk infants with viral infection, SBI was identified in 1.8%, compared with 3.1% in those without viral infection, and in high-risk infants. 5.5% has SBI when viral infection was found, compared to 16.7% in the absence of viral infection. She also proposed risk features to identify those infected with herpes simplex virus; age less than 42 days, vesicular rash, elevated alanine transaminase (ALT) and aspartate aminotransferase (AST), CSF pleocytosis, and seizure or twitching.
Greenhow et al. reported on the experience with “serious” bacterial infection in infants less than 90 days of age receiving care at Northern California Kaiser Permanente during the period 2005-2011.2 As pictured, the majority of children have UTI, and smaller numbers have bacteremia or meningitis. A small group of children with UTI have urosepsis as well; those with urosepsis can be differentiated from those with only UTI by age (less than 21 days), clinical exam (ill appearing), and elevated C reactive protein (greater than 20 mg/L) or elevated procalcitonin (greater than 0.5 ng/mL).3 Further evaluation of procalcitonin by other groups appears to validate its role in identifying children at low risk of SBI (procalcitonin less than 0.3 ng/mL).4
Currently, studies of febrile infants less than 90 days of age demonstrate that E. coli dominates in bacteremia, UTI, and meningitis, with Group B streptococcus as the next most frequent pathogen identified.2 Increasingly ampicillin resistance has been reported among E. coli isolates from both early- and late-onset disease as well as rare isolates that are resistant to third generation cephalosporins or gentamicin. Surveillance to identify changes in antimicrobial susceptibility will need to be ongoing to ensure that current approaches for initial therapy in high-risk infants aligns with current susceptibility patterns.
Dr. Pelton is chief of the section of pediatric infectious diseases and coordinator of the maternal-child HIV program at Boston Medical Center. He said he had no relevant financial disclosures. Email him at pdnews@mdedge.com.
References
1. Pediatrics. 2004 Jun;113(6):1662-6.
2. Pediatr Infect Dis J. 2014 Jun;33(6):595-9.
3. Pediatr Infect Dis J. 2015 Jan;34(1):17-21.
4. JAMA Pediatr. 2016;170(1):17-18.
5. “AAP Proposes Update to Evaluating, Managing Febrile Infants Guideline,” The Hospitalist, 2016.