User login
Nearly 80% of health care personnel stepped up for flu shots
Nearly four out of five health care personnel in the United States received a flu vaccination during the 2016-2017 flu season, but a majority of those working in long-term care settings were not vaccinated, based on data from an Internet survey of more than 2,000 individuals that was conducted by the Centers for Disease Control and Prevention.
A total of 78.6% of the survey’s respondents said they’d been vaccinated during the 2016-2017 season. Vaccination coverage for health care personnel overall has remained in the 77%-79% range in recent years, but that represents an increase from 64% in 2010-2011.
“As in previous seasons, the highest coverage was among HCP whose workplace had vaccination requirements,” noted Carla L. Black, PhD, of the CDC, and colleagues (MMWR Morb Mortal Wkly Rep. 2017 Sep 29;66[38]:1009-15). The researchers reviewed data collected from an Internet panel survey of 2,438 health care personnel between March 28, 2017, and April 19, 2017.
Physicians boasted the highest vaccination coverage in 2016-2017 (96%), followed by pharmacists (94%), nurses (93%), nurse practitioners and physician assistants (92%), other clinical providers (80%), nonclinical health care providers (74%), and aides and assistants (69%).
Flu vaccination rates were highest among HCPs working in a hospital setting (92%); 94% of survey respondents in hospitals reported either having a vaccination requirement at work or being provided at least 1 day of on-site vaccination.
Vaccination rates were lowest among health care personnel in long-term care settings (68%), where only 26% reported a workplace vaccination requirement. However, vaccination rates in long-term care rose to 90% when employers required vaccination.
The report’s findings were limited by several factors, including the use of a volunteer sample, the reliance on self-reports, and the potential differences between Internet survey results and population-based estimates of flu vaccination.
However, “in the absence of vaccination requirements, the findings in this study support the recommendations found in the Guide to Community Preventive Services, which include active promotion of on-site vaccination at no cost or low cost to increase influenza vaccination coverage among HCPs,” the researchers said.
The researchers had no financial conflicts to disclose.
Nearly four out of five health care personnel in the United States received a flu vaccination during the 2016-2017 flu season, but a majority of those working in long-term care settings were not vaccinated, based on data from an Internet survey of more than 2,000 individuals that was conducted by the Centers for Disease Control and Prevention.
A total of 78.6% of the survey’s respondents said they’d been vaccinated during the 2016-2017 season. Vaccination coverage for health care personnel overall has remained in the 77%-79% range in recent years, but that represents an increase from 64% in 2010-2011.
“As in previous seasons, the highest coverage was among HCP whose workplace had vaccination requirements,” noted Carla L. Black, PhD, of the CDC, and colleagues (MMWR Morb Mortal Wkly Rep. 2017 Sep 29;66[38]:1009-15). The researchers reviewed data collected from an Internet panel survey of 2,438 health care personnel between March 28, 2017, and April 19, 2017.
Physicians boasted the highest vaccination coverage in 2016-2017 (96%), followed by pharmacists (94%), nurses (93%), nurse practitioners and physician assistants (92%), other clinical providers (80%), nonclinical health care providers (74%), and aides and assistants (69%).
Flu vaccination rates were highest among HCPs working in a hospital setting (92%); 94% of survey respondents in hospitals reported either having a vaccination requirement at work or being provided at least 1 day of on-site vaccination.
Vaccination rates were lowest among health care personnel in long-term care settings (68%), where only 26% reported a workplace vaccination requirement. However, vaccination rates in long-term care rose to 90% when employers required vaccination.
The report’s findings were limited by several factors, including the use of a volunteer sample, the reliance on self-reports, and the potential differences between Internet survey results and population-based estimates of flu vaccination.
However, “in the absence of vaccination requirements, the findings in this study support the recommendations found in the Guide to Community Preventive Services, which include active promotion of on-site vaccination at no cost or low cost to increase influenza vaccination coverage among HCPs,” the researchers said.
The researchers had no financial conflicts to disclose.
Nearly four out of five health care personnel in the United States received a flu vaccination during the 2016-2017 flu season, but a majority of those working in long-term care settings were not vaccinated, based on data from an Internet survey of more than 2,000 individuals that was conducted by the Centers for Disease Control and Prevention.
A total of 78.6% of the survey’s respondents said they’d been vaccinated during the 2016-2017 season. Vaccination coverage for health care personnel overall has remained in the 77%-79% range in recent years, but that represents an increase from 64% in 2010-2011.
“As in previous seasons, the highest coverage was among HCP whose workplace had vaccination requirements,” noted Carla L. Black, PhD, of the CDC, and colleagues (MMWR Morb Mortal Wkly Rep. 2017 Sep 29;66[38]:1009-15). The researchers reviewed data collected from an Internet panel survey of 2,438 health care personnel between March 28, 2017, and April 19, 2017.
Physicians boasted the highest vaccination coverage in 2016-2017 (96%), followed by pharmacists (94%), nurses (93%), nurse practitioners and physician assistants (92%), other clinical providers (80%), nonclinical health care providers (74%), and aides and assistants (69%).
Flu vaccination rates were highest among HCPs working in a hospital setting (92%); 94% of survey respondents in hospitals reported either having a vaccination requirement at work or being provided at least 1 day of on-site vaccination.
Vaccination rates were lowest among health care personnel in long-term care settings (68%), where only 26% reported a workplace vaccination requirement. However, vaccination rates in long-term care rose to 90% when employers required vaccination.
The report’s findings were limited by several factors, including the use of a volunteer sample, the reliance on self-reports, and the potential differences between Internet survey results and population-based estimates of flu vaccination.
However, “in the absence of vaccination requirements, the findings in this study support the recommendations found in the Guide to Community Preventive Services, which include active promotion of on-site vaccination at no cost or low cost to increase influenza vaccination coverage among HCPs,” the researchers said.
The researchers had no financial conflicts to disclose.
FROM MMWR
Key clinical point:
Major finding: Overall flu vaccination coverage among U.S. health care personnel was 78.6% in the 2016-2017 season
Data source: The data come from an Internet survey of 2,438 health care personnel.
Disclosures: The researchers had no financial conflicts to disclose.
Hyperlipidemia diagnosis protects against breast cancer
BARCELONA – Women diagnosed with hyperlipidemia had a strikingly reduced risk of subsequently developing breast cancer in a big-data, case-control study, Paul R. Carter, MD, reported at the annual congress of the European Society of Cardiology.
Moreover, those baseline hyperlipidemic women who later got breast cancer had a 40% lower risk of all-cause mortality than did matched nonhyperlipidemic controls diagnosed with the malignancy during follow-up, according to Dr. Carter, a cardiology fellow at Cambridge (England) University.
The inference isn’t that hyperlipidemia somehow protects against the most common type of cancer in women. Indeed, preclinical evidence indicates high cholesterol drives several key steps in carcinogenesis. Rather, the strong implication is that the explanation for the observed preventive effect lies in the pleotropic effects of the statin therapy routinely prescribed in accordance with guidelines once women received the diagnosis of hyperlipidemia, he continued.
“The results of this study provide the strongest justification to date for a clinical trial evaluating the protective effect of statins in patients with breast cancer, and this is what we intend to do,” according to Dr. Carter.
He presented a retrospective longitudinal study of the Algorithm for Comorbidities, Associations, Length of Stay and Mortality database, comprising more than 1.2 million patients admitted for various reasons to selected hospitals in northern England during 2000-2014. This big-data study entailed recruitment of 16,043 women aged 40 years and older who were diagnosed with hyperlipidemia during their hospital stay along with an equal number of age-matched women with normal lipid levels. None of the participants had a breast cancer diagnosis at baseline.
The all-cause mortality rate in baseline hyperlipidemic women who later developed breast cancer was 27.4%, significantly lower than the 37.4% rate in normolipidemic women with breast cancer. This translated into an adjusted 40% relative risk reduction.
All-cause mortality occurred during follow-up in 13.7% of breast cancer–free women with baseline hyperlipidemia, compared with 23.6% of nonhyperlipidemic controls without breast cancer.
In an analysis adjusted for age, ethnicity, and the top-10 causes of death in the U.K., women with baseline hyperlipidemia were 40% less likely to die during follow-up than were women without high cholesterol.
Dr. Carter reported having no financial conflicts of interest regarding his study, which was conducted free of commercial support.
BARCELONA – Women diagnosed with hyperlipidemia had a strikingly reduced risk of subsequently developing breast cancer in a big-data, case-control study, Paul R. Carter, MD, reported at the annual congress of the European Society of Cardiology.
Moreover, those baseline hyperlipidemic women who later got breast cancer had a 40% lower risk of all-cause mortality than did matched nonhyperlipidemic controls diagnosed with the malignancy during follow-up, according to Dr. Carter, a cardiology fellow at Cambridge (England) University.
The inference isn’t that hyperlipidemia somehow protects against the most common type of cancer in women. Indeed, preclinical evidence indicates high cholesterol drives several key steps in carcinogenesis. Rather, the strong implication is that the explanation for the observed preventive effect lies in the pleotropic effects of the statin therapy routinely prescribed in accordance with guidelines once women received the diagnosis of hyperlipidemia, he continued.
“The results of this study provide the strongest justification to date for a clinical trial evaluating the protective effect of statins in patients with breast cancer, and this is what we intend to do,” according to Dr. Carter.
He presented a retrospective longitudinal study of the Algorithm for Comorbidities, Associations, Length of Stay and Mortality database, comprising more than 1.2 million patients admitted for various reasons to selected hospitals in northern England during 2000-2014. This big-data study entailed recruitment of 16,043 women aged 40 years and older who were diagnosed with hyperlipidemia during their hospital stay along with an equal number of age-matched women with normal lipid levels. None of the participants had a breast cancer diagnosis at baseline.
The all-cause mortality rate in baseline hyperlipidemic women who later developed breast cancer was 27.4%, significantly lower than the 37.4% rate in normolipidemic women with breast cancer. This translated into an adjusted 40% relative risk reduction.
All-cause mortality occurred during follow-up in 13.7% of breast cancer–free women with baseline hyperlipidemia, compared with 23.6% of nonhyperlipidemic controls without breast cancer.
In an analysis adjusted for age, ethnicity, and the top-10 causes of death in the U.K., women with baseline hyperlipidemia were 40% less likely to die during follow-up than were women without high cholesterol.
Dr. Carter reported having no financial conflicts of interest regarding his study, which was conducted free of commercial support.
BARCELONA – Women diagnosed with hyperlipidemia had a strikingly reduced risk of subsequently developing breast cancer in a big-data, case-control study, Paul R. Carter, MD, reported at the annual congress of the European Society of Cardiology.
Moreover, those baseline hyperlipidemic women who later got breast cancer had a 40% lower risk of all-cause mortality than did matched nonhyperlipidemic controls diagnosed with the malignancy during follow-up, according to Dr. Carter, a cardiology fellow at Cambridge (England) University.
The inference isn’t that hyperlipidemia somehow protects against the most common type of cancer in women. Indeed, preclinical evidence indicates high cholesterol drives several key steps in carcinogenesis. Rather, the strong implication is that the explanation for the observed preventive effect lies in the pleotropic effects of the statin therapy routinely prescribed in accordance with guidelines once women received the diagnosis of hyperlipidemia, he continued.
“The results of this study provide the strongest justification to date for a clinical trial evaluating the protective effect of statins in patients with breast cancer, and this is what we intend to do,” according to Dr. Carter.
He presented a retrospective longitudinal study of the Algorithm for Comorbidities, Associations, Length of Stay and Mortality database, comprising more than 1.2 million patients admitted for various reasons to selected hospitals in northern England during 2000-2014. This big-data study entailed recruitment of 16,043 women aged 40 years and older who were diagnosed with hyperlipidemia during their hospital stay along with an equal number of age-matched women with normal lipid levels. None of the participants had a breast cancer diagnosis at baseline.
The all-cause mortality rate in baseline hyperlipidemic women who later developed breast cancer was 27.4%, significantly lower than the 37.4% rate in normolipidemic women with breast cancer. This translated into an adjusted 40% relative risk reduction.
All-cause mortality occurred during follow-up in 13.7% of breast cancer–free women with baseline hyperlipidemia, compared with 23.6% of nonhyperlipidemic controls without breast cancer.
In an analysis adjusted for age, ethnicity, and the top-10 causes of death in the U.K., women with baseline hyperlipidemia were 40% less likely to die during follow-up than were women without high cholesterol.
Dr. Carter reported having no financial conflicts of interest regarding his study, which was conducted free of commercial support.
AT THE ESC CONGRESS 2017
Key clinical point:
Major finding: The risk of subsequent development of breast cancer was one-third lower in women diagnosed with hyperlipidemia than in controls with normal lipid levels.
Data source: A retrospective longitudinal case-control study of 16,043 U.K. women aged 40 years or older when diagnosed with hyperlipidemia and an equal number of age-matched women with normal lipids.
Disclosures: The presenter reported having no financial conflicts of interest regarding his study, which was conducted free of commercial support.
Insulin pumps associated with lower risk of ketoacidosis and severe hypoglycemia in kids
Children with type 1 diabetes mellitus who use insulin pumps are at a lower risk for ketoacidosis and severe hypoglycemia, compared with those receiving insulin by injection, according to a population-based cohort study from the University of Ulm, Germany.
The study participants were all younger than 20 years of age and were divided into the following groups for data analysis: 1.5-5 years; 6-10 years; 11-15 years; or 16-19 years. The study evaluated both primary and secondary outcomes to analyze the effectiveness of insulin pumps vs. traditional insulin injections. The primary outcomes were the rates of ketoacidosis and hypoglycemia severe enough to require assistance from another person to administer intravenous carbohydrates or induce hypoglycemic coma. The secondary outcomes were serum levels of glycated hemoglobin (HbA1c), daily insulin dose, prandial to total insulin ratio, frequency of self-monitoring blood glucose level, and body mass index, according to Beate Karges, MD, of the University of Aachen (Germany), Division of Endocrinology and Diabetes, and her colleagues.
After applying selection criteria, 30,579 patients treated for type 1 diabetes were selected for analysis. Of the 30,579, a 1 to 1 matched cohort of 19,628 patients, split into equal sized groups, was created for propensity score analysis to compare the effect of treatment between insulin pumps and injects. The matched cohort was also included in the entire cohort for another propensity score analysis. Of the entire 30,579 patients, 14,119 used pump therapy and 16,460 used traditional daily insulin injections.
In the matched cohort, event rates for both severe hypoglycemia and hypoglycemic coma were much lower with insulin pumps than with traditional insulin injections (9.55 vs. 13.97 per 100 patient-years and 2.30 vs. 2.96 per 100 patient-years, respectively). The pattern of pump therapy lowering rates of severe hypoglycemia and hypoglycemic coma was observed in entire cohort as well, according to the investigators (JAMA. 2017 Oct 10;318[14]:1358-66).
The secondary outcomes of the matched cohort did not share as consistent a pattern as the primary outcomes. HbA1c levels were lower in pump users (8.04% vs. 8.22%). Total daily insulin doses were lower in pump users, but the prandial to total insulin ratio was higher. Daily frequency of self-monitoring blood glucose level was also elevated in pump users.
“These findings provide evidence for improved clinical outcomes associated with insulin pump therapy, compared with injection therapy, in children, adolescents, and young adults with type 1 diabetes,” Dr. Karges and her colleagues wrote.
The study was funded by both the Competence Network Diabetes Mellitus and the German Center for Diabetes Research. Thomas Kapellen, MD, had received funding from the European Commission concerning closed-loop systems as well as speaking fees for pharmaceuticals companies including Abbott, Medtronic, and Novo Nordisk. No other authors reported financial conflicts.
Children with type 1 diabetes mellitus who use insulin pumps are at a lower risk for ketoacidosis and severe hypoglycemia, compared with those receiving insulin by injection, according to a population-based cohort study from the University of Ulm, Germany.
The study participants were all younger than 20 years of age and were divided into the following groups for data analysis: 1.5-5 years; 6-10 years; 11-15 years; or 16-19 years. The study evaluated both primary and secondary outcomes to analyze the effectiveness of insulin pumps vs. traditional insulin injections. The primary outcomes were the rates of ketoacidosis and hypoglycemia severe enough to require assistance from another person to administer intravenous carbohydrates or induce hypoglycemic coma. The secondary outcomes were serum levels of glycated hemoglobin (HbA1c), daily insulin dose, prandial to total insulin ratio, frequency of self-monitoring blood glucose level, and body mass index, according to Beate Karges, MD, of the University of Aachen (Germany), Division of Endocrinology and Diabetes, and her colleagues.
After applying selection criteria, 30,579 patients treated for type 1 diabetes were selected for analysis. Of the 30,579, a 1 to 1 matched cohort of 19,628 patients, split into equal sized groups, was created for propensity score analysis to compare the effect of treatment between insulin pumps and injects. The matched cohort was also included in the entire cohort for another propensity score analysis. Of the entire 30,579 patients, 14,119 used pump therapy and 16,460 used traditional daily insulin injections.
In the matched cohort, event rates for both severe hypoglycemia and hypoglycemic coma were much lower with insulin pumps than with traditional insulin injections (9.55 vs. 13.97 per 100 patient-years and 2.30 vs. 2.96 per 100 patient-years, respectively). The pattern of pump therapy lowering rates of severe hypoglycemia and hypoglycemic coma was observed in entire cohort as well, according to the investigators (JAMA. 2017 Oct 10;318[14]:1358-66).
The secondary outcomes of the matched cohort did not share as consistent a pattern as the primary outcomes. HbA1c levels were lower in pump users (8.04% vs. 8.22%). Total daily insulin doses were lower in pump users, but the prandial to total insulin ratio was higher. Daily frequency of self-monitoring blood glucose level was also elevated in pump users.
“These findings provide evidence for improved clinical outcomes associated with insulin pump therapy, compared with injection therapy, in children, adolescents, and young adults with type 1 diabetes,” Dr. Karges and her colleagues wrote.
The study was funded by both the Competence Network Diabetes Mellitus and the German Center for Diabetes Research. Thomas Kapellen, MD, had received funding from the European Commission concerning closed-loop systems as well as speaking fees for pharmaceuticals companies including Abbott, Medtronic, and Novo Nordisk. No other authors reported financial conflicts.
Children with type 1 diabetes mellitus who use insulin pumps are at a lower risk for ketoacidosis and severe hypoglycemia, compared with those receiving insulin by injection, according to a population-based cohort study from the University of Ulm, Germany.
The study participants were all younger than 20 years of age and were divided into the following groups for data analysis: 1.5-5 years; 6-10 years; 11-15 years; or 16-19 years. The study evaluated both primary and secondary outcomes to analyze the effectiveness of insulin pumps vs. traditional insulin injections. The primary outcomes were the rates of ketoacidosis and hypoglycemia severe enough to require assistance from another person to administer intravenous carbohydrates or induce hypoglycemic coma. The secondary outcomes were serum levels of glycated hemoglobin (HbA1c), daily insulin dose, prandial to total insulin ratio, frequency of self-monitoring blood glucose level, and body mass index, according to Beate Karges, MD, of the University of Aachen (Germany), Division of Endocrinology and Diabetes, and her colleagues.
After applying selection criteria, 30,579 patients treated for type 1 diabetes were selected for analysis. Of the 30,579, a 1 to 1 matched cohort of 19,628 patients, split into equal sized groups, was created for propensity score analysis to compare the effect of treatment between insulin pumps and injects. The matched cohort was also included in the entire cohort for another propensity score analysis. Of the entire 30,579 patients, 14,119 used pump therapy and 16,460 used traditional daily insulin injections.
In the matched cohort, event rates for both severe hypoglycemia and hypoglycemic coma were much lower with insulin pumps than with traditional insulin injections (9.55 vs. 13.97 per 100 patient-years and 2.30 vs. 2.96 per 100 patient-years, respectively). The pattern of pump therapy lowering rates of severe hypoglycemia and hypoglycemic coma was observed in entire cohort as well, according to the investigators (JAMA. 2017 Oct 10;318[14]:1358-66).
The secondary outcomes of the matched cohort did not share as consistent a pattern as the primary outcomes. HbA1c levels were lower in pump users (8.04% vs. 8.22%). Total daily insulin doses were lower in pump users, but the prandial to total insulin ratio was higher. Daily frequency of self-monitoring blood glucose level was also elevated in pump users.
“These findings provide evidence for improved clinical outcomes associated with insulin pump therapy, compared with injection therapy, in children, adolescents, and young adults with type 1 diabetes,” Dr. Karges and her colleagues wrote.
The study was funded by both the Competence Network Diabetes Mellitus and the German Center for Diabetes Research. Thomas Kapellen, MD, had received funding from the European Commission concerning closed-loop systems as well as speaking fees for pharmaceuticals companies including Abbott, Medtronic, and Novo Nordisk. No other authors reported financial conflicts.
FROM JAMA
Key clinical point:
Major finding: Patients using insulin pumps had lower rates of hypoglycemia (9.55 per 100 patient-years) and severe ketoacidosis (3.64 per 100 patient-years).
Data source: Population cohort study of 30,579 patients from the Diabetes Prospective Follow-up Initiative Database.
Disclosures: The study was funded by both the Competence Network Diabetes Mellitus and the German Center for Diabetes Research. Thomas Kapellen, MD, had received funding from the European Commission concerning closed-loop systems as well as speaking fees for pharmaceutical companies including Abbott, Medtronic, and Novo Nordisk. No other authors reported financial conflicts.
Revisions proposed for sexual behaviors in ICD-11
BERLIN – A move to depathologize paraphilias that do not adversely affect self or others is at the heart of changes suggested for the eleventh revision of the International Classification of Diseases and Related Health Problems.
The upcoming version of the World Health Organization document, slated for release in 2018, would reduce the number of named F65 classifications from nine to five, Richard B. Krueger, MD, said at the meeting of the World Psychiatric Association.
Three behaviors now included – fetishism, fetishistic transvestitism, and sadomasochism – would be dropped altogether, said Dr. Krueger of Columbia University, New York. Others would be streamlined into four more general diagnostic groupings (exhibitionism, voyeurism, pedophilia, and coercive sexual sadism disorderA fifth diagnosis, frotteuristic disorder, would be added in light of its fairly common presentation in clinical practice. Two new general categories would address unspecified behaviors that potentially are criminal, or which cause distress or danger to the individual who performs them, Dr. Krueger said.
The proposed changes would bring the document more in line with the DSM-5, said Dr. Krueger, who also leads the Working Group on the Classification of Sexual Disorders and Sexual Health. WHO charged the committee with reviewing the evidence and making recommendations for categories related to sexuality that are contained in the chapter of Mental and Behavioral Disorders in ICD-10.
This definition parallels the A criterion in the paraphilic definitions in the DSM-5, which identifies a pattern of “recurrent and intense sexual arousal” as well as the B criterion, which specifies that the person “has acted on these sexual urges with a nonconsenting person, or the sexual urges or fantasies cause clinically significant distress or impairment in social, occupational, or other important areas of functioning.”
The proposed revisions reflect a growing acceptance of the immense variation in human sexual behavior, and the depathologization of some of them, as long as they do not affect public health, said Dr. Krueger, a psychiatrist with expertise in forensic and sexual psychiatry.
“In a general sense, this is analogous to the situation that we faced with alcohol and drug users in the early 20th century, when the main treatment was incarceration. In the 1940s and 50s, if you admitted an alcoholic to the hospital for delirium tremens, you could risk losing your admitting privileges. This is similar to where we are now with the paraphilic disorders,” he said, with most of them still considered potentially criminal.
That paraphilic disorders will remain at all in ICD-11 is something of a compromise.
“At first, we considered outright deletion of this section. Many psychiatrists have suggested we have no business with these disorders, since paraphilias are largely dealt with in the legal system. Some feel that psychiatrists should not be involved with them at all. But there has been a lot of discussion about the public health impact of these behaviors. For example, about 50% of men incarcerated for crimes against children meet the diagnostic criteria for pedophilia, and about 10% of crimes that result in incarceration in the U.S. are sexual crimes. There is also forensic usage of these diagnoses in many countries in Europe, and in Great Britain, the U.S., and Canada, so we felt that it’s important to maintain them” as diagnosable disorders. Keeping official diagnostic codes alive also helps encourage research into epidemiology and potential treatments, he added.
The committee’s first recommendation is to change the name of the chapter from “Disorders of Sexual Preference” to “Paraphilic Disorders.”
“This better represents the content of the section, which involves atypical sexual interests,” Dr. Krueger said. “The word ‘disorders’ was added to clarify that these atypical interests have to be pathological. That is, they must result in action against a nonconsenting individual, or cause severe distress or significant risk of injury or death to the patient.”
This emphasis on a pathological aspect to the diagnoses leads directly to the elimination of the fetishism, transvestitism, and sadomasochism categories, which are considered largely benign. “These have no public health importance, generally no association with distress or functional impairment, and including them can result in stigmatization with no discernible health benefit,” Dr. Krueger said. “If you’re a sadomasochist and not bothered by it, and engage alone or with a consenting person, it’s not a problem. Why should these categories be retained if they are not associated with distress or dysfunction? If they are not, then clearly they are not disorders.”
The remaining diagnoses were not as cut-and-dried, he said. Voyeurism, exhibitionism, pedophilia, and frotteurism clearly can affect others who are either unwilling or unable to consent because of age or other circumstances – for example, children, unaware targets, and animals. These behaviors also present with different intensities, from solitary imaginings to well planned and executed acts.
Sadomasochism did retain an altered diagnostic code as a new entity: coercive sexual sadism disorder. While many practice sadomasochism with a willing partner and are not disturbed by it, the committee acknowledged that crimes of sexual sadism are a serious threat to public health and should be recognized as such.
The committee also added two general categories to cover unnamed paraphilias, of which there are many.
“Other paraphilic disorder involving nonconsenting individuals” may be used for a behavior in which the focus of arousal is unwilling or unable to consent, but not specified. Zoophilia would fall into this category, as would other potentially criminal acts. “We can capture a huge variety of paraphilic behaviors under this rubric,” Dr. Krueger said.
During discussion, some clinicians expressed concern that, in its effort to depathologize solitary behavior, the proposal shortchanges patients who suffer deeply from their sexual behaviors. An example, one British psychiatrist said, is the person who is distressed because he can’t experience sexual enjoyment without a particular object or behavior. The final diagnostic category, “Other paraphilic disorder involving solitary behavior or consenting individuals,” will serve that person’s needs, Dr. Krueger said.
“This is an attempt to address the concern if a behavior markedly distresses a person, outside of the rather normal fear of social rejection, or if the behavior poses a serious risk, such as asphyxiophilia.”
The draft proposal was published earlier this year in Archives of Sexual Behavior (2017 Jul;46[5]:1529-45).
Dr. Krueger had no financial disclosures.
msullivan@frontlinemedcom.com
On Twitter @Alz_Gal
BERLIN – A move to depathologize paraphilias that do not adversely affect self or others is at the heart of changes suggested for the eleventh revision of the International Classification of Diseases and Related Health Problems.
The upcoming version of the World Health Organization document, slated for release in 2018, would reduce the number of named F65 classifications from nine to five, Richard B. Krueger, MD, said at the meeting of the World Psychiatric Association.
Three behaviors now included – fetishism, fetishistic transvestitism, and sadomasochism – would be dropped altogether, said Dr. Krueger of Columbia University, New York. Others would be streamlined into four more general diagnostic groupings (exhibitionism, voyeurism, pedophilia, and coercive sexual sadism disorderA fifth diagnosis, frotteuristic disorder, would be added in light of its fairly common presentation in clinical practice. Two new general categories would address unspecified behaviors that potentially are criminal, or which cause distress or danger to the individual who performs them, Dr. Krueger said.
The proposed changes would bring the document more in line with the DSM-5, said Dr. Krueger, who also leads the Working Group on the Classification of Sexual Disorders and Sexual Health. WHO charged the committee with reviewing the evidence and making recommendations for categories related to sexuality that are contained in the chapter of Mental and Behavioral Disorders in ICD-10.
This definition parallels the A criterion in the paraphilic definitions in the DSM-5, which identifies a pattern of “recurrent and intense sexual arousal” as well as the B criterion, which specifies that the person “has acted on these sexual urges with a nonconsenting person, or the sexual urges or fantasies cause clinically significant distress or impairment in social, occupational, or other important areas of functioning.”
The proposed revisions reflect a growing acceptance of the immense variation in human sexual behavior, and the depathologization of some of them, as long as they do not affect public health, said Dr. Krueger, a psychiatrist with expertise in forensic and sexual psychiatry.
“In a general sense, this is analogous to the situation that we faced with alcohol and drug users in the early 20th century, when the main treatment was incarceration. In the 1940s and 50s, if you admitted an alcoholic to the hospital for delirium tremens, you could risk losing your admitting privileges. This is similar to where we are now with the paraphilic disorders,” he said, with most of them still considered potentially criminal.
That paraphilic disorders will remain at all in ICD-11 is something of a compromise.
“At first, we considered outright deletion of this section. Many psychiatrists have suggested we have no business with these disorders, since paraphilias are largely dealt with in the legal system. Some feel that psychiatrists should not be involved with them at all. But there has been a lot of discussion about the public health impact of these behaviors. For example, about 50% of men incarcerated for crimes against children meet the diagnostic criteria for pedophilia, and about 10% of crimes that result in incarceration in the U.S. are sexual crimes. There is also forensic usage of these diagnoses in many countries in Europe, and in Great Britain, the U.S., and Canada, so we felt that it’s important to maintain them” as diagnosable disorders. Keeping official diagnostic codes alive also helps encourage research into epidemiology and potential treatments, he added.
The committee’s first recommendation is to change the name of the chapter from “Disorders of Sexual Preference” to “Paraphilic Disorders.”
“This better represents the content of the section, which involves atypical sexual interests,” Dr. Krueger said. “The word ‘disorders’ was added to clarify that these atypical interests have to be pathological. That is, they must result in action against a nonconsenting individual, or cause severe distress or significant risk of injury or death to the patient.”
This emphasis on a pathological aspect to the diagnoses leads directly to the elimination of the fetishism, transvestitism, and sadomasochism categories, which are considered largely benign. “These have no public health importance, generally no association with distress or functional impairment, and including them can result in stigmatization with no discernible health benefit,” Dr. Krueger said. “If you’re a sadomasochist and not bothered by it, and engage alone or with a consenting person, it’s not a problem. Why should these categories be retained if they are not associated with distress or dysfunction? If they are not, then clearly they are not disorders.”
The remaining diagnoses were not as cut-and-dried, he said. Voyeurism, exhibitionism, pedophilia, and frotteurism clearly can affect others who are either unwilling or unable to consent because of age or other circumstances – for example, children, unaware targets, and animals. These behaviors also present with different intensities, from solitary imaginings to well planned and executed acts.
Sadomasochism did retain an altered diagnostic code as a new entity: coercive sexual sadism disorder. While many practice sadomasochism with a willing partner and are not disturbed by it, the committee acknowledged that crimes of sexual sadism are a serious threat to public health and should be recognized as such.
The committee also added two general categories to cover unnamed paraphilias, of which there are many.
“Other paraphilic disorder involving nonconsenting individuals” may be used for a behavior in which the focus of arousal is unwilling or unable to consent, but not specified. Zoophilia would fall into this category, as would other potentially criminal acts. “We can capture a huge variety of paraphilic behaviors under this rubric,” Dr. Krueger said.
During discussion, some clinicians expressed concern that, in its effort to depathologize solitary behavior, the proposal shortchanges patients who suffer deeply from their sexual behaviors. An example, one British psychiatrist said, is the person who is distressed because he can’t experience sexual enjoyment without a particular object or behavior. The final diagnostic category, “Other paraphilic disorder involving solitary behavior or consenting individuals,” will serve that person’s needs, Dr. Krueger said.
“This is an attempt to address the concern if a behavior markedly distresses a person, outside of the rather normal fear of social rejection, or if the behavior poses a serious risk, such as asphyxiophilia.”
The draft proposal was published earlier this year in Archives of Sexual Behavior (2017 Jul;46[5]:1529-45).
Dr. Krueger had no financial disclosures.
msullivan@frontlinemedcom.com
On Twitter @Alz_Gal
BERLIN – A move to depathologize paraphilias that do not adversely affect self or others is at the heart of changes suggested for the eleventh revision of the International Classification of Diseases and Related Health Problems.
The upcoming version of the World Health Organization document, slated for release in 2018, would reduce the number of named F65 classifications from nine to five, Richard B. Krueger, MD, said at the meeting of the World Psychiatric Association.
Three behaviors now included – fetishism, fetishistic transvestitism, and sadomasochism – would be dropped altogether, said Dr. Krueger of Columbia University, New York. Others would be streamlined into four more general diagnostic groupings (exhibitionism, voyeurism, pedophilia, and coercive sexual sadism disorderA fifth diagnosis, frotteuristic disorder, would be added in light of its fairly common presentation in clinical practice. Two new general categories would address unspecified behaviors that potentially are criminal, or which cause distress or danger to the individual who performs them, Dr. Krueger said.
The proposed changes would bring the document more in line with the DSM-5, said Dr. Krueger, who also leads the Working Group on the Classification of Sexual Disorders and Sexual Health. WHO charged the committee with reviewing the evidence and making recommendations for categories related to sexuality that are contained in the chapter of Mental and Behavioral Disorders in ICD-10.
This definition parallels the A criterion in the paraphilic definitions in the DSM-5, which identifies a pattern of “recurrent and intense sexual arousal” as well as the B criterion, which specifies that the person “has acted on these sexual urges with a nonconsenting person, or the sexual urges or fantasies cause clinically significant distress or impairment in social, occupational, or other important areas of functioning.”
The proposed revisions reflect a growing acceptance of the immense variation in human sexual behavior, and the depathologization of some of them, as long as they do not affect public health, said Dr. Krueger, a psychiatrist with expertise in forensic and sexual psychiatry.
“In a general sense, this is analogous to the situation that we faced with alcohol and drug users in the early 20th century, when the main treatment was incarceration. In the 1940s and 50s, if you admitted an alcoholic to the hospital for delirium tremens, you could risk losing your admitting privileges. This is similar to where we are now with the paraphilic disorders,” he said, with most of them still considered potentially criminal.
That paraphilic disorders will remain at all in ICD-11 is something of a compromise.
“At first, we considered outright deletion of this section. Many psychiatrists have suggested we have no business with these disorders, since paraphilias are largely dealt with in the legal system. Some feel that psychiatrists should not be involved with them at all. But there has been a lot of discussion about the public health impact of these behaviors. For example, about 50% of men incarcerated for crimes against children meet the diagnostic criteria for pedophilia, and about 10% of crimes that result in incarceration in the U.S. are sexual crimes. There is also forensic usage of these diagnoses in many countries in Europe, and in Great Britain, the U.S., and Canada, so we felt that it’s important to maintain them” as diagnosable disorders. Keeping official diagnostic codes alive also helps encourage research into epidemiology and potential treatments, he added.
The committee’s first recommendation is to change the name of the chapter from “Disorders of Sexual Preference” to “Paraphilic Disorders.”
“This better represents the content of the section, which involves atypical sexual interests,” Dr. Krueger said. “The word ‘disorders’ was added to clarify that these atypical interests have to be pathological. That is, they must result in action against a nonconsenting individual, or cause severe distress or significant risk of injury or death to the patient.”
This emphasis on a pathological aspect to the diagnoses leads directly to the elimination of the fetishism, transvestitism, and sadomasochism categories, which are considered largely benign. “These have no public health importance, generally no association with distress or functional impairment, and including them can result in stigmatization with no discernible health benefit,” Dr. Krueger said. “If you’re a sadomasochist and not bothered by it, and engage alone or with a consenting person, it’s not a problem. Why should these categories be retained if they are not associated with distress or dysfunction? If they are not, then clearly they are not disorders.”
The remaining diagnoses were not as cut-and-dried, he said. Voyeurism, exhibitionism, pedophilia, and frotteurism clearly can affect others who are either unwilling or unable to consent because of age or other circumstances – for example, children, unaware targets, and animals. These behaviors also present with different intensities, from solitary imaginings to well planned and executed acts.
Sadomasochism did retain an altered diagnostic code as a new entity: coercive sexual sadism disorder. While many practice sadomasochism with a willing partner and are not disturbed by it, the committee acknowledged that crimes of sexual sadism are a serious threat to public health and should be recognized as such.
The committee also added two general categories to cover unnamed paraphilias, of which there are many.
“Other paraphilic disorder involving nonconsenting individuals” may be used for a behavior in which the focus of arousal is unwilling or unable to consent, but not specified. Zoophilia would fall into this category, as would other potentially criminal acts. “We can capture a huge variety of paraphilic behaviors under this rubric,” Dr. Krueger said.
During discussion, some clinicians expressed concern that, in its effort to depathologize solitary behavior, the proposal shortchanges patients who suffer deeply from their sexual behaviors. An example, one British psychiatrist said, is the person who is distressed because he can’t experience sexual enjoyment without a particular object or behavior. The final diagnostic category, “Other paraphilic disorder involving solitary behavior or consenting individuals,” will serve that person’s needs, Dr. Krueger said.
“This is an attempt to address the concern if a behavior markedly distresses a person, outside of the rather normal fear of social rejection, or if the behavior poses a serious risk, such as asphyxiophilia.”
The draft proposal was published earlier this year in Archives of Sexual Behavior (2017 Jul;46[5]:1529-45).
Dr. Krueger had no financial disclosures.
msullivan@frontlinemedcom.com
On Twitter @Alz_Gal
AT WPA 2017
HIV Testing Low Among Transgender Adults
Transgender men and women are at high risk for HIV infection. In a recent analysis of more than 9 million CDC-funded HIV test results, transgender women had the highest percentage of confirmed positive results (2.7%) of any gender category. But this group also tends to have too-low testing numbers. In a CDC study, only 36% of transgender women and 32% of transgender men reported being tested; only 10% of both groups had been tested in the past year. By comparison, gay and bisexual men reported getting tested at roughly twice the rates (61.8% ever and 21.6% past year).
Black transgender women and men had twice the prevalence of ever testing compared with their white counterparts (63%-67% vs 31%-33%). Transgender women who had been diagnosed with a depressive disorder had the highest prevalence of getting tested for HIV (69%).
Transgender persons face “unique barriers to testing,” the CDC researchers say, such as the HIV stigma within the transgender community, gender identity stigma in health care settings, and socioeconomic marginalization. The CDC is working on “innovative approaches” to delivering HIV testing and other prevention and support services to transgender persons who are at risk for or have newly diagnosed HIV.
Transgender men and women are at high risk for HIV infection. In a recent analysis of more than 9 million CDC-funded HIV test results, transgender women had the highest percentage of confirmed positive results (2.7%) of any gender category. But this group also tends to have too-low testing numbers. In a CDC study, only 36% of transgender women and 32% of transgender men reported being tested; only 10% of both groups had been tested in the past year. By comparison, gay and bisexual men reported getting tested at roughly twice the rates (61.8% ever and 21.6% past year).
Black transgender women and men had twice the prevalence of ever testing compared with their white counterparts (63%-67% vs 31%-33%). Transgender women who had been diagnosed with a depressive disorder had the highest prevalence of getting tested for HIV (69%).
Transgender persons face “unique barriers to testing,” the CDC researchers say, such as the HIV stigma within the transgender community, gender identity stigma in health care settings, and socioeconomic marginalization. The CDC is working on “innovative approaches” to delivering HIV testing and other prevention and support services to transgender persons who are at risk for or have newly diagnosed HIV.
Transgender men and women are at high risk for HIV infection. In a recent analysis of more than 9 million CDC-funded HIV test results, transgender women had the highest percentage of confirmed positive results (2.7%) of any gender category. But this group also tends to have too-low testing numbers. In a CDC study, only 36% of transgender women and 32% of transgender men reported being tested; only 10% of both groups had been tested in the past year. By comparison, gay and bisexual men reported getting tested at roughly twice the rates (61.8% ever and 21.6% past year).
Black transgender women and men had twice the prevalence of ever testing compared with their white counterparts (63%-67% vs 31%-33%). Transgender women who had been diagnosed with a depressive disorder had the highest prevalence of getting tested for HIV (69%).
Transgender persons face “unique barriers to testing,” the CDC researchers say, such as the HIV stigma within the transgender community, gender identity stigma in health care settings, and socioeconomic marginalization. The CDC is working on “innovative approaches” to delivering HIV testing and other prevention and support services to transgender persons who are at risk for or have newly diagnosed HIV.
Immunotherapy demonstrates potential for T-cell lymphoma
Researchers have reported successful inhibition of the phosphatase SHIP1, which may be an effective approach for treating T-cell lymphoma.
The team found that intermittent treatment with a SHIP1 inhibitor prevented the immune exhaustion observed with SHIP1 deletion.
Intermittent SHIP1 inhibition enhanced the antitumor activity of natural killer (NK) cells and T cells in a mouse model of T-cell lymphoma.
The treatment also appeared to have a direct chemotherapeutic effect and induced immunological memory against lymphoma cells.
Matthew Gumbleton, MD, of SUNY Upstate Medical University in Syracuse, New York, and his colleagues reported these results in Science Signaling.
The researchers noted that previous efforts to inhibit SHIP1 have yielded disappointing results. Mice engineered to lack the SHIP1 gene had poorly responsive immune systems, potentially because overactivated cells became exhausted.
Dr Gumbleton and his colleagues found they could overcome this problem by administering a SHIP1 inhibitor—3-a-aminocholestane (3AC)—in a pulsed regimen of 2 consecutive treatment days per week.
The team tested this regimen in mouse models of colorectal cancer and T-cell lymphoma (RMA-Rae1).
In the lymphoma model, intermittent 3AC treatment increased the responsiveness of T cells and NK cells.
The treatment significantly increased the number of NK cells at the tumor site and the terminal maturation of the peripheral NK-cell compartment.
3AC also enhanced FasL-Fas–mediated killing of lymphoma cells. (NK cells induce apoptosis of target cells via Fas-FasL signaling.)
However, 3AC treatment reduced lymphoma burden in NK-cell-deficient mice as well. Therefore, the researchers believe 3AC may have a direct chemotherapeutic effect.
The team also found that intermittent 3AC treatment increased survival in lymphoma-bearing mice.
Treated mice had significantly longer survival than control mice. And some of the treated mice had long-term survival with no evidence of tumor burden, which suggests the treatment could be curative.
Additional experiments revealed that both NK cells and T cells were required to induce long-term survival in the lymphoma-bearing mice.
Finally, the researchers found evidence to suggest that 3AC treatment triggered “immunological memory capable of sustained and protective antitumor response that prevents relapse.”
The team infused hematolymphoid cells from either a naïve donor mouse or a lymphoma-challenged, 3AC-treated, long-term-surviving donor mouse into naïve host mice. The host mice were then challenged with RMA-Rae1 cells but didn’t receive 3AC.
Mice that received cells from the 3AC-treated donors had significantly better survival than mice that received cells from naive donors.
Based on these results, the researchers concluded that intermittent SHIP1 inhibition may be effective for treating and preventing relapse of T-cell lymphoma and other cancers. ![]()
Researchers have reported successful inhibition of the phosphatase SHIP1, which may be an effective approach for treating T-cell lymphoma.
The team found that intermittent treatment with a SHIP1 inhibitor prevented the immune exhaustion observed with SHIP1 deletion.
Intermittent SHIP1 inhibition enhanced the antitumor activity of natural killer (NK) cells and T cells in a mouse model of T-cell lymphoma.
The treatment also appeared to have a direct chemotherapeutic effect and induced immunological memory against lymphoma cells.
Matthew Gumbleton, MD, of SUNY Upstate Medical University in Syracuse, New York, and his colleagues reported these results in Science Signaling.
The researchers noted that previous efforts to inhibit SHIP1 have yielded disappointing results. Mice engineered to lack the SHIP1 gene had poorly responsive immune systems, potentially because overactivated cells became exhausted.
Dr Gumbleton and his colleagues found they could overcome this problem by administering a SHIP1 inhibitor—3-a-aminocholestane (3AC)—in a pulsed regimen of 2 consecutive treatment days per week.
The team tested this regimen in mouse models of colorectal cancer and T-cell lymphoma (RMA-Rae1).
In the lymphoma model, intermittent 3AC treatment increased the responsiveness of T cells and NK cells.
The treatment significantly increased the number of NK cells at the tumor site and the terminal maturation of the peripheral NK-cell compartment.
3AC also enhanced FasL-Fas–mediated killing of lymphoma cells. (NK cells induce apoptosis of target cells via Fas-FasL signaling.)
However, 3AC treatment reduced lymphoma burden in NK-cell-deficient mice as well. Therefore, the researchers believe 3AC may have a direct chemotherapeutic effect.
The team also found that intermittent 3AC treatment increased survival in lymphoma-bearing mice.
Treated mice had significantly longer survival than control mice. And some of the treated mice had long-term survival with no evidence of tumor burden, which suggests the treatment could be curative.
Additional experiments revealed that both NK cells and T cells were required to induce long-term survival in the lymphoma-bearing mice.
Finally, the researchers found evidence to suggest that 3AC treatment triggered “immunological memory capable of sustained and protective antitumor response that prevents relapse.”
The team infused hematolymphoid cells from either a naïve donor mouse or a lymphoma-challenged, 3AC-treated, long-term-surviving donor mouse into naïve host mice. The host mice were then challenged with RMA-Rae1 cells but didn’t receive 3AC.
Mice that received cells from the 3AC-treated donors had significantly better survival than mice that received cells from naive donors.
Based on these results, the researchers concluded that intermittent SHIP1 inhibition may be effective for treating and preventing relapse of T-cell lymphoma and other cancers. ![]()
Researchers have reported successful inhibition of the phosphatase SHIP1, which may be an effective approach for treating T-cell lymphoma.
The team found that intermittent treatment with a SHIP1 inhibitor prevented the immune exhaustion observed with SHIP1 deletion.
Intermittent SHIP1 inhibition enhanced the antitumor activity of natural killer (NK) cells and T cells in a mouse model of T-cell lymphoma.
The treatment also appeared to have a direct chemotherapeutic effect and induced immunological memory against lymphoma cells.
Matthew Gumbleton, MD, of SUNY Upstate Medical University in Syracuse, New York, and his colleagues reported these results in Science Signaling.
The researchers noted that previous efforts to inhibit SHIP1 have yielded disappointing results. Mice engineered to lack the SHIP1 gene had poorly responsive immune systems, potentially because overactivated cells became exhausted.
Dr Gumbleton and his colleagues found they could overcome this problem by administering a SHIP1 inhibitor—3-a-aminocholestane (3AC)—in a pulsed regimen of 2 consecutive treatment days per week.
The team tested this regimen in mouse models of colorectal cancer and T-cell lymphoma (RMA-Rae1).
In the lymphoma model, intermittent 3AC treatment increased the responsiveness of T cells and NK cells.
The treatment significantly increased the number of NK cells at the tumor site and the terminal maturation of the peripheral NK-cell compartment.
3AC also enhanced FasL-Fas–mediated killing of lymphoma cells. (NK cells induce apoptosis of target cells via Fas-FasL signaling.)
However, 3AC treatment reduced lymphoma burden in NK-cell-deficient mice as well. Therefore, the researchers believe 3AC may have a direct chemotherapeutic effect.
The team also found that intermittent 3AC treatment increased survival in lymphoma-bearing mice.
Treated mice had significantly longer survival than control mice. And some of the treated mice had long-term survival with no evidence of tumor burden, which suggests the treatment could be curative.
Additional experiments revealed that both NK cells and T cells were required to induce long-term survival in the lymphoma-bearing mice.
Finally, the researchers found evidence to suggest that 3AC treatment triggered “immunological memory capable of sustained and protective antitumor response that prevents relapse.”
The team infused hematolymphoid cells from either a naïve donor mouse or a lymphoma-challenged, 3AC-treated, long-term-surviving donor mouse into naïve host mice. The host mice were then challenged with RMA-Rae1 cells but didn’t receive 3AC.
Mice that received cells from the 3AC-treated donors had significantly better survival than mice that received cells from naive donors.
Based on these results, the researchers concluded that intermittent SHIP1 inhibition may be effective for treating and preventing relapse of T-cell lymphoma and other cancers. ![]()
AML trial placed on full clinical hold
The US Food and Drug Administration (FDA) has placed a full clinical hold on a phase 1/2 trial of SEL24, a dual PIM/FLT3 kinase inhibitor, in patients with relapsed/refractory acute myeloid leukemia (AML).
The hold is due to a fatal cerebral adverse event that is considered possibly related to SEL24.
The clinical hold means no new patients will be enrolled in the trial and enrolled patients will not receive SEL24 until the hold is lifted.
Selvita S.A., the company developing SEL24, received a clinical hold letter from the FDA on October 6 and said it plans to work with the agency to have the hold lifted.
As part of this process, Selvita will provide the FDA with additional data and analysis on patients treated with SEL24 as well as a proposed protocol amendment.
The trial began in the first quarter of 2017. The study is designed to determine the maximum tolerated dose and recommended dose of SEL24 in patients with relapsed and refractory AML. The study began with a 25 mg daily dose, which was then escalated following cohort reviews.
One AML patient started treatment with a 150 mg dose of SEL24 as the third patient in this dose cohort and received 4 doses of the drug. This patient developed a life-threatening, grade 4 venous thrombus in the brain with subsequent intracerebral hemorrhage, which required hospitalization.
The patient died in hospice 4 days later due to the cerebral event. The patient’s death was subsequently evaluated as possibly related to SEL24.
A safety report and a review of data by the trial’s data monitoring committee were submitted to the FDA. The agency then placed a clinical hold on the trial and requested more safety data on patients who have received SEL24, as well as specific protocol changes and additional guidance to the study staff.
Selvita said it plans to comply with the requests and provide additional information to the agency and clinical trial centers, in collaboration with the Menarini Group, its global development partner for SEL24.
The FDA has 30 days from the receipt of Selvita’s response to let the company know whether the clinical hold is lifted. ![]()
The US Food and Drug Administration (FDA) has placed a full clinical hold on a phase 1/2 trial of SEL24, a dual PIM/FLT3 kinase inhibitor, in patients with relapsed/refractory acute myeloid leukemia (AML).
The hold is due to a fatal cerebral adverse event that is considered possibly related to SEL24.
The clinical hold means no new patients will be enrolled in the trial and enrolled patients will not receive SEL24 until the hold is lifted.
Selvita S.A., the company developing SEL24, received a clinical hold letter from the FDA on October 6 and said it plans to work with the agency to have the hold lifted.
As part of this process, Selvita will provide the FDA with additional data and analysis on patients treated with SEL24 as well as a proposed protocol amendment.
The trial began in the first quarter of 2017. The study is designed to determine the maximum tolerated dose and recommended dose of SEL24 in patients with relapsed and refractory AML. The study began with a 25 mg daily dose, which was then escalated following cohort reviews.
One AML patient started treatment with a 150 mg dose of SEL24 as the third patient in this dose cohort and received 4 doses of the drug. This patient developed a life-threatening, grade 4 venous thrombus in the brain with subsequent intracerebral hemorrhage, which required hospitalization.
The patient died in hospice 4 days later due to the cerebral event. The patient’s death was subsequently evaluated as possibly related to SEL24.
A safety report and a review of data by the trial’s data monitoring committee were submitted to the FDA. The agency then placed a clinical hold on the trial and requested more safety data on patients who have received SEL24, as well as specific protocol changes and additional guidance to the study staff.
Selvita said it plans to comply with the requests and provide additional information to the agency and clinical trial centers, in collaboration with the Menarini Group, its global development partner for SEL24.
The FDA has 30 days from the receipt of Selvita’s response to let the company know whether the clinical hold is lifted. ![]()
The US Food and Drug Administration (FDA) has placed a full clinical hold on a phase 1/2 trial of SEL24, a dual PIM/FLT3 kinase inhibitor, in patients with relapsed/refractory acute myeloid leukemia (AML).
The hold is due to a fatal cerebral adverse event that is considered possibly related to SEL24.
The clinical hold means no new patients will be enrolled in the trial and enrolled patients will not receive SEL24 until the hold is lifted.
Selvita S.A., the company developing SEL24, received a clinical hold letter from the FDA on October 6 and said it plans to work with the agency to have the hold lifted.
As part of this process, Selvita will provide the FDA with additional data and analysis on patients treated with SEL24 as well as a proposed protocol amendment.
The trial began in the first quarter of 2017. The study is designed to determine the maximum tolerated dose and recommended dose of SEL24 in patients with relapsed and refractory AML. The study began with a 25 mg daily dose, which was then escalated following cohort reviews.
One AML patient started treatment with a 150 mg dose of SEL24 as the third patient in this dose cohort and received 4 doses of the drug. This patient developed a life-threatening, grade 4 venous thrombus in the brain with subsequent intracerebral hemorrhage, which required hospitalization.
The patient died in hospice 4 days later due to the cerebral event. The patient’s death was subsequently evaluated as possibly related to SEL24.
A safety report and a review of data by the trial’s data monitoring committee were submitted to the FDA. The agency then placed a clinical hold on the trial and requested more safety data on patients who have received SEL24, as well as specific protocol changes and additional guidance to the study staff.
Selvita said it plans to comply with the requests and provide additional information to the agency and clinical trial centers, in collaboration with the Menarini Group, its global development partner for SEL24.
The FDA has 30 days from the receipt of Selvita’s response to let the company know whether the clinical hold is lifted. ![]()
FDA rejects pegfilgrastim biosimilar
The US Food and Drug Administration (FDA) has issued a complete response letter saying the agency cannot approve MYL-1401H, a proposed biosimilar of pegfilgrastim (Neulasta).
Biocon and Mylan are seeking approval of MYL-1401H to reduce the duration of neutropenia and the incidence of febrile neutropenia in adults receiving chemotherapy to treat non-myeloid malignancies.
Biocon and Mylan filed the biologics license application for MYL-1401H in February.
The FDA had planned to issue a decision on the application by October 9.
Biocon and Mylan said the FDA’s complete response letter relates to a pending update to the application. The update involves chemistry manufacturing and control data from facility requalification activities after recent plant modifications.
The complete response letter did not raise any questions on the biosimilarity of MYL-1401H, pharmacokinetic/pharmacodynamic data, clinical data, or immunogenicity. (Results of a phase 3 study presented at ESMO 2016 Congress suggested MYL-1401H is equivalent to Neulasta.)
Biocon and Mylan said they do not expect the complete response letter for MYL-1401H to impact the commercial launch timing of the drug in the US. The companies said they are committed to working with the FDA to resolve the issues outlined in the letter. ![]()
The US Food and Drug Administration (FDA) has issued a complete response letter saying the agency cannot approve MYL-1401H, a proposed biosimilar of pegfilgrastim (Neulasta).
Biocon and Mylan are seeking approval of MYL-1401H to reduce the duration of neutropenia and the incidence of febrile neutropenia in adults receiving chemotherapy to treat non-myeloid malignancies.
Biocon and Mylan filed the biologics license application for MYL-1401H in February.
The FDA had planned to issue a decision on the application by October 9.
Biocon and Mylan said the FDA’s complete response letter relates to a pending update to the application. The update involves chemistry manufacturing and control data from facility requalification activities after recent plant modifications.
The complete response letter did not raise any questions on the biosimilarity of MYL-1401H, pharmacokinetic/pharmacodynamic data, clinical data, or immunogenicity. (Results of a phase 3 study presented at ESMO 2016 Congress suggested MYL-1401H is equivalent to Neulasta.)
Biocon and Mylan said they do not expect the complete response letter for MYL-1401H to impact the commercial launch timing of the drug in the US. The companies said they are committed to working with the FDA to resolve the issues outlined in the letter. ![]()
The US Food and Drug Administration (FDA) has issued a complete response letter saying the agency cannot approve MYL-1401H, a proposed biosimilar of pegfilgrastim (Neulasta).
Biocon and Mylan are seeking approval of MYL-1401H to reduce the duration of neutropenia and the incidence of febrile neutropenia in adults receiving chemotherapy to treat non-myeloid malignancies.
Biocon and Mylan filed the biologics license application for MYL-1401H in February.
The FDA had planned to issue a decision on the application by October 9.
Biocon and Mylan said the FDA’s complete response letter relates to a pending update to the application. The update involves chemistry manufacturing and control data from facility requalification activities after recent plant modifications.
The complete response letter did not raise any questions on the biosimilarity of MYL-1401H, pharmacokinetic/pharmacodynamic data, clinical data, or immunogenicity. (Results of a phase 3 study presented at ESMO 2016 Congress suggested MYL-1401H is equivalent to Neulasta.)
Biocon and Mylan said they do not expect the complete response letter for MYL-1401H to impact the commercial launch timing of the drug in the US. The companies said they are committed to working with the FDA to resolve the issues outlined in the letter. ![]()
Pelvic Inflammatory Disease: How to Recognize and Treat Revised
IN THIS ARTICLE
- Diagnostic tests
- Complications of PID
- CDC treatment regimens
Pelvic inflammatory disease (PID) is an ascending polymicrobial infection of the female upper reproductive tract that primarily affects sexually active women ages 15 to 29. Around 5% of sexually active women in the United States were treated for PID from 2011-2013.1 The rates and severity of PID have declined in North America and Western Europe due to overall decrease in sexually transmitted infection (STI) rates, improved screening initiatives for Chlamydia trachomatis, better treatment compliance secondary to increased access to antibiotics, and diagnostic tests with higher sensitivity.2 Despite this rate reduction, PID remains a major public health concern given the significant long-term complications, which include infertility, ectopic pregnancy, and chronic pelvic pain.3
EPIDEMIOLOGY AND PATHOGENESIS
PID is caused by sexually transmitted bacteria or enteric organisms that have spread to internal reproductive organs. Historically, the two most common pathogens identified in cases of PID have been Chlamydia trachomatis and Neisseria gonorrhoeae; however, the decline in rates of gonorrhea has led to a diminished role for N gonorrhoeae (though it continues to be associated with more severe cases).4,5
More recent studies have suggested a shift in the causative organisms; less than half of women diagnosed with acute PID test positive for either N gonorrhoeae or C trachomatis.6 Emerging infectious agents associated with PID include Mycoplasma genitalium, Gardnerella vaginalis, and bacterial vaginosis–associated bacteria.5,7,8-10
RISK FACTORS
Women ages 15 to 25 are at an increased risk for PID. The high prevalence in this age group may be attributable to high-risk behaviors, including a high number of sexual partners, high frequency of new sexual partners, and engagement in sexual intercourse without condoms.11
Taking an accurate sexual history is imperative. Clinicians should maintain a high level of suspicion for PID in women with a history of the disease, as 25% will experience recurrence.12
Clinicians should not be deterred from screening for STIs and cervical cancer in women who report having sex with other women. In addition, transgender patients should be assessed for STIs and HIV-related risks based on current anatomy sexual practices.13
PHYSICAL EXAM
While some cases of PID are asymptomatic, the typical presentation includes bilateral abdominal pain and/or pelvic pain, with onset during or shortly after menses. The pain often worsens with movement and coitus. Associated signs and symptoms include abnormal uterine bleeding or vaginal discharge; dysuria; fever and chills; frequent urination; lower back pain; and nausea and/or vomiting.14,15
All females suspected of having PID should undergo both a bimanual exam and a speculum exam. On bimanual examination, adnexal tenderness has the highest sensitivity (93% to 95.5%) for ruling out acute PID, whereas on speculum exam, purulent endocervical discharge has the highest specificity (93%).16,17 Bimanual exam findings suggestive of PID include cervical motion tenderness, uterine tenderness, and/or adnexal tenderness. Suggestive speculum exam findings include abnormal discoloration or texture of the cervix and/or endocervical mucopurulent discharge.5,16,17
One cardinal rule that should not be overlooked is that all females of reproductive age who present with abdominal pain and/or pelvic pain should take a pregnancy test to rule out ectopic pregnancy and any other pregnancy-related complications.
DIAGNOSIS
The diagnosis of PID relies on clinical judgement and a high index of suspicion.5,18 The CDC’s diagnostic criteria for acute PID include
- Sexually active female AND
- Pelvic or lower abdominal pain AND
- Cervical motion tenderness OR uterine tenderness OR adnexal tenderness.5
Additional findings that support the diagnosis include
- Abnormal cervical mucopurulent discharge or cervical friability
- Abundant white blood cells (WBCs) on saline microscopy of vaginal fluid
- Elevated C-reactive protein
- Elevated erythrocyte sedimentation rate
- Laboratory documentation of infection with C trachomatis or N gonorrhea
- Oral temperature > 101°F.5,18
The CDC notes that the first two findings (mucopurulent discharge and evidence of WBCs on microscopy) occur in most women with PID; in their absence, the diagnosis is unlikely and other sources of pain should be considered.5 The differential for PID includes acute appendicitis; adhesions; carcinoid tumor; cholecystitis; ectopic pregnancy; endometriosis; inflammatory bowel disease; and ovarian cyst.19
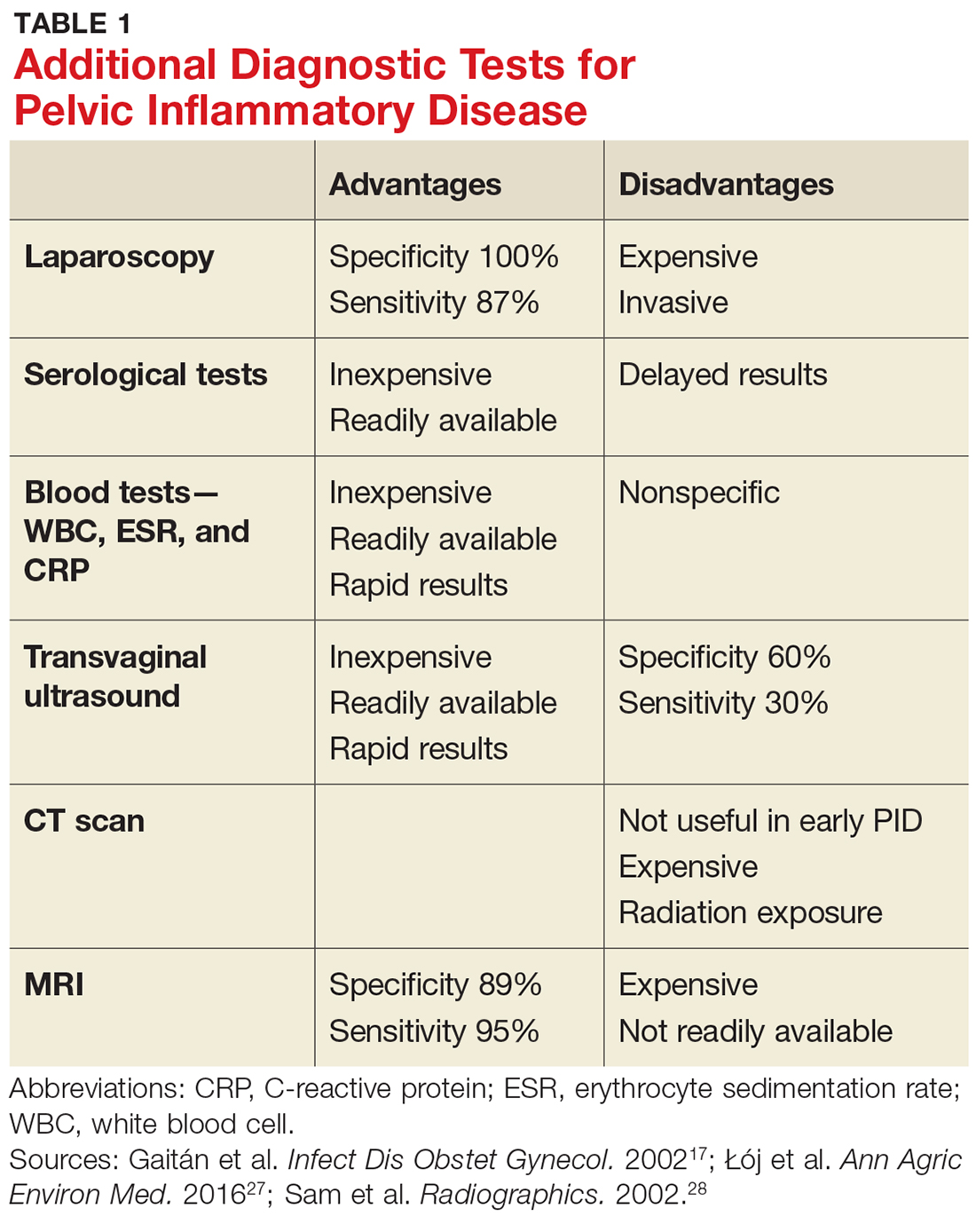
Given the variability in presentation, clinicians may find it useful to perform further diagnostic testing. There are additional laboratory tests that may be ordered for patients with a suspected diagnosis of PID (see Table 1).
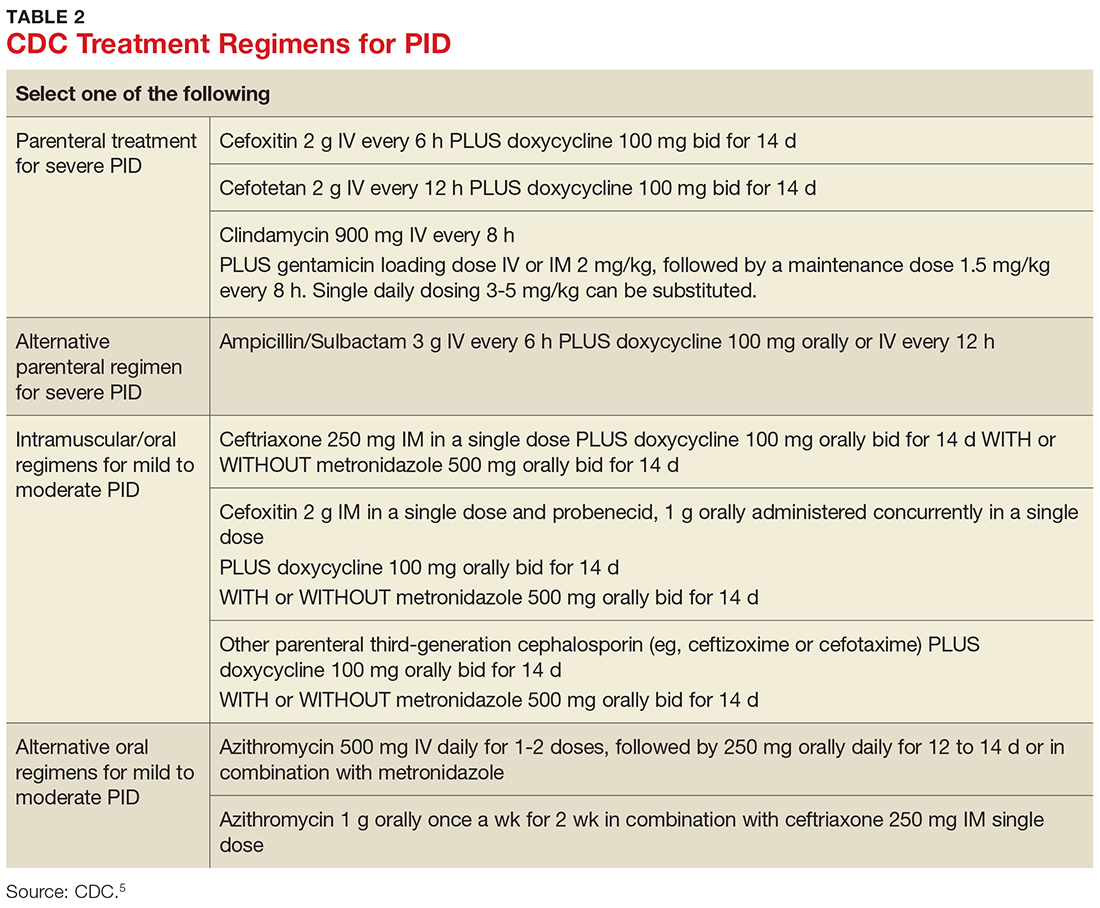
TREATMENT
According to the CDC’s 2015 treatment guidelines for PID, a negative endocervical exam and negative microbial screening do not rule out an upper reproductive tract infection. Therefore, all sexually active women who present with lower abdominal pain and/or pelvic pain and have evidence of cervical motion, uterine, or adnexal tenderness on bimanual exam should be treated immediately.5
Treatment guidelines are outlined in Table 2. The polymicrobial nature of PID requires gram-negative antibiotic coverage, such as doxycycline plus a second/third-generation cephalosporin.5 Clinicians should note that cefoxitin, a second-generation cephalosporin, is recommended as firstline therapy for inpatients, as it has better anaerobic coverage than ceftriaxone.19 A targeted change in antibiotic coverage—such as inclusion of a macrolide and/or metronidazole—might be necessary if a causative organism is identified by culture.7
Treatment is indicated for all patients with a presumptive diagnosis of PID regardless of symptoms or exam findings, as PID may be asymptomatic and long-term sequelae (eg, infertility, ectopic pregnancy) are often irreversible. At-risk patients include sexually active adolescents, women with multiple sexual partners, women with a history of STI, those whose sexual partner has an STI, and women living in communities with a high prevalence of disease.20,21
Women being treated for PID should be advised to abstain from sexual intercourse until symptoms have resolved, treatment is completed, and any sexual partners have been treated as well. It is essential to emphasize to patients (and their partners) the importance of compliance to treatment regimens and the risk for PID co-infection and reinfection, as recurrence leads to an increase in long-term complications.5
Treatment of sexual partners. The CDC instructs that a woman’s most recent partner should be treated if she had sexual intercourse within 60 days of onset of symptoms or diagnosis. Furthermore, men who have had sexual contact with a woman who has PID in the 60 days prior to onset of her symptoms should be evaluated, tested, and treated for chlamydia and gonorrhea, regardless of the etiology of PID or the pathogens isolated from the woman.5
Admission criteria. Hospitalization should be based on provider judgment despite patient age. The suggested admission criteria include surgical emergency (eg, appendicitis), tubo-ovarian abscess, pregnancy, severe illness, nausea and vomiting, high fever, inability to follow or tolerate an outpatient oral regimen, and lack of clinical response to oral antimicrobial therapy.5
Follow-up care. Clinical improvement (ie, reduction in abdominal, uterine, adnexal, and cervical motion tenderness) should occur within 72 hours of antimicrobial therapy initiation. If it does not, hospital admission or adjustment in antimicrobial regimen should be considered, as well as additional diagnostic testing (eg, laparoscopy). In addition, all women with chlamydial- or gonococcal-related PID should return in three months for surveillance testing.22
COMPLICATIONS
Long-term complications—including infertility, chronic pelvic pain, and ectopic pregnancy—may occur, even when there has been a clinical response to adequate treatment. Data from the PID Evaluation and Clinical Health (PEACH) study were analyzed to assess long-term sequelae at seven years postdiagnosis and treatment. The researchers found that about 21% of women experienced recurrent PID, 19% developed infertility, and 42% reported chronic pelvic pain.3 Other research has also shown that repeat episodes of PID and delayed treatment increase the risk for long-term complications.23,24
SCREENING AND PREVENTION
Ten percent of women with an untreated STI will go on to develop PID.4 It is imperative to educate patients on the dangers and consequences of STIs when they become sexually active. Adolescents benefit the most from preventive education; this group is twice as likely as any other age group to be diagnosed with PID due to their inclination toward risky sexual behavior. Additionally, younger women tend to have a more friable cervix, increasing their risk for infection.25,26 Providers should promote safe sexual practices, such as condom use and less frequent partner exchange, in order to reduce STI exposure.
In 2015, the rate of reported cases of C trachomatis was 645.5 per 100,000 females, and of N gonorrheae, 107.2 per 100,000 females.23 The United States Preventive Services Task Force and the CDC recommend annual screening for chlamydia and gonorrhea in all sexually active women younger than 25, as well as sexually active women ages 25 and older who are considered at increased risk.5
CONCLUSION
PID is often difficult to diagnose, since patients may be asymptomatic or present with vague symptoms. Clinicians should maintain a high level of suspicion for PID in adolescent females due to the high incidence of STI exposure in this population. The best way to prevent long-term complications of PID is to prevent the first episode of PID and/or first exposure to STIs. Therefore, clinicians should be proactive in offering STI screenings to all sexually active patients younger than 25 who request care, regardless of their chief complaint, and educating patients on the potential long-term effects of PID and STIs.
1. Leichliter JS, Chandra A, Aral SO. Correlates of self-reported pelvic inflammatory disease treatment in sexually experienced reproductive-aged women in the United States, 1995 and 2006-2010. Sex Transm Dis. 2013;40(5):413-418.
2. Owusu-Edusei K Jr, Bohm MK, Chesson HW, Kent CK. Chlamydia screening and pelvic inflammatory disease: insights from exploratory time-series analyses. Am J Prev Med. 2010;38(6):652-657.
3. Trent M, Bass D, Ness RB, Haggerty C. Recurrent PID, subsequent STI, and reproductive health outcomes: findings from the PID evaluation and clinical health (PEACH) study. Sex Transm Dis. 2011;38(9):879-881.
4. Mitchell C, Prabhu M. Pelvic inflammatory disease: current concepts in pathogenesis, diagnosis and treatment. Infect Dis Clin North Am. 2013;27(4):793-809.
5. CDC. Pelvic inflammatory disease (PID). www.cdc.gov/std/tg2015/pid.htm. Accessed July 13, 2017.
6. Burnett AM, Anderson CP, Zwank MD. Laboratory-confirmed gonorrhea and/or chlamydia rates in clinically diagnosed pelvic inflammatory disease and cervicitis. Am J Emerg Med. 2012;30:1114–1117.
7. Bjartling C, Osser S, Persson K. The association between Mycoplasma genitalium and pelvic inflammatory disease after termination of pregnancy. BJOG. 2010;117(3):361-364.
8. Ness RB, Kip KE, Hillier SL, et al. A cluster analysis of bacterial vaginosis-associated microflora and pelvic inflammatory disease. Am J Epidemiol. 2005;162(6):585-590.
9. Ness RB, Hillier SL, Kip KE, et al. Bacterial vaginosis and risk of pelvic inflammatory disease. Obstet Gynecol. 2004;104(4):761-769.
10. Cherpes TL, Wiesenfeld HC, Melan MA, et al. The associations between pelvic inflammatory disease, Trichomonas vaginalis infection, and positive herpes simplex virus type 2 serology. Sex Transm Dis. 2006;33(12):747-752.
11. Simms I, Stephenson JM, Mallinson H, et al. Risk factors associated with pelvic inflammatory disease. Sex Transm Infect. 2006;82(6):452-457.
12. Schindlbeck C, Dziura D, Mylonas I. Diagnosis of pelvic inflammatory disease (PID): intra-operative findings and comparison of vaginal and intra-abdominal cultures. Arch Gynecol Obstet. 2014;289(6):1263-1269.
13. CDC. 2015 sexually transmitted diseases treatment guidelines. www.cdc.gov/std/tg2015/default.htm. Accessed September 6, 2017.
14. Korn AP, Hessol NA, Padian NS, et al. Risk factors for plasma cell endometritis among women with cervical Neisseria gonorrhoeae, cervical Chlamydia trachomatis, or bacterial vaginosis. Am J Obstet Gynecol. 1998;178(5):987-990.
15. Wiesenfeld HC, Sweet RL, Ness RB, et al. Comparison of acute and subclinical pelvic inflammatory disease. Sex Transm Dis. 2005;32(7):400-405.
16. Peipert JF, Ness RB, Blume J, et al. Clinical predictors of endometritis in women with symptoms and signs of pelvic inflammatory disease. Am J Obstet Gynecol. 2001;184(5):856-864.
17. Gaitán H, Angel E, Diaz R, et al. Accuracy of five different diagnostic techniques in mild-to-moderate pelvic inflammatory disease. Infect Dis Obstet Gynecol. 2002;10(4):171-180.
18. Tukeva TA, Aronen HJ, Karjalainen PT, et al. MR imaging in pelvic inflammatory disease: comparison with laparoscopy and US. Radiology. 1999;210(1):209-216.
19. Morino M, Pellegrino L, Castagna E, et al. Acute nonspecific abdominal pain. Ann Surg. 2006;244(6):881-888.
20. Woods JL, Scurlock AM, Hensel DJ. Pelvic inflammatory disease in the adolescent: understanding diagnosis and treatment as a health care provider. Pediatric Emergency Care. 2013;29(6):720-725.
21. LeFevre ML; U.S. Preventive Services Task Force. Screening for chlamydia and gonorrhea: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;161(12):902-910.
22. Hosenfeld CB, Workowski KA, Berman S, et al. Repeat infection with Chlamydia and gonorrhea among females: a systematic review of the literature. Sex Transm Dis. 2009;36(8):478-489.
23. CDC. Sexually transmitted disease surveillance. www.cdc.gov/std/stats15/STD-Surveillance-2015-print.pdf. Accessed July 13, 2017.
24. Hillis SD, Joesoef R, Marchbanks PA, et al. Delayed care of pelvic inflammatory disease as a risk factor for impaired fertility. Am J Obstet Gynecol. 1993;168(5):1503-1509.
25. Goyal M, Hersh A, Luan X, et al. National trends in pelvic inflammatory disease among adolescents in the emergency department. J Adolesc Health. 2013;53(2):249-252.
26. Gray-Swain MR, Peipert JF. Pelvic inflammatory disease in adolescents. Curr Opin Obstet Gynecol. 2006;18(5):503-510.
27. Łój B, Brodowska A, Ciecwiez S, et al. The role of serological testing for Chlamydia trachomatis in differential diagnosis of pelvic pain. Ann Agric Environ Med. 2016;23(3):506-510.
28. Sam JW, Jacobs JE, Birnbaum BA. Spectrum of CT findings in acute pyogenic pelvic inflammatory disease. Radiographics. 2002;22(6):1327-1 334.
IN THIS ARTICLE
- Diagnostic tests
- Complications of PID
- CDC treatment regimens
Pelvic inflammatory disease (PID) is an ascending polymicrobial infection of the female upper reproductive tract that primarily affects sexually active women ages 15 to 29. Around 5% of sexually active women in the United States were treated for PID from 2011-2013.1 The rates and severity of PID have declined in North America and Western Europe due to overall decrease in sexually transmitted infection (STI) rates, improved screening initiatives for Chlamydia trachomatis, better treatment compliance secondary to increased access to antibiotics, and diagnostic tests with higher sensitivity.2 Despite this rate reduction, PID remains a major public health concern given the significant long-term complications, which include infertility, ectopic pregnancy, and chronic pelvic pain.3
EPIDEMIOLOGY AND PATHOGENESIS
PID is caused by sexually transmitted bacteria or enteric organisms that have spread to internal reproductive organs. Historically, the two most common pathogens identified in cases of PID have been Chlamydia trachomatis and Neisseria gonorrhoeae; however, the decline in rates of gonorrhea has led to a diminished role for N gonorrhoeae (though it continues to be associated with more severe cases).4,5
More recent studies have suggested a shift in the causative organisms; less than half of women diagnosed with acute PID test positive for either N gonorrhoeae or C trachomatis.6 Emerging infectious agents associated with PID include Mycoplasma genitalium, Gardnerella vaginalis, and bacterial vaginosis–associated bacteria.5,7,8-10
RISK FACTORS
Women ages 15 to 25 are at an increased risk for PID. The high prevalence in this age group may be attributable to high-risk behaviors, including a high number of sexual partners, high frequency of new sexual partners, and engagement in sexual intercourse without condoms.11
Taking an accurate sexual history is imperative. Clinicians should maintain a high level of suspicion for PID in women with a history of the disease, as 25% will experience recurrence.12
Clinicians should not be deterred from screening for STIs and cervical cancer in women who report having sex with other women. In addition, transgender patients should be assessed for STIs and HIV-related risks based on current anatomy sexual practices.13
PHYSICAL EXAM
While some cases of PID are asymptomatic, the typical presentation includes bilateral abdominal pain and/or pelvic pain, with onset during or shortly after menses. The pain often worsens with movement and coitus. Associated signs and symptoms include abnormal uterine bleeding or vaginal discharge; dysuria; fever and chills; frequent urination; lower back pain; and nausea and/or vomiting.14,15
All females suspected of having PID should undergo both a bimanual exam and a speculum exam. On bimanual examination, adnexal tenderness has the highest sensitivity (93% to 95.5%) for ruling out acute PID, whereas on speculum exam, purulent endocervical discharge has the highest specificity (93%).16,17 Bimanual exam findings suggestive of PID include cervical motion tenderness, uterine tenderness, and/or adnexal tenderness. Suggestive speculum exam findings include abnormal discoloration or texture of the cervix and/or endocervical mucopurulent discharge.5,16,17
One cardinal rule that should not be overlooked is that all females of reproductive age who present with abdominal pain and/or pelvic pain should take a pregnancy test to rule out ectopic pregnancy and any other pregnancy-related complications.
DIAGNOSIS
The diagnosis of PID relies on clinical judgement and a high index of suspicion.5,18 The CDC’s diagnostic criteria for acute PID include
- Sexually active female AND
- Pelvic or lower abdominal pain AND
- Cervical motion tenderness OR uterine tenderness OR adnexal tenderness.5
Additional findings that support the diagnosis include
- Abnormal cervical mucopurulent discharge or cervical friability
- Abundant white blood cells (WBCs) on saline microscopy of vaginal fluid
- Elevated C-reactive protein
- Elevated erythrocyte sedimentation rate
- Laboratory documentation of infection with C trachomatis or N gonorrhea
- Oral temperature > 101°F.5,18
The CDC notes that the first two findings (mucopurulent discharge and evidence of WBCs on microscopy) occur in most women with PID; in their absence, the diagnosis is unlikely and other sources of pain should be considered.5 The differential for PID includes acute appendicitis; adhesions; carcinoid tumor; cholecystitis; ectopic pregnancy; endometriosis; inflammatory bowel disease; and ovarian cyst.19
Given the variability in presentation, clinicians may find it useful to perform further diagnostic testing. There are additional laboratory tests that may be ordered for patients with a suspected diagnosis of PID (see Table 1).
TREATMENT
According to the CDC’s 2015 treatment guidelines for PID, a negative endocervical exam and negative microbial screening do not rule out an upper reproductive tract infection. Therefore, all sexually active women who present with lower abdominal pain and/or pelvic pain and have evidence of cervical motion, uterine, or adnexal tenderness on bimanual exam should be treated immediately.5
Treatment guidelines are outlined in Table 2. The polymicrobial nature of PID requires gram-negative antibiotic coverage, such as doxycycline plus a second/third-generation cephalosporin.5 Clinicians should note that cefoxitin, a second-generation cephalosporin, is recommended as firstline therapy for inpatients, as it has better anaerobic coverage than ceftriaxone.19 A targeted change in antibiotic coverage—such as inclusion of a macrolide and/or metronidazole—might be necessary if a causative organism is identified by culture.7
Treatment is indicated for all patients with a presumptive diagnosis of PID regardless of symptoms or exam findings, as PID may be asymptomatic and long-term sequelae (eg, infertility, ectopic pregnancy) are often irreversible. At-risk patients include sexually active adolescents, women with multiple sexual partners, women with a history of STI, those whose sexual partner has an STI, and women living in communities with a high prevalence of disease.20,21
Women being treated for PID should be advised to abstain from sexual intercourse until symptoms have resolved, treatment is completed, and any sexual partners have been treated as well. It is essential to emphasize to patients (and their partners) the importance of compliance to treatment regimens and the risk for PID co-infection and reinfection, as recurrence leads to an increase in long-term complications.5
Treatment of sexual partners. The CDC instructs that a woman’s most recent partner should be treated if she had sexual intercourse within 60 days of onset of symptoms or diagnosis. Furthermore, men who have had sexual contact with a woman who has PID in the 60 days prior to onset of her symptoms should be evaluated, tested, and treated for chlamydia and gonorrhea, regardless of the etiology of PID or the pathogens isolated from the woman.5
Admission criteria. Hospitalization should be based on provider judgment despite patient age. The suggested admission criteria include surgical emergency (eg, appendicitis), tubo-ovarian abscess, pregnancy, severe illness, nausea and vomiting, high fever, inability to follow or tolerate an outpatient oral regimen, and lack of clinical response to oral antimicrobial therapy.5
Follow-up care. Clinical improvement (ie, reduction in abdominal, uterine, adnexal, and cervical motion tenderness) should occur within 72 hours of antimicrobial therapy initiation. If it does not, hospital admission or adjustment in antimicrobial regimen should be considered, as well as additional diagnostic testing (eg, laparoscopy). In addition, all women with chlamydial- or gonococcal-related PID should return in three months for surveillance testing.22
COMPLICATIONS
Long-term complications—including infertility, chronic pelvic pain, and ectopic pregnancy—may occur, even when there has been a clinical response to adequate treatment. Data from the PID Evaluation and Clinical Health (PEACH) study were analyzed to assess long-term sequelae at seven years postdiagnosis and treatment. The researchers found that about 21% of women experienced recurrent PID, 19% developed infertility, and 42% reported chronic pelvic pain.3 Other research has also shown that repeat episodes of PID and delayed treatment increase the risk for long-term complications.23,24
SCREENING AND PREVENTION
Ten percent of women with an untreated STI will go on to develop PID.4 It is imperative to educate patients on the dangers and consequences of STIs when they become sexually active. Adolescents benefit the most from preventive education; this group is twice as likely as any other age group to be diagnosed with PID due to their inclination toward risky sexual behavior. Additionally, younger women tend to have a more friable cervix, increasing their risk for infection.25,26 Providers should promote safe sexual practices, such as condom use and less frequent partner exchange, in order to reduce STI exposure.
In 2015, the rate of reported cases of C trachomatis was 645.5 per 100,000 females, and of N gonorrheae, 107.2 per 100,000 females.23 The United States Preventive Services Task Force and the CDC recommend annual screening for chlamydia and gonorrhea in all sexually active women younger than 25, as well as sexually active women ages 25 and older who are considered at increased risk.5
CONCLUSION
PID is often difficult to diagnose, since patients may be asymptomatic or present with vague symptoms. Clinicians should maintain a high level of suspicion for PID in adolescent females due to the high incidence of STI exposure in this population. The best way to prevent long-term complications of PID is to prevent the first episode of PID and/or first exposure to STIs. Therefore, clinicians should be proactive in offering STI screenings to all sexually active patients younger than 25 who request care, regardless of their chief complaint, and educating patients on the potential long-term effects of PID and STIs.
IN THIS ARTICLE
- Diagnostic tests
- Complications of PID
- CDC treatment regimens
Pelvic inflammatory disease (PID) is an ascending polymicrobial infection of the female upper reproductive tract that primarily affects sexually active women ages 15 to 29. Around 5% of sexually active women in the United States were treated for PID from 2011-2013.1 The rates and severity of PID have declined in North America and Western Europe due to overall decrease in sexually transmitted infection (STI) rates, improved screening initiatives for Chlamydia trachomatis, better treatment compliance secondary to increased access to antibiotics, and diagnostic tests with higher sensitivity.2 Despite this rate reduction, PID remains a major public health concern given the significant long-term complications, which include infertility, ectopic pregnancy, and chronic pelvic pain.3
EPIDEMIOLOGY AND PATHOGENESIS
PID is caused by sexually transmitted bacteria or enteric organisms that have spread to internal reproductive organs. Historically, the two most common pathogens identified in cases of PID have been Chlamydia trachomatis and Neisseria gonorrhoeae; however, the decline in rates of gonorrhea has led to a diminished role for N gonorrhoeae (though it continues to be associated with more severe cases).4,5
More recent studies have suggested a shift in the causative organisms; less than half of women diagnosed with acute PID test positive for either N gonorrhoeae or C trachomatis.6 Emerging infectious agents associated with PID include Mycoplasma genitalium, Gardnerella vaginalis, and bacterial vaginosis–associated bacteria.5,7,8-10
RISK FACTORS
Women ages 15 to 25 are at an increased risk for PID. The high prevalence in this age group may be attributable to high-risk behaviors, including a high number of sexual partners, high frequency of new sexual partners, and engagement in sexual intercourse without condoms.11
Taking an accurate sexual history is imperative. Clinicians should maintain a high level of suspicion for PID in women with a history of the disease, as 25% will experience recurrence.12
Clinicians should not be deterred from screening for STIs and cervical cancer in women who report having sex with other women. In addition, transgender patients should be assessed for STIs and HIV-related risks based on current anatomy sexual practices.13
PHYSICAL EXAM
While some cases of PID are asymptomatic, the typical presentation includes bilateral abdominal pain and/or pelvic pain, with onset during or shortly after menses. The pain often worsens with movement and coitus. Associated signs and symptoms include abnormal uterine bleeding or vaginal discharge; dysuria; fever and chills; frequent urination; lower back pain; and nausea and/or vomiting.14,15
All females suspected of having PID should undergo both a bimanual exam and a speculum exam. On bimanual examination, adnexal tenderness has the highest sensitivity (93% to 95.5%) for ruling out acute PID, whereas on speculum exam, purulent endocervical discharge has the highest specificity (93%).16,17 Bimanual exam findings suggestive of PID include cervical motion tenderness, uterine tenderness, and/or adnexal tenderness. Suggestive speculum exam findings include abnormal discoloration or texture of the cervix and/or endocervical mucopurulent discharge.5,16,17
One cardinal rule that should not be overlooked is that all females of reproductive age who present with abdominal pain and/or pelvic pain should take a pregnancy test to rule out ectopic pregnancy and any other pregnancy-related complications.
DIAGNOSIS
The diagnosis of PID relies on clinical judgement and a high index of suspicion.5,18 The CDC’s diagnostic criteria for acute PID include
- Sexually active female AND
- Pelvic or lower abdominal pain AND
- Cervical motion tenderness OR uterine tenderness OR adnexal tenderness.5
Additional findings that support the diagnosis include
- Abnormal cervical mucopurulent discharge or cervical friability
- Abundant white blood cells (WBCs) on saline microscopy of vaginal fluid
- Elevated C-reactive protein
- Elevated erythrocyte sedimentation rate
- Laboratory documentation of infection with C trachomatis or N gonorrhea
- Oral temperature > 101°F.5,18
The CDC notes that the first two findings (mucopurulent discharge and evidence of WBCs on microscopy) occur in most women with PID; in their absence, the diagnosis is unlikely and other sources of pain should be considered.5 The differential for PID includes acute appendicitis; adhesions; carcinoid tumor; cholecystitis; ectopic pregnancy; endometriosis; inflammatory bowel disease; and ovarian cyst.19
Given the variability in presentation, clinicians may find it useful to perform further diagnostic testing. There are additional laboratory tests that may be ordered for patients with a suspected diagnosis of PID (see Table 1).
TREATMENT
According to the CDC’s 2015 treatment guidelines for PID, a negative endocervical exam and negative microbial screening do not rule out an upper reproductive tract infection. Therefore, all sexually active women who present with lower abdominal pain and/or pelvic pain and have evidence of cervical motion, uterine, or adnexal tenderness on bimanual exam should be treated immediately.5
Treatment guidelines are outlined in Table 2. The polymicrobial nature of PID requires gram-negative antibiotic coverage, such as doxycycline plus a second/third-generation cephalosporin.5 Clinicians should note that cefoxitin, a second-generation cephalosporin, is recommended as firstline therapy for inpatients, as it has better anaerobic coverage than ceftriaxone.19 A targeted change in antibiotic coverage—such as inclusion of a macrolide and/or metronidazole—might be necessary if a causative organism is identified by culture.7
Treatment is indicated for all patients with a presumptive diagnosis of PID regardless of symptoms or exam findings, as PID may be asymptomatic and long-term sequelae (eg, infertility, ectopic pregnancy) are often irreversible. At-risk patients include sexually active adolescents, women with multiple sexual partners, women with a history of STI, those whose sexual partner has an STI, and women living in communities with a high prevalence of disease.20,21
Women being treated for PID should be advised to abstain from sexual intercourse until symptoms have resolved, treatment is completed, and any sexual partners have been treated as well. It is essential to emphasize to patients (and their partners) the importance of compliance to treatment regimens and the risk for PID co-infection and reinfection, as recurrence leads to an increase in long-term complications.5
Treatment of sexual partners. The CDC instructs that a woman’s most recent partner should be treated if she had sexual intercourse within 60 days of onset of symptoms or diagnosis. Furthermore, men who have had sexual contact with a woman who has PID in the 60 days prior to onset of her symptoms should be evaluated, tested, and treated for chlamydia and gonorrhea, regardless of the etiology of PID or the pathogens isolated from the woman.5
Admission criteria. Hospitalization should be based on provider judgment despite patient age. The suggested admission criteria include surgical emergency (eg, appendicitis), tubo-ovarian abscess, pregnancy, severe illness, nausea and vomiting, high fever, inability to follow or tolerate an outpatient oral regimen, and lack of clinical response to oral antimicrobial therapy.5
Follow-up care. Clinical improvement (ie, reduction in abdominal, uterine, adnexal, and cervical motion tenderness) should occur within 72 hours of antimicrobial therapy initiation. If it does not, hospital admission or adjustment in antimicrobial regimen should be considered, as well as additional diagnostic testing (eg, laparoscopy). In addition, all women with chlamydial- or gonococcal-related PID should return in three months for surveillance testing.22
COMPLICATIONS
Long-term complications—including infertility, chronic pelvic pain, and ectopic pregnancy—may occur, even when there has been a clinical response to adequate treatment. Data from the PID Evaluation and Clinical Health (PEACH) study were analyzed to assess long-term sequelae at seven years postdiagnosis and treatment. The researchers found that about 21% of women experienced recurrent PID, 19% developed infertility, and 42% reported chronic pelvic pain.3 Other research has also shown that repeat episodes of PID and delayed treatment increase the risk for long-term complications.23,24
SCREENING AND PREVENTION
Ten percent of women with an untreated STI will go on to develop PID.4 It is imperative to educate patients on the dangers and consequences of STIs when they become sexually active. Adolescents benefit the most from preventive education; this group is twice as likely as any other age group to be diagnosed with PID due to their inclination toward risky sexual behavior. Additionally, younger women tend to have a more friable cervix, increasing their risk for infection.25,26 Providers should promote safe sexual practices, such as condom use and less frequent partner exchange, in order to reduce STI exposure.
In 2015, the rate of reported cases of C trachomatis was 645.5 per 100,000 females, and of N gonorrheae, 107.2 per 100,000 females.23 The United States Preventive Services Task Force and the CDC recommend annual screening for chlamydia and gonorrhea in all sexually active women younger than 25, as well as sexually active women ages 25 and older who are considered at increased risk.5
CONCLUSION
PID is often difficult to diagnose, since patients may be asymptomatic or present with vague symptoms. Clinicians should maintain a high level of suspicion for PID in adolescent females due to the high incidence of STI exposure in this population. The best way to prevent long-term complications of PID is to prevent the first episode of PID and/or first exposure to STIs. Therefore, clinicians should be proactive in offering STI screenings to all sexually active patients younger than 25 who request care, regardless of their chief complaint, and educating patients on the potential long-term effects of PID and STIs.
1. Leichliter JS, Chandra A, Aral SO. Correlates of self-reported pelvic inflammatory disease treatment in sexually experienced reproductive-aged women in the United States, 1995 and 2006-2010. Sex Transm Dis. 2013;40(5):413-418.
2. Owusu-Edusei K Jr, Bohm MK, Chesson HW, Kent CK. Chlamydia screening and pelvic inflammatory disease: insights from exploratory time-series analyses. Am J Prev Med. 2010;38(6):652-657.
3. Trent M, Bass D, Ness RB, Haggerty C. Recurrent PID, subsequent STI, and reproductive health outcomes: findings from the PID evaluation and clinical health (PEACH) study. Sex Transm Dis. 2011;38(9):879-881.
4. Mitchell C, Prabhu M. Pelvic inflammatory disease: current concepts in pathogenesis, diagnosis and treatment. Infect Dis Clin North Am. 2013;27(4):793-809.
5. CDC. Pelvic inflammatory disease (PID). www.cdc.gov/std/tg2015/pid.htm. Accessed July 13, 2017.
6. Burnett AM, Anderson CP, Zwank MD. Laboratory-confirmed gonorrhea and/or chlamydia rates in clinically diagnosed pelvic inflammatory disease and cervicitis. Am J Emerg Med. 2012;30:1114–1117.
7. Bjartling C, Osser S, Persson K. The association between Mycoplasma genitalium and pelvic inflammatory disease after termination of pregnancy. BJOG. 2010;117(3):361-364.
8. Ness RB, Kip KE, Hillier SL, et al. A cluster analysis of bacterial vaginosis-associated microflora and pelvic inflammatory disease. Am J Epidemiol. 2005;162(6):585-590.
9. Ness RB, Hillier SL, Kip KE, et al. Bacterial vaginosis and risk of pelvic inflammatory disease. Obstet Gynecol. 2004;104(4):761-769.
10. Cherpes TL, Wiesenfeld HC, Melan MA, et al. The associations between pelvic inflammatory disease, Trichomonas vaginalis infection, and positive herpes simplex virus type 2 serology. Sex Transm Dis. 2006;33(12):747-752.
11. Simms I, Stephenson JM, Mallinson H, et al. Risk factors associated with pelvic inflammatory disease. Sex Transm Infect. 2006;82(6):452-457.
12. Schindlbeck C, Dziura D, Mylonas I. Diagnosis of pelvic inflammatory disease (PID): intra-operative findings and comparison of vaginal and intra-abdominal cultures. Arch Gynecol Obstet. 2014;289(6):1263-1269.
13. CDC. 2015 sexually transmitted diseases treatment guidelines. www.cdc.gov/std/tg2015/default.htm. Accessed September 6, 2017.
14. Korn AP, Hessol NA, Padian NS, et al. Risk factors for plasma cell endometritis among women with cervical Neisseria gonorrhoeae, cervical Chlamydia trachomatis, or bacterial vaginosis. Am J Obstet Gynecol. 1998;178(5):987-990.
15. Wiesenfeld HC, Sweet RL, Ness RB, et al. Comparison of acute and subclinical pelvic inflammatory disease. Sex Transm Dis. 2005;32(7):400-405.
16. Peipert JF, Ness RB, Blume J, et al. Clinical predictors of endometritis in women with symptoms and signs of pelvic inflammatory disease. Am J Obstet Gynecol. 2001;184(5):856-864.
17. Gaitán H, Angel E, Diaz R, et al. Accuracy of five different diagnostic techniques in mild-to-moderate pelvic inflammatory disease. Infect Dis Obstet Gynecol. 2002;10(4):171-180.
18. Tukeva TA, Aronen HJ, Karjalainen PT, et al. MR imaging in pelvic inflammatory disease: comparison with laparoscopy and US. Radiology. 1999;210(1):209-216.
19. Morino M, Pellegrino L, Castagna E, et al. Acute nonspecific abdominal pain. Ann Surg. 2006;244(6):881-888.
20. Woods JL, Scurlock AM, Hensel DJ. Pelvic inflammatory disease in the adolescent: understanding diagnosis and treatment as a health care provider. Pediatric Emergency Care. 2013;29(6):720-725.
21. LeFevre ML; U.S. Preventive Services Task Force. Screening for chlamydia and gonorrhea: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;161(12):902-910.
22. Hosenfeld CB, Workowski KA, Berman S, et al. Repeat infection with Chlamydia and gonorrhea among females: a systematic review of the literature. Sex Transm Dis. 2009;36(8):478-489.
23. CDC. Sexually transmitted disease surveillance. www.cdc.gov/std/stats15/STD-Surveillance-2015-print.pdf. Accessed July 13, 2017.
24. Hillis SD, Joesoef R, Marchbanks PA, et al. Delayed care of pelvic inflammatory disease as a risk factor for impaired fertility. Am J Obstet Gynecol. 1993;168(5):1503-1509.
25. Goyal M, Hersh A, Luan X, et al. National trends in pelvic inflammatory disease among adolescents in the emergency department. J Adolesc Health. 2013;53(2):249-252.
26. Gray-Swain MR, Peipert JF. Pelvic inflammatory disease in adolescents. Curr Opin Obstet Gynecol. 2006;18(5):503-510.
27. Łój B, Brodowska A, Ciecwiez S, et al. The role of serological testing for Chlamydia trachomatis in differential diagnosis of pelvic pain. Ann Agric Environ Med. 2016;23(3):506-510.
28. Sam JW, Jacobs JE, Birnbaum BA. Spectrum of CT findings in acute pyogenic pelvic inflammatory disease. Radiographics. 2002;22(6):1327-1 334.
1. Leichliter JS, Chandra A, Aral SO. Correlates of self-reported pelvic inflammatory disease treatment in sexually experienced reproductive-aged women in the United States, 1995 and 2006-2010. Sex Transm Dis. 2013;40(5):413-418.
2. Owusu-Edusei K Jr, Bohm MK, Chesson HW, Kent CK. Chlamydia screening and pelvic inflammatory disease: insights from exploratory time-series analyses. Am J Prev Med. 2010;38(6):652-657.
3. Trent M, Bass D, Ness RB, Haggerty C. Recurrent PID, subsequent STI, and reproductive health outcomes: findings from the PID evaluation and clinical health (PEACH) study. Sex Transm Dis. 2011;38(9):879-881.
4. Mitchell C, Prabhu M. Pelvic inflammatory disease: current concepts in pathogenesis, diagnosis and treatment. Infect Dis Clin North Am. 2013;27(4):793-809.
5. CDC. Pelvic inflammatory disease (PID). www.cdc.gov/std/tg2015/pid.htm. Accessed July 13, 2017.
6. Burnett AM, Anderson CP, Zwank MD. Laboratory-confirmed gonorrhea and/or chlamydia rates in clinically diagnosed pelvic inflammatory disease and cervicitis. Am J Emerg Med. 2012;30:1114–1117.
7. Bjartling C, Osser S, Persson K. The association between Mycoplasma genitalium and pelvic inflammatory disease after termination of pregnancy. BJOG. 2010;117(3):361-364.
8. Ness RB, Kip KE, Hillier SL, et al. A cluster analysis of bacterial vaginosis-associated microflora and pelvic inflammatory disease. Am J Epidemiol. 2005;162(6):585-590.
9. Ness RB, Hillier SL, Kip KE, et al. Bacterial vaginosis and risk of pelvic inflammatory disease. Obstet Gynecol. 2004;104(4):761-769.
10. Cherpes TL, Wiesenfeld HC, Melan MA, et al. The associations between pelvic inflammatory disease, Trichomonas vaginalis infection, and positive herpes simplex virus type 2 serology. Sex Transm Dis. 2006;33(12):747-752.
11. Simms I, Stephenson JM, Mallinson H, et al. Risk factors associated with pelvic inflammatory disease. Sex Transm Infect. 2006;82(6):452-457.
12. Schindlbeck C, Dziura D, Mylonas I. Diagnosis of pelvic inflammatory disease (PID): intra-operative findings and comparison of vaginal and intra-abdominal cultures. Arch Gynecol Obstet. 2014;289(6):1263-1269.
13. CDC. 2015 sexually transmitted diseases treatment guidelines. www.cdc.gov/std/tg2015/default.htm. Accessed September 6, 2017.
14. Korn AP, Hessol NA, Padian NS, et al. Risk factors for plasma cell endometritis among women with cervical Neisseria gonorrhoeae, cervical Chlamydia trachomatis, or bacterial vaginosis. Am J Obstet Gynecol. 1998;178(5):987-990.
15. Wiesenfeld HC, Sweet RL, Ness RB, et al. Comparison of acute and subclinical pelvic inflammatory disease. Sex Transm Dis. 2005;32(7):400-405.
16. Peipert JF, Ness RB, Blume J, et al. Clinical predictors of endometritis in women with symptoms and signs of pelvic inflammatory disease. Am J Obstet Gynecol. 2001;184(5):856-864.
17. Gaitán H, Angel E, Diaz R, et al. Accuracy of five different diagnostic techniques in mild-to-moderate pelvic inflammatory disease. Infect Dis Obstet Gynecol. 2002;10(4):171-180.
18. Tukeva TA, Aronen HJ, Karjalainen PT, et al. MR imaging in pelvic inflammatory disease: comparison with laparoscopy and US. Radiology. 1999;210(1):209-216.
19. Morino M, Pellegrino L, Castagna E, et al. Acute nonspecific abdominal pain. Ann Surg. 2006;244(6):881-888.
20. Woods JL, Scurlock AM, Hensel DJ. Pelvic inflammatory disease in the adolescent: understanding diagnosis and treatment as a health care provider. Pediatric Emergency Care. 2013;29(6):720-725.
21. LeFevre ML; U.S. Preventive Services Task Force. Screening for chlamydia and gonorrhea: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;161(12):902-910.
22. Hosenfeld CB, Workowski KA, Berman S, et al. Repeat infection with Chlamydia and gonorrhea among females: a systematic review of the literature. Sex Transm Dis. 2009;36(8):478-489.
23. CDC. Sexually transmitted disease surveillance. www.cdc.gov/std/stats15/STD-Surveillance-2015-print.pdf. Accessed July 13, 2017.
24. Hillis SD, Joesoef R, Marchbanks PA, et al. Delayed care of pelvic inflammatory disease as a risk factor for impaired fertility. Am J Obstet Gynecol. 1993;168(5):1503-1509.
25. Goyal M, Hersh A, Luan X, et al. National trends in pelvic inflammatory disease among adolescents in the emergency department. J Adolesc Health. 2013;53(2):249-252.
26. Gray-Swain MR, Peipert JF. Pelvic inflammatory disease in adolescents. Curr Opin Obstet Gynecol. 2006;18(5):503-510.
27. Łój B, Brodowska A, Ciecwiez S, et al. The role of serological testing for Chlamydia trachomatis in differential diagnosis of pelvic pain. Ann Agric Environ Med. 2016;23(3):506-510.
28. Sam JW, Jacobs JE, Birnbaum BA. Spectrum of CT findings in acute pyogenic pelvic inflammatory disease. Radiographics. 2002;22(6):1327-1 334.
Can we stop worrying about the age of blood?
Blood transfusions are common in critically ill patients; two in five adults admitted to an ICU receive at least one transfusion during their hospitalization (Corwin HL, et al. Crit Care Med. 2004;32[1]:39). Recently, there has been growing concern about the potential dangers involved with prolonged blood storage. Several provocative observational and retrospective studies found that prolonged storage time (ie, the age of the blood being transfused) negatively affects clinical outcomes (Wang D, et al. Transfusion. 2012;52[6]:1184). But now, some newly published trials on blood transfusion practice, including one published in late September 2017 (Cooper DJ, et al. N Engl J Med. Published online, September 27, 2017) seem to debunk much of this literature. Was all of the concern about age of blood overblown?
The appeal of “fresh” blood is intuitive. As consumers, we’re conditioned that the fresher the better. Fresh food tastes best. Carbonated beverages go “flat” over time. The newest iPhone® device is superior to your old one. So, of course, it follows that fresh blood is also better for your health than older blood.
But, in order to have a viable transfusion service, blood has to be stored. Blood is a scarce resource, and blood banks need to keep an adequate supply on hand for expected clinical necessities, as well as for emergencies. Donors can’t be on standby, waiting in the hospital to provide immediate whole blood transfusion. Also, blood needs to be tested for infections and for potential interactions with the patient, and whole blood must be broken down into individual components for transfusion. All of this requires time and storage.
In a randomized study of 100 critically ill adults supported by mechanical ventilation, 50 were randomized to receive “fresh” blood (median storage age 4 days, interquartile range 3-5 days) and 50 were randomized to receive “standard” blood (median storage age 26.5 days, interquartile range 21-36 days) (Kor DJ, et al. Am J Respir Crit Care Med. 2012;185[8]:842). The primary outcome was gas exchange, as prolonged storage of red blood cells could potentially lead to an increased inflammatory response in patients. However, the authors found no difference in gas exchange between the two groups, and there were no differences in immunologic function or coagulation status.
The ABLE (Age of Blood Evaluation) trial was a randomized, blinded trial of transfusion practices in critically ill patients (Lacroix J, et al. N Engl J Med. 2015;372:1410). In 64 centers in Canada and Europe, 2,430 critically ill adults were randomized to receive either “fresh” blood (mean storage age 6.1 ± 4.9 days) or “standard” blood (mean storage age 22.0 ± 8.4 days). The primary outcome was 90-day mortality, with a power of 90% to detect a 5% change in mortality between the two groups. The investigators found no statistically significant difference in 90-day mortality between the “fresh” and “standard” groups (37% vs 35.3%; hazard ratio 1.1; 95% CI 0.9 – 1.2). Additionally, there were no differences in secondary outcomes, including multiorgan system dysfunction, duration of supportive care, or development of nosocomial infections.
The INFORM (Informing Fresh versus Old Red Cell Management) trial was a randomized study of patients hospitalized in six centers in Canada, Australia, Israel, and the United States (Heddle NM, et al. N Engl J Med. 2016;375[2]:1937). A total of 24,736 patients received transfusions with either “fresh” blood (median storage age 11 days) or “standard” blood (median storage age 23 days). The primary outcome was in-hospital death, with a 90% power to detect a 15% lower relative risk. When comparing the 8,215 patients who received “fresh” blood and the 16,521 patients who received “standard” blood, the authors found no difference in mortality between the two groups (9.1% vs 8.8%; odds ratio 1.04; 95% CI 0.95 to 1.14). Furthermore, there were no differences in outcomes in the high-risk subgroups that included patients with cancer, patients in the ICU, and patients undergoing cardiovascular surgery.
A meta-analysis examined 12 trials of patients who received “fresh” blood compared with those who received “older” or “standard” blood (Alexander PE, et al. Blood. 2016;127[4]:400); 5,229 patients were included in these trials, in which “fresh” blood was defined as blood stored for 3 to 10 days and “older” blood was stored for longer durations. There was no difference in mortality between the two groups (relative risk 1.04; 95% CI 0.94 - 1.14), and no difference in adverse events (relative risk 1.02; 95% CI 0.91 - 1.14). However, perhaps surprisingly, “fresh” blood was associated with an increased risk of nosocomial infections (relative risk 1.09; 95% CI 1.00 - 1.18).
So, can we stop worrying about the age of the blood that we are about to transfuse? Probably. Taken together, these studies suggest that differences in the duration of red blood cell storage allowed within current US FDA standards aren’t clinically relevant, even in critically ill patients. At least, for now, the current practices for age of blood and duration of storage appear unrelated to adverse clinical outcomes.
Dr. Carroll is Professor of Pediatrics, University of Connecticut, Division of Critical Care, Connecticut Children’s Medical Center, Hartford, Connecticut.
Blood transfusions are common in critically ill patients; two in five adults admitted to an ICU receive at least one transfusion during their hospitalization (Corwin HL, et al. Crit Care Med. 2004;32[1]:39). Recently, there has been growing concern about the potential dangers involved with prolonged blood storage. Several provocative observational and retrospective studies found that prolonged storage time (ie, the age of the blood being transfused) negatively affects clinical outcomes (Wang D, et al. Transfusion. 2012;52[6]:1184). But now, some newly published trials on blood transfusion practice, including one published in late September 2017 (Cooper DJ, et al. N Engl J Med. Published online, September 27, 2017) seem to debunk much of this literature. Was all of the concern about age of blood overblown?
The appeal of “fresh” blood is intuitive. As consumers, we’re conditioned that the fresher the better. Fresh food tastes best. Carbonated beverages go “flat” over time. The newest iPhone® device is superior to your old one. So, of course, it follows that fresh blood is also better for your health than older blood.
But, in order to have a viable transfusion service, blood has to be stored. Blood is a scarce resource, and blood banks need to keep an adequate supply on hand for expected clinical necessities, as well as for emergencies. Donors can’t be on standby, waiting in the hospital to provide immediate whole blood transfusion. Also, blood needs to be tested for infections and for potential interactions with the patient, and whole blood must be broken down into individual components for transfusion. All of this requires time and storage.
In a randomized study of 100 critically ill adults supported by mechanical ventilation, 50 were randomized to receive “fresh” blood (median storage age 4 days, interquartile range 3-5 days) and 50 were randomized to receive “standard” blood (median storage age 26.5 days, interquartile range 21-36 days) (Kor DJ, et al. Am J Respir Crit Care Med. 2012;185[8]:842). The primary outcome was gas exchange, as prolonged storage of red blood cells could potentially lead to an increased inflammatory response in patients. However, the authors found no difference in gas exchange between the two groups, and there were no differences in immunologic function or coagulation status.
The ABLE (Age of Blood Evaluation) trial was a randomized, blinded trial of transfusion practices in critically ill patients (Lacroix J, et al. N Engl J Med. 2015;372:1410). In 64 centers in Canada and Europe, 2,430 critically ill adults were randomized to receive either “fresh” blood (mean storage age 6.1 ± 4.9 days) or “standard” blood (mean storage age 22.0 ± 8.4 days). The primary outcome was 90-day mortality, with a power of 90% to detect a 5% change in mortality between the two groups. The investigators found no statistically significant difference in 90-day mortality between the “fresh” and “standard” groups (37% vs 35.3%; hazard ratio 1.1; 95% CI 0.9 – 1.2). Additionally, there were no differences in secondary outcomes, including multiorgan system dysfunction, duration of supportive care, or development of nosocomial infections.
The INFORM (Informing Fresh versus Old Red Cell Management) trial was a randomized study of patients hospitalized in six centers in Canada, Australia, Israel, and the United States (Heddle NM, et al. N Engl J Med. 2016;375[2]:1937). A total of 24,736 patients received transfusions with either “fresh” blood (median storage age 11 days) or “standard” blood (median storage age 23 days). The primary outcome was in-hospital death, with a 90% power to detect a 15% lower relative risk. When comparing the 8,215 patients who received “fresh” blood and the 16,521 patients who received “standard” blood, the authors found no difference in mortality between the two groups (9.1% vs 8.8%; odds ratio 1.04; 95% CI 0.95 to 1.14). Furthermore, there were no differences in outcomes in the high-risk subgroups that included patients with cancer, patients in the ICU, and patients undergoing cardiovascular surgery.
A meta-analysis examined 12 trials of patients who received “fresh” blood compared with those who received “older” or “standard” blood (Alexander PE, et al. Blood. 2016;127[4]:400); 5,229 patients were included in these trials, in which “fresh” blood was defined as blood stored for 3 to 10 days and “older” blood was stored for longer durations. There was no difference in mortality between the two groups (relative risk 1.04; 95% CI 0.94 - 1.14), and no difference in adverse events (relative risk 1.02; 95% CI 0.91 - 1.14). However, perhaps surprisingly, “fresh” blood was associated with an increased risk of nosocomial infections (relative risk 1.09; 95% CI 1.00 - 1.18).
So, can we stop worrying about the age of the blood that we are about to transfuse? Probably. Taken together, these studies suggest that differences in the duration of red blood cell storage allowed within current US FDA standards aren’t clinically relevant, even in critically ill patients. At least, for now, the current practices for age of blood and duration of storage appear unrelated to adverse clinical outcomes.
Dr. Carroll is Professor of Pediatrics, University of Connecticut, Division of Critical Care, Connecticut Children’s Medical Center, Hartford, Connecticut.
Blood transfusions are common in critically ill patients; two in five adults admitted to an ICU receive at least one transfusion during their hospitalization (Corwin HL, et al. Crit Care Med. 2004;32[1]:39). Recently, there has been growing concern about the potential dangers involved with prolonged blood storage. Several provocative observational and retrospective studies found that prolonged storage time (ie, the age of the blood being transfused) negatively affects clinical outcomes (Wang D, et al. Transfusion. 2012;52[6]:1184). But now, some newly published trials on blood transfusion practice, including one published in late September 2017 (Cooper DJ, et al. N Engl J Med. Published online, September 27, 2017) seem to debunk much of this literature. Was all of the concern about age of blood overblown?
The appeal of “fresh” blood is intuitive. As consumers, we’re conditioned that the fresher the better. Fresh food tastes best. Carbonated beverages go “flat” over time. The newest iPhone® device is superior to your old one. So, of course, it follows that fresh blood is also better for your health than older blood.
But, in order to have a viable transfusion service, blood has to be stored. Blood is a scarce resource, and blood banks need to keep an adequate supply on hand for expected clinical necessities, as well as for emergencies. Donors can’t be on standby, waiting in the hospital to provide immediate whole blood transfusion. Also, blood needs to be tested for infections and for potential interactions with the patient, and whole blood must be broken down into individual components for transfusion. All of this requires time and storage.
In a randomized study of 100 critically ill adults supported by mechanical ventilation, 50 were randomized to receive “fresh” blood (median storage age 4 days, interquartile range 3-5 days) and 50 were randomized to receive “standard” blood (median storage age 26.5 days, interquartile range 21-36 days) (Kor DJ, et al. Am J Respir Crit Care Med. 2012;185[8]:842). The primary outcome was gas exchange, as prolonged storage of red blood cells could potentially lead to an increased inflammatory response in patients. However, the authors found no difference in gas exchange between the two groups, and there were no differences in immunologic function or coagulation status.
The ABLE (Age of Blood Evaluation) trial was a randomized, blinded trial of transfusion practices in critically ill patients (Lacroix J, et al. N Engl J Med. 2015;372:1410). In 64 centers in Canada and Europe, 2,430 critically ill adults were randomized to receive either “fresh” blood (mean storage age 6.1 ± 4.9 days) or “standard” blood (mean storage age 22.0 ± 8.4 days). The primary outcome was 90-day mortality, with a power of 90% to detect a 5% change in mortality between the two groups. The investigators found no statistically significant difference in 90-day mortality between the “fresh” and “standard” groups (37% vs 35.3%; hazard ratio 1.1; 95% CI 0.9 – 1.2). Additionally, there were no differences in secondary outcomes, including multiorgan system dysfunction, duration of supportive care, or development of nosocomial infections.
The INFORM (Informing Fresh versus Old Red Cell Management) trial was a randomized study of patients hospitalized in six centers in Canada, Australia, Israel, and the United States (Heddle NM, et al. N Engl J Med. 2016;375[2]:1937). A total of 24,736 patients received transfusions with either “fresh” blood (median storage age 11 days) or “standard” blood (median storage age 23 days). The primary outcome was in-hospital death, with a 90% power to detect a 15% lower relative risk. When comparing the 8,215 patients who received “fresh” blood and the 16,521 patients who received “standard” blood, the authors found no difference in mortality between the two groups (9.1% vs 8.8%; odds ratio 1.04; 95% CI 0.95 to 1.14). Furthermore, there were no differences in outcomes in the high-risk subgroups that included patients with cancer, patients in the ICU, and patients undergoing cardiovascular surgery.
A meta-analysis examined 12 trials of patients who received “fresh” blood compared with those who received “older” or “standard” blood (Alexander PE, et al. Blood. 2016;127[4]:400); 5,229 patients were included in these trials, in which “fresh” blood was defined as blood stored for 3 to 10 days and “older” blood was stored for longer durations. There was no difference in mortality between the two groups (relative risk 1.04; 95% CI 0.94 - 1.14), and no difference in adverse events (relative risk 1.02; 95% CI 0.91 - 1.14). However, perhaps surprisingly, “fresh” blood was associated with an increased risk of nosocomial infections (relative risk 1.09; 95% CI 1.00 - 1.18).
So, can we stop worrying about the age of the blood that we are about to transfuse? Probably. Taken together, these studies suggest that differences in the duration of red blood cell storage allowed within current US FDA standards aren’t clinically relevant, even in critically ill patients. At least, for now, the current practices for age of blood and duration of storage appear unrelated to adverse clinical outcomes.
Dr. Carroll is Professor of Pediatrics, University of Connecticut, Division of Critical Care, Connecticut Children’s Medical Center, Hartford, Connecticut.