User login
Reducing the Burden of Cirrhosis and Hepatic Encephalopathy
Click Here to Read the Supplement.
Topics include:
- Overview of HE
- Early Identification and Management
- An Option for HE Management
Dr. William Ford, MD, SFHM
Regional Medical Director
Clinical Associate Professor
of Medicine
Abington, Jefferson Health
Philadelphia, PA
Click Here to Read the Supplement.
XIF.0129.USA.17
Click Here to Read the Supplement.
Topics include:
- Overview of HE
- Early Identification and Management
- An Option for HE Management
Dr. William Ford, MD, SFHM
Regional Medical Director
Clinical Associate Professor
of Medicine
Abington, Jefferson Health
Philadelphia, PA
Click Here to Read the Supplement.
XIF.0129.USA.17
Click Here to Read the Supplement.
Topics include:
- Overview of HE
- Early Identification and Management
- An Option for HE Management
Dr. William Ford, MD, SFHM
Regional Medical Director
Clinical Associate Professor
of Medicine
Abington, Jefferson Health
Philadelphia, PA
Click Here to Read the Supplement.
XIF.0129.USA.17
Wearable Health Device Dermatitis: A Case of Acrylate-Related Contact Allergy
Mobile health devices enable patients and clinicians to monitor the type, quantity, and quality of everyday activities and hold the promise of improving patient health and health care practices.1 In 2013, 75% of surveyed consumers in the United States owned a fitness technology product, either a dedicated fitness device, application, or portable blood pressure monitor.2 Ownership of dedicated wearable fitness devices among consumers in the United States increased from 3% in 2012 to 9% in 2013. The immense popularity of wearable fitness devices is evident in the trajectory of their reported sales, which increased from $43 million in 2009 to $854 million in 2013.2 Recognizing that “widespread adoption and use of mobile technologies is opening new and innovative ways to improve health,”3 the US Food and Drug Administration (FDA) ruled that “[technologies] that can pose a greater risk to patients will require FDA review.” One popular class of mobile technologies—activity and sleep sensors—falls outside the FDA’s regulatory guidance. To enable continuous monitoring, these sensors often are embedded into wearable devices.
Reports in the media have documented skin rashes arising in conjunction with use of one type of device,4 which may be related to nickel contact allergy, and the manufacturer has reported that the metal housing consists of surgical stainless steel that is known to contain nickel. We report a complication related to continuous use of an unregulated, commercially available, watchlike wearable sensor that was linked not to nickel but to an acrylate-containing component.
Case Report
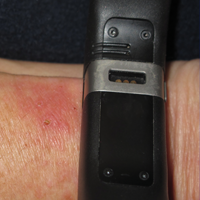
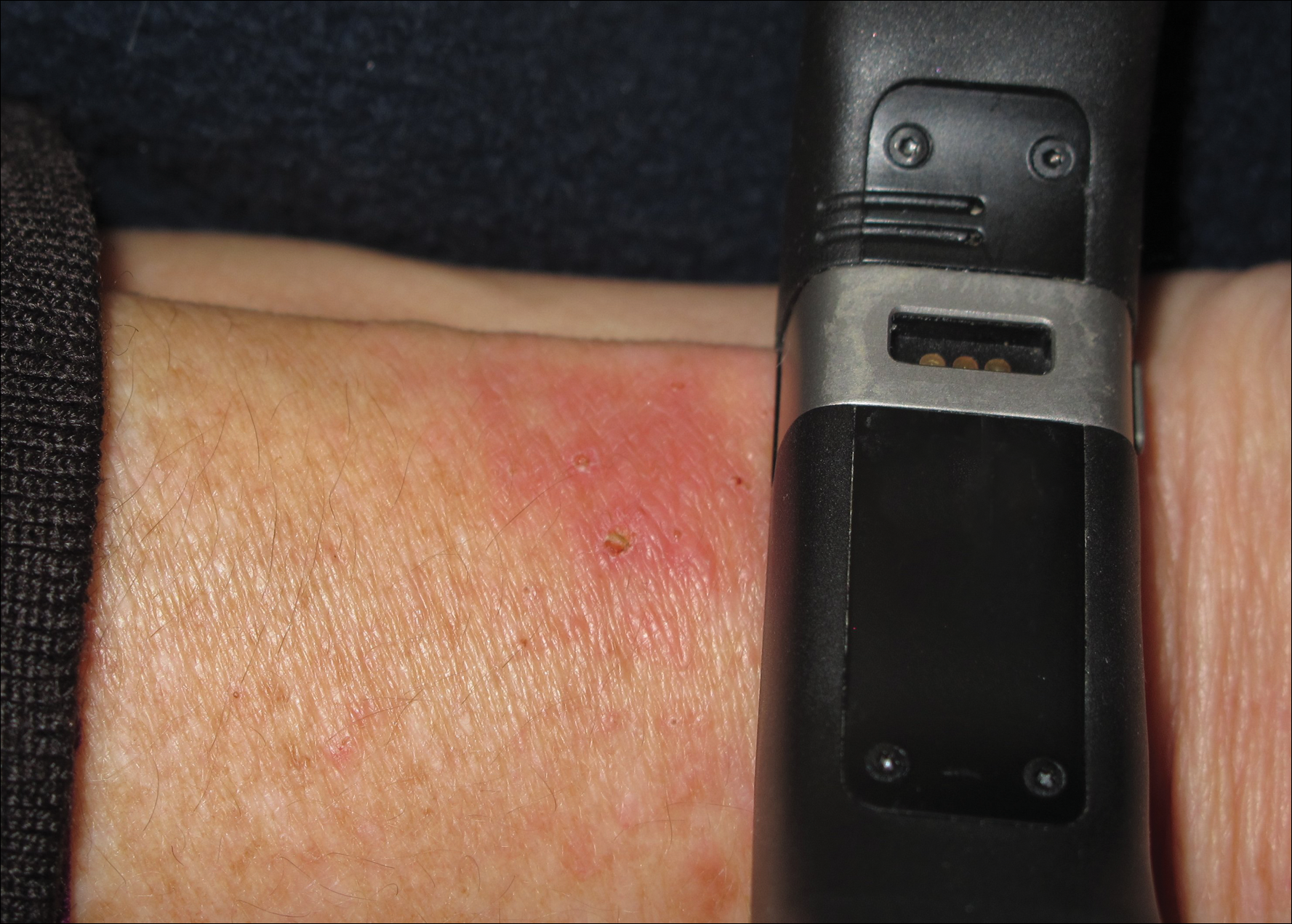
An otherwise healthy 52-year-old woman with no history of contact allergy presented with an intensely itchy eruption involving the left wrist arising 4 days after continuous use of a new watchlike wearable fitness sensor. By day 11, the eruption evolved into a well-demarcated, erythematous, scaly plaque at the location where the device’s rechargeable battery metal housing came into contact with skin (Figure 1).
Dimethylglyoxime testing of the metal housing and clips was negative, but testing of contacts within the housing was positive for nickel (Figure 2). Epicutaneous patch testing of the patient using a modified North American Contact Dermatitis Group patch test series (Table) demonstrated no reaction to nickel, instead showing a strong positive (2+) reaction at 48 and 72 hours to methyl methacrylate 2% and a positive (1+) reaction at 96 hours to ethyl acrylate 0.1% (Figure 3).


Comment
Acrylates are used as adhesives to bond metal to plastic and as part of lithium ion polymer batteries, presumably similar to the one used in this device.5 Our patient had a history of using acrylic nail polish, which may have been a source of prior sensitization. Exposure to sweat or other moisture could theoretically dissolve such a water-soluble polymer,6 allowing for skin contact. Other acrylate polymers have been reported to break down slowly in contact with water, leading to contact sensitization to the monomer.7 The manufacturer of the device was contacted for additional information but declined to provide specific details regarding the device’s composition (personal communication, January 2014).
Although not considered toxic,8 acrylate was named Allergen of the Year in 2012 by the American Contact Dermatitis Society.9-11 Nickel might be a source of allergy for some other patients who wear mobile health devices, but we concluded that this particular patient developed allergic contact dermatitis from prolonged exposure to low levels of methyl methacrylate or another acrylate due to gradual breakdown of the acrylate polymer used in the rechargeable battery housing for this wearable health device.
Given the FDA’s tailored risk approach to regulation, many wearable sensors that may contain potential contact allergens such as nickel and acrylates do not fall under the FDA regulatory framework. This case should alert physicians to the lack of regulatory oversight for many mobile technologies. They should consider a screening history for contact allergens before recommending wearable sensors and broader testing for contact allergens should exposed patients develop reactions. Future wearable sensor materials and designs should minimize exposure to allergens given prolonged contact with continuous use. In the absence of regulation, manufacturers of these devices should consider due care testing prior to commercialization.
Acknowledgment
We are indebted to Alexander S. Rattner, PhD (State College, Pennsylvania), who provided his engineering expertise and insight during conversations with the authors.
- Dobkin BH, Dorsch A. The promise of mHealth: daily activity monitoring and outcome assessments by wearable sensors. Neurorehabil Neural Repair. 2011;25:788-798.
- Consumer interest in purchasing wearable fitness devices in 2014 quadruples, according to CEA Study [press release]. Arlington, VA: Consumer Electronics Association; December 11, 2013.
- US Food and Drug Administration. Mobile medical applications. http://www.fda.gov/medicaldevices/digitalhealth/mobilemedicalapplications/default.htm. Updated September 22, 2015. Accessed July 26, 2017.
- Northrup L. Fitbit Force is an amazing device, except for my contact dermatitis. Consumerist website. http://consumerist.com/2014/01/13/fitbit-force-is-an-amazing-device-except-for-my-contact-dermatitis/. Published January 13, 2014. Accessed January 12, 2017.
- Stern B. Inside Fitbit Force. Adafruit website. http://learn.adafruit.com/fitbit-force-teardown/inside-fitbit-force. Published December 11, 2013. Updated May 4, 2015. Accessed January 12, 2017.
- Pemberton MA, Lohmann BS. Risk assessment of residual monomer migrating from acrylic polymers and causing allergic contact dermatitis during normal handling and use. Regul Toxicol Pharmacol. 2014;69:467-475.
- Guin JD, Baas K, Nelson-Adesokan P. Contact sensitization to cyanoacrylate adhesive as a cause of severe onychodystrophy. Int J Dermatol. 1998;37:31-36.
- Zondlo Fiume M. Final report on the safety assessment of Acrylates Copolymer and 33 related cosmetic ingredients. Int J Toxicol. 2002;21(suppl 3):1-50.
- Sasseville D. Acrylates. Dermatitis. 2012;23:3-5.
- Bowen C, Bidinger J, Hivnor C, et al. Allergic contact dermatitis to 2-octyl cyanoacrylate. Cutis. 2014;94:183-186.
- Spencer A, Gazzani P, Thompson DA. Acrylate and methacrylate contact allergy and allergic contact disease: a 13-year review [published online July 11, 2016]. Contact Dermatitis. 2016;75:157-164.
Mobile health devices enable patients and clinicians to monitor the type, quantity, and quality of everyday activities and hold the promise of improving patient health and health care practices.1 In 2013, 75% of surveyed consumers in the United States owned a fitness technology product, either a dedicated fitness device, application, or portable blood pressure monitor.2 Ownership of dedicated wearable fitness devices among consumers in the United States increased from 3% in 2012 to 9% in 2013. The immense popularity of wearable fitness devices is evident in the trajectory of their reported sales, which increased from $43 million in 2009 to $854 million in 2013.2 Recognizing that “widespread adoption and use of mobile technologies is opening new and innovative ways to improve health,”3 the US Food and Drug Administration (FDA) ruled that “[technologies] that can pose a greater risk to patients will require FDA review.” One popular class of mobile technologies—activity and sleep sensors—falls outside the FDA’s regulatory guidance. To enable continuous monitoring, these sensors often are embedded into wearable devices.
Reports in the media have documented skin rashes arising in conjunction with use of one type of device,4 which may be related to nickel contact allergy, and the manufacturer has reported that the metal housing consists of surgical stainless steel that is known to contain nickel. We report a complication related to continuous use of an unregulated, commercially available, watchlike wearable sensor that was linked not to nickel but to an acrylate-containing component.
Case Report
An otherwise healthy 52-year-old woman with no history of contact allergy presented with an intensely itchy eruption involving the left wrist arising 4 days after continuous use of a new watchlike wearable fitness sensor. By day 11, the eruption evolved into a well-demarcated, erythematous, scaly plaque at the location where the device’s rechargeable battery metal housing came into contact with skin (Figure 1).

Dimethylglyoxime testing of the metal housing and clips was negative, but testing of contacts within the housing was positive for nickel (Figure 2). Epicutaneous patch testing of the patient using a modified North American Contact Dermatitis Group patch test series (Table) demonstrated no reaction to nickel, instead showing a strong positive (2+) reaction at 48 and 72 hours to methyl methacrylate 2% and a positive (1+) reaction at 96 hours to ethyl acrylate 0.1% (Figure 3).


Comment
Acrylates are used as adhesives to bond metal to plastic and as part of lithium ion polymer batteries, presumably similar to the one used in this device.5 Our patient had a history of using acrylic nail polish, which may have been a source of prior sensitization. Exposure to sweat or other moisture could theoretically dissolve such a water-soluble polymer,6 allowing for skin contact. Other acrylate polymers have been reported to break down slowly in contact with water, leading to contact sensitization to the monomer.7 The manufacturer of the device was contacted for additional information but declined to provide specific details regarding the device’s composition (personal communication, January 2014).
Although not considered toxic,8 acrylate was named Allergen of the Year in 2012 by the American Contact Dermatitis Society.9-11 Nickel might be a source of allergy for some other patients who wear mobile health devices, but we concluded that this particular patient developed allergic contact dermatitis from prolonged exposure to low levels of methyl methacrylate or another acrylate due to gradual breakdown of the acrylate polymer used in the rechargeable battery housing for this wearable health device.
Given the FDA’s tailored risk approach to regulation, many wearable sensors that may contain potential contact allergens such as nickel and acrylates do not fall under the FDA regulatory framework. This case should alert physicians to the lack of regulatory oversight for many mobile technologies. They should consider a screening history for contact allergens before recommending wearable sensors and broader testing for contact allergens should exposed patients develop reactions. Future wearable sensor materials and designs should minimize exposure to allergens given prolonged contact with continuous use. In the absence of regulation, manufacturers of these devices should consider due care testing prior to commercialization.
Acknowledgment
We are indebted to Alexander S. Rattner, PhD (State College, Pennsylvania), who provided his engineering expertise and insight during conversations with the authors.
Mobile health devices enable patients and clinicians to monitor the type, quantity, and quality of everyday activities and hold the promise of improving patient health and health care practices.1 In 2013, 75% of surveyed consumers in the United States owned a fitness technology product, either a dedicated fitness device, application, or portable blood pressure monitor.2 Ownership of dedicated wearable fitness devices among consumers in the United States increased from 3% in 2012 to 9% in 2013. The immense popularity of wearable fitness devices is evident in the trajectory of their reported sales, which increased from $43 million in 2009 to $854 million in 2013.2 Recognizing that “widespread adoption and use of mobile technologies is opening new and innovative ways to improve health,”3 the US Food and Drug Administration (FDA) ruled that “[technologies] that can pose a greater risk to patients will require FDA review.” One popular class of mobile technologies—activity and sleep sensors—falls outside the FDA’s regulatory guidance. To enable continuous monitoring, these sensors often are embedded into wearable devices.
Reports in the media have documented skin rashes arising in conjunction with use of one type of device,4 which may be related to nickel contact allergy, and the manufacturer has reported that the metal housing consists of surgical stainless steel that is known to contain nickel. We report a complication related to continuous use of an unregulated, commercially available, watchlike wearable sensor that was linked not to nickel but to an acrylate-containing component.
Case Report
An otherwise healthy 52-year-old woman with no history of contact allergy presented with an intensely itchy eruption involving the left wrist arising 4 days after continuous use of a new watchlike wearable fitness sensor. By day 11, the eruption evolved into a well-demarcated, erythematous, scaly plaque at the location where the device’s rechargeable battery metal housing came into contact with skin (Figure 1).

Dimethylglyoxime testing of the metal housing and clips was negative, but testing of contacts within the housing was positive for nickel (Figure 2). Epicutaneous patch testing of the patient using a modified North American Contact Dermatitis Group patch test series (Table) demonstrated no reaction to nickel, instead showing a strong positive (2+) reaction at 48 and 72 hours to methyl methacrylate 2% and a positive (1+) reaction at 96 hours to ethyl acrylate 0.1% (Figure 3).


Comment
Acrylates are used as adhesives to bond metal to plastic and as part of lithium ion polymer batteries, presumably similar to the one used in this device.5 Our patient had a history of using acrylic nail polish, which may have been a source of prior sensitization. Exposure to sweat or other moisture could theoretically dissolve such a water-soluble polymer,6 allowing for skin contact. Other acrylate polymers have been reported to break down slowly in contact with water, leading to contact sensitization to the monomer.7 The manufacturer of the device was contacted for additional information but declined to provide specific details regarding the device’s composition (personal communication, January 2014).
Although not considered toxic,8 acrylate was named Allergen of the Year in 2012 by the American Contact Dermatitis Society.9-11 Nickel might be a source of allergy for some other patients who wear mobile health devices, but we concluded that this particular patient developed allergic contact dermatitis from prolonged exposure to low levels of methyl methacrylate or another acrylate due to gradual breakdown of the acrylate polymer used in the rechargeable battery housing for this wearable health device.
Given the FDA’s tailored risk approach to regulation, many wearable sensors that may contain potential contact allergens such as nickel and acrylates do not fall under the FDA regulatory framework. This case should alert physicians to the lack of regulatory oversight for many mobile technologies. They should consider a screening history for contact allergens before recommending wearable sensors and broader testing for contact allergens should exposed patients develop reactions. Future wearable sensor materials and designs should minimize exposure to allergens given prolonged contact with continuous use. In the absence of regulation, manufacturers of these devices should consider due care testing prior to commercialization.
Acknowledgment
We are indebted to Alexander S. Rattner, PhD (State College, Pennsylvania), who provided his engineering expertise and insight during conversations with the authors.
- Dobkin BH, Dorsch A. The promise of mHealth: daily activity monitoring and outcome assessments by wearable sensors. Neurorehabil Neural Repair. 2011;25:788-798.
- Consumer interest in purchasing wearable fitness devices in 2014 quadruples, according to CEA Study [press release]. Arlington, VA: Consumer Electronics Association; December 11, 2013.
- US Food and Drug Administration. Mobile medical applications. http://www.fda.gov/medicaldevices/digitalhealth/mobilemedicalapplications/default.htm. Updated September 22, 2015. Accessed July 26, 2017.
- Northrup L. Fitbit Force is an amazing device, except for my contact dermatitis. Consumerist website. http://consumerist.com/2014/01/13/fitbit-force-is-an-amazing-device-except-for-my-contact-dermatitis/. Published January 13, 2014. Accessed January 12, 2017.
- Stern B. Inside Fitbit Force. Adafruit website. http://learn.adafruit.com/fitbit-force-teardown/inside-fitbit-force. Published December 11, 2013. Updated May 4, 2015. Accessed January 12, 2017.
- Pemberton MA, Lohmann BS. Risk assessment of residual monomer migrating from acrylic polymers and causing allergic contact dermatitis during normal handling and use. Regul Toxicol Pharmacol. 2014;69:467-475.
- Guin JD, Baas K, Nelson-Adesokan P. Contact sensitization to cyanoacrylate adhesive as a cause of severe onychodystrophy. Int J Dermatol. 1998;37:31-36.
- Zondlo Fiume M. Final report on the safety assessment of Acrylates Copolymer and 33 related cosmetic ingredients. Int J Toxicol. 2002;21(suppl 3):1-50.
- Sasseville D. Acrylates. Dermatitis. 2012;23:3-5.
- Bowen C, Bidinger J, Hivnor C, et al. Allergic contact dermatitis to 2-octyl cyanoacrylate. Cutis. 2014;94:183-186.
- Spencer A, Gazzani P, Thompson DA. Acrylate and methacrylate contact allergy and allergic contact disease: a 13-year review [published online July 11, 2016]. Contact Dermatitis. 2016;75:157-164.
- Dobkin BH, Dorsch A. The promise of mHealth: daily activity monitoring and outcome assessments by wearable sensors. Neurorehabil Neural Repair. 2011;25:788-798.
- Consumer interest in purchasing wearable fitness devices in 2014 quadruples, according to CEA Study [press release]. Arlington, VA: Consumer Electronics Association; December 11, 2013.
- US Food and Drug Administration. Mobile medical applications. http://www.fda.gov/medicaldevices/digitalhealth/mobilemedicalapplications/default.htm. Updated September 22, 2015. Accessed July 26, 2017.
- Northrup L. Fitbit Force is an amazing device, except for my contact dermatitis. Consumerist website. http://consumerist.com/2014/01/13/fitbit-force-is-an-amazing-device-except-for-my-contact-dermatitis/. Published January 13, 2014. Accessed January 12, 2017.
- Stern B. Inside Fitbit Force. Adafruit website. http://learn.adafruit.com/fitbit-force-teardown/inside-fitbit-force. Published December 11, 2013. Updated May 4, 2015. Accessed January 12, 2017.
- Pemberton MA, Lohmann BS. Risk assessment of residual monomer migrating from acrylic polymers and causing allergic contact dermatitis during normal handling and use. Regul Toxicol Pharmacol. 2014;69:467-475.
- Guin JD, Baas K, Nelson-Adesokan P. Contact sensitization to cyanoacrylate adhesive as a cause of severe onychodystrophy. Int J Dermatol. 1998;37:31-36.
- Zondlo Fiume M. Final report on the safety assessment of Acrylates Copolymer and 33 related cosmetic ingredients. Int J Toxicol. 2002;21(suppl 3):1-50.
- Sasseville D. Acrylates. Dermatitis. 2012;23:3-5.
- Bowen C, Bidinger J, Hivnor C, et al. Allergic contact dermatitis to 2-octyl cyanoacrylate. Cutis. 2014;94:183-186.
- Spencer A, Gazzani P, Thompson DA. Acrylate and methacrylate contact allergy and allergic contact disease: a 13-year review [published online July 11, 2016]. Contact Dermatitis. 2016;75:157-164.
Practice Points
- Mobile wearable health devices are likely to become an important potential source of contact sensitization as their use increases given their often prolonged contact time with the skin.
- Mobile wearable health devices may pose a risk for allergic contact dermatitis as a result of a variety of components that come into contact with the skin, including but not limited to metals, rubber components, adhesives, and dyes.
What’s Eating You? Minute Brown Scavenger Beetle
Delusional infestation is the fixed false belief of skin infestation with a pathogen. Patients will often bring “proof” of their infestation to their visit to a physician. The presentation of a specimen was previously referred to by several names that reflected the receptacle that the patient utilized to bring the specimen (eg, a baggie or matchbox), but now the more encompassing term specimen sign is employed.1 Establishing rapport with the patient is critically important in the treatment of delusional infestation. Examining the specimen samples brought by the patient is a simple manner of communicating to a patient that the clinician is empathetic to and respectful of his/her concerns.2,3 The specimens often consist of dirt, dust, debris, fibers, and skin flakes and fragments, but they also have been reported to contain flies and insect parts.4,5 In our case, the patient captured a minute brown scavenger beetle with adhesive tape.
Case Report
A woman in her mid-30s with a history of generalized anxiety disorder presented to the dermatology clinic with a concern of bugs infesting her skin. The symptoms occurred just after she moved into a new home with her family approximately 4 months prior to presentation. She felt the home was not cleaned properly, but they could not afford to move. She reported a crawling sensation that she identified as bugs biting her all over her body. Prior to presentation in the dermatology clinic, she and her family were treated by primary care for scabies 3 times with permethrin cream, and she was prescribed 1 course of oral ivermectin. She reported seeing bugs all over her house, which led her to clean her home and clothing many times. She was more concerned now because she thought her 2 children also were starting to be affected.
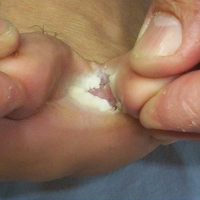
Physical examination revealed pressured speech, and the patient became tearful several times. The skin demonstrated several excoriations in various stages of healing on the breasts, legs, and upper back, as well as small scars in the same distribution. She brought several specimens stuck to clear tape to the visit. Examination of the specimens revealed fabric fibers; various debris; and a small, brown, 6-legged beetle with punctate indentations in rows along the wing covers (Figure). The head was narrower than the thorax, which was narrower than the abdomen.

We diagnosed the patient with a delusional infestation and discussed the beetle that we saw when examining the specimen the patient brought to the clinic. We provided reassurance that the minute brown scavenger beetle is not pathogenic and was present incidentally. Thus far, the patient has been resistant to initiating specific therapy for the delusional infestation, such as risperidone, olanzapine, or pimozide. We co
Comment
Minute brown scavenger beetles are arthropod members of the family Latridiidae. They also are commonly referred to as plaster or mold beetles. They are small (0.8–3.0 mm) and can be found in moist environments such as dead and rotting foliage, bird’s nests, debris, moist wallpaper/plaster, and stored products. They feed exclusively on fungus, such as mold and mildew, and pose no threat to humans.6 It is important for clinicians to recognize the appearance of the minute brown scavenger beetle so as not to mistake it for a pathogenic arthropod in patients presenting with delusional parasitosis.
- Freudenmann RW, Lepping P. Delusional infestation. Clin Microbiol Rev. 2009;22:690-732.
- Heller MM, Wong JW, Lee ES, et al. Delusional infestations: clinical presentation, diagnosis and treatment. Int J Dermatol. 2013;52:775-783.
- Patel V, Koo JY. Delusions of parasitosis; suggested dialogue between dermatologist and patient. J Dermatolog Treat. 2015;26:456-460.
- Zomer SF, De Wit RF, Van Bronswijk JE, et al. Delusions of parasitosis. a psychiatric disorder to be treated by dermatologists? an analysis of 33 patients. Br J Dermatol. 1998;138:1030-1032.
- Freudenmann RW, Kölle M, Schönfeldt-Lecuona C, et al. Delusional parasitosis and the matchbox sign revisited: the international perspective. Acta Derm Venereol. 2010;90:517-519.
- Bousquet Y. Beetles Associated With Stored Products in Canada: An identification Guide. Ottawa, Canada: Canadian Governement Publishing Centre; 1990.
Delusional infestation is the fixed false belief of skin infestation with a pathogen. Patients will often bring “proof” of their infestation to their visit to a physician. The presentation of a specimen was previously referred to by several names that reflected the receptacle that the patient utilized to bring the specimen (eg, a baggie or matchbox), but now the more encompassing term specimen sign is employed.1 Establishing rapport with the patient is critically important in the treatment of delusional infestation. Examining the specimen samples brought by the patient is a simple manner of communicating to a patient that the clinician is empathetic to and respectful of his/her concerns.2,3 The specimens often consist of dirt, dust, debris, fibers, and skin flakes and fragments, but they also have been reported to contain flies and insect parts.4,5 In our case, the patient captured a minute brown scavenger beetle with adhesive tape.
Case Report
A woman in her mid-30s with a history of generalized anxiety disorder presented to the dermatology clinic with a concern of bugs infesting her skin. The symptoms occurred just after she moved into a new home with her family approximately 4 months prior to presentation. She felt the home was not cleaned properly, but they could not afford to move. She reported a crawling sensation that she identified as bugs biting her all over her body. Prior to presentation in the dermatology clinic, she and her family were treated by primary care for scabies 3 times with permethrin cream, and she was prescribed 1 course of oral ivermectin. She reported seeing bugs all over her house, which led her to clean her home and clothing many times. She was more concerned now because she thought her 2 children also were starting to be affected.
Physical examination revealed pressured speech, and the patient became tearful several times. The skin demonstrated several excoriations in various stages of healing on the breasts, legs, and upper back, as well as small scars in the same distribution. She brought several specimens stuck to clear tape to the visit. Examination of the specimens revealed fabric fibers; various debris; and a small, brown, 6-legged beetle with punctate indentations in rows along the wing covers (Figure). The head was narrower than the thorax, which was narrower than the abdomen.

We diagnosed the patient with a delusional infestation and discussed the beetle that we saw when examining the specimen the patient brought to the clinic. We provided reassurance that the minute brown scavenger beetle is not pathogenic and was present incidentally. Thus far, the patient has been resistant to initiating specific therapy for the delusional infestation, such as risperidone, olanzapine, or pimozide. We co
Comment
Minute brown scavenger beetles are arthropod members of the family Latridiidae. They also are commonly referred to as plaster or mold beetles. They are small (0.8–3.0 mm) and can be found in moist environments such as dead and rotting foliage, bird’s nests, debris, moist wallpaper/plaster, and stored products. They feed exclusively on fungus, such as mold and mildew, and pose no threat to humans.6 It is important for clinicians to recognize the appearance of the minute brown scavenger beetle so as not to mistake it for a pathogenic arthropod in patients presenting with delusional parasitosis.
Delusional infestation is the fixed false belief of skin infestation with a pathogen. Patients will often bring “proof” of their infestation to their visit to a physician. The presentation of a specimen was previously referred to by several names that reflected the receptacle that the patient utilized to bring the specimen (eg, a baggie or matchbox), but now the more encompassing term specimen sign is employed.1 Establishing rapport with the patient is critically important in the treatment of delusional infestation. Examining the specimen samples brought by the patient is a simple manner of communicating to a patient that the clinician is empathetic to and respectful of his/her concerns.2,3 The specimens often consist of dirt, dust, debris, fibers, and skin flakes and fragments, but they also have been reported to contain flies and insect parts.4,5 In our case, the patient captured a minute brown scavenger beetle with adhesive tape.
Case Report
A woman in her mid-30s with a history of generalized anxiety disorder presented to the dermatology clinic with a concern of bugs infesting her skin. The symptoms occurred just after she moved into a new home with her family approximately 4 months prior to presentation. She felt the home was not cleaned properly, but they could not afford to move. She reported a crawling sensation that she identified as bugs biting her all over her body. Prior to presentation in the dermatology clinic, she and her family were treated by primary care for scabies 3 times with permethrin cream, and she was prescribed 1 course of oral ivermectin. She reported seeing bugs all over her house, which led her to clean her home and clothing many times. She was more concerned now because she thought her 2 children also were starting to be affected.
Physical examination revealed pressured speech, and the patient became tearful several times. The skin demonstrated several excoriations in various stages of healing on the breasts, legs, and upper back, as well as small scars in the same distribution. She brought several specimens stuck to clear tape to the visit. Examination of the specimens revealed fabric fibers; various debris; and a small, brown, 6-legged beetle with punctate indentations in rows along the wing covers (Figure). The head was narrower than the thorax, which was narrower than the abdomen.

We diagnosed the patient with a delusional infestation and discussed the beetle that we saw when examining the specimen the patient brought to the clinic. We provided reassurance that the minute brown scavenger beetle is not pathogenic and was present incidentally. Thus far, the patient has been resistant to initiating specific therapy for the delusional infestation, such as risperidone, olanzapine, or pimozide. We co
Comment
Minute brown scavenger beetles are arthropod members of the family Latridiidae. They also are commonly referred to as plaster or mold beetles. They are small (0.8–3.0 mm) and can be found in moist environments such as dead and rotting foliage, bird’s nests, debris, moist wallpaper/plaster, and stored products. They feed exclusively on fungus, such as mold and mildew, and pose no threat to humans.6 It is important for clinicians to recognize the appearance of the minute brown scavenger beetle so as not to mistake it for a pathogenic arthropod in patients presenting with delusional parasitosis.
- Freudenmann RW, Lepping P. Delusional infestation. Clin Microbiol Rev. 2009;22:690-732.
- Heller MM, Wong JW, Lee ES, et al. Delusional infestations: clinical presentation, diagnosis and treatment. Int J Dermatol. 2013;52:775-783.
- Patel V, Koo JY. Delusions of parasitosis; suggested dialogue between dermatologist and patient. J Dermatolog Treat. 2015;26:456-460.
- Zomer SF, De Wit RF, Van Bronswijk JE, et al. Delusions of parasitosis. a psychiatric disorder to be treated by dermatologists? an analysis of 33 patients. Br J Dermatol. 1998;138:1030-1032.
- Freudenmann RW, Kölle M, Schönfeldt-Lecuona C, et al. Delusional parasitosis and the matchbox sign revisited: the international perspective. Acta Derm Venereol. 2010;90:517-519.
- Bousquet Y. Beetles Associated With Stored Products in Canada: An identification Guide. Ottawa, Canada: Canadian Governement Publishing Centre; 1990.
- Freudenmann RW, Lepping P. Delusional infestation. Clin Microbiol Rev. 2009;22:690-732.
- Heller MM, Wong JW, Lee ES, et al. Delusional infestations: clinical presentation, diagnosis and treatment. Int J Dermatol. 2013;52:775-783.
- Patel V, Koo JY. Delusions of parasitosis; suggested dialogue between dermatologist and patient. J Dermatolog Treat. 2015;26:456-460.
- Zomer SF, De Wit RF, Van Bronswijk JE, et al. Delusions of parasitosis. a psychiatric disorder to be treated by dermatologists? an analysis of 33 patients. Br J Dermatol. 1998;138:1030-1032.
- Freudenmann RW, Kölle M, Schönfeldt-Lecuona C, et al. Delusional parasitosis and the matchbox sign revisited: the international perspective. Acta Derm Venereol. 2010;90:517-519.
- Bousquet Y. Beetles Associated With Stored Products in Canada: An identification Guide. Ottawa, Canada: Canadian Governement Publishing Centre; 1990.
Practice Points
- Examining the specimens brought by a patient with delusional infestation is important for the therapeutic relationship.
- Clinicians must be able to recognize nonpathogenic insects that may incidentally be present in the specimen such as the minute brown scavenger beetle.
Effect Of Inpatient Rehab Vs. Home-Based Program For TKA
Title: Inpatient rehabilitation does not improve mobility after total knee arthroplasty versus a monitored home-based program.
Clinical Question: Does initial treatment in an inpatient rehabilitation facility offer greater improvements in mobility when added to a monitored home-based program after undergoing total knee arthroplasty?
Background: Total knee arthroplasty (TKA) is common and postsurgical care varies. No randomized controlled trials have compared inpatient rehabilitation to monitored home-based programs.
Study Design: Multicenter, two intervention groups in parallel, randomized controlled trial with a third observational group.
Synopsis: 165 patients who underwent unilateral TKA were randomized to inpatient rehabilitation followed by a home-based program vs. a home-based program only. A separate observation group (patients who chose home-based program) was included in the analysis of primary outcome. Primary outcome was functional mobility at 26 weeks as measured by walking distance via the 6-minute walk test. All 165 patients were included in an intention-to-treat analysis. The primary outcome was no different among the two randomized groups (adjusted mean difference with imputation, –1.01; 95% CI, –25.56 to 23.55). The per protocol analysis of the primary outcome yielded similar results; nonadherent patients were excluded from the per protocol analysis so the sample size was smaller. There were no between-group differences in the primary outcome when the home-based program was compared to the observation group. Secondary outcomes included patient reported and observer assessed outcomes in function and quality of life. The most significant limitation was that these results are generalizable only to patients considered appropriate for discharge home.
Bottom Line: In total knee arthroplasty patients appropriate for discharge home, inpatient rehabilitation followed by a home-based program did not improve mobility as compared with a monitored home-based program alone.
Citation: Buhagiar MA, Naylor JM, Harris IA, et al. Effect of inpatient rehabilitation vs. a monitored home-based program on mobility in patients with total knee arthroplasty, the HIHO randomized clinical trial. JAMA. 2017;317(10):1037-46. doi: 10.1001/jama.2017.1224.
Dr. Burns is assistant professor in the division of hospital medicine at the University of New Mexico.
Title: Inpatient rehabilitation does not improve mobility after total knee arthroplasty versus a monitored home-based program.
Clinical Question: Does initial treatment in an inpatient rehabilitation facility offer greater improvements in mobility when added to a monitored home-based program after undergoing total knee arthroplasty?
Background: Total knee arthroplasty (TKA) is common and postsurgical care varies. No randomized controlled trials have compared inpatient rehabilitation to monitored home-based programs.
Study Design: Multicenter, two intervention groups in parallel, randomized controlled trial with a third observational group.
Synopsis: 165 patients who underwent unilateral TKA were randomized to inpatient rehabilitation followed by a home-based program vs. a home-based program only. A separate observation group (patients who chose home-based program) was included in the analysis of primary outcome. Primary outcome was functional mobility at 26 weeks as measured by walking distance via the 6-minute walk test. All 165 patients were included in an intention-to-treat analysis. The primary outcome was no different among the two randomized groups (adjusted mean difference with imputation, –1.01; 95% CI, –25.56 to 23.55). The per protocol analysis of the primary outcome yielded similar results; nonadherent patients were excluded from the per protocol analysis so the sample size was smaller. There were no between-group differences in the primary outcome when the home-based program was compared to the observation group. Secondary outcomes included patient reported and observer assessed outcomes in function and quality of life. The most significant limitation was that these results are generalizable only to patients considered appropriate for discharge home.
Bottom Line: In total knee arthroplasty patients appropriate for discharge home, inpatient rehabilitation followed by a home-based program did not improve mobility as compared with a monitored home-based program alone.
Citation: Buhagiar MA, Naylor JM, Harris IA, et al. Effect of inpatient rehabilitation vs. a monitored home-based program on mobility in patients with total knee arthroplasty, the HIHO randomized clinical trial. JAMA. 2017;317(10):1037-46. doi: 10.1001/jama.2017.1224.
Dr. Burns is assistant professor in the division of hospital medicine at the University of New Mexico.
Title: Inpatient rehabilitation does not improve mobility after total knee arthroplasty versus a monitored home-based program.
Clinical Question: Does initial treatment in an inpatient rehabilitation facility offer greater improvements in mobility when added to a monitored home-based program after undergoing total knee arthroplasty?
Background: Total knee arthroplasty (TKA) is common and postsurgical care varies. No randomized controlled trials have compared inpatient rehabilitation to monitored home-based programs.
Study Design: Multicenter, two intervention groups in parallel, randomized controlled trial with a third observational group.
Synopsis: 165 patients who underwent unilateral TKA were randomized to inpatient rehabilitation followed by a home-based program vs. a home-based program only. A separate observation group (patients who chose home-based program) was included in the analysis of primary outcome. Primary outcome was functional mobility at 26 weeks as measured by walking distance via the 6-minute walk test. All 165 patients were included in an intention-to-treat analysis. The primary outcome was no different among the two randomized groups (adjusted mean difference with imputation, –1.01; 95% CI, –25.56 to 23.55). The per protocol analysis of the primary outcome yielded similar results; nonadherent patients were excluded from the per protocol analysis so the sample size was smaller. There were no between-group differences in the primary outcome when the home-based program was compared to the observation group. Secondary outcomes included patient reported and observer assessed outcomes in function and quality of life. The most significant limitation was that these results are generalizable only to patients considered appropriate for discharge home.
Bottom Line: In total knee arthroplasty patients appropriate for discharge home, inpatient rehabilitation followed by a home-based program did not improve mobility as compared with a monitored home-based program alone.
Citation: Buhagiar MA, Naylor JM, Harris IA, et al. Effect of inpatient rehabilitation vs. a monitored home-based program on mobility in patients with total knee arthroplasty, the HIHO randomized clinical trial. JAMA. 2017;317(10):1037-46. doi: 10.1001/jama.2017.1224.
Dr. Burns is assistant professor in the division of hospital medicine at the University of New Mexico.
Successive Potassium Hydroxide Testing for Improved Diagnosis of Tinea Pedis
The gold standard for diagnosing dermatophytosis is the use of direct microscopic examination together with fungal culture.1 However, in the last 2 decades, molecular techniques that currently are available worldwide have improved the diagnosis procedure.2,3 In the practice of dermatology, potassium hydroxide (KOH) testing is a commonly used method for the diagnosis of superficial fungal infections.4 The sensitivity and specificity of KOH testing in patients with tinea pedis have been reported as 73.3% and 42.5%, respectively.5 Repetition of this test after an initial negative test result is recommended if the clinical picture strongly suggests a fungal infection.6,7 Alternatively, several repetitions of direct microscopic examinations also have been proposed for detecting other microorganisms. For example, 3 negative sputum smears traditionally are recommended to exclude a diagnosis of pulmonary tuberculosis.8 However, after numerous investigations in various regions of the world, the World Health Organization reduced the recommended number of these specimens from 3 to 2 in 2007.9
The literature suggests that successive mycological tests, both with direct microscopy and fungal cultures, improve the diagnosis of onychomycosis.1,10,11 Therefore, if such investigations are increased in number, recommendations for successive mycological tests may be more reliable. In the current study, we aimed to investigate the value of successive KOH testing in the management of patients with clinically suspected tinea pedis.
Methods
Patients and Clinical Evaluation
One hundred thirty-five consecutive patients (63 male; 72 female) with clinical symptoms suggestive of intertriginous, vesiculobullous, and/or moccasin-type tinea pedis were enrolled in this prospective study. The mean age (SD) of patients was 45.9 (14.7) years (range, 11–77 years). Almost exclusively, the clinical symptoms suggestive of tinea pedis were desquamation or maceration in the toe webs, blistering lesions on the soles, and diffuse or patchy scaling or keratosis on the soles. A single dermatologist (B.F.K.) clinically evaluated the patients and found only 1 region showing different patterns suggestive of tinea pedis in 72 patients, 2 regions in 61 patients, and 3 regions in 2 patients. Therefore, 200 lesions from the 135 patients were chosen for the KOH test. The dermatologist recorded her level of suspicion for a fungal infection as low or high for each lesion, depending on the absence or presence of signs (eg, unilateral involvement, a well-defined border). None of the patients had used topical or systemic antifungal therapy for at least 1 month prior to the study.12
Clinical Sampling and Direct Microscopic Examination
The dermatologist took 3 samples of skin scrapings from each of the 200 lesions. All 3 samples from a given lesion were obtained from sites with the same clinical symptoms in a single session. Special attention was paid to samples from the active advancing borders of the lesions and the roofs of blisters if they were present.13 Upon completion of every 15 samples from every 5 lesions, the dermatologist randomized the order of the samples (https://www.random.org/). She then gave the samples, without the identities of the patients or any clinical information, to an experienced laboratory technician for direct microscopic examination. The technician prepared and examined the samples as described elsewhere5,7,14 and recorded the results as positive if hyphal elements were present or negative if they were not. The study was reviewed and approved by the Çukurova University Faculty of Medicine Ethics Committee (Adana, Turkey). Informed consent was obtained from each patient or from his/her guardian(s) prior to initiating the study.
Statistical Analysis
Statistical analysis was conducted using the χ2 test in the SPSS software version 20.0. McNemar test was used for analysis of the paired data.
Results
Among the 135 patients, lesions were suggestive of the intertriginous type of tinea pedis in 24 patients, moccasin type in 50 patients, and both intertriginous and moccasin type in 58 patients. Among the remaining 3 patients, 1 had lesions suggestive of the vesiculobullous type, and another patient had both the vesiculobullous and intertriginous types; the last patient demonstrated lesions that were inconsistent with any of these 3 subtypes of tinea pedis, and a well-defined eczematous plaque was observed on the dorsal surface of the patient’s left foot.
Among the 200 lesions from which skin scrapings were taken for KOH testing, 83 were in the toe webs, 110 were on the soles, and 7 were on the dorsal surfaces of the feet. Of these 7 dorsal lesions, 6 were extensions from lesions on the toe webs or soles and 1 was inconsistent with the 3 subtypes of tinea pedis. Among the 200 lesions, the main clinical symptom was maceration in 38 lesions, desquamation or scaling in 132 lesions, keratosis in 28 lesions, and blistering in 2 lesions. The dermatologist recorded the level of suspicion for tinea pedis as low in 68 lesions and high in 132.
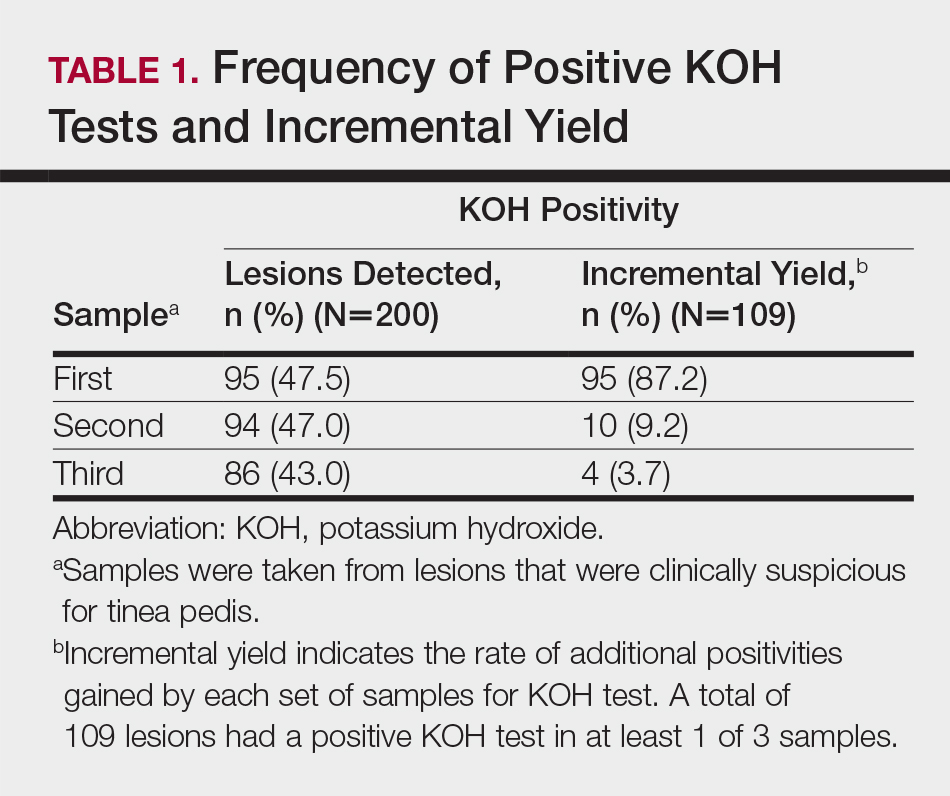
According to the order in which the dermatologist took the 3 samples from each lesion, the KOH test was positive in 95 of the first set of 200 samples, 94 of the second set, and 86 of the third set; however, from the second set, the incremental yield (ie, the number of lesions in which the first KOH test was negative and the second was positive) was 10. The number of lesions in which the first and the second tests were negative and the third was positive was only 4. Therefore, the number of lesions with a positive KOH test was significantly increased from 95 to 105 by performing the second KOH test (P=.002). This number again increased from 105 to 109 when a third test was performed; however, this increase was not statistically significant (P=.125)(Table 1).
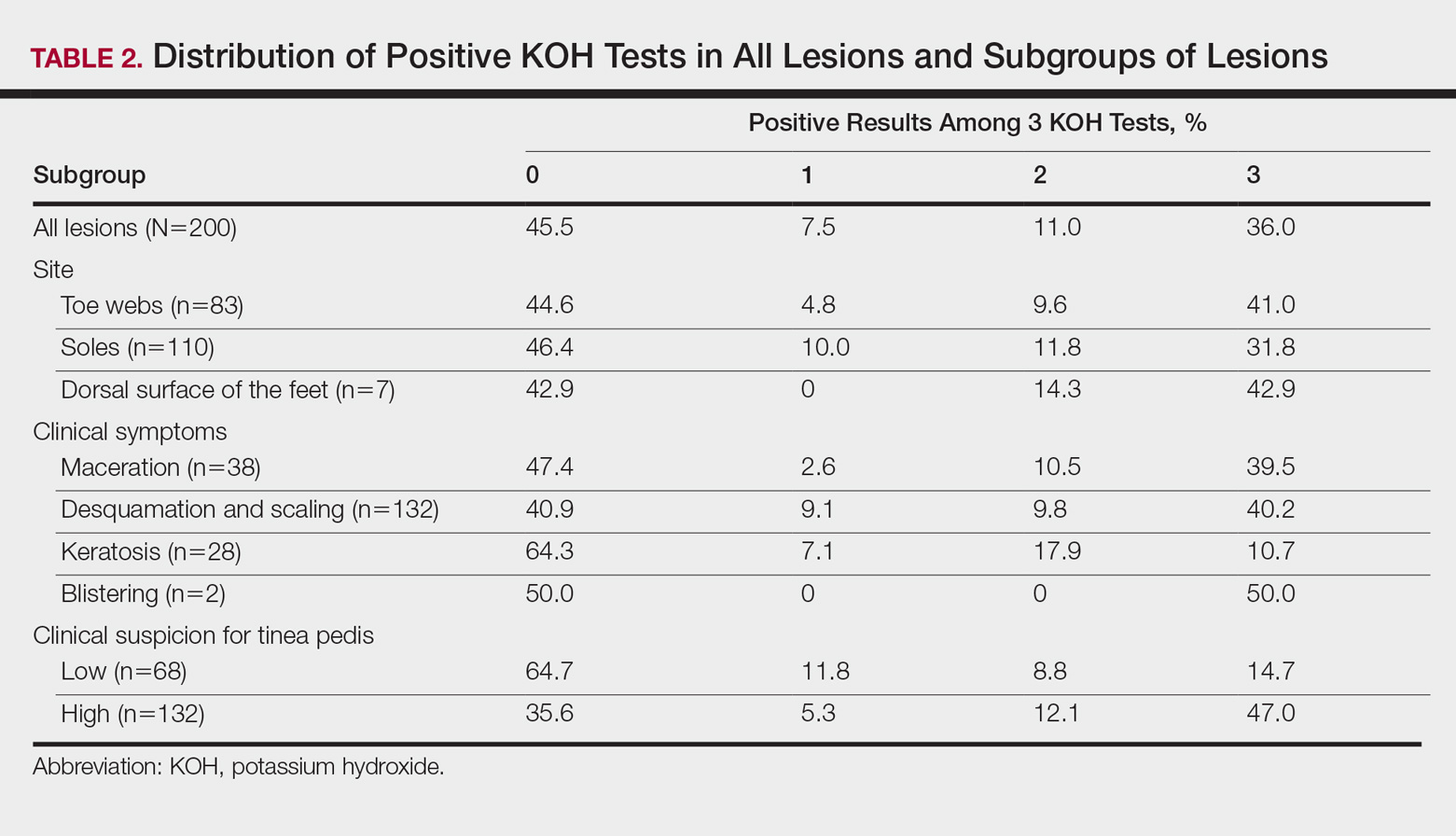
According to an evaluation that was not stratified by the dermatologist’s order of sampling, 72 lesions (36.0%) showed KOH test positivity in all 3 samples, 22 (11.0%) were positive in 2 samples, 15 (7.5%) were positive in only 1 sample, and 91 (45.5%) were positive in none of the samples (Table 2). When the data were subdivided based on the sites of the lesions, the toe web lesions (n=83) showed rates of 41.0%, 9.6%, and 4.8% for 3, 2, and 1 positive KOH tests, respectively. For the sole lesions (n=110), the rates were somewhat different at 31.8%, 11.8%, and 10.0%, respectively, but the difference was not statistically significant (P=.395).
For the subgroups based on the main clinical symptoms, the percentage of lesions having at least 1 positive KOH test from the 3 samples was 35.7% for the keratotic lesions (n=28). This rate was lower than macerated lesions (n=38) and desquamating or scaling lesions (n=132), which were 52.6% and 59.1%, respectively (Table 2). On the other hand, the percentage of lesions that produced only 1 or 2 positive KOH tests from the 3 samples was 25.0% for the keratotic lesions, which was higher than the rates for the macerated lesions and the desquamating or scaling lesions (13.1% and 18.9%, respectively). In particular, the difference between the keratotic lesions and the desquamating or scaling lesions in the distribution of the rates of 0, 1, 2, and 3 positive KOH tests was statistically significant (P=.019). The macerated, desquamating or scaling, keratotic, and blistering lesions are presented in the Figure.
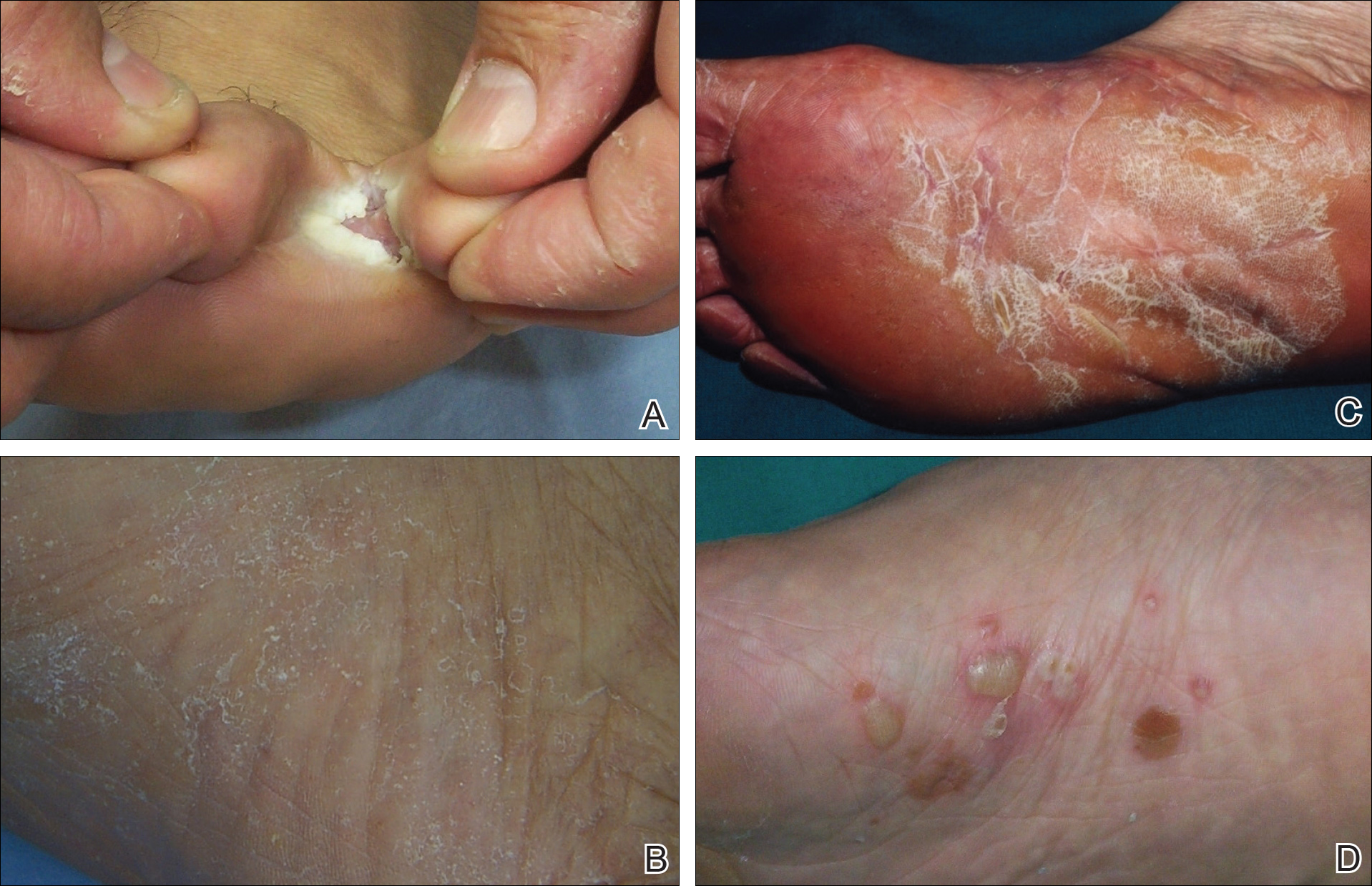
If the dermatologist indicated a high suspicion of fungal infection, it was more likely that at least 1 of 3 KOH test results was positive. The rate of at least 1 positive test was 64.4% for the highly suspicious lesions (n=132) and 35.3% for the lesions with low suspicion of a fungal infection (n=68)(Table 2). The difference was statistically significant (P<.001). Conversely, if the suspicion was low, it was more likely that only 1 or 2 KOH tests were positive. The percentages of lesions having 3, 2, or 1 positive KOH tests were 14.7%, 8.8%, and 11.8%, respectively, for the low-suspicion lesions and 47.0%, 12.1%, and 5.3%, respectively, for the high-suspicion lesions. The difference was statistically significant (P<.001).
Comment
In the current study, we aimed to investigate if successive KOH tests provide an incremental diagnostic yield in the management of patients with clinically suspected tinea pedis and if these results differ among the subgroups of patients. Both in the evaluation taking into account the order of sampling and in the evaluation disregarding this order, we found that the second sample was necessary for all subgroups, and even the third sample was necessary for patients with keratotic lesions. The main limitation of the study was that we lacked a gold-standard technique (eg, a molecular-based technique); therefore, we are unable to comment on the false-negative and false-positive results of the successive KOH testing.
Summerbell et al11 found in their study that in initial specimens of toenails with apparent lesions taken from 473 patients, the KOH test was 73.8% sensitive for dermatophytes, and this rate was only somewhat higher for cultures (74.6%). Arabatzis et al2 investigated 92 skin, nail, and hair specimens from 67 patients with suspected dermatophytosis and found that the KOH test was superior to culture for the detection of dermatophytes (43% vs 33%). Moreover and more importantly, they noted that a real-time polymerase chain reaction (PCR) assay yielded a higher detection rate (51%).2 In another study, Wisselink et al3 examined 1437 clinical samples and demonstrated a great increase in the detection of dermatophytes using a real-time PCR assay (48.5%) compared to culture (26.9%). However, PCR may not reflect active disease and could lead to false-positive results.2,3 Therefore, the aforementioned weakness of our study will be overcome in further studies investigating the benefit of successive KOH testing compared to a molecular-based assay, such as the real-time PCR assay.
In this study, repeating the KOH test provided better results for achieving the diagnosis of tinea pedis in a large number of samples from clinically suspected lesions. Additionally, the distribution of 3, 2, or 1 positive results on the 3 KOH tests was different among the subgroups of lesions. Overall, positivity was less frequent in the keratotic lesions compared to the macerated or desquamating or scaling lesions. Moreover, positivity on all 3 tests also was less frequent in the keratotic lesions. Inversely, the frequency of samples with only 1 or 2 positive results was higher in this subgroup. The necessity for the second, even the third, tests was greater in this subgroup.
Our findings were consistent with the results of the studies performed with successive mycological tests on the nail specimens. Meireles et al1 repeated 156 mycological nail tests 3 times and found the rate of positivity in the first test to be 19.9%. When the results of the first and second tests were combined, this rate increased to 28.2%, and when the results of all 3 tests were combined, it increased to 37.8%.1 Gupta10 demonstrated that even a fourth culture provided an incremental diagnostic yield in the diagnosis of onychomycosis, yet 4 cultures may not be clinically practical. Furthermore, periodic acid–Schiff staining is a more effective measure of positivity in onychomycosis.15
Although the overall rate of positivity on the 3 tests in our study was unsurprisingly higher in lesions rated highly suspicious for a fungal infection, the rate of only 1 or 2 positive tests was surprisingly somewhat higher in low-suspicion lesions, which suggested that repeating the KOH test would be beneficial, even if the clinical suspicion for tinea pedis was low. The novel contribution of this study includes the finding that mycological information was markedly improved in highly suspicious tinea pedis lesions regardless of the infection site (Table 1) by using 3 successive KOH tests; the percentage of lesions with 1, 2, or 3 positive KOH tests was 5.3%, 12.1%, and 47.0%, respectively (Table 2). A single physician from a single geographical location introduces a limitation to the study for a variety of reasons, including bias in the cases chosen and possible overrepresentation of the causative organism due to region-specific incidence. It is unknown how different causative organisms affect KOH results. The lack of fungal culture results limits the value of this information.
Conclusion
In this study, we investigated the benefit of successive KOH testing in the laboratory diagnosis of tinea pedis and found that the use of second samples in particular provided a substantial increase in diagnostic yield. In other words, the utilization of successive KOH testing remarkably improved the diagnosis of tinea pedis. Therefore, we suggest that at least 2 samples of skin scrapings should be taken for the diagnosis of tinea pedis and that the number of samples should be at least 3 for keratotic lesions. However, further study by using a gold-standard method such as a molecular-based assay as well as taking the samples in daily or weekly intervals is recommended to achieve a more reliable result.
Acknowledgment
The authors would like to thank Gökçen Şahin (Adana, Turkey) for providing technical support in direct microscopic examination.
- Meireles TE, Rocha MF, Brilhante RS, et al. Successive mycological nail tests for onychomycosis: a strategy to improve diagnosis efficiency. Braz J Infect Dis. 2008;2:333-337.
- Arabatzis M, Bruijnesteijn van Coppenraet LE, Kuijper EJ, et al. Diagnosis of dermatophyte infection by a novel multiplex real-time polymerase chain reaction detection/identification scheme. Br J Dermatol. 2007;157:681-689.
- Wisselink GJ, van Zanten E, Kooistra-Smid AM. Trapped in keratin; a comparison of dermatophyte detection in nail, skin and hair samples directly from clinical samples using culture and real-time PCR. J Microbiol Methods. 2011;85:62-66.
- Kurade SM, Amladi SA, Miskeen AK. Skin scraping and a potassium hydroxide mount. Indian J Dermatol Venereol Leprol. 2006;72:238-241.
- Levitt JO, Levitt BH, Akhavan A, et al. The sensitivity and specificity of potassium hydroxide smear and fungal culture relative to clinical assessment in the evaluation of tinea pedis: a pooled analysis [published online June 22, 2010]. Dermatol Res Pract. 2010;2010:764843.
- Brodell RT, Helms SE, Snelson ME. Office dermatologic testing: the KOH preparation. Am Fam Physicin. 1991;43:2061-2065.
- McKay M. Office techniques for dermatologic diagnosis. In: Walkers HK, Hall WD, Hurst JW, eds. Clinical Methods: The History, Physical, and Laboratory Examinations. 3rd ed. Boston, MA: Butterworths; 1990:540-543.
- Wilmer A, Bryce E, Grant J. The role of the third acid-fast bacillus smear in tuberculosis screening for infection control purposes: a controversial topic revisited. Can J Infect Dis Med Microbiol. 2011;22:E1-E3.
- World Health Organization. Same-day diagnosis of tuberculosis by microscopy: WHO policy statement. http://www.who.int/tb/publications/2011/tb_microscopy_9789241501606/en/. Published 2011. Accessed July 24, 2017.
- Gupta A. The incremental diagnostic yield of successive re-cultures in patients with a clinical diagnosis of onychomycosis. J Am Acad Dermatol. 2005;52:P129.
- Summerbell RC, Cooper E, Bunn U, et al. Onychomycosis: a critical study of techniques and criteria for confirming the etiologic significance of nondermatophytes. Med Mycol. 2005;43:39-59.
- Miller MA, Hodgson Y. Sensitivity and specificity of potassium hydroxide smears of skin scrapings for the diagnosis of tinea pedis. Arch Dermatol. 1993;129:510-511.
- Ilkit M, Durdu M. Tinea pedis: the etiology and global epidemiology of a common fungal infection. Crit Rev Microbiol. 2015;41:374-388.
- McGinnis MR. Laboratory Handbook of Medical Mycology. New York, NY: Academic Press, Inc; 1980.
- Jeelani S, Ahmed QM, Lanker AM, et al. Histopathological examination of nail clippings using PAS staining (HPE-PAS): gold-standard in diagnosis of onychomycosis. Mycoses. 2015;58:27-32.
The gold standard for diagnosing dermatophytosis is the use of direct microscopic examination together with fungal culture.1 However, in the last 2 decades, molecular techniques that currently are available worldwide have improved the diagnosis procedure.2,3 In the practice of dermatology, potassium hydroxide (KOH) testing is a commonly used method for the diagnosis of superficial fungal infections.4 The sensitivity and specificity of KOH testing in patients with tinea pedis have been reported as 73.3% and 42.5%, respectively.5 Repetition of this test after an initial negative test result is recommended if the clinical picture strongly suggests a fungal infection.6,7 Alternatively, several repetitions of direct microscopic examinations also have been proposed for detecting other microorganisms. For example, 3 negative sputum smears traditionally are recommended to exclude a diagnosis of pulmonary tuberculosis.8 However, after numerous investigations in various regions of the world, the World Health Organization reduced the recommended number of these specimens from 3 to 2 in 2007.9
The literature suggests that successive mycological tests, both with direct microscopy and fungal cultures, improve the diagnosis of onychomycosis.1,10,11 Therefore, if such investigations are increased in number, recommendations for successive mycological tests may be more reliable. In the current study, we aimed to investigate the value of successive KOH testing in the management of patients with clinically suspected tinea pedis.
Methods
Patients and Clinical Evaluation
One hundred thirty-five consecutive patients (63 male; 72 female) with clinical symptoms suggestive of intertriginous, vesiculobullous, and/or moccasin-type tinea pedis were enrolled in this prospective study. The mean age (SD) of patients was 45.9 (14.7) years (range, 11–77 years). Almost exclusively, the clinical symptoms suggestive of tinea pedis were desquamation or maceration in the toe webs, blistering lesions on the soles, and diffuse or patchy scaling or keratosis on the soles. A single dermatologist (B.F.K.) clinically evaluated the patients and found only 1 region showing different patterns suggestive of tinea pedis in 72 patients, 2 regions in 61 patients, and 3 regions in 2 patients. Therefore, 200 lesions from the 135 patients were chosen for the KOH test. The dermatologist recorded her level of suspicion for a fungal infection as low or high for each lesion, depending on the absence or presence of signs (eg, unilateral involvement, a well-defined border). None of the patients had used topical or systemic antifungal therapy for at least 1 month prior to the study.12
Clinical Sampling and Direct Microscopic Examination
The dermatologist took 3 samples of skin scrapings from each of the 200 lesions. All 3 samples from a given lesion were obtained from sites with the same clinical symptoms in a single session. Special attention was paid to samples from the active advancing borders of the lesions and the roofs of blisters if they were present.13 Upon completion of every 15 samples from every 5 lesions, the dermatologist randomized the order of the samples (https://www.random.org/). She then gave the samples, without the identities of the patients or any clinical information, to an experienced laboratory technician for direct microscopic examination. The technician prepared and examined the samples as described elsewhere5,7,14 and recorded the results as positive if hyphal elements were present or negative if they were not. The study was reviewed and approved by the Çukurova University Faculty of Medicine Ethics Committee (Adana, Turkey). Informed consent was obtained from each patient or from his/her guardian(s) prior to initiating the study.
Statistical Analysis
Statistical analysis was conducted using the χ2 test in the SPSS software version 20.0. McNemar test was used for analysis of the paired data.
Results
Among the 135 patients, lesions were suggestive of the intertriginous type of tinea pedis in 24 patients, moccasin type in 50 patients, and both intertriginous and moccasin type in 58 patients. Among the remaining 3 patients, 1 had lesions suggestive of the vesiculobullous type, and another patient had both the vesiculobullous and intertriginous types; the last patient demonstrated lesions that were inconsistent with any of these 3 subtypes of tinea pedis, and a well-defined eczematous plaque was observed on the dorsal surface of the patient’s left foot.
Among the 200 lesions from which skin scrapings were taken for KOH testing, 83 were in the toe webs, 110 were on the soles, and 7 were on the dorsal surfaces of the feet. Of these 7 dorsal lesions, 6 were extensions from lesions on the toe webs or soles and 1 was inconsistent with the 3 subtypes of tinea pedis. Among the 200 lesions, the main clinical symptom was maceration in 38 lesions, desquamation or scaling in 132 lesions, keratosis in 28 lesions, and blistering in 2 lesions. The dermatologist recorded the level of suspicion for tinea pedis as low in 68 lesions and high in 132.
According to the order in which the dermatologist took the 3 samples from each lesion, the KOH test was positive in 95 of the first set of 200 samples, 94 of the second set, and 86 of the third set; however, from the second set, the incremental yield (ie, the number of lesions in which the first KOH test was negative and the second was positive) was 10. The number of lesions in which the first and the second tests were negative and the third was positive was only 4. Therefore, the number of lesions with a positive KOH test was significantly increased from 95 to 105 by performing the second KOH test (P=.002). This number again increased from 105 to 109 when a third test was performed; however, this increase was not statistically significant (P=.125)(Table 1).
According to an evaluation that was not stratified by the dermatologist’s order of sampling, 72 lesions (36.0%) showed KOH test positivity in all 3 samples, 22 (11.0%) were positive in 2 samples, 15 (7.5%) were positive in only 1 sample, and 91 (45.5%) were positive in none of the samples (Table 2). When the data were subdivided based on the sites of the lesions, the toe web lesions (n=83) showed rates of 41.0%, 9.6%, and 4.8% for 3, 2, and 1 positive KOH tests, respectively. For the sole lesions (n=110), the rates were somewhat different at 31.8%, 11.8%, and 10.0%, respectively, but the difference was not statistically significant (P=.395).
For the subgroups based on the main clinical symptoms, the percentage of lesions having at least 1 positive KOH test from the 3 samples was 35.7% for the keratotic lesions (n=28). This rate was lower than macerated lesions (n=38) and desquamating or scaling lesions (n=132), which were 52.6% and 59.1%, respectively (Table 2). On the other hand, the percentage of lesions that produced only 1 or 2 positive KOH tests from the 3 samples was 25.0% for the keratotic lesions, which was higher than the rates for the macerated lesions and the desquamating or scaling lesions (13.1% and 18.9%, respectively). In particular, the difference between the keratotic lesions and the desquamating or scaling lesions in the distribution of the rates of 0, 1, 2, and 3 positive KOH tests was statistically significant (P=.019). The macerated, desquamating or scaling, keratotic, and blistering lesions are presented in the Figure.

If the dermatologist indicated a high suspicion of fungal infection, it was more likely that at least 1 of 3 KOH test results was positive. The rate of at least 1 positive test was 64.4% for the highly suspicious lesions (n=132) and 35.3% for the lesions with low suspicion of a fungal infection (n=68)(Table 2). The difference was statistically significant (P<.001). Conversely, if the suspicion was low, it was more likely that only 1 or 2 KOH tests were positive. The percentages of lesions having 3, 2, or 1 positive KOH tests were 14.7%, 8.8%, and 11.8%, respectively, for the low-suspicion lesions and 47.0%, 12.1%, and 5.3%, respectively, for the high-suspicion lesions. The difference was statistically significant (P<.001).
Comment
In the current study, we aimed to investigate if successive KOH tests provide an incremental diagnostic yield in the management of patients with clinically suspected tinea pedis and if these results differ among the subgroups of patients. Both in the evaluation taking into account the order of sampling and in the evaluation disregarding this order, we found that the second sample was necessary for all subgroups, and even the third sample was necessary for patients with keratotic lesions. The main limitation of the study was that we lacked a gold-standard technique (eg, a molecular-based technique); therefore, we are unable to comment on the false-negative and false-positive results of the successive KOH testing.
Summerbell et al11 found in their study that in initial specimens of toenails with apparent lesions taken from 473 patients, the KOH test was 73.8% sensitive for dermatophytes, and this rate was only somewhat higher for cultures (74.6%). Arabatzis et al2 investigated 92 skin, nail, and hair specimens from 67 patients with suspected dermatophytosis and found that the KOH test was superior to culture for the detection of dermatophytes (43% vs 33%). Moreover and more importantly, they noted that a real-time polymerase chain reaction (PCR) assay yielded a higher detection rate (51%).2 In another study, Wisselink et al3 examined 1437 clinical samples and demonstrated a great increase in the detection of dermatophytes using a real-time PCR assay (48.5%) compared to culture (26.9%). However, PCR may not reflect active disease and could lead to false-positive results.2,3 Therefore, the aforementioned weakness of our study will be overcome in further studies investigating the benefit of successive KOH testing compared to a molecular-based assay, such as the real-time PCR assay.
In this study, repeating the KOH test provided better results for achieving the diagnosis of tinea pedis in a large number of samples from clinically suspected lesions. Additionally, the distribution of 3, 2, or 1 positive results on the 3 KOH tests was different among the subgroups of lesions. Overall, positivity was less frequent in the keratotic lesions compared to the macerated or desquamating or scaling lesions. Moreover, positivity on all 3 tests also was less frequent in the keratotic lesions. Inversely, the frequency of samples with only 1 or 2 positive results was higher in this subgroup. The necessity for the second, even the third, tests was greater in this subgroup.
Our findings were consistent with the results of the studies performed with successive mycological tests on the nail specimens. Meireles et al1 repeated 156 mycological nail tests 3 times and found the rate of positivity in the first test to be 19.9%. When the results of the first and second tests were combined, this rate increased to 28.2%, and when the results of all 3 tests were combined, it increased to 37.8%.1 Gupta10 demonstrated that even a fourth culture provided an incremental diagnostic yield in the diagnosis of onychomycosis, yet 4 cultures may not be clinically practical. Furthermore, periodic acid–Schiff staining is a more effective measure of positivity in onychomycosis.15
Although the overall rate of positivity on the 3 tests in our study was unsurprisingly higher in lesions rated highly suspicious for a fungal infection, the rate of only 1 or 2 positive tests was surprisingly somewhat higher in low-suspicion lesions, which suggested that repeating the KOH test would be beneficial, even if the clinical suspicion for tinea pedis was low. The novel contribution of this study includes the finding that mycological information was markedly improved in highly suspicious tinea pedis lesions regardless of the infection site (Table 1) by using 3 successive KOH tests; the percentage of lesions with 1, 2, or 3 positive KOH tests was 5.3%, 12.1%, and 47.0%, respectively (Table 2). A single physician from a single geographical location introduces a limitation to the study for a variety of reasons, including bias in the cases chosen and possible overrepresentation of the causative organism due to region-specific incidence. It is unknown how different causative organisms affect KOH results. The lack of fungal culture results limits the value of this information.
Conclusion
In this study, we investigated the benefit of successive KOH testing in the laboratory diagnosis of tinea pedis and found that the use of second samples in particular provided a substantial increase in diagnostic yield. In other words, the utilization of successive KOH testing remarkably improved the diagnosis of tinea pedis. Therefore, we suggest that at least 2 samples of skin scrapings should be taken for the diagnosis of tinea pedis and that the number of samples should be at least 3 for keratotic lesions. However, further study by using a gold-standard method such as a molecular-based assay as well as taking the samples in daily or weekly intervals is recommended to achieve a more reliable result.
Acknowledgment
The authors would like to thank Gökçen Şahin (Adana, Turkey) for providing technical support in direct microscopic examination.
The gold standard for diagnosing dermatophytosis is the use of direct microscopic examination together with fungal culture.1 However, in the last 2 decades, molecular techniques that currently are available worldwide have improved the diagnosis procedure.2,3 In the practice of dermatology, potassium hydroxide (KOH) testing is a commonly used method for the diagnosis of superficial fungal infections.4 The sensitivity and specificity of KOH testing in patients with tinea pedis have been reported as 73.3% and 42.5%, respectively.5 Repetition of this test after an initial negative test result is recommended if the clinical picture strongly suggests a fungal infection.6,7 Alternatively, several repetitions of direct microscopic examinations also have been proposed for detecting other microorganisms. For example, 3 negative sputum smears traditionally are recommended to exclude a diagnosis of pulmonary tuberculosis.8 However, after numerous investigations in various regions of the world, the World Health Organization reduced the recommended number of these specimens from 3 to 2 in 2007.9
The literature suggests that successive mycological tests, both with direct microscopy and fungal cultures, improve the diagnosis of onychomycosis.1,10,11 Therefore, if such investigations are increased in number, recommendations for successive mycological tests may be more reliable. In the current study, we aimed to investigate the value of successive KOH testing in the management of patients with clinically suspected tinea pedis.
Methods
Patients and Clinical Evaluation
One hundred thirty-five consecutive patients (63 male; 72 female) with clinical symptoms suggestive of intertriginous, vesiculobullous, and/or moccasin-type tinea pedis were enrolled in this prospective study. The mean age (SD) of patients was 45.9 (14.7) years (range, 11–77 years). Almost exclusively, the clinical symptoms suggestive of tinea pedis were desquamation or maceration in the toe webs, blistering lesions on the soles, and diffuse or patchy scaling or keratosis on the soles. A single dermatologist (B.F.K.) clinically evaluated the patients and found only 1 region showing different patterns suggestive of tinea pedis in 72 patients, 2 regions in 61 patients, and 3 regions in 2 patients. Therefore, 200 lesions from the 135 patients were chosen for the KOH test. The dermatologist recorded her level of suspicion for a fungal infection as low or high for each lesion, depending on the absence or presence of signs (eg, unilateral involvement, a well-defined border). None of the patients had used topical or systemic antifungal therapy for at least 1 month prior to the study.12
Clinical Sampling and Direct Microscopic Examination
The dermatologist took 3 samples of skin scrapings from each of the 200 lesions. All 3 samples from a given lesion were obtained from sites with the same clinical symptoms in a single session. Special attention was paid to samples from the active advancing borders of the lesions and the roofs of blisters if they were present.13 Upon completion of every 15 samples from every 5 lesions, the dermatologist randomized the order of the samples (https://www.random.org/). She then gave the samples, without the identities of the patients or any clinical information, to an experienced laboratory technician for direct microscopic examination. The technician prepared and examined the samples as described elsewhere5,7,14 and recorded the results as positive if hyphal elements were present or negative if they were not. The study was reviewed and approved by the Çukurova University Faculty of Medicine Ethics Committee (Adana, Turkey). Informed consent was obtained from each patient or from his/her guardian(s) prior to initiating the study.
Statistical Analysis
Statistical analysis was conducted using the χ2 test in the SPSS software version 20.0. McNemar test was used for analysis of the paired data.
Results
Among the 135 patients, lesions were suggestive of the intertriginous type of tinea pedis in 24 patients, moccasin type in 50 patients, and both intertriginous and moccasin type in 58 patients. Among the remaining 3 patients, 1 had lesions suggestive of the vesiculobullous type, and another patient had both the vesiculobullous and intertriginous types; the last patient demonstrated lesions that were inconsistent with any of these 3 subtypes of tinea pedis, and a well-defined eczematous plaque was observed on the dorsal surface of the patient’s left foot.
Among the 200 lesions from which skin scrapings were taken for KOH testing, 83 were in the toe webs, 110 were on the soles, and 7 were on the dorsal surfaces of the feet. Of these 7 dorsal lesions, 6 were extensions from lesions on the toe webs or soles and 1 was inconsistent with the 3 subtypes of tinea pedis. Among the 200 lesions, the main clinical symptom was maceration in 38 lesions, desquamation or scaling in 132 lesions, keratosis in 28 lesions, and blistering in 2 lesions. The dermatologist recorded the level of suspicion for tinea pedis as low in 68 lesions and high in 132.
According to the order in which the dermatologist took the 3 samples from each lesion, the KOH test was positive in 95 of the first set of 200 samples, 94 of the second set, and 86 of the third set; however, from the second set, the incremental yield (ie, the number of lesions in which the first KOH test was negative and the second was positive) was 10. The number of lesions in which the first and the second tests were negative and the third was positive was only 4. Therefore, the number of lesions with a positive KOH test was significantly increased from 95 to 105 by performing the second KOH test (P=.002). This number again increased from 105 to 109 when a third test was performed; however, this increase was not statistically significant (P=.125)(Table 1).
According to an evaluation that was not stratified by the dermatologist’s order of sampling, 72 lesions (36.0%) showed KOH test positivity in all 3 samples, 22 (11.0%) were positive in 2 samples, 15 (7.5%) were positive in only 1 sample, and 91 (45.5%) were positive in none of the samples (Table 2). When the data were subdivided based on the sites of the lesions, the toe web lesions (n=83) showed rates of 41.0%, 9.6%, and 4.8% for 3, 2, and 1 positive KOH tests, respectively. For the sole lesions (n=110), the rates were somewhat different at 31.8%, 11.8%, and 10.0%, respectively, but the difference was not statistically significant (P=.395).
For the subgroups based on the main clinical symptoms, the percentage of lesions having at least 1 positive KOH test from the 3 samples was 35.7% for the keratotic lesions (n=28). This rate was lower than macerated lesions (n=38) and desquamating or scaling lesions (n=132), which were 52.6% and 59.1%, respectively (Table 2). On the other hand, the percentage of lesions that produced only 1 or 2 positive KOH tests from the 3 samples was 25.0% for the keratotic lesions, which was higher than the rates for the macerated lesions and the desquamating or scaling lesions (13.1% and 18.9%, respectively). In particular, the difference between the keratotic lesions and the desquamating or scaling lesions in the distribution of the rates of 0, 1, 2, and 3 positive KOH tests was statistically significant (P=.019). The macerated, desquamating or scaling, keratotic, and blistering lesions are presented in the Figure.

If the dermatologist indicated a high suspicion of fungal infection, it was more likely that at least 1 of 3 KOH test results was positive. The rate of at least 1 positive test was 64.4% for the highly suspicious lesions (n=132) and 35.3% for the lesions with low suspicion of a fungal infection (n=68)(Table 2). The difference was statistically significant (P<.001). Conversely, if the suspicion was low, it was more likely that only 1 or 2 KOH tests were positive. The percentages of lesions having 3, 2, or 1 positive KOH tests were 14.7%, 8.8%, and 11.8%, respectively, for the low-suspicion lesions and 47.0%, 12.1%, and 5.3%, respectively, for the high-suspicion lesions. The difference was statistically significant (P<.001).
Comment
In the current study, we aimed to investigate if successive KOH tests provide an incremental diagnostic yield in the management of patients with clinically suspected tinea pedis and if these results differ among the subgroups of patients. Both in the evaluation taking into account the order of sampling and in the evaluation disregarding this order, we found that the second sample was necessary for all subgroups, and even the third sample was necessary for patients with keratotic lesions. The main limitation of the study was that we lacked a gold-standard technique (eg, a molecular-based technique); therefore, we are unable to comment on the false-negative and false-positive results of the successive KOH testing.
Summerbell et al11 found in their study that in initial specimens of toenails with apparent lesions taken from 473 patients, the KOH test was 73.8% sensitive for dermatophytes, and this rate was only somewhat higher for cultures (74.6%). Arabatzis et al2 investigated 92 skin, nail, and hair specimens from 67 patients with suspected dermatophytosis and found that the KOH test was superior to culture for the detection of dermatophytes (43% vs 33%). Moreover and more importantly, they noted that a real-time polymerase chain reaction (PCR) assay yielded a higher detection rate (51%).2 In another study, Wisselink et al3 examined 1437 clinical samples and demonstrated a great increase in the detection of dermatophytes using a real-time PCR assay (48.5%) compared to culture (26.9%). However, PCR may not reflect active disease and could lead to false-positive results.2,3 Therefore, the aforementioned weakness of our study will be overcome in further studies investigating the benefit of successive KOH testing compared to a molecular-based assay, such as the real-time PCR assay.
In this study, repeating the KOH test provided better results for achieving the diagnosis of tinea pedis in a large number of samples from clinically suspected lesions. Additionally, the distribution of 3, 2, or 1 positive results on the 3 KOH tests was different among the subgroups of lesions. Overall, positivity was less frequent in the keratotic lesions compared to the macerated or desquamating or scaling lesions. Moreover, positivity on all 3 tests also was less frequent in the keratotic lesions. Inversely, the frequency of samples with only 1 or 2 positive results was higher in this subgroup. The necessity for the second, even the third, tests was greater in this subgroup.
Our findings were consistent with the results of the studies performed with successive mycological tests on the nail specimens. Meireles et al1 repeated 156 mycological nail tests 3 times and found the rate of positivity in the first test to be 19.9%. When the results of the first and second tests were combined, this rate increased to 28.2%, and when the results of all 3 tests were combined, it increased to 37.8%.1 Gupta10 demonstrated that even a fourth culture provided an incremental diagnostic yield in the diagnosis of onychomycosis, yet 4 cultures may not be clinically practical. Furthermore, periodic acid–Schiff staining is a more effective measure of positivity in onychomycosis.15
Although the overall rate of positivity on the 3 tests in our study was unsurprisingly higher in lesions rated highly suspicious for a fungal infection, the rate of only 1 or 2 positive tests was surprisingly somewhat higher in low-suspicion lesions, which suggested that repeating the KOH test would be beneficial, even if the clinical suspicion for tinea pedis was low. The novel contribution of this study includes the finding that mycological information was markedly improved in highly suspicious tinea pedis lesions regardless of the infection site (Table 1) by using 3 successive KOH tests; the percentage of lesions with 1, 2, or 3 positive KOH tests was 5.3%, 12.1%, and 47.0%, respectively (Table 2). A single physician from a single geographical location introduces a limitation to the study for a variety of reasons, including bias in the cases chosen and possible overrepresentation of the causative organism due to region-specific incidence. It is unknown how different causative organisms affect KOH results. The lack of fungal culture results limits the value of this information.
Conclusion
In this study, we investigated the benefit of successive KOH testing in the laboratory diagnosis of tinea pedis and found that the use of second samples in particular provided a substantial increase in diagnostic yield. In other words, the utilization of successive KOH testing remarkably improved the diagnosis of tinea pedis. Therefore, we suggest that at least 2 samples of skin scrapings should be taken for the diagnosis of tinea pedis and that the number of samples should be at least 3 for keratotic lesions. However, further study by using a gold-standard method such as a molecular-based assay as well as taking the samples in daily or weekly intervals is recommended to achieve a more reliable result.
Acknowledgment
The authors would like to thank Gökçen Şahin (Adana, Turkey) for providing technical support in direct microscopic examination.
- Meireles TE, Rocha MF, Brilhante RS, et al. Successive mycological nail tests for onychomycosis: a strategy to improve diagnosis efficiency. Braz J Infect Dis. 2008;2:333-337.
- Arabatzis M, Bruijnesteijn van Coppenraet LE, Kuijper EJ, et al. Diagnosis of dermatophyte infection by a novel multiplex real-time polymerase chain reaction detection/identification scheme. Br J Dermatol. 2007;157:681-689.
- Wisselink GJ, van Zanten E, Kooistra-Smid AM. Trapped in keratin; a comparison of dermatophyte detection in nail, skin and hair samples directly from clinical samples using culture and real-time PCR. J Microbiol Methods. 2011;85:62-66.
- Kurade SM, Amladi SA, Miskeen AK. Skin scraping and a potassium hydroxide mount. Indian J Dermatol Venereol Leprol. 2006;72:238-241.
- Levitt JO, Levitt BH, Akhavan A, et al. The sensitivity and specificity of potassium hydroxide smear and fungal culture relative to clinical assessment in the evaluation of tinea pedis: a pooled analysis [published online June 22, 2010]. Dermatol Res Pract. 2010;2010:764843.
- Brodell RT, Helms SE, Snelson ME. Office dermatologic testing: the KOH preparation. Am Fam Physicin. 1991;43:2061-2065.
- McKay M. Office techniques for dermatologic diagnosis. In: Walkers HK, Hall WD, Hurst JW, eds. Clinical Methods: The History, Physical, and Laboratory Examinations. 3rd ed. Boston, MA: Butterworths; 1990:540-543.
- Wilmer A, Bryce E, Grant J. The role of the third acid-fast bacillus smear in tuberculosis screening for infection control purposes: a controversial topic revisited. Can J Infect Dis Med Microbiol. 2011;22:E1-E3.
- World Health Organization. Same-day diagnosis of tuberculosis by microscopy: WHO policy statement. http://www.who.int/tb/publications/2011/tb_microscopy_9789241501606/en/. Published 2011. Accessed July 24, 2017.
- Gupta A. The incremental diagnostic yield of successive re-cultures in patients with a clinical diagnosis of onychomycosis. J Am Acad Dermatol. 2005;52:P129.
- Summerbell RC, Cooper E, Bunn U, et al. Onychomycosis: a critical study of techniques and criteria for confirming the etiologic significance of nondermatophytes. Med Mycol. 2005;43:39-59.
- Miller MA, Hodgson Y. Sensitivity and specificity of potassium hydroxide smears of skin scrapings for the diagnosis of tinea pedis. Arch Dermatol. 1993;129:510-511.
- Ilkit M, Durdu M. Tinea pedis: the etiology and global epidemiology of a common fungal infection. Crit Rev Microbiol. 2015;41:374-388.
- McGinnis MR. Laboratory Handbook of Medical Mycology. New York, NY: Academic Press, Inc; 1980.
- Jeelani S, Ahmed QM, Lanker AM, et al. Histopathological examination of nail clippings using PAS staining (HPE-PAS): gold-standard in diagnosis of onychomycosis. Mycoses. 2015;58:27-32.
- Meireles TE, Rocha MF, Brilhante RS, et al. Successive mycological nail tests for onychomycosis: a strategy to improve diagnosis efficiency. Braz J Infect Dis. 2008;2:333-337.
- Arabatzis M, Bruijnesteijn van Coppenraet LE, Kuijper EJ, et al. Diagnosis of dermatophyte infection by a novel multiplex real-time polymerase chain reaction detection/identification scheme. Br J Dermatol. 2007;157:681-689.
- Wisselink GJ, van Zanten E, Kooistra-Smid AM. Trapped in keratin; a comparison of dermatophyte detection in nail, skin and hair samples directly from clinical samples using culture and real-time PCR. J Microbiol Methods. 2011;85:62-66.
- Kurade SM, Amladi SA, Miskeen AK. Skin scraping and a potassium hydroxide mount. Indian J Dermatol Venereol Leprol. 2006;72:238-241.
- Levitt JO, Levitt BH, Akhavan A, et al. The sensitivity and specificity of potassium hydroxide smear and fungal culture relative to clinical assessment in the evaluation of tinea pedis: a pooled analysis [published online June 22, 2010]. Dermatol Res Pract. 2010;2010:764843.
- Brodell RT, Helms SE, Snelson ME. Office dermatologic testing: the KOH preparation. Am Fam Physicin. 1991;43:2061-2065.
- McKay M. Office techniques for dermatologic diagnosis. In: Walkers HK, Hall WD, Hurst JW, eds. Clinical Methods: The History, Physical, and Laboratory Examinations. 3rd ed. Boston, MA: Butterworths; 1990:540-543.
- Wilmer A, Bryce E, Grant J. The role of the third acid-fast bacillus smear in tuberculosis screening for infection control purposes: a controversial topic revisited. Can J Infect Dis Med Microbiol. 2011;22:E1-E3.
- World Health Organization. Same-day diagnosis of tuberculosis by microscopy: WHO policy statement. http://www.who.int/tb/publications/2011/tb_microscopy_9789241501606/en/. Published 2011. Accessed July 24, 2017.
- Gupta A. The incremental diagnostic yield of successive re-cultures in patients with a clinical diagnosis of onychomycosis. J Am Acad Dermatol. 2005;52:P129.
- Summerbell RC, Cooper E, Bunn U, et al. Onychomycosis: a critical study of techniques and criteria for confirming the etiologic significance of nondermatophytes. Med Mycol. 2005;43:39-59.
- Miller MA, Hodgson Y. Sensitivity and specificity of potassium hydroxide smears of skin scrapings for the diagnosis of tinea pedis. Arch Dermatol. 1993;129:510-511.
- Ilkit M, Durdu M. Tinea pedis: the etiology and global epidemiology of a common fungal infection. Crit Rev Microbiol. 2015;41:374-388.
- McGinnis MR. Laboratory Handbook of Medical Mycology. New York, NY: Academic Press, Inc; 1980.
- Jeelani S, Ahmed QM, Lanker AM, et al. Histopathological examination of nail clippings using PAS staining (HPE-PAS): gold-standard in diagnosis of onychomycosis. Mycoses. 2015;58:27-32.
Practice Points
- At least 2 samples should be taken for potassium hydroxide examination when tinea pedis is sus-pected clinically.
- The number of samples should be at least 3 if keratotic lesions are present.
Adalimumab for Hidradenitis Suppurativa
We applaud Kimball et al1 on their report that adalimumab demonstrated clinical improvement in patients with hidradenitis suppurativa (HS) versus placebo in 2 phase 3 trials. Hidradenitis suppurativa is a chronic relapsing condition with painful subcutaneous abscesses, malodorous drainage, sinus tract formation, and scarring that typically occurs in the axillae and anogenital region. It impairs the quality of life for these patients, as evidenced by higher Dermatology Life Quality Index scores compared to psoriasis, pimples, hand rash, atopic eczema, or control.2
The exact pathogenesis of HS is unknown but likely involves a complex interaction of genetic, hormonal, immunologic, and environmental factors.3 The levels of inflammatory cytokines are elevated in HS lesions, specifically IL-1β, tumor necrosis factor α, IL-10, and CXCL9, as well as monokines from IFN-γ, IL-11, and IL-17A. Additionally, the dermis of affected regions contains IL-12– and IL-23–containing macrophages along with IL-17–producing T cells.3 These findings reveal many potential therapeutic targets for the treatment of HS.
PIONEER I and PIONEER II are similarly designed 36-week phase 3 trials of 633 patients with HS who were unresponsive to oral antibiotic treatment.1 By week 12, a significantly greater proportion of patients receiving adalimumab demonstrated clinical improvement (≥50% reduction in total abscess and nodule count) compared to placebo in both trials (PIONEER I: 41.8% vs 26.0%, P=.003; PIONEER II: 58.9% vs 27.6%, P<.001). Secondary end points (inflammatory-nodule count, pain score, and disease severity) were only achieved in PIONEER II. The difference in clinical improvement between the trials is likely due to higher baseline disease severity in the HS patients in PIONEER I versus PIONEER II. No new safety risks were reported and were in accordance with prior adalimumab trials for other diseases. Notably, 10 paradoxical psoriasislike eruptions were reported.
Adalimumab is the first and only US Food and Drug Administration–approved therapy for HS. Further understanding of the pathogenesis of HS may result in additional biologic treatments for HS. We encourage the manufacturers of other biologic therapies, such as infliximab,4 ustekinumab,5 anakinra,6 secukinumab, ixekizumab, and brodalumab, to consider conducting further clinical trials in HS to enhance the therapeutic options available for this debilitating disease.
- Kimball AB, Okun MM, Williams DA, et al. Two Phase 3 trials of adalimumab for hidradenitis suppurativa. N Engl J Med. 2016;375:422-434.
- Vinding GR, Knudsen KM, Ellervik C, et al. Self-reported skin morbidities and health-related quality of life: a population-based nested case-control study. Dermatology. 2014;228:261-268.
- Deckers IE, van der Zee HH, Prens EP. Epidemiology of hidradenitis suppurativa: prevalence, pathogenesis, and factors associated with the development of HS. Curr Dermatol Rep. 2014;3:54-60.
- Ingram JR, Woo PN, Chua SL, et al. Interventions for hidradenitis suppurativa: a Cochrane systematic review incorporating GRADE assessment of evidence quality. Br J Dermatol. 2016;174:970-978.
- Blok JL, Li K, Brodmerkel C, et al. Ustekinumab in hidradenitis suppurativa: clinical results and a search for potential biomarkers in serum. Br J Dermatol. 2016;174:839-846.
- Tzanetakou V, Kanni T, Giatrakou S, et al. Safety and efficacy of anakinra in severe hidradenitis suppurativa: a randomized clinical trial. JAMA Dermatol. 2016;152:52-59.
We applaud Kimball et al1 on their report that adalimumab demonstrated clinical improvement in patients with hidradenitis suppurativa (HS) versus placebo in 2 phase 3 trials. Hidradenitis suppurativa is a chronic relapsing condition with painful subcutaneous abscesses, malodorous drainage, sinus tract formation, and scarring that typically occurs in the axillae and anogenital region. It impairs the quality of life for these patients, as evidenced by higher Dermatology Life Quality Index scores compared to psoriasis, pimples, hand rash, atopic eczema, or control.2
The exact pathogenesis of HS is unknown but likely involves a complex interaction of genetic, hormonal, immunologic, and environmental factors.3 The levels of inflammatory cytokines are elevated in HS lesions, specifically IL-1β, tumor necrosis factor α, IL-10, and CXCL9, as well as monokines from IFN-γ, IL-11, and IL-17A. Additionally, the dermis of affected regions contains IL-12– and IL-23–containing macrophages along with IL-17–producing T cells.3 These findings reveal many potential therapeutic targets for the treatment of HS.
PIONEER I and PIONEER II are similarly designed 36-week phase 3 trials of 633 patients with HS who were unresponsive to oral antibiotic treatment.1 By week 12, a significantly greater proportion of patients receiving adalimumab demonstrated clinical improvement (≥50% reduction in total abscess and nodule count) compared to placebo in both trials (PIONEER I: 41.8% vs 26.0%, P=.003; PIONEER II: 58.9% vs 27.6%, P<.001). Secondary end points (inflammatory-nodule count, pain score, and disease severity) were only achieved in PIONEER II. The difference in clinical improvement between the trials is likely due to higher baseline disease severity in the HS patients in PIONEER I versus PIONEER II. No new safety risks were reported and were in accordance with prior adalimumab trials for other diseases. Notably, 10 paradoxical psoriasislike eruptions were reported.
Adalimumab is the first and only US Food and Drug Administration–approved therapy for HS. Further understanding of the pathogenesis of HS may result in additional biologic treatments for HS. We encourage the manufacturers of other biologic therapies, such as infliximab,4 ustekinumab,5 anakinra,6 secukinumab, ixekizumab, and brodalumab, to consider conducting further clinical trials in HS to enhance the therapeutic options available for this debilitating disease.
We applaud Kimball et al1 on their report that adalimumab demonstrated clinical improvement in patients with hidradenitis suppurativa (HS) versus placebo in 2 phase 3 trials. Hidradenitis suppurativa is a chronic relapsing condition with painful subcutaneous abscesses, malodorous drainage, sinus tract formation, and scarring that typically occurs in the axillae and anogenital region. It impairs the quality of life for these patients, as evidenced by higher Dermatology Life Quality Index scores compared to psoriasis, pimples, hand rash, atopic eczema, or control.2
The exact pathogenesis of HS is unknown but likely involves a complex interaction of genetic, hormonal, immunologic, and environmental factors.3 The levels of inflammatory cytokines are elevated in HS lesions, specifically IL-1β, tumor necrosis factor α, IL-10, and CXCL9, as well as monokines from IFN-γ, IL-11, and IL-17A. Additionally, the dermis of affected regions contains IL-12– and IL-23–containing macrophages along with IL-17–producing T cells.3 These findings reveal many potential therapeutic targets for the treatment of HS.
PIONEER I and PIONEER II are similarly designed 36-week phase 3 trials of 633 patients with HS who were unresponsive to oral antibiotic treatment.1 By week 12, a significantly greater proportion of patients receiving adalimumab demonstrated clinical improvement (≥50% reduction in total abscess and nodule count) compared to placebo in both trials (PIONEER I: 41.8% vs 26.0%, P=.003; PIONEER II: 58.9% vs 27.6%, P<.001). Secondary end points (inflammatory-nodule count, pain score, and disease severity) were only achieved in PIONEER II. The difference in clinical improvement between the trials is likely due to higher baseline disease severity in the HS patients in PIONEER I versus PIONEER II. No new safety risks were reported and were in accordance with prior adalimumab trials for other diseases. Notably, 10 paradoxical psoriasislike eruptions were reported.
Adalimumab is the first and only US Food and Drug Administration–approved therapy for HS. Further understanding of the pathogenesis of HS may result in additional biologic treatments for HS. We encourage the manufacturers of other biologic therapies, such as infliximab,4 ustekinumab,5 anakinra,6 secukinumab, ixekizumab, and brodalumab, to consider conducting further clinical trials in HS to enhance the therapeutic options available for this debilitating disease.
- Kimball AB, Okun MM, Williams DA, et al. Two Phase 3 trials of adalimumab for hidradenitis suppurativa. N Engl J Med. 2016;375:422-434.
- Vinding GR, Knudsen KM, Ellervik C, et al. Self-reported skin morbidities and health-related quality of life: a population-based nested case-control study. Dermatology. 2014;228:261-268.
- Deckers IE, van der Zee HH, Prens EP. Epidemiology of hidradenitis suppurativa: prevalence, pathogenesis, and factors associated with the development of HS. Curr Dermatol Rep. 2014;3:54-60.
- Ingram JR, Woo PN, Chua SL, et al. Interventions for hidradenitis suppurativa: a Cochrane systematic review incorporating GRADE assessment of evidence quality. Br J Dermatol. 2016;174:970-978.
- Blok JL, Li K, Brodmerkel C, et al. Ustekinumab in hidradenitis suppurativa: clinical results and a search for potential biomarkers in serum. Br J Dermatol. 2016;174:839-846.
- Tzanetakou V, Kanni T, Giatrakou S, et al. Safety and efficacy of anakinra in severe hidradenitis suppurativa: a randomized clinical trial. JAMA Dermatol. 2016;152:52-59.
- Kimball AB, Okun MM, Williams DA, et al. Two Phase 3 trials of adalimumab for hidradenitis suppurativa. N Engl J Med. 2016;375:422-434.
- Vinding GR, Knudsen KM, Ellervik C, et al. Self-reported skin morbidities and health-related quality of life: a population-based nested case-control study. Dermatology. 2014;228:261-268.
- Deckers IE, van der Zee HH, Prens EP. Epidemiology of hidradenitis suppurativa: prevalence, pathogenesis, and factors associated with the development of HS. Curr Dermatol Rep. 2014;3:54-60.
- Ingram JR, Woo PN, Chua SL, et al. Interventions for hidradenitis suppurativa: a Cochrane systematic review incorporating GRADE assessment of evidence quality. Br J Dermatol. 2016;174:970-978.
- Blok JL, Li K, Brodmerkel C, et al. Ustekinumab in hidradenitis suppurativa: clinical results and a search for potential biomarkers in serum. Br J Dermatol. 2016;174:839-846.
- Tzanetakou V, Kanni T, Giatrakou S, et al. Safety and efficacy of anakinra in severe hidradenitis suppurativa: a randomized clinical trial. JAMA Dermatol. 2016;152:52-59.
Product News: 08 2017
Avène A-OXitive
Pierre Fabre Dermo-Cosmetique introduces Avène A-OXitive Antioxidant Defense Serum and Avène A-OXitive Antioxidant Water-Cream to fight against oxidative stress. Stable forms of time-released vitamin E and vitamin C work synergistically to defend against environmental aggressors without irritation. Both products contain hyaluronic acid to visibly plump and firm the skin and soothing Avène thermal spring water to soften and restore the skin’s balance. To preserve stability, each formula comes in an airless pump bottle. For more information, visit www.aveneusa.com.
Orencia
Bristol-Myers Squibb Company announces US Food and Drug Administration approval of Orencia (abatacept) for the treatment of adults with active psoriatic arthritis. Orencia is available in both intravenous and subcutaneous injection formulations. It should not be administered concomitantly with tumor necrosis factor antagonists and is not recommended for use with other biologic rheumatoid arthritis therapy. Orencia also is indicated for the treatment of adult rheumatoid arthritis and juvenile idiopathic arthritis. For more information, visit www.orenciahcp.com.
Tremfya
Janssen Biotech, Inc, announces US Food and Drug Administration approval of Tremfya (guselkumab) for the treatment of moderate to severe plaque psoriasis in adults who are candidates for systemic therapy or phototherapy. Tremfya is a biologic therapy that selectively blocks only IL-23, a cytokine that plays a key role in plaque psoriasis. Tremfya is administered as a 100-mg subcutaneous injection every 8 weeks, following 2 starter doses at weeks 0 and 4. Clinical studies documented skin clearance at week 16 and up to week 48. For more information, visit www.tremfyahcp.com.
If you would like your product included in Product News, please email a press release to the Edi
Avène A-OXitive
Pierre Fabre Dermo-Cosmetique introduces Avène A-OXitive Antioxidant Defense Serum and Avène A-OXitive Antioxidant Water-Cream to fight against oxidative stress. Stable forms of time-released vitamin E and vitamin C work synergistically to defend against environmental aggressors without irritation. Both products contain hyaluronic acid to visibly plump and firm the skin and soothing Avène thermal spring water to soften and restore the skin’s balance. To preserve stability, each formula comes in an airless pump bottle. For more information, visit www.aveneusa.com.
Orencia
Bristol-Myers Squibb Company announces US Food and Drug Administration approval of Orencia (abatacept) for the treatment of adults with active psoriatic arthritis. Orencia is available in both intravenous and subcutaneous injection formulations. It should not be administered concomitantly with tumor necrosis factor antagonists and is not recommended for use with other biologic rheumatoid arthritis therapy. Orencia also is indicated for the treatment of adult rheumatoid arthritis and juvenile idiopathic arthritis. For more information, visit www.orenciahcp.com.
Tremfya
Janssen Biotech, Inc, announces US Food and Drug Administration approval of Tremfya (guselkumab) for the treatment of moderate to severe plaque psoriasis in adults who are candidates for systemic therapy or phototherapy. Tremfya is a biologic therapy that selectively blocks only IL-23, a cytokine that plays a key role in plaque psoriasis. Tremfya is administered as a 100-mg subcutaneous injection every 8 weeks, following 2 starter doses at weeks 0 and 4. Clinical studies documented skin clearance at week 16 and up to week 48. For more information, visit www.tremfyahcp.com.
If you would like your product included in Product News, please email a press release to the Edi
Avène A-OXitive
Pierre Fabre Dermo-Cosmetique introduces Avène A-OXitive Antioxidant Defense Serum and Avène A-OXitive Antioxidant Water-Cream to fight against oxidative stress. Stable forms of time-released vitamin E and vitamin C work synergistically to defend against environmental aggressors without irritation. Both products contain hyaluronic acid to visibly plump and firm the skin and soothing Avène thermal spring water to soften and restore the skin’s balance. To preserve stability, each formula comes in an airless pump bottle. For more information, visit www.aveneusa.com.
Orencia
Bristol-Myers Squibb Company announces US Food and Drug Administration approval of Orencia (abatacept) for the treatment of adults with active psoriatic arthritis. Orencia is available in both intravenous and subcutaneous injection formulations. It should not be administered concomitantly with tumor necrosis factor antagonists and is not recommended for use with other biologic rheumatoid arthritis therapy. Orencia also is indicated for the treatment of adult rheumatoid arthritis and juvenile idiopathic arthritis. For more information, visit www.orenciahcp.com.
Tremfya
Janssen Biotech, Inc, announces US Food and Drug Administration approval of Tremfya (guselkumab) for the treatment of moderate to severe plaque psoriasis in adults who are candidates for systemic therapy or phototherapy. Tremfya is a biologic therapy that selectively blocks only IL-23, a cytokine that plays a key role in plaque psoriasis. Tremfya is administered as a 100-mg subcutaneous injection every 8 weeks, following 2 starter doses at weeks 0 and 4. Clinical studies documented skin clearance at week 16 and up to week 48. For more information, visit www.tremfyahcp.com.
If you would like your product included in Product News, please email a press release to the Edi
Recent Controversies in Pediatric Dermatology: The Usage of General Anesthesia in Young Children
Clinicians who have attempted to perform an in-office procedure on infants or young children will recognize the difficulties that arise from the developmental inability to cooperate with procedures.1 Potential problems mentioned in the literature include but are not limited to anxiety, which is identified in all age groups of patients undergoing dermatologic procedures2; limitation of pain control3; and poor outcomes due to movement by the patient.1 In one author’s experience (N.B.S.), anxious and scared children can potentially cause injury to themselves, parents/guardians, and health care professionals by flailing and kicking; children are flexible and can wriggle out of even fine grips, and some children, especially toddlers, can be strong.
The usage of topical anesthetics can only give superficial anesthesia. They can ostensibly reduce pain and are useful for anesthesia of curettage, but their use is limited in infants and young children by the minimal amount of drug that is safe for application, as risks of absorption include methemoglobinemia and seizure
General anesthesia seems to be the best alternative due to associated amnesia of the events occurring including pain; immobilization and ability to produce more accurate biopsy sampling; better immobilization leading to superior cosmetic results; and reduced risk to patients, parents/guardians, and health care professionals from a flailing child. In the field of pediatric dermatology, general anesthesia often is used for excision of larger lesions and cosmetic repairs. Operating room privileges are not always easy to obtain, but many pediatric dermatologists take advantage of outpatient surgical centers associated with their medical center. A retrospective review of 226 children receiving 681 procedures at a single institution documented low rates of complications.1
If it was that easy, most children would be anesthetized with general anesthesia. However, there are risks associated with general anesthesia. Parents/guardians often will do what they can to avoid risk and may therefore refuse general anesthesia, but it is not completely avoidable in complicated skin disease. Despite the risks, the benefit is present in a major anomaly correction such as a cleft palate in a 6-month-old but may not be there for the treatment of a wart. When procedures are nonessential or may be conducted without anesthesia, avoidance of general anesthesia is reasonable and a combination of topical and local infiltrative anesthesia can help. In the American Academy of Dermatology guidelines on in-office anesthesia, Kouba et al5 states: “Topical agents are recommended as a first-line method of anesthesia for the repair of dermal lacerations in children and for other minor dermatologic procedures, including curettage. For skin biopsy, excision, or other cases where topical agents alone are insufficient, adjunctive use of topical anesthesia to lessen the discomfort of infiltrative anesthetic should be considered.”
A new controversy recently has emerged concerning the potential risks of anesthesia on neurocognitive development in infants and young children. These concerns regardingthe labeling changes of anesthetic and sedation drugs by the US Food and Drug Administration (FDA) in December 2016 specifically focused on these risks in children younger than 3 years with prolonged (>3 hours) and repeated exposures; however, this kind of exposure is unlikely with standard pediatric dermatologic procedures.6-9
There is compelling evidence from animal studies that exposure to all anesthetic agents in clinical use induces neurotoxicity and long-term adverse neurobehavioral deficits; however, whether these findings are applicable in human infants is unknown.6-9 Most of the studies in humans showing adverse outcomes have been retrospective observational studies subject to multiple sources of bias. Two recent large clinical studies—the GAS (General Anaesthesia compared to Spinal anaesthesia) trial10 and the PANDA (Pediatric Anesthesia and Neurodevelopment Assessment) study11—have shown no evidence of abnormal neurocognitive effects with a single brief exposure before 3 years of age (PANDA) or during infancy (GAS) in otherwise-healthy children.10,11
It is important to note that the FDA labeling change warning specifically stated that “[c]onsistent with animal studies, recent human data suggest that a single, relatively short exposure to general anesthetic and sedation drugs in infants or toddlers is unlikely to have negative effects on behavior or learning.” Moreover, the FDA emphasized that “Surgeries or procedures in children younger than 3 years should not be delayed or avoided when medically necessary.”12 Taking these points into consideration, we should offer our patients in-office care when possible and postpone elective procedures when advisable but proceed when necessary for our patients’ physical and emotional health.
- Juern AM, Cassidy LD, Lyon VB. More evidence confirming the safety of general anesthesia in pediatric dermatologic surgery. Pediatr Dermatol. 2010;27:355-360.
- Gerwels JW, Bezzant JL, Le Maire L, et al. Oral transmucosal fentanyl citrate premedication in patients undergoing outpatient dermatologic procedures. J Dermatol Surg Oncol. 1994;20:823-826.
- D’Acunto C, Raone B, Neri I, et al. Outpatient pediatric dermatologic surgery: experience in 296 patients. Pediatr Dermatol. 2015;32:424-426.
- Gunter JB. Benefit and risks of local anesthetics in infants and children. Paediatr Drugs. 2002;4:649-672.
- Kouba DJ, LoPiccolo MC, Alam M, et al. Guidelines for the use of local anesthesia in office-based dermatologic surgery [published online March 4, 2016]. J Am Acad Dermatol. 2016;74:1201-1219.
- Jevtovic-Todorovic V, Hartman RE, Izumi Y, et al. Early exposure to common anesthetic agents causes widespread neurodegeneration in the developing rat brain and persistent learning deficits. J Neurosci. 2003;23:876-882.
- Brambrink AM, Evers AS, Avidan MS, et al. Isoflurane-induced neuroapoptosis in the neonatal rhesus macaque brain. Anesthesiology. 2010;112:834-841.
- Raper J, Alvarado MC, Murphy KL, et al. Multiple anesthetic exposure in infant monkeys alters emotional reactivity to an acute stressor. Anesthesiology. 2015;123:1084-1092.
- Davidson AJ. Anesthesia and neurotoxicity to the developing brain: the clinical relevance. Paediatric Anaesthesia. 2011;21:716-721.
- Davidson AJ, Disma N, de Graaff JC, et al; GAS consortium. Neurodevelopmental outcome at 2 years of age after general anaesthesia and awake-regional anaesthesia in infancy (GAS): an international multicentre, randomised controlled trial. Lancet. 2016;387:239-250.
- Sun LS, Li G, Miller TL, et al. Association between a single general anesthesia exposure before age 36 months and neurocognitive outcomes in later childhood. JAMA. 2016;315:2312-2320.
- General anesthetic and sedation drugs: drug safety communication—new warnings for young children and pregnant women. US Food and Drug Administration website. https://www.fda.gov/safety/medwatch/safetyinformation/safetyalertsforhumanmedicalproducts/ucm533195.htm. Published December 14, 2016. Accessed July 25, 2017.
Clinicians who have attempted to perform an in-office procedure on infants or young children will recognize the difficulties that arise from the developmental inability to cooperate with procedures.1 Potential problems mentioned in the literature include but are not limited to anxiety, which is identified in all age groups of patients undergoing dermatologic procedures2; limitation of pain control3; and poor outcomes due to movement by the patient.1 In one author’s experience (N.B.S.), anxious and scared children can potentially cause injury to themselves, parents/guardians, and health care professionals by flailing and kicking; children are flexible and can wriggle out of even fine grips, and some children, especially toddlers, can be strong.
The usage of topical anesthetics can only give superficial anesthesia. They can ostensibly reduce pain and are useful for anesthesia of curettage, but their use is limited in infants and young children by the minimal amount of drug that is safe for application, as risks of absorption include methemoglobinemia and seizure
General anesthesia seems to be the best alternative due to associated amnesia of the events occurring including pain; immobilization and ability to produce more accurate biopsy sampling; better immobilization leading to superior cosmetic results; and reduced risk to patients, parents/guardians, and health care professionals from a flailing child. In the field of pediatric dermatology, general anesthesia often is used for excision of larger lesions and cosmetic repairs. Operating room privileges are not always easy to obtain, but many pediatric dermatologists take advantage of outpatient surgical centers associated with their medical center. A retrospective review of 226 children receiving 681 procedures at a single institution documented low rates of complications.1
If it was that easy, most children would be anesthetized with general anesthesia. However, there are risks associated with general anesthesia. Parents/guardians often will do what they can to avoid risk and may therefore refuse general anesthesia, but it is not completely avoidable in complicated skin disease. Despite the risks, the benefit is present in a major anomaly correction such as a cleft palate in a 6-month-old but may not be there for the treatment of a wart. When procedures are nonessential or may be conducted without anesthesia, avoidance of general anesthesia is reasonable and a combination of topical and local infiltrative anesthesia can help. In the American Academy of Dermatology guidelines on in-office anesthesia, Kouba et al5 states: “Topical agents are recommended as a first-line method of anesthesia for the repair of dermal lacerations in children and for other minor dermatologic procedures, including curettage. For skin biopsy, excision, or other cases where topical agents alone are insufficient, adjunctive use of topical anesthesia to lessen the discomfort of infiltrative anesthetic should be considered.”
A new controversy recently has emerged concerning the potential risks of anesthesia on neurocognitive development in infants and young children. These concerns regardingthe labeling changes of anesthetic and sedation drugs by the US Food and Drug Administration (FDA) in December 2016 specifically focused on these risks in children younger than 3 years with prolonged (>3 hours) and repeated exposures; however, this kind of exposure is unlikely with standard pediatric dermatologic procedures.6-9
There is compelling evidence from animal studies that exposure to all anesthetic agents in clinical use induces neurotoxicity and long-term adverse neurobehavioral deficits; however, whether these findings are applicable in human infants is unknown.6-9 Most of the studies in humans showing adverse outcomes have been retrospective observational studies subject to multiple sources of bias. Two recent large clinical studies—the GAS (General Anaesthesia compared to Spinal anaesthesia) trial10 and the PANDA (Pediatric Anesthesia and Neurodevelopment Assessment) study11—have shown no evidence of abnormal neurocognitive effects with a single brief exposure before 3 years of age (PANDA) or during infancy (GAS) in otherwise-healthy children.10,11
It is important to note that the FDA labeling change warning specifically stated that “[c]onsistent with animal studies, recent human data suggest that a single, relatively short exposure to general anesthetic and sedation drugs in infants or toddlers is unlikely to have negative effects on behavior or learning.” Moreover, the FDA emphasized that “Surgeries or procedures in children younger than 3 years should not be delayed or avoided when medically necessary.”12 Taking these points into consideration, we should offer our patients in-office care when possible and postpone elective procedures when advisable but proceed when necessary for our patients’ physical and emotional health.
Clinicians who have attempted to perform an in-office procedure on infants or young children will recognize the difficulties that arise from the developmental inability to cooperate with procedures.1 Potential problems mentioned in the literature include but are not limited to anxiety, which is identified in all age groups of patients undergoing dermatologic procedures2; limitation of pain control3; and poor outcomes due to movement by the patient.1 In one author’s experience (N.B.S.), anxious and scared children can potentially cause injury to themselves, parents/guardians, and health care professionals by flailing and kicking; children are flexible and can wriggle out of even fine grips, and some children, especially toddlers, can be strong.
The usage of topical anesthetics can only give superficial anesthesia. They can ostensibly reduce pain and are useful for anesthesia of curettage, but their use is limited in infants and young children by the minimal amount of drug that is safe for application, as risks of absorption include methemoglobinemia and seizure
General anesthesia seems to be the best alternative due to associated amnesia of the events occurring including pain; immobilization and ability to produce more accurate biopsy sampling; better immobilization leading to superior cosmetic results; and reduced risk to patients, parents/guardians, and health care professionals from a flailing child. In the field of pediatric dermatology, general anesthesia often is used for excision of larger lesions and cosmetic repairs. Operating room privileges are not always easy to obtain, but many pediatric dermatologists take advantage of outpatient surgical centers associated with their medical center. A retrospective review of 226 children receiving 681 procedures at a single institution documented low rates of complications.1
If it was that easy, most children would be anesthetized with general anesthesia. However, there are risks associated with general anesthesia. Parents/guardians often will do what they can to avoid risk and may therefore refuse general anesthesia, but it is not completely avoidable in complicated skin disease. Despite the risks, the benefit is present in a major anomaly correction such as a cleft palate in a 6-month-old but may not be there for the treatment of a wart. When procedures are nonessential or may be conducted without anesthesia, avoidance of general anesthesia is reasonable and a combination of topical and local infiltrative anesthesia can help. In the American Academy of Dermatology guidelines on in-office anesthesia, Kouba et al5 states: “Topical agents are recommended as a first-line method of anesthesia for the repair of dermal lacerations in children and for other minor dermatologic procedures, including curettage. For skin biopsy, excision, or other cases where topical agents alone are insufficient, adjunctive use of topical anesthesia to lessen the discomfort of infiltrative anesthetic should be considered.”
A new controversy recently has emerged concerning the potential risks of anesthesia on neurocognitive development in infants and young children. These concerns regardingthe labeling changes of anesthetic and sedation drugs by the US Food and Drug Administration (FDA) in December 2016 specifically focused on these risks in children younger than 3 years with prolonged (>3 hours) and repeated exposures; however, this kind of exposure is unlikely with standard pediatric dermatologic procedures.6-9
There is compelling evidence from animal studies that exposure to all anesthetic agents in clinical use induces neurotoxicity and long-term adverse neurobehavioral deficits; however, whether these findings are applicable in human infants is unknown.6-9 Most of the studies in humans showing adverse outcomes have been retrospective observational studies subject to multiple sources of bias. Two recent large clinical studies—the GAS (General Anaesthesia compared to Spinal anaesthesia) trial10 and the PANDA (Pediatric Anesthesia and Neurodevelopment Assessment) study11—have shown no evidence of abnormal neurocognitive effects with a single brief exposure before 3 years of age (PANDA) or during infancy (GAS) in otherwise-healthy children.10,11
It is important to note that the FDA labeling change warning specifically stated that “[c]onsistent with animal studies, recent human data suggest that a single, relatively short exposure to general anesthetic and sedation drugs in infants or toddlers is unlikely to have negative effects on behavior or learning.” Moreover, the FDA emphasized that “Surgeries or procedures in children younger than 3 years should not be delayed or avoided when medically necessary.”12 Taking these points into consideration, we should offer our patients in-office care when possible and postpone elective procedures when advisable but proceed when necessary for our patients’ physical and emotional health.
- Juern AM, Cassidy LD, Lyon VB. More evidence confirming the safety of general anesthesia in pediatric dermatologic surgery. Pediatr Dermatol. 2010;27:355-360.
- Gerwels JW, Bezzant JL, Le Maire L, et al. Oral transmucosal fentanyl citrate premedication in patients undergoing outpatient dermatologic procedures. J Dermatol Surg Oncol. 1994;20:823-826.
- D’Acunto C, Raone B, Neri I, et al. Outpatient pediatric dermatologic surgery: experience in 296 patients. Pediatr Dermatol. 2015;32:424-426.
- Gunter JB. Benefit and risks of local anesthetics in infants and children. Paediatr Drugs. 2002;4:649-672.
- Kouba DJ, LoPiccolo MC, Alam M, et al. Guidelines for the use of local anesthesia in office-based dermatologic surgery [published online March 4, 2016]. J Am Acad Dermatol. 2016;74:1201-1219.
- Jevtovic-Todorovic V, Hartman RE, Izumi Y, et al. Early exposure to common anesthetic agents causes widespread neurodegeneration in the developing rat brain and persistent learning deficits. J Neurosci. 2003;23:876-882.
- Brambrink AM, Evers AS, Avidan MS, et al. Isoflurane-induced neuroapoptosis in the neonatal rhesus macaque brain. Anesthesiology. 2010;112:834-841.
- Raper J, Alvarado MC, Murphy KL, et al. Multiple anesthetic exposure in infant monkeys alters emotional reactivity to an acute stressor. Anesthesiology. 2015;123:1084-1092.
- Davidson AJ. Anesthesia and neurotoxicity to the developing brain: the clinical relevance. Paediatric Anaesthesia. 2011;21:716-721.
- Davidson AJ, Disma N, de Graaff JC, et al; GAS consortium. Neurodevelopmental outcome at 2 years of age after general anaesthesia and awake-regional anaesthesia in infancy (GAS): an international multicentre, randomised controlled trial. Lancet. 2016;387:239-250.
- Sun LS, Li G, Miller TL, et al. Association between a single general anesthesia exposure before age 36 months and neurocognitive outcomes in later childhood. JAMA. 2016;315:2312-2320.
- General anesthetic and sedation drugs: drug safety communication—new warnings for young children and pregnant women. US Food and Drug Administration website. https://www.fda.gov/safety/medwatch/safetyinformation/safetyalertsforhumanmedicalproducts/ucm533195.htm. Published December 14, 2016. Accessed July 25, 2017.
- Juern AM, Cassidy LD, Lyon VB. More evidence confirming the safety of general anesthesia in pediatric dermatologic surgery. Pediatr Dermatol. 2010;27:355-360.
- Gerwels JW, Bezzant JL, Le Maire L, et al. Oral transmucosal fentanyl citrate premedication in patients undergoing outpatient dermatologic procedures. J Dermatol Surg Oncol. 1994;20:823-826.
- D’Acunto C, Raone B, Neri I, et al. Outpatient pediatric dermatologic surgery: experience in 296 patients. Pediatr Dermatol. 2015;32:424-426.
- Gunter JB. Benefit and risks of local anesthetics in infants and children. Paediatr Drugs. 2002;4:649-672.
- Kouba DJ, LoPiccolo MC, Alam M, et al. Guidelines for the use of local anesthesia in office-based dermatologic surgery [published online March 4, 2016]. J Am Acad Dermatol. 2016;74:1201-1219.
- Jevtovic-Todorovic V, Hartman RE, Izumi Y, et al. Early exposure to common anesthetic agents causes widespread neurodegeneration in the developing rat brain and persistent learning deficits. J Neurosci. 2003;23:876-882.
- Brambrink AM, Evers AS, Avidan MS, et al. Isoflurane-induced neuroapoptosis in the neonatal rhesus macaque brain. Anesthesiology. 2010;112:834-841.
- Raper J, Alvarado MC, Murphy KL, et al. Multiple anesthetic exposure in infant monkeys alters emotional reactivity to an acute stressor. Anesthesiology. 2015;123:1084-1092.
- Davidson AJ. Anesthesia and neurotoxicity to the developing brain: the clinical relevance. Paediatric Anaesthesia. 2011;21:716-721.
- Davidson AJ, Disma N, de Graaff JC, et al; GAS consortium. Neurodevelopmental outcome at 2 years of age after general anaesthesia and awake-regional anaesthesia in infancy (GAS): an international multicentre, randomised controlled trial. Lancet. 2016;387:239-250.
- Sun LS, Li G, Miller TL, et al. Association between a single general anesthesia exposure before age 36 months and neurocognitive outcomes in later childhood. JAMA. 2016;315:2312-2320.
- General anesthetic and sedation drugs: drug safety communication—new warnings for young children and pregnant women. US Food and Drug Administration website. https://www.fda.gov/safety/medwatch/safetyinformation/safetyalertsforhumanmedicalproducts/ucm533195.htm. Published December 14, 2016. Accessed July 25, 2017.
Pain frequency varies by employment status
Adults who were previously employed are twice as likely to report daily or almost-daily pain than are those who are currently employed, according to the Centers for Disease Control and Prevention.
In an ongoing survey, just over 30% of adults aged 18 years and older who were previously employed reported that they experienced pain on “most days or every day” in the past 6 months, compared with 15% of those who were currently employed and 19% of those classified as never employed, investigators from the CDC estimated (MMWR. 2017 Jul 28;66[29]:796).
Adults who were previously employed are twice as likely to report daily or almost-daily pain than are those who are currently employed, according to the Centers for Disease Control and Prevention.
In an ongoing survey, just over 30% of adults aged 18 years and older who were previously employed reported that they experienced pain on “most days or every day” in the past 6 months, compared with 15% of those who were currently employed and 19% of those classified as never employed, investigators from the CDC estimated (MMWR. 2017 Jul 28;66[29]:796).
Adults who were previously employed are twice as likely to report daily or almost-daily pain than are those who are currently employed, according to the Centers for Disease Control and Prevention.
In an ongoing survey, just over 30% of adults aged 18 years and older who were previously employed reported that they experienced pain on “most days or every day” in the past 6 months, compared with 15% of those who were currently employed and 19% of those classified as never employed, investigators from the CDC estimated (MMWR. 2017 Jul 28;66[29]:796).
FROM MMWR
Advances in Hematology and Oncology (August 2017)
Table of Contents
- The Challenges of Precision Medicine and New Advances in Molecular Diagnostic Testing in Hematolymphoid Malignancies: Impact on the VHA
- Systems Automation for Cancer Surveillance: A Lean Six Sigma Project for Tracking Care of Patients With Head and Neck Cancer
- Open Clinical Trials for Patients With Renal Cell Carcinoma
- Innovations Lead to More Targeted Prostate Cancer Treatments
- Depression Screening and Treatment: A Missed Opportunity in Lung Cancer Care
- Acute Leukemia of Ambiguous Lineage in Elderly Patients: A SEER Medicare Database Analysis
- Treatment and Management of Patients With Non-Small Cell Lung Cancer
- Do Erythropoiesis-Stimulating Agents Have a Risk Evaluation and Mitigation Strategy?

Table of Contents
- The Challenges of Precision Medicine and New Advances in Molecular Diagnostic Testing in Hematolymphoid Malignancies: Impact on the VHA
- Systems Automation for Cancer Surveillance: A Lean Six Sigma Project for Tracking Care of Patients With Head and Neck Cancer
- Open Clinical Trials for Patients With Renal Cell Carcinoma
- Innovations Lead to More Targeted Prostate Cancer Treatments
- Depression Screening and Treatment: A Missed Opportunity in Lung Cancer Care
- Acute Leukemia of Ambiguous Lineage in Elderly Patients: A SEER Medicare Database Analysis
- Treatment and Management of Patients With Non-Small Cell Lung Cancer
- Do Erythropoiesis-Stimulating Agents Have a Risk Evaluation and Mitigation Strategy?

Table of Contents
- The Challenges of Precision Medicine and New Advances in Molecular Diagnostic Testing in Hematolymphoid Malignancies: Impact on the VHA
- Systems Automation for Cancer Surveillance: A Lean Six Sigma Project for Tracking Care of Patients With Head and Neck Cancer
- Open Clinical Trials for Patients With Renal Cell Carcinoma
- Innovations Lead to More Targeted Prostate Cancer Treatments
- Depression Screening and Treatment: A Missed Opportunity in Lung Cancer Care
- Acute Leukemia of Ambiguous Lineage in Elderly Patients: A SEER Medicare Database Analysis
- Treatment and Management of Patients With Non-Small Cell Lung Cancer
- Do Erythropoiesis-Stimulating Agents Have a Risk Evaluation and Mitigation Strategy?