User login
Which antibiotics should be used with caution in pregnant women with UTI?
EXPERT COMMENTARY
Lower urinary tract infection (UTI) is one of the most common medical complications of pregnancy. Approximately 5% to 10% of all pregnant women have asymptomatic bacteriuria, which usually antedates the pregnancy and is detected at the time of the first prenatal appointment. Another 2% to 3% develop acute cystitis during pregnancy. The dominant organisms that cause lower UTIs in pregnant women are Escherichia coli, Klebsiella pneumoniae, Proteus species, group B streptococci, enterococci, and Staphylococcus saprophyticus.
One goal of treating asymptomatic bacteriuria and acute cystitis is to prevent ascending infection (pyelonephritis), which can be associated with preterm delivery, sepsis, and adult respiratory distress syndrome. Another key goal is to use an antibiotic that eradicates the uropathogen without causing harm to either the mother or fetus.
In 2009, Crider and colleagues reported that 2 of the most commonly used antibiotics for UTIs, sulfonamides and nitrofurantoin, were associated with a disturbing spectrum of birth defects.1 Following that report, in 2011 the American College of Obstetricians and Gynecologists (ACOG) published a committee opinion that recommended against the use of these 2 agents in the first trimester of pregnancy unless other antibiotics were unlikely to be effective.2
Details of the study
Centers for Disease Control and Prevention investigators recently conducted a study to assess the effect of these ACOG recommendations on clinical practice. Ailes and co-workers used the Truven Health MarketScan Commercial Database to examine antibiotic prescriptions filled by pregnant women with UTIs.
The database included 482,917 pregnancies in 2014 eligible for analysis. A total of 7.2% (n = 34,864) of pregnant women were treated as outpatients for a UTI within the 90-day interval before the last menstrual period or during the pregnancy. Among these women, the most commonly prescribed antibiotics during the first trimester were nitrofurantoin (34.7%), ciprofloxacin (10.5%), cephalexin (10.3%), and trimethoprim-sulfamethoxazole (7.6%).
The authors concluded that 43% of women used an antibiotic (nitrofurantoin or trimethoprim-sulfamethoxazole) in the first trimester that had potential teratogenicity, despite the precautionary statement articulated in the ACOG committee opinion.2
Antibiotic-associated effects
Of all the antibiotics that could be used to treat a lower UTI in pregnancy, nitrofurantoin probably has the greatest appeal. The drug is highly concentrated in the urine and is very active against all the common uropathogens except Proteus species. It is not absorbed significantly outside the lower urinary tract, and thus it does not alter the natural flora of the bowel or vagina (such alteration would predispose the patient to antibiotic-associated diarrhea or vulvovaginal candidiasis). Nitrofurantoin is inexpensive and usually is very well tolerated.
In the National Birth Defects Prevention Study by Crider and colleagues, nitrofurantoin was associated with anophthalmia or microphthalmos (adjusted odds ratio [AOR], 3.7; 95% confidence interval [CI], 1.1–12.2), hypoplastic left heart syndrome (AOR, 4.2; 95% CI, 1.9–9.1), atrial septal defects (AOR, 1.9; 95% CI, 1.1–3.4), and cleft lip with cleft palate (AOR, 2.1; 95% CI, 1.2–3.9).1 Other investigations, including one published as recently as 2013, have not documented these same associations.3
Similarly, the combination of trimethoprim-sulfamethoxazole also has considerable appeal for treating lower UTIs in pregnancy because it is highly active against most uropathogens, is inexpensive, and usually is very well tolerated. The report by Crider and colleagues, however, was even more worrisome with respect to the possible teratogenicity of this antibiotic.1 The authors found that use of this antibiotic in the first trimester was associated with anencephaly (AOR, 3.4; 95% CI, 1.3–8.8), coarctation of the aorta (AOR, 2.7; 95% CI, 1.3–5.6), hypoplastic left heart (AOR, 3.2; 95% CI, 1.3–7.6), choanal atresia (AOR, 8.0; 95% CI, 2.7–23.5), transverse limb deficiency (AOR, 2.5; 95% CI, 1.0–5.9), and diaphragmatic hernia (AOR, 2.4; 95% CI, 1.1–5.4). Again, other authors, using different epidemiologic methods, have not found the same associations.3
Study strengths and weaknesses
The National Birth Defects Prevention Study by Crider and colleagues was a large, well-funded, and well-designed epidemiologic study. It included more than 13,000 patients from 10 different states.
Nevertheless, the study had certain limitations.4 The findings are subject to recall bias because the investigators questioned patients about antibiotic use after, rather than during, pregnancy. Understandably, the investigators were not able to verify the prescriptions for antibiotics by reviewing each individual medical record. In fact, one-third of study participants were unable to recall the exact name of the antibiotic they received. The authors did not precisely distinguish between single-agent sulfonamides and the combination drug, trimethoprim-sulfamethoxazole, although it seems reasonable to assume that the majority of the prescriptions were for the latter. Finally, given the observational nature of the study, the authors could not be certain that the observed associations were due to the antibiotic, the infection for which the drug was prescribed, or another confounding factor.
Pending the publication of additional investigations, I believe that the guidance outlined below is prudent.
Trimethoprim-sulfamethoxazole should not be used for treating UTIs in the first trimester of pregnancy unless no other antibiotic is likely to be effective. This drug also should be avoided just prior to expected delivery because it can displace bilirubin from protein-binding sites in the newborn and increase the risk of neonatal jaundice.
There may be instances in which trimethoprim-sulfamethoxazole should be used even early in pregnancy, such as to provide prophylaxis against Pneumocystis jiroveci infection in women with human immunodeficiency virus.
To exercise an abundance of caution, I recommend that nitrofurantoin not be used in the first trimester of pregnancy unless no other antibiotic is likely to be effective.
Alternative antibiotics that might be used in the first trimester for treatment of UTIs include ampicillin, amoxicillin, cephalexin, and amoxicillin-clavulanic acid. Substantial evidence supports the safety of these antibiotics in early pregnancy. Unless no other drug is likely to be effective, I would not recommend use of a quinolone antibiotic, such as ciprofloxacin, because of concern about the possible injurious effect of these agents on cartilaginous tissue in the developing fetus.
Neither trimethoprim-sulfamethoxazole nor nitrofurantoin should be used at any time in pregnancy in a patient who has glucose-6-phosphate dehydrogenase deficiency or who may be at increased risk for this disorder.2
-- Patrick Duff, MD
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Crider KS, Cleves MA, Reefhuis J, Berry RJ, Hobbs CA, Hu DJ. Antibacterial medication use during pregnancy and risk of birth defects: National Birth Defects Prevention Study. Arch Pediatr Adolesc Med. 2009;163(11):978–985.
- American College of Obstetricians and Gynecologists Committee on Obstetric Practice. ACOG Committee Opinion No. 494: Sulfonamides, nitrofurantoin, and risk of birth defects. Obstet Gynecol. 2011;117(6):1484–1485.
- Nordeng H, Lupattelli A, Romoren M, Koren G. Neonatal outcomes after gestational exposure to nitrofurantoin. Obstet Gynecol. 2013;121(2 pt 1):306–313.
- American College of Obstetricians and Gynecologists Committee on Obstetric Practice. ACOG Committee Opinion No. 717: Sulfonamides, nitrofurantoin, and risk of birth defects. Obstet Gynecol. 2017;130(3):e150–e152. doi:10.1097/AOG.0000000000002300.
EXPERT COMMENTARY
Lower urinary tract infection (UTI) is one of the most common medical complications of pregnancy. Approximately 5% to 10% of all pregnant women have asymptomatic bacteriuria, which usually antedates the pregnancy and is detected at the time of the first prenatal appointment. Another 2% to 3% develop acute cystitis during pregnancy. The dominant organisms that cause lower UTIs in pregnant women are Escherichia coli, Klebsiella pneumoniae, Proteus species, group B streptococci, enterococci, and Staphylococcus saprophyticus.
One goal of treating asymptomatic bacteriuria and acute cystitis is to prevent ascending infection (pyelonephritis), which can be associated with preterm delivery, sepsis, and adult respiratory distress syndrome. Another key goal is to use an antibiotic that eradicates the uropathogen without causing harm to either the mother or fetus.
In 2009, Crider and colleagues reported that 2 of the most commonly used antibiotics for UTIs, sulfonamides and nitrofurantoin, were associated with a disturbing spectrum of birth defects.1 Following that report, in 2011 the American College of Obstetricians and Gynecologists (ACOG) published a committee opinion that recommended against the use of these 2 agents in the first trimester of pregnancy unless other antibiotics were unlikely to be effective.2
Details of the study
Centers for Disease Control and Prevention investigators recently conducted a study to assess the effect of these ACOG recommendations on clinical practice. Ailes and co-workers used the Truven Health MarketScan Commercial Database to examine antibiotic prescriptions filled by pregnant women with UTIs.
The database included 482,917 pregnancies in 2014 eligible for analysis. A total of 7.2% (n = 34,864) of pregnant women were treated as outpatients for a UTI within the 90-day interval before the last menstrual period or during the pregnancy. Among these women, the most commonly prescribed antibiotics during the first trimester were nitrofurantoin (34.7%), ciprofloxacin (10.5%), cephalexin (10.3%), and trimethoprim-sulfamethoxazole (7.6%).
The authors concluded that 43% of women used an antibiotic (nitrofurantoin or trimethoprim-sulfamethoxazole) in the first trimester that had potential teratogenicity, despite the precautionary statement articulated in the ACOG committee opinion.2
Antibiotic-associated effects
Of all the antibiotics that could be used to treat a lower UTI in pregnancy, nitrofurantoin probably has the greatest appeal. The drug is highly concentrated in the urine and is very active against all the common uropathogens except Proteus species. It is not absorbed significantly outside the lower urinary tract, and thus it does not alter the natural flora of the bowel or vagina (such alteration would predispose the patient to antibiotic-associated diarrhea or vulvovaginal candidiasis). Nitrofurantoin is inexpensive and usually is very well tolerated.
In the National Birth Defects Prevention Study by Crider and colleagues, nitrofurantoin was associated with anophthalmia or microphthalmos (adjusted odds ratio [AOR], 3.7; 95% confidence interval [CI], 1.1–12.2), hypoplastic left heart syndrome (AOR, 4.2; 95% CI, 1.9–9.1), atrial septal defects (AOR, 1.9; 95% CI, 1.1–3.4), and cleft lip with cleft palate (AOR, 2.1; 95% CI, 1.2–3.9).1 Other investigations, including one published as recently as 2013, have not documented these same associations.3
Similarly, the combination of trimethoprim-sulfamethoxazole also has considerable appeal for treating lower UTIs in pregnancy because it is highly active against most uropathogens, is inexpensive, and usually is very well tolerated. The report by Crider and colleagues, however, was even more worrisome with respect to the possible teratogenicity of this antibiotic.1 The authors found that use of this antibiotic in the first trimester was associated with anencephaly (AOR, 3.4; 95% CI, 1.3–8.8), coarctation of the aorta (AOR, 2.7; 95% CI, 1.3–5.6), hypoplastic left heart (AOR, 3.2; 95% CI, 1.3–7.6), choanal atresia (AOR, 8.0; 95% CI, 2.7–23.5), transverse limb deficiency (AOR, 2.5; 95% CI, 1.0–5.9), and diaphragmatic hernia (AOR, 2.4; 95% CI, 1.1–5.4). Again, other authors, using different epidemiologic methods, have not found the same associations.3
Study strengths and weaknesses
The National Birth Defects Prevention Study by Crider and colleagues was a large, well-funded, and well-designed epidemiologic study. It included more than 13,000 patients from 10 different states.
Nevertheless, the study had certain limitations.4 The findings are subject to recall bias because the investigators questioned patients about antibiotic use after, rather than during, pregnancy. Understandably, the investigators were not able to verify the prescriptions for antibiotics by reviewing each individual medical record. In fact, one-third of study participants were unable to recall the exact name of the antibiotic they received. The authors did not precisely distinguish between single-agent sulfonamides and the combination drug, trimethoprim-sulfamethoxazole, although it seems reasonable to assume that the majority of the prescriptions were for the latter. Finally, given the observational nature of the study, the authors could not be certain that the observed associations were due to the antibiotic, the infection for which the drug was prescribed, or another confounding factor.
Pending the publication of additional investigations, I believe that the guidance outlined below is prudent.
Trimethoprim-sulfamethoxazole should not be used for treating UTIs in the first trimester of pregnancy unless no other antibiotic is likely to be effective. This drug also should be avoided just prior to expected delivery because it can displace bilirubin from protein-binding sites in the newborn and increase the risk of neonatal jaundice.
There may be instances in which trimethoprim-sulfamethoxazole should be used even early in pregnancy, such as to provide prophylaxis against Pneumocystis jiroveci infection in women with human immunodeficiency virus.
To exercise an abundance of caution, I recommend that nitrofurantoin not be used in the first trimester of pregnancy unless no other antibiotic is likely to be effective.
Alternative antibiotics that might be used in the first trimester for treatment of UTIs include ampicillin, amoxicillin, cephalexin, and amoxicillin-clavulanic acid. Substantial evidence supports the safety of these antibiotics in early pregnancy. Unless no other drug is likely to be effective, I would not recommend use of a quinolone antibiotic, such as ciprofloxacin, because of concern about the possible injurious effect of these agents on cartilaginous tissue in the developing fetus.
Neither trimethoprim-sulfamethoxazole nor nitrofurantoin should be used at any time in pregnancy in a patient who has glucose-6-phosphate dehydrogenase deficiency or who may be at increased risk for this disorder.2
-- Patrick Duff, MD
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
EXPERT COMMENTARY
Lower urinary tract infection (UTI) is one of the most common medical complications of pregnancy. Approximately 5% to 10% of all pregnant women have asymptomatic bacteriuria, which usually antedates the pregnancy and is detected at the time of the first prenatal appointment. Another 2% to 3% develop acute cystitis during pregnancy. The dominant organisms that cause lower UTIs in pregnant women are Escherichia coli, Klebsiella pneumoniae, Proteus species, group B streptococci, enterococci, and Staphylococcus saprophyticus.
One goal of treating asymptomatic bacteriuria and acute cystitis is to prevent ascending infection (pyelonephritis), which can be associated with preterm delivery, sepsis, and adult respiratory distress syndrome. Another key goal is to use an antibiotic that eradicates the uropathogen without causing harm to either the mother or fetus.
In 2009, Crider and colleagues reported that 2 of the most commonly used antibiotics for UTIs, sulfonamides and nitrofurantoin, were associated with a disturbing spectrum of birth defects.1 Following that report, in 2011 the American College of Obstetricians and Gynecologists (ACOG) published a committee opinion that recommended against the use of these 2 agents in the first trimester of pregnancy unless other antibiotics were unlikely to be effective.2
Details of the study
Centers for Disease Control and Prevention investigators recently conducted a study to assess the effect of these ACOG recommendations on clinical practice. Ailes and co-workers used the Truven Health MarketScan Commercial Database to examine antibiotic prescriptions filled by pregnant women with UTIs.
The database included 482,917 pregnancies in 2014 eligible for analysis. A total of 7.2% (n = 34,864) of pregnant women were treated as outpatients for a UTI within the 90-day interval before the last menstrual period or during the pregnancy. Among these women, the most commonly prescribed antibiotics during the first trimester were nitrofurantoin (34.7%), ciprofloxacin (10.5%), cephalexin (10.3%), and trimethoprim-sulfamethoxazole (7.6%).
The authors concluded that 43% of women used an antibiotic (nitrofurantoin or trimethoprim-sulfamethoxazole) in the first trimester that had potential teratogenicity, despite the precautionary statement articulated in the ACOG committee opinion.2
Antibiotic-associated effects
Of all the antibiotics that could be used to treat a lower UTI in pregnancy, nitrofurantoin probably has the greatest appeal. The drug is highly concentrated in the urine and is very active against all the common uropathogens except Proteus species. It is not absorbed significantly outside the lower urinary tract, and thus it does not alter the natural flora of the bowel or vagina (such alteration would predispose the patient to antibiotic-associated diarrhea or vulvovaginal candidiasis). Nitrofurantoin is inexpensive and usually is very well tolerated.
In the National Birth Defects Prevention Study by Crider and colleagues, nitrofurantoin was associated with anophthalmia or microphthalmos (adjusted odds ratio [AOR], 3.7; 95% confidence interval [CI], 1.1–12.2), hypoplastic left heart syndrome (AOR, 4.2; 95% CI, 1.9–9.1), atrial septal defects (AOR, 1.9; 95% CI, 1.1–3.4), and cleft lip with cleft palate (AOR, 2.1; 95% CI, 1.2–3.9).1 Other investigations, including one published as recently as 2013, have not documented these same associations.3
Similarly, the combination of trimethoprim-sulfamethoxazole also has considerable appeal for treating lower UTIs in pregnancy because it is highly active against most uropathogens, is inexpensive, and usually is very well tolerated. The report by Crider and colleagues, however, was even more worrisome with respect to the possible teratogenicity of this antibiotic.1 The authors found that use of this antibiotic in the first trimester was associated with anencephaly (AOR, 3.4; 95% CI, 1.3–8.8), coarctation of the aorta (AOR, 2.7; 95% CI, 1.3–5.6), hypoplastic left heart (AOR, 3.2; 95% CI, 1.3–7.6), choanal atresia (AOR, 8.0; 95% CI, 2.7–23.5), transverse limb deficiency (AOR, 2.5; 95% CI, 1.0–5.9), and diaphragmatic hernia (AOR, 2.4; 95% CI, 1.1–5.4). Again, other authors, using different epidemiologic methods, have not found the same associations.3
Study strengths and weaknesses
The National Birth Defects Prevention Study by Crider and colleagues was a large, well-funded, and well-designed epidemiologic study. It included more than 13,000 patients from 10 different states.
Nevertheless, the study had certain limitations.4 The findings are subject to recall bias because the investigators questioned patients about antibiotic use after, rather than during, pregnancy. Understandably, the investigators were not able to verify the prescriptions for antibiotics by reviewing each individual medical record. In fact, one-third of study participants were unable to recall the exact name of the antibiotic they received. The authors did not precisely distinguish between single-agent sulfonamides and the combination drug, trimethoprim-sulfamethoxazole, although it seems reasonable to assume that the majority of the prescriptions were for the latter. Finally, given the observational nature of the study, the authors could not be certain that the observed associations were due to the antibiotic, the infection for which the drug was prescribed, or another confounding factor.
Pending the publication of additional investigations, I believe that the guidance outlined below is prudent.
Trimethoprim-sulfamethoxazole should not be used for treating UTIs in the first trimester of pregnancy unless no other antibiotic is likely to be effective. This drug also should be avoided just prior to expected delivery because it can displace bilirubin from protein-binding sites in the newborn and increase the risk of neonatal jaundice.
There may be instances in which trimethoprim-sulfamethoxazole should be used even early in pregnancy, such as to provide prophylaxis against Pneumocystis jiroveci infection in women with human immunodeficiency virus.
To exercise an abundance of caution, I recommend that nitrofurantoin not be used in the first trimester of pregnancy unless no other antibiotic is likely to be effective.
Alternative antibiotics that might be used in the first trimester for treatment of UTIs include ampicillin, amoxicillin, cephalexin, and amoxicillin-clavulanic acid. Substantial evidence supports the safety of these antibiotics in early pregnancy. Unless no other drug is likely to be effective, I would not recommend use of a quinolone antibiotic, such as ciprofloxacin, because of concern about the possible injurious effect of these agents on cartilaginous tissue in the developing fetus.
Neither trimethoprim-sulfamethoxazole nor nitrofurantoin should be used at any time in pregnancy in a patient who has glucose-6-phosphate dehydrogenase deficiency or who may be at increased risk for this disorder.2
-- Patrick Duff, MD
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Crider KS, Cleves MA, Reefhuis J, Berry RJ, Hobbs CA, Hu DJ. Antibacterial medication use during pregnancy and risk of birth defects: National Birth Defects Prevention Study. Arch Pediatr Adolesc Med. 2009;163(11):978–985.
- American College of Obstetricians and Gynecologists Committee on Obstetric Practice. ACOG Committee Opinion No. 494: Sulfonamides, nitrofurantoin, and risk of birth defects. Obstet Gynecol. 2011;117(6):1484–1485.
- Nordeng H, Lupattelli A, Romoren M, Koren G. Neonatal outcomes after gestational exposure to nitrofurantoin. Obstet Gynecol. 2013;121(2 pt 1):306–313.
- American College of Obstetricians and Gynecologists Committee on Obstetric Practice. ACOG Committee Opinion No. 717: Sulfonamides, nitrofurantoin, and risk of birth defects. Obstet Gynecol. 2017;130(3):e150–e152. doi:10.1097/AOG.0000000000002300.
- Crider KS, Cleves MA, Reefhuis J, Berry RJ, Hobbs CA, Hu DJ. Antibacterial medication use during pregnancy and risk of birth defects: National Birth Defects Prevention Study. Arch Pediatr Adolesc Med. 2009;163(11):978–985.
- American College of Obstetricians and Gynecologists Committee on Obstetric Practice. ACOG Committee Opinion No. 494: Sulfonamides, nitrofurantoin, and risk of birth defects. Obstet Gynecol. 2011;117(6):1484–1485.
- Nordeng H, Lupattelli A, Romoren M, Koren G. Neonatal outcomes after gestational exposure to nitrofurantoin. Obstet Gynecol. 2013;121(2 pt 1):306–313.
- American College of Obstetricians and Gynecologists Committee on Obstetric Practice. ACOG Committee Opinion No. 717: Sulfonamides, nitrofurantoin, and risk of birth defects. Obstet Gynecol. 2017;130(3):e150–e152. doi:10.1097/AOG.0000000000002300.
MDedge Daily News: How real is triptans’ serotonin syndrome risk?
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
How real is triptans’ serotonin syndrome risk? Are free testosterone and frailty linked? Breast cancer mortality trends keep moving, and what pioglitazone may offer NASH patients.
Listen to the MDedge Daily News podcast for all the details on today’s top news.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
How real is triptans’ serotonin syndrome risk? Are free testosterone and frailty linked? Breast cancer mortality trends keep moving, and what pioglitazone may offer NASH patients.
Listen to the MDedge Daily News podcast for all the details on today’s top news.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
How real is triptans’ serotonin syndrome risk? Are free testosterone and frailty linked? Breast cancer mortality trends keep moving, and what pioglitazone may offer NASH patients.
Listen to the MDedge Daily News podcast for all the details on today’s top news.
Healthy Food May Come at Higher Price for Native Americans
Healthy foods may be available in grocery stores in rural American Indian communities, but the price can be high. Researchers from University of Washington looked at the availability and cost of 68 food items that comprise the Nutrition Environment Measures Survey in Stores (NEMS-S), which evaluates whether food items adhere to the USDA Thrifty Food Plan (TFP). The TFP “market basket” represents the minimal cost of a healthy diet for a family of 4 for 1 week. The study included 27 stores within a 90-mile radius of the town center of a large American Indian reservation: 13 convenience stores, 10 grocery stores, 3 discount/dollar stores, and 1 discount supermarket. Of the surveyed stores, 10 were on the reservation, including 4 grocery stores.
All NEMS-S foods were available at the discount supermarket, and about 97% of the foods were available at the grocery stores. Convenience and discount/dollar stores were less likely to carry the foods.
The cost of a TFP market basket ranged from 3% lower to 24% higher than the national average. It also varied among the community stores: The TFP cost > 15% at the discount supermarket than at grocery stores ($152.91 vs $179.52). However, the researchers note that the cost of foods that made up the TFP market basket varied across food groups. For instance, the mean cost of dairy products was 43% lower at the discount supermarket than at the grocery stores, while fresh fruits and vegetables were 6% higher.
Healthy foods may be available in grocery stores in rural American Indian communities, but the price can be high. Researchers from University of Washington looked at the availability and cost of 68 food items that comprise the Nutrition Environment Measures Survey in Stores (NEMS-S), which evaluates whether food items adhere to the USDA Thrifty Food Plan (TFP). The TFP “market basket” represents the minimal cost of a healthy diet for a family of 4 for 1 week. The study included 27 stores within a 90-mile radius of the town center of a large American Indian reservation: 13 convenience stores, 10 grocery stores, 3 discount/dollar stores, and 1 discount supermarket. Of the surveyed stores, 10 were on the reservation, including 4 grocery stores.
All NEMS-S foods were available at the discount supermarket, and about 97% of the foods were available at the grocery stores. Convenience and discount/dollar stores were less likely to carry the foods.
The cost of a TFP market basket ranged from 3% lower to 24% higher than the national average. It also varied among the community stores: The TFP cost > 15% at the discount supermarket than at grocery stores ($152.91 vs $179.52). However, the researchers note that the cost of foods that made up the TFP market basket varied across food groups. For instance, the mean cost of dairy products was 43% lower at the discount supermarket than at the grocery stores, while fresh fruits and vegetables were 6% higher.
Healthy foods may be available in grocery stores in rural American Indian communities, but the price can be high. Researchers from University of Washington looked at the availability and cost of 68 food items that comprise the Nutrition Environment Measures Survey in Stores (NEMS-S), which evaluates whether food items adhere to the USDA Thrifty Food Plan (TFP). The TFP “market basket” represents the minimal cost of a healthy diet for a family of 4 for 1 week. The study included 27 stores within a 90-mile radius of the town center of a large American Indian reservation: 13 convenience stores, 10 grocery stores, 3 discount/dollar stores, and 1 discount supermarket. Of the surveyed stores, 10 were on the reservation, including 4 grocery stores.
All NEMS-S foods were available at the discount supermarket, and about 97% of the foods were available at the grocery stores. Convenience and discount/dollar stores were less likely to carry the foods.
The cost of a TFP market basket ranged from 3% lower to 24% higher than the national average. It also varied among the community stores: The TFP cost > 15% at the discount supermarket than at grocery stores ($152.91 vs $179.52). However, the researchers note that the cost of foods that made up the TFP market basket varied across food groups. For instance, the mean cost of dairy products was 43% lower at the discount supermarket than at the grocery stores, while fresh fruits and vegetables were 6% higher.
Team identifies biomarkers for cGVHD in kids
SALT LAKE CITY—Researchers say they have identified prognostic biomarkers for chronic graft-vs-host disease (cGVHD) in children.
The group found that recent thymic emigrants (RTEs) and regulatory T cells expressing CD31 and CD45RA (Treg RTEs) were significantly lower at day 100 after transplant in patients who developed cGVHD.
Because prior research indicated that RTEs are higher in adults with cGVHD, the researchers concluded that thymic function may play a bigger role in cGVHD development for children than for adults.
Geoff D.E. Cuvelier, MD, of CancerCare Manitoba in Winnipeg, Canada, presented these findings at the 2018 BMT Tandem Meetings (abstract 104).
“Our group became interested in these populations of recent thymic emigrants when 2 adult studies1,2 . . . showed that higher proportions of recent thymic emigrants at day 100 were prognostic for the development of chronic graft-vs-host disease,” Dr Cuvelier said.
This led his group to evaluate whether RTEs (CD4+CD45RA+CD31+ T cells), as well as Treg RTEs—Tregs (CD4+CD25+CD127Low) that co-express naïve and recently emigrated markers (CD45RA+CD31+)—are prognostic for pediatric cGVHD at day 100 after allogeneic hematopoietic stem cell transplant (allo-HSCT).
The researchers’ study enrolled patients younger than 18 years of age who underwent allo-HSCT to treat malignant and non-malignant diseases. There were 144 patients who had 1 year of follow-up after allo-HSCT.
Thirty-seven of the patients (25.7%) had cGVHD, and 34 (23.6%) had late acute GVHD (post-day 100). The remaining 73 patients (50.7%) had neither cGVHD nor late acute GVHD, so they served as controls.
Twenty cases of cGVHD were severe, 12 were moderate, and 5 were mild. cGVHD was initially diagnosed at a mean of 192 days post-transplant. The median number of organ systems involved was 3 (range, 1-6). Seventeen patients (46%) had overlap syndrome.
The researchers found that RTEs as a percentage of CD4 T cells were significantly lower at day 100 in patients with cGVHD than in controls—7.8% and 13.5%, respectively (P=0.007). The difference between controls and patients with late acute GVHD was not significant—13.5% and 9.4%, respectively (P=0.08).
Treg RTEs as a percentage of all Tregs were also significantly lower at day 100 in patients with cGVHD than in controls—6.4% and 13.2%, respectively (P=0.002). Again, the difference between controls and patients with late acute GVHD was not significant—13.2% and 9.1%, respectively (P=0.06).
However, further analysis revealed that percentages of RTEs and Treg RTEs were significantly lower at day 100 in patients who developed any type of GVHD after day 100.
The percentage of RTEs was 13.5% in controls and 8.5% in patients with any GVHD after day 100 (P=0.009). The percentage of Treg RTEs was 13.2% and 7.6%, respectively (P=0.004).
“Both recent thymic emigrants and Treg RTEs at day 100 are prognostic biomarkers for chronic graft-vs-host disease in children in this large, multi-institutional, prospective trial,” Dr Cuvelier said in closing.
“Unlike adults, in children, RTEs are proportionally lower, not higher, in patients that later develop chronic GVHD compared to no chronic GVHD. This may suggest that thymic dysfunction and thymic output may play a greater role in chronic GVHD development in children compared to adults.”
To expand upon this research, Dr Cuvelier and his colleagues are planning to begin model building using prognostic cellular and plasma biomarkers at day 100 to determine high-risk profiles for cGVHD.
1. Greinix HT et al; CD19+CD21low B Cells and CD4+CD45RA+CD31+ T Cells Correlate with First Diagnosis of Chronic Graft-versus-Host Disease. BBMT 2015; 21(2):250-258.
2. Li AM et al. An Early Naïve T Cell Population Lacking PD1 Expression at Day 100 As A Prognostic Biomarker of Chronic GVHD. TSS 2017; 210.6.
SALT LAKE CITY—Researchers say they have identified prognostic biomarkers for chronic graft-vs-host disease (cGVHD) in children.
The group found that recent thymic emigrants (RTEs) and regulatory T cells expressing CD31 and CD45RA (Treg RTEs) were significantly lower at day 100 after transplant in patients who developed cGVHD.
Because prior research indicated that RTEs are higher in adults with cGVHD, the researchers concluded that thymic function may play a bigger role in cGVHD development for children than for adults.
Geoff D.E. Cuvelier, MD, of CancerCare Manitoba in Winnipeg, Canada, presented these findings at the 2018 BMT Tandem Meetings (abstract 104).
“Our group became interested in these populations of recent thymic emigrants when 2 adult studies1,2 . . . showed that higher proportions of recent thymic emigrants at day 100 were prognostic for the development of chronic graft-vs-host disease,” Dr Cuvelier said.
This led his group to evaluate whether RTEs (CD4+CD45RA+CD31+ T cells), as well as Treg RTEs—Tregs (CD4+CD25+CD127Low) that co-express naïve and recently emigrated markers (CD45RA+CD31+)—are prognostic for pediatric cGVHD at day 100 after allogeneic hematopoietic stem cell transplant (allo-HSCT).
The researchers’ study enrolled patients younger than 18 years of age who underwent allo-HSCT to treat malignant and non-malignant diseases. There were 144 patients who had 1 year of follow-up after allo-HSCT.
Thirty-seven of the patients (25.7%) had cGVHD, and 34 (23.6%) had late acute GVHD (post-day 100). The remaining 73 patients (50.7%) had neither cGVHD nor late acute GVHD, so they served as controls.
Twenty cases of cGVHD were severe, 12 were moderate, and 5 were mild. cGVHD was initially diagnosed at a mean of 192 days post-transplant. The median number of organ systems involved was 3 (range, 1-6). Seventeen patients (46%) had overlap syndrome.
The researchers found that RTEs as a percentage of CD4 T cells were significantly lower at day 100 in patients with cGVHD than in controls—7.8% and 13.5%, respectively (P=0.007). The difference between controls and patients with late acute GVHD was not significant—13.5% and 9.4%, respectively (P=0.08).
Treg RTEs as a percentage of all Tregs were also significantly lower at day 100 in patients with cGVHD than in controls—6.4% and 13.2%, respectively (P=0.002). Again, the difference between controls and patients with late acute GVHD was not significant—13.2% and 9.1%, respectively (P=0.06).
However, further analysis revealed that percentages of RTEs and Treg RTEs were significantly lower at day 100 in patients who developed any type of GVHD after day 100.
The percentage of RTEs was 13.5% in controls and 8.5% in patients with any GVHD after day 100 (P=0.009). The percentage of Treg RTEs was 13.2% and 7.6%, respectively (P=0.004).
“Both recent thymic emigrants and Treg RTEs at day 100 are prognostic biomarkers for chronic graft-vs-host disease in children in this large, multi-institutional, prospective trial,” Dr Cuvelier said in closing.
“Unlike adults, in children, RTEs are proportionally lower, not higher, in patients that later develop chronic GVHD compared to no chronic GVHD. This may suggest that thymic dysfunction and thymic output may play a greater role in chronic GVHD development in children compared to adults.”
To expand upon this research, Dr Cuvelier and his colleagues are planning to begin model building using prognostic cellular and plasma biomarkers at day 100 to determine high-risk profiles for cGVHD.
1. Greinix HT et al; CD19+CD21low B Cells and CD4+CD45RA+CD31+ T Cells Correlate with First Diagnosis of Chronic Graft-versus-Host Disease. BBMT 2015; 21(2):250-258.
2. Li AM et al. An Early Naïve T Cell Population Lacking PD1 Expression at Day 100 As A Prognostic Biomarker of Chronic GVHD. TSS 2017; 210.6.
SALT LAKE CITY—Researchers say they have identified prognostic biomarkers for chronic graft-vs-host disease (cGVHD) in children.
The group found that recent thymic emigrants (RTEs) and regulatory T cells expressing CD31 and CD45RA (Treg RTEs) were significantly lower at day 100 after transplant in patients who developed cGVHD.
Because prior research indicated that RTEs are higher in adults with cGVHD, the researchers concluded that thymic function may play a bigger role in cGVHD development for children than for adults.
Geoff D.E. Cuvelier, MD, of CancerCare Manitoba in Winnipeg, Canada, presented these findings at the 2018 BMT Tandem Meetings (abstract 104).
“Our group became interested in these populations of recent thymic emigrants when 2 adult studies1,2 . . . showed that higher proportions of recent thymic emigrants at day 100 were prognostic for the development of chronic graft-vs-host disease,” Dr Cuvelier said.
This led his group to evaluate whether RTEs (CD4+CD45RA+CD31+ T cells), as well as Treg RTEs—Tregs (CD4+CD25+CD127Low) that co-express naïve and recently emigrated markers (CD45RA+CD31+)—are prognostic for pediatric cGVHD at day 100 after allogeneic hematopoietic stem cell transplant (allo-HSCT).
The researchers’ study enrolled patients younger than 18 years of age who underwent allo-HSCT to treat malignant and non-malignant diseases. There were 144 patients who had 1 year of follow-up after allo-HSCT.
Thirty-seven of the patients (25.7%) had cGVHD, and 34 (23.6%) had late acute GVHD (post-day 100). The remaining 73 patients (50.7%) had neither cGVHD nor late acute GVHD, so they served as controls.
Twenty cases of cGVHD were severe, 12 were moderate, and 5 were mild. cGVHD was initially diagnosed at a mean of 192 days post-transplant. The median number of organ systems involved was 3 (range, 1-6). Seventeen patients (46%) had overlap syndrome.
The researchers found that RTEs as a percentage of CD4 T cells were significantly lower at day 100 in patients with cGVHD than in controls—7.8% and 13.5%, respectively (P=0.007). The difference between controls and patients with late acute GVHD was not significant—13.5% and 9.4%, respectively (P=0.08).
Treg RTEs as a percentage of all Tregs were also significantly lower at day 100 in patients with cGVHD than in controls—6.4% and 13.2%, respectively (P=0.002). Again, the difference between controls and patients with late acute GVHD was not significant—13.2% and 9.1%, respectively (P=0.06).
However, further analysis revealed that percentages of RTEs and Treg RTEs were significantly lower at day 100 in patients who developed any type of GVHD after day 100.
The percentage of RTEs was 13.5% in controls and 8.5% in patients with any GVHD after day 100 (P=0.009). The percentage of Treg RTEs was 13.2% and 7.6%, respectively (P=0.004).
“Both recent thymic emigrants and Treg RTEs at day 100 are prognostic biomarkers for chronic graft-vs-host disease in children in this large, multi-institutional, prospective trial,” Dr Cuvelier said in closing.
“Unlike adults, in children, RTEs are proportionally lower, not higher, in patients that later develop chronic GVHD compared to no chronic GVHD. This may suggest that thymic dysfunction and thymic output may play a greater role in chronic GVHD development in children compared to adults.”
To expand upon this research, Dr Cuvelier and his colleagues are planning to begin model building using prognostic cellular and plasma biomarkers at day 100 to determine high-risk profiles for cGVHD.
1. Greinix HT et al; CD19+CD21low B Cells and CD4+CD45RA+CD31+ T Cells Correlate with First Diagnosis of Chronic Graft-versus-Host Disease. BBMT 2015; 21(2):250-258.
2. Li AM et al. An Early Naïve T Cell Population Lacking PD1 Expression at Day 100 As A Prognostic Biomarker of Chronic GVHD. TSS 2017; 210.6.
CHMP backs bosutinib for newly diagnosed CML
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended expanding the approved use of bosutinib (BOSULIF) to include treatment of patients with newly diagnosed, chronic phase, Philadelphia chromosome-positive (Ph+) chronic myelogenous leukemia (CML).
Bosutinib is currently approved in Europe to treat patients with Ph+ CML in chronic, accelerated, or blast phase who have received one or more tyrosine kinase inhibitors and for whom imatinib, nilotinib, and dasatinib are not considered appropriate treatment options.
The CHMP’s opinion on bosutinib will be reviewed by the European Commission (EC). If the EC agrees with the CHMP, the commission will grant a centralized marketing authorization that will be valid in the European Union. Norway, Iceland, and Liechtenstein will make corresponding decisions on the basis of the EC’s decision.
The EC typically makes a decision within 67 days of the CHMP’s recommendation.
The CHMP’s recommendation for bosutinib is based on results from the BFORE trial, which were recently published in the Journal of Clinical Oncology.
The publication included data on 536 patients newly diagnosed with chronic phase CML. They were randomized 1:1 to receive bosutinib (n=268) or imatinib (n=268).
The modified intent-to-treat population included Ph+ patients with e13a2/e14a2 transcripts who had at least 12 months of follow-up. In this group, there were 246 patients in the bosutinib arm and 241 in the imatinib arm.
In the modified intent-to-treat population, the rate of major molecular response at 12 months was 47.2% in the bosutinib arm and 36.9% in the imatinib arm (P=0.02). The rate of complete cytogenetic response was 77.2% and 66.4%, respectively (P<0.008).
In the entire study population, 22.0% of patients receiving bosutinib and 26.8% of those receiving imatinib discontinued treatment—12.7% and 8.7%, respectively, due to drug-related toxicity.
Adverse events that were more common in the bosutinib arm than the imatinib arm included grade 3 or higher diarrhea (7.8% vs 0.8%), increased alanine levels (19% vs 1.5%), increased aspartate levels (9.7% vs 1.9%), cardiovascular events (3% vs 0.4%), and peripheral vascular events (1.5% vs 1.1%).
Cerebrovascular events were more common with imatinib than bosutinib (0.4% and 0%, respectively).
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended expanding the approved use of bosutinib (BOSULIF) to include treatment of patients with newly diagnosed, chronic phase, Philadelphia chromosome-positive (Ph+) chronic myelogenous leukemia (CML).
Bosutinib is currently approved in Europe to treat patients with Ph+ CML in chronic, accelerated, or blast phase who have received one or more tyrosine kinase inhibitors and for whom imatinib, nilotinib, and dasatinib are not considered appropriate treatment options.
The CHMP’s opinion on bosutinib will be reviewed by the European Commission (EC). If the EC agrees with the CHMP, the commission will grant a centralized marketing authorization that will be valid in the European Union. Norway, Iceland, and Liechtenstein will make corresponding decisions on the basis of the EC’s decision.
The EC typically makes a decision within 67 days of the CHMP’s recommendation.
The CHMP’s recommendation for bosutinib is based on results from the BFORE trial, which were recently published in the Journal of Clinical Oncology.
The publication included data on 536 patients newly diagnosed with chronic phase CML. They were randomized 1:1 to receive bosutinib (n=268) or imatinib (n=268).
The modified intent-to-treat population included Ph+ patients with e13a2/e14a2 transcripts who had at least 12 months of follow-up. In this group, there were 246 patients in the bosutinib arm and 241 in the imatinib arm.
In the modified intent-to-treat population, the rate of major molecular response at 12 months was 47.2% in the bosutinib arm and 36.9% in the imatinib arm (P=0.02). The rate of complete cytogenetic response was 77.2% and 66.4%, respectively (P<0.008).
In the entire study population, 22.0% of patients receiving bosutinib and 26.8% of those receiving imatinib discontinued treatment—12.7% and 8.7%, respectively, due to drug-related toxicity.
Adverse events that were more common in the bosutinib arm than the imatinib arm included grade 3 or higher diarrhea (7.8% vs 0.8%), increased alanine levels (19% vs 1.5%), increased aspartate levels (9.7% vs 1.9%), cardiovascular events (3% vs 0.4%), and peripheral vascular events (1.5% vs 1.1%).
Cerebrovascular events were more common with imatinib than bosutinib (0.4% and 0%, respectively).
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended expanding the approved use of bosutinib (BOSULIF) to include treatment of patients with newly diagnosed, chronic phase, Philadelphia chromosome-positive (Ph+) chronic myelogenous leukemia (CML).
Bosutinib is currently approved in Europe to treat patients with Ph+ CML in chronic, accelerated, or blast phase who have received one or more tyrosine kinase inhibitors and for whom imatinib, nilotinib, and dasatinib are not considered appropriate treatment options.
The CHMP’s opinion on bosutinib will be reviewed by the European Commission (EC). If the EC agrees with the CHMP, the commission will grant a centralized marketing authorization that will be valid in the European Union. Norway, Iceland, and Liechtenstein will make corresponding decisions on the basis of the EC’s decision.
The EC typically makes a decision within 67 days of the CHMP’s recommendation.
The CHMP’s recommendation for bosutinib is based on results from the BFORE trial, which were recently published in the Journal of Clinical Oncology.
The publication included data on 536 patients newly diagnosed with chronic phase CML. They were randomized 1:1 to receive bosutinib (n=268) or imatinib (n=268).
The modified intent-to-treat population included Ph+ patients with e13a2/e14a2 transcripts who had at least 12 months of follow-up. In this group, there were 246 patients in the bosutinib arm and 241 in the imatinib arm.
In the modified intent-to-treat population, the rate of major molecular response at 12 months was 47.2% in the bosutinib arm and 36.9% in the imatinib arm (P=0.02). The rate of complete cytogenetic response was 77.2% and 66.4%, respectively (P<0.008).
In the entire study population, 22.0% of patients receiving bosutinib and 26.8% of those receiving imatinib discontinued treatment—12.7% and 8.7%, respectively, due to drug-related toxicity.
Adverse events that were more common in the bosutinib arm than the imatinib arm included grade 3 or higher diarrhea (7.8% vs 0.8%), increased alanine levels (19% vs 1.5%), increased aspartate levels (9.7% vs 1.9%), cardiovascular events (3% vs 0.4%), and peripheral vascular events (1.5% vs 1.1%).
Cerebrovascular events were more common with imatinib than bosutinib (0.4% and 0%, respectively).
CHMP supports approval of denosumab in MM
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended expanding the current indication for denosumab (XGEVA®).
The CHMP said the drug should be approved for use in preventing skeletal related events (SREs) in adults with advanced malignancies involving bone, which includes multiple myeloma (MM).
Denosumab is currently approved in Europe to prevent SREs—defined as radiation to bone, pathologic fracture, surgery to bone, and spinal cord compression—in adults with bone metastases from solid tumors.
The drug is also approved for the treatment of adults and skeletally mature adolescents with giant cell tumor of bone that is unresectable or where surgical resection is likely to result in severe morbidity.
The CHMP’s opinion on expanding the indication for denosumab will be reviewed by the European Commission (EC).
If the EC agrees with the CHMP, the commission will grant a centralized marketing authorization that will be valid in the European Union. Norway, Iceland, and Liechtenstein will make corresponding decisions on the basis of the EC’s decision.
The EC typically makes a decision within 67 days of the CHMP’s recommendation.
The CHMP’s recommendation for denosumab is based on data from the ’482 study, which were recently published in The Lancet Oncology.
In this phase 3 trial, denosumab proved non-inferior to zoledronic acid for delaying SREs in patients with newly diagnosed MM and bone disease.
Researchers randomized 1718 patients to receive subcutaneous denosumab at 120 mg and intravenous placebo every 4 weeks (n=859) or intravenous zoledronic acid at 4 mg (adjusted for renal function at baseline) and subcutaneous placebo every 4 weeks (n=859). All patients also received investigators’ choice of first-line MM therapy.
The median time to first on-study SRE was 22.8 months for patients in the denosumab arm and 24 months for those in the zoledronic acid arm (hazard ratio=0.98; 95% confidence interval: 0.85-1.14; P non-inferiority=0.010).
There were fewer renal treatment-emergent adverse events in the denosumab arm than the zoledronic acid arm—10% and 17%, respectively.
But there were more hypocalcemia adverse events in the denosumab arm than the zoledronic acid arm—17% and 12%, respectively.
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended expanding the current indication for denosumab (XGEVA®).
The CHMP said the drug should be approved for use in preventing skeletal related events (SREs) in adults with advanced malignancies involving bone, which includes multiple myeloma (MM).
Denosumab is currently approved in Europe to prevent SREs—defined as radiation to bone, pathologic fracture, surgery to bone, and spinal cord compression—in adults with bone metastases from solid tumors.
The drug is also approved for the treatment of adults and skeletally mature adolescents with giant cell tumor of bone that is unresectable or where surgical resection is likely to result in severe morbidity.
The CHMP’s opinion on expanding the indication for denosumab will be reviewed by the European Commission (EC).
If the EC agrees with the CHMP, the commission will grant a centralized marketing authorization that will be valid in the European Union. Norway, Iceland, and Liechtenstein will make corresponding decisions on the basis of the EC’s decision.
The EC typically makes a decision within 67 days of the CHMP’s recommendation.
The CHMP’s recommendation for denosumab is based on data from the ’482 study, which were recently published in The Lancet Oncology.
In this phase 3 trial, denosumab proved non-inferior to zoledronic acid for delaying SREs in patients with newly diagnosed MM and bone disease.
Researchers randomized 1718 patients to receive subcutaneous denosumab at 120 mg and intravenous placebo every 4 weeks (n=859) or intravenous zoledronic acid at 4 mg (adjusted for renal function at baseline) and subcutaneous placebo every 4 weeks (n=859). All patients also received investigators’ choice of first-line MM therapy.
The median time to first on-study SRE was 22.8 months for patients in the denosumab arm and 24 months for those in the zoledronic acid arm (hazard ratio=0.98; 95% confidence interval: 0.85-1.14; P non-inferiority=0.010).
There were fewer renal treatment-emergent adverse events in the denosumab arm than the zoledronic acid arm—10% and 17%, respectively.
But there were more hypocalcemia adverse events in the denosumab arm than the zoledronic acid arm—17% and 12%, respectively.
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended expanding the current indication for denosumab (XGEVA®).
The CHMP said the drug should be approved for use in preventing skeletal related events (SREs) in adults with advanced malignancies involving bone, which includes multiple myeloma (MM).
Denosumab is currently approved in Europe to prevent SREs—defined as radiation to bone, pathologic fracture, surgery to bone, and spinal cord compression—in adults with bone metastases from solid tumors.
The drug is also approved for the treatment of adults and skeletally mature adolescents with giant cell tumor of bone that is unresectable or where surgical resection is likely to result in severe morbidity.
The CHMP’s opinion on expanding the indication for denosumab will be reviewed by the European Commission (EC).
If the EC agrees with the CHMP, the commission will grant a centralized marketing authorization that will be valid in the European Union. Norway, Iceland, and Liechtenstein will make corresponding decisions on the basis of the EC’s decision.
The EC typically makes a decision within 67 days of the CHMP’s recommendation.
The CHMP’s recommendation for denosumab is based on data from the ’482 study, which were recently published in The Lancet Oncology.
In this phase 3 trial, denosumab proved non-inferior to zoledronic acid for delaying SREs in patients with newly diagnosed MM and bone disease.
Researchers randomized 1718 patients to receive subcutaneous denosumab at 120 mg and intravenous placebo every 4 weeks (n=859) or intravenous zoledronic acid at 4 mg (adjusted for renal function at baseline) and subcutaneous placebo every 4 weeks (n=859). All patients also received investigators’ choice of first-line MM therapy.
The median time to first on-study SRE was 22.8 months for patients in the denosumab arm and 24 months for those in the zoledronic acid arm (hazard ratio=0.98; 95% confidence interval: 0.85-1.14; P non-inferiority=0.010).
There were fewer renal treatment-emergent adverse events in the denosumab arm than the zoledronic acid arm—10% and 17%, respectively.
But there were more hypocalcemia adverse events in the denosumab arm than the zoledronic acid arm—17% and 12%, respectively.
Maternity care: The challenge of paying for value
TriHealth of Cincinnati is testing the waters of value-based payment for maternity care.
The impetus came from a Cincinnati-based employer that contracts with TriHealth under its self-insured health care plan, according to Jennifer Pavelka, project lead for the health system’s Maternity Bundling Care Select Program.
“Employers are looking at the value proposition and are having keen interest in the achievement of the triple aim of optimal outcomes, optimal experience, both with a mindfulness on value, Ms. Pavelka said in an interview. “An employer that had extensive experience with other episodes of care [payments], particularly in the space of knee and hip replacements in the orthopedic world, wanted to dip their toe into a value-based program for maternity.”
“Many forays into value-based payments are very much code driven, saying this code, this service is included in the bundle and this is not,” Ms. Pavelka said. “Part of what makes our work really challenging and exciting is the fact that we have taken in the realm of the methodology and philosophy of bundles, we are implementing a prospective bundled payment model.”
Their engaged physician community has been a key factor in early successes, she added. “What heartens me the most is the degree to which the physicians have really led and informed that evolution of the program and the commitment on the partner side to say first and foremost what does the evidence say, what is clinically appropriate. That has always been our North Star.”
Even with the guidelines of eligibility, patient selection is at the physician’s discretion.
And doctors are helping to shape how women are included in the process.
“We actually have two tiers,” Dr. Marcotte said. “Tier one is the uncomplicated pregnancy and our first project was really to get consensus [around those patients]. There was quite a bit of engagement. We had several meetings at the beginning of our project to help build consensus and we continue to look at that as new evidence comes out to make sure that we are staying consistent with best practice.”
The second tier focuses on more complicated pregnancies.
“There was a reengagement with providers, both our subspecialists in maternal fetal medicine and our generalists [ob.gyns.], to rethink what are the essential elements of best practice care for a patient that has certain complications like hypertension or twins or some of the other things that we included in the tier two clinical care pathways.”
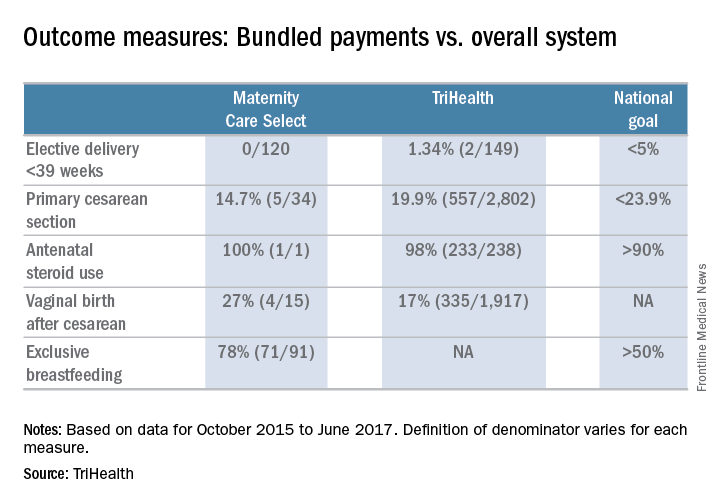
So far, very early results show that those in the bundle are in fact seeing better outcomes, something that in general is expected of a value-based payment model.
The bundled payment program also is opening the door to new service that can be provided to maternity patients.
“We are still relatively early in that journey,” Dr. Marcotte said. “We have a full-time concierge navigator who really works with this population, and we are beginning to make plans to expand that service to more patients beyond just our one population that are in the bundled care package. That’s one concrete area.”
He continued: “I think there is recognition of the need to provide more [patient] education and resources for our entire population that we are working with. Lastly, I think some of our specialized obstetric services for women with complicated pregnancies have gotten a boost. We can additionally provide those kind of services that might be extended beyond just our local community and provide attractive things that can be best in class for even those complicated pregnancies.”
TriHealth really found the key with its early and robust physician involvement, according to Malini Nijagal, MD, associate professor of ob.gyn. and reproductive sciences at the University of California, San Francisco.
“The strong sense that I have is that there is a bit of a disconnect, where it feels on the ground like people believe that value-based payment models may be good for finance and health care expenditures but they are not going to be good for patients and providers,” Dr. Nijagal said in an interview. “I totally disagree.”
Rather, value-based payment models are a way to increase provider autonomy.
“Specific to maternity care and across health care, at this point, insurers are the ones who decide what services we can provide,” she said. “They have their fee schedules and that limits what we can do. What value-based payments and episode payments allow us to do is that, as providers, if we end up saving money from avoiding unnecessary interventions and diagnostics, we then get some of that money back to provide services that may not be covered.”
Dr. Nijagal and her colleagues looked at maternity care bundles and episode payment models in state Medicaid programs, managed care plans, and self-insured employers and found that physicians and other providers can benefit from participation in four ways, by:
- Finding opportunities to improve outcomes and patient experience.
- Developing better quality metrics.
- Reducing waste in the system.
- Creating stronger health care teams.
Value-based payments can “actually bring together hospitals and health care providers onto the same team and the way they do that is by tying the payment together,” Dr. Nijagal said.
The biggest challenge, according to Dr. Nijagal, is that the conversion takes time and the patience to realize that benefits will likely not be fully realized until the system is able to get to a prospective payment model, in which the real innovation can take place (Am J Obstet Gynecol. 2018 Jan 12. doi. org/10.1016/j.ajog.2018.01.014).
“We are having to prioritize just getting providers to agree to doing them and because of that, we are not actually seeing the improvements in quality and cost that we would expect,” Dr. Nijagal said. “For example, a number of the programs that have been rolled out have been the upside risk only, which is superimportant because that is the way you are going to get people to feel more comfortable. It’s a stepping-stone, but you are not going to see the benefits.” And if you can’t prove benefit, it will be harder to get people to move to a two-sided risk model where the real innovation can take place.
To that end, TriHealth provides full transparency with the program so physicians can see how patients in the bundle are doing compared to the overall TriHealth patient population.
“We provide those to them in a dashboard on a monthly basis with transparency so they can see how the other providers in other practices are doing within the system,” Dr, Marcotte said.
TriHealth of Cincinnati is testing the waters of value-based payment for maternity care.
The impetus came from a Cincinnati-based employer that contracts with TriHealth under its self-insured health care plan, according to Jennifer Pavelka, project lead for the health system’s Maternity Bundling Care Select Program.
“Employers are looking at the value proposition and are having keen interest in the achievement of the triple aim of optimal outcomes, optimal experience, both with a mindfulness on value, Ms. Pavelka said in an interview. “An employer that had extensive experience with other episodes of care [payments], particularly in the space of knee and hip replacements in the orthopedic world, wanted to dip their toe into a value-based program for maternity.”
“Many forays into value-based payments are very much code driven, saying this code, this service is included in the bundle and this is not,” Ms. Pavelka said. “Part of what makes our work really challenging and exciting is the fact that we have taken in the realm of the methodology and philosophy of bundles, we are implementing a prospective bundled payment model.”
Their engaged physician community has been a key factor in early successes, she added. “What heartens me the most is the degree to which the physicians have really led and informed that evolution of the program and the commitment on the partner side to say first and foremost what does the evidence say, what is clinically appropriate. That has always been our North Star.”
Even with the guidelines of eligibility, patient selection is at the physician’s discretion.

And doctors are helping to shape how women are included in the process.
“We actually have two tiers,” Dr. Marcotte said. “Tier one is the uncomplicated pregnancy and our first project was really to get consensus [around those patients]. There was quite a bit of engagement. We had several meetings at the beginning of our project to help build consensus and we continue to look at that as new evidence comes out to make sure that we are staying consistent with best practice.”
The second tier focuses on more complicated pregnancies.
“There was a reengagement with providers, both our subspecialists in maternal fetal medicine and our generalists [ob.gyns.], to rethink what are the essential elements of best practice care for a patient that has certain complications like hypertension or twins or some of the other things that we included in the tier two clinical care pathways.”
So far, very early results show that those in the bundle are in fact seeing better outcomes, something that in general is expected of a value-based payment model.
The bundled payment program also is opening the door to new service that can be provided to maternity patients.
“We are still relatively early in that journey,” Dr. Marcotte said. “We have a full-time concierge navigator who really works with this population, and we are beginning to make plans to expand that service to more patients beyond just our one population that are in the bundled care package. That’s one concrete area.”
He continued: “I think there is recognition of the need to provide more [patient] education and resources for our entire population that we are working with. Lastly, I think some of our specialized obstetric services for women with complicated pregnancies have gotten a boost. We can additionally provide those kind of services that might be extended beyond just our local community and provide attractive things that can be best in class for even those complicated pregnancies.”
TriHealth really found the key with its early and robust physician involvement, according to Malini Nijagal, MD, associate professor of ob.gyn. and reproductive sciences at the University of California, San Francisco.
“The strong sense that I have is that there is a bit of a disconnect, where it feels on the ground like people believe that value-based payment models may be good for finance and health care expenditures but they are not going to be good for patients and providers,” Dr. Nijagal said in an interview. “I totally disagree.”
Rather, value-based payment models are a way to increase provider autonomy.
“Specific to maternity care and across health care, at this point, insurers are the ones who decide what services we can provide,” she said. “They have their fee schedules and that limits what we can do. What value-based payments and episode payments allow us to do is that, as providers, if we end up saving money from avoiding unnecessary interventions and diagnostics, we then get some of that money back to provide services that may not be covered.”
Dr. Nijagal and her colleagues looked at maternity care bundles and episode payment models in state Medicaid programs, managed care plans, and self-insured employers and found that physicians and other providers can benefit from participation in four ways, by:
- Finding opportunities to improve outcomes and patient experience.
- Developing better quality metrics.
- Reducing waste in the system.
- Creating stronger health care teams.
Value-based payments can “actually bring together hospitals and health care providers onto the same team and the way they do that is by tying the payment together,” Dr. Nijagal said.
The biggest challenge, according to Dr. Nijagal, is that the conversion takes time and the patience to realize that benefits will likely not be fully realized until the system is able to get to a prospective payment model, in which the real innovation can take place (Am J Obstet Gynecol. 2018 Jan 12. doi. org/10.1016/j.ajog.2018.01.014).
“We are having to prioritize just getting providers to agree to doing them and because of that, we are not actually seeing the improvements in quality and cost that we would expect,” Dr. Nijagal said. “For example, a number of the programs that have been rolled out have been the upside risk only, which is superimportant because that is the way you are going to get people to feel more comfortable. It’s a stepping-stone, but you are not going to see the benefits.” And if you can’t prove benefit, it will be harder to get people to move to a two-sided risk model where the real innovation can take place.
To that end, TriHealth provides full transparency with the program so physicians can see how patients in the bundle are doing compared to the overall TriHealth patient population.
“We provide those to them in a dashboard on a monthly basis with transparency so they can see how the other providers in other practices are doing within the system,” Dr, Marcotte said.
TriHealth of Cincinnati is testing the waters of value-based payment for maternity care.
The impetus came from a Cincinnati-based employer that contracts with TriHealth under its self-insured health care plan, according to Jennifer Pavelka, project lead for the health system’s Maternity Bundling Care Select Program.
“Employers are looking at the value proposition and are having keen interest in the achievement of the triple aim of optimal outcomes, optimal experience, both with a mindfulness on value, Ms. Pavelka said in an interview. “An employer that had extensive experience with other episodes of care [payments], particularly in the space of knee and hip replacements in the orthopedic world, wanted to dip their toe into a value-based program for maternity.”
“Many forays into value-based payments are very much code driven, saying this code, this service is included in the bundle and this is not,” Ms. Pavelka said. “Part of what makes our work really challenging and exciting is the fact that we have taken in the realm of the methodology and philosophy of bundles, we are implementing a prospective bundled payment model.”
Their engaged physician community has been a key factor in early successes, she added. “What heartens me the most is the degree to which the physicians have really led and informed that evolution of the program and the commitment on the partner side to say first and foremost what does the evidence say, what is clinically appropriate. That has always been our North Star.”
Even with the guidelines of eligibility, patient selection is at the physician’s discretion.

And doctors are helping to shape how women are included in the process.
“We actually have two tiers,” Dr. Marcotte said. “Tier one is the uncomplicated pregnancy and our first project was really to get consensus [around those patients]. There was quite a bit of engagement. We had several meetings at the beginning of our project to help build consensus and we continue to look at that as new evidence comes out to make sure that we are staying consistent with best practice.”
The second tier focuses on more complicated pregnancies.
“There was a reengagement with providers, both our subspecialists in maternal fetal medicine and our generalists [ob.gyns.], to rethink what are the essential elements of best practice care for a patient that has certain complications like hypertension or twins or some of the other things that we included in the tier two clinical care pathways.”
So far, very early results show that those in the bundle are in fact seeing better outcomes, something that in general is expected of a value-based payment model.
The bundled payment program also is opening the door to new service that can be provided to maternity patients.
“We are still relatively early in that journey,” Dr. Marcotte said. “We have a full-time concierge navigator who really works with this population, and we are beginning to make plans to expand that service to more patients beyond just our one population that are in the bundled care package. That’s one concrete area.”
He continued: “I think there is recognition of the need to provide more [patient] education and resources for our entire population that we are working with. Lastly, I think some of our specialized obstetric services for women with complicated pregnancies have gotten a boost. We can additionally provide those kind of services that might be extended beyond just our local community and provide attractive things that can be best in class for even those complicated pregnancies.”
TriHealth really found the key with its early and robust physician involvement, according to Malini Nijagal, MD, associate professor of ob.gyn. and reproductive sciences at the University of California, San Francisco.
“The strong sense that I have is that there is a bit of a disconnect, where it feels on the ground like people believe that value-based payment models may be good for finance and health care expenditures but they are not going to be good for patients and providers,” Dr. Nijagal said in an interview. “I totally disagree.”
Rather, value-based payment models are a way to increase provider autonomy.
“Specific to maternity care and across health care, at this point, insurers are the ones who decide what services we can provide,” she said. “They have their fee schedules and that limits what we can do. What value-based payments and episode payments allow us to do is that, as providers, if we end up saving money from avoiding unnecessary interventions and diagnostics, we then get some of that money back to provide services that may not be covered.”
Dr. Nijagal and her colleagues looked at maternity care bundles and episode payment models in state Medicaid programs, managed care plans, and self-insured employers and found that physicians and other providers can benefit from participation in four ways, by:
- Finding opportunities to improve outcomes and patient experience.
- Developing better quality metrics.
- Reducing waste in the system.
- Creating stronger health care teams.
Value-based payments can “actually bring together hospitals and health care providers onto the same team and the way they do that is by tying the payment together,” Dr. Nijagal said.
The biggest challenge, according to Dr. Nijagal, is that the conversion takes time and the patience to realize that benefits will likely not be fully realized until the system is able to get to a prospective payment model, in which the real innovation can take place (Am J Obstet Gynecol. 2018 Jan 12. doi. org/10.1016/j.ajog.2018.01.014).
“We are having to prioritize just getting providers to agree to doing them and because of that, we are not actually seeing the improvements in quality and cost that we would expect,” Dr. Nijagal said. “For example, a number of the programs that have been rolled out have been the upside risk only, which is superimportant because that is the way you are going to get people to feel more comfortable. It’s a stepping-stone, but you are not going to see the benefits.” And if you can’t prove benefit, it will be harder to get people to move to a two-sided risk model where the real innovation can take place.
To that end, TriHealth provides full transparency with the program so physicians can see how patients in the bundle are doing compared to the overall TriHealth patient population.
“We provide those to them in a dashboard on a monthly basis with transparency so they can see how the other providers in other practices are doing within the system,” Dr, Marcotte said.
Taming or teaching the tiger? Myths and management of childhood aggression
How to deal with aggression delivered by a child’s peers is a common concern and social dilemma for both parents and children. How does a child ward off aggressive peers without getting hurt or in trouble while also not looking weak or whiny? What can parents do to stop their child from being hurt or frightened but also not humiliate them or interfere with their learning important life skills by being over protective?
Children do not want to fight, but they do want to be treated fairly. Frustration, with its associated feelings of anger, is the most common reason for aggression. Being a child is certainly full of its frustrations because, while autonomy and desires are increasing, opportunities expand at a slower rate, particularly for children with developmental weaknesses or economic disadvantage. Fear and a lack of coping skills are other major reasons for resorting to aggressive responses.
Physical bullying affects 21% of students in grades 3-12 and is a risk factor for aggression at all ages. A full one-third of 9th-12th graders report having been in a physical fight in the last year. In grade school age and adolescence, factors known to be associated with peer aggression include the humiliation of school failure, substance use, and anger from experiencing parental or sibling aggression.
One would think a universal goal of parents would be to raise their children to get along with others without fighting. Unfortunately, some parents actually espouse childrearing methods that directly or indirectly make fighting more likely.
Essentially all toddlers and preschoolers can be aggressive at times to get things they want (instrumental) or when angry in the beginning of their second year of life; this peaks in the third year and typically declines after age 3 years. But for some 10% of children, aggression remains high. What parent and child factors set children up for such persistent aggression?
Parents have many reasons for how they raise their children, but some myths about parenting that persist promote aggression.
“My child will love me more if I am more permissive.”
Infants and toddlers develop self-regulation skills better when it is gradually expected of them with encouragement and support from their parents. Parents may feel that they are showing love to their toddler by having a “relaxed” home with few limits and no specific bedtime or rules. These parents also may “rescue” their child from frustrating situations by giving in to their demands or removing them from even mildly stressful situations.
These strategies can interfere with the progressive development of frustration tolerance, a key life skill. A lack of routines, inadequate sleep or food, overstimulation by noise, frightening experiences (including fighting in the home or neighborhood), or violent media exposure sets toddlers up to be out of control and thereby increases dysregulation. In addition, the dysregulated child may then act up, which can invoke punishment from that same parent.
Frustrating toddlers with inconsistent expectations and arbitrary punishment, a common result of low structure, makes the child feel insecure and leads to aggression. Instead, children need small doses of frustration appropriate to their age and encouragement from a supportive adult to problem solve. You can praise (or model), cheering on a child with words such as “Are you stuck? You can do it! Try again,” instead of instantly solving problems for them.
“Spare the rod and spoil the child.”
Parents may feel that they are promoting obedience when they use corporal punishment, thinking this will keep the child out of trouble in society. Instead, corporal punishment is associated with increased aggression toward peers, as well as defiance toward parents. These effects are especially strong when mothers are distant emotionally. As pediatricians, we can educate people on the importance of warm parenting, redirection instead of punishment for younger children, and using small, logical consequences or time out when needed for aggression.
“Just ignore bullies.”
It is a rare child who can follow the command to “ignore” a bully without turning red or getting tears in his or her eyes – making them appealing targets. We can coach parents and kids how to disarm bullies by standing tall, putting hands on hips, making eye contact, and asking the peer a question such as “I do not understand what you’re trying to accomplish.” Learning martial arts also teaches children that they are powerful (but not to fight outside the class) so they can present themselves in this way. Programs that encourage children to get together to confront bullies supported by a school administration that uses comprehensive assessment and habilitation strategies for aggressive students are most effective in reducing aggression in schools. Anonymous reporting (for example, by using a cell phone app, such as STOPit) empowers students to report bullying or fights to school staff without risking later retribution from the peer.
“Tough teachers help kids fall in line.”
While peer fights generally increase from 2nd to 4th grade before declining, student fighting progressively increases when teachers use reprimands, rather than praise, to manage their classes. Children look to teachers to learn more than what is in books – how to be respectful and in control without putting others down. The most effective classroom management includes clear, fair rules; any correction should be done privately to avoid shaming students. Students dealt with this way are less likely to be angry and take it out on others. Of course, appropriate services helping every child experience success in learning is the foundation of positive behavior in school.
“Children with ADHD won’t learn self-regulation if they are treated with medicine.”
Children who show “low effortful control” or higher “dysregulation” are both more aggressive and also less likely to decline in aggression in early childhood. ADHD is a neurological condition characterized by such dysregulation and low effortful control. Children with ADHD often have higher and more persistent aggression. These tendencies also result in impulsive behaviors that can irritate peers and adults and can result in correction and criticism, further increasing aggression. Children with ADHD who are better controlled, often with the help of medication, have more positive interactions at school and at home, receive more praise and less correction, and develop more reasoned interaction patterns.
“I am the parent, and my child should do what I say.”
When adults step in to stop a fight, they are rarely in a position to know what actually happened between the kids. Children may quickly learn how to entrap a sibling or peer to look like the perpetrator in order to get them in trouble and/or avoid consequences for themselves, especially if large or harsh punishments are being used.
While it can seem tricky to treat children who are very different in age or development equally, having parents elicit or at least verbalize each child’s point of view is part of how children learn respect and mediation skills. Parents who refrain from taking sides or dictating how disputes should be resolved leave the chance for the children to acquire these component skills of negotiation. This does not mean there are no consequences, just that a brief discussion comes first.
When fighting is a pediatric complaint, you have a great opportunity to educate families in evidence-based ways that can both prevent and reduce their child’s use of aggression.
In one effective 90-minute training program, parents were taught basic mediation principles: to give ground rules and ask their children to agree to them, to ask each child to describe what happened and identify their disagreements and common ground, to encourage the children to discuss their goals in the fight and feelings about the issues, and to encourage the children to come up with suggestions to resolve their disputes and help them assess the practical aspects of their ideas. Praise should be used each time a child uses even some of these skills. Parents in this program also were given communication strategies, such as active listening, reflecting, and reframing, to help children learn to take the others’ perspective. In a follow up survey a month later, children of parents in the intervention group were seen to use these skills in real situations that might otherwise have been fights.
When aggression persists, mindfulness training, cognitive-behavioral techniques, social-emotional approaches, or peer mentoring programs delivered through individual counseling or school programs are all ways of teaching kids important interaction skills to reduce peer aggression. Remember, 40% of severe adult aggression begins before age 8 years, so preventive education or early referral to mental health services is key.
Dr. Howard is assistant professor of pediatrics at Johns Hopkins University, Baltimore, and creator of CHADIS (www.CHADIS.com). She had no other relevant disclosures. Dr. Howard’s contribution to this publication was as a paid expert to Frontline Medical News. E-mail her at pdnews@frontlinemedcom.com.
How to deal with aggression delivered by a child’s peers is a common concern and social dilemma for both parents and children. How does a child ward off aggressive peers without getting hurt or in trouble while also not looking weak or whiny? What can parents do to stop their child from being hurt or frightened but also not humiliate them or interfere with their learning important life skills by being over protective?
Children do not want to fight, but they do want to be treated fairly. Frustration, with its associated feelings of anger, is the most common reason for aggression. Being a child is certainly full of its frustrations because, while autonomy and desires are increasing, opportunities expand at a slower rate, particularly for children with developmental weaknesses or economic disadvantage. Fear and a lack of coping skills are other major reasons for resorting to aggressive responses.
Physical bullying affects 21% of students in grades 3-12 and is a risk factor for aggression at all ages. A full one-third of 9th-12th graders report having been in a physical fight in the last year. In grade school age and adolescence, factors known to be associated with peer aggression include the humiliation of school failure, substance use, and anger from experiencing parental or sibling aggression.
One would think a universal goal of parents would be to raise their children to get along with others without fighting. Unfortunately, some parents actually espouse childrearing methods that directly or indirectly make fighting more likely.
Essentially all toddlers and preschoolers can be aggressive at times to get things they want (instrumental) or when angry in the beginning of their second year of life; this peaks in the third year and typically declines after age 3 years. But for some 10% of children, aggression remains high. What parent and child factors set children up for such persistent aggression?
Parents have many reasons for how they raise their children, but some myths about parenting that persist promote aggression.
“My child will love me more if I am more permissive.”
Infants and toddlers develop self-regulation skills better when it is gradually expected of them with encouragement and support from their parents. Parents may feel that they are showing love to their toddler by having a “relaxed” home with few limits and no specific bedtime or rules. These parents also may “rescue” their child from frustrating situations by giving in to their demands or removing them from even mildly stressful situations.
These strategies can interfere with the progressive development of frustration tolerance, a key life skill. A lack of routines, inadequate sleep or food, overstimulation by noise, frightening experiences (including fighting in the home or neighborhood), or violent media exposure sets toddlers up to be out of control and thereby increases dysregulation. In addition, the dysregulated child may then act up, which can invoke punishment from that same parent.
Frustrating toddlers with inconsistent expectations and arbitrary punishment, a common result of low structure, makes the child feel insecure and leads to aggression. Instead, children need small doses of frustration appropriate to their age and encouragement from a supportive adult to problem solve. You can praise (or model), cheering on a child with words such as “Are you stuck? You can do it! Try again,” instead of instantly solving problems for them.
“Spare the rod and spoil the child.”
Parents may feel that they are promoting obedience when they use corporal punishment, thinking this will keep the child out of trouble in society. Instead, corporal punishment is associated with increased aggression toward peers, as well as defiance toward parents. These effects are especially strong when mothers are distant emotionally. As pediatricians, we can educate people on the importance of warm parenting, redirection instead of punishment for younger children, and using small, logical consequences or time out when needed for aggression.
“Just ignore bullies.”
It is a rare child who can follow the command to “ignore” a bully without turning red or getting tears in his or her eyes – making them appealing targets. We can coach parents and kids how to disarm bullies by standing tall, putting hands on hips, making eye contact, and asking the peer a question such as “I do not understand what you’re trying to accomplish.” Learning martial arts also teaches children that they are powerful (but not to fight outside the class) so they can present themselves in this way. Programs that encourage children to get together to confront bullies supported by a school administration that uses comprehensive assessment and habilitation strategies for aggressive students are most effective in reducing aggression in schools. Anonymous reporting (for example, by using a cell phone app, such as STOPit) empowers students to report bullying or fights to school staff without risking later retribution from the peer.
“Tough teachers help kids fall in line.”
While peer fights generally increase from 2nd to 4th grade before declining, student fighting progressively increases when teachers use reprimands, rather than praise, to manage their classes. Children look to teachers to learn more than what is in books – how to be respectful and in control without putting others down. The most effective classroom management includes clear, fair rules; any correction should be done privately to avoid shaming students. Students dealt with this way are less likely to be angry and take it out on others. Of course, appropriate services helping every child experience success in learning is the foundation of positive behavior in school.
“Children with ADHD won’t learn self-regulation if they are treated with medicine.”
Children who show “low effortful control” or higher “dysregulation” are both more aggressive and also less likely to decline in aggression in early childhood. ADHD is a neurological condition characterized by such dysregulation and low effortful control. Children with ADHD often have higher and more persistent aggression. These tendencies also result in impulsive behaviors that can irritate peers and adults and can result in correction and criticism, further increasing aggression. Children with ADHD who are better controlled, often with the help of medication, have more positive interactions at school and at home, receive more praise and less correction, and develop more reasoned interaction patterns.
“I am the parent, and my child should do what I say.”
When adults step in to stop a fight, they are rarely in a position to know what actually happened between the kids. Children may quickly learn how to entrap a sibling or peer to look like the perpetrator in order to get them in trouble and/or avoid consequences for themselves, especially if large or harsh punishments are being used.
While it can seem tricky to treat children who are very different in age or development equally, having parents elicit or at least verbalize each child’s point of view is part of how children learn respect and mediation skills. Parents who refrain from taking sides or dictating how disputes should be resolved leave the chance for the children to acquire these component skills of negotiation. This does not mean there are no consequences, just that a brief discussion comes first.
When fighting is a pediatric complaint, you have a great opportunity to educate families in evidence-based ways that can both prevent and reduce their child’s use of aggression.
In one effective 90-minute training program, parents were taught basic mediation principles: to give ground rules and ask their children to agree to them, to ask each child to describe what happened and identify their disagreements and common ground, to encourage the children to discuss their goals in the fight and feelings about the issues, and to encourage the children to come up with suggestions to resolve their disputes and help them assess the practical aspects of their ideas. Praise should be used each time a child uses even some of these skills. Parents in this program also were given communication strategies, such as active listening, reflecting, and reframing, to help children learn to take the others’ perspective. In a follow up survey a month later, children of parents in the intervention group were seen to use these skills in real situations that might otherwise have been fights.
When aggression persists, mindfulness training, cognitive-behavioral techniques, social-emotional approaches, or peer mentoring programs delivered through individual counseling or school programs are all ways of teaching kids important interaction skills to reduce peer aggression. Remember, 40% of severe adult aggression begins before age 8 years, so preventive education or early referral to mental health services is key.
Dr. Howard is assistant professor of pediatrics at Johns Hopkins University, Baltimore, and creator of CHADIS (www.CHADIS.com). She had no other relevant disclosures. Dr. Howard’s contribution to this publication was as a paid expert to Frontline Medical News. E-mail her at pdnews@frontlinemedcom.com.
How to deal with aggression delivered by a child’s peers is a common concern and social dilemma for both parents and children. How does a child ward off aggressive peers without getting hurt or in trouble while also not looking weak or whiny? What can parents do to stop their child from being hurt or frightened but also not humiliate them or interfere with their learning important life skills by being over protective?
Children do not want to fight, but they do want to be treated fairly. Frustration, with its associated feelings of anger, is the most common reason for aggression. Being a child is certainly full of its frustrations because, while autonomy and desires are increasing, opportunities expand at a slower rate, particularly for children with developmental weaknesses or economic disadvantage. Fear and a lack of coping skills are other major reasons for resorting to aggressive responses.
Physical bullying affects 21% of students in grades 3-12 and is a risk factor for aggression at all ages. A full one-third of 9th-12th graders report having been in a physical fight in the last year. In grade school age and adolescence, factors known to be associated with peer aggression include the humiliation of school failure, substance use, and anger from experiencing parental or sibling aggression.
One would think a universal goal of parents would be to raise their children to get along with others without fighting. Unfortunately, some parents actually espouse childrearing methods that directly or indirectly make fighting more likely.
Essentially all toddlers and preschoolers can be aggressive at times to get things they want (instrumental) or when angry in the beginning of their second year of life; this peaks in the third year and typically declines after age 3 years. But for some 10% of children, aggression remains high. What parent and child factors set children up for such persistent aggression?
Parents have many reasons for how they raise their children, but some myths about parenting that persist promote aggression.
“My child will love me more if I am more permissive.”
Infants and toddlers develop self-regulation skills better when it is gradually expected of them with encouragement and support from their parents. Parents may feel that they are showing love to their toddler by having a “relaxed” home with few limits and no specific bedtime or rules. These parents also may “rescue” their child from frustrating situations by giving in to their demands or removing them from even mildly stressful situations.
These strategies can interfere with the progressive development of frustration tolerance, a key life skill. A lack of routines, inadequate sleep or food, overstimulation by noise, frightening experiences (including fighting in the home or neighborhood), or violent media exposure sets toddlers up to be out of control and thereby increases dysregulation. In addition, the dysregulated child may then act up, which can invoke punishment from that same parent.
Frustrating toddlers with inconsistent expectations and arbitrary punishment, a common result of low structure, makes the child feel insecure and leads to aggression. Instead, children need small doses of frustration appropriate to their age and encouragement from a supportive adult to problem solve. You can praise (or model), cheering on a child with words such as “Are you stuck? You can do it! Try again,” instead of instantly solving problems for them.
“Spare the rod and spoil the child.”
Parents may feel that they are promoting obedience when they use corporal punishment, thinking this will keep the child out of trouble in society. Instead, corporal punishment is associated with increased aggression toward peers, as well as defiance toward parents. These effects are especially strong when mothers are distant emotionally. As pediatricians, we can educate people on the importance of warm parenting, redirection instead of punishment for younger children, and using small, logical consequences or time out when needed for aggression.
“Just ignore bullies.”
It is a rare child who can follow the command to “ignore” a bully without turning red or getting tears in his or her eyes – making them appealing targets. We can coach parents and kids how to disarm bullies by standing tall, putting hands on hips, making eye contact, and asking the peer a question such as “I do not understand what you’re trying to accomplish.” Learning martial arts also teaches children that they are powerful (but not to fight outside the class) so they can present themselves in this way. Programs that encourage children to get together to confront bullies supported by a school administration that uses comprehensive assessment and habilitation strategies for aggressive students are most effective in reducing aggression in schools. Anonymous reporting (for example, by using a cell phone app, such as STOPit) empowers students to report bullying or fights to school staff without risking later retribution from the peer.
“Tough teachers help kids fall in line.”
While peer fights generally increase from 2nd to 4th grade before declining, student fighting progressively increases when teachers use reprimands, rather than praise, to manage their classes. Children look to teachers to learn more than what is in books – how to be respectful and in control without putting others down. The most effective classroom management includes clear, fair rules; any correction should be done privately to avoid shaming students. Students dealt with this way are less likely to be angry and take it out on others. Of course, appropriate services helping every child experience success in learning is the foundation of positive behavior in school.
“Children with ADHD won’t learn self-regulation if they are treated with medicine.”
Children who show “low effortful control” or higher “dysregulation” are both more aggressive and also less likely to decline in aggression in early childhood. ADHD is a neurological condition characterized by such dysregulation and low effortful control. Children with ADHD often have higher and more persistent aggression. These tendencies also result in impulsive behaviors that can irritate peers and adults and can result in correction and criticism, further increasing aggression. Children with ADHD who are better controlled, often with the help of medication, have more positive interactions at school and at home, receive more praise and less correction, and develop more reasoned interaction patterns.
“I am the parent, and my child should do what I say.”
When adults step in to stop a fight, they are rarely in a position to know what actually happened between the kids. Children may quickly learn how to entrap a sibling or peer to look like the perpetrator in order to get them in trouble and/or avoid consequences for themselves, especially if large or harsh punishments are being used.
While it can seem tricky to treat children who are very different in age or development equally, having parents elicit or at least verbalize each child’s point of view is part of how children learn respect and mediation skills. Parents who refrain from taking sides or dictating how disputes should be resolved leave the chance for the children to acquire these component skills of negotiation. This does not mean there are no consequences, just that a brief discussion comes first.
When fighting is a pediatric complaint, you have a great opportunity to educate families in evidence-based ways that can both prevent and reduce their child’s use of aggression.
In one effective 90-minute training program, parents were taught basic mediation principles: to give ground rules and ask their children to agree to them, to ask each child to describe what happened and identify their disagreements and common ground, to encourage the children to discuss their goals in the fight and feelings about the issues, and to encourage the children to come up with suggestions to resolve their disputes and help them assess the practical aspects of their ideas. Praise should be used each time a child uses even some of these skills. Parents in this program also were given communication strategies, such as active listening, reflecting, and reframing, to help children learn to take the others’ perspective. In a follow up survey a month later, children of parents in the intervention group were seen to use these skills in real situations that might otherwise have been fights.
When aggression persists, mindfulness training, cognitive-behavioral techniques, social-emotional approaches, or peer mentoring programs delivered through individual counseling or school programs are all ways of teaching kids important interaction skills to reduce peer aggression. Remember, 40% of severe adult aggression begins before age 8 years, so preventive education or early referral to mental health services is key.
Dr. Howard is assistant professor of pediatrics at Johns Hopkins University, Baltimore, and creator of CHADIS (www.CHADIS.com). She had no other relevant disclosures. Dr. Howard’s contribution to this publication was as a paid expert to Frontline Medical News. E-mail her at pdnews@frontlinemedcom.com.
Vaccines: Effectiveness vs. efficacy
During the influenza portion of the Feb. 21, 2018, Centers for Diseases Control and Prevention’s Advisory Committee on Immunization Practices meeting, two pleas from the audience asked the CDC/ACIP to make messages very clear about how protective influenza vaccine really is.
We hear apparently conflicting percentages from Australia, Canada, Europe, and the United States from the many stories/press releases in the news media and from official public health outlets. And the gloomiest ones get the most exposure.1 It can be confusing even for medical care providers who are supposed to advise families on such matters.
A key misunderstanding in many medical and lay news stories is about what vaccine effectiveness and vaccine efficacy really mean. What? Aren’t those the same thing? Nope. They are quite different. And are we sure of what those 95% confidence intervals (CI) mean? Let’s review the “math” so we can explain this to families.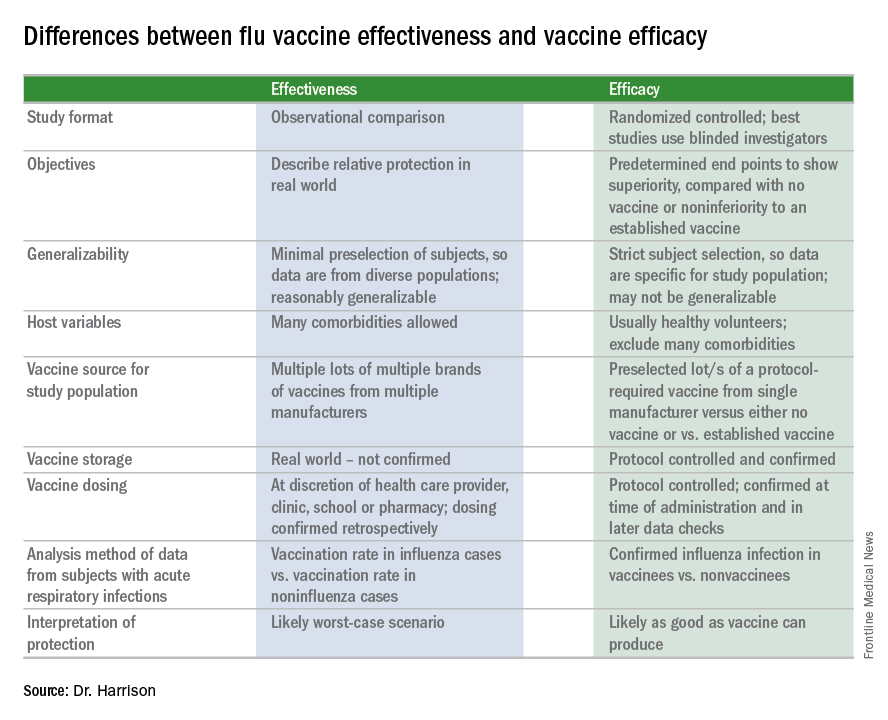
Vaccine effectiveness (VE)2,3
The first thing to know is that the CDC and similar public health agencies in other countries do not report vaccine efficacy. Instead, the percentage reported is VE during (interim estimated VE) and just after (final adjusted VE) each influenza season. This means that VE is generally a retrospective analysis of data, most of which were collected prospectively. Further, VE is likely the worst case scenario. VE is a measure of real-world benefit to patients for whom vaccine is recommended, by analyzing specific geographically diverse populations (population-based) without excluding most underlying illness or comorbidities (note that immunosuppressed persons are excluded). Subjects in VE studies receive their vaccine in the real world and, therefore, vaccinees may receive their vaccines from any number of the usual outlets (e.g., primary care provider, urgent care or emergency department, public health department, pharmacy, school, or nursing home). There are multiple lots of multiple brands from multiple vaccine manufacturers. Children who need two doses of influenza vaccine do not necessarily receive those doses according to the package insert’s schedule. VE studies do not have the capability to confirm that vaccine was stored, handled, and administered in a precisely correct manner according to manufacturer’s and CDC’s recommendations.
VE is calculated using a “test-negative” (case-control) analysis of patients presenting with acute respiratory infections (ARIs). People who are not in vaccine research can find this methodology confusing. Briefly, the VE compares the odds of vaccination in ARIs due to confirmed influenza to the odds of vaccination in ARIs not due to influenza. Additional statistical tools can adjust VE for specific factors. VE is also calculated by factors of interest, such as age, gender, pregnancy, influenza type, region of the country, presence of asthma or other comorbidity, etc. Whether the VE value is the “truth in the universe” is related to having enough subjects in each analyzed group and the degree to which the studied populations actually represent the whole country. So, VE is more accurate when there are large subject numbers.
Remember also that VE is usually calculated from outpatients, so it does not really measure all the benefits of vaccination. Prevention rates for severe influenza (such as influenza hospitalizations) are higher but usually unavailable until after the entire season.
VE studies generally measure real-world and likely worst-case-scenario benefit for the overall population being protected against outpatient influenza medical visits.
Vaccine efficacy2,3
Vaccine efficacy measures how the vaccine performs under ideal circumstances in a regimented protocol in relatively normal hosts – likely the best-case-scenario benefit. Vaccine efficacy is the percent difference in confirmed influenza episodes in vaccinees getting the “experimental” vaccine vs. episodes in nonvaccinees (or vaccinees getting an established vaccine). Vaccine efficacy, therefore, is usually calculated based on prospective well-controlled studies under ideal circumstances in subjects who received their vaccines on time per the recommended schedule. Most such studies are performed on otherwise healthy children or adults, with most comorbidities excluded. The “experimental” vaccine is generally from a single manufacturer from a single lot, and chain-of-custody is well controlled. The vaccine is administered at selected research sites according to a strict protocol; vaccine storage is ensured to be as recommended.
Confidence intervals
To assess whether the “protection” is “significant,” the calculations derive 95% confidence intervals (CI). If the 95% CI range is wide, such as many tens of percents, then there is less confidence that the calculation is correct. And if the lower CI is less than 0, then the result is not significant. For example, a VE of 20% is not highly protective, but can be significant if the 95% CI ranges from 10 to 28 (the lower value of 10 is above zero). It would not be significant if the 95% CI lower limit was –10. Values for seasons 2004-2005 and 2005-2006 were similar to this. Consider however that a VE of 55% seems great, but may not be significant if the 95% CI range is –20 to 89 (the lower value is less than zero). In the ideal world, the VE would be greater than 50% and the 95% CI range would be tight with the lower CI value far above zero; for example, VE of 70% with 95% CI ranging from 60 to 80. The 2010-2011 season was close to this.
Type and age-specific VE
Aside from overall VE, there are subset analyses that can be revealing. This year there are the concerning mid-season VE estimates of approximately 25% for the United States and 17% in Canada, for one specific type, H3N2, which unfortunately has been the dominant circulating U.S. type. That number is what everybody seems to have focused on. But remember influenza B becomes dominant late in most seasons (increasing at the time of writing this article). Interim 2017-2018 VE for influenza B was in the mid 60% range, making the box plot near 40% overall.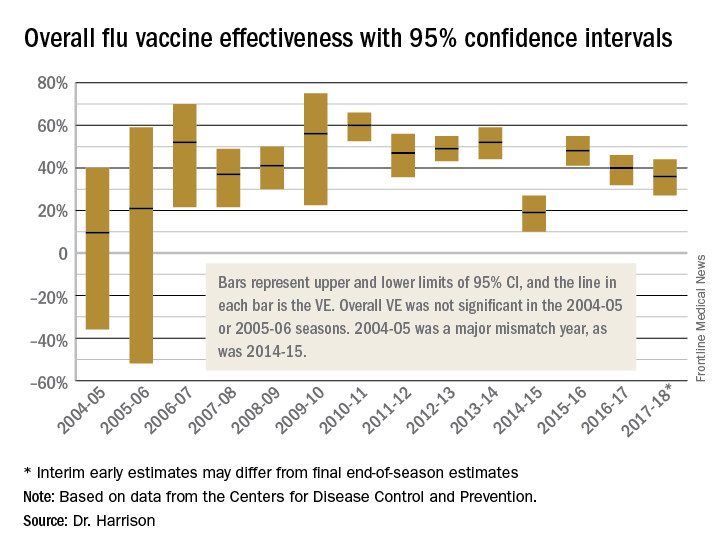
Age-related VE analysis can show difference; for example, the best benefit for H3N2 this season has been in young children and the worst in elderly and 9- to 17-year-olds.
Take-home message
The simplest way to think of overall VE is that it is the real-world, worst-case-scenario value for influenza protection by vaccine against the several circulating types of influenza.
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics, Kansas City, Mo. Children’s Mercy Hospital receives grant funding for Dr. Harrison’s work as an investigator from GSK for MMR and rotavirus vaccine studies, from Merck for in vitro and clinical antibiotic studies, from Allergan for clinical antibiotic studies, from Pfizer for pneumococcal seroepidemiology studies, and from Regeneron for RSV studies. Dr. Harrison received support for travel and to present seroepidemiology data at one meeting. Email him at pdnews@frontlinemedcom.com.
References
1. MMWR Weekly. 2017 Feb 17;66(6):167-71.
2. Dev Biol Stand. 1998;95:195-201.
3. Lancet Infect Dis. 2012 Jan;12(1):36-44.
During the influenza portion of the Feb. 21, 2018, Centers for Diseases Control and Prevention’s Advisory Committee on Immunization Practices meeting, two pleas from the audience asked the CDC/ACIP to make messages very clear about how protective influenza vaccine really is.
We hear apparently conflicting percentages from Australia, Canada, Europe, and the United States from the many stories/press releases in the news media and from official public health outlets. And the gloomiest ones get the most exposure.1 It can be confusing even for medical care providers who are supposed to advise families on such matters.
A key misunderstanding in many medical and lay news stories is about what vaccine effectiveness and vaccine efficacy really mean. What? Aren’t those the same thing? Nope. They are quite different. And are we sure of what those 95% confidence intervals (CI) mean? Let’s review the “math” so we can explain this to families.
Vaccine effectiveness (VE)2,3
The first thing to know is that the CDC and similar public health agencies in other countries do not report vaccine efficacy. Instead, the percentage reported is VE during (interim estimated VE) and just after (final adjusted VE) each influenza season. This means that VE is generally a retrospective analysis of data, most of which were collected prospectively. Further, VE is likely the worst case scenario. VE is a measure of real-world benefit to patients for whom vaccine is recommended, by analyzing specific geographically diverse populations (population-based) without excluding most underlying illness or comorbidities (note that immunosuppressed persons are excluded). Subjects in VE studies receive their vaccine in the real world and, therefore, vaccinees may receive their vaccines from any number of the usual outlets (e.g., primary care provider, urgent care or emergency department, public health department, pharmacy, school, or nursing home). There are multiple lots of multiple brands from multiple vaccine manufacturers. Children who need two doses of influenza vaccine do not necessarily receive those doses according to the package insert’s schedule. VE studies do not have the capability to confirm that vaccine was stored, handled, and administered in a precisely correct manner according to manufacturer’s and CDC’s recommendations.
VE is calculated using a “test-negative” (case-control) analysis of patients presenting with acute respiratory infections (ARIs). People who are not in vaccine research can find this methodology confusing. Briefly, the VE compares the odds of vaccination in ARIs due to confirmed influenza to the odds of vaccination in ARIs not due to influenza. Additional statistical tools can adjust VE for specific factors. VE is also calculated by factors of interest, such as age, gender, pregnancy, influenza type, region of the country, presence of asthma or other comorbidity, etc. Whether the VE value is the “truth in the universe” is related to having enough subjects in each analyzed group and the degree to which the studied populations actually represent the whole country. So, VE is more accurate when there are large subject numbers.
Remember also that VE is usually calculated from outpatients, so it does not really measure all the benefits of vaccination. Prevention rates for severe influenza (such as influenza hospitalizations) are higher but usually unavailable until after the entire season.
VE studies generally measure real-world and likely worst-case-scenario benefit for the overall population being protected against outpatient influenza medical visits.
Vaccine efficacy2,3
Vaccine efficacy measures how the vaccine performs under ideal circumstances in a regimented protocol in relatively normal hosts – likely the best-case-scenario benefit. Vaccine efficacy is the percent difference in confirmed influenza episodes in vaccinees getting the “experimental” vaccine vs. episodes in nonvaccinees (or vaccinees getting an established vaccine). Vaccine efficacy, therefore, is usually calculated based on prospective well-controlled studies under ideal circumstances in subjects who received their vaccines on time per the recommended schedule. Most such studies are performed on otherwise healthy children or adults, with most comorbidities excluded. The “experimental” vaccine is generally from a single manufacturer from a single lot, and chain-of-custody is well controlled. The vaccine is administered at selected research sites according to a strict protocol; vaccine storage is ensured to be as recommended.
Confidence intervals
To assess whether the “protection” is “significant,” the calculations derive 95% confidence intervals (CI). If the 95% CI range is wide, such as many tens of percents, then there is less confidence that the calculation is correct. And if the lower CI is less than 0, then the result is not significant. For example, a VE of 20% is not highly protective, but can be significant if the 95% CI ranges from 10 to 28 (the lower value of 10 is above zero). It would not be significant if the 95% CI lower limit was –10. Values for seasons 2004-2005 and 2005-2006 were similar to this. Consider however that a VE of 55% seems great, but may not be significant if the 95% CI range is –20 to 89 (the lower value is less than zero). In the ideal world, the VE would be greater than 50% and the 95% CI range would be tight with the lower CI value far above zero; for example, VE of 70% with 95% CI ranging from 60 to 80. The 2010-2011 season was close to this.
Type and age-specific VE
Aside from overall VE, there are subset analyses that can be revealing. This year there are the concerning mid-season VE estimates of approximately 25% for the United States and 17% in Canada, for one specific type, H3N2, which unfortunately has been the dominant circulating U.S. type. That number is what everybody seems to have focused on. But remember influenza B becomes dominant late in most seasons (increasing at the time of writing this article). Interim 2017-2018 VE for influenza B was in the mid 60% range, making the box plot near 40% overall.
Age-related VE analysis can show difference; for example, the best benefit for H3N2 this season has been in young children and the worst in elderly and 9- to 17-year-olds.
Take-home message
The simplest way to think of overall VE is that it is the real-world, worst-case-scenario value for influenza protection by vaccine against the several circulating types of influenza.
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics, Kansas City, Mo. Children’s Mercy Hospital receives grant funding for Dr. Harrison’s work as an investigator from GSK for MMR and rotavirus vaccine studies, from Merck for in vitro and clinical antibiotic studies, from Allergan for clinical antibiotic studies, from Pfizer for pneumococcal seroepidemiology studies, and from Regeneron for RSV studies. Dr. Harrison received support for travel and to present seroepidemiology data at one meeting. Email him at pdnews@frontlinemedcom.com.
References
1. MMWR Weekly. 2017 Feb 17;66(6):167-71.
2. Dev Biol Stand. 1998;95:195-201.
3. Lancet Infect Dis. 2012 Jan;12(1):36-44.
During the influenza portion of the Feb. 21, 2018, Centers for Diseases Control and Prevention’s Advisory Committee on Immunization Practices meeting, two pleas from the audience asked the CDC/ACIP to make messages very clear about how protective influenza vaccine really is.
We hear apparently conflicting percentages from Australia, Canada, Europe, and the United States from the many stories/press releases in the news media and from official public health outlets. And the gloomiest ones get the most exposure.1 It can be confusing even for medical care providers who are supposed to advise families on such matters.
A key misunderstanding in many medical and lay news stories is about what vaccine effectiveness and vaccine efficacy really mean. What? Aren’t those the same thing? Nope. They are quite different. And are we sure of what those 95% confidence intervals (CI) mean? Let’s review the “math” so we can explain this to families.
Vaccine effectiveness (VE)2,3
The first thing to know is that the CDC and similar public health agencies in other countries do not report vaccine efficacy. Instead, the percentage reported is VE during (interim estimated VE) and just after (final adjusted VE) each influenza season. This means that VE is generally a retrospective analysis of data, most of which were collected prospectively. Further, VE is likely the worst case scenario. VE is a measure of real-world benefit to patients for whom vaccine is recommended, by analyzing specific geographically diverse populations (population-based) without excluding most underlying illness or comorbidities (note that immunosuppressed persons are excluded). Subjects in VE studies receive their vaccine in the real world and, therefore, vaccinees may receive their vaccines from any number of the usual outlets (e.g., primary care provider, urgent care or emergency department, public health department, pharmacy, school, or nursing home). There are multiple lots of multiple brands from multiple vaccine manufacturers. Children who need two doses of influenza vaccine do not necessarily receive those doses according to the package insert’s schedule. VE studies do not have the capability to confirm that vaccine was stored, handled, and administered in a precisely correct manner according to manufacturer’s and CDC’s recommendations.
VE is calculated using a “test-negative” (case-control) analysis of patients presenting with acute respiratory infections (ARIs). People who are not in vaccine research can find this methodology confusing. Briefly, the VE compares the odds of vaccination in ARIs due to confirmed influenza to the odds of vaccination in ARIs not due to influenza. Additional statistical tools can adjust VE for specific factors. VE is also calculated by factors of interest, such as age, gender, pregnancy, influenza type, region of the country, presence of asthma or other comorbidity, etc. Whether the VE value is the “truth in the universe” is related to having enough subjects in each analyzed group and the degree to which the studied populations actually represent the whole country. So, VE is more accurate when there are large subject numbers.
Remember also that VE is usually calculated from outpatients, so it does not really measure all the benefits of vaccination. Prevention rates for severe influenza (such as influenza hospitalizations) are higher but usually unavailable until after the entire season.
VE studies generally measure real-world and likely worst-case-scenario benefit for the overall population being protected against outpatient influenza medical visits.
Vaccine efficacy2,3
Vaccine efficacy measures how the vaccine performs under ideal circumstances in a regimented protocol in relatively normal hosts – likely the best-case-scenario benefit. Vaccine efficacy is the percent difference in confirmed influenza episodes in vaccinees getting the “experimental” vaccine vs. episodes in nonvaccinees (or vaccinees getting an established vaccine). Vaccine efficacy, therefore, is usually calculated based on prospective well-controlled studies under ideal circumstances in subjects who received their vaccines on time per the recommended schedule. Most such studies are performed on otherwise healthy children or adults, with most comorbidities excluded. The “experimental” vaccine is generally from a single manufacturer from a single lot, and chain-of-custody is well controlled. The vaccine is administered at selected research sites according to a strict protocol; vaccine storage is ensured to be as recommended.
Confidence intervals
To assess whether the “protection” is “significant,” the calculations derive 95% confidence intervals (CI). If the 95% CI range is wide, such as many tens of percents, then there is less confidence that the calculation is correct. And if the lower CI is less than 0, then the result is not significant. For example, a VE of 20% is not highly protective, but can be significant if the 95% CI ranges from 10 to 28 (the lower value of 10 is above zero). It would not be significant if the 95% CI lower limit was –10. Values for seasons 2004-2005 and 2005-2006 were similar to this. Consider however that a VE of 55% seems great, but may not be significant if the 95% CI range is –20 to 89 (the lower value is less than zero). In the ideal world, the VE would be greater than 50% and the 95% CI range would be tight with the lower CI value far above zero; for example, VE of 70% with 95% CI ranging from 60 to 80. The 2010-2011 season was close to this.
Type and age-specific VE
Aside from overall VE, there are subset analyses that can be revealing. This year there are the concerning mid-season VE estimates of approximately 25% for the United States and 17% in Canada, for one specific type, H3N2, which unfortunately has been the dominant circulating U.S. type. That number is what everybody seems to have focused on. But remember influenza B becomes dominant late in most seasons (increasing at the time of writing this article). Interim 2017-2018 VE for influenza B was in the mid 60% range, making the box plot near 40% overall.
Age-related VE analysis can show difference; for example, the best benefit for H3N2 this season has been in young children and the worst in elderly and 9- to 17-year-olds.
Take-home message
The simplest way to think of overall VE is that it is the real-world, worst-case-scenario value for influenza protection by vaccine against the several circulating types of influenza.
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics, Kansas City, Mo. Children’s Mercy Hospital receives grant funding for Dr. Harrison’s work as an investigator from GSK for MMR and rotavirus vaccine studies, from Merck for in vitro and clinical antibiotic studies, from Allergan for clinical antibiotic studies, from Pfizer for pneumococcal seroepidemiology studies, and from Regeneron for RSV studies. Dr. Harrison received support for travel and to present seroepidemiology data at one meeting. Email him at pdnews@frontlinemedcom.com.
References
1. MMWR Weekly. 2017 Feb 17;66(6):167-71.
2. Dev Biol Stand. 1998;95:195-201.
3. Lancet Infect Dis. 2012 Jan;12(1):36-44.
Supreme Court declines to hear DACA case
The U.S. Supreme Court has declined to decide for now whether the Deferred Action for Childhood Arrivals (DACA) program should continue, turning down a request by the Trump administration to hear the case early. The Feb. 26 decision allows the DACA program to remain in effect under a district court ruling and sends the case back to the appeals court.
The Trump administration has called DACA, an Obama administration policy that protected from deportation immigrants who were brought to the United States illegally as children and authorized them to work in the United States, an “unconstitutional overreach of executive branch power” by the former administration that deliberately sought to undermine the legislative branch.
President Trump announced in September 2016 that he would phase out the DACA program and gave Congress 6 months to pass legislation that would replace DACA or preserve some of its provisions or he would terminate it. They have made little progress toward agreeing on replacement legislation for DACA since then.
A number of plaintiffs sued the Trump administration over the program’s rescission, including the University of California, several states, and a group of individuals who remain in the United States because of DACA. The plaintiffs alleged the rescission violated the Administrative Procedure Act because it was an abuse of discretion and deprived those affected by DACA of “constitutionally protected property and liberty interests without due process of law.”
In a Jan. 9 opinion, the United States District Court for the Northern District of California sided with the plaintiffs, ruling that they were likely to succeed on their claims that the rescission “was arbitrary, capricious, an abuse of discretion, or not otherwise in accordance with law.” The court ordered the government to continue accepting DACA renewal applications while the challenges continued through the court system. Rather than pursuing an appeal, the U.S. Department of Justice requested that the Supreme Court take up the case.
In its Feb. 26 order, the high court declined to hear the case, writing, “it is assumed the court of appeals will act expeditiously to decide this case.”
Jirayut New Latthivongskorn, a co-plaintiff in the case and a medical student with DACA status at the University of California, San Francisco, said the Supreme Court made the right decision. Mr. Latthivongskorn, who came to the United States from Thailand when he was 9 years old, is cofounder of Pre-Health Dreamers, a network of undocumented students who plan to pursue medical careers. He is in his third year at UCSF and is slated to graduate in 2019 after completing the university’s Program in Medical Education for the Urban Underserved.
The Department of Justice expressed disappointment at the Supreme Court’s decision not to take up the case and said it will continue to strongly defend the case.
“While we were hopeful for a different outcome, the Supreme Court very rarely grants certiorari before judgment, though in our view it was warranted for the extraordinary injunction requiring the Department of Homeland Security to maintain DACA,” DOJ spokesman Devin O’Malley said in a statement. “We will continue to defend DHS’ lawful authority to wind down DACA in an orderly manner.”
The U.S. Supreme Court has declined to decide for now whether the Deferred Action for Childhood Arrivals (DACA) program should continue, turning down a request by the Trump administration to hear the case early. The Feb. 26 decision allows the DACA program to remain in effect under a district court ruling and sends the case back to the appeals court.
The Trump administration has called DACA, an Obama administration policy that protected from deportation immigrants who were brought to the United States illegally as children and authorized them to work in the United States, an “unconstitutional overreach of executive branch power” by the former administration that deliberately sought to undermine the legislative branch.
President Trump announced in September 2016 that he would phase out the DACA program and gave Congress 6 months to pass legislation that would replace DACA or preserve some of its provisions or he would terminate it. They have made little progress toward agreeing on replacement legislation for DACA since then.
A number of plaintiffs sued the Trump administration over the program’s rescission, including the University of California, several states, and a group of individuals who remain in the United States because of DACA. The plaintiffs alleged the rescission violated the Administrative Procedure Act because it was an abuse of discretion and deprived those affected by DACA of “constitutionally protected property and liberty interests without due process of law.”
In a Jan. 9 opinion, the United States District Court for the Northern District of California sided with the plaintiffs, ruling that they were likely to succeed on their claims that the rescission “was arbitrary, capricious, an abuse of discretion, or not otherwise in accordance with law.” The court ordered the government to continue accepting DACA renewal applications while the challenges continued through the court system. Rather than pursuing an appeal, the U.S. Department of Justice requested that the Supreme Court take up the case.
In its Feb. 26 order, the high court declined to hear the case, writing, “it is assumed the court of appeals will act expeditiously to decide this case.”
Jirayut New Latthivongskorn, a co-plaintiff in the case and a medical student with DACA status at the University of California, San Francisco, said the Supreme Court made the right decision. Mr. Latthivongskorn, who came to the United States from Thailand when he was 9 years old, is cofounder of Pre-Health Dreamers, a network of undocumented students who plan to pursue medical careers. He is in his third year at UCSF and is slated to graduate in 2019 after completing the university’s Program in Medical Education for the Urban Underserved.
The Department of Justice expressed disappointment at the Supreme Court’s decision not to take up the case and said it will continue to strongly defend the case.
“While we were hopeful for a different outcome, the Supreme Court very rarely grants certiorari before judgment, though in our view it was warranted for the extraordinary injunction requiring the Department of Homeland Security to maintain DACA,” DOJ spokesman Devin O’Malley said in a statement. “We will continue to defend DHS’ lawful authority to wind down DACA in an orderly manner.”
The U.S. Supreme Court has declined to decide for now whether the Deferred Action for Childhood Arrivals (DACA) program should continue, turning down a request by the Trump administration to hear the case early. The Feb. 26 decision allows the DACA program to remain in effect under a district court ruling and sends the case back to the appeals court.
The Trump administration has called DACA, an Obama administration policy that protected from deportation immigrants who were brought to the United States illegally as children and authorized them to work in the United States, an “unconstitutional overreach of executive branch power” by the former administration that deliberately sought to undermine the legislative branch.
President Trump announced in September 2016 that he would phase out the DACA program and gave Congress 6 months to pass legislation that would replace DACA or preserve some of its provisions or he would terminate it. They have made little progress toward agreeing on replacement legislation for DACA since then.
A number of plaintiffs sued the Trump administration over the program’s rescission, including the University of California, several states, and a group of individuals who remain in the United States because of DACA. The plaintiffs alleged the rescission violated the Administrative Procedure Act because it was an abuse of discretion and deprived those affected by DACA of “constitutionally protected property and liberty interests without due process of law.”
In a Jan. 9 opinion, the United States District Court for the Northern District of California sided with the plaintiffs, ruling that they were likely to succeed on their claims that the rescission “was arbitrary, capricious, an abuse of discretion, or not otherwise in accordance with law.” The court ordered the government to continue accepting DACA renewal applications while the challenges continued through the court system. Rather than pursuing an appeal, the U.S. Department of Justice requested that the Supreme Court take up the case.
In its Feb. 26 order, the high court declined to hear the case, writing, “it is assumed the court of appeals will act expeditiously to decide this case.”
Jirayut New Latthivongskorn, a co-plaintiff in the case and a medical student with DACA status at the University of California, San Francisco, said the Supreme Court made the right decision. Mr. Latthivongskorn, who came to the United States from Thailand when he was 9 years old, is cofounder of Pre-Health Dreamers, a network of undocumented students who plan to pursue medical careers. He is in his third year at UCSF and is slated to graduate in 2019 after completing the university’s Program in Medical Education for the Urban Underserved.
The Department of Justice expressed disappointment at the Supreme Court’s decision not to take up the case and said it will continue to strongly defend the case.
“While we were hopeful for a different outcome, the Supreme Court very rarely grants certiorari before judgment, though in our view it was warranted for the extraordinary injunction requiring the Department of Homeland Security to maintain DACA,” DOJ spokesman Devin O’Malley said in a statement. “We will continue to defend DHS’ lawful authority to wind down DACA in an orderly manner.”