User login
Program reduces transfusions in leukemia, HSCT patients
New research suggests a patient blood management (PBM) program can safely reduce transfusion use in patients with acute leukemia and those undergoing hematopoietic stem cell transplant (HSCT).
The program significantly reduced the use of red blood cell (RBC) and platelet transfusions without increasing morbidity or mortality in patients who were receiving intensive chemotherapy to treat acute leukemia and in patients receiving an allogeneic or autologous HSCT.
“There has been a long-standing belief among hematologists that patients with leukemia undergoing chemotherapy should have a transfusion of red blood cells if their hemoglobin level drops below about 9 g/dL to help avoid adverse outcomes,” said study author Michael Leahy, MB ChB, a consultant hematologist at the University of Western Australia in Perth.
“Findings in this real-world, non-clinical trial setting challenge that belief.”
Dr Leahy and his colleagues published their findings in Transfusion.
The researchers said the PBM program used in this study was built around the “3 pillars” concept of PBM, which are:
- Optimize the patient’s RBC mass
- Minimize blood loss
- Harness and optimize the patient’s physiologic anemia reserve.
No specific transfusion thresholds were established. However, the hospitals did adopt a single-unit RBC transfusion policy for symptomatic anemic patients who were not actively bleeding.
Results
The study included 695 admissions to 2 major hospitals in Western Australia. Patients were admitted between July 2010 and December 2014 for treatment of acute leukemia or for autologous or allogeneic HSCT.
During this time, the patients received 3384 RBC units and 3639 units of platelets.
The mean number of platelet units transfused per hospital admission decreased 35% from baseline to the end of the study period, from 6.3 to 4.1 units (P<0.001).
The mean number of RBC units transfused decreased 39%, from 6.1 to 3.7 (P<0.001). Meanwhile, the use of single-unit RBC transfusions increased from 39% to 67% (P<0.001).
And the mean hemoglobin level prior to RBC transfusion decreased from 8.0 g/dL to 6.8 g/dL (P<0.001).
“This study suggests that patients undergoing chemotherapy with hematological disease may tolerate much lower levels of hemoglobin than previously thought,” said Shannon Farmer, an adjunct research fellow at the University of Western Australia.
“The transfusion threshold, the hemoglobin value at which a transfusion is given, dropped significantly from 8.0 g/dL at the beginning of the study to 6.8 g/dL at the end. This was associated with significant reductions in transfusion and substantial costs savings without evidence of harm to the patients. In fact, it was associated with a trend toward improved survival.”
The reduction in blood products over the study period resulted in a cost savings of AU$694,886 (US$654,007)—AU$389,537 (US$364,177) for RBCs and AU$305,349 (US$289,830) for platelets.
There were no significant changes over the study period in length of hospital stay, serious bleeding events, or in-hospital mortality.
There was a non-significant reduction in the mean length of hospital stay, from 24.5 days to 22.6 days (P=0.338). The difference was still not significant after the researchers adjusted for patient age, patient group, and comorbidities (incident rate ratio=0.88; 95% CI, 0.75-1.04).
The rate of serious bleeding increased over the study period, from 5.3% to 7.0% (P=0.582). After adjustment, the odds ratio was 1.14 (95% CI, 0.38-3.44; P=0.811).
There was a non-significant decrease in in-hospital mortality, from 5.3% to 2.0% (P=0.218). After adjustment, the odds ratio was 0.31 (95% CI, 0.06-1.56; P=0.154).
Based on these results, the researchers concluded that PBM programs could have a substantial impact in this patient population, reducing blood utilization and healthcare costs. ![]()
New research suggests a patient blood management (PBM) program can safely reduce transfusion use in patients with acute leukemia and those undergoing hematopoietic stem cell transplant (HSCT).
The program significantly reduced the use of red blood cell (RBC) and platelet transfusions without increasing morbidity or mortality in patients who were receiving intensive chemotherapy to treat acute leukemia and in patients receiving an allogeneic or autologous HSCT.
“There has been a long-standing belief among hematologists that patients with leukemia undergoing chemotherapy should have a transfusion of red blood cells if their hemoglobin level drops below about 9 g/dL to help avoid adverse outcomes,” said study author Michael Leahy, MB ChB, a consultant hematologist at the University of Western Australia in Perth.
“Findings in this real-world, non-clinical trial setting challenge that belief.”
Dr Leahy and his colleagues published their findings in Transfusion.
The researchers said the PBM program used in this study was built around the “3 pillars” concept of PBM, which are:
- Optimize the patient’s RBC mass
- Minimize blood loss
- Harness and optimize the patient’s physiologic anemia reserve.
No specific transfusion thresholds were established. However, the hospitals did adopt a single-unit RBC transfusion policy for symptomatic anemic patients who were not actively bleeding.
Results
The study included 695 admissions to 2 major hospitals in Western Australia. Patients were admitted between July 2010 and December 2014 for treatment of acute leukemia or for autologous or allogeneic HSCT.
During this time, the patients received 3384 RBC units and 3639 units of platelets.
The mean number of platelet units transfused per hospital admission decreased 35% from baseline to the end of the study period, from 6.3 to 4.1 units (P<0.001).
The mean number of RBC units transfused decreased 39%, from 6.1 to 3.7 (P<0.001). Meanwhile, the use of single-unit RBC transfusions increased from 39% to 67% (P<0.001).
And the mean hemoglobin level prior to RBC transfusion decreased from 8.0 g/dL to 6.8 g/dL (P<0.001).
“This study suggests that patients undergoing chemotherapy with hematological disease may tolerate much lower levels of hemoglobin than previously thought,” said Shannon Farmer, an adjunct research fellow at the University of Western Australia.
“The transfusion threshold, the hemoglobin value at which a transfusion is given, dropped significantly from 8.0 g/dL at the beginning of the study to 6.8 g/dL at the end. This was associated with significant reductions in transfusion and substantial costs savings without evidence of harm to the patients. In fact, it was associated with a trend toward improved survival.”
The reduction in blood products over the study period resulted in a cost savings of AU$694,886 (US$654,007)—AU$389,537 (US$364,177) for RBCs and AU$305,349 (US$289,830) for platelets.
There were no significant changes over the study period in length of hospital stay, serious bleeding events, or in-hospital mortality.
There was a non-significant reduction in the mean length of hospital stay, from 24.5 days to 22.6 days (P=0.338). The difference was still not significant after the researchers adjusted for patient age, patient group, and comorbidities (incident rate ratio=0.88; 95% CI, 0.75-1.04).
The rate of serious bleeding increased over the study period, from 5.3% to 7.0% (P=0.582). After adjustment, the odds ratio was 1.14 (95% CI, 0.38-3.44; P=0.811).
There was a non-significant decrease in in-hospital mortality, from 5.3% to 2.0% (P=0.218). After adjustment, the odds ratio was 0.31 (95% CI, 0.06-1.56; P=0.154).
Based on these results, the researchers concluded that PBM programs could have a substantial impact in this patient population, reducing blood utilization and healthcare costs. ![]()
New research suggests a patient blood management (PBM) program can safely reduce transfusion use in patients with acute leukemia and those undergoing hematopoietic stem cell transplant (HSCT).
The program significantly reduced the use of red blood cell (RBC) and platelet transfusions without increasing morbidity or mortality in patients who were receiving intensive chemotherapy to treat acute leukemia and in patients receiving an allogeneic or autologous HSCT.
“There has been a long-standing belief among hematologists that patients with leukemia undergoing chemotherapy should have a transfusion of red blood cells if their hemoglobin level drops below about 9 g/dL to help avoid adverse outcomes,” said study author Michael Leahy, MB ChB, a consultant hematologist at the University of Western Australia in Perth.
“Findings in this real-world, non-clinical trial setting challenge that belief.”
Dr Leahy and his colleagues published their findings in Transfusion.
The researchers said the PBM program used in this study was built around the “3 pillars” concept of PBM, which are:
- Optimize the patient’s RBC mass
- Minimize blood loss
- Harness and optimize the patient’s physiologic anemia reserve.
No specific transfusion thresholds were established. However, the hospitals did adopt a single-unit RBC transfusion policy for symptomatic anemic patients who were not actively bleeding.
Results
The study included 695 admissions to 2 major hospitals in Western Australia. Patients were admitted between July 2010 and December 2014 for treatment of acute leukemia or for autologous or allogeneic HSCT.
During this time, the patients received 3384 RBC units and 3639 units of platelets.
The mean number of platelet units transfused per hospital admission decreased 35% from baseline to the end of the study period, from 6.3 to 4.1 units (P<0.001).
The mean number of RBC units transfused decreased 39%, from 6.1 to 3.7 (P<0.001). Meanwhile, the use of single-unit RBC transfusions increased from 39% to 67% (P<0.001).
And the mean hemoglobin level prior to RBC transfusion decreased from 8.0 g/dL to 6.8 g/dL (P<0.001).
“This study suggests that patients undergoing chemotherapy with hematological disease may tolerate much lower levels of hemoglobin than previously thought,” said Shannon Farmer, an adjunct research fellow at the University of Western Australia.
“The transfusion threshold, the hemoglobin value at which a transfusion is given, dropped significantly from 8.0 g/dL at the beginning of the study to 6.8 g/dL at the end. This was associated with significant reductions in transfusion and substantial costs savings without evidence of harm to the patients. In fact, it was associated with a trend toward improved survival.”
The reduction in blood products over the study period resulted in a cost savings of AU$694,886 (US$654,007)—AU$389,537 (US$364,177) for RBCs and AU$305,349 (US$289,830) for platelets.
There were no significant changes over the study period in length of hospital stay, serious bleeding events, or in-hospital mortality.
There was a non-significant reduction in the mean length of hospital stay, from 24.5 days to 22.6 days (P=0.338). The difference was still not significant after the researchers adjusted for patient age, patient group, and comorbidities (incident rate ratio=0.88; 95% CI, 0.75-1.04).
The rate of serious bleeding increased over the study period, from 5.3% to 7.0% (P=0.582). After adjustment, the odds ratio was 1.14 (95% CI, 0.38-3.44; P=0.811).
There was a non-significant decrease in in-hospital mortality, from 5.3% to 2.0% (P=0.218). After adjustment, the odds ratio was 0.31 (95% CI, 0.06-1.56; P=0.154).
Based on these results, the researchers concluded that PBM programs could have a substantial impact in this patient population, reducing blood utilization and healthcare costs. ![]()
Company resubmits BLA for andexanet alfa
Portola Pharmaceuticals Inc. has resubmitted its biologics license application (BLA) for andexanet alfa (AndexXa®) to the US Food and Drug Administration (FDA).
With this BLA, Portola is seeking approval of andexanet alfa for reversal of the anticoagulant effects of apixaban and rivaroxaban in patients experiencing uncontrolled or life-threatening bleeding.
The resubmission includes supplemental information requested by the FDA in a complete response letter issued in August 2016.
At that time, Portola was seeking approval for andexanet alfa in patients treated with apixaban, rivaroxaban, edoxaban, or enoxaparin when reversal of anticoagulation is needed.
However, the FDA said it could not approve andexanet alfa for that indication. The FDA requested that Portola provide information related to manufacturing of andexanet alfa as well as additional data to support the inclusion of edoxaban and enoxaparin on andexanet alfa’s label.
The initial BLA for andexanet alfa included data from a pair of phase 3 studies—ANNEXA-A and ANNEXA-R—which were designed to assess andexanet alfa’s ability to reverse the effects of apixaban and rivaroxaban—but not edoxaban or enoxaparin—in healthy volunteers.
Results from ANNEXA-A and ANNEXA-R were published in NEJM in 2015.
The BLA also included limited adjudicated efficacy and safety data from patients enrolled in the phase 3b/4 ANNEXA-4 study. This ongoing study is enrolling patients receiving apixaban, rivaroxaban, edoxaban, and enoxaparin who present with an acute major bleed.
Preliminary results from ANNEXA-4 were presented at ESC Congress 2016 and published in NEJM. ![]()
Portola Pharmaceuticals Inc. has resubmitted its biologics license application (BLA) for andexanet alfa (AndexXa®) to the US Food and Drug Administration (FDA).
With this BLA, Portola is seeking approval of andexanet alfa for reversal of the anticoagulant effects of apixaban and rivaroxaban in patients experiencing uncontrolled or life-threatening bleeding.
The resubmission includes supplemental information requested by the FDA in a complete response letter issued in August 2016.
At that time, Portola was seeking approval for andexanet alfa in patients treated with apixaban, rivaroxaban, edoxaban, or enoxaparin when reversal of anticoagulation is needed.
However, the FDA said it could not approve andexanet alfa for that indication. The FDA requested that Portola provide information related to manufacturing of andexanet alfa as well as additional data to support the inclusion of edoxaban and enoxaparin on andexanet alfa’s label.
The initial BLA for andexanet alfa included data from a pair of phase 3 studies—ANNEXA-A and ANNEXA-R—which were designed to assess andexanet alfa’s ability to reverse the effects of apixaban and rivaroxaban—but not edoxaban or enoxaparin—in healthy volunteers.
Results from ANNEXA-A and ANNEXA-R were published in NEJM in 2015.
The BLA also included limited adjudicated efficacy and safety data from patients enrolled in the phase 3b/4 ANNEXA-4 study. This ongoing study is enrolling patients receiving apixaban, rivaroxaban, edoxaban, and enoxaparin who present with an acute major bleed.
Preliminary results from ANNEXA-4 were presented at ESC Congress 2016 and published in NEJM. ![]()
Portola Pharmaceuticals Inc. has resubmitted its biologics license application (BLA) for andexanet alfa (AndexXa®) to the US Food and Drug Administration (FDA).
With this BLA, Portola is seeking approval of andexanet alfa for reversal of the anticoagulant effects of apixaban and rivaroxaban in patients experiencing uncontrolled or life-threatening bleeding.
The resubmission includes supplemental information requested by the FDA in a complete response letter issued in August 2016.
At that time, Portola was seeking approval for andexanet alfa in patients treated with apixaban, rivaroxaban, edoxaban, or enoxaparin when reversal of anticoagulation is needed.
However, the FDA said it could not approve andexanet alfa for that indication. The FDA requested that Portola provide information related to manufacturing of andexanet alfa as well as additional data to support the inclusion of edoxaban and enoxaparin on andexanet alfa’s label.
The initial BLA for andexanet alfa included data from a pair of phase 3 studies—ANNEXA-A and ANNEXA-R—which were designed to assess andexanet alfa’s ability to reverse the effects of apixaban and rivaroxaban—but not edoxaban or enoxaparin—in healthy volunteers.
Results from ANNEXA-A and ANNEXA-R were published in NEJM in 2015.
The BLA also included limited adjudicated efficacy and safety data from patients enrolled in the phase 3b/4 ANNEXA-4 study. This ongoing study is enrolling patients receiving apixaban, rivaroxaban, edoxaban, and enoxaparin who present with an acute major bleed.
Preliminary results from ANNEXA-4 were presented at ESC Congress 2016 and published in NEJM. ![]()
Less than half of office visits involve primary care
, according to the National Center for Health Statistics.
Primary care physicians’ share of office visits fell from 66.2% in 1980 to 49.1% in 2013, the NCHS reported in “Health, United States, 2016.” The corresponding increase among specialty care physicians gave them a total of 50.9% of all office visits in 2013, up from 33.8% in 1980.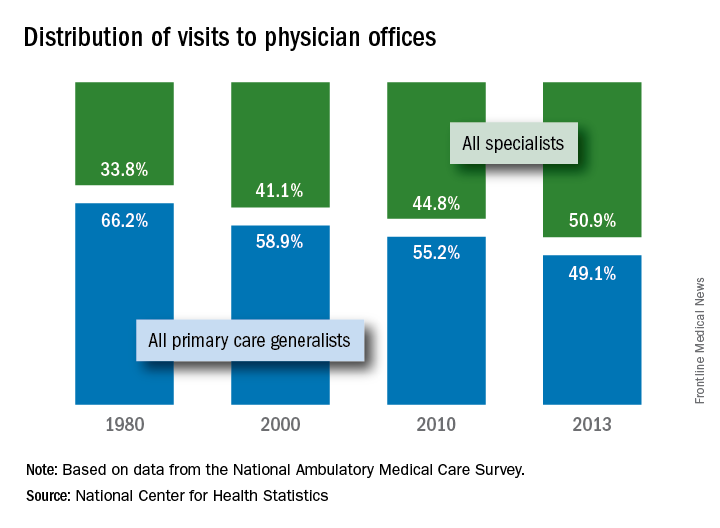
The NCHS estimates are based on data collected by the National Ambulatory Medical Care Survey, which excluded Alaska and Hawaii in 1980.
, according to the National Center for Health Statistics.
Primary care physicians’ share of office visits fell from 66.2% in 1980 to 49.1% in 2013, the NCHS reported in “Health, United States, 2016.” The corresponding increase among specialty care physicians gave them a total of 50.9% of all office visits in 2013, up from 33.8% in 1980.
The NCHS estimates are based on data collected by the National Ambulatory Medical Care Survey, which excluded Alaska and Hawaii in 1980.
, according to the National Center for Health Statistics.
Primary care physicians’ share of office visits fell from 66.2% in 1980 to 49.1% in 2013, the NCHS reported in “Health, United States, 2016.” The corresponding increase among specialty care physicians gave them a total of 50.9% of all office visits in 2013, up from 33.8% in 1980.
The NCHS estimates are based on data collected by the National Ambulatory Medical Care Survey, which excluded Alaska and Hawaii in 1980.
Sodium fusidate noninferior to linezolid for acute skin infections
NEW ORLEANS – An oral antibiotic in development in the United States, fusidic acid (oral formulation, sodium fusidate) was noninferior to linezolid based on early clinical response in a randomized, double-blind, multicenter trial of 716 people with acute bacterial skin and skin structure infections (ABSSSI), including cellulitis, wound infection, and major cutaneous abscesses.
Early clinical response was defined as a 20% or greater reduction from baseline in the surface area of redness, edema, or induration at 48-72 hours after starting treatment with the study drugs. In an intent-to-treat analysis, 87.2% of patients randomized to fusidic acid and 86.6% of the linezolid group met this primary endpoint of the phase 3 study.
“Fusidic acid showed similar efficacy and comparable safety” that persisted through treatment, said Andy Strayer, PharmD, vice president of clinical programs at Cempra Pharmaceuticals, which is developing sodium fusidate as an oral agent to treat ABSSSI patients in the United States. Leo Pharmaceuticals has marketed sodium fusidate outside the United States in various formulations for decades.
Fusidic acid has potent activity against gram-positive aerobic organisms, including methicillin-resistant Staphylococcus aureus (MRSA). “Strikingly, fusidic acid showed 100% success in patients with MRSA in the microbiologically evaluable population at the end of treatment and posttherapy evaluation time points,” Dr. Strayer said at the annual meeting of the American Society for Microbiology. “Fusidic acid may offer an important oral therapy alternative for MRSA infection.”
“Fusidic acid, a drug long used in other parts of the world, has been demonstrated in this first phase 3 trial, to be a potential new option for the treatment of MRSA skin and skin structure infections in the U.S.,” said Carrie Cardenas, MD, lead study author and a principal investigator at eStudySite, San Diego, and an internist in private practice in La Mesa, California.
There was a microbiological diagnosis established in 75% of patients. S. aureus was the most commonly detected pathogen (422 patients; 59%), and the study included 235 patients diagnosed with MRSA infection.
About two-thirds, 65%, of participants were men. Mean age was 45 years. Infections were classified as wounds in 61%, cellulitis in 26%, and abscess in 13%. Notably, 68% of the recruited participants had ABSSSI associated with intravenous drug use, a “sometimes overlooked consequence of the ongoing epidemic of IV drug use in the U.S.,” Dr. Strayer said.
In terms of safety, treatment-emergent adverse event rates were comparable between the two groups (37.9% with fusidic acid versus 36.1% with linezolid). Gastrointestinal events were the most common adverse events, 22.8% versus 18.2%, respectively.
“Considering complicated skin infections are one of the most rapidly growing reasons for hospitalizations and emergency department visits each year, we anticipate that fusidic acid, if approved, may help clinicians decrease the length of inpatient stay or avoid hospitalization altogether,” Dr. Strayer said.
Cempra sponsored the study. Dr. Strayer is a Cempra employee and shareholder. Dr. Carrie Cardenas is a principal investigator at eStudySite, San Diego, and performs research for Cempra, Paratek, Debiopharm, Motif, Durata, MicuRx, Bristol-Myers Squibb, and Bayer.
NEW ORLEANS – An oral antibiotic in development in the United States, fusidic acid (oral formulation, sodium fusidate) was noninferior to linezolid based on early clinical response in a randomized, double-blind, multicenter trial of 716 people with acute bacterial skin and skin structure infections (ABSSSI), including cellulitis, wound infection, and major cutaneous abscesses.
Early clinical response was defined as a 20% or greater reduction from baseline in the surface area of redness, edema, or induration at 48-72 hours after starting treatment with the study drugs. In an intent-to-treat analysis, 87.2% of patients randomized to fusidic acid and 86.6% of the linezolid group met this primary endpoint of the phase 3 study.
“Fusidic acid showed similar efficacy and comparable safety” that persisted through treatment, said Andy Strayer, PharmD, vice president of clinical programs at Cempra Pharmaceuticals, which is developing sodium fusidate as an oral agent to treat ABSSSI patients in the United States. Leo Pharmaceuticals has marketed sodium fusidate outside the United States in various formulations for decades.
Fusidic acid has potent activity against gram-positive aerobic organisms, including methicillin-resistant Staphylococcus aureus (MRSA). “Strikingly, fusidic acid showed 100% success in patients with MRSA in the microbiologically evaluable population at the end of treatment and posttherapy evaluation time points,” Dr. Strayer said at the annual meeting of the American Society for Microbiology. “Fusidic acid may offer an important oral therapy alternative for MRSA infection.”
“Fusidic acid, a drug long used in other parts of the world, has been demonstrated in this first phase 3 trial, to be a potential new option for the treatment of MRSA skin and skin structure infections in the U.S.,” said Carrie Cardenas, MD, lead study author and a principal investigator at eStudySite, San Diego, and an internist in private practice in La Mesa, California.
There was a microbiological diagnosis established in 75% of patients. S. aureus was the most commonly detected pathogen (422 patients; 59%), and the study included 235 patients diagnosed with MRSA infection.
About two-thirds, 65%, of participants were men. Mean age was 45 years. Infections were classified as wounds in 61%, cellulitis in 26%, and abscess in 13%. Notably, 68% of the recruited participants had ABSSSI associated with intravenous drug use, a “sometimes overlooked consequence of the ongoing epidemic of IV drug use in the U.S.,” Dr. Strayer said.
In terms of safety, treatment-emergent adverse event rates were comparable between the two groups (37.9% with fusidic acid versus 36.1% with linezolid). Gastrointestinal events were the most common adverse events, 22.8% versus 18.2%, respectively.
“Considering complicated skin infections are one of the most rapidly growing reasons for hospitalizations and emergency department visits each year, we anticipate that fusidic acid, if approved, may help clinicians decrease the length of inpatient stay or avoid hospitalization altogether,” Dr. Strayer said.
Cempra sponsored the study. Dr. Strayer is a Cempra employee and shareholder. Dr. Carrie Cardenas is a principal investigator at eStudySite, San Diego, and performs research for Cempra, Paratek, Debiopharm, Motif, Durata, MicuRx, Bristol-Myers Squibb, and Bayer.
NEW ORLEANS – An oral antibiotic in development in the United States, fusidic acid (oral formulation, sodium fusidate) was noninferior to linezolid based on early clinical response in a randomized, double-blind, multicenter trial of 716 people with acute bacterial skin and skin structure infections (ABSSSI), including cellulitis, wound infection, and major cutaneous abscesses.
Early clinical response was defined as a 20% or greater reduction from baseline in the surface area of redness, edema, or induration at 48-72 hours after starting treatment with the study drugs. In an intent-to-treat analysis, 87.2% of patients randomized to fusidic acid and 86.6% of the linezolid group met this primary endpoint of the phase 3 study.
“Fusidic acid showed similar efficacy and comparable safety” that persisted through treatment, said Andy Strayer, PharmD, vice president of clinical programs at Cempra Pharmaceuticals, which is developing sodium fusidate as an oral agent to treat ABSSSI patients in the United States. Leo Pharmaceuticals has marketed sodium fusidate outside the United States in various formulations for decades.
Fusidic acid has potent activity against gram-positive aerobic organisms, including methicillin-resistant Staphylococcus aureus (MRSA). “Strikingly, fusidic acid showed 100% success in patients with MRSA in the microbiologically evaluable population at the end of treatment and posttherapy evaluation time points,” Dr. Strayer said at the annual meeting of the American Society for Microbiology. “Fusidic acid may offer an important oral therapy alternative for MRSA infection.”
“Fusidic acid, a drug long used in other parts of the world, has been demonstrated in this first phase 3 trial, to be a potential new option for the treatment of MRSA skin and skin structure infections in the U.S.,” said Carrie Cardenas, MD, lead study author and a principal investigator at eStudySite, San Diego, and an internist in private practice in La Mesa, California.
There was a microbiological diagnosis established in 75% of patients. S. aureus was the most commonly detected pathogen (422 patients; 59%), and the study included 235 patients diagnosed with MRSA infection.
About two-thirds, 65%, of participants were men. Mean age was 45 years. Infections were classified as wounds in 61%, cellulitis in 26%, and abscess in 13%. Notably, 68% of the recruited participants had ABSSSI associated with intravenous drug use, a “sometimes overlooked consequence of the ongoing epidemic of IV drug use in the U.S.,” Dr. Strayer said.
In terms of safety, treatment-emergent adverse event rates were comparable between the two groups (37.9% with fusidic acid versus 36.1% with linezolid). Gastrointestinal events were the most common adverse events, 22.8% versus 18.2%, respectively.
“Considering complicated skin infections are one of the most rapidly growing reasons for hospitalizations and emergency department visits each year, we anticipate that fusidic acid, if approved, may help clinicians decrease the length of inpatient stay or avoid hospitalization altogether,” Dr. Strayer said.
Cempra sponsored the study. Dr. Strayer is a Cempra employee and shareholder. Dr. Carrie Cardenas is a principal investigator at eStudySite, San Diego, and performs research for Cempra, Paratek, Debiopharm, Motif, Durata, MicuRx, Bristol-Myers Squibb, and Bayer.
AT ASM MICROBE 2017
Key clinical point: Sodium fusidate, active as fusidic acid, showed noninferiority to linezolid for early clinical response in ABSSI patients.
Major finding: 87.2% of patients given sodium fusidate and 86.6% of those receiving linezolid achieved an early clinical response.
Data source: Randomized, controlled, double-blind, phase 3 study with 716 participants.
Disclosures: Cempra sponsored the study. Dr. Carrier Cardenas is a researcher for Cempra, Paratek, Debiopharm, Motif, Durata, MicuRx, Bristol-Myers Squibb, and Bayer. Dr. Strayer is a Cempra employee and shareholder.
For acute gout, corticosteroids look safer than NSAIDs
For the treatment of acute gout, corticosteroids may be as effective as nonsteroidal anti-inflammatory drugs but with fewer side effects, based on findings from a meta-analysis of six randomized, controlled trials.
“There is insufficient information to determine the comparative efficacy of corticosteroids and NSAID[s] to treat acute gout, while corticosteroids appear to have a more favorable safety profile for selected [adverse events],” Christy A. Billy, MD, of the University of Sydney and her coauthors wrote in their report published online in the Journal of Rheumatology (J Rheumatol. 2017 Aug. doi: 10.3899/jrheum.170137).
Two previous systematic reviews also suggested that corticosteroids may be therapeutically equivalent to but safer than NSAIDs, but both were based on a very small number of available studies and suffered from statistical between-trial heterogeneity.
The meta-analysis of six trials included a total of 817 patients. The trials had a mean follow-up of 15 days. Two trials were in hospitalized patients, two involved patients in the emergency department, one included outpatients, and one did not disclose the location of clinical presentation. Mean age of participants ranged from 44 years to 65.9 years, and the proportion of men ranged from 70% to 100%.
With respect to pain scores, the researchers found no significant difference between corticosteroid and NSAIDs within 7 days of treatment based on moderate-quality evidence from two randomized, controlled trials (RCTs) involving 534 patients (standardized mean difference = –0.09; 95% confidence interval, –0.26 to 0.08). There was also no difference between the two on pain after 7 or more days based on low-quality evidence from two RCTs of 506 patients (SMD = 0.32; 95% CI, –0.27 to 0.92). There was no evidence of statistical heterogeneity in the short-term trials, but there was evidence of significant heterogeneity in trials measuring treatment effects for 7 days or longer (P = .01; I2 [heterogeneity] = 85%).
Two RCTs of 173 patients gave low-quality evidence to show no difference between corticosteroids and NSAIDs in the rate of treatment response in the short term (relative risk, 1.07; 95% CI, 0.80-1.44; moderate heterogeneity, P = .15, I2 = 53%). One long-term study of the rate of treatment response provided similar results. There were also no between-group differences in joint swelling, erythema, tenderness, or activity limitations.
The investigators discovered that patients who took corticosteroids had a lower risk of indigestion in three RCTs with 526 patients (RR, 0.50; 95% CI, 0.27-0.92), nausea in three RCTs of 566 patients (RR, 0.25; 95% CI, 0.11-0.54), and vomiting in two RCTs totaling 506 patients (RR, 0.11; 95% CI, 0.02-0.56).
Patients taking corticosteroids had a higher risk of rash in two RCTs of 506 patients (RR, 4.62; 95% CI, 1.34-15.97). There was statistically significant heterogeneity in summary effects of treatment for total adverse events across all six studies (P = .04; I2 = 56%).
This meta-analysis was limited by the small number of clinical trials available for inclusion, which prevented the estimate of a number of outcomes and subgroup analyses. There was also a high risk of bias in many of the studies. Only half of the studies confirmed the diagnosis of gout by the presence of monosodium urate crystals within joint spaces. No studies reported on the effects of treatment on kidney function or injury.
The authors disclosed no source of funding or financial relationships.
For the treatment of acute gout, corticosteroids may be as effective as nonsteroidal anti-inflammatory drugs but with fewer side effects, based on findings from a meta-analysis of six randomized, controlled trials.
“There is insufficient information to determine the comparative efficacy of corticosteroids and NSAID[s] to treat acute gout, while corticosteroids appear to have a more favorable safety profile for selected [adverse events],” Christy A. Billy, MD, of the University of Sydney and her coauthors wrote in their report published online in the Journal of Rheumatology (J Rheumatol. 2017 Aug. doi: 10.3899/jrheum.170137).
Two previous systematic reviews also suggested that corticosteroids may be therapeutically equivalent to but safer than NSAIDs, but both were based on a very small number of available studies and suffered from statistical between-trial heterogeneity.
The meta-analysis of six trials included a total of 817 patients. The trials had a mean follow-up of 15 days. Two trials were in hospitalized patients, two involved patients in the emergency department, one included outpatients, and one did not disclose the location of clinical presentation. Mean age of participants ranged from 44 years to 65.9 years, and the proportion of men ranged from 70% to 100%.
With respect to pain scores, the researchers found no significant difference between corticosteroid and NSAIDs within 7 days of treatment based on moderate-quality evidence from two randomized, controlled trials (RCTs) involving 534 patients (standardized mean difference = –0.09; 95% confidence interval, –0.26 to 0.08). There was also no difference between the two on pain after 7 or more days based on low-quality evidence from two RCTs of 506 patients (SMD = 0.32; 95% CI, –0.27 to 0.92). There was no evidence of statistical heterogeneity in the short-term trials, but there was evidence of significant heterogeneity in trials measuring treatment effects for 7 days or longer (P = .01; I2 [heterogeneity] = 85%).
Two RCTs of 173 patients gave low-quality evidence to show no difference between corticosteroids and NSAIDs in the rate of treatment response in the short term (relative risk, 1.07; 95% CI, 0.80-1.44; moderate heterogeneity, P = .15, I2 = 53%). One long-term study of the rate of treatment response provided similar results. There were also no between-group differences in joint swelling, erythema, tenderness, or activity limitations.
The investigators discovered that patients who took corticosteroids had a lower risk of indigestion in three RCTs with 526 patients (RR, 0.50; 95% CI, 0.27-0.92), nausea in three RCTs of 566 patients (RR, 0.25; 95% CI, 0.11-0.54), and vomiting in two RCTs totaling 506 patients (RR, 0.11; 95% CI, 0.02-0.56).
Patients taking corticosteroids had a higher risk of rash in two RCTs of 506 patients (RR, 4.62; 95% CI, 1.34-15.97). There was statistically significant heterogeneity in summary effects of treatment for total adverse events across all six studies (P = .04; I2 = 56%).
This meta-analysis was limited by the small number of clinical trials available for inclusion, which prevented the estimate of a number of outcomes and subgroup analyses. There was also a high risk of bias in many of the studies. Only half of the studies confirmed the diagnosis of gout by the presence of monosodium urate crystals within joint spaces. No studies reported on the effects of treatment on kidney function or injury.
The authors disclosed no source of funding or financial relationships.
For the treatment of acute gout, corticosteroids may be as effective as nonsteroidal anti-inflammatory drugs but with fewer side effects, based on findings from a meta-analysis of six randomized, controlled trials.
“There is insufficient information to determine the comparative efficacy of corticosteroids and NSAID[s] to treat acute gout, while corticosteroids appear to have a more favorable safety profile for selected [adverse events],” Christy A. Billy, MD, of the University of Sydney and her coauthors wrote in their report published online in the Journal of Rheumatology (J Rheumatol. 2017 Aug. doi: 10.3899/jrheum.170137).
Two previous systematic reviews also suggested that corticosteroids may be therapeutically equivalent to but safer than NSAIDs, but both were based on a very small number of available studies and suffered from statistical between-trial heterogeneity.
The meta-analysis of six trials included a total of 817 patients. The trials had a mean follow-up of 15 days. Two trials were in hospitalized patients, two involved patients in the emergency department, one included outpatients, and one did not disclose the location of clinical presentation. Mean age of participants ranged from 44 years to 65.9 years, and the proportion of men ranged from 70% to 100%.
With respect to pain scores, the researchers found no significant difference between corticosteroid and NSAIDs within 7 days of treatment based on moderate-quality evidence from two randomized, controlled trials (RCTs) involving 534 patients (standardized mean difference = –0.09; 95% confidence interval, –0.26 to 0.08). There was also no difference between the two on pain after 7 or more days based on low-quality evidence from two RCTs of 506 patients (SMD = 0.32; 95% CI, –0.27 to 0.92). There was no evidence of statistical heterogeneity in the short-term trials, but there was evidence of significant heterogeneity in trials measuring treatment effects for 7 days or longer (P = .01; I2 [heterogeneity] = 85%).
Two RCTs of 173 patients gave low-quality evidence to show no difference between corticosteroids and NSAIDs in the rate of treatment response in the short term (relative risk, 1.07; 95% CI, 0.80-1.44; moderate heterogeneity, P = .15, I2 = 53%). One long-term study of the rate of treatment response provided similar results. There were also no between-group differences in joint swelling, erythema, tenderness, or activity limitations.
The investigators discovered that patients who took corticosteroids had a lower risk of indigestion in three RCTs with 526 patients (RR, 0.50; 95% CI, 0.27-0.92), nausea in three RCTs of 566 patients (RR, 0.25; 95% CI, 0.11-0.54), and vomiting in two RCTs totaling 506 patients (RR, 0.11; 95% CI, 0.02-0.56).
Patients taking corticosteroids had a higher risk of rash in two RCTs of 506 patients (RR, 4.62; 95% CI, 1.34-15.97). There was statistically significant heterogeneity in summary effects of treatment for total adverse events across all six studies (P = .04; I2 = 56%).
This meta-analysis was limited by the small number of clinical trials available for inclusion, which prevented the estimate of a number of outcomes and subgroup analyses. There was also a high risk of bias in many of the studies. Only half of the studies confirmed the diagnosis of gout by the presence of monosodium urate crystals within joint spaces. No studies reported on the effects of treatment on kidney function or injury.
The authors disclosed no source of funding or financial relationships.
FROM JOURNAL OF RHEUMATOLOGY
Key clinical point: Corticosteroids had a similar efficacy but a better safety profile than NSAIDs.
Major finding: Corticosteroids had efficacy similar to NSAIDs in the treatment of acute gout.
Data source: Meta-analysis of six randomized, controlled trials (n = 817).
Disclosures: The authors disclosed no source of funding or financial relationships.
Depression, PTSD double risk of dementia for older female veterans
LONDON – Women veterans with either depression or post-traumatic stress disorder face a doubling in their risk of dementia – and having both increases the risk even more, Dr. Kristine Yaffe reported at the Alzheimer’s Association International Conference.*
The risk ratios for incident dementia that Dr. Yaffe of the University of California, San Francisco, and her colleagues calculated from their analysis of a cohort of 149,000 older female veterans in the national Veterans Health Administration (VHA) database remained unchanged even when they adjusted for age, education, medical comorbidities, and other confounders.
Not only are older women veterans a growing group; they are frequently diagnosed with mental health disorders. In 2012, 45% of women veteran patients in the VHA had a mental health condition, Dr. Yaffe noted.
“Over 9% of all veterans in the U.S. are women, accounting for more than 2 million women veterans. And 30% of those are more than 55 years old. Additionally, the number of women utilizing the Veterans Healthcare Administration system has nearly doubled in the last decade.”
The study of the impact of depression and PTSD on incident dementia is the first of its kind, Dr. Yaffe noted. The cohort comprised women without dementia who had at least two VHA visits during 2005-2015. They were followed for a mean of 5 years. A diagnosis of depression or PTSD had to occur during a 2-year baseline period. Confounders considered in the analysis were demographics, medical comorbidities, and health habits, including alcohol and tobacco use. The primary outcome was time to incident dementia.
At baseline, the group was a mean of 67 years old. Most subjects (70%) were white. Hypertension was common (46%), as was diabetes (16%). About 6% had cardiovascular disease. Depression was present in 18% and PTSD in 4%.
When parsed by diagnosis, there were some significant between-group differences at baseline. Women with depression or PTSD were younger than those without (65 and 63 vs. 67 years). Women who had both disorders were the youngest group, at 62 years.
Hypertension was least common in women without depression or PTSD (41%), and most common among those with depression (65%). Diabetes was almost more common among women with depression than among those without (24% vs. 14%).
Dr. Yaffe created two regression analyses. Model 1 controlled for age, race, and education. Model 2 controlled for the factors in Model 1, plus diabetes, hypertension, and cardiovascular disease.
By the end of follow-up, 4% of the group had developed dementia. The presence of depression approximately doubled the risk of dementia (hazard ratio, 2.14), compared with women who had neither depression nor PTSD. This risk was virtually unchanged in both Model 1 and Model 2 (HRs, 2.12 and 2.00).
The risk associated with PTSD was quite similar, increasing the risk of dementia twofold (HR, 2.19). Again, this was similar after controlling for the confounders in both Model 1 (HR, 2.20) and Model 2 (HR, 2.16).
Women with both depression and PTSD had almost a tripling of risk for dementia (HR, 2.71). Adjustment for confounders did not significantly alter this risk, either in Model 1 (HR, 2.59) or Model 2 (HR, 2.42).
“A question that often comes up in these types of studies is, ‘Is this a reverse causation?’ ” Dr. Yaffe said. “In other words, are people with dementia somehow getting more depression? We conducted a lag-time analysis that allowed a 2-year lag time for dementia, and also adjusted for the number of clinic visits. The results were almost identical.”
“This consistent doubling of risk is quite high,” Dr. Yaffe said. “In our prior work with male veterans, we didn’t see this robust an association.”
The study was funded by the Department of Defense and the National Institutes of Health. Dr. Yaffe had no financial disclosures.
Correction, 8/7/17: An earlier version of this article misstated Dr. Kristine Yaffe's degree.
msullivan@frontlinemedcom.com
On Twitter @alz_gal
LONDON – Women veterans with either depression or post-traumatic stress disorder face a doubling in their risk of dementia – and having both increases the risk even more, Dr. Kristine Yaffe reported at the Alzheimer’s Association International Conference.*
The risk ratios for incident dementia that Dr. Yaffe of the University of California, San Francisco, and her colleagues calculated from their analysis of a cohort of 149,000 older female veterans in the national Veterans Health Administration (VHA) database remained unchanged even when they adjusted for age, education, medical comorbidities, and other confounders.
Not only are older women veterans a growing group; they are frequently diagnosed with mental health disorders. In 2012, 45% of women veteran patients in the VHA had a mental health condition, Dr. Yaffe noted.
“Over 9% of all veterans in the U.S. are women, accounting for more than 2 million women veterans. And 30% of those are more than 55 years old. Additionally, the number of women utilizing the Veterans Healthcare Administration system has nearly doubled in the last decade.”
The study of the impact of depression and PTSD on incident dementia is the first of its kind, Dr. Yaffe noted. The cohort comprised women without dementia who had at least two VHA visits during 2005-2015. They were followed for a mean of 5 years. A diagnosis of depression or PTSD had to occur during a 2-year baseline period. Confounders considered in the analysis were demographics, medical comorbidities, and health habits, including alcohol and tobacco use. The primary outcome was time to incident dementia.
At baseline, the group was a mean of 67 years old. Most subjects (70%) were white. Hypertension was common (46%), as was diabetes (16%). About 6% had cardiovascular disease. Depression was present in 18% and PTSD in 4%.
When parsed by diagnosis, there were some significant between-group differences at baseline. Women with depression or PTSD were younger than those without (65 and 63 vs. 67 years). Women who had both disorders were the youngest group, at 62 years.
Hypertension was least common in women without depression or PTSD (41%), and most common among those with depression (65%). Diabetes was almost more common among women with depression than among those without (24% vs. 14%).
Dr. Yaffe created two regression analyses. Model 1 controlled for age, race, and education. Model 2 controlled for the factors in Model 1, plus diabetes, hypertension, and cardiovascular disease.
By the end of follow-up, 4% of the group had developed dementia. The presence of depression approximately doubled the risk of dementia (hazard ratio, 2.14), compared with women who had neither depression nor PTSD. This risk was virtually unchanged in both Model 1 and Model 2 (HRs, 2.12 and 2.00).
The risk associated with PTSD was quite similar, increasing the risk of dementia twofold (HR, 2.19). Again, this was similar after controlling for the confounders in both Model 1 (HR, 2.20) and Model 2 (HR, 2.16).
Women with both depression and PTSD had almost a tripling of risk for dementia (HR, 2.71). Adjustment for confounders did not significantly alter this risk, either in Model 1 (HR, 2.59) or Model 2 (HR, 2.42).
“A question that often comes up in these types of studies is, ‘Is this a reverse causation?’ ” Dr. Yaffe said. “In other words, are people with dementia somehow getting more depression? We conducted a lag-time analysis that allowed a 2-year lag time for dementia, and also adjusted for the number of clinic visits. The results were almost identical.”
“This consistent doubling of risk is quite high,” Dr. Yaffe said. “In our prior work with male veterans, we didn’t see this robust an association.”
The study was funded by the Department of Defense and the National Institutes of Health. Dr. Yaffe had no financial disclosures.
Correction, 8/7/17: An earlier version of this article misstated Dr. Kristine Yaffe's degree.
msullivan@frontlinemedcom.com
On Twitter @alz_gal
LONDON – Women veterans with either depression or post-traumatic stress disorder face a doubling in their risk of dementia – and having both increases the risk even more, Dr. Kristine Yaffe reported at the Alzheimer’s Association International Conference.*
The risk ratios for incident dementia that Dr. Yaffe of the University of California, San Francisco, and her colleagues calculated from their analysis of a cohort of 149,000 older female veterans in the national Veterans Health Administration (VHA) database remained unchanged even when they adjusted for age, education, medical comorbidities, and other confounders.
Not only are older women veterans a growing group; they are frequently diagnosed with mental health disorders. In 2012, 45% of women veteran patients in the VHA had a mental health condition, Dr. Yaffe noted.
“Over 9% of all veterans in the U.S. are women, accounting for more than 2 million women veterans. And 30% of those are more than 55 years old. Additionally, the number of women utilizing the Veterans Healthcare Administration system has nearly doubled in the last decade.”
The study of the impact of depression and PTSD on incident dementia is the first of its kind, Dr. Yaffe noted. The cohort comprised women without dementia who had at least two VHA visits during 2005-2015. They were followed for a mean of 5 years. A diagnosis of depression or PTSD had to occur during a 2-year baseline period. Confounders considered in the analysis were demographics, medical comorbidities, and health habits, including alcohol and tobacco use. The primary outcome was time to incident dementia.
At baseline, the group was a mean of 67 years old. Most subjects (70%) were white. Hypertension was common (46%), as was diabetes (16%). About 6% had cardiovascular disease. Depression was present in 18% and PTSD in 4%.
When parsed by diagnosis, there were some significant between-group differences at baseline. Women with depression or PTSD were younger than those without (65 and 63 vs. 67 years). Women who had both disorders were the youngest group, at 62 years.
Hypertension was least common in women without depression or PTSD (41%), and most common among those with depression (65%). Diabetes was almost more common among women with depression than among those without (24% vs. 14%).
Dr. Yaffe created two regression analyses. Model 1 controlled for age, race, and education. Model 2 controlled for the factors in Model 1, plus diabetes, hypertension, and cardiovascular disease.
By the end of follow-up, 4% of the group had developed dementia. The presence of depression approximately doubled the risk of dementia (hazard ratio, 2.14), compared with women who had neither depression nor PTSD. This risk was virtually unchanged in both Model 1 and Model 2 (HRs, 2.12 and 2.00).
The risk associated with PTSD was quite similar, increasing the risk of dementia twofold (HR, 2.19). Again, this was similar after controlling for the confounders in both Model 1 (HR, 2.20) and Model 2 (HR, 2.16).
Women with both depression and PTSD had almost a tripling of risk for dementia (HR, 2.71). Adjustment for confounders did not significantly alter this risk, either in Model 1 (HR, 2.59) or Model 2 (HR, 2.42).
“A question that often comes up in these types of studies is, ‘Is this a reverse causation?’ ” Dr. Yaffe said. “In other words, are people with dementia somehow getting more depression? We conducted a lag-time analysis that allowed a 2-year lag time for dementia, and also adjusted for the number of clinic visits. The results were almost identical.”
“This consistent doubling of risk is quite high,” Dr. Yaffe said. “In our prior work with male veterans, we didn’t see this robust an association.”
The study was funded by the Department of Defense and the National Institutes of Health. Dr. Yaffe had no financial disclosures.
Correction, 8/7/17: An earlier version of this article misstated Dr. Kristine Yaffe's degree.
msullivan@frontlinemedcom.com
On Twitter @alz_gal
AT AAIC 2017
Key clinical point:
Major finding: Depression or PTSD both doubled the risk of dementia; both conditions together increased the risk by almost 2.5 times.
Data source: The retrospective cohort study comprised 149,000 women in the national Veterans Health Administration database.
Disclosures: The Department of Defense and National Institutes of Health Funded the study. The presenter had no financial disclosures.
Under Trump, hospitals face same penalties embraced by Obama
Amid all the turbulence over the future of the Affordable Care Act, one facet continues unchanged: President Trump’s administration is penalizing more than half the nation’s hospitals for having too many patients return within a month.
Medicare is punishing 2,573 hospitals, just two dozen short of what it did last year under President Obama, according to federal records released Aug. 2. Starting in October, the federal government will cut those hospitals’ payments by as much as 3% for a year.
Medicare docked all but 174 of those hospitals last year as well. The $564 million the government projects to save also is roughly the same as it was last year under Obama.
High rates of readmissions have been a safety concern for decades, with one in five Medicare patients historically ending up back in the hospital within 30 days. In 2011, 3.3 million adults returned to the hospital, running up medical costs estimated at $41 billion, according to the Agency for Healthcare Research and Quality.
The penalties, which begin their sixth year in October, have coincided with a nationwide decrease in hospital repeat patients. Between 2007 and 2015, the frequency of readmissions for conditions targeted by Medicare dropped from 21.5% to 17.8%, with the majority of the decrease occurring shortly after the ACA passed in 2010, according to a study conducted by Obama administration health policy experts and published in 2016 in the New England Journal of Medicine.
Some hospitals began giving impoverished patients free medications that they prescribed for their recovery, while others sent nurses to check up on patients seen as most likely to relapse in their homes. Readmissions dropped more quickly at hospitals potentially subject to the penalty than at other hospitals, another study found.
“The sum of the evidence really suggests that this program is helping people,” said Susannah Bernheim, MD, the director of quality measurement at the Yale/Yale-New Haven Hospital Center for Outcomes Research and Evaluation.
But the pace of these reductions has been leveling off in the past few years, indicating that the penalties’ ability to induce improvements may be waning.
“Presumably, hospitals made substantial changes during the implementation period but could not sustain such a high rate of reductions in the long term,” the New England Journal article said.
An analysis by Dr. Bernheim’s group found no decrease in the overall rate of readmissions between 2012 and 2015, although small drops in the medical conditions targeted by the penalties continued.
“We have indeed reached the limits of what changes in how we deliver care will allow us to do,” said Nancy Foster, vice president for quality at the American Hospital Association. “We can’t prevent every readmission. It could be that there is further room for improvement, but we just don’t know what the technique is to make that happen.”
The Hospital Readmissions Reduction Program was designed to use the purchasing power of Medicare to reward hospitals for higher quality. Those penalties, along with other ones aimed at improving hospital care, have been spared the partisan rancor over the law, and they would have continued under the GOP repeal proposals that stalled in Congress. But they have also been largely ignored.
Ashish Jha, MD, a professor at the Harvard T.H. Chan School of Public Health, Boston, said the fight over abolishing the ACA has drowned out talk about how to make the health care system more effective.
“We’ve spent the last 6 months fighting about how we’re going to pay for health insurance, which is one part of the ACA,” he said. “There’s been almost no discussion of the underlying health care delivery system changes that the ACA ushered in, and that is more important in the long run to be discussing because that’s what’s going to determine the underlying costs and outcomes of the health system.”
The readmission penalties are intended to neutralize an unintended incentive in the way Medicare pays hospitals that had profited from return patients. Medicare pays hospitals a lump sum for a patient’s stay based on the nature of the admission and other factors. Since hospitals generally are not paid extra if patients remain longer, they seek to discharge patients as soon as is medically feasible. If the patient ends up back in the hospital, it becomes a financial benefit as the hospital is paid for that second stay, filling a bed that would not have generated income if the patient had remained there continuously.
Because of the way the readmission penalty program was designed, it is not surprising that the new results are so similar to last year’s. As before, Medicare determined the penalties based on readmissions of the same six types of patients: those admitted for heart attacks, heart failure, pneumonia, chronic lung disease, hip or knee replacements, or coronary artery bypass graft surgery. Hospitals were judged on patients discharged between July 2013 and June 2016. Because the government looks at a 3-year period, 2 of those years were also examined in determining last year’s penalties.
This year, the average penalty will be 0.73% of each payment Medicare makes for a patient between Oct. 1 and Sept. 30, 2018, according to a Kaiser Health News analysis. That too was practically the same as last year. Forty-eight hospitals received the maximum punishment of a 3% reduction. Medicare did not release hospital-specific estimates for how much lost money these penalties would translate to.
More than 1,500 hospitals treating veterans, children, and psychiatric patients were exempted from penalties this year as required by law. Critical access hospitals, which Medicare also pays differently, also were excluded. So were Maryland hospitals because Congress has given that state extra leeway in how it distributes Medicare money.
Of the 3,241 hospitals whose readmissions were evaluated, Medicare penalized four out of five, the KHN analysis found. That is because the program’s methods are not very forgiving: A hospital can be penalized even if it has higher than expected readmission rates for only one of the six conditions that are targeted. Every nonexcluded hospital in Delaware and West Virginia will have their reimbursements reduced. Ninety percent or more will be punished in Arizona, Connecticut, Florida, Kentucky, Massachusetts, Minnesota, New Jersey, New York, and Virginia. Sixty percent or fewer will be penalized in Colorado, Kansas, Idaho, Montana, Oregon, South Dakota, and Utah.
Since the readmission program’s structure is set by law, the administration cannot make major changes unilaterally, even if it wanted to.
Congress last year instructed Medicare to make one future alteration in response to complaints from safety-net hospitals and major academic medical centers.
They have objected that their patients tended to be lower income than other hospitals and were more likely to return to the hospital, sometimes because they didn’t have a primary care doctor and other times because they could not afford the right medication or diet. Those hospitals argued that this was a disadvantage for them since Medicare bases its readmission targets on industry-wide trends and that it hurt them financially, depriving them of resources they could use to help those same patients.
Dr. Bernheim noted that, despite those complaints, safety-net hospitals have shown some of the greatest drops in readmission rates. In October 2018, Medicare will begin basing the penalties on how hospitals compared with their peer groups with similar numbers of poor patients. Akin Demehin, director of policy at the hospital association, said, “We expect the adjustment will provide some relief for safety-net hospitals.”
Medicare is planning to release two other rounds of recurring quality incentives for hospitals later this year. One gives out bonuses and penalties based on a mix of measures, with Medicare redistributing $1.9 billion based on how hospitals perform and improve. The other, the Hospital-Acquired Condition Reduction Program, cuts payments to roughly 750 hospitals with the highest rates of infections and other patient injuries by 1%.
Kaiser Health News is a national health policy news service that is part of the nonpartisan Henry J. Kaiser Family Foundation. KHN’s coverage related to aging & improving care of older adults is supported by The John A. Hartford Foundation.
Amid all the turbulence over the future of the Affordable Care Act, one facet continues unchanged: President Trump’s administration is penalizing more than half the nation’s hospitals for having too many patients return within a month.
Medicare is punishing 2,573 hospitals, just two dozen short of what it did last year under President Obama, according to federal records released Aug. 2. Starting in October, the federal government will cut those hospitals’ payments by as much as 3% for a year.
Medicare docked all but 174 of those hospitals last year as well. The $564 million the government projects to save also is roughly the same as it was last year under Obama.
High rates of readmissions have been a safety concern for decades, with one in five Medicare patients historically ending up back in the hospital within 30 days. In 2011, 3.3 million adults returned to the hospital, running up medical costs estimated at $41 billion, according to the Agency for Healthcare Research and Quality.
The penalties, which begin their sixth year in October, have coincided with a nationwide decrease in hospital repeat patients. Between 2007 and 2015, the frequency of readmissions for conditions targeted by Medicare dropped from 21.5% to 17.8%, with the majority of the decrease occurring shortly after the ACA passed in 2010, according to a study conducted by Obama administration health policy experts and published in 2016 in the New England Journal of Medicine.
Some hospitals began giving impoverished patients free medications that they prescribed for their recovery, while others sent nurses to check up on patients seen as most likely to relapse in their homes. Readmissions dropped more quickly at hospitals potentially subject to the penalty than at other hospitals, another study found.
“The sum of the evidence really suggests that this program is helping people,” said Susannah Bernheim, MD, the director of quality measurement at the Yale/Yale-New Haven Hospital Center for Outcomes Research and Evaluation.
But the pace of these reductions has been leveling off in the past few years, indicating that the penalties’ ability to induce improvements may be waning.
“Presumably, hospitals made substantial changes during the implementation period but could not sustain such a high rate of reductions in the long term,” the New England Journal article said.
An analysis by Dr. Bernheim’s group found no decrease in the overall rate of readmissions between 2012 and 2015, although small drops in the medical conditions targeted by the penalties continued.
“We have indeed reached the limits of what changes in how we deliver care will allow us to do,” said Nancy Foster, vice president for quality at the American Hospital Association. “We can’t prevent every readmission. It could be that there is further room for improvement, but we just don’t know what the technique is to make that happen.”
The Hospital Readmissions Reduction Program was designed to use the purchasing power of Medicare to reward hospitals for higher quality. Those penalties, along with other ones aimed at improving hospital care, have been spared the partisan rancor over the law, and they would have continued under the GOP repeal proposals that stalled in Congress. But they have also been largely ignored.
Ashish Jha, MD, a professor at the Harvard T.H. Chan School of Public Health, Boston, said the fight over abolishing the ACA has drowned out talk about how to make the health care system more effective.
“We’ve spent the last 6 months fighting about how we’re going to pay for health insurance, which is one part of the ACA,” he said. “There’s been almost no discussion of the underlying health care delivery system changes that the ACA ushered in, and that is more important in the long run to be discussing because that’s what’s going to determine the underlying costs and outcomes of the health system.”
The readmission penalties are intended to neutralize an unintended incentive in the way Medicare pays hospitals that had profited from return patients. Medicare pays hospitals a lump sum for a patient’s stay based on the nature of the admission and other factors. Since hospitals generally are not paid extra if patients remain longer, they seek to discharge patients as soon as is medically feasible. If the patient ends up back in the hospital, it becomes a financial benefit as the hospital is paid for that second stay, filling a bed that would not have generated income if the patient had remained there continuously.
Because of the way the readmission penalty program was designed, it is not surprising that the new results are so similar to last year’s. As before, Medicare determined the penalties based on readmissions of the same six types of patients: those admitted for heart attacks, heart failure, pneumonia, chronic lung disease, hip or knee replacements, or coronary artery bypass graft surgery. Hospitals were judged on patients discharged between July 2013 and June 2016. Because the government looks at a 3-year period, 2 of those years were also examined in determining last year’s penalties.
This year, the average penalty will be 0.73% of each payment Medicare makes for a patient between Oct. 1 and Sept. 30, 2018, according to a Kaiser Health News analysis. That too was practically the same as last year. Forty-eight hospitals received the maximum punishment of a 3% reduction. Medicare did not release hospital-specific estimates for how much lost money these penalties would translate to.
More than 1,500 hospitals treating veterans, children, and psychiatric patients were exempted from penalties this year as required by law. Critical access hospitals, which Medicare also pays differently, also were excluded. So were Maryland hospitals because Congress has given that state extra leeway in how it distributes Medicare money.
Of the 3,241 hospitals whose readmissions were evaluated, Medicare penalized four out of five, the KHN analysis found. That is because the program’s methods are not very forgiving: A hospital can be penalized even if it has higher than expected readmission rates for only one of the six conditions that are targeted. Every nonexcluded hospital in Delaware and West Virginia will have their reimbursements reduced. Ninety percent or more will be punished in Arizona, Connecticut, Florida, Kentucky, Massachusetts, Minnesota, New Jersey, New York, and Virginia. Sixty percent or fewer will be penalized in Colorado, Kansas, Idaho, Montana, Oregon, South Dakota, and Utah.
Since the readmission program’s structure is set by law, the administration cannot make major changes unilaterally, even if it wanted to.
Congress last year instructed Medicare to make one future alteration in response to complaints from safety-net hospitals and major academic medical centers.
They have objected that their patients tended to be lower income than other hospitals and were more likely to return to the hospital, sometimes because they didn’t have a primary care doctor and other times because they could not afford the right medication or diet. Those hospitals argued that this was a disadvantage for them since Medicare bases its readmission targets on industry-wide trends and that it hurt them financially, depriving them of resources they could use to help those same patients.
Dr. Bernheim noted that, despite those complaints, safety-net hospitals have shown some of the greatest drops in readmission rates. In October 2018, Medicare will begin basing the penalties on how hospitals compared with their peer groups with similar numbers of poor patients. Akin Demehin, director of policy at the hospital association, said, “We expect the adjustment will provide some relief for safety-net hospitals.”
Medicare is planning to release two other rounds of recurring quality incentives for hospitals later this year. One gives out bonuses and penalties based on a mix of measures, with Medicare redistributing $1.9 billion based on how hospitals perform and improve. The other, the Hospital-Acquired Condition Reduction Program, cuts payments to roughly 750 hospitals with the highest rates of infections and other patient injuries by 1%.
Kaiser Health News is a national health policy news service that is part of the nonpartisan Henry J. Kaiser Family Foundation. KHN’s coverage related to aging & improving care of older adults is supported by The John A. Hartford Foundation.
Amid all the turbulence over the future of the Affordable Care Act, one facet continues unchanged: President Trump’s administration is penalizing more than half the nation’s hospitals for having too many patients return within a month.
Medicare is punishing 2,573 hospitals, just two dozen short of what it did last year under President Obama, according to federal records released Aug. 2. Starting in October, the federal government will cut those hospitals’ payments by as much as 3% for a year.
Medicare docked all but 174 of those hospitals last year as well. The $564 million the government projects to save also is roughly the same as it was last year under Obama.
High rates of readmissions have been a safety concern for decades, with one in five Medicare patients historically ending up back in the hospital within 30 days. In 2011, 3.3 million adults returned to the hospital, running up medical costs estimated at $41 billion, according to the Agency for Healthcare Research and Quality.
The penalties, which begin their sixth year in October, have coincided with a nationwide decrease in hospital repeat patients. Between 2007 and 2015, the frequency of readmissions for conditions targeted by Medicare dropped from 21.5% to 17.8%, with the majority of the decrease occurring shortly after the ACA passed in 2010, according to a study conducted by Obama administration health policy experts and published in 2016 in the New England Journal of Medicine.
Some hospitals began giving impoverished patients free medications that they prescribed for their recovery, while others sent nurses to check up on patients seen as most likely to relapse in their homes. Readmissions dropped more quickly at hospitals potentially subject to the penalty than at other hospitals, another study found.
“The sum of the evidence really suggests that this program is helping people,” said Susannah Bernheim, MD, the director of quality measurement at the Yale/Yale-New Haven Hospital Center for Outcomes Research and Evaluation.
But the pace of these reductions has been leveling off in the past few years, indicating that the penalties’ ability to induce improvements may be waning.
“Presumably, hospitals made substantial changes during the implementation period but could not sustain such a high rate of reductions in the long term,” the New England Journal article said.
An analysis by Dr. Bernheim’s group found no decrease in the overall rate of readmissions between 2012 and 2015, although small drops in the medical conditions targeted by the penalties continued.
“We have indeed reached the limits of what changes in how we deliver care will allow us to do,” said Nancy Foster, vice president for quality at the American Hospital Association. “We can’t prevent every readmission. It could be that there is further room for improvement, but we just don’t know what the technique is to make that happen.”
The Hospital Readmissions Reduction Program was designed to use the purchasing power of Medicare to reward hospitals for higher quality. Those penalties, along with other ones aimed at improving hospital care, have been spared the partisan rancor over the law, and they would have continued under the GOP repeal proposals that stalled in Congress. But they have also been largely ignored.
Ashish Jha, MD, a professor at the Harvard T.H. Chan School of Public Health, Boston, said the fight over abolishing the ACA has drowned out talk about how to make the health care system more effective.
“We’ve spent the last 6 months fighting about how we’re going to pay for health insurance, which is one part of the ACA,” he said. “There’s been almost no discussion of the underlying health care delivery system changes that the ACA ushered in, and that is more important in the long run to be discussing because that’s what’s going to determine the underlying costs and outcomes of the health system.”
The readmission penalties are intended to neutralize an unintended incentive in the way Medicare pays hospitals that had profited from return patients. Medicare pays hospitals a lump sum for a patient’s stay based on the nature of the admission and other factors. Since hospitals generally are not paid extra if patients remain longer, they seek to discharge patients as soon as is medically feasible. If the patient ends up back in the hospital, it becomes a financial benefit as the hospital is paid for that second stay, filling a bed that would not have generated income if the patient had remained there continuously.
Because of the way the readmission penalty program was designed, it is not surprising that the new results are so similar to last year’s. As before, Medicare determined the penalties based on readmissions of the same six types of patients: those admitted for heart attacks, heart failure, pneumonia, chronic lung disease, hip or knee replacements, or coronary artery bypass graft surgery. Hospitals were judged on patients discharged between July 2013 and June 2016. Because the government looks at a 3-year period, 2 of those years were also examined in determining last year’s penalties.
This year, the average penalty will be 0.73% of each payment Medicare makes for a patient between Oct. 1 and Sept. 30, 2018, according to a Kaiser Health News analysis. That too was practically the same as last year. Forty-eight hospitals received the maximum punishment of a 3% reduction. Medicare did not release hospital-specific estimates for how much lost money these penalties would translate to.
More than 1,500 hospitals treating veterans, children, and psychiatric patients were exempted from penalties this year as required by law. Critical access hospitals, which Medicare also pays differently, also were excluded. So were Maryland hospitals because Congress has given that state extra leeway in how it distributes Medicare money.
Of the 3,241 hospitals whose readmissions were evaluated, Medicare penalized four out of five, the KHN analysis found. That is because the program’s methods are not very forgiving: A hospital can be penalized even if it has higher than expected readmission rates for only one of the six conditions that are targeted. Every nonexcluded hospital in Delaware and West Virginia will have their reimbursements reduced. Ninety percent or more will be punished in Arizona, Connecticut, Florida, Kentucky, Massachusetts, Minnesota, New Jersey, New York, and Virginia. Sixty percent or fewer will be penalized in Colorado, Kansas, Idaho, Montana, Oregon, South Dakota, and Utah.
Since the readmission program’s structure is set by law, the administration cannot make major changes unilaterally, even if it wanted to.
Congress last year instructed Medicare to make one future alteration in response to complaints from safety-net hospitals and major academic medical centers.
They have objected that their patients tended to be lower income than other hospitals and were more likely to return to the hospital, sometimes because they didn’t have a primary care doctor and other times because they could not afford the right medication or diet. Those hospitals argued that this was a disadvantage for them since Medicare bases its readmission targets on industry-wide trends and that it hurt them financially, depriving them of resources they could use to help those same patients.
Dr. Bernheim noted that, despite those complaints, safety-net hospitals have shown some of the greatest drops in readmission rates. In October 2018, Medicare will begin basing the penalties on how hospitals compared with their peer groups with similar numbers of poor patients. Akin Demehin, director of policy at the hospital association, said, “We expect the adjustment will provide some relief for safety-net hospitals.”
Medicare is planning to release two other rounds of recurring quality incentives for hospitals later this year. One gives out bonuses and penalties based on a mix of measures, with Medicare redistributing $1.9 billion based on how hospitals perform and improve. The other, the Hospital-Acquired Condition Reduction Program, cuts payments to roughly 750 hospitals with the highest rates of infections and other patient injuries by 1%.
Kaiser Health News is a national health policy news service that is part of the nonpartisan Henry J. Kaiser Family Foundation. KHN’s coverage related to aging & improving care of older adults is supported by The John A. Hartford Foundation.
Oral prophylaxis and vaginal ring effective in adolescent HIV prevention
Dapivirine vaginal ring and oral pre-exposure prophylaxis (PrEP) are effective HIV prevention measures in adolescent girls, according to two studies presented at the International AIDS Society Conference on HIV Pathogenesis and Treatment in Paris.
These solutions could be critical in lowering the rate of HIV and AIDS in girls between the ages of 15 and 25 years, a population that has proven to be particularly vulnerable to HIV infection, researchers say.
Females aged 15-24 years made up 20% of new HIV infections globally in 2015, even though they represented only 11% of the adult population, according to the National Institutes of Health (NIH).
“Adolescents and young people represent a growing share of people living with HIV worldwide,” Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases (NIAID) said in a statement released by the NIH. “Science has demonstrated that the HIV prevention needs of adolescents may be different than those of adults, which is why these new study findings are so important.”
In a phase II, double-blind, placebo-controlled trial, researchers administered a 25-mg dapivirine vaginal ring to 73 patients, with a placebo group of 23 patients, gathered from six sites across the United States once every 4 weeks over a period of 24 weeks.
Patients’ ages ranged from 15 to 17 years of age and a majority were African American, with a median of three sexual partners over the course of their lifetimes.
After the 24-week period, dapivirine residual drug levels indicated a 95% adherence rate, with a reported 42% of patients in the test group reporting never having removed the vaginal ring.
Among the patients given the ring, 63% reported never feeling the ring during intercourse, while 73% of those who said they did feel the ring reported not being bothered by it.
Overall, 93% of the study population reported not being bothered by the solution, which investigators interpreted as a positive sign for the dapivirine ring as an effective HIV prevention tool.
“We are encouraged by these results of the dapivirine ring in 15- to 17-year-old girls,” Sharon Hillier, PhD, of the NIH-funded Microbicide Trials Network (MTN), said in the NIH statement. “The study has demonstrated that the ring is safe in U.S. teens, and now we need data on the safety and acceptability of the ring in African adolescent girls. The REACH study, scheduled to launch later this year, will generate [these] data.”
In a second study presented at the conference, investigators tested the safety and acceptability of daily oral Truvada (emtricitabine/tenofovir) as a PrEP solution in 148 HIV-free adolescents aged 15-19 years from two study sites in South Africa over a span of 12 months. Truvada has not yet been approved by any national regulatory body for use as oral PrEP in adolescents.
Patients were majority female (99 girls/49 boys), with 74% of the population reporting having used a condom during their last sexual encounter.
At the start of the trial, sexually transmitted infections were present in 40% of participants, a level that remained constant throughout the study.
A total of 16 (11%) participants reported grade 2 adverse effects, including headaches, nausea, abdominal pain, and skin rashes, with another 2 patients reporting weight loss during the trial, according to Katherine Gill, MBBS, of the Desmond Tutu HIV Foundation, Cape Town, South Africa.
One instance of HIV infection was reported, although the patient in question dropped out of the program 24 weeks before diagnosis.
Overall, investigators found the Truvada PrEP program to be reasonably well tolerated, with plasma tenofovir levels detectable in 57% of participants after 12 weeks, 38% after 24 weeks and 38% at the end of the study, according to Dr. Gill and her colleagues.
Both studies were funded by NIH grants. Investigators of both studies reported no relevant financial conflicts.
ezimmerman@frontlinemedcom.com
On Twitter @eaztweets
Dapivirine vaginal ring and oral pre-exposure prophylaxis (PrEP) are effective HIV prevention measures in adolescent girls, according to two studies presented at the International AIDS Society Conference on HIV Pathogenesis and Treatment in Paris.
These solutions could be critical in lowering the rate of HIV and AIDS in girls between the ages of 15 and 25 years, a population that has proven to be particularly vulnerable to HIV infection, researchers say.
Females aged 15-24 years made up 20% of new HIV infections globally in 2015, even though they represented only 11% of the adult population, according to the National Institutes of Health (NIH).
“Adolescents and young people represent a growing share of people living with HIV worldwide,” Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases (NIAID) said in a statement released by the NIH. “Science has demonstrated that the HIV prevention needs of adolescents may be different than those of adults, which is why these new study findings are so important.”
In a phase II, double-blind, placebo-controlled trial, researchers administered a 25-mg dapivirine vaginal ring to 73 patients, with a placebo group of 23 patients, gathered from six sites across the United States once every 4 weeks over a period of 24 weeks.
Patients’ ages ranged from 15 to 17 years of age and a majority were African American, with a median of three sexual partners over the course of their lifetimes.
After the 24-week period, dapivirine residual drug levels indicated a 95% adherence rate, with a reported 42% of patients in the test group reporting never having removed the vaginal ring.
Among the patients given the ring, 63% reported never feeling the ring during intercourse, while 73% of those who said they did feel the ring reported not being bothered by it.
Overall, 93% of the study population reported not being bothered by the solution, which investigators interpreted as a positive sign for the dapivirine ring as an effective HIV prevention tool.
“We are encouraged by these results of the dapivirine ring in 15- to 17-year-old girls,” Sharon Hillier, PhD, of the NIH-funded Microbicide Trials Network (MTN), said in the NIH statement. “The study has demonstrated that the ring is safe in U.S. teens, and now we need data on the safety and acceptability of the ring in African adolescent girls. The REACH study, scheduled to launch later this year, will generate [these] data.”
In a second study presented at the conference, investigators tested the safety and acceptability of daily oral Truvada (emtricitabine/tenofovir) as a PrEP solution in 148 HIV-free adolescents aged 15-19 years from two study sites in South Africa over a span of 12 months. Truvada has not yet been approved by any national regulatory body for use as oral PrEP in adolescents.
Patients were majority female (99 girls/49 boys), with 74% of the population reporting having used a condom during their last sexual encounter.
At the start of the trial, sexually transmitted infections were present in 40% of participants, a level that remained constant throughout the study.
A total of 16 (11%) participants reported grade 2 adverse effects, including headaches, nausea, abdominal pain, and skin rashes, with another 2 patients reporting weight loss during the trial, according to Katherine Gill, MBBS, of the Desmond Tutu HIV Foundation, Cape Town, South Africa.
One instance of HIV infection was reported, although the patient in question dropped out of the program 24 weeks before diagnosis.
Overall, investigators found the Truvada PrEP program to be reasonably well tolerated, with plasma tenofovir levels detectable in 57% of participants after 12 weeks, 38% after 24 weeks and 38% at the end of the study, according to Dr. Gill and her colleagues.
Both studies were funded by NIH grants. Investigators of both studies reported no relevant financial conflicts.
ezimmerman@frontlinemedcom.com
On Twitter @eaztweets
Dapivirine vaginal ring and oral pre-exposure prophylaxis (PrEP) are effective HIV prevention measures in adolescent girls, according to two studies presented at the International AIDS Society Conference on HIV Pathogenesis and Treatment in Paris.
These solutions could be critical in lowering the rate of HIV and AIDS in girls between the ages of 15 and 25 years, a population that has proven to be particularly vulnerable to HIV infection, researchers say.
Females aged 15-24 years made up 20% of new HIV infections globally in 2015, even though they represented only 11% of the adult population, according to the National Institutes of Health (NIH).
“Adolescents and young people represent a growing share of people living with HIV worldwide,” Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases (NIAID) said in a statement released by the NIH. “Science has demonstrated that the HIV prevention needs of adolescents may be different than those of adults, which is why these new study findings are so important.”
In a phase II, double-blind, placebo-controlled trial, researchers administered a 25-mg dapivirine vaginal ring to 73 patients, with a placebo group of 23 patients, gathered from six sites across the United States once every 4 weeks over a period of 24 weeks.
Patients’ ages ranged from 15 to 17 years of age and a majority were African American, with a median of three sexual partners over the course of their lifetimes.
After the 24-week period, dapivirine residual drug levels indicated a 95% adherence rate, with a reported 42% of patients in the test group reporting never having removed the vaginal ring.
Among the patients given the ring, 63% reported never feeling the ring during intercourse, while 73% of those who said they did feel the ring reported not being bothered by it.
Overall, 93% of the study population reported not being bothered by the solution, which investigators interpreted as a positive sign for the dapivirine ring as an effective HIV prevention tool.
“We are encouraged by these results of the dapivirine ring in 15- to 17-year-old girls,” Sharon Hillier, PhD, of the NIH-funded Microbicide Trials Network (MTN), said in the NIH statement. “The study has demonstrated that the ring is safe in U.S. teens, and now we need data on the safety and acceptability of the ring in African adolescent girls. The REACH study, scheduled to launch later this year, will generate [these] data.”
In a second study presented at the conference, investigators tested the safety and acceptability of daily oral Truvada (emtricitabine/tenofovir) as a PrEP solution in 148 HIV-free adolescents aged 15-19 years from two study sites in South Africa over a span of 12 months. Truvada has not yet been approved by any national regulatory body for use as oral PrEP in adolescents.
Patients were majority female (99 girls/49 boys), with 74% of the population reporting having used a condom during their last sexual encounter.
At the start of the trial, sexually transmitted infections were present in 40% of participants, a level that remained constant throughout the study.
A total of 16 (11%) participants reported grade 2 adverse effects, including headaches, nausea, abdominal pain, and skin rashes, with another 2 patients reporting weight loss during the trial, according to Katherine Gill, MBBS, of the Desmond Tutu HIV Foundation, Cape Town, South Africa.
One instance of HIV infection was reported, although the patient in question dropped out of the program 24 weeks before diagnosis.
Overall, investigators found the Truvada PrEP program to be reasonably well tolerated, with plasma tenofovir levels detectable in 57% of participants after 12 weeks, 38% after 24 weeks and 38% at the end of the study, according to Dr. Gill and her colleagues.
Both studies were funded by NIH grants. Investigators of both studies reported no relevant financial conflicts.
ezimmerman@frontlinemedcom.com
On Twitter @eaztweets
FROM IAS 2017
FDA approves faster, pangenotypic cure for hep C virus
The first pangenotypic treatment for the hepatitis C virus, which also shaves 4 weeks off current regimens, has just been approved by the Food and Drug Administration.
Manufactured by AbbVie, glecaprevir/pibrentasvir (Mavyret) combines a nonstructural protein 3/4A protease inhibitor with a next-generation NS5A protein inhibitor for a once-daily, ribavirin-free treatment for adults with any of the major genotypes of chronic hepatitis C virus (HCV) infection.
“This approval provides a shorter treatment duration for many patients, and also a treatment option for certain patients with genotype 1 infection, the most common HCV genotype in the United States, who were not successfully treated with other direct-acting antiviral treatments in the past,” Edward Cox, MD, director of the office of antimicrobial products in the FDA’s Center for Drug Evaluation and Research, Silver Spring, Md., said in a statement.
The 8-week regimen is indicated in patients without cirrhosis or with compensated cirrhosis, who are new to treatment, and those with limited treatment options, such as patients with chronic kidney disease, including those on dialysis. The intervention also is indicated in adults with HCV genotype 1 who have been treated with either of the drugs in the combination, but not both. Glecaprevir/pibrentasvir is not recommended in patients with moderate cirrhosis and is contraindicated in patients with severe cirrhosis and in those taking the drugs atazanavir and rifampin.
The safety and efficacy of the treatment were evaluated in approximately 2,300 adults with genotype 1, 2, 3, 4, 5 or 6 HCV infection without cirrhosis or with mild cirrhosis. In the clinical trials, between 92% and 100% of patients treated with glecaprevir/pibrentasvir for 8, 12, or 16 weeks had no detectable serum levels of the virus 12 weeks after finishing treatment. The most commonly reported adverse reactions were headache, fatigue, and nausea.
The FDA directs health care professionals to test all patients for current or prior hepatitis B virus (HBV) infection prior to starting this direct-acting antiviral drug combination since HBV reactivation has been reported in adult patients coinfected with both viruses who were undergoing or had completed treatment with HCV direct-acting antivirals and who were not receiving HBV antiviral therapy.
The first pangenotypic treatment for the hepatitis C virus, which also shaves 4 weeks off current regimens, has just been approved by the Food and Drug Administration.
Manufactured by AbbVie, glecaprevir/pibrentasvir (Mavyret) combines a nonstructural protein 3/4A protease inhibitor with a next-generation NS5A protein inhibitor for a once-daily, ribavirin-free treatment for adults with any of the major genotypes of chronic hepatitis C virus (HCV) infection.
“This approval provides a shorter treatment duration for many patients, and also a treatment option for certain patients with genotype 1 infection, the most common HCV genotype in the United States, who were not successfully treated with other direct-acting antiviral treatments in the past,” Edward Cox, MD, director of the office of antimicrobial products in the FDA’s Center for Drug Evaluation and Research, Silver Spring, Md., said in a statement.
The 8-week regimen is indicated in patients without cirrhosis or with compensated cirrhosis, who are new to treatment, and those with limited treatment options, such as patients with chronic kidney disease, including those on dialysis. The intervention also is indicated in adults with HCV genotype 1 who have been treated with either of the drugs in the combination, but not both. Glecaprevir/pibrentasvir is not recommended in patients with moderate cirrhosis and is contraindicated in patients with severe cirrhosis and in those taking the drugs atazanavir and rifampin.
The safety and efficacy of the treatment were evaluated in approximately 2,300 adults with genotype 1, 2, 3, 4, 5 or 6 HCV infection without cirrhosis or with mild cirrhosis. In the clinical trials, between 92% and 100% of patients treated with glecaprevir/pibrentasvir for 8, 12, or 16 weeks had no detectable serum levels of the virus 12 weeks after finishing treatment. The most commonly reported adverse reactions were headache, fatigue, and nausea.
The FDA directs health care professionals to test all patients for current or prior hepatitis B virus (HBV) infection prior to starting this direct-acting antiviral drug combination since HBV reactivation has been reported in adult patients coinfected with both viruses who were undergoing or had completed treatment with HCV direct-acting antivirals and who were not receiving HBV antiviral therapy.
The first pangenotypic treatment for the hepatitis C virus, which also shaves 4 weeks off current regimens, has just been approved by the Food and Drug Administration.
Manufactured by AbbVie, glecaprevir/pibrentasvir (Mavyret) combines a nonstructural protein 3/4A protease inhibitor with a next-generation NS5A protein inhibitor for a once-daily, ribavirin-free treatment for adults with any of the major genotypes of chronic hepatitis C virus (HCV) infection.
“This approval provides a shorter treatment duration for many patients, and also a treatment option for certain patients with genotype 1 infection, the most common HCV genotype in the United States, who were not successfully treated with other direct-acting antiviral treatments in the past,” Edward Cox, MD, director of the office of antimicrobial products in the FDA’s Center for Drug Evaluation and Research, Silver Spring, Md., said in a statement.
The 8-week regimen is indicated in patients without cirrhosis or with compensated cirrhosis, who are new to treatment, and those with limited treatment options, such as patients with chronic kidney disease, including those on dialysis. The intervention also is indicated in adults with HCV genotype 1 who have been treated with either of the drugs in the combination, but not both. Glecaprevir/pibrentasvir is not recommended in patients with moderate cirrhosis and is contraindicated in patients with severe cirrhosis and in those taking the drugs atazanavir and rifampin.
The safety and efficacy of the treatment were evaluated in approximately 2,300 adults with genotype 1, 2, 3, 4, 5 or 6 HCV infection without cirrhosis or with mild cirrhosis. In the clinical trials, between 92% and 100% of patients treated with glecaprevir/pibrentasvir for 8, 12, or 16 weeks had no detectable serum levels of the virus 12 weeks after finishing treatment. The most commonly reported adverse reactions were headache, fatigue, and nausea.
The FDA directs health care professionals to test all patients for current or prior hepatitis B virus (HBV) infection prior to starting this direct-acting antiviral drug combination since HBV reactivation has been reported in adult patients coinfected with both viruses who were undergoing or had completed treatment with HCV direct-acting antivirals and who were not receiving HBV antiviral therapy.
Diabetes’ social determinants: What they mean in our practices
More than many other pregnancy complications, diabetes exemplifies the impact of social determinants of health.
The medical management of diabetes during pregnancy involves major lifestyle changes. Diabetes care is largely a patient-driven social experience involving complex and demanding self-care behaviors and tasks.
The pregnant woman with diabetes is placed on a diet that is often novel to her and may be in conflict with the eating patterns of her family. She is advised to exercise, read nutrition labels, and purchase and cook healthy food. She often has to pick up prescriptions, check finger sticks and log results, accurately draw up insulin, and manage strict schedules.
Management requires a tremendous amount of daily engagement during a period of time that, in and of itself, is cognitively demanding.
Outcomes, in turn, are impacted by social context and social factors – by the patient’s economic stability and the safety and characteristics of her neighborhood, for instance, as well as her work schedule, her social support, and her level of health literacy. Each of these factors can influence behaviors and decision making, and ultimately glycemic control and perinatal outcomes.
The social determinants of diabetes-related health are so individualized and impactful that they must be realized and addressed throughout our care, from the way in which we communicate at the initial prenatal checkup to the support we offer for self-management.
Barriers to diabetes self-care
While the incidence of type 2 diabetes is increasing among all social, ethnic, and racial groups, its prevalence among nonpregnant U.S. adults is greatest among racial/ethnic minorities, as well as in individuals with a low-income status. Women who enter pregnancy with preexisting diabetes are more likely to be racial/ethnic minorities.
In pregnancy, minority women (especially Hispanic, but also Asian and non-Hispanic black women), and women with low-income status are similarly predisposed to developing gestational diabetes mellitus (GDM).
Social determinants of health are interwoven with inequities stemming from race/ethnicity, income, and other factors that affect outcomes. For example, not only do non-Hispanic black women experience a greater incidence of GDM than non-Hispanic white women, but when they have GDM, they also appear to experience worse pregnancy outcomes compared with white women who also have GDM. In addition, they have a greater likelihood of developing type 2 diabetes after a pregnancy with GDM.
I care for a population that consists largely of minority, low-income women with either gestational or pregestational diabetes. Despite their best intentions and efforts – and despite seemingly high motivation levels – these women struggle to achieve the levels of glycemic control necessary for preventing maternal and fetal complications.
Several years ago, I sought to better understand the barriers to diabetes self-care and behavioral change these women face. Through a series of in-depth, semi-structured interviews with 10 English-speaking women (half with pregestational diabetes) over the course of their pregnancies, we found that the barriers to self-care related to the following: disease novelty, social and economic instability, nutrition challenges, psychological stressors, a failure of outcome expectations, and the burden of disease management (J Health Care Poor Underserved. 2015 Aug;26[3]:926-40; J Nutr Educ Behav. 2016 Mar;48[3]:170-80.e1).
Some of these barriers, such as the lack of any prior experience with diabetes (through a family member, for instance) or the inability to believe that behavior change and other treatment could impact her diabetes and her fetus’ health, echoed other limited published data. However, women in our study also appeared to be affected by barriers driven by social instability (e.g., a lack of partner or family support, family conflict, or neighborhood violence), inadequate access to healthy food, and the psychological impact of diabetes.
They often felt isolated and overwhelmed by their diabetes; the condition amplified stresses they were already experiencing and contributed to worsening mental health in those who already had depression or anxiety. In the other direction, women also described how preexisting mental health challenges affected their ability to sustain recommended behavior changes.
However, we also identified factors that empowered women in this community to succeed with their diabetes during pregnancy – these included having prior familiarity and diabetes self-efficacy, being motivated by the health of the fetus or older children, having a supportive social and physical environment, and having the ability to self-regulate or set and achieve goals (J Perinatol. 2016 Jan;36[1]:13-8).
To address these barriers, my group has undertaken a series of projects aimed at improving care for pregnant women with diabetes. We developed a diabetes-specific text message support system, for instance, and are now transitioning this support to an advanced mobile health tool that can help patients beyond our site.
What we can do
Much of what we can do in our practices to identify and address social determinants and alleviate barriers to effective diabetes management is about finding the “sweet spot” – about being able to convey the right information in the right amount, with the right timing and the right delivery.
While we can’t improve a woman’s neighborhood or resolve food instability, I believe that we can still work to improve outcomes for women who experience these problems. Here are some key strategies for optimal support of our patients:
Inquire about social factors
Identify hurdles by asking questions such as: Where do you live? Is it safe to walk in your neighborhood? If not, where’s your closest mall? What kind of job do you have, and does your employer allow breaks to take care of your health? How are things going at home? Who is at home to help you? Are you having any trouble affording food? How can we help you learn to adapt your personal or cultural food preferences to healthier options?
Look for small actions to take. I often write letters to my patients’ employers requesting that they be given short, frequent breaks to accommodate their care regimens. I also work to ensure that diet recommendations and medication/insulin regimens are customized for patients with irregular meal and sleep schedules, such as those working night shifts.
Employ a social worker if possible, especially if your practice cares for large numbers of underserved women.
Serve as a resource center, and engage your team in doing so. Be prepared to refer women for social services support, food banks, intimate partner violence support services, and other local resources.
Take a low-health-literacy approach
Health literacy is the ability to obtain and utilize health information. It has been widely investigated outside of pregnancy (and to some extent during pregnancy), and has been found to be at the root of many disparities in health care and health outcomes. Numeracy, a type of health literacy, is the ability to understand numbers, perform basic calculations, and use simple math skills in a way that helps one’s health.
The barriers created by inadequate health literacy are distinct from language barriers. I’ve had patients who can read the labels on their insulin vials but cannot distinguish the short-acting from the long-acting formulation, or who can read the words on a nutrition label but don’t know how to interpret the amount of carbohydrates and determine if a food fits the diet plan.
Moreover, while health literacy is correlated with cognitive ability, it still is a distinct skill set. Studies have shown that patients educated in a traditional sense – college-educated professionals, for instance – will not necessarily understand health-related words and instructions.
Research similarly suggests that a low-health-literacy approach that uses focused, simple, and straightforward messages benefits everyone. This type of approach involves the following:
Simple language
Teach-back techniques (“tell me you what your understanding is of what I just told you”)
Diagrams, handouts, and brochures written at a sixth-grade level.
Teaching that is limited to five to eight key messages per session, and reinforcement of these messages over time.
Promote self-efficacy
Self-efficacy is the confidence in one’s ability to perform certain health behaviors. It involves motivation as well as knowledge of the disease, the rationale for treatment, and the specific behaviors that are required for effective self-care.
Help patients understand “why it matters” – that diabetes raises the risk of macrosomia, shoulder dystocia, hypertension, long-term diabetes, and other adverse maternal and neonatal outcomes. Explain basic physiologic concepts and provide background information. This builds self-efficacy.
Do not issue recommendations for exercising and eating well without asking: How can I help you do this? What do you need to be able to eat healthy? Do you need an appointment with a nutritionist? Do you need to see a social worker?
Inquire about and help patients identify supportive family members or other “champions.” Look for ways to incorporate these support people into the patient’s care. At a minimum, encourage the patient to ask her support person to eat healthy with her and/or to understand her daily tasks so that this individual can offer reminders and be a source of support when she feels exhausted or overwhelmed.
If possible, facilitate some type of “diabetes buddy” program to offer peer support and help patients stay engaged in their care, or use group education sessions.
Piggyback on your patients’ own motivating factors. Research has shown that women are extraordinarily motivated to stop smoking during pregnancy because of the health of the fetus. This should extend as well to the difficult lifestyle changes required for diabetes self-care.
View pregnancy as a “golden opportunity” to promote healthy life changes that endure because of the often-extraordinary levels of motivation that women feel or can be encouraged to feel.
Facilitate access
The ability to attend frequent appointments and to juggle the logistics of transportation, child care, and time off work (all part of the burden of disease management) is a social determinant of health. It’s something we should ask about, and it is often something we can positively impact by modifying our practice hours and/or using telehealth or mobile health techniques.
Coordinating newborn and pediatric care with the mother’s subsequent primary care is optimal. Women often prioritize their babies’ health over their own health and they rarely miss pediatric appointments. Coordinating care through medical homes or other mechanisms may help women remain engaged and may lessen the gaps between obstetrical and subsequent primary care.
For me, facilitating doctor-to-doctor transitions sometimes entails picking up the phone or sending communication to a primary care doctor to say, for instance, “I’m worried about my patient’s lifetime risk of type 2 diabetes, and I’d like to hand off her care to you.” This is one of many small but meaningful steps we can take.
Dr. Yee is an assistant professor in the division of maternal-fetal medicine at Northwestern University, Chicago. She reported having no relevant financial disclosures.
More than many other pregnancy complications, diabetes exemplifies the impact of social determinants of health.
The medical management of diabetes during pregnancy involves major lifestyle changes. Diabetes care is largely a patient-driven social experience involving complex and demanding self-care behaviors and tasks.
The pregnant woman with diabetes is placed on a diet that is often novel to her and may be in conflict with the eating patterns of her family. She is advised to exercise, read nutrition labels, and purchase and cook healthy food. She often has to pick up prescriptions, check finger sticks and log results, accurately draw up insulin, and manage strict schedules.
Management requires a tremendous amount of daily engagement during a period of time that, in and of itself, is cognitively demanding.
Outcomes, in turn, are impacted by social context and social factors – by the patient’s economic stability and the safety and characteristics of her neighborhood, for instance, as well as her work schedule, her social support, and her level of health literacy. Each of these factors can influence behaviors and decision making, and ultimately glycemic control and perinatal outcomes.
The social determinants of diabetes-related health are so individualized and impactful that they must be realized and addressed throughout our care, from the way in which we communicate at the initial prenatal checkup to the support we offer for self-management.
Barriers to diabetes self-care
While the incidence of type 2 diabetes is increasing among all social, ethnic, and racial groups, its prevalence among nonpregnant U.S. adults is greatest among racial/ethnic minorities, as well as in individuals with a low-income status. Women who enter pregnancy with preexisting diabetes are more likely to be racial/ethnic minorities.

In pregnancy, minority women (especially Hispanic, but also Asian and non-Hispanic black women), and women with low-income status are similarly predisposed to developing gestational diabetes mellitus (GDM).
Social determinants of health are interwoven with inequities stemming from race/ethnicity, income, and other factors that affect outcomes. For example, not only do non-Hispanic black women experience a greater incidence of GDM than non-Hispanic white women, but when they have GDM, they also appear to experience worse pregnancy outcomes compared with white women who also have GDM. In addition, they have a greater likelihood of developing type 2 diabetes after a pregnancy with GDM.
I care for a population that consists largely of minority, low-income women with either gestational or pregestational diabetes. Despite their best intentions and efforts – and despite seemingly high motivation levels – these women struggle to achieve the levels of glycemic control necessary for preventing maternal and fetal complications.
Several years ago, I sought to better understand the barriers to diabetes self-care and behavioral change these women face. Through a series of in-depth, semi-structured interviews with 10 English-speaking women (half with pregestational diabetes) over the course of their pregnancies, we found that the barriers to self-care related to the following: disease novelty, social and economic instability, nutrition challenges, psychological stressors, a failure of outcome expectations, and the burden of disease management (J Health Care Poor Underserved. 2015 Aug;26[3]:926-40; J Nutr Educ Behav. 2016 Mar;48[3]:170-80.e1).
Some of these barriers, such as the lack of any prior experience with diabetes (through a family member, for instance) or the inability to believe that behavior change and other treatment could impact her diabetes and her fetus’ health, echoed other limited published data. However, women in our study also appeared to be affected by barriers driven by social instability (e.g., a lack of partner or family support, family conflict, or neighborhood violence), inadequate access to healthy food, and the psychological impact of diabetes.
They often felt isolated and overwhelmed by their diabetes; the condition amplified stresses they were already experiencing and contributed to worsening mental health in those who already had depression or anxiety. In the other direction, women also described how preexisting mental health challenges affected their ability to sustain recommended behavior changes.
However, we also identified factors that empowered women in this community to succeed with their diabetes during pregnancy – these included having prior familiarity and diabetes self-efficacy, being motivated by the health of the fetus or older children, having a supportive social and physical environment, and having the ability to self-regulate or set and achieve goals (J Perinatol. 2016 Jan;36[1]:13-8).
To address these barriers, my group has undertaken a series of projects aimed at improving care for pregnant women with diabetes. We developed a diabetes-specific text message support system, for instance, and are now transitioning this support to an advanced mobile health tool that can help patients beyond our site.
What we can do
Much of what we can do in our practices to identify and address social determinants and alleviate barriers to effective diabetes management is about finding the “sweet spot” – about being able to convey the right information in the right amount, with the right timing and the right delivery.
While we can’t improve a woman’s neighborhood or resolve food instability, I believe that we can still work to improve outcomes for women who experience these problems. Here are some key strategies for optimal support of our patients:
Inquire about social factors
Identify hurdles by asking questions such as: Where do you live? Is it safe to walk in your neighborhood? If not, where’s your closest mall? What kind of job do you have, and does your employer allow breaks to take care of your health? How are things going at home? Who is at home to help you? Are you having any trouble affording food? How can we help you learn to adapt your personal or cultural food preferences to healthier options?
Look for small actions to take. I often write letters to my patients’ employers requesting that they be given short, frequent breaks to accommodate their care regimens. I also work to ensure that diet recommendations and medication/insulin regimens are customized for patients with irregular meal and sleep schedules, such as those working night shifts.
Employ a social worker if possible, especially if your practice cares for large numbers of underserved women.
Serve as a resource center, and engage your team in doing so. Be prepared to refer women for social services support, food banks, intimate partner violence support services, and other local resources.
Take a low-health-literacy approach
Health literacy is the ability to obtain and utilize health information. It has been widely investigated outside of pregnancy (and to some extent during pregnancy), and has been found to be at the root of many disparities in health care and health outcomes. Numeracy, a type of health literacy, is the ability to understand numbers, perform basic calculations, and use simple math skills in a way that helps one’s health.
The barriers created by inadequate health literacy are distinct from language barriers. I’ve had patients who can read the labels on their insulin vials but cannot distinguish the short-acting from the long-acting formulation, or who can read the words on a nutrition label but don’t know how to interpret the amount of carbohydrates and determine if a food fits the diet plan.
Moreover, while health literacy is correlated with cognitive ability, it still is a distinct skill set. Studies have shown that patients educated in a traditional sense – college-educated professionals, for instance – will not necessarily understand health-related words and instructions.
Research similarly suggests that a low-health-literacy approach that uses focused, simple, and straightforward messages benefits everyone. This type of approach involves the following:
Simple language
Teach-back techniques (“tell me you what your understanding is of what I just told you”)
Diagrams, handouts, and brochures written at a sixth-grade level.
Teaching that is limited to five to eight key messages per session, and reinforcement of these messages over time.
Promote self-efficacy
Self-efficacy is the confidence in one’s ability to perform certain health behaviors. It involves motivation as well as knowledge of the disease, the rationale for treatment, and the specific behaviors that are required for effective self-care.
Help patients understand “why it matters” – that diabetes raises the risk of macrosomia, shoulder dystocia, hypertension, long-term diabetes, and other adverse maternal and neonatal outcomes. Explain basic physiologic concepts and provide background information. This builds self-efficacy.
Do not issue recommendations for exercising and eating well without asking: How can I help you do this? What do you need to be able to eat healthy? Do you need an appointment with a nutritionist? Do you need to see a social worker?
Inquire about and help patients identify supportive family members or other “champions.” Look for ways to incorporate these support people into the patient’s care. At a minimum, encourage the patient to ask her support person to eat healthy with her and/or to understand her daily tasks so that this individual can offer reminders and be a source of support when she feels exhausted or overwhelmed.
If possible, facilitate some type of “diabetes buddy” program to offer peer support and help patients stay engaged in their care, or use group education sessions.
Piggyback on your patients’ own motivating factors. Research has shown that women are extraordinarily motivated to stop smoking during pregnancy because of the health of the fetus. This should extend as well to the difficult lifestyle changes required for diabetes self-care.
View pregnancy as a “golden opportunity” to promote healthy life changes that endure because of the often-extraordinary levels of motivation that women feel or can be encouraged to feel.
Facilitate access
The ability to attend frequent appointments and to juggle the logistics of transportation, child care, and time off work (all part of the burden of disease management) is a social determinant of health. It’s something we should ask about, and it is often something we can positively impact by modifying our practice hours and/or using telehealth or mobile health techniques.
Coordinating newborn and pediatric care with the mother’s subsequent primary care is optimal. Women often prioritize their babies’ health over their own health and they rarely miss pediatric appointments. Coordinating care through medical homes or other mechanisms may help women remain engaged and may lessen the gaps between obstetrical and subsequent primary care.
For me, facilitating doctor-to-doctor transitions sometimes entails picking up the phone or sending communication to a primary care doctor to say, for instance, “I’m worried about my patient’s lifetime risk of type 2 diabetes, and I’d like to hand off her care to you.” This is one of many small but meaningful steps we can take.
Dr. Yee is an assistant professor in the division of maternal-fetal medicine at Northwestern University, Chicago. She reported having no relevant financial disclosures.
More than many other pregnancy complications, diabetes exemplifies the impact of social determinants of health.
The medical management of diabetes during pregnancy involves major lifestyle changes. Diabetes care is largely a patient-driven social experience involving complex and demanding self-care behaviors and tasks.
The pregnant woman with diabetes is placed on a diet that is often novel to her and may be in conflict with the eating patterns of her family. She is advised to exercise, read nutrition labels, and purchase and cook healthy food. She often has to pick up prescriptions, check finger sticks and log results, accurately draw up insulin, and manage strict schedules.
Management requires a tremendous amount of daily engagement during a period of time that, in and of itself, is cognitively demanding.
Outcomes, in turn, are impacted by social context and social factors – by the patient’s economic stability and the safety and characteristics of her neighborhood, for instance, as well as her work schedule, her social support, and her level of health literacy. Each of these factors can influence behaviors and decision making, and ultimately glycemic control and perinatal outcomes.
The social determinants of diabetes-related health are so individualized and impactful that they must be realized and addressed throughout our care, from the way in which we communicate at the initial prenatal checkup to the support we offer for self-management.
Barriers to diabetes self-care
While the incidence of type 2 diabetes is increasing among all social, ethnic, and racial groups, its prevalence among nonpregnant U.S. adults is greatest among racial/ethnic minorities, as well as in individuals with a low-income status. Women who enter pregnancy with preexisting diabetes are more likely to be racial/ethnic minorities.

In pregnancy, minority women (especially Hispanic, but also Asian and non-Hispanic black women), and women with low-income status are similarly predisposed to developing gestational diabetes mellitus (GDM).
Social determinants of health are interwoven with inequities stemming from race/ethnicity, income, and other factors that affect outcomes. For example, not only do non-Hispanic black women experience a greater incidence of GDM than non-Hispanic white women, but when they have GDM, they also appear to experience worse pregnancy outcomes compared with white women who also have GDM. In addition, they have a greater likelihood of developing type 2 diabetes after a pregnancy with GDM.
I care for a population that consists largely of minority, low-income women with either gestational or pregestational diabetes. Despite their best intentions and efforts – and despite seemingly high motivation levels – these women struggle to achieve the levels of glycemic control necessary for preventing maternal and fetal complications.
Several years ago, I sought to better understand the barriers to diabetes self-care and behavioral change these women face. Through a series of in-depth, semi-structured interviews with 10 English-speaking women (half with pregestational diabetes) over the course of their pregnancies, we found that the barriers to self-care related to the following: disease novelty, social and economic instability, nutrition challenges, psychological stressors, a failure of outcome expectations, and the burden of disease management (J Health Care Poor Underserved. 2015 Aug;26[3]:926-40; J Nutr Educ Behav. 2016 Mar;48[3]:170-80.e1).
Some of these barriers, such as the lack of any prior experience with diabetes (through a family member, for instance) or the inability to believe that behavior change and other treatment could impact her diabetes and her fetus’ health, echoed other limited published data. However, women in our study also appeared to be affected by barriers driven by social instability (e.g., a lack of partner or family support, family conflict, or neighborhood violence), inadequate access to healthy food, and the psychological impact of diabetes.
They often felt isolated and overwhelmed by their diabetes; the condition amplified stresses they were already experiencing and contributed to worsening mental health in those who already had depression or anxiety. In the other direction, women also described how preexisting mental health challenges affected their ability to sustain recommended behavior changes.
However, we also identified factors that empowered women in this community to succeed with their diabetes during pregnancy – these included having prior familiarity and diabetes self-efficacy, being motivated by the health of the fetus or older children, having a supportive social and physical environment, and having the ability to self-regulate or set and achieve goals (J Perinatol. 2016 Jan;36[1]:13-8).
To address these barriers, my group has undertaken a series of projects aimed at improving care for pregnant women with diabetes. We developed a diabetes-specific text message support system, for instance, and are now transitioning this support to an advanced mobile health tool that can help patients beyond our site.
What we can do
Much of what we can do in our practices to identify and address social determinants and alleviate barriers to effective diabetes management is about finding the “sweet spot” – about being able to convey the right information in the right amount, with the right timing and the right delivery.
While we can’t improve a woman’s neighborhood or resolve food instability, I believe that we can still work to improve outcomes for women who experience these problems. Here are some key strategies for optimal support of our patients:
Inquire about social factors
Identify hurdles by asking questions such as: Where do you live? Is it safe to walk in your neighborhood? If not, where’s your closest mall? What kind of job do you have, and does your employer allow breaks to take care of your health? How are things going at home? Who is at home to help you? Are you having any trouble affording food? How can we help you learn to adapt your personal or cultural food preferences to healthier options?
Look for small actions to take. I often write letters to my patients’ employers requesting that they be given short, frequent breaks to accommodate their care regimens. I also work to ensure that diet recommendations and medication/insulin regimens are customized for patients with irregular meal and sleep schedules, such as those working night shifts.
Employ a social worker if possible, especially if your practice cares for large numbers of underserved women.
Serve as a resource center, and engage your team in doing so. Be prepared to refer women for social services support, food banks, intimate partner violence support services, and other local resources.
Take a low-health-literacy approach
Health literacy is the ability to obtain and utilize health information. It has been widely investigated outside of pregnancy (and to some extent during pregnancy), and has been found to be at the root of many disparities in health care and health outcomes. Numeracy, a type of health literacy, is the ability to understand numbers, perform basic calculations, and use simple math skills in a way that helps one’s health.
The barriers created by inadequate health literacy are distinct from language barriers. I’ve had patients who can read the labels on their insulin vials but cannot distinguish the short-acting from the long-acting formulation, or who can read the words on a nutrition label but don’t know how to interpret the amount of carbohydrates and determine if a food fits the diet plan.
Moreover, while health literacy is correlated with cognitive ability, it still is a distinct skill set. Studies have shown that patients educated in a traditional sense – college-educated professionals, for instance – will not necessarily understand health-related words and instructions.
Research similarly suggests that a low-health-literacy approach that uses focused, simple, and straightforward messages benefits everyone. This type of approach involves the following:
Simple language
Teach-back techniques (“tell me you what your understanding is of what I just told you”)
Diagrams, handouts, and brochures written at a sixth-grade level.
Teaching that is limited to five to eight key messages per session, and reinforcement of these messages over time.
Promote self-efficacy
Self-efficacy is the confidence in one’s ability to perform certain health behaviors. It involves motivation as well as knowledge of the disease, the rationale for treatment, and the specific behaviors that are required for effective self-care.
Help patients understand “why it matters” – that diabetes raises the risk of macrosomia, shoulder dystocia, hypertension, long-term diabetes, and other adverse maternal and neonatal outcomes. Explain basic physiologic concepts and provide background information. This builds self-efficacy.
Do not issue recommendations for exercising and eating well without asking: How can I help you do this? What do you need to be able to eat healthy? Do you need an appointment with a nutritionist? Do you need to see a social worker?
Inquire about and help patients identify supportive family members or other “champions.” Look for ways to incorporate these support people into the patient’s care. At a minimum, encourage the patient to ask her support person to eat healthy with her and/or to understand her daily tasks so that this individual can offer reminders and be a source of support when she feels exhausted or overwhelmed.
If possible, facilitate some type of “diabetes buddy” program to offer peer support and help patients stay engaged in their care, or use group education sessions.
Piggyback on your patients’ own motivating factors. Research has shown that women are extraordinarily motivated to stop smoking during pregnancy because of the health of the fetus. This should extend as well to the difficult lifestyle changes required for diabetes self-care.
View pregnancy as a “golden opportunity” to promote healthy life changes that endure because of the often-extraordinary levels of motivation that women feel or can be encouraged to feel.
Facilitate access
The ability to attend frequent appointments and to juggle the logistics of transportation, child care, and time off work (all part of the burden of disease management) is a social determinant of health. It’s something we should ask about, and it is often something we can positively impact by modifying our practice hours and/or using telehealth or mobile health techniques.
Coordinating newborn and pediatric care with the mother’s subsequent primary care is optimal. Women often prioritize their babies’ health over their own health and they rarely miss pediatric appointments. Coordinating care through medical homes or other mechanisms may help women remain engaged and may lessen the gaps between obstetrical and subsequent primary care.
For me, facilitating doctor-to-doctor transitions sometimes entails picking up the phone or sending communication to a primary care doctor to say, for instance, “I’m worried about my patient’s lifetime risk of type 2 diabetes, and I’d like to hand off her care to you.” This is one of many small but meaningful steps we can take.
Dr. Yee is an assistant professor in the division of maternal-fetal medicine at Northwestern University, Chicago. She reported having no relevant financial disclosures.