User login
Pruritic Eruption on the Chest
The Diagnosis: Grover Disease
Grover disease (also known as transient acantholytic dermatosis) was first described by Ralph W. Grover in 1970 as an idiopathic, acquired, monomorphous, papulovesicular eruption. Although originally characterized by solely transient acantholytic dermatosis, over time the term Grover disease has been expanded to include persistent acantholytic dermatoses. Grover disease chiefly affects white adults older than 40 years and is more prevalent in males than females. Cases generally are self-limited but correlate with age, as older adults are more likely to have prolonged eruptions.1
Grover disease typically erupts with discrete, erythematous, edematous, acneform, red-brown or flesh-colored papules, papulovesicles, or keratotic papules that primarily are seen on the trunk and anterior portion of the chest. As the rash spreads, it can erupt on the neck and thighs. The etiology of Grover disease is unknown, but many factors have been associated with the condition in a limited number of patients, including exposure to UV radiation, excessive heat or sweating, use of sulfadoxine-pyrimethamine and recombinant human IL-4, and infection with Malassezia furfur and Demodex folliculorum.1 Grover disease also has been associated with other conditions such as asteatotic eczema, allergic contact dermatitis, and atopic dermatitis.2
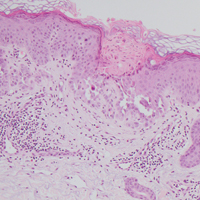
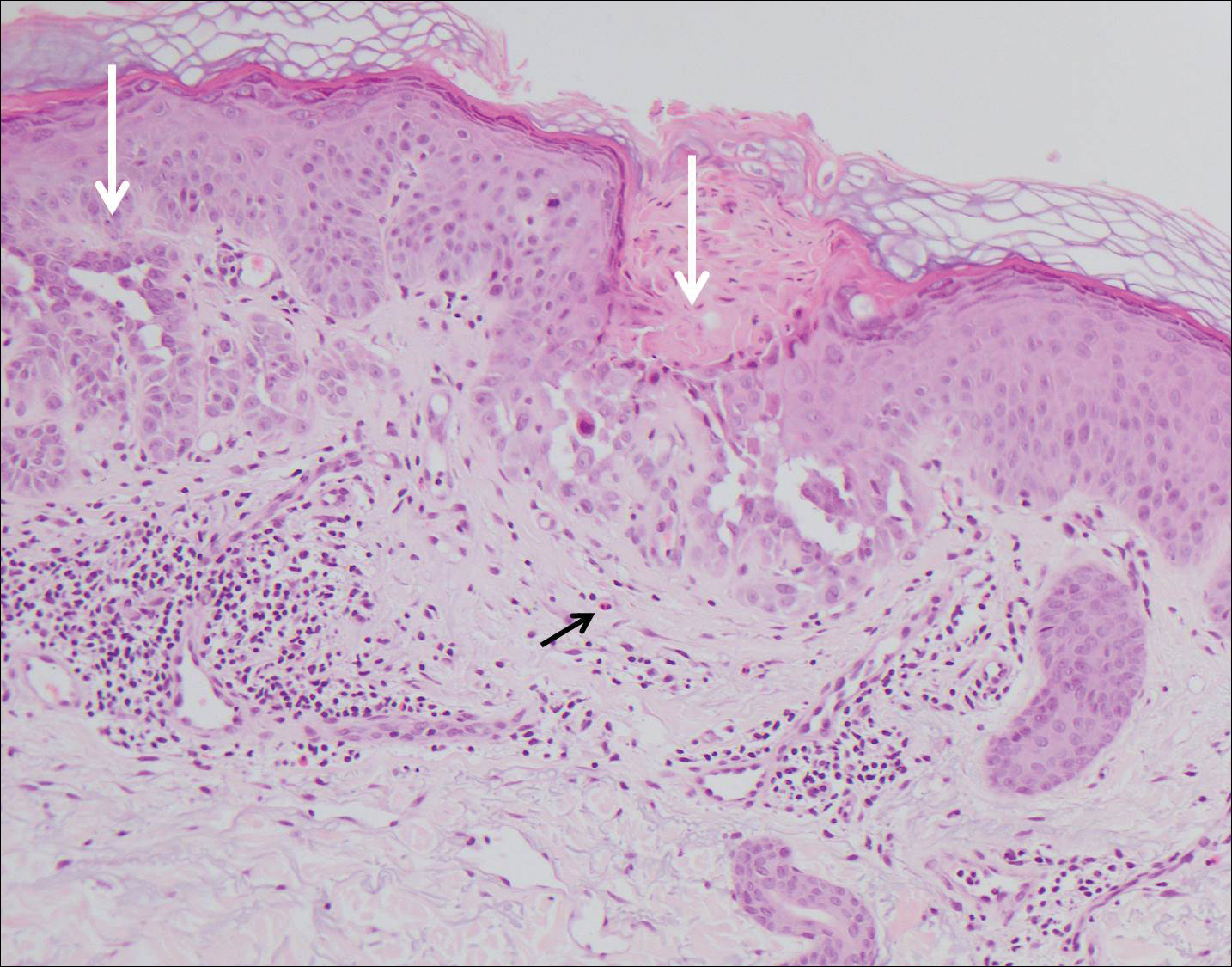
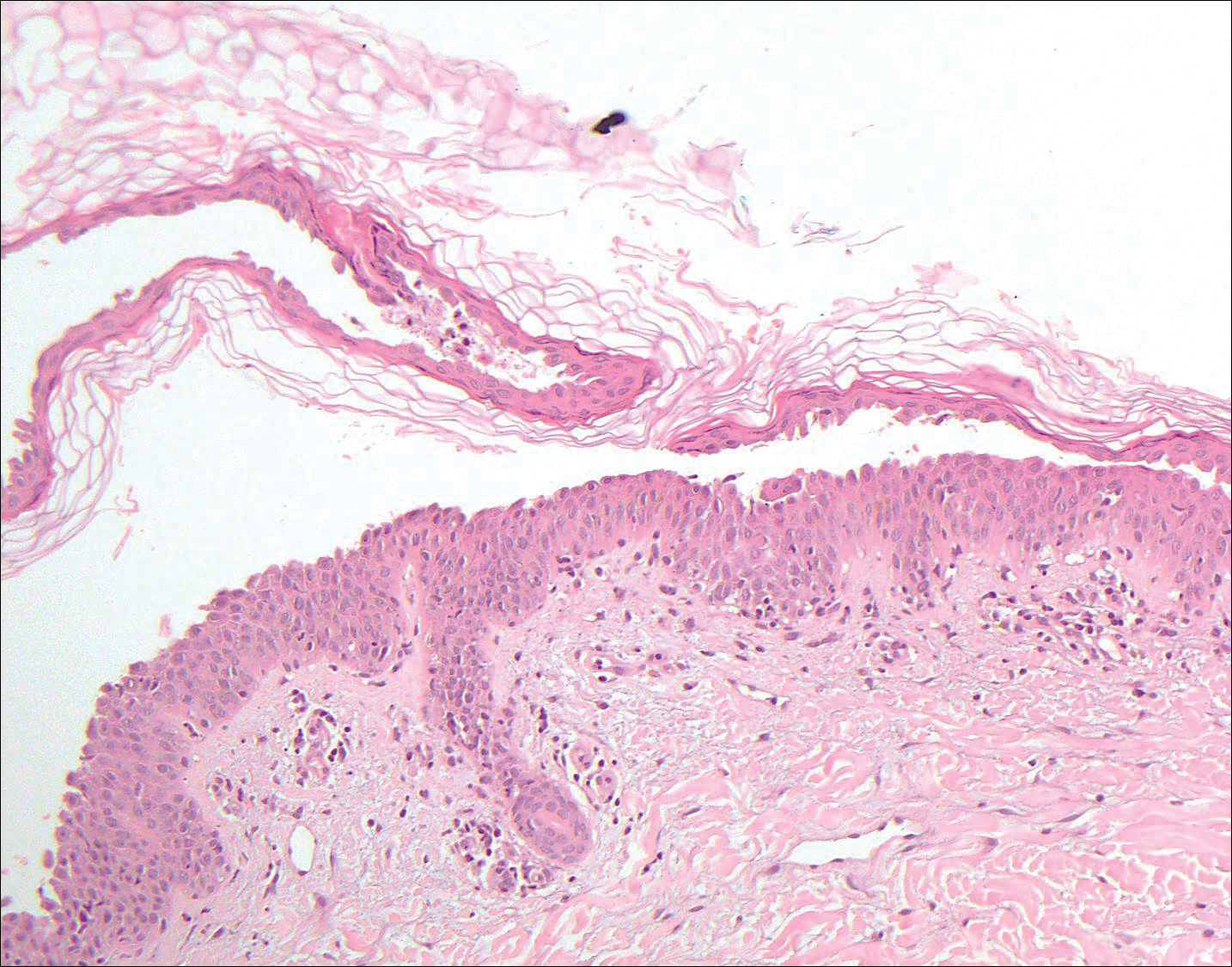
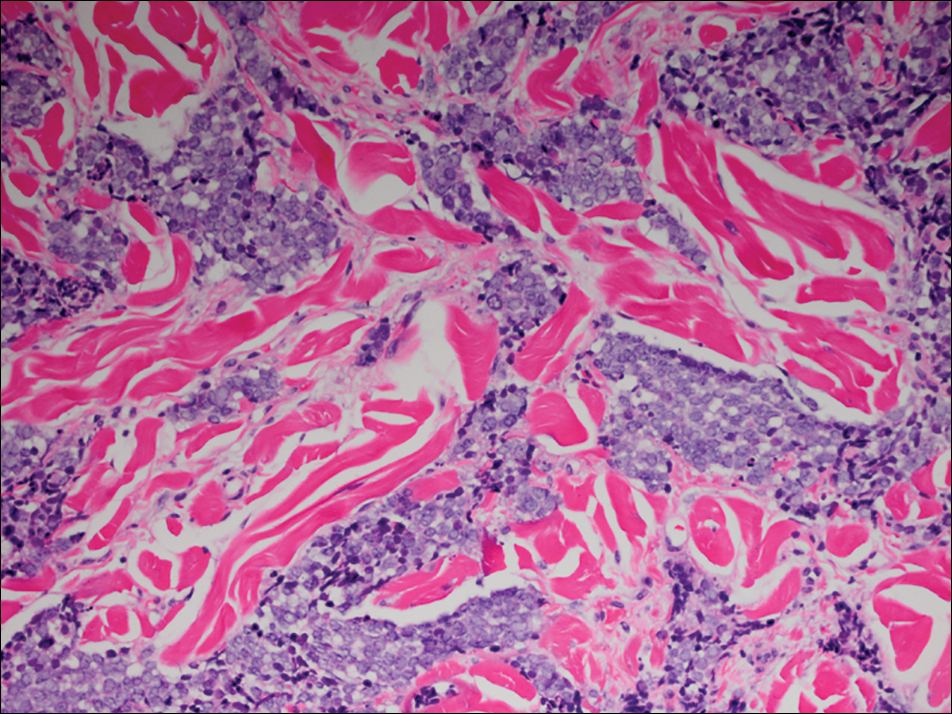
Histologically, Grover disease (Figure 1) is an acantholytic process that can exhibit dyskeratosis (corps ronds and grains). Foci often are small and multiple foci are seen on shave biopsy. There also may be spongiotic changes when associated with an eczematous element. A perivascular lymphohistiocytic infiltrate with eosinophils usually is seen.3 Basket weave keratin may be seen; however, as the lesions cause pruritus, erosions and ulcerations often are present.4
Grover disease has multiple histologic variants that may resemble Darier disease, Hailey-Hailey disease, pemphigus foliaceus, pemphigus vulgaris, and spongiotic dermatitis and can present in combination.5
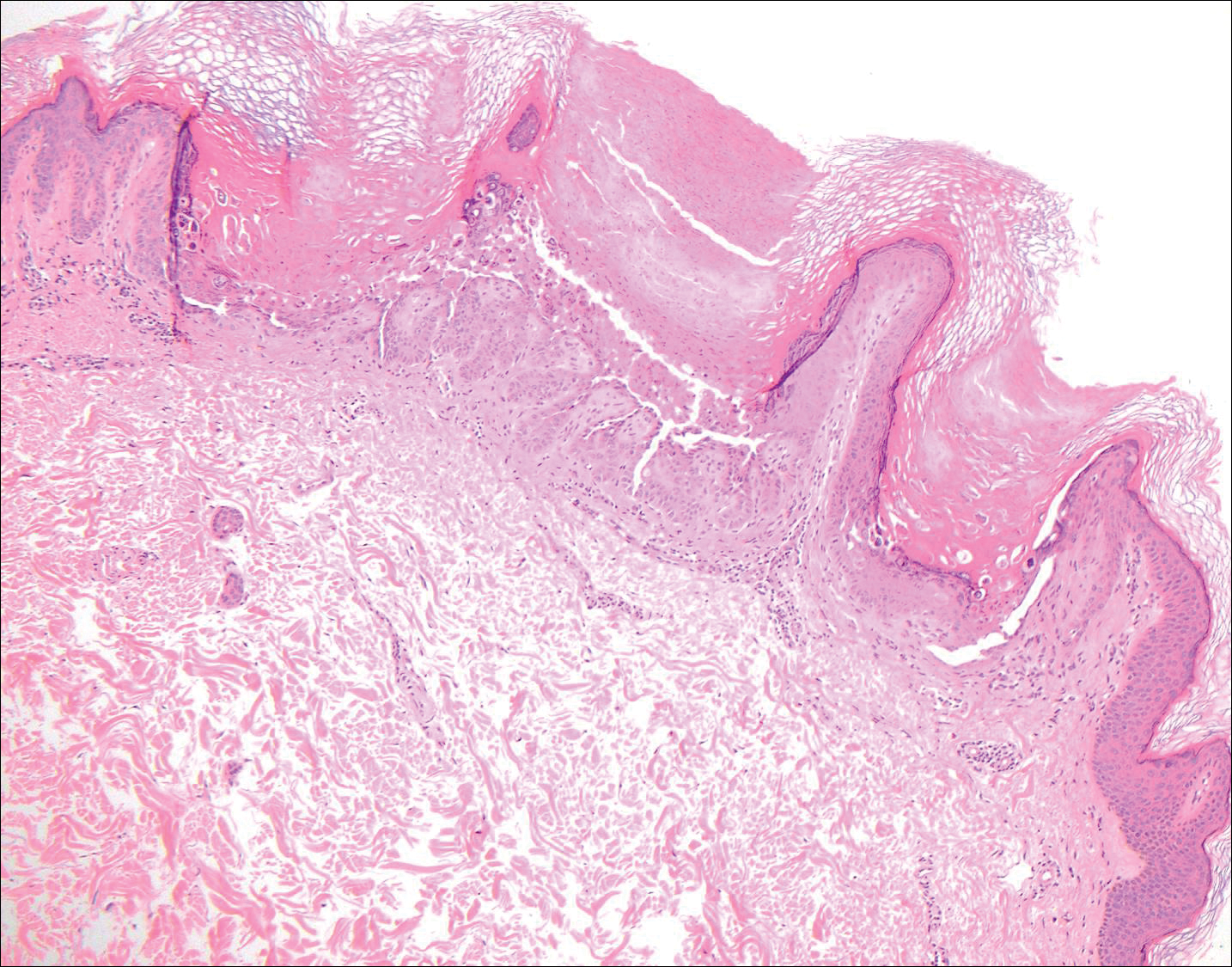
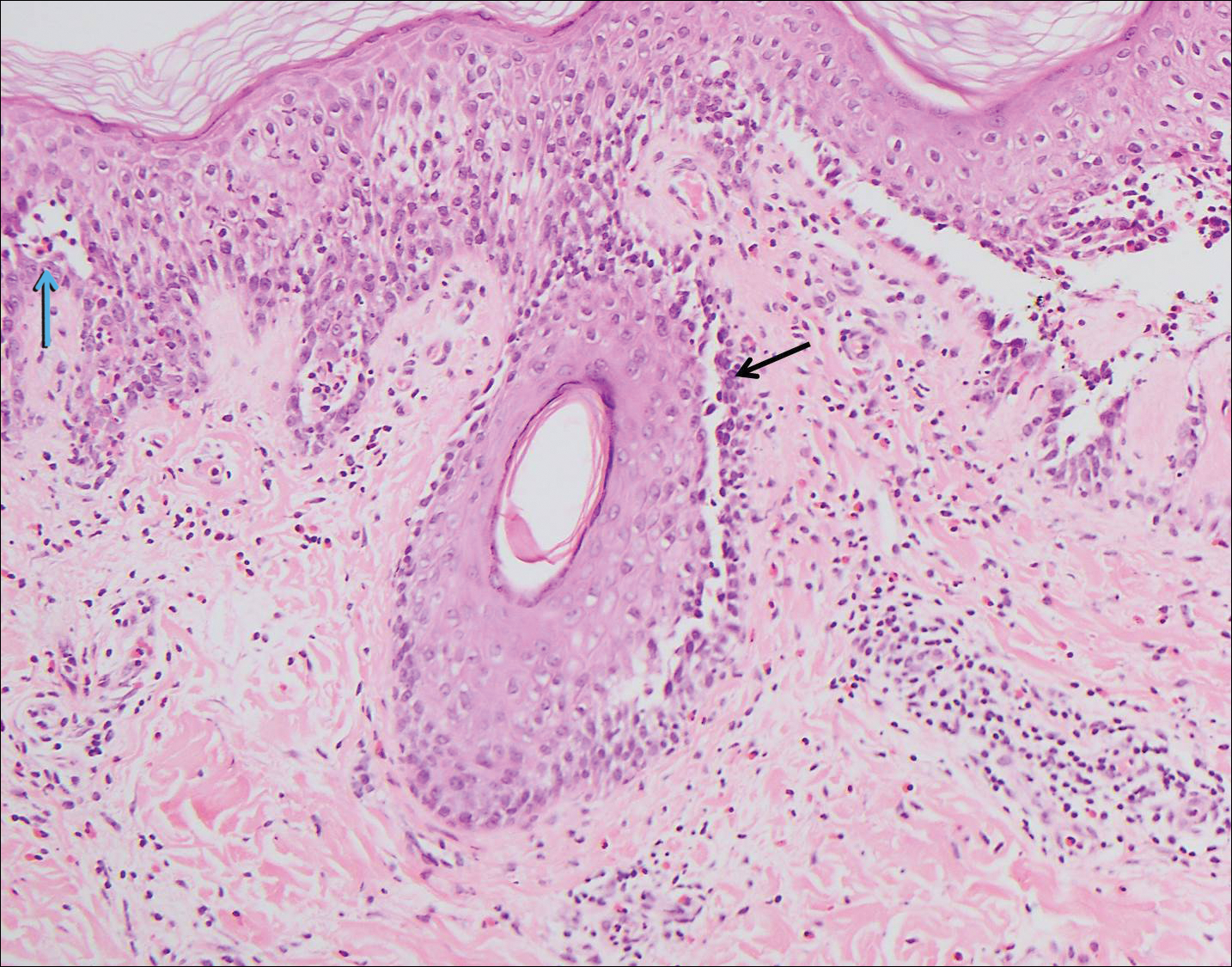
The variant of Grover disease that has a Darier-like pattern is difficult to distinguish from Darier disease, an autosomal-dominant-inherited disorder classified by small papules that emerge in seborrheic areas during childhood and adolescence. Histologically, Darier disease (Figure 2) shows broad areas of dyskeratosis and acantholysis that lead to suprabasal cleavage. Follicular extension may be present. In addition, there often is prominent vertical parakeratosis in Darier disease.6 Histologic features that favor Darier disease over the Darier-like variant of Grover disease include a broad focus of acanthotic dyskeratosis with follicular extension; the presence of a hyperkeratotic stratum corneum; and a lack of spongiosis and eosinophils, which are notably absent in Darier disease but may be present in Grover disease.4
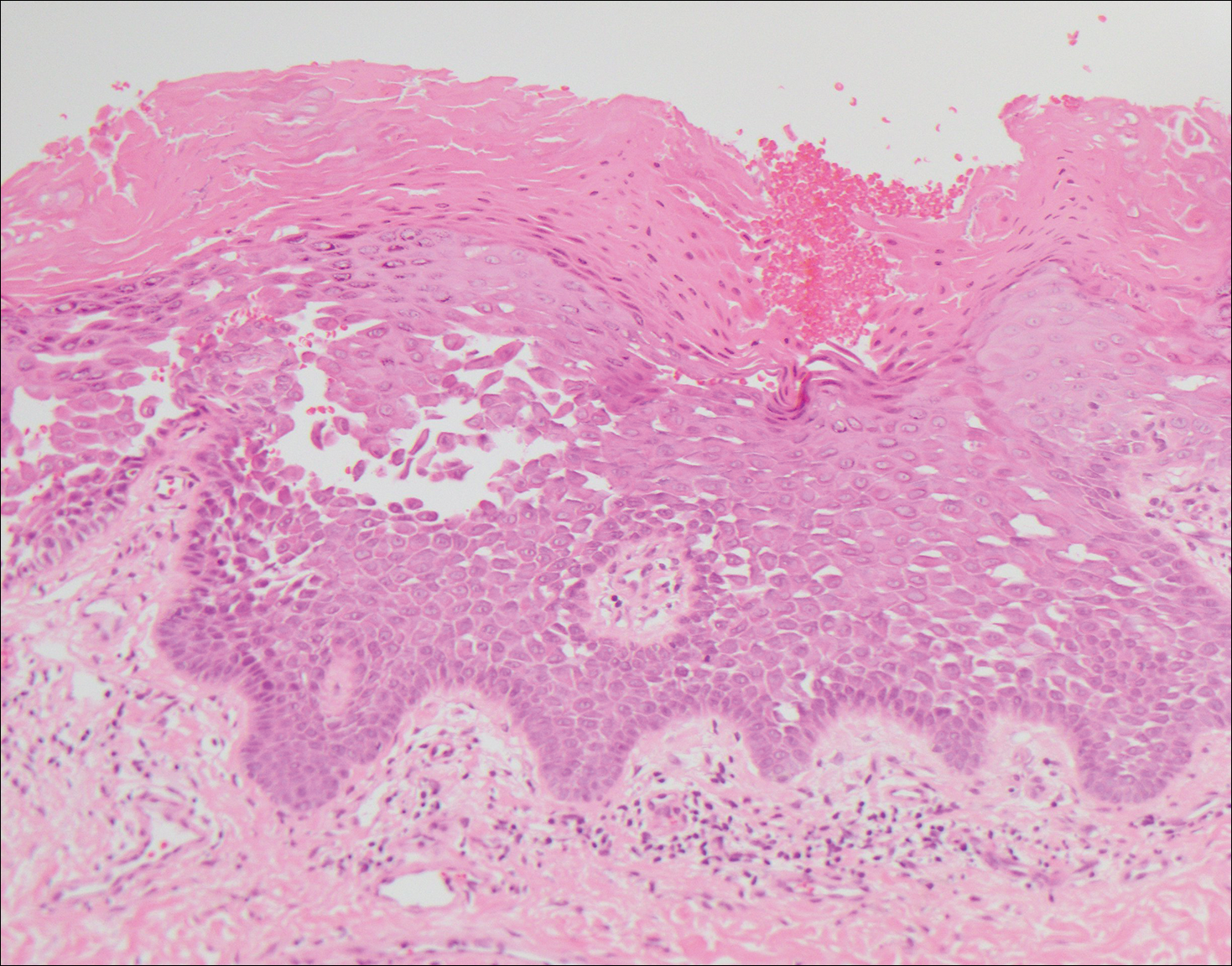
Another variant of Grover disease has a Hailey-Hailey-like pattern, which is characterized by Hailey-Hailey disease's dilapidated brick wall appearance or the diffuse suprabasal acantholysis of all epidermal layers without notable dyskeratosis.4 Hailey-Hailey disease, also known as familial benign pemphigus, is an autosomal-dominant disorder that presents with erythematous vesicular plaques in flexural areas. The plaques progress to flaccid bullae with rupture and crusting and spread peripherally.7 Pathology shows suprabasilar clefts and numerous acantholytic cells (Figure 3). Dyskeratotic keratinocytes are rare with infrequent corps ronds and rare grains. The epidermis also is less hyperplastic in Grover disease than in Hailey-Hailey disease.1
Grover disease also may present histologically with a pemphiguslike pattern, mimicking pemphigus foliaceus and pemphigus vulgaris; however, direct immunofluorescence studies are negative in Grover disease.
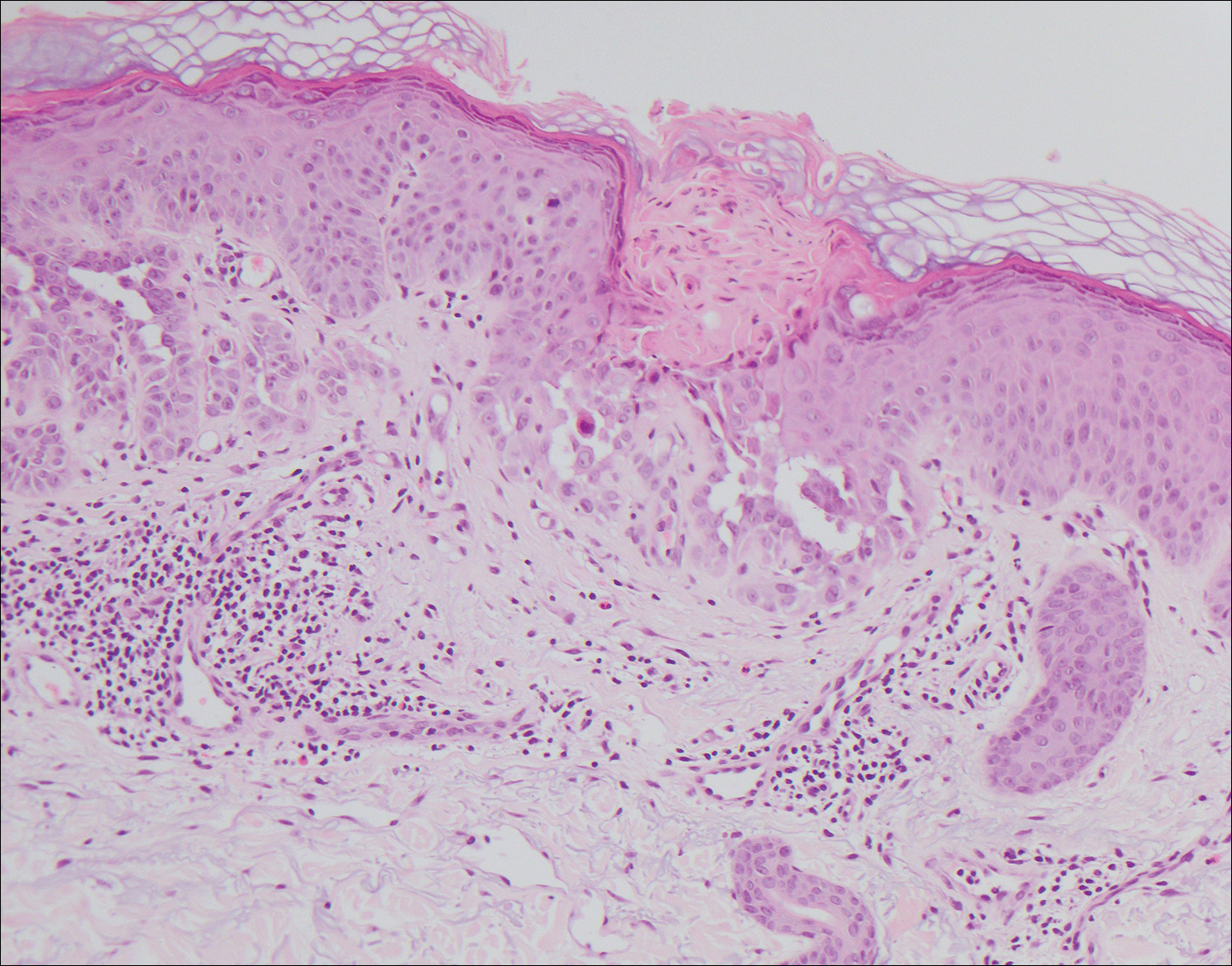
Pemphigus foliaceus is an autoimmune disorder caused by autoantibodies to desmoglein 1, which are present on the surfaces of keratinocytes, and is characterized by scaly crusts and blisters.8 Histologically, pemphigus foliaceus (Figure 4) shows a superficial epidermal blistering process. The acantholysis may be subtle and is commonly localized to the stratum granulosum, extending into the stratum corneum. Complete loss of the stratum corneum can be seen, resulting in only scattered acantholytic cells. Spongiosis also may be seen. The dermis shows a perivascular infiltrate that often contains eosinophils. Pemphigus foliaceus is confirmed by direct immunofluorescence.9
Pemphigus vulgaris is an autoimmune blistering disorder that is characterized by IgG autoantibodies to desmoglein 3, a component of desmosomes that are involved in keratinocyte-to-keratinocyte adhesion. Clinically, patients present with flaccid fragile blisters on the skin and mucous membranes that rupture easily, leading to painful erosions.10 Intraepidermal blisters are seen histologically (Figure 5) with the loss of cohesion (acantholysis) seen classically in the lower portions of the epidermis where desmoglein 3 is most prominent. When only the basal layer remains, the histology has been likened to a tombstone row.11 Extension of the blister along the adnexa is common. The underlying dermis shows a perivascular infiltrate with eosinophils. Early lesions may show only eosinophilic spongiosis. Direct immunofluorescence studies show IgG and C3 in an intercellular pattern that resembles a fish net or chicken wire.4,11
The spongioticlike pattern of Grover disease is marked by epidermal edema with separation of the keratinocytes and the revelation of their intracellular bridges,4 which manifests as vesiculation in the stratum corneum or upper layers of the epidermis.12
Grover disease is self-limited and may spontaneously resolve; however, the disease may be responsive to topical and systemic steroids. Additionally, avoidance of aggravating factors such as sunlight, heat, and sweating can improve symptoms.2
- Parsons JM. Transient acantholytic dermatosis (Grover's disease): a global perspective. J Am Acad Dermatol. 1996;35(5, pt 1):653-666; quiz 667-670.
- Quirk CJ, Heenan PJ. Grover's disease: 34 years on. Australas J Dermatol. 2004;45:83-86.
- Davis MD, Dinneen AM, Landa N, et al. Grover's disease: clinicopathologic review of 72 cases. Mayo Clin Proc. 1999;74:229-234.
- Weaver J, Bergfeld WF. Grover disease (transient acantholytic dermatosis). Arch Pathol Lab Med. 2009;133:1490-1494.
- Chalet M, Grover R, Ackerman AB. Transient acantholytic dermatosis: a reevaluation. Arch Dermatol. 1977;133:431-435.
- Takagi A, Kamijo M, Ikeda S. Darier disease. J Dermatol. 2016;43:275-279.
- Engin B, Kutlubay Z, Celik U, et al. Hailey-Hailey disease: a fold (intertriginous) dermatosis. Clin Dermatol. 2015;33:452-455.
- de Sena Nogueira Maehara L, Huizinga J, Jonkman MF. Rituximab therapy in pemphigus foliaceus: report of 12 cases and review of recent literature [published online March 31, 2015]. Br J Dermatol. 2015;172:1420-1423.
- James KA, Culton DA, Diaz LA. Diagnosis and clinical features of pemphigus foliaceus. Dermatol Clin. 2011;29:405-412.
- Black M, Mignogna MD, Scully C. Number II. pemphigus vulgaris. Oral Dis. 2005;11:119-130.
- Madke B, Doshi B, Khopkar U, et al. Appearances in dermatopathology: the diagnostic and the deceptive. Indian J Dermatol Venerol Leprol. 2013;79:338-348.
- Motaparthi K. Pseudoherpetic transient acantholytic dermatosis (Grover disease): case series and review of the literature [published online February 16, 2017]. J Cutan Pathol. 2017;44:486-489.
The Diagnosis: Grover Disease
Grover disease (also known as transient acantholytic dermatosis) was first described by Ralph W. Grover in 1970 as an idiopathic, acquired, monomorphous, papulovesicular eruption. Although originally characterized by solely transient acantholytic dermatosis, over time the term Grover disease has been expanded to include persistent acantholytic dermatoses. Grover disease chiefly affects white adults older than 40 years and is more prevalent in males than females. Cases generally are self-limited but correlate with age, as older adults are more likely to have prolonged eruptions.1
Grover disease typically erupts with discrete, erythematous, edematous, acneform, red-brown or flesh-colored papules, papulovesicles, or keratotic papules that primarily are seen on the trunk and anterior portion of the chest. As the rash spreads, it can erupt on the neck and thighs. The etiology of Grover disease is unknown, but many factors have been associated with the condition in a limited number of patients, including exposure to UV radiation, excessive heat or sweating, use of sulfadoxine-pyrimethamine and recombinant human IL-4, and infection with Malassezia furfur and Demodex folliculorum.1 Grover disease also has been associated with other conditions such as asteatotic eczema, allergic contact dermatitis, and atopic dermatitis.2
Histologically, Grover disease (Figure 1) is an acantholytic process that can exhibit dyskeratosis (corps ronds and grains). Foci often are small and multiple foci are seen on shave biopsy. There also may be spongiotic changes when associated with an eczematous element. A perivascular lymphohistiocytic infiltrate with eosinophils usually is seen.3 Basket weave keratin may be seen; however, as the lesions cause pruritus, erosions and ulcerations often are present.4

Grover disease has multiple histologic variants that may resemble Darier disease, Hailey-Hailey disease, pemphigus foliaceus, pemphigus vulgaris, and spongiotic dermatitis and can present in combination.5
The variant of Grover disease that has a Darier-like pattern is difficult to distinguish from Darier disease, an autosomal-dominant-inherited disorder classified by small papules that emerge in seborrheic areas during childhood and adolescence. Histologically, Darier disease (Figure 2) shows broad areas of dyskeratosis and acantholysis that lead to suprabasal cleavage. Follicular extension may be present. In addition, there often is prominent vertical parakeratosis in Darier disease.6 Histologic features that favor Darier disease over the Darier-like variant of Grover disease include a broad focus of acanthotic dyskeratosis with follicular extension; the presence of a hyperkeratotic stratum corneum; and a lack of spongiosis and eosinophils, which are notably absent in Darier disease but may be present in Grover disease.4

Another variant of Grover disease has a Hailey-Hailey-like pattern, which is characterized by Hailey-Hailey disease's dilapidated brick wall appearance or the diffuse suprabasal acantholysis of all epidermal layers without notable dyskeratosis.4 Hailey-Hailey disease, also known as familial benign pemphigus, is an autosomal-dominant disorder that presents with erythematous vesicular plaques in flexural areas. The plaques progress to flaccid bullae with rupture and crusting and spread peripherally.7 Pathology shows suprabasilar clefts and numerous acantholytic cells (Figure 3). Dyskeratotic keratinocytes are rare with infrequent corps ronds and rare grains. The epidermis also is less hyperplastic in Grover disease than in Hailey-Hailey disease.1

Grover disease also may present histologically with a pemphiguslike pattern, mimicking pemphigus foliaceus and pemphigus vulgaris; however, direct immunofluorescence studies are negative in Grover disease.
Pemphigus foliaceus is an autoimmune disorder caused by autoantibodies to desmoglein 1, which are present on the surfaces of keratinocytes, and is characterized by scaly crusts and blisters.8 Histologically, pemphigus foliaceus (Figure 4) shows a superficial epidermal blistering process. The acantholysis may be subtle and is commonly localized to the stratum granulosum, extending into the stratum corneum. Complete loss of the stratum corneum can be seen, resulting in only scattered acantholytic cells. Spongiosis also may be seen. The dermis shows a perivascular infiltrate that often contains eosinophils. Pemphigus foliaceus is confirmed by direct immunofluorescence.9

Pemphigus vulgaris is an autoimmune blistering disorder that is characterized by IgG autoantibodies to desmoglein 3, a component of desmosomes that are involved in keratinocyte-to-keratinocyte adhesion. Clinically, patients present with flaccid fragile blisters on the skin and mucous membranes that rupture easily, leading to painful erosions.10 Intraepidermal blisters are seen histologically (Figure 5) with the loss of cohesion (acantholysis) seen classically in the lower portions of the epidermis where desmoglein 3 is most prominent. When only the basal layer remains, the histology has been likened to a tombstone row.11 Extension of the blister along the adnexa is common. The underlying dermis shows a perivascular infiltrate with eosinophils. Early lesions may show only eosinophilic spongiosis. Direct immunofluorescence studies show IgG and C3 in an intercellular pattern that resembles a fish net or chicken wire.4,11

The spongioticlike pattern of Grover disease is marked by epidermal edema with separation of the keratinocytes and the revelation of their intracellular bridges,4 which manifests as vesiculation in the stratum corneum or upper layers of the epidermis.12
Grover disease is self-limited and may spontaneously resolve; however, the disease may be responsive to topical and systemic steroids. Additionally, avoidance of aggravating factors such as sunlight, heat, and sweating can improve symptoms.2
The Diagnosis: Grover Disease
Grover disease (also known as transient acantholytic dermatosis) was first described by Ralph W. Grover in 1970 as an idiopathic, acquired, monomorphous, papulovesicular eruption. Although originally characterized by solely transient acantholytic dermatosis, over time the term Grover disease has been expanded to include persistent acantholytic dermatoses. Grover disease chiefly affects white adults older than 40 years and is more prevalent in males than females. Cases generally are self-limited but correlate with age, as older adults are more likely to have prolonged eruptions.1
Grover disease typically erupts with discrete, erythematous, edematous, acneform, red-brown or flesh-colored papules, papulovesicles, or keratotic papules that primarily are seen on the trunk and anterior portion of the chest. As the rash spreads, it can erupt on the neck and thighs. The etiology of Grover disease is unknown, but many factors have been associated with the condition in a limited number of patients, including exposure to UV radiation, excessive heat or sweating, use of sulfadoxine-pyrimethamine and recombinant human IL-4, and infection with Malassezia furfur and Demodex folliculorum.1 Grover disease also has been associated with other conditions such as asteatotic eczema, allergic contact dermatitis, and atopic dermatitis.2
Histologically, Grover disease (Figure 1) is an acantholytic process that can exhibit dyskeratosis (corps ronds and grains). Foci often are small and multiple foci are seen on shave biopsy. There also may be spongiotic changes when associated with an eczematous element. A perivascular lymphohistiocytic infiltrate with eosinophils usually is seen.3 Basket weave keratin may be seen; however, as the lesions cause pruritus, erosions and ulcerations often are present.4

Grover disease has multiple histologic variants that may resemble Darier disease, Hailey-Hailey disease, pemphigus foliaceus, pemphigus vulgaris, and spongiotic dermatitis and can present in combination.5
The variant of Grover disease that has a Darier-like pattern is difficult to distinguish from Darier disease, an autosomal-dominant-inherited disorder classified by small papules that emerge in seborrheic areas during childhood and adolescence. Histologically, Darier disease (Figure 2) shows broad areas of dyskeratosis and acantholysis that lead to suprabasal cleavage. Follicular extension may be present. In addition, there often is prominent vertical parakeratosis in Darier disease.6 Histologic features that favor Darier disease over the Darier-like variant of Grover disease include a broad focus of acanthotic dyskeratosis with follicular extension; the presence of a hyperkeratotic stratum corneum; and a lack of spongiosis and eosinophils, which are notably absent in Darier disease but may be present in Grover disease.4

Another variant of Grover disease has a Hailey-Hailey-like pattern, which is characterized by Hailey-Hailey disease's dilapidated brick wall appearance or the diffuse suprabasal acantholysis of all epidermal layers without notable dyskeratosis.4 Hailey-Hailey disease, also known as familial benign pemphigus, is an autosomal-dominant disorder that presents with erythematous vesicular plaques in flexural areas. The plaques progress to flaccid bullae with rupture and crusting and spread peripherally.7 Pathology shows suprabasilar clefts and numerous acantholytic cells (Figure 3). Dyskeratotic keratinocytes are rare with infrequent corps ronds and rare grains. The epidermis also is less hyperplastic in Grover disease than in Hailey-Hailey disease.1

Grover disease also may present histologically with a pemphiguslike pattern, mimicking pemphigus foliaceus and pemphigus vulgaris; however, direct immunofluorescence studies are negative in Grover disease.
Pemphigus foliaceus is an autoimmune disorder caused by autoantibodies to desmoglein 1, which are present on the surfaces of keratinocytes, and is characterized by scaly crusts and blisters.8 Histologically, pemphigus foliaceus (Figure 4) shows a superficial epidermal blistering process. The acantholysis may be subtle and is commonly localized to the stratum granulosum, extending into the stratum corneum. Complete loss of the stratum corneum can be seen, resulting in only scattered acantholytic cells. Spongiosis also may be seen. The dermis shows a perivascular infiltrate that often contains eosinophils. Pemphigus foliaceus is confirmed by direct immunofluorescence.9

Pemphigus vulgaris is an autoimmune blistering disorder that is characterized by IgG autoantibodies to desmoglein 3, a component of desmosomes that are involved in keratinocyte-to-keratinocyte adhesion. Clinically, patients present with flaccid fragile blisters on the skin and mucous membranes that rupture easily, leading to painful erosions.10 Intraepidermal blisters are seen histologically (Figure 5) with the loss of cohesion (acantholysis) seen classically in the lower portions of the epidermis where desmoglein 3 is most prominent. When only the basal layer remains, the histology has been likened to a tombstone row.11 Extension of the blister along the adnexa is common. The underlying dermis shows a perivascular infiltrate with eosinophils. Early lesions may show only eosinophilic spongiosis. Direct immunofluorescence studies show IgG and C3 in an intercellular pattern that resembles a fish net or chicken wire.4,11

The spongioticlike pattern of Grover disease is marked by epidermal edema with separation of the keratinocytes and the revelation of their intracellular bridges,4 which manifests as vesiculation in the stratum corneum or upper layers of the epidermis.12
Grover disease is self-limited and may spontaneously resolve; however, the disease may be responsive to topical and systemic steroids. Additionally, avoidance of aggravating factors such as sunlight, heat, and sweating can improve symptoms.2
- Parsons JM. Transient acantholytic dermatosis (Grover's disease): a global perspective. J Am Acad Dermatol. 1996;35(5, pt 1):653-666; quiz 667-670.
- Quirk CJ, Heenan PJ. Grover's disease: 34 years on. Australas J Dermatol. 2004;45:83-86.
- Davis MD, Dinneen AM, Landa N, et al. Grover's disease: clinicopathologic review of 72 cases. Mayo Clin Proc. 1999;74:229-234.
- Weaver J, Bergfeld WF. Grover disease (transient acantholytic dermatosis). Arch Pathol Lab Med. 2009;133:1490-1494.
- Chalet M, Grover R, Ackerman AB. Transient acantholytic dermatosis: a reevaluation. Arch Dermatol. 1977;133:431-435.
- Takagi A, Kamijo M, Ikeda S. Darier disease. J Dermatol. 2016;43:275-279.
- Engin B, Kutlubay Z, Celik U, et al. Hailey-Hailey disease: a fold (intertriginous) dermatosis. Clin Dermatol. 2015;33:452-455.
- de Sena Nogueira Maehara L, Huizinga J, Jonkman MF. Rituximab therapy in pemphigus foliaceus: report of 12 cases and review of recent literature [published online March 31, 2015]. Br J Dermatol. 2015;172:1420-1423.
- James KA, Culton DA, Diaz LA. Diagnosis and clinical features of pemphigus foliaceus. Dermatol Clin. 2011;29:405-412.
- Black M, Mignogna MD, Scully C. Number II. pemphigus vulgaris. Oral Dis. 2005;11:119-130.
- Madke B, Doshi B, Khopkar U, et al. Appearances in dermatopathology: the diagnostic and the deceptive. Indian J Dermatol Venerol Leprol. 2013;79:338-348.
- Motaparthi K. Pseudoherpetic transient acantholytic dermatosis (Grover disease): case series and review of the literature [published online February 16, 2017]. J Cutan Pathol. 2017;44:486-489.
- Parsons JM. Transient acantholytic dermatosis (Grover's disease): a global perspective. J Am Acad Dermatol. 1996;35(5, pt 1):653-666; quiz 667-670.
- Quirk CJ, Heenan PJ. Grover's disease: 34 years on. Australas J Dermatol. 2004;45:83-86.
- Davis MD, Dinneen AM, Landa N, et al. Grover's disease: clinicopathologic review of 72 cases. Mayo Clin Proc. 1999;74:229-234.
- Weaver J, Bergfeld WF. Grover disease (transient acantholytic dermatosis). Arch Pathol Lab Med. 2009;133:1490-1494.
- Chalet M, Grover R, Ackerman AB. Transient acantholytic dermatosis: a reevaluation. Arch Dermatol. 1977;133:431-435.
- Takagi A, Kamijo M, Ikeda S. Darier disease. J Dermatol. 2016;43:275-279.
- Engin B, Kutlubay Z, Celik U, et al. Hailey-Hailey disease: a fold (intertriginous) dermatosis. Clin Dermatol. 2015;33:452-455.
- de Sena Nogueira Maehara L, Huizinga J, Jonkman MF. Rituximab therapy in pemphigus foliaceus: report of 12 cases and review of recent literature [published online March 31, 2015]. Br J Dermatol. 2015;172:1420-1423.
- James KA, Culton DA, Diaz LA. Diagnosis and clinical features of pemphigus foliaceus. Dermatol Clin. 2011;29:405-412.
- Black M, Mignogna MD, Scully C. Number II. pemphigus vulgaris. Oral Dis. 2005;11:119-130.
- Madke B, Doshi B, Khopkar U, et al. Appearances in dermatopathology: the diagnostic and the deceptive. Indian J Dermatol Venerol Leprol. 2013;79:338-348.
- Motaparthi K. Pseudoherpetic transient acantholytic dermatosis (Grover disease): case series and review of the literature [published online February 16, 2017]. J Cutan Pathol. 2017;44:486-489.
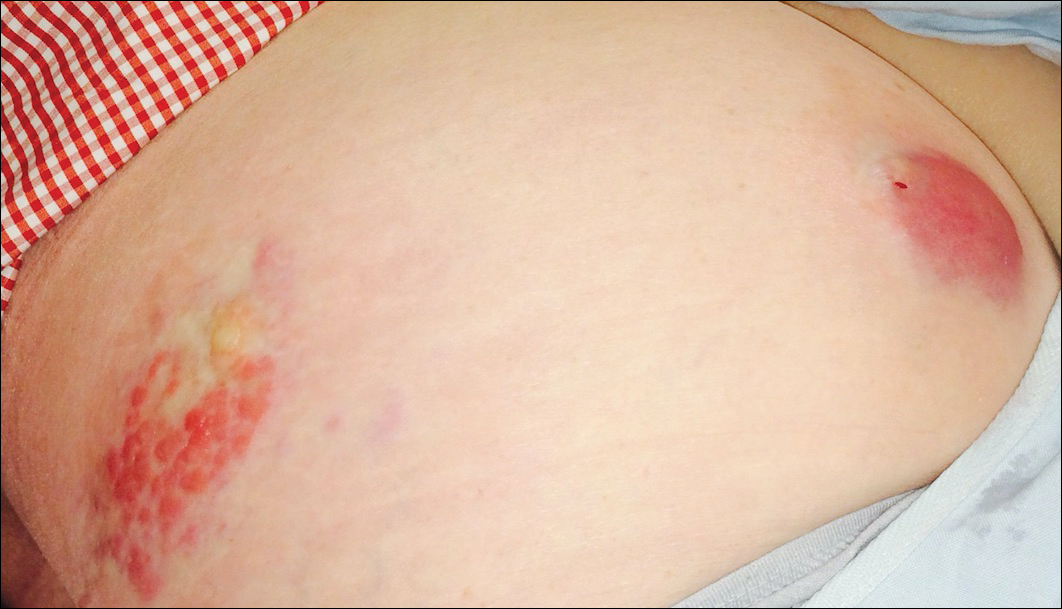
A 55-year-old man presented with small, erythematous, nonfollicular, pruritic papules on the mid chest.
Tips for navigating pityriasis lichenoides in children
CHICAGO – Pityriasis lichenoides chronica (PLC) is a benign, chronic disorder that can last from 1 to 5 years, yet no formal treatment standards exist.
“Topical corticosteroids will not alter the course of the disease, but they provide symptomatic relief when there is pruritus,” Sibel Ersoy-Evans, MD, said at the World Congress of Pediatric Dermatology. “Oral antibiotics, especially erythromycin, and phototherapy are the most common modalities used in children. Systemic immunosuppressants are rarely needed.”
Dr. Ersoy-Evans, a dermatologist at Hacettepe University in Ankara, Turkey, described pityriasis lichenoides (PL) as a spectrum with two polar ends: PLC and pityriasis lichenoides et varioliformis acuta (PLEVA). Its incidence and prevalence are unknown, but age distribution peaks at 2, 5, and 10 years of age. The average age of onset is 6.5 years, it’s slightly more common in boys, and there is no racial or ethnic predilection.
PLEVA presents with symmetrical, reddish-brown papules that later evolve into vesicular, purpuric, necrotic papules. The scalp, face, mucosa, palms, and soles are spared. Resolution occurs with varioliform scarring and dyspigmentation in 2-18 months. On the other hand, chronic PL is characterized by asymptomatic scaly papules and plaques. The lesions start de novo or evolve from PLEVA lesions and they resolve with hypo-hyperpigmentation in about 8-20 months. In what is believed to be the largest study of its kind, Dr. Ersoy-Evans and her associates respectively reviewed 124 patients with PL. They observed that 57% of patients had PLEVA, primarily those who were white, and 37% had PLC (J Am Acad Dermatol. 2007;56[2]:205-10). The median age of onset was 6 years in PLC patients, versus 5 years in those with PLEVA. Age peaks were observed at 2-3 years and 5-7 years.
They also observed that 30% of cases had a history of recent infection prior to the eruption. “Most patients had generalized distribution, and there wasn’t a significant difference in duration according to the distribution of the lesions,” she said. “Postinflammatory pigmentary changes were observed in 90% of the cases, most commonly hypopigmentation. Pruritus was the most common symptom observed, and the disease was relapsing/recurring in 77% of patients. The duration was longer in PL patients.”
A separate study of 25 children and 32 adults with PL found that hypopigmentation was more common in children (72% vs. 19%, respectively), fewer children experienced remission (20% vs. 78%), and phototherapy was more effective than oral antibiotics in children (Br J Dermatol. 2007;157[5]:941-5).
PL runs a chronic, remitting course in 25%-77% of patients, but there are infrequent reports about progression into cutaneous T-cell lymphoma. “We have not observed any lymphoma development in our cohort with a 10-year follow-up, but long-term follow-up is necessary, especially if clonality is present,” Dr. Ersoy-Evans said. The diagnosis of PL is mostly clinical, but histologic examination may be necessary in some cases. “CD8-positive cells predominate in PLEVA, whereas CD4-positive cells predominate in PLC,” she said.
In the review of 124 patients that she and her associates published in 2007, erythromycin estolate or ethylsuccinate was administered to 80% of children, and 67% of these children showed at least a partial response, with a median response time of 2 months.
In a separate study of 24 children with PL, the response rate was highest (83%) after 3.8 months of erythromycin therapy (Ped Dermatol. 2012; 29[6]:719-24). Dr. Ersoy-Evans stated that oral erythromycin should be continued at least 2 months for a response.
A separate study evaluated the use of phototherapy in 18 children with PLC for a mean of 3.7 months (Ped Dermatol 2008;25[6]:599-605). Among 12 patients who received broadband ultraviolet B radiation for a mean of 3.7 months, the response rate was 83%, which was achieved after a mean of 18 sessions. All five patients who received narrow-band UVB cleared, and the mean number of sessions for a response was 22. One patient who received psoralen and ultraviolet A therapy had no response. A larger review of the published literature found that the delivery of narrow-band UVB has the lowest recurrence rate in PL patients treated with phototherapy (Am J Clin Dermatol. 2016;17:583-91). “Given the efficacy and favorable side effects, I think that phototherapy can be a promising option for PL in children,” Dr. Ersoy-Evans said.
She reported having no financial disclosures
CHICAGO – Pityriasis lichenoides chronica (PLC) is a benign, chronic disorder that can last from 1 to 5 years, yet no formal treatment standards exist.
“Topical corticosteroids will not alter the course of the disease, but they provide symptomatic relief when there is pruritus,” Sibel Ersoy-Evans, MD, said at the World Congress of Pediatric Dermatology. “Oral antibiotics, especially erythromycin, and phototherapy are the most common modalities used in children. Systemic immunosuppressants are rarely needed.”
Dr. Ersoy-Evans, a dermatologist at Hacettepe University in Ankara, Turkey, described pityriasis lichenoides (PL) as a spectrum with two polar ends: PLC and pityriasis lichenoides et varioliformis acuta (PLEVA). Its incidence and prevalence are unknown, but age distribution peaks at 2, 5, and 10 years of age. The average age of onset is 6.5 years, it’s slightly more common in boys, and there is no racial or ethnic predilection.
PLEVA presents with symmetrical, reddish-brown papules that later evolve into vesicular, purpuric, necrotic papules. The scalp, face, mucosa, palms, and soles are spared. Resolution occurs with varioliform scarring and dyspigmentation in 2-18 months. On the other hand, chronic PL is characterized by asymptomatic scaly papules and plaques. The lesions start de novo or evolve from PLEVA lesions and they resolve with hypo-hyperpigmentation in about 8-20 months. In what is believed to be the largest study of its kind, Dr. Ersoy-Evans and her associates respectively reviewed 124 patients with PL. They observed that 57% of patients had PLEVA, primarily those who were white, and 37% had PLC (J Am Acad Dermatol. 2007;56[2]:205-10). The median age of onset was 6 years in PLC patients, versus 5 years in those with PLEVA. Age peaks were observed at 2-3 years and 5-7 years.
They also observed that 30% of cases had a history of recent infection prior to the eruption. “Most patients had generalized distribution, and there wasn’t a significant difference in duration according to the distribution of the lesions,” she said. “Postinflammatory pigmentary changes were observed in 90% of the cases, most commonly hypopigmentation. Pruritus was the most common symptom observed, and the disease was relapsing/recurring in 77% of patients. The duration was longer in PL patients.”
A separate study of 25 children and 32 adults with PL found that hypopigmentation was more common in children (72% vs. 19%, respectively), fewer children experienced remission (20% vs. 78%), and phototherapy was more effective than oral antibiotics in children (Br J Dermatol. 2007;157[5]:941-5).
PL runs a chronic, remitting course in 25%-77% of patients, but there are infrequent reports about progression into cutaneous T-cell lymphoma. “We have not observed any lymphoma development in our cohort with a 10-year follow-up, but long-term follow-up is necessary, especially if clonality is present,” Dr. Ersoy-Evans said. The diagnosis of PL is mostly clinical, but histologic examination may be necessary in some cases. “CD8-positive cells predominate in PLEVA, whereas CD4-positive cells predominate in PLC,” she said.
In the review of 124 patients that she and her associates published in 2007, erythromycin estolate or ethylsuccinate was administered to 80% of children, and 67% of these children showed at least a partial response, with a median response time of 2 months.
In a separate study of 24 children with PL, the response rate was highest (83%) after 3.8 months of erythromycin therapy (Ped Dermatol. 2012; 29[6]:719-24). Dr. Ersoy-Evans stated that oral erythromycin should be continued at least 2 months for a response.
A separate study evaluated the use of phototherapy in 18 children with PLC for a mean of 3.7 months (Ped Dermatol 2008;25[6]:599-605). Among 12 patients who received broadband ultraviolet B radiation for a mean of 3.7 months, the response rate was 83%, which was achieved after a mean of 18 sessions. All five patients who received narrow-band UVB cleared, and the mean number of sessions for a response was 22. One patient who received psoralen and ultraviolet A therapy had no response. A larger review of the published literature found that the delivery of narrow-band UVB has the lowest recurrence rate in PL patients treated with phototherapy (Am J Clin Dermatol. 2016;17:583-91). “Given the efficacy and favorable side effects, I think that phototherapy can be a promising option for PL in children,” Dr. Ersoy-Evans said.
She reported having no financial disclosures
CHICAGO – Pityriasis lichenoides chronica (PLC) is a benign, chronic disorder that can last from 1 to 5 years, yet no formal treatment standards exist.
“Topical corticosteroids will not alter the course of the disease, but they provide symptomatic relief when there is pruritus,” Sibel Ersoy-Evans, MD, said at the World Congress of Pediatric Dermatology. “Oral antibiotics, especially erythromycin, and phototherapy are the most common modalities used in children. Systemic immunosuppressants are rarely needed.”
Dr. Ersoy-Evans, a dermatologist at Hacettepe University in Ankara, Turkey, described pityriasis lichenoides (PL) as a spectrum with two polar ends: PLC and pityriasis lichenoides et varioliformis acuta (PLEVA). Its incidence and prevalence are unknown, but age distribution peaks at 2, 5, and 10 years of age. The average age of onset is 6.5 years, it’s slightly more common in boys, and there is no racial or ethnic predilection.
PLEVA presents with symmetrical, reddish-brown papules that later evolve into vesicular, purpuric, necrotic papules. The scalp, face, mucosa, palms, and soles are spared. Resolution occurs with varioliform scarring and dyspigmentation in 2-18 months. On the other hand, chronic PL is characterized by asymptomatic scaly papules and plaques. The lesions start de novo or evolve from PLEVA lesions and they resolve with hypo-hyperpigmentation in about 8-20 months. In what is believed to be the largest study of its kind, Dr. Ersoy-Evans and her associates respectively reviewed 124 patients with PL. They observed that 57% of patients had PLEVA, primarily those who were white, and 37% had PLC (J Am Acad Dermatol. 2007;56[2]:205-10). The median age of onset was 6 years in PLC patients, versus 5 years in those with PLEVA. Age peaks were observed at 2-3 years and 5-7 years.
They also observed that 30% of cases had a history of recent infection prior to the eruption. “Most patients had generalized distribution, and there wasn’t a significant difference in duration according to the distribution of the lesions,” she said. “Postinflammatory pigmentary changes were observed in 90% of the cases, most commonly hypopigmentation. Pruritus was the most common symptom observed, and the disease was relapsing/recurring in 77% of patients. The duration was longer in PL patients.”
A separate study of 25 children and 32 adults with PL found that hypopigmentation was more common in children (72% vs. 19%, respectively), fewer children experienced remission (20% vs. 78%), and phototherapy was more effective than oral antibiotics in children (Br J Dermatol. 2007;157[5]:941-5).
PL runs a chronic, remitting course in 25%-77% of patients, but there are infrequent reports about progression into cutaneous T-cell lymphoma. “We have not observed any lymphoma development in our cohort with a 10-year follow-up, but long-term follow-up is necessary, especially if clonality is present,” Dr. Ersoy-Evans said. The diagnosis of PL is mostly clinical, but histologic examination may be necessary in some cases. “CD8-positive cells predominate in PLEVA, whereas CD4-positive cells predominate in PLC,” she said.
In the review of 124 patients that she and her associates published in 2007, erythromycin estolate or ethylsuccinate was administered to 80% of children, and 67% of these children showed at least a partial response, with a median response time of 2 months.
In a separate study of 24 children with PL, the response rate was highest (83%) after 3.8 months of erythromycin therapy (Ped Dermatol. 2012; 29[6]:719-24). Dr. Ersoy-Evans stated that oral erythromycin should be continued at least 2 months for a response.
A separate study evaluated the use of phototherapy in 18 children with PLC for a mean of 3.7 months (Ped Dermatol 2008;25[6]:599-605). Among 12 patients who received broadband ultraviolet B radiation for a mean of 3.7 months, the response rate was 83%, which was achieved after a mean of 18 sessions. All five patients who received narrow-band UVB cleared, and the mean number of sessions for a response was 22. One patient who received psoralen and ultraviolet A therapy had no response. A larger review of the published literature found that the delivery of narrow-band UVB has the lowest recurrence rate in PL patients treated with phototherapy (Am J Clin Dermatol. 2016;17:583-91). “Given the efficacy and favorable side effects, I think that phototherapy can be a promising option for PL in children,” Dr. Ersoy-Evans said.
She reported having no financial disclosures
AT WCPD 2017
Hyaluronic Acid Gel Filler for Nipple Enhancement Following Breast Reconstruction
The most frequently used surgical techniques in nipple-areola complex (NAC) reconstruction involve the use of local tissue flaps and yield the fewest complications, though these techniques can be associated with up to a 75% loss in nipple projection over time.1 In a best-case scenario for both the surgeon and the patient, the NAC is preserved during mastectomy; however, even when the tissues are spared, an eventual loss of nipple projection is expected due to atrophy and contraction of the healing skin.2 Loss of nipple projection is the most common attribute that patients dislike regarding their NAC reconstruction results.Additional efforts made to restore the natural look and feel of the NAC provides undeniable benefit to the patient in the form of improved body image and psychosocial well-being.3
Augmentation with a grafted material can include cartilage or fat (autologous grafts), calcium hydroxylapatite or polymethyl methacrylate (PMMA)(alloplastic grafts), and acellular dermal matrix or biologic collagen (allografts). Among these options, successive treatment with autologous fat has been shown to provide satisfactory projections over time with minimal complications.4 However, an additional consideration associated with graft augmentation is the need for an additional surgical site (autologous grafts) or the possibility that graft material may not be compatible with subsequent breast examination techniques. For example, calcium hydroxylapatite is a radiopaque material that may interfere with the interpretation of radiography and mammography.5
The use of injectable hyaluronic acid (HA) dermal fillers to enhance nipple projection represents a noninvasive procedure with immediate and adjustable results. A variety of dermal fillers that do not interfere with subsequent breast imaging needs have already been successfully used for nipple reconstruction including HA 60% plus acrylic hydrogel 40%, PMMA microspheres in a bovine collagen 3.5% gel, and poly-L-lactic acid.5-7
The results achieved with HA 60% plus acrylic hydrogel 40% were as much as a 2.5-mm mean increase in nipple projection after 12 months for 70 nipples reconstructed using a small wedge from the labia minora.5 In these treatments, an initial injection of 0.1 to 0.3 mL of filler into each nipple along with a 0.2-mL injection at the base of each nipple was made. Further optional treatments at 2 and 4 months after the initial injection were made using up to 0.3 mL additional volume depending on filler reabsorption.5 Results achieved with PMMA microspheres in a bovine collagen 3.5% gel included a 1.6-mm mean increase in nipple projection at 9 months versus baseline for 33 nipples in 23 patients, which involved up to 2 injections at baseline and again at 3 months.6 Treatment with poly-L-lactic acid provided a 2.3-mm mean increase in nipple projection for 12 patients after 1 year of treatment, which involved 0.5-mL injections every 4 weeks over a series of 4 treatments.7
This report describes the technique and cosmetic outcome using an injectable HA gel to postoperatively restore the 3-dimensional contour of the nipple following surgical breast reconstruction. This chemically cross-linked, stabilized HA gel suspended in phosphate-buffered saline at a pH of 7 and a concentration of 20 mg/mL with lidocaine 0.3% is indicated for mid to deep dermal implantation for the correction of moderate to severe facial wrinkles and folds, such as the nasolabial folds.8
Case Report
A 49-year-old woman with a history of breast cancer with a focal, high-grade ductal carcinoma in situ underwent a complete bilateral mastectomy. The sentinel lymph nodes were negative at the time of mastectomy. One year later, the patient elected to have breast and nipple-areola (flap) reconstruction. Following the reconstructive surgery, her nipples had become visibly atrophic and flat, and she was interested in cosmetic enhancement.
After informed consent had been obtained from the patient, a baseline measurement of each nipple was made while the patient was standing. Each nipple was then injected with up to 0.1 to 0.2 mL of HA gel filler using a 30-gauge needle inserted 2-mm deep (bilaterally) into each nipple. The patient tolerated the procedure well with no pain, bleeding, or bruising. Although HA gel filler contains lidocaine 0.3% and tricaine can further be used to ensure patient comfort, the nipple reconstruction surgery left the patient with little sensation in the treatment area. Rubbing alcohol was used to prepare the skin prior to the procedure, and fractionated coconut oil spray with a nonadherent dressing was used postprocedure.
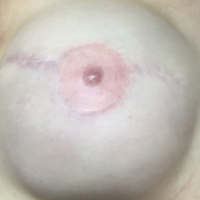
Following the injection, an immediate increase of 1.6 and 1.5 mm in nipple projection in the right and left breasts, respectively, was achieved with HA gel. The nipple projection of the right breast was 1.7 mm before injection (Figure, A) and 3.3 mm immediately postinjection (Figure, C). The nipple projection of the left breast was 1.8 mm before injection (Figure, B) and 3.3 mm immediately postinjection (Figure, D).

Comment
With a single treatment consisting of 0.2 mL or less of filler volume, the HA gel used in this procedure provided an immediate mean increase in nipple projection of 1.5 mm. Although our assessment involved a single patient evaluated at baseline and immediately post-injection of HA filler only, it is reasonable to assume that subsequent reinjections would provide results comparable to other fillers. Although other fillers that are semipermanent (acrylic hydrogel) and nonbiodegradable (PMMA) make them more durable, these properties also make the augmentation less reversible in the case of overfilling. As with all dermal fillers, rare side effects associated with injection of HA gel filler could potentially include injection-site inflammation, extrusion of filler at the needle insertion site, minimal pain or discomfort during or after injections, bruising, swelling, or delayed-type hypersensitivity reaction. Ideally, HA gel is a soft transparent filler that is reversible with hyaluronidase, an advantage not shared by other filler materials.9
Conclusion
Nipple augmentation with HA gel is a simple noninvasive
- Sisti A, Grimaldi L, Tassinari J, et al. Nipple-areola complex reconstruction techniques: a literature review. Eur J Surg Oncol. 2016;42:441-465.
- Murthy V, Chamberlain RS. Defining a place for nipple sparing mastectomy in modern breast care: an evidence based review. Breast J. 2013;19:571-581.
- Jabor MA, Shayani P, Collins DR Jr, et al. Nipple-areola reconstruction: satisfaction and clinical determinants. Plast Reconstr Surg. 2002;110:457-463.
- Kaoutzanis C, Xin M, Ballard TN, et al. Autologous fat grafting after breast reconstruction in postmastectomy patients: complications, biopsy rates, and locoregional cancer recurrence rates. Ann Plast Surg. 2016;76:270-275.
- Panettiere P, Marchetti L, Accorsi D. Filler injection enhances the projection of the reconstructed nipple: an original easy technique. Aesthet Plast Surg. 2005;29:287-294.
- McCarthy CM, Van Laeken N, Lennox P, et al. The efficacy of Artecoll injections for the augmentation of nipple projection in breast reconstruction. Eplasty. 2010;10:E7.
- Dessy LA, Troccola A, Ranno RL, et al. The use of Poly-lactic acid to improve projection of reconstructed nipple. Breast. 2011;20:220-224.
- Restylane L [package insert]. Fort Worth, TX: Galderma Laboratories, LP; 2016.
- Funt D, Pavicic T. Dermal fillers in aesthetics: an overview of adverse events and treatment approaches. Clin Cosmet Investig Dermatol. 2013;6:295-316.
The most frequently used surgical techniques in nipple-areola complex (NAC) reconstruction involve the use of local tissue flaps and yield the fewest complications, though these techniques can be associated with up to a 75% loss in nipple projection over time.1 In a best-case scenario for both the surgeon and the patient, the NAC is preserved during mastectomy; however, even when the tissues are spared, an eventual loss of nipple projection is expected due to atrophy and contraction of the healing skin.2 Loss of nipple projection is the most common attribute that patients dislike regarding their NAC reconstruction results.Additional efforts made to restore the natural look and feel of the NAC provides undeniable benefit to the patient in the form of improved body image and psychosocial well-being.3
Augmentation with a grafted material can include cartilage or fat (autologous grafts), calcium hydroxylapatite or polymethyl methacrylate (PMMA)(alloplastic grafts), and acellular dermal matrix or biologic collagen (allografts). Among these options, successive treatment with autologous fat has been shown to provide satisfactory projections over time with minimal complications.4 However, an additional consideration associated with graft augmentation is the need for an additional surgical site (autologous grafts) or the possibility that graft material may not be compatible with subsequent breast examination techniques. For example, calcium hydroxylapatite is a radiopaque material that may interfere with the interpretation of radiography and mammography.5
The use of injectable hyaluronic acid (HA) dermal fillers to enhance nipple projection represents a noninvasive procedure with immediate and adjustable results. A variety of dermal fillers that do not interfere with subsequent breast imaging needs have already been successfully used for nipple reconstruction including HA 60% plus acrylic hydrogel 40%, PMMA microspheres in a bovine collagen 3.5% gel, and poly-L-lactic acid.5-7
The results achieved with HA 60% plus acrylic hydrogel 40% were as much as a 2.5-mm mean increase in nipple projection after 12 months for 70 nipples reconstructed using a small wedge from the labia minora.5 In these treatments, an initial injection of 0.1 to 0.3 mL of filler into each nipple along with a 0.2-mL injection at the base of each nipple was made. Further optional treatments at 2 and 4 months after the initial injection were made using up to 0.3 mL additional volume depending on filler reabsorption.5 Results achieved with PMMA microspheres in a bovine collagen 3.5% gel included a 1.6-mm mean increase in nipple projection at 9 months versus baseline for 33 nipples in 23 patients, which involved up to 2 injections at baseline and again at 3 months.6 Treatment with poly-L-lactic acid provided a 2.3-mm mean increase in nipple projection for 12 patients after 1 year of treatment, which involved 0.5-mL injections every 4 weeks over a series of 4 treatments.7
This report describes the technique and cosmetic outcome using an injectable HA gel to postoperatively restore the 3-dimensional contour of the nipple following surgical breast reconstruction. This chemically cross-linked, stabilized HA gel suspended in phosphate-buffered saline at a pH of 7 and a concentration of 20 mg/mL with lidocaine 0.3% is indicated for mid to deep dermal implantation for the correction of moderate to severe facial wrinkles and folds, such as the nasolabial folds.8
Case Report
A 49-year-old woman with a history of breast cancer with a focal, high-grade ductal carcinoma in situ underwent a complete bilateral mastectomy. The sentinel lymph nodes were negative at the time of mastectomy. One year later, the patient elected to have breast and nipple-areola (flap) reconstruction. Following the reconstructive surgery, her nipples had become visibly atrophic and flat, and she was interested in cosmetic enhancement.
After informed consent had been obtained from the patient, a baseline measurement of each nipple was made while the patient was standing. Each nipple was then injected with up to 0.1 to 0.2 mL of HA gel filler using a 30-gauge needle inserted 2-mm deep (bilaterally) into each nipple. The patient tolerated the procedure well with no pain, bleeding, or bruising. Although HA gel filler contains lidocaine 0.3% and tricaine can further be used to ensure patient comfort, the nipple reconstruction surgery left the patient with little sensation in the treatment area. Rubbing alcohol was used to prepare the skin prior to the procedure, and fractionated coconut oil spray with a nonadherent dressing was used postprocedure.
Following the injection, an immediate increase of 1.6 and 1.5 mm in nipple projection in the right and left breasts, respectively, was achieved with HA gel. The nipple projection of the right breast was 1.7 mm before injection (Figure, A) and 3.3 mm immediately postinjection (Figure, C). The nipple projection of the left breast was 1.8 mm before injection (Figure, B) and 3.3 mm immediately postinjection (Figure, D).

Comment
With a single treatment consisting of 0.2 mL or less of filler volume, the HA gel used in this procedure provided an immediate mean increase in nipple projection of 1.5 mm. Although our assessment involved a single patient evaluated at baseline and immediately post-injection of HA filler only, it is reasonable to assume that subsequent reinjections would provide results comparable to other fillers. Although other fillers that are semipermanent (acrylic hydrogel) and nonbiodegradable (PMMA) make them more durable, these properties also make the augmentation less reversible in the case of overfilling. As with all dermal fillers, rare side effects associated with injection of HA gel filler could potentially include injection-site inflammation, extrusion of filler at the needle insertion site, minimal pain or discomfort during or after injections, bruising, swelling, or delayed-type hypersensitivity reaction. Ideally, HA gel is a soft transparent filler that is reversible with hyaluronidase, an advantage not shared by other filler materials.9
Conclusion
Nipple augmentation with HA gel is a simple noninvasive
The most frequently used surgical techniques in nipple-areola complex (NAC) reconstruction involve the use of local tissue flaps and yield the fewest complications, though these techniques can be associated with up to a 75% loss in nipple projection over time.1 In a best-case scenario for both the surgeon and the patient, the NAC is preserved during mastectomy; however, even when the tissues are spared, an eventual loss of nipple projection is expected due to atrophy and contraction of the healing skin.2 Loss of nipple projection is the most common attribute that patients dislike regarding their NAC reconstruction results.Additional efforts made to restore the natural look and feel of the NAC provides undeniable benefit to the patient in the form of improved body image and psychosocial well-being.3
Augmentation with a grafted material can include cartilage or fat (autologous grafts), calcium hydroxylapatite or polymethyl methacrylate (PMMA)(alloplastic grafts), and acellular dermal matrix or biologic collagen (allografts). Among these options, successive treatment with autologous fat has been shown to provide satisfactory projections over time with minimal complications.4 However, an additional consideration associated with graft augmentation is the need for an additional surgical site (autologous grafts) or the possibility that graft material may not be compatible with subsequent breast examination techniques. For example, calcium hydroxylapatite is a radiopaque material that may interfere with the interpretation of radiography and mammography.5
The use of injectable hyaluronic acid (HA) dermal fillers to enhance nipple projection represents a noninvasive procedure with immediate and adjustable results. A variety of dermal fillers that do not interfere with subsequent breast imaging needs have already been successfully used for nipple reconstruction including HA 60% plus acrylic hydrogel 40%, PMMA microspheres in a bovine collagen 3.5% gel, and poly-L-lactic acid.5-7
The results achieved with HA 60% plus acrylic hydrogel 40% were as much as a 2.5-mm mean increase in nipple projection after 12 months for 70 nipples reconstructed using a small wedge from the labia minora.5 In these treatments, an initial injection of 0.1 to 0.3 mL of filler into each nipple along with a 0.2-mL injection at the base of each nipple was made. Further optional treatments at 2 and 4 months after the initial injection were made using up to 0.3 mL additional volume depending on filler reabsorption.5 Results achieved with PMMA microspheres in a bovine collagen 3.5% gel included a 1.6-mm mean increase in nipple projection at 9 months versus baseline for 33 nipples in 23 patients, which involved up to 2 injections at baseline and again at 3 months.6 Treatment with poly-L-lactic acid provided a 2.3-mm mean increase in nipple projection for 12 patients after 1 year of treatment, which involved 0.5-mL injections every 4 weeks over a series of 4 treatments.7
This report describes the technique and cosmetic outcome using an injectable HA gel to postoperatively restore the 3-dimensional contour of the nipple following surgical breast reconstruction. This chemically cross-linked, stabilized HA gel suspended in phosphate-buffered saline at a pH of 7 and a concentration of 20 mg/mL with lidocaine 0.3% is indicated for mid to deep dermal implantation for the correction of moderate to severe facial wrinkles and folds, such as the nasolabial folds.8
Case Report
A 49-year-old woman with a history of breast cancer with a focal, high-grade ductal carcinoma in situ underwent a complete bilateral mastectomy. The sentinel lymph nodes were negative at the time of mastectomy. One year later, the patient elected to have breast and nipple-areola (flap) reconstruction. Following the reconstructive surgery, her nipples had become visibly atrophic and flat, and she was interested in cosmetic enhancement.
After informed consent had been obtained from the patient, a baseline measurement of each nipple was made while the patient was standing. Each nipple was then injected with up to 0.1 to 0.2 mL of HA gel filler using a 30-gauge needle inserted 2-mm deep (bilaterally) into each nipple. The patient tolerated the procedure well with no pain, bleeding, or bruising. Although HA gel filler contains lidocaine 0.3% and tricaine can further be used to ensure patient comfort, the nipple reconstruction surgery left the patient with little sensation in the treatment area. Rubbing alcohol was used to prepare the skin prior to the procedure, and fractionated coconut oil spray with a nonadherent dressing was used postprocedure.
Following the injection, an immediate increase of 1.6 and 1.5 mm in nipple projection in the right and left breasts, respectively, was achieved with HA gel. The nipple projection of the right breast was 1.7 mm before injection (Figure, A) and 3.3 mm immediately postinjection (Figure, C). The nipple projection of the left breast was 1.8 mm before injection (Figure, B) and 3.3 mm immediately postinjection (Figure, D).

Comment
With a single treatment consisting of 0.2 mL or less of filler volume, the HA gel used in this procedure provided an immediate mean increase in nipple projection of 1.5 mm. Although our assessment involved a single patient evaluated at baseline and immediately post-injection of HA filler only, it is reasonable to assume that subsequent reinjections would provide results comparable to other fillers. Although other fillers that are semipermanent (acrylic hydrogel) and nonbiodegradable (PMMA) make them more durable, these properties also make the augmentation less reversible in the case of overfilling. As with all dermal fillers, rare side effects associated with injection of HA gel filler could potentially include injection-site inflammation, extrusion of filler at the needle insertion site, minimal pain or discomfort during or after injections, bruising, swelling, or delayed-type hypersensitivity reaction. Ideally, HA gel is a soft transparent filler that is reversible with hyaluronidase, an advantage not shared by other filler materials.9
Conclusion
Nipple augmentation with HA gel is a simple noninvasive
- Sisti A, Grimaldi L, Tassinari J, et al. Nipple-areola complex reconstruction techniques: a literature review. Eur J Surg Oncol. 2016;42:441-465.
- Murthy V, Chamberlain RS. Defining a place for nipple sparing mastectomy in modern breast care: an evidence based review. Breast J. 2013;19:571-581.
- Jabor MA, Shayani P, Collins DR Jr, et al. Nipple-areola reconstruction: satisfaction and clinical determinants. Plast Reconstr Surg. 2002;110:457-463.
- Kaoutzanis C, Xin M, Ballard TN, et al. Autologous fat grafting after breast reconstruction in postmastectomy patients: complications, biopsy rates, and locoregional cancer recurrence rates. Ann Plast Surg. 2016;76:270-275.
- Panettiere P, Marchetti L, Accorsi D. Filler injection enhances the projection of the reconstructed nipple: an original easy technique. Aesthet Plast Surg. 2005;29:287-294.
- McCarthy CM, Van Laeken N, Lennox P, et al. The efficacy of Artecoll injections for the augmentation of nipple projection in breast reconstruction. Eplasty. 2010;10:E7.
- Dessy LA, Troccola A, Ranno RL, et al. The use of Poly-lactic acid to improve projection of reconstructed nipple. Breast. 2011;20:220-224.
- Restylane L [package insert]. Fort Worth, TX: Galderma Laboratories, LP; 2016.
- Funt D, Pavicic T. Dermal fillers in aesthetics: an overview of adverse events and treatment approaches. Clin Cosmet Investig Dermatol. 2013;6:295-316.
- Sisti A, Grimaldi L, Tassinari J, et al. Nipple-areola complex reconstruction techniques: a literature review. Eur J Surg Oncol. 2016;42:441-465.
- Murthy V, Chamberlain RS. Defining a place for nipple sparing mastectomy in modern breast care: an evidence based review. Breast J. 2013;19:571-581.
- Jabor MA, Shayani P, Collins DR Jr, et al. Nipple-areola reconstruction: satisfaction and clinical determinants. Plast Reconstr Surg. 2002;110:457-463.
- Kaoutzanis C, Xin M, Ballard TN, et al. Autologous fat grafting after breast reconstruction in postmastectomy patients: complications, biopsy rates, and locoregional cancer recurrence rates. Ann Plast Surg. 2016;76:270-275.
- Panettiere P, Marchetti L, Accorsi D. Filler injection enhances the projection of the reconstructed nipple: an original easy technique. Aesthet Plast Surg. 2005;29:287-294.
- McCarthy CM, Van Laeken N, Lennox P, et al. The efficacy of Artecoll injections for the augmentation of nipple projection in breast reconstruction. Eplasty. 2010;10:E7.
- Dessy LA, Troccola A, Ranno RL, et al. The use of Poly-lactic acid to improve projection of reconstructed nipple. Breast. 2011;20:220-224.
- Restylane L [package insert]. Fort Worth, TX: Galderma Laboratories, LP; 2016.
- Funt D, Pavicic T. Dermal fillers in aesthetics: an overview of adverse events and treatment approaches. Clin Cosmet Investig Dermatol. 2013;6:295-316.
Practice Points
- The use of injectable hyaluronic acid (HA) gel to restore 3-dimensional contour of the nipple following nipple-areola complex (NAC) reconstruction is a noninvasive procedure that contributes to patient well-being.
- The use of HA gel for NAC augmentation can be performed in an office setting and may eliminate the need for secondary reconstructive surgeries.
Aggressive Merkel Cell Carcinoma in a Liver Transplant Recipient
Merkel cell carcinoma (MCC) is a rare cutaneous neuroendocrine tumor derived from the nerve-associated Merkel cell touch receptors.1 It typically presents as a solitary, rapidly growing, red to violaceous, asymptomatic nodule, though ulcerated, acneform, and cystic lesions also have been described.2 Merkel cell carcinoma follows an aggressive clinical course with a tendency for rapid growth, local recurrence (26%–60% of cases), lymph node invasion, and distant metastases (18%–52% of cases).3
Several risk factors contribute to the development of MCC, including chronic immunosuppression, exposure to UV radiation, and infection with the Merkel cell polyomavirus. Immunosuppression has been shown to increase the risk for MCC and is associated with a worse prognosis independent of stage at diagnosis.4 Organ transplant recipients represent a subset of immunosuppressed patients who are at increased risk for the development of MCC. We report a case of metastatic MCC in a 67-year-old woman 6 years after liver transplantation.
Case Report
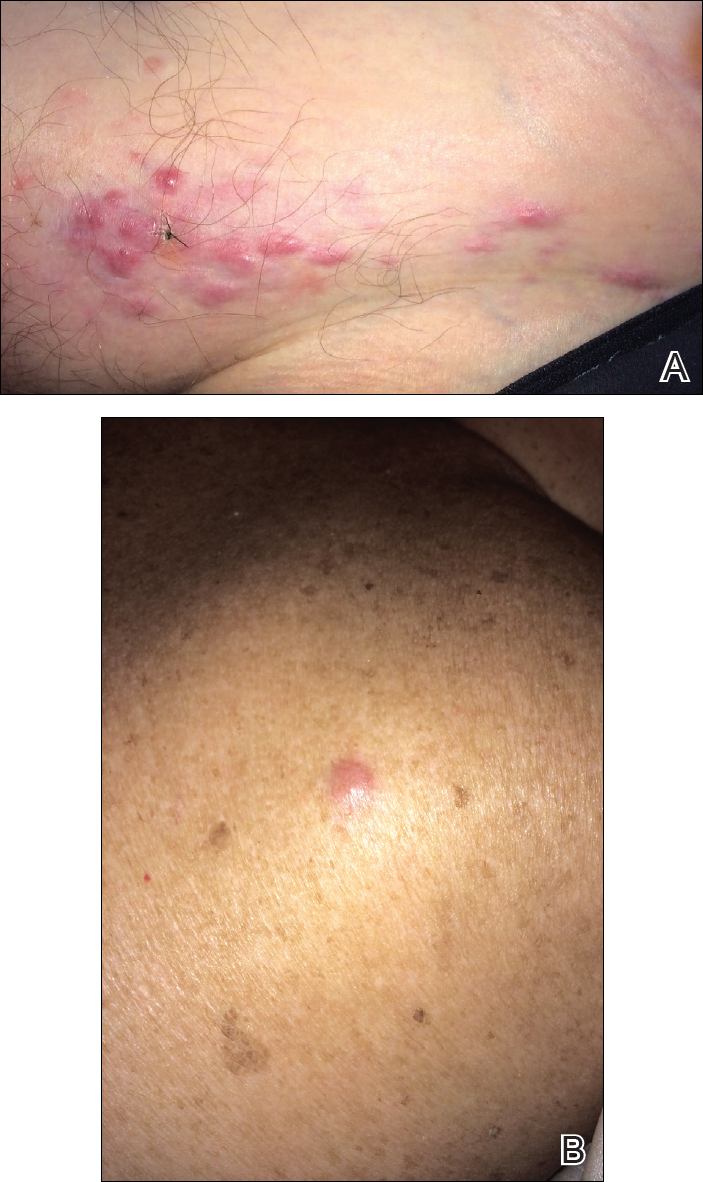
A 67-year-old woman presented to our clinic with 2 masses—1 on the left buttock and 1 on the left hip—of 4 months’ duration. The patient’s medical history was remarkable for autoimmune hepatitis requiring liver transplantation 6 years prior as well as hypertension and thyroid disorder. Her posttransplantation course was unremarkable, and she was maintained on chronic immunosuppression with tacrolimus and mycophenolate mofetil. Six years after transplantation, the patient was observed to have a 4-cm, red-violaceous, painless, dome-shaped tumor on the left buttock (Figure 1). She also was noted to have pink-red papulonodules forming a painless 8-cm plaque on the left hip that was present for 2 weeks prior to presentation (Figure 1). Both lesions were subsequently biopsied.
Microscopic examination of both lesions was consistent with the diagnosis of MCC. On histopathology, both samples exhibited a dense cellular dermis composed of atypical basophilic tumor cells with extension into superficial dilated lymphatic channels indicating lymphovascular invasion (Figure 2). Tumor cells were positive for the immunohistochemical markers pankeratin AE1/AE3, CAM 5.2, cytokeratin 20, synaptophysin, chromogranin A, and Merkel cell polyomavirus.
Total-body computed tomography and positron emission tomography revealed a hypermetabolic lobular density in the left gluteal region measuring 3.9×1.1 cm. The mass was associated with avid disease involving the left inguinal, bilateral iliac chain, and retroperitoneal lymph nodes. The patient was determined to have stage IV MCC based on the presence of distant lymph node metastases. The mass on the left hip was identified as an in-transit metastasis from the primary tumor on the left buttock.
The patient was referred to surgical and medical oncology. The decision was made to start palliative chemotherapy without surgical intervention given the extent of metastases not amenable for resection. The patient was subsequently initiated on chemotherapy with etoposide and carboplatin. After one cycle of chemotherapy, both tumors initially decreased in size; however, 4 months later, despite multiple cycles of chemotherapy, the patient was noted to have growth of existing tumors and interval development of a new 7×5-cm erythematous plaque in the left groin (Figure 3A) and a 1.1×1.0-cm smooth nodule on the right upper back (Figure 3B), both also found to be consistent with distant skin metastases of MCC upon microscopic examination after biopsy. Despite chemotherapy, the patient’s tumor continued to spread and the patient died within 8 months of diagnosis.
Comment
Transplant recipients represent a well-described cohort of immunosuppressed patients prone to the development of MCC. Merkel cell carcinoma in organ transplant recipients has been most frequently documented to occur after kidney transplantation and less frequently after heart and liver transplantations.5,6 However, the role of organ type and immunosuppressive regimen is not well characterized in the literature. Clarke et al7 investigated the risk for MCC in a large cohort of solid organ transplant recipients based on specific immunosuppression medications. They found a higher risk for MCC in patients who were maintained on cyclosporine, azathioprine, and mTOR (mechanistic target of rapamycin) inhibitors rather than tacrolimus, mycophenolate mofetil, and corticosteroids. In comparison to combination tacrolimus–mycophenolate mofetil, cyclosporine-azathioprine was associated with an increased incidence of MCC; this risk rose remarkably in patients who resided in geographic locations with a higher average of UV exposure. The authors suggested that UV radiation and immunosuppression-induced DNA damage may be synergistic in the development of MCC.7
Merkel cell carcinoma most frequently occurs on sun-exposed sites, including the face, head, and neck (55%); upper and lower extremities (40%); and truncal regions (5%).8 However, case reports highlight MCC arising in atypical locations such as the buttocks and gluteal region in organ transplant recipients.7,9 In the general population, MCC predominantly arises in elderly patients (ie, >70 years), but it is more likely to present at an earlier age in transplant recipients.6,10 In a retrospective analysis of 41 solid organ transplant recipients, 12 were diagnosed before the age of 50 years.6 Data from the US Scientific Registry of Transplant Recipients showed a median age at diagnosis of 62 years, with the highest incidence occurring 10 or more years after transplantation.7
Merkel cell carcinoma behaves aggressively and is the most common cause of skin cancer death after melanoma.11 Organ transplant recipients with MCC have a worse prognosis than MCC patients who are not transplant recipients. In a retrospective registry analysis of 45 de novo cases, Buell at al5 found a 60% mortality rate in transplant recipients, almost double the 33% mortality rate of the general population. Furthermore, Arron et al10 revealed substantially increased rates of disease progression and decreased rates of disease-specific and overall survival in solid organ transplant recipients on immunosuppression compared to immunocompetent controls. The most important factor for poor prognosis is the presence of lymph node invasion, which lowers survival rate.12
Conclusion
Merkel cell carcinoma following liver transplantation is not well described in the literature. We highlight a case of an aggressive MCC arising in a sun-protected site with rapid metastasis 6 years after liver transplantation. This case emphasizes the importance of surveillance for cutaneous malignancy in solid organ transplant recipients.
- Gould VE, Moll R, Moll I, et al. Neuroendocrine (Merkel) cells of the skin: hyperplasias, dysplasias, and neoplasms. Lab Invest. 1985;52:334-353.
- Ratner D, Nelson BR, Brown MD, et al. Merkel cell carcinoma. J Am Acad Dermatol. 1993;29(2, pt 1):143-156.
- Pectasides D, Pectasides M, Economopoulos T. Merkel cell cancer of the skin. Ann Oncol. 2006;17:1489-1495.
- Paulson KG, Iyer JG, Blom A, et al. Systemic immune suppression predicts diminished Merkel cell carcinoma-specific survival independent of stage. J Invest Dermatol. 2013;133:642-646.
- Buell JF, Trofe J, Hanaway MJ, et al. Immunosuppression and Merkel cell cancer. Transplant Proc. 2002;34:1780-1781.
- Penn I, First MR. Merkel’s cell carcinoma in organ recipients: report of 41 cases. Transplantation. 1999;68:1717-1721.
- Clarke CA, Robbins HA, Tatalovich Z, et al. Risk of Merkel cell carcinoma after solid organ transplantation. J Natl Cancer Inst. 2015;107. pii:dju382. doi:10.1093/jnci/dju382.
- Rockville Merkel Cell Carcinoma Group. Merkel cell carcinoma: recent progress and current priorities on etiology, pathogenesis and clinical management [published online July 13, 2009]. J Clin Oncol. 2009;27:4021-4026.
- Krejčí K, Tichý T, Horák P, et al. Merkel cell carcinoma of the gluteal region with ipsilateral metastasis into the pancreatic graft of a patient after combined kidney-pancreas transplantation [published online September 20, 2010]. Onkologie. 2010;33:520-524.
- Arron ST, Canavan T, Yu SS. Organ transplant recipients with Merkel cell carcinoma have reduced progression-free, overall, and disease-specific survival independent of stage at presentation [published online July 1, 2014]. J Am Acad Dermatol. 2014;71:684-690.
- Albores-Saavedra J, Batich K, Chable-Montero F, et al. Merkel cell carcinoma demographics, morphology, and survival based on 3870 cases: a population-based study [published online July 23, 2009]. J Cutan Pathol. 2010;37:20-27.
- Eng TY, Boersma MG, Fuller CD, et al. Treatment of Merkel cell carcinoma. Am J Clin Oncol. 2004;27:510-515.
Merkel cell carcinoma (MCC) is a rare cutaneous neuroendocrine tumor derived from the nerve-associated Merkel cell touch receptors.1 It typically presents as a solitary, rapidly growing, red to violaceous, asymptomatic nodule, though ulcerated, acneform, and cystic lesions also have been described.2 Merkel cell carcinoma follows an aggressive clinical course with a tendency for rapid growth, local recurrence (26%–60% of cases), lymph node invasion, and distant metastases (18%–52% of cases).3
Several risk factors contribute to the development of MCC, including chronic immunosuppression, exposure to UV radiation, and infection with the Merkel cell polyomavirus. Immunosuppression has been shown to increase the risk for MCC and is associated with a worse prognosis independent of stage at diagnosis.4 Organ transplant recipients represent a subset of immunosuppressed patients who are at increased risk for the development of MCC. We report a case of metastatic MCC in a 67-year-old woman 6 years after liver transplantation.
Case Report
A 67-year-old woman presented to our clinic with 2 masses—1 on the left buttock and 1 on the left hip—of 4 months’ duration. The patient’s medical history was remarkable for autoimmune hepatitis requiring liver transplantation 6 years prior as well as hypertension and thyroid disorder. Her posttransplantation course was unremarkable, and she was maintained on chronic immunosuppression with tacrolimus and mycophenolate mofetil. Six years after transplantation, the patient was observed to have a 4-cm, red-violaceous, painless, dome-shaped tumor on the left buttock (Figure 1). She also was noted to have pink-red papulonodules forming a painless 8-cm plaque on the left hip that was present for 2 weeks prior to presentation (Figure 1). Both lesions were subsequently biopsied.

Microscopic examination of both lesions was consistent with the diagnosis of MCC. On histopathology, both samples exhibited a dense cellular dermis composed of atypical basophilic tumor cells with extension into superficial dilated lymphatic channels indicating lymphovascular invasion (Figure 2). Tumor cells were positive for the immunohistochemical markers pankeratin AE1/AE3, CAM 5.2, cytokeratin 20, synaptophysin, chromogranin A, and Merkel cell polyomavirus.

Total-body computed tomography and positron emission tomography revealed a hypermetabolic lobular density in the left gluteal region measuring 3.9×1.1 cm. The mass was associated with avid disease involving the left inguinal, bilateral iliac chain, and retroperitoneal lymph nodes. The patient was determined to have stage IV MCC based on the presence of distant lymph node metastases. The mass on the left hip was identified as an in-transit metastasis from the primary tumor on the left buttock.
The patient was referred to surgical and medical oncology. The decision was made to start palliative chemotherapy without surgical intervention given the extent of metastases not amenable for resection. The patient was subsequently initiated on chemotherapy with etoposide and carboplatin. After one cycle of chemotherapy, both tumors initially decreased in size; however, 4 months later, despite multiple cycles of chemotherapy, the patient was noted to have growth of existing tumors and interval development of a new 7×5-cm erythematous plaque in the left groin (Figure 3A) and a 1.1×1.0-cm smooth nodule on the right upper back (Figure 3B), both also found to be consistent with distant skin metastases of MCC upon microscopic examination after biopsy. Despite chemotherapy, the patient’s tumor continued to spread and the patient died within 8 months of diagnosis.

Comment
Transplant recipients represent a well-described cohort of immunosuppressed patients prone to the development of MCC. Merkel cell carcinoma in organ transplant recipients has been most frequently documented to occur after kidney transplantation and less frequently after heart and liver transplantations.5,6 However, the role of organ type and immunosuppressive regimen is not well characterized in the literature. Clarke et al7 investigated the risk for MCC in a large cohort of solid organ transplant recipients based on specific immunosuppression medications. They found a higher risk for MCC in patients who were maintained on cyclosporine, azathioprine, and mTOR (mechanistic target of rapamycin) inhibitors rather than tacrolimus, mycophenolate mofetil, and corticosteroids. In comparison to combination tacrolimus–mycophenolate mofetil, cyclosporine-azathioprine was associated with an increased incidence of MCC; this risk rose remarkably in patients who resided in geographic locations with a higher average of UV exposure. The authors suggested that UV radiation and immunosuppression-induced DNA damage may be synergistic in the development of MCC.7
Merkel cell carcinoma most frequently occurs on sun-exposed sites, including the face, head, and neck (55%); upper and lower extremities (40%); and truncal regions (5%).8 However, case reports highlight MCC arising in atypical locations such as the buttocks and gluteal region in organ transplant recipients.7,9 In the general population, MCC predominantly arises in elderly patients (ie, >70 years), but it is more likely to present at an earlier age in transplant recipients.6,10 In a retrospective analysis of 41 solid organ transplant recipients, 12 were diagnosed before the age of 50 years.6 Data from the US Scientific Registry of Transplant Recipients showed a median age at diagnosis of 62 years, with the highest incidence occurring 10 or more years after transplantation.7
Merkel cell carcinoma behaves aggressively and is the most common cause of skin cancer death after melanoma.11 Organ transplant recipients with MCC have a worse prognosis than MCC patients who are not transplant recipients. In a retrospective registry analysis of 45 de novo cases, Buell at al5 found a 60% mortality rate in transplant recipients, almost double the 33% mortality rate of the general population. Furthermore, Arron et al10 revealed substantially increased rates of disease progression and decreased rates of disease-specific and overall survival in solid organ transplant recipients on immunosuppression compared to immunocompetent controls. The most important factor for poor prognosis is the presence of lymph node invasion, which lowers survival rate.12
Conclusion
Merkel cell carcinoma following liver transplantation is not well described in the literature. We highlight a case of an aggressive MCC arising in a sun-protected site with rapid metastasis 6 years after liver transplantation. This case emphasizes the importance of surveillance for cutaneous malignancy in solid organ transplant recipients.
Merkel cell carcinoma (MCC) is a rare cutaneous neuroendocrine tumor derived from the nerve-associated Merkel cell touch receptors.1 It typically presents as a solitary, rapidly growing, red to violaceous, asymptomatic nodule, though ulcerated, acneform, and cystic lesions also have been described.2 Merkel cell carcinoma follows an aggressive clinical course with a tendency for rapid growth, local recurrence (26%–60% of cases), lymph node invasion, and distant metastases (18%–52% of cases).3
Several risk factors contribute to the development of MCC, including chronic immunosuppression, exposure to UV radiation, and infection with the Merkel cell polyomavirus. Immunosuppression has been shown to increase the risk for MCC and is associated with a worse prognosis independent of stage at diagnosis.4 Organ transplant recipients represent a subset of immunosuppressed patients who are at increased risk for the development of MCC. We report a case of metastatic MCC in a 67-year-old woman 6 years after liver transplantation.
Case Report
A 67-year-old woman presented to our clinic with 2 masses—1 on the left buttock and 1 on the left hip—of 4 months’ duration. The patient’s medical history was remarkable for autoimmune hepatitis requiring liver transplantation 6 years prior as well as hypertension and thyroid disorder. Her posttransplantation course was unremarkable, and she was maintained on chronic immunosuppression with tacrolimus and mycophenolate mofetil. Six years after transplantation, the patient was observed to have a 4-cm, red-violaceous, painless, dome-shaped tumor on the left buttock (Figure 1). She also was noted to have pink-red papulonodules forming a painless 8-cm plaque on the left hip that was present for 2 weeks prior to presentation (Figure 1). Both lesions were subsequently biopsied.

Microscopic examination of both lesions was consistent with the diagnosis of MCC. On histopathology, both samples exhibited a dense cellular dermis composed of atypical basophilic tumor cells with extension into superficial dilated lymphatic channels indicating lymphovascular invasion (Figure 2). Tumor cells were positive for the immunohistochemical markers pankeratin AE1/AE3, CAM 5.2, cytokeratin 20, synaptophysin, chromogranin A, and Merkel cell polyomavirus.

Total-body computed tomography and positron emission tomography revealed a hypermetabolic lobular density in the left gluteal region measuring 3.9×1.1 cm. The mass was associated with avid disease involving the left inguinal, bilateral iliac chain, and retroperitoneal lymph nodes. The patient was determined to have stage IV MCC based on the presence of distant lymph node metastases. The mass on the left hip was identified as an in-transit metastasis from the primary tumor on the left buttock.
The patient was referred to surgical and medical oncology. The decision was made to start palliative chemotherapy without surgical intervention given the extent of metastases not amenable for resection. The patient was subsequently initiated on chemotherapy with etoposide and carboplatin. After one cycle of chemotherapy, both tumors initially decreased in size; however, 4 months later, despite multiple cycles of chemotherapy, the patient was noted to have growth of existing tumors and interval development of a new 7×5-cm erythematous plaque in the left groin (Figure 3A) and a 1.1×1.0-cm smooth nodule on the right upper back (Figure 3B), both also found to be consistent with distant skin metastases of MCC upon microscopic examination after biopsy. Despite chemotherapy, the patient’s tumor continued to spread and the patient died within 8 months of diagnosis.

Comment
Transplant recipients represent a well-described cohort of immunosuppressed patients prone to the development of MCC. Merkel cell carcinoma in organ transplant recipients has been most frequently documented to occur after kidney transplantation and less frequently after heart and liver transplantations.5,6 However, the role of organ type and immunosuppressive regimen is not well characterized in the literature. Clarke et al7 investigated the risk for MCC in a large cohort of solid organ transplant recipients based on specific immunosuppression medications. They found a higher risk for MCC in patients who were maintained on cyclosporine, azathioprine, and mTOR (mechanistic target of rapamycin) inhibitors rather than tacrolimus, mycophenolate mofetil, and corticosteroids. In comparison to combination tacrolimus–mycophenolate mofetil, cyclosporine-azathioprine was associated with an increased incidence of MCC; this risk rose remarkably in patients who resided in geographic locations with a higher average of UV exposure. The authors suggested that UV radiation and immunosuppression-induced DNA damage may be synergistic in the development of MCC.7
Merkel cell carcinoma most frequently occurs on sun-exposed sites, including the face, head, and neck (55%); upper and lower extremities (40%); and truncal regions (5%).8 However, case reports highlight MCC arising in atypical locations such as the buttocks and gluteal region in organ transplant recipients.7,9 In the general population, MCC predominantly arises in elderly patients (ie, >70 years), but it is more likely to present at an earlier age in transplant recipients.6,10 In a retrospective analysis of 41 solid organ transplant recipients, 12 were diagnosed before the age of 50 years.6 Data from the US Scientific Registry of Transplant Recipients showed a median age at diagnosis of 62 years, with the highest incidence occurring 10 or more years after transplantation.7
Merkel cell carcinoma behaves aggressively and is the most common cause of skin cancer death after melanoma.11 Organ transplant recipients with MCC have a worse prognosis than MCC patients who are not transplant recipients. In a retrospective registry analysis of 45 de novo cases, Buell at al5 found a 60% mortality rate in transplant recipients, almost double the 33% mortality rate of the general population. Furthermore, Arron et al10 revealed substantially increased rates of disease progression and decreased rates of disease-specific and overall survival in solid organ transplant recipients on immunosuppression compared to immunocompetent controls. The most important factor for poor prognosis is the presence of lymph node invasion, which lowers survival rate.12
Conclusion
Merkel cell carcinoma following liver transplantation is not well described in the literature. We highlight a case of an aggressive MCC arising in a sun-protected site with rapid metastasis 6 years after liver transplantation. This case emphasizes the importance of surveillance for cutaneous malignancy in solid organ transplant recipients.
- Gould VE, Moll R, Moll I, et al. Neuroendocrine (Merkel) cells of the skin: hyperplasias, dysplasias, and neoplasms. Lab Invest. 1985;52:334-353.
- Ratner D, Nelson BR, Brown MD, et al. Merkel cell carcinoma. J Am Acad Dermatol. 1993;29(2, pt 1):143-156.
- Pectasides D, Pectasides M, Economopoulos T. Merkel cell cancer of the skin. Ann Oncol. 2006;17:1489-1495.
- Paulson KG, Iyer JG, Blom A, et al. Systemic immune suppression predicts diminished Merkel cell carcinoma-specific survival independent of stage. J Invest Dermatol. 2013;133:642-646.
- Buell JF, Trofe J, Hanaway MJ, et al. Immunosuppression and Merkel cell cancer. Transplant Proc. 2002;34:1780-1781.
- Penn I, First MR. Merkel’s cell carcinoma in organ recipients: report of 41 cases. Transplantation. 1999;68:1717-1721.
- Clarke CA, Robbins HA, Tatalovich Z, et al. Risk of Merkel cell carcinoma after solid organ transplantation. J Natl Cancer Inst. 2015;107. pii:dju382. doi:10.1093/jnci/dju382.
- Rockville Merkel Cell Carcinoma Group. Merkel cell carcinoma: recent progress and current priorities on etiology, pathogenesis and clinical management [published online July 13, 2009]. J Clin Oncol. 2009;27:4021-4026.
- Krejčí K, Tichý T, Horák P, et al. Merkel cell carcinoma of the gluteal region with ipsilateral metastasis into the pancreatic graft of a patient after combined kidney-pancreas transplantation [published online September 20, 2010]. Onkologie. 2010;33:520-524.
- Arron ST, Canavan T, Yu SS. Organ transplant recipients with Merkel cell carcinoma have reduced progression-free, overall, and disease-specific survival independent of stage at presentation [published online July 1, 2014]. J Am Acad Dermatol. 2014;71:684-690.
- Albores-Saavedra J, Batich K, Chable-Montero F, et al. Merkel cell carcinoma demographics, morphology, and survival based on 3870 cases: a population-based study [published online July 23, 2009]. J Cutan Pathol. 2010;37:20-27.
- Eng TY, Boersma MG, Fuller CD, et al. Treatment of Merkel cell carcinoma. Am J Clin Oncol. 2004;27:510-515.
- Gould VE, Moll R, Moll I, et al. Neuroendocrine (Merkel) cells of the skin: hyperplasias, dysplasias, and neoplasms. Lab Invest. 1985;52:334-353.
- Ratner D, Nelson BR, Brown MD, et al. Merkel cell carcinoma. J Am Acad Dermatol. 1993;29(2, pt 1):143-156.
- Pectasides D, Pectasides M, Economopoulos T. Merkel cell cancer of the skin. Ann Oncol. 2006;17:1489-1495.
- Paulson KG, Iyer JG, Blom A, et al. Systemic immune suppression predicts diminished Merkel cell carcinoma-specific survival independent of stage. J Invest Dermatol. 2013;133:642-646.
- Buell JF, Trofe J, Hanaway MJ, et al. Immunosuppression and Merkel cell cancer. Transplant Proc. 2002;34:1780-1781.
- Penn I, First MR. Merkel’s cell carcinoma in organ recipients: report of 41 cases. Transplantation. 1999;68:1717-1721.
- Clarke CA, Robbins HA, Tatalovich Z, et al. Risk of Merkel cell carcinoma after solid organ transplantation. J Natl Cancer Inst. 2015;107. pii:dju382. doi:10.1093/jnci/dju382.
- Rockville Merkel Cell Carcinoma Group. Merkel cell carcinoma: recent progress and current priorities on etiology, pathogenesis and clinical management [published online July 13, 2009]. J Clin Oncol. 2009;27:4021-4026.
- Krejčí K, Tichý T, Horák P, et al. Merkel cell carcinoma of the gluteal region with ipsilateral metastasis into the pancreatic graft of a patient after combined kidney-pancreas transplantation [published online September 20, 2010]. Onkologie. 2010;33:520-524.
- Arron ST, Canavan T, Yu SS. Organ transplant recipients with Merkel cell carcinoma have reduced progression-free, overall, and disease-specific survival independent of stage at presentation [published online July 1, 2014]. J Am Acad Dermatol. 2014;71:684-690.
- Albores-Saavedra J, Batich K, Chable-Montero F, et al. Merkel cell carcinoma demographics, morphology, and survival based on 3870 cases: a population-based study [published online July 23, 2009]. J Cutan Pathol. 2010;37:20-27.
- Eng TY, Boersma MG, Fuller CD, et al. Treatment of Merkel cell carcinoma. Am J Clin Oncol. 2004;27:510-515.
Practice Points
- Organ transplant recipients are at an increased risk for Merkel cell carcinoma (MCC).
- Early recognition and diagnosis of MCC is important to improve morbidity and mortality.
Cases of Drug-Associated Endocarditis Multiply
As the epidemic of opioid use has expanded, so has the incidence of drug-associated endocarditis. North Carolina, where CDC researchers analyzed discharge data from 128 hospitals, saw about a 12-fold jump in hospitalizations for endocarditis combined with drug dependence between 2010-2015.
The incidence of hospitalizations sharply increased, particularly beginning in 2013, from 0.2 cases per 100,000 persons per year in 2010 to 2.7 cases per 100,000 persons in 2015. The rise was fastest among adults aged 18 to 25 years.
About one-third of the patients also were infected with hepatitis C virus (HCV), a not unexpected finding since IV drug use is a recognized risk factor for both endocarditis and HCV infection.
The financial repercussions also are evident. Between 2010 and 2015, the median hospital charge for drug dependence-associated endocarditis hospitalization jumped from $1.1 million to $22.2 million—an 18-fold increase.
The findings suggest a need to focus on preventive interventions, the researchers say, such as fact-based drug education, syringe service programs, safe injection education, and treatment programs offering opioid agonist and antagonist therapies.
As the epidemic of opioid use has expanded, so has the incidence of drug-associated endocarditis. North Carolina, where CDC researchers analyzed discharge data from 128 hospitals, saw about a 12-fold jump in hospitalizations for endocarditis combined with drug dependence between 2010-2015.
The incidence of hospitalizations sharply increased, particularly beginning in 2013, from 0.2 cases per 100,000 persons per year in 2010 to 2.7 cases per 100,000 persons in 2015. The rise was fastest among adults aged 18 to 25 years.
About one-third of the patients also were infected with hepatitis C virus (HCV), a not unexpected finding since IV drug use is a recognized risk factor for both endocarditis and HCV infection.
The financial repercussions also are evident. Between 2010 and 2015, the median hospital charge for drug dependence-associated endocarditis hospitalization jumped from $1.1 million to $22.2 million—an 18-fold increase.
The findings suggest a need to focus on preventive interventions, the researchers say, such as fact-based drug education, syringe service programs, safe injection education, and treatment programs offering opioid agonist and antagonist therapies.
As the epidemic of opioid use has expanded, so has the incidence of drug-associated endocarditis. North Carolina, where CDC researchers analyzed discharge data from 128 hospitals, saw about a 12-fold jump in hospitalizations for endocarditis combined with drug dependence between 2010-2015.
The incidence of hospitalizations sharply increased, particularly beginning in 2013, from 0.2 cases per 100,000 persons per year in 2010 to 2.7 cases per 100,000 persons in 2015. The rise was fastest among adults aged 18 to 25 years.
About one-third of the patients also were infected with hepatitis C virus (HCV), a not unexpected finding since IV drug use is a recognized risk factor for both endocarditis and HCV infection.
The financial repercussions also are evident. Between 2010 and 2015, the median hospital charge for drug dependence-associated endocarditis hospitalization jumped from $1.1 million to $22.2 million—an 18-fold increase.
The findings suggest a need to focus on preventive interventions, the researchers say, such as fact-based drug education, syringe service programs, safe injection education, and treatment programs offering opioid agonist and antagonist therapies.
Nivolumab Linked to Nephritis in Melanoma
Nivolumab and ipilimumab, new immunotherapies for metastatic melanoma, have both been linked to nephritis. Now, researchers from Centre Hospitalier Lyon-Sud and Université Claude Bernard Lyon in France, report on a patient with melanoma who developed acute interstitial immune nephritis after being treated with nivolumab—not once, but twice.
The patient, a 76-year-old woman with pulmonary metastatic melanoma, was given 4 intravenous cycles of ipilimumab as a first-line treatment. After 16 weeks, the disease was progressing; the ipilimumab was discontinued, and 8 weeks later she was started on second-line treatment with nivolumab. After 3 cycles of nivolumab, she developed acute kidney injury. The patient’s creatinine went from 69 µmol/L before nivolumab to 142 µmol/L before the fourth cycle. Immunotherapy was discontinued.
Related: Getting a Better Picture of Skin Cancer
The patient had not received any other drug that could explain the increased creatinine level, the researchers say, and she was otherwise asymptomatic. The renal failure persisted despite an adequate fluid intake over 3 days. Biopsy revealed interstitial edema.
The clinicians treated the patient with oral prednisolone, and her renal function rapidly improved, although her creatinine level remained higher than before the nivolumab.
A follow-up CT scan found a partial response to the nivolumab. Based on that reponse, the multidisciplinary staff elected to continue the treatment at the same dose. The fourth cycle was administered while the patient was still receiving daily corticosteroids.
The infusion did not cause kidney failure relapse. However, after the corticosteroids were stopped, the patient’s creatinine level increased gradually, to 158 µmol/L, and again she was hospitalized with relapse of immune interstitial nephritis. The clinicians reinstituted prednisolone, and the acute interstitial nephritis improved. Nivolumab was discontinued.
Related: Immunotherapy in Melanoma
Drug-induced acute interstitial nephritis often has been more described with nonsteroidal anti-inflammatory drugs and beta-lactams, among others, the researchers say. Immune interstitial nephritis had been reported in a patient treated with nivolumab and ipilimumab concomitantly, and 3 cases of granulomatous interstitial nephritis have been reported with ipilimumab monotherapy. To the authors’ knowledge, this is the first case of immune interstitial nephritis reported with nivolumab monotherapy in metastatic melanoma. It is important to consider, they add, that the patient had also received ipilimumab, and that due to the drug’s elimination half-life (15.4 days), they can’t exclude an “overlap” between the 2 drugs that might have increased the risk of acute interstitial nephritis.
Source:
Bottlaender L, Breton AL, de Laforcade L, Dijoud F, Thomas L, Dalle S. J Immunother Cancer. 2017;5:56.
doi: 10.1186/s40425-017-0261-2
Nivolumab and ipilimumab, new immunotherapies for metastatic melanoma, have both been linked to nephritis. Now, researchers from Centre Hospitalier Lyon-Sud and Université Claude Bernard Lyon in France, report on a patient with melanoma who developed acute interstitial immune nephritis after being treated with nivolumab—not once, but twice.
The patient, a 76-year-old woman with pulmonary metastatic melanoma, was given 4 intravenous cycles of ipilimumab as a first-line treatment. After 16 weeks, the disease was progressing; the ipilimumab was discontinued, and 8 weeks later she was started on second-line treatment with nivolumab. After 3 cycles of nivolumab, she developed acute kidney injury. The patient’s creatinine went from 69 µmol/L before nivolumab to 142 µmol/L before the fourth cycle. Immunotherapy was discontinued.
Related: Getting a Better Picture of Skin Cancer
The patient had not received any other drug that could explain the increased creatinine level, the researchers say, and she was otherwise asymptomatic. The renal failure persisted despite an adequate fluid intake over 3 days. Biopsy revealed interstitial edema.
The clinicians treated the patient with oral prednisolone, and her renal function rapidly improved, although her creatinine level remained higher than before the nivolumab.
A follow-up CT scan found a partial response to the nivolumab. Based on that reponse, the multidisciplinary staff elected to continue the treatment at the same dose. The fourth cycle was administered while the patient was still receiving daily corticosteroids.
The infusion did not cause kidney failure relapse. However, after the corticosteroids were stopped, the patient’s creatinine level increased gradually, to 158 µmol/L, and again she was hospitalized with relapse of immune interstitial nephritis. The clinicians reinstituted prednisolone, and the acute interstitial nephritis improved. Nivolumab was discontinued.
Related: Immunotherapy in Melanoma
Drug-induced acute interstitial nephritis often has been more described with nonsteroidal anti-inflammatory drugs and beta-lactams, among others, the researchers say. Immune interstitial nephritis had been reported in a patient treated with nivolumab and ipilimumab concomitantly, and 3 cases of granulomatous interstitial nephritis have been reported with ipilimumab monotherapy. To the authors’ knowledge, this is the first case of immune interstitial nephritis reported with nivolumab monotherapy in metastatic melanoma. It is important to consider, they add, that the patient had also received ipilimumab, and that due to the drug’s elimination half-life (15.4 days), they can’t exclude an “overlap” between the 2 drugs that might have increased the risk of acute interstitial nephritis.
Source:
Bottlaender L, Breton AL, de Laforcade L, Dijoud F, Thomas L, Dalle S. J Immunother Cancer. 2017;5:56.
doi: 10.1186/s40425-017-0261-2
Nivolumab and ipilimumab, new immunotherapies for metastatic melanoma, have both been linked to nephritis. Now, researchers from Centre Hospitalier Lyon-Sud and Université Claude Bernard Lyon in France, report on a patient with melanoma who developed acute interstitial immune nephritis after being treated with nivolumab—not once, but twice.
The patient, a 76-year-old woman with pulmonary metastatic melanoma, was given 4 intravenous cycles of ipilimumab as a first-line treatment. After 16 weeks, the disease was progressing; the ipilimumab was discontinued, and 8 weeks later she was started on second-line treatment with nivolumab. After 3 cycles of nivolumab, she developed acute kidney injury. The patient’s creatinine went from 69 µmol/L before nivolumab to 142 µmol/L before the fourth cycle. Immunotherapy was discontinued.
Related: Getting a Better Picture of Skin Cancer
The patient had not received any other drug that could explain the increased creatinine level, the researchers say, and she was otherwise asymptomatic. The renal failure persisted despite an adequate fluid intake over 3 days. Biopsy revealed interstitial edema.
The clinicians treated the patient with oral prednisolone, and her renal function rapidly improved, although her creatinine level remained higher than before the nivolumab.
A follow-up CT scan found a partial response to the nivolumab. Based on that reponse, the multidisciplinary staff elected to continue the treatment at the same dose. The fourth cycle was administered while the patient was still receiving daily corticosteroids.
The infusion did not cause kidney failure relapse. However, after the corticosteroids were stopped, the patient’s creatinine level increased gradually, to 158 µmol/L, and again she was hospitalized with relapse of immune interstitial nephritis. The clinicians reinstituted prednisolone, and the acute interstitial nephritis improved. Nivolumab was discontinued.
Related: Immunotherapy in Melanoma
Drug-induced acute interstitial nephritis often has been more described with nonsteroidal anti-inflammatory drugs and beta-lactams, among others, the researchers say. Immune interstitial nephritis had been reported in a patient treated with nivolumab and ipilimumab concomitantly, and 3 cases of granulomatous interstitial nephritis have been reported with ipilimumab monotherapy. To the authors’ knowledge, this is the first case of immune interstitial nephritis reported with nivolumab monotherapy in metastatic melanoma. It is important to consider, they add, that the patient had also received ipilimumab, and that due to the drug’s elimination half-life (15.4 days), they can’t exclude an “overlap” between the 2 drugs that might have increased the risk of acute interstitial nephritis.
Source:
Bottlaender L, Breton AL, de Laforcade L, Dijoud F, Thomas L, Dalle S. J Immunother Cancer. 2017;5:56.
doi: 10.1186/s40425-017-0261-2
FDA approves first treatment for cGVHD
The US Food and Drug Administration (FDA) has expanded the approved use of ibrutinib (Imbruvica) to include the treatment of adults with chronic graft-versus-host disease (cGVHD) who have failed at least one prior treatment.
This makes ibrutinib the first FDA-approved therapy for cGVHD.
Ibrutinib was previously FDA-approved to treat chronic lymphocytic leukemia/small lymphocytic lymphoma, Waldenström’s macroglobulinemia, marginal zone lymphoma, and mantle cell lymphoma.
“This approval highlights how a known treatment for cancer is finding a new use in treating a serious and life-threatening condition that may occur in patients with blood cancer who receive a stem cell transplant,” said Richard Pazdur, MD, director of the FDA’s Oncology Center of Excellence and acting director of the Office of Hematology and Oncology Products in the FDA’s Center for Drug Evaluation and Research.
The approval of ibrutinib to treat cGVHD is based on results of a phase 2 trial, which were presented at the 2016 ASH Annual Meeting.
The trial included 42 patients with cGVHD whose symptoms persisted despite standard treatment with corticosteroids. Most patients’ symptoms included mouth ulcers and skin rashes, and more than 50% had 2 or more organs affected by cGVHD.
Sixty-seven percent of patients responded to treatment with ibrutinib, experiencing improvements in their cGVHD symptoms. In 48% of patients, this improvement lasted for 5 months or longer.
Common side effects of ibrutinib in this trial were fatigue, bruising, diarrhea, thrombocytopenia, muscle spasms, swelling and stomatitis, nausea, hemorrhage, anemia, and pneumonia. ![]()
The US Food and Drug Administration (FDA) has expanded the approved use of ibrutinib (Imbruvica) to include the treatment of adults with chronic graft-versus-host disease (cGVHD) who have failed at least one prior treatment.
This makes ibrutinib the first FDA-approved therapy for cGVHD.
Ibrutinib was previously FDA-approved to treat chronic lymphocytic leukemia/small lymphocytic lymphoma, Waldenström’s macroglobulinemia, marginal zone lymphoma, and mantle cell lymphoma.
“This approval highlights how a known treatment for cancer is finding a new use in treating a serious and life-threatening condition that may occur in patients with blood cancer who receive a stem cell transplant,” said Richard Pazdur, MD, director of the FDA’s Oncology Center of Excellence and acting director of the Office of Hematology and Oncology Products in the FDA’s Center for Drug Evaluation and Research.
The approval of ibrutinib to treat cGVHD is based on results of a phase 2 trial, which were presented at the 2016 ASH Annual Meeting.
The trial included 42 patients with cGVHD whose symptoms persisted despite standard treatment with corticosteroids. Most patients’ symptoms included mouth ulcers and skin rashes, and more than 50% had 2 or more organs affected by cGVHD.
Sixty-seven percent of patients responded to treatment with ibrutinib, experiencing improvements in their cGVHD symptoms. In 48% of patients, this improvement lasted for 5 months or longer.
Common side effects of ibrutinib in this trial were fatigue, bruising, diarrhea, thrombocytopenia, muscle spasms, swelling and stomatitis, nausea, hemorrhage, anemia, and pneumonia. ![]()
The US Food and Drug Administration (FDA) has expanded the approved use of ibrutinib (Imbruvica) to include the treatment of adults with chronic graft-versus-host disease (cGVHD) who have failed at least one prior treatment.
This makes ibrutinib the first FDA-approved therapy for cGVHD.
Ibrutinib was previously FDA-approved to treat chronic lymphocytic leukemia/small lymphocytic lymphoma, Waldenström’s macroglobulinemia, marginal zone lymphoma, and mantle cell lymphoma.
“This approval highlights how a known treatment for cancer is finding a new use in treating a serious and life-threatening condition that may occur in patients with blood cancer who receive a stem cell transplant,” said Richard Pazdur, MD, director of the FDA’s Oncology Center of Excellence and acting director of the Office of Hematology and Oncology Products in the FDA’s Center for Drug Evaluation and Research.
The approval of ibrutinib to treat cGVHD is based on results of a phase 2 trial, which were presented at the 2016 ASH Annual Meeting.
The trial included 42 patients with cGVHD whose symptoms persisted despite standard treatment with corticosteroids. Most patients’ symptoms included mouth ulcers and skin rashes, and more than 50% had 2 or more organs affected by cGVHD.
Sixty-seven percent of patients responded to treatment with ibrutinib, experiencing improvements in their cGVHD symptoms. In 48% of patients, this improvement lasted for 5 months or longer.
Common side effects of ibrutinib in this trial were fatigue, bruising, diarrhea, thrombocytopenia, muscle spasms, swelling and stomatitis, nausea, hemorrhage, anemia, and pneumonia. ![]()
FDA grants drug orphan designation for PNH
The US Food and Drug Administration (FDA) has granted orphan drug designation to RA101495 for the treatment of paroxysmal nocturnal hemoglobinuria (PNH).
RA101495 is a synthetic, macrocyclic peptide being developed by Ra Pharmaceuticals.
The peptide binds complement component 5 (C5) with sub-nanomolar affinity and allosterically inhibits its cleavage into C5a and C5b upon activation of the classical, alternative, or lectin pathways.
By binding to a region of C5 corresponding to C5b, RA101495 also disrupts the interaction between C5b and C6 and prevents assembly of the membrane attack complex.
In phase 1 studies, dosing of RA101495 was well tolerated in healthy volunteers and demonstrated sustained and near complete suppression of hemolysis and complement activity.
The phase 1 results were presented at the 21st Congress of the European Hematology Association in June 2016 (abstracts LB2249 and P632).
RA101495 is currently under investigation in a phase 2 trial of patients with PNH.
“There is an urgent need for new treatment options for patients suffering from PNH,” said Doug Treco, PhD, president and chief executive officer of Ra Pharmaceuticals.
“The current standard of care requires biweekly intravenous infusions, a dosing regimen that imposes a severe burden on patients, providers, and caregivers. We have designed RA101495 for once-daily, subcutaneous self-administration, an approach which has the potential to ease this burden, improve convenience, and provide much-needed dosing flexibility.”
“We are encouraged by our initial phase 2 data in PNH patients, which showed near-complete inhibition of hemolysis and a favorable safety and tolerability profile. We look forward to advancing our PNH program and providing additional data updates around year-end.”
About orphan designation
The FDA grants orphan designation to drugs and biologics intended to treat, diagnose, or prevent diseases/disorders that affect fewer than 200,000 people in the US.
The designation provides incentives for sponsors to develop products for rare diseases. This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the product is approved. ![]()
The US Food and Drug Administration (FDA) has granted orphan drug designation to RA101495 for the treatment of paroxysmal nocturnal hemoglobinuria (PNH).
RA101495 is a synthetic, macrocyclic peptide being developed by Ra Pharmaceuticals.
The peptide binds complement component 5 (C5) with sub-nanomolar affinity and allosterically inhibits its cleavage into C5a and C5b upon activation of the classical, alternative, or lectin pathways.
By binding to a region of C5 corresponding to C5b, RA101495 also disrupts the interaction between C5b and C6 and prevents assembly of the membrane attack complex.
In phase 1 studies, dosing of RA101495 was well tolerated in healthy volunteers and demonstrated sustained and near complete suppression of hemolysis and complement activity.
The phase 1 results were presented at the 21st Congress of the European Hematology Association in June 2016 (abstracts LB2249 and P632).
RA101495 is currently under investigation in a phase 2 trial of patients with PNH.
“There is an urgent need for new treatment options for patients suffering from PNH,” said Doug Treco, PhD, president and chief executive officer of Ra Pharmaceuticals.
“The current standard of care requires biweekly intravenous infusions, a dosing regimen that imposes a severe burden on patients, providers, and caregivers. We have designed RA101495 for once-daily, subcutaneous self-administration, an approach which has the potential to ease this burden, improve convenience, and provide much-needed dosing flexibility.”
“We are encouraged by our initial phase 2 data in PNH patients, which showed near-complete inhibition of hemolysis and a favorable safety and tolerability profile. We look forward to advancing our PNH program and providing additional data updates around year-end.”
About orphan designation
The FDA grants orphan designation to drugs and biologics intended to treat, diagnose, or prevent diseases/disorders that affect fewer than 200,000 people in the US.
The designation provides incentives for sponsors to develop products for rare diseases. This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the product is approved. ![]()
The US Food and Drug Administration (FDA) has granted orphan drug designation to RA101495 for the treatment of paroxysmal nocturnal hemoglobinuria (PNH).
RA101495 is a synthetic, macrocyclic peptide being developed by Ra Pharmaceuticals.
The peptide binds complement component 5 (C5) with sub-nanomolar affinity and allosterically inhibits its cleavage into C5a and C5b upon activation of the classical, alternative, or lectin pathways.
By binding to a region of C5 corresponding to C5b, RA101495 also disrupts the interaction between C5b and C6 and prevents assembly of the membrane attack complex.
In phase 1 studies, dosing of RA101495 was well tolerated in healthy volunteers and demonstrated sustained and near complete suppression of hemolysis and complement activity.
The phase 1 results were presented at the 21st Congress of the European Hematology Association in June 2016 (abstracts LB2249 and P632).
RA101495 is currently under investigation in a phase 2 trial of patients with PNH.
“There is an urgent need for new treatment options for patients suffering from PNH,” said Doug Treco, PhD, president and chief executive officer of Ra Pharmaceuticals.
“The current standard of care requires biweekly intravenous infusions, a dosing regimen that imposes a severe burden on patients, providers, and caregivers. We have designed RA101495 for once-daily, subcutaneous self-administration, an approach which has the potential to ease this burden, improve convenience, and provide much-needed dosing flexibility.”
“We are encouraged by our initial phase 2 data in PNH patients, which showed near-complete inhibition of hemolysis and a favorable safety and tolerability profile. We look forward to advancing our PNH program and providing additional data updates around year-end.”
About orphan designation
The FDA grants orphan designation to drugs and biologics intended to treat, diagnose, or prevent diseases/disorders that affect fewer than 200,000 people in the US.
The designation provides incentives for sponsors to develop products for rare diseases. This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the product is approved. ![]()
Hand pain and rash
The FP diagnosed psoriatic arthritis and psoriasis in this patient. The psoriatic arthritis had already caused swan neck deformities of multiple fingers, consistent with the mutilans subtype of psoriatic arthritis. The FP was aware that this patient needed to see a dermatologist and/or rheumatologist due to the severity of the arthritis.
Patients like this need systemic therapy with methotrexate, apremilast, or an anti-tumor necrosis factor alpha-inhibitor on an ongoing basis to both treat the arthritis and attempt to prevent further joint destruction. This will also treat the extensive plaque psoriasis that may accompany psoriatic arthritis. Most patients with psoriatic arthritis also have nail involvement (as did this patient), and it may respond to systemic treatment, as well.
Since systemic treatment is frequently outside the FP’s scope of practice, referral to a specialist is essential. The FP can, however, start topical therapy for the plaque psoriasis. This can be initiated with mid- to high-potency topical steroids.
It may be tempting to give a patient like this oral prednisone or an intramuscular steroid, but such therapy should be avoided because it can result in rebound pustular psoriasis. (These treatments do not work well enough to take the risk.)
In this case, the FP prescribed a 60-g tube of 0.05% clobetasol ointment to be applied to the worst areas, along with a 1-lb tub (454 g) of 0.1% triamcinolone to be applied to the other plaques. The patient was glad to receive treatment for his psoriasis and looked forward to seeing a specialist for treatment of his arthritis.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Usatine R. Psoriasis. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013: 878-895.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com
The FP diagnosed psoriatic arthritis and psoriasis in this patient. The psoriatic arthritis had already caused swan neck deformities of multiple fingers, consistent with the mutilans subtype of psoriatic arthritis. The FP was aware that this patient needed to see a dermatologist and/or rheumatologist due to the severity of the arthritis.
Patients like this need systemic therapy with methotrexate, apremilast, or an anti-tumor necrosis factor alpha-inhibitor on an ongoing basis to both treat the arthritis and attempt to prevent further joint destruction. This will also treat the extensive plaque psoriasis that may accompany psoriatic arthritis. Most patients with psoriatic arthritis also have nail involvement (as did this patient), and it may respond to systemic treatment, as well.
Since systemic treatment is frequently outside the FP’s scope of practice, referral to a specialist is essential. The FP can, however, start topical therapy for the plaque psoriasis. This can be initiated with mid- to high-potency topical steroids.
It may be tempting to give a patient like this oral prednisone or an intramuscular steroid, but such therapy should be avoided because it can result in rebound pustular psoriasis. (These treatments do not work well enough to take the risk.)
In this case, the FP prescribed a 60-g tube of 0.05% clobetasol ointment to be applied to the worst areas, along with a 1-lb tub (454 g) of 0.1% triamcinolone to be applied to the other plaques. The patient was glad to receive treatment for his psoriasis and looked forward to seeing a specialist for treatment of his arthritis.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Usatine R. Psoriasis. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013: 878-895.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com
The FP diagnosed psoriatic arthritis and psoriasis in this patient. The psoriatic arthritis had already caused swan neck deformities of multiple fingers, consistent with the mutilans subtype of psoriatic arthritis. The FP was aware that this patient needed to see a dermatologist and/or rheumatologist due to the severity of the arthritis.
Patients like this need systemic therapy with methotrexate, apremilast, or an anti-tumor necrosis factor alpha-inhibitor on an ongoing basis to both treat the arthritis and attempt to prevent further joint destruction. This will also treat the extensive plaque psoriasis that may accompany psoriatic arthritis. Most patients with psoriatic arthritis also have nail involvement (as did this patient), and it may respond to systemic treatment, as well.
Since systemic treatment is frequently outside the FP’s scope of practice, referral to a specialist is essential. The FP can, however, start topical therapy for the plaque psoriasis. This can be initiated with mid- to high-potency topical steroids.
It may be tempting to give a patient like this oral prednisone or an intramuscular steroid, but such therapy should be avoided because it can result in rebound pustular psoriasis. (These treatments do not work well enough to take the risk.)
In this case, the FP prescribed a 60-g tube of 0.05% clobetasol ointment to be applied to the worst areas, along with a 1-lb tub (454 g) of 0.1% triamcinolone to be applied to the other plaques. The patient was glad to receive treatment for his psoriasis and looked forward to seeing a specialist for treatment of his arthritis.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Usatine R. Psoriasis. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013: 878-895.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com
Slow and Steady: A Worrisome Pace
ANSWER
The false statement is that most melanomas are raised (choice “a”), since most melanomas are essentially macular (flat).
DISCUSSION
Misconceptions about melanomas often delay diagnosis, costing lives. In truth, the majority of melanomas are difficult, if not impossible, to feel on palpation. This is because they arise in the skin, rather than on it.
Malignant melanomas are cancers of melanocytes (the cells that line the basal cell layer). Overexposure to UV sources damages the nuclei of these cells, compromising their ability to repair the damage. This can lead to focally unregulated cell growth.
Initially, this uncontrolled cell replication spreads horizontally. Over time, though, it can grow vertically and penetrate deep enough to invade the vasculature (roughly 1 mm deep), and spread to the liver, lung, or brain. This is why ascertaining the depth of a melanoma is critical for predicting prognosis and determining the extent of additional surgery and search for evidence of metastatic disease.
Melanomas do not typically itch or bleed until they are advanced (if at all). And most arise de novo (as new lesions, rather than pre-existing). So, contrary to popular belief, moles rarely turn into melanomas.
It is also true that approximately 80% of all melanomas arise on skin normally covered by clothing, despite the role of sunlight in the development of melanoma. For reasons not totally understood, the combination of fair, su
ANSWER
The false statement is that most melanomas are raised (choice “a”), since most melanomas are essentially macular (flat).
DISCUSSION
Misconceptions about melanomas often delay diagnosis, costing lives. In truth, the majority of melanomas are difficult, if not impossible, to feel on palpation. This is because they arise in the skin, rather than on it.
Malignant melanomas are cancers of melanocytes (the cells that line the basal cell layer). Overexposure to UV sources damages the nuclei of these cells, compromising their ability to repair the damage. This can lead to focally unregulated cell growth.
Initially, this uncontrolled cell replication spreads horizontally. Over time, though, it can grow vertically and penetrate deep enough to invade the vasculature (roughly 1 mm deep), and spread to the liver, lung, or brain. This is why ascertaining the depth of a melanoma is critical for predicting prognosis and determining the extent of additional surgery and search for evidence of metastatic disease.
Melanomas do not typically itch or bleed until they are advanced (if at all). And most arise de novo (as new lesions, rather than pre-existing). So, contrary to popular belief, moles rarely turn into melanomas.
It is also true that approximately 80% of all melanomas arise on skin normally covered by clothing, despite the role of sunlight in the development of melanoma. For reasons not totally understood, the combination of fair, su
ANSWER
The false statement is that most melanomas are raised (choice “a”), since most melanomas are essentially macular (flat).
DISCUSSION
Misconceptions about melanomas often delay diagnosis, costing lives. In truth, the majority of melanomas are difficult, if not impossible, to feel on palpation. This is because they arise in the skin, rather than on it.
Malignant melanomas are cancers of melanocytes (the cells that line the basal cell layer). Overexposure to UV sources damages the nuclei of these cells, compromising their ability to repair the damage. This can lead to focally unregulated cell growth.
Initially, this uncontrolled cell replication spreads horizontally. Over time, though, it can grow vertically and penetrate deep enough to invade the vasculature (roughly 1 mm deep), and spread to the liver, lung, or brain. This is why ascertaining the depth of a melanoma is critical for predicting prognosis and determining the extent of additional surgery and search for evidence of metastatic disease.
Melanomas do not typically itch or bleed until they are advanced (if at all). And most arise de novo (as new lesions, rather than pre-existing). So, contrary to popular belief, moles rarely turn into melanomas.
It is also true that approximately 80% of all melanomas arise on skin normally covered by clothing, despite the role of sunlight in the development of melanoma. For reasons not totally understood, the combination of fair, su
For more than two years, a 43-year-old man has had an asymptomatic lesion on his right deltoid area. His primary care provider has repeatedly assured him of its benignancy “because it is flat.” But its slow, steady, horizontal growth concerns the patient, who is otherwise healthy.
He does admit to a significant history of sun exposure, explaining that he burns easily but acquires a tan by summer’s end each year. Several members of his immediate family have had skin cancers removed.
Examination reveals a round, dark macule, measuring 1.1 cm, on the right lateral deltoid area. The lesion has poorly defined borders and a variety of colors—mostly black and brown, with flecks of red and pink. There is no palpable component, and no nodes can be detected in the area.
The rest of his type II skin has abundant evidence of UV overexposure, including solar lentigines and weathering, especially around the neck.
The lesion is anesthetized, removed by deep shave technique, and submitted to pathology. The report shows a superficial, spreading melanoma with a Breslow depth of 0.75 mm. Malignant cells extend to the dermoepidermal junction (Clark level II). Only a few mitotic cells can be seen, with no intravascular invasion or signs of ulceration.