User login
Molluscum Contagiosum in Immunocompromised Patients: AIDS Presenting as Molluscum Contagiosum in a Patient With Psoriasis on Biologic Therapy
Molluscum contagiosum (MC) is a double-stranded DNA virus of the Poxviridae family, which commonly infects human keratinocytes resulting in small, umbilicated, flesh-colored papules. The greatest incidence of MC is seen in the pediatric population and sexually active young adults, and it is considered a self-limited disease in immunocompetent individuals.1 With the emergence of the human immunodeficiency virus (HIV) and subsequent AIDS epidemic in the 1980s, a new population of immunocompromised individuals has been observed to be increasingly susceptible to MC with an atypical clinical presentation and a recalcitrant disease course.2 Although the increased prevalence of MC in the HIV population has been well-documented, it has been observed in other disease states or iatrogenically induced immunosuppression due to a deficiency in function or absolute number of T lymphocytes.
We present a case of a patient with long-standing psoriasis on biologic therapy who presented with MC with a subsequent workup that revealed AIDS. This case reiterates the importance of MC as a potential indicator of underlying immunosuppression. We review the literature to evaluate the occurrence of MC in immunosuppressed patients.
Case Report
A 33-year-old man initially presented for evaluation of severe plaque-type psoriasis associated with pain, erythema, and swelling of the joints of the hands of 10 years’ duration. He was started on methotrexate 5 mg weekly and topical corticosteroids but was unable to tolerate methotrexate due to headaches. He also had difficulty affording topical medications and adjunctive phototherapy. The patient was sporadically seen in follow-up with persistence of psoriatic plaques involving up to 60% body surface area (BSA) with the only treatment consisting of occasional topical steroids. Five years later, the patient was restarted on methotrexate 5 to 7.5 mg weekly, which resulted in moderate improvement. However, because of persistent elevation of liver enzymes, this treatment was stopped. Several months later he was evaluated for treatment with a biologic agent, and after a negative tuberculin skin test, he began treatment with etanercept 50 mg subcutaneous injection twice weekly, which provided notable improvement and allowed for reduction of dose frequency to once weekly.
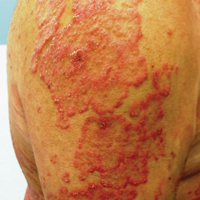
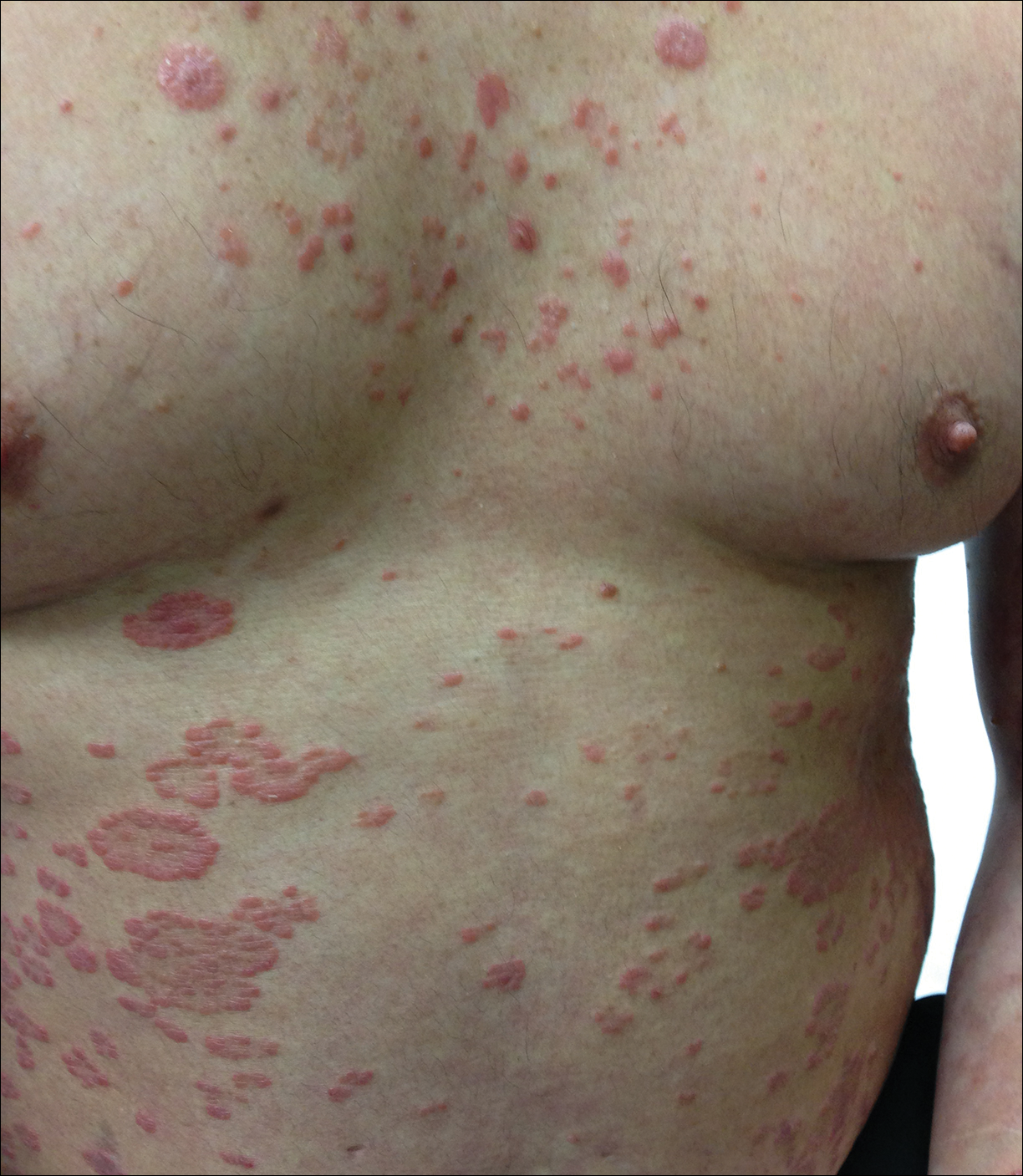
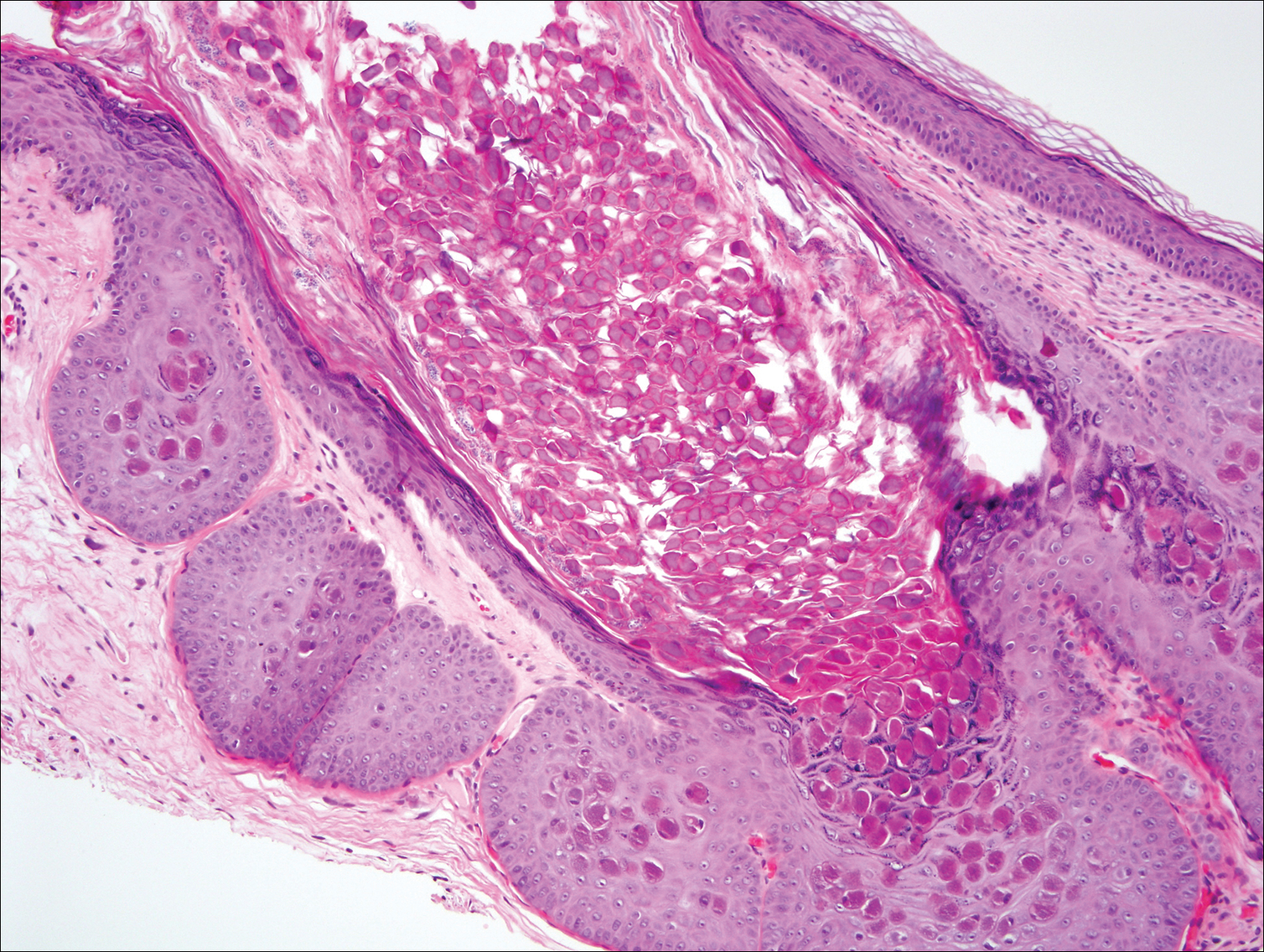
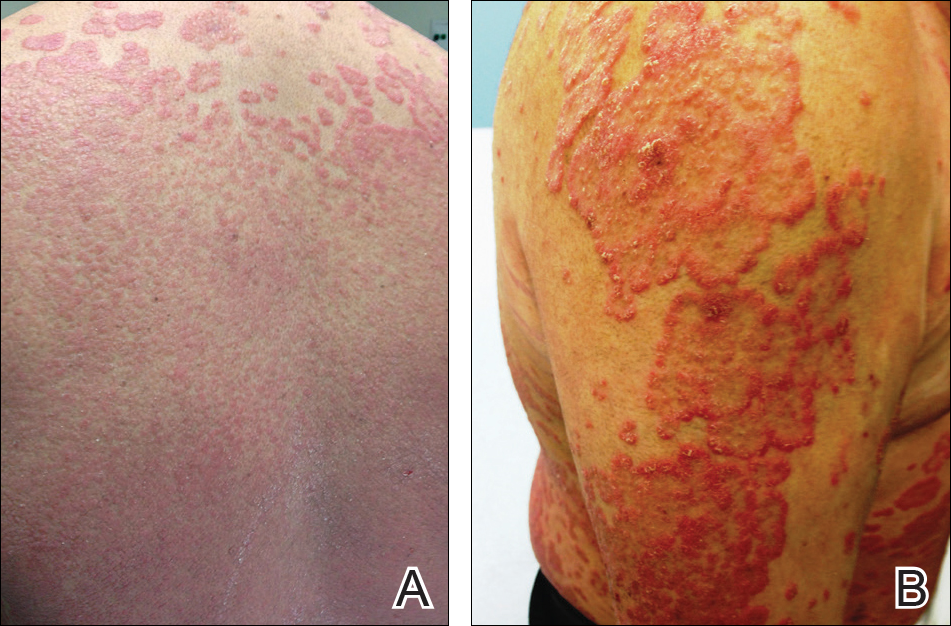
At follow-up 1 year later, the patient had continued improvement of psoriasis with approximately 30% BSA on a treatment regimen of etanercept 50 mg weekly injection and topical corticosteroids. However, on physical examination, there were multiple small semitranslucent papules with telangiectases on the chest and upper back (Figure 1). Biopsy of a representative papule on the chest revealed MC (Figure 2). The patient was subsequently advised to stop etanercept and to return immediately to the clinic for HIV testing. He returned for follow-up 3 months later with pronounced worsening of disease and a new onset of blurred vision of the right eye. Cutaneous examination revealed numerous large erythematous plaques with superficial scale and cerebriform surface on the chest, back, abdomen, and upper and lower extremities involving 80% BSA (Figure 3). Biopsy of a plaque demonstrated psoriasiform dermatitis with neutrophils and parakeratosis consistent with psoriasis. Extensive blood work was notable for reactive HIV antibody and lymphopenia, CD4 lymphocyte count of 60 cells/mm3, and an HIV viral load of 247,000 copies/mL, meeting diagnostic criteria for AIDS. Additionally, ophthalmologic evaluation revealed toxoplasma retinitis. Upon initiation of highly active antiretroviral therapy (HAART) and continued use of topical corticosteroids, the patient experienced notable improvement of disease severity with approximately 20% BSA.
Comment
Molluscum contagiosum is a common skin infection. Among patients with HIV and other types of impaired cellular immunity, the prevalence of MC is estimated to be as high as 20%.3 The MC poxvirus survives and proliferates within the epidermis by interfering with tumor necrosis factor–induced apoptosis of virally infected cells; therefore, intact cell-mediated immunity is an important component of prevention and clearance of poxvirus infections. In immunocompromised patients, the presentation of MC varies widely, and the disease is often difficult to eradicate. This review will highlight the prevalence, presentation, and treatment of MC in the context of immunosuppressed states.
HIV/AIDS
Molluscum contagiosum in HIV-positive patients was first recognized in 1983,2 and its prevalence is estimated to range from 5% to 18% in AIDS patients.3 Molluscum contagiosum is a clinical sign of HIV progression, and its incidence appears to increase with reduced immune function (ie, a CD4 cell count <200/mm3).3 In a study of 456 patients with HIV-associated skin disorders, the majority of patients with MC had notable immunosuppression with a median survival time of 12 months. Thus, MC was not an independent prognostic marker but a clinical indicator of markedly reduced immune status.4
Molluscum contagiosum is transmitted in both sexual and nonsexual patterns in HIV-positive individuals, with the distribution of the latter involving primarily the face and neck. Although it may present with typical umbilicated papules, MC has a wide range of atypical clinical presentations in patients with AIDS that can make it difficult to diagnose. Complicated cases of eyelid MC have been reported in advanced HIV in both adults and children, resulting in obstruction of vision due to large lesions (up to 2 cm) or hundreds of confluent lesions.5 Giant MC, which appears as large exophytic nodules, is another presentation that has been frequently described in patients with advanced HIV. In these patients, the lesions often are too voluminous for conservative therapy and require excision.6 Atypical MC lesions also can resemble other dermatologic conditions, including condyloma acuminatum,7 nevus sebaceous of Jadassohn, ecthyma,8 and cutaneous horns,9,10 as well as other bacterial and fungal infections in HIV-positive patients, such as cutaneous Cryptococcus neoformans,11,12 disseminated histoplasmosis,13 and infections caused by Penicillium marneffei14 and Bartonella henselae.15 In most cases of MC in HIV-positive patients, diagnosis is dependent on the examination of biopsy specimens, which maintain the same histopathologic features regardless of immune status.
The management of MC in patients with HIV/AIDS is difficult. Molluscum contagiosum has shown no evidence of spontaneous resolution in patients with HIV, and treatment with one modality is often insufficient. Treatment is most successful when a combination approach is utilized with destructive procedures (eg, curettage, cryosurgery) and adjunctive agents (eg, retinoids, cantharidin, trichloroacetic acid). Imiquimod and cidofovir have been used off label for MC in AIDS patients.16 Imiquimod, which is used to treat genital warts, another cutaneous viral infection seen in patients with HIV, has demonstrated efficacy in treating MC.16 In a randomized controlled trial comparing imiquimod cream 5% to cryotherapy for MC in healthy children, imiquimod was slow acting but better suited than cryotherapy for patients with eruptions of many small lesions.17 For HIV patients, numerous reports have described successful treatment of disseminated or recalcitrant MC with topical imiquimod.18-20 Cidofovir, an antiviral used to treat cytomegalovirus retinitis in patients with AIDS, is a promising antiviral agent against the poxvirus family. In a study of viral DNA polymerase genes of MC virus, cidofovir inhibited MC virus DNA polymerase activity.21 It has been used in both topical (1% to 3%) and intravenous form to successfully treat recalcitrant and exuberant giant MC.6,22 However, the use of cidofovir is limited by its high costs, especially when compounded into a topical formulation.23
From a systemic standpoint, numerous reports have shown that treating the underlying HIV by optimizing HAART is the most important first step in clearing MC.24-27 However, a special concern regarding the initiation of HAART in patients with MC as well as a markedly impaired immune function is the development of an inflammatory reaction called immune reconstitution inflammatory syndrome (IRIS). This reaction is thought to be a result of immune recovery in severely immunosuppressed patients. During the initial phase of reconstitution when CD4 lymphocyte counts rise and viral load decreases, IRIS occurs due to an inflammatory reaction to microbial and autoimmune antigens, leading to temporary clinical deterioration.28 The incidence has been reported in up to 25% of patients starting HAART, and 52% to 78% of IRIS cases involve dermatologic manifestations such as varicella-zoster virus, cytomegalovirus infections, genital warts, and MC.29,30 In a cohort study of 199 patients, 2% of patients developed MC within 6 months of initiating HAART.31 In a case of exuberant MC lesions after beginning HAART, the lesions spontaneously resolved with the progression of immune reconstitution.28
Malignancies
Patients with hematologic malignancies such as lymphoma and leukemia comprise another subset of patients at risk for atypical presentations of MC. Molluscum contagiosum has been described in patients with hematologic malignancies such as adult T-cell leukemia/lymphoma, multiple myeloma, chronic myeloid leukemia, acute lymphoblastic leukemia, lymphomatoid papulosis, and non-Hodgkin lymphoma. In a review of MC in children with cancer, 0.5% were diagnosed with MC.32,33 Reports also have documented eruptive MC in the presence of solid organ cancers, including lung cancer.34
In patients with malignancies, the differential diagnosis should include other common dermatologic conditions such as varicella, herpes simplex, papillomas, pyoderma, and cutaneous cryptococcosis, as well as MC. Similar to HIV-positive patients, the lesions of MC described in patients with malignancies do not tend to spontaneously resolve. In a report of a pediatric patient with acute lymphoblastic leukemia, MC presented as an ulcerated lesion without any classic features, requiring biopsy for definitive diagnosis. Only partial resolution was achieved with cryotherapy and crusting of the lesion in an attempt to slow the progression.35 In a series of 5 children with hematologic malignancies and MC, little improvement was noted after treatment with surgical scraping, liquid nitrogen, and salicylic acid ointment 5%. Similar to patients with HIV, improvement of immune status and function help clear the disease, and patients who reach remission and discontinue chemotherapeutic agents have a higher rate of spontaneous resolution of previously recalcitrant MC lesions.36
Transplant Patients
Molluscum contagiosum in transplant patients has features similar to patients with HIV/AIDS. In organ transplant recipients, there is an increased risk for cutaneous disease from iatrogenic immunosuppression or immunosuppression through infectious or neoplastic processes.37 As in other immunocompromised populations, MC often has an atypical presentation in transplant patients with more extensive involvement and recalcitrant, rapidly recurring lesions.
In a review of 145 pediatric organ transplant recipients, MC was the fourth most common skin infection after verruca vulgaris, tinea versicolor, and herpes simplex/zoster. Affecting 7% of patients, the majority of patients demonstrated clinically typical lesions; however, the disease was difficult to eradicate if multiple lesions were present.37 In other reports in adults, fulminant and giant MC have been described after renal and other solid organ transplants.38,39 Molluscum contagiosum also has been reported to mimic other skin diseases in transplant patients including tinea barbae40 and nodular basal cell carcinomas.41
The standard treatments are identical to those used in patients with HIV, including ablative methods via liquid nitrogen, electrocautery, cantharidin, trichloroacetic acid, and topical retinoids. Similar to MC in other immunocompromised states, treatment can be difficult and usually requires multiple modalities. For children, imiquimod cream 5% has been recommended due to high clearance rates (up to 92%) and the painless nature of the treatment.42,43
Other Iatrogenic Immunosuppressive States
Immunosuppression through the use of steroids, chemotherapeutic agents, and biologic drugs often is the result of treatment of various diseases. In patients with psoriasis treated with systemic immunosuppressive agents, there are numerous reports that describe the appearance of eruptive MC in association with methotrexate, cyclosporine, and biologics. Methotrexate acts as an immunosuppressive agent by binding to dihydrofolate reductase, which inhibits DNA synthesis in immunologically competent cells.44 It also may block host defense mechanisms against MC by suppressing the expression of serum inflammatory cytokines such as tumor necrosis factor α (TNF-α) and IFN-γ and suppressing the activity of TNF-α inducing apoptosis of virus-infected cells. Cyclosporine used in conjunction with methotrexate may exacerbate the insult to the immune system by inhibiting the production of IFN-γ.45 Biologics are an emerging class of drugs that have demonstrated efficacy in moderate to severe psoriasis by inhibiting TNF-α or other inflammatory molecules. Several published reports have described eruptive or atypical MC in patients on biologic medications. In one case, within 2 weeks after initiation of infliximab, a monoclonal antibody against TNF-α, a patient developed an eruption of MC involving the entire body.46 In another report, an anti–TNF-α agent for rheumatoid arthritis was associated with atypical MC with eyelid lesions.47
There are other skin disorders treated with immunosuppressive agents that also have been associated with MC. In a patient with pemphigus vulgaris treated with prednisolone, pimecrolimus, and azathioprine, MC lesions were observed on the face and within healed pemphigus vulgaris sites.48 Pimecrolimus and tacrolimus, corticosteroid-sparing agents, suppress cell-mediated immunity and inhibit inflammatory cytokines such as IL-2. The infection resolved with a gradual tapering of immunosuppressive therapy and 10 sessions of cryotherapy.48 In a case of topical pimecrolimus for pityriasis alba, the patient developed biopsy-proven MC within 2 weeks of initiating treatment in the areas that were treated with tacrolimus.49
In nontransplant patients with iatrogenic immunosuppression, MC treatment has not been documented to be as challenging as in patients with inherent immunosuppression. Most patients respond to either withdrawal of the drug alone or to simple ablative treatments such as cryotherapy.45,46,48 This important difference is most likely due to the presence of an otherwise intact immune system.
Conclusion
This case describes the appearance of MC in a patient with psoriasis treated with a TNF-α inhibitor who was ultimately diagnosed with AIDS. Although atypical MC infections have been documented in patients with psoriasis undergoing treatment with biologics, it is thought to be more common for MC to occur in more remarkably immunocompromised states such as AIDS. Thus, the persistence and progression of MC in our patient despite discontinuation of etanercept suggested a separate underlying process. Subsequent workup led to the diagnosis of AIDS along with the opportunistic ocular infection of toxoplasmosis retinitis. This clinical sequence consisting of psoriasis treated with a biologic agent, development of MC, and subsequent diagnosis of AIDS is unique and clinically significant to dermatologists. The presentation of psoriasis in patients with HIV can be diverse with different levels of severity and atypical clinical features. In many cases, HIV is known to exacerbate the classic clinical presentation of psoriasis. However, there are other particular presentations of psoriasis in HIV patients that have been observed, which include a predilection for scalp lesions, palmoplantar keratoderma, flexural involvement, and higher levels of immunodeficiency.50 Although tuberculin skin tests are required prior to initiating biologic therapy due to the potential for disease reactivation, there are no requirements for HIV antibody testing. In cases of severe recalcitrant psoriasis, an HIV test should be ordered during the workup to establish an early diagnosis so that an HIV-positive patient can avoid poor outcomes from either the disease processes, the use of certain therapeutic agents, or both. Furthermore, the benefit of avoiding possible harm to the patient and potential legal action outweighs the cost of performing surveillance HIV testing in this subset of patients. Thus, due to the potential additive immunosuppressive effect of HIV with biologic therapy, providers should always assess for risk factors and consider testing for HIV in all patients before initiating treatment with immunosuppressive agents such as biologics.
- Dohil MA, Lin P, Lee J, et al. The epidemiology of molluscum contagiosum in children. J Am Acad Dermatol. 2006;54:47-54.
- Reichert CM, O’Leary TJ, Levens DL, et al. Autopsy pathology in the acquired immune deficiency syndrome. Am J Pathol. 1983;112:357-382.
- Czelusta A, Yen-Moore A, Van der Straten M, et al. An overview of sexually transmitted diseases. Part III. Sexually transmitted diseases in HIV-infected patients. J Am Acad Dermatol. 2000;43:409-432.
- Husak R, Garbe C, Orfanos CE. Mollusca contagiosa in HIV infection. Clinical manifestation, relation to immune status and prognostic value in 39 patients [in German]. Hautarzt. 1997;48:103-109.
- Averbuch D, Jaouni T, Pe’er J, et al. Confluent molluscum contagiosum covering the eyelids of an HIV-positive child. Clin Exp Ophthalmol. 2009;37:525-527.
- Erickson C, Driscoll M, Gaspari A. Efficacy of intravenous cidofovir in the treatment of giant molluscum contagiosum in a patient with human immunodeficiency virus. Arch Dermatol. 2011;147:652-654.
- Mastrolorenzo A, Urbano FG, Salimbeni L, et al. Atypical molluscum contagiosum infection in an HIV-infected patient. Int J Dermatol. 1998;37:378-380.
- Itin PH, Gilli L. Molluscum contagiosum mimicking sebaceous nevus of Jadassohn, ecthyma and giant condylomata acuminata in HIV-infected patients. Dermatology. 1994;189:396-398.
- Sim JH, Lee ES. Molluscum contagiosum presenting as a cutaneous horn. Ann Dermatol. 2011;23:262-263.
- Manchanda Y, Sethuraman G, Paderwani PP, et al. Molluscum contagiosum presenting as penile horn in an HIV positive patient. Sex Transm Infect. 2005;81:183-184.
- Miller SJ. Cutaneous cryptococcus resembling molluscum contagiosum in a patient with acquired immunodeficiency syndrome. Cutis. 1988;41:411-412.
- Sornum A. A mistaken diagnosis of molluscum contagiosum in a HIV-positive patient in rural South Africa. BMJ Case Rep. 2012;14.
- Corti M, Villafañe MF, Palmieri O, et al. Rupioid histoplasmosis: first case reported in an AIDS patient in Argentina. Rev Inst Med Trop Sao Paulo. 2010;52:279-280.
- Saikia L, Nath R, Hazarika D, et al. Atypical cutaneous lesions of Penicillium marneffei infection as a manifestation of the immune reconstitution inflammatory syndrome after highly active antiretroviral therapy. Indian J Dermatol Venereol Leprol. 2010;76:45-48.
- de Souza JA. Molluscum or a mimic? Am J Med. 2006;119:927-929.
- Conant MA. Immunomodulatory therapy in the management of viral infections in patients with HIV infection. J Am Acad Dermatol. 2000;43:S27-S30.
- Gamble RG, Echols KF, Dellavalle RP. Imiquimod vs cryotherapy for molluscum contagiosum: a randomized controlled trial. Arch Dermatol. 2012;148:109-112.
- Brown CW Jr, O’Donoghue M, Moore J, et al. Recalcitrant molluscum contagiosum in an HIV-afflicted male treated successfully with topical imiquimod. Cutis. 2000;65:363-366.
- Strauss RM, Doyle EL, Mohsen AH, et al. Successful treatment of molluscum contagiosum with topical imiquimod in a severely immunocompromised HIV-positive patient. Int J STD AIDS. 2001;12:264-266.
- Theiler M, Kempf W, Kerl K, et al. Disseminated molluscum contagiosum in a HIV-positive child. improvement after therapy with 5% imiquimod. J Dermatol Case Rep. 2011;5:19-23.
- Watanabe T, Tamaki K. Cidofovir diphosphate inhibits molluscum contagiosum virus DNA polymerase activity. J Invest Dermatol. 2008;128:1327-1329.
- Calista D. Topical cidofovir for severe cutaneous human papillomavirus and molluscum contagiosum infections in patients with HIV/AIDS. a pilot study. J Eur Acad Dermatol Venereol. 2000;14:484-488.
- Toro JR, Sanchez S, Turiansky G, et al. Topical cidofovir for the treatment of dermatologic conditions: verruca, condyloma, intraepithelial neoplasia, herpes simplex and its potential use in smallpox. Dermatol Clin. 2003;21:301-309.
- Calista D, Boschini A, Landi G. Resolution of disseminated molluscum contagiosum with highly active anti-retroviral therapy (HAART) in patients with AIDS. Eur J Dermatol. 1999;9:211-213.
- Cattelan AM, Sasset L, Corti L, et al. A complete remission of recalcitrant molluscum contagiosum in an AIDS patient following highly active antiretroviral therapy (HAART). J Infect. 1999;38:58-60.
- Sen S, Bhaumik P. Resolution of giant molluscum contagiosum with antiretroviral therapy. Indian J Dermatol Venereol Leprol. 2008;74:267-268.
- Sen S, Goswami BK, Karjyi N, et al. Disfiguring molluscum contagiosum in a HIV-positive patient responding to antiretroviral therapy. Indian J Dermatol. 2009;54:180-182.
- Pereira B, Fernandes C, Nachiambo E, et al. Exuberant molluscum contagiosum as a manifestation of the immune reconstitution inflammatory syndrome. Dermatol Online J. 2007;13:6.
- Osei-Sekyere B, Karstaedt AS. Immune reconstitution inflammatory syndrome involving the skin. Clin Exp Dermatol. 2010;35:477-481.
- Sung KU, Lee HE, Choi WR, et al. Molluscum contagiosum as a skin manifestation of immune reconstitution inflammatory syndrome in an AIDS patient who is receiving HAART. Korean J Fam Med. 2012;33:182-185.
- Ratnam I, Chiu C, Kandala NB, et al. Incidence and risk factors for immune reconstitution inflammatory syndrome in an ethnically diverse HIV type 1-infected cohort. Clin Infect Dis. 2006;42:418-427.
- Chen KW, Yang CF, Huang CT, et al. Molluscum contagiosum in a patient with adult T-cell leukaemia/lymphoma. Br J Haematol. 2011;155:286.
- Fernandez KH, Bream M, Ali MA, et al. Investigation of molluscum contagiosum virus, orf and other parapoxviruses in lymphomatoid papulosis. J Am Acad Dermatol. 2013;68:1046-1047.
- Nakamura-Wakatsuki T, Kato Y, Miura T, et al. Eruptive molluscum contagiosums in a patient with rheumatoid arthritis and lung cancer. Rheumatol Int. 2011;31:1117-1118.
- Ozyürek E, Sentürk N, Kefeli M, et al. Ulcerating molluscum contagiosum in a boy with relapsed acute lymphoblastic leukemia. J Pediatr Hematol Oncol. 2011;33:E114-E116.
- Hughes WT, Parham DM. Molluscum contagiosum in children with cancer or acquired immunodeficiency syndrome. Pediatr Infect Dis J. 1991;10:152-156.
- Euvrard S, Kanitakis J, Cochat P, et al. Skin diseases in children with organ transplants. J Am Acad Dermatol. 2001;44:932-939.
- Gardner LS, Ormond PJ. Treatment of multiple giant molluscum contagiosum in a renal transplant patient with imiquimod 5% cream. Clin Exp Dermatol. 2006;31:452-453.
- Mansur AT, Göktay F, Gündüz S, et al. Multiple giant molluscum contagiosum in a renal transplant recipient. Transpl Infect Dis. 2004;6:120-123.
- Feldmeyer L, Kamarashev J, Boehler A, et al. Molluscum contagiosum folliculitis mimicking tinea barbae in a lung transplant recipient. J Am Acad Dermatol. 2010;63:169-171.
- Tas¸kapan O, Yenicesu M, Aksu A. A giant solitary molluscum contagiosum, resembling nodular basal cell carcinoma, in a renal transplant recipient. Acta Derm Venereol. 1996;76:247-248.
- Tan HH, Goh CL. Viral infections affecting the skin in organ transplant recipients: epidemiology and current management strategies. Am J Clin Dermatol. 2006;7:13-29.
- Al-Mutairi N, Al-Doukhi A, Al-Farag S, et al. Comparative study on the efficacy, safety, and acceptability of imiquimod 5% cream versus cryotherapy for molluscum contagiosum in children. Pediatr Dermatol. 2010;27:388-394.
- Lim KS, Foo CC. Disseminated molluscum contagiosum in a patient with chronic plaque psoriasis taking methotrexate. Clin Exp Dermatol. 2007;32:591-593.
- Fotiadou C, Lazaridou E, Lekkas D, et al. Disseminated, eruptive molluscum contagiosum lesions in a psoriasis patient under treatment with methotrexate and cyclosporine. Eur J Dermatol. 2012;22:147-148.
- Antoniou C, Kosmadaki MG, Stratigos AJ, et al. Genital HPV lesions and molluscum contagiosum occurring in patients receiving anti-TNF-alpha therapy. Dermatology. 2008;216:364-365.
- Cursiefen C, Grunke M, Dechant C, et al. Multiple bilateral eyelid molluscum contagiosum lesions associated with TNFalpha-antibody and methotrexate therapy. Am J Ophthalmol. 2002;134:270-271.
- Heng YK, Lee JS, Neoh CY. Verrucous plaques in a pemphigus vulgaris patient on immunosuppressive therapy. Int J Dermatol. 2012;51:1044-1046.
- Goksugur N, Ozbostanci B, Goksugur SB. Molluscum contagiosum infection associated with pimecrolimus use in pityriasis alba. Pediatr Dermatol. 2007;24:E63-E65.
- Fernandes S, Pinto GM, Cardoso J. Particular clinical presentations of psoriasis in HIV patients. Int J STD AIDS. 2011;22:653-654.
Molluscum contagiosum (MC) is a double-stranded DNA virus of the Poxviridae family, which commonly infects human keratinocytes resulting in small, umbilicated, flesh-colored papules. The greatest incidence of MC is seen in the pediatric population and sexually active young adults, and it is considered a self-limited disease in immunocompetent individuals.1 With the emergence of the human immunodeficiency virus (HIV) and subsequent AIDS epidemic in the 1980s, a new population of immunocompromised individuals has been observed to be increasingly susceptible to MC with an atypical clinical presentation and a recalcitrant disease course.2 Although the increased prevalence of MC in the HIV population has been well-documented, it has been observed in other disease states or iatrogenically induced immunosuppression due to a deficiency in function or absolute number of T lymphocytes.
We present a case of a patient with long-standing psoriasis on biologic therapy who presented with MC with a subsequent workup that revealed AIDS. This case reiterates the importance of MC as a potential indicator of underlying immunosuppression. We review the literature to evaluate the occurrence of MC in immunosuppressed patients.
Case Report
A 33-year-old man initially presented for evaluation of severe plaque-type psoriasis associated with pain, erythema, and swelling of the joints of the hands of 10 years’ duration. He was started on methotrexate 5 mg weekly and topical corticosteroids but was unable to tolerate methotrexate due to headaches. He also had difficulty affording topical medications and adjunctive phototherapy. The patient was sporadically seen in follow-up with persistence of psoriatic plaques involving up to 60% body surface area (BSA) with the only treatment consisting of occasional topical steroids. Five years later, the patient was restarted on methotrexate 5 to 7.5 mg weekly, which resulted in moderate improvement. However, because of persistent elevation of liver enzymes, this treatment was stopped. Several months later he was evaluated for treatment with a biologic agent, and after a negative tuberculin skin test, he began treatment with etanercept 50 mg subcutaneous injection twice weekly, which provided notable improvement and allowed for reduction of dose frequency to once weekly.
At follow-up 1 year later, the patient had continued improvement of psoriasis with approximately 30% BSA on a treatment regimen of etanercept 50 mg weekly injection and topical corticosteroids. However, on physical examination, there were multiple small semitranslucent papules with telangiectases on the chest and upper back (Figure 1). Biopsy of a representative papule on the chest revealed MC (Figure 2). The patient was subsequently advised to stop etanercept and to return immediately to the clinic for HIV testing. He returned for follow-up 3 months later with pronounced worsening of disease and a new onset of blurred vision of the right eye. Cutaneous examination revealed numerous large erythematous plaques with superficial scale and cerebriform surface on the chest, back, abdomen, and upper and lower extremities involving 80% BSA (Figure 3). Biopsy of a plaque demonstrated psoriasiform dermatitis with neutrophils and parakeratosis consistent with psoriasis. Extensive blood work was notable for reactive HIV antibody and lymphopenia, CD4 lymphocyte count of 60 cells/mm3, and an HIV viral load of 247,000 copies/mL, meeting diagnostic criteria for AIDS. Additionally, ophthalmologic evaluation revealed toxoplasma retinitis. Upon initiation of highly active antiretroviral therapy (HAART) and continued use of topical corticosteroids, the patient experienced notable improvement of disease severity with approximately 20% BSA.



Comment
Molluscum contagiosum is a common skin infection. Among patients with HIV and other types of impaired cellular immunity, the prevalence of MC is estimated to be as high as 20%.3 The MC poxvirus survives and proliferates within the epidermis by interfering with tumor necrosis factor–induced apoptosis of virally infected cells; therefore, intact cell-mediated immunity is an important component of prevention and clearance of poxvirus infections. In immunocompromised patients, the presentation of MC varies widely, and the disease is often difficult to eradicate. This review will highlight the prevalence, presentation, and treatment of MC in the context of immunosuppressed states.
HIV/AIDS
Molluscum contagiosum in HIV-positive patients was first recognized in 1983,2 and its prevalence is estimated to range from 5% to 18% in AIDS patients.3 Molluscum contagiosum is a clinical sign of HIV progression, and its incidence appears to increase with reduced immune function (ie, a CD4 cell count <200/mm3).3 In a study of 456 patients with HIV-associated skin disorders, the majority of patients with MC had notable immunosuppression with a median survival time of 12 months. Thus, MC was not an independent prognostic marker but a clinical indicator of markedly reduced immune status.4
Molluscum contagiosum is transmitted in both sexual and nonsexual patterns in HIV-positive individuals, with the distribution of the latter involving primarily the face and neck. Although it may present with typical umbilicated papules, MC has a wide range of atypical clinical presentations in patients with AIDS that can make it difficult to diagnose. Complicated cases of eyelid MC have been reported in advanced HIV in both adults and children, resulting in obstruction of vision due to large lesions (up to 2 cm) or hundreds of confluent lesions.5 Giant MC, which appears as large exophytic nodules, is another presentation that has been frequently described in patients with advanced HIV. In these patients, the lesions often are too voluminous for conservative therapy and require excision.6 Atypical MC lesions also can resemble other dermatologic conditions, including condyloma acuminatum,7 nevus sebaceous of Jadassohn, ecthyma,8 and cutaneous horns,9,10 as well as other bacterial and fungal infections in HIV-positive patients, such as cutaneous Cryptococcus neoformans,11,12 disseminated histoplasmosis,13 and infections caused by Penicillium marneffei14 and Bartonella henselae.15 In most cases of MC in HIV-positive patients, diagnosis is dependent on the examination of biopsy specimens, which maintain the same histopathologic features regardless of immune status.
The management of MC in patients with HIV/AIDS is difficult. Molluscum contagiosum has shown no evidence of spontaneous resolution in patients with HIV, and treatment with one modality is often insufficient. Treatment is most successful when a combination approach is utilized with destructive procedures (eg, curettage, cryosurgery) and adjunctive agents (eg, retinoids, cantharidin, trichloroacetic acid). Imiquimod and cidofovir have been used off label for MC in AIDS patients.16 Imiquimod, which is used to treat genital warts, another cutaneous viral infection seen in patients with HIV, has demonstrated efficacy in treating MC.16 In a randomized controlled trial comparing imiquimod cream 5% to cryotherapy for MC in healthy children, imiquimod was slow acting but better suited than cryotherapy for patients with eruptions of many small lesions.17 For HIV patients, numerous reports have described successful treatment of disseminated or recalcitrant MC with topical imiquimod.18-20 Cidofovir, an antiviral used to treat cytomegalovirus retinitis in patients with AIDS, is a promising antiviral agent against the poxvirus family. In a study of viral DNA polymerase genes of MC virus, cidofovir inhibited MC virus DNA polymerase activity.21 It has been used in both topical (1% to 3%) and intravenous form to successfully treat recalcitrant and exuberant giant MC.6,22 However, the use of cidofovir is limited by its high costs, especially when compounded into a topical formulation.23
From a systemic standpoint, numerous reports have shown that treating the underlying HIV by optimizing HAART is the most important first step in clearing MC.24-27 However, a special concern regarding the initiation of HAART in patients with MC as well as a markedly impaired immune function is the development of an inflammatory reaction called immune reconstitution inflammatory syndrome (IRIS). This reaction is thought to be a result of immune recovery in severely immunosuppressed patients. During the initial phase of reconstitution when CD4 lymphocyte counts rise and viral load decreases, IRIS occurs due to an inflammatory reaction to microbial and autoimmune antigens, leading to temporary clinical deterioration.28 The incidence has been reported in up to 25% of patients starting HAART, and 52% to 78% of IRIS cases involve dermatologic manifestations such as varicella-zoster virus, cytomegalovirus infections, genital warts, and MC.29,30 In a cohort study of 199 patients, 2% of patients developed MC within 6 months of initiating HAART.31 In a case of exuberant MC lesions after beginning HAART, the lesions spontaneously resolved with the progression of immune reconstitution.28
Malignancies
Patients with hematologic malignancies such as lymphoma and leukemia comprise another subset of patients at risk for atypical presentations of MC. Molluscum contagiosum has been described in patients with hematologic malignancies such as adult T-cell leukemia/lymphoma, multiple myeloma, chronic myeloid leukemia, acute lymphoblastic leukemia, lymphomatoid papulosis, and non-Hodgkin lymphoma. In a review of MC in children with cancer, 0.5% were diagnosed with MC.32,33 Reports also have documented eruptive MC in the presence of solid organ cancers, including lung cancer.34
In patients with malignancies, the differential diagnosis should include other common dermatologic conditions such as varicella, herpes simplex, papillomas, pyoderma, and cutaneous cryptococcosis, as well as MC. Similar to HIV-positive patients, the lesions of MC described in patients with malignancies do not tend to spontaneously resolve. In a report of a pediatric patient with acute lymphoblastic leukemia, MC presented as an ulcerated lesion without any classic features, requiring biopsy for definitive diagnosis. Only partial resolution was achieved with cryotherapy and crusting of the lesion in an attempt to slow the progression.35 In a series of 5 children with hematologic malignancies and MC, little improvement was noted after treatment with surgical scraping, liquid nitrogen, and salicylic acid ointment 5%. Similar to patients with HIV, improvement of immune status and function help clear the disease, and patients who reach remission and discontinue chemotherapeutic agents have a higher rate of spontaneous resolution of previously recalcitrant MC lesions.36
Transplant Patients
Molluscum contagiosum in transplant patients has features similar to patients with HIV/AIDS. In organ transplant recipients, there is an increased risk for cutaneous disease from iatrogenic immunosuppression or immunosuppression through infectious or neoplastic processes.37 As in other immunocompromised populations, MC often has an atypical presentation in transplant patients with more extensive involvement and recalcitrant, rapidly recurring lesions.
In a review of 145 pediatric organ transplant recipients, MC was the fourth most common skin infection after verruca vulgaris, tinea versicolor, and herpes simplex/zoster. Affecting 7% of patients, the majority of patients demonstrated clinically typical lesions; however, the disease was difficult to eradicate if multiple lesions were present.37 In other reports in adults, fulminant and giant MC have been described after renal and other solid organ transplants.38,39 Molluscum contagiosum also has been reported to mimic other skin diseases in transplant patients including tinea barbae40 and nodular basal cell carcinomas.41
The standard treatments are identical to those used in patients with HIV, including ablative methods via liquid nitrogen, electrocautery, cantharidin, trichloroacetic acid, and topical retinoids. Similar to MC in other immunocompromised states, treatment can be difficult and usually requires multiple modalities. For children, imiquimod cream 5% has been recommended due to high clearance rates (up to 92%) and the painless nature of the treatment.42,43
Other Iatrogenic Immunosuppressive States
Immunosuppression through the use of steroids, chemotherapeutic agents, and biologic drugs often is the result of treatment of various diseases. In patients with psoriasis treated with systemic immunosuppressive agents, there are numerous reports that describe the appearance of eruptive MC in association with methotrexate, cyclosporine, and biologics. Methotrexate acts as an immunosuppressive agent by binding to dihydrofolate reductase, which inhibits DNA synthesis in immunologically competent cells.44 It also may block host defense mechanisms against MC by suppressing the expression of serum inflammatory cytokines such as tumor necrosis factor α (TNF-α) and IFN-γ and suppressing the activity of TNF-α inducing apoptosis of virus-infected cells. Cyclosporine used in conjunction with methotrexate may exacerbate the insult to the immune system by inhibiting the production of IFN-γ.45 Biologics are an emerging class of drugs that have demonstrated efficacy in moderate to severe psoriasis by inhibiting TNF-α or other inflammatory molecules. Several published reports have described eruptive or atypical MC in patients on biologic medications. In one case, within 2 weeks after initiation of infliximab, a monoclonal antibody against TNF-α, a patient developed an eruption of MC involving the entire body.46 In another report, an anti–TNF-α agent for rheumatoid arthritis was associated with atypical MC with eyelid lesions.47
There are other skin disorders treated with immunosuppressive agents that also have been associated with MC. In a patient with pemphigus vulgaris treated with prednisolone, pimecrolimus, and azathioprine, MC lesions were observed on the face and within healed pemphigus vulgaris sites.48 Pimecrolimus and tacrolimus, corticosteroid-sparing agents, suppress cell-mediated immunity and inhibit inflammatory cytokines such as IL-2. The infection resolved with a gradual tapering of immunosuppressive therapy and 10 sessions of cryotherapy.48 In a case of topical pimecrolimus for pityriasis alba, the patient developed biopsy-proven MC within 2 weeks of initiating treatment in the areas that were treated with tacrolimus.49
In nontransplant patients with iatrogenic immunosuppression, MC treatment has not been documented to be as challenging as in patients with inherent immunosuppression. Most patients respond to either withdrawal of the drug alone or to simple ablative treatments such as cryotherapy.45,46,48 This important difference is most likely due to the presence of an otherwise intact immune system.
Conclusion
This case describes the appearance of MC in a patient with psoriasis treated with a TNF-α inhibitor who was ultimately diagnosed with AIDS. Although atypical MC infections have been documented in patients with psoriasis undergoing treatment with biologics, it is thought to be more common for MC to occur in more remarkably immunocompromised states such as AIDS. Thus, the persistence and progression of MC in our patient despite discontinuation of etanercept suggested a separate underlying process. Subsequent workup led to the diagnosis of AIDS along with the opportunistic ocular infection of toxoplasmosis retinitis. This clinical sequence consisting of psoriasis treated with a biologic agent, development of MC, and subsequent diagnosis of AIDS is unique and clinically significant to dermatologists. The presentation of psoriasis in patients with HIV can be diverse with different levels of severity and atypical clinical features. In many cases, HIV is known to exacerbate the classic clinical presentation of psoriasis. However, there are other particular presentations of psoriasis in HIV patients that have been observed, which include a predilection for scalp lesions, palmoplantar keratoderma, flexural involvement, and higher levels of immunodeficiency.50 Although tuberculin skin tests are required prior to initiating biologic therapy due to the potential for disease reactivation, there are no requirements for HIV antibody testing. In cases of severe recalcitrant psoriasis, an HIV test should be ordered during the workup to establish an early diagnosis so that an HIV-positive patient can avoid poor outcomes from either the disease processes, the use of certain therapeutic agents, or both. Furthermore, the benefit of avoiding possible harm to the patient and potential legal action outweighs the cost of performing surveillance HIV testing in this subset of patients. Thus, due to the potential additive immunosuppressive effect of HIV with biologic therapy, providers should always assess for risk factors and consider testing for HIV in all patients before initiating treatment with immunosuppressive agents such as biologics.
Molluscum contagiosum (MC) is a double-stranded DNA virus of the Poxviridae family, which commonly infects human keratinocytes resulting in small, umbilicated, flesh-colored papules. The greatest incidence of MC is seen in the pediatric population and sexually active young adults, and it is considered a self-limited disease in immunocompetent individuals.1 With the emergence of the human immunodeficiency virus (HIV) and subsequent AIDS epidemic in the 1980s, a new population of immunocompromised individuals has been observed to be increasingly susceptible to MC with an atypical clinical presentation and a recalcitrant disease course.2 Although the increased prevalence of MC in the HIV population has been well-documented, it has been observed in other disease states or iatrogenically induced immunosuppression due to a deficiency in function or absolute number of T lymphocytes.
We present a case of a patient with long-standing psoriasis on biologic therapy who presented with MC with a subsequent workup that revealed AIDS. This case reiterates the importance of MC as a potential indicator of underlying immunosuppression. We review the literature to evaluate the occurrence of MC in immunosuppressed patients.
Case Report
A 33-year-old man initially presented for evaluation of severe plaque-type psoriasis associated with pain, erythema, and swelling of the joints of the hands of 10 years’ duration. He was started on methotrexate 5 mg weekly and topical corticosteroids but was unable to tolerate methotrexate due to headaches. He also had difficulty affording topical medications and adjunctive phototherapy. The patient was sporadically seen in follow-up with persistence of psoriatic plaques involving up to 60% body surface area (BSA) with the only treatment consisting of occasional topical steroids. Five years later, the patient was restarted on methotrexate 5 to 7.5 mg weekly, which resulted in moderate improvement. However, because of persistent elevation of liver enzymes, this treatment was stopped. Several months later he was evaluated for treatment with a biologic agent, and after a negative tuberculin skin test, he began treatment with etanercept 50 mg subcutaneous injection twice weekly, which provided notable improvement and allowed for reduction of dose frequency to once weekly.
At follow-up 1 year later, the patient had continued improvement of psoriasis with approximately 30% BSA on a treatment regimen of etanercept 50 mg weekly injection and topical corticosteroids. However, on physical examination, there were multiple small semitranslucent papules with telangiectases on the chest and upper back (Figure 1). Biopsy of a representative papule on the chest revealed MC (Figure 2). The patient was subsequently advised to stop etanercept and to return immediately to the clinic for HIV testing. He returned for follow-up 3 months later with pronounced worsening of disease and a new onset of blurred vision of the right eye. Cutaneous examination revealed numerous large erythematous plaques with superficial scale and cerebriform surface on the chest, back, abdomen, and upper and lower extremities involving 80% BSA (Figure 3). Biopsy of a plaque demonstrated psoriasiform dermatitis with neutrophils and parakeratosis consistent with psoriasis. Extensive blood work was notable for reactive HIV antibody and lymphopenia, CD4 lymphocyte count of 60 cells/mm3, and an HIV viral load of 247,000 copies/mL, meeting diagnostic criteria for AIDS. Additionally, ophthalmologic evaluation revealed toxoplasma retinitis. Upon initiation of highly active antiretroviral therapy (HAART) and continued use of topical corticosteroids, the patient experienced notable improvement of disease severity with approximately 20% BSA.



Comment
Molluscum contagiosum is a common skin infection. Among patients with HIV and other types of impaired cellular immunity, the prevalence of MC is estimated to be as high as 20%.3 The MC poxvirus survives and proliferates within the epidermis by interfering with tumor necrosis factor–induced apoptosis of virally infected cells; therefore, intact cell-mediated immunity is an important component of prevention and clearance of poxvirus infections. In immunocompromised patients, the presentation of MC varies widely, and the disease is often difficult to eradicate. This review will highlight the prevalence, presentation, and treatment of MC in the context of immunosuppressed states.
HIV/AIDS
Molluscum contagiosum in HIV-positive patients was first recognized in 1983,2 and its prevalence is estimated to range from 5% to 18% in AIDS patients.3 Molluscum contagiosum is a clinical sign of HIV progression, and its incidence appears to increase with reduced immune function (ie, a CD4 cell count <200/mm3).3 In a study of 456 patients with HIV-associated skin disorders, the majority of patients with MC had notable immunosuppression with a median survival time of 12 months. Thus, MC was not an independent prognostic marker but a clinical indicator of markedly reduced immune status.4
Molluscum contagiosum is transmitted in both sexual and nonsexual patterns in HIV-positive individuals, with the distribution of the latter involving primarily the face and neck. Although it may present with typical umbilicated papules, MC has a wide range of atypical clinical presentations in patients with AIDS that can make it difficult to diagnose. Complicated cases of eyelid MC have been reported in advanced HIV in both adults and children, resulting in obstruction of vision due to large lesions (up to 2 cm) or hundreds of confluent lesions.5 Giant MC, which appears as large exophytic nodules, is another presentation that has been frequently described in patients with advanced HIV. In these patients, the lesions often are too voluminous for conservative therapy and require excision.6 Atypical MC lesions also can resemble other dermatologic conditions, including condyloma acuminatum,7 nevus sebaceous of Jadassohn, ecthyma,8 and cutaneous horns,9,10 as well as other bacterial and fungal infections in HIV-positive patients, such as cutaneous Cryptococcus neoformans,11,12 disseminated histoplasmosis,13 and infections caused by Penicillium marneffei14 and Bartonella henselae.15 In most cases of MC in HIV-positive patients, diagnosis is dependent on the examination of biopsy specimens, which maintain the same histopathologic features regardless of immune status.
The management of MC in patients with HIV/AIDS is difficult. Molluscum contagiosum has shown no evidence of spontaneous resolution in patients with HIV, and treatment with one modality is often insufficient. Treatment is most successful when a combination approach is utilized with destructive procedures (eg, curettage, cryosurgery) and adjunctive agents (eg, retinoids, cantharidin, trichloroacetic acid). Imiquimod and cidofovir have been used off label for MC in AIDS patients.16 Imiquimod, which is used to treat genital warts, another cutaneous viral infection seen in patients with HIV, has demonstrated efficacy in treating MC.16 In a randomized controlled trial comparing imiquimod cream 5% to cryotherapy for MC in healthy children, imiquimod was slow acting but better suited than cryotherapy for patients with eruptions of many small lesions.17 For HIV patients, numerous reports have described successful treatment of disseminated or recalcitrant MC with topical imiquimod.18-20 Cidofovir, an antiviral used to treat cytomegalovirus retinitis in patients with AIDS, is a promising antiviral agent against the poxvirus family. In a study of viral DNA polymerase genes of MC virus, cidofovir inhibited MC virus DNA polymerase activity.21 It has been used in both topical (1% to 3%) and intravenous form to successfully treat recalcitrant and exuberant giant MC.6,22 However, the use of cidofovir is limited by its high costs, especially when compounded into a topical formulation.23
From a systemic standpoint, numerous reports have shown that treating the underlying HIV by optimizing HAART is the most important first step in clearing MC.24-27 However, a special concern regarding the initiation of HAART in patients with MC as well as a markedly impaired immune function is the development of an inflammatory reaction called immune reconstitution inflammatory syndrome (IRIS). This reaction is thought to be a result of immune recovery in severely immunosuppressed patients. During the initial phase of reconstitution when CD4 lymphocyte counts rise and viral load decreases, IRIS occurs due to an inflammatory reaction to microbial and autoimmune antigens, leading to temporary clinical deterioration.28 The incidence has been reported in up to 25% of patients starting HAART, and 52% to 78% of IRIS cases involve dermatologic manifestations such as varicella-zoster virus, cytomegalovirus infections, genital warts, and MC.29,30 In a cohort study of 199 patients, 2% of patients developed MC within 6 months of initiating HAART.31 In a case of exuberant MC lesions after beginning HAART, the lesions spontaneously resolved with the progression of immune reconstitution.28
Malignancies
Patients with hematologic malignancies such as lymphoma and leukemia comprise another subset of patients at risk for atypical presentations of MC. Molluscum contagiosum has been described in patients with hematologic malignancies such as adult T-cell leukemia/lymphoma, multiple myeloma, chronic myeloid leukemia, acute lymphoblastic leukemia, lymphomatoid papulosis, and non-Hodgkin lymphoma. In a review of MC in children with cancer, 0.5% were diagnosed with MC.32,33 Reports also have documented eruptive MC in the presence of solid organ cancers, including lung cancer.34
In patients with malignancies, the differential diagnosis should include other common dermatologic conditions such as varicella, herpes simplex, papillomas, pyoderma, and cutaneous cryptococcosis, as well as MC. Similar to HIV-positive patients, the lesions of MC described in patients with malignancies do not tend to spontaneously resolve. In a report of a pediatric patient with acute lymphoblastic leukemia, MC presented as an ulcerated lesion without any classic features, requiring biopsy for definitive diagnosis. Only partial resolution was achieved with cryotherapy and crusting of the lesion in an attempt to slow the progression.35 In a series of 5 children with hematologic malignancies and MC, little improvement was noted after treatment with surgical scraping, liquid nitrogen, and salicylic acid ointment 5%. Similar to patients with HIV, improvement of immune status and function help clear the disease, and patients who reach remission and discontinue chemotherapeutic agents have a higher rate of spontaneous resolution of previously recalcitrant MC lesions.36
Transplant Patients
Molluscum contagiosum in transplant patients has features similar to patients with HIV/AIDS. In organ transplant recipients, there is an increased risk for cutaneous disease from iatrogenic immunosuppression or immunosuppression through infectious or neoplastic processes.37 As in other immunocompromised populations, MC often has an atypical presentation in transplant patients with more extensive involvement and recalcitrant, rapidly recurring lesions.
In a review of 145 pediatric organ transplant recipients, MC was the fourth most common skin infection after verruca vulgaris, tinea versicolor, and herpes simplex/zoster. Affecting 7% of patients, the majority of patients demonstrated clinically typical lesions; however, the disease was difficult to eradicate if multiple lesions were present.37 In other reports in adults, fulminant and giant MC have been described after renal and other solid organ transplants.38,39 Molluscum contagiosum also has been reported to mimic other skin diseases in transplant patients including tinea barbae40 and nodular basal cell carcinomas.41
The standard treatments are identical to those used in patients with HIV, including ablative methods via liquid nitrogen, electrocautery, cantharidin, trichloroacetic acid, and topical retinoids. Similar to MC in other immunocompromised states, treatment can be difficult and usually requires multiple modalities. For children, imiquimod cream 5% has been recommended due to high clearance rates (up to 92%) and the painless nature of the treatment.42,43
Other Iatrogenic Immunosuppressive States
Immunosuppression through the use of steroids, chemotherapeutic agents, and biologic drugs often is the result of treatment of various diseases. In patients with psoriasis treated with systemic immunosuppressive agents, there are numerous reports that describe the appearance of eruptive MC in association with methotrexate, cyclosporine, and biologics. Methotrexate acts as an immunosuppressive agent by binding to dihydrofolate reductase, which inhibits DNA synthesis in immunologically competent cells.44 It also may block host defense mechanisms against MC by suppressing the expression of serum inflammatory cytokines such as tumor necrosis factor α (TNF-α) and IFN-γ and suppressing the activity of TNF-α inducing apoptosis of virus-infected cells. Cyclosporine used in conjunction with methotrexate may exacerbate the insult to the immune system by inhibiting the production of IFN-γ.45 Biologics are an emerging class of drugs that have demonstrated efficacy in moderate to severe psoriasis by inhibiting TNF-α or other inflammatory molecules. Several published reports have described eruptive or atypical MC in patients on biologic medications. In one case, within 2 weeks after initiation of infliximab, a monoclonal antibody against TNF-α, a patient developed an eruption of MC involving the entire body.46 In another report, an anti–TNF-α agent for rheumatoid arthritis was associated with atypical MC with eyelid lesions.47
There are other skin disorders treated with immunosuppressive agents that also have been associated with MC. In a patient with pemphigus vulgaris treated with prednisolone, pimecrolimus, and azathioprine, MC lesions were observed on the face and within healed pemphigus vulgaris sites.48 Pimecrolimus and tacrolimus, corticosteroid-sparing agents, suppress cell-mediated immunity and inhibit inflammatory cytokines such as IL-2. The infection resolved with a gradual tapering of immunosuppressive therapy and 10 sessions of cryotherapy.48 In a case of topical pimecrolimus for pityriasis alba, the patient developed biopsy-proven MC within 2 weeks of initiating treatment in the areas that were treated with tacrolimus.49
In nontransplant patients with iatrogenic immunosuppression, MC treatment has not been documented to be as challenging as in patients with inherent immunosuppression. Most patients respond to either withdrawal of the drug alone or to simple ablative treatments such as cryotherapy.45,46,48 This important difference is most likely due to the presence of an otherwise intact immune system.
Conclusion
This case describes the appearance of MC in a patient with psoriasis treated with a TNF-α inhibitor who was ultimately diagnosed with AIDS. Although atypical MC infections have been documented in patients with psoriasis undergoing treatment with biologics, it is thought to be more common for MC to occur in more remarkably immunocompromised states such as AIDS. Thus, the persistence and progression of MC in our patient despite discontinuation of etanercept suggested a separate underlying process. Subsequent workup led to the diagnosis of AIDS along with the opportunistic ocular infection of toxoplasmosis retinitis. This clinical sequence consisting of psoriasis treated with a biologic agent, development of MC, and subsequent diagnosis of AIDS is unique and clinically significant to dermatologists. The presentation of psoriasis in patients with HIV can be diverse with different levels of severity and atypical clinical features. In many cases, HIV is known to exacerbate the classic clinical presentation of psoriasis. However, there are other particular presentations of psoriasis in HIV patients that have been observed, which include a predilection for scalp lesions, palmoplantar keratoderma, flexural involvement, and higher levels of immunodeficiency.50 Although tuberculin skin tests are required prior to initiating biologic therapy due to the potential for disease reactivation, there are no requirements for HIV antibody testing. In cases of severe recalcitrant psoriasis, an HIV test should be ordered during the workup to establish an early diagnosis so that an HIV-positive patient can avoid poor outcomes from either the disease processes, the use of certain therapeutic agents, or both. Furthermore, the benefit of avoiding possible harm to the patient and potential legal action outweighs the cost of performing surveillance HIV testing in this subset of patients. Thus, due to the potential additive immunosuppressive effect of HIV with biologic therapy, providers should always assess for risk factors and consider testing for HIV in all patients before initiating treatment with immunosuppressive agents such as biologics.
- Dohil MA, Lin P, Lee J, et al. The epidemiology of molluscum contagiosum in children. J Am Acad Dermatol. 2006;54:47-54.
- Reichert CM, O’Leary TJ, Levens DL, et al. Autopsy pathology in the acquired immune deficiency syndrome. Am J Pathol. 1983;112:357-382.
- Czelusta A, Yen-Moore A, Van der Straten M, et al. An overview of sexually transmitted diseases. Part III. Sexually transmitted diseases in HIV-infected patients. J Am Acad Dermatol. 2000;43:409-432.
- Husak R, Garbe C, Orfanos CE. Mollusca contagiosa in HIV infection. Clinical manifestation, relation to immune status and prognostic value in 39 patients [in German]. Hautarzt. 1997;48:103-109.
- Averbuch D, Jaouni T, Pe’er J, et al. Confluent molluscum contagiosum covering the eyelids of an HIV-positive child. Clin Exp Ophthalmol. 2009;37:525-527.
- Erickson C, Driscoll M, Gaspari A. Efficacy of intravenous cidofovir in the treatment of giant molluscum contagiosum in a patient with human immunodeficiency virus. Arch Dermatol. 2011;147:652-654.
- Mastrolorenzo A, Urbano FG, Salimbeni L, et al. Atypical molluscum contagiosum infection in an HIV-infected patient. Int J Dermatol. 1998;37:378-380.
- Itin PH, Gilli L. Molluscum contagiosum mimicking sebaceous nevus of Jadassohn, ecthyma and giant condylomata acuminata in HIV-infected patients. Dermatology. 1994;189:396-398.
- Sim JH, Lee ES. Molluscum contagiosum presenting as a cutaneous horn. Ann Dermatol. 2011;23:262-263.
- Manchanda Y, Sethuraman G, Paderwani PP, et al. Molluscum contagiosum presenting as penile horn in an HIV positive patient. Sex Transm Infect. 2005;81:183-184.
- Miller SJ. Cutaneous cryptococcus resembling molluscum contagiosum in a patient with acquired immunodeficiency syndrome. Cutis. 1988;41:411-412.
- Sornum A. A mistaken diagnosis of molluscum contagiosum in a HIV-positive patient in rural South Africa. BMJ Case Rep. 2012;14.
- Corti M, Villafañe MF, Palmieri O, et al. Rupioid histoplasmosis: first case reported in an AIDS patient in Argentina. Rev Inst Med Trop Sao Paulo. 2010;52:279-280.
- Saikia L, Nath R, Hazarika D, et al. Atypical cutaneous lesions of Penicillium marneffei infection as a manifestation of the immune reconstitution inflammatory syndrome after highly active antiretroviral therapy. Indian J Dermatol Venereol Leprol. 2010;76:45-48.
- de Souza JA. Molluscum or a mimic? Am J Med. 2006;119:927-929.
- Conant MA. Immunomodulatory therapy in the management of viral infections in patients with HIV infection. J Am Acad Dermatol. 2000;43:S27-S30.
- Gamble RG, Echols KF, Dellavalle RP. Imiquimod vs cryotherapy for molluscum contagiosum: a randomized controlled trial. Arch Dermatol. 2012;148:109-112.
- Brown CW Jr, O’Donoghue M, Moore J, et al. Recalcitrant molluscum contagiosum in an HIV-afflicted male treated successfully with topical imiquimod. Cutis. 2000;65:363-366.
- Strauss RM, Doyle EL, Mohsen AH, et al. Successful treatment of molluscum contagiosum with topical imiquimod in a severely immunocompromised HIV-positive patient. Int J STD AIDS. 2001;12:264-266.
- Theiler M, Kempf W, Kerl K, et al. Disseminated molluscum contagiosum in a HIV-positive child. improvement after therapy with 5% imiquimod. J Dermatol Case Rep. 2011;5:19-23.
- Watanabe T, Tamaki K. Cidofovir diphosphate inhibits molluscum contagiosum virus DNA polymerase activity. J Invest Dermatol. 2008;128:1327-1329.
- Calista D. Topical cidofovir for severe cutaneous human papillomavirus and molluscum contagiosum infections in patients with HIV/AIDS. a pilot study. J Eur Acad Dermatol Venereol. 2000;14:484-488.
- Toro JR, Sanchez S, Turiansky G, et al. Topical cidofovir for the treatment of dermatologic conditions: verruca, condyloma, intraepithelial neoplasia, herpes simplex and its potential use in smallpox. Dermatol Clin. 2003;21:301-309.
- Calista D, Boschini A, Landi G. Resolution of disseminated molluscum contagiosum with highly active anti-retroviral therapy (HAART) in patients with AIDS. Eur J Dermatol. 1999;9:211-213.
- Cattelan AM, Sasset L, Corti L, et al. A complete remission of recalcitrant molluscum contagiosum in an AIDS patient following highly active antiretroviral therapy (HAART). J Infect. 1999;38:58-60.
- Sen S, Bhaumik P. Resolution of giant molluscum contagiosum with antiretroviral therapy. Indian J Dermatol Venereol Leprol. 2008;74:267-268.
- Sen S, Goswami BK, Karjyi N, et al. Disfiguring molluscum contagiosum in a HIV-positive patient responding to antiretroviral therapy. Indian J Dermatol. 2009;54:180-182.
- Pereira B, Fernandes C, Nachiambo E, et al. Exuberant molluscum contagiosum as a manifestation of the immune reconstitution inflammatory syndrome. Dermatol Online J. 2007;13:6.
- Osei-Sekyere B, Karstaedt AS. Immune reconstitution inflammatory syndrome involving the skin. Clin Exp Dermatol. 2010;35:477-481.
- Sung KU, Lee HE, Choi WR, et al. Molluscum contagiosum as a skin manifestation of immune reconstitution inflammatory syndrome in an AIDS patient who is receiving HAART. Korean J Fam Med. 2012;33:182-185.
- Ratnam I, Chiu C, Kandala NB, et al. Incidence and risk factors for immune reconstitution inflammatory syndrome in an ethnically diverse HIV type 1-infected cohort. Clin Infect Dis. 2006;42:418-427.
- Chen KW, Yang CF, Huang CT, et al. Molluscum contagiosum in a patient with adult T-cell leukaemia/lymphoma. Br J Haematol. 2011;155:286.
- Fernandez KH, Bream M, Ali MA, et al. Investigation of molluscum contagiosum virus, orf and other parapoxviruses in lymphomatoid papulosis. J Am Acad Dermatol. 2013;68:1046-1047.
- Nakamura-Wakatsuki T, Kato Y, Miura T, et al. Eruptive molluscum contagiosums in a patient with rheumatoid arthritis and lung cancer. Rheumatol Int. 2011;31:1117-1118.
- Ozyürek E, Sentürk N, Kefeli M, et al. Ulcerating molluscum contagiosum in a boy with relapsed acute lymphoblastic leukemia. J Pediatr Hematol Oncol. 2011;33:E114-E116.
- Hughes WT, Parham DM. Molluscum contagiosum in children with cancer or acquired immunodeficiency syndrome. Pediatr Infect Dis J. 1991;10:152-156.
- Euvrard S, Kanitakis J, Cochat P, et al. Skin diseases in children with organ transplants. J Am Acad Dermatol. 2001;44:932-939.
- Gardner LS, Ormond PJ. Treatment of multiple giant molluscum contagiosum in a renal transplant patient with imiquimod 5% cream. Clin Exp Dermatol. 2006;31:452-453.
- Mansur AT, Göktay F, Gündüz S, et al. Multiple giant molluscum contagiosum in a renal transplant recipient. Transpl Infect Dis. 2004;6:120-123.
- Feldmeyer L, Kamarashev J, Boehler A, et al. Molluscum contagiosum folliculitis mimicking tinea barbae in a lung transplant recipient. J Am Acad Dermatol. 2010;63:169-171.
- Tas¸kapan O, Yenicesu M, Aksu A. A giant solitary molluscum contagiosum, resembling nodular basal cell carcinoma, in a renal transplant recipient. Acta Derm Venereol. 1996;76:247-248.
- Tan HH, Goh CL. Viral infections affecting the skin in organ transplant recipients: epidemiology and current management strategies. Am J Clin Dermatol. 2006;7:13-29.
- Al-Mutairi N, Al-Doukhi A, Al-Farag S, et al. Comparative study on the efficacy, safety, and acceptability of imiquimod 5% cream versus cryotherapy for molluscum contagiosum in children. Pediatr Dermatol. 2010;27:388-394.
- Lim KS, Foo CC. Disseminated molluscum contagiosum in a patient with chronic plaque psoriasis taking methotrexate. Clin Exp Dermatol. 2007;32:591-593.
- Fotiadou C, Lazaridou E, Lekkas D, et al. Disseminated, eruptive molluscum contagiosum lesions in a psoriasis patient under treatment with methotrexate and cyclosporine. Eur J Dermatol. 2012;22:147-148.
- Antoniou C, Kosmadaki MG, Stratigos AJ, et al. Genital HPV lesions and molluscum contagiosum occurring in patients receiving anti-TNF-alpha therapy. Dermatology. 2008;216:364-365.
- Cursiefen C, Grunke M, Dechant C, et al. Multiple bilateral eyelid molluscum contagiosum lesions associated with TNFalpha-antibody and methotrexate therapy. Am J Ophthalmol. 2002;134:270-271.
- Heng YK, Lee JS, Neoh CY. Verrucous plaques in a pemphigus vulgaris patient on immunosuppressive therapy. Int J Dermatol. 2012;51:1044-1046.
- Goksugur N, Ozbostanci B, Goksugur SB. Molluscum contagiosum infection associated with pimecrolimus use in pityriasis alba. Pediatr Dermatol. 2007;24:E63-E65.
- Fernandes S, Pinto GM, Cardoso J. Particular clinical presentations of psoriasis in HIV patients. Int J STD AIDS. 2011;22:653-654.
- Dohil MA, Lin P, Lee J, et al. The epidemiology of molluscum contagiosum in children. J Am Acad Dermatol. 2006;54:47-54.
- Reichert CM, O’Leary TJ, Levens DL, et al. Autopsy pathology in the acquired immune deficiency syndrome. Am J Pathol. 1983;112:357-382.
- Czelusta A, Yen-Moore A, Van der Straten M, et al. An overview of sexually transmitted diseases. Part III. Sexually transmitted diseases in HIV-infected patients. J Am Acad Dermatol. 2000;43:409-432.
- Husak R, Garbe C, Orfanos CE. Mollusca contagiosa in HIV infection. Clinical manifestation, relation to immune status and prognostic value in 39 patients [in German]. Hautarzt. 1997;48:103-109.
- Averbuch D, Jaouni T, Pe’er J, et al. Confluent molluscum contagiosum covering the eyelids of an HIV-positive child. Clin Exp Ophthalmol. 2009;37:525-527.
- Erickson C, Driscoll M, Gaspari A. Efficacy of intravenous cidofovir in the treatment of giant molluscum contagiosum in a patient with human immunodeficiency virus. Arch Dermatol. 2011;147:652-654.
- Mastrolorenzo A, Urbano FG, Salimbeni L, et al. Atypical molluscum contagiosum infection in an HIV-infected patient. Int J Dermatol. 1998;37:378-380.
- Itin PH, Gilli L. Molluscum contagiosum mimicking sebaceous nevus of Jadassohn, ecthyma and giant condylomata acuminata in HIV-infected patients. Dermatology. 1994;189:396-398.
- Sim JH, Lee ES. Molluscum contagiosum presenting as a cutaneous horn. Ann Dermatol. 2011;23:262-263.
- Manchanda Y, Sethuraman G, Paderwani PP, et al. Molluscum contagiosum presenting as penile horn in an HIV positive patient. Sex Transm Infect. 2005;81:183-184.
- Miller SJ. Cutaneous cryptococcus resembling molluscum contagiosum in a patient with acquired immunodeficiency syndrome. Cutis. 1988;41:411-412.
- Sornum A. A mistaken diagnosis of molluscum contagiosum in a HIV-positive patient in rural South Africa. BMJ Case Rep. 2012;14.
- Corti M, Villafañe MF, Palmieri O, et al. Rupioid histoplasmosis: first case reported in an AIDS patient in Argentina. Rev Inst Med Trop Sao Paulo. 2010;52:279-280.
- Saikia L, Nath R, Hazarika D, et al. Atypical cutaneous lesions of Penicillium marneffei infection as a manifestation of the immune reconstitution inflammatory syndrome after highly active antiretroviral therapy. Indian J Dermatol Venereol Leprol. 2010;76:45-48.
- de Souza JA. Molluscum or a mimic? Am J Med. 2006;119:927-929.
- Conant MA. Immunomodulatory therapy in the management of viral infections in patients with HIV infection. J Am Acad Dermatol. 2000;43:S27-S30.
- Gamble RG, Echols KF, Dellavalle RP. Imiquimod vs cryotherapy for molluscum contagiosum: a randomized controlled trial. Arch Dermatol. 2012;148:109-112.
- Brown CW Jr, O’Donoghue M, Moore J, et al. Recalcitrant molluscum contagiosum in an HIV-afflicted male treated successfully with topical imiquimod. Cutis. 2000;65:363-366.
- Strauss RM, Doyle EL, Mohsen AH, et al. Successful treatment of molluscum contagiosum with topical imiquimod in a severely immunocompromised HIV-positive patient. Int J STD AIDS. 2001;12:264-266.
- Theiler M, Kempf W, Kerl K, et al. Disseminated molluscum contagiosum in a HIV-positive child. improvement after therapy with 5% imiquimod. J Dermatol Case Rep. 2011;5:19-23.
- Watanabe T, Tamaki K. Cidofovir diphosphate inhibits molluscum contagiosum virus DNA polymerase activity. J Invest Dermatol. 2008;128:1327-1329.
- Calista D. Topical cidofovir for severe cutaneous human papillomavirus and molluscum contagiosum infections in patients with HIV/AIDS. a pilot study. J Eur Acad Dermatol Venereol. 2000;14:484-488.
- Toro JR, Sanchez S, Turiansky G, et al. Topical cidofovir for the treatment of dermatologic conditions: verruca, condyloma, intraepithelial neoplasia, herpes simplex and its potential use in smallpox. Dermatol Clin. 2003;21:301-309.
- Calista D, Boschini A, Landi G. Resolution of disseminated molluscum contagiosum with highly active anti-retroviral therapy (HAART) in patients with AIDS. Eur J Dermatol. 1999;9:211-213.
- Cattelan AM, Sasset L, Corti L, et al. A complete remission of recalcitrant molluscum contagiosum in an AIDS patient following highly active antiretroviral therapy (HAART). J Infect. 1999;38:58-60.
- Sen S, Bhaumik P. Resolution of giant molluscum contagiosum with antiretroviral therapy. Indian J Dermatol Venereol Leprol. 2008;74:267-268.
- Sen S, Goswami BK, Karjyi N, et al. Disfiguring molluscum contagiosum in a HIV-positive patient responding to antiretroviral therapy. Indian J Dermatol. 2009;54:180-182.
- Pereira B, Fernandes C, Nachiambo E, et al. Exuberant molluscum contagiosum as a manifestation of the immune reconstitution inflammatory syndrome. Dermatol Online J. 2007;13:6.
- Osei-Sekyere B, Karstaedt AS. Immune reconstitution inflammatory syndrome involving the skin. Clin Exp Dermatol. 2010;35:477-481.
- Sung KU, Lee HE, Choi WR, et al. Molluscum contagiosum as a skin manifestation of immune reconstitution inflammatory syndrome in an AIDS patient who is receiving HAART. Korean J Fam Med. 2012;33:182-185.
- Ratnam I, Chiu C, Kandala NB, et al. Incidence and risk factors for immune reconstitution inflammatory syndrome in an ethnically diverse HIV type 1-infected cohort. Clin Infect Dis. 2006;42:418-427.
- Chen KW, Yang CF, Huang CT, et al. Molluscum contagiosum in a patient with adult T-cell leukaemia/lymphoma. Br J Haematol. 2011;155:286.
- Fernandez KH, Bream M, Ali MA, et al. Investigation of molluscum contagiosum virus, orf and other parapoxviruses in lymphomatoid papulosis. J Am Acad Dermatol. 2013;68:1046-1047.
- Nakamura-Wakatsuki T, Kato Y, Miura T, et al. Eruptive molluscum contagiosums in a patient with rheumatoid arthritis and lung cancer. Rheumatol Int. 2011;31:1117-1118.
- Ozyürek E, Sentürk N, Kefeli M, et al. Ulcerating molluscum contagiosum in a boy with relapsed acute lymphoblastic leukemia. J Pediatr Hematol Oncol. 2011;33:E114-E116.
- Hughes WT, Parham DM. Molluscum contagiosum in children with cancer or acquired immunodeficiency syndrome. Pediatr Infect Dis J. 1991;10:152-156.
- Euvrard S, Kanitakis J, Cochat P, et al. Skin diseases in children with organ transplants. J Am Acad Dermatol. 2001;44:932-939.
- Gardner LS, Ormond PJ. Treatment of multiple giant molluscum contagiosum in a renal transplant patient with imiquimod 5% cream. Clin Exp Dermatol. 2006;31:452-453.
- Mansur AT, Göktay F, Gündüz S, et al. Multiple giant molluscum contagiosum in a renal transplant recipient. Transpl Infect Dis. 2004;6:120-123.
- Feldmeyer L, Kamarashev J, Boehler A, et al. Molluscum contagiosum folliculitis mimicking tinea barbae in a lung transplant recipient. J Am Acad Dermatol. 2010;63:169-171.
- Tas¸kapan O, Yenicesu M, Aksu A. A giant solitary molluscum contagiosum, resembling nodular basal cell carcinoma, in a renal transplant recipient. Acta Derm Venereol. 1996;76:247-248.
- Tan HH, Goh CL. Viral infections affecting the skin in organ transplant recipients: epidemiology and current management strategies. Am J Clin Dermatol. 2006;7:13-29.
- Al-Mutairi N, Al-Doukhi A, Al-Farag S, et al. Comparative study on the efficacy, safety, and acceptability of imiquimod 5% cream versus cryotherapy for molluscum contagiosum in children. Pediatr Dermatol. 2010;27:388-394.
- Lim KS, Foo CC. Disseminated molluscum contagiosum in a patient with chronic plaque psoriasis taking methotrexate. Clin Exp Dermatol. 2007;32:591-593.
- Fotiadou C, Lazaridou E, Lekkas D, et al. Disseminated, eruptive molluscum contagiosum lesions in a psoriasis patient under treatment with methotrexate and cyclosporine. Eur J Dermatol. 2012;22:147-148.
- Antoniou C, Kosmadaki MG, Stratigos AJ, et al. Genital HPV lesions and molluscum contagiosum occurring in patients receiving anti-TNF-alpha therapy. Dermatology. 2008;216:364-365.
- Cursiefen C, Grunke M, Dechant C, et al. Multiple bilateral eyelid molluscum contagiosum lesions associated with TNFalpha-antibody and methotrexate therapy. Am J Ophthalmol. 2002;134:270-271.
- Heng YK, Lee JS, Neoh CY. Verrucous plaques in a pemphigus vulgaris patient on immunosuppressive therapy. Int J Dermatol. 2012;51:1044-1046.
- Goksugur N, Ozbostanci B, Goksugur SB. Molluscum contagiosum infection associated with pimecrolimus use in pityriasis alba. Pediatr Dermatol. 2007;24:E63-E65.
- Fernandes S, Pinto GM, Cardoso J. Particular clinical presentations of psoriasis in HIV patients. Int J STD AIDS. 2011;22:653-654.
Practice Points
- Molluscum contagiosum (MC) is highly prevalent and can have a wide range of atypical clinical presentations in patients with impaired cellular immunity (eg, human immunodeficiency virus [HIV]).
- Treatment of MC should include destructive procedures, if possible, as well as adjunctive agents such as topical retinoids, cantharidin, trichloroacetic acid, imiquimod, or cidofovir.
- Clinicians should consider screening patients with severe recalcitrant psoriasis for HIV to avoid poor outcomes from therapeutic agents.
Barbed sutures shorten cesarean closure time, reduce blood loss
DALLAS – Using knotless barbed sutures to close the uterine incision after cesarean delivery reduced operating time and blood loss, according to a recent randomized controlled study.
“On average, knotless barbed sutures were 1 minute and 43 seconds faster,” said David Peleg, MD, discussing the study results at the meeting sponsored by the Society for Maternal-Fetal Medicine. Average uterine closure time with knotless barbed sutures was 3 minutes and 37 seconds; with smooth sutures, average closure time was 5 minutes and 20 seconds (103-second difference; 95% confidence interval, 67.69-138.47 seconds; P less than .001).
Barbed sutures, in contrast, are usually made of monofilament with barbs created by the addition of tiny diagonal cuts made just partway through the suture material. When the suture is pulled through with the angle of the barbs, it pulls smoothly, but pulling back against the barb angle causes the barbs to protrude and catch against tissue, preventing slippage and eliminating the need for knots.
The fact that the monofilament has been partially cut to create the barbs can also reduce tensile strength, but some of the other disadvantages of smooth sutures are avoided, said Dr. Peleg.
The suture material used for the study was bidirectional, with the barbs running in opposite directions from the midline and a needle swaged onto each end; other barbed suture systems have an integral loop at one end of a unidirectionally barbed length of suture material that is used to anchor the first suture. The brand used was Stratafix.
The prospective study was necessarily unblinded, and compared the knotless barbed sutures with smooth sutures using polyglactin 910 braided material (Vicryl) for use during closure of the uterine incision during cesarean section procedures.
Patients were eligible if they were having an elective cesarean section after at least 38 weeks’ gestation, or if they were having a cesarean for the usual obstetric indications after laboring. Women with previous cesareans who failed a trial of labor were eligible.
The primary outcome was the length of time to close the uterine incision, measured from the start of suturing until hemostasis was achieved; the time included hemostatic suturing.
After a small pilot study that established a baseline suturing time with polyglactin 910 sutures of 6 minutes (standard deviation, 2 minutes and 10 seconds), Dr. Peleg and his collaborators determined that they would need to enroll at least 34 women per study arm to detect a decrease of 25% in suture time – to 4.5 minutes – with barbed sutures.
“The decrease in closure time is not linear,” said Dr. Peleg, so they increased their sample size by 50%, to 51 patients in each group, to ensure statistical significance of the results.
One of the challenges of a surgical randomized controlled trial is ensuring uniform technique; for this study, all patients had epidural or spinal anesthesia and antibiotics before opening. Surgeon clinical judgment was used to determine whether a Pfannenstiel or low transverse incision was made. In either case, there was no closure of the parietal peritoneum or the rectus muscles, and subcutaneous closure was used if tissues were greater than 2 cm in depth. Subcuticular stitches were used if possible.
Looking at secondary outcomes, there was a trend toward shorter total operative time with barbed sutures that didn’t reach statistical significance (20.1 min for barbed sutures vs. 23.1 for conventional, P = .062). However, fewer hemostatic sutures were required when barbed sutures were used: Extra sutures were used in 16 of the barbed group vs. 41 who had conventional sutures (P less than .001). Those receiving barbed sutures also had significantly less blood loss and estimated total blood loss during uterine closure than the conventional suture group (P = .005 and P = .002, respectively).
No study patients experienced serious postoperative complications; there were no infections, hematomas, or other wound complications, said Dr. Peleg of Bar-Ilan University, Zefat, Israel.
On the pro side for wider implementation of the use of barbed sutures for uterine closure stand the quicker closure and better hemostasis, along with the theoretical benefits of having no knots. Additionally, said Dr. Peleg, “there’s a gentle learning curve – it’s relatively easy to get used to the technique” of using barbed sutures. And, he said, “surgeons find them satisfying to use.”
However, he acknowledged the extra expense of barbed suture material – depending on the location and supplier, he estimated the cost could run from 7- to 20-fold for the barbed sutures, which he said cost $23.50 apiece. Also, he said, though the results were statistically significant, “Are they clinically significant? Does a difference in closure time of one minute 43 seconds, and a decrease in blood loss of 47 milliliters matter?”
Other considerations, he said, will require longer-term study. Polydioxanone, used for the barbed sutures, has a longer absorption time – a factor with unknown clinical implications in this application. Other longer-term outcomes, such as vaginal birth after cesarean success rates, rates of uterine rupture, the thickness of the uterine scar, and rates of adhesions and placenta accreta, will need to be tracked for years.
The authors reported no conflicts of interest and specifically reported that they had no relevant consulting or research agreements with suture manufacturers or marketers.
SOURCE: Peleg D et al. The Pregnancy Meeting Abstract 32.
DALLAS – Using knotless barbed sutures to close the uterine incision after cesarean delivery reduced operating time and blood loss, according to a recent randomized controlled study.
“On average, knotless barbed sutures were 1 minute and 43 seconds faster,” said David Peleg, MD, discussing the study results at the meeting sponsored by the Society for Maternal-Fetal Medicine. Average uterine closure time with knotless barbed sutures was 3 minutes and 37 seconds; with smooth sutures, average closure time was 5 minutes and 20 seconds (103-second difference; 95% confidence interval, 67.69-138.47 seconds; P less than .001).
Barbed sutures, in contrast, are usually made of monofilament with barbs created by the addition of tiny diagonal cuts made just partway through the suture material. When the suture is pulled through with the angle of the barbs, it pulls smoothly, but pulling back against the barb angle causes the barbs to protrude and catch against tissue, preventing slippage and eliminating the need for knots.
The fact that the monofilament has been partially cut to create the barbs can also reduce tensile strength, but some of the other disadvantages of smooth sutures are avoided, said Dr. Peleg.
The suture material used for the study was bidirectional, with the barbs running in opposite directions from the midline and a needle swaged onto each end; other barbed suture systems have an integral loop at one end of a unidirectionally barbed length of suture material that is used to anchor the first suture. The brand used was Stratafix.
The prospective study was necessarily unblinded, and compared the knotless barbed sutures with smooth sutures using polyglactin 910 braided material (Vicryl) for use during closure of the uterine incision during cesarean section procedures.
Patients were eligible if they were having an elective cesarean section after at least 38 weeks’ gestation, or if they were having a cesarean for the usual obstetric indications after laboring. Women with previous cesareans who failed a trial of labor were eligible.
The primary outcome was the length of time to close the uterine incision, measured from the start of suturing until hemostasis was achieved; the time included hemostatic suturing.
After a small pilot study that established a baseline suturing time with polyglactin 910 sutures of 6 minutes (standard deviation, 2 minutes and 10 seconds), Dr. Peleg and his collaborators determined that they would need to enroll at least 34 women per study arm to detect a decrease of 25% in suture time – to 4.5 minutes – with barbed sutures.
“The decrease in closure time is not linear,” said Dr. Peleg, so they increased their sample size by 50%, to 51 patients in each group, to ensure statistical significance of the results.
One of the challenges of a surgical randomized controlled trial is ensuring uniform technique; for this study, all patients had epidural or spinal anesthesia and antibiotics before opening. Surgeon clinical judgment was used to determine whether a Pfannenstiel or low transverse incision was made. In either case, there was no closure of the parietal peritoneum or the rectus muscles, and subcutaneous closure was used if tissues were greater than 2 cm in depth. Subcuticular stitches were used if possible.
Looking at secondary outcomes, there was a trend toward shorter total operative time with barbed sutures that didn’t reach statistical significance (20.1 min for barbed sutures vs. 23.1 for conventional, P = .062). However, fewer hemostatic sutures were required when barbed sutures were used: Extra sutures were used in 16 of the barbed group vs. 41 who had conventional sutures (P less than .001). Those receiving barbed sutures also had significantly less blood loss and estimated total blood loss during uterine closure than the conventional suture group (P = .005 and P = .002, respectively).
No study patients experienced serious postoperative complications; there were no infections, hematomas, or other wound complications, said Dr. Peleg of Bar-Ilan University, Zefat, Israel.
On the pro side for wider implementation of the use of barbed sutures for uterine closure stand the quicker closure and better hemostasis, along with the theoretical benefits of having no knots. Additionally, said Dr. Peleg, “there’s a gentle learning curve – it’s relatively easy to get used to the technique” of using barbed sutures. And, he said, “surgeons find them satisfying to use.”
However, he acknowledged the extra expense of barbed suture material – depending on the location and supplier, he estimated the cost could run from 7- to 20-fold for the barbed sutures, which he said cost $23.50 apiece. Also, he said, though the results were statistically significant, “Are they clinically significant? Does a difference in closure time of one minute 43 seconds, and a decrease in blood loss of 47 milliliters matter?”
Other considerations, he said, will require longer-term study. Polydioxanone, used for the barbed sutures, has a longer absorption time – a factor with unknown clinical implications in this application. Other longer-term outcomes, such as vaginal birth after cesarean success rates, rates of uterine rupture, the thickness of the uterine scar, and rates of adhesions and placenta accreta, will need to be tracked for years.
The authors reported no conflicts of interest and specifically reported that they had no relevant consulting or research agreements with suture manufacturers or marketers.
SOURCE: Peleg D et al. The Pregnancy Meeting Abstract 32.
DALLAS – Using knotless barbed sutures to close the uterine incision after cesarean delivery reduced operating time and blood loss, according to a recent randomized controlled study.
“On average, knotless barbed sutures were 1 minute and 43 seconds faster,” said David Peleg, MD, discussing the study results at the meeting sponsored by the Society for Maternal-Fetal Medicine. Average uterine closure time with knotless barbed sutures was 3 minutes and 37 seconds; with smooth sutures, average closure time was 5 minutes and 20 seconds (103-second difference; 95% confidence interval, 67.69-138.47 seconds; P less than .001).
Barbed sutures, in contrast, are usually made of monofilament with barbs created by the addition of tiny diagonal cuts made just partway through the suture material. When the suture is pulled through with the angle of the barbs, it pulls smoothly, but pulling back against the barb angle causes the barbs to protrude and catch against tissue, preventing slippage and eliminating the need for knots.
The fact that the monofilament has been partially cut to create the barbs can also reduce tensile strength, but some of the other disadvantages of smooth sutures are avoided, said Dr. Peleg.
The suture material used for the study was bidirectional, with the barbs running in opposite directions from the midline and a needle swaged onto each end; other barbed suture systems have an integral loop at one end of a unidirectionally barbed length of suture material that is used to anchor the first suture. The brand used was Stratafix.
The prospective study was necessarily unblinded, and compared the knotless barbed sutures with smooth sutures using polyglactin 910 braided material (Vicryl) for use during closure of the uterine incision during cesarean section procedures.
Patients were eligible if they were having an elective cesarean section after at least 38 weeks’ gestation, or if they were having a cesarean for the usual obstetric indications after laboring. Women with previous cesareans who failed a trial of labor were eligible.
The primary outcome was the length of time to close the uterine incision, measured from the start of suturing until hemostasis was achieved; the time included hemostatic suturing.
After a small pilot study that established a baseline suturing time with polyglactin 910 sutures of 6 minutes (standard deviation, 2 minutes and 10 seconds), Dr. Peleg and his collaborators determined that they would need to enroll at least 34 women per study arm to detect a decrease of 25% in suture time – to 4.5 minutes – with barbed sutures.
“The decrease in closure time is not linear,” said Dr. Peleg, so they increased their sample size by 50%, to 51 patients in each group, to ensure statistical significance of the results.
One of the challenges of a surgical randomized controlled trial is ensuring uniform technique; for this study, all patients had epidural or spinal anesthesia and antibiotics before opening. Surgeon clinical judgment was used to determine whether a Pfannenstiel or low transverse incision was made. In either case, there was no closure of the parietal peritoneum or the rectus muscles, and subcutaneous closure was used if tissues were greater than 2 cm in depth. Subcuticular stitches were used if possible.
Looking at secondary outcomes, there was a trend toward shorter total operative time with barbed sutures that didn’t reach statistical significance (20.1 min for barbed sutures vs. 23.1 for conventional, P = .062). However, fewer hemostatic sutures were required when barbed sutures were used: Extra sutures were used in 16 of the barbed group vs. 41 who had conventional sutures (P less than .001). Those receiving barbed sutures also had significantly less blood loss and estimated total blood loss during uterine closure than the conventional suture group (P = .005 and P = .002, respectively).
No study patients experienced serious postoperative complications; there were no infections, hematomas, or other wound complications, said Dr. Peleg of Bar-Ilan University, Zefat, Israel.
On the pro side for wider implementation of the use of barbed sutures for uterine closure stand the quicker closure and better hemostasis, along with the theoretical benefits of having no knots. Additionally, said Dr. Peleg, “there’s a gentle learning curve – it’s relatively easy to get used to the technique” of using barbed sutures. And, he said, “surgeons find them satisfying to use.”
However, he acknowledged the extra expense of barbed suture material – depending on the location and supplier, he estimated the cost could run from 7- to 20-fold for the barbed sutures, which he said cost $23.50 apiece. Also, he said, though the results were statistically significant, “Are they clinically significant? Does a difference in closure time of one minute 43 seconds, and a decrease in blood loss of 47 milliliters matter?”
Other considerations, he said, will require longer-term study. Polydioxanone, used for the barbed sutures, has a longer absorption time – a factor with unknown clinical implications in this application. Other longer-term outcomes, such as vaginal birth after cesarean success rates, rates of uterine rupture, the thickness of the uterine scar, and rates of adhesions and placenta accreta, will need to be tracked for years.
The authors reported no conflicts of interest and specifically reported that they had no relevant consulting or research agreements with suture manufacturers or marketers.
SOURCE: Peleg D et al. The Pregnancy Meeting Abstract 32.
REPORTING FROM THE PREGNANCY MEETING
Key clinical point: Closure time and blood loss were less with barbed sutures closing the uterine incision.
Major finding: Average uterine incision closure time was 3:37 minutes with knotless barbed sutures and 5:20 minutes with conventional smooth sutures.
Study details: Randomized controlled trial of 102 women undergoing cesarean section.
Disclosures: The study had no external sources of funding, and the study authors reported no relevant outside support.
Source: Peleg D et al. The Pregnancy Meeting Abstract 32.
Nail-Patella Syndrome: Clinical Clues for Making the Diagnosis
Nail-patella syndrome (NPS), also known as hereditary osteo-onychodysplasia syndrome, is a rare autosomal-dominant disorder with an estimated incidence of 1 per 50,000 individuals in the United States. Nail-patella syndrome presents due to a heterozygous loss-of-function mutation in the LIM homeobox transcription factor 1 beta gene, LMX1B, on chromosome 9q34.1 LMX1B gene mutations are fully penetrant, but there is variable expressivity, even within families.2
Case Report
A 69-year-old man presented to the dermatology clinic for a routine skin cancer screening. The patient’s history was remarkable for dystrophic fingernails and toenails since birth. In his 20s he developed progressively worsening instability of the left knee and chronic back pain due to scoliosis, lumbar lordosis, and spinal disc herniation. Since then, he underwent knee surgery and 7 back surgeries for rheumatologic disease. His medical history also was remarkable for osteoporosis, hypertension, and glaucoma. Family history was notable for similar findings in the patient’s sister; mother; and maternal aunt, uncle, and grandmother, all with varying disease severity.
Physical examination was remarkable for bilateral fingernail hypoplasia that was most prominent on the thumb, with improvement in each nail on progression toward the fifth digit (Figure 1A). Triangular fingernail lunulae, longitudinal ridging, and nail splitting were present (Figure 1A and 1B). Hypoplastic crumbly toenails also were appreciated (Figure 1C). Skin creases over the distal interphalangeal joints of the fingers and toes were conspicuously absent. Limited range of motion was noted in multiple joints, with profound limitation of bilateral elbow extension. Review of prior imaging reports revealed bilateral iliac horns as well as left patellar absence and right patellar hypoplasia (Figure 2). Urinalysis was remarkable for proteinuria and microscopic hematuria. Given the constellation of examination findings and positive family history, a diagnosis of NPS was made.
Comment
Nail-patella syndrome is characterized by variable dermatologic, neurologic, nephrogenic, ophthalmologic, and orthopedic clinical manifestations.3 Almost all patients with NPS have bilateral and symmetric nail changes, including absent or hypoplastic nails with ridging, splitting, or discoloration and triangular-shaped lunulae.1,4 Nail findings are the most consistent findings of NPS, as they are present in more than 98% of patients.5 The thumb often is the most severely affected nail, with improvement appreciated on progression toward the fifth digit, as seen in our patient (Figure 1A).5 Each individual nail usually is more severely affected on its ulnar side. When toenails are involved, the abnormalities tend to be less severe, and the little toenail is most commonly affected. Distal digital changes also are observed in almost all patients. Loss of dorsal creases in the skin overlying the distal interphalangeal joints can be considered as a diagnostic clue.3,4
There are a variety of orthopedic manifestations of NPS. Hypoplastic or absent patellae leading to recurrent subluxations or dislocations is a common finding.4 Bilateral symmetric bone formations (horns) arising from the iliac crest are pathognomonic but only found on radiography 70% of the time.6 Occasionally these protuberances can be palpated on physical examination,5 though this finding was not appreciated in our patient. Dysplasia of the elbows may result in limited elbow extension and limited pronation and supination. Early degenerative arthritis, lumbar lordosis, and scoliosis also are not uncommon. In addition, skeletal integrity is compromised, leading to early osteoporosis and increased risk for fractures.5
Nephropathy develops in approximately 30% to 40% of patients and is a major determinant of mortality in these patients.2 Mutations in the LMX1B gene lead to abnormal development of podocytes and reduction in collagen in the glomerular basement membrane. The first sign of renal involvement usually is proteinuria, with or without microscopic hematuria. As in our patient, many patients develop hypertension. Patients may progress to develop nephrotic syndrome and end-stage renal failure (5%–10%).7 Death from NPS-related nephropathy has occurred, even in childhood.4,5
Primary open-angle glaucoma has been recognized as a feature of NPS.8 It is the most frequent ocular abnormality observed, followed by ocular hypertension and Lester sign of the iris.3,5 These conditions also are more common in younger patients with NPS than in the general population.5 Important neurologic findings include epilepsy, peripheral neuropathy, attention deficit disorder, major depressive disorder, and vasomotor problems.9
Our case highlights the importance of recognizing this rare condition to provide a multidisciplinary approach to care that addresses all aspects of LMX1B-associated disease in affected individuals. Nail findings may be the first clue to the need for additional screenings in these patients. Nail-patella syndrome patients should undergo thorough ophthalmologic examinations every 2 years, including measurement of intraocular pressure, examination of the optic disc, and assessment of visual fields. Given the variability in severity of joint problems and the unpredictable anatomy of the joints, magnetic resonance imaging of the joints is recommended prior to orthopedic intervention. Most importantly, physicians should recognize this genodermatosis to implement periodic screenings for renal disease, as up to 40% of NPS patients develop kidney failure. Annual blood pressure measurements, urinalysis, and measurement of the protein to creatinine ratio in the urine are recommended. For patients with end-stage renal failure, renal transplantation results in cure of nephropathy and may even result in nail regrowth.10 Further, this case is notable in that it describes a patient with NPS who is older than most other individuals presenting with the condition, thereby revealing novel information about NPS in its more advanced stages.
- Harita Y, Kitanaka S, Isojima T, et al. Spectrum of LMX1B mutations: from nail-patella syndrome to isolated nephropathy [published online July 23, 2016]. Pediatr Nephrol. doi:10.1007/s00467-016-3462-x.
- Ghoumid J, Petit F, Holder-Espinasse M, et al. Nail-patella syndrome: clinical and molecular data in 55 families raising the hypothesis of a genetic heterogeneity [published online April 22, 2015]. Eur J Hum Genet. 2016;24:44-50.
- Tong SY, Luk HM, Tong TM, et al. The nail points to the diagnosis. Fong disease or hereditary osteo-onychodysplasia. Hong Kong Med J. 2015;21:573.e3-573.e5.
- Figueroa-Silva O, Vicente A, Agudo A, et al. Nail-patella syndrome: report of 11 pediatric cases. J Eur Acad Dermatol Venereol. 2016;30:1614-1617.
- Sweeney E, Fryer A, Mountford R, et al. Nail patella syndrome: a review of the phenotype aided by developmental biology. J Med Genet. 2003;40:153-162.
- Tigchelaar S, Lenting A, Bongers EM, et al. Nail patella syndrome: knee symptoms and surgical outcomes. a questionnaire-based survey [published online November 17, 2015]. Orthop Traumatol Surg Res. 2015;101:959-962.
- Lemley KV. Kidney disease in nail-patella syndrome [published online June 6, 2008]. Pediatr Nephrol. 2009;24:2345-2354.
- Sweeney E, Hoover-Fong JE, McIntosh I. Nail-patella syndrome. In: Adam MP, Ardinger HH, Pagon RA, et al, eds. GeneReviews. Seattle, WA: University of Washington, Seattle; 2003. https://www.ncbi.nlm.nih.gov/books/NBK1132/. Updated November 13, 2014. Accessed January 30, 2018.
- Lopez-Arvizu C, Sparrow EP, Strube MJ, et al. Increased symptoms of attention deficit hyperactivity disorder and major depressive disorder symptoms in nail-patella syndrome: potential association with LMX1B loss-of-function [published online November 2, 2010]. Am J Med Genet B Neuropsychiatr Genet. 2011;156B:59-66.
- Chan PC, Chan KW, Cheng IK, et al. Living-related renal transplantation in a patient with nail-patella syndrome. Nephron. 1988;50:164-166.
Nail-patella syndrome (NPS), also known as hereditary osteo-onychodysplasia syndrome, is a rare autosomal-dominant disorder with an estimated incidence of 1 per 50,000 individuals in the United States. Nail-patella syndrome presents due to a heterozygous loss-of-function mutation in the LIM homeobox transcription factor 1 beta gene, LMX1B, on chromosome 9q34.1 LMX1B gene mutations are fully penetrant, but there is variable expressivity, even within families.2
Case Report
A 69-year-old man presented to the dermatology clinic for a routine skin cancer screening. The patient’s history was remarkable for dystrophic fingernails and toenails since birth. In his 20s he developed progressively worsening instability of the left knee and chronic back pain due to scoliosis, lumbar lordosis, and spinal disc herniation. Since then, he underwent knee surgery and 7 back surgeries for rheumatologic disease. His medical history also was remarkable for osteoporosis, hypertension, and glaucoma. Family history was notable for similar findings in the patient’s sister; mother; and maternal aunt, uncle, and grandmother, all with varying disease severity.
Physical examination was remarkable for bilateral fingernail hypoplasia that was most prominent on the thumb, with improvement in each nail on progression toward the fifth digit (Figure 1A). Triangular fingernail lunulae, longitudinal ridging, and nail splitting were present (Figure 1A and 1B). Hypoplastic crumbly toenails also were appreciated (Figure 1C). Skin creases over the distal interphalangeal joints of the fingers and toes were conspicuously absent. Limited range of motion was noted in multiple joints, with profound limitation of bilateral elbow extension. Review of prior imaging reports revealed bilateral iliac horns as well as left patellar absence and right patellar hypoplasia (Figure 2). Urinalysis was remarkable for proteinuria and microscopic hematuria. Given the constellation of examination findings and positive family history, a diagnosis of NPS was made.


Comment
Nail-patella syndrome is characterized by variable dermatologic, neurologic, nephrogenic, ophthalmologic, and orthopedic clinical manifestations.3 Almost all patients with NPS have bilateral and symmetric nail changes, including absent or hypoplastic nails with ridging, splitting, or discoloration and triangular-shaped lunulae.1,4 Nail findings are the most consistent findings of NPS, as they are present in more than 98% of patients.5 The thumb often is the most severely affected nail, with improvement appreciated on progression toward the fifth digit, as seen in our patient (Figure 1A).5 Each individual nail usually is more severely affected on its ulnar side. When toenails are involved, the abnormalities tend to be less severe, and the little toenail is most commonly affected. Distal digital changes also are observed in almost all patients. Loss of dorsal creases in the skin overlying the distal interphalangeal joints can be considered as a diagnostic clue.3,4
There are a variety of orthopedic manifestations of NPS. Hypoplastic or absent patellae leading to recurrent subluxations or dislocations is a common finding.4 Bilateral symmetric bone formations (horns) arising from the iliac crest are pathognomonic but only found on radiography 70% of the time.6 Occasionally these protuberances can be palpated on physical examination,5 though this finding was not appreciated in our patient. Dysplasia of the elbows may result in limited elbow extension and limited pronation and supination. Early degenerative arthritis, lumbar lordosis, and scoliosis also are not uncommon. In addition, skeletal integrity is compromised, leading to early osteoporosis and increased risk for fractures.5
Nephropathy develops in approximately 30% to 40% of patients and is a major determinant of mortality in these patients.2 Mutations in the LMX1B gene lead to abnormal development of podocytes and reduction in collagen in the glomerular basement membrane. The first sign of renal involvement usually is proteinuria, with or without microscopic hematuria. As in our patient, many patients develop hypertension. Patients may progress to develop nephrotic syndrome and end-stage renal failure (5%–10%).7 Death from NPS-related nephropathy has occurred, even in childhood.4,5
Primary open-angle glaucoma has been recognized as a feature of NPS.8 It is the most frequent ocular abnormality observed, followed by ocular hypertension and Lester sign of the iris.3,5 These conditions also are more common in younger patients with NPS than in the general population.5 Important neurologic findings include epilepsy, peripheral neuropathy, attention deficit disorder, major depressive disorder, and vasomotor problems.9
Our case highlights the importance of recognizing this rare condition to provide a multidisciplinary approach to care that addresses all aspects of LMX1B-associated disease in affected individuals. Nail findings may be the first clue to the need for additional screenings in these patients. Nail-patella syndrome patients should undergo thorough ophthalmologic examinations every 2 years, including measurement of intraocular pressure, examination of the optic disc, and assessment of visual fields. Given the variability in severity of joint problems and the unpredictable anatomy of the joints, magnetic resonance imaging of the joints is recommended prior to orthopedic intervention. Most importantly, physicians should recognize this genodermatosis to implement periodic screenings for renal disease, as up to 40% of NPS patients develop kidney failure. Annual blood pressure measurements, urinalysis, and measurement of the protein to creatinine ratio in the urine are recommended. For patients with end-stage renal failure, renal transplantation results in cure of nephropathy and may even result in nail regrowth.10 Further, this case is notable in that it describes a patient with NPS who is older than most other individuals presenting with the condition, thereby revealing novel information about NPS in its more advanced stages.
Nail-patella syndrome (NPS), also known as hereditary osteo-onychodysplasia syndrome, is a rare autosomal-dominant disorder with an estimated incidence of 1 per 50,000 individuals in the United States. Nail-patella syndrome presents due to a heterozygous loss-of-function mutation in the LIM homeobox transcription factor 1 beta gene, LMX1B, on chromosome 9q34.1 LMX1B gene mutations are fully penetrant, but there is variable expressivity, even within families.2
Case Report
A 69-year-old man presented to the dermatology clinic for a routine skin cancer screening. The patient’s history was remarkable for dystrophic fingernails and toenails since birth. In his 20s he developed progressively worsening instability of the left knee and chronic back pain due to scoliosis, lumbar lordosis, and spinal disc herniation. Since then, he underwent knee surgery and 7 back surgeries for rheumatologic disease. His medical history also was remarkable for osteoporosis, hypertension, and glaucoma. Family history was notable for similar findings in the patient’s sister; mother; and maternal aunt, uncle, and grandmother, all with varying disease severity.
Physical examination was remarkable for bilateral fingernail hypoplasia that was most prominent on the thumb, with improvement in each nail on progression toward the fifth digit (Figure 1A). Triangular fingernail lunulae, longitudinal ridging, and nail splitting were present (Figure 1A and 1B). Hypoplastic crumbly toenails also were appreciated (Figure 1C). Skin creases over the distal interphalangeal joints of the fingers and toes were conspicuously absent. Limited range of motion was noted in multiple joints, with profound limitation of bilateral elbow extension. Review of prior imaging reports revealed bilateral iliac horns as well as left patellar absence and right patellar hypoplasia (Figure 2). Urinalysis was remarkable for proteinuria and microscopic hematuria. Given the constellation of examination findings and positive family history, a diagnosis of NPS was made.


Comment
Nail-patella syndrome is characterized by variable dermatologic, neurologic, nephrogenic, ophthalmologic, and orthopedic clinical manifestations.3 Almost all patients with NPS have bilateral and symmetric nail changes, including absent or hypoplastic nails with ridging, splitting, or discoloration and triangular-shaped lunulae.1,4 Nail findings are the most consistent findings of NPS, as they are present in more than 98% of patients.5 The thumb often is the most severely affected nail, with improvement appreciated on progression toward the fifth digit, as seen in our patient (Figure 1A).5 Each individual nail usually is more severely affected on its ulnar side. When toenails are involved, the abnormalities tend to be less severe, and the little toenail is most commonly affected. Distal digital changes also are observed in almost all patients. Loss of dorsal creases in the skin overlying the distal interphalangeal joints can be considered as a diagnostic clue.3,4
There are a variety of orthopedic manifestations of NPS. Hypoplastic or absent patellae leading to recurrent subluxations or dislocations is a common finding.4 Bilateral symmetric bone formations (horns) arising from the iliac crest are pathognomonic but only found on radiography 70% of the time.6 Occasionally these protuberances can be palpated on physical examination,5 though this finding was not appreciated in our patient. Dysplasia of the elbows may result in limited elbow extension and limited pronation and supination. Early degenerative arthritis, lumbar lordosis, and scoliosis also are not uncommon. In addition, skeletal integrity is compromised, leading to early osteoporosis and increased risk for fractures.5
Nephropathy develops in approximately 30% to 40% of patients and is a major determinant of mortality in these patients.2 Mutations in the LMX1B gene lead to abnormal development of podocytes and reduction in collagen in the glomerular basement membrane. The first sign of renal involvement usually is proteinuria, with or without microscopic hematuria. As in our patient, many patients develop hypertension. Patients may progress to develop nephrotic syndrome and end-stage renal failure (5%–10%).7 Death from NPS-related nephropathy has occurred, even in childhood.4,5
Primary open-angle glaucoma has been recognized as a feature of NPS.8 It is the most frequent ocular abnormality observed, followed by ocular hypertension and Lester sign of the iris.3,5 These conditions also are more common in younger patients with NPS than in the general population.5 Important neurologic findings include epilepsy, peripheral neuropathy, attention deficit disorder, major depressive disorder, and vasomotor problems.9
Our case highlights the importance of recognizing this rare condition to provide a multidisciplinary approach to care that addresses all aspects of LMX1B-associated disease in affected individuals. Nail findings may be the first clue to the need for additional screenings in these patients. Nail-patella syndrome patients should undergo thorough ophthalmologic examinations every 2 years, including measurement of intraocular pressure, examination of the optic disc, and assessment of visual fields. Given the variability in severity of joint problems and the unpredictable anatomy of the joints, magnetic resonance imaging of the joints is recommended prior to orthopedic intervention. Most importantly, physicians should recognize this genodermatosis to implement periodic screenings for renal disease, as up to 40% of NPS patients develop kidney failure. Annual blood pressure measurements, urinalysis, and measurement of the protein to creatinine ratio in the urine are recommended. For patients with end-stage renal failure, renal transplantation results in cure of nephropathy and may even result in nail regrowth.10 Further, this case is notable in that it describes a patient with NPS who is older than most other individuals presenting with the condition, thereby revealing novel information about NPS in its more advanced stages.
- Harita Y, Kitanaka S, Isojima T, et al. Spectrum of LMX1B mutations: from nail-patella syndrome to isolated nephropathy [published online July 23, 2016]. Pediatr Nephrol. doi:10.1007/s00467-016-3462-x.
- Ghoumid J, Petit F, Holder-Espinasse M, et al. Nail-patella syndrome: clinical and molecular data in 55 families raising the hypothesis of a genetic heterogeneity [published online April 22, 2015]. Eur J Hum Genet. 2016;24:44-50.
- Tong SY, Luk HM, Tong TM, et al. The nail points to the diagnosis. Fong disease or hereditary osteo-onychodysplasia. Hong Kong Med J. 2015;21:573.e3-573.e5.
- Figueroa-Silva O, Vicente A, Agudo A, et al. Nail-patella syndrome: report of 11 pediatric cases. J Eur Acad Dermatol Venereol. 2016;30:1614-1617.
- Sweeney E, Fryer A, Mountford R, et al. Nail patella syndrome: a review of the phenotype aided by developmental biology. J Med Genet. 2003;40:153-162.
- Tigchelaar S, Lenting A, Bongers EM, et al. Nail patella syndrome: knee symptoms and surgical outcomes. a questionnaire-based survey [published online November 17, 2015]. Orthop Traumatol Surg Res. 2015;101:959-962.
- Lemley KV. Kidney disease in nail-patella syndrome [published online June 6, 2008]. Pediatr Nephrol. 2009;24:2345-2354.
- Sweeney E, Hoover-Fong JE, McIntosh I. Nail-patella syndrome. In: Adam MP, Ardinger HH, Pagon RA, et al, eds. GeneReviews. Seattle, WA: University of Washington, Seattle; 2003. https://www.ncbi.nlm.nih.gov/books/NBK1132/. Updated November 13, 2014. Accessed January 30, 2018.
- Lopez-Arvizu C, Sparrow EP, Strube MJ, et al. Increased symptoms of attention deficit hyperactivity disorder and major depressive disorder symptoms in nail-patella syndrome: potential association with LMX1B loss-of-function [published online November 2, 2010]. Am J Med Genet B Neuropsychiatr Genet. 2011;156B:59-66.
- Chan PC, Chan KW, Cheng IK, et al. Living-related renal transplantation in a patient with nail-patella syndrome. Nephron. 1988;50:164-166.
- Harita Y, Kitanaka S, Isojima T, et al. Spectrum of LMX1B mutations: from nail-patella syndrome to isolated nephropathy [published online July 23, 2016]. Pediatr Nephrol. doi:10.1007/s00467-016-3462-x.
- Ghoumid J, Petit F, Holder-Espinasse M, et al. Nail-patella syndrome: clinical and molecular data in 55 families raising the hypothesis of a genetic heterogeneity [published online April 22, 2015]. Eur J Hum Genet. 2016;24:44-50.
- Tong SY, Luk HM, Tong TM, et al. The nail points to the diagnosis. Fong disease or hereditary osteo-onychodysplasia. Hong Kong Med J. 2015;21:573.e3-573.e5.
- Figueroa-Silva O, Vicente A, Agudo A, et al. Nail-patella syndrome: report of 11 pediatric cases. J Eur Acad Dermatol Venereol. 2016;30:1614-1617.
- Sweeney E, Fryer A, Mountford R, et al. Nail patella syndrome: a review of the phenotype aided by developmental biology. J Med Genet. 2003;40:153-162.
- Tigchelaar S, Lenting A, Bongers EM, et al. Nail patella syndrome: knee symptoms and surgical outcomes. a questionnaire-based survey [published online November 17, 2015]. Orthop Traumatol Surg Res. 2015;101:959-962.
- Lemley KV. Kidney disease in nail-patella syndrome [published online June 6, 2008]. Pediatr Nephrol. 2009;24:2345-2354.
- Sweeney E, Hoover-Fong JE, McIntosh I. Nail-patella syndrome. In: Adam MP, Ardinger HH, Pagon RA, et al, eds. GeneReviews. Seattle, WA: University of Washington, Seattle; 2003. https://www.ncbi.nlm.nih.gov/books/NBK1132/. Updated November 13, 2014. Accessed January 30, 2018.
- Lopez-Arvizu C, Sparrow EP, Strube MJ, et al. Increased symptoms of attention deficit hyperactivity disorder and major depressive disorder symptoms in nail-patella syndrome: potential association with LMX1B loss-of-function [published online November 2, 2010]. Am J Med Genet B Neuropsychiatr Genet. 2011;156B:59-66.
- Chan PC, Chan KW, Cheng IK, et al. Living-related renal transplantation in a patient with nail-patella syndrome. Nephron. 1988;50:164-166.
Practice Points
- Nail-patella syndrome (NPS) is a multisystem disease.
- Nail findings (eg, triangular lunulae) may be the first clue to NPS and should prompt investigation of associated renal, ocular, neurologic, skeletal, and orthopedic abnormalities.
- Early intervention and a multidisciplinary approach to care can improve morbidity and mortality in patients with NPS.
These amniotic biomarkers predicted pPROM in women undergoing fetal surgery for spinal cord defect
DALLAS – Two molecular biomarkers in amniotic fluid seem to predict preterm premature rupture of membranes (pPROM) and subsequent premature delivery in women whose fetuses undergo surgical repair of spinal cord defects.
Matrix metalloproteinase–8 (MMP-8) and lactic acid levels were significantly higher in these women than in a comparator group of women who did not deliver a premature infant after the repair, Aikaterini Athanasiou, MD, said at the meeting sponsored by the Society for Maternal Fetal Medicine.
“Based on this pilot study, it appears that elevated amniotic levels of lactic acid and MMP-8 at time of surgery might identify a subset of women with increased susceptibility for pPROM and shorter time to delivery,” said Dr. Athanasiou, a fellow in obstetrics and gynecology at Cornell University, New York. She presented the work on behalf of primary author Antonio Moron, MD, PhD, of the Federal University of São Paulo.
Dr. Athanasiou was part of the Cornell team that conducted molecular assays on amniotic fluid samples drawn from 26 women carrying fetuses about to have corrective surgery for spinal defects in the fetuses. The women were all patients at the Federal Hospital of São Paulo. Samples were drawn immediately before surgery commenced, frozen, and shipped to Cornell for assay.
After surgical repair, 7 of the women (27%) later experienced pPROM and 19 did not.
At baseline, there were no significant differences between the groups. Women were a mean age of 32 years, with a mean of two prior pregnancies. There were no differences in prior cesarean and vaginal births, smoking status, and fetal gender. The defect was most commonly a myelomeningocele (about 70% of each group). Rachischisis was next most common, occurring in 27% of the pPROM group and 21% of the non-pPROM group. There were two cases of encephalocele, both in the non-pPROM group.
The length of surgery was not significantly different between those who experienced pPROM and those who did not (121 vs. 130 minutes). Wound healing time was 7 days for each group, as was the mean length of hospital stay.
Both groups went a mean of 57 days from surgery to delivery, although the fetal gestational age was almost a week younger in the pPROM group (33.7 vs. 34.4 weeks). These infants were also smaller at birth (2,247 g vs. 2,410 g).
The Cornell team examined five potential biomarkers in each amniotic fluid sample: MMP-8, MMP-9, MMP-2, lactic acid, and interleukin-6 (IL-6). The levels of IL-6, MMP-2, and MMP-9 were similar between groups.
However, lactic acid was significantly higher in the pPROM group (7.1 vs. 5.9 mU/mL). MMP-8 was also significantly elevated in the pPROM group (1.7 vs. 0.6 mcg/mL).
Dr. Athanasiou and her colleagues also observed an inverse relationship between MMP-8 levels and gestational age at delivery, which was statistically significant. There was also an inverse relationship between lactic acid levels and gestational age, but this did not reach statistical significance.
“While further investigations are needed to verify our findings, our data suggest that these differences are present before fetal surgery and that an increase in intra-amniotic anaerobic glycolysis as evidenced by elevated lactic acid may enhance MMP-8 production, which will weaken the maternal-fetal membranes,” Dr. Athanasiou said. “The mechanism may not be related to inflammation, as evidenced by the lack of association between pPROM and IL-6.”
She had no financial disclosures.
SOURCE: Moron A et al. Am J Obstet Gynecol. 2018 Jan:218(1);S64.
DALLAS – Two molecular biomarkers in amniotic fluid seem to predict preterm premature rupture of membranes (pPROM) and subsequent premature delivery in women whose fetuses undergo surgical repair of spinal cord defects.
Matrix metalloproteinase–8 (MMP-8) and lactic acid levels were significantly higher in these women than in a comparator group of women who did not deliver a premature infant after the repair, Aikaterini Athanasiou, MD, said at the meeting sponsored by the Society for Maternal Fetal Medicine.
“Based on this pilot study, it appears that elevated amniotic levels of lactic acid and MMP-8 at time of surgery might identify a subset of women with increased susceptibility for pPROM and shorter time to delivery,” said Dr. Athanasiou, a fellow in obstetrics and gynecology at Cornell University, New York. She presented the work on behalf of primary author Antonio Moron, MD, PhD, of the Federal University of São Paulo.
Dr. Athanasiou was part of the Cornell team that conducted molecular assays on amniotic fluid samples drawn from 26 women carrying fetuses about to have corrective surgery for spinal defects in the fetuses. The women were all patients at the Federal Hospital of São Paulo. Samples were drawn immediately before surgery commenced, frozen, and shipped to Cornell for assay.
After surgical repair, 7 of the women (27%) later experienced pPROM and 19 did not.
At baseline, there were no significant differences between the groups. Women were a mean age of 32 years, with a mean of two prior pregnancies. There were no differences in prior cesarean and vaginal births, smoking status, and fetal gender. The defect was most commonly a myelomeningocele (about 70% of each group). Rachischisis was next most common, occurring in 27% of the pPROM group and 21% of the non-pPROM group. There were two cases of encephalocele, both in the non-pPROM group.
The length of surgery was not significantly different between those who experienced pPROM and those who did not (121 vs. 130 minutes). Wound healing time was 7 days for each group, as was the mean length of hospital stay.
Both groups went a mean of 57 days from surgery to delivery, although the fetal gestational age was almost a week younger in the pPROM group (33.7 vs. 34.4 weeks). These infants were also smaller at birth (2,247 g vs. 2,410 g).
The Cornell team examined five potential biomarkers in each amniotic fluid sample: MMP-8, MMP-9, MMP-2, lactic acid, and interleukin-6 (IL-6). The levels of IL-6, MMP-2, and MMP-9 were similar between groups.
However, lactic acid was significantly higher in the pPROM group (7.1 vs. 5.9 mU/mL). MMP-8 was also significantly elevated in the pPROM group (1.7 vs. 0.6 mcg/mL).
Dr. Athanasiou and her colleagues also observed an inverse relationship between MMP-8 levels and gestational age at delivery, which was statistically significant. There was also an inverse relationship between lactic acid levels and gestational age, but this did not reach statistical significance.
“While further investigations are needed to verify our findings, our data suggest that these differences are present before fetal surgery and that an increase in intra-amniotic anaerobic glycolysis as evidenced by elevated lactic acid may enhance MMP-8 production, which will weaken the maternal-fetal membranes,” Dr. Athanasiou said. “The mechanism may not be related to inflammation, as evidenced by the lack of association between pPROM and IL-6.”
She had no financial disclosures.
SOURCE: Moron A et al. Am J Obstet Gynecol. 2018 Jan:218(1);S64.
DALLAS – Two molecular biomarkers in amniotic fluid seem to predict preterm premature rupture of membranes (pPROM) and subsequent premature delivery in women whose fetuses undergo surgical repair of spinal cord defects.
Matrix metalloproteinase–8 (MMP-8) and lactic acid levels were significantly higher in these women than in a comparator group of women who did not deliver a premature infant after the repair, Aikaterini Athanasiou, MD, said at the meeting sponsored by the Society for Maternal Fetal Medicine.
“Based on this pilot study, it appears that elevated amniotic levels of lactic acid and MMP-8 at time of surgery might identify a subset of women with increased susceptibility for pPROM and shorter time to delivery,” said Dr. Athanasiou, a fellow in obstetrics and gynecology at Cornell University, New York. She presented the work on behalf of primary author Antonio Moron, MD, PhD, of the Federal University of São Paulo.
Dr. Athanasiou was part of the Cornell team that conducted molecular assays on amniotic fluid samples drawn from 26 women carrying fetuses about to have corrective surgery for spinal defects in the fetuses. The women were all patients at the Federal Hospital of São Paulo. Samples were drawn immediately before surgery commenced, frozen, and shipped to Cornell for assay.
After surgical repair, 7 of the women (27%) later experienced pPROM and 19 did not.
At baseline, there were no significant differences between the groups. Women were a mean age of 32 years, with a mean of two prior pregnancies. There were no differences in prior cesarean and vaginal births, smoking status, and fetal gender. The defect was most commonly a myelomeningocele (about 70% of each group). Rachischisis was next most common, occurring in 27% of the pPROM group and 21% of the non-pPROM group. There were two cases of encephalocele, both in the non-pPROM group.
The length of surgery was not significantly different between those who experienced pPROM and those who did not (121 vs. 130 minutes). Wound healing time was 7 days for each group, as was the mean length of hospital stay.
Both groups went a mean of 57 days from surgery to delivery, although the fetal gestational age was almost a week younger in the pPROM group (33.7 vs. 34.4 weeks). These infants were also smaller at birth (2,247 g vs. 2,410 g).
The Cornell team examined five potential biomarkers in each amniotic fluid sample: MMP-8, MMP-9, MMP-2, lactic acid, and interleukin-6 (IL-6). The levels of IL-6, MMP-2, and MMP-9 were similar between groups.
However, lactic acid was significantly higher in the pPROM group (7.1 vs. 5.9 mU/mL). MMP-8 was also significantly elevated in the pPROM group (1.7 vs. 0.6 mcg/mL).
Dr. Athanasiou and her colleagues also observed an inverse relationship between MMP-8 levels and gestational age at delivery, which was statistically significant. There was also an inverse relationship between lactic acid levels and gestational age, but this did not reach statistical significance.
“While further investigations are needed to verify our findings, our data suggest that these differences are present before fetal surgery and that an increase in intra-amniotic anaerobic glycolysis as evidenced by elevated lactic acid may enhance MMP-8 production, which will weaken the maternal-fetal membranes,” Dr. Athanasiou said. “The mechanism may not be related to inflammation, as evidenced by the lack of association between pPROM and IL-6.”
She had no financial disclosures.
SOURCE: Moron A et al. Am J Obstet Gynecol. 2018 Jan:218(1);S64.
REPORTING FROM THE PREGNANCY MEETING
Key clinical point: Lactic acid and MMP-8 in amniotic fluid may have utility as biomarkers predictive of pPROM in women who undergo fetal surgery for spinal cord defects.
Major finding: Women who experienced pPROM had higher amniotic lactic acid (7.1 vs. 5.9 mU/mL) and MMP-8 (1.7 vs. 0.6 mcg/mL) at time of fetal surgery.
Study details: The prospective study comprised 26 women.
Disclosures: Dr. Athanasiou had no financial disclosures.
Source: Moron A et al. Am J Obstet Gynecol. 2018 Jan:218(1);S64.
Melkersson-Rosenthal Syndrome Successfully Treated With Adalimumab
Melkersson-Rosenthal syndrome (MRS) is a rare condition comprised of unilateral peripheral facial nerve palsy, episodic or progressive facial edema, and lingua plicata (also known as fissured tongue). Melkersson-Rosenthal syndrome is a subtype of orofacial granulomatosis and often is mistaken for angioedema or pseudoangioedema due to the swelling of the lips and eyelids. We present a case of MRS that cleared in response to adalimumab therapy.
Case Report
A 69-year-old woman presented to our dermatology clinic with facial edema and a fissured tongue of 4 years’ duration. These symptoms had failed to improve with doxycycline, tacrolimus ointment 0.1%, and cortisone injections of the upper lip, as well as a balsam-free diet, fragrance-free skin products, and flavor-free toothpaste prescribed by multiple physicians over 4 years. Two weeks prior to the current presentation the patient developed left facial nerve palsy that was diagnosed by an outside physician as Bell palsy, and the patient completed a 7-day course of prednisone 1 day prior to presentation. The patient’s medical history was remarkable for type 2 diabetes mellitus controlled with metformin, hyperlipidemia controlled with ezetimibe-simvastatin, and psoriasis. She reported no family history of autoimmune or dermatologic disorders and denied any fever, unintentional weight loss, nausea, vomiting, or diarrhea.
On physical examination, the patient had considerable perioral edema and erythema without warmth or tenderness (Figure 1A). The tongue was fissured with notable scalloping at the lateral margins (Figure 1B). There were no aphthous ulcers or lymphadenopathy, and the remainder of the neurologic examination was normal. The patient had erythematous plaques with scaling on the bilateral elbows. Cardiopulmonary, musculoskeletal, and abdominal examinations were otherwise normal.
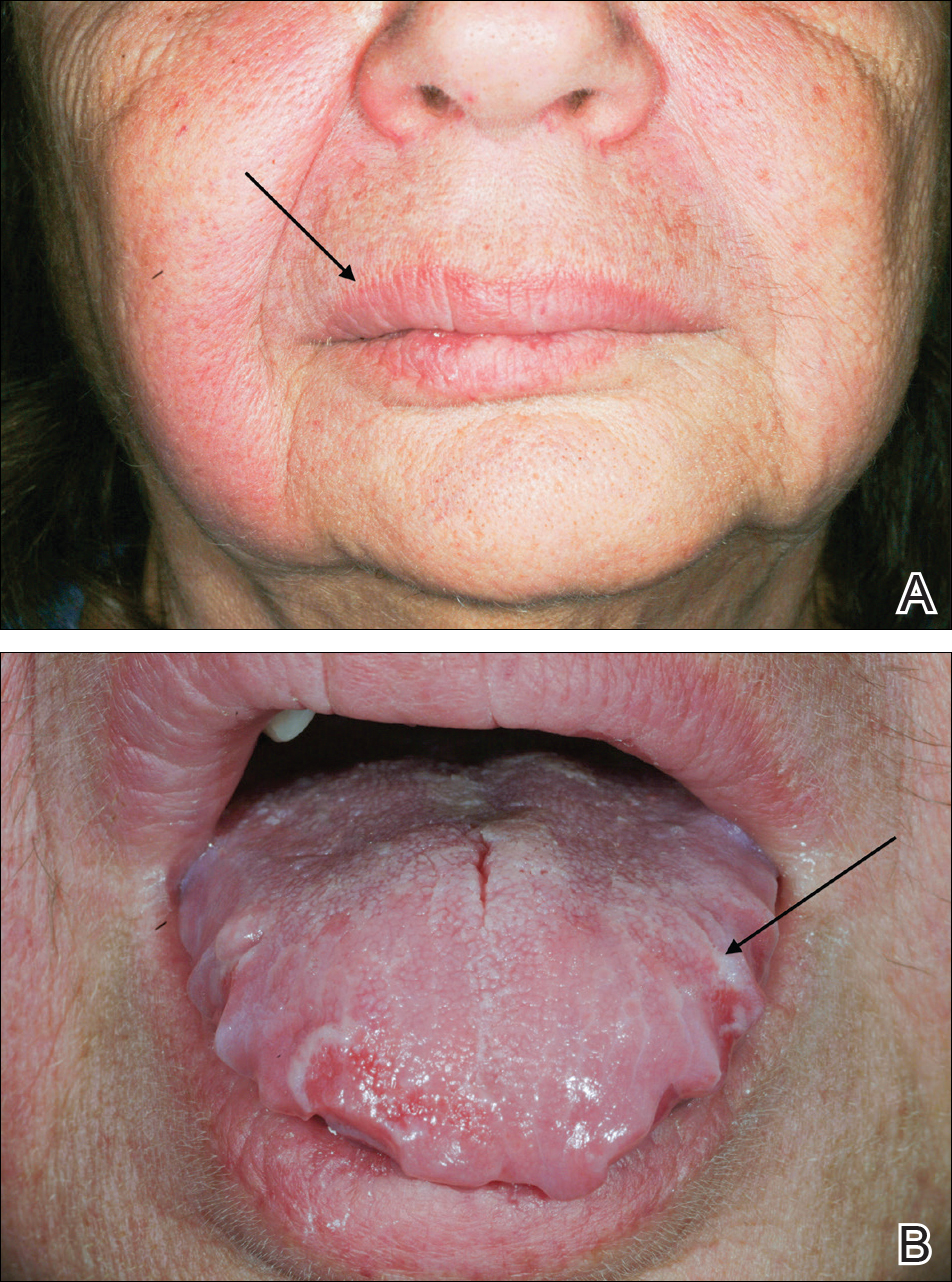
Laboratory data revealed an elevated white blood cell count of 17,500/µL (reference range, 4500–11,000/µL), an elevated absolute neutrophil count of 14,018/µL (reference range, 0–700/µL), and an absolute eosinophil count of 0/µL (reference range, 0–450/µL), with the rest of the complete blood cell count within reference range. A basic metabolic panel showed an elevated glucose level of 326 mg/dL (reference range, 70–110 mg/dL), consistent with diabetes and most likely exacerbated by the recent steroid course. A lipid panel was consistent with diagnosed hyperlipidemia (total cholesterol, 236 mg/dL [reference range, <200 mg/dL]; low-density lipoprotein, 134 mg/dL [reference range, 10–30 mg/dL]; triglycerides, 188 mg/dL [reference range, <160 mg/dL]). Hepatitis B and C tests were negative. A punch biopsy of the buccal and labial mucosa was taken, revealing a parakeratinized stratified squamous epithelium with an unusual pattern of surface keratinization with foci of intracellular and extracellular edema in the spinous layer. The underlying fibrous connective tissue was edematous with infiltrates of lymphocytes, mast cells, macrophages, and a few plasma cells. The pathology report listed the diagnosis as nonspecific “chronic mucositis,” with a list of differential diagnoses that included angioedema, hypersensitivity reaction, or other possible autoimmune disorders.
On consideration of these differential diagnoses, it was felt most likely to be MRS, which remains a primarily clinical diagnosis characterized by the triad of symptoms seen in this patient. Treatment of this condition emphasizes inflammation, and steroid therapy often is utilized, as it was in our patient. After the diagnosis of MRS was made, the patient received adalimumab 80 mg subcutaneously on day 1 and 40 mg on day 8 as a loading dose; she subsequently began a course of subcutaneous injections of adalimumab 40 mg once every other week for treatment of psoriasis with the goal of simultaneously treating the MRS. The symptoms did not completely resolve at this dose, so it was increased to 40 mg once weekly. The patient reported that the facial edema, lingua plicata, and facial nerve palsy resolved concomitantly over approximately 3 months with greater improvement at 5 months (Figure 2). The patient has had no relapses as of the last follow-up at 11 months.
Comment
Melkersson-Rosenthal syndrome usually presents sporadically, though there are reports of familial association,1-3 and only 8% to 25% of patients worldwide present with the complete triad of symptoms.4 The pathogenesis of the syndrome is controversial. Granulomatous changes have been found in patients experiencing chronic edema. However, according to Zimmer et al5 in a study of 42 MRS patients, only 46% (19/42) had granulomatous changes; 36% (15/42) had nonspecific inflammation, 11% (5/42) had incidental findings, and 7% (3/42) showed no histopathologic abnormalities. Granulomatous cheilitis is a subtype of orofacial granulomatosis, an idiopathic process that causes swelling of the face and lips as well as intraoral swelling and ulceration. Orofacial granulomatosis is referred to as granulomatous cheilitis when the lip is involved. Melkersson-Rosenthal syndrome is another subtype of orofacial granulomatosis that includes facial palsy and fissured tongue.6,7
In a clinical study of 7 patients with MRS, Liu and Yu1 found 3 (42%) patients to have dysarthria, dysphagia, and tongue muscle atrophy; 1 patient to have migrainelike headaches; 1 patient to have decreased vision and an ocular movement disorder; 1 patient to have ipsilateral hearing loss; and 1 patient to lack any other symptoms. Halevy et al8 suggested a possible association of MRS with psoriasis. In their review of 12 patients, 1 (8%) had psoriatic arthritis, 2 (17%) had skin biopsy–proven psoriasis, and 3 (25%) had a family history of psoriasis.8 Because the disease is quite rare, it is difficult to determine other symptoms that may be associated with the disease.
Tumor necrosis factor α (TNF-α) is needed for granuloma formation, and TNF-α antagonists have been used to treat a number of granulomatous conditions including Crohn disease and sarcoidosis.9-11 Two case reports indicate that infliximab, a mouse/human chimeric monoclonal antibody to TNF-α, has been used successfully to clear MRS.12,13 One report cited the use of adalimumab for maintenance therapy of MRS,12 and more recently, adalimumab has been reported for refractory MRS.14 However, there currently are no known reports regarding the efficacy of adalimumab as a first-line treatment of MRS.
Adalimumab is a fully human monoclonal antibody to TNF-α, which is administered via subcutaneous injections. Infliximab must be administered at an infusion center, making treatment logistically more difficult for patients, and can be associated with the development of infusion reactions, though the exact data on infusion reactions are difficult to estimate due to variations in reporting.15,16
In 2014, Stein et al
Conclusion
We present a case of a 69-year-old woman who presented with facial nerve palsy, facial edema, and a fissured tongue, which is the classic triad of MRS, and all 3 symptoms improved with adalimumab.
- Liu R, Yu S. Melk
ersson-Rosenthal syndrome: a review of seven patients [published online May 7, 2013]. J Clin Neurosci. 2013;20:993-995. - Sun B, Zhou C, Han Z. Facial palsy in Melkersson-Rosenthal syndrome and Bell’s palsy: familial history and recurrence tendency [published online August 13, 2014]. Ann Otol Rhinol Laryngol. 2015;124:107-109.
- Meisel-Stosiek M, Hornstein OP, Stosiek N. Family study on Melkersson-Rosenthal syndrome. some hereditary aspects of the disease and review of literature. Acta Derm Venereol. 1990;70:221-226.
- Sciubba JJ, Said-Al-Naief N. Orofacial granulomatosis: presentation, pathology and management of 13 cases. J Oral Pathol Med. 2003;32:576-585.
- Zimmer WM, Rogers
RS 3rd, Reeve CM, et al. Orofacial manifestations of Melkersson-Rosenthal syndrome. a study of 42 patients and review of 220 cases from the literature. Oral Surg Oral Med Oral Pathol. 1992;74:610-619. - Critchlow WA, Cha
ng D. Cheilitis granulomatosa: a review [published online September 22, 2013]. Head Neck Pathol. 2014;8:209-213. - Allen CM, Camisa
C. Oral disease. In: Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. Philadelphia, PA: Elsevier; 2012:1157-1160. - Halevy S, Shalom
G, Trattner A, et al. Melkersson-Rosenthal syndrome: a possible association with psoriasis. J Am Acad Dermatol. 2012;67:795-796. - Algood HM, Lin P
L, Flynn JL. Tumor necrosis factor and chemokine interactions in the formation and maintenance of granulomas in tuberculosis. Clin Infect Dis. 2005;41(suppl 3):S189-S193. - Yee AM, Pochapin
MB. Treatment of complicated sarcoidosis with infliximab anti-tumor necrosis factor-alpha therapy. Ann Intern Med. 2001;135:27-31. - Targan SR, Hanau
er SB, van Deventer SJ, et al. A short-term study of chimeric monoclonal antibody cA2 to tumor necrosis factor alpha for Crohn’s disease. Crohn’s Disease cA2 Study Group. N Engl J Med. 1997;337:1029-1035. - Kakimoto C, Spar
ks C, White AA. Melkersson-Rosenthal syndrome: a form of pseudoangioedema. Ann Allergy Asthma Immunol. 2007;99:185-189. - Wickramasinghe N
, Gunasekara CN, Fernando WS, et al. Vulvitis granulomatosa, Melkersson-Rosenthal syndrome, and Crohn’s disease: dramatic response to infliximab therapy. Int J Dermatol. 2012;51:966-968. - Stein J, Paulke
A, Schacher B, et al. An extraordinary form of the Melkersson-Rosenthal syndrome successfully treated with the tumour necrosis factor-α blocker adalimumab [published online May 14, 2014]. BMJ Case Rep. doi:10.1136/bcr-2014-204674. - Cheifetz A, Smedley M, Martin S, et al. The incidence and management of infusion reactions to infliximab: a large center experience. Am J Gastroenterol. 2003;98:1315-1324.
- Choquette D, Faraawi R, Chow A, et al. Incidence and management of infusion reactions to infliximab in a prospective real-world community registry. J Rheumatol. 2015;42:1105-1111.
- Ruiz Villaverde R, Sánchez Cano D. Successful treatment of granulomatous cheilitis with adalimumab. Int J Dermatol. 2012;51:118-120.
Melkersson-Rosenthal syndrome (MRS) is a rare condition comprised of unilateral peripheral facial nerve palsy, episodic or progressive facial edema, and lingua plicata (also known as fissured tongue). Melkersson-Rosenthal syndrome is a subtype of orofacial granulomatosis and often is mistaken for angioedema or pseudoangioedema due to the swelling of the lips and eyelids. We present a case of MRS that cleared in response to adalimumab therapy.
Case Report
A 69-year-old woman presented to our dermatology clinic with facial edema and a fissured tongue of 4 years’ duration. These symptoms had failed to improve with doxycycline, tacrolimus ointment 0.1%, and cortisone injections of the upper lip, as well as a balsam-free diet, fragrance-free skin products, and flavor-free toothpaste prescribed by multiple physicians over 4 years. Two weeks prior to the current presentation the patient developed left facial nerve palsy that was diagnosed by an outside physician as Bell palsy, and the patient completed a 7-day course of prednisone 1 day prior to presentation. The patient’s medical history was remarkable for type 2 diabetes mellitus controlled with metformin, hyperlipidemia controlled with ezetimibe-simvastatin, and psoriasis. She reported no family history of autoimmune or dermatologic disorders and denied any fever, unintentional weight loss, nausea, vomiting, or diarrhea.
On physical examination, the patient had considerable perioral edema and erythema without warmth or tenderness (Figure 1A). The tongue was fissured with notable scalloping at the lateral margins (Figure 1B). There were no aphthous ulcers or lymphadenopathy, and the remainder of the neurologic examination was normal. The patient had erythematous plaques with scaling on the bilateral elbows. Cardiopulmonary, musculoskeletal, and abdominal examinations were otherwise normal.

Laboratory data revealed an elevated white blood cell count of 17,500/µL (reference range, 4500–11,000/µL), an elevated absolute neutrophil count of 14,018/µL (reference range, 0–700/µL), and an absolute eosinophil count of 0/µL (reference range, 0–450/µL), with the rest of the complete blood cell count within reference range. A basic metabolic panel showed an elevated glucose level of 326 mg/dL (reference range, 70–110 mg/dL), consistent with diabetes and most likely exacerbated by the recent steroid course. A lipid panel was consistent with diagnosed hyperlipidemia (total cholesterol, 236 mg/dL [reference range, <200 mg/dL]; low-density lipoprotein, 134 mg/dL [reference range, 10–30 mg/dL]; triglycerides, 188 mg/dL [reference range, <160 mg/dL]). Hepatitis B and C tests were negative. A punch biopsy of the buccal and labial mucosa was taken, revealing a parakeratinized stratified squamous epithelium with an unusual pattern of surface keratinization with foci of intracellular and extracellular edema in the spinous layer. The underlying fibrous connective tissue was edematous with infiltrates of lymphocytes, mast cells, macrophages, and a few plasma cells. The pathology report listed the diagnosis as nonspecific “chronic mucositis,” with a list of differential diagnoses that included angioedema, hypersensitivity reaction, or other possible autoimmune disorders.
On consideration of these differential diagnoses, it was felt most likely to be MRS, which remains a primarily clinical diagnosis characterized by the triad of symptoms seen in this patient. Treatment of this condition emphasizes inflammation, and steroid therapy often is utilized, as it was in our patient. After the diagnosis of MRS was made, the patient received adalimumab 80 mg subcutaneously on day 1 and 40 mg on day 8 as a loading dose; she subsequently began a course of subcutaneous injections of adalimumab 40 mg once every other week for treatment of psoriasis with the goal of simultaneously treating the MRS. The symptoms did not completely resolve at this dose, so it was increased to 40 mg once weekly. The patient reported that the facial edema, lingua plicata, and facial nerve palsy resolved concomitantly over approximately 3 months with greater improvement at 5 months (Figure 2). The patient has had no relapses as of the last follow-up at 11 months.

Comment
Melkersson-Rosenthal syndrome usually presents sporadically, though there are reports of familial association,1-3 and only 8% to 25% of patients worldwide present with the complete triad of symptoms.4 The pathogenesis of the syndrome is controversial. Granulomatous changes have been found in patients experiencing chronic edema. However, according to Zimmer et al5 in a study of 42 MRS patients, only 46% (19/42) had granulomatous changes; 36% (15/42) had nonspecific inflammation, 11% (5/42) had incidental findings, and 7% (3/42) showed no histopathologic abnormalities. Granulomatous cheilitis is a subtype of orofacial granulomatosis, an idiopathic process that causes swelling of the face and lips as well as intraoral swelling and ulceration. Orofacial granulomatosis is referred to as granulomatous cheilitis when the lip is involved. Melkersson-Rosenthal syndrome is another subtype of orofacial granulomatosis that includes facial palsy and fissured tongue.6,7
In a clinical study of 7 patients with MRS, Liu and Yu1 found 3 (42%) patients to have dysarthria, dysphagia, and tongue muscle atrophy; 1 patient to have migrainelike headaches; 1 patient to have decreased vision and an ocular movement disorder; 1 patient to have ipsilateral hearing loss; and 1 patient to lack any other symptoms. Halevy et al8 suggested a possible association of MRS with psoriasis. In their review of 12 patients, 1 (8%) had psoriatic arthritis, 2 (17%) had skin biopsy–proven psoriasis, and 3 (25%) had a family history of psoriasis.8 Because the disease is quite rare, it is difficult to determine other symptoms that may be associated with the disease.
Tumor necrosis factor α (TNF-α) is needed for granuloma formation, and TNF-α antagonists have been used to treat a number of granulomatous conditions including Crohn disease and sarcoidosis.9-11 Two case reports indicate that infliximab, a mouse/human chimeric monoclonal antibody to TNF-α, has been used successfully to clear MRS.12,13 One report cited the use of adalimumab for maintenance therapy of MRS,12 and more recently, adalimumab has been reported for refractory MRS.14 However, there currently are no known reports regarding the efficacy of adalimumab as a first-line treatment of MRS.
Adalimumab is a fully human monoclonal antibody to TNF-α, which is administered via subcutaneous injections. Infliximab must be administered at an infusion center, making treatment logistically more difficult for patients, and can be associated with the development of infusion reactions, though the exact data on infusion reactions are difficult to estimate due to variations in reporting.15,16
In 2014, Stein et al
Conclusion
We present a case of a 69-year-old woman who presented with facial nerve palsy, facial edema, and a fissured tongue, which is the classic triad of MRS, and all 3 symptoms improved with adalimumab.
Melkersson-Rosenthal syndrome (MRS) is a rare condition comprised of unilateral peripheral facial nerve palsy, episodic or progressive facial edema, and lingua plicata (also known as fissured tongue). Melkersson-Rosenthal syndrome is a subtype of orofacial granulomatosis and often is mistaken for angioedema or pseudoangioedema due to the swelling of the lips and eyelids. We present a case of MRS that cleared in response to adalimumab therapy.
Case Report
A 69-year-old woman presented to our dermatology clinic with facial edema and a fissured tongue of 4 years’ duration. These symptoms had failed to improve with doxycycline, tacrolimus ointment 0.1%, and cortisone injections of the upper lip, as well as a balsam-free diet, fragrance-free skin products, and flavor-free toothpaste prescribed by multiple physicians over 4 years. Two weeks prior to the current presentation the patient developed left facial nerve palsy that was diagnosed by an outside physician as Bell palsy, and the patient completed a 7-day course of prednisone 1 day prior to presentation. The patient’s medical history was remarkable for type 2 diabetes mellitus controlled with metformin, hyperlipidemia controlled with ezetimibe-simvastatin, and psoriasis. She reported no family history of autoimmune or dermatologic disorders and denied any fever, unintentional weight loss, nausea, vomiting, or diarrhea.
On physical examination, the patient had considerable perioral edema and erythema without warmth or tenderness (Figure 1A). The tongue was fissured with notable scalloping at the lateral margins (Figure 1B). There were no aphthous ulcers or lymphadenopathy, and the remainder of the neurologic examination was normal. The patient had erythematous plaques with scaling on the bilateral elbows. Cardiopulmonary, musculoskeletal, and abdominal examinations were otherwise normal.

Laboratory data revealed an elevated white blood cell count of 17,500/µL (reference range, 4500–11,000/µL), an elevated absolute neutrophil count of 14,018/µL (reference range, 0–700/µL), and an absolute eosinophil count of 0/µL (reference range, 0–450/µL), with the rest of the complete blood cell count within reference range. A basic metabolic panel showed an elevated glucose level of 326 mg/dL (reference range, 70–110 mg/dL), consistent with diabetes and most likely exacerbated by the recent steroid course. A lipid panel was consistent with diagnosed hyperlipidemia (total cholesterol, 236 mg/dL [reference range, <200 mg/dL]; low-density lipoprotein, 134 mg/dL [reference range, 10–30 mg/dL]; triglycerides, 188 mg/dL [reference range, <160 mg/dL]). Hepatitis B and C tests were negative. A punch biopsy of the buccal and labial mucosa was taken, revealing a parakeratinized stratified squamous epithelium with an unusual pattern of surface keratinization with foci of intracellular and extracellular edema in the spinous layer. The underlying fibrous connective tissue was edematous with infiltrates of lymphocytes, mast cells, macrophages, and a few plasma cells. The pathology report listed the diagnosis as nonspecific “chronic mucositis,” with a list of differential diagnoses that included angioedema, hypersensitivity reaction, or other possible autoimmune disorders.
On consideration of these differential diagnoses, it was felt most likely to be MRS, which remains a primarily clinical diagnosis characterized by the triad of symptoms seen in this patient. Treatment of this condition emphasizes inflammation, and steroid therapy often is utilized, as it was in our patient. After the diagnosis of MRS was made, the patient received adalimumab 80 mg subcutaneously on day 1 and 40 mg on day 8 as a loading dose; she subsequently began a course of subcutaneous injections of adalimumab 40 mg once every other week for treatment of psoriasis with the goal of simultaneously treating the MRS. The symptoms did not completely resolve at this dose, so it was increased to 40 mg once weekly. The patient reported that the facial edema, lingua plicata, and facial nerve palsy resolved concomitantly over approximately 3 months with greater improvement at 5 months (Figure 2). The patient has had no relapses as of the last follow-up at 11 months.

Comment
Melkersson-Rosenthal syndrome usually presents sporadically, though there are reports of familial association,1-3 and only 8% to 25% of patients worldwide present with the complete triad of symptoms.4 The pathogenesis of the syndrome is controversial. Granulomatous changes have been found in patients experiencing chronic edema. However, according to Zimmer et al5 in a study of 42 MRS patients, only 46% (19/42) had granulomatous changes; 36% (15/42) had nonspecific inflammation, 11% (5/42) had incidental findings, and 7% (3/42) showed no histopathologic abnormalities. Granulomatous cheilitis is a subtype of orofacial granulomatosis, an idiopathic process that causes swelling of the face and lips as well as intraoral swelling and ulceration. Orofacial granulomatosis is referred to as granulomatous cheilitis when the lip is involved. Melkersson-Rosenthal syndrome is another subtype of orofacial granulomatosis that includes facial palsy and fissured tongue.6,7
In a clinical study of 7 patients with MRS, Liu and Yu1 found 3 (42%) patients to have dysarthria, dysphagia, and tongue muscle atrophy; 1 patient to have migrainelike headaches; 1 patient to have decreased vision and an ocular movement disorder; 1 patient to have ipsilateral hearing loss; and 1 patient to lack any other symptoms. Halevy et al8 suggested a possible association of MRS with psoriasis. In their review of 12 patients, 1 (8%) had psoriatic arthritis, 2 (17%) had skin biopsy–proven psoriasis, and 3 (25%) had a family history of psoriasis.8 Because the disease is quite rare, it is difficult to determine other symptoms that may be associated with the disease.
Tumor necrosis factor α (TNF-α) is needed for granuloma formation, and TNF-α antagonists have been used to treat a number of granulomatous conditions including Crohn disease and sarcoidosis.9-11 Two case reports indicate that infliximab, a mouse/human chimeric monoclonal antibody to TNF-α, has been used successfully to clear MRS.12,13 One report cited the use of adalimumab for maintenance therapy of MRS,12 and more recently, adalimumab has been reported for refractory MRS.14 However, there currently are no known reports regarding the efficacy of adalimumab as a first-line treatment of MRS.
Adalimumab is a fully human monoclonal antibody to TNF-α, which is administered via subcutaneous injections. Infliximab must be administered at an infusion center, making treatment logistically more difficult for patients, and can be associated with the development of infusion reactions, though the exact data on infusion reactions are difficult to estimate due to variations in reporting.15,16
In 2014, Stein et al
Conclusion
We present a case of a 69-year-old woman who presented with facial nerve palsy, facial edema, and a fissured tongue, which is the classic triad of MRS, and all 3 symptoms improved with adalimumab.
- Liu R, Yu S. Melk
ersson-Rosenthal syndrome: a review of seven patients [published online May 7, 2013]. J Clin Neurosci. 2013;20:993-995. - Sun B, Zhou C, Han Z. Facial palsy in Melkersson-Rosenthal syndrome and Bell’s palsy: familial history and recurrence tendency [published online August 13, 2014]. Ann Otol Rhinol Laryngol. 2015;124:107-109.
- Meisel-Stosiek M, Hornstein OP, Stosiek N. Family study on Melkersson-Rosenthal syndrome. some hereditary aspects of the disease and review of literature. Acta Derm Venereol. 1990;70:221-226.
- Sciubba JJ, Said-Al-Naief N. Orofacial granulomatosis: presentation, pathology and management of 13 cases. J Oral Pathol Med. 2003;32:576-585.
- Zimmer WM, Rogers
RS 3rd, Reeve CM, et al. Orofacial manifestations of Melkersson-Rosenthal syndrome. a study of 42 patients and review of 220 cases from the literature. Oral Surg Oral Med Oral Pathol. 1992;74:610-619. - Critchlow WA, Cha
ng D. Cheilitis granulomatosa: a review [published online September 22, 2013]. Head Neck Pathol. 2014;8:209-213. - Allen CM, Camisa
C. Oral disease. In: Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. Philadelphia, PA: Elsevier; 2012:1157-1160. - Halevy S, Shalom
G, Trattner A, et al. Melkersson-Rosenthal syndrome: a possible association with psoriasis. J Am Acad Dermatol. 2012;67:795-796. - Algood HM, Lin P
L, Flynn JL. Tumor necrosis factor and chemokine interactions in the formation and maintenance of granulomas in tuberculosis. Clin Infect Dis. 2005;41(suppl 3):S189-S193. - Yee AM, Pochapin
MB. Treatment of complicated sarcoidosis with infliximab anti-tumor necrosis factor-alpha therapy. Ann Intern Med. 2001;135:27-31. - Targan SR, Hanau
er SB, van Deventer SJ, et al. A short-term study of chimeric monoclonal antibody cA2 to tumor necrosis factor alpha for Crohn’s disease. Crohn’s Disease cA2 Study Group. N Engl J Med. 1997;337:1029-1035. - Kakimoto C, Spar
ks C, White AA. Melkersson-Rosenthal syndrome: a form of pseudoangioedema. Ann Allergy Asthma Immunol. 2007;99:185-189. - Wickramasinghe N
, Gunasekara CN, Fernando WS, et al. Vulvitis granulomatosa, Melkersson-Rosenthal syndrome, and Crohn’s disease: dramatic response to infliximab therapy. Int J Dermatol. 2012;51:966-968. - Stein J, Paulke
A, Schacher B, et al. An extraordinary form of the Melkersson-Rosenthal syndrome successfully treated with the tumour necrosis factor-α blocker adalimumab [published online May 14, 2014]. BMJ Case Rep. doi:10.1136/bcr-2014-204674. - Cheifetz A, Smedley M, Martin S, et al. The incidence and management of infusion reactions to infliximab: a large center experience. Am J Gastroenterol. 2003;98:1315-1324.
- Choquette D, Faraawi R, Chow A, et al. Incidence and management of infusion reactions to infliximab in a prospective real-world community registry. J Rheumatol. 2015;42:1105-1111.
- Ruiz Villaverde R, Sánchez Cano D. Successful treatment of granulomatous cheilitis with adalimumab. Int J Dermatol. 2012;51:118-120.
- Liu R, Yu S. Melk
ersson-Rosenthal syndrome: a review of seven patients [published online May 7, 2013]. J Clin Neurosci. 2013;20:993-995. - Sun B, Zhou C, Han Z. Facial palsy in Melkersson-Rosenthal syndrome and Bell’s palsy: familial history and recurrence tendency [published online August 13, 2014]. Ann Otol Rhinol Laryngol. 2015;124:107-109.
- Meisel-Stosiek M, Hornstein OP, Stosiek N. Family study on Melkersson-Rosenthal syndrome. some hereditary aspects of the disease and review of literature. Acta Derm Venereol. 1990;70:221-226.
- Sciubba JJ, Said-Al-Naief N. Orofacial granulomatosis: presentation, pathology and management of 13 cases. J Oral Pathol Med. 2003;32:576-585.
- Zimmer WM, Rogers
RS 3rd, Reeve CM, et al. Orofacial manifestations of Melkersson-Rosenthal syndrome. a study of 42 patients and review of 220 cases from the literature. Oral Surg Oral Med Oral Pathol. 1992;74:610-619. - Critchlow WA, Cha
ng D. Cheilitis granulomatosa: a review [published online September 22, 2013]. Head Neck Pathol. 2014;8:209-213. - Allen CM, Camisa
C. Oral disease. In: Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. Philadelphia, PA: Elsevier; 2012:1157-1160. - Halevy S, Shalom
G, Trattner A, et al. Melkersson-Rosenthal syndrome: a possible association with psoriasis. J Am Acad Dermatol. 2012;67:795-796. - Algood HM, Lin P
L, Flynn JL. Tumor necrosis factor and chemokine interactions in the formation and maintenance of granulomas in tuberculosis. Clin Infect Dis. 2005;41(suppl 3):S189-S193. - Yee AM, Pochapin
MB. Treatment of complicated sarcoidosis with infliximab anti-tumor necrosis factor-alpha therapy. Ann Intern Med. 2001;135:27-31. - Targan SR, Hanau
er SB, van Deventer SJ, et al. A short-term study of chimeric monoclonal antibody cA2 to tumor necrosis factor alpha for Crohn’s disease. Crohn’s Disease cA2 Study Group. N Engl J Med. 1997;337:1029-1035. - Kakimoto C, Spar
ks C, White AA. Melkersson-Rosenthal syndrome: a form of pseudoangioedema. Ann Allergy Asthma Immunol. 2007;99:185-189. - Wickramasinghe N
, Gunasekara CN, Fernando WS, et al. Vulvitis granulomatosa, Melkersson-Rosenthal syndrome, and Crohn’s disease: dramatic response to infliximab therapy. Int J Dermatol. 2012;51:966-968. - Stein J, Paulke
A, Schacher B, et al. An extraordinary form of the Melkersson-Rosenthal syndrome successfully treated with the tumour necrosis factor-α blocker adalimumab [published online May 14, 2014]. BMJ Case Rep. doi:10.1136/bcr-2014-204674. - Cheifetz A, Smedley M, Martin S, et al. The incidence and management of infusion reactions to infliximab: a large center experience. Am J Gastroenterol. 2003;98:1315-1324.
- Choquette D, Faraawi R, Chow A, et al. Incidence and management of infusion reactions to infliximab in a prospective real-world community registry. J Rheumatol. 2015;42:1105-1111.
- Ruiz Villaverde R, Sánchez Cano D. Successful treatment of granulomatous cheilitis with adalimumab. Int J Dermatol. 2012;51:118-120.
Practice Points
- The classical triad of Melkersson-Rosenthal syndrome (MRS), which includes facial nerve palsy, facial edema, and lingua plicata, can present gradually over time and should therefore be kept in the differential of cheilitis.
- Tumor necrosis factor α therapy may play a crucial role in rare granulomatous diseases, including MRS.
Debunking Psoriasis Myths: How Long Do Patients Have to Wait to See Results With Biologics?
Myth: Biologics Work Slowly
Biologics have demonstrated efficacy in psoriasis and often are used in psoriasis patients who have not achieved desired results with other treatments, patients who have had intolerable side effects from other treatments, and patients with concurrent diseases that preclude the use of systemic therapies. Because of the quality-of-life impact of psoriasis, patients look for quick clearance of their symptoms, but can biologics deliver fast results or do they work slowly?
Biologics such as etanercept and adalimumab block tumor necrosis factor α signaling, while ustekinumab targets IL-12 and IL-23 and others target IL-17. Some patients may begin to see improvement in skin lesions within 1 month of initiating biologic therapies because they target specific proinflammatory pathways that are critical to the pathogenesis of psoriasis, but response time varies among patients and specific therapy used.
The psoriasis area and severity index (PASI) measures psoriasis treatment success. Based on the American Academy of Dermatology’s guidelines of care for the management of psoriasis and psoriatic arthritis published in 2008, short-term response was achieved in 10 to 14 weeks for the following biologics:
- Adalimumab: 80% of patients achieved PASI 75 at week 12
- Etanercept: 49% of patients given 50 mg twice weekly achieved PASI 75 at 12 weeks; 34% of patients given 25 mg twice weekly achieved PASI 75 at 12 weeks
- Infliximab: 80% of patients achieved PASI 75 at week 10
Of the newer biologics, Premier Research recently noted that PASI 75 was achieved after 12 weeks with the following biologics:
- Brodalumab: 83% after 12 weeks
- Ixekizumab: 90% after 12 weeks
- Secukinumab: 80% after 12 weeks
- Ustekinumab: 70% after 12 weeks
There are a variety of factors to consider when determining which biologic to use for a psoriasis patient. These data may help in the decision process. However, dermatologists must educate psoriasis patients with a high body mass index that their disease may take longer to respond and may need combination therapy for optimal clearance.
Expert Commentary
All of the biologics, especially the IL-17 inhibitors, work very quickly to clear psoriasis. The only way they work “slowly” is that it may take time (usually a few days) for the payers to approve biologic prescriptions.
—Jashin J. Wu, MD (Los Angeles, California)
Biologics. DermNet New Zealand website. https://www.dermnetnz.org/topics/biologics/. Accessed February 6, 2018.
Biologics in psoriasis treatment. Premier Research website. https://premier-research.com/perspectives-biologics-psoriasis-treatment/. Published May 9, 2017. Accessed February 6, 2018.
Menter A, Gottlieb A, Feldman SR, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 1. Overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol. 2008;58:826-850.
Vilarrasa E, Notario J, Bordas X, et al. ORBIT (Outcome and Retention Rate of Biologic Treatments for Psoriasis): a retrospective observational study on biologic drug survival in daily practice. J Am Acad Dermatol. 2016;74:1066-1072.
Myth: Biologics Work Slowly
Biologics have demonstrated efficacy in psoriasis and often are used in psoriasis patients who have not achieved desired results with other treatments, patients who have had intolerable side effects from other treatments, and patients with concurrent diseases that preclude the use of systemic therapies. Because of the quality-of-life impact of psoriasis, patients look for quick clearance of their symptoms, but can biologics deliver fast results or do they work slowly?
Biologics such as etanercept and adalimumab block tumor necrosis factor α signaling, while ustekinumab targets IL-12 and IL-23 and others target IL-17. Some patients may begin to see improvement in skin lesions within 1 month of initiating biologic therapies because they target specific proinflammatory pathways that are critical to the pathogenesis of psoriasis, but response time varies among patients and specific therapy used.
The psoriasis area and severity index (PASI) measures psoriasis treatment success. Based on the American Academy of Dermatology’s guidelines of care for the management of psoriasis and psoriatic arthritis published in 2008, short-term response was achieved in 10 to 14 weeks for the following biologics:
- Adalimumab: 80% of patients achieved PASI 75 at week 12
- Etanercept: 49% of patients given 50 mg twice weekly achieved PASI 75 at 12 weeks; 34% of patients given 25 mg twice weekly achieved PASI 75 at 12 weeks
- Infliximab: 80% of patients achieved PASI 75 at week 10
Of the newer biologics, Premier Research recently noted that PASI 75 was achieved after 12 weeks with the following biologics:
- Brodalumab: 83% after 12 weeks
- Ixekizumab: 90% after 12 weeks
- Secukinumab: 80% after 12 weeks
- Ustekinumab: 70% after 12 weeks
There are a variety of factors to consider when determining which biologic to use for a psoriasis patient. These data may help in the decision process. However, dermatologists must educate psoriasis patients with a high body mass index that their disease may take longer to respond and may need combination therapy for optimal clearance.
Expert Commentary
All of the biologics, especially the IL-17 inhibitors, work very quickly to clear psoriasis. The only way they work “slowly” is that it may take time (usually a few days) for the payers to approve biologic prescriptions.
—Jashin J. Wu, MD (Los Angeles, California)
Myth: Biologics Work Slowly
Biologics have demonstrated efficacy in psoriasis and often are used in psoriasis patients who have not achieved desired results with other treatments, patients who have had intolerable side effects from other treatments, and patients with concurrent diseases that preclude the use of systemic therapies. Because of the quality-of-life impact of psoriasis, patients look for quick clearance of their symptoms, but can biologics deliver fast results or do they work slowly?
Biologics such as etanercept and adalimumab block tumor necrosis factor α signaling, while ustekinumab targets IL-12 and IL-23 and others target IL-17. Some patients may begin to see improvement in skin lesions within 1 month of initiating biologic therapies because they target specific proinflammatory pathways that are critical to the pathogenesis of psoriasis, but response time varies among patients and specific therapy used.
The psoriasis area and severity index (PASI) measures psoriasis treatment success. Based on the American Academy of Dermatology’s guidelines of care for the management of psoriasis and psoriatic arthritis published in 2008, short-term response was achieved in 10 to 14 weeks for the following biologics:
- Adalimumab: 80% of patients achieved PASI 75 at week 12
- Etanercept: 49% of patients given 50 mg twice weekly achieved PASI 75 at 12 weeks; 34% of patients given 25 mg twice weekly achieved PASI 75 at 12 weeks
- Infliximab: 80% of patients achieved PASI 75 at week 10
Of the newer biologics, Premier Research recently noted that PASI 75 was achieved after 12 weeks with the following biologics:
- Brodalumab: 83% after 12 weeks
- Ixekizumab: 90% after 12 weeks
- Secukinumab: 80% after 12 weeks
- Ustekinumab: 70% after 12 weeks
There are a variety of factors to consider when determining which biologic to use for a psoriasis patient. These data may help in the decision process. However, dermatologists must educate psoriasis patients with a high body mass index that their disease may take longer to respond and may need combination therapy for optimal clearance.
Expert Commentary
All of the biologics, especially the IL-17 inhibitors, work very quickly to clear psoriasis. The only way they work “slowly” is that it may take time (usually a few days) for the payers to approve biologic prescriptions.
—Jashin J. Wu, MD (Los Angeles, California)
Biologics. DermNet New Zealand website. https://www.dermnetnz.org/topics/biologics/. Accessed February 6, 2018.
Biologics in psoriasis treatment. Premier Research website. https://premier-research.com/perspectives-biologics-psoriasis-treatment/. Published May 9, 2017. Accessed February 6, 2018.
Menter A, Gottlieb A, Feldman SR, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 1. Overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol. 2008;58:826-850.
Vilarrasa E, Notario J, Bordas X, et al. ORBIT (Outcome and Retention Rate of Biologic Treatments for Psoriasis): a retrospective observational study on biologic drug survival in daily practice. J Am Acad Dermatol. 2016;74:1066-1072.
Biologics. DermNet New Zealand website. https://www.dermnetnz.org/topics/biologics/. Accessed February 6, 2018.
Biologics in psoriasis treatment. Premier Research website. https://premier-research.com/perspectives-biologics-psoriasis-treatment/. Published May 9, 2017. Accessed February 6, 2018.
Menter A, Gottlieb A, Feldman SR, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 1. Overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol. 2008;58:826-850.
Vilarrasa E, Notario J, Bordas X, et al. ORBIT (Outcome and Retention Rate of Biologic Treatments for Psoriasis): a retrospective observational study on biologic drug survival in daily practice. J Am Acad Dermatol. 2016;74:1066-1072.
Shades of gray
If you were born in or after the 1970s, it is very likely that you have never watched a television show on a black and white set. Although the roots of its technology extend well back into the early 20th century, the first color broadcast on a national television network didn’t occur until 1954 with NBC’s coverage of the Tournament of Roses Parade.
When we compare the popularization of color television with the rapid pace at which we adopt new technology today, the popularization of color TV was glacial. In large part because of their expense, sales of color sets did not surpass black and white sets until 1972. Our family lagged behind the curve and finally caved in and junked our black and white television around 1977.
The observable change in our viewing behavior was dramatic. While programming in black and white was interesting, the color images were magnetic. We were drawn by the visual excitement and stimulation that color offered, and our family’s viewing standards took a precipitous dip. We seemed to watch anything that was colorful and moved. The quality of the content took a back seat. Viewing in color seemed to require much less cognitive effort. Ironically what attracted our attention allowed us to invest less energy in paying attention.
As a regular reader of Letters From Maine, you know that I am convinced that sleep deprivation is a major contributor to the emergence of the ADHD phenomenon. However, I can make a similar argument that the introduction of color television is an equally potent coconspirator or confounder. The magnetism inherent in a moving color image can tempt even the most health conscious among us to stay well past a brain-friendly bedtime. The invention of the electric light may have gotten the ball rolling, but the ubiquity of moving electronic color images has certainly greased what was already a very slippery slope into an abyss of unhealthy sleep habits.
There are those who argue that smartphones and tablets can open a world of creative opportunities for even very young children. And, it is obvious that parents are struggling to find a balance as they try to decide when, where, and how often to allow their infants and toddlers access to handheld electronic devices.
Recently there has been much finger-pointing at the developers and manufacturers of smartphones and tablets. How can any company with a social conscience sell a product with such dangerous attractive potential for children without providing safeguards? Isn’t it like selling a swimming pool without a gated fence?
Of course the answer to this question goes to the heart of how our society views its responsibility to protect its children. Regardless of who makes the rules and how the responsibility is assigned, it is still the child’s parents who must make sure that the gate is locked.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.”
If you were born in or after the 1970s, it is very likely that you have never watched a television show on a black and white set. Although the roots of its technology extend well back into the early 20th century, the first color broadcast on a national television network didn’t occur until 1954 with NBC’s coverage of the Tournament of Roses Parade.
When we compare the popularization of color television with the rapid pace at which we adopt new technology today, the popularization of color TV was glacial. In large part because of their expense, sales of color sets did not surpass black and white sets until 1972. Our family lagged behind the curve and finally caved in and junked our black and white television around 1977.
The observable change in our viewing behavior was dramatic. While programming in black and white was interesting, the color images were magnetic. We were drawn by the visual excitement and stimulation that color offered, and our family’s viewing standards took a precipitous dip. We seemed to watch anything that was colorful and moved. The quality of the content took a back seat. Viewing in color seemed to require much less cognitive effort. Ironically what attracted our attention allowed us to invest less energy in paying attention.
As a regular reader of Letters From Maine, you know that I am convinced that sleep deprivation is a major contributor to the emergence of the ADHD phenomenon. However, I can make a similar argument that the introduction of color television is an equally potent coconspirator or confounder. The magnetism inherent in a moving color image can tempt even the most health conscious among us to stay well past a brain-friendly bedtime. The invention of the electric light may have gotten the ball rolling, but the ubiquity of moving electronic color images has certainly greased what was already a very slippery slope into an abyss of unhealthy sleep habits.
There are those who argue that smartphones and tablets can open a world of creative opportunities for even very young children. And, it is obvious that parents are struggling to find a balance as they try to decide when, where, and how often to allow their infants and toddlers access to handheld electronic devices.
Recently there has been much finger-pointing at the developers and manufacturers of smartphones and tablets. How can any company with a social conscience sell a product with such dangerous attractive potential for children without providing safeguards? Isn’t it like selling a swimming pool without a gated fence?
Of course the answer to this question goes to the heart of how our society views its responsibility to protect its children. Regardless of who makes the rules and how the responsibility is assigned, it is still the child’s parents who must make sure that the gate is locked.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.”
If you were born in or after the 1970s, it is very likely that you have never watched a television show on a black and white set. Although the roots of its technology extend well back into the early 20th century, the first color broadcast on a national television network didn’t occur until 1954 with NBC’s coverage of the Tournament of Roses Parade.
When we compare the popularization of color television with the rapid pace at which we adopt new technology today, the popularization of color TV was glacial. In large part because of their expense, sales of color sets did not surpass black and white sets until 1972. Our family lagged behind the curve and finally caved in and junked our black and white television around 1977.
The observable change in our viewing behavior was dramatic. While programming in black and white was interesting, the color images were magnetic. We were drawn by the visual excitement and stimulation that color offered, and our family’s viewing standards took a precipitous dip. We seemed to watch anything that was colorful and moved. The quality of the content took a back seat. Viewing in color seemed to require much less cognitive effort. Ironically what attracted our attention allowed us to invest less energy in paying attention.
As a regular reader of Letters From Maine, you know that I am convinced that sleep deprivation is a major contributor to the emergence of the ADHD phenomenon. However, I can make a similar argument that the introduction of color television is an equally potent coconspirator or confounder. The magnetism inherent in a moving color image can tempt even the most health conscious among us to stay well past a brain-friendly bedtime. The invention of the electric light may have gotten the ball rolling, but the ubiquity of moving electronic color images has certainly greased what was already a very slippery slope into an abyss of unhealthy sleep habits.
There are those who argue that smartphones and tablets can open a world of creative opportunities for even very young children. And, it is obvious that parents are struggling to find a balance as they try to decide when, where, and how often to allow their infants and toddlers access to handheld electronic devices.
Recently there has been much finger-pointing at the developers and manufacturers of smartphones and tablets. How can any company with a social conscience sell a product with such dangerous attractive potential for children without providing safeguards? Isn’t it like selling a swimming pool without a gated fence?
Of course the answer to this question goes to the heart of how our society views its responsibility to protect its children. Regardless of who makes the rules and how the responsibility is assigned, it is still the child’s parents who must make sure that the gate is locked.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.”
Use of a Small-Bore Needle Arthroscope to Diagnose Intra-Articular Knee Pathology: Comparison With Magnetic Resonance Imaging
ABSTRACT
The use of arthroscopy for purely diagnostic purposes has been largely supplanted by noninvasive technologies, such as magnetic resonance imaging (MRI). The mi-eye+TM (Trice Medical) technology is a small-bore needle unit for in-office arthroscopy. We conducted a pilot study comparing the mi-eye+TM unit with MRI, using surgical arthroscopy as a gold-standard reference. We hypothesized that the mi-eye+TM needle arthroscope, which can be used in an office setting, would be equivalent to MRI for the diagnosis of intra-articular pathology of the knee.
This prospective, multicenter, observational study was approved by the Institutional Review Board. There were 106 patients (53 males, 53 females) in the study. MRIs were interpreted by musculoskeletally trained radiologists. The study was conducted in the operating room using the mi-eye+TM device. The mi-eye+ TM device findings were compared with the MRI findings within individual pathologies, and a “per-patient” analysis was performed to compare the arthroscopic findings with those of the mi-eye+TM and the MRI. Additionally, we identified all mi-eye+TM findings and MRI findings that exactly matched the surgical arthroscopy findings.
The mi-eye+TM demonstrated complete accuracy of all pathologies for 97 (91.5%) of the 106 patients included in the study, whereas MRI demonstrated complete accuracy for 65 patients (61.3%) (P < .0001). All discrepancies between mi-eye+TM and arthroscopy were false-negative mi-eye+TM results, as the mi-eye+TM did not reveal some aspect of the knee’s pathology for 9 patients. The mi-eye+TM was more sensitive than MRI in identifying meniscal tears (92.6% vs 77.8%; P = .0035) and more specific in diagnosing these tears (100% vs 41.7%; P < .0001).
The mi-eye+TM device proved to be more sensitive and specific than MRI for intra-articular findings at time of knee arthroscopy. Certainly there are contraindications to using the mi-eye+TM, and our results do not obviate the need for MRI, but our study did demonstrate that the mi-eye+TM needle arthroscope can safely provide excellent visualization of intra-articular knee pathology.
Continue to: Surgical arthroscopy is the gold standard...
Surgical arthroscopy is the gold standard for the diagnosis of intra-articular knee pathologies. Nevertheless, the use of arthroscopy for purely diagnostic purposes has been largely supplanted by noninvasive technologies, such as magnetic resonance imaging (MRI). Although MRI is considered the standard diagnostic tool for acute and chronic soft-tissue injuries of the knee, its use is not without contraindication and some potential inconveniences. Contraindications to MRI are well documented. In terms of inconvenience, MRI usually requires a separate visit followed by another visit to the prescribing physician. In addition, required interpretation by a radiologist may lead to a delay in care and increase in cost.
In the early 1990s, in-office needle arthroscopy was described as a viable means of diagnosing pathologies and obtaining synovial biopsies from the knee.1-3 Initial results were good, and the procedures had very low complication rates. Nevertheless, in-office arthroscopy of the knee is not yet widely performed, likely given concerns about the technical difficulties of in-office arthroscopy, the potential for patient discomfort, and the cumbersomeness of in-office arthroscopy units. However, significant advances have been made in the resolution capability of small-bore needle arthroscopy, resulting in much less painful procedures. Additionally, the early hardware designs, which mimicked operating room setups using towers, fluid irrigation systems, and larger arthroscopes, have been replaced with small-needle arthroscopes that use syringes for irrigation and tablet computers for visualization (Figures 1A, 1B).
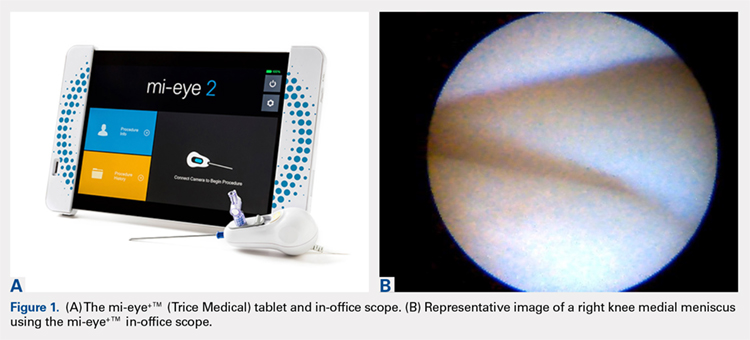
The mi-eye+TM technology (Trice Medical) is a small-bore needle unit for in-office arthroscopy with digital optics that does not need an irrigation tower. We conducted a pilot study of the sensitivity and specificity of the mi-eye+TM unit in comparison with MRI, using surgical arthroscopy as a gold-standard reference. We hypothesized that the mi-eye+TM needle arthroscope, which can be used in an office setting, would be equivalent to the standard of care (MRI) for the diagnosis of intra-articular pathology of the knee.
METHODS
Central regulatory approval for this prospective, multicenter, observational study was obtained from the Western Institutional Review Board for 3 of the sites, and 1 institution required and was granted internal Institutional Review Board approval.
The study was performed by 4 sports medicine orthopedic surgeons experienced in using the mi-eye+TM in-office arthroscope. Patients were enrolled from December 2015 through June 2016. Inclusion criteria were an indication for an arthroscopic procedure of the knee based on history, physical examination, and MRI findings. Patients were excluded from the study if there were any contraindications to completing an MRI. Acute hemarthroses of the knee or active systemic infections were also excluded. Once a patient was identified as meeting the criteria for participation, informed consent was obtained. Of the 113 patients who enrolled, 7 did not have a complete study dataset available, leaving 106 patients (53 males, 53 females) in the study. Mean age was 47 years (range, 18-82 years).
Continue to: A test result form was used...
A test result form was used to record mi-eye+TM, surgical arthroscopy, and MRI results. This form required a “positive” or “negative” result for all of several diagnoses: medial and lateral meniscal tears, intra-articular loose body, osteoarthritis (OA), osteochondritis dissecans (OCD), and tears of the anterior and posterior cruciate ligaments (ACL, PCL). MRI was performed at a variety of imaging facilities, but the images were interpreted by musculoskeletally trained radiologists.
The study was conducted in the operating room. After the patient was appropriately anesthetized, and the extremity prepared and draped, the mi-eye+TM procedure was performed immediately prior to surgical arthroscopy. A tourniquet was not used. At surgeon discretion, medial, lateral, or both approaches were used with the mi-eye+TM, and diagnostic arthroscopy was performed. During the procedure, the mi-eye+TM was advanced into the knee. Once in the synovial compartment, the external 14-gauge needle was retracted, exposing the unit’s optics. Visualization was improved by injecting normal saline through the lure lock in the mi-eye+TM needle arthroscope. An average of 20 mL of saline was used, though the amount varied with surgeon discretion. Subsequently, the surgeon visualized structures in the knee and documented all findings.
At the end of the mi-eye+TM procedure, the scheduled surgical arthroscopy was performed. After the surgical procedure, if there were no issues or complications, the patient was discharged from the study. No follow-up was required for the study, as arthroscopic findings served as the conclusive diagnosis for each patient, and no interventions were being studied. There were no complications related to use of the mi-eye+TM.
The mi-eye+TM device findings were compared with the MRI findings within individual pathologies, and a “per-patient” analysis was performed to compare the arthroscopic findings with those of the mi-eye+TM and the MRI. Additionally, we identified all mi-eye+TM findings and MRI findings that exactly matched the surgical arthroscopy findings. When a test had no false-positive or false-negative findings in comparison with surgical arthroscopy, it was identified as having complete accuracy for all intra-articular knee pathologies. For these methods, the 95% confidence interval was determined based on binomial distribution.
RESULTS
The mi-eye+ TM demonstrated complete accuracy of all pathologies for 97 (91.5%) of the 106 patients included in the study, whereas MRI demonstrated complete accuracy for 65 patients (61.3%) (P < .0001). All discrepancies between mi-eye+TM and surgical arthroscopy were false-negative mi-eye+TM results, as the mi-eye+TM did not reveal some aspect of the knee’s pathology for 9 patients. On the other hand, MRI demonstrated both false-negative and false-positive results, failing to reveal some aspect of the knee’s pathology for 31 patients, and potentially overcalling some aspect of the knee’s pathology among 18 patients.
Continue to: The pathology most frequently...
The pathology most frequently identified in the study was a meniscal tear. The mi-eye+TM was more sensitive than MRI in identifying meniscal tears (92.6% vs 77.8%; P = .0035) and more specific in diagnosing these tears (100% vs 87.5%; P < .0002). The difference in specificity resulted from the false MRI diagnosis of a meniscal tear among 24 patients, who were found to have no tear by both mi-eye+TM and surgical arthroscopy.
Table 1. Raw Data of mi-eye+TM and Magnetic Resonance Imaging Findings
| Data | True-Positive | False-Negative | False-Negative | True-Negative |
| mi-eye+TM | ||||
| Medial meniscal tear | 68 | 3 | 0 | 35 |
| Lateral meniscal tear | 32 | 5 | 0 | 69 |
| Any meniscal tear | 100 | 8 | 0 | 104 |
| Intra-articular loose body | 13 | 2 | 0 | 87 |
| Osteoarthritis | 31 | 2 | 00 | 73 |
| Osteochondritis dissecans | 8 | 2 | 0 | 97 |
| Anterior cruciate ligament tear | 16 | 0 | 0 | 90 |
| Posterior cruciate ligament tear | 0 | 0 | 0 | 106 |
| All pathologies | 168 | 14 | 0 | 557 |
| Magnetic resonance imaging | ||||
| Medial meniscal tear | 62 | 9 | 6 | 29 |
| Lateral meniscal tear | 22 | 15 | 7 | 62 |
| Any meniscal tear | 84 | 24 | 13 | 91 |
| Intra-articular loose body | 3 | 12 | 0 | 87 |
| Osteoarthritis | 26 | 7 | 8 | 65 |
| Osteochondritis dissecans | 5 | 5 | 4 | 93 |
| Anterior cruciate ligament tear | 14 | 2 | 3 | 87 |
| Posterior cruciate ligament tear | 0 | 0 | 2 | 104 |
| All pathologies | 132 | 500 | 30 | 527 |
The second most frequent pathology was an intra-articular loose body. The mi-eye+TM was more sensitive than MRI in identifying loose bodies (86.7% vs 20%; P = .0007). The specificity of the mi-eye+TM and the specificity of MRI were equivalent in diagnosing loose bodies (100%). Table 1 and Table 2 show the complete set of diagnoses and associated diagnostic profiles.
Table 2. Diagnostic Profiles: Sensitivity and Specificity of mi-eye+TM and Magnetic Resonance Imaging
| Patient Group | mi-eye+TM | MRI | |||
| Estimate, % | CI, % | Estimate, % | CI, % | Pa | |
| Sensitivity | |||||
| Medial meniscal tear | 95.77 | 88.1-99.1 | 87.32 | 77.3-94.0 | .0129 |
| Lateral meniscal tear | 86.49 | 71.2-95.5 | 59.46 | 42.1-75.3 | .0172 |
| Any meniscal tear | 92.59 | 85.9-96.8 | 77.78 | 68.8-85.2 | .0035 |
| Intra-articular loose body | 86.70 | 59.5-98.3 | 20 | 4.3-48.1 | .0006789 |
| Osteoarthritis | 93.90 | 79.8-99.3 | 78.80 | 61.1-91.0 | .1487 |
| Osteochondritis dissecans | 80.00 | 44.4-97.5 | 50 | 18.7-81.3 | .3498 |
| Anterior crucitate ligament tear | 100.00 | 79.4-100.0 | 87.50 | 61.7-98.4 | .4839 |
| Posterior cruciate ligament tear | N/A | N/A | N/A | N/A | N/A |
| Specificity | |||||
| Medial meniscal tear | 100.00 | 90.0-100.0 | 82.86 | 66.4-93.4 | .0246 |
| Lateral meniscal tear | 100.00 | 94.8-100.0 | 89.86 | 80.2-95.8 | .0133 |
| Any meniscal tear | 100.00 | 96.5-100.0 | 87.50 | 79.6-93.2 | .0002 |
| Intra-articular loose body | 100.00 | 95.9-100.0 | 100.00 | 95.9-100.0 | 1 |
| Osteoarthritis | 100.00 | 95.1-100.0 | 89.00 | 79.5-95.1 | .006382 |
| Osteochondritis dissecans | 100.00 | 96.3-100.0 | 95.90 | 89.8-98.9 | .1211 |
| Anterior cruciate ligament tear | 100.00 | 96.0-100.0 | 96.70 | 90.6-99.3 | .2458 |
| Posterior crttuciate ligament tear | 100.00 | 96.6-100.0 | 98.10 | 93.4-99.8 | .4976 |
aBold P values are significant. Abbreviations: CI, confidence interval; MRI, magnetic resonance imaging; N/A, not applicable.
DISCUSSION
The overall accuracy of the mi-eye+TM was superior to that of MRI relative to the arthroscopic gold standard in this pilot study. Other studies have demonstrated the accuracy, feasibility, and cost-efficacy of in-office arthroscopy. However, likely because of the cumbersomeness of in-office arthroscopy equipment and the potential for patient discomfort, the technique is not yet standard in the field. Recent advances in small-bore technology, digital optics, and ergonomics have addressed the difficulties associated with in-office arthroscopy, facilitating a faster and more efficient procedure. Our goal in this study was to evaluate the diagnostic capability of the mi-eye+TM in-office arthroscopy unit, which features a small bore, digital optics, and functionality without an irrigation tower.
This study of 106 patients demonstrated equivalent or better accuracy of the mi-eye+TM relative to MRI when compared with the gold standard of surgical arthroscopy. This was not surprising given that both the mi-eye+TM and surgical arthroscopy are based on direct visualization of intra-articular pathology. The mi-eye+TM unit identified more meniscal tears, intra-articular loose bodies, ACL tears, and OCD lesions than MRI did, and with enough power to demonstrate statistically significant improved sensitivity for meniscal tears and loose bodies. Furthermore, MRI demonstrated false-positive meniscal tears, ACL tears, OCD lesions, and OA, whereas the mi-eye+TM did not demonstrate any false-positive results in comparison with surgical arthroscopy. This study demonstrated statistically significant improved specificity of the mi-eye+ compared with MRI in the diagnosis of meniscal tears and OA.
There are several limitations to our study. We refer to it as a pilot study because it was performed in a standard operating room. Before taking the technology to an outpatient setting, we wanted to confirm efficacy and safety in an operating room. However, the techniques used in this study are readily transferable to the outpatient clinic setting and to date have been used in more than 2000 cases.
Continue to: The specificity of MRI...
The specificity of MRI for meniscal tears was unexpectedly low compared with previous studies, which may reflect the multi-institution, multi-surgeon, multi-radiologist involvement in MRI interpretation.4-10 MRI was performed at a variety of institutions without a standardized protocol. This lack of standardization of image capture and interpretation may have contributed to the suboptimal performance of MRI, falsely decreasing the potential ideal specificity for meniscal tears. Although this study may have underestimated the specificity of MRI for meniscal tears, we think the mi-eye+TM and MRI results reported here reflect the findings of standard practice, without the standardization usually applied in studies. For example, a study of 139 knee MRI reports at 14 different institutions confirmed arthroscopic findings and concluded that 37% of the operations supported by a significant MRI finding were unjustified.11 The authors attributed the rate of false-positive MRI findings to the wide variety of places where patients had their MRIs performed, and the subsequent variation in quality of imaging and MRI reader skill level.11
Before inserting the mi-eye+TM needle arthroscope, the surgeons had a working diagnosis of the pathology based on their clinical examination and MRI results. Clearly, this introduced a bias. Further studies will be conducted in a prospective, blinded manner to address this limitation.
Although studies of in-office arthroscopy technology date to the 1990s, there is an overall lack of data comparing in-office arthroscopy with MRI. Halbrecht and Jackson2 conducted a study of 20 knee patients with both MRI and in-office needle arthroscopy. Overall, MRI was poor in detecting cartilage defects, with sensitivity of 34.6%, using the in-office arthroscopy as the confirmatory diagnosis. Although the authors did not compare in-office diagnoses with surgical arthroscopic findings, they concluded that office arthroscopy is an accurate and cost-efficient alternative to MRI in diagnostic evaluation of knee patients. Xerogeanes and colleagues12 studied 110 patients in a prospective, blinded, multicenter trial comparing a minimally invasive office-based arthroscopy with MRI, using surgical arthroscopy as the confirmatory diagnosis. They concluded that the office-based arthroscope was statistically equivalent to diagnostic surgical arthroscopy and that it outperformed MRI in helping make accurate diagnoses. The authors applied a cost analysis to their findings and determined that office-based arthroscopy could result in an annual potential savings of $177 million for the healthcare system.12
Modern imaging sequences on high-Tesla MRI machines provide excellent visualization. Nevertheless, a significant number of patients do not undergo MRI, owing to time constraints, contraindications, body habitus, or anxiety/claustrophobia. Our study results confirmed that doctors treating such patients now have a viable alternative to help diagnose pathology.
CONCLUSION
The mi-eye+TM device proved to be more sensitive and specific than MRI for intra-articular findings at the time of knee arthroscopy. Certainly there are contraindications to using the mi-eye+TM, and our results do not obviate the need for MRI; our study did demonstrate that the mi-eye+TM needle arthroscope can safely provide excellent visualization of intra-articular knee pathology. More studies of the mi-eye+TM device in a clinical setting are warranted.
1. Baeten D, Van den Bosch F, Elewaut D, Stuer A, Veys EM, De Keyser F. Needle arthroscopy of the knee with synovial biopsy sampling: technical experience in 150 patients. Clin Rheumatol. 1999;18(6):434-441.
2. Halbrecht J, Jackson D. Office arthroscopy: a diagnostic alternative. Arthroscopy. 1992;8(3):320-326.
3. Batcheleor R, Henshaw K, Astin P, Emery P, Reece R, Leeds DM. Rheumatological needle arthroscopy: a 5-year follow up of safety and efficacy. Arthritis Rheum Ann Sci Meet Abstr. 2001;(9 suppl).
4. Barronian AD, Zoltan JD, Bucon KA. Magnetic resonance imaging of the knee: correlation with arthroscopy. Arthroscopy. 1989;5(3):187-191.
5. Crues JV 3rd, Ryu R, Morgan FW. Meniscal pathology. The expanding role of magnetic resonance imaging. Clin Orthop Relat Res. 1990;(252):80-87.
6. Raunest J, Oberle K, Leohnert J, Hoetzinger H. The clinical value of magnetic resonance imaging in the evaluation of meniscal disorders. J Bone Joint Surg Am. 1991;73(1):11-16.
7. Spiers AS, Meagher T, Ostlere SJ, Wilson DJ, Dodd CA. Can MRI of the knee affect arthroscopic practice? A prospective study of 58 patients. J Bone Joint Surg Br. 1993;75(1):49-52.
8. O’Shea KJ, Murphy KP, Heekin RD, Herzwurm PJ. The diagnostic accuracy of history, physical examination, and radiographs in the evaluation of traumatic knee disorders. Am J Sports Med. 1996;24(2):164-167.
9. Ben-Galim P, Steinberg EL, Amir H, Ash N, Dekel S, Arbel R. Accuracy of magnetic resonance imaging of the knee and unjustified surgery. Clin Orthop Relat Res. 2006;(447):100-104.
10. Gramas DA, Antounian FS, Peterfy CG, Genant HK, Lane NE. Assessment of needle arthroscopy, standard arthroscopy, physical examination, and magnetic resonance imaging in knee pain: a pilot study. J Clin Rheumatol. 1995;1(1):26-34.
11. Voigt JD, Mosier M, Huber B. In-office diagnostic arthroscopy for knee and shoulder intra-articular injuries: its potential impact on cost savings in the United States. BMC Health Serv Res. 2014;14:203.
12. Xerogeanes JW, Safran MR, Huber B, Mandelbaum BR, Robertson W, Gambardella RA. A prospective multi-center clinical trial to compare efficiency, accuracy and safety of the VisionScope imaging system compared to MRI and diagnostic arthroscopy. Orthop J Sports Med. 2014;2(2 suppl):1.
ABSTRACT
The use of arthroscopy for purely diagnostic purposes has been largely supplanted by noninvasive technologies, such as magnetic resonance imaging (MRI). The mi-eye+TM (Trice Medical) technology is a small-bore needle unit for in-office arthroscopy. We conducted a pilot study comparing the mi-eye+TM unit with MRI, using surgical arthroscopy as a gold-standard reference. We hypothesized that the mi-eye+TM needle arthroscope, which can be used in an office setting, would be equivalent to MRI for the diagnosis of intra-articular pathology of the knee.
This prospective, multicenter, observational study was approved by the Institutional Review Board. There were 106 patients (53 males, 53 females) in the study. MRIs were interpreted by musculoskeletally trained radiologists. The study was conducted in the operating room using the mi-eye+TM device. The mi-eye+ TM device findings were compared with the MRI findings within individual pathologies, and a “per-patient” analysis was performed to compare the arthroscopic findings with those of the mi-eye+TM and the MRI. Additionally, we identified all mi-eye+TM findings and MRI findings that exactly matched the surgical arthroscopy findings.
The mi-eye+TM demonstrated complete accuracy of all pathologies for 97 (91.5%) of the 106 patients included in the study, whereas MRI demonstrated complete accuracy for 65 patients (61.3%) (P < .0001). All discrepancies between mi-eye+TM and arthroscopy were false-negative mi-eye+TM results, as the mi-eye+TM did not reveal some aspect of the knee’s pathology for 9 patients. The mi-eye+TM was more sensitive than MRI in identifying meniscal tears (92.6% vs 77.8%; P = .0035) and more specific in diagnosing these tears (100% vs 41.7%; P < .0001).
The mi-eye+TM device proved to be more sensitive and specific than MRI for intra-articular findings at time of knee arthroscopy. Certainly there are contraindications to using the mi-eye+TM, and our results do not obviate the need for MRI, but our study did demonstrate that the mi-eye+TM needle arthroscope can safely provide excellent visualization of intra-articular knee pathology.
Continue to: Surgical arthroscopy is the gold standard...
Surgical arthroscopy is the gold standard for the diagnosis of intra-articular knee pathologies. Nevertheless, the use of arthroscopy for purely diagnostic purposes has been largely supplanted by noninvasive technologies, such as magnetic resonance imaging (MRI). Although MRI is considered the standard diagnostic tool for acute and chronic soft-tissue injuries of the knee, its use is not without contraindication and some potential inconveniences. Contraindications to MRI are well documented. In terms of inconvenience, MRI usually requires a separate visit followed by another visit to the prescribing physician. In addition, required interpretation by a radiologist may lead to a delay in care and increase in cost.
In the early 1990s, in-office needle arthroscopy was described as a viable means of diagnosing pathologies and obtaining synovial biopsies from the knee.1-3 Initial results were good, and the procedures had very low complication rates. Nevertheless, in-office arthroscopy of the knee is not yet widely performed, likely given concerns about the technical difficulties of in-office arthroscopy, the potential for patient discomfort, and the cumbersomeness of in-office arthroscopy units. However, significant advances have been made in the resolution capability of small-bore needle arthroscopy, resulting in much less painful procedures. Additionally, the early hardware designs, which mimicked operating room setups using towers, fluid irrigation systems, and larger arthroscopes, have been replaced with small-needle arthroscopes that use syringes for irrigation and tablet computers for visualization (Figures 1A, 1B).

The mi-eye+TM technology (Trice Medical) is a small-bore needle unit for in-office arthroscopy with digital optics that does not need an irrigation tower. We conducted a pilot study of the sensitivity and specificity of the mi-eye+TM unit in comparison with MRI, using surgical arthroscopy as a gold-standard reference. We hypothesized that the mi-eye+TM needle arthroscope, which can be used in an office setting, would be equivalent to the standard of care (MRI) for the diagnosis of intra-articular pathology of the knee.
METHODS
Central regulatory approval for this prospective, multicenter, observational study was obtained from the Western Institutional Review Board for 3 of the sites, and 1 institution required and was granted internal Institutional Review Board approval.
The study was performed by 4 sports medicine orthopedic surgeons experienced in using the mi-eye+TM in-office arthroscope. Patients were enrolled from December 2015 through June 2016. Inclusion criteria were an indication for an arthroscopic procedure of the knee based on history, physical examination, and MRI findings. Patients were excluded from the study if there were any contraindications to completing an MRI. Acute hemarthroses of the knee or active systemic infections were also excluded. Once a patient was identified as meeting the criteria for participation, informed consent was obtained. Of the 113 patients who enrolled, 7 did not have a complete study dataset available, leaving 106 patients (53 males, 53 females) in the study. Mean age was 47 years (range, 18-82 years).
Continue to: A test result form was used...
A test result form was used to record mi-eye+TM, surgical arthroscopy, and MRI results. This form required a “positive” or “negative” result for all of several diagnoses: medial and lateral meniscal tears, intra-articular loose body, osteoarthritis (OA), osteochondritis dissecans (OCD), and tears of the anterior and posterior cruciate ligaments (ACL, PCL). MRI was performed at a variety of imaging facilities, but the images were interpreted by musculoskeletally trained radiologists.
The study was conducted in the operating room. After the patient was appropriately anesthetized, and the extremity prepared and draped, the mi-eye+TM procedure was performed immediately prior to surgical arthroscopy. A tourniquet was not used. At surgeon discretion, medial, lateral, or both approaches were used with the mi-eye+TM, and diagnostic arthroscopy was performed. During the procedure, the mi-eye+TM was advanced into the knee. Once in the synovial compartment, the external 14-gauge needle was retracted, exposing the unit’s optics. Visualization was improved by injecting normal saline through the lure lock in the mi-eye+TM needle arthroscope. An average of 20 mL of saline was used, though the amount varied with surgeon discretion. Subsequently, the surgeon visualized structures in the knee and documented all findings.
At the end of the mi-eye+TM procedure, the scheduled surgical arthroscopy was performed. After the surgical procedure, if there were no issues or complications, the patient was discharged from the study. No follow-up was required for the study, as arthroscopic findings served as the conclusive diagnosis for each patient, and no interventions were being studied. There were no complications related to use of the mi-eye+TM.
The mi-eye+TM device findings were compared with the MRI findings within individual pathologies, and a “per-patient” analysis was performed to compare the arthroscopic findings with those of the mi-eye+TM and the MRI. Additionally, we identified all mi-eye+TM findings and MRI findings that exactly matched the surgical arthroscopy findings. When a test had no false-positive or false-negative findings in comparison with surgical arthroscopy, it was identified as having complete accuracy for all intra-articular knee pathologies. For these methods, the 95% confidence interval was determined based on binomial distribution.
RESULTS
The mi-eye+ TM demonstrated complete accuracy of all pathologies for 97 (91.5%) of the 106 patients included in the study, whereas MRI demonstrated complete accuracy for 65 patients (61.3%) (P < .0001). All discrepancies between mi-eye+TM and surgical arthroscopy were false-negative mi-eye+TM results, as the mi-eye+TM did not reveal some aspect of the knee’s pathology for 9 patients. On the other hand, MRI demonstrated both false-negative and false-positive results, failing to reveal some aspect of the knee’s pathology for 31 patients, and potentially overcalling some aspect of the knee’s pathology among 18 patients.
Continue to: The pathology most frequently...
The pathology most frequently identified in the study was a meniscal tear. The mi-eye+TM was more sensitive than MRI in identifying meniscal tears (92.6% vs 77.8%; P = .0035) and more specific in diagnosing these tears (100% vs 87.5%; P < .0002). The difference in specificity resulted from the false MRI diagnosis of a meniscal tear among 24 patients, who were found to have no tear by both mi-eye+TM and surgical arthroscopy.
Table 1. Raw Data of mi-eye+TM and Magnetic Resonance Imaging Findings
| Data | True-Positive | False-Negative | False-Negative | True-Negative |
| mi-eye+TM | ||||
| Medial meniscal tear | 68 | 3 | 0 | 35 |
| Lateral meniscal tear | 32 | 5 | 0 | 69 |
| Any meniscal tear | 100 | 8 | 0 | 104 |
| Intra-articular loose body | 13 | 2 | 0 | 87 |
| Osteoarthritis | 31 | 2 | 00 | 73 |
| Osteochondritis dissecans | 8 | 2 | 0 | 97 |
| Anterior cruciate ligament tear | 16 | 0 | 0 | 90 |
| Posterior cruciate ligament tear | 0 | 0 | 0 | 106 |
| All pathologies | 168 | 14 | 0 | 557 |
| Magnetic resonance imaging | ||||
| Medial meniscal tear | 62 | 9 | 6 | 29 |
| Lateral meniscal tear | 22 | 15 | 7 | 62 |
| Any meniscal tear | 84 | 24 | 13 | 91 |
| Intra-articular loose body | 3 | 12 | 0 | 87 |
| Osteoarthritis | 26 | 7 | 8 | 65 |
| Osteochondritis dissecans | 5 | 5 | 4 | 93 |
| Anterior cruciate ligament tear | 14 | 2 | 3 | 87 |
| Posterior cruciate ligament tear | 0 | 0 | 2 | 104 |
| All pathologies | 132 | 500 | 30 | 527 |
The second most frequent pathology was an intra-articular loose body. The mi-eye+TM was more sensitive than MRI in identifying loose bodies (86.7% vs 20%; P = .0007). The specificity of the mi-eye+TM and the specificity of MRI were equivalent in diagnosing loose bodies (100%). Table 1 and Table 2 show the complete set of diagnoses and associated diagnostic profiles.
Table 2. Diagnostic Profiles: Sensitivity and Specificity of mi-eye+TM and Magnetic Resonance Imaging
| Patient Group | mi-eye+TM | MRI | |||
| Estimate, % | CI, % | Estimate, % | CI, % | Pa | |
| Sensitivity | |||||
| Medial meniscal tear | 95.77 | 88.1-99.1 | 87.32 | 77.3-94.0 | .0129 |
| Lateral meniscal tear | 86.49 | 71.2-95.5 | 59.46 | 42.1-75.3 | .0172 |
| Any meniscal tear | 92.59 | 85.9-96.8 | 77.78 | 68.8-85.2 | .0035 |
| Intra-articular loose body | 86.70 | 59.5-98.3 | 20 | 4.3-48.1 | .0006789 |
| Osteoarthritis | 93.90 | 79.8-99.3 | 78.80 | 61.1-91.0 | .1487 |
| Osteochondritis dissecans | 80.00 | 44.4-97.5 | 50 | 18.7-81.3 | .3498 |
| Anterior crucitate ligament tear | 100.00 | 79.4-100.0 | 87.50 | 61.7-98.4 | .4839 |
| Posterior cruciate ligament tear | N/A | N/A | N/A | N/A | N/A |
| Specificity | |||||
| Medial meniscal tear | 100.00 | 90.0-100.0 | 82.86 | 66.4-93.4 | .0246 |
| Lateral meniscal tear | 100.00 | 94.8-100.0 | 89.86 | 80.2-95.8 | .0133 |
| Any meniscal tear | 100.00 | 96.5-100.0 | 87.50 | 79.6-93.2 | .0002 |
| Intra-articular loose body | 100.00 | 95.9-100.0 | 100.00 | 95.9-100.0 | 1 |
| Osteoarthritis | 100.00 | 95.1-100.0 | 89.00 | 79.5-95.1 | .006382 |
| Osteochondritis dissecans | 100.00 | 96.3-100.0 | 95.90 | 89.8-98.9 | .1211 |
| Anterior cruciate ligament tear | 100.00 | 96.0-100.0 | 96.70 | 90.6-99.3 | .2458 |
| Posterior crttuciate ligament tear | 100.00 | 96.6-100.0 | 98.10 | 93.4-99.8 | .4976 |
aBold P values are significant. Abbreviations: CI, confidence interval; MRI, magnetic resonance imaging; N/A, not applicable.
DISCUSSION
The overall accuracy of the mi-eye+TM was superior to that of MRI relative to the arthroscopic gold standard in this pilot study. Other studies have demonstrated the accuracy, feasibility, and cost-efficacy of in-office arthroscopy. However, likely because of the cumbersomeness of in-office arthroscopy equipment and the potential for patient discomfort, the technique is not yet standard in the field. Recent advances in small-bore technology, digital optics, and ergonomics have addressed the difficulties associated with in-office arthroscopy, facilitating a faster and more efficient procedure. Our goal in this study was to evaluate the diagnostic capability of the mi-eye+TM in-office arthroscopy unit, which features a small bore, digital optics, and functionality without an irrigation tower.
This study of 106 patients demonstrated equivalent or better accuracy of the mi-eye+TM relative to MRI when compared with the gold standard of surgical arthroscopy. This was not surprising given that both the mi-eye+TM and surgical arthroscopy are based on direct visualization of intra-articular pathology. The mi-eye+TM unit identified more meniscal tears, intra-articular loose bodies, ACL tears, and OCD lesions than MRI did, and with enough power to demonstrate statistically significant improved sensitivity for meniscal tears and loose bodies. Furthermore, MRI demonstrated false-positive meniscal tears, ACL tears, OCD lesions, and OA, whereas the mi-eye+TM did not demonstrate any false-positive results in comparison with surgical arthroscopy. This study demonstrated statistically significant improved specificity of the mi-eye+ compared with MRI in the diagnosis of meniscal tears and OA.
There are several limitations to our study. We refer to it as a pilot study because it was performed in a standard operating room. Before taking the technology to an outpatient setting, we wanted to confirm efficacy and safety in an operating room. However, the techniques used in this study are readily transferable to the outpatient clinic setting and to date have been used in more than 2000 cases.
Continue to: The specificity of MRI...
The specificity of MRI for meniscal tears was unexpectedly low compared with previous studies, which may reflect the multi-institution, multi-surgeon, multi-radiologist involvement in MRI interpretation.4-10 MRI was performed at a variety of institutions without a standardized protocol. This lack of standardization of image capture and interpretation may have contributed to the suboptimal performance of MRI, falsely decreasing the potential ideal specificity for meniscal tears. Although this study may have underestimated the specificity of MRI for meniscal tears, we think the mi-eye+TM and MRI results reported here reflect the findings of standard practice, without the standardization usually applied in studies. For example, a study of 139 knee MRI reports at 14 different institutions confirmed arthroscopic findings and concluded that 37% of the operations supported by a significant MRI finding were unjustified.11 The authors attributed the rate of false-positive MRI findings to the wide variety of places where patients had their MRIs performed, and the subsequent variation in quality of imaging and MRI reader skill level.11
Before inserting the mi-eye+TM needle arthroscope, the surgeons had a working diagnosis of the pathology based on their clinical examination and MRI results. Clearly, this introduced a bias. Further studies will be conducted in a prospective, blinded manner to address this limitation.
Although studies of in-office arthroscopy technology date to the 1990s, there is an overall lack of data comparing in-office arthroscopy with MRI. Halbrecht and Jackson2 conducted a study of 20 knee patients with both MRI and in-office needle arthroscopy. Overall, MRI was poor in detecting cartilage defects, with sensitivity of 34.6%, using the in-office arthroscopy as the confirmatory diagnosis. Although the authors did not compare in-office diagnoses with surgical arthroscopic findings, they concluded that office arthroscopy is an accurate and cost-efficient alternative to MRI in diagnostic evaluation of knee patients. Xerogeanes and colleagues12 studied 110 patients in a prospective, blinded, multicenter trial comparing a minimally invasive office-based arthroscopy with MRI, using surgical arthroscopy as the confirmatory diagnosis. They concluded that the office-based arthroscope was statistically equivalent to diagnostic surgical arthroscopy and that it outperformed MRI in helping make accurate diagnoses. The authors applied a cost analysis to their findings and determined that office-based arthroscopy could result in an annual potential savings of $177 million for the healthcare system.12
Modern imaging sequences on high-Tesla MRI machines provide excellent visualization. Nevertheless, a significant number of patients do not undergo MRI, owing to time constraints, contraindications, body habitus, or anxiety/claustrophobia. Our study results confirmed that doctors treating such patients now have a viable alternative to help diagnose pathology.
CONCLUSION
The mi-eye+TM device proved to be more sensitive and specific than MRI for intra-articular findings at the time of knee arthroscopy. Certainly there are contraindications to using the mi-eye+TM, and our results do not obviate the need for MRI; our study did demonstrate that the mi-eye+TM needle arthroscope can safely provide excellent visualization of intra-articular knee pathology. More studies of the mi-eye+TM device in a clinical setting are warranted.
ABSTRACT
The use of arthroscopy for purely diagnostic purposes has been largely supplanted by noninvasive technologies, such as magnetic resonance imaging (MRI). The mi-eye+TM (Trice Medical) technology is a small-bore needle unit for in-office arthroscopy. We conducted a pilot study comparing the mi-eye+TM unit with MRI, using surgical arthroscopy as a gold-standard reference. We hypothesized that the mi-eye+TM needle arthroscope, which can be used in an office setting, would be equivalent to MRI for the diagnosis of intra-articular pathology of the knee.
This prospective, multicenter, observational study was approved by the Institutional Review Board. There were 106 patients (53 males, 53 females) in the study. MRIs were interpreted by musculoskeletally trained radiologists. The study was conducted in the operating room using the mi-eye+TM device. The mi-eye+ TM device findings were compared with the MRI findings within individual pathologies, and a “per-patient” analysis was performed to compare the arthroscopic findings with those of the mi-eye+TM and the MRI. Additionally, we identified all mi-eye+TM findings and MRI findings that exactly matched the surgical arthroscopy findings.
The mi-eye+TM demonstrated complete accuracy of all pathologies for 97 (91.5%) of the 106 patients included in the study, whereas MRI demonstrated complete accuracy for 65 patients (61.3%) (P < .0001). All discrepancies between mi-eye+TM and arthroscopy were false-negative mi-eye+TM results, as the mi-eye+TM did not reveal some aspect of the knee’s pathology for 9 patients. The mi-eye+TM was more sensitive than MRI in identifying meniscal tears (92.6% vs 77.8%; P = .0035) and more specific in diagnosing these tears (100% vs 41.7%; P < .0001).
The mi-eye+TM device proved to be more sensitive and specific than MRI for intra-articular findings at time of knee arthroscopy. Certainly there are contraindications to using the mi-eye+TM, and our results do not obviate the need for MRI, but our study did demonstrate that the mi-eye+TM needle arthroscope can safely provide excellent visualization of intra-articular knee pathology.
Continue to: Surgical arthroscopy is the gold standard...
Surgical arthroscopy is the gold standard for the diagnosis of intra-articular knee pathologies. Nevertheless, the use of arthroscopy for purely diagnostic purposes has been largely supplanted by noninvasive technologies, such as magnetic resonance imaging (MRI). Although MRI is considered the standard diagnostic tool for acute and chronic soft-tissue injuries of the knee, its use is not without contraindication and some potential inconveniences. Contraindications to MRI are well documented. In terms of inconvenience, MRI usually requires a separate visit followed by another visit to the prescribing physician. In addition, required interpretation by a radiologist may lead to a delay in care and increase in cost.
In the early 1990s, in-office needle arthroscopy was described as a viable means of diagnosing pathologies and obtaining synovial biopsies from the knee.1-3 Initial results were good, and the procedures had very low complication rates. Nevertheless, in-office arthroscopy of the knee is not yet widely performed, likely given concerns about the technical difficulties of in-office arthroscopy, the potential for patient discomfort, and the cumbersomeness of in-office arthroscopy units. However, significant advances have been made in the resolution capability of small-bore needle arthroscopy, resulting in much less painful procedures. Additionally, the early hardware designs, which mimicked operating room setups using towers, fluid irrigation systems, and larger arthroscopes, have been replaced with small-needle arthroscopes that use syringes for irrigation and tablet computers for visualization (Figures 1A, 1B).

The mi-eye+TM technology (Trice Medical) is a small-bore needle unit for in-office arthroscopy with digital optics that does not need an irrigation tower. We conducted a pilot study of the sensitivity and specificity of the mi-eye+TM unit in comparison with MRI, using surgical arthroscopy as a gold-standard reference. We hypothesized that the mi-eye+TM needle arthroscope, which can be used in an office setting, would be equivalent to the standard of care (MRI) for the diagnosis of intra-articular pathology of the knee.
METHODS
Central regulatory approval for this prospective, multicenter, observational study was obtained from the Western Institutional Review Board for 3 of the sites, and 1 institution required and was granted internal Institutional Review Board approval.
The study was performed by 4 sports medicine orthopedic surgeons experienced in using the mi-eye+TM in-office arthroscope. Patients were enrolled from December 2015 through June 2016. Inclusion criteria were an indication for an arthroscopic procedure of the knee based on history, physical examination, and MRI findings. Patients were excluded from the study if there were any contraindications to completing an MRI. Acute hemarthroses of the knee or active systemic infections were also excluded. Once a patient was identified as meeting the criteria for participation, informed consent was obtained. Of the 113 patients who enrolled, 7 did not have a complete study dataset available, leaving 106 patients (53 males, 53 females) in the study. Mean age was 47 years (range, 18-82 years).
Continue to: A test result form was used...
A test result form was used to record mi-eye+TM, surgical arthroscopy, and MRI results. This form required a “positive” or “negative” result for all of several diagnoses: medial and lateral meniscal tears, intra-articular loose body, osteoarthritis (OA), osteochondritis dissecans (OCD), and tears of the anterior and posterior cruciate ligaments (ACL, PCL). MRI was performed at a variety of imaging facilities, but the images were interpreted by musculoskeletally trained radiologists.
The study was conducted in the operating room. After the patient was appropriately anesthetized, and the extremity prepared and draped, the mi-eye+TM procedure was performed immediately prior to surgical arthroscopy. A tourniquet was not used. At surgeon discretion, medial, lateral, or both approaches were used with the mi-eye+TM, and diagnostic arthroscopy was performed. During the procedure, the mi-eye+TM was advanced into the knee. Once in the synovial compartment, the external 14-gauge needle was retracted, exposing the unit’s optics. Visualization was improved by injecting normal saline through the lure lock in the mi-eye+TM needle arthroscope. An average of 20 mL of saline was used, though the amount varied with surgeon discretion. Subsequently, the surgeon visualized structures in the knee and documented all findings.
At the end of the mi-eye+TM procedure, the scheduled surgical arthroscopy was performed. After the surgical procedure, if there were no issues or complications, the patient was discharged from the study. No follow-up was required for the study, as arthroscopic findings served as the conclusive diagnosis for each patient, and no interventions were being studied. There were no complications related to use of the mi-eye+TM.
The mi-eye+TM device findings were compared with the MRI findings within individual pathologies, and a “per-patient” analysis was performed to compare the arthroscopic findings with those of the mi-eye+TM and the MRI. Additionally, we identified all mi-eye+TM findings and MRI findings that exactly matched the surgical arthroscopy findings. When a test had no false-positive or false-negative findings in comparison with surgical arthroscopy, it was identified as having complete accuracy for all intra-articular knee pathologies. For these methods, the 95% confidence interval was determined based on binomial distribution.
RESULTS
The mi-eye+ TM demonstrated complete accuracy of all pathologies for 97 (91.5%) of the 106 patients included in the study, whereas MRI demonstrated complete accuracy for 65 patients (61.3%) (P < .0001). All discrepancies between mi-eye+TM and surgical arthroscopy were false-negative mi-eye+TM results, as the mi-eye+TM did not reveal some aspect of the knee’s pathology for 9 patients. On the other hand, MRI demonstrated both false-negative and false-positive results, failing to reveal some aspect of the knee’s pathology for 31 patients, and potentially overcalling some aspect of the knee’s pathology among 18 patients.
Continue to: The pathology most frequently...
The pathology most frequently identified in the study was a meniscal tear. The mi-eye+TM was more sensitive than MRI in identifying meniscal tears (92.6% vs 77.8%; P = .0035) and more specific in diagnosing these tears (100% vs 87.5%; P < .0002). The difference in specificity resulted from the false MRI diagnosis of a meniscal tear among 24 patients, who were found to have no tear by both mi-eye+TM and surgical arthroscopy.
Table 1. Raw Data of mi-eye+TM and Magnetic Resonance Imaging Findings
| Data | True-Positive | False-Negative | False-Negative | True-Negative |
| mi-eye+TM | ||||
| Medial meniscal tear | 68 | 3 | 0 | 35 |
| Lateral meniscal tear | 32 | 5 | 0 | 69 |
| Any meniscal tear | 100 | 8 | 0 | 104 |
| Intra-articular loose body | 13 | 2 | 0 | 87 |
| Osteoarthritis | 31 | 2 | 00 | 73 |
| Osteochondritis dissecans | 8 | 2 | 0 | 97 |
| Anterior cruciate ligament tear | 16 | 0 | 0 | 90 |
| Posterior cruciate ligament tear | 0 | 0 | 0 | 106 |
| All pathologies | 168 | 14 | 0 | 557 |
| Magnetic resonance imaging | ||||
| Medial meniscal tear | 62 | 9 | 6 | 29 |
| Lateral meniscal tear | 22 | 15 | 7 | 62 |
| Any meniscal tear | 84 | 24 | 13 | 91 |
| Intra-articular loose body | 3 | 12 | 0 | 87 |
| Osteoarthritis | 26 | 7 | 8 | 65 |
| Osteochondritis dissecans | 5 | 5 | 4 | 93 |
| Anterior cruciate ligament tear | 14 | 2 | 3 | 87 |
| Posterior cruciate ligament tear | 0 | 0 | 2 | 104 |
| All pathologies | 132 | 500 | 30 | 527 |
The second most frequent pathology was an intra-articular loose body. The mi-eye+TM was more sensitive than MRI in identifying loose bodies (86.7% vs 20%; P = .0007). The specificity of the mi-eye+TM and the specificity of MRI were equivalent in diagnosing loose bodies (100%). Table 1 and Table 2 show the complete set of diagnoses and associated diagnostic profiles.
Table 2. Diagnostic Profiles: Sensitivity and Specificity of mi-eye+TM and Magnetic Resonance Imaging
| Patient Group | mi-eye+TM | MRI | |||
| Estimate, % | CI, % | Estimate, % | CI, % | Pa | |
| Sensitivity | |||||
| Medial meniscal tear | 95.77 | 88.1-99.1 | 87.32 | 77.3-94.0 | .0129 |
| Lateral meniscal tear | 86.49 | 71.2-95.5 | 59.46 | 42.1-75.3 | .0172 |
| Any meniscal tear | 92.59 | 85.9-96.8 | 77.78 | 68.8-85.2 | .0035 |
| Intra-articular loose body | 86.70 | 59.5-98.3 | 20 | 4.3-48.1 | .0006789 |
| Osteoarthritis | 93.90 | 79.8-99.3 | 78.80 | 61.1-91.0 | .1487 |
| Osteochondritis dissecans | 80.00 | 44.4-97.5 | 50 | 18.7-81.3 | .3498 |
| Anterior crucitate ligament tear | 100.00 | 79.4-100.0 | 87.50 | 61.7-98.4 | .4839 |
| Posterior cruciate ligament tear | N/A | N/A | N/A | N/A | N/A |
| Specificity | |||||
| Medial meniscal tear | 100.00 | 90.0-100.0 | 82.86 | 66.4-93.4 | .0246 |
| Lateral meniscal tear | 100.00 | 94.8-100.0 | 89.86 | 80.2-95.8 | .0133 |
| Any meniscal tear | 100.00 | 96.5-100.0 | 87.50 | 79.6-93.2 | .0002 |
| Intra-articular loose body | 100.00 | 95.9-100.0 | 100.00 | 95.9-100.0 | 1 |
| Osteoarthritis | 100.00 | 95.1-100.0 | 89.00 | 79.5-95.1 | .006382 |
| Osteochondritis dissecans | 100.00 | 96.3-100.0 | 95.90 | 89.8-98.9 | .1211 |
| Anterior cruciate ligament tear | 100.00 | 96.0-100.0 | 96.70 | 90.6-99.3 | .2458 |
| Posterior crttuciate ligament tear | 100.00 | 96.6-100.0 | 98.10 | 93.4-99.8 | .4976 |
aBold P values are significant. Abbreviations: CI, confidence interval; MRI, magnetic resonance imaging; N/A, not applicable.
DISCUSSION
The overall accuracy of the mi-eye+TM was superior to that of MRI relative to the arthroscopic gold standard in this pilot study. Other studies have demonstrated the accuracy, feasibility, and cost-efficacy of in-office arthroscopy. However, likely because of the cumbersomeness of in-office arthroscopy equipment and the potential for patient discomfort, the technique is not yet standard in the field. Recent advances in small-bore technology, digital optics, and ergonomics have addressed the difficulties associated with in-office arthroscopy, facilitating a faster and more efficient procedure. Our goal in this study was to evaluate the diagnostic capability of the mi-eye+TM in-office arthroscopy unit, which features a small bore, digital optics, and functionality without an irrigation tower.
This study of 106 patients demonstrated equivalent or better accuracy of the mi-eye+TM relative to MRI when compared with the gold standard of surgical arthroscopy. This was not surprising given that both the mi-eye+TM and surgical arthroscopy are based on direct visualization of intra-articular pathology. The mi-eye+TM unit identified more meniscal tears, intra-articular loose bodies, ACL tears, and OCD lesions than MRI did, and with enough power to demonstrate statistically significant improved sensitivity for meniscal tears and loose bodies. Furthermore, MRI demonstrated false-positive meniscal tears, ACL tears, OCD lesions, and OA, whereas the mi-eye+TM did not demonstrate any false-positive results in comparison with surgical arthroscopy. This study demonstrated statistically significant improved specificity of the mi-eye+ compared with MRI in the diagnosis of meniscal tears and OA.
There are several limitations to our study. We refer to it as a pilot study because it was performed in a standard operating room. Before taking the technology to an outpatient setting, we wanted to confirm efficacy and safety in an operating room. However, the techniques used in this study are readily transferable to the outpatient clinic setting and to date have been used in more than 2000 cases.
Continue to: The specificity of MRI...
The specificity of MRI for meniscal tears was unexpectedly low compared with previous studies, which may reflect the multi-institution, multi-surgeon, multi-radiologist involvement in MRI interpretation.4-10 MRI was performed at a variety of institutions without a standardized protocol. This lack of standardization of image capture and interpretation may have contributed to the suboptimal performance of MRI, falsely decreasing the potential ideal specificity for meniscal tears. Although this study may have underestimated the specificity of MRI for meniscal tears, we think the mi-eye+TM and MRI results reported here reflect the findings of standard practice, without the standardization usually applied in studies. For example, a study of 139 knee MRI reports at 14 different institutions confirmed arthroscopic findings and concluded that 37% of the operations supported by a significant MRI finding were unjustified.11 The authors attributed the rate of false-positive MRI findings to the wide variety of places where patients had their MRIs performed, and the subsequent variation in quality of imaging and MRI reader skill level.11
Before inserting the mi-eye+TM needle arthroscope, the surgeons had a working diagnosis of the pathology based on their clinical examination and MRI results. Clearly, this introduced a bias. Further studies will be conducted in a prospective, blinded manner to address this limitation.
Although studies of in-office arthroscopy technology date to the 1990s, there is an overall lack of data comparing in-office arthroscopy with MRI. Halbrecht and Jackson2 conducted a study of 20 knee patients with both MRI and in-office needle arthroscopy. Overall, MRI was poor in detecting cartilage defects, with sensitivity of 34.6%, using the in-office arthroscopy as the confirmatory diagnosis. Although the authors did not compare in-office diagnoses with surgical arthroscopic findings, they concluded that office arthroscopy is an accurate and cost-efficient alternative to MRI in diagnostic evaluation of knee patients. Xerogeanes and colleagues12 studied 110 patients in a prospective, blinded, multicenter trial comparing a minimally invasive office-based arthroscopy with MRI, using surgical arthroscopy as the confirmatory diagnosis. They concluded that the office-based arthroscope was statistically equivalent to diagnostic surgical arthroscopy and that it outperformed MRI in helping make accurate diagnoses. The authors applied a cost analysis to their findings and determined that office-based arthroscopy could result in an annual potential savings of $177 million for the healthcare system.12
Modern imaging sequences on high-Tesla MRI machines provide excellent visualization. Nevertheless, a significant number of patients do not undergo MRI, owing to time constraints, contraindications, body habitus, or anxiety/claustrophobia. Our study results confirmed that doctors treating such patients now have a viable alternative to help diagnose pathology.
CONCLUSION
The mi-eye+TM device proved to be more sensitive and specific than MRI for intra-articular findings at the time of knee arthroscopy. Certainly there are contraindications to using the mi-eye+TM, and our results do not obviate the need for MRI; our study did demonstrate that the mi-eye+TM needle arthroscope can safely provide excellent visualization of intra-articular knee pathology. More studies of the mi-eye+TM device in a clinical setting are warranted.
1. Baeten D, Van den Bosch F, Elewaut D, Stuer A, Veys EM, De Keyser F. Needle arthroscopy of the knee with synovial biopsy sampling: technical experience in 150 patients. Clin Rheumatol. 1999;18(6):434-441.
2. Halbrecht J, Jackson D. Office arthroscopy: a diagnostic alternative. Arthroscopy. 1992;8(3):320-326.
3. Batcheleor R, Henshaw K, Astin P, Emery P, Reece R, Leeds DM. Rheumatological needle arthroscopy: a 5-year follow up of safety and efficacy. Arthritis Rheum Ann Sci Meet Abstr. 2001;(9 suppl).
4. Barronian AD, Zoltan JD, Bucon KA. Magnetic resonance imaging of the knee: correlation with arthroscopy. Arthroscopy. 1989;5(3):187-191.
5. Crues JV 3rd, Ryu R, Morgan FW. Meniscal pathology. The expanding role of magnetic resonance imaging. Clin Orthop Relat Res. 1990;(252):80-87.
6. Raunest J, Oberle K, Leohnert J, Hoetzinger H. The clinical value of magnetic resonance imaging in the evaluation of meniscal disorders. J Bone Joint Surg Am. 1991;73(1):11-16.
7. Spiers AS, Meagher T, Ostlere SJ, Wilson DJ, Dodd CA. Can MRI of the knee affect arthroscopic practice? A prospective study of 58 patients. J Bone Joint Surg Br. 1993;75(1):49-52.
8. O’Shea KJ, Murphy KP, Heekin RD, Herzwurm PJ. The diagnostic accuracy of history, physical examination, and radiographs in the evaluation of traumatic knee disorders. Am J Sports Med. 1996;24(2):164-167.
9. Ben-Galim P, Steinberg EL, Amir H, Ash N, Dekel S, Arbel R. Accuracy of magnetic resonance imaging of the knee and unjustified surgery. Clin Orthop Relat Res. 2006;(447):100-104.
10. Gramas DA, Antounian FS, Peterfy CG, Genant HK, Lane NE. Assessment of needle arthroscopy, standard arthroscopy, physical examination, and magnetic resonance imaging in knee pain: a pilot study. J Clin Rheumatol. 1995;1(1):26-34.
11. Voigt JD, Mosier M, Huber B. In-office diagnostic arthroscopy for knee and shoulder intra-articular injuries: its potential impact on cost savings in the United States. BMC Health Serv Res. 2014;14:203.
12. Xerogeanes JW, Safran MR, Huber B, Mandelbaum BR, Robertson W, Gambardella RA. A prospective multi-center clinical trial to compare efficiency, accuracy and safety of the VisionScope imaging system compared to MRI and diagnostic arthroscopy. Orthop J Sports Med. 2014;2(2 suppl):1.
1. Baeten D, Van den Bosch F, Elewaut D, Stuer A, Veys EM, De Keyser F. Needle arthroscopy of the knee with synovial biopsy sampling: technical experience in 150 patients. Clin Rheumatol. 1999;18(6):434-441.
2. Halbrecht J, Jackson D. Office arthroscopy: a diagnostic alternative. Arthroscopy. 1992;8(3):320-326.
3. Batcheleor R, Henshaw K, Astin P, Emery P, Reece R, Leeds DM. Rheumatological needle arthroscopy: a 5-year follow up of safety and efficacy. Arthritis Rheum Ann Sci Meet Abstr. 2001;(9 suppl).
4. Barronian AD, Zoltan JD, Bucon KA. Magnetic resonance imaging of the knee: correlation with arthroscopy. Arthroscopy. 1989;5(3):187-191.
5. Crues JV 3rd, Ryu R, Morgan FW. Meniscal pathology. The expanding role of magnetic resonance imaging. Clin Orthop Relat Res. 1990;(252):80-87.
6. Raunest J, Oberle K, Leohnert J, Hoetzinger H. The clinical value of magnetic resonance imaging in the evaluation of meniscal disorders. J Bone Joint Surg Am. 1991;73(1):11-16.
7. Spiers AS, Meagher T, Ostlere SJ, Wilson DJ, Dodd CA. Can MRI of the knee affect arthroscopic practice? A prospective study of 58 patients. J Bone Joint Surg Br. 1993;75(1):49-52.
8. O’Shea KJ, Murphy KP, Heekin RD, Herzwurm PJ. The diagnostic accuracy of history, physical examination, and radiographs in the evaluation of traumatic knee disorders. Am J Sports Med. 1996;24(2):164-167.
9. Ben-Galim P, Steinberg EL, Amir H, Ash N, Dekel S, Arbel R. Accuracy of magnetic resonance imaging of the knee and unjustified surgery. Clin Orthop Relat Res. 2006;(447):100-104.
10. Gramas DA, Antounian FS, Peterfy CG, Genant HK, Lane NE. Assessment of needle arthroscopy, standard arthroscopy, physical examination, and magnetic resonance imaging in knee pain: a pilot study. J Clin Rheumatol. 1995;1(1):26-34.
11. Voigt JD, Mosier M, Huber B. In-office diagnostic arthroscopy for knee and shoulder intra-articular injuries: its potential impact on cost savings in the United States. BMC Health Serv Res. 2014;14:203.
12. Xerogeanes JW, Safran MR, Huber B, Mandelbaum BR, Robertson W, Gambardella RA. A prospective multi-center clinical trial to compare efficiency, accuracy and safety of the VisionScope imaging system compared to MRI and diagnostic arthroscopy. Orthop J Sports Med. 2014;2(2 suppl):1.
TAKE-HOME POINTS
- Small-bore needle arthroscopy is an effective way to diagnose intra-articular knee pathology.
- Small-bore needle arthroscopy is safe and easy to use with no complications reported in this series.
- Small-bore needle arthroscopy is a useful diagnostic tool in office settings.
- In this series, small-bore needle arthroscopy was more accurate than MRI to diagnose knee meniscal tears.
- In-office diagnostic arthroscopy can be used for other joints such as shoulder, elbow, and ankle.
Commentary—Serotonin Syndrome and Triptans
Serotonin syndrome (SS) is diagnosed by the clinical triad of dysautonomia (fever, mydriasis, diaphoresis, tachycardia), neuromuscular signs (ataxia, hyperreflexia, tremor, myoclonus), and altered mental status (seizures, delirium). Two validated criteria groups are accepted, the Hunter criteria and the Sternbach criteria. These criteria require a menu-like approach of clinical manifestations of the above signs with known addition or increase of a serotonergic medication and the absence of other possible causes, such as neuroleptics.
In 2006, the FDA issued a clinical warning titled “Potentially Life-Threatening Serotonin Syndrome With Combined Use of SSRIs or SNRIs and Triptan Medications.” Subsequently, Randolph W. Evans, MD, and others conducted a close evaluation of the cases used by the FDA as the basis for their warning. They noted that none of the initial cases met Hunter criteria, only 10 of 29 met Sternbach criteria, and a second set of 11 patients also were questionable in terms of the diagnosis of serotonin toxicity. Serotonin (5-HT) toxicity is mediated by excessive activity of 5-HT2A receptors, and triptans have no action at those receptors, only having activity at 5-HT1B, 1D, and 1F receptors.
In 2010, the American Headache Society (AHS) published a position paper on this drug-drug interaction. In it, they stated, “with only Class IV evidence available in the literature and available through the FDA registration of adverse events, …the currently available evidence does not support limiting the use of triptans with SSRIs or SNRIs, or the use of triptan monotherapy, due to concerns for serotonin syndrome (Level U).”
Confirming the lack of evidence for an interaction, Dr. Yulia Orlova from the Graham Headache Center in Boston reported from the Partners Healthcare System Research Patient Data Registry on about 48,000 patients prescribed triptans, of whom about 19,000 were also co-prescribed SSRI or SNRI antidepressants. None of the cases met Hunter and Sternbach criteria and one patient who manifested serotonin toxicity had signs that preceded triptan use. A previous trial of a cohort of 240,268 patients receiving pharmacy benefits reported that the frequency of co-prescription of triptans with SSRIs was about 20%. With the size of these reports, the absence of documented cases fulfilling both sets of criteria, and the lack of receptor plausibility as a cause for serotonin toxicity from triptans, the likelihood of the syndrome from triptan use is low, and the warning inappropriate. The co-occurrence of depression, anxiety, and migraine often makes co-prescription of triptans and antidepressants necessary, and the concern for co-prescription excessive.
—Stewart J. Tepper, MD
Professor of Neurology
Geisel School of Medicine at Dartmouth
Serotonin syndrome (SS) is diagnosed by the clinical triad of dysautonomia (fever, mydriasis, diaphoresis, tachycardia), neuromuscular signs (ataxia, hyperreflexia, tremor, myoclonus), and altered mental status (seizures, delirium). Two validated criteria groups are accepted, the Hunter criteria and the Sternbach criteria. These criteria require a menu-like approach of clinical manifestations of the above signs with known addition or increase of a serotonergic medication and the absence of other possible causes, such as neuroleptics.
In 2006, the FDA issued a clinical warning titled “Potentially Life-Threatening Serotonin Syndrome With Combined Use of SSRIs or SNRIs and Triptan Medications.” Subsequently, Randolph W. Evans, MD, and others conducted a close evaluation of the cases used by the FDA as the basis for their warning. They noted that none of the initial cases met Hunter criteria, only 10 of 29 met Sternbach criteria, and a second set of 11 patients also were questionable in terms of the diagnosis of serotonin toxicity. Serotonin (5-HT) toxicity is mediated by excessive activity of 5-HT2A receptors, and triptans have no action at those receptors, only having activity at 5-HT1B, 1D, and 1F receptors.
In 2010, the American Headache Society (AHS) published a position paper on this drug-drug interaction. In it, they stated, “with only Class IV evidence available in the literature and available through the FDA registration of adverse events, …the currently available evidence does not support limiting the use of triptans with SSRIs or SNRIs, or the use of triptan monotherapy, due to concerns for serotonin syndrome (Level U).”
Confirming the lack of evidence for an interaction, Dr. Yulia Orlova from the Graham Headache Center in Boston reported from the Partners Healthcare System Research Patient Data Registry on about 48,000 patients prescribed triptans, of whom about 19,000 were also co-prescribed SSRI or SNRI antidepressants. None of the cases met Hunter and Sternbach criteria and one patient who manifested serotonin toxicity had signs that preceded triptan use. A previous trial of a cohort of 240,268 patients receiving pharmacy benefits reported that the frequency of co-prescription of triptans with SSRIs was about 20%. With the size of these reports, the absence of documented cases fulfilling both sets of criteria, and the lack of receptor plausibility as a cause for serotonin toxicity from triptans, the likelihood of the syndrome from triptan use is low, and the warning inappropriate. The co-occurrence of depression, anxiety, and migraine often makes co-prescription of triptans and antidepressants necessary, and the concern for co-prescription excessive.
—Stewart J. Tepper, MD
Professor of Neurology
Geisel School of Medicine at Dartmouth
Serotonin syndrome (SS) is diagnosed by the clinical triad of dysautonomia (fever, mydriasis, diaphoresis, tachycardia), neuromuscular signs (ataxia, hyperreflexia, tremor, myoclonus), and altered mental status (seizures, delirium). Two validated criteria groups are accepted, the Hunter criteria and the Sternbach criteria. These criteria require a menu-like approach of clinical manifestations of the above signs with known addition or increase of a serotonergic medication and the absence of other possible causes, such as neuroleptics.
In 2006, the FDA issued a clinical warning titled “Potentially Life-Threatening Serotonin Syndrome With Combined Use of SSRIs or SNRIs and Triptan Medications.” Subsequently, Randolph W. Evans, MD, and others conducted a close evaluation of the cases used by the FDA as the basis for their warning. They noted that none of the initial cases met Hunter criteria, only 10 of 29 met Sternbach criteria, and a second set of 11 patients also were questionable in terms of the diagnosis of serotonin toxicity. Serotonin (5-HT) toxicity is mediated by excessive activity of 5-HT2A receptors, and triptans have no action at those receptors, only having activity at 5-HT1B, 1D, and 1F receptors.
In 2010, the American Headache Society (AHS) published a position paper on this drug-drug interaction. In it, they stated, “with only Class IV evidence available in the literature and available through the FDA registration of adverse events, …the currently available evidence does not support limiting the use of triptans with SSRIs or SNRIs, or the use of triptan monotherapy, due to concerns for serotonin syndrome (Level U).”
Confirming the lack of evidence for an interaction, Dr. Yulia Orlova from the Graham Headache Center in Boston reported from the Partners Healthcare System Research Patient Data Registry on about 48,000 patients prescribed triptans, of whom about 19,000 were also co-prescribed SSRI or SNRI antidepressants. None of the cases met Hunter and Sternbach criteria and one patient who manifested serotonin toxicity had signs that preceded triptan use. A previous trial of a cohort of 240,268 patients receiving pharmacy benefits reported that the frequency of co-prescription of triptans with SSRIs was about 20%. With the size of these reports, the absence of documented cases fulfilling both sets of criteria, and the lack of receptor plausibility as a cause for serotonin toxicity from triptans, the likelihood of the syndrome from triptan use is low, and the warning inappropriate. The co-occurrence of depression, anxiety, and migraine often makes co-prescription of triptans and antidepressants necessary, and the concern for co-prescription excessive.
—Stewart J. Tepper, MD
Professor of Neurology
Geisel School of Medicine at Dartmouth
Imaging methods for stroke thrombectomy eligibility yield similar results
LOS ANGELES – The benefits of mechanical thrombectomy observed in the DAWN trial for patients with acute ischemic stroke and a mismatch between core imaging and clinical presentation out to 24 hours appear to apply regardless of whether their eligibility is determined by CT perfusion or diffusion-weighted magnetic resonance imaging, according to a subanalysis of the trial data.
Diffusion-weighted magnetic resonance imaging (DW-MRI) is considered the gold standard, but it is not as widely available as CT perfusion (CTP) and previous studies have shown that MR is associated with longer times between stroke onset and treatment randomization. “Though MR was originally preferred in DAWN, it was pretty clear that CT perfusion was going to need to be employed in the trial as well,” Cathy Sila, MD, said during her presentation of the results of the subanalysis at the International Stroke Conference 2018, sponsored by the American Heart Association.
The research sought to determine if the two imaging methods perform similarly. CTP is more readily available, but it has some issues. In patients with severe heart failure, a severe proximal stenosis, or a contralateral severe stenosis, the technique may struggle to accurately image the core infarct, which has led some to wonder if the outcomes would be as good using CTP as selection criteria. “In our institution, we’ve had this conversation very frequently,” said Dr. Sila, who is a vascular neurologist and the director of the University Hospitals Systems stroke program in Cleveland.
To be eligible for DAWN, the core infarct had to correspond to at least a 30% decrease in regional blood flow in the CTP map, or an apparent diffusion coefficient of less than 620 on DW-MRI.
The researchers included all 206 patients in the DAWN study (N Engl J Med. 2018;378:11-21), separating them into DW-MRI or CTP groups based on which imaging method was used to randomize them during the trial. There were no statistically significant differences in any of the baseline characteristics between the two imaging groups.
The 26 sites participating in DAWN had clear differences in their preferences for imaging techniques; 19 exclusively used CTP, 4 used only DW-MRI, and 3 sites used a combination of both imaging methods.
There were no statistically significant differences between the two groups in any of the measured clinical outcomes, including neurologic deterioration in hospital (22.8% with CTP vs. 15.7% with DW-MRII, P = .286), symptomatic intracranial hemorrhage (4.1% with CTP vs. 4.8% with DW-MRI, P = 1.000), or death related to stroke (19.5% with CTP vs. 13.3% with DW-MRI, P = .263). Outcomes at 90 days proved to be similar between CTP and DW-MRI for achieving functional independence (29.3% vs. 34.9%, respectively; P = .445) and utility-weighted modified Rankin Scale scores (4.2 vs. 4.9, respectively; P = .172).
Multivariate analyses showed that 90-day functional independence was predicted by thrombectomy treatment, age, blood glucose level, baseline National Institutes of Health Stroke Scale score, and core lab ASPECTS (Alberta Stroke Program Early CT Score), but not the method of imaging.
“The efficacy and safety of mechanical thrombectomy for patients meeting those clinical mismatch criteria at 6-24 hours were comparable whether the small core infarcts were measured by diffusion imaging or cerebral blood flow imaging. I believe that future clinical trials aiming to extend the eligibility outside of this prespecified population should include both imaging modalities to determine whether these results are generalizable,” Dr. Sila said.
The DAWN study was funded by Stryker Neurovascular. Dr. Sila has reported receiving honoraria from Medtronic.
SOURCE: Sila C et al. ISC 2018, abstract LB11.
LOS ANGELES – The benefits of mechanical thrombectomy observed in the DAWN trial for patients with acute ischemic stroke and a mismatch between core imaging and clinical presentation out to 24 hours appear to apply regardless of whether their eligibility is determined by CT perfusion or diffusion-weighted magnetic resonance imaging, according to a subanalysis of the trial data.
Diffusion-weighted magnetic resonance imaging (DW-MRI) is considered the gold standard, but it is not as widely available as CT perfusion (CTP) and previous studies have shown that MR is associated with longer times between stroke onset and treatment randomization. “Though MR was originally preferred in DAWN, it was pretty clear that CT perfusion was going to need to be employed in the trial as well,” Cathy Sila, MD, said during her presentation of the results of the subanalysis at the International Stroke Conference 2018, sponsored by the American Heart Association.
The research sought to determine if the two imaging methods perform similarly. CTP is more readily available, but it has some issues. In patients with severe heart failure, a severe proximal stenosis, or a contralateral severe stenosis, the technique may struggle to accurately image the core infarct, which has led some to wonder if the outcomes would be as good using CTP as selection criteria. “In our institution, we’ve had this conversation very frequently,” said Dr. Sila, who is a vascular neurologist and the director of the University Hospitals Systems stroke program in Cleveland.
To be eligible for DAWN, the core infarct had to correspond to at least a 30% decrease in regional blood flow in the CTP map, or an apparent diffusion coefficient of less than 620 on DW-MRI.
The researchers included all 206 patients in the DAWN study (N Engl J Med. 2018;378:11-21), separating them into DW-MRI or CTP groups based on which imaging method was used to randomize them during the trial. There were no statistically significant differences in any of the baseline characteristics between the two imaging groups.
The 26 sites participating in DAWN had clear differences in their preferences for imaging techniques; 19 exclusively used CTP, 4 used only DW-MRI, and 3 sites used a combination of both imaging methods.
There were no statistically significant differences between the two groups in any of the measured clinical outcomes, including neurologic deterioration in hospital (22.8% with CTP vs. 15.7% with DW-MRII, P = .286), symptomatic intracranial hemorrhage (4.1% with CTP vs. 4.8% with DW-MRI, P = 1.000), or death related to stroke (19.5% with CTP vs. 13.3% with DW-MRI, P = .263). Outcomes at 90 days proved to be similar between CTP and DW-MRI for achieving functional independence (29.3% vs. 34.9%, respectively; P = .445) and utility-weighted modified Rankin Scale scores (4.2 vs. 4.9, respectively; P = .172).
Multivariate analyses showed that 90-day functional independence was predicted by thrombectomy treatment, age, blood glucose level, baseline National Institutes of Health Stroke Scale score, and core lab ASPECTS (Alberta Stroke Program Early CT Score), but not the method of imaging.
“The efficacy and safety of mechanical thrombectomy for patients meeting those clinical mismatch criteria at 6-24 hours were comparable whether the small core infarcts were measured by diffusion imaging or cerebral blood flow imaging. I believe that future clinical trials aiming to extend the eligibility outside of this prespecified population should include both imaging modalities to determine whether these results are generalizable,” Dr. Sila said.
The DAWN study was funded by Stryker Neurovascular. Dr. Sila has reported receiving honoraria from Medtronic.
SOURCE: Sila C et al. ISC 2018, abstract LB11.
LOS ANGELES – The benefits of mechanical thrombectomy observed in the DAWN trial for patients with acute ischemic stroke and a mismatch between core imaging and clinical presentation out to 24 hours appear to apply regardless of whether their eligibility is determined by CT perfusion or diffusion-weighted magnetic resonance imaging, according to a subanalysis of the trial data.
Diffusion-weighted magnetic resonance imaging (DW-MRI) is considered the gold standard, but it is not as widely available as CT perfusion (CTP) and previous studies have shown that MR is associated with longer times between stroke onset and treatment randomization. “Though MR was originally preferred in DAWN, it was pretty clear that CT perfusion was going to need to be employed in the trial as well,” Cathy Sila, MD, said during her presentation of the results of the subanalysis at the International Stroke Conference 2018, sponsored by the American Heart Association.
The research sought to determine if the two imaging methods perform similarly. CTP is more readily available, but it has some issues. In patients with severe heart failure, a severe proximal stenosis, or a contralateral severe stenosis, the technique may struggle to accurately image the core infarct, which has led some to wonder if the outcomes would be as good using CTP as selection criteria. “In our institution, we’ve had this conversation very frequently,” said Dr. Sila, who is a vascular neurologist and the director of the University Hospitals Systems stroke program in Cleveland.
To be eligible for DAWN, the core infarct had to correspond to at least a 30% decrease in regional blood flow in the CTP map, or an apparent diffusion coefficient of less than 620 on DW-MRI.
The researchers included all 206 patients in the DAWN study (N Engl J Med. 2018;378:11-21), separating them into DW-MRI or CTP groups based on which imaging method was used to randomize them during the trial. There were no statistically significant differences in any of the baseline characteristics between the two imaging groups.
The 26 sites participating in DAWN had clear differences in their preferences for imaging techniques; 19 exclusively used CTP, 4 used only DW-MRI, and 3 sites used a combination of both imaging methods.
There were no statistically significant differences between the two groups in any of the measured clinical outcomes, including neurologic deterioration in hospital (22.8% with CTP vs. 15.7% with DW-MRII, P = .286), symptomatic intracranial hemorrhage (4.1% with CTP vs. 4.8% with DW-MRI, P = 1.000), or death related to stroke (19.5% with CTP vs. 13.3% with DW-MRI, P = .263). Outcomes at 90 days proved to be similar between CTP and DW-MRI for achieving functional independence (29.3% vs. 34.9%, respectively; P = .445) and utility-weighted modified Rankin Scale scores (4.2 vs. 4.9, respectively; P = .172).
Multivariate analyses showed that 90-day functional independence was predicted by thrombectomy treatment, age, blood glucose level, baseline National Institutes of Health Stroke Scale score, and core lab ASPECTS (Alberta Stroke Program Early CT Score), but not the method of imaging.
“The efficacy and safety of mechanical thrombectomy for patients meeting those clinical mismatch criteria at 6-24 hours were comparable whether the small core infarcts were measured by diffusion imaging or cerebral blood flow imaging. I believe that future clinical trials aiming to extend the eligibility outside of this prespecified population should include both imaging modalities to determine whether these results are generalizable,” Dr. Sila said.
The DAWN study was funded by Stryker Neurovascular. Dr. Sila has reported receiving honoraria from Medtronic.
SOURCE: Sila C et al. ISC 2018, abstract LB11.
REPORTING FROM ISC 2018
Key clinical point: DW-MRI is the gold standard for imaging, but CTP is more widely available.
Major finding: Rates of neurologic deterioration in hospital, symptomatic intracranial hemorrhage, and death related to stroke were similar regardless of whether CT or MR imaging was used to assess patients’ infarcts.
Data source: A subanalysis of the DAWN randomized, controlled trial (n = 206).
Disclosures: The DAWN study was funded by Stryker Neurovascular. Dr. Sila reported receiving honoraria from Medtronic.
Source: Sila C et al. ISC 2018, abstract LB11.