User login
Breast cancer deaths projected for 2018
Female breast cancer mortality is expected to be about 25.3 per 100,000 women in 2018, with the highest rate in the District of Columbia and the lowest in Utah.
Approximately 40,920 deaths from invasive female breast cancer are predicted in the United States by the American Cancer Society (ACS) in its Cancer Facts & Figures 2018, which is based on analysis of 2001-2015 data from the National Center for Health Statistics. The death rate has declined 39% since its peak in 1989, and over the last 10 years, the annual decline has been 1.8% for white women and 1.5% for black women per year, the ACS said.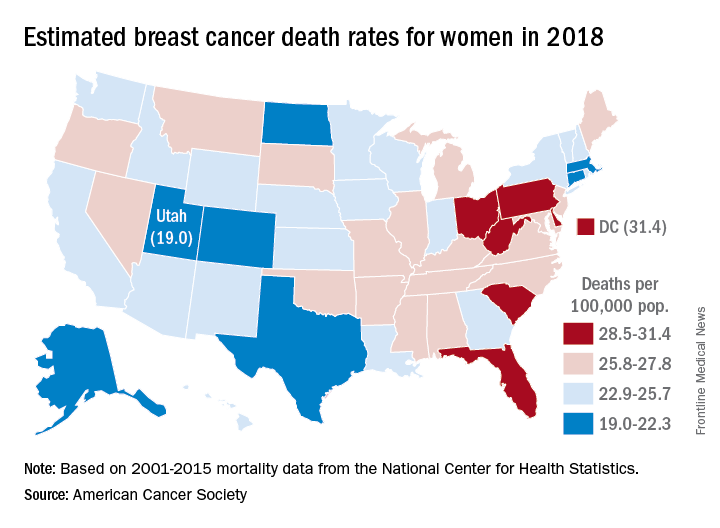
Breast cancer is the most common cancer in women, as it is expected to account for 30% of the almost 880,000 new cancer cases in 2018, compared with 13% for lung cancer, which is second. Lung cancer, however, is projected to cause more deaths among women – 70,500 – than any other cancer, the ACS reported.
Female breast cancer mortality is expected to be about 25.3 per 100,000 women in 2018, with the highest rate in the District of Columbia and the lowest in Utah.
Approximately 40,920 deaths from invasive female breast cancer are predicted in the United States by the American Cancer Society (ACS) in its Cancer Facts & Figures 2018, which is based on analysis of 2001-2015 data from the National Center for Health Statistics. The death rate has declined 39% since its peak in 1989, and over the last 10 years, the annual decline has been 1.8% for white women and 1.5% for black women per year, the ACS said.
Breast cancer is the most common cancer in women, as it is expected to account for 30% of the almost 880,000 new cancer cases in 2018, compared with 13% for lung cancer, which is second. Lung cancer, however, is projected to cause more deaths among women – 70,500 – than any other cancer, the ACS reported.
Female breast cancer mortality is expected to be about 25.3 per 100,000 women in 2018, with the highest rate in the District of Columbia and the lowest in Utah.
Approximately 40,920 deaths from invasive female breast cancer are predicted in the United States by the American Cancer Society (ACS) in its Cancer Facts & Figures 2018, which is based on analysis of 2001-2015 data from the National Center for Health Statistics. The death rate has declined 39% since its peak in 1989, and over the last 10 years, the annual decline has been 1.8% for white women and 1.5% for black women per year, the ACS said.
Breast cancer is the most common cancer in women, as it is expected to account for 30% of the almost 880,000 new cancer cases in 2018, compared with 13% for lung cancer, which is second. Lung cancer, however, is projected to cause more deaths among women – 70,500 – than any other cancer, the ACS reported.
Fluarix Quadrivalent effective in very young, simplifies flu shots for all ages
Fluarix Quadrivalent is highly effective against moderate and severe flu strains in children aged 6-35 months, and has the potential to simplify influenza vaccinations for all ages, according the results of a phase 3 clinical trial presented at a meeting of the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices.
“Fluarix Quadrivalent, at the 0.5-mL dose in young children 6 to 35 months of age, demonstrated efficacy of 63.2% against moderate to severe influenza and 49.8% against any severity influenza disease” stated Leonard Friedland, MD, director of scientific affairs and public health, Vaccines North America, GlaxoSmithKline. Dr. Friedland, a pediatrician in Pennsylvania, said that a standard 0.5-mL dose of Fluarix Quadrivalent has practice-changing implications for physicians. “The use of a 0.5-mL dose (15 mcg per strain) for all persons aged 6 months and older potentially simplifies influenza vaccination by allowing the same vaccine dose to be used for all eligible individuals.”
The high efficacy of Fluarix against almost half of all influenza strains, regardless of severity, and in preventing moderate to severe influenza, correlated with a reduction in health care utilization by pediatric influenza patients, he said. Visits to general practitioners and emergency departments decreased by 47% and 79%, respectively, in children aged 6-35 months. Influenza-associated antibiotic use in these pediatric influenza patients also decreased by 50%.
These findings were the result of D-QIV-004, a phase 3, observer-blinded, randomized trial of 12,018 children aged 6-35 months. These children were split into five cohorts, each in a different influenza season. The study spanned 13 countries and ran from October 2011 to December 2014. To determine the safety of Fluarix, the study utilized noninfluenza vaccine comparator vaccines that were age appropriate, including Prevnar 13, Havrix, and Varivax.
A majority of the children in the study (98%) were vaccine unprimed (had never received two doses of seasonal influenza vaccine) and received two doses of Fluarix. The remaining children received one dose.
On Jan. 11, 2018, the Food and Drug Administration expanded the indication of Fluarix Quadrivalent to include use in persons 6 months and older. Previously, it was approved only for persons 3 years and older.
“These study results support universal vaccination of all individuals from 6 months of age [with Fluarix] to prevent influenza.” Dr. Friedland concluded.
For live updates and information concerning influenza, visit the CDC website.
ilacy@frontlinemedcom.com
SOURCE: D-QIV-004.
Fluarix Quadrivalent is highly effective against moderate and severe flu strains in children aged 6-35 months, and has the potential to simplify influenza vaccinations for all ages, according the results of a phase 3 clinical trial presented at a meeting of the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices.
“Fluarix Quadrivalent, at the 0.5-mL dose in young children 6 to 35 months of age, demonstrated efficacy of 63.2% against moderate to severe influenza and 49.8% against any severity influenza disease” stated Leonard Friedland, MD, director of scientific affairs and public health, Vaccines North America, GlaxoSmithKline. Dr. Friedland, a pediatrician in Pennsylvania, said that a standard 0.5-mL dose of Fluarix Quadrivalent has practice-changing implications for physicians. “The use of a 0.5-mL dose (15 mcg per strain) for all persons aged 6 months and older potentially simplifies influenza vaccination by allowing the same vaccine dose to be used for all eligible individuals.”
The high efficacy of Fluarix against almost half of all influenza strains, regardless of severity, and in preventing moderate to severe influenza, correlated with a reduction in health care utilization by pediatric influenza patients, he said. Visits to general practitioners and emergency departments decreased by 47% and 79%, respectively, in children aged 6-35 months. Influenza-associated antibiotic use in these pediatric influenza patients also decreased by 50%.
These findings were the result of D-QIV-004, a phase 3, observer-blinded, randomized trial of 12,018 children aged 6-35 months. These children were split into five cohorts, each in a different influenza season. The study spanned 13 countries and ran from October 2011 to December 2014. To determine the safety of Fluarix, the study utilized noninfluenza vaccine comparator vaccines that were age appropriate, including Prevnar 13, Havrix, and Varivax.
A majority of the children in the study (98%) were vaccine unprimed (had never received two doses of seasonal influenza vaccine) and received two doses of Fluarix. The remaining children received one dose.
On Jan. 11, 2018, the Food and Drug Administration expanded the indication of Fluarix Quadrivalent to include use in persons 6 months and older. Previously, it was approved only for persons 3 years and older.
“These study results support universal vaccination of all individuals from 6 months of age [with Fluarix] to prevent influenza.” Dr. Friedland concluded.
For live updates and information concerning influenza, visit the CDC website.
ilacy@frontlinemedcom.com
SOURCE: D-QIV-004.
Fluarix Quadrivalent is highly effective against moderate and severe flu strains in children aged 6-35 months, and has the potential to simplify influenza vaccinations for all ages, according the results of a phase 3 clinical trial presented at a meeting of the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices.
“Fluarix Quadrivalent, at the 0.5-mL dose in young children 6 to 35 months of age, demonstrated efficacy of 63.2% against moderate to severe influenza and 49.8% against any severity influenza disease” stated Leonard Friedland, MD, director of scientific affairs and public health, Vaccines North America, GlaxoSmithKline. Dr. Friedland, a pediatrician in Pennsylvania, said that a standard 0.5-mL dose of Fluarix Quadrivalent has practice-changing implications for physicians. “The use of a 0.5-mL dose (15 mcg per strain) for all persons aged 6 months and older potentially simplifies influenza vaccination by allowing the same vaccine dose to be used for all eligible individuals.”
The high efficacy of Fluarix against almost half of all influenza strains, regardless of severity, and in preventing moderate to severe influenza, correlated with a reduction in health care utilization by pediatric influenza patients, he said. Visits to general practitioners and emergency departments decreased by 47% and 79%, respectively, in children aged 6-35 months. Influenza-associated antibiotic use in these pediatric influenza patients also decreased by 50%.
These findings were the result of D-QIV-004, a phase 3, observer-blinded, randomized trial of 12,018 children aged 6-35 months. These children were split into five cohorts, each in a different influenza season. The study spanned 13 countries and ran from October 2011 to December 2014. To determine the safety of Fluarix, the study utilized noninfluenza vaccine comparator vaccines that were age appropriate, including Prevnar 13, Havrix, and Varivax.
A majority of the children in the study (98%) were vaccine unprimed (had never received two doses of seasonal influenza vaccine) and received two doses of Fluarix. The remaining children received one dose.
On Jan. 11, 2018, the Food and Drug Administration expanded the indication of Fluarix Quadrivalent to include use in persons 6 months and older. Previously, it was approved only for persons 3 years and older.
“These study results support universal vaccination of all individuals from 6 months of age [with Fluarix] to prevent influenza.” Dr. Friedland concluded.
For live updates and information concerning influenza, visit the CDC website.
ilacy@frontlinemedcom.com
SOURCE: D-QIV-004.
FROM AN ACIP MEETING
Key clinical point: The high efficacy of Fluarix against almost half of all influenza strains, regardless of severity, as well as preventing moderate to severe influenza, reduced health care utilization by pediatric influenza patients.
Major finding: Fluarix Quadrivalent was effective against moderate to severe influenza in 63.2% and against any severity of influenza in 49.8% of children aged 6-35 months.
Study details: A phase 3, observer-blinded, randomized trial of 12,018 children aged 6-35 months, in which the children were split into five cohorts, each in a different influenza season from October 2011 to December 2014.
Disclosures: No disclosures were reported.
Source: The D-QIV-004 study.
Guidelines update best practices for hemorrhoid treatment
Each year, more than 2.2 million patients in the United States undergo evaluations for symptoms of hemorrhoids, according to updated guidelines on the management of hemorrhoids issued by the American Society of Colon and Rectal Surgeons.
“As a result, it is important to identify symptomatic hemorrhoids as the underlying source of the anorectal symptom and to have a clear understanding of the evaluation and management of this disease process,” wrote Bradley R. Davis, MD, FACS, chief of colon and rectal surgery at the Carolinas Medical Center, Charlotte, N.C., and the fellow members of the Clinical Practice Guidelines Committee of the ASCRS.
The guidelines are based on the ASCRS Practice Parameters for the Management of Hemorrhoids published in 2011. The 2018 update was published in the Diseases of the Colon & Rectum.
The guidelines recommend evaluation of hemorrhoids based on a disease-specific history, and a physical that emphasizes the degree and duration of symptoms and identifies risk factors. But the guideline writers note that the recommendation is a grade 1C because the supporting data mainly come from observational or case studies.
“The cardinal signs of internal hemorrhoids are painless bleeding with bowel movements with intermittent protrusion,” the committee said, also emphasizing that patients should be evaluated for fecal incontinence, which could inform surgical decision making.
In addition, the guidelines call for a complete endoscopic evaluation of the colon for patients who present with symptomatic hemorrhoids and rectal bleeding; this recommendation is based on moderately strong evidence, and presented with a grade of 1B.
Medical management of hemorrhoids may include office-based procedures or surgery, according to the guidelines.
“Most patients with grade I and II and select patients with grade III internal hemorrhoidal disease who fail medical treatment can be effectively treated with office-based procedures, such as banding, sclerotherapy, and infrared coagulation,” the committee wrote, and medical office treatment received a strong grade 1A recommendation based on high-quality evidence. Although office procedures are generally well tolerated, the condition can recur. Bleeding is the most common complication, and it is more likely after rubber-band ligation than other office-based options, the guidelines state.
The guidelines offer a weak recommendation of 2C, based on the lack of quality evidence, for the use of early surgical excision to treat patients with thrombosed external hemorrhoids. “Although most patients treated nonoperatively will experience eventual resolution of their symptoms, excision of thrombosed external hemorrhoids may result in more rapid symptom resolution, lower incidence of recurrence, and longer remission intervals,” the committee noted.
Surgical hemorrhoidectomy received the strongest possible recommendation (1A, based on high-quality evidence) for the treatment of patients with external hemorrhoids or a combination of internal and external hemorrhoids with prolapse.
Surgical options described in the recommendations include surgical excision (hemorrhoidectomy), hemorrhoidopexy, and Doppler-guided hemorrhoidectomy, with citations of studies on each procedure. Data from a meta-analysis of 18 randomized prospective studies comparing hemorrhoidectomy with office-based procedures showed that hemorrhoidectomy was “the most effective treatment for patients with grade III hemorrhoids,” but it was associated with greater pain and complication rates, according to the guidelines.
However, complications in general are low after surgical hemorrhoidectomy, with reported complication rates of 1%-2% for the most common complication of postprocedure hemorrhage, the guidelines state. After surgery, the guidelines recommend with a 1B grade (moderate quality evidence) that patients use “a multimodality pain regimen to reduce narcotic usage and promote a faster recovery.”
The committee members had no financial conflicts to disclose.
AGA offers information for your patients about hemorrhoids at http://www.gastro.org/patient-care/conditions-diseases/hemorrhoids.
SOURCE: Davis BR et al. Dis Colon Rectum. 2018; 61:284-92.
Each year, more than 2.2 million patients in the United States undergo evaluations for symptoms of hemorrhoids, according to updated guidelines on the management of hemorrhoids issued by the American Society of Colon and Rectal Surgeons.
“As a result, it is important to identify symptomatic hemorrhoids as the underlying source of the anorectal symptom and to have a clear understanding of the evaluation and management of this disease process,” wrote Bradley R. Davis, MD, FACS, chief of colon and rectal surgery at the Carolinas Medical Center, Charlotte, N.C., and the fellow members of the Clinical Practice Guidelines Committee of the ASCRS.
The guidelines are based on the ASCRS Practice Parameters for the Management of Hemorrhoids published in 2011. The 2018 update was published in the Diseases of the Colon & Rectum.
The guidelines recommend evaluation of hemorrhoids based on a disease-specific history, and a physical that emphasizes the degree and duration of symptoms and identifies risk factors. But the guideline writers note that the recommendation is a grade 1C because the supporting data mainly come from observational or case studies.
“The cardinal signs of internal hemorrhoids are painless bleeding with bowel movements with intermittent protrusion,” the committee said, also emphasizing that patients should be evaluated for fecal incontinence, which could inform surgical decision making.
In addition, the guidelines call for a complete endoscopic evaluation of the colon for patients who present with symptomatic hemorrhoids and rectal bleeding; this recommendation is based on moderately strong evidence, and presented with a grade of 1B.
Medical management of hemorrhoids may include office-based procedures or surgery, according to the guidelines.
“Most patients with grade I and II and select patients with grade III internal hemorrhoidal disease who fail medical treatment can be effectively treated with office-based procedures, such as banding, sclerotherapy, and infrared coagulation,” the committee wrote, and medical office treatment received a strong grade 1A recommendation based on high-quality evidence. Although office procedures are generally well tolerated, the condition can recur. Bleeding is the most common complication, and it is more likely after rubber-band ligation than other office-based options, the guidelines state.
The guidelines offer a weak recommendation of 2C, based on the lack of quality evidence, for the use of early surgical excision to treat patients with thrombosed external hemorrhoids. “Although most patients treated nonoperatively will experience eventual resolution of their symptoms, excision of thrombosed external hemorrhoids may result in more rapid symptom resolution, lower incidence of recurrence, and longer remission intervals,” the committee noted.
Surgical hemorrhoidectomy received the strongest possible recommendation (1A, based on high-quality evidence) for the treatment of patients with external hemorrhoids or a combination of internal and external hemorrhoids with prolapse.
Surgical options described in the recommendations include surgical excision (hemorrhoidectomy), hemorrhoidopexy, and Doppler-guided hemorrhoidectomy, with citations of studies on each procedure. Data from a meta-analysis of 18 randomized prospective studies comparing hemorrhoidectomy with office-based procedures showed that hemorrhoidectomy was “the most effective treatment for patients with grade III hemorrhoids,” but it was associated with greater pain and complication rates, according to the guidelines.
However, complications in general are low after surgical hemorrhoidectomy, with reported complication rates of 1%-2% for the most common complication of postprocedure hemorrhage, the guidelines state. After surgery, the guidelines recommend with a 1B grade (moderate quality evidence) that patients use “a multimodality pain regimen to reduce narcotic usage and promote a faster recovery.”
The committee members had no financial conflicts to disclose.
AGA offers information for your patients about hemorrhoids at http://www.gastro.org/patient-care/conditions-diseases/hemorrhoids.
SOURCE: Davis BR et al. Dis Colon Rectum. 2018; 61:284-92.
Each year, more than 2.2 million patients in the United States undergo evaluations for symptoms of hemorrhoids, according to updated guidelines on the management of hemorrhoids issued by the American Society of Colon and Rectal Surgeons.
“As a result, it is important to identify symptomatic hemorrhoids as the underlying source of the anorectal symptom and to have a clear understanding of the evaluation and management of this disease process,” wrote Bradley R. Davis, MD, FACS, chief of colon and rectal surgery at the Carolinas Medical Center, Charlotte, N.C., and the fellow members of the Clinical Practice Guidelines Committee of the ASCRS.
The guidelines are based on the ASCRS Practice Parameters for the Management of Hemorrhoids published in 2011. The 2018 update was published in the Diseases of the Colon & Rectum.
The guidelines recommend evaluation of hemorrhoids based on a disease-specific history, and a physical that emphasizes the degree and duration of symptoms and identifies risk factors. But the guideline writers note that the recommendation is a grade 1C because the supporting data mainly come from observational or case studies.
“The cardinal signs of internal hemorrhoids are painless bleeding with bowel movements with intermittent protrusion,” the committee said, also emphasizing that patients should be evaluated for fecal incontinence, which could inform surgical decision making.
In addition, the guidelines call for a complete endoscopic evaluation of the colon for patients who present with symptomatic hemorrhoids and rectal bleeding; this recommendation is based on moderately strong evidence, and presented with a grade of 1B.
Medical management of hemorrhoids may include office-based procedures or surgery, according to the guidelines.
“Most patients with grade I and II and select patients with grade III internal hemorrhoidal disease who fail medical treatment can be effectively treated with office-based procedures, such as banding, sclerotherapy, and infrared coagulation,” the committee wrote, and medical office treatment received a strong grade 1A recommendation based on high-quality evidence. Although office procedures are generally well tolerated, the condition can recur. Bleeding is the most common complication, and it is more likely after rubber-band ligation than other office-based options, the guidelines state.
The guidelines offer a weak recommendation of 2C, based on the lack of quality evidence, for the use of early surgical excision to treat patients with thrombosed external hemorrhoids. “Although most patients treated nonoperatively will experience eventual resolution of their symptoms, excision of thrombosed external hemorrhoids may result in more rapid symptom resolution, lower incidence of recurrence, and longer remission intervals,” the committee noted.
Surgical hemorrhoidectomy received the strongest possible recommendation (1A, based on high-quality evidence) for the treatment of patients with external hemorrhoids or a combination of internal and external hemorrhoids with prolapse.
Surgical options described in the recommendations include surgical excision (hemorrhoidectomy), hemorrhoidopexy, and Doppler-guided hemorrhoidectomy, with citations of studies on each procedure. Data from a meta-analysis of 18 randomized prospective studies comparing hemorrhoidectomy with office-based procedures showed that hemorrhoidectomy was “the most effective treatment for patients with grade III hemorrhoids,” but it was associated with greater pain and complication rates, according to the guidelines.
However, complications in general are low after surgical hemorrhoidectomy, with reported complication rates of 1%-2% for the most common complication of postprocedure hemorrhage, the guidelines state. After surgery, the guidelines recommend with a 1B grade (moderate quality evidence) that patients use “a multimodality pain regimen to reduce narcotic usage and promote a faster recovery.”
The committee members had no financial conflicts to disclose.
AGA offers information for your patients about hemorrhoids at http://www.gastro.org/patient-care/conditions-diseases/hemorrhoids.
SOURCE: Davis BR et al. Dis Colon Rectum. 2018; 61:284-92.
FROM DISEASES OF THE COLON & RECTUM
How to lower occupational radiation exposure in the cath lab
Adding some lead shielding in cardiac catheterization labs can cut the amount of radiation that nurse circulators and scrub technologists are exposed to, according to a single-center study of more than 750 patients.
“The simple and relatively inexpensive approach of providing staff members with a dedicated accessory lead shield during cardiac catheterization was associated with a nearly two-thirds reduction in radiation exposure among both nurses and technologists” according to Ryan Madder, MD, and his associates. “The present study is the first, to our knowledge, to observe a similar benefit among nonphysician staff members, an observation that may have important implications for occupational safety in the cardiac catheterization laboratory.”
The report was published in JACC: Cardiovascular Interventions.
SHIELD (Combining Robotic-Stenting and Proactive Shielding Techniques in the Catheterization Laboratory to Achieve Lowest Possible Radiation Exposure to Physicians and Staff) was a single-center, prospective, observational designed study to research radiation exposure levels among physicians and other staff members in cardiac catheterization labs. Dr. Madder, a cardiologist at Spectrum Health in Grand Rapids, Mich., and his colleagues collected radiation exposure information for staff members such as nurse circulators and scrub technologists during August 2015–February 2016 and measured radiation exposure in 764 consecutive cases. Radiation exposure was monitored for these staff members by dosimeters worn on either the left anterior side of the glasses or thyroid collar, and a body dosimeter underneath the lead apparel on the V-neck of their scrub shirt.
The radiation protection used in the study followed standard institutional operating procedures, which includes two shields between the patient and physician, a ceiling mounted upper body shield with a patient contour cutout, and a lower body shield attached to the side of the operating table, extending from the table to the floor. Staff members wore traditional lead garments, consisting of a lead skirt, apron, and thyroid collar. Additional radiation absorbing disposable pads were used as desired by the staff members.
The study was split into two phases to evaluate the effectiveness of accessory shields with phase 1 (401 cases) utilizing traditional protective measures. Phase 2 (363 cases) used the same material as phase I, but a dedicated accessory lead shield was used. For nurse circulators, this shield was placed between the patient and the intravenous medication pole. For scrub technologists, the shield was placed at the foot of the patient’s bed.
Radiation exposure was higher in phase 1 than phase 2 for all workers. In phase I, technologists faced an effective dose normalized to the dose-area product (EDAP) of 2.4 mcSv/(mGy x cm2) x 10-5 per case. This dropped significantly in phase 2 to an EDAP of 0.9per case, a 62.5% lower dose per case (P less than .0001).
Nurses experienced a similar decrease in exposure per case from phase 1 (EDAP 1.1) and phase 2 (EDAP 0.4). This accounted for a 63.6% lower dose per case (P less than .0001).
The contrast delivery system used at the lab did not allow technologists to maximize their distance from radiation sources, so it is unclear whether the results of this study can be extrapolated to centers where technologists do not perform contrast injections.
Dr. Madder and his colleagues pointed out that this research only begins to scratch the surface of the occupational hazards that catheterization laboratory personnel face.“The use of accessory lead shields, which might be effective to reduce staff radiation exposure, will not likely reduce other occupational hazards of working in the catheterization laboratory related to wearing heavy lead garments, including the risks for orthopedic injuries and experiencing chronic work-related pain.”
Corindus Vascular Robotics partially funded this study and has provided research support to Dr. Madder. All other researchers have no financial conflicts to report.
SOURCE: Madder R. JACC: Cardiovasc Interven. 2018 Jan 22;11[2]:206-12.
While concerns about occupational radiation exposure to cardiologists has prompted equipment, shielding, and behavioral modifications to reduce radiation exposure in cardiologists, exposure to staff members such as nurses and technologists has not received this same attention.
Considering that the shield should also absorb approximately 98% of the scatter radiation, the only real issue about its effectiveness is whether technologists and nurses can remain behind the wall while still being able to assist during a procedure.
All cardiology labs should be equipped with these shields to provide adequate radiation protection to those who work there. The work of Madder et al. should provide confidence to nurses and technologists that their occupational radiation exposure can be expected to be low, as well as their cancer risk.
Kenneth A. Fetterly, PhD , a radiologist, and Malcolm R. Bell, MD , a cardiologist and director of the ischemic heart disease program at the Mayo Clinic in Rochester, Minn., made these comments in an editorial ( JACC: Cardiovasc Interven. 2018 Jan 22;11[2]:213-4 ).
While concerns about occupational radiation exposure to cardiologists has prompted equipment, shielding, and behavioral modifications to reduce radiation exposure in cardiologists, exposure to staff members such as nurses and technologists has not received this same attention.
Considering that the shield should also absorb approximately 98% of the scatter radiation, the only real issue about its effectiveness is whether technologists and nurses can remain behind the wall while still being able to assist during a procedure.
All cardiology labs should be equipped with these shields to provide adequate radiation protection to those who work there. The work of Madder et al. should provide confidence to nurses and technologists that their occupational radiation exposure can be expected to be low, as well as their cancer risk.
Kenneth A. Fetterly, PhD , a radiologist, and Malcolm R. Bell, MD , a cardiologist and director of the ischemic heart disease program at the Mayo Clinic in Rochester, Minn., made these comments in an editorial ( JACC: Cardiovasc Interven. 2018 Jan 22;11[2]:213-4 ).
While concerns about occupational radiation exposure to cardiologists has prompted equipment, shielding, and behavioral modifications to reduce radiation exposure in cardiologists, exposure to staff members such as nurses and technologists has not received this same attention.
Considering that the shield should also absorb approximately 98% of the scatter radiation, the only real issue about its effectiveness is whether technologists and nurses can remain behind the wall while still being able to assist during a procedure.
All cardiology labs should be equipped with these shields to provide adequate radiation protection to those who work there. The work of Madder et al. should provide confidence to nurses and technologists that their occupational radiation exposure can be expected to be low, as well as their cancer risk.
Kenneth A. Fetterly, PhD , a radiologist, and Malcolm R. Bell, MD , a cardiologist and director of the ischemic heart disease program at the Mayo Clinic in Rochester, Minn., made these comments in an editorial ( JACC: Cardiovasc Interven. 2018 Jan 22;11[2]:213-4 ).
Adding some lead shielding in cardiac catheterization labs can cut the amount of radiation that nurse circulators and scrub technologists are exposed to, according to a single-center study of more than 750 patients.
“The simple and relatively inexpensive approach of providing staff members with a dedicated accessory lead shield during cardiac catheterization was associated with a nearly two-thirds reduction in radiation exposure among both nurses and technologists” according to Ryan Madder, MD, and his associates. “The present study is the first, to our knowledge, to observe a similar benefit among nonphysician staff members, an observation that may have important implications for occupational safety in the cardiac catheterization laboratory.”
The report was published in JACC: Cardiovascular Interventions.
SHIELD (Combining Robotic-Stenting and Proactive Shielding Techniques in the Catheterization Laboratory to Achieve Lowest Possible Radiation Exposure to Physicians and Staff) was a single-center, prospective, observational designed study to research radiation exposure levels among physicians and other staff members in cardiac catheterization labs. Dr. Madder, a cardiologist at Spectrum Health in Grand Rapids, Mich., and his colleagues collected radiation exposure information for staff members such as nurse circulators and scrub technologists during August 2015–February 2016 and measured radiation exposure in 764 consecutive cases. Radiation exposure was monitored for these staff members by dosimeters worn on either the left anterior side of the glasses or thyroid collar, and a body dosimeter underneath the lead apparel on the V-neck of their scrub shirt.
The radiation protection used in the study followed standard institutional operating procedures, which includes two shields between the patient and physician, a ceiling mounted upper body shield with a patient contour cutout, and a lower body shield attached to the side of the operating table, extending from the table to the floor. Staff members wore traditional lead garments, consisting of a lead skirt, apron, and thyroid collar. Additional radiation absorbing disposable pads were used as desired by the staff members.
The study was split into two phases to evaluate the effectiveness of accessory shields with phase 1 (401 cases) utilizing traditional protective measures. Phase 2 (363 cases) used the same material as phase I, but a dedicated accessory lead shield was used. For nurse circulators, this shield was placed between the patient and the intravenous medication pole. For scrub technologists, the shield was placed at the foot of the patient’s bed.
Radiation exposure was higher in phase 1 than phase 2 for all workers. In phase I, technologists faced an effective dose normalized to the dose-area product (EDAP) of 2.4 mcSv/(mGy x cm2) x 10-5 per case. This dropped significantly in phase 2 to an EDAP of 0.9per case, a 62.5% lower dose per case (P less than .0001).
Nurses experienced a similar decrease in exposure per case from phase 1 (EDAP 1.1) and phase 2 (EDAP 0.4). This accounted for a 63.6% lower dose per case (P less than .0001).
The contrast delivery system used at the lab did not allow technologists to maximize their distance from radiation sources, so it is unclear whether the results of this study can be extrapolated to centers where technologists do not perform contrast injections.
Dr. Madder and his colleagues pointed out that this research only begins to scratch the surface of the occupational hazards that catheterization laboratory personnel face.“The use of accessory lead shields, which might be effective to reduce staff radiation exposure, will not likely reduce other occupational hazards of working in the catheterization laboratory related to wearing heavy lead garments, including the risks for orthopedic injuries and experiencing chronic work-related pain.”
Corindus Vascular Robotics partially funded this study and has provided research support to Dr. Madder. All other researchers have no financial conflicts to report.
SOURCE: Madder R. JACC: Cardiovasc Interven. 2018 Jan 22;11[2]:206-12.
Adding some lead shielding in cardiac catheterization labs can cut the amount of radiation that nurse circulators and scrub technologists are exposed to, according to a single-center study of more than 750 patients.
“The simple and relatively inexpensive approach of providing staff members with a dedicated accessory lead shield during cardiac catheterization was associated with a nearly two-thirds reduction in radiation exposure among both nurses and technologists” according to Ryan Madder, MD, and his associates. “The present study is the first, to our knowledge, to observe a similar benefit among nonphysician staff members, an observation that may have important implications for occupational safety in the cardiac catheterization laboratory.”
The report was published in JACC: Cardiovascular Interventions.
SHIELD (Combining Robotic-Stenting and Proactive Shielding Techniques in the Catheterization Laboratory to Achieve Lowest Possible Radiation Exposure to Physicians and Staff) was a single-center, prospective, observational designed study to research radiation exposure levels among physicians and other staff members in cardiac catheterization labs. Dr. Madder, a cardiologist at Spectrum Health in Grand Rapids, Mich., and his colleagues collected radiation exposure information for staff members such as nurse circulators and scrub technologists during August 2015–February 2016 and measured radiation exposure in 764 consecutive cases. Radiation exposure was monitored for these staff members by dosimeters worn on either the left anterior side of the glasses or thyroid collar, and a body dosimeter underneath the lead apparel on the V-neck of their scrub shirt.
The radiation protection used in the study followed standard institutional operating procedures, which includes two shields between the patient and physician, a ceiling mounted upper body shield with a patient contour cutout, and a lower body shield attached to the side of the operating table, extending from the table to the floor. Staff members wore traditional lead garments, consisting of a lead skirt, apron, and thyroid collar. Additional radiation absorbing disposable pads were used as desired by the staff members.
The study was split into two phases to evaluate the effectiveness of accessory shields with phase 1 (401 cases) utilizing traditional protective measures. Phase 2 (363 cases) used the same material as phase I, but a dedicated accessory lead shield was used. For nurse circulators, this shield was placed between the patient and the intravenous medication pole. For scrub technologists, the shield was placed at the foot of the patient’s bed.
Radiation exposure was higher in phase 1 than phase 2 for all workers. In phase I, technologists faced an effective dose normalized to the dose-area product (EDAP) of 2.4 mcSv/(mGy x cm2) x 10-5 per case. This dropped significantly in phase 2 to an EDAP of 0.9per case, a 62.5% lower dose per case (P less than .0001).
Nurses experienced a similar decrease in exposure per case from phase 1 (EDAP 1.1) and phase 2 (EDAP 0.4). This accounted for a 63.6% lower dose per case (P less than .0001).
The contrast delivery system used at the lab did not allow technologists to maximize their distance from radiation sources, so it is unclear whether the results of this study can be extrapolated to centers where technologists do not perform contrast injections.
Dr. Madder and his colleagues pointed out that this research only begins to scratch the surface of the occupational hazards that catheterization laboratory personnel face.“The use of accessory lead shields, which might be effective to reduce staff radiation exposure, will not likely reduce other occupational hazards of working in the catheterization laboratory related to wearing heavy lead garments, including the risks for orthopedic injuries and experiencing chronic work-related pain.”
Corindus Vascular Robotics partially funded this study and has provided research support to Dr. Madder. All other researchers have no financial conflicts to report.
SOURCE: Madder R. JACC: Cardiovasc Interven. 2018 Jan 22;11[2]:206-12.
FROM JACC: CARDIOVASCULAR INTERVENTIONS
Key clinical point: Accessory lead shielding reduces radiation exposure for nurses and technologists.
Major finding: Accessory lead shielding reduced radiation exposure by over two-thirds.
Study details: A single-center, prospective, observational study of 764 cases.
Disclosures: Corindus Vascular Robotics partially funded this study and has provided research support to Dr. Madder. All other researchers have no financial conflicts to report.
Source: Madder R. JACC: Cardiovasc Interven. 2018 Jan 22;11[2]:206-12.
Dexmedetomidine: ‘Silver bullet’ for ventilator liberation?
SAN ANTONIO – Among medications to facilitate extubation, dexmedetomidine offers favorable attributes, but whether it’s the best choice for patients who have difficulty being liberated from the ventilator remains to be proven, said Gilles L. Fraser, BS Pharm, PharmD.
The current CHEST/ATS guidelines on liberation from mechanical ventilation in critically ill adults strongly suggest extubation to noninvasive mechanical ventilation in high-risk patients (Chest. 2017 Jan;151[1]:160-5. doi: 10.1016/j.chest.2016.10.037). Guideline authors also suggested protocols attempting to minimize sedation for acutely hospitalized patients ventilated for more than 24 hours, based on some evidence showing a trend toward shorter ventilation time and ICU stay, as well as lower short-term mortality.
, one of the coauthors of the guidelines, during his presentation at the Critical Care Congress sponsored by the Society for Critical Care Medicine.
“I’ll leave you up to your own devices,” he told attendees at a session on conundrums in critical care that are not addressed in current guidelines. “We use it all the time, frankly, but I don’t have any firm data to support that contention.”
Despite best practices, extubation attempts are not always successful: “If you follow the rules of the road, success is going to occur about 85% of the time,” said Dr. Fraser, who is a clinical pharmacist at Maine Medical Center, Portland, and professor of medicine at Tufts University, Boston. “That means that about 15% of our patients have difficulties in being liberated from the ventilator.”
In terms of medications to facilitate ventilator liberation, benzodiazepines, dexmedetomidine, and propofol all have roles to play, according to Dr. Fraser. Clinicians have to consider agent-specific side effects, pharmacokinetics and dynamics, and “econotoxicity,” or the cost of care, he added.
Although there are few comparative data available to guide choice of medication, Dr. Fraser and his colleagues have published a systematic review and meta-analysis of randomized trials of benzodiazepine versus nonbenzodiazepine-based sedation for mechanically ventilated, critically ill adult patients (Crit Care Med. 2013 Sep;41[9 Suppl 1]:S30-8. doi: 10.1097/CCM.0b013e3182a16898).
They found that dexmedetomidine- or propofol-based sedation regimens appeared to reduce mechanical ventilation duration and length of ICU stay versus benzodiazepine-based sedation, but they stated that larger controlled studies would be needed to further define outcomes in this setting.
More recently, other investigators reported an evaluation of 9,603 consecutive mechanical ventilation episodes (Chest. 2016 Jun;149[6]:1373-9. doi: 10.1378/chest.15-1389). In this large, real-world experience, propofol and dexmedetomidine were both associated with less time to extubation versus benzodiazepines, and dexmedetomidine was associated with less time to extubation versus propofol.
Relatively few patients (about 12%), however, received dexmedetomidine in that large series, and that was mostly in the setting of cardiac surgery, Dr. Fraser noted. Moreover, the investigators reported finding no differences between any two agents in hospital discharge or mortality hazard ratio.
“We’re not suggesting the benzodiazepines as routine sedative agents in our patient populations,” Dr. Fraser said in his presentation. “The primary reason is that they result in a longer time on the vent, typically between 1 and 2 days.”
But this doesn’t mean that the benzodiazepines are the “devil’s handiwork,” he added, noting that they may be useful in patients with anxiety related to ventilator weaning and those recovering from hemodynamic instability or at risk for GABA-agonist withdrawal.
Dexmedetomidine is opioid sparing and has a minimal effect on respiratory drive, among other advantages; however, some potential drawbacks include its hemodynamic effects and its cost, according to Dr. Fraser.
Dr. Fraser said that his institution’s daily acquisition cost for dexmedetomidine is $500, compared with $120 for propofol and $40 for benzodiazepines, but some pharmacoeconomic evaluations suggest use of dexmedetomidine may actually save between $3,000 and $9,000 per ICU admission. “At least in our place, one day in the ICU costs about $5,000, so that all makes sense … and I can argue fairly effectively that dexmedetomidine really isn’t that expensive compared to midazolam,” he said.
Dr. Fraser said that he had no disclosures related to his presentation.
SAN ANTONIO – Among medications to facilitate extubation, dexmedetomidine offers favorable attributes, but whether it’s the best choice for patients who have difficulty being liberated from the ventilator remains to be proven, said Gilles L. Fraser, BS Pharm, PharmD.
The current CHEST/ATS guidelines on liberation from mechanical ventilation in critically ill adults strongly suggest extubation to noninvasive mechanical ventilation in high-risk patients (Chest. 2017 Jan;151[1]:160-5. doi: 10.1016/j.chest.2016.10.037). Guideline authors also suggested protocols attempting to minimize sedation for acutely hospitalized patients ventilated for more than 24 hours, based on some evidence showing a trend toward shorter ventilation time and ICU stay, as well as lower short-term mortality.
, one of the coauthors of the guidelines, during his presentation at the Critical Care Congress sponsored by the Society for Critical Care Medicine.
“I’ll leave you up to your own devices,” he told attendees at a session on conundrums in critical care that are not addressed in current guidelines. “We use it all the time, frankly, but I don’t have any firm data to support that contention.”
Despite best practices, extubation attempts are not always successful: “If you follow the rules of the road, success is going to occur about 85% of the time,” said Dr. Fraser, who is a clinical pharmacist at Maine Medical Center, Portland, and professor of medicine at Tufts University, Boston. “That means that about 15% of our patients have difficulties in being liberated from the ventilator.”
In terms of medications to facilitate ventilator liberation, benzodiazepines, dexmedetomidine, and propofol all have roles to play, according to Dr. Fraser. Clinicians have to consider agent-specific side effects, pharmacokinetics and dynamics, and “econotoxicity,” or the cost of care, he added.
Although there are few comparative data available to guide choice of medication, Dr. Fraser and his colleagues have published a systematic review and meta-analysis of randomized trials of benzodiazepine versus nonbenzodiazepine-based sedation for mechanically ventilated, critically ill adult patients (Crit Care Med. 2013 Sep;41[9 Suppl 1]:S30-8. doi: 10.1097/CCM.0b013e3182a16898).
They found that dexmedetomidine- or propofol-based sedation regimens appeared to reduce mechanical ventilation duration and length of ICU stay versus benzodiazepine-based sedation, but they stated that larger controlled studies would be needed to further define outcomes in this setting.
More recently, other investigators reported an evaluation of 9,603 consecutive mechanical ventilation episodes (Chest. 2016 Jun;149[6]:1373-9. doi: 10.1378/chest.15-1389). In this large, real-world experience, propofol and dexmedetomidine were both associated with less time to extubation versus benzodiazepines, and dexmedetomidine was associated with less time to extubation versus propofol.
Relatively few patients (about 12%), however, received dexmedetomidine in that large series, and that was mostly in the setting of cardiac surgery, Dr. Fraser noted. Moreover, the investigators reported finding no differences between any two agents in hospital discharge or mortality hazard ratio.
“We’re not suggesting the benzodiazepines as routine sedative agents in our patient populations,” Dr. Fraser said in his presentation. “The primary reason is that they result in a longer time on the vent, typically between 1 and 2 days.”
But this doesn’t mean that the benzodiazepines are the “devil’s handiwork,” he added, noting that they may be useful in patients with anxiety related to ventilator weaning and those recovering from hemodynamic instability or at risk for GABA-agonist withdrawal.
Dexmedetomidine is opioid sparing and has a minimal effect on respiratory drive, among other advantages; however, some potential drawbacks include its hemodynamic effects and its cost, according to Dr. Fraser.
Dr. Fraser said that his institution’s daily acquisition cost for dexmedetomidine is $500, compared with $120 for propofol and $40 for benzodiazepines, but some pharmacoeconomic evaluations suggest use of dexmedetomidine may actually save between $3,000 and $9,000 per ICU admission. “At least in our place, one day in the ICU costs about $5,000, so that all makes sense … and I can argue fairly effectively that dexmedetomidine really isn’t that expensive compared to midazolam,” he said.
Dr. Fraser said that he had no disclosures related to his presentation.
SAN ANTONIO – Among medications to facilitate extubation, dexmedetomidine offers favorable attributes, but whether it’s the best choice for patients who have difficulty being liberated from the ventilator remains to be proven, said Gilles L. Fraser, BS Pharm, PharmD.
The current CHEST/ATS guidelines on liberation from mechanical ventilation in critically ill adults strongly suggest extubation to noninvasive mechanical ventilation in high-risk patients (Chest. 2017 Jan;151[1]:160-5. doi: 10.1016/j.chest.2016.10.037). Guideline authors also suggested protocols attempting to minimize sedation for acutely hospitalized patients ventilated for more than 24 hours, based on some evidence showing a trend toward shorter ventilation time and ICU stay, as well as lower short-term mortality.
, one of the coauthors of the guidelines, during his presentation at the Critical Care Congress sponsored by the Society for Critical Care Medicine.
“I’ll leave you up to your own devices,” he told attendees at a session on conundrums in critical care that are not addressed in current guidelines. “We use it all the time, frankly, but I don’t have any firm data to support that contention.”
Despite best practices, extubation attempts are not always successful: “If you follow the rules of the road, success is going to occur about 85% of the time,” said Dr. Fraser, who is a clinical pharmacist at Maine Medical Center, Portland, and professor of medicine at Tufts University, Boston. “That means that about 15% of our patients have difficulties in being liberated from the ventilator.”
In terms of medications to facilitate ventilator liberation, benzodiazepines, dexmedetomidine, and propofol all have roles to play, according to Dr. Fraser. Clinicians have to consider agent-specific side effects, pharmacokinetics and dynamics, and “econotoxicity,” or the cost of care, he added.
Although there are few comparative data available to guide choice of medication, Dr. Fraser and his colleagues have published a systematic review and meta-analysis of randomized trials of benzodiazepine versus nonbenzodiazepine-based sedation for mechanically ventilated, critically ill adult patients (Crit Care Med. 2013 Sep;41[9 Suppl 1]:S30-8. doi: 10.1097/CCM.0b013e3182a16898).
They found that dexmedetomidine- or propofol-based sedation regimens appeared to reduce mechanical ventilation duration and length of ICU stay versus benzodiazepine-based sedation, but they stated that larger controlled studies would be needed to further define outcomes in this setting.
More recently, other investigators reported an evaluation of 9,603 consecutive mechanical ventilation episodes (Chest. 2016 Jun;149[6]:1373-9. doi: 10.1378/chest.15-1389). In this large, real-world experience, propofol and dexmedetomidine were both associated with less time to extubation versus benzodiazepines, and dexmedetomidine was associated with less time to extubation versus propofol.
Relatively few patients (about 12%), however, received dexmedetomidine in that large series, and that was mostly in the setting of cardiac surgery, Dr. Fraser noted. Moreover, the investigators reported finding no differences between any two agents in hospital discharge or mortality hazard ratio.
“We’re not suggesting the benzodiazepines as routine sedative agents in our patient populations,” Dr. Fraser said in his presentation. “The primary reason is that they result in a longer time on the vent, typically between 1 and 2 days.”
But this doesn’t mean that the benzodiazepines are the “devil’s handiwork,” he added, noting that they may be useful in patients with anxiety related to ventilator weaning and those recovering from hemodynamic instability or at risk for GABA-agonist withdrawal.
Dexmedetomidine is opioid sparing and has a minimal effect on respiratory drive, among other advantages; however, some potential drawbacks include its hemodynamic effects and its cost, according to Dr. Fraser.
Dr. Fraser said that his institution’s daily acquisition cost for dexmedetomidine is $500, compared with $120 for propofol and $40 for benzodiazepines, but some pharmacoeconomic evaluations suggest use of dexmedetomidine may actually save between $3,000 and $9,000 per ICU admission. “At least in our place, one day in the ICU costs about $5,000, so that all makes sense … and I can argue fairly effectively that dexmedetomidine really isn’t that expensive compared to midazolam,” he said.
Dr. Fraser said that he had no disclosures related to his presentation.
EXPERT ANALYSIS FROM CCC47
Combo therapy does not improve outcomes for A. Baumannii
, based on data from 406 patients.
In a study published online in The Lancet Infectious Diseases, Mical Paul, MD, of Rambam Health Care Campus, Haifa, Israel, and colleagues randomized 198 patients to colistin alone and 208 to colistin plus meropenem (Lancet Infect Dis. 2018 Feb 15. doi: 10.1016/S1473-3099[18]30099-9).
The demographics were similar between the groups and approximately 77% of patients in each group were infected with A. baumannii.
The results highlight “the necessity of assessing combination therapy in randomized trials before adopting it into clinical use,” the researchers said.
The study was not designed to examine the effect of the two types of therapy on bacteria other than A. baumannii, the researchers noted. However, based on the findings, “we recommend against the routine use of carbapenems for the treatment of carbapenem-resistant A. baumannii infections,” they said.
The study was supported by EU AIDA grant Health-F3-2011-278348. Dr. Paul had no financial conflicts to disclose.
SOURCE: Paul M et al. Lancet Infect Dis. 2018 Feb 15. doi: 10.1016/S1473-3099(18)30099-9.
, based on data from 406 patients.
In a study published online in The Lancet Infectious Diseases, Mical Paul, MD, of Rambam Health Care Campus, Haifa, Israel, and colleagues randomized 198 patients to colistin alone and 208 to colistin plus meropenem (Lancet Infect Dis. 2018 Feb 15. doi: 10.1016/S1473-3099[18]30099-9).
The demographics were similar between the groups and approximately 77% of patients in each group were infected with A. baumannii.
The results highlight “the necessity of assessing combination therapy in randomized trials before adopting it into clinical use,” the researchers said.
The study was not designed to examine the effect of the two types of therapy on bacteria other than A. baumannii, the researchers noted. However, based on the findings, “we recommend against the routine use of carbapenems for the treatment of carbapenem-resistant A. baumannii infections,” they said.
The study was supported by EU AIDA grant Health-F3-2011-278348. Dr. Paul had no financial conflicts to disclose.
SOURCE: Paul M et al. Lancet Infect Dis. 2018 Feb 15. doi: 10.1016/S1473-3099(18)30099-9.
, based on data from 406 patients.
In a study published online in The Lancet Infectious Diseases, Mical Paul, MD, of Rambam Health Care Campus, Haifa, Israel, and colleagues randomized 198 patients to colistin alone and 208 to colistin plus meropenem (Lancet Infect Dis. 2018 Feb 15. doi: 10.1016/S1473-3099[18]30099-9).
The demographics were similar between the groups and approximately 77% of patients in each group were infected with A. baumannii.
The results highlight “the necessity of assessing combination therapy in randomized trials before adopting it into clinical use,” the researchers said.
The study was not designed to examine the effect of the two types of therapy on bacteria other than A. baumannii, the researchers noted. However, based on the findings, “we recommend against the routine use of carbapenems for the treatment of carbapenem-resistant A. baumannii infections,” they said.
The study was supported by EU AIDA grant Health-F3-2011-278348. Dr. Paul had no financial conflicts to disclose.
SOURCE: Paul M et al. Lancet Infect Dis. 2018 Feb 15. doi: 10.1016/S1473-3099(18)30099-9.
FROM THE LANCET INFECTIOUS DISEASES
Commentary—Finding Important Nutrients in Unexpected Places
While you might not typically put chopped or blended, unsalted, boiled canned oysters on your usual list of recommended infant and toddler foods, maybe you should.
The American Academy of Pediatrics just published a new policy statement on advocacy to improve child nutrition in the first 1,000 days (from conception to age 2). The statement emphasizes the importance of nutrition to optimal brain development. Pediatricians are encouraged to be familiar with community services to support optimal nutrition such as the Special Supplemental Nutrition Program for Women, Infants, and Children, the Supplemental Nutrition Assistance Program, the Child and Adult Care Food Program, and food pantries and soup kitchens, but also to get beyond recommending a “good diet” to something more specific that is high in key nutrients important for brain development such as protein; zinc; iron; choline; folate; iodine; vitamins A, D, B6, and B12; and polyunsaturated fatty acids. That’s where the boiled oysters, a decent source of the listed nutrients and especially loaded with zinc, iron, and vitamin B12, come in. While not everyone is going to rush out to buy their babies such an unexpected (and for many, unfamiliar) food, the statement reminds pediatricians to recommend foods that are good sources of the nutrients that babies and toddlers need most. Other foods that fit the bill include oatmeal, meat and poultry, fish like salmon and tuna, eggs, tofu and soybeans, and other legumes and beans like chickpeas and lentils.
—Natalie D. Muth, MD
Pediatrician
Children's Primary Care Medical Group
Carlsbad, California
While you might not typically put chopped or blended, unsalted, boiled canned oysters on your usual list of recommended infant and toddler foods, maybe you should.
The American Academy of Pediatrics just published a new policy statement on advocacy to improve child nutrition in the first 1,000 days (from conception to age 2). The statement emphasizes the importance of nutrition to optimal brain development. Pediatricians are encouraged to be familiar with community services to support optimal nutrition such as the Special Supplemental Nutrition Program for Women, Infants, and Children, the Supplemental Nutrition Assistance Program, the Child and Adult Care Food Program, and food pantries and soup kitchens, but also to get beyond recommending a “good diet” to something more specific that is high in key nutrients important for brain development such as protein; zinc; iron; choline; folate; iodine; vitamins A, D, B6, and B12; and polyunsaturated fatty acids. That’s where the boiled oysters, a decent source of the listed nutrients and especially loaded with zinc, iron, and vitamin B12, come in. While not everyone is going to rush out to buy their babies such an unexpected (and for many, unfamiliar) food, the statement reminds pediatricians to recommend foods that are good sources of the nutrients that babies and toddlers need most. Other foods that fit the bill include oatmeal, meat and poultry, fish like salmon and tuna, eggs, tofu and soybeans, and other legumes and beans like chickpeas and lentils.
—Natalie D. Muth, MD
Pediatrician
Children's Primary Care Medical Group
Carlsbad, California
While you might not typically put chopped or blended, unsalted, boiled canned oysters on your usual list of recommended infant and toddler foods, maybe you should.
The American Academy of Pediatrics just published a new policy statement on advocacy to improve child nutrition in the first 1,000 days (from conception to age 2). The statement emphasizes the importance of nutrition to optimal brain development. Pediatricians are encouraged to be familiar with community services to support optimal nutrition such as the Special Supplemental Nutrition Program for Women, Infants, and Children, the Supplemental Nutrition Assistance Program, the Child and Adult Care Food Program, and food pantries and soup kitchens, but also to get beyond recommending a “good diet” to something more specific that is high in key nutrients important for brain development such as protein; zinc; iron; choline; folate; iodine; vitamins A, D, B6, and B12; and polyunsaturated fatty acids. That’s where the boiled oysters, a decent source of the listed nutrients and especially loaded with zinc, iron, and vitamin B12, come in. While not everyone is going to rush out to buy their babies such an unexpected (and for many, unfamiliar) food, the statement reminds pediatricians to recommend foods that are good sources of the nutrients that babies and toddlers need most. Other foods that fit the bill include oatmeal, meat and poultry, fish like salmon and tuna, eggs, tofu and soybeans, and other legumes and beans like chickpeas and lentils.
—Natalie D. Muth, MD
Pediatrician
Children's Primary Care Medical Group
Carlsbad, California
New device cuts postoperative pulmonary complications
SAN ANTONIO – according to results of a nonrandomized study including high-risk patients undergoing elective surgical procedures.
“For certain types of surgical procedures, this therapy (MetaNeb, Hill-Rom) may provide a benefit for high-risk patients in terms of reducing their pulmonary complications and their hospital stay,” said Toan Huynh, MD, lead investigator and director of trauma research at Carolinas HealthCare System, Charlotte, N.C., at the Critical Care Congress sponsored by the Society for Critical Care Medicine.
Currently, aggressive management of high-risk patients with strategies such as optimal analgesia, early ambulation, secretion mobilization, and lung expansion are used to try to reduce the incidence of postoperative pulmonary complications, noted Dr. Huynh, in an interview.
In this study, Dr. Huynh and his colleagues from the University of Pennsylvania, Philadelphia, and the Lahey Hospital & Medical Center, Burlington, Mass., sought to evaluate the efficacy of the MetaNeb system, which delivers continuous high-frequency oscillation, continuous positive expiratory pressure, and in-line aerosol flow in one combined unit. To estimate usual postoperative pulmonary complication rates, they first queried CPT and ICD-9-CM codes to identify a total of 210 patients who had undergone thoracic, upper-abdominal, or aortic open surgical procedures. Then, in the second stage of the study, the investigators prospectively enrolled 209 subjects who underwent those types of surgery with the MetaNeb system in addition to a standard postoperative respiratory regimen. All patients were high risk as defined by having either an American Society of Anesthesiologists classification of at least 3 or an ASA classification of 2 along with one or more comorbidities, such as COPD or recent smoking history.
Among the patients managed with MetaNeb, 33 (15.8%) experienced one or more pulmonary complications, compared with 48 (22.9%) in the retrospective cohort (P = 0.06). For intubated patients, at least one complication was seen in 22 patients (36.7%) in the MetaNeb group, compared with 37 (69.8%) in the comparison group (P less than .05). Time on mechanical ventilation was 8.5 hours in the MetaNeb group versus 23.7 hours in the comparison group (P less than .05).
Use of the device was also associated with decreased length of hospital stay, but the difference between lengths of stay was not statistically significant. Hospital length of stay was 6.8 days in the MetaNeb versus 8.4 days in the comparison groups.“In the current day and age of value-based health care, I think any kind of reduction in expenditure related to health care costs would be compelling for clinicians,” Dr. Huynh said in the interview.
Further study may be needed to better define the role of the combined modality system in clinical practice, according to Dr. Huynh.
“This is sort of a ‘before and after’ nonrandomized trial,” Dr. Huynh explained. “I think, ideally, if we can do a truly controlled, randomized trial, that will be much more powerful.”
The study was sponsored by Hill-Rom, which manufactures the device under study. Dr. Huynh said he and coinvestigators had no financial conflicts related to the research.
SOURCE: Huynh T et al. Critical Care Congress, Abstract 17.
SAN ANTONIO – according to results of a nonrandomized study including high-risk patients undergoing elective surgical procedures.
“For certain types of surgical procedures, this therapy (MetaNeb, Hill-Rom) may provide a benefit for high-risk patients in terms of reducing their pulmonary complications and their hospital stay,” said Toan Huynh, MD, lead investigator and director of trauma research at Carolinas HealthCare System, Charlotte, N.C., at the Critical Care Congress sponsored by the Society for Critical Care Medicine.
Currently, aggressive management of high-risk patients with strategies such as optimal analgesia, early ambulation, secretion mobilization, and lung expansion are used to try to reduce the incidence of postoperative pulmonary complications, noted Dr. Huynh, in an interview.
In this study, Dr. Huynh and his colleagues from the University of Pennsylvania, Philadelphia, and the Lahey Hospital & Medical Center, Burlington, Mass., sought to evaluate the efficacy of the MetaNeb system, which delivers continuous high-frequency oscillation, continuous positive expiratory pressure, and in-line aerosol flow in one combined unit. To estimate usual postoperative pulmonary complication rates, they first queried CPT and ICD-9-CM codes to identify a total of 210 patients who had undergone thoracic, upper-abdominal, or aortic open surgical procedures. Then, in the second stage of the study, the investigators prospectively enrolled 209 subjects who underwent those types of surgery with the MetaNeb system in addition to a standard postoperative respiratory regimen. All patients were high risk as defined by having either an American Society of Anesthesiologists classification of at least 3 or an ASA classification of 2 along with one or more comorbidities, such as COPD or recent smoking history.
Among the patients managed with MetaNeb, 33 (15.8%) experienced one or more pulmonary complications, compared with 48 (22.9%) in the retrospective cohort (P = 0.06). For intubated patients, at least one complication was seen in 22 patients (36.7%) in the MetaNeb group, compared with 37 (69.8%) in the comparison group (P less than .05). Time on mechanical ventilation was 8.5 hours in the MetaNeb group versus 23.7 hours in the comparison group (P less than .05).
Use of the device was also associated with decreased length of hospital stay, but the difference between lengths of stay was not statistically significant. Hospital length of stay was 6.8 days in the MetaNeb versus 8.4 days in the comparison groups.“In the current day and age of value-based health care, I think any kind of reduction in expenditure related to health care costs would be compelling for clinicians,” Dr. Huynh said in the interview.
Further study may be needed to better define the role of the combined modality system in clinical practice, according to Dr. Huynh.
“This is sort of a ‘before and after’ nonrandomized trial,” Dr. Huynh explained. “I think, ideally, if we can do a truly controlled, randomized trial, that will be much more powerful.”
The study was sponsored by Hill-Rom, which manufactures the device under study. Dr. Huynh said he and coinvestigators had no financial conflicts related to the research.
SOURCE: Huynh T et al. Critical Care Congress, Abstract 17.
SAN ANTONIO – according to results of a nonrandomized study including high-risk patients undergoing elective surgical procedures.
“For certain types of surgical procedures, this therapy (MetaNeb, Hill-Rom) may provide a benefit for high-risk patients in terms of reducing their pulmonary complications and their hospital stay,” said Toan Huynh, MD, lead investigator and director of trauma research at Carolinas HealthCare System, Charlotte, N.C., at the Critical Care Congress sponsored by the Society for Critical Care Medicine.
Currently, aggressive management of high-risk patients with strategies such as optimal analgesia, early ambulation, secretion mobilization, and lung expansion are used to try to reduce the incidence of postoperative pulmonary complications, noted Dr. Huynh, in an interview.
In this study, Dr. Huynh and his colleagues from the University of Pennsylvania, Philadelphia, and the Lahey Hospital & Medical Center, Burlington, Mass., sought to evaluate the efficacy of the MetaNeb system, which delivers continuous high-frequency oscillation, continuous positive expiratory pressure, and in-line aerosol flow in one combined unit. To estimate usual postoperative pulmonary complication rates, they first queried CPT and ICD-9-CM codes to identify a total of 210 patients who had undergone thoracic, upper-abdominal, or aortic open surgical procedures. Then, in the second stage of the study, the investigators prospectively enrolled 209 subjects who underwent those types of surgery with the MetaNeb system in addition to a standard postoperative respiratory regimen. All patients were high risk as defined by having either an American Society of Anesthesiologists classification of at least 3 or an ASA classification of 2 along with one or more comorbidities, such as COPD or recent smoking history.
Among the patients managed with MetaNeb, 33 (15.8%) experienced one or more pulmonary complications, compared with 48 (22.9%) in the retrospective cohort (P = 0.06). For intubated patients, at least one complication was seen in 22 patients (36.7%) in the MetaNeb group, compared with 37 (69.8%) in the comparison group (P less than .05). Time on mechanical ventilation was 8.5 hours in the MetaNeb group versus 23.7 hours in the comparison group (P less than .05).
Use of the device was also associated with decreased length of hospital stay, but the difference between lengths of stay was not statistically significant. Hospital length of stay was 6.8 days in the MetaNeb versus 8.4 days in the comparison groups.“In the current day and age of value-based health care, I think any kind of reduction in expenditure related to health care costs would be compelling for clinicians,” Dr. Huynh said in the interview.
Further study may be needed to better define the role of the combined modality system in clinical practice, according to Dr. Huynh.
“This is sort of a ‘before and after’ nonrandomized trial,” Dr. Huynh explained. “I think, ideally, if we can do a truly controlled, randomized trial, that will be much more powerful.”
The study was sponsored by Hill-Rom, which manufactures the device under study. Dr. Huynh said he and coinvestigators had no financial conflicts related to the research.
SOURCE: Huynh T et al. Critical Care Congress, Abstract 17.
AT THE CRITICAL CARE CONGRESS
Key clinical point: A device that combines lung expansion, secretion clearance, and aerosol delivery (MetaNeb) appears to reduce postoperative pulmonary complications and resource use.Major finding: Pulmonary complications occurred in 36.7% of intubated patients, compared with 69.8% for a prospectively evaluated reference population (P less than 0.05).
Data source: A prospective, nonrandomized, two-stage study including 417 subjects who underwent thoracic, upper-abdominal, or aortic open surgical procedure at one of three centers.
Disclosures: The study was sponsored by Hill-Rom, which manufactures the MetaNeb device. Investigators had no financial conflicts related to the study.
Source: Huynh T et al. Critical Care Congress, Abstract 17.
Adenotonsillectomy reduced hypertension in OSA subgroup
after surgery, according to a retrospective analysis.
This is one of the few studies to have ever examined whether adenotonsillectomy for children with OSA had any effects on blood pressure (BP) and was based on “one of the largest cohorts for evaluating postoperative BP changes in nonobese children with OSA,” noted Cho-Hsueh Lee, MD, and colleagues. The report was published in JAMA Otolaryngology–Head & Neck Surgery. Among the previous studies that evaluated BP in children with OSA before and after having this surgery, the results varied, they added.
The researchers analyzed the medical records of 240 nonobese children with clinical symptoms and polysomnography-confirmed OSA (having an apnea-hypopnea index of greater than 1) who underwent adenotonsillectomy. Prior to surgery, 169 patients (70.4%) of the patients were classified as nonhypertensive, while 71 (29.6%) were classified as hypertensive. The children had a mean age of 7.3 years, and 160 were males.
Patients participated in full-night polysomnography (PSG) before surgery and at 3-6 months after adenotonsillectomy in the National Taiwan University Hospital Sleep Center. Apnea episodes were defined as a 90% decrease in airflow for two consecutive breaths. Sleep center staff measured the study participants’ systolic and diastolic BP in a sleep center using an electronic sphygmomanometer, in the evening, prior to the PSG study, and in the morning. Pediatric hypertension was based on the nocturnal BP measurement and was defined as having mean systolic and diastolic BP greater or equal to the 95th percentile for age, sex, and height.
“Postoperatively, hypertensive children had a significant decrease in all BP measures, including nocturnal and morning [systolic] BP ... A total of 47 hypertensive patients (66.2%) became nonhypertensive after surgery,” the researchers said.
For patients who were hypertensive before surgery, the average nocturnal (before PSG) preop systolic BP was 114.3 mm Hg, versus 107.5 mm Hg after surgery. The mean nocturnal diastolic BP for this same group of patients decreased to 65.1 mm Hg from 74.3 mm Hg. Similarly, the average morning (after PSG) systolic BP and diastolic BP were 106.0 mm Hg and 64.4 mm Hg after these patients underwent adenotonsillectomy, compared with 111.8 mm Hg and 71.7 mm Hg prior to surgery, respectively.
The adenotonsillectomy didn’t improve all patients’ BP. For some who were nonhypertensive before surgery, blood pressure increased, with 36 (21.3%) of this group having become hypersensitive after surgery, the researchers acknowledged.
Overall, the cohort experienced significant improvements in several PSG measures, including the average apnea-hypopnea index, which decreased from 12.1 events per hour to 1.7. The total arousal index also declined, going from 6.1 events per hour to 4.2. In addition, the mean oxygen saturation improved from 96.8% to 97.7%.
The investigators described several limitations of the study, including their inability to collect patients’ arterial stiffness, carotid intima thickness, and other cardiovascular measures beyond BP.
They recommended a follow-up study. “Although we observed improvements in BP measures within 6 months after surgery for hypertensive children with OSA, the long-term effects of surgery on BP remain uncertain,” they explained.
The study was supported by grants from the Ministry of Science and Technology, Republic of China (Taiwan). The researchers disclosed no potential conflicts of interest.
SOURCE: Lee, C-H et al. JAMA Otolaryngol Head Neck Surg. 2018 Feb 15. doi: 10.1001/jamaoto.2017.3127.
after surgery, according to a retrospective analysis.
This is one of the few studies to have ever examined whether adenotonsillectomy for children with OSA had any effects on blood pressure (BP) and was based on “one of the largest cohorts for evaluating postoperative BP changes in nonobese children with OSA,” noted Cho-Hsueh Lee, MD, and colleagues. The report was published in JAMA Otolaryngology–Head & Neck Surgery. Among the previous studies that evaluated BP in children with OSA before and after having this surgery, the results varied, they added.
The researchers analyzed the medical records of 240 nonobese children with clinical symptoms and polysomnography-confirmed OSA (having an apnea-hypopnea index of greater than 1) who underwent adenotonsillectomy. Prior to surgery, 169 patients (70.4%) of the patients were classified as nonhypertensive, while 71 (29.6%) were classified as hypertensive. The children had a mean age of 7.3 years, and 160 were males.
Patients participated in full-night polysomnography (PSG) before surgery and at 3-6 months after adenotonsillectomy in the National Taiwan University Hospital Sleep Center. Apnea episodes were defined as a 90% decrease in airflow for two consecutive breaths. Sleep center staff measured the study participants’ systolic and diastolic BP in a sleep center using an electronic sphygmomanometer, in the evening, prior to the PSG study, and in the morning. Pediatric hypertension was based on the nocturnal BP measurement and was defined as having mean systolic and diastolic BP greater or equal to the 95th percentile for age, sex, and height.
“Postoperatively, hypertensive children had a significant decrease in all BP measures, including nocturnal and morning [systolic] BP ... A total of 47 hypertensive patients (66.2%) became nonhypertensive after surgery,” the researchers said.
For patients who were hypertensive before surgery, the average nocturnal (before PSG) preop systolic BP was 114.3 mm Hg, versus 107.5 mm Hg after surgery. The mean nocturnal diastolic BP for this same group of patients decreased to 65.1 mm Hg from 74.3 mm Hg. Similarly, the average morning (after PSG) systolic BP and diastolic BP were 106.0 mm Hg and 64.4 mm Hg after these patients underwent adenotonsillectomy, compared with 111.8 mm Hg and 71.7 mm Hg prior to surgery, respectively.
The adenotonsillectomy didn’t improve all patients’ BP. For some who were nonhypertensive before surgery, blood pressure increased, with 36 (21.3%) of this group having become hypersensitive after surgery, the researchers acknowledged.
Overall, the cohort experienced significant improvements in several PSG measures, including the average apnea-hypopnea index, which decreased from 12.1 events per hour to 1.7. The total arousal index also declined, going from 6.1 events per hour to 4.2. In addition, the mean oxygen saturation improved from 96.8% to 97.7%.
The investigators described several limitations of the study, including their inability to collect patients’ arterial stiffness, carotid intima thickness, and other cardiovascular measures beyond BP.
They recommended a follow-up study. “Although we observed improvements in BP measures within 6 months after surgery for hypertensive children with OSA, the long-term effects of surgery on BP remain uncertain,” they explained.
The study was supported by grants from the Ministry of Science and Technology, Republic of China (Taiwan). The researchers disclosed no potential conflicts of interest.
SOURCE: Lee, C-H et al. JAMA Otolaryngol Head Neck Surg. 2018 Feb 15. doi: 10.1001/jamaoto.2017.3127.
after surgery, according to a retrospective analysis.
This is one of the few studies to have ever examined whether adenotonsillectomy for children with OSA had any effects on blood pressure (BP) and was based on “one of the largest cohorts for evaluating postoperative BP changes in nonobese children with OSA,” noted Cho-Hsueh Lee, MD, and colleagues. The report was published in JAMA Otolaryngology–Head & Neck Surgery. Among the previous studies that evaluated BP in children with OSA before and after having this surgery, the results varied, they added.
The researchers analyzed the medical records of 240 nonobese children with clinical symptoms and polysomnography-confirmed OSA (having an apnea-hypopnea index of greater than 1) who underwent adenotonsillectomy. Prior to surgery, 169 patients (70.4%) of the patients were classified as nonhypertensive, while 71 (29.6%) were classified as hypertensive. The children had a mean age of 7.3 years, and 160 were males.
Patients participated in full-night polysomnography (PSG) before surgery and at 3-6 months after adenotonsillectomy in the National Taiwan University Hospital Sleep Center. Apnea episodes were defined as a 90% decrease in airflow for two consecutive breaths. Sleep center staff measured the study participants’ systolic and diastolic BP in a sleep center using an electronic sphygmomanometer, in the evening, prior to the PSG study, and in the morning. Pediatric hypertension was based on the nocturnal BP measurement and was defined as having mean systolic and diastolic BP greater or equal to the 95th percentile for age, sex, and height.
“Postoperatively, hypertensive children had a significant decrease in all BP measures, including nocturnal and morning [systolic] BP ... A total of 47 hypertensive patients (66.2%) became nonhypertensive after surgery,” the researchers said.
For patients who were hypertensive before surgery, the average nocturnal (before PSG) preop systolic BP was 114.3 mm Hg, versus 107.5 mm Hg after surgery. The mean nocturnal diastolic BP for this same group of patients decreased to 65.1 mm Hg from 74.3 mm Hg. Similarly, the average morning (after PSG) systolic BP and diastolic BP were 106.0 mm Hg and 64.4 mm Hg after these patients underwent adenotonsillectomy, compared with 111.8 mm Hg and 71.7 mm Hg prior to surgery, respectively.
The adenotonsillectomy didn’t improve all patients’ BP. For some who were nonhypertensive before surgery, blood pressure increased, with 36 (21.3%) of this group having become hypersensitive after surgery, the researchers acknowledged.
Overall, the cohort experienced significant improvements in several PSG measures, including the average apnea-hypopnea index, which decreased from 12.1 events per hour to 1.7. The total arousal index also declined, going from 6.1 events per hour to 4.2. In addition, the mean oxygen saturation improved from 96.8% to 97.7%.
The investigators described several limitations of the study, including their inability to collect patients’ arterial stiffness, carotid intima thickness, and other cardiovascular measures beyond BP.
They recommended a follow-up study. “Although we observed improvements in BP measures within 6 months after surgery for hypertensive children with OSA, the long-term effects of surgery on BP remain uncertain,” they explained.
The study was supported by grants from the Ministry of Science and Technology, Republic of China (Taiwan). The researchers disclosed no potential conflicts of interest.
SOURCE: Lee, C-H et al. JAMA Otolaryngol Head Neck Surg. 2018 Feb 15. doi: 10.1001/jamaoto.2017.3127.
FROM JAMA OTOLARYNGOLOGY-HEAD & NECK SURGERY
Key clinical point: Hypertensive children with obstructive sleep apnea (OSA) who had an adenotonsillectomy experienced significant improvements in their blood pressure after surgery.
Major finding: Sixty-six percent of hypertensive patients with OSA became nonhypertensive after adenotonsillectomy.
Study details: A retrospective analysis of 240 nonobese children with OSA who underwent adenotonsillectomy.
Disclosures: The study was supported by grants from the Ministry of Science and Technology, Republic of China (Taiwan). The researchers disclosed no potential conflicts of interest.
Source: Lee, C-H et al. JAMA Otolaryngol Head Neck Surg. 2018 Feb 15. doi: 10.1001/jamaoto.2017.3127.
Patient-Specific Guides/Instrumentation in Shoulder Arthroplasty
ABSTRACT
Optimal outcomes following total shoulder arthroplasty TSA and reverse shoulder arthroplasty RSA are dependent on proper implant position. Multiple cadaver studies have demonstrated improved accuracy of implant positioning with use of patient-specific guides/instrumentation compared to traditional methods. At this time, there are 3 commercially available single use patient-specific instrumentation systems and 1 commercially available reusable patient-specific instrumentation system. Currently though, there are no studies comparing the clinical outcomes of patient-specific guides to those of traditional methods of glenoid placement, and limited research has been done comparing the accuracy of each system’s 3-dimensional planning software. Future work is necessary to elucidate the ideal indications for the use of patient-specific guides and instrumentation, but it is likely, particularly in the setting of advanced glenoid deformity, that these systems will improve a surgeon's ability to put the implant in the best position possible.
Continue to: Optimal functional recovery...
Optimal functional recovery and implant longevity following both total shoulder arthroplasty (TSA) and reverse shoulder arthroplasty (RSA) depend, in large part, on proper placement of the glenoid component. Glenoid component malpositioning has an adverse effect on shoulder stability, range of motion (ROM), impingement, and glenoid implant longevity.
Traditionally, glenoid component positioning has been done manually by the surgeons based on their review of preoperative films and knowledge of glenoid anatomy. Anatomic studies have demonstrated high individual variability in the version of the native glenoid, thus making ideal placement of the initial glenoid guide pin difficult using standard guide pin guides.1
The following 2 methods have been described for improving the accuracy of glenoid guide pin insertion and subsequent glenoid implant placement: (1) computerized navigation and (2) patient-specific guides/instrumentation. Although navigated shoulder systems have demonstrated improved accuracy in glenoid placement compared with traditional methods, navigated systems require often large and expensive systems for implementation. The majority of them also require placement of guide pins or arrays on scapular bony landmarks, likely leading to an increase in operative time and possible iatrogenic complications, including fracture and pin site infections.
This review focuses on the use of patient-specific guides/instrumentation in shoulder arthroplasty. This includes the topic of proper glenoid and glenosphere placement as well as patient-specific guides/instrumentation and their accuracy.
GLENOID PLACEMENT
Glenohumeral osteoarthritis is the most common indication for TSA2 and commonly results in glenoid deformity. Using computed tomography (CT) scans of 45 arthritic shoulders and 19 normal shoulders, Mullaji and colleagues3 reported that the anteroposterior dimensions of the glenoid were increased by an average of 5 mm to 8 mm in osteoarthritic shoulders and by an average of 6 mm in rheumatoid arthritic shoulders compared to those in normal shoulders. A retrospective review of serial CT scans performed preoperatively on 113 osteoarthritic shoulders by Walch and colleagues4 demonstrated an average retroversion of 16°, and it has been the basis for the commonly used Walch classification of glenoid wear in osteoarthritis. Increased glenoid wear and increased glenoid retroversion make the proper restoration of glenoid version, inclination, and offset during shoulder arthroplasty more difficult and lead to increased glenoid component malpositioning.
Continue to: The ideal placement of the glenoid...
The ideal placement of the glenoid to maximize function, ROM, and implant longevity is in a mechanically neutral alignment with no superoinferior inclination1 and neutral version with respect to the transverse axis of the scapula.5
Improper glenoid positioning has an adverse effect on the functional results of shoulder arthroplasty. Yian and colleagues6 evaluated 47 cemented, pegged glenoids using standard radiography and CT scans at a mean follow-up of 40 months. They observed a significant correlation between increased glenoid component retroversion and lower Constant scores. Hasan and colleagues7 evaluated 139 consecutive patients who were dissatisfied with the result of their primary arthroplasty and found that 28% of them had at least 1 substantially malpositioned component identified either on radiography or during a revision surgery. They also found a significant correlation between stiffness, instability, and component malposition in their cohort.
Glenoid longevity is also dependent on proper component positioning, with the worst outcomes coming if the glenoid is malaligned with either superior or inferior inclination. Hasan and colleagues7 found that of their 74 patients with failed TSAs, 44 patients (59%) demonstrated mechanical loosening of their glenoid components either radiographically or during revision surgery, and 10 of their 44 patients with loose glenoids (23%) also had a malpositioned component. Using finite element analysis, Hopkins and colleagues8 analyzed the stresses through the cement mantle in glenoid prostheses that were centrally aligned, superiorly inclined, inferiorly inclined, anteverted, and retroverted. They found that malalignment of the glenoid increases the stresses through the cement mantle, leading to increased likelihood of mantle failure compared to that of centrally aligned glenoids, especially if there is malalignment with superior or inferior inclination or retroversion.
The accuracy of traditional methods of glenoid placement using an initial guide pin is limited and decreases with increasing amounts of glenoid deformity and retroversion. Iannotti and colleagues 9 investigated 13 patients undergoing TSA with an average preoperative retroversion of 13° and evaluated them using a 3-dimensional (3-D) surgical simulator. They found that the postoperative glenoid version was within 5° of ideal version in only 7 of their 13 patients (54%) and within 10° of ideal version in only 10 of their 13 patients (77%). In their study, the ideal version was considered to be the version as close to perpendicular to the plane of the scapula as possible with complete contact of the back side of the component on glenoid bone and maintenance of the center peg of the component within bone. In addition, they found that of their 7 patients with preoperative retroversion >10°, only 1 patient (14%) had a postoperative glenoid with <10° of retroversion with regard to the plane of the scapula and that all 6 of their patients with preoperative glenoid retroversion of <10° had a postoperative glenoid version of <10°.
Preoperative CT scans are much more accurate at determining glenoid version and thus how much glenoid correction is required to reestablish neutral version than plain radiography. Nyffeler and colleagues10 compared CT scans with axillary views for comparing glenoid version in 25 patients with no shoulder prosthesis present and 25 patients with a TSA in place. They found that glenoid retroversion was overestimated on plain radiographs in 86% of their patients with an average difference between CT and plain radiography of 6.4° and a maximum difference of 21°. They also found poor interobserver reliability in the plain radiography group and good interobserver reliability in the CT group, with coefficients of correlation of 0.77 for the plain radiography group and 0.93 for the CT group. Thus, they concluded that glenoid version cannot be accurately measured by plain radiography and that CT should be used. Hoenecke and colleagues11 subsequently evaluated 33 patients scheduled for TSA and found that CT version measurements made on 2-dimensional (2-D) CT slices compared with 3-D-reconstructed models of the same CT slices differed by an average of 5.1° because the axial CT slices were most often made perpendicular to the axis of the patient’s torso and not perpendicular to the body of the scapula. Accurate version assessment is critically important in planning for the degree of correction required to restore neutral glenoid version, and differences of 6.4° between CT assessment and plain radiography, and 5.1° between 2-D and 3-D CT scan assessments may lead to inadequate version correction intraoperatively and inferior postoperative results.
Continue to: GLENOSPHERE PLACEMENT
GLENOSPHERE PLACEMENT
The most common indication for reverse TSA is rotator cuff arthropathy characterized by rotator cuff dysfunction and end-stage glenohumeral arthritis.12 These patients require accurate and reproducible glenoid placement to optimize their postoperative range of motion and stability and minimize scapular notching.
Ideal glenosphere placement is the location and orientation that maximizes impingement-free ROM and stability while avoiding notching. Individual patient anatomy determines ideal placement; however, several guidelines for placement include inferior translation on the glenoid with neutral to inferior inclination. Gutiérrez and colleagues13 developed a computer model to assess the hierarchy of surgical factors affecting the ROM after a reverse TSA. They found that lateralizing the center of rotation gave the largest increase in impingement-free abduction, followed closely by inferior translation of the glenosphere on the glenoid.
Avoiding scapular notching is also a very important factor in ideal glenosphere placement. Scapular notching can be described as impingement of the humeral cup against the scapular neck during arm adduction and/or humeral rotation. Gutiérrez and colleagues13 also found that decreasing the neck shaft angle to create a more varus proximal humerus was the most important factor in increasing the impingement-free adduction. Roche and colleagues14 reviewed the radiographs of 151 patients who underwent primary reverse TSA at a mean follow-up of 28.3 months postoperatively; they found that 13.2% of their patients had a notch and that, on average, their patients who had no scapular notch had significantly more inferior glenosphere overhang than those who had a scapular notch. Poon and colleagues15 found that a glenosphere overhang of >3.5 mm prevented notching in their randomized control trial comparing concentrically and eccentrically placed glenospheres. Multiple other studies have demonstrated similar results and recommended inferior glenoid translation and inferior glenoid inclination to avoid scapular notching.16,17 Lévigne and colleagues18 retrospectively reviewed 337 reverse TSAs and observed a correlation between scapular notching and radiolucencies around the glenosphere component, with 14% of patients with scapular notching displaying radiolucencies vs 4% of patients without scapula notching displaying radiolucencies.
Several studies have also focused on the ideal amount of inferior glenoid inclination to maximize impingement-free ROM. Li and colleagues17 performed a computer simulation study on the Comprehensive Reverse Shoulder System (Zimmer Biomet) to determine impingement-free internal and external ROM with varying amounts of glenosphere offset, translation, and inclination. They found that progressive glenosphere inferior inclination up to 30° improved impingement-free rotational ROM at all degrees of scaption. Gutiérrez and colleagues19 used computer modeling to compare concentrically placed glenospheres in neutral inclination with eccentrically placed glenospheres in varying degrees of inclination. They found that the lowest forces across the baseplate occurred in the lateralized and inferiorly inclined glenospheres, and the highest forces occurred in the lateralized and superiorly inclined glenospheres. Together, these studies show that inferior glenoid inclination increases impingement-free ROM and, combined with lateralization, may result in improved glenosphere longevity due to significantly decreased forces at the RSA glenoid baseplate when compared to that at superiorly inclined glenoids.
The ideal amount of mediolateral glenosphere offset has not been well defined. Grammont design systems place the center of rotation of the glenosphere medial to the glenoid baseplate together with valgus humeral component neck shaft angles of around 155°. These design elements are believed to decrease shear stresses through the glenoid baseplate to the glenoid interface and improve shoulder stability, but they are also associated with reduced impingement-free ROM and increased rates of scapular notching.13 This effect is accentuated in patients with preexisting glenoid bone loss and/or congenitally short scapular necks that further medialize the glenosphere. Medialization of the glenosphere may also shorten the remaining rotator cuff muscles and result in decreased implant stability and external rotation strength. Several implant systems have options to vary the amount of lateral offset. The correct amount of lateral offset for each patient requires the understanding that improving patients’ impingement-free ROM by increasing the amount of lateral offset comes at the price of increasing the shear forces experienced by the interface between the glenoid baseplate and the glenoid. As glenoid fixation technology improves increased lateralization of glenospheres without increased rates of glenoid baseplate, loosening should improve the ROM after reverse TSA.
Continue to: Regardless of the intraoperative goals...
Regardless of the intraoperative goals for placement and orientation of the glenosphere components, it is vitally important to accurately and consistently meet those goals for achieving optimal patient outcomes. Verborgt and colleagues20 implanted 7 glenospheres in cadaveric specimens without any glenohumeral arthritis using standard techniques to evaluate the accuracy of glenosphere version and inclination. Their goal was to place components in neutral version and with 10° of inferior inclination. Their average glenoid version postoperatively was 8.7° of anteversion, and their average inclination was 0.9° of superior inclination. Throckmorton and colleagues21 randomized 35 cadaveric shoulders to receive either an anatomic or a reverse total shoulder prosthesis from high-, mid-, and low-volume surgeons. They found that components placed using traditional guides averaged 6° of deviation in version and 5° of deviation in inclination from their target values, with no significant differences between surgeons of different volumes.
PATIENT-SPECIFIC GUIDES/INSTRUMENTATION
Patient-specific guides/instrumentation and intraoperative navigation are the 2 techniques that have been used to improve the accuracy of glenoid and glenosphere placement. Both techniques require the use of high-resolution CT scans and computer software to determine the proper position for glenoid or glenosphere placement based on the patient’s individual anatomy. Patient-specific guides and instrumentation use the data acquired from a CT scan to generate a preoperative plan for the location and orientation of the glenoid baseplate. Once the surgeon approves the preoperative plan, a patient-specific guide is created using the patient’s glenoid as a reference for the location and orientation of the central guide pin. The location of the central guide pin on the glenoid determines the center of the glenoid baseplate, and the guide pin’s orientation determines the version and inclination of the glenoid or the glenosphere. Once the guide pin is placed in the glenoid, the remainder of the glenoid implantation uses the guide pin as a reference, and, in that way, patient-specific guides control the orientation of the glenoid at the time of surgery.
Intraoperative navigation uses an optical tracking system to determine the location and orientation of the central guide pin. Navigation systems require intraoperative calibration of the optical tracking system before they can track the location of implantation relative to bony landmarks on the patient’s scapula. Their advantage over patient-specific instrumentation (PSI) is that they do not require the manufacture of a custom guide; however, they may add significantly increased cost and surgical time due to the need for calibration prior to use and the cost of the navigation system along with any disposable components associated with it. Kircher and colleagues22 performed a prospective randomized clinical study of navigation-aided TSA compared with conventional TSA and found that operating time was significantly increased for the navigated group with an average operating room time of 169.5 minutes compared to 138 minutes for the conventional group. They also found that navigation had to be abandoned in 37.5% of their navigated patients due to technical errors during glenoid referencing.
COMMERCIAL PATIENT-SPECIFIC INSTRUMENTATION SYSTEMS
The 2 types of PSI that are currently available are single-use PSI and reusable PSI. The single-use PSI involves the fabrication of unique guides based on surgeon-approved preoperative plans generated by computer-software-processed preoperative CT scans. The guides are fabricated to rest on the glenoid articular surface and direct the guide pin to the correct location and in the correct direction to place the glenoid baseplate in the desired position with the desired version and inclination. Most of these systems also provide a 3-D model of the patient’s glenoid so that surgeons can visualize glenoid deformities and the correct guide placement on the glenoid. Single-use PSI systems are available from DJO Global, Wright Medical Group, and Zimmer Biomet. The second category of PSI is reusable and is available from Arthrex. The guide pin for this system is adjusted to fit individual patient anatomy and guide the guide pin into the glenoid in a location and orientation preplanned on the CT-scan-based computer software or using a 3-D model of the patient’s glenoid (Table).
Table. Details of Available Patient-Specific Instrumentation Systems
| System | Manufacturer | Single-Use/Reusable | Guides |
| MatchPoint System | DJO Global | Single-use | Central guide pin |
| Blueprint 3D Planning + PSI | Wright Medical Group | Single-use | Central guide pin |
| Zimmer Patient Specific Instruments Shoulder | Zimmer Biomet | Single-use | Central guide pin, reaming guide, roll guide, screw drill guide |
| Virtual Implant Positioning System | Arthrex | Reusable | Central guide pin |
The DJO Global patient-specific guide is termed as the MatchPoint System. This system creates 3-D renderings of the scapula and allows the surgeon to manipulate the glenoid baseplate on the scapula. The surgeon chooses the glenoid baseplate, location, version, and inclination on the computerized 3-D model. The system then fabricates a guide pin matching the computerized template that references the patient’s glenoid surface with a hook to orient it against the coracoid. A 3-D model of the glenoid is also provided along with the customized guide pin.
Continue to: Blueprint 3D Planning + PSI...
Blueprint 3D Planning + PSI (Wright Medical Group) allows custom placement of the glenoid version, inclination, and position on computerized 3-D models of the patient’s scapula. This PSI references the glenoid with 4 feet that captures the edge of the patient’s glenoid at specific locations and is unique because it allows the surgeon to control where on the glenoid edge to 4 feet contact as long as 1 foot is placed on the posterior edge of the glenoid and the remaining 3 feet are placed on the anterior edge of the glenoid. A 3-D model of the glenoid is also provided with this guide.
The Zimmer Biomet patient-specific guide is termed as the Zimmer Patient Specific Instruments Shoulder. Its computer software allows custom placement of the glenoid as well, but it also includes computerized customization of the reaming depth, screw angles, and screw lengths to optimize fixation. Their system includes a central guide pin to set the glenoid baseplate’s location and orientation, a reaming guide to control reaming depth and direction, a roll guide to control the glenoid baseplate’s rotation, and a drill guide to control the screw direction. They also provide a 3-D model of the glenoid.
The Arthrex Virtual Implant Positioning (VIP) System is similar to other systems in that its 3-D planning software is based on CT images uploaded by the surgeon. The unique aspect of this system is that the guide pin is adjusted by the surgeon for each individual patient based on instructions generated by the planning software; however, after use, the instruments are resterilized and reused on subsequent patients (Figures 1A, 1B). In this manner, their instruments are reusable and allow custom adjustment for each patient with the ability to set the pin location and glenoid version in a patient-specific manner. This has the potential benefit of keeping costs down. For more complex deformity cases, the Arthrex VIP System can also 3-D-print a sterile model of the glenoid to help surgeons appreciate the deformity better (Figure 2).

DATA ON PATIENT-SPECIFIC INSTRUMENTS
Several studies have measured the accuracy of patient-specific guides and have compared the accuracy of patient-specific guides to that of traditional methods. Levy and colleagues23 investigated the accuracy of single-use patient-specific guides compared to that of preoperative plans. They used patient-specific guides on 14 cadaveric shoulders based on plans developed by virtual preoperative 3-D planning system using CT images. Once the guide pin was drilled using the patient-specific guide, they obtained a second CT scan to compare the accuracy of the patient-specific guide to the surgical plan generated preoperatively. They found that the translational accuracy of the starting point for the guide pin averaged 1.2 mm ± 0.7 mm, the accuracy of the inferior inclination was 1.2° ± 1.2°, and the accuracy of the glenoid version was 2.6° ± 1.7°. They concluded that patient-specific guides were highly accurate in reproducing the starting point, inclination, and version set on preoperative guides.
Walch and colleagues24 subsequently performed a similar study using 15 cadaveric scapulae without any other shoulder soft tissue or bone attached. They also used CT-scan-based 3-D planning software to plan their glenoid placement with a subsequently fabricated single-use patient-specific guide used to place a guide pin. They obtained a second CT scan after guide pin implantation and compared the preoperative plan with the subsequent guide pin. They found a mean entry point position error of 1.05 mm ± 0.31 mm, a mean inclination error of 1.42° ± 1.37°, and a mean version error of 1.64° ± 1.01°.
Continue to: Throckmorton and colleagues...
Throckmorton and colleagues21 used 70 cadaveric shoulders with radiographically confirmed arthritis and randomized them to undergo either anatomic or reverse TSA using either a patient-specific guide or standard instrumentation. Postoperative CT scans were used to evaluate the glenoid inclination, version, and starting point. They found that glenoid components implanted using patient-specific guides were more accurate than those placed using traditional instrumentation. The average deviation from intended inclination was 3° for patient-specific guides and 7° for traditional instrumentation, the average deviation from intended version was 5° for patient-specific guides and 8° for traditional instrumentation, and the average deviation in intended starting point was 2 mm for patient-specific guides and 3 mm for traditional instrumentation. They also analyzed significantly malpositioned components as defined by a variation in version or inclination of >10° or >4 mm in starting point. They found that 6 of their 35 glenoids using patient-specific guides were significantly malpositioned compared to 23 of 35 glenoids using traditional instrumentation. They concluded that patient-specific guides were more accurate and reduced the number of significantly malpositioned implants when compared with traditional instrumentation.
Early and colleagues25 analyzed the effect of severe glenoid bone defects on the accuracy of patient-specific guides compared with traditional guides. Using 10 cadaveric shoulders, they created anterior, central, or posterior glenoid defects using a reamer and chisel to erode the bone past the coracoid base. Subsequent CT scans were performed on the specimens, and patient-specific guides were fabricated and used for reverse TSA in 5 of the 10 specimens. A reverse TSA was performed using traditional instrumentation in the remaining 5 specimens. They found that the average deviation in inclination and version from preoperative plan was more accurate in the patient-specific guide cohort than that in the traditional instrument cohort, with an average deviation in inclination and version of 1.2° ± 1.2° and 1.8° ± 1.2° respectively for the cohort using patient-specific instruments vs 2.8° ± 1.8° and 3.5° ± 3° for the cohort using traditional instruments. They also found that their total bone screw lengths were longer in the patient-specific guide group than those in the traditional group, with screws averaging 52% of preoperatively planned length in the traditional instrument cohort vs 89% of preoperatively planned length in the patient-specific instrument cohort.
Gauci and colleagues26 measured the accuracy of patient-specific guides in vivo in 17 patients receiving TSA. Preoperative CT scans were used to fabricate patient-specific guides, and postoperative CT scans were used to measure version, inclination, and error of entry in comparison with the templated goals used to create patient-specific guides. They found a mean error in version and inclination of 3.4° and 1.8°, respectively, and a mean error in entry of 0.9 mm of translation on the glenoid. Dallalana and colleagues27 performed a very similar study on 20 patients and found a mean deviation in glenoid version of 1.8° ± 1.9°, a mean deviation in glenoid inclination of 1.3° ± 1.0°, a mean translation in anterior-posterior plane of 0.5 mm ± 0.3 mm, and a mean translation in the superior-inferior plane of 0.8 mm ± 0.5 mm.
Hendel and colleagues28 performed a randomized prospective clinical trial comparing patient-specific guides with traditional methods for glenoid insertion. They randomized 31 patients to receive a glenoid implant using either a patient-specific guide or traditional methods and compared glenoid retroversion and inclination with their preoperative plan. They found an average version deviation of 6.9° in the traditional method cohort and 4.3° in the patient-specific guide cohort. Their average deviation in inclination was 11.6° in the traditional method cohort and 2.9° in the patient-specific guide cohort. For patients with preoperative retroversion >16°, the average deviation was 10° in the standard surgical cohort and 1.2° in the patient-specific instrument cohort. Their data suggest that increasing preoperative retroversion leads to an increased version variation from preoperative plan.
Iannotti and colleagues29 randomly assigned 46 patients to preoperatively undergo either CT scan with 3-D templating of glenoid component without patient-specific guide fabrication or CT scan with 3-D templating and patient-specific guide fabrication prior to receiving a TSA. They recorded the postoperative inclination and version for each patient and compared them to those of a nonrandomized control group of 17 patients who underwent TSA using standard instrumentation. They found no difference between the cohorts with or without patient-specific guide use with regard to implant location, inclination, or version; however, they did find a difference between the combined 3-D templating cohort compared with their standard instrumentation cohort. They concluded that 3-D templating significantly improved the surgeons’ ability to correctly position the glenoid component with or without the fabrication and the use of a patient-specific guide.
Continue to: Denard and colleagues...
Denard and colleagues30 compared the preoperative glenoid version and inclination measurements obtained using the Blueprint 3D Planning + PSI software and the VIP System 3D planning software. They analyzed the preoperative CT scans of 63 consecutive patients undergoing either TSA or reverse TSA using both the Blueprint and the VIP System 3D planning software and compared the resulting native glenoid version and inclination measured by the software. They found a statistically significant difference (P = 0.04) in the version measurements provided by the different planning software; however, the differences found in inclination did not reach statistical significance (P = 0.463). In 19 of the 63 patients (30%), the version measurements between the systems were >5°, and in 29 of the 63 patients (46%), the inclination measurements between the systems were 5° or greater. In addition, 12 of the 63 patients (19%) had both version and inclination measurement differences of >5° between the systems. In total, they found that 35 of the 63 patients had at least 1 measurement that varied by >5° between the systems, and that in 15 patients (24%), 1 measurement varied by >10°. Their data demonstrate considerable variability in the preoperative measurements provided by different 3-D planning software systems, and that further study of each commercially available 3-D planning software system is needed to evaluate their accuracy.
CONCLUSION
Optimal outcomes following TSA and reverse TSA are dependent on proper implant position. Multiple studies have demonstrated improved accuracy in implant positioning with the use of patient-specific guides compared to that with traditional methods. Currently, there are no studies comparing the clinical outcomes of patient-specific guides to those of traditional methods of glenoid placement, and limited research had been done comparing the accuracy of each system’s 3-D planning software with each other and with standardized measurements of glenoid version and inclination. Further research is required to determine the accuracy of each commercially available 3-D planning software system as well as the clinical benefit of patient-specific guides in shoulder arthroplasty.
1. Churchill RS, Brems JJ, Kotschi H. Glenoid size, inclination, and version: an anatomic study. J Shoulder Elbow Surg. 2001;10(4):327-332. doi:10.1067/mse.2001.115269.
2. Norris TR, Iannotti JP. Functional outcome after shoulder arthroplasty for primary osteoarthritis: a multicenter study. J Shoulder Elbow Surg. 2002;11(2):130-135. doi:10.1067/mse.2002.121146.
3. Mullaji AB, Beddow FH, Lamb GH. CT measurement of glenoid erosion in arthritis. J Bone Joint Surg Br. 1994;76(3):384-388.
4. Walch G, Badet R, Boulahia A, Khoury A. Morphologic study of the glenoid in primary glenohumeral osteoarthritis. J Arthroplasty. 1999;14(6):756-760.
5. RJ Friedman, KB Hawthorne, BM Genez. The use of computerized tomography in the measurement of glenoid version. J Bone Joint Surg. 1992;74(7):1032-1037. doi:10.2106/00004623-199274070-00009.
6. Yian EH, Werner CM, Nyffeler RW, et al. Radiographic and computed tomography analysis of cemented pegged polyethylene glenoid components in total shoulder replacement. J Bone Joint Surg. 2005;87(9):1928-1936. doi:10.2106/00004623-200509000-00004.
7. Hasan SS, Leith JM, Campbell B, Kapil R, Smith KL, Matsen FA. Characteristics of unsatisfactory shoulder arthroplasties. J Shoulder Elbow Surg. 2002;11(5):431-441.
8. Hopkins AR, Hansen UN, Amis AA, Emery R. The effects of glenoid component alignment variations on cement mantle stresses in total shoulder arthroplasty. J Shoulder Elbow Surg. 2004;13(6):668-675. doi:10.1016/S1058274604001399.
9. Iannotti JP, Greeson C, Downing D, Sabesan V, Bryan JA. Effect of glenoid deformity on glenoid component placement in primary shoulder arthroplasty. J Shoulder Elbow Surg. 2012;21(1):48-55. doi:10.1016/j.jse.2011.02.011.
10. Nyffeler RW, Jost B, Pfirrmann CWA, Gerber C. Measurement of glenoid version: conventional radiographs versus computed tomography scans. J Shoulder Elbow Surg. 2003;12(5):493-496. doi:10.1016/S1058274603001812.
11. Hoenecke HR, Hermida JC, Flores-Hernandez C, D'Lima DD. Accuracy of CT-based measurements of glenoid version for total shoulder arthroplasty. J Shoulder Elbow Surg. 2010;19(2):166-171. doi:10.1016/j.jse.2009.08.009.
12. Wall B, Nové-Josserand L, O'Connor DP, Edwards TB, Walch G. Reverse total shoulder arthroplasty: a review of results according to etiology. J Bone Joint Surg Am. 2007;89(7):1476-1485. doi:10.2106/JBJS.F.00666.
13. Gutiérrez S, Comiskey 4, Charles A, Luo Z, Pupello DR, Frankle MA. Range of impingement-free abduction and adduction deficit after reverse shoulder arthroplasty. hierarchy of surgical and implant-design-related factors. J Bone Joint Surg Am. 2008;90(12):2606-2615. doi:10.2106/JBJS.H.00012.
14. Roche CP, Marczuk Y, Wright TW, et al. Scapular notching and osteophyte formation after reverse shoulder replacement: Radiological analysis of implant position in male and female patients. Bone Joint J. 2013;95-B(4):530-535. doi:10.1302/0301-620X.95B4.30442.
15. Poon PC, Chou J, Young SW, Astley T. A comparison of concentric and eccentric glenospheres in reverse shoulder arthroplasty: a randomized controlled trial. J Bone Joint Surg Am. 2014;96(16):e138. doi:10.2106/JBJS.M.00941.
16. Nyffeler RW, Werner CML, Gerber C. Biomechanical relevance of glenoid component positioning in the reverse delta III total shoulder prosthesis. J Shoulder Elbow Surg. 2005;14(5):524-528. doi:10.1016/j.jse.2004.09.010.
17. Li X, Knutson Z, Choi D, et al. Effects of glenosphere positioning on impingement-free internal and external rotation after reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22(6):807-813. doi:10.1016/j.jse.2012.07.013.
18. Lévigne C, Boileau P, Favard L, et al. Scapular notching in reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2008;17(6):925-935. doi:10.1016/j.jse.2008.02.010.
19. Gutiérrez S, Walker M, Willis M, Pupello DR, Frankle MA. Effects of tilt and glenosphere eccentricity on baseplate/bone interface forces in a computational model, validated by a mechanical model, of reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2011;20(5):732-739. doi:10.1016/j.jse.2010.10.035.
20. Verborgt O, De Smedt T, Vanhees M, Clockaerts S, Parizel PM, Van Glabbeek F. Accuracy of placement of the glenoid component in reversed shoulder arthroplasty with and without navigation. J Shoulder Elbow Surg. 2011;20(1):21-26. doi:10.1016/j.jse.2010.07.014.
21. Throckmorton TW, Gulotta LV, Bonnarens FO, et al. Patient-specific targeting guides compared with traditional instrumentation for glenoid component placement in shoulder arthroplasty: A multi-surgeon study in 70 arthritic cadaver specimens. J Shoulder Elbow Surg. 2015;24(6):965-971. doi:10.1016/j.jse.2014.10.013.
22. Kircher J, Wiedemann M, Magosch P, Lichtenberg S, Habermeyer P. Improved accuracy of glenoid positioning in total shoulder arthroplasty with intraoperative navigation: a prospective-randomized clinical study. J Shoulder Elbow Surg. 2009;18(4):515-520. doi:10.1016/j.jse.2009.03.014.
23. Levy JC, Everding NG, Frankle MA, Keppler LJ. Accuracy of patient-specific guided glenoid baseplate positioning for reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(10):1563-1567. doi:10.1016/j.jse.2014.01.051.
24. Walch G, Vezeridis PS, Boileau P, Deransart P, Chaoui J. Three-dimensional planning and use of patient-specific guides improve glenoid component position: an in vitro study. J Shoulder Elbow Surg. 2015;24(2):302-309. doi:10.1016/j.jse.2014.05.029.
25. Eraly K, Stoffelen D, Vander Sloten J, Jonkers I, Debeer P. A patient-specific guide for optimizing custom-made glenoid implantation in cases of severe glenoid defects: an in vitro study. J Shoulder Elbow Surg. 2016;25(5):837-845. doi:10.1016/j.jse.2015.09.034.
26. Gauci MO, Boileau P, Baba M, Chaoui J, Walch G. Patient-specific glenoid guides provide accuracy and reproducibility in total shoulder arthroplasty. Bone Joint J. 2016;98-B(8):1080-1085. doi:10.1302/0301-620X.98B8.37257.
27. Dallalana RJ, McMahon RA, East B, Geraghty L. Accuracy of patient-specific instrumentation in anatomic and reverse total shoulder arthroplasty. Int J Shoulder Surg. 2016;10(2):59-66. doi:10.4103/09736042.180717.
28. Hendel MD, Bryan JA, Barsoum WK, et al. Comparison of patient-specific instruments with standard surgical instruments in determining glenoid component position: a randomized prospective clinical trial. J Bone Joint Surg. 2012;94(23):2167-2175. doi:10.2106/JBJS.K.01209.
29. Iannotti JP, Weiner S, Rodriguez E, et al. Three-dimensional imaging and templating improve glenoid implant positioning. J Bone Joint Surg. 2015;97(8):651-658. doi:10.2106/JBJS.N.00493.
30. Denard PJ, Provencher MT, Lädermann A, Romeo AA, Dines JS. Version and inclination obtained with 3D planning in total shoulder arthroplasty: do different programs produce the same results? SECEC-ESSSE Congress, Berlin 2017. 2017.
ABSTRACT
Optimal outcomes following total shoulder arthroplasty TSA and reverse shoulder arthroplasty RSA are dependent on proper implant position. Multiple cadaver studies have demonstrated improved accuracy of implant positioning with use of patient-specific guides/instrumentation compared to traditional methods. At this time, there are 3 commercially available single use patient-specific instrumentation systems and 1 commercially available reusable patient-specific instrumentation system. Currently though, there are no studies comparing the clinical outcomes of patient-specific guides to those of traditional methods of glenoid placement, and limited research has been done comparing the accuracy of each system’s 3-dimensional planning software. Future work is necessary to elucidate the ideal indications for the use of patient-specific guides and instrumentation, but it is likely, particularly in the setting of advanced glenoid deformity, that these systems will improve a surgeon's ability to put the implant in the best position possible.
Continue to: Optimal functional recovery...
Optimal functional recovery and implant longevity following both total shoulder arthroplasty (TSA) and reverse shoulder arthroplasty (RSA) depend, in large part, on proper placement of the glenoid component. Glenoid component malpositioning has an adverse effect on shoulder stability, range of motion (ROM), impingement, and glenoid implant longevity.
Traditionally, glenoid component positioning has been done manually by the surgeons based on their review of preoperative films and knowledge of glenoid anatomy. Anatomic studies have demonstrated high individual variability in the version of the native glenoid, thus making ideal placement of the initial glenoid guide pin difficult using standard guide pin guides.1
The following 2 methods have been described for improving the accuracy of glenoid guide pin insertion and subsequent glenoid implant placement: (1) computerized navigation and (2) patient-specific guides/instrumentation. Although navigated shoulder systems have demonstrated improved accuracy in glenoid placement compared with traditional methods, navigated systems require often large and expensive systems for implementation. The majority of them also require placement of guide pins or arrays on scapular bony landmarks, likely leading to an increase in operative time and possible iatrogenic complications, including fracture and pin site infections.
This review focuses on the use of patient-specific guides/instrumentation in shoulder arthroplasty. This includes the topic of proper glenoid and glenosphere placement as well as patient-specific guides/instrumentation and their accuracy.
GLENOID PLACEMENT
Glenohumeral osteoarthritis is the most common indication for TSA2 and commonly results in glenoid deformity. Using computed tomography (CT) scans of 45 arthritic shoulders and 19 normal shoulders, Mullaji and colleagues3 reported that the anteroposterior dimensions of the glenoid were increased by an average of 5 mm to 8 mm in osteoarthritic shoulders and by an average of 6 mm in rheumatoid arthritic shoulders compared to those in normal shoulders. A retrospective review of serial CT scans performed preoperatively on 113 osteoarthritic shoulders by Walch and colleagues4 demonstrated an average retroversion of 16°, and it has been the basis for the commonly used Walch classification of glenoid wear in osteoarthritis. Increased glenoid wear and increased glenoid retroversion make the proper restoration of glenoid version, inclination, and offset during shoulder arthroplasty more difficult and lead to increased glenoid component malpositioning.
Continue to: The ideal placement of the glenoid...
The ideal placement of the glenoid to maximize function, ROM, and implant longevity is in a mechanically neutral alignment with no superoinferior inclination1 and neutral version with respect to the transverse axis of the scapula.5
Improper glenoid positioning has an adverse effect on the functional results of shoulder arthroplasty. Yian and colleagues6 evaluated 47 cemented, pegged glenoids using standard radiography and CT scans at a mean follow-up of 40 months. They observed a significant correlation between increased glenoid component retroversion and lower Constant scores. Hasan and colleagues7 evaluated 139 consecutive patients who were dissatisfied with the result of their primary arthroplasty and found that 28% of them had at least 1 substantially malpositioned component identified either on radiography or during a revision surgery. They also found a significant correlation between stiffness, instability, and component malposition in their cohort.
Glenoid longevity is also dependent on proper component positioning, with the worst outcomes coming if the glenoid is malaligned with either superior or inferior inclination. Hasan and colleagues7 found that of their 74 patients with failed TSAs, 44 patients (59%) demonstrated mechanical loosening of their glenoid components either radiographically or during revision surgery, and 10 of their 44 patients with loose glenoids (23%) also had a malpositioned component. Using finite element analysis, Hopkins and colleagues8 analyzed the stresses through the cement mantle in glenoid prostheses that were centrally aligned, superiorly inclined, inferiorly inclined, anteverted, and retroverted. They found that malalignment of the glenoid increases the stresses through the cement mantle, leading to increased likelihood of mantle failure compared to that of centrally aligned glenoids, especially if there is malalignment with superior or inferior inclination or retroversion.
The accuracy of traditional methods of glenoid placement using an initial guide pin is limited and decreases with increasing amounts of glenoid deformity and retroversion. Iannotti and colleagues 9 investigated 13 patients undergoing TSA with an average preoperative retroversion of 13° and evaluated them using a 3-dimensional (3-D) surgical simulator. They found that the postoperative glenoid version was within 5° of ideal version in only 7 of their 13 patients (54%) and within 10° of ideal version in only 10 of their 13 patients (77%). In their study, the ideal version was considered to be the version as close to perpendicular to the plane of the scapula as possible with complete contact of the back side of the component on glenoid bone and maintenance of the center peg of the component within bone. In addition, they found that of their 7 patients with preoperative retroversion >10°, only 1 patient (14%) had a postoperative glenoid with <10° of retroversion with regard to the plane of the scapula and that all 6 of their patients with preoperative glenoid retroversion of <10° had a postoperative glenoid version of <10°.
Preoperative CT scans are much more accurate at determining glenoid version and thus how much glenoid correction is required to reestablish neutral version than plain radiography. Nyffeler and colleagues10 compared CT scans with axillary views for comparing glenoid version in 25 patients with no shoulder prosthesis present and 25 patients with a TSA in place. They found that glenoid retroversion was overestimated on plain radiographs in 86% of their patients with an average difference between CT and plain radiography of 6.4° and a maximum difference of 21°. They also found poor interobserver reliability in the plain radiography group and good interobserver reliability in the CT group, with coefficients of correlation of 0.77 for the plain radiography group and 0.93 for the CT group. Thus, they concluded that glenoid version cannot be accurately measured by plain radiography and that CT should be used. Hoenecke and colleagues11 subsequently evaluated 33 patients scheduled for TSA and found that CT version measurements made on 2-dimensional (2-D) CT slices compared with 3-D-reconstructed models of the same CT slices differed by an average of 5.1° because the axial CT slices were most often made perpendicular to the axis of the patient’s torso and not perpendicular to the body of the scapula. Accurate version assessment is critically important in planning for the degree of correction required to restore neutral glenoid version, and differences of 6.4° between CT assessment and plain radiography, and 5.1° between 2-D and 3-D CT scan assessments may lead to inadequate version correction intraoperatively and inferior postoperative results.
Continue to: GLENOSPHERE PLACEMENT
GLENOSPHERE PLACEMENT
The most common indication for reverse TSA is rotator cuff arthropathy characterized by rotator cuff dysfunction and end-stage glenohumeral arthritis.12 These patients require accurate and reproducible glenoid placement to optimize their postoperative range of motion and stability and minimize scapular notching.
Ideal glenosphere placement is the location and orientation that maximizes impingement-free ROM and stability while avoiding notching. Individual patient anatomy determines ideal placement; however, several guidelines for placement include inferior translation on the glenoid with neutral to inferior inclination. Gutiérrez and colleagues13 developed a computer model to assess the hierarchy of surgical factors affecting the ROM after a reverse TSA. They found that lateralizing the center of rotation gave the largest increase in impingement-free abduction, followed closely by inferior translation of the glenosphere on the glenoid.
Avoiding scapular notching is also a very important factor in ideal glenosphere placement. Scapular notching can be described as impingement of the humeral cup against the scapular neck during arm adduction and/or humeral rotation. Gutiérrez and colleagues13 also found that decreasing the neck shaft angle to create a more varus proximal humerus was the most important factor in increasing the impingement-free adduction. Roche and colleagues14 reviewed the radiographs of 151 patients who underwent primary reverse TSA at a mean follow-up of 28.3 months postoperatively; they found that 13.2% of their patients had a notch and that, on average, their patients who had no scapular notch had significantly more inferior glenosphere overhang than those who had a scapular notch. Poon and colleagues15 found that a glenosphere overhang of >3.5 mm prevented notching in their randomized control trial comparing concentrically and eccentrically placed glenospheres. Multiple other studies have demonstrated similar results and recommended inferior glenoid translation and inferior glenoid inclination to avoid scapular notching.16,17 Lévigne and colleagues18 retrospectively reviewed 337 reverse TSAs and observed a correlation between scapular notching and radiolucencies around the glenosphere component, with 14% of patients with scapular notching displaying radiolucencies vs 4% of patients without scapula notching displaying radiolucencies.
Several studies have also focused on the ideal amount of inferior glenoid inclination to maximize impingement-free ROM. Li and colleagues17 performed a computer simulation study on the Comprehensive Reverse Shoulder System (Zimmer Biomet) to determine impingement-free internal and external ROM with varying amounts of glenosphere offset, translation, and inclination. They found that progressive glenosphere inferior inclination up to 30° improved impingement-free rotational ROM at all degrees of scaption. Gutiérrez and colleagues19 used computer modeling to compare concentrically placed glenospheres in neutral inclination with eccentrically placed glenospheres in varying degrees of inclination. They found that the lowest forces across the baseplate occurred in the lateralized and inferiorly inclined glenospheres, and the highest forces occurred in the lateralized and superiorly inclined glenospheres. Together, these studies show that inferior glenoid inclination increases impingement-free ROM and, combined with lateralization, may result in improved glenosphere longevity due to significantly decreased forces at the RSA glenoid baseplate when compared to that at superiorly inclined glenoids.
The ideal amount of mediolateral glenosphere offset has not been well defined. Grammont design systems place the center of rotation of the glenosphere medial to the glenoid baseplate together with valgus humeral component neck shaft angles of around 155°. These design elements are believed to decrease shear stresses through the glenoid baseplate to the glenoid interface and improve shoulder stability, but they are also associated with reduced impingement-free ROM and increased rates of scapular notching.13 This effect is accentuated in patients with preexisting glenoid bone loss and/or congenitally short scapular necks that further medialize the glenosphere. Medialization of the glenosphere may also shorten the remaining rotator cuff muscles and result in decreased implant stability and external rotation strength. Several implant systems have options to vary the amount of lateral offset. The correct amount of lateral offset for each patient requires the understanding that improving patients’ impingement-free ROM by increasing the amount of lateral offset comes at the price of increasing the shear forces experienced by the interface between the glenoid baseplate and the glenoid. As glenoid fixation technology improves increased lateralization of glenospheres without increased rates of glenoid baseplate, loosening should improve the ROM after reverse TSA.
Continue to: Regardless of the intraoperative goals...
Regardless of the intraoperative goals for placement and orientation of the glenosphere components, it is vitally important to accurately and consistently meet those goals for achieving optimal patient outcomes. Verborgt and colleagues20 implanted 7 glenospheres in cadaveric specimens without any glenohumeral arthritis using standard techniques to evaluate the accuracy of glenosphere version and inclination. Their goal was to place components in neutral version and with 10° of inferior inclination. Their average glenoid version postoperatively was 8.7° of anteversion, and their average inclination was 0.9° of superior inclination. Throckmorton and colleagues21 randomized 35 cadaveric shoulders to receive either an anatomic or a reverse total shoulder prosthesis from high-, mid-, and low-volume surgeons. They found that components placed using traditional guides averaged 6° of deviation in version and 5° of deviation in inclination from their target values, with no significant differences between surgeons of different volumes.
PATIENT-SPECIFIC GUIDES/INSTRUMENTATION
Patient-specific guides/instrumentation and intraoperative navigation are the 2 techniques that have been used to improve the accuracy of glenoid and glenosphere placement. Both techniques require the use of high-resolution CT scans and computer software to determine the proper position for glenoid or glenosphere placement based on the patient’s individual anatomy. Patient-specific guides and instrumentation use the data acquired from a CT scan to generate a preoperative plan for the location and orientation of the glenoid baseplate. Once the surgeon approves the preoperative plan, a patient-specific guide is created using the patient’s glenoid as a reference for the location and orientation of the central guide pin. The location of the central guide pin on the glenoid determines the center of the glenoid baseplate, and the guide pin’s orientation determines the version and inclination of the glenoid or the glenosphere. Once the guide pin is placed in the glenoid, the remainder of the glenoid implantation uses the guide pin as a reference, and, in that way, patient-specific guides control the orientation of the glenoid at the time of surgery.
Intraoperative navigation uses an optical tracking system to determine the location and orientation of the central guide pin. Navigation systems require intraoperative calibration of the optical tracking system before they can track the location of implantation relative to bony landmarks on the patient’s scapula. Their advantage over patient-specific instrumentation (PSI) is that they do not require the manufacture of a custom guide; however, they may add significantly increased cost and surgical time due to the need for calibration prior to use and the cost of the navigation system along with any disposable components associated with it. Kircher and colleagues22 performed a prospective randomized clinical study of navigation-aided TSA compared with conventional TSA and found that operating time was significantly increased for the navigated group with an average operating room time of 169.5 minutes compared to 138 minutes for the conventional group. They also found that navigation had to be abandoned in 37.5% of their navigated patients due to technical errors during glenoid referencing.
COMMERCIAL PATIENT-SPECIFIC INSTRUMENTATION SYSTEMS
The 2 types of PSI that are currently available are single-use PSI and reusable PSI. The single-use PSI involves the fabrication of unique guides based on surgeon-approved preoperative plans generated by computer-software-processed preoperative CT scans. The guides are fabricated to rest on the glenoid articular surface and direct the guide pin to the correct location and in the correct direction to place the glenoid baseplate in the desired position with the desired version and inclination. Most of these systems also provide a 3-D model of the patient’s glenoid so that surgeons can visualize glenoid deformities and the correct guide placement on the glenoid. Single-use PSI systems are available from DJO Global, Wright Medical Group, and Zimmer Biomet. The second category of PSI is reusable and is available from Arthrex. The guide pin for this system is adjusted to fit individual patient anatomy and guide the guide pin into the glenoid in a location and orientation preplanned on the CT-scan-based computer software or using a 3-D model of the patient’s glenoid (Table).
Table. Details of Available Patient-Specific Instrumentation Systems
| System | Manufacturer | Single-Use/Reusable | Guides |
| MatchPoint System | DJO Global | Single-use | Central guide pin |
| Blueprint 3D Planning + PSI | Wright Medical Group | Single-use | Central guide pin |
| Zimmer Patient Specific Instruments Shoulder | Zimmer Biomet | Single-use | Central guide pin, reaming guide, roll guide, screw drill guide |
| Virtual Implant Positioning System | Arthrex | Reusable | Central guide pin |
The DJO Global patient-specific guide is termed as the MatchPoint System. This system creates 3-D renderings of the scapula and allows the surgeon to manipulate the glenoid baseplate on the scapula. The surgeon chooses the glenoid baseplate, location, version, and inclination on the computerized 3-D model. The system then fabricates a guide pin matching the computerized template that references the patient’s glenoid surface with a hook to orient it against the coracoid. A 3-D model of the glenoid is also provided along with the customized guide pin.
Continue to: Blueprint 3D Planning + PSI...
Blueprint 3D Planning + PSI (Wright Medical Group) allows custom placement of the glenoid version, inclination, and position on computerized 3-D models of the patient’s scapula. This PSI references the glenoid with 4 feet that captures the edge of the patient’s glenoid at specific locations and is unique because it allows the surgeon to control where on the glenoid edge to 4 feet contact as long as 1 foot is placed on the posterior edge of the glenoid and the remaining 3 feet are placed on the anterior edge of the glenoid. A 3-D model of the glenoid is also provided with this guide.
The Zimmer Biomet patient-specific guide is termed as the Zimmer Patient Specific Instruments Shoulder. Its computer software allows custom placement of the glenoid as well, but it also includes computerized customization of the reaming depth, screw angles, and screw lengths to optimize fixation. Their system includes a central guide pin to set the glenoid baseplate’s location and orientation, a reaming guide to control reaming depth and direction, a roll guide to control the glenoid baseplate’s rotation, and a drill guide to control the screw direction. They also provide a 3-D model of the glenoid.

The Arthrex Virtual Implant Positioning (VIP) System is similar to other systems in that its 3-D planning software is based on CT images uploaded by the surgeon. The unique aspect of this system is that the guide pin is adjusted by the surgeon for each individual patient based on instructions generated by the planning software; however, after use, the instruments are resterilized and reused on subsequent patients (Figures 1A, 1B). In this manner, their instruments are reusable and allow custom adjustment for each patient with the ability to set the pin location and glenoid version in a patient-specific manner. This has the potential benefit of keeping costs down. For more complex deformity cases, the Arthrex VIP System can also 3-D-print a sterile model of the glenoid to help surgeons appreciate the deformity better (Figure 2).

DATA ON PATIENT-SPECIFIC INSTRUMENTS
Several studies have measured the accuracy of patient-specific guides and have compared the accuracy of patient-specific guides to that of traditional methods. Levy and colleagues23 investigated the accuracy of single-use patient-specific guides compared to that of preoperative plans. They used patient-specific guides on 14 cadaveric shoulders based on plans developed by virtual preoperative 3-D planning system using CT images. Once the guide pin was drilled using the patient-specific guide, they obtained a second CT scan to compare the accuracy of the patient-specific guide to the surgical plan generated preoperatively. They found that the translational accuracy of the starting point for the guide pin averaged 1.2 mm ± 0.7 mm, the accuracy of the inferior inclination was 1.2° ± 1.2°, and the accuracy of the glenoid version was 2.6° ± 1.7°. They concluded that patient-specific guides were highly accurate in reproducing the starting point, inclination, and version set on preoperative guides.
Walch and colleagues24 subsequently performed a similar study using 15 cadaveric scapulae without any other shoulder soft tissue or bone attached. They also used CT-scan-based 3-D planning software to plan their glenoid placement with a subsequently fabricated single-use patient-specific guide used to place a guide pin. They obtained a second CT scan after guide pin implantation and compared the preoperative plan with the subsequent guide pin. They found a mean entry point position error of 1.05 mm ± 0.31 mm, a mean inclination error of 1.42° ± 1.37°, and a mean version error of 1.64° ± 1.01°.
Continue to: Throckmorton and colleagues...
Throckmorton and colleagues21 used 70 cadaveric shoulders with radiographically confirmed arthritis and randomized them to undergo either anatomic or reverse TSA using either a patient-specific guide or standard instrumentation. Postoperative CT scans were used to evaluate the glenoid inclination, version, and starting point. They found that glenoid components implanted using patient-specific guides were more accurate than those placed using traditional instrumentation. The average deviation from intended inclination was 3° for patient-specific guides and 7° for traditional instrumentation, the average deviation from intended version was 5° for patient-specific guides and 8° for traditional instrumentation, and the average deviation in intended starting point was 2 mm for patient-specific guides and 3 mm for traditional instrumentation. They also analyzed significantly malpositioned components as defined by a variation in version or inclination of >10° or >4 mm in starting point. They found that 6 of their 35 glenoids using patient-specific guides were significantly malpositioned compared to 23 of 35 glenoids using traditional instrumentation. They concluded that patient-specific guides were more accurate and reduced the number of significantly malpositioned implants when compared with traditional instrumentation.
Early and colleagues25 analyzed the effect of severe glenoid bone defects on the accuracy of patient-specific guides compared with traditional guides. Using 10 cadaveric shoulders, they created anterior, central, or posterior glenoid defects using a reamer and chisel to erode the bone past the coracoid base. Subsequent CT scans were performed on the specimens, and patient-specific guides were fabricated and used for reverse TSA in 5 of the 10 specimens. A reverse TSA was performed using traditional instrumentation in the remaining 5 specimens. They found that the average deviation in inclination and version from preoperative plan was more accurate in the patient-specific guide cohort than that in the traditional instrument cohort, with an average deviation in inclination and version of 1.2° ± 1.2° and 1.8° ± 1.2° respectively for the cohort using patient-specific instruments vs 2.8° ± 1.8° and 3.5° ± 3° for the cohort using traditional instruments. They also found that their total bone screw lengths were longer in the patient-specific guide group than those in the traditional group, with screws averaging 52% of preoperatively planned length in the traditional instrument cohort vs 89% of preoperatively planned length in the patient-specific instrument cohort.
Gauci and colleagues26 measured the accuracy of patient-specific guides in vivo in 17 patients receiving TSA. Preoperative CT scans were used to fabricate patient-specific guides, and postoperative CT scans were used to measure version, inclination, and error of entry in comparison with the templated goals used to create patient-specific guides. They found a mean error in version and inclination of 3.4° and 1.8°, respectively, and a mean error in entry of 0.9 mm of translation on the glenoid. Dallalana and colleagues27 performed a very similar study on 20 patients and found a mean deviation in glenoid version of 1.8° ± 1.9°, a mean deviation in glenoid inclination of 1.3° ± 1.0°, a mean translation in anterior-posterior plane of 0.5 mm ± 0.3 mm, and a mean translation in the superior-inferior plane of 0.8 mm ± 0.5 mm.
Hendel and colleagues28 performed a randomized prospective clinical trial comparing patient-specific guides with traditional methods for glenoid insertion. They randomized 31 patients to receive a glenoid implant using either a patient-specific guide or traditional methods and compared glenoid retroversion and inclination with their preoperative plan. They found an average version deviation of 6.9° in the traditional method cohort and 4.3° in the patient-specific guide cohort. Their average deviation in inclination was 11.6° in the traditional method cohort and 2.9° in the patient-specific guide cohort. For patients with preoperative retroversion >16°, the average deviation was 10° in the standard surgical cohort and 1.2° in the patient-specific instrument cohort. Their data suggest that increasing preoperative retroversion leads to an increased version variation from preoperative plan.
Iannotti and colleagues29 randomly assigned 46 patients to preoperatively undergo either CT scan with 3-D templating of glenoid component without patient-specific guide fabrication or CT scan with 3-D templating and patient-specific guide fabrication prior to receiving a TSA. They recorded the postoperative inclination and version for each patient and compared them to those of a nonrandomized control group of 17 patients who underwent TSA using standard instrumentation. They found no difference between the cohorts with or without patient-specific guide use with regard to implant location, inclination, or version; however, they did find a difference between the combined 3-D templating cohort compared with their standard instrumentation cohort. They concluded that 3-D templating significantly improved the surgeons’ ability to correctly position the glenoid component with or without the fabrication and the use of a patient-specific guide.
Continue to: Denard and colleagues...
Denard and colleagues30 compared the preoperative glenoid version and inclination measurements obtained using the Blueprint 3D Planning + PSI software and the VIP System 3D planning software. They analyzed the preoperative CT scans of 63 consecutive patients undergoing either TSA or reverse TSA using both the Blueprint and the VIP System 3D planning software and compared the resulting native glenoid version and inclination measured by the software. They found a statistically significant difference (P = 0.04) in the version measurements provided by the different planning software; however, the differences found in inclination did not reach statistical significance (P = 0.463). In 19 of the 63 patients (30%), the version measurements between the systems were >5°, and in 29 of the 63 patients (46%), the inclination measurements between the systems were 5° or greater. In addition, 12 of the 63 patients (19%) had both version and inclination measurement differences of >5° between the systems. In total, they found that 35 of the 63 patients had at least 1 measurement that varied by >5° between the systems, and that in 15 patients (24%), 1 measurement varied by >10°. Their data demonstrate considerable variability in the preoperative measurements provided by different 3-D planning software systems, and that further study of each commercially available 3-D planning software system is needed to evaluate their accuracy.
CONCLUSION
Optimal outcomes following TSA and reverse TSA are dependent on proper implant position. Multiple studies have demonstrated improved accuracy in implant positioning with the use of patient-specific guides compared to that with traditional methods. Currently, there are no studies comparing the clinical outcomes of patient-specific guides to those of traditional methods of glenoid placement, and limited research had been done comparing the accuracy of each system’s 3-D planning software with each other and with standardized measurements of glenoid version and inclination. Further research is required to determine the accuracy of each commercially available 3-D planning software system as well as the clinical benefit of patient-specific guides in shoulder arthroplasty.
ABSTRACT
Optimal outcomes following total shoulder arthroplasty TSA and reverse shoulder arthroplasty RSA are dependent on proper implant position. Multiple cadaver studies have demonstrated improved accuracy of implant positioning with use of patient-specific guides/instrumentation compared to traditional methods. At this time, there are 3 commercially available single use patient-specific instrumentation systems and 1 commercially available reusable patient-specific instrumentation system. Currently though, there are no studies comparing the clinical outcomes of patient-specific guides to those of traditional methods of glenoid placement, and limited research has been done comparing the accuracy of each system’s 3-dimensional planning software. Future work is necessary to elucidate the ideal indications for the use of patient-specific guides and instrumentation, but it is likely, particularly in the setting of advanced glenoid deformity, that these systems will improve a surgeon's ability to put the implant in the best position possible.
Continue to: Optimal functional recovery...
Optimal functional recovery and implant longevity following both total shoulder arthroplasty (TSA) and reverse shoulder arthroplasty (RSA) depend, in large part, on proper placement of the glenoid component. Glenoid component malpositioning has an adverse effect on shoulder stability, range of motion (ROM), impingement, and glenoid implant longevity.
Traditionally, glenoid component positioning has been done manually by the surgeons based on their review of preoperative films and knowledge of glenoid anatomy. Anatomic studies have demonstrated high individual variability in the version of the native glenoid, thus making ideal placement of the initial glenoid guide pin difficult using standard guide pin guides.1
The following 2 methods have been described for improving the accuracy of glenoid guide pin insertion and subsequent glenoid implant placement: (1) computerized navigation and (2) patient-specific guides/instrumentation. Although navigated shoulder systems have demonstrated improved accuracy in glenoid placement compared with traditional methods, navigated systems require often large and expensive systems for implementation. The majority of them also require placement of guide pins or arrays on scapular bony landmarks, likely leading to an increase in operative time and possible iatrogenic complications, including fracture and pin site infections.
This review focuses on the use of patient-specific guides/instrumentation in shoulder arthroplasty. This includes the topic of proper glenoid and glenosphere placement as well as patient-specific guides/instrumentation and their accuracy.
GLENOID PLACEMENT
Glenohumeral osteoarthritis is the most common indication for TSA2 and commonly results in glenoid deformity. Using computed tomography (CT) scans of 45 arthritic shoulders and 19 normal shoulders, Mullaji and colleagues3 reported that the anteroposterior dimensions of the glenoid were increased by an average of 5 mm to 8 mm in osteoarthritic shoulders and by an average of 6 mm in rheumatoid arthritic shoulders compared to those in normal shoulders. A retrospective review of serial CT scans performed preoperatively on 113 osteoarthritic shoulders by Walch and colleagues4 demonstrated an average retroversion of 16°, and it has been the basis for the commonly used Walch classification of glenoid wear in osteoarthritis. Increased glenoid wear and increased glenoid retroversion make the proper restoration of glenoid version, inclination, and offset during shoulder arthroplasty more difficult and lead to increased glenoid component malpositioning.
Continue to: The ideal placement of the glenoid...
The ideal placement of the glenoid to maximize function, ROM, and implant longevity is in a mechanically neutral alignment with no superoinferior inclination1 and neutral version with respect to the transverse axis of the scapula.5
Improper glenoid positioning has an adverse effect on the functional results of shoulder arthroplasty. Yian and colleagues6 evaluated 47 cemented, pegged glenoids using standard radiography and CT scans at a mean follow-up of 40 months. They observed a significant correlation between increased glenoid component retroversion and lower Constant scores. Hasan and colleagues7 evaluated 139 consecutive patients who were dissatisfied with the result of their primary arthroplasty and found that 28% of them had at least 1 substantially malpositioned component identified either on radiography or during a revision surgery. They also found a significant correlation between stiffness, instability, and component malposition in their cohort.
Glenoid longevity is also dependent on proper component positioning, with the worst outcomes coming if the glenoid is malaligned with either superior or inferior inclination. Hasan and colleagues7 found that of their 74 patients with failed TSAs, 44 patients (59%) demonstrated mechanical loosening of their glenoid components either radiographically or during revision surgery, and 10 of their 44 patients with loose glenoids (23%) also had a malpositioned component. Using finite element analysis, Hopkins and colleagues8 analyzed the stresses through the cement mantle in glenoid prostheses that were centrally aligned, superiorly inclined, inferiorly inclined, anteverted, and retroverted. They found that malalignment of the glenoid increases the stresses through the cement mantle, leading to increased likelihood of mantle failure compared to that of centrally aligned glenoids, especially if there is malalignment with superior or inferior inclination or retroversion.
The accuracy of traditional methods of glenoid placement using an initial guide pin is limited and decreases with increasing amounts of glenoid deformity and retroversion. Iannotti and colleagues 9 investigated 13 patients undergoing TSA with an average preoperative retroversion of 13° and evaluated them using a 3-dimensional (3-D) surgical simulator. They found that the postoperative glenoid version was within 5° of ideal version in only 7 of their 13 patients (54%) and within 10° of ideal version in only 10 of their 13 patients (77%). In their study, the ideal version was considered to be the version as close to perpendicular to the plane of the scapula as possible with complete contact of the back side of the component on glenoid bone and maintenance of the center peg of the component within bone. In addition, they found that of their 7 patients with preoperative retroversion >10°, only 1 patient (14%) had a postoperative glenoid with <10° of retroversion with regard to the plane of the scapula and that all 6 of their patients with preoperative glenoid retroversion of <10° had a postoperative glenoid version of <10°.
Preoperative CT scans are much more accurate at determining glenoid version and thus how much glenoid correction is required to reestablish neutral version than plain radiography. Nyffeler and colleagues10 compared CT scans with axillary views for comparing glenoid version in 25 patients with no shoulder prosthesis present and 25 patients with a TSA in place. They found that glenoid retroversion was overestimated on plain radiographs in 86% of their patients with an average difference between CT and plain radiography of 6.4° and a maximum difference of 21°. They also found poor interobserver reliability in the plain radiography group and good interobserver reliability in the CT group, with coefficients of correlation of 0.77 for the plain radiography group and 0.93 for the CT group. Thus, they concluded that glenoid version cannot be accurately measured by plain radiography and that CT should be used. Hoenecke and colleagues11 subsequently evaluated 33 patients scheduled for TSA and found that CT version measurements made on 2-dimensional (2-D) CT slices compared with 3-D-reconstructed models of the same CT slices differed by an average of 5.1° because the axial CT slices were most often made perpendicular to the axis of the patient’s torso and not perpendicular to the body of the scapula. Accurate version assessment is critically important in planning for the degree of correction required to restore neutral glenoid version, and differences of 6.4° between CT assessment and plain radiography, and 5.1° between 2-D and 3-D CT scan assessments may lead to inadequate version correction intraoperatively and inferior postoperative results.
Continue to: GLENOSPHERE PLACEMENT
GLENOSPHERE PLACEMENT
The most common indication for reverse TSA is rotator cuff arthropathy characterized by rotator cuff dysfunction and end-stage glenohumeral arthritis.12 These patients require accurate and reproducible glenoid placement to optimize their postoperative range of motion and stability and minimize scapular notching.
Ideal glenosphere placement is the location and orientation that maximizes impingement-free ROM and stability while avoiding notching. Individual patient anatomy determines ideal placement; however, several guidelines for placement include inferior translation on the glenoid with neutral to inferior inclination. Gutiérrez and colleagues13 developed a computer model to assess the hierarchy of surgical factors affecting the ROM after a reverse TSA. They found that lateralizing the center of rotation gave the largest increase in impingement-free abduction, followed closely by inferior translation of the glenosphere on the glenoid.
Avoiding scapular notching is also a very important factor in ideal glenosphere placement. Scapular notching can be described as impingement of the humeral cup against the scapular neck during arm adduction and/or humeral rotation. Gutiérrez and colleagues13 also found that decreasing the neck shaft angle to create a more varus proximal humerus was the most important factor in increasing the impingement-free adduction. Roche and colleagues14 reviewed the radiographs of 151 patients who underwent primary reverse TSA at a mean follow-up of 28.3 months postoperatively; they found that 13.2% of their patients had a notch and that, on average, their patients who had no scapular notch had significantly more inferior glenosphere overhang than those who had a scapular notch. Poon and colleagues15 found that a glenosphere overhang of >3.5 mm prevented notching in their randomized control trial comparing concentrically and eccentrically placed glenospheres. Multiple other studies have demonstrated similar results and recommended inferior glenoid translation and inferior glenoid inclination to avoid scapular notching.16,17 Lévigne and colleagues18 retrospectively reviewed 337 reverse TSAs and observed a correlation between scapular notching and radiolucencies around the glenosphere component, with 14% of patients with scapular notching displaying radiolucencies vs 4% of patients without scapula notching displaying radiolucencies.
Several studies have also focused on the ideal amount of inferior glenoid inclination to maximize impingement-free ROM. Li and colleagues17 performed a computer simulation study on the Comprehensive Reverse Shoulder System (Zimmer Biomet) to determine impingement-free internal and external ROM with varying amounts of glenosphere offset, translation, and inclination. They found that progressive glenosphere inferior inclination up to 30° improved impingement-free rotational ROM at all degrees of scaption. Gutiérrez and colleagues19 used computer modeling to compare concentrically placed glenospheres in neutral inclination with eccentrically placed glenospheres in varying degrees of inclination. They found that the lowest forces across the baseplate occurred in the lateralized and inferiorly inclined glenospheres, and the highest forces occurred in the lateralized and superiorly inclined glenospheres. Together, these studies show that inferior glenoid inclination increases impingement-free ROM and, combined with lateralization, may result in improved glenosphere longevity due to significantly decreased forces at the RSA glenoid baseplate when compared to that at superiorly inclined glenoids.
The ideal amount of mediolateral glenosphere offset has not been well defined. Grammont design systems place the center of rotation of the glenosphere medial to the glenoid baseplate together with valgus humeral component neck shaft angles of around 155°. These design elements are believed to decrease shear stresses through the glenoid baseplate to the glenoid interface and improve shoulder stability, but they are also associated with reduced impingement-free ROM and increased rates of scapular notching.13 This effect is accentuated in patients with preexisting glenoid bone loss and/or congenitally short scapular necks that further medialize the glenosphere. Medialization of the glenosphere may also shorten the remaining rotator cuff muscles and result in decreased implant stability and external rotation strength. Several implant systems have options to vary the amount of lateral offset. The correct amount of lateral offset for each patient requires the understanding that improving patients’ impingement-free ROM by increasing the amount of lateral offset comes at the price of increasing the shear forces experienced by the interface between the glenoid baseplate and the glenoid. As glenoid fixation technology improves increased lateralization of glenospheres without increased rates of glenoid baseplate, loosening should improve the ROM after reverse TSA.
Continue to: Regardless of the intraoperative goals...
Regardless of the intraoperative goals for placement and orientation of the glenosphere components, it is vitally important to accurately and consistently meet those goals for achieving optimal patient outcomes. Verborgt and colleagues20 implanted 7 glenospheres in cadaveric specimens without any glenohumeral arthritis using standard techniques to evaluate the accuracy of glenosphere version and inclination. Their goal was to place components in neutral version and with 10° of inferior inclination. Their average glenoid version postoperatively was 8.7° of anteversion, and their average inclination was 0.9° of superior inclination. Throckmorton and colleagues21 randomized 35 cadaveric shoulders to receive either an anatomic or a reverse total shoulder prosthesis from high-, mid-, and low-volume surgeons. They found that components placed using traditional guides averaged 6° of deviation in version and 5° of deviation in inclination from their target values, with no significant differences between surgeons of different volumes.
PATIENT-SPECIFIC GUIDES/INSTRUMENTATION
Patient-specific guides/instrumentation and intraoperative navigation are the 2 techniques that have been used to improve the accuracy of glenoid and glenosphere placement. Both techniques require the use of high-resolution CT scans and computer software to determine the proper position for glenoid or glenosphere placement based on the patient’s individual anatomy. Patient-specific guides and instrumentation use the data acquired from a CT scan to generate a preoperative plan for the location and orientation of the glenoid baseplate. Once the surgeon approves the preoperative plan, a patient-specific guide is created using the patient’s glenoid as a reference for the location and orientation of the central guide pin. The location of the central guide pin on the glenoid determines the center of the glenoid baseplate, and the guide pin’s orientation determines the version and inclination of the glenoid or the glenosphere. Once the guide pin is placed in the glenoid, the remainder of the glenoid implantation uses the guide pin as a reference, and, in that way, patient-specific guides control the orientation of the glenoid at the time of surgery.
Intraoperative navigation uses an optical tracking system to determine the location and orientation of the central guide pin. Navigation systems require intraoperative calibration of the optical tracking system before they can track the location of implantation relative to bony landmarks on the patient’s scapula. Their advantage over patient-specific instrumentation (PSI) is that they do not require the manufacture of a custom guide; however, they may add significantly increased cost and surgical time due to the need for calibration prior to use and the cost of the navigation system along with any disposable components associated with it. Kircher and colleagues22 performed a prospective randomized clinical study of navigation-aided TSA compared with conventional TSA and found that operating time was significantly increased for the navigated group with an average operating room time of 169.5 minutes compared to 138 minutes for the conventional group. They also found that navigation had to be abandoned in 37.5% of their navigated patients due to technical errors during glenoid referencing.
COMMERCIAL PATIENT-SPECIFIC INSTRUMENTATION SYSTEMS
The 2 types of PSI that are currently available are single-use PSI and reusable PSI. The single-use PSI involves the fabrication of unique guides based on surgeon-approved preoperative plans generated by computer-software-processed preoperative CT scans. The guides are fabricated to rest on the glenoid articular surface and direct the guide pin to the correct location and in the correct direction to place the glenoid baseplate in the desired position with the desired version and inclination. Most of these systems also provide a 3-D model of the patient’s glenoid so that surgeons can visualize glenoid deformities and the correct guide placement on the glenoid. Single-use PSI systems are available from DJO Global, Wright Medical Group, and Zimmer Biomet. The second category of PSI is reusable and is available from Arthrex. The guide pin for this system is adjusted to fit individual patient anatomy and guide the guide pin into the glenoid in a location and orientation preplanned on the CT-scan-based computer software or using a 3-D model of the patient’s glenoid (Table).
Table. Details of Available Patient-Specific Instrumentation Systems
| System | Manufacturer | Single-Use/Reusable | Guides |
| MatchPoint System | DJO Global | Single-use | Central guide pin |
| Blueprint 3D Planning + PSI | Wright Medical Group | Single-use | Central guide pin |
| Zimmer Patient Specific Instruments Shoulder | Zimmer Biomet | Single-use | Central guide pin, reaming guide, roll guide, screw drill guide |
| Virtual Implant Positioning System | Arthrex | Reusable | Central guide pin |
The DJO Global patient-specific guide is termed as the MatchPoint System. This system creates 3-D renderings of the scapula and allows the surgeon to manipulate the glenoid baseplate on the scapula. The surgeon chooses the glenoid baseplate, location, version, and inclination on the computerized 3-D model. The system then fabricates a guide pin matching the computerized template that references the patient’s glenoid surface with a hook to orient it against the coracoid. A 3-D model of the glenoid is also provided along with the customized guide pin.
Continue to: Blueprint 3D Planning + PSI...
Blueprint 3D Planning + PSI (Wright Medical Group) allows custom placement of the glenoid version, inclination, and position on computerized 3-D models of the patient’s scapula. This PSI references the glenoid with 4 feet that captures the edge of the patient’s glenoid at specific locations and is unique because it allows the surgeon to control where on the glenoid edge to 4 feet contact as long as 1 foot is placed on the posterior edge of the glenoid and the remaining 3 feet are placed on the anterior edge of the glenoid. A 3-D model of the glenoid is also provided with this guide.
The Zimmer Biomet patient-specific guide is termed as the Zimmer Patient Specific Instruments Shoulder. Its computer software allows custom placement of the glenoid as well, but it also includes computerized customization of the reaming depth, screw angles, and screw lengths to optimize fixation. Their system includes a central guide pin to set the glenoid baseplate’s location and orientation, a reaming guide to control reaming depth and direction, a roll guide to control the glenoid baseplate’s rotation, and a drill guide to control the screw direction. They also provide a 3-D model of the glenoid.

The Arthrex Virtual Implant Positioning (VIP) System is similar to other systems in that its 3-D planning software is based on CT images uploaded by the surgeon. The unique aspect of this system is that the guide pin is adjusted by the surgeon for each individual patient based on instructions generated by the planning software; however, after use, the instruments are resterilized and reused on subsequent patients (Figures 1A, 1B). In this manner, their instruments are reusable and allow custom adjustment for each patient with the ability to set the pin location and glenoid version in a patient-specific manner. This has the potential benefit of keeping costs down. For more complex deformity cases, the Arthrex VIP System can also 3-D-print a sterile model of the glenoid to help surgeons appreciate the deformity better (Figure 2).

DATA ON PATIENT-SPECIFIC INSTRUMENTS
Several studies have measured the accuracy of patient-specific guides and have compared the accuracy of patient-specific guides to that of traditional methods. Levy and colleagues23 investigated the accuracy of single-use patient-specific guides compared to that of preoperative plans. They used patient-specific guides on 14 cadaveric shoulders based on plans developed by virtual preoperative 3-D planning system using CT images. Once the guide pin was drilled using the patient-specific guide, they obtained a second CT scan to compare the accuracy of the patient-specific guide to the surgical plan generated preoperatively. They found that the translational accuracy of the starting point for the guide pin averaged 1.2 mm ± 0.7 mm, the accuracy of the inferior inclination was 1.2° ± 1.2°, and the accuracy of the glenoid version was 2.6° ± 1.7°. They concluded that patient-specific guides were highly accurate in reproducing the starting point, inclination, and version set on preoperative guides.
Walch and colleagues24 subsequently performed a similar study using 15 cadaveric scapulae without any other shoulder soft tissue or bone attached. They also used CT-scan-based 3-D planning software to plan their glenoid placement with a subsequently fabricated single-use patient-specific guide used to place a guide pin. They obtained a second CT scan after guide pin implantation and compared the preoperative plan with the subsequent guide pin. They found a mean entry point position error of 1.05 mm ± 0.31 mm, a mean inclination error of 1.42° ± 1.37°, and a mean version error of 1.64° ± 1.01°.
Continue to: Throckmorton and colleagues...
Throckmorton and colleagues21 used 70 cadaveric shoulders with radiographically confirmed arthritis and randomized them to undergo either anatomic or reverse TSA using either a patient-specific guide or standard instrumentation. Postoperative CT scans were used to evaluate the glenoid inclination, version, and starting point. They found that glenoid components implanted using patient-specific guides were more accurate than those placed using traditional instrumentation. The average deviation from intended inclination was 3° for patient-specific guides and 7° for traditional instrumentation, the average deviation from intended version was 5° for patient-specific guides and 8° for traditional instrumentation, and the average deviation in intended starting point was 2 mm for patient-specific guides and 3 mm for traditional instrumentation. They also analyzed significantly malpositioned components as defined by a variation in version or inclination of >10° or >4 mm in starting point. They found that 6 of their 35 glenoids using patient-specific guides were significantly malpositioned compared to 23 of 35 glenoids using traditional instrumentation. They concluded that patient-specific guides were more accurate and reduced the number of significantly malpositioned implants when compared with traditional instrumentation.
Early and colleagues25 analyzed the effect of severe glenoid bone defects on the accuracy of patient-specific guides compared with traditional guides. Using 10 cadaveric shoulders, they created anterior, central, or posterior glenoid defects using a reamer and chisel to erode the bone past the coracoid base. Subsequent CT scans were performed on the specimens, and patient-specific guides were fabricated and used for reverse TSA in 5 of the 10 specimens. A reverse TSA was performed using traditional instrumentation in the remaining 5 specimens. They found that the average deviation in inclination and version from preoperative plan was more accurate in the patient-specific guide cohort than that in the traditional instrument cohort, with an average deviation in inclination and version of 1.2° ± 1.2° and 1.8° ± 1.2° respectively for the cohort using patient-specific instruments vs 2.8° ± 1.8° and 3.5° ± 3° for the cohort using traditional instruments. They also found that their total bone screw lengths were longer in the patient-specific guide group than those in the traditional group, with screws averaging 52% of preoperatively planned length in the traditional instrument cohort vs 89% of preoperatively planned length in the patient-specific instrument cohort.
Gauci and colleagues26 measured the accuracy of patient-specific guides in vivo in 17 patients receiving TSA. Preoperative CT scans were used to fabricate patient-specific guides, and postoperative CT scans were used to measure version, inclination, and error of entry in comparison with the templated goals used to create patient-specific guides. They found a mean error in version and inclination of 3.4° and 1.8°, respectively, and a mean error in entry of 0.9 mm of translation on the glenoid. Dallalana and colleagues27 performed a very similar study on 20 patients and found a mean deviation in glenoid version of 1.8° ± 1.9°, a mean deviation in glenoid inclination of 1.3° ± 1.0°, a mean translation in anterior-posterior plane of 0.5 mm ± 0.3 mm, and a mean translation in the superior-inferior plane of 0.8 mm ± 0.5 mm.
Hendel and colleagues28 performed a randomized prospective clinical trial comparing patient-specific guides with traditional methods for glenoid insertion. They randomized 31 patients to receive a glenoid implant using either a patient-specific guide or traditional methods and compared glenoid retroversion and inclination with their preoperative plan. They found an average version deviation of 6.9° in the traditional method cohort and 4.3° in the patient-specific guide cohort. Their average deviation in inclination was 11.6° in the traditional method cohort and 2.9° in the patient-specific guide cohort. For patients with preoperative retroversion >16°, the average deviation was 10° in the standard surgical cohort and 1.2° in the patient-specific instrument cohort. Their data suggest that increasing preoperative retroversion leads to an increased version variation from preoperative plan.
Iannotti and colleagues29 randomly assigned 46 patients to preoperatively undergo either CT scan with 3-D templating of glenoid component without patient-specific guide fabrication or CT scan with 3-D templating and patient-specific guide fabrication prior to receiving a TSA. They recorded the postoperative inclination and version for each patient and compared them to those of a nonrandomized control group of 17 patients who underwent TSA using standard instrumentation. They found no difference between the cohorts with or without patient-specific guide use with regard to implant location, inclination, or version; however, they did find a difference between the combined 3-D templating cohort compared with their standard instrumentation cohort. They concluded that 3-D templating significantly improved the surgeons’ ability to correctly position the glenoid component with or without the fabrication and the use of a patient-specific guide.
Continue to: Denard and colleagues...
Denard and colleagues30 compared the preoperative glenoid version and inclination measurements obtained using the Blueprint 3D Planning + PSI software and the VIP System 3D planning software. They analyzed the preoperative CT scans of 63 consecutive patients undergoing either TSA or reverse TSA using both the Blueprint and the VIP System 3D planning software and compared the resulting native glenoid version and inclination measured by the software. They found a statistically significant difference (P = 0.04) in the version measurements provided by the different planning software; however, the differences found in inclination did not reach statistical significance (P = 0.463). In 19 of the 63 patients (30%), the version measurements between the systems were >5°, and in 29 of the 63 patients (46%), the inclination measurements between the systems were 5° or greater. In addition, 12 of the 63 patients (19%) had both version and inclination measurement differences of >5° between the systems. In total, they found that 35 of the 63 patients had at least 1 measurement that varied by >5° between the systems, and that in 15 patients (24%), 1 measurement varied by >10°. Their data demonstrate considerable variability in the preoperative measurements provided by different 3-D planning software systems, and that further study of each commercially available 3-D planning software system is needed to evaluate their accuracy.
CONCLUSION
Optimal outcomes following TSA and reverse TSA are dependent on proper implant position. Multiple studies have demonstrated improved accuracy in implant positioning with the use of patient-specific guides compared to that with traditional methods. Currently, there are no studies comparing the clinical outcomes of patient-specific guides to those of traditional methods of glenoid placement, and limited research had been done comparing the accuracy of each system’s 3-D planning software with each other and with standardized measurements of glenoid version and inclination. Further research is required to determine the accuracy of each commercially available 3-D planning software system as well as the clinical benefit of patient-specific guides in shoulder arthroplasty.
1. Churchill RS, Brems JJ, Kotschi H. Glenoid size, inclination, and version: an anatomic study. J Shoulder Elbow Surg. 2001;10(4):327-332. doi:10.1067/mse.2001.115269.
2. Norris TR, Iannotti JP. Functional outcome after shoulder arthroplasty for primary osteoarthritis: a multicenter study. J Shoulder Elbow Surg. 2002;11(2):130-135. doi:10.1067/mse.2002.121146.
3. Mullaji AB, Beddow FH, Lamb GH. CT measurement of glenoid erosion in arthritis. J Bone Joint Surg Br. 1994;76(3):384-388.
4. Walch G, Badet R, Boulahia A, Khoury A. Morphologic study of the glenoid in primary glenohumeral osteoarthritis. J Arthroplasty. 1999;14(6):756-760.
5. RJ Friedman, KB Hawthorne, BM Genez. The use of computerized tomography in the measurement of glenoid version. J Bone Joint Surg. 1992;74(7):1032-1037. doi:10.2106/00004623-199274070-00009.
6. Yian EH, Werner CM, Nyffeler RW, et al. Radiographic and computed tomography analysis of cemented pegged polyethylene glenoid components in total shoulder replacement. J Bone Joint Surg. 2005;87(9):1928-1936. doi:10.2106/00004623-200509000-00004.
7. Hasan SS, Leith JM, Campbell B, Kapil R, Smith KL, Matsen FA. Characteristics of unsatisfactory shoulder arthroplasties. J Shoulder Elbow Surg. 2002;11(5):431-441.
8. Hopkins AR, Hansen UN, Amis AA, Emery R. The effects of glenoid component alignment variations on cement mantle stresses in total shoulder arthroplasty. J Shoulder Elbow Surg. 2004;13(6):668-675. doi:10.1016/S1058274604001399.
9. Iannotti JP, Greeson C, Downing D, Sabesan V, Bryan JA. Effect of glenoid deformity on glenoid component placement in primary shoulder arthroplasty. J Shoulder Elbow Surg. 2012;21(1):48-55. doi:10.1016/j.jse.2011.02.011.
10. Nyffeler RW, Jost B, Pfirrmann CWA, Gerber C. Measurement of glenoid version: conventional radiographs versus computed tomography scans. J Shoulder Elbow Surg. 2003;12(5):493-496. doi:10.1016/S1058274603001812.
11. Hoenecke HR, Hermida JC, Flores-Hernandez C, D'Lima DD. Accuracy of CT-based measurements of glenoid version for total shoulder arthroplasty. J Shoulder Elbow Surg. 2010;19(2):166-171. doi:10.1016/j.jse.2009.08.009.
12. Wall B, Nové-Josserand L, O'Connor DP, Edwards TB, Walch G. Reverse total shoulder arthroplasty: a review of results according to etiology. J Bone Joint Surg Am. 2007;89(7):1476-1485. doi:10.2106/JBJS.F.00666.
13. Gutiérrez S, Comiskey 4, Charles A, Luo Z, Pupello DR, Frankle MA. Range of impingement-free abduction and adduction deficit after reverse shoulder arthroplasty. hierarchy of surgical and implant-design-related factors. J Bone Joint Surg Am. 2008;90(12):2606-2615. doi:10.2106/JBJS.H.00012.
14. Roche CP, Marczuk Y, Wright TW, et al. Scapular notching and osteophyte formation after reverse shoulder replacement: Radiological analysis of implant position in male and female patients. Bone Joint J. 2013;95-B(4):530-535. doi:10.1302/0301-620X.95B4.30442.
15. Poon PC, Chou J, Young SW, Astley T. A comparison of concentric and eccentric glenospheres in reverse shoulder arthroplasty: a randomized controlled trial. J Bone Joint Surg Am. 2014;96(16):e138. doi:10.2106/JBJS.M.00941.
16. Nyffeler RW, Werner CML, Gerber C. Biomechanical relevance of glenoid component positioning in the reverse delta III total shoulder prosthesis. J Shoulder Elbow Surg. 2005;14(5):524-528. doi:10.1016/j.jse.2004.09.010.
17. Li X, Knutson Z, Choi D, et al. Effects of glenosphere positioning on impingement-free internal and external rotation after reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22(6):807-813. doi:10.1016/j.jse.2012.07.013.
18. Lévigne C, Boileau P, Favard L, et al. Scapular notching in reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2008;17(6):925-935. doi:10.1016/j.jse.2008.02.010.
19. Gutiérrez S, Walker M, Willis M, Pupello DR, Frankle MA. Effects of tilt and glenosphere eccentricity on baseplate/bone interface forces in a computational model, validated by a mechanical model, of reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2011;20(5):732-739. doi:10.1016/j.jse.2010.10.035.
20. Verborgt O, De Smedt T, Vanhees M, Clockaerts S, Parizel PM, Van Glabbeek F. Accuracy of placement of the glenoid component in reversed shoulder arthroplasty with and without navigation. J Shoulder Elbow Surg. 2011;20(1):21-26. doi:10.1016/j.jse.2010.07.014.
21. Throckmorton TW, Gulotta LV, Bonnarens FO, et al. Patient-specific targeting guides compared with traditional instrumentation for glenoid component placement in shoulder arthroplasty: A multi-surgeon study in 70 arthritic cadaver specimens. J Shoulder Elbow Surg. 2015;24(6):965-971. doi:10.1016/j.jse.2014.10.013.
22. Kircher J, Wiedemann M, Magosch P, Lichtenberg S, Habermeyer P. Improved accuracy of glenoid positioning in total shoulder arthroplasty with intraoperative navigation: a prospective-randomized clinical study. J Shoulder Elbow Surg. 2009;18(4):515-520. doi:10.1016/j.jse.2009.03.014.
23. Levy JC, Everding NG, Frankle MA, Keppler LJ. Accuracy of patient-specific guided glenoid baseplate positioning for reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(10):1563-1567. doi:10.1016/j.jse.2014.01.051.
24. Walch G, Vezeridis PS, Boileau P, Deransart P, Chaoui J. Three-dimensional planning and use of patient-specific guides improve glenoid component position: an in vitro study. J Shoulder Elbow Surg. 2015;24(2):302-309. doi:10.1016/j.jse.2014.05.029.
25. Eraly K, Stoffelen D, Vander Sloten J, Jonkers I, Debeer P. A patient-specific guide for optimizing custom-made glenoid implantation in cases of severe glenoid defects: an in vitro study. J Shoulder Elbow Surg. 2016;25(5):837-845. doi:10.1016/j.jse.2015.09.034.
26. Gauci MO, Boileau P, Baba M, Chaoui J, Walch G. Patient-specific glenoid guides provide accuracy and reproducibility in total shoulder arthroplasty. Bone Joint J. 2016;98-B(8):1080-1085. doi:10.1302/0301-620X.98B8.37257.
27. Dallalana RJ, McMahon RA, East B, Geraghty L. Accuracy of patient-specific instrumentation in anatomic and reverse total shoulder arthroplasty. Int J Shoulder Surg. 2016;10(2):59-66. doi:10.4103/09736042.180717.
28. Hendel MD, Bryan JA, Barsoum WK, et al. Comparison of patient-specific instruments with standard surgical instruments in determining glenoid component position: a randomized prospective clinical trial. J Bone Joint Surg. 2012;94(23):2167-2175. doi:10.2106/JBJS.K.01209.
29. Iannotti JP, Weiner S, Rodriguez E, et al. Three-dimensional imaging and templating improve glenoid implant positioning. J Bone Joint Surg. 2015;97(8):651-658. doi:10.2106/JBJS.N.00493.
30. Denard PJ, Provencher MT, Lädermann A, Romeo AA, Dines JS. Version and inclination obtained with 3D planning in total shoulder arthroplasty: do different programs produce the same results? SECEC-ESSSE Congress, Berlin 2017. 2017.
1. Churchill RS, Brems JJ, Kotschi H. Glenoid size, inclination, and version: an anatomic study. J Shoulder Elbow Surg. 2001;10(4):327-332. doi:10.1067/mse.2001.115269.
2. Norris TR, Iannotti JP. Functional outcome after shoulder arthroplasty for primary osteoarthritis: a multicenter study. J Shoulder Elbow Surg. 2002;11(2):130-135. doi:10.1067/mse.2002.121146.
3. Mullaji AB, Beddow FH, Lamb GH. CT measurement of glenoid erosion in arthritis. J Bone Joint Surg Br. 1994;76(3):384-388.
4. Walch G, Badet R, Boulahia A, Khoury A. Morphologic study of the glenoid in primary glenohumeral osteoarthritis. J Arthroplasty. 1999;14(6):756-760.
5. RJ Friedman, KB Hawthorne, BM Genez. The use of computerized tomography in the measurement of glenoid version. J Bone Joint Surg. 1992;74(7):1032-1037. doi:10.2106/00004623-199274070-00009.
6. Yian EH, Werner CM, Nyffeler RW, et al. Radiographic and computed tomography analysis of cemented pegged polyethylene glenoid components in total shoulder replacement. J Bone Joint Surg. 2005;87(9):1928-1936. doi:10.2106/00004623-200509000-00004.
7. Hasan SS, Leith JM, Campbell B, Kapil R, Smith KL, Matsen FA. Characteristics of unsatisfactory shoulder arthroplasties. J Shoulder Elbow Surg. 2002;11(5):431-441.
8. Hopkins AR, Hansen UN, Amis AA, Emery R. The effects of glenoid component alignment variations on cement mantle stresses in total shoulder arthroplasty. J Shoulder Elbow Surg. 2004;13(6):668-675. doi:10.1016/S1058274604001399.
9. Iannotti JP, Greeson C, Downing D, Sabesan V, Bryan JA. Effect of glenoid deformity on glenoid component placement in primary shoulder arthroplasty. J Shoulder Elbow Surg. 2012;21(1):48-55. doi:10.1016/j.jse.2011.02.011.
10. Nyffeler RW, Jost B, Pfirrmann CWA, Gerber C. Measurement of glenoid version: conventional radiographs versus computed tomography scans. J Shoulder Elbow Surg. 2003;12(5):493-496. doi:10.1016/S1058274603001812.
11. Hoenecke HR, Hermida JC, Flores-Hernandez C, D'Lima DD. Accuracy of CT-based measurements of glenoid version for total shoulder arthroplasty. J Shoulder Elbow Surg. 2010;19(2):166-171. doi:10.1016/j.jse.2009.08.009.
12. Wall B, Nové-Josserand L, O'Connor DP, Edwards TB, Walch G. Reverse total shoulder arthroplasty: a review of results according to etiology. J Bone Joint Surg Am. 2007;89(7):1476-1485. doi:10.2106/JBJS.F.00666.
13. Gutiérrez S, Comiskey 4, Charles A, Luo Z, Pupello DR, Frankle MA. Range of impingement-free abduction and adduction deficit after reverse shoulder arthroplasty. hierarchy of surgical and implant-design-related factors. J Bone Joint Surg Am. 2008;90(12):2606-2615. doi:10.2106/JBJS.H.00012.
14. Roche CP, Marczuk Y, Wright TW, et al. Scapular notching and osteophyte formation after reverse shoulder replacement: Radiological analysis of implant position in male and female patients. Bone Joint J. 2013;95-B(4):530-535. doi:10.1302/0301-620X.95B4.30442.
15. Poon PC, Chou J, Young SW, Astley T. A comparison of concentric and eccentric glenospheres in reverse shoulder arthroplasty: a randomized controlled trial. J Bone Joint Surg Am. 2014;96(16):e138. doi:10.2106/JBJS.M.00941.
16. Nyffeler RW, Werner CML, Gerber C. Biomechanical relevance of glenoid component positioning in the reverse delta III total shoulder prosthesis. J Shoulder Elbow Surg. 2005;14(5):524-528. doi:10.1016/j.jse.2004.09.010.
17. Li X, Knutson Z, Choi D, et al. Effects of glenosphere positioning on impingement-free internal and external rotation after reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22(6):807-813. doi:10.1016/j.jse.2012.07.013.
18. Lévigne C, Boileau P, Favard L, et al. Scapular notching in reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2008;17(6):925-935. doi:10.1016/j.jse.2008.02.010.
19. Gutiérrez S, Walker M, Willis M, Pupello DR, Frankle MA. Effects of tilt and glenosphere eccentricity on baseplate/bone interface forces in a computational model, validated by a mechanical model, of reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2011;20(5):732-739. doi:10.1016/j.jse.2010.10.035.
20. Verborgt O, De Smedt T, Vanhees M, Clockaerts S, Parizel PM, Van Glabbeek F. Accuracy of placement of the glenoid component in reversed shoulder arthroplasty with and without navigation. J Shoulder Elbow Surg. 2011;20(1):21-26. doi:10.1016/j.jse.2010.07.014.
21. Throckmorton TW, Gulotta LV, Bonnarens FO, et al. Patient-specific targeting guides compared with traditional instrumentation for glenoid component placement in shoulder arthroplasty: A multi-surgeon study in 70 arthritic cadaver specimens. J Shoulder Elbow Surg. 2015;24(6):965-971. doi:10.1016/j.jse.2014.10.013.
22. Kircher J, Wiedemann M, Magosch P, Lichtenberg S, Habermeyer P. Improved accuracy of glenoid positioning in total shoulder arthroplasty with intraoperative navigation: a prospective-randomized clinical study. J Shoulder Elbow Surg. 2009;18(4):515-520. doi:10.1016/j.jse.2009.03.014.
23. Levy JC, Everding NG, Frankle MA, Keppler LJ. Accuracy of patient-specific guided glenoid baseplate positioning for reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(10):1563-1567. doi:10.1016/j.jse.2014.01.051.
24. Walch G, Vezeridis PS, Boileau P, Deransart P, Chaoui J. Three-dimensional planning and use of patient-specific guides improve glenoid component position: an in vitro study. J Shoulder Elbow Surg. 2015;24(2):302-309. doi:10.1016/j.jse.2014.05.029.
25. Eraly K, Stoffelen D, Vander Sloten J, Jonkers I, Debeer P. A patient-specific guide for optimizing custom-made glenoid implantation in cases of severe glenoid defects: an in vitro study. J Shoulder Elbow Surg. 2016;25(5):837-845. doi:10.1016/j.jse.2015.09.034.
26. Gauci MO, Boileau P, Baba M, Chaoui J, Walch G. Patient-specific glenoid guides provide accuracy and reproducibility in total shoulder arthroplasty. Bone Joint J. 2016;98-B(8):1080-1085. doi:10.1302/0301-620X.98B8.37257.
27. Dallalana RJ, McMahon RA, East B, Geraghty L. Accuracy of patient-specific instrumentation in anatomic and reverse total shoulder arthroplasty. Int J Shoulder Surg. 2016;10(2):59-66. doi:10.4103/09736042.180717.
28. Hendel MD, Bryan JA, Barsoum WK, et al. Comparison of patient-specific instruments with standard surgical instruments in determining glenoid component position: a randomized prospective clinical trial. J Bone Joint Surg. 2012;94(23):2167-2175. doi:10.2106/JBJS.K.01209.
29. Iannotti JP, Weiner S, Rodriguez E, et al. Three-dimensional imaging and templating improve glenoid implant positioning. J Bone Joint Surg. 2015;97(8):651-658. doi:10.2106/JBJS.N.00493.
30. Denard PJ, Provencher MT, Lädermann A, Romeo AA, Dines JS. Version and inclination obtained with 3D planning in total shoulder arthroplasty: do different programs produce the same results? SECEC-ESSSE Congress, Berlin 2017. 2017.
TAKE-HOME POINTS
- Optimal outcomes following TSA and RSA are dependent on proper implant position.
- Patient-specific guides/instrumentation result in improved accuracy of implant positioning compared to traditional methods.
- Currently, there are no clinical studies demonstrating superiority of patient-specific guide/instrumentation use on patient outcomes.
- At this time there are 3 commercially available single use patient-specific instrumentation systems (DJO Global, Wright Medical Group, and Zimmer Biomet) and 1 commercially available reusable patient-specific instrumentation system (Arthrex).
- Limited research is available comparing the accuracy of different commercially available 3-D planning systems.