User login
ALL therapies grow, so do the complexities of choosing the order of treatments
SAN FRANCISCO – A growing number of immunotherapy options for adults with acute lymphocytic leukemia (ALL) – rituximab, inotuzumab ozogamicin, blinatumomab and chimeric antigen receptor (CAR) T-cell therapy – have improved remission rates, but their collective effects on patient outcomes remain to be seen, David Maloney, MD, PhD, said at the National Comprehensive Cancer Network Annual Congress: Hematologic Malignancies.
The main challenge for the field is deciding when and how to use a variety of therapies, he said. “How are we going to put these together? What’s the order?” he asked. “Are we going to be able to decrease the need for allogeneic stem cell transplant? And, obviously, that’s the goal.”
About 30%-50% of adults with ALL exhibit CD20-positive cells, making them potentially treatable with rituximab. Data show a better event-free survival rate and a reduced relapse rate when rituximab is added to standard chemotherapy as compared with standard chemotherapy alone, Dr. Maloney of the clinical research division at the Fred Hutchinson Cancer Research Center, Seattle, noted (N Engl J Med. 2016 Sep 15;375[11]:1044-53). But the improvement was only “modest,” he said.
The anti-CD22 antibody inotuzumab ozogamicin has produced complete remission in 81% of relapsed or refractory ALL patients, compared with those getting standard therapy (N Engl J Med. 2016 Aug 25;375:740-53). Dr. Maloney said it seems well tolerated, but there is concern about an increase in veno-occlusive disease in patients who have undergone or will undergo an allogeneic stem cell transplant.
Blinatumomab produces moderate response rates and minimal residual disease–negative remissions, but delivery of the drug is “cumbersome,” requiring a 4-week continuous infusion, he said. The drug seems to be more effective in those with a lower burden of disease, he noted.
CAR T-cell therapy has produced MRD-negative complete responses in 94% of patients, based on results from a clinical trial at Fred Hutchinson. And using the chemotherapy drug fludarabine in combination with this therapy “dramatically” boosts the peak number of the CAR T cells and how long they persist, Dr. Maloney said. Still, CAR T-cell therapy is a work-intensive treatment requiring cells harvested from the patient, and the procedure often brings on cytokine-release syndrome and neurotoxicity, though both adverse events are typically reversible, he said.
It may be that using fewer CAR T cells can reduce toxicity without compromising treatment response, he said.
Questions remain over whether to transplant patients who are in remission after CAR T-cell therapy. “This is a hot debate,” he said. The decision will likely depend on their prior therapy, whether they’ve had a prior transplant, and the how robust the CAR T-cell expansion has been, he said.
Dr. Maloney reports financial relationships with Celgene, Gilead Sciences, Kite Pharma, and Roche.
SAN FRANCISCO – A growing number of immunotherapy options for adults with acute lymphocytic leukemia (ALL) – rituximab, inotuzumab ozogamicin, blinatumomab and chimeric antigen receptor (CAR) T-cell therapy – have improved remission rates, but their collective effects on patient outcomes remain to be seen, David Maloney, MD, PhD, said at the National Comprehensive Cancer Network Annual Congress: Hematologic Malignancies.
The main challenge for the field is deciding when and how to use a variety of therapies, he said. “How are we going to put these together? What’s the order?” he asked. “Are we going to be able to decrease the need for allogeneic stem cell transplant? And, obviously, that’s the goal.”
About 30%-50% of adults with ALL exhibit CD20-positive cells, making them potentially treatable with rituximab. Data show a better event-free survival rate and a reduced relapse rate when rituximab is added to standard chemotherapy as compared with standard chemotherapy alone, Dr. Maloney of the clinical research division at the Fred Hutchinson Cancer Research Center, Seattle, noted (N Engl J Med. 2016 Sep 15;375[11]:1044-53). But the improvement was only “modest,” he said.
The anti-CD22 antibody inotuzumab ozogamicin has produced complete remission in 81% of relapsed or refractory ALL patients, compared with those getting standard therapy (N Engl J Med. 2016 Aug 25;375:740-53). Dr. Maloney said it seems well tolerated, but there is concern about an increase in veno-occlusive disease in patients who have undergone or will undergo an allogeneic stem cell transplant.
Blinatumomab produces moderate response rates and minimal residual disease–negative remissions, but delivery of the drug is “cumbersome,” requiring a 4-week continuous infusion, he said. The drug seems to be more effective in those with a lower burden of disease, he noted.
CAR T-cell therapy has produced MRD-negative complete responses in 94% of patients, based on results from a clinical trial at Fred Hutchinson. And using the chemotherapy drug fludarabine in combination with this therapy “dramatically” boosts the peak number of the CAR T cells and how long they persist, Dr. Maloney said. Still, CAR T-cell therapy is a work-intensive treatment requiring cells harvested from the patient, and the procedure often brings on cytokine-release syndrome and neurotoxicity, though both adverse events are typically reversible, he said.
It may be that using fewer CAR T cells can reduce toxicity without compromising treatment response, he said.
Questions remain over whether to transplant patients who are in remission after CAR T-cell therapy. “This is a hot debate,” he said. The decision will likely depend on their prior therapy, whether they’ve had a prior transplant, and the how robust the CAR T-cell expansion has been, he said.
Dr. Maloney reports financial relationships with Celgene, Gilead Sciences, Kite Pharma, and Roche.
SAN FRANCISCO – A growing number of immunotherapy options for adults with acute lymphocytic leukemia (ALL) – rituximab, inotuzumab ozogamicin, blinatumomab and chimeric antigen receptor (CAR) T-cell therapy – have improved remission rates, but their collective effects on patient outcomes remain to be seen, David Maloney, MD, PhD, said at the National Comprehensive Cancer Network Annual Congress: Hematologic Malignancies.
The main challenge for the field is deciding when and how to use a variety of therapies, he said. “How are we going to put these together? What’s the order?” he asked. “Are we going to be able to decrease the need for allogeneic stem cell transplant? And, obviously, that’s the goal.”
About 30%-50% of adults with ALL exhibit CD20-positive cells, making them potentially treatable with rituximab. Data show a better event-free survival rate and a reduced relapse rate when rituximab is added to standard chemotherapy as compared with standard chemotherapy alone, Dr. Maloney of the clinical research division at the Fred Hutchinson Cancer Research Center, Seattle, noted (N Engl J Med. 2016 Sep 15;375[11]:1044-53). But the improvement was only “modest,” he said.
The anti-CD22 antibody inotuzumab ozogamicin has produced complete remission in 81% of relapsed or refractory ALL patients, compared with those getting standard therapy (N Engl J Med. 2016 Aug 25;375:740-53). Dr. Maloney said it seems well tolerated, but there is concern about an increase in veno-occlusive disease in patients who have undergone or will undergo an allogeneic stem cell transplant.
Blinatumomab produces moderate response rates and minimal residual disease–negative remissions, but delivery of the drug is “cumbersome,” requiring a 4-week continuous infusion, he said. The drug seems to be more effective in those with a lower burden of disease, he noted.
CAR T-cell therapy has produced MRD-negative complete responses in 94% of patients, based on results from a clinical trial at Fred Hutchinson. And using the chemotherapy drug fludarabine in combination with this therapy “dramatically” boosts the peak number of the CAR T cells and how long they persist, Dr. Maloney said. Still, CAR T-cell therapy is a work-intensive treatment requiring cells harvested from the patient, and the procedure often brings on cytokine-release syndrome and neurotoxicity, though both adverse events are typically reversible, he said.
It may be that using fewer CAR T cells can reduce toxicity without compromising treatment response, he said.
Questions remain over whether to transplant patients who are in remission after CAR T-cell therapy. “This is a hot debate,” he said. The decision will likely depend on their prior therapy, whether they’ve had a prior transplant, and the how robust the CAR T-cell expansion has been, he said.
Dr. Maloney reports financial relationships with Celgene, Gilead Sciences, Kite Pharma, and Roche.
EXPERT ANALYSIS FROM THE NCCN ANNUAL CONGRESS: HEMATOLOGIC MALIGNANCIES
Obesity increases risk of complications with hernia repair
Both obese and underweight patients undergoing ventral hernia repair have a significantly greater risk of complications, particularly if they have strangulated/reducible hernias, according to data published online in Surgery.
In a retrospective analysis, researchers examined data from 102,191 patients – 58.5% of whom were obese – who underwent ventral hernia repair, and found a J-shaped curve in the association between complication rates and body mass index.
Underweight patients with a BMI less than 18.5kg/m2 had a 10% risk of complications, while those of normal BMI (18.5-24.99) had the lowest complication risk: 7.7%. Complication rates then increased steadily with increasing BMI; 8.2% for overweight patients, 9.7% for the obese, 12.2% for the severely obese, 16.1% for the morbidly obese, and 19.9% for the super obese (Surgery 2017, Sep 27. http://dx.doi.org/10.1016/j.surg.2017.07.025).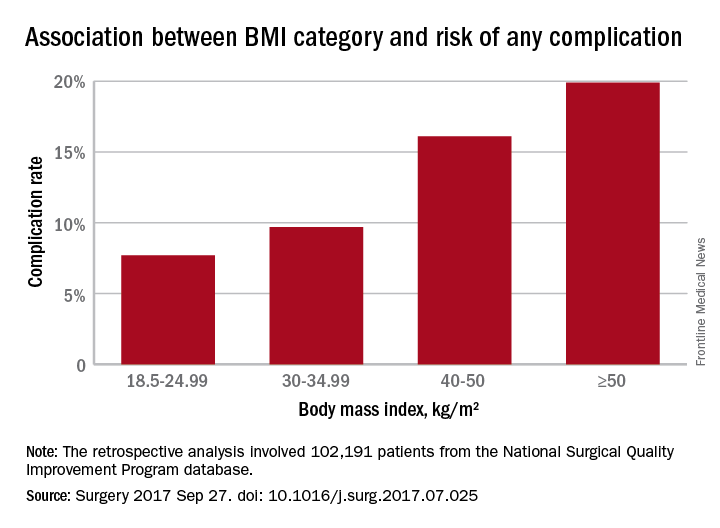
Analysis by individual medical complications showed that postoperative pneumonia, pulmonary embolism, acute renal failure, and urinary tract infection were all statistically significantly more common with increasing BMI. The risk of myocardial infarction did not differ significantly with BMI.
The researchers also examined the effects of different hernia types. The 70.3% of patients who had reducible hernias had lower complication rates overall, as well as lower rates of complications in each category of operative, medical, and respiratory, compared with the 29.7% of patients with strangulated/incarcerated hernias.
Nearly one-quarter of the patients in the study were undergoing recurrent ventral hernia repair, and these patients were more likely to have a higher BMI.
After taking into account variables such as age, smoking comorbidities, type of hernia, and type of repair, the authors concluded that the odds of having any complication increased significantly above a BMI of 30 kg/m2, using normal weight BMI as a reference. The odds were 22% higher in those with BMIs in the 30-34.99 range, 54% higher for those in the 35-39.99 range, twofold higher for those with a BMI between 40 and 50, and 2.6-fold higher above 50 kg/m2.
“Surgeons are presented with increasing numbers of obese patients, and the best way to manage ventral hernias in this population remains unclear,” the authors wrote, although they raised the possibility that laparoscopic procedures reduce the risk of some complications.
“Although the majority of VHRs performed utilize an open technique, studies have found decreased duration of stay, morbidity, and, in selected studies, even decreased recurrence using the laparoscopic approach.”
No conflicts of interest were declared.
Both obese and underweight patients undergoing ventral hernia repair have a significantly greater risk of complications, particularly if they have strangulated/reducible hernias, according to data published online in Surgery.
In a retrospective analysis, researchers examined data from 102,191 patients – 58.5% of whom were obese – who underwent ventral hernia repair, and found a J-shaped curve in the association between complication rates and body mass index.
Underweight patients with a BMI less than 18.5kg/m2 had a 10% risk of complications, while those of normal BMI (18.5-24.99) had the lowest complication risk: 7.7%. Complication rates then increased steadily with increasing BMI; 8.2% for overweight patients, 9.7% for the obese, 12.2% for the severely obese, 16.1% for the morbidly obese, and 19.9% for the super obese (Surgery 2017, Sep 27. http://dx.doi.org/10.1016/j.surg.2017.07.025).
Analysis by individual medical complications showed that postoperative pneumonia, pulmonary embolism, acute renal failure, and urinary tract infection were all statistically significantly more common with increasing BMI. The risk of myocardial infarction did not differ significantly with BMI.
The researchers also examined the effects of different hernia types. The 70.3% of patients who had reducible hernias had lower complication rates overall, as well as lower rates of complications in each category of operative, medical, and respiratory, compared with the 29.7% of patients with strangulated/incarcerated hernias.
Nearly one-quarter of the patients in the study were undergoing recurrent ventral hernia repair, and these patients were more likely to have a higher BMI.
After taking into account variables such as age, smoking comorbidities, type of hernia, and type of repair, the authors concluded that the odds of having any complication increased significantly above a BMI of 30 kg/m2, using normal weight BMI as a reference. The odds were 22% higher in those with BMIs in the 30-34.99 range, 54% higher for those in the 35-39.99 range, twofold higher for those with a BMI between 40 and 50, and 2.6-fold higher above 50 kg/m2.
“Surgeons are presented with increasing numbers of obese patients, and the best way to manage ventral hernias in this population remains unclear,” the authors wrote, although they raised the possibility that laparoscopic procedures reduce the risk of some complications.
“Although the majority of VHRs performed utilize an open technique, studies have found decreased duration of stay, morbidity, and, in selected studies, even decreased recurrence using the laparoscopic approach.”
No conflicts of interest were declared.
Both obese and underweight patients undergoing ventral hernia repair have a significantly greater risk of complications, particularly if they have strangulated/reducible hernias, according to data published online in Surgery.
In a retrospective analysis, researchers examined data from 102,191 patients – 58.5% of whom were obese – who underwent ventral hernia repair, and found a J-shaped curve in the association between complication rates and body mass index.
Underweight patients with a BMI less than 18.5kg/m2 had a 10% risk of complications, while those of normal BMI (18.5-24.99) had the lowest complication risk: 7.7%. Complication rates then increased steadily with increasing BMI; 8.2% for overweight patients, 9.7% for the obese, 12.2% for the severely obese, 16.1% for the morbidly obese, and 19.9% for the super obese (Surgery 2017, Sep 27. http://dx.doi.org/10.1016/j.surg.2017.07.025).
Analysis by individual medical complications showed that postoperative pneumonia, pulmonary embolism, acute renal failure, and urinary tract infection were all statistically significantly more common with increasing BMI. The risk of myocardial infarction did not differ significantly with BMI.
The researchers also examined the effects of different hernia types. The 70.3% of patients who had reducible hernias had lower complication rates overall, as well as lower rates of complications in each category of operative, medical, and respiratory, compared with the 29.7% of patients with strangulated/incarcerated hernias.
Nearly one-quarter of the patients in the study were undergoing recurrent ventral hernia repair, and these patients were more likely to have a higher BMI.
After taking into account variables such as age, smoking comorbidities, type of hernia, and type of repair, the authors concluded that the odds of having any complication increased significantly above a BMI of 30 kg/m2, using normal weight BMI as a reference. The odds were 22% higher in those with BMIs in the 30-34.99 range, 54% higher for those in the 35-39.99 range, twofold higher for those with a BMI between 40 and 50, and 2.6-fold higher above 50 kg/m2.
“Surgeons are presented with increasing numbers of obese patients, and the best way to manage ventral hernias in this population remains unclear,” the authors wrote, although they raised the possibility that laparoscopic procedures reduce the risk of some complications.
“Although the majority of VHRs performed utilize an open technique, studies have found decreased duration of stay, morbidity, and, in selected studies, even decreased recurrence using the laparoscopic approach.”
No conflicts of interest were declared.
FROM SURGERY
Key clinical point: Obesity, as well as underweight, are associated with significantly higher rates of complications with ventral repair.
Major finding: Complication rates increased with increasing BMI; 8.2% for overweight patients, 9.7% for the obese, 12.2% for the severely obese, 16.1% for the morbidly obese, and 19.9% for the super obese.
Data source: Retrospective analysis of 102,191 patients who underwent ventral hernia repair.
Disclosures: No conflicts of interest were declared.
To boost HCV testing in baby boomers, offer the option
Rates of hepatitis C testing increased among New York adults born between 1945 and 1965 after the state passed a law mandating that health care providers offer HCV testing to people of that age, according to a report from the Centers for Disease Control and Prevention.
In 2013, the year before the new law became effective on Jan. 1, 2014, the total of specimens collected for HCV testing from the 106 clinics that reported data for both 2013 and 2014 was 538,229. In the following year after the law became effective, 813,492 samples were collected from the same clinics, an increase of 51.1% over 2013. The rate of increase for New York Medicaid recipients was similar at 52%.
“This report highlights the potential for state laws to promote HCV testing and the utility of HCV surveillance and Medicaid claims data to monitor the quality of HCV testing and linkage to care for HCV-infected persons,” the CDC investigators concluded.
Find the full report in the MMWR (doi: 10.15585/mmwr.mm6638a3).
Rates of hepatitis C testing increased among New York adults born between 1945 and 1965 after the state passed a law mandating that health care providers offer HCV testing to people of that age, according to a report from the Centers for Disease Control and Prevention.
In 2013, the year before the new law became effective on Jan. 1, 2014, the total of specimens collected for HCV testing from the 106 clinics that reported data for both 2013 and 2014 was 538,229. In the following year after the law became effective, 813,492 samples were collected from the same clinics, an increase of 51.1% over 2013. The rate of increase for New York Medicaid recipients was similar at 52%.
“This report highlights the potential for state laws to promote HCV testing and the utility of HCV surveillance and Medicaid claims data to monitor the quality of HCV testing and linkage to care for HCV-infected persons,” the CDC investigators concluded.
Find the full report in the MMWR (doi: 10.15585/mmwr.mm6638a3).
Rates of hepatitis C testing increased among New York adults born between 1945 and 1965 after the state passed a law mandating that health care providers offer HCV testing to people of that age, according to a report from the Centers for Disease Control and Prevention.
In 2013, the year before the new law became effective on Jan. 1, 2014, the total of specimens collected for HCV testing from the 106 clinics that reported data for both 2013 and 2014 was 538,229. In the following year after the law became effective, 813,492 samples were collected from the same clinics, an increase of 51.1% over 2013. The rate of increase for New York Medicaid recipients was similar at 52%.
“This report highlights the potential for state laws to promote HCV testing and the utility of HCV surveillance and Medicaid claims data to monitor the quality of HCV testing and linkage to care for HCV-infected persons,” the CDC investigators concluded.
Find the full report in the MMWR (doi: 10.15585/mmwr.mm6638a3).
FROM THE MMWR
Clinical trial: Mesh weights compared for ventral hernia repair
A clinical trial comparing heavy- and medium-weight surgical mesh for ventral hernia repairs is recruiting patients.
The Long-term Results of Heavy Weight Versus Medium Weight Mesh in Ventral Hernia Repair trial will determine if mesh weight has an impact on postoperative pain, ventral hernia recurrence, incidence of deep wound infection, and overall quality of life following ventral hernia repair with mesh.
Patients will be included if they have a ventral hernia, are 18 years of age or older, have a defect classified as CDC wound class 1, are able to achieve midline fascial closure, have a hernia defect width less than or equal to 20 cm, can tolerate general anesthesia, and can give informed consent. Patients will be excluded if they have undergone emergent ventral hernia repair, undergone laparoscopic or robotic ventral hernia repair, undergone staged repair of their ventral hernia, or are pregnant at the time of the surgery.
The primary outcome of this trial is pain that will be measured via the NIH Promis 3A Pain instrument in 1 year postoperatively. Other outcomes include hernia recurrence, to be determined via the Ventral Hernia Recurrence Inventory; the occurrence of a deep wound infection, to be determined by physical examination and/or computed tomography scanning; and quality of life measured by the HerQLes questionnaire.
Find more information at clinicaltrials.gov.
A clinical trial comparing heavy- and medium-weight surgical mesh for ventral hernia repairs is recruiting patients.
The Long-term Results of Heavy Weight Versus Medium Weight Mesh in Ventral Hernia Repair trial will determine if mesh weight has an impact on postoperative pain, ventral hernia recurrence, incidence of deep wound infection, and overall quality of life following ventral hernia repair with mesh.
Patients will be included if they have a ventral hernia, are 18 years of age or older, have a defect classified as CDC wound class 1, are able to achieve midline fascial closure, have a hernia defect width less than or equal to 20 cm, can tolerate general anesthesia, and can give informed consent. Patients will be excluded if they have undergone emergent ventral hernia repair, undergone laparoscopic or robotic ventral hernia repair, undergone staged repair of their ventral hernia, or are pregnant at the time of the surgery.
The primary outcome of this trial is pain that will be measured via the NIH Promis 3A Pain instrument in 1 year postoperatively. Other outcomes include hernia recurrence, to be determined via the Ventral Hernia Recurrence Inventory; the occurrence of a deep wound infection, to be determined by physical examination and/or computed tomography scanning; and quality of life measured by the HerQLes questionnaire.
Find more information at clinicaltrials.gov.
A clinical trial comparing heavy- and medium-weight surgical mesh for ventral hernia repairs is recruiting patients.
The Long-term Results of Heavy Weight Versus Medium Weight Mesh in Ventral Hernia Repair trial will determine if mesh weight has an impact on postoperative pain, ventral hernia recurrence, incidence of deep wound infection, and overall quality of life following ventral hernia repair with mesh.
Patients will be included if they have a ventral hernia, are 18 years of age or older, have a defect classified as CDC wound class 1, are able to achieve midline fascial closure, have a hernia defect width less than or equal to 20 cm, can tolerate general anesthesia, and can give informed consent. Patients will be excluded if they have undergone emergent ventral hernia repair, undergone laparoscopic or robotic ventral hernia repair, undergone staged repair of their ventral hernia, or are pregnant at the time of the surgery.
The primary outcome of this trial is pain that will be measured via the NIH Promis 3A Pain instrument in 1 year postoperatively. Other outcomes include hernia recurrence, to be determined via the Ventral Hernia Recurrence Inventory; the occurrence of a deep wound infection, to be determined by physical examination and/or computed tomography scanning; and quality of life measured by the HerQLes questionnaire.
Find more information at clinicaltrials.gov.
FROM CLINICALTRIALS.GOV
Gap in osteoporosis diagnosis and treatment stirs concern
DENVER – A recent study of Medicare recipients who experienced a hip fracture found that just 19% of them had been receiving a bone-active osteoporosis treatment before the fracture occurred. That number reveals an alarming trend of underdiagnosis of osteoporosis.
But that number – from a 2016 study in JAMA Internal Medicine – is just the start. After the fracture, the percentage of women receiving treatment barely changed, rising to just 21% (JAMA Intern Med. 2016 Oct 1;176[10]:1531-8).
This trend of under-diagnosis and treatment of osteoporosis has occurred in spite of the fact that effective therapy exists to reduce future fractures. In fact, a single dose of zoledronic acid has been shown to reduce clinical fractures by 35%, and mortality by 28% over an average 2-year follow-up (N Engl J Med. 2007;357:1799-1809).
“To me, this is really a shame,” Douglas Bauer, MD, professor of medicine at the University of California, San Francisco, said at a session at the annual meeting of the American Society for Bone and Mineral Research that was dedicated to the issue.
It remains unclear why the fracture rate declined despite low levels of diagnosis and treatment. Some researchers, such as Bo Abrahamsen, MD, PhD, suggest there is a population effect. At the ASBMR annual meeting, Dr. Abrahamsen of the University of Southern Denmark, Odense, noted that in the western world, women born in the 1930s appear to be less prone to fractures than other birth cohorts, and it could be that this group contributed to the decline in fracture rates. A Danish study, he said, seems to confirm this idea. As other birth cohorts age, the numbers could well get worse.
And that’s worrying, because a gap in treatment and diagnosis of osteoporosis could lead to an epidemic of new fractures. “We need to address this crisis in health care for a very preventable disease,” Meryl LeBoff, MD, director of the skeletal health and osteoporosis center and bone density unit at Brigham and Women’s Hospital, Boston, said in an interview.
There are several likely causes of declining treatment and diagnosis rates. In 2003, a report surfaced that osteonecrosis of the jaw occurred in cancer patients taking bisphosphonates to prevent metastasis to bone. Those patients received doses that were far higher than the typical osteoporosis patient, but the reports spooked patients. In 2005, researchers pinned another rare side effect on bisphosphonates – atypical femur fractures.
Other factors contributed. In 2007, the Centers for Medicare & Medicaid Services cut the reimbursement rate for bone densitometry (DXA) testing, which has led to a drop in the number of tests, from a peak of 17.9% of women over age 65 years having been tested in 2009 to 14.8% in 2014. That has complicated efforts to diagnose osteoporosis, and may be affecting patients’ willingness to undergo therapy. A patient may go to the hospital for a hip fracture, but without a bone density test to raise concerns, she may write the fracture off as an accident. A DXA test that returns an abnormal value can change that. “If you have low BMD and a fracture, you have a worse prognosis and a higher risk of the next fracture. It’s much easier to convince patients [to begin therapy],” said John Carey, MD, a rheumatologist at Galway (Ireland) University Hospitals and president of the International Society for Clinical Densitometry.
Others emphasized the need to get DXA reimbursement raised, at least to a point where physicians can break even on the test. “Unfortunately, what’s happening is that as DXA reimbursement goes down, physicians are investing less in the education of their staff according to current guidelines,” Dr. Lewiecki said. He noted that there is draft legislation in Congress to raise DXA reimbursement (H.R. 1898), although it is currently in committee.
The forces that create the treatment and diagnostic gap aren’t simple, and no single strategy is likely to fix the problem. A number have been proposed.
FLS programs seek to quickly identify and provide treatment and monitoring for individuals who are at high risk for additional fractures. The programs identify patients who have suffered a fragility fracture and place them into a coordinated care model. For example, if a radiologist identified a patient of concern, she can be immediately referred to the FLS for quick follow-up, to include bone density scans and other steps to ensure proper diagnosis and treatment. In the absence of an FLS, it could be months before a patient is seen again, if a radiologist refers her at all. “You lose patients. But if they are tied into a coordination of care model, the patient is brought in immediately and they get the attention and awareness. They’re not just lost,” said Debbie Zeldow, executive director of the National Bone Health Alliance (NBHA).
Despite their effectiveness, FLS programs could be a tough sell for the upfront investment they require. To help organizations determine the cost-effectiveness of an FLS, the National Bone Health Alliance has developed a return on investment calculator.
And in fact, FLS programs are gaining traction, according to Ms. Zeldow. NBHA has posted a variety of resources for establishing an FLS on its website, which contains webinars and other resources, including the text of patient flyers produced by Kaiser Permanente translated into many languages. “There’s been a huge jump in interest in implementation. I’m getting calls on a daily basis from sites that want to implement an FLS. People are being pinged for readmission, and they’re looking for ways for their hospital or practice to improve outcomes. It’s definitely a way for hospitals to differentiate themselves around care,” Ms. Zeldow said in an interview.
FLS programs reduce secondary fractures, but the ultimate goal is to catch osteoporosis earlier and prevent even first-time fractures. That will require better communication with primary care providers, who bear the brunt of osteoporosis diagnosis and care.
“We need to do a better job of reducing barriers for primary care doctors, to try to minimize unnecessary treatment complexity, and address the fact that this is one of the many prevention issues that physicians are asked to manage, and all that in an increasingly time-constrained world,” Dr. Bauer said.
With that goal in mind, the American College of Physicians released new guidelines in May (Ann Intern Med. 2017;166[11]:818-39). They were intended to identify first-line therapies and simplify matters for primary care physicians, but they drew the ire of many endocrinologists for being too general. NBHA has formed an ACP guideline working group that aims to refine those guidelines. The committee is drafting a statement and a document for patients to help clarify the guidelines, particularly with respect to high-risk patients. The committee also seeks to avoid any rancor. “We’ve made a concerted effort to include primary care doctors, to make sure there’s not a disconnect between experts and general practitioners. We want to make sure it’s a useful tool and we’re not just bashing the guidelines,” Ms. Zeldow said.
Another way to help overburdened primary care providers is to provide training for physicians, especially in underserved areas. The National Osteoporosis Foundation’s TeleECHO program is a remote training service that can help interested local providers elevate their knowledge so they can become a local osteoporosis expert. “Patients can get better care, at more convenience and at lower cost, rather than having to travel to a specialist center far away,” Dr. Lewiecki said.
Patient concerns about side effects, the changing health care climate, and the challenges facing primary care providers add up to difficult environment for countering the reductions in diagnosis and treatment of osteoporosis, but Dr. Lewiecki is hopeful. “Fracture liaison services combined with better education of health care providers through new educational methods I think have great promise. It’s just a matter of getting those things online,” he said.
Dr. Carey has been a speaker for Roche, Pfizer, and AbbVie. Dr. Lewiecki has consulted for Amgen. Dr. Leder has received funding from Lilly and Amgen, and has been a consultant for Lilly, Amgen, and Radius. Dr. Bauer and Ms. Zeldow reported having no financial disclosures.
DENVER – A recent study of Medicare recipients who experienced a hip fracture found that just 19% of them had been receiving a bone-active osteoporosis treatment before the fracture occurred. That number reveals an alarming trend of underdiagnosis of osteoporosis.
But that number – from a 2016 study in JAMA Internal Medicine – is just the start. After the fracture, the percentage of women receiving treatment barely changed, rising to just 21% (JAMA Intern Med. 2016 Oct 1;176[10]:1531-8).
This trend of under-diagnosis and treatment of osteoporosis has occurred in spite of the fact that effective therapy exists to reduce future fractures. In fact, a single dose of zoledronic acid has been shown to reduce clinical fractures by 35%, and mortality by 28% over an average 2-year follow-up (N Engl J Med. 2007;357:1799-1809).
“To me, this is really a shame,” Douglas Bauer, MD, professor of medicine at the University of California, San Francisco, said at a session at the annual meeting of the American Society for Bone and Mineral Research that was dedicated to the issue.
It remains unclear why the fracture rate declined despite low levels of diagnosis and treatment. Some researchers, such as Bo Abrahamsen, MD, PhD, suggest there is a population effect. At the ASBMR annual meeting, Dr. Abrahamsen of the University of Southern Denmark, Odense, noted that in the western world, women born in the 1930s appear to be less prone to fractures than other birth cohorts, and it could be that this group contributed to the decline in fracture rates. A Danish study, he said, seems to confirm this idea. As other birth cohorts age, the numbers could well get worse.
And that’s worrying, because a gap in treatment and diagnosis of osteoporosis could lead to an epidemic of new fractures. “We need to address this crisis in health care for a very preventable disease,” Meryl LeBoff, MD, director of the skeletal health and osteoporosis center and bone density unit at Brigham and Women’s Hospital, Boston, said in an interview.
There are several likely causes of declining treatment and diagnosis rates. In 2003, a report surfaced that osteonecrosis of the jaw occurred in cancer patients taking bisphosphonates to prevent metastasis to bone. Those patients received doses that were far higher than the typical osteoporosis patient, but the reports spooked patients. In 2005, researchers pinned another rare side effect on bisphosphonates – atypical femur fractures.
Other factors contributed. In 2007, the Centers for Medicare & Medicaid Services cut the reimbursement rate for bone densitometry (DXA) testing, which has led to a drop in the number of tests, from a peak of 17.9% of women over age 65 years having been tested in 2009 to 14.8% in 2014. That has complicated efforts to diagnose osteoporosis, and may be affecting patients’ willingness to undergo therapy. A patient may go to the hospital for a hip fracture, but without a bone density test to raise concerns, she may write the fracture off as an accident. A DXA test that returns an abnormal value can change that. “If you have low BMD and a fracture, you have a worse prognosis and a higher risk of the next fracture. It’s much easier to convince patients [to begin therapy],” said John Carey, MD, a rheumatologist at Galway (Ireland) University Hospitals and president of the International Society for Clinical Densitometry.
Others emphasized the need to get DXA reimbursement raised, at least to a point where physicians can break even on the test. “Unfortunately, what’s happening is that as DXA reimbursement goes down, physicians are investing less in the education of their staff according to current guidelines,” Dr. Lewiecki said. He noted that there is draft legislation in Congress to raise DXA reimbursement (H.R. 1898), although it is currently in committee.
The forces that create the treatment and diagnostic gap aren’t simple, and no single strategy is likely to fix the problem. A number have been proposed.
FLS programs seek to quickly identify and provide treatment and monitoring for individuals who are at high risk for additional fractures. The programs identify patients who have suffered a fragility fracture and place them into a coordinated care model. For example, if a radiologist identified a patient of concern, she can be immediately referred to the FLS for quick follow-up, to include bone density scans and other steps to ensure proper diagnosis and treatment. In the absence of an FLS, it could be months before a patient is seen again, if a radiologist refers her at all. “You lose patients. But if they are tied into a coordination of care model, the patient is brought in immediately and they get the attention and awareness. They’re not just lost,” said Debbie Zeldow, executive director of the National Bone Health Alliance (NBHA).
Despite their effectiveness, FLS programs could be a tough sell for the upfront investment they require. To help organizations determine the cost-effectiveness of an FLS, the National Bone Health Alliance has developed a return on investment calculator.
And in fact, FLS programs are gaining traction, according to Ms. Zeldow. NBHA has posted a variety of resources for establishing an FLS on its website, which contains webinars and other resources, including the text of patient flyers produced by Kaiser Permanente translated into many languages. “There’s been a huge jump in interest in implementation. I’m getting calls on a daily basis from sites that want to implement an FLS. People are being pinged for readmission, and they’re looking for ways for their hospital or practice to improve outcomes. It’s definitely a way for hospitals to differentiate themselves around care,” Ms. Zeldow said in an interview.
FLS programs reduce secondary fractures, but the ultimate goal is to catch osteoporosis earlier and prevent even first-time fractures. That will require better communication with primary care providers, who bear the brunt of osteoporosis diagnosis and care.
“We need to do a better job of reducing barriers for primary care doctors, to try to minimize unnecessary treatment complexity, and address the fact that this is one of the many prevention issues that physicians are asked to manage, and all that in an increasingly time-constrained world,” Dr. Bauer said.
With that goal in mind, the American College of Physicians released new guidelines in May (Ann Intern Med. 2017;166[11]:818-39). They were intended to identify first-line therapies and simplify matters for primary care physicians, but they drew the ire of many endocrinologists for being too general. NBHA has formed an ACP guideline working group that aims to refine those guidelines. The committee is drafting a statement and a document for patients to help clarify the guidelines, particularly with respect to high-risk patients. The committee also seeks to avoid any rancor. “We’ve made a concerted effort to include primary care doctors, to make sure there’s not a disconnect between experts and general practitioners. We want to make sure it’s a useful tool and we’re not just bashing the guidelines,” Ms. Zeldow said.
Another way to help overburdened primary care providers is to provide training for physicians, especially in underserved areas. The National Osteoporosis Foundation’s TeleECHO program is a remote training service that can help interested local providers elevate their knowledge so they can become a local osteoporosis expert. “Patients can get better care, at more convenience and at lower cost, rather than having to travel to a specialist center far away,” Dr. Lewiecki said.
Patient concerns about side effects, the changing health care climate, and the challenges facing primary care providers add up to difficult environment for countering the reductions in diagnosis and treatment of osteoporosis, but Dr. Lewiecki is hopeful. “Fracture liaison services combined with better education of health care providers through new educational methods I think have great promise. It’s just a matter of getting those things online,” he said.
Dr. Carey has been a speaker for Roche, Pfizer, and AbbVie. Dr. Lewiecki has consulted for Amgen. Dr. Leder has received funding from Lilly and Amgen, and has been a consultant for Lilly, Amgen, and Radius. Dr. Bauer and Ms. Zeldow reported having no financial disclosures.
DENVER – A recent study of Medicare recipients who experienced a hip fracture found that just 19% of them had been receiving a bone-active osteoporosis treatment before the fracture occurred. That number reveals an alarming trend of underdiagnosis of osteoporosis.
But that number – from a 2016 study in JAMA Internal Medicine – is just the start. After the fracture, the percentage of women receiving treatment barely changed, rising to just 21% (JAMA Intern Med. 2016 Oct 1;176[10]:1531-8).
This trend of under-diagnosis and treatment of osteoporosis has occurred in spite of the fact that effective therapy exists to reduce future fractures. In fact, a single dose of zoledronic acid has been shown to reduce clinical fractures by 35%, and mortality by 28% over an average 2-year follow-up (N Engl J Med. 2007;357:1799-1809).
“To me, this is really a shame,” Douglas Bauer, MD, professor of medicine at the University of California, San Francisco, said at a session at the annual meeting of the American Society for Bone and Mineral Research that was dedicated to the issue.
It remains unclear why the fracture rate declined despite low levels of diagnosis and treatment. Some researchers, such as Bo Abrahamsen, MD, PhD, suggest there is a population effect. At the ASBMR annual meeting, Dr. Abrahamsen of the University of Southern Denmark, Odense, noted that in the western world, women born in the 1930s appear to be less prone to fractures than other birth cohorts, and it could be that this group contributed to the decline in fracture rates. A Danish study, he said, seems to confirm this idea. As other birth cohorts age, the numbers could well get worse.
And that’s worrying, because a gap in treatment and diagnosis of osteoporosis could lead to an epidemic of new fractures. “We need to address this crisis in health care for a very preventable disease,” Meryl LeBoff, MD, director of the skeletal health and osteoporosis center and bone density unit at Brigham and Women’s Hospital, Boston, said in an interview.
There are several likely causes of declining treatment and diagnosis rates. In 2003, a report surfaced that osteonecrosis of the jaw occurred in cancer patients taking bisphosphonates to prevent metastasis to bone. Those patients received doses that were far higher than the typical osteoporosis patient, but the reports spooked patients. In 2005, researchers pinned another rare side effect on bisphosphonates – atypical femur fractures.
Other factors contributed. In 2007, the Centers for Medicare & Medicaid Services cut the reimbursement rate for bone densitometry (DXA) testing, which has led to a drop in the number of tests, from a peak of 17.9% of women over age 65 years having been tested in 2009 to 14.8% in 2014. That has complicated efforts to diagnose osteoporosis, and may be affecting patients’ willingness to undergo therapy. A patient may go to the hospital for a hip fracture, but without a bone density test to raise concerns, she may write the fracture off as an accident. A DXA test that returns an abnormal value can change that. “If you have low BMD and a fracture, you have a worse prognosis and a higher risk of the next fracture. It’s much easier to convince patients [to begin therapy],” said John Carey, MD, a rheumatologist at Galway (Ireland) University Hospitals and president of the International Society for Clinical Densitometry.
Others emphasized the need to get DXA reimbursement raised, at least to a point where physicians can break even on the test. “Unfortunately, what’s happening is that as DXA reimbursement goes down, physicians are investing less in the education of their staff according to current guidelines,” Dr. Lewiecki said. He noted that there is draft legislation in Congress to raise DXA reimbursement (H.R. 1898), although it is currently in committee.
The forces that create the treatment and diagnostic gap aren’t simple, and no single strategy is likely to fix the problem. A number have been proposed.
FLS programs seek to quickly identify and provide treatment and monitoring for individuals who are at high risk for additional fractures. The programs identify patients who have suffered a fragility fracture and place them into a coordinated care model. For example, if a radiologist identified a patient of concern, she can be immediately referred to the FLS for quick follow-up, to include bone density scans and other steps to ensure proper diagnosis and treatment. In the absence of an FLS, it could be months before a patient is seen again, if a radiologist refers her at all. “You lose patients. But if they are tied into a coordination of care model, the patient is brought in immediately and they get the attention and awareness. They’re not just lost,” said Debbie Zeldow, executive director of the National Bone Health Alliance (NBHA).
Despite their effectiveness, FLS programs could be a tough sell for the upfront investment they require. To help organizations determine the cost-effectiveness of an FLS, the National Bone Health Alliance has developed a return on investment calculator.
And in fact, FLS programs are gaining traction, according to Ms. Zeldow. NBHA has posted a variety of resources for establishing an FLS on its website, which contains webinars and other resources, including the text of patient flyers produced by Kaiser Permanente translated into many languages. “There’s been a huge jump in interest in implementation. I’m getting calls on a daily basis from sites that want to implement an FLS. People are being pinged for readmission, and they’re looking for ways for their hospital or practice to improve outcomes. It’s definitely a way for hospitals to differentiate themselves around care,” Ms. Zeldow said in an interview.
FLS programs reduce secondary fractures, but the ultimate goal is to catch osteoporosis earlier and prevent even first-time fractures. That will require better communication with primary care providers, who bear the brunt of osteoporosis diagnosis and care.
“We need to do a better job of reducing barriers for primary care doctors, to try to minimize unnecessary treatment complexity, and address the fact that this is one of the many prevention issues that physicians are asked to manage, and all that in an increasingly time-constrained world,” Dr. Bauer said.
With that goal in mind, the American College of Physicians released new guidelines in May (Ann Intern Med. 2017;166[11]:818-39). They were intended to identify first-line therapies and simplify matters for primary care physicians, but they drew the ire of many endocrinologists for being too general. NBHA has formed an ACP guideline working group that aims to refine those guidelines. The committee is drafting a statement and a document for patients to help clarify the guidelines, particularly with respect to high-risk patients. The committee also seeks to avoid any rancor. “We’ve made a concerted effort to include primary care doctors, to make sure there’s not a disconnect between experts and general practitioners. We want to make sure it’s a useful tool and we’re not just bashing the guidelines,” Ms. Zeldow said.
Another way to help overburdened primary care providers is to provide training for physicians, especially in underserved areas. The National Osteoporosis Foundation’s TeleECHO program is a remote training service that can help interested local providers elevate their knowledge so they can become a local osteoporosis expert. “Patients can get better care, at more convenience and at lower cost, rather than having to travel to a specialist center far away,” Dr. Lewiecki said.
Patient concerns about side effects, the changing health care climate, and the challenges facing primary care providers add up to difficult environment for countering the reductions in diagnosis and treatment of osteoporosis, but Dr. Lewiecki is hopeful. “Fracture liaison services combined with better education of health care providers through new educational methods I think have great promise. It’s just a matter of getting those things online,” he said.
Dr. Carey has been a speaker for Roche, Pfizer, and AbbVie. Dr. Lewiecki has consulted for Amgen. Dr. Leder has received funding from Lilly and Amgen, and has been a consultant for Lilly, Amgen, and Radius. Dr. Bauer and Ms. Zeldow reported having no financial disclosures.
AT ASBMR
Dabrafenib/trametinib bests docetaxel for advanced NSCLC in indirect comparison
CHICAGO – Compared with docetaxel in matched external controls, combination therapy with dabrafenib and trametinib was associated with significantly prolonged progression-free and overall survival in previously treated patients with metastatic non–small cell lung cancer in a phase 2 trial.
Median progression-free survival (PFS) was 9.7 months in 57 patients in an open-label, multicenter phase 2 trial that investigated dabrafenib/trametinib treatment for metastatic BRAF V600E–mutated NSCLC, compared with 4.2 months in 290 patients treated with docetaxel in the randomized phase 3 CheckMate057 trial, which compared nivolumab and docetaxel in similar patients (hazard ratio, 0.32). Overall survival in the groups was 19.2 vs. 9.3 months, respectively (HR, 0.41), Junlong Li, MD, of Analysis Group, Boston, reported at the Chicago Multidisciplinary Symposium in Thoracic Oncology.
Patients treated with the combination of dabrafenib and trametinib also had a significantly higher overall response rate (61% vs. 12%) and disease control rate (77% vs. 55%), Dr. Li said.
Patient-level data for the combination therapy patients and summary data for the docetaxel-treated patients were used for the current analysis. Patients and controls were matched based on age, sex, race, smoking history, performance score, tumor histology, prior regimens, prior radiotherapy, and prior maintenance therapy. The two trials used for the analysis (NCT01336634 and CheckMate 057) were comparable in design and inclusion/exclusion criteria, and both used RECIST v1.1 to evaluate response to therapy.
“In the absence of head-to-head trials ... this study contributes some comparative efficacy evidence in this area,” Dr. Li concluded.
Invited discussant, Thomas Eldridge Stinchcombe, MD, of Duke University, Durham, N.C., said that the findings are unsurprising but important in that they are confirmatory.
Dr. Li is a consultant for Novartis, which sponsored the analysis.
CHICAGO – Compared with docetaxel in matched external controls, combination therapy with dabrafenib and trametinib was associated with significantly prolonged progression-free and overall survival in previously treated patients with metastatic non–small cell lung cancer in a phase 2 trial.
Median progression-free survival (PFS) was 9.7 months in 57 patients in an open-label, multicenter phase 2 trial that investigated dabrafenib/trametinib treatment for metastatic BRAF V600E–mutated NSCLC, compared with 4.2 months in 290 patients treated with docetaxel in the randomized phase 3 CheckMate057 trial, which compared nivolumab and docetaxel in similar patients (hazard ratio, 0.32). Overall survival in the groups was 19.2 vs. 9.3 months, respectively (HR, 0.41), Junlong Li, MD, of Analysis Group, Boston, reported at the Chicago Multidisciplinary Symposium in Thoracic Oncology.
Patients treated with the combination of dabrafenib and trametinib also had a significantly higher overall response rate (61% vs. 12%) and disease control rate (77% vs. 55%), Dr. Li said.
Patient-level data for the combination therapy patients and summary data for the docetaxel-treated patients were used for the current analysis. Patients and controls were matched based on age, sex, race, smoking history, performance score, tumor histology, prior regimens, prior radiotherapy, and prior maintenance therapy. The two trials used for the analysis (NCT01336634 and CheckMate 057) were comparable in design and inclusion/exclusion criteria, and both used RECIST v1.1 to evaluate response to therapy.
“In the absence of head-to-head trials ... this study contributes some comparative efficacy evidence in this area,” Dr. Li concluded.
Invited discussant, Thomas Eldridge Stinchcombe, MD, of Duke University, Durham, N.C., said that the findings are unsurprising but important in that they are confirmatory.
Dr. Li is a consultant for Novartis, which sponsored the analysis.
CHICAGO – Compared with docetaxel in matched external controls, combination therapy with dabrafenib and trametinib was associated with significantly prolonged progression-free and overall survival in previously treated patients with metastatic non–small cell lung cancer in a phase 2 trial.
Median progression-free survival (PFS) was 9.7 months in 57 patients in an open-label, multicenter phase 2 trial that investigated dabrafenib/trametinib treatment for metastatic BRAF V600E–mutated NSCLC, compared with 4.2 months in 290 patients treated with docetaxel in the randomized phase 3 CheckMate057 trial, which compared nivolumab and docetaxel in similar patients (hazard ratio, 0.32). Overall survival in the groups was 19.2 vs. 9.3 months, respectively (HR, 0.41), Junlong Li, MD, of Analysis Group, Boston, reported at the Chicago Multidisciplinary Symposium in Thoracic Oncology.
Patients treated with the combination of dabrafenib and trametinib also had a significantly higher overall response rate (61% vs. 12%) and disease control rate (77% vs. 55%), Dr. Li said.
Patient-level data for the combination therapy patients and summary data for the docetaxel-treated patients were used for the current analysis. Patients and controls were matched based on age, sex, race, smoking history, performance score, tumor histology, prior regimens, prior radiotherapy, and prior maintenance therapy. The two trials used for the analysis (NCT01336634 and CheckMate 057) were comparable in design and inclusion/exclusion criteria, and both used RECIST v1.1 to evaluate response to therapy.
“In the absence of head-to-head trials ... this study contributes some comparative efficacy evidence in this area,” Dr. Li concluded.
Invited discussant, Thomas Eldridge Stinchcombe, MD, of Duke University, Durham, N.C., said that the findings are unsurprising but important in that they are confirmatory.
Dr. Li is a consultant for Novartis, which sponsored the analysis.
AT A SYMPOSIUM IN THORACIC ONCOLOGY
Key clinical point:
Major finding: Median PFS with dabrafenib and trametinib vs. docetaxel: 9.7 vs. 4.2 months (HR, 0.32); overall survival: 19.2 vs. 9.3 months (HR, 0.41).
Data source: An adjusted indirect comparison of data from 347 patients from two separate studies.
Disclosures: Dr. Li is a consultant for Novartis, which sponsored the analysis.
Sneak Peek: Journal of Hospital Medicine – Oct. 2017
BACKGROUND: Hospitalized patients frequently report poor sleep, partly due to the inpatient environment. In-hospital sound and light levels are not well described on non–intensive care unit (non-ICU) wards. Although non-ICU wards may have lower average and peak noise levels, sound level changes (SLCs), which are important in disrupting sleep, may still be a substantial problem.
OBJECTIVE: To compare ambient sound and light levels, including SLCs, in ICU and non-ICU environments.
DESIGN: Observational study.
SETTING: Tertiary-care hospital.
MEASUREMENTS: Sound measurements of 0.5 Hz were analyzed to provide average hourly sound levels, sound peaks, and SLCs greater than or equal to 17.5 decibels (dB). For light data, measurements taken at 2-minute intervals provided average and maximum light levels.
RESULTS: The ICU rooms were louder than non-ICU wards; hourly averages ranged from 56.1 plus or minus 1.3 dB to 60.3 plus or minus 1.7 dB in the ICU, 47.3 plus or minus 3.7 dB to 55.1 plus or minus 3.7 dB on the telemetry floor, and 44.6 plus or minus 2.1 dB to 53.7 plus or minus 3.6 dB on the general ward. However, SLCs greater than or equal to 17.5 dB were not statistically different (ICU, 203.9 plus or minus 28.8 times; non-ICU, 270.9 plus or minus 39.5; P = 0.11). In both ICU and non-ICU wards, average daytime light levels were less than 250 lux, and peak light levels occurred in the afternoon and early evening.
CONCLUSIONS: While quieter, non-ICU wards have as many SLCs as ICUs do, which has implications for quality improvement measurements. Efforts to further reduce average noise levels might be counterproductive. Light levels in the hospital (ICU and non-ICU) may not be optimal for maintenance of a normal circadian rhythm for most people.
Read the entire article in the Journal of Hospital Medicine.
Also in JHM this month
Associations of physician empathy with patient anxiety and ratings of communication in hospital admission encounters
AUTHORS: Rachel Weiss, MD, Eric Vittinghoff, PhD, MPH, Margaret C. Fang, MD, MPH, Jenica E. W. Cimino, Kristen Adams Chasteen, MD, Robert M. Arnold, MD, Andrew D. Auerbach, MD, Wendy G. Anderson, MD, MS
A concise tool for measuring care coordination from the provider’s perspective in the hospital setting
AUTHORS: Christine M. Weston, PhD, and Sehyo Yune, MD, Eric B. Bass, MD, MPH, Scott A. Berkowitz, MD, MBA, Daniel J. Brotman, MD, Amy Deutschendorf, MS, RN, ACNS-BC, Eric E. Howell, MD, Melissa B. Richardson, MBA Carol Sylvester, RN, MS, Albert W. Wu, MD, MPH
Post–intensive care unit psychiatric comorbidity and quality of life
AUTHORS: Sophia Wang, MD, and Chris Mosher, MD, Anthony J. Perkins, MS, Sujuan Gao, PhD, Sue Lasiter, RN, PhD, Sikandar Khan, MD, Malaz Boustani, MD, MPH, Babar Khan, MD, MS
An opportunity to improve Medicare’s planned readmissions measure
AUTHORS: Chad Ellimoottil, MD, MS, Roger K. Khouri Jr., MD, Apoorv Dhir, BA, Hechuan Hou, MS, David C. Miller, MD, MPH, James M. Dupree, MD, MPH
Against medical advice discharges
AUTHORS: David Alfandre, MD, MSPH, Jay Brenner, MD, Eberechukwu Onukwugha, MS, PhD
BACKGROUND: Hospitalized patients frequently report poor sleep, partly due to the inpatient environment. In-hospital sound and light levels are not well described on non–intensive care unit (non-ICU) wards. Although non-ICU wards may have lower average and peak noise levels, sound level changes (SLCs), which are important in disrupting sleep, may still be a substantial problem.
OBJECTIVE: To compare ambient sound and light levels, including SLCs, in ICU and non-ICU environments.
DESIGN: Observational study.
SETTING: Tertiary-care hospital.
MEASUREMENTS: Sound measurements of 0.5 Hz were analyzed to provide average hourly sound levels, sound peaks, and SLCs greater than or equal to 17.5 decibels (dB). For light data, measurements taken at 2-minute intervals provided average and maximum light levels.
RESULTS: The ICU rooms were louder than non-ICU wards; hourly averages ranged from 56.1 plus or minus 1.3 dB to 60.3 plus or minus 1.7 dB in the ICU, 47.3 plus or minus 3.7 dB to 55.1 plus or minus 3.7 dB on the telemetry floor, and 44.6 plus or minus 2.1 dB to 53.7 plus or minus 3.6 dB on the general ward. However, SLCs greater than or equal to 17.5 dB were not statistically different (ICU, 203.9 plus or minus 28.8 times; non-ICU, 270.9 plus or minus 39.5; P = 0.11). In both ICU and non-ICU wards, average daytime light levels were less than 250 lux, and peak light levels occurred in the afternoon and early evening.
CONCLUSIONS: While quieter, non-ICU wards have as many SLCs as ICUs do, which has implications for quality improvement measurements. Efforts to further reduce average noise levels might be counterproductive. Light levels in the hospital (ICU and non-ICU) may not be optimal for maintenance of a normal circadian rhythm for most people.
Read the entire article in the Journal of Hospital Medicine.
Also in JHM this month
Associations of physician empathy with patient anxiety and ratings of communication in hospital admission encounters
AUTHORS: Rachel Weiss, MD, Eric Vittinghoff, PhD, MPH, Margaret C. Fang, MD, MPH, Jenica E. W. Cimino, Kristen Adams Chasteen, MD, Robert M. Arnold, MD, Andrew D. Auerbach, MD, Wendy G. Anderson, MD, MS
A concise tool for measuring care coordination from the provider’s perspective in the hospital setting
AUTHORS: Christine M. Weston, PhD, and Sehyo Yune, MD, Eric B. Bass, MD, MPH, Scott A. Berkowitz, MD, MBA, Daniel J. Brotman, MD, Amy Deutschendorf, MS, RN, ACNS-BC, Eric E. Howell, MD, Melissa B. Richardson, MBA Carol Sylvester, RN, MS, Albert W. Wu, MD, MPH
Post–intensive care unit psychiatric comorbidity and quality of life
AUTHORS: Sophia Wang, MD, and Chris Mosher, MD, Anthony J. Perkins, MS, Sujuan Gao, PhD, Sue Lasiter, RN, PhD, Sikandar Khan, MD, Malaz Boustani, MD, MPH, Babar Khan, MD, MS
An opportunity to improve Medicare’s planned readmissions measure
AUTHORS: Chad Ellimoottil, MD, MS, Roger K. Khouri Jr., MD, Apoorv Dhir, BA, Hechuan Hou, MS, David C. Miller, MD, MPH, James M. Dupree, MD, MPH
Against medical advice discharges
AUTHORS: David Alfandre, MD, MSPH, Jay Brenner, MD, Eberechukwu Onukwugha, MS, PhD
BACKGROUND: Hospitalized patients frequently report poor sleep, partly due to the inpatient environment. In-hospital sound and light levels are not well described on non–intensive care unit (non-ICU) wards. Although non-ICU wards may have lower average and peak noise levels, sound level changes (SLCs), which are important in disrupting sleep, may still be a substantial problem.
OBJECTIVE: To compare ambient sound and light levels, including SLCs, in ICU and non-ICU environments.
DESIGN: Observational study.
SETTING: Tertiary-care hospital.
MEASUREMENTS: Sound measurements of 0.5 Hz were analyzed to provide average hourly sound levels, sound peaks, and SLCs greater than or equal to 17.5 decibels (dB). For light data, measurements taken at 2-minute intervals provided average and maximum light levels.
RESULTS: The ICU rooms were louder than non-ICU wards; hourly averages ranged from 56.1 plus or minus 1.3 dB to 60.3 plus or minus 1.7 dB in the ICU, 47.3 plus or minus 3.7 dB to 55.1 plus or minus 3.7 dB on the telemetry floor, and 44.6 plus or minus 2.1 dB to 53.7 plus or minus 3.6 dB on the general ward. However, SLCs greater than or equal to 17.5 dB were not statistically different (ICU, 203.9 plus or minus 28.8 times; non-ICU, 270.9 plus or minus 39.5; P = 0.11). In both ICU and non-ICU wards, average daytime light levels were less than 250 lux, and peak light levels occurred in the afternoon and early evening.
CONCLUSIONS: While quieter, non-ICU wards have as many SLCs as ICUs do, which has implications for quality improvement measurements. Efforts to further reduce average noise levels might be counterproductive. Light levels in the hospital (ICU and non-ICU) may not be optimal for maintenance of a normal circadian rhythm for most people.
Read the entire article in the Journal of Hospital Medicine.
Also in JHM this month
Associations of physician empathy with patient anxiety and ratings of communication in hospital admission encounters
AUTHORS: Rachel Weiss, MD, Eric Vittinghoff, PhD, MPH, Margaret C. Fang, MD, MPH, Jenica E. W. Cimino, Kristen Adams Chasteen, MD, Robert M. Arnold, MD, Andrew D. Auerbach, MD, Wendy G. Anderson, MD, MS
A concise tool for measuring care coordination from the provider’s perspective in the hospital setting
AUTHORS: Christine M. Weston, PhD, and Sehyo Yune, MD, Eric B. Bass, MD, MPH, Scott A. Berkowitz, MD, MBA, Daniel J. Brotman, MD, Amy Deutschendorf, MS, RN, ACNS-BC, Eric E. Howell, MD, Melissa B. Richardson, MBA Carol Sylvester, RN, MS, Albert W. Wu, MD, MPH
Post–intensive care unit psychiatric comorbidity and quality of life
AUTHORS: Sophia Wang, MD, and Chris Mosher, MD, Anthony J. Perkins, MS, Sujuan Gao, PhD, Sue Lasiter, RN, PhD, Sikandar Khan, MD, Malaz Boustani, MD, MPH, Babar Khan, MD, MS
An opportunity to improve Medicare’s planned readmissions measure
AUTHORS: Chad Ellimoottil, MD, MS, Roger K. Khouri Jr., MD, Apoorv Dhir, BA, Hechuan Hou, MS, David C. Miller, MD, MPH, James M. Dupree, MD, MPH
Against medical advice discharges
AUTHORS: David Alfandre, MD, MSPH, Jay Brenner, MD, Eberechukwu Onukwugha, MS, PhD
Verrucoid Lesion on the Eyelid
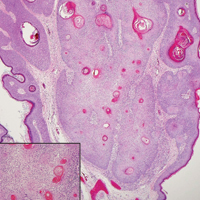
The Diagnosis: Inverted Follicular Keratosis
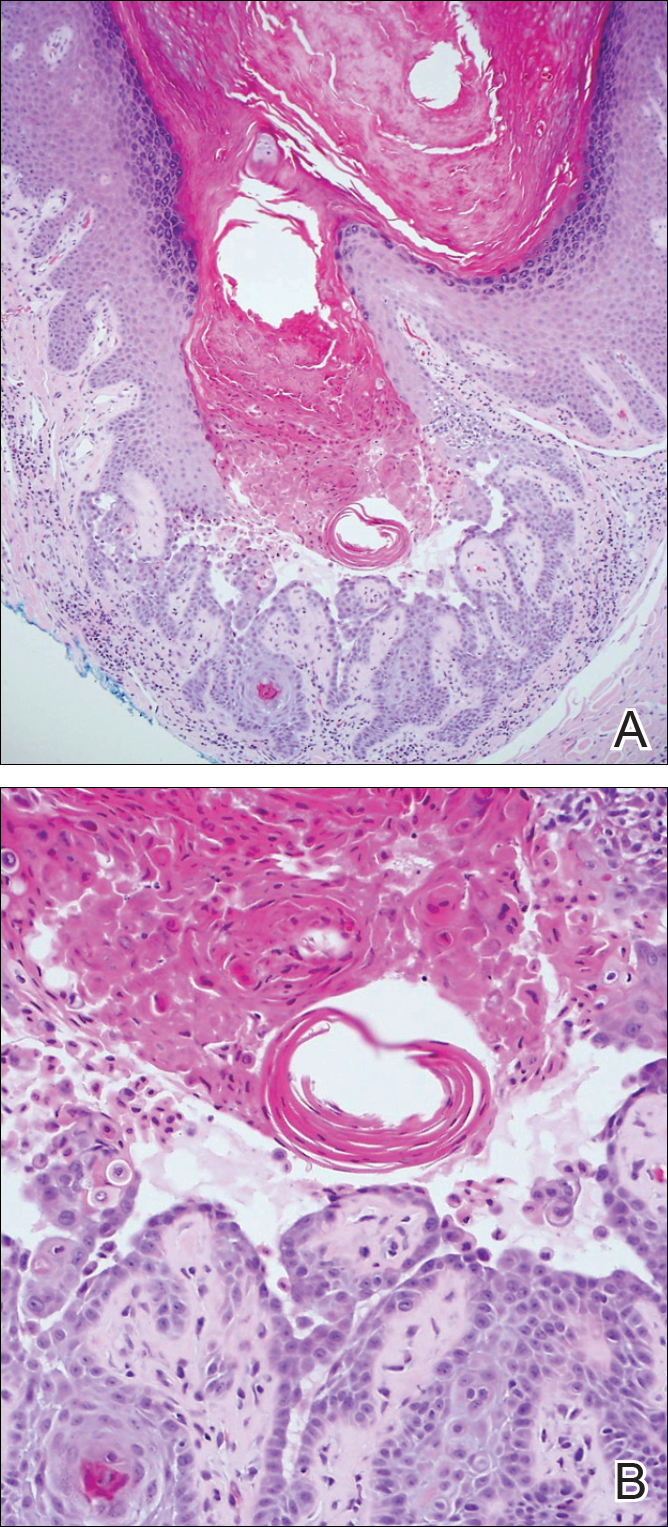
The differential diagnosis for endophytic squamous neoplasms encompasses benign and malignant entities. The histologic findings of our patient's lesion were compatible with the diagnosis of inverted follicular keratosis (IFK), a benign neoplasm that usually presents as a keratotic papule on the head or neck. Histologically, IFK is characterized by an endophytic growth pattern with squamous eddies (quiz images). Inverted follicular keratosis may represent an irritated seborrheic keratosis or a distinct neoplasm derived from the infundibular portion of the hair follicle; the exact etiology is uncertain.1,2 No relationship between IFK and human papillomavirus (HPV) has been established.3 Inverted follicular keratosis can mimic squamous cell carcinoma (SCC). Important clues to the diagnosis of IFK are the presence of squamous eddies and the lack of squamous pearls or cytologic atypia.4 Squamous eddies consist of whorled keratinocytes without keratinization or atypia. Superficial shave biopsies may fail to demonstrate the characteristic well-circumscribed architecture and may lead to an erroneous diagnosis.
Acantholytic SCC is characterized by atypical keratinocytes that have lost cohesive properties, resulting in acantholysis (Figure 1).5 This histologic variant was once categorized as an aggressive variant of SCC, but studies have failed to support this assertion.5,6 Acantholytic SCC has a discohesive nature producing a pseudoglandular appearance sometimes mistaken for adenosquamous carcinoma or metastatic carcinoma. Recent literature has suggested that acantholytic SCCs, similar to IFKs, are derived from the follicular infundibulum.5,6 Also similar to IFKs, acantholytic SCCs often are located on the face. The invasive architecture and atypical cytology of acantholytic SCCs can differentiate them from IFKs. Acantholytic SCCs can contain keratin pearls with concentric keratinocytes showing incomplete keratinization centrally, often with retained nuclei, but rare to no squamous eddies unless irritated.

Trichilemmoma is an endophytic benign neoplasm derived from the outer sheath of the pilosebaceous follicle characterized by lobules of clear cells hanging from the epidermis.7 A study investigating the relationship between HPV and trichilemmomas failed to definitively detect HPV in trichilemmomas and this relationship remains unclear.8 Desmoplastic trichilemmoma is a subtype histologically characterized by jagged islands of epithelial cells separated by dense pink stroma and encased in a glassy basement membrane (Figure 2). The presence of desmoplasia and a jagged growth pattern can mimic invasive SCC, but the absence of cytologic atypia and the surrounding basement membrane differs from SCC.4,7 Trichilemmomas typically are solitary, but multiple lesions are associated with Cowden syndrome. Cowden syndrome is a rare autosomal-dominant condition characterized by the presence of benign hamartomas and a predisposition to the development of malignancies including breast, endometrial, and thyroid cancers.9,10 There is no such association with desmoplastic trichilemmomas.11

Pilar sheath acanthoma is a benign neoplasm that clinically presents as a solitary flesh-colored nodule with a central pore containing keratin.12 Histologically, pilar sheath acanthoma is similar to a dilated pore of Winer with the addition of acanthotic epidermal projections (Figure 3).

Warty dyskeratoma (WD) is a benign endophytic neoplasm traditionally seen as a solitary lesion histologically similar to Darier disease. Warty dyskeratomas are known to occur both on the skin and oral mucosa.13 Histologically, WD is characterized as a cup-shaped lesion with numerous villi at the base of the lesion along with acantholysis and dyskeratosis (Figure 4). The dyskeratotic cells in WD consist of corps ronds, which are cells with abundant pink cytoplasm, and small nuclei along with grains, which are flattened basophilic cells. These dyskeratotic cells help differentiate WD from IFK. Although they are endophytic neoplasms, WDs are well circumscribed and should not be confused with SCC. Despite this entity's name and histologic similarity to verrucae, no relationship with HPV has been established.14
- Ruhoy SM, Thomas D, Nuovo GJ. Multiple inverted follicular keratoses as a presenting sign of Cowden's syndrome: case report with human papillomavirus studies. J Am Acad Dermatol. 2004;51:411-415.
- Lever WF. Inverted follicular keratosis is an irritated seborrheic keratosis. Am J Dermatopathol. 1983;5:474.
- Kambiz KH, Kaveh D, Maede D, et al. Human papillomavirus deoxyribonucleic acid may not be detected in non-genital benign papillomatous skin lesions by polymerase chain reaction. Indian J Dermatol. 2014;59:334-338.
- Tan KB, Tan SH, Aw DC, et al. Simulators of squamous cell carcinoma of the skin: diagnostic challenges on small biopsies and clinicopathological correlation [published online June 25, 2013]. J Skin Cancer. 2013;2013:752864.
- Ogawa T, Kiuru M, Konia TH, et al. Acantholytic squamous cell carcinoma is usually associated with hair follicles, not acantholytic actinic keratosis, and is not "high risk": diagnosis, management, and clinical outcomes in a series of 115 cases. J Am Acad Dermatol. 2017;76:327-333.
- Motaparthi K, Kapil JP, Velazquez EF. Cutaneous squamous cell carcinoma: review of the eighth edition of the American Joint Committee on Cancer staging guidelines, prognostic factors, and histopathologic variants. Adv Anat Pathol. 2017;24:171-194.
- Sano DT, Yang JJ, Tebcherani AJ, et al. A rare clinical presentation of desmoplastic trichilemmoma mimicking invasive carcinoma. An Bras Dermatol. 2014;89:796-798.
- Stierman S, Chen S, Nuovo G, et al. Detection of human papillomavirus infection in trichilemmomas and verrucae using in situ hybridization. J Cutan Pathol. 2010;37:75-80.
- Ngeow J, Eng C. PTEN hamartoma tumor syndrome: clinical risk assessment and management protocol [published online October 22, 2014]. Methods. 2015;77-78:11-19.
- Molvi M, Sharma YK, Dash K. Cowden syndrome: case report, update and proposed diagnostic and surveillance routines. Indian J Dermatol. 2015;60:255-259.
- Jin M, Hampel H, Pilarski R, et al. Phosphatase and tensin homolog immunohistochemical staining and clinical criteria for Cowden syndrome in patients with trichilemmoma or associated lesions. Am J Dermatopathol. 2013;35:637-640.
- Mehregan AH, Brownstein MH. Pilar sheath acanthoma. Arch Dermatol. 1978;114:1495-1497.
- Newland JR, Leventon GS. Warty dyskeratoma of the oral mucosa. correlated light and electron microscopic study. Oral Surg Oral Med Oral Pathol. 1984;58:176-183.
- Kaddu S, Dong H, Mayer G, et al. Warty dyskeratoma--"follicular dyskeratoma": analysis of clinicopathologic features of a distinctive follicular adnexal neoplasm. J Am Acad Dermatol. 2002;47:423-428.
The Diagnosis: Inverted Follicular Keratosis
The differential diagnosis for endophytic squamous neoplasms encompasses benign and malignant entities. The histologic findings of our patient's lesion were compatible with the diagnosis of inverted follicular keratosis (IFK), a benign neoplasm that usually presents as a keratotic papule on the head or neck. Histologically, IFK is characterized by an endophytic growth pattern with squamous eddies (quiz images). Inverted follicular keratosis may represent an irritated seborrheic keratosis or a distinct neoplasm derived from the infundibular portion of the hair follicle; the exact etiology is uncertain.1,2 No relationship between IFK and human papillomavirus (HPV) has been established.3 Inverted follicular keratosis can mimic squamous cell carcinoma (SCC). Important clues to the diagnosis of IFK are the presence of squamous eddies and the lack of squamous pearls or cytologic atypia.4 Squamous eddies consist of whorled keratinocytes without keratinization or atypia. Superficial shave biopsies may fail to demonstrate the characteristic well-circumscribed architecture and may lead to an erroneous diagnosis.
Acantholytic SCC is characterized by atypical keratinocytes that have lost cohesive properties, resulting in acantholysis (Figure 1).5 This histologic variant was once categorized as an aggressive variant of SCC, but studies have failed to support this assertion.5,6 Acantholytic SCC has a discohesive nature producing a pseudoglandular appearance sometimes mistaken for adenosquamous carcinoma or metastatic carcinoma. Recent literature has suggested that acantholytic SCCs, similar to IFKs, are derived from the follicular infundibulum.5,6 Also similar to IFKs, acantholytic SCCs often are located on the face. The invasive architecture and atypical cytology of acantholytic SCCs can differentiate them from IFKs. Acantholytic SCCs can contain keratin pearls with concentric keratinocytes showing incomplete keratinization centrally, often with retained nuclei, but rare to no squamous eddies unless irritated.

Trichilemmoma is an endophytic benign neoplasm derived from the outer sheath of the pilosebaceous follicle characterized by lobules of clear cells hanging from the epidermis.7 A study investigating the relationship between HPV and trichilemmomas failed to definitively detect HPV in trichilemmomas and this relationship remains unclear.8 Desmoplastic trichilemmoma is a subtype histologically characterized by jagged islands of epithelial cells separated by dense pink stroma and encased in a glassy basement membrane (Figure 2). The presence of desmoplasia and a jagged growth pattern can mimic invasive SCC, but the absence of cytologic atypia and the surrounding basement membrane differs from SCC.4,7 Trichilemmomas typically are solitary, but multiple lesions are associated with Cowden syndrome. Cowden syndrome is a rare autosomal-dominant condition characterized by the presence of benign hamartomas and a predisposition to the development of malignancies including breast, endometrial, and thyroid cancers.9,10 There is no such association with desmoplastic trichilemmomas.11

Pilar sheath acanthoma is a benign neoplasm that clinically presents as a solitary flesh-colored nodule with a central pore containing keratin.12 Histologically, pilar sheath acanthoma is similar to a dilated pore of Winer with the addition of acanthotic epidermal projections (Figure 3).

Warty dyskeratoma (WD) is a benign endophytic neoplasm traditionally seen as a solitary lesion histologically similar to Darier disease. Warty dyskeratomas are known to occur both on the skin and oral mucosa.13 Histologically, WD is characterized as a cup-shaped lesion with numerous villi at the base of the lesion along with acantholysis and dyskeratosis (Figure 4). The dyskeratotic cells in WD consist of corps ronds, which are cells with abundant pink cytoplasm, and small nuclei along with grains, which are flattened basophilic cells. These dyskeratotic cells help differentiate WD from IFK. Although they are endophytic neoplasms, WDs are well circumscribed and should not be confused with SCC. Despite this entity's name and histologic similarity to verrucae, no relationship with HPV has been established.14

The Diagnosis: Inverted Follicular Keratosis
The differential diagnosis for endophytic squamous neoplasms encompasses benign and malignant entities. The histologic findings of our patient's lesion were compatible with the diagnosis of inverted follicular keratosis (IFK), a benign neoplasm that usually presents as a keratotic papule on the head or neck. Histologically, IFK is characterized by an endophytic growth pattern with squamous eddies (quiz images). Inverted follicular keratosis may represent an irritated seborrheic keratosis or a distinct neoplasm derived from the infundibular portion of the hair follicle; the exact etiology is uncertain.1,2 No relationship between IFK and human papillomavirus (HPV) has been established.3 Inverted follicular keratosis can mimic squamous cell carcinoma (SCC). Important clues to the diagnosis of IFK are the presence of squamous eddies and the lack of squamous pearls or cytologic atypia.4 Squamous eddies consist of whorled keratinocytes without keratinization or atypia. Superficial shave biopsies may fail to demonstrate the characteristic well-circumscribed architecture and may lead to an erroneous diagnosis.
Acantholytic SCC is characterized by atypical keratinocytes that have lost cohesive properties, resulting in acantholysis (Figure 1).5 This histologic variant was once categorized as an aggressive variant of SCC, but studies have failed to support this assertion.5,6 Acantholytic SCC has a discohesive nature producing a pseudoglandular appearance sometimes mistaken for adenosquamous carcinoma or metastatic carcinoma. Recent literature has suggested that acantholytic SCCs, similar to IFKs, are derived from the follicular infundibulum.5,6 Also similar to IFKs, acantholytic SCCs often are located on the face. The invasive architecture and atypical cytology of acantholytic SCCs can differentiate them from IFKs. Acantholytic SCCs can contain keratin pearls with concentric keratinocytes showing incomplete keratinization centrally, often with retained nuclei, but rare to no squamous eddies unless irritated.

Trichilemmoma is an endophytic benign neoplasm derived from the outer sheath of the pilosebaceous follicle characterized by lobules of clear cells hanging from the epidermis.7 A study investigating the relationship between HPV and trichilemmomas failed to definitively detect HPV in trichilemmomas and this relationship remains unclear.8 Desmoplastic trichilemmoma is a subtype histologically characterized by jagged islands of epithelial cells separated by dense pink stroma and encased in a glassy basement membrane (Figure 2). The presence of desmoplasia and a jagged growth pattern can mimic invasive SCC, but the absence of cytologic atypia and the surrounding basement membrane differs from SCC.4,7 Trichilemmomas typically are solitary, but multiple lesions are associated with Cowden syndrome. Cowden syndrome is a rare autosomal-dominant condition characterized by the presence of benign hamartomas and a predisposition to the development of malignancies including breast, endometrial, and thyroid cancers.9,10 There is no such association with desmoplastic trichilemmomas.11

Pilar sheath acanthoma is a benign neoplasm that clinically presents as a solitary flesh-colored nodule with a central pore containing keratin.12 Histologically, pilar sheath acanthoma is similar to a dilated pore of Winer with the addition of acanthotic epidermal projections (Figure 3).

Warty dyskeratoma (WD) is a benign endophytic neoplasm traditionally seen as a solitary lesion histologically similar to Darier disease. Warty dyskeratomas are known to occur both on the skin and oral mucosa.13 Histologically, WD is characterized as a cup-shaped lesion with numerous villi at the base of the lesion along with acantholysis and dyskeratosis (Figure 4). The dyskeratotic cells in WD consist of corps ronds, which are cells with abundant pink cytoplasm, and small nuclei along with grains, which are flattened basophilic cells. These dyskeratotic cells help differentiate WD from IFK. Although they are endophytic neoplasms, WDs are well circumscribed and should not be confused with SCC. Despite this entity's name and histologic similarity to verrucae, no relationship with HPV has been established.14

- Ruhoy SM, Thomas D, Nuovo GJ. Multiple inverted follicular keratoses as a presenting sign of Cowden's syndrome: case report with human papillomavirus studies. J Am Acad Dermatol. 2004;51:411-415.
- Lever WF. Inverted follicular keratosis is an irritated seborrheic keratosis. Am J Dermatopathol. 1983;5:474.
- Kambiz KH, Kaveh D, Maede D, et al. Human papillomavirus deoxyribonucleic acid may not be detected in non-genital benign papillomatous skin lesions by polymerase chain reaction. Indian J Dermatol. 2014;59:334-338.
- Tan KB, Tan SH, Aw DC, et al. Simulators of squamous cell carcinoma of the skin: diagnostic challenges on small biopsies and clinicopathological correlation [published online June 25, 2013]. J Skin Cancer. 2013;2013:752864.
- Ogawa T, Kiuru M, Konia TH, et al. Acantholytic squamous cell carcinoma is usually associated with hair follicles, not acantholytic actinic keratosis, and is not "high risk": diagnosis, management, and clinical outcomes in a series of 115 cases. J Am Acad Dermatol. 2017;76:327-333.
- Motaparthi K, Kapil JP, Velazquez EF. Cutaneous squamous cell carcinoma: review of the eighth edition of the American Joint Committee on Cancer staging guidelines, prognostic factors, and histopathologic variants. Adv Anat Pathol. 2017;24:171-194.
- Sano DT, Yang JJ, Tebcherani AJ, et al. A rare clinical presentation of desmoplastic trichilemmoma mimicking invasive carcinoma. An Bras Dermatol. 2014;89:796-798.
- Stierman S, Chen S, Nuovo G, et al. Detection of human papillomavirus infection in trichilemmomas and verrucae using in situ hybridization. J Cutan Pathol. 2010;37:75-80.
- Ngeow J, Eng C. PTEN hamartoma tumor syndrome: clinical risk assessment and management protocol [published online October 22, 2014]. Methods. 2015;77-78:11-19.
- Molvi M, Sharma YK, Dash K. Cowden syndrome: case report, update and proposed diagnostic and surveillance routines. Indian J Dermatol. 2015;60:255-259.
- Jin M, Hampel H, Pilarski R, et al. Phosphatase and tensin homolog immunohistochemical staining and clinical criteria for Cowden syndrome in patients with trichilemmoma or associated lesions. Am J Dermatopathol. 2013;35:637-640.
- Mehregan AH, Brownstein MH. Pilar sheath acanthoma. Arch Dermatol. 1978;114:1495-1497.
- Newland JR, Leventon GS. Warty dyskeratoma of the oral mucosa. correlated light and electron microscopic study. Oral Surg Oral Med Oral Pathol. 1984;58:176-183.
- Kaddu S, Dong H, Mayer G, et al. Warty dyskeratoma--"follicular dyskeratoma": analysis of clinicopathologic features of a distinctive follicular adnexal neoplasm. J Am Acad Dermatol. 2002;47:423-428.
- Ruhoy SM, Thomas D, Nuovo GJ. Multiple inverted follicular keratoses as a presenting sign of Cowden's syndrome: case report with human papillomavirus studies. J Am Acad Dermatol. 2004;51:411-415.
- Lever WF. Inverted follicular keratosis is an irritated seborrheic keratosis. Am J Dermatopathol. 1983;5:474.
- Kambiz KH, Kaveh D, Maede D, et al. Human papillomavirus deoxyribonucleic acid may not be detected in non-genital benign papillomatous skin lesions by polymerase chain reaction. Indian J Dermatol. 2014;59:334-338.
- Tan KB, Tan SH, Aw DC, et al. Simulators of squamous cell carcinoma of the skin: diagnostic challenges on small biopsies and clinicopathological correlation [published online June 25, 2013]. J Skin Cancer. 2013;2013:752864.
- Ogawa T, Kiuru M, Konia TH, et al. Acantholytic squamous cell carcinoma is usually associated with hair follicles, not acantholytic actinic keratosis, and is not "high risk": diagnosis, management, and clinical outcomes in a series of 115 cases. J Am Acad Dermatol. 2017;76:327-333.
- Motaparthi K, Kapil JP, Velazquez EF. Cutaneous squamous cell carcinoma: review of the eighth edition of the American Joint Committee on Cancer staging guidelines, prognostic factors, and histopathologic variants. Adv Anat Pathol. 2017;24:171-194.
- Sano DT, Yang JJ, Tebcherani AJ, et al. A rare clinical presentation of desmoplastic trichilemmoma mimicking invasive carcinoma. An Bras Dermatol. 2014;89:796-798.
- Stierman S, Chen S, Nuovo G, et al. Detection of human papillomavirus infection in trichilemmomas and verrucae using in situ hybridization. J Cutan Pathol. 2010;37:75-80.
- Ngeow J, Eng C. PTEN hamartoma tumor syndrome: clinical risk assessment and management protocol [published online October 22, 2014]. Methods. 2015;77-78:11-19.
- Molvi M, Sharma YK, Dash K. Cowden syndrome: case report, update and proposed diagnostic and surveillance routines. Indian J Dermatol. 2015;60:255-259.
- Jin M, Hampel H, Pilarski R, et al. Phosphatase and tensin homolog immunohistochemical staining and clinical criteria for Cowden syndrome in patients with trichilemmoma or associated lesions. Am J Dermatopathol. 2013;35:637-640.
- Mehregan AH, Brownstein MH. Pilar sheath acanthoma. Arch Dermatol. 1978;114:1495-1497.
- Newland JR, Leventon GS. Warty dyskeratoma of the oral mucosa. correlated light and electron microscopic study. Oral Surg Oral Med Oral Pathol. 1984;58:176-183.
- Kaddu S, Dong H, Mayer G, et al. Warty dyskeratoma--"follicular dyskeratoma": analysis of clinicopathologic features of a distinctive follicular adnexal neoplasm. J Am Acad Dermatol. 2002;47:423-428.
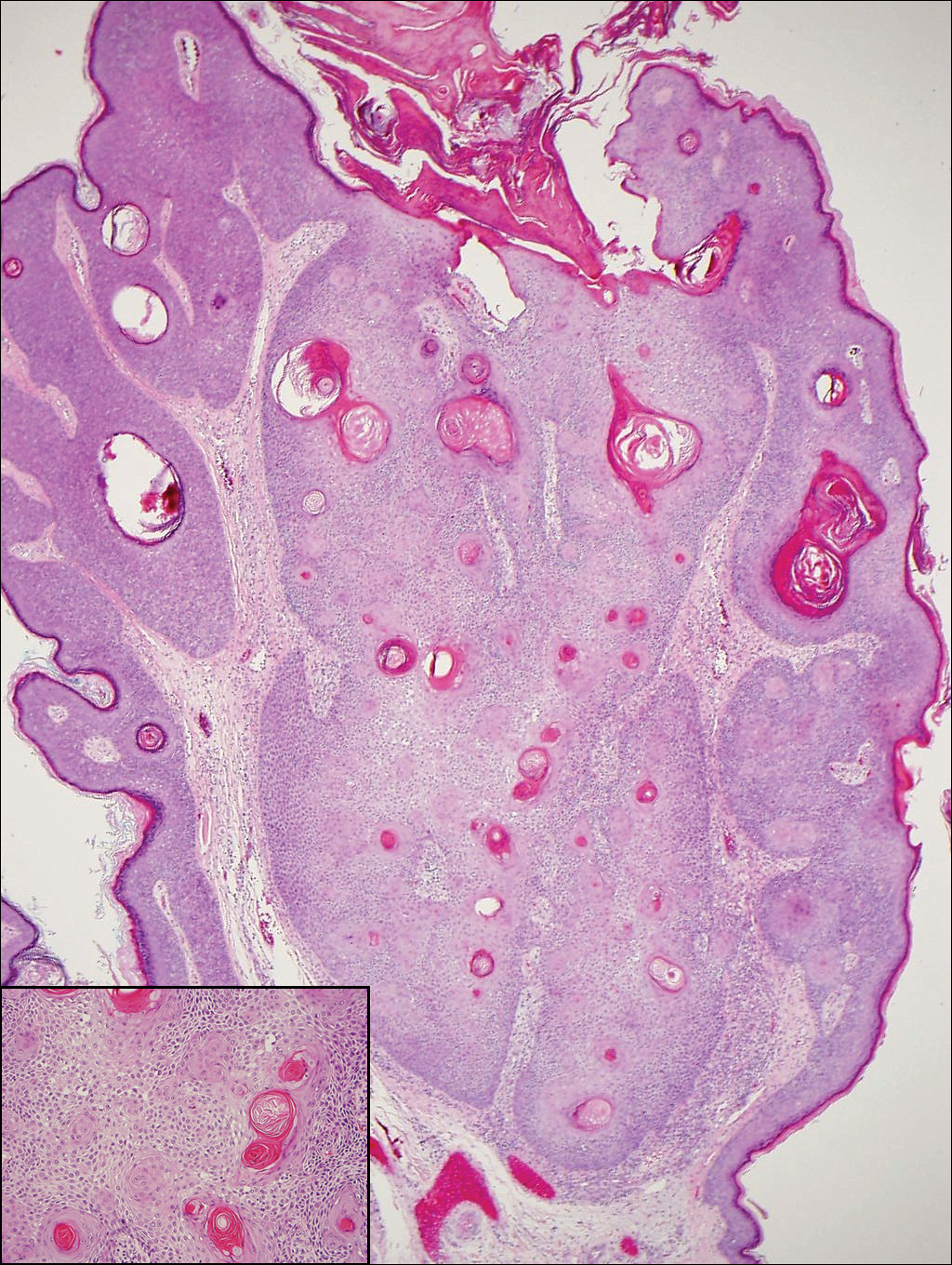
A 60-year-old man presented with a 3-mm verrucous papule on the right upper eyelid of 2 years' duration.
Cyanosis of the Foot
The Diagnosis: Antiphospholipid Antibody Syndrome
A biopsy demonstrated scattered intravascular thrombi in the dermis and subcutis, intact vascular walls, and scant lymphocytic inflammation in a background of stasis (Figure 1). A periodic acid-Schiff stain was negative for fungal elements and highlighted the intravascular thrombi. Histologic findings were consistent with thrombotic vasculopathy. On further laboratory workup, lupus anticoagulant studies, including a mixing study, diluted Russell viper venom test, and hexagonal phase phospholipid neutralization test, were abnormal. Titers of anticardiolipin and β2-glycoprotein I antibodies were elevated (anticardiolipin IgG, 137.7 calculated units [normal, <15 calculated units]; β2-glycoprotein I IgG, 256.4 calculated units [normal, <20 calculated units]). Tissue cultures showed no growth of microorganisms and studies for cryoglobulinemia were negative.

The patient was diagnosed with primary antiphospholipid syndrome (APS). He remained on anticoagulation therapy with fondaparinux as an inpatient and was treated with pulse-dose intravenous (IV) corticosteroids followed by a slow oral taper, daily plasmapheresis for 1 week, IV immunoglobulin (0.5 g/kg) for 3 doses, and 4 weekly doses of rituximab (375 mg/m2). His cutaneous findings slowly improved over the next several weeks (Figure 2).
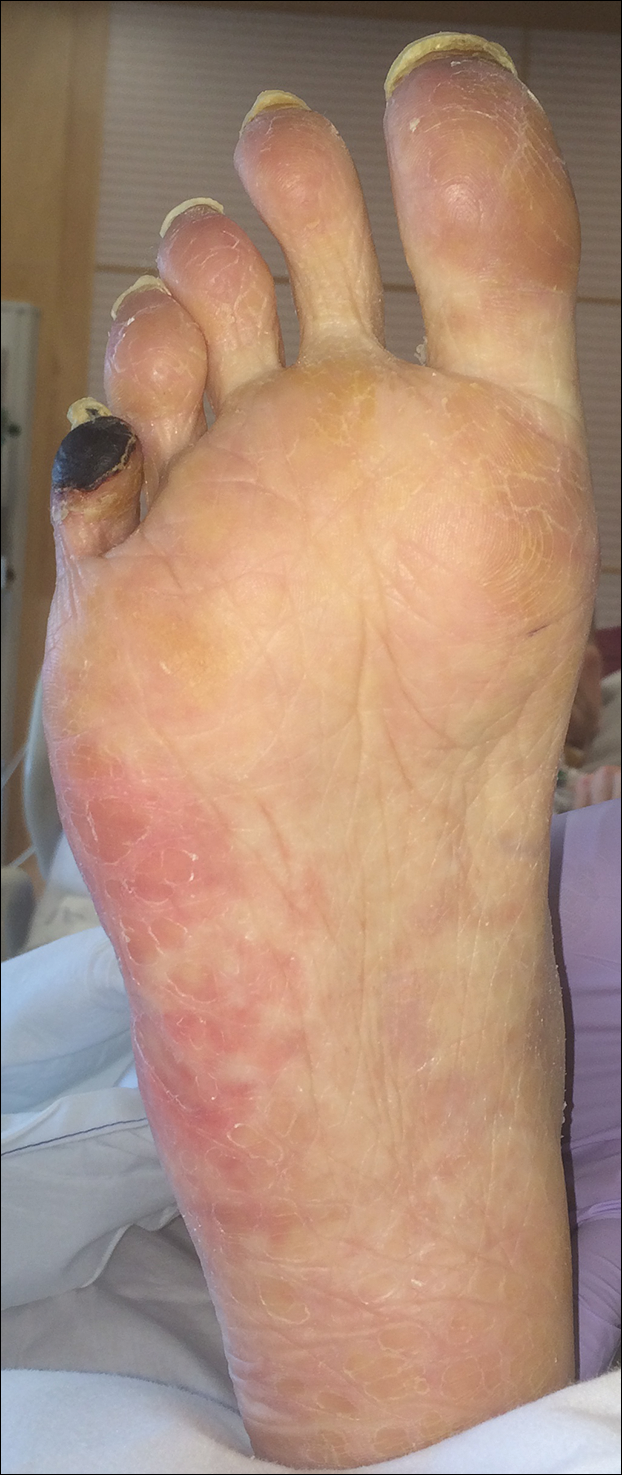
Antiphospholipid syndrome is an autoimmune disorder characterized by thrombotic events and the presence of autoantibodies. The syndrome is defined by 2 major criteria: (1) the occurrence of at least 1 clinical feature of either an episode of vascular thrombosis or pregnancy morbidity such as unexplained fetal death beyond 10 weeks of gestation or recurrent unexplained pregnancy losses; and (2) the presence of at least 1 type of autoantibody, including lupus anticoagulant, anticardiolipin, or β2-glycoprotein antibodies, on 2 separate occasions at least 12 weeks apart.1 Antiphospholipid syndrome can either be primary with no identifiable associated rheumatologic disease or secondary to another autoimmune disease such as systemic lupus erythematosus. Cutaneous manifestations are common and frequently are the first sign of disease in 30% to 40% of patients.2 The most common skin finding is persistent livedo reticularis, which can be seen in 20% to 25% of patients. Patients also may develop skin necrosis, ulcerations, digital gangrene, splinter hemorrhages, and livedoid vasculopathy.2 Systemic manifestations of APS include thrombocytopenia, nephropathy, cognitive dysfunction, and cardiac valve abnormalities.
The exact pathogenesis of APS remains unknown. It is thought to be due to the combination of an inflammatory stimulus that has yet to be characterized in conjunction with autoantibodies that affect multiple target cells including monocytes, platelets, and endothelial cells, which results in activation of the complement system and clotting cascade.3 In rare cases, the disorder can progress to catastrophic antiphospholipid syndrome (CAPS), which requires fulfillment of 4 criteria: (1) evidence of involvement of 3 organs, tissues, or systems; (2) development of manifestations simultaneously or in less than 1 week; (3) laboratory confirmation of the presence of antiphospholipid antibodies; and (4) confirmation by histopathology of small vessel occlusion.4 Probable CAPS is diagnosed when 3 of 4 criteria are present. Our patient met criteria for probable CAPS, as his antibody titers remained elevated 15 weeks after initial presentation. Precipitating factors that can lead to CAPS are thought to include infection, surgical procedures, medications, or discontinuation of anticoagulation drugs.2 Although the mainstay of management of APS is anticoagulation therapy with warfarin and antiplatelet agents such as aspirin, first-line treatment of CAPS involves high-dose systemic glucocorticoids and plasma exchange. Intravenous immunoglobulin also may be employed in treatment. Data from the CAPS registry demonstrate a role for rituximab, an anti-CD20 antibody, at 375 mg/m2 weekly for 4 weeks (the regimen described in our case) or 1 g every 14 days for 2 sessions.5 A majority of the registry patients treated with rituximab recovered (75% [15/20]) and had no recurrent thrombosis (87% [13/15]) at follow-up.5 Data also are emerging on the role of eculizumab, an anti-C5 antibody that inhibits the terminal complement cascade, as a therapy in difficult-to-treat or refractory CAPS.6-8 The prognosis for CAPS patients without treatment is poor, and mortality has been reported in up to 44% of patients. However, with intervention mortality is reduced by more than 2-fold.9,10
It is important to recognize that acral cyanosis with persistent livedo reticularis and digital gangrene can be a presenting manifestation of APS. These cutaneous manifestations should prompt histologic evaluation for thrombotic vasculopathy in addition to serologic tests for APS autoantibodies. Although APS may be treated with anticoagulants and antiplatelet agents, CAPS may require more aggressive therapy with systemic steroids, plasma exchange, IV immunoglobulin, rituximab, and/or eculizumab.
- Wilson WA, Gharavi AE, Koike T, et al. International consensus statement on preliminary classification criteria for definite antiphospholipid syndrome: report of an international workshop. Arthritis Rheum. 1999;42:1309-1311.
- Pinto-Almeida T, Caetano M, Sanches M, et al. Cutaneous manifestations of antiphospholipid syndrome: a review of the clinical features, diagnosis and management. Acta Reumatol Port. 2013;38:10-18.
- Meroni PL, Chighizola CB, Rovelli F, et al. Antiphospholipid syndrome in 2014: more clinical manifestations, novel pathogenic players and emerging biomarkers. Arthritis Res Ther. 2014;16:209.
- Asherson RA, Cervera R, de Grott PG, et al; Catastrophic Antiphospholipid Syndrome Registry Project Group. Catastrophic antiphospholipid syndrome: international consensus statement on classification criteria and treatment guidelines. Lupus. 2003;12:530-534.
- Berman H, Rodríguez-Pintó I, Cervera R, et al. Rituximab use in the catastrophic antiphospholipid syndrome: descriptive analysis of the CAPS registry patients receiving rituximab [published online June 15, 2013]. Autoimmun Rev. 2013;12:1085-1090.
- Shapira I, Andrade D, Allen SL, et al. Brief report: induction of sustained remission in recurrent catastrophic antiphospholipid syndrome via inhibition of terminal complement with eculizumab. Arthritis Rheum. 2012;64:2719-2723.
- Strakhan M, Hurtado-Sbordoni M, Galeas N, et al. 36-year-old female with catastrophic antiphospholipid syndrome treated with eculizumab: a case report and review of literature. Case Rep Hematol. 2014;2014:704371.
- Lonze BE, Zachary AA, Magro CM, et al. Eculizumab prevents recurrent antiphospholipid antibody syndrome and enables successful renal transplantation. Am J Transplant. 2014;14:459-465.
- Bucciarelli S, Espinosa G, Cervera R, et al. Mortality in the catastrophic antiphospholipid syndrome: causes of death and prognostic factors in a series of 250 patients. Arthritis Rheum. 2006;54:2568-2576.
- Asherson RA, Cervera R, Piette JC, et al. Catastrophic antiphospholipid syndrome. clinical and laboratory features of 50 patients. Medicine (Baltimore). 1998;77:195-207.
The Diagnosis: Antiphospholipid Antibody Syndrome
A biopsy demonstrated scattered intravascular thrombi in the dermis and subcutis, intact vascular walls, and scant lymphocytic inflammation in a background of stasis (Figure 1). A periodic acid-Schiff stain was negative for fungal elements and highlighted the intravascular thrombi. Histologic findings were consistent with thrombotic vasculopathy. On further laboratory workup, lupus anticoagulant studies, including a mixing study, diluted Russell viper venom test, and hexagonal phase phospholipid neutralization test, were abnormal. Titers of anticardiolipin and β2-glycoprotein I antibodies were elevated (anticardiolipin IgG, 137.7 calculated units [normal, <15 calculated units]; β2-glycoprotein I IgG, 256.4 calculated units [normal, <20 calculated units]). Tissue cultures showed no growth of microorganisms and studies for cryoglobulinemia were negative.

The patient was diagnosed with primary antiphospholipid syndrome (APS). He remained on anticoagulation therapy with fondaparinux as an inpatient and was treated with pulse-dose intravenous (IV) corticosteroids followed by a slow oral taper, daily plasmapheresis for 1 week, IV immunoglobulin (0.5 g/kg) for 3 doses, and 4 weekly doses of rituximab (375 mg/m2). His cutaneous findings slowly improved over the next several weeks (Figure 2).

Antiphospholipid syndrome is an autoimmune disorder characterized by thrombotic events and the presence of autoantibodies. The syndrome is defined by 2 major criteria: (1) the occurrence of at least 1 clinical feature of either an episode of vascular thrombosis or pregnancy morbidity such as unexplained fetal death beyond 10 weeks of gestation or recurrent unexplained pregnancy losses; and (2) the presence of at least 1 type of autoantibody, including lupus anticoagulant, anticardiolipin, or β2-glycoprotein antibodies, on 2 separate occasions at least 12 weeks apart.1 Antiphospholipid syndrome can either be primary with no identifiable associated rheumatologic disease or secondary to another autoimmune disease such as systemic lupus erythematosus. Cutaneous manifestations are common and frequently are the first sign of disease in 30% to 40% of patients.2 The most common skin finding is persistent livedo reticularis, which can be seen in 20% to 25% of patients. Patients also may develop skin necrosis, ulcerations, digital gangrene, splinter hemorrhages, and livedoid vasculopathy.2 Systemic manifestations of APS include thrombocytopenia, nephropathy, cognitive dysfunction, and cardiac valve abnormalities.
The exact pathogenesis of APS remains unknown. It is thought to be due to the combination of an inflammatory stimulus that has yet to be characterized in conjunction with autoantibodies that affect multiple target cells including monocytes, platelets, and endothelial cells, which results in activation of the complement system and clotting cascade.3 In rare cases, the disorder can progress to catastrophic antiphospholipid syndrome (CAPS), which requires fulfillment of 4 criteria: (1) evidence of involvement of 3 organs, tissues, or systems; (2) development of manifestations simultaneously or in less than 1 week; (3) laboratory confirmation of the presence of antiphospholipid antibodies; and (4) confirmation by histopathology of small vessel occlusion.4 Probable CAPS is diagnosed when 3 of 4 criteria are present. Our patient met criteria for probable CAPS, as his antibody titers remained elevated 15 weeks after initial presentation. Precipitating factors that can lead to CAPS are thought to include infection, surgical procedures, medications, or discontinuation of anticoagulation drugs.2 Although the mainstay of management of APS is anticoagulation therapy with warfarin and antiplatelet agents such as aspirin, first-line treatment of CAPS involves high-dose systemic glucocorticoids and plasma exchange. Intravenous immunoglobulin also may be employed in treatment. Data from the CAPS registry demonstrate a role for rituximab, an anti-CD20 antibody, at 375 mg/m2 weekly for 4 weeks (the regimen described in our case) or 1 g every 14 days for 2 sessions.5 A majority of the registry patients treated with rituximab recovered (75% [15/20]) and had no recurrent thrombosis (87% [13/15]) at follow-up.5 Data also are emerging on the role of eculizumab, an anti-C5 antibody that inhibits the terminal complement cascade, as a therapy in difficult-to-treat or refractory CAPS.6-8 The prognosis for CAPS patients without treatment is poor, and mortality has been reported in up to 44% of patients. However, with intervention mortality is reduced by more than 2-fold.9,10
It is important to recognize that acral cyanosis with persistent livedo reticularis and digital gangrene can be a presenting manifestation of APS. These cutaneous manifestations should prompt histologic evaluation for thrombotic vasculopathy in addition to serologic tests for APS autoantibodies. Although APS may be treated with anticoagulants and antiplatelet agents, CAPS may require more aggressive therapy with systemic steroids, plasma exchange, IV immunoglobulin, rituximab, and/or eculizumab.
The Diagnosis: Antiphospholipid Antibody Syndrome
A biopsy demonstrated scattered intravascular thrombi in the dermis and subcutis, intact vascular walls, and scant lymphocytic inflammation in a background of stasis (Figure 1). A periodic acid-Schiff stain was negative for fungal elements and highlighted the intravascular thrombi. Histologic findings were consistent with thrombotic vasculopathy. On further laboratory workup, lupus anticoagulant studies, including a mixing study, diluted Russell viper venom test, and hexagonal phase phospholipid neutralization test, were abnormal. Titers of anticardiolipin and β2-glycoprotein I antibodies were elevated (anticardiolipin IgG, 137.7 calculated units [normal, <15 calculated units]; β2-glycoprotein I IgG, 256.4 calculated units [normal, <20 calculated units]). Tissue cultures showed no growth of microorganisms and studies for cryoglobulinemia were negative.

The patient was diagnosed with primary antiphospholipid syndrome (APS). He remained on anticoagulation therapy with fondaparinux as an inpatient and was treated with pulse-dose intravenous (IV) corticosteroids followed by a slow oral taper, daily plasmapheresis for 1 week, IV immunoglobulin (0.5 g/kg) for 3 doses, and 4 weekly doses of rituximab (375 mg/m2). His cutaneous findings slowly improved over the next several weeks (Figure 2).

Antiphospholipid syndrome is an autoimmune disorder characterized by thrombotic events and the presence of autoantibodies. The syndrome is defined by 2 major criteria: (1) the occurrence of at least 1 clinical feature of either an episode of vascular thrombosis or pregnancy morbidity such as unexplained fetal death beyond 10 weeks of gestation or recurrent unexplained pregnancy losses; and (2) the presence of at least 1 type of autoantibody, including lupus anticoagulant, anticardiolipin, or β2-glycoprotein antibodies, on 2 separate occasions at least 12 weeks apart.1 Antiphospholipid syndrome can either be primary with no identifiable associated rheumatologic disease or secondary to another autoimmune disease such as systemic lupus erythematosus. Cutaneous manifestations are common and frequently are the first sign of disease in 30% to 40% of patients.2 The most common skin finding is persistent livedo reticularis, which can be seen in 20% to 25% of patients. Patients also may develop skin necrosis, ulcerations, digital gangrene, splinter hemorrhages, and livedoid vasculopathy.2 Systemic manifestations of APS include thrombocytopenia, nephropathy, cognitive dysfunction, and cardiac valve abnormalities.
The exact pathogenesis of APS remains unknown. It is thought to be due to the combination of an inflammatory stimulus that has yet to be characterized in conjunction with autoantibodies that affect multiple target cells including monocytes, platelets, and endothelial cells, which results in activation of the complement system and clotting cascade.3 In rare cases, the disorder can progress to catastrophic antiphospholipid syndrome (CAPS), which requires fulfillment of 4 criteria: (1) evidence of involvement of 3 organs, tissues, or systems; (2) development of manifestations simultaneously or in less than 1 week; (3) laboratory confirmation of the presence of antiphospholipid antibodies; and (4) confirmation by histopathology of small vessel occlusion.4 Probable CAPS is diagnosed when 3 of 4 criteria are present. Our patient met criteria for probable CAPS, as his antibody titers remained elevated 15 weeks after initial presentation. Precipitating factors that can lead to CAPS are thought to include infection, surgical procedures, medications, or discontinuation of anticoagulation drugs.2 Although the mainstay of management of APS is anticoagulation therapy with warfarin and antiplatelet agents such as aspirin, first-line treatment of CAPS involves high-dose systemic glucocorticoids and plasma exchange. Intravenous immunoglobulin also may be employed in treatment. Data from the CAPS registry demonstrate a role for rituximab, an anti-CD20 antibody, at 375 mg/m2 weekly for 4 weeks (the regimen described in our case) or 1 g every 14 days for 2 sessions.5 A majority of the registry patients treated with rituximab recovered (75% [15/20]) and had no recurrent thrombosis (87% [13/15]) at follow-up.5 Data also are emerging on the role of eculizumab, an anti-C5 antibody that inhibits the terminal complement cascade, as a therapy in difficult-to-treat or refractory CAPS.6-8 The prognosis for CAPS patients without treatment is poor, and mortality has been reported in up to 44% of patients. However, with intervention mortality is reduced by more than 2-fold.9,10
It is important to recognize that acral cyanosis with persistent livedo reticularis and digital gangrene can be a presenting manifestation of APS. These cutaneous manifestations should prompt histologic evaluation for thrombotic vasculopathy in addition to serologic tests for APS autoantibodies. Although APS may be treated with anticoagulants and antiplatelet agents, CAPS may require more aggressive therapy with systemic steroids, plasma exchange, IV immunoglobulin, rituximab, and/or eculizumab.
- Wilson WA, Gharavi AE, Koike T, et al. International consensus statement on preliminary classification criteria for definite antiphospholipid syndrome: report of an international workshop. Arthritis Rheum. 1999;42:1309-1311.
- Pinto-Almeida T, Caetano M, Sanches M, et al. Cutaneous manifestations of antiphospholipid syndrome: a review of the clinical features, diagnosis and management. Acta Reumatol Port. 2013;38:10-18.
- Meroni PL, Chighizola CB, Rovelli F, et al. Antiphospholipid syndrome in 2014: more clinical manifestations, novel pathogenic players and emerging biomarkers. Arthritis Res Ther. 2014;16:209.
- Asherson RA, Cervera R, de Grott PG, et al; Catastrophic Antiphospholipid Syndrome Registry Project Group. Catastrophic antiphospholipid syndrome: international consensus statement on classification criteria and treatment guidelines. Lupus. 2003;12:530-534.
- Berman H, Rodríguez-Pintó I, Cervera R, et al. Rituximab use in the catastrophic antiphospholipid syndrome: descriptive analysis of the CAPS registry patients receiving rituximab [published online June 15, 2013]. Autoimmun Rev. 2013;12:1085-1090.
- Shapira I, Andrade D, Allen SL, et al. Brief report: induction of sustained remission in recurrent catastrophic antiphospholipid syndrome via inhibition of terminal complement with eculizumab. Arthritis Rheum. 2012;64:2719-2723.
- Strakhan M, Hurtado-Sbordoni M, Galeas N, et al. 36-year-old female with catastrophic antiphospholipid syndrome treated with eculizumab: a case report and review of literature. Case Rep Hematol. 2014;2014:704371.
- Lonze BE, Zachary AA, Magro CM, et al. Eculizumab prevents recurrent antiphospholipid antibody syndrome and enables successful renal transplantation. Am J Transplant. 2014;14:459-465.
- Bucciarelli S, Espinosa G, Cervera R, et al. Mortality in the catastrophic antiphospholipid syndrome: causes of death and prognostic factors in a series of 250 patients. Arthritis Rheum. 2006;54:2568-2576.
- Asherson RA, Cervera R, Piette JC, et al. Catastrophic antiphospholipid syndrome. clinical and laboratory features of 50 patients. Medicine (Baltimore). 1998;77:195-207.
- Wilson WA, Gharavi AE, Koike T, et al. International consensus statement on preliminary classification criteria for definite antiphospholipid syndrome: report of an international workshop. Arthritis Rheum. 1999;42:1309-1311.
- Pinto-Almeida T, Caetano M, Sanches M, et al. Cutaneous manifestations of antiphospholipid syndrome: a review of the clinical features, diagnosis and management. Acta Reumatol Port. 2013;38:10-18.
- Meroni PL, Chighizola CB, Rovelli F, et al. Antiphospholipid syndrome in 2014: more clinical manifestations, novel pathogenic players and emerging biomarkers. Arthritis Res Ther. 2014;16:209.
- Asherson RA, Cervera R, de Grott PG, et al; Catastrophic Antiphospholipid Syndrome Registry Project Group. Catastrophic antiphospholipid syndrome: international consensus statement on classification criteria and treatment guidelines. Lupus. 2003;12:530-534.
- Berman H, Rodríguez-Pintó I, Cervera R, et al. Rituximab use in the catastrophic antiphospholipid syndrome: descriptive analysis of the CAPS registry patients receiving rituximab [published online June 15, 2013]. Autoimmun Rev. 2013;12:1085-1090.
- Shapira I, Andrade D, Allen SL, et al. Brief report: induction of sustained remission in recurrent catastrophic antiphospholipid syndrome via inhibition of terminal complement with eculizumab. Arthritis Rheum. 2012;64:2719-2723.
- Strakhan M, Hurtado-Sbordoni M, Galeas N, et al. 36-year-old female with catastrophic antiphospholipid syndrome treated with eculizumab: a case report and review of literature. Case Rep Hematol. 2014;2014:704371.
- Lonze BE, Zachary AA, Magro CM, et al. Eculizumab prevents recurrent antiphospholipid antibody syndrome and enables successful renal transplantation. Am J Transplant. 2014;14:459-465.
- Bucciarelli S, Espinosa G, Cervera R, et al. Mortality in the catastrophic antiphospholipid syndrome: causes of death and prognostic factors in a series of 250 patients. Arthritis Rheum. 2006;54:2568-2576.
- Asherson RA, Cervera R, Piette JC, et al. Catastrophic antiphospholipid syndrome. clinical and laboratory features of 50 patients. Medicine (Baltimore). 1998;77:195-207.
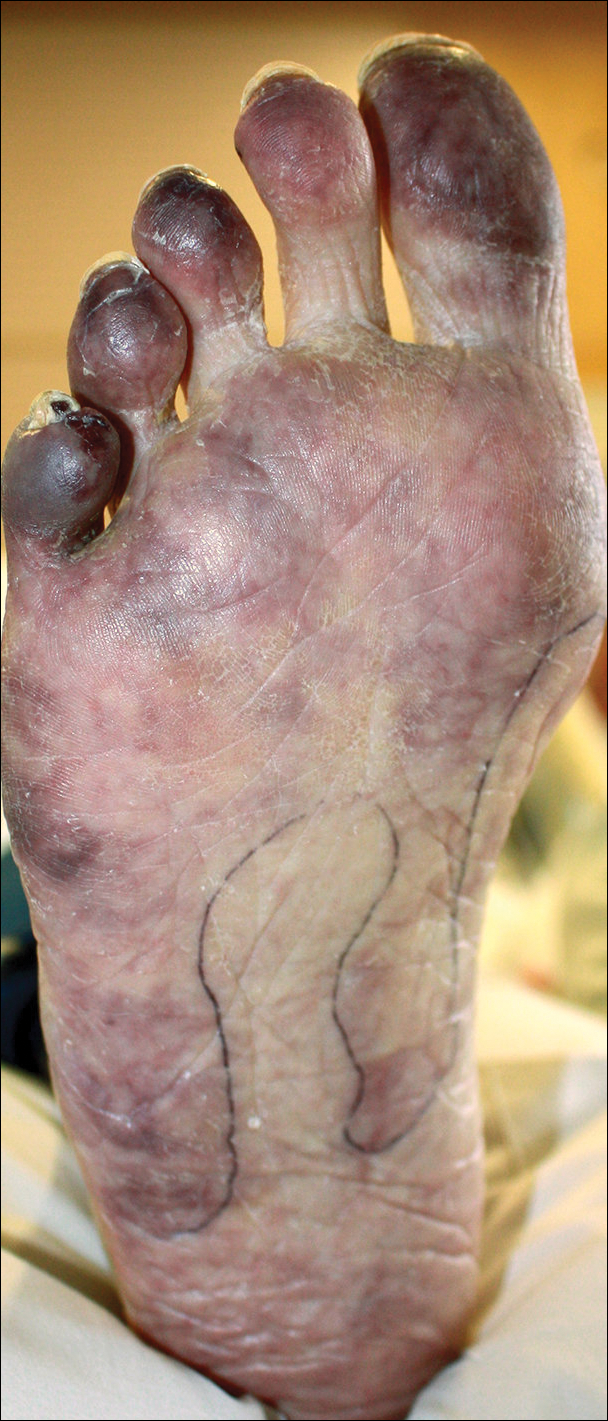
A man in his 50s with a medical history of arterial thrombosis of the right arm, multiple deep vein thromboses (DVTs) of the legs on long-term warfarin, ischemic stroke, atrial fibrillation, and peripheral arterial disease presented with discoloration of the right foot and increasing tenderness of 1 month's duration. There was no history of trauma or recent change in outpatient medications. A family history was notable for an aunt and 2 cousins with DVTs and protein S deficiency. Physical examination revealed livedo reticularis on the sole and lateral aspect of the right foot. There was violaceous discoloration of the volar aspects of all 5 toes and a focal area of ulceration on the fifth toe. Pulses were palpable bilaterally. Initial laboratory evaluation was notable for thrombocytopenia, and preliminary blood cultures revealed no growth of bacterial or fungal organisms. Imaging studies revealed increased arterial stenosis of the right leg as well as DVT of the right great saphenous vein. A punch biopsy of the right medial foot was performed for hematoxylin and eosin stain as well as tissue culture.
SVS Coding Workshop is Oct. 13-14
Don't let the federal government keep money -- in the form of Medicare reimbursements -- to which you are entitled!
Learn all about coding and reimbursement, from the essentials to modifiers to future initiatives, at the SVS Coding and Reimbursement Workshop, Oct. 13-14, in Chicago. Cost is $880 for an SVS member or staff, $955 for a non-member and $250 for residents and trainees. Cost for an optional session is $100 for an SVS member or staff, $215 for a non-member and $50 for residents and trainees.
Learn more, register and access the full agenda here.
Don't let the federal government keep money -- in the form of Medicare reimbursements -- to which you are entitled!
Learn all about coding and reimbursement, from the essentials to modifiers to future initiatives, at the SVS Coding and Reimbursement Workshop, Oct. 13-14, in Chicago. Cost is $880 for an SVS member or staff, $955 for a non-member and $250 for residents and trainees. Cost for an optional session is $100 for an SVS member or staff, $215 for a non-member and $50 for residents and trainees.
Learn more, register and access the full agenda here.
Don't let the federal government keep money -- in the form of Medicare reimbursements -- to which you are entitled!
Learn all about coding and reimbursement, from the essentials to modifiers to future initiatives, at the SVS Coding and Reimbursement Workshop, Oct. 13-14, in Chicago. Cost is $880 for an SVS member or staff, $955 for a non-member and $250 for residents and trainees. Cost for an optional session is $100 for an SVS member or staff, $215 for a non-member and $50 for residents and trainees.
Learn more, register and access the full agenda here.