User login
Study reveals potential target for AML treatment
New research has revealed a potential therapeutic target for acute myeloid leukemia (AML)—the methyl transferase enzyme METTL3.
Researchers found that inhibiting METTL3 destroys human and mouse AML cells without harming non-leukemic blood cells.
The team also discovered why METTL3 is required for AML cell survival by deciphering the mechanism it uses to regulate several other leukemia genes.
The researchers described this work in Nature.
“New treatments for AML are desperately needed, and we have been looking for genes that would be good drug targets,” said study author Tony Kouzarides, PhD, of University of Cambridge in the UK.
“We identified the methyl transferase enzyme METTL3 as a highly viable target against AML. Our study will inspire pharmaceutical efforts to find drugs that specifically inhibit METTL3 to treat AML.”
In their attempt to find therapeutic targets for AML, Dr Kouzarides and his colleagues used CRISPR-Cas9 to screen AML cells for vulnerable points.
The researchers created mouse leukemia cells with mutations in genes that may be targeted in human AML cells and systematically tested each gene, finding which were essential for AML survival.
The team ended up with 46 likely candidate genes, many of which produce proteins that could modify RNA. Among these, METTL3 was one of the genes with the strongest effect.
Experiments revealed that METTL3 was essential for the survival of AML cells, but it was not required for healthy blood cells.
Having found a potential target in METTL3, the researchers investigated how it worked.
They discovered that the protein produced by METTL3 bound to the beginning of 126 different genes, including several required for AML cell survival.
As RNAs were produced, the METTL3 protein added methyl groups to their middle section, something which had not been previously observed. These middle methyl groups increased the ability of the RNAs to be translated into proteins.
The researchers then found that when METTL3 was inhibited, no methyl groups were added to the RNA. This prevented the production of their essential proteins, so the AML cells started dying.
“This study uncovered an entirely new mechanism of gene regulation in AML that operates through modifications of RNA,” said study author Konstantinos Tzelepis, PhD, of Wellcome Trust Sanger Institute in Cambridge, UK.
“We discovered that inhibiting the methyl transferase activity of METTL3 would stop the translation of a whole set of proteins that the leukemia needs. This mechanism shows that a drug to inhibit methylation could be effective against AML without affecting normal cells.” ![]()
New research has revealed a potential therapeutic target for acute myeloid leukemia (AML)—the methyl transferase enzyme METTL3.
Researchers found that inhibiting METTL3 destroys human and mouse AML cells without harming non-leukemic blood cells.
The team also discovered why METTL3 is required for AML cell survival by deciphering the mechanism it uses to regulate several other leukemia genes.
The researchers described this work in Nature.
“New treatments for AML are desperately needed, and we have been looking for genes that would be good drug targets,” said study author Tony Kouzarides, PhD, of University of Cambridge in the UK.
“We identified the methyl transferase enzyme METTL3 as a highly viable target against AML. Our study will inspire pharmaceutical efforts to find drugs that specifically inhibit METTL3 to treat AML.”
In their attempt to find therapeutic targets for AML, Dr Kouzarides and his colleagues used CRISPR-Cas9 to screen AML cells for vulnerable points.
The researchers created mouse leukemia cells with mutations in genes that may be targeted in human AML cells and systematically tested each gene, finding which were essential for AML survival.
The team ended up with 46 likely candidate genes, many of which produce proteins that could modify RNA. Among these, METTL3 was one of the genes with the strongest effect.
Experiments revealed that METTL3 was essential for the survival of AML cells, but it was not required for healthy blood cells.
Having found a potential target in METTL3, the researchers investigated how it worked.
They discovered that the protein produced by METTL3 bound to the beginning of 126 different genes, including several required for AML cell survival.
As RNAs were produced, the METTL3 protein added methyl groups to their middle section, something which had not been previously observed. These middle methyl groups increased the ability of the RNAs to be translated into proteins.
The researchers then found that when METTL3 was inhibited, no methyl groups were added to the RNA. This prevented the production of their essential proteins, so the AML cells started dying.
“This study uncovered an entirely new mechanism of gene regulation in AML that operates through modifications of RNA,” said study author Konstantinos Tzelepis, PhD, of Wellcome Trust Sanger Institute in Cambridge, UK.
“We discovered that inhibiting the methyl transferase activity of METTL3 would stop the translation of a whole set of proteins that the leukemia needs. This mechanism shows that a drug to inhibit methylation could be effective against AML without affecting normal cells.” ![]()
New research has revealed a potential therapeutic target for acute myeloid leukemia (AML)—the methyl transferase enzyme METTL3.
Researchers found that inhibiting METTL3 destroys human and mouse AML cells without harming non-leukemic blood cells.
The team also discovered why METTL3 is required for AML cell survival by deciphering the mechanism it uses to regulate several other leukemia genes.
The researchers described this work in Nature.
“New treatments for AML are desperately needed, and we have been looking for genes that would be good drug targets,” said study author Tony Kouzarides, PhD, of University of Cambridge in the UK.
“We identified the methyl transferase enzyme METTL3 as a highly viable target against AML. Our study will inspire pharmaceutical efforts to find drugs that specifically inhibit METTL3 to treat AML.”
In their attempt to find therapeutic targets for AML, Dr Kouzarides and his colleagues used CRISPR-Cas9 to screen AML cells for vulnerable points.
The researchers created mouse leukemia cells with mutations in genes that may be targeted in human AML cells and systematically tested each gene, finding which were essential for AML survival.
The team ended up with 46 likely candidate genes, many of which produce proteins that could modify RNA. Among these, METTL3 was one of the genes with the strongest effect.
Experiments revealed that METTL3 was essential for the survival of AML cells, but it was not required for healthy blood cells.
Having found a potential target in METTL3, the researchers investigated how it worked.
They discovered that the protein produced by METTL3 bound to the beginning of 126 different genes, including several required for AML cell survival.
As RNAs were produced, the METTL3 protein added methyl groups to their middle section, something which had not been previously observed. These middle methyl groups increased the ability of the RNAs to be translated into proteins.
The researchers then found that when METTL3 was inhibited, no methyl groups were added to the RNA. This prevented the production of their essential proteins, so the AML cells started dying.
“This study uncovered an entirely new mechanism of gene regulation in AML that operates through modifications of RNA,” said study author Konstantinos Tzelepis, PhD, of Wellcome Trust Sanger Institute in Cambridge, UK.
“We discovered that inhibiting the methyl transferase activity of METTL3 would stop the translation of a whole set of proteins that the leukemia needs. This mechanism shows that a drug to inhibit methylation could be effective against AML without affecting normal cells.” ![]()
Inhibitor exhibits activity against range of lymphomas
Preclinical research suggests the dual PI3K/mTOR inhibitor PQR309 has activity against several types of lymphoma and works well in combination with other agents.
PQR309 exhibited anti-lymphoma activity as a single agent and in combination with venetoclax, panobinostat, ibrutinib, lenalidomide, ARV-825, marizomib, and rituximab.
PQR309 demonstrated greater activity against B-cell lymphoma than T-cell lymphoma, and the inhibitor was able to overcome both primary and acquired resistance to idelalisib.
Francesco Bertoni, MD, of the Institute of Oncology Research in Bellinzona, Switzerland, and his colleagues conducted this research and reported the results in Clinical Cancer Research.
The work was funded by PIQUR Therapeutics AG, the company developing PQR309, and some study authors are PIQUR employees.
The researchers tested PQR309 in 49 human lymphoma cell lines—7 activated B-cell-like (ABC) diffuse large B-cell lymphoma (DLBCL), 18 germinal center B-cell (GCB) DLBCL, 10 mantle cell lymphoma (MCL), 3 splenic marginal zone lymphoma (SMZL), 2 chronic lymphocytic leukemia (CLL), 4 Hodgkin lymphoma, and 5 anaplastic large-cell lymphoma (ALCL).
In most cell lines, PQR309 halted proliferation, mainly due to cell-cycle arrest with a block in G1. However, PQR309 induced apoptosis in 2 cell lines tested—SU-DHL-4 and TMD8.
The researchers noted that PQR309 was significantly more active in the B-cell lymphoma cell lines (DLBCL, MCL, CLL, and SMZL) than in the T-cell lymphoma cell line ALCL (P=0.028).
PQR309 exhibited similar activity in ABC and GCB DLBCL cell lines, de novo DLBCL, and DLBCL derived from transformed follicular lymphoma. TP53, MYC, and BCL2 status also had no significant effect on PQR309 activity.
The researchers compared cell lines that were very sensitive to PQR309 to those with low sensitivity to the drug and identified differences.
The team said that transcripts preferentially expressed in PQR309-sensitive cell lines were significantly enriched of genes involved in BCR pathway/signaling and BLIMP1 targets. Transcripts associated with less sensitive cell lines were enriched of members of proteasome pathway, response to unfolded proteins, MYC targets, XBP1 targets, genes downregulated by mTOR inhibitors, and genes involved in oxidative phosphorylation.
PQR309 demonstrated synergistic effects when combined with the BTK inhibitor ibrutinib, the immunomodulatory drug lenalidomide, the anti-CD20 monoclonal antibody rituximab, and the proteasome inhibitor marizomib.
PQR309 demonstrated synergistic or additive effects when combined with the BCL2 inhibitor venetoclax, the HDAC inhibitor panobinostat, and the PROTAC BET inhibitor ARV-825.
In addition, PQR309 was active in lymphoma cell lines with primary and secondary resistance to the PI3K inhibitor idelalisib.
The researchers believe the results of this study, together with ongoing clinical studies of PQR309, can lead to better treatments for lymphoma patients and better understanding of the mechanisms of anti-lymphoma agents. ![]()
Preclinical research suggests the dual PI3K/mTOR inhibitor PQR309 has activity against several types of lymphoma and works well in combination with other agents.
PQR309 exhibited anti-lymphoma activity as a single agent and in combination with venetoclax, panobinostat, ibrutinib, lenalidomide, ARV-825, marizomib, and rituximab.
PQR309 demonstrated greater activity against B-cell lymphoma than T-cell lymphoma, and the inhibitor was able to overcome both primary and acquired resistance to idelalisib.
Francesco Bertoni, MD, of the Institute of Oncology Research in Bellinzona, Switzerland, and his colleagues conducted this research and reported the results in Clinical Cancer Research.
The work was funded by PIQUR Therapeutics AG, the company developing PQR309, and some study authors are PIQUR employees.
The researchers tested PQR309 in 49 human lymphoma cell lines—7 activated B-cell-like (ABC) diffuse large B-cell lymphoma (DLBCL), 18 germinal center B-cell (GCB) DLBCL, 10 mantle cell lymphoma (MCL), 3 splenic marginal zone lymphoma (SMZL), 2 chronic lymphocytic leukemia (CLL), 4 Hodgkin lymphoma, and 5 anaplastic large-cell lymphoma (ALCL).
In most cell lines, PQR309 halted proliferation, mainly due to cell-cycle arrest with a block in G1. However, PQR309 induced apoptosis in 2 cell lines tested—SU-DHL-4 and TMD8.
The researchers noted that PQR309 was significantly more active in the B-cell lymphoma cell lines (DLBCL, MCL, CLL, and SMZL) than in the T-cell lymphoma cell line ALCL (P=0.028).
PQR309 exhibited similar activity in ABC and GCB DLBCL cell lines, de novo DLBCL, and DLBCL derived from transformed follicular lymphoma. TP53, MYC, and BCL2 status also had no significant effect on PQR309 activity.
The researchers compared cell lines that were very sensitive to PQR309 to those with low sensitivity to the drug and identified differences.
The team said that transcripts preferentially expressed in PQR309-sensitive cell lines were significantly enriched of genes involved in BCR pathway/signaling and BLIMP1 targets. Transcripts associated with less sensitive cell lines were enriched of members of proteasome pathway, response to unfolded proteins, MYC targets, XBP1 targets, genes downregulated by mTOR inhibitors, and genes involved in oxidative phosphorylation.
PQR309 demonstrated synergistic effects when combined with the BTK inhibitor ibrutinib, the immunomodulatory drug lenalidomide, the anti-CD20 monoclonal antibody rituximab, and the proteasome inhibitor marizomib.
PQR309 demonstrated synergistic or additive effects when combined with the BCL2 inhibitor venetoclax, the HDAC inhibitor panobinostat, and the PROTAC BET inhibitor ARV-825.
In addition, PQR309 was active in lymphoma cell lines with primary and secondary resistance to the PI3K inhibitor idelalisib.
The researchers believe the results of this study, together with ongoing clinical studies of PQR309, can lead to better treatments for lymphoma patients and better understanding of the mechanisms of anti-lymphoma agents. ![]()
Preclinical research suggests the dual PI3K/mTOR inhibitor PQR309 has activity against several types of lymphoma and works well in combination with other agents.
PQR309 exhibited anti-lymphoma activity as a single agent and in combination with venetoclax, panobinostat, ibrutinib, lenalidomide, ARV-825, marizomib, and rituximab.
PQR309 demonstrated greater activity against B-cell lymphoma than T-cell lymphoma, and the inhibitor was able to overcome both primary and acquired resistance to idelalisib.
Francesco Bertoni, MD, of the Institute of Oncology Research in Bellinzona, Switzerland, and his colleagues conducted this research and reported the results in Clinical Cancer Research.
The work was funded by PIQUR Therapeutics AG, the company developing PQR309, and some study authors are PIQUR employees.
The researchers tested PQR309 in 49 human lymphoma cell lines—7 activated B-cell-like (ABC) diffuse large B-cell lymphoma (DLBCL), 18 germinal center B-cell (GCB) DLBCL, 10 mantle cell lymphoma (MCL), 3 splenic marginal zone lymphoma (SMZL), 2 chronic lymphocytic leukemia (CLL), 4 Hodgkin lymphoma, and 5 anaplastic large-cell lymphoma (ALCL).
In most cell lines, PQR309 halted proliferation, mainly due to cell-cycle arrest with a block in G1. However, PQR309 induced apoptosis in 2 cell lines tested—SU-DHL-4 and TMD8.
The researchers noted that PQR309 was significantly more active in the B-cell lymphoma cell lines (DLBCL, MCL, CLL, and SMZL) than in the T-cell lymphoma cell line ALCL (P=0.028).
PQR309 exhibited similar activity in ABC and GCB DLBCL cell lines, de novo DLBCL, and DLBCL derived from transformed follicular lymphoma. TP53, MYC, and BCL2 status also had no significant effect on PQR309 activity.
The researchers compared cell lines that were very sensitive to PQR309 to those with low sensitivity to the drug and identified differences.
The team said that transcripts preferentially expressed in PQR309-sensitive cell lines were significantly enriched of genes involved in BCR pathway/signaling and BLIMP1 targets. Transcripts associated with less sensitive cell lines were enriched of members of proteasome pathway, response to unfolded proteins, MYC targets, XBP1 targets, genes downregulated by mTOR inhibitors, and genes involved in oxidative phosphorylation.
PQR309 demonstrated synergistic effects when combined with the BTK inhibitor ibrutinib, the immunomodulatory drug lenalidomide, the anti-CD20 monoclonal antibody rituximab, and the proteasome inhibitor marizomib.
PQR309 demonstrated synergistic or additive effects when combined with the BCL2 inhibitor venetoclax, the HDAC inhibitor panobinostat, and the PROTAC BET inhibitor ARV-825.
In addition, PQR309 was active in lymphoma cell lines with primary and secondary resistance to the PI3K inhibitor idelalisib.
The researchers believe the results of this study, together with ongoing clinical studies of PQR309, can lead to better treatments for lymphoma patients and better understanding of the mechanisms of anti-lymphoma agents. ![]()
FDA grants drug orphan designation for AML, MDS
The US Food and Drug Administration (FDA) has granted orphan drug designation to AMV564, a CD33/CD3 bispecific antibody, for the treatment of acute myeloid leukemia (AML) and myelodysplastic syndromes (MDS).
AMV564 is a T-cell engager, derived from human protein sequences, that binds both CD33 and CD3 to mediate T-cell directed lysis of CD33-positive cancer cells.
Amphivena Therapeutics Inc., is currently conducting a phase 1 trial of AMV564 in relapsed or refractory AML. The company plans to launch a phase 1 trial in patients with MDS in early 2018.
According to Amphivena, AMV564 has demonstrated “potent activity” in AML patient samples, and that activity was independent of CD33 expression level, disease stage, and cytogenetic risk.
AMV564 also eliminated nearly all blasts from the bone marrow and spleen in a stringent AML patient-derived xenograft murine model.
In addition, Amphivena established a therapeutic window for AMV564 in cynomolgus monkeys, with rapid and sustained elimination of CD33-expressing cells during AMV564 dosing and rapid hematopoietic recovery following dosing.
About orphan designation
The FDA grants orphan designation to products intended to treat, diagnose, or prevent diseases/disorders that affect fewer than 200,000 people in the US.
The designation provides incentives for sponsors to develop products for rare diseases. This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the product is approved. ![]()
The US Food and Drug Administration (FDA) has granted orphan drug designation to AMV564, a CD33/CD3 bispecific antibody, for the treatment of acute myeloid leukemia (AML) and myelodysplastic syndromes (MDS).
AMV564 is a T-cell engager, derived from human protein sequences, that binds both CD33 and CD3 to mediate T-cell directed lysis of CD33-positive cancer cells.
Amphivena Therapeutics Inc., is currently conducting a phase 1 trial of AMV564 in relapsed or refractory AML. The company plans to launch a phase 1 trial in patients with MDS in early 2018.
According to Amphivena, AMV564 has demonstrated “potent activity” in AML patient samples, and that activity was independent of CD33 expression level, disease stage, and cytogenetic risk.
AMV564 also eliminated nearly all blasts from the bone marrow and spleen in a stringent AML patient-derived xenograft murine model.
In addition, Amphivena established a therapeutic window for AMV564 in cynomolgus monkeys, with rapid and sustained elimination of CD33-expressing cells during AMV564 dosing and rapid hematopoietic recovery following dosing.
About orphan designation
The FDA grants orphan designation to products intended to treat, diagnose, or prevent diseases/disorders that affect fewer than 200,000 people in the US.
The designation provides incentives for sponsors to develop products for rare diseases. This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the product is approved. ![]()
The US Food and Drug Administration (FDA) has granted orphan drug designation to AMV564, a CD33/CD3 bispecific antibody, for the treatment of acute myeloid leukemia (AML) and myelodysplastic syndromes (MDS).
AMV564 is a T-cell engager, derived from human protein sequences, that binds both CD33 and CD3 to mediate T-cell directed lysis of CD33-positive cancer cells.
Amphivena Therapeutics Inc., is currently conducting a phase 1 trial of AMV564 in relapsed or refractory AML. The company plans to launch a phase 1 trial in patients with MDS in early 2018.
According to Amphivena, AMV564 has demonstrated “potent activity” in AML patient samples, and that activity was independent of CD33 expression level, disease stage, and cytogenetic risk.
AMV564 also eliminated nearly all blasts from the bone marrow and spleen in a stringent AML patient-derived xenograft murine model.
In addition, Amphivena established a therapeutic window for AMV564 in cynomolgus monkeys, with rapid and sustained elimination of CD33-expressing cells during AMV564 dosing and rapid hematopoietic recovery following dosing.
About orphan designation
The FDA grants orphan designation to products intended to treat, diagnose, or prevent diseases/disorders that affect fewer than 200,000 people in the US.
The designation provides incentives for sponsors to develop products for rare diseases. This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the product is approved. ![]()
Prenatal vitamin D supplementation plagued by lack of evidence
Prenatal vitamin D supplementation may reduce the risk of small for gestational age and early wheeze in infants, but most of the evidence comes from small, low-quality trials, according to a systematic review and meta-analysis.
Researchers reported on a meta-analysis of 43 randomized controlled trials, involving 8,406 participants, which examined the effects of vitamin D supplementation during pregnancy (BMJ. 2017;359:j5237. doi: 10.1136/bmj.j5237).
The most commonly reported outcomes involved fetal growth and preterm birth. A pooling of 37 comparisons suggested that vitamin D supplementation increased mean birth weight by an average of 58 g, compared with low-dose vitamin D, no vitamin D, or placebo.
Two high-quality trials using a regular dose of vitamin D, which were conducted in high-income countries, found a 19% reduction in the risk of persistent/recurrent wheeze by age 3, which the authors said was consistent with other data suggesting a beneficial effect of vitamin D in adults with asthma. However, there were no other respiratory effects, such as on the risk of upper or lower respiratory tract infections.
There were few studies that reported on maternal clinical outcomes, and those that did showed no evidence of benefit.
“Though some observational studies have shown associations between maternal vitamin D deficiency and gestational diabetes and preeclampsia, we did not find robust corroborating evidence from randomised controlled trials,” wrote Daniel E. Roth, MD, and his colleagues at the Hospital for Sick Children, Toronto, and the University of Toronto.
They did report significantly higher maternal and cord blood concentrations of 25-hydroxyvitamin D in the intervention groups, compared with controls.
The median sample size of the studies was 133, and the researchers found that only 8 of the 43 trials had an overall low risk of bias. They also noted that there were wide variations in baseline maternal vitamin D levels.
“Though trials of prenatal vitamin D supplementation are being published at an accelerating pace, randomised controlled trials published up to 2017 were generally small, low quality, and rarely designed to examine clinical outcomes,” the researchers wrote.
The study was supported by the Hospital for Sick Children. No conflicts of interest were declared.
Prenatal vitamin D supplementation may reduce the risk of small for gestational age and early wheeze in infants, but most of the evidence comes from small, low-quality trials, according to a systematic review and meta-analysis.
Researchers reported on a meta-analysis of 43 randomized controlled trials, involving 8,406 participants, which examined the effects of vitamin D supplementation during pregnancy (BMJ. 2017;359:j5237. doi: 10.1136/bmj.j5237).
The most commonly reported outcomes involved fetal growth and preterm birth. A pooling of 37 comparisons suggested that vitamin D supplementation increased mean birth weight by an average of 58 g, compared with low-dose vitamin D, no vitamin D, or placebo.
Two high-quality trials using a regular dose of vitamin D, which were conducted in high-income countries, found a 19% reduction in the risk of persistent/recurrent wheeze by age 3, which the authors said was consistent with other data suggesting a beneficial effect of vitamin D in adults with asthma. However, there were no other respiratory effects, such as on the risk of upper or lower respiratory tract infections.
There were few studies that reported on maternal clinical outcomes, and those that did showed no evidence of benefit.
“Though some observational studies have shown associations between maternal vitamin D deficiency and gestational diabetes and preeclampsia, we did not find robust corroborating evidence from randomised controlled trials,” wrote Daniel E. Roth, MD, and his colleagues at the Hospital for Sick Children, Toronto, and the University of Toronto.
They did report significantly higher maternal and cord blood concentrations of 25-hydroxyvitamin D in the intervention groups, compared with controls.
The median sample size of the studies was 133, and the researchers found that only 8 of the 43 trials had an overall low risk of bias. They also noted that there were wide variations in baseline maternal vitamin D levels.
“Though trials of prenatal vitamin D supplementation are being published at an accelerating pace, randomised controlled trials published up to 2017 were generally small, low quality, and rarely designed to examine clinical outcomes,” the researchers wrote.
The study was supported by the Hospital for Sick Children. No conflicts of interest were declared.
Prenatal vitamin D supplementation may reduce the risk of small for gestational age and early wheeze in infants, but most of the evidence comes from small, low-quality trials, according to a systematic review and meta-analysis.
Researchers reported on a meta-analysis of 43 randomized controlled trials, involving 8,406 participants, which examined the effects of vitamin D supplementation during pregnancy (BMJ. 2017;359:j5237. doi: 10.1136/bmj.j5237).
The most commonly reported outcomes involved fetal growth and preterm birth. A pooling of 37 comparisons suggested that vitamin D supplementation increased mean birth weight by an average of 58 g, compared with low-dose vitamin D, no vitamin D, or placebo.
Two high-quality trials using a regular dose of vitamin D, which were conducted in high-income countries, found a 19% reduction in the risk of persistent/recurrent wheeze by age 3, which the authors said was consistent with other data suggesting a beneficial effect of vitamin D in adults with asthma. However, there were no other respiratory effects, such as on the risk of upper or lower respiratory tract infections.
There were few studies that reported on maternal clinical outcomes, and those that did showed no evidence of benefit.
“Though some observational studies have shown associations between maternal vitamin D deficiency and gestational diabetes and preeclampsia, we did not find robust corroborating evidence from randomised controlled trials,” wrote Daniel E. Roth, MD, and his colleagues at the Hospital for Sick Children, Toronto, and the University of Toronto.
They did report significantly higher maternal and cord blood concentrations of 25-hydroxyvitamin D in the intervention groups, compared with controls.
The median sample size of the studies was 133, and the researchers found that only 8 of the 43 trials had an overall low risk of bias. They also noted that there were wide variations in baseline maternal vitamin D levels.
“Though trials of prenatal vitamin D supplementation are being published at an accelerating pace, randomised controlled trials published up to 2017 were generally small, low quality, and rarely designed to examine clinical outcomes,” the researchers wrote.
The study was supported by the Hospital for Sick Children. No conflicts of interest were declared.
FROM BMJ
Key clinical point:
Major finding: Prenatal vitamin D supplementation is associated with a 40% reduction in the risk of having an SGA infant.
Data source: A systematic review and meta-analysis of 43 randomized controlled trials.
Disclosures: The study was supported by the Hospital for Sick Children, Toronto. No conflicts of interest were declared.
Drug prices a key focus of Senate HELP examination of Azar nomination
WASHINGTON Escalating drug prices topped the agenda as members of the Senate Health, Education, Labor & Pensions Committee interviewed Alex Azar regarding his nomination as secretary of the Department of Health & Human Services.
Mr. Azar, a former HHS deputy secretary and general counsel during the Bush Administration and a former president of Eli Lilly’s U.S. operations, outlined his priorities to the Senate HELP committee during the Nov. 29 hearing.
Drug prices were the focus of many senators’ questions, and while many contentious questions came from panel Democrats, Sen. Rand Paul (R-Ky.) signaled he was not yet on board with his approval for Mr. Azar’s nomination.
“I think many [Americans] perceive [that drug companies use] their economic might to manipulate the system to maximize profits,” Sen. Paul said. “It’s not like they are selling a cheaper product to more people. They are using government to maximize their profits. Do you acknowledge that, under the current system, Big Pharma uses their economic clout to manipulate the patent system to increase drug prices?”
“There are clearly abuses, Senator, in the system, and that is why one of the steps that I mentioned ... that I believe we have to go after, is the gaming of that,” Mr. Azar responded. He suggested that although Hatch-Waxman rules give innovators a time frame to exclusively sell products “there should be a certain moment” when full generic competition should begin.
Sen. Paul also challenged Mr. Azar on the notion of drug importation.
There has not been a successful path to certify that drugs being imported are “safe and reliable,” Mr. Azar noted.
Sen. Paul countered that “you would have to sit there and say that the European Union has unsafe drugs. It would be unsafe for Americans to buy drugs from the European Union or from Canada or Australia. It’s just frankly not true.”
Sen. Paul told Mr. Azar that if he cannot come up with a way to reimport drugs as a means of addressing the high cost of pharmaceuticals in the United States, “I can’t support you.”
Sen. Paul continued that a lot of people have talked about how they are going to change the system, particularly patent issues that stand in the way of generic competition, and “you’ve got some convincing to make me believe that you are going to represent the American people and not Big Pharma, and I know that’s insulting, and I don’t mean it to be because I am sure you are an honest and upright person. But we all have our doubts because Big Pharma manipulates the system to keep prices high. ... We’ve got to fix it. We can’t tepidly go at it. We have to really fix it, and you need to convince those of us who are skeptical that you will be part of fixing it and won’t be beholden to Big Pharma.”
Regarding his other priorities, Mr. Azar noted that, through his “experience helping to implement [Medicare] Part D and with my extensive knowledge of how insurance, manufacturers, pharmacy, and government programs work together, I believe I can bring the skills and experiences to the table that can help us address these issues, while still encouraging discovery so Americans have access to high-quality care.”
He called for making health care “more affordable, more available, and more tailored to what individuals want and need. … Under the status quo, premiums have been skyrocketing year after year, and choices have been dwindling. We must address these challenges for those who have insurance coverage and for those who have been pushed out or left out of the insurance market by the Affordable Care Act.”
Mr. Azar signaled that he will continue the push toward value-based care and will use the power of Medicare to lead the rest of the health care delivery system to follow suit.
“We can better channel the power of health information technology and leverage what is best in our programs and in the private competitive marketplace to ensure the individual patient is the center of decision making and his or her needs are being met with greater transparency and accountability.”
Regarding the opioid crisis, Mr. Azar said that “we must heed President Trump’s call to action and tackle the scourge of the opioid epidemic that is destroying so many individuals, families, and communities. We need aggressive prevention, education, regulatory, and enforcement efforts to stop overprescribing and overuse of these legal and illegal drugs. And we need compassionate treatment for those suffering from dependence and addiction.”
Mr. Azar also was challenged on women’s health issues, particularly the ability of employers to exclude health insurance coverage of contraception because of religious objections. He noted that there needs to be a balance between the medical needs of the patient and the rights of an organization to follow its conscience.
When queried about making contraception available over the counter, he noted that the regulations regarding OTC conversion are outdated, and he was encouraged that FDA Commissioner Scott Gottlieb, MD, is looking into that.
Mr. Azar also committed during the hearing to working with improving interoperability of electronic health records as well as working with physicians to reduce the associated documentation burden.
He voiced his support of reforming the Affordable Care Act, adding that, “if it remains the law, my goal is to implement a way that leads to affordable insurance, leads to choice of insurance that leads to real access and not a meaningless insurance care, and insurance that has the benefits that people want, not what we say in D.C. for them.”
He also expressed support for the use of block grants to help fund Medicaid.
Mr. Azar’s appearance before the HELP committee was a courtesy as the Senate Finance Committee holds jurisdiction over his nomination. No confirmation hearing had been scheduled at press time.
WASHINGTON Escalating drug prices topped the agenda as members of the Senate Health, Education, Labor & Pensions Committee interviewed Alex Azar regarding his nomination as secretary of the Department of Health & Human Services.
Mr. Azar, a former HHS deputy secretary and general counsel during the Bush Administration and a former president of Eli Lilly’s U.S. operations, outlined his priorities to the Senate HELP committee during the Nov. 29 hearing.
Drug prices were the focus of many senators’ questions, and while many contentious questions came from panel Democrats, Sen. Rand Paul (R-Ky.) signaled he was not yet on board with his approval for Mr. Azar’s nomination.
“I think many [Americans] perceive [that drug companies use] their economic might to manipulate the system to maximize profits,” Sen. Paul said. “It’s not like they are selling a cheaper product to more people. They are using government to maximize their profits. Do you acknowledge that, under the current system, Big Pharma uses their economic clout to manipulate the patent system to increase drug prices?”
“There are clearly abuses, Senator, in the system, and that is why one of the steps that I mentioned ... that I believe we have to go after, is the gaming of that,” Mr. Azar responded. He suggested that although Hatch-Waxman rules give innovators a time frame to exclusively sell products “there should be a certain moment” when full generic competition should begin.
Sen. Paul also challenged Mr. Azar on the notion of drug importation.
There has not been a successful path to certify that drugs being imported are “safe and reliable,” Mr. Azar noted.
Sen. Paul countered that “you would have to sit there and say that the European Union has unsafe drugs. It would be unsafe for Americans to buy drugs from the European Union or from Canada or Australia. It’s just frankly not true.”
Sen. Paul told Mr. Azar that if he cannot come up with a way to reimport drugs as a means of addressing the high cost of pharmaceuticals in the United States, “I can’t support you.”
Sen. Paul continued that a lot of people have talked about how they are going to change the system, particularly patent issues that stand in the way of generic competition, and “you’ve got some convincing to make me believe that you are going to represent the American people and not Big Pharma, and I know that’s insulting, and I don’t mean it to be because I am sure you are an honest and upright person. But we all have our doubts because Big Pharma manipulates the system to keep prices high. ... We’ve got to fix it. We can’t tepidly go at it. We have to really fix it, and you need to convince those of us who are skeptical that you will be part of fixing it and won’t be beholden to Big Pharma.”
Regarding his other priorities, Mr. Azar noted that, through his “experience helping to implement [Medicare] Part D and with my extensive knowledge of how insurance, manufacturers, pharmacy, and government programs work together, I believe I can bring the skills and experiences to the table that can help us address these issues, while still encouraging discovery so Americans have access to high-quality care.”
He called for making health care “more affordable, more available, and more tailored to what individuals want and need. … Under the status quo, premiums have been skyrocketing year after year, and choices have been dwindling. We must address these challenges for those who have insurance coverage and for those who have been pushed out or left out of the insurance market by the Affordable Care Act.”
Mr. Azar signaled that he will continue the push toward value-based care and will use the power of Medicare to lead the rest of the health care delivery system to follow suit.
“We can better channel the power of health information technology and leverage what is best in our programs and in the private competitive marketplace to ensure the individual patient is the center of decision making and his or her needs are being met with greater transparency and accountability.”
Regarding the opioid crisis, Mr. Azar said that “we must heed President Trump’s call to action and tackle the scourge of the opioid epidemic that is destroying so many individuals, families, and communities. We need aggressive prevention, education, regulatory, and enforcement efforts to stop overprescribing and overuse of these legal and illegal drugs. And we need compassionate treatment for those suffering from dependence and addiction.”
Mr. Azar also was challenged on women’s health issues, particularly the ability of employers to exclude health insurance coverage of contraception because of religious objections. He noted that there needs to be a balance between the medical needs of the patient and the rights of an organization to follow its conscience.
When queried about making contraception available over the counter, he noted that the regulations regarding OTC conversion are outdated, and he was encouraged that FDA Commissioner Scott Gottlieb, MD, is looking into that.
Mr. Azar also committed during the hearing to working with improving interoperability of electronic health records as well as working with physicians to reduce the associated documentation burden.
He voiced his support of reforming the Affordable Care Act, adding that, “if it remains the law, my goal is to implement a way that leads to affordable insurance, leads to choice of insurance that leads to real access and not a meaningless insurance care, and insurance that has the benefits that people want, not what we say in D.C. for them.”
He also expressed support for the use of block grants to help fund Medicaid.
Mr. Azar’s appearance before the HELP committee was a courtesy as the Senate Finance Committee holds jurisdiction over his nomination. No confirmation hearing had been scheduled at press time.
WASHINGTON Escalating drug prices topped the agenda as members of the Senate Health, Education, Labor & Pensions Committee interviewed Alex Azar regarding his nomination as secretary of the Department of Health & Human Services.
Mr. Azar, a former HHS deputy secretary and general counsel during the Bush Administration and a former president of Eli Lilly’s U.S. operations, outlined his priorities to the Senate HELP committee during the Nov. 29 hearing.
Drug prices were the focus of many senators’ questions, and while many contentious questions came from panel Democrats, Sen. Rand Paul (R-Ky.) signaled he was not yet on board with his approval for Mr. Azar’s nomination.
“I think many [Americans] perceive [that drug companies use] their economic might to manipulate the system to maximize profits,” Sen. Paul said. “It’s not like they are selling a cheaper product to more people. They are using government to maximize their profits. Do you acknowledge that, under the current system, Big Pharma uses their economic clout to manipulate the patent system to increase drug prices?”
“There are clearly abuses, Senator, in the system, and that is why one of the steps that I mentioned ... that I believe we have to go after, is the gaming of that,” Mr. Azar responded. He suggested that although Hatch-Waxman rules give innovators a time frame to exclusively sell products “there should be a certain moment” when full generic competition should begin.
Sen. Paul also challenged Mr. Azar on the notion of drug importation.
There has not been a successful path to certify that drugs being imported are “safe and reliable,” Mr. Azar noted.
Sen. Paul countered that “you would have to sit there and say that the European Union has unsafe drugs. It would be unsafe for Americans to buy drugs from the European Union or from Canada or Australia. It’s just frankly not true.”
Sen. Paul told Mr. Azar that if he cannot come up with a way to reimport drugs as a means of addressing the high cost of pharmaceuticals in the United States, “I can’t support you.”
Sen. Paul continued that a lot of people have talked about how they are going to change the system, particularly patent issues that stand in the way of generic competition, and “you’ve got some convincing to make me believe that you are going to represent the American people and not Big Pharma, and I know that’s insulting, and I don’t mean it to be because I am sure you are an honest and upright person. But we all have our doubts because Big Pharma manipulates the system to keep prices high. ... We’ve got to fix it. We can’t tepidly go at it. We have to really fix it, and you need to convince those of us who are skeptical that you will be part of fixing it and won’t be beholden to Big Pharma.”
Regarding his other priorities, Mr. Azar noted that, through his “experience helping to implement [Medicare] Part D and with my extensive knowledge of how insurance, manufacturers, pharmacy, and government programs work together, I believe I can bring the skills and experiences to the table that can help us address these issues, while still encouraging discovery so Americans have access to high-quality care.”
He called for making health care “more affordable, more available, and more tailored to what individuals want and need. … Under the status quo, premiums have been skyrocketing year after year, and choices have been dwindling. We must address these challenges for those who have insurance coverage and for those who have been pushed out or left out of the insurance market by the Affordable Care Act.”
Mr. Azar signaled that he will continue the push toward value-based care and will use the power of Medicare to lead the rest of the health care delivery system to follow suit.
“We can better channel the power of health information technology and leverage what is best in our programs and in the private competitive marketplace to ensure the individual patient is the center of decision making and his or her needs are being met with greater transparency and accountability.”
Regarding the opioid crisis, Mr. Azar said that “we must heed President Trump’s call to action and tackle the scourge of the opioid epidemic that is destroying so many individuals, families, and communities. We need aggressive prevention, education, regulatory, and enforcement efforts to stop overprescribing and overuse of these legal and illegal drugs. And we need compassionate treatment for those suffering from dependence and addiction.”
Mr. Azar also was challenged on women’s health issues, particularly the ability of employers to exclude health insurance coverage of contraception because of religious objections. He noted that there needs to be a balance between the medical needs of the patient and the rights of an organization to follow its conscience.
When queried about making contraception available over the counter, he noted that the regulations regarding OTC conversion are outdated, and he was encouraged that FDA Commissioner Scott Gottlieb, MD, is looking into that.
Mr. Azar also committed during the hearing to working with improving interoperability of electronic health records as well as working with physicians to reduce the associated documentation burden.
He voiced his support of reforming the Affordable Care Act, adding that, “if it remains the law, my goal is to implement a way that leads to affordable insurance, leads to choice of insurance that leads to real access and not a meaningless insurance care, and insurance that has the benefits that people want, not what we say in D.C. for them.”
He also expressed support for the use of block grants to help fund Medicaid.
Mr. Azar’s appearance before the HELP committee was a courtesy as the Senate Finance Committee holds jurisdiction over his nomination. No confirmation hearing had been scheduled at press time.
AT A SENATE HELP COMMITTEE HEARING
HealthCare.gov seeing more action this fall
Four weeks into the open enrollment for 2018, the number of health insurance plans selected through HealthCare.gov is up by 30% over the first 4 weeks of the 2017 sign-up period, according to data from the Centers for Medicare & Medicaid Services.
From Nov. 1 to Nov. 25 of this year, 2.78 million plans for 2018 were selected on the 39 state marketplaces that use the HealthCare.gov platform, the CMS reported Nov. 29, compared with the 2.14 million plans for 2017 selected from Nov. 1 to Nov. 26 of last year.
Last year, the enrollment numbers were released only every 2 weeks, so direct week-to-week comparisons are not possible. Looking at 2-week periods, however, shows that, despite a drop in the number of selections from the first to the second biweekly period this year, weeks 3 and 4 were still up considerably over last year.
“The final number of plan selections associated with enrollment activity during a reporting period may change due to plan modifications or cancellations,” the CMS noted. Also, “the weekly snapshot only reports new plan selections and active plan renewals and does not report the number of consumers who have paid premiums to effectuate their enrollment.”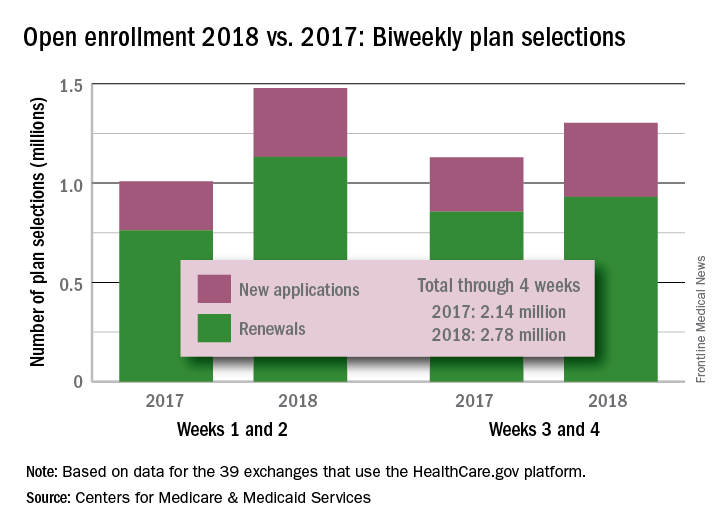
Four weeks into the open enrollment for 2018, the number of health insurance plans selected through HealthCare.gov is up by 30% over the first 4 weeks of the 2017 sign-up period, according to data from the Centers for Medicare & Medicaid Services.
From Nov. 1 to Nov. 25 of this year, 2.78 million plans for 2018 were selected on the 39 state marketplaces that use the HealthCare.gov platform, the CMS reported Nov. 29, compared with the 2.14 million plans for 2017 selected from Nov. 1 to Nov. 26 of last year.
Last year, the enrollment numbers were released only every 2 weeks, so direct week-to-week comparisons are not possible. Looking at 2-week periods, however, shows that, despite a drop in the number of selections from the first to the second biweekly period this year, weeks 3 and 4 were still up considerably over last year.
“The final number of plan selections associated with enrollment activity during a reporting period may change due to plan modifications or cancellations,” the CMS noted. Also, “the weekly snapshot only reports new plan selections and active plan renewals and does not report the number of consumers who have paid premiums to effectuate their enrollment.”
Four weeks into the open enrollment for 2018, the number of health insurance plans selected through HealthCare.gov is up by 30% over the first 4 weeks of the 2017 sign-up period, according to data from the Centers for Medicare & Medicaid Services.
From Nov. 1 to Nov. 25 of this year, 2.78 million plans for 2018 were selected on the 39 state marketplaces that use the HealthCare.gov platform, the CMS reported Nov. 29, compared with the 2.14 million plans for 2017 selected from Nov. 1 to Nov. 26 of last year.
Last year, the enrollment numbers were released only every 2 weeks, so direct week-to-week comparisons are not possible. Looking at 2-week periods, however, shows that, despite a drop in the number of selections from the first to the second biweekly period this year, weeks 3 and 4 were still up considerably over last year.
“The final number of plan selections associated with enrollment activity during a reporting period may change due to plan modifications or cancellations,” the CMS noted. Also, “the weekly snapshot only reports new plan selections and active plan renewals and does not report the number of consumers who have paid premiums to effectuate their enrollment.”
VIDEO: Team approach boosts effective blood pressure control
ANAHEIM, CALIF. – Using a multidisciplinary team of clinicians “could be one of the most effective measures we have to improve blood pressure control,” Tracy Y. Wang, MD, said in a video interview during the American Heart Association scientific sessions.
Speaking a day after the release of revised guidelines for the prevention, detection, evaluation, and management of high blood pressure in adults (J Am Coll Cardiol. 2017 Nov 13;doi: 10.1016/j.jacc.2017.11.006), Dr. Wang particularly highlighted the strong recommendation the guidelines made for a team-based approach for managing hypertension.
“I’m absolutely ecstatic that team management is embedded firmly in the new guidelines,” commented Dr. Wang, a cardiologist at Duke University in Durham, N.C. who has studied methods to optimize evidence-based treatment of cardiovascular diseases. “Endorsement of a team approach for blood pressure control was long overdue,” she said.
Dr. Wang discussed some approaches she believes would help better integrate team-based care into the routine management of patients with hypertension.
Dr. Wang has received honoraria from AstraZeneca, Eli Lilly, and Premier, and she has received research funding from several companies.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
ANAHEIM, CALIF. – Using a multidisciplinary team of clinicians “could be one of the most effective measures we have to improve blood pressure control,” Tracy Y. Wang, MD, said in a video interview during the American Heart Association scientific sessions.
Speaking a day after the release of revised guidelines for the prevention, detection, evaluation, and management of high blood pressure in adults (J Am Coll Cardiol. 2017 Nov 13;doi: 10.1016/j.jacc.2017.11.006), Dr. Wang particularly highlighted the strong recommendation the guidelines made for a team-based approach for managing hypertension.
“I’m absolutely ecstatic that team management is embedded firmly in the new guidelines,” commented Dr. Wang, a cardiologist at Duke University in Durham, N.C. who has studied methods to optimize evidence-based treatment of cardiovascular diseases. “Endorsement of a team approach for blood pressure control was long overdue,” she said.
Dr. Wang discussed some approaches she believes would help better integrate team-based care into the routine management of patients with hypertension.
Dr. Wang has received honoraria from AstraZeneca, Eli Lilly, and Premier, and she has received research funding from several companies.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
ANAHEIM, CALIF. – Using a multidisciplinary team of clinicians “could be one of the most effective measures we have to improve blood pressure control,” Tracy Y. Wang, MD, said in a video interview during the American Heart Association scientific sessions.
Speaking a day after the release of revised guidelines for the prevention, detection, evaluation, and management of high blood pressure in adults (J Am Coll Cardiol. 2017 Nov 13;doi: 10.1016/j.jacc.2017.11.006), Dr. Wang particularly highlighted the strong recommendation the guidelines made for a team-based approach for managing hypertension.
“I’m absolutely ecstatic that team management is embedded firmly in the new guidelines,” commented Dr. Wang, a cardiologist at Duke University in Durham, N.C. who has studied methods to optimize evidence-based treatment of cardiovascular diseases. “Endorsement of a team approach for blood pressure control was long overdue,” she said.
Dr. Wang discussed some approaches she believes would help better integrate team-based care into the routine management of patients with hypertension.
Dr. Wang has received honoraria from AstraZeneca, Eli Lilly, and Premier, and she has received research funding from several companies.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
EXPERT ANALYSIS FROM THE AHA SCIENTIFIC SESSIONS
Empagliflozin’s heart failure benefits linked to volume drop
ANAHEIM, CALIF. – When results from the EMPA-REG OUTCOME trial came out 2 years ago and showed a dramatic decrease in heart failure hospitalizations and deaths linked to treatment with the oral diabetes drug empagliflozin, some experts suggested that a completely hypothetical effect of empagliflozin on reducing fluid volume may have largely caused these unexpected clinical benefits.
New analyses of the trial results show this hypothesis may be at least partially correct.
Results from a post hoc analysis of data collected in Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients (EMPA-REG OUTCOME) suggest that perhaps half the heart failure benefit was attributable to what appears to have been a roughly 7% drop in plasma volume in patients treated with empagliflozin (Jardiance), which began soon after treatment started and continued through the balance of the study, David Fitchett, MD, said at the American Heart Association scientific sessions.
“Markers of change in plasma volume were important mediators of the reduction in risk of hospitalization for heart failure or death from heart failure,” said Dr Fitchett, a cardiologist at St. Michael’s Hospital in Toronto and a coinvestigator of EMPA-REG OUTCOME (N Engl J Med. 2015 Nov 26;373[22]:2117-28).
The analysis also showed that a “modest” effect from a reduction in uric acid might explain about 20%-25% of the observed heart failure benefit, he reported. In contrast, none of the traditional cardiovascular disease risk factors examined in the analysis – including lipids, blood pressure, obesity, and hemoglobin A1c – appeared to have any relationship to the heart failure effects of empagliflozin.
Dr. Fitchett and his associates assessed the possible impact of a list of potential mediators with a statistical method that performed an unadjusted, univariate analysis of the time-dependent change in each of several variables relative to the observed changes in heart failure outcomes.
This analysis showed that on-treatment changes in two markers of plasma volume, hematocrit and hemoglobin, each showed changes that appeared to mediate about half of the heart failure effects. A third marker of plasma volume, albumin level, appeared to mediate about a quarter of the heart failure effects.
The changes in both hematocrit and hemoglobin first appeared within a few weeks of treatment onset, and soon reached a plateau that remained sustained through the balance of the study. For example, during the first 12 weeks of treatment, the average hematocrit level rose from about 41% at baseline to about 44%. This 3% net rise corresponds to about a 7% drop in plasma volume, Dr. Fitchett said.
In addition to reflecting a potentially beneficial decrease in fluid volume, this effect would also boost the oxygen-carrying capacity of a patient’s blood that could be beneficial for patients with ischemic heart disease and those with reduced left ventricular function, he noted.
The EMPA-REG OUTCOME trial was sponsored by Boehringer Ingelheim and Eli Lilly, which jointly market empagliflozin (Jardiance). Dr. Fitchett has received honoraria from those companies and also from Amgen, AstraZeneca, Merck, and Sanofi.
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
ANAHEIM, CALIF. – When results from the EMPA-REG OUTCOME trial came out 2 years ago and showed a dramatic decrease in heart failure hospitalizations and deaths linked to treatment with the oral diabetes drug empagliflozin, some experts suggested that a completely hypothetical effect of empagliflozin on reducing fluid volume may have largely caused these unexpected clinical benefits.
New analyses of the trial results show this hypothesis may be at least partially correct.
Results from a post hoc analysis of data collected in Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients (EMPA-REG OUTCOME) suggest that perhaps half the heart failure benefit was attributable to what appears to have been a roughly 7% drop in plasma volume in patients treated with empagliflozin (Jardiance), which began soon after treatment started and continued through the balance of the study, David Fitchett, MD, said at the American Heart Association scientific sessions.
“Markers of change in plasma volume were important mediators of the reduction in risk of hospitalization for heart failure or death from heart failure,” said Dr Fitchett, a cardiologist at St. Michael’s Hospital in Toronto and a coinvestigator of EMPA-REG OUTCOME (N Engl J Med. 2015 Nov 26;373[22]:2117-28).
The analysis also showed that a “modest” effect from a reduction in uric acid might explain about 20%-25% of the observed heart failure benefit, he reported. In contrast, none of the traditional cardiovascular disease risk factors examined in the analysis – including lipids, blood pressure, obesity, and hemoglobin A1c – appeared to have any relationship to the heart failure effects of empagliflozin.
Dr. Fitchett and his associates assessed the possible impact of a list of potential mediators with a statistical method that performed an unadjusted, univariate analysis of the time-dependent change in each of several variables relative to the observed changes in heart failure outcomes.
This analysis showed that on-treatment changes in two markers of plasma volume, hematocrit and hemoglobin, each showed changes that appeared to mediate about half of the heart failure effects. A third marker of plasma volume, albumin level, appeared to mediate about a quarter of the heart failure effects.
The changes in both hematocrit and hemoglobin first appeared within a few weeks of treatment onset, and soon reached a plateau that remained sustained through the balance of the study. For example, during the first 12 weeks of treatment, the average hematocrit level rose from about 41% at baseline to about 44%. This 3% net rise corresponds to about a 7% drop in plasma volume, Dr. Fitchett said.
In addition to reflecting a potentially beneficial decrease in fluid volume, this effect would also boost the oxygen-carrying capacity of a patient’s blood that could be beneficial for patients with ischemic heart disease and those with reduced left ventricular function, he noted.
The EMPA-REG OUTCOME trial was sponsored by Boehringer Ingelheim and Eli Lilly, which jointly market empagliflozin (Jardiance). Dr. Fitchett has received honoraria from those companies and also from Amgen, AstraZeneca, Merck, and Sanofi.
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
ANAHEIM, CALIF. – When results from the EMPA-REG OUTCOME trial came out 2 years ago and showed a dramatic decrease in heart failure hospitalizations and deaths linked to treatment with the oral diabetes drug empagliflozin, some experts suggested that a completely hypothetical effect of empagliflozin on reducing fluid volume may have largely caused these unexpected clinical benefits.
New analyses of the trial results show this hypothesis may be at least partially correct.
Results from a post hoc analysis of data collected in Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients (EMPA-REG OUTCOME) suggest that perhaps half the heart failure benefit was attributable to what appears to have been a roughly 7% drop in plasma volume in patients treated with empagliflozin (Jardiance), which began soon after treatment started and continued through the balance of the study, David Fitchett, MD, said at the American Heart Association scientific sessions.
“Markers of change in plasma volume were important mediators of the reduction in risk of hospitalization for heart failure or death from heart failure,” said Dr Fitchett, a cardiologist at St. Michael’s Hospital in Toronto and a coinvestigator of EMPA-REG OUTCOME (N Engl J Med. 2015 Nov 26;373[22]:2117-28).
The analysis also showed that a “modest” effect from a reduction in uric acid might explain about 20%-25% of the observed heart failure benefit, he reported. In contrast, none of the traditional cardiovascular disease risk factors examined in the analysis – including lipids, blood pressure, obesity, and hemoglobin A1c – appeared to have any relationship to the heart failure effects of empagliflozin.
Dr. Fitchett and his associates assessed the possible impact of a list of potential mediators with a statistical method that performed an unadjusted, univariate analysis of the time-dependent change in each of several variables relative to the observed changes in heart failure outcomes.
This analysis showed that on-treatment changes in two markers of plasma volume, hematocrit and hemoglobin, each showed changes that appeared to mediate about half of the heart failure effects. A third marker of plasma volume, albumin level, appeared to mediate about a quarter of the heart failure effects.
The changes in both hematocrit and hemoglobin first appeared within a few weeks of treatment onset, and soon reached a plateau that remained sustained through the balance of the study. For example, during the first 12 weeks of treatment, the average hematocrit level rose from about 41% at baseline to about 44%. This 3% net rise corresponds to about a 7% drop in plasma volume, Dr. Fitchett said.
In addition to reflecting a potentially beneficial decrease in fluid volume, this effect would also boost the oxygen-carrying capacity of a patient’s blood that could be beneficial for patients with ischemic heart disease and those with reduced left ventricular function, he noted.
The EMPA-REG OUTCOME trial was sponsored by Boehringer Ingelheim and Eli Lilly, which jointly market empagliflozin (Jardiance). Dr. Fitchett has received honoraria from those companies and also from Amgen, AstraZeneca, Merck, and Sanofi.
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
AT THE AHA SCIENTIFIC SESSIONS
Key clinical point: About according to post hoc analysis of the EMPA-REG OUTCOME study.
Major finding: About half of the observed heart failure benefit was tied to a roughly 3% rise in average hematocrit level.
Data source: Post hoc analysis of data from the 7,028 patients enrolled in the EMPA-REG OUTCOME trial.
Disclosures: The EMPA-REG OUTCOME trial was sponsored by Boehringer Ingelheim and Eli Lilly, the two companies that market empagliflozin (Jardiance). Dr. Fitchett has received honoraria from those companies and also from Amgen, AstraZeneca, Merck, and Sanofi.
Fremanezumab may reduce chronic migraine frequency
Subcutaneous injections of fremanezumab, a humanized monoclonal antibody, reduced headache frequency, compared with placebo, in patients with chronic migraine, according to results of a randomized, double-blind, placebo-controlled trial.
Beneficial effects of fremanezumab were seen within 4 weeks of the initial treatment, according to Stephen D. Silberstein, MD, director of the Jefferson Headache Center at Thomas Jefferson University, Philadelphia, and his coauthors.
“Expert opinion has been that patients with chronic migraine should receive preventive treatment,” they wrote. “However, these treatments may be underused, not adhered to, associated with side effects, or ineffective.”
Fremanezumab targets calcitonin gene-related peptide, which is “involved in central and peripheral pathophysiological events of migraine,” according to the investigators.
The trial comprised 1,130 patients with chronic migraine, defined as occurring at least 8 days per month and headache of any severity at least 15 days per month. They were randomly assigned to receive fremanezumab on a planned quarterly regimen (a single dose at baseline, followed by placebo injections at weeks 4 and 8), fremanezumab monthly (a single dose at baseline, followed by lower doses at weeks 4 and 8), or matching placebo.
For the 12-week period after the first dose, the average number of headache days per month dropped by 4.3 from a baseline mean of 13.2 in the group of patients receiving treatment quarterly, and by 4.6 from a baseline of 12.8 in patients on monthly treatment, compared with a reduction of only 2.5 from a baseline of 13.3 in the placebo-treated patient group (P less than .001 for both fremanezumab groups vs. placebo).
Migraine days also declined significantly more among patients receiving quarterly and monthly fremanezumab by 12 weeks (from a mean of 16.2 to 11.3 and from 16.0 to 11.0, respectively) when compared with placebo (from 16.4 to 13.2; P less than .001 for both comparisons).
The number of patients experiencing a reduction of at least 50% in average number of headache days was higher in both fremanezumab groups at 38% for the quarterly dosing and 41% for monthly dosing, compared with placebo at 18% (P less than .001 for comparisons of fremanezumab to placebo).
Injection site pain was the most common adverse event in the trial, occurring in 30% and 26% of the fremanezumab quarterly and monthly groups, respectively, and 28% of the placebo group, according to the reported data.
Serious adverse events occurred in 1% of patients in the quarterly treatment group, 2% of the monthly group, and 2% of the placebo group, the data showed.
An ongoing extension of the trial will provide “further insights” on the safety and efficacy of treating chronic migraine with fremanezumab over a longer term, the investigators said.
Teva Pharmaceuticals funded the study. Dr. Silberstein and some of his coauthors reported receiving consulting fees from Teva and others. Many coauthors were employees of Teva.
Subcutaneous injections of fremanezumab, a humanized monoclonal antibody, reduced headache frequency, compared with placebo, in patients with chronic migraine, according to results of a randomized, double-blind, placebo-controlled trial.
Beneficial effects of fremanezumab were seen within 4 weeks of the initial treatment, according to Stephen D. Silberstein, MD, director of the Jefferson Headache Center at Thomas Jefferson University, Philadelphia, and his coauthors.
“Expert opinion has been that patients with chronic migraine should receive preventive treatment,” they wrote. “However, these treatments may be underused, not adhered to, associated with side effects, or ineffective.”
Fremanezumab targets calcitonin gene-related peptide, which is “involved in central and peripheral pathophysiological events of migraine,” according to the investigators.
The trial comprised 1,130 patients with chronic migraine, defined as occurring at least 8 days per month and headache of any severity at least 15 days per month. They were randomly assigned to receive fremanezumab on a planned quarterly regimen (a single dose at baseline, followed by placebo injections at weeks 4 and 8), fremanezumab monthly (a single dose at baseline, followed by lower doses at weeks 4 and 8), or matching placebo.
For the 12-week period after the first dose, the average number of headache days per month dropped by 4.3 from a baseline mean of 13.2 in the group of patients receiving treatment quarterly, and by 4.6 from a baseline of 12.8 in patients on monthly treatment, compared with a reduction of only 2.5 from a baseline of 13.3 in the placebo-treated patient group (P less than .001 for both fremanezumab groups vs. placebo).
Migraine days also declined significantly more among patients receiving quarterly and monthly fremanezumab by 12 weeks (from a mean of 16.2 to 11.3 and from 16.0 to 11.0, respectively) when compared with placebo (from 16.4 to 13.2; P less than .001 for both comparisons).
The number of patients experiencing a reduction of at least 50% in average number of headache days was higher in both fremanezumab groups at 38% for the quarterly dosing and 41% for monthly dosing, compared with placebo at 18% (P less than .001 for comparisons of fremanezumab to placebo).
Injection site pain was the most common adverse event in the trial, occurring in 30% and 26% of the fremanezumab quarterly and monthly groups, respectively, and 28% of the placebo group, according to the reported data.
Serious adverse events occurred in 1% of patients in the quarterly treatment group, 2% of the monthly group, and 2% of the placebo group, the data showed.
An ongoing extension of the trial will provide “further insights” on the safety and efficacy of treating chronic migraine with fremanezumab over a longer term, the investigators said.
Teva Pharmaceuticals funded the study. Dr. Silberstein and some of his coauthors reported receiving consulting fees from Teva and others. Many coauthors were employees of Teva.
Subcutaneous injections of fremanezumab, a humanized monoclonal antibody, reduced headache frequency, compared with placebo, in patients with chronic migraine, according to results of a randomized, double-blind, placebo-controlled trial.
Beneficial effects of fremanezumab were seen within 4 weeks of the initial treatment, according to Stephen D. Silberstein, MD, director of the Jefferson Headache Center at Thomas Jefferson University, Philadelphia, and his coauthors.
“Expert opinion has been that patients with chronic migraine should receive preventive treatment,” they wrote. “However, these treatments may be underused, not adhered to, associated with side effects, or ineffective.”
Fremanezumab targets calcitonin gene-related peptide, which is “involved in central and peripheral pathophysiological events of migraine,” according to the investigators.
The trial comprised 1,130 patients with chronic migraine, defined as occurring at least 8 days per month and headache of any severity at least 15 days per month. They were randomly assigned to receive fremanezumab on a planned quarterly regimen (a single dose at baseline, followed by placebo injections at weeks 4 and 8), fremanezumab monthly (a single dose at baseline, followed by lower doses at weeks 4 and 8), or matching placebo.
For the 12-week period after the first dose, the average number of headache days per month dropped by 4.3 from a baseline mean of 13.2 in the group of patients receiving treatment quarterly, and by 4.6 from a baseline of 12.8 in patients on monthly treatment, compared with a reduction of only 2.5 from a baseline of 13.3 in the placebo-treated patient group (P less than .001 for both fremanezumab groups vs. placebo).
Migraine days also declined significantly more among patients receiving quarterly and monthly fremanezumab by 12 weeks (from a mean of 16.2 to 11.3 and from 16.0 to 11.0, respectively) when compared with placebo (from 16.4 to 13.2; P less than .001 for both comparisons).
The number of patients experiencing a reduction of at least 50% in average number of headache days was higher in both fremanezumab groups at 38% for the quarterly dosing and 41% for monthly dosing, compared with placebo at 18% (P less than .001 for comparisons of fremanezumab to placebo).
Injection site pain was the most common adverse event in the trial, occurring in 30% and 26% of the fremanezumab quarterly and monthly groups, respectively, and 28% of the placebo group, according to the reported data.
Serious adverse events occurred in 1% of patients in the quarterly treatment group, 2% of the monthly group, and 2% of the placebo group, the data showed.
An ongoing extension of the trial will provide “further insights” on the safety and efficacy of treating chronic migraine with fremanezumab over a longer term, the investigators said.
Teva Pharmaceuticals funded the study. Dr. Silberstein and some of his coauthors reported receiving consulting fees from Teva and others. Many coauthors were employees of Teva.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point:
Major finding: The average number of headache days was reduced by 4.3 and 4.6 for fremanezumab quarterly and monthly, respectively, compared with 2.5 for placebo (P less than .001 for both comparisons of fremanezumab to placebo).
Data source: A randomized, double-blind, placebo-controlled, parallel-group study of 1,130 patients with chronic migraine who received 12 weeks of treatment.
Disclosures: Teva Pharmaceuticals funded the study. Dr. Silberstein and some of his coauthors reported receiving consulting fees from Teva and others. Many coauthors were employees of Teva.
Vasopressin stimulates red blood cell production
The hormone vasopressin, well known for its antidiuretic effects, also appears to stimulate proliferation and differentiation of red blood cell (RBC) precursors, results of a series of preclinical investigations suggest.
Treating anemic mice with an arginine vasopressin (AVP) receptor agonist increased hematocrit and reticulocyte counts significantly, compared with controls, according to the results published in Science Translational Medicine (2017 Nov 29;9:eaao1632).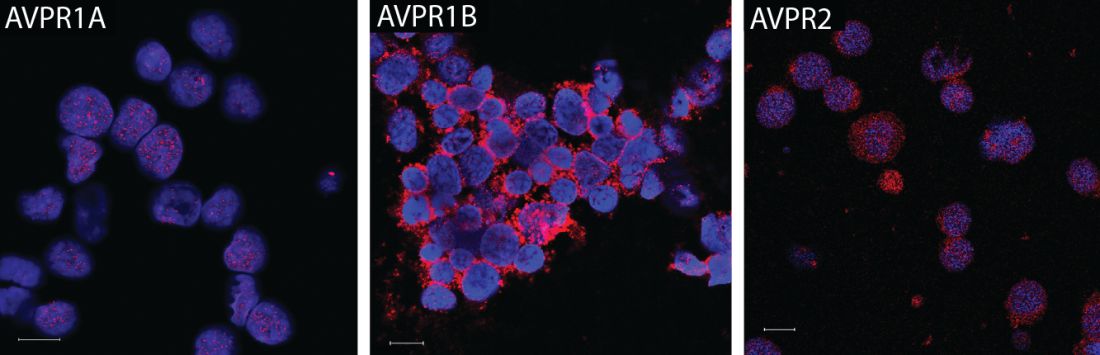
That finding could have implications for the development of new treatments designed to stimulate RBC production after bleeding, chemotherapy, or drug toxicity, according to the investigators.
“Currently, EPO is the only agent that is used clinically to stimulate erythropoiesis, but there are patients who do not respond to EPO or who cannot take the drug because it stimulates tumor growth,” the investigators wrote. “AVP appears to be an EPO-independent, fast-acting agent that increases RBC numbers after anemia.”
Dr. Mayer and his colleagues initially asked whether AVP might play a role in RBC production after observing that patients with central diabetes insipidus (CDI), who lack the antidiuretic hormone, are frequently anemic. A review of patient records from an NIH database revealed that 60% of CDI patients were anemic despite treatment with desmopressin.
They subsequently found that all three AVP receptor subtypes are expressed in human and mouse hematopoietic stem and progenitor cells. In particular, the AVPR1B subtype appeared to play the most important role in regulating erythropoiesis.
Accordingly, they tested the ability of both AVP and a AVPR1B-specific agonist to stimulate production of RBCs in mice that had anemia induced by bleeding or irradiation. They found significant improvements in both hematocrit and reticulocyte numbers as early as 2 days after treatment started.
Subsequent experiments were designed to determine whether the effect of AVP on RBC production was caused by EPO release. In fact, the effects of AVP occurred “long before an effect of EPO was observed,” investigators wrote.
The research was supported by the NIH. Some of the study authors are listed as inventors on a patent application held by the U.S. Department of Health and Human Services covering methods for modulating erythropoiesis with arginine vasopressin receptor 1b molecules.
The hormone vasopressin, well known for its antidiuretic effects, also appears to stimulate proliferation and differentiation of red blood cell (RBC) precursors, results of a series of preclinical investigations suggest.
Treating anemic mice with an arginine vasopressin (AVP) receptor agonist increased hematocrit and reticulocyte counts significantly, compared with controls, according to the results published in Science Translational Medicine (2017 Nov 29;9:eaao1632).
That finding could have implications for the development of new treatments designed to stimulate RBC production after bleeding, chemotherapy, or drug toxicity, according to the investigators.
“Currently, EPO is the only agent that is used clinically to stimulate erythropoiesis, but there are patients who do not respond to EPO or who cannot take the drug because it stimulates tumor growth,” the investigators wrote. “AVP appears to be an EPO-independent, fast-acting agent that increases RBC numbers after anemia.”
Dr. Mayer and his colleagues initially asked whether AVP might play a role in RBC production after observing that patients with central diabetes insipidus (CDI), who lack the antidiuretic hormone, are frequently anemic. A review of patient records from an NIH database revealed that 60% of CDI patients were anemic despite treatment with desmopressin.
They subsequently found that all three AVP receptor subtypes are expressed in human and mouse hematopoietic stem and progenitor cells. In particular, the AVPR1B subtype appeared to play the most important role in regulating erythropoiesis.
Accordingly, they tested the ability of both AVP and a AVPR1B-specific agonist to stimulate production of RBCs in mice that had anemia induced by bleeding or irradiation. They found significant improvements in both hematocrit and reticulocyte numbers as early as 2 days after treatment started.
Subsequent experiments were designed to determine whether the effect of AVP on RBC production was caused by EPO release. In fact, the effects of AVP occurred “long before an effect of EPO was observed,” investigators wrote.
The research was supported by the NIH. Some of the study authors are listed as inventors on a patent application held by the U.S. Department of Health and Human Services covering methods for modulating erythropoiesis with arginine vasopressin receptor 1b molecules.
The hormone vasopressin, well known for its antidiuretic effects, also appears to stimulate proliferation and differentiation of red blood cell (RBC) precursors, results of a series of preclinical investigations suggest.
Treating anemic mice with an arginine vasopressin (AVP) receptor agonist increased hematocrit and reticulocyte counts significantly, compared with controls, according to the results published in Science Translational Medicine (2017 Nov 29;9:eaao1632).
That finding could have implications for the development of new treatments designed to stimulate RBC production after bleeding, chemotherapy, or drug toxicity, according to the investigators.
“Currently, EPO is the only agent that is used clinically to stimulate erythropoiesis, but there are patients who do not respond to EPO or who cannot take the drug because it stimulates tumor growth,” the investigators wrote. “AVP appears to be an EPO-independent, fast-acting agent that increases RBC numbers after anemia.”
Dr. Mayer and his colleagues initially asked whether AVP might play a role in RBC production after observing that patients with central diabetes insipidus (CDI), who lack the antidiuretic hormone, are frequently anemic. A review of patient records from an NIH database revealed that 60% of CDI patients were anemic despite treatment with desmopressin.
They subsequently found that all three AVP receptor subtypes are expressed in human and mouse hematopoietic stem and progenitor cells. In particular, the AVPR1B subtype appeared to play the most important role in regulating erythropoiesis.
Accordingly, they tested the ability of both AVP and a AVPR1B-specific agonist to stimulate production of RBCs in mice that had anemia induced by bleeding or irradiation. They found significant improvements in both hematocrit and reticulocyte numbers as early as 2 days after treatment started.
Subsequent experiments were designed to determine whether the effect of AVP on RBC production was caused by EPO release. In fact, the effects of AVP occurred “long before an effect of EPO was observed,” investigators wrote.
The research was supported by the NIH. Some of the study authors are listed as inventors on a patent application held by the U.S. Department of Health and Human Services covering methods for modulating erythropoiesis with arginine vasopressin receptor 1b molecules.
FROM SCIENCE TRANSLATIONAL MEDICINE
Key clinical point:
Major finding: In anemic mice, treatment with a vasopressin or a vasopressin receptor agonist significantly increased hematocrit and reticulocyte number vs. controls.
Data source: A series of in vitro and in vivo experiments, plus a retrospective review of anemia incidence data in patients with central diabetes insipidus.
Disclosures: The research was supported by the National Institutes of Health. Some of the study authors are listed as inventors on a patent application held by the U.S. Department of Health and Human Services covering methods for modulating erythropoiesis with arginine vasopressin receptor 1b molecules.